
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 140
POLYCHLORINATED BIPHENYLS AND TERPHENYLS
(SECOND EDITION)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr S. Dobson, Institute of Terrestrial
Ecology, United Kingdom, and Dr G.J. van Esch, Bilthoven, The
Netherlands
World Health Organization
Geneva, 1993
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organization, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Polychlorinated Biphenyls and Terphenyls. -- 2nd ed.
(Environmental health criteria; 140)
1.Environmental exposure 2.Environmental pollutants 3.Polychlorinated
biphenyls -- adverse effects 4.Polychlorinated biphenyls -- toxicity
5.Polychloroterphenyl compounds -- adverse effects
6.Polychloroterphenyl compounds -- toxicity I.Series
ISBN 92 4 157140 3 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1993
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. SUMMARY AND EVALUATION, CONCLUSIONS, RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Introduction
1.1.2 Identity, physical, and chemical properties
1.1.3 Analytical methods
1.1.4 Production and uses
1.1.5 Environmental transport, distribution, and transformation
1.1.6 Environmental levels and human exposure
1.1.7 Kinetics and metabolism
1.1.8 Effects on organisms in the environment
1.1.8.1 Laboratory studies
1.1.8.2 Field studies
1.1.9 Effects on experimental animals and in vitro systems
1.1.9.1 Single exposure
1.1.9.2 Short-term exposure
1.1.10 Reproduction, embryotoxicity, and teratogenicity
1.1.11 Mutagenicity
1.1.12 Carcinogenicity
1.1.13 Special studies
1.1.14 Factors modifying toxicity, mode of action
1.1.15 Effects on humans
1.2 Conclusions
1.2.1 Distribution
1.2.2 Effects on experimental animals
1.2.3 Effects on humans
1.2.4 Effects on the environment
1.3 Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Chemical formula and structure
2.1.2 Relative molecular mass
2.1.3 Common name
2.1.4 Chemical composition
2.1.5 Technical product
2.1.6 Purity and impurities
2.2 Physical and chemical properties
2.2.1 Log n-octanol/water partition coefficient
2.2.2 Conversion factors
2.3 Analytical methods
2.3.1 Sampling strategy and sampling methods
2.3.1.1 Extraction procedures
2.3.1.2 Sample clean-up
2.3.2 Separation and identification
2.3.2.1 Chromatographic separation
2.3.2.2 Gas-liquid chromatography
2.3.3 Quantification
2.3.4 Accuracy of PCB determinations
2.3.5 Confirmation
2.3.6 Detection limits
2.4 Codex questionnaire on analytical methods
2.4.1 Interpretation and comparability of data
2.5 Activities of the WHO Regional Office for Europe
2.6 Appraisal
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.2.1 Production levels and processes, uses
3.2.1.1 World production figures
3.2.1.2 Manufacturing processes
3.2.2 Uses
3.2.2.1 Completely closed systems
3.2.2.2 Nominally closed systems
3.2.2.3 Open-ended applications
3.2.2.4 Contamination of other compounds
3.2.3 Loss into the environment
3.2.3.1 Routes of environmental pollution
3.2.3.2 Release of PCBs into the atmosphere
3.2.3.3 Leakage and disposal of PCBs in industry
3.2.4 Thermal decomposition of PCBs
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Transport in air
4.1.1.1 Dry deposition
4.1.1.2 Precipitation deposition
4.1.2 Transport in soil
4.1.3 Transport in water
4.1.4 Transport between media
4.2 Biotransformation
4.2.1 Biodegradation
4.2.1.1 Bacteria
4.2.2 Biodegradation; individual congeners
4.2.2.1 Bacteria
4.2.2.2 Fungi
4.2.3 Photodegradation
4.2.4 Bioaccumulation, distribution in organisms, and elimination
4.2.4.1 Microorganisms
4.2.4.2 Plants
4.2.4.3 Aquatic invertebrates
4.2.4.4 Fish
4.2.4.5 Birds
4.2.4.6 Mammals
4.2.5 Appraisal
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Levels in the environment
5.1.1 Air
5.1.1.1 Rain and snow
5.1.1.2 Natural gas
5.1.2 Water
5.1.3 Soil
5.1.4 Aquatic and terrestrial organisms
5.1.4.1 Effect of dredging-contaminated sediment on organisms
5.1.4.2 Relationship to lipid content of organisms
5.1.4.3 Residues in different trophic levels and effects of diets
5.1.4.4 Effects of age, sex, and reproductive status on uptake and elimination
5.1.4.5 Time trends in residues
5.1.4.6 Seasonal patterns in residues
5.1.5 Appraisal
5.2 Levels in animal feed
5.3 Levels in human food
5.3.1 General
5.3.2 Drinking-water
5.3.3 Dairy products
5.3.4 Fish and shellfish
5.3.5 Influence of food processing
5.3.6 Food contamination by packaging materials
5.3.7 Appraisal
5.4 General population exposure
5.4.1 Air
5.4.2 Drinking-water
5.4.3 Intake by infants through mother's milk
5.4.4 Infant and toddler total diet
5.4.5 Total intake by adults via food
5.4.6 Total diet/market-basket studies
5.4.7 Total intake of major congeners by adults via food
5.4.8 Time trends in different matrices
5.5 Concentrations in the body tissues of the general population
5.5.1 Adipose tissue
5.5.1.1 PCBs in the fetus
5.5.1.2 Congeners in adipose tissue
5.5.2 Blood of the general population
5.5.3 Human milk
5.5.3.1 Major PCB congeners in human milk
5.5.3.2 Factors that influence the intake of PCBs with milk
5.5.4 Other tissues
5.6 Accidental exposures (Yusho and Yu-Cheng)
5.7 Occupational exposure
5.7.1 Accidental exposure
5.7.2 Occupational exposure during manufacture and use
5.7.2.1 Adipose tissue
5.7.2.2 Blood
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Inhalation
6.1.2 Dermal
6.1.3 Oral
6.2 Distribution
6.2.1 Inhalation (rat)
6.2.2 Oral (rat)
6.2.3 Oral (monkey)
6.2.4 Oral (humans)
6.2.5 Individual congeners of PCBs
6.2.6 Appraisal
6.3 Placental transport
6.3.1 Laboratory animals
6.3.2 Wildlife
6.3.3 Humans
6.4 Excretion and elimination
6.4.1 Following oral dosing
6.4.2 Following parenteral dosing
6.4.3 Humans
6.4.4 Elimination via milk (animals)
6.4.4.1 Elimination via breast milk
6.5 Metabolic transformation
6.5.1 PCBs
6.5.2 Dichlorobiphenyls
6.5.3 Tetrachlorobiphenyls
6.5.4 Hexachlorobiphenyls and higher chlorinated compounds
6.5.5 Retention and turnover
6.5.6 Appraisal
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Toxicity for microorganisms
7.1.1 Freshwater microorganisms
7.1.2 Marine and estuarine microorganisms
7.1.3 Soil microorganisms
7.1.4 Plankton communities
7.1.5 Interactions with other chemicals
7.1.6 Tolerance
7.2 Toxicity for aquatic organisms
7.2.1 Aquatic plants
7.2.2 Aquatic invertebrates
7.2.2.1 Short- and long-term toxicity
7.2.2.2 Response to temperature and salinity
7.2.2.3 Reproduction
7.2.2.4 Moulting
7.2.2.5 Behaviour
7.2.2.6 Population structure
7.2.2.7 Interactions with other chemicals
7.2.3 Fish
7.2.3.1 Short- and long-term toxicity
7.2.3.2 Carcinogenicity
7.2.3.3 Effects on developmental stages and reproduction
7.2.3.4 Physiological and biochemical effects
7.2.3.5 Behavioural effects
7.2.3.6 Interactions with other chemicals
7.2.4 Amphibians
7.2.5 Aquatic mammals
7.3 Toxicity for terrestrial organisms
7.3.1 Plants
7.3.2 Terrestrial invertebrates
7.3.3 Birds
7.3.3.1 Short-term toxicity
7.3.3.2 Egg production
7.3.3.3 Hatchability and embryotoxicity
7.3.3.4 Eggshell thinning
7.3.3.5 Effects on the male
7.3.3.6 The effects of stress
7.3.3.7 Physiological, biochemical, and behavioural effects
7.3.3.8 Interactive effects with other chemicals
7.3.4 Terrestrial mammals
7.3.4.1 Short-term toxicity
7.3.4.2 Reproductive effects
7.3.4.3 Physiological effects
7.4 Effects on organisms in the field
7.4.1 Plants
7.4.2 Fish
7.4.3 Birds
7.4.4 Mammals
7.4.4.1 Appraisal
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.1.1 Oral
8.1.2 Inhalation
8.1.3 Dermal
8.1.4 Other routes
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Aroclors
8.2.1.2 Individual congeners
8.2.2 Intraperitoneal: reconstituted PCB mixtures
8.2.3 Dermal exposure
8.2.4 Appraisal
8.3 Skin and eye irritation, sensitization
8.4 Reproduction, embryotoxicity, and teratogenicity
8.4.1 Reproduction and embryotoxicity
8.4.1.1 Oral
8.4.2 Teratogenicity
8.4.2.1 Aroclors (oral)
8.4.2.2 Aroclors (subcutaneous)
8.4.2.3 Individual congeners (oral)
8.4.3 Appraisal
8.4.4 Mutagenicity and related end-points
8.4.4.1 DNA damage
8.4.4.2 Mutagenicity tests
8.4.4.3 Cell transformation
8.4.4.4 Cell to cell communication
8.4.4.5 Interaction
8.4.4.6 Cell division parameters
8.5 Carcinogenicity
8.5.1 Long-term toxicity/carcinogenicity
8.5.2 Tumour promotion/anticarcinogenic effects
8.5.3 Initiation, promotion, and other special studies on individual congeners
8.5.4 Skin carcinogenicity
8.5.5 Appraisal
8.6 Special studies: target-organ effects
8.6.1 Liver
8.6.1.1 PCB mixtures
8.6.1.2 Individual congeners
8.6.2 Enzyme induction
8.6.2.1 Effects on liver enzymes of PCBs
8.6.2.2 Effects on liver enzymes of "biologically filtered" PCB mixtures
8.6.2.3 Effects of individual congeners on liver enzymes
8.6.2.4 Appraisal
8.6.3 Effects on vitamins and mineral metabolism
8.6.3.1 Effects of PCB mixtures
8.6.3.2 Effects of individual congeners
8.6.4 Effects on the gastrointestinal tract
8.6.5 Effects on lipid metabolism
8.6.5.1 Effects of PCB mixtures
8.6.5.2 Effects of individual congeners
8.6.6 Effects on porphyrin metabolism
8.6.6.1 Effects of PCB mixtures
8.6.6.2 Effects of individual congeners
8.6.7 Effects on the endocrine system
8.6.7.1 Effects of PCB mixtures
8.6.7.2 Effects of individual congeners
8.6.8 Immunotoxicity
8.6.8.1 Effects of PCB mixtures
8.6.8.2 Effects of individual congeners
8.6.8.3 Appraisal
8.6.9 Neurotoxic effects
8.6.10 Skin effects
8.6.11 Effects on the lung
8.6.12 Miscellaneous
8.7 Factors modifying toxicity; mode of action
8.7.1 Factors modifying toxicity
8.7.2 Mechanisms of toxicity
8.7.3 Toxicity of impurities in commercial PCBs
9. EFFECTS ON HUMANS
9.1 General population exposure
9.1.1 Acute effects - poisoning incidents
9.1.2 Effects of short- and long-term exposure
9.1.2.1 Yusho and Yu-Cheng accidents
9.1.2.2 Effects of PCBs on babies and infants
9.1.3 Appraisal
9.2 Occupational exposure
9.2.1 Acute toxicity - poisoning incidents
9.2.1.1 Acute dermal effects
9.2.2 Effects of short- and long-term exposure
9.2.3 Appraisal
9.2.4 Special studies (target organ effects)
9.2.4.1 Effects on the liver
9.2.4.2 Immunotoxicity
9.2.4.3 Effects on the respiratory system
9.2.4.4 Neurotoxicity
9.2.4.5 Blood pressure
9.2.5 Mortality studies
9.2.6 Appraisal
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
POLYCHLORINATED TERPHENYLS
1. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
1.1 Identity
1.2 Physical and chemical properties
1.3 Analytical methods
2. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 Residues in the environment
4.2 Residues in food
4.3 Concentrations in adipose tissue
4.4 Concentrations in blood
5. KINETICS AND METABOLISM
5.1 Absorption
5.2 Distribution
5.3 Biotransformation
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Marine and estuarine organisms
6.2 Terrestrial invertebrates
6.3 Birds
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single oral exposures
7.2 Short-term oral exposures
7.2.1 Rat
7.2.2 Monkey
7.3 Teratogenicity
7.4 Carcinogenicity
7.5 Miscellaneous effects
REFERENCES
ANNEX 1
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR POLYCHLORINATED
BIPHENYLS (PCBs) AND POLYCHLORINATED TERPHENYLS (PCTs)
Members
Dr L.A. Albert, Consultores Ambientales Asociados, Xalapa, Veracruz,
Mexico
Professor U.G. Ahlborg, Institute of Environmental Medicine,
Karolinska Institute, Stockholm, Sweden
Dr V. Benes, Department of Toxicology and Reference Laboratory,
Institute of Hygiene and Epidemiology, Prague, Czechoslovakia
(Vice-Chairman)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, United Kingdom
(Chairman)
Dr Yuzo Hayashi, Division of Pathology, National Institute of Hygienic
Sciences, Tokyo, Japan
Dr T. Lakhanisky, Division of Toxicology, Institute of Hygiene and
Epidemiology, Brussels, Belgium
Dr J. McKinney, US Environmental Protection Agency, Research Triangle
Park, North Carolina, USA
Dr Pang Ying Fa, Chinese Academy of Preventive Medicine, Beijing,
China
Dr T. Vermeire, National Institute of Public Health and Environmental
Protection, Bilthoven, Netherlands (Co-Rapporteur)
Dr E. Yrjänheikki, Regional Institute of Occupational Health, Oulu,
Finland
Observers
Dr M. Martens (Representative from ECETOC), Monsanto Services
International, Brussels, Belgium
Mrs H. B. Sundmark (Representative from ECETOC), Norsk Hydro a.s.
Porsgrunn, Research Centre, Porsgrunn, Norway
Secretariat:
Dr G.J. van Esch, Bilthoven, Netherlands (Co-Rapporteur and
Secretary)
Dr M. Kogevinas, Unit of Analytical Epidemiology, International Agency
for Research on Cancer (IARC), Lyon, France
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda, which
will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone no. 7988400/7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR PCBs AND PCTs
A WHO Task Group on Environmental Health Criteria for PCBs and PCTs
met in Brussels from 28 May to 1 June 1990. The meeting was convened
in the Institute of Hygiene and Epidemiology in Brussels and sponsored
by the Belgian Ministry of Health. Mrs A.-M. Sacré-Bestin of the
Ministry opened the meeting and welcomed the participants on behalf of
the host country. Dr G.J. van Esch welcomed the participants on behalf
of the Heads of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Group reviewed and revised the draft Environmental
Health Criteria monograph and the companion Health and Safety Guide
and made an evaluation of the risks for human health and the
environment from exposure to PCBs and PCTs.
The first draft of the EHC monograph was prepared by Dr S. Dobson
(environmental aspects) and Dr G.J. van Esch (other sections) and was
based on contributions from several authors and countries. It was
prepared in close cooperation with the WHO Regional Office for Europe,
in Copenhagen.
The second draft was prepared by Dr G.J. van Esch, incorporating
comments received following the circulation of the first draft to the
IPCS contact points for Environmental Health Criteria monographs.
Dr K. Jager, Central Unit, IPCS, was responsible for the scientific
content of the final monograph and Mrs M.O. Head, Oxford, for the
editing.
The efforts of all who helped in the preparation and finalization of
the documents are gratefully acknowledged.
INTRODUCTION
The commercial production of the polychlorinated biphenyls (PCBs)
began in 1930, and, during the 1930s, cases of poisoning were reported
among men engaged in their manufacture. The nature of this
occupational disease was characterized by a skin affection with
acneiform eruptions; occasionally the liver was involved, in some
cases with fatal consequences. Subsequent safety precautions appear
largely to have prevented further outbreaks of this disease in
connection with the manufacture of PCBs, but, since 1953, cases have
been reported in Japanese factories manufacturing condensers.
The distribution of PCBs in the environment was not recognized until
Jensen started an investigation in 1964 to ascertain the origins of
unknown peaks, observed during the gas-liquid chromatographic
separation of organochlorine pesticides from wildlife samples. In
1966, he and his colleagues succeeded in attributing these to the
presence of PCBs. Since then, investigations in many parts of the
world have revealed the widespread distribution of PCBs in
environmental samples.
The serious outbreaks of poisoning in humans and in domestic animals
from the ingestion of food, accidentally contaminated with PCBs, have
stimulated investigations into the toxic effects of PCBs on animals
and on nutritional food chains. This has resulted in the limitation of
the commercial exploitation of PCBs and polychlorinated terphenyls
(PCTs), and in regulations to limit the residues in human and animal
food.
In recent years, many industrial nations have taken steps to control
the flow of PCBs into the environment. PCBs and PCB-containing
formulations are restricted (an exception is sometimes made for mono-
and dichloro-PCBs) for most uses. Now they are almost entirely
restricted to use in closed systems, such as isolating oils in
transformers, capacitors, and other electrical systems, and as a heat
transfer medium and hydraulic liquid. The most influential forces
leading to these restrictions have probably been the 1973 and 1987
decision-recommendations from the Organisation for Economic
Co-operation and Development (OECD).
The environmental impact of the PCBs and PCTs has been discussed at a
number of regional and international meetings and has been the subject
of several reviews, including: ATSDR (1989), DFG (1988), IARC (1978),
IRPTC (1988), Kimbrough (1987), Lorenz & Neumeier (1983a,b), NIOSH
(1987), NTIS (1972), OECD (1982), Slorach & Vaz (1983), WHO (1985a,b,
1986a,b) & WHO/EUR (1987).
In 1976, the World Health Organization published Environmental Health
Criteria 2: Polychlorinated biphenyls (PCBs) and terphenyls (PCTs)
(WHO, 1976), discussing and evaluating the data then available on
exposure levels and the effects of PCBs and PCTs on human beings, and,
to a lesser extent, on the environment.
Since then, a wealth of new information has become available.
The IPCS decided to update the above-mentioned EHC and also to produce
a Health and Safety Guide (HSG) and to do this in close coordination
with the WHO Regional Office for Europe, which prepared "PCBs, PCDDs
and PCDFs, prevention and control of accidental and environmental
exposures" as No. 23 of their Environmental Health Series (WHO/EURO,
1987). This publication includes a set of guidelines to assist Member
States in the development of strategies to reduce the probability of
accidents involving the environmental release of PCBs, PCDDS, and
PCDFs and also the severity of their hazardous effects, should such
accidents occur. In particular, it is intended to guide occupational
safety and health personnel and other staff, in workplaces and
environments where PCBs and/or PCB-containing equipment are in use, to
develop adequate safety measures, contingency planning, effective and
relevant accident response, and appropriate rehabilitation.
Within the scope of the present EHC on PCBs and PCTs, the PCDDs and
PCDFs have been mentioned where relevant. Full discussion of these
compounds and evaluation, however, can be found in the IPCS EHC 88:
Polychlorinated dibenzo- para-dioxins and dibenzofurans (WHO, 1989).
1. SUMMARY AND EVALUATION, CONCLUSIONS, RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Introduction
Polychlorinated biphenyls (PCBs) were discovered before the turn of
the century and their usefulness for industry, because of their
physical properties, was recognized early. The PCBs have been used
commercially, since 1930, as dielectric and heat-exchange fluids and
in a variety of other applications. They have become widely
distributed in the environment throughout the world, and are
persistent and accumulate in food webs. Human exposure to PCBs has
resulted largely from the consumption of contaminated food, but also
from inhalation and skin absorption in work environments. PCBs
accumulate in the fatty tissues of humans and other animals and have
caused toxic effects in both, particularly if repeated exposure
occurs. The skin and liver are the major sites of pathology, but the
gastrointestinal tract, the immune system, and the nervous system are
also targets. Polychlorinated dibenzofurans (PCDFs), which are
contaminants in commercial PCB mixtures, contribute significantly to
their toxicity. The results of studies on rodents suggest that some
PCB congeners may be carcinogenic and that they can promote the
carcinogenicity of other chemicals.
It is clear from available data on polychlorinated biphenyls (PCBs)
and polychlorinated terphenyls (PCTs) that, in an ideal situation, it
would be preferable not to have these compounds in food at any level.
However, it is equally clear that the reduction of PCBs or PCTs
exposure from food sources to "zero" or to a level approaching zero,
would mean the elimination (prohibition of the consumption) of large
amounts of important food items, such as fish, but more importantly
breast milk. National and international scientific committees have to
decide where the proper balance lies between providing an adequate
degree of public health protection and avoiding excessive losses of
food.
No levels of PCBs or PCTs exposure that can provide an absolute
assurance of safety can be identified on the basis of the available
data.
1.1.2 Identity, physical, and chemical properties
PCBs are mixtures of aromatic chemicals, manufactured by the
chlorination of biphenyl in the presence of a suitable catalyst. The
chemical formula of PCBs can be presented as C12H10-nCln, where n is
a number of chlorine atoms within the range of 1-10.
Theoretically, 209 congeners are possible, but only about 130
congeners are likely to occur in commercial products. In addition,
PCBs may contain polychlorinated dibenzofurans (PCDFs) and chlorinated
quarterphenyls as impurities. These impurities are relatively stable
and resistant to chemical reactions, under normal conditions. All
congeners of PCBs are lipophilic and have a very low water solubility.
As a result, they easily enter the food chain and accumulate in fatty
tissues.
Commercial PCB mixtures contain PCDFs at levels ranging from a few
mg/kg up to 40 mg/kg. Polychlorinated dibenzo- p-dioxins (PCDDs), are
not found in commercial PCBs. However, when PCBs are mixed with other
chlorinated compounds, such as the chloro-benzenes used in
transformers, PCDDs can be found in the case of accidental fires and
during incineration.
Commercial PCB mixtures are light yellow or dark yellow in colour.
They do not crystallize, even at low temperatures, but turn into solid
resins. PCBs are, in practice, fire resistant, with rather high flash
points. They form vapours heavier than air, but they do not form any
explosive mixtures with air. They have very low electrical
conductivity, rather high thermal conductivity, and extremely high
resistance to thermal break-down. PCBs are chemically very stable
under normal conditions; however, when heated, other toxic compounds,
such as PCDFs, can be produced.
1.1.3 Analytical methods
In 1966, the discovery of PCBs in environmental samples raised
interest in the analysis of these compounds and their toxicity for
human beings and their environment.
Because of differences in the analytical methodology used, existing
data are not directly comparable; nevertheless, they can be used for
the establishment of control and preventive measures and for the
preliminary assessment of health and environmental risks associated
with these chemicals.
PCBs have been determined using gas chromatography (GC) techniques
with electron capture detection, often using packed columns, though
more sophisticated methods, such as capillary column and GC coupled
with mass-spectrometry (GC-MS), have been used in recent studies to
identify the individual congeners, to improve the comparability of the
analytical data from different sources, and to establish a basis for
toxicity assessment.
An extensive quality assurance programme is required for these
analyses and intercalibration studies have been implemented and
recommended. The quality and utility of the analytical data depend
critically on the validity of the sample and the adequacy of the
sampling. Furthermore, it is essential to have a planned and well
documented sampling programme; a detailed sampling procedure is
described in WHO/EURO (1987).
1.1.4 Production and uses
The commercial production of the PCBs began in 1930. They have been
widely used in electrical equipment, and smaller volumes of PCBs are
used as fire-resistant liquid in nominally closed systems.
By the end of 1980, the total world production of PCBs was in excess
of 1 million tonnes and, since then, production has continued in some
countries. Despite increasing withdrawal of the use, and restrictions
on the production, of PCBs, very large amounts of these compounds
continue to be present in the environment, either in use or as waste.
In recent years, many industrialized countries have taken steps to
control and restrict the flow of PCBs into the environment. The most
influential force leading to these restrictions has probably been a
1973 recommendation from the Organisation for Economic Co-operation
and Development (OECD) (WHO, 1976; IARC, 1978; OECD, 1982). Since
then, the 24 OECD member countries have restricted the manufacture,
sales, importation, exportation, and use of PCBs, as well as
establishing a labelling system for these compounds.
Current sources of PCB release include volatilization from landfills
containing transformer, capacitor, and other PCB-wastes, sewage
sludge, spills, and dredge spoils, and improper (or illegal) disposal
to open areas. Pollution may occur during the incineration of
industrial and municipal waste. Most municipal incinerators are not
effective in destroying PCBs. Explosions or overheating of
transformers and capacitors may release significant amounts of PCBs
into the local environment.
PCBs can be converted to PCDFs under pyrolytic conditions. The highest
yield of PCDFs under laboratory conditions was obtained at a
temperature between 550 and 700°C. Thus, the uncontrolled burning of
PCBs can be an important source of hazardous PCDFs. It is therefore
recommended that destruction of PCB-contaminated waste should be
carefully controlled, especially with regard to the burning
temperature (above 1000°C), residence time, and turbulence.
1.1.5 Environmental transport, distribution, and transformation
In the atmosphere, PCBs exist primarily in the vapour phase; the
tendency to adsorb on particulates increases with the degree of
chlorination. The virtually universal distribution of PCBs suggests
transport in air.
At present, the major source of PCB exposure in the general
environment appears to be the redistribution of PCBs, previously
introduced into the environment. This redistribution involves
volatilization from soil and water into the atmosphere with subsequent
transport in air and removal from the atmosphere via wet/dry
deposition (of PCBs bound to particulates) and then re-volatilization.
Concentrations of PCBs in precipitation range from 0.001 to
0.25 µg/litre. Since the volatilization and degradation rates of PCBs
vary between congeners, this redistribution leads to an alteration in
the composition of PCB mixtures in the environment.
In water, PCBs are adsorbed on sediments and other organic matter;
experimental and monitoring data have shown that PCB concentrations in
sediment and suspended matter are higher than those in associated
water columns. Strong adsorption on sediment, especially in the case
of the higher chlorinated PCBs, decreases the rate of volatilization.
On the basis of their water solubilities and n-octanol-water
partition coefficients, the lower chlorinated PCB congeners will sorb
less strongly than the higher chlorinated isomers. Although adsorption
can immobilize PCBs for relatively long periods in the aquatic
environment, desorption into the water column has been shown to occur
by both abiotic and biotic routes. The substantial quantities of PCBs
in aquatic sediments can therefore act as both an environmental sink
and a reservoir of PCBs for organisms. Most of the environmental load
of PCBs has been estimated to be in aquatic sediment.
The low solubility and the strong adsorption of PCBs on soil particles
limits leaching in soil; lower chlorinated PCBs will tend to leach
more than the highly chlorinated PCBs.
Degradation of PCBs in the environment is dependent on the degree of
chlorination of the biphenyl. In general, persistence of PCB congeners
increases as the degree of chlorination increases. In the atmosphere,
the vapour phase reaction of PCBs with hydroxyl radicals (which are
photochemically formed by sunlight) may be the dominant transformation
process. Estimated half-lives for this reaction in the atmosphere
range from about 10 days for a monochlorobiphenyl to 1.5 years for a
heptachlorobiphenyl.
In the aquatic environment, hydrolysis and oxidation do not
significantly degrade PCBs. Photolysis appears to be the only viable
abiotic degradation process in water; however, available experimental
data are not sufficient to determine its rate or importance in the
environment.
Microorganisms degrade mono-, di-, and trichlorinated biphenyls
relatively rapidly and tetrachlorobiphenyls slowly, whilst higher
chlorinated biphenyls are resistant to biodegradation. Chlorine
substitution positions on the biphenyl ring appear to be important in
determining the biodegradation rate. PCBs containing chlorine atoms in
the para positions are preferentially biodegraded. Higher
chlorinated congeners are biotransformed anaerobically, by a reductive
dechlorination, to lower chlorinated PCBs, which may then be
biodegradable by aerobic processes.
Several factors determine the degree of bioaccumulation in adipose
tissues: the duration and level of exposure, the chemical structure of
the compound, and the position and pattern of substitution. In
general, the higher chlorinated congeners are accumulated more
readily.
Experimentally determined bioconcentration factors of various PCBs in
aquatic species (fish, shrimp, oyster) range from 200 up to 70 000 or
more. In the open ocean, there is bioaccumulation of PCBs in higher
trophic levels with an increased proportion of higher chlorinated
biphenyls in higher ranking predators.
Transfer of PCBs from soil to vegetation takes place mainly by
adsorption on the external surfaces of terrestrial plants; little
translocation takes place.
1.1.6 Environmental levels and human exposure
Because of their high persistence, and their other physical and
chemical properties, PCBs are present in the environment all over the
world.
Globally, PCBs are found in air concentrations of 0.002 up to
15 ng/m3. In industrial areas, levels are higher (up to µg/m3). In
rain water and snow, PCBs are found in the range of nd (1 ng)-
250 ng/litre.
Under occupational conditions, the levels in the air may be much
higher. Under certain conditions, for instance, in the manufacturing
of transformers or capacitors, levels of up to 1000 µg/m3 have been
observed. In acute emergencies, concentrations of up to 16 mg/m3 have
been measured. In case of fires and/or explosions, soot may be
produced that contains high levels of PCBs. Levels of 8000 mg PCBs/kg
soot have been found. In the latter situation, PCDFs will also be
present. Polychlorinated dioxins (PCDDs) will be found in accidents
with transformers containing chlorinated benzenes, as well as PCBs.
In these emergency situations, ingestion, skin contamination, or
inhalation of soot particles may occur and result in serious exposure
of personnel. However, the exposure of the general population via air
will be very low.
Surface water may be contaminated by PCBs from atmospheric fallout,
from direct emissions from point sources, or from waste disposal.
Under certain conditions, levels of up to 100-500 ng/litre water have
been measured. In the oceans, levels of 0.05-0.6 ng/litre have been
found.
In non-contaminated areas, drinking-water contains less than 1 ng
PCBs/litre, but levels of up to 5 ng/litre have been reported. Soil
and sediments in different areas and depending on local conditions,
contain levels of PCBs ranging from <0.01 up to 2.0 mg/kg. In
polluted areas, the levels have been much higher, i.e., up to
500 mg/kg.
In past years, many thousands of samples of different foodstuffs have
been analysed in several countries for contaminants including PCBs.
Most samples have been taken from individual food items, especially
fish and other foods of animal origin, such as meat and milk. Human
food has become contaminated with PCBs by 3 main routes:
(a) uptake from the environment by fish, birds, livestock (via
food-chains), and crops;
(b) migration from packaging materials into food (mainly below
1 mg/kg, but, in some cases, up to 10 mg/kg);
(c) direct contamination of food or animal feed by an industrial
accident.
The levels for the most important PCB-containing food items were:
animal fat, 20-240 µg/kg; cow's milk, 5-200 µg/kg; butter,
30-80 µg/kg; fish, 10-500 µg/kg, on a fat basis. Certain fish species
(eel) or fish products (fish liver and fish oils) contain much higher
levels, up to 10 mg/kg. Vegetables, cereals, fruits, and a number of
other products contained levels of <10 µg/kg. The major foods in
which contamination with PCBs needs consideration are fish, shellfish,
meat, milk, and other dairy products. Median levels in fish, reported
in various countries, are of the order of 100 µg/kg (on a fat basis).
When comparisons have been made, it appears that the levels of PCBs in
fish are slowly decreasing.
PCBs concentrate in human adipose tissue and breast milk. The
concentrations of PCBs in the different organs and tissues depend on
their lipid contents, with the exception of the brain. PCB residues in
the adipose tissue of the general population in industrialized
countries range from less than 1 up to 5 mg/kg, on a fat basis.
The average concentrations of total PCBs in human milk fat are in the
range of 0.5-1.5 mg/kg fat, depending on the donor's residence,
life-style, and the analytical methods used. Women who live in
heavily-industrialized, urban areas, or who consume a lot of fish,
especially from heavily-contaminated waters, may have higher PCB
concentrations in their breast milk.
The composition of most PCB extracts from environmental samples does
not resemble that of the commercial PCB mixtures. It has also been
shown, using high-resolution gas chromatography analysis, that the
congener composition and the relative concentrations of the individual
components in adipose tissues and breast milk differ markedly from
those in the commercial PCBs. The GC patterns of PCBs in human adipose
tissue and breast milk contain relatively high concentrations of
mainly the higher chlorinated PCBs, such as: 2,4,5,3',4'-pentachloro
biphenyl; 2,4,5,2',4',5'-hexachlorobiphenyl, and 2,3,4,2',4',5'-
hexachlorobiphenyl; 2,3,4,5,2',4',5'-hepta- and 2,3,4,5,2',3',4'-
heptachlorobiphenyl. A few other PCB congeners are present in
much lower quantities, such as the most toxic, coplanar PCBs:
3,4,3',4'-tetra-, 3,4,5,3',4'-penta-, and 3,4,5,3',4',5'-
hexachlorobiphenyl.
It has been calculated that the daily intake of PCBs by infants from
breast milk, is of the order of 4.2 µg/kg body weight (5.2 µg/100 Kcal
consumed) (WHO/EURO, 1988). The average total of ingested PCBs from
breast milk, during the first 6 months of life, is 4.5 mg compared
with the calculated intake of 357 mg of PCBs over the subsequent
life-time (0.2 µg/kg per day from the diet of a 70-kg person over a
70-year life-time). Therefore, the nursing period contributes about
1.3% of the life-time intake, which is not large, in the light of the
benefits of breast-feeding (WHO/EURO, 1988).
On the basis of the evaluated background data, for adults the average
dietary intake of PCBs amounts to a maximum of 100 µg per week, or
approximately 14 µg/person per day. For a 70-kg person, this is an
intake equivalent to a maximum of 0.2 µg/kg body weight per day
(WHO/EURO, 1988).
1.1.7 Kinetics and metabolism
Animal studies have been reported involving mainly oral, inhalation,
and dermal exposures to both PCB mixtures and individual congeners. In
general, PCBs appear to be rapidly absorbed, particularly by the
gastrointestinal tract after oral exposure. It is clear that
absorption does occur in humans, but information on the rates of human
absorption of PCBs is limited.
From the available studies, the data on the distribution of PCBs,
suggest a biphasic kinetic process with rapid clearance from the blood
and accumulation in the liver and the adipose tissue of various
organs. There is also evidence of placental transport, fetal
accumulation, and distribution to milk. In some human studies, the
skin contained a high concentration of PCBs, but the concentration in
the brain was lower than that expected on the basis of the lipid
content.
Mobilization of PCBs from fat appears to depend largely on the rates
of metabolism of the individual PCB congeners. Excretion depends on
the metabolism of PCBs to more polar compounds, such as phenols,
conjugates of thiol compounds, and other water-soluble derivatives.
Metabolic pathways include hydroxylation, and conjugation with thiols
and other water-soluble derivatives, some of which can involve
reactive intermediates, such as the arene oxides. Rates of metabolism
have been shown to depend on the PCB structure and reflect both the
degree and position of chlorine substituents. The polar metabolites of
the more highly chlorinated PCBs appear to be eliminated primarily in
the faeces, but excretion in the urine can also be significant. An
important elimination route, is via (breast) milk. Certain PCB
congeners can also be eliminated via hair.
The available kinetic studies indicate that there is a wide divergence
in biological half-life among the individual congeners and this can
reflect differences in structure-dependent metabolism, tissue
affinities, and other factors affecting mobilization from storage
sites. Persistence in tissues is not always correlated with high
toxicity, and differences in toxicity between PCB congeners may be
associated with specific metabolites and/or their intermediates.
1.1.8 Effects on organisms in the environment
PCBs are universal, environmental contaminants and are present in most
environmental compartments, abiotic and biotic, throughout the world.
Since many countries have controlled both use and release, new input
into the environment is on a reduced scale compared with the past.
However, the available evidence suggests that the cycling of PCBs is
causing a gradual redistribution of some congeners towards the marine
environment. There is a trend for the highest chlorinated congeners to
accumulate preferentially. While much of the PCB is adsorbed on to
particulates in sediment, it is still bioavailable to organisms and
will continue to be accumulated in higher trophic levels.
1.1.8.1 Laboratory studies
Effects of PCB mixtures on microorganisms are highly variable with
some species adversely affected by a level of 0.1 mg/litre and others
unaffected by 100 mg/litre; effects on different species do not vary
consistently with the degree of chlorination of the mixtures. Almost
all of the studies of the effects of PCBs on aquatic organisms have
been concerned with Aroclor mixtures. Results have been extremely
variable with no consistent relationship between percentage
chlorination or environmental conditions and toxicity, even with
closely-related organisms. Over 96 h, under static conditions, LC50
values have ranged between 12 µg/litre and >10 mg/litre for various
aquatic invertebrate species and different Aroclor mixtures.
Flow-through conditions increased the toxicity of the PCBs. Generally,
the most toxic mixtures were Aroclors in the mid-range of
chlorination; low and high percentage chlorination mixtures were less
toxic. This was also true for sub-lethal effects, such as reproduction
effects in Daphnia. Crustaceans seem to be more susceptible to PCBs
during moult. In model populations, the community structure of
estuarine species changed on exposure to Aroclor 1254, with the
numbers of amphipods, bryozoans, crabs, and molluscs decreasing and
those of annelids, brachyopods, coelenterates, echinoderms, and
nemerines unaffected. Too few of the groups have been included in
acute tests to determine whether the results represent variation in
susceptibility to PCBs or differences in interaction between species.
There is a similar variation in the toxicity of PCB mixtures for fish,
with 96-h LC50s varying between 0.008 and >100 mg/litre. Long-term
tests have shown that acute exposure, particularly in static
conditions, considerably underestimates the toxicity of the PCB.
Rainbow trout was particularly susceptible, with embryo-larval stages
showing a 22-day LC50 of 0.32 µg/litre for Aroclor 1254 and a
no-observed-effect level (NOEL) over 22 days of 0.01 µg/litre for
Aroclors 1016, 1242, and 1254.
Freshwater fathead minnow showed NOELs of 5.4, 0.1, 1.8, and
1.3 µg/litre for Aroclors 1242, 1248, 1254, and 1260, respectively;
the estuarine sheephead minnow showed NOELs of 3.4 and 0.06 µg/litre
for Aroclors 1016 and 1254, respectively.
Experimental evidence has confirmed field observations demonstrating
reproductive impairment in seals fed on fish containing PCBs
accumulated in the wild. The effect occurs late in reproduction,
preventing implantation of the embryo in the uterine wall.
In short-term tests, the toxicity of Aroclor for birds increased with
increasing percentage chlorination; 5-day dietary LC50s ranged from
604 to >6000 mg/kg diet. The main reproductive effects of PCBs on
birds were reduced hatchability of eggs and embryotoxicity. These
effects continued after dosing ended, as the hens reduced their PCB
load via the eggs. There is no evidence that Aroclors cause egg-shell
thinning, directly; effects on the food consumption and body weight of
hens have an indirect effect on shell thickness. Sub-lethal effects on
behaviour and hormone secretion have been reported.
The acute toxicity of Aroclors for mink decreases with increasing
percentage chlorination, acute oral LD50s varying between >750 and
4000 mg/kg body weight; the ferret is less sensitive. Aroclor reduces
food consumption and, thus, the growth rate of young mink.
Reproduction of mink is reduced or eliminated by Aroclors, either
given directly or as natural contaminants in fish. Higher percentage
chlorinated Aroclors (notably 1254) have a greater effect. The
reproductive rate returns to normal after cessation of feeding with
Aroclor.
Bats are susceptible to Aroclor released from fat during migration.
Because the great majority of laboratory tests on aquatic and
terrestrial organisms were carried out using PCB mixtures, it is not
possible to identify which specific components of the mixtures were
responsible for effects. Similarly, because tests were conducted in
environmentally unrealistic conditions (e.g., beyond the solubility of
congeners and without sediment present in aquatic tests), it is
difficult to extrapolate from laboratory to field. However, it can
reasonably be assumed that any effects on populations of organisms,
likely to occur more generally in the environment in the future, will
already have been observed in local populations exposed to high PCB
levels in the past.
1.1.8.2 Field studies
Results suggesting effects of PCBs on fish populations in the field
are inconclusive. Interpretation of field data on birds is difficult,
since residues of many different organochlorines are also present.
Most authors have shown a correlation between effects (embryotoxicity)
and total organochlorine residues. Of the organochlorine compounds
present, PCB residues correlate best with the effects on embryos, but
the results cannot be regarded as proved field effects of the PCBs.
There is evidence (confirmed in laboratory studies) that PCBs reduce
the reproductive capacity of sea mammals. The effect is on the
implantation of the embryo, but there can also be physical changes in
the female reproductive tract.
Extrapolation from laboratory, acute and short-term tests to effects
at the population level in the field is not possible. Uncertainties
about which components of the PCB mixtures cause effects, the specific
congeners present in the environment, and the bioavailability of PCB
components to organisms, all combine to make estimates of likely
environmental exposures and effects difficult. The effects on sea
mammal populations can be regarded as proved, but the component(s) of
the PCB mixtures that are responsible are not yet known.
Given the trends towards increased contamination of the marine
environment, attention should be concentrated on the effects on marine
organisms. There is clear laboratory and field evidence of
reproductive effects on populations of sea mammals in heavily-polluted
areas. The residues and effects of PCBs on other populations of sea
mammals are likely to increase in the future. It is less clear whether
effects will be seen in other organisms, such as birds feeding on
marine prey.
Population and community effects on lower organisms, phytoplankton,
and zooplankton, would be expected to occur on the basis of laboratory
experiments. Both the extent and significance of such changes are
difficult to assess. From currently available information, effects on
fish populations would not be expected, though fish will act as a
route of exposure of fish-eating mammals and birds.
Previously reported effects on terrestrial species, fish-eating,
freshwater mammals and migratory bats, for example, should be less
evident as residues of PCBs are redistributed. Residues in terrestrial
biota currently show little decline overall, but information on
changes in congeners is scarce or absent. Declines in higher
chlorinated congeners would be expected to be slow.
1.1.9 Effects on experimental animals and in vitro systems
1.1.9.1 Single exposure
The acute toxicity of Aroclors, after a single oral exposure, is
generally low in rats. Young animals appear to be more sensitive
(LD50: 1.3-2.5 g/kg body weight) than adults (LD50: 4-11 g/kg body
weight). The lowest LD50 reported for Aroclor 1254 in adult rats was
1.0 g/kg body weight. No differences between the sexes were observed.
The dermal LD50 in rabbits ranged from >1.26 to <2 g/kg body weight
for Aroclor 1260 (in corn oil) and from 0.79 to <3.17 g/kg body
weight for some other undiluted PCB mixtures. With intravenous
application, an LD50 of 0.4 g/kg body weight for Aroclor 1254 was
shown in rats; the LD50 after intraperitoneal injection in the mouse
varied from 0.9 to 1.2 g/kg body weight.
1.1.9.2 Short-term exposure
The main targets in mammals, with short-term, oral exposure to PCB
mixtures or congeners, were the liver, the skin, the immune system,
and the reproductive system. The Rhesus monkey was the most sensitive
species tested, females being more sensitive than males. Adult female
Rhesus monkeys exposed to a diet containing Aroclor 1248 at a level of
2.5 mg/kg, or 0.09 mg/kg body weight per day, for 6 months, showed an
increased mortality rate, growth retardation, alopecia, acne, swelling
of the Meibomian glands, and possibly immunosuppression.
Microscopically, enlarged fatty liver with focal necrosis, and
epithelial hyperplasia, and keratinization of hair follicles were
found. At higher exposure levels, microscopic changes have also been
observed in other epithelial tissues, such as the sebaceous and
Meibomian glands, the gastric mucosa, gall bladder, bile duct, nail
beds, and the ameloblast. Serum levels of total lipid triglycerides
and cholesterol were decreased. Short-term exposure to commercial PCB
mixtures induced an increase in the concentrations of total lipids,
triglycerides, cholesterol, and/or phospholipids in the liver. Among
the PCB congeners, 3,4,3',4'-tetrachlorobiphenyl 3,4,5,3',4',5'-, and
2,4,6,2',4',6'-hexachlorobiphenyl were the most potent. Aroclor 1254,
at a dose level of 0.2 mg/kg body weight per day, also showed several
other effects, such as lymphoreticular lesions, fingernail detachment,
and gingival effects, but no acne and alopecia. A NOEL for the general
toxicity of Aroclor 1242 of 0.04 mg/kg body weight per day was
established in Rhesus monkeys. Relatively mild effects were shown in
suckling Rhesus monkeys, exposed to a much higher dose of Aroclor 1248
of 35 mg/kg body weight per day. Effects in the liver have been best
investigated in rats and include hypertrophy, fatty degeneration,
proliferation of the endoplasmic reticulum, porphyria, adenofibrosis,
bile-duct hyperplasia, cysts, and preneoplastic and neoplastic
changes. In studies on rats and mice, individual PCB congeners caused
effects in the liver, spleen, and thymus, the planar congeners being
most toxic. In monkeys, planar congeners, at doses of 1-3 mg/kg diet,
induced effects similar in character and severity to those produced by
Aroclor 1242, at a dose of 100 mg/kg diet, and Aroclor 1248, at a dose
of 25 mg/kg diet.
Following dermal exposure of rabbits and mice, PCB mixtures and some
congeners caused effects on the skin and liver, similar to those found
after oral exposure. In rabbits, thymic atrophy, a reduction of
germinal centres of the lymph nodes, and leukopenia were also
observed.
1.1.10 Reproduction, embryotoxicity, and teratogenicity
1.1.10.1 Reproduction and embryotoxicity
Comprehensive reproduction and teratogenicity studies have not been
conducted. In a 2-generation reproduction study on rats, a NOEL of
0.32 mg/kg body weight, based on reproductive parameters (Aroclor
1254) and a NOEL of 7.5 mg/kg body weight (Aroclor 1260) were
established. However, the lowest tested dose of 0.06 mg/kg body weight
resulted in increased relative liver weights in weanlings.
In Rhesus monkeys exposed to Aroclor 1016, a NOEL of 0.03 mg/kg body
weight was established, on the basis of reproductive parameters.
However, at this level, decreased birth weight was observed and the
lowest dose tested, of 0.01 mg/kg body weight, resulted in skin
hyperpigmentation.
For Aroclor 1248 (containing PCDFs), a NOEL of 0.09 mg/kg body weight
was established in Rhesus monkeys, 1 year after exposure ceased.
1.1.10.2 Teratogenicity
Available studies on rats and monkeys did not indicate any teratogenic
effects, when animals were dosed orally during organogenesis. A NOEL
of 50 mg/kg body weight for Aroclor 1254 was demonstrated in rats with
regard to pup weight, and a LOEL of 2.5 mg/kg body weight, on the
basis of fetotoxicity (lesion in thyroid follicular cells) could be
assumed.
In teratogenicity tests with individual congeners on mice, rats, and
Rhesus monkeys, no NOEL was demonstrated. In Rhesus monkeys a dose of
0.07 mg/kg body weight resulted in maternal toxic effects
(3,4,3',4'-tetrachlorobiphenyl).
1.1.11 Mutagenicity
PCB mixtures did not cause mutation or chromosomal damage in a variety
of test systems. Chromosome breakage was induced in human lymphocytes
in vitro by 3,4,3',4'-tetrachlorobiphenyl. High concentrations of
PCB mixtures may cause primary DNA damage, as evidenced by DNA single
strand breaks in alkaline elution assays.
1.1.12 Carcinogenicity
The interpretation of the available animal data involving commercial
PCB mixtures is often complicated by lack of information concerning
the presence, or contribution, of chlorinated dibenzofuran impurities
as well as variations in congener composition.
A number of long-term carcinogenicity studies have been carried out on
mice and rats. The PCB mixtures used were Kanechlors 300, 400, and
500, Aroclors 1254 and 1260, and Clophens A30 and A60. The Clophens
were reported to be free of PCDFs, but no data were provided on the
purity of the other PCB mixtures.
A significant increase in hepatocellular adenomas and/or carcinomas
was observed in mice fed a diet containing Kanechlor 500 and Aroclor
1254 at dose levels of approximately 15-25 mg/kg body weight. No
neoplasms could be detected in mice treated with Kanechlors 300 and
400.
In rats, an increase in hepatocellular adenomas and/or carcinomas was
noted in studies on Aroclors 1254 and 1260, and Clophen A30, with an
exposure period of more than one year. The increase in the incidence
of tumour-bearing animals in these studies was not considered to be
statistically significant, however, it was in the case of 2 other
studies. An increase in the incidence of hepatocellular (trabecular)
carcinomas and adenocarcinomas was demonstrated with Aroclor 1260 and
Clophen A60 administered at a dose level of approximately 5 mg/kg body
weight.
The liver tumours concerned were considered to be non-aggressive
(benign or of low malignancy, no metastasis) and not life shortening.
Adenofibrosis, a preneoplastic lesion and/or neoplastic nodules in the
liver were reported in some of the studies. In one test with Aroclor
1254, a dose-related increase in intestinal metaplasia and
adenocarcinomas of the glandular stomach was demonstrated in the rat.
There is a substantial body of evidence to support the enhancing
effects of PCBs on liver carcinogenesis in rodents pretreated with
hepatocarcinogens. There is weak evidence for the initiating activity
of PCB-mixtures in rodents. From the genotoxicity studies reported, it
can be concluded that PCB-mixtures can be regarded as non-genotoxic.
These results imply that the association of liver tumours with the
administration of PCBs in rodents is attributable to some epigenetic
mechanisms involving enforcement of cell proliferation in the liver
and other manifestations of liver toxicity, hence a threshold approach
can be followed in the evaluation of PCB toxicity. The possibility
that PCBs might enhance carcinogenesis in tissues other than the
liver, in animals pre-exposed to various tissue-specific carcinogens,
needs to be addressed. The anticarcinogenic activity of PCBs shown in
some studies, where PCBs were given to animals during, and prior to,
the administration of carcinogens, may be related to the microsomal,
enzyme-inducing properties of PCBs resulting in an increase in
detoxification.
Overall, there is reason to exercise caution in extrapolating the
available animal data on the carcinogenic potential of PCBs to humans.
1.1.13 Special studies
Lesions induced after exposure to PCB mixtures or individual congeners
concern the liver, skin, immune system, reproductive system, oedema
and disturbances of the gastrointestinal tract, and thyroid gland.
PCBs are able to induce various enzymes in the liver. This has been
demonstrated, in rats, mice, guinea-pigs, rabbits, dogs, and monkeys,
for Aroclors 1248, 1254, 1260, and Kanechlor 400 (induction of
cytochrome P450 and P448). The inducing ability increases with the
chlorine content in the molecule. It is also dependent on the congener
composition, congeners with chlorine in the para- and meta-
position inducing the P450 enzyme. For AHH induction, the position of
the chlorine seems to be more important than the degree of
chlorination. Congeners with both para- and at least two meta-
positions substituted by chlorine, are the most potent inducers of
AHH. Distinct inter-species variations have been demonstrated. The
lowest NOEL (0.025 mg/kg body weight) was found for Aroclor 1260 in
Osborn-Mendel rats.
Effects on the endocrine system are seen as alterations in hormonal
receptor binding and in steroid hormone balance. Direct and indirect
evidence for a weak estrogenic activity was observed for various
Aroclors. Decreased levels of gonadal hormones and increased relative
testes weight were found in rats exposed to 75 mg Aroclor 1242/kg diet
for 36 weeks. Decreased plasma corticosteroid levels without increased
adrenal weight, was found in female mice exposed to Aroclor 1254
(25 mg/kg diet) for 3 weeks. Increased adrenal weight was found in
another strain given a diet containing 200 mg/kg for 2 weeks.
PCB mixtures have shown an immunosuppressive effect in various animal
species, monkeys and rabbits being the most sensitive. The lowest NOEL
in monkeys was 0.1 mg/kg body weight, and, in rabbits, 0.18 mg/kg body
weight.
Depressed motor-activity was seen in mice administered a single oral
dose of 500 mg Aroclor 1254/kg body weight. This was probably in
relation to inhibition of the uptake and release of neurotransmitters.
PCB mixtures were found to decrease the levels of vitamins A and B1
in the blood and liver of rats. Decreased levels of vitamins A, B1,
B2, and B6 were seen in rats and mice exposed to PCB mixtures.
1.1.14 Factors modifying toxicity, mode of action
Commercial PCBs show a spectrum of toxic responses, partly resembling
that of PCDDs and PCDFs. In addition, the analogous structure-activity
relations of PCB congeners, with respect to most of their toxic
responses and to their potency in inducing P448-dependent AHH,
indicate that PCB congeners that are approximate stereoisomers of
2,3,7,8,-TCDD are the most active. These findings suggest a common
mechanism of action based on the affinity of these compounds for the
cytosolic AH-receptor protein. Toxic equivalence factors relating to
2,3,7,8-TCDD have been proposed for these coplanar PCB congeners. The
nature of the likely interactions between PCBs, PCDFs, and PCDDs has
not been adequately investigated. As PCBs can stimulate microsomal
enzyme activity, they can influence the action of other chemicals that
undergo microsomal metabolism. Other so-called, non-planar PCB
congeners may cause other more subtle toxicities. In addition, PCB
congeners, especially the lower chlorinated ones, may be metabolized
through arene oxide intermediates and methylsulfonyl metabolites.
1.1.15 Effects on humans
The toxicological evaluation of PCBs presents many problems. PCBs
usually occur as mixtures of many congeners, and many of the data on
the toxicity of the PCBs are based on the testing of these mixtures.
Some components of the mixtures are more easily degraded in the
environment than others. Thus, the general population may be exposed
to mixtures that are different from those to which workers, working
with PCBs, are exposed.
The general population is exposed to PCBs mainly through contaminated
food (aquatic organisms, meat and dairy products). The daily intake of
PCBs is of the order of some micrograms per person for most of the
industrialized countries. Such exposures have not been associated with
disease. The infant is exposed to PCBs through its mother's milk.
Daily intake of PCBs may be some micrograms/kg body weight.
There are great difficulties in assessing human health effects
separately for PCBs, PCDFs, or PCDDs, since, quite frequently, PCB
mixtures contain PCDFs. The presence of PCDDs has also been seen
occasionally, in accidents with certain mixtures. Commercial PCBs have
been shown to be contaminated with PCDFs and, therefore, in many
cases, it is not clear which effects are attributable to the PCBs
themselves and which to the much more toxic PCDFs. Thus, much of the
data that can be retrieved from large episodes of intoxication in
humans, e.g., the Yusho, Yu-Cheng, and other intoxications, probably
reflect effects of exposure to both PCDFs and PCBs.
The signs of intoxication in Yusho and Yu-Cheng patients were
hypersecretion of the Meibomian glands of the eyes, swelling of the
eyelids and pigmentation of the nails and mucous membranes,
occasionally associated with fatigue, nausea, and vomiting. This was
usually followed by hyperkeratosis and darkening of the skin with
follicular enlargement and acneiform eruptions. Furthermore, oedema of
the arms and legs, liver enlargement and liver disorders, central
nervous disturbances, respiratory problems e.g., bronchitis-like
disturbances, and changes in the immune status of the patients were
also observed. In children of Yusho- and Yu-Cheng patients, diminished
growth, dark pigmentation of the skin and mucous membranes, gingival
hyperplasia, xenophthalmic oedematous eyes, dentition at birth,
abnormal calcification of the skull, rocker bottom heel, and a high
incidence of low birth weight were observed. Whether or not a
correlation existed between the exposure and the occurrence of
malignant neoplasms in these patients could not be definitely
concluded, because the number of deaths was too small. However, a
statistically significant increase was observed in male patients, with
regard to mortality from all neoplasms, liver and lung cancer.
Under occupational conditions, skin rashes occurred a few hours after
acute exposure. Furthermore, itching, burning sensations, irritation
of the conjunctivae, pigmentation the fingers and nails, and chloracne
were found after exposure to high PCB concentrations. Chloracne is one
of the most prevalent findings among PCB-exposed workers. Besides
these dermal signs of intoxication, different authors have found liver
disturbances, immunosuppressive changes, transient irritation of the
mucous membranes of the respiratory tract, neurological and unspecific
psychological or psychosomatic effects, such as headache, dizziness,
depression, sleep and memory disturbances, nervousness, fatigue, and
impotence. The overall conclusion is that continuous occupational
exposure to high PCB and PCDF concentrations may result in effects on
the skin and liver.
Two large mortality studies were carried out on cohorts of workers.
When exposure to Aroclor 1254, 1242, and 1016 occurred, increased
mortality from cancer of the liver and gall bladder was observed in
one study and from neoplasms and cancer of the gastrointestinal tract
in the other. None of the available epidemiological studies provide
conclusive evidence of an association between PCB exposure and
increased cancer mortality, because of the small number of deaths in
exposed populations, the lack of dose-response relationships, and the
problem of contaminants in the PCB mixtures.
1.2 Conclusions
1.2.1 Distribution
Because of their physical and chemical properties, PCBs have become
dispersed globally, throughout the environment.
PCBs are almost universally present in organisms in the environment
and are readily bioaccumulated. Biomagnification in food chains has
also been demonstrated.
Higher chlorinated congeners accumulate preferentially.
1.2.2 Effects on experimental animals
The results of animal studies suggest that PCBs are immunosuppressive,
as assessed by alterations in gross measures of immune function
(spleen weight, thymus weight, and lymphocyte counts). NOELs in
monkeys have been estimated at 100 µg/kg for Aroclor 1248 and
<100 µg/kg body weight for Aroclor 1254. Immunosuppression appears to
be a congener-specific effect.
Reproductive toxicity is, in general, only seen at doses producing
systemic toxicity in the mother. Neonates feeding on contaminated
mother's milk (in monkeys and other animal species, used as models)
appear to be particularly sensitive to PCBs and show reduced growth
with other toxic symptoms. The NOEL for Aroclor 1016 on reproductive
effects is 30 µg/kg body weight for monkeys; no NOEL could be
established for the reproductive effects of Aroclor 1248.
PCBs are not genotoxic and there is inconclusive evidence for action
as tumour initiators. PCBs do act as tumour promoters. It can be
concluded that the toxicity of PCB mixtures can be evaluated on a
threshold basis.
1.2.3 Effects on humans
Exposure of the general population to PCBs will be principally through
food items. Babies will be exposed through the mother's milk.
Two large episodes of intoxication in humans have occurred in Japan
(Yusho) and Province of Taiwan (Yu-Cheng). The main symptoms in Yusho
and Yu-Cheng patients have frequently been attributed to contaminants
in the PCB mixtures, specifically, to PCDFs. The Task Group concluded
that symptoms may have been caused by the combined exposure to PCBs
and PCDFs. However, some of the symptoms, principally, the chronic
respiratory effects, may have been caused specifically by the
methylsulfone metabolites of certain PCB congeners.
1.2.4 Effects on the environment
While there have been reports of effects on local populations of
birds, the most important effect of PCBs on organisms in the
environment has been reproductive failure in sea mammals. This has
been observed principally in semi-enclosed seas and has led to
population declines, locally. The prediction that residues of PCBs in
the environment will gradually be redistributed towards the marine
environment indicates an increasing hazard for sea mammals in the
future.
1.3 Recommendations
* International agreement on analytical procedures to improve the
comparability of results of monitoring programmes is recommended.
Methodology for congener-specific analysis should continue to be
developed, though the value of analysis based on mixtures is
recognized.
* In order to ensure the reliability of analytical data,
inter-laboratory quality control studies are strongly recommended.
It is also recommended that an international network of technical
support and supervision is established, to allow developing
countries to participate in monitoring.
* Long-term studies using specific congeners, and studies on the
mechanism of action of constituents of PCBs mixtures, with special
regard to tumour promotion, are recommended to improve the
precision of the risk assessment of PCBs.
* Epidemiological studies to better assess the risk to neonates are
required, since new-born infants appear to be the most vulnerable
sector of the general population, because of high exposure through
milk.
* Sensitive and specific biomarkers for some of the more subtle
types of PCB toxicity (such as reproductive, immunological, and
neural toxicity) should be developed for use in future
epidemiological studies.
* Disposal of PCBs should be carried out by incineration in properly
designed and run facilities that can guarantee the constant high
temperatures (above 1000°C), residence time, and turbulence
necessary to ensure complete breakdown.
* Methods to remove PCBs already contained in landfills should be
investigated.
* Monitoring of PCBs in the environment and in wildlife should be
encouraged globally, to follow the expected redistribution of
residues already present.
* Marine mammals are susceptible to reproductive failure as a result
of PCB contamination. Studies on the population size and
reproductive success of cetaceans should be encouraged, together
with further research to establish which congeners are responsible
for the effects.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Chemical formula and structure
The chlorination of biphenyl can lead to the replacement of 1-10
hydrogen atoms by chlorine; the conventional numbering of substituent
positions is shown in the diagram:
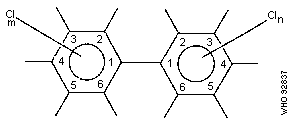 The chemical formula can be presented as C12H10-nCln, where n, the
number of chlorine atoms in the molecule, can range from 1 to 10.
2.1.2 Relative molecular mass
The relative molecular mass depends on the degree of substitution.
Monochlorobiphenyl has a relative molecular mass of 188, while
completely chlorinated biphenyl (C12Cl10) has a relative molecular
mass of 494 (US EPA, 1980).
2.1.3 Common name
Common name: polychlorinated biphenyls (PCBs)
CAS Registry number: 1336-36-3
RTECS Registry number: TQ 1350000
2.1.4 Chemical composition
The PCBs are chlorinated hydrocarbons, manufactured commercially by
the progressive chlorination of biphenyl in the presence of a suitable
catalyst (e.g., iron chloride). Depending on the reaction conditions,
the degree of chlorination can vary between 21 and 68% (w/w). The
yield is always a mixture of different isomers and congeners. Thus, a
total of 209 theoretically different chemical components exist, but
only about 130 of these are likely to occur in commercial products or
mixtures of such compounds (Safe, 1990).
Seventy-eight out of the possible 209 PCB congeners can exist as
rotational isomers that are enantiomeric to each other. Nineteen PCBs,
of which 9 are components of commercial PCB formulations, have been
predicted to be stable at room temperature (Kaiser, 1974).
Puttmann et al. (1988) separated the atropisomers of
2,3,4,6,2',4'-hexachlorobiphenyl and demonstrated that they possess
different biological effects with regard to in vivo enzyme induction
(aminopyrine N-demethylase, aldrin epoxidase, cytochrome P-450
content, morphine UDP-glucuronosyl transferase) in Sprague-Dawley
rats.
Unlike the dioxins or dibenzofurans, the phenyl rings of a PCB are not
constrained through ring fusions and have relatively unconstrained
rotational freedom. Chlorines at the ortho (2,2', 6,6') positions
introduce constraints on rotational freedom that can hinder
coplanarity of the phenyl rings. X-ray crystallographic studies
(McKinney & Singh, 1981) indicate that the preferred conformation for
all PCBs, including those without ortho-substituents, is
noncoplanar. The proportion of molecules of a particular congener
assuming a coplanar configuration becomes increasingly small as the
degree of ortho-substitution and the energetic cost of conforming
increases. However, PCBs without ortho-substitution are often
referred to in the biological literature as the planar (or coplanar)
PCBs and all others as the nonplanar (or noncoplanar) PCBs. This
terminology, though somewhat misleading, is also used throughout this
publication for convenience and ease of referring back to the
published literature. It is widely recognized that certain biological
activities of the PCBs vary, at least quantitatively, with
stereochemical differences in the congeners.
Individual manufacturers have their own system of identification for
their products. In the Aroclor series, a 4-digit code is used;
biphenyls are generally indicated by 12 in the first 2 positions,
while the last 2 numbers indicate the percentage by weight of chlorine
in the mixture; thus, Aroclor 1260 is a polychlorinated-biphenyl
mixture containing 60% of chlorine. An exception to this
generalization is Aroclor 1016, which is a distillation product of
Aroclor 1242 containing only 1% of components with 5 or more chlorine
atoms (Burse et al., 1974). With other commercial products, the codes
may indicate the approximate mean number of chlorine atoms in the
components; thus Clophen A60, Phenochlor DP6, and Kanechlor 600 are
biphenyls with an average of about 6 chlorine atoms per molecule
(equivalent to 59% chlorine by weight).
Ballschmiter & Zell (1980) proposed a numbering system for the PCB
congeners, that was later adopted by the International Union of Pure
and Applied Chemists (IUPAC). The number, structure, and isomer group
are given for each congener in the paper of McFarland & Clarke (1989)
(see Appendix A). In the literature, the structure of a congener is
given in 2 ways; for example 2,2',5,5' or 2,5,2',5' (No 52).
Individual PCBs have been synthesized for use as reference samples in
the identification of gas-liquid chromatographic peaks, for
toxicological investigations, and for studying their metabolic fate in
living organisms, for which purpose they have been prepared labelled
with carbon-14 (Hutzinger et al., 1971; Jensen & Sundström, 1974a;
Sundström & Wachtmeister, 1975).
The proportions of PCBs with 1-9 chlorine substituents in the Aroclors
are shown in Table 1.
It is apparent, from gas chromatographic analyses of commercial
products, that PCB mixtures differ with respect to the individual
congeners present and their relative concentrations (Jensen &
Sundström, 1974a; Albro & Parker, 1979; Ballschmiter & Zell, 1980;
Albro et al., 1981; Mullin et al., 1984; Safe et al., 1985a;
Alford-Stevens, 1986).
There have been several investigations to identify individual PCBs in
commercial products. The components of the Aroclors were separated by
column and gas-liquid chromatography and many of the peaks
characterized by high-resolution mass spectrometry and nuclear
magnetic resonance, and also by comparison with synthesized PCBs
(Table 2) (see also DFG, 1988).
Jensen & Sundström (1974a) recognized that conventional gas-liquid
chromatography was not suitable for separating all the components, so
they devised a preliminary fractionation on a charcoal column, which
separated the component PCBs according to the number of chlorines in
the 2,6,2' or 6' positions in the molecule ( o-chlorines). They
compared the gas-liquid chromatographic peaks with those of 90
synthesized PCBs, and were able to characterize and quantify 60
components of Clophens A50 and A60.
Table 1. Approximate percentages (w/v) of Aroclors with different degrees of
chlorinationa
Number of Chlorine
chlorine weight Aroclor
atoms in (%)
molecule 1221 1232 1016 1242 1248 1254 1260
0 0 10 - -
1 18.8 50 26 2 3
2 31.8 35 29 19 13 2
3 41.3 4 24 57 28 18
4 48.6 1 15 22 30 40 11
5 54.4 22 36 49 12
6 59.0 4 4 34 38
7 62.8 6 41
8 66.0 8
9 68.8 1
a From: WHO/EURO (1987).
2.1.5 Technical product
Major trade names
The PCBs manufactured commercially are known by a variety of trade
names including: Aroclor, Pyranol, Pyroclor (USA), Phenoclor, Pyralene
(France), Clophen, Elaol (Germany), Kanechlor, Santotherm (Japan),
Fenchlor, Apirolio (Italy), and Sovol (USSR). Table 3 contains the
most common trade names for commercial products, some of which are not
in use any more (Brinkman & De Kok, 1980; WHO/EURO, 1987).
2.1.6 Purity and impurities
Commercial PCBs are not sold according to a composition specification,
but according to their physical properties. The composition of
Aroclors and Clophens has been presented in recent papers; the
composition of 5 Aroclors is shown in Tables 1 and 2. In Table 1, the
approximate composition is expressed as the percentage of chlorine
weight, and, in Table 2, the composition of the chlorine substitution
pattern is expressed in mol % (Albro & Parker, 1979; Albro et al.,
1981; Jones, 1988). The composition of the chlorine substitution
pattern for 4 Clophens is described by Duinker & Hillebrand (1983) and
Jones (1988). It should be kept in mind that nothing can be said about
the variations in the different lots of these mixtures. Impurities
known to be present in commercial PCBs are chlorinated dibenzofurans
and chlorinated naphthalenes (Vos et al., 1970; Bowes et al., 1975;
Albro & Parker, 1979; Albro et al., 1981; Duinker & Hillebrand, 1983;
Rappe et al., 1985a). The concentrations of PCDFs in Aroclor, Clophen,
Phenoclor, and Kanechlor are summarized in Tables 4 and 5.
Different authors have examined the presence of PCDFs in PCB mixtures.
Bowes et al. (1975) found 0.8-2.0 mg/kg in samples of Aroclor 1248 and
1260, but none in Aroclor 1016, 8.4 mg/kg in Clophen A60, and
13.6 mg/kg in Phenoclor DP-6. Rappe et al. (1985a) and Bentley (1983)
found levels of PCDFs up to 40 mg/kg in a number of commercial PCBs.
Recently, Wakimoto et al. (1988) found a number of extremely toxic
PCDFs in several Japanese and American commercial PCB preparations.
These isomer-specific analyses revealed the 2,3,7,8-tetra-,
1,2,4,7,8-penta-, 1,2,3,7,8-penta-, 2,3,4,7,8-penta-, and
1,2,3,6,7,8-hexachlorodibenzofurans. The concentrations in unused
Kanechlor 300, 400, 500, and 600, were 7.5, 26, 7.2, and 5.4 mg/kg,
respectively, and those in Aroclors 1242, 1248, 1254, and 1260, were
0.6, 3.7, 4.2, and 7.5 mg/kg, respectively. Brown et al. (1988) found
that the electrical use of PCB dielectric fluids in transformers and
capacitors did not increase the PCDFs content significantly.
More data about the occurrence of PCDFs in the different commercial
PCB mixtures are summarized in WHO/EURO (1987).
There are no reports on the presence of PCDDs in commercial mixtures
(Bowes et al., 1975). Wakimoto et al. (1988) could not find PCDDs in
the above samples of Kanechlors and Aroclors with a detection limit of
<2 µg/kg.
2.2 Physical and chemical properties
Individual pure PCB congeners are colourless, often crystalline
compounds, but commercial PCBs are mixtures of these congeners with a
clear, light yellow or dark colour. They do not crystallize at low
temperatures, but turn into solid resins. Because of the chlorine
atoms in the molecule, their density is rather high. PCBs are, in
practice, fire resistant with rather high flash-points (170-380°C).
They form vapours heavier than air, but do not form any explosive
mixtures with air. They possess very low electrical conductivity and
an extremely high resistance to thermal breakdown, and it is on the
basis of these properties that they are used as cooling liquids in
electrical equipment (US EPA, 1980; WHO/EURO, 1987; DFG, 1988).
Table 2. PCB compositions of aroclors in mol %a
IUPAC Chlorine Aroclor
No. substitution
pattern 1242 1016 1248 1254 1260
BP 0.01 0.50
1 2 0.68 0.80
2 3 0.04 0.10
3 4 0.22 1.00
4 2.2' 3.99 4.36 0.25
6 2.3' 1.24 1.37 0.69 0.07
7 2.4 1.04 1.16
8 2.4' 8.97 10.30 0.18
9 2.5 0.31 0.34 trace
10 2.6 0.13 0.20
12 3.4 0.09 0.11
13 3.4' 0.12 0.12
14 3.5 0.35 0.37
15 4.4' 0.99 1.07
16 2.3.2' 3.25 3.50 0.84
17 2.4.2' 2.92 3.14 0.19
18 2.5.2' 9.36 10.87 9.95 0.07
19 2.6.2' 0.97 1.08
20 2.3.3' 3.64 3.99
22 2.3.4' 2.64 2.80 1.24 trace trace
25 2.4.3' 1.68 1.79
26 2.5.3' 0.55 0.62 0.75
27 2.6.3' 0.54 0.58
28 2.4.4' 13.30 14.48 trace
31 2.5.4' 4.53 4.72 9.31 0.72
32 2.6.4' 2.15 2.31 1.46
33 3.4.2' 2.83 3.08
35 3.4.3' 0.66 0.38
37 3.4.4' 1.62 1.89 1.28 0.20 0.09
39 3.5.4' 1.03 1.08
40 2.3.2'.3' 0.15 0.18 1.12 0.26 0.04
41 2.3.4.2' 1.67 2.00
42 2.3.2'.4' 7.05 2.18 0.66
43 2.3.5.2' 0.44 0.47
44 2.3.2'.5' 1.06 1.14
45 2.3.6.2' 0.90 1.00 5.73 0.15
46 2.3.2'.6' 0.31 0.33
47 2.4.2'.4' 1.65 1.8 3.18 0.52 0.88
48 2.4.5.2' 1.33 1.41
Table 2. (cont'd).
IUPAC Chlorine Aroclor
No. substitution
pattern 1242 1016 1248 1254 1260
? 2.5.2'.4' - - 3.81 1.63 0.44
49 2.4.2'.5' 3.28 3.48
52 2.5.2'.5' 4.08 4.35 8.36 4.36 1.91
53 2.5.2'.6' 0.97 1.07 6.30 0.13
54 2.6.2'.6' 0.17 0.19
55 2.3.4.3' 0.11 0.43 0.12
56 2.3.3'.4' 0.60 trace 0.18 0.03
60 2.3.4.4' 0.21
66 2.4.3'.4' 0.81 0.14 4.95 2.24 0.22
70 2.5.3'.4' 1.11 6.38 4.75 0.85
71 2.6.3'.4' 0.65
72 2.5.3'.5' 0.33 2.10 1.01 0.28
74 2.4.5.4' 2.02 1.35 0.25 0.30 0.09
75 2.4.6.4' 2.18 2.40
76 3.4.5.2' trace trace 0.18 0.01
77 3.4.3'.4' 0.34 0.47 0.12 0.04
78 3.4.5.3' 0.52
79 3.4.3'.5' 0.24 trace 0.23 0.04
80 3.5.3'.5' trace trace trace
81 3.4.5.4' 0.28
83 2.3.5.2'.3' trace 0.32 0.09
84 2.3.6.2'.3' 0.38 0.01 0.71 1.72 0.69
85 2.3.4.2'.4' 0.40 0.55 2.15 0.31
? 2.3.4.3'.5' 0.02 0.55 0.14
87 2.3.4.2'.5' 0.09 1.05 3.81 1.10
91 2.3.6.2'.4' trace 1.78 5.00 3.22
92 2.3.5.2'.5' 0.12 0.20 0.63 0.21
95 2.3.6.2'.5' 0.53 0.18
97 2.4.5.2'.3' 0.78 2.59 0.63
98 2.4.6.2'.3' 0.13 0.04
99 2.4.5.2'.4' 0.55 2.52 6.10 0.82
101 2.4.5.2'.5' 0.27 1.50 6.98 5.04
102 2.4.5.2'.6' trace trace trace
105 2.3.4.3'.4' 0.25
106 2.3.4.5.3' 0.40 0.06
108 2.3.4.3'.5' 0.46 0.16
110 2.3.6.3'.4' 1.69 8.51 3.57
113 2.3.6.3'.5' 0.39 0.01 3.10 trace 0.01
114 2.3.4.5.4' 0.25 0.03
118 2.4.5.3'.4' 8.09 2.00
120 2.4.5.3'.5' 0.31 trace 0.15 3.01
121 2.4.6.3'.5' 0.92 4.32 3.51 0.57
Table 2. (cont'd).
IUPAC Chlorine Aroclor
No. substitution
pattern 1242 1016 1248 1254 1260
123 3.4.5.2'.4' 0.36
? 3.4.5.2'.3' trace 0.76 1.88
126 3.4.5.3'.4' 0.03 0.16 1.59
127 3.4.5.3'.5' 0.05
128 2.3.4.2'.3'.4' 1.31 0.47
131 2.3.4.6.2'.3' 0.14 0.01
132 2.3.4.2'.3'.6' trace 2.00 2.77
133 2.3.5.2'.3'.5' 1.13 0.03 0.06
134 2.3.5.6.2'.3' 0.11 0.38 1.01
135 2.3.5.2'.3'.6' 0.20 0.29
136 2.3.6.2'.3'.6' 0.20 0.34 1.12
138 2.3.4.2'.4'.5' 0.08 0.19 4.17 5.01
143 2.3.4.5.2'.6' 0.07
148 2.3.5.2'.4'.6' 0.12 0.07 0.06
149 2.4.5.2'.3'.6' 0.77 3.59 9.52
151 2.3.5.6.2'.5' trace 0.33 0.06
153 2.4.5.2'.4'.5' 0.02 0.13 3.32 8.22
154 2.4.5.4'.6' 0.14
156 2.3.4.5.3'.4' 0.41
157 2.3.4.3'.4'.5' 0.18 0.03
158 2.3.4.6.3'.4' 0.46 0.18
159 2.4.5.2'.3'.5' 0.75 1.48
163 2.3.5.6.3'.4' trace
167 2.4.5.3'.4'.5' 0.21 0.17
168 2.4.6.3'.4'.5' 0.56 4.23 0.59
170 2.3.4.5.2'.3'.4' 0.43 0.62
171 2.3.4.6.2'.3'.4' 0.30 4.31
174 2.3.4.5.2'.3'.6' trace 0.09
176 2.3.4.6.2'.3'.6' 0.09 trace 0.57
177 2.3.5.6.2'.3'.4' trace
179 2.3.5.6.2'.3'.6' 0.56 0.83
180 2.3.4.5.2'.4'.5' 0.76 7.20
181 2.3.4.5.6.2'.4' 0.28 2.72
182 2.3.4.5.2'.4'.6' trace 0.47
183 2.3.4.6.2'.4'.5' 1.16 2.58
185 2.3.4.5.6.2'.5' 1.11 5.65
186 2.3.4.5.6.2'.6' trace trace 0.37
187 2.3.5.6.2'.4'.5' 0.48 1.12
189 2.3.4.5.3'.4'.5' 0.13
190 2.3.4.5.6.3'.4' 0.02
192 2.3.4.5.6.3'.5' 0.20 0.97
Table 2. (cont'd).
IUPAC Chlorine Aroclor
No. substitution
pattern 1242 1016 1248 1254 1260
193 2.3.5.6.3'.4'.5' 2.30
194 2.3.4.5.2'.3'.4'.5' 2.21
195 2.3.4.5.6.2'.3'.4' trace
196 2.3.4.5.2'.3'.4'.6' 0.79
197 2.3.4.6.2'.3'.4'.6' 0.30
198 2.3.4.5.6.2'.3'.5' 1.00 0.15
199 2.3.4.5.6.2'.3'.6' 0.38
200 2.3.4.6.2'.3'.5'.6' trace 0.15
202 2.3.5.6.2'.3'.5'.6' trace 0.31
203 2.3.4.5.6.2'.4'.5' 0.08
204 2.3.4.5.6.2'.4'.6' trace 0.13
205 2.3.4.5.6.3'.4'.5' 0.01
206 2.3.4.5.6.2'.3'.4'.5' 0.51
207 2.3.4.5.6.2'3'.4'.6' 1.15
208 2.3.4.5.6.2'.3'.5'.6' 1.64
? 2.3.4.5.6.2'.3'.5'.6' 0.18
a From: Albro & Parker (1979); Albro et el. (1981).
Table 3. The trade marks of PCB products and mixtures containing PCBsa
Aceclor (t) Disconon (c) PCBs
Apirolio (t,c) Dk (t,c) Phenoclor (t,c)
Aroclor (t,c) Duconol (c) Polychlorinated biphenyl
Arubren Dykanol (t,c) Polychlorobiphenyl
Asbestol (t,c) EEC-18 Pydraulc
Askarel Elemex (t,c) Pyralene (t,c)
Bakola 131 (t,c) Eucarel Pyranol (t,c)
Biclor (c) Fenchlor (t,c) Pyroclor (t)
Chlorextol (t) Hivar (c) Saf-T-Kuhl (t,c)
Chlorinated Biphenyl Hydol (t,c) Santotherm FRb
Chlorinated Diphenyl Inclor Santovac 1 and 2
Chlorinol Inerteen (t,c) Siclonyl (c)
Chlorobiphenyl Kanechlor (t,c) Solvol (t,c)
Clophen (t,c) Kennechlor Sovol
Clorphen (t) Montar Therminol FRb
Delor Nepolin
Diaclor (t,c) No-Flamol (t,c)
Dialor (c) PCB
a From: WHO/EURO (1987).
b Previous products (FR-series) used as pressure oil contained PCBs, but current
products are a different series and do not contain PCBs.
c Previous products (A-series) e.g., PYDRAUL A-200 contained PCBs, but current
commercial products are B, C, or D-series and do not contain any chlorinated
compounds.
(t) Used in transformers.
(c) Used in capacitors.
Table 4. Concentrations of chlorinated dibenzofuransa in Aroclor, Clophen, and
Phenoclorb
PCB 4-Cl 5-Cl 6-Cl Total
Aroclor 1248 (1969) 0.5 (25) 1.2 (60) 0.3 (15) 2.0
Aroclor 1254 (1969) 0.1 (6) 0.2 (12) 1.4 (82) 1.7
Aroclor 1254 (1970) 0.2 (13) 0.4 (27) 0.9 (60) 1.5
Aroclor 1260 (1969) 0.1 (10) 0.4 (40) 0.5 (50) 1.0
Aroclor 1260 (lot AK3) 0.2 (25) 0.3 (38) 0.3 (38) 0.8
Aroclor 1016 (1972) ND ND ND
Clophen A-60 1.4 (17) 5.0 (59) 2.2 (26) 8.4
Phenoclor DP-6 0.7 (5) 10.0 (74) 2.9 (21) 13.6
a Expressed as mg PCB/kg. Values in parentheses represent quantity as percentage
of total dibenzofurans.
b From: Bowes et al. (1975).
ND = not detected (0.001 mg/kg).
Table 5. Concentrations of chlorinated dibenzofurans in Kanechlorsa
Kanechlor Chlorodibenzofurans Concentration
(mg/kg)
Di- Tri- Tetra- Penta- Hexa- Hepta- b c
300 + + 1 1.5
400 + + + + 18 17
500 + + + + 4 2.5
600 + + + + 5 3
a From: Nagayama et al. (1975).
b Calculated from peak heights.
c Calculated by perchlorination method.
PCBs have a high degree of chemical stability under normal conditions.
They are very resistant to a range of different oxidants and other
chemicals. According to laboratory tests, they stay chemically
unchanged, even in the presence of oxygen or some active metals at
high temperatures (up to 170°C) and for protracted periods (WHO/EURO,
1987).
PCBs are practically insoluble in water, whereas they dissolve easily
in hydrocarbons, fats, and other organic compounds and they are
readily absorbed by fatty tissues (WHO/EURO, 1987).
Some physical and chemical data for a number of Aroclors are presented
in Table 6.
Foreman & Bidleman (1985) estimated the liquid phase vapour pressures,
at 25°C, of 134 PCB congeners found in 5 Aroclor fluids, using a
capillary gas chromatographic method in conjunction with published
retention indices of PCBs.
Burkhard et al. (1985) predicted Henry's Law Constants from the ratio
of the liquid (or subcooled liquid) vapour pressure and aqueous
solubility for PCB congeners. The predicted values were in fair
agreement with experimental values and the error for these constants
was estimated to be a factor of 5 in the temperature range of 0-40°C.
For the PCB congeners, Henry's Law Constants were independent of the
relative molecular mass and increased approximately an order of
magnitude with a 25°C increase in temperature.
Aqueous solubility is considered an essential parameter for predicting
the fate and transport of organic chemicals in the environment. As
already stated, some physical and chemical data are given for 6
Aroclor mixtures in Table 6 (Alford-Stevens, 1986). However, during
the last 5 years, much more information on aqueous solubility, melting
points, entropies of melting, Henry's law constants, and vapour
pressures has become available. This information concerns not only PCB
mixtures but also individual congeners.
Opperhuizen et al. (1988) studied the aqueous solubilities of 45
chlorinated biphenyls and the relationships between activity
coefficient and chemical structure parameters (total surface area
(TSA) and total molecular volume (TMV)) of hydrophobic chemicals, to
understand the nature of hydrophobicity. The aqueous solubilities of
PCBs showed a linear relationship between logarithms of aqueous
activity coefficients or TSA and TMV.
Table 6. Physical and chemical properties of a number of Aroclorsa
Substance Water Vapour Density Appearance Henry's Law Refractive index Boiling point
Aroclor solubility pressure (g/cm3) constant (distillation
(mg/litre) (torr) 25°C 25°C (atm-m3/mol range) (750
25°C at 25°C)b torr, °C)
1016 0.42 4.0 × 10-4 1.33 Clear, mobile oil 2.9 × 10-4 1.6215-1.6235 325-356
(at 25°C)
1221 0.59c 6.7 × 10-3 1.15 Clear, mobile oil 3.5 × 10-3 1.617-1.618 (at 20°C) 275-320
1232 0.45 4.1 × 10-3 1.24 Clear, mobile oil unknown unknown 290-325
1242 0.24 4.1 × 10-3 1.35 Clear, mobile oil 5.2 × 10-4 1.627-1.629 (at 20°C) 325-366
1248 0.054 4.9 × 10-4 1.41 Clear, mobile oil 2.8 × 10-3 unknown 340-375
1254 0.021 7.7 × 10-5 1.50 Light yellow 2.0 × 10-3 1.6375-1.6415 365-390
viscous oil (at 25°C)
1260 0.0027 4.0 × 10-5 1.58 Light yellow 4.6 × 10-3 unknown 385-420
sticky resin
a From: IARC (1978); WHO/EURO (1987); ATSDR (1989).
b These Henry's Law Constants were estimated by dividing the vapour pressure by the water solubility. The first water solubility
given in this table was used for the calculation. The resulting estimated Henry's law constant is only an average for the
entire mixture; the individual chlorobiphenyl isomers may vary significantly from the average. Burkhard et al. (1985)
estimated the following Henry's Law Constants (atm-m3/mol) for various Aroclors at 25°C: 1221 (2.28 × 10-4), 1242 (3.43 × 10-4),
1248 (4.4 × 10-4), 1254 (2.83 × 10-4), 1260 (4.15 × 10-4).
c At 24°C.
Dickhut et al. (1986) studied the solubilities of 6 higher chlorinated
biphenyl congeners at different temperatures and found that the
solubility increased exponentially with temperature in the range of
0.4-80°C. From the temperature dependence of solubility, enthalpies of
solution were calculated. The same results were found by Doucette &
Andren (1988), who determined the aqueous solubilities of a few PCBs,
using a generator-column technique, at temperatures of 4.0, 25.0, and
40.0°C.
The dissolution of extremely hydrophobic chemicals that may be
associated with a relatively constant endothermic enthalpy of solution
and an endothermic enthalpy of fusion that is proportional to the
solute's melting point is discussed by Opperhuizen et al. (1987) and
Dickhut et al. (1987).
Dunnivant & Elzerman (1988) estimated the aqueous solubilities and
Henry's Law Constants (HLC) for 26 selected PCB congeners for the
evaluation of quantitative structure-property relationships (QSPRs).
Aqueous solubilities (as solids at 25°C, column generation technique),
determined for the 26 congeners, ranged from 1.08 × 10-5 to
9.69 × 10-10 mol/litre and generally decreased with relative molecular
mass. HLCs (25°C, gas purge technique), determined for 20 congeners,
ranged from 0.3 × 10-4 to 8.97 × 10-4 atm.m3/mol. Measured HLCs were
not correlated with relative molecular mass, but increased with the
degree of ortho-chlorine substitution within a relative molecular
mass class.
Vapour pressures calculated from the product of solubility (mol/m3)
and HLC (atm-m3/mol) data, generally decreased with relative
molecular mass and increased with increasing degree of
ortho-chlorine substitution (Dunnivant & Elzerman, 1988; Hawker,
1989). Westcott et al. (1981) used a semimicro gas saturation method
to determine the vapour pressures of 3 PCB isomers and 2 Aroclor
mixtures.
Experimental data were tabulated and the relationships between the
environmentally relevant physical chemical properties of the PCBs
critically reviewed by Shui & Mackay (1986). Aqueous solubility,
vapour pressure, Henry's law constant, and octanol-water partition
coefficient were discussed and recommended values given for 42 of the
209 congeners; procedures were suggested for estimating the properties
of the other congeners.
2.2.1 Log n-octanol/water partition coefficient
The environmental fate of PCBs is governed primarily by the
partitioning process. Partitioning processes that are of particular
interest with regard to environmental problems include: the octanol/
water partition coefficient and the aqueous solubility. The octanol/
water partition coefficient is a measure of the hydrophobicity of a
substance and, in this respect, it has been used to predict the extent
of bioconcentration of organic pollutants in organisms. Miller et al.
(1984) studied the octanol/water partition coefficients for 16 PCBs
and Hawker & Connell (1988) for 13 PCB congeners, using the generator
column method. These partition coefficients were used to confirm a
highly significant linear relationship between log Kow and the
logarithm of the relative retention time on a nonselective gas
chromatographic stationary phase. The total surface areas (TSA) for
all the PCB congeners were determined by assuming planar molecules,
van der Waal's radii for component atoms, and appropriate values for
solvent radius, bond angles, and distances. The TSA was highly
significantly correlated with log Kow and the relationship was used
to calculate log Kow values for all the PCB congeners. In the report
of Hawker & Connell (1988), log Kow values are summarized for all 209
PCB congeners. These log Kow values range from 4.46 to 8.18.
2.2.2 Conversion factorsa
Aroclor
1016 1 mg/m3 = 0.095 ppm
1221 1 mg/m3 = 0.12 ppm
1232 1 mg/m3 = 0.105 ppm
1242 1 mg/m3 = 0.092 ppm
1248 1 mg/m3 = 0.008 ppm
1254 1 mg/m3 = 0.075 ppm
1260 1 mg/m3 = 0.065 ppm
2.3 Analytical methods
Reviews have been published on the methods used for the determination
of organochlorine compounds including PCBs in environmental samples
(Panel on Hazardous Trace Substances, 1972; Holden, 1973; US DHEW,
1978; Slorach & Vaz, 1983; Jensen, 1984, 1985; Erickson 1985;
Alford-Stevens, 1986; NIOSH, 1987; DFG, 1988; WHO/EURO, 1987, 1988).
a These air conversion factors were calculated by using the average
molecular mass at 25°C.
No two laboratories used identical methods, though all the methods
have features in common. The techniques appear to be those previously
developed for the determination of organochlorine pesticides, with
appropriate modifications for the presence of PCBs, and the studies on
PCBs sometimes form part of a wider programme for monitoring
persistent organochlorine compounds in the environment. In the past,
the major difficulty in the determination of PCBs was to obtain a
single quantitative figure from a variable mixture of components. The
PCBs were chlorinated with antimony pentachloride to decachloro-
biphenyl, which was measured as a single peak (Greve & Wegman, 1983;
Tuinstra, 1983). At the moment, chemists and toxicologists are no
longer trying to derive a single quantitative figure, preferring
instead to quantify individual congeners. The legislation in certain
countries is now based on quantifying a few selected congeners,
instead of reporting "total PCBs". It is also felt that for
pinpointing areas with high levels of contamination, in order to rank
them into low, medium, or high priority areas for action, highly
accurate laboratory analyses are not necessary; instead, analytical
competence and the use of adequate controls and standards, resulting
in consistent, reasonably accurate results would be enough. Of course,
for complicated research, especially involving laboratories in
different countries, standardization of techniques through
collaborative and comparative studies would be necessary.
Jones (1988) and Safe et al. (1985a) studied the occurrence of
specific PCB congeners in commercial formulations or mixtures. The
congener composition of commercial formulations differs from
batch-to-batch, between manufacturing processes, and with the level of
chlorination. The presence of congeners in the environment will depend
on the eventual use of commercial formulations, the quantity of each
formulation manufactured, as well as on the isomer composition of the
source.
On the basis of a literature review of the occurrence of PCB congeners
in environmental and biological samples and human tissues, and
consideration of the relative toxicity and persistence of the
congeners, suggestions were made by Jones (1988), with regard to the
most relevant components to be quantified in human foodstuffs and
tissues, using a selective analytical approach.
The congeners reported (Safe et al., 1985a; Duinker et al., 1988;
McFarland & Clarke, 1989) as being the most abundant in human tissues
and which are most important, are compounds with IUPAC numbers 28, 52,
74, 77, 99, 101, 105, 118, 126, 128, 138, 153, 156, 169, 170, 179, and
180 (comprising >70% of total PCBs and being of greatest
toxicological significance). Because of their reported occurrence or
toxicity, congeners with IUPAC numbers 8, 37, 44, 49, 60, 66, 70, 82,
87, 114, 158, 166, 183, 187, and 189 might also be considered. Duinker
et al. (1988) were also of the opinion that toxicity should be
considered as a criterion for the selection of PCB congeners for
analysis in environmental samples. Most of these congeners can be
accurately determined with the application of the multidimensional,
high-resolution GC-ECD techniques.
PCB reference materials are necessary for the qualitative and
quantitative calibration of analytical apparatus and methods (e.g.,
determination of retention times, response factors, and reference
spectra in chromatographic and spectroscopic analyses) and for the
study of biological activity. Lindsey & Wagstaffe (1989) described the
production and certification of 10 high-purity PCBs with IUPAC numbers
8, 20, 28, 35, 52, 101, 118, 138, 153, and 180.
Mes et al. (1989a) described an analytical method to determine 34
isomers of PCB congeners in fatty foods. A sample was extracted with
an acetone:hexane mixture and the extracts washed and dried; this was
followed by a clean-up and determination by gas chromatography. GC/MS
was used for confirmation.
Environmental PCB residues are often expressed in terms of relative
Aroclor composition. Schwartz et al. (1987) assessed the similarity of
Aroclors with class models derived for fish and turtles, to ascertain
if the PCB residues in the samples could be described by an Aroclor or
Aroclor mixture. The PCB residues in fish and turtles were analysed
with Soft Independent Modelling of Class Analogy, a principal
components analysis (PCA) technique. Using PCA, it was inappropriate
to report these samples as an Aroclor or Aroclor mixture.
2.3.1 Sampling strategy and sampling methods
The quality and usefulness of analytical data, especially in the
microgram-nanogram range, or even lower, depend critically on the
validity of the sample and the adequacy of the sampling programme. The
purpose of sampling is to obtain specimens that represent the
situation being studied. Sampling plans may require that systematic
samples be obtained at specified times and places, or simple random
sampling may be necessary. Generally, the sample should be an unbiased
representative of the situation of interest (WHO/EURO, 1987). Slorach
(1984) described the problems encountered with the sampling and
determination of PCBs in breast milk (see also WHO/EURO, 1985, 1988).
All aspects of a sampling programme should be planned and documented
in detail, and the expected relationship of the sampling protocol to
the analytical result should be defined. A sampling programme should
include reasons for choosing sampling sites, the number and type of
samples, the timing of sample acquisition, and the sampling equipment
used. A detailed sampling procedure should include a description of
the sampling situation, the sampling methodology, labelling of
samples, field blank preparation, pretreatment procedures,
transportation, and storage (WHO/EURO, 1987).
The quality assurance programme should include means to demonstrate
that containers or storage procedures do not alter the qualitative or
quantitative composition of the sample. Special transportation and
storage procedures (refrigeration or exclusion of light) should be
described (WHO/EURO, 1987).
Because environmental samples are typically heterogeneous, a
sufficiently large number of samples (10 or more) must be analysed to
obtain meaningful composition data. The number of individual samples
that should be analysed will depend on the kind of information
required. If an average composition value is required, a number of
randomly selected individual samples may be obtained, combined, and
blended to provide a homogeneous composite sample, from which a
sufficient number of subsamples are analysed. If composition profiles,
time trends, or the variability of the sample population is of
interest, many samples need to be collected and analysed individually.
If field blanks are not available, efforts should be made to obtain
blank samples that best simulate a sample that does not contain the
analyte. In addition, measurements should be made to ascertain
whether, and to what extent, any reagent or solvent used may
contribute or interfere with the analytical results (laboratory and
solvent blanks). The recovery tests are frequently used and are
necessary to evaluate the analytical methodology. Uncontaminated
samples from control sites that have been spiked with the analyte of
interest provide the best information, because they simulate any
matrix effect. When feasible, isotopically labelled (13C, 37Cl)
analytes spiked into the sample provide the greatest accuracy, since
they are subjected to the same matrix effects as the analytes. The
13C-labelled compounds can be used to:
(a) validate sampling (sampling surrogate);
(b) validate analytical waste (clean-up surrogate);
(c) validate quantification (internal standard).
Only a small number of laboratories in the world have access to, and
experience in working with, these complicated analyses. In order to be
able to compare data generated in different laboratories, the same
quantitative standard compounds should be used. Interlaboratory
calibrations, or "round-robin" studies, have been performed in a few
cases (WHO/EURO, 1987).
2.3.1.1 Extraction procedures
Air
The sampling device used to collect and determine PCBs in air consists
of a glass fibre filter and a Florisil stick. The glass fibre filter,
held in a stainless steel holder, removes particles larger than
0.3 µm. The air passes from the filter to the Florisil stick, which is
made in 2 sections, to provide information on migration and trapping
efficiency for PCBs. Each section contains 0.4 g of Florisil preceded
and followed by a glass wool plug. The front and back sections are
separated by 2 plugs of glass wool. The front is spiked with 0.1 µg of
p,p'-DDE as a surrogate for recovery measurement and as an indication
of analyte migration. The detection limit for PCBs in air is reported
to be 0.3 ng/m3 (Anon., 1985; WHO/EURO, 1987; NIOSH, 1987).
Particulate fallout from air has been trapped on 200 µm nylon net
coated with silicone oil, and the PCBs then extracted with hexane
(Södergren, 1972). Separate determinations of particulate and vapour
phase PCBs in air have been made by passing a large volume of air
through a filter followed by an impinger containing hexane or toluene
(Rappe et al., 1985c), a polyurethane plug (Bidleman & Olney, 1974),
or ceramic saddles coated with OV 17 silicone (Harvey & Steinhauer,
1974) to absorb the vapour.
Surface sampling
Surface sampling of PCBs can be carried out using a wet-wipe procedure
with a cotton gauze pad that has been dampened with hexane before
collecting the sample. The sampled area is 0.25 m2. The wet-wipe
sampling procedure collects both the contaminants from the surface and
the contaminants that can be extracted from pores in the material.
Materials such as waxes and plasticizers may interfere with the
chemical analysis (WHO/EURO, 1987).
Another sampling method has been described by Rappe et al. (1985c),
where a dry filter paper or Kleenex tissue is used first, for wiping,
followed by a wet wipe with water-dampened material.
Water
PCBs have been extracted from water by passing a sample through a
filter of undecane and Carbowax 400 monostearate supported on
Chromosorb W (Ahling & Jensen, 1970) or a porous plug of polyurethane
coated with a suitable gas-liquid chromatographic stationary phase, or
Amberlite XAD-2 resin (Harvey et al., 1973) followed by elution of the
PCBs with a solvent. Ahnoff & Josefsson (1974, 1975) have described
liquid-liquid extraction into cyclohexane.
Soil and sediment
In a study by Huckins et al. (1988), sediment samples were thawed at
room temperature and placed in a hexane-rinsed foil pan and air dried
for 5 days. The sediment was broken up, homogenized, and mixed with
anhydrous disodium sulfate until dry, for column extraction. The
samples were extracted with methylene chloride. PCB residues were
enriched by adsorption column chromatography on silica gel and
sulfuric acid silica gel. Prior to GC analysis, nitric acid-rinsed
copper wool was added to the sediment extract to remove elemental
sulfur. An aliquot of the PCB residues was diluted in a mixture
methylene chloride: cyclohexane (1:1) and the bulk of the o,o-Cl
substituted PCB components eliminated by eluting the column with
different solvents. The different PCB congeners were determined by
GC-ECD.
The feasibility of cleaning PCB-contaminated soils using a solvent
extraction method was studied by Reilly et al. (1986). Compared with
direct incineration of the sludge, the solvent extraction route has a
number of shortcomings; the detailed design of the extraction plant as
well as its operation will be quite challenging as an extremely
leak-tight operation is essential, considering the nature of the
material handled. Direct incineration will clean the solids much more
thoroughly than is feasible by solvent extraction under ambient
conditions. Furthermore, it is inevitable that some residual solvent
will remain in the solids after processing. The solvent extraction
process costs essentially the same as direct incineration.
Biological samples
Most analysts have used standard methods, developed for organochlorine
pesticides, in which the PCBs are extracted together with the fat; the
sample is ground with anhydrous sodium sulfate and extracted with
petroleum ether or hexane. Porter et al. (1970) studied the optimal
conditions for this procedure. A dehydrating solvent may be included
to facilitate the breakdown of cell structures; ethanol (Norén &
Westöö, 1968) and acetone (Jensen et al., 1973) have been used.
Reznicek (1987) described a method to extract and determine PCBs in
blood. The sensitivity of the method was 10 µg/litre.
2.3.1.2 Sample clean-up
Diverse extraction and clean-up procedures have been devised to
preferentially remove co-extractives that are present in different
matrices and interfere with routine quantitative gas chromatographic
and gas chromatographic-mass spectrometric analysis.
The analysis of lipid-containing matrices for residues of
organochlorine pesticides and PCBs is a common procedure. All the
methods require the separation of the residues from the lipids prior
to the determination of the PCBs by gas chromatography. The removal of
the lipids is usually carried out by low-resolution column
chromatography using an adsorbent, such as silica, alumina, or
Florisil as the stationary phase. Low-resolution gel permeation
chromatography has also been used. An electron-capture detector is the
most commonly used detector, but clean-up procedures may still leave
electron-capturing species in the extract, so the identities of the
eluting peaks must be confirmed. In order to overcome some of these
problems, perchlorination of the PCBs has been used, giving rise to
one GC peak (decachlorobiphenyl), which is well removed from most
interfering peaks, but this technique has been found to be
qualitatively and quantitatively unreliable and unsatisfactory.
Seymour et al. (1986b,c) attempted to simplify clean-up procedures by
using high performance liquid chromatography (HPLC) coupled with gas
chromatography-mass spectroscopy. This latter technique is less
expensive than it used to be and is the only technique that can
possibly identify each peak as a PCB before quantification is carried
out, thereby improving the quality of the result. It is also capable,
when used in the selective ion monitoring mode (SIM), of detecting
only PCBs, even in the presence of pesticides, so that sample clean-up
is further simplified.
Seymour et al. (1986a) described a clean-up procedure, with a
preparative, high-performance liquid chromatographic (HPLC) separation
method for selected pairs of chlorobiphenyl isomers, produced by
Cadogen coupling in the preparation of individual congeners, to be
used as standards in congener-specific determination using capillary
GC methods.
A routine method for the determination of PCBs in breast milk,
described by Seymour et al. (1987), is less labour-intensive and more
cost effective than the traditional methods. These advantages were
achieved by adsorption of the milk on a polar substrate prior to
Soxhlet extraction, using a polymeric HPLC column for the clean-up of
the extract, followed by highly selective capillary GC-MS analysis.
Methods for the removal of fat from the extract include solvent
partitioning between hexane and acetonitrile or dimethylformamide, or
treatment with strong sulfuric acid or ethanolic potassium hydroxide.
Gel permeation has also been used (Stalling et al., 1972), and Holden
& Marsden (1969) removed fat on dry, partially deactivated, alumina
columns. Certain pesticides, such as dieldrin, are destroyed by the
sulfuric acid treatment, so this method cannot be used if such
pesticides are to be determined together with PCBs (Jensen et al.,
1973).
Huckins et al. (1988) described the clean-up of fish samples. Tissue
samples were thawed, mixed, dried with sodium sulfate, and extracted
in glass columns with methylene chloride. The extract was evaporated
and the lipid content was determined gravimetrically. Gel permeation
chromatography was used for removal of lipid from fish sample
extracts. PCB residues were enriched by adsorption column
chromatography on silica gel and sulfuric acid silica gel, eluted with
a mixture of methylene chloride and cyclohexane, and determined by
GC-ECD.
PCBs can be separated from organochlorine pesticides by column
chromatography on Florisil (Mulhern et al., 1971), silica gel (Holden
& Marsden, 1969; Armour & Burke, 1970; Collins et al., 1972) or on
charcoal (Berg et al., 1972; Jensen & Sundström, 1974a). Several
laboratories have reported difficulties in repeating results obtained
by other investigators; the ease of separation appears to depend on
the characteristics of the absorbent, of the eluting solvent, and of
the sample extract, though there does not appear to be any difficulty
in separating all interfering substances, except DDE, a metabolite of
DDT. Thin-layer chromatography has been used for separation by Norén &
Westöö (1968), Bagley et al. (1970), and Reinke et al. (1973).
In many environmental samples, DDE is present in larger amounts than
the PCBs, and must be removed before their quantitative determination.
Oxidation procedures have been used to convert DDE to dichlorobenzo-
phenone; recommended oxidants are potassium dichromate and sulfuric
acid (Westöö & Norén, 1970b) and chromium (II)oxide and acetic acid
(Mulhern et al., 1971). Jensen & Sundström (1974a), who were
interested in determining DDT/PCB ratios in environmental samples,
preferred sodium dichromate in acetic acid with a trace of sulfuric
acid. They claimed that this does not destroy DDT and its metabolite
DDD, which may be present in extracts after clean-up with strong
sulfuric acid, and that using this mixture makes possible the
quantitative determination of the dichlorobenzophenone from the
oxidation of DDE.
Conversion of DDT to DDE can be achieved by treatment with ethanolic
potassium hydroxide, which also removes interference from elemental
sulfur (Ahling & Jensen, 1970). Sulfur may also be removed by
activated Raney nickel (Ahnoff & Josefsson, 1975) or by metallic
mercury.
Beck & Mathar (1985) used gel permeation chromatography to clean
extracts of food of animal origin.
2.3.2 Separation and identification
2.3.2.1 Chromatographic separation
Numerous gas chromatographic studies using packed or capillary columns
have confirmed the complexity of all commercial PCB formulations. The
accuracy in determining PCB levels is highly variable and matrix
dependent. Many factors including: the water solubility, volatility,
and biodegradability of individual PCBs, will alter the composition of
a commercial PCB preparation introduced as a pollutant into the
environment. Thus, the composition of PCB extracts from environmental
matrices will vary widely and often do not resemble any commercial
mixture. Quantitative analyses on these mixtures is usually determined
by pattern- or peak-matching methods, using artificially reconstituted
mixtures of different commercial formulations. High-resolution, glass
capillary gas chromatographic analysis can provide a solution.
Capillary gas chromatography columns, currently in use, are made of
fused silica, chemically bonded with various stationary phases, to
achieve a range of different selectivities towards complex samples. In
general, packed columns have been replaced by capillary columns,
because of their far superior efficiency. The identities of the
individual peaks must then be determined by using synthetic standards
and by retention index addition methods. This latter technique
predicts the relative retention times (RRT) of specific PCBs and has
been used to assign the structures of individual PCB congeners. The
method relies on the RRT values that have been determined for
synthetic PCB standards. On this basis, Safe et al. (1985a) reported
the first congener-specific analysis of a PCB preparation and PCBs in
human milk.
Some workers use GC with mass selective detection (MSD), which
quantifies the level of chlorination in a sample extract
(Alford-Stevens, 1986). Tanabe et al. (1987) and Kannan et al. (1987)
described a method to determine the 3 toxic, non- ortho-chlorine-
substituted, coplanar PCBs, 3,4,3',4'-tetra, 3,4,5,3',4'-penta-,
and 3,4,5,3',4',5'-hexachlorobiphenyl, which are biologically active
congeners. The method comprised alkali digestion, carbon
chromatography, and high-resolution gas-chromatography. Using
this method, it is possible to determine ppt levels of these toxic
residues in biological samples. Duinker et al. (1988) used
multidimensional gas chromatography with ECD to determine levels of
all congeners in some Clophen and Aroclor mixtures and found
considerable differences between their composition of congeners and
those in an extract of a seal blubber sample. Using this technique,
congeners were identified that had, hitherto, been undetected, using
other analytical techniques. It was possible to identify the toxic
congeners in the samples studied, even when the relative contribution
of each congener to the cluster was as low as 0.01%.
2.3.2.2 Gas-liquid chromatography
Most analysts use gas-liquid chromatography with an electron-capture
detector for the separation of PCBs from the extract after clean-up.
Stationary phases commonly used are silicones or their derivatives,
for example, DC 200, SF 96, OV 1, and QF 1, or Apiezon L. Jensen &
Sundström (1974a) stated that, with a mixture of SF 96 and QF 1, 14
peaks could be obtained from Clophen A50, but that Apiezon L gave much
better resolution. They obtained better peak separation by prior
fractionation on a charcoal column, which separated the PCBs according
to the number of o-chlorine substituents; they regarded such
refinements as unnecessary in PCB residue analysis, but they may be of
value in the study of the selective, environmental degradation of
PCBs. Column temperatures used ranged between 170°C and 230°C. Glass
capillary columns are superior to packed columns giving better
separation of closely-related congeners; they also give good
separation of PCBs from DDT and its metabolites (Zell et al., 1977;
Dunn et al., 1984; Beck & Mathar, 1985; Alford-Stevens, 1986; Tanabe
et al., 1987; Duinker et al., 1988).
A gas chromatography/electron impact mass spectrometry (GC/EIMS)
method was used by Erickson et al. (1988) for the determination of
by-product (non-Aroclor) PCBs. In this method, the recovery of 4
13C-labelled PCBs was measured to assure adequate recovery of the
native PCBs from diverse matrices. The complexity of the matrices and
the high probability of chlorinated organic interferents precluded the
use of GC/ECD. The best available technique for universal application
to commercial products, and associated waste, is GC/EIMS. During the
validation work, the anticipated difficulty of qualitative and
quantitative data interpretation was confirmed. In addition to the
inherent problems resulting from extrapolation from 11 standards to
209 analytes, interpretation of the complex peak clusters is tedious.
2.3.3 Quantification
An electron-capture detector (ECD) is the most commonly used
instrument for the quantification of PCBs. However, the response of
this detector varies according to the number and location of the
chlorine atoms in the PCB molecule, resulting in difficulties when the
sample under investigation contains PCBs that have degraded (Zitko et
al., 1971).
Various principles have been used to quantify PCB residues:
* comparison of a single peak in the residue with the corresponding
peak in a commercial reference PCB (Aroclor, Clophen);
* comparison of the total response for several peaks in the residue
with the total response of the corresponding peaks in a reference
standard;
* comparison of the response of all peaks in the sample with those
in the reference standard;
* perchlorination of PCBs to decachlorobiphenyl followed by
quantification of this single compound.
The results obtained using these various methods differ; consequently,
the precision in these analyses is not very good. Recently, Dunn et
al. (1984) described a method for the quantification of PCBs using gas
chromatography data, based on a pattern recognition technique and
partial least squares in latent variables. The data to which it was
applied were gas chromatograms of Aroclor 1242, 1248, 1252, and 1260.
This technique also allows the classification of unknown samples
(WHO/EURO, 1987).
Fait et al. (1989) investigated whether the results obtained for total
PCBs using FSCGC/ECD (see section 2.3), differed significantly from
those determined using packed column gas chromatography electron
capture (PCGC/ECD) techniques, within 3 exposure groups. The
concentrations of individual PCBs were determined in both the serum
and adipose tissue from 35 transformer repair workers and 17 previous
repair workers, exposed mainly to Aroclor 1260, in comparison with 56
non-exposed workers. Eighty-nine PCB peaks were identified. The total
serum PCBs determined by FSCGC/ECD greatly exceeded that from standard
PCGC/ECD. The median concentrations in serum were: 43.7, 30.0, and
16.1 µg/litre, and the median concentrations in adipose tissue were:
3180, 888, and 821 µg/kg, respectively. In all workers,
hexachlorinated and heptachlorinated congeners predominated followed
by octachlorinated and pentachlorinated species. The 7 major peaks in
serum and adipose tissue were 2,3,5,6,3',4',5'/ 2,3,4,5,2',4',5'/
2,3,4,5,2',3',4'-heptachloro-; 2,3,4,2',3',5'-hexachloro-;
2,4,6,3',4',5'/ 2,4,5,2',4',5'-hexachloro-; 2,3,4,5,2',3',5',6'/
2,3,4,5,6,2',3',5'-octachloro-; 2,4,5,3',4'/ 3,4,5,2',3'-pentachloro-
and 2,3,4,2',3',4'/ 2,3,5,6,2',4',5'/ 2,3,4,5,2',4',6'
multichlorobiphenyls.
The response of the electron capture detector is not equal for all PCB
components, being much affected by the degree of chlorination, as
already mentioned (Zitko et al., 1971). This does not lead to
difficulties when the sample under investigation has been directly
contaminated by a commercial PCB mixture, as this mixture can be used
as a standard. Difficulties are encountered when the PCBs in the
sample have undergone selective environmental degradation. Several
investigators have noted that the pattern of peaks from such samples
resembles fairly closely that of one or other of the higher
chlorinated PCB mixtures, such as Aroclor 1254, and they have compared
the total area of the peaks with that of the nearest commercial
product, in order to determine the amount of PCBs in the sample
(Armour & Burke, 1970; Tuinstra, 1983). Collins et al. (1972) observed
that, under their conditions, the area of peaks usually encountered in
extracts of tissue samples was very similar to that of an equivalent
amount of DDE, thus, DDE could be used for calibration. In order to
overcome the uncertainties of these procedures, Rote & Murphy (1971)
divided the peaks into groups according to the number of chlorine
atoms in the molecule, as determined from mass spectrographic data,
and calculated the PCB content of each group from the theoretical
response of the detector to chlorine content. Jensen et al. (1973)
selected a commercial PCB that included all the peaks from the
extract; they determined the PCB content of each peak by combined mass
spectrometry and coulometry, and determined the total PCBs in the
sample by comparing the height of each peak obtained with the extract
with those obtained with the reference sample. Simpler methods have
been used including that of Koeman et al. (1969), who compared the
height of a single peak, obtained with the extract, with that of a
peak with the same retention time obtained with a commercial PCB
mixture, and those of others who averaged out more than one peak for
this calculation (Reynolds, 1971; Reinke et al., 1973). Rote & Murphy
(1971) calculated that such procedures may give more than double the
values obtained by a more accurate method.
In the characterization of PCB components in PCB mixtures, the
retention properties of the components of the mixtures, as well as a
great number of synthesized components, were used to predict a
complete analysis of mixtures as Aroclors 1242, 1254, and 1260. Jensen
& Sundström (1974a) synthesized a large number of reference substances
and were able to identify almost 60 components in Clophen A50 and A60.
Attempting to account for unidentified peaks, authors have used the
chromatographic retention indices of available components to calculate
such data for missing ones. The identity of many peaks could not,
however, be determined unambiguously. Some of these uncertainties have
been resolved by the application of techniques other than the
comparison of retention times e.g., MS, NMR, and IR. The efficiency of
packed columns in GLC is not sufficient to allow their use for the
accurate analysis of complex mixtures, in most cases. Another approach
to the use of packed columns involves the use of columns with various
selectivities. In this way, complete analysis of all components in
Aroclors has been claimed with the use of up to 12 columns. The
strongly increased GLC separation offered by capillary columns has
been used to advantage in the analysis of technical formulations, in
some cases the eluate was analysed by MS. To identify individual
congeners, gas-liquid (using glass capillaries with different
coatings) chromatography (GLC) was used by Albro & Parker (1979) and
Albro et al. (1981). Hydrogen flame ionization detection (HFID) and
electron capture detection (ECD) and MS were used by Duinker &
Hillebrand (1983).
2.3.4 Accuracy of PCB determinations
A group of 8 analysts, engaged in an investigation of pollution in the
North Sea, undertook a collaborative study to determine the PCB
content of a sample of fish oil, using the methods currently employed
in their laboratories (International Council for the Exploration of
the Sea, 1974). The PCB values obtained ranged from 1.0 to 3.9 mg/kg
with a mean of 1.97 mg/kg and a standard deviation of 0.93 mg/kg.
Better agreement was obtained with the same fish oil fortified with
PCBs at a concentration of 10 mg/kg; the mean of the results for the
fortified sample was 10.0 mg/kg with a standard deviation of
1.1 mg/kg.
A probable source of error is incomplete initial extraction of PCBs
from a sample (Holden & Marsden, 1969). Another source of variation
between laboratories lies in the method used to quantify gas-liquid
chromatographic peaks; Van Hove Holdrinet (1975) considered this to be
the major source of error.
It is evident that caution should be exercised in accepting the
analytical results from a laboratory, particularly for samples with a
low PCB content, until the competence of the laboratory has been
established by an inter-laboratory collaborative study (Tuinstra,
1983).
Schulte & Malisch (1984) described a method to determine the real PCB
contents of environmental samples. A technical PCB mixture of known
composition was used for calibration. The PCB concentrations were
determined in samples of human milk and butter and the calculated
contents were 50% and 40% lower, respectively, than the values
obtained by the usual calculation based on evaluation of some higher
peaks of technical PCB mixtures.
2.3.5 Confirmation
Since Jensen first identified as PCBs hitherto unknown substances that
interfered in the glass-liquid chromatographic determination of
organochlorine pesticides using mass spectrographic data, other
investigators have confirmed the presence of PCBs in environmental
samples by combining gas-liquid chromatography with mass spectrometry
(Bagley et al., 1970) and with coulometry, to measure the chlorine
content. The conversion of PCBs to bicyclohexyl and decachlorobiphenyl
is further confirmation (Berg et al., 1972). The widespread
distribution of PCBs is now well established, and, as adequate methods
are available to remove interference from organochlorine pesticides,
there is no evidence of the presence of other interfering substances
in the types of sample that have so far been analysed, down to a limit
of detection of around 0.01 mg/kg. This does not necessarily apply to
other types of sample, particularly when very low levels are being
sought; Ahnoff & Josefsson (1973) reported a number of unknown
interfering substances, when measuring PCBs in water at levels below
1 ng/litre. One of these substances was subsequently identified as
elemental sulfur. They recommend confirmation by mass fragmentography
for such samples.
2.3.6 Detection limits
The limits of determination using low or high resolution mass
spectrometry are 0.01-1 pg per injection of each congener. The
detection levels in samples depend on the sample size and matrix.
Using an air sampling device described by Rappe et al. (1985b), a
detection level of 0.05 pg/m3 per congener could be determined in
ambient air (WHO/EURO, 1987).
In general, other substances are not considered to interfere at levels
of about 0.01 mg/kg. In river water and air, levels of 1 ng/litre and
0.3 ng/m3, respectively, are reported to be the detection limits of
PCBs (WHO/EURO, 1987). Tuinstra (1983) found a limit of detection for
individual chlorobiphenyls in environmental and biological samples, of
less than 1 µg/kg (see Table 7).
The results for sewage sludge, eel, grass, cow's milk, and human fat
are given in Table 7 (Tuinstra, 1983). Individual chlorobiphenyls were
also estimated in the monitoring programme for environmental and
biological samples in the Netherlands.
2.4 Codex questionnaire on analytical methods
2.4.1 Interpretation and comparability of data
Monitoring data are available from many sources in many countries.
They have been obtained using various methodologies, such as different
sampling techniques and different methods of analysis and
quantification. Limits of determination reported vary by a factor of
1000 or more.
Given this situation, data on levels of PCBs have to be interpreted
with the greatest care. Comparisons can only be made between data from
the same laboratory, using the same validated technique over a long
period. Comparisons between data from different laboratories have to
be limited to the very few cases, where very strict inter-laboratory
checks have been made on the basis of the same sampling and analytical
techniques. Indications about trends can only be obtained when taking
into account these basic considerations (Beck & Mathar, 1985; Tuinstra
et al., 1985b,c).
In June 1985, a questionnaire was distributed to all Codex Contact
Points with the aim of providing background information on PCBs for
the ad hoc working group on contaminants to compare such factors as
methods of analysis, quantification, monitoring, etc. Eighteen out of
22 countries responded to the questionnaire.
In some cases, the information given was incomplete, but it is
apparent that a variety of clean-up methods is employed. Where good
laboratory practices are followed and tests indicate close to 100%
recovery of standards from spiked samples, the main effect of
different clean-up procedures will be on the limit of detection.
For gas chromatography, 6 countries reported that they used capillary
columns as alternative or confirmatory systems. Among the respondents,
the Netherlands and the Federal Republic of Germany routinely used
capillary columns and specific PCB isomers as regulatory standards.
The types of packed column materials used varied considerably. With
respect to quantification, pattern comparison with standards of
various PCB formulations was the method most favoured, though some
countries specified the use of certain combinations of peaks. In
several cases, the methods being used were stated to have been
collaboratively tested, or checked by inter-laboratory ring tests.
During the sixties, packed column chromatography was the most widely
used method in the determination of PCBs. Results obtained with this
technique varied widely between laboratories, and were much influenced
by the method of quantification chosen and by the PCB mixture used as
a standard. Chemical conversion methods, especially perchlorination,
have also been used. These methods are quite sensitive, but do not
allow for peak pattern identification. Another drawback of
perchlorination is that conversion of less chlorinated biphenyls is
not quantitative.
Sensitivity is sufficient, if adequate clean-up methods are used.
Combined gas chromatography/mass spectrometry has a somewhat lower
sensitivity, needs more expensive equipment, and is not considered
suitable for routine work. The results obtained using these techniques
may vary widely and most of them can only be used as rough estimates.
When capillary columns are used with temperature programming, almost
all PCB isomers and congeners normally present in samples can be
identified. This method is now considered to be the best available
technique. However, it is important to decide which isomers should be
used as guiding substances.
Table 7. Typical values of individual chlorobiphenyls in Dutch environmental and
biological samples. Peak numbering according to IUPAC rulesa
PCB Structure Sewage Eel Grass Cow's Human
compound sludge milk fat
µg/kg µg/kg µg/kg µg/kg µg/kg
(dm)b product (dm)b,c fatc fat
28d 2,4,4' 60 35 -c -c 45
52d 2,5,2'5' 22 110 0.4 2.1 10
44 2,3,2'5' 20 34 0.2 0.9 10
95 2,3,6,2'5' 58 130 0.7 1.6 30
101d 2,4,5,2'5' 30 85 0.6 3.1 15
151 2,3,5,6,2'5' 9 24 0.2 0.6 10
149 2,3,6,2'4'5' 42 90 0.6 2.5 15
118 2,4,5,3'4' 20 110 0.3 -c 80
153d 2,4,5,2'4'5' 54 180 0.7 13 295
141 2,3,4,5,2'5' 10 40 0.2 0.6 <5
138d 2,3,4,2'4'5' 45 200 0.7 11 235
128 2,3,4,2'3'4' 7 20 <0.1 1.2 15
180d 2,3,4,5,2'4'5' 33 80 0.5 6.4 205
170 2,3,4,5,2'3'4' 10 30 0.2 1.8 90
201 2,3,4,5,2'3'5'6' <5 10 <0.1 <0.5 20
a From: Tuinstra (1983).
b dm = dry matter.
c nd = not determined.
d Monitoring compounds.
2.5 Activities of the WHO Regional Office for Europe
The WHO Regional Office for Europe (WHO/EURO) has an ongoing programme
related to PCBs, as well as to other chlorinated hydrocarbons,
including polychlorinated- para-dibenzodioxins (PCDDs) and
polychlorinated dibenzofurans (PCDFs). Within this programme,
practical guidelines to prevent and control accidental and
environmental exposures to these chemicals have been published in the
Environmental Health Series of WHO/EURO (1987). The other important
project within this programme dealt with the assessment of the health
risks to infants associated with contamination of mother's milk. This
assessment was completed by a WHO/EURO Expert Consultation held in
Abano Terme, Italy, in 1987, and the output of this consultation has
been published in the Environmental Health Series of WHO/EURO (1988).
In order to produce more data on exposure levels through human milk,
WHO/EURO has been coordinating analytical field studies in which
several countries have participated. The results of these studies have
been published in the Environmental Health Series of WHO/EURO (1989).
This document also includes the results of interlaboratory quality
control studies on levels of PCBs, PCDFs, and PCDDs in human milk. In
the first series of studies, 12 laboratories were involved. The second
round of the quality control studies has been completed, with the
participation of additional laboratories, and the results will be
published. Furthermore, the repetition of the analytical field studies
on the levels of PCBs, PCDFs, and PCDDs in human milk will be
implemented in 1991 and coordinated at WHO/EURO.
2.6 Appraisal
Since the congener composition and relative concentrations of the
individual components in PCB extracts from environmental and
biological samples differ markedly from those in commercial PCB
mixtures, the quantitative determination of the PCB contents of such
samples presents a special problem. Various approaches to the
quantitative determination of PCBs have been reported including:
attempts to determine the total PCB concentration through
perchlorination of the mixture; identification of selected
chromatographic peaks through gas chromatographic techniques with
packed columns using certain commercial products as standards; as well
as attempts to carry out congener-specific analysis, based on high
resolution chromatographic separation followed by identification and
quantification by mass spectrometry using synthetic standards. This
last method is considered the best at present, though it is not
feasible for all laboratories. Although the concentration values
obtained from the various methods might be similar, such comparison
will be limited and is of questionable value for most purposes. The
occurrence of specific PCB congeners in various samples and a
consideration of the relative toxicity and persistence of the
congeners have been suggested as a basis for a congener-specific
analytical approach. While this approach can be useful, particularly
in risk/hazard assessment exercises, it must be realized that it is
based on the present knowledge about the occurrence, persistence, and
toxicity of specific congeners. It does not take into consideration
potentially unrecognized toxicities associated with the same or
different congeners, which may be present in a sample, also it is not
feasible in some countries. Therefore, further research in this area
should continue to improve the basis for monitoring programmes and for
a congener-specific approach.
In the selection of areas with high levels of contamination, in order
to establish priorities for action, it is considered that analytical
competence and the use of adequate controls and standards is more
important than highly accurate laboratory analysis. Also, the quality
and usefulness of analytical data depend critically on the validity of
the samples and the adequacy of the sampling programme. A quality
assurance programme and collaborative studies should be part of any
long-term study on PCBs, since there are several possible sources of
error. In this situation, data on levels of PCBs have to be
interpreted with the greatest care and, in general, definitive
comparison can only be made between data from laboratories using the
same techniques and interpretation of results.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Polychlorinated biphenyls are aromatic chemicals that do not occur
naturally in the environment.
3.2 Man-made sources
3.2.1 Production levels and processes, uses
The first chlorinated biphenyl was synthesized in 1864, but it was not
until 1929/1930 that the PCBs were produced commercially for use:
(a) as dielectrics in transformers and large capacitors;
(b) in heat transfer and hydraulic systems;
(c) in the formulation of lubricating and cutting oils and wax
extenders;
(d) as plasticizers in paints, and as ink solvent/carriers in
carbonless copy paper, adhesives, sealants, flame retardants, and
plastics (Hutzinger et al., 1974; Pomerantz et al., 1978).
An extensive review of the uses of PCBs is given in DFG (1988).
3.2.1.1 World production figures
Over one million tonnes of PCBs have been produced commercially under
a number of trade names, such as Aroclor, Fenchlor, Clophen, and
Kanechlor.
Details of the production and uses of PCBs in the USA have been
released, and have been summarized by Nisbet & Sarofim (1972). Annual
production increased steadily from 1930 and reached a maximum in 1970
of 33 000 tonnes. Of this, 56% was used as a dielectric (36% in
capacitors and 20% in transformers). Various plasticizer outlets
accounted for 30%, hydraulic fluids and lubricants, 12%, and heat
transfer liquids, 1.5%. During this peak year, 65% of the production
was of the 42% chlorinated type, 25% was less chlorinated, and the
remainder more chlorinated. After 1970, production decreased sharply
owing to the voluntary limitation of sales by the Monsanto Company,
the major manufacturer in the USA.
Following the restriction of sales for dissipative uses, the
percentage of PCBs sold as dielectrics rose to 77% in 1971 and the
proportion of highly chlorinated products was considerably reduced;
Aroclor 1016 replaced Aroclor 1242. In Japan, 44 800 tonnes of PCBs
were used from 1962 to 1971; of this, 65.4% was used in the electrical
industry, 11.3% in heat exchangers, 17.9% in carbonless copying paper,
and 5.4% for other dissipative uses (Ishi, 1972).
During the period 1980-84, the production in EEC member states was as
follows: France, 16 200; Federal Republic of Germany, 24 200; Italy,
4500; and Spain, 3400 tonnes. After 1984, production was continued
only in France and Spain (Bletchly, 1985; WHO/EURO, 1987).
By the end of 1980, the total amount of PCBs produced was 1 054 800
tonnes (of which approximately half was used in transformers and
capacitors, see Table 8), divided between the following countries (in
tonnes): USA, 647 700; Federal Republic of Germany, 130 800; France,
101 600; United Kingdom, 66 800; Japan, 59 300; Spain, 25 100; and
Italy, 23 500 (Bletchly, 1983).
In addition, Czechoslovakia and the USSR have manufactured PCBs for
their domestic market under the trade names of Delor and Sovol,
respectively, but the data on production quantities are not available.
According to an OECD report, transformers and capacitors provided the
major outlets for PCBs in most OECD countries in 1971. In 1972,
several countries restricted sales; in Sweden the importation and use
of PCBs were restricted by law; in the United Kingdom, as in the USA,
sales were voluntarily restricted to the lower chlorinated PCBs for
use as dielectrics in enclosed systems, and, in the USA in 1979,
manufacture, use, handling, storage, and disposal were promulgated. As
late as 1985, a final rule concerning the restriction and conditions
on the use of PCB transformers was published (USEPA, 1985). In Japan,
the production and use of PCBs were banned in 1972.
The 24 OECD countries adopted a Decision in 1973, limiting the use of
PCBs to certain specific applications and asking for the control of
the manufacture, import, and export of bulk PCBs, for adequate waste
treatment and for a special labelling system for PCBs and
PCB-containing products. On 13 February 1987, the Council of the
Organization for Economic Co-operation and Development (OECD) adopted
a further Decision-Recommendation (C(87)2(final)) on "Further measures
for the protection of the environment by control of polychlorinated
Table 8. Estimated usage of PCBs in transformers and large capacitors in
a number of OECD countries in 1930-80 (in tonnes)a
Country Usage in Usage in Total
transformers capacitors
France 50 700 8 800 59 500
Federal Republic 44 400 17 700 62 100
of Germany
Italy 10 400 1 500 11 900
Japan 37 200b 37 200
Spain 20 100 3 400 23 500
United Kingdom 5 800 8 100 13 900
United States 125 800 130 400 256 200
of America
Total 294 400 169 900 464 300
a From: WHO/EURO (1987).
b Includes the usage in both transformers and capacitors
biphenyls". With this Decision-Recommendation, the OECD Member
countries committed themselves to ban virtually all new uses of PCBs,
accelerate the phasing out of PCBs from existing uses, control PCBs in
contaminated products, articles, or equipment, and ensure appropriate
disposal methods for PCB-containing waste. The uses of PCBs have been
virtually restricted to those in "closed systems". In 1976, an EEG
Directive made the limitations of the use compulsory for the EEG
Member States. Other Directives, such as those on waste treatment and
disposal, followed (van der Kolk, 1984a, Personal communication).
3.2.1.2 Manufacturing processes
Industrial manufacturing of PCBs is based on the chlorination of
biphenyl by anhydrous chlorine, under heated reaction conditions and
in the presence of suitable catalysts (e.g., iron-chloride). Depending
on the reaction conditions, a degree of chlorination varying between
21% and 68% (weight percentage, w/w) can be achieved.
The yield is always a mixture of different compounds and congeners.
Commercial mixtures generally have been purified by filtration and
fractional distillation, but, in spite of this, they have been found
to contain many impurities (WHO/EURO, 1987). In general, commercial
PCB products contain impurities, mainly polychlorinated dibenzofurans
(PCDFs).
Rappe et al. (1985d) cf. WHO/EURO (1987) analysed a series of
commercial PCBs, using a new clean-up technique based on reverse-phase
chromatography on a carbon column followed by a fluorosil column. In
all PCB products, PCDFs were found at levels varying from a few mg/kg
up to 40 mg/kg. The chlorination pattern of the PCDFs was found to
vary with the chlorination level of the PCBs. In most products,
2,3,7,8-substituted tetra-, penta-, and hexa-CDFs were the major
constituents.
3.2.2 Uses
PCBs have been widely used in electrical equipment, such as capacitors
and transformers. These have often been considered to be closed
systems, though small amounts of PCBs can frequently be found on the
outer metal surface of such equipment.
Smaller volumes of PCBs have often been used as fire-resistant liquid
in nominally closed systems, such as hydraulic and heat exchange
systems (WHO/EURO, 1988).
Broadhurst (1972) reviewed the many technical applications of PCBs
that appear in the literature and in patent specifications, and
indicate the possibility of a widespread, non-occupational, low-level
exposure to PCBs, other than that derived from the diet. PCBs are used
in the home in ballast capacitors for fluorescent lighting, and
exposure from pressure-sensitive copying paper has not been limited to
office workers. The valuable properties of PCBs as plasticizers has
led to their use in furnishings, interior decoration, and building
construction; examples are surface treatment for textiles, adhesive
for waterproof wall coatings, paints, and sealant putties. PCBs have
been used as plasticizers for plastic materials and in the formulation
of printing inks.
The value of PCBs for industrial applications depends on their
chemical inertness, resistance to heat, non-flammability, low vapour
pressure (particularly with the higher chlorinated compounds), and
high dielectric constant.
Data on the usage of technical PCB mixtures in Europe are scarce. In
the 1960s and early 1970s, PCBs were used in (WHO/EURO, 1987):
(a) completely closed systems;
(b) nominally closed systems;
(c) open-ended applications.
3.2.2.1 Completely closed systems
PCBs have been widely used in electrical equipment, such as capacitors
and transformers, which are considered to be completely closed
systems. Historically, capacitors are the single largest PCB-use
category. The PCB mixtures used for this purpose are, for example,
Pyralene 3010, Aroclor 1016, 1221, and, earlier, also Aroclor 1242 and
1254. The amounts used in a number of OECD countries are presented in
Table 8 (OECD, 1982; Bletchly, 1983; Callahan et al., 1983).
Since the late 1970s and the beginning of the 1980s, PCB-filled
capacitors have largely been superseded by capacitors with a non-PCB
dielectric fluid. The tendency for this substitution varies from
country to country, for example, it started in Sweden and Finland in
1982, and in Norway in 1985.
The technical PCB mixtures used in transformers are mostly highly
chlorinated like Aroclor 1254 and 1260. In general, the PCBs are used
in combination with tri- and tetrachlorobenzenes as mixtures called
Askarel.
The amounts of PCBs used in transformers differ in different
countries. In France, where most transformers are placed indoors, the
major dielectric fluid is PCBs or Askarels, which are both flame
retardants, while in Scandinavia, where most capacitors are placed
outdoors, mineral oils (with a lower melting point) are frequently
used.
During the 1980s, there has been a marked interest in replacing the
PCBs, mainly in indoor transformers, as a result of serious accidents,
for example, in Binghamton, San Francisco, Miami in the USA, and Reims
in France. Various products are used for this exchange, such as
mineral oils, silicone oils, perchloroethylene, and other chlorinated
products (WHO/EURO, 1987).
3.2.2.2 Nominally closed systems
Smaller volumes of PCBs have frequently been used as fire-resistant
liquid in nominally closed systems, such as hydraulic and heat
transfer exchange systems (for example, trade names Pydraul and
Therminol FR, containing Aroclor 1242, 1248, 1254, and 1260). PCBs are
used as a working fluid in vacuum pumps (Aroclor 1248, 1254), which
can also be considered as nominally closed systems (WHO/EURO, 1987).
3.2.2.3 Open-ended applications
With open-ended applications of PCB, both the emissions into the
environment and the levels of occupational exposure are more
pronounced. The major open-ended applications include use as a
plasticizer (in PVC, neoprene, and other artificial chlorinated
rubbers). Other open-ended uses, such as surface coatings, paints,
inks, adhesives, pesticide extenders, microencapsulation of dyes, and
carbonless copy paper contribute smaller volumes into the environment.
PCBs have also been used in immersion oils for microscopes, as
catalysts in the chemical industry, in casting waxes in the iron/steel
industry (decachlorobiphenyl), and in cutting and lubricating oils
(WHO/EURO, 1987).
3.2.2.4 Contamination of other compounds
In addition to the above uses of PCBs, numerous halogenated compounds
may contain PCBs in small amounts as a contaminant (US EPA, 1983).
3.2.3 Loss into the environment
PCBs are dispersed into the environment through atmospheric transport
and, on a more regional scale, following release into water. PCBs are
also mobilized in the soil or landfills, but the rates of dispersion
and subsequent transfer to biota and humans are difficult to estimate.
More highly chlorinated forms become most prevalent in compartments
further along the pathway chains. The analytical methods used to
quantify PCBs in the environment and biota vary greatly within, and
between, countries. Thus, comparisons can only be made in a very broad
sense and could, to some extent, be erroneous (WHO/EURO, 1988).
An overview of prevention and control measures of accidental and
environmental exposures is given in WHO/EURO (1987).
3.2.3.1 Routes of environmental pollution
Surveys of the sources of environmental pollution with PCBs were made
before production and use became limited, and the information
available may not now apply in North America and elsewhere. Only 20%
of the annual production in the USA can be regarded as a net increase
in current usage, and the remainder is balanced by a loss to the
environment. More than one-half of this entered dumps and landfills
and it has been calculated that 0.3 million tonnes of PCBs have
accumulated in such locations in North America, since 1930 (Nisbet &
Sarofim, 1972). Much of this was originally enclosed in containers,
such as capacitors, or was in plasticized resins and will not be
released until the containing medium decays. The diffusion of PCBs
from landfills is likely to be slow, on account of their low
volatility and low water solubility. Carnes et al. (1973) found little
leaching from the one site that they tested.
The concentration of PCBs in emissions from several municipal sanitary
landfills and refuse and sewage sludge incinerators were determined in
the Midwest of the USA. Sanitary landfills continuously emit the
gaseous products of anaerobic fermentation together with other
volatile materials into the atmosphere. A projection, based on the
amount of methane generated annually from landfills and a PCB to
methane ratio of 0.3 µg PCBs/m3 of methane found from the landfills
sampled, indicates that the annual PCB emissions from sanitary
landfills in the USA are of the order of 10-100 kg/year. The
concentrations of PCBs from the incinerator stacks ranged from
0.3-3 µg/m3 and the annual emissions per stack were 0.25 kg/year.
These estimates are very small in comparison with the 900 000 kg
PCBs/year estimated to cycle through the atmosphere over the USA,
annually (Murphy et al., 1985).
Scrap transformer fluid containing PCBs has been used in the USA in
amounts of about 10 tonnes/year in pesticide formulations (Panel on
Hazardous Trace Substances, 1972, cf. WHO/EURO, 1988), and this
unauthorized use has led to the local contamination of milk supplies.
Pressure sensitive duplicating paper (carbonless copying paper)
containing PCBs has found its way into waste paper supplies and has
been recycled into paper and board used as food packaging materials,
but not since 1970; paints for coating the bottom of ships contained
3-5% of PCBs, about 3% of the annual quantity imported into Sweden has
been used for this purpose, and this has been a source of plankton
contamination (Jensen et al., 1972a).
Schecter (1987) described the contamination of drinking-water by the
use of submersible water pumps which, in certain instances, contained
PCBs in the oil. When the pumps leak, PCBs may be released into the
drinking-water.
In addition, the US EPA, in 1980, estimated that over 1 000 000 wells
in the USA may have PCB capacitors in the well motors. Levels recorded
in drinking-water range from 0.26 to 57 µg/litre compared with
1 µg/litre considered safe in the guidelines for New York State. The
oil from these pumps contained 630 000-24 000 000 µg/kg of PCBs.
Stehr et al. (1985) studied the possibility of contamination with PCBs
of oils and oil-filled devices used by amateur radio operators. Two of
77 oil samples contained more than 50 mg/kg.
3.2.3.2 Release of PCBs into the atmosphere
There appears to be little atmospheric contamination during the
manufacture and processing of PCBs, but this can occur during their
subsequent use and disposal. Although PCBs have a low volatility,
there may be an appreciable loss to the atmosphere during the lifetime
of a PCB-plasticized resin, particularly of the lower chlorinated
products. Further pollution may occur during the incineration of
industrial and municipal waste. Most municipal incinerators are not
very effective in destroying PCBs; efficient incinerators can be
designed for this purpose (Oehme et al., 1987), though the higher
chlorinated PCBs are more resistant to pyrolysis. Secondary sources of
atmospheric pollution are volatilization from soil, and the drying of
sewage sludge. Furthermore, there is evidence that, even at ambient
temperatures, PCBs will enter the atmosphere by volatilization from
soils and water bodies, landfill sites etc. (section 4.1.1).
3.2.3.3 Leakage and disposal of PCBs in industry
Eschenroeder et al. (1986) analysed PCB risks using estimates of human
intake of PCBs originating from accidental spills from electrical
equipment. Equipment spills without controls resulted in a human
intake of PCBs of, at the most, 2 ng/day via the water exposure
pathway. This was negligible in comparison with the intakes calculated
on the basis of fish consumption. The inhalation exposure of
approximately 100 persons living in the vicinity of a spill in
Southern California was determined to equal the PCB intakes of a
fish-eating population.
3.2.4 Thermal decomposition of PCBs
It has been found by Buser et al. (1978a,b) that PCBs can be converted
to PCDFs under pyrolytic conditions. The pyrolysis of a commercial PCB
mixture in a sealed quartz ampoule, in the presence of air, yielded a
mixture including about 30 major and more than 30 minor PCDF
congeners.
Buser & Rappe (1979) studied the pyrolysis (at 600°C) of 15 individual
PCB isomers and demonstrated the presence of PCDFs via intramolecular
cyclizations, where m + n varies from 4 to 8 (Fig. 1). The
thermochemical generation of PCDFs from PCBs was found to follow 4
general reaction routes including loss of ortho-Cl; loss of HCl
involving a 2,3-chlorine shift at the benzene nucleus; loss of
ortho-HCl and loss of ortho-H (Buser, 1985; Hutzinger et al.,
1985).
The chemical formula can be presented as C12H10-nCln, where n, the
number of chlorine atoms in the molecule, can range from 1 to 10.
2.1.2 Relative molecular mass
The relative molecular mass depends on the degree of substitution.
Monochlorobiphenyl has a relative molecular mass of 188, while
completely chlorinated biphenyl (C12Cl10) has a relative molecular
mass of 494 (US EPA, 1980).
2.1.3 Common name
Common name: polychlorinated biphenyls (PCBs)
CAS Registry number: 1336-36-3
RTECS Registry number: TQ 1350000
2.1.4 Chemical composition
The PCBs are chlorinated hydrocarbons, manufactured commercially by
the progressive chlorination of biphenyl in the presence of a suitable
catalyst (e.g., iron chloride). Depending on the reaction conditions,
the degree of chlorination can vary between 21 and 68% (w/w). The
yield is always a mixture of different isomers and congeners. Thus, a
total of 209 theoretically different chemical components exist, but
only about 130 of these are likely to occur in commercial products or
mixtures of such compounds (Safe, 1990).
Seventy-eight out of the possible 209 PCB congeners can exist as
rotational isomers that are enantiomeric to each other. Nineteen PCBs,
of which 9 are components of commercial PCB formulations, have been
predicted to be stable at room temperature (Kaiser, 1974).
Puttmann et al. (1988) separated the atropisomers of
2,3,4,6,2',4'-hexachlorobiphenyl and demonstrated that they possess
different biological effects with regard to in vivo enzyme induction
(aminopyrine N-demethylase, aldrin epoxidase, cytochrome P-450
content, morphine UDP-glucuronosyl transferase) in Sprague-Dawley
rats.
Unlike the dioxins or dibenzofurans, the phenyl rings of a PCB are not
constrained through ring fusions and have relatively unconstrained
rotational freedom. Chlorines at the ortho (2,2', 6,6') positions
introduce constraints on rotational freedom that can hinder
coplanarity of the phenyl rings. X-ray crystallographic studies
(McKinney & Singh, 1981) indicate that the preferred conformation for
all PCBs, including those without ortho-substituents, is
noncoplanar. The proportion of molecules of a particular congener
assuming a coplanar configuration becomes increasingly small as the
degree of ortho-substitution and the energetic cost of conforming
increases. However, PCBs without ortho-substitution are often
referred to in the biological literature as the planar (or coplanar)
PCBs and all others as the nonplanar (or noncoplanar) PCBs. This
terminology, though somewhat misleading, is also used throughout this
publication for convenience and ease of referring back to the
published literature. It is widely recognized that certain biological
activities of the PCBs vary, at least quantitatively, with
stereochemical differences in the congeners.
Individual manufacturers have their own system of identification for
their products. In the Aroclor series, a 4-digit code is used;
biphenyls are generally indicated by 12 in the first 2 positions,
while the last 2 numbers indicate the percentage by weight of chlorine
in the mixture; thus, Aroclor 1260 is a polychlorinated-biphenyl
mixture containing 60% of chlorine. An exception to this
generalization is Aroclor 1016, which is a distillation product of
Aroclor 1242 containing only 1% of components with 5 or more chlorine
atoms (Burse et al., 1974). With other commercial products, the codes
may indicate the approximate mean number of chlorine atoms in the
components; thus Clophen A60, Phenochlor DP6, and Kanechlor 600 are
biphenyls with an average of about 6 chlorine atoms per molecule
(equivalent to 59% chlorine by weight).
Ballschmiter & Zell (1980) proposed a numbering system for the PCB
congeners, that was later adopted by the International Union of Pure
and Applied Chemists (IUPAC). The number, structure, and isomer group
are given for each congener in the paper of McFarland & Clarke (1989)
(see Appendix A). In the literature, the structure of a congener is
given in 2 ways; for example 2,2',5,5' or 2,5,2',5' (No 52).
Individual PCBs have been synthesized for use as reference samples in
the identification of gas-liquid chromatographic peaks, for
toxicological investigations, and for studying their metabolic fate in
living organisms, for which purpose they have been prepared labelled
with carbon-14 (Hutzinger et al., 1971; Jensen & Sundström, 1974a;
Sundström & Wachtmeister, 1975).
The proportions of PCBs with 1-9 chlorine substituents in the Aroclors
are shown in Table 1.
It is apparent, from gas chromatographic analyses of commercial
products, that PCB mixtures differ with respect to the individual
congeners present and their relative concentrations (Jensen &
Sundström, 1974a; Albro & Parker, 1979; Ballschmiter & Zell, 1980;
Albro et al., 1981; Mullin et al., 1984; Safe et al., 1985a;
Alford-Stevens, 1986).
There have been several investigations to identify individual PCBs in
commercial products. The components of the Aroclors were separated by
column and gas-liquid chromatography and many of the peaks
characterized by high-resolution mass spectrometry and nuclear
magnetic resonance, and also by comparison with synthesized PCBs
(Table 2) (see also DFG, 1988).
Jensen & Sundström (1974a) recognized that conventional gas-liquid
chromatography was not suitable for separating all the components, so
they devised a preliminary fractionation on a charcoal column, which
separated the component PCBs according to the number of chlorines in
the 2,6,2' or 6' positions in the molecule ( o-chlorines). They
compared the gas-liquid chromatographic peaks with those of 90
synthesized PCBs, and were able to characterize and quantify 60
components of Clophens A50 and A60.
Table 1. Approximate percentages (w/v) of Aroclors with different degrees of
chlorinationa
Number of Chlorine
chlorine weight Aroclor
atoms in (%)
molecule 1221 1232 1016 1242 1248 1254 1260
0 0 10 - -
1 18.8 50 26 2 3
2 31.8 35 29 19 13 2
3 41.3 4 24 57 28 18
4 48.6 1 15 22 30 40 11
5 54.4 22 36 49 12
6 59.0 4 4 34 38
7 62.8 6 41
8 66.0 8
9 68.8 1
a From: WHO/EURO (1987).
2.1.5 Technical product
Major trade names
The PCBs manufactured commercially are known by a variety of trade
names including: Aroclor, Pyranol, Pyroclor (USA), Phenoclor, Pyralene
(France), Clophen, Elaol (Germany), Kanechlor, Santotherm (Japan),
Fenchlor, Apirolio (Italy), and Sovol (USSR). Table 3 contains the
most common trade names for commercial products, some of which are not
in use any more (Brinkman & De Kok, 1980; WHO/EURO, 1987).
2.1.6 Purity and impurities
Commercial PCBs are not sold according to a composition specification,
but according to their physical properties. The composition of
Aroclors and Clophens has been presented in recent papers; the
composition of 5 Aroclors is shown in Tables 1 and 2. In Table 1, the
approximate composition is expressed as the percentage of chlorine
weight, and, in Table 2, the composition of the chlorine substitution
pattern is expressed in mol % (Albro & Parker, 1979; Albro et al.,
1981; Jones, 1988). The composition of the chlorine substitution
pattern for 4 Clophens is described by Duinker & Hillebrand (1983) and
Jones (1988). It should be kept in mind that nothing can be said about
the variations in the different lots of these mixtures. Impurities
known to be present in commercial PCBs are chlorinated dibenzofurans
and chlorinated naphthalenes (Vos et al., 1970; Bowes et al., 1975;
Albro & Parker, 1979; Albro et al., 1981; Duinker & Hillebrand, 1983;
Rappe et al., 1985a). The concentrations of PCDFs in Aroclor, Clophen,
Phenoclor, and Kanechlor are summarized in Tables 4 and 5.
Different authors have examined the presence of PCDFs in PCB mixtures.
Bowes et al. (1975) found 0.8-2.0 mg/kg in samples of Aroclor 1248 and
1260, but none in Aroclor 1016, 8.4 mg/kg in Clophen A60, and
13.6 mg/kg in Phenoclor DP-6. Rappe et al. (1985a) and Bentley (1983)
found levels of PCDFs up to 40 mg/kg in a number of commercial PCBs.
Recently, Wakimoto et al. (1988) found a number of extremely toxic
PCDFs in several Japanese and American commercial PCB preparations.
These isomer-specific analyses revealed the 2,3,7,8-tetra-,
1,2,4,7,8-penta-, 1,2,3,7,8-penta-, 2,3,4,7,8-penta-, and
1,2,3,6,7,8-hexachlorodibenzofurans. The concentrations in unused
Kanechlor 300, 400, 500, and 600, were 7.5, 26, 7.2, and 5.4 mg/kg,
respectively, and those in Aroclors 1242, 1248, 1254, and 1260, were
0.6, 3.7, 4.2, and 7.5 mg/kg, respectively. Brown et al. (1988) found
that the electrical use of PCB dielectric fluids in transformers and
capacitors did not increase the PCDFs content significantly.
More data about the occurrence of PCDFs in the different commercial
PCB mixtures are summarized in WHO/EURO (1987).
There are no reports on the presence of PCDDs in commercial mixtures
(Bowes et al., 1975). Wakimoto et al. (1988) could not find PCDDs in
the above samples of Kanechlors and Aroclors with a detection limit of
<2 µg/kg.
2.2 Physical and chemical properties
Individual pure PCB congeners are colourless, often crystalline
compounds, but commercial PCBs are mixtures of these congeners with a
clear, light yellow or dark colour. They do not crystallize at low
temperatures, but turn into solid resins. Because of the chlorine
atoms in the molecule, their density is rather high. PCBs are, in
practice, fire resistant with rather high flash-points (170-380°C).
They form vapours heavier than air, but do not form any explosive
mixtures with air. They possess very low electrical conductivity and
an extremely high resistance to thermal breakdown, and it is on the
basis of these properties that they are used as cooling liquids in
electrical equipment (US EPA, 1980; WHO/EURO, 1987; DFG, 1988).
Table 2. PCB compositions of aroclors in mol %a
IUPAC Chlorine Aroclor
No. substitution
pattern 1242 1016 1248 1254 1260
BP 0.01 0.50
1 2 0.68 0.80
2 3 0.04 0.10
3 4 0.22 1.00
4 2.2' 3.99 4.36 0.25
6 2.3' 1.24 1.37 0.69 0.07
7 2.4 1.04 1.16
8 2.4' 8.97 10.30 0.18
9 2.5 0.31 0.34 trace
10 2.6 0.13 0.20
12 3.4 0.09 0.11
13 3.4' 0.12 0.12
14 3.5 0.35 0.37
15 4.4' 0.99 1.07
16 2.3.2' 3.25 3.50 0.84
17 2.4.2' 2.92 3.14 0.19
18 2.5.2' 9.36 10.87 9.95 0.07
19 2.6.2' 0.97 1.08
20 2.3.3' 3.64 3.99
22 2.3.4' 2.64 2.80 1.24 trace trace
25 2.4.3' 1.68 1.79
26 2.5.3' 0.55 0.62 0.75
27 2.6.3' 0.54 0.58
28 2.4.4' 13.30 14.48 trace
31 2.5.4' 4.53 4.72 9.31 0.72
32 2.6.4' 2.15 2.31 1.46
33 3.4.2' 2.83 3.08
35 3.4.3' 0.66 0.38
37 3.4.4' 1.62 1.89 1.28 0.20 0.09
39 3.5.4' 1.03 1.08
40 2.3.2'.3' 0.15 0.18 1.12 0.26 0.04
41 2.3.4.2' 1.67 2.00
42 2.3.2'.4' 7.05 2.18 0.66
43 2.3.5.2' 0.44 0.47
44 2.3.2'.5' 1.06 1.14
45 2.3.6.2' 0.90 1.00 5.73 0.15
46 2.3.2'.6' 0.31 0.33
47 2.4.2'.4' 1.65 1.8 3.18 0.52 0.88
48 2.4.5.2' 1.33 1.41
Table 2. (cont'd).
IUPAC Chlorine Aroclor
No. substitution
pattern 1242 1016 1248 1254 1260
? 2.5.2'.4' - - 3.81 1.63 0.44
49 2.4.2'.5' 3.28 3.48
52 2.5.2'.5' 4.08 4.35 8.36 4.36 1.91
53 2.5.2'.6' 0.97 1.07 6.30 0.13
54 2.6.2'.6' 0.17 0.19
55 2.3.4.3' 0.11 0.43 0.12
56 2.3.3'.4' 0.60 trace 0.18 0.03
60 2.3.4.4' 0.21
66 2.4.3'.4' 0.81 0.14 4.95 2.24 0.22
70 2.5.3'.4' 1.11 6.38 4.75 0.85
71 2.6.3'.4' 0.65
72 2.5.3'.5' 0.33 2.10 1.01 0.28
74 2.4.5.4' 2.02 1.35 0.25 0.30 0.09
75 2.4.6.4' 2.18 2.40
76 3.4.5.2' trace trace 0.18 0.01
77 3.4.3'.4' 0.34 0.47 0.12 0.04
78 3.4.5.3' 0.52
79 3.4.3'.5' 0.24 trace 0.23 0.04
80 3.5.3'.5' trace trace trace
81 3.4.5.4' 0.28
83 2.3.5.2'.3' trace 0.32 0.09
84 2.3.6.2'.3' 0.38 0.01 0.71 1.72 0.69
85 2.3.4.2'.4' 0.40 0.55 2.15 0.31
? 2.3.4.3'.5' 0.02 0.55 0.14
87 2.3.4.2'.5' 0.09 1.05 3.81 1.10
91 2.3.6.2'.4' trace 1.78 5.00 3.22
92 2.3.5.2'.5' 0.12 0.20 0.63 0.21
95 2.3.6.2'.5' 0.53 0.18
97 2.4.5.2'.3' 0.78 2.59 0.63
98 2.4.6.2'.3' 0.13 0.04
99 2.4.5.2'.4' 0.55 2.52 6.10 0.82
101 2.4.5.2'.5' 0.27 1.50 6.98 5.04
102 2.4.5.2'.6' trace trace trace
105 2.3.4.3'.4' 0.25
106 2.3.4.5.3' 0.40 0.06
108 2.3.4.3'.5' 0.46 0.16
110 2.3.6.3'.4' 1.69 8.51 3.57
113 2.3.6.3'.5' 0.39 0.01 3.10 trace 0.01
114 2.3.4.5.4' 0.25 0.03
118 2.4.5.3'.4' 8.09 2.00
120 2.4.5.3'.5' 0.31 trace 0.15 3.01
121 2.4.6.3'.5' 0.92 4.32 3.51 0.57
Table 2. (cont'd).
IUPAC Chlorine Aroclor
No. substitution
pattern 1242 1016 1248 1254 1260
123 3.4.5.2'.4' 0.36
? 3.4.5.2'.3' trace 0.76 1.88
126 3.4.5.3'.4' 0.03 0.16 1.59
127 3.4.5.3'.5' 0.05
128 2.3.4.2'.3'.4' 1.31 0.47
131 2.3.4.6.2'.3' 0.14 0.01
132 2.3.4.2'.3'.6' trace 2.00 2.77
133 2.3.5.2'.3'.5' 1.13 0.03 0.06
134 2.3.5.6.2'.3' 0.11 0.38 1.01
135 2.3.5.2'.3'.6' 0.20 0.29
136 2.3.6.2'.3'.6' 0.20 0.34 1.12
138 2.3.4.2'.4'.5' 0.08 0.19 4.17 5.01
143 2.3.4.5.2'.6' 0.07
148 2.3.5.2'.4'.6' 0.12 0.07 0.06
149 2.4.5.2'.3'.6' 0.77 3.59 9.52
151 2.3.5.6.2'.5' trace 0.33 0.06
153 2.4.5.2'.4'.5' 0.02 0.13 3.32 8.22
154 2.4.5.4'.6' 0.14
156 2.3.4.5.3'.4' 0.41
157 2.3.4.3'.4'.5' 0.18 0.03
158 2.3.4.6.3'.4' 0.46 0.18
159 2.4.5.2'.3'.5' 0.75 1.48
163 2.3.5.6.3'.4' trace
167 2.4.5.3'.4'.5' 0.21 0.17
168 2.4.6.3'.4'.5' 0.56 4.23 0.59
170 2.3.4.5.2'.3'.4' 0.43 0.62
171 2.3.4.6.2'.3'.4' 0.30 4.31
174 2.3.4.5.2'.3'.6' trace 0.09
176 2.3.4.6.2'.3'.6' 0.09 trace 0.57
177 2.3.5.6.2'.3'.4' trace
179 2.3.5.6.2'.3'.6' 0.56 0.83
180 2.3.4.5.2'.4'.5' 0.76 7.20
181 2.3.4.5.6.2'.4' 0.28 2.72
182 2.3.4.5.2'.4'.6' trace 0.47
183 2.3.4.6.2'.4'.5' 1.16 2.58
185 2.3.4.5.6.2'.5' 1.11 5.65
186 2.3.4.5.6.2'.6' trace trace 0.37
187 2.3.5.6.2'.4'.5' 0.48 1.12
189 2.3.4.5.3'.4'.5' 0.13
190 2.3.4.5.6.3'.4' 0.02
192 2.3.4.5.6.3'.5' 0.20 0.97
Table 2. (cont'd).
IUPAC Chlorine Aroclor
No. substitution
pattern 1242 1016 1248 1254 1260
193 2.3.5.6.3'.4'.5' 2.30
194 2.3.4.5.2'.3'.4'.5' 2.21
195 2.3.4.5.6.2'.3'.4' trace
196 2.3.4.5.2'.3'.4'.6' 0.79
197 2.3.4.6.2'.3'.4'.6' 0.30
198 2.3.4.5.6.2'.3'.5' 1.00 0.15
199 2.3.4.5.6.2'.3'.6' 0.38
200 2.3.4.6.2'.3'.5'.6' trace 0.15
202 2.3.5.6.2'.3'.5'.6' trace 0.31
203 2.3.4.5.6.2'.4'.5' 0.08
204 2.3.4.5.6.2'.4'.6' trace 0.13
205 2.3.4.5.6.3'.4'.5' 0.01
206 2.3.4.5.6.2'.3'.4'.5' 0.51
207 2.3.4.5.6.2'3'.4'.6' 1.15
208 2.3.4.5.6.2'.3'.5'.6' 1.64
? 2.3.4.5.6.2'.3'.5'.6' 0.18
a From: Albro & Parker (1979); Albro et el. (1981).
Table 3. The trade marks of PCB products and mixtures containing PCBsa
Aceclor (t) Disconon (c) PCBs
Apirolio (t,c) Dk (t,c) Phenoclor (t,c)
Aroclor (t,c) Duconol (c) Polychlorinated biphenyl
Arubren Dykanol (t,c) Polychlorobiphenyl
Asbestol (t,c) EEC-18 Pydraulc
Askarel Elemex (t,c) Pyralene (t,c)
Bakola 131 (t,c) Eucarel Pyranol (t,c)
Biclor (c) Fenchlor (t,c) Pyroclor (t)
Chlorextol (t) Hivar (c) Saf-T-Kuhl (t,c)
Chlorinated Biphenyl Hydol (t,c) Santotherm FRb
Chlorinated Diphenyl Inclor Santovac 1 and 2
Chlorinol Inerteen (t,c) Siclonyl (c)
Chlorobiphenyl Kanechlor (t,c) Solvol (t,c)
Clophen (t,c) Kennechlor Sovol
Clorphen (t) Montar Therminol FRb
Delor Nepolin
Diaclor (t,c) No-Flamol (t,c)
Dialor (c) PCB
a From: WHO/EURO (1987).
b Previous products (FR-series) used as pressure oil contained PCBs, but current
products are a different series and do not contain PCBs.
c Previous products (A-series) e.g., PYDRAUL A-200 contained PCBs, but current
commercial products are B, C, or D-series and do not contain any chlorinated
compounds.
(t) Used in transformers.
(c) Used in capacitors.
Table 4. Concentrations of chlorinated dibenzofuransa in Aroclor, Clophen, and
Phenoclorb
PCB 4-Cl 5-Cl 6-Cl Total
Aroclor 1248 (1969) 0.5 (25) 1.2 (60) 0.3 (15) 2.0
Aroclor 1254 (1969) 0.1 (6) 0.2 (12) 1.4 (82) 1.7
Aroclor 1254 (1970) 0.2 (13) 0.4 (27) 0.9 (60) 1.5
Aroclor 1260 (1969) 0.1 (10) 0.4 (40) 0.5 (50) 1.0
Aroclor 1260 (lot AK3) 0.2 (25) 0.3 (38) 0.3 (38) 0.8
Aroclor 1016 (1972) ND ND ND
Clophen A-60 1.4 (17) 5.0 (59) 2.2 (26) 8.4
Phenoclor DP-6 0.7 (5) 10.0 (74) 2.9 (21) 13.6
a Expressed as mg PCB/kg. Values in parentheses represent quantity as percentage
of total dibenzofurans.
b From: Bowes et al. (1975).
ND = not detected (0.001 mg/kg).
Table 5. Concentrations of chlorinated dibenzofurans in Kanechlorsa
Kanechlor Chlorodibenzofurans Concentration
(mg/kg)
Di- Tri- Tetra- Penta- Hexa- Hepta- b c
300 + + 1 1.5
400 + + + + 18 17
500 + + + + 4 2.5
600 + + + + 5 3
a From: Nagayama et al. (1975).
b Calculated from peak heights.
c Calculated by perchlorination method.
PCBs have a high degree of chemical stability under normal conditions.
They are very resistant to a range of different oxidants and other
chemicals. According to laboratory tests, they stay chemically
unchanged, even in the presence of oxygen or some active metals at
high temperatures (up to 170°C) and for protracted periods (WHO/EURO,
1987).
PCBs are practically insoluble in water, whereas they dissolve easily
in hydrocarbons, fats, and other organic compounds and they are
readily absorbed by fatty tissues (WHO/EURO, 1987).
Some physical and chemical data for a number of Aroclors are presented
in Table 6.
Foreman & Bidleman (1985) estimated the liquid phase vapour pressures,
at 25°C, of 134 PCB congeners found in 5 Aroclor fluids, using a
capillary gas chromatographic method in conjunction with published
retention indices of PCBs.
Burkhard et al. (1985) predicted Henry's Law Constants from the ratio
of the liquid (or subcooled liquid) vapour pressure and aqueous
solubility for PCB congeners. The predicted values were in fair
agreement with experimental values and the error for these constants
was estimated to be a factor of 5 in the temperature range of 0-40°C.
For the PCB congeners, Henry's Law Constants were independent of the
relative molecular mass and increased approximately an order of
magnitude with a 25°C increase in temperature.
Aqueous solubility is considered an essential parameter for predicting
the fate and transport of organic chemicals in the environment. As
already stated, some physical and chemical data are given for 6
Aroclor mixtures in Table 6 (Alford-Stevens, 1986). However, during
the last 5 years, much more information on aqueous solubility, melting
points, entropies of melting, Henry's law constants, and vapour
pressures has become available. This information concerns not only PCB
mixtures but also individual congeners.
Opperhuizen et al. (1988) studied the aqueous solubilities of 45
chlorinated biphenyls and the relationships between activity
coefficient and chemical structure parameters (total surface area
(TSA) and total molecular volume (TMV)) of hydrophobic chemicals, to
understand the nature of hydrophobicity. The aqueous solubilities of
PCBs showed a linear relationship between logarithms of aqueous
activity coefficients or TSA and TMV.
Table 6. Physical and chemical properties of a number of Aroclorsa
Substance Water Vapour Density Appearance Henry's Law Refractive index Boiling point
Aroclor solubility pressure (g/cm3) constant (distillation
(mg/litre) (torr) 25°C 25°C (atm-m3/mol range) (750
25°C at 25°C)b torr, °C)
1016 0.42 4.0 × 10-4 1.33 Clear, mobile oil 2.9 × 10-4 1.6215-1.6235 325-356
(at 25°C)
1221 0.59c 6.7 × 10-3 1.15 Clear, mobile oil 3.5 × 10-3 1.617-1.618 (at 20°C) 275-320
1232 0.45 4.1 × 10-3 1.24 Clear, mobile oil unknown unknown 290-325
1242 0.24 4.1 × 10-3 1.35 Clear, mobile oil 5.2 × 10-4 1.627-1.629 (at 20°C) 325-366
1248 0.054 4.9 × 10-4 1.41 Clear, mobile oil 2.8 × 10-3 unknown 340-375
1254 0.021 7.7 × 10-5 1.50 Light yellow 2.0 × 10-3 1.6375-1.6415 365-390
viscous oil (at 25°C)
1260 0.0027 4.0 × 10-5 1.58 Light yellow 4.6 × 10-3 unknown 385-420
sticky resin
a From: IARC (1978); WHO/EURO (1987); ATSDR (1989).
b These Henry's Law Constants were estimated by dividing the vapour pressure by the water solubility. The first water solubility
given in this table was used for the calculation. The resulting estimated Henry's law constant is only an average for the
entire mixture; the individual chlorobiphenyl isomers may vary significantly from the average. Burkhard et al. (1985)
estimated the following Henry's Law Constants (atm-m3/mol) for various Aroclors at 25°C: 1221 (2.28 × 10-4), 1242 (3.43 × 10-4),
1248 (4.4 × 10-4), 1254 (2.83 × 10-4), 1260 (4.15 × 10-4).
c At 24°C.
Dickhut et al. (1986) studied the solubilities of 6 higher chlorinated
biphenyl congeners at different temperatures and found that the
solubility increased exponentially with temperature in the range of
0.4-80°C. From the temperature dependence of solubility, enthalpies of
solution were calculated. The same results were found by Doucette &
Andren (1988), who determined the aqueous solubilities of a few PCBs,
using a generator-column technique, at temperatures of 4.0, 25.0, and
40.0°C.
The dissolution of extremely hydrophobic chemicals that may be
associated with a relatively constant endothermic enthalpy of solution
and an endothermic enthalpy of fusion that is proportional to the
solute's melting point is discussed by Opperhuizen et al. (1987) and
Dickhut et al. (1987).
Dunnivant & Elzerman (1988) estimated the aqueous solubilities and
Henry's Law Constants (HLC) for 26 selected PCB congeners for the
evaluation of quantitative structure-property relationships (QSPRs).
Aqueous solubilities (as solids at 25°C, column generation technique),
determined for the 26 congeners, ranged from 1.08 × 10-5 to
9.69 × 10-10 mol/litre and generally decreased with relative molecular
mass. HLCs (25°C, gas purge technique), determined for 20 congeners,
ranged from 0.3 × 10-4 to 8.97 × 10-4 atm.m3/mol. Measured HLCs were
not correlated with relative molecular mass, but increased with the
degree of ortho-chlorine substitution within a relative molecular
mass class.
Vapour pressures calculated from the product of solubility (mol/m3)
and HLC (atm-m3/mol) data, generally decreased with relative
molecular mass and increased with increasing degree of
ortho-chlorine substitution (Dunnivant & Elzerman, 1988; Hawker,
1989). Westcott et al. (1981) used a semimicro gas saturation method
to determine the vapour pressures of 3 PCB isomers and 2 Aroclor
mixtures.
Experimental data were tabulated and the relationships between the
environmentally relevant physical chemical properties of the PCBs
critically reviewed by Shui & Mackay (1986). Aqueous solubility,
vapour pressure, Henry's law constant, and octanol-water partition
coefficient were discussed and recommended values given for 42 of the
209 congeners; procedures were suggested for estimating the properties
of the other congeners.
2.2.1 Log n-octanol/water partition coefficient
The environmental fate of PCBs is governed primarily by the
partitioning process. Partitioning processes that are of particular
interest with regard to environmental problems include: the octanol/
water partition coefficient and the aqueous solubility. The octanol/
water partition coefficient is a measure of the hydrophobicity of a
substance and, in this respect, it has been used to predict the extent
of bioconcentration of organic pollutants in organisms. Miller et al.
(1984) studied the octanol/water partition coefficients for 16 PCBs
and Hawker & Connell (1988) for 13 PCB congeners, using the generator
column method. These partition coefficients were used to confirm a
highly significant linear relationship between log Kow and the
logarithm of the relative retention time on a nonselective gas
chromatographic stationary phase. The total surface areas (TSA) for
all the PCB congeners were determined by assuming planar molecules,
van der Waal's radii for component atoms, and appropriate values for
solvent radius, bond angles, and distances. The TSA was highly
significantly correlated with log Kow and the relationship was used
to calculate log Kow values for all the PCB congeners. In the report
of Hawker & Connell (1988), log Kow values are summarized for all 209
PCB congeners. These log Kow values range from 4.46 to 8.18.
2.2.2 Conversion factorsa
Aroclor
1016 1 mg/m3 = 0.095 ppm
1221 1 mg/m3 = 0.12 ppm
1232 1 mg/m3 = 0.105 ppm
1242 1 mg/m3 = 0.092 ppm
1248 1 mg/m3 = 0.008 ppm
1254 1 mg/m3 = 0.075 ppm
1260 1 mg/m3 = 0.065 ppm
2.3 Analytical methods
Reviews have been published on the methods used for the determination
of organochlorine compounds including PCBs in environmental samples
(Panel on Hazardous Trace Substances, 1972; Holden, 1973; US DHEW,
1978; Slorach & Vaz, 1983; Jensen, 1984, 1985; Erickson 1985;
Alford-Stevens, 1986; NIOSH, 1987; DFG, 1988; WHO/EURO, 1987, 1988).
a These air conversion factors were calculated by using the average
molecular mass at 25°C.
No two laboratories used identical methods, though all the methods
have features in common. The techniques appear to be those previously
developed for the determination of organochlorine pesticides, with
appropriate modifications for the presence of PCBs, and the studies on
PCBs sometimes form part of a wider programme for monitoring
persistent organochlorine compounds in the environment. In the past,
the major difficulty in the determination of PCBs was to obtain a
single quantitative figure from a variable mixture of components. The
PCBs were chlorinated with antimony pentachloride to decachloro-
biphenyl, which was measured as a single peak (Greve & Wegman, 1983;
Tuinstra, 1983). At the moment, chemists and toxicologists are no
longer trying to derive a single quantitative figure, preferring
instead to quantify individual congeners. The legislation in certain
countries is now based on quantifying a few selected congeners,
instead of reporting "total PCBs". It is also felt that for
pinpointing areas with high levels of contamination, in order to rank
them into low, medium, or high priority areas for action, highly
accurate laboratory analyses are not necessary; instead, analytical
competence and the use of adequate controls and standards, resulting
in consistent, reasonably accurate results would be enough. Of course,
for complicated research, especially involving laboratories in
different countries, standardization of techniques through
collaborative and comparative studies would be necessary.
Jones (1988) and Safe et al. (1985a) studied the occurrence of
specific PCB congeners in commercial formulations or mixtures. The
congener composition of commercial formulations differs from
batch-to-batch, between manufacturing processes, and with the level of
chlorination. The presence of congeners in the environment will depend
on the eventual use of commercial formulations, the quantity of each
formulation manufactured, as well as on the isomer composition of the
source.
On the basis of a literature review of the occurrence of PCB congeners
in environmental and biological samples and human tissues, and
consideration of the relative toxicity and persistence of the
congeners, suggestions were made by Jones (1988), with regard to the
most relevant components to be quantified in human foodstuffs and
tissues, using a selective analytical approach.
The congeners reported (Safe et al., 1985a; Duinker et al., 1988;
McFarland & Clarke, 1989) as being the most abundant in human tissues
and which are most important, are compounds with IUPAC numbers 28, 52,
74, 77, 99, 101, 105, 118, 126, 128, 138, 153, 156, 169, 170, 179, and
180 (comprising >70% of total PCBs and being of greatest
toxicological significance). Because of their reported occurrence or
toxicity, congeners with IUPAC numbers 8, 37, 44, 49, 60, 66, 70, 82,
87, 114, 158, 166, 183, 187, and 189 might also be considered. Duinker
et al. (1988) were also of the opinion that toxicity should be
considered as a criterion for the selection of PCB congeners for
analysis in environmental samples. Most of these congeners can be
accurately determined with the application of the multidimensional,
high-resolution GC-ECD techniques.
PCB reference materials are necessary for the qualitative and
quantitative calibration of analytical apparatus and methods (e.g.,
determination of retention times, response factors, and reference
spectra in chromatographic and spectroscopic analyses) and for the
study of biological activity. Lindsey & Wagstaffe (1989) described the
production and certification of 10 high-purity PCBs with IUPAC numbers
8, 20, 28, 35, 52, 101, 118, 138, 153, and 180.
Mes et al. (1989a) described an analytical method to determine 34
isomers of PCB congeners in fatty foods. A sample was extracted with
an acetone:hexane mixture and the extracts washed and dried; this was
followed by a clean-up and determination by gas chromatography. GC/MS
was used for confirmation.
Environmental PCB residues are often expressed in terms of relative
Aroclor composition. Schwartz et al. (1987) assessed the similarity of
Aroclors with class models derived for fish and turtles, to ascertain
if the PCB residues in the samples could be described by an Aroclor or
Aroclor mixture. The PCB residues in fish and turtles were analysed
with Soft Independent Modelling of Class Analogy, a principal
components analysis (PCA) technique. Using PCA, it was inappropriate
to report these samples as an Aroclor or Aroclor mixture.
2.3.1 Sampling strategy and sampling methods
The quality and usefulness of analytical data, especially in the
microgram-nanogram range, or even lower, depend critically on the
validity of the sample and the adequacy of the sampling programme. The
purpose of sampling is to obtain specimens that represent the
situation being studied. Sampling plans may require that systematic
samples be obtained at specified times and places, or simple random
sampling may be necessary. Generally, the sample should be an unbiased
representative of the situation of interest (WHO/EURO, 1987). Slorach
(1984) described the problems encountered with the sampling and
determination of PCBs in breast milk (see also WHO/EURO, 1985, 1988).
All aspects of a sampling programme should be planned and documented
in detail, and the expected relationship of the sampling protocol to
the analytical result should be defined. A sampling programme should
include reasons for choosing sampling sites, the number and type of
samples, the timing of sample acquisition, and the sampling equipment
used. A detailed sampling procedure should include a description of
the sampling situation, the sampling methodology, labelling of
samples, field blank preparation, pretreatment procedures,
transportation, and storage (WHO/EURO, 1987).
The quality assurance programme should include means to demonstrate
that containers or storage procedures do not alter the qualitative or
quantitative composition of the sample. Special transportation and
storage procedures (refrigeration or exclusion of light) should be
described (WHO/EURO, 1987).
Because environmental samples are typically heterogeneous, a
sufficiently large number of samples (10 or more) must be analysed to
obtain meaningful composition data. The number of individual samples
that should be analysed will depend on the kind of information
required. If an average composition value is required, a number of
randomly selected individual samples may be obtained, combined, and
blended to provide a homogeneous composite sample, from which a
sufficient number of subsamples are analysed. If composition profiles,
time trends, or the variability of the sample population is of
interest, many samples need to be collected and analysed individually.
If field blanks are not available, efforts should be made to obtain
blank samples that best simulate a sample that does not contain the
analyte. In addition, measurements should be made to ascertain
whether, and to what extent, any reagent or solvent used may
contribute or interfere with the analytical results (laboratory and
solvent blanks). The recovery tests are frequently used and are
necessary to evaluate the analytical methodology. Uncontaminated
samples from control sites that have been spiked with the analyte of
interest provide the best information, because they simulate any
matrix effect. When feasible, isotopically labelled (13C, 37Cl)
analytes spiked into the sample provide the greatest accuracy, since
they are subjected to the same matrix effects as the analytes. The
13C-labelled compounds can be used to:
(a) validate sampling (sampling surrogate);
(b) validate analytical waste (clean-up surrogate);
(c) validate quantification (internal standard).
Only a small number of laboratories in the world have access to, and
experience in working with, these complicated analyses. In order to be
able to compare data generated in different laboratories, the same
quantitative standard compounds should be used. Interlaboratory
calibrations, or "round-robin" studies, have been performed in a few
cases (WHO/EURO, 1987).
2.3.1.1 Extraction procedures
Air
The sampling device used to collect and determine PCBs in air consists
of a glass fibre filter and a Florisil stick. The glass fibre filter,
held in a stainless steel holder, removes particles larger than
0.3 µm. The air passes from the filter to the Florisil stick, which is
made in 2 sections, to provide information on migration and trapping
efficiency for PCBs. Each section contains 0.4 g of Florisil preceded
and followed by a glass wool plug. The front and back sections are
separated by 2 plugs of glass wool. The front is spiked with 0.1 µg of
p,p'-DDE as a surrogate for recovery measurement and as an indication
of analyte migration. The detection limit for PCBs in air is reported
to be 0.3 ng/m3 (Anon., 1985; WHO/EURO, 1987; NIOSH, 1987).
Particulate fallout from air has been trapped on 200 µm nylon net
coated with silicone oil, and the PCBs then extracted with hexane
(Södergren, 1972). Separate determinations of particulate and vapour
phase PCBs in air have been made by passing a large volume of air
through a filter followed by an impinger containing hexane or toluene
(Rappe et al., 1985c), a polyurethane plug (Bidleman & Olney, 1974),
or ceramic saddles coated with OV 17 silicone (Harvey & Steinhauer,
1974) to absorb the vapour.
Surface sampling
Surface sampling of PCBs can be carried out using a wet-wipe procedure
with a cotton gauze pad that has been dampened with hexane before
collecting the sample. The sampled area is 0.25 m2. The wet-wipe
sampling procedure collects both the contaminants from the surface and
the contaminants that can be extracted from pores in the material.
Materials such as waxes and plasticizers may interfere with the
chemical analysis (WHO/EURO, 1987).
Another sampling method has been described by Rappe et al. (1985c),
where a dry filter paper or Kleenex tissue is used first, for wiping,
followed by a wet wipe with water-dampened material.
Water
PCBs have been extracted from water by passing a sample through a
filter of undecane and Carbowax 400 monostearate supported on
Chromosorb W (Ahling & Jensen, 1970) or a porous plug of polyurethane
coated with a suitable gas-liquid chromatographic stationary phase, or
Amberlite XAD-2 resin (Harvey et al., 1973) followed by elution of the
PCBs with a solvent. Ahnoff & Josefsson (1974, 1975) have described
liquid-liquid extraction into cyclohexane.
Soil and sediment
In a study by Huckins et al. (1988), sediment samples were thawed at
room temperature and placed in a hexane-rinsed foil pan and air dried
for 5 days. The sediment was broken up, homogenized, and mixed with
anhydrous disodium sulfate until dry, for column extraction. The
samples were extracted with methylene chloride. PCB residues were
enriched by adsorption column chromatography on silica gel and
sulfuric acid silica gel. Prior to GC analysis, nitric acid-rinsed
copper wool was added to the sediment extract to remove elemental
sulfur. An aliquot of the PCB residues was diluted in a mixture
methylene chloride: cyclohexane (1:1) and the bulk of the o,o-Cl
substituted PCB components eliminated by eluting the column with
different solvents. The different PCB congeners were determined by
GC-ECD.
The feasibility of cleaning PCB-contaminated soils using a solvent
extraction method was studied by Reilly et al. (1986). Compared with
direct incineration of the sludge, the solvent extraction route has a
number of shortcomings; the detailed design of the extraction plant as
well as its operation will be quite challenging as an extremely
leak-tight operation is essential, considering the nature of the
material handled. Direct incineration will clean the solids much more
thoroughly than is feasible by solvent extraction under ambient
conditions. Furthermore, it is inevitable that some residual solvent
will remain in the solids after processing. The solvent extraction
process costs essentially the same as direct incineration.
Biological samples
Most analysts have used standard methods, developed for organochlorine
pesticides, in which the PCBs are extracted together with the fat; the
sample is ground with anhydrous sodium sulfate and extracted with
petroleum ether or hexane. Porter et al. (1970) studied the optimal
conditions for this procedure. A dehydrating solvent may be included
to facilitate the breakdown of cell structures; ethanol (Norén &
Westöö, 1968) and acetone (Jensen et al., 1973) have been used.
Reznicek (1987) described a method to extract and determine PCBs in
blood. The sensitivity of the method was 10 µg/litre.
2.3.1.2 Sample clean-up
Diverse extraction and clean-up procedures have been devised to
preferentially remove co-extractives that are present in different
matrices and interfere with routine quantitative gas chromatographic
and gas chromatographic-mass spectrometric analysis.
The analysis of lipid-containing matrices for residues of
organochlorine pesticides and PCBs is a common procedure. All the
methods require the separation of the residues from the lipids prior
to the determination of the PCBs by gas chromatography. The removal of
the lipids is usually carried out by low-resolution column
chromatography using an adsorbent, such as silica, alumina, or
Florisil as the stationary phase. Low-resolution gel permeation
chromatography has also been used. An electron-capture detector is the
most commonly used detector, but clean-up procedures may still leave
electron-capturing species in the extract, so the identities of the
eluting peaks must be confirmed. In order to overcome some of these
problems, perchlorination of the PCBs has been used, giving rise to
one GC peak (decachlorobiphenyl), which is well removed from most
interfering peaks, but this technique has been found to be
qualitatively and quantitatively unreliable and unsatisfactory.
Seymour et al. (1986b,c) attempted to simplify clean-up procedures by
using high performance liquid chromatography (HPLC) coupled with gas
chromatography-mass spectroscopy. This latter technique is less
expensive than it used to be and is the only technique that can
possibly identify each peak as a PCB before quantification is carried
out, thereby improving the quality of the result. It is also capable,
when used in the selective ion monitoring mode (SIM), of detecting
only PCBs, even in the presence of pesticides, so that sample clean-up
is further simplified.
Seymour et al. (1986a) described a clean-up procedure, with a
preparative, high-performance liquid chromatographic (HPLC) separation
method for selected pairs of chlorobiphenyl isomers, produced by
Cadogen coupling in the preparation of individual congeners, to be
used as standards in congener-specific determination using capillary
GC methods.
A routine method for the determination of PCBs in breast milk,
described by Seymour et al. (1987), is less labour-intensive and more
cost effective than the traditional methods. These advantages were
achieved by adsorption of the milk on a polar substrate prior to
Soxhlet extraction, using a polymeric HPLC column for the clean-up of
the extract, followed by highly selective capillary GC-MS analysis.
Methods for the removal of fat from the extract include solvent
partitioning between hexane and acetonitrile or dimethylformamide, or
treatment with strong sulfuric acid or ethanolic potassium hydroxide.
Gel permeation has also been used (Stalling et al., 1972), and Holden
& Marsden (1969) removed fat on dry, partially deactivated, alumina
columns. Certain pesticides, such as dieldrin, are destroyed by the
sulfuric acid treatment, so this method cannot be used if such
pesticides are to be determined together with PCBs (Jensen et al.,
1973).
Huckins et al. (1988) described the clean-up of fish samples. Tissue
samples were thawed, mixed, dried with sodium sulfate, and extracted
in glass columns with methylene chloride. The extract was evaporated
and the lipid content was determined gravimetrically. Gel permeation
chromatography was used for removal of lipid from fish sample
extracts. PCB residues were enriched by adsorption column
chromatography on silica gel and sulfuric acid silica gel, eluted with
a mixture of methylene chloride and cyclohexane, and determined by
GC-ECD.
PCBs can be separated from organochlorine pesticides by column
chromatography on Florisil (Mulhern et al., 1971), silica gel (Holden
& Marsden, 1969; Armour & Burke, 1970; Collins et al., 1972) or on
charcoal (Berg et al., 1972; Jensen & Sundström, 1974a). Several
laboratories have reported difficulties in repeating results obtained
by other investigators; the ease of separation appears to depend on
the characteristics of the absorbent, of the eluting solvent, and of
the sample extract, though there does not appear to be any difficulty
in separating all interfering substances, except DDE, a metabolite of
DDT. Thin-layer chromatography has been used for separation by Norén &
Westöö (1968), Bagley et al. (1970), and Reinke et al. (1973).
In many environmental samples, DDE is present in larger amounts than
the PCBs, and must be removed before their quantitative determination.
Oxidation procedures have been used to convert DDE to dichlorobenzo-
phenone; recommended oxidants are potassium dichromate and sulfuric
acid (Westöö & Norén, 1970b) and chromium (II)oxide and acetic acid
(Mulhern et al., 1971). Jensen & Sundström (1974a), who were
interested in determining DDT/PCB ratios in environmental samples,
preferred sodium dichromate in acetic acid with a trace of sulfuric
acid. They claimed that this does not destroy DDT and its metabolite
DDD, which may be present in extracts after clean-up with strong
sulfuric acid, and that using this mixture makes possible the
quantitative determination of the dichlorobenzophenone from the
oxidation of DDE.
Conversion of DDT to DDE can be achieved by treatment with ethanolic
potassium hydroxide, which also removes interference from elemental
sulfur (Ahling & Jensen, 1970). Sulfur may also be removed by
activated Raney nickel (Ahnoff & Josefsson, 1975) or by metallic
mercury.
Beck & Mathar (1985) used gel permeation chromatography to clean
extracts of food of animal origin.
2.3.2 Separation and identification
2.3.2.1 Chromatographic separation
Numerous gas chromatographic studies using packed or capillary columns
have confirmed the complexity of all commercial PCB formulations. The
accuracy in determining PCB levels is highly variable and matrix
dependent. Many factors including: the water solubility, volatility,
and biodegradability of individual PCBs, will alter the composition of
a commercial PCB preparation introduced as a pollutant into the
environment. Thus, the composition of PCB extracts from environmental
matrices will vary widely and often do not resemble any commercial
mixture. Quantitative analyses on these mixtures is usually determined
by pattern- or peak-matching methods, using artificially reconstituted
mixtures of different commercial formulations. High-resolution, glass
capillary gas chromatographic analysis can provide a solution.
Capillary gas chromatography columns, currently in use, are made of
fused silica, chemically bonded with various stationary phases, to
achieve a range of different selectivities towards complex samples. In
general, packed columns have been replaced by capillary columns,
because of their far superior efficiency. The identities of the
individual peaks must then be determined by using synthetic standards
and by retention index addition methods. This latter technique
predicts the relative retention times (RRT) of specific PCBs and has
been used to assign the structures of individual PCB congeners. The
method relies on the RRT values that have been determined for
synthetic PCB standards. On this basis, Safe et al. (1985a) reported
the first congener-specific analysis of a PCB preparation and PCBs in
human milk.
Some workers use GC with mass selective detection (MSD), which
quantifies the level of chlorination in a sample extract
(Alford-Stevens, 1986). Tanabe et al. (1987) and Kannan et al. (1987)
described a method to determine the 3 toxic, non- ortho-chlorine-
substituted, coplanar PCBs, 3,4,3',4'-tetra, 3,4,5,3',4'-penta-,
and 3,4,5,3',4',5'-hexachlorobiphenyl, which are biologically active
congeners. The method comprised alkali digestion, carbon
chromatography, and high-resolution gas-chromatography. Using
this method, it is possible to determine ppt levels of these toxic
residues in biological samples. Duinker et al. (1988) used
multidimensional gas chromatography with ECD to determine levels of
all congeners in some Clophen and Aroclor mixtures and found
considerable differences between their composition of congeners and
those in an extract of a seal blubber sample. Using this technique,
congeners were identified that had, hitherto, been undetected, using
other analytical techniques. It was possible to identify the toxic
congeners in the samples studied, even when the relative contribution
of each congener to the cluster was as low as 0.01%.
2.3.2.2 Gas-liquid chromatography
Most analysts use gas-liquid chromatography with an electron-capture
detector for the separation of PCBs from the extract after clean-up.
Stationary phases commonly used are silicones or their derivatives,
for example, DC 200, SF 96, OV 1, and QF 1, or Apiezon L. Jensen &
Sundström (1974a) stated that, with a mixture of SF 96 and QF 1, 14
peaks could be obtained from Clophen A50, but that Apiezon L gave much
better resolution. They obtained better peak separation by prior
fractionation on a charcoal column, which separated the PCBs according
to the number of o-chlorine substituents; they regarded such
refinements as unnecessary in PCB residue analysis, but they may be of
value in the study of the selective, environmental degradation of
PCBs. Column temperatures used ranged between 170°C and 230°C. Glass
capillary columns are superior to packed columns giving better
separation of closely-related congeners; they also give good
separation of PCBs from DDT and its metabolites (Zell et al., 1977;
Dunn et al., 1984; Beck & Mathar, 1985; Alford-Stevens, 1986; Tanabe
et al., 1987; Duinker et al., 1988).
A gas chromatography/electron impact mass spectrometry (GC/EIMS)
method was used by Erickson et al. (1988) for the determination of
by-product (non-Aroclor) PCBs. In this method, the recovery of 4
13C-labelled PCBs was measured to assure adequate recovery of the
native PCBs from diverse matrices. The complexity of the matrices and
the high probability of chlorinated organic interferents precluded the
use of GC/ECD. The best available technique for universal application
to commercial products, and associated waste, is GC/EIMS. During the
validation work, the anticipated difficulty of qualitative and
quantitative data interpretation was confirmed. In addition to the
inherent problems resulting from extrapolation from 11 standards to
209 analytes, interpretation of the complex peak clusters is tedious.
2.3.3 Quantification
An electron-capture detector (ECD) is the most commonly used
instrument for the quantification of PCBs. However, the response of
this detector varies according to the number and location of the
chlorine atoms in the PCB molecule, resulting in difficulties when the
sample under investigation contains PCBs that have degraded (Zitko et
al., 1971).
Various principles have been used to quantify PCB residues:
* comparison of a single peak in the residue with the corresponding
peak in a commercial reference PCB (Aroclor, Clophen);
* comparison of the total response for several peaks in the residue
with the total response of the corresponding peaks in a reference
standard;
* comparison of the response of all peaks in the sample with those
in the reference standard;
* perchlorination of PCBs to decachlorobiphenyl followed by
quantification of this single compound.
The results obtained using these various methods differ; consequently,
the precision in these analyses is not very good. Recently, Dunn et
al. (1984) described a method for the quantification of PCBs using gas
chromatography data, based on a pattern recognition technique and
partial least squares in latent variables. The data to which it was
applied were gas chromatograms of Aroclor 1242, 1248, 1252, and 1260.
This technique also allows the classification of unknown samples
(WHO/EURO, 1987).
Fait et al. (1989) investigated whether the results obtained for total
PCBs using FSCGC/ECD (see section 2.3), differed significantly from
those determined using packed column gas chromatography electron
capture (PCGC/ECD) techniques, within 3 exposure groups. The
concentrations of individual PCBs were determined in both the serum
and adipose tissue from 35 transformer repair workers and 17 previous
repair workers, exposed mainly to Aroclor 1260, in comparison with 56
non-exposed workers. Eighty-nine PCB peaks were identified. The total
serum PCBs determined by FSCGC/ECD greatly exceeded that from standard
PCGC/ECD. The median concentrations in serum were: 43.7, 30.0, and
16.1 µg/litre, and the median concentrations in adipose tissue were:
3180, 888, and 821 µg/kg, respectively. In all workers,
hexachlorinated and heptachlorinated congeners predominated followed
by octachlorinated and pentachlorinated species. The 7 major peaks in
serum and adipose tissue were 2,3,5,6,3',4',5'/ 2,3,4,5,2',4',5'/
2,3,4,5,2',3',4'-heptachloro-; 2,3,4,2',3',5'-hexachloro-;
2,4,6,3',4',5'/ 2,4,5,2',4',5'-hexachloro-; 2,3,4,5,2',3',5',6'/
2,3,4,5,6,2',3',5'-octachloro-; 2,4,5,3',4'/ 3,4,5,2',3'-pentachloro-
and 2,3,4,2',3',4'/ 2,3,5,6,2',4',5'/ 2,3,4,5,2',4',6'
multichlorobiphenyls.
The response of the electron capture detector is not equal for all PCB
components, being much affected by the degree of chlorination, as
already mentioned (Zitko et al., 1971). This does not lead to
difficulties when the sample under investigation has been directly
contaminated by a commercial PCB mixture, as this mixture can be used
as a standard. Difficulties are encountered when the PCBs in the
sample have undergone selective environmental degradation. Several
investigators have noted that the pattern of peaks from such samples
resembles fairly closely that of one or other of the higher
chlorinated PCB mixtures, such as Aroclor 1254, and they have compared
the total area of the peaks with that of the nearest commercial
product, in order to determine the amount of PCBs in the sample
(Armour & Burke, 1970; Tuinstra, 1983). Collins et al. (1972) observed
that, under their conditions, the area of peaks usually encountered in
extracts of tissue samples was very similar to that of an equivalent
amount of DDE, thus, DDE could be used for calibration. In order to
overcome the uncertainties of these procedures, Rote & Murphy (1971)
divided the peaks into groups according to the number of chlorine
atoms in the molecule, as determined from mass spectrographic data,
and calculated the PCB content of each group from the theoretical
response of the detector to chlorine content. Jensen et al. (1973)
selected a commercial PCB that included all the peaks from the
extract; they determined the PCB content of each peak by combined mass
spectrometry and coulometry, and determined the total PCBs in the
sample by comparing the height of each peak obtained with the extract
with those obtained with the reference sample. Simpler methods have
been used including that of Koeman et al. (1969), who compared the
height of a single peak, obtained with the extract, with that of a
peak with the same retention time obtained with a commercial PCB
mixture, and those of others who averaged out more than one peak for
this calculation (Reynolds, 1971; Reinke et al., 1973). Rote & Murphy
(1971) calculated that such procedures may give more than double the
values obtained by a more accurate method.
In the characterization of PCB components in PCB mixtures, the
retention properties of the components of the mixtures, as well as a
great number of synthesized components, were used to predict a
complete analysis of mixtures as Aroclors 1242, 1254, and 1260. Jensen
& Sundström (1974a) synthesized a large number of reference substances
and were able to identify almost 60 components in Clophen A50 and A60.
Attempting to account for unidentified peaks, authors have used the
chromatographic retention indices of available components to calculate
such data for missing ones. The identity of many peaks could not,
however, be determined unambiguously. Some of these uncertainties have
been resolved by the application of techniques other than the
comparison of retention times e.g., MS, NMR, and IR. The efficiency of
packed columns in GLC is not sufficient to allow their use for the
accurate analysis of complex mixtures, in most cases. Another approach
to the use of packed columns involves the use of columns with various
selectivities. In this way, complete analysis of all components in
Aroclors has been claimed with the use of up to 12 columns. The
strongly increased GLC separation offered by capillary columns has
been used to advantage in the analysis of technical formulations, in
some cases the eluate was analysed by MS. To identify individual
congeners, gas-liquid (using glass capillaries with different
coatings) chromatography (GLC) was used by Albro & Parker (1979) and
Albro et al. (1981). Hydrogen flame ionization detection (HFID) and
electron capture detection (ECD) and MS were used by Duinker &
Hillebrand (1983).
2.3.4 Accuracy of PCB determinations
A group of 8 analysts, engaged in an investigation of pollution in the
North Sea, undertook a collaborative study to determine the PCB
content of a sample of fish oil, using the methods currently employed
in their laboratories (International Council for the Exploration of
the Sea, 1974). The PCB values obtained ranged from 1.0 to 3.9 mg/kg
with a mean of 1.97 mg/kg and a standard deviation of 0.93 mg/kg.
Better agreement was obtained with the same fish oil fortified with
PCBs at a concentration of 10 mg/kg; the mean of the results for the
fortified sample was 10.0 mg/kg with a standard deviation of
1.1 mg/kg.
A probable source of error is incomplete initial extraction of PCBs
from a sample (Holden & Marsden, 1969). Another source of variation
between laboratories lies in the method used to quantify gas-liquid
chromatographic peaks; Van Hove Holdrinet (1975) considered this to be
the major source of error.
It is evident that caution should be exercised in accepting the
analytical results from a laboratory, particularly for samples with a
low PCB content, until the competence of the laboratory has been
established by an inter-laboratory collaborative study (Tuinstra,
1983).
Schulte & Malisch (1984) described a method to determine the real PCB
contents of environmental samples. A technical PCB mixture of known
composition was used for calibration. The PCB concentrations were
determined in samples of human milk and butter and the calculated
contents were 50% and 40% lower, respectively, than the values
obtained by the usual calculation based on evaluation of some higher
peaks of technical PCB mixtures.
2.3.5 Confirmation
Since Jensen first identified as PCBs hitherto unknown substances that
interfered in the glass-liquid chromatographic determination of
organochlorine pesticides using mass spectrographic data, other
investigators have confirmed the presence of PCBs in environmental
samples by combining gas-liquid chromatography with mass spectrometry
(Bagley et al., 1970) and with coulometry, to measure the chlorine
content. The conversion of PCBs to bicyclohexyl and decachlorobiphenyl
is further confirmation (Berg et al., 1972). The widespread
distribution of PCBs is now well established, and, as adequate methods
are available to remove interference from organochlorine pesticides,
there is no evidence of the presence of other interfering substances
in the types of sample that have so far been analysed, down to a limit
of detection of around 0.01 mg/kg. This does not necessarily apply to
other types of sample, particularly when very low levels are being
sought; Ahnoff & Josefsson (1973) reported a number of unknown
interfering substances, when measuring PCBs in water at levels below
1 ng/litre. One of these substances was subsequently identified as
elemental sulfur. They recommend confirmation by mass fragmentography
for such samples.
2.3.6 Detection limits
The limits of determination using low or high resolution mass
spectrometry are 0.01-1 pg per injection of each congener. The
detection levels in samples depend on the sample size and matrix.
Using an air sampling device described by Rappe et al. (1985b), a
detection level of 0.05 pg/m3 per congener could be determined in
ambient air (WHO/EURO, 1987).
In general, other substances are not considered to interfere at levels
of about 0.01 mg/kg. In river water and air, levels of 1 ng/litre and
0.3 ng/m3, respectively, are reported to be the detection limits of
PCBs (WHO/EURO, 1987). Tuinstra (1983) found a limit of detection for
individual chlorobiphenyls in environmental and biological samples, of
less than 1 µg/kg (see Table 7).
The results for sewage sludge, eel, grass, cow's milk, and human fat
are given in Table 7 (Tuinstra, 1983). Individual chlorobiphenyls were
also estimated in the monitoring programme for environmental and
biological samples in the Netherlands.
2.4 Codex questionnaire on analytical methods
2.4.1 Interpretation and comparability of data
Monitoring data are available from many sources in many countries.
They have been obtained using various methodologies, such as different
sampling techniques and different methods of analysis and
quantification. Limits of determination reported vary by a factor of
1000 or more.
Given this situation, data on levels of PCBs have to be interpreted
with the greatest care. Comparisons can only be made between data from
the same laboratory, using the same validated technique over a long
period. Comparisons between data from different laboratories have to
be limited to the very few cases, where very strict inter-laboratory
checks have been made on the basis of the same sampling and analytical
techniques. Indications about trends can only be obtained when taking
into account these basic considerations (Beck & Mathar, 1985; Tuinstra
et al., 1985b,c).
In June 1985, a questionnaire was distributed to all Codex Contact
Points with the aim of providing background information on PCBs for
the ad hoc working group on contaminants to compare such factors as
methods of analysis, quantification, monitoring, etc. Eighteen out of
22 countries responded to the questionnaire.
In some cases, the information given was incomplete, but it is
apparent that a variety of clean-up methods is employed. Where good
laboratory practices are followed and tests indicate close to 100%
recovery of standards from spiked samples, the main effect of
different clean-up procedures will be on the limit of detection.
For gas chromatography, 6 countries reported that they used capillary
columns as alternative or confirmatory systems. Among the respondents,
the Netherlands and the Federal Republic of Germany routinely used
capillary columns and specific PCB isomers as regulatory standards.
The types of packed column materials used varied considerably. With
respect to quantification, pattern comparison with standards of
various PCB formulations was the method most favoured, though some
countries specified the use of certain combinations of peaks. In
several cases, the methods being used were stated to have been
collaboratively tested, or checked by inter-laboratory ring tests.
During the sixties, packed column chromatography was the most widely
used method in the determination of PCBs. Results obtained with this
technique varied widely between laboratories, and were much influenced
by the method of quantification chosen and by the PCB mixture used as
a standard. Chemical conversion methods, especially perchlorination,
have also been used. These methods are quite sensitive, but do not
allow for peak pattern identification. Another drawback of
perchlorination is that conversion of less chlorinated biphenyls is
not quantitative.
Sensitivity is sufficient, if adequate clean-up methods are used.
Combined gas chromatography/mass spectrometry has a somewhat lower
sensitivity, needs more expensive equipment, and is not considered
suitable for routine work. The results obtained using these techniques
may vary widely and most of them can only be used as rough estimates.
When capillary columns are used with temperature programming, almost
all PCB isomers and congeners normally present in samples can be
identified. This method is now considered to be the best available
technique. However, it is important to decide which isomers should be
used as guiding substances.
Table 7. Typical values of individual chlorobiphenyls in Dutch environmental and
biological samples. Peak numbering according to IUPAC rulesa
PCB Structure Sewage Eel Grass Cow's Human
compound sludge milk fat
µg/kg µg/kg µg/kg µg/kg µg/kg
(dm)b product (dm)b,c fatc fat
28d 2,4,4' 60 35 -c -c 45
52d 2,5,2'5' 22 110 0.4 2.1 10
44 2,3,2'5' 20 34 0.2 0.9 10
95 2,3,6,2'5' 58 130 0.7 1.6 30
101d 2,4,5,2'5' 30 85 0.6 3.1 15
151 2,3,5,6,2'5' 9 24 0.2 0.6 10
149 2,3,6,2'4'5' 42 90 0.6 2.5 15
118 2,4,5,3'4' 20 110 0.3 -c 80
153d 2,4,5,2'4'5' 54 180 0.7 13 295
141 2,3,4,5,2'5' 10 40 0.2 0.6 <5
138d 2,3,4,2'4'5' 45 200 0.7 11 235
128 2,3,4,2'3'4' 7 20 <0.1 1.2 15
180d 2,3,4,5,2'4'5' 33 80 0.5 6.4 205
170 2,3,4,5,2'3'4' 10 30 0.2 1.8 90
201 2,3,4,5,2'3'5'6' <5 10 <0.1 <0.5 20
a From: Tuinstra (1983).
b dm = dry matter.
c nd = not determined.
d Monitoring compounds.
2.5 Activities of the WHO Regional Office for Europe
The WHO Regional Office for Europe (WHO/EURO) has an ongoing programme
related to PCBs, as well as to other chlorinated hydrocarbons,
including polychlorinated- para-dibenzodioxins (PCDDs) and
polychlorinated dibenzofurans (PCDFs). Within this programme,
practical guidelines to prevent and control accidental and
environmental exposures to these chemicals have been published in the
Environmental Health Series of WHO/EURO (1987). The other important
project within this programme dealt with the assessment of the health
risks to infants associated with contamination of mother's milk. This
assessment was completed by a WHO/EURO Expert Consultation held in
Abano Terme, Italy, in 1987, and the output of this consultation has
been published in the Environmental Health Series of WHO/EURO (1988).
In order to produce more data on exposure levels through human milk,
WHO/EURO has been coordinating analytical field studies in which
several countries have participated. The results of these studies have
been published in the Environmental Health Series of WHO/EURO (1989).
This document also includes the results of interlaboratory quality
control studies on levels of PCBs, PCDFs, and PCDDs in human milk. In
the first series of studies, 12 laboratories were involved. The second
round of the quality control studies has been completed, with the
participation of additional laboratories, and the results will be
published. Furthermore, the repetition of the analytical field studies
on the levels of PCBs, PCDFs, and PCDDs in human milk will be
implemented in 1991 and coordinated at WHO/EURO.
2.6 Appraisal
Since the congener composition and relative concentrations of the
individual components in PCB extracts from environmental and
biological samples differ markedly from those in commercial PCB
mixtures, the quantitative determination of the PCB contents of such
samples presents a special problem. Various approaches to the
quantitative determination of PCBs have been reported including:
attempts to determine the total PCB concentration through
perchlorination of the mixture; identification of selected
chromatographic peaks through gas chromatographic techniques with
packed columns using certain commercial products as standards; as well
as attempts to carry out congener-specific analysis, based on high
resolution chromatographic separation followed by identification and
quantification by mass spectrometry using synthetic standards. This
last method is considered the best at present, though it is not
feasible for all laboratories. Although the concentration values
obtained from the various methods might be similar, such comparison
will be limited and is of questionable value for most purposes. The
occurrence of specific PCB congeners in various samples and a
consideration of the relative toxicity and persistence of the
congeners have been suggested as a basis for a congener-specific
analytical approach. While this approach can be useful, particularly
in risk/hazard assessment exercises, it must be realized that it is
based on the present knowledge about the occurrence, persistence, and
toxicity of specific congeners. It does not take into consideration
potentially unrecognized toxicities associated with the same or
different congeners, which may be present in a sample, also it is not
feasible in some countries. Therefore, further research in this area
should continue to improve the basis for monitoring programmes and for
a congener-specific approach.
In the selection of areas with high levels of contamination, in order
to establish priorities for action, it is considered that analytical
competence and the use of adequate controls and standards is more
important than highly accurate laboratory analysis. Also, the quality
and usefulness of analytical data depend critically on the validity of
the samples and the adequacy of the sampling programme. A quality
assurance programme and collaborative studies should be part of any
long-term study on PCBs, since there are several possible sources of
error. In this situation, data on levels of PCBs have to be
interpreted with the greatest care and, in general, definitive
comparison can only be made between data from laboratories using the
same techniques and interpretation of results.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Polychlorinated biphenyls are aromatic chemicals that do not occur
naturally in the environment.
3.2 Man-made sources
3.2.1 Production levels and processes, uses
The first chlorinated biphenyl was synthesized in 1864, but it was not
until 1929/1930 that the PCBs were produced commercially for use:
(a) as dielectrics in transformers and large capacitors;
(b) in heat transfer and hydraulic systems;
(c) in the formulation of lubricating and cutting oils and wax
extenders;
(d) as plasticizers in paints, and as ink solvent/carriers in
carbonless copy paper, adhesives, sealants, flame retardants, and
plastics (Hutzinger et al., 1974; Pomerantz et al., 1978).
An extensive review of the uses of PCBs is given in DFG (1988).
3.2.1.1 World production figures
Over one million tonnes of PCBs have been produced commercially under
a number of trade names, such as Aroclor, Fenchlor, Clophen, and
Kanechlor.
Details of the production and uses of PCBs in the USA have been
released, and have been summarized by Nisbet & Sarofim (1972). Annual
production increased steadily from 1930 and reached a maximum in 1970
of 33 000 tonnes. Of this, 56% was used as a dielectric (36% in
capacitors and 20% in transformers). Various plasticizer outlets
accounted for 30%, hydraulic fluids and lubricants, 12%, and heat
transfer liquids, 1.5%. During this peak year, 65% of the production
was of the 42% chlorinated type, 25% was less chlorinated, and the
remainder more chlorinated. After 1970, production decreased sharply
owing to the voluntary limitation of sales by the Monsanto Company,
the major manufacturer in the USA.
Following the restriction of sales for dissipative uses, the
percentage of PCBs sold as dielectrics rose to 77% in 1971 and the
proportion of highly chlorinated products was considerably reduced;
Aroclor 1016 replaced Aroclor 1242. In Japan, 44 800 tonnes of PCBs
were used from 1962 to 1971; of this, 65.4% was used in the electrical
industry, 11.3% in heat exchangers, 17.9% in carbonless copying paper,
and 5.4% for other dissipative uses (Ishi, 1972).
During the period 1980-84, the production in EEC member states was as
follows: France, 16 200; Federal Republic of Germany, 24 200; Italy,
4500; and Spain, 3400 tonnes. After 1984, production was continued
only in France and Spain (Bletchly, 1985; WHO/EURO, 1987).
By the end of 1980, the total amount of PCBs produced was 1 054 800
tonnes (of which approximately half was used in transformers and
capacitors, see Table 8), divided between the following countries (in
tonnes): USA, 647 700; Federal Republic of Germany, 130 800; France,
101 600; United Kingdom, 66 800; Japan, 59 300; Spain, 25 100; and
Italy, 23 500 (Bletchly, 1983).
In addition, Czechoslovakia and the USSR have manufactured PCBs for
their domestic market under the trade names of Delor and Sovol,
respectively, but the data on production quantities are not available.
According to an OECD report, transformers and capacitors provided the
major outlets for PCBs in most OECD countries in 1971. In 1972,
several countries restricted sales; in Sweden the importation and use
of PCBs were restricted by law; in the United Kingdom, as in the USA,
sales were voluntarily restricted to the lower chlorinated PCBs for
use as dielectrics in enclosed systems, and, in the USA in 1979,
manufacture, use, handling, storage, and disposal were promulgated. As
late as 1985, a final rule concerning the restriction and conditions
on the use of PCB transformers was published (USEPA, 1985). In Japan,
the production and use of PCBs were banned in 1972.
The 24 OECD countries adopted a Decision in 1973, limiting the use of
PCBs to certain specific applications and asking for the control of
the manufacture, import, and export of bulk PCBs, for adequate waste
treatment and for a special labelling system for PCBs and
PCB-containing products. On 13 February 1987, the Council of the
Organization for Economic Co-operation and Development (OECD) adopted
a further Decision-Recommendation (C(87)2(final)) on "Further measures
for the protection of the environment by control of polychlorinated
Table 8. Estimated usage of PCBs in transformers and large capacitors in
a number of OECD countries in 1930-80 (in tonnes)a
Country Usage in Usage in Total
transformers capacitors
France 50 700 8 800 59 500
Federal Republic 44 400 17 700 62 100
of Germany
Italy 10 400 1 500 11 900
Japan 37 200b 37 200
Spain 20 100 3 400 23 500
United Kingdom 5 800 8 100 13 900
United States 125 800 130 400 256 200
of America
Total 294 400 169 900 464 300
a From: WHO/EURO (1987).
b Includes the usage in both transformers and capacitors
biphenyls". With this Decision-Recommendation, the OECD Member
countries committed themselves to ban virtually all new uses of PCBs,
accelerate the phasing out of PCBs from existing uses, control PCBs in
contaminated products, articles, or equipment, and ensure appropriate
disposal methods for PCB-containing waste. The uses of PCBs have been
virtually restricted to those in "closed systems". In 1976, an EEG
Directive made the limitations of the use compulsory for the EEG
Member States. Other Directives, such as those on waste treatment and
disposal, followed (van der Kolk, 1984a, Personal communication).
3.2.1.2 Manufacturing processes
Industrial manufacturing of PCBs is based on the chlorination of
biphenyl by anhydrous chlorine, under heated reaction conditions and
in the presence of suitable catalysts (e.g., iron-chloride). Depending
on the reaction conditions, a degree of chlorination varying between
21% and 68% (weight percentage, w/w) can be achieved.
The yield is always a mixture of different compounds and congeners.
Commercial mixtures generally have been purified by filtration and
fractional distillation, but, in spite of this, they have been found
to contain many impurities (WHO/EURO, 1987). In general, commercial
PCB products contain impurities, mainly polychlorinated dibenzofurans
(PCDFs).
Rappe et al. (1985d) cf. WHO/EURO (1987) analysed a series of
commercial PCBs, using a new clean-up technique based on reverse-phase
chromatography on a carbon column followed by a fluorosil column. In
all PCB products, PCDFs were found at levels varying from a few mg/kg
up to 40 mg/kg. The chlorination pattern of the PCDFs was found to
vary with the chlorination level of the PCBs. In most products,
2,3,7,8-substituted tetra-, penta-, and hexa-CDFs were the major
constituents.
3.2.2 Uses
PCBs have been widely used in electrical equipment, such as capacitors
and transformers. These have often been considered to be closed
systems, though small amounts of PCBs can frequently be found on the
outer metal surface of such equipment.
Smaller volumes of PCBs have often been used as fire-resistant liquid
in nominally closed systems, such as hydraulic and heat exchange
systems (WHO/EURO, 1988).
Broadhurst (1972) reviewed the many technical applications of PCBs
that appear in the literature and in patent specifications, and
indicate the possibility of a widespread, non-occupational, low-level
exposure to PCBs, other than that derived from the diet. PCBs are used
in the home in ballast capacitors for fluorescent lighting, and
exposure from pressure-sensitive copying paper has not been limited to
office workers. The valuable properties of PCBs as plasticizers has
led to their use in furnishings, interior decoration, and building
construction; examples are surface treatment for textiles, adhesive
for waterproof wall coatings, paints, and sealant putties. PCBs have
been used as plasticizers for plastic materials and in the formulation
of printing inks.
The value of PCBs for industrial applications depends on their
chemical inertness, resistance to heat, non-flammability, low vapour
pressure (particularly with the higher chlorinated compounds), and
high dielectric constant.
Data on the usage of technical PCB mixtures in Europe are scarce. In
the 1960s and early 1970s, PCBs were used in (WHO/EURO, 1987):
(a) completely closed systems;
(b) nominally closed systems;
(c) open-ended applications.
3.2.2.1 Completely closed systems
PCBs have been widely used in electrical equipment, such as capacitors
and transformers, which are considered to be completely closed
systems. Historically, capacitors are the single largest PCB-use
category. The PCB mixtures used for this purpose are, for example,
Pyralene 3010, Aroclor 1016, 1221, and, earlier, also Aroclor 1242 and
1254. The amounts used in a number of OECD countries are presented in
Table 8 (OECD, 1982; Bletchly, 1983; Callahan et al., 1983).
Since the late 1970s and the beginning of the 1980s, PCB-filled
capacitors have largely been superseded by capacitors with a non-PCB
dielectric fluid. The tendency for this substitution varies from
country to country, for example, it started in Sweden and Finland in
1982, and in Norway in 1985.
The technical PCB mixtures used in transformers are mostly highly
chlorinated like Aroclor 1254 and 1260. In general, the PCBs are used
in combination with tri- and tetrachlorobenzenes as mixtures called
Askarel.
The amounts of PCBs used in transformers differ in different
countries. In France, where most transformers are placed indoors, the
major dielectric fluid is PCBs or Askarels, which are both flame
retardants, while in Scandinavia, where most capacitors are placed
outdoors, mineral oils (with a lower melting point) are frequently
used.
During the 1980s, there has been a marked interest in replacing the
PCBs, mainly in indoor transformers, as a result of serious accidents,
for example, in Binghamton, San Francisco, Miami in the USA, and Reims
in France. Various products are used for this exchange, such as
mineral oils, silicone oils, perchloroethylene, and other chlorinated
products (WHO/EURO, 1987).
3.2.2.2 Nominally closed systems
Smaller volumes of PCBs have frequently been used as fire-resistant
liquid in nominally closed systems, such as hydraulic and heat
transfer exchange systems (for example, trade names Pydraul and
Therminol FR, containing Aroclor 1242, 1248, 1254, and 1260). PCBs are
used as a working fluid in vacuum pumps (Aroclor 1248, 1254), which
can also be considered as nominally closed systems (WHO/EURO, 1987).
3.2.2.3 Open-ended applications
With open-ended applications of PCB, both the emissions into the
environment and the levels of occupational exposure are more
pronounced. The major open-ended applications include use as a
plasticizer (in PVC, neoprene, and other artificial chlorinated
rubbers). Other open-ended uses, such as surface coatings, paints,
inks, adhesives, pesticide extenders, microencapsulation of dyes, and
carbonless copy paper contribute smaller volumes into the environment.
PCBs have also been used in immersion oils for microscopes, as
catalysts in the chemical industry, in casting waxes in the iron/steel
industry (decachlorobiphenyl), and in cutting and lubricating oils
(WHO/EURO, 1987).
3.2.2.4 Contamination of other compounds
In addition to the above uses of PCBs, numerous halogenated compounds
may contain PCBs in small amounts as a contaminant (US EPA, 1983).
3.2.3 Loss into the environment
PCBs are dispersed into the environment through atmospheric transport
and, on a more regional scale, following release into water. PCBs are
also mobilized in the soil or landfills, but the rates of dispersion
and subsequent transfer to biota and humans are difficult to estimate.
More highly chlorinated forms become most prevalent in compartments
further along the pathway chains. The analytical methods used to
quantify PCBs in the environment and biota vary greatly within, and
between, countries. Thus, comparisons can only be made in a very broad
sense and could, to some extent, be erroneous (WHO/EURO, 1988).
An overview of prevention and control measures of accidental and
environmental exposures is given in WHO/EURO (1987).
3.2.3.1 Routes of environmental pollution
Surveys of the sources of environmental pollution with PCBs were made
before production and use became limited, and the information
available may not now apply in North America and elsewhere. Only 20%
of the annual production in the USA can be regarded as a net increase
in current usage, and the remainder is balanced by a loss to the
environment. More than one-half of this entered dumps and landfills
and it has been calculated that 0.3 million tonnes of PCBs have
accumulated in such locations in North America, since 1930 (Nisbet &
Sarofim, 1972). Much of this was originally enclosed in containers,
such as capacitors, or was in plasticized resins and will not be
released until the containing medium decays. The diffusion of PCBs
from landfills is likely to be slow, on account of their low
volatility and low water solubility. Carnes et al. (1973) found little
leaching from the one site that they tested.
The concentration of PCBs in emissions from several municipal sanitary
landfills and refuse and sewage sludge incinerators were determined in
the Midwest of the USA. Sanitary landfills continuously emit the
gaseous products of anaerobic fermentation together with other
volatile materials into the atmosphere. A projection, based on the
amount of methane generated annually from landfills and a PCB to
methane ratio of 0.3 µg PCBs/m3 of methane found from the landfills
sampled, indicates that the annual PCB emissions from sanitary
landfills in the USA are of the order of 10-100 kg/year. The
concentrations of PCBs from the incinerator stacks ranged from
0.3-3 µg/m3 and the annual emissions per stack were 0.25 kg/year.
These estimates are very small in comparison with the 900 000 kg
PCBs/year estimated to cycle through the atmosphere over the USA,
annually (Murphy et al., 1985).
Scrap transformer fluid containing PCBs has been used in the USA in
amounts of about 10 tonnes/year in pesticide formulations (Panel on
Hazardous Trace Substances, 1972, cf. WHO/EURO, 1988), and this
unauthorized use has led to the local contamination of milk supplies.
Pressure sensitive duplicating paper (carbonless copying paper)
containing PCBs has found its way into waste paper supplies and has
been recycled into paper and board used as food packaging materials,
but not since 1970; paints for coating the bottom of ships contained
3-5% of PCBs, about 3% of the annual quantity imported into Sweden has
been used for this purpose, and this has been a source of plankton
contamination (Jensen et al., 1972a).
Schecter (1987) described the contamination of drinking-water by the
use of submersible water pumps which, in certain instances, contained
PCBs in the oil. When the pumps leak, PCBs may be released into the
drinking-water.
In addition, the US EPA, in 1980, estimated that over 1 000 000 wells
in the USA may have PCB capacitors in the well motors. Levels recorded
in drinking-water range from 0.26 to 57 µg/litre compared with
1 µg/litre considered safe in the guidelines for New York State. The
oil from these pumps contained 630 000-24 000 000 µg/kg of PCBs.
Stehr et al. (1985) studied the possibility of contamination with PCBs
of oils and oil-filled devices used by amateur radio operators. Two of
77 oil samples contained more than 50 mg/kg.
3.2.3.2 Release of PCBs into the atmosphere
There appears to be little atmospheric contamination during the
manufacture and processing of PCBs, but this can occur during their
subsequent use and disposal. Although PCBs have a low volatility,
there may be an appreciable loss to the atmosphere during the lifetime
of a PCB-plasticized resin, particularly of the lower chlorinated
products. Further pollution may occur during the incineration of
industrial and municipal waste. Most municipal incinerators are not
very effective in destroying PCBs; efficient incinerators can be
designed for this purpose (Oehme et al., 1987), though the higher
chlorinated PCBs are more resistant to pyrolysis. Secondary sources of
atmospheric pollution are volatilization from soil, and the drying of
sewage sludge. Furthermore, there is evidence that, even at ambient
temperatures, PCBs will enter the atmosphere by volatilization from
soils and water bodies, landfill sites etc. (section 4.1.1).
3.2.3.3 Leakage and disposal of PCBs in industry
Eschenroeder et al. (1986) analysed PCB risks using estimates of human
intake of PCBs originating from accidental spills from electrical
equipment. Equipment spills without controls resulted in a human
intake of PCBs of, at the most, 2 ng/day via the water exposure
pathway. This was negligible in comparison with the intakes calculated
on the basis of fish consumption. The inhalation exposure of
approximately 100 persons living in the vicinity of a spill in
Southern California was determined to equal the PCB intakes of a
fish-eating population.
3.2.4 Thermal decomposition of PCBs
It has been found by Buser et al. (1978a,b) that PCBs can be converted
to PCDFs under pyrolytic conditions. The pyrolysis of a commercial PCB
mixture in a sealed quartz ampoule, in the presence of air, yielded a
mixture including about 30 major and more than 30 minor PCDF
congeners.
Buser & Rappe (1979) studied the pyrolysis (at 600°C) of 15 individual
PCB isomers and demonstrated the presence of PCDFs via intramolecular
cyclizations, where m + n varies from 4 to 8 (Fig. 1). The
thermochemical generation of PCDFs from PCBs was found to follow 4
general reaction routes including loss of ortho-Cl; loss of HCl
involving a 2,3-chlorine shift at the benzene nucleus; loss of
ortho-HCl and loss of ortho-H (Buser, 1985; Hutzinger et al.,
1985).
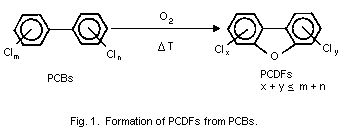 The maximum yield of PCDFs was about 10%, calculated on the amount of
PCBs decomposed, and the optimal temperature was between 550 and
650/700°C (Bentley, 1983). Thus, the uncontrolled burning of PCBs can
be an important occupational and environmental source of toxic and
hazardous PCDFs and it is recommended that all destruction of
PCB-contaminated waste should be carefully controlled, especially with
regard to the burning temperature (above 1000°C), residence time, and
turbulence (Bentley, 1983; WHO/EURO, 1987).
In the temperature range 300-400°C, Morita et al. (1978) reported that
the yield of conversion seemed to be in the mg/kg range. However,
Nagayama et al. (1981) reported a dramatic increase in the levels of
PCDFs at these rather low temperatures, in the presence of stainless
steel or nickel.
No, or very low levels of, PCDDs have been reported from the pyrolysis
of PCBs. However, pyrolysis of a mixture of PCBs and chlorobenzenes
(product Askarel) can yield both PCDFs and PCDDs (Buser, 1979).
Rappe et al. (1985b) found that various types of industrial
incinerators, such as copper smelters and steel mills generate PCDFs
and PCDDs. Pyrolysis of chlorinated polymers like polyvinylchloride
(PVC) and Saran also generate these compounds and exhaust gases of
motor cars and their motor oil may contain PCDDs and PCDFs (WHO/EURO,
1987).
In a State Office Building in the centre of Binghamton, New York, a
fire, in conjunction with several explosions, occurred in the basement
mechanical room, in 1981. Approximately 750 litres of Askarel, a
dielectric fluid composed of 65% PCBs (Aroclor 1254) and 35%
polychlorinated benzenes, leaked from a transformer and caught fire.
Pyrolysis of the Askarel led to the formation of a fine oily soot
that spread throughout the building via 2 ventilation shafts. Samples
taken several days after the fire showed average concentrations of
PCBs in the air of the building of 1.5 µg/m3. The average result for
surfaces ranged from 4.6 to 162.2 µg/m2. TCDFs and PCDDs were also
present. The soot samples were analysed for pyrolysis products. They
contained average levels of 3 mg TCDD/kg and 199 mg 2,3,7,8-TCDF/kg
(Fitzgerald et al., 1989). Achilles (1983) reported the following
levels in the deposited smut; 2160 mg PCDFs/kg and 20 mg PCDDs/kg
(including 0.6 mg 2,3,7,8-TCDD/kg).
In the soot from the Binghamton, Reims, and Stockholm accidents, high
levels of polychlorinated biphenylenes (PCBPs) were identified as well
as the PCDFs (Fig. 2) (Rappe et al., 1982, 1985).
Between 1981 and 1985, a number of accidents in electrical equipment
were reported from different countries; 28 accidents were mentioned in
WHO/EURO (1987) including actual capacitor explosions, capacitor
fires, and transformer accidents. In all eases, the accident site was
contaminated by PCDFs, average levels of total PCDFs being in the
range of 1-5 µg/m2.
The maximum yield of PCDFs was about 10%, calculated on the amount of
PCBs decomposed, and the optimal temperature was between 550 and
650/700°C (Bentley, 1983). Thus, the uncontrolled burning of PCBs can
be an important occupational and environmental source of toxic and
hazardous PCDFs and it is recommended that all destruction of
PCB-contaminated waste should be carefully controlled, especially with
regard to the burning temperature (above 1000°C), residence time, and
turbulence (Bentley, 1983; WHO/EURO, 1987).
In the temperature range 300-400°C, Morita et al. (1978) reported that
the yield of conversion seemed to be in the mg/kg range. However,
Nagayama et al. (1981) reported a dramatic increase in the levels of
PCDFs at these rather low temperatures, in the presence of stainless
steel or nickel.
No, or very low levels of, PCDDs have been reported from the pyrolysis
of PCBs. However, pyrolysis of a mixture of PCBs and chlorobenzenes
(product Askarel) can yield both PCDFs and PCDDs (Buser, 1979).
Rappe et al. (1985b) found that various types of industrial
incinerators, such as copper smelters and steel mills generate PCDFs
and PCDDs. Pyrolysis of chlorinated polymers like polyvinylchloride
(PVC) and Saran also generate these compounds and exhaust gases of
motor cars and their motor oil may contain PCDDs and PCDFs (WHO/EURO,
1987).
In a State Office Building in the centre of Binghamton, New York, a
fire, in conjunction with several explosions, occurred in the basement
mechanical room, in 1981. Approximately 750 litres of Askarel, a
dielectric fluid composed of 65% PCBs (Aroclor 1254) and 35%
polychlorinated benzenes, leaked from a transformer and caught fire.
Pyrolysis of the Askarel led to the formation of a fine oily soot
that spread throughout the building via 2 ventilation shafts. Samples
taken several days after the fire showed average concentrations of
PCBs in the air of the building of 1.5 µg/m3. The average result for
surfaces ranged from 4.6 to 162.2 µg/m2. TCDFs and PCDDs were also
present. The soot samples were analysed for pyrolysis products. They
contained average levels of 3 mg TCDD/kg and 199 mg 2,3,7,8-TCDF/kg
(Fitzgerald et al., 1989). Achilles (1983) reported the following
levels in the deposited smut; 2160 mg PCDFs/kg and 20 mg PCDDs/kg
(including 0.6 mg 2,3,7,8-TCDD/kg).
In the soot from the Binghamton, Reims, and Stockholm accidents, high
levels of polychlorinated biphenylenes (PCBPs) were identified as well
as the PCDFs (Fig. 2) (Rappe et al., 1982, 1985).
Between 1981 and 1985, a number of accidents in electrical equipment
were reported from different countries; 28 accidents were mentioned in
WHO/EURO (1987) including actual capacitor explosions, capacitor
fires, and transformer accidents. In all eases, the accident site was
contaminated by PCDFs, average levels of total PCDFs being in the
range of 1-5 µg/m2.
 Hutzinger et al. (1985) also mentioned the presence of polychlorinated
pyrenes (PCPYs).
In the period 1977-85, particulates and flue gas from municipal
incinerators and hazardous waste incinerators in Canada, Denmark,
Netherlands, Sweden, and Switzerland were investigated. It was found
that emissions from incinerators contained many different PCDF and
PCDD isomers. The total levels ranged from ng/m3 to µg/m3. Fly-ash
contained levels of 0.1-0.6 mg/kg (Buser & Bosshardt, 1978; Rappe et
al., 1985c; WHO/EURO, 1987).
Rappe et al. (1985b) studied the emissions of the municipal solid
waste incinerator in Umea, Sweden. The levels of PCDDs and PCDFs
varied under different burning conditions. The amount of dioxins
formed seems to be dependent on the chlorine content in the waste, as
well as the construction of the incinerator. The critical parameters
seem to be temperature, residence time, turbulence, and excess air
(oxygen).
The 2,3,7,8-tetra-CDD was always found to be a very minor constituent,
whereas the 1,2,3,7,8-penta-CDD in all samples gave a medium-sized
peak. The 2,3,7,8-substituted PCDFs were always middle or major
components (WHO/EURO, 1987).
The fact that PCBs may be thermally converted to PCDFs has raised
concern that similar conversions might occur in electrical equipment,
such as capacitors and transformers, in which the dielectric fluids
used are subjected to modest temperature rises accompanied by
electrical stress. Brown et al. (1988) investigated the presence of
PCDFs in both used and unused capacitors and transformers and did not
find any evidence of an increase in PCDFs levels in the heavily used
capacitor or the transformer PCBs compared with levels in unused
samples.
For a number of years, concern has been expressed regarding the
release of PCBs and other dangerous compounds when fluorescent light
ballasts "burn out". The breakdown products may contain vapours and
condensed particles of PCBs and asphalt. In response to concern at a
school, the US EPA met with officials of Blaine Elementary School,
because of material leaking from some fluorescent light fixtures. It
was determined that the leaking material ("oil") contained PCBs
(Aroclor 1242 or 1260). Air samples collected following the burn out
of such lights, at different distances from the light fixture, gave
concentrations of 0.166 and 0.012 mg/m3, respectively, 1 and 6 m from
the light. Three days later, levels of 0.004-0.001 mg/m3 were still
found. In a second series of tests, both burn-out and non-burn-out
ballasts were heated to 150°C, 300°C, and 400°C, in a chamber. No PCBs
were detected at 150°C. At 300°C, concentrations ranged from 0.55 to
1.70 mg/m3 and, at 400°C, 2.54 to 28.2 mg/m3. Wipe samples were
taken in schoolrooms after burn-outs; average concentrations of
Aroclor of 0.34 and 1.22 µg/cm2 were found. It is obvious that PCBs
and asphalt contamination, both surface and atmospheric, can occur
when fluorescent lamp ballasts burn out.
The most serious potential contamination results when thermal runaway
takes place. Thermal runaway volatilizes the asphalt potting compound
and may rupture the capacitor. When the potting compound and the PCBs
are exposed to high temperatures, some of both materials vapourizes.
As the vapours pass through the atmosphere they condense into freely
divided aerosols, less than 1 µm in diameter. Much of the visible
fumes results from volatilization of the asphalt (Anon., 1987).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
A more detailed review of transport mechanisms can be found in Jury et
al. (1987).
4.1.1 Transport in air
The virtually universal distribution of PCBs throughout the world,
including the arctic and other remote areas, suggests that PCBs are
transported in air (Risebrough & de Lappe, 1972). The ability of PCBs
to co-distill, volatilize from landfills into the atmosphere
(adsorption to aerosols with particle size of less than 0.05-20 µm),
and resist degradation at low incinerating temperatures, makes
atmospheric transport the primary mode of global distribution within
the troposphere and stratosphere (Nisbet & Sarofim, 1972; Eisenreich
et al., 1981). PCBs have been measured in air samples at Eniwetok
Atoll in the North Pacific Ocean (Atlas & Giam, 1981), over the North
Atlantic (Giam et al., 1978), and in the Gulf of Mexico (Giam et al.,
1978, 1980). Murphy et al. (1985) estimated that approximately
18 000 kg of PCBs are present in the atmosphere over the USA, at any
given time. The authors also estimated that, if these PCBs had an
atmospheric residence time of one week, then about 900 000 kg/year of
PCBs cycle through the atmosphere of the USA.
Nisbet & Sarofim (1972) suggested that most of the airborne PCBs will
be adsorbed on any particles present. The half-life of particles in
the air will depend greatly on the size of the particles and the
extent of atmospheric precipitation. Most will be deposited within 2-3
days in their areas of origin (usually urban), the small amount
attached to fine particles will last in the atmosphere for longer
periods and can be transported to more remote regions.
Södergren (1972) collected airborne fallout in southern Sweden and
found regional differences in PCB levels, with mean monthly levels
ranging from 620 ng/m2 per month to 10 510 ng/m2 per month. The
lowest level was in a remote forest area. Industrialized areas had
high levels but so too did some agricultural regions. Higher levels
were generally found in the western part of the study region,
suggesting that some PCB fallout may have originated from further
afield and be dependent on the prevailing winds. Seasonal variations
in fallout correlated well with precipitation. Lower levels of PCB
precipitation were found in Iceland by Bengtson & Södergren (1974).
The highest level was found in Northern Iceland at 1050 ng/m2 per
month and, like other sites sampled, showed a seasonal trend with
highest levels in the summer.
Harvey & Steinhauer (1974) measured PCBs in the atmosphere over the
western North Atlantic. They found that concentrations decreased
exponentially with distance from land and concluded that wind
transport is the major method of transport over the oceans. They also
suggested that PCBs are transported primarily in the vapour phase.
4.1.1.1 Dry deposition
Atmospheric input into the Great Lakes has been studied extensively,
because the lakes, as a whole, represent the largest surface area of
any freshwater body in the world, with the lake surface area
comprising from 27% (Ontario) to 64% (Superior) of the total basin
area, and ranging from 19 000 km2 (Ontario) to 82 100 km2
(Superior). Eisenreich et al. (1981) estimated that more than 80% of
the annual mean total input of PCBs in Lake Michigan originated from
the atmosphere. They estimated that approximately 56% of the
9000 kg/year of PCB input in Lake Michigan was in the form of wet
deposition and that 30% of the 6600-8300 kg/year input in Lake
Superior was also in this form. However, Andren (1982) calculated a
precipitation input of 650 kg/year for Lake Michigan, again assuming
that all PCBs were on 0.5 µm airborne particles. Even assuming the
lowest estimate for the annual input of PCBs into the lake,
approximately 60% of the total input might be atmospheric deposition.
Andren (1982) also measured the input of PCB into an isolated lake
(Crystal Lake, Wisconsin), to calibrate a dry deposition model. The
model was then applied to Lake Michigan and the author concluded that,
assuming all particulate inputs of PCB are associated with 0.5 mm
particles, dry deposition inputs were significantly less than wet
inputs.
Manchester-Neesvig & Andren (1989) collected and analysed air samples
from a remote site in the Great Lakes watershed during 1984 and 1985.
Total PCB concentrations varied from 1.82 ng/m3 in the summer to
0.135 ng/m3 in the winter. They found that, on average, 92% of the
PCBs detected were in the vapour phase. When these data were compared
with data collected over the previous 7 years, no significant changes
in PCB concentrations were found. The authors concluded that, on the
basis of the short residence time and the relatively constant annual
average levels of PCBs, repeated cycling between earth and atmosphere
takes place.
Murphy (1984) reviewing data from the Great Lakes region on the
relative distribution of airborne PCBs between particulate matter and
vapour, concluded that they are transported predominantly in vapour.
He stated that there was reasonable evidence to suggest that the
atmosphere is the major source of the PCBs found in Lakes Michigan,
Superior, and Huron, Siskiwit Lake on Isle Royale, and probably in the
upper Great Lakes too.
Using liquid-coated collecting plates in near-shore areas of Lakes
Huron and Michigan, close to urban centres, more PCBs were found on
the upper plates suggesting that much of the dry deposit of PCBs was
associated with large particles (20 µm). This sampling technique also
indicated that, for the areas studied, dry deposition inputs were
higher than wet inputs (Murphy, 1984).
Duinker & Bouchertall (1989) analysed filtered air, particulates, and
rain, in the city of Kiel, Federal Republic of Germany for 14
different PCB congeners. They found that congeners with a low degree
of chlorination were dominant in filtered air, whereas, congeners with
a high degree of chlorination dominated in aerosols and rainfall. The
vapour phase represented up to 99% of the more volatile congeners
(i.e., those with a lower degree of chlorination). The particulates
were found to carry relatively more of the less volatile congeners.
Particle scavenging was the dominant source of PCBs in rain water
despite the small contribution of particulate PCBs to the overall
atmospheric concentration of PCBs (only 1 or 2%).
In a study by Södergren (1973), most of the PCB deposited on a south
Swedish lake was in the form of dry deposit, with 11% as particulate
matter in the precipitation and 2% from precipitation water. McClure
(1976) stated that, on the basis of flux measurements and model
calculations, most of the PCB fallout is in the form of dry deposition
and that most of the dry deposition of aerosol PCB introduced into the
troposphere falls within 100 km of its source.
4.1.1.2 Precipitation deposition
Precipitation scavenging of chlorinated hydrocarbons in the atmosphere
is complex. Scavenging of particles by cloud droplets and by rain
drops in, and below, clouds, and the scavenging of the vapour phase by
rain occurs (Murphy 1984). Thus chlorinated hydrocarbons are
concentrated in precipitation rather than in the atmosphere, resulting
in rainfall levels of many ng/litre. Swain (1978) and Strachan &
Huneault (1979) measured levels in rainfall ranging between 0 (not
detectable) and 230 ng/litre in the Great lakes area.
Murphy (1984) pointed out that variables, such as the amount of
particulate material and PCBs in the atmosphere, the type of rain, and
the rate of rainfall, will affect the precision of precipitation
estimates.
Levels of PCBs in the rainfall throughout Canada during 1984 were
monitored by Strachan (1988). Levels ranged from nd to 17 ng/litre, no
geographical trends were apparent.
4.1.2 Transport in soil
PCBs in soil, derive from particulate deposition (often concentrated
in urban areas), wet deposition, the use of sewage sludge as a
fertilizer, and leaching from landfill sites.
Significant amounts of PCBs are deposited on soil by particulate
deposition (see previous section). Fujiwara (1975) analysed soil
samples in Japan, and found that the main sources of PCB contamination
of agricultural soils are the industries using PCBs. Other sources
include treatment of soil with sewage sludge and accidental spills.
The 15% of soil samples in Indiana (USA) that contained more than
50 mg/kg had been treated with PCB-contaminated dried sludge (Bergh &
People, 1977).
Tucker et al. (1975a) found that, during a 4-month period following
the addition of Aroclor 1016 to soil, the PCBs were not readily
leached by percolating water and that only the lower chlorinated
isomers were leached. The ease of leaching from different soils was in
the order sandy loam > silty loam > silty clay loam.
The behaviour of 14C-labelled PCB in flooded soils was studied by
Ogiso et al. (1976). The amounts of PCB volatilized occurred in the
following order: water > subsoil > soil. The addition of compost
powder to soil reduced the amount that volatilized.
Haque et al. (1974) studied the adsorption of Aroclor 1254 on various
soil particle types in an aqueous solution of 56 µg PCB/litre.
Delmonte sand and silica gel did not adsorb any PCB. Woodburn soil
adsorbed the highest amount followed by illite, montmorillonite, and
kaolinite clays, in decreasing order. The high adsorptive capacity of
Woodburn soil was attributed to the presence of organic matter and
lipophilic or hydrophobic materials. Moza et al. (1976a) found that, 2
years after the application of 14C-labelled dichlorobiphenyl to a
loamy sand soil at 1 mg/kg, most of the detectable PCB was in the top
10 cm of the soil and only 0.2% had reached a depth of 40 cm. In
another study, Suzuki et al. (1977) found that Aroclors 1242 and 1254
did not move upwards through uncontaminated sand deposited over
contaminated soil. The leaching of water from soil may lead to a
downward movement of PCBs, depending on the soil type and clay content
(Pal et al., 1980).
A large spill of Askarel (containing 70% Aroclor 1254 and 30% tri-
and tetrachlorobenzenes) occurred at a transformer-manufacturing
facility in Canada, in 1976. Condie silt from near the site of the
spill was studied with respect to the sorption partition coefficients
and the transport retardation factors. The sorption partition
coefficient values for 2,5,2',5'-tetrachloro-, 2,4,5,2',5'-penta-
chloro-, and 2,4,5,2',4',5'-hexachlorobiphenyl were 5000, 9400, and
26 000, respectively. The mean transport retardation factors for these
3 congeners were 2.7 E + 04, 5.0 E + 04, and 1.4 E + 05, respectively.
This implies that dissolved PCBs will move only very slowly through
unfractured Condie silt (Anderson & Pankow, 1986).
4.1.3 Transport in water
PCBs enter water mainly from discharge points of industrial and urban
wastes into rivers, lakes, and coastal waters. In static water, PCBs
are more concentrated in the surface micro-layer than in subsurface
samples (Bidleman & Olney, 1974). This is probably due to deposition
from the air rather than redistribution in the water. On account of
their low water solubility and high specific activity, it is expected
that most of the PCBs discharged will be adsorbed by sediment at the
bottom of rivers or lakes and transport will be mainly via waterborne
particles (Nisbet & Sarofim, 1972). The bulk of the PCBs will sink to
the bottom sediments. The sinking rate of PCBs from the surface to
deeper layers in the open ocean is relatively slower in tropical
waters than in high-latitude waters (Tanabe, 1985).
Oloffs et al. (1973) added 0.1 mg Aroclor 1260/litre to water samples
in the presence of sediment. After 6 weeks, all of the PCBs had been
adsorbed by the sediment, none being given off to the atmosphere. The
degree of PCBs sorption is inversely related to the size of the
particles (Haque et al., 1974) and the solubility of PCBs in water
(Haque & Schmedding, 1975). Smaller particles have a relatively larger
surface area and so adsorb more PCBs (Steen et al., 1978). Nau-Ritter
et al. (1982) found the adsorption and retention of PCBs to be
directly related to the particle organic content. A significant
correlation was found by Larsen et al. (1985) between PCB levels and
total organic carbon in the deepwater sediments of the Gulf of Maine,
PCBs were concentrated on finer grain particles. Organic carbon and,
therefore, the PCB concentration were also correlated with depth.
Wildish et al. (1980) found that estuarine sediments, especially those
containing higher levels of organic matter, readily adsorbed Aroclor
1254. The PCBs were found to be tightly bound to the sediment with
virtually no desorption. Horzempa & Di Toro (1983) found that the
adsorption of hexachlorobiphenyl was correlated with both sediment
surface area and organic content. Adsorption was found to be
significantly greater at 40°C than at 1°C. Hexachlorobiphenyl is
strongly adsorbed on sediment and weakly desorbed. There is no simple
reversible reaction.
Fisher et al. (1983) found that the rate of release of PCBs from
contaminated sediment was a function of sediment PCB concentration,
chlorine substitution pattern, and degree of chlorination. In the
absence of disturbance, even very low deposition rates of new sediment
will quickly remove PCB-contaminated sediments from diffusional
communication with overlying water. Little change was found (Nimmo et
al., 1971a) in the PCB concentration in sediment at a point downstream
of a contamination source over a period of 9 months. The very small
amounts of PCBs leached from sediment into overlying water may be
taken up by organisms.
Hom et al. (1974) stated that the annual inputs of PCBs into the
southern California bight from waste water and from surface runoff in
1970-71 were estimated to be 10 and 0.25 tonnes, respectively.
Sewage treatment appears to remove PCBs from waste water,
concentrating them in the sludge. However, often, the sludge is then
discharged into open water (Ahling & Jensen, 1970). Holden (1970)
found an average of 3 mg PCBs/kg in wet sewage sludge dumped in the
Clyde estuary, in the United Kingdom, and calculated that this would
be equivalent to approximately one tonne per year. A similar annual
discharge of PCBs in the sludge on the Californian coast was
calculated by Schmidt et al., (1971).
Dredging of inland rivers and harbours may lead to a significant
transfer of PCBs from contaminated sediments, especially when dumped
at sea (Nisbet & Sarofim, 1972). Rice & White (1987) found that there
was an increase in water concentrations of PCBs immediately following
the dredging of sediment in the Shiawassee River, Michigan. The
availability of PCBs for clams and fish, as measured by an increase in
uptake, was found for up to 6 months following dredging.
4.1.4 Transport between media
In a model ecosystem, Södergren & Larsson (1982) found that the
presence of bottom-living organisms, such as Chironomus and
Tubifex, resulted not only in the uptake of PCBs from the sediment
but also in the release of PCBs into the water and to the surface
microlayer, compared with a system without organisms. PCBs were
transported to the air via jet drops from bursting bubbles in the
surface microlayer.
A similar pattern was found using large outdoor artificial ponds
(Larsson, 1985a). Following the addition of Clophen A50 to sediment,
the transport of PCBs from sediment to water followed a seasonal
cycle, with higher levels in the summer than in the winter. The
processes that transfer PCBs across the sediment/water interface
(bioturbation, desorption, and gas convection) are positively related
to temperature. Transfer from water to air was probably dominated by
volatilization with maximum concentrations of PCBs in air at the
highest water concentrations, lower chlorinated biphenyls achieving
the highest concentrations in air. The majority of the airborne phase
was presumed to be in the gaseous phase as it passed through particle
filters. In the same ponds, Larsson & Okla (1987) measured the rate at
which PCBs volatilized from water to air. PCB compounds volatilized at
a rate of 0.9 to 9.6 ng/m2 per h, the rate increasing with the
temperature of the water and the concentration of PCBs. The transport
rate during the day exceeded the rate at night and was positively
correlated with the air temperature (Okla & Larsson, 1987).
Larsson (1985b) added Clophen A50 to the sediment in a model ecosystem
comprising sediment, water, benthic macroinvertebrates, and fish. PCBs
were detected in the water. The transport of PCBs from the water to
air included at least 2 routes, volatilization and jet drop transport.
Both routes were of the same magnitude (0.2-1.0 µg/week). However,
though the PCBs transported by volatilization consisted of lower
chlorinated isomers, those transported by jet drops were identical to
those in the sediment and water.
In an earlier study, Larsson (1984) measured the uptake of PCBs from
sediment by chironomid midge larvae and the concentrations of PCBs
from larva to adult. In the field, chironomid larvae contained
114 µg/kg fresh weight at a sediment concentration of 39 µg/kg wet
weight. Different sediments affected the amount of PCBs available to
the organisms. Adult chironomids sampled near a sewage plant contained
251 µg/kg fresh weight. The chironomid larval population was estimated
to be 9900 per m2 and the authors calculated that these would move
20 µg PCB/m2 per year into the terrestrial compartment of the
environment.
A model, based on the fugacity concept, was described and illustrated
by applying it to the time-varying fate of PCBs in Lake Ontario over
the period 1940-2000. Expressions are included for a great number of
variables, such as loadings and the partitioning of the contaminant
between the phases of air, aerosols, water, suspended and bottom
sediments, various trophic levels of aquatic organisms, and gull eggs.
Also included are expressions for transformation rates, and transport
rates for diffusion between water and sediment, and water and air wet
and dry atmospheric deposition, sediment deposition, burial, and
resuspension, and water and the inflow and outflow of suspended
matter. The results obtained by numerical integration and by assuming
reasonable loading and air concentrations were in accordance with
data. It was shown that PCBs cycle appreciably between the atmosphere
and water by wet and dry deposition and volatilization, and between
water and sediment by deposition, resuspension, and diffusion.
Biomonitors were shown to be particularly valuable indicators of
contamination levels in the ecosystem (MacKay, 1989).
4.2 Biotransformation
4.2.1 Biodegradation
Nissen (1973) did not find any alteration in Aroclor 1254 after a
9-week incubation period in soil. Iwata et al. (1973) added Aroclor
1254 to various soil types. They did not find any change after one
year in soils containing high amounts of organic matter (10.8-19.5%).
Biotransformation had occurred, causing the disappearance of the lower
chlorinated biphenyls, in soils with a low organic matter content
(0.1-3.3%), as diverse as loamy sand and clay. The authors concluded
that, after one year, the material remaining in loamy sand (0.1%
organic matter) consisted of mainly penta- and hexachlorobiphenyl
isomers.
4.2.1.1 Bacteria
The biodegradation of PCB isomers, which is possible with some aerobic
bacteria, depends on the degree of chlorination and the position of
chlorine substitution. Degradation decreases with increasing
chlorination. Dechlorination of PCBs occurs in anaerobic sediments.
Here bacterial activity is preferentially targeted towards PCB
congeners with higher levels of chlorination. Products of
dechlorination are, therefore, more readily degraded by aerobic
systems.
Early experiments were carried out to study the biodegradation of PCBs
using activated sludge inocula; some degradation was found (Baxter et
al., 1975). However, the presence of PCBs in sewage sludge shows that
they are not all readily transformed by microorganisms. Fries (1972)
analysed silage containing PCBs (Aroclor 1254) that had undergone
normal fermentation. The gas chromatogram of the standard was
identical to that of the silage sample. The authors suggested that, if
anaerobic degradation had taken place, it would have been unlikely to
have been uniform for all components. They stated, however, that this
test may not have been a good indication of possible anaerobic
degradation because DDT showed much less degradation, under the same
conditions, compared with other degradation test systems.
Lunt & Evans (1970) postulated a metabolic pathway, used by
microorganisms, for biphenyl oxidation, which was later confirmed by
the findings of Gibson et al. (1973) using a bacterium isolated from a
polluted stream. Lunt & Evans (1970) found that a Gram-negative
bacterium oxidized biphenyl to phenylpyruvic acid with the
intermediary formation of 2,3-dihydroxybiphenyl and
alpha-hydroxy-ß-phenylmuconic semialdehyde. Catelani et al. (1971)
found that the metabolism of biphenyl by Pseudomonas putida was
different, in that, though the intermediate products were the same,
benzoic acid was isolated, not phenylpyruvic acid. Ahmed & Focht
(1973a) isolated 2 species of Achromobacter from sewage effluent
using biphenyl and p-chlorobiphenyl as the sole carbon source. They
found that both sources were rapidly degraded, biphenyl being oxidized
to benzoic acid and both mono and dichlorinated biphenyls to
p-chlorobenzoic acid. In a second study, Ahmed & Focht (1973b)
investigated the biodegradation of other isomers of PCBs, with 2-5
chlorine atoms. The extent of oxidation seemed to be somewhat
dependent on the presence of unsubstituted biphenyl rings. Because of
the absence of chloride in all the supernatants, they concluded that
the bacterium was unable to dechlorinate the PCBs. The fact that
increasing chlorine substitution rendered the molecule more resistant
to microbial attack was used to support this argument. However, Kaiser
& Wong (1974), studying the degradation of Aroclor 1242 by a bacterial
culture, isolated from lake water, showed that the PCBs were degraded
into several metabolites (aliphatic and aromatic hydrocarbons), none
of which contained chlorine. Dechlorination had already taken place at
an early stage of metabolism.
Wong & Kaiser (1975) found that lake water bacteria could use both
Aroclor 1221 and 1242, but not 1254, as a sole carbon source for
growth, but that only 1% of the bacterial culture had this ability.
The authors then followed the degradation of Aroclor 1221. After one
month, the mixture had been totally degraded to several compounds of
low relative molecular mass. Unchlorinated biphenyls were degraded
faster than chlorinated forms.
Tucker et al. (1975b) observed the degradation rates of Aroclors 1221,
1016, 1242, and 1254, and MCS 1043 (a non-commercial mixture). They
found a clear relationship between the level of chlorination and the
relative degradability, when degradation rate was plotted against
percentage chlorine by weight. Volatilization rates fell within the
95% confidence limits of overall disappearance rates and so could be
ruled out. Analysis of the Aroclors, following exposure to the
activated sludge, revealed a redistribution of the dominant PCBs. For
example, the chromatograms for Aroclor 1221 and 1242 were very similar
showing that the lower chlorinated biphenyls were more rapidly
degraded. Furthermore, since Aroclor 1221 was found to be rapidly
degraded, a closer study was performed that showed that most of the
degradation occurred within 24 h.
The degradation of polychlorinated biphenyls by either Nocardia spp.
or Pseudomonas spp. was studied by Baxter et al. (1975). They found
that, under experimental conditions, many of the lower chlorinated
biphenyls (<3 chlorine atoms/molecule) were degraded very readily
and some biphenyls containing as many as 6 chlorine atoms could be
degraded, if the conditions were suitable. When PCB mixtures Aroclor
1016 and 1242 were used, a different pattern of degradation was
observed with an enhanced ability of the microorganisms to degrade.
For example, 4,4'-dichlorobiphenyl degraded to 50% in about 2 days,
when presented to Nocardia spp. as a component of Aroclor 1242, but
it was virtually unaffected after 12 days exposure as the pure isomer.
The authors suggested that mutual solubilization might play some part.
Sayler et al. (1977) found that an estuarine Pseudomonas sp. was
able to degrade both mixtures of PCBs (Aroclor 1254) and pure isomers
of hexachlorobiphenyl. Degradation was dependent on incubation time
and the purity and degree of chlorination of the biphenyl. Appreciable
degradation occurred at all substrate concentrations of the Aroclor
(10, 100, and 1000 µg/litre) within 22 days. Although, over this
22-day period, only 9% had been degraded at the lowest concentration
compared with 30-40% for the other concentrations, after 60 days, this
was reversed with 84% being degraded at 10 µg/litre, 70% at
100 µg/litre, and 63% at 1000 µg/litre. When compared with the pure
isomer, degradation of the Aroclor mixture proceeded at a slower rate.
Even though average chlorination was less, the authors speculated that
this could be owing to the substitution positions of the chlorines.
Chromatographic tracings showed that degradation of the lower
chlorinated components of the Aroclor occurs before degradation of the
more highly chlorinated biphenyls.
Furukawa et al. (1978a,b) examined 31 PCB isomers (mono to
pentachlorobiphenyl) for biodegradability by 2 bacterial species,
Alcaligenes and Acinetobacter. They found the following
relationship between chlorine substitution and biodegradability.
i. Degradation decreased as chlorine substitution increased.
ii. Isomers containing two chlorines at the ortho position of
either a single ring or on both rings showed very poor
degradability.
iii. Isomers, in which all the chlorines were on one ring, were
generally degraded faster.
iv. Molecules with non-chlorinated rings or rings with few chlorines
underwent preferential ring fission.
v. The 4'-chloro-substituted PCBs formed and accumulated a yellow
intermediate during degradation.
vi. Only with respect to 2,4,6-trichlorobiphenyl was there a
significant difference in ability to degrade between the 2
bacteria. This compound was mostly metabolized within 1 h by
Acinetobacter, but was degraded very slowly by Alcaligenes.
It was demonstrated by Carey & Harvey (1978) that mixed cultures of
marine bacteria were capable of metabolizing both pure isomers (tri-
and tetrachlorobiphenyl) and mixtures (Aroclor 1254). They isolated
and partially characterized an acid lactone metabolite. They did not
find any change in the chromatogram trace for the Aroclor but
suggested that this might be related to the insensitivity of the
method, since even if each of the isomers in the mixture had been
metabolized to the same extent as pure isomers, this would still not
have been detectable on the trace. The authors also found that no
metabolism occurred when a chlorobiphenyl isomer in an anaerobic
marine mud was incubated for 6 weeks. Degradation of Aroclor 1242 by
mixed microbial cultures, isolated from soil and river water samples,
was demonstrated by Clark et al. (1979). The predominant organisms in
the cultures were Alcaligenes odorans, Alcaligenes denitrificans,
and an unidentified bacterium. The lower chlorinated isomers were not
only degraded at a faster rate but were also more completely utilized
by the bacteria. In general, the rate of degradation was much faster
than in previous studies. Co-metabolism in the presence of sodium
acetate was studied; greatly enhanced degradation was found for the
more highly chlorinated isomers. Liu (1980) found that sodium
ligninsulfonate also greatly enhanced the biodegradation of commercial
PCB mixtures.
The same author found that a Pseudomonas sp. could oxidize Aroclors
1221, 1016, 1242, and 1254, at a rapid rate. A kinetic study using
resting cells revealed that Aroclor 1221 was degraded much faster
(980 µg/h per mg cell dry weight) than Aroclor 1254 (43 µg/h per mg
cell dry weight). The degradation of the higher chlorinated PCB
(Aroclor 1254) could be enhanced by the addition of Aroclor 1221. Liu
(1981) observed that the oxidation of Aroclor 1221 by the bacteria was
10 times faster than with sewage. Two possible explanations for this
difference were that the sewage contained toxic chemicals that
inhibited the bacteria, but this was found not to be the case, or, the
bacteria preferred Aroclor 1221 to the other substrates. This second
explanation is a possibility, for glucose, a substrate used readily by
most bacteria was poorly oxidized by this bacterium. Pseudomonas
oxidized Aroclor 1221 readily between 15 and 35°C, the rate increasing
with temperature. Reducing the temperature to 4 and 10°C drastically
retarded, but did not halt, degradation. Adjusting the concentrations
of phosphorus and nitrogen from 2 mg to 20 mg/litre (the lower
concentration being that found normally in sewage) did not alter the
rate of degradation by Pseudomonas spp. in raw sewage. But
increasing nitrogen and phosphorus gave more reproducible results,
suggesting that the compounds are on the border of limiting
degradation rates in raw sewage. The oxygen content was found not to
affect degradation at concentrations over 1 mg/litre (oxygen levels
are generally maintained at between 2 and 3 mg/litre in activated
sludge reactors, under the operational conditions of sewage-treatment
plants). Liu (1982) found that, under a limited substrate supply,
Pseudomonas spp. degraded all 7 of the major components of Aroclor
1221. However, with excessive amounts of nutrient, preferential
degradation of certain components was observed. The author stated that
one of the main factors influencing this selective biodegradability
was the position of chlorine substitution on the biphenyl.
4.2.2 Biodegradation; individual congeners
4.2.2.1 Bacteria
In a study by Parsons & Sijm (1988), the co-metabolism was
investigated of several different mono-, di- and tetrachlorobiphenyls
in chemostat continuous cultures of a Pseudomonas strain (JB1). They
found that chemostat conditions favoured degradation compared with
exposure of the Pseudomonos in batch culture, where little or no
degradation was recorded. Using benzoate as the carbon source, results
varied widely, with repeat incubations showing different degrees of
degradation of chlorobiphenyls and, sometimes, no breakdown at all. In
cultures that did degrade the materials, the monosubstituted
4-chlorobiphenyl was rapidly degraded. Of the disubstituted
dibiphenyls, 3,5-dichlorobiphenyl was more readily broken down than
2,5-dichlorobiphenyl. Changing the carbon source available to the
Pseudomonas sp. improved the reproducibility of the results. The
authors reviewed the literature relative to their own findings and
concluded that repeated culture on benzoate leads to the loss of the
ability of the Pseudomonas sp. to degrade biphenyl by meta
cleavage; ortho cleavage is retained. Coding for the meta cleavage
resides on plasmids, which can be lost, whereas coding for the ortho
cleavage is chromosomal. Growth of the Pseudomonas sp. on a
3-methylbenzoate substrate improved degradation of the biphenyls.
3-Methylbenzoate can only be degraded by a meta cleavage favouring
retention of the plasmid. Comparison of degradation of 4
tetrachlorobiphenyls showed the influence of the positions of the
chlorine substitutions. The relative degradability of the 5 compounds,
shown in Fig. 3, was: 2,3,2',3'-tetrachloro- >2,5,3',4'-tetrachloro-
> 2,5,2',5'-tetrachloro- approx. 2,6,2',6'-tetrachloro- approx.
3,4,3',4'-tetrachlorobiphenyl. The authors stated, from the
literature, that the first reaction in the degradation of
chlorobiphenyls is, in most cases, 2,3-dioxygenation, eventually
leading to the formation of chlorobenzoates. Chlorines in the ortho
and meta positions will, therefore, offer steric hindrance to this
reaction.
The low degradation rate of 3,4,3',4'-tetrachlorobiphenyl is not
explained by this mechanism, since it has 2 adjacent unoccupied 2,3
positions, but is more likely explained by its toxicity. Steric
influence on enzyme binding is offered as an explanation in this case.
Similarly, Furukawa et al. (1978a) did not find any degradation of
this compound in initial studies, though they did find degradation to
a dichlorobenzoic acid by Acinetobacter in a later study (Furukawa
et al., 1978b; Rogers, undated(a)).
Hutzinger et al. (1985) also mentioned the presence of polychlorinated
pyrenes (PCPYs).
In the period 1977-85, particulates and flue gas from municipal
incinerators and hazardous waste incinerators in Canada, Denmark,
Netherlands, Sweden, and Switzerland were investigated. It was found
that emissions from incinerators contained many different PCDF and
PCDD isomers. The total levels ranged from ng/m3 to µg/m3. Fly-ash
contained levels of 0.1-0.6 mg/kg (Buser & Bosshardt, 1978; Rappe et
al., 1985c; WHO/EURO, 1987).
Rappe et al. (1985b) studied the emissions of the municipal solid
waste incinerator in Umea, Sweden. The levels of PCDDs and PCDFs
varied under different burning conditions. The amount of dioxins
formed seems to be dependent on the chlorine content in the waste, as
well as the construction of the incinerator. The critical parameters
seem to be temperature, residence time, turbulence, and excess air
(oxygen).
The 2,3,7,8-tetra-CDD was always found to be a very minor constituent,
whereas the 1,2,3,7,8-penta-CDD in all samples gave a medium-sized
peak. The 2,3,7,8-substituted PCDFs were always middle or major
components (WHO/EURO, 1987).
The fact that PCBs may be thermally converted to PCDFs has raised
concern that similar conversions might occur in electrical equipment,
such as capacitors and transformers, in which the dielectric fluids
used are subjected to modest temperature rises accompanied by
electrical stress. Brown et al. (1988) investigated the presence of
PCDFs in both used and unused capacitors and transformers and did not
find any evidence of an increase in PCDFs levels in the heavily used
capacitor or the transformer PCBs compared with levels in unused
samples.
For a number of years, concern has been expressed regarding the
release of PCBs and other dangerous compounds when fluorescent light
ballasts "burn out". The breakdown products may contain vapours and
condensed particles of PCBs and asphalt. In response to concern at a
school, the US EPA met with officials of Blaine Elementary School,
because of material leaking from some fluorescent light fixtures. It
was determined that the leaking material ("oil") contained PCBs
(Aroclor 1242 or 1260). Air samples collected following the burn out
of such lights, at different distances from the light fixture, gave
concentrations of 0.166 and 0.012 mg/m3, respectively, 1 and 6 m from
the light. Three days later, levels of 0.004-0.001 mg/m3 were still
found. In a second series of tests, both burn-out and non-burn-out
ballasts were heated to 150°C, 300°C, and 400°C, in a chamber. No PCBs
were detected at 150°C. At 300°C, concentrations ranged from 0.55 to
1.70 mg/m3 and, at 400°C, 2.54 to 28.2 mg/m3. Wipe samples were
taken in schoolrooms after burn-outs; average concentrations of
Aroclor of 0.34 and 1.22 µg/cm2 were found. It is obvious that PCBs
and asphalt contamination, both surface and atmospheric, can occur
when fluorescent lamp ballasts burn out.
The most serious potential contamination results when thermal runaway
takes place. Thermal runaway volatilizes the asphalt potting compound
and may rupture the capacitor. When the potting compound and the PCBs
are exposed to high temperatures, some of both materials vapourizes.
As the vapours pass through the atmosphere they condense into freely
divided aerosols, less than 1 µm in diameter. Much of the visible
fumes results from volatilization of the asphalt (Anon., 1987).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
A more detailed review of transport mechanisms can be found in Jury et
al. (1987).
4.1.1 Transport in air
The virtually universal distribution of PCBs throughout the world,
including the arctic and other remote areas, suggests that PCBs are
transported in air (Risebrough & de Lappe, 1972). The ability of PCBs
to co-distill, volatilize from landfills into the atmosphere
(adsorption to aerosols with particle size of less than 0.05-20 µm),
and resist degradation at low incinerating temperatures, makes
atmospheric transport the primary mode of global distribution within
the troposphere and stratosphere (Nisbet & Sarofim, 1972; Eisenreich
et al., 1981). PCBs have been measured in air samples at Eniwetok
Atoll in the North Pacific Ocean (Atlas & Giam, 1981), over the North
Atlantic (Giam et al., 1978), and in the Gulf of Mexico (Giam et al.,
1978, 1980). Murphy et al. (1985) estimated that approximately
18 000 kg of PCBs are present in the atmosphere over the USA, at any
given time. The authors also estimated that, if these PCBs had an
atmospheric residence time of one week, then about 900 000 kg/year of
PCBs cycle through the atmosphere of the USA.
Nisbet & Sarofim (1972) suggested that most of the airborne PCBs will
be adsorbed on any particles present. The half-life of particles in
the air will depend greatly on the size of the particles and the
extent of atmospheric precipitation. Most will be deposited within 2-3
days in their areas of origin (usually urban), the small amount
attached to fine particles will last in the atmosphere for longer
periods and can be transported to more remote regions.
Södergren (1972) collected airborne fallout in southern Sweden and
found regional differences in PCB levels, with mean monthly levels
ranging from 620 ng/m2 per month to 10 510 ng/m2 per month. The
lowest level was in a remote forest area. Industrialized areas had
high levels but so too did some agricultural regions. Higher levels
were generally found in the western part of the study region,
suggesting that some PCB fallout may have originated from further
afield and be dependent on the prevailing winds. Seasonal variations
in fallout correlated well with precipitation. Lower levels of PCB
precipitation were found in Iceland by Bengtson & Södergren (1974).
The highest level was found in Northern Iceland at 1050 ng/m2 per
month and, like other sites sampled, showed a seasonal trend with
highest levels in the summer.
Harvey & Steinhauer (1974) measured PCBs in the atmosphere over the
western North Atlantic. They found that concentrations decreased
exponentially with distance from land and concluded that wind
transport is the major method of transport over the oceans. They also
suggested that PCBs are transported primarily in the vapour phase.
4.1.1.1 Dry deposition
Atmospheric input into the Great Lakes has been studied extensively,
because the lakes, as a whole, represent the largest surface area of
any freshwater body in the world, with the lake surface area
comprising from 27% (Ontario) to 64% (Superior) of the total basin
area, and ranging from 19 000 km2 (Ontario) to 82 100 km2
(Superior). Eisenreich et al. (1981) estimated that more than 80% of
the annual mean total input of PCBs in Lake Michigan originated from
the atmosphere. They estimated that approximately 56% of the
9000 kg/year of PCB input in Lake Michigan was in the form of wet
deposition and that 30% of the 6600-8300 kg/year input in Lake
Superior was also in this form. However, Andren (1982) calculated a
precipitation input of 650 kg/year for Lake Michigan, again assuming
that all PCBs were on 0.5 µm airborne particles. Even assuming the
lowest estimate for the annual input of PCBs into the lake,
approximately 60% of the total input might be atmospheric deposition.
Andren (1982) also measured the input of PCB into an isolated lake
(Crystal Lake, Wisconsin), to calibrate a dry deposition model. The
model was then applied to Lake Michigan and the author concluded that,
assuming all particulate inputs of PCB are associated with 0.5 mm
particles, dry deposition inputs were significantly less than wet
inputs.
Manchester-Neesvig & Andren (1989) collected and analysed air samples
from a remote site in the Great Lakes watershed during 1984 and 1985.
Total PCB concentrations varied from 1.82 ng/m3 in the summer to
0.135 ng/m3 in the winter. They found that, on average, 92% of the
PCBs detected were in the vapour phase. When these data were compared
with data collected over the previous 7 years, no significant changes
in PCB concentrations were found. The authors concluded that, on the
basis of the short residence time and the relatively constant annual
average levels of PCBs, repeated cycling between earth and atmosphere
takes place.
Murphy (1984) reviewing data from the Great Lakes region on the
relative distribution of airborne PCBs between particulate matter and
vapour, concluded that they are transported predominantly in vapour.
He stated that there was reasonable evidence to suggest that the
atmosphere is the major source of the PCBs found in Lakes Michigan,
Superior, and Huron, Siskiwit Lake on Isle Royale, and probably in the
upper Great Lakes too.
Using liquid-coated collecting plates in near-shore areas of Lakes
Huron and Michigan, close to urban centres, more PCBs were found on
the upper plates suggesting that much of the dry deposit of PCBs was
associated with large particles (20 µm). This sampling technique also
indicated that, for the areas studied, dry deposition inputs were
higher than wet inputs (Murphy, 1984).
Duinker & Bouchertall (1989) analysed filtered air, particulates, and
rain, in the city of Kiel, Federal Republic of Germany for 14
different PCB congeners. They found that congeners with a low degree
of chlorination were dominant in filtered air, whereas, congeners with
a high degree of chlorination dominated in aerosols and rainfall. The
vapour phase represented up to 99% of the more volatile congeners
(i.e., those with a lower degree of chlorination). The particulates
were found to carry relatively more of the less volatile congeners.
Particle scavenging was the dominant source of PCBs in rain water
despite the small contribution of particulate PCBs to the overall
atmospheric concentration of PCBs (only 1 or 2%).
In a study by Södergren (1973), most of the PCB deposited on a south
Swedish lake was in the form of dry deposit, with 11% as particulate
matter in the precipitation and 2% from precipitation water. McClure
(1976) stated that, on the basis of flux measurements and model
calculations, most of the PCB fallout is in the form of dry deposition
and that most of the dry deposition of aerosol PCB introduced into the
troposphere falls within 100 km of its source.
4.1.1.2 Precipitation deposition
Precipitation scavenging of chlorinated hydrocarbons in the atmosphere
is complex. Scavenging of particles by cloud droplets and by rain
drops in, and below, clouds, and the scavenging of the vapour phase by
rain occurs (Murphy 1984). Thus chlorinated hydrocarbons are
concentrated in precipitation rather than in the atmosphere, resulting
in rainfall levels of many ng/litre. Swain (1978) and Strachan &
Huneault (1979) measured levels in rainfall ranging between 0 (not
detectable) and 230 ng/litre in the Great lakes area.
Murphy (1984) pointed out that variables, such as the amount of
particulate material and PCBs in the atmosphere, the type of rain, and
the rate of rainfall, will affect the precision of precipitation
estimates.
Levels of PCBs in the rainfall throughout Canada during 1984 were
monitored by Strachan (1988). Levels ranged from nd to 17 ng/litre, no
geographical trends were apparent.
4.1.2 Transport in soil
PCBs in soil, derive from particulate deposition (often concentrated
in urban areas), wet deposition, the use of sewage sludge as a
fertilizer, and leaching from landfill sites.
Significant amounts of PCBs are deposited on soil by particulate
deposition (see previous section). Fujiwara (1975) analysed soil
samples in Japan, and found that the main sources of PCB contamination
of agricultural soils are the industries using PCBs. Other sources
include treatment of soil with sewage sludge and accidental spills.
The 15% of soil samples in Indiana (USA) that contained more than
50 mg/kg had been treated with PCB-contaminated dried sludge (Bergh &
People, 1977).
Tucker et al. (1975a) found that, during a 4-month period following
the addition of Aroclor 1016 to soil, the PCBs were not readily
leached by percolating water and that only the lower chlorinated
isomers were leached. The ease of leaching from different soils was in
the order sandy loam > silty loam > silty clay loam.
The behaviour of 14C-labelled PCB in flooded soils was studied by
Ogiso et al. (1976). The amounts of PCB volatilized occurred in the
following order: water > subsoil > soil. The addition of compost
powder to soil reduced the amount that volatilized.
Haque et al. (1974) studied the adsorption of Aroclor 1254 on various
soil particle types in an aqueous solution of 56 µg PCB/litre.
Delmonte sand and silica gel did not adsorb any PCB. Woodburn soil
adsorbed the highest amount followed by illite, montmorillonite, and
kaolinite clays, in decreasing order. The high adsorptive capacity of
Woodburn soil was attributed to the presence of organic matter and
lipophilic or hydrophobic materials. Moza et al. (1976a) found that, 2
years after the application of 14C-labelled dichlorobiphenyl to a
loamy sand soil at 1 mg/kg, most of the detectable PCB was in the top
10 cm of the soil and only 0.2% had reached a depth of 40 cm. In
another study, Suzuki et al. (1977) found that Aroclors 1242 and 1254
did not move upwards through uncontaminated sand deposited over
contaminated soil. The leaching of water from soil may lead to a
downward movement of PCBs, depending on the soil type and clay content
(Pal et al., 1980).
A large spill of Askarel (containing 70% Aroclor 1254 and 30% tri-
and tetrachlorobenzenes) occurred at a transformer-manufacturing
facility in Canada, in 1976. Condie silt from near the site of the
spill was studied with respect to the sorption partition coefficients
and the transport retardation factors. The sorption partition
coefficient values for 2,5,2',5'-tetrachloro-, 2,4,5,2',5'-penta-
chloro-, and 2,4,5,2',4',5'-hexachlorobiphenyl were 5000, 9400, and
26 000, respectively. The mean transport retardation factors for these
3 congeners were 2.7 E + 04, 5.0 E + 04, and 1.4 E + 05, respectively.
This implies that dissolved PCBs will move only very slowly through
unfractured Condie silt (Anderson & Pankow, 1986).
4.1.3 Transport in water
PCBs enter water mainly from discharge points of industrial and urban
wastes into rivers, lakes, and coastal waters. In static water, PCBs
are more concentrated in the surface micro-layer than in subsurface
samples (Bidleman & Olney, 1974). This is probably due to deposition
from the air rather than redistribution in the water. On account of
their low water solubility and high specific activity, it is expected
that most of the PCBs discharged will be adsorbed by sediment at the
bottom of rivers or lakes and transport will be mainly via waterborne
particles (Nisbet & Sarofim, 1972). The bulk of the PCBs will sink to
the bottom sediments. The sinking rate of PCBs from the surface to
deeper layers in the open ocean is relatively slower in tropical
waters than in high-latitude waters (Tanabe, 1985).
Oloffs et al. (1973) added 0.1 mg Aroclor 1260/litre to water samples
in the presence of sediment. After 6 weeks, all of the PCBs had been
adsorbed by the sediment, none being given off to the atmosphere. The
degree of PCBs sorption is inversely related to the size of the
particles (Haque et al., 1974) and the solubility of PCBs in water
(Haque & Schmedding, 1975). Smaller particles have a relatively larger
surface area and so adsorb more PCBs (Steen et al., 1978). Nau-Ritter
et al. (1982) found the adsorption and retention of PCBs to be
directly related to the particle organic content. A significant
correlation was found by Larsen et al. (1985) between PCB levels and
total organic carbon in the deepwater sediments of the Gulf of Maine,
PCBs were concentrated on finer grain particles. Organic carbon and,
therefore, the PCB concentration were also correlated with depth.
Wildish et al. (1980) found that estuarine sediments, especially those
containing higher levels of organic matter, readily adsorbed Aroclor
1254. The PCBs were found to be tightly bound to the sediment with
virtually no desorption. Horzempa & Di Toro (1983) found that the
adsorption of hexachlorobiphenyl was correlated with both sediment
surface area and organic content. Adsorption was found to be
significantly greater at 40°C than at 1°C. Hexachlorobiphenyl is
strongly adsorbed on sediment and weakly desorbed. There is no simple
reversible reaction.
Fisher et al. (1983) found that the rate of release of PCBs from
contaminated sediment was a function of sediment PCB concentration,
chlorine substitution pattern, and degree of chlorination. In the
absence of disturbance, even very low deposition rates of new sediment
will quickly remove PCB-contaminated sediments from diffusional
communication with overlying water. Little change was found (Nimmo et
al., 1971a) in the PCB concentration in sediment at a point downstream
of a contamination source over a period of 9 months. The very small
amounts of PCBs leached from sediment into overlying water may be
taken up by organisms.
Hom et al. (1974) stated that the annual inputs of PCBs into the
southern California bight from waste water and from surface runoff in
1970-71 were estimated to be 10 and 0.25 tonnes, respectively.
Sewage treatment appears to remove PCBs from waste water,
concentrating them in the sludge. However, often, the sludge is then
discharged into open water (Ahling & Jensen, 1970). Holden (1970)
found an average of 3 mg PCBs/kg in wet sewage sludge dumped in the
Clyde estuary, in the United Kingdom, and calculated that this would
be equivalent to approximately one tonne per year. A similar annual
discharge of PCBs in the sludge on the Californian coast was
calculated by Schmidt et al., (1971).
Dredging of inland rivers and harbours may lead to a significant
transfer of PCBs from contaminated sediments, especially when dumped
at sea (Nisbet & Sarofim, 1972). Rice & White (1987) found that there
was an increase in water concentrations of PCBs immediately following
the dredging of sediment in the Shiawassee River, Michigan. The
availability of PCBs for clams and fish, as measured by an increase in
uptake, was found for up to 6 months following dredging.
4.1.4 Transport between media
In a model ecosystem, Södergren & Larsson (1982) found that the
presence of bottom-living organisms, such as Chironomus and
Tubifex, resulted not only in the uptake of PCBs from the sediment
but also in the release of PCBs into the water and to the surface
microlayer, compared with a system without organisms. PCBs were
transported to the air via jet drops from bursting bubbles in the
surface microlayer.
A similar pattern was found using large outdoor artificial ponds
(Larsson, 1985a). Following the addition of Clophen A50 to sediment,
the transport of PCBs from sediment to water followed a seasonal
cycle, with higher levels in the summer than in the winter. The
processes that transfer PCBs across the sediment/water interface
(bioturbation, desorption, and gas convection) are positively related
to temperature. Transfer from water to air was probably dominated by
volatilization with maximum concentrations of PCBs in air at the
highest water concentrations, lower chlorinated biphenyls achieving
the highest concentrations in air. The majority of the airborne phase
was presumed to be in the gaseous phase as it passed through particle
filters. In the same ponds, Larsson & Okla (1987) measured the rate at
which PCBs volatilized from water to air. PCB compounds volatilized at
a rate of 0.9 to 9.6 ng/m2 per h, the rate increasing with the
temperature of the water and the concentration of PCBs. The transport
rate during the day exceeded the rate at night and was positively
correlated with the air temperature (Okla & Larsson, 1987).
Larsson (1985b) added Clophen A50 to the sediment in a model ecosystem
comprising sediment, water, benthic macroinvertebrates, and fish. PCBs
were detected in the water. The transport of PCBs from the water to
air included at least 2 routes, volatilization and jet drop transport.
Both routes were of the same magnitude (0.2-1.0 µg/week). However,
though the PCBs transported by volatilization consisted of lower
chlorinated isomers, those transported by jet drops were identical to
those in the sediment and water.
In an earlier study, Larsson (1984) measured the uptake of PCBs from
sediment by chironomid midge larvae and the concentrations of PCBs
from larva to adult. In the field, chironomid larvae contained
114 µg/kg fresh weight at a sediment concentration of 39 µg/kg wet
weight. Different sediments affected the amount of PCBs available to
the organisms. Adult chironomids sampled near a sewage plant contained
251 µg/kg fresh weight. The chironomid larval population was estimated
to be 9900 per m2 and the authors calculated that these would move
20 µg PCB/m2 per year into the terrestrial compartment of the
environment.
A model, based on the fugacity concept, was described and illustrated
by applying it to the time-varying fate of PCBs in Lake Ontario over
the period 1940-2000. Expressions are included for a great number of
variables, such as loadings and the partitioning of the contaminant
between the phases of air, aerosols, water, suspended and bottom
sediments, various trophic levels of aquatic organisms, and gull eggs.
Also included are expressions for transformation rates, and transport
rates for diffusion between water and sediment, and water and air wet
and dry atmospheric deposition, sediment deposition, burial, and
resuspension, and water and the inflow and outflow of suspended
matter. The results obtained by numerical integration and by assuming
reasonable loading and air concentrations were in accordance with
data. It was shown that PCBs cycle appreciably between the atmosphere
and water by wet and dry deposition and volatilization, and between
water and sediment by deposition, resuspension, and diffusion.
Biomonitors were shown to be particularly valuable indicators of
contamination levels in the ecosystem (MacKay, 1989).
4.2 Biotransformation
4.2.1 Biodegradation
Nissen (1973) did not find any alteration in Aroclor 1254 after a
9-week incubation period in soil. Iwata et al. (1973) added Aroclor
1254 to various soil types. They did not find any change after one
year in soils containing high amounts of organic matter (10.8-19.5%).
Biotransformation had occurred, causing the disappearance of the lower
chlorinated biphenyls, in soils with a low organic matter content
(0.1-3.3%), as diverse as loamy sand and clay. The authors concluded
that, after one year, the material remaining in loamy sand (0.1%
organic matter) consisted of mainly penta- and hexachlorobiphenyl
isomers.
4.2.1.1 Bacteria
The biodegradation of PCB isomers, which is possible with some aerobic
bacteria, depends on the degree of chlorination and the position of
chlorine substitution. Degradation decreases with increasing
chlorination. Dechlorination of PCBs occurs in anaerobic sediments.
Here bacterial activity is preferentially targeted towards PCB
congeners with higher levels of chlorination. Products of
dechlorination are, therefore, more readily degraded by aerobic
systems.
Early experiments were carried out to study the biodegradation of PCBs
using activated sludge inocula; some degradation was found (Baxter et
al., 1975). However, the presence of PCBs in sewage sludge shows that
they are not all readily transformed by microorganisms. Fries (1972)
analysed silage containing PCBs (Aroclor 1254) that had undergone
normal fermentation. The gas chromatogram of the standard was
identical to that of the silage sample. The authors suggested that, if
anaerobic degradation had taken place, it would have been unlikely to
have been uniform for all components. They stated, however, that this
test may not have been a good indication of possible anaerobic
degradation because DDT showed much less degradation, under the same
conditions, compared with other degradation test systems.
Lunt & Evans (1970) postulated a metabolic pathway, used by
microorganisms, for biphenyl oxidation, which was later confirmed by
the findings of Gibson et al. (1973) using a bacterium isolated from a
polluted stream. Lunt & Evans (1970) found that a Gram-negative
bacterium oxidized biphenyl to phenylpyruvic acid with the
intermediary formation of 2,3-dihydroxybiphenyl and
alpha-hydroxy-ß-phenylmuconic semialdehyde. Catelani et al. (1971)
found that the metabolism of biphenyl by Pseudomonas putida was
different, in that, though the intermediate products were the same,
benzoic acid was isolated, not phenylpyruvic acid. Ahmed & Focht
(1973a) isolated 2 species of Achromobacter from sewage effluent
using biphenyl and p-chlorobiphenyl as the sole carbon source. They
found that both sources were rapidly degraded, biphenyl being oxidized
to benzoic acid and both mono and dichlorinated biphenyls to
p-chlorobenzoic acid. In a second study, Ahmed & Focht (1973b)
investigated the biodegradation of other isomers of PCBs, with 2-5
chlorine atoms. The extent of oxidation seemed to be somewhat
dependent on the presence of unsubstituted biphenyl rings. Because of
the absence of chloride in all the supernatants, they concluded that
the bacterium was unable to dechlorinate the PCBs. The fact that
increasing chlorine substitution rendered the molecule more resistant
to microbial attack was used to support this argument. However, Kaiser
& Wong (1974), studying the degradation of Aroclor 1242 by a bacterial
culture, isolated from lake water, showed that the PCBs were degraded
into several metabolites (aliphatic and aromatic hydrocarbons), none
of which contained chlorine. Dechlorination had already taken place at
an early stage of metabolism.
Wong & Kaiser (1975) found that lake water bacteria could use both
Aroclor 1221 and 1242, but not 1254, as a sole carbon source for
growth, but that only 1% of the bacterial culture had this ability.
The authors then followed the degradation of Aroclor 1221. After one
month, the mixture had been totally degraded to several compounds of
low relative molecular mass. Unchlorinated biphenyls were degraded
faster than chlorinated forms.
Tucker et al. (1975b) observed the degradation rates of Aroclors 1221,
1016, 1242, and 1254, and MCS 1043 (a non-commercial mixture). They
found a clear relationship between the level of chlorination and the
relative degradability, when degradation rate was plotted against
percentage chlorine by weight. Volatilization rates fell within the
95% confidence limits of overall disappearance rates and so could be
ruled out. Analysis of the Aroclors, following exposure to the
activated sludge, revealed a redistribution of the dominant PCBs. For
example, the chromatograms for Aroclor 1221 and 1242 were very similar
showing that the lower chlorinated biphenyls were more rapidly
degraded. Furthermore, since Aroclor 1221 was found to be rapidly
degraded, a closer study was performed that showed that most of the
degradation occurred within 24 h.
The degradation of polychlorinated biphenyls by either Nocardia spp.
or Pseudomonas spp. was studied by Baxter et al. (1975). They found
that, under experimental conditions, many of the lower chlorinated
biphenyls (<3 chlorine atoms/molecule) were degraded very readily
and some biphenyls containing as many as 6 chlorine atoms could be
degraded, if the conditions were suitable. When PCB mixtures Aroclor
1016 and 1242 were used, a different pattern of degradation was
observed with an enhanced ability of the microorganisms to degrade.
For example, 4,4'-dichlorobiphenyl degraded to 50% in about 2 days,
when presented to Nocardia spp. as a component of Aroclor 1242, but
it was virtually unaffected after 12 days exposure as the pure isomer.
The authors suggested that mutual solubilization might play some part.
Sayler et al. (1977) found that an estuarine Pseudomonas sp. was
able to degrade both mixtures of PCBs (Aroclor 1254) and pure isomers
of hexachlorobiphenyl. Degradation was dependent on incubation time
and the purity and degree of chlorination of the biphenyl. Appreciable
degradation occurred at all substrate concentrations of the Aroclor
(10, 100, and 1000 µg/litre) within 22 days. Although, over this
22-day period, only 9% had been degraded at the lowest concentration
compared with 30-40% for the other concentrations, after 60 days, this
was reversed with 84% being degraded at 10 µg/litre, 70% at
100 µg/litre, and 63% at 1000 µg/litre. When compared with the pure
isomer, degradation of the Aroclor mixture proceeded at a slower rate.
Even though average chlorination was less, the authors speculated that
this could be owing to the substitution positions of the chlorines.
Chromatographic tracings showed that degradation of the lower
chlorinated components of the Aroclor occurs before degradation of the
more highly chlorinated biphenyls.
Furukawa et al. (1978a,b) examined 31 PCB isomers (mono to
pentachlorobiphenyl) for biodegradability by 2 bacterial species,
Alcaligenes and Acinetobacter. They found the following
relationship between chlorine substitution and biodegradability.
i. Degradation decreased as chlorine substitution increased.
ii. Isomers containing two chlorines at the ortho position of
either a single ring or on both rings showed very poor
degradability.
iii. Isomers, in which all the chlorines were on one ring, were
generally degraded faster.
iv. Molecules with non-chlorinated rings or rings with few chlorines
underwent preferential ring fission.
v. The 4'-chloro-substituted PCBs formed and accumulated a yellow
intermediate during degradation.
vi. Only with respect to 2,4,6-trichlorobiphenyl was there a
significant difference in ability to degrade between the 2
bacteria. This compound was mostly metabolized within 1 h by
Acinetobacter, but was degraded very slowly by Alcaligenes.
It was demonstrated by Carey & Harvey (1978) that mixed cultures of
marine bacteria were capable of metabolizing both pure isomers (tri-
and tetrachlorobiphenyl) and mixtures (Aroclor 1254). They isolated
and partially characterized an acid lactone metabolite. They did not
find any change in the chromatogram trace for the Aroclor but
suggested that this might be related to the insensitivity of the
method, since even if each of the isomers in the mixture had been
metabolized to the same extent as pure isomers, this would still not
have been detectable on the trace. The authors also found that no
metabolism occurred when a chlorobiphenyl isomer in an anaerobic
marine mud was incubated for 6 weeks. Degradation of Aroclor 1242 by
mixed microbial cultures, isolated from soil and river water samples,
was demonstrated by Clark et al. (1979). The predominant organisms in
the cultures were Alcaligenes odorans, Alcaligenes denitrificans,
and an unidentified bacterium. The lower chlorinated isomers were not
only degraded at a faster rate but were also more completely utilized
by the bacteria. In general, the rate of degradation was much faster
than in previous studies. Co-metabolism in the presence of sodium
acetate was studied; greatly enhanced degradation was found for the
more highly chlorinated isomers. Liu (1980) found that sodium
ligninsulfonate also greatly enhanced the biodegradation of commercial
PCB mixtures.
The same author found that a Pseudomonas sp. could oxidize Aroclors
1221, 1016, 1242, and 1254, at a rapid rate. A kinetic study using
resting cells revealed that Aroclor 1221 was degraded much faster
(980 µg/h per mg cell dry weight) than Aroclor 1254 (43 µg/h per mg
cell dry weight). The degradation of the higher chlorinated PCB
(Aroclor 1254) could be enhanced by the addition of Aroclor 1221. Liu
(1981) observed that the oxidation of Aroclor 1221 by the bacteria was
10 times faster than with sewage. Two possible explanations for this
difference were that the sewage contained toxic chemicals that
inhibited the bacteria, but this was found not to be the case, or, the
bacteria preferred Aroclor 1221 to the other substrates. This second
explanation is a possibility, for glucose, a substrate used readily by
most bacteria was poorly oxidized by this bacterium. Pseudomonas
oxidized Aroclor 1221 readily between 15 and 35°C, the rate increasing
with temperature. Reducing the temperature to 4 and 10°C drastically
retarded, but did not halt, degradation. Adjusting the concentrations
of phosphorus and nitrogen from 2 mg to 20 mg/litre (the lower
concentration being that found normally in sewage) did not alter the
rate of degradation by Pseudomonas spp. in raw sewage. But
increasing nitrogen and phosphorus gave more reproducible results,
suggesting that the compounds are on the border of limiting
degradation rates in raw sewage. The oxygen content was found not to
affect degradation at concentrations over 1 mg/litre (oxygen levels
are generally maintained at between 2 and 3 mg/litre in activated
sludge reactors, under the operational conditions of sewage-treatment
plants). Liu (1982) found that, under a limited substrate supply,
Pseudomonas spp. degraded all 7 of the major components of Aroclor
1221. However, with excessive amounts of nutrient, preferential
degradation of certain components was observed. The author stated that
one of the main factors influencing this selective biodegradability
was the position of chlorine substitution on the biphenyl.
4.2.2 Biodegradation; individual congeners
4.2.2.1 Bacteria
In a study by Parsons & Sijm (1988), the co-metabolism was
investigated of several different mono-, di- and tetrachlorobiphenyls
in chemostat continuous cultures of a Pseudomonas strain (JB1). They
found that chemostat conditions favoured degradation compared with
exposure of the Pseudomonos in batch culture, where little or no
degradation was recorded. Using benzoate as the carbon source, results
varied widely, with repeat incubations showing different degrees of
degradation of chlorobiphenyls and, sometimes, no breakdown at all. In
cultures that did degrade the materials, the monosubstituted
4-chlorobiphenyl was rapidly degraded. Of the disubstituted
dibiphenyls, 3,5-dichlorobiphenyl was more readily broken down than
2,5-dichlorobiphenyl. Changing the carbon source available to the
Pseudomonas sp. improved the reproducibility of the results. The
authors reviewed the literature relative to their own findings and
concluded that repeated culture on benzoate leads to the loss of the
ability of the Pseudomonas sp. to degrade biphenyl by meta
cleavage; ortho cleavage is retained. Coding for the meta cleavage
resides on plasmids, which can be lost, whereas coding for the ortho
cleavage is chromosomal. Growth of the Pseudomonas sp. on a
3-methylbenzoate substrate improved degradation of the biphenyls.
3-Methylbenzoate can only be degraded by a meta cleavage favouring
retention of the plasmid. Comparison of degradation of 4
tetrachlorobiphenyls showed the influence of the positions of the
chlorine substitutions. The relative degradability of the 5 compounds,
shown in Fig. 3, was: 2,3,2',3'-tetrachloro- >2,5,3',4'-tetrachloro-
> 2,5,2',5'-tetrachloro- approx. 2,6,2',6'-tetrachloro- approx.
3,4,3',4'-tetrachlorobiphenyl. The authors stated, from the
literature, that the first reaction in the degradation of
chlorobiphenyls is, in most cases, 2,3-dioxygenation, eventually
leading to the formation of chlorobenzoates. Chlorines in the ortho
and meta positions will, therefore, offer steric hindrance to this
reaction.
The low degradation rate of 3,4,3',4'-tetrachlorobiphenyl is not
explained by this mechanism, since it has 2 adjacent unoccupied 2,3
positions, but is more likely explained by its toxicity. Steric
influence on enzyme binding is offered as an explanation in this case.
Similarly, Furukawa et al. (1978a) did not find any degradation of
this compound in initial studies, though they did find degradation to
a dichlorobenzoic acid by Acinetobacter in a later study (Furukawa
et al., 1978b; Rogers, undated(a)).
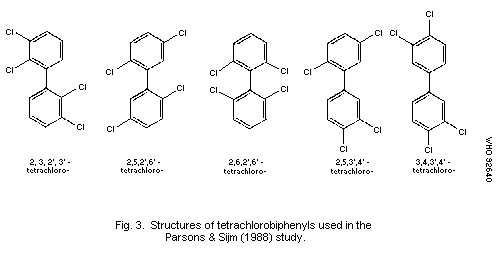 Brown et al. (1987a,b) examined patterns of PCB congeners remaining in
sediments after spills of commercial mixtures of Aroclor. Sediment
from 5 different sites was examined. Shifts in gas chromatographic
peak distribution were indicative of dechlorination of congeners by
anaerobic bacteria in the sediment. Analysis of sediment from
different depths indicated less difference from the original traces in
superficial layers and the greatest shift in deeper layers of the
sediment cores. They concluded that dechlorination had taken place and
deduced several different processes involved by comparison between
sites. Six of these processes have been characterized in detail, each
presumed to be mediated by different populations of anaerobic
bacteria, with different selectivity for different congeners in the
PCB mixture. The point of most interest was that congeners with high
degrees of chlorination were selectively dechlorinated by these
anaerobic organisms. Whilst dechlorination still leaves the mass of
PCB intact, congeners with lower chlorination can be more readily
degraded by aerobic bacteria. This anaerobic dechlorination,
therefore, enables further degradation to take place elsewhere and
contributes significantly to the detoxification of the PCBs. While the
combined meta- para selective dechlorinating/oxidizing action of
sediment microbes for PCB residues is likely to be detoxifying, with
respect to dioxin-like effects, there are reservations about whether
this action would be detoxifying in respect of other, more subtle
toxic effects of PCBs and their degradation products, known (such as
the potential reproductive toxicity of the hydroxylated,
ortho-enriched PCBs from sediment microbe action) and unknown. This
is why it is important to study not only the disappearance of PCBs,
but also the exact nature and amounts of the degradation products
(McKinney et al., 1990). Two broad categories of transformation have
been observed: the first dechlorinates in the ortho, meta, and para
positions and the potential for the dechlorination of biphenyls is
related to the reduction potential of the compound, the second
dechlorinates only in the meta and para positions, and the
reactivities of the congeners relate to the molecular shape. The
second category suggested to the authors an active site on a
dechlorinating agent that would be roughly conical with a reducing or
hydrogenating site at the apex. In this schema, para-substituted
molecules could enter the site directly, enough rotation of the
molecule would be possible for the accommodation of meta, but not
ortho, substitution. Quensen et al. (1988) demonstrated this
dechlorinating capacity of anaerobic bacteria from Hudson River
sediments in the laboratory. Dechlorination occurred primarily from
the meta and para positions; ortho-substituted congeners
accumulated selectively. The fastest rate of dechlorination occurred
at the highest exposure used (700 mg Aroclor 1242/kg); 53% of the
total chlorine was removed over a 16-week incubation period. During
incubation, the proportion of mono- and dichlorobiphenyls increased
from 9 to 88%. The authors believed that a sequential anaerobic to
aerobic system could be devised for the biological degradation of
PCBs.
4.2.2.2 Fungi
Wallnofer et al. (1973) incubated a soil fungus Rhizopus japonicus
in a medium containing 3H-labelled 4-chlorobiphenyl or
4,4'-dichlorobiphenyl. After incubation for 1 week, the fungal
mycelium was filtered out. Scans of TLC plates indicated a
hydroxybiphenyl derivative present in the filtrate of both cultures.
To further identify the metabolite, larger amounts of unlabelled
4-chlorobiphenyl were added to a similar culture. The NMR and mass
spectra were identical to a synthetic sample of 4- chloro-4'-hydroxy-
biphenyl; mixed melting point determination showed no depression.
Further positive identification of the product was not possible,
because of limited material, but the experiment indicates the
probability of degradation of biphenyl to a hydroxy derivative by a
fungus.
4.2.3 Photodegradation
Several authors have reported that simple chlorinated biphenyls, as
well as complex commercial PCB mixtures, undergo photoreduction in
organic solvents (Safe & Hutzinger, 1971; Hustert & Korte, 1972; Ruzo
et al., 1972, 1974, 1975; Sawai & Sawai, 1973; Koshioka et al., 1987)
and aqueous systems (Crosby & Moilanen, 1973; Bunce, 1978) in the
laboratory. Herring et al. (1972) found that PCBs degraded faster in
hexane solution than in aqueous solution and slower in benzene
solution.
Bunce et al. (1978) posed the question of the environmental
significance of the photodegradation of PCBs and tried to estimate the
likely degree of photolysis under real environmental conditions,
rather than in solution in organic solvents at high concentrations.
The current best estimate suggests that significant amounts,
particularly of higher chlorinated PCB congeners, might be degraded in
water by the action of sunlight.
4.2.4 Bioaccumulation, distribution in organisms, and elimination
Polychlorinated biphenyls accumulate in almost all organisms, because
of their high lipid solubility and slow rate of metabolism and
elimination. They accumulate preferentially in fat-rich tissues.
Bioconcentration factors (BCFs) should be interpreted with caution,
since they are simple ratios. The exposure concentration, therefore,
makes a marked difference to the BCF obtained; very low exposure
concentrations are likely to lead to high BCFs, since all the PCBs are
absorbed, whilst high exposure concentration will tend to minimize the
BCFs.
Experimental data on the bioconcentration of PCB mixtures and pure
chlorinated biphenyls are presented in Table 9 for microorganisms,
Table 10 for aquatic organisms, and Table 11 for plants, birds, and
mammals.
4.2.4.1 Microorganisms
Uptake of both pure chlorinated biphenyl isomers and commercial PCB
mixtures by microorganisms is rapid, and high bioconcentration factors
are achieved. While there is a suggestion in studies on some species
that PCB congeners with higher levels of chlorination are taken up
preferentially, in the majority of studies, all PCBs appear to be
taken up equally. Uptake is true absorption; adsorption onto the
surface of the organisms represents little of the uptake. Since
resistant forms of microorganisms take up less PCBs than sensitive
forms and dead cells accumulate more PCBs than live ones, there is
some capacity to exclude the compounds.
Harding & Phillips (1978b) studied the uptake of 14C-labelled
2,4,5,2',5'-pentachlorobiphenyl, at concentrations of 0.31 or
9.86 µg/litre water, by 11 marine phytoplankton species including:
diatoms, green algae, chrysophytes, haptophytes, and dinoflagellates.
The cell density of each culture was maintained at 106-109 cells/
litre. Equilibrium between water and cell concentrations of biphenyl
was reached very rapidly after 0.5-2 h; small motile forms reached
equilibrium within 1 h and large centric diatoms after approximately
2 h. Exposure concentration and cell density, within the range given
above, had little effect on the time-course of uptake. Substantial
interspecies differences in adsorptive capacity were shown by
differences in the Freundlich adsorption constant (log K). A large
centric diatom, Coscinodiscus sp., had the highest log K. Nitzschia
longissima, a penate diatom that has been shown to be resistant to
PCBs (Harding & Phillips, 1978a), had the lowest log K value. The
flagellates, with the exception of Monochrysis lutheri, which has
been shown to be very sensitive to the effects of PCBs, had much lower
log K values than diatoms. Concentration factors, calculated from the
Freundlich adsorption isotherms, ranged between 12 300 and 2 410 000.
Biggs et al. (1980) exposed mixed species of estuarine phytoplankton
(numerically dominated by the diatom Skeletonema costatum) to
14C-labelled PCB (approximately 54% chlorine by weight) at
concentrations of 5.8 or 11.6 µg/litre. At a particle concentration of
25 mg/litre, 19-22% of the labelled-PCB was sorbed on the particles
after a 1-h exposure, with 70-72% in the water. At 4 times the above
particle concentration, 66-69% was sorbed on particles and only 22-23%
was retained in the water. Doubling the amount of 14C-PCB doubled the
mean amount of labelled-PCB in both the particles and the water. The
authors calculated an index of sorption (the ratio of 14C-PCB sorbed
on particles to that in an equal volume of water) at an average of
2 ± 1 × 104. The authors suggested that the higher uptake (88%) of
PCBs found by Södergren (1971) was probably the result of an
unnaturally high cell concentration. Phytoplankton sampled in the
surface waters of Long Island Sound, USA, varied seasonally in
concentration from about 0.5 to 30 mg/litre.
Lederman & Rhee (1982) calculated bioconcentration factors for 3
species of Great Lakes planktonic algae (Table 9). In the case of
Fragilaria crotonensis, the uptake of hexachlorobiphenyl into the
frustule (the siliceous wall of the diatom) was investigated. The
bioconcentration factors for frustules were lower by an order of
magnitude than the factors for live and dead cells. It appears,
therefore, that adsorption on the cell surface contributes only a
little to the bioaccumulation of hexachlorobiphenyl.
Table 9. Bioaccumulation of PCBs: Microorganisms
Organism Biomass Temperature PCB type Duration Exposure Bioconcentration Reference
(cells/ml) (°C) (µg/litre) factora
Green alga 2 × 106 20-25 TeCB 1 h 10 3200 Urey et al. (1976)
Chlorella 2 × 106 20-25 HeCB 1 h 10 7000 Urey et al. (1976)
pyrenoidosa 2 × 106 20-25 OcCB 1 h 10 1600 Urey et al. (1976)
2 × 106 20-25 DeCB 1 h 10 5200 Urey et al. (1976)
Algae 3.2 × 105 HeCB 19 h 1 117 000b Lederman & Rhee
Fragilaria 1.6 × 105 HeCB 19 h 1 313 000b (1982)
crotonensis Lederman & Rhee
(1982)
Algae 3.4 × 105 HeCB 6 h 1 619 000b Lederman & Rhee
Ankistrodesmus 1.7 × 105 HeCB 6 h 1 959 000b (1982)
falcatus 8.5 × 104 HeCB 6 h 1 1 207 000b Lederman & Rhee
(1982)
Lederman & Rhee
(1982)
Algae 1.1 × 106 HeCB 6 h 1 129 000b Lederman & Rhee
Mycrocystis sp. 5.5 × 105 HeCB 6 h 1 170 000b (1982)
2.8 × 105 HeCB 6 h 1 264 000b Lederman & Rhee
(1982)
Lederman & Rhee
(1982)
Table 9. (cont'd).
Organism Biomass Temperature PCB type Duration Exposure Bioconcentration Reference
(cells/ml) (°C) (µg/litre) factora
Fungus 22-25 Aroclor 1254 24 h 0.007 mg/kg 1327b,c Pinkney et al. (1985)
Fusarium 22-25 Aroclor 1254 48 h 0.007 mg/kg 1144b,c Pinkney et al. (1985)
oxysporum
a Concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration factors calculated on a wet weight
basis unless otherwise stated.
b Calculated on a dry weight basis.
c Radioactive isotope used to calculate bioconcentration factor.
Table 10. Bioaccumulation of PCBs: Aquatic organisms
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
American oyster flow WB Aroclor 1016 96 h 0.6 6666 Hansen et al. (1974b)
Crassostrea WB Aroclor 1254 56 d 0.01 165 000 Parrish (1973)
virginica WB Aroclor 1254 392 d 0.01 89 000 Parrish (1973)
Polychaete stat WB Aroclor 1254 5 d 1.1 236 Courtney & Langston
Arenicola marina stat WB Aroclor 1254 5 d 1 mg/kgd 0.24 (1978)
Polychaete stat WB Aroclor 1254 5 d 1.1 373 Courtney & Langston
Nereis stat WB Aroclor 1254 5 d 1 mg/kgd 0.36 (1978)
diversicolor
Water flea flow WB 20-22 Aroclor 1254 96 h 1.1 47 000e* Sanders & Chandler
Daphnia magna (1972)
Amphipod (M) statf WB Aroclor 1254 24 h 0.03 8700 Pinkney et al. (1985)
Gammarus statf WB Aroclor 1254 24 h 195.8 mg/kg 0.118 Pinkney et al. (1985)
tigrinus
Scud flow WB 20-22 Aroclor 1254 96 h 1.6 24 000e* Sanders & Chandler
Gammarus flow WB 20-22 Aroclor 1254 21 d 1.6 27 000e (1972)
pseudolimnaeus
Glass shrimp flow WB 20-22 Aroclor 1254 96 h 1.3 12 300e* Sanders & Chandler
Palaemonetes flow WB 20-22 Aroclor 1254 21 d 1.3 16 600e* (1972)
kadiekensis
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Brown shrimp flow WB Aroclor 1016 96 h 0.9 4222 Hansen et al. (1974b)
Penaeus aztecus
Grass shrimp flow WB 17-28 Aroclor 1254 7 d 2.3 11 000 Nimmo et al. (1974)
Palaemonetes flow WB 17-28 Aroclor 1254 16 d 1.3 14 000 Nimmo et al. (1974)
pugio flow WB 17-28 Aroclor 1254 28 d 0.62 17 450 Nimmo et al. (1974)
flow WB 17-28 Aroclor 1254 35 d 0.62 26 580 Nimmo et al. (1974)
flow WB Aroclor 1016 96 h 0.4 2750 Hansen et al. (1974b)
Crayfish flow WB 20-22 Aroclor 1254 96 h 1.2 1700e* Sanders & Chandler
Orconectes nais flow WB 20-22 Aroclor 1254 21 d 1.2 5100e* (1972)
Stonefly flow WB 20-22 Aroclor 1254 96 h 2.8 2500e* Sanders & Chandler
Pteronarcys flow WB 20-22 Aroclor 1254 21 d 2.8 2800e* (1972)
dorsata
Dobsonfly flow WB 20-22 Aroclor 1254 96 h 1.1 4600e* Sanders & Chandler
Corydalus flow WB 20-22 Aroclor 1254 21 d 1.1 6800e* (1972)
cornutus
Phantom midge flow WB 20-22 Aroclor 1254 96 h 1.3 23 600e* Sanders & Chandler
Chaoboruspuncti (1972)
pennis
Mosquito larvae flow WB 20-22 Aroclor 1254 96 h 1.5 18 000e* Sanders & Chandler
Culex tarsalis (1972)
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Mayfly flow WB 8 Clophen A50 6 d 0.526 2940 Södergren &
Ephemera danica Svensson (1973)
Pinfish flow WB Aroclor 1016 96 h 0.8 2750 Hansen et al. (1974b)
Lagodon flow WB Aroclor 1016 28 d 1 n 25 000 Hansen et al. (1974b)
rhomboides flow WB Aroclor 1016 56 d 1 n 17 000 Hansen et al. (1974b)
Sheepshead flowg WB Aroclor 1016 33 d 1 n 26 000 Hansen et al. (1975)
minnow flowg WB Aroclor 1016 28 d 1 n 54 000 Hansen et al. (1975)
Cyprinodon flowg WB Aroclor 1016 28 d 1 n 22 000 Hansen et al. (1975)
variegatus
Spot flow WB Aroclor 1254 7 d 1 n 7200 Hansen et al. (1971)
Leiostomus flow WB Aroclor 1254 14 d 1 n 17 000 Hansen et al. (1971)
xanthurus flow WB Aroclor 1254 28 d 1 n 37 000 Hansen et al. (1971)
flow WB Aroclor 1254 56 d 1 n 27 000 Hansen et al. (1971)
Atlantic salmon flow WB 10-15 Aroclor 1254 33 d 10 mg/kg 0.39 Zitko (1974)
Salmo salar
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Coho salmon flow WB 17 Aroclor 1254 112 d 0.048 mg/kg 9.79 Mayer et al. (1977)
Oncorhynchus flow WB 17 Aroclor 1254 112 d 4.8 mg/kg 0.79 Mayer et al. (1977)
kisutch WB TeCB 17 d 1 mg/kg 0.144 Gruger et al. (1976)
WB TeCB 35 d 1 mg/kg 0.139 Gruger et al. (1976)
WB PeCB 35 d 1 mg/kg 0.162 Gruger et al. (1976)
WB HeCB 35 d 1 mg/kg 0.151 Gruger et al. (1976)
Channel catfish flow WB 26 Aroclor 1232 150 d 2.4 mg/kg 1.875 Mayer et al. (1977)
Ictalurus flow WB 26 Aroclor 1232 193 d 2.4 mg/kg 1.3 Mayer et al. (1977)
punctatus flow WB 26 Aroclor 1248 193 d 2.4 mg/kg 0.79 Mayer et al. (1977)
flow WB 26 Aroclor 1254 193 d 2.4 mg/kg 2 Mayer et al. (1977)
flow WB 26 Aroclor 1260 193 d 2.4 mg/kg 1.46 Mayer et al. (1977)
flow WBh 24-26 Aroclor 1242 130 d 20 mg/kg 0.72 Hansen et al. (1976a)
flow WB Aroclor 1248 77 d 5.8 56 370* Mayer et al. (1977)
flow WB Aroclor 1254 77 d 2.4 61 190* Mayer et al. (1977)
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Fathead (M) flow WB 25 Aroclor 1248 250 d 3 approx. 60 000 DeFoe et al. (1978)
minnow (M) flow WB 25 Aroclor 1260 250 d 2.1 approx. 160 000 DeFoe et al. (1978)
Pimephales (F) flow WB 25 Aroclor 1248 250 d 3 approx. 120 000 DeFoe et al. (1978)
promelas (F) flow WB 25 Aroclor 1260 250 d 2.1 approx. 270 000 DeFoe et al. (1978)
d = Days; M = Male; F = Female; DiCB = dichlorobiphenyl; TeCB = tetrachlorobiphenyl; PeCB = pentachlorobiphenyl;
HeCB = hexachlorobiphenyl; OcCB = octachlorobiphenyl; DeCB = decachlorobiphenyl.
a Stat = static conditions (water unchanged for duration of experiment); flow = flow-through conditions (PCB concentration
in water continously maintained).
b WB = whole body.
c Bioconcentration factor = concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration
factors calculated on a wet weight basis unless otherwise stated. * Radioactive isotope used to calculate
bioconcentration factor.
d Sediment.
e Calculated on a dry weight basis.
f Static conditions, but test solution changed at intervals.
g Intermittent flow-through conditions.
h Not including stomach.
Södergren (1971) maintained the unicellular freshwater green alga
Chlorella pyrenoidosa in water (at a cell concentration of
approximately 900 mg/litre) with added nutrient medium containing
3.7 µg Clophen A50/litre, over a period of 7 days. By the end of the
experiment, 88% of the PCBs had been taken up by the alga. The
remaining PCBs were detected in the water, none being found in the air
samples taken. In another study, Urey et al. (1976) found that both
tetrachloro- and hexachlorobiphenyl isomers, at 10 µg/litre, were
concentrated by dead Chlorella pyrenoidosa cells by 6000 and 15 000
times, respectively, after a 1-h exposure. These concentration factors
are approximately twice those for living cells (Table 9). Similar
findings have been noted with other species of algae (Biggs et al.,
1980; Lederman & Rhee, 1982).
The ciliate Tetrahymena pyriformis was exposed to Aroclors 1248
(0.01, 0.1, and 1 mg/litre) and 1260 (0.001, 0.01, 0.1, and
1 mg/litre) for 7 days (Cooley et al., 1973). Uptake of the toxicant
increased linearly with increasing concentration. Concentration
factors ranged from 14.8 to 40.6 for Aroclor 1248 and from 21 to 79
for Aroclor 1260. Approximately 15-20% of Aroclor 1248 was absorbed at
each concentration compared with means of 37-53%, with increasing
concentration, for Aroclor 1260. If the data from Cooley et al. (1972)
on the uptake from Aroclor 1254 is included, it is clear that
T. pyriformis accumulates more PCBs with increasing degree of
chlorination.
Dive et al. (1976) studied the accumulation of 16 pure isomers of PCB
and one commercial product, Pyralene 3010, by the ciliate protozoan
Colpidium campylum, at concentrations of 0.1, 1, or 10 mg/litre for
43 h. The amount of PCBs taken up at 0.1 mg/litre was very similar for
each of the PCB isomers and the commercial product, ranging from 29.4
to 49%. The percentage uptake did not change greatly for the higher
exposures.
4.2.4.2 Plants
Uptake of PCBs into plants from soil is positively correlated with the
soil concentration of the PCBs. Roots accumulate more than stems and
foliage. Bioconcentration factors are low. More lower chlorinated
congeners of the PCBs are taken up, probably because of their greater
mobility in the soil. Much of the uptake is adsorption on the surfaces
of roots and there is little translocation. PCBs found in leaves have
volatilized from the soil. Uptake on root surfaces can be reduced or
eliminated by adding activated charcoal to the soil.
Lawrence & Tosine (1977) found that plants took up significant amounts
of PCBs (30-140% of the applied PCB concentration) from soil treated
with sewage sludge. In a waste PCB spill besides a North Carolina
highway, levels as high as 4700 mg/kg were recorded in the top 3 cm of
soil. Seven months later, the PCB concentrations were unchanged; the
authors believed that this was because the PCBs were bound to
activated carbon that had been used to treat the spill (Pal et al.,
1980).
Strek & Weber (1982) analysed statistically the data from several
literature sources (Iwata et al., 1974; Wallnofer & Koniger, 1974;
Wallnofer et al., 1975; Iwata & Gunther, 1976; Moza et al., 1976a,
1979a,b; Weber & Mrozek, 1979) on PCB uptake by plants, with the
following conclusions.
i. The PCB content of the plant is significantly dependent on the
soil PCB concentration.
ii. There is a significant difference between plant species,
carrots taking up more PCBs than other plants.
iii. There appears to be a limit of the PCB concentration in the
soil at which no detectable PCBs are taken up by the plants.
iv. Roots take up more PCBs than tops.
v. most of the PCBs in roots may, in fact, be adsorbed on the
surface and not actually taken up.
vi. There is a general trend of increasing PCB content with
decreasing chlorination, for pure PCB congeners.
vii. The amount of chlorination seems to have an effect on the
mobility of PCBs within plant parts. Since lower chlorinated
PCBs have been reported to be more mobile in soils than highly
chlorinated PCBs, they may be more readily transported and
available for plant uptake.
Larsson (1987) maintained the macroalga Cladophora glomerata in a
flowing-water, outdoor pool. Sediment contaminated with Clophen A50 at
2.7 mg/kg dry weight was added and PCB residues in the alga were
monitored. The algal concentration was 3.55 mg/kg dry weight within 3
months. Residues had fallen a year later to 0.2 mg/kg, reflecting the
water levels of PCBs. The authors concluded that a partitioning
process governed the uptake of PCBs by C. glomerata in this
experiment, because the alga accumulated the same PCBs and the same
proportion of PCBs that were present in the water.
Table 11. Bioaccumulation, of PCBs: Plants, birds, and mammals
Organism Organ PCB type Duration Exposure Bioconcentration Reference
(mg/kg) factora
Soilb
Beet (Beta vulgaris) plant top Aroclor 1254 39 days 20 0.041c Strek et al. (1981)
Sorghum (Sorghum bicolor) plant top Aroclor 1254 39 days 20 0.003c Strek et al. (1981)
Peanut (Arachis hypogaea) plant top Aroclor 1254 78 days 20 0.024c Strek et al. (1981)
Corn (Zea mays) plant top Aroclor 1254 13 days 20 0.001c Strek et al. (1981)
Carrot root DiCBd 112 days 0.118 2c Moza et al. (1976a)
leaves DiCBd 112 days 0.118 0.92c Moza et al. (1976a)
Food
White pelican carcase Aroclor 1254 70 days 144 14.8 Greichus et al. (1975)
(Pelecanus erythrorhynchos)
Table 11. (cont'd).
Organism Organ PCB type Duration Exposure Bioconcentration Reference
(mg/kg) factora
Chicken fat Aroclor 1242 28 days 100 2.83 Harris & Rose (1972)
fat Aroclor 1254 28 days 100 5.15 Harris & Rose (1972)
fat Aroclor 1260 28 days 100 4.82 Harris & Rose (1972)
Big brown bat carcase Aroclor 1254 37 days 9.4 6.6 Clark & Prouty
(Eptesicus fuscus) (1977)
Mink fat Aroclor 1254 approx. 56 days 1.5 16.5 Hornshaw et al.
(Mustela vison) fat Aroclor 1254 approx. 126 days 1.5 28.5 (1983)
Hornshaw et al.
(1983)
a Bioconcentration factor = concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration
factors calculated on a wet weight basis, unless otherwise stated.
b Calculated on a dry weight basis.
c Radioactive isotope used to calculate bioconcentration factor.
d DiCB = dichlorobiphenyl.
Red mangrove (Rhizophora mangle) seedlings were grown for 6 weeks in
soil treated with Aroclor 1242 at concentrations of between 0.038 and
6 mg/kg (Walsh et al., 1974). Low levels (detection limit was
0.1 mg/kg) of the PCBs were detected in the roots at exposure
concentrations of 3 or 6 mg/kg, during the exposure period, but no
residues were found in the stems. Residues were detected in both the
hypocotyls and leaves at application rates of 0.3 mg/kg or more. Leaf
residues did not change with time, but PCB concentrations in the
hypocotyls showed an increase. The highest mean residues of 1.5 mg/kg
were found in the hypocotyl in the highest exposure group.
Iwata et al. (1974) treated soil with Aroclor 1254, at a concentration
of 100 mg/kg, and sowed carrots in the plot 7 months later. The
carrots were harvested 3 or 4 months after seeding. The authors found
that the lower chlorinated biphenyls were more readily taken up from
the soil into the carrot root. Analysis of the carrot peel revealed
approximately 97% of the PCB residue, showing that there is little
translocation within the plant; 23 months after sowing carrots in soil
contaminated with 100 mg/kg Aroclor 1254, dissipation from soil
appeared to parallel the degree of chlorination (Iwata & Gunther,
1976). Analysis of the soil revealed that the lower chlorinated
biphenyls were slowly dissipated while the more highly chlorinated
biphenyls appeared to be unaffected. Small amounts of PCBs were found
in carrot foliage and the authors suggested that the PCB composition
indicated that they came from soil dust. Suzuki et al. (1977) also
found that lower chlorinated biphenyls were preferentially taken up by
plants, following exposure of soybean sprouts to soil contaminated
with Aroclor 1254 or 1242 at 100 mg/kg.
Moza et al. (1976a) found that carrot bioconcentrated
2,2'-dichlorobiphenyl (0.118 mg/kg soil) from soil by a factor of 2
(Table 11). No bioaccumulation was found in sugar beet, but the soil
residue was only 0.029 mg/kg. Carrots were grown in soil amended with
either 14C-labelled 2,5,4'-trichlorobiphenyl at 1.28 kg/ha or
2,4,2',4',6-pentachlorobiphenyl at 1.12 kg/ha for one season (Moza et
al., (1979a). Only 32.5% of the trichlorobiphenyl was recovered, the
rest being lost through volatilization. The carrots had taken up 3.1%
of the applied 14C, representing a concentration factor of 2.8. For
the pentachlorobiphenyl, 58.5% was recovered, 1.4% of which had been
taken up by the carrots. Sugar beet grown in the soil the following
year accumulated only 0.4% of the applied 14C.
In a study by Weber & Mrozek (1979), 14C-labelled Aroclor 1254 was
applied to Lakeland soil at a rate of 20 mg/kg. Activated carbon was
mixed with half the pots at a rate of 3.7 t/ha (3333 mg/kg). The pots
were seeded with either soybean or fescue. After harvesting at 16 days
for soybean and 50 days for fescue, the amounts of labelled-PCBs,
recovered from the plant tops, were 0.016% and 0.17% for the 2
species, respectively. The addition of activated carbon to the soil
reduced the uptake of 14C-PCBs, the recovery of labelled-PCBs being
0.001% and 0% for soybean and fescue, respectively. Strek et al.
(1981) also applied 14C-labelled Aroclor 1254, at the same rate, to
Lakeland soil; several species of crop plants were grown in the soil
and bioaccumulation factors, calculated (Table 11). Addition of
activated carbon (3.7 t/ha), equivalent to 3333 mg/kg to replicate
pots, reduced the uptake of the labelled-PCBs by 80-100%.
When approximately 1 mg 14C-labelled Aroclor 1254/kg was applied to
the centre leaflet of the first trifoliate leaf of 18-day-old soybean
plants, only 6.7% was recovered from the plant after 12 days, 76% of
which was still present in the treated leaf (Weber & Mrozek, 1979).
Mrozek & Leidy (1981) transferred the marsh plant Spartina
alterniflora from the field into soil containing 1 mg Aroclor
1254/kg (dry weight) and harvested the plants after a growth period of
90 days. The plants were found to take up selectively the lesser
chlorinated biphenyls. The authors stated that a further shifting of
the chromatographic pattern of PCBs towards the lesser chlorinated
components in aerial tissues suggested that some alteration of the PCB
mixture occurs in the plant. Mrozek et al. (1982) also found that
Spartina accumulates PCBs from both contaminated sand and mud-soil
systems. The total 14C-radioactivity accumulated in plants grown in
sand systems was higher than that in plants grown in mud. The level of
radioactivity accumulated in the green parts of the plants was similar
in both soil systems.
Moza et al. (1976b) applied 76 mg/kg of 14C-labelled 2,5,4'-tri-
chlorobiphenyl or 133 mg/kg of labelled 2,4,6,2',4'-pentachloro-
biphenyl to the leaves of the marsh plant Veronica beccabunga. Six
weeks later, the total recovery from plant, water, and soil was 3.7
and 18.3%, respectively, 86 and 95% of which was recovered from the
plant. In an earlier study, Moza et al. (1974) applied 14C-labelled
2,2'-dichlorobiphenyl in water or soil to 2 higher water plant species
( Ranunculus fluitans and Callitriche sp.) at concentrations of
13.7 and 14.5 mg/kg, respectively. Four weeks after application, the
results showed that the dichlorobiphenyl was metabolized more readily
after addition to water; the authors suggested the involvement of
aquatic bacteria. When applied in soil, 1.2% of the dichlorobiphenyl
was metabolized. This was contributed to the plant.
Moza et al. (1979b) grew 3-year-old spruce trees (Picea abies) in
soil containing 14C-labelled PCBs at approximately 4.2 mg/litre in
sewage sludge. When analysed 4 years later, only 0.8% (0.5% in
needles, 0.3% in stems) of the applied radioactivity was found in the
trees. Leaching of radioactive substances from the soil was less than
0.1% in the first 2 years and undetectable for the remainder of the
study.
In another study, Fries & Marrow (1981) grew soybean (Glycine max)
in pots, to determine residue contamination in plant tops from
14C-labelled 2,5,2'-trichlorobiphenyl, 2,5,2',5'-tetrachlorobiphenyl
or 2,4,5,2',5'-pentachlorobiphenyl, applied to the surface or
subsurface soil. Each compound was added to the soil at a rate of
2-3 mg/kg and the plants were harvested after a period of 52 days.
Detectable residues were only found in plants grown in surface-treated
soil, and concentrations in the plants increased with increasing
chlorination. Little of the labelled PCBs was lost from
subsurface-treated soil, but 20-30% of the surface-treated PCBs were
lost through volatilization. The authors concluded that the PCB
residues in the plant tops were, therefore, due to foliar
contamination from vapour rather than the uptake from the soil via the
roots. Miyazaki et al. (1975) came to the same conclusion when they
found no absorption or translocation of PCBs in sesame or rice seeds,
following the application of 4 types of Kanechlor (KC300, 400, 500,
and 600) at rates of between 0.1 and 100 mg/kg. But the rice straws
contained PCB levels of 0.02-0.08 mg/kg, which were the same as levels
found in plants from untreated soils.
Beets ( Beta vulgaris L.), turnips ( Brassica rapa L.), and beans
( Phaseolus vulgaris L.) were grown (Sawhney & Hankin, 1984) in soil
to which lake sediment contaminated with PCBs had been added. The
plants were exposed to Aroclor 1248 at a concentration of 80 µg/kg,
Aroclor 1254 at 1880 µg/kg, and Aroclor 1260 at 14 440 µg/kg. When
beets and turnips were grown in the soil for 6 months, the plants
showed greater uptake in the leaves than in the roots. For example,
beet roots contained 15, 16, and 35 µg/kg of Aroclors 1248, 1254, and
1260, respectively, while beet leaves contained 22, 94, and 52 µg/kg,
respectively. Total concentrations of the 3 Aroclors in beet roots and
leaves and in turnip roots and leaves were 66 and 168 µg/kg,
respectively, and 66 and 99 µg/kg, respectively. During a second
growing season, turnips and beans were grown for 6 months without any
additional PCB-contaminated sediment. Comparing PCB levels in turnips
between the 2 growing seasons showed a decrease in Aroclor 1248 uptake
relative to Aroclors 1254 and 1260. This was primarily because of a
large reduction in the amount of Aroclor 1248 in the soil after 1
year, due to degradation and volatilization. In beans, higher PCB
levels were found in the leaves and pods than in the stems and seeds.
Ten sludge application sites were selected within the Ontario area to
determine background heavy metal and PCB concentrations in the soils
and crops. Control sites (without sludge application) were adjacent to
the sludge application sites. Grab samples of liquid sludges applied
at each of the sites were taken for analysis. The soil samples were
taken at a depth of 15 cm. Twenty core samples were taken at 20-m
intervals and combined to form 1 sample. Eight of the application
sites were cropped with corn, one with oats, and one was left without
a crop. At the control sites, 7 were cropped with corn, 1 with oats,
and 2 left without a crop. PCB concentrations in the sludges ranged
from 0.13 to 1.61 mg/kg dry solids. PCB concentrations were in the
range of 0.007-0.025 mg/kg in the soil without sludges, and in the
range of 0.018-0.453 mg/kg air-dry weight in the soil with sludges.
The PCB levels in the crops were close to the control values (Webber
et al., 1983).
Bacci & Gaggi (1985) assessed the influence of translocation on the
concentrations of PCBs in the foliage of different plant species.
Beans, broad beans, tomatoes, and cucumbers were grown, either in soil
with a nominal added concentration of 500 mg/kg Fenclor 64 (similar to
Aroclor 1260), or in clean sand, for 28 days, enclosed in a glass box
with a constant turnover of air. The plants grown in clean sands were
exposed to PCBs by volatilization from other pots containing PCBs,
which were in the same growing box. The PCB peak pattern of both sand
and roots was similar to that of Fenclor 64, whereas the peak pattern
for foliage and air had moved towards lesser chlorinated congeners.
The concentrations of PCBs in the roots of tomatoes grown in
contaminated soil ranged from 105 to 168 mg/kg dry weight. But
translocation through the plants does not seem to be very likely since
there was no significant difference in foliage uptake of PCBs between
plants grown in contaminated soil and plants grown in clean soil.
Foliar uptake ranged from 13.8 to 42.6 mg PCB/kg (dry weight) for the
different species in PCB-fortified soil and from 11.8 to 47.1 mg/kg
for plants grown in clean soil.
4.2.4.3 Aquatic invertebrates
Bioconcentration factors are high for PCBs taken up by aquatic
invertebrates exposed to either pure chlorinated biphenyl isomers or
commercial mixtures in the water. Since PCBs are strongly bound to
sediments, this method of exposure is unrealistic. Addition of
sediment to test tanks decreases the uptake of PCBs, particularly by
organisms living in the upper water. However, there is clear evidence
that PCBs can also be readily absorbed into invertebrates from both
sediment and food. For organisms living on or in, sediment, uptake can
take place from the sediment, via food organisms that have absorbed
the PCBs, and from interstitial water or water immediately above the
sediment layer. A high content of organic matter in sediment decreases
the availability of PCBs for organisms. Uptake is rapid in most cases
and equilibrium is often reached in hours, though it may take weeks in
other examples. Uptake increases with increasing temperature. The
route of uptake is often via the gills, but varies among species. Loss
of PCBs is slow, but residues do decrease on cessation of exposure.
PCB uptake by aquatic invertebrates is transferred to predators and
can also be transferred to the terrestrial environment.
(a) Uptake from water
Vreeland (1974) exposed American oysters (Crassostrea virginica) to
various PCB isomers at concentrations of 5.5, 17, or 60 ng/litre
(which is within the range found in coastal waters) for 65 days.
Equilibrium was reached after approximately 1 month of exposure, with
concentration factors ranging from 1200 to 48 000 for PCB isomers with
2-6 chlorine atoms/molecule. The PCB concentration, after equilibrium
had been reached, was directly proportional to the amount of PCBs
added to the water. Lowe et al. (1972) found a linear pattern of
uptake in young American oysters exposed to Aroclor 1254 at 5 µg/litre
for 24 weeks, followed by a further 32 weeks in clean water. The
oysters already contained 17 mg/kg from a previous exposure and, by
the end of the 24-week exposure period, had accumulated 425 mg/kg (a
steady state was not established). By the end of the 32-week period in
clean water, no PCB residues could be detected. In another study on
uncontaminated young oysters, concentration factors of up to 101 000
were achieved after a 25-week exposure to 1 µg Aroclor 1254/litre.
After 12 weeks in clean water, whole-body residues were reduced to
0.2 mg/kg.
Courtney & Denton (1976) fed hard clams (Mercenaria mercenaria)
Aroclor 1254 adsorbed on the surface of alumina particles, at 1.25 and
12.5 µg/litre, for 21 days. The maximum concentration factor was 1800
for visceral mass, when the clams had been exposed to 1.25 µg/litre
for 18 days. The visceral mass accumulated a 1.4-5.3 times greater
concentration of PCBs per unit time than the muscular foot. Tissue
samples contained relatively more lower chlorinated isomers than the
Aroclor 1254 standard and, faeces and mud samples contained more
higher chlorinated isomers. Following exposure, clams from the lowest
dose group showed little change in PCB content after 3 months in clean
seawater. However, at the higher dose level, there was a significant
reduction in the PCB levels found in the foot after 1 month, but PCB
residues in the visceral mass remained unchanged for 6 months.
Pink shrimp (Penaeus duorarum) were exposed to Aroclor 1254 at a
concentration of 2.5 µg/litre, in flowing water, for 22 days (Nimmo et
al., 1971b). Accumulation was linear for the hepatopancreas and whole
body, but a plateau was reached after 2 days in muscle. Residues in
the hepatopancreas reached 510 mg/kg over the exposure period,
representing a concentration factor of 204 000; over the same period,
50% of the shrimps died. In a separate study, the shrimps were exposed
to 7.5 µg/litre for 16 days followed by an elimination period of 5
weeks in clean water. When calculated on the basis of the total tissue
burden of PCBs, an 80% reduction in the hepatopancreas was found,
concomitant with a doubling of the PCB levels in remaining tissues.
However, when data were presented as a concentration, a linear loss
from the hepatopancreas was seen, with the concentration in other
tissues remaining constant. The authors calculated a half-life for
loss of PCBs from the hepatopancreas of 17 days.
Nimmo et al. (1975) sampled shrimp from Pensacola estuary, USA, and
measured the relative concentration of PCBs in the various tissues.
The hepatopancreas contained the greatest amounts (50-75%) followed by
the ventral nerve. The authors studied the uptake of PCBs by pink
shrimp, experimentally, using various regimes with dosed food or dosed
water. They found the same tissue distribution in pink shrimp that had
been exposed to 0.2 µg Aroclor 1254/litre, in water, for 50 days. They
concluded that most of the PCBs were taken up directly from the water
in both the "wild" and laboratory situation. However, they did not
exclude the possibility of some PCBs being taken up from food, which
was found under some of the laboratory regimes.
To determine whether there was a concentration below which shrimps
would be unable to accumulate PCBs, grass shrimp (Palaemonetes pugio)
were exposed to flowing water concentrations of 0.04, 0.09, or
0.62 µg/litre. Whole-body residues of 0.2, 1.0, and 10 mg/kg,
respectively, were accumulated within 3-5 weeks. Even at the lowest
dose, shrimps accumulated more PCBs than the residues found in control
shrimp. Concentrations in the shrimp did not reach equilibrium during
the 5-week exposure, but the rate of accumulation decreased towards
the end of the exposure. When transferred to clean water, the shrimps
lost most of the PCBs within 4 weeks (Nimmo et al., 1975).
Gammarus oceanus were exposed by Wildish & Zitko (1971) to Aroclor
1254 concentrations of 2.5, 10, or 20 mg/litre for up to 6 h. Uptake
increased with increasing PCB concentration. Uptake decreased to half
of the initial rate after 4-6 h exposure at 20 mg/litre. There was
little or no uptake by dead animals. Although uptake was related to
branchial surface area, branchiae were not necessary sites of uptake,
since uptake could occur at an unchanged rate following branchial
removal. The authors did not find any change in the rate of uptake
during the intermoult stage.
Zhang et al. (1983) exposed Daphnia magna to 14C-labelled
2,2'-dichlorobiphenyl, 2,5,4'-trichlorobiphenyl, 2,4,6,2'-tetra-
chlorobiphenyl, or 2,4,6,2',4'-pentachlorobiphenyl at 50 µg/litre.
Equilibrium was reached after 20 h for all except the pentachloro-
biphenyl, which had not reached equilibrium within 24 h.
Bioaccumulation factors at equilibrium ranged from 3741, for the
dichlorobiphenyl, to 18 144, for the trichlorobiphenyl. Concentration
factors were not significantly related to the water solubility or
chlorine content of the biphenyl, but there was a tendency for the
bioaccumulation factor to increase with chlorine content and
decreasing water solubility. The authors studied the rate of
depuration and found it to increase with increasing water temperature
between 2 and 22°C. The rate of depuration was also faster for the
dichlorobiphenyl than for the pentachlorobiphenyl; after 48 h, the
amount of PCBs remaining in Daphnia was 22% and 77% (at 10-11°C) for
the 2 chlorobiphenyls, respectively.
(b) Uptake from sediment
Sediment was collected from the field and spiked with Phenochlor DP-5
to achieve a final PCB concentration of 0.65 mg/kg dry weight,
compared with 0.2 mg/kg in unspiked sediment (Elder et al., 1979).
Worms (Nereis diversicolor) were then added to aquaria containing
the sediment under flowing seawater. Equilibrium was reached within
40-60 days, by which time both groups had concentrated the PCBs by 3.5
times. Upon transfer from spiked to unspiked sediment, the worms took
2 months to attain body levels of PCBs comparable with those of the
unspiked group. A half-life of approximately 27 days was calculated
for incorporated PCBs.
Fowler et al. (1978) exposed Nereis diversicolor to spiked sediment
containing 9.3 or 80 mg Phenochlor DP-5/kg (dry weight), for 120 days,
compared with 0.11 mg PCB/kg in unspiked sediment. At the beginning of
the study, worms in the unspiked sediment had body residues of
0.59 mg/kg dry weight and reached a steady state at 1.2 mg/kg. Those
exposed to spiked sediment reached a steady state after a period of
approximately 2 months, with concentration factors ranging from 3 to
4. The worms maintained at the highest level of PCBs all died within a
90-day exposure period. When transferred to unspiked sediment for a
2-month period, the worms that had taken up PCBs from the unspiked
sediment lost PCBs exponentially. In a separate study, worms were
exposed to PCBs in water alone at a concentration of 0.57 µg/litre. A
steady state was reached much more quickly (2 weeks) than it was in
the presence of sediment, with a concentration factor of approximately
800. By comparing these results with field monitoring, the authors
calculated the relative importance of the 2 media. They stated that
approximately 99% of the PCBs in these studies was taken up from the
sediment. When the water overlying the spiked sediment was monitored,
28 ng PCBs/litre was measured (not leached, but a contaminant in the
experimental system) reducing the figure of uptake from sediment to
89%.
In a study by Courtney & Langston (1978), 1.1 mg Aroclor 1254/kg was
incorporated into intertidal sand. Specimens of 2 intertidal
polychaetes (Arenicola marina and Nereis diversicolor) containing
mean residues of 0.017 and 0.11 mg PCBs/kg (wet weight), respectively,
were collected. After 5 days in the spiked sediment, they contained
0.24 and 0.36 mg/kg, and, after a further 5 days, 0.39 and 0.49 mg/kg,
respectively. During a 3-week post-exposure period, there was no
significant loss of these PCB residues. The authors achieved
comparable PCB residues in these polychaetes after exposure to
1 µg/litre water or 1 mg/kg sediment.
McLeese et al. (1980) exposed the polychaete worm (Nereis virens)
and the shrimp (Crangon septemspinosa) to sediment containing
0.016-0.58 mg Aroclor 1254/kg (dry weight) for 32 days. Uptake was
found to be dependent on the exposure concentration and, in the case
of the worms, on the exposure period. The accumulation of PCBs was
inversely related to animal size; at 32 days, concentration factors
for worms ranged from 10.8 for 0.6-g worms to 3.8 for 4.7-g worms
following exposure to 0.17 mg PCB/kg. Factors of 3.5 and 1.9 were
found for shrimps weighing 0.1 and 2.9 g, respectively, after exposure
to 0.13 mg Aroclor/kg. Shrimps were found to accumulate, on average,
60% less PCBs than worms per unit weight. During the 26 days following
exposure, there was not any obvious loss of PCBs from the worms.
Rubinstein et al. (1983) collected sediments containing various levels
of pollutants (PCBs, 0.46-7.28 mg/kg dry weight; Cd; Hg) and organic
matter (5.5-22.3%). During a 100-day exposure period, only small
increases in PCB concentrations were detected in hard clam
(Mercenaria mercenaria) and grass shrimp (Palaemonetes pugio).
Higher concentrations of PCBs were accumulated by Nereis virens.
Uptake was found to be more dependent on the organic content of the
sediment than on the exposure concentration. Concentration factors
ranged from 1.59 in a low organic sediment to 0.15 in a high organic
sediment. The authors also calculated the maximum water exposure
concentration eluted from each of the sediments. On the basis of a
concentration factor of 800, calculated by Fowler et al. (1978) for
the uptake from water of Nereis sp., body residues of between 0.007
and 0.034 mg PCBs/kg (wet weight) would have been expected if
accumulation were dependent purely on direct partitioning from water.
However, whole-body residues of PCBs were found to be 0.4-0.63 mg/kg,
suggesting that pathways other than direct uptake from water (e.g.,
ingestion and sorption) contributed significantly to the accumulation
of PCBs by the polychaete.
Freshwater prawns (Macrobrachium rosenbergii) and clams (Corbicula
fluminea) were exposed to contaminated sediments for 48-50 days
(Tatem, 1986). Prawns were exposed to sediment containing
approximately 62 mg PCBs/kg (dry weight) and to the same sediment
diluted with sand to 50 and 10% of the original. Clams were exposed to
100, 50, or 10% of another sediment containing approximately 2 mg
PCBs/kg at 100%. The amount of PCBs accumulated was related to the
exposure concentration, with the highest concentration factors at the
lowest exposure (10%) level. Bioaccumulation factors for prawns ranged
from 0.1 to 0.9 for Aroclor 1242 and from 0.2 to 2.4 for Aroclor 1254,
relative to sediment concentrations. Exposed clams accumulated PCBs
(Aroclors 1242 and 1254) at concentration factors of 0.54-12.52,
relative to sediment. When tissues were analysed for Aroclor 1242 and
1254, maximum concentrations in prawns were attained at 7 and 40 days
for the 2 Aroclors, respectively. Exposure of prawns at 100 and 50%
dilution of sediment killed all the animals after 62 and 70 days,
respectively. Clams survived exposure.
Clark et al. (1986) investigated the accumulation of sediment-bound
PCBs by fiddler crabs (Uca pugilator) and (Uca minax). Mud and
mud/sand sediments were used; both were naturally contaminated with
PCBs and no further PCBs were added. Both species were exposed to a
mud sediment containing 1.04 mg PCBs/kg and to a mud/sand sediment
containing 0.37 mg/kg (dry weight). Concentration factors, after a
28-day exposure, were 0.19 and 0.79, for U. minax, and 0.2 and 0.59,
for U. pugilator, for the 2 sediments, respectively. In a second
study, using mud with 0.97 mg PCBs/kg and mud/sand with 0.55 mg
PCBs/kg, U. pugilator showed concentration factors of 0.58 and 0.71,
respectively, after 28 days. The authors did not find any detectable
PCBs in the overlying water, suggesting that the PCBs are tightly
bound to the sediment and leach out only very slowly. Following
transfer to uncontaminated sediment on day 42, no PCB residues were
detected in U. pugilator on day 56, or in U. minax on day 63.
Lynch & Johnson (1982) exposed the amphipod (Gammarus pseudolimnaeus)
to 2,4,5,2',4',5'-hexachlorobiphenyl added to sediment in flow-through
bioassays. Water overflowing from the tank containing the contaminated
sediment was directed into a second tank where further amphipods were
exposed without sediment. The hexachlorobiphenyl was labelled with
14C and added to the sediment at 1 mg/kg; the system was allowed to
equilibrate for 7-15 days prior to addition of amphipods, which were
sampled from the tanks after 24, 48, 96, and 192 h. In the initial
studies, the specific activity of the labelled hexachlorobiphenyl was
insufficient to detect the hexachlorobiphenyl concentrations in water.
However, it was clear that amphipods in the tank with the sediment
accumulated more hexachlorobiphenyl than animals exposed only to the
water overflow (8.8-10.5 times more PCBs). Removal of organic matter
from the sediment, by combustion, before addition of the PCB,
increased uptake of the hexachlorobiphenyl by increasing the
availability of the material to the Gammarus. In later studies,
specific activity was increased and water concentrations could be
measured. These were very low, ranging between 11 and 35 ng/litre in
the upper tank and 9 and 25 ng/litre in the lower tank. The lower end
of this range was found later in the exposure period suggesting that
less hexachlorobiphenyl was released over time. There was little
difference in concentration between water taken from the surface and
that sampled close to the sediment suggesting rapid mixing of the
overlying water. In this later series of studies, the authors
demonstrated that both the organic matter content of the sediment and
the presence of smaller particle sizes (silt and clay) reduced uptake
of hexachlorobiphenyl by the amphipods. Organic matter was the more
important factor. Adding maple leaves, to give about 70% organic
content in the sediment, reduced hexachlorobiphenyl uptake to between
10 and 20% of that in sediment without organic matter. Very high
bioconcentration factors were calculated relative to the very low
water concentrations of hexachlorobiphenyl (ranging between 27 000 and
1 000 000 in the upper tank and 2000 and 460 000 in the lower tank,
increasing with exposure period). These factors would be very low
relative to sediment concentrations of the PCB. However, it is clear
that the amphipod can accumulate hexachlorobiphenyl, leaching in very
small amounts from contaminated sediment.
Cores of lake sediment complete with overlying water were taken by
Larsson (1984) and transported back to the laboratory, still in the
sampling tube. PCBs were introduced at different dose levels by
injection through silicon septa in the walls of the tubes and spread
evenly 10 mm below the surface. The cores were allowed to stabilize in
the dark for 1 week at which time 80-100 chironomid larvae were
introduced. After 8 weeks, the systems were moved and kept at 20°C in
the light. After 2 days, the chironomid larvae began to pupate and
emerge. The study was terminated after 10 weeks. PCBs were measured in
sediment, larvae, adults, and exuviae (discarded skins after
emergence). Ranges of PCBs in sediment were between 0.5 and 14 mg/kg
giving rise to residues in larvae, exuviae, and adults directly
related to sediment concentrations. There was "biomagnification"
between larvae and adult. There was loss of body weight between the
final larval stage and the adult, but little loss of PCBs (only 17%
was retained in the exuviae). The author stated that the low variation
in uptake between animals is an indication of passive physicochemical
factors being involved in the handling of PCBs by chironomids. Active
uptake via ingestion would be expected to lead to more variation in
results. Meier & Rediske (1984) also monitored the uptake of PCBs from
contaminated sediment into chironomid larvae (Glyptotendipes
barbipes). Concentration factors for Aroclor 1242 from sediment
ranged between 20 and 130 for exposures of between 0.01 and 1.0 mg/kg,
considerably lower than concentration factors relative to water
(10 000 for these organisms) (Sanders & Chandler, 1972). Addition of
oil, commonly found in polluted areas where PCBs spills are likely,
reduced the uptake of PCBs from the sediment.
(c) Uptake from food
A detritus diet containing 17 µg Aroclor 1242/kg (wet weight) was fed
to male fiddler crabs (Uca pugnax) for 34 days (Marinucci & Bartha,
1982). The Spartina detritus was placed in the culture system at the
start of the study and, because of rapid depletion, was renewed after
19 days of exposure. Since PCBs leached continually from the food
source into the water, a second study was carried out to examine the
uptake of PCBs from water alone. Contaminated detritus was mixed
thoroughly with water and allowed to equilibrate for 24 h. Water
levels were found to be 14-15 µg/litre. Aroclor 1242 was accumulated
at a more rapid rate from PCB-laden detritus than from water alone.
The linear accumulation rate from litter was calculated to be 1 µg
PCBs/day per animal whereas, from water alone, the uptake was 0.1 µg
PCBs/day per animal. Aroclor 1242 was highly concentrated in the
hepatopancreatic tissue. It was found that the PCB residue in the
crabs was inversely related to their weight. Comparison of the
concentrations of PCBs in animals of the same weight shows that, at
the end of the 34 day exposure, those exposed to water alone had taken
up approximately half of the PCBs of those exposed to detritus. The
authors concluded that the crabs in the study accumulated a similar
amount of PCBs from both the food and the water.
Pinkney et al. (1985) exposed the amphipod Gammarus tigrinus to
Aroclor 1254 (14C-labelled) in fungus (Fusarium oxysporum) as a
food item. The fungus contained 195.8 mg Aroclor/kg dry weight.
Accumulation of PCBs was rapid, reaching a constant level in the
amphipods of 23 mg/kg after 9-24 h. Similar exposure of the amphipods,
but with exclusion from direct contact with the fungus by Teflon mesh
(to monitor uptake of PCBs leached into the water), resulted in
residues of between 0.16 and 3.3 mg/kg (from concentrations in the
water at 0.03 µg/litre), representing between 0.6 and 13.9% of uptake
from water and food combined. The PCB residues in the amphipods were
also monitored over 144 h on uncontaminated food to measure the
elimination rate. The water was changed every 24 h. Within this
period, 57% of the accumulated PCBs was eliminated.
(d) Comparison of different routes of uptake
In a study by Wyman & O'Connors (1980), the uptake by the marine
copepods Acartia tonsa and Acartia clausi of 14C-labelled Aroclor
1254 from water, inorganic sediment, and food, was monitored over a
period of 48 h. Acartia were exposed to water concentrations of
10 µg PCBs/litre. An asymptotic uptake curve was observed; equilibrium
was reached after 36 h, corresponding to whole-body residues of 248 mg
PCBs/kg (dry weight) for A. tonsa and 223 mg/kg for A. clausi.
During exposure, water concentrations fell rapidly to 5 or 6 µg/litre.
A similar pattern of uptake was found after exposure to sediment
contaminated with 20 mg PCBs/kg with maximum levels of PCBs in
A. tonsa of 22 mg/kg after 30 h. As in the water exposure, levels of
PCBs in sediment fell rapidly from 20 mg/kg to 14 mg/kg and then
slowly to 7 mg/kg at the end of the study. Water levels were initially
0.62 µg/litre and fell to 0.15 µg/litre. Uptake of PCBs by A. tonsa
from phytoplankton contaminated with 80 mg PCBs/kg (wet weight) was
very rapid and reached a maximum after 5 h at 61 mg/kg, but
subsequently declined after exhaustion of the food supply. PCB
concentrations in water were similar to those found when copepods were
exposed to contaminated sediment, copepods exposed to these water
concentrations alone accumulated significantly less PCBs than those
fed PCB-dosed phytoplankton.
McManus et al. (1983) exposed the marine copepod Acartia tonsa to
14C-Aroclor 1254 either in the food, as phytoplankton containing
approximately 1.3 mg PCBs, or in water at 1.5 µg/litre, for a period
of 30 h. For copepods exposed to contaminated phytoplankton, PCB
levels ranged from 117 to 163 mg/kg dry weight. For copepods exposed
to contaminated water alone, levels ranged from 82 to 104 mg/kg. When
transferred to clean water, the authors found that copepods lost PCBs
at a significantly faster rate if they were fed during depuration;
after 36 h, PCB concentrations in copepods fed during deputation were
10 mg/copepod whereas those starved contained 30 mg/copepod. No
significant difference in depuration rate was found between those
exposed via food and those exposed via water. In a second study,
elimination in males and females was compared. Although both sexes
contained similar residues at the start of depuration (117 mg/kg and
95 mg/kg, respectively), after 36 h, females contained significantly
lower levels of PCBs than males. During depuration, faecal pellets and
eggs were analysed; similar levels of PCBs were found in both male and
female faecal pellets during this period, but levels of PCBs more than
four times that in the females were found in eggs (407.5 mg/kg dry
weight after 4 h), indicating that egg production is an important
route for PCB elimination.
4.2.4.4 Fish
Fish of all life stages have been shown to take up PCBs readily from
water; bioconcentration factors are high. Time taken to reach
equilibrium is variable, but often long, in excess of 100 days. PCBs
with greater chlorination are more readily taken up and retained. PCB
body burden tends to increase with age and levels are higher in fish
with a greater lipid content. The accumulated PCBs are concentrated in
lipid-rich tissues. PCBs of lower chlorination are eliminated more
rapidly. Loss of PCBs is evident when exposure ends; an initial rapid
loss is followed by a slower rate of loss. Half-life estimates,
therefore, vary greatly, from a few weeks to several years.
Reproduction, with the production of a large mass of eggs or sperm,
allows loss of substantial amounts of the PCB residue. Depending on
the species, habitat, and behaviour, PCBs can be taken up from water,
sediment, or food to different degrees.
(a) Uptake from water
Califano et al. (1980) maintained larval striped bass (Morone
saxatilis) in Hudson river water (filtered and unfiltered)
contaminated with 14C-Aroclor 1254 at 1.36 µg/litre for a period of
48 h. Whole-body residues for filtered and unfiltered water were not
significantly different at 5 mg/kg and 5.9 mg/kg, respectively. Uptake
between 34 and 48 h was very slow, suggesting a steady state had
already been reached. Exposure of fish for a further 72 h in
unfiltered water, supported this theory. Elimination was slow, only
18% being lost in 48 h following a 24-h exposure.
The PCB uptake pattern in lake trout (Salvelinus namaycush) sac fry
was studied by Mac & Seelye (1981) by exposing them to a nominal
concentration of 50 ng Aroclor 1254/litre for 48 days. Patterns of
accumulation were similar, regardless of how the data were expressed
(wet weight, dry weight, or body burden). PCBs levels increased
slowly, reaching a peak after 32 days (just before completion of yolk
absorption), and then decreased by day 48.
Hansen et al. (1975) exposed different life-stages of sheepshead
minnow (Cyprinodon variegatus) to Aroclor 1016 (Table 10). After a
4-week exposure to nominal concentrations of 1, 3.2, or 10 µg/litre,
adult fish laid eggs containing on average 4.2, 17, and 66 mg/kg,
respectively. DeFoe et al. (1978) exposed fathead minnow (Pimephales
promelas) to Aroclor 1248 or 1260 at concentrations of
0.1-3 µg/litre, for 240 days (life cycle). Bioconcentration factors for
the uptake of PCBs were independent of the PCB concentration in the
water. Residues in the fish reached an apparent steady state within
about 100 days of exposure and growth. Females accumulated about twice
as much PCBs as males, because of their higher body lipid content. The
variability of residues in females reflected the variability of their
lipid content. Although mechanisms for uptake were similar for both
Aroclors, greater body burdens were always achieved with exposure to
Aroclor 1260. Bioconcentration factors ranged from 60 000 to 160 000
for males and from 120 000 to 270 000 for females. After transfer to
clean water, 18% of Aroclor 1248 was lost within 28 days and 15% of
Aroclor 1260 in 42 days. The authors stated that, because of
variations between fish, this 10-20% decline in total body burden of
PCBs was insufficient to indicate definite PCB elimination over this
period.
De Kock & Lord (1988) exposed an estuarine fish, the Cape stumpnose
(Rhabdosargus holubi) to a flowing water concentration of 1 µg
Aroclor 1260/litre for 90 days followed by a 90-day period in clean
water. Equilibrium was reached at 90 days with a concentration factor
of 24 000. The depuration rate was calculated to be 0.014 days,
producing a half-life of 50 days.
Goldfish (Carassius auratus) were exposed to Clophen A50 at levels
of 0.01, 0.05, 0.1, or 0.5 mg/litre for 18 days (Hattula & Karlog,
1973). Rapid uptake was observed with concentration factors of over
1000 at 18 days, but equilibrium was not achieved within this period.
Nearly all the fish exposed to 0.5 mg/litre died within 7 days. After
transfer to clean water, fish that had been exposed to 0.1 mg/litre
for 13 days and had attained body residues of 70 mg/kg lost half of
the PCBs within 3 weeks, but still retained levels of approximately
15 mg/kg, after 70 days.
Yoshida et al. (1973) exposed carp (Cyprinus carpio) to 14C-PCBs
(equivalent to Aroclor 1254) in water or in food. By measuring the
radioactivity, they found similar tissue patterns of uptake from both
water and diet. PCBs were localized in the gall bladder, adipose
tissue, and hepatopancreas and, in particular, the adipose tissue of
the skull.
Hansen et al. (1971) exposed spot (Leiostomus xanthurus) to Aroclor
1254 at 1 µg/litre, for 56 days. Maximum tissue levels of PCBs were
achieved between days 14 and 28. Highest levels were found in the
liver (210 mg/kg, after 28 days) followed by the gills, whole fish,
heart, brain, and muscle. Aroclor 1254 was slowly lost from tissues;
after 84 days in clean water, levels of PCBs had dropped by 73%.
In a study by Braun & Meyhofer (1977), rainbow trout (Salmo
gairdneri) fingerlings were exposed to water concentrations of 2 or
20 µg Clophen C/litre, for 8 weeks. Tissue PCB concentrations for
gills, muscle, and liver were found to be 0.62, 0.82, and 3.47 mg/kg,
respectively, for the lower dose and 12.3, 7.6, and 10.6 mg/kg, for
the higher dose. When fish were held in clean water for 10 weeks,
following exposure to 2 µg/litre for 8 weeks, residues decreased by
half in the liver and had disappeared completely from the gills, but
there was no change in the PCB levels in muscle.
Rainbow trout (Salmo gairdneri) were exposed by Guiney et al. (1977)
to 14C-labelled 2,5,2',5'-tetrachlorobiphenyl at 0.5 mg/litre for
36 h. The tissue distribution of 14C was measured at regular
intervals after transfer to clean water. Carcase, muscle, skin, lower
gastrointestinal tract, and fat contained most of the radioactivity
(88%). During the first 14 days after exposure, radioactivity
increased in adipose tissue, carcase, and eyes. Elimination from most
tissues appeared to be biphasic with a 30% loss within 2 weeks
followed by a loss of only 6% in the following 126 days. Losses from
the bile and blood were very rapid and nearly complete within 14 days.
Based on the initial rate of loss, the authors calculated a half-life
of 1.55 days, however, the second phase of eliminated PCBs suggested a
half-life at 2.66 years. In a similar study, Guiney et al. (1979)
calculated half-lives of 1.76 and 1.43 years for female and male
rainbow trout, respectively, based on fish sampled 2-34 weeks after
exposure. For both sexes the half-life of elimination was recalculated
to 0.52 and 0.54 years between weeks 38 and 52 after exposure (the
spawning season). The increased elimination appeared to be because of
loss via eggs and sperm. Vodicnik & Peterson (1985) found a similar
result after dosing yellow perch (Perca flavescens); an elimination
half-life of 22 weeks was calculated. This was later recalculated to
be <0.7 weeks during spawning, returning to 16.3 weeks after the
completion of spawning.
(b) Uptake from sediment
The uptake of Aroclor 1254 from suspended solids by juvenile Atlantic
salmon (Salmo salar) was studied by Zitko (1974). Aroclor 1254 was
mixed with suspended solids (simulated by SilicAR CC7) in hexane at
5 mg/ml. Fish were exposed to contaminated solids at 1 g/litre for up
to 144 days. Over this exposure period, the salmon accumulated 134 mg
Aroclor 1254/kg.
Stein et al. (1984) exposed English sole (Parophrys vetulus) to a
sediment concentration of 1 mg 14C-Aroclor 1254/kg (dry weight).
Seawater was allowed to flow over the sediment for 6 days before the
fish were added. A steady state of PCBs accumulated in the tissues of
the fish was achieved after 10 days of exposure. Highest residue
concentrations were found in the bile and the liver. Concentration
factors were 10 for the bile and 4 for the liver, with other tissues
individually concentrating PCBs by factors of 3 or less. Simultaneous
exposure of sole to PCBs and 3H-benzo[ a]pyrene (3 mg/kg, dry
weight) reduced the amount of PCBs accumulated. Stein et al. (1987)
collected urban sediment containing aromatic hydrocarbons and PCBs at
32 mg/kg and 2.2 mg/kg dry weight, respectively. English sole
accumulated hepatic concentrations of 1.4 mg PCBs/kg (wet weight) over
a period of 108 days exposure to the urban sediment. This was 8 times
the PCBs accumulated by sole exposed to the control sediment, which
did not contain any detectable PCBs. In another study, the same
authors added a 14C-labelled PCBs tracer to the urban sediment. The
concentration of PCB-derived radioactivity in the liver reached a
steady state after 14 days of exposure; the steady state concentration
in the carcase was found to be significantly lower.
(c) Uptake from food
Lieb et al. (1974) fed rainbow trout Salmo gairdneri on a diet
containing 15 mg Aroclor 1254/kg for 16 or 32 weeks. PCB levels in the
lipid fraction increased rapidly for the first 8 weeks, reaching
equilibrium at about 95 mg/kg. The absolute quantity of PCBs continued
to increase as the fish grew. The trout had retained 68% of the total
PCBs ingested at equilibrium. No elimination was found after transfer
to uncontaminated food at 16 weeks (for a period of 16 weeks), or
after starving the fish for 8 weeks following exposure for 32 weeks.
Reductions in PCB levels were found, but these were cancelled out by
concomitant reductions in lipid content.
Coho salmon (Oncorhynchus kisutch) parr were fed 10 mg chloro-
biphenyls/kg (containing equal parts by weight of 3,4,3',4'-tetra-
chlorobiphenyl, 2,4,5,2',4',5'-hexachlorobiphenyl, and
2,4,6,2',4',6'-hexachlorobiphenyl) for up to 165 days (Gruger et al.,
1975). Most of the PCBs were accumulated in the adipose tissue of the
salmon (51.1 mg/kg total chlorobiphenyls after 165 days). Tissue
levels of tetrachlorobiphenyl were about half those of either of the
two hexachlorobiphenyls throughout the exposure period. When fish were
starved for 48 days, the data indicate mobilization or transformation,
with, for example, chlorobiphenyls in the spleens lowered by half and
in adipose tissue increased 5-fold. Most tissues showed an increase in
PCB levels, especially blood levels. In contrast, when a second group
of salmon were fed on a clean diet, chlorobiphenyls were released from
adipose tissue and levels increased in some other tissues, such as the
lateral line dark muscle tissue. The ratio of the different
chlorobiphenyls remained unchanged during both of these post-exposure
treatments. Gruger et al. (1976) fed juvenile coho salmon diets
containing a mixture of 2,5,2',5'-tetrachlorobiphenyl, 2,4,5,2',5'-
pentachlorobiphenyl, and 2,4,5,2',4',5'-hexachlorobiphenyl
at 1, 2, and 12 mg/kg, for up to 72 days. A steady state appeared to
have been reached between 17 and 35 days at the lowest dose (a whole
body concentration of approximately 0.45 µg/kg (wet weight)); steady
state was not achieved at the other 2 dose levels. All 3
chlorobiphenyls were accumulated to similar levels. Comparing these
data with the study by Gruger et al. (1975), suggests that the
position of the chlorine substitution is an important factor.
Hansen et al. (1976a) fed channel catfish (Ictalurus punctatus) on a
diet contaminated with 20 mg Aroclor 1242/kg. The total burden of PCBs
(µg PCB/fish) increased exponentially with exposure time. When fish
were placed on a clean diet (from day 84 for 56 days) a slight net
decrease in body burden was observed, but levels remained constant
when fish were placed on a clean diet for 56 days after 140 days
exposure. On return to a PCB-contaminated diet, accumulation rates
returned to those previously observed. The authors noted that, during
PCB-free periods, there was a shift in residues from edible carcase to
offal.
Mayer et al. (1977) fed fingerling coho salmon with Aroclor 1254 at
concentrations ranging between 1.45 and 14 500 µg/kg body weight.
Equilibrium was reached after 112 days at concentrations of 1.45,
14.5, and 145 µg/kg, with whole body residues of 0.47, 0.5, and
3.8 mg/kg, respectively. A steady state was reached at the 2 highest
dose levels of 1450 and 14 500 µg/kg after 200 days, with
corresponding residues of 57 and 659 mg/kg. In another study, channel
catfish (Ictalurus punctatus) were exposed to Aroclors 1232, 1248,
1254, and 1260 in the diet at concentrations of 48 or 480 µg/kg body
weight, for 193 days. Equilibrium was only achieved at the lowest
exposure dose of Aroclor 1232, within 150 days, with a whole-body
burden of 4.5 mg/kg. Similar whole-body residues were achieved at the
lowest dose of the other Aroclors, but no steady state was reached. At
the higher dose, accumulation increased in the order Aroclor 1232 =
1248 < 1254 < 1260, with residues ranging from 13 to 32 mg/kg after
193 days.
When Zitko (1974) fed juvenile Atlantic salmon (Salmo salar) diets
containing 10 or 100 mg Aroclor 1254/kg, accumulation reached
equilibrium within 30 days at the lower dose, with a whole-body
residue of approximately 3.8 mg/kg. Equilibrium was not reached within
200 days at 100 mg PCBs/kg. A whole-body residue of 30 mg/kg was
recorded at 181 days.
Zinck & Addison (1974) administered a mixture of 2-, 3-, and
4-chlorobiphenyl to thorny skate (Raja radiata) and winter skate
(Raja ocellata) by intravenous injection. All three congeners were
cleared rapidly from blood plasma, 3-chlorobiphenyl consistently being
cleared more rapidly than the other two. Less than 6% of
3-chlorobiphenyl remained in the plasma after 15 min compared with 30%
for the other chlorobiphenyls. All three accumulated in the other
tissues of R. radiata, principally in the liver and muscle. Tissue
levels of 3-chlorobiphenyl were consistently less than the others
during the 53-h sampling period.
In a study by Guiney & Peterson (1980), both yellow perch (a non-fatty
fish) and rainbow trout (a fatty fish) were dosed with 0.8 µg of
14C-labelled 2,5,2',5'-tetrachlorobiphenyl, either orally or by
intraperitoneal injection. Whole-body elimination was found to be
similar for both species and routes. A 20-30% elimination was observed
after 3-4 days with virtually no more PCBs being eliminated during the
rest of the 32-day sampling period. Tissue distribution varied between
the 2 species; uptake in the perch was mainly concentrated in the
viscera and carcase, whereas, in the trout, skeletal muscle and
carcase were the major sites of uptake.
Niimi & Oliver (1983) calculated the biological half-life of 31
dichloro- to decachlorobiphenyl congeners, 105 days after a single
oral dose of 46-261 mg/kg was administered to rainbow trout (Salmo
gairdneri). Whole-body half-lives increased from 5 days to >1000
days as the number of chlorines on the biphenyl increased. From
structure-activity analysis of half-lives in whole fish, the authors
concluded that elimination was enhanced for congeners with a lower
chlorine content and no chlorine substitutions in the ortho
positions, and for those with 2 unsubstituted carbons adjacent on the
biphenyl.
4.2.4.5 Birds
PCBs are taken up from contaminated food or water and concentrated in
the fatty tissues of birds. PCBs of higher chlorination levels are
accumulated to a greater extent. Egg-laying females can lose
substantial amounts of PCBs from body tissues by transferring the PCBs
to the eggs. Redistribution of residues occurs on starvation (of
significance during the migration of birds in the wild). Expressed as
a whole-body concentration, PCB residues fall during starvation.
However, expressed as a concentration in fat, residues rise. Most
critically, PCB residues in the brain increase during starvation and
this may kill the birds without further intake of PCBs.
Brunström et al. (1982a) injected the yolk of developing hens' eggs,
on day 4 of incubation, with 14C-labelled 2,4,2',5'-tetra-
chlorobiphenyl at a concentration of 5 mg/kg. One hour after
injection, radioactivity was found in the sub-blastodermic fluid, the
highest concentrations being in amniotic membranes. None was present
in the yolk, albumen, or embryonic tissues. Uptake was uniform
throughout the embryo, after one day, and, as tissues developed,
became concentrated in certain of them, such as the liver, kidney, and
fluid brain vesicles, by day 7. 14C was found uniformly in the yolk
after 11-14 days and was highly concentrated in the first bile
produced on day 11. The labelled PCBs accumulated in fatty tissue as
it developed from day 14 onwards. In the hatched chick, large amounts
of radioactivity were found to be concentrated in the gall bladder,
intestine, cloaca, and the coiling of the gizzard. When either
3,4,3',4'-tetrachlorobiphenyl or 2,4,2',5'-tetrachlorobiphenyl was
injected into the air sac of hens' eggs on day 14 of incubation at
0.4 mg/kg, no difference in distribution pattern was observed 1-5 days
later (Brunström & Darnerud, 1983). The highest amounts of
radioactivity were found in the fatty tissue, liver, kidneys, and the
gall bladder, 14C was also found in the bone marrow, the adrenals,
and the gonads, but to a lesser extent. The yolk contained less
radioactivity than the yolk analysed in the previous study by
Brunström et al. (1982a), because the PCBs were administered via the
air sac.
White leghorn hens were exposed to 50 mg Aroclor 1254/litre in their
water for 6 weeks (Tumasonis et al., 1973). PCB residues in the yolks
of eggs laid increased during the exposure period to a peak, after 6
weeks, of approximately 205 mg/kg. When hens were given clean water,
the yolk levels of PCBs quickly dropped within 5 weeks to
approximately 100 mg/kg, and then more slowly until, after 20 weeks
without Aroclor 1254 in their water, the hens laid eggs containing
0.7 mg/kg.
During a 4-week exposure to Aroclor 1242, 1254, or 1260, in the feed
of one-day-old chicks, Harris & Rose (1972) found that PCBs
accumulated in the fat and that this accumulation increased with
increasing exposure concentrations of 100, 200, and 400 mg/kg. At the
2 highest dose levels, the hens accumulated more of Aroclor 1260 than
of the other 2 Aroclors (i.e., 482, 1427, and 2151 mg Aroclor 1260/kg
at the 3 exposure concentrations, respectively). At the highest dose,
there was high mortality during exposure to Aroclor 1242 and 1254 and
this might have affected the residues found.
Greichus et al. (1975) fed white pelicans (Pelecanus erythrorhynchos)
on a fish diet containing 100 mg Aroclor 1254/day, for 10 weeks. PCB
residues were measured in the carcase, liver, feathers, and brain;
mean residues found were 2130, 290, 120, and 110 mg/kg wet weight,
respectively.
In a study by Dahlgren et al. (1972), 11-week-old pheasant (Phasianus
colchicus) were dosed with one capsule per day containing 210 mg of
Aroclor 1254. Birds that died between days 1 and 5 contained, on
average, PCB residues of 520 mg/kg in the brain, 2500 mg/kg in the
liver, and 140 mg/kg in muscle. Birds that were sacrificed over the
same period had mean brain, liver, and muscle PCB levels of 370, 1900,
and 83 mg/kg, respectively. All birds dosed with only 10 mg of Aroclor
1254 per day died within 180 days and contained average brain and
liver residues of 360 and 1200 mg/kg, respectively.
Södergren & Ulfstrand (1972) fed robins (Erithacus rubecula)
mealworms containing 1 µg of Clophen A50/day for 15 days. Brain,
breast muscle, and carcase were analysed and contained mean PCB
residues of 0.35, 0.55, and 4.5 mg/kg fresh weight, respectively. A
second group of robins was starved following dosing and all died
within 48 h. PCB levels were higher in the brain and breast muscle at
1.1 and 1.3 mg/kg, respectively, but carcase PCB levels were lower on
a fresh weight basis at 2.6 mg/kg. When the carcase lost some of its
fat content during starvation, PCB levels in terms of fresh weight
decreased. Consequently, because of the low remaining fat content,
residue levels in terms of fat weight increased. Another group of
birds were fed both PCBs and DDT (10.5 µg/day) for 15 days and then
starved. PCB levels in all 3 tissues analysed were higher than those
in birds administered PCBs alone followed by starvation; residues
were: brain, 9.3 mg/kg fresh weight, breast muscle, 8.8 mg/kg, and
carcase, 4.5 mg/kg.
Cormorants (Phalacrocorax carbosinensis) were kept on a fish diet
contaminated with PCBs for one month, followed by gelatin capsules of
PCBs administered daily for the remainder of the exposure (Koeman et
al., 1973). After 14 weeks, the dose rate of Clophen A60 was increased
periodically during the exposure period from 200 to 500 mg/kg. The
birds died between days 55 and 124, and overall residues of PCBs
increased in the tissues, the longer the birds survived. Total-body
residues ranged from 850 to 2750 mg PCBs/kg (wet weight) at death.
Brain and liver residues ranged from 76 to 180 mg/kg and from 210 to
290 mg/kg, respectively. The fat of 2 birds was analysed for PCBs and
was found to contain 10 300 and 20 500 mg/kg.
Harris & Osborn (1981) dosed wild puffins (Fratercula arctica) by
implantation with 30-35 mg of Aroclor 1254. PCBs were quickly taken up
in fat, with concentrations rising to 10-14 times that in control
birds (highest fat residue 654 mg/kg wet weight), and remaining at
this level for up to 10 months. Levels slowly declined, but were still
twice those of controls after 34 months. PCB concentrations in the
liver and muscle tissue were highest shortly after dosing (48.4 and
25.2 mg/kg, respectively) and declined until, after 16 months, no PCBs
were detectable. Levels of PCBs in the kidneys and brain were variable
with no consistent trends.
Common grackles (Quiscalus quiscula), starlings (Sturnus vulgaris),
red-winged blackbirds (Agelaius phoeniceus), and brown-headed
cowbirds (Molothrus ater), were fed diets containing 1500 mg Aroclor
1254/kg over an 8-day period (Stickel et al., 1984). PCB residues in
the brains of birds that died were found to be higher than those in
birds that were sacrificed over a similar period. PCB residues ranged
from 349 to 763 mg/kg in birds that died and from 54 to 301 mg/kg in
birds sacrificed. Liver and whole-body residues tended to be higher in
birds that died, but they overlapped to a large extent. PCB residues
in whole bodies on a lipid basis showed the most clear-cut difference,
ranging from 22 600 to 98 600 mg/kg for birds that died and from 6690
to 22 500 mg/kg for those sacrificed. PCB residues in grackles
declined slowly, when the birds were placed on a clean diet. From a
whole-body level of 1300 mg/kg, residues declined to 169 mg/kg, 224
days later. The rate of decline was irregular, but a half-life was
estimated at 89 days over this period of loss.
4.2.4.6 Mammals
Olsson et al. (1979) fed mink (Mustela vison) on a diet containing
11 mg PCBs/kg for 66 days. Mink accumulated 310 mg PCBs/kg in
extractable fat over the exposure period. Control mink were found to
contain 14 mg PCBs/kg, and, when the control feed was analysed, it was
found to contain 0.05 mg PCBs/kg. The authors also found a significant
increase in cadmium uptake in the kidneys of PCB-treated animals
compared with controls. In another study on mink (Mustela vison),
Hornshaw et al. (1983) administered various PCB-contaminated fish
diets containing between 0.21 and 1.5 mg PCBs/kg. Adipose tissue
samples were taken after 6-8 weeks and after 18 weeks exposure (Table
11). The amount of PCBs accumulated was directly related to the amount
of PCBs in the diet; mean PCB residues ranging from 4 to 24.8 mg/kg
after 6-8 weeks and from 8.1 to 42.8 mg/kg after 18 weeks. When
expressed as individual congeners, it can be seen that the mink showed
the highest accumulation of the PCBs with the chromatographic peak
corresponding to 2,4,5,2',4',5'-hexachlorobiphenyl. To determine the
rate of PCB elimination, male mink that had been on a fish diet
containing 1.5 mg PCBs/kg for 10 weeks were transferred to a control
diet. Over this period, adipose tissue residues of 32 mg PCBs/kg had
accumulated. Over the 16-week elimination period, 60.3% of the total
PCB burden of the adipose tissue was eliminated. This consisted of a
loss of 87.2% of 2,5,2',5'-tetrachlorobiphenyl, 88.9% of
2,3,6,2',5'-pentachlorobiphenyl, and 55.4% of the hexachlorobiphenyl.
The half-life for total PCBs in mink adipose tissue was calculated to
be 98 days.
Wren et al. (1987a,b) fed mink on a commercial mink food supplemented
with 1 mg Aroclor 1254/kg for a period of 6 months. Male mink had
liver residues of 1.98 mg PCBs/kg after 118 days and 2.8 mg/kg after
183 days exposure. The liver of a female, analysed on day 161
contained a residue of 3.1 mg PCBs/kg. Liver PCB levels in 5-week-old
kits were similar to those in adult mink fed the experimental diet for
several months. Bleavins et al. (1981) measured the relative
importance of placental transfer and milk in the transfer of PCB
residues from mother mink to offspring. Newborn kits contained less
than 0.1% of a dose of PCBs injected into the mother mink. At 2 weeks
of age, the kits contained 1.2% of the dose given to the mother,
suggesting that lactation is a major route of exposing the young to
PCBs and a major route for the loss of PCBs from the mother. Placental
transfer of PCBs was greater in the ferret than in the mink (Bleavins
et al., 1984). The ratio of placental to mammary transfer was 1:15 for
offspring whose mothers were dosed during the first trimester of
pregnancy and 1:7 for mothers exposed during the last trimester.
Big brown bats (Eptesicus fuscus) were fed on mealworm diets
containing 9.4 mg Aroclor 1254/kg for up to 37 days (Clark & Prouty,
1977). In bats sacrificed on day 37, residues ranged from 29 to 121 mg
PCBs/kg (wet weight) for the carcase and from not detectable to
4.2 mg/kg in the brain. Bats that were starved following exposure
showed a significant correlation between increasing brain PCB
concentrations and carcase lipid concentrations. The authors stated
that PCBs increased in brain tissue as carcase fat was metabolized.
Clark (1978) exposed pregnant big brown bats to a mealworm diet
containing 6.36 mg Aroclor 1260/kg for approximately 18-28 days, until
the young were born. Mean carcase levels of PCBs were 20.34 mg/kg in
parent females and 4.38 mg/kg in litters. Levels of PCBs in both
adults and young continued to rise throughout the sampling period; the
longer the gestation time, the higher the PCB level in the sample.
4.2.5 Appraisal
Experimental work on mammals has been concentrated on terrestrial
species. Problems with PCB toxicity are important for marine mammals,
but these are less convenient for experimental study. Results in this
section, therefore, have to be related to field observations on marine
species.
Mink take up more chlorinated components of PCB mixtures and can
accumulate large residues of PCBs. On cessation of exposure, more
tetrachloro- and pentachlorobiphenyls were eliminated than
hexachlorobiphenyl. The half-life for total PCBs was calculated to be
98 days. PCB residues are transferred from mother to offspring. The
relative importance of transplacental transfer and transfer in milk
varies between species. Redistribution of residues takes place on
starvation, which is of significance for migratory species; brain
residues, which may be fatal with no further intake of PCBs, increase
as animals are starved.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Levels in the environment
PCBs were detected in the environment in the late 1960s (Risebrough et
al., 1968; Jensen et al., 1969) and, within a short time, were
reported as contaminants in almost every component of the global
ecosystem including air, water, soil, fish, wildlife, human blood,
adipose tissues, and milk (Holdrinet et al., 1977; Wassermann et al.,
1979; Ballschmitter et al., 1981; Buckley, 1982; Safe, 1982b; Bush et
al., 1985; Kannan et al., 1988; Tanabe, 1988).
The lipophilic properties of PCBs are the basis of the bioaccumulation
and biomagnification that has been demonstrated and, thus, numerous
sources within the environment can lead to human exposure.
High-resolution, gas-chromatographic analysis has shown that the
congener composition and relative concentrations of the individual
components in many PCB extracts from environmental samples differ
markedly from those in the commercial PCBs (Jensen & Sundström, 1974a;
Wolff et al., 1982a; Safe et al., 1985a; Brown et al., 1987a,b).
A major problem with data concerning PCB levels in environmental
samples is that they normally are only available for "total PCBs" and
that there are much fewer data on actual "PCB patterns". Moreover,
when comparing results produced from different laboratories or from
the same laboratory at different times, an additional difficulty may
arise from differences in the sampling and analytical techniques used.
It is difficult, if not impossible, to compare data obtained with
different analytical methods, from different laboratories, and
countries. Nowadays, the older data seem less reliable, especially in
the light of the use of improved analytical methods and better
sampling techniques (WHO/EURO, 1987). A comprehensive review of world
PCB levels was published by Wassermann et al. (1979).
5.1.1 Air
PCB concentrations in air differ markedly from location to location,
with the lower levels found over the oceans or over non-industrialized
regions, such as the Canadian Northwest territories. In general,
levels over industrialized areas or over landfills are the highest.
Apparently, these levels influence PCB levels in rainwater and there
is a gradient of values in air from industrial to rural areas. Some
typical values can be found in Table 12.
MacLeod (1981) described a method for the analysis of PCBs using
low-volume, indoor air sampling to estimate the presence of PCBs in
indoor air in work-places and homes in the USA. Three facilities, an
industrial research facility, an academic facility, and a shopping
complex were sampled. The periods of sampling ranged from 2 days up to
6 months. The average concentrations (calculated as Aroclor 1242 plus
Aroclor 1254) ranged from 44 up to 240 ng/m3. Outdoor levels of up to
18 ng/mg3 were found. In the homes, air samples from 14 areas (of
which 9 were kitchens) were also analysed. The average concentrations
in the kitchens ranged from 150 up to 500 ng/m3 and, in the other
rooms, from 39 to 170 ng/m3. In a library, a level of 400 ng/m3 was
found.
The levels of PCB exposure that may occur in buildings in the USA were
determined by Oatman & Roy (1986). Air samples and surface wipe
samples were taken in 5 state-owned, office buildings and 2 elementary
schools. The average levels of airborne PCBs in buildings with PCB
transformers were nearly twice the levels in buildings without
transformers, i.e., 457 ± 223 and 229 ± 106 ng/m3, respectively. The
mean of the surface wipes taken in buildings without PCB transformers
was 0.17 and that in the buildings with transformers 0.23 µg/100 cm2.
There was a wide variation between the different buildings and, as
shown above, the presence of transformers influenced the indoor PCB
concentrations.
5.1.1.1 Rain and snow
In the Netherlands, at Bilthoven, the PCB-concentrations in rainwater
ranged from 0.01 to 1.5 µg/litre (van Zorge, cf. WHO/EURO, 1985). In
the Federal Republic of Germany, concentrations of 0-4 ng/litre were
found (DFG, 1988).
Table 12. PCB levels in air in several countries
Country Location and/or type of sample PCB levels References
average and/or range
Canada Northwestern territories 0.002-0.07 ng/m3 Bidleman et al. (1978)
Germany Industrial area (Ruhr area) 3.3 ng/m3 DFG (1988)
Non-contaminated area 0.003 ng/m3
Japan Within industrial plants: - PCB vapours 13-540 µg/m3 Tatsukawa & Watanabe
- PCBs on airborne particulates 4-650 µg/m3 (1972)
North Pacific, South Pacific, Indian, 0.1-0.3 ng/m3 Tatsukawa & Tanabe
Antarctic and South Atlantic Oceans (1983)
North Atlantic Ocean 0.5 ng/m3 Tatsukawa & Tanabe
(1983)
Sweden Several locations 0.8a-3.9 ng/m3 Ekstedt & Odén (1974)
USA Near the North-East Coast 5 ng/m3 Harvey & Steinhauer
Over the Atlantic Ocean, 2000 km away 0.05 ng/m3 (1974)
from the industrial complex
several locations 1-50 ng/m3 Panel on Hazardous
Substances (1972)
cf WHO/EURO (1988)
Yugoslavia Bela Krajina: - 300 m from an industrial plant 4-7 µg/m3 Jan et al. (1988b)
- air near a waste landfill 45 µg/m3
- over the River Kruga 2-5 µg/m3
a Limit of determination.
5.1.1.2 Natural gas
PCBs were first identified in gas pipelines in January 1981, when a
PCB-containing oil condensate was found in the gas meters of some
residential customers in Long Island, New York. Voluntary monitoring
of condensate and natural gas by 33 transmission companies, showed the
presence of PCBs in 12 companies. PCBs were also found in gas
pipelines. Condensate is a mixture of heavier hydrocarbons and other
liquids, such as water, that condenses, because the gas is transmitted
under pressure. This condensate tends to collect in pools in the
pipes. In the period 1981-83, 1841 samples of condensate from gas
pipelines were analysed: 659 (35.8%) of the samples contained
< 25 mg/kg; 65.8% of the samples contained <1000 mg/kg, and 0.4%,
> 10 000 mg/kg. The maximum level that was found was 42 394 mg/kg
(Versar Inc., 1984).
In the period 1981-83, 138 samples of natural gas in transmission were
analysed. In 29 samples, PCBs were found with a minimum concentration
of <0.004 µg/m3 and a maximum concentration of 1050 µg/m3. Natural
gas in distribution lines was also analysed in the same period. Out of
528 samples, 224 did not contain any PCBs. The levels ranged from
<0.02 to 51 µg/m3.
Indoor concentrations (kitchens, etc.) were measured in 419 samples in
the period 1981-83. No PCBs could be detected in 49 samples, but, in
the others, levels ranged from <0.01 to 1.08 µg/m3 (Versar Inc.,
1984).
5.1.2 Water
Surface water may become contaminated with PCBs from atmospheric
fall-out or from direct emissions from point sources. Because of
adsorption on suspended particles, PCB concentrations in heavily
contaminated waters may be several times greater than their
solubility. Södergren (1973) reported a seasonal variation, which was
attributed to aerial fall-out.
It has been shown that polluted rivers, lakes, and estuaries have
higher PCB values than non-polluted waters (Table 13). On the basis of
scanty information on PCBs and reinforced by extensive analogue
information on DDT, it has been estimated that, for the Great Lakes of
North America, non-polluted freshwaters might contain less than
5 ng/litre, moderately polluted rivers and estuaries, 50 ng/litre, and
highly polluted rivers, 500 ng/litre. These values can be used to
evaluate those reported by several authors and presented in Table 13.
Table 13. PCB levels in water in several countries
Country Location and/or type of PCB levels References
sample average and/or range
Germany Several rivers 5-103 ng/litre Lorenz & Neumeier (1983)
Netherlands River Rhine (1976/1977) 100-500 ng/litre Wegman & Greve (1980)
Sweden Water entering a treatment 0.5 ng/litre Ahling & Jensen (1970)
plant
Tap water produced at the plant 0.33 ng/litre Ahling & Jensen (1970)
Several rivers 0.1-0.3 ng/litre Ahnoff & Josefsson (1974)
USA Polluted coastal area 100-450 ng/litre Panel on Trace Hazardous
Lake Michigan (1970)a Substances (1972) (cf.
WHO/EURO, 1988)
Distribution system feeding the Fort Edwards reservoirs < 12-160 ng/litre Brinkman et al. (1980, 1981)
in New York (1978)
Hudson River at Fort Edward up to 530 ng/litre Brinkman et al. (1980, 1981)
a Followed by a marked decrease in 1971.
5.1.3 Soil
Soil may become contaminated with PCBs from atmospheric fall-out or
from direct emissions from point sources. The presence and behaviour
of these compounds in the soil depend on substance (congener)-specific
characteristics and on a number of soil parameters. Sorption and
condensation processes in the soil also play a role in the removal of
PCBs. Some values of PCB levels in soil can be found in Table 14.
Klein (1983) found that PCBs accumulate in the sediments of rivers and
lakes in the Federal Republic of Germany and that these levels
indirectly reflect the contamination of water by PCBs. Some values for
PCBs in sediments can also be found in Table 14.
An important, though localized, source of PCB contamination of soil,
can be the use of sewage sludge as a fertilizer in agriculture. PCB
levels varying from 0.1 to 765 mg/kg (dry weight) have been reported
in sewage sludge from different countries, the usual range being 0.1
to 9.0 mg/kg (WHO/EURO, 1987). In the USA, 16 sewage sludge samples
from cities contained a mean Aroclor 1254 concentration of 5.2 mg/kg
dry weight (range 0.01-23.1 mg/kg). Other authors reported a range of
1.5-27.3 µg/litre in 36 raw sewage sludges. Some levels that have been
found for PCBs in sludges are presented in Table 14 (WHO/EURO, 1987).
Five sediment samples were collected from the Waukegan Harbour of Lake
Michigan, Illinois, in 1978. Residues of 3,4,3',4'-tetrachlorobiphenyl
ranged from 0.005 to 27.5 mg/kg and residues of 2,3,4,3',4'-penta-
chlorobiphenyl, from 0.102-131 mg/kg. The total PCB contents of the
sediment ranged from 10.6 to 13 360 mg/kg (Huckins et al., 1988).
5.1.4 Aquatic and terrestrial organisms
PCBs have been measured in a wide variety of biota from many different
locations throughout the world. Only a few illustrative examples are
given here, more comprehensive lists of PCB residues can be found in
reviews by Risebrough et al. (1968); Peakall (1975); and Eisler
(1986). Tanabe et al. (1987) reported that the highly toxic, coplanar
PCBs are as widely spread as general PCB pollution.
In the biota of a small upstate New York public water supply system,
which is near the polluted section of the Hudson River and a disposal
site of PCB-containing waste, PCBs were found in detectable
concentrations (Table 13). Five samples of algae showed Aroclor 1254
levels of <25 (nd)-120 µg/kg dry weight, macro-invertebrates showed
levels between <200 and 3800 µg/kg and vertebrates, between <25 and
1100 µg/kg dry weight (Brinkman et al., 1980, 1981).
Table 14. PCB levels in soils, sediments, and sewage sludge in several countries
Country Location and/or type of sample PCB levels References
average and/or range
Germany Soil without sewage sludge 0.02-0.08 mg/kga Markard (1988)
Soil with sewage sludge 0.05-3.0 mg/kga
Sewage sludge ndb-19 mg/kg
Sediments of contaminated waters 0.1-1.0 mg/kga Klein (1983)
Sediments of several rivers 0.16-0.59 mg/kg DFG (1988)
Agricultural soil 0.03 mg/kg DFG (1988)
Japan Agricultural soil < 1 mg/kg Fukada et al. (1973)
Soil near a factory making electrical components 510 mg/kg Fukada et al. (1973)
Netherlands Sediments from several surface waters < 0.01-1.2 mg/kga Greve & Wegman (1983)
United Soil from a waste disposal area with chemical treatment 4.5-44.8 µg/kg Eduljee et al. (1986);
Kingdom and incineration facilities Badsha et al. (1986);
(Scotland) Grass samples from the same area (foliage) 2.9-64.7 µg/kg Badsha & Eduljee (1986)
Soil of rural areas 8 µg/kga (1-23)
Grass of rural areas 9 µg/kga (7-16)
Soil of urban areas 52 µg/kg (11-141)
Soil of industrial locations 41 µg/kg (20-67)
United Surface soil 2.5 µg/kg Jones (1989)
Kingdom (0.2-12.2)
(Wales)
Table 14. (cont'd).
Country Location and/or type of sample PCB levels References
average and/or range
USA Sediments near a point of accidental release of PCBs 1.4-61 mg/kg Nimmo et al. (1971a)
(Florida)
Escambia Sediments 16 km downstream of this point 0.6 mg/kg
river
Escambia Soil samples from the bank, 6.5 km downstream from the 1.4-1.7 mg/kg
Bay point
a Dry weight
b Not detectable
Serious environmental contamination has been documented in enclosed
water bodies close to urban and industrialized areas, such as the
Great Lakes, the Baltic Sea, and Tokyo Bay. PCB levels in aquatic
organisms reflect these localized high concentrations.
Nimmo et al. (1971a) reported that PCB levels in shrimp from Escambia
Bay, Florida (contaminated by an industrial plant on the Escambia
River) contained between 0.6 and 120 mg Aroclor 1254/kg in 1969 and
fiddler crabs, collected in 1970, contained 0.45-1.5 mg/kg.
When fish, sampled throughout the USA, were analysed by Schmitt et al.
(1983, 1985), the highest levels of PCBs were found in the
North-eastern industrialized areas. Delfino (1979) reported
concentrations ranging from 26 to almost 1000 mg PCBs/kg in fish
collected from the Sheboygan River, Wisconsin, contaminated by a
die-casting plant.
Wiemeyer et al. (1975) analysed osprey eggs in 1968-69 and found
average levels of 2.6 mg/kg in Maryland compared with an average level
of 15 mg/kg in eggs from Connecticut. PCB residues in Connecticut eggs
had not changed significantly compared with those collected in 1964.
Buckley (1982) analysed aspen, sumac, and golden rod plants growing at
various distances (< 1200 m) and in different directions from a PCB
dump in New York State, USA. All the plants were growing beyond a
natural drainage ravine, which prevented contamination of soil and
water by PCBs. Downwind of the site, PCB levels in the plants were
found to be approximately 100 mg/kg dry weight (over 600 times
background levels in plants). Levels above background concentrations
were also found in directions from the site less obviously
contaminated by airborne dust.
Eggs of terrestrial birds collected in a rural environment in Canada
contained lower PCB levels than those sampled from urban areas (Frank
et al., 1975).
In the Great Lakes, the highest levels of PCBs were found in Lakes
Michigan and Ontario for fish (Delfino, 1979) and Lake Ontario for
birds (Weseloh et al., 1979); both lakes receive input from industrial
and urban sites. Glooschenko et al. (1976) found concentrations of up
to 8.1 mg/kg in microorganisms from the middle of Lake Huron.
Weseloh et al. (1983) found that the PCB levels in double-crested
cormorant eggs, collected from Lake Superior during 1972 (average of
23.8 mg/kg fresh weight), were higher than those in cormorant eggs
analysed in other Canadian colonies. Mineau et al. (1984) found that
the locations of herring gull colonies with the greatest mean levels
of PCBs, in each of the Great Lakes, corresponded with the locations
of major sources of the contaminant, as indicated by elevated residues
in sediment.
Muir et al. (1988) determined PCB levels in pooled Arctic cod muscle
(Boreogadus saida) and polar bear fat (Ursus maritimus), and in
the blubber and liver of ringed seals (Phoca hispida) from 3
locations in the East/Central Canadian Arctic. The mean arithmetic
concentrations of total-PCBs in the muscle of Arctic cod of 2
locations were 3 and 5 µg/kg wet weight. The mean concentrations shown
in the tabulation below were found in the blubber and liver of ringed
seals.
Year Number of Sex Arithmetic mean ± SD
samples (µg/kg wet weight)
(blubber)
1972 3 female 639 ± 249
1975/76 5 female 600 ± 99
1983 10 male 794 ± 879
16 female 308 ± 138
1984 19 male 568 ± 287
14 female 375 ± 172
(liver)
1984 19 male 6 ± 4
14 female 4 ± 3
The presence of PCBs in polar bears (Ursus maritimus) was studied by
Norström et al. (1988) in the Northwest territories of Canada. Liver
and adipose tissue specimens were obtained by Inuit hunters from 12
zones over the period 1982-84. A total of 121 samples was obtained.
The mean concentrations of total PCBs in pooled samples ranged from
3.24 to 8.25 mg/kg, on a lipid weight basis. The adipose tissue of
polar bear (10 pooled samples collected in 1982 and 10 samples, in
1984) contained 4.42 and 4.57 mg/kg wet weight, respectively. From
these results, biomagnification factors for the food-chain of the
Arctic cod/ringed seal/polar bears were calculated. For total PCBs,
these factors ranged from 3.7 to 8.8 for fish to seal; from 7.4 to
13.9 for seal to bear, and 49.2 for fish to bear. For individual PCB
homologues, for instance, for fish to bear, these factors ranged from
<0.5 (tetrachlorinated PCBs) to 263.4 for heptachlorinated PCBs.
Niimi & Oliver (1989b) monitored the presence of 92 monochloro- to
decachlorobiphenyl congeners in brown and lake trout, small and large
rainbow trout, and small and large coho salmon from Lake Ontario. Each
sample consisted of 8-12 fish. The highest concentrations were among
the penta- and hexachlorobiphenyl homologues, with 2,4,5,2',4',5'-
hexachlorobiphenyl the most common congener.
Total congener concentrations ranged from 1 to 10 mg/kg in whole fish
and from 0.3 to 4 mg/kg in muscle. The 10 most common PCB isomers were
84, 87/97, 101, 110, 118, 138, 149, 153, and 180, and represented 52%
of the total content. This value did not appear to be influenced by
species or by total concentration.
Huckins et al. (1988) collected fish (1-6 fish of 7 species) from the
Waukegan Harbour of Lake Michigan, Illinois in 1978. The fish samples
were analysed for the presence of 3,4,3',4'-tetrachloro- and
2,3,4,3',4'-pentachlorobiphenyl. Total PCB congener residues averaged
33.4 (2.4-56.6) mg/kg. The concentrations of 3,4,3',4'-tetra-
chlorobiphenyl averaged 45.3 µg/kg (2-89 µg/kg) in the whole body. The
concentrations for 2,3,4,3',4'-pentachlorobiphenyl averaged 229 µg/kg
(80-483 µg/kg).
Five times as much PCBs were found in herrings caught in
industrialized areas of Sweden (near Stockholm) compared with those
caught in the cleaner waters off the Swedish west coast. Levels in
plankton fell progressively with increasing distance from
industrialized areas (Jensen et al., 1972a).
Holden (1973) found levels of up to 235 mg/kg in the blubber of seals
sampled in the polluted coastal areas of the United Kingdom compared
with lower levels (2 mg/kg) from unpolluted areas. Higher levels, (up
to 88 mg/kg) were found in the blubber of toothed whales sampled in
the North Sea, but none was detectable in similar species sampled off
New Zealand and Surinam (Koeman et al., 1972).
Peakall (1975) mapped out the global distribution of PCB levels in
marine plankton. The values for the open North Atlantic (300-450 mg/kg
lipid) were found to be very similar to those collected from polluted
areas, such as the Baltic sea and the Firth of Clyde, in the United
Kingdom. Values in the South Atlantic (12-64 mg/kg) were considerably
lower. The highest values shown were for the Eastern coast of the USA
(up to 3050 mg/kg). There were no values for the Pacific Ocean.
When monitoring PCB levels in fish from the Mediterranean, Albaiges et
al. (1987) found that territorial species reflected local inputs of
the pollutant, but migratory species had baseline levels.
Risebrough & de Lappe (1972) reported PCB levels higher than 3 mg/kg
in fish from the industrialized areas of Tokyo Bay and New York Sound.
Tanabe et al. (1986a) analysed Antarctic minke whales and found that
they contained lower PCB levels than those caught in the Northern
hemisphere (Tanabe et al., 1983). McClurg (1984) also found low levels
of PCB in the Antarctic; Ross seals contained 0.09 mg/kg (in blubber).
Mean levels of 0.69 mg PCB/kg (wet weight), found by Smillie & Waid
(1987) in Australian fur seal blubber, were much lower than levels
found in seals from the temperate Northern hemisphere. Similarly,
Antarctic fish had very low PCB residues, ranging from 0.08 to
0.77 µg/kg wet weight (Subramanian et al., 1983).
PCB residues in biota are usually highest near industrial sources, but
this geographical distribution is becoming less pronounced. In fact,
O'Shea et al. (1980) and Tanabe et al. (1988) found PCB levels in
small oceanic cetaceans to be higher than those reported for
terrestrial mammals and birds. For example, Tanabe et al. (1988) found
the mean level of PCBs in the fatty tissue of the striped dolphin to
be 36 mg/kg wet weight.
Subramanian et al. (1986) analysed subcutaneous fat from Adelie
penguins from the Antarctic and found PCB levels of 0.05 mg/kg fat
weight. This is a factor of 100 lower than that in auks caught in the
northern North Pacific (Tanaka & Ogi, 1984) and a factor of 10 000
lower than residues found in the pectoral muscle (on a lipid weight
basis) of herring gulls in the Baltic (Lemmetyinen et al., 1982).
5.1.4.1 Effect of dredging-contaminated sediment on organisms
Dredging to remove contaminated sediments from the Shiawassee River,
Michigan, increased the availability of PCBs, and, thus, residue
levels, in freshwater clams (64.5-88 mg/kg dry weight) and in fish
(fathead minnow; 13.8-18.3 mg/kg), both during dredging and up to 6
months afterwards (Rice & White, 1987).
5.1.4.2 Relationship to lipid content of organisms
PCBs are accumulated in lipid-rich tissues and care must be taken when
interpreting results between species with different amounts of body
fat. Jensen et al. (1969) found that PCB levels in herring and cod,
from the same area of the Baltic Sea, were 0.27 and 0.033 mg/kg, on a
wet weight basis, respectively, even though the cod is at a higher
trophic level. The 2 species were found to have body fat contents of
4.4 and 0.32%, respectively, and when the PCB residues were
recalculated on a lipid weight basis, herring contained 6.8 mg/kg and
cod, 11 mg/kg.
PCBs are particularly accumulated in animals with large amounts of
fat, such as seals, dolphins, porpoises, and whales (Tanabe, 1988) and
in Arctic and Antarctic birds and mammals. Subramanian et al. (1986)
found PCBs in all Adelie penguins sampled in the Antarctic, an area
known to be relatively low in PCBs; the PCBs were mainly concentrated
in fat-rich tissues. Kawai et al. (1988) measured PCBs in striped
dolphins and found that the tissue level of PCBs depended entirely on
their lipid content and, especially, on the amount of triglycerides in
tissues.
Redistribution of PCBs, from fat to other tissues, occurs in animals
during periods of enforced starvation, such as seasonal food shortage,
hibernation, migration, incubation, and the feeding of offspring.
Subramanian et al. (1986) found that, as individuals Adelie penguins
starved during incubation, residues of PCBs increased with declining
fat reserves concomitant with tissue redistribution. Llorente et al.
(1987) found that migratory duck species had a smaller percentage of
the body burden of PCBs in adipose tissue than a resident species. A
similar redistribution during starvation has been shown in the
laboratory in European robins (Södergren & Ulfstrand, 1972) and big
brown bats (Clark & Prouty, 1977) (see sections 4.2.4.5 and 4.2.4.6).
5.1.4.3 Residues in different trophic levels and effects of diets
In a study by Shaw & Connell (1982), bioaccumulation was increasingly
evident in upper trophic level organisms, such as gulls and pelicans,
in an Australian estuary compared with organisms from lower trophic
levels. Veith et al. (1977) found typical PCB concentrations in Lake
Superior biota to be 0.1 mg/kg for large zooplankton, 0.3 mg/kg for
bottom fish, such as sculpins, and 1 mg/kg for pelagic fish.
When various insects were sampled for PCB residues (Morse et al.,
1987), levels in honey bees ranged from <0.1 to 1.5 mg/kg dry weight.
PCB residues in other species ranged from <0.1 to 2.6 mg/kg, with
predatory wasps containing the highest residues.
Prestt et al. (1970) analysed the livers from various bird species in
the United Kingdom. The highest PCB residues were found in freshwater,
fish-eating species (up to approximately 900 mg/kg). The authors did
not find any geographical pattern of distribution of PCBs in the
species studied.
Frank et al. (1975) collected birds' eggs from the Niagara peninsula
in 1971. Eggs from carnivorous species of birds at the top of the
aquatic food chain contained the highest levels of PCBs
(3.5- 74 mg/kg). Terrestrial carnivores contained lower, but still
relatively high, residues (0.2-1 mg/kg). Eggs from herbivorous and
insectivorous birds contained much lower residues of PCBs. Again, eggs
from terrestrial birds tended to contain lower levels (0.05-2 mg/kg)
than those feeding on aquatic prey (0.14-4 mg/kg). Focardi et al.
(1988) compared the PCB residues in the eggs of 8 species of water
bird. The residues were found to be higher in fish-eating birds than
in invertebrate feeders. The invertebrate feeders tended to contain
higher percentages of the lower chlorinated congeners. Bird species
that fed on other birds or fish had higher liver residues of PCBs than
those feeding on mammals (Cooke et al., 1982). Peregrine falcons,
herons, sparrowhawks, kingfishers, and great crested grebes had
relatively high residues of PCBs. By contrast, golden eagles were only
very lightly contaminated with PCBs.
Bowes & Jonkel (1975) found a similar pattern in Arctic and subarctic
food chains with PCB levels following the pattern: Arctic charfish
< seals < adult polar bears < polar bear cubs.
Mean PCB concentrations of 0.0018 mg/kg were found by Tanabe et al.
(1984) in zooplankton, 0.048 mg/kg in myctophid, 0.068 mg/kg in squid,
and 3.7 mg/kg in striped dolphin (all based on a whole-body, wet
weight basis) sampled from the western North Pacific. The authors
concluded that the bioaccumulation of chlorinated hydrocarbons was
dependent on physical and chemical factors, such as water solubility
and lipophilicity, in the lower trophic levels, whereas, in higher
trophic levels, accumulation was affected by biochemical factors, such
as the biodegradability of pollutants and the metabolizing capability
of the organism.
5.1.4.4 Effects of age, sex, and reproductive status on uptake and
elimination
Bache et al. (1972) found that the burden of PCBs increased with age
in lake trout from Cayuga lake, Ithaca, New York, sampled in 1970
(residues ranged from 0.6 to 30.4 mg PCBs/kg). An age- and
length-related increase in PCBs was found in striped bass from the
Hudson River and Long Island Sound; the author (Connell, 1987) stated
that this observed relationship was due to the slow rate of
bioaccumulation of the PCBs, particularly the higher chlorinated
congeners.
PCBs have been shown to accumulate with age in marine mammals, such as
pinnipeds (Addison et al., 1973; Frank et al., 1973; Helle et al.,
1983) and cetaceans (Gaskin et al., 1983; Aguilar & Borrell 1988;
Subramanian et al., 1988). Helle et al. (1983) found mean levels of
5.1 mg PCBs/kg (in extractable fat of blubber) in newly-born ringed
seal pups, 17.3 mg/kg in seals of 2-4 months of age, and 65.3 mg/kg in
sexually mature adults (4-12 years). However, lower levels of PCBs
have been found in females compared with males (Martineau et al.,
1987) and the age-related increase has often not been found in females
(Addison & Smith, 1974). In many studies, while levels of PCBs in
males have increased with age, those measured in females have fallen
(Born et al., 1981; Gaskin et al., 1983; Aguilar & Borrell, 1988).
Gaskin et al. (1983) found that PCB levels in the blubber of male
harbour porpoises increased from 48.4 mg/kg at birth to 161 mg/kg
after 8 years, whereas, in females, levels fell from 51 to 14.7 mg/kg.
A significant decrease in the PCB levels was found by Subramanian et
al. (1988) in female Dall's porpoises from 2 years of age onwards; 2
years is required for the animals to reach sexual maturity. Excretion
of PCBs during reproduction is known, from the laboratory, to be an
important means of females losing residues. This PCB loss has been
shown to be because of the transfer of PCBs to offspring via milk
during lactation (Addison & Brodie, 1977). Addison & Brodie (1977)
calculated that female grey seals excreted about 15% of their body
burden of PCBs via lactation. In striped dolphins, females transferred
between 72 and 98% of their body burden to the offspring (Fukushima &
Kawai, 1981; Tanabe et al., 1982). It was suggested by Tanabe (1988)
that such large transfer was because of the very high lipid content of
the milk. Relocation of the PCB burden during pregnancy is generally
thought not to be as important; in grey seals, the mother transfers
only about 1% of her body burden to her offspring (Donkin et al.,
1981) and in striped dolphins, only 4-9% (Fukushima & Kawai, 1981;
Tanabe et al., 1982). However, Duinker & Hillebrand (1979) suggested
that a much bigger percentage of female body burden (up to 15%) could
be transferred to the fetus across the placenta of Harbour porpoise.
Clark & Lamont (1976) calculated that female big brown bats
transferred between 17 and 32% of their body burden of PCBs to their
young, during gestation. The concentration of PCBs in adult females
plus their litters declined with increasing age of the female. PCB
levels were 0.83-3.6 mg Aroclor 1260/kg (wet weight) in adults and
0.22-3.3 mg/kg in litters.
When Passino & Kramer (1980) measured PCBs in deepwater ciscoes from
Lake Superior, male fish contained significantly higher levels of PCBs
(2.3 mg/kg wet weight) than females (1.2 mg/kg), eggs containing
0.51 mg/kg. Lemmetyinen et al. (1982) found annual rates of
elimination via egg production of 45% in the female Arctic tern and
24% in the herring gull. Adelie penguins eliminated only 4% of their
PCB body burden after laying their annual clutch of 2 eggs (Tanabe et
al., 1986b). Elimination was thought to be dependent on the relative
weights of the egg and mother.
5.1.4.5 Time trends in residues
Buckley (1983) analysed various species of terrestrial plants from New
York state. Total decreases of 42% in PCB residues were found between
1978 and 1980.
PCB levels in fish in the Hudson River, New York declined between 1977
and 1981. The PCB levels were much higher in the Upper Hudson River
(4217-1431 mg/kg of lipid), near to a major discharge of PCBs, than in
the Lower Hudson River (1604-319 mg/kg) (Sloan et al., 1983).
Frank et al. (1978) measured PCB levels in various fish species from
Lakes Huron and Superior during the period 1968-76. PCB residues
declined in lake trout and lake whitefish in Lake Superior between
1971 and 1975, but increased slightly over the same period in bloaters
and white sucker. In Lake Huron, PCB levels decreased between 1968 and
1971, and, in alewife, rainbow smelt, and walleye, between 1975 and
1976. In some of the study areas, residues increased in cisco, yellow
perch, coho salmon, and splake but, at most locations, and, for other
species analysed, no trends in PCB levels were found. St Amant et al.
(1984) analysed fish from Lake Michigan between 1971 and 1981. An
overall decrease in PCB levels was found for all species monitored
except the walleye. Levels decreased from a maximum of 22.4 mg/kg at
the beginning of the study to 3.8 mg/kg or less in 1981.
Fish from all over the USA were analysed in 1980-81 by Schmitt et al.
(1985) who found a significant downward trend (0.88-0.53 mg/kg PCB;
wet weight) when mean residues were compared with fish collected
between 1976 and 1977 (Schmitt et al., 1983). A similar downward
pattern in residues was found in the Baltic when Moilanen et al.
(1982) compared residues found in pike and herring caught between 1978
and 1982 with those in fish sampled between 1972 and 1978 (Paasivirta
& Linko, 1980). Haahti & Perttila (1988) found a continued decline in
PCB residues between 1979 and 1986, when residues in herring muscle
tissue decreased from 2.7-3.7 mg/kg to 0.3-1.1 mg/kg.
An overall fall in PCB levels was found by Newton & Bogan (1978) in
sparrowhawk eggs during the period 1971-74. Cooke et al. (1982)
analysed liver samples from grey herons, kestrels, and barn owls for
PCB residues during the period 1967-77. They found a significant
decline in PCB residues over the sampling period in all 3 species. The
mean residues in heron, kestrel, and barn owl for the period 1967-71
were 5.77, 1.57, and 0.44 mg/kg, respectively, and for 1977, 0.56,
0.6, and 0.15 mg/kg, respectively. However, Newton et al. (1986), when
analysing sparrowhawk eggs from 1971-80, found that, although levels
had fallen in the early 1970s, they had risen again in the late 1970s
(mean PCB residues in eggs ranged from 16 to 293 mg/kg in lipid). Data
on PCB residues in the livers of kestrel, sparrowhawk, heron,
kingfisher, and the great crested grebe, collected from the late 1960s
up to 1987, were analysed statistically by Newton & Haas (1989). For
the great crested grebe, a significant overall decline in PCB residues
was found when comparing data from 1987 with that from the 1960s. For
the other species, there was no significant difference. Spitzer et al.
(1978) reported that there was no significant change in PCB levels in
osprey eggs collected from the Connecticut-New York area during the
period 1969-76. Similarly, Wiemeyer et al. (1987) did not find any
change in the carcase levels of PCBs in ospreys from the Eastern
United States when comparing the 1971-73 and 1975-82 periods. They did
find that adults contained significantly higher concentrations of PCBs
than immature ospreys.
Blus et al. (1979) analysed brown pelican eggs from South Carolina and
Florida between 1969 and 1976. The highest levels of PCBs were found
in South Carolina (means ranged from 5.25 to 7.63 mg/kg wet weight),
but no significant trend was found during the study period. In
Florida, the authors did not find any significant change in eggs
collected from colonies in Florida Bay and on the Gulf Coast over the
study period (means ranged from 0.62 to 1.18 mg/kg), but the Atlantic
coastal colony showed a significant increase in PCB residues (from a
mean of 2.68 to 6.12 mg/kg) between 1969 and 1976.
In analysing herring gull eggs from the Great Lakes between 1974 and
1978, Weseloh et al. (1979) found a significant decline in PCB
residues from colonies on all the lakes. Lake Ontario, the most
contaminated, showed the biggest decline from 170 to 75 mg PCBs/kg at
one of the colonies, with other less contaminated Lakes, Huron,
Superior, and Erie, showing levels in the range of 50-86 mg/kg in 1974
and 32-46 mg/kg in 1978.
Moksnes & Norheim (1986) analysed herring gull eggs collected from the
Norwegian Coast between 1979 and 1981 and found that the PCB levels
were not significantly different from those in eggs collected in 1969;
mean PCB residues ranged from 1.2 to 6.7 mg/kg wet weight. They found
a small but significant increase in the most persistent congeners and
a significant decrease in DDE and the DDE/PCB ratio, but not in total
PCB levels.
An analysis of the eggs of double-crested cormorant (an inshore-
subsurface feeder), Leach's storm petrel (an offshore-surface
feeder) and Atlantic puffin (an offshore-subsurface feeder) was
carried by Pearce et al. (1989), every 4 years, between 1968 and 1984.
In the Bay of Fundy, Canada, PCB levels declined significantly during
this period in all 3 species. PCB levels in the cormorant were
consistently higher throughout than those in the other 2 species,
ranging from 4 to 29.5 mg/kg (wet weight). Petrel and puffin eggs
collected from the Atlantic Coast of Newfoundland showed lower levels
than those in eggs from both the Bay of Fundy and the St Lawrence
River estuary; as in the St Lawrence River, no significant trend in
PCB levels was observed. A significant decline in PCB residues was
found in gannet eggs collected during the same period from the gulf of
St Lawrence (Elliott et al., 1988).
The frequency of occurrence of measurable PCB residues has increased
in large-scale sampling exercises; PCBs in mallard wings increased
from 39% in 1976-77 (White, 1979) to 95% in 1979-80 (Cain, 1981). Cain
& Bunck (1983) found that, in 1976, 21% of European starlings
collected in the USA contained PCBs compared with 83% in 1979.
Addison et al. (1986) analysed the blubber of Arctic ringed seals
(Phoca hispida) from Holman Island, NWT, Canada, in 1981. They found
mean PCB levels of 0.58 mg/kg (wet weight) in the females and
1.28 mg/kg in the males. These concentrations were significantly lower
than those detected in the same species from this area in 1972. Over
this same period, pp'-DDE levels, although at lower levels, also
fell significantly, but it should be noted that total DDT levels in
blubber are much lower than PCB levels and have not changed
significantly.
5.1.4.6 Seasonal patterns in residues
Jensen et al. (1969) observed that there was considerable seasonal
variation in the fat content of herring caught in the Baltic Sea,
ranging from 1% in the spring to 10% in the autumn and that this
seasonal change in fat content led to seasonal changes in the tissue
levels of PCBs.
Cooke et al. (1982) found a seasonal pattern of PCB levels in European
kestrels. Residues in both fat and liver were low in the autumn, but
increased from about January, with a peak almost invariably occurring
during the second quarter of the year (April, May, or June). Seasonal
patterns were based on samples collected over a 10-year period.
Similar trends were found in sparrowhawks and barn owls, but fewer
samples were available.
5.1.5 Appraisal
PCB contamination is widespread and has been measured in a wide
variety of biota between the 1960s and the present day. They are
present throughout the world and, although initially concentrated in
areas of high industrial activity, are now found in organisms living
in remote areas, such as the oceans and the polar regions. In the
past, PCB levels were positively correlated with areas of heavy
industry and consequent discharge but, with the implementation of PCB
controls, in some countries, these geographical differences are
becoming less clear. Generally, levels of PCBs are declining in areas
previously high in PCBs. However, time-trend analysis for the general
environment shows little change in total PCBs since the late 1960s.
The ratio of congeners is, as would be expected, changing, with lower
chlorinated isomers disappearing and the more highly chlorinated ones
becoming more dominant in environmental samples.
PCBs are persistent and bioaccumulate in many organisms, because of
their high lipid solubility and low biodegradability, and usually
enter food-chains from water containing industrial discharge and by
precipitation.
Because of their hydrophobic nature, PCBs are associated with both
oildrop-like aggregates in the surface microlayer of water and with
sediment on the bottom.
They are accumulated by micro- and macroplankton organisms that live
in the surface microlayer and by bottom-living organisms.
5.2 Levels in animal feed
The effects of pollution are seen in the use of fish-meal in poultry
and fish farming. Kolbye (1972) sated that this may contain PCB levels
of 0.6-4.5 mg/kg.
Hansen et al. (1981) studied the transfer of PCBs in swine foraging on
sewage sludge amended soils in 1975-76. Sixteen Berkshire sows were
overwintered for 2 seasons on 4 experimental plots that had been
treated with 0, 126, 252, or 504 tonnes/hectare (on a dry solids
basis) of Chicago sewage sludge for the 8 preceding years. The
estimated PCB residues in the soils of the 4 plots (average of 3-4
samples) were 1.62, 1.88, 2.13, and 2.81 mg/kg dry weight (mean values
of 3-4 samples/plot). The mean concentrations in fat of 3-4 sows per
plot were 36 ± 9, 106 ± 64, 191 ± 97 and 389 ± 118 µg/kg fat basis. Of
the 12 individual congeners that were present in the fat, 3 accounted
for more than 50% of the congeners, e.g., 2,3,4,2',4',5'-,
2,4,5,2',4',5'-hexachlorobiphenyl and 2,3,4,5,2',4',5'-
heptachlorobiphenyl.
In vegetable animal feed (155 samples) originating from 5 areas of the
world, samples, collected in 1984/85, contained PCB levels of 0.0009
(Africa) up to 0.0093 mg/kg dry weight (Europe). In feed from North
and South America and the Far-East, the levels were between 0.0024 and
0.0066 mg/kg. Different types of feed originating from agriculture in
the Federal Republic in Germany, collected in 1985, contained PCB
levels of the order of 0.02 mg/kg dry weight. In feed (301 samples)
originating from animals (exclusive fish meals), collected in 1985,
0.021-0.036 mg/kg dry weight was found (DFG, 1988).
Levels of 10-100 µg/kg are given for groats, soybeans, and cotton
seed, and a mean value of 18 µg/kg is given for mixed feedstuffs. Fish
meal contained levels of 110-330 µg/kg (Klein, 1983).
Samples of fish meal from different areas of the world, collected in
1985, were analysed for the presence of PCBs. In 323 samples, the PCB
contents varied between 0.006 and 0.055 mg/kg dry weight. The PCB
congeners numbers 28, 138, and 153 were present in the highest
quantities (DFG, 1988).
Samples of fish meal from different areas of the world, collected in
1985, were analysed for the presence of PCBs. In 323 samples, the PCB
contents varied between 0.006 and 0.055 mg/kg dry weight. The PCB
congeners numbers 28, 138, and 153 were present in the highest
quantities (DFG, 1988).
5.3 Levels in human food
5.3.1 General
Two general reviews of PCB residues in food, animal feed, human milk,
plants, soils, and packaging materials have been published by Khan et
al. (1976) and Sawhney & Hankin (1985).
The PCB contents of a variety of foods on the Swedish market has been
measured by Westöö & Norén (1970a) and Westöö et al. (1971). Less than
0.1 mg/kg was found in samples of butter, margarine, vegetable oils,
eggs, beef, lamb, chicken, bread, biscuits, and baby food; one sample
of pork out of more than 100 had a PCB content of <0.5 mg/kg.
In the period 1980-81, 5270 food samples were drawn at wholesale or
production levels or at the site of importation including: butter,
cheese, eggs, kidneys from pigs and cattle, and fat of poultry. Levels
in Danish butter (99.4% of the samples) were below 0.05 mg/kg and
those in imported butter (100%), below 0.125 mg/kg; Danish cheese
(100% of the samples) levels were below 0.05 mg/kg and, in imported
cheese, 82.4% of samples had levels below 0.125 mg/kg and the other
17.6%, below 0.2 mg/kg; 100% of eggs had levels below 0.05 mg/kg, and
100% of kidneys of pigs and cattle were below 0.15 mg/kg; 96% of
poultry fat samples had levels below 0.15, and 4%, below 0.20 mg/kg,
on a fat basis (not stated) (Statens Levnedsmiddelinstitut, Danmark,
undated).
Mes et al. (1989b) studied the presence of specific isomers of PCB
congeners in fatty foods of the Canadian diet. A total of 93 food
composites from the cities of Ottawa and Halifax were analysed for 34
PCB isomers, as part of a revised total diet programme. Each market
basket comprised approximately 200 different food types collected from
each of 4 major supermarkets in Ottawa during September 1985 and
January 1986, and, in Halifax, in September 1986. Foods were used
per se, or prepared and cooked in a manner ready for consumption,
then composited to give 112 composites from each market basket.
Thirty-one selected composites, representing the fatty foods were
analysed from each market basket.
PCB isomers 118, 138, 153, and 180 were found in all dairy products,
except skimmed milk. Cheese and butter contained the highest levels of
PCB residues. The residue level of isomer 118 (2,4,5,3',4'-
pentachlorobiphenyl) in butter was the highest e.g., 0.7 µg/kg, of all
PCB isomers found in dairy products. Almost all meat, fish, and
poultry contained PCB isomers 183 and 187. Occasionally, isomers 49,
87, 185, and 189 were also present, but isomer 105 (2,3,4,3'4'-
pentachlorobiphenyl), present in most dairy products, was only found
in some beef samples. Fresh water fish contained most PCB isomers (28
out of 34 selected PCB isomers), at levels considerably higher than
those in any other meat, fish, or poultry samples. The level of isomer
110 in fresh water fish was 3.05 µg/kg. PCB isomers 138, 153, 180, and
187 were present in almost all samples of meat and fish products,
fats, oils, and soups. Cooking fats, salad oils, and margarine
contained relatively low levels of PCB residues. PCB isomers 37, 49,
87, 105, and 185 were not detected in meat and fish products, fats,
oils, or soups.
The calculated sum of all PCB isomer residues found in selected food
commodities (except fish) ranged from 0.03 to 1.98 µg/kg on a wet
basis, and from 0.07 to 10.71 µg/kg on a lipid basis, with mean values
of 0.60 and 3.91 µg/kg, respectively. However, the mean residue levels
of fish and fish products were considerably higher, i.e., 10 and
194 µg/kg on a wet and lipid basis, respectively.
The major PCB isomers in fatty foods were isomers 37, 52, 99, 110,
118, 138, 153, 180, and 187.
The PCB levels obtained in an extensive study by the US Food and Drug
Administration are shown in Table 15. These values are considerably
higher than those reported from Sweden, but they are probably biased,
as they include samples originating from areas previously suspected of
having been subject to local pollution.
In a Canadian survey, PCB levels of less than 0.01 mg/kg were found in
eggs (Mes et al., 1974) and a mean of 0.042 mg/kg was found in
domestic and imported cheese with a maximum of 0.27 mg/kg (Villeneuve
et al., 1973b).
A preliminary study was carried out to estimate the dietary intake of
PCBs in fresh food composites grown in Ontario in 1985. The following
5 food composites: fresh meat and eggs, root vegetables (including
potatoes), fresh fruit, leafy and other above-ground vegetables, and
cow's milk were analysed. The concentrations in the different food
composites were below 0.0005 mg/kg. The annual dietary intake of PCBs
was estimated to be 32.6 µg (Davies, 1988).
In Japan, a similar range of PCB contents has been reported for most
foods; however, some high levels have been reported for rice and
vegetables harvested in fields polluted with PCBs (Environmental
Sanitation Bureau, 1973). The PCB content of most fish on the market
was less than 3 mg/kg.
Table 15. PCB levels in food in the USAa
Food % Positive Level in positive samples (mg/kg)
(0.1 mg/kg)
Mean Maximum
Cheese 6 0.25 1.0
Milk 7 2.3 27.8
Eggs 29 0.55 3.7
Fish 54 1.87 35.3
a From: Kolbye (1972).
Cantoni et al. (1988) analysed different food items, in 1985-87, in
Italy, taking 20-60 samples per item. Different types of meat were
analysed and the median concentrations were 0.25-0.50 mg/kg, on a fat
basis. Twenty to 50% of the samples were positive. Poultry contained
0.028 mg/kg, cow's milk 0.05 mg/kg, cream 0.027 mg/kg, butter
0.065 mg/kg and fish 1.105 mg/kg, on a fat basis; 71% of fish samples
contained PCBs.
When the fat of poultry (42 samples) and 44 eggs was analysed, PCB
values were below 0.3 mg/kg (Dutch Agricultural Advisory Commission,
1983).
In the Federal Republic of Germany, wheat was analysed during the
period 1972-82. The mean concentrations for 1972-78 ranged from 10 to
30 µg/kg; in the period 1980-82, the range was < 2.0-18 µg/kg (Klein,
1983). In wheat and rye (total 850 samples), median levels of
0.4-1 µg/kg product were found in 1984 (Codex Alimentarius, 1986). The
concentrations found in other food items are summarized in Table 16.
Samples of canned ham exported from Czechoslovakia to the USA in 1983
contained PCBs levels of up to 4.8 mg/kg (Anon., 1983a,b).
Table 16. PCBs in food (1982) in the Federal Republic of Germanya
Food Total no. Number of Variation Mean
of samples min-max (µg/kg)
samples below (µg/kg)
detection
limita
Milk 854 234 < 2-3000 126.7 (FB)
Beef 76 43 < 10-687 72.4 (FB)
Pork 58 36 < 10-458 58.1 (FB)
Poultry 64 61 < 10-85 7.3 (FB)c
Meat products 185 86 < 4-2700 114.2 (FB)
Eggs 82 67 < 5-230 9.1 (FW)
Fish (only 70 - 40-87 41.1 (FW)
cod, herring,
plaice)
Food of plant origin
Oil 167 139 < 5-65 7.1 (FB)
Cereals 345 44 < 2-30 6.7 (FW)
Potatoes 106 106 < 2 -
a From: Klein (1983).
b Not stated.
c Only 3 samples.
FB = fat basis
FW = fresh weight.
5.3.2 Drinking-water
Ruoff et al. (1988) examined 83 drinking-water samples from the
Federal Republic of Germany and from 5 other European countries for
their contents of the PCB congeners 28, 52, 101, 138, 153, and 180.
The average total content of the 6 congeners was 0.002 µg/litre water.
The average concentrations of the above-mentioned PCB congeners in the
drinking-water of 6 countries were 0.0001, 0.001, 0.00018, 0.00035,
0.00037, and 0.00042 µg/litre. The variation between the 6 countries
was quite small.
The highest concentration of PCBs reported in domestic tap water was
0.1 µg/litre in the Kyoto area of Japan (Panel on Hazardous Trace
Substances, 1972 cf. WHO/EURO, 1988), but, levels, more likely to be
encountered, should not exceed 0.001 µg/litre.
In the FAO/WHO collaborating centres for the food contamination
monitoring programme, the median levels were:
Cereals below 10 µg/kg
Vegetable fat/oils below 5 µg/kg
Fresh fruit and vegetables 0.5-5 µg/kg
Animal fat (depending on type of
animal and origin) 20-240 µg/kg
Whole fluid cow's milk (depending
on country) 10-200 µg/kg
(on fat basis)
Butter 30-80 µg/kg
Whole dried cow's milk 20-50 µg/kg
Hen eggs < 10 µg/kg
Fresh finfish 10-200 µg/kg
(WHO, 1985b).
The contamination of a drinking-water system in Pickens County, South
Carolina by PCBs discharged from a manufacturing facility was
described by Billings et al. (1978). They observed that PCBs
discharged by a capacitor manufacturing plant resulted in levels as
high as 0.818 µg/litre in finished potable water.
5.3.3 Dairy products
A number of data on food-producing animals have recently become
available within the framework of the Joint FAO/WHO Food Contamination
Monitoring Programme (JFCMP, 1985). All reported median values of PCBs
in animal fat (excluding milk fat) were below the respective limits of
detection, which varied from 0.001 mg/kg in the United Kingdom to a
high of 0.5 mg/kg in Thailand and the USA. Data on PCB levels in cow's
milk fat were supplied by the Federal Republic of Germany, Japan, the
Netherlands, the United Kingdom, and the USA. The United Kingdom and
the USA reported that median concentrations in cow's milk were below
the detection limits of 0.5 µg/kg and 0.5 mg/kg, respectively.
The available data are summarized in Table 17.
From the end of 1982 to the beginning of 1983, high levels of PCBs
were detected in milk from several dairy farms in Switzerland. The
investigations showed that the silo coatings and consequently the
silage from the silos were the origin of the contamination of the
milk. The PCB levels were between 0.80 and 3.80 mg/kg fat. PCB
dissolution in acid juice, mechanical erosion of the coatings, and
volatilization of the coating surface seemed to be the principal
mechanisms explaining the migration of PCBs into the silage
(Alencastro et al., 1984).
Forty-two samples of cow's milk (14 samples in 1976, 14 in 1983, and
14 in 1986) and 41 samples of market milk (10 in 1976, 16 in 1983, and
15 in 1986) were analysed for PCBs, in Israel. During this period, a
change was observed in the PCB distribution in the milk samples. The
percentage of hexachlorobiphenyl decreased with time and the
pentachlorobiphenyl increased (Pines et al., 1988).
The monitoring data for dairy products from all over the world for
1980-83 have been summarized by the Joint FAO/WHO Food Contamination
Monitoring Programme (WHO, 1986a,b).
5.3.4 Fish and shellfish
A summary of the monitoring data on fish from all over the world for
1980-83 has been published by the Joint FAO/WHO Food Contamination
Monitoring Programme (WHO, 1986a,b).
As might be expected, the PCB values found in fish depended on the fat
content and the pollution of the fishing area (Westöö & Norén, 1970a;
Berglund, 1972).
In a collaborative study by 7 national laboratories (International
Council for the Exploration of the Sea, 1974), the PCB contents in the
muscle tissue of fish taken from the North Sea were measured. A mean
of 0.01 mg/kg was found in cod, while herring contained up to
0.48 mg/kg, with most samples in the range of 0.1-0.2 mg/kg; plaice
contained 0.1 mg/kg or less. Similar values were reported by Zitko
(1974) for fish taken from the North Atlantic.
Risebrough & de Lappe (1972) reported levels higher than 3 mg/kg in
fish from New York Sound and Tokyo Bay, both very polluted areas. Even
higher levels of PCBs were found in fish from polluted lakes and
inland waterways, a level of 20 mg/kg being found in fish from Lake
Ontario, and levels over 200 mg/kg in fish from the Hudson River
(Stalling & Mayer, 1972). Similar correlations between pollution and
PCB levels have been reported from the United Kingdom in fish
(Portmann, 1970), and in mussels (Holdgate, 1971).
Table 17. Occurrence of PCBs in dairy products
Country Year Product Number of Mean concentrations Reference
samples in mg/kg on fat basis
(range)
North America
USA 1973-1974 milk (bulk) 198 (9 positive) 1.91 (0.32-4.99) Willett (1980)
Europe
Germany 1982-1986 milk 3279 0.09-0.14a DFG (1988)
(3 areas) 1983-1986 butter/cheese 2088 0.05-0.11
Westphalian area 1972-1974 butter - 0.38 (0.25-0.54) Claus & Acker (1975)
Northern part 1978-1980 milk - 0.17-0.20 Codex Alimentarius
1984 milk 3510 0.013 (1986)
Northern part - butter 1836 0.0077c Codex Alimentarius
(1986)
- meat and fat 957 (about 3/4 0.01b DFG (1988)
positive)
cows entrails 51 0.149b
Sweden 1972-1977 beef, pork and meat 232 (217 < 0.001-0.01 Vaz et al. (1982)
products (domestic negative) (whole product)
and imported)
Denmark 1981-1982 milk - 0.10-0.13 Jensen (1983b)
Table 17. (cont'd).
Country Year Product Number of Mean concentrations Reference
samples in mg/kg on fat basis
(range)
Netherlands 1975-1977 milk 315 0.16 (0.06-0.33) Gezondheidsraad
1980-1983 milk - 0.07-0.13 (1985)
1978-1984 milk 2319 < 0.1-0.2 Olling (1984)
1977-1981 cattle fat - 0.11b (< 0.05-0.55) Greve & Wegman
pork - 0.07 (< 0.05-0.66) (1983)
1983 fat of cattle, pork, 40-45 < 0.03b Dutch Agric. Adv.
calves Comm. (1983)
sheep 22 < 0.03b
Switzerland - milk 6 0.034-0.144 Rappe et al. (1987)
(6 locations)
a Major congeners were Nos. 138 and 153.
b Median value.
c Arithmetic mean.
Jensen et al. (1969) found PCB levels of 0.27 mg/kg and 0.33 mg/kg,
respectively, in the muscle tissue of herring and cod from the same
area of the Baltic, though the cod is at a higher trophic stage. The 2
species had 4.4 and 0.32% of extractable fat, respectively, and, when
the PCB level was calculated on the fat content, values of 6.8 mg/kg
for the herring and 11 mg/kg for the cod were obtained. Cod liver has
a much higher fat content than cod muscle, and Jensen (1973) reported
the ratio of PCB concentrations in cod liver and muscle to be over
100, the maximum in liver being 59 mg/kg. Jensen et al. (1969)
remarked that the considerable seasonal variation in the fat content
of the herring, rising from 1% in spring to 10% in autumn, influenced
the tissue level of PCBs.
There are many examples of different PCB levels in similar species
collected from areas of high and low pollution. Jensen et al. (1972b)
found 5 times as much PCBs in herrings caught in waters off
industrialized areas near Stockholm, as in herrings from the cleaner
waters of the west coast of Sweden.
Different freshwater and seawater fish were analysed for PCB contents,
during the period 1981-83, in the Netherlands. Eel from different
places over the period 1971-81 contained 0.2-13 mg/kg on a product
basis (in the edible part). The median value was between 1 and
2 mg/kg. Sea fish from the North Sea, such as herring and mackerel,
contained 0.1-0.2 mg/kg, on a fat basis. The same level was found in
shrimps and mussels (Freudenthal & Greve, 1973; Greve & Wegman, 1983;
van der Kolk, personal communication, 1984a).
The mean PCB contents in the liver of cod from the North Sea, North
Atlantic, and Baltic Sea, were 2.1-5.7, 0.48, and 10.4-12.8 mg/kg,
respectively (Klein, 1983).
When fish from the North Atlantic, North Sea, and Baltic Sea, were
collected in 1985, PCB concentrations of 0.098-0.123 mg/kg fillet
weight were found in fish from the North Atlantic and North Sea and
0.338 mg/kg fillet weight in fish from the Baltic Sea. In total, 60
samples were analysed. The PCBs 101, 138, and 153 were the major
congeners (DFG, 1988).
The PCB concentration in freshwater fish of the River Rhine was found
to be more than 2 mg/kg. The mean PCBs levels decreased, however, over
the period 1976-81 from 1.92 to 0.38 mg/kg (fresh weight) (Klein,
1983).
In 1984, PCB concentrations in freshwater fish (59 samples) collected
in the River Rhine ranged from 0.742 to 1.017 mg/kg fillet weight. In
this case, the major congeners were 138 and 153, but numbers 28, 52,
101, 180 were also present. In total, 199 samples of eel were
collected in a number of surface waters and analysed for the presence
of PCBs. The levels ranged from 1.42 to 6.51 mg/kg fresh weight. In
studies reported by DFG (1988), the highest levels of PCBs were found
in the River Rhine.
In the United Kingdom, fish and shellfish were analysed for PCBs
during the period 1982-84 (HMSO, 1986). The results are summarized in
Table 18.
Table 18. PCB levels in marine fish and shellfisha
Year Product Tissue No. of Range (mg/kg)
samples
1982 Marine fish (from England) liver 381 0.3-4.1
(7 types of fish)
1982 Marine fish (from England) muscle 326 0.03-0.13
(7 types of fish)
1983 Marine fish (imported) muscle 102 nd-0.06
(5 types of fish)
1983 Shellfish (imported) muscle 53 nd-0.06
(4 types of shellfish)
1984 Fish oils 16 0.11-2.3
a From: HMSO (1986).
Different types of marine fish and shellfish from different areas in
the United Kingdom were analysed during the period 1977-84. Those from
the North Sea coast contained concentrations in the range of 0.04-5.7
and < 0.001-0.058 mg/kg, respectively, while those from the English
channel contained < 0.05-6.9 and < 0.006-0.1 mg/kg, respectively,
and those from the West coast, < 0.002-8.4 and < 0.001-0.25 mg/kg
wet weight. PCB concentrations in fish livers of 0.2 up to 12.9 mg/kg
wet weight were found during this period (Franklin, 1987).
When samples of fish of different species, collected from major USA
watersheds in 1976, were analysed, PCBs were found in 93% of the
samples. Fifty-eight of the samples had levels exceeding 5 mg/kg, on a
whole fish basis. The PCB concentrations ranged from less than 0.3 to
140 mg/kg, on a whole fish basis (Veith et al., 1979).
Maack & Sonzogni (1988) analysed 98 fish (14 species) of different
sizes from Wisconsin waters, for the presence of PCB congeners. Among
the most prominent congeners were numbers 153/132, 138, 66/95, 110,
180, 70/76, 146, 28/31, 149, 118, and 105. The total PCBs (determined
by adding individual congener concentrations) ranged from 0.070 to
7.0 mg/kg. The mean concentration was 1.3 mg/kg.
Blue crabs ( Callinectes sapidus, an important member of the
estuarine food web), collected from Campbell Creek and surroundings in
South Carolina, were analysed for PCBs in 1985. The highest mean total
concentration was 0.861 mg/kg muscle tissue. In 1986, the mean
concentrations in blue crab collected by 8 stations in the same area
ranged from 0.026 to 0.361 mg/kg muscle tissue. Blue crab (15 samples)
collected from the coast of South Carolina, contained concentrations
of < 0.020-0.372 mg/kg tissue (Marcus & Mathews, 1987).
PCBs concentrations in sea fish were determined in 1971-77 in Japan.
In-shore fish (90 samples) showed concentrations of 0.2-0.72 mg/kg
fresh weight and pelagic fish (112 samples), 0.005-0.265 mg/kg fresh
weight (Watanabe et al., 1979).
Data on individual species of fish, submitted by Japan, showed the
following median levels: barracuda, 70 µg/kg; conger eel, 290 µg/kg;
croaker, 200 µg/kg; flounder (yellow-tail), 90 µg/kg; hair-tail,
100 µg/kg; mullet, 84 µg/kg; and seabass, 110 µg/kg. Median levels for
other species of fish, such as cod, mackerel, pacific saury, rockfish,
salmon, and sardines, were below 100 µg/kg (WHO, 1986b).
Using a very sensitive analytical method, Tanabe et al. (1987) found
the toxic non- ortho-substituted coplanar 3,4,3',4'-tetrachloro-,
3,4,5,3',4'-pentachloro-, and 3,4,5,3',4',5'-hexachlorobiphenyl in
finless porpoise, at concentrations of 13.5, 0.89, and 0.64 µg/kg,
respectively.
Blue mussel (Mytilus edulis) was collected from coastal areas near
Osaka and Hokkaido, Japan, in 1984-86. Depending on the site of
collection, the average PCB concentrations (11-13 samples) ranged from
0.56 to 65.0 µg/kg (Miyata et al., 1987).
5.3.5 Influence of food processing
Fifty striped bass (Morone saxatilis) were analysed for the presence
of PCBs in the fish fillets before, and after, boiling, steaming,
baking, frying, microwaving, or poaching, to study the possible
reduction of the PCB residues by these cooking procedures. PCB
contents were reduced by approximately 10%, by all 6 methods of
cooking. No significant reductions were observed with the other
cooking methods (Armbruster et al., 1987).
5.3.6 Food contamination by packaging materials
When Villeneuve et al. (1973a) analysed packaged food in Canada, they
found that 66.7% of the samples contained PCB levels of less than
0.01 mg/kg, 30.7% contained between 0.01 and 1 mg/kg, and 2.6%
contained more than 1 mg/kg. The highest PCB levels were in a rice
sample (2.1 mg/kg), where the packaging material contained 31 mg/kg,
and in a dried fruit sample (4.5 mg/kg), in a container containing
76 mg/kg. In a survey of packaging containers, approximately 80% were
found to contain PCB levels of less than 1 mg/kg, while about 4%
contained levels higher than 10 mg/kg. The most likely source of PCBs
in packaging materials was the recycling of waste paper containing
pressure-sensitive duplicating paper (carbonless copying paper)
(Masuda et al., 1972).
Relatively high PCB levels in some packaged foods in Sweden, mainly of
imported origin, could be attributed to migration from the packaging
material (Westöö et al., 1971). The highest level encountered was
11 mg/kg in a childrens' breakfast cereal; PCB levels of 70 mg/kg and
700 mg/kg were found in the material of the inner bag containing this
product and in the outer cardboard container, respectively. Up to
2000 mg/kg was found in cartons of other samples.
In the United Kingdom, levels in imported waste-paper, which could be
contaminated with PCBs from carbonless copying paper and subsequently
used to manufacture food contact paper and board materials, were found
to be low, compared with the 10 mg/kg limit for PCBs recommended by
the British Paper and Board Industry Federation for food contact
materials (HMSO, 1989).
5.3.7 Appraisal
Foods have become contaminated with PCBs by 3 main routes:
* accumulation of PCBs in the different food-chains in the
environment and consumption of fish, birds, or other animals and
crops;
* direct contamination of food or animal feed by an industrial
accident;
* migration from packaging materials into food.
During the past years, many thousands of samples of different
foodstuffs have been analysed for PCB contamination. The most common
foodstuffs analysed have been fish, meat, and milk. Many fish samples
have been taken in an effort to monitor aquatic pollution. In
addition, samples have been taken, for regulatory or similar purposes,
from sources suspected of being relatively highly contaminated. The
fact that most samples have not been taken at random, jeopardizes the
proper assessment of the exposure of the general population.
5.4 General population exposure
5.4.1 Air
Relatively high levels of PCBs have been detected in indoor air,
especially in kitchens and offices with electric installations
(Jensen, 1983a) (section 3.2.4 and 5.1.1).
Results from the US EPA indicate PCB concentrations in the air ranging
from 1 up to 50 ng/m3; similar results have been reported from Japan
(WHO, 1976). Assuming a level of 5 ng PCBs/m3 in urban air, a
breathing rate of 22 m3/day, retention and absorption of inhaled
particles/vapour of 50%, and a mean residence time of PCBs in the body
of 3 years, air would contribute 0.8 µg/kg to the PCB concentration in
the body. Higher concentrations of PCBs in indoor air could increase
this estimate (WHO/EURO, 1988).
Van der Kolk (1985) calculated air intake through inhalation for the
Dutch population of about 36 ng/day, a quantity approximately 1000
times lower than the intake with food.
During the manufacture, formulation, or use of PCBs, where levels in
the workroom air correspond to exposure limit values, varying between
0.1 mg/m3 and 1 mg/m3, the calculated mean intakes would range
between 1 and 10 mg during an 8-h workshift. In some occupational
situations, much higher concentrations have been measured and the
estimates of intakes would be higher (WHO/EURO, 1987).
5.4.2 Drinking-water
Levels reported in drinking-water are typically between 0.1 and
0.5 ng/litre. Even assuming a PCB level of 2 ng/litre in
drinking-water, consumption of 2 litre/day contributes 0.04 µg/kg body
weight to the PCB concentration in the body. This additional quantity
is negligible in comparison with the intake via food (WHO/EURO, 1988).
5.4.3 Intake by infants through mother's milk
The daily intake of PCBs was calculated in breast-fed infants in the
countries participating in a monitoring study by Slorach & Vaz (1983,
1985) (Table 19).
The intakes in EEC countries were calculated to range from 3 to
11 µg/kg body weight per day, compared with 0.12-0.3 µg/kg body weight
for bottle-fed infants in Denmark (WHO/EURO, 1985).
In Yusho infants with clinical symptoms of poisoning, the daily intake
of PCBs with breast milk was calculated to be 70 µg/kg body weight
(Jensen, 1983b) (see section 9.1.2.2).
5.4.4 Infant and toddler total diet
Johnson et al. (1979) analysed the average diet of 6-month-old infants
and 2-year-old toddlers for the presence of PCBs. Ten market baskets
were collected in 10 cities in the USA. The foods were prepared in the
manner in which they would be prepared and served in the home. Trace
amounts of PCBs were detected in only one infant and one toddler diet.
In the USA, Gartrell et al. (1986b) found a daily intake of 0.011 µg
PCBs/kg body weight in infants consuming infant diets in 1978. In the
years 1979, 1980, and 1981/82, the intake was below the detection
level. The intake by toddlers was 0.099 µg/kg body weight in 1978 and
not detectable in the following 3 years.
Tuinstra et al. (1985a) analysed samples of infant food from the Dutch
market and found average PCB levels of 0.1-0.2 µg/kg food (the maximum
level found was 1.1 µg/kg).
5.4.5 Total intake by adults via food
The oral consumption of contaminated products is presumed to be the
main route of exposure to the PCBs.
Table 19. Calculated daily intakes of PCBs by breast-fed infants (µg/kg body weight)a
Country/area Year(s) Calculation according Calculation according
to US FDA methodb to national methodc
median maximum median maximum
Belgium,
Brussels 1982 3.6 10.4 NR NR
China,
Beijing 1982 NR NR 0.45d 0.45d
Israel,
Jerusalem 1981/82 2.0 9.5 NR NR
Germany,
Hanau 1981 NR NR 9.5 45
Japan,
Osaka 1980/81 1.6 4.4 2.3 6.3
Sweden,
Uppsala 1981 4.4 8.1 5.9 11
USA
22 states 1979 4.5e 13.5 4.5e 22.5
Yugoslavia,
Zagreb 1981/82 2.8 7.2 2.8 7.7
a Assuming a milk consumption of ca 130 g/kg body weight and a milk fat content of
3.5% (w/w). Calculations based on data for all mothers studied. Results for
different methods of PCB analysis shown separately.
From: Slorach & Vaz (1983, 1985); Van der Kolk (1984b).
b Sawyer method.
c "Own method".
d PCB level below limit of detection (0.1 mg/kg fat) in milk samples.
e PCB level below limit of detection (1 mg/kg fat) in milk samples.
NR = No data on levels in milk reported.
It has been stated that the major part of the human dietary intake of
PCBs is from fish (Berglund, 1972; Hammond, 1972). This may well be
true in areas such as Japan or certain localities near the North
American Great Lakes, where fish from polluted waters may form a
relatively large part of the diet. Several investigators from Japan
have measured the daily intake of PCBs in food; the highest mean value
recorded was 48 µg/day, of which 90% was from fish (Kobayashi, 1972);
the lowest was 8 µg/day (Ushio et al., 1974).
In much of Europe and North America, however, the daily intake of fish
is in the region of 30-40 g, and most of the fish is taken from waters
of low pollution with PCB levels in the fish not exceeding 0.1 mg/kg.
Berglund (1972) has estimated that the daily intake of PCBs from fish
in Sweden is in the region of 1 µg, though if the fish consumed were
solely Baltic herring, the intake would be about 10 µg/person. It is
difficult to make an assessment of the PCB intake from foods other
than fish. Westöö et al. (1971) in their extensive study of the
Swedish diet, reported that most foods contained PCB levels of less
than 0.1 mg/kg; and concluded that this corresponds to a daily intake
of less than 100 µg.
Weekly intakes in the range of 23-889 µg/person have been reported
from the USA (OTA, 1979). The higher range concerns people consuming
more than 12 kg/year of Lake Michigan fish.
The intake of total PCBs by the general adult population depends
greatly on the geographical area and food habits.
5.4.6 Total diet/market-basket studies
Data on total-diet studies of PCBs have been reported from a few
countries. These reported intakes show a wide variation, which can
partially be explained by methodological factors, such as the ways in
which samples below the limits of determination are considered,
especially when noting the different limits of determination.
Considering the available data, an average intake of 5-15 µg/day for
the non-occupationally exposed population in industrialized countries
may be the best available estimation.
These estimates apply to the average diet of an average adult citizen.
In practice, few people are really "average" in their consumption
pattern. Given the widespread nature of the contamination, however, a
higher intake in one food group is more or less balanced by a lower
intake in another food group with an equal calorie intake. Total
intake will certainly be higher for diets with a more than average
calorie content (van der Kolk, 1985).
Gartrell et al. (1985) determined the total intake of PCBs by 16- to
19-year-old males in the USA. The samples represent a typical 14-day
diet. Approximately 120 individual food items (of 12 food groups),
including drinking-water, were collected for each market-basket sample
in 20 cities in the period 1979-80. Only 2 samples of meat, fish, and
poultry contained PCBs with an average concentration of 0.002 mg/kg.
Gartrell et al. (1985, 1986a) reported a daily intake in the USA of
0.016, 0.027, 0.014, 0.008, and 0.003 µg PCBs/kg body weight during
the years 1977, 1978, 1979, 1980, and 1981/82, respectively.
Manske & Johnson (1975) collected 35 market baskets in 32 cities over
the period 1971-72. PCB residues were found in the range of
0.035-0.15 mg/kg in 51 composites. Fish and oils, fats, and
shortenings contained the highest levels. The same authors (Manske &
Johnson, 1977) carried out a market-basket study representing the
basic 2-week diet of a 16- to 19-year-old male. The various foods were
prepared in the manner in which they would normally be served and
eaten. Thirty market-baskets, containing 12 classes of foods (in total
360 composites) were collected in 30 cities in the period 1973-74. A
trace of PCB was found once in whole milk, ground beef, and fish
fillet.
The FDA revised the concept of the Total Diet Study in 1982. As
discussed by Gunderson (1988b), the Total Diet Study conducted before
1982 was based on a "composite sample approach", regardless of the
diet involved. The revised study is based on updated dietary survey
information and allows the "total diet" of the US population to be
represented by a relatively small number of food items for a greater
number of age/sex groups. The daily intake expressed in ng/kg body
weight per day for PCBs (Aroclor 1221, 1242, and 1254) in 1982-84 for
the age groups 6-11 months, 2 years, 14-16-year females, 14-16-year
males, 25-30-year females, 25-30-year males, 60-65-year females and
60-65-year males were: 0.8, 1.2, 0.4, 0.5, 0.5, 0.6, 0.4, and
0.5 ng/kg body weight per day, respectively (Gunderson, 1988b).
Foods, representative of Canadian eating habits, as determined by a
national nutritional survey, were prepared for eating, categorized,
and blended into 11 different composites representing the dietary
intake for 5 cities over the period 1976-78. It concerned 194 samples,
collected in winter and in summer. The average dietary intake was
0.001 µg PCB/kg body weight (McLeod et al., 1980).
Over a period of 2 years, 126 different food items of a market-basket
of 16- to 18-year-old males were purchased every 2 months in the
period 1976-78, in the Netherlands. The foodstuffs were prepared for
eating and were combined in 12 commodity groups. The mean
concentration and range of PCBs in 5 food classes was:
Class Mean concentration Range
(mg/kg on fat basis)
Meat, poultry, and eggs - 0.13-0.17 (2)a
Fish 0.07 0.04-0.24 (7)
Dairy products - 0.04-0.06 (2)
Sugar and sweets - 0.08 (1)
Drinks, drinking-water - 0.035 (1)
a In parentheses: number of positive composites.
The authors calculated a daily intake of PCBs of 15 µg/person (a
maximum level was 90 µg/person (de Vos et al., 1984). In the period
May-July 1976, 100 total diets (summer meals) were collected and
besides organochlorine pesticides, PCBs were determined as
decachlorobiphenyl, after perchloration, and calculated as Aroclor
1260. The mean intake of PCBs/person per day was 11.6 µg with a range
of 3-71 µg (Greve & van Hulst, 1977; Greve & Wegman, 1983; van der
Kolk, 1985).
In 1978, another survey was carried out with 100 total diets during
the winter (winter meals). It was estimated that the daily intake was
6 µg/person (range 1-19 µg).
Zimmerli & Marek (1973) studied the total human intake of PCBs from
prepared meals in 1971-72 in Bern, Switzerland. Five typical total
diets were composed and analysed. The intake of PCBs, especially with
daily diets containing cheese, meat, fish, or fat, ranged from 6 to
84 µg.
According to a calculation by Summerman et al. (1978), the average
weekly intake of PCBs in the Federal Republic of Germany was about 215
and 268 µg/week for females and males, respectively. Much lower
figures, 36-44 µg/week, were calculated by Klein (1983).
A survey of the daily PCB intake from the total diet of Japanese women
(number of samples varied from 18 to 60) was performed for the years
1972-76. The daily intake of PCBs averaged approximately 10 µg/person
(range 2.8-21.2 µg). The main source of PCBs in the diet of Japan was
in-shore fish. There was no clear change in daily intake over the
5-year period studied (Watanabe et al., 1979).
Ushio & Doguchi (1977) studied the dietary intake of PCBs in Tokyo.
They found an average daily intake of PCBs of 6.3 µg/person (range,
trace-17 µg/person). It was concluded that the dietary daily intake of
PCBs for the majority of the population of Tokyo rarely exceeded
20 µg/person, when no heavily contaminated fish were consumed.
Yakushiji et al. (1977) found that the PCB daily intake through meals
of unexposed adults living in Osaka prefecture, was 3-20 µg/day.
Data for PCBs in the diets of Canada, Guatamala, Japan, the United
Kingdom, and the USA over the period 1972-83 were summarized by
Gorchev & Jelinek (1985). The mean dietary intake reported was at, or
below, 0.06 µg/kg body weight, the mean intake per person ranged from
< 0.01 to 0.12 µg/kg body weight (Slorach et al., 1982; WHO, 1986b).
5.4.7 Total intake of major congeners by adults via food
In the Federal Republic of Germany, the daily intake of the 3 PCB
congeners numbers 138, 153, and 180, together with the different food
items, was calculated. The intake (µg/day) with meat and meat products
was 0.30; with fish and fish products 0.36; eggs and egg products
0.008; milk and milk products 0.40; cheese 0.11; butter 0.39; fats and
oil 0.098; bread and pastries 0.17; potatoes 0.081; vegetables 0.11
and fruits 0.082 (DFG, 1988).
5.4.8 Time trends in different matrices
Although many countries introduced severe restrictions on the
manufacture, use, and disposal of PCBs many years ago, it is difficult
to discern any marked decline in the levels in human milk fat, from
the published data.
Levels of PCBs were estimated in 1085 samples of different cereals,
collected in the Federal Republic of Germany over the period
1972/74-1984. The levels, which were the highest in 1972/74 0.04 mg/kg
(0.005-0.12 mg/kg), decreased during the years to 0.004-0.005 mg/kg
dry weight in 1984 (DFG, 1988).
Data from the Federal Republic of Germany showed no clear trend in PCB
levels in human milk during 1975-79 (Slorach et al., 1982). The same
was found in the Netherlands over the period 1974-83 (Greve & Wegman,
1984).
Japanese data showed a decline in PCB levels in the fat of whole cow's
milk during the period 1972-79. A decline was also found in PCB levels
in finfish from coastal waters and in total marine fish (Slorach et
al., 1982).
A downward trend was found in human milk from Japan over the period
1972-80. Each year, a large number of samples (361-877 samples/year)
were analysed. In 1972, the median level was about 0.8 mg/kg and, in
1980, 0.5 mg/kg, on a fat basis. A gradual decline was observed
(Slorach & Vaz, 1983).
In Canada, human milk and adipose tissue from Ontario residents were
analysed over the period 1969-74. The values found did not indicate a
trend in this period.
The mean total PCB intakes determined in the FDA Total Diet Study, for
the period 1971-87, for a typical "adult" diet, represented in Fig. 4,
reflect that of a 14- to 16-year-old male during 1982-87. A clear
decline was shown from approximately 7 µg/person per day to less than
0.1 µg/person per day (Gunderson, 1988a).
The daily intake of PCBs, expressed as ng/kg body weight per day, by
6-month-old and 2-year-old children in the years 1980, 1981/82, and
1982/84 did not show a trend, while, in adults, a decrease from 8 to
0.5 ng/kg body weight per day was observed over the same years
(Gunderson, 1988b).
5.5 Concentrations in the body tissues of the general population
The PCB levels in body tissues are a good indication of the overall
and total exposure of the body to PCBs.
Several factors may influence the concentrations of PCBs in body
tissues, including duration and level of exposure, the route and
pattern of exposure, the chemical structure of the PCB (degree and
position of chlorination in the molecule), the amount of adipose
tissue, other simultaneous exposures, as well as other biological
parameters.
5.5.1 Adipose tissue
In general, while highly chlorinated congeners accumulate more easily,
a lower degree of substitution provides more possibilities for
hydroxylation and facilitates excretion. Factors other than the degree
of substitution also affect accumulation, particularly the position
and pattern of substitution (WHO/EURO, 1987).
The available information on the occurrence of PCBs in the body fat of
the general population is summarized in Table 20.
Brown et al. (1987a,b) examined patterns of PCB congeners remaining in
sediments after spills of commercial mixtures of Aroclor. Sediment
from 5 different sites was examined. Shifts in gas chromatographic
peak distribution were indicative of dechlorination of congeners by
anaerobic bacteria in the sediment. Analysis of sediment from
different depths indicated less difference from the original traces in
superficial layers and the greatest shift in deeper layers of the
sediment cores. They concluded that dechlorination had taken place and
deduced several different processes involved by comparison between
sites. Six of these processes have been characterized in detail, each
presumed to be mediated by different populations of anaerobic
bacteria, with different selectivity for different congeners in the
PCB mixture. The point of most interest was that congeners with high
degrees of chlorination were selectively dechlorinated by these
anaerobic organisms. Whilst dechlorination still leaves the mass of
PCB intact, congeners with lower chlorination can be more readily
degraded by aerobic bacteria. This anaerobic dechlorination,
therefore, enables further degradation to take place elsewhere and
contributes significantly to the detoxification of the PCBs. While the
combined meta- para selective dechlorinating/oxidizing action of
sediment microbes for PCB residues is likely to be detoxifying, with
respect to dioxin-like effects, there are reservations about whether
this action would be detoxifying in respect of other, more subtle
toxic effects of PCBs and their degradation products, known (such as
the potential reproductive toxicity of the hydroxylated,
ortho-enriched PCBs from sediment microbe action) and unknown. This
is why it is important to study not only the disappearance of PCBs,
but also the exact nature and amounts of the degradation products
(McKinney et al., 1990). Two broad categories of transformation have
been observed: the first dechlorinates in the ortho, meta, and para
positions and the potential for the dechlorination of biphenyls is
related to the reduction potential of the compound, the second
dechlorinates only in the meta and para positions, and the
reactivities of the congeners relate to the molecular shape. The
second category suggested to the authors an active site on a
dechlorinating agent that would be roughly conical with a reducing or
hydrogenating site at the apex. In this schema, para-substituted
molecules could enter the site directly, enough rotation of the
molecule would be possible for the accommodation of meta, but not
ortho, substitution. Quensen et al. (1988) demonstrated this
dechlorinating capacity of anaerobic bacteria from Hudson River
sediments in the laboratory. Dechlorination occurred primarily from
the meta and para positions; ortho-substituted congeners
accumulated selectively. The fastest rate of dechlorination occurred
at the highest exposure used (700 mg Aroclor 1242/kg); 53% of the
total chlorine was removed over a 16-week incubation period. During
incubation, the proportion of mono- and dichlorobiphenyls increased
from 9 to 88%. The authors believed that a sequential anaerobic to
aerobic system could be devised for the biological degradation of
PCBs.
4.2.2.2 Fungi
Wallnofer et al. (1973) incubated a soil fungus Rhizopus japonicus
in a medium containing 3H-labelled 4-chlorobiphenyl or
4,4'-dichlorobiphenyl. After incubation for 1 week, the fungal
mycelium was filtered out. Scans of TLC plates indicated a
hydroxybiphenyl derivative present in the filtrate of both cultures.
To further identify the metabolite, larger amounts of unlabelled
4-chlorobiphenyl were added to a similar culture. The NMR and mass
spectra were identical to a synthetic sample of 4- chloro-4'-hydroxy-
biphenyl; mixed melting point determination showed no depression.
Further positive identification of the product was not possible,
because of limited material, but the experiment indicates the
probability of degradation of biphenyl to a hydroxy derivative by a
fungus.
4.2.3 Photodegradation
Several authors have reported that simple chlorinated biphenyls, as
well as complex commercial PCB mixtures, undergo photoreduction in
organic solvents (Safe & Hutzinger, 1971; Hustert & Korte, 1972; Ruzo
et al., 1972, 1974, 1975; Sawai & Sawai, 1973; Koshioka et al., 1987)
and aqueous systems (Crosby & Moilanen, 1973; Bunce, 1978) in the
laboratory. Herring et al. (1972) found that PCBs degraded faster in
hexane solution than in aqueous solution and slower in benzene
solution.
Bunce et al. (1978) posed the question of the environmental
significance of the photodegradation of PCBs and tried to estimate the
likely degree of photolysis under real environmental conditions,
rather than in solution in organic solvents at high concentrations.
The current best estimate suggests that significant amounts,
particularly of higher chlorinated PCB congeners, might be degraded in
water by the action of sunlight.
4.2.4 Bioaccumulation, distribution in organisms, and elimination
Polychlorinated biphenyls accumulate in almost all organisms, because
of their high lipid solubility and slow rate of metabolism and
elimination. They accumulate preferentially in fat-rich tissues.
Bioconcentration factors (BCFs) should be interpreted with caution,
since they are simple ratios. The exposure concentration, therefore,
makes a marked difference to the BCF obtained; very low exposure
concentrations are likely to lead to high BCFs, since all the PCBs are
absorbed, whilst high exposure concentration will tend to minimize the
BCFs.
Experimental data on the bioconcentration of PCB mixtures and pure
chlorinated biphenyls are presented in Table 9 for microorganisms,
Table 10 for aquatic organisms, and Table 11 for plants, birds, and
mammals.
4.2.4.1 Microorganisms
Uptake of both pure chlorinated biphenyl isomers and commercial PCB
mixtures by microorganisms is rapid, and high bioconcentration factors
are achieved. While there is a suggestion in studies on some species
that PCB congeners with higher levels of chlorination are taken up
preferentially, in the majority of studies, all PCBs appear to be
taken up equally. Uptake is true absorption; adsorption onto the
surface of the organisms represents little of the uptake. Since
resistant forms of microorganisms take up less PCBs than sensitive
forms and dead cells accumulate more PCBs than live ones, there is
some capacity to exclude the compounds.
Harding & Phillips (1978b) studied the uptake of 14C-labelled
2,4,5,2',5'-pentachlorobiphenyl, at concentrations of 0.31 or
9.86 µg/litre water, by 11 marine phytoplankton species including:
diatoms, green algae, chrysophytes, haptophytes, and dinoflagellates.
The cell density of each culture was maintained at 106-109 cells/
litre. Equilibrium between water and cell concentrations of biphenyl
was reached very rapidly after 0.5-2 h; small motile forms reached
equilibrium within 1 h and large centric diatoms after approximately
2 h. Exposure concentration and cell density, within the range given
above, had little effect on the time-course of uptake. Substantial
interspecies differences in adsorptive capacity were shown by
differences in the Freundlich adsorption constant (log K). A large
centric diatom, Coscinodiscus sp., had the highest log K. Nitzschia
longissima, a penate diatom that has been shown to be resistant to
PCBs (Harding & Phillips, 1978a), had the lowest log K value. The
flagellates, with the exception of Monochrysis lutheri, which has
been shown to be very sensitive to the effects of PCBs, had much lower
log K values than diatoms. Concentration factors, calculated from the
Freundlich adsorption isotherms, ranged between 12 300 and 2 410 000.
Biggs et al. (1980) exposed mixed species of estuarine phytoplankton
(numerically dominated by the diatom Skeletonema costatum) to
14C-labelled PCB (approximately 54% chlorine by weight) at
concentrations of 5.8 or 11.6 µg/litre. At a particle concentration of
25 mg/litre, 19-22% of the labelled-PCB was sorbed on the particles
after a 1-h exposure, with 70-72% in the water. At 4 times the above
particle concentration, 66-69% was sorbed on particles and only 22-23%
was retained in the water. Doubling the amount of 14C-PCB doubled the
mean amount of labelled-PCB in both the particles and the water. The
authors calculated an index of sorption (the ratio of 14C-PCB sorbed
on particles to that in an equal volume of water) at an average of
2 ± 1 × 104. The authors suggested that the higher uptake (88%) of
PCBs found by Södergren (1971) was probably the result of an
unnaturally high cell concentration. Phytoplankton sampled in the
surface waters of Long Island Sound, USA, varied seasonally in
concentration from about 0.5 to 30 mg/litre.
Lederman & Rhee (1982) calculated bioconcentration factors for 3
species of Great Lakes planktonic algae (Table 9). In the case of
Fragilaria crotonensis, the uptake of hexachlorobiphenyl into the
frustule (the siliceous wall of the diatom) was investigated. The
bioconcentration factors for frustules were lower by an order of
magnitude than the factors for live and dead cells. It appears,
therefore, that adsorption on the cell surface contributes only a
little to the bioaccumulation of hexachlorobiphenyl.
Table 9. Bioaccumulation of PCBs: Microorganisms
Organism Biomass Temperature PCB type Duration Exposure Bioconcentration Reference
(cells/ml) (°C) (µg/litre) factora
Green alga 2 × 106 20-25 TeCB 1 h 10 3200 Urey et al. (1976)
Chlorella 2 × 106 20-25 HeCB 1 h 10 7000 Urey et al. (1976)
pyrenoidosa 2 × 106 20-25 OcCB 1 h 10 1600 Urey et al. (1976)
2 × 106 20-25 DeCB 1 h 10 5200 Urey et al. (1976)
Algae 3.2 × 105 HeCB 19 h 1 117 000b Lederman & Rhee
Fragilaria 1.6 × 105 HeCB 19 h 1 313 000b (1982)
crotonensis Lederman & Rhee
(1982)
Algae 3.4 × 105 HeCB 6 h 1 619 000b Lederman & Rhee
Ankistrodesmus 1.7 × 105 HeCB 6 h 1 959 000b (1982)
falcatus 8.5 × 104 HeCB 6 h 1 1 207 000b Lederman & Rhee
(1982)
Lederman & Rhee
(1982)
Algae 1.1 × 106 HeCB 6 h 1 129 000b Lederman & Rhee
Mycrocystis sp. 5.5 × 105 HeCB 6 h 1 170 000b (1982)
2.8 × 105 HeCB 6 h 1 264 000b Lederman & Rhee
(1982)
Lederman & Rhee
(1982)
Table 9. (cont'd).
Organism Biomass Temperature PCB type Duration Exposure Bioconcentration Reference
(cells/ml) (°C) (µg/litre) factora
Fungus 22-25 Aroclor 1254 24 h 0.007 mg/kg 1327b,c Pinkney et al. (1985)
Fusarium 22-25 Aroclor 1254 48 h 0.007 mg/kg 1144b,c Pinkney et al. (1985)
oxysporum
a Concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration factors calculated on a wet weight
basis unless otherwise stated.
b Calculated on a dry weight basis.
c Radioactive isotope used to calculate bioconcentration factor.
Table 10. Bioaccumulation of PCBs: Aquatic organisms
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
American oyster flow WB Aroclor 1016 96 h 0.6 6666 Hansen et al. (1974b)
Crassostrea WB Aroclor 1254 56 d 0.01 165 000 Parrish (1973)
virginica WB Aroclor 1254 392 d 0.01 89 000 Parrish (1973)
Polychaete stat WB Aroclor 1254 5 d 1.1 236 Courtney & Langston
Arenicola marina stat WB Aroclor 1254 5 d 1 mg/kgd 0.24 (1978)
Polychaete stat WB Aroclor 1254 5 d 1.1 373 Courtney & Langston
Nereis stat WB Aroclor 1254 5 d 1 mg/kgd 0.36 (1978)
diversicolor
Water flea flow WB 20-22 Aroclor 1254 96 h 1.1 47 000e* Sanders & Chandler
Daphnia magna (1972)
Amphipod (M) statf WB Aroclor 1254 24 h 0.03 8700 Pinkney et al. (1985)
Gammarus statf WB Aroclor 1254 24 h 195.8 mg/kg 0.118 Pinkney et al. (1985)
tigrinus
Scud flow WB 20-22 Aroclor 1254 96 h 1.6 24 000e* Sanders & Chandler
Gammarus flow WB 20-22 Aroclor 1254 21 d 1.6 27 000e (1972)
pseudolimnaeus
Glass shrimp flow WB 20-22 Aroclor 1254 96 h 1.3 12 300e* Sanders & Chandler
Palaemonetes flow WB 20-22 Aroclor 1254 21 d 1.3 16 600e* (1972)
kadiekensis
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Brown shrimp flow WB Aroclor 1016 96 h 0.9 4222 Hansen et al. (1974b)
Penaeus aztecus
Grass shrimp flow WB 17-28 Aroclor 1254 7 d 2.3 11 000 Nimmo et al. (1974)
Palaemonetes flow WB 17-28 Aroclor 1254 16 d 1.3 14 000 Nimmo et al. (1974)
pugio flow WB 17-28 Aroclor 1254 28 d 0.62 17 450 Nimmo et al. (1974)
flow WB 17-28 Aroclor 1254 35 d 0.62 26 580 Nimmo et al. (1974)
flow WB Aroclor 1016 96 h 0.4 2750 Hansen et al. (1974b)
Crayfish flow WB 20-22 Aroclor 1254 96 h 1.2 1700e* Sanders & Chandler
Orconectes nais flow WB 20-22 Aroclor 1254 21 d 1.2 5100e* (1972)
Stonefly flow WB 20-22 Aroclor 1254 96 h 2.8 2500e* Sanders & Chandler
Pteronarcys flow WB 20-22 Aroclor 1254 21 d 2.8 2800e* (1972)
dorsata
Dobsonfly flow WB 20-22 Aroclor 1254 96 h 1.1 4600e* Sanders & Chandler
Corydalus flow WB 20-22 Aroclor 1254 21 d 1.1 6800e* (1972)
cornutus
Phantom midge flow WB 20-22 Aroclor 1254 96 h 1.3 23 600e* Sanders & Chandler
Chaoboruspuncti (1972)
pennis
Mosquito larvae flow WB 20-22 Aroclor 1254 96 h 1.5 18 000e* Sanders & Chandler
Culex tarsalis (1972)
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Mayfly flow WB 8 Clophen A50 6 d 0.526 2940 Södergren &
Ephemera danica Svensson (1973)
Pinfish flow WB Aroclor 1016 96 h 0.8 2750 Hansen et al. (1974b)
Lagodon flow WB Aroclor 1016 28 d 1 n 25 000 Hansen et al. (1974b)
rhomboides flow WB Aroclor 1016 56 d 1 n 17 000 Hansen et al. (1974b)
Sheepshead flowg WB Aroclor 1016 33 d 1 n 26 000 Hansen et al. (1975)
minnow flowg WB Aroclor 1016 28 d 1 n 54 000 Hansen et al. (1975)
Cyprinodon flowg WB Aroclor 1016 28 d 1 n 22 000 Hansen et al. (1975)
variegatus
Spot flow WB Aroclor 1254 7 d 1 n 7200 Hansen et al. (1971)
Leiostomus flow WB Aroclor 1254 14 d 1 n 17 000 Hansen et al. (1971)
xanthurus flow WB Aroclor 1254 28 d 1 n 37 000 Hansen et al. (1971)
flow WB Aroclor 1254 56 d 1 n 27 000 Hansen et al. (1971)
Atlantic salmon flow WB 10-15 Aroclor 1254 33 d 10 mg/kg 0.39 Zitko (1974)
Salmo salar
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Coho salmon flow WB 17 Aroclor 1254 112 d 0.048 mg/kg 9.79 Mayer et al. (1977)
Oncorhynchus flow WB 17 Aroclor 1254 112 d 4.8 mg/kg 0.79 Mayer et al. (1977)
kisutch WB TeCB 17 d 1 mg/kg 0.144 Gruger et al. (1976)
WB TeCB 35 d 1 mg/kg 0.139 Gruger et al. (1976)
WB PeCB 35 d 1 mg/kg 0.162 Gruger et al. (1976)
WB HeCB 35 d 1 mg/kg 0.151 Gruger et al. (1976)
Channel catfish flow WB 26 Aroclor 1232 150 d 2.4 mg/kg 1.875 Mayer et al. (1977)
Ictalurus flow WB 26 Aroclor 1232 193 d 2.4 mg/kg 1.3 Mayer et al. (1977)
punctatus flow WB 26 Aroclor 1248 193 d 2.4 mg/kg 0.79 Mayer et al. (1977)
flow WB 26 Aroclor 1254 193 d 2.4 mg/kg 2 Mayer et al. (1977)
flow WB 26 Aroclor 1260 193 d 2.4 mg/kg 1.46 Mayer et al. (1977)
flow WBh 24-26 Aroclor 1242 130 d 20 mg/kg 0.72 Hansen et al. (1976a)
flow WB Aroclor 1248 77 d 5.8 56 370* Mayer et al. (1977)
flow WB Aroclor 1254 77 d 2.4 61 190* Mayer et al. (1977)
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Fathead (M) flow WB 25 Aroclor 1248 250 d 3 approx. 60 000 DeFoe et al. (1978)
minnow (M) flow WB 25 Aroclor 1260 250 d 2.1 approx. 160 000 DeFoe et al. (1978)
Pimephales (F) flow WB 25 Aroclor 1248 250 d 3 approx. 120 000 DeFoe et al. (1978)
promelas (F) flow WB 25 Aroclor 1260 250 d 2.1 approx. 270 000 DeFoe et al. (1978)
d = Days; M = Male; F = Female; DiCB = dichlorobiphenyl; TeCB = tetrachlorobiphenyl; PeCB = pentachlorobiphenyl;
HeCB = hexachlorobiphenyl; OcCB = octachlorobiphenyl; DeCB = decachlorobiphenyl.
a Stat = static conditions (water unchanged for duration of experiment); flow = flow-through conditions (PCB concentration
in water continously maintained).
b WB = whole body.
c Bioconcentration factor = concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration
factors calculated on a wet weight basis unless otherwise stated. * Radioactive isotope used to calculate
bioconcentration factor.
d Sediment.
e Calculated on a dry weight basis.
f Static conditions, but test solution changed at intervals.
g Intermittent flow-through conditions.
h Not including stomach.
Södergren (1971) maintained the unicellular freshwater green alga
Chlorella pyrenoidosa in water (at a cell concentration of
approximately 900 mg/litre) with added nutrient medium containing
3.7 µg Clophen A50/litre, over a period of 7 days. By the end of the
experiment, 88% of the PCBs had been taken up by the alga. The
remaining PCBs were detected in the water, none being found in the air
samples taken. In another study, Urey et al. (1976) found that both
tetrachloro- and hexachlorobiphenyl isomers, at 10 µg/litre, were
concentrated by dead Chlorella pyrenoidosa cells by 6000 and 15 000
times, respectively, after a 1-h exposure. These concentration factors
are approximately twice those for living cells (Table 9). Similar
findings have been noted with other species of algae (Biggs et al.,
1980; Lederman & Rhee, 1982).
The ciliate Tetrahymena pyriformis was exposed to Aroclors 1248
(0.01, 0.1, and 1 mg/litre) and 1260 (0.001, 0.01, 0.1, and
1 mg/litre) for 7 days (Cooley et al., 1973). Uptake of the toxicant
increased linearly with increasing concentration. Concentration
factors ranged from 14.8 to 40.6 for Aroclor 1248 and from 21 to 79
for Aroclor 1260. Approximately 15-20% of Aroclor 1248 was absorbed at
each concentration compared with means of 37-53%, with increasing
concentration, for Aroclor 1260. If the data from Cooley et al. (1972)
on the uptake from Aroclor 1254 is included, it is clear that
T. pyriformis accumulates more PCBs with increasing degree of
chlorination.
Dive et al. (1976) studied the accumulation of 16 pure isomers of PCB
and one commercial product, Pyralene 3010, by the ciliate protozoan
Colpidium campylum, at concentrations of 0.1, 1, or 10 mg/litre for
43 h. The amount of PCBs taken up at 0.1 mg/litre was very similar for
each of the PCB isomers and the commercial product, ranging from 29.4
to 49%. The percentage uptake did not change greatly for the higher
exposures.
4.2.4.2 Plants
Uptake of PCBs into plants from soil is positively correlated with the
soil concentration of the PCBs. Roots accumulate more than stems and
foliage. Bioconcentration factors are low. More lower chlorinated
congeners of the PCBs are taken up, probably because of their greater
mobility in the soil. Much of the uptake is adsorption on the surfaces
of roots and there is little translocation. PCBs found in leaves have
volatilized from the soil. Uptake on root surfaces can be reduced or
eliminated by adding activated charcoal to the soil.
Lawrence & Tosine (1977) found that plants took up significant amounts
of PCBs (30-140% of the applied PCB concentration) from soil treated
with sewage sludge. In a waste PCB spill besides a North Carolina
highway, levels as high as 4700 mg/kg were recorded in the top 3 cm of
soil. Seven months later, the PCB concentrations were unchanged; the
authors believed that this was because the PCBs were bound to
activated carbon that had been used to treat the spill (Pal et al.,
1980).
Strek & Weber (1982) analysed statistically the data from several
literature sources (Iwata et al., 1974; Wallnofer & Koniger, 1974;
Wallnofer et al., 1975; Iwata & Gunther, 1976; Moza et al., 1976a,
1979a,b; Weber & Mrozek, 1979) on PCB uptake by plants, with the
following conclusions.
i. The PCB content of the plant is significantly dependent on the
soil PCB concentration.
ii. There is a significant difference between plant species,
carrots taking up more PCBs than other plants.
iii. There appears to be a limit of the PCB concentration in the
soil at which no detectable PCBs are taken up by the plants.
iv. Roots take up more PCBs than tops.
v. most of the PCBs in roots may, in fact, be adsorbed on the
surface and not actually taken up.
vi. There is a general trend of increasing PCB content with
decreasing chlorination, for pure PCB congeners.
vii. The amount of chlorination seems to have an effect on the
mobility of PCBs within plant parts. Since lower chlorinated
PCBs have been reported to be more mobile in soils than highly
chlorinated PCBs, they may be more readily transported and
available for plant uptake.
Larsson (1987) maintained the macroalga Cladophora glomerata in a
flowing-water, outdoor pool. Sediment contaminated with Clophen A50 at
2.7 mg/kg dry weight was added and PCB residues in the alga were
monitored. The algal concentration was 3.55 mg/kg dry weight within 3
months. Residues had fallen a year later to 0.2 mg/kg, reflecting the
water levels of PCBs. The authors concluded that a partitioning
process governed the uptake of PCBs by C. glomerata in this
experiment, because the alga accumulated the same PCBs and the same
proportion of PCBs that were present in the water.
Table 11. Bioaccumulation, of PCBs: Plants, birds, and mammals
Organism Organ PCB type Duration Exposure Bioconcentration Reference
(mg/kg) factora
Soilb
Beet (Beta vulgaris) plant top Aroclor 1254 39 days 20 0.041c Strek et al. (1981)
Sorghum (Sorghum bicolor) plant top Aroclor 1254 39 days 20 0.003c Strek et al. (1981)
Peanut (Arachis hypogaea) plant top Aroclor 1254 78 days 20 0.024c Strek et al. (1981)
Corn (Zea mays) plant top Aroclor 1254 13 days 20 0.001c Strek et al. (1981)
Carrot root DiCBd 112 days 0.118 2c Moza et al. (1976a)
leaves DiCBd 112 days 0.118 0.92c Moza et al. (1976a)
Food
White pelican carcase Aroclor 1254 70 days 144 14.8 Greichus et al. (1975)
(Pelecanus erythrorhynchos)
Table 11. (cont'd).
Organism Organ PCB type Duration Exposure Bioconcentration Reference
(mg/kg) factora
Chicken fat Aroclor 1242 28 days 100 2.83 Harris & Rose (1972)
fat Aroclor 1254 28 days 100 5.15 Harris & Rose (1972)
fat Aroclor 1260 28 days 100 4.82 Harris & Rose (1972)
Big brown bat carcase Aroclor 1254 37 days 9.4 6.6 Clark & Prouty
(Eptesicus fuscus) (1977)
Mink fat Aroclor 1254 approx. 56 days 1.5 16.5 Hornshaw et al.
(Mustela vison) fat Aroclor 1254 approx. 126 days 1.5 28.5 (1983)
Hornshaw et al.
(1983)
a Bioconcentration factor = concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration
factors calculated on a wet weight basis, unless otherwise stated.
b Calculated on a dry weight basis.
c Radioactive isotope used to calculate bioconcentration factor.
d DiCB = dichlorobiphenyl.
Red mangrove (Rhizophora mangle) seedlings were grown for 6 weeks in
soil treated with Aroclor 1242 at concentrations of between 0.038 and
6 mg/kg (Walsh et al., 1974). Low levels (detection limit was
0.1 mg/kg) of the PCBs were detected in the roots at exposure
concentrations of 3 or 6 mg/kg, during the exposure period, but no
residues were found in the stems. Residues were detected in both the
hypocotyls and leaves at application rates of 0.3 mg/kg or more. Leaf
residues did not change with time, but PCB concentrations in the
hypocotyls showed an increase. The highest mean residues of 1.5 mg/kg
were found in the hypocotyl in the highest exposure group.
Iwata et al. (1974) treated soil with Aroclor 1254, at a concentration
of 100 mg/kg, and sowed carrots in the plot 7 months later. The
carrots were harvested 3 or 4 months after seeding. The authors found
that the lower chlorinated biphenyls were more readily taken up from
the soil into the carrot root. Analysis of the carrot peel revealed
approximately 97% of the PCB residue, showing that there is little
translocation within the plant; 23 months after sowing carrots in soil
contaminated with 100 mg/kg Aroclor 1254, dissipation from soil
appeared to parallel the degree of chlorination (Iwata & Gunther,
1976). Analysis of the soil revealed that the lower chlorinated
biphenyls were slowly dissipated while the more highly chlorinated
biphenyls appeared to be unaffected. Small amounts of PCBs were found
in carrot foliage and the authors suggested that the PCB composition
indicated that they came from soil dust. Suzuki et al. (1977) also
found that lower chlorinated biphenyls were preferentially taken up by
plants, following exposure of soybean sprouts to soil contaminated
with Aroclor 1254 or 1242 at 100 mg/kg.
Moza et al. (1976a) found that carrot bioconcentrated
2,2'-dichlorobiphenyl (0.118 mg/kg soil) from soil by a factor of 2
(Table 11). No bioaccumulation was found in sugar beet, but the soil
residue was only 0.029 mg/kg. Carrots were grown in soil amended with
either 14C-labelled 2,5,4'-trichlorobiphenyl at 1.28 kg/ha or
2,4,2',4',6-pentachlorobiphenyl at 1.12 kg/ha for one season (Moza et
al., (1979a). Only 32.5% of the trichlorobiphenyl was recovered, the
rest being lost through volatilization. The carrots had taken up 3.1%
of the applied 14C, representing a concentration factor of 2.8. For
the pentachlorobiphenyl, 58.5% was recovered, 1.4% of which had been
taken up by the carrots. Sugar beet grown in the soil the following
year accumulated only 0.4% of the applied 14C.
In a study by Weber & Mrozek (1979), 14C-labelled Aroclor 1254 was
applied to Lakeland soil at a rate of 20 mg/kg. Activated carbon was
mixed with half the pots at a rate of 3.7 t/ha (3333 mg/kg). The pots
were seeded with either soybean or fescue. After harvesting at 16 days
for soybean and 50 days for fescue, the amounts of labelled-PCBs,
recovered from the plant tops, were 0.016% and 0.17% for the 2
species, respectively. The addition of activated carbon to the soil
reduced the uptake of 14C-PCBs, the recovery of labelled-PCBs being
0.001% and 0% for soybean and fescue, respectively. Strek et al.
(1981) also applied 14C-labelled Aroclor 1254, at the same rate, to
Lakeland soil; several species of crop plants were grown in the soil
and bioaccumulation factors, calculated (Table 11). Addition of
activated carbon (3.7 t/ha), equivalent to 3333 mg/kg to replicate
pots, reduced the uptake of the labelled-PCBs by 80-100%.
When approximately 1 mg 14C-labelled Aroclor 1254/kg was applied to
the centre leaflet of the first trifoliate leaf of 18-day-old soybean
plants, only 6.7% was recovered from the plant after 12 days, 76% of
which was still present in the treated leaf (Weber & Mrozek, 1979).
Mrozek & Leidy (1981) transferred the marsh plant Spartina
alterniflora from the field into soil containing 1 mg Aroclor
1254/kg (dry weight) and harvested the plants after a growth period of
90 days. The plants were found to take up selectively the lesser
chlorinated biphenyls. The authors stated that a further shifting of
the chromatographic pattern of PCBs towards the lesser chlorinated
components in aerial tissues suggested that some alteration of the PCB
mixture occurs in the plant. Mrozek et al. (1982) also found that
Spartina accumulates PCBs from both contaminated sand and mud-soil
systems. The total 14C-radioactivity accumulated in plants grown in
sand systems was higher than that in plants grown in mud. The level of
radioactivity accumulated in the green parts of the plants was similar
in both soil systems.
Moza et al. (1976b) applied 76 mg/kg of 14C-labelled 2,5,4'-tri-
chlorobiphenyl or 133 mg/kg of labelled 2,4,6,2',4'-pentachloro-
biphenyl to the leaves of the marsh plant Veronica beccabunga. Six
weeks later, the total recovery from plant, water, and soil was 3.7
and 18.3%, respectively, 86 and 95% of which was recovered from the
plant. In an earlier study, Moza et al. (1974) applied 14C-labelled
2,2'-dichlorobiphenyl in water or soil to 2 higher water plant species
( Ranunculus fluitans and Callitriche sp.) at concentrations of
13.7 and 14.5 mg/kg, respectively. Four weeks after application, the
results showed that the dichlorobiphenyl was metabolized more readily
after addition to water; the authors suggested the involvement of
aquatic bacteria. When applied in soil, 1.2% of the dichlorobiphenyl
was metabolized. This was contributed to the plant.
Moza et al. (1979b) grew 3-year-old spruce trees (Picea abies) in
soil containing 14C-labelled PCBs at approximately 4.2 mg/litre in
sewage sludge. When analysed 4 years later, only 0.8% (0.5% in
needles, 0.3% in stems) of the applied radioactivity was found in the
trees. Leaching of radioactive substances from the soil was less than
0.1% in the first 2 years and undetectable for the remainder of the
study.
In another study, Fries & Marrow (1981) grew soybean (Glycine max)
in pots, to determine residue contamination in plant tops from
14C-labelled 2,5,2'-trichlorobiphenyl, 2,5,2',5'-tetrachlorobiphenyl
or 2,4,5,2',5'-pentachlorobiphenyl, applied to the surface or
subsurface soil. Each compound was added to the soil at a rate of
2-3 mg/kg and the plants were harvested after a period of 52 days.
Detectable residues were only found in plants grown in surface-treated
soil, and concentrations in the plants increased with increasing
chlorination. Little of the labelled PCBs was lost from
subsurface-treated soil, but 20-30% of the surface-treated PCBs were
lost through volatilization. The authors concluded that the PCB
residues in the plant tops were, therefore, due to foliar
contamination from vapour rather than the uptake from the soil via the
roots. Miyazaki et al. (1975) came to the same conclusion when they
found no absorption or translocation of PCBs in sesame or rice seeds,
following the application of 4 types of Kanechlor (KC300, 400, 500,
and 600) at rates of between 0.1 and 100 mg/kg. But the rice straws
contained PCB levels of 0.02-0.08 mg/kg, which were the same as levels
found in plants from untreated soils.
Beets ( Beta vulgaris L.), turnips ( Brassica rapa L.), and beans
( Phaseolus vulgaris L.) were grown (Sawhney & Hankin, 1984) in soil
to which lake sediment contaminated with PCBs had been added. The
plants were exposed to Aroclor 1248 at a concentration of 80 µg/kg,
Aroclor 1254 at 1880 µg/kg, and Aroclor 1260 at 14 440 µg/kg. When
beets and turnips were grown in the soil for 6 months, the plants
showed greater uptake in the leaves than in the roots. For example,
beet roots contained 15, 16, and 35 µg/kg of Aroclors 1248, 1254, and
1260, respectively, while beet leaves contained 22, 94, and 52 µg/kg,
respectively. Total concentrations of the 3 Aroclors in beet roots and
leaves and in turnip roots and leaves were 66 and 168 µg/kg,
respectively, and 66 and 99 µg/kg, respectively. During a second
growing season, turnips and beans were grown for 6 months without any
additional PCB-contaminated sediment. Comparing PCB levels in turnips
between the 2 growing seasons showed a decrease in Aroclor 1248 uptake
relative to Aroclors 1254 and 1260. This was primarily because of a
large reduction in the amount of Aroclor 1248 in the soil after 1
year, due to degradation and volatilization. In beans, higher PCB
levels were found in the leaves and pods than in the stems and seeds.
Ten sludge application sites were selected within the Ontario area to
determine background heavy metal and PCB concentrations in the soils
and crops. Control sites (without sludge application) were adjacent to
the sludge application sites. Grab samples of liquid sludges applied
at each of the sites were taken for analysis. The soil samples were
taken at a depth of 15 cm. Twenty core samples were taken at 20-m
intervals and combined to form 1 sample. Eight of the application
sites were cropped with corn, one with oats, and one was left without
a crop. At the control sites, 7 were cropped with corn, 1 with oats,
and 2 left without a crop. PCB concentrations in the sludges ranged
from 0.13 to 1.61 mg/kg dry solids. PCB concentrations were in the
range of 0.007-0.025 mg/kg in the soil without sludges, and in the
range of 0.018-0.453 mg/kg air-dry weight in the soil with sludges.
The PCB levels in the crops were close to the control values (Webber
et al., 1983).
Bacci & Gaggi (1985) assessed the influence of translocation on the
concentrations of PCBs in the foliage of different plant species.
Beans, broad beans, tomatoes, and cucumbers were grown, either in soil
with a nominal added concentration of 500 mg/kg Fenclor 64 (similar to
Aroclor 1260), or in clean sand, for 28 days, enclosed in a glass box
with a constant turnover of air. The plants grown in clean sands were
exposed to PCBs by volatilization from other pots containing PCBs,
which were in the same growing box. The PCB peak pattern of both sand
and roots was similar to that of Fenclor 64, whereas the peak pattern
for foliage and air had moved towards lesser chlorinated congeners.
The concentrations of PCBs in the roots of tomatoes grown in
contaminated soil ranged from 105 to 168 mg/kg dry weight. But
translocation through the plants does not seem to be very likely since
there was no significant difference in foliage uptake of PCBs between
plants grown in contaminated soil and plants grown in clean soil.
Foliar uptake ranged from 13.8 to 42.6 mg PCB/kg (dry weight) for the
different species in PCB-fortified soil and from 11.8 to 47.1 mg/kg
for plants grown in clean soil.
4.2.4.3 Aquatic invertebrates
Bioconcentration factors are high for PCBs taken up by aquatic
invertebrates exposed to either pure chlorinated biphenyl isomers or
commercial mixtures in the water. Since PCBs are strongly bound to
sediments, this method of exposure is unrealistic. Addition of
sediment to test tanks decreases the uptake of PCBs, particularly by
organisms living in the upper water. However, there is clear evidence
that PCBs can also be readily absorbed into invertebrates from both
sediment and food. For organisms living on or in, sediment, uptake can
take place from the sediment, via food organisms that have absorbed
the PCBs, and from interstitial water or water immediately above the
sediment layer. A high content of organic matter in sediment decreases
the availability of PCBs for organisms. Uptake is rapid in most cases
and equilibrium is often reached in hours, though it may take weeks in
other examples. Uptake increases with increasing temperature. The
route of uptake is often via the gills, but varies among species. Loss
of PCBs is slow, but residues do decrease on cessation of exposure.
PCB uptake by aquatic invertebrates is transferred to predators and
can also be transferred to the terrestrial environment.
(a) Uptake from water
Vreeland (1974) exposed American oysters (Crassostrea virginica) to
various PCB isomers at concentrations of 5.5, 17, or 60 ng/litre
(which is within the range found in coastal waters) for 65 days.
Equilibrium was reached after approximately 1 month of exposure, with
concentration factors ranging from 1200 to 48 000 for PCB isomers with
2-6 chlorine atoms/molecule. The PCB concentration, after equilibrium
had been reached, was directly proportional to the amount of PCBs
added to the water. Lowe et al. (1972) found a linear pattern of
uptake in young American oysters exposed to Aroclor 1254 at 5 µg/litre
for 24 weeks, followed by a further 32 weeks in clean water. The
oysters already contained 17 mg/kg from a previous exposure and, by
the end of the 24-week exposure period, had accumulated 425 mg/kg (a
steady state was not established). By the end of the 32-week period in
clean water, no PCB residues could be detected. In another study on
uncontaminated young oysters, concentration factors of up to 101 000
were achieved after a 25-week exposure to 1 µg Aroclor 1254/litre.
After 12 weeks in clean water, whole-body residues were reduced to
0.2 mg/kg.
Courtney & Denton (1976) fed hard clams (Mercenaria mercenaria)
Aroclor 1254 adsorbed on the surface of alumina particles, at 1.25 and
12.5 µg/litre, for 21 days. The maximum concentration factor was 1800
for visceral mass, when the clams had been exposed to 1.25 µg/litre
for 18 days. The visceral mass accumulated a 1.4-5.3 times greater
concentration of PCBs per unit time than the muscular foot. Tissue
samples contained relatively more lower chlorinated isomers than the
Aroclor 1254 standard and, faeces and mud samples contained more
higher chlorinated isomers. Following exposure, clams from the lowest
dose group showed little change in PCB content after 3 months in clean
seawater. However, at the higher dose level, there was a significant
reduction in the PCB levels found in the foot after 1 month, but PCB
residues in the visceral mass remained unchanged for 6 months.
Pink shrimp (Penaeus duorarum) were exposed to Aroclor 1254 at a
concentration of 2.5 µg/litre, in flowing water, for 22 days (Nimmo et
al., 1971b). Accumulation was linear for the hepatopancreas and whole
body, but a plateau was reached after 2 days in muscle. Residues in
the hepatopancreas reached 510 mg/kg over the exposure period,
representing a concentration factor of 204 000; over the same period,
50% of the shrimps died. In a separate study, the shrimps were exposed
to 7.5 µg/litre for 16 days followed by an elimination period of 5
weeks in clean water. When calculated on the basis of the total tissue
burden of PCBs, an 80% reduction in the hepatopancreas was found,
concomitant with a doubling of the PCB levels in remaining tissues.
However, when data were presented as a concentration, a linear loss
from the hepatopancreas was seen, with the concentration in other
tissues remaining constant. The authors calculated a half-life for
loss of PCBs from the hepatopancreas of 17 days.
Nimmo et al. (1975) sampled shrimp from Pensacola estuary, USA, and
measured the relative concentration of PCBs in the various tissues.
The hepatopancreas contained the greatest amounts (50-75%) followed by
the ventral nerve. The authors studied the uptake of PCBs by pink
shrimp, experimentally, using various regimes with dosed food or dosed
water. They found the same tissue distribution in pink shrimp that had
been exposed to 0.2 µg Aroclor 1254/litre, in water, for 50 days. They
concluded that most of the PCBs were taken up directly from the water
in both the "wild" and laboratory situation. However, they did not
exclude the possibility of some PCBs being taken up from food, which
was found under some of the laboratory regimes.
To determine whether there was a concentration below which shrimps
would be unable to accumulate PCBs, grass shrimp (Palaemonetes pugio)
were exposed to flowing water concentrations of 0.04, 0.09, or
0.62 µg/litre. Whole-body residues of 0.2, 1.0, and 10 mg/kg,
respectively, were accumulated within 3-5 weeks. Even at the lowest
dose, shrimps accumulated more PCBs than the residues found in control
shrimp. Concentrations in the shrimp did not reach equilibrium during
the 5-week exposure, but the rate of accumulation decreased towards
the end of the exposure. When transferred to clean water, the shrimps
lost most of the PCBs within 4 weeks (Nimmo et al., 1975).
Gammarus oceanus were exposed by Wildish & Zitko (1971) to Aroclor
1254 concentrations of 2.5, 10, or 20 mg/litre for up to 6 h. Uptake
increased with increasing PCB concentration. Uptake decreased to half
of the initial rate after 4-6 h exposure at 20 mg/litre. There was
little or no uptake by dead animals. Although uptake was related to
branchial surface area, branchiae were not necessary sites of uptake,
since uptake could occur at an unchanged rate following branchial
removal. The authors did not find any change in the rate of uptake
during the intermoult stage.
Zhang et al. (1983) exposed Daphnia magna to 14C-labelled
2,2'-dichlorobiphenyl, 2,5,4'-trichlorobiphenyl, 2,4,6,2'-tetra-
chlorobiphenyl, or 2,4,6,2',4'-pentachlorobiphenyl at 50 µg/litre.
Equilibrium was reached after 20 h for all except the pentachloro-
biphenyl, which had not reached equilibrium within 24 h.
Bioaccumulation factors at equilibrium ranged from 3741, for the
dichlorobiphenyl, to 18 144, for the trichlorobiphenyl. Concentration
factors were not significantly related to the water solubility or
chlorine content of the biphenyl, but there was a tendency for the
bioaccumulation factor to increase with chlorine content and
decreasing water solubility. The authors studied the rate of
depuration and found it to increase with increasing water temperature
between 2 and 22°C. The rate of depuration was also faster for the
dichlorobiphenyl than for the pentachlorobiphenyl; after 48 h, the
amount of PCBs remaining in Daphnia was 22% and 77% (at 10-11°C) for
the 2 chlorobiphenyls, respectively.
(b) Uptake from sediment
Sediment was collected from the field and spiked with Phenochlor DP-5
to achieve a final PCB concentration of 0.65 mg/kg dry weight,
compared with 0.2 mg/kg in unspiked sediment (Elder et al., 1979).
Worms (Nereis diversicolor) were then added to aquaria containing
the sediment under flowing seawater. Equilibrium was reached within
40-60 days, by which time both groups had concentrated the PCBs by 3.5
times. Upon transfer from spiked to unspiked sediment, the worms took
2 months to attain body levels of PCBs comparable with those of the
unspiked group. A half-life of approximately 27 days was calculated
for incorporated PCBs.
Fowler et al. (1978) exposed Nereis diversicolor to spiked sediment
containing 9.3 or 80 mg Phenochlor DP-5/kg (dry weight), for 120 days,
compared with 0.11 mg PCB/kg in unspiked sediment. At the beginning of
the study, worms in the unspiked sediment had body residues of
0.59 mg/kg dry weight and reached a steady state at 1.2 mg/kg. Those
exposed to spiked sediment reached a steady state after a period of
approximately 2 months, with concentration factors ranging from 3 to
4. The worms maintained at the highest level of PCBs all died within a
90-day exposure period. When transferred to unspiked sediment for a
2-month period, the worms that had taken up PCBs from the unspiked
sediment lost PCBs exponentially. In a separate study, worms were
exposed to PCBs in water alone at a concentration of 0.57 µg/litre. A
steady state was reached much more quickly (2 weeks) than it was in
the presence of sediment, with a concentration factor of approximately
800. By comparing these results with field monitoring, the authors
calculated the relative importance of the 2 media. They stated that
approximately 99% of the PCBs in these studies was taken up from the
sediment. When the water overlying the spiked sediment was monitored,
28 ng PCBs/litre was measured (not leached, but a contaminant in the
experimental system) reducing the figure of uptake from sediment to
89%.
In a study by Courtney & Langston (1978), 1.1 mg Aroclor 1254/kg was
incorporated into intertidal sand. Specimens of 2 intertidal
polychaetes (Arenicola marina and Nereis diversicolor) containing
mean residues of 0.017 and 0.11 mg PCBs/kg (wet weight), respectively,
were collected. After 5 days in the spiked sediment, they contained
0.24 and 0.36 mg/kg, and, after a further 5 days, 0.39 and 0.49 mg/kg,
respectively. During a 3-week post-exposure period, there was no
significant loss of these PCB residues. The authors achieved
comparable PCB residues in these polychaetes after exposure to
1 µg/litre water or 1 mg/kg sediment.
McLeese et al. (1980) exposed the polychaete worm (Nereis virens)
and the shrimp (Crangon septemspinosa) to sediment containing
0.016-0.58 mg Aroclor 1254/kg (dry weight) for 32 days. Uptake was
found to be dependent on the exposure concentration and, in the case
of the worms, on the exposure period. The accumulation of PCBs was
inversely related to animal size; at 32 days, concentration factors
for worms ranged from 10.8 for 0.6-g worms to 3.8 for 4.7-g worms
following exposure to 0.17 mg PCB/kg. Factors of 3.5 and 1.9 were
found for shrimps weighing 0.1 and 2.9 g, respectively, after exposure
to 0.13 mg Aroclor/kg. Shrimps were found to accumulate, on average,
60% less PCBs than worms per unit weight. During the 26 days following
exposure, there was not any obvious loss of PCBs from the worms.
Rubinstein et al. (1983) collected sediments containing various levels
of pollutants (PCBs, 0.46-7.28 mg/kg dry weight; Cd; Hg) and organic
matter (5.5-22.3%). During a 100-day exposure period, only small
increases in PCB concentrations were detected in hard clam
(Mercenaria mercenaria) and grass shrimp (Palaemonetes pugio).
Higher concentrations of PCBs were accumulated by Nereis virens.
Uptake was found to be more dependent on the organic content of the
sediment than on the exposure concentration. Concentration factors
ranged from 1.59 in a low organic sediment to 0.15 in a high organic
sediment. The authors also calculated the maximum water exposure
concentration eluted from each of the sediments. On the basis of a
concentration factor of 800, calculated by Fowler et al. (1978) for
the uptake from water of Nereis sp., body residues of between 0.007
and 0.034 mg PCBs/kg (wet weight) would have been expected if
accumulation were dependent purely on direct partitioning from water.
However, whole-body residues of PCBs were found to be 0.4-0.63 mg/kg,
suggesting that pathways other than direct uptake from water (e.g.,
ingestion and sorption) contributed significantly to the accumulation
of PCBs by the polychaete.
Freshwater prawns (Macrobrachium rosenbergii) and clams (Corbicula
fluminea) were exposed to contaminated sediments for 48-50 days
(Tatem, 1986). Prawns were exposed to sediment containing
approximately 62 mg PCBs/kg (dry weight) and to the same sediment
diluted with sand to 50 and 10% of the original. Clams were exposed to
100, 50, or 10% of another sediment containing approximately 2 mg
PCBs/kg at 100%. The amount of PCBs accumulated was related to the
exposure concentration, with the highest concentration factors at the
lowest exposure (10%) level. Bioaccumulation factors for prawns ranged
from 0.1 to 0.9 for Aroclor 1242 and from 0.2 to 2.4 for Aroclor 1254,
relative to sediment concentrations. Exposed clams accumulated PCBs
(Aroclors 1242 and 1254) at concentration factors of 0.54-12.52,
relative to sediment. When tissues were analysed for Aroclor 1242 and
1254, maximum concentrations in prawns were attained at 7 and 40 days
for the 2 Aroclors, respectively. Exposure of prawns at 100 and 50%
dilution of sediment killed all the animals after 62 and 70 days,
respectively. Clams survived exposure.
Clark et al. (1986) investigated the accumulation of sediment-bound
PCBs by fiddler crabs (Uca pugilator) and (Uca minax). Mud and
mud/sand sediments were used; both were naturally contaminated with
PCBs and no further PCBs were added. Both species were exposed to a
mud sediment containing 1.04 mg PCBs/kg and to a mud/sand sediment
containing 0.37 mg/kg (dry weight). Concentration factors, after a
28-day exposure, were 0.19 and 0.79, for U. minax, and 0.2 and 0.59,
for U. pugilator, for the 2 sediments, respectively. In a second
study, using mud with 0.97 mg PCBs/kg and mud/sand with 0.55 mg
PCBs/kg, U. pugilator showed concentration factors of 0.58 and 0.71,
respectively, after 28 days. The authors did not find any detectable
PCBs in the overlying water, suggesting that the PCBs are tightly
bound to the sediment and leach out only very slowly. Following
transfer to uncontaminated sediment on day 42, no PCB residues were
detected in U. pugilator on day 56, or in U. minax on day 63.
Lynch & Johnson (1982) exposed the amphipod (Gammarus pseudolimnaeus)
to 2,4,5,2',4',5'-hexachlorobiphenyl added to sediment in flow-through
bioassays. Water overflowing from the tank containing the contaminated
sediment was directed into a second tank where further amphipods were
exposed without sediment. The hexachlorobiphenyl was labelled with
14C and added to the sediment at 1 mg/kg; the system was allowed to
equilibrate for 7-15 days prior to addition of amphipods, which were
sampled from the tanks after 24, 48, 96, and 192 h. In the initial
studies, the specific activity of the labelled hexachlorobiphenyl was
insufficient to detect the hexachlorobiphenyl concentrations in water.
However, it was clear that amphipods in the tank with the sediment
accumulated more hexachlorobiphenyl than animals exposed only to the
water overflow (8.8-10.5 times more PCBs). Removal of organic matter
from the sediment, by combustion, before addition of the PCB,
increased uptake of the hexachlorobiphenyl by increasing the
availability of the material to the Gammarus. In later studies,
specific activity was increased and water concentrations could be
measured. These were very low, ranging between 11 and 35 ng/litre in
the upper tank and 9 and 25 ng/litre in the lower tank. The lower end
of this range was found later in the exposure period suggesting that
less hexachlorobiphenyl was released over time. There was little
difference in concentration between water taken from the surface and
that sampled close to the sediment suggesting rapid mixing of the
overlying water. In this later series of studies, the authors
demonstrated that both the organic matter content of the sediment and
the presence of smaller particle sizes (silt and clay) reduced uptake
of hexachlorobiphenyl by the amphipods. Organic matter was the more
important factor. Adding maple leaves, to give about 70% organic
content in the sediment, reduced hexachlorobiphenyl uptake to between
10 and 20% of that in sediment without organic matter. Very high
bioconcentration factors were calculated relative to the very low
water concentrations of hexachlorobiphenyl (ranging between 27 000 and
1 000 000 in the upper tank and 2000 and 460 000 in the lower tank,
increasing with exposure period). These factors would be very low
relative to sediment concentrations of the PCB. However, it is clear
that the amphipod can accumulate hexachlorobiphenyl, leaching in very
small amounts from contaminated sediment.
Cores of lake sediment complete with overlying water were taken by
Larsson (1984) and transported back to the laboratory, still in the
sampling tube. PCBs were introduced at different dose levels by
injection through silicon septa in the walls of the tubes and spread
evenly 10 mm below the surface. The cores were allowed to stabilize in
the dark for 1 week at which time 80-100 chironomid larvae were
introduced. After 8 weeks, the systems were moved and kept at 20°C in
the light. After 2 days, the chironomid larvae began to pupate and
emerge. The study was terminated after 10 weeks. PCBs were measured in
sediment, larvae, adults, and exuviae (discarded skins after
emergence). Ranges of PCBs in sediment were between 0.5 and 14 mg/kg
giving rise to residues in larvae, exuviae, and adults directly
related to sediment concentrations. There was "biomagnification"
between larvae and adult. There was loss of body weight between the
final larval stage and the adult, but little loss of PCBs (only 17%
was retained in the exuviae). The author stated that the low variation
in uptake between animals is an indication of passive physicochemical
factors being involved in the handling of PCBs by chironomids. Active
uptake via ingestion would be expected to lead to more variation in
results. Meier & Rediske (1984) also monitored the uptake of PCBs from
contaminated sediment into chironomid larvae (Glyptotendipes
barbipes). Concentration factors for Aroclor 1242 from sediment
ranged between 20 and 130 for exposures of between 0.01 and 1.0 mg/kg,
considerably lower than concentration factors relative to water
(10 000 for these organisms) (Sanders & Chandler, 1972). Addition of
oil, commonly found in polluted areas where PCBs spills are likely,
reduced the uptake of PCBs from the sediment.
(c) Uptake from food
A detritus diet containing 17 µg Aroclor 1242/kg (wet weight) was fed
to male fiddler crabs (Uca pugnax) for 34 days (Marinucci & Bartha,
1982). The Spartina detritus was placed in the culture system at the
start of the study and, because of rapid depletion, was renewed after
19 days of exposure. Since PCBs leached continually from the food
source into the water, a second study was carried out to examine the
uptake of PCBs from water alone. Contaminated detritus was mixed
thoroughly with water and allowed to equilibrate for 24 h. Water
levels were found to be 14-15 µg/litre. Aroclor 1242 was accumulated
at a more rapid rate from PCB-laden detritus than from water alone.
The linear accumulation rate from litter was calculated to be 1 µg
PCBs/day per animal whereas, from water alone, the uptake was 0.1 µg
PCBs/day per animal. Aroclor 1242 was highly concentrated in the
hepatopancreatic tissue. It was found that the PCB residue in the
crabs was inversely related to their weight. Comparison of the
concentrations of PCBs in animals of the same weight shows that, at
the end of the 34 day exposure, those exposed to water alone had taken
up approximately half of the PCBs of those exposed to detritus. The
authors concluded that the crabs in the study accumulated a similar
amount of PCBs from both the food and the water.
Pinkney et al. (1985) exposed the amphipod Gammarus tigrinus to
Aroclor 1254 (14C-labelled) in fungus (Fusarium oxysporum) as a
food item. The fungus contained 195.8 mg Aroclor/kg dry weight.
Accumulation of PCBs was rapid, reaching a constant level in the
amphipods of 23 mg/kg after 9-24 h. Similar exposure of the amphipods,
but with exclusion from direct contact with the fungus by Teflon mesh
(to monitor uptake of PCBs leached into the water), resulted in
residues of between 0.16 and 3.3 mg/kg (from concentrations in the
water at 0.03 µg/litre), representing between 0.6 and 13.9% of uptake
from water and food combined. The PCB residues in the amphipods were
also monitored over 144 h on uncontaminated food to measure the
elimination rate. The water was changed every 24 h. Within this
period, 57% of the accumulated PCBs was eliminated.
(d) Comparison of different routes of uptake
In a study by Wyman & O'Connors (1980), the uptake by the marine
copepods Acartia tonsa and Acartia clausi of 14C-labelled Aroclor
1254 from water, inorganic sediment, and food, was monitored over a
period of 48 h. Acartia were exposed to water concentrations of
10 µg PCBs/litre. An asymptotic uptake curve was observed; equilibrium
was reached after 36 h, corresponding to whole-body residues of 248 mg
PCBs/kg (dry weight) for A. tonsa and 223 mg/kg for A. clausi.
During exposure, water concentrations fell rapidly to 5 or 6 µg/litre.
A similar pattern of uptake was found after exposure to sediment
contaminated with 20 mg PCBs/kg with maximum levels of PCBs in
A. tonsa of 22 mg/kg after 30 h. As in the water exposure, levels of
PCBs in sediment fell rapidly from 20 mg/kg to 14 mg/kg and then
slowly to 7 mg/kg at the end of the study. Water levels were initially
0.62 µg/litre and fell to 0.15 µg/litre. Uptake of PCBs by A. tonsa
from phytoplankton contaminated with 80 mg PCBs/kg (wet weight) was
very rapid and reached a maximum after 5 h at 61 mg/kg, but
subsequently declined after exhaustion of the food supply. PCB
concentrations in water were similar to those found when copepods were
exposed to contaminated sediment, copepods exposed to these water
concentrations alone accumulated significantly less PCBs than those
fed PCB-dosed phytoplankton.
McManus et al. (1983) exposed the marine copepod Acartia tonsa to
14C-Aroclor 1254 either in the food, as phytoplankton containing
approximately 1.3 mg PCBs, or in water at 1.5 µg/litre, for a period
of 30 h. For copepods exposed to contaminated phytoplankton, PCB
levels ranged from 117 to 163 mg/kg dry weight. For copepods exposed
to contaminated water alone, levels ranged from 82 to 104 mg/kg. When
transferred to clean water, the authors found that copepods lost PCBs
at a significantly faster rate if they were fed during depuration;
after 36 h, PCB concentrations in copepods fed during deputation were
10 mg/copepod whereas those starved contained 30 mg/copepod. No
significant difference in depuration rate was found between those
exposed via food and those exposed via water. In a second study,
elimination in males and females was compared. Although both sexes
contained similar residues at the start of depuration (117 mg/kg and
95 mg/kg, respectively), after 36 h, females contained significantly
lower levels of PCBs than males. During depuration, faecal pellets and
eggs were analysed; similar levels of PCBs were found in both male and
female faecal pellets during this period, but levels of PCBs more than
four times that in the females were found in eggs (407.5 mg/kg dry
weight after 4 h), indicating that egg production is an important
route for PCB elimination.
4.2.4.4 Fish
Fish of all life stages have been shown to take up PCBs readily from
water; bioconcentration factors are high. Time taken to reach
equilibrium is variable, but often long, in excess of 100 days. PCBs
with greater chlorination are more readily taken up and retained. PCB
body burden tends to increase with age and levels are higher in fish
with a greater lipid content. The accumulated PCBs are concentrated in
lipid-rich tissues. PCBs of lower chlorination are eliminated more
rapidly. Loss of PCBs is evident when exposure ends; an initial rapid
loss is followed by a slower rate of loss. Half-life estimates,
therefore, vary greatly, from a few weeks to several years.
Reproduction, with the production of a large mass of eggs or sperm,
allows loss of substantial amounts of the PCB residue. Depending on
the species, habitat, and behaviour, PCBs can be taken up from water,
sediment, or food to different degrees.
(a) Uptake from water
Califano et al. (1980) maintained larval striped bass (Morone
saxatilis) in Hudson river water (filtered and unfiltered)
contaminated with 14C-Aroclor 1254 at 1.36 µg/litre for a period of
48 h. Whole-body residues for filtered and unfiltered water were not
significantly different at 5 mg/kg and 5.9 mg/kg, respectively. Uptake
between 34 and 48 h was very slow, suggesting a steady state had
already been reached. Exposure of fish for a further 72 h in
unfiltered water, supported this theory. Elimination was slow, only
18% being lost in 48 h following a 24-h exposure.
The PCB uptake pattern in lake trout (Salvelinus namaycush) sac fry
was studied by Mac & Seelye (1981) by exposing them to a nominal
concentration of 50 ng Aroclor 1254/litre for 48 days. Patterns of
accumulation were similar, regardless of how the data were expressed
(wet weight, dry weight, or body burden). PCBs levels increased
slowly, reaching a peak after 32 days (just before completion of yolk
absorption), and then decreased by day 48.
Hansen et al. (1975) exposed different life-stages of sheepshead
minnow (Cyprinodon variegatus) to Aroclor 1016 (Table 10). After a
4-week exposure to nominal concentrations of 1, 3.2, or 10 µg/litre,
adult fish laid eggs containing on average 4.2, 17, and 66 mg/kg,
respectively. DeFoe et al. (1978) exposed fathead minnow (Pimephales
promelas) to Aroclor 1248 or 1260 at concentrations of
0.1-3 µg/litre, for 240 days (life cycle). Bioconcentration factors for
the uptake of PCBs were independent of the PCB concentration in the
water. Residues in the fish reached an apparent steady state within
about 100 days of exposure and growth. Females accumulated about twice
as much PCBs as males, because of their higher body lipid content. The
variability of residues in females reflected the variability of their
lipid content. Although mechanisms for uptake were similar for both
Aroclors, greater body burdens were always achieved with exposure to
Aroclor 1260. Bioconcentration factors ranged from 60 000 to 160 000
for males and from 120 000 to 270 000 for females. After transfer to
clean water, 18% of Aroclor 1248 was lost within 28 days and 15% of
Aroclor 1260 in 42 days. The authors stated that, because of
variations between fish, this 10-20% decline in total body burden of
PCBs was insufficient to indicate definite PCB elimination over this
period.
De Kock & Lord (1988) exposed an estuarine fish, the Cape stumpnose
(Rhabdosargus holubi) to a flowing water concentration of 1 µg
Aroclor 1260/litre for 90 days followed by a 90-day period in clean
water. Equilibrium was reached at 90 days with a concentration factor
of 24 000. The depuration rate was calculated to be 0.014 days,
producing a half-life of 50 days.
Goldfish (Carassius auratus) were exposed to Clophen A50 at levels
of 0.01, 0.05, 0.1, or 0.5 mg/litre for 18 days (Hattula & Karlog,
1973). Rapid uptake was observed with concentration factors of over
1000 at 18 days, but equilibrium was not achieved within this period.
Nearly all the fish exposed to 0.5 mg/litre died within 7 days. After
transfer to clean water, fish that had been exposed to 0.1 mg/litre
for 13 days and had attained body residues of 70 mg/kg lost half of
the PCBs within 3 weeks, but still retained levels of approximately
15 mg/kg, after 70 days.
Yoshida et al. (1973) exposed carp (Cyprinus carpio) to 14C-PCBs
(equivalent to Aroclor 1254) in water or in food. By measuring the
radioactivity, they found similar tissue patterns of uptake from both
water and diet. PCBs were localized in the gall bladder, adipose
tissue, and hepatopancreas and, in particular, the adipose tissue of
the skull.
Hansen et al. (1971) exposed spot (Leiostomus xanthurus) to Aroclor
1254 at 1 µg/litre, for 56 days. Maximum tissue levels of PCBs were
achieved between days 14 and 28. Highest levels were found in the
liver (210 mg/kg, after 28 days) followed by the gills, whole fish,
heart, brain, and muscle. Aroclor 1254 was slowly lost from tissues;
after 84 days in clean water, levels of PCBs had dropped by 73%.
In a study by Braun & Meyhofer (1977), rainbow trout (Salmo
gairdneri) fingerlings were exposed to water concentrations of 2 or
20 µg Clophen C/litre, for 8 weeks. Tissue PCB concentrations for
gills, muscle, and liver were found to be 0.62, 0.82, and 3.47 mg/kg,
respectively, for the lower dose and 12.3, 7.6, and 10.6 mg/kg, for
the higher dose. When fish were held in clean water for 10 weeks,
following exposure to 2 µg/litre for 8 weeks, residues decreased by
half in the liver and had disappeared completely from the gills, but
there was no change in the PCB levels in muscle.
Rainbow trout (Salmo gairdneri) were exposed by Guiney et al. (1977)
to 14C-labelled 2,5,2',5'-tetrachlorobiphenyl at 0.5 mg/litre for
36 h. The tissue distribution of 14C was measured at regular
intervals after transfer to clean water. Carcase, muscle, skin, lower
gastrointestinal tract, and fat contained most of the radioactivity
(88%). During the first 14 days after exposure, radioactivity
increased in adipose tissue, carcase, and eyes. Elimination from most
tissues appeared to be biphasic with a 30% loss within 2 weeks
followed by a loss of only 6% in the following 126 days. Losses from
the bile and blood were very rapid and nearly complete within 14 days.
Based on the initial rate of loss, the authors calculated a half-life
of 1.55 days, however, the second phase of eliminated PCBs suggested a
half-life at 2.66 years. In a similar study, Guiney et al. (1979)
calculated half-lives of 1.76 and 1.43 years for female and male
rainbow trout, respectively, based on fish sampled 2-34 weeks after
exposure. For both sexes the half-life of elimination was recalculated
to 0.52 and 0.54 years between weeks 38 and 52 after exposure (the
spawning season). The increased elimination appeared to be because of
loss via eggs and sperm. Vodicnik & Peterson (1985) found a similar
result after dosing yellow perch (Perca flavescens); an elimination
half-life of 22 weeks was calculated. This was later recalculated to
be <0.7 weeks during spawning, returning to 16.3 weeks after the
completion of spawning.
(b) Uptake from sediment
The uptake of Aroclor 1254 from suspended solids by juvenile Atlantic
salmon (Salmo salar) was studied by Zitko (1974). Aroclor 1254 was
mixed with suspended solids (simulated by SilicAR CC7) in hexane at
5 mg/ml. Fish were exposed to contaminated solids at 1 g/litre for up
to 144 days. Over this exposure period, the salmon accumulated 134 mg
Aroclor 1254/kg.
Stein et al. (1984) exposed English sole (Parophrys vetulus) to a
sediment concentration of 1 mg 14C-Aroclor 1254/kg (dry weight).
Seawater was allowed to flow over the sediment for 6 days before the
fish were added. A steady state of PCBs accumulated in the tissues of
the fish was achieved after 10 days of exposure. Highest residue
concentrations were found in the bile and the liver. Concentration
factors were 10 for the bile and 4 for the liver, with other tissues
individually concentrating PCBs by factors of 3 or less. Simultaneous
exposure of sole to PCBs and 3H-benzo[ a]pyrene (3 mg/kg, dry
weight) reduced the amount of PCBs accumulated. Stein et al. (1987)
collected urban sediment containing aromatic hydrocarbons and PCBs at
32 mg/kg and 2.2 mg/kg dry weight, respectively. English sole
accumulated hepatic concentrations of 1.4 mg PCBs/kg (wet weight) over
a period of 108 days exposure to the urban sediment. This was 8 times
the PCBs accumulated by sole exposed to the control sediment, which
did not contain any detectable PCBs. In another study, the same
authors added a 14C-labelled PCBs tracer to the urban sediment. The
concentration of PCB-derived radioactivity in the liver reached a
steady state after 14 days of exposure; the steady state concentration
in the carcase was found to be significantly lower.
(c) Uptake from food
Lieb et al. (1974) fed rainbow trout Salmo gairdneri on a diet
containing 15 mg Aroclor 1254/kg for 16 or 32 weeks. PCB levels in the
lipid fraction increased rapidly for the first 8 weeks, reaching
equilibrium at about 95 mg/kg. The absolute quantity of PCBs continued
to increase as the fish grew. The trout had retained 68% of the total
PCBs ingested at equilibrium. No elimination was found after transfer
to uncontaminated food at 16 weeks (for a period of 16 weeks), or
after starving the fish for 8 weeks following exposure for 32 weeks.
Reductions in PCB levels were found, but these were cancelled out by
concomitant reductions in lipid content.
Coho salmon (Oncorhynchus kisutch) parr were fed 10 mg chloro-
biphenyls/kg (containing equal parts by weight of 3,4,3',4'-tetra-
chlorobiphenyl, 2,4,5,2',4',5'-hexachlorobiphenyl, and
2,4,6,2',4',6'-hexachlorobiphenyl) for up to 165 days (Gruger et al.,
1975). Most of the PCBs were accumulated in the adipose tissue of the
salmon (51.1 mg/kg total chlorobiphenyls after 165 days). Tissue
levels of tetrachlorobiphenyl were about half those of either of the
two hexachlorobiphenyls throughout the exposure period. When fish were
starved for 48 days, the data indicate mobilization or transformation,
with, for example, chlorobiphenyls in the spleens lowered by half and
in adipose tissue increased 5-fold. Most tissues showed an increase in
PCB levels, especially blood levels. In contrast, when a second group
of salmon were fed on a clean diet, chlorobiphenyls were released from
adipose tissue and levels increased in some other tissues, such as the
lateral line dark muscle tissue. The ratio of the different
chlorobiphenyls remained unchanged during both of these post-exposure
treatments. Gruger et al. (1976) fed juvenile coho salmon diets
containing a mixture of 2,5,2',5'-tetrachlorobiphenyl, 2,4,5,2',5'-
pentachlorobiphenyl, and 2,4,5,2',4',5'-hexachlorobiphenyl
at 1, 2, and 12 mg/kg, for up to 72 days. A steady state appeared to
have been reached between 17 and 35 days at the lowest dose (a whole
body concentration of approximately 0.45 µg/kg (wet weight)); steady
state was not achieved at the other 2 dose levels. All 3
chlorobiphenyls were accumulated to similar levels. Comparing these
data with the study by Gruger et al. (1975), suggests that the
position of the chlorine substitution is an important factor.
Hansen et al. (1976a) fed channel catfish (Ictalurus punctatus) on a
diet contaminated with 20 mg Aroclor 1242/kg. The total burden of PCBs
(µg PCB/fish) increased exponentially with exposure time. When fish
were placed on a clean diet (from day 84 for 56 days) a slight net
decrease in body burden was observed, but levels remained constant
when fish were placed on a clean diet for 56 days after 140 days
exposure. On return to a PCB-contaminated diet, accumulation rates
returned to those previously observed. The authors noted that, during
PCB-free periods, there was a shift in residues from edible carcase to
offal.
Mayer et al. (1977) fed fingerling coho salmon with Aroclor 1254 at
concentrations ranging between 1.45 and 14 500 µg/kg body weight.
Equilibrium was reached after 112 days at concentrations of 1.45,
14.5, and 145 µg/kg, with whole body residues of 0.47, 0.5, and
3.8 mg/kg, respectively. A steady state was reached at the 2 highest
dose levels of 1450 and 14 500 µg/kg after 200 days, with
corresponding residues of 57 and 659 mg/kg. In another study, channel
catfish (Ictalurus punctatus) were exposed to Aroclors 1232, 1248,
1254, and 1260 in the diet at concentrations of 48 or 480 µg/kg body
weight, for 193 days. Equilibrium was only achieved at the lowest
exposure dose of Aroclor 1232, within 150 days, with a whole-body
burden of 4.5 mg/kg. Similar whole-body residues were achieved at the
lowest dose of the other Aroclors, but no steady state was reached. At
the higher dose, accumulation increased in the order Aroclor 1232 =
1248 < 1254 < 1260, with residues ranging from 13 to 32 mg/kg after
193 days.
When Zitko (1974) fed juvenile Atlantic salmon (Salmo salar) diets
containing 10 or 100 mg Aroclor 1254/kg, accumulation reached
equilibrium within 30 days at the lower dose, with a whole-body
residue of approximately 3.8 mg/kg. Equilibrium was not reached within
200 days at 100 mg PCBs/kg. A whole-body residue of 30 mg/kg was
recorded at 181 days.
Zinck & Addison (1974) administered a mixture of 2-, 3-, and
4-chlorobiphenyl to thorny skate (Raja radiata) and winter skate
(Raja ocellata) by intravenous injection. All three congeners were
cleared rapidly from blood plasma, 3-chlorobiphenyl consistently being
cleared more rapidly than the other two. Less than 6% of
3-chlorobiphenyl remained in the plasma after 15 min compared with 30%
for the other chlorobiphenyls. All three accumulated in the other
tissues of R. radiata, principally in the liver and muscle. Tissue
levels of 3-chlorobiphenyl were consistently less than the others
during the 53-h sampling period.
In a study by Guiney & Peterson (1980), both yellow perch (a non-fatty
fish) and rainbow trout (a fatty fish) were dosed with 0.8 µg of
14C-labelled 2,5,2',5'-tetrachlorobiphenyl, either orally or by
intraperitoneal injection. Whole-body elimination was found to be
similar for both species and routes. A 20-30% elimination was observed
after 3-4 days with virtually no more PCBs being eliminated during the
rest of the 32-day sampling period. Tissue distribution varied between
the 2 species; uptake in the perch was mainly concentrated in the
viscera and carcase, whereas, in the trout, skeletal muscle and
carcase were the major sites of uptake.
Niimi & Oliver (1983) calculated the biological half-life of 31
dichloro- to decachlorobiphenyl congeners, 105 days after a single
oral dose of 46-261 mg/kg was administered to rainbow trout (Salmo
gairdneri). Whole-body half-lives increased from 5 days to >1000
days as the number of chlorines on the biphenyl increased. From
structure-activity analysis of half-lives in whole fish, the authors
concluded that elimination was enhanced for congeners with a lower
chlorine content and no chlorine substitutions in the ortho
positions, and for those with 2 unsubstituted carbons adjacent on the
biphenyl.
4.2.4.5 Birds
PCBs are taken up from contaminated food or water and concentrated in
the fatty tissues of birds. PCBs of higher chlorination levels are
accumulated to a greater extent. Egg-laying females can lose
substantial amounts of PCBs from body tissues by transferring the PCBs
to the eggs. Redistribution of residues occurs on starvation (of
significance during the migration of birds in the wild). Expressed as
a whole-body concentration, PCB residues fall during starvation.
However, expressed as a concentration in fat, residues rise. Most
critically, PCB residues in the brain increase during starvation and
this may kill the birds without further intake of PCBs.
Brunström et al. (1982a) injected the yolk of developing hens' eggs,
on day 4 of incubation, with 14C-labelled 2,4,2',5'-tetra-
chlorobiphenyl at a concentration of 5 mg/kg. One hour after
injection, radioactivity was found in the sub-blastodermic fluid, the
highest concentrations being in amniotic membranes. None was present
in the yolk, albumen, or embryonic tissues. Uptake was uniform
throughout the embryo, after one day, and, as tissues developed,
became concentrated in certain of them, such as the liver, kidney, and
fluid brain vesicles, by day 7. 14C was found uniformly in the yolk
after 11-14 days and was highly concentrated in the first bile
produced on day 11. The labelled PCBs accumulated in fatty tissue as
it developed from day 14 onwards. In the hatched chick, large amounts
of radioactivity were found to be concentrated in the gall bladder,
intestine, cloaca, and the coiling of the gizzard. When either
3,4,3',4'-tetrachlorobiphenyl or 2,4,2',5'-tetrachlorobiphenyl was
injected into the air sac of hens' eggs on day 14 of incubation at
0.4 mg/kg, no difference in distribution pattern was observed 1-5 days
later (Brunström & Darnerud, 1983). The highest amounts of
radioactivity were found in the fatty tissue, liver, kidneys, and the
gall bladder, 14C was also found in the bone marrow, the adrenals,
and the gonads, but to a lesser extent. The yolk contained less
radioactivity than the yolk analysed in the previous study by
Brunström et al. (1982a), because the PCBs were administered via the
air sac.
White leghorn hens were exposed to 50 mg Aroclor 1254/litre in their
water for 6 weeks (Tumasonis et al., 1973). PCB residues in the yolks
of eggs laid increased during the exposure period to a peak, after 6
weeks, of approximately 205 mg/kg. When hens were given clean water,
the yolk levels of PCBs quickly dropped within 5 weeks to
approximately 100 mg/kg, and then more slowly until, after 20 weeks
without Aroclor 1254 in their water, the hens laid eggs containing
0.7 mg/kg.
During a 4-week exposure to Aroclor 1242, 1254, or 1260, in the feed
of one-day-old chicks, Harris & Rose (1972) found that PCBs
accumulated in the fat and that this accumulation increased with
increasing exposure concentrations of 100, 200, and 400 mg/kg. At the
2 highest dose levels, the hens accumulated more of Aroclor 1260 than
of the other 2 Aroclors (i.e., 482, 1427, and 2151 mg Aroclor 1260/kg
at the 3 exposure concentrations, respectively). At the highest dose,
there was high mortality during exposure to Aroclor 1242 and 1254 and
this might have affected the residues found.
Greichus et al. (1975) fed white pelicans (Pelecanus erythrorhynchos)
on a fish diet containing 100 mg Aroclor 1254/day, for 10 weeks. PCB
residues were measured in the carcase, liver, feathers, and brain;
mean residues found were 2130, 290, 120, and 110 mg/kg wet weight,
respectively.
In a study by Dahlgren et al. (1972), 11-week-old pheasant (Phasianus
colchicus) were dosed with one capsule per day containing 210 mg of
Aroclor 1254. Birds that died between days 1 and 5 contained, on
average, PCB residues of 520 mg/kg in the brain, 2500 mg/kg in the
liver, and 140 mg/kg in muscle. Birds that were sacrificed over the
same period had mean brain, liver, and muscle PCB levels of 370, 1900,
and 83 mg/kg, respectively. All birds dosed with only 10 mg of Aroclor
1254 per day died within 180 days and contained average brain and
liver residues of 360 and 1200 mg/kg, respectively.
Södergren & Ulfstrand (1972) fed robins (Erithacus rubecula)
mealworms containing 1 µg of Clophen A50/day for 15 days. Brain,
breast muscle, and carcase were analysed and contained mean PCB
residues of 0.35, 0.55, and 4.5 mg/kg fresh weight, respectively. A
second group of robins was starved following dosing and all died
within 48 h. PCB levels were higher in the brain and breast muscle at
1.1 and 1.3 mg/kg, respectively, but carcase PCB levels were lower on
a fresh weight basis at 2.6 mg/kg. When the carcase lost some of its
fat content during starvation, PCB levels in terms of fresh weight
decreased. Consequently, because of the low remaining fat content,
residue levels in terms of fat weight increased. Another group of
birds were fed both PCBs and DDT (10.5 µg/day) for 15 days and then
starved. PCB levels in all 3 tissues analysed were higher than those
in birds administered PCBs alone followed by starvation; residues
were: brain, 9.3 mg/kg fresh weight, breast muscle, 8.8 mg/kg, and
carcase, 4.5 mg/kg.
Cormorants (Phalacrocorax carbosinensis) were kept on a fish diet
contaminated with PCBs for one month, followed by gelatin capsules of
PCBs administered daily for the remainder of the exposure (Koeman et
al., 1973). After 14 weeks, the dose rate of Clophen A60 was increased
periodically during the exposure period from 200 to 500 mg/kg. The
birds died between days 55 and 124, and overall residues of PCBs
increased in the tissues, the longer the birds survived. Total-body
residues ranged from 850 to 2750 mg PCBs/kg (wet weight) at death.
Brain and liver residues ranged from 76 to 180 mg/kg and from 210 to
290 mg/kg, respectively. The fat of 2 birds was analysed for PCBs and
was found to contain 10 300 and 20 500 mg/kg.
Harris & Osborn (1981) dosed wild puffins (Fratercula arctica) by
implantation with 30-35 mg of Aroclor 1254. PCBs were quickly taken up
in fat, with concentrations rising to 10-14 times that in control
birds (highest fat residue 654 mg/kg wet weight), and remaining at
this level for up to 10 months. Levels slowly declined, but were still
twice those of controls after 34 months. PCB concentrations in the
liver and muscle tissue were highest shortly after dosing (48.4 and
25.2 mg/kg, respectively) and declined until, after 16 months, no PCBs
were detectable. Levels of PCBs in the kidneys and brain were variable
with no consistent trends.
Common grackles (Quiscalus quiscula), starlings (Sturnus vulgaris),
red-winged blackbirds (Agelaius phoeniceus), and brown-headed
cowbirds (Molothrus ater), were fed diets containing 1500 mg Aroclor
1254/kg over an 8-day period (Stickel et al., 1984). PCB residues in
the brains of birds that died were found to be higher than those in
birds that were sacrificed over a similar period. PCB residues ranged
from 349 to 763 mg/kg in birds that died and from 54 to 301 mg/kg in
birds sacrificed. Liver and whole-body residues tended to be higher in
birds that died, but they overlapped to a large extent. PCB residues
in whole bodies on a lipid basis showed the most clear-cut difference,
ranging from 22 600 to 98 600 mg/kg for birds that died and from 6690
to 22 500 mg/kg for those sacrificed. PCB residues in grackles
declined slowly, when the birds were placed on a clean diet. From a
whole-body level of 1300 mg/kg, residues declined to 169 mg/kg, 224
days later. The rate of decline was irregular, but a half-life was
estimated at 89 days over this period of loss.
4.2.4.6 Mammals
Olsson et al. (1979) fed mink (Mustela vison) on a diet containing
11 mg PCBs/kg for 66 days. Mink accumulated 310 mg PCBs/kg in
extractable fat over the exposure period. Control mink were found to
contain 14 mg PCBs/kg, and, when the control feed was analysed, it was
found to contain 0.05 mg PCBs/kg. The authors also found a significant
increase in cadmium uptake in the kidneys of PCB-treated animals
compared with controls. In another study on mink (Mustela vison),
Hornshaw et al. (1983) administered various PCB-contaminated fish
diets containing between 0.21 and 1.5 mg PCBs/kg. Adipose tissue
samples were taken after 6-8 weeks and after 18 weeks exposure (Table
11). The amount of PCBs accumulated was directly related to the amount
of PCBs in the diet; mean PCB residues ranging from 4 to 24.8 mg/kg
after 6-8 weeks and from 8.1 to 42.8 mg/kg after 18 weeks. When
expressed as individual congeners, it can be seen that the mink showed
the highest accumulation of the PCBs with the chromatographic peak
corresponding to 2,4,5,2',4',5'-hexachlorobiphenyl. To determine the
rate of PCB elimination, male mink that had been on a fish diet
containing 1.5 mg PCBs/kg for 10 weeks were transferred to a control
diet. Over this period, adipose tissue residues of 32 mg PCBs/kg had
accumulated. Over the 16-week elimination period, 60.3% of the total
PCB burden of the adipose tissue was eliminated. This consisted of a
loss of 87.2% of 2,5,2',5'-tetrachlorobiphenyl, 88.9% of
2,3,6,2',5'-pentachlorobiphenyl, and 55.4% of the hexachlorobiphenyl.
The half-life for total PCBs in mink adipose tissue was calculated to
be 98 days.
Wren et al. (1987a,b) fed mink on a commercial mink food supplemented
with 1 mg Aroclor 1254/kg for a period of 6 months. Male mink had
liver residues of 1.98 mg PCBs/kg after 118 days and 2.8 mg/kg after
183 days exposure. The liver of a female, analysed on day 161
contained a residue of 3.1 mg PCBs/kg. Liver PCB levels in 5-week-old
kits were similar to those in adult mink fed the experimental diet for
several months. Bleavins et al. (1981) measured the relative
importance of placental transfer and milk in the transfer of PCB
residues from mother mink to offspring. Newborn kits contained less
than 0.1% of a dose of PCBs injected into the mother mink. At 2 weeks
of age, the kits contained 1.2% of the dose given to the mother,
suggesting that lactation is a major route of exposing the young to
PCBs and a major route for the loss of PCBs from the mother. Placental
transfer of PCBs was greater in the ferret than in the mink (Bleavins
et al., 1984). The ratio of placental to mammary transfer was 1:15 for
offspring whose mothers were dosed during the first trimester of
pregnancy and 1:7 for mothers exposed during the last trimester.
Big brown bats (Eptesicus fuscus) were fed on mealworm diets
containing 9.4 mg Aroclor 1254/kg for up to 37 days (Clark & Prouty,
1977). In bats sacrificed on day 37, residues ranged from 29 to 121 mg
PCBs/kg (wet weight) for the carcase and from not detectable to
4.2 mg/kg in the brain. Bats that were starved following exposure
showed a significant correlation between increasing brain PCB
concentrations and carcase lipid concentrations. The authors stated
that PCBs increased in brain tissue as carcase fat was metabolized.
Clark (1978) exposed pregnant big brown bats to a mealworm diet
containing 6.36 mg Aroclor 1260/kg for approximately 18-28 days, until
the young were born. Mean carcase levels of PCBs were 20.34 mg/kg in
parent females and 4.38 mg/kg in litters. Levels of PCBs in both
adults and young continued to rise throughout the sampling period; the
longer the gestation time, the higher the PCB level in the sample.
4.2.5 Appraisal
Experimental work on mammals has been concentrated on terrestrial
species. Problems with PCB toxicity are important for marine mammals,
but these are less convenient for experimental study. Results in this
section, therefore, have to be related to field observations on marine
species.
Mink take up more chlorinated components of PCB mixtures and can
accumulate large residues of PCBs. On cessation of exposure, more
tetrachloro- and pentachlorobiphenyls were eliminated than
hexachlorobiphenyl. The half-life for total PCBs was calculated to be
98 days. PCB residues are transferred from mother to offspring. The
relative importance of transplacental transfer and transfer in milk
varies between species. Redistribution of residues takes place on
starvation, which is of significance for migratory species; brain
residues, which may be fatal with no further intake of PCBs, increase
as animals are starved.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Levels in the environment
PCBs were detected in the environment in the late 1960s (Risebrough et
al., 1968; Jensen et al., 1969) and, within a short time, were
reported as contaminants in almost every component of the global
ecosystem including air, water, soil, fish, wildlife, human blood,
adipose tissues, and milk (Holdrinet et al., 1977; Wassermann et al.,
1979; Ballschmitter et al., 1981; Buckley, 1982; Safe, 1982b; Bush et
al., 1985; Kannan et al., 1988; Tanabe, 1988).
The lipophilic properties of PCBs are the basis of the bioaccumulation
and biomagnification that has been demonstrated and, thus, numerous
sources within the environment can lead to human exposure.
High-resolution, gas-chromatographic analysis has shown that the
congener composition and relative concentrations of the individual
components in many PCB extracts from environmental samples differ
markedly from those in the commercial PCBs (Jensen & Sundström, 1974a;
Wolff et al., 1982a; Safe et al., 1985a; Brown et al., 1987a,b).
A major problem with data concerning PCB levels in environmental
samples is that they normally are only available for "total PCBs" and
that there are much fewer data on actual "PCB patterns". Moreover,
when comparing results produced from different laboratories or from
the same laboratory at different times, an additional difficulty may
arise from differences in the sampling and analytical techniques used.
It is difficult, if not impossible, to compare data obtained with
different analytical methods, from different laboratories, and
countries. Nowadays, the older data seem less reliable, especially in
the light of the use of improved analytical methods and better
sampling techniques (WHO/EURO, 1987). A comprehensive review of world
PCB levels was published by Wassermann et al. (1979).
5.1.1 Air
PCB concentrations in air differ markedly from location to location,
with the lower levels found over the oceans or over non-industrialized
regions, such as the Canadian Northwest territories. In general,
levels over industrialized areas or over landfills are the highest.
Apparently, these levels influence PCB levels in rainwater and there
is a gradient of values in air from industrial to rural areas. Some
typical values can be found in Table 12.
MacLeod (1981) described a method for the analysis of PCBs using
low-volume, indoor air sampling to estimate the presence of PCBs in
indoor air in work-places and homes in the USA. Three facilities, an
industrial research facility, an academic facility, and a shopping
complex were sampled. The periods of sampling ranged from 2 days up to
6 months. The average concentrations (calculated as Aroclor 1242 plus
Aroclor 1254) ranged from 44 up to 240 ng/m3. Outdoor levels of up to
18 ng/mg3 were found. In the homes, air samples from 14 areas (of
which 9 were kitchens) were also analysed. The average concentrations
in the kitchens ranged from 150 up to 500 ng/m3 and, in the other
rooms, from 39 to 170 ng/m3. In a library, a level of 400 ng/m3 was
found.
The levels of PCB exposure that may occur in buildings in the USA were
determined by Oatman & Roy (1986). Air samples and surface wipe
samples were taken in 5 state-owned, office buildings and 2 elementary
schools. The average levels of airborne PCBs in buildings with PCB
transformers were nearly twice the levels in buildings without
transformers, i.e., 457 ± 223 and 229 ± 106 ng/m3, respectively. The
mean of the surface wipes taken in buildings without PCB transformers
was 0.17 and that in the buildings with transformers 0.23 µg/100 cm2.
There was a wide variation between the different buildings and, as
shown above, the presence of transformers influenced the indoor PCB
concentrations.
5.1.1.1 Rain and snow
In the Netherlands, at Bilthoven, the PCB-concentrations in rainwater
ranged from 0.01 to 1.5 µg/litre (van Zorge, cf. WHO/EURO, 1985). In
the Federal Republic of Germany, concentrations of 0-4 ng/litre were
found (DFG, 1988).
Table 12. PCB levels in air in several countries
Country Location and/or type of sample PCB levels References
average and/or range
Canada Northwestern territories 0.002-0.07 ng/m3 Bidleman et al. (1978)
Germany Industrial area (Ruhr area) 3.3 ng/m3 DFG (1988)
Non-contaminated area 0.003 ng/m3
Japan Within industrial plants: - PCB vapours 13-540 µg/m3 Tatsukawa & Watanabe
- PCBs on airborne particulates 4-650 µg/m3 (1972)
North Pacific, South Pacific, Indian, 0.1-0.3 ng/m3 Tatsukawa & Tanabe
Antarctic and South Atlantic Oceans (1983)
North Atlantic Ocean 0.5 ng/m3 Tatsukawa & Tanabe
(1983)
Sweden Several locations 0.8a-3.9 ng/m3 Ekstedt & Odén (1974)
USA Near the North-East Coast 5 ng/m3 Harvey & Steinhauer
Over the Atlantic Ocean, 2000 km away 0.05 ng/m3 (1974)
from the industrial complex
several locations 1-50 ng/m3 Panel on Hazardous
Substances (1972)
cf WHO/EURO (1988)
Yugoslavia Bela Krajina: - 300 m from an industrial plant 4-7 µg/m3 Jan et al. (1988b)
- air near a waste landfill 45 µg/m3
- over the River Kruga 2-5 µg/m3
a Limit of determination.
5.1.1.2 Natural gas
PCBs were first identified in gas pipelines in January 1981, when a
PCB-containing oil condensate was found in the gas meters of some
residential customers in Long Island, New York. Voluntary monitoring
of condensate and natural gas by 33 transmission companies, showed the
presence of PCBs in 12 companies. PCBs were also found in gas
pipelines. Condensate is a mixture of heavier hydrocarbons and other
liquids, such as water, that condenses, because the gas is transmitted
under pressure. This condensate tends to collect in pools in the
pipes. In the period 1981-83, 1841 samples of condensate from gas
pipelines were analysed: 659 (35.8%) of the samples contained
< 25 mg/kg; 65.8% of the samples contained <1000 mg/kg, and 0.4%,
> 10 000 mg/kg. The maximum level that was found was 42 394 mg/kg
(Versar Inc., 1984).
In the period 1981-83, 138 samples of natural gas in transmission were
analysed. In 29 samples, PCBs were found with a minimum concentration
of <0.004 µg/m3 and a maximum concentration of 1050 µg/m3. Natural
gas in distribution lines was also analysed in the same period. Out of
528 samples, 224 did not contain any PCBs. The levels ranged from
<0.02 to 51 µg/m3.
Indoor concentrations (kitchens, etc.) were measured in 419 samples in
the period 1981-83. No PCBs could be detected in 49 samples, but, in
the others, levels ranged from <0.01 to 1.08 µg/m3 (Versar Inc.,
1984).
5.1.2 Water
Surface water may become contaminated with PCBs from atmospheric
fall-out or from direct emissions from point sources. Because of
adsorption on suspended particles, PCB concentrations in heavily
contaminated waters may be several times greater than their
solubility. Södergren (1973) reported a seasonal variation, which was
attributed to aerial fall-out.
It has been shown that polluted rivers, lakes, and estuaries have
higher PCB values than non-polluted waters (Table 13). On the basis of
scanty information on PCBs and reinforced by extensive analogue
information on DDT, it has been estimated that, for the Great Lakes of
North America, non-polluted freshwaters might contain less than
5 ng/litre, moderately polluted rivers and estuaries, 50 ng/litre, and
highly polluted rivers, 500 ng/litre. These values can be used to
evaluate those reported by several authors and presented in Table 13.
Table 13. PCB levels in water in several countries
Country Location and/or type of PCB levels References
sample average and/or range
Germany Several rivers 5-103 ng/litre Lorenz & Neumeier (1983)
Netherlands River Rhine (1976/1977) 100-500 ng/litre Wegman & Greve (1980)
Sweden Water entering a treatment 0.5 ng/litre Ahling & Jensen (1970)
plant
Tap water produced at the plant 0.33 ng/litre Ahling & Jensen (1970)
Several rivers 0.1-0.3 ng/litre Ahnoff & Josefsson (1974)
USA Polluted coastal area 100-450 ng/litre Panel on Trace Hazardous
Lake Michigan (1970)a Substances (1972) (cf.
WHO/EURO, 1988)
Distribution system feeding the Fort Edwards reservoirs < 12-160 ng/litre Brinkman et al. (1980, 1981)
in New York (1978)
Hudson River at Fort Edward up to 530 ng/litre Brinkman et al. (1980, 1981)
a Followed by a marked decrease in 1971.
5.1.3 Soil
Soil may become contaminated with PCBs from atmospheric fall-out or
from direct emissions from point sources. The presence and behaviour
of these compounds in the soil depend on substance (congener)-specific
characteristics and on a number of soil parameters. Sorption and
condensation processes in the soil also play a role in the removal of
PCBs. Some values of PCB levels in soil can be found in Table 14.
Klein (1983) found that PCBs accumulate in the sediments of rivers and
lakes in the Federal Republic of Germany and that these levels
indirectly reflect the contamination of water by PCBs. Some values for
PCBs in sediments can also be found in Table 14.
An important, though localized, source of PCB contamination of soil,
can be the use of sewage sludge as a fertilizer in agriculture. PCB
levels varying from 0.1 to 765 mg/kg (dry weight) have been reported
in sewage sludge from different countries, the usual range being 0.1
to 9.0 mg/kg (WHO/EURO, 1987). In the USA, 16 sewage sludge samples
from cities contained a mean Aroclor 1254 concentration of 5.2 mg/kg
dry weight (range 0.01-23.1 mg/kg). Other authors reported a range of
1.5-27.3 µg/litre in 36 raw sewage sludges. Some levels that have been
found for PCBs in sludges are presented in Table 14 (WHO/EURO, 1987).
Five sediment samples were collected from the Waukegan Harbour of Lake
Michigan, Illinois, in 1978. Residues of 3,4,3',4'-tetrachlorobiphenyl
ranged from 0.005 to 27.5 mg/kg and residues of 2,3,4,3',4'-penta-
chlorobiphenyl, from 0.102-131 mg/kg. The total PCB contents of the
sediment ranged from 10.6 to 13 360 mg/kg (Huckins et al., 1988).
5.1.4 Aquatic and terrestrial organisms
PCBs have been measured in a wide variety of biota from many different
locations throughout the world. Only a few illustrative examples are
given here, more comprehensive lists of PCB residues can be found in
reviews by Risebrough et al. (1968); Peakall (1975); and Eisler
(1986). Tanabe et al. (1987) reported that the highly toxic, coplanar
PCBs are as widely spread as general PCB pollution.
In the biota of a small upstate New York public water supply system,
which is near the polluted section of the Hudson River and a disposal
site of PCB-containing waste, PCBs were found in detectable
concentrations (Table 13). Five samples of algae showed Aroclor 1254
levels of <25 (nd)-120 µg/kg dry weight, macro-invertebrates showed
levels between <200 and 3800 µg/kg and vertebrates, between <25 and
1100 µg/kg dry weight (Brinkman et al., 1980, 1981).
Table 14. PCB levels in soils, sediments, and sewage sludge in several countries
Country Location and/or type of sample PCB levels References
average and/or range
Germany Soil without sewage sludge 0.02-0.08 mg/kga Markard (1988)
Soil with sewage sludge 0.05-3.0 mg/kga
Sewage sludge ndb-19 mg/kg
Sediments of contaminated waters 0.1-1.0 mg/kga Klein (1983)
Sediments of several rivers 0.16-0.59 mg/kg DFG (1988)
Agricultural soil 0.03 mg/kg DFG (1988)
Japan Agricultural soil < 1 mg/kg Fukada et al. (1973)
Soil near a factory making electrical components 510 mg/kg Fukada et al. (1973)
Netherlands Sediments from several surface waters < 0.01-1.2 mg/kga Greve & Wegman (1983)
United Soil from a waste disposal area with chemical treatment 4.5-44.8 µg/kg Eduljee et al. (1986);
Kingdom and incineration facilities Badsha et al. (1986);
(Scotland) Grass samples from the same area (foliage) 2.9-64.7 µg/kg Badsha & Eduljee (1986)
Soil of rural areas 8 µg/kga (1-23)
Grass of rural areas 9 µg/kga (7-16)
Soil of urban areas 52 µg/kg (11-141)
Soil of industrial locations 41 µg/kg (20-67)
United Surface soil 2.5 µg/kg Jones (1989)
Kingdom (0.2-12.2)
(Wales)
Table 14. (cont'd).
Country Location and/or type of sample PCB levels References
average and/or range
USA Sediments near a point of accidental release of PCBs 1.4-61 mg/kg Nimmo et al. (1971a)
(Florida)
Escambia Sediments 16 km downstream of this point 0.6 mg/kg
river
Escambia Soil samples from the bank, 6.5 km downstream from the 1.4-1.7 mg/kg
Bay point
a Dry weight
b Not detectable
Serious environmental contamination has been documented in enclosed
water bodies close to urban and industrialized areas, such as the
Great Lakes, the Baltic Sea, and Tokyo Bay. PCB levels in aquatic
organisms reflect these localized high concentrations.
Nimmo et al. (1971a) reported that PCB levels in shrimp from Escambia
Bay, Florida (contaminated by an industrial plant on the Escambia
River) contained between 0.6 and 120 mg Aroclor 1254/kg in 1969 and
fiddler crabs, collected in 1970, contained 0.45-1.5 mg/kg.
When fish, sampled throughout the USA, were analysed by Schmitt et al.
(1983, 1985), the highest levels of PCBs were found in the
North-eastern industrialized areas. Delfino (1979) reported
concentrations ranging from 26 to almost 1000 mg PCBs/kg in fish
collected from the Sheboygan River, Wisconsin, contaminated by a
die-casting plant.
Wiemeyer et al. (1975) analysed osprey eggs in 1968-69 and found
average levels of 2.6 mg/kg in Maryland compared with an average level
of 15 mg/kg in eggs from Connecticut. PCB residues in Connecticut eggs
had not changed significantly compared with those collected in 1964.
Buckley (1982) analysed aspen, sumac, and golden rod plants growing at
various distances (< 1200 m) and in different directions from a PCB
dump in New York State, USA. All the plants were growing beyond a
natural drainage ravine, which prevented contamination of soil and
water by PCBs. Downwind of the site, PCB levels in the plants were
found to be approximately 100 mg/kg dry weight (over 600 times
background levels in plants). Levels above background concentrations
were also found in directions from the site less obviously
contaminated by airborne dust.
Eggs of terrestrial birds collected in a rural environment in Canada
contained lower PCB levels than those sampled from urban areas (Frank
et al., 1975).
In the Great Lakes, the highest levels of PCBs were found in Lakes
Michigan and Ontario for fish (Delfino, 1979) and Lake Ontario for
birds (Weseloh et al., 1979); both lakes receive input from industrial
and urban sites. Glooschenko et al. (1976) found concentrations of up
to 8.1 mg/kg in microorganisms from the middle of Lake Huron.
Weseloh et al. (1983) found that the PCB levels in double-crested
cormorant eggs, collected from Lake Superior during 1972 (average of
23.8 mg/kg fresh weight), were higher than those in cormorant eggs
analysed in other Canadian colonies. Mineau et al. (1984) found that
the locations of herring gull colonies with the greatest mean levels
of PCBs, in each of the Great Lakes, corresponded with the locations
of major sources of the contaminant, as indicated by elevated residues
in sediment.
Muir et al. (1988) determined PCB levels in pooled Arctic cod muscle
(Boreogadus saida) and polar bear fat (Ursus maritimus), and in
the blubber and liver of ringed seals (Phoca hispida) from 3
locations in the East/Central Canadian Arctic. The mean arithmetic
concentrations of total-PCBs in the muscle of Arctic cod of 2
locations were 3 and 5 µg/kg wet weight. The mean concentrations shown
in the tabulation below were found in the blubber and liver of ringed
seals.
Year Number of Sex Arithmetic mean ± SD
samples (µg/kg wet weight)
(blubber)
1972 3 female 639 ± 249
1975/76 5 female 600 ± 99
1983 10 male 794 ± 879
16 female 308 ± 138
1984 19 male 568 ± 287
14 female 375 ± 172
(liver)
1984 19 male 6 ± 4
14 female 4 ± 3
The presence of PCBs in polar bears (Ursus maritimus) was studied by
Norström et al. (1988) in the Northwest territories of Canada. Liver
and adipose tissue specimens were obtained by Inuit hunters from 12
zones over the period 1982-84. A total of 121 samples was obtained.
The mean concentrations of total PCBs in pooled samples ranged from
3.24 to 8.25 mg/kg, on a lipid weight basis. The adipose tissue of
polar bear (10 pooled samples collected in 1982 and 10 samples, in
1984) contained 4.42 and 4.57 mg/kg wet weight, respectively. From
these results, biomagnification factors for the food-chain of the
Arctic cod/ringed seal/polar bears were calculated. For total PCBs,
these factors ranged from 3.7 to 8.8 for fish to seal; from 7.4 to
13.9 for seal to bear, and 49.2 for fish to bear. For individual PCB
homologues, for instance, for fish to bear, these factors ranged from
<0.5 (tetrachlorinated PCBs) to 263.4 for heptachlorinated PCBs.
Niimi & Oliver (1989b) monitored the presence of 92 monochloro- to
decachlorobiphenyl congeners in brown and lake trout, small and large
rainbow trout, and small and large coho salmon from Lake Ontario. Each
sample consisted of 8-12 fish. The highest concentrations were among
the penta- and hexachlorobiphenyl homologues, with 2,4,5,2',4',5'-
hexachlorobiphenyl the most common congener.
Total congener concentrations ranged from 1 to 10 mg/kg in whole fish
and from 0.3 to 4 mg/kg in muscle. The 10 most common PCB isomers were
84, 87/97, 101, 110, 118, 138, 149, 153, and 180, and represented 52%
of the total content. This value did not appear to be influenced by
species or by total concentration.
Huckins et al. (1988) collected fish (1-6 fish of 7 species) from the
Waukegan Harbour of Lake Michigan, Illinois in 1978. The fish samples
were analysed for the presence of 3,4,3',4'-tetrachloro- and
2,3,4,3',4'-pentachlorobiphenyl. Total PCB congener residues averaged
33.4 (2.4-56.6) mg/kg. The concentrations of 3,4,3',4'-tetra-
chlorobiphenyl averaged 45.3 µg/kg (2-89 µg/kg) in the whole body. The
concentrations for 2,3,4,3',4'-pentachlorobiphenyl averaged 229 µg/kg
(80-483 µg/kg).
Five times as much PCBs were found in herrings caught in
industrialized areas of Sweden (near Stockholm) compared with those
caught in the cleaner waters off the Swedish west coast. Levels in
plankton fell progressively with increasing distance from
industrialized areas (Jensen et al., 1972a).
Holden (1973) found levels of up to 235 mg/kg in the blubber of seals
sampled in the polluted coastal areas of the United Kingdom compared
with lower levels (2 mg/kg) from unpolluted areas. Higher levels, (up
to 88 mg/kg) were found in the blubber of toothed whales sampled in
the North Sea, but none was detectable in similar species sampled off
New Zealand and Surinam (Koeman et al., 1972).
Peakall (1975) mapped out the global distribution of PCB levels in
marine plankton. The values for the open North Atlantic (300-450 mg/kg
lipid) were found to be very similar to those collected from polluted
areas, such as the Baltic sea and the Firth of Clyde, in the United
Kingdom. Values in the South Atlantic (12-64 mg/kg) were considerably
lower. The highest values shown were for the Eastern coast of the USA
(up to 3050 mg/kg). There were no values for the Pacific Ocean.
When monitoring PCB levels in fish from the Mediterranean, Albaiges et
al. (1987) found that territorial species reflected local inputs of
the pollutant, but migratory species had baseline levels.
Risebrough & de Lappe (1972) reported PCB levels higher than 3 mg/kg
in fish from the industrialized areas of Tokyo Bay and New York Sound.
Tanabe et al. (1986a) analysed Antarctic minke whales and found that
they contained lower PCB levels than those caught in the Northern
hemisphere (Tanabe et al., 1983). McClurg (1984) also found low levels
of PCB in the Antarctic; Ross seals contained 0.09 mg/kg (in blubber).
Mean levels of 0.69 mg PCB/kg (wet weight), found by Smillie & Waid
(1987) in Australian fur seal blubber, were much lower than levels
found in seals from the temperate Northern hemisphere. Similarly,
Antarctic fish had very low PCB residues, ranging from 0.08 to
0.77 µg/kg wet weight (Subramanian et al., 1983).
PCB residues in biota are usually highest near industrial sources, but
this geographical distribution is becoming less pronounced. In fact,
O'Shea et al. (1980) and Tanabe et al. (1988) found PCB levels in
small oceanic cetaceans to be higher than those reported for
terrestrial mammals and birds. For example, Tanabe et al. (1988) found
the mean level of PCBs in the fatty tissue of the striped dolphin to
be 36 mg/kg wet weight.
Subramanian et al. (1986) analysed subcutaneous fat from Adelie
penguins from the Antarctic and found PCB levels of 0.05 mg/kg fat
weight. This is a factor of 100 lower than that in auks caught in the
northern North Pacific (Tanaka & Ogi, 1984) and a factor of 10 000
lower than residues found in the pectoral muscle (on a lipid weight
basis) of herring gulls in the Baltic (Lemmetyinen et al., 1982).
5.1.4.1 Effect of dredging-contaminated sediment on organisms
Dredging to remove contaminated sediments from the Shiawassee River,
Michigan, increased the availability of PCBs, and, thus, residue
levels, in freshwater clams (64.5-88 mg/kg dry weight) and in fish
(fathead minnow; 13.8-18.3 mg/kg), both during dredging and up to 6
months afterwards (Rice & White, 1987).
5.1.4.2 Relationship to lipid content of organisms
PCBs are accumulated in lipid-rich tissues and care must be taken when
interpreting results between species with different amounts of body
fat. Jensen et al. (1969) found that PCB levels in herring and cod,
from the same area of the Baltic Sea, were 0.27 and 0.033 mg/kg, on a
wet weight basis, respectively, even though the cod is at a higher
trophic level. The 2 species were found to have body fat contents of
4.4 and 0.32%, respectively, and when the PCB residues were
recalculated on a lipid weight basis, herring contained 6.8 mg/kg and
cod, 11 mg/kg.
PCBs are particularly accumulated in animals with large amounts of
fat, such as seals, dolphins, porpoises, and whales (Tanabe, 1988) and
in Arctic and Antarctic birds and mammals. Subramanian et al. (1986)
found PCBs in all Adelie penguins sampled in the Antarctic, an area
known to be relatively low in PCBs; the PCBs were mainly concentrated
in fat-rich tissues. Kawai et al. (1988) measured PCBs in striped
dolphins and found that the tissue level of PCBs depended entirely on
their lipid content and, especially, on the amount of triglycerides in
tissues.
Redistribution of PCBs, from fat to other tissues, occurs in animals
during periods of enforced starvation, such as seasonal food shortage,
hibernation, migration, incubation, and the feeding of offspring.
Subramanian et al. (1986) found that, as individuals Adelie penguins
starved during incubation, residues of PCBs increased with declining
fat reserves concomitant with tissue redistribution. Llorente et al.
(1987) found that migratory duck species had a smaller percentage of
the body burden of PCBs in adipose tissue than a resident species. A
similar redistribution during starvation has been shown in the
laboratory in European robins (Södergren & Ulfstrand, 1972) and big
brown bats (Clark & Prouty, 1977) (see sections 4.2.4.5 and 4.2.4.6).
5.1.4.3 Residues in different trophic levels and effects of diets
In a study by Shaw & Connell (1982), bioaccumulation was increasingly
evident in upper trophic level organisms, such as gulls and pelicans,
in an Australian estuary compared with organisms from lower trophic
levels. Veith et al. (1977) found typical PCB concentrations in Lake
Superior biota to be 0.1 mg/kg for large zooplankton, 0.3 mg/kg for
bottom fish, such as sculpins, and 1 mg/kg for pelagic fish.
When various insects were sampled for PCB residues (Morse et al.,
1987), levels in honey bees ranged from <0.1 to 1.5 mg/kg dry weight.
PCB residues in other species ranged from <0.1 to 2.6 mg/kg, with
predatory wasps containing the highest residues.
Prestt et al. (1970) analysed the livers from various bird species in
the United Kingdom. The highest PCB residues were found in freshwater,
fish-eating species (up to approximately 900 mg/kg). The authors did
not find any geographical pattern of distribution of PCBs in the
species studied.
Frank et al. (1975) collected birds' eggs from the Niagara peninsula
in 1971. Eggs from carnivorous species of birds at the top of the
aquatic food chain contained the highest levels of PCBs
(3.5- 74 mg/kg). Terrestrial carnivores contained lower, but still
relatively high, residues (0.2-1 mg/kg). Eggs from herbivorous and
insectivorous birds contained much lower residues of PCBs. Again, eggs
from terrestrial birds tended to contain lower levels (0.05-2 mg/kg)
than those feeding on aquatic prey (0.14-4 mg/kg). Focardi et al.
(1988) compared the PCB residues in the eggs of 8 species of water
bird. The residues were found to be higher in fish-eating birds than
in invertebrate feeders. The invertebrate feeders tended to contain
higher percentages of the lower chlorinated congeners. Bird species
that fed on other birds or fish had higher liver residues of PCBs than
those feeding on mammals (Cooke et al., 1982). Peregrine falcons,
herons, sparrowhawks, kingfishers, and great crested grebes had
relatively high residues of PCBs. By contrast, golden eagles were only
very lightly contaminated with PCBs.
Bowes & Jonkel (1975) found a similar pattern in Arctic and subarctic
food chains with PCB levels following the pattern: Arctic charfish
< seals < adult polar bears < polar bear cubs.
Mean PCB concentrations of 0.0018 mg/kg were found by Tanabe et al.
(1984) in zooplankton, 0.048 mg/kg in myctophid, 0.068 mg/kg in squid,
and 3.7 mg/kg in striped dolphin (all based on a whole-body, wet
weight basis) sampled from the western North Pacific. The authors
concluded that the bioaccumulation of chlorinated hydrocarbons was
dependent on physical and chemical factors, such as water solubility
and lipophilicity, in the lower trophic levels, whereas, in higher
trophic levels, accumulation was affected by biochemical factors, such
as the biodegradability of pollutants and the metabolizing capability
of the organism.
5.1.4.4 Effects of age, sex, and reproductive status on uptake and
elimination
Bache et al. (1972) found that the burden of PCBs increased with age
in lake trout from Cayuga lake, Ithaca, New York, sampled in 1970
(residues ranged from 0.6 to 30.4 mg PCBs/kg). An age- and
length-related increase in PCBs was found in striped bass from the
Hudson River and Long Island Sound; the author (Connell, 1987) stated
that this observed relationship was due to the slow rate of
bioaccumulation of the PCBs, particularly the higher chlorinated
congeners.
PCBs have been shown to accumulate with age in marine mammals, such as
pinnipeds (Addison et al., 1973; Frank et al., 1973; Helle et al.,
1983) and cetaceans (Gaskin et al., 1983; Aguilar & Borrell 1988;
Subramanian et al., 1988). Helle et al. (1983) found mean levels of
5.1 mg PCBs/kg (in extractable fat of blubber) in newly-born ringed
seal pups, 17.3 mg/kg in seals of 2-4 months of age, and 65.3 mg/kg in
sexually mature adults (4-12 years). However, lower levels of PCBs
have been found in females compared with males (Martineau et al.,
1987) and the age-related increase has often not been found in females
(Addison & Smith, 1974). In many studies, while levels of PCBs in
males have increased with age, those measured in females have fallen
(Born et al., 1981; Gaskin et al., 1983; Aguilar & Borrell, 1988).
Gaskin et al. (1983) found that PCB levels in the blubber of male
harbour porpoises increased from 48.4 mg/kg at birth to 161 mg/kg
after 8 years, whereas, in females, levels fell from 51 to 14.7 mg/kg.
A significant decrease in the PCB levels was found by Subramanian et
al. (1988) in female Dall's porpoises from 2 years of age onwards; 2
years is required for the animals to reach sexual maturity. Excretion
of PCBs during reproduction is known, from the laboratory, to be an
important means of females losing residues. This PCB loss has been
shown to be because of the transfer of PCBs to offspring via milk
during lactation (Addison & Brodie, 1977). Addison & Brodie (1977)
calculated that female grey seals excreted about 15% of their body
burden of PCBs via lactation. In striped dolphins, females transferred
between 72 and 98% of their body burden to the offspring (Fukushima &
Kawai, 1981; Tanabe et al., 1982). It was suggested by Tanabe (1988)
that such large transfer was because of the very high lipid content of
the milk. Relocation of the PCB burden during pregnancy is generally
thought not to be as important; in grey seals, the mother transfers
only about 1% of her body burden to her offspring (Donkin et al.,
1981) and in striped dolphins, only 4-9% (Fukushima & Kawai, 1981;
Tanabe et al., 1982). However, Duinker & Hillebrand (1979) suggested
that a much bigger percentage of female body burden (up to 15%) could
be transferred to the fetus across the placenta of Harbour porpoise.
Clark & Lamont (1976) calculated that female big brown bats
transferred between 17 and 32% of their body burden of PCBs to their
young, during gestation. The concentration of PCBs in adult females
plus their litters declined with increasing age of the female. PCB
levels were 0.83-3.6 mg Aroclor 1260/kg (wet weight) in adults and
0.22-3.3 mg/kg in litters.
When Passino & Kramer (1980) measured PCBs in deepwater ciscoes from
Lake Superior, male fish contained significantly higher levels of PCBs
(2.3 mg/kg wet weight) than females (1.2 mg/kg), eggs containing
0.51 mg/kg. Lemmetyinen et al. (1982) found annual rates of
elimination via egg production of 45% in the female Arctic tern and
24% in the herring gull. Adelie penguins eliminated only 4% of their
PCB body burden after laying their annual clutch of 2 eggs (Tanabe et
al., 1986b). Elimination was thought to be dependent on the relative
weights of the egg and mother.
5.1.4.5 Time trends in residues
Buckley (1983) analysed various species of terrestrial plants from New
York state. Total decreases of 42% in PCB residues were found between
1978 and 1980.
PCB levels in fish in the Hudson River, New York declined between 1977
and 1981. The PCB levels were much higher in the Upper Hudson River
(4217-1431 mg/kg of lipid), near to a major discharge of PCBs, than in
the Lower Hudson River (1604-319 mg/kg) (Sloan et al., 1983).
Frank et al. (1978) measured PCB levels in various fish species from
Lakes Huron and Superior during the period 1968-76. PCB residues
declined in lake trout and lake whitefish in Lake Superior between
1971 and 1975, but increased slightly over the same period in bloaters
and white sucker. In Lake Huron, PCB levels decreased between 1968 and
1971, and, in alewife, rainbow smelt, and walleye, between 1975 and
1976. In some of the study areas, residues increased in cisco, yellow
perch, coho salmon, and splake but, at most locations, and, for other
species analysed, no trends in PCB levels were found. St Amant et al.
(1984) analysed fish from Lake Michigan between 1971 and 1981. An
overall decrease in PCB levels was found for all species monitored
except the walleye. Levels decreased from a maximum of 22.4 mg/kg at
the beginning of the study to 3.8 mg/kg or less in 1981.
Fish from all over the USA were analysed in 1980-81 by Schmitt et al.
(1985) who found a significant downward trend (0.88-0.53 mg/kg PCB;
wet weight) when mean residues were compared with fish collected
between 1976 and 1977 (Schmitt et al., 1983). A similar downward
pattern in residues was found in the Baltic when Moilanen et al.
(1982) compared residues found in pike and herring caught between 1978
and 1982 with those in fish sampled between 1972 and 1978 (Paasivirta
& Linko, 1980). Haahti & Perttila (1988) found a continued decline in
PCB residues between 1979 and 1986, when residues in herring muscle
tissue decreased from 2.7-3.7 mg/kg to 0.3-1.1 mg/kg.
An overall fall in PCB levels was found by Newton & Bogan (1978) in
sparrowhawk eggs during the period 1971-74. Cooke et al. (1982)
analysed liver samples from grey herons, kestrels, and barn owls for
PCB residues during the period 1967-77. They found a significant
decline in PCB residues over the sampling period in all 3 species. The
mean residues in heron, kestrel, and barn owl for the period 1967-71
were 5.77, 1.57, and 0.44 mg/kg, respectively, and for 1977, 0.56,
0.6, and 0.15 mg/kg, respectively. However, Newton et al. (1986), when
analysing sparrowhawk eggs from 1971-80, found that, although levels
had fallen in the early 1970s, they had risen again in the late 1970s
(mean PCB residues in eggs ranged from 16 to 293 mg/kg in lipid). Data
on PCB residues in the livers of kestrel, sparrowhawk, heron,
kingfisher, and the great crested grebe, collected from the late 1960s
up to 1987, were analysed statistically by Newton & Haas (1989). For
the great crested grebe, a significant overall decline in PCB residues
was found when comparing data from 1987 with that from the 1960s. For
the other species, there was no significant difference. Spitzer et al.
(1978) reported that there was no significant change in PCB levels in
osprey eggs collected from the Connecticut-New York area during the
period 1969-76. Similarly, Wiemeyer et al. (1987) did not find any
change in the carcase levels of PCBs in ospreys from the Eastern
United States when comparing the 1971-73 and 1975-82 periods. They did
find that adults contained significantly higher concentrations of PCBs
than immature ospreys.
Blus et al. (1979) analysed brown pelican eggs from South Carolina and
Florida between 1969 and 1976. The highest levels of PCBs were found
in South Carolina (means ranged from 5.25 to 7.63 mg/kg wet weight),
but no significant trend was found during the study period. In
Florida, the authors did not find any significant change in eggs
collected from colonies in Florida Bay and on the Gulf Coast over the
study period (means ranged from 0.62 to 1.18 mg/kg), but the Atlantic
coastal colony showed a significant increase in PCB residues (from a
mean of 2.68 to 6.12 mg/kg) between 1969 and 1976.
In analysing herring gull eggs from the Great Lakes between 1974 and
1978, Weseloh et al. (1979) found a significant decline in PCB
residues from colonies on all the lakes. Lake Ontario, the most
contaminated, showed the biggest decline from 170 to 75 mg PCBs/kg at
one of the colonies, with other less contaminated Lakes, Huron,
Superior, and Erie, showing levels in the range of 50-86 mg/kg in 1974
and 32-46 mg/kg in 1978.
Moksnes & Norheim (1986) analysed herring gull eggs collected from the
Norwegian Coast between 1979 and 1981 and found that the PCB levels
were not significantly different from those in eggs collected in 1969;
mean PCB residues ranged from 1.2 to 6.7 mg/kg wet weight. They found
a small but significant increase in the most persistent congeners and
a significant decrease in DDE and the DDE/PCB ratio, but not in total
PCB levels.
An analysis of the eggs of double-crested cormorant (an inshore-
subsurface feeder), Leach's storm petrel (an offshore-surface
feeder) and Atlantic puffin (an offshore-subsurface feeder) was
carried by Pearce et al. (1989), every 4 years, between 1968 and 1984.
In the Bay of Fundy, Canada, PCB levels declined significantly during
this period in all 3 species. PCB levels in the cormorant were
consistently higher throughout than those in the other 2 species,
ranging from 4 to 29.5 mg/kg (wet weight). Petrel and puffin eggs
collected from the Atlantic Coast of Newfoundland showed lower levels
than those in eggs from both the Bay of Fundy and the St Lawrence
River estuary; as in the St Lawrence River, no significant trend in
PCB levels was observed. A significant decline in PCB residues was
found in gannet eggs collected during the same period from the gulf of
St Lawrence (Elliott et al., 1988).
The frequency of occurrence of measurable PCB residues has increased
in large-scale sampling exercises; PCBs in mallard wings increased
from 39% in 1976-77 (White, 1979) to 95% in 1979-80 (Cain, 1981). Cain
& Bunck (1983) found that, in 1976, 21% of European starlings
collected in the USA contained PCBs compared with 83% in 1979.
Addison et al. (1986) analysed the blubber of Arctic ringed seals
(Phoca hispida) from Holman Island, NWT, Canada, in 1981. They found
mean PCB levels of 0.58 mg/kg (wet weight) in the females and
1.28 mg/kg in the males. These concentrations were significantly lower
than those detected in the same species from this area in 1972. Over
this same period, pp'-DDE levels, although at lower levels, also
fell significantly, but it should be noted that total DDT levels in
blubber are much lower than PCB levels and have not changed
significantly.
5.1.4.6 Seasonal patterns in residues
Jensen et al. (1969) observed that there was considerable seasonal
variation in the fat content of herring caught in the Baltic Sea,
ranging from 1% in the spring to 10% in the autumn and that this
seasonal change in fat content led to seasonal changes in the tissue
levels of PCBs.
Cooke et al. (1982) found a seasonal pattern of PCB levels in European
kestrels. Residues in both fat and liver were low in the autumn, but
increased from about January, with a peak almost invariably occurring
during the second quarter of the year (April, May, or June). Seasonal
patterns were based on samples collected over a 10-year period.
Similar trends were found in sparrowhawks and barn owls, but fewer
samples were available.
5.1.5 Appraisal
PCB contamination is widespread and has been measured in a wide
variety of biota between the 1960s and the present day. They are
present throughout the world and, although initially concentrated in
areas of high industrial activity, are now found in organisms living
in remote areas, such as the oceans and the polar regions. In the
past, PCB levels were positively correlated with areas of heavy
industry and consequent discharge but, with the implementation of PCB
controls, in some countries, these geographical differences are
becoming less clear. Generally, levels of PCBs are declining in areas
previously high in PCBs. However, time-trend analysis for the general
environment shows little change in total PCBs since the late 1960s.
The ratio of congeners is, as would be expected, changing, with lower
chlorinated isomers disappearing and the more highly chlorinated ones
becoming more dominant in environmental samples.
PCBs are persistent and bioaccumulate in many organisms, because of
their high lipid solubility and low biodegradability, and usually
enter food-chains from water containing industrial discharge and by
precipitation.
Because of their hydrophobic nature, PCBs are associated with both
oildrop-like aggregates in the surface microlayer of water and with
sediment on the bottom.
They are accumulated by micro- and macroplankton organisms that live
in the surface microlayer and by bottom-living organisms.
5.2 Levels in animal feed
The effects of pollution are seen in the use of fish-meal in poultry
and fish farming. Kolbye (1972) sated that this may contain PCB levels
of 0.6-4.5 mg/kg.
Hansen et al. (1981) studied the transfer of PCBs in swine foraging on
sewage sludge amended soils in 1975-76. Sixteen Berkshire sows were
overwintered for 2 seasons on 4 experimental plots that had been
treated with 0, 126, 252, or 504 tonnes/hectare (on a dry solids
basis) of Chicago sewage sludge for the 8 preceding years. The
estimated PCB residues in the soils of the 4 plots (average of 3-4
samples) were 1.62, 1.88, 2.13, and 2.81 mg/kg dry weight (mean values
of 3-4 samples/plot). The mean concentrations in fat of 3-4 sows per
plot were 36 ± 9, 106 ± 64, 191 ± 97 and 389 ± 118 µg/kg fat basis. Of
the 12 individual congeners that were present in the fat, 3 accounted
for more than 50% of the congeners, e.g., 2,3,4,2',4',5'-,
2,4,5,2',4',5'-hexachlorobiphenyl and 2,3,4,5,2',4',5'-
heptachlorobiphenyl.
In vegetable animal feed (155 samples) originating from 5 areas of the
world, samples, collected in 1984/85, contained PCB levels of 0.0009
(Africa) up to 0.0093 mg/kg dry weight (Europe). In feed from North
and South America and the Far-East, the levels were between 0.0024 and
0.0066 mg/kg. Different types of feed originating from agriculture in
the Federal Republic in Germany, collected in 1985, contained PCB
levels of the order of 0.02 mg/kg dry weight. In feed (301 samples)
originating from animals (exclusive fish meals), collected in 1985,
0.021-0.036 mg/kg dry weight was found (DFG, 1988).
Levels of 10-100 µg/kg are given for groats, soybeans, and cotton
seed, and a mean value of 18 µg/kg is given for mixed feedstuffs. Fish
meal contained levels of 110-330 µg/kg (Klein, 1983).
Samples of fish meal from different areas of the world, collected in
1985, were analysed for the presence of PCBs. In 323 samples, the PCB
contents varied between 0.006 and 0.055 mg/kg dry weight. The PCB
congeners numbers 28, 138, and 153 were present in the highest
quantities (DFG, 1988).
Samples of fish meal from different areas of the world, collected in
1985, were analysed for the presence of PCBs. In 323 samples, the PCB
contents varied between 0.006 and 0.055 mg/kg dry weight. The PCB
congeners numbers 28, 138, and 153 were present in the highest
quantities (DFG, 1988).
5.3 Levels in human food
5.3.1 General
Two general reviews of PCB residues in food, animal feed, human milk,
plants, soils, and packaging materials have been published by Khan et
al. (1976) and Sawhney & Hankin (1985).
The PCB contents of a variety of foods on the Swedish market has been
measured by Westöö & Norén (1970a) and Westöö et al. (1971). Less than
0.1 mg/kg was found in samples of butter, margarine, vegetable oils,
eggs, beef, lamb, chicken, bread, biscuits, and baby food; one sample
of pork out of more than 100 had a PCB content of <0.5 mg/kg.
In the period 1980-81, 5270 food samples were drawn at wholesale or
production levels or at the site of importation including: butter,
cheese, eggs, kidneys from pigs and cattle, and fat of poultry. Levels
in Danish butter (99.4% of the samples) were below 0.05 mg/kg and
those in imported butter (100%), below 0.125 mg/kg; Danish cheese
(100% of the samples) levels were below 0.05 mg/kg and, in imported
cheese, 82.4% of samples had levels below 0.125 mg/kg and the other
17.6%, below 0.2 mg/kg; 100% of eggs had levels below 0.05 mg/kg, and
100% of kidneys of pigs and cattle were below 0.15 mg/kg; 96% of
poultry fat samples had levels below 0.15, and 4%, below 0.20 mg/kg,
on a fat basis (not stated) (Statens Levnedsmiddelinstitut, Danmark,
undated).
Mes et al. (1989b) studied the presence of specific isomers of PCB
congeners in fatty foods of the Canadian diet. A total of 93 food
composites from the cities of Ottawa and Halifax were analysed for 34
PCB isomers, as part of a revised total diet programme. Each market
basket comprised approximately 200 different food types collected from
each of 4 major supermarkets in Ottawa during September 1985 and
January 1986, and, in Halifax, in September 1986. Foods were used
per se, or prepared and cooked in a manner ready for consumption,
then composited to give 112 composites from each market basket.
Thirty-one selected composites, representing the fatty foods were
analysed from each market basket.
PCB isomers 118, 138, 153, and 180 were found in all dairy products,
except skimmed milk. Cheese and butter contained the highest levels of
PCB residues. The residue level of isomer 118 (2,4,5,3',4'-
pentachlorobiphenyl) in butter was the highest e.g., 0.7 µg/kg, of all
PCB isomers found in dairy products. Almost all meat, fish, and
poultry contained PCB isomers 183 and 187. Occasionally, isomers 49,
87, 185, and 189 were also present, but isomer 105 (2,3,4,3'4'-
pentachlorobiphenyl), present in most dairy products, was only found
in some beef samples. Fresh water fish contained most PCB isomers (28
out of 34 selected PCB isomers), at levels considerably higher than
those in any other meat, fish, or poultry samples. The level of isomer
110 in fresh water fish was 3.05 µg/kg. PCB isomers 138, 153, 180, and
187 were present in almost all samples of meat and fish products,
fats, oils, and soups. Cooking fats, salad oils, and margarine
contained relatively low levels of PCB residues. PCB isomers 37, 49,
87, 105, and 185 were not detected in meat and fish products, fats,
oils, or soups.
The calculated sum of all PCB isomer residues found in selected food
commodities (except fish) ranged from 0.03 to 1.98 µg/kg on a wet
basis, and from 0.07 to 10.71 µg/kg on a lipid basis, with mean values
of 0.60 and 3.91 µg/kg, respectively. However, the mean residue levels
of fish and fish products were considerably higher, i.e., 10 and
194 µg/kg on a wet and lipid basis, respectively.
The major PCB isomers in fatty foods were isomers 37, 52, 99, 110,
118, 138, 153, 180, and 187.
The PCB levels obtained in an extensive study by the US Food and Drug
Administration are shown in Table 15. These values are considerably
higher than those reported from Sweden, but they are probably biased,
as they include samples originating from areas previously suspected of
having been subject to local pollution.
In a Canadian survey, PCB levels of less than 0.01 mg/kg were found in
eggs (Mes et al., 1974) and a mean of 0.042 mg/kg was found in
domestic and imported cheese with a maximum of 0.27 mg/kg (Villeneuve
et al., 1973b).
A preliminary study was carried out to estimate the dietary intake of
PCBs in fresh food composites grown in Ontario in 1985. The following
5 food composites: fresh meat and eggs, root vegetables (including
potatoes), fresh fruit, leafy and other above-ground vegetables, and
cow's milk were analysed. The concentrations in the different food
composites were below 0.0005 mg/kg. The annual dietary intake of PCBs
was estimated to be 32.6 µg (Davies, 1988).
In Japan, a similar range of PCB contents has been reported for most
foods; however, some high levels have been reported for rice and
vegetables harvested in fields polluted with PCBs (Environmental
Sanitation Bureau, 1973). The PCB content of most fish on the market
was less than 3 mg/kg.
Table 15. PCB levels in food in the USAa
Food % Positive Level in positive samples (mg/kg)
(0.1 mg/kg)
Mean Maximum
Cheese 6 0.25 1.0
Milk 7 2.3 27.8
Eggs 29 0.55 3.7
Fish 54 1.87 35.3
a From: Kolbye (1972).
Cantoni et al. (1988) analysed different food items, in 1985-87, in
Italy, taking 20-60 samples per item. Different types of meat were
analysed and the median concentrations were 0.25-0.50 mg/kg, on a fat
basis. Twenty to 50% of the samples were positive. Poultry contained
0.028 mg/kg, cow's milk 0.05 mg/kg, cream 0.027 mg/kg, butter
0.065 mg/kg and fish 1.105 mg/kg, on a fat basis; 71% of fish samples
contained PCBs.
When the fat of poultry (42 samples) and 44 eggs was analysed, PCB
values were below 0.3 mg/kg (Dutch Agricultural Advisory Commission,
1983).
In the Federal Republic of Germany, wheat was analysed during the
period 1972-82. The mean concentrations for 1972-78 ranged from 10 to
30 µg/kg; in the period 1980-82, the range was < 2.0-18 µg/kg (Klein,
1983). In wheat and rye (total 850 samples), median levels of
0.4-1 µg/kg product were found in 1984 (Codex Alimentarius, 1986). The
concentrations found in other food items are summarized in Table 16.
Samples of canned ham exported from Czechoslovakia to the USA in 1983
contained PCBs levels of up to 4.8 mg/kg (Anon., 1983a,b).
Table 16. PCBs in food (1982) in the Federal Republic of Germanya
Food Total no. Number of Variation Mean
of samples min-max (µg/kg)
samples below (µg/kg)
detection
limita
Milk 854 234 < 2-3000 126.7 (FB)
Beef 76 43 < 10-687 72.4 (FB)
Pork 58 36 < 10-458 58.1 (FB)
Poultry 64 61 < 10-85 7.3 (FB)c
Meat products 185 86 < 4-2700 114.2 (FB)
Eggs 82 67 < 5-230 9.1 (FW)
Fish (only 70 - 40-87 41.1 (FW)
cod, herring,
plaice)
Food of plant origin
Oil 167 139 < 5-65 7.1 (FB)
Cereals 345 44 < 2-30 6.7 (FW)
Potatoes 106 106 < 2 -
a From: Klein (1983).
b Not stated.
c Only 3 samples.
FB = fat basis
FW = fresh weight.
5.3.2 Drinking-water
Ruoff et al. (1988) examined 83 drinking-water samples from the
Federal Republic of Germany and from 5 other European countries for
their contents of the PCB congeners 28, 52, 101, 138, 153, and 180.
The average total content of the 6 congeners was 0.002 µg/litre water.
The average concentrations of the above-mentioned PCB congeners in the
drinking-water of 6 countries were 0.0001, 0.001, 0.00018, 0.00035,
0.00037, and 0.00042 µg/litre. The variation between the 6 countries
was quite small.
The highest concentration of PCBs reported in domestic tap water was
0.1 µg/litre in the Kyoto area of Japan (Panel on Hazardous Trace
Substances, 1972 cf. WHO/EURO, 1988), but, levels, more likely to be
encountered, should not exceed 0.001 µg/litre.
In the FAO/WHO collaborating centres for the food contamination
monitoring programme, the median levels were:
Cereals below 10 µg/kg
Vegetable fat/oils below 5 µg/kg
Fresh fruit and vegetables 0.5-5 µg/kg
Animal fat (depending on type of
animal and origin) 20-240 µg/kg
Whole fluid cow's milk (depending
on country) 10-200 µg/kg
(on fat basis)
Butter 30-80 µg/kg
Whole dried cow's milk 20-50 µg/kg
Hen eggs < 10 µg/kg
Fresh finfish 10-200 µg/kg
(WHO, 1985b).
The contamination of a drinking-water system in Pickens County, South
Carolina by PCBs discharged from a manufacturing facility was
described by Billings et al. (1978). They observed that PCBs
discharged by a capacitor manufacturing plant resulted in levels as
high as 0.818 µg/litre in finished potable water.
5.3.3 Dairy products
A number of data on food-producing animals have recently become
available within the framework of the Joint FAO/WHO Food Contamination
Monitoring Programme (JFCMP, 1985). All reported median values of PCBs
in animal fat (excluding milk fat) were below the respective limits of
detection, which varied from 0.001 mg/kg in the United Kingdom to a
high of 0.5 mg/kg in Thailand and the USA. Data on PCB levels in cow's
milk fat were supplied by the Federal Republic of Germany, Japan, the
Netherlands, the United Kingdom, and the USA. The United Kingdom and
the USA reported that median concentrations in cow's milk were below
the detection limits of 0.5 µg/kg and 0.5 mg/kg, respectively.
The available data are summarized in Table 17.
From the end of 1982 to the beginning of 1983, high levels of PCBs
were detected in milk from several dairy farms in Switzerland. The
investigations showed that the silo coatings and consequently the
silage from the silos were the origin of the contamination of the
milk. The PCB levels were between 0.80 and 3.80 mg/kg fat. PCB
dissolution in acid juice, mechanical erosion of the coatings, and
volatilization of the coating surface seemed to be the principal
mechanisms explaining the migration of PCBs into the silage
(Alencastro et al., 1984).
Forty-two samples of cow's milk (14 samples in 1976, 14 in 1983, and
14 in 1986) and 41 samples of market milk (10 in 1976, 16 in 1983, and
15 in 1986) were analysed for PCBs, in Israel. During this period, a
change was observed in the PCB distribution in the milk samples. The
percentage of hexachlorobiphenyl decreased with time and the
pentachlorobiphenyl increased (Pines et al., 1988).
The monitoring data for dairy products from all over the world for
1980-83 have been summarized by the Joint FAO/WHO Food Contamination
Monitoring Programme (WHO, 1986a,b).
5.3.4 Fish and shellfish
A summary of the monitoring data on fish from all over the world for
1980-83 has been published by the Joint FAO/WHO Food Contamination
Monitoring Programme (WHO, 1986a,b).
As might be expected, the PCB values found in fish depended on the fat
content and the pollution of the fishing area (Westöö & Norén, 1970a;
Berglund, 1972).
In a collaborative study by 7 national laboratories (International
Council for the Exploration of the Sea, 1974), the PCB contents in the
muscle tissue of fish taken from the North Sea were measured. A mean
of 0.01 mg/kg was found in cod, while herring contained up to
0.48 mg/kg, with most samples in the range of 0.1-0.2 mg/kg; plaice
contained 0.1 mg/kg or less. Similar values were reported by Zitko
(1974) for fish taken from the North Atlantic.
Risebrough & de Lappe (1972) reported levels higher than 3 mg/kg in
fish from New York Sound and Tokyo Bay, both very polluted areas. Even
higher levels of PCBs were found in fish from polluted lakes and
inland waterways, a level of 20 mg/kg being found in fish from Lake
Ontario, and levels over 200 mg/kg in fish from the Hudson River
(Stalling & Mayer, 1972). Similar correlations between pollution and
PCB levels have been reported from the United Kingdom in fish
(Portmann, 1970), and in mussels (Holdgate, 1971).
Table 17. Occurrence of PCBs in dairy products
Country Year Product Number of Mean concentrations Reference
samples in mg/kg on fat basis
(range)
North America
USA 1973-1974 milk (bulk) 198 (9 positive) 1.91 (0.32-4.99) Willett (1980)
Europe
Germany 1982-1986 milk 3279 0.09-0.14a DFG (1988)
(3 areas) 1983-1986 butter/cheese 2088 0.05-0.11
Westphalian area 1972-1974 butter - 0.38 (0.25-0.54) Claus & Acker (1975)
Northern part 1978-1980 milk - 0.17-0.20 Codex Alimentarius
1984 milk 3510 0.013 (1986)
Northern part - butter 1836 0.0077c Codex Alimentarius
(1986)
- meat and fat 957 (about 3/4 0.01b DFG (1988)
positive)
cows entrails 51 0.149b
Sweden 1972-1977 beef, pork and meat 232 (217 < 0.001-0.01 Vaz et al. (1982)
products (domestic negative) (whole product)
and imported)
Denmark 1981-1982 milk - 0.10-0.13 Jensen (1983b)
Table 17. (cont'd).
Country Year Product Number of Mean concentrations Reference
samples in mg/kg on fat basis
(range)
Netherlands 1975-1977 milk 315 0.16 (0.06-0.33) Gezondheidsraad
1980-1983 milk - 0.07-0.13 (1985)
1978-1984 milk 2319 < 0.1-0.2 Olling (1984)
1977-1981 cattle fat - 0.11b (< 0.05-0.55) Greve & Wegman
pork - 0.07 (< 0.05-0.66) (1983)
1983 fat of cattle, pork, 40-45 < 0.03b Dutch Agric. Adv.
calves Comm. (1983)
sheep 22 < 0.03b
Switzerland - milk 6 0.034-0.144 Rappe et al. (1987)
(6 locations)
a Major congeners were Nos. 138 and 153.
b Median value.
c Arithmetic mean.
Jensen et al. (1969) found PCB levels of 0.27 mg/kg and 0.33 mg/kg,
respectively, in the muscle tissue of herring and cod from the same
area of the Baltic, though the cod is at a higher trophic stage. The 2
species had 4.4 and 0.32% of extractable fat, respectively, and, when
the PCB level was calculated on the fat content, values of 6.8 mg/kg
for the herring and 11 mg/kg for the cod were obtained. Cod liver has
a much higher fat content than cod muscle, and Jensen (1973) reported
the ratio of PCB concentrations in cod liver and muscle to be over
100, the maximum in liver being 59 mg/kg. Jensen et al. (1969)
remarked that the considerable seasonal variation in the fat content
of the herring, rising from 1% in spring to 10% in autumn, influenced
the tissue level of PCBs.
There are many examples of different PCB levels in similar species
collected from areas of high and low pollution. Jensen et al. (1972b)
found 5 times as much PCBs in herrings caught in waters off
industrialized areas near Stockholm, as in herrings from the cleaner
waters of the west coast of Sweden.
Different freshwater and seawater fish were analysed for PCB contents,
during the period 1981-83, in the Netherlands. Eel from different
places over the period 1971-81 contained 0.2-13 mg/kg on a product
basis (in the edible part). The median value was between 1 and
2 mg/kg. Sea fish from the North Sea, such as herring and mackerel,
contained 0.1-0.2 mg/kg, on a fat basis. The same level was found in
shrimps and mussels (Freudenthal & Greve, 1973; Greve & Wegman, 1983;
van der Kolk, personal communication, 1984a).
The mean PCB contents in the liver of cod from the North Sea, North
Atlantic, and Baltic Sea, were 2.1-5.7, 0.48, and 10.4-12.8 mg/kg,
respectively (Klein, 1983).
When fish from the North Atlantic, North Sea, and Baltic Sea, were
collected in 1985, PCB concentrations of 0.098-0.123 mg/kg fillet
weight were found in fish from the North Atlantic and North Sea and
0.338 mg/kg fillet weight in fish from the Baltic Sea. In total, 60
samples were analysed. The PCBs 101, 138, and 153 were the major
congeners (DFG, 1988).
The PCB concentration in freshwater fish of the River Rhine was found
to be more than 2 mg/kg. The mean PCBs levels decreased, however, over
the period 1976-81 from 1.92 to 0.38 mg/kg (fresh weight) (Klein,
1983).
In 1984, PCB concentrations in freshwater fish (59 samples) collected
in the River Rhine ranged from 0.742 to 1.017 mg/kg fillet weight. In
this case, the major congeners were 138 and 153, but numbers 28, 52,
101, 180 were also present. In total, 199 samples of eel were
collected in a number of surface waters and analysed for the presence
of PCBs. The levels ranged from 1.42 to 6.51 mg/kg fresh weight. In
studies reported by DFG (1988), the highest levels of PCBs were found
in the River Rhine.
In the United Kingdom, fish and shellfish were analysed for PCBs
during the period 1982-84 (HMSO, 1986). The results are summarized in
Table 18.
Table 18. PCB levels in marine fish and shellfisha
Year Product Tissue No. of Range (mg/kg)
samples
1982 Marine fish (from England) liver 381 0.3-4.1
(7 types of fish)
1982 Marine fish (from England) muscle 326 0.03-0.13
(7 types of fish)
1983 Marine fish (imported) muscle 102 nd-0.06
(5 types of fish)
1983 Shellfish (imported) muscle 53 nd-0.06
(4 types of shellfish)
1984 Fish oils 16 0.11-2.3
a From: HMSO (1986).
Different types of marine fish and shellfish from different areas in
the United Kingdom were analysed during the period 1977-84. Those from
the North Sea coast contained concentrations in the range of 0.04-5.7
and < 0.001-0.058 mg/kg, respectively, while those from the English
channel contained < 0.05-6.9 and < 0.006-0.1 mg/kg, respectively,
and those from the West coast, < 0.002-8.4 and < 0.001-0.25 mg/kg
wet weight. PCB concentrations in fish livers of 0.2 up to 12.9 mg/kg
wet weight were found during this period (Franklin, 1987).
When samples of fish of different species, collected from major USA
watersheds in 1976, were analysed, PCBs were found in 93% of the
samples. Fifty-eight of the samples had levels exceeding 5 mg/kg, on a
whole fish basis. The PCB concentrations ranged from less than 0.3 to
140 mg/kg, on a whole fish basis (Veith et al., 1979).
Maack & Sonzogni (1988) analysed 98 fish (14 species) of different
sizes from Wisconsin waters, for the presence of PCB congeners. Among
the most prominent congeners were numbers 153/132, 138, 66/95, 110,
180, 70/76, 146, 28/31, 149, 118, and 105. The total PCBs (determined
by adding individual congener concentrations) ranged from 0.070 to
7.0 mg/kg. The mean concentration was 1.3 mg/kg.
Blue crabs ( Callinectes sapidus, an important member of the
estuarine food web), collected from Campbell Creek and surroundings in
South Carolina, were analysed for PCBs in 1985. The highest mean total
concentration was 0.861 mg/kg muscle tissue. In 1986, the mean
concentrations in blue crab collected by 8 stations in the same area
ranged from 0.026 to 0.361 mg/kg muscle tissue. Blue crab (15 samples)
collected from the coast of South Carolina, contained concentrations
of < 0.020-0.372 mg/kg tissue (Marcus & Mathews, 1987).
PCBs concentrations in sea fish were determined in 1971-77 in Japan.
In-shore fish (90 samples) showed concentrations of 0.2-0.72 mg/kg
fresh weight and pelagic fish (112 samples), 0.005-0.265 mg/kg fresh
weight (Watanabe et al., 1979).
Data on individual species of fish, submitted by Japan, showed the
following median levels: barracuda, 70 µg/kg; conger eel, 290 µg/kg;
croaker, 200 µg/kg; flounder (yellow-tail), 90 µg/kg; hair-tail,
100 µg/kg; mullet, 84 µg/kg; and seabass, 110 µg/kg. Median levels for
other species of fish, such as cod, mackerel, pacific saury, rockfish,
salmon, and sardines, were below 100 µg/kg (WHO, 1986b).
Using a very sensitive analytical method, Tanabe et al. (1987) found
the toxic non- ortho-substituted coplanar 3,4,3',4'-tetrachloro-,
3,4,5,3',4'-pentachloro-, and 3,4,5,3',4',5'-hexachlorobiphenyl in
finless porpoise, at concentrations of 13.5, 0.89, and 0.64 µg/kg,
respectively.
Blue mussel (Mytilus edulis) was collected from coastal areas near
Osaka and Hokkaido, Japan, in 1984-86. Depending on the site of
collection, the average PCB concentrations (11-13 samples) ranged from
0.56 to 65.0 µg/kg (Miyata et al., 1987).
5.3.5 Influence of food processing
Fifty striped bass (Morone saxatilis) were analysed for the presence
of PCBs in the fish fillets before, and after, boiling, steaming,
baking, frying, microwaving, or poaching, to study the possible
reduction of the PCB residues by these cooking procedures. PCB
contents were reduced by approximately 10%, by all 6 methods of
cooking. No significant reductions were observed with the other
cooking methods (Armbruster et al., 1987).
5.3.6 Food contamination by packaging materials
When Villeneuve et al. (1973a) analysed packaged food in Canada, they
found that 66.7% of the samples contained PCB levels of less than
0.01 mg/kg, 30.7% contained between 0.01 and 1 mg/kg, and 2.6%
contained more than 1 mg/kg. The highest PCB levels were in a rice
sample (2.1 mg/kg), where the packaging material contained 31 mg/kg,
and in a dried fruit sample (4.5 mg/kg), in a container containing
76 mg/kg. In a survey of packaging containers, approximately 80% were
found to contain PCB levels of less than 1 mg/kg, while about 4%
contained levels higher than 10 mg/kg. The most likely source of PCBs
in packaging materials was the recycling of waste paper containing
pressure-sensitive duplicating paper (carbonless copying paper)
(Masuda et al., 1972).
Relatively high PCB levels in some packaged foods in Sweden, mainly of
imported origin, could be attributed to migration from the packaging
material (Westöö et al., 1971). The highest level encountered was
11 mg/kg in a childrens' breakfast cereal; PCB levels of 70 mg/kg and
700 mg/kg were found in the material of the inner bag containing this
product and in the outer cardboard container, respectively. Up to
2000 mg/kg was found in cartons of other samples.
In the United Kingdom, levels in imported waste-paper, which could be
contaminated with PCBs from carbonless copying paper and subsequently
used to manufacture food contact paper and board materials, were found
to be low, compared with the 10 mg/kg limit for PCBs recommended by
the British Paper and Board Industry Federation for food contact
materials (HMSO, 1989).
5.3.7 Appraisal
Foods have become contaminated with PCBs by 3 main routes:
* accumulation of PCBs in the different food-chains in the
environment and consumption of fish, birds, or other animals and
crops;
* direct contamination of food or animal feed by an industrial
accident;
* migration from packaging materials into food.
During the past years, many thousands of samples of different
foodstuffs have been analysed for PCB contamination. The most common
foodstuffs analysed have been fish, meat, and milk. Many fish samples
have been taken in an effort to monitor aquatic pollution. In
addition, samples have been taken, for regulatory or similar purposes,
from sources suspected of being relatively highly contaminated. The
fact that most samples have not been taken at random, jeopardizes the
proper assessment of the exposure of the general population.
5.4 General population exposure
5.4.1 Air
Relatively high levels of PCBs have been detected in indoor air,
especially in kitchens and offices with electric installations
(Jensen, 1983a) (section 3.2.4 and 5.1.1).
Results from the US EPA indicate PCB concentrations in the air ranging
from 1 up to 50 ng/m3; similar results have been reported from Japan
(WHO, 1976). Assuming a level of 5 ng PCBs/m3 in urban air, a
breathing rate of 22 m3/day, retention and absorption of inhaled
particles/vapour of 50%, and a mean residence time of PCBs in the body
of 3 years, air would contribute 0.8 µg/kg to the PCB concentration in
the body. Higher concentrations of PCBs in indoor air could increase
this estimate (WHO/EURO, 1988).
Van der Kolk (1985) calculated air intake through inhalation for the
Dutch population of about 36 ng/day, a quantity approximately 1000
times lower than the intake with food.
During the manufacture, formulation, or use of PCBs, where levels in
the workroom air correspond to exposure limit values, varying between
0.1 mg/m3 and 1 mg/m3, the calculated mean intakes would range
between 1 and 10 mg during an 8-h workshift. In some occupational
situations, much higher concentrations have been measured and the
estimates of intakes would be higher (WHO/EURO, 1987).
5.4.2 Drinking-water
Levels reported in drinking-water are typically between 0.1 and
0.5 ng/litre. Even assuming a PCB level of 2 ng/litre in
drinking-water, consumption of 2 litre/day contributes 0.04 µg/kg body
weight to the PCB concentration in the body. This additional quantity
is negligible in comparison with the intake via food (WHO/EURO, 1988).
5.4.3 Intake by infants through mother's milk
The daily intake of PCBs was calculated in breast-fed infants in the
countries participating in a monitoring study by Slorach & Vaz (1983,
1985) (Table 19).
The intakes in EEC countries were calculated to range from 3 to
11 µg/kg body weight per day, compared with 0.12-0.3 µg/kg body weight
for bottle-fed infants in Denmark (WHO/EURO, 1985).
In Yusho infants with clinical symptoms of poisoning, the daily intake
of PCBs with breast milk was calculated to be 70 µg/kg body weight
(Jensen, 1983b) (see section 9.1.2.2).
5.4.4 Infant and toddler total diet
Johnson et al. (1979) analysed the average diet of 6-month-old infants
and 2-year-old toddlers for the presence of PCBs. Ten market baskets
were collected in 10 cities in the USA. The foods were prepared in the
manner in which they would be prepared and served in the home. Trace
amounts of PCBs were detected in only one infant and one toddler diet.
In the USA, Gartrell et al. (1986b) found a daily intake of 0.011 µg
PCBs/kg body weight in infants consuming infant diets in 1978. In the
years 1979, 1980, and 1981/82, the intake was below the detection
level. The intake by toddlers was 0.099 µg/kg body weight in 1978 and
not detectable in the following 3 years.
Tuinstra et al. (1985a) analysed samples of infant food from the Dutch
market and found average PCB levels of 0.1-0.2 µg/kg food (the maximum
level found was 1.1 µg/kg).
5.4.5 Total intake by adults via food
The oral consumption of contaminated products is presumed to be the
main route of exposure to the PCBs.
Table 19. Calculated daily intakes of PCBs by breast-fed infants (µg/kg body weight)a
Country/area Year(s) Calculation according Calculation according
to US FDA methodb to national methodc
median maximum median maximum
Belgium,
Brussels 1982 3.6 10.4 NR NR
China,
Beijing 1982 NR NR 0.45d 0.45d
Israel,
Jerusalem 1981/82 2.0 9.5 NR NR
Germany,
Hanau 1981 NR NR 9.5 45
Japan,
Osaka 1980/81 1.6 4.4 2.3 6.3
Sweden,
Uppsala 1981 4.4 8.1 5.9 11
USA
22 states 1979 4.5e 13.5 4.5e 22.5
Yugoslavia,
Zagreb 1981/82 2.8 7.2 2.8 7.7
a Assuming a milk consumption of ca 130 g/kg body weight and a milk fat content of
3.5% (w/w). Calculations based on data for all mothers studied. Results for
different methods of PCB analysis shown separately.
From: Slorach & Vaz (1983, 1985); Van der Kolk (1984b).
b Sawyer method.
c "Own method".
d PCB level below limit of detection (0.1 mg/kg fat) in milk samples.
e PCB level below limit of detection (1 mg/kg fat) in milk samples.
NR = No data on levels in milk reported.
It has been stated that the major part of the human dietary intake of
PCBs is from fish (Berglund, 1972; Hammond, 1972). This may well be
true in areas such as Japan or certain localities near the North
American Great Lakes, where fish from polluted waters may form a
relatively large part of the diet. Several investigators from Japan
have measured the daily intake of PCBs in food; the highest mean value
recorded was 48 µg/day, of which 90% was from fish (Kobayashi, 1972);
the lowest was 8 µg/day (Ushio et al., 1974).
In much of Europe and North America, however, the daily intake of fish
is in the region of 30-40 g, and most of the fish is taken from waters
of low pollution with PCB levels in the fish not exceeding 0.1 mg/kg.
Berglund (1972) has estimated that the daily intake of PCBs from fish
in Sweden is in the region of 1 µg, though if the fish consumed were
solely Baltic herring, the intake would be about 10 µg/person. It is
difficult to make an assessment of the PCB intake from foods other
than fish. Westöö et al. (1971) in their extensive study of the
Swedish diet, reported that most foods contained PCB levels of less
than 0.1 mg/kg; and concluded that this corresponds to a daily intake
of less than 100 µg.
Weekly intakes in the range of 23-889 µg/person have been reported
from the USA (OTA, 1979). The higher range concerns people consuming
more than 12 kg/year of Lake Michigan fish.
The intake of total PCBs by the general adult population depends
greatly on the geographical area and food habits.
5.4.6 Total diet/market-basket studies
Data on total-diet studies of PCBs have been reported from a few
countries. These reported intakes show a wide variation, which can
partially be explained by methodological factors, such as the ways in
which samples below the limits of determination are considered,
especially when noting the different limits of determination.
Considering the available data, an average intake of 5-15 µg/day for
the non-occupationally exposed population in industrialized countries
may be the best available estimation.
These estimates apply to the average diet of an average adult citizen.
In practice, few people are really "average" in their consumption
pattern. Given the widespread nature of the contamination, however, a
higher intake in one food group is more or less balanced by a lower
intake in another food group with an equal calorie intake. Total
intake will certainly be higher for diets with a more than average
calorie content (van der Kolk, 1985).
Gartrell et al. (1985) determined the total intake of PCBs by 16- to
19-year-old males in the USA. The samples represent a typical 14-day
diet. Approximately 120 individual food items (of 12 food groups),
including drinking-water, were collected for each market-basket sample
in 20 cities in the period 1979-80. Only 2 samples of meat, fish, and
poultry contained PCBs with an average concentration of 0.002 mg/kg.
Gartrell et al. (1985, 1986a) reported a daily intake in the USA of
0.016, 0.027, 0.014, 0.008, and 0.003 µg PCBs/kg body weight during
the years 1977, 1978, 1979, 1980, and 1981/82, respectively.
Manske & Johnson (1975) collected 35 market baskets in 32 cities over
the period 1971-72. PCB residues were found in the range of
0.035-0.15 mg/kg in 51 composites. Fish and oils, fats, and
shortenings contained the highest levels. The same authors (Manske &
Johnson, 1977) carried out a market-basket study representing the
basic 2-week diet of a 16- to 19-year-old male. The various foods were
prepared in the manner in which they would normally be served and
eaten. Thirty market-baskets, containing 12 classes of foods (in total
360 composites) were collected in 30 cities in the period 1973-74. A
trace of PCB was found once in whole milk, ground beef, and fish
fillet.
The FDA revised the concept of the Total Diet Study in 1982. As
discussed by Gunderson (1988b), the Total Diet Study conducted before
1982 was based on a "composite sample approach", regardless of the
diet involved. The revised study is based on updated dietary survey
information and allows the "total diet" of the US population to be
represented by a relatively small number of food items for a greater
number of age/sex groups. The daily intake expressed in ng/kg body
weight per day for PCBs (Aroclor 1221, 1242, and 1254) in 1982-84 for
the age groups 6-11 months, 2 years, 14-16-year females, 14-16-year
males, 25-30-year females, 25-30-year males, 60-65-year females and
60-65-year males were: 0.8, 1.2, 0.4, 0.5, 0.5, 0.6, 0.4, and
0.5 ng/kg body weight per day, respectively (Gunderson, 1988b).
Foods, representative of Canadian eating habits, as determined by a
national nutritional survey, were prepared for eating, categorized,
and blended into 11 different composites representing the dietary
intake for 5 cities over the period 1976-78. It concerned 194 samples,
collected in winter and in summer. The average dietary intake was
0.001 µg PCB/kg body weight (McLeod et al., 1980).
Over a period of 2 years, 126 different food items of a market-basket
of 16- to 18-year-old males were purchased every 2 months in the
period 1976-78, in the Netherlands. The foodstuffs were prepared for
eating and were combined in 12 commodity groups. The mean
concentration and range of PCBs in 5 food classes was:
Class Mean concentration Range
(mg/kg on fat basis)
Meat, poultry, and eggs - 0.13-0.17 (2)a
Fish 0.07 0.04-0.24 (7)
Dairy products - 0.04-0.06 (2)
Sugar and sweets - 0.08 (1)
Drinks, drinking-water - 0.035 (1)
a In parentheses: number of positive composites.
The authors calculated a daily intake of PCBs of 15 µg/person (a
maximum level was 90 µg/person (de Vos et al., 1984). In the period
May-July 1976, 100 total diets (summer meals) were collected and
besides organochlorine pesticides, PCBs were determined as
decachlorobiphenyl, after perchloration, and calculated as Aroclor
1260. The mean intake of PCBs/person per day was 11.6 µg with a range
of 3-71 µg (Greve & van Hulst, 1977; Greve & Wegman, 1983; van der
Kolk, 1985).
In 1978, another survey was carried out with 100 total diets during
the winter (winter meals). It was estimated that the daily intake was
6 µg/person (range 1-19 µg).
Zimmerli & Marek (1973) studied the total human intake of PCBs from
prepared meals in 1971-72 in Bern, Switzerland. Five typical total
diets were composed and analysed. The intake of PCBs, especially with
daily diets containing cheese, meat, fish, or fat, ranged from 6 to
84 µg.
According to a calculation by Summerman et al. (1978), the average
weekly intake of PCBs in the Federal Republic of Germany was about 215
and 268 µg/week for females and males, respectively. Much lower
figures, 36-44 µg/week, were calculated by Klein (1983).
A survey of the daily PCB intake from the total diet of Japanese women
(number of samples varied from 18 to 60) was performed for the years
1972-76. The daily intake of PCBs averaged approximately 10 µg/person
(range 2.8-21.2 µg). The main source of PCBs in the diet of Japan was
in-shore fish. There was no clear change in daily intake over the
5-year period studied (Watanabe et al., 1979).
Ushio & Doguchi (1977) studied the dietary intake of PCBs in Tokyo.
They found an average daily intake of PCBs of 6.3 µg/person (range,
trace-17 µg/person). It was concluded that the dietary daily intake of
PCBs for the majority of the population of Tokyo rarely exceeded
20 µg/person, when no heavily contaminated fish were consumed.
Yakushiji et al. (1977) found that the PCB daily intake through meals
of unexposed adults living in Osaka prefecture, was 3-20 µg/day.
Data for PCBs in the diets of Canada, Guatamala, Japan, the United
Kingdom, and the USA over the period 1972-83 were summarized by
Gorchev & Jelinek (1985). The mean dietary intake reported was at, or
below, 0.06 µg/kg body weight, the mean intake per person ranged from
< 0.01 to 0.12 µg/kg body weight (Slorach et al., 1982; WHO, 1986b).
5.4.7 Total intake of major congeners by adults via food
In the Federal Republic of Germany, the daily intake of the 3 PCB
congeners numbers 138, 153, and 180, together with the different food
items, was calculated. The intake (µg/day) with meat and meat products
was 0.30; with fish and fish products 0.36; eggs and egg products
0.008; milk and milk products 0.40; cheese 0.11; butter 0.39; fats and
oil 0.098; bread and pastries 0.17; potatoes 0.081; vegetables 0.11
and fruits 0.082 (DFG, 1988).
5.4.8 Time trends in different matrices
Although many countries introduced severe restrictions on the
manufacture, use, and disposal of PCBs many years ago, it is difficult
to discern any marked decline in the levels in human milk fat, from
the published data.
Levels of PCBs were estimated in 1085 samples of different cereals,
collected in the Federal Republic of Germany over the period
1972/74-1984. The levels, which were the highest in 1972/74 0.04 mg/kg
(0.005-0.12 mg/kg), decreased during the years to 0.004-0.005 mg/kg
dry weight in 1984 (DFG, 1988).
Data from the Federal Republic of Germany showed no clear trend in PCB
levels in human milk during 1975-79 (Slorach et al., 1982). The same
was found in the Netherlands over the period 1974-83 (Greve & Wegman,
1984).
Japanese data showed a decline in PCB levels in the fat of whole cow's
milk during the period 1972-79. A decline was also found in PCB levels
in finfish from coastal waters and in total marine fish (Slorach et
al., 1982).
A downward trend was found in human milk from Japan over the period
1972-80. Each year, a large number of samples (361-877 samples/year)
were analysed. In 1972, the median level was about 0.8 mg/kg and, in
1980, 0.5 mg/kg, on a fat basis. A gradual decline was observed
(Slorach & Vaz, 1983).
In Canada, human milk and adipose tissue from Ontario residents were
analysed over the period 1969-74. The values found did not indicate a
trend in this period.
The mean total PCB intakes determined in the FDA Total Diet Study, for
the period 1971-87, for a typical "adult" diet, represented in Fig. 4,
reflect that of a 14- to 16-year-old male during 1982-87. A clear
decline was shown from approximately 7 µg/person per day to less than
0.1 µg/person per day (Gunderson, 1988a).
The daily intake of PCBs, expressed as ng/kg body weight per day, by
6-month-old and 2-year-old children in the years 1980, 1981/82, and
1982/84 did not show a trend, while, in adults, a decrease from 8 to
0.5 ng/kg body weight per day was observed over the same years
(Gunderson, 1988b).
5.5 Concentrations in the body tissues of the general population
The PCB levels in body tissues are a good indication of the overall
and total exposure of the body to PCBs.
Several factors may influence the concentrations of PCBs in body
tissues, including duration and level of exposure, the route and
pattern of exposure, the chemical structure of the PCB (degree and
position of chlorination in the molecule), the amount of adipose
tissue, other simultaneous exposures, as well as other biological
parameters.
5.5.1 Adipose tissue
In general, while highly chlorinated congeners accumulate more easily,
a lower degree of substitution provides more possibilities for
hydroxylation and facilitates excretion. Factors other than the degree
of substitution also affect accumulation, particularly the position
and pattern of substitution (WHO/EURO, 1987).
The available information on the occurrence of PCBs in the body fat of
the general population is summarized in Table 20.
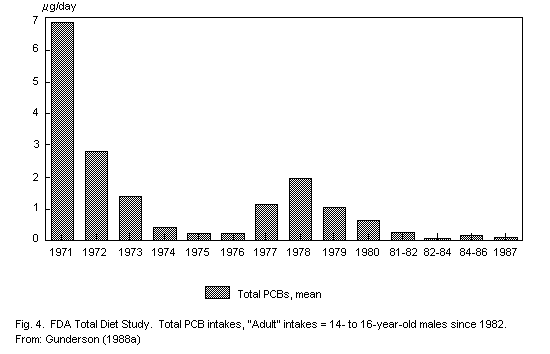 Table 20. Concentrations of PCBs in the body fat of the general population
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
North America
USA (18 states) - 637 < 1 (68.9%)e Yobs (1972)
< 1-2 (25.9%)e Price & Welch (1972)
> 2 (5.2%)f
Northeast Louisiana 1980 8 1.04 (0.38-2.33) Holt et al. (1986)
1984 10 1.23 (0.65-1.44)
Texas 1969-1972 88 (15 positive) 1.7 (0.6-9.9) Burns (1974)
New York - 101 (women) 3.4 ± 1.1 Bush et al. (1984)
(urban and rural
vicinity)
Canada - 99 0.94 (0.04-6.8)a Mes et al. (1982)
Ontario 1976 and 570 2.1-2.2 Frank et al. (1988)
1984
Table 20. (cont'd).
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
Asia
Japan (Kochi area) - - 2.86 (maximum 7.5) Nishimoto et al. (1972a,b)
Japan 1971-1982 - 0.5-6.0a Katsunuma et al. (1985)
Tokyo 1974 30 1.04 (0.38-2.5) Fukano & Doguchi (1977)
Japan - 241 0.30-1.48 Curley et al. (1973b)
New Zealand - 51 0.82 Solly & Shanks (1974)
Africa
South Africa 1982 63 0.15-5.18 van Dijk et al. (1987)
Europe
Austria (Vienna area) - 32 0.3-7.3 Pesendorfer et al. (1973)
Finland - 105 0.2 Mussalo-Rauhamaa et al.
(1984)
Germany, Federal - 20 5.7 Acker & Schulte (1970)
Republic of - 282 8.3 Acker & Schulte (1974)
1982-1983 50b 0.5-1.5 Niessen et al. (1984)
Italy (Siena) 1983-1984 26 1.75c (dry weight) Focardi et al. (1986)
Table 20. (cont'd).
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
Netherlands 1973-1983 24-78 per year 1.6-2.5d Greve & van Harten
(1983a);
Greve & Wegman (1983,
1984)
Norway (Oslo) - 40 1.6 Bjerk (1972)
Spain 1985-1987 14 1.68 Camps et al. (1989)
United Kingdom - 201 < 1.0 Abbott et al. (1972)
1976-1977 236 0.7 (nd-10) HMSO (1986)
1982-1983 187 0.9 (0.1-6.9)
a Wet weight.
b 34 infants, 14 children, and 2 older children.
c About 60% included only five congeners: Nos. 118, 138, 153, 170, 180.
d Median.
e Percentage of samples.
5.5.1.1 PCBs in the fetus
PCBs are also present in serum and all organs of the body in
proportion to their fat content. PCBs pass more, or less (depending on
structure and chlorination), through the placenta into the fetus.
Since the fetus has little adipose tissue until 7 months of age, PCB
concentrations may be higher in vital organs, such as the adrenal
gland, but available data suggest somewhat lower levels in the brain
(Masuda et al., 1978a; Kodama & Ota, 1980).
Masuda et al. (1978a) found PCB levels of 270-960 µg/kg fat in adipose
tissue samples of fetuses beyond 7 months of gestation. Levels in the
adipose tissue of adult females from the same geographical area ranged
from 270 to 1360 µg/kg fat. The mean concentrations were 470 µg/kg for
fetuses and 780 µg/kg for adult females. However, since the ranges
showed an overlap and the number of samples was small, it is not clear
whether this represents a true difference.
5.5.1.2 Congeners in adipose tissue
Wegman & Berkhoff (1986) investigated the presence of the different
congeners in 24 human fat samples, collected in 1984. The following
congeners were present at the highest levels: 2,4,4'-trichloro-,
2,4,5,2',5'-pentachloro-, 2,4,5,3',4'-pentachloro-, 2,3,4,2',3',4'-
hexachloro, 2,3,4,2',4',5'-hexachloro-, 2,4,5,2',4',5'-hexachloro-,
2,3,4,5,2',4',5'-heptachloro, 2,3,4,5,2',3',4',5'-octachloro, and
2,3,5,6,2',3',5',6'-octachlorobiphenyl.
Focardi & Romei (1987) analysed 30 samples of adipose tissue,
obtained from patients in Siena, Italy, in 1986, for the presence of
19 PCB congeners. The results indicate that the mean PCB (as sum of
the congeners) concentration was 1063 µg/kg dry weight (range
391-1918 mg/kg). The major constituents of the PCBs (about 60%) were
the isomers 99, 138, 153, 170, and 180.
Human adipose tissue was analysed for 3 non- ortho chlorine
substituted coplanar congeners: 3,4,3',4'-tetrachloro-, 3,4,5,3',4'-
pentachloro- and 3,4,5,3',4',5'-hexachlorobiphenyl (Kannan et al.,
1988). Twelve samples, from 7 male and 5 female persons were obtained
from hospitals. The average total PCB concentrations were 1.22 and
1.02 mg/kg (wet weight basis), respectively. The concentrations of the
3 congeners were 94-860, 120-730, and 36-200 ng/kg, on a wet weight
basis, respectively.
5.5.2 Blood of the general population
Finklea et al. (1972) studied human plasma of different races of the
population (723 volunteers with ages ranging up to 60 years) of urban
and rural areas of South Carolina. The average concentration was
5 µg/litre (range 0-29 µg/litre). No age effect was found, but ethnic
differences and ethnic residence interactions were significant. Kreiss
(1985) found mean serum concentrations in the non-occupationally
exposed population in the USA, of between 4 and 8 µg/litre, with 95%
of the individuals having serum PCB concentrations of less than
20 µg/litre. More data are summarized in Table 21.
Maternal blood and fetal cord blood were collected from volunteers
from an urban and rural vicinity in upstate New York. Whole blood
samples were taken from 101 women (26 ± 4 years) entering maternity
facilities. Maternal blood contained 3.4 ± 1.1 µg PCBs/kg and fetal
cord blood contained 2.4 ± 1.0 µg/kg whole blood. The PCB congeners
making up these totals were surprisingly few; 38% of the total residue
in the maternal blood and 21% of the fetal cord blood comprised only 4
components, 2,4,4'-trichlorobiphenyl, 2,4,5,2',4',5'-hexachloro-,
2,3,4,2',4',5'-hexachloro-, and 2,3,5,6,2',3',6'-heptachlorobiphenyl.
The congener 2,5,2',5'-tetrachlorobiphenyl crossed the placenta
preferentially (Bush et al., 1984).
The concentrations of PCBs were determined in blood samples from 120
women hospitalized for miscarriages and 120 full-term pregnancy
controls. The average PCB level was higher in women with miscarriages
than in control women (8.65 µg/litre and 6.89 µg/litre, respectively,
as Fenclor 54 and 14.81 and 14.90 µg/litre, respectively, as
decachlorobiphenyl). The reproductive history of each woman was
assessed together with confounding variables and with environmental
exposure and food intake. Food consumption did not indicate diet as
the main source of PCB intake (Leoni et al., 1989).
A cross section of the population of Michigan was studied following an
accidental exposure in 1978. Five years after the accident, PCB and
PBB residues were measured in adipose tissue and serum. Serum levels
of PCB were measured in 1681 adults and 1462 children. Children (430)
were found to have uniform levels throughout the state (mean
concentration 4 ± 2 µg/litre). In adults, the serum PCB levels were
higher in the area with highest PBB levels. The mean serum PCB level
was 21 µg/litre, compared with control levels for the rest of the
state of 9 µg/litre. No sex difference was found (Wolff et al.,
1982a).
Table 21. Concentrations of PCBs in whole blood of the general population
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Canada
Ontario area 1975-1976 118 18 Frank et al. (1988)
(patients suspected 1980-1981
of being exposed 1984
dermally)
Japan
- - 3.2 Doguchi & Fukano (1975)
- 28 (women) 2.6 Kuwabara et al. (1978)
(Osaka area) 1976 16 (women) 2.8 (1.7-4.6) Kuwabara et al. (1979)
1972-1977 - 3-4 Yakushiji et al. (1977)
farmers 1978-1983 - trace-21.4b Katsunuma et al. (1985)
Tokyo 1973 27 3.19 (2.2-5.1) Fukano & Doguchi (1977)
1975 10 2.59 (1.8-3.8)
Table 21. (cont'd).
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Finland - 3.1-12 Karppanen & Kolho (1973)
Netherlands - 34 (women) 4.5 (nd-11.6) Blok et al. (1984)
31 (men) 4.8 (1.0-17.1)
1978 48-127 3.1e Greve & Wegman (1983,
1980 samples/year 3.5 1984)
1981 4.4
1982 4.4
North America
South Carolina 1968 723 5 (4.2-5.5)a Finklea et al. (1972)
(urban and rural
area)
Michigan 1973 1100 56c Kreiss (1985)
(areas of Lake 1979-1981 17.2-23.6c
Michigan)
Lake Michigan 1985 196 5.5 ± 3.7 Schwartz et al. (1983)
(high fish
consumption)
Table 21. (cont'd).
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Yugoslavia 1984-1986 10f 155 (35-480)d Jan & Tratnik (1988a)
(residents around 19g 11 (6-18)
River Krupa; 4h 5 (2-7)
contamination by a
plant using PCBs)
a Plasma.
b Serum.
c Geometric mean.
d Arithmetic mean.
e Median concentration.
f Living close to plant.
g Living 1-3 km from plant.
h Non-exposed other areas.
Specific PCB isomer levels in the blood of 30 children, ages 2-5
years, residing in an area of PCB-contaminated soil in Canada, were
compared with those of 25 children in a non-contaminated area. The sum
of individual PCB isomer levels in the exposed and non-exposed group
were not significantly different, e.g., 0.54 µg/litre (range
0.22-0.99 µg/litre) and 0.88 µg/litre (range 0.28-2.30 µg/litre). The
major component in both groups was 2,4,5,2',4',5'-hexa-chlorobiphenyl
(Mes, 1987).
High levels of PCBs were found in the blood (up to 100 µg/litre) in
patients with severe weight loss (Hesselberg & Scherr, 1974). This was
attributed to the release of PCBs from the mobilization of fat.
Greve & van Harten (1983b) studied the relationship between the levels
of PCBs in the adipose tissue and in the blood of the same persons. A
total of 48 persons were involved in this study. A concentration
factor (concentration in adipose tissue divided by concentration in
blood) of 660 was found.
5.5.3 Human milk
Human milk is the major source of exposure for breast-fed infants. The
amount of human milk secreted varies widely. The composition of the
milk is related to the amount secreted, the stage of lactation, the
timing of withdrawal (early or late in feeding) and to individual
variations among lactating women. The individual variations depend on
maternal age, health, social class, and diet. The concentration of
PCBs depends primarily on the lipid concentration in milk. Wide
variations in published results are caused by inaccuracies inherent in
the analytical methods used for the quantification of lipids, and
whether the milk sample is collected early or late during the feeding
period. The fat content increases during emptying, and the fat content
of milk from the 2 breasts may differ. According to a recent
determination, the fat level in human milk averages 2.6-4.5%
(WHO/EURO, 1988).
Whether the differences in concentration in various countries are
merely a function of the analytical methods used and the type of
samples collected or whether true differences in body burden exist, is
not clear at present. For instance, some countries have reported
levels of PCBs in human milk fat ranging from nondetectable to
14 mg/kg, while, in other countries, the highest levels found have
been around 3 mg/kg. Because of these variations, calculating an
average dose for nursing infants is difficult. The same difficulties
exist when attempts are made to investigate trends over time
(WHO/EURO, 1988).
The results of the older studies have been obtained with a less
sophisticated method using packed column GC. With this method only a
dozen peaks can be separated. The quantitative results are reported as
"total PCB values", though different techniques of quantification and
different types of calculations were used.
In contrast with the situation with many organochlorine insecticides,
the levels of PCBs in human milk fat are higher in European countries,
Japan, and the USA than in China (Slorach & Vaz, 1983, 1985), and are
significant, particularly in the highly industrialized countries.
Results from a large number of countries have been summarized by
Jensen (1983a, 1985, 1987), Acker et al., (1984), Katsunuma et al.
(1985) (especially Japanese data; period 1972-83); and WHO/EURO,
(1987, 1988). The countries concerned are: Argentina, Austria,
Belgium, Canada, Finland, France, Federal Republic of Germany (Klein,
1983), German Democratic Republic, Israel, Japan, the Netherlands,
Norway, Poland, Romania, South Africa, Sweden, Switzerland, Turkey,
United Kingdom, USA, USSR, and Yugoslavia. The average levels of PCBs
in human milk do not appear to differ very much between the
industrialized countries and range between 0.5 and 2 mg/kg milk fat,
except in Czechoslovakia, the Federal Republic of Germany, India,
Denmark and Italy, where levels up to 3 mg/kg milk fat were found
(Jensen, 1983b; Acker et al., 1984) (Table 22).
The variation in residue levels in human milk during lactation was
investigated in 5 women in the Federal Republic of Germany. Month-mix
samples, composed of breast milk samples collected weekly, were
analysed over a lactation period of between 5 and 9 months. The ages
of the women ranged from 23 to 36 years. The PCB concentrations were
between 0.61 and 2.20 mg/kg, on a fat basis. While the concentrations
remained relatively constant, some fluctuations were seen but no trend
was observed over the lactation period investigated (Fooken & Butte,
1987).
Breast milk samples from 16 women in Canada were analysed for PCBs at
8 intervals (7, 14, 28, 42, 56, 70, 84, and 98 days) during the
lactation period. The average PCB concentrations in breast milk varied
between 22.8 and 29.7 µg/kg whole milk. No clear decrease or increase
was observed. The average milk/blood ratio for PCBs was 23 and
remained relatively constant during lactation (Mes et al., 1984).
Wolff (1983) reported the half-life of PCBs (percentage chlorine not
specified) in breast milk to be 5-8 months and found that the
concentration of PCBs in breast milk was 4-10 times that in the
maternal blood. Similar results were reported by Jacobson et al.
(1984b).
Table 22. Concentrations of PCBs in breast milk of the general population
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
North America
USA (Michigan) 1977-1978 1057 1.5 (maximum 5.1) Wickizer et al. (1981);
Wickizer & Brilliant (1981)
Canada (Quebec) - 154 0.84 (nd-4.34) Dillon et al. (1981)
Ontario 1971-1974 - 1.2 (0.1-3.0) Atkinson (1979)
1978 215 0.6 ± 0.3
Ontario 1975-1985 348 0.023 (0.016-0.033)a Frank et al. (1988)
Five regions across Canada 1982 210 0.697 Mes et al. (1986)
Regina, Saskatchewan 1979 80 0.0052 (0.001-0.019)a Qureshi & Robertson (1987)
Table 22. (cont'd).
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
Asia
Japan (Osaka) 1972-1977 - 0.030-0.040a Yakushiji et al. (1977)
1969-1976 19-52 each year 1-2 Yakushiji et al. (1979)
India (Ahmedabad) 1981-1982 50 not present Jani et al. (1988)
Hawaii (different islands) 1979-1980 54 0.80 ± 0.43 (0.13-2.2) Takei et al. (1983)
Europe
Germany, since 1970 several thousands 1.0-2.5 (98% of samples Acker et al. (1984);
Federal Republic of between 0.001-7.2) Cetinkaya et al. (1984);
Heeschen et al. (1986);
Lorenz & Neumeier (1983)
- 2709 1.77 Fooken & Butte (1987)
Netherlands 1983 278 0.72 (0.27-2.20)b Greve et al. (1985);
(11 centres country-wide) Greve & Wegman (1984)
1977-1979, 2649 2.1 Olling (1984)
1981
United Kingdom (Scotland) 1979-1980 30 0.01 (nd-0.04) HMSO (1986)
1983-1984 30 < 0.01 (nd-0.02)
Table 22. (cont'd).
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
Italy (Rome) 1983-1985 65 0.070 (0.007-0.176)a,c Dommarco et al. (1987)
Finland (different parts) 1984-1985 183 (165 of 0.57 (0.05-10.7) Mussalo-Rauhamaa et al.
women) (1988)
Sweden (5 regions) - 300e 1.06-1.18 (four regions) Noren (1983)
1.44 (one region)
1972 227d 1.05 Noren (1988)
1976 245 0.99
1980 340 0.78
1984-1985 102 0.60
Austria (Vienna) - 22 1.54 (0.58-3.78) Pesendorfer (1975)
Other regions 9 1.29 (0.95-1.57) Pesendorfer (1975)
a Whole milk.
b Median concentration.
c Arithmetic mean.
d Number of mothers that provided 4-7 samples each (samples were pooled).
e In each region, 300 mothers gave breast milk 3-5 days after parturition.
In a study by Kuwabara et al. (1978), the relationship was
investigated between breast-feeding and PCB residues in the blood of
children whose mothers were occupationally exposed to PCBs. The
children ingested their mother's milk for periods of < 1 to 3 years.
The age of the children at the time of the study ranged up to 13
years. The data provide evidence that PCBs are retained in the
children's body for many years and that longer intake of mother's milk
tends to increase PCB levels in the blood of the children. The PCB
levels in the blood of the 20 occupationally-exposed women and their
39 children ranged from 8.3 to 84.5 and 0.8 to 93.2 µg/litre,
respectively.
The results suggest that the PCB levels in the blood of children are
much more influenced by the transportation of PCBs through the
mother's milk than through the placenta. Furthermore, it was found
that the gas chromatographic patterns of the blood PCBs of the
children, breast fed for a long time, were different from those of
their mothers. Blood from 16 non-occupationally exposed mothers and
their children (17), showed that, as the length of the breast-feeding
period increased, there was an increase in the PCB levels in the blood
of the children. The mean blood PCB level in mothers was 2.8 ±
0.8 µg/litre; in children, it was 3.8 ± 3.6 µg/litre. In this study,
no clear change in blood PCBs patterns between mothers and children
was observed (Kuwabara et al., 1979).
Samples of maternal blood, milk, and umbilical cord blood were
collected from 43 mothers giving birth to their first or second child;
all the mothers had lived in Oslo during the previous 2 years. Blood
samples were collected immediately after delivery, either by Caesarean
section (16 Norwegians) or normally (20 Norwegians and 7 immigrants).
Subcutaneous fat samples were obtained during the operation. Samples
of colostrum and milk were obtained 3 and 5 days postpartum. PCBs were
found in 135 of the total 168 samples. In the Norwegian women and
infants, PCBs were the major contaminants, whereas only traces of PCBs
were found in the samples of immigrants. The average concentrations in
the maternal serum, cord serum, colostrum, and breast milk of
Norwegian women (Caesarean and normally delivered taken together)
were: 10, 3-5, 18-21, 20-23 µg/kg wet weight (Skaare et al., 1988).
5.5.3.1 Major PCB congeners in human milk
Commercial PCB preparations consist of complex mixtures of
environmentally stable compounds with a wide range of chlorine
contents. PCBs are transferred to breast-fed infants with the fat of
the mother's milk. Thus, infants nurtured on maternal milk are exposed
to relatively high concentrations of the higher chlorinated PCBs in
the short period preceding the full functioning of certain organs,
e.g., the liver (Jensen, 1983b; Slorach & Vaz, 1983; Gezondheidsraad,
1985).
Three major congeners were present in breast milk, e.g., PCB congener
numbers 138, 153, and 180 (DFG, 1988).
Slorach & Vaz (1983) reported that the GC patterns of PCBs in breast
milk samples from different countries were similar. The peaks denoted
146, 174, and 180 were dominant in the gas chromatograms. The total
levels of PCBs and the concentrations of certain congeners in Swedish
human milk, sampled in 1972-89, were studied by Noren et al. (1990).
Minor changes in the distribution of the congeners were found over the
period of study. The most abundant of the non- ortho coplanar PCBs in
Swedish human milk was 3,4,5,3',4'-pentachlorobiphenyl (126), with
levels decreasing from 0.35 µg/kg milk fat (1972) to about 0.10 µg/kg
(1989).
Safe et al. (1985a) analysed a sample of breast milk using the
congener-specific PCB method and found the following major components:
2,4,4'-trichloro-; 2,4,5,4'-tetrachloro-; 2,4,5,2',4'-pentachloro-;
2,4,5,3',4'-pentachloro-; 2,3,4,5,2',5'-hexachloro-;2,4,5,2',4',5'-
hexachloro; 2,3,4,5,2',3',4'-heptachloro-; and 2,3,4,5,2',4',5'-
heptachlorobiphenyls.
The major PCB congeners in the breast milk of Japanese women from the
general population were: 2,4,4'-trichloro-; 2,4,3',4'-tetrachloro-;
2,4,5,3',4'-pentachloro-; 2,3,4,2',3',4'-hexachloro-;2,3,4,5,2',4'-
hexachloro-; and 2,3,4,5,2',4',5'-heptachlorobiphenyls. The congeners
were present in 5% or more samples; a few other congeners were present
in only 1-3% (Gyorkos et al., 1985; Jensen, 1983b).
Sixty-eight breast milk samples collected in the Netherlands were used
to determine the congener distribution. The indicator congeners,
present in the highest concentrations, were: 2,4,4'-trichloro-,
2,4,5,2',5'-pentachloro-, 2,4,5,3',4'-pentachloro-,
2,3,4,2',4',5'-hexachloro-, 2,4,5,2',4',5'-hexachloro-,
2,3,4,5,2',4',5'-heptachlorobiphenyl (Wegman & Berkhoff, 1986).
Schecter et al. (1989a) analysed a total of 17 samples of human milk
from Thailand and Vietnam, for the presence of PCB congeners. The main
congeners that were present were 138, 153, and 180 (each in the range
of 8-31 µg/litre). The other congeners, normally present, were all
below the detection limit of 2 µg/litre.
In a study on pooled human milk samples from a 1982 nation-wide survey
in Canada, Mes & Marchand (1987) compared the relative amounts of 29
selected PCB isomers with amounts in milk samples of unexposed Rhesus
monkeys. In the pooled milk sample, 397 µg PCBs/litre, on a fat basis,
were found and the PCB isomer numbers 74, 99, 118, 138, 153, and 180
were the main contributors. Most of the predominant PCB isomers in
human milk were also observed in monkey's milk, but monkey's milk had
relatively low levels of PCB isomers numbers 74 and 99.
In another study, Davies & Mes (1987) analysed breast milk samples
from Canadian, Indian, and Inuit (Eskimo) mothers in Canada. The 18
samples were received from 5 Indian and Inuit nursing zones. The
combined total PCB isomer level (on a whole-milk basis) of the native
population was comparable with that of the national population. Even
the levels of the 5 largest PCB congeners (Nos. 74, 118, 138, 153, and
180) were comparable.
Individual congeners in the blood of Yusho- and Yu-Cheng patients are
discussed in section 5.6.
5.5.3.2 Factors that influence the intake of PCBs with milk
Present data suggest that the PCB content of human milk varies
considerably from individual to individual.
Many factors affect the level of PCBs and other organochlorine
compounds in breast milk including the fat content of the milk; time
from start of lactation; mother's age; mother's body weight; parity;
number of children previously breast-fed; origin and residence; eating
habits; season; smoking; use of household products; amount of milk;
and exposure at work (WHO/EURO, 1985, 1988).
In a given woman's milk, there are fluctuation in the PCB levels in
whole milk and in milk fat during one nursing session and during the
day (Jensen, 1983b). A decrease of PCB levels in both milk and milk
fat has been found during the lactation period. Furthermore, the PCB
concentration in human whole milk and milk fat increases with the age
of donor. Another confounding factor is that the PCB levels decrease
with increasing numbers of deliveries and lactations (Greve et al.
1985); lactation serves as a period for the biological elimination of
PCBs (Jensen, 1983b). The PCB levels in human milk are higher in
heavily populated and industrialized areas than in rural areas.
Furthermore, in general, the PCB levels in the breast milk of women
from developing countries are lower (Jensen, 1983b).
Cetinkaya et al. (1984) studied the PCB levels in human milk samples
from all over the Federal Republic of Germany. At the same time, data
were collected by means of a detailed questionnaire on residency,
workplace, smoking, drinking and eating habits, and the age of
participating individuals.
The breast milk of 45 women consuming lacto-vegetarian food was
compared with that of 41 women consuming conventional food in the
Federal Republic of Germany in the period 1979-81. The PCB
concentration was comparable, e.g., 2.2 and 2.5 mg/kg, on a fat basis,
respectively (Acker et al., 1984).
Fish consumption was positively correlated with PCB levels in maternal
serum and breast milk. PCB levels in serum increased with age, but
were unrelated to social class, parity, or body weight (Schwartz et
al., 1983).
Eight hundred and one Wisconsin anglers were surveyed for fishing and
consumption habits in 1985. The mean annual number of sport-caught
fish meals was 18 (range 7.1 to 33.3). The mean number of
non-sport-caught fish meals was 24. The median PCB serum congener sum
level for 192 anglers was 1.3 µg/litre (range, nd to 27.1 µg/litre).
Statistically significant positive Spearman correlations were observed
between sport-caught fish meals and PCB levels in serum and between kg
of fish caught and PCB levels in serum (Fiore et al., 1989).
PCBs were measured in maternal serum, cord blood, placenta, and serial
samples of breast milk and colostrum, from 868 women in North Carolina
(USA). Forty-three per cent of the women were primiparous. Breast milk
was collected at 6 weeks, 3 months, and 6 months, and, in a few cases,
up to 18 months postpartum. The median PCB concentration in breast
milk decreased during the sampling period from 1.77 to 1.02 mg/kg, on
a fat basis. The PCB concentration dropped by about 20% over 6 months
and 40% over 18 months. This implies that excretion in milk is a major
factor in lessening the mother's body burden; however, it also implies
substantial exposure of the child. Colostrum contained a median value
of 1.74 mg/kg. PCBs concentrations were higher in milk than in serum
and higher in maternal serum than in the placenta. The levels in cord
blood were almost always below the limit of quantification. Older
women and women who regularly drank alcohol had higher PCB levels in
their milk; blacks had higher levels than whites. In general, women
had higher levels in their first lactation and in the earlier samples
of a given lactation, and levels declined both with time spent
breast-feeding and with number of children nursed (Rogan et al.,
1986a).
Two hundred and forty-two newborn infants of mothers who consumed
moderate quantities of contaminated lake fish and 71 infants whose
mothers did not eat such fish were examined during the immediate post
partum period. PCB exposure was correlated with lower birth weight and
smaller head circumference, and the authors claimed that these effects
were not attributable to any of 37 potential confounding variables,
including socioeconomic status, maternal age, smoking, etc. (Fein et
al., 1984).
The mother's diet may be an important determinant of the PCB levels in
her milk. In some areas of the world, the intake of PCBs from eating
contaminated fish has been claimed to be the most important source of
PCBs in human milk. Dairy products and meat may be contaminated via
natural food or feedstuffs (WHO/EURO, 1988).
In a pilot study on the course of the PCB concentration in human milk
during 6 months of lactation, some PCB determinants were studied in 23
women and their infants. The average PCB concentration in the milk of
14 mothers during a 6-month period amounted to 0.66 ± 0.12 mg/kg, on a
fat basis. In univariate analyses, the PCB concentration on a fat
basis was strongly associated with pre- versus post-pregnancy weight
gain, age, and occupation. After multiple regression analysis, the PCB
concentration on a fat basis remained significantly associated with
changes in weight gain. The pre-pregnancy Quetelet Index of the mother
(height/weight) and the estimated PCB content of the diet (fish) were
correlated with the PCB concentration, on a milk basis (Drijver et
al., 1988).
5.5.4 Other tissues
Schecter et al. (1989b) analysed the tissues of 3 patients from the
North American continent, with no known history of chemical exposure,
for the presence of PCB isomers. The total PCB concentrations in the 9
tissues studied were different. The highest levels were found in
adipose tissue, subcutaneous fat (range 86-423 µg/kg), adrenals
(25-103 µg/kg), liver (3-149 µg/kg), bone marrow (26 µg/kg), kidneys
(2-31 µg/kg); levels in the spleen, lung, and testes were below
12 µg/kg. Congeners present in the highest concentrations were numbers
28, 74, 118, 153, 105, 138, 183, and 180.
5.6 Accidental exposures (Yusho- and Yu-Cheng)
In 1968, a large number of persons in Japan were accidentally poisoned
by the consumption of a batch of rice oil contaminated with Kanechlor
400. A similar accident happened in the Province of Taiwan in 1979,
where the affected persons had also consumed rice-bran oil
contaminated with PCBs. The 2 cases of poisoning were called Yusho and
Yu-Cheng accidents, respectively (see section 9.1.2.1).
The average PCB concentration in the plasma of Yusho children was
6 µg/litre, compared with 3.7 µg/litre in controls. Breast-fed Yusho
children had higher levels than children not breast-fed (Abe et al.,
1975).
The concentrations of PCBs in the adipose tissue, liver, and blood of
Yusho patients, about 5 years after the outbreak, were 1.9 ±
1.4 mg/kg, 0.08 ± 0.06 mg/kg, and 6.7 ± .3 µg/litre, respectively.
These values were only about twice those of controls. The mean blood
PCB level of 278 persons involved in the Yu-Cheng accident was
89.1 µg/litre (range 3-1156 µg/litre). Six months after the exposure,
the concentrations of PCBs in the blood had decreased to
12-50 µg/litre. The mean blood concentration of 165 patients,
9-18 months after the onset of poisoning, was 38 µg/litre (range
10-720 µg/litre) (see section 9.1.2.1). The blood PCB level of some
Yu-Cheng patients (99 ± 163 µg/litre), was much higher than that of
the Taiwanese population (1.2 ± 0.7 µg/litre), one year after the
outbreak of the intoxication.
Chen et al. (1985) analysed the blood of 165 Yu-Cheng patients, 9-18
months after the onset of poisoning, and found 10-720 µg PCBs/litre
with a mean value of 38 µg/litre. The blood of 10 patients, 9-27
months after poisoning, contained 0.02-0.2 µg PCDFs/litre. The
PCDF-congeners found in tissues were the same as those found by Masuda
et al. (1985).
Seven PCB congeners including: 2,4,5,3',4'-pentachloro-; 2,3,4,3',4'-
pentachloro-; 2,4,5,2',4',5'-hexachloro-; 2,3,4,2',4',5'-hexachloro-;
2,3,4,5,3',4'-hexachloro-; 2,3,4,5,2',4',5'-heptachloro-; and
2,3,4,5,2',3',4'-heptachlorobiphenyls, were identified in the blood
and tissues of Yusho, Yu-Cheng patients and controls.
Major PCDF congeners identified in the tissues and blood of Yusho and
Yu-Cheng patients were 2,3,6,8-tetrachloro-; 2,3,7,8-tetrachloro-;
1,2,4,7,8-pentachloro-; 2,3,4,7,8-pentachloro-; and 1,2,3,4,7,8-
hexachlorodibenzofurans. The 2,3,4,7,8-pentachloro-compound was
predominant. The concentrations of PCDFs in the adipose tissue and
liver of Yusho patients were 6-13 µg and 3-25 µg/kg tissue,
respectively. No PCDFs could be detected in the controls. Besides PCBs
and PCDFs, 4-methylthio-2,5,2',5'-tetrachlorobiphenyl (concentrations
ranging from 0.1 to 1.4 µg/kg tissue) and 4-methylsulfone-
2,5,2',5'-tetrachlorobiphenyl (range 0.3-2.5 µg/kg tissue) were also
found (Masuda et al., 1985).
5.7 Occupational exposure
5.7.1 Accidental exposure
Though the volatility of the PCBs is low, they are found in rather
high concentrations in the workroom air in both the long-term open use
of PCBs and in temporary or acute events where evaporation into the
air is possible. The measured air concentrations of PCBs in long-term
exposure situations, such as the manufacturing of transformers or
capacitors, varied from 30 to 1000 µg/m3, depending on the year of
measurement and the factory concerned (Silbergeld, 1983).
In discontinuous work, such as the inspection and repair of
transformers and capacitors, levels of between 0.1 and 60 µg/m3 have
been observed (Wolff, 1985). PCB concentrations in the breathing zone
of workers in transformer repair and maintenance work varied between
0.01 and 24.0 µg/m3 (Moseley et al., 1982).
In the atmosphere of an electroindustrial plant in Bela Krajina,
levels in the manufacturing room, where the autoclave was emptied,
averaged 2000 µg/m3 (range, 1400-3200 µg/m3); an average of
80 µg/m3 (range 40-120 µg/m3) was found in the working environment
in capacitor manufacture (Jan et al., 1988b).
Digernes & Astrup (1982) determined the concentrations of PCBs in the
atmosphere of the workplace of data screen operators, because skin
rashes and eczema had been reported among the workers. The PCB
concentrations in the working atmosphere (3 samples: concentrations
ranging from 0.056 to 0.081 µg/m3) were about 50-80 times higher than
the maximum level of PCBs in 3 samples collected outside the building
(0.0005-0.001 µg/m3). The indoor and outdoor samples also differed
qualitatively. The indoor samples contained only Aroclor 1242, while
outdoor samples contained a mixture of Aroclor 1242 and 1254.
Acute emergency events may cause extremely high concentrations of PCBs
in the air, particularly in cases when PCBs are burnt or heated (fire,
short circuit with electric arcing, burning in welding, etc.). Levels
of up to 10 000-16 000 µg/m3 have been measured. In the case of
extensive leaks of unheated PCBs from capacitors, concentrations of
1900 µg/m3 have been measured in workroom air (Elo et al., 1985;
WHO/EURO, 1987).
In connection with fires and electrical explosions, due to short
circuits, PCBs may be decomposed at elevated temperatures varying from
a few hundred to 2000°C. Soot may be produced in large amounts,
consisting of particles that may contain PCB concentrations up to
5000-8000 mg/kg of soot (Elo et al., 1985; O'Keefe et al., 1985;
WHO/EURO, 1987).
When evaluating PCB exposure, it is important to take into account
skin absorption from surfaces and tools, in addition to exposure via
inhalation. Surface concentrations of PCBs in capacitor factories have
varied between 4 and 60 µg/m2, and, where PCB leaks have occurred,
levels of up to 30 mg/m2 have been measured. Where PCBs have been
used long-term, contamination levels of 1-2 µg/cm2 have been found on
tools and tables.
A transformer was found to have overheated and released an oily mist
containing PCBs and their pyrolysis by-products, in a Department
building in New Mexico. The transformer contained Askarel (87% Aroclor
1260 and 13% of a mixture of tri- and tetrachlorinated benzenes). The
3-storey building was extensively contaminated via the following ways:
* mist entered 2 rooms, adjacent to the basement in which the
transformer was located;
* direct spread of mist and fumes through stairways;
* air drafts created by open windows and exhaust fans, spreading
fumes throughout the building;
* foot traffic by employees and other persons;
* the exhaust vent of the transformer room, located near the intake
vents for the building's air-conditioning system.
Air samples obtained up to 14 h after the incident showed levels of
48 µg/m in the transformer vault and 20 µg/m3 in the room above the
vault. Wipe samples of surfaces showed PCB levels ranging from 30
million µg/m2 for grossly contaminated surfaces to 4700 µg/m2 for
surfaces without visible contamination.
Five to 7 days later, air and surface samples were analysed for
2,3,7,8-tetrachlorodibenzofuran (TCDF), which was found to be present
in the air at an average level of 48 µg/m3 in most contaminated
areas. In wipe samples, the levels ranged from 5 ng/m2 to
41.224 ng/m2. 2,3,7,8-Tetrachlorodibenzo- p-dioxin (TCDD) was not
detectable in either air samples (detection limit, 0.5-5.0 pg/m3 air)
or wipe samples (detection limit 180 ng/m2) (Anon., 1985).
Very high concentrations of these toxic chemicals may be found in soot
emitted in connection with fires and explosions in capacitors.
Thus, skin contamination, and the ingestion and inhalation of soot
particles, may result in serious exposure in PCB accidents and
emergencies.
A short-term, follow-up study was performed on 55 workers in a gear
plant, whose work did not involve the use of PCBs. Exposure was to the
total residual PCB left behind by a capacitor company that had
formerly (3 years before) used the site. Air samples contained
< 10 µg/m3 and mean concentrations in wipe samples ranged from 23 to
161 µg/100 cm2. The 38 workers had a mean PCB concentration in serum
of 14.4 and the 17 office workers, 4.8 µg/litre. When the PCB
determinations were repeated in the 2 following years, no clear
decrease was observed (Christiani et al., 1986).
5.7.2 Occupational exposure during manufacture and use
Occupational exposure occurs during the manufacture of PCBs as well as
during their use by the electrical industry. It may also be widespread
among mechanics in contact with lubricating oils and hydraulic fluids,
among workers exposed to varnishes and paints, and among office
workers who have contact with pressure-sensitive duplicating paper
(carbonless copying paper), some brands of which readily transferred
PCBs to skin (Kuratsune & Masuda, 1972).
5.7.2.1 Adipose tissue
Levels of PCBs in the adipose tissue of occupationally exposed workers
have been found to vary between 26 and 50 mg/kg (range, 2.2-290 mg/kg).
There is a strong correlation between the blood PCB concentration and
PCB levels in adipose tissue, but the distribution of the various
congeners between plasma and adipose tissue is not the same, as
described above.
Emmett (1985) found the following congeners in the adipose tissue of
present and past transformer workers exposed to Aroclor 1242 and 1254:
2,4,3',4',5'-pentachloro-, 2,3,4,3',4'-pentachloro-, 2,3,4,5,2',4'-
hexachloro-, 2,3,4,6,3',4'-hexachloro-, 2,4,5,3',4',5'-hexachloro-,
2,3,4,5,2',3',4'-heptachloro-, and 2,3,4,5,6,3',4'-hepta-
chlorobiphenyl.
5.7.2.2 Blood
Karppanen & Kolho (1973) analysed the blood of 26 persons, 9
non-exposed, 6 persons handling PCBs, and 11 persons employed for 4
years in a capacitor-manufacturing plant in Finland. In the latter
case, Aroclor 1242 was used. The average concentrations in the blood
of the 3 groups were 7.1 µg/kg (3.1-12 µg/kg), 49.5 µg/kg
(36-63 µg/kg), and 440 µg/kg (70-1900 µg/kg), on a wet weight basis.
More recent results of a Finnish control group of workers indicated
serum PCB levels of 1.2 ± 0.6 µg/litre in an industrial area (Luotamo
et al., 1985; WHO/EURO, 1987). With acute exposure to high
concentrations of PCBs in air (8000-16 000 µg/m3), for a short
period, blood PCB concentrations rose to levels of 30 µg/litre; a
return to the normal level of 3 µg/litre was achieved, 4 weeks after
termination of exposure (Elo et al., 1985; WHO/EURO, 1987).
Similar plasma values were found in workers from Japanese capacitor
factories, but, here, skin lesions were noted (Hasegawa et al.,
1972a). In this same study, it was reported that air levels of PCBs of
10-50 µg/m3 were measured in a factory where KC-300 was used in the
manufacture of electric condensers. PCB levels in the serum of workers
ranged from 100 to 650 µg/litre. One month after the use of PCBs had
been suspended, serum levels remained unchanged (90-740 µg/litre).
However, in another factory making electric condensers, serum levels
decreased from an average of 800 to 300 µg/litre, within 3 months of
the use of PCBs being discontinued (Kitamura et al., 1973). According
to Hara et al. (1974), the half-time of PCBs in the blood of workers,
engaged in the manufacture of electric condensers for less than 5
years, was several months, while that of workers employed for more
than 10 years was 2-3 years.
Kuwabara et al. (1978) reported mean PCB levels of 36.8 µg/litre
(range 8.3-84.5 µg/litre) blood in 20 PCB-workers, 39 children had
blood levels of 14.3 µg/litre (0.8-93.2 µg/litre), and 12 Yusho
patients, 4.2 µg/litre (1.8-8.6 µg/litre).
Fact-finding surveys of 63 workers, who were occupationally exposed to
PCBs (Kanechlor 500) in the production of silk thread or of paint,
were carried out in Japan in 1974-75; some of them and their families
were also surveyed again in 1975-82. Nineteen per cent of them showed
PCB levels higher than 50 µg/litre plasma. These persons did not show
the typical clinical findings of Yusho patients. During 7 years, no
clear decline was observed (Takamatsu et al., 1984).
There is clear evidence that relatively high PCB levels persist in the
blood of workers whose "external" exposure ceased several months or
years previously. The blood PCB concentrations in capacitor
manufacturing workers, who had been exposed for 1-24 years, varied
between 24.4 and 192 µg/litre; this was higher than levels in the
blood of a reference population (0.5-33 µg/litre) (Maroni et al.,
1981a).
In Japan, Yakushiji et al. (1984a) studied the rate of decrease and
the half-life of PCBs in the blood of children (aged 1-13 years) and
their mothers, who were occupationally exposed to PCBs, over a 5-year
period (1975-79). The mean concentration of 121 blood samples from
50 children was 17.4 ± 22.9 µg/litre and that in 65 samples from 29
mothers was 32.3 ± 20.6 µg/litre. The concentrations of PCBs in the
blood of the children varied over a wide range, because of differences
in the duration of breast-feeding. The rate of decrease of the PCB
concentration in the blood in both 18 children and 8 mothers was
relatively constant and independent of the PCB concentrations. A
one-compartment model equation was sufficient to represent the
decrease in the concentration of PCBs in the blood. The mean rate
constant of the decrease for the children was 24.2% per year,
approximately 2.6 times higher than that of the mothers (9.2%),
equivalent to half-lives of 2.8 ± 1.1 and 7.1 ± 2.7 years,
respectively. The dilution effect due to the increase in body weight
was the most important factor that affected the reduction of the PCB
concentrations in the children.
A total of 118 blood samples, mainly from employees in industries
using PCBs, were collected in the period 1975-85. In 64 blood samples,
an average level of 17 µg/litre (range nd-110 µg/litre) was found
(Frank et al., 1988).
Brown & Lawton (1984) studied the partitioning of PCBs between adipose
tissue and serum in a population of 173 capacitor workers, who were
occupationally exposed to Aroclors 1254, 1242, and 1016 for various
periods of time. The serum levels of PCBs were significantly dependent
on the level of lipids in the serum, but not on that in the albumin.
The apparent contribution of cholesterol and its esters to PCB
transport is nearly equal to their contribution to the total serum
neutral lipids. The level of serum lipids PCBs must be equal to the
adipose fat PCBs level.
Yakushiji et al. (1984b) studied the relationship between
breast-feeding and the PCB levels in the blood. The blood samples of
50 children (121 samples) and of 29 occupationally exposed mothers (65
samples) were analysed during the period 1975-79. The PCB levels in
the blood of the children were greatly influenced by the duration of
breast-feeding, but showed little relationship to the PCBs levels in
maternal blood.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Inhalation
Studies on rats (6 per group) showed that an aerosol containing a PCB
mixture (Pydraul A200: 42% chlorine), particle size 0.5-3.0 µm, at a
concentration of 30.4 ± 3.4 g/m3 for 30 min, was readily absorbed
through the lungs. The PCB concentration in the liver, 15 min after
cessation of exposure, was 50% of the maximum concentration attained
after 2 h (70 mg/kg tissue) (Benthe et al., 1972).
6.1.2 Dermal
Vos & Beems (1971) and Vos & Notenboom-Ram (1972) applied Aroclor 1260
to the shaved backs of rabbits and found systemic effects in the
kidneys, indicating that PCBs can penetrate the skin (see section
8.2.5).
Nishizumi (1976), using tritium-labelled PCBs (40% chlorine), found
evidence for the dermal absorption of PCBs in rats.
In a study of the occupational exposure of electrical workers to PCBs
(Pyralen 3010 and Apirolio, 42% chlorine content), Maroni et al.
(1981a) concluded that absorption of PCBs occurred through the human
skin. Quantitative data were not available.
6.1.3 Oral
When polychlorobiphenyl isomers were administered orally, by gavage,
to rats, at levels of 5, 50, or 100 mg/kg body weight for the lower
chlorinated compounds and up to 5 mg/kg for the higher chlorinated
compounds, 90% of the compounds were rapidly absorbed by the
gastrointestinal tract (Albro & Fishbein, 1972; Berlin et al., 1973;
Melvås & Brandt, 1973).
Using Rhesus monkeys, Allen et al. (1974a,b) determined that > 90% of
a single oral dose of 1.5 or 3.0 g Aroclor 1248/kg body weight was
absorbed over a period of 2 weeks. Drill et al. (1981) and US EPA
(1985) reviewed a number of studies indicating that PCBs are readily
absorbed from the gastrointestinal tract following oral
administration.
Bleavins et al. (1984) found that, over a period of 5 weeks, European
ferrets absorbed 85.4% of a single dose of 14C-labelled Aroclor 1254
(0.05 mg) given in food.
In contrast to the above studies, Norback et al. (1978) claimed that
59.3-87% of a single oral dose of 2,4,5,2',4',5'-hexachlorobiphenyl
passed unabsorbed through the intestines of monkeys, the first week
after dosing.
6.2 Distribution
6.2.1 Inhalation (rat)
Maximum PCB concentrations in the liver and brain of rats occurred 2
and 24 h, respectively, after a single, 30-min exposure to 30.4 ±
3.4 g/m3 of Pydraul A200 aerosol (42% chlorine content). The
concentrations in these tissues declined, while concentrations in
adipose tissues reached a maximum after 48 h (Benthe et al., 1972).
6.2.2 Oral (rat)
As in the case of other lipophilic substances, the absorption and
distribution of PCBs will, in all probability, take place via the
lymphatic system (by the chylomicrones) (DFG, 1988).
Following absorption, the clearance of PCBs from the blood and tissues
follows a biphasic pattern. The compounds rapidly clear from the blood
and accumulate in the liver and adipose tissue or are metabolized in
the liver to metabolites that are excreted in the urine and/or bile
(Drill et al., 1981).
Kurachi & Mio (1983) exposed mice to Kaneclor 400 at 100 mg/kg diet,
for 5-20 days. High levels were found in the gonads, skin, adipose
tissue, adrenals, and kidneys.
In a study by Grant et al. (1971a), 4 days after an oral dose of
Aroclor 1254 was given to rats at 500 mg/kg, the concentrations of
PCBs in the fat, liver, and brain were 996, 116, and 40 mg/kg,
respectively. Similar results showing that the highest concentration
was in the fat, were obtained in rats given Aroclor 1254 in the diet
(Curley et al., 1971), in boars (Platonow et al., 1972), cows
(Platonow & Chen, 1973), and in pigeons and quail (Bailey & Bunyan,
1972). In the studies of Curley et al. (1971), the tissue
concentrations initially showed a rapid rise and then a slow increase
while the PCB diet was being administered; Grant et al. (1974) fed
diets containing Aroclor 1254 at 0.2, 20, and 100 mg/kg to rats for 8
months, during which period the tissue concentrations reached a steady
state that was dose-dependent (Table 23). Similar tissue distribution
data for Aroclors 1016 and 1242 have been reported by Burse et al.
(1974) and for Kanechlor-400 by Yoshimura et al. (1971).
Table 23. Tissue distribution of PCBs (mg/kg wet weight) in rats fed Aroclor 1254,
Aroclor 1242, or Aroclor 1016 at 100 mg/kg for about 6 months
Tissue Aroclor 1254a Aroclor 1242b Aroclor 1016b
Blood 0.40 0.53 (plasma) 0.38 (plasma)
Liver 16 4.21 7.86
Brain 3.4 1.69 2.98
Kidneys 1.89 3.21
Heart 7.3
Fat 32.0 110 236
Urine 0.03 0.28
a From: Grant et al. (1974).
b From: Burse et al. (1974).
The study by Burse et al. (1974) showed that, with continuous feeding
of 3 types of Aroclor (see Table 23) at 100 mg/kg diet, a steady state
was not reached for 6-8 months and that the decline of stored PCBs in
adipose tissue (when the animals were kept on a PCB-free diet) was
slow and did not reach zero during a recovery period of 5-6 months.
This is surprising because the Aroclor sample used should not have
contained appreciable amounts of hexachlorobiphenyls and higher
isomers. Mizutani et al. (1977), discussing this aspect, came to the
conclusion that mobilization from storage sites rather than metabolism
constitutes the rate-limiting step in the depletion of the body burden
of PCBs.
It was demonstrated that, as the number of chlorine atoms on the
biphenyl rings increased from 1 to 6, the tissue/blood ratio tended to
increase. This increase was also proportional to the amount of lipid
in the tissues with, consequently, a higher degree of bioaccumulation
(Matthews, 1983, cf. WHO/EURO, 1988).
The fat-plasma partition-coefficients for the different PCB congeners
range from 50 up to 310 (DFG, 1988).
6.2.3 Oral (monkey)
Feeding studies were carried out on female rhesus monkeys given doses
of 0, 5, 20, 40, or 80 µg Aroclor 1254/kg body weight per day, for a
period of 37 months (Arnold et al., 1984). Eighty monkeys were divided
into 5 groups, each of 16 animals. The mean body weight of the monkeys
at the start of the study was 6.44 kg. The Aroclor 1254 was dissolved
in corn oil with glycerol as sweetener and fed to the monkeys in
gelatin capsules. Samples of blood, adipose tissue, and faeces were
collected every month and the presence of PCBs, determined. After 27
weeks, levels of 1, 2-3, 9, 18, and 37 mg PCBs/kg fat were found in
the 5 groups; after 47 weeks, blood levels were 1-3, 12, 35, 73, and
129 µg/litre respectively. PCB concentrations in whole blood increased
more rapidly during the first 10 months of the study than in the
remaining 27 months, in all groups. Concentrations in adipose tissue
(fat) increased continuously during the 37 months. The ratio profiles
of PCB levels in blood/adipose tissue, remained relatively static
between the second and twenty-seventh month of feeding. The data in
terms of relative concentrations (concentration/dose) suggest that the
bioaccumulation or retention of PCBs may be dose-dependent,
particularly for adipose tissue. The data available from PCBs in
faeces indicate a dose-dependent PCB absorption.
6.2.4 Oral (humans)
According to the study by Nishimura et al. (1976) cf. Katsunuma et al.
(1985), the PCBs within a human fetus are not evenly distributed. The
concentrations of PCBs were highest in the skin and lowest in the
brain among the 5 major organs (cerebrum, heart, liver, kidneys, and
skin). That the highest level was found in the skin might have been
because of the high solubility of the compounds in adipose tissue. In
other words, PCBs accumulate increasingly as the body fat of a fetus
increases. The authors stated that the low residue levels in the brain
were likely to be because PCBs have a poor affinity for the brain
lipids.
6.2.5 Individual congeners of PCBs
More detailed information on the tissue distribution of PCBs and their
metabolites has been obtained by the administration of pure
14C-labelled compounds, using both whole-body autoradiography and
scintillation counting of tissue samples. Berlin et al. (1975)
demonstrated that, after a single oral dose of 14C-labelled
2,5,2',4',5'-pentachlorobiphenyl, radioactivity rapidly entered the
circulation of mice and was distributed in the tissues, particularly
in the liver, kidneys, lungs, and adrenals. Subsequently, the
radioactivity in the body fat increased, rising to a maximum within
4-24 h. In most other tissues, the radioactivity decreased rapidly
after dosing, but the authors noted a special affinity for the skin,
the bronchiolar epithelium of the lungs, and certain glandular
secreting tissues. Soon after administration of the dose,
radioactivity appeared in the bile and was eliminated in the faeces.
Similar results were obtained by Melvås & Brandt (1973) in mice
treated with 2,4,2',4'-tetrachlorobiphenyl, which possessed a high
affinity for the adrenal cortex, the corpora lutea, and glandular
secreting tissue. In quail treated with 2,4,2',3'- and
2,4,3',4'-tetrachlorobiphenyl, the radioactivity in the egg yolk was
high, exceeding that in the fat. Gage & Holm (1976) determined
concentrations in the abdominal fat of mice, 7 and 21 days after they
were administered a single dose (13-165 µg/mouse) of one of 14 PCB
congeners, by gavage. Relatively low levels (< 10 ng/g per µg dose)
were found at 7 days for 4,4'-dichloro-; 3,2',4',6'-tetrachloro, and
2,3,4,2',4',6'-hexachlorobiphenyl with relatively high levels (>
100 ng/g per µg dose) for 2,4,5,2',4',5'-hexachloro-, and the
4,2',4',6'-, and 2,4,2',4'- tetrachlorobiphenyls.
Muehleback & Bickel (1981) treated rats, by gavage, with a single dose
of 14C-2,4,5,2',4',5'-hexachlorobiphenyl at 0.6 or 3.6 mg/kg body
weight. The rats were examined 1 h, 24 h, 6 weeks, 20 weeks, or 40
weeks after dosing. The highest levels of PCBs were found in the
muscle, liver, adipose tissue, and skin, early in the study. By the
end of the study, the highest PCB levels were found in the adipose
tissue followed by the skin, muscle, and liver. During the 40-week
study period, only 16% of the total dose was excreted.
The pharmacokinetics of individual monochloro-, dichloro-,
tetrachloro-, pentachloro-, and hexachlorobiphenyls were studied by
Matthews & Anderson (1975a,b), Lutz et al. (1977), and Tuey & Matthews
(1977). The mono- and dichlorobiphenyls were largely removed from
adipose tissue within 4-7 days, the 3 higher chlorinated biphenyls
were eliminated much more slowly. The half-life for the
tetrachlorobiphenyl from adipose tissue was 15 days. Skin effects were
more or less comparable.
Beran et al. (1983) studied the distribution of 14C-labelled
2,5,4'-tri-, 2,4,5,2',4',5'-hexa-, and 2,3,4,5,2',3',4',6'-
octachlorobiphenyl in the haematopoietic tissues of squirrel monkeys
(Saimiri scureus) and C67Bl mice using whole-body autoradiography.
An accumulation of radioactivity was observed in the bone marrow of
one monkey after iv injection (substances dissolved in DMSO) of the
tri- or hexachlorobiphenyl. The same was found in 3 normal mice
treated with the octachlorobiphenyl. A study using whole-body
autoradiography and spleen-colony assay in supralethally irradiated
mice, implanted with syngenic bone-marrow cells, indicated that the
major part of the radioactivity was localized outside the bone-marrow
haemic compartment, probably in the fat. Nevertheless, the trichloro-
and octachlorobiphenyls were found to inhibit the in vitro formation
of granulocytic colonies from mouse progenitor cells. Very low uptake
of labelled chlorobiphenyls was observed in the thymus, spleen, and
lymph nodes.
14C-labelled-2,4,2',4'-tetrachloro- and 3,4,3',4'-tetrachlorobiphenyl
were each administered orally to male Sprague-Dawley rats in a single
dose at 0.54 mg/kg and 0.51 mg/kg, body weight, respectively.
Distribution and covalent binding were studied. The accumulation of
2,4,2',4'-tetrachlorobiphenyl in adipose tissue was much higher than
that of 3,4,3',4'-tetrachlorobiphenyl, though the level in the blood
was consistently higher in the 3,4,3',4'-tetrachlorobiphenyl-treated
rats. The radioactivity bound in covalent linkages with cellular
macromolecules in several tissues was determined. The data indicated
that covalent binding was higher in 3,4,3',4'-tetrachloro-
biphenyl-treated rats than in those treated with 2,4,2',4'-
tetrachloro-biphenyl, particularly in the liver and blood components.
These results suggest that the 2 tetrachlorobiphenyl isomers have
different pharmacokinetic properties in rats and that the association
of covalent binding with 3,4,3',4'-tetrachlorobiphenyl induced
toxicities might be important. The microsomal enzyme system is likely
to play an important role in the in vivo covalent binding of
tetrachlorobiphenyls (Shimada & Sawabe, 1984).
In pharmacokinetic studies, 11 groups of 3 male ICR mice/group were
administered daily doses of 100 mg 2,5,2',5'-tetrachlorobiphenyl/kg
body weight dissolved in corn-oil/acetone (9:1), by gavage, for 8
consecutive days. Thirteen groups of 3 mice were administered (by
gavage) 8 mg 3,4,3',4'-tetrachlorobiphenyl/kg in the same vehicle
every other day for 10 doses. One group was sacrificed just before
each of the last 3 doses, the other groups were sacrificed at
intervals of 0.5-336 h after dosing. After dosing to an apparent
steady-state, 2,5,2',5'-tetrachlorobiphenyl was found to have a tissue
elimination half-life of between 39.5 and 70 h. The half-life of
3,4,3',4'-tetrachlorobiphenyl was 26-62.5 h. The 3,4,3',4'-
tetrachlorobiphenyl had a substantially greater partitioning from
serum into adipose tissue, liver, and thymic tissues. Studies were
undertaken to compare the toxic potency of these 2
tetrachlorobiphenyls, when similar tissue concentrations of the 2
isomers were achieved in target and storage tissues. The studies
demonstrated that thymic atrophy occurs at lower doses and tissue
concentrations of 3,4,3'4'-tetrachlorobiphenyl than those required to
produce hepatotoxicity. These two organ toxicities were produced only
by 3,4,3'4'-tetrachlorobiphenyl, despite the fact that equivalent or
higher tissue concentrations of 2,5,2',5'-tetrachlorobiphenyl were
achieved in vivo, in all tissues. The conclusion was that the
in vivo difference in the toxic potency of these tetrachloro-
biphenyl isomers does not result from the differences in their tissue
disposition, elimination, and ultimate bioaccumulation (Clevenger et
al., 1989).
6.2.6 Appraisal
Matthews & Dedrick (1984), in a review, concluded that the
pharmacokinetics of PCBs are complicated by numerous factors, not
least of which is the existence of 209 different chlorinated
biphenyls. While all PCB congeners are highly lipophilic and most are
readily absorbed and rapidly distributed to all tissues, PCBs are
cleared from the tissues at very different rates, and the same
congeners may be cleared at different rates by different species. With
the exception of special situations in which PCBs may be passively
eliminated in lipid sinks, e.g., milk or eggs, clearance is minimal
prior to metabolism to more polar compounds. Rates of PCB metabolism
vary greatly with species and with the degree and positions of
chlorination. Mammals metabolize these compounds most rapidly, but,
even among mammalian species, the rates of metabolism vary greatly. In
all species studied, the more readily metabolized chlorinated
biphenyls have adjacent unsubstituted carbon atoms in the 3-4
positions. Congeners that do not have adjacent unsubstituted carbon
atoms may be metabolized very slowly and therefore cleared very
slowly. PCBs that are not readily cleared concentrate in adipose
tissue.
6.3 Placental transport
6.3.1 Laboratory animals
The results of a number of animal studies have demonstrated that PCBs
and specific congeners can cross the placental barrier and accumulate
in the tissues of fetuses (US EPA, 1987). In studies in which monkeys
were exposed prior to, and during, gestation, signs of PCB
intoxication were observed in nursing, but not in newborn offspring
(Allen & Barsotti, 1976; Iatropoulos et al., 1978). Results such as
these have led to the conclusion that transfer through nursing may
account for higher exposure of the young than placental transfer.
Groups of pregnant ddN mice were fed diets containing Kanechlor 500
(mainly comprising pentachloro- and hexachlorobiphenyls) at 0.01
(controls), 0.94, or 86 mg/kg diet from day 1 to 18 of pregnancy.
Regardless of the dietary level of PCBs, whole-body levels in the
fetuses were only 0.1-0.2% of the total maternal intake, indicating
limited transplacental transfer (Masuda et al., 1978a). Two groups of
ddN mice were fed Kanechlor 500 (mainly containing pentachloro- and
hexachlorobiphenyls at 0 or 0.94 mg/kg diet) from the day of
insemination throughout gestation and for 5 weeks after delivery of
offspring. Total PCBs were 100 times greater in the suckling animal
than in the fetuses at term, from dams fed the same amount, indicating
a considerable transfer of PCBs during lactation (Masuda et al.,
1978a).
Masuda et al. (1979) fed female ddN-mice diets containing
polychlorinated biphenyls: 2,4,4'-trichloro-; 2,5,3',4'-tetrachloro-;
2,4,5,2',5'-pentachloro-; 2,3,4,2',4',5'-hexachloro-;2,4,5,2',4',5'-
hexachloro-; 2,3,4,5,6,2',5'-heptachloro-; and 2,3,4,5,2',3',4',5'-
octachlorobiphenyl at levels of 0.32, 0.42, 0.42, 0.44, 0.44,
0.16, and 0.23 mg/kg diet, respectively, for 18 days prior
to, or after, mating. Animals were either sacrificed on day 18 of
gestation or allowed to deliver and the offspring maintained for 5
weeks on a normal diet. All the PCBs were qualitatively transferred
across the placenta and through the milk. The amount transferred
during lactation was greater than that transferred transplacentally.
The transfer of 2,4,5,2',4',5'-hexachlorobi[14C-]phenyl across the
placenta during the course of pregnancy in Sprague-Dawley mice was
studied by Vodicnik & Lech (1980). The PCB was injected
intraperitoneally at 100 mg/kg body weight, in corn oil, 2 weeks prior
to mating. The concentrations of 14C-PCB in the fetuses from 12- and
18-day pregnant animals were 0.71 and 2.45 mg/kg tissue, respectively.
At birth, the total carcass concentration for all newborn animals was
less than 3 mg/kg tissue, which represents less than 3% of the dose
present in the mothers at birth.
Placental transfer of polychlorinated biphenyls has also been reported
in the mouse by Berlin et al. (1975) and Melvås & Brandt (1973).
Curley et al. (1973a) found some placental transport of Aroclor 1254
in the rat.
Groups of pregnant and non-pregnant Wistar rats received a dose of
14C-2,4,5,2',4',5'-hexachlorobiphenyl (2.1 µC/kg), intraperitoneally.
The amount of radioactivity transferred through the placenta was 2.7%
of the administered dose, whereas 39.2% of the original dose was
transferred through the milk (Ando et al., 1978).
Aroclors 1221 and 1254 were found to cross the placenta of rabbits,
when administered orally to does during gestation. The concentration
in fetal tissues was dose-dependent and much lower with Aroclor 1221
than with Aroclor 1254; the concentration of the latter in the fetal
liver was greater than that in the maternal liver (Grant et al.,
1971b).
Bleavins et al. (1984) fed female European ferrets a single dose of
14C-labelled Aroclor 1254 in the diet (0.05 mg), early (day 14) or
late (day 35) in gestation, and determined the placental transfer of
PCBs. Placental transfer to the kits was 0.01% (per kit) of the
maternal dose, when the dams were exposed early in gestation, and,
0.04%, when the dams were exposed late in gestation. Placental
transfer of PCBs was considerably less than mammary transfer, with a
ratio at 1 week of lactation of 1:15 and 1:7 for offspring of dams
dosed early or late in gestation, respectively.
Groups of lactating mother Rhesus monkeys, between 1 and 3 months post
partum, received 16 mg Clophen A-30/kg per day for 30 days. One
mother/infant pair served as a control. Clophen A-30 concentrations in
the serum of both mother and infant and the milk were determined on
days -14, -7, 0, 1, 2, 4, 8 and at weekly intervals thereafter. One
mother and all infants were killed and tissues taken for PCB analysis.
The concentration of Clophen A-30 in milk was 20 times higher than
maternal serum levels. Infant serum levels were 2-5 times higher than
their mothers. Tissue levels were generally higher in the infants.
Clophen A-30 tended to concentrate in the infant fat, bone marrow, and
adrenals (Bailey et al., 1980).
Groups of 24 Rhesus monkeys were maintained on diets that provided
Aroclor 1016 at doses of 0, 4.5, or 18.1 mg/kg body weight per day
throughout gestation and a 4-month nursing period. At birth, the
concentrations of PCBs in the skin of infants were similar to
concentrations in the subcutaneous fat of the mothers. At weaning, the
PCB content in the mesenteric fat of the infants was 4-7 times greater
than that in the subcutaneous fat of the mothers. Gas chromatographic
patterns showed that the adult adipose tissue did not include the
total spectrum of peaks observed in the Aroclor 1016 standard, and
that all of the peaks in the mesenteric fat of the infants at weaning
and 4 months after weaning were qualitatively similar to those in the
adult adipose tissue. According to the authors, these data suggested
an inability of the fetus to metabolize and excrete certain congeners
that are more readily metabolized and eliminated by adults and older
infants (Barsotti & Van Miller, 1984).
6.3.2 Wildlife
A 6 1/2-year-old desert bighorn (Ovis canadensis cremnobates) ewe
and her term ram fetus were used to study the distribution and
concentrations of PCBs in different organs and tissues. Fourteen
maternal and 13 fetal tissues were analysed for their presence of
organochlorine hydrocarbons. PCBs averaged 85 and 88% of the total
residue loads for maternal and fetal tissues, respectively. It is
remarkable that the "natural" PCB levels in the different organs and
tissues were nearly the same, i.e., in maternal organs and tissues
between 0.37 and 0.44 mg/kg, and, in fetal organs and tissues, between
0.30 and 0.35 mg/kg, on a fat basis (Turner, 1979).
6.3.3 Humans
Four studies of placental passage in humans, based on small samples
drawn from the general Japanese population, have yielded inconsistent
results (Yoshimura, 1974; Akiyama et al., 1975; Kodama & Ota, 1977;
Masuda et al., 1978a).
PCBs were detected in the umbilical tissues, umbilical blood, amniotic
fluid, and baby's blood from a woman who was occupationally exposed to
Kanechlors 300 and 500 in a capacitor factory (Yakushiji et al.,
1978). PCB levels in these tissues and fluids were considerably lower
than that in the mother's blood.
Jacobson et al. (1984b) examined maternal and cord serum (196 or 198
samples each) for the presence of PCBs, in women who resided in the
Michigan area (USA), where, in 1973, a PBB-incident occurred. Mean
concentrations of maternal and cord serum were 4.7 µg/litre (1.1-
14.3 µg/litre) and 2.0 µg/litre (0.1-7.2 µg/litre), respectively.
Placental passage was indicated by a significant maternal to cord
serum correlation for PCBs. The fact that cord serum levels were lower
than those in maternal serum is consistent with the notion that the
placenta may function as a partial barrier. The transfer rate of PCBs
in maternal blood through the placenta to cord blood may vary,
depending on the chemical nature of each PCB isomer (Ando et al.,
1984). Ando et al. (1985) examined the PCB concentrations in the
maternal blood, breast milk, and the placenta of 6 Japanese women.
They found that the congeners present were more typical of Kanechlor
500 than Kanechlor 300, 400, or 600. The results indicated that, as
the chlorine content of the PCB congeners increased, the correlation
between the placental content of congeners and those in the maternal
blood and breast milk also increased. The same was found in laboratory
animals (Allen & Barsotti, 1976; Masuda et al., 1978b).
A study on the transfer of PCBs to infants from their mothers was
carried out in Japan from 1974 to 1976 by Kodama & Ota (1977). When
the cord blood was considered as the infant blood at birth, the level
of PCBs in the blood of breast-fed infants rose gradually with
ingestion of breast milk, exceeded the level in the blood of their
mothers after 3 months, continued to increase up to the age of 1 year
and then significantly decreased, 2 years after birth. The PCB
concentrations in the blood of non-breast-fed infants remained low
(Table 24).
6.4 Excretion and elimination
6.4.1 Following oral dosing
The excretion of PCBs is, to a large extent, dependent on the
metabolism of PCBs to form more polar compounds (US EPA, 1987). At
equilibrium, the elimination of PCBs from all tissues will be
dependent on the structure-dependent metabolism rates of the
individual PCB congeners. For example, the biological half-lives in
the rat range from 1.15 days for 2,2'-dichlorobiphenyl to
approximately 460 days for 2,4,5,2',4',5',-hexachlorobiphenyl (Tanabe
et al., 1981; Wyss et al., 1986). Metabolites of the more highly
chlorinated congeners are eliminated primarily via the faeces (Goto et
al., 1974; WHO/EURO, 1987).
When the analysis of faeces is limited to the determination of
unchanged PCBs, the recovery of the dose administered is incomplete;
in boars receiving single or repeated doses of Aroclor 1254, not more
than 16% of the dose was recovered from the faeces and less than 1% in
the urine (Platonow et al., 1972). Better recoveries have been
obtained with PCB labelled with radioactive isotopes. Yoshimura et al.
(1971) found 70% of the activity from a dose of tritium-labelled
Kanechlor 400 in the faeces and 2% in the urine, over a 4-week period.
Berlin et al. (1973, 1975) found over 75% of the activity from
14C-labelled pentachloro- and hexachlorobiphenyls in the faeces and
less than 2% in the urine; most of the faecal elimination consisted of
PCB metabolites. Similar results were obtained by Melvås & Brandt
(1973) with tetrachlorobiphenyls.
Hashimoto et al. (1976) examined the excretion of 14C-PCB compounds
given to rats by gavage, at a total dose of 6.35-7.85 mg/kg body
weight, over a period of 5-50 days. The PCBs studied were
predominantly tetra- and hexachlorinated isomers. The results
indicated that 1.9-4.9% of the dose of tetrachlorobiphenyls was
excreted in the urine, with higher amounts excreted in rats treated
for longer periods. In rats treated with hexachlorobiphenyls, only
0.3% of the dose was excreted in urine. About 47-68% of the dose of
both tetrachloro- and hexachloro-isomers was eliminated in the faeces.
Table 24. Level of PCBs in mothers' and babies' blood (average over
3 years in µg/litre)a
Maternal blood 4.5 (0.8-15.5)
Cord blood 1.1 (nd-5.6)
Mother's blood (breast-feeding) 2.5 (nd-10.8)
Babies' blood (3 months old) 3.6 (0.2-10.9)
Babies' blood (1 year old) 4.7 (0.8-17.7)
Mother's blood (bottle feeding) 2.7 (0.6-8.7)
Babies' blood (3 months old) 1.6 (nd-7.6)
Babies' blood (1 year old) 0.7 (nd-2.1)
a From: Kodama & Ota (1977).
Bleavins et al. (1984) found 22.1% and 1.8% in the faeces and urine,
respectively, during the first week following dosing of 0.05 mg
14C-labelled Aroclor 1254 to female European ferrets.
A biological half-life of about 200 days was recorded in the fat of
rats after feeding with Aroclor 1254 (Grant et al., 1974). Berlin et
al. (1975) noted that, in mice dosed with a pentachlorobiphenyl, there
was an initial rapid elimination from the liver while liver PCB levels
were high, followed by a slower elimination when most of the PCB was
located in the fat. The author suggested that the mobilization of PCBs
from fat, and, therefore, their half-life in the body, depends upon
their rates of metabolism. Berlin et al. (1973) investigated the
hypothesis that the ability of a PCB to be readily degraded with a
half-life of a few days depended on the presence of 2 adjacent
unsubstituted carbon atoms in the molecule, rather than on the number
of chlorine atoms, though the presence of such unsubstituted pairs
depends to a large extent on the degree of chlorination. They came to
the conclusion that this hypothesis probably applied to unsubstituted
pairs in the 3,4-position, but that in the 2,3-position, their
susceptibility to metabolic degradation was influenced more by the
presence of chlorines in the o-position of the ring bridge.
Sprague-Dawley rats, white Swiss mice, and Rhesus monkeys were
administered a single dose of 14C-2,2'-dichlorobiphenyl, by gavage.
Within 6 days, mice eliminated a total of approximately 46% of the PCB
(urine, 20%; faeces, 26%). There was no clear difference between male
and female mice. In the rat, the total elimination was 51-56% after 9
days, mainly via the biliary/faecal route. The monkeys had the highest
elimination rate, a total of 68.6% (urine, 54%; faeces, 14.6%), within
10 days (Milling et al., 1979).
Male and female Wistar rats were administered a daily dose of
14C-2,5,4'-trichlorobiphenyl for 14 days. The animals were killed 5
days after receiving the last dose. The compound was rapidly
eliminated primarily with the faeces. Most of the trichlorobiphenyl
was metabolized (78.5%) and the major metabolites excreted were
identified as hydroxy-, dichloro-, and conjugated derivatives (Lay et
al., 1979).
The elimination of tetrachlorobiphenyl isomers in mice fed diets
containing a single isomer, at 10 mg/kg diet for 20 days, was studied
by Mizutani et al. (1977). Biological half-lives for the isomers
2,3,2',3'-tetrachloro-, 2,4,2',4'-tetrachloro-, 2,5,2',5'-
tetrachloro-, 3,4,3',4'-tetrachloro-, and 3,5,3',5'-tetrachloro-
biphenyl, were 0.9, 9.2, 3.4, 0.9, and 2.1 days, respectively.
Gage & Holm (1976) studied the influence of molecular structure on the
excretion of 14 PCB congeners in mice. They found that the
4,4'-dichloro-; 3,3',4',6'-tetrachloro-; 2,3,2',4',6'-pentachloro-;
and 2,3,4,2',4',5'-hexachloro-isomers were eliminated most rapidly.
These compounds had at least one pair of unsubstituted ortho-meta,
vicinal carbon atoms, a configuration thought to be important for
rapid metabolism and excretion. The most slowly eliminated compounds
were 2,4,5,2',4',5'- and 2,3,4,2',4',5'-hexachlorobiphenyl.
2,4,5,2',4',5'-Hexachlorobiphenyl was the PCB congener found in the
highest concentration in human adipose tissue, while
2,4,6,2',4',6'-hexachlorobiphenyl was not detected (Jensen &
Sundström, 1974a). As both of these compounds are found in commercial
PCB mixtures and in the environment, the presence of the
2,4,5,2',4',5'-hexachlorobiphenyl in adipose tissue appears to be
related to resistance to metabolism (US EPA, 1987). That this congener
is not, or is only minimally, metabolized is also indicated by the
finding that the blood concentration of this congener decreased only
10% over 300-500 days (Chen et al., 1982) and by the results of
in vitro metabolism studies with human liver microsomes (Schnellman
et al., 1984a,b).
Felt et al. (1977) examined the elimination of 14C-2,5,4'-trichloro-
biphenyl in rhesus monkeys. The monkeys were fed 550 mg of the
compound in fruit, daily, for 84 days. On the basis of total excretion
and recovered radioactivity, the half-life was found to be 4.5-4.8
days.
Male and female Sprague-Dawley rats were administered 14C-labelled
2,4,6,2',4'-pentachlorobiphenyl by gavage and the urine and faeces
were collected. After 8 days, the animals were killed. The elimination
of the PCBs followed a bi-exponential rate expression with a-phase
half-lives of 0.90 and 0.95 days and b-phase half-lives of 4.2 and 3.8
days, for males and females, respectively (Felt et al., 1979).
6.4.2 Following parenteral dosing
The results of injection studies indicate that PCBs can be excreted
unmetabolized into the gastrointestinal tract. Yoshimura & Yamamoto
(1975) recovered unchanged tetrachlorobiphenyl from the duodenal
contents of rats injected intravenously with tetrachlorobiphenyl.
Daily excretion for 4 days ranged from 0.5 to 0.8% of the total
dose/day. Goto et al. (1974) found that 4.7-23.2% of injected PCBs
were excreted unchanged into the gastrointestinal tract by day 10
after dosing, with the excretion of a penta-isomer greater than the
excretion of di-, tri-, or tetra-isomers.
Adult male Sprague-Dawley rats received doses of 4 symmetrical
hexachlorobiphenyl 14C-isomers, i.e., 2,3,5,2',3',5'-hexachloro-,
2,3,6,2',3',6'-hexachloro-, 2,4,5,2',4',5'-hexachloro-, and
2,4,6,2',4',6'-hexachlorobiphenyl, by intravenous injection. Most of
the radioactivity was eliminated in the faeces with less than 1% found
in the urine. The metabolites showed evidence of dechlorination,
chlorine shifts, and possible metabolism by direct insertion of a
hydroxyl group. There was also evidence supporting the intermediate
step of an arene-oxide as a predominant mechanism of PCB metabolism
(Kato et al., 1980).
The disposition of 2 symmetrical 14C-labelled 2,3,6,2',3',6'-
hexachloro- and 2,4,5,2',4',5'-hexachlorobiphenyl was studied in
24-month-old, male, Sprague-Dawley rats, after iv treatment. More than
50% of the 2,3,6,2',3',6'-hexachlorobiphenyl was metabolized and
excreted via the bile into the faeces within 2 days, and only 2% was
excreted in urine. More than 90% was eliminated as metabolites. In
contrast, 2,4,5,2',4',5'-hexachlorobiphenyl was redistributed from the
liver, muscle, and skin to the adipose tissue, where it accumulated
without being metabolized. Only 2% of the total dose was eliminated,
primarily in the faeces, within 21 days. In 2- to 3-month-old rats,
the general pattern of disposition of these hexachlorobiphenyls did
not change with age; however, there were differences in the rates of
elimination and in the tissue levels. There was enhanced metabolic
retention in the muscle, skin, and adipose tissue of older rats, which
suggested an age-related decrease in tissue clearance. The larger
volume of adipose tissue could not explain this observation. In
general, there were few changes in decay rates from tissues or in
biliary excretion, so age had a greater effect on the disposition of
the "persistent" 2,4,5,2',4',5'-hexachlorobiphenyl than on the
metabolizable 2,3,6,2',3',6'-hexachlorobiphenyl (Birnbaum, 1983).
Ethane exhalation was increased in male Sprague-Dawley rats, 30 days
after a single ip injection of Aroclor 1254 (500 mg/kg body weight).
Before day 30, there was no increase in ethane production. Parallel
increases in hepatic malondialdehyde levels were found. A single ip
injection of 3,4,3',4'-tetrachloro-, 2,3,4,5,4'-pentachloro, and
2,4,5,2',4',5'-hexachlorobiphenyl (300 µmol/kg) also increased (after
30 days) the production of malondialdehyde and ethane, indicators of
in vivo lipid peroxidation. These effects were not reflected in
increased diene conjugation (Dogra et al., 1988).
Sipes et al. (1980, 1982a,b) studied the distribution, metabolism, and
excretion of 14C-labelled 4,4-dichloro-, 2,4,5,2'4'5'-hexachloro-, or
2,3,6,2',3',6'-hexachlorobiphenyl in beagle dogs and cynomolgus
monkeys, after a single intravenous dose. The elimination of the test
substances from the blood of both species was shown to be biphasic.
The results for dichlorobiphenyl showed that the dog eliminated 50% of
the dose (urine, 7%; faeces, 43%) within 24 h, while the remainder was
found mainly in the adipose tissue. By 5 days, 90% had been
eliminated. The monkey eliminated less than 15% of the dose within
24 h, with less than 1% in the faeces. The remainder was found in the
adipose tissue. Within 28 days, 59% of the dose had been eliminated,
chiefly in the urine. Biliary excretion after 24 h was shown to be 33%
in the dog and only 0.4% in the monkey.
The data for 2,4,5,2',4',5'-hexachlorobiphenyl showed that the dog
eliminated 66% (urine, 3%; faeces, 63%) within 3 days; the monkey
eliminated 18% of the dose (of which 17% was in the faeces), 90 days
following administration. The remainder was found in the adipose
tissue. In the studies with 2,3,6,2',3',6'-hexachlorobiphenyl, the dog
eliminated 52% of the dose within 24 h (urine, 11%; faeces, 41%) and
70% in 3 days. The monkey eliminated 19% during the first 24 h,
divided equally between urine and faeces. By 15 days, 61% had been
eliminated, primarily in the faeces. The 24-h biliary excretion was
26% and 2.4% in the dog and the monkey, respectively.
6.4.3 Humans
Chen et al. (1982, 1985) studied the presence of PCBs in the blood of
human beings, in the Province of Taiwan, after they had consumed
rice-bran oil contaminated with Kanechlor 500 and PCDFs. Blood samples
from 17 patients were examined, with 2-3 samples taken from each
patient, 2-17 months apart. The results indicated that the
tetrachloro- and some pentachloro- isomers tended to be eliminated
more rapidly than the other pentachloro- and the hexachloro- and
heptachloro- isomers. Half-lives for the 2,4,5,2',4'- and 2,3,4,3',4'-
pentachloro- isomers in the blood were 9.8 and 8.7 months,
respectively. Two adjacent unsubstituted carbon atoms at the meta,
para positions facilitated metabolism and the subsequent elimination
from the blood. PCBs containing adjacent unsubstituted carbon atoms at
the ortho and meta positions of the biphenyl ring are eliminated
very slowly and will accumulate.
Buhler et al. (1988) administered a uniformly 13C-labelled PCB
mixture similar to Aroclor 1254 to a volunteer. A single dose of
329 µg/kg body weight was ingested; blood samples taken over a period
of 260 days were analysed for 13C- and 12C-PCBs using GC/MS and
GC/ECD. Elimination of the isomers followed a first order kinetics.
The half-lives for the isomers 2,3,4,2',4',5'-hexachlorobiphenyl,
2,4,5,2',4',5'-hexachlorobiphenyl, and 2,3,4,5,2',4',5'-hepta-
chlorobiphenyl were 321, 338, and 124 days, respectively.
6.4.4 Elimination via milk (animals)
Vodicnik (1986) studied the disposition of 14C-2,4,2',4'-
tetrachlorobiphenyl (150 mg/kg body weight administered
intraperitoneally) as a function of non-pregnant body weight in
virgin, late pregnant, and early post partum ICR mice and their
offspring. The highest concentrations were observed in adipose tissue
and the mammary glands, regardless of reproductive state. The
concentrations of the tetrachlorobiphenyl equivalents in the tissues
differed among the 3 groups, possibly because of the alterations in
lipid deposition/mobilization associated with pregnancy and lactation.
Approximately 20% of 14C-activity was eliminated from the carcass of
virgin mice, 4 days after administration, but no decrease was seen in
late-pregnant animals. Minimal transplacental transfer of
14C-activity occurred (approximately 1%), but the tetrachlorobiphenyl
was rapidly eliminated in breast milk to nursing offspring. Ninety per
cent of the total-carcass 14C-activity was eliminated from lactating
mice over a 4-day period, approximately 75% of which could be
accounted for in neonatal carcasses.
Saschenbrecker et al. (1972) found that, after oral administration of
doses of Aroclor 1254 of 10 or 100 mg/kg to cows, 6.27 and
74.5 mg/litre, respectively, appeared in the milk after 24 h. These
levels were reduced to less than one-half within 3 days, but traces
still remained at 50 days. Cows receiving 200 mg/day of Aroclor 1254
reached a steady state concentration of 61 mg/kg in the milk fat and
42 mg/kg in the body fat, after 10 days (Fries et al., 1973).
The "carry-over factor" from animal feed into the cow's milk showed
that the lower (tri-, tetra-, and penta-) chlorinated biphenyls have a
lower carry-over factor than the higher (hexa- and hepta-) chlorinated
biphenyls. Thus, it is the latter that are particularly concentrated
in cow's milk fat. From studies in the Federal Republic of Germany, it
was found that the major congeners in cow's milk were numbers 138,
153, and 180 (DFG, 1988).
6.4.4.1 Elimination via breast milk
The composition of common commercial PCB mixtures clearly differs from
the composition of the PCB contents of human fat or human breast milk,
because of the preferential elimination of certain PCB congeners
containing 3 or 4 ortho substituents and the retention of PCBs with
1 or 2 ortho substituents (Kuroki & Masuda, 1977; Watanabe et al.,
1979; Yakushiji et al., 1979).
The major PCB components (and average relative concentrations) that
have been identified in breast milk in the Osaka area in Japan
include: 2,4,4'-trichlorobiphenyl (8.4%); 2,5,2',5'-tetrachloro-
biphenyl (2.0%); 2,4,5,4'-tetrachlorobiphenyl (19%); 2,4,5,2',5'-
pentachlorobiphenyl (2.8%); 2,4,5,3',4'-pentachlorobiphenyl (11.8%);
2,4,5,2',4',5'-hexachlorobiphenyl (15.5%); 2,3,4,2',4',5'-
hexachlorobiphenyl (15.8%); 2,3,4,3',4',5'-hexachlorobiphenyl (2.3%);
2,3,4,6,2',4',5'-heptachlorobiphenyl (1.6%); 2,3,5,6,2',4',5'-
heptachlorobiphenyl (3.2%). These PCB-congeners constituted at least
95% of the PCBs in the breast milk of the women examined in Osaka.
In recent studies, the contents of PCBs in human milk and maternal
blood were compared for US citizens (Bush et al., 1984, 1985). Eight
individual PCB congeners comprised 52% of the total PCB residues in
the milk and 48.5% in the blood. The mean concentrations for total
PCBs were 26.5 µg/kg for whole milk and 3.5 µg/kg for blood. The
percentages of the different congeners are given in Table 25.
6.5 Metabolic transformation
6.5.1 PCBs
The metabolism of PCBs has been investigated in numerous studies on
animals and reviewed by Drill et al. (1981) and the US EPA (1987). The
PCBs were usually administered by the oral or parenteral route.
Phenolic products are the major PCB metabolites, though
sulfur-containing metabolites, trans-dihydrodiols, polyhydroxylated
PCBs, and methyl ether derivatives have also been identified. Although
the effects of the chlorine substitution pattern on sites of oxidation
have not been studied systematically, US EPA (1987) suggested the
following:
* hydroxylation is favoured at the para position in the least
chlorinated phenyl ring, unless this site is sterically hindered
(i.e., 3,5-dichloro-substitution);
* in the lower chlorinated biphenyls the para position of both
biphenyl rings and carbon atoms that are para to the chloro
substituent are all readily hydroxylated (Sparling et al., 1980);
* the availability of 2 vicinal unsubstituted carbon atoms
(particularly C5 and C4 in the biphenyl nucleus) also facilitates
the oxidative metabolism of the PCB substrate, but is not a
necessary requirement for metabolism;
Table 25. Concentrations of most abundant PCB congeners present in whole breast milk and maternal blooda
Congener Milk Maternal blood Ratio
(40 samples) (101 samples) milk/blood
µg/litre % of µg/litre % of
total PCBs total PCBs
2,4,5,2',4',5'-hexachlorobiphenyl 3.2 12 0.31 8.8 10
2,3,5,6,2',3',6-heptachlorobiphenyl 2.5 9.4 0.27 8.0 9.2
2,4,5,2',3',4'-hexachlorobiphenyl 2.1 7.8 0.58 17 3.5
2,5,3'4'-tetrachlorobiphenyl 1.7 6.6 0.01 - 500
2,3,4,5,2'4'5'-heptachlorobiphenyl 1.2 4.5 0.03 3.7 9.4
2,3,4,5,3',4'-hexachlorobiphenyl 1.0 4.0 0.01 - 125
2,4,5,2',4'-pentachlorobiphenyl 1.1 4.0 0.12 3.4 8.9
2,3,4,3',4'-pentachlorobiphenyl 0.97 3.7 0.25 7.6 3.8
Total PCBs 26.5 - 3.5 - 7.5
a Modified from: Bush et al. (1985).
* as the rate of chlorination increases on both phenyl rings, the
rate of metabolism decreases;
* the metabolism of specific PCB isomers by different species can
result in considerable variations in metabolic pattern.
Kannan et al. (1989) studied the possible involvement of frontier
(pi) electrons in the metabolism of polychlorinated biphenyls. The
electron density, at each carbon atom, of the highest occupied pi
orbital of 13 PCB molecules was calculated and the result was compared
with their in vitro and/or in vivo metabolism. It was found that:
* the carbon position at which the frontier electron density was the
highest was most readily hydroxylated or sulfonated;
* if the carbon with the highest frontier (pi) electrons was
occupied by chlorine, either a replacement occurred or the carbon
with the next highest electron density was activated for
metabolism;
* because of steric hindrance, "ortho" carbons were least
preferred for such reactions, in spite of possessing favourable
electron density;
* this was applicable to both phenobarbital (PB)-type and
3-methylcholanthrene (3-MC)-type PCB inducers.
The authors suggested that frontier (pi) electron density could be
an easy guide for understanding the metabolic products of persistent
and toxic environmental pollutants in vitro and in vivo, and for
understanding their environmental fate.
There appears to be little metabolism of PCBs with 6 or more chlorine
substituents (Matthews & Anderson, 1975b). When between 2 and 5
chlorine substituent PCBs are metabolized, the metabolic products are
primarily hydroxylated compounds, frequently found as glucuronide
conjugates (hydroxymethoxy derivatives) and partially dechlorinated
metabolites. In some cases, smaller amounts of dihydrohydroxy
compounds and related substances are also found.
The parent compound is also eliminated in various quantities in
faeces, hair, and maternal milk, but very little unmetabolized
compound is excreted in the urine. This pattern is not unusual for
lipophilic xenobiotics.
PCB metabolism has been examined in primates (monkeys) by Greb et al.
(1975), Hsu et al. (1975a,b), and Allen & Norback (1976); in ungulates
(cows, pigs, and goats) by Platanow & Chen (1973), Safe et al. (1975),
and Gardner et al. (1976); in rats by Grant et al. (1971a), Hutzinger
et al. (1972), Yoshimura et al. (1973), Goto et al. (1973, 1974,
1975), Safe et al. (1974), Matthews & Anderson (1975b), van Miller et
al. (1975), Sundström & Jansson (1975), Sundström et al. (1976a), Lay
et al. (1975, 1979), Chen et al. (1976), Kamal et al. (1976), and
Norback et al. (1976); in mice by Berlin et al. (1973), Yamamoto &
Yoshimura (1973), and Sundström & Jansson (1975); in rabbits by Grant
et al. (1971b), Hutzinger et al. (1974), Sundström & Wachmeister
(1975), and Sundström et al. (1976b); in pigeons, and quails by Koeman
et al. (1969), Hutzinger et al. (1972), Bailey & Bunyan (1972), and
Sundström & Jansson (1975); and in trout by Hutzinger et al. (1972).
The different metabolic products formed from pure isomers in these
various species have been catalogued in an NAS report (1979) and in a
review by Sundström et al. (1976a). Neither of these reports is
complete, but, together, they cover most of the studies up to 1979.
In the rat, monochloro-, dichloro-, trichloro-, tetrachloro-,
pentachloro-, and at least one hexachlorobiphenyl, yielded at least
one hydroxylated metabolite. Some isomers produced as many as 5
different hydroxylated metabolites including both mono- and dihydroxy-
derivatives. Most of the hexachloro-, octachloro-, and
decachlorobiphenyls did not yield detectable levels of hydroxylated
products.
Similar hydroxylated derivatives were also produced in other species,
but the ability to metabolize PCBs is not absolutely uniform in all
species. In the rabbit, dichloro-, tetrachloro-, and
hexachlorobiphenyls were metabolized, while further down the
phylogenetic scale, the pigeon only metabolized monochloro- and
dichlorobiphenyls and the trout failed to metabolize any of the
chlorinated biphenyls tested. Table 26 shows the PCBs tested in
different species and indicates whether or not the organism was able
to metabolize the compound. Although different species may metabolize
a given isomer, the metabolic products are not necessarily identical.
An example of this is found in the simple 4,4'-dichlorobiphenyl which
is metabolized by the rat, rabbit, and goat, but does not give
identical products in these species; all 3 species produce
4,4'-dichloro-3-hydroxybiphenyl as a metabolite, but, in addition, the
rat produces 4,4'-dichloro-, 2,3-dihydroxybiphenyl, and the goat
produces 3,4'-dichloro-4-hydroxybiphenyl as a metabolite.
However, a product such as the 3,4'-dichloro-4-hydroxybiphenyl found
in the goat involves a chlorine shift, which may be indicative of a
more toxic intermediate.
Many different pathways of metabolism have been described as
summarized in Fig. 5 (Safe, 1984; WHO/EURO, 1987).
These pathways include hydroxylation, and conjugation with thiols and
other water-soluble derivatives. The most important pathway seems to
be through hydroxylation and subsequent conjugation. Rats and mice
that were exposed to dichloro-, tetrachloro-, or pentachlorobiphenyls
by intraperitoneal injection or diet, eliminated metabolites as
glutathione conjugates and other sulfur-containing compounds (Kurachi,
1983; Kurachi & Mio, 1983). Mammalian metabolism of many individual
PCBs may proceed via oxide intermediates, which have not been
isolated, but are presumed to be precursors of some of the major
metabolites identified. One type of metabolite is the methylsulfone
PCB metabolite that has been identified in environmental samples by
Jansson et al. (1975) and WHO/EURO (1987), and in human milk by
Yoshida & Nakamura (1979).
The formation of xenobiotic thioether derivatives, including
glutathione, cysteinylglycine, cysteine, and N-acetylcysteine
(mercapturic acid) conjugates, is generally considered a pathway for
the detoxification of reactive intermediates. Mio & Sumino (1985)
detected methylsulfonyl metabolites, by using GC/MS/COM, from the
adipose tissues of mice treated with Kanechlor 300, 400, 500, or
2,5,2',5'-tetrachlorobiphenyl. Metabolites were detected in the faeces
of mice treated with 2,5,2',5'-tetrachlorobiphenyl, e.g., 6
sulfur-containing and 5 non-sulfur-containing metabolites. The
elimination rates for one week were 1.7% and 43%, respectively. The
methylsulfonyl metabolites accumulated in the liver, adipose tissue,
and lungs. Mio & Sumino (1985) proposed the methylsulfonyl metabolic
pathway of 2,5,2',5'-tetrachlorobiphenyl.
Klasson-Wehler et al. (1987) administered a single dose of 2,3,6,4'-
tetrachlorobiphenyl to 3 groups of 5 female C57B1 mice at 0, 10, or
100 mg/kg body weight. 35S-cysteine was administered by ip injections
4 times at 12-h intervals. The animals were sacrificed and the organs
analysed on day 12. Methyl [35S]sulfonyl-tetrachlorobiphenyl was
found in the lungs, kidneys, and fat of the treated mice, as well as
minor amounts of tetrachlorobiphenyl and traces of
methylthiotetrachlorobiphenyl.
The formation of serial methylsulfonyl metabolites can be summarized
as follows: the glutathione conjugate is converted, by cleavage, to a
cysteine or thiol conjugate and translocated into the liver. The thiol
conjugate from the cysteine moiety is transmethylated by
thiol- S-methyltransferase and is oxygenated by cytochromes P-450 and
P-448 oxidase or is glucuronidated by UDP-glucuronyl-transferase in
the liver, resulting in methylsulfonyl derivatives.
Table 26. Metabolism of various PCBs in different organisms
Compound Species
Chlorobiphenyl Trout Pigeon Mouse Rat Rabbit Monkey
4-mono- - + + +
4,4'-di- - + + +
2,2' +
2,4' + +
2,5,2'-tri +
2,4,2',4'-tetra- +
2,5,2',5' - - + + +
3,4,3',4' -
2,3,4,5,6,-penta- +
2,4,5,2',5' + +
2,4,6,2',4' +
2,4,6,2',6' +
2,4,6,3',5' +
2,3,4,6,4' +
2,3,4,3',4' -
2,4,5,2',4',5',-hexa- - - (±)a +
2,4,6,2',4',6' (±)a
2,3,5,6,2',3',5',6'-octa- -
2,3,4,5,6,2',3',4',5',6'-deca -
a These compounds were reported by Sundström et al. (1976a) as failing to produce
hydroxylated derivatives, but were positive in an IARC report referred to in an
NAS report (NAS, 1979).
+ = Compound is metabolized; - = Compound not metabolized: (blank) not tested.
Table 20. Concentrations of PCBs in the body fat of the general population
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
North America
USA (18 states) - 637 < 1 (68.9%)e Yobs (1972)
< 1-2 (25.9%)e Price & Welch (1972)
> 2 (5.2%)f
Northeast Louisiana 1980 8 1.04 (0.38-2.33) Holt et al. (1986)
1984 10 1.23 (0.65-1.44)
Texas 1969-1972 88 (15 positive) 1.7 (0.6-9.9) Burns (1974)
New York - 101 (women) 3.4 ± 1.1 Bush et al. (1984)
(urban and rural
vicinity)
Canada - 99 0.94 (0.04-6.8)a Mes et al. (1982)
Ontario 1976 and 570 2.1-2.2 Frank et al. (1988)
1984
Table 20. (cont'd).
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
Asia
Japan (Kochi area) - - 2.86 (maximum 7.5) Nishimoto et al. (1972a,b)
Japan 1971-1982 - 0.5-6.0a Katsunuma et al. (1985)
Tokyo 1974 30 1.04 (0.38-2.5) Fukano & Doguchi (1977)
Japan - 241 0.30-1.48 Curley et al. (1973b)
New Zealand - 51 0.82 Solly & Shanks (1974)
Africa
South Africa 1982 63 0.15-5.18 van Dijk et al. (1987)
Europe
Austria (Vienna area) - 32 0.3-7.3 Pesendorfer et al. (1973)
Finland - 105 0.2 Mussalo-Rauhamaa et al.
(1984)
Germany, Federal - 20 5.7 Acker & Schulte (1970)
Republic of - 282 8.3 Acker & Schulte (1974)
1982-1983 50b 0.5-1.5 Niessen et al. (1984)
Italy (Siena) 1983-1984 26 1.75c (dry weight) Focardi et al. (1986)
Table 20. (cont'd).
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
Netherlands 1973-1983 24-78 per year 1.6-2.5d Greve & van Harten
(1983a);
Greve & Wegman (1983,
1984)
Norway (Oslo) - 40 1.6 Bjerk (1972)
Spain 1985-1987 14 1.68 Camps et al. (1989)
United Kingdom - 201 < 1.0 Abbott et al. (1972)
1976-1977 236 0.7 (nd-10) HMSO (1986)
1982-1983 187 0.9 (0.1-6.9)
a Wet weight.
b 34 infants, 14 children, and 2 older children.
c About 60% included only five congeners: Nos. 118, 138, 153, 170, 180.
d Median.
e Percentage of samples.
5.5.1.1 PCBs in the fetus
PCBs are also present in serum and all organs of the body in
proportion to their fat content. PCBs pass more, or less (depending on
structure and chlorination), through the placenta into the fetus.
Since the fetus has little adipose tissue until 7 months of age, PCB
concentrations may be higher in vital organs, such as the adrenal
gland, but available data suggest somewhat lower levels in the brain
(Masuda et al., 1978a; Kodama & Ota, 1980).
Masuda et al. (1978a) found PCB levels of 270-960 µg/kg fat in adipose
tissue samples of fetuses beyond 7 months of gestation. Levels in the
adipose tissue of adult females from the same geographical area ranged
from 270 to 1360 µg/kg fat. The mean concentrations were 470 µg/kg for
fetuses and 780 µg/kg for adult females. However, since the ranges
showed an overlap and the number of samples was small, it is not clear
whether this represents a true difference.
5.5.1.2 Congeners in adipose tissue
Wegman & Berkhoff (1986) investigated the presence of the different
congeners in 24 human fat samples, collected in 1984. The following
congeners were present at the highest levels: 2,4,4'-trichloro-,
2,4,5,2',5'-pentachloro-, 2,4,5,3',4'-pentachloro-, 2,3,4,2',3',4'-
hexachloro, 2,3,4,2',4',5'-hexachloro-, 2,4,5,2',4',5'-hexachloro-,
2,3,4,5,2',4',5'-heptachloro, 2,3,4,5,2',3',4',5'-octachloro, and
2,3,5,6,2',3',5',6'-octachlorobiphenyl.
Focardi & Romei (1987) analysed 30 samples of adipose tissue,
obtained from patients in Siena, Italy, in 1986, for the presence of
19 PCB congeners. The results indicate that the mean PCB (as sum of
the congeners) concentration was 1063 µg/kg dry weight (range
391-1918 mg/kg). The major constituents of the PCBs (about 60%) were
the isomers 99, 138, 153, 170, and 180.
Human adipose tissue was analysed for 3 non- ortho chlorine
substituted coplanar congeners: 3,4,3',4'-tetrachloro-, 3,4,5,3',4'-
pentachloro- and 3,4,5,3',4',5'-hexachlorobiphenyl (Kannan et al.,
1988). Twelve samples, from 7 male and 5 female persons were obtained
from hospitals. The average total PCB concentrations were 1.22 and
1.02 mg/kg (wet weight basis), respectively. The concentrations of the
3 congeners were 94-860, 120-730, and 36-200 ng/kg, on a wet weight
basis, respectively.
5.5.2 Blood of the general population
Finklea et al. (1972) studied human plasma of different races of the
population (723 volunteers with ages ranging up to 60 years) of urban
and rural areas of South Carolina. The average concentration was
5 µg/litre (range 0-29 µg/litre). No age effect was found, but ethnic
differences and ethnic residence interactions were significant. Kreiss
(1985) found mean serum concentrations in the non-occupationally
exposed population in the USA, of between 4 and 8 µg/litre, with 95%
of the individuals having serum PCB concentrations of less than
20 µg/litre. More data are summarized in Table 21.
Maternal blood and fetal cord blood were collected from volunteers
from an urban and rural vicinity in upstate New York. Whole blood
samples were taken from 101 women (26 ± 4 years) entering maternity
facilities. Maternal blood contained 3.4 ± 1.1 µg PCBs/kg and fetal
cord blood contained 2.4 ± 1.0 µg/kg whole blood. The PCB congeners
making up these totals were surprisingly few; 38% of the total residue
in the maternal blood and 21% of the fetal cord blood comprised only 4
components, 2,4,4'-trichlorobiphenyl, 2,4,5,2',4',5'-hexachloro-,
2,3,4,2',4',5'-hexachloro-, and 2,3,5,6,2',3',6'-heptachlorobiphenyl.
The congener 2,5,2',5'-tetrachlorobiphenyl crossed the placenta
preferentially (Bush et al., 1984).
The concentrations of PCBs were determined in blood samples from 120
women hospitalized for miscarriages and 120 full-term pregnancy
controls. The average PCB level was higher in women with miscarriages
than in control women (8.65 µg/litre and 6.89 µg/litre, respectively,
as Fenclor 54 and 14.81 and 14.90 µg/litre, respectively, as
decachlorobiphenyl). The reproductive history of each woman was
assessed together with confounding variables and with environmental
exposure and food intake. Food consumption did not indicate diet as
the main source of PCB intake (Leoni et al., 1989).
A cross section of the population of Michigan was studied following an
accidental exposure in 1978. Five years after the accident, PCB and
PBB residues were measured in adipose tissue and serum. Serum levels
of PCB were measured in 1681 adults and 1462 children. Children (430)
were found to have uniform levels throughout the state (mean
concentration 4 ± 2 µg/litre). In adults, the serum PCB levels were
higher in the area with highest PBB levels. The mean serum PCB level
was 21 µg/litre, compared with control levels for the rest of the
state of 9 µg/litre. No sex difference was found (Wolff et al.,
1982a).
Table 21. Concentrations of PCBs in whole blood of the general population
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Canada
Ontario area 1975-1976 118 18 Frank et al. (1988)
(patients suspected 1980-1981
of being exposed 1984
dermally)
Japan
- - 3.2 Doguchi & Fukano (1975)
- 28 (women) 2.6 Kuwabara et al. (1978)
(Osaka area) 1976 16 (women) 2.8 (1.7-4.6) Kuwabara et al. (1979)
1972-1977 - 3-4 Yakushiji et al. (1977)
farmers 1978-1983 - trace-21.4b Katsunuma et al. (1985)
Tokyo 1973 27 3.19 (2.2-5.1) Fukano & Doguchi (1977)
1975 10 2.59 (1.8-3.8)
Table 21. (cont'd).
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Finland - 3.1-12 Karppanen & Kolho (1973)
Netherlands - 34 (women) 4.5 (nd-11.6) Blok et al. (1984)
31 (men) 4.8 (1.0-17.1)
1978 48-127 3.1e Greve & Wegman (1983,
1980 samples/year 3.5 1984)
1981 4.4
1982 4.4
North America
South Carolina 1968 723 5 (4.2-5.5)a Finklea et al. (1972)
(urban and rural
area)
Michigan 1973 1100 56c Kreiss (1985)
(areas of Lake 1979-1981 17.2-23.6c
Michigan)
Lake Michigan 1985 196 5.5 ± 3.7 Schwartz et al. (1983)
(high fish
consumption)
Table 21. (cont'd).
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Yugoslavia 1984-1986 10f 155 (35-480)d Jan & Tratnik (1988a)
(residents around 19g 11 (6-18)
River Krupa; 4h 5 (2-7)
contamination by a
plant using PCBs)
a Plasma.
b Serum.
c Geometric mean.
d Arithmetic mean.
e Median concentration.
f Living close to plant.
g Living 1-3 km from plant.
h Non-exposed other areas.
Specific PCB isomer levels in the blood of 30 children, ages 2-5
years, residing in an area of PCB-contaminated soil in Canada, were
compared with those of 25 children in a non-contaminated area. The sum
of individual PCB isomer levels in the exposed and non-exposed group
were not significantly different, e.g., 0.54 µg/litre (range
0.22-0.99 µg/litre) and 0.88 µg/litre (range 0.28-2.30 µg/litre). The
major component in both groups was 2,4,5,2',4',5'-hexa-chlorobiphenyl
(Mes, 1987).
High levels of PCBs were found in the blood (up to 100 µg/litre) in
patients with severe weight loss (Hesselberg & Scherr, 1974). This was
attributed to the release of PCBs from the mobilization of fat.
Greve & van Harten (1983b) studied the relationship between the levels
of PCBs in the adipose tissue and in the blood of the same persons. A
total of 48 persons were involved in this study. A concentration
factor (concentration in adipose tissue divided by concentration in
blood) of 660 was found.
5.5.3 Human milk
Human milk is the major source of exposure for breast-fed infants. The
amount of human milk secreted varies widely. The composition of the
milk is related to the amount secreted, the stage of lactation, the
timing of withdrawal (early or late in feeding) and to individual
variations among lactating women. The individual variations depend on
maternal age, health, social class, and diet. The concentration of
PCBs depends primarily on the lipid concentration in milk. Wide
variations in published results are caused by inaccuracies inherent in
the analytical methods used for the quantification of lipids, and
whether the milk sample is collected early or late during the feeding
period. The fat content increases during emptying, and the fat content
of milk from the 2 breasts may differ. According to a recent
determination, the fat level in human milk averages 2.6-4.5%
(WHO/EURO, 1988).
Whether the differences in concentration in various countries are
merely a function of the analytical methods used and the type of
samples collected or whether true differences in body burden exist, is
not clear at present. For instance, some countries have reported
levels of PCBs in human milk fat ranging from nondetectable to
14 mg/kg, while, in other countries, the highest levels found have
been around 3 mg/kg. Because of these variations, calculating an
average dose for nursing infants is difficult. The same difficulties
exist when attempts are made to investigate trends over time
(WHO/EURO, 1988).
The results of the older studies have been obtained with a less
sophisticated method using packed column GC. With this method only a
dozen peaks can be separated. The quantitative results are reported as
"total PCB values", though different techniques of quantification and
different types of calculations were used.
In contrast with the situation with many organochlorine insecticides,
the levels of PCBs in human milk fat are higher in European countries,
Japan, and the USA than in China (Slorach & Vaz, 1983, 1985), and are
significant, particularly in the highly industrialized countries.
Results from a large number of countries have been summarized by
Jensen (1983a, 1985, 1987), Acker et al., (1984), Katsunuma et al.
(1985) (especially Japanese data; period 1972-83); and WHO/EURO,
(1987, 1988). The countries concerned are: Argentina, Austria,
Belgium, Canada, Finland, France, Federal Republic of Germany (Klein,
1983), German Democratic Republic, Israel, Japan, the Netherlands,
Norway, Poland, Romania, South Africa, Sweden, Switzerland, Turkey,
United Kingdom, USA, USSR, and Yugoslavia. The average levels of PCBs
in human milk do not appear to differ very much between the
industrialized countries and range between 0.5 and 2 mg/kg milk fat,
except in Czechoslovakia, the Federal Republic of Germany, India,
Denmark and Italy, where levels up to 3 mg/kg milk fat were found
(Jensen, 1983b; Acker et al., 1984) (Table 22).
The variation in residue levels in human milk during lactation was
investigated in 5 women in the Federal Republic of Germany. Month-mix
samples, composed of breast milk samples collected weekly, were
analysed over a lactation period of between 5 and 9 months. The ages
of the women ranged from 23 to 36 years. The PCB concentrations were
between 0.61 and 2.20 mg/kg, on a fat basis. While the concentrations
remained relatively constant, some fluctuations were seen but no trend
was observed over the lactation period investigated (Fooken & Butte,
1987).
Breast milk samples from 16 women in Canada were analysed for PCBs at
8 intervals (7, 14, 28, 42, 56, 70, 84, and 98 days) during the
lactation period. The average PCB concentrations in breast milk varied
between 22.8 and 29.7 µg/kg whole milk. No clear decrease or increase
was observed. The average milk/blood ratio for PCBs was 23 and
remained relatively constant during lactation (Mes et al., 1984).
Wolff (1983) reported the half-life of PCBs (percentage chlorine not
specified) in breast milk to be 5-8 months and found that the
concentration of PCBs in breast milk was 4-10 times that in the
maternal blood. Similar results were reported by Jacobson et al.
(1984b).
Table 22. Concentrations of PCBs in breast milk of the general population
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
North America
USA (Michigan) 1977-1978 1057 1.5 (maximum 5.1) Wickizer et al. (1981);
Wickizer & Brilliant (1981)
Canada (Quebec) - 154 0.84 (nd-4.34) Dillon et al. (1981)
Ontario 1971-1974 - 1.2 (0.1-3.0) Atkinson (1979)
1978 215 0.6 ± 0.3
Ontario 1975-1985 348 0.023 (0.016-0.033)a Frank et al. (1988)
Five regions across Canada 1982 210 0.697 Mes et al. (1986)
Regina, Saskatchewan 1979 80 0.0052 (0.001-0.019)a Qureshi & Robertson (1987)
Table 22. (cont'd).
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
Asia
Japan (Osaka) 1972-1977 - 0.030-0.040a Yakushiji et al. (1977)
1969-1976 19-52 each year 1-2 Yakushiji et al. (1979)
India (Ahmedabad) 1981-1982 50 not present Jani et al. (1988)
Hawaii (different islands) 1979-1980 54 0.80 ± 0.43 (0.13-2.2) Takei et al. (1983)
Europe
Germany, since 1970 several thousands 1.0-2.5 (98% of samples Acker et al. (1984);
Federal Republic of between 0.001-7.2) Cetinkaya et al. (1984);
Heeschen et al. (1986);
Lorenz & Neumeier (1983)
- 2709 1.77 Fooken & Butte (1987)
Netherlands 1983 278 0.72 (0.27-2.20)b Greve et al. (1985);
(11 centres country-wide) Greve & Wegman (1984)
1977-1979, 2649 2.1 Olling (1984)
1981
United Kingdom (Scotland) 1979-1980 30 0.01 (nd-0.04) HMSO (1986)
1983-1984 30 < 0.01 (nd-0.02)
Table 22. (cont'd).
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
Italy (Rome) 1983-1985 65 0.070 (0.007-0.176)a,c Dommarco et al. (1987)
Finland (different parts) 1984-1985 183 (165 of 0.57 (0.05-10.7) Mussalo-Rauhamaa et al.
women) (1988)
Sweden (5 regions) - 300e 1.06-1.18 (four regions) Noren (1983)
1.44 (one region)
1972 227d 1.05 Noren (1988)
1976 245 0.99
1980 340 0.78
1984-1985 102 0.60
Austria (Vienna) - 22 1.54 (0.58-3.78) Pesendorfer (1975)
Other regions 9 1.29 (0.95-1.57) Pesendorfer (1975)
a Whole milk.
b Median concentration.
c Arithmetic mean.
d Number of mothers that provided 4-7 samples each (samples were pooled).
e In each region, 300 mothers gave breast milk 3-5 days after parturition.
In a study by Kuwabara et al. (1978), the relationship was
investigated between breast-feeding and PCB residues in the blood of
children whose mothers were occupationally exposed to PCBs. The
children ingested their mother's milk for periods of < 1 to 3 years.
The age of the children at the time of the study ranged up to 13
years. The data provide evidence that PCBs are retained in the
children's body for many years and that longer intake of mother's milk
tends to increase PCB levels in the blood of the children. The PCB
levels in the blood of the 20 occupationally-exposed women and their
39 children ranged from 8.3 to 84.5 and 0.8 to 93.2 µg/litre,
respectively.
The results suggest that the PCB levels in the blood of children are
much more influenced by the transportation of PCBs through the
mother's milk than through the placenta. Furthermore, it was found
that the gas chromatographic patterns of the blood PCBs of the
children, breast fed for a long time, were different from those of
their mothers. Blood from 16 non-occupationally exposed mothers and
their children (17), showed that, as the length of the breast-feeding
period increased, there was an increase in the PCB levels in the blood
of the children. The mean blood PCB level in mothers was 2.8 ±
0.8 µg/litre; in children, it was 3.8 ± 3.6 µg/litre. In this study,
no clear change in blood PCBs patterns between mothers and children
was observed (Kuwabara et al., 1979).
Samples of maternal blood, milk, and umbilical cord blood were
collected from 43 mothers giving birth to their first or second child;
all the mothers had lived in Oslo during the previous 2 years. Blood
samples were collected immediately after delivery, either by Caesarean
section (16 Norwegians) or normally (20 Norwegians and 7 immigrants).
Subcutaneous fat samples were obtained during the operation. Samples
of colostrum and milk were obtained 3 and 5 days postpartum. PCBs were
found in 135 of the total 168 samples. In the Norwegian women and
infants, PCBs were the major contaminants, whereas only traces of PCBs
were found in the samples of immigrants. The average concentrations in
the maternal serum, cord serum, colostrum, and breast milk of
Norwegian women (Caesarean and normally delivered taken together)
were: 10, 3-5, 18-21, 20-23 µg/kg wet weight (Skaare et al., 1988).
5.5.3.1 Major PCB congeners in human milk
Commercial PCB preparations consist of complex mixtures of
environmentally stable compounds with a wide range of chlorine
contents. PCBs are transferred to breast-fed infants with the fat of
the mother's milk. Thus, infants nurtured on maternal milk are exposed
to relatively high concentrations of the higher chlorinated PCBs in
the short period preceding the full functioning of certain organs,
e.g., the liver (Jensen, 1983b; Slorach & Vaz, 1983; Gezondheidsraad,
1985).
Three major congeners were present in breast milk, e.g., PCB congener
numbers 138, 153, and 180 (DFG, 1988).
Slorach & Vaz (1983) reported that the GC patterns of PCBs in breast
milk samples from different countries were similar. The peaks denoted
146, 174, and 180 were dominant in the gas chromatograms. The total
levels of PCBs and the concentrations of certain congeners in Swedish
human milk, sampled in 1972-89, were studied by Noren et al. (1990).
Minor changes in the distribution of the congeners were found over the
period of study. The most abundant of the non- ortho coplanar PCBs in
Swedish human milk was 3,4,5,3',4'-pentachlorobiphenyl (126), with
levels decreasing from 0.35 µg/kg milk fat (1972) to about 0.10 µg/kg
(1989).
Safe et al. (1985a) analysed a sample of breast milk using the
congener-specific PCB method and found the following major components:
2,4,4'-trichloro-; 2,4,5,4'-tetrachloro-; 2,4,5,2',4'-pentachloro-;
2,4,5,3',4'-pentachloro-; 2,3,4,5,2',5'-hexachloro-;2,4,5,2',4',5'-
hexachloro; 2,3,4,5,2',3',4'-heptachloro-; and 2,3,4,5,2',4',5'-
heptachlorobiphenyls.
The major PCB congeners in the breast milk of Japanese women from the
general population were: 2,4,4'-trichloro-; 2,4,3',4'-tetrachloro-;
2,4,5,3',4'-pentachloro-; 2,3,4,2',3',4'-hexachloro-;2,3,4,5,2',4'-
hexachloro-; and 2,3,4,5,2',4',5'-heptachlorobiphenyls. The congeners
were present in 5% or more samples; a few other congeners were present
in only 1-3% (Gyorkos et al., 1985; Jensen, 1983b).
Sixty-eight breast milk samples collected in the Netherlands were used
to determine the congener distribution. The indicator congeners,
present in the highest concentrations, were: 2,4,4'-trichloro-,
2,4,5,2',5'-pentachloro-, 2,4,5,3',4'-pentachloro-,
2,3,4,2',4',5'-hexachloro-, 2,4,5,2',4',5'-hexachloro-,
2,3,4,5,2',4',5'-heptachlorobiphenyl (Wegman & Berkhoff, 1986).
Schecter et al. (1989a) analysed a total of 17 samples of human milk
from Thailand and Vietnam, for the presence of PCB congeners. The main
congeners that were present were 138, 153, and 180 (each in the range
of 8-31 µg/litre). The other congeners, normally present, were all
below the detection limit of 2 µg/litre.
In a study on pooled human milk samples from a 1982 nation-wide survey
in Canada, Mes & Marchand (1987) compared the relative amounts of 29
selected PCB isomers with amounts in milk samples of unexposed Rhesus
monkeys. In the pooled milk sample, 397 µg PCBs/litre, on a fat basis,
were found and the PCB isomer numbers 74, 99, 118, 138, 153, and 180
were the main contributors. Most of the predominant PCB isomers in
human milk were also observed in monkey's milk, but monkey's milk had
relatively low levels of PCB isomers numbers 74 and 99.
In another study, Davies & Mes (1987) analysed breast milk samples
from Canadian, Indian, and Inuit (Eskimo) mothers in Canada. The 18
samples were received from 5 Indian and Inuit nursing zones. The
combined total PCB isomer level (on a whole-milk basis) of the native
population was comparable with that of the national population. Even
the levels of the 5 largest PCB congeners (Nos. 74, 118, 138, 153, and
180) were comparable.
Individual congeners in the blood of Yusho- and Yu-Cheng patients are
discussed in section 5.6.
5.5.3.2 Factors that influence the intake of PCBs with milk
Present data suggest that the PCB content of human milk varies
considerably from individual to individual.
Many factors affect the level of PCBs and other organochlorine
compounds in breast milk including the fat content of the milk; time
from start of lactation; mother's age; mother's body weight; parity;
number of children previously breast-fed; origin and residence; eating
habits; season; smoking; use of household products; amount of milk;
and exposure at work (WHO/EURO, 1985, 1988).
In a given woman's milk, there are fluctuation in the PCB levels in
whole milk and in milk fat during one nursing session and during the
day (Jensen, 1983b). A decrease of PCB levels in both milk and milk
fat has been found during the lactation period. Furthermore, the PCB
concentration in human whole milk and milk fat increases with the age
of donor. Another confounding factor is that the PCB levels decrease
with increasing numbers of deliveries and lactations (Greve et al.
1985); lactation serves as a period for the biological elimination of
PCBs (Jensen, 1983b). The PCB levels in human milk are higher in
heavily populated and industrialized areas than in rural areas.
Furthermore, in general, the PCB levels in the breast milk of women
from developing countries are lower (Jensen, 1983b).
Cetinkaya et al. (1984) studied the PCB levels in human milk samples
from all over the Federal Republic of Germany. At the same time, data
were collected by means of a detailed questionnaire on residency,
workplace, smoking, drinking and eating habits, and the age of
participating individuals.
The breast milk of 45 women consuming lacto-vegetarian food was
compared with that of 41 women consuming conventional food in the
Federal Republic of Germany in the period 1979-81. The PCB
concentration was comparable, e.g., 2.2 and 2.5 mg/kg, on a fat basis,
respectively (Acker et al., 1984).
Fish consumption was positively correlated with PCB levels in maternal
serum and breast milk. PCB levels in serum increased with age, but
were unrelated to social class, parity, or body weight (Schwartz et
al., 1983).
Eight hundred and one Wisconsin anglers were surveyed for fishing and
consumption habits in 1985. The mean annual number of sport-caught
fish meals was 18 (range 7.1 to 33.3). The mean number of
non-sport-caught fish meals was 24. The median PCB serum congener sum
level for 192 anglers was 1.3 µg/litre (range, nd to 27.1 µg/litre).
Statistically significant positive Spearman correlations were observed
between sport-caught fish meals and PCB levels in serum and between kg
of fish caught and PCB levels in serum (Fiore et al., 1989).
PCBs were measured in maternal serum, cord blood, placenta, and serial
samples of breast milk and colostrum, from 868 women in North Carolina
(USA). Forty-three per cent of the women were primiparous. Breast milk
was collected at 6 weeks, 3 months, and 6 months, and, in a few cases,
up to 18 months postpartum. The median PCB concentration in breast
milk decreased during the sampling period from 1.77 to 1.02 mg/kg, on
a fat basis. The PCB concentration dropped by about 20% over 6 months
and 40% over 18 months. This implies that excretion in milk is a major
factor in lessening the mother's body burden; however, it also implies
substantial exposure of the child. Colostrum contained a median value
of 1.74 mg/kg. PCBs concentrations were higher in milk than in serum
and higher in maternal serum than in the placenta. The levels in cord
blood were almost always below the limit of quantification. Older
women and women who regularly drank alcohol had higher PCB levels in
their milk; blacks had higher levels than whites. In general, women
had higher levels in their first lactation and in the earlier samples
of a given lactation, and levels declined both with time spent
breast-feeding and with number of children nursed (Rogan et al.,
1986a).
Two hundred and forty-two newborn infants of mothers who consumed
moderate quantities of contaminated lake fish and 71 infants whose
mothers did not eat such fish were examined during the immediate post
partum period. PCB exposure was correlated with lower birth weight and
smaller head circumference, and the authors claimed that these effects
were not attributable to any of 37 potential confounding variables,
including socioeconomic status, maternal age, smoking, etc. (Fein et
al., 1984).
The mother's diet may be an important determinant of the PCB levels in
her milk. In some areas of the world, the intake of PCBs from eating
contaminated fish has been claimed to be the most important source of
PCBs in human milk. Dairy products and meat may be contaminated via
natural food or feedstuffs (WHO/EURO, 1988).
In a pilot study on the course of the PCB concentration in human milk
during 6 months of lactation, some PCB determinants were studied in 23
women and their infants. The average PCB concentration in the milk of
14 mothers during a 6-month period amounted to 0.66 ± 0.12 mg/kg, on a
fat basis. In univariate analyses, the PCB concentration on a fat
basis was strongly associated with pre- versus post-pregnancy weight
gain, age, and occupation. After multiple regression analysis, the PCB
concentration on a fat basis remained significantly associated with
changes in weight gain. The pre-pregnancy Quetelet Index of the mother
(height/weight) and the estimated PCB content of the diet (fish) were
correlated with the PCB concentration, on a milk basis (Drijver et
al., 1988).
5.5.4 Other tissues
Schecter et al. (1989b) analysed the tissues of 3 patients from the
North American continent, with no known history of chemical exposure,
for the presence of PCB isomers. The total PCB concentrations in the 9
tissues studied were different. The highest levels were found in
adipose tissue, subcutaneous fat (range 86-423 µg/kg), adrenals
(25-103 µg/kg), liver (3-149 µg/kg), bone marrow (26 µg/kg), kidneys
(2-31 µg/kg); levels in the spleen, lung, and testes were below
12 µg/kg. Congeners present in the highest concentrations were numbers
28, 74, 118, 153, 105, 138, 183, and 180.
5.6 Accidental exposures (Yusho- and Yu-Cheng)
In 1968, a large number of persons in Japan were accidentally poisoned
by the consumption of a batch of rice oil contaminated with Kanechlor
400. A similar accident happened in the Province of Taiwan in 1979,
where the affected persons had also consumed rice-bran oil
contaminated with PCBs. The 2 cases of poisoning were called Yusho and
Yu-Cheng accidents, respectively (see section 9.1.2.1).
The average PCB concentration in the plasma of Yusho children was
6 µg/litre, compared with 3.7 µg/litre in controls. Breast-fed Yusho
children had higher levels than children not breast-fed (Abe et al.,
1975).
The concentrations of PCBs in the adipose tissue, liver, and blood of
Yusho patients, about 5 years after the outbreak, were 1.9 ±
1.4 mg/kg, 0.08 ± 0.06 mg/kg, and 6.7 ± .3 µg/litre, respectively.
These values were only about twice those of controls. The mean blood
PCB level of 278 persons involved in the Yu-Cheng accident was
89.1 µg/litre (range 3-1156 µg/litre). Six months after the exposure,
the concentrations of PCBs in the blood had decreased to
12-50 µg/litre. The mean blood concentration of 165 patients,
9-18 months after the onset of poisoning, was 38 µg/litre (range
10-720 µg/litre) (see section 9.1.2.1). The blood PCB level of some
Yu-Cheng patients (99 ± 163 µg/litre), was much higher than that of
the Taiwanese population (1.2 ± 0.7 µg/litre), one year after the
outbreak of the intoxication.
Chen et al. (1985) analysed the blood of 165 Yu-Cheng patients, 9-18
months after the onset of poisoning, and found 10-720 µg PCBs/litre
with a mean value of 38 µg/litre. The blood of 10 patients, 9-27
months after poisoning, contained 0.02-0.2 µg PCDFs/litre. The
PCDF-congeners found in tissues were the same as those found by Masuda
et al. (1985).
Seven PCB congeners including: 2,4,5,3',4'-pentachloro-; 2,3,4,3',4'-
pentachloro-; 2,4,5,2',4',5'-hexachloro-; 2,3,4,2',4',5'-hexachloro-;
2,3,4,5,3',4'-hexachloro-; 2,3,4,5,2',4',5'-heptachloro-; and
2,3,4,5,2',3',4'-heptachlorobiphenyls, were identified in the blood
and tissues of Yusho, Yu-Cheng patients and controls.
Major PCDF congeners identified in the tissues and blood of Yusho and
Yu-Cheng patients were 2,3,6,8-tetrachloro-; 2,3,7,8-tetrachloro-;
1,2,4,7,8-pentachloro-; 2,3,4,7,8-pentachloro-; and 1,2,3,4,7,8-
hexachlorodibenzofurans. The 2,3,4,7,8-pentachloro-compound was
predominant. The concentrations of PCDFs in the adipose tissue and
liver of Yusho patients were 6-13 µg and 3-25 µg/kg tissue,
respectively. No PCDFs could be detected in the controls. Besides PCBs
and PCDFs, 4-methylthio-2,5,2',5'-tetrachlorobiphenyl (concentrations
ranging from 0.1 to 1.4 µg/kg tissue) and 4-methylsulfone-
2,5,2',5'-tetrachlorobiphenyl (range 0.3-2.5 µg/kg tissue) were also
found (Masuda et al., 1985).
5.7 Occupational exposure
5.7.1 Accidental exposure
Though the volatility of the PCBs is low, they are found in rather
high concentrations in the workroom air in both the long-term open use
of PCBs and in temporary or acute events where evaporation into the
air is possible. The measured air concentrations of PCBs in long-term
exposure situations, such as the manufacturing of transformers or
capacitors, varied from 30 to 1000 µg/m3, depending on the year of
measurement and the factory concerned (Silbergeld, 1983).
In discontinuous work, such as the inspection and repair of
transformers and capacitors, levels of between 0.1 and 60 µg/m3 have
been observed (Wolff, 1985). PCB concentrations in the breathing zone
of workers in transformer repair and maintenance work varied between
0.01 and 24.0 µg/m3 (Moseley et al., 1982).
In the atmosphere of an electroindustrial plant in Bela Krajina,
levels in the manufacturing room, where the autoclave was emptied,
averaged 2000 µg/m3 (range, 1400-3200 µg/m3); an average of
80 µg/m3 (range 40-120 µg/m3) was found in the working environment
in capacitor manufacture (Jan et al., 1988b).
Digernes & Astrup (1982) determined the concentrations of PCBs in the
atmosphere of the workplace of data screen operators, because skin
rashes and eczema had been reported among the workers. The PCB
concentrations in the working atmosphere (3 samples: concentrations
ranging from 0.056 to 0.081 µg/m3) were about 50-80 times higher than
the maximum level of PCBs in 3 samples collected outside the building
(0.0005-0.001 µg/m3). The indoor and outdoor samples also differed
qualitatively. The indoor samples contained only Aroclor 1242, while
outdoor samples contained a mixture of Aroclor 1242 and 1254.
Acute emergency events may cause extremely high concentrations of PCBs
in the air, particularly in cases when PCBs are burnt or heated (fire,
short circuit with electric arcing, burning in welding, etc.). Levels
of up to 10 000-16 000 µg/m3 have been measured. In the case of
extensive leaks of unheated PCBs from capacitors, concentrations of
1900 µg/m3 have been measured in workroom air (Elo et al., 1985;
WHO/EURO, 1987).
In connection with fires and electrical explosions, due to short
circuits, PCBs may be decomposed at elevated temperatures varying from
a few hundred to 2000°C. Soot may be produced in large amounts,
consisting of particles that may contain PCB concentrations up to
5000-8000 mg/kg of soot (Elo et al., 1985; O'Keefe et al., 1985;
WHO/EURO, 1987).
When evaluating PCB exposure, it is important to take into account
skin absorption from surfaces and tools, in addition to exposure via
inhalation. Surface concentrations of PCBs in capacitor factories have
varied between 4 and 60 µg/m2, and, where PCB leaks have occurred,
levels of up to 30 mg/m2 have been measured. Where PCBs have been
used long-term, contamination levels of 1-2 µg/cm2 have been found on
tools and tables.
A transformer was found to have overheated and released an oily mist
containing PCBs and their pyrolysis by-products, in a Department
building in New Mexico. The transformer contained Askarel (87% Aroclor
1260 and 13% of a mixture of tri- and tetrachlorinated benzenes). The
3-storey building was extensively contaminated via the following ways:
* mist entered 2 rooms, adjacent to the basement in which the
transformer was located;
* direct spread of mist and fumes through stairways;
* air drafts created by open windows and exhaust fans, spreading
fumes throughout the building;
* foot traffic by employees and other persons;
* the exhaust vent of the transformer room, located near the intake
vents for the building's air-conditioning system.
Air samples obtained up to 14 h after the incident showed levels of
48 µg/m in the transformer vault and 20 µg/m3 in the room above the
vault. Wipe samples of surfaces showed PCB levels ranging from 30
million µg/m2 for grossly contaminated surfaces to 4700 µg/m2 for
surfaces without visible contamination.
Five to 7 days later, air and surface samples were analysed for
2,3,7,8-tetrachlorodibenzofuran (TCDF), which was found to be present
in the air at an average level of 48 µg/m3 in most contaminated
areas. In wipe samples, the levels ranged from 5 ng/m2 to
41.224 ng/m2. 2,3,7,8-Tetrachlorodibenzo- p-dioxin (TCDD) was not
detectable in either air samples (detection limit, 0.5-5.0 pg/m3 air)
or wipe samples (detection limit 180 ng/m2) (Anon., 1985).
Very high concentrations of these toxic chemicals may be found in soot
emitted in connection with fires and explosions in capacitors.
Thus, skin contamination, and the ingestion and inhalation of soot
particles, may result in serious exposure in PCB accidents and
emergencies.
A short-term, follow-up study was performed on 55 workers in a gear
plant, whose work did not involve the use of PCBs. Exposure was to the
total residual PCB left behind by a capacitor company that had
formerly (3 years before) used the site. Air samples contained
< 10 µg/m3 and mean concentrations in wipe samples ranged from 23 to
161 µg/100 cm2. The 38 workers had a mean PCB concentration in serum
of 14.4 and the 17 office workers, 4.8 µg/litre. When the PCB
determinations were repeated in the 2 following years, no clear
decrease was observed (Christiani et al., 1986).
5.7.2 Occupational exposure during manufacture and use
Occupational exposure occurs during the manufacture of PCBs as well as
during their use by the electrical industry. It may also be widespread
among mechanics in contact with lubricating oils and hydraulic fluids,
among workers exposed to varnishes and paints, and among office
workers who have contact with pressure-sensitive duplicating paper
(carbonless copying paper), some brands of which readily transferred
PCBs to skin (Kuratsune & Masuda, 1972).
5.7.2.1 Adipose tissue
Levels of PCBs in the adipose tissue of occupationally exposed workers
have been found to vary between 26 and 50 mg/kg (range, 2.2-290 mg/kg).
There is a strong correlation between the blood PCB concentration and
PCB levels in adipose tissue, but the distribution of the various
congeners between plasma and adipose tissue is not the same, as
described above.
Emmett (1985) found the following congeners in the adipose tissue of
present and past transformer workers exposed to Aroclor 1242 and 1254:
2,4,3',4',5'-pentachloro-, 2,3,4,3',4'-pentachloro-, 2,3,4,5,2',4'-
hexachloro-, 2,3,4,6,3',4'-hexachloro-, 2,4,5,3',4',5'-hexachloro-,
2,3,4,5,2',3',4'-heptachloro-, and 2,3,4,5,6,3',4'-hepta-
chlorobiphenyl.
5.7.2.2 Blood
Karppanen & Kolho (1973) analysed the blood of 26 persons, 9
non-exposed, 6 persons handling PCBs, and 11 persons employed for 4
years in a capacitor-manufacturing plant in Finland. In the latter
case, Aroclor 1242 was used. The average concentrations in the blood
of the 3 groups were 7.1 µg/kg (3.1-12 µg/kg), 49.5 µg/kg
(36-63 µg/kg), and 440 µg/kg (70-1900 µg/kg), on a wet weight basis.
More recent results of a Finnish control group of workers indicated
serum PCB levels of 1.2 ± 0.6 µg/litre in an industrial area (Luotamo
et al., 1985; WHO/EURO, 1987). With acute exposure to high
concentrations of PCBs in air (8000-16 000 µg/m3), for a short
period, blood PCB concentrations rose to levels of 30 µg/litre; a
return to the normal level of 3 µg/litre was achieved, 4 weeks after
termination of exposure (Elo et al., 1985; WHO/EURO, 1987).
Similar plasma values were found in workers from Japanese capacitor
factories, but, here, skin lesions were noted (Hasegawa et al.,
1972a). In this same study, it was reported that air levels of PCBs of
10-50 µg/m3 were measured in a factory where KC-300 was used in the
manufacture of electric condensers. PCB levels in the serum of workers
ranged from 100 to 650 µg/litre. One month after the use of PCBs had
been suspended, serum levels remained unchanged (90-740 µg/litre).
However, in another factory making electric condensers, serum levels
decreased from an average of 800 to 300 µg/litre, within 3 months of
the use of PCBs being discontinued (Kitamura et al., 1973). According
to Hara et al. (1974), the half-time of PCBs in the blood of workers,
engaged in the manufacture of electric condensers for less than 5
years, was several months, while that of workers employed for more
than 10 years was 2-3 years.
Kuwabara et al. (1978) reported mean PCB levels of 36.8 µg/litre
(range 8.3-84.5 µg/litre) blood in 20 PCB-workers, 39 children had
blood levels of 14.3 µg/litre (0.8-93.2 µg/litre), and 12 Yusho
patients, 4.2 µg/litre (1.8-8.6 µg/litre).
Fact-finding surveys of 63 workers, who were occupationally exposed to
PCBs (Kanechlor 500) in the production of silk thread or of paint,
were carried out in Japan in 1974-75; some of them and their families
were also surveyed again in 1975-82. Nineteen per cent of them showed
PCB levels higher than 50 µg/litre plasma. These persons did not show
the typical clinical findings of Yusho patients. During 7 years, no
clear decline was observed (Takamatsu et al., 1984).
There is clear evidence that relatively high PCB levels persist in the
blood of workers whose "external" exposure ceased several months or
years previously. The blood PCB concentrations in capacitor
manufacturing workers, who had been exposed for 1-24 years, varied
between 24.4 and 192 µg/litre; this was higher than levels in the
blood of a reference population (0.5-33 µg/litre) (Maroni et al.,
1981a).
In Japan, Yakushiji et al. (1984a) studied the rate of decrease and
the half-life of PCBs in the blood of children (aged 1-13 years) and
their mothers, who were occupationally exposed to PCBs, over a 5-year
period (1975-79). The mean concentration of 121 blood samples from
50 children was 17.4 ± 22.9 µg/litre and that in 65 samples from 29
mothers was 32.3 ± 20.6 µg/litre. The concentrations of PCBs in the
blood of the children varied over a wide range, because of differences
in the duration of breast-feeding. The rate of decrease of the PCB
concentration in the blood in both 18 children and 8 mothers was
relatively constant and independent of the PCB concentrations. A
one-compartment model equation was sufficient to represent the
decrease in the concentration of PCBs in the blood. The mean rate
constant of the decrease for the children was 24.2% per year,
approximately 2.6 times higher than that of the mothers (9.2%),
equivalent to half-lives of 2.8 ± 1.1 and 7.1 ± 2.7 years,
respectively. The dilution effect due to the increase in body weight
was the most important factor that affected the reduction of the PCB
concentrations in the children.
A total of 118 blood samples, mainly from employees in industries
using PCBs, were collected in the period 1975-85. In 64 blood samples,
an average level of 17 µg/litre (range nd-110 µg/litre) was found
(Frank et al., 1988).
Brown & Lawton (1984) studied the partitioning of PCBs between adipose
tissue and serum in a population of 173 capacitor workers, who were
occupationally exposed to Aroclors 1254, 1242, and 1016 for various
periods of time. The serum levels of PCBs were significantly dependent
on the level of lipids in the serum, but not on that in the albumin.
The apparent contribution of cholesterol and its esters to PCB
transport is nearly equal to their contribution to the total serum
neutral lipids. The level of serum lipids PCBs must be equal to the
adipose fat PCBs level.
Yakushiji et al. (1984b) studied the relationship between
breast-feeding and the PCB levels in the blood. The blood samples of
50 children (121 samples) and of 29 occupationally exposed mothers (65
samples) were analysed during the period 1975-79. The PCB levels in
the blood of the children were greatly influenced by the duration of
breast-feeding, but showed little relationship to the PCBs levels in
maternal blood.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Inhalation
Studies on rats (6 per group) showed that an aerosol containing a PCB
mixture (Pydraul A200: 42% chlorine), particle size 0.5-3.0 µm, at a
concentration of 30.4 ± 3.4 g/m3 for 30 min, was readily absorbed
through the lungs. The PCB concentration in the liver, 15 min after
cessation of exposure, was 50% of the maximum concentration attained
after 2 h (70 mg/kg tissue) (Benthe et al., 1972).
6.1.2 Dermal
Vos & Beems (1971) and Vos & Notenboom-Ram (1972) applied Aroclor 1260
to the shaved backs of rabbits and found systemic effects in the
kidneys, indicating that PCBs can penetrate the skin (see section
8.2.5).
Nishizumi (1976), using tritium-labelled PCBs (40% chlorine), found
evidence for the dermal absorption of PCBs in rats.
In a study of the occupational exposure of electrical workers to PCBs
(Pyralen 3010 and Apirolio, 42% chlorine content), Maroni et al.
(1981a) concluded that absorption of PCBs occurred through the human
skin. Quantitative data were not available.
6.1.3 Oral
When polychlorobiphenyl isomers were administered orally, by gavage,
to rats, at levels of 5, 50, or 100 mg/kg body weight for the lower
chlorinated compounds and up to 5 mg/kg for the higher chlorinated
compounds, 90% of the compounds were rapidly absorbed by the
gastrointestinal tract (Albro & Fishbein, 1972; Berlin et al., 1973;
Melvås & Brandt, 1973).
Using Rhesus monkeys, Allen et al. (1974a,b) determined that > 90% of
a single oral dose of 1.5 or 3.0 g Aroclor 1248/kg body weight was
absorbed over a period of 2 weeks. Drill et al. (1981) and US EPA
(1985) reviewed a number of studies indicating that PCBs are readily
absorbed from the gastrointestinal tract following oral
administration.
Bleavins et al. (1984) found that, over a period of 5 weeks, European
ferrets absorbed 85.4% of a single dose of 14C-labelled Aroclor 1254
(0.05 mg) given in food.
In contrast to the above studies, Norback et al. (1978) claimed that
59.3-87% of a single oral dose of 2,4,5,2',4',5'-hexachlorobiphenyl
passed unabsorbed through the intestines of monkeys, the first week
after dosing.
6.2 Distribution
6.2.1 Inhalation (rat)
Maximum PCB concentrations in the liver and brain of rats occurred 2
and 24 h, respectively, after a single, 30-min exposure to 30.4 ±
3.4 g/m3 of Pydraul A200 aerosol (42% chlorine content). The
concentrations in these tissues declined, while concentrations in
adipose tissues reached a maximum after 48 h (Benthe et al., 1972).
6.2.2 Oral (rat)
As in the case of other lipophilic substances, the absorption and
distribution of PCBs will, in all probability, take place via the
lymphatic system (by the chylomicrones) (DFG, 1988).
Following absorption, the clearance of PCBs from the blood and tissues
follows a biphasic pattern. The compounds rapidly clear from the blood
and accumulate in the liver and adipose tissue or are metabolized in
the liver to metabolites that are excreted in the urine and/or bile
(Drill et al., 1981).
Kurachi & Mio (1983) exposed mice to Kaneclor 400 at 100 mg/kg diet,
for 5-20 days. High levels were found in the gonads, skin, adipose
tissue, adrenals, and kidneys.
In a study by Grant et al. (1971a), 4 days after an oral dose of
Aroclor 1254 was given to rats at 500 mg/kg, the concentrations of
PCBs in the fat, liver, and brain were 996, 116, and 40 mg/kg,
respectively. Similar results showing that the highest concentration
was in the fat, were obtained in rats given Aroclor 1254 in the diet
(Curley et al., 1971), in boars (Platonow et al., 1972), cows
(Platonow & Chen, 1973), and in pigeons and quail (Bailey & Bunyan,
1972). In the studies of Curley et al. (1971), the tissue
concentrations initially showed a rapid rise and then a slow increase
while the PCB diet was being administered; Grant et al. (1974) fed
diets containing Aroclor 1254 at 0.2, 20, and 100 mg/kg to rats for 8
months, during which period the tissue concentrations reached a steady
state that was dose-dependent (Table 23). Similar tissue distribution
data for Aroclors 1016 and 1242 have been reported by Burse et al.
(1974) and for Kanechlor-400 by Yoshimura et al. (1971).
Table 23. Tissue distribution of PCBs (mg/kg wet weight) in rats fed Aroclor 1254,
Aroclor 1242, or Aroclor 1016 at 100 mg/kg for about 6 months
Tissue Aroclor 1254a Aroclor 1242b Aroclor 1016b
Blood 0.40 0.53 (plasma) 0.38 (plasma)
Liver 16 4.21 7.86
Brain 3.4 1.69 2.98
Kidneys 1.89 3.21
Heart 7.3
Fat 32.0 110 236
Urine 0.03 0.28
a From: Grant et al. (1974).
b From: Burse et al. (1974).
The study by Burse et al. (1974) showed that, with continuous feeding
of 3 types of Aroclor (see Table 23) at 100 mg/kg diet, a steady state
was not reached for 6-8 months and that the decline of stored PCBs in
adipose tissue (when the animals were kept on a PCB-free diet) was
slow and did not reach zero during a recovery period of 5-6 months.
This is surprising because the Aroclor sample used should not have
contained appreciable amounts of hexachlorobiphenyls and higher
isomers. Mizutani et al. (1977), discussing this aspect, came to the
conclusion that mobilization from storage sites rather than metabolism
constitutes the rate-limiting step in the depletion of the body burden
of PCBs.
It was demonstrated that, as the number of chlorine atoms on the
biphenyl rings increased from 1 to 6, the tissue/blood ratio tended to
increase. This increase was also proportional to the amount of lipid
in the tissues with, consequently, a higher degree of bioaccumulation
(Matthews, 1983, cf. WHO/EURO, 1988).
The fat-plasma partition-coefficients for the different PCB congeners
range from 50 up to 310 (DFG, 1988).
6.2.3 Oral (monkey)
Feeding studies were carried out on female rhesus monkeys given doses
of 0, 5, 20, 40, or 80 µg Aroclor 1254/kg body weight per day, for a
period of 37 months (Arnold et al., 1984). Eighty monkeys were divided
into 5 groups, each of 16 animals. The mean body weight of the monkeys
at the start of the study was 6.44 kg. The Aroclor 1254 was dissolved
in corn oil with glycerol as sweetener and fed to the monkeys in
gelatin capsules. Samples of blood, adipose tissue, and faeces were
collected every month and the presence of PCBs, determined. After 27
weeks, levels of 1, 2-3, 9, 18, and 37 mg PCBs/kg fat were found in
the 5 groups; after 47 weeks, blood levels were 1-3, 12, 35, 73, and
129 µg/litre respectively. PCB concentrations in whole blood increased
more rapidly during the first 10 months of the study than in the
remaining 27 months, in all groups. Concentrations in adipose tissue
(fat) increased continuously during the 37 months. The ratio profiles
of PCB levels in blood/adipose tissue, remained relatively static
between the second and twenty-seventh month of feeding. The data in
terms of relative concentrations (concentration/dose) suggest that the
bioaccumulation or retention of PCBs may be dose-dependent,
particularly for adipose tissue. The data available from PCBs in
faeces indicate a dose-dependent PCB absorption.
6.2.4 Oral (humans)
According to the study by Nishimura et al. (1976) cf. Katsunuma et al.
(1985), the PCBs within a human fetus are not evenly distributed. The
concentrations of PCBs were highest in the skin and lowest in the
brain among the 5 major organs (cerebrum, heart, liver, kidneys, and
skin). That the highest level was found in the skin might have been
because of the high solubility of the compounds in adipose tissue. In
other words, PCBs accumulate increasingly as the body fat of a fetus
increases. The authors stated that the low residue levels in the brain
were likely to be because PCBs have a poor affinity for the brain
lipids.
6.2.5 Individual congeners of PCBs
More detailed information on the tissue distribution of PCBs and their
metabolites has been obtained by the administration of pure
14C-labelled compounds, using both whole-body autoradiography and
scintillation counting of tissue samples. Berlin et al. (1975)
demonstrated that, after a single oral dose of 14C-labelled
2,5,2',4',5'-pentachlorobiphenyl, radioactivity rapidly entered the
circulation of mice and was distributed in the tissues, particularly
in the liver, kidneys, lungs, and adrenals. Subsequently, the
radioactivity in the body fat increased, rising to a maximum within
4-24 h. In most other tissues, the radioactivity decreased rapidly
after dosing, but the authors noted a special affinity for the skin,
the bronchiolar epithelium of the lungs, and certain glandular
secreting tissues. Soon after administration of the dose,
radioactivity appeared in the bile and was eliminated in the faeces.
Similar results were obtained by Melvås & Brandt (1973) in mice
treated with 2,4,2',4'-tetrachlorobiphenyl, which possessed a high
affinity for the adrenal cortex, the corpora lutea, and glandular
secreting tissue. In quail treated with 2,4,2',3'- and
2,4,3',4'-tetrachlorobiphenyl, the radioactivity in the egg yolk was
high, exceeding that in the fat. Gage & Holm (1976) determined
concentrations in the abdominal fat of mice, 7 and 21 days after they
were administered a single dose (13-165 µg/mouse) of one of 14 PCB
congeners, by gavage. Relatively low levels (< 10 ng/g per µg dose)
were found at 7 days for 4,4'-dichloro-; 3,2',4',6'-tetrachloro, and
2,3,4,2',4',6'-hexachlorobiphenyl with relatively high levels (>
100 ng/g per µg dose) for 2,4,5,2',4',5'-hexachloro-, and the
4,2',4',6'-, and 2,4,2',4'- tetrachlorobiphenyls.
Muehleback & Bickel (1981) treated rats, by gavage, with a single dose
of 14C-2,4,5,2',4',5'-hexachlorobiphenyl at 0.6 or 3.6 mg/kg body
weight. The rats were examined 1 h, 24 h, 6 weeks, 20 weeks, or 40
weeks after dosing. The highest levels of PCBs were found in the
muscle, liver, adipose tissue, and skin, early in the study. By the
end of the study, the highest PCB levels were found in the adipose
tissue followed by the skin, muscle, and liver. During the 40-week
study period, only 16% of the total dose was excreted.
The pharmacokinetics of individual monochloro-, dichloro-,
tetrachloro-, pentachloro-, and hexachlorobiphenyls were studied by
Matthews & Anderson (1975a,b), Lutz et al. (1977), and Tuey & Matthews
(1977). The mono- and dichlorobiphenyls were largely removed from
adipose tissue within 4-7 days, the 3 higher chlorinated biphenyls
were eliminated much more slowly. The half-life for the
tetrachlorobiphenyl from adipose tissue was 15 days. Skin effects were
more or less comparable.
Beran et al. (1983) studied the distribution of 14C-labelled
2,5,4'-tri-, 2,4,5,2',4',5'-hexa-, and 2,3,4,5,2',3',4',6'-
octachlorobiphenyl in the haematopoietic tissues of squirrel monkeys
(Saimiri scureus) and C67Bl mice using whole-body autoradiography.
An accumulation of radioactivity was observed in the bone marrow of
one monkey after iv injection (substances dissolved in DMSO) of the
tri- or hexachlorobiphenyl. The same was found in 3 normal mice
treated with the octachlorobiphenyl. A study using whole-body
autoradiography and spleen-colony assay in supralethally irradiated
mice, implanted with syngenic bone-marrow cells, indicated that the
major part of the radioactivity was localized outside the bone-marrow
haemic compartment, probably in the fat. Nevertheless, the trichloro-
and octachlorobiphenyls were found to inhibit the in vitro formation
of granulocytic colonies from mouse progenitor cells. Very low uptake
of labelled chlorobiphenyls was observed in the thymus, spleen, and
lymph nodes.
14C-labelled-2,4,2',4'-tetrachloro- and 3,4,3',4'-tetrachlorobiphenyl
were each administered orally to male Sprague-Dawley rats in a single
dose at 0.54 mg/kg and 0.51 mg/kg, body weight, respectively.
Distribution and covalent binding were studied. The accumulation of
2,4,2',4'-tetrachlorobiphenyl in adipose tissue was much higher than
that of 3,4,3',4'-tetrachlorobiphenyl, though the level in the blood
was consistently higher in the 3,4,3',4'-tetrachlorobiphenyl-treated
rats. The radioactivity bound in covalent linkages with cellular
macromolecules in several tissues was determined. The data indicated
that covalent binding was higher in 3,4,3',4'-tetrachloro-
biphenyl-treated rats than in those treated with 2,4,2',4'-
tetrachloro-biphenyl, particularly in the liver and blood components.
These results suggest that the 2 tetrachlorobiphenyl isomers have
different pharmacokinetic properties in rats and that the association
of covalent binding with 3,4,3',4'-tetrachlorobiphenyl induced
toxicities might be important. The microsomal enzyme system is likely
to play an important role in the in vivo covalent binding of
tetrachlorobiphenyls (Shimada & Sawabe, 1984).
In pharmacokinetic studies, 11 groups of 3 male ICR mice/group were
administered daily doses of 100 mg 2,5,2',5'-tetrachlorobiphenyl/kg
body weight dissolved in corn-oil/acetone (9:1), by gavage, for 8
consecutive days. Thirteen groups of 3 mice were administered (by
gavage) 8 mg 3,4,3',4'-tetrachlorobiphenyl/kg in the same vehicle
every other day for 10 doses. One group was sacrificed just before
each of the last 3 doses, the other groups were sacrificed at
intervals of 0.5-336 h after dosing. After dosing to an apparent
steady-state, 2,5,2',5'-tetrachlorobiphenyl was found to have a tissue
elimination half-life of between 39.5 and 70 h. The half-life of
3,4,3',4'-tetrachlorobiphenyl was 26-62.5 h. The 3,4,3',4'-
tetrachlorobiphenyl had a substantially greater partitioning from
serum into adipose tissue, liver, and thymic tissues. Studies were
undertaken to compare the toxic potency of these 2
tetrachlorobiphenyls, when similar tissue concentrations of the 2
isomers were achieved in target and storage tissues. The studies
demonstrated that thymic atrophy occurs at lower doses and tissue
concentrations of 3,4,3'4'-tetrachlorobiphenyl than those required to
produce hepatotoxicity. These two organ toxicities were produced only
by 3,4,3'4'-tetrachlorobiphenyl, despite the fact that equivalent or
higher tissue concentrations of 2,5,2',5'-tetrachlorobiphenyl were
achieved in vivo, in all tissues. The conclusion was that the
in vivo difference in the toxic potency of these tetrachloro-
biphenyl isomers does not result from the differences in their tissue
disposition, elimination, and ultimate bioaccumulation (Clevenger et
al., 1989).
6.2.6 Appraisal
Matthews & Dedrick (1984), in a review, concluded that the
pharmacokinetics of PCBs are complicated by numerous factors, not
least of which is the existence of 209 different chlorinated
biphenyls. While all PCB congeners are highly lipophilic and most are
readily absorbed and rapidly distributed to all tissues, PCBs are
cleared from the tissues at very different rates, and the same
congeners may be cleared at different rates by different species. With
the exception of special situations in which PCBs may be passively
eliminated in lipid sinks, e.g., milk or eggs, clearance is minimal
prior to metabolism to more polar compounds. Rates of PCB metabolism
vary greatly with species and with the degree and positions of
chlorination. Mammals metabolize these compounds most rapidly, but,
even among mammalian species, the rates of metabolism vary greatly. In
all species studied, the more readily metabolized chlorinated
biphenyls have adjacent unsubstituted carbon atoms in the 3-4
positions. Congeners that do not have adjacent unsubstituted carbon
atoms may be metabolized very slowly and therefore cleared very
slowly. PCBs that are not readily cleared concentrate in adipose
tissue.
6.3 Placental transport
6.3.1 Laboratory animals
The results of a number of animal studies have demonstrated that PCBs
and specific congeners can cross the placental barrier and accumulate
in the tissues of fetuses (US EPA, 1987). In studies in which monkeys
were exposed prior to, and during, gestation, signs of PCB
intoxication were observed in nursing, but not in newborn offspring
(Allen & Barsotti, 1976; Iatropoulos et al., 1978). Results such as
these have led to the conclusion that transfer through nursing may
account for higher exposure of the young than placental transfer.
Groups of pregnant ddN mice were fed diets containing Kanechlor 500
(mainly comprising pentachloro- and hexachlorobiphenyls) at 0.01
(controls), 0.94, or 86 mg/kg diet from day 1 to 18 of pregnancy.
Regardless of the dietary level of PCBs, whole-body levels in the
fetuses were only 0.1-0.2% of the total maternal intake, indicating
limited transplacental transfer (Masuda et al., 1978a). Two groups of
ddN mice were fed Kanechlor 500 (mainly containing pentachloro- and
hexachlorobiphenyls at 0 or 0.94 mg/kg diet) from the day of
insemination throughout gestation and for 5 weeks after delivery of
offspring. Total PCBs were 100 times greater in the suckling animal
than in the fetuses at term, from dams fed the same amount, indicating
a considerable transfer of PCBs during lactation (Masuda et al.,
1978a).
Masuda et al. (1979) fed female ddN-mice diets containing
polychlorinated biphenyls: 2,4,4'-trichloro-; 2,5,3',4'-tetrachloro-;
2,4,5,2',5'-pentachloro-; 2,3,4,2',4',5'-hexachloro-;2,4,5,2',4',5'-
hexachloro-; 2,3,4,5,6,2',5'-heptachloro-; and 2,3,4,5,2',3',4',5'-
octachlorobiphenyl at levels of 0.32, 0.42, 0.42, 0.44, 0.44,
0.16, and 0.23 mg/kg diet, respectively, for 18 days prior
to, or after, mating. Animals were either sacrificed on day 18 of
gestation or allowed to deliver and the offspring maintained for 5
weeks on a normal diet. All the PCBs were qualitatively transferred
across the placenta and through the milk. The amount transferred
during lactation was greater than that transferred transplacentally.
The transfer of 2,4,5,2',4',5'-hexachlorobi[14C-]phenyl across the
placenta during the course of pregnancy in Sprague-Dawley mice was
studied by Vodicnik & Lech (1980). The PCB was injected
intraperitoneally at 100 mg/kg body weight, in corn oil, 2 weeks prior
to mating. The concentrations of 14C-PCB in the fetuses from 12- and
18-day pregnant animals were 0.71 and 2.45 mg/kg tissue, respectively.
At birth, the total carcass concentration for all newborn animals was
less than 3 mg/kg tissue, which represents less than 3% of the dose
present in the mothers at birth.
Placental transfer of polychlorinated biphenyls has also been reported
in the mouse by Berlin et al. (1975) and Melvås & Brandt (1973).
Curley et al. (1973a) found some placental transport of Aroclor 1254
in the rat.
Groups of pregnant and non-pregnant Wistar rats received a dose of
14C-2,4,5,2',4',5'-hexachlorobiphenyl (2.1 µC/kg), intraperitoneally.
The amount of radioactivity transferred through the placenta was 2.7%
of the administered dose, whereas 39.2% of the original dose was
transferred through the milk (Ando et al., 1978).
Aroclors 1221 and 1254 were found to cross the placenta of rabbits,
when administered orally to does during gestation. The concentration
in fetal tissues was dose-dependent and much lower with Aroclor 1221
than with Aroclor 1254; the concentration of the latter in the fetal
liver was greater than that in the maternal liver (Grant et al.,
1971b).
Bleavins et al. (1984) fed female European ferrets a single dose of
14C-labelled Aroclor 1254 in the diet (0.05 mg), early (day 14) or
late (day 35) in gestation, and determined the placental transfer of
PCBs. Placental transfer to the kits was 0.01% (per kit) of the
maternal dose, when the dams were exposed early in gestation, and,
0.04%, when the dams were exposed late in gestation. Placental
transfer of PCBs was considerably less than mammary transfer, with a
ratio at 1 week of lactation of 1:15 and 1:7 for offspring of dams
dosed early or late in gestation, respectively.
Groups of lactating mother Rhesus monkeys, between 1 and 3 months post
partum, received 16 mg Clophen A-30/kg per day for 30 days. One
mother/infant pair served as a control. Clophen A-30 concentrations in
the serum of both mother and infant and the milk were determined on
days -14, -7, 0, 1, 2, 4, 8 and at weekly intervals thereafter. One
mother and all infants were killed and tissues taken for PCB analysis.
The concentration of Clophen A-30 in milk was 20 times higher than
maternal serum levels. Infant serum levels were 2-5 times higher than
their mothers. Tissue levels were generally higher in the infants.
Clophen A-30 tended to concentrate in the infant fat, bone marrow, and
adrenals (Bailey et al., 1980).
Groups of 24 Rhesus monkeys were maintained on diets that provided
Aroclor 1016 at doses of 0, 4.5, or 18.1 mg/kg body weight per day
throughout gestation and a 4-month nursing period. At birth, the
concentrations of PCBs in the skin of infants were similar to
concentrations in the subcutaneous fat of the mothers. At weaning, the
PCB content in the mesenteric fat of the infants was 4-7 times greater
than that in the subcutaneous fat of the mothers. Gas chromatographic
patterns showed that the adult adipose tissue did not include the
total spectrum of peaks observed in the Aroclor 1016 standard, and
that all of the peaks in the mesenteric fat of the infants at weaning
and 4 months after weaning were qualitatively similar to those in the
adult adipose tissue. According to the authors, these data suggested
an inability of the fetus to metabolize and excrete certain congeners
that are more readily metabolized and eliminated by adults and older
infants (Barsotti & Van Miller, 1984).
6.3.2 Wildlife
A 6 1/2-year-old desert bighorn (Ovis canadensis cremnobates) ewe
and her term ram fetus were used to study the distribution and
concentrations of PCBs in different organs and tissues. Fourteen
maternal and 13 fetal tissues were analysed for their presence of
organochlorine hydrocarbons. PCBs averaged 85 and 88% of the total
residue loads for maternal and fetal tissues, respectively. It is
remarkable that the "natural" PCB levels in the different organs and
tissues were nearly the same, i.e., in maternal organs and tissues
between 0.37 and 0.44 mg/kg, and, in fetal organs and tissues, between
0.30 and 0.35 mg/kg, on a fat basis (Turner, 1979).
6.3.3 Humans
Four studies of placental passage in humans, based on small samples
drawn from the general Japanese population, have yielded inconsistent
results (Yoshimura, 1974; Akiyama et al., 1975; Kodama & Ota, 1977;
Masuda et al., 1978a).
PCBs were detected in the umbilical tissues, umbilical blood, amniotic
fluid, and baby's blood from a woman who was occupationally exposed to
Kanechlors 300 and 500 in a capacitor factory (Yakushiji et al.,
1978). PCB levels in these tissues and fluids were considerably lower
than that in the mother's blood.
Jacobson et al. (1984b) examined maternal and cord serum (196 or 198
samples each) for the presence of PCBs, in women who resided in the
Michigan area (USA), where, in 1973, a PBB-incident occurred. Mean
concentrations of maternal and cord serum were 4.7 µg/litre (1.1-
14.3 µg/litre) and 2.0 µg/litre (0.1-7.2 µg/litre), respectively.
Placental passage was indicated by a significant maternal to cord
serum correlation for PCBs. The fact that cord serum levels were lower
than those in maternal serum is consistent with the notion that the
placenta may function as a partial barrier. The transfer rate of PCBs
in maternal blood through the placenta to cord blood may vary,
depending on the chemical nature of each PCB isomer (Ando et al.,
1984). Ando et al. (1985) examined the PCB concentrations in the
maternal blood, breast milk, and the placenta of 6 Japanese women.
They found that the congeners present were more typical of Kanechlor
500 than Kanechlor 300, 400, or 600. The results indicated that, as
the chlorine content of the PCB congeners increased, the correlation
between the placental content of congeners and those in the maternal
blood and breast milk also increased. The same was found in laboratory
animals (Allen & Barsotti, 1976; Masuda et al., 1978b).
A study on the transfer of PCBs to infants from their mothers was
carried out in Japan from 1974 to 1976 by Kodama & Ota (1977). When
the cord blood was considered as the infant blood at birth, the level
of PCBs in the blood of breast-fed infants rose gradually with
ingestion of breast milk, exceeded the level in the blood of their
mothers after 3 months, continued to increase up to the age of 1 year
and then significantly decreased, 2 years after birth. The PCB
concentrations in the blood of non-breast-fed infants remained low
(Table 24).
6.4 Excretion and elimination
6.4.1 Following oral dosing
The excretion of PCBs is, to a large extent, dependent on the
metabolism of PCBs to form more polar compounds (US EPA, 1987). At
equilibrium, the elimination of PCBs from all tissues will be
dependent on the structure-dependent metabolism rates of the
individual PCB congeners. For example, the biological half-lives in
the rat range from 1.15 days for 2,2'-dichlorobiphenyl to
approximately 460 days for 2,4,5,2',4',5',-hexachlorobiphenyl (Tanabe
et al., 1981; Wyss et al., 1986). Metabolites of the more highly
chlorinated congeners are eliminated primarily via the faeces (Goto et
al., 1974; WHO/EURO, 1987).
When the analysis of faeces is limited to the determination of
unchanged PCBs, the recovery of the dose administered is incomplete;
in boars receiving single or repeated doses of Aroclor 1254, not more
than 16% of the dose was recovered from the faeces and less than 1% in
the urine (Platonow et al., 1972). Better recoveries have been
obtained with PCB labelled with radioactive isotopes. Yoshimura et al.
(1971) found 70% of the activity from a dose of tritium-labelled
Kanechlor 400 in the faeces and 2% in the urine, over a 4-week period.
Berlin et al. (1973, 1975) found over 75% of the activity from
14C-labelled pentachloro- and hexachlorobiphenyls in the faeces and
less than 2% in the urine; most of the faecal elimination consisted of
PCB metabolites. Similar results were obtained by Melvås & Brandt
(1973) with tetrachlorobiphenyls.
Hashimoto et al. (1976) examined the excretion of 14C-PCB compounds
given to rats by gavage, at a total dose of 6.35-7.85 mg/kg body
weight, over a period of 5-50 days. The PCBs studied were
predominantly tetra- and hexachlorinated isomers. The results
indicated that 1.9-4.9% of the dose of tetrachlorobiphenyls was
excreted in the urine, with higher amounts excreted in rats treated
for longer periods. In rats treated with hexachlorobiphenyls, only
0.3% of the dose was excreted in urine. About 47-68% of the dose of
both tetrachloro- and hexachloro-isomers was eliminated in the faeces.
Table 24. Level of PCBs in mothers' and babies' blood (average over
3 years in µg/litre)a
Maternal blood 4.5 (0.8-15.5)
Cord blood 1.1 (nd-5.6)
Mother's blood (breast-feeding) 2.5 (nd-10.8)
Babies' blood (3 months old) 3.6 (0.2-10.9)
Babies' blood (1 year old) 4.7 (0.8-17.7)
Mother's blood (bottle feeding) 2.7 (0.6-8.7)
Babies' blood (3 months old) 1.6 (nd-7.6)
Babies' blood (1 year old) 0.7 (nd-2.1)
a From: Kodama & Ota (1977).
Bleavins et al. (1984) found 22.1% and 1.8% in the faeces and urine,
respectively, during the first week following dosing of 0.05 mg
14C-labelled Aroclor 1254 to female European ferrets.
A biological half-life of about 200 days was recorded in the fat of
rats after feeding with Aroclor 1254 (Grant et al., 1974). Berlin et
al. (1975) noted that, in mice dosed with a pentachlorobiphenyl, there
was an initial rapid elimination from the liver while liver PCB levels
were high, followed by a slower elimination when most of the PCB was
located in the fat. The author suggested that the mobilization of PCBs
from fat, and, therefore, their half-life in the body, depends upon
their rates of metabolism. Berlin et al. (1973) investigated the
hypothesis that the ability of a PCB to be readily degraded with a
half-life of a few days depended on the presence of 2 adjacent
unsubstituted carbon atoms in the molecule, rather than on the number
of chlorine atoms, though the presence of such unsubstituted pairs
depends to a large extent on the degree of chlorination. They came to
the conclusion that this hypothesis probably applied to unsubstituted
pairs in the 3,4-position, but that in the 2,3-position, their
susceptibility to metabolic degradation was influenced more by the
presence of chlorines in the o-position of the ring bridge.
Sprague-Dawley rats, white Swiss mice, and Rhesus monkeys were
administered a single dose of 14C-2,2'-dichlorobiphenyl, by gavage.
Within 6 days, mice eliminated a total of approximately 46% of the PCB
(urine, 20%; faeces, 26%). There was no clear difference between male
and female mice. In the rat, the total elimination was 51-56% after 9
days, mainly via the biliary/faecal route. The monkeys had the highest
elimination rate, a total of 68.6% (urine, 54%; faeces, 14.6%), within
10 days (Milling et al., 1979).
Male and female Wistar rats were administered a daily dose of
14C-2,5,4'-trichlorobiphenyl for 14 days. The animals were killed 5
days after receiving the last dose. The compound was rapidly
eliminated primarily with the faeces. Most of the trichlorobiphenyl
was metabolized (78.5%) and the major metabolites excreted were
identified as hydroxy-, dichloro-, and conjugated derivatives (Lay et
al., 1979).
The elimination of tetrachlorobiphenyl isomers in mice fed diets
containing a single isomer, at 10 mg/kg diet for 20 days, was studied
by Mizutani et al. (1977). Biological half-lives for the isomers
2,3,2',3'-tetrachloro-, 2,4,2',4'-tetrachloro-, 2,5,2',5'-
tetrachloro-, 3,4,3',4'-tetrachloro-, and 3,5,3',5'-tetrachloro-
biphenyl, were 0.9, 9.2, 3.4, 0.9, and 2.1 days, respectively.
Gage & Holm (1976) studied the influence of molecular structure on the
excretion of 14 PCB congeners in mice. They found that the
4,4'-dichloro-; 3,3',4',6'-tetrachloro-; 2,3,2',4',6'-pentachloro-;
and 2,3,4,2',4',5'-hexachloro-isomers were eliminated most rapidly.
These compounds had at least one pair of unsubstituted ortho-meta,
vicinal carbon atoms, a configuration thought to be important for
rapid metabolism and excretion. The most slowly eliminated compounds
were 2,4,5,2',4',5'- and 2,3,4,2',4',5'-hexachlorobiphenyl.
2,4,5,2',4',5'-Hexachlorobiphenyl was the PCB congener found in the
highest concentration in human adipose tissue, while
2,4,6,2',4',6'-hexachlorobiphenyl was not detected (Jensen &
Sundström, 1974a). As both of these compounds are found in commercial
PCB mixtures and in the environment, the presence of the
2,4,5,2',4',5'-hexachlorobiphenyl in adipose tissue appears to be
related to resistance to metabolism (US EPA, 1987). That this congener
is not, or is only minimally, metabolized is also indicated by the
finding that the blood concentration of this congener decreased only
10% over 300-500 days (Chen et al., 1982) and by the results of
in vitro metabolism studies with human liver microsomes (Schnellman
et al., 1984a,b).
Felt et al. (1977) examined the elimination of 14C-2,5,4'-trichloro-
biphenyl in rhesus monkeys. The monkeys were fed 550 mg of the
compound in fruit, daily, for 84 days. On the basis of total excretion
and recovered radioactivity, the half-life was found to be 4.5-4.8
days.
Male and female Sprague-Dawley rats were administered 14C-labelled
2,4,6,2',4'-pentachlorobiphenyl by gavage and the urine and faeces
were collected. After 8 days, the animals were killed. The elimination
of the PCBs followed a bi-exponential rate expression with a-phase
half-lives of 0.90 and 0.95 days and b-phase half-lives of 4.2 and 3.8
days, for males and females, respectively (Felt et al., 1979).
6.4.2 Following parenteral dosing
The results of injection studies indicate that PCBs can be excreted
unmetabolized into the gastrointestinal tract. Yoshimura & Yamamoto
(1975) recovered unchanged tetrachlorobiphenyl from the duodenal
contents of rats injected intravenously with tetrachlorobiphenyl.
Daily excretion for 4 days ranged from 0.5 to 0.8% of the total
dose/day. Goto et al. (1974) found that 4.7-23.2% of injected PCBs
were excreted unchanged into the gastrointestinal tract by day 10
after dosing, with the excretion of a penta-isomer greater than the
excretion of di-, tri-, or tetra-isomers.
Adult male Sprague-Dawley rats received doses of 4 symmetrical
hexachlorobiphenyl 14C-isomers, i.e., 2,3,5,2',3',5'-hexachloro-,
2,3,6,2',3',6'-hexachloro-, 2,4,5,2',4',5'-hexachloro-, and
2,4,6,2',4',6'-hexachlorobiphenyl, by intravenous injection. Most of
the radioactivity was eliminated in the faeces with less than 1% found
in the urine. The metabolites showed evidence of dechlorination,
chlorine shifts, and possible metabolism by direct insertion of a
hydroxyl group. There was also evidence supporting the intermediate
step of an arene-oxide as a predominant mechanism of PCB metabolism
(Kato et al., 1980).
The disposition of 2 symmetrical 14C-labelled 2,3,6,2',3',6'-
hexachloro- and 2,4,5,2',4',5'-hexachlorobiphenyl was studied in
24-month-old, male, Sprague-Dawley rats, after iv treatment. More than
50% of the 2,3,6,2',3',6'-hexachlorobiphenyl was metabolized and
excreted via the bile into the faeces within 2 days, and only 2% was
excreted in urine. More than 90% was eliminated as metabolites. In
contrast, 2,4,5,2',4',5'-hexachlorobiphenyl was redistributed from the
liver, muscle, and skin to the adipose tissue, where it accumulated
without being metabolized. Only 2% of the total dose was eliminated,
primarily in the faeces, within 21 days. In 2- to 3-month-old rats,
the general pattern of disposition of these hexachlorobiphenyls did
not change with age; however, there were differences in the rates of
elimination and in the tissue levels. There was enhanced metabolic
retention in the muscle, skin, and adipose tissue of older rats, which
suggested an age-related decrease in tissue clearance. The larger
volume of adipose tissue could not explain this observation. In
general, there were few changes in decay rates from tissues or in
biliary excretion, so age had a greater effect on the disposition of
the "persistent" 2,4,5,2',4',5'-hexachlorobiphenyl than on the
metabolizable 2,3,6,2',3',6'-hexachlorobiphenyl (Birnbaum, 1983).
Ethane exhalation was increased in male Sprague-Dawley rats, 30 days
after a single ip injection of Aroclor 1254 (500 mg/kg body weight).
Before day 30, there was no increase in ethane production. Parallel
increases in hepatic malondialdehyde levels were found. A single ip
injection of 3,4,3',4'-tetrachloro-, 2,3,4,5,4'-pentachloro, and
2,4,5,2',4',5'-hexachlorobiphenyl (300 µmol/kg) also increased (after
30 days) the production of malondialdehyde and ethane, indicators of
in vivo lipid peroxidation. These effects were not reflected in
increased diene conjugation (Dogra et al., 1988).
Sipes et al. (1980, 1982a,b) studied the distribution, metabolism, and
excretion of 14C-labelled 4,4-dichloro-, 2,4,5,2'4'5'-hexachloro-, or
2,3,6,2',3',6'-hexachlorobiphenyl in beagle dogs and cynomolgus
monkeys, after a single intravenous dose. The elimination of the test
substances from the blood of both species was shown to be biphasic.
The results for dichlorobiphenyl showed that the dog eliminated 50% of
the dose (urine, 7%; faeces, 43%) within 24 h, while the remainder was
found mainly in the adipose tissue. By 5 days, 90% had been
eliminated. The monkey eliminated less than 15% of the dose within
24 h, with less than 1% in the faeces. The remainder was found in the
adipose tissue. Within 28 days, 59% of the dose had been eliminated,
chiefly in the urine. Biliary excretion after 24 h was shown to be 33%
in the dog and only 0.4% in the monkey.
The data for 2,4,5,2',4',5'-hexachlorobiphenyl showed that the dog
eliminated 66% (urine, 3%; faeces, 63%) within 3 days; the monkey
eliminated 18% of the dose (of which 17% was in the faeces), 90 days
following administration. The remainder was found in the adipose
tissue. In the studies with 2,3,6,2',3',6'-hexachlorobiphenyl, the dog
eliminated 52% of the dose within 24 h (urine, 11%; faeces, 41%) and
70% in 3 days. The monkey eliminated 19% during the first 24 h,
divided equally between urine and faeces. By 15 days, 61% had been
eliminated, primarily in the faeces. The 24-h biliary excretion was
26% and 2.4% in the dog and the monkey, respectively.
6.4.3 Humans
Chen et al. (1982, 1985) studied the presence of PCBs in the blood of
human beings, in the Province of Taiwan, after they had consumed
rice-bran oil contaminated with Kanechlor 500 and PCDFs. Blood samples
from 17 patients were examined, with 2-3 samples taken from each
patient, 2-17 months apart. The results indicated that the
tetrachloro- and some pentachloro- isomers tended to be eliminated
more rapidly than the other pentachloro- and the hexachloro- and
heptachloro- isomers. Half-lives for the 2,4,5,2',4'- and 2,3,4,3',4'-
pentachloro- isomers in the blood were 9.8 and 8.7 months,
respectively. Two adjacent unsubstituted carbon atoms at the meta,
para positions facilitated metabolism and the subsequent elimination
from the blood. PCBs containing adjacent unsubstituted carbon atoms at
the ortho and meta positions of the biphenyl ring are eliminated
very slowly and will accumulate.
Buhler et al. (1988) administered a uniformly 13C-labelled PCB
mixture similar to Aroclor 1254 to a volunteer. A single dose of
329 µg/kg body weight was ingested; blood samples taken over a period
of 260 days were analysed for 13C- and 12C-PCBs using GC/MS and
GC/ECD. Elimination of the isomers followed a first order kinetics.
The half-lives for the isomers 2,3,4,2',4',5'-hexachlorobiphenyl,
2,4,5,2',4',5'-hexachlorobiphenyl, and 2,3,4,5,2',4',5'-hepta-
chlorobiphenyl were 321, 338, and 124 days, respectively.
6.4.4 Elimination via milk (animals)
Vodicnik (1986) studied the disposition of 14C-2,4,2',4'-
tetrachlorobiphenyl (150 mg/kg body weight administered
intraperitoneally) as a function of non-pregnant body weight in
virgin, late pregnant, and early post partum ICR mice and their
offspring. The highest concentrations were observed in adipose tissue
and the mammary glands, regardless of reproductive state. The
concentrations of the tetrachlorobiphenyl equivalents in the tissues
differed among the 3 groups, possibly because of the alterations in
lipid deposition/mobilization associated with pregnancy and lactation.
Approximately 20% of 14C-activity was eliminated from the carcass of
virgin mice, 4 days after administration, but no decrease was seen in
late-pregnant animals. Minimal transplacental transfer of
14C-activity occurred (approximately 1%), but the tetrachlorobiphenyl
was rapidly eliminated in breast milk to nursing offspring. Ninety per
cent of the total-carcass 14C-activity was eliminated from lactating
mice over a 4-day period, approximately 75% of which could be
accounted for in neonatal carcasses.
Saschenbrecker et al. (1972) found that, after oral administration of
doses of Aroclor 1254 of 10 or 100 mg/kg to cows, 6.27 and
74.5 mg/litre, respectively, appeared in the milk after 24 h. These
levels were reduced to less than one-half within 3 days, but traces
still remained at 50 days. Cows receiving 200 mg/day of Aroclor 1254
reached a steady state concentration of 61 mg/kg in the milk fat and
42 mg/kg in the body fat, after 10 days (Fries et al., 1973).
The "carry-over factor" from animal feed into the cow's milk showed
that the lower (tri-, tetra-, and penta-) chlorinated biphenyls have a
lower carry-over factor than the higher (hexa- and hepta-) chlorinated
biphenyls. Thus, it is the latter that are particularly concentrated
in cow's milk fat. From studies in the Federal Republic of Germany, it
was found that the major congeners in cow's milk were numbers 138,
153, and 180 (DFG, 1988).
6.4.4.1 Elimination via breast milk
The composition of common commercial PCB mixtures clearly differs from
the composition of the PCB contents of human fat or human breast milk,
because of the preferential elimination of certain PCB congeners
containing 3 or 4 ortho substituents and the retention of PCBs with
1 or 2 ortho substituents (Kuroki & Masuda, 1977; Watanabe et al.,
1979; Yakushiji et al., 1979).
The major PCB components (and average relative concentrations) that
have been identified in breast milk in the Osaka area in Japan
include: 2,4,4'-trichlorobiphenyl (8.4%); 2,5,2',5'-tetrachloro-
biphenyl (2.0%); 2,4,5,4'-tetrachlorobiphenyl (19%); 2,4,5,2',5'-
pentachlorobiphenyl (2.8%); 2,4,5,3',4'-pentachlorobiphenyl (11.8%);
2,4,5,2',4',5'-hexachlorobiphenyl (15.5%); 2,3,4,2',4',5'-
hexachlorobiphenyl (15.8%); 2,3,4,3',4',5'-hexachlorobiphenyl (2.3%);
2,3,4,6,2',4',5'-heptachlorobiphenyl (1.6%); 2,3,5,6,2',4',5'-
heptachlorobiphenyl (3.2%). These PCB-congeners constituted at least
95% of the PCBs in the breast milk of the women examined in Osaka.
In recent studies, the contents of PCBs in human milk and maternal
blood were compared for US citizens (Bush et al., 1984, 1985). Eight
individual PCB congeners comprised 52% of the total PCB residues in
the milk and 48.5% in the blood. The mean concentrations for total
PCBs were 26.5 µg/kg for whole milk and 3.5 µg/kg for blood. The
percentages of the different congeners are given in Table 25.
6.5 Metabolic transformation
6.5.1 PCBs
The metabolism of PCBs has been investigated in numerous studies on
animals and reviewed by Drill et al. (1981) and the US EPA (1987). The
PCBs were usually administered by the oral or parenteral route.
Phenolic products are the major PCB metabolites, though
sulfur-containing metabolites, trans-dihydrodiols, polyhydroxylated
PCBs, and methyl ether derivatives have also been identified. Although
the effects of the chlorine substitution pattern on sites of oxidation
have not been studied systematically, US EPA (1987) suggested the
following:
* hydroxylation is favoured at the para position in the least
chlorinated phenyl ring, unless this site is sterically hindered
(i.e., 3,5-dichloro-substitution);
* in the lower chlorinated biphenyls the para position of both
biphenyl rings and carbon atoms that are para to the chloro
substituent are all readily hydroxylated (Sparling et al., 1980);
* the availability of 2 vicinal unsubstituted carbon atoms
(particularly C5 and C4 in the biphenyl nucleus) also facilitates
the oxidative metabolism of the PCB substrate, but is not a
necessary requirement for metabolism;
Table 25. Concentrations of most abundant PCB congeners present in whole breast milk and maternal blooda
Congener Milk Maternal blood Ratio
(40 samples) (101 samples) milk/blood
µg/litre % of µg/litre % of
total PCBs total PCBs
2,4,5,2',4',5'-hexachlorobiphenyl 3.2 12 0.31 8.8 10
2,3,5,6,2',3',6-heptachlorobiphenyl 2.5 9.4 0.27 8.0 9.2
2,4,5,2',3',4'-hexachlorobiphenyl 2.1 7.8 0.58 17 3.5
2,5,3'4'-tetrachlorobiphenyl 1.7 6.6 0.01 - 500
2,3,4,5,2'4'5'-heptachlorobiphenyl 1.2 4.5 0.03 3.7 9.4
2,3,4,5,3',4'-hexachlorobiphenyl 1.0 4.0 0.01 - 125
2,4,5,2',4'-pentachlorobiphenyl 1.1 4.0 0.12 3.4 8.9
2,3,4,3',4'-pentachlorobiphenyl 0.97 3.7 0.25 7.6 3.8
Total PCBs 26.5 - 3.5 - 7.5
a Modified from: Bush et al. (1985).
* as the rate of chlorination increases on both phenyl rings, the
rate of metabolism decreases;
* the metabolism of specific PCB isomers by different species can
result in considerable variations in metabolic pattern.
Kannan et al. (1989) studied the possible involvement of frontier
(pi) electrons in the metabolism of polychlorinated biphenyls. The
electron density, at each carbon atom, of the highest occupied pi
orbital of 13 PCB molecules was calculated and the result was compared
with their in vitro and/or in vivo metabolism. It was found that:
* the carbon position at which the frontier electron density was the
highest was most readily hydroxylated or sulfonated;
* if the carbon with the highest frontier (pi) electrons was
occupied by chlorine, either a replacement occurred or the carbon
with the next highest electron density was activated for
metabolism;
* because of steric hindrance, "ortho" carbons were least
preferred for such reactions, in spite of possessing favourable
electron density;
* this was applicable to both phenobarbital (PB)-type and
3-methylcholanthrene (3-MC)-type PCB inducers.
The authors suggested that frontier (pi) electron density could be
an easy guide for understanding the metabolic products of persistent
and toxic environmental pollutants in vitro and in vivo, and for
understanding their environmental fate.
There appears to be little metabolism of PCBs with 6 or more chlorine
substituents (Matthews & Anderson, 1975b). When between 2 and 5
chlorine substituent PCBs are metabolized, the metabolic products are
primarily hydroxylated compounds, frequently found as glucuronide
conjugates (hydroxymethoxy derivatives) and partially dechlorinated
metabolites. In some cases, smaller amounts of dihydrohydroxy
compounds and related substances are also found.
The parent compound is also eliminated in various quantities in
faeces, hair, and maternal milk, but very little unmetabolized
compound is excreted in the urine. This pattern is not unusual for
lipophilic xenobiotics.
PCB metabolism has been examined in primates (monkeys) by Greb et al.
(1975), Hsu et al. (1975a,b), and Allen & Norback (1976); in ungulates
(cows, pigs, and goats) by Platanow & Chen (1973), Safe et al. (1975),
and Gardner et al. (1976); in rats by Grant et al. (1971a), Hutzinger
et al. (1972), Yoshimura et al. (1973), Goto et al. (1973, 1974,
1975), Safe et al. (1974), Matthews & Anderson (1975b), van Miller et
al. (1975), Sundström & Jansson (1975), Sundström et al. (1976a), Lay
et al. (1975, 1979), Chen et al. (1976), Kamal et al. (1976), and
Norback et al. (1976); in mice by Berlin et al. (1973), Yamamoto &
Yoshimura (1973), and Sundström & Jansson (1975); in rabbits by Grant
et al. (1971b), Hutzinger et al. (1974), Sundström & Wachmeister
(1975), and Sundström et al. (1976b); in pigeons, and quails by Koeman
et al. (1969), Hutzinger et al. (1972), Bailey & Bunyan (1972), and
Sundström & Jansson (1975); and in trout by Hutzinger et al. (1972).
The different metabolic products formed from pure isomers in these
various species have been catalogued in an NAS report (1979) and in a
review by Sundström et al. (1976a). Neither of these reports is
complete, but, together, they cover most of the studies up to 1979.
In the rat, monochloro-, dichloro-, trichloro-, tetrachloro-,
pentachloro-, and at least one hexachlorobiphenyl, yielded at least
one hydroxylated metabolite. Some isomers produced as many as 5
different hydroxylated metabolites including both mono- and dihydroxy-
derivatives. Most of the hexachloro-, octachloro-, and
decachlorobiphenyls did not yield detectable levels of hydroxylated
products.
Similar hydroxylated derivatives were also produced in other species,
but the ability to metabolize PCBs is not absolutely uniform in all
species. In the rabbit, dichloro-, tetrachloro-, and
hexachlorobiphenyls were metabolized, while further down the
phylogenetic scale, the pigeon only metabolized monochloro- and
dichlorobiphenyls and the trout failed to metabolize any of the
chlorinated biphenyls tested. Table 26 shows the PCBs tested in
different species and indicates whether or not the organism was able
to metabolize the compound. Although different species may metabolize
a given isomer, the metabolic products are not necessarily identical.
An example of this is found in the simple 4,4'-dichlorobiphenyl which
is metabolized by the rat, rabbit, and goat, but does not give
identical products in these species; all 3 species produce
4,4'-dichloro-3-hydroxybiphenyl as a metabolite, but, in addition, the
rat produces 4,4'-dichloro-, 2,3-dihydroxybiphenyl, and the goat
produces 3,4'-dichloro-4-hydroxybiphenyl as a metabolite.
However, a product such as the 3,4'-dichloro-4-hydroxybiphenyl found
in the goat involves a chlorine shift, which may be indicative of a
more toxic intermediate.
Many different pathways of metabolism have been described as
summarized in Fig. 5 (Safe, 1984; WHO/EURO, 1987).
These pathways include hydroxylation, and conjugation with thiols and
other water-soluble derivatives. The most important pathway seems to
be through hydroxylation and subsequent conjugation. Rats and mice
that were exposed to dichloro-, tetrachloro-, or pentachlorobiphenyls
by intraperitoneal injection or diet, eliminated metabolites as
glutathione conjugates and other sulfur-containing compounds (Kurachi,
1983; Kurachi & Mio, 1983). Mammalian metabolism of many individual
PCBs may proceed via oxide intermediates, which have not been
isolated, but are presumed to be precursors of some of the major
metabolites identified. One type of metabolite is the methylsulfone
PCB metabolite that has been identified in environmental samples by
Jansson et al. (1975) and WHO/EURO (1987), and in human milk by
Yoshida & Nakamura (1979).
The formation of xenobiotic thioether derivatives, including
glutathione, cysteinylglycine, cysteine, and N-acetylcysteine
(mercapturic acid) conjugates, is generally considered a pathway for
the detoxification of reactive intermediates. Mio & Sumino (1985)
detected methylsulfonyl metabolites, by using GC/MS/COM, from the
adipose tissues of mice treated with Kanechlor 300, 400, 500, or
2,5,2',5'-tetrachlorobiphenyl. Metabolites were detected in the faeces
of mice treated with 2,5,2',5'-tetrachlorobiphenyl, e.g., 6
sulfur-containing and 5 non-sulfur-containing metabolites. The
elimination rates for one week were 1.7% and 43%, respectively. The
methylsulfonyl metabolites accumulated in the liver, adipose tissue,
and lungs. Mio & Sumino (1985) proposed the methylsulfonyl metabolic
pathway of 2,5,2',5'-tetrachlorobiphenyl.
Klasson-Wehler et al. (1987) administered a single dose of 2,3,6,4'-
tetrachlorobiphenyl to 3 groups of 5 female C57B1 mice at 0, 10, or
100 mg/kg body weight. 35S-cysteine was administered by ip injections
4 times at 12-h intervals. The animals were sacrificed and the organs
analysed on day 12. Methyl [35S]sulfonyl-tetrachlorobiphenyl was
found in the lungs, kidneys, and fat of the treated mice, as well as
minor amounts of tetrachlorobiphenyl and traces of
methylthiotetrachlorobiphenyl.
The formation of serial methylsulfonyl metabolites can be summarized
as follows: the glutathione conjugate is converted, by cleavage, to a
cysteine or thiol conjugate and translocated into the liver. The thiol
conjugate from the cysteine moiety is transmethylated by
thiol- S-methyltransferase and is oxygenated by cytochromes P-450 and
P-448 oxidase or is glucuronidated by UDP-glucuronyl-transferase in
the liver, resulting in methylsulfonyl derivatives.
Table 26. Metabolism of various PCBs in different organisms
Compound Species
Chlorobiphenyl Trout Pigeon Mouse Rat Rabbit Monkey
4-mono- - + + +
4,4'-di- - + + +
2,2' +
2,4' + +
2,5,2'-tri +
2,4,2',4'-tetra- +
2,5,2',5' - - + + +
3,4,3',4' -
2,3,4,5,6,-penta- +
2,4,5,2',5' + +
2,4,6,2',4' +
2,4,6,2',6' +
2,4,6,3',5' +
2,3,4,6,4' +
2,3,4,3',4' -
2,4,5,2',4',5',-hexa- - - (±)a +
2,4,6,2',4',6' (±)a
2,3,5,6,2',3',5',6'-octa- -
2,3,4,5,6,2',3',4',5',6'-deca -
a These compounds were reported by Sundström et al. (1976a) as failing to produce
hydroxylated derivatives, but were positive in an IARC report referred to in an
NAS report (NAS, 1979).
+ = Compound is metabolized; - = Compound not metabolized: (blank) not tested.
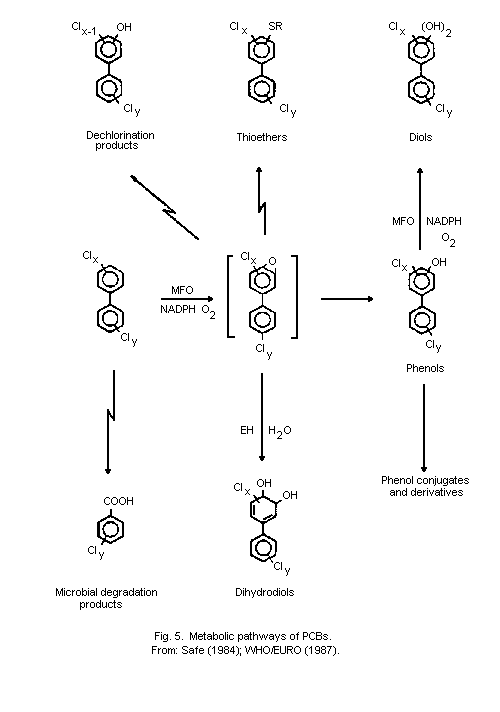 The occurrence of trans-dihydrodiol metabolites suggests that the
metabolism of PCBs proceeds through the formation of arene oxide
intermediates (US EPA, 1987). Arene oxides are potential electrophiles
that have been implicated in cellular necrosis, mutagenicity, and
carcinogenicity (Safe et al., 1975; Sundström et al., 1976a).
While the arene oxide pathway is important in carcinogenic
considerations, it may not be the primary pathway for the metabolism
of PCBs, in most cases. So far, most discussions about the metabolism
of PCB isomers have focused on the position and number of the chlorine
substituents.
The metabolic products of dichloro-, trichloro-, tetrachloro-,
pentachloro-, and hexachlorobiphenyl appear to reflect direct
hydroxylation at the meta and/or para positions, relative to the
position of the phenyl-phenyl bond. In a few instances, a methoxy
group is found instead of a second hydroxyl. This direct mechanism
appears to operate, therefore, irrespective of the degree of
chlorination, and, for the most part, irrespective of the position of
the chlorine substituents. In the rabbit, some exceptions have been
found that involve: a chlorine shift and the removal of a chlorine, in
the case of the 4,4'-dichlorobiphenyl, the formation of a dihydrodiol
at the meta and para positions, in the case of 2,5,2',5'-tetra-
chlorobiphenyl, and the removal of a chlorine from one of the rings,
in the case of 2,4,5,2',4',5'-hexachlorobiphenyl (Sundström et al.,
1976a).
Studies carried out by Matthew & Anderson (1975b) and Tuey & Matthews
(1977) showed that monochloro- and dichlorobiphenyls were rapidly
metabolized and excreted and that pentachloro- and hexachlorobiphenyls
were poorly metabolized and retained longer in the adipose tissue and
skin. The situation for the tetrachlorobiphenyls is more complicated.
The following analysis is based on the data on metabolites identified
and reported in the review by Sundström et al. (1976a).
6.5.2 Dichlorobiphenyls
Consideration of the various dichloro- isomers shows that, when
chlorines are only on one ring, hydroxylation occurs on the
nonchlorinated ring. Single hydroxylation occurs para to the
phenyl-phenyl bond; if another hydroxylation occurs, it is always
meta to the phenyl-phenyl bond. This holds true for the 3 different
isomers tested: 2,3-; 2,4-; and 3,4-dichlorobiphenyls. When the
dichloro-compounds are symmetrically chlorinated on each ring, as in
2,2'-; 3,3'-; and 4,4'-dichloro compounds, the same pattern applies
generally, but with a variation on the theme and an exception in the
case of 4,4'-dichlorobiphenyl. In the case of 2,2'- and 3,3'-dichloro
compounds, monohydroxylation occurs meta and para to the
phenyl-phenyl ring, respectively. In both cases, double hydroxylation
involves both meta and para positions on the same ring, (that is,
meta and para to the phenyl-phenyl bond). In the case of
4,4'-dichlorobiphenyl, there appears to be a difference in the rat.
The monohydroxy- derivative is meta, but the dihydroxy derivative is
ortho and meta to the phenyl-phenyl bond. Not only is the rat
metabolism of the 4,4'-compound an exception, but the rabbit also
shows an unusual response to this compound. In the rabbit, the
monohydroxy-derivative is the same meta hydroxy found in the rat,
but, instead of a dihydroxy- compound, the rabbit produces a chlorine
shift and a single hydroxy group in the para position as well as
dechlorination and hydroxylation in the para position. These latter
products have been considered as characteristic of the arene oxide
intermediate pathway. Nevertheless, among the 6 different dichloro-
isomers examined, all produced a monohydroxy- derivative, either meta
or para to the phenyl-phenyl bond, and all but the 4,4' produced
dihydroxy- derivatives were meta and para on the same ring to the
phenyl-phenyl bond.
The absence of a substitution at 4,4'- with vicinal unsubstituted
positions cannot be correlated with rapid metabolism, since this
property is also shared by both a rapid and slowly metabolized isomer.
This is in direct contradiction to the often repeated statement "the
presence of at least two adjacent unsubstituted carbons, particularly
in positions 3,4-, or 5- or 3',4'- or 5'- is required for rapid
metabolism of chloro-biphenyl" (Jensen & Sundström, 1974b; Berlin et
al., 1975; Safe et al., 1975; Matthews & Anderson, 1976; NIOSH, 1977;
Matthews & Tuey, 1980).
6.5.3 Tetrachlorobiphenyls
Examination of the 2 different tetrachloro- isomers, 2,3,5,6,-
tetrachloro- and 2,5,2',5',-tetrachlorobiphenyl, showed that, in the
case of the molecule with all 4 chlorines on one ring, the products
were monohydroxy- derivatives meta or para, dihydroxy- derivatives
meta and para, and a para hydroxy- plus a meta methoxy- group
or a meta hydroxy- and a para methoxy- group, all on the
unsubstituted ring. The symmetrical 2,5,2',5'-tetrachlorobiphenyl in
the rat gave the meta hydroxy-, but in the rabbit a para hydroxy-,
and also in the rabbit a dihydro-dihydroxy-, on the meta and para
positions. The asymmetric 2,4,3',4'-tetrachlorobiphenyl gave
monohydroxy-derivatives, both in the meta position, either in the
three or five position. It seems that an alternative enzyme pathway is
available in the rabbit.
The level of retention was highest for 2,4,2'4'-tetrachlorobiphenyl
descending in the following order; 2,5,2',5'-, 3,5,3',5'-, 3,4,3',4'-,
2,3,2',3'-, and 2,6,2',6'-tetrachlorobiphenyl. Since no PCBs were
detected in the liver, and only small amounts of 2,3,2',3'- and
3,4,3',4'-tetrachlorobiphenyls in the carcass, it can be concluded
that these 2 isomers were readily metabolized and excreted. Both
compounds have unsubstituted vicinal positions. However, the compounds
slowest to be metabolized were 2,4,2',4'- and 2,5,2',5'-tetra-
chlorobiphenyls, which also have unsubstituted vicinal positions.
Metabolic restriction cannot be entirely attributed to substitution at
the 4,4'- positions (Kato et al., 1980), since this also occurred in
3,4,3',4'-tetrachlorobiphenyl, which was removed relatively rapidly.
The excretion of the monohydroxy metabolites of 3,4,3',4'-tetra-
chlorobiphenyl and 2,4,3',4'-tetrachlorobiphenyl (orally administered)
in rats has been demonstrated by Yoshimura et al. (1973); Yamamoto &
Yoshimura (1973); Yoshimura & Yamamoto (1975); and Yoshimura et al.
(1974). They demonstrated that the metabolites of the first isomer
were 2-hydroxy- or 5-hydroxy- compounds, while the metabolites of the
second isomer were 5-hydroxy- and 3-hydroxy- compounds. All hydroxy
metabolites were excreted non-conjugated via the bile and no parent
isomers were found in the bile. Yoshimura & Yamamoto (1975) found that
unchanged 2,4,3',4'-tetrachlorobiphenyl was excreted through the
intestine, when it was intravenously injected in rats with the bile
duct ligated, while no metabolite of this isomer was excreted by this
route.
The results with the 2,5,2',5'- molecule are probably related to an
arene oxide pathway. Direct evidence for this was reported by Forgue
et al. (1980), who showed that 3,3,3,-trichloropropene-1,2-oxide,
which is an inhibitor of epoxide hydrase, blocked the formation of the
suspected arene oxide metabolites. The arene oxide mechanism
supposedly operates in rabbits and monkeys for 2,5,2',5'-tetrachloro-
biphenyl and, possibly, also in rats for 2,4,5,2',4',5'-hexachloro-
biphenyl. Isomers that may utilize the arene oxide pathway, to some
extent, are: 4,4'-dichloro-; 2,5,2',5'-tetrachloro-; and
2,4,5,2',4',5'-hexachlorobiphenyl.
6.5.4 Hexachlorobiphenyls and higher chlorinated compounds
The symmetrical hexachlorobiphenyls were used in a study by Matthews &
Tuey (1980), in which Sprague-Dawley rats were injected intravenously
with the PCBs and killed at increasing time intervals from 15 min to
42 days. The 2,3,6,2',3',6'- isomer was rapidly metabolized and
excreted compared with the other isomers, which were slowly
metabolized and excreted with much longer half-lives. The results
indicated that the metabolism of hexachlorobiphenyls is slow, when the
position of the chlorine atoms is such that arene oxide formation is
inhibited.
2,3,6,2',3',6'-Hexachlorobiphenyl produces only one metabolite in the
rat: 2,3,6,2',3',6'-hexachloro-4'-hydroxybiphenyl, which is believed
to be the result of arene oxide formation. All commercial mixtures of
PCBs will contain congeners that could be metabolized via the arene
oxide pathway. However, it does not seem to be the major pathway of
metabolism for most of the components of the commercial products,
since most of the higher congeners will not have vicinal unsubstituted
carbons.
The metabolic data on individual isomers shows that, at least up to
hexachloro- compounds, ordinary hydroxylation can take place. It is
reasonable to consider that it is not only as a consequence of poor
metabolism that pentachloro- and hexachloro- compounds are persistent
in the tissues, but rather that they are not metabolized as readily,
because they are sequestered from tissues in which the bulk of the
metabolism takes place. In support of this position, it has been shown
by Matthews & Anderson (1975b) that, when animals are caused to lose a
substantial portion of body weight, the stored higher chlorinated
compounds can, indeed, be metabolized. Octachloro- and
decachlorobiphenyls would not be expected to be easily metabolized,
simply because there are few or no sites for hydroxylation to take
place. It was found (Vodicnick & Lech, 1980; Vodicnick et al., 1980)
that almost the entire body burden of 2,4,5,2',4',5'-hexa-
chlorobiphenyl was removed from mothers given this PCB and that it was
transferred to their offspring via the nursing mother's milk. In mice,
the preferential distribution of this PCB in milk reflects the high
fat content of mouse milk.
Virgin, female, Sprague-Dawley mice, injected ip with 50 or 100 mg
[14C] 2,4,5,2',4', 5' -hexachlorobiphenyl/kg body weight in corn oil
for 2 weeks, prior to mating, eliminated virtually their entire body
burden of the compound through milk during one lactation cycle
(Gallenberg & Vodicnick, 1987).
Storage is caused by lack of metabolism and also implies that the
availability of adjacent unsubstituted carbons is the determinant for
metabolism. Direct hydroxylation reactions do not require
unsubstituted adjacent carbons. Rapid storage in fat is the
rate-limiting factor in the removal of most PCBs, with the exception
of isomers that might be very rapidly metabolized by arene oxide
formation, such as 2,3,6,2',3',6'-hexachlorobiphenyl.
Matthews & Anderson (1975b) extended their study to include a fasting
period to reduce the weight of the test rats and showed that severe
fasting mobilized stored PCBs and brought them into the metabolic
pool.
6.5.5 Retention and turnover
Mizutani et al. (1977) studied the pharmacokinetic behaviour of 6
different tetrachlorobiphenyls. They administered mice the 6 isomers,
at 10 mg/kg body weight, for 20 days. The isomer concentrations in the
liver and the remainder of the carcass were determined at various
times during the recovery period. They found that the accumulated body
burden was a function of both storage ratio and biological half-life.
The results of Mizutani et al. (1977) suggest that the correlations
claimed between the position of the chlorine substituents and storage
or metabolic activity (Kato et al., 1980; Matthews & Tuey, 1980) are
not simply explained.
The hypothesis that the position of the chlorine atoms alone
determines the rates of metabolism, accumulation, and excretion does
not appear to be entirely supported. This idea has been used to
support the notion that PCBs with unsubstituted vicinal carbon atoms
favour metabolism by arene oxide formation.
6.5.6 Appraisal
The results of most studies suggest that PCBs are absorbed by the
organ systems (gastrointestinal tract, lung, and liver), representing
the likely routes of entry into the body. This is particularly true
for the gastrointestinal tract where absorption is rapid. PCBs, once
absorbed, are usually distributed in a biphasic manner and are rapidly
cleared from the blood and accumulated in the liver and adipose
tissue, or they can be metabolized in the liver, to form metabolites
that are excreted in the urine and bile. In some studies on humans,
the skin, an organ rich in adipose tissue, had a high PCB content,
whereas the brain content was low. This distribution can also include
the fetus and human milk, an extension of the adipose tissue system in
the body. Mobilization of PCBs from fat appears to depend on their
rates of metabolism. Metabolic pathways include hydroxylation, and
conjugation with thiols and other water-soluble derivatives, some of
which can involve reactive intermediates, such as the arene oxides.
The most important pathway seems to be hydroxylation and subsequent
conjugation. This pathway is facilitated by the presence of at least
one pair of unoccupied vicinal carbon atoms in the PCB structure.
Persistence in tissue is not correlated with high toxicity.
Differences in toxicities among PCBs may be associated with specific
metabolites and/or their associated intermediates.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Toxicity for microorganisms
7.1.1 Freshwater microorganisms
Zullei & Benecke (1978) used the motility of filamentous blue-green
algae (Cyanophyceae) of the genus Phormium as a bioassay for the
rapid determination of the toxicity of various compounds. They tested
the relative toxicity of purified, specific chlorobiphenyls and of PCB
commercial mixtures (Clophen). Inhibition of motility was greatest in
the presence of chlorobiphenyls of low chlorination. All mono- and
dichlorinated isomers were inhibitory at the test concentration of
100 µg per test spot of algae. Tetra- and hexachlorobiphenyls did not
have any effects; the effects of trichlorobiphenyl isomers varied, the
2,5,2' isomer not producing any effects and the 2,4,4' and 3,4,4'
isomers being inhibitory. Tests using the commercial mixtures
confirmed the greater toxicity of low chlorination levels; Clophen A30
was more toxic than Clophen A60, though the presence of more than
expected, low-chlorinated compounds in the Clophen A60 reduced the
difference in toxicity.
Cultures of the green alga Chlorella pyrenoidosa were incubated with
1 mg/litre of Aroclors 1242, 1254, and 1268 (Hawes et al., 1976a).
The initial culture was 5 days old with a cell density of 27 ×
106 cells/ml. After 8 h of incubation with the PCBs, cell densities
in the cultures were 64% lower than controls for Aroclor 1242, 45%
lower for Aroclor 1254, and 36% lower for Aroclor 1268. As the study
progressed, the cell densities in the cultures improved relative to
the controls; cell density in the Aroclor 1254 culture was equal to
that in the control by 129 h and the density in the Aroclor 1268
culture was equal to the control by 59 h. Although the density in the
Aroclor 1242 culture remained much lower than that in the control
throughout the culture period, there was evidence of recovery with
this compound. The toxicity of the Aroclors was inversely proportional
to their degree of chlorination. A concurrent investigation of the
primary productivity of the alga (Hawes et al., 1976b), suggested that
the productivity of individual cells was stimulated by the Aroclors,
with a positive relationship between the level of chlorination and the
effect. However, this did not take into account the differences in
density between cultures (PCBs reduce growth through cell division)
and the authors pointed out that the response of the alga to the PCBs
was not simple. They stated that the density, culture age, and Aroclor
type were all factors that influenced response.
Larsson & Tillberg (1975) cultured the green alga Scenedesmus
obtusiusculus, in a liquid medium, in concentrations of Aroclor 1242
ranging between 10 and 1000 µg/litre. Growth was reduced at
concentrations of 300 µg Aroclor/litre or more; viability was only
affected at the highest concentration of 1000 µg/litre. Reduced
phosphate uptake, which was nearly identical in light or in darkness,
was reduced from 300 µg/litre upwards. The authors regarded this as a
result of an effect on the plasmalemma. At 800 µg PCBs/litre, the
results of some studies suggested an effect of the uncoupling of
oxidative phosphorylation; at 1000 µg/litre, both respiration and
oxygen evolution were inhibited.
The effects of various Aroclors on the respiration and photosynthesis
of the green alga Chlorella vulgaris were examined by Sinclair et
al. (1977). Aroclor 1221, at a final concentration in the medium at
10-4 mol/litre (= 192 mg/litre based on an average relative molecular
mass for the Aroclor at 192), produced a rate of oxygen production in
the light of 43% of control levels and a rate of oxygen uptake in the
dark of 59% of control levels. The Aroclors were dissolved using
dimethylformamide (DMF) as a solubilising agent; whilst this had some
effects on the parameters measured, they were very little compared
with the effects of the Aroclors (94% of control levels). A
dose-response curve of the effect of Aroclor 1221 on the respiratory
uptake of oxygen in the dark, in the presence of glucose, showed that
there was already a marked effect at a concentration of
10-7 mol/litre and that a maximum (at 50% inhibition) was reached at
a concentration of 10-6 mol/litre (= 1.92 mg/litre).
A further series of studies was performed to investigate the
individual processes of oxygen exchange in the alga. Respiration in
the dark was investigated in the absence of added glucose, to monitor
"endogenous" respiration, which was found to be stimulated by Aroclor
1221 at concentrations of 10-4 mol/litre or more. Net oxygen
production in the light (photosynthetic oxygen production minus
respiratory oxygen usage) was reduced at a concentration of
10-4 mol/litre. At this concentration of Aroclor 1221, there was
approximately 50% stimulation of endogenous respiration and
approximately 50% inhibition of net oxygen production. Calculated
photosynthetic rate was unaffected by the Aroclor. Increasing light
intensity reduced the effect of Aroclor 1221 on net oxygen production;
intensities of 8.2 × 104 ergs/cm2 virtually eliminated the effect.
Other measures directly or indirectly associated with photosynthesis
(fluorescence, oxygen evolution in flashing light, the Emerson
enhancement phenomenon) were not affected by the Aroclor. The authors
suggested that the photosynthetic apparatus of Chlorella was
unaffected by Aroclor 1221; the major, and probably the only, effect
of the PCBs being a stimulation of endogenous respiration rate.
Results with Aroclors 1242 and 1268 were consistent with those for
Aroclor 1221; both inhibited net oxygen production in the light and
glucose-driven respiration in the dark, but stimulated endogenous
respiration in the dark (Sinclair et al., 1977).
Luard (1973) reported inhibition of 14C uptake by the green alga
Scenedesmus quadricauda at concentrations of Aroclor 1254 as low as
0.1 µg/litre. At this concentration, the Aroclor caused a 20%
inhibition of 14C uptake, which rose to 65% inhibition at 1 mg/litre.
A marked effect of the initial number of cells in the incubation tube
on the toxicity of Aroclor 1254 for the green alga Chlorella
pyrenoidosa was demonstrated by Cole & Plapp (1974). At a constant
concentration of 1 mg Aroclor 1254/litre, the numbers of algal cells
in the initial incubation medium were as varied as 1, 10, 100, or
1000 µg alga/ml of medium. At the highest inoculation rate, the growth
of the alga was unaffected by the Aroclor. At lower initial
inoculation, the rate of growth was reduced to between 11 and 55% of
control levels. A comparable effect was found using 14C fixation as
the parameter; with inoculation rates of 1000 µg alga/ml medium, the
Aroclor did not have any effects, whereas lower inoculation rates
reduced carbon fixation to between 6 and 13% of control levels.
Mosser et al. (1972) showed that 2 species of freshwater algae
(Euglena gracilis and Chlamydomonas reinhardtii) were unaffected
by 100 µg PCBs/litre (type unspecified). Ewald et al. (1976)
determined the 48-h EC50 on the growth of Euglena gracilis to be
4.4 mg/litre for Aroclor 1221 and 55 mg/litre for Aroclor 1232.
Aroclor 1242 showed no inhibition at 100 mg/litre. Aroclor 1221, at
the EC50 concentration of 4.4 mg/litre, significantly depressed
carbon fixation and chlorophyll levels, but did not affect oxygen
consumption. Uptake of L-leucine was increased 2-fold, but
incorporation was not affected. Uridine uptake was significantly
decreased, but thymidine uptake and incorporation were not affected.
In studies by Glooschenko & Glooschenko (1975), 3 algal species from
the Great Lakes were cultured with Aroclor at concentrations of 1, 5,
10, 20, or 50 µg/litre. Cell numbers of the diatom Synedra acus were
reduced in culture from day 3 of treatment with Aroclor 1242, at 10,
20, or 50 µg/litre, and from day 7, with 1 or 5 µg/litre. The green
alga Scenedesmus quadricauda showed a lag phase of 3 days in all
concentrations of Aroclor 1242. After 3 days, exponential growth
occurred in all treatments, except for the highest dose levels (20 and
50 µg/litre) which showed little or no cell division. A second green
alga (Ankistrodesmus falcatus) was more sensitive, showing
significantly reduced cell numbers at all dose levels of Aroclor 1242.
Ankistrodesmus was used to examine the relative toxicities of
different Aroclors. Cell numbers were 57, 66, 36, and 53% of control
levels for Aroclors 1016, 1221, 1242, and 1248, respectively, after 2
days of culture. Carbon fixation, estimated as uptake of 14C from
solution, was 73, 98, 51, and 59% of control levels for the 4
Aroclors, respectively.
Dive et al. (1976) cultured the ciliate protozoan Colpidium campylum
with purified chlorobiphenyls and with a commercial mixture (Pyralene
3010). None of the 16 isomers affected the ciliate's growth and
reproduction at concentrations of 0.01 or 0.1 mg/litre; similarly,
Pyralene 3010 was not toxic at these concentrations. 2-Mono-
chlorobiphenyl showed little toxicity for the organism at 1 mg/litre
and little or no toxicity was demonstrated by the tetrachloro-,
pentachloro-, and hexachlorobiphenyls at this concentration or at
10 mg/litre (with the exception of 2,5,2',5'-tetrachlorobiphenyl,
which inhibited growth considerably, limiting it to about 10% of
controls at 10 mg/litre). 4,4'-Dichlorobiphenyl was not toxic at any
concentration tested (up to 10 mg/litre) but both 2,3- and
2,5-dichlorobiphenyls were toxic, killing all organisms at both 1 and
10 mg/litre. These results are comparable with other reports that the
lower chlorinated biphenyls are the most toxic for microorganisms;
differences in toxicity could not be explained by the differential
uptake of the different isomers.
It was reported by French (1976) that the EC50 for growth inhibition
of Aroclor 1254 on the flagellated protozoan Crithidia fasciculata
was 10.5 mg/litre. The PCBs slightly inhibited (after 6 h) and then
increased (after 24 h) the carbon dioxide evolution of cultures
utilizing D-glucose. After 6 h exposure, the uptake and incorporation
of thymidine and uridine (but not L-leucine) were inhibited;
inhibition was transient and returned to normal after 12 or 24 h. Fine
structural changes were noted after exposure to PCBs including:
deterioration of the kinetoplast, mitochondrial or cellular swelling,
and the presence of concentric membrane arrays. It was concluded that
cell population growth inhibition was due to disruption of uptake,
incorporation of nucleic acids, and loss of cell regulatory capacity.
7.1.2 Marine and estuarine microorganisms
Bourquin & Cassidy (1975) and Bourquin & Kiefer (1975) investigated
the effects of Aroclors 1016 and 1242 on 85 bacterial isolates from
various estuarine environments near Pensacola, Florida. Twenty six of
the 85 isolates were inhibited to various extents by 0.5 mg of either
of the PCBs, applied to a disc placed on the surface of an agar plate
on which the bacteria were growing. Zones of inhibition ranged from 14
to 20 mm in diameter. Cultures that showed sensitivity to Aroclor 1242
were also inhibited by Aroclor 1016. Sixty five percent of isolates
inhibited by 0.5 mg of Aroclor 1242 were still sensitive at 0.1 mg,
and 58% of those sensitive to Aroclor 1016 at 0.5 mg were still
sensitive to 0.1 mg. Four isolates were examined in a liquid medium.
Inhibition of the cultures was characterized by a greatly extended lag
phase (extended from 2 h to at least 14 h); when growth occurred,
growth curves were parallel with those of the controls. The
physiological activity of sensitive and insensitive isolates were
investigated to try to explain the reasons behind sensitivity. More of
the sensitive isolates were amylase and gelatinase producers (76 and
86%, respectively, compared with 33 and 42%, respectively, for the
whole range of isolates). The significance of this observation is
unclear. Because of the method of exposure in this screening exercise,
it is difficult to relate the results to exposure in natural waters
and to draw conclusions about likely hazards for aquatic bacteria.
Kleppel & McLaughlin (1980) determined the toxic threshold of Aroclor
1254 for the estuarine diatom Skeletonema costatum to be between
3 × 10-9 and 3 × 10-8 µg/cell. When the effect of cell density on the
toxicity of the Aroclor for the organism was examined, maximum
inhibition occurred with the lowest inoculum rates.
Michaels et al. (1982) estimated the effects of Aroclor 1254 on
photosynthesis in the marine diatom Thalassiosira pseudonana
( = Cyclotella nana) by monitoring the uptake of 14C-carbon
dioxide. The numbers of viable cells in the culture were also
estimated. Total cell numbers were estimated at regular intervals
during the 48 h of the experiment and the minimum number of viable
cells required to produce the increase in numbers between periods
calculated. This gave an estimate of the viable numbers,
retrospectively, for each period. Inhibition of 14C-uptake per
culture, per cell, and per viable cell was evident within 1 h of the
start of the incubation. By 48 h, the 14C uptake per culture was
reduced to 0.2% of control levels, per cell, to 13% of control levels,
and per viable cell, to 34% of control levels. The authors concluded
that the effect of Aroclor 1254 on the diatom is a combination of
inhibition of carbon assimilation by individual cells and inhibition
of cell division.
In an earlier study, Fisher & Wurster (1973) exposed 2 estuarine
diatoms (Thalassiosira pseudonana and Rhizosolenia setigera) and
an estuarine green alga (Dunaliella tertiolecta) to Aroclor 1254 at
0.1 or 10 µg/litre, cultured over 100 h. There was no effect on the
growth of the alga. T. pseudonana was unaffected by the PCB at
0.1 µg/litre. The effect of the Aroclor on T. pseudonana at
10 µg/-litre was dependent on temperature; there was a 17% reduction
in growth rate at 25°C, a 44% reduction in growth rate at 18°C, and a
58% reduction in growth rate at 12°C. The growth of control cultures
was greatest at the highest temperature. The growth of R. setigera
was completely stopped by all concentrations of PCB tested, for the
first 48 h of culture. When growth resumed, the degree of inhibition
was greater at 10°C than at 15°C. Fisher et al. (1976) exposed the
marine diatom Thalassiosira pseudonana to 1 µg Aroclor 1254/litre in
cultures containing various levels of nitrate nutrient. The toxic
effects of the PCBs on the growth of the diatom were greatest at low
nitrogen levels. Analysis of variance showed the diatom to be
significantly dependent for growth on nitrogen concentration and on
PCB concentration, and that the PCB effect was significantly nitrogen
dependent. The authors pointed out that marine phytoplankton are often
nitrogen-limited in nature and suggested that the effects of
pollutants, such as PCBs, may, therefore, vary with season. The
greatest effects are likely to occur during bloom conditions, when
competition for nutrients is greatest. Fisher (1975) considered that
the presence of PCBs, even at concentrations far above the maximum
recorded level in sea water, would not affect the overall carbon
fixation by phytoplankton. Although the cell division of some
organisms was adversely affected, enough insensitive species existed
to compensate for the sensitive species. Species diversity and
community structure were likely to be affected.
In a study by Craigie & Hutzinger (1975), 6 marine algae were cultured
for 6 days in the presence of commercial mixtures of PCBs (Aroclors
1221 to 1262; Phenoclors DP3 to DP6), PCTs (Aroclor 5460), and
specific chlorobiphenyls. Each compound or mixture was applied to the
culture medium at 2 concentrations (1 and 100 mg/litre). The algae
were representatives of 6 different classes: Bacillariophyceae -
Skeletonema costatum, Thalassiosira fluviatilis; Chlorophyceae -
Dunaliella tertiolecta; Chrysophyceae - Monochrysis lutheri;
Prainophyceae - Platymonas sp.; Rhodophyceae - Porphyridium sp.;
Xanthophyceae - Olisthodiscus sp. All were cultured at 20°C. Growth
was estimated by turbidity. The response to particular Aroclors was
species dependent; the least sensitive species was Dunaliella and
the most sensitive was Olisthodiscus. At 1 mg/litre of the PCB
mixtures, there was relatively little inhibition of Dunaliella,
Platymonas, Skeletonema, or Thalassiosira. Olisthodiscus was
completely inhibited by Aroclors 1248, 1254, 1260, and Phenoclor DP4.
The Phenoclor series was more toxic for Olisthodiscus. than the
Aroclors. Porphyridium and Monochrysis showed intermediate
sensitivity. Generally, the Aroclors with higher chlorination levels
were less toxic for all species than the Aroclors with lower
chlorination levels. Results with pure, specific chlorinated biphenyls
confirmed that highly chlorinated compounds are less toxic than those
with only 1-4 chlorine atoms per molecule. Experiments with biphenyls
containing identical percentages of chlorine showed that biological
response was highly dependent on the structure of the molecule. The
2,4,2',4'-tetrachlorobiphenyl was more toxic for both Dunaliella and
Olisthodiscus than the 2,5,2',5'- isomer, and both were more toxic
than either the 2,3,4,5- or the 3,4,3',4'- isomers. The last was not
toxic for any of the algae, even when added at 100 mg/litre.
Luard (1973) reported a significant reduction in 14C uptake by the
estuarine green alga Dunaliella tertiolecta in the presence of
Aroclor 1254, at 100 µg/litre. The culture showed 14C uptake at 65%
of control levels. At 1000 µg/litre, uptake was further reduced to 59%
of controls. The 14C uptake was unaffected at 10 µg/litre.
7.1.3 Soil microorganisms
When Murado et al. (1976) added Aroclors to a liquid or solid medium
on which the soil microfungus Aspergillus flavus was cultured,
mycelial growth was reduced progressively as the dose of Aroclor 1254
increased from 5 to 50 mg/litre in liquid culture. At 25 mg/litre, the
dry weight of the mycelium was reduced to 1.4, 3.4, 3.9, 3.3, and
54.6% of control levels by Aroclors 1232, 1242, 1248, 1254, and 1260,
respectively. At the same time, the relative RNA content of the
mycelium increased, rising from a control level of 5.9 µg RNA/mg dry
weight to between 13.2 and 18.6 µg RNA/mg dry weight for Aroclors 1232
to 1254. Aroclor 1260 had no marked effect on RNA. The DNA content was
not affected by any treatment. Cultures on solid medium showed a delay
in sporulation and a decrease in the diameter of colonies at doses up
to 20 µg/cm2.
Glooschenko & Glooschenko (1975) cultured the soil alga Navicula
pelliculosa with Aroclors 1016, 1221, 1242, and 1248 at a
concentration of 20 µg/litre. The numbers of cells in the culture,
after 2 days, were reduced to 66, 53, 46, and 56% of control levels
for the 4 Aroclors, respectively.
7.1.4 Plankton communities
Phytoplankton communities from 2 lakes, one oligotrophic and one
eutrophic, were exposed to PCBs, as Clophen A50, at 26 µg/litre
(Södergren & Gelin, 1983). In the community from the eutrophic lake
with a greater biomass of phytoplankton, measurement of 14C uptake,
monitored immediately after the addition of the PCBs, showed a
reduction of 34% compared with the controls. Monitoring carbon
fixation 16 h later showed the phytoplankton recovering from the
effect of the PCBs, with only 21% inhibition relative to the controls.
The results differed in the community from the oligotrophic lake. 14C
uptake immediately after the addition of the Clophen was 70% less than
that in the controls; 16 h later, the effect was greater, at 84%
inhibition. The authors pointed out that these results parallel
findings in pure culture showing that a high density of organisms
reduces the effects of PCBs.
Mosser et al. (1972) first demonstrated the effects of PCBs on both
single and mixed cultures of marine phytoplankton. Two organisms, a
marine diatom (Thalassiosira pseudonana) and a marine green alga
(Dunaliella tertiolecta) were used. The diatom is sensitive and the
alga insensitive to PCBs. The application of PCBs (not specified) to
mixed cultures changed the usual dominance of the diatom into
dominance of the alga, even at concentrations of the PCBs (1 and
10 µg/litre) that had no discernible effects on the diatom in pure
culture.
The effect of Aroclor 1254 on the relative biomass of 2 species of
marine diatoms Phaeodactylum tricornutum and Cyclotella cryptica
was examined by Lundy et al. (1984). The diatoms were cultured
together in either 10 or 20 µg PCBs/litre for 6 days. At both
concentrations of Aroclor, the ratio between the species was shifted
in favour of Phaeodactylum. After 6 days, the ratio of
Phaeodactylum: Cyclotella was 0.8 in the controls and 2.63 in the
treated cultures (10 µg Aroclor 1254/litre).
Biggs et al. (1979) exposed a mixed culture of 2 marine algae to PCBs
(Aroclor 1254) at 50 µg/litre. In the control culture, Thalassiosira
pseudonana became the dominant organism over Dunaliella
tertiolecta. After exposure to the PCBs, the Thalassiosira was
affected, but the Dunaliella was not. By day 2 of Aroclor 1254
exposure, Dunaliella was the dominant species. Fisher et al. (1974)
showed a similar effect with the greater effects of PCBs on the
sensitive diatom Thalassiosira pseudonana when the organism was in
competition with other organisms. The effect was also demonstrated
using natural communities of phytoplankton, when Thalassiosira was
also selectively affected.
Biggs et al. (1978) exposed a natural community of marine
phytoplankton (from which large detritus and zooplankton had been
filtered) to Aroclor 1254 at either 5 or 10 µg/litre. Cell division,
chlorophyll-a synthesis, and 14C uptake were monitored, as well as
particle size. Treatment with the Aroclor reduced community growth
rates by 20-50%, compared with controls. Growth had not fully
recovered after 10 days. The 14C uptake was reduced for 6 days. The
control cultures became dominated by algae larger than 8 µm in
diameter. The PCB-treated cultures showed strong inhibition of these
larger algal cells and the culture became dominated by small cells.
In a study by Iseki et al. (1981), a natural community of plankton was
exposed, in situ, in a marine environment, to Aroclor 1254 in large
bags holding 68 m3 of seawater. The Aroclor was added to the bag
giving an initial concentration in the upper layers of about
40 µg/litre (15 µg/litre at 10 m depth). Over time, the levels of PCB
fell in the upper layer and increased in the lower layer in the bag.
Six days after addition, the concentrations at all depths were less
than 15 µg/litre. Immediately after addition of the PCBs, the rate of
sedimentation of the particles increased; these particles were thought
to be dead or senescent large cells, such as diatoms, and this
sedimentation was assumed to be responsible for the major part of the
loss of the PCBs from the water. Zooplankters were eliminated from the
bags by this level of Aroclor; no recovery was seen over the 21 days
of the experiment. Large diatoms were selectively eliminated from the
bags and replaced by small flagellates as the dominant organism.
Moore & Harriss (1972, 1974) exposed a natural community of plankton
to PCBs (as Aroclor 1242) at 10 or 25 µg/litre. The population was
collected from natural water, placed in glass bottles suspended
in situ, and monitored for uptake of 14C, added to the bottles as
bicarbonate. After incubation, the community was separated into small
"nannoplankton" and larger "net-plankton" by filtration at a mesh size
of 53 µm. Nannoplankton radiocarbon uptake accounted for 72.6% of the
total community carbon uptake and was not affected by either of the
concentrations of Aroclor 1242. The net-plankton uptake of 14C was
reduced by 56% at 10 µg Aroclor/litre and by 58% at 25 µg/litre. The
authors suggested that PCBs at levels found in natural waters would
alter the species diversity or community structure of microorganisms
and that this might affect higher levels of the food chain with
specialist feeders utilizing one type of prey. O'Connors et al. (1978)
exposed natural communities of phytoplankton, in situ, in dialysis
bags in a salt marsh. Large zooplankton herbivores were removed by
filtration through a mesh. Aroclor 1254 was added to the bags to give
water concentrations of 1-10 µg/litre. Larger diatoms were selectively
inhibited by the Aroclor, even at the lower dose (1 µg/litre). The
authors suggested that, not only would phytoplankton communities be
affected by PCBs in natural waters, but that the effect would be
carried forward through the food chain. Gelatinous predators, such as
jellyfish, could be selected at the expense of fish, since fish tend
to depend directly, or indirectly, on the larger phytoplankton.
Natural communities of phytoplankton from a stream and a reservoir
were cultured with Aroclors 1232 and 1254 by Kricher et al. (1979).
Although the algal communities were different in composition, both
Aroclors exerted similar effects on both communities; primary
productivity was reduced in a dose-dependent manner. Aroclor 1232 was
more toxic than Aroclor 1254 at 1 mg/litre. Algal species within the
populations were differentially affected by the Aroclors; diatoms were
particularly susceptible and treatment produced disproportionate
numbers of blue-green algae, such as Anacystis. The authors pointed
out that the insensitive species still accumulated PCBs and,
therefore, formed the basis for the accumulation of the Aroclors in
aquatic food chains.
7.1.5 Interactions with other chemicals
Mosser et al. (1974) investigated the interactions between Aroclor
1254, DDT, and DDE in a marine diatom Thalassiosira pseudonana. The
diatom was cultured for 4 days with either 10 or 50 µg Aroclor
1254/litre, 100 µg DDE/litre or 500 µg DDT/litre (with each chemical
alone or in combination). The PCBs alone (at 10 µg/litre) and the DDE
alone had little effect on the growth of the diatom; when combined
these treatments were synergistic, growth being less than half that of
the control culture. Higher concentrations of either compound
increased the inhibitory effect. In contrast, DDT reduced the toxic
effects of PCBs at higher concentrations (50 µg/litre). Treatment with
the Aroclor alone at 50 µg/litre almost stopped the growth of the
diatom. Simultaneous treatment with DDT at 500 µg/litre restored
growth to 60-70% of control levels. Lower concentrations of DDT had a
comparable, but reduced, effect. Addition of the DDT to the medium, 12
or 24 h after culture had begun in the presence of PCBs, also reversed
the inhibitory effect.
7.1.6 Tolerance
Fisher et al. (1973) showed that strains of diatoms, isolated from the
Sargasso Sea, were more sensitive to the effects of PCBs than isolates
of the same species, obtained from estuaries or the continental shelf.
It was suggested by the authors that the difference in sensitivity was
derived from the variable environment of the estuarine strains; these
strains are able to cope with wide variations in their living
conditions, not experienced in the open ocean of the Sargasso, and,
therefore, were better able to cope with stress from chemical
pollutants.
When Cosper et al. (1984) compared the sensitivity of clones of 2
species of diatom (Asterionella japonica and Ditylum brightwelii)
from polluted and unpolluted sites, Asterionella was less sensitive
than Ditylum to the action of PCBs (Aroclor 1254); some strains of
the former were tolerant of 25 µg Aroclor/litre whereas no strains of
the latter could tolerate this concentration. There was evidence that
strains of Asterionella from the polluted site were more tolerant
than the same species from unpolluted sites. One strain, from the
polluted site, was tolerant to Aroclor 1254 at 50 µg/litre.
7.2 Toxicity for aquatic organisms
7.2.1 Aquatic plants
Mahanty (1975) grew the aquatic angiosperm Spirodela oligorhiza in a
sterile culture solution, to which had been added Aroclor 1242, at
concentrations of 5-100 mg/litre. The numbers of colonies were counted
throughout the 14-day exposure. The highest dose (100 mg/litre) was
found to be lethal. At both 25 and 50 mg/litre, though there was some
growth, the colonies were small and showed morphological differences
from control colonies including: smaller fronds, in larger numbers
than usual, and a characteristic striped pattern of chlorosis on the
fronds. Even at 5 mg/litre, growth was reduced by 50% (as recorded by
the number of colonies and the fresh weight). Mahanty & McWha (1976),
using only the 5 mg/litre dose, found reduced growth, an unusual
striped pattern of chlorosis, and a reduction in the levels of
chlorophyll and total RNA, but no change in the levels of DNA. When
Mahanty & Fineran (1976) studied treated (5 mg/litre) and untreated
fronds of Spirodela by electron microscopy, they found almost
complete disorganization of the internal structure of the chloroplasts
in chlorotic tissue. Organization of other cell components was largely
unaffected.
7.2.2 Aquatic invertebrates
The acute toxicity of PCBs for aquatic invertebrates is summarized in
Tables 27 and 28. Toxicity is very variable between species, even
closely-related species. For most aquatic invertebrates, there is an
effect of degree of chlorination of the PCBs, but this is not a direct
correlation, either negative or positive, the most toxic PCBs often
being in the mid range of chlorination. Under flow-through conditions,
the toxicity of PCBs appears to be much higher. Over 96 h, under
static conditions, LC50 values ranged between 12 µg/litre and
> 10 mg/litre for different organisms and different PCBs.
MATC (maximum acceptable toxicant concentrations) were set for various
PCBs by the US EPA (1980). These are expressed as a range between
no-observed-effect values and lowest concentration tested that
produced a measurable effect. For Daphnia magna, these values were
1.2 and 3.5 µg/litre for Aroclor 1248, and 2.5 and 7.5 µg/litre for
Aroclor 1254. For the scud (Gammarus pseudolimnaeus) values for
Aroclor 1242 were 2.8 and 8.7 µg/litre and values for Aroclor 1248
were 2.5 and 5.1 µg/litre. Aquatic larvae of the midge (Tanytarsus
dissimilis) had a no-observed-effect level of 0.5 µg/litre and a
lowest effective concentration at 1.2 µg/litre.
7.2.2.1 Short- and long-term toxicity
Roberts (1975) exposed the common mussel (Mytilus edulis) to
Aroclors 1242 and 1254 and studied byssus formation. The 24- and 48-h
EC50s for a reduction in the number of mussels byssally-attaching
were 2.2 and 3.0 mg/litre, respectively, for Aroclor 1254. EC50s
after exposure to Aroclor 1242 were 0.9 mg/litre for 24 h and
1.0 mg/litre for 48 h. Duke et al. (1970) maintained oysters
Table 27. Acute toxicity of PCB mixtures for freshwater invertebrates
Organism Size/age Stat/ Temperature Hardness pH PCB type Parameter Concentration Reference
flowa (°C) (mg/litre)b (Aroclor) (mg/litre)
Scud mature flow 15 272 7.4 1242 96 h - LC50 0.01 Mayer & Ellersieck
(Gammarus (1986)
pseudolimnaeus)
juvenile flow 18 1248 96 h - LC50 0.029 Nebeker & Puglisi
juvenile flow 18 1242 96 h - LC50 0.073 (1974)
Scud mature stat 21 44 7.1 1248 96 h - LC50 0.052 Mayer & Ellersieck
(Gammarus mature stat 21 44 7.1 1254 96 h - LC50 2.4 (1986)
fasciatus)
Glass shrimp mature flow 15 272 7.4 1254 168 h - LC50 0.003 Mayer & Ellersieck
(Palaemonetes (1986)
kadiakensis)
Crayfish early stat 21 44 7.1 1242 168 h - LC50 0.03 Mayer & Ellersieck
(Orconectes nais) instar (1986)
Crayfish early stat 21 44 7.1 1254 168 h - LC50 0.1 Mayer & Ellersieck
(Procambarus sp.) instar (1986)
immature stat 12 44 7.5 1254 96 h - LC50 > 0.55
Table 27. (cont'd)
Organism Size/age Stat/ Temperature Hardness pH PCB type Parameter Concentration Reference
flowa (°C) (mg/litre)b (Aroclor) (mg/litre)
Stonefly first stat 10 170 7.2 1016 96 h - LC50 0.61 Mayer & Ellersieck
(Pteronarcella year (0.42-0.88) (1986)
badia)
Damselfly late flow 15 272 7.4 1242 96 h - LC50 0.4 Mayer & Ellersieck
(Ischnura instar flow 15 272 7.4 1254 96 h - LC50 0.2 (1986)
verticalis)
Dragonfly late stat 21 44 7.1 1242 168 h - LC50 0.8 Mayer & Ellersieck
(Macromia sp.) instar stat 21 44 7.1 1254 168 h - LC50 0.8 (1986)
a Stat = static conditions: water not changed during the exposure; flow = flow-through conditions; concentration of
toxicant continuously maintained.
b Hardness expressed as mg CaCO3/litre, unless otherwise stated.
Table 28. Acute toxicity of PCB mixtures for marine invertebrates
Organism Size/age Stat/ Temperature Salinity PCB type Parameter Concentration Reference
flowa (°C) (%) (mg/litre)
Cockle adult stat 15 Aroclor 1248 48 h - LC50 > 10 Portmann &
(Cardium adult stat 15 Aroclor 1254 48 h - LC50 > 10 Wilson (1971)
edule) adult stat 15 Aroclor 1260 48 h - LC50 > 10
adult stat 15 Aroclor 1262 48 h - LC50 > 10
adult stat 15 Clophen A30 48 h - LC50 3.0
adult stat 15 Clophen A60 48 h - LC50 > 10
Eastern oyster adult flow 28 28 Aroclor 1016 96 h - EC50 0.01 Mayer (1987)
(Crassostrea
virginica)
Brown shrimp adult flow 30 29 Aroclor 1016 96 h - LC50 0.01 Mayer (1987)
(Penaeus
aztecus)
Brown shrimp adult stat 15 Clophen A60 48 h - EC50 > 10 Portmann &
(Crangon adult stat 15 Aroclor 1242 48 h - LC50 1.0 Wilson (1971)
crangon) adult stat 15 Aroclor 1248 48 h - LC50 0.3-1.0
adult stat 15 Aroclor 1254 48 h - LC50 3.0-10.0
adult stat 15 Aroclor 1260 48 h - LC50 > 10
adult stat 15 Aroclor 1262 48 h - LC50 > 10
adult stat 15 Clophen A40 48 h - LC50 0.3-1.0
adult stat 15 Clophen A30 48 h - LC50 1.0-3.3
adult stat 15 Clophen A50 48 h - LC50 3.3-10.0
Table 28. (cont'd).
Organism Size/age Stat/ Temperature Salinity PCB type Parameter Concentration Reference
flowa (°C) (%) (mg/litre)
Grass shrimp 1 day stat 25 25 Aroclor 1016 96 h - LC50 0.15 Mayer (1987)
(Palaemonets 3 days stat 25 25 Aroclor 1016 96 h - LC50 0.021
pugio) 6 days stat 25 25 Aroclor 1016 96 h - LC50 0.017
9 days stat 25 25 Aroclor 1016 96 h - LC50 0.019
12 days stat 25 25 Aroclor 1016 96 h - LC50 0.021
15 days stat 25 25 Aroclor 1016 96 h - LC50 0.024
18 days stat 25 25 Aroclor 1016 96 h - LC50 0.037
30 days stat 25 25 Aroclor 1016 96 h - LC50 0.044
adult stat 25 25 Aroclor 1016 96 h - LC50 0.052 (0.046-0.057)
adult flow 30 28 Aroclor 1016 96 h - LC50 0.012
1 day stat 25 25 Aroclor 1242 96 h - LC50 0.015
3 days stat 25 25 Aroclor 1242 96 h - LC50 0.019
6 days stat 25 25 Aroclor 1242 96 h - LC50 0.015
9 days stat 25 25 Aroclor 1242 96 h - LC50 0.017
12 days stat 25 25 Aroclor 1242 96 h - LC50 0.016
15 days stat 25 25 Aroclor 1242 96 h - LC50 0.024
18 days stat 25 25 Aroclor 1242 96 h - LC50 0.034
30 days stat 25 25 Aroclor 1242 96 h - LC50 0.041
adult stat 25 25 Aroclor 1242 96 h - LC50 0.057 (0.048-0.062)
a stat = static conditions: water not changed during the exposure; flow = flow-through conditions; concentration
of toxicant continuously maintained.
(Crassostrea virginica) at concentrations of 1, 10, and 100 µg
Aroclor 1254/litre and monitored shell growth over a period of 96 h;
the rates of shell growth were decreased by 19, 41, and 100%,
respectively. Lowe et al. (1972) found the growth rate (height and wet
weight) of young oysters (Crassostrea virginica) to be significantly
reduced after exposure, in flowing sea water, to 5 µg Aroclor
1254/litre, for 24 weeks. No effects on growth were observed at
1 µg/litre over a period of 30 weeks. Oysters exposed to 5 µg/litre
showed atrophy of the digestive diverticular epithelium and
degeneration of the vesicular connective tissues of the hepatopancreas
together with leukocytic infiltration. There was complete tissue
recovery after 12 weeks in clean water.
After exposing Daphnia magna to Aroclor 1254, over a period of 14
days under static renewal procedures, Maki & Johnson (1975) calculated
an LC50 of 24 µg/litre.
Nebeker & Puglisi (1974) calculated 3-week LC50 values, under static
conditions, for a range of Aroclors on Daphnia magna. Aroclors were
dissolved in acetone and triton X100 to maintain the PCBs in solution.
Tests were performed in raw Lake Superior water. Results are presented
in Table 29. The Aroclors most toxic for Daphnia had between 48 and
62% chlorination; the most toxic Aroclor was 1248. Under flow-through
conditions, renewing the original test concentration of the Aroclor
continuously, the Aroclors were much more toxic. Two-week LC50 values
for Aroclors 1248 and 1254 were 2.6 and 1.8 µg/litre, respectively,
while the 3-week LC50 for Aroclor 1254 was 1.3 µg/litre. Groups of 40
scud (Gammarus pseudolimnaeus) were exposed to various concentrations
of Aroclor 1242 under flow-through conditions. No animals survived
exposure for 2 months, at Aroclor concentrations of 26 µg/litre or
more.
Survival at lower exposures was 52% at 8.7 µg/litre and 77% at
2.8 µg/litre (control survival was low at 48%).
Duke et al. (1970) conducted acute, flowing-water bioassays on pink
shrimp (Penaeus duorarum). At 0.1 mg/litre, 100% of the shrimps were
killed within 48 h of exposure to Aroclor 1254. There was no mortality
after 48 h of exposure to 0.01 mg/litre Aroclor. In long-term
flowing-water bioassays, Nimmo et al. (1971a) found that Aroclor 1254,
at a concentration of 0.94 µg/litre killed 51% of juvenile pink
shrimps within 15 days. Juveniles were found to be more sensitive than
adults; 50% of adults were killed after exposure to 3.5 µg/litre over
35 days. There were no apparent symptoms of poisoning prior to death.
Table 29. Toxicity of various Aroclors for Daphnia magna in static testsa
Aroclor 3-week Confidence limits (95%)
LC50 (µg/litre)
1221 180 (158.0-205.0)
1232 72 (62.6-82.8)
1242 67 (55.4-81.0)
1248 25 (21.4-29.2)
1254 31 (25.8-37.2)
1260 36 (27.7-46.8)
1262 43 (37.0-49.9)
1268 253 (222.0-288.0)
a From: Nebeker & Puglisi (1974).
Striped hermit crabs (Clibanarius vittatus) were kept in static
seawater solutions containing 3, 5, 10, 15, 20, 25, or 30 µg/litre of
Aroclor 1254 for 96 h (Stahl, 1979). No deaths were reported, though
the crabs exposed to the higher concentrations (20, 25, and
30 µg/litre) were less active. Six crabs already exposed to 30 µg
PCBs/litre were then placed in solutions containing 300 µg/litre for a
further 96 h; there were still no deaths.
Vernberg et al. (1977) exposed fiddler crab (Uca pugilator) larvae
to concentrations of 0.1, 1.0, 5, 10, 50, or 500 µg Aroclor
1254/litre, for 96 h. They did not find any effects on survival at 0.1
or 1.0 µg/litre; Aroclor 1254 at 5 µg/litre increased deaths by 20%,
but the increase was not statistically significant. Exposure to 10 µg
PCBs/litre resulted in a 57% reduction in survival of larvae.
Increasing the PCB concentration to 50 µg/litre did not greatly
increase the effect. The 500 µg/litre concentration killed all larvae.
Increasing exposure time, at 5 µg/litre, produced a significant
reduction in survival after 14 days. Exposure of larvae to Aroclors
1016 or 1254 at 0.1, 1, or 5 µg/litre for periods of up to 168 h, was
then investigated. No significant effects were found on survival with
any concentration of Aroclor 1016, for up to 120 h of exposure. After
168 h, survival rates of larvae exposed to 1 and 5 µg/litre were
reduced to 61 and 66%, respectively. There was no effect at
0.1 µg/litre. Aroclor 1254 did not have any effects on survival at
concentrations of 0.1 or 1 µg/litre. Survival was reduced after
exposure to 5 µg Aroclor 1254/litre for more than 96 h, increasing
from 60-81% up to 168 h. Exposure to 10 µg Aroclor 1254/litre caused
55% deaths after 120 h; there were no further deaths after 168 h.
Fifty per cent of adult male crabs, exposed to Aroclor 1254 or Aroclor
1016 at 50 µg/litre, died after 2 days and after 4-6 days,
respectively. Females (50%) exposed to 50 µg/litre survived for 7 days
after exposure to Aroclor 1016 but for only 4 days after exposure to
Aroclor 1254.
Neff & Giam (1977) exposed juvenile horseshoe crabs (Limulus
polyphemus) to concentrations of Aroclor 1016 of 10, 20, 40, or
80 µg/litre, for up to 96 days. The crabs were divided into 2 groups:
group A consisted of juveniles at the late first tailed stage and
group B, of juveniles at the early second tailed stage. The authors
calculated LT50s (LT50: median survival time for a given exposure
concentration) of 20.8 days at 40 µg/litre and 20.3 days at
80 µg/litre for group A juveniles. The LT50 for group B crabs at
80 µg/litre was 61 days, but less than 50% had died within 96 days at
40 µg/litre.
7.2.2.2 Response to temperature and salinity
In a study by Vernberg et al. (1977), larval fiddler crabs
(Uca pugilator) were exposed to "sub-lethal" concentrations of
Aroclor 1254 and 1016 and the conditions of temperature (15-30°C) and
salinity (15-36%) varied. Exposure to 0.01 µg Aroclor/litre showed no
consistent effects of temperature or salinity, though the organisms
exposed under conditions furthest from the optimum (25°C and 30%) were
more likely to differ significantly from the controls. At the optimum
temperature and salinity, no deaths were recorded in adult crabs
exposed to 0.1, 1, 10, or 100 µg Aroclor 1254/litre for up to 3 weeks.
Lowering the salinity or increasing the temperature did not have any
effects on survival with Aroclor 1254 or 1016 at 5 µg/litre. Lowering
both salinity and temperature (to 5% and 10°C) caused 50% of crabs to
die between 21 and 28 days of exposure to Aroclor 1016 at 5 µg/litre,
but there were no lethal effects of Aroclor 1254 at the same
concentration. Lowering the temperature further (7°C and 5%) reduced
the 50% survival time to between 5 and 8 days for both Aroclors.
Nimmo & Barrier (1974) reported some deaths in adult brown shrimp
(Penaeus aztecus) exposed to a "sub-lethal" concentration of Aroclor
1254 (3 µg/litre), for 7 days, after subjection to salinity shock.
The shrimp normally adapts readily to the wide range of salinity
found in its natural estuarine habitat. Roesijadi et al. (1976a)
exposed the adult grass shrimp (Palaemonetes pugio) to sub-lethal
(6.3-8.8 µg/litre) and lethal (57.6-76.4 µg/litre) concentrations of
Aroclor 1254 for 96 h, at various salinities. They found little effect
on haemolymph chloride concentration or osmolarity, chloride space
(the apparent volume of distribution of chloride ions), or chloride
exchange kinetics. The shrimp showed an adaptive altered permeability
to chloride ions at a salinity of 17%, the isotonic point. PCBs did
not affect this permeability change in adult shrimp. In the juvenile
grass shrimp, there was a reduction in haemolymph chloride levels at
low salinities in non-steady state exposures; the PCBs delayed the
permeability change. This disruption of haemolymph chloride was
associated with high numbers of deaths, even at the "sub-lethal"
exposure concentration. It was concluded that juveniles died from
salinity shock, because of delayed adaptive response. In another study
(Roesijadi et al., 1976b), grass shrimp were exposed to Aroclor 1254
at 29.4 µg/litre for 96 h at various salinities. No appreciable effect
was observed on total free amino acid levels in abdominal muscle,
indicating that intracellular osmoregulation was not a major
consequence of PCB toxicity, though changes in individual amino acid
concentrations suggested an altered metabolic state. The authors found
that blood glycine levels showed large decreases after the transfer of
the shrimps to clean water, a delayed response to the Aroclor
exposure.
7.2.2.3 Reproduction
Sea urchin (Arbacia punctulata) eggs were exposed to concentrations
of Aroclor 1254 of 0.5, 1.0, 5.0, and 10.0 mg/litre (Adams, 1983).
There was no effect on percentage fertilization, percentage pluteus
development, or percentage mortality when eggs were exposed at
fertilization. However, when eggs were exposed 1 h prior to
fertilization, there was a significant reduction in fertilization
efficiency at all doses. At all but the lowest dose, there was a
significant increase in mortality and a significant depression in
successful pluteus development.
Maki & Johnson (1975) calculated EC50s for total young produced,
average brood size, and percentage of days reproducing, during a
14-day exposure of Daphnia magna to Aroclor 1254; the results were
19, 23, and 25 µg/litre, respectively. Daphnia magna were exposed to
a range of Aroclors, by Nebeker & Puglisi (1974) who estimated
reproductive impairment, measured as a percentage of surviving young
relative to controls. The study was conducted over 3 weeks under
static conditions. Results are presented in Table 30. Reproductive
impairment matches lethality of the Aroclors (see Table 29); there was
no indication of reproductive effects of the Aroclors at
concentrations below those leading to the death of adults or young. No
young were produced by scud (Gammarus pseudolimnaeus) exposed to
8.7 µg Aroclor 1242/litre. Scud exposed at 2.8 µg/litre produced fewer
young per surviving adult (4.2), compared with controls (6.8).
Table 30. Reproductive impairment of Daphnia magna exposed to Aroclors under
static conditionsa
Aroclor Concentration (µg/litre) producing reproductive impairment
50% 16%
1221 125 89
1232 66 53
1242 63 48
1248 24 16
1254 28 18
1260 33 22
1262 41 24
1268 206 162
a From: Nebeker & Puglisi (1974).
7.2.2.4 Moulting
Several authors have suggested that crustaceans may be more
susceptible to the toxic effects of PCBs during moult (Duke et al.,
1970; Wildish, 1970; Nimmo et al., 1971a,b). Fingerman & Fingerman
(1979) exposed 2 groups of fiddler crabs (Uca pugilator) at 8 mg
Aroclor 1242/litre, one group for 40 days, and the other for only the
first 14 of the 40 days. Eye-stalks were removed on day 15 to increase
moulting activity. Controls underwent rapid ecdysis, with more than
50% of the population completing moult within 40 days. Crabs exposed
to the Aroclor for the full 40 days showed less moulting, with no more
than 10% of the population completing moult. Crabs exposed for 14 days
showed 20% of the population successfully moulting. Removal of
eye-stalks on day 1 of the study produced similar results, in terms of
numbers of crabs moulting in each treatment, but speeded-up moult in
the controls. No more Aroclor-exposed crabs moulted after eye-stalk
removal. In an earlier study, Fingerman & Fingerman (1977) exposed
fiddler crabs to Aroclor 1242 at 8 mg/litre for 38 days. Either
eye-stalks or 4 walking legs were removed to stimulate moulting. Both
control groups underwent ecdysis rapidly. Treated crabs without
eye-stalks did not undergo any moult. Moulting in those with legs
removed was much slower than in the controls. The authors also exposed
crabs to dibenzofuran (1,2,3,4,5,6,7,8-octachlorodibenzofuran) at
16 ng/litre. This is equivalent to the maximum reported dibenzofuran
contamination of the Aroclor with the dose equivalent to (in terms of
the dibenzofuran) the same concentration (8 mg/litre) in the PCB
mixture. There was only a slight inhibition of moulting caused by the
dibenzofuran.
Neff & Giam (1977) exposed juvenile horseshoe crabs (Limulus
polyphemus) to concentrations of Aroclor 1016 of 10, 20, 40, or
80 µg/litre, for up to 96 days. The crabs were divided into 2 groups:
group A consisted of juveniles at the late first tailed stage and
group B of juveniles at the early second tailed stage. ET50s (median
time for moulting to begin) were calculated between the start of the
study and the first moult (ET50-1) and between subsequent moults
(ET50-2 to -n). No moult occurred within 96 days at concentrations of
Aroclor of 40 or 80 µg/litre in either group of crabs. The ET50-1, in
group A at 10 and 20 µg/litre, was not different from controls; the
ET50-2 was slightly decreased. In group B, 10 and 20 µg/litre did not
affect the ET50-1; 40 and 80 µg/litre delayed the onset of the first
moult by 7 and 9 days, respectively. The ET50-3 were substantially
decreased at 10, 20, and 40 µg Aroclor/litre.
7.2.2.5 Behaviour
Hansen et al. (1974a) studied the avoidance response of the pink
shrimp (Penaeus duorarum) and the grass shrimp (Palaemonetes pugio)
given a choice between clean water and water containing Aroclor 1254,
at concentrations of between 0.001 and 10 mg/litre. Pink shrimp did
not avoid any of the concentrations used; grass shrimp only
significantly avoided the highest dose.
7.2.2.6 Population structure
The composition of communities of estuarine animals, in aquaria, were
studied under different exposures to Aroclor 1254 (0.1, 1.0, and
10.0 µg/litre) for 4 months (Hansen, 1974). The author found that, in
control groups and the group at the lowest concentration, the
community was mainly comprised (>75%) of arthropods, mostly the
amphipod Corophium volutator. At 1 and 10 µg Aroclor 1254/litre, the
numbers of arthropods decreased and the numbers of chordates
increased; at 10 µg/litre, over 75% of the animals were tunicates. The
highest concentration of Aroclor decreased the numbers of phyla,
species, and individuals represented (particularly of amphipods,
bryozoans, crabs, and molluscs), whereas the numbers of annelids,
brachypods, coelenterates, echinoderms, and nemertines were
unaffected.
7.2.2.7 Interactions with other chemicals
Maki & Johnson (1975) exposed Daphnia magna to various combinations
of DDT (0.2-0.75 µg/litre) and Aroclor 1254 (2-24 µg/litre). They
studied adult mortality, total young produced, average brood size, and
percentage days reproducing, during a 14-day exposure period. The
effects of one toxicant significantly enhanced the action of the other
for all test parameters. In the presence of a no-observed-effect level
of PCB (12 µg/litre), the susceptibility of Daphnia to DDT increased
by one third. When combined with 0.5 µg DDT/litre, the toxicity of
Aroclor 1254 was doubled.
In a study by Nimmo & Bahner (1976), the pink shrimp (Penaeus
duorarum) was exposed to various combinations of Aroclor 1254
(0.7-1.1 µg/litre), cadmium (640-829 µg/litre), and methoxychlor
(0.9-1.0 µg/litre) and numbers of shrimps dying were monitored. The
results showed no evidence of synergism or potentiation in any
combination.
7.2.3 Fish
The acute toxicity of PCBs for fish is summarized in Table 31. Values
for 96-h LC50s vary between 0.008 mg/litre, for the fry of the
fathead minnow, to > 100 mg/litre for channel catfish. This
considerable variation is dependent on species and on the PCB mixture
but appears to depend little on test conditions, such as temperature
and water hardness. The toxicity of PCBs appears much greater in
flow-through tests, where the water concentration of the PCBs is
constantly maintained.
MATC (maximum acceptable toxicant concentrations) were set by the US
EPA (1980) and are expressed as a range between the no-observed-effect
level (based on the results of long-term studies and the sub-lethal as
well as the lethal effect) and the lowest concentration showing a
measurable effect. These values, for the fathead minnow, were 5.4 and
15.0 µg/litre for Aroclor 1242; 0.1 and 0.4 µg/litre for Aroclor 1248;
1.8 and 4.6 µg/litre for Aroclor 1254, and 1.3 and 4.0 µg/litre for
Aroclor 1260. The early life stage of the estuarine sheepshead minnow
gave values of 3.4 and 15.0 µg/litre for Aroclor 1016 and 0.06 and
0.16 µg/litre for Aroclor 1254.
7.2.3.1 Short- and long-term toxicity
The toxicity of Aroclors for 3 species of freshwater fish, over
exposure of up to 30 days, was systematically investigated by Mayer et
al. (1977) under flow-through conditions. Results are presented in
Table 32. Short-term tests consistently underestimate the toxicity of
PCBs.
Table 31. Acute toxicity of PCB and PCT mixtures for fish
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Channel catfish 0.60 g stat 20 40 7.4 Aroclor 1016 96-h LC50 > 100
(Ictalurus yolk-sac stat 25 272 7.4 Aroclor 1016 96-h LC50 0.44 (0.34-0.56)
punctatus) 2.80 g flow 17 272 7.4 Aroclor 1242 96-h LC50 >0.10
Mayer & 2.80 g flow 22 272 7.4 Aroclor 1248 96-h LC50 >0.1
Ellersieck 2.80 g flow 22 272 7.4 Aroclor 1254 96-h LC50 >0.20
(1986) 2.80 g flow 22 272 7.4 Aroclor 1260 96-h LC50 >0.40
Atlantic salmon 5.60 g flow 17 314 7.6 Aroclor 1016 96-h LC50 0.13 (0.11-0.16)
(Salmo salar)
Mayer &
Ellersieck (1986)
Brook trout 3.0 g flow 12 314 7.6 Aroclor 1016 96-h LC50 >0.80
(Salvelinus
fontinalis)
Mayer &
Ellersieck (1986)
Brown trout 4.60 g flow 12 314 7.6 Aroclor 1016 96-h LC50 0.14 (0.11-0.18)
(Salmo trutta) 1.10 g stat 13 44 7.4 Aroclor 1260 96-h LC50 > 24.0
Mayer &
Ellersieck (1986)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Cutthroat trout 2.70 g stat 9 159 162 7.4 Aroclor 1221 96-h LC50 1.17 (0.96-1.43)
(Salmo clarki) 2.20 g stat 9 159 162 7.4 Aroclor 1232 96-h LC50 2.5 (1.72-3.08)
Mayer & 2.40 g stat 9 159 162 7.4 Aroclor 1242 96-h LC50 5.42 (3.82-7.68)
Ellersieck 2.50 g stat 9 159 162 7.4 Aroclor 1248 96-h LC50 5.75 (5.1-6.5)
(1986) 2.50 g stat 9 159 162 7.4 Aroclor 1254 96-h LC50 42.5 (38.7-46.7)
2.70 g stat 9 159 162 7.4 Aroclor 1260 96-h LC50 60.9 (55.4-67.0)
2.40 g stat 9 159 162 7.4 Aroclor 1262 96-h LC50 > 50
2.20 g stat 9 159 162 7.4 Aroclor 1268 96-h LC50 > 50
2.70 g stat 9 162 7.4 Aroclor 4465 96-h LC50 > 65
2.10 g stat 9 162 7.4 Aroclor 5442 96-h LC50 > 50
2.90 g stat 9 162 7.4 Aroclor 5460 96-h LC50 > 50
Lake trout fry stat 10 170 7.2 Aroclor 1016 96-h LC50 0.48 (0.39-0.60)
(Salvelinus yolk-sac stat 10 170 7.2 Aroclor 1016 96-h LC50 0.89 (0.69-1.15)
namaycush)
Mayer &
Ellersieck (1986)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Rainbow trout 0.50 g stat 12 44 7.4 Aroclor 1016 96-h LC50 0.14 (0.11-0.16)
(Salmo gairdneri) 2.50 g flow 10 272 7.4 Aroclor 1016 96-h LC50 0.62 (0.42-0.90)
Mayer & fry flow 12 314 7.6 Aroclor 1016 96-h LC50 0.44 (0.37-0.53)
Ellersieck 1.80 g flow 17 272 7.4 Aroclor 1242 120-h LC50 0.07
(1986) 1.80 g flow 17 272 7.4 Aroclor 1248 120-h LC50 0.05
1.80 g flow 17 272 7.4 Aroclor 1254 120-h LC50 0.14
1.80 g flow 17 272 7.4 Aroclor 1260 96-h LC50 >0.23
Harlequin fish 10-30 mm flow 20 20 8.1 Aroclor 1221 96-h LC50 1.05
(Rasbora 10-30 mm flow 20 20 8.1 Aroclor 1232 96-h LC50 0.32
heteromorpha) 10-30 mm flow 20 20 8.1 Aroclor 1242 96-h LC50 0.37
Tooby et al. 10-30 mm flow 20 20 8.1 Aroclor 1254 96-h LC50 1.1
(1975) 10-30 mm flow 20 20 8.1 Aroclor 1262 96-h LC50 >100
Bluegill sunfish 0.90 g stat 12 44 7.4 Aroclor 1016 96-h LC50 0.60
(Lepomis 1.80 g flow 20 272 7.4 Aroclor 1016 96-h LC50 0.46 (0.39-0.54)
macrochirus) 2.20 g flow 17 272 7.4 Aroclor 1242 120-h LC50 0.13
Mayer & 0.80 g stat 18 44 7.1 Aroclor 1248 96-h LC50 0.69 (0.48-0.99)
Ellersieck 2.20 g flow 22 272 7.4 Aroclor 1248 120-h LC50 0.14
(1986) 0.80 g stat 18 44 7.1 Aroclor 1254 96-h LC50 2.74 (1.29-5.81)
2.20 g flow 22 272 7.4 Aroclor 1254 96-h LC50 0.20
2.20 g flow 22 272 7.4 Aroclor 1260 96-h LC50 0.40
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Longnose sucker finger flow 12 314 7.5 Aroclor 1016 96-h LC50 0.33 (0.22-0.49)
(Catostomus
catostomus)
Mayer &
Ellersieck (1986)
Yellow perch 1.20 g flow 17 314 7.6 Aroclor 1242 96-h LC50 >0.15
(Perca flavescens) 1.10 g flow 17 314 7.6 Aroclor 1248 96-h LC50 >0.1
Mayer & 1.00 g flow 17 314 7.6 Aroclor 1254 96-h LC50 >0.15
Ellersieck 1.20 g flow 17 314 7.6 Aroclor 1260 96-h LC50 >0.20
(1986)
Fathead minnow fry flow 24 Aroclor 1254 96-h LC50 0.008
(Pimephales fry flow 24 Aroclor 1242 96-h LC50 0.015
promelas) 3 months flow 24 Aroclor 1242 96-h LC50 0.30
Nebeker
et al. (1974)
Cisco (chub) 22 days flow 7 30-35 40-48 Aroclor 1254 96-h LC50 >10
(Coregonus sp.) 22 days flow 7 30-35 40-48 Aroclor 1254 120-h LC50 3.2 (1.9-5.5)
Passino &
Kramer (1980)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Carp (Cyprinus fry stat 23-25 Kanechlor 300 96-h LC50 1.45
carpio)
Kimura et al.
(1974)
Guppy (Lebistes fry stat 24-25 Kanechlor 300 96-h LC50 0.9
reticulatus) 0.35 g stat 24-25 Kanechlor 300 96-h LC50 3.2
Kimura et al. (1974)
a stat = static conditions: water not changed during the exposure;
flow = flow-through conditions; concentration of toxicant continuously maintained.
b Alkalinity and hardness expressed as mg/litre CaCO3, unless otherwise stated.
Table 32. Toxicity of Aroclors for fish (LC50s in µg/litre) at 17°Ca
Aroclor Exposure (days)
5 10 15 20 25 30
Rainbow trout
1242 67 48 18 10 12 -
1248 54 38 16 6.4 3.4 -
1254 - 160 64 39 27 -
1260 - 326 143 78 49 51
Bluegill sunfish
1242 - - 164 125 120 84
1248 136 115 111 106 100 78
1254 - - 303 260 239 177
1260 - - - - - 400
Channel catfish
1242 - - 219 150 132 87
1248 - 121 121 115 104 75
1254 - 303 286 293 181 139
1260 - 535 482 512 465 433
a From: Mayer et al. (1977).
Duke et al. (1970) kept juvenile pinfish (Lagodon rhomboides) in
seawater containing 1, 10, or 100 µg Aroclor 1254/litre for up to
48 h. There were no deaths at any concentration. It was suggested by
Nimmo et al. (1975) that acute toxicity tests underestimated the true
sensitivity of marine species; in bioassays lasting 1 week or more,
Aroclor proved to be 100 times more toxic than acute exposure
suggested. In tests lasting 2 weeks or longer, Aroclor 1254 was lethal
for longnose killifish (Fundulus similis) at 1 µg/litre and for
pinfish and spot (Leiostomus xanthurus) at 5 µg/litre. Hansen et al.
(1974b) did not find any significant lethal effects in pinfish exposed
to 100 µg Aroclor 1016/litre for 96 h but significant mortality (50%)
was observed after 33 days at 32 µg/litre, and, after 18 days, at
100 µg/litre. Nebeker et al. (1974) exposed the flagfish (Jordanella
floridae) to Aroclor 1248 for 40 days. No fish survived at a
concentration of 18 µg/litre and only 35% survived at 5.1 µg/litre.
The fish at these two concentrations almost completely lost their fins
and tails. Fish survival was not affected at concentrations of
2.2 µg/litre or less. In a study by Defoe et al. (1978), fathead
minnow (Pimephales promelas) larvae were exposed, in flow-through
bioassays, to Aroclors 1248 and 1260 for 30 days; the LC50s were
calculated to be 4.7 and 3.3 µg/litre, respectively.
Hansen et al. (1976a) fed fingerling channel catfish (Ictalurus
punctatus) a diet containing 20 mg Aroclor 1242/kg for 20 weeks. The
fish showed a reduced weight gain and hypertrophy of the liver. When
the treated fish were transferred to a control diet for 8 weeks and
then back to the dosed diet for a further 8 weeks, weight gain and
liver weights returned to normal levels. No histopathological lesions
were observed in any of the fish fed PCBs.
Rainbow trout (Salmo gairdneri) were fed a diet containing 1, 10, or
100 mg Aroclor 1254/kg over a period of 330 days (Nestel & Budd,
1975). No effects on growth rates were seen, but renal lesions were
observed at all doses; however, they were not dose-related. Foci of
renal necrosis, with cellular or granular cast formation were seen. A
significant increase in the number of hepatocytes per unit area in the
liver was observed at all doses and appeared to be dose-related. A
reduction of 'white pulp' (lymphatic elements) in the spleen was
observed at 10 and 100 mg/kg diet. Fish with renal necrosis also had
reduced splenic white pulp and a reduced white cell count.
7.2.3.2 Carcinogenicity
Hendricks et al. (1977) studied the combined effects of Aroclor 1254
(100 mg/kg diet) and aflatoxin B1 (6 mg/kg diet) in rainbow trout. A
significantly reduced incidence of liver tumours was observed in the
combination. There was no retardation of growth in the treated
animals, but they showed glycogen depletion in hepatocytes,
hyperaemia, and white pulp depletion in the spleen.
PCB administration prior to aflatoxin B1 treatment also decreased the
liver tumour incidence, whereas when Aroclor 1254 was fed after
exposure of trout embryo to aflatoxin B1, there was no effect on the
formation of liver tumours (Shelton et al., 1984b).
When rainbow trout were fed 0, 1, 4, or 8 mg aflatoxin B1/kg diet or
aflatoxin B1 at these dose levels plus 50 mg Aroclor 1254/kg diet,
the incidence of hepatic tumours was lower in the groups receiving
aflatoxin combined with PCBs (Shelton et al., 1984a). The inhibition
of aflatoxin B1-mediated carcinogenicity was also correlated with the
decreased bacterial mutagenicity of this compound in the presence of
an Aroclor 1254-induced drug metabolizing enzyme fraction from fish.
The potential mechanisms of this interaction were further investigated
by Shelton et al. (1986) by studying the effects of Aroclor 1254 on
aflatoxin B1 distribution, metabolism, and adduct formation. The
results from the in vivo studies showed that PCB treatment resulted
in a marked increase in the metabolism of aflatoxin B1 to aflatoxin
M1 and their glucuronide conjugates. The DNA-adduct levels in the
PCB-treated fish were 48-96% lower than those in the controls. The
results in the fish model using aflatoxin B1 as the carcinogen were
associated with the activity of Aroclor as an inducer of cytochrome
P-450-dependent monooxygenases (Halverson et al., 1985; Shelton et
al., 1986).
7.2.3.3 Effects on developmental stages and reproduction
Birge et al. (1978) determined LC50s and LC1s for 4 species of fish
from the fertilization of the eggs to 4 days after hatching. Exposure
times varied with species dependent on the time taken to hatching of
the eggs; hatching took 22 days for rainbow trout and 3-4 days for the
other species. In most species, eggs were considerably less sensitive
to the toxic effects of Aroclors than larvae. The major exception was
the rainbow trout for which the duration of exposure of the eggs until
hatching was considerably longer than for other species. LC50s ranged
from 0.32 to 11.16 µg/litre for the 4 species (up to 4 days after
hatching) and 4 different PCB mixtures; LC1s ranged from 0.009 to
0.26 µg/litre. Results are presented in Table 33. The rainbow trout
was the most sensitive species tested.
Adult minnows (Phoxinus phoxinus) were fed Clophen A50 at 20, 200,
or 2000 mg/kg diet (on a dry weight basis), for 40 days (Bengtsson,
1980). Growth was monitored from day 46 to day 79; a significant
increase in growth (relative to controls) was seen in fish fed the
highest dose. Other doses caused increases in growth, but these were
not significant. Stimulated growth had been observed in previous
studies with minnows fed Clophen A50 at 0.88-78 mg/kg diet (Bengtsson,
1979). Between days 127 and 166, the swimming performance of the fish
was tested using a rotary flow technique. Although the PCBs impaired
swimming performance, this was not statistically significant.
Reproduction was monitored from day 235 (first day of spawning) to day
300. Spawning was delayed in the treated groups by 1 day, 1 week, and
3 weeks for the 3 treatments (20, 200, and 2000 mg/kg diet),
respectively. Only the highest dose affected hatchability of eggs,
which was reduced by approximately 80%.
Snarski & Puglisi (1976) did not find any adverse effects on survival,
growth, or reproduction of brook trout (Salvelinus fontinalis)
exposed to concentrations of Aroclor 1254 of up to 0.94 µg/litre, for
71 weeks. Survival and growth of alevin-juveniles from exposed parents
were unaffected for up to 90 days.
Continuous-flow bioassays were conducted over 8 months on fathead
minnow (Pimephales promelas), exposing the fish to either Aroclor
1242 or 1254 (Nebeker et al., 1974). Reproduction occurred at, and
below, 5.4 µg Aroclor 1242/litre, but spawning and egg production
were very variable. With Aroclor 1254, reproduction occurred at, and
below, 1.8 µg/litre. Spawning occurred at 1.8 µg/litre, but was
significantly less than spawning at the lower concentrations of 0.23
and 0.52 µg/litre. Egg hatchability and fry survival were good at
1.8 µg/litre. Eggs were more resistant than fry at 15 and 51 µg
Aroclor 1242/litre; with Aroclor 1254 at a concentration of
15 µg/litre, eggs hatched readily, but all fry were dead within 96 h.
Halter & Johnson (1974) kept coho salmon (Oncorhynchus kisutch) eggs
in solutions containing 4.4-56.4 µg Aroclor 1254/litre. Exposure
continued for 2 weeks before, and 4 weeks after hatching, until the
young were alevins. Hatchability was reduced by 30% at the highest
concentration of 56.4 µg/litre. Survival of alevins was markedly
higher when eggs were transferred to clean water prior to hatching,
but there was still 58% mortality in alevins hatched from eggs at the
highest dose. When the alevins were exposed to the PCBs for 4 weeks
after hatching, survival was inversely related to exposure
concentration. No group survived as well as the controls (for example,
18% died at 4.4 µg/litre and 90% at 26 µg/litre, or more).
In a study by Defoe et al. (1978), fathead minnow (Pimephales
promelas) were maintained in flow-through bioassays in solutions of
Aroclors 1248 or 1260, for 240 days (a full life-cycle test).
Reproduction occurred at all concentrations tested (up to 3 µg/litre
for Aroclor 1248 and up to 2.1 µg/litre for Aroclor 1260). The authors
concluded that PCBs did not produce major effects on reproduction at
concentrations up to the 30-day LC50 and that reduced populations
after long-term exposure are largely due to larval mortality.
Weis & Weis (1982) exposed eggs of the mummichog (Fundulus
heteroclitus) to concentrations of Aroclor 1254 of 0.01-10 mg/litre
(after cleavage had begun). No effect was found on embryonic
development or hatching and embryonic mortality was negligible.
Seven-day larval tests also showed no effect on mortality up to the
highest concentration. However, the authors found approximately 20%
larval mortality, when larvae were exposed to 5 mg/litre for 72 h,
after hatching from eggs at the highest exposure concentration. In a
second group of studies, eggs were exposed to 10 mg Aroclor
1242/litre; no malformations were found, though there was a consistent
retardation of hatching. There was a positive correlation between
hatching rate and female length (length being related to age). Larvae
exposed to 5 mg/litre showed an average of 45% mortality within 72 h.
Pre-exposure as eggs to 10 mg/litre greatly increased larval
mortality; pre-exposure to 1 mg/litre resulted in an intermediate
response. There was no mortality in control larvae, even when they had
been pre-exposed as eggs.
Eggs of brook trout (Salvelinus fontinalis) were exposed to Aroclor
1254 (0.043-13 µg/litre) for 10 days before hatching, and the fry for
118 days after hatching (Mauck et al., 1978). Median hatching time,
egg hatchability, and sac-fry survival were not affected by the PCBs.
Significantly decreased survival (32% survived) was seen after 48 days
at 3 µg/litre. There was significant mortality of fry after 118 days
with exposure to concentrations of 3.1 µg/litre and above. Growth of
the trout, as measured by weight, was significantly decreased after 48
days at concentrations of 1.5 µg/litre or more. By the end of the
study (118 days), no significant differences in weight were seen
between surviving fry on different treatments. Analysis of the
backbone composition at this time showed that hydroxyproline and
phosphorus were significantly decreased by concentrations of
0.43 µg/litre or more and that, at 0.69 µg or more/litre, calcium
levels were significantly increased. Although collagen was
significantly decreased at 0.69, 3.1, and 6.2 µg/litre, it was
unaffected at 1.5 µg/litre; no explanation for the anomaly was
suggested.
Schimmel et al. (1974) exposed sheepshead minnow (Cyprinodon
variegatus) eggs, immediately after fertilization, to Aroclor 1254
at concentrations of between 0.1 and 10 µg/litre. The fertility of
eggs was unaffected. Hatching was significantly reduced (by 30%) only
at the highest exposure. Survival of fry to 2 weeks was significantly
reduced at concentrations of 0.32 µg/litre or above (30% survival at
0.32 µg/litre and 9% survival at 10 µg/litre). The 3-week LC50 for
embryo-fry was calculated to be 0.93 µg/litre for Aroclor 1254. Many
of the dying fish exhibited fin rot. Exposure of juveniles and adults
to the same concentrations of the Aroclor produced 24% mortality in
juveniles at the highest exposure rate and no mortality in adults.
Some fin rot was seen in adults.
Table 33. Toxicity of PCBs for the embryolarval stages of fisha,b
Organism Aroclor 1016 Aroclor 1242 Aroclor 1254
LC1 LC50 LC1 LC50 LC1 LC50
(µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre)
Channel catfish 0.08 11.16 0.14 4.24 0.05 1.76
(Ictalurus punctatus) (9.93-12.97) (0.07-0.23) (3.32-5.34) (0.02-0.09) (1.36-2.24)
Goldfish 0.10 13.21 0.04 2.64 0.02 1.18
(Carassius auratus) (10.63-16.43) (0.01-0.08) (1.89-3.61) (0.01-0.04) (0.84-1.61)
Rainbow trout 0.011 1.08 0.01 1.03 0.009 0.32
(Salmo gairdneri) (0.003-0.027) (0.7-1.56) (0.002-0.025) (0.67-1.51) (0.003-0.02) (0.22-0.45)
Redear sunfish 0.26 7.82 0.19 3.56 0.02 0.53
(Lepomis microlophus) (0.1-0.51) (5.74-10.35) (0.08-0.35) (0.65-4.66) (0.01-0.04) (0.39-0.7)
a From: Birge et al. (1978).
b Exposure under static conditions, but with water renewed every 12 h. Exposure was initiated 2-6 h after spawning (except
for rainbow trout where exposure was initiated 15 min after fertilization) and continued to 4 days post-hatching. Hatching
times varied: 22 days for rainbow trout (13.5-14.3°C); 3 days for catfish (29-31°C); 3-4 days for goldfish and sunfish
(20-24°C); hardness 90-115 mg/litre; pH 7.6-8.1.
In another study, Hansen et al. (1975) exposed embryos, fry,
juveniles, and adults of sheepshead minnow (Cyprinodon variegatus)
to concentrations of Aroclor 1016 of 0.1-10 µg/litre, for 28 days, in
intermittent flow bioassays. No effects on survival were observed
during this period. When exposed to concentrations of 32 or
100 µg/litre, there was high mortality in eggs, juveniles, and adults;
all were killed at 100 µg/litre. The authors calculated that the
28-day LC50s for juveniles and adults were 20 and 19 µg/litre,
respectively.
Freeman et al. (1982) fed Atlantic cod (Gadus morrhua) diets
containing 1-50 mg Aroclor 1254/kg for 5.5 months. Altered steroid
biosynthetic patterns in vitro were observed in the testes and head
kidneys (adrenal equivalent) of dosed fish. Histological examination
revealed abnormalities in the testes, gills, and livers. Testicular
abnormalities included derangement of lobules, hyperplasia of lobule
walls, and disintegration and/or fatty necrosis of spermatogenic
elements. In fish fed at dietary rates of 5-50 mg/kg, hyperplasia of
the epithelial layer of the secondary lamellae of the gills was noted.
Fatty degeneration of the liver was observed in all treated fish.
Similar testicular abnormalities were observed by Sangalang et al.
(1981), but only in sexually mature individuals or at a stage of rapid
spermatogenic proliferation.
7.2.3.4 Physiological and biochemical effects
Coho salmon (Oncorhynchus kisutch) were fed diets containing a
mixture of PCBs (1:4, Aroclor 1242:1254) at a concentration of 50 or
500 mg/kg dry feed (Leatherland & Sonstegard, 1978). Serum
triiodothyronine (T3) levels were significantly reduced after 3
months, in fish fed the highest dose. Thyroxine (T4) levels were not
affected. After 3 months, the T3:T4 ratio was significantly higher in
fish fed the highest dose than in control and low-dose fish. Fish on
500 mg/kg had significantly lower body weights than controls by the
end of the study. A mixture of 50 mg PCBs/kg and 5 mg mirex/kg
significantly reduced serum triiodothyronine and thyroxine levels over
a period of 3 months, but did not affect the T3:T4 ratio. Leatherland
& Sonstegard (1979) fed rainbow trout (Salmo gairdneri) diets
containing Aroclor 1254 at 500 mg/kg dry feed, for up to 2 months. The
PCBs did not have any significant effects on thyroid histology or on
serum thyroid hormone levels. Liver weights, total liver lipid
content, and carcase lipid content were significantly greater in
treated fish. Mayer et al. (1977) fed fingerling coho salmon
(Oncorhynchus kisutch) 1.45-14 500 µg Aroclor 1254/kg body weight
per day, for 260 days. Channel catfish (Ictalurus punctatus) were
fed the same Aroclor at rates of 48 and 480 µg/kg body weight per day
for 193 days. Thyroid activity (as measured by 125I uptake) was
significantly stimulated at dose rates of 14.5 µg/kg per day, and
above, in coho salmon. Stimulation ranged from 52%, at 14.5 µg/kg per
day, to 119%, at 14 500 µg/kg per day, compared with controls. In
catfish, both dose rates of Aroclor 1254 caused significant increases
in thyroid activity, whereas other Aroclors (1232, 1248, and 1260) did
not have any significant effects on the thyroid at the same dose
rates. Folmar et al. (1982) injected yearling coho salmon
(Oncorhynchus kisutch) intraperitoneally with a total of 150 µg
Aroclor 1254/kg body weight (2 injections, 10 days apart), prior to
smoltification. Over a 6-week period, the authors did not find any
significant effects on gill Na-K ATPase activity. However, the PCBs
did alter the normal developmental patterns of thyroxine; there was a
delay in the normal increase in circulating thyroxine levels.
Triiodothyronine levels were significantly elevated after
approximately 3 weeks, but then fell to well below control levels
after 6 weeks. Fish were transferred to seawater; there was no
significant effect on the gill Na-K ATPase activity in the treated
group, but there was a significant increase in mortality (6%). Ten per
cent of fish, dosed with PCBs and placed in sea water containing No 2
fuel oil at 700 µg/litre, died; this was an additive effect.
Fingerman (1980) kept Gulf killifish (Fundulus grandis) in a
seawater solution containing Aroclor 1242, 1254, or 1268 at
8 mg/litre, for up to 28 days. The author removed the lower half of
the caudal fin to study fin regeneration. No significant effects were
observed with either Aroclor 1242 or 1254. With Aroclor 1268, a
significant decrease in regeneration rate was observed after 28 days,
when the study was conducted in the spring, and after 7 days, in the
autumn. No differences were found at other sampling times.
Rainbow trout (Salmo gairdneri) were administered capsules
containing 0.173 g Aroclor 1254 every second day over a 6-day period
(Kiessling et al., 1983). Two to 4 weeks after the last capsule,
isolated gills were perfused. There was no significant difference in
adrenergic response in the gill vascular bed and no significant
difference in the "oxygen transfer factor" (% changes in oxygen in
saline from dorsal aorta before, and after, addition of adrenaline).
Similarly, there was no effect on muscle glycogen content.
Johansson et al. (1972) dosed brown trout (Salmo trutta) twice, 4
days apart, with 5 mg Clophen A50/kg body weight, either by capsule or
by intramuscular injection. The fish were fed for 43 days, starved for
116 days, and fed again for another 87 days. Metabolic analysis of the
fish was undertaken on day 43 and at the end of the study. A
significant increase in body weight was noted at the end of the study,
but not after 43 days. Blood glucose, muscle glycogen, and the
liver-somatic index (liver weight as a ratio of body weight), which
had all increased significantly after 43 days, decreased significantly
by the end of the study (compared with controls). Both haematocrit and
haemoglobin levels had significantly decreased after 43 days but, by
the end of the study, were not significantly different from those of
the controls.
In a study by Camp et al. (1974), fingerling catfish (Ictalurus
punctatus) were kept in water containing Aroclor 1254 at 8 mg/litre.
There was a significant increase in the serum transaminase activity of
the fish after 4 h. The cortisol content of the serum was depressed,
but not significantly so. The sodium:potassium ratio was constant.
Merkins & Kinter (1971) exposed the killifish (Fundulus heteroclitus)
to concentrations of Aroclor 1221 of 7.5, 25, or 75 mg/litre. No fish
died, at the lowest concentration, over a period of 4 days; 50% died
at 25 mg/litre within 24 h and 88% at 75 mg/litre, within the same
period. Serum osmolality, and serum ion levels (Na and K) were then
measured. Within 6 h at 75 mg/litre, blood osmolality significantly
increased, but sodium and potassium ions in the blood were not
affected. After 24 h at 75 mg/litre, there was also a significant
increase in sodium ions, but no effects on potassium ions. None of
these parameters were affected by exposure at 25 mg/litre for 24 h.
7.2.3.5 Behavioural effects
Fingerman & Russell (1980) exposed male Gulf killifish (Fundulus
grandis) to Aroclor 1242 at 4 mg/litre for 24 h. A significant
reduction in whole-brain levels of both noradrenalin and dopamine were
reported over this period. The swimming activity of the fish was
monitored by counting the number of times they crossed lines marked on
the bottom of the tank within a 10-min period, after exposure to the
Aroclor for 24 h. Activity was significantly increased and remained so
for a further 2 days.
The avoidance response was studied by Hansen et al. (1974a) in the
sheepshead minnow (Cyprinodon variegatus), the pinfish (Lagodon
rhomboides), and the mosquito fish (Gambusia affinis), when given
a choice between clean water and water containing Aroclor 1254 at
0.001, 0.01, 0.1, 1.0, or 10 µg/litre. Sheepshead minnow did not avoid
any concentration; pinfish avoided only the highest concentration of
the Aroclor. Mosquitofish significantly avoided concentrations of 0.1,
1.0, and 10 µg/litre.
Peterson (1973) did not find any effect on temperature selection in
Atlantic salmon (Salmo salar) exposed to 2 mg Aroclor 1254/litre,
for 24 h prior to a horizontal temperature gradient test. Similarly,
Miller & Ogilvie (1975) did not find any effect of Aroclor 1254 on
temperature selection when brook trout (Salvelinus fontinalis) were
exposed to a water concentration of 25-100 mg/litre, for 24 h, prior
to temperature gradient tests. Even at a concentration of 100 mg/litre
for 48 h, which was sufficient to cause some mortality, temperature
selection was still unaffected.
7.2.3.6 Interactions with other chemicals
Halter & Johnson (1974) found that the median survival time for coho
salmon (Oncorhynchus kisutch) fry, exposed to Aroclor 1254 at
32.2 µg/litre, was greater than 336 h. When fry were exposed to
mixtures of Aroclor and DDT, for 2 weeks, the survival times were
always similar to the more rapid reaction time found for DDT alone.
The authors suggested that this indicated the lack of an additive
effect.
7.2.4 Amphibians
Tadpoles (Rana chensinenis) were maintained in water containing PCBs
(as Kanechlor 300) at 0.5, 5.0, 50, or 500 µg/litre. At the 2 highest
doses, all individuals died rapidly. The time of onset of lethality
was related to dose level; at 5 µg/litre, death occurred between 15
and 21 days, and, at 0.5 µg/litre, on the thirty-second day after
first exposure. Growth at 0.5 and 5.0 µg/litre did not differ from
that of controls. Tail abnormalities were found, but there was no
correlation with PCB concentrations and a NOEL could not be
established. The mechanism by which PCBs caused tail malformation was
not known (Hasegawa, 1973).
Birge et al. (1978) conducted embryo-larval bioassays on 3 species of
amphibia. Exposure to various PCBs was maintained from 2-6 h after
spawning to 4 days after hatching, using static renewal procedures
(Table 34). Toxicity increased with increasing chlorination, the
leopard frog being the most sensitive species with an LC50 of
1.03 µg/litre after exposure to Aroclor 1254. The authors also
calculated an LC1 value from the same study; the leopard frog and
American toad were equally sensitive at 0.02 µg/litre. The eggs were
much less sensitive to the PCBs than the hatched larvae, with LC50s
ranging from 3.5 to 250 µg/litre, for 3 different Aroclors and
Capacitor 21, and 3 species of tadpole.
Table 34. Toxicity of PCBs for the tadpoles of amphibiansa,b
Organism Aroclor 1016 Aroclor 1242 Aroclor 1254 Capacitor 21
LC1 LC50 LC1 LC50 LC1 LC50 LC1 LC50
(µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre)
American toad 0.35 7.16 0.03 2.71 0.02 2.02 0.21 9.97
(Bufo americanus) (0.15-0.64) (5.39-9.34) (0.01-0.06) (1.91-3.75) (0.01-0.05) (1.44-2.77) (0.08-0.42) (7.21-13.53)
Fowler's toad 0.18 27.72 0.22 12.09 0.07 3.74 0.55 28.02
(Bufo fowleri) (0.09-0.33) (21.77-35.08) (0.12-0.36) (9.74-14.91) (0.04-0.11) (2.98-4.64) (0.3-0.91) (22.59-34.47)
Leopard frog 0.1 6.19 0.04 2.13 0.02 1.03 0.03 2.87
(Rana pipiens) (0.05-0.16) (4.95-7.69) (0.02-0.06) (1.72-2.63) (0.01-0.03) (0.83-1.27) (0.02-0.06) (2.29-3.57)
a From: Birge et al. (1978).
b Exposure under static conditions, but with water renewed every 12 h. Exposure was initiated 2-6 h after spawning and continued to
4 days post-hatching. Hatching times varied between 3 and 4 days, therefore, exposure varied between 7 and 8 days.
Temperature 20-24°C; hardness 90-115 mg/litre; pH 7.6-8.1.
7.2.5 Aquatic mammals
Following-up on field reports of the reproductive effects of PCBs on
seal reproduction (see section 7.4.4), Reijnders (1986) conducted a
study on captive common seals (Phoca vitulina) fed fish contaminated
with PCBs. The contaminated diet produced an average daily intake of
PCBs of 1.5 mg compared with the control level of 0.22 mg/day. Twelve
female seals were used as controls and 12 as the treated group. Blood
samples were taken regularly and assayed for circulating steroid
hormones progesterone and estradiol. Females were mated with undosed
males. Of the 12 females in the control group, 10 became pregnant; all
12 ovulated. Only 4 females became pregnant out of the 12 fed the
PCB-contaminated diet; again all 12 ovulated. Throughout the breeding
cycle, no significant differences were found between the hormonal
profiles of pregnant animals in the treated and control groups. No
significant differences were observed in progesterone levels in the
treated and control groups, despite the fact that many fewer treated
females became pregnant. However, a rise in estradiol levels in
non-pregnant females in the control group was not found in
non-pregnant females in the treated group, suggesting a difference in
non-pregnancy in treated females. The effects of PCBs occurred only
late in the breeding cycle at the time of implantation of the embryo.
Seals, like mink, show delayed implantation of the embryo as a normal
component of the annual reproductive cycle. No conclusions about the
mechanism of action could be drawn.
Brouwer et al. (1989) fed common seals (Phoca vitulina) on a diet of
polychlorinated biphenyl-contaminated fish (average daily intake
1.5 mg PCBs) for almost 2 years. Significant reductions in levels of
plasma total and free thyroxine, triiodothyronin, and retinol were
found compared with those in seals maintained on a "low" contaminated
diet (average daily intake 0.22 mg PCBs). It should be noted that the
diet consisted of fish contaminated in the environment and not dosed.
No attempt was made to analyse levels of retinol in the different fish
diets. The "high" contamination group were caught in the Wadden sea
and the "low" contamination group in the north-east Atlantic. When the
fish were analysed for other likely contaminants, it was found that
pp'-DDE also showed higher levels in the "high" contamination group
than the "low" contamination group; average daily intakes of pp'-DDE
were estimated to be 0.4 mg and 0.13 mg, respectively.
7.3 Toxicity for terrestrial organisms
7.3.1 Plants
Aroclor 1254 was applied to soil at rates of 10, 100, or 1000 mg/kg
(Weber & Mrozek, 1979). Both soybean (Glycine max) and rescue
(Fescue arundinacea) were grown in the soil, from seed, for up to 26
and 42 days, respectively. The height of the soybean plants and the
fresh top weights of both plants were measured. PCBs applied to the
soil significantly reduced height and fresh top weight of soybean
plants, only at the highest rate of application. Low rates were
inhibitory, but not significantly so. Aroclor applied to the soil also
reduced the fresh top weight of rescue at the highest rate of
application (1000 mg/kg); lower application rates of the Aroclor did
not have any effects. The addition of activated carbon to the soil
(3.7 tonnes/ha; approximately 3333 mg/kg) annulled the inhibitory
effect of PCBs. The Aroclor also inhibited the uptake of water by the
soybean in proportion to the dose applied to the soil; water uptake
was monitored between 21 and 25 days after sowing of the seed. The
reduction in water uptake over this 5-day period was 12%, with the
application of 1 mg Aroclor 1254/kg soil, rising to 52% at 1000 mg/kg
soil. Again, the effect on water uptake was eliminated by the addition
of carbon to the soil (1% rising to 4% inhibition over the dose
range).
Continuation of the experiment through a second and third crop of
soybeans on the same soil, without further addition of Aroclor 1254
(Strek et al., 1981), showed similar effects on the height, top fresh
weight, and water uptake of the plants, reflecting the persistence of
the Aroclor. However, there were no significant effects at doses lower
than 1000 mg/kg, with the exception of reduced height in the third
crop, seen at all dose levels. All effects were eliminated by the
addition of activated carbon to the soil. The same authors found that
beet (Beta vulgaris) was significantly affected by 1000 mg Aroclor
1254/kg, using the same parameters of water uptake, height, and fresh
top weight between 14 and 56 days after sowing. Doses of 100 mg/kg or
less did not have any significant effects. Effects at the highest dose
were again eliminated by the addition of activated carbon to the soil.
Growth parameters, taken at harvest, showed no apparent inhibition of
corn (Zea mays) or sorghum (Sorghum bicolor) by Aroclor 1254 over
the same dose range. There was, however, a reduction of plant height
over the first 5 days of growth at 100 and 1000 mg/kg in corn, but the
plants recovered.
Mrozek et al. (1983) grew Spartina alterniflora plants in mud or
sandy soils in the presence of 2.2 µg PCBs/kg (54% chlorine similar to
Aroclor 1254), admixed with the soil, over a 6-week period. Plants
grown in sand showed significantly reduced values for cumulative
change in height (approx. 30%) and the number of live leaves per stem
(approx. 25%), and increased values for the number of stems per plant
(approx. 300%), whereas plants grown in mud showed a significantly
reduced value for cumulative change in the number of stems per plant
(approx. 75%). Mud-grown plants also exhibited an altered biomass
distribution, as indicated by the aerial:below ground biomass ratio,
which increased from 1.2 to 1.5, on a dry weight basis.
7.3.2 Terrestrial invertebrates
Hatch & Allen (1979) observed the behaviour of the snail (Cepeae
(=Helix) nemoralis), with regard to the rasping of conspecifics'
shells to obtain calcium. On a low calcium diet (0.53 mg calcium/kg),
snails showed an increased tendency to rasp the shells of other
snails, in order to obtain calcium. The best indicator of this
behaviour was found to be the counting of holes bored completely
through the shell. This behaviour was not seen with a high calcium
diet (250 mg calcium/kg). The addition of PCBs, as a mixture of
Aroclors 1016 and 1254, to the high calcium diet at a rate of 0.5,
1.0, or 5.0 mg/kg increased the number of snail shells penetrated by
other snails. Penetration increased in a dose-dependent manner with 2,
5, and 7% penetration for the 3 dose rates, respectively. Damage to
shells, without actual penetration, also increased with PCB treatment
from the low level found on the control diet to between 16 and 21% on
the PCB diet. No clear dose-dependent effect was seen using this
method of assessing damage. Fourth instar nymphs of the grasshopper
(Chorthippus brunneus), were dosed topically with the PCB mixture
Aroclor 1254 (Moriarty, 1969). A single dose of either 12.5, 50, or
200 µg/insect was applied in a volume of 1 µl of 1,4-dioxan. No
sublethal effects were detected on either development or reproductive
potential. At the highest dose, there appeared to be a latent toxicity
that could be correlated with the mobilization of lipids at moult;
more moulted males died than unmoulted males over the test period.
Females took longer to moult than males and showed a distinctly
bimodal distribution in time to death, with a similar correlation
between toxicity and moult. Males showed 46% mortality and females,
41%, after treatment with 200 µg Aroclor 1254. Fungal infection
affected insects on the lower doses and mortality figures are,
therefore, unreliable.
Lichtenstein et al. (1969) exposed Drosophila melanogaster to the
dry residue of various PCBs. They exposed flies to Aroclors 1221,
1232, 1242, and 1248 at 200 or 800 µg. No mortality was observed after
48 h at 200 µg. At 800 µg, there was an increase in mortality with
decreasing chlorination (after a 48-h exposure to Aroclor 1221, 92%
had died; only 45% died after exposure to Aroclor 1248 over the same
length of time). No mortality was observed after a 48-h exposure to
2000 µg of Aroclors 1254, 1260, 1262, or 1268. In a separate study,
the authors treated houseflies (Musca domestica) topically with
either 10 or 20 µg (in 2 µl of acetone); mortality was assessed after
24 h. Results were comparable with those from the study on
Drosophila; deaths were dose related and occurred with Aroclors up
to 1254, where mortality was 10% at the higher dose. Aroclors of
higher chlorination than 1254 had no effect. Lower chlorinated
Aroclors had the greatest effect with more deaths at the highest dose
(20 µg) and lowest chlorination (Aroclor 1221) than with any other
treatment (43% killed). Plapp (1972) found that the 24-h LC50 for
Aroclor 1254 in the housefly (Musca domestica) was > 3000 µg/jar
where the Aroclor was added to a container in acetone, which was dried
before the addition of the flies. This was true for both
DDT-susceptible and DDT-tolerant strains. The same author reported a
powerful synergistic effect between carbaryl and Aroclor 1254; the
LC50 with carbaryl alone was calculated to be 1386 µg/jar and that
for carbaryl:PCB in the ratio of 1:5, 96 µg/jar. The Aroclor was as
powerful a synergizing agent as piperonyl butoxide.
Youssef et al. (1974) hatched eggs of the housefly (Musca domestica)
on a medium of paper tissue dosed with 0.808 g of Aroclor 1254 per
200 g of tissue. The adult flies hatching from the eggs were examined
using the electron microscope for effects on the male reproductive
tissue. The PCBs induced nuclear and flagellar abnormalities in
developing spermatids. Spermatid nuclei failed to elongate and
membranes originating from the nuclear envelope formed invaginations
into the nucleus. These resulted in the appearance of cytoplasmic
inclusions in the nucleus. Spermatid flagellae contained an abnormal
number of axonemes and mitochondrial derivatives; abnormal spermatids
did not coil and degenerated.
In a study by Wasilewska et al. (1975), female nematodes
(Acrobeloides nanus) were exposed to Aroclor 1254. Initially, 60 µg
of the Aroclor were added to a petri dish (on the surface of agar) in
which there were 20 nematode worms. The nematodes fed on a culture of
bacteria introduced to the agar at the same time as the worms. After 5
days of exposure, eggs and adult nematodes were counted and adult
weights determined. No significant effects were found. In a second
study, over a longer period (10 days), nematodes were exposed to 15,
30, or 60 µg of the Aroclor. Adverse effects increased with dose, and
even at the lowest dose, the number of adults was reduced from 123 to
76, the number of eggs from 539 to 288, and the weight of adults from
18.9 to 9.4 µg. At the highest dose of 60 µg per dish, the number of
adults was 32, the number of eggs, 37, and the weight of adults,
4.9 µg.
7.3.3 Birds
Five-day dietary LC50s for PCBs in birds ranged from 604 to >
6000 mg/kg diet (Table 35). Generally, the oral single dose LD50 and
the dietary LC50 data are similar to those for mammals. PCBs are less
toxic for birds than other organochlorines, such as DDT and its
metabolites and the chlorinated cyclodienes.
The toxicity of Aroclors in birds increases with the percentage
chlorination (generally reflected in the final 2 digits of the Aroclor
number), according to the data of Hill et al. (1975) and Hill &
Camardese (1986). Hill et al. (1974) noted that the toxicity of
Aroclors is not simply a reflection of the chlorine content of the
different Aroclors. They adjusted the dietary content of the Aroclors
to a constant dietary chlorine level and found the same increased
toxicity with higher Aroclor numbers.
Dahlgren et al. (1972) reported some mortality in sub-adult pheasants
after regular oral doses of Aroclor 1254 ranging from 10 to 210 mg.
Mortality was related to both dose and body weight; heavier birds
lived longer, though they lost a greater proportion of body weight. A
sudden heavy intake of PCBs led to high brain residues. Brain residues
were best correlated with death; residues in the brain of about
300-400 mg/kg were considered by the authors to be diagnostic of acute
poisoning and death (Dahlgren et al., 1972). Stickel et al. (1984)
concluded that similar levels in the brain killed red-winged
blackbirds, starlings, brown-headed cowbirds, and grackles. Intake of
lower doses of PCBs over long periods does not lead to such high brain
residues; the cause of death after long-term exposure appears to be
oedema and related symptoms.
7.3.3.1 Short-term toxicity
Hurst et al. (1973) observed differential toxicity of PCBs between
bobwhite quail hens and cocks. This differential was eliminated when
the tests were conducted on birds not in the breeding condition and
with short daylengths. Females survived better than males, only when
they were laying eggs, and survival was well correlated with the
numbers of eggs produced. The authors concluded that females reduce
their exposure to PCBs by eliminating the compound in the eggs.
When Koeman et al. (1969) fed Japanese quail a diet containing 2000 mg
Phenochlor DP6/kg, all the dosed birds died between 6 and 55 days of
dosing. The quail developed hydropericardia at this dose level. Vos &
Koeman (1970) fed one-day-old cockerels a diet containing PCBs at
400 mg/kg, for 60 days; the PCBs were in one of the following forms,
Phenochlor DP6, Clophen A60, or Aroclor 1260 (all 3 are 60%
chlorinated). The mean survival time was calculated to be 24.3 days
for Phenochlor and 20.5 days for Clophen, only 3 out of 20 birds died
on the diet containing the Aroclor. Microscopically, centrolobular
liver necrosis was found in chicks fed the first 2 compounds. Atrophy
of the spleen and porphyria were observed in all dosed groups.
Table 35. Toxicity of PCBs for birds
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Bobwhite quail 10 days diet Aroclor 1221 5-d LC50 >6000 Hill et al. (1975)
(Colinus virginianus) 10 days diet Aroclor 1232 5-d LC50 3002 (2577-3501) Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 2098 (1706-2610) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 1175 (966-1440) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 604 (410-840) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 747 (577-937) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 871 (702-1069) Hill et al. (1975)
male 1 year oral Aroclor 1268 acute LD50 >2000 Hudson et al. (1984)
Japanese quail 14 days diet Aroclor 1221 5-d LC50 >5000 Hill & Camardese (1986)
(Coturnix coturnix 14 days diet Aroclor 1232 5-d LC50 >5000 Hill & Camardese (1986)
japonica) 14 days diet Aroclor 1242 5-d LC50 >6000 Hill & Camardese (1986)
14 days diet Aroclor 1248 5-d LC50 4819 (4267-5443) Hill & Camardese (1986)
14 days diet Aroclor 1254 5-d LC50 2929 (2516-3409) Hill & Camardese (1986)
14 days diet Aroclor 1260 5-d LC50 2195 (1861-2589) Hill & Camardese (1986)
14 days diet Aroclor 1262 5-d LC50 2304 (1978-2684) Hill & Camardese (1986)
Table 35. (cont'd).
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Mallard 10 days diet Aroclor 1221 5-d LC50 >5000 Hill et al. (1975)
(Anas platyrhynchos) 10 days diet Aroclor 1232 5-d LC50 >6000 Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 3182 (2613-3879) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 2798 (2264-3422) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 2699 (2159-3309) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 1975 (1363-2749) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 3008 (2461-3634) Hill et al. (1975)
male 8-9 months oral Aroclor 1242 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1254 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1260 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1268 acute LD50 >2000 Hudson et al. (1984)
Red-winged blackbird diet Aroclor 1254 6-d LC50 1500 Stickel et al. (1984)
(Agelaius phoeniceus)
Ring-necked pheasant 10 days diet Aroclor 1221 5-d LC50 >5000 Hill et al. (1975)
(Phasianus colchicus) 10 days diet Aroclor 1232 5-d LC50 3146 (2626-3948) Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 2078 (1843-3879) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 1312 (1166-1477) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 1091 (968-1228) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 1260 (1106-1433) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 1234 (1086-1402) Hill et al. (1975)
Table 35. (cont'd).
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Starling diet Aroclor 1254 4-d LC50 1500 Stickel et al. (1984)
(Sturnus vulgaris)
Brown-headed cowbird diet Aroclor 1254 7-d LC50 1500 Stickel et al. (1984)
(Molothrus ater)
Grackle diet Aroclor 1254 8-d LC50 1500 Stickel et al. (1984)
(Quiscalus quiscula)
a oral = acute oral test (result expressed as mg/kg body weight); diet = dietary test (result expressed as mg/kg diet).
Miranda et al. (1987) studied the effects of acute oral exposure of
Japanese quail to Aroclor 1242 (100, 250, or 500 mg/kg), or
2,4,2',4'-tetrachlorobiphenyl and 3,4,3',4'-tetrachlorobiphenyl (both
87.6 mg/kg) in corn oil. Control birds received only the corn oil. The
birds were killed after 48 h. All the PCB compounds caused a
significant increase in porphyrin content and delta- aminolevulinic
acid synthetase (ALA-S) activity in the small intestine and liver. All
the compounds increased the cytochrome P-450 content of the liver. In
the intestine, the P-450 content was only increased by Aroclor 1242
and 2,4,2',4'-tetrachlorobiphenyl. The activity of 7-ethoxyresorufin
O-deethylase was increased by all compounds in both the intestines
and liver. In the liver, 7-ethoxycoumarin O-deethylase (ECOD)
activity was unchanged or decreased, but, in the intestines, ECOD
activity increased with dose. No tissue differences in ECOD activity
were found after treatment with 2,4,2',4'-tetrachlorobiphenyl and
3,4,3',4'-tetrachlorobiphenyl. It was concluded that the small
intestine was more responsive than the liver to the porphyrinogenic
effect of a single oral dose of PCBs, and, that the induction of drug
metabolizing enzymes in the quail was tissue-specific, depending on
the PCB preparation used.
Day-old chicks were fed on a diet containing 500 mg PCBs/kg. All the
birds died between the third and the tenth week of dosing; a reduction
in the dosage to 250 mg/kg delayed the onset of death until the
thirteenth week (Platonow & Funnell, 1971). Mortality did not occur in
chickens dosed at 200 mg/kg over a period of 3 weeks (Flick et al.,
1965). Harris & Rose (1972) fed one-day-old broiler chicks diets
containing 100, 200, or 400 mg PCBs/kg (Aroclors 1242, 1254, and
1260). No mortality occurred at doses of up to 100 mg/kg, over a
period of 4 weeks. Over the same period, all the birds on Aroclor
1242, 60% of the birds on Aroclor 1254, and none of the birds on
Aroclor 1260, died, all at a dosage of 400 mg/kg. Holleman et al.
(1976) fed day-old broiler chicks and turkey poults on diets
containing Aroclor 1242 at 38, 75, or 150 mg/kg for 4 weeks. The
authors found increased mortality at 75 mg/kg (21% mortality) with the
chicks, but this was not significantly different from controls;
however, there was a significant increase in deaths at 150 mg/kg (75%
mortality). Significantly increased mortality was not found in the
turkeys at any dose level, but the mortality rate in the controls was
33%. Both the 75 and 150 mg/kg diets produced oedema and other lesions
attributed to PCB toxicity. Prestt et al. (1970) maintained Bengalese
finches on a diet containing various concentrations of Aroclor 1254.
The estimated dose rate for 50% mortality, over 56 days, was 254 mg/kg
per day.
7.3.3.2 Egg production
Most studies demonstrating the lowering of egg production by PCBs were
conducted on chickens. The most severe effects came from dosing with
Aroclors in the middle of the range of chlorination (Aroclors
1232-1254). The literature has been reviewed by Stendall (1976).
Platonow & Reinhart (1973) fed chickens with Aroclor 1254 at either 5
or 50 mg/kg diet over 39 weeks. Egg production was erratically reduced
with the lower doses and sharply reduced on 50 mg/kg diet. A dietary
dose of 2 mg/kg did not have any reproductive effects on chickens over
9 weeks (Lillie et al., 1974) or after 39 weeks (Platonow & Reinhart,
1973). Scott et al. (1975) showed a 10% reduction in egg production in
chickens related to egg residues of PCBs (Aroclor 1248) of 3 mg/kg.
When egg residues reached 4.5 mg/kg, the production rate was further
reduced. A significant reduction in egg production was demonstrated by
Call & Harrell (1974) after dosing Japanese quail with 3 different
Aroclors at 62.5-5000 mg/kg, over 33-264 days.
7.3.3.3 Hatchability and embryotoxicity
Aroclors reduced the hatchability of chicken eggs. In 2 studies,
Lillie et al. (1974) examined the effects on hatchability of Aroclors
1221, 1232, 1242, 1248, and 1268, all fed at 2 or 20 mg/kg diet, over
9 weeks. Aroclors 1221 and 1268, with low and high chlorination,
respectively, showed no effects at 20 mg/kg diet. Aroclor 1248
produced some adult mortality at 20 mg/kg diet and nearly eliminated
hatching of the eggs produced. Aroclor 1242 showed similar, but
slightly less severe, effects; there was even less effect with Aroclor
1232. Cecil et al. (1974) tested a similar range of PCBs at the same
dosages (2 and 20 mg/kg diet). They also reported no effects for
Aroclors 1221 and 1268. Aroclors 1254, 1232, 1242, and 1248 reduced
hatchability, as in the previous study. PCBs that reduced hatchability
also produced abnormalities in the chicks. The fertility of eggs was
not affected by any of the treatments. Females were artificially
inseminated with semen collected from males fed a similar diet of
PCBs. Scott (1977) dosed chickens with 0.5, 1, 10, or 20 mg PCBs/kg
diet and found no effect at the 2 lowest doses. Hatchability was
reduced at 10-20 mg Aroclor 1248/kg diet. Kosutzky et al. (1979) dosed
chickens with 2 other PCBs (Delor 103 and 105) (42 and 54%
chlorination, respectively), at 5 mg/kg diet, for 6 weeks; there was
little effect on hatchability, which returned to control levels soon
after a return to a clean diet. Solomon et al. (1973) studied the
effects of PCBs (Aroclor 1254) on pheasants. The birds were dosed at
weekly intervals, for 17 weeks, with gelatin capsules containing
50 mg/bird for the hens or 25 mg/bird for the cock birds. No effects
were observed on fertility and there was no increase in the numbers of
abnormal embryos. In another study, Ax & Hansen (1975) maintained
white leghorn pullets on a diet containing Aroclor 1242 or 1254 at
20 mg/kg, or 2,4,5,3',4'-pentachlorobiphenyl, for a period of 10
weeks. Average embryonic mortality was found to be significantly
increased, i.e., 54.7, 59.2, and 74% for the 3 compounds,
respectively. In the same study, the authors found that average
embryonic mortality in eggs laid by birds dosed with
2,5,2'-trichloro-, 2,5,2',5'-tetrachloro-, or 2,4,5,2',4',5'-
hexachlorobiphenyl was not significantly different from that in the
controls. When both broiler breeder hens and leghorn hens were fed
diets containing either 20 or 50 mg Aroclor 1242/kg, for 1 week, the
hatchability of the eggs laid was reduced by 67.3 and 26.8%,
respectively, of control levels, on the 50 mg/kg diet (Briggs &
Harris, 1973). Hatchability also was reduced at 20 mg/kg diet, but the
time required to achieve the same depression as that found with the
higher dose was doubled. Even after dosing had finished,
embryotoxicity continued and, in fact, increased until, after 6 weeks,
hatchability was between 0 and 10% of controls for both birds at both
doses.
Chickens given Aroclor 1254 at 50 mg/litre in the drinking-water, for
6 weeks, showed progressive reduction in egg hatchability. This fell
to zero after 3 weeks (Bush et al., 1974). Hatchability remained at
almost zero for the first 8 weeks of the chickens receiving control
water following dosing, but returned to normal after a further 8
weeks.
Platonow & Reinhart (1973) fed chickens on a diet containing 50 mg
Aroclor 1254/kg for 39 weeks. There was some adult mortality; egg
production and hatchability fell almost to zero. Residues of Aroclor
in the last eggs produced ranged between 25 and 50 mg/kg. After 6
weeks of uncontaminated food, egg residues dropped and hatchability
improved. The authors reported that egg residues of less than 5 mg/kg
had no effect on hatchability, whereas residues greater than
10-15 mg/kg led to embryotoxic effects. Scott et al. (1975) related
hatchability to egg residues. At residue levels of 3 mg/kg,
hatchability was reduced by 44%, and, at residue levels of 4.5 mg/kg,
it was reduced to almost zero.
The yolk-sacs of eggs from pheasant, mallard, goldeneye duck, and
black-headed gull were injected with 3,4,3',4'-tetrachlorobiphenyl at
0.1 mg/kg (pheasant and mallard) or 1.0 mg/kg (pheasant, goldeneye
duck and black-headed gull) after 4 or 5 days of incubation (Brunström
& Reutergardh, 1986). A significant decrease in the hatching rate was
seen only in pheasants, at the highest dose. At a dose of 1 mg/kg, all
the embryos died before hatching, but, at 0.1 mg/kg, no effect on
hatching was observed. No gross abnormalities were noted in either
hatched chicks or dead embryos. A great difference was noted by the
authors between the avian embryos in this study and chicken embryos,
with regard to sensitivity towards tetrachlorobiphenyl. In chicken
embryos, a dose of 0.004 mg/kg, administered on day 4 of incubation,
gave a significant reduction in hatching. At 0.02 mg/kg, no embryos
survived to hatching (Brunström & Darnerud, 1983). Carlson & Duby
(1973) injected Aroclors directly into chicken eggs on the first day
of incubation, or 9 days later. Aroclor 1242 severely limited
hatchability at levels of more than 2.5 mg/kg. Aroclors 1254 and 1260
had no effect at 10 mg/kg. Delaying the injection until day 9 of
incubation reduced the effect of Aroclor 1242. With 5 mg/kg injected
at day zero, hatchability was 8.3%; when the same dose was given at
day 9, 82% of eggs hatched. These results are not compatible with
those of Scott et al. (1971) who reported that most embryonic deaths
occurred late in incubation. However, these authors were not dosing
the eggs directly, but measuring the residues in eggs from dosed
females. PCBs administered directly into the eggs may not produce
effects comparable with those produced by the same material received
from the mother hen, because of different distribution in the egg.
Platonow & Reinhart (1973) dosed hens at 50 mg/kg diet. Early in the
study, the majority of embryo deaths occurred late in incubation. As
the study progressed, the time of embryo death moved to earlier in the
incubation period. Bush et al. (1974) showed that there was greater
mortality for any given egg residue as the period of dosing the mother
hen progressed. Their experimental chickens were dosed for 6 weeks at
50 mg/litre drinking-water and then kept for a further 20 weeks on
clean water. On day 11 of the study, an egg yolk residue of 50 mg/kg
was associated with 50% mortality in the embryos. On day 131, 50%
mortality was associated with an egg residue of only 10 mg/kg. The
greatest toxicity of PCBs for chicken embryos occurred after 11 weeks
of clean water, that is 17 weeks into the study. At this stage, the
eggs would be receiving doses of PCBs from material stored within the
hen. Late in the study, residues of between 6 and 8 mg/kg in egg yolks
(equivalent to 3.6 mg/kg whole egg) were correlated with between 14
and 36% mortality. Platonow & Reinhart (1973) reported that egg
residues greater than 10-15 mg/kg caused embryotoxic effects whereas
low residues of less than 5 mg/kg did not produce any effects.
Abnormalities were reported by Cecil et al. (1974) in 34% of 843
embryos that died during their study. The most common abnormality was
oedema, which was seen in 50% of all chicks showing any abnormality.
Tumasonis et al. (1973) also reported deformities in chicks.
7.3.3.4 Eggshell thinning
Since the 1950s, thin eggshells have been characteristic of many wild
bird populations, though the effect was not noticed until some time
afterwards. Thin shells has been a contributory factor to reduced
reproductive capacity, particularly in birds of prey. The main
chemical causing thin shells appears to be DDE, a metabolite of DDT.
It has been shown to cause thin eggshells in laboratory experiments,
as well as through the correlation of field data. Literature on thin
eggshells has been reviewed by Cooke (1973). Most experimental studies
using PCBs have shown no effect on shell thickness. Peakall (1971), in
the first controlled study, dosed ring doves at 10 mg PCBs (Aroclor
1254)/kg diet or at 25 mg (equivalent to 160 mg/kg body weight)
injected ip. Shells were ashed and weighed. Two separate studies on
dietary dosing showed no effect on shell weight. In the first study, 2
groups of birds were compared, in the second the same birds were used,
comparing their eggs before and after dosing. Injection of PCBs, 1-4
days prior to egg laying, also had no effect. Studies on mallard dosed
with Aroclor 1254 at 25 mg/kg diet and bobwhite quail dosed at
50 mg/kg diet over 2 years and also on mallard dosed at up to
500 mg/kg diet for 5 weeks (Heath et al., 1972) showed no effects on
shell thickness. There was an apparent shell thickening of about 6% at
the highest dose, which could not be statistically confirmed. The same
authors outlined results from a study on white leghorn chickens.
Aroclor 1242 at 10 or 100 mg/kg diet or Aroclor 1254 at 100 mg/kg diet
did significantly reduce shell thickness. There were no measurable
effects of: Aroclor 1242 at 1 mg/kg diet, Aroclor 1254 at 10 mg/kg, or
Aroclor 1260 at 100 mg/kg diet (Heath et al., 1972). Experimental
details and detailed results were not given for the work on chickens.
Lillie et al. (1974) dosed chickens with a range of Aroclors, at 2 or
25 mg/kg diet, for 9 weeks. Aroclors 1248 and 1242 greatly reduced the
hatchability of eggs and caused some adult mortality, but failed to
cause thinning of the eggshells. When Britton & Huston (1972) fed
single comb White Leghorns Aroclor 1242 at 80 mg/kg diet, for 6 weeks,
no effects on shell thickness were observed. Dahlgren & Linder (1971)
failed to demonstrate any deleterious effects on the eggshells of
pheasants, dosed by gelatin capsule, once a week for 17 weeks, with
doses of Aroclor 1254 up to 50 mg. Call & Harrell (1974) fed various
Aroclors in the diet to Japanese quail for 21 days. Very significant
shell thinning was found with Aroclors 1254 and 1260 at doses of 1250
and 1000 mg/kg diet, respectively. At these high doses, egg production
was severely diminished and shell dimensions were based on very few
eggs. Adult mortality might have occurred at these doses; the paper
does not make it clear whether this actually happened. At lower doses
of 78.1 and 62.5 mg/kg diet of Aroclors 1254 and 1260, respectively,
there was also significant egg-shell thinning and reduced egg
production. Aroclor 1242 was tested at 312.5 and 5000 mg/kg diet and
both doses caused shell thinning, though this was to a lesser degree
than with other Aroclors at similar doses. Risebrough & Anderson
(1975) showed that eggshells thinned by dietary DDE were not further
affected by adding PCB (Aroclor 1254) to the experimental DDE diet.
Results on shell thinning are, therefore, not completely clear. It is
generally agreed that PCBs do not affect birds in this way and the few
results suggesting shell effects are regarded as anomalous or
difficult to interpret, because of experimental design. Shell thinning
can occur because of several different direct and indirect factors.
DDE and sulfanilamide have direct effects on the deposition of calcium
in the shell or on its mobilization from the skeleton, which acts as a
calcium store. PCBs are more likely to affect shells indirectly by
reducing food consumption; none of the studies cited above reported
whether individual birds took less food because of the dosing with
Aroclors. Haseltine & Prouty (1980) fed 24 pairs of mallard with
Aroclor 1242 at 0 or 150 mg/kg diet for 12 weeks and reported a
reduction in shell thickness of 8.9%. They pointed out that all
females laying thin-shelled eggs showed a significant depression in
body weight. This, they regarded as sufficient explanation for the
shell thinning. Much of the shell thinning found in Japanese quail
eggs, laid by females given a single oral dose of Aroclor 1254 of
500 mg/kg body weight, was thought to be due to reduced food
consumption (Haegele & Tucker, 1974).
Biessmann (1982) did not find any effects on eggshell thickness on
dosing Japanese quail with Clophen A60 at levels of up to 150 mg/kg
diet. However, the breaking strength of the eggs was reduced.
Hill et al. (1976) fed 6-month-old laying Japanese quail hens a diet
containing 10 mg Aroclor 1242/kg, for 40 days. Eggs were collected and
measured and, after 40 days, were found to have significantly thinner
shells (5.2%) than the controls. The authors stated that the handling
of the birds and the diet had no effect on food consumption or hen
weights during the test. This is the only study showing shell thinning
at moderate dose levels without an effect on food consumption. The
question of whether PCBs can cause shell thinning, therefore, remains
open.
7.3.3.5 Effects on the male
Platonow & Funnell (1971) kept day-old, white leghorn cockerels on a
diet containing 250 mg Aroclor 1254/kg, for up to 13 weeks. They found
a significant reduction in the weight of both combs and testes after 9
and 13 weeks and, a reduction in comb weight, only, after 6 weeks of
dosing. In a later study, Platonow & Funnell (1972) found a more
severe effect at 500 mg/kg; the comb was significantly reduced in
weight after just one week of dosing and the testicular weight
significantly reduced after 4 weeks, relative to the controls. The
control combs and testes increased in weight during the course of the
study; treated birds failed to develop either comb or testes.
Lillie et al. (1974) did not find any effects on weight gain, food
intake, or semen characteristics in leghorn cockerels fed Aroclor 1248
at 10 or 20 mg/kg diet for 8 weeks. They also did not find any effects
on fertility or hatchability of fertile eggs laid by similarly dosed
females. Liver weights were significantly increased at both dose
levels, and heart weights were significantly decreased at the highest
dose.
7.3.3.6 The effects of stress
Stress, imposed in various ways, increases the sensitivity of birds to
PCBs. Stress seems to have its effect by increasing the mobilization
of fat. Lower fat storage decreases the attenuation of PCB toxicity
seen when fat uptake of the material acts as an effective temporary
detoxification mechanism. Dahlgren et al. (1972) showed that brain
residues were higher in pheasants subject to starvation stress than in
unstressed birds dosed at the same rate. deFreitas et al. (1972)
obtained similar results using cold stress or starvation in pigeons.
As a corollary, biochemical adaptation to stress is inhibited by
exposure to PCBs. This is presumed to be due to residues in non-lipid
tissues (Dieter, 1974).
7.3.3.7 Physiological, biochemical, and behavioural effects
Jefferies & Parslow (1972) dosed young lesser blackbacked gulls
(Larus fuscus) with daily gelatin capsules containing Aroclor 1254
at 50, 100, 200, or 400 mg/kg body weight, for 8 weeks. Mean
individual thyroid weights were significantly increased by 32% (taking
all dosed birds as a single group). There was also an increase in the
mean cross-sectional area of the thyroid. There was, however, no
dose-related effect of PCBs on thyroid weight. The same authors
(Jefferies & Parslow, 1976) showed a similar effect of increased
thyroid weight when they dosed guillemots (Uria aalge) for 45 days
with Aroclor 1254 at 12 or 25 mg/kg body weight. Hurst et al. (1974)
also found a significant stimulation of thyroid growth after feeding
bobwhite quail a diet containing 5, 50, or 500 mg Aroclor 1260/kg for
4 months. Spear & Moon (1985) raised ring doves on either a low iodine
or normal diet. Insufficient iodine caused thyroid hyperplasia. This
hyperplasia was reversed within 7 days by a single dose of
3,4,3',4'-tetrachlorobiphenyl at 60 mg/kg body weight. The PCB
treatment also caused a significant decrease in core body temperature
and serum total thyroxine (T4) and triiodothyronine (T3). No effect,
other than decreased serum T3 and T4, was caused by dosing doves with
PCBs on a diet containing normal iodine levels.
Behavioural effects of PCBs have been noted by several authors.
Peakall & Peakall (1973) reported decreased parental attentiveness in
ring doves dosed at 10 mg Aroclor 1254/kg diet. Kreitzer & Heinz
(1974) measured the avoidance response (from a moving silhouette) in
Japanese quail chicks, for 14 days before, and 8 days after, dosing
with Aroclor 1254 at 200 mg/kg diet. After dosing, the avoidance
response was significantly reduced. Normal responsiveness to the
silhouette was not recovered after 6 days on a clean diet. Two
examples of hyperactivity in birds were also noted. European robins
fed one mealworm/day containing 5 µg Clophen A50, for 11-13 days,
showed increased migratory restlessness (Ulfstrand et al., 1971).
There were similar tendencies in redstart fed one mealworm/day,
containing 11µg Clophen A50, for 12 days, it was estimated that the
birds had ingested 132 µg of PCB overall (Karlsson et al., 1974).
A reduction was reported by Dobson (1981)in the nest-building activity
of pigeons (Columba livia), dosed orally, by gelatin capsule, with
15 mg Aroclor 1254/day, throughout a courtship cycle. The birds
produced a nest but the number of twigs used was reduced compared with
the controls. Reproductive and thyroid hormones were measured in blood
plasma samples, taken each day during the courtship cycle. While the
patterns of hormone secretion remained the same in both control and
treated birds (rises and falls of hormone levels occurred at
comparable times in the 2 groups) the absolute circulating levels of
the hormones were changed by the treatment. Both thyroxine and
luteinising hormone levels in the treated birds were elevated relative
to the controls. The levels were significantly higher, except at the
beginning and the end of the cycle. It was concluded that hormone
levels were unaffected, except when they would naturally be changing,
suggesting an interference with the feedback control of hormone
secretion and a central nervous site of action. Tori & Peterle (1983)
kept mourning doves (Zenaida macroura carolinensis) on a diet
containing Aroclor 1254 at 10 or 40 mg/kg for 42 days. The doves were
then paired and observed each day for 30 days. Both treatments
significantly increased the mean number of days in the courtship
phase; only 4 out of 8 pairs on 10 mg PCBs/kg completed this phase and
moved onto the nesting phase; none of the birds on 40 mg/kg had
completed the courtship phase within 30 days. Behaviour was scored to
measure intensity and, at both doses, this was significantly reduced
overall. Although dosed birds formed pair-bonds approximately 4 days
sooner than controls, there was no significant difference in the
length of the pair-bond formation period or in behaviour scores during
this period in doves fed 10 mg/kg. The length of time spent in the
courtship period was extended significantly (by 8.5 days) by PCBs at
10 mg/kg. Behaviour scores were not significantly affected, but dosed
birds averaged 32% lower scores. Of the birds reaching the nesting
phase, there was no significant difference between controls and dosed
birds with regard to length of time spent nesting or behaviour scores
in the nesting phase. PCBs, however, significantly delayed the onset
of nest initiation (by approximately 7 days) and, therefore, egg
laying.
Japanese quail were dosed with Clophen A60 in the feed at 150 mg/kg,
from the first week of life up to 42 days of age, while the birds were
developing sexually and becoming reproductively mature (Biessmann,
1982). In females, progesterone levels were not greatly affected by
the PCBs, but estradiol levels in blood plasma were lower before
sexual maturity and were less stable during egg laying. In males,
levels of testosterone and dihydrotestosterone (the primary
metabolite) were not affected. Quail fed up to 150 mg Clophen A60/kg
diet during the time of sexual maturation (second to fourth weeks of
age) showed delayed onset of egglaying and a diminished capacity to
lay eggs. Hormone levels in both males and females were not
significantly different from those in the controls.
A possible mechanism for central nervous effects was provided from
studies on neurotransmitters. Dopamine and noradrenalin were depleted
in the brain of the ring dove in a dose-related manner with increasing
brain residues of PCBs (Heinz et al., 1980).
7.3.3.8 Interactive effects with other chemicals
The only information on the interaction between PCBs and other
chemicals in birds has shown PCBs to be additive and not synergistic.
Kreitzer & Spann (1973) carried out tests on several pairs of
chemicals to study pesticidal synergism in young pheasants and
Japanese quail. Two PCBs were used in the study. Aroclor 1262 and
malathion showed additive results, when fed in the diet to 16-day-old
Japanese quail. Aroclor 1254 and DDE were also additive, when given in
the diet to 9-day-old quail. Another study on the possible interactive
effects of PCBs was conducted by Heath et al. (1972), who found that
feeding Aroclor 1254 and DDE in the diet to 14-day-old Japanese quail
gave additive results. There was no evidence of mutual potentiation or
antagonism.
7.3.4 Terrestrial mammals
Acute oral LD50 values reported for PCBs in mink ranged from >750 to
4000 mg/kg body weight. Acute LD50 values for 3 Aroclors in mink were
determined by Aulerich & Ringer (1977) after administration orally, by
gavage, or by intraperitoneal injection. Mortality was assessed 4 days
after i.p. administration and 14 days after oral administration. The
lethality of the Aroclors was found to be inversely related to the
chlorine content; Aroclor 1221 was most toxic and Aroclor 1254 least
toxic (Table 36). This is in marked contrast to the situation in
birds, where toxicity was correlated positively with chlorine content
of the PCBs (see section 7.3.3).
Table 36. Acute toxicity of Aroclors for minka
Aroclor LD50 (mg/kg body weight)
Intraperitoneal Oral
Aroclor 1221 >500-<750 >750-<1000
Aroclor 1242 1000 >3000
Aroclor 1254 >1250-<2250 4000
a From: Aulerich & Ringer (1977).
7.3.4.1 Short-term toxicity
Ferrets (Mustela putorius furo), fed a diet containing 20 mg of
Aroclor 1242/kg, for 8 months, developed enlarged, thickened, and
deformed toe-nails with hyperkeratosis at the junction of the skin and
sponchium, and dysplasia of the root of the nail and the matrix. The
same diet containing Aroclor 1016 did not produce these effects
(Bleavins et al., 1982).
Bleavins et al. (1980) showed the ferret to be less sensitive to PCBs
than the mink, though LD50 values were not determined. Aroclor 1242
at 20 mg/kg diet killed all mink (3 males and 12 females) to which it
was fed. The same diet did not kill any ferrets, though it did cause
reproductive failure.
No mortality occurred in mink fed a diet containing 1 mg PCBs/kg over
183 days (Wren et al., 1987a).
Hornshaw et al. (1986) conducted 28-day LC50 tests on mink, using
Aroclor 1254, in a study to investigate the effects of age, season,
and diet on the toxicity of PCBs; no effects were noted on any of
these parameters. In replicate tests, the calculated LC50 values
varied between 79 and 84 mg/kg diet (48-132, range of confidence
limits). The authors noted that the period of observation after dosing
was critical in assessing the results, since mortality continued after
dosing had stopped and the PCBs were persistent in the body. Taking
total mortality over 28 days of dosing and a further 7 days of
observation, the LC50 values fell to between 47 and 58 mg/kg diet.
A consistent finding among various studies is an effect of PCBs on
food consumption and, therefore, on body weight. The most detailed
analysis of food consumption during the feeding of Aroclor 1254 to
mink is presented by Hornshaw et al. (1986). Young mink fed the
Aroclor over 28 days showed a dose-dependent decrease in the amount of
food consumed. The cumulative weight of food consumed over 5 weeks
(1 week predosing and 4 weeks dosing) for controls was 7574 g. This
was reduced progressively with increasing dose of Aroclor 1254 at 10,
18, 32.4, 58.3, and 105 mg/kg diet to 6447, 6153, 4816, 3556, and
2723 g, respectively. This led to loss of original body weight over
the study period rising to more than 40% at the highest dose. Similar
effects were seen in adults of both sexes; females, with a smaller
initial body weight, were more severely affected than males. This
effect has implications for the interpretation of results of dietary
toxicity tests. The effects seen are a combination of the direct toxic
effects of the compound and the indirect effects of progressive
starvation. The doses of PCBs to which the animals are exposed must
also be calculated with reduced food intake in mind; apparent doses
are higher than real exposure as dose rates increase.
Organ weights (expressed as a percentage of brain weight) were
unaffected by Aroclor 1254 at doses up to 105 mg/kg diet, with the
exception of the heart and the adrenal glands. Heart weight was
reduced in both adult and young mink fed Aroclor 1254 at 58 mg/kg diet
or more; adrenal weights were increased by doses of 13 mg/kg diet or
more (Hornshaw et al., 1986).
Female minks received 0.1 or 0.5 mg of 3,4,5,3',4',5'-
hexachlorobiphenyl/kg diet, or 2.5 or 5.0 mg of 2,4,6,2',4',6'- and
2,3,6,2',3',6'-hexachlorobiphenyl/kg diet for 12.5-14.5 weeks. In both
studies, 3,4,5,3',4',5'-hexachlorobiphenyl was the most toxic isomer,
causing high mortality and reduced body weights (Aulerich et al.,
1985).
Clark & Prouty (1977) fed female big brown bats (Eptesicus fuscus)
on a diet of meal-worms containing 10 mg Aroclor 1254/kg. After the
feeding period of 54 days, the bats were starved to simulate loss of
body fat during the period of migration (when the animals do not
feed). Two out of 12 bats died. The brain residues of 20 mg PCBs/kg at
the end of the study were considered to be sub-lethal, since no
neurotoxic symptoms were observed before death.
7.3.4.2 Reproductive effects
Experimental investigations of mink reproduction in relation to
environmental pollution were carried out as a result of the reduced
reproductive success seen after feeding farm mink with fish from the
Great Lakes. Early studies, therefore, involved the analysis of fish
for pollutants and the experimental feeding of both the fish and of
mixtures of chemicals contained in various fish in the Great Lakes.
Aulerich & Ringer (1977) performed a comprehensive series of feeding
studies using coho salmon from 2 of the Great Lakes (Michigan and
Erie), other fish species from the same source, salmon from the west
coast of the USA, and various combinations of organochlorine
contaminants.
In their first study, ocean fish (perch or whiting) were used as
control diets and the reproductive performances were compared of dosed
and undosed female mink mated with undosed males. Lake Michigan coho
salmon, as 30% of the diet, had the most severe effect on
reproduction, i.e., total reproductive failure, as measured by numbers
of live kits surviving 4 weeks after parturition. Coho salmon from
Lake Erie also reduced reproductive success; 12 females produced only
7 kits still alive 4 weeks after birth. Two other species of fish from
Lake Michigan produced less severe effects, 5 kits being produced on a
diet of 30% bloater chub and 15 kits, on a diet of yellow perch.
Controls produced more than 40 kits over the same period. Kits
produced on Lake Michigan or Lake Erie fish, other than salmon, and
surviving to 4 weeks of age showed significantly lower body weights
than the controls. The small numbers of surviving kits from mothers
fed Lake Erie salmon also showed reduced body weight at 4 weeks of
age. No kits were produced after feeding Lake Michigan salmon diets to
females (Aulerich & Ringer, 1977).
In a long-term, low-level feeding study, 4 different Aroclors (1016,
1221, 1242, and 1254) were included in the diet of mink at a rate of
2 mg/kg. Groups of 8 female and 2 male animals were given this, or a
control, diet for 11 months, from August to June. The reproductive
performance of the animals on different diets is summarized, together
with mortality, in Table 37. Body weight, haemoglobin levels, and
haematocrit were monitored at monthly intervals during the study and
no significant effects of PCBs were noted. Only one of the test diets
(containing Aroclor 1254) adversely affected reproduction, with only 1
live birth in the study period. This single kit was considerably
lighter at birth than the controls and failed to survive 4 weeks after
birth (Aulerich & Ringer, 1977).
The reproductive effects of either Lake Michigan coho salmon or
Aroclor 1254 were reversible, when animals were transferred to a
control diet. Eleven females fed salmon as 30% of the diet for a year,
and then given control food for a further year, produced young with an
average litter size of 3.5 kits per mated female, in the second year
of the study. No young had been produced in the first year of the
study, during dosing. Similarly, 3 females given a year of control
food following a year on a diet containing 5 mg Aroclor 1254/kg,
produced an average of 4.3 young per mated female in the second year
of observation (Aulerich & Ringer, 1977).
In a later study, Bleavins et al. (1980) fed 2 different Aroclors at
various dose levels to mink and ferrets. Aroclor 1242 was fed to mink
at doses ranging from 5 to 40 mg/kg diet; Aroclor 1016 was given at
only 20 mg/kg diet. Results for mortality and reproductive effects are
summarized in Table 38, together with some data for Aroclor 1254 taken
from Aulerich & Ringer (1977).
There was a clear reproductive effect of Aroclor 1242 at a dose of
5 mg/kg diet, but no significant mortality. Aroclor 1242 caused 66%
mortality at 10 mg/kg diet and 100% mortality at 20 mg/kg diet.
Aroclor 1016, at 20 mg/kg diet, caused some deaths of adults and
reduced birth-weight and survival of kits, but the reproductive
effects were considerably less severe than those caused by Aroclor
1242 at 5 mg/kg diet. The authors (Bleavins et al., 1980) calculated
dietary LC50 values for Aroclors 1242 and 1254, using their own data
and data from Aulerich & Ringer (1977), to be 8.6 and 6.7 mg/kg diet,
respectively. It is clear that reproductive effects are less marked at
lower levels of chlorination of Aroclors.
Table 37. Effects of Aroclors on mortality and reproduction in minka
Aroclorb Adult females Kits
Number Number Number Number born Whelped/ Alive at Average weight
died (%) mated whelped female 4 (g ± SE) at
live dead mated weeks birth
Control 0 8 8 28 5 4.1 18 9.9 ± 0.32
1016 0 8 8 28 8 4.5 16 9.2 ± 0.33
1221 12 7 7 43 1 6.3 37 9.6 ± 0.22
1242 12 7 7 35 4 5.6 32 9.3 ± 0.27
1254 12 7 2 1 1 0.3 0 5.4
a From: Aulerich & Ringer (1977).
b Aroclors all fed at 2 mg/kg diet.
Reproductive effects on mink were reported by Jensen et al. (1977),
who fed groups of 10 females at 0.05, 3.3, or 11 mg PCBs/kg diet (type
unspecified), for 66 days. The highest dose eliminated successful
reproduction, whereas 3.3 mg/kg severely reduced the number of kits
born per female.
Table 38. Summary of mortality and reproduction in mink fed various dietary
levels of Aroclors
Treatment level Period fed Number dead/ Number of kits/
(mg/kg diet) (days) total number female
Aroclor 1254a
0 280 1/7 5.0
0 297 0/8 4.1
2 297 1/8 0.3
5 280 2/7 0.0
10 280 5/7 0.0
Aroclor 1242b
0 247 3/30 4.9
2 297 1/8 5.6
5 247 1/15 0.0
10 247 10/15 0.0
20 192c 15/15 0.0
40 138d 15/15 0.0
Aroclor 1016b
0 247 3/30 4.9
2 297 0/8 4.5
20 247 3/15 6.3
a From Aulerich & Ringer (1977).
b From: Bleavins et al. (1980).
c All mink died within 192 days on diet.
d All mink died within 138 days on diet: no females survived to whelping.
Wren et al. (1987b) did not find any significant effects on numbers of
kits produced or surviving to weaning age (5 weeks) after feeding mink
with Aroclor 1254 at 1.0 mg/kg diet, over 183 days. The dosing period
covered the seasonal period when the animals came into breeding
condition as well as a period of giving birth to the young. Although
the weights of kits born to dosed females were not significantly
different at 1 week postpartum, the weight gain of kits was then
affected and weights were significantly different from those of the
controls at ages 3 and 5 weeks. At age 5 weeks, when the kits were
weaned, the mean body weight of kits of the controls was 227.8 g,
while the mean body weight of kits fed by dosed mothers was 161.2 g.
Similar effects on the reproduction of female mink fed PCBs (type
unspecified) were observed by Jensen et al. (1977), i.e., a reduction
in the numbers of whelps born per pregnant female. The authors killed
the females after they had given birth and examined the numbers of
implantation sites in the uterus. This did not differ statistically
between groups (on average, 6.6 in control females, 6.1, in females
fed 5 mg/kg diet, and 4.5, in females fed 15 mg/kg diet PCBs).
However, the number of kits born to the same females showed a marked
effect of the PCBs: 5.1 (on average) born to control mothers; 2.9, to
mothers fed 5 mg/kg diet PCBs, and 0, to mothers fed 15 mg PCBs/kg
diet. The authors concluded that the effects of PCBs occur at the time
of implantation or later, causing resorption of implanted embryos.
Male mink seemed unaffected by doses of Aroclor that caused
reproductive effects in females. Males, dosed and mated with undosed
females, fathered normal numbers of kits (Aulerich & Ringer, 1977).
Wren et al. (1987b) did not note any effects of Aroclor 1254, fed to
male mink over 183 days at a rate of 1 mg/kg diet. Testicular size and
testicular histology were unaffected by the PCBs at any stage of the
reproductive cycle.
Treatment of adult, male, white-footed and white mice with Aroclor
1254 in the diet at a level of 400 or 200 mg/kg (equivalent to 57 or
29 mg/kg body weight), for 2 weeks resulted in a reduced testicular
spermatozoan concentration and, in the white-looted mice, a reduced
absolute weight of the seminal vesicles. In both strains, the absolute
weights of the testes and the final body weights were unchanged
(Sanders & Kirkpatrick, 1975; Sanders et al., 1977).
The reproductive performance of 27 pairs of white-looted mice (44-222
days of age), was compared with that of 26 control pairs, within 60
days of exposure to Aroclor 1254 at a dietary level of 200 mg/kg.
One-third of the exposed pairs did not survive the exposure but
produced at least one litter. The number of pairs producing at least
one litter was reduced by 65% and the number producing 2 or more
litters was reduced by 91%. Litter size was not affected. No offspring
survived to weaning in the 7 first litters of PCB-fed pairs (Merson &
Kirkpatrick, 1976).
A group of 10 pairs of wild-caught, white-footed, mice and groups of
18 (12 weeks of age) and 19 (16 weeks of age) pairs of
laboratory-raised, white-looted mice received Aroclor 1254 in the diet
at 10 mg/kg. Control groups comprised 10, 15, and 20 pairs,
respectively. The reproductive performance of the first group was
recorded for 18 months. The duration of the other studies varied from
7 to 15 months. The number of young per litter, 28 days after birth,
was lower in all treated groups. In laboratory-raised mice paired at
12 weeks of age, the birth interval was increased and the number of
young per litter at birth reduced (Linzey, 1987a). The second
generation of mice, maintained on the same diet as their parents, did
not differ in weight at birth, but were significantly smaller at 4, 8,
and 12 weeks of age. A similar trend was observed in the few young of
the third generation. The uterus, ovaries, and accessory glands, but
not the testes, weighed less in exposed groups than in the controls
(Linzey, 1987b). Linzey (1987a) reported similar reproductive effects
of Aroclor 1254 at a much lower dose.
The author suggested that the major consistent effect on the survival
of the young being fed milk was the result of much higher levels of
PCBs being transported via lactation than via the placenta.
Cottontail rabbits (Sylvilagus floridanus) were fed Aroclor 1254 at
10 mg/kg diet for 12 weeks, and then transferred to a clean diet and
allowed to breed. No effects were observed on any reproductive
parameters, and reduction in food availability did not change this
lack of effect (Zepp & Kirkpatrick, 1976).
7.3.4.3 Physiological effects
Wren et al. (1987a) examined histologically various organs in male and
female mink, dosed for 183 days with Aroclor 1254 in the diet. At
autopsy, no effects were seen on the histology of the pituitary and
adrenal glands. Brain histology also appeared normal. Thyroid
follicles gave the general appearance of minimal activity but did not
differ between treatments. Measurement of plasma thyroid hormones
(T3 -triiodothyronine; T4 -thyroxine) did not show any significant
differences between treatments in male mink. Females showed reduced
circulating T3 in a single sample, in January, but no other
differences were seen at other stages of the study. Thyroxine levels
were not affected at any time.
7.4 Effects on organisms in the field
The acute toxicity of PCBs is relatively low for most species and will
not, therefore, kill enough individuals to affect populations.
However, because of the high potential for bioaccumulation sufficient
residues of PCBs may build up to cause direct lethal effects over
time. Although PCBs are almost universally present in the tissues of
organisms in the environment, there are relatively few examples of
proved effects of these residues on populations of the organisms.
Sublethal effects, affecting populations by reducing reproduction or
growth, are possible, but difficult to prove, because PCBs are always
present with other environmental contaminants. Many possible effects
of PCBs in the environment have been suggested in the literature, but
few have actually been investigated in the field. It has proved
difficult, if not impossible, to relate residues of PCBs in tissues to
possible sublethal toxic effects; residues found after laboratory
dosing cannot be directly related to the field situation.
7.4.1 Plants
Klekowski (1982) studied the ostrich fern (Matreuccia struthiopteris)
growing in the flood plain of the Housatonic River, Massachusetts,
USA. This area of the river is contaminated with PCBs from land-fill
sites containing waste materials from the manufacture of transformers
in the nearby city of Pittsfield. Contamination with PCBs (principally
Aroclor 1254) had been a regular feature of the river area for a
period of more than 40 years. The frequency of somatic mutations in
the fern population was compared to a control population from an
uncontaminated area. The levels of PCBs in river sediments ranged from
1.4 to 139 mg/kg dry weight; at the site where the majority of fern
spores were collected, the level of PCBs was 26.3 mg/kg. The somatic
mutation frequency for the contaminated population was 5.2-6.2 times
higher than that for the controls. It is not known whether similar
genetic damage had occurred in other inhabitants of this contaminated
habitat. No other studies seem to have been conducted on the possible
effects of PCBs, from land-fill sites, on plants.
7.4.2 Fish
There have been many suggestions in the literature that PCBs might
affect populations of fish in the wild. Studies attempting to
demonstrate such an effect are few and, generally, inconclusive or
negative.
Olofsson & Lindahl (1979) used the ability of the cod (Gadus morrhua)
to react to different velocities of water under rotary flow, to
examine the effects of water pollution. Cod sampled from polluted
waters off the Swedish Coast were compared with cod sampled from
unpolluted areas. The ability of the fish to react to rotary flow was
significantly reduced in animals from polluted areas. However, the
authors were unable to relate the reduced reaction of the fish to
levels of various pollutants measured in muscle tissue. Experimental
studies showed that PCBs affected the reactions of the fish; the
residue level in muscle that was associated with this effect was
1.8 mg/kg. This was 30 times greater than the actual residues of PCBs
measured in the cod from the polluted area. While the authors stated
that the distribution of the PCBs in experimental fish and fish taken
from the wild would almost certainly have been different, that PCBs
exert the above effect in the field must be regarded as not proved.
Zitko & Saunders (1979) collected eggs of Atlantic salmon (Salmo
salar) from various areas and measured the PCB contents of the eggs
that proved infertile. The hatchability of different batches of eggs
was tested in the laboratory. No correlation was found between
residues of PCBs and the hatchability of the eggs; in fact the batch
of eggs showing the lowest hatchability also showed very low residues
of PCBs. However, it should be stated that hatchability was seldom
affected by PCBs in laboratory experiments; effects were more usually
seen on the developing young. Hogan & Brauhn (1975) related the
survival of fry hatched from rainbow trout (Salmo gairdneri) eggs to
the contamination of the eggs with PCBs. Five batches of eggs hatched
in 1971 had shown percentage mortalities, 30 days after hatching,
ranging from 10 to 28%. The eggs contained total organochlorine
residues of between 0.31 and 1.30 mg/kg, the majority of which was
PCBs. A batch of eggs collected in 1972 showed 75% mortality, 30 days
after hatching. Many of the fish hatched with such deformities as
scoliosis, lordosis, kyphosis, absence of caudal vertebrae, cranial
deformities, and projecting mandibles. This batch of eggs contained
2.7 mg PCBs/kg and 0.09 mg total DDT/kg (metabolites present not
stated).
Westin et al. (1983) investigated the effects of PCBs, passed on to
the eggs of striped bass (Morone saxatilis) by the female fish, and
of PCBs in food organisms fed to the hatched larvae. The hatchlings
were fed on brine shrimp ( Artemia sp.) from 2 sources, one
contaminated with PCBs and the other not. No effects of either
maternal PCBs or PCBs from the food were found. Residues of PCBs in
young fish decreased consistently throughout the study, which was
conducted in water free of PCBs. The authors suggested that PCBs from
the mother and from food are unlikely to affect the offspring in the
wild. A similar conclusion was drawn about the survival of lake trout
(Salvelinus namaycush) fry, hatched from eggs contaminated with PCBs
in the wild (Willford, 1980). Eggs taken from the wild and hatched in
clean water showed good survival of the offspring (suggesting that
residues of PCBs in the eggs were not responsible for the failure of
the species in Lake Michigan). However, experimental exposure of eggs,
and hatched larvae/fry to levels of PCBs in water and food, similar to
those found in the lake, led to high mortality and the increased
occurrence of deformities in fry. The author concluded that the levels
of PCBs in lake water and food items would be sufficient to lead to
the population decline seen in the lake. It should, however, be
pointed out that other factors in Lake Michigan could also have
contributed to the failure of the lake trout. Sea lampreys had
increased in number in the lake and could have caused population
decline by predation.
The relationships between the occurrence of hepatic diseases and
specific chemicals present in sediment were studied by Malins et al.
(1987). The concentrations of PCBs in the sediments from 4 urban and 2
non-urban areas in Puget Sound USA, were determined. In 3 of the
sites, the concentrations were <0.01 and, in the other 3, the
concentrations ranged from 0.11 to 0.53 mg/kg. Over 900 individual
organic compounds were found. To study the fish diseases, English sole
(Parophrys vetulus), rock sole (Lepidopsetta bilineata), and
Pacific staghorn sculpin (Leptocottus armatus) were collected. The
organs of fish containing the greatest number of lesions were the
liver, kidneys, and gills. Liver cell adenoma, hepatocellular
carcinoma, cholangiocellular carcinoma, haemangioma, and fibroma
constituted major types of liver lesions. Statistically significant
correlations between levels of chemicals in sediment and hepatic
neoplasms in the bottom-dwelling fish suggest a general
cause-and-effect relationship, but there is little firm evidence about
the actual cause of these neoplasms, in particular, in this case,
whether PCBs were involved.
7.4.3 Birds
In studies on chickens and different PCB-mixtures, Vos et al. (1970)
and Vos & Koeman (1970) found that the induction of subcutaneous and
abdominal oedema, centrilobular liver necrosis, hydropericardium, and
higher mortality was more or less related to the presence of
tetrachloro- and pentachlorodibenzofurans, as impurities in the PCB
samples.
From the middle of February to the end of March 1968, an epizootic
disease closely resembling chicken oedema disease occurred in Japan.
Two million chickens were involved, of which 400 000 (20%) died. The
clinical signs were laboured breathing, droopiness, ruffled feathers,
high mortality, and decreased egg production. Autopsy revealed marked
subcutaneous oedema, hydropericardium, ascites, pulmonary oedema,
muscular ecchymosis in the thorax or inside of the thigh, and
yellowish mottled appearance of the liver. The cause of the disease
was found to be Kanechlor 400 contamination of the feed. Experimental
reproduction of these symptoms with Kanechlor 400 was successful. The
remaining sample chicken feed contained 1300 mg of Kanechlor 400/kg
feed (Kuratsune et al., 1972).
Administration of PCBs leads to an atrophy of lymphoid tissue in
chickens (Flick et al., 1965; Vos & Koeman, 1970), and in pheasants
(Dahlgren et al., 1972). Vos & de Roij (1972) and Vos & van
Driel-Grootenhuis (1972) came to the conclusion that these effects
could be attributed to an immunosuppressive effect of PCBs. Vos & de
Roij (1972) suggested that the ability of PCBs to increase the
susceptibility of ducklings to duck hepatitis virus (Friend & Trainer,
1970) and of fish to fungal disease (Hansen et al., 1971) could be
attributed to this immunosuppressive effect.
Koeman et al. (1973) analysed 6 adult cormorants (Phalacrocorax carbo
sinenis), found dead in the wild, for PCB residues. Three additional
birds were shot and 6 fully grown nestlings were also taken. The
authors also carried out a study on 5 cormorants that were dosed with
the PCB, Clophen A60 (a 60% chlorinated PCB mixture that seemed to
correspond most closely with the PCBs profile of material found in
dead birds taken from the wild). The birds were initially dosed with
the PCBs in the diet, but, subsequently, the PCBs were administered
orally in gelatin capsules, until they died. PCB levels in the brains
and livers of the dead birds found in the wild were higher, overall,
than the levels obtained by dosing. The authors thought it highly
probable that this was indicative of PCB poisoning of the birds in the
wild. Without further detailed studies, this conclusion can only be
implied. The higher residues in dead birds from the wild suggest that
a large body burden was taken up, relatively safely in fat, and
released quickly on starvation, prior to death.
A field study, to show the effects of a sub-lethal dose of PCBs on
puffin (Fratercula arctica) breeding success and survival, was
conducted by Harris & Osborn (1981). A total of 150 puffins, trapped
on the Isle-of-May National Nature Reserve, Fife, Scotland, were
implanted, 108 with between 30 and 35 mg of PCBs (Aroclor 1254) and 42
with sucrose as controls. The test chemicals were implanted in
open-ended silastic tubes into the peritoneum. All birds were then
marked and released back into the wild. The same birds returned in
successive years to breed on the island. Breeding and survival of the
implanted birds were monitored through the breeding seasons of 1977,
1978, and 1979. Observations on survival were also made in 1980. Some
implanted birds were killed on recapture, and analysed for PCBs, in
each of the years of observation. No effects on survival or breeding
were seen. PCB levels in fat increased by a factor of between 10 and
14 compared with levels in birds that had not been implanted with the
Aroclor.
Herring gull (Larus argentatus) reproductive success in the area of
the Great Lakes declined with increasing residues of organochlorines
in the birds. Breeding success improved as these residues fell in the
1970s (Weseloh et al., 1979). The species was chosen as an indicator
of environmental pollution in the area and has been extensively
studied. Poor nesting success in the species is related to high
embryonic mortality (Gilbertson & Hale, 1974). Abnormal chicks have
been reported for herring gulls and also for other fish-eating species
from the area including: night herons, ring-billed gulls, common
terns, and Caspian terns (Gilbertson et al., 1976). Eggs from these
species contained residues of PCBs, but it was not possible to
directly relate these residues to chick abnormalities. Gilbertson &
Fox (1977) found a correlation between total organochlorine content
and the hatchability of herring gull eggs. While it cannot be shown
that PCBs were directly responsible for the overall effect, PCB
residues in the livers of hatched chicks, were the only contaminant
that was significantly correlated with the presence of pericardial
oedema. Weseloh et al. (1979) concluded that the reproductive success
of the herring gull can only safely be correlated with total
organochlorine residues and that the possible effects of PCBs cannot
be isolated. Shell thinning usually attributed to DDE alone, does not
occur significantly in these gulls. Hays & Risebrough (1972) suggested
that PCB residues in terns were responsible for the high percentage of
abnormal young in a colony from Long Island Sound.
Twenty-four black-crowned night heron (Nycticorax nycticorax) eggs
were collected at the San Francisco Bay National Wildlife Refuge in
1983. Twelve of these were collected from separate nests, when
late-stage embryos were pipping and an additional egg was randomly
collected from each nest for organochlorine analysis. Other anomalies
and skeletal defects were not apparent. Embryonic weights (with
partially absorbed yolk sacs removed) were 15% lower in comparison
with controls collected at Patuxent Wildlife Research Center.
Crown-rump length and femur length were shorter in the San Francisco
Bay embryos. The geometric mean PCB concentration was 4.1 mg/kg wet
weight with a range of 0.8-52.0 mg/kg. A negative correlation existed
between embryonic weight and log-transformed PCB residues in whole
eggs, suggesting a possible impact of PCBs on embryonic growth. DDE
did not show such a correlation (Hoffman et al., 1986).
Klaas & Swineford (1976) found low PCB residues (0.26-3.4 mg/kg wet
weight) in 35 screech owl (Otus asio) eggs (16 of which were known
to be addled) taken from the wild; there was no relationship between
the presence of residues and hatching failure. Dosing captive screech
owls with 3 mg Aroclor 1248/kg (McLane & Hughes, 1980) showed no
effects on eggshell thickness, number of eggs produced, young hatched,
or young fledged. Residues of PCBs measured in the eggs ranged between
3.9 and 17.8 mg/kg. This range covers the egg residue levels that were
clearly associated with effects in experimental chickens and
pheasants.
Eggs from 315 clutches of sparrowhawks (Accipiter nisus) from 9
sites in Scotland were examined by Newton & Bogan (1978) and Newton
(1979). Eggs that failed to hatch were collected and analysed for
PCBs, DDE, and for aldrin and dieldrin. Statistical analysis showed
little variation in residues within a single clutch, but wide
variation between clutches, even clutches from the same female in the
same area in different years. On this basis, analysis of single eggs
was taken as representative of the complete clutch. Analysis of
variance and multiple regression were used to try to relate particular
effects, such as shell thinning, addling of eggs, and late-embryo
mortality, with particular organochlorines. Results showed that
addling of eggs was significantly correlated with levels of both DDE
and PCBs. Because of the close correlation between residues of DDE and
PCBs in eggs of sparrowhawks, it is not possible to tell whether only
one, or both, organochlorines were involved in egg addling. PCBs
showed the strongest relationship with addling. Shell thinning was
more directly linked with DDE. Newton et al. (1982) concluded that the
level of PCB residues in merlin (Falco columbarius) was not
sufficiently high to have exerted any effect on the breeding success
of this species in Britain. Hodson (1975) did not find any effects of
PCB residues in Richardson's merlin in Canada and attributed all
effects to DDE. A relationship between DDE residues and shell thinning
and the breeding success of the bald eagle (Haliaetus leucocephalus)
was demonstrated by Wiemeyer et al. (1984). PCB residues were
correlated with DDE residues. The authors concluded that contaminants
other than DDE contributed no more than a minor role in reproductive
effects on this species in the field. It was considered that PCBs
might have contributed to reproductive problems, but that the evidence
was unclear. Newton et al. (1986) presented a statistical analysis of
residues in sparrowhawks related to reproductive success. Study
populations from many sites throughout the British Isles showed
different reproductive success. Some of this variation could be
related to levels of organochlorine compounds in eggs. When shell
thickness index was related to PCBs alone, a significant negative
correlation was detected. However, once DDE residues were included,
PCBs did not improve the model. It was concluded that DDE alone could
account for all of the reproductive effects recorded and that PCBs, at
the levels of contamination found, probably did not play a role in
reduced breeding performance. Newton et al. (1988) examined new data
on residues of organochlorine compounds in the eggs of the peregrine
falcon (Falco peregrinus) and also reassessed older data. Total
information considered included information on residues and breeding
success covering the period 1963-86. The PCB residues found were not
considered to have had a significant effect on breeding success in
this species. In earlier studies, Wiemeyer et al. (1975, 1987) could
not isolate any single organochlorine compound as responsible for the
reduced breeding success of the fish-eating osprey (Pandion
haliaetus).
Heinz et al. (1983) examined the breeding success of groups of
red-breasted mergansers (Mergus serrator) on islands in northwestern
Lake Michigan. This is a fish-eating species and, since eating fish
from the Great Lakes had been shown to affect captive mink, the
authors investigated the possible effects of contaminants (measured in
blood samples taken from the birds) on reproduction. Other,
non-contaminant effects were also examined. Many organochlorine
contaminants of the environment were found in eggs including 14
different organochlorine compounds; there was a correlation between
the levels of many of these compounds. No relationship could be
established between levels of PCBs, or any other contaminant, and the
breeding success of the birds. The hatching success of dabbling ducks
also seemed to be largely unaffected by feeding in Lake Michigan,
despite the presence of residues of PCBs and other contaminants in the
eggs (Haseltine et al., 1981).
Appraisal
Effects of PCBs have been shown in laboratory studies on many domestic
species of birds, but few wild species. However, there are many
studies showing measurable residues of PCBs in wild birds. PCBs have
been implicated in population decline in several bird species in
different parts of the world, but it is seldom easy to demonstrate
directly the effects of PCBs on populations of birds in the field.
This is largely because PCB residues invariably occur together with
other organochlorine compounds, such as DDE and dieldrin. Separating
out individual effects can only be done completely satisfactorily when
laboratory data are available for the same species and each individual
pollutant. Some idea of likely effects can be obtained from
statistical manipulation of data from the field, though, in this case,
correlation is the best that can be achieved. In practice, few field
studies give the results that have been predicted from laboratory
studies on other species; that is direct extrapolation from laboratory
to field and species to species is not straightforward.
7.4.4 Mammals
Laboratory studies involving the dosing of non-laboratory mammals with
PCBs are limited to a few species. Field studies have been conducted
on a wider range of species.
Norström et al. (1988) studied liver and adipose tissue specimens of
polar bears (121 samples) obtained by Inuit hunters from 12 zones in
the Canadian Arctic Archipelago in 1982-84 (see section 5.1.4). Six
PCB congeners (Nos 99, 153, 138, 180, 170, and 194) constituted
approximately 93% of total PCBs. The major PCBs accumulated belong to
the group formed by combinations of 2,4-dichloro-, 2,4,5-,
2,3,4-trichloro-, and 2,3,4,5-tetrachloro- substitution on each ring.
Congeners with 2,4-, 3,4-dichloro-, 2,3,4-, 2,3,5-trichloro-,
2,3,4,6-, 2,3,5,6-tetrachloro- or 2,3,4,5,6-pentachloro- substitution
on one ring, such as PCB Nos 118, 138, 187, 183 and 196, which usually
bioaccumulate readily in mammals, were all at lower levels compared
with congeners with only 2,4,5-trichloro- and 2,3,4,5-tetrachloro-
substitution in ringed seals, such as PCB Nos 153, 180, and 170. The
last 3 congeners accounted for 71% of total PCBs in polar bears. Thus,
it appears that the polar bear is able to metabolize PCB congeners in
which there are nonchlorinated para positions, adjacent
nonchlorinated ortho-meta positions, or both ortho positions
chlorinated in one ring.
DeLong et al. (1973) measured various organochlorine pollutants (DDT,
DDD, DDE, dieldrin, and PCBs) in sea lions from the Channel Islands
off the Californian coast. Previous observations had recorded a high
incidence of premature births in the population and organochlorine
residues from females showing premature or normally-timed parturition
were compared. Both DDT and PCB residues (measured against a standard
of Aroclor 1254) were significantly higher in the females producing
premature pups. Residues were estimated in the blubber, liver, and
brain, and, for the first 2 tissues, the ranges of values, for the 2
groups of females, did not overlap. Previous studies on both
substances indicated possible reproductive effects in mammals,
corresponding to some extent with those observed in the sea lions.
Cause and effect could not, therefore, be directly established for
each of the pollutants alone. Helle et al. (1976a) collected ringed
seals from Simo, on the northern Bothnian Bay area of the Baltic Sea,
in October/November. This population of seals shows reduced
reproductive capacity and the sampled population showed only 27% of
females pregnant, compared with other reports of 62.5% and 85-90% for
the same species elsewhere. Residues of DDT and PCBs were compared
with those in seals from other areas of the Baltic and found to be
lower (Table 39). The seals sampled from Simo were divided into
pregnant and non-pregnant groups (n = 15 and 26, respectively).
DDT and PCBs levels were both significantly higher in non-pregnant
animals (Table 40). All females from both groups showed a corpus
luteum in one ovary, indicating that all had ovulated. About half of
the non-pregnant females showed indications of an embryo having been
implanted, which had subsequently aborted or resorbed. Again cause and
effect could not be established directly. The authors pointed out that
normally breeding Californian sea lions had DDT levels as high as
those in Baltic seals showing reproductive failure and proposed this
as an indication that PCBs were the causative agent.
Table 39. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable fat of
blubber from ringed seal from the Baltic Seaa
Area Number DDT PCBs
Northern most part 40 110 ± 10b 69 ± 4.4b
of the Bothnian Bay
Gulf of Bothnia 33 200 ± 28 110 ± 15
a Data from: Helle et al. (1976a).
b Value significantly different from those in the Gulf of Bothnia (P < 0.05).
Table 40. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable
fat of blubber in non-pregnant and pregnant ringed seal of
reproductive agea
Number DDT PCBs
Non-pregnant 26 130 ± 13b 77 ± 5.2b
Pregnant 15 75 ± 11 56 ± 6
a Data from: Helle et al. (1976a).
b Values for non-pregnant females significantly higher than
those of pregnant females. DDT P < 0.01; PCBs P < 0.05.
It was further pointed out that the group of non-pregnant females
would include some animals that were not pregnant for reasons other
than the presence of organochlorine compounds. In a later paper (Helle
et al., 1976b), the same authors reported that some of the
non-pregnant females showed abnormal uteri with stenosis or occlusion
of the uterine horns. They, therefore, subdivided the non-pregnant
females in their second sample into those showing the anatomical
abnormality and those not. Both DDT and PCBs were significantly higher
in the occluded group than in pregnant animals. Non-pregnant females,
without occlusions, showed residues of DDT and PCBs not significantly
different from those in pregnant animals, and significantly lower than
those females with occlusions. Residues in males were comparable with
the highest residues found in females (Table 41).
There is some indication of a positive correlation between PCB
residues and age in male seals but not in females (Addison et al.,
1973; Addison & Smith, 1974). These observations are presumed to
indicate that females excrete some of their body burden of PCBs in the
milk.
These field observations have been confirmed in a study in which
captive seals were fed on fish from the Wadden Sea (where reproductive
problems had been found in the wild seal population) and compared with
controls fed fish from the Atlantic Ocean. Seals eating the
contaminated fish, which differed from the control fish only in the
PCB content, showed the same failure to carry the fetus successfully
to term seen in the wild (Reijnders, 1986; see section 7.2.5).
Table 41. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable fat from
blubber of ringed seal from Simo, Bothnian Baya
Group Sample description Number DDT PCBs
I pregnant females 24 88 ± 9.7 73 ± 6.6
II non-pregnant females 29 130 ± 10 110 ± 7.8
with stenoses and
occlusions
III non-pregnant females 8 100 ± 15 89 ± 11
with normal uteri
IV fetuses 24 62 ± 4.3 49 ± 3.0
V males 24 130 ± 18 100 ± 13
probability of similarity between test groups (t-test)
I-II P < 0.01b P < 0.01b
I-III P > 0.05 P > 0.05
I-IV P < 0.05c P < 0.01b
a Data from: Helle et al. (1976b).
b Groups significantly different at the 99% level.
c Groups significantly different at the 95% level.
Subramanian et al. (1987) collected samples of blubber from male
Dall's porpoise (Phocoenoides dalli) in the northwestern Pacific
Ocean and analysed the samples for organochlorine content. Blood
samples taken from the same animals were analysed for testosterone, a
male sex steroid, and for aldosterone, another steroid hormone,
responsible for blood electrolyte balance. Testosterone levels in
blood were correlated with blubber levels of DDT and PCBs; as the
organochlorine content increased, testosterone levels decreased. There
was no relationship between blubber organochlorine levels and blood
levels of aldosterone. The number of samples was small (n = 12) and,
though the relationship between DDT and testosterone was significant,
the apparent relationship with PCBs was not. The animals were sampled
outside the breeding season, when testosterone levels would be
expected to be low.
In a study by Clark & Lamont (1976), 26 pregnant big brown bats
(Eptesicus fuscus) were collected from the field and maintained on a
control diet, in captivity, until they gave birth to young. Levels of
PCBs were measured in both the adults and the offspring. The
concentrations of PCBs in litters with dead young were significantly
greater than in litters where both young were born alive (mean for
litters with dead young was 2.44 mg/kg; mean for all other litters was
0.34 mg/kg). The contents of PCBs ranged between 1.07 and 1.96 mg/kg
wet weight in adults and between 0.28 and 1.69 mg/kg in the young.
7.4.4.1 Appraisal
PCBs have been implicated in population declines of seals and sea
lions. Population decreases and reproductive failure have been
observed in seals from the Baltic Sea, the Wadden Sea (southeastern
North Sea), and the Gulf of St. Lawrence and in sea lions off the
Californian coast. Poor reproduction has been correlated with residues
of PCBs in the affected animals. A major problem in such studies is
the occurrence of more than one chemical pollutant in the animals.
PCBs are found together with other organochlorine compounds and heavy
metals. Conclusions, therefore, have often relied on making the best
correlation between observed effects and residues and checking cause
and effect relationships on animals more amenable to laboratory study.
A study on captive seals confirmed field observations on the effects
of PCBs on these marine mammals (see section 7.2.5).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Evaluation of the toxicity of Aroclors and other commercial PCB
mixtures is complicated by numerous factors, including isomer and
congener composition, differences in species susceptibility,
quantitatively inconsistent data, and various degrees of contamination
with toxic compounds, such as chlorinated dibenzofurans. Because of
these factors and a lack of data for some of the Aroclors (most
studies have been conducted on the higher chlorinated Aroclors), it is
assumed that effects resulting from exposure to a specific Aroclor are
representative of effects that may be produced by the other Aroclors.
Many of the literature sources do not give details of the composition
of the PCB mixture used in the studies.
Reviews on the literature concerning the toxicity of PCBs are given by
ATSDR (1989), Kimbrough (1980, 1987), Safe (1984), NIH (1985), and
Lorenz & Neumeier (1983).
8.1 Single exposures
8.1.1 Oral
(a) Aroclors
The acute oral LD50 values for a number of Aroclors are presented in
Table 42. The lowest oral LD50 in the rat was 1.0 g/kg body weight
for Aroclor 1254 (Garthoff et al., 1981).
In Sherman rats, lethal doses of Aroclor 1254 and 1260 in peanut oil
caused ulceration in the duodenum and glandular stomach (Kimbrough et
al., 1972). Osborne-Mendel rats, receiving a single oral dose of
50 mg/kg body weight of Aroclor 1254 in corn oil, showed an increased
relative liver weight together with the induction of microsomal
enzymes, within 12 h (Litterst et al., 1974).
Monkeys, sacrificed 4 days after gastric intubation of Aroclor 1248 at
1.5 or 3.0 g/kg body weight (no vehicle reported), exhibited enlarged
livers with proliferation of the endoplasmic reticulum and hypertrophy
and hyperplasia of the gastric mucosa (Allen et al., 1974a).
(b) Individual congeners
Estimated oral LD50 values in 30 days for individual congeners in
corn oil in Hartley guinea-pigs (probably single application) were
0.5 mg/kg body weight for 3,4,5,3',4',5'-hexachlorobiphenyl, less than
1 mg/kg body weight for 3,4,3',4',-tetrachlorobiphenyl, more than
3 mg/kg body weight for 2,3,4,5,3',4',5'-heptachlorobiphenyl, and more
than 10 mg/kg body weight for 2,4,5,2',4',5'-hexachlorobiphenyl
(McKinney et al., 1985).
Table 42. Acute oral LD50s of Aroclorsa
Aroclor Species/strain Sex/ageb LD50 (g/kg body weight) References
1254 rat/Wistar male/30 days 1.3 Grant & Phillips (1974)
female/30 days 1.4
male/60 days 1.4
female/60 days 1.4
male/120 days 2.0
female/120 days 2.5
rat/Sherman male/weanling 1.295 Linder et al. (1974);
NR/adult 4-10 Kimbrough et al. (1972);
rat/Osborne-Mendel male/adult 1.01 (single dose) Garthoff et al. (1981)
1.53 (5 doses over 2 1/2 weeks)
1.99 (5 doses, 1 day/week)
1221 rat/Sherman female/NR 4.0 Nelson et al. (1972)
1260 rat/Sherman NR/adult 4-10 Linder et al. (1974)
M/weanling 1.315
1242 rat/Sprague-Dawley male/adult 4.25 Bruckner et al. (1973)
1262 rat NR 11.3 Panel on Hazardous Trace
Substances (1972)
a References: ATSDR (1989); WHO (1976).
b NR = not reported.
Information on solvents used was not available.
8.1.2 Inhalation
No acute data were available.
8.1.3 Dermal
Median lethal doses for a single application of Aroclors to the skin
of rabbits ranged from >0.79 to <1.27 g/kg body weight for Aroclors
1242 and 1248 in 50% corn oil, to >1.0 to <3.17 g/kg body weight for
undiluted Aroclor 1221, and >1.26 to <2.0 g/kg body weight for
Aroclor 1260 (Fishbein, 1974).
8.1.4 Other routes
(a) Aroclors
With a single intravenous dose of Aroclor 1254 in a 1% lecithin-saline
suspension, the LD50 for adult female Sherman rats was 0.4 g/kg body
weight (Linder et al., 1974). The LD50s for Aroclor 1254 in DMSO for
various mouse strains, following a single intraperitoneal injection,
varied between 0.9 and 1.2 g/kg body weight (Lewin et al., 1972).
(b) Individual congeners
The intraperitoneal LD50 for 2,4,3',4'-tetrachlorobiphenyl in CF-1
mice was 2.15 g/kg body weight, while that of its main metabolite,
(5-hydroxy-2,4,3',4'-tetrachlorobiphenyl) was 0.43 g/kg body weight,
which suggests that the acute toxicity of 2,4,3',4'-tetra-
chlorobiphenyl might be attributable to this phenolic metabolite
(Yamamoto & Yoshimura, 1973).
In male Wistar and Charles-River CD rats, a single intraperitoneal
dose of 1 mg 3,4,5,3',4',-pentachlorobiphenyl/kg body weight or a
single oral dose of 50 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body
weight caused significant liver enlargement accompanied by fatty
changes, atrophy of the thymus, and decreased relative spleen weights.
Mono- ortho substituted biphenyls, such as 2,3,4,5,3',4'-hexa-
chlorobiphenyl and 2,4,5,3',4'-pentachlorobiphenyl, induced these
effects in the liver and thymus to a minor degree following an
intraperitoneal dose of 50 mg/kg body weight, while di- ortho
substituted hexachloro- and heptachlorobiphenyls did not cause adverse
effects at this dose level (Kohli et al., 1979; Yoshihara et al.,
1979).
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Aroclors
(a) Mouse
While oral intoxications by PCB mixtures in monkeys are easily
recognizable by their effects on the skin, skin lesions in female ddN
mice were only observed after 2-3 months of daily oral exposure to
1.6 mg of a technical PCB mixture (48% chlorine) per mouse (80 mg/kg
body weight per day) in olive oil. The lesions included alopecia,
erosions, ulcerations, and eczematous changes around the eyelids. No
increase in mortality and only slight growth retardation were seen
after 26 weeks of exposure (Nishizumi, 1970).
Koller (1977) found histological changes in the liver of mice exposed
to 37.5 mg Aroclor 1254/kg diet for 6 months. A dose level of
3.75 mg/kg diet was without effect.
The oral toxicities of Kanechlor 400, 500, and 600 were compared by
feeding male mice diets containing 300 mg PCBs/kg for 14 weeks
(Kawanishi et al., 1975). Fatty degeneration and accumulation of
pigment in the liver were observed in the mice fed Kanechlor 600.
(b) Rat
At lethal, oral doses of undiluted Aroclor 1242 (100 mg/kg body
weight, every 2 days, for 3 weeks), Sprague-Dawley rats showed reduced
body weight, thymus atrophy, chromodacryorrhea, progressive
dehydration, and central nervous system depression with terminal
ataxia and coma. Fatty changes were observed in the liver and kidneys
(Bruckner et al., 1973).
Male and female Sherman rats (10 animals of each sex) were fed diets
containing 0, 20, 100, 500, or 1000 mg of Aroclor 1260 or Aroclor
1254/kg (equivalent to 0, 1.5, 7, 36, or 72 mg/kg body weight,
respectively) for 8 months. The animals receiving the 2 highest dose
levels showed reduced growth. Female rats fed Aroclor 1260 at 500 and
1000 mg/kg diet showed a high mortality rate. However, only 3 rats fed
Aroclor 1254 at 500 mg/kg diet died. Significant increases in relative
liver/body weight ratios were found for both Aroclors at all doses
tested. Microscopically enlarged hepatocytes, cytoplasmic inclusions,
increased lipid levels, and foamy cytoplasm were all found
consistently. Adenofibrosis was found at higher doses (500 and
1000 mg/kg diet) and corresponded to the glistering white areas seen
on gross inspection. These areas also showed cholangiofibrosis
(according to Kimbrough, synonymous for bile duct proliferation, bile
duct adenomatosis, and fibroadenoma) (Kimbrough et al., 1972).
Growth and mortality rates were unaffected in male Sprague-Dawley rats
exposed for 1 year to a diet containing Aroclors 1248, 1254, or 1262
at 100 mg/kg diet (equivalent to 5 mg/kg body weight) compared with
controls (Allen et al., 1976).
Female Charles-River CD rats were fed for 20 weeks on a diet
containing 0, 10, 30, or 100 mg Aroclor 1254/kg (equivalent to 0, 0.5,
1.5, and 5 mg/kg body weight, respectively). No increase in mortality
rates occurred but growth inhibition was seen at 30 mg/kg from month 2
onwards and at 100 mg/kg diet from week 2 onwards. Skin lesions,
initially on the ears, were found after 10-20 weeks of exposure to
Aroclor 1254 at all dose levels. The lesions, including alopecia and
reddened and thickened skin with hyperkeratosis, subcutaneous oedema,
and infiltration by polymorphonuclear leukocytes, also involved the
nose, tail, and feet at the highest exposure level (Zinkl, 1977).
Effects on the liver following exposure to PCB mixtures have mainly
been investigated in rats (see section 8.6.1.1).
(c) Rabbit
Four groups of 5 male and 5 female New Zealand White rabbits were
administered 300 mg Aroclor 1221, 1242, or 1254 in corn oil, via a
stomach tube, once a week, for 14 weeks. The fourth group received
only the vehicle.
The mortality rate was increased and the body weight gain was reduced
after 14 weeks of oral exposure to Aroclor 1254, but not after
exposure to Aroclor 1221 or 1242. Liver/body weight ratio and SGOT and
SGPT activities were increased in the animals treated with Aroclor
1242 or 1254, but not in those treated with Aroclor 1221. In the
animals treated with Aroclor 1254, the smooth endoplasmic reticulum
was condensed in the liver cell and formed hyalin inclusions which
might have been accompanied by a loss of enzyme activity. Lipid
accumulation, pigment deposition, nuclear changes, and necrosis were
also found (Koller & Zinkl, 1973).
(d) Pig
Pigs, fed Aroclor 1242 or 1254 at a dietary level of 20 mg/kg
(equivalent to 0.8 mg/kg body weight) for 91 days, showed gastric
lesions, consisting of erosions and necrosis. Two pigs fed a high-dose
regimen of 100 mg of Aroclor 1254/kg body weight for 11 days, also
showed hypertrophy and hyperplasia of the gastric mucosa; this was
also found in monkeys at low exposure levels (Hansen et al., 1976b).
(e) Cow
Holstein cows were studied throughout a complete lactation period, a
non-lactating period, and 42 days of a subsequent lactation period for
overt responses to Aroclor 1254. Four cows received daily doses of 0,
10, 100, or 1000 mg/cow (1000 mg/cow is equivalent to 1.67 mg/kg body
weight) over 60 days. The mean daily milk production and net energy of
a complete lactation period did not differ between control and
PCB-treated animals. At the end of the study, the concentrations of
PCBs were 0.005, 0.021, 0.14 in blood plasma; 1.9, 10.9, 91.3 in milk
fat, and 1.4, 6.9, 70.0 µg/kg in adipose tissue for the 10, 100, and
1000 mg PCB groups, respectively. No signs of impaired health,
productivity, or changes in blood and urine chemistry were observed
(Willett et al., 1987).
(f) Monkey
Rhesus monkey
(i) Aroclor 1242
Becker et al. (1979) exposed 6, 7-8-month-old, male Rhesus monkeys to
Aroclor 1242 at levels of 0, 3, 10, 30, or 100 mg/kg diet (equivalent
to 0, 0.12, 0.4, 0.4, 1.2, or 4.0 mg/kg body weight, respectively) to
study changes in the stomach mucosa. At 3, 10, 30, and 100 mg/kg diet,
4 monkeys died after 245, 146, 92, and 137 days of exposure,
respectively. All exposed monkeys showed a decreased body weight gain.
At all dose levels, changes were observed in the folds of the
suborbital facial skin, and eyelids became swollen and red. From day
71 at 3 mg/kg diet, days 69 and 77 at 10 mg/kg diet, and day 12 at the
higher exposure levels, stomach biopsies revealed an apparent arrest
of the differentiation of generative cells of the isthmus and neck
regions into parietal and zymogenic cells. Mature parietal and
zymogenic cells, which were found only in the bases of the glands,
showed signs of injury, such as dilatation of the rough endoplasmic
reticulum on the zymogenic cells, irregularity of the mitochondria in
parietal cells, and irregular luminal membranes and an increase in the
number of autophagic vesicles in both type of cells. The severity of
the lesions was directly correlated with both duration and level
exposure. A no-effect level was not obtained in this study.
A group of 5 Rhesus monkeys, 1-2.5 years old, was exposed to a diet
containing 1 mg of Aroclor 1242/kg (equivalent to 0.04 mg/kg body
weight) for 133 days. A control group contained 4 monkeys. No adverse
effects were found (McNulty et al., 1980).
Characteristic lesions, metaplasia in epithelial structures, such as
sebaceous glands, nail beds, gastric mucosa, and ameloblast
surrounding unerupted teeth were reported to have developed in Rhesus
monkeys, 13 months after a 40-day diet containing 400 mg Aroclor
1242/kg (equivalent to 16 mg/kg body weight) (McNulty, 1985).
(ii) Aroclor 1248
A group of 5 (4 males and 1 female), 1-month-old Rhesus monkeys,
without previous exposure, were administered 30 daily doses of 35 mg
of Aroclor 1248/kg body weight, by gavage, in corn oil. Four animals
received only corn oil. No mortality or clinical signs were observed,
except for a decrease in body weight gain, slightly reduced food
consumption, and anaemia. Mild microscopic changes were observed in
the thymus, bone marrow, eye, skin, stomach mucosa, and liver
(Abrahamson & Allen, 1973).
Six male Rhesus monkeys, aged 1 1/2-2 years were fed a diet containing
300 mg Aroclor 1248/kg for 3 months. Three animals served as controls.
A decrease in body weight was observed. Within one month, all the
animals fed PCBs had alopecia, subcutaneous oedema, particularly of
the face, which manifested as swollen eyelids, erythema, and acneiform
lesions involving the areas devoid of hair (Allen & Norback, 1972 cf.
Hayes (1987)).
Six adult, female Rhesus monkeys were fed 25 mg Aroclor 1248/kg diet
for 2 months. Facial oedema, alopecia, and acneiform changes developed
within 1 month and 1 animal died 2 months after removal from the
experimental diet. In addition to the above changes, this animal
showed anaemia, hypoproteinaemia, hypertrophy, and hyperplasia with
invasion through the muscularis mucosa, focal haemorrhages and
ulcerations of the gastric mucosa, and bone marrow hypoplasia. Eight
months later, the 5 surviving animals continued to show clinical signs
of intoxication. The PCB concentration in body fat, which, after 2
months of treatment, averaged 127 mg/kg, had decreased 8 months later
to 34 mg/kg (Allen et al., 1974a).
Groups of 9 adult, female Rhesus monkeys (weight approximately
5.6 kg), were fed a diet containing 0, 2.5, or 5.0 mg Aroclor 1248/kg
(equivalent to 0, 0.1, or 0.2 mg/kg body weight, respectively) for an
average period of 18.2 months. An additional group of 4 males was fed
5.0 mg/kg diet. Control groups contained 12 female and 6 male monkeys.
The Aroclor 1248 contained 4.4-8.7 ng polychlorinated dibenzofurans/kg
diet. The female monkeys were mated with control males after 6 months
of exposure (the reproductive effects are discussed in section
8.4.1.3). Levels of Aroclor 1248 in adipose tissue reached a plateau
after 1 year at 2.5 mg/kg diet and after 6 months at 5 mg/kg diet.
Exposed males showed slight to moderate periorbital oedema and
congestion of the eyes. Females showed an average 15% loss in body
weight over the first 5 months of both exposures, while food
consumption was normal. At 6 months, they all showed loss of hair,
acne of the face and neck, and erythema and swelling of the eyelids.
Skin biopsies revealed keratinization of the affected hair follicles.
One female monkey died after 173 days of exposure to 2.5 mg/kg diet
and one female died after 310 days of exposure to 5.0 mg/kg diet. Both
animals developed terminal enteritis due to Shigellosis, which was
resistant to treatment. At autopsy, the animals showed generalized
alopecia, subcutaneous oedema, and acne. Microscopically, follicular
epithelial hyperplasia with inflammation of the surrounding tissue,
and keratinization of the hair follicles were observed. The livers
showed focal areas of necrosis, enlarged hepatocytes, and fatty
changes (Barsotti et al., 1976).
The surviving females in the former study, 8 per dose level, were
placed on a control diet for approximately 1 year after an average
total intake of 270 and 498 mg Aroclor 1248, respectively. There was a
gradual improvement in their physical condition and, within one year,
their gross appearance was no longer different from that of the
controls. However, their breeding performance was still affected, as
outlined in section 8.4.1.3 (Allen et al., 1980).
(iii) Aroclor 1254
Several pilot studies were carried out before this major study to
characterize the toxicity of Aroclor 1254 and to compare the toxic
findings in the Rhesus and Cynomolgus monkey (Tryphonas et al., 1984,
1986b; see section 8.2.1.6). In these pilot studies, Mes & Marchand
(1987) and Mes et al. (1989a) measured the concentrations of PCBs in
the blood, adipose tissue, and faeces.
A preliminary report describes the results of an ongoing study after
54 weeks of daily oral administration of gelatin capsules containing
0, 5, 20, 40, or 80 µg Aroclor 1254/kg body weight, in corn
oil-glycerol, to groups of 16 adult, female Rhesus monkeys (Macaca
mulatta). At this stage, a slight decrease in body weight gain was
observed in the exposed monkeys together with a decrease in water
consumption, but not in food consumption. In week 52, the incidences
of prominent nail beds, of nails separated from the beds, and of
prominent tarsal glands increased in the animals exposed at 80 µg/kg
body weight (Arnold et al., 1984).
Aroclor 1254, at a dose level equivalent to 280 µg/kg body weight was
given for 5 days per week to Rhesus monkeys (Macaca mulatta) over a
period of 27-28 months. Four animals were treated as described and 4
animals served as controls. The Aroclor 1254 was administered in
apple-juice-gelatin-corn oil emulsion. The weight of the monkeys at
the beginning of the study was approximately 4 kg. Terminal clinical
signs of varying severity included finger nail detachment, exuberant
nail beds, weight loss, stomatitis, and normocytic anaemia. At
necropsy, the bone marrow was hypocellular with cytoplasmic vacuoles
in erythroid precursor cells. Histopathological lesions included
dilatation of the tarsal gland ducts, atrophy, or absence of, splenic
and lymphonodal germinal centres, bone marrow depletion, gingival
erosion and ulceration, moderate mucinous hypertrophic gastropathy
with cystic dilatation of occasional gastric glands, hepatocellular
enlargement and necrosis, hypertrophy of biliary duct epithelium,
hyperplasia of biliary ducts, hypertrophy of the gall bladder
epithelium, and an equivocal increase in the number of lysosomes in
the thyroid follicular epithelial cells. The terminal PCBs
concentrations in a number of organs were as follows: adipose tissue
106.7-2073.2 mg/kg (in control animals 0.26-0.65 mg/kg); brain,
31.2-252.0 mg/kg; kidneys, 85.4-964.1 mg/kg; and liver,
255.1-828.1 mg/kg tissue. The PCB concentrations were expressed on a
mg/kg fat basis. It was concluded that skin appendicular lesions are
good clinical indices of PCB exposure in monkeys and that
lymphoreticular lesions (atrophy and absence of lymphoid follicular
centres) are good indicators of impending or active immunological
crisis (Tryphonas et al., 1986a).
(iv) Miscellaneous studies
Accidental exposure of a colony of 256 Rhesus monkeys to PCBs in a
concrete sealant produced a disease characterized by high mortality,
gradual weight loss, behavioural changes, alopecia, acne, facial
oedema, swollen eyelids, diarrhoea, anaemia, poor breeding
performance, and high incidences of abortions and still births.
Samples of the concrete slabs within several buildings were obtained
and analysed. Significant levels of PCBs (5280 mg PCB/kg sample) were
found (Altman et al., 1979; McConnell et al., 1979).
The effects of exposure to PCBs on the eyes were investigated by
Ohnishi & Kohno (1979), who administered a banana injected with 0.5 mg
of PCBs/kg body weight, daily, to 8 adult Rhesus monkeys of both sexes
for 1-5 months. Two out of this group were fed PCBs with
polychlorinated dibenzofurans (2.5 µg/kg body weight). Four untreated
animals were used as controls. One month after the onset of the
exposures there was a reduction in body weight. When pressure was
applied to the eyelids of treated monkeys, white secretions extruded.
Within 3 months alopecia, swelling of the eyelids, and acne-form
eruptions developed. The retina and choroid were normal. The
histopathological changes in the eyelids were comparable in both
groups of exposed monkeys and included the appearance of keratinous
cysts and atrophy of the Meibomian glands with hyperkeratosis and
hyperplasia of the ductal epithelium.
Cynomolgus monkey
Groups of 5 or 6 adult, female Cynomolgus monkeys (Macaca
fascicularis) were exposed to 3, weekly doses of 4.7 mg of Aroclor
1248/kg body weight (equivalent to 2 mg/kg per day) or to 3-weekly
doses of 11.7 mg of Aroclor 1254/kg body weight (equivalent to 5 mg/kg
per day) in an apple juice-corn oil emulsion. The monkeys were exposed
until necropsy at day 30-164 in dead or moribund condition. A control
group contained 5 monkeys. In both exposed groups, body weight loss,
emaciation, facial oedema, finger-nail loss, and lacrimation were
observed. Common histopathological lesions were: dilated Meibomian
gland ducts, mucinous hyperplasia and hypertrophy of the gastric
mucosa, enlargement, fatty degeneration, and necrosis of hepatocytes,
bile duct and gall bladder epithelial cell hypertrophy and
hyperplasia, and thyroid changes in follicular cell size and the
number of intracytoplasmic lysozomes.
The onset of the signs and lesions of toxicity was not as rapid and
uniform as that in Rhesus monkeys. Aroclor 1248 appeared more toxic
than Aroclor 1254 (Tryphonas et al., 1984).
Groups of 4 adult, Rhesus and 4 adult, Cynomolgus female monkeys,
weighing 3.2-4.5 and 3.2-5.2 kg, respectively, received doses of
Aroclor 1254 at 0 or 280 µg/kg body weight for 5 days/week (equivalent
to 200 µg/kg per day) in an apple-juice-gelatin-corn oil emulsion for
27-28 and 12-13 months, respectively. This study showed that the
Rhesus monkey is more susceptible to PCB toxicity than the Cynomolgus
monkey (Tryphonas et al., 1986b).
Hori et al. (1982) exposed 1 female, Cynomolgus monkey to daily doses
of Kanechlor 400 (without detectable quantities of polychlorinated
dibenzofurans) at 2 mg/kg body weight, in olive oil. They also exposed
3 monkeys to a PCB mixture with a chromatographic pattern similar to
that of the Yusho mixture (2 or 4 mg/kg body weight), and 1 monkey to
2 mg/kg body weight of the same mixture, without detectable quantities
of polychlorinated dibenzofurans (detection limit not given). The
doses were administered 6 times per week in a piece of banana. Two
controls received only the vehicle. The 2 monkeys receiving the Yusho
mixture at 4 mg/kg body weight died within 4 and 8 weeks,
respectively. The other monkeys were kept for 20 weeks. In all monkeys
exposed to the Yusho mixture, toxic effects were similar to those
already described above. In addition, there was cytoplasmic
vacuolation and dilatation of the convoluted tubules with cytoplasmic
casts in the kidneys. The other 2 mixtures induced less severe
reductions in body weight gain, immunosuppression and
histopathological alterations in liver, kidneys, and periorbital skin.
The effects in the animals fed a diet with dibenzofurans yielded
enhanced decreases in body weight, immunosuppression, fatty liver and
histological changes, in addition to hair loss, acne-form eruptions,
oedema of the eyelids and cornification of the skin, compared with the
other test substances.
8.2.1.2 Individual congeners
(a) Monkey
Rhesus monkeys have been exposed to various congeners, as outlined in
Table 43.
Table 43. Toxicity of PCB-congeners in Rhesus monkeysa
Congenerb No. of Exposure Time clinical Deaths
animals/ toxicity
group mg/kg period first noted
diet (days) (day)
2,5,4'-TriCB 4 5 84 - 0
3,4,3',4'-TCB 3 3=>1c 215 14-21 3
5 1 38 27 1
2,5,2',5'-TCB 3 3=>1c 215 - 0
5 1=>5d 200 - 0
3,4,5,3',4',5'-HCB 1 0.1 127 117 1
4 0.5 63 28-30 4
1 1 57 20 1
2,4,5,2',4',5'-HCB 4 15 122 - 0
1 65 63 - 0
2,4,6,2',4',6'-HCB 4 15 122 - 0
1 65 64 - 0
2,3,6,2',3',6'-HCB 4 15 122 - 0
a From: McNulty et al. (1980); McNulty (1985); Iatropoulos et al. (1977).
b TriCB = trichlorobiphenyl; TCB = tetrachlorobiphenyl; HCB = hexachlorobiphenyl.
c Dietary level reduced after 23 days.
d Dietary level raised after 133 days.
No toxicity could be demonstrated either by clinical appearance or by
histopathological examination for isomers with 2 or 4 chlorine atoms
ortho to the biphenyl bridge. The clinical signs observed in monkeys
exposed to 3,4,3',4'-tetrachlorobiphenyl (up to 3 mg/kg diet) and
3,4,5,3',4',5'-hexachlorobiphenyl (at 1 mg/kg diet) were similar in
character and severity to those produced by Aroclor 1242 (at 100 mg/kg
diet) and Aroclor 1248 (at 25 mg/kg diet) in monkeys (Allen et al.,
1974a). The same histopathological lesions were found. The lesions of
the skin and eyes were described as an expression of squamous atrophy
or squamous cyst formation of the sebaceous glands. The epithelial
changes in the skin and nails were found to be reversible. In this
study, 2,5,2',5'-tetrachlorobiphenyl did not produce any clinical or
pathological lesions at 3 mg/kg diet. Aroclor 1242 and 1248 were
reported to contain about 0.24 and 0.34% of 3,4,3',4'-tetra-
chlorobiphenyl, and it was concluded that this congener could account
for some of the toxicity of the commercial mixtures (McNulty et al.,
1980; McNulty, 1985).
Rhesus monkeys exposed to 2,5,4'-trichlorobiphenyl showed a reversible
primary injury of the arterioles, capillaries, and venules in the
adrenal glands, kidneys, liver, brain, and lungs (Iatropoulos et al.,
1977).
(b) Other animal species
In studies on rats and mice, individual PCB-congeners caused adverse
effects on the liver, spleen, and thymus. The most toxic compounds
were the planar congeners 3,4,3',4'-tetrachlorobiphenyl,
3,4,5,3',4'-pentachlorobiphenyl and 3,4,5,3',4',5'-hexachlorobiphenyl.
Biocca et al. (1981) and Aulerich et al. (1985) compared the
toxicities of various hexachlorobiphenyl isomers in mice. Male C57
BL/6 mice were exposed via the diet to 0.3, 1, 3, 10, 30, 100, or
300 mg 3,4,5,3',4',5'-hexachlorobiphenyl; 10, 30, 100, or 300 mg
2,4,5,2',4',5'-hexachlorobiphenyl, 2,3,6,2',3',6'-hexachlorobiphenyl,
or 2,4,6,2',4',6'-hexachlorobiphenyl/kg diet for 28 days. There were
marked differences in dose response and in the severity of the
pathological effects among the isomers. 3,4,5,3',4',5'-Hexa-
chlorobiphenyl was the most toxic isomer causing mortality, and body
and organ weight effects at all dose levels and was the only isomer
that produced excess porphyrin accumulation. It was also the isomer
that occurred in the highest concentration in the fat and the liver.
3,4,5,3',4',5'-Hexachlorobiphenyl caused subcutaneous oedema,
enlargement of the liver with accentuated hepatic lobular markings,
fatty infiltration, hepatocellular swelling and necrosis, and atrophy
of the thymus. The other 2 isomers caused the same lesions, but to a
lesser extent.
In the mice, the overall order of toxicity was 3,4,5,3',4',5'-
hexa-chlorobiphenyl > 2,4,6,2',4',6'-hexachlorobiphenyl >
2,4,5,2',4',5'-hexachlorobiphenyl > 2,3,6,2',3',6'-hexachlorobiphenyl,
based on the effects on mortality and growth, and on histopathology.
8.2.2 Intraperitoneal: reconstituted PCB mixtures
Bandiera et al. (1984) administered reconstituted mixtures of PCDFs
and reconstituted mixtures of PCBs, by intraperitoneal injection, to
immature Wistar rats, to determine the effects on weight loss, thymic
atrophy, and the induction of P-448 dependent monooxygenases. The
mixtures consisted of compounds that persisted in the blood and liver
of Yusho patients. From the results, it was clear that the PCDFs were
600 to 2100 times more toxic than the PCBs.
A group of 4, one-month-old, male Wistar rats received
intraperitoneally a reconstituted PCB mixture containing 13 of the
major congeners that have been identified in human milk, at the
corresponding relative concentrations. The mixture was injected on
days 1 and 3 in corn oil at dose-levels of 0, 0.45, 0.90, 4.5, or
45 mg/kg body weight. The rats were killed on day 6 for
histopathological studies. At the highest exposure level, increased
relative liver weights and enlarged and vacuolated hepatocytes were
observed together with changes in nuclei. In the thyroid, a mild
reduction in follicular size, focal collapse, and changes in nuclei
were found. No changes were seen with 0.9 mg/kg body weight (Gyorkos
et al., 1985) (see section 8.8.1.1).
8.2.3 Dermal exposure
(a) Aroclors
Solutions of Phenoclor DP6, Clophen A60, or Aroclor 1260 in
isopropanol were applied in doses of 118 mg on 50 cm2 of the shaved
back skin of groups of 4 New Zealand rabbits, 5 times per week, for 38
days. A group of 4 rabbits received the vehicle only. After initial
reddening, transverse wrinkling and thickening of the skin developed
with hyperplasia and hyperkeratosis of the epidermal and follicular
epithelium. These effects were more marked with Clophen and Phenoclor
than with Aroclors. During the study, deaths occurred in the Clophen-
and Phenoclor-treated groups. Body weights and relative kidney weights
were decreased in the Aroclor-treated rabbits. Histological liver
changes were least marked in the Aroclor-treated rabbits. Treated
rabbits had fluorescing livers and bone under UV radiation and also
showed other evidence of porphyria. In the kidneys, hydropic
degeneration of the convoluted tubuli, and tubular dilatation were
found. There was atrophy of the thymic cortex and a reduction of
germinal centres of the lymph nodes, as well as leukopenia, and some
animals in all groups showed oedema of the abdominal and thoracic
cavities, subcutaneous tissue, and pericardium. Faecal elimination of
copro- and protoporphyrine was increased by all 3 PCBs, but was lowest
with Aroclor 1260 (Vos & Beems, 1971).
Puhvel et al. (1982) exposed groups of 3 female, hairless mice of 2
strains, (Skh:HR-1 and HRS/J), topically to Aroclor 1254 (4 doses of 1
or 8 mg/week, for 6 weeks) or Phenoclor 54 (5 doses of 0.2 mg/week,
for 10 weeks) in acetone or an acetone-mineral oil-Tween 80 emulsion,
or to the vehicle only. Punch biopsies of the skin were taken
regularly. The skin of treated mice appeared grossly normal after the
exposures, but examination of microscopic skin samples of
Phenoclor-treated mice showed hyperkeratosis of the stratum corneum,
epidermal hyperplasia, disappearance of sebaceous glands, and the
presence of numerous keratinous cysts. No histological changes were
observed in the internal organs.
(b) Individual congeners
In the study of Puhvel et al. (1982), described above, groups of 3
hairless mice of both strains were also exposed topically to 5 doses
of 0.2 mg 3,4,3',4'-tetrachlorobiphenyl/week, for 10 weeks. Grossly
there were no visible changes. The histological changes induced in
HRS/J mice were similar to those found after Phenoclor treatment.
However, identical changes induced in Skh:HR-1 mice were already
observed by 4 weeks. After 8 weeks, these lesions were more marked and
also included hyperkeratosis of the sebaceous follicles and
hyperkeratinization of intradermal pilar cysts. Often these cysts
ruptured into the dermis leading to dense infiltrates of
polymorphonuclear leukocytes. The treated mice showed weight gain,
primarily because of large intra-abdominal fat deposits.
In a dermal toxicity study on rabbits, with a protocol identical to
that of the study of Vos & Beems (1971), skin lesions on animals
treated with 2,4,5,2',4',5'-hexachlorobiphenyl were less severe than
those on Aroclor 1260-treated animals. The liver damage observed
histologically was essentially the same after treatment with either
Aroclor 1260 or 2,4,5,2',4',5'-hexachlorobiphenyl, but the individual
congener was more porphyrogenic (Vos & Nootenboom-Ram, 1972). In
another study, 4 applications of a 25% solution of 3,4,3',4'-
tetrachlorobiphenyl in olive oil to the inner surface of the ears
of rabbits resulted in the same lesions that were found after 2
applications of undiluted Kanechlor 400 or 500. The lesions included
hyperkeratosis, dilatation of hair follicles, and the formation of
keratinous cysts (Komatsu & Kikuchi, 1972).
8.2.4 Appraisal
Rhesus monkey is the most sensitive test species with regard to the
general toxicity of PCBs, both as a mixture and as individual
congeners. The toxicity of mixtures may be confounded by the presence
of impurities, such as PCDFs, which are, or may have been, present in
the mixtures tested. PCBs induce some of the biological and
toxicological effects qualitatively similar to those induced by PCDFs.
Another confounding variable in these studies is the difference in the
composition of the mixtures (Aroclor 1242, 1248, 1254) used.
Beating the above in mind, the available data show that Aroclor 1248,
containing 4.4-8.7 ng of PCDFs/kg, still showed adverse general toxic
effects in Rhesus monkeys at a dose of 0.1 mg/kg diet per day
(0.09 mg/kg body weight per day) administered for an average of 18.2
months (Bowman et al., 1981). A NOEL for general toxicity was not
established for Aroclor 1248. The NOEL for the general toxicity of
Aroclor 1242 was 0.04 mg/kg body weight per day, as established after
dietary exposure for 133 days. The main effects induced by Aroclor
1248 at 0.09 mg/kg body weight per day were an increased mortality
rate, growth retardation, alopecia, acne, swelling of the Meibomian
glands, and, possibly, immunotoxicity. Microscopically, enlarged
hepatocytes, fatty liver with focal necrosis, and epithelial
hyperplasia and keratinization of hair follicles were found. These
effects appeared reversible. Aroclor 1254 at a dose level of
0.200 mg/kg body weight per day showed several effects, not reported
for 1248 (lymforeticular lesions, finger-nail detachment, gingival
effects) and vice-versa (acne, alopecia), which could be related to
the confounding variables noted above. Several effects observed in the
monkeys exposed to Aroclor 1254 (hypertrophic gastropathy, bone marrow
hyperplasia) were also observed in monkeys exposed to Aroclor 1248,
but at a higher dose (4 mg/kg body weight per day). In contrast with
the severe effects observed in adult Rhesus monkeys at low doses,
relatively mild effects were shown by suckling Rhesus monkeys exposed
to much higher doses.
8.3 Skin and eye irritation, sensitization
The injury to skin and eyelids following oral and/or dermal exposure
to PCBs has been discussed in sections 8.2.1 and 8.2.3.
8.4 Reproduction, embryotoxicity, and teratogenicity
8.4.1 Reproduction and embryotoxicity
8.4.1.1 Oral
(a) Mouse
In castrated, mature, male NMRI mice (10-13 per group) that received
28 daily doses of 0.25 mg Aroclor 1254/mouse, in peanut oil, the
weight of the seminal vesicles was decreased, but this was not seen in
intact mice (Orberg & Lundberg, 1974).
When 23 adult female NMRI mice were mated with 22 control males and
orally intubated with 0.025 mg Clophen A60/day, in peanut oil, for 62
days prior to mating and up to days 8-10 of gestation, blastocytes
failed to implant. Twenty-five (14 experimental and 11 control
animals) out of the 45 animals were used to study the effects of PCBs
on the estrous cycle. Effects, such as prolonged estrous cycle and
less frequent periods of sexual receptivity and a decline in the
number of implanted ova, were found (Orberg & Kihlström, 1973).
In order to study the effects of PCBs on the development of sexual
functions in the early postnatal period, these authors also mated mice
that had been suckled by mothers dosed with Clophen A60 during the
lactation period. A decrease in the frequency of implanted ova was
noted, when both parents of the couple had been suckled with milk
containing PCBs. When adult female NMRI mice received 50 mg of Clophen
A60/kg body weight once per week, subcutaneously, during lactation,
the same effect was observed in the offspring after mating with
similarly exposed males (Kihlström et al., 1975).
(b) Rat
In a 2-generation reproduction study, groups of 10 male and 20 female
Sherman weanling rats were fed diets containing 1, 5, 20, or 100 mg of
Aroclor 1254/kg (equivalent to 0.06, 0.32, 1.5, and 7.6 mg/kg body
weight, respectively), or diets containing 5, 20, or 100 mg/kg of
Aroclor 1260 (equivalent to 0.39, 1.5, and 7.5 mg/kg body weight,
respectively). Control groups comprised 20 male and 40 female rats.
The F0 rats were started on the diet at 3-4 weeks of age and the F1
rats, at weaning. The F0 rats were pair-mated when 3 and 7 months old
to produce the F1a and F1b generations, respectively. Breeding-stock
F1b rats were selected at weaning and pair-mated when 3 months old to
produce the F2a, and, when 8 months old, the F2b generation. Rats
exposed to Aroclor 1254 at 20 mg/kg diet or more showed a reduced
litter size at birth, but not when exposed at weaning, in the F1b and
F2 generations. At 100 mg/kg diet, the number of litters in the F2
generation was decreased. In 2 F2a and 2 F2b litters no live offspring
were found at birth, while pup survival at weaning was reduced in the
F2a generation. At weaning, exposed F1a pups weighed less than their
controls. Increased relative liver weights were found in male F1a
weanlings at 1 mg Aroclor 1254/kg diet and in all weanlings at 5 mg/kg
diet or more. Adult rats showed increased relative liver weights at
levels of 20 mg/kg diet or more. No reproductive effects were found
with 5 mg Aroclor 1254/kg diet. In groups treated with Aroclor 1260,
increased liver weights were found in all weanlings at 5 mg/kg diet or
more, but no effects on reproduction were seen, even at 100 mg/kg diet
(Linder et al., 1974).
The only effect observed in Holtzman rats fed Aroclor 1254 at a level
of 500 mg/kg diet, for 3 weeks, was an increase in relative testes
weights (Garthoff et al., 1977). Increased absolute testes weights and
unchanged body weights were found in 6-month-old offspring of
Sprague-Dawley dams exposed to daily doses of 30 mg Aroclor 1260/kg
body weight in ethanol-sesame oil on days 14-20 of gestation. No
effects on testes weight were found in animals treated with Aroclor
1221 or Aroclor 1242 (Gellert & Wilson, 1979).
Sager (1983) evaluated the effects on the reproductive function of
adult male Holtzman rats following exposure of their mothers to doses
of 8, 32, or 64 mg Aroclor 1254/kg body weight in peanut oil, on days
1-3, 5, 7, or 9 of lactation. At all dose levels, 165-day-old males
showed a decreased relative ventral prostate weight, and, at the 2
higher doses, a decreased relative weight of seminal vesicles and
testes as well as decreased body weight. In the ventral prostate,
alveoli were decreased in number and showed folding of the mucosa and
flattened epithelial cells. At 130 days of age, the male offspring at
all 3 dose levels showed a reluctance to mate with control breeders,
leading to a decreased number of pregnancies. Moreover, at the 2
higher dose-levels, the females showed a reduced number of
implantations and an increased resorption rate. The litters showed a
reduced weight gain up to 11 days of age.
This study was repeated with an evaluation of the reproductive
performance of the second generation male rats from 130 days of age,
following mating with normal females. In the first study, autopsy on
pregnant females was carried out on day 11 or 12 of gestation. The
females had fewer implants, fewer embryos, and a reduced proportion of
ovulated eggs that implanted, compared with controls. The effects were
dose-related. In a second study, females mated to the same males were
autopsied on day 2 or 4 after mating. Sperm counts were not affected.
At the 2 highest doses, fewer females had eggs in the expected state
of development, the average number of blastocytes found in one uterine
horn on day 4 was reduced, and an increased incidence of abnormally
developed embryos was observed (Sager et al., 1987).
Female Wistar rats exposed to daily doses of 10 mg Aroclor 1254/kg
body weight, for at least one month, showed a prolonged estrous cycle,
decrease in sexual receptivity, delay in timing of copulation, vaginal
bleeding during gestation, decrease in litter size, and delay in the
time of parturition. After mating of the rats to control males, the
female offspring, exposed in utero and during lactation, showed a
slower rate of body weight gain, higher mortality, earlier vaginal
opening, and a delay in the appearance of the first estrous cycle
(Brezner et al., 1984).
(c) Monkey
Groups of 9 adult female Rhesus monkeys were exposed to a diet
containing Aroclor 1248 (containing polychlorinated dibenzofurans) at
levels of 2.5 or 5.0 mg/kg (Barsotti et al., 1976; Allen et al., 1979,
1980). The study and the maternal toxicity data have already been
described in section 8.2.1.6. Within 4 months, menstrual bleeding and
the duration of the menstrual cycle were increased. Flattening and
prolongation of the serum progesterone peak during the menstrual cycle
was observed. After 6 months of exposure, the females were mated with
control males. Reproductive dysfunction was obvious as shown in Table
44. Following the total exposure period of 18.2 months, the mothers
were put on a control diet. Their menstrual cycles and serum
progesterone levels returned to pre-exposure values. One year after
exposure ceased, the females were again mated with control males and
showed a recovery of their reproductive status (Table 44; Allen et
al., 1980).
Other groups of adult, female Rhesus monkeys were continuously fed
diets with 0, 0.25, and 1.0 mg Aroclor 1016/kg (equivalent to 0, 0.01
and 0.04 mg/kg body weight, respectively), in which no polychlorinated
dibenzofurans were detected (no details). No maternal toxicity was
noted at these levels. In this study, the females were mated with
control males after 7 months of exposure. Reproductive dysfunction
was not observed. Decreased birth weights were found in the offspring
of mothers exposed to Aroclor 1016 at 1.0 mg/kg diet. Skin
hyper-pigmentation occurred in both exposure groups (Barsotti & Van
Miller, 1984). Preliminary reports have indicated possible effects on
learning and behavioural tasks. The milk contained an average of 1.45
and 3.92 mg/kg fat at 0.25 and 1.0 mg/kg, respectively, whereas the
serum of the mothers contained 0.012 and 0.027 mg/litre, respectively
(Heironimus et al., 1981; Levin & Bowman, 1983).
Table 44. Reproductive status of Rhesus monkeys exposed to dietary levels of Aroclor 1016 or 1248
PCB Exposure level Total intake Conceptions Abortions Stillborn Live Reference
mixture at and births
(Aroclor) Diet Body conception resorptions
(mg/kg) weight (mg/kg)
(mg/kg)
1016 0 0 0 8/8 0/8 0/8 8/8 Barsotti &
0.25 0.01 8c 8/8 0/8 0/8 8/8 van Miller
1.0 0.04 30c 8/8 0/8 0/8 8/8 (1984)
1248 0 0 0 12/12 0/12 0/12 12/12 Barsotti et al.
2.5 0.09a 105 8/8 3/8 0/8 5/8 (1976)
5.0 0.2 210 6/8 4/8 1/8 1/8
1248 0 0 0 8/8 0/8 0/8 8/8 Allen et al.
2.5 0.09a 270 8/8b 1/8 0/8 7/8 (1980)
5.0 0.2 498 7/7b 1/7 2/7 4/7
a From: Bowman et al. (1981) amended.
b Breeding 1 year after exposure.
c Calculated assuming a body weight of 5 kg.
In these studies, the monkeys were maintained on the diets during the
gestation and lactation of the first generation. PCBs are known to
cross the placenta and to be excreted via breast milk (see section
6.3). The 6 infants born to monkeys during exposure at 2.5 or 5.0 mg
Aroclor 1248/kg diet showed decreased birth weights, a small stature,
and a decreased body weight gain during nursing. Within 2 months,
signs of intoxication appeared including acne, increased skin
pigmentation, swelling of the eyelids, and loss of eyelashes. In 3
milk samples, values ranging from 0.154 to 0.397 mg PCBs/kg milk were
measured, and, 1 milk sample contained 16.44 mg PCBs/kg fat. Three
infants died. Necropsy and histopathology revealed atrophy of the
thymus and lymph nodes, hypocellular bone marrow, moderate fatty
infiltration of the liver, hypertrophic Meibomian glands, and
keratinization of hair follicles. One dead infant showed hyperplasia
of the gastric mucosa. The 3 surviving infants were weaned and
subsequently showed marked improvements in their physical status
(Allen & Barsotti, 1976; Allen et al., 1979, 1980). At 6 and 12 months
of age, they were found to be hyperactive in a locomotor activity test
and, between 7 and 24 months of age, they did not learn reversal tasks
as readily as the controls. The PCB body burdens of these infants
ranged between 11 and 27 mg/kg body fat, at the age of 8 months, and
dropped to 0-1.6 mg/kg, at the age of 23 months. However, using the
same apparatus, these infants showed hypolocomotor activity at 44
months of age in comparison with the same controls (Bowman et al.,
1978; Bowman & Heironimus, 1981; Bowman et al., 1982; Levin & Bowman,
1983).
The infants delivered by the same adult females, after 1 year on a
control diet, showed a reduced body weight and signs of intoxication
similar to those observed in their siblings of the first breed.
Two infants in each exposed group died. Milk samples contained
0.02-0.19 mg PCBs/kg milk (Allen et al., 1980). At 12 months of age,
when the PCB body burdens were only slightly higher than those of the
controls, the infants showed hyperlocomotor activity (Bowman et al.,
1982).
Groups of 4 or 6 Rhesus monkeys, which had been exposed to 0 or 2.5 mg
Aroclor 1248/kg diet in utero and during nursing until 4 months
after birth, were tested at 4-6 years of age on delayed spatial
alternation (DSA), a spatial learning and memory task. Deficits in
performance accuracy were detected in 2 cohorts of monkeys, whose
mothers had been fed 2.5 mg Aroclor 1248/kg diet for an 18-month
period ending at least 12 months prior to pregnancy. The deficit was
most apparent at the shorter delays, suggesting impairments in
association or attention processes were involved rather than memory
impairment. Such a deficit was also found in monkeys fed 1.0 mg
Aroclor 1016/kg diet, but the effect was less pronounced. The
appearance of a PCB-induced cognitive deficit more than 3 years after
the end of exposure indicated the existence of long-term adverse
consequences of perinatal PCB exposure (Levin et al., 1988).
Clophen A30, which did not contain detectable levels of
polychlorinated dibenzofurans (limit of detection <1 mg/kg), was
given by gavage in a 1% solution of methylcellulose in water, once
daily for 30 days, to 3 lactating Rhesus monkeys and their offspring
at a level of 16 mg/kg body weight. PCB concentrations were measured
in the serum of both mothers and infants and in the milk, on days -14,
-7, 0, 1, 2, 4, 8, and then at weekly intervals until the end of the
study. The mean PCB concentrations in the serum of mothers and infants
were between 0.13 and 1.16 mg/litre and 0.07 and 2.67 mg/litre,
respectively (before treatment days -14 and -7). The mean PCB levels
in milk ranged from 0.63 mg/kg on day 1 of exposure to 18.90 mg/kg. On
days -14 and -7, the concentrations in milk were 0.14 and 0.34 mg/kg
(wet weight). Five control pairs were used. One dam and her offspring
were sacrificed on day 22, exhibiting symptoms of anorexia,
depression, lethargy, and ataxia. Two of 3 infants showed a decreased
body weight gain. At autopsy of the infants, after the exposure
period, slight degenerative changes were seen in the liver and the
kidneys, together with slight demyelination of the central nervous
system, slight to moderate gliosis of the cerebrum, and slight
granular cell layer thinning of the cerebellum. On the basis of
earlier work with adults in which the degenerative changes described
above were considerable, the authors concluded that the nursing
infants seemed to be less susceptible than the adults under the
conditions of the studies (Iatropoulos et al., 1978; Bailey et al.,
1980).
8.4.2 Teratogenicity
8.4.2.1 Aroclors (oral)
(a) Mouse
Haake et al. (1987) reported that treatment of pregnant C57Bl/6 mice
with Aroclor 1254, by gavage, at 224 mg/kg body weight, on day 9 of
gestation, did not result in any fetuses with cleft palate (see
section 8.6.6).
(b) Rat
Wistar rats were exposed to dietary levels of Kanechlor 400 of up to
250 mg/kg from day 1 to day 21 of gestation (Mizunoya et al., 1974).
Fetuses showed decreased body weights from 10 mg/kg diet onward
(equivalent to 0.67 mg/kg body weight), but did not show any increased
incidence of malformations. Maternal toxicity was not observed and
litter size and the number of litters were unaffected. Decreased pup
survival was noted at dietary levels from 50 mg/kg (equivalent to
3.5 mg/kg body weight) upwards. The 28-day-old offspring showed
decreased body weight and increased relative liver weight from
10 mg/kg.
Commercial Kanechlor 300 or 500 was mixed with food and administered
to pregnant Sprague-Dawley rats, throughout gestation, at levels of
20, 100, or 500 mg/kg diet. On day 21, about three-quarters of the
pregnant females were sacrificed; the remainder were allowed to litter
naturally and the postnatal development of the pups was observed.
Kanechlor 500 at a concentration of 500 mg/kg resulted in decreased
maternal weight gain and decreased food consumption. At 20 and 500 mg
Kanechlor 300/kg, and 500 mg Kanechlor 500/kg, the fetal weight
decreased significantly. Resorption and malformations were not
increased by treatment with Kanechlors. The Kanechlors did not show a
teratogenic potential in this study (Shiota, 1976a).
Offspring of Sprague-Dawley rats that received 20 mg of Kanechlor
500/kg body weight on days 15-21 of gestation were slower than
controls in achieving the water maze test at the age of 12-13 weeks,
but did not perform worse in the open field test and in the swimming
test (Shiota, 1976b). Behavioural effects were also observed in the
offspring of ICR dams, 23-27 days of age, exposed to Aroclor 1254 in
the diet at levels of 11 or 82 mg/kg (equivalent to 1.7 and 12 mg/kg
body weight) from 3 days before mating up to weaning. The offspring
were maintained on the same diet. PCB exposure did not have any
effects on the ability to learn an avoidance response, but increased
the latency to make such a response. Moreover, the young mice
exhibited slower habituation to an open field (Storm et al., 1981).
The effects of Fenchlor 42 (trichloro- 63%, tetrachloro- 33%, and
small amounts of dichlorobiphenyl; purity 97.5%) exposure of Fischer
344 male and female rats were studied through assessment of the
behavioural development of their F1 progeny. Female rats were
administered 5 daily ip injections of corn oil or 5-10 mg Fenchlor
42/kg body weight per day, 2 weeks prior to mating. Another group
received 2-4 mg/kg per day during gestation (days 6-15 of pregnancy)
and a third group of 8 previously treated lactating females received
corn oil or 1-2 mg/kg per day on postnatal days 1-21. The total doses
in the 3 groups were 25-50, 20-40 and 20-40 mg/kg body weight.
Dose-dependent differences in behaviour were found in the offspring of
the PCB-treated animals. Differences in the development of cliff
avoidance reflexive behaviour, swimming ability, and open field
activity were evident. The PCB exposure of female animals during
gestation and lactation resulted in impaired acquisition of the active
avoidance behaviour, while preconception PCB exposure affected active
avoidance performance, as reflected in an increased number of
avoidance responses to reach criterion for extinction (Pantaleoni et
al., 1988).
Doses of 0, 6.25, 12.5, 25, 50, or 100 mg/kg body weight per day of
Aroclor 1254 were administered to rats, by gavage, on days 6-15 of
gestation. Average pup weights were reduced at 100 mg/kg, though total
litter weight (average weight times number of fetuses) did not differ
from controls. There were no skeletal or visceral abnormalities or
effects on conception, resorptions, litter size or number, or average
litter weight in any of the treated groups (Villeneuve et al., 1971b).
Spencer (1982) exposed Holtzman rats to a diet containing Aroclor 1254
at levels of 25 up to 900 mg/kg diet from day 6 to day 15 of gestation
and found reduced maternal body weight gain and decreased fetal
survival at birth from 300 mg/kg diet (equivalent to 18 mg/kg body
weight) upwards, and decreased fetal weights at birth from 100 mg/kg
(equivalent to 8 mg/kg body weight) upwards. No visceral or skeletal
data were available.
When Sherman rats were exposed to doses of Aroclor 1254 of up to
100 mg/kg body weight, in peanut oil, from day 7 to day 15 of
gestation, a decrease in the survival of the pups was found. At
weaning, the survival and body weight of the grossly normal pups were
reduced at the 100 mg/kg dose level, but not at 50 mg/kg body weight.
No effects were observed at 100 mg Aroclor 1260/kg body weight (Linder
et al., 1974).
(c) Monkey
Two pregnant Cynomolgus monkeys (Macaca fascicularis) were dosed
with Aroclor 1254 at 100 mg/kg body weight per day and 1 monkey, at
400 mg/kg body weight per day, from day 60 of gestation. One control
animal received the vehicle, corn oil. The 2 monkeys fed 100 mg/kg
delivered dead male infants after 105 and 108 days of dosing, and the
female fed 400 mg/kg delivered a female infant (with no overt clinical
signs of toxicity) that died at 139 days of age with an acute
bronchopneumonia. The breast milk of the monkey fed 400 mg/kg
contained, over a period of 5-75 days after parturition,
concentrations of 73.7 up to 139.4 mg/kg, on a fat basis. No overt
signs of toxicity were observed in the adult animals, with exception
of finger nail loss. All 3 treated monkeys showed impaired
immunological capacity, assessed at approximately 50 days postpartum
(148 days of treatment) (Truelove et al., 1982).
(d) Rabbit
Rabbits were exposed to 0, 1.0, or 10.0 mg Aroclor 1254/kg body weight
and in another study to 0, 12.5, 25.0, or 50 mg/kg body weight (purity
not reported), by gavage, on days 1-28 of pregnancy. Abortions, still
births, and maternal deaths occurred at 12.5 mg/kg body weight or
more, but no teratogenic effects were found (Villeneuve et al.,
1971a,b).
8.4.2.2 Aroclors (subcutaneous)
(a) Mouse
A possible teratogenic effect was observed in ddy mice subcutaneously
exposed to doses of 1-5 mg Kanechlor 500/mouse (equivalent to
40-200 mg/kg body weight) in 95% ethanol from day 6 to day 15 of
gestation. A dose-related increase in maternal mortality was observed
from a dose of 3 mg/mouse onwards. Some dams showed skin lesions,
alopecia, or swelling of the liver, but no effects on body weight
gain. A slight increase was noted in the incidence of dead and
resorbed fetuses. The incidence of cleft palate was increased in a
dose-related manner from the lowest dose (Watanabe & Sugahara, 1981).
8.4.2.3 Individual congeners (oral)
(a) Mouse
Orberg (1978) fed groups of 20-56 pregnant female NMRI-mice 0, 0.05,
or 0.5 mg 2,5,4'-trichloro- or 2,4,5,2',4',5'-hexachlorobiphenyl,
dissolved in peanut oil, per animal, from days 1 to 6 of gestation. A
significant decrease in the pregnancy of implanted ova was found with
the 0.5 mg treatment. No effects on percentage of pregnancies and mean
number of corpora lutea were found. There were no effects at the lower
dose level.
A dose-related increase in embryotoxicity and the incidence of
malformed fetuses, mainly showing cleft palate and hydronephrosis, was
found in pregnant CD-1 mice after exposure to daily doses of 2, 4, 8,
or 16 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body weight, in
cotton-seed oil, on days 6-15 of gestation. Lower dose levels, e.g.,
0.1 and 1 mg/kg body weight were without effects. No dibenzofurans
were detectable (no details) in the test compound. The dams showed a
decreased body weight gain at 8 mg/kg body weight. The authors
reported that 3,4,3',4'-tetrachlorobiphenyl and 2,3,4,2',3',4'-hexa-
chlorobiphenyl also produced the same teratogenic effects, though they
were less potent than those of 3,4,5,3',4',5'-hexachlorobiphenyl
(Marks et al., 1981).
A neurobehavioural "spinning" syndrome (a syndrome characterized by
the fact that the mice rotate or spin in a circular motion when held
by the tail) and hydronephrosis developed in CD-1 mouse weanlings,
whose dams received, by gavage, 32 mg 3,4,3',4'-tetrachlorobiphenyl/kg
body weight, in corn oil, on days 10-16 of gestation. Maternal
neurotoxicity was not observed. Histological and ultrastructural
examination of the CNS of affected mice revealed longitudinal
projections of the cylindrical CNS in the ventral and dorsal roots
and, to a lesser extent, in cranial nerve roots. The effect was
possibly related to an observed altered development of striatal
synapses (Chou et al., 1979; Tilson et al., 1979; Agrawal et al.,
1981).
(b) Rat
The congener 3,4,3',4'-tetrachlorobiphenyl was found to be embyrotoxic
and caused accumulation of blood in the amniotic fluid and the
gastrointestinal tract of fetuses from Sprague-Dawley rats treated on
days 6-18 of gestation with doses of 3 or 10 mg/kg body weight in corn
oil. Decreased fetal growth was also observed (Wardell et al., 1982).
When the rats were allowed to deliver, high perinatal mortality was
observed, which appeared to be related to an increase in gestational
length and to be independent of the smaller total litter size. In
addition, pup weights were found to be lower than those of controls
(Rands et al., 1982; White et al., 1983).
(c) Guinea-pig, mouse
Neither embryotoxicity nor teratogenicity were found in Dunkin Hartley
guinea-pigs, and CBA mice exposed in utero to 2,4,5,2',4',5'-
hexa-chlorobiphenyl during gestation (Mattsson et al., 1981; Brunström
et al., 1982; Aulerich et al., 1985) and in C57BL/6N mice similarly
exposed to 2,4,5,2',4',5'- or 2,3,4,5,3',4'-hexachlorobiphenyl
(Birnbaum et al., 1985).
Pregnant guinea-pigs received a total dose of 100 mg technical grade
Clophen A50 orally, in peanut oil, from day 16 to day 60 of gestation,
25 mg 2,4,5,2',4',5'-hexachlorobiphenyl from day 16 to day 60 of
gestation, or 100 mg from day 22 to day 60 of gestation. The
administration of Clophen A50 resulted in fetal deaths, but no
maternal deaths. In contrast, 2,4,5,2',4',5'-hexachlorobiphenyl did
not cause fetal deaths. Prenatal weight of live fetuses was increased
by a dose of 25 mg, but not by 100 mg of 2,4,5,2',4',5'-hexa-
chlorobiphenyl (Brunström et al., 1982).
(d) Monkey
In a briefly reported study, 6 female Rhesus monkeys received 9 doses
of 70 or 350 µg 3,4,3',4'-tetrachlorobiphenyl/kg body weight by
gavage, in corn oil, from day 20 to day 40 of gestation. A control
group comprised 12 animals. Maternal toxicity (not specified) but no
mortality, was reported in all exposed monkeys from day 31 following
exposure. Between days 17 and 35 following exposure all exposed
fetuses and 3 out of 12 control fetuses aborted (McNulty, 1985).
8.4.3 Appraisal
The Rhesus monkey is the most sensitive species with regard to general
toxicity (see section 8.2) and particularly with regard to
reproductive toxicity. The presence of PCDFs and the variation in PCB
composition may be confounding factors in determining the reproductive
toxicity of PCB mixtures. Aroclor 1248, containing 4.4-8.7 µg
PCDFs/kg, adversely affected the reproductive performance of female
Rhesus monkeys, mated with control males after 6 months of dietary
exposure to a toxic dose of 0.09 mg/kg body weight per day and
continuation of the exposure for an average of up to 10 months. This
effect was reversible after an exposure-free period of 1 year. No
effect on reproduction was found in female Rhesus monkeys exposed to a
non-toxic dose of 0.01 or 0.03 mg Aroclor 1016 (reported not to
contain PCDFs)/kg body weight per day and mated after 7 months with
control males.
Neonates of the nursing mothers exposed to Aroclor 1248 showed adverse
effects similar to those seen in their mothers and, in addition,
persistent behavioural disturbances, atrophy of the thymus and lymph
nodes, bone marrow hypoplasia, and hyperplasia of the gastric mucosa.
Neonates of the mothers after the recovery period still showed adverse
effects, as well as the neonates of the mothers exposed to Aroclor
1016. These effects were caused by PCBs, with or claimed to be
without, PCDFs, transmitted via the placenta during gestation and
later via the breast milk. Neonates have much greater susceptibility
to PCB toxicity when exposed via the mothers compared with suckling
monkeys orally exposed. Reproductive toxicity has also been observed
in the mink, rabbit, and rat. The changes seemed to be related to
alterations in the serum levels of gonadal steroid hormones, as a
result of enzyme induction. PCBs may also bind to the cytoplasmic
estrogen receptor. Effects have also been observed on the estrus cycle
of female rats, minks, and monkeys, on the sex organs of male rats,
and on the implantation rate of fertilized ova following exposure of
female mice or male rats.
Comprehensive teratological examinations have not been conducted;
however, the available studies indicated that the Aroclors were not
teratogenic in rats and nonhuman primates, when tested via the oral
route during the critical periods of organogenesis at doses that
produced fetotoxicity and/or maternal toxicity. Although the
fetotoxicity of Aroclors is documented in several species of animals,
the possibility that contaminants (e.g., PCDFs) might be (partly)
responsible for the effects should be recognized.
The results of the reproduction and teratogenicity studies are
summarized in Tables 45, 46, and 47.
8.4.4 Mutagenicity and related end-points
8.4.4.1 DNA damage
PCBs have been shown to interact with the proteins, RNA and DNA, after
metabolic activation. The potential of readily metabolizable
PCB-congeners to cause primary DNA damage was indicated by the
activity of 2,5,2',5'-tetrachlorobiphenyl and its metabolites in
causing DNA, single-strand breaks in an alkaline elution assay with
L-929 cells in vitro (Stadnicki et al., 1979). Furthermore,
unscheduled DNA synthesis was elicited by 4-chlorobiphenyl in vitro
in Chinese hamster ovary cells (Wong et al., 1979). No unscheduled DNA
synthesis was elicited by Aroclor 1254 in rat hepatocytes in vitro
(Probst et al., 1981).
DNA-breaking activity was found in an alkaline elution assay with
hepatocytes of intact rats treated in vivo with a single, high dose
(500 mg Aroclor 1254/kg body weight, intraperitoneally, or 1295 mg/kg
body weight, orally) with complete repair of the damage within 48 h
(Robbiano & Pino, 1981). Aroclor 1254 was also shown to be a
DNA-breaking agent in an alkaline elution assay in vitro with rat
hepatocytes (Sina et al., 1983). An alkaline sedimentation assay
showed the DNA-breaking activity of Aroclor 1254 in rats treated
in vivo with a single intraperitoneal dose of 500 mg/kg body weight.
In this assay, the same Aroclor 1254 pretreatment of the rats in vivo
elevated the DNA-breaking activity of the direct-acting, alkylating
N-methyl- N'-nitro- N-nitrosoguanidine and the carcinogens,
dimethylnitrosamine and benzo (a)pyrene, in vitro (Mendoza-Figueroa
et al., 1985).
8.4.4.2 Mutagenicity tests
Many mutagenicity tests have been carried out over the years, with
different PCB mixtures. Most of these were commercial mixtures the
composition of which was not described. Only a few studies are
available on specific congeners. Besides studies on microorganisms,
mammalian cell point mutation, dominant lethal assays, micronucleus
tests, chromosome and cytogenicity studies, and DNA repair studies
were carried out. With a few exceptions the results of most of the
studies with PCB mixtures were negative (see Table 48).
Aroclor mixtures and the congener 2,5,2',5'-tetrachlorobiphenyl and
its metabolites did not induce point mutations in Salmonella
typhimurium TA 98, TA 100, TA 1535, TA 1537, and TA 1538 with, and
without, metabolic activation (Wyndham et al., 1976; Hsia et al.,
1978; Bruce & Heddle, 1979; Schoeny et al., 1979; Shahin et al.,
1979), neither did 2,4,2',4'- and 3,4,3',4'-tetrachlorobiphenyl and
2,4,6,2',4',6'-hexachlorobiphenyl in the strains TA 98 and TA 100
(Schoeny, 1982).
However, Wyndham et al. (1976) found a dose-related mutagenic activity
of 4-chlorobiphenyl and, to a lesser extent, of Aroclor 1221 in the
strain TA 1538, after metabolic activation by the S9 liver fraction of
uninduced rabbits. The study was repeated 3 times in the same
laboratory, but the results of Wyndham et al. (1976) could not be
confirmed (Safe, 1980). Schoeny (1982) could not detect any mutagenic
activity of 4-chlorobiphenyl in the same dose range in the strains TA
98, TA 100, TA 1535, and TA 1537, with, or without, the S9 liver
fraction of induced rats.
Table 45. PCBs: reproduction and embryo toxicity
Animal (strain, PCB mixture Exposure period NOAEL LOAEL Parameters, effects Reference
sex) (oral) (mg/kg body (mg/kg body
weight) weight)
Rat (Sherman, Aroclor 1254 continuous up to 0.06 (1254) increased relative liver Linder et al.
10 male, Aroclor 1260 weaning weights in male F1A (1974)
20 female) weanlings
0.32 (1254)
former effect in all weanlings
0.32 (1254)
7.5 (1260) reproduction parameters
reproduction parameters
Rhesus monkey Aroclor 1016 7 months 0.03 (0.04) reproduction parameters Barsotti &
(female) [0.03(0.04): decreased birth van Miller
weight] [0.01: skin (1984)
hyperpigmentation]
Rhesus monkey Aroclor 1248 6 months 0.09 abortions, resorptions, live Barsotti et al.
(female) births (1976)
1 year after 0.09 0.2 Allen et al.
exposure stillborn, live births (1980)
Table 46. PCBs: teratogenicity
Animal (strain) PCB-mixture Exposure period NOAEL LOAEL Parameters, effects Reference
(oral) (mg/kg body (mg/kg body
weight) weight)
Rat Aroclor 1254 days 6 to 15 of 50 100 reduced average pup Villeneuve et al.
gestation weight (1971b)
Rat (Holtzman) Aroclor 1254 days 6 to 15 of < 8 8 decreased fetal weight at Spencer (1982)
gestation birth
Rat (Sherman) Aroclor 1254 days 7 to 15 of 100 reproduction effects Spencer (1982)
gestation
Rabbit Aroclor 1254 days 1 to 28 of 10 12.5 abortions, still births, Villeneuve et al.
gestation maternal deaths (1971 a,b)
Aroclor 1221 days 1 to 28 of 25 fetotoxicity
gestation
Table 47. PCBs: teratogenicity of individual congeners
Animal PCB-mixture Exposure period NOAEL LOAEL Parameters, effects Reference
(strain) (oral) (mg/kg body (mg/kg body
weight) weight)
Mice 2,5,4'-TCB day 1 to 6 of 0.05/animal 0.5/animal decrease in the number Örberg (1978)
(NMRI) 2,4,5,2',4', gestation of implant/dams
5'-HCB
Mice 3,4,5,3',4', day 6 to 15 of 2 embryotoxicity, malformations Marks et al.
(CD-1) 5'-HCB gestation (cleft palate, hydronephrosis) (1981)
3,4,3',4'-TCB 8 maternal toxicity, less potent
2,3,4,2', than that of 3,4,5,3',4',5'-HCB
3'4'-HCB
Rat 3,4,3',4'-TCB day 6 to 18 of 3 embryotoxicity Wardell et al.
(Sprague- gestation (1982)
Dawley)
Rhesus 3,4,3',4'-TCB day 20 to 40 of 0.07 maternal toxicity, total abortions McNulty (1985)
monkey gestation
Table 48. Results of mutagenicity, and related, tests
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
2,5,2',5'- Salmonella TA 1538 200 µg/plate modified - Wyndham et al.
tetrachloro typhimurium 100 µg/plate microsomal - (1976)
biphenyl 50 µg/plate fraction -
10 µg/plate from rabbits -
Aroclor 1268 Salmonella TA 1538 200 µg/plate modified - Wyndham et al.
typhimurium 100 µg/plate microsomal - (1976)
50 µg/plate fraction -
10 µg/plate from rabbits -
Aroclor 1254 Salmonella TA 1535 4 different both with, and - Heddle & Bruce
typhimurium TA 1537 concentrations, without, S-9 in - (1977)
TA 98 figures not all cases -
TA 100 given -
Aroclor 1254 Salmonella TA 1535 8 different both with, and - Schoeny et al.
typhimurium TA 1537 concentrations without, S-9 - (1979)
TA 98 from 0.5 to -
TA 100 500 µg/plate -
Aroclor 1254 Salmonella TA 1535 0.05, 0.5, both with, and - Bruce & Heddle
typhimurium TA 1537 5, 50, and without, S-9 - (1979)
TA 98 500 µg/plate -
TA 100 -
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Aroclor 1254 Salmonella TA 1538 50, 100, 500, both with, and - Shahin et al.
typhimurium TA 98 1000, 2000, without, S-9 - (1979)
5000 µg/plate
Aroclor 1242 V79 50, 100, and without - Hattula (1985)
Clophen A60 hamster cells 150 µg/ml metabolizing -
co-cultivated cells
with lethally
irridatiated
hepatocytes
Aroclor 1254 Micronucleus 4 different - Heddle & Bruce
test concentrations, (1977)
(erythrocytes) figures not
given
Aroclor 1254 Micronucleus (C57B1/6 × (approximately) - Bruce & Heddle
test C3H/He) LD50, 1/2, 1/4, (at all doses) (1979)
F1 mice and 1/8 of the
highest dose
(5 consecutive
days, ip)
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Kanechlor 500 Micronucleus ddY-mice 100 mg/kg in - - Watanabe et al.
test with corn oil orally (1982)
polychromatic and 100 mg/kg
erythrocytes 95% ethanol
subcutaneously
Aroclor 1254 Chromosomal (embryonic 0 or 10 mg/kg inconclusive Peakall et al.
aberrations Ring diet (1972)
Doves)
Aroclor 1254 Chromosomal (human 100 mg/litre - Hoopingarner
aberrations lymphocytes) culture medium et al. (1972)
Aroclor 1254 Chromosomal male 5, 50, 500 mg/kg negative at all Garthoff et al.
abnormalities in Holtzmann diet doses (1977)
bone marrow and rats
spermatogonial
cells
Aroclor 1242 Chromosomal Osborne- 5000 mg/kg × 1a - Green et al.
abnormalities Mendel 2500 mg/kg × 1 - (1975a)
in bone marrow rats 1250 mg/kg × 1 -
cells and 500 mg/kg × 4 -
spermatogonial
cells
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Clophen A30 Drosophila 62.5, 125, 250, -b Nilsson & Ramel
melanogaster and 500 mg/litre (1974)
(adults or larvae) substrate
Clophen A50 Drosophila 25, 50, 100, -b Nilsson & Ramel
melanogaster and 200 mg/litre (1974)
(adults or larvae) substrate
Aroclor 1242 Dominant Lethal Osborne- 2500 mg/kg × 1a - Green et al.
test Mendel 1250 mg/kg × 1 - (1975b)
rats 625 mg/kg × 1 -
250 mg/kg × 5 -
125 mg/kg × 5 -
Aroclor 1254 Dominant Lethal Osborne- 150 mg/kg × 5a - Green et al.
test Mendel 75 mg/kg × 5 - (1975b)
rats
Aroclor 1254 Dominant Lethal Osborne- 25, 100 mg/kg - Green et al.
test Mendel diet for 70 days - (1975b)
rats
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Aroclor 1254 Sperm (C57 B1/6 × (approximately)LD50, negative at all Bruce & Heddle
Abnormality C3H/He)F1 1/2, 1/4, 1/8 top dose doses (1979)
mice on 5 consecutive
days, ip
Aroclor 1254 mitotic index human 100 mg/litre mitotic index Hoopingarner
lymphocytes culture medium equivocal et al. (1972)
4-chloro- DNA repair and Chinese 10-5 mmol/litre covalent- Wong et al.
biphenyl and unscheduled hamster 3H-4-chloro- binding to (1979)
metabolites synthesis ovary cells biphenyl, 24 h protein, RNA,
(hydroxyurea and DNA
addition suppress Increase
DNA synthesis) specific
activity with
DNA
a Means single dose (× 1) or 5 doses in 5 days (× 5).
b No loss or non-disjunction of sex-chromosomes.
SD=Significant decrease.
Dose-related chromosome breakage was found in human lymphocytes
exposed to the planar PCB congener, 3,4,3',4'-tetrachlorobiphenyl, at
0.1-10-4 µg/ml (Sargent et al. (1989). In contrast, the non-planar
2,5,2',5'-tetrachlorobiphenyl did not cause chromosome damage in a
comparable test, even at concentrations as high as 1 µg/ml. However, a
combination of 3,4,3',4'-tetrachlorobiphenyl at a concentration of
10-5 µg/ml with 2,5,2',5'-tetrachlorobiphenyl caused chromosomal
damage that was far in excess of what might be expected from higher
doses of 3,4,3',4'-tetrachlorobiphenyl alone. The results suggest that
some PCB congeners may interact to cause synergistic effects.
Peakall et al. (1972) carried out cytogenic studies on Ring dove
embryos (Streptopelia risoria); 6 embryos were from dove pairs not
fed PCBs (controls) and 17 embryos were from PCB-fed (10 mg/kg diet)
pairs. The frequencies of chromosome aberrations were recorded for
chromosome pairs occurring in metaphase cells of allantoic sac and
limb bud origin. Mean aberration rates were as follows: control 0.8%
(range 0-2.0%) and PCB-treated 1.8% (range 0-9.4%). It was concluded
by the authors that these results were indicative of a possible
clastogenic action of PCBs.
A DNA repair assay was carried out by Wong et al. (1979) using CHO
cells and measuring the effects of 4-chlorobiphenyl (10 mol/litre) on
the unscheduled DNA synthesis (UDS) in the presence of hydroxyurea
(HU), a chemical agent that suppresses normal replicative DNA
synthesis. The quantification of DNA synthesis was determined by the
uptake of [H3]-thymidine into the cellular DNA. A significant
(1.6-fold) enhancement of UDS was found when the cells were incubated
for 2.5 h in the presence of HU, 4-chlorobiphenyl, and thymidine.
8.4.4.3 Cell transformation
Aroclor 1254 did not cause an increase in benzo (a)pyrene-induced
transformation in a test using C3H10 T1/2 CL8 mouse embryo fibroblasts
(Nesnow et al., 1981).
Aroclor 1254 also failed to transform Golden Syrian hamster cells
76-582 in culture at 50 µl/ml (Pienta, 1980).
8.4.4.4 Cell to cell communication
The congener 2,4,5,2',4',5'-hexachlorobiphenyl inhibited one form of
intercellular communication in V79 Chinese hamster cells, i.e.,
metabolic cooperation by mutant rescue at non-cytotoxic levels, while
3,4,5,3',4',5'-hexachlorobiphenyl was inactive (Tsushimoto et al.,
1983).
8.4.4.5 Interaction
Grolier et al. (1989) studied the effects of Vitamin A dietary intake
(2 and 20 IU/g of food) on the mutagenicity of benzo (a)pyrene
(B (a)P) towards Salmonella typhymurium TA 98, either in control
rats or in animals treated with 2,4,5,2',4',5'- hexachlorobiphenyl and
3,4,3',4'-tetrachlorobiphenyl. The planar tetrachlorobiphenyl strongly
increased B (a)P-monooxygenase activity and glutathione transferase,
while the non planar hexachlorobiphenyl was a strong inducer of
epoxide hydrolase and a weak inducer of B (a)P-monooxygenase. Enzyme
induction was not modified by changes in Vitamin A intake. A greater
mutagenic effect was observed in the tetrachlorobiphenyl group than in
the hexachlorobiphenyl group. This could be related to the specific
form of cytochrome P-450 induced by the tetrachlorobiphenyl congener.
In PCB-treated rats, the mutagenic activity of B (a)P was higher in
the 20-IU group than in the 2-IU group.
8.4.4.6 Cell division parameters
Tests on Osborne-Mendel rats gave various results, but may provide the
most important clue to the mechanism of action of PCBs in
carcinogenesis. At high doses (5000 mg Aroclor 1242/kg given once, and
500 mg/kg, given in a series of 4 daily doses), there were significant
decreases in the numbers of spermatogonial cells in mitosis. Single
dose levels of 1250 or 2500 mg/kg gave negative results (Green et al.,
1975a). Garthoff et al. (1977) also found negative results in male
Holtzmann rats treated with 0, 5, 50, or 500 mg Aroclor 1254/kg diet
for 5 weeks with regard to the mitotic indices of bone marrow and
spermatogonial cells. The data of Hoopingarner et al. (1972) showed an
increase in mitotic index in human lymphocytes exposed to Aroclor
1254.
The above-mentioned studies provide evidence that Aroclors can enhance
cell proliferation, and this is of special interest because it
suggests that the Aroclors may act to promote carcinogenesis to a
greater extent than to initiate it. The effect on cell proliferation
requires further examination in a variety of systems.
8.5 Carcinogenicity
Hayes (1987) critically reviewed the available evidence for, and
against, the view that environmental PCBs present a significant
potential carcinogenic hazard for humans.
8.5.1 Long-term toxicity/carcinogenicity
(a) Mouse
Nagasaki et al. (1974) exposed 10 groups of dd mice (6-12 of each sex)
to Kanechlor 300, 400, or 500 in the diet at levels of 0, 100, 250, or
500 mg/kg (equivalent to 0, 5, 12.5, and 25 mg/kg body weight,
respectively) for 32 weeks. Nine nodular hyperplasia and 7
hepatocellular carcinomas were found in 17 male mice, and 4 cases of
liver hypertrophy in 17 female mice, after exposure to 500 mg
Kanechlor 500/kg diet. No neoplasms were found in the other groups.
In another mouse study, groups of male BALB/cJ mice were fed 0 or
300 mg Aroclor 1254/kg diet (equivalent to 50 mg/kg body weight) for 6
or 11 months. Adenofibrosis was observed in the livers of all 22 mice
fed Aroclor 1254 for 11 months, but not in those of the 24 mice
exposed for 6 months. Hepatomas were noted in 9/22 mice exposed for 11
months and in 1/24 mice exposed for 6 months. No tumours were found in
the controls (Kimbrough & Linder, 1974).
(b) Rat
In a preliminary study, liver tumours (multiple adenomatous nodules)
were induced by Kanechlor 400 (containing 2,4,3',4'-; 2,5,3,3'-;
2,3,4,4'-; and 3,4,3',4'-tetrachlorobiphenyls) in 6/10 females, but
not in male Donryu rats, in 400 days. The dietary exposure was
periodically adjusted according to animal weights and ranged from 38.5
to 616 mg/kg diet. The latter dose level was administered for 275
days. The number of animals used was small (10 treated and 5 control
rats of each sex). Increased incidences of pneumonia, and lung and
intracranial abscesses were found in rats on a diet containing
Kanechlor 400, possibly because of lowered resistance to infection
(Kimura & Baba, 1973).
Ito et al. (1974) fed 10 groups of 29 male Wistar rats with Kanechlor
300, 400, or 500 at dose levels of 100, 500, or 1000 mg/kg diet
(equivalent to 5, 25, and 50 mg/kg body weight, respectively) for
28-52 weeks. Another group received the control diet. A number of
animals died in all groups (4 up to 21 animals); within the treated
groups, deaths were more or less dose related. Adenofibrosis was
observed in the livers of rats fed 1000 mg/kg diet of all 3 mixtures.
Kanechlor 500 produced nodular hyperplasia at all dose-levels and at a
higher incidence than the mixtures with a lower chlorine content. No
neoplastic nodules were observed in the controls. Kanechlor 300 and
500 (100 mg/kg diet) did not show significant growth inhibition or
increases in liver weight. No fibrosis or cholangiofibrosis, bile duct
proliferations, fatty changes, or cellular hypertrophy in the liver
were found. The liver nodular hyperplasia, designated as preneoplastic
by the investigators, was found in 3/25 of the Kanechlor 500
(100 mg/kg diet) treated animals, 1/22 of the Kanechlor 300 (100 mg/kg
diet) treated animals, and 0/18 controls. The 1/22 (4.5% incidence in
the Kanechlor 300, 100 mg/kg diet) is not significant and the 2 higher
dose levels of this product did not induce such changes. It can be
concluded that Kanechlor 300 did not induce neoplasia at dietary
levels of up to 1000 mg/kg over a 52-week period, in this study. At
the 1000 mg/kg level, Kanechlor 300 did produce other evidence of
chronic liver toxicity including oval cell and bile-duct
proliferation, fatty changes, and cellular hypertrophy and, possibly,
cholangiofibrosis (2/15). In the case of Kanechlor 500 (100 mg/kg
diet), essentially the same picture was obtained. In this group, 3/25
cases of nodular hyperplasia were found. In the case of Kanechlor 400
with the dietary levels of 100 and 1000 mg/kg, 2/16 and 3/10 of the
animals had nodular hyperplasia in the liver, respectively.
In a preliminary study on Sherman rats (10 animals of each sex/dose),
Aroclor 1254 at dietary levels of 0, 20, 100, or 500 mg/kg, and
Aroclor 1260 at levels of 0, 20, 100, 500, or 1000 mg/kg, for 8
months, did not give neoplastic nodules or hepatocellular carcinoma.
At 500 mg/kg, Aroclor 1254 produced adenofibrosis in 10/10 male
animals and at 100 and 500 mg/kg, in 7/10 and 9/10 females,
respectively. This change was only seen in 2/10 male and 4/10 female
animals with Aroclor 1260. The authors stated that hepatocellular
adenofibrosis is a persistent progressive lesion that consists of a
marked proliferation of fibrous tissue and epithelial glandular cells
that are well differentiated in mice, but appear atypical in rats
(Kimbrough et al., 1972).
A group of 200 female Sherman rats was given Aroclor 1260 at an
average dietary level of 100 mg/kg (range, 70-107 mg/kg), for 21
months. The PCB intake declined from 11.6 mg/kg per day during the
first week to 6.1 mg/kg at 3 months and to 4.3 mg/kg body weight per
day, later on. The control group also comprised 200 rats. The survival
rate and the food intake were not affected and no treatment-related
signs of toxicity were observed. Body weight gain was decreased from 3
months after the onset of the exposures. Hepatocellular carcinomas
were present in the liver of 26/184 (14%) exposed rats and 1/173
(0.58%) control rats. The livers of most of the remaining exposed rats
(144/184) showed hyperplastic nodules, while none were found in
control rats. A total of 182 exposed rats and 28 control rats had
livers with foci or areas of cytoplasmic alteration. A few livers of
exposed rats showed adenofibrosis. No induction of tumours in other
organs and no metastases from the liver tumours were found (Kimbrough
et al., 1975).
Calandra (1976) reported the findings from several long-term studies,
performed by a commercial laboratory for Monsanto. (These studies have
never been published). In these studies, 1000 rats were divided into
10 groups of 100 animals (50 of each sex) and 9 of the groups were
exposed to Aroclors 1242, 1254, or 1260 at dietary levels of 1, 10, or
100 mg/kg diet. Apparently, 5 animals of each sex were sacrificed at
3, 6, and 12 months with about 35 animals killed at the end of the
2-year studies. In the animals sacrificed early, only one nodular
hyperplasia was observed and it was in the group fed 100 mg Aroclor
1260/kg for 12 months. Mortality in these studies was high and
approximately one-third of the 105 animals anticipated to be exposed
for 2 years at each dietary level died. Hepatomas were observed in
7/25 livers from animals fed 100 mg Aroclor 1260/kg, in 4/26 fed
Aroclor 1254, 3/19 fed Aroclor 1242, and only in 1/168 animals
receiving the 1-10 mg/kg diets. Nodular hyperplasia was twice as
prevalent as hepatomas in the high-dose animals, particularly in the
Aroclor 1254 group (Harbison et al., 1987).
In a limited study, groups of 24 Fischer 344 rats of each sex received
a diet containing Aroclor 1254 at 0, 25, 50, or 100 mg/kg diet
(equivalent to 0, 1.2, 2.5, and 5 mg/kg body weight, respectively) for
104-105 weeks. The survival rate decreased with a dose-related trend
in male, but not in female rats (92, 83, 58, and 46%, respectively).
From 10 weeks of exposure onwards, the body weight gain of all rats,
except that of the low-dose males, decreased. In the groups receiving
the 2 highest dose levels, alopecia, facial oedema, exophthalmos, and
cyanosis were observed. Foci of hepatocellular alterations, which were
dose-related, were found at all dose levels, but not in the controls.
The incidence of non-neoplastic hyperplastic nodules in male rats with
25, 50, or 100 mg/kg diet was 5/24, 8/24, and 12/24 and in female rats
6/24, 9/22, and 17/24, respectively (US EPA, 1980). None was found in
the controls. Hepatocellular adenoma and carcinomas were found in 1/24
males and 1/24 females on the 50 mg/kg diet and in 3/24 males and in
2/24 females on the 100 mg/kg diet. Non-neoplastic liver lesions
included degenerative changes and aggregates of macrophages with
crystalline cytoplasmic structures and pigment granules. An apparently
dose-related increase in the incidence of intestinal metaplasia was
observed in both sexes and 0, 1, 3, and 2 adenocarcinomas located in
the pyloric region of the glandular stomach were found at 0, 25, 50,
and 100 mg/kg diet, respectively. Morgan et al. (1981) and Ward (1985)
reexamined the NCI (1978) data with respect to gastric
adenocarcinomas, hepatocellular adenomas, and carcinomas. Morgan et
al. (1981) found incidences of focal stomach lesions, mostly
metaplasia, of 6, 10, 17, and 35% in rats receiving 0, 25, 50, or
100 mg Aroclor 1254/kg diet, respectively. Adenomas were found in 6
treated rats. When compared with the incidences of stomach
adenocarcinomas in historical controls (1/3548), the incidence of
6/144 was statistically significantly increased (NCI, 1978; Morgan et
al., 1981; Ward, 1985).
Groups of 70 Sprague-Dawley rats of each sex received a diet
containing Aroclor 1260 in corn oil at a concentration of 100 mg/kg
diet (equivalent to 5 mg/kg body weight) for 16 months and 50 mg/kg
diet (equivalent to 2.5 mg/kg body weight) for an additional 8 months.
All surviving rats received a basal diet from month 25 to month 29.
The control group comprising 63 rats of each sex, received the basal
diet with corn oil for 18 months and the basal diet alone for an
additional 5 months. All surviving rats received the basal diet from
the 25th month to the 29th month. Data on growth were not available.
Groups of 2 control or 3 PCB-treated rats of each sex were partially
hepatectomized at 1, 3, 6, 9, 12, 15, and 18 months. At 24 months, a
similar group was sacrificed; after 29 months all remaining animals
were sacrificed. The mortality rate was not affected by the exposure.
In the livers of exposed rats, centrolobular hypertrophy was apparent
at 1 month, foci at 3 months, and areas of cellular alteration after 6
months, neoplastic nodules after 12 months, trabecular carcinomas
after 15 months, and adenocarcinomas after 24 months. Metastases in
the lung were not found. In exposed rats that survived 18 months or
longer, hepatocellular carcinomas were present in 43/47 females and in
2/46 males, but were absent in 81 controls. Simple and cystic
cholangioma at 18 and 23 months, respectively, and adenofibrosis at 22
months were present in the treated rats (Norback & Weltman, 1985).
Rao & Banerji (1988b) fed 3 groups of 32 weanling Wistar rats a
protein diet containing 0 (coconut oil), 50, or 100 mg Aroclor 1260/kg
diet, for 120 days. The incidence of neoplastic nodules in the liver
was 0/32, 24/32 and 16/32, respectively. Adenofibrosis was also
observed in the treated animals.
In order to exclude possible effects of dibenzofurans and to
investigate the effect of the degree of biphenyl-chlorination, groups
of 152 and 144 male Wistar rats were exposed to Clophen A30 and
Clophen A60, respectively, not containing detectable quantities
(detection limit not stated) of dibenzofurans, for 800 days at a dose
of 100 mg/kg diet. A group of 139 rats received a control diet. After
800 days, randomly selected rats were killed daily, until all
survivors had been examined by day 832. The survival rate of the
remaining exposed rats was increased by day 800. In rats autopsied by
day 800 and in rats autopsied later, the incidence of hepatocellular
carcinoma was increased by exposure to Clophen A60 (9/129 and 52/85,
respectively) relative to controls (0/131 and 1/53, respectively), but
not by exposure to Clophen A30 (1/138 and 3/87, respectively). There
was a marked trend from foci of hepatocellular alteration to
neoplastic nodule to carcinoma with increasing time and degree of
biphenyl chlorination. Controls mainly showed foci. Non-neoplastic
liver lesions with increased incidences of bile duct hyperplasia were
found in rats receiving Clophen A30 and A60, and cysts in rats
receiving Clophen A60. The incidence of adenofibrosis in the liver was
decreased, compared with that in the controls, in all exposed rats
that were killed after exposure (Schaeffer et al., 1984).
8.5.2 Tumour promotion/anticarcinogenic effects
(a) Mice
Tatematsu et al. (1979) studied the effects of inducers of liver
microsomal enzymes on the induction of hyperplastic liver nodules by
N-2-fluorenylacetamide (2-FAA) in male F344 rats. The rats were fed
a diet containing 200 mg 2-FAA/kg diet for 2 weeks and then given 500
or 1000 mg Kanechlor 500/kg diet for the following 8 weeks. Partial
hepatectomies were performed at the end of the third week of the
study. Kanechlor 500 in the dose levels applied showed a promoting
effect.
Ito et al. (1973) and Nagasaki et al. (1974, 1975) exposed groups of
20-38 male dd mice to diets containing Kanechlor 400 or 500 at 100 or
250 mg/kg for 24 weeks. Control groups consisted of 20 mice. Combined
exposure to Kanechlor 500 at 100 and 250 mg and either 50, 100, or
250 mg alpha- or ß-BHC (hexachlorocyclohexane)/kg diet enhanced the
development of nodular hyperplasia and hepatocellular carcinomas. A
combination of Kanechlor 500 and gamma-hexachlorocyclohexane did not
produce tumours. Dosing with Kanechlor 500 alone at a dietary level of
100 and 250 mg/kg, and ß- or gamma-hexachlorocyclohexane at dietary
levels of up to 500 mg/kg did not produce tumours. However,
alpha-hexachlorocyclohexane (250 mg/kg diet) produced 10/38
hepatocellular carcinomas and 30/38 hyperplastic nodules.
In another study, both inhibition and promotion were observed
following transplacental and transmammary exposure of mice to PCBs
prior to, or simultaneously with, exposure to dimethylnitrosamine
(DMNA). In this study, Aroclor 1254 was administered intraperitoneally
to female Swiss CD-1 mice on the 19th day of gestation, at a dose of
500 mg/kg body weight. Groups of 17-31 sucklings of these mice and of
controls were then treated intraperitoneally with DMNA on postnatal
day 4 or 14, or remained untreated. The progeny were killed at 28
weeks or at 18 months of age. Aroclor 1254 exposure decreased the
incidence of tumours in the liver and lung, induced by DMNA
administered at postnatal day 14. The average numbers of lung tumours
per mouse were also decreased. However, Aroclor 1254 increased the
liver tumour-bearing mice with extensive DMNA-initiated liver tumours
at 18 months of age, especially when DMNA was administered on
postnatal day 4. Mice exposed only to Aroclor 1254 did not show tumour
incidences higher than those of controls (Anderson et al., 1983). The
authors also reported that 2 higher chlorinated biphenyls,
2,4,5,2',4',5'-hexachlorobiphenyl and 2,3,5,2',3',5'-hexa-
chlorobiphenyl, were the dominant congeners persisting in the tissues
of the PCB-treated mice. It should be noted that only the more highly
chlorinated PCBs (>50% by weight) were reported as promoters of
hepatocarcinogenesis in rodents. The promoting activities of the lower
chlorinated PCBs have not been determined so far.
(b) Rat
As shown in Table 49, administration of PCBs to rats after exposure to
several initiating agents promoted the development of neoplastic
lesions of the liver. In these studies, treatment of the rats with a
control diet of PCBs alone did not usually lead to neoplastic changes
in the liver. Preston et al. (1981) compared the promoting effect of
exposure of rats to Aroclor 1254 with that of exposure to Aroclor 1254
from which the polychlorinated dibenzofuran moieties had been removed,
and did not find any significant differences. The results of this
study suggested that the promoting effect of commercial PCBs cannot be
ascribed entirely to the presence of chlorinated dibenzofurans.
Tatematsu et al. (1979) showed a dose-effect relationship in the
observed promoting effect of Kanechlor 500.
Inhibition, rather than promotion, of liver neoplasms was observed
when female Donryu rats were exposed to Kanechlor 400 preceding, or
simultaneously with, exposure to 3'-methyl-4-dimethylaminoazobenzene
(3'-Me-DAB) (Kimura et al., 1976). Simultaneous dietary exposure of
groups of 16-24 male Sprague-Dawley rats to Kanechlor 500, at a
dose-level of 500 mg/kg (25 mg/kg body weight per day), and to
3'-Me-DAB, or N-2-fluorenylacetamide (2-FAA), or diethylnitrosamine
(DENA), or combinations of these carcinogens, for 20 weeks, showed
almost complete inhibition of neoplastic nodules and hepatocarcinomas
(Makiura et al., 1974). It should be noted that the results of this
study may be influenced by the extreme toxicity, as shown by the
weight records, especially when the substances were combined.
The antitumour activity of Aroclor 1254 was demonstrated in male
Sprague-Dawley rats, inoculated with Walker 256 tumour cells. Groups
of 16 rats received PCB doses of 50, 100, or 200 mg/kg body weight for
2 weeks, once in 2 days, starting on the day of tumour cell injection.
A group of 16 rats did not receive PCBs. The inhibition of tumour
growth and transplantability were dose-related (Kerkvliet & Kimeldorf,
1977). The antitumour activity of Phenoclor DP5 was observed in groups
of 20 female Swiss mice inoculated with Ehrlich's tumoral ascites
liquid, after receiving PCBs in the diet at levels of 0, 10, 50, or
250 mg/kg, for 120 days (Keck, 1982).
Nishizumi (1980) showed that placentally transferred PCBs inhibited
diethylnitrosamine (DENA)-induced liver tumours. Groups of ten,
10-week-old female Wistar rats were treated with 40 or 200 mg
Kanechlor 500/kg body weight, by gavage, on days 5, 10, and 15 of
gestation. One F1 offspring from each litter was killed for
quantification of liver PCBs. The remaining F1 offspring were exposed
to 50 mg DENA/litre drinking-water, continuously for 5 weeks. At 16,
20, and 24 weeks after the beginning of the DENA exposure, 6-8 rats of
Table 49. Promotion of liver neoplasms in rats exposed to PCBs after treatment with carcinogenic substances
Strain Sex Group size Carcinogenic PCBs Doseb Exposure Neoplasms Reference
substancesa (mg/kg diet) period promoted by
(weeks) PCBs
Donryu female 25 3'-Me-DAB Kanechlor 400 400 26 carcinoma Kimura et al.
(1976)
Wistar male 20-24 DENA Kanechlor 500 2 × 15 4 nodules Nishizumi (1976)
mg/week carcinoma
F 344 male 15-16 2-FAA Kanechlor 500 500 and 8 hyperplasia Tatematsu et al.
1000 nodules (1979)
F 344 male 20 EHEN Kanechlor 500 32 carcinoma Hirose et al.
(1981)
Sprague- male 40 DENA Aroclor 1254c 100 18 carcinoma Preston et al.
Dawley (1981)
a 3'-Me-DAB = 3'-methyl-4-dimethylaminoazobenzene; DENA = diethylnitrosamine; 2-FAA = N-2-fluorenylacetamide;
EHEN = N-ethyl-N-hydroxyethylnitrosamine.
b Unless otherwise specified.
c With and without PCDFs.
each sex from each treatment group were killed and examined
histologically. A significant reduction in tumour incidence occurred
only in male offspring in the 200-mg group. The liver-PCB values in
28-day-old F1 mice were < 1, 18 ± 7, and 360 ± 30 mg/kg tissue for
the controls, 40, and 200 mg/kg groups, respectively. It was suggested
that placental transfer of PCBs protected the treated rats from
DENA-induced liver tumours.
The inhibitory effect of PCBs on tumour initiators following
simultaneous exposure has been explained by an enhanced metabolism of
the initiator by PCB-induced mixed function oxygenase.
Tests for putatively preneoplastic enzyme-altered foci in the liver of
rats have shown a dose-related increase in the promotion of such foci
by intraperitoneal exposure to Aroclor 1254 in tricaprylin or by oral
exposure to Clophen A50 in olive oil, following oral administration of
diethylnitrosamine (Deml & Oesterle, 1982; Pereira et al., 1982;
Oesterle & Deml, 1983).
The highest oral dose of Clophen A50 not enhancing the number and area
of enzyme-altered loci in female Sprague-Dawley rats, treated for 11
weeks with doses of 0, 0.1, 0.5, 1, 5, or 10 mg/kg body weight after
initiation by a single dose of 8 mg/kg body weight of
diethylnitrosamine, was 0.5 mg/kg body weight (Deml & Oesterle, 1987).
8.5.3 Initiation, promotion, and other special studies on individual
congeners
2,5,2',5'-Tetrachlorobiphenyl and its metabolite 2,5,2',5'-
tetrachlorobiphenyl-3,4-oxide were tested in a pulmonary tumour
induction assay with intraperitoneally injected A/T mice of both
sexes, and in a two-stage skin carcinogenicity assay with
dermally-exposed female SENCAR mice. Pulmonary adenomas and skin
papillomas were not induced (Preston et al., 1985).
Female Harlan-Sprague-Dawley rats received 2,4,2',4'-
tetrachlorobiphenyl or 2,5,2',5'-tetrachlorobiphenyl at 100 mg/kg
diet for 28 weeks, 1 week after oral exposure to diethylnitrosamine
(DENA). Both congeners showed a promoting effect on the development of
foci of hepatocellular alteration. The effect was approximately
10-fold greater in rats receiving 2,4,2',4'-tetrachlorobiphenyl
(Preston et al., 1985).
The effects of PCB mixtures and selected congeners have also been
investigated by Hayes et al. (1985, 1986) using the resistant
hepatocyte model developed by Farber and coworkers (Solt & Farber,
1976; Tsuda et al., 1980; Farber, 1984a,b, 1986). The ability of
2,4,2',4' and 2,5,2',5'-tetrachlorobiphenyl, 2,4,5,2',4',5'-
hexachlorobiphenyl, and a mixture of PCB-congeners (the composition of
which resembled that ascertained in human breast milk) to initiate
enzyme-altered hepatocellular nodules was investigated in
proliferating hepatocytes of neonatal or partially hepatectomized
adult rats (the PCB-congeners did not contain detectable levels of
dibenzofurans or dioxins). Neonatal rats were exposed 3 times in 3
weeks and adult rats once. After several weeks, the rats received a
selection regimen of 2-acetylaminofluorene followed by partial
hepatectomy (neonates) or necrotizing carbon tetrachloride (adults).
None of the PCB exposures generated nodules in contrast to known
initiators (Hayes et al., 1985).
Subsequent studies by Hayes et al. (1986) using the afore-mentioned
compounds and 3,4,3',4'-tetrachlorobiphenyl (a typical MC-type inducer
of cytochrome P-450-dependent monooxygenases) showed that these PCBs
(50 µmol/kg body weight), given 10 days after a dose of the initiator,
DENA, and 7 days before 2-AAF, all reduced the size of the
2-AAF-selected gamma-glutamyltranspeptidase-positive nodules. These
results show that, in contrast to the previous studies, PCBs also
exhibit "anti-promoting" activities in this Farber-model, which
utilizes 2-AAF as a mito-inhibitory toxin.
8.5.4 Skin carcinogenicity
Aroclor 1254 (100 µg/mouse) administered 18 h prior to the initiator,
7,12-dimethylbenz (a)anthracene (DMBA), significantly decreased the
incidence of papilloma formation in female Charles-River CD-1 mice.
2,4,5,2',4',5'-Hexachlorobiphenyl (625 µg/mouse), a PCB congener that
resembles phenobarbital in its mode of induction of drug-metabolizing
enzymes, did not act as an anticarcinogen, whereas 3,4,3',4'-
tetrachlorobiphenyl was more active than Aroclor 1254 as an
inhibitor. 3,4,3',4'-Tetrachlorobiphenyl resembled methylcholanthrene
in their mode of cytochrome P-450 induction (Parkinson et al., 1983)
and decreased the number of DMBA-initiated papillomas/mouse. Although
the PCB treatment did not modulate the incidence of papillomas caused
by benzo (a)pyrene, it was suggested that the anticarcinogenic
effects of PCBs on mouse skin tumours initiated by DMBA were due to
altered metabolism and DNA binding of the carcinogen by the
PCB-induced skin monooxygenases (DiGiovanni et al., 1979).
Berry et al. (1978, 1979) studied the tumour-promoting activity of
Aroclor 1254 in groups of 30 female CD-1 mice initiated with
0.2 mmol/litre (equivalent to 51 µg) DMBA. One week later, a positive
control group received 2 µg tetradecanoylphorbolacetate (TPA) and an
experimental group received 100 µg Aroclor 1254 in acetone. The TPA
and Aroclor applications were made twice weekly for 30 weeks. The TPA
promotion resulted in 92% of the animals developing papillomas, while
none developed in the Aroclor-treated animals. It was concluded that
Aroclor 1254 was not a skin tumour promoter at the dose used in this
study. Inhibition of skin papilloma was observed when Aroclor 1254 was
administered 18 to 72 h prior to DMBA treatment and promotion by TPA.
Poland et al. (1982) used HRS/J hairless mice to study the tumour
promotion activity of Aroclor 1254 in combination with N-methyl-
N'-nitro- N-nitrosoguanidine (MNNG). Twenty female mice per group
received a single administration of MNNG (5 micromol in acetone) or
acetone alone applied on the skin, and were then treated topically,
twice weekly, with 1 mg Aroclor/mouse, dissolved in acetone, for 20
weeks. No tumours were induced in the control group; in the
MNNG-treated mice, 4/19 had papillomas. It was concluded that Aroclor
had a weak promoting effect.
8.5.5 Appraisal
In summarizing the potential carcinogenic activity of PCBs, it is
perhaps more informative to express it in terms of what is known about
the mechanisms of chemical carcinogenicity (Hayes, 1987). In other
words, what the evidence is to support the carcinogenicity of PCBs
through genotoxic/initiating, cocarcinogenic, promotional/
antipromotional, and progressional activities. There is no evidence to
support genotoxic activity for PCBs and, in in vitro studies,
evidence for initiating activity through direct interactions with DNA
is weak. Poor initiating activity is consistent with the demonstrated
lack of mutagenic activity in various short-term tests. There is
evidence to suggest that PCBs can potentiate the activity of known
carcinogens (or act as cocarcinogens) in in vitro systems, but the
opposite result is often seen in in vivo studies, suggesting that
certain protecting enzyme systems present in the intact systems are
absent in the in vitro systems. There is a substantial body of
evidence to support the promotional activity of PCBs, particularly the
more highly chlorinated ones, in rodent liver, and this activity may
depend on the sequence in which the chemicals are administered in
experimental animal studies. In addition, promotional activity is
correlated closely, but not consistently, with the induction of MFO
activity. The hyperplastic effect (stimulation of cell proliferation)
of PCB inducers could promote preneoplastic growth. This type of
activity may possibly involve a threshold, suggesting that it may not
be a factor in low-level exposures to PCBs. The anticarcinogenic
activity of PCBs may also depend on the sequence of events in
experimental animal studies, and it may be related more to the
antipromotional properties of PCBs, possibly functioning as
protectants of mito-inhibitory toxicity. The interpretation of the
available animal data involving the commercial PCB mixtures is often
complicated by lack of information concerning the presence and
contribution of chlorinated dibenzofuran impurities, as well as
variations in congener composition to toxicity. In structure-activity
terms, a key factor in determining promotional activity appears to be
the degree of chlorination, which may reflect increased resistance to
metabolism and elimination and possibly also higher degrees of
ortho-substitution among the congeners present. Ortho-substituted
PCBs (possibly acting as persistent PB type inducers) have been shown
to be effective tumour promoters, and, at least in the case of the
closely related PBBs, it has been shown that a non-toxic and
non-promoting dose of a non- ortho-substituted congener in
combination with a promoting dose of a highly ortho-substituted
congener has a synergistic effect. This result may explain why the
mixtures can have greater promoting ability than the individual
congeners involved. This result might also suggest multiple pathways
for promoting activity, possibly involving the Ah receptor as well as
the putative receptor for phenobarbital. The possibility that PCBs
might promote carcinogenesis in tissues, other than liver, in animals
exposed to various tissue-specific, initiating agents, needs to be
addressed. Nevertheless, the potential for human liver cancer from
exposure to PCBs cannot be reliably predicted from animal studies.
Overall, there is reason to exercise caution in extrapolating the
available animal data on the carcinogenic potential of PCBs for
humans.
8.6 Special studies: target-organ effects
The lesions commonly introduced in animals after acute, short-term, or
long-term administration/application of PCB mixtures and/or individual
congeners concern the liver, skin, immune system, reproductive system,
oedema at various sites, as well as disturbances of the
gastrointestinal tract and the thyroid gland.
8.6.1 Liver
8.6.1.1 PCB mixtures
The toxic effects of PCBs result both directly and indirectly from
their presence in certain organs, such as the liver, where they
induce, in various degrees, a variety of liver enzymes. Some of these
enzymes are active in the metabolism of the PCBs themselves, while
others involve activation, deactivation, detoxication, etc., of other
compounds.
In itself, induction of enzymes by xenobiotics does not represent a
toxic manifestation, rather it is the ordinary response to such
foreign chemicals, which results generally in their detoxication and
ultimate modification, enabling them to be excreted from the organism.
In this sense, the response of the liver to such compounds is a
biological protective mechanism. Since some compounds, including PCBs,
are capable of inducing not only enzymes that result in their own
detoxication, but others as well, and the level of enzyme induction
may be high enough to cause liver pathology, a line cannot be drawn
that clearly separates a normal biological function from a toxic
manifestation. Superimposed on this situation is the direct toxic
action of the compounds on liver tissue, because of the properties of
the parent compound or its metabolites.
An enlarged liver and increased absolute and relative liver weights
are commonly reported as gross effects of PCB administration. The
lowest-observed-effect levels, in studies on different rat strains,
exposed to a diet containing Aroclor 1254, for a (dose-related)
increase in relative liver weights, vary between 20 and 100 mg/kg diet
(equivalent to 1 and 5 mg/kg body weight, respectively) (Kimbrough et
al., 1972; Bruckner et al., 1974; Burse et al., 1974; Grant et al.,
1974; Allen et al., 1976; Zinkl, 1977; Hinton et al., 1978; Kasza et
al., 1978b; Jonsson et al., 1981; Baumann et al., 1983).
The liver hypertrophy is microscopically visible as enlarged,
pleiomorphic hepatocytes, sometimes multinucleated or with enlarged
nuclei. A liver alteration observed by many investigators after
exposure of rats to various PCB-mixtures is fatty degeneration,
characterized by fat vacuolation and/or a foamy appearance of the
cytoplasm. Ultrastructurally, an increase in the number and size of
cytoplasmic lipid droplets and liposomes (membrane-associated lipid
droplets) can be observed. Fatty degeneration of the liver was already
observed after exposure of male Sprague-Dawley rats to a diet
containing 5 mg Aroclor 1242/kg diet (equivalent to 0.25 mg/kg body
weight) for 2-6 months (Bruckner et al., 1974) and after exposure of
male Holtzman rats to a diet containing 5 mg Aroclor 1254/kg for 5
weeks (Kasza et al., 1978b). Chu et al. (1977) found a comparable
effect on the liver with >20 mg Aroclor 1254 or 1260/kg diet for 28
days. Another characteristic change is the appearance of eosinophilic,
lamellar, cytoplasmic inclusions (Kimbrough et al., 1972; Kasza et
al., 1978b) or "hyaline-like material" (Grant et al., 1974). Electron
microscopy revealed that these changes corresponded to concentric
laminated membranes of smooth endoplasmic reticulum ("whorls"; "myelin
figures") (Vos & Beems, 1971; Kimbrough et al., 1972; Allen et al.,
1976; Kasza et al., 1978b; Jonsson et al., 1981). Proliferation of the
smooth endoplasmic reticulum and a decrease in rough endoplasmic
reticulum are commonly observed in rats exposed to dietary levels of
PCB mixtures that also induce fatty degeneration. Kasza et al. (1978b)
further observed a marked proliferation of Golgi condensing vesicles
containing lipoprotein in the livers of male Holtzman rats fed Aroclor
1254, for 5 weeks, at 5 mg/kg diet. A decreased number of these
vesicles was seen at 50 and 500 mg/kg diet (equivalent to 2.5 and
25 mg/kg body weight, respectively), together with a marked increase
in the smooth endoplasmic reticulum and lysosomes. The above decrease
in Golgi vesicles was also observed in Sprague-Dawley rats by Hinton
et al. (1978). The proliferative changes of the endoplasmic reticulum
are closely related to the observed induction of microsomal enzymes,
as discussed in section 8.6.1.2. Atypical mitochondria (Burse et al.,
1974; Kasza et al., 1978a,b), and single cell and focal necrosis
(Grant et al., 1974; Allen et al., 1976; Jonsson et al., 1981; Baumann
et al., 1983) have also been described.
Hypobilirubinaemia was produced in rats by Bastomsky et al. (1975),
who investigated the mechanism by administering daily intraperitoneal
injections of Aroclor 1254 (25 mg/kg body weight, in corn oil) to
female rats for 4 days, before measuring bilirubin glucuronide
formation by hepatic microsomes in vitro. PCB treatment was not
effective in increasing UDP-glucuronosyltransferase (EC 2.4.1.7)
activity. Serum bilirubin levels in Gunn rats were also significantly
decreased by PCB treatment; the rats are genetically deficient in
UDP-glucuronosyltransferase (2.4.1.7) activity.
The fluorescing of livers on exposure to UV radiation, consistent with
the presence of porphyrin, and accumulation of brown pigment, positive
for iron, especially in Kupffer cells and perivascular macrophages
have also been reported (Kimbrough et al., 1972; Burse et al., 1974;
Zinkl, 1977; Jonsson et al., 1981). The changes described in the
livers of mice (Nishizumi, 1970) and rabbits (Koller & Zinkl, 1973),
following exposure to PCB mixtures, are comparable with those in rats.
8.6.1.2 Individual congeners
The effects of chlorination and the chemical composition of PCBs, with
regard to the dose-effects relations in liver toxicity after
short-term exposure, are indicated by the data of Biocca et al.
(1981). In this study, hepatotoxic effects were observed in mice after
5 weeks of maintenance on diets containing 0.3 mg of 3,4,5,3',4',5'-
hexachlorobiphenyl, while similar effects were observed only after
30 mg of 2,4,5,2',4',5'-hexachlorobiphenyl and 100 mg of 2,4,6,2',4',6'-
hexachlorobiphenyl/kg diet. No effects were found with 300 mg of
2,3,6,2',3',6'-hexachlorobiphenyl/kg diet. Similar dependence of liver
toxicity on the chemical composition of the PCB mixture would be
anticipated following long-term exposure in mice and other species.
8.6.2 Enzyme induction
8.6.2.1 Effects on liver enzymes of PCBs
Proliferation of the smooth endoplasmic reticulum is a common
observation in the liver cells of experimental animals following
exposure to PCB mixtures. This effect is accompanied by an increase in
microsomal protein and the induction of cytochrome P-450, cytochrome
P-448, and drug-metabolizing enzymes, including the microsomal
monooxygenases (EC 1.14.14.1), epoxide hydrolases (EC 3.3.2.3),
UDP-glucuronosyltransferases (EC 2.4.1.17), NADPH-cytochrome c
reductase (EC 1.6.2.4) and esterases (EC 3.1.1.1), and the cytosolic
glutathione S-transferase (EC 2.5.1.18). The subject has been
reviewed by Safe (1984).
The spectral, enzymatic, and electrophoretic properties of the
microsomal enzymes, induced by Aroclor 1248, 1254, 1260, and Kanechlor
400, are consistent with the inducing properties of both the
phenobarbital (PB) and 3-methylcholanthrene (MC) classes of inducers.
They induce both cytochromes P-450 and P-448 and associated enzymes
(Alvares & Kappas, 1977; Goldstein et al., 1977; Yoshimura et al.,
1978; Iverson et al., 1982; Lashneva & Tutelyan, 1984; Khan et al.,
1985; Tutelyan et al., 1986). Aroclor 1016, administered to
Sprague-Dawley rats at 50 mg/kg per day for 4 days, intraperitoneally,
elicited a barbiturate type of inducing effect on the hepatic
microsomal oxidative enzyme system. Aroclor 1016 caused increases in
liver cytochrome P-450 content, microsomal protein, and its
ethylmorphine N-demethylase activity. It did not induce cytochrome
P-448 in liver microsomes.
A dose-related induction of hepatic and, in some cases, extrahepatic
microsomal enzymes was observed in several animal species including
the rat, rabbit, mouse, ferret, guinea-pig, hamster (Safe, 1984), mink
(Shull et al., 1982; Aulerich et al., 1985) and monkey (Iverson et
al., 1982). Distinct interspecies variations have been demonstrated.
For example, while 6 daily intraperitoneal doses of 25 mg of Aroclor
1254/kg body weight caused a potent induction of benzo (a)pyrene
hydroxylase in adult, male Sprague-Dawley rats, no, or a minimal,
induction of this monooxygenase was observed in adult, male Swiss mice
after 4 daily intraperitoneal doses of 50 mg/kg body weight and in
male New Zealand rabbits after 2 intraperitoneal doses of 100 mg/kg
body weight on days 1 and 4 (Alvares et al., 1982). Furthermore, when
comparing the inducing potency of Aroclor 1242 in the mink and the
genetically related ferret, Shull et al. (1982) measured a greater
induction of cytochrome P-448 and MC-type monooxygenases and no toxic
effects in the ferret at a dosing regime that resulted in toxic
effects in the mink (100 mg/kg body weight on day 1,200 mg/kg body
weight on day 5, sacrifice on day 10). The authors considered the
observed induction moderate in both species compared with that
observed in the rat. Earlier, it had been found that male
Sprague-Dawley rats were indeed more sensitive than ferrets with
respect to the inducing effect of Aroclor 1254 following a single
intraperitoneal dose of 500 mg/kg body weight, though the responses of
both species were comparable qualitatively (Lake et al., 1979).
Moreover, pretreatment of rats with Aroclor 1254 resulted in the
induction of microsomal cytochromes P-450 c, P-450 d (MC-inducible),
P-450 b and P-450 e (PB-inducible) (Ryan et al., 1979a,b, 1982). In
general, the extent of induction of microsomal enzymes by PCB-mixtures
increased with increasing chlorine content up to 54%. The effect has
also been demonstrated with single pure PCBs administered orally
(Ecobichon & Comeau, 1975). The results are summarized in Table 50 and
show a greater degree of enzyme induction with the higher chlorinated
compounds (see section 8.6.1.2).
Table 50. Stimulation of microsomal enzyme activity by single chlorinated biphenylsa
Hepatic microsomal enzyme activity
Chlorine O-Demethylation N-Demethylation Aniline Nitro-
substituents hydroxylation reduction
4 0 0 0 0
2,2' 0 + + 0
2,4' 0 0 + 0
4,4' + + + + + + +
2,5,2',5' 0 + + + +
2,4,2',4' + + + + + + 0
2,4,5,2',4',5' + + + + + + + +
2,3,5,2',3',5' + + + + + + + +
2,4,6,2',4',6' + + + + + + + +
2,3,4,5,2',3',4',5' + + + + + + + +
a From: Johnstone et al. (1974).
0 = No activity.
+ = Slight activity.
+ + = Marked activity.
Litterst et al. (1972) exposed groups of 6 male Osborn-Mendel rats to
Aroclors 1242, 1248, 1254, or 1260 in the diet, at concentrations of
0, 0.5, 5.0, 50, or 500 mg/kg diet, for 4 weeks. Increased microsomal
nitroreductase and demethylase activities occurred at 0.5 mg/kg or
more, increased pentobarbital hydroxylation and increased relative
liver weight occurred at 5.0 mg/kg or more, and increased liver
triglycerides occurred at 50 mg/kg diet. An inducing activity similar
to, or lower than, that of Aroclor 1254 has often been found for more
chlorinated mixtures (Villeneuve et al., 1971a, 1972; Bickers et al.,
1972; Chen & Dubois, 1973; Ecobichon & Comeau, 1974; Schmoldt et al.,
1974; Sawyer et al., 1984). Aroclor 1016 and 1242, both containing 42%
chlorine but differing in congener composition, showed qualitative and
quantitative differences in inducing effects. For example, Aroclor
1254 enhanced ethylmorphine N-demethylase activity 3-fold, while the
maximum increase produced by Aroclor 1016 was only 40%. The 2 Aroclors
also differed in their induction of the various forms of cytochrome
P-450 (Alvares et al., 1982). Adult, male Sprague-Dawley rats were
administered, intraperitoneally, a dosage of 0 or 50 mg Aroclor 1016
in corn oil/kg body weight, for 4 days. Aroclor 1016 was a potent
inducer of N-methylase but a poor inducer of benzo (a)pyrene
hydroxylase. Administration of 100 mg Aroclor 1016 in corn oil/kg body
weight per day to adult male New Zealand White rabbits, for 4 days,
resulted in an increase in liver cytochrome P-450 activity and
decreases in benzphetamine- N-demethylase and benzo (a)pyrene
hydroxylase activities compared with the controls. 7-Ethoxycoumarine-
O-deethylase and 7-ethoxyresorufin- O-deethylase activities were
comparable with those in the controls (Ueng & Alvares, 1985).
Therefore, it can be concluded that the degree and type of induction
not only depends on the chlorine content of the mixture, but is also a
function of the congener composition, as will be discussed further in
the next section.
Not only species specificity, but also marked tissue specificity has
been observed. While Aroclor 1254 was found to be a potent inducer of
cytochrome P-450 content and benzo (a)pyrene hydroxylase activity in
the rat lung and liver (Alvares & Kappas, 1977), it caused a 46%
decrease in cytochrome P-450 content, a 31% decrease in
benzo (a)pyrene hydroxylase activity, and a 61% decrease in
ethylmorphine N-demethylase activity in the rabbit lung. Aroclor
1254 caused induction of cytochrome P-450 and both enzymes in the
kidneys of these rabbits, but benzo (a)pyrene hydroxylase was not
induced in the liver (Alvares et al., 1982).
The inducing effect of PCB mixtures on the monooxygenase system has
been observed in the livers of both male and female rats (Chen &
Dubois, 1973; Grant & Phillips, 1974), minks and ferrets (Lake et al.,
1979; Shull et al., 1982), in the livers of pregnant rats (Alvares,
1977) and rabbits (Villeneuve et al., 1971a), in the placenta of rats
(Alvares & Kappas, 1975), in fetal and neonatal rat livers (Alvares &
Kappas, 1975; Baker et al., 1977; Inoue et al., 1981; Jannetti &
Anderson, 1981; Lashneva et al., 1987), in immature rat livers (Chen &
Du Bois, 1973; Narbonne, 1980), and in mature and senescent rat livers
(Birnbaum & Baird, 1978).
The lowest-observed-adverse-effect levels and the no-observed-effect
levels for enzyme induction, found in short-term diet studies on rats,
are presented in Table 51.
Bruckner et al. (1977) showed that when Aroclor 1254 was administered
in the diet, at a level equivalent to 0.25 mg/kg body weight,
microsomal enzyme activity was induced after 1 day of exposure.
Narbonne (1980) found a significant induction with 0.1 mg Phenoclor
DP6/kg body weight, given in the diet, after 3-5 days.
After a few weeks of exposure to Aroclor 1254 or 1260, at low dietary
levels of between 0.25 and 1.25 mg/kg body weight, a plateau in
microsomal enzyme activity was reached that was maintained over
several months of exposure (Chen & Du Bois, 1973; Grant et al., 1974;
Bruckner et al., 1977). Following short-term exposure, dietary levels
of between 5 and 25 mg/kg may stimulate microsomal enzymes for up to 4
months (Grant et al., 1974; Bruckner et al., 1977). Levels of 0.05 or
0.1 mg/kg body weight failed to produce any effects or the periods of
induction at these levels were long (Grant et al., 1974; Chen & Du
Bois, 1973).
A marked induction of the liver monooxygenase system was observed in
the offspring of female rats given a single oral dose of a mixture of
PCBs (Sovol) (500 mg/kg body weight) on the 14th day of pregnancy. In
one-day-old rats, an increase in cytochrome P-450 content associated
with an increase in the cytochrome b 5 level, an increase in NADPH-
cytochrome c reductase activity, an increased rate of aminopyrine-
N-demethylation activity in microsomes and also increased
3,4-benzo (a)pyrene hydroxylation, 7-ethoxycoumarin O-deethylation,
and NADPH-dependent lipid peroxidation were found. The activity of the
cytochrome P-450 system in the young rats remained elevated during the
early postnatal period (Lashneva et al., 1987).
8.6.2.2 Effects on liver enzymes of "biologically filtered" PCB
mixtures
Young Sprague-Dawley rats were administered a total of 38 oral doses
of a PCB mixture in olive oil at 0, 0.25, 1.0, 4.0, 16.0, 64.0, 256,
or 1025 µg/kg body weight, twice a day, over 1 month; the mixture
contained 55% chlorine and had a gas-chromatographic profile very
similar to that of the congeners found in the breast milk of Japanese
women. A dose-related induction of liver aminopyrine demethylase and
benzo (a)pyrene hydroxylase (EC 1.14.14.1) was found with doses of
1.0 µg/kg body weight or more. PCB-binding to liver microsomes was
increased at doses of 4.0 µg/kg body weight or more (Shimada & Ugawa,
1978). In another study, 1-month-old, male Wistar rats received
(intraperitoneally) doses of 0, 1, 10, 25, 50, or 100 mg/kg body
weight of a reconstituted PCB-mixture in corn oil, containing average
levels of 13 of the major congeners found in the breast milk of
Japanese women (purity > 98.5%). Each dose was administered in 2
portions on days 1 and 3. The same dose regimen of Kanechlor 500 was
also tested. The rats were killed on day 6. The reconstituted PCB
mixture and Kanechlor 500 caused dose-related increases in liver
Table 51. Microsomal enzyme induction by PCB-mixtures in rats
PCB-mixture Rat strain Sex Exposure No-effect-level Lowest-observed- Reference
male/ period mg/kg body adverse effect level,
female weight mg/kg body weight
Aroclor 1016 Sprague-Dawley male 3 weeksa 0.1 1 Iverson et al. (1975)
Aroclor 1242 Sprague-Dawley male 3 weeksa 0.1 1 Iverson et al. (1975)
Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Sprague-Dawley male 2-6 months < 0.25 0.25 Bruckner et al.
(1974, 1977)
Aroclor 1248 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Aroclor 1254 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Wistar male 2-8 months < 0.1 0.1c Grant et al. (1974)
Wistar male 2 weeks 0.25 0.5 Den Tonkelaar &
Wistar male 12 weeks 0.05 0.5 van Esch (1974)
Sprague-Dawley male 0-20 weeks 0.05 0.25 Turner & Green (1974)
Holtzman male 3 weeks < 0.25 0.25 Bruckner et al. (1977)
Garthoff et al. (1977)
Table 51. (cont'd).
PCB-mixture Rat strain Sex Exposure No-effect-level Lowest-observed- Reference
male/ period mg/kg body adverse effect level,
female weight mg/kg body weight
Aroclor 1260 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Holtzman male 1-13 weeks < 0.05 0.05 Chen & DuBois (1973)
female 1-13 weeks 0.05 0.25
Phenoclor DP6 Sprague- male 3 days < 0.1 0.1 Narbonne (1979, 1980)
Dawleyb
a Daily dosing by gavage; the other studies are all diet studies.
b Immature rats (60-65 g).
c Significant effect after months 4 and 6, but not after months 2 and 8.
benzo (a)pyrene hydroxylase activity that were 3.5 and 2.2 times the
control value, respectively, at 1 mg/kg body weight. The ED50 of the
reconstituted breast milk PCB mixture for the induction of rat hepatic
microsomal aryl hydroxylase (AHH) was 7 times lower than that of
Kanechlor 500. The authors concluded that the increased potency of the
breast milk-PCB mixture reflected the preferential bioconcentration of
the relatively toxic congeners 2,4,5,3',4'-penta-, 2,3,4,3',4'-penta-,
and 2,3,4,5,3',4'-hexachlorobiphenyl (Parkinson, et al., 1980b). When
Gyorkos et al. (1985) repeated the studies, the reconstituted mixture
was inactive at the lowest dose level but exhibited mixed-type
microsomal enzyme induction characteristics at the higher dose levels.
Increases in the activities of several hepatic microsomal
monooxygenases, including dimethylaminoantipyrine N-demethylase,
aldrin epoxidase, benzo (a)pyrene hydroxylase and
ethoxyresorufin- O-deethylase were found.
8.6.2.3 Effects of individual congeners on liver enzymes
The enzyme-inducing potencies of individual PCB congeners have been
studied extensively and reviews have been published (Goldstein, 1980;
Safe, 1984; Safe et al., 1985b), in which the following
structure-activity relationships are proposed. The most active
congeners with respect to the induction of aryl hydrocarbon
hydroxylase (and toxicity), 3,4,5,4'-tetrachloro-, 3,4,3',4'-
tetrachloro-, 3,4,5,3',4'-pentachloro-, and 3,4,5,3',4',5'-
hexachlorobiphenyl, are substituted at both para positions, at 2 or
more meta positions, but not at ortho positions. These congeners
can assume coplanar conformations and are approximate stereoisomers of
2,3,7,8-tetrachlorodibenzo- para-dioxin. They resemble
3-methylcholanthrene and 2,3,7,8-tetrachlorodibenzo- para-dioxin in
their mode of hepatic enzyme induction, inducing hepatic microsomal
benzo (a)pyrene hydroxylase, ethoxyresorufin- O-deethylase, and the
cytochromes P-450 a, P-450 c, and P-450 d.
These congeners are only present as trace compounds in commercial PCB
mixtures, but appear in significant quantities in breast milk (Noren
et al., 1990).
The least active of these 4 coplanar congeners, 3,4,5,4'-
tetrachlorobiphenyl, also shows a phenobarbital type of hepatic
microsomal enzyme induction, inducing dimethylaminoantipyrine,
ethylmorphine and related N-dealkylases, biphenyl-4-hydroxylase,
aldrin epoxidase, several O-dealkylases, and the cytochromes P-450 a,
P-450 b, and P-450 e. This "mixed-type" induction pattern is also
shown by 3,4,4'-trichlorobiphenyl, and by all the mono- ortho, and at
least 7 di- ortho, substituted analogues of the coplanar PCB
congeners. Several of these congeners, e.g., 2,4,5,3',4'-penta,
2,3,3',4,4'-penta, 2,3,4,5,3',4'-hexa-, and 2,3,4,5,2',4'-
hexachlorobiphenyl are components of commercial PCB mixtures and have
been identified in breast milk.
Studies have revealed that 4,4'-dichlorobiphenyl, with no meta
substituents exhibits a PB-type induction pattern in rats. Adding
meta substituents as in 3,4,4'-tri-, and 3,4,5,4'-tetrachloro-
biphenyl will give a mixed PB- and 3-MC-type induction pattern. While
3,4,3',4'-tetrachlorobiphenyl is a potent inducer of microsomal
hepatic aryl hydrocarbon hydroxylase (AHH), it did not significantly
increase the activities of benzo (a)pyrene-hydroxylase, [3H]-4-
chlorobiphenyl-hydroxylase, and ethoxyresofurin- O-deethylase (EROD)
at a dose level of 10 µmol/kg (Andres et al., 1983).
Most other PCB congeners are phenobarbital-type inducers or are
inactive. In general, the more highly chlorinated of these congeners
are more active inducers than the lower chlorinated ones, probably
reflecting the relative half-lives of these compounds. The
non-availability of 2 adjacent unhalogenated carbon atoms and
para-substitution are 2 factors that decrease the degradability of
the congeners and increase their inducing activity.
Various congeners were tested for their inducing activity in
responsive C57BL/6J mice, i.e., mice containing the Ah receptor
protein, and in non-responsive DBA/2J mice, lacking this receptor,
after single intraperitoneal (Robertson et al., 1984; Silkworth et
al., 1984) or oral (Kohli et al., 1980) doses in corn oil and
cottonseed oil, respectively. The coplanar PCBs and their mono-ortho
substituted analogues all induced benzo (a)pyrene hydroxylase or
ethoxyresorufin deethylase in responsive mice, but not, or only to a
minor degree, in non-responsive mice. Most tested mono-ortho
substituted analogues of coplanar PCBs slightly induced aminopyrine
N-demethylase in both strains, while the coplanar congeners did not
induce this enzyme.
The above structure-activity relationships were confirmed in a few
limited studies on monkeys. Hepatic aryl hydrocarbon hydroxylase was
induced in 3 young, male Rhesus monkeys after a single oral dose of
1 mg of 3,4,3',4'-tetrachlorobiphenyl/kg body weight and in 3 young
male monkeys during continued feeding of a diet containing 0.5 mg of
3,4,5,3',4',5'-hexachlorobiphenyl/kg (McNulty, 1985). A single oral
dose of 18 mg of 2,5,2',5',-tetrachlorobiphenyl/kg body weight,
administered to male Rhesus monkeys, in corn oil, elevated hepatic
cytochrome P-450 levels, while no change was noted in the activities
of several microsomal enzymes (Allen et al., 1975b). Mono- ortho or
di- ortho substituted analogues of coplanar PCB congeners have not
been tested in monkeys.
Vodicnik et al. (1980) studied the effect of 2,4,5,2',4',5'-
hexa-chlorobiphenyl on hepatic microsomal monooxygenase activity in
virgin or pregnant and lactating Sprague-Dawley mice and their
offspring. A single intraperitoneal dose of 100 mg/kg body weight, was
administered 14 days before mating. Prior to, and during early
pregnancy, hepatic monooxygenase activity in pretreated mice was
greater than that in the controls. No differences were found between
pregnant and virgin mice. Mothers, pretreated with the
hexachlorobiphenyl and sacrificed on the day of birth, had lower
microsomal monooxygenase activity and cytochrome P-450 content than
PCB-pretreated virgins sacrificed concurrently. No differences were
noted between these groups of animals during lactation. Hepatic enzyme
activities and cytochrome P-450 content were not different between
newborn offspring of corn oil- and PCB-pretreated mothers. However,
these parameters were elevated in 5- to 20-day postpartum nursing
offspring from pretreated mothers, compared with those from corn
oil-pretreated mothers suggesting the transfer of hexachlorobiphenyl
through the breast milk in quantities sufficient to affect hepatic
microsomal monooxygenase activity.
In a study on ICR mice, Vodicnik (1986) administered 150 mg
14C-2,4,2',4'-tetrachlorobiphenyl intraperitoneally, and compared
hepatic microsomal ethoxycoumarin- O-deethylase activity and liver
concentrations of 14C-activity. It was shown that pregnant mice were
less responsive to the inducing effects of the tetrachlorobiphenyl
than virgin or postpartum mice. This diminution in response may be, in
part, responsible for the lack of elimination of the
tetrachlorobiphenyl equivalents from the late pregnant animal during
the 4-day experimental period (see section 6.4.3).
Hardwick et al. (1985) studied both the time course and dose-response
for the induction of the 2 isoenzymes and their respective mRNAs after
administration of 3,4,5,3',4',5'-hexachlorobiphenyl to rats. It was
concluded that the congener under study induced 2 major 3-MC-inducible
isoenzymes of cytochrome P-450 and their mRNAs in a coordinated
manner, probably via a common mechanism. The data are consistent with
the hypothesis that both genes are probably regulated by a single
receptor in the rat. The magnitude of the increase in the isoenzymes
was greater than the increase in the amount of translationally active
mRNA in polysomes, suggesting that other factors may also influence
the relative induction of these P-450 isoenzymes.
In this study, the BP-type inducers, 2,4,6,2',4',6'- and
2,4,5,2',4',5'-hexachlorobiphenyl, both of which have been reported to
increase the hepatic cytosolic receptor level and consequently enhance
enzyme induction in vivo, were not able to enhance EROD or AHH
induction by 3,4,5,3',4'-pentachlorobiphenyl in vitro.
The mixed-type inducer 2,3,4,2',4',5'-hexachlorobiphenyl inhibited
enzyme induction by 3,4,5,3',4'-pentachlorobiphenyl in vitro, when
used in concentrations of at least 400 times higher than that of the
3,4,5,3',4'-pentachlorobiphenyl. Enzyme induction by 3,4,3',4'-
tetrachlorobiphenyl was inhibited by 2,3,4,2',4',5'-hexachlorobiphenyl
at concentrations at least 40 times higher, and enzyme induction by
3,4,5,3',4',5'-hexachlorobiphenyl was inhibited by 2,3,4,2',4',5'-
hexachlorobiphenyl at concentrations at least 8 times higher. Since
the concentration of 2,3,4,2',4',5'-hexachlorobiphenyl found in human
adipose tissue is about 300 times higher than those of the 3 coplanar
PCBs, this inhibition of enzyme induction probably occurs after
natural exposure to PCB mixtures. If enzyme induction by
3,4,3',4'-tetrachlorobiphenyl and 3,4,5,3',4',5'-hexachlorobiphenyl is
also inhibited by various concentrations of 2,3,4,2',3',4'-
hexachlorobiphenyl, inhibition of enzyme induction after natural
exposure to a mixture of PCBs is to be expected.
It was concluded that the in vitro enzyme induction by mixtures of
PCBs cannot be determined by the simple addition of the induction by
the individual PCBs. Possibly, in vivo enzyme induction seems
additive, because some compounds increase the receptor level, while
other compounds inhibit enzyme induction (van Vliet, 1990).
8.6.2.4 Appraisal
The liver is the organ most often implicated in the toxicity of PCBs
in animals. Hepatotoxicity has been observed in numerous studies with
exposed mice, rats, guinea-pigs, rabbits, dogs, and monkeys. The
effects, which appear to be reversible at low doses, are similar among
the species and include enzyme induction, liver enlargement, fat
deposition, and necrosis. Enzyme induction is the most sensitive
indicator of hepatic effects, but few studies have been designed to
define the minimum effective doses of PCB mixtures. The liver
enlargement is associated with hepatocyte enlargement and an increase
in smooth endoplasmic reticulum and/or increased enzymatic activity.
Proliferative lesions in the liver have been attributed to Aroclor
treatment. The hepatic effects of Aroclors in animals appear to be
typical of chlorinated hydrocarbons. Histologically-documented liver
damage is a consistent finding among PCB-exposed animals.
8.6.3 Effects on vitamins and mineral metabolism
8.6.3.1 Effects of PCB mixtures
PCB mixtures have been found to decrease levels of retinol (Vitamin A)
in the liver of rats (Innami et al., 1976; Kato et al., 1978; Hudecova
et al., 1979), rabbits (Villeneuve et al., 1971a), and in the plasma
of pigs (Guoth et al., 1984). Levels of thiamine (Vitamin B1) were
decreased in the blood, liver, and sciatic nerve of rats (Yagi et al.,
1979) and levels of pyridoxal phosphate (Vitamin B6) were decreased in
several tissues of rats, while riboflavin (Vitamin B2) levels remained
unaffected (Fujiwara & Kuriyama, 1977). The changes in the levels of
retinol and thiamine were thought to be secondary to the induction of
metabolizing enzymes (Yagi et al., 1979; Saito et al., 1982). The
induction of these enzymes was also found to be responsible for the
increased de novo synthesis of L-ascorbic acid that was observed in
the plasma, tissues, and urine of PCB-treated rats (Fujiwara &
Kuriyama, 1977; Chakraborty et al., 1978; Chow et al., 1979; Saito et
al., 1983). Lipid peroxidation was increased in the liver of
PCB-treated rats and Saito et al. (1983) suggested that ascorbic acid
may have initiated the peroxidation.
It was shown that induction of NADP-cytochrome c reductase (EC
1.6.2.4) and insufficiency of lipid peroxide scavengers, such as
alpha-tocopherol (Vitamin E) and glutathione peroxidase (EC 1.11.1.9),
could also be involved in the enhancement of lipid peroxidation by
PCBs (Saito et al., 1982, 1983; Kamohara et al., 1984).
PCB mixtures decreased the activity of both sodium/potassium- and
magnesium-dependent adenosinetriphosphatase (EC 3.6.1.3) in the
tissues of rats (Narbonne et al., 1978; La Rocca & Carlson, 1979). The
results of in vitro studies on isolated rat mitochondria showed that
PCB mixtures may act as inhibitors of respiration and uncouplers of
oxidative phosphorylation (Sivalingan et al., 1973; Nishihara, 1983,
1985). However, contradictory results have been obtained in vivo
with respect to the NAD/NADH ratio, the ADP/O ratio, and state 3 and
state 4 respiration rates (Mehlman et al., 1974; Chesney & Allen,
1974; Garthoff et al., 1977).
Byrne & Sepkovic (1987) studied the in vitro incorporation of
monovalent cations into rat erythrocytes as a model for evaluating the
impairment of electrogenic transport by PCBs. Female, Sprague-Dawley
rats were fed 50 mg Aroclor 1242 or 1254/kg diet for 7 months. The
uptake of 86Rb by erythrocytes in the Aroclor 1254 group was
depressed compared with that in the control group in K+ - depleted
culture media. No changes were observed with Aroclor 1242. A reduction
in 86Rb incorporation was also seen in erythrocytes from the Aroclor
1254 group in a Na+ -depleted medium. Ouabain did not have any
effect in the Aroclor 1254 group, because Aroclor 1254 suppressed the
cationic transport maximally. This study provides evidence that PCBs
(Aroclor 1254) can damage the cell sufficiently to decrease the active
transport of monovalent cations.
Male Fischer 344 rats were dosed daily, intragastrically, for 5, 10,
or 15 weeks with 0, 0.1, 1, 10, or 25 mg Aroclor 1254/kg body weight
in corn oil, to investigate the effects on calcium metabolism, femur
morphometry, and nephrotoxicity. The relative liver weights were
increased significantly with doses of 1.0 mg/kg or more after 5 weeks
treatment. The relative kidney weights were increased after 15 weeks
treatment in the 10 and 25 mg/kg groups. Hypercalcaemia was present in
the 25 mg/kg group after 5 and 10 weeks treatment, but not after 15
weeks. Serum triglyceride levels were elevated after 5 weeks
treatment, but decreased after 10 and 15 weeks. Serum cholesterol
levels were increased at the 2 higher dose levels with all 3 lengths
of treatment. Urinary alkaline phosphatase and lactate dehydrogenase
activities were elevated at 5, 10, and 15 weeks of treatment. Femur
density was increased at the 10 mg/kg dose level after 5 weeks, and at
all dose levels after 10 and 15 weeks. Cross-sectional, medullary, and
cortical areas of the midpoint of the femur were significantly
decreased at the higher dose levels after 10 and 15 weeks of exposure.
The per cent medullary area was decreased after 10 and 15 weeks
treatment indicating a decrease in medullary size and also a decrease
relative to the cortical bone area. The result was weaker bones after
15 weeks at the highest dose level. Thus, PCB exposure affects calcium
metabolism and bone morphometry (Andrews, 1989).
8.6.3.2 Effects of individual congeners
3,4,3',4'-Tetrachlorobiphenyl induced a decrease in serum and liver
retinol and retinyl palmitate in C57BL/Rij mice. In "non-responsive"
DBA/2 mice, only serum retinol was decreased. The time and
dose-responses observed suggested that the difference in aryl
hydrocarbon hydroxylase responsiveness was not directly involved in
the effects on retinoid levels (Brouwer et al., 1985).
Powers et al. (1987) administered female, Sprague-Dawley rats single,
intraperitoneal injections of 1, 5, or 15 mg 3,4,3',4'-
tetrachlorobiphenyl/kg body weight and found a dose-related
depression of plasma retinol levels, 24 h after treatment. The loss of
plasma retinol appeared to be a function of depressed levels of the
retinol-binding protein (RBP)-transthyretin ternary complex. No free
retinol was observed in the plasma. Hepatic retinyl palmitate
hydrolase (RPH) activity was depressed and highly and positively
correlated with the plasma retinol levels. Doses of either
2,4,5,2',4',5'- and 3,4,5,3',4',5'-hexachlorobiphenyl, equimolar to
the 15 mg/kg tetrachlorobiphenyl dose, failed to cause a similar
depression in plasma retinol in treated female rats.
A study was carried out to investigate the effects of PCBs on retinoid
homeostasis in Sprague-Dawley rats. Female Sprague-Dawley/Rij rats
were fed a Vitamin A-deficient diet for 12-16 weeks. Serum retinol
concentrations at the end of this period were decreased to
approximately 10% of the normal retinol level. The rats were repleted
with radiolabelled [3H]retinol by feeding a diet containing 18.5 MBq
(8000 IU) of retinol/kg diet for 14 days. Saturation in the blood was
reached after 6 days [3H]retinol repletion. On day 7, the rats were
either treated with an intraperitoneal dose of 3,4,3',4'-
tetrachlorobiphenyl (15 mg/kg) in corn oil, or corn oil alone.
Exposure to tetrachlorobiphenyl resulted in significant reductions in
both retinol and retinyl ester concentrations in the liver and lung to
25% and 44% of the controls, respectively, and a reduction of retinol
in the heart of 35% of the controls. No changes in concentrations were
observed in the skin and kidneys (Brouwer et al., 1988).
Female WAG/Rij rats received a single ip injection of corn oil, or 15
or 200 mg 3,4,3',4'-tetrachlorobiphenyl/kg body weight and were killed
on days 1,3,7, or 14 to study the effects on serum and hepatic
retinoid contents and liver morphology. There was a significant
increase in liver weight at the highest dose level after 3, 7, and 14
days. There was a rapid increase in the 3H-tetrachlorobiphenyl levels
present after 7 days, after which a rapid decline occurred.
Tetrachlorobiphenyl induced a significant decrease in serum retinol
content in the 200 mg tetrachlorobiphenyl group on days 3 and 7. The
same was found for the retinol and retinyl palmitate contents of the
liver. Ultrastructural alterations in the hepatocytes, such as
proliferation and vesiculation of the endoplasmic reticulum and
mitochondrial enlargement with inclusions, were found (Durham &
Brouwer, 1989).
2,2',5,5'-Tetrachlorobiphenyl caused inhibition of Ca/Mg- and
Mg-dependent adenosinetriphosphatase in the liver of rats (Lin et al.,
1979). In vitro, several PCB congeners inhibited Na/K- and
Mg-dependent adenosinetriphosphatase. Although a general trend towards
increased inhibition, paralleling increased chlorination, was
observed, no correlation was evident between chlorine substitution
patterns and inhibitory activity (La Rocca & Carlson, 1979).
8.6.4 Effects on the gastrointestinal tract
Effects on the stomach have been studied or observed by Allen &
Norback (1973); Allen et al. (1974a); Allen (1975); Becker et al.
(1979) and Tryphonas et al. (1986a) in monkeys. Oral administration of
Aroclor 1242, 1248, or 1254 to monkeys produced gastritis, which
progressed to hypertrophy and hyperplasia of the gastric mucosa.
Related effects included mucous-filled cysts that penetrated the
muscularis mucosa. These effects were initiated by exposure as low
and/or short as a single gavage dose of 1.5 g Aroclor 1248/kg body
weight, 25 mg Aroclor 1248/kg diet for up to 1 year, 3 mg of Aroclor
1242/kg diet, for 71 days, or 280 µg/kg body weight for 28 months.
In studies on monkeys, Becker et al. (1979) carried out stomach
biopsies and found microscopically apparent arrest of the
differentiation of generative cells of the isthmus and neck into
parietal and zymogenic cells. Mature parietal and zymogenic cells,
which were found only in the bases of the glands, showed signs of
injury, such as dilatation of the rough endoplasmic reticulum on the
zymogenic cells, irregularity of the mitochondria and irregular
luminal membranes in parietal cells, and an increase in the number of
autophagic vesicles on both types of cell (see 8.2.1.6).
The Aroclor-induced gastric lesions, which occurred mainly along the
greater curvature of the stomach (not in the cardiac or pyloric
regions) and did not occur in other sections of the gastrointestinal
tract, have only been observed in pigs and monkeys (Hansen et al.,
1976b; Becker et al., 1979; Drill et al., 1981). The gastric effects
may therefore be species specific. Aroclor 1254 induced metaplasia and
adenocarcinoma in the glandular stomach of F344 rats (see section
8.7.1.2).
8.6.5 Effects on lipid metabolism
8.6.5.1 Effects of PCB mixtures
Consistent with the histopathological observation of fatty
degeneration in the liver (see section 8.2.1), short-term exposure to
commercial mixtures of PCBs induced increases in the contents and
concentrations of total lipids, triglycerides, cholesterol, and/or
phospholipids in this organ of the rat and rabbit (Litterst et al.,
1972; Bruckner et al., 1974; Itokawa et al., 1976; Garthoff et al.,
1977; Hinton et al., 1978; Ishidate et al., 1978; Dzogbefia et al.,
1978; Yagi, 1980; Kato & Yoshida, 1980, 1981; Kato et al., 1982).
Litterst et al. (1972) exposed male Osborne-Mendel rats, for 4 weeks,
to diets containing Aroclor 1242, 1248, 1254, or 1260 at levels of, or
between, 0.5, 5.0, 50, and 500 mg/kg (equivalent to 0.025, 0.25, 2.5,
and 25 mg/kg body weight). Aroclor 1248 caused the highest
dose-related increase in the triglyceride concentration in the liver,
which was significant at 500 mg/kg diet (equivalent to 25 mg/kg body
weight). The lowest-observed-effect level was reported by Bruckner et
al. (1974), who exposed male Sprague-Dawley rats to 0, 5, or 25 mg of
Aroclor 1242/kg diet (equivalent to 0, 0.3, and 1.5 mg/kg body weight,
respectively), for 2, 4, or 6 months and found a slight increase in
the concentrations of total lipids in the liver at both exposure
levels.
Levels of total lipids, triglycerides, and/or cholesterol in the serum
of rats and rabbits, exposed to PCB mixtures, were found to be
increased (Koller & Zinkl, 1973; Allen et al., 1976; Itokawa et al.,
1976; Garthoff et al., 1977; Zinkl, 1977; Kato & Yoshida, 1980, 1981;
Yagi, 1980; Kato et al., 1982; Baumann et al., 1983; Hladkà et al.,
1983; Carter, 1985). Wistar rats received Clophen A50, twice weekly,
by gavage, at levels of 2, 10, 50, 150, or 250 mg/kg body weight, for
6 weeks. Serum triglyceride and cholesterol levels were increased in a
dose-related manner at 50 and 2 mg/kg body weight, respectively
(Baumann et al., 1983). Decreased serum triglyceride levels were
reported by Kato et al. (1982). Fischer rats exposed for 8 days to
Aroclor 1254 in the diet at levels of 8 mg/kg (0.4 mg/kg body weight)
or more showed a dose-related increase in serum total cholesterol
concentrations. Hypercholesterolaemia was not found at 4 mg/kg diet
(Carter, 1985). Studies on monkeys exposed to Aroclors 1248 or 1254
for 1-2 years revealed lowered serum levels of total lipids,
triglycerides, and cholesterol (Barsotti et al., 1976; Arnold et al.,
1984). The cause of these changes may be an altered synthesis and/or
lipoprotein transport in the liver. No increase in the rate of
synthesis of liver triglycerides was found following intraperitoneal
exposure of rats to 3-8 daily doses of 50 mg of Aroclor 1254/kg body
weight (Hinton et al., 1978; Sandberg & Glaumann, 1980). The observed
increases in the half-lives of liver triglycerides and phospholipids
(Hinton et al., 1978) and the observed increase in the number of Very
Low Density Lipoproteins (VLDL) in the liver, without a change in
lipid composition (Sandberg & Glaumann, 1980), seems to be indicative
of impaired transport of these lipids from the liver to the blood.
This was also demonstrated by the repression in serum VLDL and in the
incorporation of tritiated water in serum total lipids following
tritiated water injection, found in rats exposed for 24 days to a
low-protein diet containing 1000 mg of Aroclor 1248/kg (50 mg/kg body
weight) (Kato et al., 1982). Sandberg & Glaumann (1980) observed an
impaired transport of VLDL from the endoplasmic reticulum to the Golgi
apparatus. This compares well with the observed flattening of the
Golgi apparatus, which also lacks secretory vesicles with lipoprotein
particles (Hinton et al., 1978). No explanation was found in the
available literature for the observed increase in serum triglyceride
levels in rats.
Ishidate et al. (1978) measured a decreased rate of synthesis of
phospholipids, especially of phosphatidyl choline, in the liver of
rats that had received 2 daily doses of a PCB mixture at 100 mg/kg
body weight, composed mainly of tetrachlorobiphenyl isomers. Decreased
phospholipid synthesis was also observed by Hinton et al. (1978). The
accumulation of phospholipids in the proliferated endoplasmic
reticulum was ascribed to the observed depression of the secretion of
lipoproteins into blood (Hinton et al., 1978; Ishidate et al., 1978;
Sandberg & Glaumann, 1980) and to a depressed catabolism of liver
phospholipids (Ishidate et al., 1978).
The synthesis of cholesterol in the rat liver may be increased by PCB
mixtures considering the increased concentration of labelled
cholesterol in the liver following 3H20-injection (Kato et al.,
1982) or 14C-glucose or 14C-acetate administration (Yagi, 1980) in
rats that had been exposed for 3-5 weeks to diets containing 1000 mg
of Aroclor 1248/kg diet or 500 mg Kanechlor 500/kg diet, respectively.
It was also shown that the activity of 3-hydroxy-3-methylglutaryl
Coenzyme A reductase (EC 1.1.1.34) was increased in rats following a
6-day exposure to a diet containing 1000 mg Aroclor 1248/kg diet
(equivalent to 50 mg/kg body weight) (Kato & Yoshida, 1980). The
enhanced synthesis of cholesterol has to compete with an enhanced
degradation, as Aroclor 1248 has been shown to induce cholesterol
7-alpha-hydroxylase (EC 1.14.14.1) in rats (Quazi et al., 1984).
Decreased biosynthesis of liver cholesterol was found in rats exposed
for 30 days to Aroclor 1254 at a dietary level of 500 mg/kg (Kling &
Gamble, 1982). Hypercholesterolaemia in PCB-exposed rats can be
explained partly by an increased synthesis of cholesterol and/or an
increase in serum high density lipoprotein cholesterol, which was
observed in several studies (Ishikawa et al., 1978; Yagi, 1980; Kato &
Yoshida, 1981; Carter, 1985).
Isolated hepatocytes were capable of secreting protein and
triacylglycerol in the form of VLDL into serum-free media. Eighty per
cent of 2,4,5,2',4',5'-hexachlorobiphenyl released from hepatocytes
was in association with VLDL, the remainder being in association with
protein (Gallenberg & Vodicnik, 1987).
8.6.5.2 Effects of individual congeners
Charles-River CD rats received a single oral dose of 3,4,5,3',4',5'-,
2,4,5,2',4',5'-, or 2,3,5,2',3',5'-hexachlorobiphenyl in cotton-seed
oil. After 72 h, all isomers had increased the levels of total lipids
in the liver. 3,4,5,3',4',5'-Hexachlorobiphenyl had the most
pronounced effect. This isomer was the only one that increased the
levels of total cholesterol, cholesterol esters, and triglycerides in
the liver, while the other 2 isomers slightly increased the content of
liver phospholipids (Kohli et al., 1979).
Shireman (1988) studied the lipoprotein-mediated transfer of
2,4,5,2',4',5'-hexachlorobiphenyl into cultured human fibroblasts, and
found that the plasma lipoproteins may play a role in the distribution
of this hexachlorobiphenyl to peripheral cells. Using normal skin
fibroblasts incubated with medium containing serum LDL or high density
lipoproteins (HDL) labelled with the 14C-hexachlorobiphenyl, the
author characterized the cellular incorporation, and efflux from
cells, of this congener and concluded that HDL might be involved in
the delivery of hexachlorobiphenyl to cells and not, as generally
thought, in the transport from cells.
2,4,5,2',4',5'-Hexachlorobiphenyl was shown to be distributed among
rat and human plasma lipoproteins and protein in vitro. It was
readily transferred among plasma constituents and its distribution was
related to the triacylglycerol:protein ratio in the plasma. One h
following intravenous administration of 70 µg labelled
hexachlorobiphenyl to virgin, female Sprague-Dawley rats, the
hexachlorobiphenyl was primarily distributed to low density
lipoprotein (LDL) with the hypertriglyceridemia of late pregnancy;
more than 70% of circulating hexachlorobiphenyl was associated with
very low density lipoproteins (VLDL). VLDL is a major substrate for
mammary gland lipoprotein lipase, which is elevated during lactation.
When hexachlorobiphenyl was complexed with human VLDL and injected
intravenously into late pregnant mice, mammary gland concentrations of
the compound exceeded those in the adipose tissue at all sacrifice
times between 5 min and 6 h (Gallenberg & Vodicnik, 1987; Gallenberg
et al., 1987).
8.6.6 Effects on porphyrin metabolism
8.6.6.1 Effects of PCB mixtures
Hepatic porphyria has been induced by a number of commercial PCB
mixtures (Clophen A60; Phenochlor DP6; Aroclor 1016, 1232, 1242, 1254,
and 1260; Kanechlor 400, 500, and 600) in mice, rats, rabbits,
chickens, and Japanese quail. Young rats, guinea-pigs, and minks seem
to be less sensitive (Strik, 1973). The porphyria was characterized by
the presence of pigment in the liver which fluoresced red under UV
radiation (Vos & Beems, 1971; Kimbrough et al., 1972; Vos &
Notenboom-Ram, 1972; Zinkl, 1977; Honda et al., 1983), an increase in
the concentration of porphyrins in the liver (Goldstein et al., 1974,
1975; Grote et al., 1975; Iverson et al., 1975; Kawanishi et al.,
1975) and an increase in the concentrations of delta-aminolevulinic
acid, porphobilinogen, and porphyrins in the urine or faeces (Vos &
Beems, 1971; Vos & Notenboom-Ram, 1972; Goldstein et al., 1974, 1975;
Baumann et al., 1983; Honda et al., 1983). Vos & Beems (1971) found
increased faecal elimination of coproporphyrin and protoporphyrin in
rabbits dermally treated with 118 mg Aroclor 1260/day (free of PCDFs),
5 days/week, for 36 days and Vos & Notenboom-Ram (1972) found the same
results when female, New Zealand rabbits received a 120 mg application
of Aroclor 1260 on the shaved skin, 5 days/week, for 4 weeks.
When Sherman rats were exposed for up to 13 months to a diet
containing 100 mg Aroclor 1254/kg or for up to 26 weeks to Aroclor
1242 at 100 or 500 mg/kg diet (equivalent to 5 and 25 mg/kg body
weight, respectively) a delayed onset of porphyria was noted after 2-7
months of exposure. The porphyria was mainly characterized by the
excretion and hepatic storage of uroporphyrin and heptacarboxy-
porphyrin, resembling human porphyria cutanea tarda (Goldstein et al.,
1974, 1975). A dose-dependent increase in the concentration of liver
porphyrins was observed in female, Sprague-Dawley rats receiving 21
daily doses of Aroclor 1242 (by gavage) in corn oil at 10 or 100 mg/kg
body weight, but not at 1 mg/kg body weight. Female rats were more
sensitive than male rats and Aroclor 1016 at the same dietary level
had less effect than Aroclor 1242 (Iverson et al., 1975). Others also
noted the greater effect of higher chlorinated PCB mixtures on liver
and urinary levels of porphyrins (Goldstein et al., 1974, 1975;
Kawanishi et al., 1975). Kawanishi et al. (1973, 1974) showed that
administration, in the diet, of Kanechlors KC-300 and KC-500 to rats
at 500 mg/kg produced a marked increase in urinary excretion of copro-
and uroporphyrins, and in faecal elimination of protoporphyrin, but no
increases were observed with Kanechlor KC-400.
Increased urinary coproporphyrin levels were found in male
Sprague-Dawley rats exposed for 2, 4, or 6 months to a diet containing
Aroclor 1242 at 5 or 25 mg/kg (equivalent to 0.25 and 1.25 mg/kg body
weight) (Bruckner et al., 1974).
Porphyria in rats and rabbits has been associated with the observed
stimulation of delta-aminolevulinate synthase (EC 2.3.1.37), the
rate-limiting enzyme in the haem synthesis of porphyrins (Goldstein et
al., 1974, 1975; Grote et al., 1975; Drill et al., 1981; Hill, 1985),
and with the inhibition of uroporphyrin decarboxylase (EC 4.1.1.37),
as measured in chick embryo cells and chicken erythrocytes in vitro
(Kawanishi et al., 1983; Sano et al., 1985). Seki et al. (1987)
observed 80% inhibition of liver uroporphyrin decarboxylase together
with a 15-fold increase in the activity of liver delta-aminolevulinate
synthase and accumulation in the liver of a large amount of
uroporphyrin in C57BL/6 mice exposed for 3 weeks to Kanechlor 500 at a
dietary dose of 500 mg/kg. Liver microsomal cytochrome P-450 was
increased and induction of microsomal enzymes was observed. The
effects were less outstanding in ddY mice whereas liver cytosol levels
of the PCBs were comparable in both strains. The authors postulated
that the development of porphyria is causally related to the
inhibition of uroporphyrin decarboxylase rather than the induction of
drug metabolizing function. Porphyria would develop only when the
ratio of hepatic uroporphyrin decarboxylase and delta-aminolevulinate
synthase decreased to less than 1.0.
8.6.6.2 Effects of individual congeners
The levels of coproporphyrin and protoporphyrin found in the faeces of
rabbits, dermally exposed to 5 doses/week of 120 mg of
2,4,5,2',4',5'-hexachlorobiphenyl (no dibenzofurans detected) in
isopropanol, for 4 weeks, were more elevated than those in the faeces
of rabbits exposed similarly to Aroclor 1260 (Vos & Notenboom-Ram,
1972). Koss et al. (1980) also found 2,4,5,2',4',5'-hexachlorobiphenyl
highly effective in inducing porphyria in female rats receiving,
orally, 64 mg of this PCB-congener/kg body weight in oil, once every 2
days, for 10 weeks. In mice receiving a diet containing 300 mg of one
of various tetrachlorobiphenyls, hexachlorobiphenyls, or
Kanechlors/kg, for 14 weeks, the most pronounced increases in the
levels of coproporphyrin and protoporphyrin in the liver were found in
animals fed 3,4,5,3',4',5'- and 2,4,6,2',4',6'-hexachlorobiphenyl, and
Kanechlor 600, followed by animals fed 3,5,3',5'- and 2,5,2',5'-
tetra-chlorobiphenyls and Kanechlor 500. No porphyrinogenic action was
found in mice fed 3,4,3',4'-, 2,4,2',4'-, 2,3,2',3'-, or 2,6,2',6'-
tetra-chlorobiphenyl, 2,3,4,2',3',4'-hexachlorobiphenyl, or Kanechlor
400 (Kawanishi et al., 1975). Accumulation of uroporphyrins was
observed in the livers of "responsive" C57BL/6 mice treated with
3,4,5,3',4',5'-hexachlorobiphenyl, but not in the livers of
"non-responsive" ddY mice. It was suggested that induction of
apocytochrome P-450 may take part in inducing porphyrin synthesis
(Sano et al., 1985).
Sano et al. (1985) studied the mechanism of the porphyrinogenic
activity of PCBs using cultured chick embryo liver cells to examine
the relationship between the induction of delta-aminolaevulinic acid
(ALA) synthetase and the inhibition of uroporphyrinogen dicarboxylase.
The porphyrinogenic effect of PCBs exhibited a defined
structure-activity relationship in that only 3,4,3',4'-tetrachloro-
and 3,4,5,3',4',5'-hexachlorobiphenyl out of 9 biphenyls produced a
marked accumulation of uroporphyrin in the liver cells. In
ALA-supplemented cultures, these 2 congeners led to the accumulation
of a large amount of uroporphyrin III, whereas with the other PCBs
(which were weak inducers of porphyrin synthesis) the accumulated
porphyrin was mostly protoporphyrin. These results suggested that the
active inducers of porphyrin synthesis also inhibit uroporphyrinogen
decarboxylase, in 2 steps, i.e., first, in the formation of
hexacarboxylic porphyrinogen III from heptacarboxylic porphyrinogen
III, and, second, in the formation of heptacarboxylic porphyrinogen
III from uroporphyrinogen III. The inhibition of uroporphyrinogen
decarboxylase leads to a depletion of haem. In addition, induction of
apocytochrome P-450 by PCBs may contribute to a decrease of haem. As a
result, synthesis of ALA synthetase increases, leading to an
accumulation of uroporphyrin in liver.
8.6.7 Effects on the endocrine system
8.6.7.1 Effects of PCB mixtures
The underlying cause of the reproductive toxicity of PCBs, described
in section 8.4, may be alterations in hormonal receptor binding and/or
alterations in the steroid hormone balance through effects on
metabolism and excretion.
Precocious vaginal opening was observed in neonatal Sprague-Dawley
rats receiving a subcutaneous dose of 10 mg of Aroclor 1221
(2000 mg/kg body weight) in sesame oil on days 2 and 3 of life. At 6
months of age, these females showed persistent vaginal estrus and
anovulation, despite no further exposure to Aroclor 1221. Doses of
Aroclor 1221, 1242, 1254, or 1260 at 1 mg/kg were without effect.
Groups of 22-day-old Sprague-Dawley rats were injected subcutaneously
with Aroclor 1221 or 1242 at 1, 10, 100, or 1000 mg/kg body weight or
Aroclor 1254 or 1260 mixed in sesame oil at 1, 10, or 100 mg/kg body
weight. Uteri were weighed. New-born female pups were injected
subcutaneously on the second and third day postpartum with Aroclor
1221 at 1 or 10 mg/day or Aroclor 1242, 1254, or 1260 in sesame oil or
dimethylsulfoxide at 1 mg/day. Pups were weaned at 21 days and
examined daily from the 25th day of puberty. Animals were sacrificed
at 7 or 8 months, at which time organs were examined. A significant
uterotrophic response was noted with 1000 mg Aroclor 1221/kg, but not
with the other PCBs (Gellert, 1978).
Indirect evidence for a weak estrogenic activity of PCBs was found for
various Aroclors by the glycogen response of immature rat uterus
(Bitman & Cecil, 1970; Bitman et al., 1972; Ecobichon & Mackenzie,
1974) or the less sensitive uterotropic response, observed in immature
rats exposed to Aroclor 1221, 1232, or 1248, but not in immature rats
exposed to Aroclor 1254 or 1260 (Ecobichon & Mackenzie, 1974; Gellert,
1978). More direct evidence is the inhibition in vitro of the
binding of labelled 17-beta-estradiol to the rat uterine receptor by
Aroclors 1221 and 1254 (Nelson, 1974).
Pregnant mares' serum was administered to immature female outbred rats
on day 29 postpartum, and, 60 h later, rats were injected with human
chorionic gonadotrophin. On day 34, the animals were divided into
groups and treated orally with sesame oil (control), or 20 mg PCBs/kg
(Clophen A30). Two days later they were killed and the ovaries removed
and analysed for in vitro synthesis of progesteron (unincubated,
incubated, and incubated with luteinizing hormone). The addition of
luteinizing hormone resulted in an approximately 100% increase in
progesterone synthesis above basal level with tissue exposed to PCBs.
With control tissue there was a 31% increase with luteinizing hormone
(Fuller et al., 1980).
PCBs induced a decrease in gonadal steroid hormone levels in rats,
minks, seals, and monkeys. When, after confirmed ovulation, mature
female Rhesus monkeys were exposed during the following cycle to daily
gavage doses of 4, 16, or 64 mg of Clophen A30/kg body weight, for 28
days, ovulation was blocked in 2 out of 4 treated monkeys. One out of
16 controls was anovulatory. The levels of luteinizing hormone and
follicle-stimulating hormone were not changed by the treatment (Muller
et al., 1978).
Plasma progesterone levels were decreased in female rats exposed for
36 weeks to a dietary level of Aroclor 1242 of 75 mg/kg (equivalent to
3.7 mg/kg body weight) (Jonsson et al., 1976), and in female minks
exposed for 12.5-14.5 months to a dietary level of 2.5 mg Aroclor
1254/kg (equivalent to 0.25 mg/kg body weight) (Aulerich et al.,
1985). The decreased levels of gonadal hormones can be explained by
enhanced metabolism of steroids, which are normal substrates for
microsomal enzymes. Increases in the formation of the metabolites of
progesterone and/or testosterone were measured in rats
intraperitoneally exposed 1-5 times to Aroclor 1260, Aroclor 1254, or
Kanechlor 400 (Krogh Derr, 1978; Lin et al., 1982; Yoshihara et al.,
1982). In contrast with these findings, increased testosterone levels
were found in male piglets exposed for 6-12 weeks to Aroclor 1232,
1242, or 1254 at 250 mg/diet. This increased production of
testosterone was related to increased relative testes weights
(Platonow et al., 1976).
Female rhesus monkeys (Macaca mulatta) were administered gelatin
capsules containing daily doses of 0, 5, 20, 40, or 80 µg Aroclor
1254/kg body weight, dissolved in corn oil plus glycerol. After
approximately 2 years of dosing, when the monkeys were considered to
be in a state approaching adipose-tissue PCBs equilibrium, each dose
group of 16 animals was divided into 2 test groups. Daily blood
samples from both test groups were acquired for estrogen and
progesteron analysis during one menstrual cycle. Serum estrogen and
progesteron concentrations in PCB-dosed monkeys were comparable with
those in the controls, except the luteal phase progesterone levels in
monkeys dosed with 20 and 80 µg/kg. There were no apparent
treatment-related differences in the incidence of anovulatory cycles
or in the temporal relationship between the estrogen peak and mensus
onset, mensus end, or the progesterone peak. Mean PCB concentrations
in the blood and adipose tissue for the different dose levels
administered were as follows: blood, 1, 11, 37, 74, and 125 µg/litre
and for adipose tissue 0.79, 7.88, 22.62, 47.6 and 85.3 mg/kg tissue,
respectively (Truelove et al., 1987).
Effects on plasma corticosteroid levels have also been observed. The
levels were decreased in female mice exposed to a diet containing
25 mg Aroclor 1254/kg (equivalent to 3.7 mg/kg body weight) for 3
weeks, and in male mice exposed to a diet containing 400 mg Aroclor
1254/kg (equivalent to 57 mg/kg body weight) for 2 weeks. No effects
were found on adrenal weight (Sanders & Kirkpatrick, 1975). However,
increased levels of plasma corticosterone and enlarged adrenal glands
were observed in male mice of another strain exposed to a diet
containing 200 mg Aroclor 1254/kg, for 2 weeks (Sanders et al., 1977).
Wasserman et al. (1973) reported increased plasma corticosterone
levels in rats receiving Aroclor 1221 in the drinking-water, at a
concentration of 250 mg/litre, for 10 weeks. This finding complies
with morphological features of hyperfunction of the adrenal zona
fasciculata found in rats that had received 200 mg Aroclor 1221/litre
drinking-water, for 6 weeks.
When female rats were exposed to Aroclor 1254, in their diet at doses
of 0, 1, 5, 10, or 50 mg/kg (equivalent to 0, 0.05, 0.25, 0.5, and
2.5 mg/kg body weight/day, respectively) for 5-7 months, relative
adrenal weights as well as serum levels of corticosterone,
dehydro-epiandrosterone, and dehydro-epiandrosterone sulfate were
decreased in a dose- and time-related manner. In the same studies, the
effects with less chlorinated Aroclors were less pronounced (Byrne et
al., 1988).
In addition, the ultrastructure of beta-cells of the pancreas of rats
was found to be changed after 13 months of exposure to 200 mg Aroclor
1254/litre drinking-water. These changes included marked dilatation
and vesiculation of the rough endoplasmic reticulum, hyperplastic
Golgi complexes with a reduction in the number of secretory granules,
and an increase in the number of beta-acinar and acinar-beta cells.
The changes in the pancreas were suggested to be secondary to the
increase in the level of glucocorticoids (Wasserman et al., 1975). An
increased relative adrenal weight was observed in pigs fed Aroclor
1242 or 1254/kg at 20 mg/kg diet (equivalent to 0.8 mg/kg body weight)
for 91 days (Hansen et al., 1976b).
Thyroid hormone levels were decreased in rats exposed to Aroclor 1254
at levels of 50 mg/kg diet or more for 4-12 weeks (Collins et al.,
1977; Collins & Capen, 1980a). Two explanations were offered. One was
the observed increase in biliary excretion of thyroxine and
triiodothyroxine (Bastomsky, 1974; Collins & Capen, 1980b) and the
larger proportion of biliary thyroxine present as glucuronide
(Bastomsky, 1974), most likely as a result of induction of microsomal
uridine diphosphate-glucuronosyltransferase (EC 2.4.1.17) (Bastomsky &
Murthy, 1976). The other explanation was a direct effect of PCBs on
thyroid follicular cells. When male Holtzman or Osborne-Mendel rats
were fed a diet containing Aroclor 1254 at a level of 0, 5, 50, or
500 mg/kg (equivalent to 0, 0.25, 2.5, and 25 mg/kg body weight),
thyroid follicular cells exhibited a dose-dependent hypertrophy and
hyperplasia. An abnormal accumulation of large colloid droplets and
irregular lysosomes in the follicular cells were observed at 5 mg/kg
diet or more and reduced serum thyroxine occurred at 50 mg/kg diet or
more. A no-observed-effect level could not be established. Microvilli
were decreased in number, shortened, and irregularly branched (Collins
et al., 1977; Kasza et al., 1978a,b; Collins & Capen, 1980a, 1980b).
The hypothalamus-pituitary axis seems not to be affected in view of
the observed increase in the serum level of thyroid-stimulating
hormone and in the iodine uptake by the thyroid following PCB exposure
(Bastomsky, 1974, 1977; Collins & Capen, 1980a).
Collins & Capen (1980a) suggested that the well-documented
PCBs-related disturbances in reproduction, growth, and development may
be related to alterations in thyroid structure and function in the
dam, fetus, or neonate. The lowering of serum thyroxine appears to be
the combined result of a direct effect on thyroid follicular cells
with an interference in hormone secretion plus an enhanced peripheral
metabolism of thyroxine.
8.6.7.2 Effects of individual congeners
Exposure of rats to various congeners produced different responses in
steroid metabolism. The most marked effects were observed after
exposure to 2,4,5,2',4',5'-hexachlorobiphenyl, which was found to
decrease the half-life of progesterone (Örberg & Ingvast, 1977), to
increase hydroxylation of progesterone, testosterone, and
androstenedione, and to decrease the 5-alpha-reduction of progesterone
and testosterone (Dieringer et al., 1979; Yoshihara et al., 1982).
3,4,5,3',4'-Pentachlorobiphenyl was found to depress the total
microsomal metabolism of progesterone and testosterone, though the
7-alpha-hydroxylation of these steroids was markedly stimulated
(Yoshihara et al., 1982). No, or very slight, effects on steroid
metabolism were found in rats exposed to chlorobiphenyls with 4
chlorine atoms or less (Örberg & Ingvast, 1977; Dieringer et al.,
1979).
Yoshimura et al. (1985) described a marked induction of liver
microsomal cytochrome P-450 and cytosolic DT-diaphorase as a cause of
a possible disorder of steroid homeostasis and promotion of
carcinogenicity of 4-nitroquinoline N-oxide (4-NQO) in rats
pretreated with 3,4,5,3',4'-pentachlorobiphenyl. The animals were
sacrificed 5 days after pretreatment. The results of the studies
showed that 7-alpha-hydroxylation of both progesterone and
testosterone in liver microsomes was increased, but hydroxylation at
the 2-alpha-, 6 alpha-, and 16 alpha-positions were depressed,
together with 5 alpha-reduction. The induced isoenzyme P-452 was most
responsible for the 7-alpha-hydroxylation of testosterone.
The major component (32 mol %) of the Aroclor 1221 mixture is
2-chlorobiphenyl. The major metabolite (4,4'-dihydroxy-2-
chlorobiphenyl) of 2-chlorobiphenyl has been shown to have a
significant binding activity with the soluble uterine estrogen
receptor protein in the rat, suggesting a possible explanation for the
unique estrogenic activity of Aroclor 1221 in the rat (Korach et al.,
1987).
8.6.8 Immunotoxicity
Some of the studies described below are summarized in Table 52.
8.6.8.1 Effects of PCB mixtures
(a) Mouse
Relative thymus and spleen weights of C57BL/6 mice were unaffected by
exposure to Aroclor 1016, for 3-41 weeks, at a dietary level of
167 mg/kg (Silkworth & Loose, 1979). Dietary exposure of outbred mice
(Mus musculus) to Aroclor 1248 at 50, 100, 500, or 1000 mg/kg diet
(equivalent to 7.1 up to 143 mg/kg body weight), for 3 or 5 weeks, did
not elicit gross signs of immunotoxicity (Thomas & Hinsdill, 1978).
BALB/c mice fed Aroclor 1242 at a dose-level of 0 or 167 mg/kg diet
(equivalent to 0 and 29 mg/kg body weight) for 3-9 weeks, did not show
adverse effects on the thymus, spleen, and lymph nodes (Loose et al.,
1977, 1978).
In addition, Carter & Clancy (1980) observed an increased graft versus
host response in a decreased number of spleen cells in 4BALB/c mice,
which were exposed to a single intraperitoneal dose of 1000 mg Aroclor
1242/kg body weight in corn oil. Spleen enlargement and lymphocyte
depletion were observed.
Offspring of Swiss-Webster mice, exposed via the dams which were fed
Aroclor 1254 at dietary levels of 10, 100, or 250 mg/kg, did not
exhibit an altered hypersensitivity reaction to oxazoline, an altered
anti-bovine serum albumin antibody titre or an altered degree of
phagocytosis of sheep red blood cells by peritoneal macrophages,
compared with controls (Talcott & Koller, 1983).
Pathogen-free ICR/JCL mice (aged 4 weeks) were intubated orally, once
a week, for 4 weeks, with 0, 10, or 100 µg Kanechlor 500/kg body
weight. Two days after the final treatment, half of the animals of
each group were injected intraperitoneally with 0, 50, 250, or 500 µg
E. coli endotoxin/mouse. Sensitivity to endotoxin was determined by
24-h mortality rate. The oral administration of Kanechlor 500 up to a
dose level of 100 µg/kg body weight did not have any effect on the
sensitivity to the endotoxin (Oishi & Hiraga, 1980).
The relative potencies of PCB mixtures Aroclors 1260, 1254, 1248,
1242, 1016, and 1232 to inhibit the murine, splenic, plaque-forming
cell response to sheep red blood cells was determined for C57Bl/6
mice. The ED50 values for the reduction in the splenic,
plaque-forming cells were 104, 118, 190, 391, 408, and 464 mg/kg body
weight, respectively. It was apparent that the higher PCBs (Aroclors
1260, 1254, and 1248) were more potent than the lower chlorinated
mixtures.
Previous studies have shown that a subeffective dose of Aroclor 1254
(25 mg/kg), interacted with an immunotoxic dose of TCDD (3.7 nmol/kg),
resulting in, a significant antagonism of the toxicity of the latter
compound. Co-treatment of mice with a dose of all these PCB mixtures
at 25 mg/kg and a reconstituted PCB mixture, as occurs in breast milk,
in combination with TCDD (3.7 nmol/kg) showed that all (except Aroclor
1232) significantly antagonized the TCDD-mediated inhibition of the
splenic, plaque-forming cell response in C56Bl/6 mice (Davis & Safe,
1989).
Table 52. The humoral and cell-mediated immunotoxicity of PCBs administered via the diet in short-term studies
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Monkey
Rhesus Aroclor 44 anti-SRBC antibody titre D 5.0 (d) 2.5 (d) Thomas &
1248 anti-tetanus toxicoid Hinsdill
antibody titre NE - 5.0 (d) (1978)
serum gamma-globulin
fraction D 5.0 (d) 2.5 (d)
Cynomolgus Kanechlor 20 anti-SRBC antibody titre D 2 (bw) Hori et al.
400 serum gamma-globulin (1982)
(purified) fraction D 2 (bw)
Cynomolgus Aroclor 21 anti-SRBC antibody titre D 2.5 (d) Truelove
1254 anti-tetanus toxicoid et al. (1982)
antibody titre NE 10 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Rabbit
New Zealand Aroclor 5 anti-SRBC antibody titre NE 6.54 (bw) Street &
1254 serum gamma-globulin Sharma
levels NE 6.54 (bw) (1975)
delayed hypersensitivity
reaction to tuberculin NE 6.54 (bw)
popliteal lymph node
antibody-forming cells D 0.92 (bw) 0.18 (bw)
Guinea-pig
Clophen 4-7 anti-tetanus toxicoid Vos & van
A60 and antibody titre D 50 (d) 10 (d) Driel-
Aroclor delayed hypersensitivity Grootenhuis
1260 reaction to tuberculin D 50 (d) 10 (d) (1972)
anti-tetanus toxicoid
producing cells in
popliteal lymph nodes D 50 (d) 10 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Rat
Sprague- Aroclor 1 mitogen response to Bonnyns &
Dawley 1254 phytohaemagglutinin I 250 (d) Bastomsky
response to poke-weed (1976)
mitogen NE 250 (d)
serum gamma-globulin
fraction D 250 (d)
Sprague- Aroclor 10 interleukin 2 production Exon et al.
Dawley 1254 induction by KLH D 50 (d) (1985)
natural killer cell
cytotoxicity D 50 (d)
anti-KLH antibody titer D 50 (d)
Mouse
ICR Kanechlor 3 host-resistance to herpes Imanishi
500 simplex virus D 33 (bw) 18 (bw) et al. (1980)
host-resistance to
ectomelia virus D 33 (bw) 18 (bw)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
ICR Kanechlor 3 host-resistance to Imanishi
500 influenza virus D 400 (d) 200 (d) et al. (1984)
host-resistance to
Staphylococcus aureus D 100 (d)
ICR/JCL Kanechlor 4 sensitivity to NE - 100 µg/kg Oishi &
500 E. coli endotoxin (bw) Hiraga
(1980)
Swiss-Webster Aroclor 12 hypersensitivity reaction NE - > 250 (d) Talcott &
1254 to oxazoline, anti-bovine Koller
serum albumine antibody (1983)
titre and phagocytosis
of SRBC by macrophages
BALB/c Aroclor 3-6 host-resistance to Loose et al.
1242 endotoxin D 167 (d) (1978)
host-resistance to
malaria D 167 (d)
BALB/c Aroclor 6 spleen cellularity NE 167 (d) Loose et al.
1242 spleen PFC D 167 (d) (1977)
serum immunoglobulins
G1, A, M D 167 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
C57BL/6 Aroclor 3-41 graft versus host response NE 167 (d) Silkworth &
1016 mixed lymphocyte Loose (1979)
response I 167 (d)
mitogen response to
lipopolysaccharide I 167 (d)
mitogen response to
concanavalin A I 167 (d)
Mus musculus Aroclor 5 host-resistance to Thomas &
1248 Salmonella typhimurium D 1000 (d) Hinsdill
host-resistance to (1978)
endotoxin D 1000 (d)
a Up to day of primary immunization.
b SRBC = Sheep red blood cells; KLH = Keyhole limpet haemocyanin; PFC = Plaque forming cells.
c I = Increased; D = Decreased; NE = No effect found.
d LOEL = Lowest-observed-effect-level; NOEL = No-observed-effect level: in mg/kg diet (d)
or mg/kg body weight per day (bw).
A single administration of 500 mg Aroclor 1254/kg, intraperitoneally,
inhibited the plaque-forming (PFC) response to subsequent challenge
with sheep erythrocytes in Ah locus positive (C57Bl/6N or B6C3F1N)
mice. However, Aroclor 1254 did not give induction in the Ah locus
negative DBA/2N mice. When B6C3F1 mice were challenged with sheep red
blood cells, 6 or 16 weeks after Aroclor 1524 treatment, substantial
recovery of a PFC response was observed. In older (76-week-old) B6C3F1
mice severe depression of the PFC response was observed.
In contrast with its profound depression of a PFC response, Aroclor
1254 (up to 1250 mg/kg) caused a slight increase in lymphocyte
proliferation induced by either T or B cell mitogens. A single
500 mg/kg dose of this Aroclor also suppressed the ability of
recipient B6C3F1 animals to reject a challenge with either the
syngenic fibrosarcoma (PYB6) or the gram negative pathogen Listeria
monocytogenes (Lubet et al., 1986).
Heinzow et al. (1988) studied the effect of 2,4,5,2',4',5'-hexa-
chlorobiphenyl in the E rosette formation with sheep red blood cells
(SRBC) as one of the characteristics of human T-lymphocytes. The
minimal concentration eliciting a significant monoclonal CD2 receptor
antibody sparing effect was 1.5 × 10-10 mol/litre.
C3H/HeN mice were treated, twice a week, for 2 or 3 weeks prior to
mating, with olive oil or with Kanechlor 500 at an oral dose of
50 mg/kg body weight. The offspring, some of which were nursed by
unexposed dams, were tested for immunocompetence, 4-15 weeks after
birth. The dams did not show any adverse effects on body weight,
absolute spleen weight, and spleen cellularity. The B-cell activity of
the offspring was comparable with that of controls. The helper T-cell
activity was reduced up to 7-11 weeks after birth: the effect was more
pronounced in prenatally-exposed groups (Takagi et al., 1989).
(b) Rabbit
Rabbits appear most sensitive with respect to the immunotoxicity of
PCBs. Street & Sharma (1975) exposed groups of 5-7 New Zealand rabbits
to Aroclor 1254 at dietary levels of 0, 3.7, 20, 45.8, or 170 mg/kg
(equivalent to 0, 0.18, 0.92, 2.1, or 6.54 mg/kg body weight) for
44-57 days. When compared with control animals, an increased degree of
thymus atrophy was observed at all dose levels except for 20.0 mg/kg.
At the 2 higher dose-levels, relative spleen weights were decreased
and the number of germinal centres reduced. When 8 female New Zealand
rabbits received 118 mg of Phenochlor DP6, Clophen A60, or Aroclor
1260 (free of PCDFs), on the back skin, 5 times per week, for 38 weeks
they showed leukopenia, thymus atrophy, and loss of germinal centres
in the spleen and lymph nodes. No such changes were observed in the 4
controls (Vos & Beems, 1971). Vos & Notenboom-Ram (1972) found the
same effects in rabbits administered 120 mg Aroclor 1260 (free of
PCDFs)/day, 5 days/week, for 4 weeks. No adverse gross immunotoxic
effects were observed in groups of 10 New Zealand rabbits fed various
Aroclors at dose levels of 150 mg/kg body weight, once a week, for
12-14 weeks (Koller & Thigpen, 1973).
When New Zealand rabbits were exposed orally, via intubation, to
Aroclor 1242 at a dose of 150 mg/kg body weight, once a week, for 11
weeks, the anti-pseudo rabies virus antibody titre and serum
gamma-globulin levels were decreased (Koller & Thigpen, 1973). The
overall picture is one of immunosuppression, though in 2 diet studies
an increased activation of the cell-mediated immune response was
observed (Bonnyns & Bastomsky, 1976; Silkworth & Loose, 1979).
New Zealand rabbit offspring, exposed via the dams fed 0, 10, 100, or
250 mg of Aroclor 1248/kg diet, showed a decrease in the delayed
hypersensitivity reaction to dinitrofluorobenzene in the highest dose
group. No effects were observed on the splenic, plaque-forming cell
response, the antibody titre against sheep red blood cells, and the
mitogen responses to Concanavalin A and Phytohaemagglutinin (Thomas &
Hinsdill, 1980).
(c) Guinea-pig
Atrophy of the thymus has been reported in female guinea-pigs exposed
to Clophen A60 or Aroclor 1260 for 4-7 weeks at a dietary level of
50 mg/kg (equivalent to 2 mg/kg body weight). Following stimulation
with tetanus toxoid, the authors found a lower antitoxin titre and a
lower count of antitoxin-producing cells in comparison with control
guinea-pigs, resulting in a significant reduction in immunoglobulins.
The skin reaction after tuberculination in animals immunized with
Freund's complete adjuvant (as a parameter of cell-mediated immunity)
was also depressed at the 50 mg/kg dose level (Vos & van
Driel-Grootenhuis, 1972).
Female guinea-pigs fed diets containing 50 mg Aroclor 1260/kg for 6
weeks had significantly lowered tetanus antitoxin titres, circulating
leukocytes and lymphocytes, and thymus atrophy (Vos & van Genderen,
1973). Also treatment with 10 mg Aroclor 1260/kg diet for 8 weeks
produced splenic atrophy (Vos & de Roij, 1972). The Aroclor 1260 was
free from PCDFs (no details).
(d) Monkey
Offspring of Rhesus monkeys appeared very sensitive to the toxic
effects of PCB exposure during gestation and nursing, as already
discussed in section 8.4.1. Thymic atrophy, loss of germinal centres
and of lymph nodules of the spleen, and bone marrow hypocellularity
were found in 3 out of 6 offspring that died during their first year
of life due to exposure to PCBs via their mothers. The mothers, 9-12
per group, were fed diets containing 0, 2.5, or 5.0 mg of Aroclor
1248/kg (equivalent to 0, 0.09, and 0.2 mg/kg body weight), for 18
months and were bred after 6 months of exposure. One mother in each
exposed group died during exposure, showing an increased
susceptibility to Shigella flexneri. At autopsy, no lesions were
observed in lymphoid organs and tissues (Allen & Barsotti, 1976;
Barsotti et al., 1976). When the surviving mothers were placed on a
control diet for approximately 1 year after exposure and then rebred,
there was a decided improvement in their health, but their infants
were still severely affected by PCBs. In the 2 offspring in each
exposed group that died after weaning at the age of 4 months, the
effects on the thymus, spleen, and bone marrow were similar to those
described for the first generation (Allen et al., 1980).
Groups of mature, female Rhesus monkeys received diets containing 0,
2.5, or 5.0 mg Aroclor 1248/kg. After 11 months, all monkeys received
intravenous injections of sheep red blood cells (SRBC) as well as an
intramuscular injection of tetatus toxin (TT). Booster injections and
a second TT injection were given after a number of weeks. Blood
samples were taken over a period of 20 weeks after immunization.
The anti-sheep red blood cells (SRBC) antibody titres, and antibody
response to TT were not clearly affected. The gamma-globulin-levels
were lower in the PCB-treated animals. After 6 months, the PCB-treated
monkeys developed chloracne, alopecia, and facial oedema (Thomas &
Hinsdill, 1978).
Hori et al. (1982) also found immunosuppression in monkeys exposed to
PCB mixtures (without detectable quantities of PCDFs), compared with 2
control monkeys. A more severe immunosuppression was observed in
another monkey exposed to a comparable PCB mixture with PCDFs.
In a pilot study, one infant Cynomolgus monkey, the mother of which
had been exposed to Aroclor 1254 at a dose-level of 400 µg/kg body
weight, showed a decreased anti-sheep erythrocyte antibody titre
following primary immunization in comparison with one control infant
(Truelove et al., 1982).
Atrophy and loss of germinal centres in the spleen and other lymphoid
tissues were observed in groups of 5-6 female Cynomolgus monkeys
following exposure to Aroclor 1254 or 1248, in an apple juice-corn oil
emulsion, 3 times per week, at dose levels of 5 and 2 mg/kg body
weight, respectively, until death at day 29-164. The monkeys exposed
to Aroclor 1254 showed bone marrow hypocellularity and leukopenia.
Lesions seen in control monkeys were similar to those described as
spontaneous (Tryphonas et al., 1984; see also section 8.2.1).
Limited data exist on the humoral or cell-mediated responses in
infants, exposed to PCBs via their mothers.
8.6.8.2 Effects of individual congeners
Thymus atrophy was observed in monkeys exposed for 1-6 months to
3,4,3',4'-tetrachlorobiphenyl, but not in monkeys exposed to
2,5,2',5'-tetrachlorobiphenyl (McNulty et al., 1980).
Decreased relative weights of the thymus and increased or decreased
relative weights of the spleen were induced by single intraperitoneal
doses of the planar congeners 3,4,3',4'-tetrachlorobiphenyl (at
10 mg/kg body weight) and 3,4,5,3',4'-pentachlorobiphenyl (at
245 mg/kg body weight) in "responsive" C57BL/6 mice, i.e., mice
possessing the cytosolic Ah receptor protein, but not, or only to a
minor degree, in "non-responsive" DBA/2 mice (lacking this receptor).
The tetrachlorobiphenyl further decreased the number of cells per
spleen and the number of splenic, plaque-forming cells at 10 and
100 mg/kg body weight, respectively. Further chlorination at the
ortho-position decreased the toxicity of these congeners. No adverse
effects were induced by 2,4,5,3',4'- and 3,4,5,2',4'-pentachloro-
biphenyl (at 490 mg/kg body weight), 2,3,4,5,3',4',5'-heptachloro-
biphenyl (at 593 mg/kg body weight) and the di- ortho substituted
tetrachlorobiphenyls (at 100 mg/kg body weight (Silkworth & Grabstein,
1982; Robertson et al., 1984; Silkworth et al., 1984).
Similar trends were observed in Wistar rats with respect to the
induction of decreased relative thymus and spleen weights (see section
8.2.1.1). Biocca et al. (1981) exposed C57BL/6J mice to various
hexachlorobiphenyl isomers in the feed for 28 days. The most toxic
isomer tested was 3,4,5,3',4',5'- hexachlorobiphenyl which, among
others, caused a marked thymus atrophy and a moderate depletion of
lymphocytes in the spleen. 2,4,6,2',4',6'-Hexachlorobiphenyl caused
the same lesions, albeit at much higher dose-levels, while the
2,4,5,2',4',5'- and 2,3,6,2',3',6'-hexachlorobiphenyls were virtually
inactive.
The in vivo generation of cytotoxic T-lymphocytes (CTL) in response
to allogeneic tumour challenge is sensitive to suppression by
3,4,5,3',4',5'-hexachlorobiphenyl, a poorly metabolized, Ah
receptor-binding PCB isomer. Groups of 5-8 C57Bl/5 mice treated with a
single oral dose of 0, 10, or 100 mg 3,4,5,3',4',5'-hexachloro-
biphenyl/kg body weight, 2 days prior to the intraperitoneal injection
of allogeneic P815 tumour cells, exhibited a dose-dependent reduction
in peak CTL activity in the spleen. When examined on a kinetic basis,
the TCL response was reduced in magnitude with no evidence for a shift
in the kinetics of the response induced by 3,4,5,3',4',5'-hexachloro-
biphenyl.
3,4,5,3',4',5'-Hexachlorobiphenyl exposure, prior to antigen challenge
(day -14, -7, or -1 relative to P815 injection on day 0), produced
significant suppression of the CTL response. 3,4,5,3',4',5'-
Hexa-chlorobiphenyl treatment (10 mg/kg body weight), 6 weeks prior to
such a challenge, was still significantly suppressive, though the
reduced degree of suppression suggested that recovery was in progress.
When 3,4,5,3',4',5'-hexachlorobiphenyl exposure occurred after antigen
challenge, significant suppression was produced only when exposure
occurred within the first 3 days of the response, suggesting that, as
the CTL matured, their sensitivity to 3,4,5,3',4',5'-hexachloro-
biphenyl diminished. Clearance of the allogeneic tumour cells from the
peritoneal cavity was delayed in 3,4,5,3',4',5'-hexachlorobiphenyl-
treated mice and was associated with an altered composition of the
white blood cell infiltrate in this cavity. Symptoms of overt
toxicity, as well as immunotoxicity, were apparent at lower doses of
3,4,5,3',4',5'-hexachlorobiphenyl in male compared with female mice.
In addition, interactive effects of 3,4,5,3',4',5'-hexachlorobiphenyl
exposure and P815 antigen challenge on body weight and thymic
involution were observed in both male and female mice (Kerkvliet &
Baecher-Steppan, 1988a).
A modest dose-dependent suppression of the proliferative response to
alloantigen in mixed lymphocyte culture (MLC) was observed with
lymphocytes from C57Bl/6 mice (groups of 5-6 mice) exposed to 10 or
100 mg 3,4,5,3',4',5'-hexachlorobiphenyl, while the cytotoxic
T-lymphocytes CTL response generated in MLC was significantly
suppressed only with 100 mg/kg. The amount of time between treatment
with 3,4,5,3',4',5'-hexachlorobiphenyl and sacrifice, which ranged
from 2 to 23 days, did not appear to influence the degree of
immunosuppression produced by 3,4,5,3',4',5'-hexachlorobiphenyl
exposure. Mitomycin C-treated lymphocytes from C57Bl/6 mice treated
with 10 or 100 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body weight,
were not suppressive when added as third party cells to an independent
MLC. However, if the mice were alloimmune, lymphocyte-mediated
suppression of the MLC response was observed and directly correlated
with the magnitude of the CTL response present in the same population.
Thus, 3,4,5,3',4',5'-hexachlorobiphenyl-treated mice that had less CTL
activity compared with vehicle-treated mice also had less suppressor
activity. Avoidance of stimulator cells lysis by using H-2
incompatible MLC stimulator cells revealed the existence of
antigen-nonspecific suppressor activity that was greater with
lymphocytes from vehicle-treated mice than from 3,4,5,3',4',5'-
hexachlorobiphenyl-treated mice, suggesting that both CTL and
suppressor cell activities were suppressed by 3,4,5,3',4',5'-
hexa-chlorobiphenyl exposure. Direct addition of 3,4,5,3',4',5'-
hexachloro-biphenyl to lymphocyte cultures in vitro indicated
a lack of direct toxicity of 3,4,5,3',4',5'-hexachlorobiphenyl on
lymphoproliferative responses to mitogen or alloantigen at
concentrations equal to or less than 1 × 10-6 mol/litre. Thus,
the in vitro functional integrity of lymphocytes obtained from
3,4,5,3',4',5'-hexachlorobiphenyl-treated mice coupled with the lack
of a direct lymphocytic effect in vitro suggest an indirect
mechanism of action for the 3,4,5,3',4',5'-hexachlorobiphenyl-mediated
suppression of CTL activity in vivo. Previous reports implicating
suppressor cell induction and/or activation by Ah-receptor-binding,
halogenated, aromatic hydrocarbons that mediate the inhibition of CTL
generation were not confirmed (Kerkvliet & Baecher-Steppan, 1988b).
8.6.8.3 Appraisal
The alterations in gross measures of immunological function (spleen
and thymus weights, lymphocyte counts, histology of lymphoid organs
and tissues) in mammals are highly suggestive of an immunosuppressive
effect of PCB mixtures and some higher chlorinated congeners. More
direct evidence of an immunodepressive effect has been obtained by
methods that detect functional alterations in the humoral and
cell-mediated immunity in mammals. One study on monkeys demonstrated a
more severe immunosuppression by a PCDF-contaminated PCB mixture
compared with a non-contaminated PCB mixture. Rabbits and monkeys are
the most sensitive species. No-observed-effect levels are 100 µg
Aroclor 1248/kg body weight per day and <100 µg of Aroclor 1254/kg
body weight per day for monkeys and 180 µg of Aroclor 1254/kg body
weight per day for rabbits.
8.6.9 Neurotoxic effects
Depressed spontaneous motor activity was shown by male CD-mice exposed
to a single oral dose of 500 mg Aroclor 1254/kg body weight in
Emulphor:saline. No effects were found in motor coordination tests and
on pentenylene-tetrazol-induced seizures. Neurochemical tests with
isolated mouse brain synaptosomes showed inhibition of the uptake of
neurotransmitters and precursors, and stimulation of the release of
neurotransmitters (Rosin & Martin, 1981).
Male Wistar rats exposed to doses of 500 or 1000 mg Aroclor 1254 and
1260/kg body weight, in corn oil, showed a reduced norepinephrine
concentration in the frontal cortex and hippocampus. No changes were
measured in the hypothalamus and brainstem. The neurochemical effects
appeared to be associated with the actual presence of PCBs in the
tissues (Seegal et al., 1985).
8.6.10 Skin effects
Cutaneous effects occurred in Rhesus monkeys fed diets that contained
Aroclors, for short periods (Allen & Norback, 1973; Allen et al.,
1974a; Allen, 1975; Barsotti et al., 1976; Thomas & Hinsdill, 1978;
Allen et al., 1979; Altman et al., 1979; Becker et al., 1979;
McConnell et al., 1979; McNulty et al., 1980). The effects included
facial (particularly periorbital) oedema, purulent discharge from the
eyes, chloracne, and alopecia. The effects, which appeared to be
reversible, were produced by doses as low as 2.5 mg Aroclor 1248/kg
for 1-6 months, and 1 mg Aroclor 1242/kg (equivalent to 0.04 mg/kg
body weight) for 6 months. Rats exposed to Aroclor 1254 in the diet
developed alopecia and facial oedema after 104 weeks at 50 mg/kg, and
exophthalamos after 72 weeks at 50 mg/kg. These effects did not occur
after 104 weeks at 25 mg/kg (equivalent to 1.25 mg/kg body weight)
(NCI, 1978).
8.6.11 Effects on the lung
Many PCBs are metabolized to methylthio derivatives in mice (Mio et
al., 1976), seals (Jensen & Jansson, 1976) and humans (Yoshida &
Nakamura, 1979). The metabolic pathways leading to the generation of
these metabolites have been shown to involve glutathione conjugation
of an arenoxide intermediate (Preston et al., 1984). Lund et al.
(1986) studied the interactions of these metabolites with the lung. It
was found that the PCB metabolite, 4,4'-bis-(methylsulfonyl)-
2,5,2',5'-tetrachlorobiphenyl ((MeSO2)2TCB), selectively accumulates
in the Clara cells of the bronchiolar epithelium and in the secretory
contents of the bronchiolar lumen. In vitro characterization of this
interaction of tritiated (MeSO2)2TCB with lung suggests that this
selective accumulation is due to the presence of a secreted
(MeSO2)2TCB-binding protein in the respiratory tract of rats, mice,
and humans. The protein appears to be an almost globular,
low-relative-molecular-mass acidic protein that binds with
methylsulfonyl-PCBs (Lund et al., 1986).
In a study on 3 groups of C57Bl mice, administered orally 0, 10, or
100 mg 2,3,6,4'-tetrachlorobiphenyl/kg body weight with repeated
administration of 35S-cysteine, Klasson-Wehler et al. (1987) found
that the major compound present in the lung was 4-methylsulfonyl-
tetrachlorobiphenyl, indicating the presence of specific binding sites
for this metabolite in lung tissue, mainly in the tracheo-bronchial
mucosa.
Groups of 20 male SD rats were given (gastric intubation) 0 or 25 mg
Kanechlor 400 in edible oil once, and, in 2 other groups, the same
doses 4 times per week. Groups of 5 animals were killed 2, 7, 14, and
28 days after the last ingestion, and tissues were studied with light
and electron microscopy. The lungs of the rats particularly showed
peribronchiolar cell infiltrations, and electron microscopy revealed
lipid vacuoles and altered lamellar bodies or lysosomes in type II
alveolar cells and alveolar macrophages. These changes were most
marked 7 days after the last ingestion and were more severe in the
short-term application (Shigematsu et al., 1978).
8.6.12 Miscellaneous
Haake et al. (1987) used mature, male C57Bl/6J mice and virgin, female
C57Bl/6N mice to study the influence of Aroclor 1254 on the
2,3,7,8,-TCDD induction of teratogenic abnormalities. Dams were
treated by oral gavage with either corn oil, Aroclor 1254 (244 mg/kg),
or TCDD (20 µg/kg) or Aroclor followed by TCDD or Aroclor followed by
dexamethasone (90 mg/kg). Aroclor 1254 alone was administered on day
9, corn oil and TCDD on day 10, and dexamethasone on day 13. In the
combinations, the Aroclor was given the day before the TCDD or
dexamethasone. TCDD induced 61.8 ± 23.1% cleft palate per litter;
Aroclor with TCDD, 8.2 ± 1.5%; Aroclor alone, 0%; dexamethasone alone,
69.9 ± 18.2%, and Aroclor followed by dexamethasone, 85.8 ± 29.1%.
Previous studies have shown that Aroclor 1254 can act as a partial
antagonist of the microsomal enzyme induction and immunotoxic effects
of TCDD in the mice strain used and, in this study, it was shown that
Aroclor 1254 also antagonizes TCDD-mediated teratogenicity. It did not
have any effect on the effects mediated by dexamethasone.
Wölfle et al. (1988) found that treatment of male Wistar rats with
200 mg 3,4,3',4'-tetrachlorobiphenyl/kg body weight, injected
intraperitoneally, markedly stimulated growth of enzyme-altered liver
foci and [3H]-thymidine incorporation into nuclear DNA. In the liver,
enlarged hepatocytes, due to hypertrophy, and fine-to-medium
fatty-droplet deposition in hepatocytes were found, but no liver
necrosis. Hence, it was concluded, that post-necrotic regenerative
growth as the cause of the tetrachlorobiphenyl-mediated stimulation of
hepatocytes proliferation, could be excluded. The treatment with
tetrachlorobiphenyl in vivo, markedly increased EGF-stimulated
autophosphorylation of the EGF-receptor (a plasma membrane protein) in
liver plasma membranes. These results suggest that altered growth
control is due to a direct effect of tetrachlorobiphenyl on
hepatocytes.
8.7 Factors modifying toxicity; mode of action
8.7.1 Factors modifying toxicity
As PCBs can stimulate microsomal enzyme activity, it can be expected
that they may potentiate the action of other chemicals that undergo
microsomal activation, and antagonize the action of those that are
detoxified. Antagonism was for example observed in studies on rodents
with drugs like pentobarbital (Villeneuve et al., 1972; Sanders et
al., 1974; Sanders & Kirkpatrick, 1975; Rosin & Martin, 1983),
hexobarbital (Bickers et al., 1972; Tanaka & Komatsu, 1972), and
zoxazolamine (Bickers et al., 1972).
Lashneva et al. (1985) and Khan et al. (1985) found potentiation of
the rate of microsomal enzyme induction in rats with a combination of
50 mg Sovol (mixture of PCBs)/kg body weight and 500 mg 2,6-ditert-
butyl-4-methylphenol (ionol)/kg body weight.
Villeneuve et al. (1972) demonstrated the antagonistic effect by the
reduction of phenobarbital sleeping time in rats receiving Aroclors
1242, 1254, and 1260 in their diet, but not in those receiving Aroclor
1221. This was confirmed by Johnstone et al. (1974) with a series of
single PCBs. Tanaka & Komatsu (1972) found that the hexobarbital-
induced sleeping time in female rats was reduced to 49% of the control
value by daily oral doses of Kanechlor 500 of 2 mg/kg for 3 days
(total 6 mg/kg). When a daily dose of 0.4 mg/kg was given for 15 days
(total 6 mg/kg), no reduction in sleeping time was observed. When this
small dose was continued for 45 and 53 days, the reduction remained at
12-13%. Phillips et al. (1972) did not find any potentiation of the
cholinesterase-inhibitory action of parathion in rats dosed with
Aroclors 1221 and 1254; this does not necessarily imply that there was
no enhanced activation of parathion, as a stimulation of detoxication
may have occurred concurrently. A stimulation of parathion
detoxication, but not of activation, has been demonstrated in rabbit
microsomes (Villeneuve et al., 1971a). Lichtenstein (1972) reported a
potentiation by PCBs of the toxicity of parathion for flies.
Aroclor 1254 at 160 mg/kg diet fed to 5-week-old male and female
Fischer-344 rats, for 8 weeks, reduced mortality due to feeding
hexachlorophene at a concentration of 600 mg/kg diet, from 77% to 7%
and completely prevented the paralysis that was observed in all
animals on the hexachlorophene diet alone. However, histological
changes in the brain characteristic of hexachlorophene exposure were
still apparent in the animals on the combined treatment, and the
possibility of delayed toxicity beyond the 8 weeks of the study could
not be eliminated. The protective effect of Aroclor 1254 was explained
by its capacity to enhance detoxication by means of hepatic microsomal
enzyme induction (Jones et al., 1974).
Coté et al. (1985) studied a mixture of 15 "persistent" chemicals,
including Aroclor 1254, in Sprague-Dawley rats at dose levels of 0, 1,
10, 100, and 1000 times the Canadian water quality objectives (WQO) of
each chemical. The PCB (Aroclor 1254) treatments were 0, 0.001, 0.01,
0.1, or 1.0 µg/kg diet for 90 days. No influence on food intake, body
weight, organ weights, clinical chemistry, haematology, or
histopathology were observed.
As these polychlorinated hydrocarbons seem to have the same mechanism
of action, questions arise on the possible interactions between these
compounds. In one reported teratogenicity study, groups of 18-21
pregnant C57BL/6N mice received (by gavage) the vehicle corn oil, or
daily doses of 3 µg 2,3,7,8-TCDD/kg body weight, 10 or 20 mg
2,3,4,5,3',4'-hexachlorobiphenyl/kg body weight, 25 or 50 mg
2,4,5,2',4',5'-hexachlorobiphenyl/kg body weight, or combinations of
TCDD and hexachlorobiphenyl at these dose-levels in corn oil, from day
10 to day 13 of gestation. All chemicals were more than 98.9% pure and
the hexachlorobiphenyls did not contain detectable levels of
dibenzofurans or TCDD. TCDD alone caused a low incidence of cleft
palate and moderate hydronephrosis, 2,3,4,5,3',4'-hexachlorobiphenyl
only caused mild hydronephrosis, and 2,4,5,2',4',5'-hexachlorobiphenyl
did not produce any effects. However, treatment of pregnant mice with
a combination of TCDD and 2,3,4,5,3',4'-hexachlorobiphenyl caused
5- and 10-fold increases in the incidence of cleft palate at 10 and
20 mg of hexachlorobiphenyl/kg body weight, respectively. No
enhancement of TCDD-induced hydronephrosis was observed, and the
incidence of TCDD-induced cleft palate was not affected by
simultaneous 2,4,5,2',4',5'-HCB treatment (Birnbaum et al., 1985).
Male Sprague-Dawley rats were given a regimen consisting of PCBs,
1 mg/day; polychlorinated quarterphenyls (PCQs), 1 mg/day; PCDFs,
10 µg/day or a mixture of PCBs, PCQs, and PCDFs (1 mg + 1 mg +
10 µg/day) in olive oil, orally, for 22 days. The congeners and ratios
in the PCBs, PCQs, and PCDFs were the same as those in Japanese Yusho
oil (see section 9.1.2.1). The PCB-treated rats showed hepatic
hypertrophy, immunosuppression, and increased drug-metabolizing enzyme
activities in hepatic microsomes. PCQ treatment did not produce any
significant effects. PCDF and the mixture PCBs + PCDFs caused
hypertrophy of the liver, immunosuppression, increased and
drug-metabolizing enzyme activity to a much greater extent than that
found for PCBs (more than 100 times more) and weight loss and thymic
atrophy (Kunita et al., 1985).
Female Cynomolgus monkeys were administered PCBs (5 mg), PCQs (5 mg),
or a mixture containing 5 mg PCBs + 20 µg PCDFs in olive oil injected
in a piece of banana, daily, for 20 weeks. The PCBs and PCDFs
comprised the same congeners as those in Japanese Yusho oil. The
PCB-treated monkeys showed hepatic hypertrophy, immunosuppression, and
increased drug-metabolizing enzyme activities in hepatic microsomes,
but were devoid of the dermal symptoms characteristic of Yusho. PCQs
caused an increase in drug-metabolizing enzyme activities in hepatic
microsomes and immunosuppression, but these effects were much less
severe than those of PCBs. The mixture with PCDFs caused hypertrophy
of the liver, immunosuppression, increase in drug-metabolizing enzyme
activities (more than 100 times that of PCBs) and weight loss and
thymic atrophy. Dermal symptoms characteristic of Yusho patients were
also found, but not with PCBs or PCQs alone (Kunita et al., 1985).
8.7.2 Mechanisms of toxicity
The analogous structure-activity relations of individual PCB congeners
with respect to most of their toxic responses and to their potency in
inducing cytochrome P-448-dependent aryl hydrocarbon hydroxylase,
indicate that the most active PCB congeners (the coplanar congeners)
are those that are approximate stereoisomers of 2,3,7,8,-tetra-
chlorodibenzo- p-dioxin (TCDD). These findings suggest a common
mechanism of action.
As is proposed for 2,3,7,8-TCDD, this mechanism is based on the
binding affinity of PCB congeners to the cytosolic Ah-receptor
protein, a product of the regulator Ah gene (Poland & Glover, 1977;
Parkinson & Safe, 1981; Bandiera et al., 1982). The induction is
dependent on the position and number of chlorine atoms in the molecule
and the congeners that bind most strongly to the Ah-receptor show the
strongest induction of monooxygenases and the highest toxicity
(effects on the liver including increase in liver weight, increase in
liver enzyme activity and lipid content, porphyria, atrophy of the
thymus, and effects on reproduction) (Ecobichon & Comeau, 1975;
Goldstein, 1980). These very active congeners are the non
ortho-substituted PCBs 3,4,3',4'- tetrachloro-, 3,4,5,3',4'-
pentachloro-, and 3,4,5,3',4',5'-hexachlorobiphenyl, which are at
least twice substituted in the meta and para positions.
In this model, the inducer-receptor complex is translocated into the
nucleus, interacts with DNA, and eventually triggers the pleiotropic
responses observed. The role of the receptor protein in the mechanism
of action of PCBs is further substantiated by the differential effects
of the congeners in non-responsive DBA/2J mice and responsive C57Bl/6J
mice. Furthermore, there is a good relationship between the aryl
hydrocarbon hydroxylase and ethoxyresorufin O-deethylase induction
potencies in rat hepatoma H-4-II-E cells in vitro (Sawyer & Safe,
1982) and their relative binding affinities for the male Wistar rat
hepatic cytosol receptor protein (Bandiera et al., 1982; Safe et al.,
1985b).
Thus, the coplanar PCBs have mechanisms of action similar to those of
the polychlorinated dioxins (PCDDs) and dibenzofurans (PCDFs) (see
also WHO, 1989).
On the basis of the comparative toxic and biochemical potencies of
coplanar and mono ortho coplanar PCBs, Safe (1990) suggested toxic
equivalent factors TEFs (relating to 2,3,7,8-tetrachloro-dibenzo-
p-dioxin, TCDD, see WHO/EURO, 1987) for these compounds. See Table
53. Although there are certain limitations and uncertainties
associated with the use of TEFs (WHO/EURO, 1987), they may be useful
for an attempt to assess the risk of the combined exposure to coplanar
PCBs and PCDDs/PCDFs.
Table 53. Proposed Toxic Equivalent Factor (TEF) values for the
coplanar and mono ortho coplanar PCB congenersa
Congener TEF value Relative potency range
1. Coplanar PCBs
3,4,5,3',4'-PeCB 0.1 0.3-0.0006
3,4,5,3',4',5'-HxCB 0.05 0.1-0.0012
3,4,3',4',-TCB 0.01 0.009-0.00008
2. Mono ortho coplanar
2,4,5,3',4'-PeCB 0.001 0.0004-0.000006
2,3,4,3',4'-PeCB 0.001 0.0008-0.00006
3,4,5,2',4'-PeCB 0.001 0.00013-0.000018
2,3,4,5,4'-PeCB 0.001 0.00045-0.000074
2,3,4,3',4',5'-HxCB 0.001 0.0005-0.0000014
2,3,4,5,3',4'-HxCB 0.001 0.0004-0.0000065
2,4,5,3',4',5'-HxCB 0.001 0.0000055
2,3,4,5,3',4',5'-HpCB 0.001 no data available
a From: Safe (1990).
The frequently occurring mixed type, the PB-type, and the
weak/noninducing type congeners with some degree of ortho
substitution may also exert other more subtle toxic effects, either
directly via conversion to hydroxylated derivatives, or indirectly
through environmental transformations involving regiospecific
dechlorination followed by hydroxylation.
In addition, some PCBs, particularly the lower chlorinated ones, can
be more readily metabolized through arene oxide intermediates that may
be directly genotoxic/carcinogenic. Arene oxide intermediates are also
involved in the formation of the methylsulfonyl metabolites of PCBs,
which selectively accumulate in the Clara cells of the bronchiolar
epithelium and in the secretory contents of the bronchiolar lumen of
the lungs. This apparently also involves specific binding proteins
that may be important in the expression of certain types of pulmonary
toxicity.
Finally, there are other forms of toxicity associated with PCBs that
appear to involve certain non-receptor, protein-binding interactions.
8.7.3 Toxicity of impurities in commercial PCBs
In many toxicological studies on the effects of commercial PCB
mixtures, the quantitative contribution of impurities to the toxic
responses found is largely unknown (WHO, 1989; WHO/EURO, 1987).
9. EFFECTS ON HUMANS
The toxicological evaluation of PCBs presents many problems. PCBs
usually occur as mixtures of many congeners, and many of the data on
the toxicity of the PCBs are based on the testing of these mixtures.
Some components of the mixtures are more easily degraded in the
environment than others. Thus, the general population may be exposed
to mixtures that are different from those to which workers, working
with PCBs, are exposed.
There are great difficulties in assessing human health effects
separately for PCBs, since, quite frequently, PCDFs have been present
in the PCB mixtures to which humans have been exposed. The presence of
PCDDs has occasionally been seen in accidents with certain
PCB/chlorobenzene mixtures. Commercial PCBs have been shown to be
contaminated with PCDFs and, therefore, in many cases it is unclear
whether effects were attributable to the PCBs themselves or to the
much more toxic PCDFs.
Because PCBs are ubiquitous and very persistent in the environment,
humans have been, and will continue to be, exposed to them,
particularly in industrialized countries. PCBs may be inhaled in small
amounts through the air or ingested through food. People are primarily
exposed to PCBs by consuming fish from contaminated water, but they
can be also exposed via other food.
Furthermore, exposures have occurred through accidents and
occupational exposure; in the latter case, for example, during the
repair of transformers, capacitors, or during the handling of toxic
wastes.
Since PCBs are lipophilic, they are preferentially stored in adipose
tissue. They are also present, to a smaller extent, in serum, organs
and tissues, and human milk. The concentrations of PCBs in the
different organs depend on the lipid content of such organs, with the
exception of the brain, where the concentration is lower than the
lipid content would indicate. PCBs pass, to a certain extent,
(depending on chlorination and structure) through the placenta. They
are primarily excreted through the bile and milk. In addition to the
lipid content, the ratios between adipose tissue, blood, and organs
are influenced by exposure level, sex, age, duration of exposure, and
also by whether exposure is current (see section 5).
Since human milk is relatively easy to obtain, it has been used to
monitor human exposure. Results of the many monitoring studies carried
out in many countries all over the world have shown that average
levels of total PCBs are below 2 mg/kg milk fat, though women living
in heavily industrialized urban areas or who consume large quantities
of fish from heavily contaminated areas, may have higher levels.
9.1 General population exposure
The general population is exposed to PCBs primarily by the oral route,
e.g., by consumption of fish from contaminated waters. The monitoring
data on adipose tissues, blood, and breast milk indicate that PCBs are
absorbed via the gastrointestinal tract, but do not provide
information regarding the extent of the absorption.
9.1.1 Acute effects -- poisoning incidents
No data available.
9.1.2 Effects of short- and long-term exposure
9.1.2.1 Yusho and Yu-Cheng accidents
(a) Yusho accident
In June 1968, patients appeared at the Dermatology Clinic of Kyushu
University Hospital, Fukuoka, Japan, suffering from chloracne. A group
at the University undertook intensive clinical, chemical, and
epidemiological investigations and found that the disease originated
from the consumption of a batch of rice oil supplied in February 1968;
the disease was called Yusho (rice oil disease) (Katsuki, 1969). This
batch of rice oil was found to be contaminated with Kanechlor 400, a
48% chlorinated biphenyl, at 2000-3000 mg/kg, which entered the oil
through a leak in a heat exchanger (Tsukamoto, 1969). Chlorinated
dibenzofurans at 5 mg/kg were found in 3 samples of the toxic rice oil
that contained PCB levels of about 1000 mg/kg (Nagayama et al., 1976).
The Japanese literature on this incident has been summarized in
English by Kuratsune et al. (1976). The average estimated intake was
633 mg PCBs, 3.4 mg PCDFs, and 596 mg PCQs, roughly equivalent to
157 µg PCBs/kg per day, 0.9 µg PCDFs/kg per day, and 148 µg PCQs/kg
body weight per day (Chen et al., 1985; Masuda et al., 1985).
The symptoms and signs of Yusho were described by Goto & Higuchi
(1969) and by Okumura & Katsuki (1969). The earliest signs were
enlargement and hypersecretion of the Meibomian glands of the eyes,
swelling of the eyelids, and pigmentation of the nails and mucous
membranes, occasionally associated with fatigue, nausea, and vomiting.
This was usually followed by hyperkeratosis and darkening of the skin
with follicular enlargement and acneiform eruptions, frequently with a
secondary staphylococcal infection. These skin changes were most often
seen on the neck and upper chest, but, in severe cases, extended to
the whole body. It was estimated that the mean length of the latency
period between exposure and the onset of clinical illness was 71 days,
with a range of from 20 to 190 days (Kuratsune et al., 1972).
Biopsy skin samples showed hyperkeratosis, dilation of the follicles,
and an accumulation of melanin in the basal cells of the epidermis;
melanin granules have also been observed in biopsy samples of the
conjunctiva. Oedema of the arms and legs was also seen in some
patients. There were no definite signs of liver enlargement or liver
disorders (Okumura & Katsuki, 1969), but slight rises in serum
transaminases and in alkaline phosphatase were detected, and a liver
sample from a Yusho patient showed an increase in the smooth
endoplasmic reticulum (Hirayama et al., 1969). The majority of the
patients were found to have respiratory symptoms, and suffered from a
chronic bronchitis-like disturbance that persisted for several years
(Shigematsu et al., 1971, 1978).
Kikuchi (1984) described the autopsy findings, up to July 1982, of 12
patients with Yusho including 2 stillborn babies. Characteristic
pathological changes were acne-like eruptions and cutaneous
pigmentation with histological features of follicular hyperkeratosis,
dilated hair follicles, and an increase of melanin pigment in the
epidermis. In addition, multiplication of the duct epithelium of the
oesophageal glands was found in 6 patients. Twenty-four Yusho patients
were observed clinically over the period 1968-78. During this decade,
the various clinical symptoms of the Yusho patients gradually
diminished. However, some of the symptoms and signs, such as
pigmentation of the skin, conjunctiva, and gingiva, eye discharge, and
various non-specific symptoms still remained in a number of severely
ill patients (Okumura, 1984).
Nakanishi et al. (1985) carried out clinical and experimental studies
on respiratory involvement and alterations in the immune status. PCBs
were not taken up by the bronchi, but were evenly distributed
throughout the lung parenchyma. However, specific dose dependence and
structural requirements of PCBs were shown to exist for accumulation
in bronchial mucosa. A large amount of expectoration at an early stage
of the disease may be related to this. Pathophysiological findings in
Yusho patients revealed that respiratory involvement was mainly small
airways disease, which may be caused by involvement of the cellular
component (Clara cells) in the bronchioles and/or associated with
viral or bacterial infections.
Changes in the immune status in these Yusho patients were decreases in
IgA and IgM in the serum at an early stage of the disease and then a
return to normal and suppression of cellular immunity. The changes in
immune status in these Japanese patients were comparable with the
findings in the Taiwanese patients (see below).
Hirayama et al. (1974) also reported that the serum bilirubin levels
of patients were significantly lower than the normal level and that it
was negatively correlated with the blood level of PCBs and the serum
triglyceride level.
A considerable number of patients had elevated serum triglyceride
levels, up to 4 times the normal values, though this was not
correlated with the severity of the symptoms; these high values were
maintained for 3 years in many patients (Uzawa et al., 1972). There
were no marked abnormalities in serum cholesterol and phospholipid
levels (Okumura & Katsuki, 1969; Uzawa et al., 1969). Nagai et al.
(1969) reported an increase in urinary 17-ketosteroids excretion.
Kusuda (1971) also observed changes in the menstrual cycle in
approximately 60% of 81 female Yusho patients, when compared with
their cycles prior to exposure. A positive correlation was observed by
Okumura et al. (1974) between the blood levels of triglycerides and
PCBs in 42 patients.
Shigematsu et al. (1971) examined serum immunoglobulin levels in 38
patients, 2 years after the onset of the disease, and observed a
decrease in IgA and IgM and an increase in IgG. Lower IgM levels were
reported in patients showing chloracne (Saito et al., 1972) (see also
section 9.2.4.2).
Yusho patients did not appear to suffer from central nervous effects,
but some complained of numbness of the arms and legs. Murai & Kuroiwa
(1971) found a decrease in the conduction velocity in peripheral
sensory nerves.
Determinations of PCB concentrations in the tissues of Yusho patients
were made several months after the ingestion of the oil, using an
X-ray fluorescence method for organic chlorine (Goto & Higuchi, 1969).
Abdominal fat contained 13.1 mg/kg, subcutaneous fat 75.5 mg/kg, and
nails 59 mg/kg. The mesenteric adipose tissue in 6 Yusho patients,
analysed by gas-liquid chromatography 1-3 years after the occurrence
of intoxication, contained an average PCB level of 2.5 mg/kg, which
was considerably higher than the normal value (Masuda et al., 1974a).
The mean blood level of PCBs in patients was 6 or 7 µg/litre
(3 µg/litre for the general population), 5 years after exposure
(Masuda et al., 1974b; Takamatsu et al., 1974). These authors also
noted a specific gas-liquid chromatographic pattern that was peculiar
to Yusho patients.
Eleven years after exposure, a mean concentrations of 6 µg PCB/litre
and 2 mg PCQs/litre, but no PCDFs, were found (Kashimoto et al.,
1985).
Urabe (1974) reported that the total number of Yusho patients had
reached 1200 by September 1973 and that 22 of them had died. At the
end of 1982, the number of identified patients was 1788 (Urabe &
Asahi, 1985). Mucocutaneous signs had decreased year by year, but
neurological and respiratory signs and symptoms and various
complaints, such as general fatigue, anorexia, abdominal pain, and
headache, had become more prominent among the patients. Over time, the
severity and the extent of the skin lesions decreased considerably in
the exposed population. Fifteen years after the accident, only a few
patients still had extensive chloracne.
By 1979, 31 Yusho patients had died, 11 (35.4%) of these from
malignant neoplasms. Only 21.1% of all deaths in this Japanese
prefecture would be expected from malignant neoplasms, but no clear
correlation between the occurrence of Yusho and increased deaths from
malignant neoplasms could be made, because of the small number of
deaths observed and the unknown latency period.
By the end of 1983, 120 Yusho patients had died, 41 of these from
malignant neoplasms. These included 8 stomach cancers, 11 liver
cancers, and 8 neoplasms of the lung. A statistically significant
excess mortality was seen for malignant neoplasms, cancer of the liver
and cancer of the lung, trachea, and bronchi in males, but no such
excess was noted in females. The excess from liver cancer deaths was
seen mainly in Fukuoka prefecture, while no excess was seen in the
Nagasaki prefecture (Ikeda et al., 1987).
(b) Yu-Cheng accident
In 1979, a similar incident occurred in Taiwan, the number of persons
involved, by the end of 1980, was 1843. In the course of 3.5 years,
2061 persons were determined to be victims of PCB poisoning. The
incident has been referred to as Yu-Cheng (Chang et al. 1980a,b, 1981;
Chen et al., 1980, 1981; Hsu et al., 1985). The affected persons had
consumed rice-bran oil contaminated with PCBs that was used as a heat
transfer medium in the manufacture of the oil. The PCB intake was
estimated to be 0.7-1.84 g and the latent period from the time of
intake to the onset of clinical manifestations was approximately 3-4
months. The average estimated PCDFs intake was 3.8 mg and 586 mg PCQs
(Chen et al., 1985a). Blood PCB levels ranged from 3 to 1156 µg/litre:
44.3% of 613 patients had levels of 51-100 µg/litre, and 27.6%, blood
levels over 100 µg/litre. Six months after the exposure, the
concentrations of PCBs, PCDFs, and PCQs were 12-50, 0.062-0.24, and
1.7-11 µg/litre. These blood levels were much higher than in the Yusho
incident.
The concentrations of PCBs, PCDFs, and PCQs in 6 samples of rice-bran
oil were 53-99 mg/kg, 0.18-0.40 mg/kg, and 25-53 mg/kg oil,
respectively (Chen et al., 1981; Hsu et al., 1985; Chen et al.,
1985a). Miyata et al. (1985) found averages of 62 mg PCB/kg, 140 µg
PCDFs/kg, and 20 mg PCQs/kg in 5 samples of oil. The levels of toxic
compounds in rice-oil samples collected from the factory and school
cafeterias and the families of the intoxicated patients in Taiwan were
in the range of 53-99 mg PCBs/litre (except for one sample with
405 mg/litre), 0.18-0.40 mg PCDFs/litre, and 25-53 mg PCQs/litre,
respectively. The most toxic PCB reported in commercial PCB
preparations was 3,4,3',4'-tetrachlorobiphenyl (Chen et al., 1984).
One hundred and thirty patients (46 males and 84 females), exposed
accidentally to PCBs in Taiwan, were examined for ocular
manifestations in 1979-80. Eye discharge was present in 80.5%,
swelling of the upper lids in 60.4%, pigmentation of conjunctiva in
67.6%, hypersecretion and cystic swelling of the Meibomian glands in
70.7%. Heavy pigmentation of conjunctiva, abnormal cystic formation
and hypersecretion of the Meibomian glands occurred in patients whose
blood PCBs concentration was above 40 µg/litre. There was a
correlation of the ocular effects and the blood concentration of PCBs
(Fu, 1984).
Wong et al. (1985), determined the enzyme activity in placental
tissue, obtained from 4 women who were exposed to contaminated
rice-oil in Yu-Cheng 3-4 years before conception. Placental
homogenates showed increases in monooxygenase enzymes, including aryl
hydrocarbon hydroxylase, 7-ethoxycoumarin O-deethylase, and diol,
quinone and phenolic metabolites of benzo (a)pyrene.
Lu & Wong (1984) described, in detail, the dermatological, medical,
and laboratory findings on 829 patients (half males and half females)
in Taiwan, poisoned with PCBs and related compounds. The ages of the
patients ranged from 7 days to 78 years. A grading of the clinical
severity of these cases was tried, and a possible association with the
PCB concentrations in their blood was examined, but could not be
demonstrated. The mean PCB concentration in 278 patients was 89.1 ±
0.9 µg/litre (median value 55 µg/litre); the maximum level was
1156 µg/litre and the minimum, 3 µg/litre.
One hundred and ten patients were studied within one year of the
exposure. The mean blood PCB level was 39.3 ± 16.6 µg/litre, and the
mean blood PCDFs and PCQs levels were 0.076 ± 0.038 and 8.6 ±
4.8 µg/litre, respectively. Both the sensory and motor nerve
conduction velocities of the patients were significantly lower than
those of the controls (cases studied in the past who did not have
neurological diseases) (Chen et al., 1985b).
Thirty-five patients out of 2000 cases of PCB poisoning in Taiwan were
examined neurologically 2 years after the accident. The neurological
manifestations included clinical, peripheral sensory neuropathy,
headache, and dizziness. There was no relationship between the blood
PCB concentrations in patients with neurological manifestations and
those without. Sensory nerve conduction velocity was reduced and motor
nerve conduction was delayed in about one-third to one-half of the
patients (Chia & Chu, 1984).
The blood samples of 165 patients, collected 9-18 months after the
onset of poisoning, contained 10-720 µg PCBs/litre with a mean value
of 38 µg/litre (Chen et al., 1984).
It is worth noting that the highly toxic 2,3,7,8-tetrachloro-,
2,3,4,7,8-pentachloro-, and 1,2,3,4,7,8-hexachlorodibenzofuran isomers
were present in samples from both the Japanese (Yusho) and the
Taiwanese (Yu-Cheng) incidents.
The most common symptoms noticed were acneiform eruptions and
follicular accentuation, skin and nail pigmentation, swelling of the
eyelids and increased discharge from the eyes; headache, nausea, and
numbness of the limbs. The major blood disorders were decreased
erythrocyte counts, haemoglobin concentration, and gamma-immunoglobin,
and increased white blood cell counts, serum triglyceride levels, and
SGOT, SGPT, and serum alkaline phosphatase activities. Decreased
concentrations of delta-aminolaevulinic acid and uroporphyrin were
also observed (Chang et al., 1980a,b).
9.1.2.2 Effects of PCBs on babies and infants
Yoshimura (1971) reported diminished growth in boys, but not in girls,
who had consumed the oil. Babies born to Yusho mothers were smaller
than normal. Newborn babies showed a dark brown skin pigmentation that
disappeared after a few months (Taki et al., 1969; Yagamuchi et al.,
1971). Funatsu et al. (1972) found spotted and sporadic ossification
of the skull and facial oedema with exophthalmia in 4 babies, but
there was no evidence of any teratogenic action. Pregnant Yusho
mothers delivered babies with a peculiar clinical manifestation, which
was called Fetal PCB Syndrome (FPS). In total, 36 babies showed this
syndrome. It consisted of dark brown pigmentation of the skin and
mucous membranes, gingival hyperplasia, exophthalmic oedematous eye,
dentition at birth, abnormal calcification of the skull (as
demonstrated by X-ray), rocker bottom heel, and a high incidence of
low birth weight babies. It was suggested by the authors that a
possible alteration in calcium metabolism in FPS might be related to
the action of PCBs (PCDFs) on female hormones. There was no evidence
of hypoadrenocorticism which would explain dark pigmentation in FPS
children (Yamashita & Hayashi, 1985).
Jensen (1983b) calculated that the daily intake of PCBs by Yusho
infants with clinical symptoms of poisoning was of the order of
70 µg/kg body weight.
Kuratsune et al. (1972) investigated whether Yusho disturbed
children's growth. The affected school children, 23 boys and 19 girls,
were compared in 1967, 1968, and 1969, with 719 healthy classmates
matched by sex. The gains of the affected boys in both height and
weight decreased significantly after the poisoning, while the affected
girls did not show any changes in these respects.
Studies were carried out by Fein et al. (1984), Fein (1984), and
Jacobson et al. (1984a,b) on 242 newborn infants whose mothers had
consumed moderate quantities of contaminated lake fish and 71 newborn
infants whose mothers had not eaten such fish, during the immediate
postpartum period. PCB exposure (measured by both contaminated fish
consumption and cord serum PCB levels) predicted lower birth weight,
shorter length of gestation, and smaller head circumference. Both
maternal consumption of fish and levels of PCBs in cord serum were
positively correlated with lower birth weight, shorter gestation and
smaller head circumference, and with impaired autonomic maturity and
increased numbers of abnormal reflexes.
In the studies by Schwartz et al. (1983), Fein et al. (1984), and
Jacobson et al. (1984a), the influence of important variables, such as
smoking and alcohol use, were not studied extensively enough. The
Brazelton test (Brazelton, 1973) was used in these studies. However,
this test was never intended to be used to evaluate neurological
conditions (Prechtl, 1982). The value of this test to predict
behavioural abnormalities in infants is small. The Public Health
Council of the Netherlands (1985) concluded, therefore, that the
reported changes could not be interpreted by the Brazelton test. The
important confounding factors "smoking" and "alcohol" were not studied
or not well studied, while it is known that these factors can result
in such changes. Furthermore, there was an indication that women
consuming more fish also consumed more alcohol and coffee and used
more medical drugs than those who were not fish eaters.
Jacobson et al. (1985) examined visual recognition memory in
7-month-old infants of women who had consumed contaminated Lake
Michigan fish. The authors reported a statistically significant
correlation between cord serum PCB levels and impairment of visual
recognition memory. It should be mentioned, however, that
interpretation of these test results is difficult. In view of the
variability associated with the measurements of fixation time (no
standard deviations were reported), it is unclear whether any of the
group means are statistically different. Moreover, the clinical
meaning of the differences noted is not known.
Neonatal effects of transplacental exposure to PCBs (and DDE) were
examined in a study on 912 children born between 1978 and 1982 in
North Carolina. When the infants were born, samples of placenta,
maternal and cord serum, and milk/colostrum were collected. Physical
examination of each infant was performed and the Brazelton test
(Brazelton, 1973) was applied. Fifty-nine per cent of the examinations
were carried out in the first week, 20% in the second week, and 16% in
the third week. The PCB levels in milk fat at birth (866 samples)
ranged from nd to 4.0 mg/kg. There was no association between PCB
levels and birth weight, head circumference, and hyperbilirubinaemia
(neonatal jaundice). For the Brazelton test, the only cluster scores
to be significantly affected by PCBs were the tonicity and reflex
scores, with higher PCB levels (above 3.5 mg/kg milk fat). The results
showed that higher PCB levels were associated with hypotonicity and
hyporeflexia while higher DDE levels (4 mg/kg milk fat) were
associated with hyporeflexia (DDE concentrations in milk fat ranged
from nd to >6 mg/kg milk fat) (Rogan et al., 1986a). As a follow-up
study, Rogan et al. (1987) followed 858 children in the USA, from
birth to one year of age, to determine whether the presence of PCBs in
breast milk affected their growth or health. The PCB concentrations in
breast milk ranged from 0.49 to 15.80 mg/kg, on a fat basis, and the
DDE concentrations from 0.31 to 23.8 mg/kg milk fat. The lactation
period varied from 13 (mothers with 4.00-15.80 mg PCBs/kg in their
milk) to 26 weeks. No adverse effects on body weight or the frequency
of visits for various illnesses were observed. There was no difference
between bottle-fed and breast-fed children. In 1985, about 6 years
after the mass poisoning in Taiwan, 117 children born to affected
women ( in utero exposure during, or after, the period of oil
contamination) and 108 unexposed controls were examined (Rogan et al.,
1988). The exposed children were shorter and lighter than the
controls; they had more frequent abnormalities of the gingiva, skin,
nails, teeth, and lungs than control children. The exposed children
showed delay in developmental milestones, deficits on formal
development testing, and abnormalities in behavioural assessment.
A follow-up study was carried out to determine the relationship
between PCBs in mother's serum and breast milk and the health and
development of the born infants, in Sheboygan, Wisconsin, USA, in the
period 1980-81. Seventy-three mothers gave birth to 62 infants that
were breast-fed and 11 that were bottle-fed. The ages of the mothers
ranged from 18 to 36 years. The mean serum PCB level for the study
population was 5.76 µg/litre (range, 1.29-14.9 µg/litre). Breast milk
contained a mean PCB level of 1.13 mg/kg (range, 0.29-4.02 mg/kg) on a
fat basis. The mother's blood serum PCB level during pregnancy was
positively associated with the number and type of infectious illnesses
the infants suffered later, such as colds, earache, and influenza
during the first 4 months of life. The development and growth of the
infant up to the age of 4 months was normal and was not affected by
PCB levels (Smith, 1984).
Lan et al. (1989) selected 18 exposed children (9 males and 9
females), and a reference group of 44 unexposed children (26 males and
18 females), to study the congenital absence of permanent teeth. Among
9 transplacental Yu-Cheng girls and 9 boys, the permanent teeth germ
was missing due to congenital factors in 4 girls and one boy. In the
control group, one boy showed this phenomenon. Fukuyama et al. cf. Lan
et al. (1989) had already reported this effect in 1979.
Gladen et al. (1988a) investigated whether PCBs, either transplacental
or through breast feeding, affected the scores on the Bayley Scales of
infant development at 6 or 12 months of age, in 802 infants. Higher
transplacental exposure to PCBs was associated with lower psychomotor
scores at both 6 and 12 months of age. Exposure to PCBs through
breast-feeding was apparently unrelated to Bayley scores.
The urine of 75 children born to mothers exposed to contaminated rice
oil in Taiwan (1979), 74 controls, and 12 siblings of the children
exposed between 1978 and 1985, was analysed for the presence of
porphyrins. Total porphyrin excretion was elevated in the exposed
children in comparison with the other 2 groups (exposed group,
95.2 µg/litre, control group, 80.7 µg/litre, and siblings,
72.6 µg/litre. The exposed children did not appear to have symptoms
directly attributable to their porphyria, but the authors concluded
that a mild disturbance in their porphyria metabolism appeared to be
related to their intrauterine exposure (Gladen et al. 1988b).
Thirty-nine babies showing hyperpigmentation were born to PCB-poisoned
mothers. In the orally exposed population of the Yu-Cheng episode, 24
deaths were reported and as many as 12 cases of hepatic disease
including hepatomas, which was more than expected (Hsu et al., 1985).
9.1.3 Appraisal
Several Japanese research groups have concluded that the main signs
and symptoms involved in the Yusho intoxications were caused by
contaminants in the PCB-mixture, i.e., mainly PCDFs (Masuda et al.,
1985; Kunita et al., 1985). This conclusion has mainly been based on
the following observations:
(a) Blood levels of PCBs in the victims were not very different from
those in the general population and several occupationally-exposed
groups had higher PCB blood levels in the absence of any recognizable
adverse health effects.
(b) There was an unusually high level of PCDF-contamination in the
PCBs that contaminated the rice oil.
(c) Signs and symptoms did agree with what could be expected from
exposure to PCBs and/or PCDFs (PCDDs).
However, blood levels in the Yusho victims were determined 5 years
after exposure. Consequently, at the time of the intoxication, blood
levels might have been much higher.
Furthermore, later studies on the biological potency of the
dioxin-like coplanar PCBs indicated that the occurrence of these might
have added significantly to the overall toxicity of the PCDFs (Safe,
1990).
In the case of the Yu-Cheng intoxication, blood levels of PCBs were
determined within 1 year of the accident and were found to be much
higher (i.e., about 70 µg/litre) than in the Yusho intoxication.
However, even at this time, some elimination of PCBs, especially lower
chlorinated PCB congeners, can be assumed to have taken place. Thus,
blood levels in the Yu-Cheng intoxication might also have been higher
initially.
In summary, it can be concluded that the main symptoms of the Yusho
and Yu-Cheng intoxications might have been caused mainly by combined
exposure to PCBs (mainly the coplanar ones) and PCDFs. However, some
of the symptoms, especially the chronic respiratory effects, may have
been caused specifically by the methylsulfone metabolites of certain
PCB congeners.
9.2 Occupational exposure
9.2.1 Acute toxicity -- poisoning incidents
9.2.1.1 Acute dermal effects
Skin rash has occurred within a few hours after acute exposure to
PCBs. Furthermore, itching, burning sensations, smarting, and sweating
have been reported. Irritation of the conjunctiva was a constant
symptom in acute exposure to high concentrations (Elo et al., 1985;
Schecter & Tiernan, 1985). A few weeks or months after acute exposure
(lasting a few hours) to high concentrations of PCBs (10-16 mg/m3),
several skin symptoms were observed in some of the accidentally
exposed population, such as slight pigmentation, ridges on the nails,
and the worsening of ache vulgaris (Elo et al., 1985). Skin wipe tests
on PCB-exposed workers were carded out by Maroni et al. (1981a), Smith
et al. (1982), and Wolff (1984) for capacitor manufacturers,
electrical equipment manufacturers, and transformer inspectors.
Concentrations varying between 2 and 28 000 µg/m2 of skin were
measured. Considerable concentrations of PCBs were also found on the
surfaces of hand tools in the factories (Maroni et al., 1981a;
WHO/EURO, 1987).
9.2.2 Effects of short- and long-term exposure
Exposure through ingestion is possible in the working environment
through the direct ingestion of soot particles or through the
contamination of cigarettes or food by hands. Maroni et al. (1981a)
measured high concentrations of PCBs in the palmar skin of capacitor
workers; this might lead to the oral ingestion of PCBs, in addition to
exposure via the dermal route.
Skin exposure is important in the case of long-term exposure, even
though the ambient concentrations may be low. According to
calculations made by Wolff (1985), in long-term exposure situations,
skin may be responsible for up to 20% of the total body uptake of PCBs
in workers exposed in capacitor manufacturing (WHO/EURO, 1987).
Symptoms similar to those of Yusho have been observed in workers in a
Japanese condenser factory, including pigmentation of the fingers and
nails, and acneiform eruptions on the jaw, hack, and thighs. It was
thought that these effects arose from local contact with PCBs; when
the use of PCBs ceased, the symptoms disappeared (Hasegawa et al.,
1972b). Chloracne is one of the most prevalent findings among
PCB-exposed workers and particularly among those exposed to highly
chlorinated compounds. Hara (1985) found prevalences of comedones and
acne of 40%, and skin irritation and erythema of 13% in workers
exposed for 1-24 years to Kanechlor 300 and 500. The blood PCB levels
were 21-117 µg/litre. The degree of skin pathology was correlated with
the blood PCB concentrations. In the production of capacitors,
(oculo-) dermatological abnormalities were found in 37% of the cases,
but typical PCB-associated changes were less prevalent. Fischbein et
al. (1979) suggested that these signs and symptoms were due to
exposure to PCDFs and/or PCDDs.
Fischbein et al. (1982) evaluated the dermatological effects of
long-term (< 5 - > 20 years) occupational exposure to PCBs, in a
cross-sectional clinical survey of 326 capacitor manufacturing
workers. Air PCB levels varied in the plant from 0.007 to 11.0 mg/m3.
A high prevalence (37%) of a wide spectrum of dermatological
abnormalities was found, such as rashes, burning sensation of the
skin, and chloracne, in most cases associated with typical comedones
(6%), but the occurrence was less than that observed in Yusho
patients, even though the serum PCB levels in the workers were much
higher.
Two persons were reported with dermatological abnormalities suggestive
of, but not specific for, chloracne, after occupational exposure to
PCBs (Fischbein & Wolff, 1987). They had raised serum PCB
concentrations (of the order of 80-100 µg/litre). Their wives also had
increased blood PCB levels, with the same PCB pattern as their
husbands. It was suggested by the authors that it would seem prudent
to take appropriate industrial hygiene measures, to prevent the
transmission of PCBs from the occupational environment into the home.
A cross-sectional study on 120 male workers was conducted to determine
the prevalence of increased PCB absorption, as well as the presence of
potentially-related clinical and metabolic abnormalities. Three groups
were used: an exposed group (86), a nominally exposed (15), and an
unexposed group (19 subjects). The exposed group had direct contact
with PCB-containing transformer fluids, while the nominally exposed
group worked in the same facility, but without direct contact, and the
unexposed group was employed elsewhere. The average length of
employment was 17 years (range, few months-40 years), 3 years and 9
months, and 4 years and 3 months, for the 3 groups, respectively. The
average plasma PCB levels were 33.4, 14.2, and 12.0 µg/litre, and the
average concentrations in adipose tissue were 5.6, 1.4, and 1.3 mg/kg,
respectively. There were no statistically significant differences
among the groups in levels of triglycerides, cholesterol, high-density
lipoproteins, and SGOT. A significant correlation was demonstrated
between plasma PCBs and triglycerides and SGOT values, but not SGPT
and gamma-GTP values (Chase et al., 1982).
To investigate the prevalence of oculo-dermatological findings, such
as hypersecretion of the Meibomian glands, swelling of the upper
eyelids, and hyperpigmentation of the conjunctiva (typical Yusho
symptoms), in a population with long-term occupational exposure to
PCBs, a group of 246 workers employed in 2 capacitor manufacturing
facilities were studied in 1976, and 181 of these workers, again in
1979. The median plasma values of lower chlorinated PCBs were
63 µg/litre in 1976 and 49 µg/litre in 1979. For the higher
chlorinated PCBs, these values were 18 and 17.5 µg/litre,
respectively. The prevalences of oculo-dermatological findings,
potentially related to the effects of PCBs, were 9.4 and 13.3%, at the
two examinations. There was no significant association between such
abnormalities and blood plasma/serum concentrations of PCBs (Fischbein
et al., 1985).
Lees et al. (1987) studied the hypothesis that the dermal route of PCB
exposure is a major contributor to the total body burden of PCBs in
workers. The investigation was conducted simultaneously with a
clinical study on switchgear workers engaged in transformer
maintenance and repair operations. The geometric means in the serum
and adipose tissue of exposed workers, previously exposed workers, and
comparison group were, respectively: (serum) 12.2, 5.9, and
4.6 µg/litre, (adipose tissue) 2.1, 0.83, and 0.6 mg/kg. The geometric
mean 8-h TWA concentrations in the different work areas (55 samples)
ranged from 0.5 to 6.1 µg/m3. The geometric mean surface
concentrations (102 samples) ranged from 0.007 to 1.075 µg/m2. From
the available data, it was calculated that exposure by the dermal
route (i.e., skin absorption) was considerable in comparison with
respiratory exposure. The daily calculated total dose through
inhalation ranged from 4.0 to 48.8 µg in the different work areas and
via the dermal route, from 1.2 to 215.0 µg. It was considered though
not conclusively, that the dermal and dermal/oral routes of exposure
are the predominant contributors to body burden.
Shalat et al. (1989) reported 3 cases of kidney adenocarcinomas among
young male utility workers who were responsible for maintaining
electrical transmission equipment, including transformers. The
duration of exposure ranged from 5 to 35 years.
The occurrence of chloracne and abnormal hepatic function as a result
of occupational exposure to PCBs had already been reported by Jones &
Alden (1936), Schwartz (1943), and Meigs et al. (1954).
Effects, such as chloracne, skin rashes, and burning eyes and skin,
have been associated with occupational exposure to Aroclors and
Kanechlors (Ouw et al., 1976; NIOSH, 1977; Fischbein et al., 1979,
1982, 1985; Baker et al., 1980; Drill et al., 1981; Kimbrough, 1987;
US EPA, 1987). In these studies, monitoring data did not adequately
characterize exposure levels, consequently correlations between the
occurrence of skin lesions and the duration of exposure, or blood
concentrations of the PCBs, are poor or nonexistent. Furthermore, the
contamination of the PCBs with PCDFs and PCQs may be partly the cause
of these skin changes.
Other effects reported in human exposure have been considered in a
criteria document for recommended standards for occupational exposure
to PCBs (NIOSH, 1977) and include several instances of chloracne that
resulted from exposure to PCB vapours in various work situations.
Other symptoms noted were sore throat, gastrointestinal disorders, and
eye disturbances.
Fischbein et al. (1979) examined 168 male and 158 female workers at a
capacitor plant, where they were exposed for <5 up to 25 years to
Aroclors 1254, 1242, 1016, and 1221. TWAs for 8 h with ranges of
0.07-0.40, 0.40-0.60, and 0.60-11.0 mg/m3 were considered low,
medium, and high, respectively. Among work-related symptoms they found
upper respiratory irritation and decreased rectal capacity,
gastrointestinal, neurological, and dermatological symptoms. The
dermatological symptoms occurred among 45% of the males and 55% of the
females and are comparable with the symptoms found in Yusho victims.
There was a significant correlation between plasma PCB levels and SGOT
levels, though changes in most liver tests were not prevalent.
Maroni et al. (1981b) reported blood PCB concentrations of
41-1319 µg/litre in 80 electrical workers (half males, half females)
employed in electric capacitor manufacture and testing plants.
Sixty-seven persons were exposed to Pyralone 3010 and 13, to Apirolio
(both PCBs containing 42% of chlorine). The mean age of the workers
was 37 ± 8 years, and the mean duration of employment was 12 ± 6
years. There were 6 cases of chloracne among the 80 workers. Sixteen
of the males had liver abnormalities, including hepatomegaly and
increased serum enzymatic activities; for 20% of these, the PCB
concentration in the blood was < 200 µg/litre. The females included 2
cases of bleeding haemangioma, one of whom also had chronic myelocytic
leukaemia, but none of the females had liver abnormalities.
Ouw et al. (1976) studied 34 electrical industry workers exposed to
0.32-2.22 mg Aroclor 1242 (free from impurities)/m3 compared with 30
control workers. The electrical workers consisted of 15 males (6
months-23 years employment) and 19 females (1 month-7 years
employment). No clear indications of liver changes were found. Major
complaints were burning of the eyes, face, and skin. One worker had
chloracne without systemic effects and 5 workers had eczematous hand
and leg rashes. These dermatological effects occurred at an air
concentration of <1 mg PCBs/m3. There were no significant health
effects in workers at, or below, a blood PCB level of 200 µg/litre.
Drill et al. (1981) concluded that individuals with blood levels of
>200 µg/litre have an increased risk of chloracne and that
chloracne may occur more frequently in workers exposed to PCBs that
have been heated (presence of PCDFs) and to PCBs that have a >54%
of chlorine.
A study was conducted to determine whether exposure to fumes or oil at
the transformer incident site at New Mexico had caused illness.
Exposed persons of different disciplines and unexposed employees were
asked to complete a questionnaire. Eighty of the 101 persons with
known exposure completed the questionnaire. The most common symptoms
were: nausea (27.5%), eye irritation (22.5%), sore throat (21.2%),
nose irritation (18.8%), chest tightness (15.0%), and headache
(15.0%). The symptoms were transient and usually resolved as soon as
the person left the site. Fifty-six exposed persons submitted sera for
PCB analysis as did 20 controls. All but 4 persons had levels below
10 µg/litre. The medium for exposed persons was 4.1 µg/litre (range,
1.2-41.8 µg/litre compared with 2.4 µg/litre (range 0.9-8.0 µg/litre)
for the controls (Anon., 1985).
A follow-up study on capacitor-manufacturing workers, exposed to PCBs,
and their children, was conducted over the period 1973-79. The PCBs
that were used were Kanechlor 300 and 500. PCB levels in the blood (up
to 120 µg/litre), as well as in breast milk, were 10-100 times higher
than those of non-exposed Japanese persons. The levels in 8 women
ranged from less than 50 µg/litre to about 400 µg/litre in whole milk.
Blood PCB levels were correlated with the duration of PCB handling and
breast milk PCB levels. The blood PCB levels ranged from 18.7 to
117 µg/litre in this population, 1 year after the use of PCBs was
discontinued. The rate of decline of blood PCB levels, as well as the
changes in the gas chromatography of blood PCBs over 7 years varied
with the kind of PCB handled. The blood PCB levels tended to be higher
(<3 up to >10 µg/litre) in children fed PCB-contaminated breast milk
for a long period. The great majority of the workers had
dermatological complaints, but these symptoms gradually disappeared
with discontinuation of contact with PCBs. The blood chemistry of the
workers showed only a correlation between PCB blood levels and serum
triglycerides. Several of the children fed breast milk for a long
period, showed the same medical findings as in Yusho (itchy skin,
eczema, red eye, fever, catching cold, carious teeth). However, they
were not diagnosed as suffering from PCB-poisoning, because the
findings were neither serious nor related to the blood PCB levels
(Hara, 1985).
Workers occupationally exposed to Kanechlor 500 or 600 showed higher
PCB levels in plasma (2-251 µg/litre) than Japanese Yusho patients.
Gas chromatographic patterns of their PCBs corresponded to the
patterns of PCBs to which they were exposed, but, with time, the PCB
pattern in plasma is changing. PCQs could not be detected in the
plasma of the workers (Takamatsu et al., 1985).
Lawton et al. (1985) studied a group of 194 workers in capacitor
manufacturing, exposed to Aroclor 1016, 1242 and/or 1254 before (1976)
and after (1979). The use of PCBs in the operations was discontinued
in 1977. The geometric mean serum levels and 5-95% ranges were: lower
chlorinated PCBs, 363 µg/litre (57-2270 µg/litre) and 68 µg/litre
(12-392 µg/litre), higher chlorinated PCBs, 30 µg/litre
(6-142 µg/litre) and 19 µg/litre (4-108 µg/litre), respectively. The
statistical findings were a depression in serum bilirubin and
elevations in serum gamma-glutamyltranspeptidase (GGTP) and lymphocyte
levels, at the time of the first examination, and only an elevation of
monocytes at the second.
In 1982, a survey was conducted in an electrical capacitor factory in
the USA, using Aroclor 1242 from 1941 to 1971, with a change to
Aroclor 1016 from 1971 to 1977. Of approximately 500 current employees
(with an average of 12.9 years of employment) 205 took part in the
survey. The geometrical mean PCB value for serum was 18.2 µg/litre
(range, nd-424 µg/litre). Only 39% of the workers ever worked in areas
with potential PCB exposure. More than 70% had serum levels below
30 µg/litre. There were no indications of acute PCB-related clinical
effects. The workers' serum PCB levels were a function of duration of
employment, cumulative occupational exposure, cumulative fish
consumption, and cholesterol level (Acquavella et al., 1986).
The blood of women occupationally exposed to PCBs (Kanechlor 300 and
500) was analysed over the period 1975-79. Sixty-five samples were
taken from 29 mothers. The PCB concentrations varied between 6.4 and
52.6 µg/kg (mean concentration 32.3 µg/kg). Clinical symptoms observed
during the use of PCBs were minor in comparison with those of Yusho
patients (Yakushiji et al., 1984b).
Guo et al. (1987) studied the influence of serum cholesterol and
albumin on the partitioning of PCB congeners between human serum and
adipose tissue. Fifty-five repair workers, who were either currently
or had been previously exposed, and 56 comparison workers without
exposure to PCBs were used. Seven PCB congeners, which had been
quantified in both serum and adipose tissue in at least one-third of
the selected populations, were evaluated. The effects of serum
cholesterol in modifying the serum PCB concentrations are likely to be
apparent in groups exposed to PCBs containing higher chlorinated
congeners, such as Aroclors 1254 and 1260, rather than those
containing lower chlorinated congeners, such as Aroclors 1242 and
1221.
A selected group of 51 workers (25 males and 26 females) with a mean
length of exposure of 10 years (range 1-30 years) were compared with 2
groups consisting of 74 subjects (37 males and 37 females) and the
same reference group of 67 workers (30 males and 37 females) used in
another study, residing in the same areas, but without exposure to
PCBs. The PCB concentrations in the blood of 28 out of the 51 subjects
ranged from 88 to 1359 µg/litre. A statistically significant increase
was found in serum GGT activity, urinary D-glucaric acid (GLA), and
porphyrin excretion, when compared with the respective control groups.
Although the PCB workers had an average urinary excretion of
porphyrins almost twice as high as those of the control groups, no
dose-response relationship was found between urinary porphyrin
excretion and blood PCB concentrations (Maroni et al., 1984).
Steele et al. (1986) found a gradual decrease in the body burden of
the more highly chlorinated PCBs, as well as a more rapid decrease of
the less chlorinated congeners over the period 1977-84 in groups of 5
current and retired workers, in comparison with 6 subjects without
current exposure. The authors calculated the half-life for the lower
chlorinated PCBs to be 6-7 months, and, for the higher chlorinated
PCBs, 33-34 months. The evidence of different half-life estimates for
serum PCBs, depending on the degree of chlorination, is consistent
with the present knowledge of the pharmacokinetics.
Hola & Reznicek (1985) carried out a cytogenetic analysis of the
peripheral lymphocytes of 48 employees at a precoated gravel plant
using Delor 103 (a mixture of mainly trichlorobiphenyls) in comparison
with 24 workers not exposed and 13 workers exposed to PCBs during the
impregnation of condensers. The frequency of aberrant cells,
percentage of blastic transformation, mitotic index, and proliferation
index in peripheral lymphocytes, and a number of biochemical
parameters were determined. In the 48 workers at the precoated gravel
plant, there were 2.87% aberrations (PCB concentrations in plasma,
107 ± 104 µg/litre); 3.14% in the 13 PCB-exposed workers (PCB
concentration in plasma 308 ± 253 µg/litre), and 2.04% in 24
non-exposed workers; 1.50% of aberrations were found in 20 controls.
Emmett (1985) studied a total of 55 workers (currently exposed (38)
and 17 past transformer repair workers) in comparison with 56
unexposed workers. PCB exposures occurred from the air and
contaminated surfaces, predominantly to Aroclor 1260, but there was
some exposure to Aroclor 1242. There was widespread PCB contamination
of the workplace surfaces and the 8-h time-weighted average
concentration was between 0.7 and 24.0 µg/m3, depending on the task
of the worker. The bulk oils and air from the transformer were
analysed revealing that PCDFs (13-116 µg/kg) were present as well as
PCBs. In one of the samples, 2,3,7,8-TCDF was found at 31 µg/kg. Eye
irritation and tearing were more prevalent in the exposed group, but
the symptoms were mild and/or transient. Ocular symptoms were also
found, possibly caused by 1,1,1-trichloroethane and/or
trichlorobenzene. Chloracne was not found. Two exposed workers
reported a history of melanoma; none were reported in the control
group. However, the difference was not statistically significant.
Adipose tissue and serum PCB geometrical mean concentrations in
exposed workers were 2.1 mg and 12.2 µg/kg, respectively, those in
unexposed workers were 0.6 mg and 4.6 µg/kg, and those in previously
exposed workers, 0.83 mg and 5.9 µg/kg. No correlations were observed
between liver function tests and either adipose tissue or serum PCB
concentrations. A significant negative correlation was found, after
adjustment for confounding variables, between adipose tissue PCB
levels and 24-h urinary 17-hydroxycorticosteroid excretion and a
positive correlation between serum PCB levels and serum-GGT. No
correlation was found between adipose tissue PCB concentrations and
any serum lipid component (Emmett et al., 1988a,b).
Bercovici et al. (1983) collected blood from 17 women with recent
missed abortions, 7 women who had experienced one or more missed
abortions in the past, and 7 women with normal, second trimester
pregnancies, and estimated the serum levels of PCBs and other
organochlorine pesticides. The range of serum PCB levels in recent
missed abortions was 10.9-416.5 µg/litre, and that of the control
group, 12.2-40.0 µg/litre. Forty-seven per cent of the recent missed
abortion group had PCB levels of the same magnitude as the controls
(range, 10.90-42.8 µg/litre) and 53% had higher levels. In the former
missed abortion group, PCB levels in the range of 45.3-109.1 µg/litre
were found. The number of women examined in the different groups was
small. Furthermore, half of the women with missed abortions had PCB
levels in serum comparable with those of the controls. The authors
also did not control for many variables that could significantly
influence the incidence of abortions. It seems, therefore, that missed
abortions, either recent or in the past, may be associated with PCB
exposure.
PCB concentrations were determined in the blood from 10 women with
normal pregnancies (controls) and from 17 women with premature
deliveries. A significant difference was seen in blood serum
concentrations of PCBs between the women with normal and those with
premature deliveries. When the premature delivery group was split into
a high-serum- (8/17) and a low-serum-PCB group (9/17), the
high-serum-PCB group had a significantly higher serum PCB
concentration than the control group. The values were 128 mg/litre in
the high group, 19.3 µg/litre in the control group, and 21.4 µg/litre
in the low group. In the high-PCB, premature delivery group, the mean
serum concentration of tetrachloro-isomers was lower than that of the
control group (0.6 vs 1.86 µg/litre), while the mean serum
concentrations of pentachloro and hexachloro-isomers were higher
(78.2 vs 15.67 µg/litre and 48.9 vs 1.72 µg/litre, respectively)
(Wassermann et al., 1982). The indications that higher serum PCB
levels may be associated with an increase in incomplete abortions and
premature deliveries (Bercovici et al., 1983; Wassermann et al., 1982)
could not be established as a definitive causal relationship.
Taylor et al. (1984) studied the relation of Aroclor 1254, 1242,
and/or 1016 exposure to birth weight and gestational age in the
offspring of women working in 2 capacitor plants. Fifty-one infants,
born to 39 women employed at the 2 capacitor manufacturing facilities,
had a mean birth weight of 153 g less than 337 infants born to 280
women employed in areas of the facilities without direct exposure.
Mean gestational age in the first group was reduced by 6.6 days
compared with the latter group. It was concluded that the small
decrease in mean birth weight seemed likely to have resulted from a
shortening of the gestation period rather than a retardation of
intrauterine growth. Smoking and alcohol consumption by the mothers
were not considered, and whether the socio-economic status of this
group of women was similar to that of the control group is not clear.
In an update of this study, 200 women with direct exposure and 205
women without direct exposure, were used to study the relation of PCBs
to birth weight and gestational age in the offspring. The authors
concluded that these data indicated that there was a significant
relationship between an increased serum PCB level and decreased birth
weight and gestational age, and that the decrease in birth weight was,
at least partially, related to shortened gestational age (Taylor et
al., 1989).
9.2.3 Appraisal
A discussion on the occupational epidemiological data on the basis of
dose-response considerations needs an acceptable indicator of the
degree of exposure and, in the particular case of PCB mixtures, a
discussion on the nature of the congeners present. For most
epidemiological studies reported, there are some, albeit often
limited, data on levels of total PCBs in blood, which could be used as
indicators for PCB exposure. It is recognized that the analytical
procedures used are different. The blood of continuously exposed
workers will contain absolutely and relatively more of the lower
chlorinated congeners than that of human beings with background
exposure only, or with past exposures (e.g., Yusho and Yu-Cheng
populations). Consequently, the toxicological profile for these 2
types of exposure will differ (see also the appraisal on
Yusho/Yu-Cheng).
In some cases, the epidemiology of the continuously exposed workers
shows a possible association between elevated exposure to PCB mixtures
and the occurrence of liver enzyme alterations, hepatomegaly, and
dermatological abnormalities, such as rashes and acne. In many
studies, no associations were found. In some of these studies, limited
end-points were investigated. Within the positive studies, there is a
poor or non-existent correlation between the incidence and degree of
effects and blood concentrations. Among the positive studies, adverse
effects are predominantly reported in the studies with the higher
blood levels. Possible contamination of used PCBs and PCB mixtures
(particularly in transformers), with PCDFs and PCQs, may contribute
to, or even determine, the toxicity observed. The overall conclusion
is that continuous exposure to high concentrations of PCBs and PCDFs
may result in effects on the skin and liver.
9.2.4 Special studies (target organ effects)
9.2.4.1 Liver
The liver is considered to be one of the most important target organs
for PCB toxicity. Acute exposures to PCBs cause alterations in liver
enzyme activities. Smith et al. (1982) found a statistically
significant correlation between elevated liver enzyme activities and
blood PCB concentrations, in workers exposed to PCBs in electrical
equipment manufacturing or maintenance. A negative correlation was
found in relation to the HDL cholesterol concentration and serum PCBs.
Positive correlations were found between the liver enzyme activities
of serum alanine aminotransferase (S-ALAT), serum aspartate
aminotransferase (S-ASAT), serum gamma-glutamyltranspeptidase
(S-Gamma-GT) and SGOT, and blood PCB concentrations, among workers
exposed in an Italian capacitor factory. Hepatomegaly was also
detected in most of the cases studied by Maroni et al. (1981a,b).
Workers occupationally exposed to PCBs, but mostly also exposed to
PCDDs and/or PCDFs, showed significantly increased S-ASAT, S-ALAT, and
gamma-GT activities. Sometimes elevated serum triglyceride values were
also found. Recovery of these disturbances in liver function and
morphology requires several weeks or months (Elo et al., 1985;
Schecter et al., 1985). Fischbein (1985) reported the results of liver
function tests in a population engaged in the manufacture of
capacitors and transformers. A low prevalence of abnormal liver
function tests was found and mean values for all tests were within
normal ranges in 5 workers occupationally exposed to Aroclor 1016.
Plasma antipyrine half-life was significantly lower than in matched
controls, suggesting induction of hepatic mixed-function oxidases
(Alvares et al., 1977). At the initial examination (in 1976, when PCBs
were still being used), statistically significant correlations were
found between log LDH and plasma levels of log HPCB (higher PCB
congeners) and log TPCB (total PCBs) among female workers, while
log-gamma-GTP was significantly correlated only with log HPCB among
male workers. A significant increase to abnormal levels of gamma-GTP
was noted at the follow-up examination (1979, 2.5 years after the use
of PCBs had been discontinued) in both male and female workers, and
preliminary results indicated significant correlations between
gamma-GTP and serum levels of PCBs among male workers. In
occupationally exposed individuals, the serum or plasma PCB levels
were higher than those found in patients in the Yusho and Yu-Cheng
incidents. An effect transmitted via liver activity was hyperlipaemia,
in which triglycerides and, in some instances, also cholesterol levels
in the blood were elevated (Smith et al., 1982).
Hepatotoxicity is suggested in occupationally-exposed humans (Drill et
al., 1981; US EPA, 1987). Drill et al. (1981) concluded that SGOT
and/or GGPT appear to be the most sensitive indicators of PCB exposure
in humans, and that changes in liver enzymes occur at levels below
those at which chloracne occurs.
9.2.4.2 Immunotoxicity
Reports on the immunological effects of long-term, occupational
exposures are sparse. Fischbein et al. (1979) found a suggestive
increase in the occurrence of trivial infections in exposed workers.
Immunological responses were found to be affected in Yu-Cheng victims
(Lu & Wu, 1985) and in Yusho patients (Nakanishi et al., 1985). Acute
accidental exposures of workers to PCBs and PCDFs have been studied
for immunological responses and immunosuppressive changes have been
found. The most important alterations were decreased numbers of
T-lymphocytes and lowered T-helper/ T-suppressor cell ratios.
Responses of lymphocytes to phytohaemagglutinin, concavalin A, and
pokeweed mitogen were also lowered. The observed changes persisted for
6 months after the acute exposure. No quantitative changes were
observed for immunoglobulins (Elo et al., 1985; WHO/EURO, 1987, 1988).
Lu & Wu (1985) found that PCB patients suffered from various kinds of
infections. Most frequent were those of the respiratory tract and
skin, including pyoderma, tinea versicolor, dermatophytosis and warts.
The low resistance of the patients suggested some degree of
immunosuppression. The function of the immune system was tested in 143
patients. Examination during the first year revealed: decreased
concentrations of IgM and IgA, but not of IgG; decreased percentages
of total T-cells, active T-cells, and helper T-cells, but normal
percentages of B-cells and suppressor T-cells; suppression of delayed
type response to recalling antigens; enhancement of lymphocyte
spontaneous proliferation; and enhancement of lymphocyte proliferation
with phytohaemagglutinin, pokeweed mitogen, and tuberculin
stimulation, but not with concanavalin A. After 3 years, the positive
rate of the tuberculin test recovered somewhat with time. The total
numbers of T-cells and B-cells were normal, the number of suppressor
T-cells (OKT-8) increased, but the number of helper T-cells (OKT 4)
was still lower, so the immuno-regulating index (OKT 4/OKT8) was still
very low. The lymphocyte proliferation stimulated by various mitogens
was also enhanced.
In patients with PCB poisoning, IgA and IgM levels in serum apparently
decreased for 2 years after the onset of the disease, but returned to
normal in most cases, in spite of the persistence of the respiratory
symptoms (Shigematsu et al., 1978, see section 9.2.4.3).
9.2.4.3 Respiratory system
In long-term exposure, up to 0.3-10 mg of PCBs may be inhaled in an
8-h working day. The respiratory tract is certainly the most important
route of exposure in the case of acute emergency situations, where
unprotected personnel working in areas containing such PCB
concentrations may, in theory, inhale a total dose of up to 10 mg/day.
This may imply a considerable cumulation of PCBs during long-term
exposure (WHO/EURO, 1987, 1988).
Transient irritation of the mucous membranes of the respiratory tract
has been reported in emergency situations, as well as difficulty in
breathing at high concentrations. It has not been confirmed that
short-term exposure causes other important respiratory effects, though
increased susceptibility and a high risk of contracting chronic
bronchitis have been suggested (Kimbrough, 1980; Elo et al., 1985;
WHO/EURO, 1987).
In the case of unheated commercial PCBs, the amount of PCDFs inhaled
might be very low, if any at all. The situation is totally different
in the case of acute exposures to heated or decomposed PCBs, in which
the inhaled total concentrations might be several orders of magnitude
higher than above, though the irritative effect may prevent breathing
in such contaminated rooms. Since the soot often contains a
considerable fraction of particles, a few microns in size, it is
partly breathed in and, thus, may lead to alveolar retention of both
soot and adsorbed chemicals. Carbon particles can accumulate in the
lung tissues and regional lymph nodes. Inhalation of soot particles
containing high concentrations of both PCBs and PCDFs would, in
practice, be the most important mechanism of exposure (Parkes, 1982;
WHO/EURO, 1987).
Warshaw et al. (1979) and Smith et al. (1982) found a correlation
between serum PCB concentration and respiratory tract symptoms among
workers exposed long-term to PCBs. An increase in the occurrence of
chronic bronchitis was possibly due to a decrease in the immunological
defence mechanism. No increase in mortality from respiratory system
diseases was found. An indication for acute and chronic irritation of
the respiratory tract was found by Brown & Jones (1981).
Shigematsu et al. (1978) studied the clinical, laboratory, and
pathological findings on respiratory involvement in PCB poisoning in
401 patients. Respiratory symptoms included expectoration in 40% of
the 289 non-smoking patients with PCB poisoning and mild wheezing in
2%. The incidence and severity of the symptoms was well correlated
with the concentrations of PCBs in blood and sputa. The clinical
examinations revealed bronchiolitis and pneumonia or atelectasis in
about one-tenth of the patients with reticulo-linear shadows. The PCB
concentrations in the blood and sputa were 27 and 8 µg/litre,
respectively. The presence of PCBs in sputum may have been associated
with the excretion from bronchial cells and/or with lipid II cells of
the lung, phagocytosed in alveolar macrophages and expectorated.
9.2.4.4 Neurotoxicity
Acute and long-term exposures to PCBs have been reported to cause
neurological and unspecific psychological or psychosomatic effects,
such as headache, dizziness, nausea, depression, sleep and memory
disturbances, nervousness, fatigue, and impotence (Smith et al., 1982;
Elo et al., 1985; Hara, 1985; Schecter et al., 1985; Takamatsu et al.,
1985; WHO/EURO, 1987).
Fischbein et al. (1979) reported the occurrence of these symptoms in
39% of male and 58% of female capacitor-manufacturing workers, exposed
to PCBs for long periods (over 5 years). To what extent these symptoms
were the direct consequences of exposure to PCBs and related compounds
and how much they were dependent on general conditions in an emergency
situation remains unclear.
Seppalainen et al. (1985) examined 16 men who were exposed to fumes
resulting from the explosion of capacitors containing Clophen A30. Air
concentrations of PCBs, measured 5.5 h after the explosion, were
8-16 mg/m3 air (PCDFs and other compounds, such as monochloropyrenes
and dichloropyrenes, were also formed). Most of the men had a
transient sensory neuropathy in their lower extremities (WHO/EURO,
1987, 1988).
9.2.4.5 Blood pressure
Kreiss et al. (1981) examined 458 volunteers (>12 years of age) from
Triana (Alabama) and correlated serum PCB levels (Aroclor 1260) with
blood pressure. This population was excessively exposed to DDT
residues through the consumption of contaminated fish. The residents
of this small rural town also had elevated PCB body burdens that were
positively correlated with fish consumption. The mean serum PCB level
was 17.2 µg/litre. The incidence of borderline (systolic of 140-159 mm
Hg and diastolic of 90-94 mm Hg) and definite hypertension (systolic
of >160 mm Hg and diastolic of >95 mm Hg) was 30% more than
would be expected for a general population of the same age, race, and
sex composition. However, this study did not have a control group in
its design, and there were more confounding factors that make the
study inadequate to conclude an association between blood PCB levels
and hypertension.
A study was carried out to test the association of serum PCB levels
and elevated blood pressure in 840 residents of New Bedford, Acushnet,
Dartmouth and Fairhaven, Canada, in the period 1984-87. The mean PCB
levels (as Aroclor 1254) were 5.9 and 5.8 µg/litre in 391 males and
449 females, respectively. The range in serum PCB levels for the total
group was 0.38-154.2 µg/litre. There was a relationship of serum PCB
level to age among the 840 individuals. In the 5 age groups: 18-24,
25-34, 35-44, 45-54, and 55-64 years, the mean serum PCB levels were
2.59, 3.84, 5.30, 8.18, and 8.96 µg/litre, respectively. Blood
pressure levels did not appear to be correlated with serum PCB levels.
The mean systolic readings, taken at 3 different times, for the 840
individuals were 115.26 ± 18.85, 113.69 ± 17.62, and 114.28 ± 11.41.
The diastolic readings were 72.19 ± 10.94, 73.19 ± 10.95, and 73.17 ±
19.94. There was no between the sexes difference (Massachusetts Dept
Public Health, 1987).
Akagi & Okumura (1985) studied the correlation of blood PCBs levels or
PCB patterns and blood pressure in 59 Yusho patients (more than 40
years old). In spite of the passage of 13 years from the onset of the
disease, 52.5% of the patients still had PCB levels higher than those
of the general population. The frequency of hypertension in these
patients was 16.9%, a value similar to that found in the general
population of the same age and sex. Blood pressure was not associated
with blood PCB levels or PCB pattern, but was associated with the well
known factors influencing blood pressure, such as age, obesity, and
habitual alcohol intake.
9.2.5 Mortality studies
Davidorf & Knupp (1979) conducted an epidemiological study on ocular
melanoma incidence in Ohio from 1967 to 1977, attempting to associate
Ohio counties with known high concentrations of PCBs and those with
industries that might use PCBs with an increased incidence of ocular
melanoma. The authors concluded that there was no causal relationship
between PCB exposure and an increased annual occurrence of ocular
melanoma in Ohio counties in the period 1967-77. Bahn et al. (1976)
reported on 31 research and development employees subjected to "heavy"
Aroclor exposure (quantity not reported) in a US petrochemical plant.
Two had malignant melanomas, and according to the standard of the
Third National Cancer Survey, incidence rates of only 0.04 would be
expected among 31 persons (NCI, 1975). The retrospective cohort
mortality studies of Brown & Jones (1981) and Brown (1987) reported
data on PCB-exposed individuals who had worked in 2 electrical
capacitor plants, one in New York, and the other in Massachusetts.
Both plants had produced this type of capacitor for more than 30
years. The PCBs used were Aroclor 1254, Aroclor 1242, and Aroclor
1916. A combined total of 2588 exposed workers from both factories,
with 3 or more months of exposure, were studied. The overall mortality
(295 deaths) was lower than expected (318) and the mortality for
cancer deaths (62 observed) was also lower than expected (80). A
statistically significant excess in deaths was observed in the disease
category that includes cancer of the liver (primary and unspecified),
gall bladder, and biliary tract (5 observed vs 1.9 expected). Most of
the excess was observed in women employed in one plant. According to
the authors, because of the small number of deaths and the variability
of specific causes of death within this category, it remains difficult
to interpret these findings with regard to PCB exposure.
At the first plant, there were 2 different facilities, a power
capacitor manufacturing facility, and a small capacitor manufacturing
facility. At the power capacitor facility, the TWAs for personal air
samples were between 24 and 393 µg/m3 for various jobs: in the
winding work area and soldering work area, they were as low as
3 µg/m3 and as high as 476 µg/m3, respectively. At the second plant,
where a few cases of rectal and liver cancer were found (see above)
the PCB levels were much higher. Degreasers and solderers, for
example, had TWAs for personal air of 1.260 and 1.060 µg/m3,
respectively, and heat soak operators and tankers had TWAs of 630 and
850 µg/m3, respectively. The work area air samples contained levels
as high as a TWA of 810 µg/m3. The duration of exposure, without
information concerning the level of exposure, when the levels varied
so widely, weakens the statement about lack of correlation between
duration of exposure and cancer mortality. In fact, the observed
cancer cases for both the liver and rectum were markedly increased in
the factory that had, at the time of measurement, the higher PCB
levels. Notwithstanding the absence of detailed information, the data
presented are suggestive of a dose-related increased incidence of
mortality from rectal cancer and possibly liver cancer.
Although there was no correlation between the latent period and cancer
mortality or between the duration of employment and cancer mortality,
most of the cancers occurred in the second plant, which had the higher
levels of PCBs at the time that the measurements were made. PCB levels
were monitored in 1977 for personal air and work area air. Since
procedures and processes were somewhat different during the years in
which most of the workers were exposed, the figures on PCB levels do
not necessarily indicate the exposure levels of the subjects.
Nevertheless, the figures given do indicate the wide variation in PCB
levels in air, with a 15-fold difference between the lowest and
highest levels among the different jobs. Although the different levels
of dust and particulate matter are not known, it would be anticipated
that an equally wide variation would exist.
A medical surveillance programme has been established for 482 persons,
who were potentially exposed to PCBs, PCDFs, and PCDDs from an
electrical transformer fire in Binghamton in 1981. Mean serum PCB
concentrations (98% of the samples) were below 20 µg/litre, a value
typical of a population with no unusual exposure. Mortality,
symptomatology, cancer incidence, and reproduction events were
assessed through 1984. The numbers of deaths, cases of cancer, fetal
deaths, and infants with low birth weight or congenital malformations,
were similar to those expected on the basis of age and sex-specific
rates for upstate New York and other comparison populations. One-third
of the fire-fighters and a number of persons, who were in the building
during the first 24 h (or longer) reported a rash or itching skin, but
no chloracne (Fitzgerald et al., 1989).
Bertazzi et al. (1981) reported the results of a mortality study on
PCB-exposed workers, who were employed in the manufacture of
electrical capacitors in an industrial area near Milan. The PCBs used
over the period from 1946 to 1970 were Aroclor 1254, Pyralene 1476
(54% chlorine), and Pyralene 3010 and 3011 (42% chlorine). In 1954, a
few measurements in the air were performed and the values of Aroclor
1254 were 5200-6800 µg/m3. In 1977, airborne concentrations of
Pyralene 3010 ranged from 48 to 275 µg/m3. The minimum and maximum
values of PCBs recovered from workplace surfaces and worker's hands
were, 0.2-159 and 0.3-9.2 µg/cm2, in 1977, and, in 1982 (2 years
after the ban on production), 0.003-6.3 and 0.09-1.5 µg/cm2,
respectively. The mortality study spanned the 25-year period from 1954
to 1978. The control mortality rates were for subjects from the city
in which the plant was located. There were 1310 workers (1020 females
and 290 males) and the vital status was obtained for 98% of both
sexes.
The study was enlarged and extended to include 2100 workers and to
cover the period 1946-82. Vital status was ascertained for over 99% of
the subjects and death certificates were obtained for all deceased
persons. Expected deaths were calculated using 2 sets of mortality
rates, national and local. Among male workers, cancer deaths (14
observed vs 7.6 expected) were significantly increased as were deaths
owing to cancer of the gastrointestinal tract (6 observed vs 2.2
expected). Also, mortality from haematological neoplasms (3 observed),
and lung cancer (3 observed) was higher than expected. However, the
excess was not statistically significant. Female workers exhibited an
overall mortality that was significantly increased above expectations.
Cancer deaths (12 observed vs 5.3 expected) and haematological
neoplasms (4 observed vs 1.1 expected) were significantly higher than
expected when compared with the local population. Interpretation of
the results is limited by the small number of deaths: however, it is
of interest that the gastrointestinal tract and the lymphatic and
haematopoietic tissue seem to be the most probable human target sites
for PCB carcinogenic activity (Bertazzi et al., 1987).
A cohort study on 142 male Swedish capacitor-manufacturing workers was
performed between 1960 and 1978. The PCB was 42% chlorinated and
contained different PCDFs totalling about 1400 µg/kg. The mean
exposure time of the workers was 6.5 years. In 1973, 0.1 mg PCBs/m3
was found in the air. Mortality was investigated for the period
1965-82 and cancer incidence from 1965 to 1980. Twenty-one deaths and
7 cancers were observed, which was in agreement with the anticipated
numbers calculated from national statistics (Gustavsson et al., 1986).
Zack & Musch (1979) examined a small cohort of workers (89) with
occupational exposure to PCBs. No liver cancer was reported among the
30 deaths that occurred in this study. There were increases for all
malignancies (8 observed vs 4.4 expected, SMR=179) and elevated lung
cancer (4 observed vs 1.44 expected). Adjustment for the confounding
variables due to multiple exposure to other agents was not made. By
1979, 31 Yusho patients had died, 11 (35.4%) of these from malignant
neoplasms. Only 21.1% of all deaths in this Japanese prefecture would
be expected from malignant neoplasms, but no clear correlation between
the occurrence of Yusho and increased deaths from malignant neoplasms
could be made, because of the small number of deaths observed and the
unknown latency period.
By the end of 1983, 120 Yusho patients had died, 41 of these from
malignant neoplasms. These included 8 stomach cancers, 11 liver
cancers, and 8 neoplasms of the lung. A statistically significant
excess mortality was seen for malignant neoplasms, cancer of the
liver, and cancer of the lung, trachea, and bronchus, in males, but no
such excess was noted in females. The excess from liver cancer deaths
was seen mainly in Fukuoka prefecture, while no excess was seen in
Nagasaki prefecture (Ikeda et al., 1987).
9.2.6 Appraisal
Some epidemiological studies on occupationally exposed workers and
Yusho patients indicate an association between PCB exposure and
cancer, especially with regard to hepatobiliary tumours. However, no
definite conclusions can be drawn from available data, because of the
small numbers of deaths in the population studies, the lack of clear
dose-response relationships in the occupational studies, and the
difficulty in evaluating the effects of other compounds present in
PCBs.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The PCBs were evaluated by IARC in 1978 and 1987 (IARC, 1978; 1987).
In 1987, IARC concluded that, because the role of impurities in PCBs
in the carcinogenicity could not be excluded, and, because of the lack
of knowledge on dose-response relationships, the evidence from
epidemiological studies is limited. However, the evidence of
carcinogenicity in laboratory animals is sufficient. Taking the
combined evidence from human and experimental animal studies, the IARC
Group concluded that PCBs are probably carcinogenic for humans (IARC,
1987).
Many countries and intergovernmental organizations have banned or
severely restricted the production, use, handling, transport, and
disposal of PCBs and PCTs. For an overview of these measures and
regulations we refer to the Health and Safety Guide on PCBs and PCTs
(WHO, 1992; IRPTC, 1986b).
At the meeting of the Joint FAO/WHO Expert Committee on Food Additives
(WHO, 1990), particular attention was paid to the possible health
consequences of the intake of PCBs by the suckling infant. It was not
anticipated that adverse health effects would occur as a result of
consuming breast milk. It should also be kept in mind that the infant
consumes breast milk for a short period (1-2% of its total life span).
In addition, other factors need to be considered:
* the benefits of breast milk and breast-feeding, including the
nutritional, immunological, and other properties of the milk, as
well as the psychological advantages, should not be discounted;
* the disadvantages of breast milk substitutes, because of the
potential contamination due to infective agents, incorrect
preparation, inadequate hygiene, etc.
For these reasons, JECFA was of the opinion that the advantages to the
infant of breast-feeding outweigh any potential hazards due to the PCB
content of breast milk, and advises that there is absolutely no
justification for discouraging this practice.
The monitoring data have indicated, up to now, that the occurrence of
PCBs in human milk persists at about the same levels during the years,
with slight decreases or increases in the PCB concentration in breast
milk in certain countries. Since the PCB levels in human milk are
still too high, every effort should be made to prevent the entry of
PCBs into the environment and to control their occurrence in the food
supply. The Committee was reassured by the observation that the
production of PCBs has largely ceased. Thus, it is expected that the
levels of PCBs in the environment and food, and consequently in breast
milk, will decrease with time (WHO, 1986b).
On the basis of the evaluated background data, an average dietary
intake of PCBs for adults was estimated to amount to a maximum of
100 µg/week, or approximately 14 µg/day. For a 70-kg person, this is
an intake equivalent to a maximum quantity of 0.2 µg/kg body weight
per day (WHO/EURO, 1988).
The above data suggest that the main exposure of the general
population to PCBs occurs through food. The daily intake of these
compounds by breast-fed infants is about 1-2 orders of magnitude
higher than for the rest of the population, compared either on the
basis of body weight or energy consumption. However, compared with
lifetime intake, a 6-month, breast-feeding period contributes less
than 5% of the total body burden from lifetime exposure (WHO/EURO,
1988).
POLYCHLORINATED TERPHENYLS
(Data relating specifically to polychlorinated terphenyls are scarce.
Nevertheless, they are presented separately in this section.)
1. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
1.1 Identity
The chemical formula of the polychlorinated terphenyls (PCTs) can be
given as C18H14-nCln, in which n is the number of chlorine atoms,
which can range from 1-14.
The chemical structure is:
The occurrence of trans-dihydrodiol metabolites suggests that the
metabolism of PCBs proceeds through the formation of arene oxide
intermediates (US EPA, 1987). Arene oxides are potential electrophiles
that have been implicated in cellular necrosis, mutagenicity, and
carcinogenicity (Safe et al., 1975; Sundström et al., 1976a).
While the arene oxide pathway is important in carcinogenic
considerations, it may not be the primary pathway for the metabolism
of PCBs, in most cases. So far, most discussions about the metabolism
of PCB isomers have focused on the position and number of the chlorine
substituents.
The metabolic products of dichloro-, trichloro-, tetrachloro-,
pentachloro-, and hexachlorobiphenyl appear to reflect direct
hydroxylation at the meta and/or para positions, relative to the
position of the phenyl-phenyl bond. In a few instances, a methoxy
group is found instead of a second hydroxyl. This direct mechanism
appears to operate, therefore, irrespective of the degree of
chlorination, and, for the most part, irrespective of the position of
the chlorine substituents. In the rabbit, some exceptions have been
found that involve: a chlorine shift and the removal of a chlorine, in
the case of the 4,4'-dichlorobiphenyl, the formation of a dihydrodiol
at the meta and para positions, in the case of 2,5,2',5'-tetra-
chlorobiphenyl, and the removal of a chlorine from one of the rings,
in the case of 2,4,5,2',4',5'-hexachlorobiphenyl (Sundström et al.,
1976a).
Studies carried out by Matthew & Anderson (1975b) and Tuey & Matthews
(1977) showed that monochloro- and dichlorobiphenyls were rapidly
metabolized and excreted and that pentachloro- and hexachlorobiphenyls
were poorly metabolized and retained longer in the adipose tissue and
skin. The situation for the tetrachlorobiphenyls is more complicated.
The following analysis is based on the data on metabolites identified
and reported in the review by Sundström et al. (1976a).
6.5.2 Dichlorobiphenyls
Consideration of the various dichloro- isomers shows that, when
chlorines are only on one ring, hydroxylation occurs on the
nonchlorinated ring. Single hydroxylation occurs para to the
phenyl-phenyl bond; if another hydroxylation occurs, it is always
meta to the phenyl-phenyl bond. This holds true for the 3 different
isomers tested: 2,3-; 2,4-; and 3,4-dichlorobiphenyls. When the
dichloro-compounds are symmetrically chlorinated on each ring, as in
2,2'-; 3,3'-; and 4,4'-dichloro compounds, the same pattern applies
generally, but with a variation on the theme and an exception in the
case of 4,4'-dichlorobiphenyl. In the case of 2,2'- and 3,3'-dichloro
compounds, monohydroxylation occurs meta and para to the
phenyl-phenyl ring, respectively. In both cases, double hydroxylation
involves both meta and para positions on the same ring, (that is,
meta and para to the phenyl-phenyl bond). In the case of
4,4'-dichlorobiphenyl, there appears to be a difference in the rat.
The monohydroxy- derivative is meta, but the dihydroxy derivative is
ortho and meta to the phenyl-phenyl bond. Not only is the rat
metabolism of the 4,4'-compound an exception, but the rabbit also
shows an unusual response to this compound. In the rabbit, the
monohydroxy-derivative is the same meta hydroxy found in the rat,
but, instead of a dihydroxy- compound, the rabbit produces a chlorine
shift and a single hydroxy group in the para position as well as
dechlorination and hydroxylation in the para position. These latter
products have been considered as characteristic of the arene oxide
intermediate pathway. Nevertheless, among the 6 different dichloro-
isomers examined, all produced a monohydroxy- derivative, either meta
or para to the phenyl-phenyl bond, and all but the 4,4' produced
dihydroxy- derivatives were meta and para on the same ring to the
phenyl-phenyl bond.
The absence of a substitution at 4,4'- with vicinal unsubstituted
positions cannot be correlated with rapid metabolism, since this
property is also shared by both a rapid and slowly metabolized isomer.
This is in direct contradiction to the often repeated statement "the
presence of at least two adjacent unsubstituted carbons, particularly
in positions 3,4-, or 5- or 3',4'- or 5'- is required for rapid
metabolism of chloro-biphenyl" (Jensen & Sundström, 1974b; Berlin et
al., 1975; Safe et al., 1975; Matthews & Anderson, 1976; NIOSH, 1977;
Matthews & Tuey, 1980).
6.5.3 Tetrachlorobiphenyls
Examination of the 2 different tetrachloro- isomers, 2,3,5,6,-
tetrachloro- and 2,5,2',5',-tetrachlorobiphenyl, showed that, in the
case of the molecule with all 4 chlorines on one ring, the products
were monohydroxy- derivatives meta or para, dihydroxy- derivatives
meta and para, and a para hydroxy- plus a meta methoxy- group
or a meta hydroxy- and a para methoxy- group, all on the
unsubstituted ring. The symmetrical 2,5,2',5'-tetrachlorobiphenyl in
the rat gave the meta hydroxy-, but in the rabbit a para hydroxy-,
and also in the rabbit a dihydro-dihydroxy-, on the meta and para
positions. The asymmetric 2,4,3',4'-tetrachlorobiphenyl gave
monohydroxy-derivatives, both in the meta position, either in the
three or five position. It seems that an alternative enzyme pathway is
available in the rabbit.
The level of retention was highest for 2,4,2'4'-tetrachlorobiphenyl
descending in the following order; 2,5,2',5'-, 3,5,3',5'-, 3,4,3',4'-,
2,3,2',3'-, and 2,6,2',6'-tetrachlorobiphenyl. Since no PCBs were
detected in the liver, and only small amounts of 2,3,2',3'- and
3,4,3',4'-tetrachlorobiphenyls in the carcass, it can be concluded
that these 2 isomers were readily metabolized and excreted. Both
compounds have unsubstituted vicinal positions. However, the compounds
slowest to be metabolized were 2,4,2',4'- and 2,5,2',5'-tetra-
chlorobiphenyls, which also have unsubstituted vicinal positions.
Metabolic restriction cannot be entirely attributed to substitution at
the 4,4'- positions (Kato et al., 1980), since this also occurred in
3,4,3',4'-tetrachlorobiphenyl, which was removed relatively rapidly.
The excretion of the monohydroxy metabolites of 3,4,3',4'-tetra-
chlorobiphenyl and 2,4,3',4'-tetrachlorobiphenyl (orally administered)
in rats has been demonstrated by Yoshimura et al. (1973); Yamamoto &
Yoshimura (1973); Yoshimura & Yamamoto (1975); and Yoshimura et al.
(1974). They demonstrated that the metabolites of the first isomer
were 2-hydroxy- or 5-hydroxy- compounds, while the metabolites of the
second isomer were 5-hydroxy- and 3-hydroxy- compounds. All hydroxy
metabolites were excreted non-conjugated via the bile and no parent
isomers were found in the bile. Yoshimura & Yamamoto (1975) found that
unchanged 2,4,3',4'-tetrachlorobiphenyl was excreted through the
intestine, when it was intravenously injected in rats with the bile
duct ligated, while no metabolite of this isomer was excreted by this
route.
The results with the 2,5,2',5'- molecule are probably related to an
arene oxide pathway. Direct evidence for this was reported by Forgue
et al. (1980), who showed that 3,3,3,-trichloropropene-1,2-oxide,
which is an inhibitor of epoxide hydrase, blocked the formation of the
suspected arene oxide metabolites. The arene oxide mechanism
supposedly operates in rabbits and monkeys for 2,5,2',5'-tetrachloro-
biphenyl and, possibly, also in rats for 2,4,5,2',4',5'-hexachloro-
biphenyl. Isomers that may utilize the arene oxide pathway, to some
extent, are: 4,4'-dichloro-; 2,5,2',5'-tetrachloro-; and
2,4,5,2',4',5'-hexachlorobiphenyl.
6.5.4 Hexachlorobiphenyls and higher chlorinated compounds
The symmetrical hexachlorobiphenyls were used in a study by Matthews &
Tuey (1980), in which Sprague-Dawley rats were injected intravenously
with the PCBs and killed at increasing time intervals from 15 min to
42 days. The 2,3,6,2',3',6'- isomer was rapidly metabolized and
excreted compared with the other isomers, which were slowly
metabolized and excreted with much longer half-lives. The results
indicated that the metabolism of hexachlorobiphenyls is slow, when the
position of the chlorine atoms is such that arene oxide formation is
inhibited.
2,3,6,2',3',6'-Hexachlorobiphenyl produces only one metabolite in the
rat: 2,3,6,2',3',6'-hexachloro-4'-hydroxybiphenyl, which is believed
to be the result of arene oxide formation. All commercial mixtures of
PCBs will contain congeners that could be metabolized via the arene
oxide pathway. However, it does not seem to be the major pathway of
metabolism for most of the components of the commercial products,
since most of the higher congeners will not have vicinal unsubstituted
carbons.
The metabolic data on individual isomers shows that, at least up to
hexachloro- compounds, ordinary hydroxylation can take place. It is
reasonable to consider that it is not only as a consequence of poor
metabolism that pentachloro- and hexachloro- compounds are persistent
in the tissues, but rather that they are not metabolized as readily,
because they are sequestered from tissues in which the bulk of the
metabolism takes place. In support of this position, it has been shown
by Matthews & Anderson (1975b) that, when animals are caused to lose a
substantial portion of body weight, the stored higher chlorinated
compounds can, indeed, be metabolized. Octachloro- and
decachlorobiphenyls would not be expected to be easily metabolized,
simply because there are few or no sites for hydroxylation to take
place. It was found (Vodicnick & Lech, 1980; Vodicnick et al., 1980)
that almost the entire body burden of 2,4,5,2',4',5'-hexa-
chlorobiphenyl was removed from mothers given this PCB and that it was
transferred to their offspring via the nursing mother's milk. In mice,
the preferential distribution of this PCB in milk reflects the high
fat content of mouse milk.
Virgin, female, Sprague-Dawley mice, injected ip with 50 or 100 mg
[14C] 2,4,5,2',4', 5' -hexachlorobiphenyl/kg body weight in corn oil
for 2 weeks, prior to mating, eliminated virtually their entire body
burden of the compound through milk during one lactation cycle
(Gallenberg & Vodicnick, 1987).
Storage is caused by lack of metabolism and also implies that the
availability of adjacent unsubstituted carbons is the determinant for
metabolism. Direct hydroxylation reactions do not require
unsubstituted adjacent carbons. Rapid storage in fat is the
rate-limiting factor in the removal of most PCBs, with the exception
of isomers that might be very rapidly metabolized by arene oxide
formation, such as 2,3,6,2',3',6'-hexachlorobiphenyl.
Matthews & Anderson (1975b) extended their study to include a fasting
period to reduce the weight of the test rats and showed that severe
fasting mobilized stored PCBs and brought them into the metabolic
pool.
6.5.5 Retention and turnover
Mizutani et al. (1977) studied the pharmacokinetic behaviour of 6
different tetrachlorobiphenyls. They administered mice the 6 isomers,
at 10 mg/kg body weight, for 20 days. The isomer concentrations in the
liver and the remainder of the carcass were determined at various
times during the recovery period. They found that the accumulated body
burden was a function of both storage ratio and biological half-life.
The results of Mizutani et al. (1977) suggest that the correlations
claimed between the position of the chlorine substituents and storage
or metabolic activity (Kato et al., 1980; Matthews & Tuey, 1980) are
not simply explained.
The hypothesis that the position of the chlorine atoms alone
determines the rates of metabolism, accumulation, and excretion does
not appear to be entirely supported. This idea has been used to
support the notion that PCBs with unsubstituted vicinal carbon atoms
favour metabolism by arene oxide formation.
6.5.6 Appraisal
The results of most studies suggest that PCBs are absorbed by the
organ systems (gastrointestinal tract, lung, and liver), representing
the likely routes of entry into the body. This is particularly true
for the gastrointestinal tract where absorption is rapid. PCBs, once
absorbed, are usually distributed in a biphasic manner and are rapidly
cleared from the blood and accumulated in the liver and adipose
tissue, or they can be metabolized in the liver, to form metabolites
that are excreted in the urine and bile. In some studies on humans,
the skin, an organ rich in adipose tissue, had a high PCB content,
whereas the brain content was low. This distribution can also include
the fetus and human milk, an extension of the adipose tissue system in
the body. Mobilization of PCBs from fat appears to depend on their
rates of metabolism. Metabolic pathways include hydroxylation, and
conjugation with thiols and other water-soluble derivatives, some of
which can involve reactive intermediates, such as the arene oxides.
The most important pathway seems to be hydroxylation and subsequent
conjugation. This pathway is facilitated by the presence of at least
one pair of unoccupied vicinal carbon atoms in the PCB structure.
Persistence in tissue is not correlated with high toxicity.
Differences in toxicities among PCBs may be associated with specific
metabolites and/or their associated intermediates.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Toxicity for microorganisms
7.1.1 Freshwater microorganisms
Zullei & Benecke (1978) used the motility of filamentous blue-green
algae (Cyanophyceae) of the genus Phormium as a bioassay for the
rapid determination of the toxicity of various compounds. They tested
the relative toxicity of purified, specific chlorobiphenyls and of PCB
commercial mixtures (Clophen). Inhibition of motility was greatest in
the presence of chlorobiphenyls of low chlorination. All mono- and
dichlorinated isomers were inhibitory at the test concentration of
100 µg per test spot of algae. Tetra- and hexachlorobiphenyls did not
have any effects; the effects of trichlorobiphenyl isomers varied, the
2,5,2' isomer not producing any effects and the 2,4,4' and 3,4,4'
isomers being inhibitory. Tests using the commercial mixtures
confirmed the greater toxicity of low chlorination levels; Clophen A30
was more toxic than Clophen A60, though the presence of more than
expected, low-chlorinated compounds in the Clophen A60 reduced the
difference in toxicity.
Cultures of the green alga Chlorella pyrenoidosa were incubated with
1 mg/litre of Aroclors 1242, 1254, and 1268 (Hawes et al., 1976a).
The initial culture was 5 days old with a cell density of 27 ×
106 cells/ml. After 8 h of incubation with the PCBs, cell densities
in the cultures were 64% lower than controls for Aroclor 1242, 45%
lower for Aroclor 1254, and 36% lower for Aroclor 1268. As the study
progressed, the cell densities in the cultures improved relative to
the controls; cell density in the Aroclor 1254 culture was equal to
that in the control by 129 h and the density in the Aroclor 1268
culture was equal to the control by 59 h. Although the density in the
Aroclor 1242 culture remained much lower than that in the control
throughout the culture period, there was evidence of recovery with
this compound. The toxicity of the Aroclors was inversely proportional
to their degree of chlorination. A concurrent investigation of the
primary productivity of the alga (Hawes et al., 1976b), suggested that
the productivity of individual cells was stimulated by the Aroclors,
with a positive relationship between the level of chlorination and the
effect. However, this did not take into account the differences in
density between cultures (PCBs reduce growth through cell division)
and the authors pointed out that the response of the alga to the PCBs
was not simple. They stated that the density, culture age, and Aroclor
type were all factors that influenced response.
Larsson & Tillberg (1975) cultured the green alga Scenedesmus
obtusiusculus, in a liquid medium, in concentrations of Aroclor 1242
ranging between 10 and 1000 µg/litre. Growth was reduced at
concentrations of 300 µg Aroclor/litre or more; viability was only
affected at the highest concentration of 1000 µg/litre. Reduced
phosphate uptake, which was nearly identical in light or in darkness,
was reduced from 300 µg/litre upwards. The authors regarded this as a
result of an effect on the plasmalemma. At 800 µg PCBs/litre, the
results of some studies suggested an effect of the uncoupling of
oxidative phosphorylation; at 1000 µg/litre, both respiration and
oxygen evolution were inhibited.
The effects of various Aroclors on the respiration and photosynthesis
of the green alga Chlorella vulgaris were examined by Sinclair et
al. (1977). Aroclor 1221, at a final concentration in the medium at
10-4 mol/litre (= 192 mg/litre based on an average relative molecular
mass for the Aroclor at 192), produced a rate of oxygen production in
the light of 43% of control levels and a rate of oxygen uptake in the
dark of 59% of control levels. The Aroclors were dissolved using
dimethylformamide (DMF) as a solubilising agent; whilst this had some
effects on the parameters measured, they were very little compared
with the effects of the Aroclors (94% of control levels). A
dose-response curve of the effect of Aroclor 1221 on the respiratory
uptake of oxygen in the dark, in the presence of glucose, showed that
there was already a marked effect at a concentration of
10-7 mol/litre and that a maximum (at 50% inhibition) was reached at
a concentration of 10-6 mol/litre (= 1.92 mg/litre).
A further series of studies was performed to investigate the
individual processes of oxygen exchange in the alga. Respiration in
the dark was investigated in the absence of added glucose, to monitor
"endogenous" respiration, which was found to be stimulated by Aroclor
1221 at concentrations of 10-4 mol/litre or more. Net oxygen
production in the light (photosynthetic oxygen production minus
respiratory oxygen usage) was reduced at a concentration of
10-4 mol/litre. At this concentration of Aroclor 1221, there was
approximately 50% stimulation of endogenous respiration and
approximately 50% inhibition of net oxygen production. Calculated
photosynthetic rate was unaffected by the Aroclor. Increasing light
intensity reduced the effect of Aroclor 1221 on net oxygen production;
intensities of 8.2 × 104 ergs/cm2 virtually eliminated the effect.
Other measures directly or indirectly associated with photosynthesis
(fluorescence, oxygen evolution in flashing light, the Emerson
enhancement phenomenon) were not affected by the Aroclor. The authors
suggested that the photosynthetic apparatus of Chlorella was
unaffected by Aroclor 1221; the major, and probably the only, effect
of the PCBs being a stimulation of endogenous respiration rate.
Results with Aroclors 1242 and 1268 were consistent with those for
Aroclor 1221; both inhibited net oxygen production in the light and
glucose-driven respiration in the dark, but stimulated endogenous
respiration in the dark (Sinclair et al., 1977).
Luard (1973) reported inhibition of 14C uptake by the green alga
Scenedesmus quadricauda at concentrations of Aroclor 1254 as low as
0.1 µg/litre. At this concentration, the Aroclor caused a 20%
inhibition of 14C uptake, which rose to 65% inhibition at 1 mg/litre.
A marked effect of the initial number of cells in the incubation tube
on the toxicity of Aroclor 1254 for the green alga Chlorella
pyrenoidosa was demonstrated by Cole & Plapp (1974). At a constant
concentration of 1 mg Aroclor 1254/litre, the numbers of algal cells
in the initial incubation medium were as varied as 1, 10, 100, or
1000 µg alga/ml of medium. At the highest inoculation rate, the growth
of the alga was unaffected by the Aroclor. At lower initial
inoculation, the rate of growth was reduced to between 11 and 55% of
control levels. A comparable effect was found using 14C fixation as
the parameter; with inoculation rates of 1000 µg alga/ml medium, the
Aroclor did not have any effects, whereas lower inoculation rates
reduced carbon fixation to between 6 and 13% of control levels.
Mosser et al. (1972) showed that 2 species of freshwater algae
(Euglena gracilis and Chlamydomonas reinhardtii) were unaffected
by 100 µg PCBs/litre (type unspecified). Ewald et al. (1976)
determined the 48-h EC50 on the growth of Euglena gracilis to be
4.4 mg/litre for Aroclor 1221 and 55 mg/litre for Aroclor 1232.
Aroclor 1242 showed no inhibition at 100 mg/litre. Aroclor 1221, at
the EC50 concentration of 4.4 mg/litre, significantly depressed
carbon fixation and chlorophyll levels, but did not affect oxygen
consumption. Uptake of L-leucine was increased 2-fold, but
incorporation was not affected. Uridine uptake was significantly
decreased, but thymidine uptake and incorporation were not affected.
In studies by Glooschenko & Glooschenko (1975), 3 algal species from
the Great Lakes were cultured with Aroclor at concentrations of 1, 5,
10, 20, or 50 µg/litre. Cell numbers of the diatom Synedra acus were
reduced in culture from day 3 of treatment with Aroclor 1242, at 10,
20, or 50 µg/litre, and from day 7, with 1 or 5 µg/litre. The green
alga Scenedesmus quadricauda showed a lag phase of 3 days in all
concentrations of Aroclor 1242. After 3 days, exponential growth
occurred in all treatments, except for the highest dose levels (20 and
50 µg/litre) which showed little or no cell division. A second green
alga (Ankistrodesmus falcatus) was more sensitive, showing
significantly reduced cell numbers at all dose levels of Aroclor 1242.
Ankistrodesmus was used to examine the relative toxicities of
different Aroclors. Cell numbers were 57, 66, 36, and 53% of control
levels for Aroclors 1016, 1221, 1242, and 1248, respectively, after 2
days of culture. Carbon fixation, estimated as uptake of 14C from
solution, was 73, 98, 51, and 59% of control levels for the 4
Aroclors, respectively.
Dive et al. (1976) cultured the ciliate protozoan Colpidium campylum
with purified chlorobiphenyls and with a commercial mixture (Pyralene
3010). None of the 16 isomers affected the ciliate's growth and
reproduction at concentrations of 0.01 or 0.1 mg/litre; similarly,
Pyralene 3010 was not toxic at these concentrations. 2-Mono-
chlorobiphenyl showed little toxicity for the organism at 1 mg/litre
and little or no toxicity was demonstrated by the tetrachloro-,
pentachloro-, and hexachlorobiphenyls at this concentration or at
10 mg/litre (with the exception of 2,5,2',5'-tetrachlorobiphenyl,
which inhibited growth considerably, limiting it to about 10% of
controls at 10 mg/litre). 4,4'-Dichlorobiphenyl was not toxic at any
concentration tested (up to 10 mg/litre) but both 2,3- and
2,5-dichlorobiphenyls were toxic, killing all organisms at both 1 and
10 mg/litre. These results are comparable with other reports that the
lower chlorinated biphenyls are the most toxic for microorganisms;
differences in toxicity could not be explained by the differential
uptake of the different isomers.
It was reported by French (1976) that the EC50 for growth inhibition
of Aroclor 1254 on the flagellated protozoan Crithidia fasciculata
was 10.5 mg/litre. The PCBs slightly inhibited (after 6 h) and then
increased (after 24 h) the carbon dioxide evolution of cultures
utilizing D-glucose. After 6 h exposure, the uptake and incorporation
of thymidine and uridine (but not L-leucine) were inhibited;
inhibition was transient and returned to normal after 12 or 24 h. Fine
structural changes were noted after exposure to PCBs including:
deterioration of the kinetoplast, mitochondrial or cellular swelling,
and the presence of concentric membrane arrays. It was concluded that
cell population growth inhibition was due to disruption of uptake,
incorporation of nucleic acids, and loss of cell regulatory capacity.
7.1.2 Marine and estuarine microorganisms
Bourquin & Cassidy (1975) and Bourquin & Kiefer (1975) investigated
the effects of Aroclors 1016 and 1242 on 85 bacterial isolates from
various estuarine environments near Pensacola, Florida. Twenty six of
the 85 isolates were inhibited to various extents by 0.5 mg of either
of the PCBs, applied to a disc placed on the surface of an agar plate
on which the bacteria were growing. Zones of inhibition ranged from 14
to 20 mm in diameter. Cultures that showed sensitivity to Aroclor 1242
were also inhibited by Aroclor 1016. Sixty five percent of isolates
inhibited by 0.5 mg of Aroclor 1242 were still sensitive at 0.1 mg,
and 58% of those sensitive to Aroclor 1016 at 0.5 mg were still
sensitive to 0.1 mg. Four isolates were examined in a liquid medium.
Inhibition of the cultures was characterized by a greatly extended lag
phase (extended from 2 h to at least 14 h); when growth occurred,
growth curves were parallel with those of the controls. The
physiological activity of sensitive and insensitive isolates were
investigated to try to explain the reasons behind sensitivity. More of
the sensitive isolates were amylase and gelatinase producers (76 and
86%, respectively, compared with 33 and 42%, respectively, for the
whole range of isolates). The significance of this observation is
unclear. Because of the method of exposure in this screening exercise,
it is difficult to relate the results to exposure in natural waters
and to draw conclusions about likely hazards for aquatic bacteria.
Kleppel & McLaughlin (1980) determined the toxic threshold of Aroclor
1254 for the estuarine diatom Skeletonema costatum to be between
3 × 10-9 and 3 × 10-8 µg/cell. When the effect of cell density on the
toxicity of the Aroclor for the organism was examined, maximum
inhibition occurred with the lowest inoculum rates.
Michaels et al. (1982) estimated the effects of Aroclor 1254 on
photosynthesis in the marine diatom Thalassiosira pseudonana
( = Cyclotella nana) by monitoring the uptake of 14C-carbon
dioxide. The numbers of viable cells in the culture were also
estimated. Total cell numbers were estimated at regular intervals
during the 48 h of the experiment and the minimum number of viable
cells required to produce the increase in numbers between periods
calculated. This gave an estimate of the viable numbers,
retrospectively, for each period. Inhibition of 14C-uptake per
culture, per cell, and per viable cell was evident within 1 h of the
start of the incubation. By 48 h, the 14C uptake per culture was
reduced to 0.2% of control levels, per cell, to 13% of control levels,
and per viable cell, to 34% of control levels. The authors concluded
that the effect of Aroclor 1254 on the diatom is a combination of
inhibition of carbon assimilation by individual cells and inhibition
of cell division.
In an earlier study, Fisher & Wurster (1973) exposed 2 estuarine
diatoms (Thalassiosira pseudonana and Rhizosolenia setigera) and
an estuarine green alga (Dunaliella tertiolecta) to Aroclor 1254 at
0.1 or 10 µg/litre, cultured over 100 h. There was no effect on the
growth of the alga. T. pseudonana was unaffected by the PCB at
0.1 µg/litre. The effect of the Aroclor on T. pseudonana at
10 µg/-litre was dependent on temperature; there was a 17% reduction
in growth rate at 25°C, a 44% reduction in growth rate at 18°C, and a
58% reduction in growth rate at 12°C. The growth of control cultures
was greatest at the highest temperature. The growth of R. setigera
was completely stopped by all concentrations of PCB tested, for the
first 48 h of culture. When growth resumed, the degree of inhibition
was greater at 10°C than at 15°C. Fisher et al. (1976) exposed the
marine diatom Thalassiosira pseudonana to 1 µg Aroclor 1254/litre in
cultures containing various levels of nitrate nutrient. The toxic
effects of the PCBs on the growth of the diatom were greatest at low
nitrogen levels. Analysis of variance showed the diatom to be
significantly dependent for growth on nitrogen concentration and on
PCB concentration, and that the PCB effect was significantly nitrogen
dependent. The authors pointed out that marine phytoplankton are often
nitrogen-limited in nature and suggested that the effects of
pollutants, such as PCBs, may, therefore, vary with season. The
greatest effects are likely to occur during bloom conditions, when
competition for nutrients is greatest. Fisher (1975) considered that
the presence of PCBs, even at concentrations far above the maximum
recorded level in sea water, would not affect the overall carbon
fixation by phytoplankton. Although the cell division of some
organisms was adversely affected, enough insensitive species existed
to compensate for the sensitive species. Species diversity and
community structure were likely to be affected.
In a study by Craigie & Hutzinger (1975), 6 marine algae were cultured
for 6 days in the presence of commercial mixtures of PCBs (Aroclors
1221 to 1262; Phenoclors DP3 to DP6), PCTs (Aroclor 5460), and
specific chlorobiphenyls. Each compound or mixture was applied to the
culture medium at 2 concentrations (1 and 100 mg/litre). The algae
were representatives of 6 different classes: Bacillariophyceae -
Skeletonema costatum, Thalassiosira fluviatilis; Chlorophyceae -
Dunaliella tertiolecta; Chrysophyceae - Monochrysis lutheri;
Prainophyceae - Platymonas sp.; Rhodophyceae - Porphyridium sp.;
Xanthophyceae - Olisthodiscus sp. All were cultured at 20°C. Growth
was estimated by turbidity. The response to particular Aroclors was
species dependent; the least sensitive species was Dunaliella and
the most sensitive was Olisthodiscus. At 1 mg/litre of the PCB
mixtures, there was relatively little inhibition of Dunaliella,
Platymonas, Skeletonema, or Thalassiosira. Olisthodiscus was
completely inhibited by Aroclors 1248, 1254, 1260, and Phenoclor DP4.
The Phenoclor series was more toxic for Olisthodiscus. than the
Aroclors. Porphyridium and Monochrysis showed intermediate
sensitivity. Generally, the Aroclors with higher chlorination levels
were less toxic for all species than the Aroclors with lower
chlorination levels. Results with pure, specific chlorinated biphenyls
confirmed that highly chlorinated compounds are less toxic than those
with only 1-4 chlorine atoms per molecule. Experiments with biphenyls
containing identical percentages of chlorine showed that biological
response was highly dependent on the structure of the molecule. The
2,4,2',4'-tetrachlorobiphenyl was more toxic for both Dunaliella and
Olisthodiscus than the 2,5,2',5'- isomer, and both were more toxic
than either the 2,3,4,5- or the 3,4,3',4'- isomers. The last was not
toxic for any of the algae, even when added at 100 mg/litre.
Luard (1973) reported a significant reduction in 14C uptake by the
estuarine green alga Dunaliella tertiolecta in the presence of
Aroclor 1254, at 100 µg/litre. The culture showed 14C uptake at 65%
of control levels. At 1000 µg/litre, uptake was further reduced to 59%
of controls. The 14C uptake was unaffected at 10 µg/litre.
7.1.3 Soil microorganisms
When Murado et al. (1976) added Aroclors to a liquid or solid medium
on which the soil microfungus Aspergillus flavus was cultured,
mycelial growth was reduced progressively as the dose of Aroclor 1254
increased from 5 to 50 mg/litre in liquid culture. At 25 mg/litre, the
dry weight of the mycelium was reduced to 1.4, 3.4, 3.9, 3.3, and
54.6% of control levels by Aroclors 1232, 1242, 1248, 1254, and 1260,
respectively. At the same time, the relative RNA content of the
mycelium increased, rising from a control level of 5.9 µg RNA/mg dry
weight to between 13.2 and 18.6 µg RNA/mg dry weight for Aroclors 1232
to 1254. Aroclor 1260 had no marked effect on RNA. The DNA content was
not affected by any treatment. Cultures on solid medium showed a delay
in sporulation and a decrease in the diameter of colonies at doses up
to 20 µg/cm2.
Glooschenko & Glooschenko (1975) cultured the soil alga Navicula
pelliculosa with Aroclors 1016, 1221, 1242, and 1248 at a
concentration of 20 µg/litre. The numbers of cells in the culture,
after 2 days, were reduced to 66, 53, 46, and 56% of control levels
for the 4 Aroclors, respectively.
7.1.4 Plankton communities
Phytoplankton communities from 2 lakes, one oligotrophic and one
eutrophic, were exposed to PCBs, as Clophen A50, at 26 µg/litre
(Södergren & Gelin, 1983). In the community from the eutrophic lake
with a greater biomass of phytoplankton, measurement of 14C uptake,
monitored immediately after the addition of the PCBs, showed a
reduction of 34% compared with the controls. Monitoring carbon
fixation 16 h later showed the phytoplankton recovering from the
effect of the PCBs, with only 21% inhibition relative to the controls.
The results differed in the community from the oligotrophic lake. 14C
uptake immediately after the addition of the Clophen was 70% less than
that in the controls; 16 h later, the effect was greater, at 84%
inhibition. The authors pointed out that these results parallel
findings in pure culture showing that a high density of organisms
reduces the effects of PCBs.
Mosser et al. (1972) first demonstrated the effects of PCBs on both
single and mixed cultures of marine phytoplankton. Two organisms, a
marine diatom (Thalassiosira pseudonana) and a marine green alga
(Dunaliella tertiolecta) were used. The diatom is sensitive and the
alga insensitive to PCBs. The application of PCBs (not specified) to
mixed cultures changed the usual dominance of the diatom into
dominance of the alga, even at concentrations of the PCBs (1 and
10 µg/litre) that had no discernible effects on the diatom in pure
culture.
The effect of Aroclor 1254 on the relative biomass of 2 species of
marine diatoms Phaeodactylum tricornutum and Cyclotella cryptica
was examined by Lundy et al. (1984). The diatoms were cultured
together in either 10 or 20 µg PCBs/litre for 6 days. At both
concentrations of Aroclor, the ratio between the species was shifted
in favour of Phaeodactylum. After 6 days, the ratio of
Phaeodactylum: Cyclotella was 0.8 in the controls and 2.63 in the
treated cultures (10 µg Aroclor 1254/litre).
Biggs et al. (1979) exposed a mixed culture of 2 marine algae to PCBs
(Aroclor 1254) at 50 µg/litre. In the control culture, Thalassiosira
pseudonana became the dominant organism over Dunaliella
tertiolecta. After exposure to the PCBs, the Thalassiosira was
affected, but the Dunaliella was not. By day 2 of Aroclor 1254
exposure, Dunaliella was the dominant species. Fisher et al. (1974)
showed a similar effect with the greater effects of PCBs on the
sensitive diatom Thalassiosira pseudonana when the organism was in
competition with other organisms. The effect was also demonstrated
using natural communities of phytoplankton, when Thalassiosira was
also selectively affected.
Biggs et al. (1978) exposed a natural community of marine
phytoplankton (from which large detritus and zooplankton had been
filtered) to Aroclor 1254 at either 5 or 10 µg/litre. Cell division,
chlorophyll-a synthesis, and 14C uptake were monitored, as well as
particle size. Treatment with the Aroclor reduced community growth
rates by 20-50%, compared with controls. Growth had not fully
recovered after 10 days. The 14C uptake was reduced for 6 days. The
control cultures became dominated by algae larger than 8 µm in
diameter. The PCB-treated cultures showed strong inhibition of these
larger algal cells and the culture became dominated by small cells.
In a study by Iseki et al. (1981), a natural community of plankton was
exposed, in situ, in a marine environment, to Aroclor 1254 in large
bags holding 68 m3 of seawater. The Aroclor was added to the bag
giving an initial concentration in the upper layers of about
40 µg/litre (15 µg/litre at 10 m depth). Over time, the levels of PCB
fell in the upper layer and increased in the lower layer in the bag.
Six days after addition, the concentrations at all depths were less
than 15 µg/litre. Immediately after addition of the PCBs, the rate of
sedimentation of the particles increased; these particles were thought
to be dead or senescent large cells, such as diatoms, and this
sedimentation was assumed to be responsible for the major part of the
loss of the PCBs from the water. Zooplankters were eliminated from the
bags by this level of Aroclor; no recovery was seen over the 21 days
of the experiment. Large diatoms were selectively eliminated from the
bags and replaced by small flagellates as the dominant organism.
Moore & Harriss (1972, 1974) exposed a natural community of plankton
to PCBs (as Aroclor 1242) at 10 or 25 µg/litre. The population was
collected from natural water, placed in glass bottles suspended
in situ, and monitored for uptake of 14C, added to the bottles as
bicarbonate. After incubation, the community was separated into small
"nannoplankton" and larger "net-plankton" by filtration at a mesh size
of 53 µm. Nannoplankton radiocarbon uptake accounted for 72.6% of the
total community carbon uptake and was not affected by either of the
concentrations of Aroclor 1242. The net-plankton uptake of 14C was
reduced by 56% at 10 µg Aroclor/litre and by 58% at 25 µg/litre. The
authors suggested that PCBs at levels found in natural waters would
alter the species diversity or community structure of microorganisms
and that this might affect higher levels of the food chain with
specialist feeders utilizing one type of prey. O'Connors et al. (1978)
exposed natural communities of phytoplankton, in situ, in dialysis
bags in a salt marsh. Large zooplankton herbivores were removed by
filtration through a mesh. Aroclor 1254 was added to the bags to give
water concentrations of 1-10 µg/litre. Larger diatoms were selectively
inhibited by the Aroclor, even at the lower dose (1 µg/litre). The
authors suggested that, not only would phytoplankton communities be
affected by PCBs in natural waters, but that the effect would be
carried forward through the food chain. Gelatinous predators, such as
jellyfish, could be selected at the expense of fish, since fish tend
to depend directly, or indirectly, on the larger phytoplankton.
Natural communities of phytoplankton from a stream and a reservoir
were cultured with Aroclors 1232 and 1254 by Kricher et al. (1979).
Although the algal communities were different in composition, both
Aroclors exerted similar effects on both communities; primary
productivity was reduced in a dose-dependent manner. Aroclor 1232 was
more toxic than Aroclor 1254 at 1 mg/litre. Algal species within the
populations were differentially affected by the Aroclors; diatoms were
particularly susceptible and treatment produced disproportionate
numbers of blue-green algae, such as Anacystis. The authors pointed
out that the insensitive species still accumulated PCBs and,
therefore, formed the basis for the accumulation of the Aroclors in
aquatic food chains.
7.1.5 Interactions with other chemicals
Mosser et al. (1974) investigated the interactions between Aroclor
1254, DDT, and DDE in a marine diatom Thalassiosira pseudonana. The
diatom was cultured for 4 days with either 10 or 50 µg Aroclor
1254/litre, 100 µg DDE/litre or 500 µg DDT/litre (with each chemical
alone or in combination). The PCBs alone (at 10 µg/litre) and the DDE
alone had little effect on the growth of the diatom; when combined
these treatments were synergistic, growth being less than half that of
the control culture. Higher concentrations of either compound
increased the inhibitory effect. In contrast, DDT reduced the toxic
effects of PCBs at higher concentrations (50 µg/litre). Treatment with
the Aroclor alone at 50 µg/litre almost stopped the growth of the
diatom. Simultaneous treatment with DDT at 500 µg/litre restored
growth to 60-70% of control levels. Lower concentrations of DDT had a
comparable, but reduced, effect. Addition of the DDT to the medium, 12
or 24 h after culture had begun in the presence of PCBs, also reversed
the inhibitory effect.
7.1.6 Tolerance
Fisher et al. (1973) showed that strains of diatoms, isolated from the
Sargasso Sea, were more sensitive to the effects of PCBs than isolates
of the same species, obtained from estuaries or the continental shelf.
It was suggested by the authors that the difference in sensitivity was
derived from the variable environment of the estuarine strains; these
strains are able to cope with wide variations in their living
conditions, not experienced in the open ocean of the Sargasso, and,
therefore, were better able to cope with stress from chemical
pollutants.
When Cosper et al. (1984) compared the sensitivity of clones of 2
species of diatom (Asterionella japonica and Ditylum brightwelii)
from polluted and unpolluted sites, Asterionella was less sensitive
than Ditylum to the action of PCBs (Aroclor 1254); some strains of
the former were tolerant of 25 µg Aroclor/litre whereas no strains of
the latter could tolerate this concentration. There was evidence that
strains of Asterionella from the polluted site were more tolerant
than the same species from unpolluted sites. One strain, from the
polluted site, was tolerant to Aroclor 1254 at 50 µg/litre.
7.2 Toxicity for aquatic organisms
7.2.1 Aquatic plants
Mahanty (1975) grew the aquatic angiosperm Spirodela oligorhiza in a
sterile culture solution, to which had been added Aroclor 1242, at
concentrations of 5-100 mg/litre. The numbers of colonies were counted
throughout the 14-day exposure. The highest dose (100 mg/litre) was
found to be lethal. At both 25 and 50 mg/litre, though there was some
growth, the colonies were small and showed morphological differences
from control colonies including: smaller fronds, in larger numbers
than usual, and a characteristic striped pattern of chlorosis on the
fronds. Even at 5 mg/litre, growth was reduced by 50% (as recorded by
the number of colonies and the fresh weight). Mahanty & McWha (1976),
using only the 5 mg/litre dose, found reduced growth, an unusual
striped pattern of chlorosis, and a reduction in the levels of
chlorophyll and total RNA, but no change in the levels of DNA. When
Mahanty & Fineran (1976) studied treated (5 mg/litre) and untreated
fronds of Spirodela by electron microscopy, they found almost
complete disorganization of the internal structure of the chloroplasts
in chlorotic tissue. Organization of other cell components was largely
unaffected.
7.2.2 Aquatic invertebrates
The acute toxicity of PCBs for aquatic invertebrates is summarized in
Tables 27 and 28. Toxicity is very variable between species, even
closely-related species. For most aquatic invertebrates, there is an
effect of degree of chlorination of the PCBs, but this is not a direct
correlation, either negative or positive, the most toxic PCBs often
being in the mid range of chlorination. Under flow-through conditions,
the toxicity of PCBs appears to be much higher. Over 96 h, under
static conditions, LC50 values ranged between 12 µg/litre and
> 10 mg/litre for different organisms and different PCBs.
MATC (maximum acceptable toxicant concentrations) were set for various
PCBs by the US EPA (1980). These are expressed as a range between
no-observed-effect values and lowest concentration tested that
produced a measurable effect. For Daphnia magna, these values were
1.2 and 3.5 µg/litre for Aroclor 1248, and 2.5 and 7.5 µg/litre for
Aroclor 1254. For the scud (Gammarus pseudolimnaeus) values for
Aroclor 1242 were 2.8 and 8.7 µg/litre and values for Aroclor 1248
were 2.5 and 5.1 µg/litre. Aquatic larvae of the midge (Tanytarsus
dissimilis) had a no-observed-effect level of 0.5 µg/litre and a
lowest effective concentration at 1.2 µg/litre.
7.2.2.1 Short- and long-term toxicity
Roberts (1975) exposed the common mussel (Mytilus edulis) to
Aroclors 1242 and 1254 and studied byssus formation. The 24- and 48-h
EC50s for a reduction in the number of mussels byssally-attaching
were 2.2 and 3.0 mg/litre, respectively, for Aroclor 1254. EC50s
after exposure to Aroclor 1242 were 0.9 mg/litre for 24 h and
1.0 mg/litre for 48 h. Duke et al. (1970) maintained oysters
Table 27. Acute toxicity of PCB mixtures for freshwater invertebrates
Organism Size/age Stat/ Temperature Hardness pH PCB type Parameter Concentration Reference
flowa (°C) (mg/litre)b (Aroclor) (mg/litre)
Scud mature flow 15 272 7.4 1242 96 h - LC50 0.01 Mayer & Ellersieck
(Gammarus (1986)
pseudolimnaeus)
juvenile flow 18 1248 96 h - LC50 0.029 Nebeker & Puglisi
juvenile flow 18 1242 96 h - LC50 0.073 (1974)
Scud mature stat 21 44 7.1 1248 96 h - LC50 0.052 Mayer & Ellersieck
(Gammarus mature stat 21 44 7.1 1254 96 h - LC50 2.4 (1986)
fasciatus)
Glass shrimp mature flow 15 272 7.4 1254 168 h - LC50 0.003 Mayer & Ellersieck
(Palaemonetes (1986)
kadiakensis)
Crayfish early stat 21 44 7.1 1242 168 h - LC50 0.03 Mayer & Ellersieck
(Orconectes nais) instar (1986)
Crayfish early stat 21 44 7.1 1254 168 h - LC50 0.1 Mayer & Ellersieck
(Procambarus sp.) instar (1986)
immature stat 12 44 7.5 1254 96 h - LC50 > 0.55
Table 27. (cont'd)
Organism Size/age Stat/ Temperature Hardness pH PCB type Parameter Concentration Reference
flowa (°C) (mg/litre)b (Aroclor) (mg/litre)
Stonefly first stat 10 170 7.2 1016 96 h - LC50 0.61 Mayer & Ellersieck
(Pteronarcella year (0.42-0.88) (1986)
badia)
Damselfly late flow 15 272 7.4 1242 96 h - LC50 0.4 Mayer & Ellersieck
(Ischnura instar flow 15 272 7.4 1254 96 h - LC50 0.2 (1986)
verticalis)
Dragonfly late stat 21 44 7.1 1242 168 h - LC50 0.8 Mayer & Ellersieck
(Macromia sp.) instar stat 21 44 7.1 1254 168 h - LC50 0.8 (1986)
a Stat = static conditions: water not changed during the exposure; flow = flow-through conditions; concentration of
toxicant continuously maintained.
b Hardness expressed as mg CaCO3/litre, unless otherwise stated.
Table 28. Acute toxicity of PCB mixtures for marine invertebrates
Organism Size/age Stat/ Temperature Salinity PCB type Parameter Concentration Reference
flowa (°C) (%) (mg/litre)
Cockle adult stat 15 Aroclor 1248 48 h - LC50 > 10 Portmann &
(Cardium adult stat 15 Aroclor 1254 48 h - LC50 > 10 Wilson (1971)
edule) adult stat 15 Aroclor 1260 48 h - LC50 > 10
adult stat 15 Aroclor 1262 48 h - LC50 > 10
adult stat 15 Clophen A30 48 h - LC50 3.0
adult stat 15 Clophen A60 48 h - LC50 > 10
Eastern oyster adult flow 28 28 Aroclor 1016 96 h - EC50 0.01 Mayer (1987)
(Crassostrea
virginica)
Brown shrimp adult flow 30 29 Aroclor 1016 96 h - LC50 0.01 Mayer (1987)
(Penaeus
aztecus)
Brown shrimp adult stat 15 Clophen A60 48 h - EC50 > 10 Portmann &
(Crangon adult stat 15 Aroclor 1242 48 h - LC50 1.0 Wilson (1971)
crangon) adult stat 15 Aroclor 1248 48 h - LC50 0.3-1.0
adult stat 15 Aroclor 1254 48 h - LC50 3.0-10.0
adult stat 15 Aroclor 1260 48 h - LC50 > 10
adult stat 15 Aroclor 1262 48 h - LC50 > 10
adult stat 15 Clophen A40 48 h - LC50 0.3-1.0
adult stat 15 Clophen A30 48 h - LC50 1.0-3.3
adult stat 15 Clophen A50 48 h - LC50 3.3-10.0
Table 28. (cont'd).
Organism Size/age Stat/ Temperature Salinity PCB type Parameter Concentration Reference
flowa (°C) (%) (mg/litre)
Grass shrimp 1 day stat 25 25 Aroclor 1016 96 h - LC50 0.15 Mayer (1987)
(Palaemonets 3 days stat 25 25 Aroclor 1016 96 h - LC50 0.021
pugio) 6 days stat 25 25 Aroclor 1016 96 h - LC50 0.017
9 days stat 25 25 Aroclor 1016 96 h - LC50 0.019
12 days stat 25 25 Aroclor 1016 96 h - LC50 0.021
15 days stat 25 25 Aroclor 1016 96 h - LC50 0.024
18 days stat 25 25 Aroclor 1016 96 h - LC50 0.037
30 days stat 25 25 Aroclor 1016 96 h - LC50 0.044
adult stat 25 25 Aroclor 1016 96 h - LC50 0.052 (0.046-0.057)
adult flow 30 28 Aroclor 1016 96 h - LC50 0.012
1 day stat 25 25 Aroclor 1242 96 h - LC50 0.015
3 days stat 25 25 Aroclor 1242 96 h - LC50 0.019
6 days stat 25 25 Aroclor 1242 96 h - LC50 0.015
9 days stat 25 25 Aroclor 1242 96 h - LC50 0.017
12 days stat 25 25 Aroclor 1242 96 h - LC50 0.016
15 days stat 25 25 Aroclor 1242 96 h - LC50 0.024
18 days stat 25 25 Aroclor 1242 96 h - LC50 0.034
30 days stat 25 25 Aroclor 1242 96 h - LC50 0.041
adult stat 25 25 Aroclor 1242 96 h - LC50 0.057 (0.048-0.062)
a stat = static conditions: water not changed during the exposure; flow = flow-through conditions; concentration
of toxicant continuously maintained.
(Crassostrea virginica) at concentrations of 1, 10, and 100 µg
Aroclor 1254/litre and monitored shell growth over a period of 96 h;
the rates of shell growth were decreased by 19, 41, and 100%,
respectively. Lowe et al. (1972) found the growth rate (height and wet
weight) of young oysters (Crassostrea virginica) to be significantly
reduced after exposure, in flowing sea water, to 5 µg Aroclor
1254/litre, for 24 weeks. No effects on growth were observed at
1 µg/litre over a period of 30 weeks. Oysters exposed to 5 µg/litre
showed atrophy of the digestive diverticular epithelium and
degeneration of the vesicular connective tissues of the hepatopancreas
together with leukocytic infiltration. There was complete tissue
recovery after 12 weeks in clean water.
After exposing Daphnia magna to Aroclor 1254, over a period of 14
days under static renewal procedures, Maki & Johnson (1975) calculated
an LC50 of 24 µg/litre.
Nebeker & Puglisi (1974) calculated 3-week LC50 values, under static
conditions, for a range of Aroclors on Daphnia magna. Aroclors were
dissolved in acetone and triton X100 to maintain the PCBs in solution.
Tests were performed in raw Lake Superior water. Results are presented
in Table 29. The Aroclors most toxic for Daphnia had between 48 and
62% chlorination; the most toxic Aroclor was 1248. Under flow-through
conditions, renewing the original test concentration of the Aroclor
continuously, the Aroclors were much more toxic. Two-week LC50 values
for Aroclors 1248 and 1254 were 2.6 and 1.8 µg/litre, respectively,
while the 3-week LC50 for Aroclor 1254 was 1.3 µg/litre. Groups of 40
scud (Gammarus pseudolimnaeus) were exposed to various concentrations
of Aroclor 1242 under flow-through conditions. No animals survived
exposure for 2 months, at Aroclor concentrations of 26 µg/litre or
more.
Survival at lower exposures was 52% at 8.7 µg/litre and 77% at
2.8 µg/litre (control survival was low at 48%).
Duke et al. (1970) conducted acute, flowing-water bioassays on pink
shrimp (Penaeus duorarum). At 0.1 mg/litre, 100% of the shrimps were
killed within 48 h of exposure to Aroclor 1254. There was no mortality
after 48 h of exposure to 0.01 mg/litre Aroclor. In long-term
flowing-water bioassays, Nimmo et al. (1971a) found that Aroclor 1254,
at a concentration of 0.94 µg/litre killed 51% of juvenile pink
shrimps within 15 days. Juveniles were found to be more sensitive than
adults; 50% of adults were killed after exposure to 3.5 µg/litre over
35 days. There were no apparent symptoms of poisoning prior to death.
Table 29. Toxicity of various Aroclors for Daphnia magna in static testsa
Aroclor 3-week Confidence limits (95%)
LC50 (µg/litre)
1221 180 (158.0-205.0)
1232 72 (62.6-82.8)
1242 67 (55.4-81.0)
1248 25 (21.4-29.2)
1254 31 (25.8-37.2)
1260 36 (27.7-46.8)
1262 43 (37.0-49.9)
1268 253 (222.0-288.0)
a From: Nebeker & Puglisi (1974).
Striped hermit crabs (Clibanarius vittatus) were kept in static
seawater solutions containing 3, 5, 10, 15, 20, 25, or 30 µg/litre of
Aroclor 1254 for 96 h (Stahl, 1979). No deaths were reported, though
the crabs exposed to the higher concentrations (20, 25, and
30 µg/litre) were less active. Six crabs already exposed to 30 µg
PCBs/litre were then placed in solutions containing 300 µg/litre for a
further 96 h; there were still no deaths.
Vernberg et al. (1977) exposed fiddler crab (Uca pugilator) larvae
to concentrations of 0.1, 1.0, 5, 10, 50, or 500 µg Aroclor
1254/litre, for 96 h. They did not find any effects on survival at 0.1
or 1.0 µg/litre; Aroclor 1254 at 5 µg/litre increased deaths by 20%,
but the increase was not statistically significant. Exposure to 10 µg
PCBs/litre resulted in a 57% reduction in survival of larvae.
Increasing the PCB concentration to 50 µg/litre did not greatly
increase the effect. The 500 µg/litre concentration killed all larvae.
Increasing exposure time, at 5 µg/litre, produced a significant
reduction in survival after 14 days. Exposure of larvae to Aroclors
1016 or 1254 at 0.1, 1, or 5 µg/litre for periods of up to 168 h, was
then investigated. No significant effects were found on survival with
any concentration of Aroclor 1016, for up to 120 h of exposure. After
168 h, survival rates of larvae exposed to 1 and 5 µg/litre were
reduced to 61 and 66%, respectively. There was no effect at
0.1 µg/litre. Aroclor 1254 did not have any effects on survival at
concentrations of 0.1 or 1 µg/litre. Survival was reduced after
exposure to 5 µg Aroclor 1254/litre for more than 96 h, increasing
from 60-81% up to 168 h. Exposure to 10 µg Aroclor 1254/litre caused
55% deaths after 120 h; there were no further deaths after 168 h.
Fifty per cent of adult male crabs, exposed to Aroclor 1254 or Aroclor
1016 at 50 µg/litre, died after 2 days and after 4-6 days,
respectively. Females (50%) exposed to 50 µg/litre survived for 7 days
after exposure to Aroclor 1016 but for only 4 days after exposure to
Aroclor 1254.
Neff & Giam (1977) exposed juvenile horseshoe crabs (Limulus
polyphemus) to concentrations of Aroclor 1016 of 10, 20, 40, or
80 µg/litre, for up to 96 days. The crabs were divided into 2 groups:
group A consisted of juveniles at the late first tailed stage and
group B, of juveniles at the early second tailed stage. The authors
calculated LT50s (LT50: median survival time for a given exposure
concentration) of 20.8 days at 40 µg/litre and 20.3 days at
80 µg/litre for group A juveniles. The LT50 for group B crabs at
80 µg/litre was 61 days, but less than 50% had died within 96 days at
40 µg/litre.
7.2.2.2 Response to temperature and salinity
In a study by Vernberg et al. (1977), larval fiddler crabs
(Uca pugilator) were exposed to "sub-lethal" concentrations of
Aroclor 1254 and 1016 and the conditions of temperature (15-30°C) and
salinity (15-36%) varied. Exposure to 0.01 µg Aroclor/litre showed no
consistent effects of temperature or salinity, though the organisms
exposed under conditions furthest from the optimum (25°C and 30%) were
more likely to differ significantly from the controls. At the optimum
temperature and salinity, no deaths were recorded in adult crabs
exposed to 0.1, 1, 10, or 100 µg Aroclor 1254/litre for up to 3 weeks.
Lowering the salinity or increasing the temperature did not have any
effects on survival with Aroclor 1254 or 1016 at 5 µg/litre. Lowering
both salinity and temperature (to 5% and 10°C) caused 50% of crabs to
die between 21 and 28 days of exposure to Aroclor 1016 at 5 µg/litre,
but there were no lethal effects of Aroclor 1254 at the same
concentration. Lowering the temperature further (7°C and 5%) reduced
the 50% survival time to between 5 and 8 days for both Aroclors.
Nimmo & Barrier (1974) reported some deaths in adult brown shrimp
(Penaeus aztecus) exposed to a "sub-lethal" concentration of Aroclor
1254 (3 µg/litre), for 7 days, after subjection to salinity shock.
The shrimp normally adapts readily to the wide range of salinity
found in its natural estuarine habitat. Roesijadi et al. (1976a)
exposed the adult grass shrimp (Palaemonetes pugio) to sub-lethal
(6.3-8.8 µg/litre) and lethal (57.6-76.4 µg/litre) concentrations of
Aroclor 1254 for 96 h, at various salinities. They found little effect
on haemolymph chloride concentration or osmolarity, chloride space
(the apparent volume of distribution of chloride ions), or chloride
exchange kinetics. The shrimp showed an adaptive altered permeability
to chloride ions at a salinity of 17%, the isotonic point. PCBs did
not affect this permeability change in adult shrimp. In the juvenile
grass shrimp, there was a reduction in haemolymph chloride levels at
low salinities in non-steady state exposures; the PCBs delayed the
permeability change. This disruption of haemolymph chloride was
associated with high numbers of deaths, even at the "sub-lethal"
exposure concentration. It was concluded that juveniles died from
salinity shock, because of delayed adaptive response. In another study
(Roesijadi et al., 1976b), grass shrimp were exposed to Aroclor 1254
at 29.4 µg/litre for 96 h at various salinities. No appreciable effect
was observed on total free amino acid levels in abdominal muscle,
indicating that intracellular osmoregulation was not a major
consequence of PCB toxicity, though changes in individual amino acid
concentrations suggested an altered metabolic state. The authors found
that blood glycine levels showed large decreases after the transfer of
the shrimps to clean water, a delayed response to the Aroclor
exposure.
7.2.2.3 Reproduction
Sea urchin (Arbacia punctulata) eggs were exposed to concentrations
of Aroclor 1254 of 0.5, 1.0, 5.0, and 10.0 mg/litre (Adams, 1983).
There was no effect on percentage fertilization, percentage pluteus
development, or percentage mortality when eggs were exposed at
fertilization. However, when eggs were exposed 1 h prior to
fertilization, there was a significant reduction in fertilization
efficiency at all doses. At all but the lowest dose, there was a
significant increase in mortality and a significant depression in
successful pluteus development.
Maki & Johnson (1975) calculated EC50s for total young produced,
average brood size, and percentage of days reproducing, during a
14-day exposure of Daphnia magna to Aroclor 1254; the results were
19, 23, and 25 µg/litre, respectively. Daphnia magna were exposed to
a range of Aroclors, by Nebeker & Puglisi (1974) who estimated
reproductive impairment, measured as a percentage of surviving young
relative to controls. The study was conducted over 3 weeks under
static conditions. Results are presented in Table 30. Reproductive
impairment matches lethality of the Aroclors (see Table 29); there was
no indication of reproductive effects of the Aroclors at
concentrations below those leading to the death of adults or young. No
young were produced by scud (Gammarus pseudolimnaeus) exposed to
8.7 µg Aroclor 1242/litre. Scud exposed at 2.8 µg/litre produced fewer
young per surviving adult (4.2), compared with controls (6.8).
Table 30. Reproductive impairment of Daphnia magna exposed to Aroclors under
static conditionsa
Aroclor Concentration (µg/litre) producing reproductive impairment
50% 16%
1221 125 89
1232 66 53
1242 63 48
1248 24 16
1254 28 18
1260 33 22
1262 41 24
1268 206 162
a From: Nebeker & Puglisi (1974).
7.2.2.4 Moulting
Several authors have suggested that crustaceans may be more
susceptible to the toxic effects of PCBs during moult (Duke et al.,
1970; Wildish, 1970; Nimmo et al., 1971a,b). Fingerman & Fingerman
(1979) exposed 2 groups of fiddler crabs (Uca pugilator) at 8 mg
Aroclor 1242/litre, one group for 40 days, and the other for only the
first 14 of the 40 days. Eye-stalks were removed on day 15 to increase
moulting activity. Controls underwent rapid ecdysis, with more than
50% of the population completing moult within 40 days. Crabs exposed
to the Aroclor for the full 40 days showed less moulting, with no more
than 10% of the population completing moult. Crabs exposed for 14 days
showed 20% of the population successfully moulting. Removal of
eye-stalks on day 1 of the study produced similar results, in terms of
numbers of crabs moulting in each treatment, but speeded-up moult in
the controls. No more Aroclor-exposed crabs moulted after eye-stalk
removal. In an earlier study, Fingerman & Fingerman (1977) exposed
fiddler crabs to Aroclor 1242 at 8 mg/litre for 38 days. Either
eye-stalks or 4 walking legs were removed to stimulate moulting. Both
control groups underwent ecdysis rapidly. Treated crabs without
eye-stalks did not undergo any moult. Moulting in those with legs
removed was much slower than in the controls. The authors also exposed
crabs to dibenzofuran (1,2,3,4,5,6,7,8-octachlorodibenzofuran) at
16 ng/litre. This is equivalent to the maximum reported dibenzofuran
contamination of the Aroclor with the dose equivalent to (in terms of
the dibenzofuran) the same concentration (8 mg/litre) in the PCB
mixture. There was only a slight inhibition of moulting caused by the
dibenzofuran.
Neff & Giam (1977) exposed juvenile horseshoe crabs (Limulus
polyphemus) to concentrations of Aroclor 1016 of 10, 20, 40, or
80 µg/litre, for up to 96 days. The crabs were divided into 2 groups:
group A consisted of juveniles at the late first tailed stage and
group B of juveniles at the early second tailed stage. ET50s (median
time for moulting to begin) were calculated between the start of the
study and the first moult (ET50-1) and between subsequent moults
(ET50-2 to -n). No moult occurred within 96 days at concentrations of
Aroclor of 40 or 80 µg/litre in either group of crabs. The ET50-1, in
group A at 10 and 20 µg/litre, was not different from controls; the
ET50-2 was slightly decreased. In group B, 10 and 20 µg/litre did not
affect the ET50-1; 40 and 80 µg/litre delayed the onset of the first
moult by 7 and 9 days, respectively. The ET50-3 were substantially
decreased at 10, 20, and 40 µg Aroclor/litre.
7.2.2.5 Behaviour
Hansen et al. (1974a) studied the avoidance response of the pink
shrimp (Penaeus duorarum) and the grass shrimp (Palaemonetes pugio)
given a choice between clean water and water containing Aroclor 1254,
at concentrations of between 0.001 and 10 mg/litre. Pink shrimp did
not avoid any of the concentrations used; grass shrimp only
significantly avoided the highest dose.
7.2.2.6 Population structure
The composition of communities of estuarine animals, in aquaria, were
studied under different exposures to Aroclor 1254 (0.1, 1.0, and
10.0 µg/litre) for 4 months (Hansen, 1974). The author found that, in
control groups and the group at the lowest concentration, the
community was mainly comprised (>75%) of arthropods, mostly the
amphipod Corophium volutator. At 1 and 10 µg Aroclor 1254/litre, the
numbers of arthropods decreased and the numbers of chordates
increased; at 10 µg/litre, over 75% of the animals were tunicates. The
highest concentration of Aroclor decreased the numbers of phyla,
species, and individuals represented (particularly of amphipods,
bryozoans, crabs, and molluscs), whereas the numbers of annelids,
brachypods, coelenterates, echinoderms, and nemertines were
unaffected.
7.2.2.7 Interactions with other chemicals
Maki & Johnson (1975) exposed Daphnia magna to various combinations
of DDT (0.2-0.75 µg/litre) and Aroclor 1254 (2-24 µg/litre). They
studied adult mortality, total young produced, average brood size, and
percentage days reproducing, during a 14-day exposure period. The
effects of one toxicant significantly enhanced the action of the other
for all test parameters. In the presence of a no-observed-effect level
of PCB (12 µg/litre), the susceptibility of Daphnia to DDT increased
by one third. When combined with 0.5 µg DDT/litre, the toxicity of
Aroclor 1254 was doubled.
In a study by Nimmo & Bahner (1976), the pink shrimp (Penaeus
duorarum) was exposed to various combinations of Aroclor 1254
(0.7-1.1 µg/litre), cadmium (640-829 µg/litre), and methoxychlor
(0.9-1.0 µg/litre) and numbers of shrimps dying were monitored. The
results showed no evidence of synergism or potentiation in any
combination.
7.2.3 Fish
The acute toxicity of PCBs for fish is summarized in Table 31. Values
for 96-h LC50s vary between 0.008 mg/litre, for the fry of the
fathead minnow, to > 100 mg/litre for channel catfish. This
considerable variation is dependent on species and on the PCB mixture
but appears to depend little on test conditions, such as temperature
and water hardness. The toxicity of PCBs appears much greater in
flow-through tests, where the water concentration of the PCBs is
constantly maintained.
MATC (maximum acceptable toxicant concentrations) were set by the US
EPA (1980) and are expressed as a range between the no-observed-effect
level (based on the results of long-term studies and the sub-lethal as
well as the lethal effect) and the lowest concentration showing a
measurable effect. These values, for the fathead minnow, were 5.4 and
15.0 µg/litre for Aroclor 1242; 0.1 and 0.4 µg/litre for Aroclor 1248;
1.8 and 4.6 µg/litre for Aroclor 1254, and 1.3 and 4.0 µg/litre for
Aroclor 1260. The early life stage of the estuarine sheepshead minnow
gave values of 3.4 and 15.0 µg/litre for Aroclor 1016 and 0.06 and
0.16 µg/litre for Aroclor 1254.
7.2.3.1 Short- and long-term toxicity
The toxicity of Aroclors for 3 species of freshwater fish, over
exposure of up to 30 days, was systematically investigated by Mayer et
al. (1977) under flow-through conditions. Results are presented in
Table 32. Short-term tests consistently underestimate the toxicity of
PCBs.
Table 31. Acute toxicity of PCB and PCT mixtures for fish
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Channel catfish 0.60 g stat 20 40 7.4 Aroclor 1016 96-h LC50 > 100
(Ictalurus yolk-sac stat 25 272 7.4 Aroclor 1016 96-h LC50 0.44 (0.34-0.56)
punctatus) 2.80 g flow 17 272 7.4 Aroclor 1242 96-h LC50 >0.10
Mayer & 2.80 g flow 22 272 7.4 Aroclor 1248 96-h LC50 >0.1
Ellersieck 2.80 g flow 22 272 7.4 Aroclor 1254 96-h LC50 >0.20
(1986) 2.80 g flow 22 272 7.4 Aroclor 1260 96-h LC50 >0.40
Atlantic salmon 5.60 g flow 17 314 7.6 Aroclor 1016 96-h LC50 0.13 (0.11-0.16)
(Salmo salar)
Mayer &
Ellersieck (1986)
Brook trout 3.0 g flow 12 314 7.6 Aroclor 1016 96-h LC50 >0.80
(Salvelinus
fontinalis)
Mayer &
Ellersieck (1986)
Brown trout 4.60 g flow 12 314 7.6 Aroclor 1016 96-h LC50 0.14 (0.11-0.18)
(Salmo trutta) 1.10 g stat 13 44 7.4 Aroclor 1260 96-h LC50 > 24.0
Mayer &
Ellersieck (1986)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Cutthroat trout 2.70 g stat 9 159 162 7.4 Aroclor 1221 96-h LC50 1.17 (0.96-1.43)
(Salmo clarki) 2.20 g stat 9 159 162 7.4 Aroclor 1232 96-h LC50 2.5 (1.72-3.08)
Mayer & 2.40 g stat 9 159 162 7.4 Aroclor 1242 96-h LC50 5.42 (3.82-7.68)
Ellersieck 2.50 g stat 9 159 162 7.4 Aroclor 1248 96-h LC50 5.75 (5.1-6.5)
(1986) 2.50 g stat 9 159 162 7.4 Aroclor 1254 96-h LC50 42.5 (38.7-46.7)
2.70 g stat 9 159 162 7.4 Aroclor 1260 96-h LC50 60.9 (55.4-67.0)
2.40 g stat 9 159 162 7.4 Aroclor 1262 96-h LC50 > 50
2.20 g stat 9 159 162 7.4 Aroclor 1268 96-h LC50 > 50
2.70 g stat 9 162 7.4 Aroclor 4465 96-h LC50 > 65
2.10 g stat 9 162 7.4 Aroclor 5442 96-h LC50 > 50
2.90 g stat 9 162 7.4 Aroclor 5460 96-h LC50 > 50
Lake trout fry stat 10 170 7.2 Aroclor 1016 96-h LC50 0.48 (0.39-0.60)
(Salvelinus yolk-sac stat 10 170 7.2 Aroclor 1016 96-h LC50 0.89 (0.69-1.15)
namaycush)
Mayer &
Ellersieck (1986)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Rainbow trout 0.50 g stat 12 44 7.4 Aroclor 1016 96-h LC50 0.14 (0.11-0.16)
(Salmo gairdneri) 2.50 g flow 10 272 7.4 Aroclor 1016 96-h LC50 0.62 (0.42-0.90)
Mayer & fry flow 12 314 7.6 Aroclor 1016 96-h LC50 0.44 (0.37-0.53)
Ellersieck 1.80 g flow 17 272 7.4 Aroclor 1242 120-h LC50 0.07
(1986) 1.80 g flow 17 272 7.4 Aroclor 1248 120-h LC50 0.05
1.80 g flow 17 272 7.4 Aroclor 1254 120-h LC50 0.14
1.80 g flow 17 272 7.4 Aroclor 1260 96-h LC50 >0.23
Harlequin fish 10-30 mm flow 20 20 8.1 Aroclor 1221 96-h LC50 1.05
(Rasbora 10-30 mm flow 20 20 8.1 Aroclor 1232 96-h LC50 0.32
heteromorpha) 10-30 mm flow 20 20 8.1 Aroclor 1242 96-h LC50 0.37
Tooby et al. 10-30 mm flow 20 20 8.1 Aroclor 1254 96-h LC50 1.1
(1975) 10-30 mm flow 20 20 8.1 Aroclor 1262 96-h LC50 >100
Bluegill sunfish 0.90 g stat 12 44 7.4 Aroclor 1016 96-h LC50 0.60
(Lepomis 1.80 g flow 20 272 7.4 Aroclor 1016 96-h LC50 0.46 (0.39-0.54)
macrochirus) 2.20 g flow 17 272 7.4 Aroclor 1242 120-h LC50 0.13
Mayer & 0.80 g stat 18 44 7.1 Aroclor 1248 96-h LC50 0.69 (0.48-0.99)
Ellersieck 2.20 g flow 22 272 7.4 Aroclor 1248 120-h LC50 0.14
(1986) 0.80 g stat 18 44 7.1 Aroclor 1254 96-h LC50 2.74 (1.29-5.81)
2.20 g flow 22 272 7.4 Aroclor 1254 96-h LC50 0.20
2.20 g flow 22 272 7.4 Aroclor 1260 96-h LC50 0.40
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Longnose sucker finger flow 12 314 7.5 Aroclor 1016 96-h LC50 0.33 (0.22-0.49)
(Catostomus
catostomus)
Mayer &
Ellersieck (1986)
Yellow perch 1.20 g flow 17 314 7.6 Aroclor 1242 96-h LC50 >0.15
(Perca flavescens) 1.10 g flow 17 314 7.6 Aroclor 1248 96-h LC50 >0.1
Mayer & 1.00 g flow 17 314 7.6 Aroclor 1254 96-h LC50 >0.15
Ellersieck 1.20 g flow 17 314 7.6 Aroclor 1260 96-h LC50 >0.20
(1986)
Fathead minnow fry flow 24 Aroclor 1254 96-h LC50 0.008
(Pimephales fry flow 24 Aroclor 1242 96-h LC50 0.015
promelas) 3 months flow 24 Aroclor 1242 96-h LC50 0.30
Nebeker
et al. (1974)
Cisco (chub) 22 days flow 7 30-35 40-48 Aroclor 1254 96-h LC50 >10
(Coregonus sp.) 22 days flow 7 30-35 40-48 Aroclor 1254 120-h LC50 3.2 (1.9-5.5)
Passino &
Kramer (1980)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Carp (Cyprinus fry stat 23-25 Kanechlor 300 96-h LC50 1.45
carpio)
Kimura et al.
(1974)
Guppy (Lebistes fry stat 24-25 Kanechlor 300 96-h LC50 0.9
reticulatus) 0.35 g stat 24-25 Kanechlor 300 96-h LC50 3.2
Kimura et al. (1974)
a stat = static conditions: water not changed during the exposure;
flow = flow-through conditions; concentration of toxicant continuously maintained.
b Alkalinity and hardness expressed as mg/litre CaCO3, unless otherwise stated.
Table 32. Toxicity of Aroclors for fish (LC50s in µg/litre) at 17°Ca
Aroclor Exposure (days)
5 10 15 20 25 30
Rainbow trout
1242 67 48 18 10 12 -
1248 54 38 16 6.4 3.4 -
1254 - 160 64 39 27 -
1260 - 326 143 78 49 51
Bluegill sunfish
1242 - - 164 125 120 84
1248 136 115 111 106 100 78
1254 - - 303 260 239 177
1260 - - - - - 400
Channel catfish
1242 - - 219 150 132 87
1248 - 121 121 115 104 75
1254 - 303 286 293 181 139
1260 - 535 482 512 465 433
a From: Mayer et al. (1977).
Duke et al. (1970) kept juvenile pinfish (Lagodon rhomboides) in
seawater containing 1, 10, or 100 µg Aroclor 1254/litre for up to
48 h. There were no deaths at any concentration. It was suggested by
Nimmo et al. (1975) that acute toxicity tests underestimated the true
sensitivity of marine species; in bioassays lasting 1 week or more,
Aroclor proved to be 100 times more toxic than acute exposure
suggested. In tests lasting 2 weeks or longer, Aroclor 1254 was lethal
for longnose killifish (Fundulus similis) at 1 µg/litre and for
pinfish and spot (Leiostomus xanthurus) at 5 µg/litre. Hansen et al.
(1974b) did not find any significant lethal effects in pinfish exposed
to 100 µg Aroclor 1016/litre for 96 h but significant mortality (50%)
was observed after 33 days at 32 µg/litre, and, after 18 days, at
100 µg/litre. Nebeker et al. (1974) exposed the flagfish (Jordanella
floridae) to Aroclor 1248 for 40 days. No fish survived at a
concentration of 18 µg/litre and only 35% survived at 5.1 µg/litre.
The fish at these two concentrations almost completely lost their fins
and tails. Fish survival was not affected at concentrations of
2.2 µg/litre or less. In a study by Defoe et al. (1978), fathead
minnow (Pimephales promelas) larvae were exposed, in flow-through
bioassays, to Aroclors 1248 and 1260 for 30 days; the LC50s were
calculated to be 4.7 and 3.3 µg/litre, respectively.
Hansen et al. (1976a) fed fingerling channel catfish (Ictalurus
punctatus) a diet containing 20 mg Aroclor 1242/kg for 20 weeks. The
fish showed a reduced weight gain and hypertrophy of the liver. When
the treated fish were transferred to a control diet for 8 weeks and
then back to the dosed diet for a further 8 weeks, weight gain and
liver weights returned to normal levels. No histopathological lesions
were observed in any of the fish fed PCBs.
Rainbow trout (Salmo gairdneri) were fed a diet containing 1, 10, or
100 mg Aroclor 1254/kg over a period of 330 days (Nestel & Budd,
1975). No effects on growth rates were seen, but renal lesions were
observed at all doses; however, they were not dose-related. Foci of
renal necrosis, with cellular or granular cast formation were seen. A
significant increase in the number of hepatocytes per unit area in the
liver was observed at all doses and appeared to be dose-related. A
reduction of 'white pulp' (lymphatic elements) in the spleen was
observed at 10 and 100 mg/kg diet. Fish with renal necrosis also had
reduced splenic white pulp and a reduced white cell count.
7.2.3.2 Carcinogenicity
Hendricks et al. (1977) studied the combined effects of Aroclor 1254
(100 mg/kg diet) and aflatoxin B1 (6 mg/kg diet) in rainbow trout. A
significantly reduced incidence of liver tumours was observed in the
combination. There was no retardation of growth in the treated
animals, but they showed glycogen depletion in hepatocytes,
hyperaemia, and white pulp depletion in the spleen.
PCB administration prior to aflatoxin B1 treatment also decreased the
liver tumour incidence, whereas when Aroclor 1254 was fed after
exposure of trout embryo to aflatoxin B1, there was no effect on the
formation of liver tumours (Shelton et al., 1984b).
When rainbow trout were fed 0, 1, 4, or 8 mg aflatoxin B1/kg diet or
aflatoxin B1 at these dose levels plus 50 mg Aroclor 1254/kg diet,
the incidence of hepatic tumours was lower in the groups receiving
aflatoxin combined with PCBs (Shelton et al., 1984a). The inhibition
of aflatoxin B1-mediated carcinogenicity was also correlated with the
decreased bacterial mutagenicity of this compound in the presence of
an Aroclor 1254-induced drug metabolizing enzyme fraction from fish.
The potential mechanisms of this interaction were further investigated
by Shelton et al. (1986) by studying the effects of Aroclor 1254 on
aflatoxin B1 distribution, metabolism, and adduct formation. The
results from the in vivo studies showed that PCB treatment resulted
in a marked increase in the metabolism of aflatoxin B1 to aflatoxin
M1 and their glucuronide conjugates. The DNA-adduct levels in the
PCB-treated fish were 48-96% lower than those in the controls. The
results in the fish model using aflatoxin B1 as the carcinogen were
associated with the activity of Aroclor as an inducer of cytochrome
P-450-dependent monooxygenases (Halverson et al., 1985; Shelton et
al., 1986).
7.2.3.3 Effects on developmental stages and reproduction
Birge et al. (1978) determined LC50s and LC1s for 4 species of fish
from the fertilization of the eggs to 4 days after hatching. Exposure
times varied with species dependent on the time taken to hatching of
the eggs; hatching took 22 days for rainbow trout and 3-4 days for the
other species. In most species, eggs were considerably less sensitive
to the toxic effects of Aroclors than larvae. The major exception was
the rainbow trout for which the duration of exposure of the eggs until
hatching was considerably longer than for other species. LC50s ranged
from 0.32 to 11.16 µg/litre for the 4 species (up to 4 days after
hatching) and 4 different PCB mixtures; LC1s ranged from 0.009 to
0.26 µg/litre. Results are presented in Table 33. The rainbow trout
was the most sensitive species tested.
Adult minnows (Phoxinus phoxinus) were fed Clophen A50 at 20, 200,
or 2000 mg/kg diet (on a dry weight basis), for 40 days (Bengtsson,
1980). Growth was monitored from day 46 to day 79; a significant
increase in growth (relative to controls) was seen in fish fed the
highest dose. Other doses caused increases in growth, but these were
not significant. Stimulated growth had been observed in previous
studies with minnows fed Clophen A50 at 0.88-78 mg/kg diet (Bengtsson,
1979). Between days 127 and 166, the swimming performance of the fish
was tested using a rotary flow technique. Although the PCBs impaired
swimming performance, this was not statistically significant.
Reproduction was monitored from day 235 (first day of spawning) to day
300. Spawning was delayed in the treated groups by 1 day, 1 week, and
3 weeks for the 3 treatments (20, 200, and 2000 mg/kg diet),
respectively. Only the highest dose affected hatchability of eggs,
which was reduced by approximately 80%.
Snarski & Puglisi (1976) did not find any adverse effects on survival,
growth, or reproduction of brook trout (Salvelinus fontinalis)
exposed to concentrations of Aroclor 1254 of up to 0.94 µg/litre, for
71 weeks. Survival and growth of alevin-juveniles from exposed parents
were unaffected for up to 90 days.
Continuous-flow bioassays were conducted over 8 months on fathead
minnow (Pimephales promelas), exposing the fish to either Aroclor
1242 or 1254 (Nebeker et al., 1974). Reproduction occurred at, and
below, 5.4 µg Aroclor 1242/litre, but spawning and egg production
were very variable. With Aroclor 1254, reproduction occurred at, and
below, 1.8 µg/litre. Spawning occurred at 1.8 µg/litre, but was
significantly less than spawning at the lower concentrations of 0.23
and 0.52 µg/litre. Egg hatchability and fry survival were good at
1.8 µg/litre. Eggs were more resistant than fry at 15 and 51 µg
Aroclor 1242/litre; with Aroclor 1254 at a concentration of
15 µg/litre, eggs hatched readily, but all fry were dead within 96 h.
Halter & Johnson (1974) kept coho salmon (Oncorhynchus kisutch) eggs
in solutions containing 4.4-56.4 µg Aroclor 1254/litre. Exposure
continued for 2 weeks before, and 4 weeks after hatching, until the
young were alevins. Hatchability was reduced by 30% at the highest
concentration of 56.4 µg/litre. Survival of alevins was markedly
higher when eggs were transferred to clean water prior to hatching,
but there was still 58% mortality in alevins hatched from eggs at the
highest dose. When the alevins were exposed to the PCBs for 4 weeks
after hatching, survival was inversely related to exposure
concentration. No group survived as well as the controls (for example,
18% died at 4.4 µg/litre and 90% at 26 µg/litre, or more).
In a study by Defoe et al. (1978), fathead minnow (Pimephales
promelas) were maintained in flow-through bioassays in solutions of
Aroclors 1248 or 1260, for 240 days (a full life-cycle test).
Reproduction occurred at all concentrations tested (up to 3 µg/litre
for Aroclor 1248 and up to 2.1 µg/litre for Aroclor 1260). The authors
concluded that PCBs did not produce major effects on reproduction at
concentrations up to the 30-day LC50 and that reduced populations
after long-term exposure are largely due to larval mortality.
Weis & Weis (1982) exposed eggs of the mummichog (Fundulus
heteroclitus) to concentrations of Aroclor 1254 of 0.01-10 mg/litre
(after cleavage had begun). No effect was found on embryonic
development or hatching and embryonic mortality was negligible.
Seven-day larval tests also showed no effect on mortality up to the
highest concentration. However, the authors found approximately 20%
larval mortality, when larvae were exposed to 5 mg/litre for 72 h,
after hatching from eggs at the highest exposure concentration. In a
second group of studies, eggs were exposed to 10 mg Aroclor
1242/litre; no malformations were found, though there was a consistent
retardation of hatching. There was a positive correlation between
hatching rate and female length (length being related to age). Larvae
exposed to 5 mg/litre showed an average of 45% mortality within 72 h.
Pre-exposure as eggs to 10 mg/litre greatly increased larval
mortality; pre-exposure to 1 mg/litre resulted in an intermediate
response. There was no mortality in control larvae, even when they had
been pre-exposed as eggs.
Eggs of brook trout (Salvelinus fontinalis) were exposed to Aroclor
1254 (0.043-13 µg/litre) for 10 days before hatching, and the fry for
118 days after hatching (Mauck et al., 1978). Median hatching time,
egg hatchability, and sac-fry survival were not affected by the PCBs.
Significantly decreased survival (32% survived) was seen after 48 days
at 3 µg/litre. There was significant mortality of fry after 118 days
with exposure to concentrations of 3.1 µg/litre and above. Growth of
the trout, as measured by weight, was significantly decreased after 48
days at concentrations of 1.5 µg/litre or more. By the end of the
study (118 days), no significant differences in weight were seen
between surviving fry on different treatments. Analysis of the
backbone composition at this time showed that hydroxyproline and
phosphorus were significantly decreased by concentrations of
0.43 µg/litre or more and that, at 0.69 µg or more/litre, calcium
levels were significantly increased. Although collagen was
significantly decreased at 0.69, 3.1, and 6.2 µg/litre, it was
unaffected at 1.5 µg/litre; no explanation for the anomaly was
suggested.
Schimmel et al. (1974) exposed sheepshead minnow (Cyprinodon
variegatus) eggs, immediately after fertilization, to Aroclor 1254
at concentrations of between 0.1 and 10 µg/litre. The fertility of
eggs was unaffected. Hatching was significantly reduced (by 30%) only
at the highest exposure. Survival of fry to 2 weeks was significantly
reduced at concentrations of 0.32 µg/litre or above (30% survival at
0.32 µg/litre and 9% survival at 10 µg/litre). The 3-week LC50 for
embryo-fry was calculated to be 0.93 µg/litre for Aroclor 1254. Many
of the dying fish exhibited fin rot. Exposure of juveniles and adults
to the same concentrations of the Aroclor produced 24% mortality in
juveniles at the highest exposure rate and no mortality in adults.
Some fin rot was seen in adults.
Table 33. Toxicity of PCBs for the embryolarval stages of fisha,b
Organism Aroclor 1016 Aroclor 1242 Aroclor 1254
LC1 LC50 LC1 LC50 LC1 LC50
(µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre)
Channel catfish 0.08 11.16 0.14 4.24 0.05 1.76
(Ictalurus punctatus) (9.93-12.97) (0.07-0.23) (3.32-5.34) (0.02-0.09) (1.36-2.24)
Goldfish 0.10 13.21 0.04 2.64 0.02 1.18
(Carassius auratus) (10.63-16.43) (0.01-0.08) (1.89-3.61) (0.01-0.04) (0.84-1.61)
Rainbow trout 0.011 1.08 0.01 1.03 0.009 0.32
(Salmo gairdneri) (0.003-0.027) (0.7-1.56) (0.002-0.025) (0.67-1.51) (0.003-0.02) (0.22-0.45)
Redear sunfish 0.26 7.82 0.19 3.56 0.02 0.53
(Lepomis microlophus) (0.1-0.51) (5.74-10.35) (0.08-0.35) (0.65-4.66) (0.01-0.04) (0.39-0.7)
a From: Birge et al. (1978).
b Exposure under static conditions, but with water renewed every 12 h. Exposure was initiated 2-6 h after spawning (except
for rainbow trout where exposure was initiated 15 min after fertilization) and continued to 4 days post-hatching. Hatching
times varied: 22 days for rainbow trout (13.5-14.3°C); 3 days for catfish (29-31°C); 3-4 days for goldfish and sunfish
(20-24°C); hardness 90-115 mg/litre; pH 7.6-8.1.
In another study, Hansen et al. (1975) exposed embryos, fry,
juveniles, and adults of sheepshead minnow (Cyprinodon variegatus)
to concentrations of Aroclor 1016 of 0.1-10 µg/litre, for 28 days, in
intermittent flow bioassays. No effects on survival were observed
during this period. When exposed to concentrations of 32 or
100 µg/litre, there was high mortality in eggs, juveniles, and adults;
all were killed at 100 µg/litre. The authors calculated that the
28-day LC50s for juveniles and adults were 20 and 19 µg/litre,
respectively.
Freeman et al. (1982) fed Atlantic cod (Gadus morrhua) diets
containing 1-50 mg Aroclor 1254/kg for 5.5 months. Altered steroid
biosynthetic patterns in vitro were observed in the testes and head
kidneys (adrenal equivalent) of dosed fish. Histological examination
revealed abnormalities in the testes, gills, and livers. Testicular
abnormalities included derangement of lobules, hyperplasia of lobule
walls, and disintegration and/or fatty necrosis of spermatogenic
elements. In fish fed at dietary rates of 5-50 mg/kg, hyperplasia of
the epithelial layer of the secondary lamellae of the gills was noted.
Fatty degeneration of the liver was observed in all treated fish.
Similar testicular abnormalities were observed by Sangalang et al.
(1981), but only in sexually mature individuals or at a stage of rapid
spermatogenic proliferation.
7.2.3.4 Physiological and biochemical effects
Coho salmon (Oncorhynchus kisutch) were fed diets containing a
mixture of PCBs (1:4, Aroclor 1242:1254) at a concentration of 50 or
500 mg/kg dry feed (Leatherland & Sonstegard, 1978). Serum
triiodothyronine (T3) levels were significantly reduced after 3
months, in fish fed the highest dose. Thyroxine (T4) levels were not
affected. After 3 months, the T3:T4 ratio was significantly higher in
fish fed the highest dose than in control and low-dose fish. Fish on
500 mg/kg had significantly lower body weights than controls by the
end of the study. A mixture of 50 mg PCBs/kg and 5 mg mirex/kg
significantly reduced serum triiodothyronine and thyroxine levels over
a period of 3 months, but did not affect the T3:T4 ratio. Leatherland
& Sonstegard (1979) fed rainbow trout (Salmo gairdneri) diets
containing Aroclor 1254 at 500 mg/kg dry feed, for up to 2 months. The
PCBs did not have any significant effects on thyroid histology or on
serum thyroid hormone levels. Liver weights, total liver lipid
content, and carcase lipid content were significantly greater in
treated fish. Mayer et al. (1977) fed fingerling coho salmon
(Oncorhynchus kisutch) 1.45-14 500 µg Aroclor 1254/kg body weight
per day, for 260 days. Channel catfish (Ictalurus punctatus) were
fed the same Aroclor at rates of 48 and 480 µg/kg body weight per day
for 193 days. Thyroid activity (as measured by 125I uptake) was
significantly stimulated at dose rates of 14.5 µg/kg per day, and
above, in coho salmon. Stimulation ranged from 52%, at 14.5 µg/kg per
day, to 119%, at 14 500 µg/kg per day, compared with controls. In
catfish, both dose rates of Aroclor 1254 caused significant increases
in thyroid activity, whereas other Aroclors (1232, 1248, and 1260) did
not have any significant effects on the thyroid at the same dose
rates. Folmar et al. (1982) injected yearling coho salmon
(Oncorhynchus kisutch) intraperitoneally with a total of 150 µg
Aroclor 1254/kg body weight (2 injections, 10 days apart), prior to
smoltification. Over a 6-week period, the authors did not find any
significant effects on gill Na-K ATPase activity. However, the PCBs
did alter the normal developmental patterns of thyroxine; there was a
delay in the normal increase in circulating thyroxine levels.
Triiodothyronine levels were significantly elevated after
approximately 3 weeks, but then fell to well below control levels
after 6 weeks. Fish were transferred to seawater; there was no
significant effect on the gill Na-K ATPase activity in the treated
group, but there was a significant increase in mortality (6%). Ten per
cent of fish, dosed with PCBs and placed in sea water containing No 2
fuel oil at 700 µg/litre, died; this was an additive effect.
Fingerman (1980) kept Gulf killifish (Fundulus grandis) in a
seawater solution containing Aroclor 1242, 1254, or 1268 at
8 mg/litre, for up to 28 days. The author removed the lower half of
the caudal fin to study fin regeneration. No significant effects were
observed with either Aroclor 1242 or 1254. With Aroclor 1268, a
significant decrease in regeneration rate was observed after 28 days,
when the study was conducted in the spring, and after 7 days, in the
autumn. No differences were found at other sampling times.
Rainbow trout (Salmo gairdneri) were administered capsules
containing 0.173 g Aroclor 1254 every second day over a 6-day period
(Kiessling et al., 1983). Two to 4 weeks after the last capsule,
isolated gills were perfused. There was no significant difference in
adrenergic response in the gill vascular bed and no significant
difference in the "oxygen transfer factor" (% changes in oxygen in
saline from dorsal aorta before, and after, addition of adrenaline).
Similarly, there was no effect on muscle glycogen content.
Johansson et al. (1972) dosed brown trout (Salmo trutta) twice, 4
days apart, with 5 mg Clophen A50/kg body weight, either by capsule or
by intramuscular injection. The fish were fed for 43 days, starved for
116 days, and fed again for another 87 days. Metabolic analysis of the
fish was undertaken on day 43 and at the end of the study. A
significant increase in body weight was noted at the end of the study,
but not after 43 days. Blood glucose, muscle glycogen, and the
liver-somatic index (liver weight as a ratio of body weight), which
had all increased significantly after 43 days, decreased significantly
by the end of the study (compared with controls). Both haematocrit and
haemoglobin levels had significantly decreased after 43 days but, by
the end of the study, were not significantly different from those of
the controls.
In a study by Camp et al. (1974), fingerling catfish (Ictalurus
punctatus) were kept in water containing Aroclor 1254 at 8 mg/litre.
There was a significant increase in the serum transaminase activity of
the fish after 4 h. The cortisol content of the serum was depressed,
but not significantly so. The sodium:potassium ratio was constant.
Merkins & Kinter (1971) exposed the killifish (Fundulus heteroclitus)
to concentrations of Aroclor 1221 of 7.5, 25, or 75 mg/litre. No fish
died, at the lowest concentration, over a period of 4 days; 50% died
at 25 mg/litre within 24 h and 88% at 75 mg/litre, within the same
period. Serum osmolality, and serum ion levels (Na and K) were then
measured. Within 6 h at 75 mg/litre, blood osmolality significantly
increased, but sodium and potassium ions in the blood were not
affected. After 24 h at 75 mg/litre, there was also a significant
increase in sodium ions, but no effects on potassium ions. None of
these parameters were affected by exposure at 25 mg/litre for 24 h.
7.2.3.5 Behavioural effects
Fingerman & Russell (1980) exposed male Gulf killifish (Fundulus
grandis) to Aroclor 1242 at 4 mg/litre for 24 h. A significant
reduction in whole-brain levels of both noradrenalin and dopamine were
reported over this period. The swimming activity of the fish was
monitored by counting the number of times they crossed lines marked on
the bottom of the tank within a 10-min period, after exposure to the
Aroclor for 24 h. Activity was significantly increased and remained so
for a further 2 days.
The avoidance response was studied by Hansen et al. (1974a) in the
sheepshead minnow (Cyprinodon variegatus), the pinfish (Lagodon
rhomboides), and the mosquito fish (Gambusia affinis), when given
a choice between clean water and water containing Aroclor 1254 at
0.001, 0.01, 0.1, 1.0, or 10 µg/litre. Sheepshead minnow did not avoid
any concentration; pinfish avoided only the highest concentration of
the Aroclor. Mosquitofish significantly avoided concentrations of 0.1,
1.0, and 10 µg/litre.
Peterson (1973) did not find any effect on temperature selection in
Atlantic salmon (Salmo salar) exposed to 2 mg Aroclor 1254/litre,
for 24 h prior to a horizontal temperature gradient test. Similarly,
Miller & Ogilvie (1975) did not find any effect of Aroclor 1254 on
temperature selection when brook trout (Salvelinus fontinalis) were
exposed to a water concentration of 25-100 mg/litre, for 24 h, prior
to temperature gradient tests. Even at a concentration of 100 mg/litre
for 48 h, which was sufficient to cause some mortality, temperature
selection was still unaffected.
7.2.3.6 Interactions with other chemicals
Halter & Johnson (1974) found that the median survival time for coho
salmon (Oncorhynchus kisutch) fry, exposed to Aroclor 1254 at
32.2 µg/litre, was greater than 336 h. When fry were exposed to
mixtures of Aroclor and DDT, for 2 weeks, the survival times were
always similar to the more rapid reaction time found for DDT alone.
The authors suggested that this indicated the lack of an additive
effect.
7.2.4 Amphibians
Tadpoles (Rana chensinenis) were maintained in water containing PCBs
(as Kanechlor 300) at 0.5, 5.0, 50, or 500 µg/litre. At the 2 highest
doses, all individuals died rapidly. The time of onset of lethality
was related to dose level; at 5 µg/litre, death occurred between 15
and 21 days, and, at 0.5 µg/litre, on the thirty-second day after
first exposure. Growth at 0.5 and 5.0 µg/litre did not differ from
that of controls. Tail abnormalities were found, but there was no
correlation with PCB concentrations and a NOEL could not be
established. The mechanism by which PCBs caused tail malformation was
not known (Hasegawa, 1973).
Birge et al. (1978) conducted embryo-larval bioassays on 3 species of
amphibia. Exposure to various PCBs was maintained from 2-6 h after
spawning to 4 days after hatching, using static renewal procedures
(Table 34). Toxicity increased with increasing chlorination, the
leopard frog being the most sensitive species with an LC50 of
1.03 µg/litre after exposure to Aroclor 1254. The authors also
calculated an LC1 value from the same study; the leopard frog and
American toad were equally sensitive at 0.02 µg/litre. The eggs were
much less sensitive to the PCBs than the hatched larvae, with LC50s
ranging from 3.5 to 250 µg/litre, for 3 different Aroclors and
Capacitor 21, and 3 species of tadpole.
Table 34. Toxicity of PCBs for the tadpoles of amphibiansa,b
Organism Aroclor 1016 Aroclor 1242 Aroclor 1254 Capacitor 21
LC1 LC50 LC1 LC50 LC1 LC50 LC1 LC50
(µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre)
American toad 0.35 7.16 0.03 2.71 0.02 2.02 0.21 9.97
(Bufo americanus) (0.15-0.64) (5.39-9.34) (0.01-0.06) (1.91-3.75) (0.01-0.05) (1.44-2.77) (0.08-0.42) (7.21-13.53)
Fowler's toad 0.18 27.72 0.22 12.09 0.07 3.74 0.55 28.02
(Bufo fowleri) (0.09-0.33) (21.77-35.08) (0.12-0.36) (9.74-14.91) (0.04-0.11) (2.98-4.64) (0.3-0.91) (22.59-34.47)
Leopard frog 0.1 6.19 0.04 2.13 0.02 1.03 0.03 2.87
(Rana pipiens) (0.05-0.16) (4.95-7.69) (0.02-0.06) (1.72-2.63) (0.01-0.03) (0.83-1.27) (0.02-0.06) (2.29-3.57)
a From: Birge et al. (1978).
b Exposure under static conditions, but with water renewed every 12 h. Exposure was initiated 2-6 h after spawning and continued to
4 days post-hatching. Hatching times varied between 3 and 4 days, therefore, exposure varied between 7 and 8 days.
Temperature 20-24°C; hardness 90-115 mg/litre; pH 7.6-8.1.
7.2.5 Aquatic mammals
Following-up on field reports of the reproductive effects of PCBs on
seal reproduction (see section 7.4.4), Reijnders (1986) conducted a
study on captive common seals (Phoca vitulina) fed fish contaminated
with PCBs. The contaminated diet produced an average daily intake of
PCBs of 1.5 mg compared with the control level of 0.22 mg/day. Twelve
female seals were used as controls and 12 as the treated group. Blood
samples were taken regularly and assayed for circulating steroid
hormones progesterone and estradiol. Females were mated with undosed
males. Of the 12 females in the control group, 10 became pregnant; all
12 ovulated. Only 4 females became pregnant out of the 12 fed the
PCB-contaminated diet; again all 12 ovulated. Throughout the breeding
cycle, no significant differences were found between the hormonal
profiles of pregnant animals in the treated and control groups. No
significant differences were observed in progesterone levels in the
treated and control groups, despite the fact that many fewer treated
females became pregnant. However, a rise in estradiol levels in
non-pregnant females in the control group was not found in
non-pregnant females in the treated group, suggesting a difference in
non-pregnancy in treated females. The effects of PCBs occurred only
late in the breeding cycle at the time of implantation of the embryo.
Seals, like mink, show delayed implantation of the embryo as a normal
component of the annual reproductive cycle. No conclusions about the
mechanism of action could be drawn.
Brouwer et al. (1989) fed common seals (Phoca vitulina) on a diet of
polychlorinated biphenyl-contaminated fish (average daily intake
1.5 mg PCBs) for almost 2 years. Significant reductions in levels of
plasma total and free thyroxine, triiodothyronin, and retinol were
found compared with those in seals maintained on a "low" contaminated
diet (average daily intake 0.22 mg PCBs). It should be noted that the
diet consisted of fish contaminated in the environment and not dosed.
No attempt was made to analyse levels of retinol in the different fish
diets. The "high" contamination group were caught in the Wadden sea
and the "low" contamination group in the north-east Atlantic. When the
fish were analysed for other likely contaminants, it was found that
pp'-DDE also showed higher levels in the "high" contamination group
than the "low" contamination group; average daily intakes of pp'-DDE
were estimated to be 0.4 mg and 0.13 mg, respectively.
7.3 Toxicity for terrestrial organisms
7.3.1 Plants
Aroclor 1254 was applied to soil at rates of 10, 100, or 1000 mg/kg
(Weber & Mrozek, 1979). Both soybean (Glycine max) and rescue
(Fescue arundinacea) were grown in the soil, from seed, for up to 26
and 42 days, respectively. The height of the soybean plants and the
fresh top weights of both plants were measured. PCBs applied to the
soil significantly reduced height and fresh top weight of soybean
plants, only at the highest rate of application. Low rates were
inhibitory, but not significantly so. Aroclor applied to the soil also
reduced the fresh top weight of rescue at the highest rate of
application (1000 mg/kg); lower application rates of the Aroclor did
not have any effects. The addition of activated carbon to the soil
(3.7 tonnes/ha; approximately 3333 mg/kg) annulled the inhibitory
effect of PCBs. The Aroclor also inhibited the uptake of water by the
soybean in proportion to the dose applied to the soil; water uptake
was monitored between 21 and 25 days after sowing of the seed. The
reduction in water uptake over this 5-day period was 12%, with the
application of 1 mg Aroclor 1254/kg soil, rising to 52% at 1000 mg/kg
soil. Again, the effect on water uptake was eliminated by the addition
of carbon to the soil (1% rising to 4% inhibition over the dose
range).
Continuation of the experiment through a second and third crop of
soybeans on the same soil, without further addition of Aroclor 1254
(Strek et al., 1981), showed similar effects on the height, top fresh
weight, and water uptake of the plants, reflecting the persistence of
the Aroclor. However, there were no significant effects at doses lower
than 1000 mg/kg, with the exception of reduced height in the third
crop, seen at all dose levels. All effects were eliminated by the
addition of activated carbon to the soil. The same authors found that
beet (Beta vulgaris) was significantly affected by 1000 mg Aroclor
1254/kg, using the same parameters of water uptake, height, and fresh
top weight between 14 and 56 days after sowing. Doses of 100 mg/kg or
less did not have any significant effects. Effects at the highest dose
were again eliminated by the addition of activated carbon to the soil.
Growth parameters, taken at harvest, showed no apparent inhibition of
corn (Zea mays) or sorghum (Sorghum bicolor) by Aroclor 1254 over
the same dose range. There was, however, a reduction of plant height
over the first 5 days of growth at 100 and 1000 mg/kg in corn, but the
plants recovered.
Mrozek et al. (1983) grew Spartina alterniflora plants in mud or
sandy soils in the presence of 2.2 µg PCBs/kg (54% chlorine similar to
Aroclor 1254), admixed with the soil, over a 6-week period. Plants
grown in sand showed significantly reduced values for cumulative
change in height (approx. 30%) and the number of live leaves per stem
(approx. 25%), and increased values for the number of stems per plant
(approx. 300%), whereas plants grown in mud showed a significantly
reduced value for cumulative change in the number of stems per plant
(approx. 75%). Mud-grown plants also exhibited an altered biomass
distribution, as indicated by the aerial:below ground biomass ratio,
which increased from 1.2 to 1.5, on a dry weight basis.
7.3.2 Terrestrial invertebrates
Hatch & Allen (1979) observed the behaviour of the snail (Cepeae
(=Helix) nemoralis), with regard to the rasping of conspecifics'
shells to obtain calcium. On a low calcium diet (0.53 mg calcium/kg),
snails showed an increased tendency to rasp the shells of other
snails, in order to obtain calcium. The best indicator of this
behaviour was found to be the counting of holes bored completely
through the shell. This behaviour was not seen with a high calcium
diet (250 mg calcium/kg). The addition of PCBs, as a mixture of
Aroclors 1016 and 1254, to the high calcium diet at a rate of 0.5,
1.0, or 5.0 mg/kg increased the number of snail shells penetrated by
other snails. Penetration increased in a dose-dependent manner with 2,
5, and 7% penetration for the 3 dose rates, respectively. Damage to
shells, without actual penetration, also increased with PCB treatment
from the low level found on the control diet to between 16 and 21% on
the PCB diet. No clear dose-dependent effect was seen using this
method of assessing damage. Fourth instar nymphs of the grasshopper
(Chorthippus brunneus), were dosed topically with the PCB mixture
Aroclor 1254 (Moriarty, 1969). A single dose of either 12.5, 50, or
200 µg/insect was applied in a volume of 1 µl of 1,4-dioxan. No
sublethal effects were detected on either development or reproductive
potential. At the highest dose, there appeared to be a latent toxicity
that could be correlated with the mobilization of lipids at moult;
more moulted males died than unmoulted males over the test period.
Females took longer to moult than males and showed a distinctly
bimodal distribution in time to death, with a similar correlation
between toxicity and moult. Males showed 46% mortality and females,
41%, after treatment with 200 µg Aroclor 1254. Fungal infection
affected insects on the lower doses and mortality figures are,
therefore, unreliable.
Lichtenstein et al. (1969) exposed Drosophila melanogaster to the
dry residue of various PCBs. They exposed flies to Aroclors 1221,
1232, 1242, and 1248 at 200 or 800 µg. No mortality was observed after
48 h at 200 µg. At 800 µg, there was an increase in mortality with
decreasing chlorination (after a 48-h exposure to Aroclor 1221, 92%
had died; only 45% died after exposure to Aroclor 1248 over the same
length of time). No mortality was observed after a 48-h exposure to
2000 µg of Aroclors 1254, 1260, 1262, or 1268. In a separate study,
the authors treated houseflies (Musca domestica) topically with
either 10 or 20 µg (in 2 µl of acetone); mortality was assessed after
24 h. Results were comparable with those from the study on
Drosophila; deaths were dose related and occurred with Aroclors up
to 1254, where mortality was 10% at the higher dose. Aroclors of
higher chlorination than 1254 had no effect. Lower chlorinated
Aroclors had the greatest effect with more deaths at the highest dose
(20 µg) and lowest chlorination (Aroclor 1221) than with any other
treatment (43% killed). Plapp (1972) found that the 24-h LC50 for
Aroclor 1254 in the housefly (Musca domestica) was > 3000 µg/jar
where the Aroclor was added to a container in acetone, which was dried
before the addition of the flies. This was true for both
DDT-susceptible and DDT-tolerant strains. The same author reported a
powerful synergistic effect between carbaryl and Aroclor 1254; the
LC50 with carbaryl alone was calculated to be 1386 µg/jar and that
for carbaryl:PCB in the ratio of 1:5, 96 µg/jar. The Aroclor was as
powerful a synergizing agent as piperonyl butoxide.
Youssef et al. (1974) hatched eggs of the housefly (Musca domestica)
on a medium of paper tissue dosed with 0.808 g of Aroclor 1254 per
200 g of tissue. The adult flies hatching from the eggs were examined
using the electron microscope for effects on the male reproductive
tissue. The PCBs induced nuclear and flagellar abnormalities in
developing spermatids. Spermatid nuclei failed to elongate and
membranes originating from the nuclear envelope formed invaginations
into the nucleus. These resulted in the appearance of cytoplasmic
inclusions in the nucleus. Spermatid flagellae contained an abnormal
number of axonemes and mitochondrial derivatives; abnormal spermatids
did not coil and degenerated.
In a study by Wasilewska et al. (1975), female nematodes
(Acrobeloides nanus) were exposed to Aroclor 1254. Initially, 60 µg
of the Aroclor were added to a petri dish (on the surface of agar) in
which there were 20 nematode worms. The nematodes fed on a culture of
bacteria introduced to the agar at the same time as the worms. After 5
days of exposure, eggs and adult nematodes were counted and adult
weights determined. No significant effects were found. In a second
study, over a longer period (10 days), nematodes were exposed to 15,
30, or 60 µg of the Aroclor. Adverse effects increased with dose, and
even at the lowest dose, the number of adults was reduced from 123 to
76, the number of eggs from 539 to 288, and the weight of adults from
18.9 to 9.4 µg. At the highest dose of 60 µg per dish, the number of
adults was 32, the number of eggs, 37, and the weight of adults,
4.9 µg.
7.3.3 Birds
Five-day dietary LC50s for PCBs in birds ranged from 604 to >
6000 mg/kg diet (Table 35). Generally, the oral single dose LD50 and
the dietary LC50 data are similar to those for mammals. PCBs are less
toxic for birds than other organochlorines, such as DDT and its
metabolites and the chlorinated cyclodienes.
The toxicity of Aroclors in birds increases with the percentage
chlorination (generally reflected in the final 2 digits of the Aroclor
number), according to the data of Hill et al. (1975) and Hill &
Camardese (1986). Hill et al. (1974) noted that the toxicity of
Aroclors is not simply a reflection of the chlorine content of the
different Aroclors. They adjusted the dietary content of the Aroclors
to a constant dietary chlorine level and found the same increased
toxicity with higher Aroclor numbers.
Dahlgren et al. (1972) reported some mortality in sub-adult pheasants
after regular oral doses of Aroclor 1254 ranging from 10 to 210 mg.
Mortality was related to both dose and body weight; heavier birds
lived longer, though they lost a greater proportion of body weight. A
sudden heavy intake of PCBs led to high brain residues. Brain residues
were best correlated with death; residues in the brain of about
300-400 mg/kg were considered by the authors to be diagnostic of acute
poisoning and death (Dahlgren et al., 1972). Stickel et al. (1984)
concluded that similar levels in the brain killed red-winged
blackbirds, starlings, brown-headed cowbirds, and grackles. Intake of
lower doses of PCBs over long periods does not lead to such high brain
residues; the cause of death after long-term exposure appears to be
oedema and related symptoms.
7.3.3.1 Short-term toxicity
Hurst et al. (1973) observed differential toxicity of PCBs between
bobwhite quail hens and cocks. This differential was eliminated when
the tests were conducted on birds not in the breeding condition and
with short daylengths. Females survived better than males, only when
they were laying eggs, and survival was well correlated with the
numbers of eggs produced. The authors concluded that females reduce
their exposure to PCBs by eliminating the compound in the eggs.
When Koeman et al. (1969) fed Japanese quail a diet containing 2000 mg
Phenochlor DP6/kg, all the dosed birds died between 6 and 55 days of
dosing. The quail developed hydropericardia at this dose level. Vos &
Koeman (1970) fed one-day-old cockerels a diet containing PCBs at
400 mg/kg, for 60 days; the PCBs were in one of the following forms,
Phenochlor DP6, Clophen A60, or Aroclor 1260 (all 3 are 60%
chlorinated). The mean survival time was calculated to be 24.3 days
for Phenochlor and 20.5 days for Clophen, only 3 out of 20 birds died
on the diet containing the Aroclor. Microscopically, centrolobular
liver necrosis was found in chicks fed the first 2 compounds. Atrophy
of the spleen and porphyria were observed in all dosed groups.
Table 35. Toxicity of PCBs for birds
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Bobwhite quail 10 days diet Aroclor 1221 5-d LC50 >6000 Hill et al. (1975)
(Colinus virginianus) 10 days diet Aroclor 1232 5-d LC50 3002 (2577-3501) Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 2098 (1706-2610) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 1175 (966-1440) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 604 (410-840) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 747 (577-937) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 871 (702-1069) Hill et al. (1975)
male 1 year oral Aroclor 1268 acute LD50 >2000 Hudson et al. (1984)
Japanese quail 14 days diet Aroclor 1221 5-d LC50 >5000 Hill & Camardese (1986)
(Coturnix coturnix 14 days diet Aroclor 1232 5-d LC50 >5000 Hill & Camardese (1986)
japonica) 14 days diet Aroclor 1242 5-d LC50 >6000 Hill & Camardese (1986)
14 days diet Aroclor 1248 5-d LC50 4819 (4267-5443) Hill & Camardese (1986)
14 days diet Aroclor 1254 5-d LC50 2929 (2516-3409) Hill & Camardese (1986)
14 days diet Aroclor 1260 5-d LC50 2195 (1861-2589) Hill & Camardese (1986)
14 days diet Aroclor 1262 5-d LC50 2304 (1978-2684) Hill & Camardese (1986)
Table 35. (cont'd).
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Mallard 10 days diet Aroclor 1221 5-d LC50 >5000 Hill et al. (1975)
(Anas platyrhynchos) 10 days diet Aroclor 1232 5-d LC50 >6000 Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 3182 (2613-3879) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 2798 (2264-3422) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 2699 (2159-3309) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 1975 (1363-2749) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 3008 (2461-3634) Hill et al. (1975)
male 8-9 months oral Aroclor 1242 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1254 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1260 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1268 acute LD50 >2000 Hudson et al. (1984)
Red-winged blackbird diet Aroclor 1254 6-d LC50 1500 Stickel et al. (1984)
(Agelaius phoeniceus)
Ring-necked pheasant 10 days diet Aroclor 1221 5-d LC50 >5000 Hill et al. (1975)
(Phasianus colchicus) 10 days diet Aroclor 1232 5-d LC50 3146 (2626-3948) Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 2078 (1843-3879) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 1312 (1166-1477) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 1091 (968-1228) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 1260 (1106-1433) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 1234 (1086-1402) Hill et al. (1975)
Table 35. (cont'd).
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Starling diet Aroclor 1254 4-d LC50 1500 Stickel et al. (1984)
(Sturnus vulgaris)
Brown-headed cowbird diet Aroclor 1254 7-d LC50 1500 Stickel et al. (1984)
(Molothrus ater)
Grackle diet Aroclor 1254 8-d LC50 1500 Stickel et al. (1984)
(Quiscalus quiscula)
a oral = acute oral test (result expressed as mg/kg body weight); diet = dietary test (result expressed as mg/kg diet).
Miranda et al. (1987) studied the effects of acute oral exposure of
Japanese quail to Aroclor 1242 (100, 250, or 500 mg/kg), or
2,4,2',4'-tetrachlorobiphenyl and 3,4,3',4'-tetrachlorobiphenyl (both
87.6 mg/kg) in corn oil. Control birds received only the corn oil. The
birds were killed after 48 h. All the PCB compounds caused a
significant increase in porphyrin content and delta- aminolevulinic
acid synthetase (ALA-S) activity in the small intestine and liver. All
the compounds increased the cytochrome P-450 content of the liver. In
the intestine, the P-450 content was only increased by Aroclor 1242
and 2,4,2',4'-tetrachlorobiphenyl. The activity of 7-ethoxyresorufin
O-deethylase was increased by all compounds in both the intestines
and liver. In the liver, 7-ethoxycoumarin O-deethylase (ECOD)
activity was unchanged or decreased, but, in the intestines, ECOD
activity increased with dose. No tissue differences in ECOD activity
were found after treatment with 2,4,2',4'-tetrachlorobiphenyl and
3,4,3',4'-tetrachlorobiphenyl. It was concluded that the small
intestine was more responsive than the liver to the porphyrinogenic
effect of a single oral dose of PCBs, and, that the induction of drug
metabolizing enzymes in the quail was tissue-specific, depending on
the PCB preparation used.
Day-old chicks were fed on a diet containing 500 mg PCBs/kg. All the
birds died between the third and the tenth week of dosing; a reduction
in the dosage to 250 mg/kg delayed the onset of death until the
thirteenth week (Platonow & Funnell, 1971). Mortality did not occur in
chickens dosed at 200 mg/kg over a period of 3 weeks (Flick et al.,
1965). Harris & Rose (1972) fed one-day-old broiler chicks diets
containing 100, 200, or 400 mg PCBs/kg (Aroclors 1242, 1254, and
1260). No mortality occurred at doses of up to 100 mg/kg, over a
period of 4 weeks. Over the same period, all the birds on Aroclor
1242, 60% of the birds on Aroclor 1254, and none of the birds on
Aroclor 1260, died, all at a dosage of 400 mg/kg. Holleman et al.
(1976) fed day-old broiler chicks and turkey poults on diets
containing Aroclor 1242 at 38, 75, or 150 mg/kg for 4 weeks. The
authors found increased mortality at 75 mg/kg (21% mortality) with the
chicks, but this was not significantly different from controls;
however, there was a significant increase in deaths at 150 mg/kg (75%
mortality). Significantly increased mortality was not found in the
turkeys at any dose level, but the mortality rate in the controls was
33%. Both the 75 and 150 mg/kg diets produced oedema and other lesions
attributed to PCB toxicity. Prestt et al. (1970) maintained Bengalese
finches on a diet containing various concentrations of Aroclor 1254.
The estimated dose rate for 50% mortality, over 56 days, was 254 mg/kg
per day.
7.3.3.2 Egg production
Most studies demonstrating the lowering of egg production by PCBs were
conducted on chickens. The most severe effects came from dosing with
Aroclors in the middle of the range of chlorination (Aroclors
1232-1254). The literature has been reviewed by Stendall (1976).
Platonow & Reinhart (1973) fed chickens with Aroclor 1254 at either 5
or 50 mg/kg diet over 39 weeks. Egg production was erratically reduced
with the lower doses and sharply reduced on 50 mg/kg diet. A dietary
dose of 2 mg/kg did not have any reproductive effects on chickens over
9 weeks (Lillie et al., 1974) or after 39 weeks (Platonow & Reinhart,
1973). Scott et al. (1975) showed a 10% reduction in egg production in
chickens related to egg residues of PCBs (Aroclor 1248) of 3 mg/kg.
When egg residues reached 4.5 mg/kg, the production rate was further
reduced. A significant reduction in egg production was demonstrated by
Call & Harrell (1974) after dosing Japanese quail with 3 different
Aroclors at 62.5-5000 mg/kg, over 33-264 days.
7.3.3.3 Hatchability and embryotoxicity
Aroclors reduced the hatchability of chicken eggs. In 2 studies,
Lillie et al. (1974) examined the effects on hatchability of Aroclors
1221, 1232, 1242, 1248, and 1268, all fed at 2 or 20 mg/kg diet, over
9 weeks. Aroclors 1221 and 1268, with low and high chlorination,
respectively, showed no effects at 20 mg/kg diet. Aroclor 1248
produced some adult mortality at 20 mg/kg diet and nearly eliminated
hatching of the eggs produced. Aroclor 1242 showed similar, but
slightly less severe, effects; there was even less effect with Aroclor
1232. Cecil et al. (1974) tested a similar range of PCBs at the same
dosages (2 and 20 mg/kg diet). They also reported no effects for
Aroclors 1221 and 1268. Aroclors 1254, 1232, 1242, and 1248 reduced
hatchability, as in the previous study. PCBs that reduced hatchability
also produced abnormalities in the chicks. The fertility of eggs was
not affected by any of the treatments. Females were artificially
inseminated with semen collected from males fed a similar diet of
PCBs. Scott (1977) dosed chickens with 0.5, 1, 10, or 20 mg PCBs/kg
diet and found no effect at the 2 lowest doses. Hatchability was
reduced at 10-20 mg Aroclor 1248/kg diet. Kosutzky et al. (1979) dosed
chickens with 2 other PCBs (Delor 103 and 105) (42 and 54%
chlorination, respectively), at 5 mg/kg diet, for 6 weeks; there was
little effect on hatchability, which returned to control levels soon
after a return to a clean diet. Solomon et al. (1973) studied the
effects of PCBs (Aroclor 1254) on pheasants. The birds were dosed at
weekly intervals, for 17 weeks, with gelatin capsules containing
50 mg/bird for the hens or 25 mg/bird for the cock birds. No effects
were observed on fertility and there was no increase in the numbers of
abnormal embryos. In another study, Ax & Hansen (1975) maintained
white leghorn pullets on a diet containing Aroclor 1242 or 1254 at
20 mg/kg, or 2,4,5,3',4'-pentachlorobiphenyl, for a period of 10
weeks. Average embryonic mortality was found to be significantly
increased, i.e., 54.7, 59.2, and 74% for the 3 compounds,
respectively. In the same study, the authors found that average
embryonic mortality in eggs laid by birds dosed with
2,5,2'-trichloro-, 2,5,2',5'-tetrachloro-, or 2,4,5,2',4',5'-
hexachlorobiphenyl was not significantly different from that in the
controls. When both broiler breeder hens and leghorn hens were fed
diets containing either 20 or 50 mg Aroclor 1242/kg, for 1 week, the
hatchability of the eggs laid was reduced by 67.3 and 26.8%,
respectively, of control levels, on the 50 mg/kg diet (Briggs &
Harris, 1973). Hatchability also was reduced at 20 mg/kg diet, but the
time required to achieve the same depression as that found with the
higher dose was doubled. Even after dosing had finished,
embryotoxicity continued and, in fact, increased until, after 6 weeks,
hatchability was between 0 and 10% of controls for both birds at both
doses.
Chickens given Aroclor 1254 at 50 mg/litre in the drinking-water, for
6 weeks, showed progressive reduction in egg hatchability. This fell
to zero after 3 weeks (Bush et al., 1974). Hatchability remained at
almost zero for the first 8 weeks of the chickens receiving control
water following dosing, but returned to normal after a further 8
weeks.
Platonow & Reinhart (1973) fed chickens on a diet containing 50 mg
Aroclor 1254/kg for 39 weeks. There was some adult mortality; egg
production and hatchability fell almost to zero. Residues of Aroclor
in the last eggs produced ranged between 25 and 50 mg/kg. After 6
weeks of uncontaminated food, egg residues dropped and hatchability
improved. The authors reported that egg residues of less than 5 mg/kg
had no effect on hatchability, whereas residues greater than
10-15 mg/kg led to embryotoxic effects. Scott et al. (1975) related
hatchability to egg residues. At residue levels of 3 mg/kg,
hatchability was reduced by 44%, and, at residue levels of 4.5 mg/kg,
it was reduced to almost zero.
The yolk-sacs of eggs from pheasant, mallard, goldeneye duck, and
black-headed gull were injected with 3,4,3',4'-tetrachlorobiphenyl at
0.1 mg/kg (pheasant and mallard) or 1.0 mg/kg (pheasant, goldeneye
duck and black-headed gull) after 4 or 5 days of incubation (Brunström
& Reutergardh, 1986). A significant decrease in the hatching rate was
seen only in pheasants, at the highest dose. At a dose of 1 mg/kg, all
the embryos died before hatching, but, at 0.1 mg/kg, no effect on
hatching was observed. No gross abnormalities were noted in either
hatched chicks or dead embryos. A great difference was noted by the
authors between the avian embryos in this study and chicken embryos,
with regard to sensitivity towards tetrachlorobiphenyl. In chicken
embryos, a dose of 0.004 mg/kg, administered on day 4 of incubation,
gave a significant reduction in hatching. At 0.02 mg/kg, no embryos
survived to hatching (Brunström & Darnerud, 1983). Carlson & Duby
(1973) injected Aroclors directly into chicken eggs on the first day
of incubation, or 9 days later. Aroclor 1242 severely limited
hatchability at levels of more than 2.5 mg/kg. Aroclors 1254 and 1260
had no effect at 10 mg/kg. Delaying the injection until day 9 of
incubation reduced the effect of Aroclor 1242. With 5 mg/kg injected
at day zero, hatchability was 8.3%; when the same dose was given at
day 9, 82% of eggs hatched. These results are not compatible with
those of Scott et al. (1971) who reported that most embryonic deaths
occurred late in incubation. However, these authors were not dosing
the eggs directly, but measuring the residues in eggs from dosed
females. PCBs administered directly into the eggs may not produce
effects comparable with those produced by the same material received
from the mother hen, because of different distribution in the egg.
Platonow & Reinhart (1973) dosed hens at 50 mg/kg diet. Early in the
study, the majority of embryo deaths occurred late in incubation. As
the study progressed, the time of embryo death moved to earlier in the
incubation period. Bush et al. (1974) showed that there was greater
mortality for any given egg residue as the period of dosing the mother
hen progressed. Their experimental chickens were dosed for 6 weeks at
50 mg/litre drinking-water and then kept for a further 20 weeks on
clean water. On day 11 of the study, an egg yolk residue of 50 mg/kg
was associated with 50% mortality in the embryos. On day 131, 50%
mortality was associated with an egg residue of only 10 mg/kg. The
greatest toxicity of PCBs for chicken embryos occurred after 11 weeks
of clean water, that is 17 weeks into the study. At this stage, the
eggs would be receiving doses of PCBs from material stored within the
hen. Late in the study, residues of between 6 and 8 mg/kg in egg yolks
(equivalent to 3.6 mg/kg whole egg) were correlated with between 14
and 36% mortality. Platonow & Reinhart (1973) reported that egg
residues greater than 10-15 mg/kg caused embryotoxic effects whereas
low residues of less than 5 mg/kg did not produce any effects.
Abnormalities were reported by Cecil et al. (1974) in 34% of 843
embryos that died during their study. The most common abnormality was
oedema, which was seen in 50% of all chicks showing any abnormality.
Tumasonis et al. (1973) also reported deformities in chicks.
7.3.3.4 Eggshell thinning
Since the 1950s, thin eggshells have been characteristic of many wild
bird populations, though the effect was not noticed until some time
afterwards. Thin shells has been a contributory factor to reduced
reproductive capacity, particularly in birds of prey. The main
chemical causing thin shells appears to be DDE, a metabolite of DDT.
It has been shown to cause thin eggshells in laboratory experiments,
as well as through the correlation of field data. Literature on thin
eggshells has been reviewed by Cooke (1973). Most experimental studies
using PCBs have shown no effect on shell thickness. Peakall (1971), in
the first controlled study, dosed ring doves at 10 mg PCBs (Aroclor
1254)/kg diet or at 25 mg (equivalent to 160 mg/kg body weight)
injected ip. Shells were ashed and weighed. Two separate studies on
dietary dosing showed no effect on shell weight. In the first study, 2
groups of birds were compared, in the second the same birds were used,
comparing their eggs before and after dosing. Injection of PCBs, 1-4
days prior to egg laying, also had no effect. Studies on mallard dosed
with Aroclor 1254 at 25 mg/kg diet and bobwhite quail dosed at
50 mg/kg diet over 2 years and also on mallard dosed at up to
500 mg/kg diet for 5 weeks (Heath et al., 1972) showed no effects on
shell thickness. There was an apparent shell thickening of about 6% at
the highest dose, which could not be statistically confirmed. The same
authors outlined results from a study on white leghorn chickens.
Aroclor 1242 at 10 or 100 mg/kg diet or Aroclor 1254 at 100 mg/kg diet
did significantly reduce shell thickness. There were no measurable
effects of: Aroclor 1242 at 1 mg/kg diet, Aroclor 1254 at 10 mg/kg, or
Aroclor 1260 at 100 mg/kg diet (Heath et al., 1972). Experimental
details and detailed results were not given for the work on chickens.
Lillie et al. (1974) dosed chickens with a range of Aroclors, at 2 or
25 mg/kg diet, for 9 weeks. Aroclors 1248 and 1242 greatly reduced the
hatchability of eggs and caused some adult mortality, but failed to
cause thinning of the eggshells. When Britton & Huston (1972) fed
single comb White Leghorns Aroclor 1242 at 80 mg/kg diet, for 6 weeks,
no effects on shell thickness were observed. Dahlgren & Linder (1971)
failed to demonstrate any deleterious effects on the eggshells of
pheasants, dosed by gelatin capsule, once a week for 17 weeks, with
doses of Aroclor 1254 up to 50 mg. Call & Harrell (1974) fed various
Aroclors in the diet to Japanese quail for 21 days. Very significant
shell thinning was found with Aroclors 1254 and 1260 at doses of 1250
and 1000 mg/kg diet, respectively. At these high doses, egg production
was severely diminished and shell dimensions were based on very few
eggs. Adult mortality might have occurred at these doses; the paper
does not make it clear whether this actually happened. At lower doses
of 78.1 and 62.5 mg/kg diet of Aroclors 1254 and 1260, respectively,
there was also significant egg-shell thinning and reduced egg
production. Aroclor 1242 was tested at 312.5 and 5000 mg/kg diet and
both doses caused shell thinning, though this was to a lesser degree
than with other Aroclors at similar doses. Risebrough & Anderson
(1975) showed that eggshells thinned by dietary DDE were not further
affected by adding PCB (Aroclor 1254) to the experimental DDE diet.
Results on shell thinning are, therefore, not completely clear. It is
generally agreed that PCBs do not affect birds in this way and the few
results suggesting shell effects are regarded as anomalous or
difficult to interpret, because of experimental design. Shell thinning
can occur because of several different direct and indirect factors.
DDE and sulfanilamide have direct effects on the deposition of calcium
in the shell or on its mobilization from the skeleton, which acts as a
calcium store. PCBs are more likely to affect shells indirectly by
reducing food consumption; none of the studies cited above reported
whether individual birds took less food because of the dosing with
Aroclors. Haseltine & Prouty (1980) fed 24 pairs of mallard with
Aroclor 1242 at 0 or 150 mg/kg diet for 12 weeks and reported a
reduction in shell thickness of 8.9%. They pointed out that all
females laying thin-shelled eggs showed a significant depression in
body weight. This, they regarded as sufficient explanation for the
shell thinning. Much of the shell thinning found in Japanese quail
eggs, laid by females given a single oral dose of Aroclor 1254 of
500 mg/kg body weight, was thought to be due to reduced food
consumption (Haegele & Tucker, 1974).
Biessmann (1982) did not find any effects on eggshell thickness on
dosing Japanese quail with Clophen A60 at levels of up to 150 mg/kg
diet. However, the breaking strength of the eggs was reduced.
Hill et al. (1976) fed 6-month-old laying Japanese quail hens a diet
containing 10 mg Aroclor 1242/kg, for 40 days. Eggs were collected and
measured and, after 40 days, were found to have significantly thinner
shells (5.2%) than the controls. The authors stated that the handling
of the birds and the diet had no effect on food consumption or hen
weights during the test. This is the only study showing shell thinning
at moderate dose levels without an effect on food consumption. The
question of whether PCBs can cause shell thinning, therefore, remains
open.
7.3.3.5 Effects on the male
Platonow & Funnell (1971) kept day-old, white leghorn cockerels on a
diet containing 250 mg Aroclor 1254/kg, for up to 13 weeks. They found
a significant reduction in the weight of both combs and testes after 9
and 13 weeks and, a reduction in comb weight, only, after 6 weeks of
dosing. In a later study, Platonow & Funnell (1972) found a more
severe effect at 500 mg/kg; the comb was significantly reduced in
weight after just one week of dosing and the testicular weight
significantly reduced after 4 weeks, relative to the controls. The
control combs and testes increased in weight during the course of the
study; treated birds failed to develop either comb or testes.
Lillie et al. (1974) did not find any effects on weight gain, food
intake, or semen characteristics in leghorn cockerels fed Aroclor 1248
at 10 or 20 mg/kg diet for 8 weeks. They also did not find any effects
on fertility or hatchability of fertile eggs laid by similarly dosed
females. Liver weights were significantly increased at both dose
levels, and heart weights were significantly decreased at the highest
dose.
7.3.3.6 The effects of stress
Stress, imposed in various ways, increases the sensitivity of birds to
PCBs. Stress seems to have its effect by increasing the mobilization
of fat. Lower fat storage decreases the attenuation of PCB toxicity
seen when fat uptake of the material acts as an effective temporary
detoxification mechanism. Dahlgren et al. (1972) showed that brain
residues were higher in pheasants subject to starvation stress than in
unstressed birds dosed at the same rate. deFreitas et al. (1972)
obtained similar results using cold stress or starvation in pigeons.
As a corollary, biochemical adaptation to stress is inhibited by
exposure to PCBs. This is presumed to be due to residues in non-lipid
tissues (Dieter, 1974).
7.3.3.7 Physiological, biochemical, and behavioural effects
Jefferies & Parslow (1972) dosed young lesser blackbacked gulls
(Larus fuscus) with daily gelatin capsules containing Aroclor 1254
at 50, 100, 200, or 400 mg/kg body weight, for 8 weeks. Mean
individual thyroid weights were significantly increased by 32% (taking
all dosed birds as a single group). There was also an increase in the
mean cross-sectional area of the thyroid. There was, however, no
dose-related effect of PCBs on thyroid weight. The same authors
(Jefferies & Parslow, 1976) showed a similar effect of increased
thyroid weight when they dosed guillemots (Uria aalge) for 45 days
with Aroclor 1254 at 12 or 25 mg/kg body weight. Hurst et al. (1974)
also found a significant stimulation of thyroid growth after feeding
bobwhite quail a diet containing 5, 50, or 500 mg Aroclor 1260/kg for
4 months. Spear & Moon (1985) raised ring doves on either a low iodine
or normal diet. Insufficient iodine caused thyroid hyperplasia. This
hyperplasia was reversed within 7 days by a single dose of
3,4,3',4'-tetrachlorobiphenyl at 60 mg/kg body weight. The PCB
treatment also caused a significant decrease in core body temperature
and serum total thyroxine (T4) and triiodothyronine (T3). No effect,
other than decreased serum T3 and T4, was caused by dosing doves with
PCBs on a diet containing normal iodine levels.
Behavioural effects of PCBs have been noted by several authors.
Peakall & Peakall (1973) reported decreased parental attentiveness in
ring doves dosed at 10 mg Aroclor 1254/kg diet. Kreitzer & Heinz
(1974) measured the avoidance response (from a moving silhouette) in
Japanese quail chicks, for 14 days before, and 8 days after, dosing
with Aroclor 1254 at 200 mg/kg diet. After dosing, the avoidance
response was significantly reduced. Normal responsiveness to the
silhouette was not recovered after 6 days on a clean diet. Two
examples of hyperactivity in birds were also noted. European robins
fed one mealworm/day containing 5 µg Clophen A50, for 11-13 days,
showed increased migratory restlessness (Ulfstrand et al., 1971).
There were similar tendencies in redstart fed one mealworm/day,
containing 11µg Clophen A50, for 12 days, it was estimated that the
birds had ingested 132 µg of PCB overall (Karlsson et al., 1974).
A reduction was reported by Dobson (1981)in the nest-building activity
of pigeons (Columba livia), dosed orally, by gelatin capsule, with
15 mg Aroclor 1254/day, throughout a courtship cycle. The birds
produced a nest but the number of twigs used was reduced compared with
the controls. Reproductive and thyroid hormones were measured in blood
plasma samples, taken each day during the courtship cycle. While the
patterns of hormone secretion remained the same in both control and
treated birds (rises and falls of hormone levels occurred at
comparable times in the 2 groups) the absolute circulating levels of
the hormones were changed by the treatment. Both thyroxine and
luteinising hormone levels in the treated birds were elevated relative
to the controls. The levels were significantly higher, except at the
beginning and the end of the cycle. It was concluded that hormone
levels were unaffected, except when they would naturally be changing,
suggesting an interference with the feedback control of hormone
secretion and a central nervous site of action. Tori & Peterle (1983)
kept mourning doves (Zenaida macroura carolinensis) on a diet
containing Aroclor 1254 at 10 or 40 mg/kg for 42 days. The doves were
then paired and observed each day for 30 days. Both treatments
significantly increased the mean number of days in the courtship
phase; only 4 out of 8 pairs on 10 mg PCBs/kg completed this phase and
moved onto the nesting phase; none of the birds on 40 mg/kg had
completed the courtship phase within 30 days. Behaviour was scored to
measure intensity and, at both doses, this was significantly reduced
overall. Although dosed birds formed pair-bonds approximately 4 days
sooner than controls, there was no significant difference in the
length of the pair-bond formation period or in behaviour scores during
this period in doves fed 10 mg/kg. The length of time spent in the
courtship period was extended significantly (by 8.5 days) by PCBs at
10 mg/kg. Behaviour scores were not significantly affected, but dosed
birds averaged 32% lower scores. Of the birds reaching the nesting
phase, there was no significant difference between controls and dosed
birds with regard to length of time spent nesting or behaviour scores
in the nesting phase. PCBs, however, significantly delayed the onset
of nest initiation (by approximately 7 days) and, therefore, egg
laying.
Japanese quail were dosed with Clophen A60 in the feed at 150 mg/kg,
from the first week of life up to 42 days of age, while the birds were
developing sexually and becoming reproductively mature (Biessmann,
1982). In females, progesterone levels were not greatly affected by
the PCBs, but estradiol levels in blood plasma were lower before
sexual maturity and were less stable during egg laying. In males,
levels of testosterone and dihydrotestosterone (the primary
metabolite) were not affected. Quail fed up to 150 mg Clophen A60/kg
diet during the time of sexual maturation (second to fourth weeks of
age) showed delayed onset of egglaying and a diminished capacity to
lay eggs. Hormone levels in both males and females were not
significantly different from those in the controls.
A possible mechanism for central nervous effects was provided from
studies on neurotransmitters. Dopamine and noradrenalin were depleted
in the brain of the ring dove in a dose-related manner with increasing
brain residues of PCBs (Heinz et al., 1980).
7.3.3.8 Interactive effects with other chemicals
The only information on the interaction between PCBs and other
chemicals in birds has shown PCBs to be additive and not synergistic.
Kreitzer & Spann (1973) carried out tests on several pairs of
chemicals to study pesticidal synergism in young pheasants and
Japanese quail. Two PCBs were used in the study. Aroclor 1262 and
malathion showed additive results, when fed in the diet to 16-day-old
Japanese quail. Aroclor 1254 and DDE were also additive, when given in
the diet to 9-day-old quail. Another study on the possible interactive
effects of PCBs was conducted by Heath et al. (1972), who found that
feeding Aroclor 1254 and DDE in the diet to 14-day-old Japanese quail
gave additive results. There was no evidence of mutual potentiation or
antagonism.
7.3.4 Terrestrial mammals
Acute oral LD50 values reported for PCBs in mink ranged from >750 to
4000 mg/kg body weight. Acute LD50 values for 3 Aroclors in mink were
determined by Aulerich & Ringer (1977) after administration orally, by
gavage, or by intraperitoneal injection. Mortality was assessed 4 days
after i.p. administration and 14 days after oral administration. The
lethality of the Aroclors was found to be inversely related to the
chlorine content; Aroclor 1221 was most toxic and Aroclor 1254 least
toxic (Table 36). This is in marked contrast to the situation in
birds, where toxicity was correlated positively with chlorine content
of the PCBs (see section 7.3.3).
Table 36. Acute toxicity of Aroclors for minka
Aroclor LD50 (mg/kg body weight)
Intraperitoneal Oral
Aroclor 1221 >500-<750 >750-<1000
Aroclor 1242 1000 >3000
Aroclor 1254 >1250-<2250 4000
a From: Aulerich & Ringer (1977).
7.3.4.1 Short-term toxicity
Ferrets (Mustela putorius furo), fed a diet containing 20 mg of
Aroclor 1242/kg, for 8 months, developed enlarged, thickened, and
deformed toe-nails with hyperkeratosis at the junction of the skin and
sponchium, and dysplasia of the root of the nail and the matrix. The
same diet containing Aroclor 1016 did not produce these effects
(Bleavins et al., 1982).
Bleavins et al. (1980) showed the ferret to be less sensitive to PCBs
than the mink, though LD50 values were not determined. Aroclor 1242
at 20 mg/kg diet killed all mink (3 males and 12 females) to which it
was fed. The same diet did not kill any ferrets, though it did cause
reproductive failure.
No mortality occurred in mink fed a diet containing 1 mg PCBs/kg over
183 days (Wren et al., 1987a).
Hornshaw et al. (1986) conducted 28-day LC50 tests on mink, using
Aroclor 1254, in a study to investigate the effects of age, season,
and diet on the toxicity of PCBs; no effects were noted on any of
these parameters. In replicate tests, the calculated LC50 values
varied between 79 and 84 mg/kg diet (48-132, range of confidence
limits). The authors noted that the period of observation after dosing
was critical in assessing the results, since mortality continued after
dosing had stopped and the PCBs were persistent in the body. Taking
total mortality over 28 days of dosing and a further 7 days of
observation, the LC50 values fell to between 47 and 58 mg/kg diet.
A consistent finding among various studies is an effect of PCBs on
food consumption and, therefore, on body weight. The most detailed
analysis of food consumption during the feeding of Aroclor 1254 to
mink is presented by Hornshaw et al. (1986). Young mink fed the
Aroclor over 28 days showed a dose-dependent decrease in the amount of
food consumed. The cumulative weight of food consumed over 5 weeks
(1 week predosing and 4 weeks dosing) for controls was 7574 g. This
was reduced progressively with increasing dose of Aroclor 1254 at 10,
18, 32.4, 58.3, and 105 mg/kg diet to 6447, 6153, 4816, 3556, and
2723 g, respectively. This led to loss of original body weight over
the study period rising to more than 40% at the highest dose. Similar
effects were seen in adults of both sexes; females, with a smaller
initial body weight, were more severely affected than males. This
effect has implications for the interpretation of results of dietary
toxicity tests. The effects seen are a combination of the direct toxic
effects of the compound and the indirect effects of progressive
starvation. The doses of PCBs to which the animals are exposed must
also be calculated with reduced food intake in mind; apparent doses
are higher than real exposure as dose rates increase.
Organ weights (expressed as a percentage of brain weight) were
unaffected by Aroclor 1254 at doses up to 105 mg/kg diet, with the
exception of the heart and the adrenal glands. Heart weight was
reduced in both adult and young mink fed Aroclor 1254 at 58 mg/kg diet
or more; adrenal weights were increased by doses of 13 mg/kg diet or
more (Hornshaw et al., 1986).
Female minks received 0.1 or 0.5 mg of 3,4,5,3',4',5'-
hexachlorobiphenyl/kg diet, or 2.5 or 5.0 mg of 2,4,6,2',4',6'- and
2,3,6,2',3',6'-hexachlorobiphenyl/kg diet for 12.5-14.5 weeks. In both
studies, 3,4,5,3',4',5'-hexachlorobiphenyl was the most toxic isomer,
causing high mortality and reduced body weights (Aulerich et al.,
1985).
Clark & Prouty (1977) fed female big brown bats (Eptesicus fuscus)
on a diet of meal-worms containing 10 mg Aroclor 1254/kg. After the
feeding period of 54 days, the bats were starved to simulate loss of
body fat during the period of migration (when the animals do not
feed). Two out of 12 bats died. The brain residues of 20 mg PCBs/kg at
the end of the study were considered to be sub-lethal, since no
neurotoxic symptoms were observed before death.
7.3.4.2 Reproductive effects
Experimental investigations of mink reproduction in relation to
environmental pollution were carried out as a result of the reduced
reproductive success seen after feeding farm mink with fish from the
Great Lakes. Early studies, therefore, involved the analysis of fish
for pollutants and the experimental feeding of both the fish and of
mixtures of chemicals contained in various fish in the Great Lakes.
Aulerich & Ringer (1977) performed a comprehensive series of feeding
studies using coho salmon from 2 of the Great Lakes (Michigan and
Erie), other fish species from the same source, salmon from the west
coast of the USA, and various combinations of organochlorine
contaminants.
In their first study, ocean fish (perch or whiting) were used as
control diets and the reproductive performances were compared of dosed
and undosed female mink mated with undosed males. Lake Michigan coho
salmon, as 30% of the diet, had the most severe effect on
reproduction, i.e., total reproductive failure, as measured by numbers
of live kits surviving 4 weeks after parturition. Coho salmon from
Lake Erie also reduced reproductive success; 12 females produced only
7 kits still alive 4 weeks after birth. Two other species of fish from
Lake Michigan produced less severe effects, 5 kits being produced on a
diet of 30% bloater chub and 15 kits, on a diet of yellow perch.
Controls produced more than 40 kits over the same period. Kits
produced on Lake Michigan or Lake Erie fish, other than salmon, and
surviving to 4 weeks of age showed significantly lower body weights
than the controls. The small numbers of surviving kits from mothers
fed Lake Erie salmon also showed reduced body weight at 4 weeks of
age. No kits were produced after feeding Lake Michigan salmon diets to
females (Aulerich & Ringer, 1977).
In a long-term, low-level feeding study, 4 different Aroclors (1016,
1221, 1242, and 1254) were included in the diet of mink at a rate of
2 mg/kg. Groups of 8 female and 2 male animals were given this, or a
control, diet for 11 months, from August to June. The reproductive
performance of the animals on different diets is summarized, together
with mortality, in Table 37. Body weight, haemoglobin levels, and
haematocrit were monitored at monthly intervals during the study and
no significant effects of PCBs were noted. Only one of the test diets
(containing Aroclor 1254) adversely affected reproduction, with only 1
live birth in the study period. This single kit was considerably
lighter at birth than the controls and failed to survive 4 weeks after
birth (Aulerich & Ringer, 1977).
The reproductive effects of either Lake Michigan coho salmon or
Aroclor 1254 were reversible, when animals were transferred to a
control diet. Eleven females fed salmon as 30% of the diet for a year,
and then given control food for a further year, produced young with an
average litter size of 3.5 kits per mated female, in the second year
of the study. No young had been produced in the first year of the
study, during dosing. Similarly, 3 females given a year of control
food following a year on a diet containing 5 mg Aroclor 1254/kg,
produced an average of 4.3 young per mated female in the second year
of observation (Aulerich & Ringer, 1977).
In a later study, Bleavins et al. (1980) fed 2 different Aroclors at
various dose levels to mink and ferrets. Aroclor 1242 was fed to mink
at doses ranging from 5 to 40 mg/kg diet; Aroclor 1016 was given at
only 20 mg/kg diet. Results for mortality and reproductive effects are
summarized in Table 38, together with some data for Aroclor 1254 taken
from Aulerich & Ringer (1977).
There was a clear reproductive effect of Aroclor 1242 at a dose of
5 mg/kg diet, but no significant mortality. Aroclor 1242 caused 66%
mortality at 10 mg/kg diet and 100% mortality at 20 mg/kg diet.
Aroclor 1016, at 20 mg/kg diet, caused some deaths of adults and
reduced birth-weight and survival of kits, but the reproductive
effects were considerably less severe than those caused by Aroclor
1242 at 5 mg/kg diet. The authors (Bleavins et al., 1980) calculated
dietary LC50 values for Aroclors 1242 and 1254, using their own data
and data from Aulerich & Ringer (1977), to be 8.6 and 6.7 mg/kg diet,
respectively. It is clear that reproductive effects are less marked at
lower levels of chlorination of Aroclors.
Table 37. Effects of Aroclors on mortality and reproduction in minka
Aroclorb Adult females Kits
Number Number Number Number born Whelped/ Alive at Average weight
died (%) mated whelped female 4 (g ± SE) at
live dead mated weeks birth
Control 0 8 8 28 5 4.1 18 9.9 ± 0.32
1016 0 8 8 28 8 4.5 16 9.2 ± 0.33
1221 12 7 7 43 1 6.3 37 9.6 ± 0.22
1242 12 7 7 35 4 5.6 32 9.3 ± 0.27
1254 12 7 2 1 1 0.3 0 5.4
a From: Aulerich & Ringer (1977).
b Aroclors all fed at 2 mg/kg diet.
Reproductive effects on mink were reported by Jensen et al. (1977),
who fed groups of 10 females at 0.05, 3.3, or 11 mg PCBs/kg diet (type
unspecified), for 66 days. The highest dose eliminated successful
reproduction, whereas 3.3 mg/kg severely reduced the number of kits
born per female.
Table 38. Summary of mortality and reproduction in mink fed various dietary
levels of Aroclors
Treatment level Period fed Number dead/ Number of kits/
(mg/kg diet) (days) total number female
Aroclor 1254a
0 280 1/7 5.0
0 297 0/8 4.1
2 297 1/8 0.3
5 280 2/7 0.0
10 280 5/7 0.0
Aroclor 1242b
0 247 3/30 4.9
2 297 1/8 5.6
5 247 1/15 0.0
10 247 10/15 0.0
20 192c 15/15 0.0
40 138d 15/15 0.0
Aroclor 1016b
0 247 3/30 4.9
2 297 0/8 4.5
20 247 3/15 6.3
a From Aulerich & Ringer (1977).
b From: Bleavins et al. (1980).
c All mink died within 192 days on diet.
d All mink died within 138 days on diet: no females survived to whelping.
Wren et al. (1987b) did not find any significant effects on numbers of
kits produced or surviving to weaning age (5 weeks) after feeding mink
with Aroclor 1254 at 1.0 mg/kg diet, over 183 days. The dosing period
covered the seasonal period when the animals came into breeding
condition as well as a period of giving birth to the young. Although
the weights of kits born to dosed females were not significantly
different at 1 week postpartum, the weight gain of kits was then
affected and weights were significantly different from those of the
controls at ages 3 and 5 weeks. At age 5 weeks, when the kits were
weaned, the mean body weight of kits of the controls was 227.8 g,
while the mean body weight of kits fed by dosed mothers was 161.2 g.
Similar effects on the reproduction of female mink fed PCBs (type
unspecified) were observed by Jensen et al. (1977), i.e., a reduction
in the numbers of whelps born per pregnant female. The authors killed
the females after they had given birth and examined the numbers of
implantation sites in the uterus. This did not differ statistically
between groups (on average, 6.6 in control females, 6.1, in females
fed 5 mg/kg diet, and 4.5, in females fed 15 mg/kg diet PCBs).
However, the number of kits born to the same females showed a marked
effect of the PCBs: 5.1 (on average) born to control mothers; 2.9, to
mothers fed 5 mg/kg diet PCBs, and 0, to mothers fed 15 mg PCBs/kg
diet. The authors concluded that the effects of PCBs occur at the time
of implantation or later, causing resorption of implanted embryos.
Male mink seemed unaffected by doses of Aroclor that caused
reproductive effects in females. Males, dosed and mated with undosed
females, fathered normal numbers of kits (Aulerich & Ringer, 1977).
Wren et al. (1987b) did not note any effects of Aroclor 1254, fed to
male mink over 183 days at a rate of 1 mg/kg diet. Testicular size and
testicular histology were unaffected by the PCBs at any stage of the
reproductive cycle.
Treatment of adult, male, white-footed and white mice with Aroclor
1254 in the diet at a level of 400 or 200 mg/kg (equivalent to 57 or
29 mg/kg body weight), for 2 weeks resulted in a reduced testicular
spermatozoan concentration and, in the white-looted mice, a reduced
absolute weight of the seminal vesicles. In both strains, the absolute
weights of the testes and the final body weights were unchanged
(Sanders & Kirkpatrick, 1975; Sanders et al., 1977).
The reproductive performance of 27 pairs of white-looted mice (44-222
days of age), was compared with that of 26 control pairs, within 60
days of exposure to Aroclor 1254 at a dietary level of 200 mg/kg.
One-third of the exposed pairs did not survive the exposure but
produced at least one litter. The number of pairs producing at least
one litter was reduced by 65% and the number producing 2 or more
litters was reduced by 91%. Litter size was not affected. No offspring
survived to weaning in the 7 first litters of PCB-fed pairs (Merson &
Kirkpatrick, 1976).
A group of 10 pairs of wild-caught, white-footed, mice and groups of
18 (12 weeks of age) and 19 (16 weeks of age) pairs of
laboratory-raised, white-looted mice received Aroclor 1254 in the diet
at 10 mg/kg. Control groups comprised 10, 15, and 20 pairs,
respectively. The reproductive performance of the first group was
recorded for 18 months. The duration of the other studies varied from
7 to 15 months. The number of young per litter, 28 days after birth,
was lower in all treated groups. In laboratory-raised mice paired at
12 weeks of age, the birth interval was increased and the number of
young per litter at birth reduced (Linzey, 1987a). The second
generation of mice, maintained on the same diet as their parents, did
not differ in weight at birth, but were significantly smaller at 4, 8,
and 12 weeks of age. A similar trend was observed in the few young of
the third generation. The uterus, ovaries, and accessory glands, but
not the testes, weighed less in exposed groups than in the controls
(Linzey, 1987b). Linzey (1987a) reported similar reproductive effects
of Aroclor 1254 at a much lower dose.
The author suggested that the major consistent effect on the survival
of the young being fed milk was the result of much higher levels of
PCBs being transported via lactation than via the placenta.
Cottontail rabbits (Sylvilagus floridanus) were fed Aroclor 1254 at
10 mg/kg diet for 12 weeks, and then transferred to a clean diet and
allowed to breed. No effects were observed on any reproductive
parameters, and reduction in food availability did not change this
lack of effect (Zepp & Kirkpatrick, 1976).
7.3.4.3 Physiological effects
Wren et al. (1987a) examined histologically various organs in male and
female mink, dosed for 183 days with Aroclor 1254 in the diet. At
autopsy, no effects were seen on the histology of the pituitary and
adrenal glands. Brain histology also appeared normal. Thyroid
follicles gave the general appearance of minimal activity but did not
differ between treatments. Measurement of plasma thyroid hormones
(T3 -triiodothyronine; T4 -thyroxine) did not show any significant
differences between treatments in male mink. Females showed reduced
circulating T3 in a single sample, in January, but no other
differences were seen at other stages of the study. Thyroxine levels
were not affected at any time.
7.4 Effects on organisms in the field
The acute toxicity of PCBs is relatively low for most species and will
not, therefore, kill enough individuals to affect populations.
However, because of the high potential for bioaccumulation sufficient
residues of PCBs may build up to cause direct lethal effects over
time. Although PCBs are almost universally present in the tissues of
organisms in the environment, there are relatively few examples of
proved effects of these residues on populations of the organisms.
Sublethal effects, affecting populations by reducing reproduction or
growth, are possible, but difficult to prove, because PCBs are always
present with other environmental contaminants. Many possible effects
of PCBs in the environment have been suggested in the literature, but
few have actually been investigated in the field. It has proved
difficult, if not impossible, to relate residues of PCBs in tissues to
possible sublethal toxic effects; residues found after laboratory
dosing cannot be directly related to the field situation.
7.4.1 Plants
Klekowski (1982) studied the ostrich fern (Matreuccia struthiopteris)
growing in the flood plain of the Housatonic River, Massachusetts,
USA. This area of the river is contaminated with PCBs from land-fill
sites containing waste materials from the manufacture of transformers
in the nearby city of Pittsfield. Contamination with PCBs (principally
Aroclor 1254) had been a regular feature of the river area for a
period of more than 40 years. The frequency of somatic mutations in
the fern population was compared to a control population from an
uncontaminated area. The levels of PCBs in river sediments ranged from
1.4 to 139 mg/kg dry weight; at the site where the majority of fern
spores were collected, the level of PCBs was 26.3 mg/kg. The somatic
mutation frequency for the contaminated population was 5.2-6.2 times
higher than that for the controls. It is not known whether similar
genetic damage had occurred in other inhabitants of this contaminated
habitat. No other studies seem to have been conducted on the possible
effects of PCBs, from land-fill sites, on plants.
7.4.2 Fish
There have been many suggestions in the literature that PCBs might
affect populations of fish in the wild. Studies attempting to
demonstrate such an effect are few and, generally, inconclusive or
negative.
Olofsson & Lindahl (1979) used the ability of the cod (Gadus morrhua)
to react to different velocities of water under rotary flow, to
examine the effects of water pollution. Cod sampled from polluted
waters off the Swedish Coast were compared with cod sampled from
unpolluted areas. The ability of the fish to react to rotary flow was
significantly reduced in animals from polluted areas. However, the
authors were unable to relate the reduced reaction of the fish to
levels of various pollutants measured in muscle tissue. Experimental
studies showed that PCBs affected the reactions of the fish; the
residue level in muscle that was associated with this effect was
1.8 mg/kg. This was 30 times greater than the actual residues of PCBs
measured in the cod from the polluted area. While the authors stated
that the distribution of the PCBs in experimental fish and fish taken
from the wild would almost certainly have been different, that PCBs
exert the above effect in the field must be regarded as not proved.
Zitko & Saunders (1979) collected eggs of Atlantic salmon (Salmo
salar) from various areas and measured the PCB contents of the eggs
that proved infertile. The hatchability of different batches of eggs
was tested in the laboratory. No correlation was found between
residues of PCBs and the hatchability of the eggs; in fact the batch
of eggs showing the lowest hatchability also showed very low residues
of PCBs. However, it should be stated that hatchability was seldom
affected by PCBs in laboratory experiments; effects were more usually
seen on the developing young. Hogan & Brauhn (1975) related the
survival of fry hatched from rainbow trout (Salmo gairdneri) eggs to
the contamination of the eggs with PCBs. Five batches of eggs hatched
in 1971 had shown percentage mortalities, 30 days after hatching,
ranging from 10 to 28%. The eggs contained total organochlorine
residues of between 0.31 and 1.30 mg/kg, the majority of which was
PCBs. A batch of eggs collected in 1972 showed 75% mortality, 30 days
after hatching. Many of the fish hatched with such deformities as
scoliosis, lordosis, kyphosis, absence of caudal vertebrae, cranial
deformities, and projecting mandibles. This batch of eggs contained
2.7 mg PCBs/kg and 0.09 mg total DDT/kg (metabolites present not
stated).
Westin et al. (1983) investigated the effects of PCBs, passed on to
the eggs of striped bass (Morone saxatilis) by the female fish, and
of PCBs in food organisms fed to the hatched larvae. The hatchlings
were fed on brine shrimp ( Artemia sp.) from 2 sources, one
contaminated with PCBs and the other not. No effects of either
maternal PCBs or PCBs from the food were found. Residues of PCBs in
young fish decreased consistently throughout the study, which was
conducted in water free of PCBs. The authors suggested that PCBs from
the mother and from food are unlikely to affect the offspring in the
wild. A similar conclusion was drawn about the survival of lake trout
(Salvelinus namaycush) fry, hatched from eggs contaminated with PCBs
in the wild (Willford, 1980). Eggs taken from the wild and hatched in
clean water showed good survival of the offspring (suggesting that
residues of PCBs in the eggs were not responsible for the failure of
the species in Lake Michigan). However, experimental exposure of eggs,
and hatched larvae/fry to levels of PCBs in water and food, similar to
those found in the lake, led to high mortality and the increased
occurrence of deformities in fry. The author concluded that the levels
of PCBs in lake water and food items would be sufficient to lead to
the population decline seen in the lake. It should, however, be
pointed out that other factors in Lake Michigan could also have
contributed to the failure of the lake trout. Sea lampreys had
increased in number in the lake and could have caused population
decline by predation.
The relationships between the occurrence of hepatic diseases and
specific chemicals present in sediment were studied by Malins et al.
(1987). The concentrations of PCBs in the sediments from 4 urban and 2
non-urban areas in Puget Sound USA, were determined. In 3 of the
sites, the concentrations were <0.01 and, in the other 3, the
concentrations ranged from 0.11 to 0.53 mg/kg. Over 900 individual
organic compounds were found. To study the fish diseases, English sole
(Parophrys vetulus), rock sole (Lepidopsetta bilineata), and
Pacific staghorn sculpin (Leptocottus armatus) were collected. The
organs of fish containing the greatest number of lesions were the
liver, kidneys, and gills. Liver cell adenoma, hepatocellular
carcinoma, cholangiocellular carcinoma, haemangioma, and fibroma
constituted major types of liver lesions. Statistically significant
correlations between levels of chemicals in sediment and hepatic
neoplasms in the bottom-dwelling fish suggest a general
cause-and-effect relationship, but there is little firm evidence about
the actual cause of these neoplasms, in particular, in this case,
whether PCBs were involved.
7.4.3 Birds
In studies on chickens and different PCB-mixtures, Vos et al. (1970)
and Vos & Koeman (1970) found that the induction of subcutaneous and
abdominal oedema, centrilobular liver necrosis, hydropericardium, and
higher mortality was more or less related to the presence of
tetrachloro- and pentachlorodibenzofurans, as impurities in the PCB
samples.
From the middle of February to the end of March 1968, an epizootic
disease closely resembling chicken oedema disease occurred in Japan.
Two million chickens were involved, of which 400 000 (20%) died. The
clinical signs were laboured breathing, droopiness, ruffled feathers,
high mortality, and decreased egg production. Autopsy revealed marked
subcutaneous oedema, hydropericardium, ascites, pulmonary oedema,
muscular ecchymosis in the thorax or inside of the thigh, and
yellowish mottled appearance of the liver. The cause of the disease
was found to be Kanechlor 400 contamination of the feed. Experimental
reproduction of these symptoms with Kanechlor 400 was successful. The
remaining sample chicken feed contained 1300 mg of Kanechlor 400/kg
feed (Kuratsune et al., 1972).
Administration of PCBs leads to an atrophy of lymphoid tissue in
chickens (Flick et al., 1965; Vos & Koeman, 1970), and in pheasants
(Dahlgren et al., 1972). Vos & de Roij (1972) and Vos & van
Driel-Grootenhuis (1972) came to the conclusion that these effects
could be attributed to an immunosuppressive effect of PCBs. Vos & de
Roij (1972) suggested that the ability of PCBs to increase the
susceptibility of ducklings to duck hepatitis virus (Friend & Trainer,
1970) and of fish to fungal disease (Hansen et al., 1971) could be
attributed to this immunosuppressive effect.
Koeman et al. (1973) analysed 6 adult cormorants (Phalacrocorax carbo
sinenis), found dead in the wild, for PCB residues. Three additional
birds were shot and 6 fully grown nestlings were also taken. The
authors also carried out a study on 5 cormorants that were dosed with
the PCB, Clophen A60 (a 60% chlorinated PCB mixture that seemed to
correspond most closely with the PCBs profile of material found in
dead birds taken from the wild). The birds were initially dosed with
the PCBs in the diet, but, subsequently, the PCBs were administered
orally in gelatin capsules, until they died. PCB levels in the brains
and livers of the dead birds found in the wild were higher, overall,
than the levels obtained by dosing. The authors thought it highly
probable that this was indicative of PCB poisoning of the birds in the
wild. Without further detailed studies, this conclusion can only be
implied. The higher residues in dead birds from the wild suggest that
a large body burden was taken up, relatively safely in fat, and
released quickly on starvation, prior to death.
A field study, to show the effects of a sub-lethal dose of PCBs on
puffin (Fratercula arctica) breeding success and survival, was
conducted by Harris & Osborn (1981). A total of 150 puffins, trapped
on the Isle-of-May National Nature Reserve, Fife, Scotland, were
implanted, 108 with between 30 and 35 mg of PCBs (Aroclor 1254) and 42
with sucrose as controls. The test chemicals were implanted in
open-ended silastic tubes into the peritoneum. All birds were then
marked and released back into the wild. The same birds returned in
successive years to breed on the island. Breeding and survival of the
implanted birds were monitored through the breeding seasons of 1977,
1978, and 1979. Observations on survival were also made in 1980. Some
implanted birds were killed on recapture, and analysed for PCBs, in
each of the years of observation. No effects on survival or breeding
were seen. PCB levels in fat increased by a factor of between 10 and
14 compared with levels in birds that had not been implanted with the
Aroclor.
Herring gull (Larus argentatus) reproductive success in the area of
the Great Lakes declined with increasing residues of organochlorines
in the birds. Breeding success improved as these residues fell in the
1970s (Weseloh et al., 1979). The species was chosen as an indicator
of environmental pollution in the area and has been extensively
studied. Poor nesting success in the species is related to high
embryonic mortality (Gilbertson & Hale, 1974). Abnormal chicks have
been reported for herring gulls and also for other fish-eating species
from the area including: night herons, ring-billed gulls, common
terns, and Caspian terns (Gilbertson et al., 1976). Eggs from these
species contained residues of PCBs, but it was not possible to
directly relate these residues to chick abnormalities. Gilbertson &
Fox (1977) found a correlation between total organochlorine content
and the hatchability of herring gull eggs. While it cannot be shown
that PCBs were directly responsible for the overall effect, PCB
residues in the livers of hatched chicks, were the only contaminant
that was significantly correlated with the presence of pericardial
oedema. Weseloh et al. (1979) concluded that the reproductive success
of the herring gull can only safely be correlated with total
organochlorine residues and that the possible effects of PCBs cannot
be isolated. Shell thinning usually attributed to DDE alone, does not
occur significantly in these gulls. Hays & Risebrough (1972) suggested
that PCB residues in terns were responsible for the high percentage of
abnormal young in a colony from Long Island Sound.
Twenty-four black-crowned night heron (Nycticorax nycticorax) eggs
were collected at the San Francisco Bay National Wildlife Refuge in
1983. Twelve of these were collected from separate nests, when
late-stage embryos were pipping and an additional egg was randomly
collected from each nest for organochlorine analysis. Other anomalies
and skeletal defects were not apparent. Embryonic weights (with
partially absorbed yolk sacs removed) were 15% lower in comparison
with controls collected at Patuxent Wildlife Research Center.
Crown-rump length and femur length were shorter in the San Francisco
Bay embryos. The geometric mean PCB concentration was 4.1 mg/kg wet
weight with a range of 0.8-52.0 mg/kg. A negative correlation existed
between embryonic weight and log-transformed PCB residues in whole
eggs, suggesting a possible impact of PCBs on embryonic growth. DDE
did not show such a correlation (Hoffman et al., 1986).
Klaas & Swineford (1976) found low PCB residues (0.26-3.4 mg/kg wet
weight) in 35 screech owl (Otus asio) eggs (16 of which were known
to be addled) taken from the wild; there was no relationship between
the presence of residues and hatching failure. Dosing captive screech
owls with 3 mg Aroclor 1248/kg (McLane & Hughes, 1980) showed no
effects on eggshell thickness, number of eggs produced, young hatched,
or young fledged. Residues of PCBs measured in the eggs ranged between
3.9 and 17.8 mg/kg. This range covers the egg residue levels that were
clearly associated with effects in experimental chickens and
pheasants.
Eggs from 315 clutches of sparrowhawks (Accipiter nisus) from 9
sites in Scotland were examined by Newton & Bogan (1978) and Newton
(1979). Eggs that failed to hatch were collected and analysed for
PCBs, DDE, and for aldrin and dieldrin. Statistical analysis showed
little variation in residues within a single clutch, but wide
variation between clutches, even clutches from the same female in the
same area in different years. On this basis, analysis of single eggs
was taken as representative of the complete clutch. Analysis of
variance and multiple regression were used to try to relate particular
effects, such as shell thinning, addling of eggs, and late-embryo
mortality, with particular organochlorines. Results showed that
addling of eggs was significantly correlated with levels of both DDE
and PCBs. Because of the close correlation between residues of DDE and
PCBs in eggs of sparrowhawks, it is not possible to tell whether only
one, or both, organochlorines were involved in egg addling. PCBs
showed the strongest relationship with addling. Shell thinning was
more directly linked with DDE. Newton et al. (1982) concluded that the
level of PCB residues in merlin (Falco columbarius) was not
sufficiently high to have exerted any effect on the breeding success
of this species in Britain. Hodson (1975) did not find any effects of
PCB residues in Richardson's merlin in Canada and attributed all
effects to DDE. A relationship between DDE residues and shell thinning
and the breeding success of the bald eagle (Haliaetus leucocephalus)
was demonstrated by Wiemeyer et al. (1984). PCB residues were
correlated with DDE residues. The authors concluded that contaminants
other than DDE contributed no more than a minor role in reproductive
effects on this species in the field. It was considered that PCBs
might have contributed to reproductive problems, but that the evidence
was unclear. Newton et al. (1986) presented a statistical analysis of
residues in sparrowhawks related to reproductive success. Study
populations from many sites throughout the British Isles showed
different reproductive success. Some of this variation could be
related to levels of organochlorine compounds in eggs. When shell
thickness index was related to PCBs alone, a significant negative
correlation was detected. However, once DDE residues were included,
PCBs did not improve the model. It was concluded that DDE alone could
account for all of the reproductive effects recorded and that PCBs, at
the levels of contamination found, probably did not play a role in
reduced breeding performance. Newton et al. (1988) examined new data
on residues of organochlorine compounds in the eggs of the peregrine
falcon (Falco peregrinus) and also reassessed older data. Total
information considered included information on residues and breeding
success covering the period 1963-86. The PCB residues found were not
considered to have had a significant effect on breeding success in
this species. In earlier studies, Wiemeyer et al. (1975, 1987) could
not isolate any single organochlorine compound as responsible for the
reduced breeding success of the fish-eating osprey (Pandion
haliaetus).
Heinz et al. (1983) examined the breeding success of groups of
red-breasted mergansers (Mergus serrator) on islands in northwestern
Lake Michigan. This is a fish-eating species and, since eating fish
from the Great Lakes had been shown to affect captive mink, the
authors investigated the possible effects of contaminants (measured in
blood samples taken from the birds) on reproduction. Other,
non-contaminant effects were also examined. Many organochlorine
contaminants of the environment were found in eggs including 14
different organochlorine compounds; there was a correlation between
the levels of many of these compounds. No relationship could be
established between levels of PCBs, or any other contaminant, and the
breeding success of the birds. The hatching success of dabbling ducks
also seemed to be largely unaffected by feeding in Lake Michigan,
despite the presence of residues of PCBs and other contaminants in the
eggs (Haseltine et al., 1981).
Appraisal
Effects of PCBs have been shown in laboratory studies on many domestic
species of birds, but few wild species. However, there are many
studies showing measurable residues of PCBs in wild birds. PCBs have
been implicated in population decline in several bird species in
different parts of the world, but it is seldom easy to demonstrate
directly the effects of PCBs on populations of birds in the field.
This is largely because PCB residues invariably occur together with
other organochlorine compounds, such as DDE and dieldrin. Separating
out individual effects can only be done completely satisfactorily when
laboratory data are available for the same species and each individual
pollutant. Some idea of likely effects can be obtained from
statistical manipulation of data from the field, though, in this case,
correlation is the best that can be achieved. In practice, few field
studies give the results that have been predicted from laboratory
studies on other species; that is direct extrapolation from laboratory
to field and species to species is not straightforward.
7.4.4 Mammals
Laboratory studies involving the dosing of non-laboratory mammals with
PCBs are limited to a few species. Field studies have been conducted
on a wider range of species.
Norström et al. (1988) studied liver and adipose tissue specimens of
polar bears (121 samples) obtained by Inuit hunters from 12 zones in
the Canadian Arctic Archipelago in 1982-84 (see section 5.1.4). Six
PCB congeners (Nos 99, 153, 138, 180, 170, and 194) constituted
approximately 93% of total PCBs. The major PCBs accumulated belong to
the group formed by combinations of 2,4-dichloro-, 2,4,5-,
2,3,4-trichloro-, and 2,3,4,5-tetrachloro- substitution on each ring.
Congeners with 2,4-, 3,4-dichloro-, 2,3,4-, 2,3,5-trichloro-,
2,3,4,6-, 2,3,5,6-tetrachloro- or 2,3,4,5,6-pentachloro- substitution
on one ring, such as PCB Nos 118, 138, 187, 183 and 196, which usually
bioaccumulate readily in mammals, were all at lower levels compared
with congeners with only 2,4,5-trichloro- and 2,3,4,5-tetrachloro-
substitution in ringed seals, such as PCB Nos 153, 180, and 170. The
last 3 congeners accounted for 71% of total PCBs in polar bears. Thus,
it appears that the polar bear is able to metabolize PCB congeners in
which there are nonchlorinated para positions, adjacent
nonchlorinated ortho-meta positions, or both ortho positions
chlorinated in one ring.
DeLong et al. (1973) measured various organochlorine pollutants (DDT,
DDD, DDE, dieldrin, and PCBs) in sea lions from the Channel Islands
off the Californian coast. Previous observations had recorded a high
incidence of premature births in the population and organochlorine
residues from females showing premature or normally-timed parturition
were compared. Both DDT and PCB residues (measured against a standard
of Aroclor 1254) were significantly higher in the females producing
premature pups. Residues were estimated in the blubber, liver, and
brain, and, for the first 2 tissues, the ranges of values, for the 2
groups of females, did not overlap. Previous studies on both
substances indicated possible reproductive effects in mammals,
corresponding to some extent with those observed in the sea lions.
Cause and effect could not, therefore, be directly established for
each of the pollutants alone. Helle et al. (1976a) collected ringed
seals from Simo, on the northern Bothnian Bay area of the Baltic Sea,
in October/November. This population of seals shows reduced
reproductive capacity and the sampled population showed only 27% of
females pregnant, compared with other reports of 62.5% and 85-90% for
the same species elsewhere. Residues of DDT and PCBs were compared
with those in seals from other areas of the Baltic and found to be
lower (Table 39). The seals sampled from Simo were divided into
pregnant and non-pregnant groups (n = 15 and 26, respectively).
DDT and PCBs levels were both significantly higher in non-pregnant
animals (Table 40). All females from both groups showed a corpus
luteum in one ovary, indicating that all had ovulated. About half of
the non-pregnant females showed indications of an embryo having been
implanted, which had subsequently aborted or resorbed. Again cause and
effect could not be established directly. The authors pointed out that
normally breeding Californian sea lions had DDT levels as high as
those in Baltic seals showing reproductive failure and proposed this
as an indication that PCBs were the causative agent.
Table 39. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable fat of
blubber from ringed seal from the Baltic Seaa
Area Number DDT PCBs
Northern most part 40 110 ± 10b 69 ± 4.4b
of the Bothnian Bay
Gulf of Bothnia 33 200 ± 28 110 ± 15
a Data from: Helle et al. (1976a).
b Value significantly different from those in the Gulf of Bothnia (P < 0.05).
Table 40. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable
fat of blubber in non-pregnant and pregnant ringed seal of
reproductive agea
Number DDT PCBs
Non-pregnant 26 130 ± 13b 77 ± 5.2b
Pregnant 15 75 ± 11 56 ± 6
a Data from: Helle et al. (1976a).
b Values for non-pregnant females significantly higher than
those of pregnant females. DDT P < 0.01; PCBs P < 0.05.
It was further pointed out that the group of non-pregnant females
would include some animals that were not pregnant for reasons other
than the presence of organochlorine compounds. In a later paper (Helle
et al., 1976b), the same authors reported that some of the
non-pregnant females showed abnormal uteri with stenosis or occlusion
of the uterine horns. They, therefore, subdivided the non-pregnant
females in their second sample into those showing the anatomical
abnormality and those not. Both DDT and PCBs were significantly higher
in the occluded group than in pregnant animals. Non-pregnant females,
without occlusions, showed residues of DDT and PCBs not significantly
different from those in pregnant animals, and significantly lower than
those females with occlusions. Residues in males were comparable with
the highest residues found in females (Table 41).
There is some indication of a positive correlation between PCB
residues and age in male seals but not in females (Addison et al.,
1973; Addison & Smith, 1974). These observations are presumed to
indicate that females excrete some of their body burden of PCBs in the
milk.
These field observations have been confirmed in a study in which
captive seals were fed on fish from the Wadden Sea (where reproductive
problems had been found in the wild seal population) and compared with
controls fed fish from the Atlantic Ocean. Seals eating the
contaminated fish, which differed from the control fish only in the
PCB content, showed the same failure to carry the fetus successfully
to term seen in the wild (Reijnders, 1986; see section 7.2.5).
Table 41. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable fat from
blubber of ringed seal from Simo, Bothnian Baya
Group Sample description Number DDT PCBs
I pregnant females 24 88 ± 9.7 73 ± 6.6
II non-pregnant females 29 130 ± 10 110 ± 7.8
with stenoses and
occlusions
III non-pregnant females 8 100 ± 15 89 ± 11
with normal uteri
IV fetuses 24 62 ± 4.3 49 ± 3.0
V males 24 130 ± 18 100 ± 13
probability of similarity between test groups (t-test)
I-II P < 0.01b P < 0.01b
I-III P > 0.05 P > 0.05
I-IV P < 0.05c P < 0.01b
a Data from: Helle et al. (1976b).
b Groups significantly different at the 99% level.
c Groups significantly different at the 95% level.
Subramanian et al. (1987) collected samples of blubber from male
Dall's porpoise (Phocoenoides dalli) in the northwestern Pacific
Ocean and analysed the samples for organochlorine content. Blood
samples taken from the same animals were analysed for testosterone, a
male sex steroid, and for aldosterone, another steroid hormone,
responsible for blood electrolyte balance. Testosterone levels in
blood were correlated with blubber levels of DDT and PCBs; as the
organochlorine content increased, testosterone levels decreased. There
was no relationship between blubber organochlorine levels and blood
levels of aldosterone. The number of samples was small (n = 12) and,
though the relationship between DDT and testosterone was significant,
the apparent relationship with PCBs was not. The animals were sampled
outside the breeding season, when testosterone levels would be
expected to be low.
In a study by Clark & Lamont (1976), 26 pregnant big brown bats
(Eptesicus fuscus) were collected from the field and maintained on a
control diet, in captivity, until they gave birth to young. Levels of
PCBs were measured in both the adults and the offspring. The
concentrations of PCBs in litters with dead young were significantly
greater than in litters where both young were born alive (mean for
litters with dead young was 2.44 mg/kg; mean for all other litters was
0.34 mg/kg). The contents of PCBs ranged between 1.07 and 1.96 mg/kg
wet weight in adults and between 0.28 and 1.69 mg/kg in the young.
7.4.4.1 Appraisal
PCBs have been implicated in population declines of seals and sea
lions. Population decreases and reproductive failure have been
observed in seals from the Baltic Sea, the Wadden Sea (southeastern
North Sea), and the Gulf of St. Lawrence and in sea lions off the
Californian coast. Poor reproduction has been correlated with residues
of PCBs in the affected animals. A major problem in such studies is
the occurrence of more than one chemical pollutant in the animals.
PCBs are found together with other organochlorine compounds and heavy
metals. Conclusions, therefore, have often relied on making the best
correlation between observed effects and residues and checking cause
and effect relationships on animals more amenable to laboratory study.
A study on captive seals confirmed field observations on the effects
of PCBs on these marine mammals (see section 7.2.5).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Evaluation of the toxicity of Aroclors and other commercial PCB
mixtures is complicated by numerous factors, including isomer and
congener composition, differences in species susceptibility,
quantitatively inconsistent data, and various degrees of contamination
with toxic compounds, such as chlorinated dibenzofurans. Because of
these factors and a lack of data for some of the Aroclors (most
studies have been conducted on the higher chlorinated Aroclors), it is
assumed that effects resulting from exposure to a specific Aroclor are
representative of effects that may be produced by the other Aroclors.
Many of the literature sources do not give details of the composition
of the PCB mixture used in the studies.
Reviews on the literature concerning the toxicity of PCBs are given by
ATSDR (1989), Kimbrough (1980, 1987), Safe (1984), NIH (1985), and
Lorenz & Neumeier (1983).
8.1 Single exposures
8.1.1 Oral
(a) Aroclors
The acute oral LD50 values for a number of Aroclors are presented in
Table 42. The lowest oral LD50 in the rat was 1.0 g/kg body weight
for Aroclor 1254 (Garthoff et al., 1981).
In Sherman rats, lethal doses of Aroclor 1254 and 1260 in peanut oil
caused ulceration in the duodenum and glandular stomach (Kimbrough et
al., 1972). Osborne-Mendel rats, receiving a single oral dose of
50 mg/kg body weight of Aroclor 1254 in corn oil, showed an increased
relative liver weight together with the induction of microsomal
enzymes, within 12 h (Litterst et al., 1974).
Monkeys, sacrificed 4 days after gastric intubation of Aroclor 1248 at
1.5 or 3.0 g/kg body weight (no vehicle reported), exhibited enlarged
livers with proliferation of the endoplasmic reticulum and hypertrophy
and hyperplasia of the gastric mucosa (Allen et al., 1974a).
(b) Individual congeners
Estimated oral LD50 values in 30 days for individual congeners in
corn oil in Hartley guinea-pigs (probably single application) were
0.5 mg/kg body weight for 3,4,5,3',4',5'-hexachlorobiphenyl, less than
1 mg/kg body weight for 3,4,3',4',-tetrachlorobiphenyl, more than
3 mg/kg body weight for 2,3,4,5,3',4',5'-heptachlorobiphenyl, and more
than 10 mg/kg body weight for 2,4,5,2',4',5'-hexachlorobiphenyl
(McKinney et al., 1985).
Table 42. Acute oral LD50s of Aroclorsa
Aroclor Species/strain Sex/ageb LD50 (g/kg body weight) References
1254 rat/Wistar male/30 days 1.3 Grant & Phillips (1974)
female/30 days 1.4
male/60 days 1.4
female/60 days 1.4
male/120 days 2.0
female/120 days 2.5
rat/Sherman male/weanling 1.295 Linder et al. (1974);
NR/adult 4-10 Kimbrough et al. (1972);
rat/Osborne-Mendel male/adult 1.01 (single dose) Garthoff et al. (1981)
1.53 (5 doses over 2 1/2 weeks)
1.99 (5 doses, 1 day/week)
1221 rat/Sherman female/NR 4.0 Nelson et al. (1972)
1260 rat/Sherman NR/adult 4-10 Linder et al. (1974)
M/weanling 1.315
1242 rat/Sprague-Dawley male/adult 4.25 Bruckner et al. (1973)
1262 rat NR 11.3 Panel on Hazardous Trace
Substances (1972)
a References: ATSDR (1989); WHO (1976).
b NR = not reported.
Information on solvents used was not available.
8.1.2 Inhalation
No acute data were available.
8.1.3 Dermal
Median lethal doses for a single application of Aroclors to the skin
of rabbits ranged from >0.79 to <1.27 g/kg body weight for Aroclors
1242 and 1248 in 50% corn oil, to >1.0 to <3.17 g/kg body weight for
undiluted Aroclor 1221, and >1.26 to <2.0 g/kg body weight for
Aroclor 1260 (Fishbein, 1974).
8.1.4 Other routes
(a) Aroclors
With a single intravenous dose of Aroclor 1254 in a 1% lecithin-saline
suspension, the LD50 for adult female Sherman rats was 0.4 g/kg body
weight (Linder et al., 1974). The LD50s for Aroclor 1254 in DMSO for
various mouse strains, following a single intraperitoneal injection,
varied between 0.9 and 1.2 g/kg body weight (Lewin et al., 1972).
(b) Individual congeners
The intraperitoneal LD50 for 2,4,3',4'-tetrachlorobiphenyl in CF-1
mice was 2.15 g/kg body weight, while that of its main metabolite,
(5-hydroxy-2,4,3',4'-tetrachlorobiphenyl) was 0.43 g/kg body weight,
which suggests that the acute toxicity of 2,4,3',4'-tetra-
chlorobiphenyl might be attributable to this phenolic metabolite
(Yamamoto & Yoshimura, 1973).
In male Wistar and Charles-River CD rats, a single intraperitoneal
dose of 1 mg 3,4,5,3',4',-pentachlorobiphenyl/kg body weight or a
single oral dose of 50 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body
weight caused significant liver enlargement accompanied by fatty
changes, atrophy of the thymus, and decreased relative spleen weights.
Mono- ortho substituted biphenyls, such as 2,3,4,5,3',4'-hexa-
chlorobiphenyl and 2,4,5,3',4'-pentachlorobiphenyl, induced these
effects in the liver and thymus to a minor degree following an
intraperitoneal dose of 50 mg/kg body weight, while di- ortho
substituted hexachloro- and heptachlorobiphenyls did not cause adverse
effects at this dose level (Kohli et al., 1979; Yoshihara et al.,
1979).
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Aroclors
(a) Mouse
While oral intoxications by PCB mixtures in monkeys are easily
recognizable by their effects on the skin, skin lesions in female ddN
mice were only observed after 2-3 months of daily oral exposure to
1.6 mg of a technical PCB mixture (48% chlorine) per mouse (80 mg/kg
body weight per day) in olive oil. The lesions included alopecia,
erosions, ulcerations, and eczematous changes around the eyelids. No
increase in mortality and only slight growth retardation were seen
after 26 weeks of exposure (Nishizumi, 1970).
Koller (1977) found histological changes in the liver of mice exposed
to 37.5 mg Aroclor 1254/kg diet for 6 months. A dose level of
3.75 mg/kg diet was without effect.
The oral toxicities of Kanechlor 400, 500, and 600 were compared by
feeding male mice diets containing 300 mg PCBs/kg for 14 weeks
(Kawanishi et al., 1975). Fatty degeneration and accumulation of
pigment in the liver were observed in the mice fed Kanechlor 600.
(b) Rat
At lethal, oral doses of undiluted Aroclor 1242 (100 mg/kg body
weight, every 2 days, for 3 weeks), Sprague-Dawley rats showed reduced
body weight, thymus atrophy, chromodacryorrhea, progressive
dehydration, and central nervous system depression with terminal
ataxia and coma. Fatty changes were observed in the liver and kidneys
(Bruckner et al., 1973).
Male and female Sherman rats (10 animals of each sex) were fed diets
containing 0, 20, 100, 500, or 1000 mg of Aroclor 1260 or Aroclor
1254/kg (equivalent to 0, 1.5, 7, 36, or 72 mg/kg body weight,
respectively) for 8 months. The animals receiving the 2 highest dose
levels showed reduced growth. Female rats fed Aroclor 1260 at 500 and
1000 mg/kg diet showed a high mortality rate. However, only 3 rats fed
Aroclor 1254 at 500 mg/kg diet died. Significant increases in relative
liver/body weight ratios were found for both Aroclors at all doses
tested. Microscopically enlarged hepatocytes, cytoplasmic inclusions,
increased lipid levels, and foamy cytoplasm were all found
consistently. Adenofibrosis was found at higher doses (500 and
1000 mg/kg diet) and corresponded to the glistering white areas seen
on gross inspection. These areas also showed cholangiofibrosis
(according to Kimbrough, synonymous for bile duct proliferation, bile
duct adenomatosis, and fibroadenoma) (Kimbrough et al., 1972).
Growth and mortality rates were unaffected in male Sprague-Dawley rats
exposed for 1 year to a diet containing Aroclors 1248, 1254, or 1262
at 100 mg/kg diet (equivalent to 5 mg/kg body weight) compared with
controls (Allen et al., 1976).
Female Charles-River CD rats were fed for 20 weeks on a diet
containing 0, 10, 30, or 100 mg Aroclor 1254/kg (equivalent to 0, 0.5,
1.5, and 5 mg/kg body weight, respectively). No increase in mortality
rates occurred but growth inhibition was seen at 30 mg/kg from month 2
onwards and at 100 mg/kg diet from week 2 onwards. Skin lesions,
initially on the ears, were found after 10-20 weeks of exposure to
Aroclor 1254 at all dose levels. The lesions, including alopecia and
reddened and thickened skin with hyperkeratosis, subcutaneous oedema,
and infiltration by polymorphonuclear leukocytes, also involved the
nose, tail, and feet at the highest exposure level (Zinkl, 1977).
Effects on the liver following exposure to PCB mixtures have mainly
been investigated in rats (see section 8.6.1.1).
(c) Rabbit
Four groups of 5 male and 5 female New Zealand White rabbits were
administered 300 mg Aroclor 1221, 1242, or 1254 in corn oil, via a
stomach tube, once a week, for 14 weeks. The fourth group received
only the vehicle.
The mortality rate was increased and the body weight gain was reduced
after 14 weeks of oral exposure to Aroclor 1254, but not after
exposure to Aroclor 1221 or 1242. Liver/body weight ratio and SGOT and
SGPT activities were increased in the animals treated with Aroclor
1242 or 1254, but not in those treated with Aroclor 1221. In the
animals treated with Aroclor 1254, the smooth endoplasmic reticulum
was condensed in the liver cell and formed hyalin inclusions which
might have been accompanied by a loss of enzyme activity. Lipid
accumulation, pigment deposition, nuclear changes, and necrosis were
also found (Koller & Zinkl, 1973).
(d) Pig
Pigs, fed Aroclor 1242 or 1254 at a dietary level of 20 mg/kg
(equivalent to 0.8 mg/kg body weight) for 91 days, showed gastric
lesions, consisting of erosions and necrosis. Two pigs fed a high-dose
regimen of 100 mg of Aroclor 1254/kg body weight for 11 days, also
showed hypertrophy and hyperplasia of the gastric mucosa; this was
also found in monkeys at low exposure levels (Hansen et al., 1976b).
(e) Cow
Holstein cows were studied throughout a complete lactation period, a
non-lactating period, and 42 days of a subsequent lactation period for
overt responses to Aroclor 1254. Four cows received daily doses of 0,
10, 100, or 1000 mg/cow (1000 mg/cow is equivalent to 1.67 mg/kg body
weight) over 60 days. The mean daily milk production and net energy of
a complete lactation period did not differ between control and
PCB-treated animals. At the end of the study, the concentrations of
PCBs were 0.005, 0.021, 0.14 in blood plasma; 1.9, 10.9, 91.3 in milk
fat, and 1.4, 6.9, 70.0 µg/kg in adipose tissue for the 10, 100, and
1000 mg PCB groups, respectively. No signs of impaired health,
productivity, or changes in blood and urine chemistry were observed
(Willett et al., 1987).
(f) Monkey
Rhesus monkey
(i) Aroclor 1242
Becker et al. (1979) exposed 6, 7-8-month-old, male Rhesus monkeys to
Aroclor 1242 at levels of 0, 3, 10, 30, or 100 mg/kg diet (equivalent
to 0, 0.12, 0.4, 0.4, 1.2, or 4.0 mg/kg body weight, respectively) to
study changes in the stomach mucosa. At 3, 10, 30, and 100 mg/kg diet,
4 monkeys died after 245, 146, 92, and 137 days of exposure,
respectively. All exposed monkeys showed a decreased body weight gain.
At all dose levels, changes were observed in the folds of the
suborbital facial skin, and eyelids became swollen and red. From day
71 at 3 mg/kg diet, days 69 and 77 at 10 mg/kg diet, and day 12 at the
higher exposure levels, stomach biopsies revealed an apparent arrest
of the differentiation of generative cells of the isthmus and neck
regions into parietal and zymogenic cells. Mature parietal and
zymogenic cells, which were found only in the bases of the glands,
showed signs of injury, such as dilatation of the rough endoplasmic
reticulum on the zymogenic cells, irregularity of the mitochondria in
parietal cells, and irregular luminal membranes and an increase in the
number of autophagic vesicles in both type of cells. The severity of
the lesions was directly correlated with both duration and level
exposure. A no-effect level was not obtained in this study.
A group of 5 Rhesus monkeys, 1-2.5 years old, was exposed to a diet
containing 1 mg of Aroclor 1242/kg (equivalent to 0.04 mg/kg body
weight) for 133 days. A control group contained 4 monkeys. No adverse
effects were found (McNulty et al., 1980).
Characteristic lesions, metaplasia in epithelial structures, such as
sebaceous glands, nail beds, gastric mucosa, and ameloblast
surrounding unerupted teeth were reported to have developed in Rhesus
monkeys, 13 months after a 40-day diet containing 400 mg Aroclor
1242/kg (equivalent to 16 mg/kg body weight) (McNulty, 1985).
(ii) Aroclor 1248
A group of 5 (4 males and 1 female), 1-month-old Rhesus monkeys,
without previous exposure, were administered 30 daily doses of 35 mg
of Aroclor 1248/kg body weight, by gavage, in corn oil. Four animals
received only corn oil. No mortality or clinical signs were observed,
except for a decrease in body weight gain, slightly reduced food
consumption, and anaemia. Mild microscopic changes were observed in
the thymus, bone marrow, eye, skin, stomach mucosa, and liver
(Abrahamson & Allen, 1973).
Six male Rhesus monkeys, aged 1 1/2-2 years were fed a diet containing
300 mg Aroclor 1248/kg for 3 months. Three animals served as controls.
A decrease in body weight was observed. Within one month, all the
animals fed PCBs had alopecia, subcutaneous oedema, particularly of
the face, which manifested as swollen eyelids, erythema, and acneiform
lesions involving the areas devoid of hair (Allen & Norback, 1972 cf.
Hayes (1987)).
Six adult, female Rhesus monkeys were fed 25 mg Aroclor 1248/kg diet
for 2 months. Facial oedema, alopecia, and acneiform changes developed
within 1 month and 1 animal died 2 months after removal from the
experimental diet. In addition to the above changes, this animal
showed anaemia, hypoproteinaemia, hypertrophy, and hyperplasia with
invasion through the muscularis mucosa, focal haemorrhages and
ulcerations of the gastric mucosa, and bone marrow hypoplasia. Eight
months later, the 5 surviving animals continued to show clinical signs
of intoxication. The PCB concentration in body fat, which, after 2
months of treatment, averaged 127 mg/kg, had decreased 8 months later
to 34 mg/kg (Allen et al., 1974a).
Groups of 9 adult, female Rhesus monkeys (weight approximately
5.6 kg), were fed a diet containing 0, 2.5, or 5.0 mg Aroclor 1248/kg
(equivalent to 0, 0.1, or 0.2 mg/kg body weight, respectively) for an
average period of 18.2 months. An additional group of 4 males was fed
5.0 mg/kg diet. Control groups contained 12 female and 6 male monkeys.
The Aroclor 1248 contained 4.4-8.7 ng polychlorinated dibenzofurans/kg
diet. The female monkeys were mated with control males after 6 months
of exposure (the reproductive effects are discussed in section
8.4.1.3). Levels of Aroclor 1248 in adipose tissue reached a plateau
after 1 year at 2.5 mg/kg diet and after 6 months at 5 mg/kg diet.
Exposed males showed slight to moderate periorbital oedema and
congestion of the eyes. Females showed an average 15% loss in body
weight over the first 5 months of both exposures, while food
consumption was normal. At 6 months, they all showed loss of hair,
acne of the face and neck, and erythema and swelling of the eyelids.
Skin biopsies revealed keratinization of the affected hair follicles.
One female monkey died after 173 days of exposure to 2.5 mg/kg diet
and one female died after 310 days of exposure to 5.0 mg/kg diet. Both
animals developed terminal enteritis due to Shigellosis, which was
resistant to treatment. At autopsy, the animals showed generalized
alopecia, subcutaneous oedema, and acne. Microscopically, follicular
epithelial hyperplasia with inflammation of the surrounding tissue,
and keratinization of the hair follicles were observed. The livers
showed focal areas of necrosis, enlarged hepatocytes, and fatty
changes (Barsotti et al., 1976).
The surviving females in the former study, 8 per dose level, were
placed on a control diet for approximately 1 year after an average
total intake of 270 and 498 mg Aroclor 1248, respectively. There was a
gradual improvement in their physical condition and, within one year,
their gross appearance was no longer different from that of the
controls. However, their breeding performance was still affected, as
outlined in section 8.4.1.3 (Allen et al., 1980).
(iii) Aroclor 1254
Several pilot studies were carried out before this major study to
characterize the toxicity of Aroclor 1254 and to compare the toxic
findings in the Rhesus and Cynomolgus monkey (Tryphonas et al., 1984,
1986b; see section 8.2.1.6). In these pilot studies, Mes & Marchand
(1987) and Mes et al. (1989a) measured the concentrations of PCBs in
the blood, adipose tissue, and faeces.
A preliminary report describes the results of an ongoing study after
54 weeks of daily oral administration of gelatin capsules containing
0, 5, 20, 40, or 80 µg Aroclor 1254/kg body weight, in corn
oil-glycerol, to groups of 16 adult, female Rhesus monkeys (Macaca
mulatta). At this stage, a slight decrease in body weight gain was
observed in the exposed monkeys together with a decrease in water
consumption, but not in food consumption. In week 52, the incidences
of prominent nail beds, of nails separated from the beds, and of
prominent tarsal glands increased in the animals exposed at 80 µg/kg
body weight (Arnold et al., 1984).
Aroclor 1254, at a dose level equivalent to 280 µg/kg body weight was
given for 5 days per week to Rhesus monkeys (Macaca mulatta) over a
period of 27-28 months. Four animals were treated as described and 4
animals served as controls. The Aroclor 1254 was administered in
apple-juice-gelatin-corn oil emulsion. The weight of the monkeys at
the beginning of the study was approximately 4 kg. Terminal clinical
signs of varying severity included finger nail detachment, exuberant
nail beds, weight loss, stomatitis, and normocytic anaemia. At
necropsy, the bone marrow was hypocellular with cytoplasmic vacuoles
in erythroid precursor cells. Histopathological lesions included
dilatation of the tarsal gland ducts, atrophy, or absence of, splenic
and lymphonodal germinal centres, bone marrow depletion, gingival
erosion and ulceration, moderate mucinous hypertrophic gastropathy
with cystic dilatation of occasional gastric glands, hepatocellular
enlargement and necrosis, hypertrophy of biliary duct epithelium,
hyperplasia of biliary ducts, hypertrophy of the gall bladder
epithelium, and an equivocal increase in the number of lysosomes in
the thyroid follicular epithelial cells. The terminal PCBs
concentrations in a number of organs were as follows: adipose tissue
106.7-2073.2 mg/kg (in control animals 0.26-0.65 mg/kg); brain,
31.2-252.0 mg/kg; kidneys, 85.4-964.1 mg/kg; and liver,
255.1-828.1 mg/kg tissue. The PCB concentrations were expressed on a
mg/kg fat basis. It was concluded that skin appendicular lesions are
good clinical indices of PCB exposure in monkeys and that
lymphoreticular lesions (atrophy and absence of lymphoid follicular
centres) are good indicators of impending or active immunological
crisis (Tryphonas et al., 1986a).
(iv) Miscellaneous studies
Accidental exposure of a colony of 256 Rhesus monkeys to PCBs in a
concrete sealant produced a disease characterized by high mortality,
gradual weight loss, behavioural changes, alopecia, acne, facial
oedema, swollen eyelids, diarrhoea, anaemia, poor breeding
performance, and high incidences of abortions and still births.
Samples of the concrete slabs within several buildings were obtained
and analysed. Significant levels of PCBs (5280 mg PCB/kg sample) were
found (Altman et al., 1979; McConnell et al., 1979).
The effects of exposure to PCBs on the eyes were investigated by
Ohnishi & Kohno (1979), who administered a banana injected with 0.5 mg
of PCBs/kg body weight, daily, to 8 adult Rhesus monkeys of both sexes
for 1-5 months. Two out of this group were fed PCBs with
polychlorinated dibenzofurans (2.5 µg/kg body weight). Four untreated
animals were used as controls. One month after the onset of the
exposures there was a reduction in body weight. When pressure was
applied to the eyelids of treated monkeys, white secretions extruded.
Within 3 months alopecia, swelling of the eyelids, and acne-form
eruptions developed. The retina and choroid were normal. The
histopathological changes in the eyelids were comparable in both
groups of exposed monkeys and included the appearance of keratinous
cysts and atrophy of the Meibomian glands with hyperkeratosis and
hyperplasia of the ductal epithelium.
Cynomolgus monkey
Groups of 5 or 6 adult, female Cynomolgus monkeys (Macaca
fascicularis) were exposed to 3, weekly doses of 4.7 mg of Aroclor
1248/kg body weight (equivalent to 2 mg/kg per day) or to 3-weekly
doses of 11.7 mg of Aroclor 1254/kg body weight (equivalent to 5 mg/kg
per day) in an apple juice-corn oil emulsion. The monkeys were exposed
until necropsy at day 30-164 in dead or moribund condition. A control
group contained 5 monkeys. In both exposed groups, body weight loss,
emaciation, facial oedema, finger-nail loss, and lacrimation were
observed. Common histopathological lesions were: dilated Meibomian
gland ducts, mucinous hyperplasia and hypertrophy of the gastric
mucosa, enlargement, fatty degeneration, and necrosis of hepatocytes,
bile duct and gall bladder epithelial cell hypertrophy and
hyperplasia, and thyroid changes in follicular cell size and the
number of intracytoplasmic lysozomes.
The onset of the signs and lesions of toxicity was not as rapid and
uniform as that in Rhesus monkeys. Aroclor 1248 appeared more toxic
than Aroclor 1254 (Tryphonas et al., 1984).
Groups of 4 adult, Rhesus and 4 adult, Cynomolgus female monkeys,
weighing 3.2-4.5 and 3.2-5.2 kg, respectively, received doses of
Aroclor 1254 at 0 or 280 µg/kg body weight for 5 days/week (equivalent
to 200 µg/kg per day) in an apple-juice-gelatin-corn oil emulsion for
27-28 and 12-13 months, respectively. This study showed that the
Rhesus monkey is more susceptible to PCB toxicity than the Cynomolgus
monkey (Tryphonas et al., 1986b).
Hori et al. (1982) exposed 1 female, Cynomolgus monkey to daily doses
of Kanechlor 400 (without detectable quantities of polychlorinated
dibenzofurans) at 2 mg/kg body weight, in olive oil. They also exposed
3 monkeys to a PCB mixture with a chromatographic pattern similar to
that of the Yusho mixture (2 or 4 mg/kg body weight), and 1 monkey to
2 mg/kg body weight of the same mixture, without detectable quantities
of polychlorinated dibenzofurans (detection limit not given). The
doses were administered 6 times per week in a piece of banana. Two
controls received only the vehicle. The 2 monkeys receiving the Yusho
mixture at 4 mg/kg body weight died within 4 and 8 weeks,
respectively. The other monkeys were kept for 20 weeks. In all monkeys
exposed to the Yusho mixture, toxic effects were similar to those
already described above. In addition, there was cytoplasmic
vacuolation and dilatation of the convoluted tubules with cytoplasmic
casts in the kidneys. The other 2 mixtures induced less severe
reductions in body weight gain, immunosuppression and
histopathological alterations in liver, kidneys, and periorbital skin.
The effects in the animals fed a diet with dibenzofurans yielded
enhanced decreases in body weight, immunosuppression, fatty liver and
histological changes, in addition to hair loss, acne-form eruptions,
oedema of the eyelids and cornification of the skin, compared with the
other test substances.
8.2.1.2 Individual congeners
(a) Monkey
Rhesus monkeys have been exposed to various congeners, as outlined in
Table 43.
Table 43. Toxicity of PCB-congeners in Rhesus monkeysa
Congenerb No. of Exposure Time clinical Deaths
animals/ toxicity
group mg/kg period first noted
diet (days) (day)
2,5,4'-TriCB 4 5 84 - 0
3,4,3',4'-TCB 3 3=>1c 215 14-21 3
5 1 38 27 1
2,5,2',5'-TCB 3 3=>1c 215 - 0
5 1=>5d 200 - 0
3,4,5,3',4',5'-HCB 1 0.1 127 117 1
4 0.5 63 28-30 4
1 1 57 20 1
2,4,5,2',4',5'-HCB 4 15 122 - 0
1 65 63 - 0
2,4,6,2',4',6'-HCB 4 15 122 - 0
1 65 64 - 0
2,3,6,2',3',6'-HCB 4 15 122 - 0
a From: McNulty et al. (1980); McNulty (1985); Iatropoulos et al. (1977).
b TriCB = trichlorobiphenyl; TCB = tetrachlorobiphenyl; HCB = hexachlorobiphenyl.
c Dietary level reduced after 23 days.
d Dietary level raised after 133 days.
No toxicity could be demonstrated either by clinical appearance or by
histopathological examination for isomers with 2 or 4 chlorine atoms
ortho to the biphenyl bridge. The clinical signs observed in monkeys
exposed to 3,4,3',4'-tetrachlorobiphenyl (up to 3 mg/kg diet) and
3,4,5,3',4',5'-hexachlorobiphenyl (at 1 mg/kg diet) were similar in
character and severity to those produced by Aroclor 1242 (at 100 mg/kg
diet) and Aroclor 1248 (at 25 mg/kg diet) in monkeys (Allen et al.,
1974a). The same histopathological lesions were found. The lesions of
the skin and eyes were described as an expression of squamous atrophy
or squamous cyst formation of the sebaceous glands. The epithelial
changes in the skin and nails were found to be reversible. In this
study, 2,5,2',5'-tetrachlorobiphenyl did not produce any clinical or
pathological lesions at 3 mg/kg diet. Aroclor 1242 and 1248 were
reported to contain about 0.24 and 0.34% of 3,4,3',4'-tetra-
chlorobiphenyl, and it was concluded that this congener could account
for some of the toxicity of the commercial mixtures (McNulty et al.,
1980; McNulty, 1985).
Rhesus monkeys exposed to 2,5,4'-trichlorobiphenyl showed a reversible
primary injury of the arterioles, capillaries, and venules in the
adrenal glands, kidneys, liver, brain, and lungs (Iatropoulos et al.,
1977).
(b) Other animal species
In studies on rats and mice, individual PCB-congeners caused adverse
effects on the liver, spleen, and thymus. The most toxic compounds
were the planar congeners 3,4,3',4'-tetrachlorobiphenyl,
3,4,5,3',4'-pentachlorobiphenyl and 3,4,5,3',4',5'-hexachlorobiphenyl.
Biocca et al. (1981) and Aulerich et al. (1985) compared the
toxicities of various hexachlorobiphenyl isomers in mice. Male C57
BL/6 mice were exposed via the diet to 0.3, 1, 3, 10, 30, 100, or
300 mg 3,4,5,3',4',5'-hexachlorobiphenyl; 10, 30, 100, or 300 mg
2,4,5,2',4',5'-hexachlorobiphenyl, 2,3,6,2',3',6'-hexachlorobiphenyl,
or 2,4,6,2',4',6'-hexachlorobiphenyl/kg diet for 28 days. There were
marked differences in dose response and in the severity of the
pathological effects among the isomers. 3,4,5,3',4',5'-Hexa-
chlorobiphenyl was the most toxic isomer causing mortality, and body
and organ weight effects at all dose levels and was the only isomer
that produced excess porphyrin accumulation. It was also the isomer
that occurred in the highest concentration in the fat and the liver.
3,4,5,3',4',5'-Hexachlorobiphenyl caused subcutaneous oedema,
enlargement of the liver with accentuated hepatic lobular markings,
fatty infiltration, hepatocellular swelling and necrosis, and atrophy
of the thymus. The other 2 isomers caused the same lesions, but to a
lesser extent.
In the mice, the overall order of toxicity was 3,4,5,3',4',5'-
hexa-chlorobiphenyl > 2,4,6,2',4',6'-hexachlorobiphenyl >
2,4,5,2',4',5'-hexachlorobiphenyl > 2,3,6,2',3',6'-hexachlorobiphenyl,
based on the effects on mortality and growth, and on histopathology.
8.2.2 Intraperitoneal: reconstituted PCB mixtures
Bandiera et al. (1984) administered reconstituted mixtures of PCDFs
and reconstituted mixtures of PCBs, by intraperitoneal injection, to
immature Wistar rats, to determine the effects on weight loss, thymic
atrophy, and the induction of P-448 dependent monooxygenases. The
mixtures consisted of compounds that persisted in the blood and liver
of Yusho patients. From the results, it was clear that the PCDFs were
600 to 2100 times more toxic than the PCBs.
A group of 4, one-month-old, male Wistar rats received
intraperitoneally a reconstituted PCB mixture containing 13 of the
major congeners that have been identified in human milk, at the
corresponding relative concentrations. The mixture was injected on
days 1 and 3 in corn oil at dose-levels of 0, 0.45, 0.90, 4.5, or
45 mg/kg body weight. The rats were killed on day 6 for
histopathological studies. At the highest exposure level, increased
relative liver weights and enlarged and vacuolated hepatocytes were
observed together with changes in nuclei. In the thyroid, a mild
reduction in follicular size, focal collapse, and changes in nuclei
were found. No changes were seen with 0.9 mg/kg body weight (Gyorkos
et al., 1985) (see section 8.8.1.1).
8.2.3 Dermal exposure
(a) Aroclors
Solutions of Phenoclor DP6, Clophen A60, or Aroclor 1260 in
isopropanol were applied in doses of 118 mg on 50 cm2 of the shaved
back skin of groups of 4 New Zealand rabbits, 5 times per week, for 38
days. A group of 4 rabbits received the vehicle only. After initial
reddening, transverse wrinkling and thickening of the skin developed
with hyperplasia and hyperkeratosis of the epidermal and follicular
epithelium. These effects were more marked with Clophen and Phenoclor
than with Aroclors. During the study, deaths occurred in the Clophen-
and Phenoclor-treated groups. Body weights and relative kidney weights
were decreased in the Aroclor-treated rabbits. Histological liver
changes were least marked in the Aroclor-treated rabbits. Treated
rabbits had fluorescing livers and bone under UV radiation and also
showed other evidence of porphyria. In the kidneys, hydropic
degeneration of the convoluted tubuli, and tubular dilatation were
found. There was atrophy of the thymic cortex and a reduction of
germinal centres of the lymph nodes, as well as leukopenia, and some
animals in all groups showed oedema of the abdominal and thoracic
cavities, subcutaneous tissue, and pericardium. Faecal elimination of
copro- and protoporphyrine was increased by all 3 PCBs, but was lowest
with Aroclor 1260 (Vos & Beems, 1971).
Puhvel et al. (1982) exposed groups of 3 female, hairless mice of 2
strains, (Skh:HR-1 and HRS/J), topically to Aroclor 1254 (4 doses of 1
or 8 mg/week, for 6 weeks) or Phenoclor 54 (5 doses of 0.2 mg/week,
for 10 weeks) in acetone or an acetone-mineral oil-Tween 80 emulsion,
or to the vehicle only. Punch biopsies of the skin were taken
regularly. The skin of treated mice appeared grossly normal after the
exposures, but examination of microscopic skin samples of
Phenoclor-treated mice showed hyperkeratosis of the stratum corneum,
epidermal hyperplasia, disappearance of sebaceous glands, and the
presence of numerous keratinous cysts. No histological changes were
observed in the internal organs.
(b) Individual congeners
In the study of Puhvel et al. (1982), described above, groups of 3
hairless mice of both strains were also exposed topically to 5 doses
of 0.2 mg 3,4,3',4'-tetrachlorobiphenyl/week, for 10 weeks. Grossly
there were no visible changes. The histological changes induced in
HRS/J mice were similar to those found after Phenoclor treatment.
However, identical changes induced in Skh:HR-1 mice were already
observed by 4 weeks. After 8 weeks, these lesions were more marked and
also included hyperkeratosis of the sebaceous follicles and
hyperkeratinization of intradermal pilar cysts. Often these cysts
ruptured into the dermis leading to dense infiltrates of
polymorphonuclear leukocytes. The treated mice showed weight gain,
primarily because of large intra-abdominal fat deposits.
In a dermal toxicity study on rabbits, with a protocol identical to
that of the study of Vos & Beems (1971), skin lesions on animals
treated with 2,4,5,2',4',5'-hexachlorobiphenyl were less severe than
those on Aroclor 1260-treated animals. The liver damage observed
histologically was essentially the same after treatment with either
Aroclor 1260 or 2,4,5,2',4',5'-hexachlorobiphenyl, but the individual
congener was more porphyrogenic (Vos & Nootenboom-Ram, 1972). In
another study, 4 applications of a 25% solution of 3,4,3',4'-
tetrachlorobiphenyl in olive oil to the inner surface of the ears
of rabbits resulted in the same lesions that were found after 2
applications of undiluted Kanechlor 400 or 500. The lesions included
hyperkeratosis, dilatation of hair follicles, and the formation of
keratinous cysts (Komatsu & Kikuchi, 1972).
8.2.4 Appraisal
Rhesus monkey is the most sensitive test species with regard to the
general toxicity of PCBs, both as a mixture and as individual
congeners. The toxicity of mixtures may be confounded by the presence
of impurities, such as PCDFs, which are, or may have been, present in
the mixtures tested. PCBs induce some of the biological and
toxicological effects qualitatively similar to those induced by PCDFs.
Another confounding variable in these studies is the difference in the
composition of the mixtures (Aroclor 1242, 1248, 1254) used.
Beating the above in mind, the available data show that Aroclor 1248,
containing 4.4-8.7 ng of PCDFs/kg, still showed adverse general toxic
effects in Rhesus monkeys at a dose of 0.1 mg/kg diet per day
(0.09 mg/kg body weight per day) administered for an average of 18.2
months (Bowman et al., 1981). A NOEL for general toxicity was not
established for Aroclor 1248. The NOEL for the general toxicity of
Aroclor 1242 was 0.04 mg/kg body weight per day, as established after
dietary exposure for 133 days. The main effects induced by Aroclor
1248 at 0.09 mg/kg body weight per day were an increased mortality
rate, growth retardation, alopecia, acne, swelling of the Meibomian
glands, and, possibly, immunotoxicity. Microscopically, enlarged
hepatocytes, fatty liver with focal necrosis, and epithelial
hyperplasia and keratinization of hair follicles were found. These
effects appeared reversible. Aroclor 1254 at a dose level of
0.200 mg/kg body weight per day showed several effects, not reported
for 1248 (lymforeticular lesions, finger-nail detachment, gingival
effects) and vice-versa (acne, alopecia), which could be related to
the confounding variables noted above. Several effects observed in the
monkeys exposed to Aroclor 1254 (hypertrophic gastropathy, bone marrow
hyperplasia) were also observed in monkeys exposed to Aroclor 1248,
but at a higher dose (4 mg/kg body weight per day). In contrast with
the severe effects observed in adult Rhesus monkeys at low doses,
relatively mild effects were shown by suckling Rhesus monkeys exposed
to much higher doses.
8.3 Skin and eye irritation, sensitization
The injury to skin and eyelids following oral and/or dermal exposure
to PCBs has been discussed in sections 8.2.1 and 8.2.3.
8.4 Reproduction, embryotoxicity, and teratogenicity
8.4.1 Reproduction and embryotoxicity
8.4.1.1 Oral
(a) Mouse
In castrated, mature, male NMRI mice (10-13 per group) that received
28 daily doses of 0.25 mg Aroclor 1254/mouse, in peanut oil, the
weight of the seminal vesicles was decreased, but this was not seen in
intact mice (Orberg & Lundberg, 1974).
When 23 adult female NMRI mice were mated with 22 control males and
orally intubated with 0.025 mg Clophen A60/day, in peanut oil, for 62
days prior to mating and up to days 8-10 of gestation, blastocytes
failed to implant. Twenty-five (14 experimental and 11 control
animals) out of the 45 animals were used to study the effects of PCBs
on the estrous cycle. Effects, such as prolonged estrous cycle and
less frequent periods of sexual receptivity and a decline in the
number of implanted ova, were found (Orberg & Kihlström, 1973).
In order to study the effects of PCBs on the development of sexual
functions in the early postnatal period, these authors also mated mice
that had been suckled by mothers dosed with Clophen A60 during the
lactation period. A decrease in the frequency of implanted ova was
noted, when both parents of the couple had been suckled with milk
containing PCBs. When adult female NMRI mice received 50 mg of Clophen
A60/kg body weight once per week, subcutaneously, during lactation,
the same effect was observed in the offspring after mating with
similarly exposed males (Kihlström et al., 1975).
(b) Rat
In a 2-generation reproduction study, groups of 10 male and 20 female
Sherman weanling rats were fed diets containing 1, 5, 20, or 100 mg of
Aroclor 1254/kg (equivalent to 0.06, 0.32, 1.5, and 7.6 mg/kg body
weight, respectively), or diets containing 5, 20, or 100 mg/kg of
Aroclor 1260 (equivalent to 0.39, 1.5, and 7.5 mg/kg body weight,
respectively). Control groups comprised 20 male and 40 female rats.
The F0 rats were started on the diet at 3-4 weeks of age and the F1
rats, at weaning. The F0 rats were pair-mated when 3 and 7 months old
to produce the F1a and F1b generations, respectively. Breeding-stock
F1b rats were selected at weaning and pair-mated when 3 months old to
produce the F2a, and, when 8 months old, the F2b generation. Rats
exposed to Aroclor 1254 at 20 mg/kg diet or more showed a reduced
litter size at birth, but not when exposed at weaning, in the F1b and
F2 generations. At 100 mg/kg diet, the number of litters in the F2
generation was decreased. In 2 F2a and 2 F2b litters no live offspring
were found at birth, while pup survival at weaning was reduced in the
F2a generation. At weaning, exposed F1a pups weighed less than their
controls. Increased relative liver weights were found in male F1a
weanlings at 1 mg Aroclor 1254/kg diet and in all weanlings at 5 mg/kg
diet or more. Adult rats showed increased relative liver weights at
levels of 20 mg/kg diet or more. No reproductive effects were found
with 5 mg Aroclor 1254/kg diet. In groups treated with Aroclor 1260,
increased liver weights were found in all weanlings at 5 mg/kg diet or
more, but no effects on reproduction were seen, even at 100 mg/kg diet
(Linder et al., 1974).
The only effect observed in Holtzman rats fed Aroclor 1254 at a level
of 500 mg/kg diet, for 3 weeks, was an increase in relative testes
weights (Garthoff et al., 1977). Increased absolute testes weights and
unchanged body weights were found in 6-month-old offspring of
Sprague-Dawley dams exposed to daily doses of 30 mg Aroclor 1260/kg
body weight in ethanol-sesame oil on days 14-20 of gestation. No
effects on testes weight were found in animals treated with Aroclor
1221 or Aroclor 1242 (Gellert & Wilson, 1979).
Sager (1983) evaluated the effects on the reproductive function of
adult male Holtzman rats following exposure of their mothers to doses
of 8, 32, or 64 mg Aroclor 1254/kg body weight in peanut oil, on days
1-3, 5, 7, or 9 of lactation. At all dose levels, 165-day-old males
showed a decreased relative ventral prostate weight, and, at the 2
higher doses, a decreased relative weight of seminal vesicles and
testes as well as decreased body weight. In the ventral prostate,
alveoli were decreased in number and showed folding of the mucosa and
flattened epithelial cells. At 130 days of age, the male offspring at
all 3 dose levels showed a reluctance to mate with control breeders,
leading to a decreased number of pregnancies. Moreover, at the 2
higher dose-levels, the females showed a reduced number of
implantations and an increased resorption rate. The litters showed a
reduced weight gain up to 11 days of age.
This study was repeated with an evaluation of the reproductive
performance of the second generation male rats from 130 days of age,
following mating with normal females. In the first study, autopsy on
pregnant females was carried out on day 11 or 12 of gestation. The
females had fewer implants, fewer embryos, and a reduced proportion of
ovulated eggs that implanted, compared with controls. The effects were
dose-related. In a second study, females mated to the same males were
autopsied on day 2 or 4 after mating. Sperm counts were not affected.
At the 2 highest doses, fewer females had eggs in the expected state
of development, the average number of blastocytes found in one uterine
horn on day 4 was reduced, and an increased incidence of abnormally
developed embryos was observed (Sager et al., 1987).
Female Wistar rats exposed to daily doses of 10 mg Aroclor 1254/kg
body weight, for at least one month, showed a prolonged estrous cycle,
decrease in sexual receptivity, delay in timing of copulation, vaginal
bleeding during gestation, decrease in litter size, and delay in the
time of parturition. After mating of the rats to control males, the
female offspring, exposed in utero and during lactation, showed a
slower rate of body weight gain, higher mortality, earlier vaginal
opening, and a delay in the appearance of the first estrous cycle
(Brezner et al., 1984).
(c) Monkey
Groups of 9 adult female Rhesus monkeys were exposed to a diet
containing Aroclor 1248 (containing polychlorinated dibenzofurans) at
levels of 2.5 or 5.0 mg/kg (Barsotti et al., 1976; Allen et al., 1979,
1980). The study and the maternal toxicity data have already been
described in section 8.2.1.6. Within 4 months, menstrual bleeding and
the duration of the menstrual cycle were increased. Flattening and
prolongation of the serum progesterone peak during the menstrual cycle
was observed. After 6 months of exposure, the females were mated with
control males. Reproductive dysfunction was obvious as shown in Table
44. Following the total exposure period of 18.2 months, the mothers
were put on a control diet. Their menstrual cycles and serum
progesterone levels returned to pre-exposure values. One year after
exposure ceased, the females were again mated with control males and
showed a recovery of their reproductive status (Table 44; Allen et
al., 1980).
Other groups of adult, female Rhesus monkeys were continuously fed
diets with 0, 0.25, and 1.0 mg Aroclor 1016/kg (equivalent to 0, 0.01
and 0.04 mg/kg body weight, respectively), in which no polychlorinated
dibenzofurans were detected (no details). No maternal toxicity was
noted at these levels. In this study, the females were mated with
control males after 7 months of exposure. Reproductive dysfunction
was not observed. Decreased birth weights were found in the offspring
of mothers exposed to Aroclor 1016 at 1.0 mg/kg diet. Skin
hyper-pigmentation occurred in both exposure groups (Barsotti & Van
Miller, 1984). Preliminary reports have indicated possible effects on
learning and behavioural tasks. The milk contained an average of 1.45
and 3.92 mg/kg fat at 0.25 and 1.0 mg/kg, respectively, whereas the
serum of the mothers contained 0.012 and 0.027 mg/litre, respectively
(Heironimus et al., 1981; Levin & Bowman, 1983).
Table 44. Reproductive status of Rhesus monkeys exposed to dietary levels of Aroclor 1016 or 1248
PCB Exposure level Total intake Conceptions Abortions Stillborn Live Reference
mixture at and births
(Aroclor) Diet Body conception resorptions
(mg/kg) weight (mg/kg)
(mg/kg)
1016 0 0 0 8/8 0/8 0/8 8/8 Barsotti &
0.25 0.01 8c 8/8 0/8 0/8 8/8 van Miller
1.0 0.04 30c 8/8 0/8 0/8 8/8 (1984)
1248 0 0 0 12/12 0/12 0/12 12/12 Barsotti et al.
2.5 0.09a 105 8/8 3/8 0/8 5/8 (1976)
5.0 0.2 210 6/8 4/8 1/8 1/8
1248 0 0 0 8/8 0/8 0/8 8/8 Allen et al.
2.5 0.09a 270 8/8b 1/8 0/8 7/8 (1980)
5.0 0.2 498 7/7b 1/7 2/7 4/7
a From: Bowman et al. (1981) amended.
b Breeding 1 year after exposure.
c Calculated assuming a body weight of 5 kg.
In these studies, the monkeys were maintained on the diets during the
gestation and lactation of the first generation. PCBs are known to
cross the placenta and to be excreted via breast milk (see section
6.3). The 6 infants born to monkeys during exposure at 2.5 or 5.0 mg
Aroclor 1248/kg diet showed decreased birth weights, a small stature,
and a decreased body weight gain during nursing. Within 2 months,
signs of intoxication appeared including acne, increased skin
pigmentation, swelling of the eyelids, and loss of eyelashes. In 3
milk samples, values ranging from 0.154 to 0.397 mg PCBs/kg milk were
measured, and, 1 milk sample contained 16.44 mg PCBs/kg fat. Three
infants died. Necropsy and histopathology revealed atrophy of the
thymus and lymph nodes, hypocellular bone marrow, moderate fatty
infiltration of the liver, hypertrophic Meibomian glands, and
keratinization of hair follicles. One dead infant showed hyperplasia
of the gastric mucosa. The 3 surviving infants were weaned and
subsequently showed marked improvements in their physical status
(Allen & Barsotti, 1976; Allen et al., 1979, 1980). At 6 and 12 months
of age, they were found to be hyperactive in a locomotor activity test
and, between 7 and 24 months of age, they did not learn reversal tasks
as readily as the controls. The PCB body burdens of these infants
ranged between 11 and 27 mg/kg body fat, at the age of 8 months, and
dropped to 0-1.6 mg/kg, at the age of 23 months. However, using the
same apparatus, these infants showed hypolocomotor activity at 44
months of age in comparison with the same controls (Bowman et al.,
1978; Bowman & Heironimus, 1981; Bowman et al., 1982; Levin & Bowman,
1983).
The infants delivered by the same adult females, after 1 year on a
control diet, showed a reduced body weight and signs of intoxication
similar to those observed in their siblings of the first breed.
Two infants in each exposed group died. Milk samples contained
0.02-0.19 mg PCBs/kg milk (Allen et al., 1980). At 12 months of age,
when the PCB body burdens were only slightly higher than those of the
controls, the infants showed hyperlocomotor activity (Bowman et al.,
1982).
Groups of 4 or 6 Rhesus monkeys, which had been exposed to 0 or 2.5 mg
Aroclor 1248/kg diet in utero and during nursing until 4 months
after birth, were tested at 4-6 years of age on delayed spatial
alternation (DSA), a spatial learning and memory task. Deficits in
performance accuracy were detected in 2 cohorts of monkeys, whose
mothers had been fed 2.5 mg Aroclor 1248/kg diet for an 18-month
period ending at least 12 months prior to pregnancy. The deficit was
most apparent at the shorter delays, suggesting impairments in
association or attention processes were involved rather than memory
impairment. Such a deficit was also found in monkeys fed 1.0 mg
Aroclor 1016/kg diet, but the effect was less pronounced. The
appearance of a PCB-induced cognitive deficit more than 3 years after
the end of exposure indicated the existence of long-term adverse
consequences of perinatal PCB exposure (Levin et al., 1988).
Clophen A30, which did not contain detectable levels of
polychlorinated dibenzofurans (limit of detection <1 mg/kg), was
given by gavage in a 1% solution of methylcellulose in water, once
daily for 30 days, to 3 lactating Rhesus monkeys and their offspring
at a level of 16 mg/kg body weight. PCB concentrations were measured
in the serum of both mothers and infants and in the milk, on days -14,
-7, 0, 1, 2, 4, 8, and then at weekly intervals until the end of the
study. The mean PCB concentrations in the serum of mothers and infants
were between 0.13 and 1.16 mg/litre and 0.07 and 2.67 mg/litre,
respectively (before treatment days -14 and -7). The mean PCB levels
in milk ranged from 0.63 mg/kg on day 1 of exposure to 18.90 mg/kg. On
days -14 and -7, the concentrations in milk were 0.14 and 0.34 mg/kg
(wet weight). Five control pairs were used. One dam and her offspring
were sacrificed on day 22, exhibiting symptoms of anorexia,
depression, lethargy, and ataxia. Two of 3 infants showed a decreased
body weight gain. At autopsy of the infants, after the exposure
period, slight degenerative changes were seen in the liver and the
kidneys, together with slight demyelination of the central nervous
system, slight to moderate gliosis of the cerebrum, and slight
granular cell layer thinning of the cerebellum. On the basis of
earlier work with adults in which the degenerative changes described
above were considerable, the authors concluded that the nursing
infants seemed to be less susceptible than the adults under the
conditions of the studies (Iatropoulos et al., 1978; Bailey et al.,
1980).
8.4.2 Teratogenicity
8.4.2.1 Aroclors (oral)
(a) Mouse
Haake et al. (1987) reported that treatment of pregnant C57Bl/6 mice
with Aroclor 1254, by gavage, at 224 mg/kg body weight, on day 9 of
gestation, did not result in any fetuses with cleft palate (see
section 8.6.6).
(b) Rat
Wistar rats were exposed to dietary levels of Kanechlor 400 of up to
250 mg/kg from day 1 to day 21 of gestation (Mizunoya et al., 1974).
Fetuses showed decreased body weights from 10 mg/kg diet onward
(equivalent to 0.67 mg/kg body weight), but did not show any increased
incidence of malformations. Maternal toxicity was not observed and
litter size and the number of litters were unaffected. Decreased pup
survival was noted at dietary levels from 50 mg/kg (equivalent to
3.5 mg/kg body weight) upwards. The 28-day-old offspring showed
decreased body weight and increased relative liver weight from
10 mg/kg.
Commercial Kanechlor 300 or 500 was mixed with food and administered
to pregnant Sprague-Dawley rats, throughout gestation, at levels of
20, 100, or 500 mg/kg diet. On day 21, about three-quarters of the
pregnant females were sacrificed; the remainder were allowed to litter
naturally and the postnatal development of the pups was observed.
Kanechlor 500 at a concentration of 500 mg/kg resulted in decreased
maternal weight gain and decreased food consumption. At 20 and 500 mg
Kanechlor 300/kg, and 500 mg Kanechlor 500/kg, the fetal weight
decreased significantly. Resorption and malformations were not
increased by treatment with Kanechlors. The Kanechlors did not show a
teratogenic potential in this study (Shiota, 1976a).
Offspring of Sprague-Dawley rats that received 20 mg of Kanechlor
500/kg body weight on days 15-21 of gestation were slower than
controls in achieving the water maze test at the age of 12-13 weeks,
but did not perform worse in the open field test and in the swimming
test (Shiota, 1976b). Behavioural effects were also observed in the
offspring of ICR dams, 23-27 days of age, exposed to Aroclor 1254 in
the diet at levels of 11 or 82 mg/kg (equivalent to 1.7 and 12 mg/kg
body weight) from 3 days before mating up to weaning. The offspring
were maintained on the same diet. PCB exposure did not have any
effects on the ability to learn an avoidance response, but increased
the latency to make such a response. Moreover, the young mice
exhibited slower habituation to an open field (Storm et al., 1981).
The effects of Fenchlor 42 (trichloro- 63%, tetrachloro- 33%, and
small amounts of dichlorobiphenyl; purity 97.5%) exposure of Fischer
344 male and female rats were studied through assessment of the
behavioural development of their F1 progeny. Female rats were
administered 5 daily ip injections of corn oil or 5-10 mg Fenchlor
42/kg body weight per day, 2 weeks prior to mating. Another group
received 2-4 mg/kg per day during gestation (days 6-15 of pregnancy)
and a third group of 8 previously treated lactating females received
corn oil or 1-2 mg/kg per day on postnatal days 1-21. The total doses
in the 3 groups were 25-50, 20-40 and 20-40 mg/kg body weight.
Dose-dependent differences in behaviour were found in the offspring of
the PCB-treated animals. Differences in the development of cliff
avoidance reflexive behaviour, swimming ability, and open field
activity were evident. The PCB exposure of female animals during
gestation and lactation resulted in impaired acquisition of the active
avoidance behaviour, while preconception PCB exposure affected active
avoidance performance, as reflected in an increased number of
avoidance responses to reach criterion for extinction (Pantaleoni et
al., 1988).
Doses of 0, 6.25, 12.5, 25, 50, or 100 mg/kg body weight per day of
Aroclor 1254 were administered to rats, by gavage, on days 6-15 of
gestation. Average pup weights were reduced at 100 mg/kg, though total
litter weight (average weight times number of fetuses) did not differ
from controls. There were no skeletal or visceral abnormalities or
effects on conception, resorptions, litter size or number, or average
litter weight in any of the treated groups (Villeneuve et al., 1971b).
Spencer (1982) exposed Holtzman rats to a diet containing Aroclor 1254
at levels of 25 up to 900 mg/kg diet from day 6 to day 15 of gestation
and found reduced maternal body weight gain and decreased fetal
survival at birth from 300 mg/kg diet (equivalent to 18 mg/kg body
weight) upwards, and decreased fetal weights at birth from 100 mg/kg
(equivalent to 8 mg/kg body weight) upwards. No visceral or skeletal
data were available.
When Sherman rats were exposed to doses of Aroclor 1254 of up to
100 mg/kg body weight, in peanut oil, from day 7 to day 15 of
gestation, a decrease in the survival of the pups was found. At
weaning, the survival and body weight of the grossly normal pups were
reduced at the 100 mg/kg dose level, but not at 50 mg/kg body weight.
No effects were observed at 100 mg Aroclor 1260/kg body weight (Linder
et al., 1974).
(c) Monkey
Two pregnant Cynomolgus monkeys (Macaca fascicularis) were dosed
with Aroclor 1254 at 100 mg/kg body weight per day and 1 monkey, at
400 mg/kg body weight per day, from day 60 of gestation. One control
animal received the vehicle, corn oil. The 2 monkeys fed 100 mg/kg
delivered dead male infants after 105 and 108 days of dosing, and the
female fed 400 mg/kg delivered a female infant (with no overt clinical
signs of toxicity) that died at 139 days of age with an acute
bronchopneumonia. The breast milk of the monkey fed 400 mg/kg
contained, over a period of 5-75 days after parturition,
concentrations of 73.7 up to 139.4 mg/kg, on a fat basis. No overt
signs of toxicity were observed in the adult animals, with exception
of finger nail loss. All 3 treated monkeys showed impaired
immunological capacity, assessed at approximately 50 days postpartum
(148 days of treatment) (Truelove et al., 1982).
(d) Rabbit
Rabbits were exposed to 0, 1.0, or 10.0 mg Aroclor 1254/kg body weight
and in another study to 0, 12.5, 25.0, or 50 mg/kg body weight (purity
not reported), by gavage, on days 1-28 of pregnancy. Abortions, still
births, and maternal deaths occurred at 12.5 mg/kg body weight or
more, but no teratogenic effects were found (Villeneuve et al.,
1971a,b).
8.4.2.2 Aroclors (subcutaneous)
(a) Mouse
A possible teratogenic effect was observed in ddy mice subcutaneously
exposed to doses of 1-5 mg Kanechlor 500/mouse (equivalent to
40-200 mg/kg body weight) in 95% ethanol from day 6 to day 15 of
gestation. A dose-related increase in maternal mortality was observed
from a dose of 3 mg/mouse onwards. Some dams showed skin lesions,
alopecia, or swelling of the liver, but no effects on body weight
gain. A slight increase was noted in the incidence of dead and
resorbed fetuses. The incidence of cleft palate was increased in a
dose-related manner from the lowest dose (Watanabe & Sugahara, 1981).
8.4.2.3 Individual congeners (oral)
(a) Mouse
Orberg (1978) fed groups of 20-56 pregnant female NMRI-mice 0, 0.05,
or 0.5 mg 2,5,4'-trichloro- or 2,4,5,2',4',5'-hexachlorobiphenyl,
dissolved in peanut oil, per animal, from days 1 to 6 of gestation. A
significant decrease in the pregnancy of implanted ova was found with
the 0.5 mg treatment. No effects on percentage of pregnancies and mean
number of corpora lutea were found. There were no effects at the lower
dose level.
A dose-related increase in embryotoxicity and the incidence of
malformed fetuses, mainly showing cleft palate and hydronephrosis, was
found in pregnant CD-1 mice after exposure to daily doses of 2, 4, 8,
or 16 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body weight, in
cotton-seed oil, on days 6-15 of gestation. Lower dose levels, e.g.,
0.1 and 1 mg/kg body weight were without effects. No dibenzofurans
were detectable (no details) in the test compound. The dams showed a
decreased body weight gain at 8 mg/kg body weight. The authors
reported that 3,4,3',4'-tetrachlorobiphenyl and 2,3,4,2',3',4'-hexa-
chlorobiphenyl also produced the same teratogenic effects, though they
were less potent than those of 3,4,5,3',4',5'-hexachlorobiphenyl
(Marks et al., 1981).
A neurobehavioural "spinning" syndrome (a syndrome characterized by
the fact that the mice rotate or spin in a circular motion when held
by the tail) and hydronephrosis developed in CD-1 mouse weanlings,
whose dams received, by gavage, 32 mg 3,4,3',4'-tetrachlorobiphenyl/kg
body weight, in corn oil, on days 10-16 of gestation. Maternal
neurotoxicity was not observed. Histological and ultrastructural
examination of the CNS of affected mice revealed longitudinal
projections of the cylindrical CNS in the ventral and dorsal roots
and, to a lesser extent, in cranial nerve roots. The effect was
possibly related to an observed altered development of striatal
synapses (Chou et al., 1979; Tilson et al., 1979; Agrawal et al.,
1981).
(b) Rat
The congener 3,4,3',4'-tetrachlorobiphenyl was found to be embyrotoxic
and caused accumulation of blood in the amniotic fluid and the
gastrointestinal tract of fetuses from Sprague-Dawley rats treated on
days 6-18 of gestation with doses of 3 or 10 mg/kg body weight in corn
oil. Decreased fetal growth was also observed (Wardell et al., 1982).
When the rats were allowed to deliver, high perinatal mortality was
observed, which appeared to be related to an increase in gestational
length and to be independent of the smaller total litter size. In
addition, pup weights were found to be lower than those of controls
(Rands et al., 1982; White et al., 1983).
(c) Guinea-pig, mouse
Neither embryotoxicity nor teratogenicity were found in Dunkin Hartley
guinea-pigs, and CBA mice exposed in utero to 2,4,5,2',4',5'-
hexa-chlorobiphenyl during gestation (Mattsson et al., 1981; Brunström
et al., 1982; Aulerich et al., 1985) and in C57BL/6N mice similarly
exposed to 2,4,5,2',4',5'- or 2,3,4,5,3',4'-hexachlorobiphenyl
(Birnbaum et al., 1985).
Pregnant guinea-pigs received a total dose of 100 mg technical grade
Clophen A50 orally, in peanut oil, from day 16 to day 60 of gestation,
25 mg 2,4,5,2',4',5'-hexachlorobiphenyl from day 16 to day 60 of
gestation, or 100 mg from day 22 to day 60 of gestation. The
administration of Clophen A50 resulted in fetal deaths, but no
maternal deaths. In contrast, 2,4,5,2',4',5'-hexachlorobiphenyl did
not cause fetal deaths. Prenatal weight of live fetuses was increased
by a dose of 25 mg, but not by 100 mg of 2,4,5,2',4',5'-hexa-
chlorobiphenyl (Brunström et al., 1982).
(d) Monkey
In a briefly reported study, 6 female Rhesus monkeys received 9 doses
of 70 or 350 µg 3,4,3',4'-tetrachlorobiphenyl/kg body weight by
gavage, in corn oil, from day 20 to day 40 of gestation. A control
group comprised 12 animals. Maternal toxicity (not specified) but no
mortality, was reported in all exposed monkeys from day 31 following
exposure. Between days 17 and 35 following exposure all exposed
fetuses and 3 out of 12 control fetuses aborted (McNulty, 1985).
8.4.3 Appraisal
The Rhesus monkey is the most sensitive species with regard to general
toxicity (see section 8.2) and particularly with regard to
reproductive toxicity. The presence of PCDFs and the variation in PCB
composition may be confounding factors in determining the reproductive
toxicity of PCB mixtures. Aroclor 1248, containing 4.4-8.7 µg
PCDFs/kg, adversely affected the reproductive performance of female
Rhesus monkeys, mated with control males after 6 months of dietary
exposure to a toxic dose of 0.09 mg/kg body weight per day and
continuation of the exposure for an average of up to 10 months. This
effect was reversible after an exposure-free period of 1 year. No
effect on reproduction was found in female Rhesus monkeys exposed to a
non-toxic dose of 0.01 or 0.03 mg Aroclor 1016 (reported not to
contain PCDFs)/kg body weight per day and mated after 7 months with
control males.
Neonates of the nursing mothers exposed to Aroclor 1248 showed adverse
effects similar to those seen in their mothers and, in addition,
persistent behavioural disturbances, atrophy of the thymus and lymph
nodes, bone marrow hypoplasia, and hyperplasia of the gastric mucosa.
Neonates of the mothers after the recovery period still showed adverse
effects, as well as the neonates of the mothers exposed to Aroclor
1016. These effects were caused by PCBs, with or claimed to be
without, PCDFs, transmitted via the placenta during gestation and
later via the breast milk. Neonates have much greater susceptibility
to PCB toxicity when exposed via the mothers compared with suckling
monkeys orally exposed. Reproductive toxicity has also been observed
in the mink, rabbit, and rat. The changes seemed to be related to
alterations in the serum levels of gonadal steroid hormones, as a
result of enzyme induction. PCBs may also bind to the cytoplasmic
estrogen receptor. Effects have also been observed on the estrus cycle
of female rats, minks, and monkeys, on the sex organs of male rats,
and on the implantation rate of fertilized ova following exposure of
female mice or male rats.
Comprehensive teratological examinations have not been conducted;
however, the available studies indicated that the Aroclors were not
teratogenic in rats and nonhuman primates, when tested via the oral
route during the critical periods of organogenesis at doses that
produced fetotoxicity and/or maternal toxicity. Although the
fetotoxicity of Aroclors is documented in several species of animals,
the possibility that contaminants (e.g., PCDFs) might be (partly)
responsible for the effects should be recognized.
The results of the reproduction and teratogenicity studies are
summarized in Tables 45, 46, and 47.
8.4.4 Mutagenicity and related end-points
8.4.4.1 DNA damage
PCBs have been shown to interact with the proteins, RNA and DNA, after
metabolic activation. The potential of readily metabolizable
PCB-congeners to cause primary DNA damage was indicated by the
activity of 2,5,2',5'-tetrachlorobiphenyl and its metabolites in
causing DNA, single-strand breaks in an alkaline elution assay with
L-929 cells in vitro (Stadnicki et al., 1979). Furthermore,
unscheduled DNA synthesis was elicited by 4-chlorobiphenyl in vitro
in Chinese hamster ovary cells (Wong et al., 1979). No unscheduled DNA
synthesis was elicited by Aroclor 1254 in rat hepatocytes in vitro
(Probst et al., 1981).
DNA-breaking activity was found in an alkaline elution assay with
hepatocytes of intact rats treated in vivo with a single, high dose
(500 mg Aroclor 1254/kg body weight, intraperitoneally, or 1295 mg/kg
body weight, orally) with complete repair of the damage within 48 h
(Robbiano & Pino, 1981). Aroclor 1254 was also shown to be a
DNA-breaking agent in an alkaline elution assay in vitro with rat
hepatocytes (Sina et al., 1983). An alkaline sedimentation assay
showed the DNA-breaking activity of Aroclor 1254 in rats treated
in vivo with a single intraperitoneal dose of 500 mg/kg body weight.
In this assay, the same Aroclor 1254 pretreatment of the rats in vivo
elevated the DNA-breaking activity of the direct-acting, alkylating
N-methyl- N'-nitro- N-nitrosoguanidine and the carcinogens,
dimethylnitrosamine and benzo (a)pyrene, in vitro (Mendoza-Figueroa
et al., 1985).
8.4.4.2 Mutagenicity tests
Many mutagenicity tests have been carried out over the years, with
different PCB mixtures. Most of these were commercial mixtures the
composition of which was not described. Only a few studies are
available on specific congeners. Besides studies on microorganisms,
mammalian cell point mutation, dominant lethal assays, micronucleus
tests, chromosome and cytogenicity studies, and DNA repair studies
were carried out. With a few exceptions the results of most of the
studies with PCB mixtures were negative (see Table 48).
Aroclor mixtures and the congener 2,5,2',5'-tetrachlorobiphenyl and
its metabolites did not induce point mutations in Salmonella
typhimurium TA 98, TA 100, TA 1535, TA 1537, and TA 1538 with, and
without, metabolic activation (Wyndham et al., 1976; Hsia et al.,
1978; Bruce & Heddle, 1979; Schoeny et al., 1979; Shahin et al.,
1979), neither did 2,4,2',4'- and 3,4,3',4'-tetrachlorobiphenyl and
2,4,6,2',4',6'-hexachlorobiphenyl in the strains TA 98 and TA 100
(Schoeny, 1982).
However, Wyndham et al. (1976) found a dose-related mutagenic activity
of 4-chlorobiphenyl and, to a lesser extent, of Aroclor 1221 in the
strain TA 1538, after metabolic activation by the S9 liver fraction of
uninduced rabbits. The study was repeated 3 times in the same
laboratory, but the results of Wyndham et al. (1976) could not be
confirmed (Safe, 1980). Schoeny (1982) could not detect any mutagenic
activity of 4-chlorobiphenyl in the same dose range in the strains TA
98, TA 100, TA 1535, and TA 1537, with, or without, the S9 liver
fraction of induced rats.
Table 45. PCBs: reproduction and embryo toxicity
Animal (strain, PCB mixture Exposure period NOAEL LOAEL Parameters, effects Reference
sex) (oral) (mg/kg body (mg/kg body
weight) weight)
Rat (Sherman, Aroclor 1254 continuous up to 0.06 (1254) increased relative liver Linder et al.
10 male, Aroclor 1260 weaning weights in male F1A (1974)
20 female) weanlings
0.32 (1254)
former effect in all weanlings
0.32 (1254)
7.5 (1260) reproduction parameters
reproduction parameters
Rhesus monkey Aroclor 1016 7 months 0.03 (0.04) reproduction parameters Barsotti &
(female) [0.03(0.04): decreased birth van Miller
weight] [0.01: skin (1984)
hyperpigmentation]
Rhesus monkey Aroclor 1248 6 months 0.09 abortions, resorptions, live Barsotti et al.
(female) births (1976)
1 year after 0.09 0.2 Allen et al.
exposure stillborn, live births (1980)
Table 46. PCBs: teratogenicity
Animal (strain) PCB-mixture Exposure period NOAEL LOAEL Parameters, effects Reference
(oral) (mg/kg body (mg/kg body
weight) weight)
Rat Aroclor 1254 days 6 to 15 of 50 100 reduced average pup Villeneuve et al.
gestation weight (1971b)
Rat (Holtzman) Aroclor 1254 days 6 to 15 of < 8 8 decreased fetal weight at Spencer (1982)
gestation birth
Rat (Sherman) Aroclor 1254 days 7 to 15 of 100 reproduction effects Spencer (1982)
gestation
Rabbit Aroclor 1254 days 1 to 28 of 10 12.5 abortions, still births, Villeneuve et al.
gestation maternal deaths (1971 a,b)
Aroclor 1221 days 1 to 28 of 25 fetotoxicity
gestation
Table 47. PCBs: teratogenicity of individual congeners
Animal PCB-mixture Exposure period NOAEL LOAEL Parameters, effects Reference
(strain) (oral) (mg/kg body (mg/kg body
weight) weight)
Mice 2,5,4'-TCB day 1 to 6 of 0.05/animal 0.5/animal decrease in the number Örberg (1978)
(NMRI) 2,4,5,2',4', gestation of implant/dams
5'-HCB
Mice 3,4,5,3',4', day 6 to 15 of 2 embryotoxicity, malformations Marks et al.
(CD-1) 5'-HCB gestation (cleft palate, hydronephrosis) (1981)
3,4,3',4'-TCB 8 maternal toxicity, less potent
2,3,4,2', than that of 3,4,5,3',4',5'-HCB
3'4'-HCB
Rat 3,4,3',4'-TCB day 6 to 18 of 3 embryotoxicity Wardell et al.
(Sprague- gestation (1982)
Dawley)
Rhesus 3,4,3',4'-TCB day 20 to 40 of 0.07 maternal toxicity, total abortions McNulty (1985)
monkey gestation
Table 48. Results of mutagenicity, and related, tests
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
2,5,2',5'- Salmonella TA 1538 200 µg/plate modified - Wyndham et al.
tetrachloro typhimurium 100 µg/plate microsomal - (1976)
biphenyl 50 µg/plate fraction -
10 µg/plate from rabbits -
Aroclor 1268 Salmonella TA 1538 200 µg/plate modified - Wyndham et al.
typhimurium 100 µg/plate microsomal - (1976)
50 µg/plate fraction -
10 µg/plate from rabbits -
Aroclor 1254 Salmonella TA 1535 4 different both with, and - Heddle & Bruce
typhimurium TA 1537 concentrations, without, S-9 in - (1977)
TA 98 figures not all cases -
TA 100 given -
Aroclor 1254 Salmonella TA 1535 8 different both with, and - Schoeny et al.
typhimurium TA 1537 concentrations without, S-9 - (1979)
TA 98 from 0.5 to -
TA 100 500 µg/plate -
Aroclor 1254 Salmonella TA 1535 0.05, 0.5, both with, and - Bruce & Heddle
typhimurium TA 1537 5, 50, and without, S-9 - (1979)
TA 98 500 µg/plate -
TA 100 -
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Aroclor 1254 Salmonella TA 1538 50, 100, 500, both with, and - Shahin et al.
typhimurium TA 98 1000, 2000, without, S-9 - (1979)
5000 µg/plate
Aroclor 1242 V79 50, 100, and without - Hattula (1985)
Clophen A60 hamster cells 150 µg/ml metabolizing -
co-cultivated cells
with lethally
irridatiated
hepatocytes
Aroclor 1254 Micronucleus 4 different - Heddle & Bruce
test concentrations, (1977)
(erythrocytes) figures not
given
Aroclor 1254 Micronucleus (C57B1/6 × (approximately) - Bruce & Heddle
test C3H/He) LD50, 1/2, 1/4, (at all doses) (1979)
F1 mice and 1/8 of the
highest dose
(5 consecutive
days, ip)
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Kanechlor 500 Micronucleus ddY-mice 100 mg/kg in - - Watanabe et al.
test with corn oil orally (1982)
polychromatic and 100 mg/kg
erythrocytes 95% ethanol
subcutaneously
Aroclor 1254 Chromosomal (embryonic 0 or 10 mg/kg inconclusive Peakall et al.
aberrations Ring diet (1972)
Doves)
Aroclor 1254 Chromosomal (human 100 mg/litre - Hoopingarner
aberrations lymphocytes) culture medium et al. (1972)
Aroclor 1254 Chromosomal male 5, 50, 500 mg/kg negative at all Garthoff et al.
abnormalities in Holtzmann diet doses (1977)
bone marrow and rats
spermatogonial
cells
Aroclor 1242 Chromosomal Osborne- 5000 mg/kg × 1a - Green et al.
abnormalities Mendel 2500 mg/kg × 1 - (1975a)
in bone marrow rats 1250 mg/kg × 1 -
cells and 500 mg/kg × 4 -
spermatogonial
cells
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Clophen A30 Drosophila 62.5, 125, 250, -b Nilsson & Ramel
melanogaster and 500 mg/litre (1974)
(adults or larvae) substrate
Clophen A50 Drosophila 25, 50, 100, -b Nilsson & Ramel
melanogaster and 200 mg/litre (1974)
(adults or larvae) substrate
Aroclor 1242 Dominant Lethal Osborne- 2500 mg/kg × 1a - Green et al.
test Mendel 1250 mg/kg × 1 - (1975b)
rats 625 mg/kg × 1 -
250 mg/kg × 5 -
125 mg/kg × 5 -
Aroclor 1254 Dominant Lethal Osborne- 150 mg/kg × 5a - Green et al.
test Mendel 75 mg/kg × 5 - (1975b)
rats
Aroclor 1254 Dominant Lethal Osborne- 25, 100 mg/kg - Green et al.
test Mendel diet for 70 days - (1975b)
rats
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Aroclor 1254 Sperm (C57 B1/6 × (approximately)LD50, negative at all Bruce & Heddle
Abnormality C3H/He)F1 1/2, 1/4, 1/8 top dose doses (1979)
mice on 5 consecutive
days, ip
Aroclor 1254 mitotic index human 100 mg/litre mitotic index Hoopingarner
lymphocytes culture medium equivocal et al. (1972)
4-chloro- DNA repair and Chinese 10-5 mmol/litre covalent- Wong et al.
biphenyl and unscheduled hamster 3H-4-chloro- binding to (1979)
metabolites synthesis ovary cells biphenyl, 24 h protein, RNA,
(hydroxyurea and DNA
addition suppress Increase
DNA synthesis) specific
activity with
DNA
a Means single dose (× 1) or 5 doses in 5 days (× 5).
b No loss or non-disjunction of sex-chromosomes.
SD=Significant decrease.
Dose-related chromosome breakage was found in human lymphocytes
exposed to the planar PCB congener, 3,4,3',4'-tetrachlorobiphenyl, at
0.1-10-4 µg/ml (Sargent et al. (1989). In contrast, the non-planar
2,5,2',5'-tetrachlorobiphenyl did not cause chromosome damage in a
comparable test, even at concentrations as high as 1 µg/ml. However, a
combination of 3,4,3',4'-tetrachlorobiphenyl at a concentration of
10-5 µg/ml with 2,5,2',5'-tetrachlorobiphenyl caused chromosomal
damage that was far in excess of what might be expected from higher
doses of 3,4,3',4'-tetrachlorobiphenyl alone. The results suggest that
some PCB congeners may interact to cause synergistic effects.
Peakall et al. (1972) carried out cytogenic studies on Ring dove
embryos (Streptopelia risoria); 6 embryos were from dove pairs not
fed PCBs (controls) and 17 embryos were from PCB-fed (10 mg/kg diet)
pairs. The frequencies of chromosome aberrations were recorded for
chromosome pairs occurring in metaphase cells of allantoic sac and
limb bud origin. Mean aberration rates were as follows: control 0.8%
(range 0-2.0%) and PCB-treated 1.8% (range 0-9.4%). It was concluded
by the authors that these results were indicative of a possible
clastogenic action of PCBs.
A DNA repair assay was carried out by Wong et al. (1979) using CHO
cells and measuring the effects of 4-chlorobiphenyl (10 mol/litre) on
the unscheduled DNA synthesis (UDS) in the presence of hydroxyurea
(HU), a chemical agent that suppresses normal replicative DNA
synthesis. The quantification of DNA synthesis was determined by the
uptake of [H3]-thymidine into the cellular DNA. A significant
(1.6-fold) enhancement of UDS was found when the cells were incubated
for 2.5 h in the presence of HU, 4-chlorobiphenyl, and thymidine.
8.4.4.3 Cell transformation
Aroclor 1254 did not cause an increase in benzo (a)pyrene-induced
transformation in a test using C3H10 T1/2 CL8 mouse embryo fibroblasts
(Nesnow et al., 1981).
Aroclor 1254 also failed to transform Golden Syrian hamster cells
76-582 in culture at 50 µl/ml (Pienta, 1980).
8.4.4.4 Cell to cell communication
The congener 2,4,5,2',4',5'-hexachlorobiphenyl inhibited one form of
intercellular communication in V79 Chinese hamster cells, i.e.,
metabolic cooperation by mutant rescue at non-cytotoxic levels, while
3,4,5,3',4',5'-hexachlorobiphenyl was inactive (Tsushimoto et al.,
1983).
8.4.4.5 Interaction
Grolier et al. (1989) studied the effects of Vitamin A dietary intake
(2 and 20 IU/g of food) on the mutagenicity of benzo (a)pyrene
(B (a)P) towards Salmonella typhymurium TA 98, either in control
rats or in animals treated with 2,4,5,2',4',5'- hexachlorobiphenyl and
3,4,3',4'-tetrachlorobiphenyl. The planar tetrachlorobiphenyl strongly
increased B (a)P-monooxygenase activity and glutathione transferase,
while the non planar hexachlorobiphenyl was a strong inducer of
epoxide hydrolase and a weak inducer of B (a)P-monooxygenase. Enzyme
induction was not modified by changes in Vitamin A intake. A greater
mutagenic effect was observed in the tetrachlorobiphenyl group than in
the hexachlorobiphenyl group. This could be related to the specific
form of cytochrome P-450 induced by the tetrachlorobiphenyl congener.
In PCB-treated rats, the mutagenic activity of B (a)P was higher in
the 20-IU group than in the 2-IU group.
8.4.4.6 Cell division parameters
Tests on Osborne-Mendel rats gave various results, but may provide the
most important clue to the mechanism of action of PCBs in
carcinogenesis. At high doses (5000 mg Aroclor 1242/kg given once, and
500 mg/kg, given in a series of 4 daily doses), there were significant
decreases in the numbers of spermatogonial cells in mitosis. Single
dose levels of 1250 or 2500 mg/kg gave negative results (Green et al.,
1975a). Garthoff et al. (1977) also found negative results in male
Holtzmann rats treated with 0, 5, 50, or 500 mg Aroclor 1254/kg diet
for 5 weeks with regard to the mitotic indices of bone marrow and
spermatogonial cells. The data of Hoopingarner et al. (1972) showed an
increase in mitotic index in human lymphocytes exposed to Aroclor
1254.
The above-mentioned studies provide evidence that Aroclors can enhance
cell proliferation, and this is of special interest because it
suggests that the Aroclors may act to promote carcinogenesis to a
greater extent than to initiate it. The effect on cell proliferation
requires further examination in a variety of systems.
8.5 Carcinogenicity
Hayes (1987) critically reviewed the available evidence for, and
against, the view that environmental PCBs present a significant
potential carcinogenic hazard for humans.
8.5.1 Long-term toxicity/carcinogenicity
(a) Mouse
Nagasaki et al. (1974) exposed 10 groups of dd mice (6-12 of each sex)
to Kanechlor 300, 400, or 500 in the diet at levels of 0, 100, 250, or
500 mg/kg (equivalent to 0, 5, 12.5, and 25 mg/kg body weight,
respectively) for 32 weeks. Nine nodular hyperplasia and 7
hepatocellular carcinomas were found in 17 male mice, and 4 cases of
liver hypertrophy in 17 female mice, after exposure to 500 mg
Kanechlor 500/kg diet. No neoplasms were found in the other groups.
In another mouse study, groups of male BALB/cJ mice were fed 0 or
300 mg Aroclor 1254/kg diet (equivalent to 50 mg/kg body weight) for 6
or 11 months. Adenofibrosis was observed in the livers of all 22 mice
fed Aroclor 1254 for 11 months, but not in those of the 24 mice
exposed for 6 months. Hepatomas were noted in 9/22 mice exposed for 11
months and in 1/24 mice exposed for 6 months. No tumours were found in
the controls (Kimbrough & Linder, 1974).
(b) Rat
In a preliminary study, liver tumours (multiple adenomatous nodules)
were induced by Kanechlor 400 (containing 2,4,3',4'-; 2,5,3,3'-;
2,3,4,4'-; and 3,4,3',4'-tetrachlorobiphenyls) in 6/10 females, but
not in male Donryu rats, in 400 days. The dietary exposure was
periodically adjusted according to animal weights and ranged from 38.5
to 616 mg/kg diet. The latter dose level was administered for 275
days. The number of animals used was small (10 treated and 5 control
rats of each sex). Increased incidences of pneumonia, and lung and
intracranial abscesses were found in rats on a diet containing
Kanechlor 400, possibly because of lowered resistance to infection
(Kimura & Baba, 1973).
Ito et al. (1974) fed 10 groups of 29 male Wistar rats with Kanechlor
300, 400, or 500 at dose levels of 100, 500, or 1000 mg/kg diet
(equivalent to 5, 25, and 50 mg/kg body weight, respectively) for
28-52 weeks. Another group received the control diet. A number of
animals died in all groups (4 up to 21 animals); within the treated
groups, deaths were more or less dose related. Adenofibrosis was
observed in the livers of rats fed 1000 mg/kg diet of all 3 mixtures.
Kanechlor 500 produced nodular hyperplasia at all dose-levels and at a
higher incidence than the mixtures with a lower chlorine content. No
neoplastic nodules were observed in the controls. Kanechlor 300 and
500 (100 mg/kg diet) did not show significant growth inhibition or
increases in liver weight. No fibrosis or cholangiofibrosis, bile duct
proliferations, fatty changes, or cellular hypertrophy in the liver
were found. The liver nodular hyperplasia, designated as preneoplastic
by the investigators, was found in 3/25 of the Kanechlor 500
(100 mg/kg diet) treated animals, 1/22 of the Kanechlor 300 (100 mg/kg
diet) treated animals, and 0/18 controls. The 1/22 (4.5% incidence in
the Kanechlor 300, 100 mg/kg diet) is not significant and the 2 higher
dose levels of this product did not induce such changes. It can be
concluded that Kanechlor 300 did not induce neoplasia at dietary
levels of up to 1000 mg/kg over a 52-week period, in this study. At
the 1000 mg/kg level, Kanechlor 300 did produce other evidence of
chronic liver toxicity including oval cell and bile-duct
proliferation, fatty changes, and cellular hypertrophy and, possibly,
cholangiofibrosis (2/15). In the case of Kanechlor 500 (100 mg/kg
diet), essentially the same picture was obtained. In this group, 3/25
cases of nodular hyperplasia were found. In the case of Kanechlor 400
with the dietary levels of 100 and 1000 mg/kg, 2/16 and 3/10 of the
animals had nodular hyperplasia in the liver, respectively.
In a preliminary study on Sherman rats (10 animals of each sex/dose),
Aroclor 1254 at dietary levels of 0, 20, 100, or 500 mg/kg, and
Aroclor 1260 at levels of 0, 20, 100, 500, or 1000 mg/kg, for 8
months, did not give neoplastic nodules or hepatocellular carcinoma.
At 500 mg/kg, Aroclor 1254 produced adenofibrosis in 10/10 male
animals and at 100 and 500 mg/kg, in 7/10 and 9/10 females,
respectively. This change was only seen in 2/10 male and 4/10 female
animals with Aroclor 1260. The authors stated that hepatocellular
adenofibrosis is a persistent progressive lesion that consists of a
marked proliferation of fibrous tissue and epithelial glandular cells
that are well differentiated in mice, but appear atypical in rats
(Kimbrough et al., 1972).
A group of 200 female Sherman rats was given Aroclor 1260 at an
average dietary level of 100 mg/kg (range, 70-107 mg/kg), for 21
months. The PCB intake declined from 11.6 mg/kg per day during the
first week to 6.1 mg/kg at 3 months and to 4.3 mg/kg body weight per
day, later on. The control group also comprised 200 rats. The survival
rate and the food intake were not affected and no treatment-related
signs of toxicity were observed. Body weight gain was decreased from 3
months after the onset of the exposures. Hepatocellular carcinomas
were present in the liver of 26/184 (14%) exposed rats and 1/173
(0.58%) control rats. The livers of most of the remaining exposed rats
(144/184) showed hyperplastic nodules, while none were found in
control rats. A total of 182 exposed rats and 28 control rats had
livers with foci or areas of cytoplasmic alteration. A few livers of
exposed rats showed adenofibrosis. No induction of tumours in other
organs and no metastases from the liver tumours were found (Kimbrough
et al., 1975).
Calandra (1976) reported the findings from several long-term studies,
performed by a commercial laboratory for Monsanto. (These studies have
never been published). In these studies, 1000 rats were divided into
10 groups of 100 animals (50 of each sex) and 9 of the groups were
exposed to Aroclors 1242, 1254, or 1260 at dietary levels of 1, 10, or
100 mg/kg diet. Apparently, 5 animals of each sex were sacrificed at
3, 6, and 12 months with about 35 animals killed at the end of the
2-year studies. In the animals sacrificed early, only one nodular
hyperplasia was observed and it was in the group fed 100 mg Aroclor
1260/kg for 12 months. Mortality in these studies was high and
approximately one-third of the 105 animals anticipated to be exposed
for 2 years at each dietary level died. Hepatomas were observed in
7/25 livers from animals fed 100 mg Aroclor 1260/kg, in 4/26 fed
Aroclor 1254, 3/19 fed Aroclor 1242, and only in 1/168 animals
receiving the 1-10 mg/kg diets. Nodular hyperplasia was twice as
prevalent as hepatomas in the high-dose animals, particularly in the
Aroclor 1254 group (Harbison et al., 1987).
In a limited study, groups of 24 Fischer 344 rats of each sex received
a diet containing Aroclor 1254 at 0, 25, 50, or 100 mg/kg diet
(equivalent to 0, 1.2, 2.5, and 5 mg/kg body weight, respectively) for
104-105 weeks. The survival rate decreased with a dose-related trend
in male, but not in female rats (92, 83, 58, and 46%, respectively).
From 10 weeks of exposure onwards, the body weight gain of all rats,
except that of the low-dose males, decreased. In the groups receiving
the 2 highest dose levels, alopecia, facial oedema, exophthalmos, and
cyanosis were observed. Foci of hepatocellular alterations, which were
dose-related, were found at all dose levels, but not in the controls.
The incidence of non-neoplastic hyperplastic nodules in male rats with
25, 50, or 100 mg/kg diet was 5/24, 8/24, and 12/24 and in female rats
6/24, 9/22, and 17/24, respectively (US EPA, 1980). None was found in
the controls. Hepatocellular adenoma and carcinomas were found in 1/24
males and 1/24 females on the 50 mg/kg diet and in 3/24 males and in
2/24 females on the 100 mg/kg diet. Non-neoplastic liver lesions
included degenerative changes and aggregates of macrophages with
crystalline cytoplasmic structures and pigment granules. An apparently
dose-related increase in the incidence of intestinal metaplasia was
observed in both sexes and 0, 1, 3, and 2 adenocarcinomas located in
the pyloric region of the glandular stomach were found at 0, 25, 50,
and 100 mg/kg diet, respectively. Morgan et al. (1981) and Ward (1985)
reexamined the NCI (1978) data with respect to gastric
adenocarcinomas, hepatocellular adenomas, and carcinomas. Morgan et
al. (1981) found incidences of focal stomach lesions, mostly
metaplasia, of 6, 10, 17, and 35% in rats receiving 0, 25, 50, or
100 mg Aroclor 1254/kg diet, respectively. Adenomas were found in 6
treated rats. When compared with the incidences of stomach
adenocarcinomas in historical controls (1/3548), the incidence of
6/144 was statistically significantly increased (NCI, 1978; Morgan et
al., 1981; Ward, 1985).
Groups of 70 Sprague-Dawley rats of each sex received a diet
containing Aroclor 1260 in corn oil at a concentration of 100 mg/kg
diet (equivalent to 5 mg/kg body weight) for 16 months and 50 mg/kg
diet (equivalent to 2.5 mg/kg body weight) for an additional 8 months.
All surviving rats received a basal diet from month 25 to month 29.
The control group comprising 63 rats of each sex, received the basal
diet with corn oil for 18 months and the basal diet alone for an
additional 5 months. All surviving rats received the basal diet from
the 25th month to the 29th month. Data on growth were not available.
Groups of 2 control or 3 PCB-treated rats of each sex were partially
hepatectomized at 1, 3, 6, 9, 12, 15, and 18 months. At 24 months, a
similar group was sacrificed; after 29 months all remaining animals
were sacrificed. The mortality rate was not affected by the exposure.
In the livers of exposed rats, centrolobular hypertrophy was apparent
at 1 month, foci at 3 months, and areas of cellular alteration after 6
months, neoplastic nodules after 12 months, trabecular carcinomas
after 15 months, and adenocarcinomas after 24 months. Metastases in
the lung were not found. In exposed rats that survived 18 months or
longer, hepatocellular carcinomas were present in 43/47 females and in
2/46 males, but were absent in 81 controls. Simple and cystic
cholangioma at 18 and 23 months, respectively, and adenofibrosis at 22
months were present in the treated rats (Norback & Weltman, 1985).
Rao & Banerji (1988b) fed 3 groups of 32 weanling Wistar rats a
protein diet containing 0 (coconut oil), 50, or 100 mg Aroclor 1260/kg
diet, for 120 days. The incidence of neoplastic nodules in the liver
was 0/32, 24/32 and 16/32, respectively. Adenofibrosis was also
observed in the treated animals.
In order to exclude possible effects of dibenzofurans and to
investigate the effect of the degree of biphenyl-chlorination, groups
of 152 and 144 male Wistar rats were exposed to Clophen A30 and
Clophen A60, respectively, not containing detectable quantities
(detection limit not stated) of dibenzofurans, for 800 days at a dose
of 100 mg/kg diet. A group of 139 rats received a control diet. After
800 days, randomly selected rats were killed daily, until all
survivors had been examined by day 832. The survival rate of the
remaining exposed rats was increased by day 800. In rats autopsied by
day 800 and in rats autopsied later, the incidence of hepatocellular
carcinoma was increased by exposure to Clophen A60 (9/129 and 52/85,
respectively) relative to controls (0/131 and 1/53, respectively), but
not by exposure to Clophen A30 (1/138 and 3/87, respectively). There
was a marked trend from foci of hepatocellular alteration to
neoplastic nodule to carcinoma with increasing time and degree of
biphenyl chlorination. Controls mainly showed foci. Non-neoplastic
liver lesions with increased incidences of bile duct hyperplasia were
found in rats receiving Clophen A30 and A60, and cysts in rats
receiving Clophen A60. The incidence of adenofibrosis in the liver was
decreased, compared with that in the controls, in all exposed rats
that were killed after exposure (Schaeffer et al., 1984).
8.5.2 Tumour promotion/anticarcinogenic effects
(a) Mice
Tatematsu et al. (1979) studied the effects of inducers of liver
microsomal enzymes on the induction of hyperplastic liver nodules by
N-2-fluorenylacetamide (2-FAA) in male F344 rats. The rats were fed
a diet containing 200 mg 2-FAA/kg diet for 2 weeks and then given 500
or 1000 mg Kanechlor 500/kg diet for the following 8 weeks. Partial
hepatectomies were performed at the end of the third week of the
study. Kanechlor 500 in the dose levels applied showed a promoting
effect.
Ito et al. (1973) and Nagasaki et al. (1974, 1975) exposed groups of
20-38 male dd mice to diets containing Kanechlor 400 or 500 at 100 or
250 mg/kg for 24 weeks. Control groups consisted of 20 mice. Combined
exposure to Kanechlor 500 at 100 and 250 mg and either 50, 100, or
250 mg alpha- or ß-BHC (hexachlorocyclohexane)/kg diet enhanced the
development of nodular hyperplasia and hepatocellular carcinomas. A
combination of Kanechlor 500 and gamma-hexachlorocyclohexane did not
produce tumours. Dosing with Kanechlor 500 alone at a dietary level of
100 and 250 mg/kg, and ß- or gamma-hexachlorocyclohexane at dietary
levels of up to 500 mg/kg did not produce tumours. However,
alpha-hexachlorocyclohexane (250 mg/kg diet) produced 10/38
hepatocellular carcinomas and 30/38 hyperplastic nodules.
In another study, both inhibition and promotion were observed
following transplacental and transmammary exposure of mice to PCBs
prior to, or simultaneously with, exposure to dimethylnitrosamine
(DMNA). In this study, Aroclor 1254 was administered intraperitoneally
to female Swiss CD-1 mice on the 19th day of gestation, at a dose of
500 mg/kg body weight. Groups of 17-31 sucklings of these mice and of
controls were then treated intraperitoneally with DMNA on postnatal
day 4 or 14, or remained untreated. The progeny were killed at 28
weeks or at 18 months of age. Aroclor 1254 exposure decreased the
incidence of tumours in the liver and lung, induced by DMNA
administered at postnatal day 14. The average numbers of lung tumours
per mouse were also decreased. However, Aroclor 1254 increased the
liver tumour-bearing mice with extensive DMNA-initiated liver tumours
at 18 months of age, especially when DMNA was administered on
postnatal day 4. Mice exposed only to Aroclor 1254 did not show tumour
incidences higher than those of controls (Anderson et al., 1983). The
authors also reported that 2 higher chlorinated biphenyls,
2,4,5,2',4',5'-hexachlorobiphenyl and 2,3,5,2',3',5'-hexa-
chlorobiphenyl, were the dominant congeners persisting in the tissues
of the PCB-treated mice. It should be noted that only the more highly
chlorinated PCBs (>50% by weight) were reported as promoters of
hepatocarcinogenesis in rodents. The promoting activities of the lower
chlorinated PCBs have not been determined so far.
(b) Rat
As shown in Table 49, administration of PCBs to rats after exposure to
several initiating agents promoted the development of neoplastic
lesions of the liver. In these studies, treatment of the rats with a
control diet of PCBs alone did not usually lead to neoplastic changes
in the liver. Preston et al. (1981) compared the promoting effect of
exposure of rats to Aroclor 1254 with that of exposure to Aroclor 1254
from which the polychlorinated dibenzofuran moieties had been removed,
and did not find any significant differences. The results of this
study suggested that the promoting effect of commercial PCBs cannot be
ascribed entirely to the presence of chlorinated dibenzofurans.
Tatematsu et al. (1979) showed a dose-effect relationship in the
observed promoting effect of Kanechlor 500.
Inhibition, rather than promotion, of liver neoplasms was observed
when female Donryu rats were exposed to Kanechlor 400 preceding, or
simultaneously with, exposure to 3'-methyl-4-dimethylaminoazobenzene
(3'-Me-DAB) (Kimura et al., 1976). Simultaneous dietary exposure of
groups of 16-24 male Sprague-Dawley rats to Kanechlor 500, at a
dose-level of 500 mg/kg (25 mg/kg body weight per day), and to
3'-Me-DAB, or N-2-fluorenylacetamide (2-FAA), or diethylnitrosamine
(DENA), or combinations of these carcinogens, for 20 weeks, showed
almost complete inhibition of neoplastic nodules and hepatocarcinomas
(Makiura et al., 1974). It should be noted that the results of this
study may be influenced by the extreme toxicity, as shown by the
weight records, especially when the substances were combined.
The antitumour activity of Aroclor 1254 was demonstrated in male
Sprague-Dawley rats, inoculated with Walker 256 tumour cells. Groups
of 16 rats received PCB doses of 50, 100, or 200 mg/kg body weight for
2 weeks, once in 2 days, starting on the day of tumour cell injection.
A group of 16 rats did not receive PCBs. The inhibition of tumour
growth and transplantability were dose-related (Kerkvliet & Kimeldorf,
1977). The antitumour activity of Phenoclor DP5 was observed in groups
of 20 female Swiss mice inoculated with Ehrlich's tumoral ascites
liquid, after receiving PCBs in the diet at levels of 0, 10, 50, or
250 mg/kg, for 120 days (Keck, 1982).
Nishizumi (1980) showed that placentally transferred PCBs inhibited
diethylnitrosamine (DENA)-induced liver tumours. Groups of ten,
10-week-old female Wistar rats were treated with 40 or 200 mg
Kanechlor 500/kg body weight, by gavage, on days 5, 10, and 15 of
gestation. One F1 offspring from each litter was killed for
quantification of liver PCBs. The remaining F1 offspring were exposed
to 50 mg DENA/litre drinking-water, continuously for 5 weeks. At 16,
20, and 24 weeks after the beginning of the DENA exposure, 6-8 rats of
Table 49. Promotion of liver neoplasms in rats exposed to PCBs after treatment with carcinogenic substances
Strain Sex Group size Carcinogenic PCBs Doseb Exposure Neoplasms Reference
substancesa (mg/kg diet) period promoted by
(weeks) PCBs
Donryu female 25 3'-Me-DAB Kanechlor 400 400 26 carcinoma Kimura et al.
(1976)
Wistar male 20-24 DENA Kanechlor 500 2 × 15 4 nodules Nishizumi (1976)
mg/week carcinoma
F 344 male 15-16 2-FAA Kanechlor 500 500 and 8 hyperplasia Tatematsu et al.
1000 nodules (1979)
F 344 male 20 EHEN Kanechlor 500 32 carcinoma Hirose et al.
(1981)
Sprague- male 40 DENA Aroclor 1254c 100 18 carcinoma Preston et al.
Dawley (1981)
a 3'-Me-DAB = 3'-methyl-4-dimethylaminoazobenzene; DENA = diethylnitrosamine; 2-FAA = N-2-fluorenylacetamide;
EHEN = N-ethyl-N-hydroxyethylnitrosamine.
b Unless otherwise specified.
c With and without PCDFs.
each sex from each treatment group were killed and examined
histologically. A significant reduction in tumour incidence occurred
only in male offspring in the 200-mg group. The liver-PCB values in
28-day-old F1 mice were < 1, 18 ± 7, and 360 ± 30 mg/kg tissue for
the controls, 40, and 200 mg/kg groups, respectively. It was suggested
that placental transfer of PCBs protected the treated rats from
DENA-induced liver tumours.
The inhibitory effect of PCBs on tumour initiators following
simultaneous exposure has been explained by an enhanced metabolism of
the initiator by PCB-induced mixed function oxygenase.
Tests for putatively preneoplastic enzyme-altered foci in the liver of
rats have shown a dose-related increase in the promotion of such foci
by intraperitoneal exposure to Aroclor 1254 in tricaprylin or by oral
exposure to Clophen A50 in olive oil, following oral administration of
diethylnitrosamine (Deml & Oesterle, 1982; Pereira et al., 1982;
Oesterle & Deml, 1983).
The highest oral dose of Clophen A50 not enhancing the number and area
of enzyme-altered loci in female Sprague-Dawley rats, treated for 11
weeks with doses of 0, 0.1, 0.5, 1, 5, or 10 mg/kg body weight after
initiation by a single dose of 8 mg/kg body weight of
diethylnitrosamine, was 0.5 mg/kg body weight (Deml & Oesterle, 1987).
8.5.3 Initiation, promotion, and other special studies on individual
congeners
2,5,2',5'-Tetrachlorobiphenyl and its metabolite 2,5,2',5'-
tetrachlorobiphenyl-3,4-oxide were tested in a pulmonary tumour
induction assay with intraperitoneally injected A/T mice of both
sexes, and in a two-stage skin carcinogenicity assay with
dermally-exposed female SENCAR mice. Pulmonary adenomas and skin
papillomas were not induced (Preston et al., 1985).
Female Harlan-Sprague-Dawley rats received 2,4,2',4'-
tetrachlorobiphenyl or 2,5,2',5'-tetrachlorobiphenyl at 100 mg/kg
diet for 28 weeks, 1 week after oral exposure to diethylnitrosamine
(DENA). Both congeners showed a promoting effect on the development of
foci of hepatocellular alteration. The effect was approximately
10-fold greater in rats receiving 2,4,2',4'-tetrachlorobiphenyl
(Preston et al., 1985).
The effects of PCB mixtures and selected congeners have also been
investigated by Hayes et al. (1985, 1986) using the resistant
hepatocyte model developed by Farber and coworkers (Solt & Farber,
1976; Tsuda et al., 1980; Farber, 1984a,b, 1986). The ability of
2,4,2',4' and 2,5,2',5'-tetrachlorobiphenyl, 2,4,5,2',4',5'-
hexachlorobiphenyl, and a mixture of PCB-congeners (the composition of
which resembled that ascertained in human breast milk) to initiate
enzyme-altered hepatocellular nodules was investigated in
proliferating hepatocytes of neonatal or partially hepatectomized
adult rats (the PCB-congeners did not contain detectable levels of
dibenzofurans or dioxins). Neonatal rats were exposed 3 times in 3
weeks and adult rats once. After several weeks, the rats received a
selection regimen of 2-acetylaminofluorene followed by partial
hepatectomy (neonates) or necrotizing carbon tetrachloride (adults).
None of the PCB exposures generated nodules in contrast to known
initiators (Hayes et al., 1985).
Subsequent studies by Hayes et al. (1986) using the afore-mentioned
compounds and 3,4,3',4'-tetrachlorobiphenyl (a typical MC-type inducer
of cytochrome P-450-dependent monooxygenases) showed that these PCBs
(50 µmol/kg body weight), given 10 days after a dose of the initiator,
DENA, and 7 days before 2-AAF, all reduced the size of the
2-AAF-selected gamma-glutamyltranspeptidase-positive nodules. These
results show that, in contrast to the previous studies, PCBs also
exhibit "anti-promoting" activities in this Farber-model, which
utilizes 2-AAF as a mito-inhibitory toxin.
8.5.4 Skin carcinogenicity
Aroclor 1254 (100 µg/mouse) administered 18 h prior to the initiator,
7,12-dimethylbenz (a)anthracene (DMBA), significantly decreased the
incidence of papilloma formation in female Charles-River CD-1 mice.
2,4,5,2',4',5'-Hexachlorobiphenyl (625 µg/mouse), a PCB congener that
resembles phenobarbital in its mode of induction of drug-metabolizing
enzymes, did not act as an anticarcinogen, whereas 3,4,3',4'-
tetrachlorobiphenyl was more active than Aroclor 1254 as an
inhibitor. 3,4,3',4'-Tetrachlorobiphenyl resembled methylcholanthrene
in their mode of cytochrome P-450 induction (Parkinson et al., 1983)
and decreased the number of DMBA-initiated papillomas/mouse. Although
the PCB treatment did not modulate the incidence of papillomas caused
by benzo (a)pyrene, it was suggested that the anticarcinogenic
effects of PCBs on mouse skin tumours initiated by DMBA were due to
altered metabolism and DNA binding of the carcinogen by the
PCB-induced skin monooxygenases (DiGiovanni et al., 1979).
Berry et al. (1978, 1979) studied the tumour-promoting activity of
Aroclor 1254 in groups of 30 female CD-1 mice initiated with
0.2 mmol/litre (equivalent to 51 µg) DMBA. One week later, a positive
control group received 2 µg tetradecanoylphorbolacetate (TPA) and an
experimental group received 100 µg Aroclor 1254 in acetone. The TPA
and Aroclor applications were made twice weekly for 30 weeks. The TPA
promotion resulted in 92% of the animals developing papillomas, while
none developed in the Aroclor-treated animals. It was concluded that
Aroclor 1254 was not a skin tumour promoter at the dose used in this
study. Inhibition of skin papilloma was observed when Aroclor 1254 was
administered 18 to 72 h prior to DMBA treatment and promotion by TPA.
Poland et al. (1982) used HRS/J hairless mice to study the tumour
promotion activity of Aroclor 1254 in combination with N-methyl-
N'-nitro- N-nitrosoguanidine (MNNG). Twenty female mice per group
received a single administration of MNNG (5 micromol in acetone) or
acetone alone applied on the skin, and were then treated topically,
twice weekly, with 1 mg Aroclor/mouse, dissolved in acetone, for 20
weeks. No tumours were induced in the control group; in the
MNNG-treated mice, 4/19 had papillomas. It was concluded that Aroclor
had a weak promoting effect.
8.5.5 Appraisal
In summarizing the potential carcinogenic activity of PCBs, it is
perhaps more informative to express it in terms of what is known about
the mechanisms of chemical carcinogenicity (Hayes, 1987). In other
words, what the evidence is to support the carcinogenicity of PCBs
through genotoxic/initiating, cocarcinogenic, promotional/
antipromotional, and progressional activities. There is no evidence to
support genotoxic activity for PCBs and, in in vitro studies,
evidence for initiating activity through direct interactions with DNA
is weak. Poor initiating activity is consistent with the demonstrated
lack of mutagenic activity in various short-term tests. There is
evidence to suggest that PCBs can potentiate the activity of known
carcinogens (or act as cocarcinogens) in in vitro systems, but the
opposite result is often seen in in vivo studies, suggesting that
certain protecting enzyme systems present in the intact systems are
absent in the in vitro systems. There is a substantial body of
evidence to support the promotional activity of PCBs, particularly the
more highly chlorinated ones, in rodent liver, and this activity may
depend on the sequence in which the chemicals are administered in
experimental animal studies. In addition, promotional activity is
correlated closely, but not consistently, with the induction of MFO
activity. The hyperplastic effect (stimulation of cell proliferation)
of PCB inducers could promote preneoplastic growth. This type of
activity may possibly involve a threshold, suggesting that it may not
be a factor in low-level exposures to PCBs. The anticarcinogenic
activity of PCBs may also depend on the sequence of events in
experimental animal studies, and it may be related more to the
antipromotional properties of PCBs, possibly functioning as
protectants of mito-inhibitory toxicity. The interpretation of the
available animal data involving the commercial PCB mixtures is often
complicated by lack of information concerning the presence and
contribution of chlorinated dibenzofuran impurities, as well as
variations in congener composition to toxicity. In structure-activity
terms, a key factor in determining promotional activity appears to be
the degree of chlorination, which may reflect increased resistance to
metabolism and elimination and possibly also higher degrees of
ortho-substitution among the congeners present. Ortho-substituted
PCBs (possibly acting as persistent PB type inducers) have been shown
to be effective tumour promoters, and, at least in the case of the
closely related PBBs, it has been shown that a non-toxic and
non-promoting dose of a non- ortho-substituted congener in
combination with a promoting dose of a highly ortho-substituted
congener has a synergistic effect. This result may explain why the
mixtures can have greater promoting ability than the individual
congeners involved. This result might also suggest multiple pathways
for promoting activity, possibly involving the Ah receptor as well as
the putative receptor for phenobarbital. The possibility that PCBs
might promote carcinogenesis in tissues, other than liver, in animals
exposed to various tissue-specific, initiating agents, needs to be
addressed. Nevertheless, the potential for human liver cancer from
exposure to PCBs cannot be reliably predicted from animal studies.
Overall, there is reason to exercise caution in extrapolating the
available animal data on the carcinogenic potential of PCBs for
humans.
8.6 Special studies: target-organ effects
The lesions commonly introduced in animals after acute, short-term, or
long-term administration/application of PCB mixtures and/or individual
congeners concern the liver, skin, immune system, reproductive system,
oedema at various sites, as well as disturbances of the
gastrointestinal tract and the thyroid gland.
8.6.1 Liver
8.6.1.1 PCB mixtures
The toxic effects of PCBs result both directly and indirectly from
their presence in certain organs, such as the liver, where they
induce, in various degrees, a variety of liver enzymes. Some of these
enzymes are active in the metabolism of the PCBs themselves, while
others involve activation, deactivation, detoxication, etc., of other
compounds.
In itself, induction of enzymes by xenobiotics does not represent a
toxic manifestation, rather it is the ordinary response to such
foreign chemicals, which results generally in their detoxication and
ultimate modification, enabling them to be excreted from the organism.
In this sense, the response of the liver to such compounds is a
biological protective mechanism. Since some compounds, including PCBs,
are capable of inducing not only enzymes that result in their own
detoxication, but others as well, and the level of enzyme induction
may be high enough to cause liver pathology, a line cannot be drawn
that clearly separates a normal biological function from a toxic
manifestation. Superimposed on this situation is the direct toxic
action of the compounds on liver tissue, because of the properties of
the parent compound or its metabolites.
An enlarged liver and increased absolute and relative liver weights
are commonly reported as gross effects of PCB administration. The
lowest-observed-effect levels, in studies on different rat strains,
exposed to a diet containing Aroclor 1254, for a (dose-related)
increase in relative liver weights, vary between 20 and 100 mg/kg diet
(equivalent to 1 and 5 mg/kg body weight, respectively) (Kimbrough et
al., 1972; Bruckner et al., 1974; Burse et al., 1974; Grant et al.,
1974; Allen et al., 1976; Zinkl, 1977; Hinton et al., 1978; Kasza et
al., 1978b; Jonsson et al., 1981; Baumann et al., 1983).
The liver hypertrophy is microscopically visible as enlarged,
pleiomorphic hepatocytes, sometimes multinucleated or with enlarged
nuclei. A liver alteration observed by many investigators after
exposure of rats to various PCB-mixtures is fatty degeneration,
characterized by fat vacuolation and/or a foamy appearance of the
cytoplasm. Ultrastructurally, an increase in the number and size of
cytoplasmic lipid droplets and liposomes (membrane-associated lipid
droplets) can be observed. Fatty degeneration of the liver was already
observed after exposure of male Sprague-Dawley rats to a diet
containing 5 mg Aroclor 1242/kg diet (equivalent to 0.25 mg/kg body
weight) for 2-6 months (Bruckner et al., 1974) and after exposure of
male Holtzman rats to a diet containing 5 mg Aroclor 1254/kg for 5
weeks (Kasza et al., 1978b). Chu et al. (1977) found a comparable
effect on the liver with >20 mg Aroclor 1254 or 1260/kg diet for 28
days. Another characteristic change is the appearance of eosinophilic,
lamellar, cytoplasmic inclusions (Kimbrough et al., 1972; Kasza et
al., 1978b) or "hyaline-like material" (Grant et al., 1974). Electron
microscopy revealed that these changes corresponded to concentric
laminated membranes of smooth endoplasmic reticulum ("whorls"; "myelin
figures") (Vos & Beems, 1971; Kimbrough et al., 1972; Allen et al.,
1976; Kasza et al., 1978b; Jonsson et al., 1981). Proliferation of the
smooth endoplasmic reticulum and a decrease in rough endoplasmic
reticulum are commonly observed in rats exposed to dietary levels of
PCB mixtures that also induce fatty degeneration. Kasza et al. (1978b)
further observed a marked proliferation of Golgi condensing vesicles
containing lipoprotein in the livers of male Holtzman rats fed Aroclor
1254, for 5 weeks, at 5 mg/kg diet. A decreased number of these
vesicles was seen at 50 and 500 mg/kg diet (equivalent to 2.5 and
25 mg/kg body weight, respectively), together with a marked increase
in the smooth endoplasmic reticulum and lysosomes. The above decrease
in Golgi vesicles was also observed in Sprague-Dawley rats by Hinton
et al. (1978). The proliferative changes of the endoplasmic reticulum
are closely related to the observed induction of microsomal enzymes,
as discussed in section 8.6.1.2. Atypical mitochondria (Burse et al.,
1974; Kasza et al., 1978a,b), and single cell and focal necrosis
(Grant et al., 1974; Allen et al., 1976; Jonsson et al., 1981; Baumann
et al., 1983) have also been described.
Hypobilirubinaemia was produced in rats by Bastomsky et al. (1975),
who investigated the mechanism by administering daily intraperitoneal
injections of Aroclor 1254 (25 mg/kg body weight, in corn oil) to
female rats for 4 days, before measuring bilirubin glucuronide
formation by hepatic microsomes in vitro. PCB treatment was not
effective in increasing UDP-glucuronosyltransferase (EC 2.4.1.7)
activity. Serum bilirubin levels in Gunn rats were also significantly
decreased by PCB treatment; the rats are genetically deficient in
UDP-glucuronosyltransferase (2.4.1.7) activity.
The fluorescing of livers on exposure to UV radiation, consistent with
the presence of porphyrin, and accumulation of brown pigment, positive
for iron, especially in Kupffer cells and perivascular macrophages
have also been reported (Kimbrough et al., 1972; Burse et al., 1974;
Zinkl, 1977; Jonsson et al., 1981). The changes described in the
livers of mice (Nishizumi, 1970) and rabbits (Koller & Zinkl, 1973),
following exposure to PCB mixtures, are comparable with those in rats.
8.6.1.2 Individual congeners
The effects of chlorination and the chemical composition of PCBs, with
regard to the dose-effects relations in liver toxicity after
short-term exposure, are indicated by the data of Biocca et al.
(1981). In this study, hepatotoxic effects were observed in mice after
5 weeks of maintenance on diets containing 0.3 mg of 3,4,5,3',4',5'-
hexachlorobiphenyl, while similar effects were observed only after
30 mg of 2,4,5,2',4',5'-hexachlorobiphenyl and 100 mg of 2,4,6,2',4',6'-
hexachlorobiphenyl/kg diet. No effects were found with 300 mg of
2,3,6,2',3',6'-hexachlorobiphenyl/kg diet. Similar dependence of liver
toxicity on the chemical composition of the PCB mixture would be
anticipated following long-term exposure in mice and other species.
8.6.2 Enzyme induction
8.6.2.1 Effects on liver enzymes of PCBs
Proliferation of the smooth endoplasmic reticulum is a common
observation in the liver cells of experimental animals following
exposure to PCB mixtures. This effect is accompanied by an increase in
microsomal protein and the induction of cytochrome P-450, cytochrome
P-448, and drug-metabolizing enzymes, including the microsomal
monooxygenases (EC 1.14.14.1), epoxide hydrolases (EC 3.3.2.3),
UDP-glucuronosyltransferases (EC 2.4.1.17), NADPH-cytochrome c
reductase (EC 1.6.2.4) and esterases (EC 3.1.1.1), and the cytosolic
glutathione S-transferase (EC 2.5.1.18). The subject has been
reviewed by Safe (1984).
The spectral, enzymatic, and electrophoretic properties of the
microsomal enzymes, induced by Aroclor 1248, 1254, 1260, and Kanechlor
400, are consistent with the inducing properties of both the
phenobarbital (PB) and 3-methylcholanthrene (MC) classes of inducers.
They induce both cytochromes P-450 and P-448 and associated enzymes
(Alvares & Kappas, 1977; Goldstein et al., 1977; Yoshimura et al.,
1978; Iverson et al., 1982; Lashneva & Tutelyan, 1984; Khan et al.,
1985; Tutelyan et al., 1986). Aroclor 1016, administered to
Sprague-Dawley rats at 50 mg/kg per day for 4 days, intraperitoneally,
elicited a barbiturate type of inducing effect on the hepatic
microsomal oxidative enzyme system. Aroclor 1016 caused increases in
liver cytochrome P-450 content, microsomal protein, and its
ethylmorphine N-demethylase activity. It did not induce cytochrome
P-448 in liver microsomes.
A dose-related induction of hepatic and, in some cases, extrahepatic
microsomal enzymes was observed in several animal species including
the rat, rabbit, mouse, ferret, guinea-pig, hamster (Safe, 1984), mink
(Shull et al., 1982; Aulerich et al., 1985) and monkey (Iverson et
al., 1982). Distinct interspecies variations have been demonstrated.
For example, while 6 daily intraperitoneal doses of 25 mg of Aroclor
1254/kg body weight caused a potent induction of benzo (a)pyrene
hydroxylase in adult, male Sprague-Dawley rats, no, or a minimal,
induction of this monooxygenase was observed in adult, male Swiss mice
after 4 daily intraperitoneal doses of 50 mg/kg body weight and in
male New Zealand rabbits after 2 intraperitoneal doses of 100 mg/kg
body weight on days 1 and 4 (Alvares et al., 1982). Furthermore, when
comparing the inducing potency of Aroclor 1242 in the mink and the
genetically related ferret, Shull et al. (1982) measured a greater
induction of cytochrome P-448 and MC-type monooxygenases and no toxic
effects in the ferret at a dosing regime that resulted in toxic
effects in the mink (100 mg/kg body weight on day 1,200 mg/kg body
weight on day 5, sacrifice on day 10). The authors considered the
observed induction moderate in both species compared with that
observed in the rat. Earlier, it had been found that male
Sprague-Dawley rats were indeed more sensitive than ferrets with
respect to the inducing effect of Aroclor 1254 following a single
intraperitoneal dose of 500 mg/kg body weight, though the responses of
both species were comparable qualitatively (Lake et al., 1979).
Moreover, pretreatment of rats with Aroclor 1254 resulted in the
induction of microsomal cytochromes P-450 c, P-450 d (MC-inducible),
P-450 b and P-450 e (PB-inducible) (Ryan et al., 1979a,b, 1982). In
general, the extent of induction of microsomal enzymes by PCB-mixtures
increased with increasing chlorine content up to 54%. The effect has
also been demonstrated with single pure PCBs administered orally
(Ecobichon & Comeau, 1975). The results are summarized in Table 50 and
show a greater degree of enzyme induction with the higher chlorinated
compounds (see section 8.6.1.2).
Table 50. Stimulation of microsomal enzyme activity by single chlorinated biphenylsa
Hepatic microsomal enzyme activity
Chlorine O-Demethylation N-Demethylation Aniline Nitro-
substituents hydroxylation reduction
4 0 0 0 0
2,2' 0 + + 0
2,4' 0 0 + 0
4,4' + + + + + + +
2,5,2',5' 0 + + + +
2,4,2',4' + + + + + + 0
2,4,5,2',4',5' + + + + + + + +
2,3,5,2',3',5' + + + + + + + +
2,4,6,2',4',6' + + + + + + + +
2,3,4,5,2',3',4',5' + + + + + + + +
a From: Johnstone et al. (1974).
0 = No activity.
+ = Slight activity.
+ + = Marked activity.
Litterst et al. (1972) exposed groups of 6 male Osborn-Mendel rats to
Aroclors 1242, 1248, 1254, or 1260 in the diet, at concentrations of
0, 0.5, 5.0, 50, or 500 mg/kg diet, for 4 weeks. Increased microsomal
nitroreductase and demethylase activities occurred at 0.5 mg/kg or
more, increased pentobarbital hydroxylation and increased relative
liver weight occurred at 5.0 mg/kg or more, and increased liver
triglycerides occurred at 50 mg/kg diet. An inducing activity similar
to, or lower than, that of Aroclor 1254 has often been found for more
chlorinated mixtures (Villeneuve et al., 1971a, 1972; Bickers et al.,
1972; Chen & Dubois, 1973; Ecobichon & Comeau, 1974; Schmoldt et al.,
1974; Sawyer et al., 1984). Aroclor 1016 and 1242, both containing 42%
chlorine but differing in congener composition, showed qualitative and
quantitative differences in inducing effects. For example, Aroclor
1254 enhanced ethylmorphine N-demethylase activity 3-fold, while the
maximum increase produced by Aroclor 1016 was only 40%. The 2 Aroclors
also differed in their induction of the various forms of cytochrome
P-450 (Alvares et al., 1982). Adult, male Sprague-Dawley rats were
administered, intraperitoneally, a dosage of 0 or 50 mg Aroclor 1016
in corn oil/kg body weight, for 4 days. Aroclor 1016 was a potent
inducer of N-methylase but a poor inducer of benzo (a)pyrene
hydroxylase. Administration of 100 mg Aroclor 1016 in corn oil/kg body
weight per day to adult male New Zealand White rabbits, for 4 days,
resulted in an increase in liver cytochrome P-450 activity and
decreases in benzphetamine- N-demethylase and benzo (a)pyrene
hydroxylase activities compared with the controls. 7-Ethoxycoumarine-
O-deethylase and 7-ethoxyresorufin- O-deethylase activities were
comparable with those in the controls (Ueng & Alvares, 1985).
Therefore, it can be concluded that the degree and type of induction
not only depends on the chlorine content of the mixture, but is also a
function of the congener composition, as will be discussed further in
the next section.
Not only species specificity, but also marked tissue specificity has
been observed. While Aroclor 1254 was found to be a potent inducer of
cytochrome P-450 content and benzo (a)pyrene hydroxylase activity in
the rat lung and liver (Alvares & Kappas, 1977), it caused a 46%
decrease in cytochrome P-450 content, a 31% decrease in
benzo (a)pyrene hydroxylase activity, and a 61% decrease in
ethylmorphine N-demethylase activity in the rabbit lung. Aroclor
1254 caused induction of cytochrome P-450 and both enzymes in the
kidneys of these rabbits, but benzo (a)pyrene hydroxylase was not
induced in the liver (Alvares et al., 1982).
The inducing effect of PCB mixtures on the monooxygenase system has
been observed in the livers of both male and female rats (Chen &
Dubois, 1973; Grant & Phillips, 1974), minks and ferrets (Lake et al.,
1979; Shull et al., 1982), in the livers of pregnant rats (Alvares,
1977) and rabbits (Villeneuve et al., 1971a), in the placenta of rats
(Alvares & Kappas, 1975), in fetal and neonatal rat livers (Alvares &
Kappas, 1975; Baker et al., 1977; Inoue et al., 1981; Jannetti &
Anderson, 1981; Lashneva et al., 1987), in immature rat livers (Chen &
Du Bois, 1973; Narbonne, 1980), and in mature and senescent rat livers
(Birnbaum & Baird, 1978).
The lowest-observed-adverse-effect levels and the no-observed-effect
levels for enzyme induction, found in short-term diet studies on rats,
are presented in Table 51.
Bruckner et al. (1977) showed that when Aroclor 1254 was administered
in the diet, at a level equivalent to 0.25 mg/kg body weight,
microsomal enzyme activity was induced after 1 day of exposure.
Narbonne (1980) found a significant induction with 0.1 mg Phenoclor
DP6/kg body weight, given in the diet, after 3-5 days.
After a few weeks of exposure to Aroclor 1254 or 1260, at low dietary
levels of between 0.25 and 1.25 mg/kg body weight, a plateau in
microsomal enzyme activity was reached that was maintained over
several months of exposure (Chen & Du Bois, 1973; Grant et al., 1974;
Bruckner et al., 1977). Following short-term exposure, dietary levels
of between 5 and 25 mg/kg may stimulate microsomal enzymes for up to 4
months (Grant et al., 1974; Bruckner et al., 1977). Levels of 0.05 or
0.1 mg/kg body weight failed to produce any effects or the periods of
induction at these levels were long (Grant et al., 1974; Chen & Du
Bois, 1973).
A marked induction of the liver monooxygenase system was observed in
the offspring of female rats given a single oral dose of a mixture of
PCBs (Sovol) (500 mg/kg body weight) on the 14th day of pregnancy. In
one-day-old rats, an increase in cytochrome P-450 content associated
with an increase in the cytochrome b 5 level, an increase in NADPH-
cytochrome c reductase activity, an increased rate of aminopyrine-
N-demethylation activity in microsomes and also increased
3,4-benzo (a)pyrene hydroxylation, 7-ethoxycoumarin O-deethylation,
and NADPH-dependent lipid peroxidation were found. The activity of the
cytochrome P-450 system in the young rats remained elevated during the
early postnatal period (Lashneva et al., 1987).
8.6.2.2 Effects on liver enzymes of "biologically filtered" PCB
mixtures
Young Sprague-Dawley rats were administered a total of 38 oral doses
of a PCB mixture in olive oil at 0, 0.25, 1.0, 4.0, 16.0, 64.0, 256,
or 1025 µg/kg body weight, twice a day, over 1 month; the mixture
contained 55% chlorine and had a gas-chromatographic profile very
similar to that of the congeners found in the breast milk of Japanese
women. A dose-related induction of liver aminopyrine demethylase and
benzo (a)pyrene hydroxylase (EC 1.14.14.1) was found with doses of
1.0 µg/kg body weight or more. PCB-binding to liver microsomes was
increased at doses of 4.0 µg/kg body weight or more (Shimada & Ugawa,
1978). In another study, 1-month-old, male Wistar rats received
(intraperitoneally) doses of 0, 1, 10, 25, 50, or 100 mg/kg body
weight of a reconstituted PCB-mixture in corn oil, containing average
levels of 13 of the major congeners found in the breast milk of
Japanese women (purity > 98.5%). Each dose was administered in 2
portions on days 1 and 3. The same dose regimen of Kanechlor 500 was
also tested. The rats were killed on day 6. The reconstituted PCB
mixture and Kanechlor 500 caused dose-related increases in liver
Table 51. Microsomal enzyme induction by PCB-mixtures in rats
PCB-mixture Rat strain Sex Exposure No-effect-level Lowest-observed- Reference
male/ period mg/kg body adverse effect level,
female weight mg/kg body weight
Aroclor 1016 Sprague-Dawley male 3 weeksa 0.1 1 Iverson et al. (1975)
Aroclor 1242 Sprague-Dawley male 3 weeksa 0.1 1 Iverson et al. (1975)
Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Sprague-Dawley male 2-6 months < 0.25 0.25 Bruckner et al.
(1974, 1977)
Aroclor 1248 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Aroclor 1254 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Wistar male 2-8 months < 0.1 0.1c Grant et al. (1974)
Wistar male 2 weeks 0.25 0.5 Den Tonkelaar &
Wistar male 12 weeks 0.05 0.5 van Esch (1974)
Sprague-Dawley male 0-20 weeks 0.05 0.25 Turner & Green (1974)
Holtzman male 3 weeks < 0.25 0.25 Bruckner et al. (1977)
Garthoff et al. (1977)
Table 51. (cont'd).
PCB-mixture Rat strain Sex Exposure No-effect-level Lowest-observed- Reference
male/ period mg/kg body adverse effect level,
female weight mg/kg body weight
Aroclor 1260 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Holtzman male 1-13 weeks < 0.05 0.05 Chen & DuBois (1973)
female 1-13 weeks 0.05 0.25
Phenoclor DP6 Sprague- male 3 days < 0.1 0.1 Narbonne (1979, 1980)
Dawleyb
a Daily dosing by gavage; the other studies are all diet studies.
b Immature rats (60-65 g).
c Significant effect after months 4 and 6, but not after months 2 and 8.
benzo (a)pyrene hydroxylase activity that were 3.5 and 2.2 times the
control value, respectively, at 1 mg/kg body weight. The ED50 of the
reconstituted breast milk PCB mixture for the induction of rat hepatic
microsomal aryl hydroxylase (AHH) was 7 times lower than that of
Kanechlor 500. The authors concluded that the increased potency of the
breast milk-PCB mixture reflected the preferential bioconcentration of
the relatively toxic congeners 2,4,5,3',4'-penta-, 2,3,4,3',4'-penta-,
and 2,3,4,5,3',4'-hexachlorobiphenyl (Parkinson, et al., 1980b). When
Gyorkos et al. (1985) repeated the studies, the reconstituted mixture
was inactive at the lowest dose level but exhibited mixed-type
microsomal enzyme induction characteristics at the higher dose levels.
Increases in the activities of several hepatic microsomal
monooxygenases, including dimethylaminoantipyrine N-demethylase,
aldrin epoxidase, benzo (a)pyrene hydroxylase and
ethoxyresorufin- O-deethylase were found.
8.6.2.3 Effects of individual congeners on liver enzymes
The enzyme-inducing potencies of individual PCB congeners have been
studied extensively and reviews have been published (Goldstein, 1980;
Safe, 1984; Safe et al., 1985b), in which the following
structure-activity relationships are proposed. The most active
congeners with respect to the induction of aryl hydrocarbon
hydroxylase (and toxicity), 3,4,5,4'-tetrachloro-, 3,4,3',4'-
tetrachloro-, 3,4,5,3',4'-pentachloro-, and 3,4,5,3',4',5'-
hexachlorobiphenyl, are substituted at both para positions, at 2 or
more meta positions, but not at ortho positions. These congeners
can assume coplanar conformations and are approximate stereoisomers of
2,3,7,8-tetrachlorodibenzo- para-dioxin. They resemble
3-methylcholanthrene and 2,3,7,8-tetrachlorodibenzo- para-dioxin in
their mode of hepatic enzyme induction, inducing hepatic microsomal
benzo (a)pyrene hydroxylase, ethoxyresorufin- O-deethylase, and the
cytochromes P-450 a, P-450 c, and P-450 d.
These congeners are only present as trace compounds in commercial PCB
mixtures, but appear in significant quantities in breast milk (Noren
et al., 1990).
The least active of these 4 coplanar congeners, 3,4,5,4'-
tetrachlorobiphenyl, also shows a phenobarbital type of hepatic
microsomal enzyme induction, inducing dimethylaminoantipyrine,
ethylmorphine and related N-dealkylases, biphenyl-4-hydroxylase,
aldrin epoxidase, several O-dealkylases, and the cytochromes P-450 a,
P-450 b, and P-450 e. This "mixed-type" induction pattern is also
shown by 3,4,4'-trichlorobiphenyl, and by all the mono- ortho, and at
least 7 di- ortho, substituted analogues of the coplanar PCB
congeners. Several of these congeners, e.g., 2,4,5,3',4'-penta,
2,3,3',4,4'-penta, 2,3,4,5,3',4'-hexa-, and 2,3,4,5,2',4'-
hexachlorobiphenyl are components of commercial PCB mixtures and have
been identified in breast milk.
Studies have revealed that 4,4'-dichlorobiphenyl, with no meta
substituents exhibits a PB-type induction pattern in rats. Adding
meta substituents as in 3,4,4'-tri-, and 3,4,5,4'-tetrachloro-
biphenyl will give a mixed PB- and 3-MC-type induction pattern. While
3,4,3',4'-tetrachlorobiphenyl is a potent inducer of microsomal
hepatic aryl hydrocarbon hydroxylase (AHH), it did not significantly
increase the activities of benzo (a)pyrene-hydroxylase, [3H]-4-
chlorobiphenyl-hydroxylase, and ethoxyresofurin- O-deethylase (EROD)
at a dose level of 10 µmol/kg (Andres et al., 1983).
Most other PCB congeners are phenobarbital-type inducers or are
inactive. In general, the more highly chlorinated of these congeners
are more active inducers than the lower chlorinated ones, probably
reflecting the relative half-lives of these compounds. The
non-availability of 2 adjacent unhalogenated carbon atoms and
para-substitution are 2 factors that decrease the degradability of
the congeners and increase their inducing activity.
Various congeners were tested for their inducing activity in
responsive C57BL/6J mice, i.e., mice containing the Ah receptor
protein, and in non-responsive DBA/2J mice, lacking this receptor,
after single intraperitoneal (Robertson et al., 1984; Silkworth et
al., 1984) or oral (Kohli et al., 1980) doses in corn oil and
cottonseed oil, respectively. The coplanar PCBs and their mono-ortho
substituted analogues all induced benzo (a)pyrene hydroxylase or
ethoxyresorufin deethylase in responsive mice, but not, or only to a
minor degree, in non-responsive mice. Most tested mono-ortho
substituted analogues of coplanar PCBs slightly induced aminopyrine
N-demethylase in both strains, while the coplanar congeners did not
induce this enzyme.
The above structure-activity relationships were confirmed in a few
limited studies on monkeys. Hepatic aryl hydrocarbon hydroxylase was
induced in 3 young, male Rhesus monkeys after a single oral dose of
1 mg of 3,4,3',4'-tetrachlorobiphenyl/kg body weight and in 3 young
male monkeys during continued feeding of a diet containing 0.5 mg of
3,4,5,3',4',5'-hexachlorobiphenyl/kg (McNulty, 1985). A single oral
dose of 18 mg of 2,5,2',5',-tetrachlorobiphenyl/kg body weight,
administered to male Rhesus monkeys, in corn oil, elevated hepatic
cytochrome P-450 levels, while no change was noted in the activities
of several microsomal enzymes (Allen et al., 1975b). Mono- ortho or
di- ortho substituted analogues of coplanar PCB congeners have not
been tested in monkeys.
Vodicnik et al. (1980) studied the effect of 2,4,5,2',4',5'-
hexa-chlorobiphenyl on hepatic microsomal monooxygenase activity in
virgin or pregnant and lactating Sprague-Dawley mice and their
offspring. A single intraperitoneal dose of 100 mg/kg body weight, was
administered 14 days before mating. Prior to, and during early
pregnancy, hepatic monooxygenase activity in pretreated mice was
greater than that in the controls. No differences were found between
pregnant and virgin mice. Mothers, pretreated with the
hexachlorobiphenyl and sacrificed on the day of birth, had lower
microsomal monooxygenase activity and cytochrome P-450 content than
PCB-pretreated virgins sacrificed concurrently. No differences were
noted between these groups of animals during lactation. Hepatic enzyme
activities and cytochrome P-450 content were not different between
newborn offspring of corn oil- and PCB-pretreated mothers. However,
these parameters were elevated in 5- to 20-day postpartum nursing
offspring from pretreated mothers, compared with those from corn
oil-pretreated mothers suggesting the transfer of hexachlorobiphenyl
through the breast milk in quantities sufficient to affect hepatic
microsomal monooxygenase activity.
In a study on ICR mice, Vodicnik (1986) administered 150 mg
14C-2,4,2',4'-tetrachlorobiphenyl intraperitoneally, and compared
hepatic microsomal ethoxycoumarin- O-deethylase activity and liver
concentrations of 14C-activity. It was shown that pregnant mice were
less responsive to the inducing effects of the tetrachlorobiphenyl
than virgin or postpartum mice. This diminution in response may be, in
part, responsible for the lack of elimination of the
tetrachlorobiphenyl equivalents from the late pregnant animal during
the 4-day experimental period (see section 6.4.3).
Hardwick et al. (1985) studied both the time course and dose-response
for the induction of the 2 isoenzymes and their respective mRNAs after
administration of 3,4,5,3',4',5'-hexachlorobiphenyl to rats. It was
concluded that the congener under study induced 2 major 3-MC-inducible
isoenzymes of cytochrome P-450 and their mRNAs in a coordinated
manner, probably via a common mechanism. The data are consistent with
the hypothesis that both genes are probably regulated by a single
receptor in the rat. The magnitude of the increase in the isoenzymes
was greater than the increase in the amount of translationally active
mRNA in polysomes, suggesting that other factors may also influence
the relative induction of these P-450 isoenzymes.
In this study, the BP-type inducers, 2,4,6,2',4',6'- and
2,4,5,2',4',5'-hexachlorobiphenyl, both of which have been reported to
increase the hepatic cytosolic receptor level and consequently enhance
enzyme induction in vivo, were not able to enhance EROD or AHH
induction by 3,4,5,3',4'-pentachlorobiphenyl in vitro.
The mixed-type inducer 2,3,4,2',4',5'-hexachlorobiphenyl inhibited
enzyme induction by 3,4,5,3',4'-pentachlorobiphenyl in vitro, when
used in concentrations of at least 400 times higher than that of the
3,4,5,3',4'-pentachlorobiphenyl. Enzyme induction by 3,4,3',4'-
tetrachlorobiphenyl was inhibited by 2,3,4,2',4',5'-hexachlorobiphenyl
at concentrations at least 40 times higher, and enzyme induction by
3,4,5,3',4',5'-hexachlorobiphenyl was inhibited by 2,3,4,2',4',5'-
hexachlorobiphenyl at concentrations at least 8 times higher. Since
the concentration of 2,3,4,2',4',5'-hexachlorobiphenyl found in human
adipose tissue is about 300 times higher than those of the 3 coplanar
PCBs, this inhibition of enzyme induction probably occurs after
natural exposure to PCB mixtures. If enzyme induction by
3,4,3',4'-tetrachlorobiphenyl and 3,4,5,3',4',5'-hexachlorobiphenyl is
also inhibited by various concentrations of 2,3,4,2',3',4'-
hexachlorobiphenyl, inhibition of enzyme induction after natural
exposure to a mixture of PCBs is to be expected.
It was concluded that the in vitro enzyme induction by mixtures of
PCBs cannot be determined by the simple addition of the induction by
the individual PCBs. Possibly, in vivo enzyme induction seems
additive, because some compounds increase the receptor level, while
other compounds inhibit enzyme induction (van Vliet, 1990).
8.6.2.4 Appraisal
The liver is the organ most often implicated in the toxicity of PCBs
in animals. Hepatotoxicity has been observed in numerous studies with
exposed mice, rats, guinea-pigs, rabbits, dogs, and monkeys. The
effects, which appear to be reversible at low doses, are similar among
the species and include enzyme induction, liver enlargement, fat
deposition, and necrosis. Enzyme induction is the most sensitive
indicator of hepatic effects, but few studies have been designed to
define the minimum effective doses of PCB mixtures. The liver
enlargement is associated with hepatocyte enlargement and an increase
in smooth endoplasmic reticulum and/or increased enzymatic activity.
Proliferative lesions in the liver have been attributed to Aroclor
treatment. The hepatic effects of Aroclors in animals appear to be
typical of chlorinated hydrocarbons. Histologically-documented liver
damage is a consistent finding among PCB-exposed animals.
8.6.3 Effects on vitamins and mineral metabolism
8.6.3.1 Effects of PCB mixtures
PCB mixtures have been found to decrease levels of retinol (Vitamin A)
in the liver of rats (Innami et al., 1976; Kato et al., 1978; Hudecova
et al., 1979), rabbits (Villeneuve et al., 1971a), and in the plasma
of pigs (Guoth et al., 1984). Levels of thiamine (Vitamin B1) were
decreased in the blood, liver, and sciatic nerve of rats (Yagi et al.,
1979) and levels of pyridoxal phosphate (Vitamin B6) were decreased in
several tissues of rats, while riboflavin (Vitamin B2) levels remained
unaffected (Fujiwara & Kuriyama, 1977). The changes in the levels of
retinol and thiamine were thought to be secondary to the induction of
metabolizing enzymes (Yagi et al., 1979; Saito et al., 1982). The
induction of these enzymes was also found to be responsible for the
increased de novo synthesis of L-ascorbic acid that was observed in
the plasma, tissues, and urine of PCB-treated rats (Fujiwara &
Kuriyama, 1977; Chakraborty et al., 1978; Chow et al., 1979; Saito et
al., 1983). Lipid peroxidation was increased in the liver of
PCB-treated rats and Saito et al. (1983) suggested that ascorbic acid
may have initiated the peroxidation.
It was shown that induction of NADP-cytochrome c reductase (EC
1.6.2.4) and insufficiency of lipid peroxide scavengers, such as
alpha-tocopherol (Vitamin E) and glutathione peroxidase (EC 1.11.1.9),
could also be involved in the enhancement of lipid peroxidation by
PCBs (Saito et al., 1982, 1983; Kamohara et al., 1984).
PCB mixtures decreased the activity of both sodium/potassium- and
magnesium-dependent adenosinetriphosphatase (EC 3.6.1.3) in the
tissues of rats (Narbonne et al., 1978; La Rocca & Carlson, 1979). The
results of in vitro studies on isolated rat mitochondria showed that
PCB mixtures may act as inhibitors of respiration and uncouplers of
oxidative phosphorylation (Sivalingan et al., 1973; Nishihara, 1983,
1985). However, contradictory results have been obtained in vivo
with respect to the NAD/NADH ratio, the ADP/O ratio, and state 3 and
state 4 respiration rates (Mehlman et al., 1974; Chesney & Allen,
1974; Garthoff et al., 1977).
Byrne & Sepkovic (1987) studied the in vitro incorporation of
monovalent cations into rat erythrocytes as a model for evaluating the
impairment of electrogenic transport by PCBs. Female, Sprague-Dawley
rats were fed 50 mg Aroclor 1242 or 1254/kg diet for 7 months. The
uptake of 86Rb by erythrocytes in the Aroclor 1254 group was
depressed compared with that in the control group in K+ - depleted
culture media. No changes were observed with Aroclor 1242. A reduction
in 86Rb incorporation was also seen in erythrocytes from the Aroclor
1254 group in a Na+ -depleted medium. Ouabain did not have any
effect in the Aroclor 1254 group, because Aroclor 1254 suppressed the
cationic transport maximally. This study provides evidence that PCBs
(Aroclor 1254) can damage the cell sufficiently to decrease the active
transport of monovalent cations.
Male Fischer 344 rats were dosed daily, intragastrically, for 5, 10,
or 15 weeks with 0, 0.1, 1, 10, or 25 mg Aroclor 1254/kg body weight
in corn oil, to investigate the effects on calcium metabolism, femur
morphometry, and nephrotoxicity. The relative liver weights were
increased significantly with doses of 1.0 mg/kg or more after 5 weeks
treatment. The relative kidney weights were increased after 15 weeks
treatment in the 10 and 25 mg/kg groups. Hypercalcaemia was present in
the 25 mg/kg group after 5 and 10 weeks treatment, but not after 15
weeks. Serum triglyceride levels were elevated after 5 weeks
treatment, but decreased after 10 and 15 weeks. Serum cholesterol
levels were increased at the 2 higher dose levels with all 3 lengths
of treatment. Urinary alkaline phosphatase and lactate dehydrogenase
activities were elevated at 5, 10, and 15 weeks of treatment. Femur
density was increased at the 10 mg/kg dose level after 5 weeks, and at
all dose levels after 10 and 15 weeks. Cross-sectional, medullary, and
cortical areas of the midpoint of the femur were significantly
decreased at the higher dose levels after 10 and 15 weeks of exposure.
The per cent medullary area was decreased after 10 and 15 weeks
treatment indicating a decrease in medullary size and also a decrease
relative to the cortical bone area. The result was weaker bones after
15 weeks at the highest dose level. Thus, PCB exposure affects calcium
metabolism and bone morphometry (Andrews, 1989).
8.6.3.2 Effects of individual congeners
3,4,3',4'-Tetrachlorobiphenyl induced a decrease in serum and liver
retinol and retinyl palmitate in C57BL/Rij mice. In "non-responsive"
DBA/2 mice, only serum retinol was decreased. The time and
dose-responses observed suggested that the difference in aryl
hydrocarbon hydroxylase responsiveness was not directly involved in
the effects on retinoid levels (Brouwer et al., 1985).
Powers et al. (1987) administered female, Sprague-Dawley rats single,
intraperitoneal injections of 1, 5, or 15 mg 3,4,3',4'-
tetrachlorobiphenyl/kg body weight and found a dose-related
depression of plasma retinol levels, 24 h after treatment. The loss of
plasma retinol appeared to be a function of depressed levels of the
retinol-binding protein (RBP)-transthyretin ternary complex. No free
retinol was observed in the plasma. Hepatic retinyl palmitate
hydrolase (RPH) activity was depressed and highly and positively
correlated with the plasma retinol levels. Doses of either
2,4,5,2',4',5'- and 3,4,5,3',4',5'-hexachlorobiphenyl, equimolar to
the 15 mg/kg tetrachlorobiphenyl dose, failed to cause a similar
depression in plasma retinol in treated female rats.
A study was carried out to investigate the effects of PCBs on retinoid
homeostasis in Sprague-Dawley rats. Female Sprague-Dawley/Rij rats
were fed a Vitamin A-deficient diet for 12-16 weeks. Serum retinol
concentrations at the end of this period were decreased to
approximately 10% of the normal retinol level. The rats were repleted
with radiolabelled [3H]retinol by feeding a diet containing 18.5 MBq
(8000 IU) of retinol/kg diet for 14 days. Saturation in the blood was
reached after 6 days [3H]retinol repletion. On day 7, the rats were
either treated with an intraperitoneal dose of 3,4,3',4'-
tetrachlorobiphenyl (15 mg/kg) in corn oil, or corn oil alone.
Exposure to tetrachlorobiphenyl resulted in significant reductions in
both retinol and retinyl ester concentrations in the liver and lung to
25% and 44% of the controls, respectively, and a reduction of retinol
in the heart of 35% of the controls. No changes in concentrations were
observed in the skin and kidneys (Brouwer et al., 1988).
Female WAG/Rij rats received a single ip injection of corn oil, or 15
or 200 mg 3,4,3',4'-tetrachlorobiphenyl/kg body weight and were killed
on days 1,3,7, or 14 to study the effects on serum and hepatic
retinoid contents and liver morphology. There was a significant
increase in liver weight at the highest dose level after 3, 7, and 14
days. There was a rapid increase in the 3H-tetrachlorobiphenyl levels
present after 7 days, after which a rapid decline occurred.
Tetrachlorobiphenyl induced a significant decrease in serum retinol
content in the 200 mg tetrachlorobiphenyl group on days 3 and 7. The
same was found for the retinol and retinyl palmitate contents of the
liver. Ultrastructural alterations in the hepatocytes, such as
proliferation and vesiculation of the endoplasmic reticulum and
mitochondrial enlargement with inclusions, were found (Durham &
Brouwer, 1989).
2,2',5,5'-Tetrachlorobiphenyl caused inhibition of Ca/Mg- and
Mg-dependent adenosinetriphosphatase in the liver of rats (Lin et al.,
1979). In vitro, several PCB congeners inhibited Na/K- and
Mg-dependent adenosinetriphosphatase. Although a general trend towards
increased inhibition, paralleling increased chlorination, was
observed, no correlation was evident between chlorine substitution
patterns and inhibitory activity (La Rocca & Carlson, 1979).
8.6.4 Effects on the gastrointestinal tract
Effects on the stomach have been studied or observed by Allen &
Norback (1973); Allen et al. (1974a); Allen (1975); Becker et al.
(1979) and Tryphonas et al. (1986a) in monkeys. Oral administration of
Aroclor 1242, 1248, or 1254 to monkeys produced gastritis, which
progressed to hypertrophy and hyperplasia of the gastric mucosa.
Related effects included mucous-filled cysts that penetrated the
muscularis mucosa. These effects were initiated by exposure as low
and/or short as a single gavage dose of 1.5 g Aroclor 1248/kg body
weight, 25 mg Aroclor 1248/kg diet for up to 1 year, 3 mg of Aroclor
1242/kg diet, for 71 days, or 280 µg/kg body weight for 28 months.
In studies on monkeys, Becker et al. (1979) carried out stomach
biopsies and found microscopically apparent arrest of the
differentiation of generative cells of the isthmus and neck into
parietal and zymogenic cells. Mature parietal and zymogenic cells,
which were found only in the bases of the glands, showed signs of
injury, such as dilatation of the rough endoplasmic reticulum on the
zymogenic cells, irregularity of the mitochondria and irregular
luminal membranes in parietal cells, and an increase in the number of
autophagic vesicles on both types of cell (see 8.2.1.6).
The Aroclor-induced gastric lesions, which occurred mainly along the
greater curvature of the stomach (not in the cardiac or pyloric
regions) and did not occur in other sections of the gastrointestinal
tract, have only been observed in pigs and monkeys (Hansen et al.,
1976b; Becker et al., 1979; Drill et al., 1981). The gastric effects
may therefore be species specific. Aroclor 1254 induced metaplasia and
adenocarcinoma in the glandular stomach of F344 rats (see section
8.7.1.2).
8.6.5 Effects on lipid metabolism
8.6.5.1 Effects of PCB mixtures
Consistent with the histopathological observation of fatty
degeneration in the liver (see section 8.2.1), short-term exposure to
commercial mixtures of PCBs induced increases in the contents and
concentrations of total lipids, triglycerides, cholesterol, and/or
phospholipids in this organ of the rat and rabbit (Litterst et al.,
1972; Bruckner et al., 1974; Itokawa et al., 1976; Garthoff et al.,
1977; Hinton et al., 1978; Ishidate et al., 1978; Dzogbefia et al.,
1978; Yagi, 1980; Kato & Yoshida, 1980, 1981; Kato et al., 1982).
Litterst et al. (1972) exposed male Osborne-Mendel rats, for 4 weeks,
to diets containing Aroclor 1242, 1248, 1254, or 1260 at levels of, or
between, 0.5, 5.0, 50, and 500 mg/kg (equivalent to 0.025, 0.25, 2.5,
and 25 mg/kg body weight). Aroclor 1248 caused the highest
dose-related increase in the triglyceride concentration in the liver,
which was significant at 500 mg/kg diet (equivalent to 25 mg/kg body
weight). The lowest-observed-effect level was reported by Bruckner et
al. (1974), who exposed male Sprague-Dawley rats to 0, 5, or 25 mg of
Aroclor 1242/kg diet (equivalent to 0, 0.3, and 1.5 mg/kg body weight,
respectively), for 2, 4, or 6 months and found a slight increase in
the concentrations of total lipids in the liver at both exposure
levels.
Levels of total lipids, triglycerides, and/or cholesterol in the serum
of rats and rabbits, exposed to PCB mixtures, were found to be
increased (Koller & Zinkl, 1973; Allen et al., 1976; Itokawa et al.,
1976; Garthoff et al., 1977; Zinkl, 1977; Kato & Yoshida, 1980, 1981;
Yagi, 1980; Kato et al., 1982; Baumann et al., 1983; Hladkà et al.,
1983; Carter, 1985). Wistar rats received Clophen A50, twice weekly,
by gavage, at levels of 2, 10, 50, 150, or 250 mg/kg body weight, for
6 weeks. Serum triglyceride and cholesterol levels were increased in a
dose-related manner at 50 and 2 mg/kg body weight, respectively
(Baumann et al., 1983). Decreased serum triglyceride levels were
reported by Kato et al. (1982). Fischer rats exposed for 8 days to
Aroclor 1254 in the diet at levels of 8 mg/kg (0.4 mg/kg body weight)
or more showed a dose-related increase in serum total cholesterol
concentrations. Hypercholesterolaemia was not found at 4 mg/kg diet
(Carter, 1985). Studies on monkeys exposed to Aroclors 1248 or 1254
for 1-2 years revealed lowered serum levels of total lipids,
triglycerides, and cholesterol (Barsotti et al., 1976; Arnold et al.,
1984). The cause of these changes may be an altered synthesis and/or
lipoprotein transport in the liver. No increase in the rate of
synthesis of liver triglycerides was found following intraperitoneal
exposure of rats to 3-8 daily doses of 50 mg of Aroclor 1254/kg body
weight (Hinton et al., 1978; Sandberg & Glaumann, 1980). The observed
increases in the half-lives of liver triglycerides and phospholipids
(Hinton et al., 1978) and the observed increase in the number of Very
Low Density Lipoproteins (VLDL) in the liver, without a change in
lipid composition (Sandberg & Glaumann, 1980), seems to be indicative
of impaired transport of these lipids from the liver to the blood.
This was also demonstrated by the repression in serum VLDL and in the
incorporation of tritiated water in serum total lipids following
tritiated water injection, found in rats exposed for 24 days to a
low-protein diet containing 1000 mg of Aroclor 1248/kg (50 mg/kg body
weight) (Kato et al., 1982). Sandberg & Glaumann (1980) observed an
impaired transport of VLDL from the endoplasmic reticulum to the Golgi
apparatus. This compares well with the observed flattening of the
Golgi apparatus, which also lacks secretory vesicles with lipoprotein
particles (Hinton et al., 1978). No explanation was found in the
available literature for the observed increase in serum triglyceride
levels in rats.
Ishidate et al. (1978) measured a decreased rate of synthesis of
phospholipids, especially of phosphatidyl choline, in the liver of
rats that had received 2 daily doses of a PCB mixture at 100 mg/kg
body weight, composed mainly of tetrachlorobiphenyl isomers. Decreased
phospholipid synthesis was also observed by Hinton et al. (1978). The
accumulation of phospholipids in the proliferated endoplasmic
reticulum was ascribed to the observed depression of the secretion of
lipoproteins into blood (Hinton et al., 1978; Ishidate et al., 1978;
Sandberg & Glaumann, 1980) and to a depressed catabolism of liver
phospholipids (Ishidate et al., 1978).
The synthesis of cholesterol in the rat liver may be increased by PCB
mixtures considering the increased concentration of labelled
cholesterol in the liver following 3H20-injection (Kato et al.,
1982) or 14C-glucose or 14C-acetate administration (Yagi, 1980) in
rats that had been exposed for 3-5 weeks to diets containing 1000 mg
of Aroclor 1248/kg diet or 500 mg Kanechlor 500/kg diet, respectively.
It was also shown that the activity of 3-hydroxy-3-methylglutaryl
Coenzyme A reductase (EC 1.1.1.34) was increased in rats following a
6-day exposure to a diet containing 1000 mg Aroclor 1248/kg diet
(equivalent to 50 mg/kg body weight) (Kato & Yoshida, 1980). The
enhanced synthesis of cholesterol has to compete with an enhanced
degradation, as Aroclor 1248 has been shown to induce cholesterol
7-alpha-hydroxylase (EC 1.14.14.1) in rats (Quazi et al., 1984).
Decreased biosynthesis of liver cholesterol was found in rats exposed
for 30 days to Aroclor 1254 at a dietary level of 500 mg/kg (Kling &
Gamble, 1982). Hypercholesterolaemia in PCB-exposed rats can be
explained partly by an increased synthesis of cholesterol and/or an
increase in serum high density lipoprotein cholesterol, which was
observed in several studies (Ishikawa et al., 1978; Yagi, 1980; Kato &
Yoshida, 1981; Carter, 1985).
Isolated hepatocytes were capable of secreting protein and
triacylglycerol in the form of VLDL into serum-free media. Eighty per
cent of 2,4,5,2',4',5'-hexachlorobiphenyl released from hepatocytes
was in association with VLDL, the remainder being in association with
protein (Gallenberg & Vodicnik, 1987).
8.6.5.2 Effects of individual congeners
Charles-River CD rats received a single oral dose of 3,4,5,3',4',5'-,
2,4,5,2',4',5'-, or 2,3,5,2',3',5'-hexachlorobiphenyl in cotton-seed
oil. After 72 h, all isomers had increased the levels of total lipids
in the liver. 3,4,5,3',4',5'-Hexachlorobiphenyl had the most
pronounced effect. This isomer was the only one that increased the
levels of total cholesterol, cholesterol esters, and triglycerides in
the liver, while the other 2 isomers slightly increased the content of
liver phospholipids (Kohli et al., 1979).
Shireman (1988) studied the lipoprotein-mediated transfer of
2,4,5,2',4',5'-hexachlorobiphenyl into cultured human fibroblasts, and
found that the plasma lipoproteins may play a role in the distribution
of this hexachlorobiphenyl to peripheral cells. Using normal skin
fibroblasts incubated with medium containing serum LDL or high density
lipoproteins (HDL) labelled with the 14C-hexachlorobiphenyl, the
author characterized the cellular incorporation, and efflux from
cells, of this congener and concluded that HDL might be involved in
the delivery of hexachlorobiphenyl to cells and not, as generally
thought, in the transport from cells.
2,4,5,2',4',5'-Hexachlorobiphenyl was shown to be distributed among
rat and human plasma lipoproteins and protein in vitro. It was
readily transferred among plasma constituents and its distribution was
related to the triacylglycerol:protein ratio in the plasma. One h
following intravenous administration of 70 µg labelled
hexachlorobiphenyl to virgin, female Sprague-Dawley rats, the
hexachlorobiphenyl was primarily distributed to low density
lipoprotein (LDL) with the hypertriglyceridemia of late pregnancy;
more than 70% of circulating hexachlorobiphenyl was associated with
very low density lipoproteins (VLDL). VLDL is a major substrate for
mammary gland lipoprotein lipase, which is elevated during lactation.
When hexachlorobiphenyl was complexed with human VLDL and injected
intravenously into late pregnant mice, mammary gland concentrations of
the compound exceeded those in the adipose tissue at all sacrifice
times between 5 min and 6 h (Gallenberg & Vodicnik, 1987; Gallenberg
et al., 1987).
8.6.6 Effects on porphyrin metabolism
8.6.6.1 Effects of PCB mixtures
Hepatic porphyria has been induced by a number of commercial PCB
mixtures (Clophen A60; Phenochlor DP6; Aroclor 1016, 1232, 1242, 1254,
and 1260; Kanechlor 400, 500, and 600) in mice, rats, rabbits,
chickens, and Japanese quail. Young rats, guinea-pigs, and minks seem
to be less sensitive (Strik, 1973). The porphyria was characterized by
the presence of pigment in the liver which fluoresced red under UV
radiation (Vos & Beems, 1971; Kimbrough et al., 1972; Vos &
Notenboom-Ram, 1972; Zinkl, 1977; Honda et al., 1983), an increase in
the concentration of porphyrins in the liver (Goldstein et al., 1974,
1975; Grote et al., 1975; Iverson et al., 1975; Kawanishi et al.,
1975) and an increase in the concentrations of delta-aminolevulinic
acid, porphobilinogen, and porphyrins in the urine or faeces (Vos &
Beems, 1971; Vos & Notenboom-Ram, 1972; Goldstein et al., 1974, 1975;
Baumann et al., 1983; Honda et al., 1983). Vos & Beems (1971) found
increased faecal elimination of coproporphyrin and protoporphyrin in
rabbits dermally treated with 118 mg Aroclor 1260/day (free of PCDFs),
5 days/week, for 36 days and Vos & Notenboom-Ram (1972) found the same
results when female, New Zealand rabbits received a 120 mg application
of Aroclor 1260 on the shaved skin, 5 days/week, for 4 weeks.
When Sherman rats were exposed for up to 13 months to a diet
containing 100 mg Aroclor 1254/kg or for up to 26 weeks to Aroclor
1242 at 100 or 500 mg/kg diet (equivalent to 5 and 25 mg/kg body
weight, respectively) a delayed onset of porphyria was noted after 2-7
months of exposure. The porphyria was mainly characterized by the
excretion and hepatic storage of uroporphyrin and heptacarboxy-
porphyrin, resembling human porphyria cutanea tarda (Goldstein et al.,
1974, 1975). A dose-dependent increase in the concentration of liver
porphyrins was observed in female, Sprague-Dawley rats receiving 21
daily doses of Aroclor 1242 (by gavage) in corn oil at 10 or 100 mg/kg
body weight, but not at 1 mg/kg body weight. Female rats were more
sensitive than male rats and Aroclor 1016 at the same dietary level
had less effect than Aroclor 1242 (Iverson et al., 1975). Others also
noted the greater effect of higher chlorinated PCB mixtures on liver
and urinary levels of porphyrins (Goldstein et al., 1974, 1975;
Kawanishi et al., 1975). Kawanishi et al. (1973, 1974) showed that
administration, in the diet, of Kanechlors KC-300 and KC-500 to rats
at 500 mg/kg produced a marked increase in urinary excretion of copro-
and uroporphyrins, and in faecal elimination of protoporphyrin, but no
increases were observed with Kanechlor KC-400.
Increased urinary coproporphyrin levels were found in male
Sprague-Dawley rats exposed for 2, 4, or 6 months to a diet containing
Aroclor 1242 at 5 or 25 mg/kg (equivalent to 0.25 and 1.25 mg/kg body
weight) (Bruckner et al., 1974).
Porphyria in rats and rabbits has been associated with the observed
stimulation of delta-aminolevulinate synthase (EC 2.3.1.37), the
rate-limiting enzyme in the haem synthesis of porphyrins (Goldstein et
al., 1974, 1975; Grote et al., 1975; Drill et al., 1981; Hill, 1985),
and with the inhibition of uroporphyrin decarboxylase (EC 4.1.1.37),
as measured in chick embryo cells and chicken erythrocytes in vitro
(Kawanishi et al., 1983; Sano et al., 1985). Seki et al. (1987)
observed 80% inhibition of liver uroporphyrin decarboxylase together
with a 15-fold increase in the activity of liver delta-aminolevulinate
synthase and accumulation in the liver of a large amount of
uroporphyrin in C57BL/6 mice exposed for 3 weeks to Kanechlor 500 at a
dietary dose of 500 mg/kg. Liver microsomal cytochrome P-450 was
increased and induction of microsomal enzymes was observed. The
effects were less outstanding in ddY mice whereas liver cytosol levels
of the PCBs were comparable in both strains. The authors postulated
that the development of porphyria is causally related to the
inhibition of uroporphyrin decarboxylase rather than the induction of
drug metabolizing function. Porphyria would develop only when the
ratio of hepatic uroporphyrin decarboxylase and delta-aminolevulinate
synthase decreased to less than 1.0.
8.6.6.2 Effects of individual congeners
The levels of coproporphyrin and protoporphyrin found in the faeces of
rabbits, dermally exposed to 5 doses/week of 120 mg of
2,4,5,2',4',5'-hexachlorobiphenyl (no dibenzofurans detected) in
isopropanol, for 4 weeks, were more elevated than those in the faeces
of rabbits exposed similarly to Aroclor 1260 (Vos & Notenboom-Ram,
1972). Koss et al. (1980) also found 2,4,5,2',4',5'-hexachlorobiphenyl
highly effective in inducing porphyria in female rats receiving,
orally, 64 mg of this PCB-congener/kg body weight in oil, once every 2
days, for 10 weeks. In mice receiving a diet containing 300 mg of one
of various tetrachlorobiphenyls, hexachlorobiphenyls, or
Kanechlors/kg, for 14 weeks, the most pronounced increases in the
levels of coproporphyrin and protoporphyrin in the liver were found in
animals fed 3,4,5,3',4',5'- and 2,4,6,2',4',6'-hexachlorobiphenyl, and
Kanechlor 600, followed by animals fed 3,5,3',5'- and 2,5,2',5'-
tetra-chlorobiphenyls and Kanechlor 500. No porphyrinogenic action was
found in mice fed 3,4,3',4'-, 2,4,2',4'-, 2,3,2',3'-, or 2,6,2',6'-
tetra-chlorobiphenyl, 2,3,4,2',3',4'-hexachlorobiphenyl, or Kanechlor
400 (Kawanishi et al., 1975). Accumulation of uroporphyrins was
observed in the livers of "responsive" C57BL/6 mice treated with
3,4,5,3',4',5'-hexachlorobiphenyl, but not in the livers of
"non-responsive" ddY mice. It was suggested that induction of
apocytochrome P-450 may take part in inducing porphyrin synthesis
(Sano et al., 1985).
Sano et al. (1985) studied the mechanism of the porphyrinogenic
activity of PCBs using cultured chick embryo liver cells to examine
the relationship between the induction of delta-aminolaevulinic acid
(ALA) synthetase and the inhibition of uroporphyrinogen dicarboxylase.
The porphyrinogenic effect of PCBs exhibited a defined
structure-activity relationship in that only 3,4,3',4'-tetrachloro-
and 3,4,5,3',4',5'-hexachlorobiphenyl out of 9 biphenyls produced a
marked accumulation of uroporphyrin in the liver cells. In
ALA-supplemented cultures, these 2 congeners led to the accumulation
of a large amount of uroporphyrin III, whereas with the other PCBs
(which were weak inducers of porphyrin synthesis) the accumulated
porphyrin was mostly protoporphyrin. These results suggested that the
active inducers of porphyrin synthesis also inhibit uroporphyrinogen
decarboxylase, in 2 steps, i.e., first, in the formation of
hexacarboxylic porphyrinogen III from heptacarboxylic porphyrinogen
III, and, second, in the formation of heptacarboxylic porphyrinogen
III from uroporphyrinogen III. The inhibition of uroporphyrinogen
decarboxylase leads to a depletion of haem. In addition, induction of
apocytochrome P-450 by PCBs may contribute to a decrease of haem. As a
result, synthesis of ALA synthetase increases, leading to an
accumulation of uroporphyrin in liver.
8.6.7 Effects on the endocrine system
8.6.7.1 Effects of PCB mixtures
The underlying cause of the reproductive toxicity of PCBs, described
in section 8.4, may be alterations in hormonal receptor binding and/or
alterations in the steroid hormone balance through effects on
metabolism and excretion.
Precocious vaginal opening was observed in neonatal Sprague-Dawley
rats receiving a subcutaneous dose of 10 mg of Aroclor 1221
(2000 mg/kg body weight) in sesame oil on days 2 and 3 of life. At 6
months of age, these females showed persistent vaginal estrus and
anovulation, despite no further exposure to Aroclor 1221. Doses of
Aroclor 1221, 1242, 1254, or 1260 at 1 mg/kg were without effect.
Groups of 22-day-old Sprague-Dawley rats were injected subcutaneously
with Aroclor 1221 or 1242 at 1, 10, 100, or 1000 mg/kg body weight or
Aroclor 1254 or 1260 mixed in sesame oil at 1, 10, or 100 mg/kg body
weight. Uteri were weighed. New-born female pups were injected
subcutaneously on the second and third day postpartum with Aroclor
1221 at 1 or 10 mg/day or Aroclor 1242, 1254, or 1260 in sesame oil or
dimethylsulfoxide at 1 mg/day. Pups were weaned at 21 days and
examined daily from the 25th day of puberty. Animals were sacrificed
at 7 or 8 months, at which time organs were examined. A significant
uterotrophic response was noted with 1000 mg Aroclor 1221/kg, but not
with the other PCBs (Gellert, 1978).
Indirect evidence for a weak estrogenic activity of PCBs was found for
various Aroclors by the glycogen response of immature rat uterus
(Bitman & Cecil, 1970; Bitman et al., 1972; Ecobichon & Mackenzie,
1974) or the less sensitive uterotropic response, observed in immature
rats exposed to Aroclor 1221, 1232, or 1248, but not in immature rats
exposed to Aroclor 1254 or 1260 (Ecobichon & Mackenzie, 1974; Gellert,
1978). More direct evidence is the inhibition in vitro of the
binding of labelled 17-beta-estradiol to the rat uterine receptor by
Aroclors 1221 and 1254 (Nelson, 1974).
Pregnant mares' serum was administered to immature female outbred rats
on day 29 postpartum, and, 60 h later, rats were injected with human
chorionic gonadotrophin. On day 34, the animals were divided into
groups and treated orally with sesame oil (control), or 20 mg PCBs/kg
(Clophen A30). Two days later they were killed and the ovaries removed
and analysed for in vitro synthesis of progesteron (unincubated,
incubated, and incubated with luteinizing hormone). The addition of
luteinizing hormone resulted in an approximately 100% increase in
progesterone synthesis above basal level with tissue exposed to PCBs.
With control tissue there was a 31% increase with luteinizing hormone
(Fuller et al., 1980).
PCBs induced a decrease in gonadal steroid hormone levels in rats,
minks, seals, and monkeys. When, after confirmed ovulation, mature
female Rhesus monkeys were exposed during the following cycle to daily
gavage doses of 4, 16, or 64 mg of Clophen A30/kg body weight, for 28
days, ovulation was blocked in 2 out of 4 treated monkeys. One out of
16 controls was anovulatory. The levels of luteinizing hormone and
follicle-stimulating hormone were not changed by the treatment (Muller
et al., 1978).
Plasma progesterone levels were decreased in female rats exposed for
36 weeks to a dietary level of Aroclor 1242 of 75 mg/kg (equivalent to
3.7 mg/kg body weight) (Jonsson et al., 1976), and in female minks
exposed for 12.5-14.5 months to a dietary level of 2.5 mg Aroclor
1254/kg (equivalent to 0.25 mg/kg body weight) (Aulerich et al.,
1985). The decreased levels of gonadal hormones can be explained by
enhanced metabolism of steroids, which are normal substrates for
microsomal enzymes. Increases in the formation of the metabolites of
progesterone and/or testosterone were measured in rats
intraperitoneally exposed 1-5 times to Aroclor 1260, Aroclor 1254, or
Kanechlor 400 (Krogh Derr, 1978; Lin et al., 1982; Yoshihara et al.,
1982). In contrast with these findings, increased testosterone levels
were found in male piglets exposed for 6-12 weeks to Aroclor 1232,
1242, or 1254 at 250 mg/diet. This increased production of
testosterone was related to increased relative testes weights
(Platonow et al., 1976).
Female rhesus monkeys (Macaca mulatta) were administered gelatin
capsules containing daily doses of 0, 5, 20, 40, or 80 µg Aroclor
1254/kg body weight, dissolved in corn oil plus glycerol. After
approximately 2 years of dosing, when the monkeys were considered to
be in a state approaching adipose-tissue PCBs equilibrium, each dose
group of 16 animals was divided into 2 test groups. Daily blood
samples from both test groups were acquired for estrogen and
progesteron analysis during one menstrual cycle. Serum estrogen and
progesteron concentrations in PCB-dosed monkeys were comparable with
those in the controls, except the luteal phase progesterone levels in
monkeys dosed with 20 and 80 µg/kg. There were no apparent
treatment-related differences in the incidence of anovulatory cycles
or in the temporal relationship between the estrogen peak and mensus
onset, mensus end, or the progesterone peak. Mean PCB concentrations
in the blood and adipose tissue for the different dose levels
administered were as follows: blood, 1, 11, 37, 74, and 125 µg/litre
and for adipose tissue 0.79, 7.88, 22.62, 47.6 and 85.3 mg/kg tissue,
respectively (Truelove et al., 1987).
Effects on plasma corticosteroid levels have also been observed. The
levels were decreased in female mice exposed to a diet containing
25 mg Aroclor 1254/kg (equivalent to 3.7 mg/kg body weight) for 3
weeks, and in male mice exposed to a diet containing 400 mg Aroclor
1254/kg (equivalent to 57 mg/kg body weight) for 2 weeks. No effects
were found on adrenal weight (Sanders & Kirkpatrick, 1975). However,
increased levels of plasma corticosterone and enlarged adrenal glands
were observed in male mice of another strain exposed to a diet
containing 200 mg Aroclor 1254/kg, for 2 weeks (Sanders et al., 1977).
Wasserman et al. (1973) reported increased plasma corticosterone
levels in rats receiving Aroclor 1221 in the drinking-water, at a
concentration of 250 mg/litre, for 10 weeks. This finding complies
with morphological features of hyperfunction of the adrenal zona
fasciculata found in rats that had received 200 mg Aroclor 1221/litre
drinking-water, for 6 weeks.
When female rats were exposed to Aroclor 1254, in their diet at doses
of 0, 1, 5, 10, or 50 mg/kg (equivalent to 0, 0.05, 0.25, 0.5, and
2.5 mg/kg body weight/day, respectively) for 5-7 months, relative
adrenal weights as well as serum levels of corticosterone,
dehydro-epiandrosterone, and dehydro-epiandrosterone sulfate were
decreased in a dose- and time-related manner. In the same studies, the
effects with less chlorinated Aroclors were less pronounced (Byrne et
al., 1988).
In addition, the ultrastructure of beta-cells of the pancreas of rats
was found to be changed after 13 months of exposure to 200 mg Aroclor
1254/litre drinking-water. These changes included marked dilatation
and vesiculation of the rough endoplasmic reticulum, hyperplastic
Golgi complexes with a reduction in the number of secretory granules,
and an increase in the number of beta-acinar and acinar-beta cells.
The changes in the pancreas were suggested to be secondary to the
increase in the level of glucocorticoids (Wasserman et al., 1975). An
increased relative adrenal weight was observed in pigs fed Aroclor
1242 or 1254/kg at 20 mg/kg diet (equivalent to 0.8 mg/kg body weight)
for 91 days (Hansen et al., 1976b).
Thyroid hormone levels were decreased in rats exposed to Aroclor 1254
at levels of 50 mg/kg diet or more for 4-12 weeks (Collins et al.,
1977; Collins & Capen, 1980a). Two explanations were offered. One was
the observed increase in biliary excretion of thyroxine and
triiodothyroxine (Bastomsky, 1974; Collins & Capen, 1980b) and the
larger proportion of biliary thyroxine present as glucuronide
(Bastomsky, 1974), most likely as a result of induction of microsomal
uridine diphosphate-glucuronosyltransferase (EC 2.4.1.17) (Bastomsky &
Murthy, 1976). The other explanation was a direct effect of PCBs on
thyroid follicular cells. When male Holtzman or Osborne-Mendel rats
were fed a diet containing Aroclor 1254 at a level of 0, 5, 50, or
500 mg/kg (equivalent to 0, 0.25, 2.5, and 25 mg/kg body weight),
thyroid follicular cells exhibited a dose-dependent hypertrophy and
hyperplasia. An abnormal accumulation of large colloid droplets and
irregular lysosomes in the follicular cells were observed at 5 mg/kg
diet or more and reduced serum thyroxine occurred at 50 mg/kg diet or
more. A no-observed-effect level could not be established. Microvilli
were decreased in number, shortened, and irregularly branched (Collins
et al., 1977; Kasza et al., 1978a,b; Collins & Capen, 1980a, 1980b).
The hypothalamus-pituitary axis seems not to be affected in view of
the observed increase in the serum level of thyroid-stimulating
hormone and in the iodine uptake by the thyroid following PCB exposure
(Bastomsky, 1974, 1977; Collins & Capen, 1980a).
Collins & Capen (1980a) suggested that the well-documented
PCBs-related disturbances in reproduction, growth, and development may
be related to alterations in thyroid structure and function in the
dam, fetus, or neonate. The lowering of serum thyroxine appears to be
the combined result of a direct effect on thyroid follicular cells
with an interference in hormone secretion plus an enhanced peripheral
metabolism of thyroxine.
8.6.7.2 Effects of individual congeners
Exposure of rats to various congeners produced different responses in
steroid metabolism. The most marked effects were observed after
exposure to 2,4,5,2',4',5'-hexachlorobiphenyl, which was found to
decrease the half-life of progesterone (Örberg & Ingvast, 1977), to
increase hydroxylation of progesterone, testosterone, and
androstenedione, and to decrease the 5-alpha-reduction of progesterone
and testosterone (Dieringer et al., 1979; Yoshihara et al., 1982).
3,4,5,3',4'-Pentachlorobiphenyl was found to depress the total
microsomal metabolism of progesterone and testosterone, though the
7-alpha-hydroxylation of these steroids was markedly stimulated
(Yoshihara et al., 1982). No, or very slight, effects on steroid
metabolism were found in rats exposed to chlorobiphenyls with 4
chlorine atoms or less (Örberg & Ingvast, 1977; Dieringer et al.,
1979).
Yoshimura et al. (1985) described a marked induction of liver
microsomal cytochrome P-450 and cytosolic DT-diaphorase as a cause of
a possible disorder of steroid homeostasis and promotion of
carcinogenicity of 4-nitroquinoline N-oxide (4-NQO) in rats
pretreated with 3,4,5,3',4'-pentachlorobiphenyl. The animals were
sacrificed 5 days after pretreatment. The results of the studies
showed that 7-alpha-hydroxylation of both progesterone and
testosterone in liver microsomes was increased, but hydroxylation at
the 2-alpha-, 6 alpha-, and 16 alpha-positions were depressed,
together with 5 alpha-reduction. The induced isoenzyme P-452 was most
responsible for the 7-alpha-hydroxylation of testosterone.
The major component (32 mol %) of the Aroclor 1221 mixture is
2-chlorobiphenyl. The major metabolite (4,4'-dihydroxy-2-
chlorobiphenyl) of 2-chlorobiphenyl has been shown to have a
significant binding activity with the soluble uterine estrogen
receptor protein in the rat, suggesting a possible explanation for the
unique estrogenic activity of Aroclor 1221 in the rat (Korach et al.,
1987).
8.6.8 Immunotoxicity
Some of the studies described below are summarized in Table 52.
8.6.8.1 Effects of PCB mixtures
(a) Mouse
Relative thymus and spleen weights of C57BL/6 mice were unaffected by
exposure to Aroclor 1016, for 3-41 weeks, at a dietary level of
167 mg/kg (Silkworth & Loose, 1979). Dietary exposure of outbred mice
(Mus musculus) to Aroclor 1248 at 50, 100, 500, or 1000 mg/kg diet
(equivalent to 7.1 up to 143 mg/kg body weight), for 3 or 5 weeks, did
not elicit gross signs of immunotoxicity (Thomas & Hinsdill, 1978).
BALB/c mice fed Aroclor 1242 at a dose-level of 0 or 167 mg/kg diet
(equivalent to 0 and 29 mg/kg body weight) for 3-9 weeks, did not show
adverse effects on the thymus, spleen, and lymph nodes (Loose et al.,
1977, 1978).
In addition, Carter & Clancy (1980) observed an increased graft versus
host response in a decreased number of spleen cells in 4BALB/c mice,
which were exposed to a single intraperitoneal dose of 1000 mg Aroclor
1242/kg body weight in corn oil. Spleen enlargement and lymphocyte
depletion were observed.
Offspring of Swiss-Webster mice, exposed via the dams which were fed
Aroclor 1254 at dietary levels of 10, 100, or 250 mg/kg, did not
exhibit an altered hypersensitivity reaction to oxazoline, an altered
anti-bovine serum albumin antibody titre or an altered degree of
phagocytosis of sheep red blood cells by peritoneal macrophages,
compared with controls (Talcott & Koller, 1983).
Pathogen-free ICR/JCL mice (aged 4 weeks) were intubated orally, once
a week, for 4 weeks, with 0, 10, or 100 µg Kanechlor 500/kg body
weight. Two days after the final treatment, half of the animals of
each group were injected intraperitoneally with 0, 50, 250, or 500 µg
E. coli endotoxin/mouse. Sensitivity to endotoxin was determined by
24-h mortality rate. The oral administration of Kanechlor 500 up to a
dose level of 100 µg/kg body weight did not have any effect on the
sensitivity to the endotoxin (Oishi & Hiraga, 1980).
The relative potencies of PCB mixtures Aroclors 1260, 1254, 1248,
1242, 1016, and 1232 to inhibit the murine, splenic, plaque-forming
cell response to sheep red blood cells was determined for C57Bl/6
mice. The ED50 values for the reduction in the splenic,
plaque-forming cells were 104, 118, 190, 391, 408, and 464 mg/kg body
weight, respectively. It was apparent that the higher PCBs (Aroclors
1260, 1254, and 1248) were more potent than the lower chlorinated
mixtures.
Previous studies have shown that a subeffective dose of Aroclor 1254
(25 mg/kg), interacted with an immunotoxic dose of TCDD (3.7 nmol/kg),
resulting in, a significant antagonism of the toxicity of the latter
compound. Co-treatment of mice with a dose of all these PCB mixtures
at 25 mg/kg and a reconstituted PCB mixture, as occurs in breast milk,
in combination with TCDD (3.7 nmol/kg) showed that all (except Aroclor
1232) significantly antagonized the TCDD-mediated inhibition of the
splenic, plaque-forming cell response in C56Bl/6 mice (Davis & Safe,
1989).
Table 52. The humoral and cell-mediated immunotoxicity of PCBs administered via the diet in short-term studies
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Monkey
Rhesus Aroclor 44 anti-SRBC antibody titre D 5.0 (d) 2.5 (d) Thomas &
1248 anti-tetanus toxicoid Hinsdill
antibody titre NE - 5.0 (d) (1978)
serum gamma-globulin
fraction D 5.0 (d) 2.5 (d)
Cynomolgus Kanechlor 20 anti-SRBC antibody titre D 2 (bw) Hori et al.
400 serum gamma-globulin (1982)
(purified) fraction D 2 (bw)
Cynomolgus Aroclor 21 anti-SRBC antibody titre D 2.5 (d) Truelove
1254 anti-tetanus toxicoid et al. (1982)
antibody titre NE 10 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Rabbit
New Zealand Aroclor 5 anti-SRBC antibody titre NE 6.54 (bw) Street &
1254 serum gamma-globulin Sharma
levels NE 6.54 (bw) (1975)
delayed hypersensitivity
reaction to tuberculin NE 6.54 (bw)
popliteal lymph node
antibody-forming cells D 0.92 (bw) 0.18 (bw)
Guinea-pig
Clophen 4-7 anti-tetanus toxicoid Vos & van
A60 and antibody titre D 50 (d) 10 (d) Driel-
Aroclor delayed hypersensitivity Grootenhuis
1260 reaction to tuberculin D 50 (d) 10 (d) (1972)
anti-tetanus toxicoid
producing cells in
popliteal lymph nodes D 50 (d) 10 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Rat
Sprague- Aroclor 1 mitogen response to Bonnyns &
Dawley 1254 phytohaemagglutinin I 250 (d) Bastomsky
response to poke-weed (1976)
mitogen NE 250 (d)
serum gamma-globulin
fraction D 250 (d)
Sprague- Aroclor 10 interleukin 2 production Exon et al.
Dawley 1254 induction by KLH D 50 (d) (1985)
natural killer cell
cytotoxicity D 50 (d)
anti-KLH antibody titer D 50 (d)
Mouse
ICR Kanechlor 3 host-resistance to herpes Imanishi
500 simplex virus D 33 (bw) 18 (bw) et al. (1980)
host-resistance to
ectomelia virus D 33 (bw) 18 (bw)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
ICR Kanechlor 3 host-resistance to Imanishi
500 influenza virus D 400 (d) 200 (d) et al. (1984)
host-resistance to
Staphylococcus aureus D 100 (d)
ICR/JCL Kanechlor 4 sensitivity to NE - 100 µg/kg Oishi &
500 E. coli endotoxin (bw) Hiraga
(1980)
Swiss-Webster Aroclor 12 hypersensitivity reaction NE - > 250 (d) Talcott &
1254 to oxazoline, anti-bovine Koller
serum albumine antibody (1983)
titre and phagocytosis
of SRBC by macrophages
BALB/c Aroclor 3-6 host-resistance to Loose et al.
1242 endotoxin D 167 (d) (1978)
host-resistance to
malaria D 167 (d)
BALB/c Aroclor 6 spleen cellularity NE 167 (d) Loose et al.
1242 spleen PFC D 167 (d) (1977)
serum immunoglobulins
G1, A, M D 167 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
C57BL/6 Aroclor 3-41 graft versus host response NE 167 (d) Silkworth &
1016 mixed lymphocyte Loose (1979)
response I 167 (d)
mitogen response to
lipopolysaccharide I 167 (d)
mitogen response to
concanavalin A I 167 (d)
Mus musculus Aroclor 5 host-resistance to Thomas &
1248 Salmonella typhimurium D 1000 (d) Hinsdill
host-resistance to (1978)
endotoxin D 1000 (d)
a Up to day of primary immunization.
b SRBC = Sheep red blood cells; KLH = Keyhole limpet haemocyanin; PFC = Plaque forming cells.
c I = Increased; D = Decreased; NE = No effect found.
d LOEL = Lowest-observed-effect-level; NOEL = No-observed-effect level: in mg/kg diet (d)
or mg/kg body weight per day (bw).
A single administration of 500 mg Aroclor 1254/kg, intraperitoneally,
inhibited the plaque-forming (PFC) response to subsequent challenge
with sheep erythrocytes in Ah locus positive (C57Bl/6N or B6C3F1N)
mice. However, Aroclor 1254 did not give induction in the Ah locus
negative DBA/2N mice. When B6C3F1 mice were challenged with sheep red
blood cells, 6 or 16 weeks after Aroclor 1524 treatment, substantial
recovery of a PFC response was observed. In older (76-week-old) B6C3F1
mice severe depression of the PFC response was observed.
In contrast with its profound depression of a PFC response, Aroclor
1254 (up to 1250 mg/kg) caused a slight increase in lymphocyte
proliferation induced by either T or B cell mitogens. A single
500 mg/kg dose of this Aroclor also suppressed the ability of
recipient B6C3F1 animals to reject a challenge with either the
syngenic fibrosarcoma (PYB6) or the gram negative pathogen Listeria
monocytogenes (Lubet et al., 1986).
Heinzow et al. (1988) studied the effect of 2,4,5,2',4',5'-hexa-
chlorobiphenyl in the E rosette formation with sheep red blood cells
(SRBC) as one of the characteristics of human T-lymphocytes. The
minimal concentration eliciting a significant monoclonal CD2 receptor
antibody sparing effect was 1.5 × 10-10 mol/litre.
C3H/HeN mice were treated, twice a week, for 2 or 3 weeks prior to
mating, with olive oil or with Kanechlor 500 at an oral dose of
50 mg/kg body weight. The offspring, some of which were nursed by
unexposed dams, were tested for immunocompetence, 4-15 weeks after
birth. The dams did not show any adverse effects on body weight,
absolute spleen weight, and spleen cellularity. The B-cell activity of
the offspring was comparable with that of controls. The helper T-cell
activity was reduced up to 7-11 weeks after birth: the effect was more
pronounced in prenatally-exposed groups (Takagi et al., 1989).
(b) Rabbit
Rabbits appear most sensitive with respect to the immunotoxicity of
PCBs. Street & Sharma (1975) exposed groups of 5-7 New Zealand rabbits
to Aroclor 1254 at dietary levels of 0, 3.7, 20, 45.8, or 170 mg/kg
(equivalent to 0, 0.18, 0.92, 2.1, or 6.54 mg/kg body weight) for
44-57 days. When compared with control animals, an increased degree of
thymus atrophy was observed at all dose levels except for 20.0 mg/kg.
At the 2 higher dose-levels, relative spleen weights were decreased
and the number of germinal centres reduced. When 8 female New Zealand
rabbits received 118 mg of Phenochlor DP6, Clophen A60, or Aroclor
1260 (free of PCDFs), on the back skin, 5 times per week, for 38 weeks
they showed leukopenia, thymus atrophy, and loss of germinal centres
in the spleen and lymph nodes. No such changes were observed in the 4
controls (Vos & Beems, 1971). Vos & Notenboom-Ram (1972) found the
same effects in rabbits administered 120 mg Aroclor 1260 (free of
PCDFs)/day, 5 days/week, for 4 weeks. No adverse gross immunotoxic
effects were observed in groups of 10 New Zealand rabbits fed various
Aroclors at dose levels of 150 mg/kg body weight, once a week, for
12-14 weeks (Koller & Thigpen, 1973).
When New Zealand rabbits were exposed orally, via intubation, to
Aroclor 1242 at a dose of 150 mg/kg body weight, once a week, for 11
weeks, the anti-pseudo rabies virus antibody titre and serum
gamma-globulin levels were decreased (Koller & Thigpen, 1973). The
overall picture is one of immunosuppression, though in 2 diet studies
an increased activation of the cell-mediated immune response was
observed (Bonnyns & Bastomsky, 1976; Silkworth & Loose, 1979).
New Zealand rabbit offspring, exposed via the dams fed 0, 10, 100, or
250 mg of Aroclor 1248/kg diet, showed a decrease in the delayed
hypersensitivity reaction to dinitrofluorobenzene in the highest dose
group. No effects were observed on the splenic, plaque-forming cell
response, the antibody titre against sheep red blood cells, and the
mitogen responses to Concanavalin A and Phytohaemagglutinin (Thomas &
Hinsdill, 1980).
(c) Guinea-pig
Atrophy of the thymus has been reported in female guinea-pigs exposed
to Clophen A60 or Aroclor 1260 for 4-7 weeks at a dietary level of
50 mg/kg (equivalent to 2 mg/kg body weight). Following stimulation
with tetanus toxoid, the authors found a lower antitoxin titre and a
lower count of antitoxin-producing cells in comparison with control
guinea-pigs, resulting in a significant reduction in immunoglobulins.
The skin reaction after tuberculination in animals immunized with
Freund's complete adjuvant (as a parameter of cell-mediated immunity)
was also depressed at the 50 mg/kg dose level (Vos & van
Driel-Grootenhuis, 1972).
Female guinea-pigs fed diets containing 50 mg Aroclor 1260/kg for 6
weeks had significantly lowered tetanus antitoxin titres, circulating
leukocytes and lymphocytes, and thymus atrophy (Vos & van Genderen,
1973). Also treatment with 10 mg Aroclor 1260/kg diet for 8 weeks
produced splenic atrophy (Vos & de Roij, 1972). The Aroclor 1260 was
free from PCDFs (no details).
(d) Monkey
Offspring of Rhesus monkeys appeared very sensitive to the toxic
effects of PCB exposure during gestation and nursing, as already
discussed in section 8.4.1. Thymic atrophy, loss of germinal centres
and of lymph nodules of the spleen, and bone marrow hypocellularity
were found in 3 out of 6 offspring that died during their first year
of life due to exposure to PCBs via their mothers. The mothers, 9-12
per group, were fed diets containing 0, 2.5, or 5.0 mg of Aroclor
1248/kg (equivalent to 0, 0.09, and 0.2 mg/kg body weight), for 18
months and were bred after 6 months of exposure. One mother in each
exposed group died during exposure, showing an increased
susceptibility to Shigella flexneri. At autopsy, no lesions were
observed in lymphoid organs and tissues (Allen & Barsotti, 1976;
Barsotti et al., 1976). When the surviving mothers were placed on a
control diet for approximately 1 year after exposure and then rebred,
there was a decided improvement in their health, but their infants
were still severely affected by PCBs. In the 2 offspring in each
exposed group that died after weaning at the age of 4 months, the
effects on the thymus, spleen, and bone marrow were similar to those
described for the first generation (Allen et al., 1980).
Groups of mature, female Rhesus monkeys received diets containing 0,
2.5, or 5.0 mg Aroclor 1248/kg. After 11 months, all monkeys received
intravenous injections of sheep red blood cells (SRBC) as well as an
intramuscular injection of tetatus toxin (TT). Booster injections and
a second TT injection were given after a number of weeks. Blood
samples were taken over a period of 20 weeks after immunization.
The anti-sheep red blood cells (SRBC) antibody titres, and antibody
response to TT were not clearly affected. The gamma-globulin-levels
were lower in the PCB-treated animals. After 6 months, the PCB-treated
monkeys developed chloracne, alopecia, and facial oedema (Thomas &
Hinsdill, 1978).
Hori et al. (1982) also found immunosuppression in monkeys exposed to
PCB mixtures (without detectable quantities of PCDFs), compared with 2
control monkeys. A more severe immunosuppression was observed in
another monkey exposed to a comparable PCB mixture with PCDFs.
In a pilot study, one infant Cynomolgus monkey, the mother of which
had been exposed to Aroclor 1254 at a dose-level of 400 µg/kg body
weight, showed a decreased anti-sheep erythrocyte antibody titre
following primary immunization in comparison with one control infant
(Truelove et al., 1982).
Atrophy and loss of germinal centres in the spleen and other lymphoid
tissues were observed in groups of 5-6 female Cynomolgus monkeys
following exposure to Aroclor 1254 or 1248, in an apple juice-corn oil
emulsion, 3 times per week, at dose levels of 5 and 2 mg/kg body
weight, respectively, until death at day 29-164. The monkeys exposed
to Aroclor 1254 showed bone marrow hypocellularity and leukopenia.
Lesions seen in control monkeys were similar to those described as
spontaneous (Tryphonas et al., 1984; see also section 8.2.1).
Limited data exist on the humoral or cell-mediated responses in
infants, exposed to PCBs via their mothers.
8.6.8.2 Effects of individual congeners
Thymus atrophy was observed in monkeys exposed for 1-6 months to
3,4,3',4'-tetrachlorobiphenyl, but not in monkeys exposed to
2,5,2',5'-tetrachlorobiphenyl (McNulty et al., 1980).
Decreased relative weights of the thymus and increased or decreased
relative weights of the spleen were induced by single intraperitoneal
doses of the planar congeners 3,4,3',4'-tetrachlorobiphenyl (at
10 mg/kg body weight) and 3,4,5,3',4'-pentachlorobiphenyl (at
245 mg/kg body weight) in "responsive" C57BL/6 mice, i.e., mice
possessing the cytosolic Ah receptor protein, but not, or only to a
minor degree, in "non-responsive" DBA/2 mice (lacking this receptor).
The tetrachlorobiphenyl further decreased the number of cells per
spleen and the number of splenic, plaque-forming cells at 10 and
100 mg/kg body weight, respectively. Further chlorination at the
ortho-position decreased the toxicity of these congeners. No adverse
effects were induced by 2,4,5,3',4'- and 3,4,5,2',4'-pentachloro-
biphenyl (at 490 mg/kg body weight), 2,3,4,5,3',4',5'-heptachloro-
biphenyl (at 593 mg/kg body weight) and the di- ortho substituted
tetrachlorobiphenyls (at 100 mg/kg body weight (Silkworth & Grabstein,
1982; Robertson et al., 1984; Silkworth et al., 1984).
Similar trends were observed in Wistar rats with respect to the
induction of decreased relative thymus and spleen weights (see section
8.2.1.1). Biocca et al. (1981) exposed C57BL/6J mice to various
hexachlorobiphenyl isomers in the feed for 28 days. The most toxic
isomer tested was 3,4,5,3',4',5'- hexachlorobiphenyl which, among
others, caused a marked thymus atrophy and a moderate depletion of
lymphocytes in the spleen. 2,4,6,2',4',6'-Hexachlorobiphenyl caused
the same lesions, albeit at much higher dose-levels, while the
2,4,5,2',4',5'- and 2,3,6,2',3',6'-hexachlorobiphenyls were virtually
inactive.
The in vivo generation of cytotoxic T-lymphocytes (CTL) in response
to allogeneic tumour challenge is sensitive to suppression by
3,4,5,3',4',5'-hexachlorobiphenyl, a poorly metabolized, Ah
receptor-binding PCB isomer. Groups of 5-8 C57Bl/5 mice treated with a
single oral dose of 0, 10, or 100 mg 3,4,5,3',4',5'-hexachloro-
biphenyl/kg body weight, 2 days prior to the intraperitoneal injection
of allogeneic P815 tumour cells, exhibited a dose-dependent reduction
in peak CTL activity in the spleen. When examined on a kinetic basis,
the TCL response was reduced in magnitude with no evidence for a shift
in the kinetics of the response induced by 3,4,5,3',4',5'-hexachloro-
biphenyl.
3,4,5,3',4',5'-Hexachlorobiphenyl exposure, prior to antigen challenge
(day -14, -7, or -1 relative to P815 injection on day 0), produced
significant suppression of the CTL response. 3,4,5,3',4',5'-
Hexa-chlorobiphenyl treatment (10 mg/kg body weight), 6 weeks prior to
such a challenge, was still significantly suppressive, though the
reduced degree of suppression suggested that recovery was in progress.
When 3,4,5,3',4',5'-hexachlorobiphenyl exposure occurred after antigen
challenge, significant suppression was produced only when exposure
occurred within the first 3 days of the response, suggesting that, as
the CTL matured, their sensitivity to 3,4,5,3',4',5'-hexachloro-
biphenyl diminished. Clearance of the allogeneic tumour cells from the
peritoneal cavity was delayed in 3,4,5,3',4',5'-hexachlorobiphenyl-
treated mice and was associated with an altered composition of the
white blood cell infiltrate in this cavity. Symptoms of overt
toxicity, as well as immunotoxicity, were apparent at lower doses of
3,4,5,3',4',5'-hexachlorobiphenyl in male compared with female mice.
In addition, interactive effects of 3,4,5,3',4',5'-hexachlorobiphenyl
exposure and P815 antigen challenge on body weight and thymic
involution were observed in both male and female mice (Kerkvliet &
Baecher-Steppan, 1988a).
A modest dose-dependent suppression of the proliferative response to
alloantigen in mixed lymphocyte culture (MLC) was observed with
lymphocytes from C57Bl/6 mice (groups of 5-6 mice) exposed to 10 or
100 mg 3,4,5,3',4',5'-hexachlorobiphenyl, while the cytotoxic
T-lymphocytes CTL response generated in MLC was significantly
suppressed only with 100 mg/kg. The amount of time between treatment
with 3,4,5,3',4',5'-hexachlorobiphenyl and sacrifice, which ranged
from 2 to 23 days, did not appear to influence the degree of
immunosuppression produced by 3,4,5,3',4',5'-hexachlorobiphenyl
exposure. Mitomycin C-treated lymphocytes from C57Bl/6 mice treated
with 10 or 100 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body weight,
were not suppressive when added as third party cells to an independent
MLC. However, if the mice were alloimmune, lymphocyte-mediated
suppression of the MLC response was observed and directly correlated
with the magnitude of the CTL response present in the same population.
Thus, 3,4,5,3',4',5'-hexachlorobiphenyl-treated mice that had less CTL
activity compared with vehicle-treated mice also had less suppressor
activity. Avoidance of stimulator cells lysis by using H-2
incompatible MLC stimulator cells revealed the existence of
antigen-nonspecific suppressor activity that was greater with
lymphocytes from vehicle-treated mice than from 3,4,5,3',4',5'-
hexachlorobiphenyl-treated mice, suggesting that both CTL and
suppressor cell activities were suppressed by 3,4,5,3',4',5'-
hexa-chlorobiphenyl exposure. Direct addition of 3,4,5,3',4',5'-
hexachloro-biphenyl to lymphocyte cultures in vitro indicated
a lack of direct toxicity of 3,4,5,3',4',5'-hexachlorobiphenyl on
lymphoproliferative responses to mitogen or alloantigen at
concentrations equal to or less than 1 × 10-6 mol/litre. Thus,
the in vitro functional integrity of lymphocytes obtained from
3,4,5,3',4',5'-hexachlorobiphenyl-treated mice coupled with the lack
of a direct lymphocytic effect in vitro suggest an indirect
mechanism of action for the 3,4,5,3',4',5'-hexachlorobiphenyl-mediated
suppression of CTL activity in vivo. Previous reports implicating
suppressor cell induction and/or activation by Ah-receptor-binding,
halogenated, aromatic hydrocarbons that mediate the inhibition of CTL
generation were not confirmed (Kerkvliet & Baecher-Steppan, 1988b).
8.6.8.3 Appraisal
The alterations in gross measures of immunological function (spleen
and thymus weights, lymphocyte counts, histology of lymphoid organs
and tissues) in mammals are highly suggestive of an immunosuppressive
effect of PCB mixtures and some higher chlorinated congeners. More
direct evidence of an immunodepressive effect has been obtained by
methods that detect functional alterations in the humoral and
cell-mediated immunity in mammals. One study on monkeys demonstrated a
more severe immunosuppression by a PCDF-contaminated PCB mixture
compared with a non-contaminated PCB mixture. Rabbits and monkeys are
the most sensitive species. No-observed-effect levels are 100 µg
Aroclor 1248/kg body weight per day and <100 µg of Aroclor 1254/kg
body weight per day for monkeys and 180 µg of Aroclor 1254/kg body
weight per day for rabbits.
8.6.9 Neurotoxic effects
Depressed spontaneous motor activity was shown by male CD-mice exposed
to a single oral dose of 500 mg Aroclor 1254/kg body weight in
Emulphor:saline. No effects were found in motor coordination tests and
on pentenylene-tetrazol-induced seizures. Neurochemical tests with
isolated mouse brain synaptosomes showed inhibition of the uptake of
neurotransmitters and precursors, and stimulation of the release of
neurotransmitters (Rosin & Martin, 1981).
Male Wistar rats exposed to doses of 500 or 1000 mg Aroclor 1254 and
1260/kg body weight, in corn oil, showed a reduced norepinephrine
concentration in the frontal cortex and hippocampus. No changes were
measured in the hypothalamus and brainstem. The neurochemical effects
appeared to be associated with the actual presence of PCBs in the
tissues (Seegal et al., 1985).
8.6.10 Skin effects
Cutaneous effects occurred in Rhesus monkeys fed diets that contained
Aroclors, for short periods (Allen & Norback, 1973; Allen et al.,
1974a; Allen, 1975; Barsotti et al., 1976; Thomas & Hinsdill, 1978;
Allen et al., 1979; Altman et al., 1979; Becker et al., 1979;
McConnell et al., 1979; McNulty et al., 1980). The effects included
facial (particularly periorbital) oedema, purulent discharge from the
eyes, chloracne, and alopecia. The effects, which appeared to be
reversible, were produced by doses as low as 2.5 mg Aroclor 1248/kg
for 1-6 months, and 1 mg Aroclor 1242/kg (equivalent to 0.04 mg/kg
body weight) for 6 months. Rats exposed to Aroclor 1254 in the diet
developed alopecia and facial oedema after 104 weeks at 50 mg/kg, and
exophthalamos after 72 weeks at 50 mg/kg. These effects did not occur
after 104 weeks at 25 mg/kg (equivalent to 1.25 mg/kg body weight)
(NCI, 1978).
8.6.11 Effects on the lung
Many PCBs are metabolized to methylthio derivatives in mice (Mio et
al., 1976), seals (Jensen & Jansson, 1976) and humans (Yoshida &
Nakamura, 1979). The metabolic pathways leading to the generation of
these metabolites have been shown to involve glutathione conjugation
of an arenoxide intermediate (Preston et al., 1984). Lund et al.
(1986) studied the interactions of these metabolites with the lung. It
was found that the PCB metabolite, 4,4'-bis-(methylsulfonyl)-
2,5,2',5'-tetrachlorobiphenyl ((MeSO2)2TCB), selectively accumulates
in the Clara cells of the bronchiolar epithelium and in the secretory
contents of the bronchiolar lumen. In vitro characterization of this
interaction of tritiated (MeSO2)2TCB with lung suggests that this
selective accumulation is due to the presence of a secreted
(MeSO2)2TCB-binding protein in the respiratory tract of rats, mice,
and humans. The protein appears to be an almost globular,
low-relative-molecular-mass acidic protein that binds with
methylsulfonyl-PCBs (Lund et al., 1986).
In a study on 3 groups of C57Bl mice, administered orally 0, 10, or
100 mg 2,3,6,4'-tetrachlorobiphenyl/kg body weight with repeated
administration of 35S-cysteine, Klasson-Wehler et al. (1987) found
that the major compound present in the lung was 4-methylsulfonyl-
tetrachlorobiphenyl, indicating the presence of specific binding sites
for this metabolite in lung tissue, mainly in the tracheo-bronchial
mucosa.
Groups of 20 male SD rats were given (gastric intubation) 0 or 25 mg
Kanechlor 400 in edible oil once, and, in 2 other groups, the same
doses 4 times per week. Groups of 5 animals were killed 2, 7, 14, and
28 days after the last ingestion, and tissues were studied with light
and electron microscopy. The lungs of the rats particularly showed
peribronchiolar cell infiltrations, and electron microscopy revealed
lipid vacuoles and altered lamellar bodies or lysosomes in type II
alveolar cells and alveolar macrophages. These changes were most
marked 7 days after the last ingestion and were more severe in the
short-term application (Shigematsu et al., 1978).
8.6.12 Miscellaneous
Haake et al. (1987) used mature, male C57Bl/6J mice and virgin, female
C57Bl/6N mice to study the influence of Aroclor 1254 on the
2,3,7,8,-TCDD induction of teratogenic abnormalities. Dams were
treated by oral gavage with either corn oil, Aroclor 1254 (244 mg/kg),
or TCDD (20 µg/kg) or Aroclor followed by TCDD or Aroclor followed by
dexamethasone (90 mg/kg). Aroclor 1254 alone was administered on day
9, corn oil and TCDD on day 10, and dexamethasone on day 13. In the
combinations, the Aroclor was given the day before the TCDD or
dexamethasone. TCDD induced 61.8 ± 23.1% cleft palate per litter;
Aroclor with TCDD, 8.2 ± 1.5%; Aroclor alone, 0%; dexamethasone alone,
69.9 ± 18.2%, and Aroclor followed by dexamethasone, 85.8 ± 29.1%.
Previous studies have shown that Aroclor 1254 can act as a partial
antagonist of the microsomal enzyme induction and immunotoxic effects
of TCDD in the mice strain used and, in this study, it was shown that
Aroclor 1254 also antagonizes TCDD-mediated teratogenicity. It did not
have any effect on the effects mediated by dexamethasone.
Wölfle et al. (1988) found that treatment of male Wistar rats with
200 mg 3,4,3',4'-tetrachlorobiphenyl/kg body weight, injected
intraperitoneally, markedly stimulated growth of enzyme-altered liver
foci and [3H]-thymidine incorporation into nuclear DNA. In the liver,
enlarged hepatocytes, due to hypertrophy, and fine-to-medium
fatty-droplet deposition in hepatocytes were found, but no liver
necrosis. Hence, it was concluded, that post-necrotic regenerative
growth as the cause of the tetrachlorobiphenyl-mediated stimulation of
hepatocytes proliferation, could be excluded. The treatment with
tetrachlorobiphenyl in vivo, markedly increased EGF-stimulated
autophosphorylation of the EGF-receptor (a plasma membrane protein) in
liver plasma membranes. These results suggest that altered growth
control is due to a direct effect of tetrachlorobiphenyl on
hepatocytes.
8.7 Factors modifying toxicity; mode of action
8.7.1 Factors modifying toxicity
As PCBs can stimulate microsomal enzyme activity, it can be expected
that they may potentiate the action of other chemicals that undergo
microsomal activation, and antagonize the action of those that are
detoxified. Antagonism was for example observed in studies on rodents
with drugs like pentobarbital (Villeneuve et al., 1972; Sanders et
al., 1974; Sanders & Kirkpatrick, 1975; Rosin & Martin, 1983),
hexobarbital (Bickers et al., 1972; Tanaka & Komatsu, 1972), and
zoxazolamine (Bickers et al., 1972).
Lashneva et al. (1985) and Khan et al. (1985) found potentiation of
the rate of microsomal enzyme induction in rats with a combination of
50 mg Sovol (mixture of PCBs)/kg body weight and 500 mg 2,6-ditert-
butyl-4-methylphenol (ionol)/kg body weight.
Villeneuve et al. (1972) demonstrated the antagonistic effect by the
reduction of phenobarbital sleeping time in rats receiving Aroclors
1242, 1254, and 1260 in their diet, but not in those receiving Aroclor
1221. This was confirmed by Johnstone et al. (1974) with a series of
single PCBs. Tanaka & Komatsu (1972) found that the hexobarbital-
induced sleeping time in female rats was reduced to 49% of the control
value by daily oral doses of Kanechlor 500 of 2 mg/kg for 3 days
(total 6 mg/kg). When a daily dose of 0.4 mg/kg was given for 15 days
(total 6 mg/kg), no reduction in sleeping time was observed. When this
small dose was continued for 45 and 53 days, the reduction remained at
12-13%. Phillips et al. (1972) did not find any potentiation of the
cholinesterase-inhibitory action of parathion in rats dosed with
Aroclors 1221 and 1254; this does not necessarily imply that there was
no enhanced activation of parathion, as a stimulation of detoxication
may have occurred concurrently. A stimulation of parathion
detoxication, but not of activation, has been demonstrated in rabbit
microsomes (Villeneuve et al., 1971a). Lichtenstein (1972) reported a
potentiation by PCBs of the toxicity of parathion for flies.
Aroclor 1254 at 160 mg/kg diet fed to 5-week-old male and female
Fischer-344 rats, for 8 weeks, reduced mortality due to feeding
hexachlorophene at a concentration of 600 mg/kg diet, from 77% to 7%
and completely prevented the paralysis that was observed in all
animals on the hexachlorophene diet alone. However, histological
changes in the brain characteristic of hexachlorophene exposure were
still apparent in the animals on the combined treatment, and the
possibility of delayed toxicity beyond the 8 weeks of the study could
not be eliminated. The protective effect of Aroclor 1254 was explained
by its capacity to enhance detoxication by means of hepatic microsomal
enzyme induction (Jones et al., 1974).
Coté et al. (1985) studied a mixture of 15 "persistent" chemicals,
including Aroclor 1254, in Sprague-Dawley rats at dose levels of 0, 1,
10, 100, and 1000 times the Canadian water quality objectives (WQO) of
each chemical. The PCB (Aroclor 1254) treatments were 0, 0.001, 0.01,
0.1, or 1.0 µg/kg diet for 90 days. No influence on food intake, body
weight, organ weights, clinical chemistry, haematology, or
histopathology were observed.
As these polychlorinated hydrocarbons seem to have the same mechanism
of action, questions arise on the possible interactions between these
compounds. In one reported teratogenicity study, groups of 18-21
pregnant C57BL/6N mice received (by gavage) the vehicle corn oil, or
daily doses of 3 µg 2,3,7,8-TCDD/kg body weight, 10 or 20 mg
2,3,4,5,3',4'-hexachlorobiphenyl/kg body weight, 25 or 50 mg
2,4,5,2',4',5'-hexachlorobiphenyl/kg body weight, or combinations of
TCDD and hexachlorobiphenyl at these dose-levels in corn oil, from day
10 to day 13 of gestation. All chemicals were more than 98.9% pure and
the hexachlorobiphenyls did not contain detectable levels of
dibenzofurans or TCDD. TCDD alone caused a low incidence of cleft
palate and moderate hydronephrosis, 2,3,4,5,3',4'-hexachlorobiphenyl
only caused mild hydronephrosis, and 2,4,5,2',4',5'-hexachlorobiphenyl
did not produce any effects. However, treatment of pregnant mice with
a combination of TCDD and 2,3,4,5,3',4'-hexachlorobiphenyl caused
5- and 10-fold increases in the incidence of cleft palate at 10 and
20 mg of hexachlorobiphenyl/kg body weight, respectively. No
enhancement of TCDD-induced hydronephrosis was observed, and the
incidence of TCDD-induced cleft palate was not affected by
simultaneous 2,4,5,2',4',5'-HCB treatment (Birnbaum et al., 1985).
Male Sprague-Dawley rats were given a regimen consisting of PCBs,
1 mg/day; polychlorinated quarterphenyls (PCQs), 1 mg/day; PCDFs,
10 µg/day or a mixture of PCBs, PCQs, and PCDFs (1 mg + 1 mg +
10 µg/day) in olive oil, orally, for 22 days. The congeners and ratios
in the PCBs, PCQs, and PCDFs were the same as those in Japanese Yusho
oil (see section 9.1.2.1). The PCB-treated rats showed hepatic
hypertrophy, immunosuppression, and increased drug-metabolizing enzyme
activities in hepatic microsomes. PCQ treatment did not produce any
significant effects. PCDF and the mixture PCBs + PCDFs caused
hypertrophy of the liver, immunosuppression, increased and
drug-metabolizing enzyme activity to a much greater extent than that
found for PCBs (more than 100 times more) and weight loss and thymic
atrophy (Kunita et al., 1985).
Female Cynomolgus monkeys were administered PCBs (5 mg), PCQs (5 mg),
or a mixture containing 5 mg PCBs + 20 µg PCDFs in olive oil injected
in a piece of banana, daily, for 20 weeks. The PCBs and PCDFs
comprised the same congeners as those in Japanese Yusho oil. The
PCB-treated monkeys showed hepatic hypertrophy, immunosuppression, and
increased drug-metabolizing enzyme activities in hepatic microsomes,
but were devoid of the dermal symptoms characteristic of Yusho. PCQs
caused an increase in drug-metabolizing enzyme activities in hepatic
microsomes and immunosuppression, but these effects were much less
severe than those of PCBs. The mixture with PCDFs caused hypertrophy
of the liver, immunosuppression, increase in drug-metabolizing enzyme
activities (more than 100 times that of PCBs) and weight loss and
thymic atrophy. Dermal symptoms characteristic of Yusho patients were
also found, but not with PCBs or PCQs alone (Kunita et al., 1985).
8.7.2 Mechanisms of toxicity
The analogous structure-activity relations of individual PCB congeners
with respect to most of their toxic responses and to their potency in
inducing cytochrome P-448-dependent aryl hydrocarbon hydroxylase,
indicate that the most active PCB congeners (the coplanar congeners)
are those that are approximate stereoisomers of 2,3,7,8,-tetra-
chlorodibenzo- p-dioxin (TCDD). These findings suggest a common
mechanism of action.
As is proposed for 2,3,7,8-TCDD, this mechanism is based on the
binding affinity of PCB congeners to the cytosolic Ah-receptor
protein, a product of the regulator Ah gene (Poland & Glover, 1977;
Parkinson & Safe, 1981; Bandiera et al., 1982). The induction is
dependent on the position and number of chlorine atoms in the molecule
and the congeners that bind most strongly to the Ah-receptor show the
strongest induction of monooxygenases and the highest toxicity
(effects on the liver including increase in liver weight, increase in
liver enzyme activity and lipid content, porphyria, atrophy of the
thymus, and effects on reproduction) (Ecobichon & Comeau, 1975;
Goldstein, 1980). These very active congeners are the non
ortho-substituted PCBs 3,4,3',4'- tetrachloro-, 3,4,5,3',4'-
pentachloro-, and 3,4,5,3',4',5'-hexachlorobiphenyl, which are at
least twice substituted in the meta and para positions.
In this model, the inducer-receptor complex is translocated into the
nucleus, interacts with DNA, and eventually triggers the pleiotropic
responses observed. The role of the receptor protein in the mechanism
of action of PCBs is further substantiated by the differential effects
of the congeners in non-responsive DBA/2J mice and responsive C57Bl/6J
mice. Furthermore, there is a good relationship between the aryl
hydrocarbon hydroxylase and ethoxyresorufin O-deethylase induction
potencies in rat hepatoma H-4-II-E cells in vitro (Sawyer & Safe,
1982) and their relative binding affinities for the male Wistar rat
hepatic cytosol receptor protein (Bandiera et al., 1982; Safe et al.,
1985b).
Thus, the coplanar PCBs have mechanisms of action similar to those of
the polychlorinated dioxins (PCDDs) and dibenzofurans (PCDFs) (see
also WHO, 1989).
On the basis of the comparative toxic and biochemical potencies of
coplanar and mono ortho coplanar PCBs, Safe (1990) suggested toxic
equivalent factors TEFs (relating to 2,3,7,8-tetrachloro-dibenzo-
p-dioxin, TCDD, see WHO/EURO, 1987) for these compounds. See Table
53. Although there are certain limitations and uncertainties
associated with the use of TEFs (WHO/EURO, 1987), they may be useful
for an attempt to assess the risk of the combined exposure to coplanar
PCBs and PCDDs/PCDFs.
Table 53. Proposed Toxic Equivalent Factor (TEF) values for the
coplanar and mono ortho coplanar PCB congenersa
Congener TEF value Relative potency range
1. Coplanar PCBs
3,4,5,3',4'-PeCB 0.1 0.3-0.0006
3,4,5,3',4',5'-HxCB 0.05 0.1-0.0012
3,4,3',4',-TCB 0.01 0.009-0.00008
2. Mono ortho coplanar
2,4,5,3',4'-PeCB 0.001 0.0004-0.000006
2,3,4,3',4'-PeCB 0.001 0.0008-0.00006
3,4,5,2',4'-PeCB 0.001 0.00013-0.000018
2,3,4,5,4'-PeCB 0.001 0.00045-0.000074
2,3,4,3',4',5'-HxCB 0.001 0.0005-0.0000014
2,3,4,5,3',4'-HxCB 0.001 0.0004-0.0000065
2,4,5,3',4',5'-HxCB 0.001 0.0000055
2,3,4,5,3',4',5'-HpCB 0.001 no data available
a From: Safe (1990).
The frequently occurring mixed type, the PB-type, and the
weak/noninducing type congeners with some degree of ortho
substitution may also exert other more subtle toxic effects, either
directly via conversion to hydroxylated derivatives, or indirectly
through environmental transformations involving regiospecific
dechlorination followed by hydroxylation.
In addition, some PCBs, particularly the lower chlorinated ones, can
be more readily metabolized through arene oxide intermediates that may
be directly genotoxic/carcinogenic. Arene oxide intermediates are also
involved in the formation of the methylsulfonyl metabolites of PCBs,
which selectively accumulate in the Clara cells of the bronchiolar
epithelium and in the secretory contents of the bronchiolar lumen of
the lungs. This apparently also involves specific binding proteins
that may be important in the expression of certain types of pulmonary
toxicity.
Finally, there are other forms of toxicity associated with PCBs that
appear to involve certain non-receptor, protein-binding interactions.
8.7.3 Toxicity of impurities in commercial PCBs
In many toxicological studies on the effects of commercial PCB
mixtures, the quantitative contribution of impurities to the toxic
responses found is largely unknown (WHO, 1989; WHO/EURO, 1987).
9. EFFECTS ON HUMANS
The toxicological evaluation of PCBs presents many problems. PCBs
usually occur as mixtures of many congeners, and many of the data on
the toxicity of the PCBs are based on the testing of these mixtures.
Some components of the mixtures are more easily degraded in the
environment than others. Thus, the general population may be exposed
to mixtures that are different from those to which workers, working
with PCBs, are exposed.
There are great difficulties in assessing human health effects
separately for PCBs, since, quite frequently, PCDFs have been present
in the PCB mixtures to which humans have been exposed. The presence of
PCDDs has occasionally been seen in accidents with certain
PCB/chlorobenzene mixtures. Commercial PCBs have been shown to be
contaminated with PCDFs and, therefore, in many cases it is unclear
whether effects were attributable to the PCBs themselves or to the
much more toxic PCDFs.
Because PCBs are ubiquitous and very persistent in the environment,
humans have been, and will continue to be, exposed to them,
particularly in industrialized countries. PCBs may be inhaled in small
amounts through the air or ingested through food. People are primarily
exposed to PCBs by consuming fish from contaminated water, but they
can be also exposed via other food.
Furthermore, exposures have occurred through accidents and
occupational exposure; in the latter case, for example, during the
repair of transformers, capacitors, or during the handling of toxic
wastes.
Since PCBs are lipophilic, they are preferentially stored in adipose
tissue. They are also present, to a smaller extent, in serum, organs
and tissues, and human milk. The concentrations of PCBs in the
different organs depend on the lipid content of such organs, with the
exception of the brain, where the concentration is lower than the
lipid content would indicate. PCBs pass, to a certain extent,
(depending on chlorination and structure) through the placenta. They
are primarily excreted through the bile and milk. In addition to the
lipid content, the ratios between adipose tissue, blood, and organs
are influenced by exposure level, sex, age, duration of exposure, and
also by whether exposure is current (see section 5).
Since human milk is relatively easy to obtain, it has been used to
monitor human exposure. Results of the many monitoring studies carried
out in many countries all over the world have shown that average
levels of total PCBs are below 2 mg/kg milk fat, though women living
in heavily industrialized urban areas or who consume large quantities
of fish from heavily contaminated areas, may have higher levels.
9.1 General population exposure
The general population is exposed to PCBs primarily by the oral route,
e.g., by consumption of fish from contaminated waters. The monitoring
data on adipose tissues, blood, and breast milk indicate that PCBs are
absorbed via the gastrointestinal tract, but do not provide
information regarding the extent of the absorption.
9.1.1 Acute effects -- poisoning incidents
No data available.
9.1.2 Effects of short- and long-term exposure
9.1.2.1 Yusho and Yu-Cheng accidents
(a) Yusho accident
In June 1968, patients appeared at the Dermatology Clinic of Kyushu
University Hospital, Fukuoka, Japan, suffering from chloracne. A group
at the University undertook intensive clinical, chemical, and
epidemiological investigations and found that the disease originated
from the consumption of a batch of rice oil supplied in February 1968;
the disease was called Yusho (rice oil disease) (Katsuki, 1969). This
batch of rice oil was found to be contaminated with Kanechlor 400, a
48% chlorinated biphenyl, at 2000-3000 mg/kg, which entered the oil
through a leak in a heat exchanger (Tsukamoto, 1969). Chlorinated
dibenzofurans at 5 mg/kg were found in 3 samples of the toxic rice oil
that contained PCB levels of about 1000 mg/kg (Nagayama et al., 1976).
The Japanese literature on this incident has been summarized in
English by Kuratsune et al. (1976). The average estimated intake was
633 mg PCBs, 3.4 mg PCDFs, and 596 mg PCQs, roughly equivalent to
157 µg PCBs/kg per day, 0.9 µg PCDFs/kg per day, and 148 µg PCQs/kg
body weight per day (Chen et al., 1985; Masuda et al., 1985).
The symptoms and signs of Yusho were described by Goto & Higuchi
(1969) and by Okumura & Katsuki (1969). The earliest signs were
enlargement and hypersecretion of the Meibomian glands of the eyes,
swelling of the eyelids, and pigmentation of the nails and mucous
membranes, occasionally associated with fatigue, nausea, and vomiting.
This was usually followed by hyperkeratosis and darkening of the skin
with follicular enlargement and acneiform eruptions, frequently with a
secondary staphylococcal infection. These skin changes were most often
seen on the neck and upper chest, but, in severe cases, extended to
the whole body. It was estimated that the mean length of the latency
period between exposure and the onset of clinical illness was 71 days,
with a range of from 20 to 190 days (Kuratsune et al., 1972).
Biopsy skin samples showed hyperkeratosis, dilation of the follicles,
and an accumulation of melanin in the basal cells of the epidermis;
melanin granules have also been observed in biopsy samples of the
conjunctiva. Oedema of the arms and legs was also seen in some
patients. There were no definite signs of liver enlargement or liver
disorders (Okumura & Katsuki, 1969), but slight rises in serum
transaminases and in alkaline phosphatase were detected, and a liver
sample from a Yusho patient showed an increase in the smooth
endoplasmic reticulum (Hirayama et al., 1969). The majority of the
patients were found to have respiratory symptoms, and suffered from a
chronic bronchitis-like disturbance that persisted for several years
(Shigematsu et al., 1971, 1978).
Kikuchi (1984) described the autopsy findings, up to July 1982, of 12
patients with Yusho including 2 stillborn babies. Characteristic
pathological changes were acne-like eruptions and cutaneous
pigmentation with histological features of follicular hyperkeratosis,
dilated hair follicles, and an increase of melanin pigment in the
epidermis. In addition, multiplication of the duct epithelium of the
oesophageal glands was found in 6 patients. Twenty-four Yusho patients
were observed clinically over the period 1968-78. During this decade,
the various clinical symptoms of the Yusho patients gradually
diminished. However, some of the symptoms and signs, such as
pigmentation of the skin, conjunctiva, and gingiva, eye discharge, and
various non-specific symptoms still remained in a number of severely
ill patients (Okumura, 1984).
Nakanishi et al. (1985) carried out clinical and experimental studies
on respiratory involvement and alterations in the immune status. PCBs
were not taken up by the bronchi, but were evenly distributed
throughout the lung parenchyma. However, specific dose dependence and
structural requirements of PCBs were shown to exist for accumulation
in bronchial mucosa. A large amount of expectoration at an early stage
of the disease may be related to this. Pathophysiological findings in
Yusho patients revealed that respiratory involvement was mainly small
airways disease, which may be caused by involvement of the cellular
component (Clara cells) in the bronchioles and/or associated with
viral or bacterial infections.
Changes in the immune status in these Yusho patients were decreases in
IgA and IgM in the serum at an early stage of the disease and then a
return to normal and suppression of cellular immunity. The changes in
immune status in these Japanese patients were comparable with the
findings in the Taiwanese patients (see below).
Hirayama et al. (1974) also reported that the serum bilirubin levels
of patients were significantly lower than the normal level and that it
was negatively correlated with the blood level of PCBs and the serum
triglyceride level.
A considerable number of patients had elevated serum triglyceride
levels, up to 4 times the normal values, though this was not
correlated with the severity of the symptoms; these high values were
maintained for 3 years in many patients (Uzawa et al., 1972). There
were no marked abnormalities in serum cholesterol and phospholipid
levels (Okumura & Katsuki, 1969; Uzawa et al., 1969). Nagai et al.
(1969) reported an increase in urinary 17-ketosteroids excretion.
Kusuda (1971) also observed changes in the menstrual cycle in
approximately 60% of 81 female Yusho patients, when compared with
their cycles prior to exposure. A positive correlation was observed by
Okumura et al. (1974) between the blood levels of triglycerides and
PCBs in 42 patients.
Shigematsu et al. (1971) examined serum immunoglobulin levels in 38
patients, 2 years after the onset of the disease, and observed a
decrease in IgA and IgM and an increase in IgG. Lower IgM levels were
reported in patients showing chloracne (Saito et al., 1972) (see also
section 9.2.4.2).
Yusho patients did not appear to suffer from central nervous effects,
but some complained of numbness of the arms and legs. Murai & Kuroiwa
(1971) found a decrease in the conduction velocity in peripheral
sensory nerves.
Determinations of PCB concentrations in the tissues of Yusho patients
were made several months after the ingestion of the oil, using an
X-ray fluorescence method for organic chlorine (Goto & Higuchi, 1969).
Abdominal fat contained 13.1 mg/kg, subcutaneous fat 75.5 mg/kg, and
nails 59 mg/kg. The mesenteric adipose tissue in 6 Yusho patients,
analysed by gas-liquid chromatography 1-3 years after the occurrence
of intoxication, contained an average PCB level of 2.5 mg/kg, which
was considerably higher than the normal value (Masuda et al., 1974a).
The mean blood level of PCBs in patients was 6 or 7 µg/litre
(3 µg/litre for the general population), 5 years after exposure
(Masuda et al., 1974b; Takamatsu et al., 1974). These authors also
noted a specific gas-liquid chromatographic pattern that was peculiar
to Yusho patients.
Eleven years after exposure, a mean concentrations of 6 µg PCB/litre
and 2 mg PCQs/litre, but no PCDFs, were found (Kashimoto et al.,
1985).
Urabe (1974) reported that the total number of Yusho patients had
reached 1200 by September 1973 and that 22 of them had died. At the
end of 1982, the number of identified patients was 1788 (Urabe &
Asahi, 1985). Mucocutaneous signs had decreased year by year, but
neurological and respiratory signs and symptoms and various
complaints, such as general fatigue, anorexia, abdominal pain, and
headache, had become more prominent among the patients. Over time, the
severity and the extent of the skin lesions decreased considerably in
the exposed population. Fifteen years after the accident, only a few
patients still had extensive chloracne.
By 1979, 31 Yusho patients had died, 11 (35.4%) of these from
malignant neoplasms. Only 21.1% of all deaths in this Japanese
prefecture would be expected from malignant neoplasms, but no clear
correlation between the occurrence of Yusho and increased deaths from
malignant neoplasms could be made, because of the small number of
deaths observed and the unknown latency period.
By the end of 1983, 120 Yusho patients had died, 41 of these from
malignant neoplasms. These included 8 stomach cancers, 11 liver
cancers, and 8 neoplasms of the lung. A statistically significant
excess mortality was seen for malignant neoplasms, cancer of the liver
and cancer of the lung, trachea, and bronchi in males, but no such
excess was noted in females. The excess from liver cancer deaths was
seen mainly in Fukuoka prefecture, while no excess was seen in the
Nagasaki prefecture (Ikeda et al., 1987).
(b) Yu-Cheng accident
In 1979, a similar incident occurred in Taiwan, the number of persons
involved, by the end of 1980, was 1843. In the course of 3.5 years,
2061 persons were determined to be victims of PCB poisoning. The
incident has been referred to as Yu-Cheng (Chang et al. 1980a,b, 1981;
Chen et al., 1980, 1981; Hsu et al., 1985). The affected persons had
consumed rice-bran oil contaminated with PCBs that was used as a heat
transfer medium in the manufacture of the oil. The PCB intake was
estimated to be 0.7-1.84 g and the latent period from the time of
intake to the onset of clinical manifestations was approximately 3-4
months. The average estimated PCDFs intake was 3.8 mg and 586 mg PCQs
(Chen et al., 1985a). Blood PCB levels ranged from 3 to 1156 µg/litre:
44.3% of 613 patients had levels of 51-100 µg/litre, and 27.6%, blood
levels over 100 µg/litre. Six months after the exposure, the
concentrations of PCBs, PCDFs, and PCQs were 12-50, 0.062-0.24, and
1.7-11 µg/litre. These blood levels were much higher than in the Yusho
incident.
The concentrations of PCBs, PCDFs, and PCQs in 6 samples of rice-bran
oil were 53-99 mg/kg, 0.18-0.40 mg/kg, and 25-53 mg/kg oil,
respectively (Chen et al., 1981; Hsu et al., 1985; Chen et al.,
1985a). Miyata et al. (1985) found averages of 62 mg PCB/kg, 140 µg
PCDFs/kg, and 20 mg PCQs/kg in 5 samples of oil. The levels of toxic
compounds in rice-oil samples collected from the factory and school
cafeterias and the families of the intoxicated patients in Taiwan were
in the range of 53-99 mg PCBs/litre (except for one sample with
405 mg/litre), 0.18-0.40 mg PCDFs/litre, and 25-53 mg PCQs/litre,
respectively. The most toxic PCB reported in commercial PCB
preparations was 3,4,3',4'-tetrachlorobiphenyl (Chen et al., 1984).
One hundred and thirty patients (46 males and 84 females), exposed
accidentally to PCBs in Taiwan, were examined for ocular
manifestations in 1979-80. Eye discharge was present in 80.5%,
swelling of the upper lids in 60.4%, pigmentation of conjunctiva in
67.6%, hypersecretion and cystic swelling of the Meibomian glands in
70.7%. Heavy pigmentation of conjunctiva, abnormal cystic formation
and hypersecretion of the Meibomian glands occurred in patients whose
blood PCBs concentration was above 40 µg/litre. There was a
correlation of the ocular effects and the blood concentration of PCBs
(Fu, 1984).
Wong et al. (1985), determined the enzyme activity in placental
tissue, obtained from 4 women who were exposed to contaminated
rice-oil in Yu-Cheng 3-4 years before conception. Placental
homogenates showed increases in monooxygenase enzymes, including aryl
hydrocarbon hydroxylase, 7-ethoxycoumarin O-deethylase, and diol,
quinone and phenolic metabolites of benzo (a)pyrene.
Lu & Wong (1984) described, in detail, the dermatological, medical,
and laboratory findings on 829 patients (half males and half females)
in Taiwan, poisoned with PCBs and related compounds. The ages of the
patients ranged from 7 days to 78 years. A grading of the clinical
severity of these cases was tried, and a possible association with the
PCB concentrations in their blood was examined, but could not be
demonstrated. The mean PCB concentration in 278 patients was 89.1 ±
0.9 µg/litre (median value 55 µg/litre); the maximum level was
1156 µg/litre and the minimum, 3 µg/litre.
One hundred and ten patients were studied within one year of the
exposure. The mean blood PCB level was 39.3 ± 16.6 µg/litre, and the
mean blood PCDFs and PCQs levels were 0.076 ± 0.038 and 8.6 ±
4.8 µg/litre, respectively. Both the sensory and motor nerve
conduction velocities of the patients were significantly lower than
those of the controls (cases studied in the past who did not have
neurological diseases) (Chen et al., 1985b).
Thirty-five patients out of 2000 cases of PCB poisoning in Taiwan were
examined neurologically 2 years after the accident. The neurological
manifestations included clinical, peripheral sensory neuropathy,
headache, and dizziness. There was no relationship between the blood
PCB concentrations in patients with neurological manifestations and
those without. Sensory nerve conduction velocity was reduced and motor
nerve conduction was delayed in about one-third to one-half of the
patients (Chia & Chu, 1984).
The blood samples of 165 patients, collected 9-18 months after the
onset of poisoning, contained 10-720 µg PCBs/litre with a mean value
of 38 µg/litre (Chen et al., 1984).
It is worth noting that the highly toxic 2,3,7,8-tetrachloro-,
2,3,4,7,8-pentachloro-, and 1,2,3,4,7,8-hexachlorodibenzofuran isomers
were present in samples from both the Japanese (Yusho) and the
Taiwanese (Yu-Cheng) incidents.
The most common symptoms noticed were acneiform eruptions and
follicular accentuation, skin and nail pigmentation, swelling of the
eyelids and increased discharge from the eyes; headache, nausea, and
numbness of the limbs. The major blood disorders were decreased
erythrocyte counts, haemoglobin concentration, and gamma-immunoglobin,
and increased white blood cell counts, serum triglyceride levels, and
SGOT, SGPT, and serum alkaline phosphatase activities. Decreased
concentrations of delta-aminolaevulinic acid and uroporphyrin were
also observed (Chang et al., 1980a,b).
9.1.2.2 Effects of PCBs on babies and infants
Yoshimura (1971) reported diminished growth in boys, but not in girls,
who had consumed the oil. Babies born to Yusho mothers were smaller
than normal. Newborn babies showed a dark brown skin pigmentation that
disappeared after a few months (Taki et al., 1969; Yagamuchi et al.,
1971). Funatsu et al. (1972) found spotted and sporadic ossification
of the skull and facial oedema with exophthalmia in 4 babies, but
there was no evidence of any teratogenic action. Pregnant Yusho
mothers delivered babies with a peculiar clinical manifestation, which
was called Fetal PCB Syndrome (FPS). In total, 36 babies showed this
syndrome. It consisted of dark brown pigmentation of the skin and
mucous membranes, gingival hyperplasia, exophthalmic oedematous eye,
dentition at birth, abnormal calcification of the skull (as
demonstrated by X-ray), rocker bottom heel, and a high incidence of
low birth weight babies. It was suggested by the authors that a
possible alteration in calcium metabolism in FPS might be related to
the action of PCBs (PCDFs) on female hormones. There was no evidence
of hypoadrenocorticism which would explain dark pigmentation in FPS
children (Yamashita & Hayashi, 1985).
Jensen (1983b) calculated that the daily intake of PCBs by Yusho
infants with clinical symptoms of poisoning was of the order of
70 µg/kg body weight.
Kuratsune et al. (1972) investigated whether Yusho disturbed
children's growth. The affected school children, 23 boys and 19 girls,
were compared in 1967, 1968, and 1969, with 719 healthy classmates
matched by sex. The gains of the affected boys in both height and
weight decreased significantly after the poisoning, while the affected
girls did not show any changes in these respects.
Studies were carried out by Fein et al. (1984), Fein (1984), and
Jacobson et al. (1984a,b) on 242 newborn infants whose mothers had
consumed moderate quantities of contaminated lake fish and 71 newborn
infants whose mothers had not eaten such fish, during the immediate
postpartum period. PCB exposure (measured by both contaminated fish
consumption and cord serum PCB levels) predicted lower birth weight,
shorter length of gestation, and smaller head circumference. Both
maternal consumption of fish and levels of PCBs in cord serum were
positively correlated with lower birth weight, shorter gestation and
smaller head circumference, and with impaired autonomic maturity and
increased numbers of abnormal reflexes.
In the studies by Schwartz et al. (1983), Fein et al. (1984), and
Jacobson et al. (1984a), the influence of important variables, such as
smoking and alcohol use, were not studied extensively enough. The
Brazelton test (Brazelton, 1973) was used in these studies. However,
this test was never intended to be used to evaluate neurological
conditions (Prechtl, 1982). The value of this test to predict
behavioural abnormalities in infants is small. The Public Health
Council of the Netherlands (1985) concluded, therefore, that the
reported changes could not be interpreted by the Brazelton test. The
important confounding factors "smoking" and "alcohol" were not studied
or not well studied, while it is known that these factors can result
in such changes. Furthermore, there was an indication that women
consuming more fish also consumed more alcohol and coffee and used
more medical drugs than those who were not fish eaters.
Jacobson et al. (1985) examined visual recognition memory in
7-month-old infants of women who had consumed contaminated Lake
Michigan fish. The authors reported a statistically significant
correlation between cord serum PCB levels and impairment of visual
recognition memory. It should be mentioned, however, that
interpretation of these test results is difficult. In view of the
variability associated with the measurements of fixation time (no
standard deviations were reported), it is unclear whether any of the
group means are statistically different. Moreover, the clinical
meaning of the differences noted is not known.
Neonatal effects of transplacental exposure to PCBs (and DDE) were
examined in a study on 912 children born between 1978 and 1982 in
North Carolina. When the infants were born, samples of placenta,
maternal and cord serum, and milk/colostrum were collected. Physical
examination of each infant was performed and the Brazelton test
(Brazelton, 1973) was applied. Fifty-nine per cent of the examinations
were carried out in the first week, 20% in the second week, and 16% in
the third week. The PCB levels in milk fat at birth (866 samples)
ranged from nd to 4.0 mg/kg. There was no association between PCB
levels and birth weight, head circumference, and hyperbilirubinaemia
(neonatal jaundice). For the Brazelton test, the only cluster scores
to be significantly affected by PCBs were the tonicity and reflex
scores, with higher PCB levels (above 3.5 mg/kg milk fat). The results
showed that higher PCB levels were associated with hypotonicity and
hyporeflexia while higher DDE levels (4 mg/kg milk fat) were
associated with hyporeflexia (DDE concentrations in milk fat ranged
from nd to >6 mg/kg milk fat) (Rogan et al., 1986a). As a follow-up
study, Rogan et al. (1987) followed 858 children in the USA, from
birth to one year of age, to determine whether the presence of PCBs in
breast milk affected their growth or health. The PCB concentrations in
breast milk ranged from 0.49 to 15.80 mg/kg, on a fat basis, and the
DDE concentrations from 0.31 to 23.8 mg/kg milk fat. The lactation
period varied from 13 (mothers with 4.00-15.80 mg PCBs/kg in their
milk) to 26 weeks. No adverse effects on body weight or the frequency
of visits for various illnesses were observed. There was no difference
between bottle-fed and breast-fed children. In 1985, about 6 years
after the mass poisoning in Taiwan, 117 children born to affected
women ( in utero exposure during, or after, the period of oil
contamination) and 108 unexposed controls were examined (Rogan et al.,
1988). The exposed children were shorter and lighter than the
controls; they had more frequent abnormalities of the gingiva, skin,
nails, teeth, and lungs than control children. The exposed children
showed delay in developmental milestones, deficits on formal
development testing, and abnormalities in behavioural assessment.
A follow-up study was carried out to determine the relationship
between PCBs in mother's serum and breast milk and the health and
development of the born infants, in Sheboygan, Wisconsin, USA, in the
period 1980-81. Seventy-three mothers gave birth to 62 infants that
were breast-fed and 11 that were bottle-fed. The ages of the mothers
ranged from 18 to 36 years. The mean serum PCB level for the study
population was 5.76 µg/litre (range, 1.29-14.9 µg/litre). Breast milk
contained a mean PCB level of 1.13 mg/kg (range, 0.29-4.02 mg/kg) on a
fat basis. The mother's blood serum PCB level during pregnancy was
positively associated with the number and type of infectious illnesses
the infants suffered later, such as colds, earache, and influenza
during the first 4 months of life. The development and growth of the
infant up to the age of 4 months was normal and was not affected by
PCB levels (Smith, 1984).
Lan et al. (1989) selected 18 exposed children (9 males and 9
females), and a reference group of 44 unexposed children (26 males and
18 females), to study the congenital absence of permanent teeth. Among
9 transplacental Yu-Cheng girls and 9 boys, the permanent teeth germ
was missing due to congenital factors in 4 girls and one boy. In the
control group, one boy showed this phenomenon. Fukuyama et al. cf. Lan
et al. (1989) had already reported this effect in 1979.
Gladen et al. (1988a) investigated whether PCBs, either transplacental
or through breast feeding, affected the scores on the Bayley Scales of
infant development at 6 or 12 months of age, in 802 infants. Higher
transplacental exposure to PCBs was associated with lower psychomotor
scores at both 6 and 12 months of age. Exposure to PCBs through
breast-feeding was apparently unrelated to Bayley scores.
The urine of 75 children born to mothers exposed to contaminated rice
oil in Taiwan (1979), 74 controls, and 12 siblings of the children
exposed between 1978 and 1985, was analysed for the presence of
porphyrins. Total porphyrin excretion was elevated in the exposed
children in comparison with the other 2 groups (exposed group,
95.2 µg/litre, control group, 80.7 µg/litre, and siblings,
72.6 µg/litre. The exposed children did not appear to have symptoms
directly attributable to their porphyria, but the authors concluded
that a mild disturbance in their porphyria metabolism appeared to be
related to their intrauterine exposure (Gladen et al. 1988b).
Thirty-nine babies showing hyperpigmentation were born to PCB-poisoned
mothers. In the orally exposed population of the Yu-Cheng episode, 24
deaths were reported and as many as 12 cases of hepatic disease
including hepatomas, which was more than expected (Hsu et al., 1985).
9.1.3 Appraisal
Several Japanese research groups have concluded that the main signs
and symptoms involved in the Yusho intoxications were caused by
contaminants in the PCB-mixture, i.e., mainly PCDFs (Masuda et al.,
1985; Kunita et al., 1985). This conclusion has mainly been based on
the following observations:
(a) Blood levels of PCBs in the victims were not very different from
those in the general population and several occupationally-exposed
groups had higher PCB blood levels in the absence of any recognizable
adverse health effects.
(b) There was an unusually high level of PCDF-contamination in the
PCBs that contaminated the rice oil.
(c) Signs and symptoms did agree with what could be expected from
exposure to PCBs and/or PCDFs (PCDDs).
However, blood levels in the Yusho victims were determined 5 years
after exposure. Consequently, at the time of the intoxication, blood
levels might have been much higher.
Furthermore, later studies on the biological potency of the
dioxin-like coplanar PCBs indicated that the occurrence of these might
have added significantly to the overall toxicity of the PCDFs (Safe,
1990).
In the case of the Yu-Cheng intoxication, blood levels of PCBs were
determined within 1 year of the accident and were found to be much
higher (i.e., about 70 µg/litre) than in the Yusho intoxication.
However, even at this time, some elimination of PCBs, especially lower
chlorinated PCB congeners, can be assumed to have taken place. Thus,
blood levels in the Yu-Cheng intoxication might also have been higher
initially.
In summary, it can be concluded that the main symptoms of the Yusho
and Yu-Cheng intoxications might have been caused mainly by combined
exposure to PCBs (mainly the coplanar ones) and PCDFs. However, some
of the symptoms, especially the chronic respiratory effects, may have
been caused specifically by the methylsulfone metabolites of certain
PCB congeners.
9.2 Occupational exposure
9.2.1 Acute toxicity -- poisoning incidents
9.2.1.1 Acute dermal effects
Skin rash has occurred within a few hours after acute exposure to
PCBs. Furthermore, itching, burning sensations, smarting, and sweating
have been reported. Irritation of the conjunctiva was a constant
symptom in acute exposure to high concentrations (Elo et al., 1985;
Schecter & Tiernan, 1985). A few weeks or months after acute exposure
(lasting a few hours) to high concentrations of PCBs (10-16 mg/m3),
several skin symptoms were observed in some of the accidentally
exposed population, such as slight pigmentation, ridges on the nails,
and the worsening of ache vulgaris (Elo et al., 1985). Skin wipe tests
on PCB-exposed workers were carded out by Maroni et al. (1981a), Smith
et al. (1982), and Wolff (1984) for capacitor manufacturers,
electrical equipment manufacturers, and transformer inspectors.
Concentrations varying between 2 and 28 000 µg/m2 of skin were
measured. Considerable concentrations of PCBs were also found on the
surfaces of hand tools in the factories (Maroni et al., 1981a;
WHO/EURO, 1987).
9.2.2 Effects of short- and long-term exposure
Exposure through ingestion is possible in the working environment
through the direct ingestion of soot particles or through the
contamination of cigarettes or food by hands. Maroni et al. (1981a)
measured high concentrations of PCBs in the palmar skin of capacitor
workers; this might lead to the oral ingestion of PCBs, in addition to
exposure via the dermal route.
Skin exposure is important in the case of long-term exposure, even
though the ambient concentrations may be low. According to
calculations made by Wolff (1985), in long-term exposure situations,
skin may be responsible for up to 20% of the total body uptake of PCBs
in workers exposed in capacitor manufacturing (WHO/EURO, 1987).
Symptoms similar to those of Yusho have been observed in workers in a
Japanese condenser factory, including pigmentation of the fingers and
nails, and acneiform eruptions on the jaw, hack, and thighs. It was
thought that these effects arose from local contact with PCBs; when
the use of PCBs ceased, the symptoms disappeared (Hasegawa et al.,
1972b). Chloracne is one of the most prevalent findings among
PCB-exposed workers and particularly among those exposed to highly
chlorinated compounds. Hara (1985) found prevalences of comedones and
acne of 40%, and skin irritation and erythema of 13% in workers
exposed for 1-24 years to Kanechlor 300 and 500. The blood PCB levels
were 21-117 µg/litre. The degree of skin pathology was correlated with
the blood PCB concentrations. In the production of capacitors,
(oculo-) dermatological abnormalities were found in 37% of the cases,
but typical PCB-associated changes were less prevalent. Fischbein et
al. (1979) suggested that these signs and symptoms were due to
exposure to PCDFs and/or PCDDs.
Fischbein et al. (1982) evaluated the dermatological effects of
long-term (< 5 - > 20 years) occupational exposure to PCBs, in a
cross-sectional clinical survey of 326 capacitor manufacturing
workers. Air PCB levels varied in the plant from 0.007 to 11.0 mg/m3.
A high prevalence (37%) of a wide spectrum of dermatological
abnormalities was found, such as rashes, burning sensation of the
skin, and chloracne, in most cases associated with typical comedones
(6%), but the occurrence was less than that observed in Yusho
patients, even though the serum PCB levels in the workers were much
higher.
Two persons were reported with dermatological abnormalities suggestive
of, but not specific for, chloracne, after occupational exposure to
PCBs (Fischbein & Wolff, 1987). They had raised serum PCB
concentrations (of the order of 80-100 µg/litre). Their wives also had
increased blood PCB levels, with the same PCB pattern as their
husbands. It was suggested by the authors that it would seem prudent
to take appropriate industrial hygiene measures, to prevent the
transmission of PCBs from the occupational environment into the home.
A cross-sectional study on 120 male workers was conducted to determine
the prevalence of increased PCB absorption, as well as the presence of
potentially-related clinical and metabolic abnormalities. Three groups
were used: an exposed group (86), a nominally exposed (15), and an
unexposed group (19 subjects). The exposed group had direct contact
with PCB-containing transformer fluids, while the nominally exposed
group worked in the same facility, but without direct contact, and the
unexposed group was employed elsewhere. The average length of
employment was 17 years (range, few months-40 years), 3 years and 9
months, and 4 years and 3 months, for the 3 groups, respectively. The
average plasma PCB levels were 33.4, 14.2, and 12.0 µg/litre, and the
average concentrations in adipose tissue were 5.6, 1.4, and 1.3 mg/kg,
respectively. There were no statistically significant differences
among the groups in levels of triglycerides, cholesterol, high-density
lipoproteins, and SGOT. A significant correlation was demonstrated
between plasma PCBs and triglycerides and SGOT values, but not SGPT
and gamma-GTP values (Chase et al., 1982).
To investigate the prevalence of oculo-dermatological findings, such
as hypersecretion of the Meibomian glands, swelling of the upper
eyelids, and hyperpigmentation of the conjunctiva (typical Yusho
symptoms), in a population with long-term occupational exposure to
PCBs, a group of 246 workers employed in 2 capacitor manufacturing
facilities were studied in 1976, and 181 of these workers, again in
1979. The median plasma values of lower chlorinated PCBs were
63 µg/litre in 1976 and 49 µg/litre in 1979. For the higher
chlorinated PCBs, these values were 18 and 17.5 µg/litre,
respectively. The prevalences of oculo-dermatological findings,
potentially related to the effects of PCBs, were 9.4 and 13.3%, at the
two examinations. There was no significant association between such
abnormalities and blood plasma/serum concentrations of PCBs (Fischbein
et al., 1985).
Lees et al. (1987) studied the hypothesis that the dermal route of PCB
exposure is a major contributor to the total body burden of PCBs in
workers. The investigation was conducted simultaneously with a
clinical study on switchgear workers engaged in transformer
maintenance and repair operations. The geometric means in the serum
and adipose tissue of exposed workers, previously exposed workers, and
comparison group were, respectively: (serum) 12.2, 5.9, and
4.6 µg/litre, (adipose tissue) 2.1, 0.83, and 0.6 mg/kg. The geometric
mean 8-h TWA concentrations in the different work areas (55 samples)
ranged from 0.5 to 6.1 µg/m3. The geometric mean surface
concentrations (102 samples) ranged from 0.007 to 1.075 µg/m2. From
the available data, it was calculated that exposure by the dermal
route (i.e., skin absorption) was considerable in comparison with
respiratory exposure. The daily calculated total dose through
inhalation ranged from 4.0 to 48.8 µg in the different work areas and
via the dermal route, from 1.2 to 215.0 µg. It was considered though
not conclusively, that the dermal and dermal/oral routes of exposure
are the predominant contributors to body burden.
Shalat et al. (1989) reported 3 cases of kidney adenocarcinomas among
young male utility workers who were responsible for maintaining
electrical transmission equipment, including transformers. The
duration of exposure ranged from 5 to 35 years.
The occurrence of chloracne and abnormal hepatic function as a result
of occupational exposure to PCBs had already been reported by Jones &
Alden (1936), Schwartz (1943), and Meigs et al. (1954).
Effects, such as chloracne, skin rashes, and burning eyes and skin,
have been associated with occupational exposure to Aroclors and
Kanechlors (Ouw et al., 1976; NIOSH, 1977; Fischbein et al., 1979,
1982, 1985; Baker et al., 1980; Drill et al., 1981; Kimbrough, 1987;
US EPA, 1987). In these studies, monitoring data did not adequately
characterize exposure levels, consequently correlations between the
occurrence of skin lesions and the duration of exposure, or blood
concentrations of the PCBs, are poor or nonexistent. Furthermore, the
contamination of the PCBs with PCDFs and PCQs may be partly the cause
of these skin changes.
Other effects reported in human exposure have been considered in a
criteria document for recommended standards for occupational exposure
to PCBs (NIOSH, 1977) and include several instances of chloracne that
resulted from exposure to PCB vapours in various work situations.
Other symptoms noted were sore throat, gastrointestinal disorders, and
eye disturbances.
Fischbein et al. (1979) examined 168 male and 158 female workers at a
capacitor plant, where they were exposed for <5 up to 25 years to
Aroclors 1254, 1242, 1016, and 1221. TWAs for 8 h with ranges of
0.07-0.40, 0.40-0.60, and 0.60-11.0 mg/m3 were considered low,
medium, and high, respectively. Among work-related symptoms they found
upper respiratory irritation and decreased rectal capacity,
gastrointestinal, neurological, and dermatological symptoms. The
dermatological symptoms occurred among 45% of the males and 55% of the
females and are comparable with the symptoms found in Yusho victims.
There was a significant correlation between plasma PCB levels and SGOT
levels, though changes in most liver tests were not prevalent.
Maroni et al. (1981b) reported blood PCB concentrations of
41-1319 µg/litre in 80 electrical workers (half males, half females)
employed in electric capacitor manufacture and testing plants.
Sixty-seven persons were exposed to Pyralone 3010 and 13, to Apirolio
(both PCBs containing 42% of chlorine). The mean age of the workers
was 37 ± 8 years, and the mean duration of employment was 12 ± 6
years. There were 6 cases of chloracne among the 80 workers. Sixteen
of the males had liver abnormalities, including hepatomegaly and
increased serum enzymatic activities; for 20% of these, the PCB
concentration in the blood was < 200 µg/litre. The females included 2
cases of bleeding haemangioma, one of whom also had chronic myelocytic
leukaemia, but none of the females had liver abnormalities.
Ouw et al. (1976) studied 34 electrical industry workers exposed to
0.32-2.22 mg Aroclor 1242 (free from impurities)/m3 compared with 30
control workers. The electrical workers consisted of 15 males (6
months-23 years employment) and 19 females (1 month-7 years
employment). No clear indications of liver changes were found. Major
complaints were burning of the eyes, face, and skin. One worker had
chloracne without systemic effects and 5 workers had eczematous hand
and leg rashes. These dermatological effects occurred at an air
concentration of <1 mg PCBs/m3. There were no significant health
effects in workers at, or below, a blood PCB level of 200 µg/litre.
Drill et al. (1981) concluded that individuals with blood levels of
>200 µg/litre have an increased risk of chloracne and that
chloracne may occur more frequently in workers exposed to PCBs that
have been heated (presence of PCDFs) and to PCBs that have a >54%
of chlorine.
A study was conducted to determine whether exposure to fumes or oil at
the transformer incident site at New Mexico had caused illness.
Exposed persons of different disciplines and unexposed employees were
asked to complete a questionnaire. Eighty of the 101 persons with
known exposure completed the questionnaire. The most common symptoms
were: nausea (27.5%), eye irritation (22.5%), sore throat (21.2%),
nose irritation (18.8%), chest tightness (15.0%), and headache
(15.0%). The symptoms were transient and usually resolved as soon as
the person left the site. Fifty-six exposed persons submitted sera for
PCB analysis as did 20 controls. All but 4 persons had levels below
10 µg/litre. The medium for exposed persons was 4.1 µg/litre (range,
1.2-41.8 µg/litre compared with 2.4 µg/litre (range 0.9-8.0 µg/litre)
for the controls (Anon., 1985).
A follow-up study on capacitor-manufacturing workers, exposed to PCBs,
and their children, was conducted over the period 1973-79. The PCBs
that were used were Kanechlor 300 and 500. PCB levels in the blood (up
to 120 µg/litre), as well as in breast milk, were 10-100 times higher
than those of non-exposed Japanese persons. The levels in 8 women
ranged from less than 50 µg/litre to about 400 µg/litre in whole milk.
Blood PCB levels were correlated with the duration of PCB handling and
breast milk PCB levels. The blood PCB levels ranged from 18.7 to
117 µg/litre in this population, 1 year after the use of PCBs was
discontinued. The rate of decline of blood PCB levels, as well as the
changes in the gas chromatography of blood PCBs over 7 years varied
with the kind of PCB handled. The blood PCB levels tended to be higher
(<3 up to >10 µg/litre) in children fed PCB-contaminated breast milk
for a long period. The great majority of the workers had
dermatological complaints, but these symptoms gradually disappeared
with discontinuation of contact with PCBs. The blood chemistry of the
workers showed only a correlation between PCB blood levels and serum
triglycerides. Several of the children fed breast milk for a long
period, showed the same medical findings as in Yusho (itchy skin,
eczema, red eye, fever, catching cold, carious teeth). However, they
were not diagnosed as suffering from PCB-poisoning, because the
findings were neither serious nor related to the blood PCB levels
(Hara, 1985).
Workers occupationally exposed to Kanechlor 500 or 600 showed higher
PCB levels in plasma (2-251 µg/litre) than Japanese Yusho patients.
Gas chromatographic patterns of their PCBs corresponded to the
patterns of PCBs to which they were exposed, but, with time, the PCB
pattern in plasma is changing. PCQs could not be detected in the
plasma of the workers (Takamatsu et al., 1985).
Lawton et al. (1985) studied a group of 194 workers in capacitor
manufacturing, exposed to Aroclor 1016, 1242 and/or 1254 before (1976)
and after (1979). The use of PCBs in the operations was discontinued
in 1977. The geometric mean serum levels and 5-95% ranges were: lower
chlorinated PCBs, 363 µg/litre (57-2270 µg/litre) and 68 µg/litre
(12-392 µg/litre), higher chlorinated PCBs, 30 µg/litre
(6-142 µg/litre) and 19 µg/litre (4-108 µg/litre), respectively. The
statistical findings were a depression in serum bilirubin and
elevations in serum gamma-glutamyltranspeptidase (GGTP) and lymphocyte
levels, at the time of the first examination, and only an elevation of
monocytes at the second.
In 1982, a survey was conducted in an electrical capacitor factory in
the USA, using Aroclor 1242 from 1941 to 1971, with a change to
Aroclor 1016 from 1971 to 1977. Of approximately 500 current employees
(with an average of 12.9 years of employment) 205 took part in the
survey. The geometrical mean PCB value for serum was 18.2 µg/litre
(range, nd-424 µg/litre). Only 39% of the workers ever worked in areas
with potential PCB exposure. More than 70% had serum levels below
30 µg/litre. There were no indications of acute PCB-related clinical
effects. The workers' serum PCB levels were a function of duration of
employment, cumulative occupational exposure, cumulative fish
consumption, and cholesterol level (Acquavella et al., 1986).
The blood of women occupationally exposed to PCBs (Kanechlor 300 and
500) was analysed over the period 1975-79. Sixty-five samples were
taken from 29 mothers. The PCB concentrations varied between 6.4 and
52.6 µg/kg (mean concentration 32.3 µg/kg). Clinical symptoms observed
during the use of PCBs were minor in comparison with those of Yusho
patients (Yakushiji et al., 1984b).
Guo et al. (1987) studied the influence of serum cholesterol and
albumin on the partitioning of PCB congeners between human serum and
adipose tissue. Fifty-five repair workers, who were either currently
or had been previously exposed, and 56 comparison workers without
exposure to PCBs were used. Seven PCB congeners, which had been
quantified in both serum and adipose tissue in at least one-third of
the selected populations, were evaluated. The effects of serum
cholesterol in modifying the serum PCB concentrations are likely to be
apparent in groups exposed to PCBs containing higher chlorinated
congeners, such as Aroclors 1254 and 1260, rather than those
containing lower chlorinated congeners, such as Aroclors 1242 and
1221.
A selected group of 51 workers (25 males and 26 females) with a mean
length of exposure of 10 years (range 1-30 years) were compared with 2
groups consisting of 74 subjects (37 males and 37 females) and the
same reference group of 67 workers (30 males and 37 females) used in
another study, residing in the same areas, but without exposure to
PCBs. The PCB concentrations in the blood of 28 out of the 51 subjects
ranged from 88 to 1359 µg/litre. A statistically significant increase
was found in serum GGT activity, urinary D-glucaric acid (GLA), and
porphyrin excretion, when compared with the respective control groups.
Although the PCB workers had an average urinary excretion of
porphyrins almost twice as high as those of the control groups, no
dose-response relationship was found between urinary porphyrin
excretion and blood PCB concentrations (Maroni et al., 1984).
Steele et al. (1986) found a gradual decrease in the body burden of
the more highly chlorinated PCBs, as well as a more rapid decrease of
the less chlorinated congeners over the period 1977-84 in groups of 5
current and retired workers, in comparison with 6 subjects without
current exposure. The authors calculated the half-life for the lower
chlorinated PCBs to be 6-7 months, and, for the higher chlorinated
PCBs, 33-34 months. The evidence of different half-life estimates for
serum PCBs, depending on the degree of chlorination, is consistent
with the present knowledge of the pharmacokinetics.
Hola & Reznicek (1985) carried out a cytogenetic analysis of the
peripheral lymphocytes of 48 employees at a precoated gravel plant
using Delor 103 (a mixture of mainly trichlorobiphenyls) in comparison
with 24 workers not exposed and 13 workers exposed to PCBs during the
impregnation of condensers. The frequency of aberrant cells,
percentage of blastic transformation, mitotic index, and proliferation
index in peripheral lymphocytes, and a number of biochemical
parameters were determined. In the 48 workers at the precoated gravel
plant, there were 2.87% aberrations (PCB concentrations in plasma,
107 ± 104 µg/litre); 3.14% in the 13 PCB-exposed workers (PCB
concentration in plasma 308 ± 253 µg/litre), and 2.04% in 24
non-exposed workers; 1.50% of aberrations were found in 20 controls.
Emmett (1985) studied a total of 55 workers (currently exposed (38)
and 17 past transformer repair workers) in comparison with 56
unexposed workers. PCB exposures occurred from the air and
contaminated surfaces, predominantly to Aroclor 1260, but there was
some exposure to Aroclor 1242. There was widespread PCB contamination
of the workplace surfaces and the 8-h time-weighted average
concentration was between 0.7 and 24.0 µg/m3, depending on the task
of the worker. The bulk oils and air from the transformer were
analysed revealing that PCDFs (13-116 µg/kg) were present as well as
PCBs. In one of the samples, 2,3,7,8-TCDF was found at 31 µg/kg. Eye
irritation and tearing were more prevalent in the exposed group, but
the symptoms were mild and/or transient. Ocular symptoms were also
found, possibly caused by 1,1,1-trichloroethane and/or
trichlorobenzene. Chloracne was not found. Two exposed workers
reported a history of melanoma; none were reported in the control
group. However, the difference was not statistically significant.
Adipose tissue and serum PCB geometrical mean concentrations in
exposed workers were 2.1 mg and 12.2 µg/kg, respectively, those in
unexposed workers were 0.6 mg and 4.6 µg/kg, and those in previously
exposed workers, 0.83 mg and 5.9 µg/kg. No correlations were observed
between liver function tests and either adipose tissue or serum PCB
concentrations. A significant negative correlation was found, after
adjustment for confounding variables, between adipose tissue PCB
levels and 24-h urinary 17-hydroxycorticosteroid excretion and a
positive correlation between serum PCB levels and serum-GGT. No
correlation was found between adipose tissue PCB concentrations and
any serum lipid component (Emmett et al., 1988a,b).
Bercovici et al. (1983) collected blood from 17 women with recent
missed abortions, 7 women who had experienced one or more missed
abortions in the past, and 7 women with normal, second trimester
pregnancies, and estimated the serum levels of PCBs and other
organochlorine pesticides. The range of serum PCB levels in recent
missed abortions was 10.9-416.5 µg/litre, and that of the control
group, 12.2-40.0 µg/litre. Forty-seven per cent of the recent missed
abortion group had PCB levels of the same magnitude as the controls
(range, 10.90-42.8 µg/litre) and 53% had higher levels. In the former
missed abortion group, PCB levels in the range of 45.3-109.1 µg/litre
were found. The number of women examined in the different groups was
small. Furthermore, half of the women with missed abortions had PCB
levels in serum comparable with those of the controls. The authors
also did not control for many variables that could significantly
influence the incidence of abortions. It seems, therefore, that missed
abortions, either recent or in the past, may be associated with PCB
exposure.
PCB concentrations were determined in the blood from 10 women with
normal pregnancies (controls) and from 17 women with premature
deliveries. A significant difference was seen in blood serum
concentrations of PCBs between the women with normal and those with
premature deliveries. When the premature delivery group was split into
a high-serum- (8/17) and a low-serum-PCB group (9/17), the
high-serum-PCB group had a significantly higher serum PCB
concentration than the control group. The values were 128 mg/litre in
the high group, 19.3 µg/litre in the control group, and 21.4 µg/litre
in the low group. In the high-PCB, premature delivery group, the mean
serum concentration of tetrachloro-isomers was lower than that of the
control group (0.6 vs 1.86 µg/litre), while the mean serum
concentrations of pentachloro and hexachloro-isomers were higher
(78.2 vs 15.67 µg/litre and 48.9 vs 1.72 µg/litre, respectively)
(Wassermann et al., 1982). The indications that higher serum PCB
levels may be associated with an increase in incomplete abortions and
premature deliveries (Bercovici et al., 1983; Wassermann et al., 1982)
could not be established as a definitive causal relationship.
Taylor et al. (1984) studied the relation of Aroclor 1254, 1242,
and/or 1016 exposure to birth weight and gestational age in the
offspring of women working in 2 capacitor plants. Fifty-one infants,
born to 39 women employed at the 2 capacitor manufacturing facilities,
had a mean birth weight of 153 g less than 337 infants born to 280
women employed in areas of the facilities without direct exposure.
Mean gestational age in the first group was reduced by 6.6 days
compared with the latter group. It was concluded that the small
decrease in mean birth weight seemed likely to have resulted from a
shortening of the gestation period rather than a retardation of
intrauterine growth. Smoking and alcohol consumption by the mothers
were not considered, and whether the socio-economic status of this
group of women was similar to that of the control group is not clear.
In an update of this study, 200 women with direct exposure and 205
women without direct exposure, were used to study the relation of PCBs
to birth weight and gestational age in the offspring. The authors
concluded that these data indicated that there was a significant
relationship between an increased serum PCB level and decreased birth
weight and gestational age, and that the decrease in birth weight was,
at least partially, related to shortened gestational age (Taylor et
al., 1989).
9.2.3 Appraisal
A discussion on the occupational epidemiological data on the basis of
dose-response considerations needs an acceptable indicator of the
degree of exposure and, in the particular case of PCB mixtures, a
discussion on the nature of the congeners present. For most
epidemiological studies reported, there are some, albeit often
limited, data on levels of total PCBs in blood, which could be used as
indicators for PCB exposure. It is recognized that the analytical
procedures used are different. The blood of continuously exposed
workers will contain absolutely and relatively more of the lower
chlorinated congeners than that of human beings with background
exposure only, or with past exposures (e.g., Yusho and Yu-Cheng
populations). Consequently, the toxicological profile for these 2
types of exposure will differ (see also the appraisal on
Yusho/Yu-Cheng).
In some cases, the epidemiology of the continuously exposed workers
shows a possible association between elevated exposure to PCB mixtures
and the occurrence of liver enzyme alterations, hepatomegaly, and
dermatological abnormalities, such as rashes and acne. In many
studies, no associations were found. In some of these studies, limited
end-points were investigated. Within the positive studies, there is a
poor or non-existent correlation between the incidence and degree of
effects and blood concentrations. Among the positive studies, adverse
effects are predominantly reported in the studies with the higher
blood levels. Possible contamination of used PCBs and PCB mixtures
(particularly in transformers), with PCDFs and PCQs, may contribute
to, or even determine, the toxicity observed. The overall conclusion
is that continuous exposure to high concentrations of PCBs and PCDFs
may result in effects on the skin and liver.
9.2.4 Special studies (target organ effects)
9.2.4.1 Liver
The liver is considered to be one of the most important target organs
for PCB toxicity. Acute exposures to PCBs cause alterations in liver
enzyme activities. Smith et al. (1982) found a statistically
significant correlation between elevated liver enzyme activities and
blood PCB concentrations, in workers exposed to PCBs in electrical
equipment manufacturing or maintenance. A negative correlation was
found in relation to the HDL cholesterol concentration and serum PCBs.
Positive correlations were found between the liver enzyme activities
of serum alanine aminotransferase (S-ALAT), serum aspartate
aminotransferase (S-ASAT), serum gamma-glutamyltranspeptidase
(S-Gamma-GT) and SGOT, and blood PCB concentrations, among workers
exposed in an Italian capacitor factory. Hepatomegaly was also
detected in most of the cases studied by Maroni et al. (1981a,b).
Workers occupationally exposed to PCBs, but mostly also exposed to
PCDDs and/or PCDFs, showed significantly increased S-ASAT, S-ALAT, and
gamma-GT activities. Sometimes elevated serum triglyceride values were
also found. Recovery of these disturbances in liver function and
morphology requires several weeks or months (Elo et al., 1985;
Schecter et al., 1985). Fischbein (1985) reported the results of liver
function tests in a population engaged in the manufacture of
capacitors and transformers. A low prevalence of abnormal liver
function tests was found and mean values for all tests were within
normal ranges in 5 workers occupationally exposed to Aroclor 1016.
Plasma antipyrine half-life was significantly lower than in matched
controls, suggesting induction of hepatic mixed-function oxidases
(Alvares et al., 1977). At the initial examination (in 1976, when PCBs
were still being used), statistically significant correlations were
found between log LDH and plasma levels of log HPCB (higher PCB
congeners) and log TPCB (total PCBs) among female workers, while
log-gamma-GTP was significantly correlated only with log HPCB among
male workers. A significant increase to abnormal levels of gamma-GTP
was noted at the follow-up examination (1979, 2.5 years after the use
of PCBs had been discontinued) in both male and female workers, and
preliminary results indicated significant correlations between
gamma-GTP and serum levels of PCBs among male workers. In
occupationally exposed individuals, the serum or plasma PCB levels
were higher than those found in patients in the Yusho and Yu-Cheng
incidents. An effect transmitted via liver activity was hyperlipaemia,
in which triglycerides and, in some instances, also cholesterol levels
in the blood were elevated (Smith et al., 1982).
Hepatotoxicity is suggested in occupationally-exposed humans (Drill et
al., 1981; US EPA, 1987). Drill et al. (1981) concluded that SGOT
and/or GGPT appear to be the most sensitive indicators of PCB exposure
in humans, and that changes in liver enzymes occur at levels below
those at which chloracne occurs.
9.2.4.2 Immunotoxicity
Reports on the immunological effects of long-term, occupational
exposures are sparse. Fischbein et al. (1979) found a suggestive
increase in the occurrence of trivial infections in exposed workers.
Immunological responses were found to be affected in Yu-Cheng victims
(Lu & Wu, 1985) and in Yusho patients (Nakanishi et al., 1985). Acute
accidental exposures of workers to PCBs and PCDFs have been studied
for immunological responses and immunosuppressive changes have been
found. The most important alterations were decreased numbers of
T-lymphocytes and lowered T-helper/ T-suppressor cell ratios.
Responses of lymphocytes to phytohaemagglutinin, concavalin A, and
pokeweed mitogen were also lowered. The observed changes persisted for
6 months after the acute exposure. No quantitative changes were
observed for immunoglobulins (Elo et al., 1985; WHO/EURO, 1987, 1988).
Lu & Wu (1985) found that PCB patients suffered from various kinds of
infections. Most frequent were those of the respiratory tract and
skin, including pyoderma, tinea versicolor, dermatophytosis and warts.
The low resistance of the patients suggested some degree of
immunosuppression. The function of the immune system was tested in 143
patients. Examination during the first year revealed: decreased
concentrations of IgM and IgA, but not of IgG; decreased percentages
of total T-cells, active T-cells, and helper T-cells, but normal
percentages of B-cells and suppressor T-cells; suppression of delayed
type response to recalling antigens; enhancement of lymphocyte
spontaneous proliferation; and enhancement of lymphocyte proliferation
with phytohaemagglutinin, pokeweed mitogen, and tuberculin
stimulation, but not with concanavalin A. After 3 years, the positive
rate of the tuberculin test recovered somewhat with time. The total
numbers of T-cells and B-cells were normal, the number of suppressor
T-cells (OKT-8) increased, but the number of helper T-cells (OKT 4)
was still lower, so the immuno-regulating index (OKT 4/OKT8) was still
very low. The lymphocyte proliferation stimulated by various mitogens
was also enhanced.
In patients with PCB poisoning, IgA and IgM levels in serum apparently
decreased for 2 years after the onset of the disease, but returned to
normal in most cases, in spite of the persistence of the respiratory
symptoms (Shigematsu et al., 1978, see section 9.2.4.3).
9.2.4.3 Respiratory system
In long-term exposure, up to 0.3-10 mg of PCBs may be inhaled in an
8-h working day. The respiratory tract is certainly the most important
route of exposure in the case of acute emergency situations, where
unprotected personnel working in areas containing such PCB
concentrations may, in theory, inhale a total dose of up to 10 mg/day.
This may imply a considerable cumulation of PCBs during long-term
exposure (WHO/EURO, 1987, 1988).
Transient irritation of the mucous membranes of the respiratory tract
has been reported in emergency situations, as well as difficulty in
breathing at high concentrations. It has not been confirmed that
short-term exposure causes other important respiratory effects, though
increased susceptibility and a high risk of contracting chronic
bronchitis have been suggested (Kimbrough, 1980; Elo et al., 1985;
WHO/EURO, 1987).
In the case of unheated commercial PCBs, the amount of PCDFs inhaled
might be very low, if any at all. The situation is totally different
in the case of acute exposures to heated or decomposed PCBs, in which
the inhaled total concentrations might be several orders of magnitude
higher than above, though the irritative effect may prevent breathing
in such contaminated rooms. Since the soot often contains a
considerable fraction of particles, a few microns in size, it is
partly breathed in and, thus, may lead to alveolar retention of both
soot and adsorbed chemicals. Carbon particles can accumulate in the
lung tissues and regional lymph nodes. Inhalation of soot particles
containing high concentrations of both PCBs and PCDFs would, in
practice, be the most important mechanism of exposure (Parkes, 1982;
WHO/EURO, 1987).
Warshaw et al. (1979) and Smith et al. (1982) found a correlation
between serum PCB concentration and respiratory tract symptoms among
workers exposed long-term to PCBs. An increase in the occurrence of
chronic bronchitis was possibly due to a decrease in the immunological
defence mechanism. No increase in mortality from respiratory system
diseases was found. An indication for acute and chronic irritation of
the respiratory tract was found by Brown & Jones (1981).
Shigematsu et al. (1978) studied the clinical, laboratory, and
pathological findings on respiratory involvement in PCB poisoning in
401 patients. Respiratory symptoms included expectoration in 40% of
the 289 non-smoking patients with PCB poisoning and mild wheezing in
2%. The incidence and severity of the symptoms was well correlated
with the concentrations of PCBs in blood and sputa. The clinical
examinations revealed bronchiolitis and pneumonia or atelectasis in
about one-tenth of the patients with reticulo-linear shadows. The PCB
concentrations in the blood and sputa were 27 and 8 µg/litre,
respectively. The presence of PCBs in sputum may have been associated
with the excretion from bronchial cells and/or with lipid II cells of
the lung, phagocytosed in alveolar macrophages and expectorated.
9.2.4.4 Neurotoxicity
Acute and long-term exposures to PCBs have been reported to cause
neurological and unspecific psychological or psychosomatic effects,
such as headache, dizziness, nausea, depression, sleep and memory
disturbances, nervousness, fatigue, and impotence (Smith et al., 1982;
Elo et al., 1985; Hara, 1985; Schecter et al., 1985; Takamatsu et al.,
1985; WHO/EURO, 1987).
Fischbein et al. (1979) reported the occurrence of these symptoms in
39% of male and 58% of female capacitor-manufacturing workers, exposed
to PCBs for long periods (over 5 years). To what extent these symptoms
were the direct consequences of exposure to PCBs and related compounds
and how much they were dependent on general conditions in an emergency
situation remains unclear.
Seppalainen et al. (1985) examined 16 men who were exposed to fumes
resulting from the explosion of capacitors containing Clophen A30. Air
concentrations of PCBs, measured 5.5 h after the explosion, were
8-16 mg/m3 air (PCDFs and other compounds, such as monochloropyrenes
and dichloropyrenes, were also formed). Most of the men had a
transient sensory neuropathy in their lower extremities (WHO/EURO,
1987, 1988).
9.2.4.5 Blood pressure
Kreiss et al. (1981) examined 458 volunteers (>12 years of age) from
Triana (Alabama) and correlated serum PCB levels (Aroclor 1260) with
blood pressure. This population was excessively exposed to DDT
residues through the consumption of contaminated fish. The residents
of this small rural town also had elevated PCB body burdens that were
positively correlated with fish consumption. The mean serum PCB level
was 17.2 µg/litre. The incidence of borderline (systolic of 140-159 mm
Hg and diastolic of 90-94 mm Hg) and definite hypertension (systolic
of >160 mm Hg and diastolic of >95 mm Hg) was 30% more than
would be expected for a general population of the same age, race, and
sex composition. However, this study did not have a control group in
its design, and there were more confounding factors that make the
study inadequate to conclude an association between blood PCB levels
and hypertension.
A study was carried out to test the association of serum PCB levels
and elevated blood pressure in 840 residents of New Bedford, Acushnet,
Dartmouth and Fairhaven, Canada, in the period 1984-87. The mean PCB
levels (as Aroclor 1254) were 5.9 and 5.8 µg/litre in 391 males and
449 females, respectively. The range in serum PCB levels for the total
group was 0.38-154.2 µg/litre. There was a relationship of serum PCB
level to age among the 840 individuals. In the 5 age groups: 18-24,
25-34, 35-44, 45-54, and 55-64 years, the mean serum PCB levels were
2.59, 3.84, 5.30, 8.18, and 8.96 µg/litre, respectively. Blood
pressure levels did not appear to be correlated with serum PCB levels.
The mean systolic readings, taken at 3 different times, for the 840
individuals were 115.26 ± 18.85, 113.69 ± 17.62, and 114.28 ± 11.41.
The diastolic readings were 72.19 ± 10.94, 73.19 ± 10.95, and 73.17 ±
19.94. There was no between the sexes difference (Massachusetts Dept
Public Health, 1987).
Akagi & Okumura (1985) studied the correlation of blood PCBs levels or
PCB patterns and blood pressure in 59 Yusho patients (more than 40
years old). In spite of the passage of 13 years from the onset of the
disease, 52.5% of the patients still had PCB levels higher than those
of the general population. The frequency of hypertension in these
patients was 16.9%, a value similar to that found in the general
population of the same age and sex. Blood pressure was not associated
with blood PCB levels or PCB pattern, but was associated with the well
known factors influencing blood pressure, such as age, obesity, and
habitual alcohol intake.
9.2.5 Mortality studies
Davidorf & Knupp (1979) conducted an epidemiological study on ocular
melanoma incidence in Ohio from 1967 to 1977, attempting to associate
Ohio counties with known high concentrations of PCBs and those with
industries that might use PCBs with an increased incidence of ocular
melanoma. The authors concluded that there was no causal relationship
between PCB exposure and an increased annual occurrence of ocular
melanoma in Ohio counties in the period 1967-77. Bahn et al. (1976)
reported on 31 research and development employees subjected to "heavy"
Aroclor exposure (quantity not reported) in a US petrochemical plant.
Two had malignant melanomas, and according to the standard of the
Third National Cancer Survey, incidence rates of only 0.04 would be
expected among 31 persons (NCI, 1975). The retrospective cohort
mortality studies of Brown & Jones (1981) and Brown (1987) reported
data on PCB-exposed individuals who had worked in 2 electrical
capacitor plants, one in New York, and the other in Massachusetts.
Both plants had produced this type of capacitor for more than 30
years. The PCBs used were Aroclor 1254, Aroclor 1242, and Aroclor
1916. A combined total of 2588 exposed workers from both factories,
with 3 or more months of exposure, were studied. The overall mortality
(295 deaths) was lower than expected (318) and the mortality for
cancer deaths (62 observed) was also lower than expected (80). A
statistically significant excess in deaths was observed in the disease
category that includes cancer of the liver (primary and unspecified),
gall bladder, and biliary tract (5 observed vs 1.9 expected). Most of
the excess was observed in women employed in one plant. According to
the authors, because of the small number of deaths and the variability
of specific causes of death within this category, it remains difficult
to interpret these findings with regard to PCB exposure.
At the first plant, there were 2 different facilities, a power
capacitor manufacturing facility, and a small capacitor manufacturing
facility. At the power capacitor facility, the TWAs for personal air
samples were between 24 and 393 µg/m3 for various jobs: in the
winding work area and soldering work area, they were as low as
3 µg/m3 and as high as 476 µg/m3, respectively. At the second plant,
where a few cases of rectal and liver cancer were found (see above)
the PCB levels were much higher. Degreasers and solderers, for
example, had TWAs for personal air of 1.260 and 1.060 µg/m3,
respectively, and heat soak operators and tankers had TWAs of 630 and
850 µg/m3, respectively. The work area air samples contained levels
as high as a TWA of 810 µg/m3. The duration of exposure, without
information concerning the level of exposure, when the levels varied
so widely, weakens the statement about lack of correlation between
duration of exposure and cancer mortality. In fact, the observed
cancer cases for both the liver and rectum were markedly increased in
the factory that had, at the time of measurement, the higher PCB
levels. Notwithstanding the absence of detailed information, the data
presented are suggestive of a dose-related increased incidence of
mortality from rectal cancer and possibly liver cancer.
Although there was no correlation between the latent period and cancer
mortality or between the duration of employment and cancer mortality,
most of the cancers occurred in the second plant, which had the higher
levels of PCBs at the time that the measurements were made. PCB levels
were monitored in 1977 for personal air and work area air. Since
procedures and processes were somewhat different during the years in
which most of the workers were exposed, the figures on PCB levels do
not necessarily indicate the exposure levels of the subjects.
Nevertheless, the figures given do indicate the wide variation in PCB
levels in air, with a 15-fold difference between the lowest and
highest levels among the different jobs. Although the different levels
of dust and particulate matter are not known, it would be anticipated
that an equally wide variation would exist.
A medical surveillance programme has been established for 482 persons,
who were potentially exposed to PCBs, PCDFs, and PCDDs from an
electrical transformer fire in Binghamton in 1981. Mean serum PCB
concentrations (98% of the samples) were below 20 µg/litre, a value
typical of a population with no unusual exposure. Mortality,
symptomatology, cancer incidence, and reproduction events were
assessed through 1984. The numbers of deaths, cases of cancer, fetal
deaths, and infants with low birth weight or congenital malformations,
were similar to those expected on the basis of age and sex-specific
rates for upstate New York and other comparison populations. One-third
of the fire-fighters and a number of persons, who were in the building
during the first 24 h (or longer) reported a rash or itching skin, but
no chloracne (Fitzgerald et al., 1989).
Bertazzi et al. (1981) reported the results of a mortality study on
PCB-exposed workers, who were employed in the manufacture of
electrical capacitors in an industrial area near Milan. The PCBs used
over the period from 1946 to 1970 were Aroclor 1254, Pyralene 1476
(54% chlorine), and Pyralene 3010 and 3011 (42% chlorine). In 1954, a
few measurements in the air were performed and the values of Aroclor
1254 were 5200-6800 µg/m3. In 1977, airborne concentrations of
Pyralene 3010 ranged from 48 to 275 µg/m3. The minimum and maximum
values of PCBs recovered from workplace surfaces and worker's hands
were, 0.2-159 and 0.3-9.2 µg/cm2, in 1977, and, in 1982 (2 years
after the ban on production), 0.003-6.3 and 0.09-1.5 µg/cm2,
respectively. The mortality study spanned the 25-year period from 1954
to 1978. The control mortality rates were for subjects from the city
in which the plant was located. There were 1310 workers (1020 females
and 290 males) and the vital status was obtained for 98% of both
sexes.
The study was enlarged and extended to include 2100 workers and to
cover the period 1946-82. Vital status was ascertained for over 99% of
the subjects and death certificates were obtained for all deceased
persons. Expected deaths were calculated using 2 sets of mortality
rates, national and local. Among male workers, cancer deaths (14
observed vs 7.6 expected) were significantly increased as were deaths
owing to cancer of the gastrointestinal tract (6 observed vs 2.2
expected). Also, mortality from haematological neoplasms (3 observed),
and lung cancer (3 observed) was higher than expected. However, the
excess was not statistically significant. Female workers exhibited an
overall mortality that was significantly increased above expectations.
Cancer deaths (12 observed vs 5.3 expected) and haematological
neoplasms (4 observed vs 1.1 expected) were significantly higher than
expected when compared with the local population. Interpretation of
the results is limited by the small number of deaths: however, it is
of interest that the gastrointestinal tract and the lymphatic and
haematopoietic tissue seem to be the most probable human target sites
for PCB carcinogenic activity (Bertazzi et al., 1987).
A cohort study on 142 male Swedish capacitor-manufacturing workers was
performed between 1960 and 1978. The PCB was 42% chlorinated and
contained different PCDFs totalling about 1400 µg/kg. The mean
exposure time of the workers was 6.5 years. In 1973, 0.1 mg PCBs/m3
was found in the air. Mortality was investigated for the period
1965-82 and cancer incidence from 1965 to 1980. Twenty-one deaths and
7 cancers were observed, which was in agreement with the anticipated
numbers calculated from national statistics (Gustavsson et al., 1986).
Zack & Musch (1979) examined a small cohort of workers (89) with
occupational exposure to PCBs. No liver cancer was reported among the
30 deaths that occurred in this study. There were increases for all
malignancies (8 observed vs 4.4 expected, SMR=179) and elevated lung
cancer (4 observed vs 1.44 expected). Adjustment for the confounding
variables due to multiple exposure to other agents was not made. By
1979, 31 Yusho patients had died, 11 (35.4%) of these from malignant
neoplasms. Only 21.1% of all deaths in this Japanese prefecture would
be expected from malignant neoplasms, but no clear correlation between
the occurrence of Yusho and increased deaths from malignant neoplasms
could be made, because of the small number of deaths observed and the
unknown latency period.
By the end of 1983, 120 Yusho patients had died, 41 of these from
malignant neoplasms. These included 8 stomach cancers, 11 liver
cancers, and 8 neoplasms of the lung. A statistically significant
excess mortality was seen for malignant neoplasms, cancer of the
liver, and cancer of the lung, trachea, and bronchus, in males, but no
such excess was noted in females. The excess from liver cancer deaths
was seen mainly in Fukuoka prefecture, while no excess was seen in
Nagasaki prefecture (Ikeda et al., 1987).
9.2.6 Appraisal
Some epidemiological studies on occupationally exposed workers and
Yusho patients indicate an association between PCB exposure and
cancer, especially with regard to hepatobiliary tumours. However, no
definite conclusions can be drawn from available data, because of the
small numbers of deaths in the population studies, the lack of clear
dose-response relationships in the occupational studies, and the
difficulty in evaluating the effects of other compounds present in
PCBs.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The PCBs were evaluated by IARC in 1978 and 1987 (IARC, 1978; 1987).
In 1987, IARC concluded that, because the role of impurities in PCBs
in the carcinogenicity could not be excluded, and, because of the lack
of knowledge on dose-response relationships, the evidence from
epidemiological studies is limited. However, the evidence of
carcinogenicity in laboratory animals is sufficient. Taking the
combined evidence from human and experimental animal studies, the IARC
Group concluded that PCBs are probably carcinogenic for humans (IARC,
1987).
Many countries and intergovernmental organizations have banned or
severely restricted the production, use, handling, transport, and
disposal of PCBs and PCTs. For an overview of these measures and
regulations we refer to the Health and Safety Guide on PCBs and PCTs
(WHO, 1992; IRPTC, 1986b).
At the meeting of the Joint FAO/WHO Expert Committee on Food Additives
(WHO, 1990), particular attention was paid to the possible health
consequences of the intake of PCBs by the suckling infant. It was not
anticipated that adverse health effects would occur as a result of
consuming breast milk. It should also be kept in mind that the infant
consumes breast milk for a short period (1-2% of its total life span).
In addition, other factors need to be considered:
* the benefits of breast milk and breast-feeding, including the
nutritional, immunological, and other properties of the milk, as
well as the psychological advantages, should not be discounted;
* the disadvantages of breast milk substitutes, because of the
potential contamination due to infective agents, incorrect
preparation, inadequate hygiene, etc.
For these reasons, JECFA was of the opinion that the advantages to the
infant of breast-feeding outweigh any potential hazards due to the PCB
content of breast milk, and advises that there is absolutely no
justification for discouraging this practice.
The monitoring data have indicated, up to now, that the occurrence of
PCBs in human milk persists at about the same levels during the years,
with slight decreases or increases in the PCB concentration in breast
milk in certain countries. Since the PCB levels in human milk are
still too high, every effort should be made to prevent the entry of
PCBs into the environment and to control their occurrence in the food
supply. The Committee was reassured by the observation that the
production of PCBs has largely ceased. Thus, it is expected that the
levels of PCBs in the environment and food, and consequently in breast
milk, will decrease with time (WHO, 1986b).
On the basis of the evaluated background data, an average dietary
intake of PCBs for adults was estimated to amount to a maximum of
100 µg/week, or approximately 14 µg/day. For a 70-kg person, this is
an intake equivalent to a maximum quantity of 0.2 µg/kg body weight
per day (WHO/EURO, 1988).
The above data suggest that the main exposure of the general
population to PCBs occurs through food. The daily intake of these
compounds by breast-fed infants is about 1-2 orders of magnitude
higher than for the rest of the population, compared either on the
basis of body weight or energy consumption. However, compared with
lifetime intake, a 6-month, breast-feeding period contributes less
than 5% of the total body burden from lifetime exposure (WHO/EURO,
1988).
POLYCHLORINATED TERPHENYLS
(Data relating specifically to polychlorinated terphenyls are scarce.
Nevertheless, they are presented separately in this section.)
1. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
1.1 Identity
The chemical formula of the polychlorinated terphenyls (PCTs) can be
given as C18H14-nCln, in which n is the number of chlorine atoms,
which can range from 1-14.
The chemical structure is:
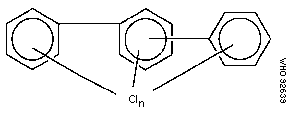 The number of different PCTs theoretically possible is orders of
magnitude higher than that for PCBs, but, in practice, as for PCBs,
PCTs are not sold on a composition specification, but on their
physical properties, which depend on the degree of chlorination.
Common Polychlorinated terphenyls - PCTs
name:
Major trade The trade names are generally similar to those
names: given for PCBs. In the Aroclor series, terphenyls are
indicated by 54 in the first two places of the four
digit code. In Japan, the PCTs are coded Kanechlor
KC-C.
1.2 Physical and chemical properties
The physical and chemical properties of PCTs are very close to those
of PCBs, and depend on the degree of chlorination.
1.3 Analytical methods
Extraction and clean-up procedures are similar to those used for PCBs
(sections 2.3.1.1 and 2.3.1.2).
The gas-liquid chromatographic details are different from those of
PCBs, because of the lower volatility of the PCTs. Zitko et al. (1972)
used 3% OV 210 as the stationary phase with a column temperature of
200°C. Thomas & Reynolds (1973) also used OV 210 with a column
temperature of 250°C and another system with 3% Dexsil as a stationary
phase at 300°C with a 63Ni electron capture detector; this was also
used by Addison et al. (1972). Sosa-Lucero et al. (1973) used OV 210
and SE 30 at 255°C and Freudenthal & Greve (1973) used OV 17 with a
temperature programmed from 200°C to 285°C. Thomas & Reynolds (1973)
confirmed the identity by chlorination to tetradecachloroterphenyl
with antimony pentachloride.
2. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
No specific information available. See PCBs.
3. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
Atmospheric input into the Great Lakes has been studied, because the
lakes, as a whole, represent the largest surface area of any
freshwater body in the world. Wingender & Williams (1984) found that
atmospheric transport was a major pathway for the deposition of
polychlorinated terphenyls into the Great Lakes (see section 4.1.1.1).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 Residues in the environment
Relatively few studies have been carried out to determine
polychlorinated terphenyls in biota. Freudenthal & Greve (1973) found
levels of 0.12 mg/kg (wet weight) in oysters and 0.4 mg/kg (fat basis)
in eels from the Netherlands. Levels of PCTs were generally lower than
PCBs in the same samples. Renberg et al. (1981) analysed biota from
the Baltic Sea and found levels of 2.8-17.2 mg/kg (wet weight) in
white-tailed eagles, 0.5-1 mg/kg in grey seals, and 0.08 mg/kg in
eels. In fish, Jan & Malnersic (1978) found PCT levels of
0.003-0.005 mg/kg in trout from the Soca River, Yugoslavia. Mean PCT
levels of 0.0025 mg/kg (Doguchi, 1977) and 0.01 mg/kg (Takai et al.,
1979) have been found in the freshwater and marine environment of
Japan.
Several bird species have been monitored for PCTs; levels of
0.03-2.2 mg/kg have been found in Japanese birds (Doguchi, 1977).
Zitko et al. (1972) found levels of 1.4 mg/kg fat (wet weight) and
0.1 mg/kg in eggs of herring gulls from the Bay of Fundy, Canada.
Hassell & Holmes (1977) analysed the livers of various birds of prey
in the United Kingdom; residues ranged from <0.05 to 1.2 mg/kg. PCT
levels of between 0.61 and 10.51 mg/kg were found in the fat of gulls
from Italy, (Vannucchi et al., 1978) and black-headed gulls from the
Baltic contained mean residues of 1.8 mg/kg in adipose tissue
(Falandysz, 1980).
PCTs were also measured in the monitoring programme carried out all
over Japan in the period 1974-81. In 1974, 1976, and 1978 no PCTs were
found in 60, 156, and 75 samples of water (limit of determination
0.0001 mg/litre). No PCTs were found in sediment samples in 1974, but,
in 1976 and 1978, 21/151 and 37/75 samples were positive, with PCT
levels of 0.001-0.2 and 0.001-1.0 mg/kg, respectively. In fish in
1974, 1976, and 1978, PCTs were found in 3/11, 0/39, and 3/66 samples,
in concentrations of 0.0002-0.2 mg/kg (Environment Agency Japan,
1983).
4.2 Residues in food
Ushio & Doguchi (1977) analysed cereal products, vegetable products
including vegetable oils, seasonings, and seaweed, marine animal
products, and terrestrial animal products including milk and eggs, for
the presence of PCTs. Only the vegetable products contained average
concentration of 0.05 µg/kg. Other authors referred to by Ushio &
Doguchi failed to detect PCTs in edible oil, vegetables, meat, or
fish.
No PCTs could be detected in a Canadian survey on eggs, domestic and
imported cheese (Villeneuve et al., 1973b).
In Japan, the PCT contents of a number of foods were determined. The
PCT contents of fish were lower than the PCB contents (Fukano et al.,
1974). Villeneuve et al. (1973a) analysed packaged food in Canada and
found that 94.5% of the samples contained less than 0.01 mg PCTs/kg
and 5.5% contained 0.01-0.05 mg PCTs/kg.
4.3 Concentrations in adipose tissue
In Japan, Doguchi et al. (1974) found an average PCT level of
0.6 mg/kg in human fat, with a range of 0.1-2.1 mg/kg. In the same
country, Takizawa & Minagawa (1974) found PCT levels of 0.02 mg/kg in
the human liver (n = 6), 0.01 mg/kg in the kidney (n = 2), 0.02 mg/kg
in the brain (n = 3), and 0.04 mg/kg in the pancreas (n = 1). Thirty
samples of adipose tissue (from 18 males and 12 females), obtained in
Tokyo in 1974, were analysed for PCTs. The average level of PCTs was
1.11 mg/kg (range 0.04-9.20 mg/kg), on a fat basis (Fukano & Doguchi,
1977). In the Netherlands, PCTs were found in human fat at levels of
0-1 mg/kg (Freudenthal & Greve, 1973).
4.4 Concentrations in blood
An average PCT level of 5.0 µg/litre was recorded in the blood of
non-occupationally exposed volunteers in Japan (Doguchi & Fukano,
1975). Human blood samples were collected from 10 subjects in Tokyo in
1975 out of 27 subjects from whom blood had been obtained in 1973. The
average concentration of PCTs in whole blood was 6.45 µg/litre
(0.7-19.6 µg/litre) in 1973, and 5.32 µg/litre (1.1-9.4 µg/litre) in
1975 (Fukano & Doguchi, 1977).
5. KINETICS AND METABOLISM
5.1 Absorption
PCTs have been shown to be absorbed from the intestinal tract
(Sosa-Lucero et al., 1973), but very little information is available
on the rate of absorption.
5.2 Distribution
Diets containing Aroclor 5460 at levels of 10, 100, or 1000 mg/kg were
administered to rats for 7 days. The greatest concentration (611 mg/kg
at 1000 mg/kg diet) was in the liver, while the blood level was
5.85 mg/litre at 1000 mg/kg diet. PCT administration did not affect
body weight, but a significant increase in liver weight occurred in
the rats fed 1000 mg/kg diet (Sosa-Lucero et al., 1973). Table 54
shows the tissue distribution obtained in this study in rats fed with
Aroclor 5460 and in another study using Aroclor 1254 (Curley et al.,
1971).
Addison et al. (1972) dosed cod Gadus morhua by gavage with the
polychlorinated terphenyl (PCT) Aroclor 5460 (in herring oil) at
0.5 g/ml. After one week of starvation, the PCTs were present in all
the tissues analysed. Uptake efficiency appeared to be low with a
total of 1-10 mg of Aroclor 5460 being distributed through all tissues
out of 1 g administered. Liver was found to be the organ richest in
PCTs, and probably contained most of the absorbed material. In a
separate group of fish, analysed 70 days later (fish were fed during
this period), PCT residues were not significantly lower.
Table 54. Tissue distribution (mg/kg wet weight) of PCTs
(Aroclor 5460) in rats fed dietary levels of 100 mg/kg
for 7 days and fed PCBs (Aroclor 1254) at 100 mg/kg
for 9 daysa
Tissue Aroclor 5460 Aroclor 1254
Blood 1.32 0.1
Liver 47 6
Brain 5.1 4
Kidney 15.1 5
Heart 21.5 -
Fat - 180
a From: Curley et al. (1971); Sosa-Lucero et al. (1973).
5.3 Biotransformation
There is little information on the biotransformation of PCTs. Addison
et al. (1972), using gas-liquid chromatography, noted a loss of PCTs
with a shorter retention time in the excreta of a cod dosed orally
with Aroclor 5460; the same loss was observed in rat faeces after the
administration of a diet containing Aroclor 5460 (Sosa-Lucero et al.,
1973).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Marine and estuarine organisms
PCTs, Aroclor 5460, did not show any toxic effects at either 1 or
100 mg/litre on Dunaliella, Olisthodiscus, or Thalassiorsira, the
only 3 species tested with this mixture (Craigie & Hutzinger, 1975).
6.2 Terrestrial invertebrates
Lichtenstein et al. (1969) exposed Drosophila melanogaster to the
dry residues of various PCTs. No mortality was observed after a 48-h
exposure to 2999 µg of Aroclor 4465, 5442, or 5460.
6.3 Birds
A single study on the toxicity of Aroclor 5442, produced a 5-day LC50
of 4477 mg/kg (1301-15402 mg/kg) in Japanese quail (aged 14 days)
(Coturnix coturnix) (Hill & Camardese, 1986).
Cecil et al. (1974) found that Aroclor 5442 at a dose level of
20 mg/kg diet did not change the hatchability of chicken eggs.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single oral exposure
Early data, reported in abstract, indicated that the approximate oral
LD50 values of the PCT-mixtures Aroclor 5442 and 5460, in corn oil,
in rats were 10.6 and 19.2 g/kg body weight, respectively. For 3:1
mixtures of PCBs and PCTs, Aroclor 4465 and 2562, in corn oil, the
LD50 values in rats were 16 and 6.3 g/kg body weight, respectively
(US FDA, 1970).
7.2 Short-term oral exposure
7.2.1 Rat
Modifications in the liver were studied in groups of male
Sprague-Dawley rats fed a diet containing Aroclor 5460 at a level of 0
or 10 000 mg/kg diet (equivalent to 0 or 400 mg/kg body weight). Body
weights were slightly decreased after 3 weeks of exposure. The
enlarged livers showed proliferation of the endoplasmic reticulum and
formation of large concentric membrane arrays. Evidence of fatty
degeneration was observed by Toftgard et al. (1980). Biochemical
changes included an increase in microsomal protein and phospholipid,
and a decrease in RNA and cholesterol. The specific esterase
activities, N-demethylase and nitroreductase, were increased and
those of glucose-6-phosphatase and aryl hydrocarbon hydroxylase
decreased (Norback & Allen, 1972).
Sosa-Lucero et al. (1973) did not observe any signs of toxicity in
groups of male Wistar rats exposed to a diet containing Aroclor 5460
at levels of up to 1000 mg/kg diet (equivalent to 50 mg/kg body
weight) for 7 days. At 1000 mg/kg diet, relative liver weights were
increased as well as microsomal protein, cytochrome P-450, and the
specific activities of aniline hydroxylase and aminopyrine-
N-demethylase. Mixed type induction of hepatic microsomal enzymes in
rats exposed to PCTs has been observed by several investigators
(Ahotupa & Aitio, 1980; Toftgard et al., 1980; Nilsen & Toftgard,
1981).
Kiriyama et al. (1974) fed groups of male Wistar rats a control diet
or diets with ortho-, meta-, or para-PCTs at a level of 2000 mg/kg
diet (equivalent to 100 mg/kg body weight) for 2 weeks. Ortho- and
meta-PCTs reduced growth and increased relative kidney weights,
while only meta-PCTs decreased food intake and increased the
relative liver weights. All mixtures increased plasma, but not liver,
cholesterol levels. There was evidence of adrenal hypertrophy.
7.2.2 Monkey
A dietary level of Aroclor 5460 of 5000 mg/kg (equivalent to 200 mg/kg
body weight) over 3 months caused growth retardation and increased
relative liver weights in 6 Rhesus monkeys compared with 3 controls.
After 6 weeks of exposure, the toxic signs observed were similar to
those found within 1 month in a group of monkeys exposed to 300 mg of
Aroclor 1248/kg diet (equivalent to 12 mg/kg body weight), i.e.,
alopecia, facial oedema, swollen eyelids and lips, and purulent eye
discharge. After exposure of both groups for 3 months, proliferation
of the smooth endoplasmic reticulum was observed as well as
hypertrophy and hyperplasia of the gastric mucosa (Allen & Norback,
1973).
7.3 Teratogenicity
Groups of 15 or 16 pregnant ddY mice were fed diets containing 0, 100,
500, or 2500 mg PCTs/kg (not specified) during gestation. The animals
were sacrificed on day 18 and examined for embryonic effects. The
fetuses of dams receiving the 500 and 2500 mg/kg diet showed a higher
incidence of cleft palate in comparison with the controls. Pregnant
ddY mice were administered 0, 50, or 100 mg PCTs/kg with
corticosterone administered subcutaneously on days 11, 12 and 13. A
significant increase was seen in corticosterone levels in the plasma
in the PCT-treated animals on day 14. Furthermore, when pregnant ddY
mice were adrenalectomized on day 10, it did not suppress the
development of cleft palate, but metapyrone, an inhibitor of
corticosterone synthesis, significantly reduced the incidence of cleft
palate in the fetuses. The results suggest that cleft palate induced
by PCTs is not due to a direct effect, but that an increase in the
corticosterone level in the maternal plasma is involved in the
mechanism of its development (Kaneko, 1988).
Pregnant Wistar rats were fed PCTs at levels of 0, 500, or 2500 mg/kg
diet during gestation and the animals were sacrificed on day 20.
Systemic oedema was observed in the fetuses of the animals fed 500 and
2500 mg PCTs/kg diet, but no cleft palate was found (Kaneko, 1988).
7.4 Carcinogenicity
Groups of 35 male ICR mice received a diet containing Kanechlor C
(a mixture of 95% PCTs and 5% PCBs) at levels of 0, 250, or 500 mg/kg
(equivalent to 0, 36, and 70 mg/kg body weight), for 24 weeks. The
mice were sacrificed following 16 exposure-free weeks. Survivors
numbering 28, 28, and 21 mice at 0, 250, and 500 mg/kg diet,
respectively, were autopsied. A dose-related reduction in body weight
gain and a dose-related increase in absolute liver weights were
observed. Neoplastic nodules (nodular hyperplasia) were found in the
livers of 3/28 mice at 250 mg/kg diet and 6/21 mice at 500 mg/kg diet.
Hepatocellular carcinomas were observed in 3/21 mice at 500 mg/kg
diet. No neoplastic nodules were noted in the controls. The increases
at the higher dose level were statistically significant (Shirai et
al., 1978).
7.5 Miscellaneous effects
Evidence for the estrogenic activity of Aroclor 5442 was found using
the glycogen response of the immature rat uterus. Aroclor 5460 was
inactive in this test (Bitman & Cecil, 1970; Bitman et al., 1972).
The mixed type inducer, Aroclor 5460, increased the metabolism of
4-androstene-3,17-dione in male Sprague-Dawley rats intraperitoneally
injected with 4 doses of 300 mg/kg body weight in 4 days (Nilsen &
Toftgard, 1981). Reproductive effects have not been investigated.
Groups of pregnant ddY mice received a control diet or diets
containing PCTs (not specified) at levels of 50 or 500 mg/kg diet
(equivalent to 7 or 70 mg/kg body weight). Increased incidence of
cleft palate and other malformations was reported in the fetuses. In
neonates, reduced growth and survival as well as hyperactivity were
observed (Kimura & Miyake, 1976). (No details available).
REFERENCES
ABBOTT, D.C., COLLIN, G.B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom, 1969-71. Br.
med. J., 2: 553-556.
ABE, S., INOU, Y., & TAKAMATSU, M. (1975) [Polychlorinated biphenyl
residues in plasma of Yusho children born to mothers who had
consumed oil contaminated by PCBs.] Fukuoka Acta med., 66: 605-609
(in Japanese).
ABRAHAMSON, L.J. & ALLEN, J.R. (1973) The biological response of
infant nonhuman primates to a polychlorinated biphenyl. Environ.
health Perspect., 4: 81-86.
ACHILLES, A. (1983) Fire hazard of polychlorinated biphenyls. In:
Barros, M.C., Könemann H., & Visser, R., ed. Proceedings of the PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 187-191.
ACKER, L. & SCHULTE, E. (1970) [The occurrence of chlorinated
biphenyls and hexachlorobenzene alongside chlorinated insecticides
in breastmilk and human fatty tissue.] Naturwissenschaften, 57: 497
(in German).
ACKER, L. & SCHULTE, E. (1974) [Chlorinated hydrocarbons in human
fatty tissue.] Naturwissenschaften, 61: 1-4 (in German).
ACKER, L., BARKE, E., HAPKE, H.-J., HEESCHEN, W., KORANSKY, W.,
KUBLER, W., & REINHARDT, D. (1984) [Residues and impurities in
breast milk. German Association for the Encouragement of Research:
Communication XII of the Committee on Testing of Residues in
Foods], Weinheim, Verlag Chemie, pp. 1-97.
ACQUAVELLA, J.F., HANIS, N.M., NICOLIC, M.J., & PHILLIPS, S.C.
(1986) Assessment of clinical, metabolic, dietary, and occupational
correlations with serum polychlorinated biphenyl levels among
employees at an electrical capacitor manufacturing plant. J. occup.
Med., 28(11): 1177-1180.
ADAMS, J.A. (1983) Effect of PCB (Aroclor 1254) on early
development and mortality in Arbacia eggs. Water air soil
Pollut., 20: 1-5.
ADDISON, R.F. & BRODIE, P.F. (1977) Organochlorine residues in
maternal blubber, milk, and pup blubber from grey seals
(Halichoerus grypus) from Sable Island, Nova Scotia. J. Fish Res.
Board Can., 34: 937-941.
ADDISON, R.F. & SMITH, T.G. (1974) Organochlorine residue levels in
Arctic ringed seals: variation with age and sex. Oikos,
25: 335-337.
ADDISON, R.F., FLETCHER, G.L., RAY, S., & DOANE, J. (1972) Analysis
of a chlorinated terphenyl (Aroclor 5460) and its deposition in
tissues of cod (Gadus morhua). Bull. environ. Contam. Toxicol.,
8: 52-60.
ADDISON, R.F., KERR, S.R., DALE, J., & SERGEANT, D.E. (1973)
Variation of organochlorine residue levels with age in Gulf of St.
Lawrence harp seals (Pagophilus groenlandicus). J. Fish. Res.
Board Can., 30: 595-600.
ADDISON, R.F., ZINCK, M.E., & SMITH, T.G. (1986) PCBs have declined
more than DDT-group residues in Arctic ringed seals (Phoca
hispida) between 1972 and 1981. Environ. Sci. Technol.,
20: 253-256.
AGRAWAL, A.K., TILSON, H.A., & BONDY, S.C. (1981)
3,4,3',4'-tetrachlorobiphenyl given to mice prenatally produces
long-term decreases in striatal dopamine and receptor binding sites
in the caudate nucleus. Toxicol. Lett., 7: 417-424.
AGUILAR, A. & BORRELL, A. (1988) Age- and sex-related changes in
organochlorine compound levels in fin whales (Balaenoptera
physalus) from the Eastern North Atlantic. Mar. environ. Res.,
25: 195-211.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of polychlorinated biphenyl (PCB) and chlorinated
pesticides in water. Anal. Chem., 42: 1483-1486.
AHMED, M. & FOCHT, D.D. (1973a) Degradation of polychlorinated
biphenyls by two species of Achromobacter. Can. J. Microbiol.,
19: 47-52.
AHMED, M. & FOCHT, D.D. (1973b) Oxidation of polychlorinated
biphenyls by Achromobacter PCB. Bull. environ. Contam. Toxicol.,
10: 70-72.
AHNOFF, M. & JOSEFSSON, B. (1973) Confirmation studies on
polychlorinated biphenyls (PCBs) from river waters using mass
fragmentography. Anal. Lett., 6: 1083-1093.
AHNOFF, M. & JOSEFSSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AHNOFF, M. & JOSEFSSON, B. (1975) Clean-up procedures for PCB
analysis on river water extracts. Bull. environ. Contam. Toxicol.,
13: 159-166.
AHOTUPA, M. & AITIO, A. (1980) Effect of chlorinated naphthalenes
and terphenyl on the activities of drug metabolizing enzymes in rat
liver. Biochem. biophys. Res. Commun., 93: 250-257.
AKAGI, K. & OKUMURA, M. (1985) Association of blood pressure and
PCB level in Yusho patients. Environ. health Perspect., 59: 37-39.
AKIYAMA, K., OHI, G., FUJITANI, K., & YAGYU, H. (1975)
Polychlorinated biphenyl residues in maternal and cord blood in
Tokyo metropolitan area. Bull. environ. Contam. Toxicol.,
14: 588-592.
ALBAIGES, J., FARRAN, A., SOLER, M., GALLIFA, A., & MARTIN, P.
(1987) Accumulation and distribution of biogenic and pollutant
hydrocarbons, PCBs and DDT in tissues of Western Mediterranean
fishes. Mar. environ. Res., 22: 1-18.
ALBRO, P.W. & FISHBEIN, L. (1972) Intestinal absorption of
polychlorinated biphenyls in rats. Bull. environ. Contam. Toxicol.,
8: 26-31.
ALBRO, P.W. & PARKER, C.E. (1979) Comparison of the compositions of
Aroclor 1242 and Aroclor 1016. J. Chromatogr., 169: 161-166.
ALBRO, P.W., CORBETT, J.T., & SCHROEDER, J.L. (1981) Quantitative
characterization of polychlorinated biphenyl mixtures (Aroclors
1248, 1254 and 1260) by gas chromatography using capillary columns.
J. Chromatogr., 205: 103-111.
ALENCASTRO, L.F. DE, PRELAZ, V., & TARRADELLAS, J. (1984)
Contamination of silos in Switzerland by PCB residues in coatings.
Bull. environ. Contam. Toxicol., 33(3): 270-276.
ALFORD-STEVENS, A.L. (1986) Analyzing PCBs. Environ. Sci. Technol.,
20(12): 1194-1199.
ALLEN, J.R. (1975) Response of the non-human primate to
polychlorinated biphenyl exposure. Fed. Proc., 34: 1675-1679.
ALLEN, J.R. & ABRAHAMSON, L.J. (1973) Morphological and biochemical
changes in the liver of rats fed polychlorinated biphenyls. Arch.
environ. Contam. Toxicol., 1: 265-280.
ALLEN, J.R. & BARSOTTI, D.A. (1976) The effects of transplacental
and mammary movement of PCBs on infant rhesus monkeys. Toxicology,
6: 331-340.
ALLEN, J.R. & NORBACK, D.H. (1973) Polychlorinated biphenyl- and
triphenyl-induced gastric mucosal hyperplasia in primates. Science,
179: 498-499.
ALLEN, J.R. & NORBACK, D.H. (1976) Pathobiological responses of
primates to polychlorinated biphenyl exposure. In: Proceedings of
the National Conference on Polychlorinated Biphenyls, Chicago,
19-21 November 1975, Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances, pp. 43-49 (EPA-560/6-75-004).
ALLEN, J.R., CARSTENS, L.A., & BARSOTTI, D.A. (1974) Residual
effects of short-term low level exposures of nonhuman primates to
polychlorinated biphenyls. Toxicol. appl. Pharmacol., 30: 440-451.
ALLEN, J.R., CARSTENS, L.A., ABRAHAMSON, L.J., & MARLAR, R.J.
(1975) Responses of rats and non-human primates to
2,5,2',5',-tetrachlorobiphenyl. Environ. Res., 9: 265-273.
ALLEN, J.R., CARSTENS, L.A., & ABRAHAMSON, L.J. (1976) Responses of
rats to polychlorinated biphenyls for fifty-two weeks. Arch.
environ. Contam., 4: 404-419.
ALLEN, J.R., BARSOTTI, D.A., LAMBRECHT, L.K., & VAN MILLER, J.P.
(1979) Reproduction effects of halogenated aromatic hydrocarbons on
nonhuman primates. Ann. NY Acad. Sci., 320: 419-425.
ALLEN, J.R., BARSOTTI, D.A., & CARSTENS, L.A. (1980) Residual
effects of polychlorinated biphenyls on adult nonhuman primates and
their offspring. J. Toxicol. environ. Health, 6: 55-66.
ALTMAN, N.H., NEW, A.E., MCCONNELL, E.E., & FERRELL, T.L. (1979) A
spontaneous outbreak of polychlorinated biphenyl (PCB) toxicity in
Rhesus monkeys (Macaca mulatta): Clinical observations. Lab.
anim. Sci., 29: 661-665.
ALVARES, A.P. (1977) Stimulatory effects of polychlorinated
biphenyls (PCB) on cytochromes P-450 and P-448 mediated microsomal
oxidations. In: Microsomes and drug oxidations, Oxford, New York,
Pergamon Press, pp. 476-483.
ALVARES, A.P. & KAPPAS, A. (1975) Induction of aryl hydrocarbon
hydroxylase by polychlorinated biphenyls in the foeto-placental
unit and neonatal livers during lactation. FEBS Lett., 50: 172-174.
ALVARES, A.P. & KAPPAS, A. (1977) Heterogenicity of cytochrome
P-450s induced by polychlorinated biphenyls. J. biol. Chem.,
252: 6373-6378.
ALVARES, A.P., FISCHBEIN, A., ANDERSON, K.E., & KAPPAS, A. (1977)
Alterations in drug metabolism in workers exposed to
polychlorinated biphenyls. Clin. Pharmacol. Ther., 22(2): 140-145.
ALVARES, A.P., EISEMAN, J.L., UENG, T.-H., & KAPPAS, A. (1982)
Polychlorinated biphenyls: species and tissue specifications of the
induction of monooxygenases in rats, mice, rabbits, and humans. In:
Proceedings of the Workshop on the Combined Effects of Xenobiotics,
Ottawa, 22-23 June 1981, Ottawa, National Research Council of
Canada, pp. 127-149 (Publication No. NRCC 18978).
ANDERSON, M.R. & PANKOW, J.F. (1986) A case study of a chemical
spill: Polychlorinated biphenyls (PCBs). 3. PCB sorption and
retardation in soil underlying the site. Water Resour. Res.,
22(7): 1051-1057.
ANDERSON, L.M., VAN HAVERE, K., & BUDINGER, J.M. (1983) Effects of
polychlorinated biphenyls on lung and liver tumours initiated in
suckling mice by N-nitrosodimethylamine. J. Natl Cancer Inst.,
71: 157-163.
ANDO, M. (1978) Transfer of 2,4,5,2',4',5'-hexachlorobiphenyl and
2,2-bis(p-chlorophenyl), 1,1,1 -trichloroethane ( p-p'DDT) from
maternal to newborn and suckling rats. Arch. Toxicol., 41: 179-186.
ANDO, M., SAITO, H., & WAKISAKA, I. (1984) [Transfer of PCBs from
mother to newborn baby through placenta and milk.] Res. Rep. Natl
Inst. Environ. Stud. Jpn, 67: 333-345 (in Japanese).
ANDO, M., SAITO, H., & WAKISAKA, I. (1985) Transfer of
polychlorinated biphenyls to newborn infants through the placenta
and mother's milk. Arch. environ. Contam. Toxicol., 14(1): 51-57.
ANDREN, A.W. (1982) Processes determining the flux of PCBs across
air/water interfaces. In: Mackay, D., ed. Physical behavior of PCBs
in the Great Lakes; Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., pp. 127-140.
ANDRES, J., LAMBERT, I., ROBERTSON, L., BANDIERA, S., SAWYER, T.,
LOVERING, S., & SAFE, S. (1983) The comparative biologic and toxic
potencies of polychlorinated biphenyls and polybrominated
biphenyls. Toxicol. appl. Pharmacol., 70: 204-215.
ANDREWS, J.E. (1989) Polychlorinated biphenyl (Aroclor 1254)
induced changes in femur morphometry calcium metabolism and
nephrotoxicity. Toxicology, 57: 83-96.
ANON. (1983a) PCB found in Czechoslovakian canned hams during each
of last four years. Food Chem. News, 18 April: 32.
ANON. (1983b) USDA blocks all Czechoslovakian meat imports because
of PCB residues. Food Chem. News, 2 May: 30-31.
ANON. (1985a) Summary of results of the first test to determine
residual contaminants in the air and on surface in floors 2 through
18 of the Binghamton State Office Building. Test report,
Springfield, Virginia, Versar Inc.
ANON. (1985b) Polychlorinated biphenyl transformer incident New
Mexico. Morb. Mortal. wkly Rep., 34(36): 557-559.
ANON. (1987) Polychlorinated biphenyl in fluorescent lighting.
Environ. Health Saf. News, 33(1): 3-18.
ARMOUR, J.A. & BURKE, J.A. (1970) Method for separating
polychlorinated biphenyls from DDT and its analogs. J. Assoc. Off.
Anal. Chem., 53: 761-768.
ARMBRUSTER, G., GEROW, K.G., GUTENMANN, W.H., LITTMAN, C.B., &
LISK, D.J. (1987) The effects of several methods of fish
preparation on residues of polychlorinated biphenyls and sensory
characteristics in striped bass. J. Food Saf., 8: 235-243.
ARNOLD, D.L., MES, J., ZAWIDZKA, Z.Z., & KARPINSKI, K. (1984)
Toxicity of PCB (Aroclor 1254) as a consequence of continuous
exposure during pregnancy and nursing (PA-24). Presented at the
27th Annual Meeting of the Canadian Federation of Biological
Societies, University of Saskatchewan, Canada, 18-22 June 1984.
ATKINSON, S.A. (1979) Chemical contamination of human milk: A
review of current knowledge. J. Can. Diet. Assoc., 40(3): 223-226.
ATLAS, E. & GIAM, C.S. (1981) Global transport of organic
pollutants: Ambient concentrations in the marine atmosphere.
Science, 211: 163-165.
ATSDR (1989) Toxicological profile for selected PCBs (Aroclor 1260,
1254, 1248, 1242, 1232, 1221 and 1016), Atlanta, Georgia, Agency
for Toxic Substances and Disease Registry (ATSDR/TP-88/21).
AULERICH, R.J. & RINGER, R.K. (1977) Current status of PCB toxicity
to mink and effect on their reproduction. Arch. environ. Contam.
Toxicol., 6: 279-292.
AULERICH, R.J., BURSIAN, S.J., BRESLIN, W.J., OLSON, B.A., &
RINGER, R.K. (1985) Toxicological manifestations of
2,4,5,2',4',5',-; 2,3,6,2',3',6',-; and 3,4,5,3',4',5',-
hexachlorobiphenyl and Aroclor 1254 in mink. J. Toxicol. environ.
Health., 15: 63-79.
AX, R.L. & HANSEN, L.G. (1975) Effects of purified polychlorinated
biphenyl analogs on chicken reproduction. Poult. Sci., 54: 895-900.
BACCI, E. & GAGGI, C. (1985) Polychlorinated biphenyls in plant
foliage: Translocation or volatilization from contaminated soils?
Bull. environ. Contam. Toxicol., 35: 673-681.
BACHE, C.A., SERUM, J.W., YOUNGS, W.D., & LISK, D.J. (1972)
Polychlorinated biphenyl residues: Accumulation in Cayuga lake
trout with age. Science, 177: 1191-1192.
BADSHA, K. & EDULJEE, G. (1986) PCB in the UK environment - A
preliminary survey. Chemosphere, 15(2): 211-215.
BADSHA, K., EDULJEE, G., & SCUDAMORE, N. (1986) Environmental
monitoring for PCB and trace metals in the vicinity of a chemical
waste disposal facility - III. Chemosphere, 15(7): 947-957.
BAGLEY, G.E., REICHEL, W.L., & CROMARTIE, E. (1970) Identification
of polychlorinated biphenyls in two bald eagles by combined
gas-liquid chromatography-mass spectroscopy. J. Assoc. Off. Anal.
Chem., 53: 251-261.
BAHN, A.K., ROSENWAIKE, I., HERRMAN, N., GROVER, P., STELLMAN, J.,
& O'LEARY, K. (1976) Melanomas after exposure to PCBs. New Engl. J.
Med., 295: 450.
BAILEY, S. & BUNYAN, P.J. (1972) Interpretation of persistence and
effects of polychlorinated biphenyls in birds. Nature (Lond.),
236: 34-36.
BAILEY, J., KNAUF, V., MUELLER, W., & HOBSON, W. (1980) Transfer of
hexachlorobenzene and polychlorinated biphenyls to nursing infant
Rhesus monkeys: enhanced toxicity. Environ. Res., 21: 190-196.
BAKER, F.D., BUSH, B., TUMASONIS, C.F., & LO, F.-C. (1977) Toxicity
and persistence of low-level PCB in adult Wistar rats, fetuses, and
young. Arch. environ. Contam. Toxicol., 5: 143-156.
BAKER, E.L., Jr., LANDRIGUN, P.J., GLUECK, C.J., ZACK, M.M., Jr.,
LIDDLE, J.A., BURSE, V.W., HOUSEWORTH, W.J., & NEEDHAM, L.L. (1980)
Metabolic consequences of exposure to polychlorinated biphenyls
(PCBs) in sewage sludge. Am. J. Epidemiol., 112(4): 553-563.
BALLSCHMITER, K. & ZELL, M. (1980) Analysis of polychlorinated
biphenyls (PCB) by glass capillary gas chromatography. Composition
of technical Aroclor- and Clophen-PCB mixtures. Fresenius' Z. anal.
Chem., 302: 20-31.
BALLSCHMITER, K., BUCHERT, H., BIHLER, S., SCHOTT, P., RÖPER, H.P.,
& PACHUR, H.J. (1981) Organochlorine pollutant analysis of
contaminated and uncontaminated lake sediments by high resolution
gas chromatography. Chemosphere, 10: 945-956.
BANDIERA, S., SAFE, S., & OKEY, A.B. (1982) Binding of
polychlorinated biphenyls classified as either phenobarbitone-,
3-methylcholanthrene-, or mixed-type inducers to cytosolic Ah
receptor. Chem. biol. Interact., 39: 259-277.
BANDIERA, S., FARRELL, K., MASON, G., KELLEY, M., ROMKES, M.,
BANNISTER, R., & SAFE, S. (1984) Comparative toxicities of the
polychlorinated dibenzofuran (PCDF) and biphenyl (PCB) mixtures
which persist in Yusho victims. Chemosphere, 13(4): 507-512.
BARSOTTI, D.A. & VAN MILLER, J.P. (1984) Accumulation of a
commercial polychlorinated biphenyl mixture (Aroclor 1016) in adult
Rhesus monkeys and their nursing infants. Toxicology, 30: 31-44.
BARSOTTI, D.A., MARLAR, R.J., & ALLEN, J.R. (1976) Reproductive
dysfunction in Rhesus monkeys exposed to low levels of
polychlorinated biphenyls (Aroclor 1248). Food Cosmet. Toxicol.,
14: 99-103.
BASTOMSKY, C.H. (1974) Effects of polychlorinated biphenyl mixture
(Aroclor 1254) and DDT on biliary excretion in rats. Endocrinology,
95: 1150-1155.
BASTOMSKY, C.H. (1977) Goitres in rats fed polychlorinated
biphenyls. Can. J. Physiol. Pharmacol., 55: 288-292.
BASTOMSKY, C.H. & MURTHY, P.V.N. (1976) Enhanced in vitro hepatic
glucuronidation of thyroxine in rats following cutaneous
application or ingestion of polychlorinated biphenyls. Can. J.
Physiol. Pharmacol., 54: 23-26.
BASTOMSKY, C.H., SOLYMOSS, B., ZSIGMOND, G., & WYSE, J.M. (1975) On
the mechanism of polychlorinated biphenyl-induced hypobili-
rubinaemia. Clin. chim. Acta, 61: 171-174.
BAUMANN, M., DEML, E., SCHAEFFER, E., & GREIM, H. (1983) Effects of
polychlorinated biphenyls at low dose levels in rats. Arch.
environ. Contam. Toxicol., 12: 509-515.
BAXTER, R.A., GILBERT, P.E., LIDGETT, R.A., MAINPRIZE, J.H., &
VODDEN, H.A. (1975) The degradation of polychlorinated biphenyls by
microorganisms. Sci. total Environ., 4: 53-61.
BECK, H. & MATHAR, W. (1985) [Analytical procedures for the
determination of selected components of PCBs in foodstuffs.]
Bundesggesundheitsblatt, 28(1): 1-12 (in German).
BECKER, G.M., MCNULTY, W.P., & BELL, M. (1979) Polychlorinated
biphenyl-induced morphologic changes in the gastric mucosa of the
Rhesus monkey. Lab. Invest., 40: 373-383.
BENGTSON, S.A. & SÖDERGREN, A. (1974) DDT and PCB residues in
airborne fallout and animals in Iceland. Ambio, 3: 84-86.
BENGTSSON, B.E. (1979) Increased growth in minnows exposed to PCBs.
Ambio, 8: 169-170.
BENGTSSON, B.E. (1980) Long-term effects of PCB (Clophen A50) on
growth, reproduction and swimming performance in the minnow,
Phoxinus phoxinus. Water Res., 14: 681-687.
BENTHE, H.F., KNOP, J., & SCHMOLDT, A. (1972) [Absorption and
distribution of polychlorinated biphenyls (PCB) after inhalatory
application.] Arch. Toxikol., 29: 85-95 (in German).
BENTLEY, J. (1983) Incineration of PCBs. In: Barros, M.C.,
Könemann, H., & Visser, R., ed. Proceedings of the PCB Seminar, The
Hague, 28-30 September 1983, The Hague, Ministry of the
Environment, pp. 281-288.
BERAN, M., BRANDT, I., & SLANINA, P. (1983) Distribution and effect
of some polychlorinated biphenyls in the hemopoietic tissues. J.
Toxicol. environ. Health., 12: 521-532.
BERCOVICI, B., WASSERMANN, M., CUCOS, S., RON, M., WASSERMAN, D.,
& PINES, A. (1983) Serum levels of polychlorinated biphenyls and
some organochlorine insecticides in women with recent and former
missed abortions. Environ. Res., 30(1): 169-174.
BERG, O.W., DIOSADY, P.L., & REES, G.A.V. (1972) Column
chromatographic separation of polychlorinated biphenyls from
chlorinated hydrocarbon pesticides and their subsequent gas
chromatographic quantitation in terms of derivatives. Bull.
environ. Contam. Toxicol., 7: 338-347.
BERGH, A.K. & PEOPLE, R.S. (1977) PCB distribution in sewage wastes
and their environmental and community effects. Proceedings of the
1977 National Conference on Treatment and Disposal of Industrial
Wastewaters and Residues, Houston, Texas, pp. 4-6.
BERGLUND, F. (1972) Levels of polychlorinated biphenyls in foods in
Sweden. Environ. health Perspect., 1: 67-69.
BERLIN, M., GAGE, J.C., & HOLM, S. (1973) The metabolism and
distribution of 2,4,5,2',5'-pentachlorobiphenyl in the mouse. In:
Proceedings of the Polychlorinated Biphenyls II Conference,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 101-108 (Publication No. 4E).
BERLIN, M., GAGE, J.C., & HOLM, S. (1975) Distribution and
metabolism of polychlorobiphenyls. In: Proceedings of an
International Symposium on Recent Advances in the Assessment of the
Health Effects of Environmental Pollution, Paris, 24-26 June 1974,
Luxembourg, Commission of the European Communities, Vol. 2,
pp. 895-902.
BERRY, D.L., SLAGA, T.J., DIGIOVANNI, J., & JUCHAU, M.R. (1979)
Studies with chlorinated dibenzo- p-dioxins, polybrominated
biphenyls, and polychlorinated biphenyls in a two-stage system of
mouse skin tumorigenesis: potent anticarcinogenic effects. Ann. NY
Acad. Sci., 320: 405-414.
BERRY, D.L., DIGIOVANNI, J., JUCHAU, M.R., BRACKEN, W.M., GLAESON,
G.L., & SLAGA, T.J. (1978) Lack of tumour promoting ability of
certain environmental chemicals in a two-stage mouse skin
tumorigenesis assay. Res. Commun. chem. Pathol. Pharmacol.,
20: 101-108.
BERTAZZI, P.A., ZOCHETTI, C., GUERCILENA, S., FOGLIA, M.D.,
PESATORI, A., & RIBOLDI, L. (1982) Mortality study of male and
female workers exposed to PCBs. In: Prevention of Occupational
Cancer: International Symposium, Geneva, International Labour
Office, pp. 242-248 (Occupational Safety and Health Series No. 46).
BERTAZZI, P.A., RIBOLDI, L., PESATORI, A., RADICE, L., & ZOCHETTI,
C. (1987) Cancer mortality of capacitor manufacturing workers. Am.
J. ind. Med., 11(2): 165-176.
BICKERS, D.R., HARBER, L.C., KAPPAS, A., & ALVARES, A.P. (1972)
Polychlorinated biphenyls: Comparative effects of high and low
chlorine containing Aroclors on hepatic mixed function oxidase.
Res. Commun. Chem. Pathol. Pharmacol., 3: 505-512.
BIDLEMAN, T.F. & OLNEY, C.E. (1974) Chlorinated hydrocarbons in the
Sargasso Sea atmosphere and surface water. Science, 183: 516-518.
BIDLEMAN, T.F., RICE, C.P., & OLNEY, C.E. (1978) High molecular
weight chlorinated hydrocarbons in the air and sea: Rates and
mechanisms of air/sea transfer. In: Windom, H. & Duce, R.A., ed.
Marine pollutant transfer, Lexington, D.C. Heath and Co.,
pp. 323-351.
BIESSMANN, A. (1982) Effects of PCBs on gonads, sex hormone balance
and reproduction processes of Japanese quail Coturnix coturnix
japonica after ingestion during sexual maturation. Environ.
Pollut., 27: 15-30.
BIGGS, D.C., ROWLAND, R.G., POWERS, C.D., O'CONNERS, H., & WURSTER,
C. (1978) A comparison of the effects of chlordane and PCB on the
growth, photosynthesis, and cell size of estuarine phytoplankton.
Environ. Pollut., 15: 253-263.
BIGGS, D.C., ROWLAND, R.G., & WURSTER, C.F. (1979) Effects of
trichloroethylene, hexachlorobenzene and polychlorinated biphenyls
on the growth and cell size of marine phytoplankton. Bull. environ.
Contam. Toxicol., 21: 196-201.
BIGGS, D.C., POWERS, C.D., ROWLAND, R.G., O'CONNORS, H.B., &
WURSTER, C.F. (1980) Uptake of polychlorinated biphenyls by natural
phytoplankton assemblages: field and laboratory determinations of
14C-PCB particle-water index of sorption. Environ. Pollut.,
22: 101-110.
BILLINGS, W.N., BIDLEMAN, T.F., & VERNBERG, W.B. (1978) Movement of
PCB from a contaminated reservoir into a drinking water supply.
Bull. environ. Contam. Toxicol., 19: 215-222.
BIOCCA, M., GUPTA, B.N., CHAE, K., MCKINNEY, J.D., & MOORE, J.A.
(1981) Toxicity of selected symmetrical hexachlorobiphenyl isomers
in the mouse. Toxicol. appl. Pharmacol., 58: 461-474.
BIRGE, W.J., BLACK, J.A., & WESTERMAN, A.G. (1978) Effects of
polychlorinated biphenyl compounds and proposed PCB-replacement
products on embryo-larval stages of fish and amphibians,
Washington, DC, US Department of the Interior, p. 33 (Research
Report No. 118).
BIRNBAUM, L.S. (1983) Distribution and excretion of 2,3,6,2',3',6'-
and 2,4,5,2',4',5'-hexachlorobiphenyl in senescent rats. Toxicol.
appl. Pharmacol., 70: 262-272.
BIRNBAUM, L. & BAIRD, M.B. (1978) Induction of hepatic mixed
function oxidases in senescent rodents-II. Effect of
polychlorinated biphenyls. Exp. Gerontol., 13: 469-477.
BIRNBAUM, L.S., WEBER, H., HARRIS, M.W., LAMB, J.C., IV, &
MCKINNEY, J.D. (1985) Toxic interaction of specific polychlorinated
biphenyls and 2,3,7,8-tetrachlorodibenzo- p-dioxin: increased
incidence of cleft palate in mice. Toxicol. appl. Pharmacol.,
77: 292-302.
BITMAN, J. & CECIL, H.C. (1970) Estrogenic activity of DDT analogs
and polychlorinated biphenyls. J. agric. food Chem., 18: 1108-1112.
BITMAN, J., CECIL, H.C., & HARRIS, S.J. (1972) Biological effects
of polychlorinated biphenyl in rats and quail. Environ. health
Perspect., 1: 145-149.
BJERK, J.E. (1972) [Residues of DDT and polychlorinated biphenyls
in Norwegian human material.] Tidsskr. Nor. Laegeforen., 92: 15-19
(in Norwegian).
BLEAVINS, M.R., AULERICH, R.J., & RINGER, R.K. (1980)
Polychlorinated biphenyls (Aroclors 1016 and 1242): Effects on
survival and reproduction in mink and ferrets. Arch. environ.
Contam. Toxicol., 9: 627-635.
BLEAVINS, M.R., AULERICH, R.J., RINGER, R.K., & BELL, T.G. (1982)
Excessive nail growth in the European ferret induced by Aroclor
1242. Arch. environ. Contam. Toxicol., 11: 305-312.
BLEAVINS, M.R., AULERICH, R.J., & RINGER, R.K. (1981) Placental and
mammary transfer of polychlorinated and polybrominated biphenyls in
the mink and ferret. In: Proceedings of the 2nd Conference on Avian
and Mammalian Wildlife Toxicology, Philadelphia, Pennsylvania,
American Society for Testing and Material, pp. 121-131
(ASTM STP 757).
BLEAVINS, M.R., BRESLIN, W.J., AULERICH, R.J., & RINGER, R.K.
(1984) Placental and mammary transfer of a polychlorinated biphenyl
mixture (Aroclor 1254) in the European ferret (Mustela putorius
furo). Environ. Toxicol. Chem., 3: 637-644.
BLETCHLY, J.D. (1983) Polychlorinated biphenyls. Production,
current use, and possible rates of future disposal in OECD
countries. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of PCB Seminar, The Hague, 28-30 September 1983, The
Hague, Ministry of the Environment, pp. 343-365.
BLETCHLY, J.D. (1985) Report to the Commission of the European
Communities on a study of measures to avoid dispersion into the
environment of polychlorinated biphenyls (PCBs) and polychlorinated
terphenyls (PCTs) from existing installations, Luxembourg,
Commission of the European Communities, pp. 22-33.
BLOK, S.M.G., GREVE, P.A., SANGSTER, B., SAVELKOUL, T.J.F., &
WEGMAN, R.C.C. (1984) [Study of normally occurring values of a
number of organochlorine pesticides and related compounds and their
metabolites, of polychlorine biphenyls and of chlorophenols in the
blood or plasma of healthy volunteers.] Bilthoven, The Netherlands,
National Institute of Public Health and Environmental Hygiene
(Unpublished report No. 638101001) (in Dutch).
BLUS, L.J., LAMONT, T.G., & NEELY, B.S. (1979) Fish, wildlife, and
estuaries: Effects of organochlorine residues on eggshell
thickness, reproduction, and population status of Brown Pelicans
(Pelecanus occidentalis) in South Carolina and Florida, 1969-76.
Pestic. monit. J., 12: 172-184.
BONNYNS, M. & BASTOMSKY, C.H. (1976) Polychlorinated
biphenyl-induced modification of lymphocyte response to plant
mitogens in rats. Experientia (Basel), 32: 522-523.
BORN, E.W., KRAUL, I., & KRISTENSEN, T. (1981) Mercury, DDT, and
PCB in the Atlantic Walrus (Odobenus rosmarus rosmarus) from the
Thule District, North Greenland. Arctic, 34: 255-260.
BOURQUIN, A.W. & CASSIDY, S. (1975) Effect of polychlorinated
biphenyl formulations on the growth of estuarine bacteria. Appl.
microbiol., 29: 125-127.
BOURQUIN, A.W. & KIEFER, L.A. (1975) Inhibition of estuarine
microorganisms by polychlorinated biphenyls. Dev. ind. Microbiol.,
16: 256-261.
BOWES, G.W. & JONKEL, C.J. (1975) Presence and distribution of
polychlorinated biphenyls (PCBs) in arctic and subarctic marine
food chains. J. Fish Res. Board Can., 32: 2111-2123.
BOWES, G.W., MULVIHILL, M.J., SIMONEIT, B.R.T., BURLINGAME, A.L.,
& RISEBROUGH, R.W. (1975) Identification of chlorinated
dibenzofurans in American polychlorinated biphenyls. Nature
(Lond.), 256: 305-307.
BOWMAN, R.E. & HEIRONIMUS, M.P. (1981) Hypoactivity in adolescent
monkeys perinatally exposed to PCBs and hyperactive as juveniles.
Neurobehav. Toxicol. Teratol., 3: 15-18.
BOWMAN, R.E., HEIRONIMUS, M.P., & ALLEN, J.R. (1978) Correlation of
PCB body burden with behavioural toxicology in monkeys. Pharmacol.
Biochem. Behav., 9: 49-56.
BOWMAN, R.E., HEIRONIMUS, M.P., & BARSOTTI, D.A. (1982) Locomotor
hyperactivity in PCB-exposed monkeys. Neurotoxicology, 2: 251-268.
BRANDT-RAUF, P.W. & NIMAN, H.L. (1988) Serum screening for oncogene
proteins in workers exposed to PCBs. Br. J. ind. Med.,
45(10): 689-693.
BRAUN, F. & MEYHOFER, B. (1977) [Investigations on the
concentration of polychlorinated biphenyls (Clophen C) in fish
organs under laboratory conditions.] Fisch Umwelt., 3: 1-11
(in German).
BRAZELTON, T.B. (1973) Neonatal behavioural assessment scale,
Philadelphia, Pennsylvania, J.B. Lippincott.
BREZNER, E., TERKEL, J., & PERRY, A.S. (1984) The effect of Aroclor
1254 (PCB) on the physiology of reproduction in the female rat-I.
Comp. Biochem. Physiol., 77C: 65-70.
BRIGGS, D.M. & HARRIS, J.R. (1973) Polychlorinated biphenyls
influence on hatchability. Poult. Sci., 52: 1291-1294.
BRINKMAN, U.A. & DE KOK, A. (1980) Production properties and usage.
In: Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins
and related products, Amsterdam, Elsevier Biomedical Press,
pp. 1-40.
BRINKMAN, M., FOGELMAN, K., HOEFLEIN, J., LINDH, T., PATEL, M.,
TRENCH, W.C., & AIKENS, D.A. (1980) Distribution of polychlorinated
biphenyls in the Fort Edward, New York, water system. Environ.
Manage., 4(6): 511-520.
BRINKMAN, M., FOGELMAN, K., HOEFLEIN, J., LINDH, T., PATEL, M.,
TRENCH, W.C., & AIKENS, D.A. (1981) Levels of polychlorinated
biphenyls in the Fort Edward, New York, water system. Adv. Identif.
Anal. Org. Pollut. Water, 2(50): 1001-1015.
BRITTON, W.M. & HUSTON, T.M. (1972) Yolk content and hatchability
of egg from hens fed Aroclor 1242. Poult. Sci., 51: 1869.
BROADHURST, M.G. (1972) Use and replaceability of polychlorinated
biphenyls. Environ. health Perspect., 2: 81-102.
BROUWER, A., VAN DEN BERG, K.J., & KUKLER, A. (1985) Time and dose
responses of the reduction in retinoid concentrations in C57BL/Rij
and DBA/2 mice induced by 3,4,3',4'-tetrachlorobiphenyl. Toxico.
appl. Pharmacol., 78: 180-189.
BROUWER, A., KUKLER, A., & VAN DEN BERG, K.J. (1988) Alterations in
retinoid concentrations in several extrahepatic organs of rats by
3,4,3',4'-tetrachlorobiphenyl. Toxicology, 50: 317-330.
BROUWER, A., REIJNDERS, P.J.H., & KOEMAN, J.H. (1989)
Polychlorinated biphenyl (PCB)-contaminated fish induces vitamin A
and thyroid hormone deficiency in the common seal (Phoca
vitulina). Aquat. Toxicol., 15: 99-106.
BROWN, D.P. (1987) Mortality of workers exposed to polychlorinated
biphenyls - An update. Arch. environ. Health, 42(6): 333-339.
BROWN, D.P. & JONES, M. (1981) Mortality and industrial hygiene
study of workers exposed to polychlorinated biphenyls. Arch.
environ. Health, 36: 120-129.
BROWN, J.F. & LAWTON, R.W. (1984) Polychlorinated biphenyl (PCB)
partitioning between adipose tissue and serum. Bull. environ.
Contam. Toxicol., 33: 277-280.
BROWN, J.F., BEDARD, D.L., BRENNAN, M.J., CARNAHAN, J.C., FENG, H.,
& WAGNER, R.E. (1987a) Polychlorinated biphenyl dechlorination in
aquatic sediments. Science, 236: 709-712.
BROWN, J.F., WAGNER, R.E., FENG, H., BEDARD, D.L., BRENNAN, M.J.,
CARNAHAN, J.C., & MAY, R.J. (1987b) Environmental dechlorination of
PCBs. Environ. Toxicol. Chem., 6: 579-593.
BROWN, J.F., CARNAHAN, J.C., DORN, S.B., GROVES, J.T., LIGON, W.V.,
MAY, R.J., WAGNER, R.E., & HAMILTON, S.B. (1988) Levels of
bioactive PCDF congeners in PCB dieletric fluids from capacitors
and transformers. Chemosphere, 17(9): 1697-1702.
BRUCE, R.W. & HEDDLE, J.A. (1979) The mutagenic activity of 61
agents as determined by the micronucleus, Salmonella, and sperm
abnormality assays. Can. J. Genet. Cytol., 21: 319-334.
BRUCKNER, J.V., KHANNA, K.L., & CORNISH, H.H. (1973) Biological
responses of the rat to polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 24: 434-448.
BRUCKNER, J.V., KHANNA, K.L., & CORNISH, H.H. (1974) Effect of
prolonged ingestion of polychlorinated biphenyls on the rat. Food
Cosmet. Toxicol., 12: 323-330.
BRUCKNER, J.V., JIANG, W.-D., BROWN, J.M., PUTCHA, L., CHU, C.K.,
& STELLA, V.J. (1977) The influence of ingestion of environmentally
encountered levels of a commercial polychlorinated biphenyl mixture
(Aroclor 1254) on drug metabolism in the rat. J. Pharmacol. exp.
Ther., 202: 22-31.
BRUNSTROM, B. & DARNERUD, P.O. (1983) Toxicity and distribution in
chick embryos of 3,3,4,4-tetrachlorobiphenyl injected into the
eggs. Toxicology, 27: 103-110.
BRUNSTROM, B. & REUTERGARDH, L. (1986) Differences in sensitivity
of some avian species to the embryotoxicity of a PCB,
3,3',4,4'-tetrachlorobiphenyl, injected into the eggs. Environ.
Pollut., 42: 37-45.
BRUNSTROM, B., KIHLSTROM, I., & LUNDKVIST, U. (1982) Studies of
foetal death and foetal weight in guinea-pigs fed polychlorinated
biphenyls (PCB). Acta pharmacol. toxicol., 50: 100-103.
BRUNSTRÖM, B., DARNERUD, P.O., BRANDT, I., & ORBERG, J. (1982a)
Distribution, metabolism and toxicity of 2,2,4,5-tetrachloro-
biphenyl after injection into the yolk of embryonated hens' eggs.
Ambio, 11: 212-214.
BRUNSTRÖM, B., KIHLSTRÖM, I., & LUNDKVIST, U. (1982b) Studies of
foetal death and foetal weight in guinea-pigs fed polychlorinated
biphenyls (PCB). Acta pharmacol. toxicol., 50: 100-103.
BUCKLEY, E.H. (1982) Accumulation of airborne polychlorinated
biphenyls in foliage. Science, 216: 520-522.
BUCKLEY, E.H. (1983) Decline of background PCB concentrations in
vegetation in New York state. Northeast. environ. Sci., 2: 181-187.
BUHLER, F., SCHMID, P., & SCHLATTER, Ch. (1988) Kinetics of PCB
elimination in man. Chemosphere, 17(9): 1717-1726.
BUNCE, N.J. (1978) Photolysis of 2-chlorobiphenyl in aqueous
acetonitrile. Chemosphere, 7: 653-656.
BUNCE, N.J., KUMAR, Y., & BROWNLEE, B.G. (1978) An assessment of
the impact of solar degradation of polychlorinated biphenyls in the
aquatic environment. Chemosphere, 7: 155-164.
BURKHARD, L.P., ARMSTRONG, D.E., & ANDREN, A.W. (1985) Henry's Law
Constants for the polychlorinated biphenyls. Environ. Sci.
Technol., 19(7): 590-596.
BURNS, J.E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue-Texas 1969-72.
Pestic. monit. J., 7(3/4): 122-126.
BURSE, V.W., KIMBROUGH, R.D., VILLANUEVA, E.C., JENNINGS, R.W.,
LINDER, R.E., & SOVOCOOL, G.W. (1974) Polychlorinated biphenyls;
storage, distribution, excretion, and recovery: liver morphology
after prolonged dietary ingestion. Arch. environ. Health,
29: 301-307.
BUSER, H.R. (1979) Formation of polychlorinated dibenzofurans
(PCDFs) and dibenzo- p-dioxins (PCDDs) from the pyrolysis of
chlorobenzenes. Chemosphere, 8: 415-424.
BUSER, H.R. (1985) Formation, occurrence, and analysis of
polychlorinated dibenzofurans, dioxins and related compounds.
Environ. health Perspect., 60: 259-267.
BUSER, H.R. & BOSSHARDT, H.P. (1978) [Polychlorinated
dibenzo- p-dioxins, dibenzofurans and benzenes in the ash of
community and industrial incineration plants.] Mitt. Geb. Lebensm.
Hyg., 69: 191-199 (in German).
BUSER, H.R. & RAPPE, C. (1979) Formation of polychlorinated
dibenzofurans (PCDFs) from the pyrolysis of individual PCB isomers.
Chemosphere, 3: 157-174.
BUSER, H.R., BOSSHARDT, H.P., & RAPPE, C. (1978a) Formation of
polychlorinated dibenzofurans (PCDF's) from the pyrolysis of PCBs.
Chemosphere, 7: 109-119.
BUSER, H.R., BOSSHARDT, H.P., RAPPE, C., & LINDAHL, R. (1978b)
Identification of polychlorinated dibenzofuran isomers in fly ash
and PCB pyrolysis. Chemosphere, 7: 419-429.
BUSH, B., TUMASONIS, C.F., & BAKER, F.D. (1974) Toxicity and
persistence of PCB homologs and isomers in the avian system. Arch.
environ. Contam. Toxicol., 2: 195-212.
BUSH, B., SNOW, J., & KOBLINTZ, R. (1984) Polychlorobiphenyl (PCB)
congeners, p,p'-DDE, and hexachlorobenzene in maternal and fetal
cord blood from mothers in upstate New York. Arch. environ. Contam.
Toxicol., 13(5): 517-527.
BUSH, B., SNOW, J., CONNOR, S., & KOBLINTZ, R. (1985)
Polychlorinated biphenyl congeners (PCBs), p,p'-DDE and
hexachlorobenzene in human milk in three areas of upstate New York.
Arch. environ. Contam. Toxicol., 14: 443-450.
BUSH, B., SIMPSON, K.W., SHANE, L., & KOBLINTZ, R.R. (1985) PCB
congener analysis of water and caddisfly larvae (Insecta:
Trichoptera) in the Upper Hudson river by glass capillary
chromatography. Bull. environ. Contam. Toxicol., 34: 96-105.
BYRNE, J.J. & SEPKOVIC, D.W. (1987) Inhibition of monovalent cation
transport across the cell membrane by polychlorinated biphenyl but
not by polybrominated biphenyl. Arch. environ. Contam. Toxicol.,
16: 573-577.
BYRNE, J.J., CARBONE, J.P., & PEPE, M.G. (1988) Suppression of
serum adrenal cortex hormones by chronic low-dose polychloro-
biphenyl of polybromobiphenyl treatments. Arch. environ. Contam.
Toxicol., 17: 47-53.
CAIN, B.W. (1981) Nationwide residues of organochlorine compounds
in wings of adult mallards and black ducks, 1979-80. Pestic. monit.
J., 15: 128-134.
CAIN, B.W. & BUNCK, C.M. (1983) Residues of organochlorine
compounds in starlings (Sturnus vulgaris), 1979. Environ. monit.
Assess., 3: 161-172.
CALANDRA, J.C. (1976) Summary of toxicological studies on
commercial PCBs. In: Proceedings of the National Conference of
polychlorinated biphenyls, Washington, DC, US Environmental
Protection Agency (560/6-75-004).
CALIFANO, R.J., O'CONNOR, J.M., & PETERS, L.S. (1980) Uptake,
retention, and elimination of PCB (Aroclor 1254) by larval striped
bass (Morone saxatilis). Bull. environ. Contam. Toxicol.,
24: 467-472.
CALL, D.J. & HARRELL, B.E. (1974) Effects of dieldrin and PCBs upon
the production and morphology of Japanese quail eggs. Bull.
environ. Contam. Toxicol., 11: 70-77.
CALLAHAN, M.A.A., HAMMERSTROM, K.A., & SCHWEER, G. (1983) Present
PCB uses and their potential for release to the environment. In:
Barros, M.C., Könemann, H., & Visser, R., ed. Proceedings of PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 152-172.
CAMP, B.J., HEJTMANCIK, E., ARMOUR, C., & LEWIS, D.H. (1974) Acute
effects of Aroclor 1254 (PCB) on Ictalurus punctatus (catfish).
Bull. environ. Contam. Toxicol., 12: 204-208.
CAMPS, M., PLANAS, J., GOMEZ-CATALAN, J., SABROSO, M., TO-FIGUERAS,
J., & CORBELLA, J. (1989) Organochlorine residues in human adipose
tissue in Spain: Study of an agrarian area. Bull. environ. Contam.
Toxicol., 42: 195-201.
CANTONI, C., FABBRIS, F., ROGLEDI, R., & CAMPAGNARI, A. (1988)
[Organochlorine pesticides found in foods of animal origin in the
1985-1987 biennium.] Ind. Aliment., XXVII(1): 6-8 (in Italian).
CARLSON, R.W. & DUBY, R.T. (1973) Embryotoxic effects of three
PCB's in the chicken. Bull. environ. Contam. Toxicol., 9: 261-266.
CARNES, R.A., DOERGER, J.U., & SPARKS, H.L. (1973) Polychlorinated
biphenyls in solid waste and solid-waste-related materials. Arch.
environ. Contam. Toxicol., 1: 27-35.
CAREY, A.E. & HARVEY, G.R. (1978) Metabolism of polychlorinated
biphenyls by marine bacteria. Bull. environ. Contam. Toxicol.,
20: 527-534.
CARTER, J.W. (1985) Effects of dietary in PCBs (Aroclor 1254) on
serum levels of lipoprotein cholesterol in Fischer rats. Bull.
environ. Contam. Toxicol., 34: 427-431.
CARTER, J.W. & CLANCY, J. (1980) Acutely administered
polychlorinated biphenyls (PCBs) decrease splenic cellularity but
increase its ability to cause graft-versus host reactions in BALB/c
mice. Immunopharmacology, 2: 341-347.
CATELANI, D., SORLINI, C., & TRECCANI, V. (1971) The metabolism of
biphenyl by Pseudomonas putida. Experientia (Basel),
27: 1173-1174.
CECIL, H.C., BITMAN, J., LILLIE, R.J., FRIES, G.F., & VERRETT, J.
(1974) Embryotoxic and teratogenic effects in unhatched fertile
eggs from hens fed polychlorinated biphenyls (PCBs). Bull. environ.
Contam. Toxicol., 11: 489-495.
CETINKAYA, M., GABEL, B., PODBIELSKI, A., & THIEMANN, W. (1984)
[Investigation on the association between nutrition and living
conditions of breastfeeding mothers and the contamination of
breastmilk with organochlorine compounds of low volatility.] Akt.
Ernähr., 9: 157-162 (in German).
CHAKRABORTY, D., BHATTACHARYYA, A., CHATTERJEE, J., CHATTERJEE, K.,
SEN, A., CHATTERJEE, S., MAJUMDAR, K., & CHATTERJEE, G.C. (1978)
Biochemical studies on polychlorinated biphenyl toxicity in rats:
manipulation by vitamin C. Int. J. Vitam. Nutr. Res., 48: 22-31.
CHANG, K.-T., CHENG, J.-S., HUANG, P.-C., & TUNG, T.-C. (1980a)
Study of patients with PCB poisoning. J. Formosan Med. Assoc.,
79: 304-313.
CHANG, K.-J., LU, F.-J., TUNG, T.-C., & LEE, T.-P. (1980b) Studies
on patients with PCB poisoning. Determination of urinary
coproporphyrin, uroporphyrin, delta-aminolaevulinic acid and
prophobilinogen. Res. Commun. chem. Pathol. Pharmacol.,
30(3): 547-554.
CHANG, K.-J., HSIEH, K.-H., LEE, T.-P., TANG, S.-Y., & TUNG, T.-C.
(1981) Immunologic evaluation of patients with polychlorinated
biphenyls poisoning. Determination of lymphocyte subpopulations.
Toxicol. appl. Pharmacol., 61: 58-63.
CHASE, K.H., WONG, O., THOMAS, D., BERNEY, B.W., & SIMON, R.K.
(1982) Clinical and metabolic abnormalities associated with
occupational exposure to polychlorinated biphenyls (PCBs). J.
occup. Med., 24(2): 109-114.
CHEN, P.H., GRAW, J.M., WONG, C.K., & CHEN, C.J. (1980) Levels and
gas chromatography patterns of PCBs in blood of patients after PCB
poisoning in Taiwan. Bull. environ. Contam. Toxicol., 25: 325-329.
CHEN, P.H., CHANG, K.T., & LU, Y.D. (1981) Polychlorinated
biphenyls and polychlorinated benzofurans in the toxic rice-bran
oil caused PCB poisoning in Taichyung. Bull. environ. Contam.
Toxicol., 26(4): 489-495.
CHEN, P.H., LUO, M.L., WONG, C.K., & CHEN, C.J. (1982) Comparative
rates of elimination of some individual polychlorinated biphenyls
from the blood of PCB-poisoned patients in Taiwan. Food chem.
Toxicol., 20(4): 417-425.
CHEN, P.H., WONG, C.-K., RAPPE, C., & NYGREN, M. (1985)
Polychlorinated biphenyls, dibenzofurans and quaterphenyls in toxic
rice-bran oil and in the blood and tissues of patients with PCB
poisoning (Yu-Cheng) in Taiwan. Environ. Health Perspect.,
59: 59-65.
CHEN, P.R., MCKINNEY, J.D., & MATTHEWS, H.B. (1976) Metabolism of
2,4,5,2',5'-pentachlorobiphenyl in the rat. Drug. Metab. Dispos.,
4: 362-367.
CHEN, R.-C., TANG, S.-Y., MIYATA, H., KASHIMOTO, T., CHANG, Y.-C.,
CHANG, K.-J., & TUNG, T.-C. (1985) Polychlorinated biphenyl
poisoning: Correlation of sensory and motor nerve conduction,
neurologic symptoms and blood levels of polychlorinated biphenyls,
quaterphenyls, and dibenzofurans. Environ. Res., 37: 340-348.
CHEN, T.S. & DUBOIS, K.P. (1973) Studies on the enzyme inducing
effects of polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
26: 504-512.
CHEN HSI-SUNG, P., LUO, M.-L., WONG, C.-K., & CHEN, C.-J. (1984)
Polychlorinated biphenyls, dibenzofurans, and quaterphenyls in the
toxic rice-bran oil and PCBs in the blood of patients with PCB
poisoning in Taiwan. Am. J. ind. Med., 5: 133-145.
CHESNEY, C.F. & ALLEN, J.R. (1974) Oxidative phosphorylation and
respiration by liver mitochondria from polychlorinated
biphenyl-intoxicated rats. Biochem. Pharmacol., 23: 1577-1582.
CHIA, L.-G. & CHU, F.-L. (1984) Neurological studies on
polychlorinated biphenyl (PCB)-poisoned patients. Am. J. ind. Med.,
5: 117-126.
CHOU, S.M., MIKE, T., PAYNE, W.M., & DAVIS, G.J. (1979)
Neuropathology of "spinning syndrome" induced by prenatal
intoxication with a PCB in mice. Ann. NY Acad. Sci., 320: 373-395.
CHOW, C.K., THACKER, R., & GAIROLA, C.C. (1979) Increased level of
L-ascorbic acid in the plasma of polychlorobiphenyls-treated rats
and its inhibition by dietary vitamin E. Res. Commun. chem. Pathol.
Pharmacol., 26: 605-608.
CHRISTIANI, D.C., KRIEBEL, D., FOX, N.J., & BAKER, E.L. (1986)
Persistently elevated polychlorinated biphenyl levels from residual
contamination of workplace surfaces. Am. J. ind. Med., 10: 143-151.
CHU, C.K., STELLA, V.J., BRUCKNER, J.V., & JIANG, W.D. (1977)
Effects of long-term exposure to environmental levels of
polychlorinated biphenyls on pharmacokinetics of pentobarbital in
rats. J. pharm. Sci., 66(2): 238-241.
CLARK, D.R. (1978) Uptake of dietary PCB by pregnant Big Brown Bats
(Eptesicus fuscus) and their fetuses. Bull. environ. Contam.
Toxicol., 19: 707-714.
CLARK, D.R. & LAMONT, T.G. (1976) Organochlorine residues and
reproduction in the big brown bat. J. Wildl. Manage., 40: 249-254.
CLARK, D.R. & PROUTY, R.M. (1977) Experimental feeding of DDT and
PCB to female Big Brown Bats (Eptesicus fuscus). J. Toxicol.
environ. Health, 2: 917-928.
CLARK, R.R., CHIAN, E.S.K., & GRIFFIN, R.A. (1979) Degradation of
polychlorinated biphenyls by mixed microbial cultures. Appl.
environ. Microbiol., 37: 680-685.
CLARK, J.R., PATRICK, J.M., MOORE, J.C., & FORESTER, J. (1986)
Accumulation of sediment-bound PCBs by Fiddler crabs. Bull.
environ. Contam. Toxicol., 36: 571-578.
CLAUS, B. & ACKER, L. (1975) [Contamination of milk and milk
products with chlorinated hydrocarbons in Westphalia. II. Results
and discussion.] Z. Lebensmittelunters. Forsch., 159(3): 129-137
(in German).
CLEVENGER, M.A., ROBERTS, S.M., LATTIN, D.L., HARBINSON, R.D., &
JAMES, R.C. (1989) The pharmacokinetics of 2,2,5,5'-tetrachloro-
biphenyl and 3,3',4,4'-tetrachlorobiphenyl and its relationship to
toxicity. Toxicol. appl. Pharmacol., 100: 315-327.
COLE, D.R. & PLAPP, F.W. (1974) Inhibition of growth and
photosynthesis in Chlorella pyrenoidosa by a polychlorinated
biphenyl and several insecticides. Environ. Entomol., 3: 217-220.
COLLINS, W.T. & CAPEN, C.C. (1980a) Ultrastructural and functional
alterations of the rat's thyroid gland produced by polychlorinated
biphenyls compared with iodide excess and deficiency, and
thyrotropin and thyroxine administration. Virchows Arch. cell.
Pathol., B33: 213-231.
COLLINS, W.T. & CAPEN, C.C. (1980b) Biliary excretion of 125
I-thyroxine and fine structural alterations in the thyroid glands
of Gunn rats fed polychlorinated biphenyls (PCB). Lab. Invest.,
43: 158-164.
COLLINS, G.B., HOLMES, D.C., & JACKSON, F.J. (1972) The estimation
of polychlorobiphenyls. J. Chromatogr., 71: 443-449.
COLLINS, W.T., CAPEN, C.C., KASZA, L., CARTER, C., & DAILEY, R.E.
(1977) Effect of polychlorinated biphenyl (PCB) on the thyroid
gland of rats. Am. J. Pathol., 89: 119-136.
CONNELL, D.W. (1987) Age to PCB concentration relationship with the
striped bass (Morone saxatilis) in the Hudson River and Long
Island Sound. Chemosphere, 16: 1469-1474.
COOKE, A.S. (1973) Shell thinning in avian eggs by environmental
pollutants. Environ. Pollut., 4: 85-152.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides and pollution, Huntingdon, United Kingdom, Natural
Environment Research Council, Institute of Terrestrial Ecology,
74 pp.
COOLEY, N.R., KELTNER, J.M., & FORESTER, J. (1972) Mirex and
Aroclor 1254: Effect on accumulation by Tetrahymena pyriformis
strain W. J. Protozool., 19: 636-638.
COOLEY, N.R., KELTNER, J.M., & FORESTER, J. (1973) The
polychlorinated biphenyls, Aroclors 1248 and 1260: Effect on and
accumulation by Tetrahymena pyriformis. J. Protozool.,
20: 443-445.
COSPER, E.M., WURSTER, C.F., & ROWLAND, R.G. (1984) PCB resistance
within phytoplankton populations in polluted and unpolluted marine
environments. Mar. environ. Res., 12: 209-223.
COTE, M.G., PLAA, G.L., VALLI, V.E., & VILLENEUVE, D.C. (1985)
Subchronic effects of a mixture of "persistent" chemicals found in
the Great Lakes. Bull. environ. Contam. Toxicol., 34: 285-290.
COURTNEY, W.A.M. & DENTON, G.R.W. (1976) Persistence of
polychlorinated biphenyls in the hard-clam (Mercenaria mercenaria)
and the effect upon the distribution of these pollutants in the
estuarine environment. Environ. Pollut., 10: 55-64.
COURTNEY, W.A.M. & LANGSTON, W.J. (1978) Uptake of polychlorinated
biphenyl (Aroclor 1254) from sediment and seawater in two
intertidal polychaetes. Environ. Pollut., 15: 303-309.
CRAIGIE, J.S. & HUTZINGER, O. (1975) Effects of commercial
chlorinated hydrocarbons and specific chlorobiphenyls on the growth
of seven species of marine phytoplankton. Chemosphere, 3: 139-144.
CROSBY, D.G. & MOILANEN, K.W. (1973) Photodecomposition of
chlorinated biphenyls and dibenzofurans. Bull. environ. Contam.
Toxicol., 10: 372-377.
CURLEY, A., BURSE, V.W., GRIM, M.E., JENNINGS, R.W., & LINDER, R.E.
(1971) Polychlorinated biphenyls: Distribution and storage in body
fluids and tissues of Sherman rats. Environ. Res., 4: 481-495.
CURLEY, A., BURSE, V.W., & GRIM, M.E. (1973a) Polychlorinated
biphenyls: Evidence of transplacental passage in the Sherman rats.
Food Cosmet. Toxicol., 11: 471-476.
CURLEY, A., BURSE, V.W., JENNINGS, R.W., VILLANUEVA, E.C., TOMATIS,
L., & AKAZAKI, K. (1973b) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond.), 242: 338-340.
DAHLGREN, R.B. & LINDER, R.L. (1971) Effects of polychlorinated
biphenyls on pheasant reproduction, behavior, and survival. J.
Wildl. Manage., 35: 315-319.
DAHLGREN, R.B., LINDER, R.L., & CARLSON, C.W. (1972)
Polychlorinated biphenyls: Their effects on penned pheasants.
Environ. health Perspect., 1: 89-101.
DAHLGREN, R.B., BURY, R.J., LINDER, R.L., & REIDINGER, R.F. (1972)
Residue levels and histopathology in Pheasants given
polychlorinated biphenyls. J. Wildl. Manage., 36: 524-533.
DAVIDORF, F.H. & KNUPP, J.A. (1979) Epidemiology of ocular
melanoma. Incidence and geographic relationship in Ohio
(1967-1977). Ohio State med. J., 75: 561-564.
DAVIES, K. (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17(2): 263-276.
DAVIES, D. & MES, J. (1987) Comparison of the residue levels of
some organochlorine compounds in breast milk of the general and
indigenous Canadian populations. Bull. environ. Contam. Toxicol.,
39: 743-749.
DAVIS, D. & SAFE, S. (1989) Dose-response immunotoxicities of
commercial polychlorinated biphenyls (PCBs) and their interaction
with 2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol. Lett.,
48: 35-43.
DEFOE, D.L., VEITH, G.D., & CARLSON, R.W. (1978) Effects of Aroclor
1248 and 1260 on the Fathead Minnow (Pimephales promelas). J.
Fish Res. Board Can., 35: 997-1002.
DEFREITAS, A.S.W., NORSTRÖM, R.J., & HUTZINGER, O. (1972) Dynamics
and metabolism of polychlorinated biphenyls (PCBs) in cold-exposed
pigeons. Am. Chem. Soc., 12: 118-123.
DE KOCK, A.C. & LORD, D.A. (1988) Kinetics of the uptake and
elimination of polychlorinated biphenyls by an estuarine fish
species (Rhabdosargus holubi) after aqueous exposure.
Chemosphere, 17: 2381-2390.
DELFINO, J.J. (1979) Toxic substances in the Great Lakes. Environ.
Sci. Technol., 13: 1462-1468.
DELONG, R.L., GILMARTIN, W.G., & SIMPSON, J.G. (1973) Premature
births in California sea lions: Association with high
organochlorine pollutant residue levels. Science, 181: 1168-1169.
DEML, E. & OESTERLE, D. (1982) Sex-dependent promoting effect of
polychlorinated biphenyls on enzyme-altered islands induced by
diethylnitrosamine in rat liver. Carcinogenesis, 3: 1449-1453.
DEML, E. & OESTERLE, D. (1987) Dose-response of promotion by
polychlorinated biphenyls and chloroform in rat liver foci
bioassay. Arch. Toxicol., 60: 209-211.
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels of
organochlorine pesticides on microsomal liver enzymes in short-term
toxicity experiments. Toxicology, 2: 371-380.
DE VOS, R.H. & PEET, E.W. (1971) Thin-layer chromatography of
polychlorinated biphenyls. Bull. environ. Contam. Toxicol.,
6: 164-170.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRIJNS, J.K., MUYS,
T., & VAN DER POLL, J.M. (1984) Pesticides and other chemical
residues in Dutch total diet samples (June 1976-July 1978). Food
Chem. Toxicol., 22(1): 11-21.
DFG (1988) [Polychlorinated biphenyls. Stocktaking on analysis,
occurrence, kinetics and toxicology.] German Association for the
Encouragement of Research: Communication XIII of the Government
Committee on the Testing of Residues in Foods], Weinheim, Verlag
Chemie (in German).
DICKHUT, R.M., ANDREN, A.W., & ARMSTRONG, D.E. (1986) Aqueous
solubilities of six polychlorinated biphenyl congeners at four
temperatures. Environ. Sci. Technol., 20(8): 807-810.
DICKHUT, R.M., ANDREN, A.W., & ARMSTRONG, D.E. (1987) Comment on
"Aqueous solubilities of six polychlorinated biphenyl congeners at
four temperatures". Environ. Sci. Technol., 21(9): 926-928.
DIERINGER, C.S., LAMARTINIERE, C.A., SCHILLER, C.M., & LUCIER, G.W.
(1979) Altered ontogeny of hepatic steroid-metabolizing enzymes by
pure polychlorinated biphenyl congeners. Biochem. Pharmacol.,
28: 2511-2514.
DIETER, M.P. (1974) Plasma enzyme activities in Coturnix quail fed
graded doses of DDE, polychlorinated biphenyls, malathion, and
mercuric chloride. Toxicol. appl. Pharmacol., 27: 86-98.
DIGERNES, V. & ASTRUP, E.G. (1982) Are datascreen terminals a
source of increased PCB-concentrations in the working atmosphere?
Int. Arch. occup. Health, 49: 193-197.
DIGIOVANNI, J., BERRY, D.L., SLAGA, T.J., & JUCHAU, M.R. (1979)
Studies on the relationship between induction of biotransformation
and tumour-initiating activity of 7,12-dimethylbenz(a)anthracene in
mouse skin. In: Jones, P.W. & Leber, P., ed. Polynuclear aromatic
hydrocarbons, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 553-568.
DILLON, J.C., MARTIN, G.B., & O'BRIEN, H.T. (1981) Pesticide
residues in human milk. Food Cosmet. Toxicol., 19: 437-442.
DIVE, D., ERB, F., LECLERC, H., PRIEM, M.N., & COLEIN, M.P. (1976)
Toxicité et bioaccumulation d'isomers de polychlorobiphenyles par
le protozoaire cilie Colpidium campylum. Eur. J. Toxicol.
environ. Hyg., 9: 105-111.
DOBSON, S. (1981) Physiological and behavioural effects of
organochlorines on pigeons. Oekol. Voegel, 3: 39-43.
DOGRA, S., FILSER, J.G., COJOCEL, C., GREIM, H., REGEL, U., OESCH,
F., & ROBERTSON, L.W. (1988) Long-term effects of commercial and
congeneric polychlorinated biphenyls on ethane production and
malondialdehyde levels, indicators of in vivo lipid peroxidation.
Arch. Toxicol., 62: 369-374.
DOGUCHI, M. (1977) Polychlorinated terphenyls as an environmental
pollutant in Japan. Ecotoxicol. environ. Saf., 1: 239-248.
DOGUCHI, M. & FUKANO, S. (1975) Residue levels of polychlorinated
terphenyls, polychlorinated biphenyls and DDT in human blood. Bull.
environ. Contam. Toxicol., 13: 57-63.
DOGUCHI, M., FUKANO, S., & USHIO, F. (1974) Polychlorinated
terphenyls in the human fat. Bull. environ. Contam. Toxicol.,
11(2): 157-158.
DOMMARCO, R., DI MUCCIO, A., COMONI, I., & GIGLI, B. (1987)
Organochlorine pesticide and polychlorinated biphenyl residues in
human milk from Rome (Italy) and surroundings. Bull. environ.
Contam. Toxicol., 39: 919-925.
DONKIN, P., MANN, S., & HAMILTON, E.I. (1981) Polychlorinated
biphenyl, DDT, and dieldrin residues in grey seal (Halichoerus
grypus) males, females and mother-foetus pairs sampled at the
Farne islands, England, during the breeding season. Sci. total
Environ., 19: 121-142.
DOUCETTE, W.J. & ANDREN, A.W. (1988) Aqueous solubility of selected
biphenyl, furan and dioxin congeners. Chemosphere, 17(2): 243-252.
DRIJVER, M., DUIJKERS, T.J., KROMHOUT, D., VISSER, T.J., MULDER,
P., & LOUW, R. (1988) Determinations of polychlorinated biphenyls
(PCBs) in human milk. Acta paediatr. Scand., 77: 30-36.
DRILL, V.A., FREISS, S.L., HAYS, H.W., LOOMIS, T.A., & SCHAFFER,
C.B. (1981) Potential health effects in the human from exposure to
polychlorinated biphenyls (PCBs) and related impurities, Arlington,
Virginia, Drill, Freiss, Hays, Loomis and Schaffer, Inc.
(Unpublished report).
DUINKER, J.C. & BOUCHERTALL, F. (1989) On the distribution of
atmospheric polychlorinated biphenyl congeners between vapor phase,
aerosols and rain. Environ. Sci. Technol., 23: 57-62.
DUINKER, J.C. & HILLEBRAND, M.T.J. (1979) Mobilization of
organochlorines from female lipid tissue and transplacental
transfer to fetus in a harbour porpoise (Phocoena phocoena) in a
contaminated area. Bull. environ. Contam. Toxicol., 23: 728-732.
DUINKER, J.C. & HILLEBRAND, M.T.J. (1983) Characterization of PCB
components in Clophen formulations by capillary GC-MS and GC-ECD
techniques. Environ. Sci. Technol., 17: 449-456.
DUINKER, J.C., SCHULTZ, D.E., & PETRICK, G. (1988) Selection of
chlorinated biphenyl congeners for analysis in environmental
samples. Mar. Pollut. Bull., 19(1): 19-25.
DUKE, T.W., LOWE, J.I., & WILSON, A.J. (1970) A polychlorinated
biphenyl (Aroclor 1254) in the water, sediment, and biota of
Escambia Bay, Florida. Bull. environ. Contam. Toxicol., 5: 171-180.
DUNN, W.I., III, STELLING, D.L., SCHWARTZ, T.R., HOGAN, J.W.,
PETTY, J.D., JOHANSSON, E., & WOLD, S. (1984) Pattern recognition
for classification and determination of polychlorinated biphenyls
in environmental samples. Anal. Chem., 56: 1308-1313.
DUNNIVANT, F.M. & ELZERMAN, A.W. (1988) Aqueous solubility and
Henry's Law Constant data for PCB congeners for evaluation of
quantitative structure-property relationships (QSPRs). Chemosphere,
17(3): 525-541.
DURHAM, S.K. & BROUWER, A. (1989) 3,4,3',4'-tetrachloro-
biphenyl-induced effects in the rat liver. I. Serum and hepatic
retinoid reduction and morphologic changes. Toxicol. Pathol.,
17(3): 536-544.
DUTCH AGRICULTURAL ADVISORY COMMISSION ON ENVIRONMENTAL POLLUTANTS
(1983) Annual report, The Hague, Ministry of Agriculture,
Management of Nature, and Fisheries (Unpublished).
DZOGBEFIA, V.P., KLING, D., & GAMBLE, W. (1978) Polychlorinated
biphenyls: in vivo and in vitro modifications of phospholipid
and glyceride biosynthesis. J. environ. Pathol. Toxicol.,
1: 841-856.
ECOBICHON, D.J. & COMEAU, A.M. (1974) Comparative effects of
commercial Aroclor on rat liver enzyme activities. Chem. biol.
Interact., 9: 341-350.
ECOBICHON, D.J. & COMEAU, A.M. (1975) Isomerically pure
chlorobiphenyl congeners and hepatic function in the rat. Influence
of position and degree of chlorination. Toxicol. appl. Pharmacol.,
33: 94-105.
ECOBICHON, D.J. & MACKENZIE, D.O. (1974) The uterotropic activity
of commercial and isomerically-pure chlorobiphenyls in the rat.
Res. Commun. chem. Pathol. Pharmacol., 9: 85-95.
EDULJEE, G., BADSHA, K., & SCUDAMORE, N. (1986) Environmental
monitoring for PCB and trace metals in the vicinity of a chemical
waste disposal facility - II. Chemosphere, 15(1): 81-93.
EISENREICH, S.J., LOONEY, B.B., & THORNTON, J.D. (1981) Airborne
organic contaminants in the Great Lakes ecosystem. Environ. Sci.
Technol., 15: 30-38.
EISLER, R. (1986) Polychlorinated biphenyl hazards to fish,
wildlife, and invertebrates: A synoptic review, Washington, DC, US
Department of Interior, Fish & Wildlife Service, 72 pp (Biology
Report No. 85).
EKSTEDT, J. & ODEN, S. (1974) Chlorinated hydrocarbons in the lower
atmosphere in Sweden, Uppsala, Sweden, Royal Agricultural College,
Department of Soil Science, pp. 1-16.
ELDER, D.L., FOWLER, S.W., & POLIKARPOV, G.G. (1979) Remobilization
of sediment-associated PCBs by the worm Nereis diversicolor.
Bull. environ. Contam. Toxicol., 21: 448-452.
ELLIOTT, J.E., NORSTRÖM, R.J., & KEITH, J.A. (1988) Organochlorines
and eggshell thinning in Northern Gannets (Sula bassanus) from
Eastern Canada, 1968-1984. Environ. Pollut., 52: 81-102.
ELO, O., VUOJOLAHTI, P., JANHUNEN, H., & RANTANEN, J. (1985) Recent
PCB accidents in Finland. Environ. health Perspect., 60: 315-319.
EMMETT, E.A. (1985) Polychlorinated biphenyl exposure and effects
in transformer repair workers. Environ. health Perspect.,
60: 185-192.
EMMETT, E.A., MARONI, M., SCHMITH, J.M., LEVIN, B.K., & JEFFERYS,
J. (1988a) Studies of transformer repair workers exposed to PCBs:
I. Study design, PCB concentrations, Questionnaire, and clinical
examination results. Am. J. ind. Med., 13: 415-427.
EMMETT, E.A., MARONI, M., JEFFERYS, J., SCHMITH, J., LEVIN, B.K.,
& ALVARES, A. (1988b) Studies of transformer repair workers exposed
to PCBs: II. Results of clinical laboratory investigations. Am. J.
ind. Med., 14: 47-62.
ERICKSON, M.D. (1985) The analytical chemistry of PCBs, Boston,
Massachusetts, Butterworth.
ERICKSON, M.D., STANLEY, J.S., TURMAN, J.K., GOING, J.E., REDFORD,
D.P., & HEGGEM, D.T. (1988) Determination of byproduct
polychlorobiphenyls in commercial products and wastes by
high-resolution gas chromatography/electron impact mass
spectrometry. Environ. Sci. Technol., 22(1): 71-76.
ESCHENROEDER, A.Q., DOYLE, C.P., & FAEDER, E.J. (1986) Health risks
of PCB spills from electrical equipment. Risk Anal., 6(2): 213-221.
EWALD, W.G., FRENCH, J.E., & CHAMP, M.A. (1976) Toxicity of
polychlorinated biphenyls (PCBs) to Euglena gracilis: Cell
population growth, carbon fixation, chlorophyll level, oxygen
consumption, and protein and nucleic acid synthesis. Bull. environ.
Contam. Toxicol., 16: 71-80.
EXON, J.H., TALCOTT, P.A., & KOLLER, L.D. (1985) Effect of lead,
polychlorinated biphenyls, and cyclophosphamide on rat natural
killer cells, interleukine 2, and antibody synthesis. Fundam. appl.
Toxicol., 5: 158-164.
FAIT, A., GROSSMAN, E., SELF, S., FEFFRIES, J., PELLIZZARI, E.D.,
& EMMETT, E.A. (1989) Polychlorinated biphenyl congeners in adipose
tissue lipid and serum of past and present transformer repair
workers and a comparison group. Fundam. appl. Toxicol., 12: 42-55.
FALANDYSZ, J. (1980) Chlorinated hydrocarbons in gulls from the
Baltic south coast. Mar. Pollut. Bull., 11: 75-80.
FARBER, E. (1984a) Perspectives in cancer research. The multistep
nature of cancer development. Cancer Res., 44: 4217-4223.
FARBER, E. (1984b) Cellular biochemistry of the step-wise
development of cancer with chemicals. G.H. Clowes Memorial Lecture.
Cancer Res., 44: 5463-5474.
FARBER, E. (1986) Some emerging general principles in the
pathogenesis of hepatocellular carcinoma. Cancer Surv., 5: 695-718.
FDA (1970) Status report on the chemistry and toxicology of
polychlorinated biphenyls (PCBs) or Aroclors as of 1 June 1970,
Washington, DC, Food and Drug Administration, 16 pp.
FDA (1979) Polychlorinated biphenyls (PCBs): Reduction of
tolerances, Fed. Reg., 44(127): 126-136.
FEIN, G.G. (1984) Intrauterine exposure of humans to PCBs: newborn
effects, Duluth, Minnesota, US Environmental Protection Agency
(EPA 600/53-84-060).
FEIN, G.G., JACOBSON, J.L., JACOBSON, S.W., SCHWARTZ, P.M., &
DOWLER, J.K. (1984) Prenatal exposure to polychlorinated biphenyls:
Effects on birth size and gestational age. J. Pediatr.,
105(2): 315-320.
FELT, G.R., MUELLER, W.F., IATROPOULOS, M.J., COULSTON, F., &
KORTE, F. (1977) Chronic toxicity of 2,5,4'-trichlorobiphenyl in
young rhesus monkeys. I. Body distribution, elimination and
metabolism. Toxicol. appl. Pharmacol., 41(3): 619-627.
FELT, G.R., MUELLER, W.F., COULSTON, F., & KORTE, F. (1979)
Distribution and excretion of 2,2',4,4',6-pentachlorobiphenyl in
the rat. Bull. environ. Contam. Toxicol., 22: 582-585.
FINGERMAN, S.W. (1980) Differences in the effects of fuel oil, an
oil dispersant, and three polychlorinated biphenyls on fin
regeneration in the Gulf Coast kill fish, Fundulus grandis. Bull.
environ. Contam. Toxicol., 25: 234-240.
FINGERMAN, S.W. & FINGERMAN, M. (1977) Effects of a polychlorinated
biphenyl and a polychlorinated dibenzofuran on molting of the
fiddler crab, Uca pugilator. Bull. environ. Contam. Toxicol.,
18: 138-142.
FINGERMAN, S.W. & FINGERMAN, M. (1979) Comparison of the effects of
fourteen-day and chronic exposures to a polychlorinated biphenyl,
Aroclor 1242, on molting of the fiddler crab, Uca pugilator.
Bull. environ. Contam. Toxicol., 21: 352-357.
FINGERMAN, S.W. & RUSSELL, L.C. (1980) Effects of the
polychlorinated biphenyl Aroclor 1242 on locomotor activity and on
the neurotransmitters dopamine and norepinephrine in the brain of
the gulf killifish, Fundulus grandis. Bull. environ. Contam.
Toxicol., 25: 682-687.
FINKLEA, J., PRIESTER, L.E., CREASON, J.P., HAUSER, T., HINNERS,
T., & HAMMER, D.I. (1972) Polychlorinated biphenyl residues in
human plasma expose a major urban pollution problem. Am. J. public
Health, 62(5): 645-651.
FIORE, B.J., ANDERSON, H.A., HANRAHAN, L.P., OLSON, L.J., &
SONZOGNI, W.C. (1989) Sport fish consumption and body burden levels
of chlorinated hydrocarbons: A study of Wisconsin anglers. Arch.
environ. Health, 44(2): 82-88.
FISCHBEIN, A. (1985) Liver function tests in workers with
occupational exposure to polychlorinated biphenyls (PCBs):
Comparison with Yusho and Yu-Cheng. Environ. health Perspect.,
60: 145-150.
FISCHBEIN, A. & WOLFF, M.S. (1987) Conjugal exposure to
polychlorinated biphenyls (PCBs). Br. J. ind. Med., 44: 284-286.
FISCHBEIN, A., WOLFF, M.S., LILIS, R., THORNTON, J., & SELIKOFF,
I.J. (1979) Clinical findings among PCB-exposed workers in a
capacitor manufacturing facility. Ann. NY Acad. Sci., 320: 703-715.
FISCHBEIN, A., WOLFF, M.S., BERNSTEIN, J., SELIKOFF, I.J., &
THORNTON, J. (1982) Dermatological findings in capacitor
manufacturing workers exposed to dielectric fluids containing
polychlorinated biphenyls (PCBs). Arch. environ. Health,
37(2): 69-74.
FISCHBEIN, A., RIZZO, J.N., SOLOMON, S.J., & WOLFF, M.S. (1985)
Oculodermatological findings in workers with occupational exposure
to polychlorinated biphenyls (PCBs). Br. J. ind. Med., 42: 426-430.
FISHBEIN, L. (1974) Toxicity of chlorinated biphenyls. Annu. Rev.
Pharmacol., 14: 139-156.
FISHER, N.S. (1975) Chlorinated hydrocarbon pollutants and
photosynthesis of marine phytoplankton: A reassessment. Science,
189: 463-464.
FISHER, N.S. & WURSTER, C.F. (1973) Individual and combined effects
of temperature and polychlorinated biphenyls on the growth of three
species of phytoplankton. Environ. Pollut., 5: 205-212.
FISHER, N.S., GRAHAM, L.B., & CARPENTER, E.J. (1973) Geographic
differences in phytoplankton sensitivity to PCBs. Nature (Lond.),
241: 548-549.
FISHER, N.S., REMSEN, C.C., WURSTER, C.R., & CARPENTER, E.J. (1974)
Effects of PCB on interspecific competition in natural and
gnotobiotic phytoplankton communities in continuous and batch
cultures. Microbiol. Ecol., 1: 39-50.
FISHER, N.S., GUILLARD, R.R., & WURSTER, C.R. (1976) Effects of a
chlorinated hydrocarbon pollutant on the growth kinetics of a
marine diatom. In: Modeling biochemical processes in aquatic
ecosystems, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 305-317.
FISHER, J.B., PETTY, R.L., & LUCK, W. (1983) Release of
polychlorinated biphenyls from contaminated lake sediments: Flux
and apparent diffusivities of four individual PCBs. Environ.
Pollut., B5: 121-132.
FITZGERALD, E.F., WEINSTEIN, A.L., YOUNGBLOOD, L.G., STANDFAST,
S.J., & MELIUS, J.M. (1989) Health effects three years after
potential exposure to the toxic contaminants of an electrical
transformer fire. Arch. environ. Health, 44(4): 214-221.
FLICK, D.F., O'DELL, R.G., & CHILDS, V.A. (1965) Studies of the
chick oedema disease. 3. Similarity of symptoms produced by feeding
chlorinated biphenyl. Poult. Sci., 44: 1460-1465.
FOCARDI, S. & ROMEI, R. (1987) Fingerprint of polychlorinated
biphenyl congeners in samples of human subcutaneous adipose tissue.
Chemosphere, 16(10/12): 2315-2320.
FOCARDI, S., FOSSI, C., LEONZIO, C., & ROMEI, R. (1986) PCB
congeners, hexachlorobenzene, and organochlorine insecticides in
human fat in Italy. Bull. environ. Contam. Toxicol., 36: 644-650.
FOCARDI, S., LEONZIO, C., & FOSSI, C. (1988) Variations in
polychlorinated biphenyl congener composition in eggs of
Mediterranean water birds in relation to their position in the food
chain. Environ. Pollut., 52: 243-255.
FOLMAR, L.C., DICKHOFF, W.W., ZAUGG, W.S., & HODGKINS, H.O. (1982)
The effects of Aroclor 1254 and No. 2 fuel oil on smoltification
and sea-water adaptation of Coho salmon (Oncorhynchus kisutch).
Aquat. Toxicol., 2: 291-299.
FOOKEN, C. & BUTTE, W. (1987) Organochlorine pesticides and
polychlorinated biphenyls in human milk during lactation.
Chemosphere, 16(6): 1301-1309.
FOREMAN, W.T. & BIDLEMAN, T.F. (1985) Vapor pressure estimates of
individual polychlorinated biphenyls and commercial fluids using
gas chromatographic retention data. J. Chromatogr., 330: 203-216.
FORGUE, S.T., PRESTON, B.D., HARGRAVES, W.A., REICH, I.L. & ALLEN,
J.R. (1980) Direct evidence that an arene oxide is a metabolic
intermediate of 2,2',5,5'-tetrachlorobiphenyl. In: Abstracts of the
Nineteenth Annual Meeting of the Society of Toxicology (Abstract
No. 383).
FOWLER, S.W., POLIKARPOV, G.G., ELDER, D.L., PARSI, P., &
VILLENEUVE, J.P. (1978) Polychlorinated biphenyls: Accumulation
from contaminated sediments and water by the polychaete Nereis
diversicolor. Mar. Biol., 48: 303-309.
FRANK, R., RONALD, K., & BRAUN, H.E. (1973) Organochlorine residues
in harp seals (Pagophilus groenlandicus) caught in Eastern
Canadian waters. J. Fish Res. Board Can., 30: 1053-1063.
FRANK, R., HOLDRINET, M.V.H., & RAPLEY, W.A. (1975) Residue of
organochlorine compounds and mercury in birds' eggs from the
Niagara Peninsula, Ontario. Arch. environ. Contam. Toxicol.,
3: 205-218.
FRANK, R., HOLDRINET, M., BRAUN, H.E., DODGE, D.P., & SPRANGLER,
G.E. (1978) Residues of organochlorine insecticides and
polychlorinated biphenyls in fish from Lakes Huron and Superior,
Canada, 1968-76. Pestic. monit. J., 12: 60-68.
FRANK, R., RASPER, J., SMOUT, M.S., & BRAUN, H.E. (1988)
Organochlorine residues in adipose tissues, blood and milk from
Ontario residents, 1976-1985. Can. J. public Health, 79: 150-158.
FRANKLIN, A. (1987) The concentration of metals, organochlorine
pesticide and PCB residues in marine fish and shellfish: Results
from MAFF fish and shellfish monitoring programmes, 1977-1984,
London, Ministry of Agriculture, Fisheries and Food, Directorate of
Fisheries Research (Aquatic Environment Monitoring Report No. 16).
FREEMAN, H.C., SANGALANG, G., & FLEMMING, B. (1982) The sublethal
effects of a polychlorinated biphenyl (Aroclor 1254) diet on the
Atlantic cod (Gadus morhua). Sci. total Environ., 24: 1-11.
FRENCH, J.E. (1976) The effect of the chlorinated polycyclic
hydrocarbons, p,p'-DDT and polychlorinated biphenyls (PCBs) on the
flagellated protozoan, Crithidia fasciculata. Diss. Abstr. Int.
Sci. Eng., B37: 96-97.
FREUDENTHAL, J. & GREVE, P.A. (1973) Polychlorinated terphenyls in
the environment. Bull. environ. Contam. Toxicol., 10: 108-111.
FRIEND M. & TRAINER, D.O. (1970) Polychlorinated biphenyl:
interaction with duck hepatitis virus. Science, 170: 1314-1316.
FRIES, G.F. (1972) Degradation of chlorinated hydrocarbons under
anaerobic conditions. Adv. Chem. Ser., 111: 256-270.
FRIES, G.F. & MARROW, G.S. (1981) Chlorobiphenyl movement from soil
to soybean plants. J. agric. food Chem., 29: 757-759.
FRIES, G.F., MARROW, G.S., Jr, & GORDON, C.H. (1973) Long-term
studies of residue retention and excretion by cows fed a
polychlorinated biphenyl (Aroclor 1254). J. agric. food Chem.,
21: 117-121.
FU, Y.A. (1984) Ocular manifestation of polychlorinated biphenyls
intoxication. Am. J. ind. Med., 5: 127-132.
FUJIWARA, K. (1975) Environmental and food contamination with PCB's
in Japan. Sci. total Environ., 4: 219-247.
FUJIWARA, M. & KURIYAMA, K. (1977) Effect of PCB (polychloro-
biphenyls) on L-ascorbic acid, pyridoxal phosphate and riboflavin
contents in various organs and on hepatic metabolism of L-ascorbic
acid in the rat. Jpn. J. Pharmacol., 27: 621-627.
FUKADA, K., INUYAMA, Y., TAKESHITA, T., & YAMAMOTO, S. (1973)
[Present state of environmental pollution by PCB in the Shimane
Prefecture.] Shimare Igaku, 5: 1-25 (in Japanese).
FUKANO, S. & DOGUCHI, M. (1977) PCT, PCB and pesticide residues in
human fat and blood. Bull. environ. Contam. Toxicol.,
17(5): 613-617.
FUKANO, S., USHIO, F., & DOGUCHI, M. (1974) [PCB, PCT and pesticide
residues in fish collected from the Tama river.] Annu. Rep. Tokyo
Metrop. Res. Lab. P.H., 25: 297-305 (in Japanese).
FUKUSHIMA, M. & KAWAI, S. (1981) Variation of organochlorine
concentration and burden in striped dolphin (Stenella
coeruleoalba) with growth. In: Fujiyama, T., ed. Studies on the
levels of organochlorine compounds and heavy metals in the marine
organisms, Ryukyus, University of Ryukyus, pp. 97-114.
FULLER, G.B., KNAUF, V., MUELLER, W., & HOBSON, W.C. (1980) PCB
augments LH induced progesteron synthesis. Bull. environ. Contam.
Toxicol., 25: 65-68.
FUNATSU, I., YAMASHITA, F., ITO, Y., TZUGAWA, S., FUNATSU, T.,
YOSHIKANE, T., HAYASHI, M., KATO, T., YAKUSHIJI, M., OKAMOTO, G.,
YAMASAKI, S., ARIMA, T., KUNO, T., IDE, H., & IBE, I. (1972) PCB
induced fetopathy. I. Clinical observation. Kurume med. J.,
919: 43-51.
FURUKAWA, K., TONOMURA, K., & KAMIBAYASHI, A. (1978a) Effect of
chlorine substitution on the biodegradability of polychlorinated
biphenyls. Appl. environ. Microbiol., 35: 223-227.
FURUKAWA, K., TOMIZUKA, N., & KAMIBAYASHI, A. (1978b) Effect of
chlorine substitution on the bacterial metabolism of various
polychlorinated biphenyls. Appl. environ. Microbiol., 38: 301-310.
GAGE, J.C. & HOLM, S. (1976) The influence of molecular structure
on the retention and excretion of polychlorinated biphenyls by the
mouse. Toxicol. appl. Pharmacol., 36: 555-560.
GALLENBERG, L.A. & VODICNIK, M.J. (1987) Potential mechanisms for
redistribution of polychlorinated biphenyls during pregnancy and
lactation. Xenobiotica, 17(3): 299-310.
GALLENBERG, L.A., RING, B.J., & VODICNIK, M.J. (1987) Influence of
lipolysis on the mobilization of 2,4,5,2',4',5'-hexachlorobiphenyl
from adipocytes in vitro. J. Toxicol. environ. Health,
20: 163-171.
GARDNER, A.M., RICHTER, H.F., & ROACH, J.A.G. (1976) Excretion of
hydroxylated polychlorinated biphenyl metabolites in cow's milk. J.
Assoc. Off. Anal. Chem., 59: 273-277.
GARTHOFF, L.H., FRIEDMAN, L., FARBER, T.M., LOCKE, K.K., SOBOTKA,
T.J., GREEN, S., HURLEY, N.E., PETERS, E.L., STORY, G.E., MORELAND,
F.M., GRAHAM, C.H., KEYS, J.E., TAYLOR, M.J., SCALERA, J.V.,
ROTHLEIN, J.E., MARKS, E.M., CERRA, F.E., RODI, S.B., & SPORN, E.M.
(1977) Biochemical and cytogenetic effects in rats caused by
short-term ingestion of Aroclor 1254 or Firemaster BP6. J. Toxicol.
environ. Health, 3: 769-796.
GARTHOFF, L.H., CERRA, F.E., & MARKS, E.M. (1981) Blood chemistry
alterations in rats after single and multiple gavage administration
of polychlorinated biphenyl. Toxicol. appl. Pharmacol., 60: 33-44.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985) Pesticides, selected elements and other chemicals in adult
total diet samples, October 1979-September 1980. J. Assoc. Off.
Anal. Chem., 68(6): 1184-1195.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements and other chemicals in adult
total diet samples, October 1980-March 1982. J. Assoc. Off. Anal.
Chem., 69(1): 146-161.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements and other chemicals in infant
and toddler total diet samples, October 1980-March 1982. J. Assoc.
Off. Anal. Chem., 69(1): 123-145.
GASKIN, D.E., FRANK, R., & HOLDRINET, M. (1983) Polychlorinated
biphenyls in harbor porpoises Phocoena phocoena (L.) from the Bay
of Fundy, Canada and adjacent waters, with some information on
chlordane and hexachlorobenzene levels. Arch. environ. Contam.
Toxicol., 12: 211-219.
GELLERT, R.J. (1978) Uterotropic activity of polychlorinated
biphenyls (PCB) and induction of precocious reproductive aging in
neonatally treated female rats. Environ. Res., 16: 123-130.
GELLERT, R.J. & WILSON, C. (1979) Reproductive function in rats
exposed prenatally to pesticides and polychlorinated biphenyls
(PCBs). Environ. Res., 18: 437-443.
GEZONDHEIDSRAAD (1985) [First recommendation on the quality of
breastmilk. Contamination of breastmilk with polychlorinated
biphenyls (PCBs). Recommendation to the Minister and the Secretary
of State for Welfare, Public Health and Culture], Rijswijk, Public
Health Council of the Netherlands (in Dutch).
GIAM, C.S., CHAN, H.S., NEFF, G.S., & ATLAS, E.L. (1978) Phthalate
ester plasticizers: A new class of marine pollutant. Science,
199: 419-420.
GIAM, C.S., ATLAS, E., CHAN, H.S., & NEFF, G. (1980) Phthalate
esters, PCB and DDT residues in the Gulf of Mexico atmosphere.
Atmos. Environ., 14: 65-69.
GIBSON, D.T., ROBERTS, R.L., WELLS, M.C., & KOBAL, V.M. (1973)
Oxidation of biphenyl by a Beijerinckia species. Biochem.
biophys. Res. Commun., 50: 211-219.
GILBERTSON, M. & FOX, G.A. (1977) Pollutant-associated embryonic
mortality of Great Lakes herring gulls. Environ. Pollut.,
12: 211-216.
GILBERTSON, M. & HALE, R. (1974) Characteristics of the breeding
failure of a colony of herring gulls in Lake Ontario. Can.
Field-Nat., 88: 356-358.
GILBERTSON, M., MORRIS, R.D., & HUNTER, R.A. (1976) Abnormal chicks
and PCB residue levels in eggs of colonial birds on the lower Great
Lakes (1971-73). Auk, 93: 434-442.
GLADEN, B.C., ROGAN, W.J., RAGAN, N.B., & SPIERTO, F.W. (1988a)
Urinary porphyrins in children exposed transplacentally to
polyhalogenated aromatics in Taiwan. Arch. environ. Health,
43(1): 54-58.
GLADEN, B.C., ROGAN, W.J., HARDY, P., THULLEN, J., TINGELSTAD, J.,
& TULLY, M. (1988b) Development after exposure to polychlorinated
biphenyls and dichlorodiphenyl dichloroethane transplacentally and
through human milk. J. Pediatr., 113(6): 991-995.
GLOOSCHENKO, V. & GLOOSCHENKO, W. (1975) Effect of polychlorinated
biphenyl compounds on growth of great lakes phytoplankton. Can. J.
Bot., 53: 653-659.
GLOOSHENKO, W.A., STRACHAN, W.M., & SAMPSON, R.C.J. (1976) Residues
in water distribution of pesticides and polychlorinated biphenyls
in water, sediments and session of the Upper Great Lakes. Pestic.
monit. J., 10: 61-67.
GOLDSTEIN, J.A. (1980) Structure-activity relationships for the
biochemical effects and the relationships to toxicity. In:
Kimbrough, R.D., ed. Halogenated biphenyls, terphenyls,
naphthalenes, dibenzodioxins and related products, Amsterdam,
Elsevier/North-Holland Biomedical Press, pp. 151-190 (Topics in
Environmental Health).
GOLDSTEIN, J.A., HICKMAN, P., & JUE, D.L. (1974) Experimental
hepatic porphyria induced by polychlorinated biphenyls. Toxicol.
appl. Pharmacol., 27: 437-448.
GOLDSTEIN, J.A., HICKMAN, P., BURSE, V.W., & BERGMAN, H. (1975) A
comparative study of two polychlorinated biphenyl mixtures
(Aroclors 1242 and 1016) containing 42% chlorine on induction of
hepatic porphyria and drug metabolizing enzymes. Toxicol. appl.
Pharmacol., 32: 461-473.
GOLDSTEIN, J.A., HICKMAN, P., BERGMAN, H., MCKINNEY, J.D., &
WALKER, M.P. (1977) Separation of pure polychlorinated biphenyl
isomers into two types of inducers on the basis of induction of
cytochrome P-450 or P-448. Chem.-biol. Interact., 17: 69-87.
GORCHEV, H.G. & JELINEK, C.F. (1985) A review of the dietary
intakes of chemical contaminants. Bull. World Health Organ.,
63(5): 945-962.
GOTO, M. & HIGUCHI, K. (1969) The symptomatology of Yusho
(chlorobiphenyls poisoning), in dermatology. Fuoka Act. Med.,
60: 409-431.
GOTO, M., SUGIURA, K., HATTORI, M., MIYAGAWA, T., & OKAMURA, M.
(1973) Hydroxylation of dichlorobiphenyls in rats. In: New
collection of papers presented at the Research Conference on New
Methodology in Ecological Chemistry, Susono, Japan, 23-25 November,
Tokyo, International Academic Printing Co. Ltd, pp. 299-302.
GOTO, M., SUGIURA, K., HATTORI, M., MIYAGAWA, T., & OKAMURA, M.
(1974) Metabolism of 2,3-dichlorobiphenyl-14C and
2,3,6-trichlorobiphenyl-14C in the rat. Chemosphere, 3: 227-232.
GOTO, M., HATTORI, M., & SUGIURA, K. (1975) Metabolism of
pentachloro- and hexachlorobiphenyls in the rat. Chemosphere,
4: 177-180.
GRANT, D.L. & PHILLIPS, W.E.J. (1974) The effect of age and sex on
the toxicity of Aroclor 1254, a polychlorinated biphenyl, in the
rat. Bull. environ. Contam. Toxicol., 12(2): 145-152.
GRANT, D.L., PHILLIPS, W.E.J., & VILLENEUVE, D.C. (1971a)
Metabolism of polychlorinated biphenyl (Aroclor 1254) mixture in
the rat. Bull. environ. Contam. Toxicol., 6: 102-112.
GRANT, D.L., VILLENEUVE, D.C., MCCULLY, K.A., & PHILLIPS, W.E.J.
(1971b) Placental transfer of polychlorinated biphenyls in the
rabbit. Environ. Physiol., 1: 61-66.
GRANT, D.L., MOODIE, C.A., & PHILLIPS, W.E.J. (1974) Toxicodynamics
of Aroclor 1254 in the male rat. Environ. Physiol. Biochem.,
4: 214-225.
GREB, W., KLEIN, W., COULSTON, F., GOLBERG, L., & KORTE, F. (1975)
Metabolism of lower polychlorinated biphenyls-C-14 in the Rhesus
monkey. Bull. environ. Contam. Toxicol., 13: 471-76.
GREEN, S., CARR, J.V., PALMER, K.A., & OSWALD, E.J. (1975a) Lack of
cytogenetic effects in bone marrow and spermatogonial cells in rats
treated with polychlorinated biphenyls (Aroclors 1242 and 1254).
Bull. environ. Contam. Toxicol., 13: 14-22.
GREEN, S., SAURO, F.M., & FRIEDMAN, L. (1975b) Lack of dominant
lethality in rats treated with polychlorinated biphenyls (Aroclors
1242 and 1254). Food Cosmet. Toxicol., 13: 507-510.
GREICHUS, Y.A., CALL, D.J., & AMMANN, B.M. (1975) Physiological
effects of polychlorinated biphenyls or a combination of DDT, DDD,
and DDE in penned white Pelicans. Arch. environ. Contam. Toxicol.,
3: 330-343.
GREIG, R.A. & SENNEFELDER, G. (1987) PCB concentration in winter
flounder from Long Island Sound, 1984-1986. Bull. environ. Contam.
Toxicol., 39(5): 863-868.
GREVE, P.A. & VAN HULST, S.J. (1977) [Organochlorine pesticides and
PCBs in total diets], Bilthoven, The Netherlands, National
Institute of Public Health (Report No. 192/77 Tox-Rob) (in Dutch).
GREVE, P.A. & VAN HARTEN, D.C. (1983a) [Organochlorine pesticides
and polychlorobiphenyls in the fatty tissue of the Dutch population
(period 1980).] Bilthoven, The Netherlands, National Institute of
Public Health (Report No. 638205001) (in Dutch).
GREVE, P.A. & VAN HARTEN, D.C. (1983b) [Association between
organochlorine pesticide and PCB contents in fat and blood in man],
Bilthoven, The Netherlands, National Institute of Public Health
(Report No. 638219001) (in Dutch).
GREVE, P.A. & WEGMAN, R.C.C. (1983) PCB residues in animal fats,
human tissues, duplicate 24-hours' diets, eel and sediments. In:
Barros, M.C., Könemann, H., & Visser, R., ed. Proceedings of the
PCB Seminar, The Hague, 28-30 September 1983, The Hague, Ministry
of the Environment, pp. 54-66.
GREVE, P.A. & WEGMAN, R.C.C. (1984) Organochlorine compounds in
human milk: data from a recent investigation in the Netherlands.
In: Report on the WHO Consultation on Organochlorine Compounds in
Human Milk and Related Hazards, Bilthoven, 9-11 January 1985,
Copenhagen World Health Organization, Regional Office for Europe,
Annex 8.
GREVE, P.A., VAN HARTEN, D.C., HEUSINKVELD, H.A.G., LEUSSINK, A.B.,
& VERSCHRAAGEN, C. (1985) [Chemical contaminants in breastmilk.
Section 2: PCBs (determination of total)], Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Hygiene (Report No. 638307002) (in Dutch).
GROLIER, P., CASSAND, P., ANTIGNAC, E., NARBONNE, J.F., ALBRECHT,
R., AZAIS, V., ROBERTSON, L.W., & OESCH, F. (1989) Effects of
prototypic PCBs on benzo(a)pyrene mutagenic activity related to
vitamin A intake. Mutat. Res., 211: 139-145.
GROTE, W., SCHMOLDT, A., & BENTHE, H.F. (1975) Hepatic porphyrin
synthesis in rat after pretreatment with polychlorinated biphenyls.
Acta. pharmacol. toxicol., 36: 215-224.
GRUGER, E.H., KARRICK, N.L., DAVIDSON, A.I., & HRUBY, T. (1975)
Accumulation of 3,4,3',4'-tetrachlorobiphenyl and 2,4,5,2',4',5'-,
and 2,4,6,2',4',6'-hexachlorobiphenyl in juvenile coho salmon.
Environ. Sci. Technol., 9: 121-127.
GRUGER, E.H., HRUBY, T., & KARRICK, N.L. (1976) Sublethal effects
of structurally related tetrachloro-, pentachloro-, and
hexachlorobiphenyl on juvenile Coho Salmon. Environ. Sci. Technol.,
10: 1033-1037.
GUINEY, P.D. & PETERSON, R.E. (1980) Distribution and elimination
of a polychlorinated biphenyl after dietary exposure in yellow
perch and rainbow trout. Arch. environ. Contam. Toxicol.,
9: 667-674.
GUINEY, P.D., PETERSON, R.E., MELANCON, M.J., & LECH, J.J. (1977)
The distribution and elimination of 2,5,2,5-[14C]Tetrachloro-
biphenyl in Rainbow Trout (Salmo gairdneri). Toxicol. appl.
Pharmacol., 39: 329-338.
GUINEY, P.D., MELANCON, M.J., LECH, J.J., & PETERSON, R.E. (1979)
Effects of egg and sperm maturation and spawning on the
distribution and elimination of a polychlorinated biphenyl in
rainbow Trout (Salmo gairdneri). Toxicol. appl. Pharmacol.,
47: 261-272.
GUNDERSON, E.L. (1988b) FDA total diet study, April 1982-April
1984, dietary intakes of pesticides, selected elements and other
chemicals. J. Assoc. Off. Anal. Chem., 71(6): 1200-1209.
GUO, Y.L., EMMETT, E.A., PELLIZZARI, E.D., & ROHDE, C.A. (1987)
Influence of serum cholesterol and albumine on partitioning of PCB
congeners between human serum and adipose tissue. Toxicol. appl.
Pharmacol., 87: 48-56.
GUOTH, J., KACHMAR, P., TELEHA, M., & VASIL, M. (1984) [The
influence of polychlorinated biphenyls (PCB) on the activity of
liver aniline hydroxylase and on some metabolic parameters in pig
blood.] Vet. Med. (Prague), 29: 29-38 (in Czech).
GUSTAVSSON, P., HOGSTEDT, C., & RAPPE, C. (1986) Short-term
mortality and cancer incidence in capacitor manufacturing workers
exposed to polychlorinated biphenyls (PCBs). Am. J. ind. Med.,
10: 341-344.
GYORKOS, J., DENOMME, M.A., LEECE, B., HOMONKO, K., VALLI, V.E., &
SAFE, S. (1985) Reconstituted halogenated hydrocarbon pesticide and
pollutant mixtures found in human tissues: effects on the immature
male Wistar rat after short-term exposure. Can. J. Physiol.
Pharmacol., 63(1): 36-43.
HAAHTI, H. & PERTTILA, M. (1988) Levels and trends of
organochlorines in Cod and Herring in the Northern Baltic. Mar.
Pollut. Bull., 19: 29-32.
HAAKE, J.M., SAFE, S., MAYURA, K., & PHILLIPS, T.D. (1987) Aroclor
1254 as an antagonist of the teratogenicity of 2,3,7,8-tetrachloro-
dibenzo- p-dioxin. Toxicol. Lett., 38: 299-306.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
Coturnix. Bull. environ. Contam. Toxicol., 11: 98-101.
HALTER, M.T. & JOHNSON, H.E. (1974) Acute toxicities of a
polychlorinated biphenyl (PCB) and DDT alone and in combination to
early life stages of Coho salmon (Oncorhynchus kisutch). J. Fish
Res. Board Can., 31: 1543-1547.
HALVERSON, M.R., PHILLIPS, T.D., SAFE, S.H., & ROBERTSON, L.W.
(1985) Metabolism of aflatoxin B1 by rat hepatic microsomes induced
by polyhalogenated biphenyl congeners. Appl. environ. Microbiol.,
49: 882-886.
HAMMOND, A.L. (1972) Chemical pollution: Polychlorinated biphenyls.
Science, 175: 155-156.
HANSEN, D.J., PARRISH, P.R., LOWE, J.I., & WILSON, A.J. (1971)
Chronic toxicity, uptake, and retention of Aroclor 1254 in two
estuarine fishes. Bull. environ. Contam. Toxicol., 6: 113-119.
HANSEN, D.J. (1974) Aroclor 1254: Effect on composition of
developing estuarine animal communities in the laboratory. Contrib.
mar. Sci., 18: 19-33.
HANSEN, D.J., SCHIMMEL, S.C., & MATTHEWS, E. (1974a) Avoidance of
Aroclor 1254 by shrimp and fishes. Bull. environ. Contam. Toxicol.,
12: 253-256.
HANSEN, D.J., PARRISH, P.R., & FORESTER, J. (1974b) Aroclor 1016:
Toxicity to and uptake by estuarine animals. Environ. Res.,
7: 363-373.
HANSEN, D.J., SCHIMMEL, S.C., & FORESTER, J. (1975) Effects of
Aroclor 1016 on embryos, fry, juveniles, and adults of sheepshead
minnow (Cyprinodon variegatus). Trans. Am. Fish Soc.,
104: 584-588.
HANSEN, L.G., WIEKHORST, W.B., & SIMON, J. (1976a) Effects of
dietary Aroclor 1242 on channel catfish (Ictalurus punctatus) and
the selective accumulation of PCB components. J. Fish Res. Board
Can., 33: 1343-1352.
HANSEN, L.G., WILSON, D.W., & BYERLY, C.S. (1976b) Effects on
growing swine and sheep of two polychlorinated biphenyls. Am. J.
vet. Res., 37: 1021-1024.
HANSEN, L.G., WASHKO, P.W., TUINSTRA, L.G.M.TH., DORN, S.B., &
HINESLY, T.D. (1981) Polychlorinated biphenyl, pesticide, and heavy
metal residues in swine foraging on sewage sludge amended soils. J.
agric. food Chem., 29: 1012-1017.
HAQUE, R. & SCHMEDDING, D.W. (1975) A method of measuring the water
solubility of hydrophobic chemicals: Solubility of five
polychlorinated biphenyls. Bull. environ. Contam. Toxicol.,
14: 13-18.
HAQUE, R., SCHMEDDING, D.W., & FREED, V.H. (1974) Aqueous
solubility adsorption and vapor behaviour of polychlorinated
biphenyl Aroclor 1254. Environ. Sci. Technol., 8: 139-142.
HARA, I. (1985) Health status and PCBs in blood of workers exposed
to PCBs and their children. Environ. health Perspect., 59: 85-90.
HARA, I., HARADA, H., KIMURA, S., ENDO, T., & KAWANO, K. (1974)
[Follow-up health examination in an electric condenser factory
after cessation of PCBs usage (1st report).] Jpn. J. ind. Health,
16: 365-366 (in Japanese).
HARBISON, R.D., JAMES, R.C., & ROBERTS, S.M. (1987) Biological data
relevant to the evaluation of carcinogenic risk to humans, Little
Rock, Arkansas, University of Arkansas, Division of
Interdisciplinary Toxicology, (Prepared for Scientific Advisory
Panel, Safe Drinking Water and Toxic Enforcement Act, State of
California).
HARDING, L.W. & PHILLIPS, J.H. (1978a) Polychlorinated biphenyl
(PCB) effects on marine phytoplankton photosynthesis and cell
division. Mar. Biol., 49: 93-101.
HARDING, L.W. & PHILLIPS, J.H. (1978b) Polychlorinated biphenyl
(PCB) uptake by marine phytoplankton. Mar. Biol., 49: 103-111.
HARDWICK, J.P., LINKO, P., & GOLDSTEIN, J.A. (1985) Dose response
for induction of two cytochrome P-450 isoenzymes and their mRNAs by
3,4,5,3',4',5'-hexachlorobiphenyl indicating coordinate regulation
in rat liver. Mol. Pharmacol., 27: 676-682.
HARRIS, M.P. & OSBORN, D. (1981) Effect of a polychlorinated
biphenyl on the survival and breeding of puffins. J. appl. Ecol.,
18: 471-479.
HARRIS, J.R. & ROSE, L. (1972) Toxicity of polychlorinated
biphenyls in poultry. J. Am. Vet. Med. Assoc., 161: 1584-1586.
HARVEY, G.R. & STEINHAUER, W.G. (1974) Atmospheric transport of
polychlorobiphenyls to the North Atlantic. Atmos. Environ.,
8: 777-782.
HARVEY, G.R., STEINHAUER, W.G., & TEAL, J.M. (1973)
Polychlorobiphenyls in North Atlantic Ocean water. Science,
180: 643-644.
HASEGAWA, M. (1973) [Studies on the wild animals as
"Contamination-index". Part 1 - The effects of PCB on frog tadpoles
(Rana chensinensis).] Hokkaidoritsu Eisei Kenkyushoho, 23: 6-9
(in Japanese).
HASEGAWA, H., SATO, M., & TSURUTA, H. (1972a) [PCB concentration in
the blood of workers handling PCB.] Occup. Health, 13(10): 50-55
(in Japanese).
HASEGAWA, H., SATO, M., & TSURUTA, H. (1972b) [PCB concentration in
air of PCB-using plants and health examination of workers.] In:
[Report on special research on prevention of environmental
pollution by PCB-like substances], Tokyo, Science and Technology
Agency, Research Co-ordination Bureau, pp. 141-149 (in Japanese).
HASELTINE, S.D. & PROUTY, R.M. (1980) Aroclor 1242 and reproductive
success of adult mallards (Anas platyrhynchos). Environ. Res.,
23: 29-34.
HASELTINE, S.D., HEINZ, G.H., REICHEL, W.L., & MOORE, J.F. (1981)
Organochlorine and metal residues in eggs of wildfowl nesting on
islands in Lake Michigan off Door county, Wisconsin, 1977-78.
Pestic. monit. J., 15: 90-97.
HASHIMOTO, K., AKASAKA, S., TAKAGI, Y., KATAOKA, M., OTAKA, T.,
MURATA, Y., ABURADA, S., KITAURA, T., & UDA, H. (1976) Distribution
and excretion of (14C)polychlorinated biphenyls after their
prolonged administration to male rats. Toxicol. appl. Pharmacol.,
37: 415-423.
HASSELL, K.D. & HOLMES, D.C. (1977) Polychlorinated terphenyls
(PCT) in some British birds. Bull. environ. Contam. Toxicol.,
17: 618-621.
HATCH, W.I. & ALLEN, D.W. (1979) Alterations in calcium
accumulation behavior in response to calcium availability and
polychlorinated biphenyl administration. Bull. environ. Contam.
Toxicol., 22: 172-174.
HATTULA, M.L. (1985) Mutagenicity of PCBs and their pyrosynthetic
derivatives in cell-mediated assay. Environ. health Perspect.,
60: 255-257.
HATTULA, M.L. & KARLOG, O. (1973) Absorption and elimination of
polychlorinated biphenyls (PCB) in goldfish. Acta pharmacol.
toxicol., 32: 237-245.
HAWES, M.L., KRICHER, J.C., & UREY, J.C. (1976a) The effects of
various Aroclor fractions on the population growth of Chlorella
pyrenoidosa. Bull. environ. Contam. Toxicol., 15: 14-18.
HAWES, M.L., KRICHER, J.C., & UREY, J.C. (1976b) The effects of
various Aroclor fractions on the productivity of Chlorella
pyrenoidosa. Bull. environ. Contam. Toxicol., 15: 588-590.
HAWKER, D.W. (1989) Vapour pressures and Henry's Law Constants of
polychlorinated biphenyls. Environ. Sci. Technol., 23: 1250-1253.
HAWKER, D.W. & CONNELL, D.W. (1988) Octanol-water partition
coefficients of polychlorinated biphenyl congeners. Environ. Sci.
Technol., 22: 382-387.
HAYES, M.A., SAFE, S.H., ARMSTRONG, D., & CAMERON, R.G. (1985)
Influence of cell proliferation on initiating activity of pure
polychlorinated biphenyls and complex mixtures in resistant
hepatocyte in vivo assays for carcinogenicity. J. Natl Cancer
Inst., 74: 1037-1041.
HAYES, M.A., ROBERTS, E., SAFE, S.H., FARBER, E., & CAMERON, R.G.
(1986) Influences of different polychlorinated biphenyls on
cytocidal, mitiinhibitory and nodule-selecting activities of
N-2-fluorenylacetamide in rat liver. J. Natl Cancer Inst.,
76: 683-691.
HAYES, M.A. (1987) Carcinogenic and mutagenic effects of PCBs. In:
Safe, S., ed. Polychlorinated biphenyls (PCBs): Mammalian and
environmental toxicology, Berlin, Heidelberg, New York,
Springer-Verlag, pp. 77-95 (Environmental Toxin Series, Vol. I).
HAYS, H. & RISEBROUGH, R.W. (1972) Pollutant concentrations in
abnormal young terns from Long Island Sound. Auk, 89: 19-35.
HEATH, R.G., SPANN, J.W., KREITZER, J.F., & VANCE, C. (1972)
Effects of polychlorinated biphenyls on birds. In: Proceedings of
the XVth International Ornithological Congress, The Hague, 30
August-5 September 1970, pp. 475-485.
HEDDLE, J.A. & BRUCE, W.R. (1977) Comparison of tests for
mutagenicity or carcinogenicity using assays for sperm
abnormalities, formation of micronuclei and mutation in Salmonella.
In: Origins of human cancer, Book C: Human risk assessment, Section
16. Short-term assays - Predictive value, Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp. 1549-1557.
HEESCHEN, W., BLUTHGEN, A., & NIJHUIS, H. (1986) [Milk hygiene:
Trends in the residue situation.] Kieler Milchwirtsch
Forschungsber., 38(2): 131-145 (in German).
HEINZ, G.H., HILL, E.F., & CONTERA, J.F. (1980) Dopamine and
norepinephrine depletion in ring doves fed DDE, dieldrin, and
Aroclor 1254. Toxicol. appl. Pharmacol., 53: 75-82.
HEINZ, G.H., HASELTINE, S.D., REICHEL, W.L., & HENSLER, G.L. (1983)
Relationships of environmental contaminants to reproductive success
in red-breasted mergansers Mergus serrator from Lake Michigan.
Environ. Pollut., 32: 211-232.
HEINZOW, B.G.J., TINNEBERG, H.R., & BOIE, C. (1988) Effects of some
lipophilic xenobiotics on T-lymphocytes using a modified
erythrocyte rosette inhibition test. Res. Commun. chem. Patho.
Pharmacol., 61(2): 277-280.
HEIRONIMUS, M.P., LAUGHLIN, N.K., & BOWMAN, R.E. (1981) Effects of
early exposure to PCBs on learned irrelevancy of cues in Rhesus
monkeys. An incidental learning paradigm. Teratology, 24: 55A.
HELLE, E., OLSSON, M., & JENSEN, S. (1976a) DDT and PCB levels and
reproduction in ringed seals from the Bothnian bay. Ambio,
5: 188-189.
HELLE, E., OLSSON, M., & JENSEN, S. (1976b) PCB levels correlated
with pathological changes in seal uteri. Ambio, 5: 261-263.
HELLE, E., HYVARINEN, H., PYYSALO, H., & WICKSTROM, K. (1983)
Levels of organochlorine compounds in an inland seal population in
Eastern Finland. Mar. Pollut. Bull., 14: 256-260.
HENDRICKS, D., PUTNAM, T.P., BILLS, D.D., & SINNHUBER, R.O. (1977)
Inhibitory effect of a polychlorinated biphenyl (Aroclor 1254) on
aflatoxin B1 carcinogenesis in rainbow trout (Salmo gairdneri).
J. Natl Cancer Inst., 59: 1545-1551.
HERRING, J.L., HANNAN, E.J., & BILLS, D.D. (1972) UV irradiation of
Aroclor 1254. Bull. environ. Contam. Toxicol., 8: 153-157.
HESSELBERG, R.J. & SCHERR, D.D. (1974) PCBs and p,p' DDE in the
blood of cachectic patients. Bull. environ. Contam. Toxicol.,
11: 202-205.
HILL, H.R., Jr, (1985) Effects of polyhalogenated aromatic
compounds on porphyrin metabolism. Environ. health Perspect.,
60: 139-143.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington,
DC, US Department of the Interior, Fish and Wildlife Service, 147
pp (Fish and Wildlife Technical Report No. 2).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1974)
Polychlorinated biphenyl toxicity to Japanese quail as related to
degree of chlorination. Poult. Sci., 53: 597-604.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 61 pp (Special Scientific Report No. 191).
HILL, E.F., HEATH, R.G., & WILLIAMS, J.D. (1976) Effect of dieldrin
and Aroclor 1242 on Japanese quail eggshell thickness. Bull.
environ. Contam. Toxicol., 16: 445-453.
HINTON, D.E., GLAUMANN, H., & TRUMP, B.F. (1978) Studies on the
cellular toxicity of polychlorinated biphenyls (PCBs) I. Effect of
PCBs on microsomal enzymes and on synthesis and turnover of
microsomal and cytoplasmic lipids of rat liver - A morphological
and biochemical study. Virchows Arch. cell. Pathol., B27: 279-306.
HIRAYAMA, C., IRISA, T., & YAMAMOTO, T. (1969) Fine structural
changes of the liver in a patient with chlorobiphenyls
intoxication. Fukuoka Acta med., 60: 445-461.
HIRAYAMA, C., OKUMURA, M., NAGAI, J., & MASUDA, Y. (1974)
Hypobilirubinemia in patients with polychlorinated biphenyls
poisoning. Clin. Chim. Acta., 55: 97-100.
HIROSE, M., SHIRAI, T., TSUCA, H., FUKUSHIMA, S., OGISO, T., & ITO,
N. (1981) Effect of phenobarbital, polychlorinated biphenyl and
sodium saccharin on hepatic and renal carcinogenesis in
unilaterally nephrectomized rats given n-ethyl- N-hydroxy-
ethylnitrosamine orally. Carcinogenesis, 2: 1299-1302.
HLADKA, A., TAKACHOVA, T., & LISHKA, D. (1983) Exposure to
polychlorinated biphenyls and its effect on selected biochemical
functions. Czech. Med., 5: 8-14.
HODSON, K. (1975) Some aspects of the nesting ecology of
Richardson's merlin (Falco columbarius richardsonii) on the
Canadian prairies, Columbia, Vancouver, University of British
Columbia (MSc Thesis).
HOFFMAN, D.J., RATTNER, B.A., BUNCK, C.M., & KRYNITSKY, A. (1986)
Association between PCBs and lower embryonic weight in
black-crowned night herons in San Francisco Bay. J. Toxicol.
environ. Health, 19: 383-391.
HOGAN, J.W. & BRAUHN, J.L. (1975) Abnormal rainbow trout fry from
eggs containing high residues of a PCB (Aroclor 1242). Prog.
Fish-Cult., 37: 229-230.
HOLA, N. & REZNICEK, J. (1985) [The genetic hazard involved in
occupational exposure to high concentrations of polychlorinated
biphenyls.] Prac. Lek., 37: 386-391 (in Czech).
HOLDEN, A.V. (1970) Source of polychlorinated contamination in the
marine environment. Nature (Lond.), 228: 1220-1221.
HOLDEN, A.V. (1973) Monitoring PCBs in water and wildlife. In:
Proceedings of the Polychlorinated Biphenyl II Conference,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 23-33 (Publication No. 4E).
HOLDEN, A.V. & MARSDEN, K. (1969) Single-stage clean-up of animal
tissue extracts for organochlorine residue analysis. J.
Chromatogr., 44: 481-492.
HOLDGATE, M.W. (1971) The sea bird wreck in the Irish Sea. Autumn
1969, London, Natural Environment Research Council (Publication
Series C4).
HOLDRINET, M.V., BRAUN, H.E., FRANK, R., STOPPS, G.J., SMOUT, M.S.,
& MCWADE, J.W. (1977) Organochlorine residues in human adipose
tissue and milk from Ontario residents 1969-1974. Can. J. public
Health, 68: 74-80.
HOLLEMAN, K.A., BARNETT, B.D., & WICKER, G.W. (1976) Response of
chicks and turkey poults to Aroclor 1242. Poult. Sci.,
55: 2354-2356.
HOLT, R.L., CRUSE, S., & DREER, E.S. (1986) Pesticide and
polychlorinated biphenyl residues in human adipose tissue from
Northeast Louisiana. Bull. environ. Contam. Toxicol., 36: 651-655.
HOM, W., RISEBROUGH, R.W., SOUTAR, A., & YOUNG, D.R. (1974)
Deposition of DDE and polychlorinated biphenyls in dated sediments
of the Santa Barbara Basin. Science, 184: 1197-1199.
HONDA, T., NONAKA, S., MURAYAMA, F., OHGAMI, T., SHIMOYAMA, T., &
YOSHIDA, H. (1983) Effects of KC-400 (polychlorinated biphenyls) on
porphyrin metabolism. J. Dermatol., 10: 259-265.
HOOPINGARNER, R., SAMUEL, A., & KRAUSE, D. (1972) Polychlorinated
biphenyl interactions with tissue culture cells. Environ. health
Perspect., 1: 155-158.
HORI, S., OBANA, H., KASHIMOTO, T., OTAKE, T., NISHIMURA, H.,
IKEGAMI, N., KUNITA, N., & UDA, H. (1982) Effect of polychlorinated
biphenyls and polychlorinated quarterphenyls in Cynomolgus monkey
(Macaca fascicularis). Toxicology, 24: 123-139.
HORNSHAW, T.C., AULERICH, R.J., & JOHNSON, H.E. (1983) Feeding
great lakes fish to mink: Effects on mink and accumulation and
elimination of PCBs by mink. J. Toxicol. environ. Health,
11: 933-946.
HORNSHAW, T.C., SAFRONOFF, J., RINGER, R.K., & AULERICH, R.J.
(1986) LC50 test results in polychlorinated biphenyl-fed mink: Age,
season, and diet comparisons. Arch. Environ. Contam. Toxicol.,
15: 717-723.
HORZEMPA, L.M. & DI TORO, D.M. (1983) The extent of reversibility
of polychlorinated biphenyl adsorption. Water Res., 17: 851-859.
HSIA, M.T.S., LIN, F.S.D., & ALLEN, J.R. (1978) Comparative
mutagenicity and toxic effects of 2,2',5,5'-tetrachlorobiphenyl and
its metabolites in bacterial and mammalian test systems. Res.
Commun. chem. Pathol. Pharmacol., 21: 485-496.
HSU, I.C., VAN MILLER, J.P., SEYMOUR, J.L., & ALLEN, J.R. (1975a)
Urinary metabolites of 2,5,2',5'-tetrachlorobiphenyl in the
non-human primate. Proc. Soc. Exp. Biol. Med., 150: 185-188.
HSU, I.C., VAN MILLER, J.P., & ALLEN, J.R. (1975b) Metabolic fate
of 3H 2,5,2',5'-tetrachlorobiphenyls in infant non-human primates.
Bull. environ. Contam. Toxicol., 14: 233-240.
HSU, S.-T., MA, C.-I., HSU, S.K.-H., WU, S.-S., HSU, N.H.-M., YEH,
C.-C., & WU, S.-B. (1985) Discovery and epidemiology of PCB
poisoning in Taiwan: A four-year follow-up. Environ. health
Perspect., 59: 5-10.
HUCKINS, J.N., SCHWARTZ, T.R., PETTY, J.D. & SMITH, L.M. (1988)
Determination, fate and potential significance of PCBs in fish and
sediment samples with emphasis on selected AHH-inducing congeners.
Chemosphere, 17(10): 1995-2016.
HUDECOVA, A., KOSHINOVA, A., & MADARICH, A. (1979) [Investigations
of the effect of polychlorinated biphenyls on the dynamics of the
changes of the vitamin A and E content in rat liver.] Czech. Hyg.,
24: 59-64 (in Czech).
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife, Washington, DC, US Department
of the Interior, Fish and Wildlife Service, 90 pp (Resource
Publication No. 153).
HURST, J.G., NEWCOMER, W.S., & MORRISON, J.A. (1973) The effects of
polychlorinated biphenyl on longevity of bobwhite quail (Collinus
virginianus): A sex differential. Proc. Soc. Exp. Biol. Med.,
144: 431-435.
HURST, J.G., NEWCOMER, W.S., & MORRISON, J.A. (1974) Some effects
of DDT, toxaphene and polychlorinated biphenyl on thyroid function
in bobwhite quail. Poult. Sci., 53: 125-133.
HUSTERT, K. & KORTE, F. (1972) [Contributions to ecological
chemistry. XXXVIII: Synthesis of polychlorinated biphenyls and
their reactions to UV irradiation.] Chemosphere, 1: 7-10
(in German).
HUTZINGER, O., SAFE, S., & ZITKO, V. (1971) Polychlorinated
biphenyls: photolysis of 2,4,6,2',4',6'-hexachlorobiphenyl,
chlorobiphenyl. Nature (Lond.), 232: 15.
HUTZINGER, O., NASH, D.M., SAFE, S., DEFREITAS, A.S.W., NORSTRÖM,
R.J., WILDISH, D.J., & ZITKO, V. (1972) Polychlorinated
biphenyls-metabolic behaviour of pure isomers in pigeons, rats, and
brook trout. Science, 178: 312-314.
HUTZINGER, O., SAFE, S., & ZITKO, V. (1974) The chemistry of PCBs,
Cleveland, Ohio, CRC Press.
HUTZINGER, O., CHOUDHRY, G.G., CHITTIM, B.G., & JOHNSTON, L.E.
(1985) Formation of polychlorinated dibenzofurans and dioxins
during combustion, electrical equipment fires, and PCB
incineration. Environ. health Perspect., 60: 3-9.
IARC (1978) Polychlorinated biphenyls and polybrominated biphenyls,
Lyon, International Agency for Research on Cancer, pp. 43-103 (IARC
Monographs on the Evaluation of the Carcinogenic Risks of Chemicals
to Humans, Volume 18).
IARC (1987) Overall evaluation of carcinogenicity: An updating of
IARC monographs Volumes 1 to 42, Lyon, International Agency for
Research on Cancer, (IARC Monographs on the Evaluation of the
Carcinogenic Risks of Chemicals to Humans, Supplement 7).
IATROPOULOS, M.J., FELT, G.R., ADAMS, H.P., KORTE, F., & COULSTON,
F. (1977) Chronic toxicity of 2,5,4'-trichlorobiphenyl in young
Rhesus monkeys. II. Histopathology. Toxicol. appl. Pharmacol., 41:
629-638.
IATROPOULOS, M.J., BAILEY, J., ADAMS, H.P., COULSTON, F., & HOBSON,
W. (1978) Response of nursing infant Rhesus to Clophen A30 or
hexachlorobenzene given to lactating mothers. Environ. Res.,
16: 38-47.
IKEDA, M., KURATSUNE, M., NAKAMURA, Y., & HIROHATA, T. (1987) A
cohort study on mortality of Yusho patients - a preliminary report.
Fukuoka Acta med., 78: 297-300.
IMANISHI, J., NOMURA, H., MATSUBARA, M., KITA, M., WON, S.-J.,
MIZUTANI, T., & KISHIDA, T. (1980) Effect of polychlorinated
biphenyl on viral infections in mice. Infect. Immun., 29: 275-277.
IMANISHI, J., OKU, T., OISHI, K., NOMURA, H., & MIZUTANI, T. (1984)
Reduced resistance to experimental viral and bacterial infections
of mice treated with polychlorinated biphenyl. Biken J.,
27: 195-198.
INNAMI, S., NAKAMURA, A., MIYAZAKI, M., NAGAYAMA, S., & NISHIDE, E.
(1976) Further studies on the reduction of vitamin A content in the
livers of rats given polychlorinated biphenyls. J. nutr. Sci.
Vitaminol., 22: 409-418.
INOUE, K., TAKANAKA, A., MIZOKAMI, K., FUJIMORI, K., SUNOUCHI, M.,
KASUYA, Y., & OMORI, Y. (1981) Effects of polychlorinated biphenyls
on the monooxygenase system in fetal livers of rats. Toxicol. appl.
Pharmacol., 59: 540-547.
INTERNATIONAL COUNCIL FOR THE EXPLORATION OF THE SEA (1974) Report
of a working group for the international study of the pollution of
the North Sea and its effects on living resources and their
exploitation, Charlottenlund, Denmark, International Council for
the Exploration of the Sea (Co-operative Research Report No. 39).
IRPTC (1986) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme.
ISEKI, K., TAKAHASHI, M., BAUERFEIND, E., & WONG, C.S. (1981)
Effects of polychlorinated biphenyls (PCBs) on a marine plankton
population and sedimentation in controlled ecosystem enclosures.
Mar. Ecol. Prog. Ser., 5: 207-214.
ISHI, H. (1972) PCB pollution in Japan, Tokyo, Japanese Public
Health Association, pp. 13-28 (Environmental Health Report No. 14).
ISHIDATE, K., YOSHIDA, M., & NAKAZAWA, Y. (1978) Effect of typical
inducers of microsomal drug-metabolizing enzymes on phospholipid
metabolism in rat liver. Biochem. Pharmacol., 27: 2595-2603.
ISHIKAWA, T.T., MCNEELY, S., STEINER, P.M., GLUECK, C.J., MELLIES,
M., GARTSIDE, P.S., & MCMILLIN, C. (1978) Effects of chlorinated
hydrocarbons on plasma alpha-lipoprotein cholesterol in rats.
Metabolism, 27: 89-96.
ITO, N., NAGASAKI, H., ARAI, M., MAKIURA, S., SUGIHARA, S., &
HIRAO, K. (1973) Histopathologic studies on liver tumorigenesis
induced in mice by technical polychlorinated biphenyl and its
promoting effect on liver tumours induced by benzenehexachloride.
J. Natl Cancer Inst., 51(5): 1637-1646.
ITO, N., NAGASAKI, H., MAKIURA, S., & ARAI, M. (1974) Histological
studies on liver tumorigenesis in rats treated with polychlorinated
biphenyls. Gann., 65: 545-549.
ITOKAWA, Y., YAGI, N., KAITO, H., KAMOHARA, K., & FUJIWARA, K.
(1976) Influence of diet on the induction of hepatic ceroid pigment
in rats by polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
36: 131-141.
IVERSON, F., VILLENEUVE, D.C., GRANT, D.L., & HATINA, G.V. (1975)
Effect of Aroclor 1016 and 1242 on selected enzyme systems in the
rat. Bull. environ. Contam. Toxicol., 13: 456-463.
IVERSON, F., TRUELOVE, J., & HIERLIHY, S.L. (1982) Hepatic
microsomal enzyme induction by Aroclors 1248 and 1254 in Cynomolgus
monkeys. Food chem. Toxicol., 20: 307-310.
IWATA, Y. & GUNTHER, F.A. (1976) Translocation of the
polychlorinated biphenyl Aroclor 1254 from soil into carrots under
field conditions. Arch. environ. Contam. Toxicol., 4: 44-59.
IWATA, Y., WESTLAKE, W.E., & GUNTHER, F.A. (1973) Varying
persistence of polychlorinated biphenyls in six California soils
under laboratory conditions. Bull. environ. Contam. Toxicol.,
9: 204-211.
IWATA, Y., GUNTHER, F.A., & WESTLAKE, W.E. (1974) Uptake of a PCB
(Aroclor 1254) from soil by carrots under field conditions. Bull.
environ. Contam. Toxicol., 11: 523-528.
JACOBSON, J.L., JACOBSON, S.W., SCHWARTZ, P.M., FEIN, G.G., &
DOWLER, J.K. (1984a) Prenatal exposure to an environmental toxic:
A test of the multiple effects model. Dev. Psychol., 20: 523-532.
JACOBSON, J.L., FEIN, G.G., JACOBSON, S.W., SCHWARTZ, P.M., &
DOWLER, J.K. (1984b) The transfer of polychlorinated biphenyls
(PCBs) and polybrominated biphenyls-(PBBs) across the human
placenta and into maternal milk. Am. J. public Health,
74(4): 378-379.
JACOBSON, S.W., FEIN, G.G., JACOBSON, J.L., SCHWARTZ, P.M., &
DOWLER, J.K. (1985) The effect of intrauterine PCB exposure on
visual recognition memory. Child Dev., 56: 856-860.
JAN, J. & MALNERSIC, S. (1978) Determination of PCB and PCT
residues in fish by tissue acid hydrolysis and destructive clean-up
of the extract. Bull. environ. Contam. Toxicol., 19: 772-780.
JAN, J. & TRATNIK, M. (1988a) Polychlorinated biphenyls in
residents around the River Krupa, Slovenia, Yugoslavia. Bull.
environ. Contam. Toxicol., 41: 809-814.
JAN, J., TRATNIK, M., & KENDA, A. (1988b) Atmospheric contamination
with polychlorinated biphenyls in Bela Krajina (Yugoslavia);
Emissions from industrial plant, landfill and river areas.
Chemosphere, 17(4): 809-813.
JANI, J.P., PATEL, J.S., SHAH, M.P., GUPTA, S.K., & KASHYAP, S.K.
(1988) Levels of organochlorine pesticides in human milk in
Ahmedabad, India. Int. Arch. occup. environ. Health., 60: 111-113.
JANNETTI, R.A. & ANDERSON, L.M. (1981) Dimethylnitrosamine
demethylase activity in fetal, suckling, and maternal mouse liver
and its transplacental and transmammary induction by
polychlorinated biphenyls. J. Natl Cancer Inst., 67: 461-466.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTRÖM, G., &
VAZ, R. (1975) Identification by GC-MS of phenolic metabolites of
PCB and p,p'-DDE isolated from Baltic guillemot and seal. Ambio,
4: 93-97.
JAPAN ENVIRONMENT AGENCY (1983) Environmental monitoring of
chemicals; Environmental survey report of F.Y. 1980 and 1981.
Tokyo, Japan Environment Agency, Department of Environmental
Health, Offices of Health Studies.
JAPAN ENVIRONMENTAL SANITATION BUREAU (1973) [Survey on food
contamination: Report of a comprehensive investigation on the
prevention of pollution by PCBs], Tokyo, Ministry of Health &
Welfare, Environmental Sanitation Bureau, pp. 191-210 (in
Japanese).
JEFFERIES, D.J. & PARSLOW, J.L.F. (1972) Effect of one
polychlorinated biphenyl on size and activity of the gull thyroid.
Bull. environ. Contam. Toxicol., 8: 306-310.
JEFFERIES, D.J. & PARSLOW, J.L.F. (1976) Thyroid changes in
PCB-dosed guillemots and their indication of one of the mechanisms
of action of these materials. Environ. Pollut., 10: 293-311.
JENSEN, A.A. (1983a) Chemical contaminants in human milk. Res.
Rev., 89: 1-128.
JENSEN, A.A. (1983b) PCB in human milk: An Overview. In: Barros,
M.C., Könemann, H., & Visser, R., ed. Proceedings of the PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 81-98.
JENSEN, A.A. (1984a) Current data of levels of organohalogen
compounds in human milk in countries outside CEC. In: Report on the
WHO Consultation on Organohalogen Compounds in Human Milk and
Related Hazards, Bilthoven, 9-11 January 1985, Copenhagen, World
Health Organization, Regional Office for Europe, Annex 3.
JENSEN, A.A. (1984b) Data extracted from document on "Assessment of
the presence of potentially toxic substances in breast milk with
special emphasis on the European Community" submitted by the
Commission of the European Communities (CEC Study No. 830465,
CEC/V/E/2/lux/35/84). In: Report on the WHO Consultation on
Organohalogen Compounds in Human Milk and Related Hazards,
Bilthoven, 9-11 January 1985, Copenhagen, World Health
Organization, Regional Office for Europe, Annex 7.
JENSEN, A.A. (1987) Polychlorobiphenyls (PCBs),
polychlorodibenzo- p-dioxins (PCDDs) and polychlorodibenzofurans
(PCDFs) in human milk, blood, and adipose tissue. Sci. total
Environ., 64: 259-293.
JENSEN, S. & JANSSON, B. (1976) Methylsulfone metabolites of PCB
and DDE in seals from the Baltic. Ambio, 5: 257-260.
JENSEN, S. & SUNDSTROM, G. (1974a) Structures and levels of most
chlorobiphenyls in two technical PCB products and in human adipose
tissue. Ambio, 3: 70-76.
JENSEN, S. & SUNDSTROM, G. (1974b) Metabolic hydroxylation of a
chlorobiphenyl containing only isolated unsubstituted
positions-2,2',4,4',5,5'-hexachlorobiphenyl. Nature (Lond.),
251: 219-220.
JENSEN, S., JOHNELS, A.G., OLSSON, M., & OTTERLIND, G. (1969) DDT
and PCB in marine animals from Swedish waters. Nature (Lond.),
224: 247-250.
JENSEN, S., JOHNELS, A.G., OLSSON, M., & OTTERLIND, G. (1972a) DDT
and PCB in herring and cod from the Baltic, the Kattegat and the
Skaggerrak. Ambio, 1(Spec. Rep.): 71-85.
JENSEN, S., JOHNELS, A.G., OLSSEN, M., & WESTERMARK, T. (1972b) The
avifauna of Sweden as indicators of environmental contamination
with mercury and chlorinated hydrocarbons. In: Proceedings of the
15th International Ornithology Congress, Leiden, pp. 455-465.
JENSEN, S., RENBERG, L., & VAZ, R. (1973) Problems in
quantification of PCB in biological material. In: Proceedings of
the Polychlorinated Biphenyls Conference II, Stockholm, 1972,
Solna, Sweden, National Environmental Protection Board, pp. 7-13
(Publication No. 4E).
JENSEN, S., ORBERG, J., KIHLSTROM, J.E., OLSSON, M., & LUNDBERG, C.
(1977) Effects of PCB and DDT on mink (Mustela vision) during the
reproductive season. Ambio, 6: 239.
JFCMP (1985) FAO/WHO Collaborating Centres for Food Contamination
Monitoring. PCB's residues in food, Rome, Food and Agriculture
Organization of the United Nations, FAO/WHO Food Contamination
Monitoring Programme (Document EFP/85,3 prepared for the 17th
Session of the Codex Committee on Pesticide Residues).
JOHANSSON, N., LARSSON, A., & LEWANDER, K. (1972) Metabolic effects
of PCB (Polychlorinated biphenyls) on the brown trout (Salmo
trutta). Comp. gen. Pharmacol., 3: 310-314.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1979)
Pesticides and other chemical residues in infant and toddler total
diet samples (I), August 1974-July 1975. Pestic. monit. J.,
13(3): 87-98.
JOHNSTONE, G.J., ECOBICHON, D.J., & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, D.C.L., DAVIS, W.E., Jr, NEWELL, G.W., SASMORE, D.P., &
ROSEN, V.J. (1974) Modification of hexachlorophene toxicity by
dieldrin and Aroclor 1254. Toxicology, 2: 309-318.
JONES, K.C. (1988) Determination of polychlorinated biphenyls in
human foodstuffs and tissues: suggestions for a selective congener
analytical approach. Sci. total Environ., 68: 141-159.
JONES, K.C. (1989) Polychlorinated biphenyls in Welsh soils: a
survey of typical levels. Chemosphere, 18(7-8): 1665-1672.
JONES, J.W. & ALDEN, H.S. (1936) An acneform dermatergosis. Arch.
Dermato. Syphilol., 33: 1022-1034.
JONSSON, H.T., KEIL, J.E., GADDY, R.G., LOADHOLT, C.B., HENNIGAR,
G.R., & WALKER, E.M. (1976) Prolonged ingestion of commercial DDT
and PCB; effects on progesterone levels and reproduction in the
mature female rat. Arch. environ. Contam. Toxicol., 3: 479-490.
JONSSON, H.T., Jr, WALKER, E.M., Jr, GREENE, W.B., HUGHSON, M.D.,
& HENNIGAR, G.R. (1981) Effects of prolonged exposure to dietary
DDT and PCB on rat liver morphology. Arch. environ. Contam.
Toxicol., 10: 171-183.
JURY, W.A., WINER, A.M., SPENCER, W.F., & FOCHT, D.D. (1987)
Transport and transformations of organic chemicals in the
soil-air-water ecosystem. Rev. environ. Contam. Toxicol.,
99: 119-164.
KAISER, K.L.E. (1974) On the optical activity of polychlorinated
biphenyls. Environ. Pollut., 7: 93-101.
KAISER, K.L.E. & WONG, P.T.S. (1974) Bacterial degradation of
polychlorinated biphenyls. I. Identification of some metabolic
products from Aroclor 1242. Bull. environ. Contam. Toxicol.,
11: 291-296.
KAMAL, M., KLEIN, M., & KORTE, F. (1976) Isolation and
identification of metabolites after long-term feeding of
2,2'-dichlorobiphenyl-C-14. Chemosphere, 5: 349-356.
KAMOHARA, T., YAGI, N., & ITOKAWA, Y. (1984) Mechanism of lipid
peroxide formation in polychlorinated biphenyls (PCB) and
dichlorodiphenyltrichloroethane (DDT)-poisoned rats. Environ. Res.,
34: 18-23.
KANEKO, T. (1988) [Polychlorinated terphenyls (PCTs). A study on
the induction of cleft palate by polychlorinated terphenyls (PCTs)
administered maternally with special reference to the role of
corticosterone.] Pharmacometrics, 36(4): 309-327 (in Japanese).
KANNAN, N., TANABE, S., WAKIMOTO, T., & TATSUKAWA, R. (1987)
Coplanar polychlorinated biphenyls in Aroclor and Kanechlor
mixtures. J. Assoc. Off. Anal. Chem., 70(3): 451-454.
KANNAN, N., TANABE, S., & TATSUKAWA, R. (1988) Potentially
hazardous residues of non- ortho chlorine substituted coplanar
PCBs in human adipose tissue. Arch. environ. Health, 43(1): 11-14.
KANNAN, N., WAKIMOTO, T., & TATSUKAWA, R. (1989) Possible
involvement of frontier (n) electrons in the metabolism of
polychlorinated biphenyls (PCBs). Chemosphere, 18(9/10): 1955-1963.
KARLSSON, B., PERSSON, B., SÖDERGREN, A., & ULFSTRAND, S. (1974)
Locomotory and dehydrogenase activities of redstarts Phoenicurus
phoenicurus L. (Aves) given PCB and DDT. Environ. Pollut.,
7: 53-63.
KARPPANEN, E. & KOLHO, L. (1973) The concentration of PCB in human
blood and adipose tissue in three different research groups.
Presented at the Polychlorinated Biphenyls Conference II,
Stockholm, 1972 (Unpublished document).
KASHIMOTO, T., MIYATA, H., FUKUSHIMA, S., KUNITA, N., OHI, G., &
TUNG, T.-C. (1985) PCBs, PCQs, and PCDFs in blood of Yusho and
Yu-Cheng patients. Environ. health Perspect., 59: 73-78.
KASZA, L., WEINBERGER, M.A., HINTON, D.E., TRUMP, B.F., PATEL, C.,
FRIEDMAN, L., & GARTHOFF, L.H. (1978a) Comparative toxicity of
polychlorinated biphenyl and polybrominated biphenyl in the rat
liver: light and electron microscopic alterations after subacute
dietary exposure. J. environ. Pathol. Toxicol., 1: 241-257.
KASZA, L., COLLINS, W.T., CAPEN, C.C., GARTHOFF, L.H., & FRIEDMAN,
L. (1978b) Comparative toxicity of polychlorinated biphenyl and
polybrominated biphenyl in the rat thyroid gland: light and
electron microscopic alterations after subacute dietary exposure.
J. environ. Pathol. Toxicol., 1: 587-599.
KATO, N. & YOSHIDA, A. (1980) Effect of dietary PCB on hepatic
cholesterogenesis in rats. Nutr. Rep. Int., 21: 107-112.
KATO, N. & YOSHIDA, A. (1981) Effect of various dietary xenobiotics
on serum total cholesterol and high density lipoprotein cholesterol
in rats. Nutr. Rep. Int., 23: 825-831.
KATO, N., KATO, M., KIMURA, T., & YOSHIDA, A. (1978) Effect of
dietary addition of PCB, DDT, or BHT and dietary protein on vitamin
A and cholesterol metabolism. Nutr. Rep. Int., 18: 437-446.
KATO, N., KAWAI, K., & YOSHIDA, A. (1982) Effects of dietary
polychlorinated biphenyls and protein level on liver and serum
lipid metabolism of rats. Agric. biol. Chem., 46: 703-707.
KATO, S., MCKINNEY, J.D., & MATTHEWS, H.B. (1980) Metabolism of
symmetrical hexachlorobiphenyl isomers in the rat. Toxicol. appl.
Pharmacol., 53: 389-398.
KATSUKI, S. (1969) Foreword. Fukuoka Acta med., 60: 403-407.
KAWAI, S., FUKUSHIMA, M., MIYAZAKI, N., & TATSUKAWA, R. (1988)
Relationship between lipid composition and organochlorine levels in
the tissues of striped Dolphin. Mar. Pollut. Bull., 19: 129-133.
KAWANISHI, S., SANO, S., MIZUTANI, T., & MATSUMOTO M. (1973)
[Experimental porphyria induced by polychlorinated biphenyls.] Jpn.
J. Hyg., 28: 84 (in Japanese).
KAWANISHI, S., SANO, S., MIZUTANI, T., & MATSUMOTO M. (1974)
[Experimental studies on toxicity of synthetic tetrachlorobiphenyl
isomers.] Jpn. J. Hyg., 29: 81 (in Japanese).
KAWANISHI, S., SANO, S., & MIZUTANI, T. (1975) [A toxicological
study on synthetic hexachlorobiphenyl isomers.] Jpn. J. Hyg.,
30: 124 (in Japanese).
KAWANISHI, S., SEKI, Y., & SANO, S. (1983) Uroporphyrinogen
decarboxylase. Purification, properties, and inhibition by
polychlorinated biphenyl isomers. J. biol. Chem., 258: 4285-4292.
KECK, G. (1981) Effets de la contamination par les
polychlorobiphényles (PCBs) sur le développement de la tumeur
d'Ehrlich chez la souris Swiss. Toxicol. Eur. Res., 3(5): 229-236.
KERKVLIET, N.I. & BAECHER-STEPPAN, L. (1988a) Suppression of
allograft immunity by 3,4,5,3',4',5-hexachlorobiphenyl. I. Effects
of exposure on tumor rejection and cytotoxic T-cell activity
in vivo. Immunopharmacology, 16: 1-12.
KERKVLIET, N.I. & BAECHER-STEPPAN, L. (1988b) Suppression of
allograft immunity by 3,4,5,3',4',5-hexachlorobiphenyl. II. Effects
of exposure on mixed lymphocyte reactivity and induction of
suppressor cell activity in vitro. Immunopharmacology, 16: 13-23.
KERKVLIET, N.I. & KIMELDORF, D.J. (1977) Anti-tumour activity of a
polychlorinate biphenyl mixture, Aroclor 1254, in rats inoculated
with Walker 256 carcinosarcoma cells. J. Natl Cancer Inst.,
59: 951-955.
KHAN, M.A., RAO, R.M., & NOVAK, A.F. (1976) Polychlorinated
biphenyls (PCBs) in food. January 1976. Crit. Rev. food Sci. Nutr.,
January: 103-145.
KHAN, A.V., LASHNEVA, N.V., KUMOK, S.T., GURVICH, YA.A., &
TUTELYAN, V.A. (1985) Study into the effect of ionol on cytochrome
P-450 induction in rat liver by polychlorinated diphenyls. Vopr.
pitan., 4(1): 66-69.
KIESSLING, A., PART, P., RING, O., & LINDAHL-KIESSLING, K. (1983)
Effects of PCB on the adrenergic response in perfused gills and on
levels of muscle glycogen in rainbow trout (Salmo gairdneri
Rich.). Bull. environ. Contam. Toxicol., 31: 712-718.
KIHLSTROM, J.E., LUNDBERG, C., ORBERG, J., DANIELSSON, P.O., &
SYDHOFF, J. (1975) Sexual functions of mice neonatally exposed to
DDT or PCB. Environ. Physiol. Biochem., 5: 54-57.
KIKUCHI, M. (1984) Autopsy of patients with Yusho. Am. J. ind.
Med., 5: 19-30.
KIMBROUGH, R.D. (1980) Occupational exposure. In: Halogenated
biphenyls, terphenyls, naphthalenes, dibenzodioxins and related
products, Amsterdam, Elsevier, North-Holland Biomedical Press,
pp. 373-395 (Topics in Environmental Health, Vol. 4).
KIMBROUGH, R.D. (1987) Human health effects of polychlorinated
biphenyls (PCBs) and polybrominated biphenyls (PBBs). Annu. Rev.
Pharmacol. Toxicol., 27: 87-111.
KIMBROUGH, R.D., LINDER, R.E., & GAINES, T.B. (1972) Morphological
changes in livers of rats fed polychlorinated biphenyls. Light
microscopy and ultrastructure. Arch. environ. Health, 25: 354-364.
KIMBROUGH, R.D. & LINDER, R.E. (1974) Induction of adenofibrosis
and hepatomas of the liver in BALB/cJ mice by polychlorinated
biphenyls (Aroclor 1254). J. Natl Cancer Inst., 53: 547-552.
KIMBROUGH, R.D., SQUIRE, R.A., LINDER, R.E., STRANBERG, J.D.,
MONTALI, R.J., & BURSE, V.W. (1975) Induction of liver tumours in
Sherman strain female rats by polychlorinated biphenyl Aroclor
1260. J. Natl Cancer Inst., 55: 1453-1459.
KIMURA, N.T. & BABA, T. (1973) Neoplastic changes in the rat liver
induced by polychlorinated biphenyls. Gann, 64: 105-108.
KIMURA, I. & MIYAKE, T. (1976) Teratogenic and postnatal growth-
suppressive effects of polychloro-triphenyl (PCT) in dd/Y mice.
Teratology, 14: 243-244 (Abstract).
KIMURA, S., KUMADA, H., & MATIDA, Y. (1974) [Acute toxicity and
accumulation of PCB (KC 300) in freshwater fish. Bull. freshwater
Fish Res. Lab. (Tokyo), 23: 115-123 (in Japanese).
KIMURA, N.T., KANEMATSU, T., & BABA, T. (1976) Polychlorinated
biphenyl(s) as a promoter in experimental hepatocarcinogenesis in
rats. Z. Krebsforsch., 87: 257-266.
KIRIYAMA, S., BANJO, M., & MATSUSHIMA, H. (1974) Effect of
polychlorinated biphenyls (PCB) and related compounds on the weight
of various organs and plasma and liver cholesterol in the rat.
Nutr. Rep. Int., 10: 79-88.
KITAMURA, M., TSUKAMOTO, T., SUMINO, K., HAYAKAWA, K., SHIBITA, T.,
& HIRANO, I. (1973) [The PCB levels in the blood of workers
employed in a condenser factory.] Jpn. J. ind. Health, 47: 354-355
(in Japanese).
KLAAS, E.E. & SWINEFORD, D.M. (1976) Chemical residue content and
hatchability of screech owl eggs. Wilson Bull., 88: 421-426.
KLASSON-WEHLER, E., BERGMAN, A., KOWALSKI, B., & BRANDT, I. (1987)
Metabolism of 2,3,4'6,-tetrachlorobiphenyl: Formation and tissue
localization of mercapturic acid pathway metabolites in mice.
Xenobiotica, 17(4): 477-486.
KLEIN, H. (1983) The PCB situation in the Federal Republic of
Germany. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of PCB Seminar, The Hague, 28-30 September 1983, The
Hague, Ministry of the Environment, pp. 66-79.
KLEKOWSKI, E.J. (1982) Mutation in ferns growing in an environment
contaminated with polychlorinated biphenyls, Amherst,
Massachusetts, University of Massachusetts, Water Resources
Research Center, 23 pp ((NTIS) PB83-150011).
KLEPPEL, G.S. & MCLAUGHLIN, J.J.A. (1980) PCB toxicity to
phytoplankton: Effects of dose and density-dependent recovery
responses. Bull. environ. Contam. Toxicol., 24: 696-703.
KLING, D. & GAMBLE, W. (1982) Cholesterol biosynthesis in
polychlorinated biphenyl-treated rats. Environ. Res., 27: 10-15.
KOBAYASHI, Y. (1972) [Answer report to the questionary paper on
"Regulation on residual levels in foods".] Biol. Pollut., 4: 93-116
(in Japanese).
KODAMA, H. & OTA, H. (1977) Studies on the transfer of PCB to
infants from their mothers (1). Jpn. J. Hyg., 32: 567-573.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated biphenyls
to infants from mothers. Arch. environ. Health, 35: 95-100.
KOEMAN, J.H., TEN HOEVER DE BRAUW, M.C., & DE VOS, R.H. (1969)
Chlorinated biphenyls in fish, mussels and birds from the River
Rhine and the Netherlands coastal area. Nature (Lond.),
221: 1126-1128.
KOEMAN, J.H., PEETERS, W.H.M., SMIT, C.J., TJIOE, P.S., & DE GOEIJ,
J.J.M. (1972) Persistent chemicals in marine mammals. TNO-nieuws,
27: 570-578.
KOEMAN, J.H., VAN VELZEN-BLAD, H.C.W., DE VRIES, R., & VOS, J.G.
(1973) Effects of PCB and DDE in cormorants and evaluation of PCB
residues from an experimental study. J. Reprod. Fertil.,
19(Suppl.): 353-364.
KOHLI, K.K., GUPTA, B.N., ALBRO, P.W., MUKHTAR, H., & MCKINNEY,
J.D. (1979) Biochemical effects of pure isomers of hexachloro-
biphenyl: fatty livers and cell structure. Chem. biol. Interact.,
25: 139-156.
KOHLI, K.K., PHILPOT, R.M., ALBRO, P.W., & MCKINNEY, J.D. (1980)
Induction of different species of cytochrome P-450 by coplanar and
noncoplanar isomers of hexachlorobiphenyl. Life Sci., 26: 945-952.
KOLBYE, A.C., Jr (1972) Food exposure to polychlorinated biphenyls.
Environ. health Perspect., 1: 85-88.
KOLLER, L.D. (1977) Enhanced polychlorinated biphenyls lesions in
Moloney leukemia virus-infected mice. Clin. Toxicol.,
11(1): 107-116.
KOLLER, L.D. & THIGPEN, J.E. (1973) Biphenyl-exposed rabbits. Am.
J. vet. Res., 34: 1605-1606.
KOLLER, L.D. & ZINKL, J.G. (1973) Pathology of polychlorinated
biphenyls in rabbits. Am. J. Pathol., 70: 363-378.
KOMATSU, F. & KIKUCHI, M. (1972) [Skin lesion by 3,4,3',4'-
tetrachlorobiphenyl in rabbits.] Fukuoka Acta med., 63: 384-386
(in Japanese).
KORACH, K.S., SARVER, P., CHAE, K., MCLACHLAN, J.A., & MCKINNEY,
J.D. (1987) Estrogen receptor-binding activity of polychlorinated
hydroxybiphenyls: Conformationally restricted structural probes.
Mol. Pharmacol., 33: 120-126.
KOSHIOKA, M., KANAZAWA, J., IIZUKA, H., & MURAI, T. (1987)
Photodegradation of decachlorobiphenyl. Bull. environ. Contam.
Toxicol., 38: 409-415.
KOSS, G., SEUBERT, S., SEUBERT, A., HERBERT, M., KORANSKY, W., &
IPPEN, H. (1980) Hexachlorobenzene and 2,4,5,2',4',5'-hexachloro-
biphenyl - A comparison of their distribution, biotransformation
and prophyrinogenic action in female rats. In: Holmstedt, B.,
Lauwerys, R., Mercier, M., & Roberfroid, M., ed. Mechanisms of
toxicity and hazard evaluation, Amsterdam, Elsevier/North-Holland
Biomedical Press, pp. 517-520.
KOSUTZKY, J., ADAMEC, O., & BOBAKOVA, E. (1979) Effects of
polychlorinated biphenyls on poultry reproduction. Bull. environ.
Contam. Toxicol., 21: 737-742.
KREISS, K. (1985) Studies on populations exposed to polychlorinated
biphenyls. Environ. health Perspect., 60: 193-199.
KREISS, K., ZACK, M.M., KIMBROUGH, R.D., NEEDHAM, L.L., SMRED,
A.L., & JONES, B.T. (1981) Association of blood pressure and
polychlorinated biphenyl levels. J. Am. Med. Assoc.,
245(24): 2505-2509.
KREITZER, J.F. & HEINZ, G.H. (1974) The effect of sublethal dosages
of five pesticides and a polychlorinated biphenyl on the avoidance
response of Coturnix quail chicks. Environ. Pollut., 6: 21-29.
KREITZER, J.F. & SPANN, J.W. (1973) Tests of pesticidal synergism
with young pheasants and Japanese quail. Bull. environ. Contam.
Toxicol., 9: 250-256.
KRICHER, J.C., BAYER, C.L., & MARTIN, D.A. (1979) Effects of two
Aroclor fractions on the productivity and diversity of algae from
two lentic ecosystems. Int. J. environ. Stud., 13: 159-167.
KROGH DERR, S. (1978) In vivo metabolism of exogenous
progesterone by PCB treated female rats. Bull. environ. Contam.
Toxicol., 19: 729-732.
KUNITA, N., HORI, S., OBANA, H., OTAKE, T., NISHIMURA, H.,
KASHIMOTO, T., & IKEGAMI, N. (1985) Biological effects of PCBs PCQs
and PCDFs present in the oil causing Yusho and Yu-Cheng. Environ.
Health Perspect., 59: 79-84.
KURACHI, M. (1983) A new sulfur-containing derivative and
possibility of conjugate formation of PCBs in mice or rats. Agric.
biol. Med., 47(6): 1183-1191.
KURACHI, M. & MIO, T. (1983) On fluctuation of PCBs under various
unnatural conditions in mice. Agric. biol. Med., 47(6): 1173-1181.
KURATSUNE, M. & MASUDA, Y. (1972) Polychlorinated biphenyls in
non-carbon copypaper. Environ. health Perspect., 1: 61-62.
KURATSUNE, M., YOSHIMURA, T., MATSUZAKA, J., & YAMAGUCHI, A. (1972)
Epidemiologic study on Yusho, a poisoning caused by ingestion of
rice-oil contaminated with a commercial brand of polychlorinated
biphenyls. Environ. health Perspect., 1: 119-128.
KURATSUNE, M., MASUDA, Y., & NAGAYAMA, J. (1976) Some of the recent
findings concerning Yusho. In: Proceedings of the National
Conference on Polychlorinated Biphenyls, Chicago, 19-21 November
1975, Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances, pp. 14-29 (EPA-560/6-75-004).
KUROKI, H. & MASUDA, Y. (1977) Structures and concentrations of the
main components of polychlorinated biphenyls retained in patients
with Yusho. Chemosphere, 6: 469-474.
KUSUDA, M. (1971) [Study on the female sexual function suffering
from the chlorobiphenyls poisoning.] Sanka Fujinka, 4: 1063-1072
(in Japanese).
KUWABARA, K., YAKUSHIJI, T., WATANABE, I., YOSHIDA, S., KOYAMA, K.,
KUNITA, N., & HARA, I. (1978) Relationship between breast feeding
and PCB residues in blood of the children whose mothers were
occupationally exposed to PCBs. Int. Arch. occup. environ. Health,
41: 189-197.
KUWABARA, K., YAKUSHIJI, T., WATANABE, I., YOSHIDA, S., KOYAMA, K.,
& KUNITA, N. (1979) Levels of polychlorinated biphenyls in blood of
breast-fed children whose mothers are non-occupationally exposed to
PCBs. Bull. environ. Contam. Toxicol., 21: 458-462.
LAKE, B.G., COLLINS, M.A., HARRIS, R.A., & GANGOLLI, S.D. (1979)
The induction of hepatic and extrahepatic xenobiotic metabolism in
the rat and ferret by a polychlorinated biphenyl mixture (Aroclor
1254). Xenobiotica, 9: 723-731.
LAN, S.-J., YEN, Y.-Y., KO, Y.-C., & CHEN, E.-R. (1989) Growth and
development of permanent teeth germ of transplacental Yu-Cheng
babies in Taiwan. Bull. environ. Contam. Toxicol., 42: 931-934.
LA ROCCA, P. & CARLSON, G.P. (1979) The effect of polychlorinated
biphenyls on adenosine triphosphatase activity. Toxicol. appl.
Pharmacol., 48: 185-192.
LARSEN, P.F., GADBOIS, D.F., & JOHNSON, A.C. (1985) Observations on
the distribution of PCBs in the deepwater sediments of the Gulf of
Maine. Mar. Pollut. Bull., 16: 439-442.
LARSSON, R. (1984) Transport of PCBs from aquatic to terrestrial
environments by emerging chironomids. Environ. Pollut.,
34: 283-289.
LARSSON, P. (1985a) Contaminated sediments of lakes and oceans act
as sources of chlorinated hydrocarbons for release to water and
atmosphere. Nature (Lond.), 317: 347-349.
LARSSON, P. (1985b) Change in PCB (Clophen A 50) composition when
transported from sediment to air in aquatic model systems. Environ.
Pollut., B9: 81-94.
LARSSON, P. (1987) Uptake of polychlorinated biphenyls (PCBs) by
the macroalga, Cladophora glomerata. Bull. environ. Contam.
Toxicol., 38: 58-62.
LARSSON, P. & OKLA, L. (1987) An attempt to measure the flow of
chlorinated hydrocarbons, such as PCBs, from water to air in the
field. Environ. Pollut., 44: 219-225.
LARSSON, C.M. & TILLBERG, J.E. (1975) Effects of the commercial
polychlorinated biphenyl mixture Aroclor 1242 on growth, viability,
phosphate uptake, respiration and oxygen evolution in Scenedesmus.
Physiol. Plant., 33: 256-260.
LASHNEVA, N.V. & TUTELYAN, V.A. (1984) Induction of cytochrome
P-450 in rat liver by polychlorinated diphenyls. Farmakol.
Toksikol., 6: 77-80.
LASHNEVA, N.V., KHAN, A.V., & TUTELYAN, V.A. (1985) The functional
state of monooxygenase system in rat liver tissue after effect of
ionol and polychlorinated diphenyls. Vopr. med. khim. 5: 7-22.
LASHNEVA, N.V., CHICHILANOVA, G.V., SOROKOVAYA, G.K., & TUTELYAN,
V.A. (1987) Effects of polychlorinated biphenyls on rat liver
cytochrome P-450 system formation at early postnatal period. In:
Abstracts of the USSR-All-Union Conference on Cytochrome P-450 and
Environment, Novosibirsk, 27-31 July 1987, Novosibirsk, USSR
Academy of Medical Sciences, Siberian Division.
LAWRENCE, J. & TOSINE, H.M. (1977) Polychlorinated biphenyl
concentrations in sewage and sludges of some waste treatment plants
in Southern Ontario. Bull. environ. Contam. Toxicol., 17: 49-56.
LAWTON, R.W., ROSS, M.R., FEINGOLD, J., & BROWN, J.F., Jr (1985)
Effects of PCB exposure on biochemical and hematological findings
in capacitor workers. Environ. health Perspect., 60: 165-184.
LAY, J.P., KLEIN, W., & KORTE, F. (1975) Excretion, storage, and
metabolism of 2,3,6,2',4'-pentachlorobiphenyl-14-C after a
long-term feeding experiment on rats. Chemosphere, 4: 161-68.
LAY, J.P., KAMAL, M., KLEIN, W., & KORTE, F. (1979) Fate of
2.5.4'-trichlorobiphenyl in rats. Xenobiotica, 12: 713-721.
LEATHERLAND, J.F. & SONSTEGARD, R.A. (1978) Lowering of serum
thyroxine and triiodothyronine levels in yearling coho salmon,
Oncorhynchus kisutch, by dietary mirex and PCBs. J. Fish Res.
Board Can., 35: 1285-1289.
LEATHERLAND, J.F. & SONSTEGARD, R.A. (1979) Effect of dietary mirex
and PCB (Aroclor 1254) on thyroid activity and lipid reserves in
rainbow trout Salmo gairdneri Richardson. J. Fish Dis., 2: 43-48.
LEDERMAN, T.C. & RHEE, G.-Y. (1982) Bioconcentration of a
hexachlorobiphenyl in Great Lakes planktonic algae. Can. J. Fish.
aquat. Sci., 39: 380-387.
LEECE, B., DENOMME, M.A., TOWNER, R., & LI, S.M.A. (1985)
Polychlorinated biphenyls: correlation between in vivo and
in vitro quantitative structure-activity relationships (QSARs).
J. Toxicol. environ. Health, 16: 379-388.
LEES, P.S.J., CORN, M., & BREYSSE, P.N. (1987) Evidence for dermal
absorption as the major route of body entry during exposure of
transformer maintenance and repairmen to PCBs. Am. Ind. Hyg. Assoc.
J., 48(3): 257-264.
LEMMETYINEN, R., RANTAMAKI, P., & KARLIN, A. (1982) Levels of DDT
and PCB's in different stages of life cycle of the Arctic tern
Sterna paradisaea and the herring gull Larus argentatus.
Chemosphere, 11: 1059-1068.
LEONI, V., FABIANI, L., MARINELLI, G., PUCCETTI, G., TARSITANI,
G.F., DE CAROLIS, A., VESCIA, N., MORINI, A., ALEANDRI, V., POZZI,
V., CAPPA, F., & BARBATI, D. (1989) PCB and other organochlorine
compounds in blood of women with or without miscarriage: A
hypothesis of correlation. Ecotoxicol. environ. Saf., 17: 1-11.
LEVIN, B.D. & BOWMAN, R.E. (1983) Polychlorinated biphenyl effects
on delayed spatial alternation in monkeys. Toxicologist, 3: 68.
LEVIN, E.D., SCHANTZ, S.L., & BOWMAN, R.E. (1988) Delayed spatial
alteration deficits resulting from perinatal PCB exposure in
monkeys. Arch. Toxicol., 62: 267-273.
LEWIN, V., MCBLAIN, W.A., & WOLFE, F.H. (1972) Acute
intraperitoneal toxicity of DDT and PCBs in mice using two
solvents. Bull. environ. Contam. Toxicol., 8(4): 245-250.
LICHTENSTEIN, E.P., SCHULZ, K.R., FUHREMANN, T.W., & LIANG, T.T.
(1969) Biological interaction between plasticizers and
insecticides. J. econ. Entomol., 62: 761-765.
LICHTENSTEIN, E.P. (1972) PCBs and interactions with insecticides.
Environ. health Perspect., 1: 151-153.
LIEB, A.J., BILLS, D.D., & SINNHUBER, R.O. (1974) Accumulation of
dietary polychlorinated biphenyls (Aroclor 1254) by rainbow trout
(Salmo gairdneri). J. agric. food Chem., 22: 638-642.
LILLIE, R.J., HARRIS, S.J., CECIL, H.C., & BITMAN, J. (1974) Normal
reproductive performance of mature cockerels fed Aroclor 1248.
Poult. Sci., 53: 1604-1607.
LIN, F.S., HSIA, M.T., & ALLEN, J.R. (1979) Acute hepatotoxicity of
a tetrachlorobiphenyl-changes in the hepatocyte ultrastructure and
plasma membrane-bound enzymes. Arch. environ. Contam. Toxicol.,
8: 321-333.
LIN, P.S., NYQUIST, S.E., & HACLERODE, J. (1982) Interactions of
polychlorinated biphenyls and delta-9-tetrahydrocannabinol with
testosterone hydroxylation and the hepatic microsomal system in the
rat. Proc. Pa. Acad. Sci., 56: 31-35.
LINDER, R.E., GAINES, T.B., & KIMBROUGH, R.D. (1974) The effect of
polychlorinated biphenyls on rat reproduction. Food Cosmet.
Toxicol., 12: 63-76.
LINDSEY, A.S. & WAGSTAFFE, P.J. (1989) Production and certification
of ten high-purity polychlorinated biphenyls as reference
materials. Analyst, 114(5): 553-557.
LINZEY, A.V. (1987a) Effects of chronic polychlorinated biphenyls
exposure on the reproductive success of white-footed mice
(Peromyscus leucopus). Arch. environ. Contam. Toxicol.,
16: 455-460.
LINZEY, A.V. (1987b) Effects of chronic polychlorinated biphenyls
exposure on growth and reproduction of second generation
white-footed mice (Peromyscus leucopus). Arch. environ. Contam.
Toxicol., 17: 39-45.
LITTERST, C.L. & VAN LOON, E.J. (1974) Time-course of induction of
microsomal enzymes following treatment with polychlorinated
biphenyl. Bull. environ. Contam. Toxicol., 11: 206-212.
LITTERST, C.L., FARBER, T.M., BAKER, A.M., & VAN LOON, E.J. (1972)
Effect of polychlorinated biphenyls on hepatic microsomal enzymes
in the rat. Toxicol. appl. Pharmacol., 23: 112-122.
LIU, D. (1980) Enhancement of PCBs biodegradation by sodium
ligninsulfonate. Water Res., 14: 1467-1475.
LIU, D. (1981) Biodegradation of Aroclor 1221 type PCBs in sewage
wastewater. Bull. environ. Contam. Toxicol., 27: 695-703.
LIU, D. (1982) Assessment of continuous biodegradation of
commercial PCB formulations. Bull. environ. Contam. Toxicol.,
29: 200-207.
LLORENTE, G.A., FARRAN, A., RUIZ, X., & ALBAIGES, J. (1987)
Accumulation and distribution of hydrocarbons, polychlorobiphenyls,
and DDT in tissues of three species of Anatidae from the Ebro Delta
(Spain). Arch. environ. Contam. Toxicol., 16: 563-572.
LOOSE, L.D., PITTMAN, K.A., BENITZ, K.-F., & SILKWORTH, J.B. (1977)
Polychlorinated biphenyl and hexachlorobenzene induced humoral
immunosuppression. J. Reticulendothel. Soc., 22: 253-271.
LOOSE, L.D., SILKWORTH, J.B., PITTMAN, K.A., BENITZ, K.-F., &
MUELLER, W. (1978) Impaired host resistance to endotoxin and
malaria in polychlorinated biphenyl- and hexachlorobenzene-treated
mice. Infect. Immun., 20: 30-35.
LORENZ, H. & NEUMEIER, G. (1983) [Polychlorinated biphenyls (PCBs).
A joint report of the Federal Health Office and the Federal
Environment Office.] Munich, MMV Medizin Press (BGA Publication
No. 4/83) (in German).
LOWE, J.I., PARRISH, P.R., PATRICK, J.M., & FORESTER, J. (1972)
Effects of the polychlorinated biphenyl Aroclor 1254 on the
American oyster Crassostrea virginica. Mar. Biol., 17: 209-214.
LU, Y.-C & WONG P.-N (1984) Dermatological, medical and laboratory
findings of patients in Taiwan and their treatments. Am. J. ind.
Med., 5: 81-115.
LU, Y.-C. & WU, Y.-C. (1985) Clinical findings and immunological
abnormalities in Yu-Cheng patients. Environ. health Perspect.,
59: 17-29.
LUARD, E.J. (1973) Sensitivity of Dunaliella and Scenedesmus
(Chlorophyceae) to chlorinated hydrocarbons. Phycologia,
12: 29-33.
LUBET, R.A., LEMAIRE, B.N., AVERY, D., & KOURI, R.E. (1986)
Induction of immunotoxicity in mice by polychlorinated biphenyls.
Arch. Toxicol., 59: 71-77.
LUND, J., ANDERSSON, O., POELLINGER, L., & GUSTAFSSON, J.A. (1986)
In vitro characterization of possible mechanisms underlying the
selective in vivo accumulation of the PCB metabolite
4,4'-bis(methyl-sulphonyl)-2,5,2',5'-tetrachlorobiphenyl in the
lung. Food chem. Toxicol., 24(6/7): 563-566.
LUNDY, P., WURSTER, C.F., & ROWLAND, R.G. (1984) A two-species
marine algal bioassay for detecting aquatic toxicity of chemical
pollutants. Water Res., 18: 187-194.
LUNT, D. & EVANS, W.C. (1970) Microbial metabolism of biphenyl.
Biochem. J., 118: 54-55.
LUOTAMO, M., JÄRVISALO, J., & AITIO, A. (1985) Analysis of
polychlorinated biphenyls (PCBs) in human serum. Environ. health
Perspect., 60: 327-332.
LUTZ, R.J., DEDRICK, R.L., MATTHEWS, H.B., ELING, T., & ANDERSON,
M.W. (1977) A preliminary pharmacokinetic model for several
chlorinated biphenyls in rat. Drug Metab. Dispos., 5: 386-396.
LYNCH, T.R. & JOHNSON, H.E. (1982) Availability of a
hexachlorobiphenyl isomer to benthic amphipods from experimentally
contaminated natural sediments, Philadelphia, Pennsylvania,
American Society for Testing Materials, Aquatic Toxicology and
Hazard Assessment, pp. 273-287 (STP No. 76).
MAACK, L. & SONZOGNI, W.C. (1988) Analysis of polychlorobiphenyl
congeners in Wisconsin fish. Arch. environ. Contam. Toxicol.,
17: 711-719.
MAC, M.J. & SEELYE, J.G. (1981) Patterns of PCB accumulation by fry
of Lake Trout. Bull. environ. Contam. Toxicol., 27: 368-375.
MCCLURE, V.E. (1976) Transport of heavy chlorinated hydrocarbons in
the atmosphere. Environ. Sci. Technol., 10: 1223-1228.
MCCLURG, T.P. (1984) Trace metals and chlorinated hydrocarbons in
Ross seals from Antarctica. Mar. Pollut. Bull., 15: 384-389.
MCCONNELL, E.E., HASS, J.R., ALTMAN, N., & MOORE, J.A. (1979) A
spontaneous outbreak of polychlorinated biphenyl (PCB) toxicity in
Rhesus monkeys (Macaca mulatta): toxicopathology. Lab. anim.
Sci., 29: 666-673.
MCFARLAND, V.A. & CLARKE, J.U. (1989) Environmental occurrence,
abundance and potential toxicity of polychlorinated biphenyl
congeners: Considerations for a congener-specific analysis.
Environ. health Perspect., 81: 225-239.
MACKAY, D. (1989) Modeling the long-term behavior of an organic
contaminant in a large lake: Application to PCBs in Lake Ontario.
J. Great Lakes Res., 15(2): 283-297.
MCKINNEY, J.D. & SINGH, P. (1981) Structure-activity relationships
in halogenated biphenyls: unifying hypothesis for structural
specificity. Chem. biol. Interact., 33: 271-283.
MCKINNEY, J.D., CHAE, K., MCCONNELL, E.E., & BIRNBAUM, L.S. (1985)
Structure-induction versus structure-toxicity relationships for
polychlorinated biphenyls and related aromatic hydrocarbons.
Environ. Health Perspect., 60: 57-68.
MCKINNEY, J.D., KORACH, K.S., & MCLACHLAN, J.A. (1990)
Detoxification of polychlorinated biphenyls. January 27. Lancet,
335: 222-223.
MCLANE, M.A.R. & HUGHES, D.L. (1980) Reproductive success of
screech owls fed Aroclor 1248. Arch. environ. Contam. Toxicol.,
9: 661-665.
MCLEESE, D.W., METCALFE, C.D., & PEZZACK, D.S. (1980) Uptake of
PCBs from sediment by Nereis virens and Crangon septemspinosa.
Arch. environ. Contam. Toxicol., 9: 507-518.
MACLEOD, H.A., SMITH, D.C., & BLUMAN, N. (1980) Pesticide residues
in the total diet in Canada, V: 1976 to 1978. J. Food Saf.,
2: 141-164.
MCMANUS, G.B., WYMAN, K.D., PETERSON, W.T., & WURSTER, C.F. (1983)
Factors affecting the elimination of PCBs in the marine copepod
Acartia tonsa. Estuarine coastal Shelf Sci., 17: 421-430.
MCNULTY, W.P. (1985) Toxicity and fetotoxicity of TCDD, TCDF and
PCB isomers in Rhesus macaques (Macaca mulatta). Environ. Health
Perspect., 60: 77-88.
MCNULTY, W.P., BECKER, G.M., & CORY, H.T. (1980) Chronic toxicity
of 3,4,3',4'- and 2,5,2',5'-tetrachlorobiphenyls in Rhesus
macaques. Toxicol. appl. Pharmacol., 56: 182-190.
MACLEOD, K.E. (1981) Polychlorinated biphenyls in indoor air.
Environ. Sci. Technol., 15(8): 926-928.
MAFF (1986) Report of file working party on pesticide residues
(1982-1985): 16th report of the Steering Group on Food
Surveillance, London, Ministry of Agriculture, Fisheries, and Food,
Her Majesty's Stationery Office, (Food Surveillance Paper No. 16).
MAFF (1989) Migration of substances from food contact materials
into food: 26th report of the Steering Group on Food Surveillance.
Progress report of the Working Party on Chemical Contaminants from
Food, London, Ministry of Agriculture, Fisheries, and Food, Her
Majesty's Stationery Office, 58 pp (Food Surveillance Paper
No. 26).
MAHANTY, H.K. (1975) A study of the effects of polychlorinated
biphenyl (Aroclor 1242) on an aquatic plant Spirodela oligorrhiza
(Kurz) Hegelm. Bull. environ. Contam. Toxicol., 14: 558-565.
MAHANTY, H.K. & FINERAN, B.A. (1976) Effects of a polychlorinated
biphenyl (Aroclor 1242) on the ultrastructure of frond cells in the
aquatic plant Spirodela oligorrhiza (Kurz) Hegelm. N. Z. J. Bot.,
14: 13-18.
MAHANTY, H.K. & MCWHA, J.A. (1976) Sensitivity of Spirodela
oligorrhiza (Kurz) Hegelm. to a polychlorinated biphenyl (Aroclor
1242). N. Z. J. Bot., 14: 9-12.
MAKI, A.W. & JOHNSON, H.E. (1975) Effects of PCB (Aroclor 1254) and
p,p'DDT on production and survival of Daphnia magna Strauss.
Bull. environ. Contam. Toxicol., 13: 412-416.
MAKIURA, S., AOE, H., SUGIHARA, S., HIARO, K., ARAI, M., & ITO, N.
(1974) Inhibitory effect of polychlorinated biphenyls on liver
tumorigenesis in rats treated with 3'-methyl-4-dimethyl-
aminobenzene, N-2-fluorenylacetamide and diethylnitrosamine. J.
Natl Cancer Inst., 53: 1253-1257.
MALINS, D.C., MCCAIN, B.B., BROWN, D.W., VARANASI, U., KRAHN, M.M.,
MYERS, M.S., & CHAN, S.-L. (1987) Sediment-associated contaminants
and liver diseases in bottom-dwelling fish. Hydrobiologia,
149(1): 64-74.
MANCHESTER-NEESVIG, J.B. & ANDREN, A.W. (1989) Seasonal variation
in the atmospheric concentration of polychlorinated biphenyl
congeners. Environ. Sci. Technol., 23: 1138-1148.
MANSKE, D.D. & JOHNSON, R.D. (1975) Pesticide residues in total
diet samples (VIII). Pestic. monit. J., 9(2): 94-105.
MANSKE, D.D. & JOHNSON, R.D. (1977) Pesticide and other chemical
residues in total diet samples (X). Pestic. monit. J.,
10(4): 134-148.
MARCUS, J.M. & MATHEWS, T.D. (1987) Polychlorinated biphenyls in
blue crabs from South Carolina. Bull. environ. Contam. Toxicol.,
39(5): 857-862.
MARINUCCI, A.C. & BARTHA, R. (1982) Accumulation of the
polychlorinated biphenyl Aroclor 1242 from contaminated detritus
and water by the saltmarsh detritivore, Uca pugnax. Bull.
environ. Contam. Toxicol., 29: 326-333.
MARKARD, C. (1988) [Organic substances in sewage sludge - a danger
to the food chain?] Korresp. Abwasser, 35(5): 449-455 (in German).
MARKS, T.A., KIMMEL, G.L., & STAPLES, R.E. (1981) Influence of
symmetrical polychlorinated biphenyl isomers on embryo and fetal
development in mice I. Teratogenicity of 3,3',4,4',5,5'-
hexachlorobiphenyl. Toxicol. appl. Pharmacol., 61: 269-276.
MARONI, M., COLUMBI, A., CANTONI, S., FERIOLI, E., & FOA, V.
(1981a) Occupational exposure to polychlorinated biphenyls in
electrical workers. I. Environmental and blood polychlorinated
biphenyl concentrations. Br. J. ind. Med., 38(1): 49-54.
MARONI, M., COLUMBI, A., ARBOSTI, G., CANTONI, S., & FOA, V.
(1981b) Occupational exposure to polychlorinated biphenyls in
electrical workers II. Health effects. Br. J. ind. Med.,
38(1): 55-60.
MARONI, M., COLOMBI, A., FERIOLI, A., & FOA, V. (1984) Evaluation
of porphyrinogenesis and enzyme induction in workers exposed to
PCB. Med. Lav., 75(3): 188-199.
MARTINEAU, D., BELAND, P., DESJARDINS, C., & LAGACE, A. (1987)
Levels of organochlorine chemicals in tissues of beluga whales
(Delphinapterus leucas) from the St. Lawrence Estuary, Quebec,
Canada. Arch. environ. Contam. Toxicol., 16: 137-147.
MASSACHUSETTS DEPARTMENT OF PUBLIC HEALTH (1987) The greater New
Bedford PCB health effects study 1984-1987, Boston, Massachusetts,
Massachusetts Department of Public Health.
MASUDA, Y., KAGAWA, R., & KURATSUNE, M. (1972) Polychlorinated
biphenyls in carbonless copying paper. Nature (Lond.), 237: 41-42.
MASUDA, Y., KAGAWA, R., & KURATSUNE, M. (1974a) [Polychlorinated
biphenyls in Yusho patients and ordinary persons.] Fukuoka Acta
med., 65: 17-24 (in Japanese).
MASUDA, Y., KAGAWA, R., SHIMAMURA, K., TAKADA, M., & KURATSUNE, M.
(1974b) [Polychlorinated biphenyls in the blood of Yusho patients
and ordinary persons.] Fukuoka Acta. med., 65: 25-27 (in Japanese).
MASUDA, Y., KAGAWA, R., KUROKI, H., KURATSUNE, M., YOSHIMURA, T.,
TAKI, I., KUSUDA, M., YAMASHITA, F., & HAYASHI, M. (1978a) Transfer
of polychlorinated biphenyls from mothers to foetuses and infants.
Bull. environ. Contam. Toxicol., 16: 543-546.
MASUDA, Y., KAGAWA, R., TOKUDOME, S., & KURATSUNE, M. (1978b)
Transfer of polychlorinated biphenyls to the foetuses and offspring
of mice. Toxicology, 6: 331-340.
MASUDA, Y., KAGAWA, R., KUROKI, H., TOKUDOME, S. & KURATSUNE, M.
(1979) Transfer of various polychlorinated biphenyls to the fetuses
and offspring of mice. Food Cosmet. Toxicol., 17(6): 623-627.
MASUDA, Y., KUROKI, H., HARAGUCHI, K., & NAGAYAMA, J. (1985) PCB
and PCDF congeners in the blood and tissues of Yusho and Yu-Cheng
patients. Environ. health Perspect., 59: 53-58.
MATTHEWS, H.B. & ANDERSON, M.W. (1975a) Effect of chlorination on
the distribution and excretion of polychlorinated biphenyls. Drug
Metab. Dispos., 3: 371-380.
MATTHEWS, H.B. & ANDERSON, M.W. (1975b) The distribution and
excretion of 2,4,5,2',5'-pentachlorobiphenyl in the rat. Drug
Metab. Dispos., 3: 211-219.
MATTHEWS, H.B. & ANDERSON, M.W. (1976) PCB chlorination versus PCB
distribution and excretion. In: Proceedings of the National
Conference on Polychlorinated Biphenyls, Chicago, 19-21 November
1975, Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances, pp. 50-56 (EPA-560/6-75-004).
MATTHEWS, H.B. & DEDRICK, R.L. (1984) Pharmacokinetics of PCBs.
Annu. Rev. Pharmacol. Toxicol., 24: 85-103.
MATTHEWS, H.B. & TUEY, D.B. (1980) The effect of chlorine position
on the distribution and excretion of four hexachlorobiphenyl
isomers. Toxicol. appl. Pharmacol., 53: 377-388.
MATTSSON, R., MATTSSON, A., KIHLSTRÖM, J.E., & LINDAHL-KIESSLING,
K. (1981) Effects of a hexachlorinated biphenyl on lymphoid organs
and resorption of foetuses in pregnant mice. Arch. environ. Contam.
Toxicol., 10: 281-288.
MAUCK, W.L., MEHRLE, P.M., & MAYER, F.L. (1978) Effects of the
polychlorinated biphenyl Aroclor 1254 on growth, survival, and bone
development in brook trout (Salvelinus fontinalis). J. Fish Res.
Board Can., 35: 1084-1088.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Washington, DC, US Department of Commerce,
National Technical Information Service, 274 pp ((NTIS)
PB87-188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior,
Fish & Wildlife Service, pp. 506-553 (Resource Publication
No. 160).
MAYER, F.L, MEHRLE, P.M., & SANDERS, H.O. (1977) Residue dynamics
and biological effects of polychlorinated biphenyls in aquatic
organisms. Arch. environ. Contam. Toxicol., 5: 501-511.
MEHLMAN, M.A., YIN, L., & NIELSEN, R.C. (1974) Metabolic changes in
"frozen-clamped" livers of rats caused by ingestion of
polychlorinated biphenyls. Toxicol. appl. Pharmacol., 27: 300-307.
MEIER, P.G. & REDISKE, R.R. (1984) Oil and PCB interactions on the
uptake and excretion in midges. Bull. environ. Contam. Toxicol.,
33: 223-232.
MEIGS, J.W., ALBOM, J.J., & KARTIN, B.L. (1954) Chloracne from an
unusual exposure to Aroclor. J. Am. Med. Assoc., 154: 1417-1418.
MELVÅS, B. & BRANDT, I. (1973) The distribution and metabolism of
labelled polychlorinated biphenyls in mice and quails. In:
Proceedings of the Polychlorinated Biphenyls Conference II,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 87-90 (Publication No. 4E).
MENDOZA-FIGUEROA, T., LOPEZ-REVILLA, R., & VILLA-TREVINO, S. (1985)
Aroclor 1254 increases the genotoxicity of several carcinogens to
liver primary cell cultures. J. Toxicol. environ. Health,
15: 245-254.
MERKENS, L.S. & KINTER, W.B. (1971) Acute toxicity of a mixture of
polychlorinated biphenyls (Aroclor 1221) and DDT in a marine
teleost (Fundulus heteroclitus) and effect on serum osmolality,
Na+ and K+. Bull. Mt Desert Isl. biol. Lab., 11: 64-68.
MERSON, M.H. & KIRKPATRICK, R.L. (1976) Reproductive performance of
Captive White-footed mice fed a PCB. Bull. environ. Contam.
Toxicol., 16: 392-398.
MES, J. (1987) Polychlorobiphenyl in children's blood. Environ.
Res., 44: 213-220.
MES, J. & MARCHAND, L. (1987) Comparison of some specific
polychlorinated biphenyl isomers in human and monkey milk. Bull.
environ. Contam. Toxicol., 39: 736-742.
MES, J., COFFIN, D.E., & CAMPBELL, D. (1974) Polychlorinated
biphenyl and organochlorine pesticide residues in Canadian chicken
eggs. Pestic. monit. J., 8: 8-11.
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 28: 97-104.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine pesticides in
milk and blood of Canadian women during lactation. Arch. environ.
Contam. Toxicol., 13: 217-223.
MES, J., DAVIES, D.J., TURTON, D., & SUN, W.-F. (1986) Levels and
trends of chlorinated hydrocarbon contaminants in the breast milk
of Canadian women. Food Addit. Contam., 3(4): 313-322.
MES, J., ARNOLD, D.L., BRYCE, F., DAVIES, D.J., & KARPINSKI, K.
(1989a) The effect of long-term feeding of Aroclor 1254 to female
Rhesus monkeys on their polychlorinated biphenyl tissue levels.
Arch. environ. Contam. Toxicol., 18: 858-865.
MES, J., NEWSOME, W.H., & CONACHER, H.B.S. (1989b) Determination of
some specific isomers of polychlorinated biphenyl congeners in
fatty foods of the Canadian diet. Food Addit. Contam.,
6(3): 365-375.
MICHAELS, R.A., ROWLAND, G., & WURSTER, C.F. (1982) Polychlorinated
biphenyls (PCB) inhibit photosynthesis per cell in the marine
diatom Thalassiosira pseudonana. Environ. Pollut., 27: 9-14.
MILLER, D.L. & OGILVIE, D.M. (1975) Temperature selection in brook
trout (Salvelinus fontinalis) following exposure to DDT, PCB, or
phenol. Bull. environ. Contam. Toxicol., 14: 545-551.
MILLER, M.M., GHODBANE, S., WASIK, S.P., TEWARI, Y.B., & MARTIRE,
D.E. (1984) Aqueous solubilities, octanol/water partition
coefficients, and entropies of melting of chlorinated benzenes and
biphenyls. J. chem. Eng. Data, 29: 184-190.
MILLING, A., MULLER, W.F., COULSTON, F., & KORTE, F. (1979)
Comparative metabolism of 2,2'-dichlorobiphenyl-14C in mice, rats
and Rhesus monkeys after a single oral application. Chemosphere,
1: 15-19.
MINEAU, P., FOX, G.A., NORSTROM, R.J., WESELOH, D.V., HALLETT,
D.J., & ELLENTON, J.A. (1984) Using the herring gull to monitor
levels and effects of organochlorine contamination in the Canadian
Great Lakes. In: Nriagu, J.O., Nriagu, J.O., & Simmons, M.S., ed.
Toxic contaminants in the Great Lakes, New York, John Wiley and
Sons, pp. 425-452.
MIO, T. & SUMINO, K. (1985) Mechanism of biosynthesis of
methylsulfones from PCBs and related compounds. Environ. Health
Perspect., 59: 129-135.
MIO, T., SUMINO K., & MIZUTANI, T. (1976) Sulfur-containing
metabolites of 2,5,2',5'-tetrachlorobiphenyl, a major component of
commercial PCBs. Chem. pharm. Bull., 24: 1958-1960.
MIRANDA, C.L., HENDERSON, M.C., WANG, J.L., NAKAUE, H.S., & BUHLER,
D.R. (1987) Effects of polychlorinated biphenyls on porphyrin
synthesis and cytochrome P-450-dependent mono-oxygenase in small
intestine and liver of Japanese quail. J. Toxicol. environ. Health,
20: 27-35.
MIYATA, H., FUKUSHIMA, S., KASHIMOTO, T., & HUNITA, N. (1985) PCBs,
PCQs, and PCDFs in tissues of Yusho and Yu-Cheng patients. Environ.
Health Perspect., 59: 67-72.
MIYATA, H., TAKAYAMA, K., OGAKI, J., KASHIMOTO, T., & FUKUSHIMA, S.
(1987) Polychlorinated dibenzo- p-dioxins in blue mussel from
marine coastal water in Japan. Bull. environ. Contam. Toxicol.,
39(5): 877-883.
MIYAZAKI, A., HOTTA, T., KATAYAMA, J., & KIMURA, Y. (1975)
Absorption and translocation of PCB into crops. Bull. Osaka agric.
Res. Cent., 12: 135-142.
MIZUNOYA, Y., TANIGUCHI, S., KUSUMOTO, K., MORITA, S., YAMADA, A.,
BABA, T., & OGAKI, S. (1974) [Effects of polychlorinated biphenyls
on fetuses and offspring in rats.] J. Food Hyg. Soc. Jpn,
15: 252-260 (in Japanese).
MIZUTANI, T., HIDAKA, K., OHE, T., & MATSUMOTO, M. (1977) A
comparative study on accumulation and elimination of
tetrachlorobiphenyl isomers in mice. Bull. environ. Contam.
Toxicol., 18: 452-461.
MOILANEN, R., PYYSALO, H., WICKSTROM, K., & LINKO, R. (1982) Time
trends of chlordane, DDT, and PCB concentrations in pike (Esox
lucius) and Baltic herring (Clupea harengus) in the Turku
Archipelago, Northern Baltic Sea for the period 1971-1982. Bull.
environ. Contam. Toxicol., 29: 334-340.
MOKSNES, M.T. & NORHEIM, G. (1986) Levels of chlorinated
hydrocarbons and composition of PCB in herring gull Larus
argentatus eggs collected in Norway in 1969 compared to 1979-81.
Environ. Pollut., B11: 109-116.
MOORE, S.A. & HARRISS, R.C. (1972) Effects of polychlorinated
biphenyl on marine phytoplankton communities. Nature (Lond.),
240: 356-357.
MOORE, S.A. & HARRISS, R.C. (1974) Differential sensitivity to PCB
by phytoplankton. Mar. Pollut. Bull., 5: 174-176.
MORGAN, R.W., WARD, J.M., & HARTMAN, P.E. (1981) Aroclor
1254-induced intestinal metaplasia and adenocarcinoma in the
glandular stomach of F344 rats. Cancer Res., 41: 5052-5059.
MORIARTY, F. (1969) The effects of polychlorobiphenyls on
Chorthippus brunneus (Saltatoria: Acrididae). Entomol. Exp.
Appl., 12: 206-210.
MORITA, M., NAKAGAVA J., & RAPPE, C. (1978) Polychlorinated
dibenzofuran (PCDF) formation from PCB mixture by heat and oxygen.
Bull. environ. Contam. Toxicol., 19: 665-670.
MORSE, R.A., CULLINEY, T.W., GUTENMANN, W.H., LITTMAN, C.B., &
LISK, D.J. (1987) Polychlorinated biphenyls in honey bees. Bull.
environ. Contam. Toxicol., 38: 271-276.
MOSELEY, C.L., GERACI, C.L., & BURG, J. (1982) Polychlorinated
biphenyl exposure in transformer maintenance operations. Am. Ind.
Hyg. Assoc. J., 43: 170-174.
MOSSER, J.L., TENG, T., FISHERR, N.S., & WURSTER, C.F. (1972)
Polychlorinated biphenyls: Toxicity to certain phytoplankters.
Science, 175: 191-192.
MOSSER, J.L., TENG, T., WALTHER, W.G., & WURSTER, C.F. (1974)
Interactions of PCBs, DDT, and DDE in a marine diatom. Bull.
environ. Contam. Toxicol., 12: 665-668.
MOZA, P., WEISBERGER, I., KLEIN, W., & KORTE, F. (1974) Metabolism
of 2,2'-dichlorobiphenyl-14C in two plant-water-soil-systems. Bull.
environ. Contam. Toxicol., 12: 541-546.
MOZA, P., WEISBERGER, I., & KLEIN, W. (1976a) Fate of
2,2'-dichlorobiphenyl-14C in carrots, sugar beet, and soil under
outdoor conditions. J. agric. food Chem., 24: 881-885.
MOZA, P., KILZER, L., WEISGERBER, I., & KLEIN, W. (1976b)
Contribution to ecological chemistry CXV. Metabolism of
2,5,4'-trichlorobiphenyl-14C and 2,4,6,2',4'-pentachloro-
biphenyl-14C in the marsh plant Veronica beccabunga. Bull.
environ. Contam. Toxicol., 16: 454-463.
MOZA, P., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1979a) Studies
with 2,4,5-trichlorobiphenyl-14C and 2,2,4,4,6-penta-
chlorobiphenyl-14C in carrots, sugar, beet, and soil. J. agric.
food Chem., 27: 1120-1124.
MOZA, P.N., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1979b) Long-term
uptake of lower chlorinated biphenyls and their conversion products
by spruce trees (Picea abies) from soil treated with sewage
sludge. Chemosphere, 8: 373-375.
MROZEK, E. & LEIDY, R.B. (1981) Investigation of selective uptake
of polychlorinated biphenyls by Spartina alterniflora Loisel.
Bull. environ. Contam. Toxicol., 27: 481-488.
MROZEK, E., SENECA, E.D., & HOBBS, L.L. (1982) Polychlorinated
biphenyl uptake and translation by Spartina alterniflora Loisel.
Water Air Soil Pollut., 17: 3-15.
MROZEK, E., QUEEN, W.H., & HOBBS, L.L. (1983) Effects of
polychlorinated biphenyls on growth of Spartina alterniflora
Loisel. Environ. exp. Bot., 23: 285-292.
MUEHLEBACK, S. & BICKEL, M.H. (1981) Pharmacokinetics in rats of
2,4,5,2',4',5'-hexachlorobiphenyl an unmetabolisable lipophilic
model compound. Xenobiotica, 11: 249-259.
MUIR, D.C.G., NORSTROM, R.J., & SIMON, M. (1988) Organochlorine
contaminants in Arctic marine food-chains: Accumulation of specific
polychlorinated biphenyls and chlordane-related compounds. Environ.
Sci. Technol., 22: 1071-1077.
MULHERN, B.M., CROMARTIE, E., REICHEL, W.L., & BELISLE, A.A. (1971)
Semiquantitative determination of polychlorinated biphenyls in
tissue samples by thin layer chromatography. J. Assoc. Off. Agric.
Chem., 54: 548-550.
MULLER, W.F., HOBSON, W., FULLER, G.B., KNAUF, W., COULSTON, F., &
KORTE, F. (1978) Endocrine effects of chlorinated hydrocarbons in
Rhesus monkeys. Ecotoxicol. Environ. Saf., 2: 161-172.
MULLIN, M.D., POCHINI, C.M., MCCRINDLE, S., ROMKES, M., SAFE, S.H.,
& SAFE, L.M. (1984) High-resolution PCB analysis: Synthesis and
chromatographic properties of all 209 PCB congeners. Environ. Sci.
Technol., 18: 468-476.
MURADO, M.A., TEJEDOR, M.C., & BALUJA, G. (1976) Interactions
between polychlorinated biphenyls (PCBs) and soil microfungi.
Effects of Aroclor 1254 and other PCBs on Aspergillus flavus
cultures. Bull. environ. Contam. Toxicol., 15: 768-774.
MURAI, Y. & KUROIWA, Y. (1971) Peripheral neuropathy in
chlorobiphenyl poisoning. Neurology, 21: 1173-1176.
MURPHY, T.J. (1984) Atmospheric inputs of chlorinated hydrocarbons
to the Great Lakes. In: Nriagu, J.O., Nriagu, J.O., & Simmons,
M.S., ed. Toxic contaminants in the Great Lakes, New York, John
Wiley and Sons, pp. 53-79.
MURPHY, T.J., FORMANSKI, L.J., BROWNAWELL, B., & MEYER, J.A. (1985)
Polychlorinated biphenyl emissions to the atmosphere in the Great
Lakes region. Municipal landfills and incinerators. Environ. Sci.
Technol., 19(10): 942-946.
MUSSALO-RAUHAMAA, H., PYYSALO, H., & MOILANEN, R. (1984) Influence
of diet and other factors on the levels of organochlorine compounds
in human adipose tissue in Finland. J. Toxicol. environ. Health,
13: 689-704.
MUSSALO-RAUHAMAA, H., PYYSALO, H., & ANTERVO, K. (1988) Relation
between the content of organochlorine compounds in Finnish human
milk and characteristics of the mothers. J. Toxicol. environ.
Health, 25: 1-19.
NAGAI, J., FURUKAWA, M., YAE, Y., & IKEDA, Y. (1969)
[Clinicochemical investigation of chlorobiphenyls poisoning.
Especially on the serum lipid analysis of the patients.] Fukuoka
Acta med., 60: 475-488 (in Japanese).
NAGASAKI, H., TOMII, S., MEGA, T., SUGIHARA, S., MIYATA, Y., & ITO,
N. (1974) [Analysis of various factors on liver carcinogenesis in
mice induced by benzene hexachloride (BHC) and technical
polychlorinated biphenyls (PCBs).] J. Nara Med. Assoc., 25: 635-648
(in Japanese).
NAGASAKI, H., KAWABATA, H., MIYATA, Y., INOUE, K., AOE, H., & ITO,
N. (1975) Effects of various factors on induction of liver tumours
in animals by the alpha-isomer of benzenehexachloride. Gann,
66: 185-191.
NAGAYAMA, J. (1975) Chlorinated dibenzofurans in Kanechlors and
rice oils used by patients with Yusho. Fukuoka Acta med., 66: 593.
NAGAYAMA, L., KURATSUNE, M., & MASUDA, Y. (1976) Determination of
chlorinated dibenzofurans in Kanechlors and "Yusho oil". Bull.
environ. Contam. Toxicol., 15(1): 9-13.
NAGAYAMA, J., KURATSUNE, M., & MASUDA, Y. (1981) Formation of
polychlorinated dibenzofurans by heating polychlorinated biphenyls.
Fukuoka Acta med., 72: 136-141.
NAKANISHI, Y., SHIGEMATSU, N., KURITA, Y., MATSUBA, K., KANEGAE,
H., ISHIMURA, S., & KAWAZOE, Y. (1985) Respiratory involvement and
immune status in Yusho patients. Environ. health Perspect.,
59: 31-36.
NARBONNE, J.F. (1979) Determination of the "no effect level" of
Phenoclor DP6 on five microsomal parameters in rat livers.
Toxicology, 14: 91-93.
NARBONNE, J.F. (1980) Time course of induction of microsomal
enzymes following dietary administration of a polychlorinated
biphenyl (Phenoclor DP6). Toxicol. appl. Pharmacol., 56: 1-7.
NARBONNE, J.F., BOURDICHON, M., GALLIS, J.L., & DAUBEZE, M. (1978)
Polychlorinated biphenyls: effect of diet level on ATPase activity
in rats. Bull. environ. Contam. Toxicol., 20: 184-190.
NAS (1979) Polychlorinated biphenyls, Washington, DC, National
Academy of Sciences, 182 pp.
NAU-RITTER, G.M., WURSTER, C.F., & ROWLAND, R.G. (1982)
Partitioning of [14C]PCB between water and particulates with
various organic contents. Water Res., 16: 1615-1618.
NCI (1975) Third national cancer survey, Bethesda, Maryland,
National Cancer Institute.
NCI (1978) Bioassay of Aroclor (trademark) 1254 for possible
carcinogenicity (CAS 27323-18-8). National Cancer Institute
(Carcinogenesis Technical Report Series No. 38 - DHEW Publication
(NIH) 78-838).
NEBEKER, A.V. & PUGLISI, F.A. (1974) Effect of polychlorinated
biphenyls (PCBs) on survival and reproduction of Daphnia,
Gammarus, and Tanytarsus. Trans Am. Fish. Soc., 103: 722-728.
NEBEKER, A.V., PUGLISI, F.A., & DEFOE, D.L. (1974) Effect of
polychlorinated biphenyl compounds on survival and reproduction of
the fathead minnow and flagfish. Trans Am. Fish. Soc.,
103: 562-568.
NEFF, J.M. & GIAM, C.S. (1977) Effects of Aroclor 1016 and Halowax
1099 on juvenile horseshoe crabs Limulus polyphemus. In:
Vernberg, F.J., Calabrese, A., Thurberg, F.P., & Vernberg, W.B.,
ed. Physiological responses of marine biota to pollution, New York,
London, Academic Press, pp. 21-35.
NELSON, J.A. (1974) Effects of dichlorodiphenyltrichloroethane
(DDT) analogs and polychlorinated biphenyl (PCB) on 17
beta-[3H]estradiol binding to rat uterine receptor. Biochem.
Pharmacol., 23: 447-451.
NELSON, N.N., HAMMON, P.B., NISBET, I.C.T, SAROFIM, A.F., & DRURY,
W.H. (1972) Polychlorinated biphenyls - environmental impact.
Environ. Res., 5: 249-362.
NESNOW, S., LEAVITT, S., GARLAND, H., VAUGHAN, T.O., HYATT, B.,
MONTGOMERY, L., & CUDAK, C. (1981) Identification of cocarcinogens
and their potential mechanisms of action using C3H10T1/2CL8 mouse
embryo fibroblasts. Can. Res., 41: 3071-3076.
NESTEL, H. & BUDD, J. (1975) Chronic oral exposure of rainbow trout
(Salmo gairdneri) to a polychlorinated biphenyl (Aroclor 1254):
Pathological effects. Can. J. comp. Med., 39: 208-215.
NEWTON, I. (1979) The ecology of raptors, Berkhamsted, United
Kingdom, T. & A.D. Poyser, 399 pp.
NEWTON, I. & BOGAN, J. (1978) The role of different organo-chlorine
compounds in the breeding of British sparrowhawks. J. appl. Ecol.,
15: 105-116.
NEWTON, I. & HAAS, M.B. (in press) Long-term trends in
organochlorine and mercury residues in some raptorial and
fish-eating birds in Britain. Environ. Pollut.
NEWTON, I., BOGAN, J., MEEK, E., & LITTLE, B. (1982) Organochlorine
compounds and shell-thinning in British merlins Falco columbarius.
Ibis, 124: 328-335.
NEWTON, I., BOGAN, J.A., & ROTHERY, P. (1986) Trends and effects of
organochlorine compounds in sparrowhawk eggs. J. appl. Ecol.,
23: 461-478.
NEWTON, I., BOGAN, J.A., & HAAS, M.B. (1988) Organochlorine and
mercury in the eggs of British peregrine Falco peregrinus. Ibis,
131: 355-376.
NIESSEN, K.H., RAMOLLA, J., BINDER, M., BRÜGMANN, G., & HOFMANN, U.
(1984) Chlorinated hydrocarbons in adipose tissue of infants and
toddlers: Inventory and studies on their association with intake of
mother's milk. Eur. J. Pediatr., 142: 238-243.
NIEHS (NATIONAL INSTITUTE OF ENVIRONMENTAL HEALTH SCIENCES) (1985)
Potential health effects of polychlorinated biphenyls and related
persistent halogenated hydrocarbons: US Symposium, 12-14 September
1983. Environ. health Perspect., 60: 1-431.
NIIMI, A.J. & OLIVER, B.G. (1983) Biological half-lives of
polychlorinated biphenyl (PCB) congeners in whole fish and muscle
of rainbow trout (Salmo gairdneri). Can. J. Fish Aquat. Sci.,
40: 1388-1394.
NIIMI, A.J. & OLIVER, B.G. (1989a) Assessment of relative toxicity
of chlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in
Lake Ontario salmonids to mammalian systems using toxic equivalent
factors (TEF). Chemosphere, 18(7-8): 1413-1423.
NIIMI, A.J. & OLIVER, B.G. (1989b) Distribution of polychlorinated
biphenyl congeners and other halocarbons in whole fish and muscle
among Lake Ontario salmonids. Environ. Sci. Technol., 23: 83-88.
NILSEN, O.G. & TOFTGARD, R. (1981) Effects of polychlorinated
terphenyls and paraffins on rat liver microsomal cytochrome P-450
and in vitro metabolic activities. Arch. Toxicol., 47: 1-11.
NILSSON, B. & RAMEL, C. (1974) Genetic test on Drosophila
melanogaster with polychlorinated biphenyls (PCB). Hereditas,
77: 319-322.
NIMMO, D.R. & BAHNER, L.H. (1974) Some physiological consequences
of polychlorinated biphenyl- and salinity-stress in penaeid shrimp.
In: Vernberg, F.J. & Vernberg, W.B., ed. Pollution and physiology
of marine organisms, New York, London, Academic Press, pp. 427-443.
NIMMO, D.W.R. & BAHNER, L.H. (1976) Metals, pesticides and PCBs:
Toxicities to shrimp singly and in combination. In: Estuarine
processes. Proceedings of the 3rd International Estuarine Research
Conference, Galveston, Texas, October 1975, Estuarine Research
Federation, Vol. 1., pp. 523-532.
NIMMO, D.R., WILSON, P.D., BLACKMAN, R.R., & WILSON, A.J. (1971a)
Polychlorinated biphenyl absorbed from sediments by fiddler crabs
and pink shrimp. Nature, (Lond.), 231: 50-52.
NIMMO, D.R., BLACKMAN, R.R., WILSON, A.J., & FORESTER, J. (1971b)
Toxicity and distribution of Aroclor 1254 in the pink shrimp
Penaeus duorarum. Mar. Biol., 11: 191-197.
NIMMO, D.R., FORESTER, J., HEITMULLER, P.T., & COOK, G.H. (1974)
Accumulation of Aroclor 1254 in grass shrimp (Palaemonetes pugio)
in laboratory and field exposures. Bull. environ. Contam. Toxicol.,
11: 303-308.
NIMMO, D.R., HANSEN, D.J., COUCH, J.A., COOLEY, N.R., PARRISH,
P.R., & LOWE, J.I. (1975) Toxicity of Aroclor 1254 and its
physiological activity in several estuarine organisms. Arch.
environ. Contam. Toxicol., 3: 22-39.
NIOSH (1977) Criteria for a recommended standard. Occupational
exposure to polychlorinated biphenyls (PCBs), Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 224 pp
(NIOSH Publication No. 77-225).
NIOSH (1987) NIOSH manual of analytical methods: Polychlorinated
biphenyls - Method 5503, Cincinnati, Ohio, National Institute for
Occupational Safety and Health, pp. 5503-5503/4.
NISBET, I.C.T. & SAROFIM, A.F. (1972) Rates and routes of transport
of PCBs in the environment. Environ. health Perspect., 1: 21-38.
NISHIHARA, Y. (1983) Effects of polychlorinated biphenyls
(Kanechlor-400) on isolated rat liver mitochondria. Arch. environ.
Contam. Toxicol., 12: 517-522.
NISHIHARA, Y. (1985) Comparative study of the effects of biphenyl
and Kanechlor 400 on the respiratory and energy linked activities
of rat liver mitochondria. Br. J. ind. Med., 42: 128-132.
NISHIMOTO, T., UEDAM, M., TAUL, S., & CHIKAZAWA, K. (1972a)
[Organochlorine pesticide residues and PCB in breast milk.] Igaku
Ayumi, 82: 574-575 (in Japanese).
NISHIMOTO, T., UETA, M., TAUL, S., CHIKAZAURA, K., NISHIUCHI, I.,
& KONDO, K. (1972b) [Deposition of organochlorine pesticide
residues and PCB in human body fat.] Igaku Ayumi, 82: 515-516
(in Japanese).
NISHIZUMI, M. (1970) Light and electron microscope study of
chlorobiphenyl poisoning in mouse and monkey liver. Arch. environ.
Health, 21: 620-632.
NISHIZUMI, M. (1976) Enhancement of diethylnitrosomine
hepatocarcinogenesis in rats by exposure to polychlorinated
biphenyls or phenobarbital. Can. Lett., 2: 11-16.
NISHIZUMI, M. (1980) Reduction of diethylnitrosamine-induced
hepatoma in rats exposed to polychlorinated biphenyls through their
dams. Gann, 71(6): 910-912.
NISHIZUMI, M. (1985) Effect of PCBs on DMH-induced colon
tumorigenesis in rats. Fukuoka Acta med., 76: 204-207.
NISSEN, T.V. (1973) Stability of PCB in soil. Tidsskr. Planteavl,
77: 533-539.
NORBACK, D.H., & WELTMAN, R.H. (1985) Polychlorinated biphenyl
induction of hepatocellular carcinoma in the Sprague-Dawley rat.
Environ. health Perspect., 60: 97-105.
NORBACK, D.H., & ALLEN, J.R. (1972) Chlorinated aromatic
hydrocarbon induced modifications of the hepatic endoplasmic
reticulum: concentric membrane arrays. Environ. health Perspect.,
1: 137-143.
NORBACK, D.H., SEYMOUR, J.L., KNIERIEM, K.M., PETERSON, R.E., &
ALLEN, J.R. (1976) Biliary metabolites of 2,5,2',5'-tetrachloro-
biphenyl in rat. Res. Commun. chem. Pathol. Pharmacol., 14: 527-33.
NORBACK, D.H. MACK, E., BLOMQUIST, K.A., & ALLEN, J.R. (1978)
Metabolic study of 2,4,5,2',4',5'-hexachlorobiphenyl in rhesus
monkeys. Toxicol. appl. Pharmacol., 45: 331 (Abstract).
NOREN, K. (1983) Levels of organochlorine contaminants in human
milk from different parts of Sweden. Ambio, 12(1): 44-46.
NOREN, K. (1988) Changes in the levels of organochlorine
pesticides, polychlorinated biphenyls, dibenzo- p-dioxins and
dibenzofurans in human milk from Stockholm, 1972-1985. Chemosphere,
17(1): 39-49.
NOREN, K. & WESTOO, G. (1968) Determination of some chlorinated
pesticides in vegetable oils, margarine, butter, milk, eggs, meat
and fish by gas chromatography and thin-layer chromatography. Acta
chem. Scand., 22: 2289-2293.
NOREN, K., LUNDEN, A., SJOVALL, J., & BERGMAN, A. (in press)
Coplanar polychlorinated biphenyls in Swedish human milk.
Chemosphere.
NORSTROM, R.J., SIMON, M., MUIR, D.C.G., & SCHWEINSBURG, R.E.
(1988) Organochlorine contaminants in arctic marine food chains:
Identification, geographical distribution, and temporal trends in
polar bears. Environ. Sci. Technol., 22: 1063-1071.
OATMAN, L. & ROY, R. (1986) Surface and indoor air levels of
polychlorinated biphenyls in public buildings. Bull. environ.
Contam. Toxicol., 37: 461-466.
O'CONNORS, H.B., WURSTER, C.F., POWERS, C.D., BIGGS, D.C., &
ROWLAND, R.G. (1978) Polychlorinated biphenyls may alter trophic
pathways by reducing phytoplankton size and production. Science,
201: 737-740.
OECD (1982) Report on the implementation by member countries of the
decision by the Council on the Protection of the Environment by
control of polychlorinated biphenyls, Paris, Organisation of
Economic Co-operation and Development (ENV/CHEM/81.2).
OEHME, M., MANO, S., & MIKALSEN, A. (1987) Formation and presence
of polychlorinated and polycyclic compounds in the emissions of
small and large scale municipal waste incinerators. Chemosphere,
16(1): 143-153.
OESTERLE, D. & DEML, E. (1983) Promoting effects of polychlorinated
biphenyls on development of enzyme-altered islands in livers of
adult and weaning rats. J. Cancer Res. clin. Oncol., 105: 141-147.
OGISO, M., TOYOTA, I., IDO, Y., & INAGAKI, I. (1976) [Behavior of
14C-PCB in flooded soils II.] Aichi-Ken Nogyo Sogo Shikenjo Kenkyu
Hokoku, 8: 109-111 (in Japanese).
OHNISHI, Y. & KOHNO, T. (1979) Polychlorinated biphenyls poisoning
in monkey eye. Invest. Ophthalmol. visual Sci., 18: 981-984.
OISHI, S. & HIRAGA, K. (1980) Effect of polychlorinated biphenyl,
dibenzofuran and dibenzo- p-dioxin in the susceptibility of male
mice to endotoxin. J. environ. Sci. Health, B15(1): 77-85.
O'KEEFE, P.W., SILKWORTH, J.B., GIERTHY, J.F., SMITH, R.M.,
DECAPRIO, A.P., TURNER, J.N., EADON, G., HILKER, D.R., ALDOUS,
K.M., KAMINSKY, L.S., & COLLINS, D.N. (1985) Chemical and
biological investigation of a transformer accident at Binghamton,
NY. Environ. health Perspect., 60: 201-209.
OKLA, L. & LARSSON, P. (1987) Day-night differences in
volatilization rates of polychlorinated biphenyls from water to
air. Environ. Toxicol. Chem., 6: 659-662.
OKUMURA, M. (1984) Past and current medical states of Yusho
patients. Am. J. ind. Med., 5: 13-18.
OKUMURA, M. & KATSUKI, S. (1969) [Clinical observation on Yusho
(chlorobiphenyls poisoning).] Fukuoka Acta med., 60: 440-446
(in Japanese).
OKUMURA, M., MASUDA, Y., & NAKAMUTA, S. (1974) [Correlation between
blood PCB and serum glyceride levels in patients with PCB
poisoning.] Fukuoka Acta med., 65: 84-87 (in Japanese).
OLLING, C.C.Y. (1984) [PCB's in infant foods and other dairy
products.] Jeugdgezondheidszorg, 16(6): 91-93 (in Dutch).
OLOFFS, P.C., ALBRIGHT, L.J., SZETO, S.Y., & LAU, J. (1973) Factors
affecting the behavior of five chlorinated hydrocarbons in two
natural waters and their sediments. J. Fish Res. Board Can.,
30: 1619-1923.
OLOFSSON, S. & LINDAHL, P.E. (1979) Decreased fitness of cod
(Gadus morrhua L.) from polluted waters. Mar. environ. Res.,
2: 33-45.
OLSSON, M., KIHLSTROM, J.E., JENSEN, S., & ORBERG, J. (1979)
Cadmium and mercury concentrations in mink (Mustela vison) after
exposure to PCBs. Ambio, 8: 25.
OPPERHUIZEN, A., BENECKE, J.I., & PARSONS, J.R. (1987) Comment on
"Aqueous solubilities of six polychlorinated biphenyl congeners at
four temperatures". Environ. Sci. Technol., 21: 925-926.
OPPERHUIZEN, A., GOBAS, F.A.P.C., & VAN DER STEEN, J.M.D. (1988)
Aqueous solubility of polychlorinated biphenyls related to
molecular structure. Environ. Sci. Technol., 22: 638-646.
ORBERG, J. (1978) Effects of pure chlorobiphenyls (2,4',5-
trichlorobiphenyl and 2,2',4,4',5,5'-hexachlorobiphenyl) on the
reproductive capacity in female mice. Acta pharmacol. toxicol.,
42: 323-327.
ORBERG, J. & INGVAST, C. (1977) Effects of pure chlorobiphenyls
(2,4',5-trichlorobiphenyl or 2,2',4,4',5,5'-hexachlorobiphenyl) on
the disappearance of 14C from the blood plasma after intravenous
injection of 4-14C-progesterone and on the hepatic drug
metabolizing system in the female rat. Acta pharmacol. toxicol.,
41: 11-17.
ORBERG, J. & KIHLSTROM, J.E. (1973) Effects of long-term feeding of
polychlorinated biphenyls (PCB, Clophen A60) on the length of the
oestrus cycle and the frequency of implanted ova in the mouse.
Environ. Res., 6: 176-179.
ORBERG, J. & LUNDBERG, C. (1974) Some effects of DDT and PCB on the
hormonal system in the male mouse. Environ. Physiol. Biochem.,
4: 116-120.
O'SHEA, S.T.J., BROWNELL, R.L., CLARK, D.R., WALKER, W.A., GAY,
M.L., & LAMONT, T.G. (1980) Fish, wildlife, and estuaries.
Organochlorine pollutants in small cetaceans from the Pacific and
South Atlantic Oceans, November 1968-June 1976. Pestic. monit. J.,
14: 35-46.
OTA (1979) Environmental contaminants in food, Washington, DC,
Office of Technology Assessment (OTA/F-103).
OUW, H.K., SIMPSON, G.R., & SIYALI, D.S. (1976) Use and health
effects of Aroclor 1242, a PCB in an electrical industry. Arch.
environ. Health, 31: 189-196.
PAASIVIRTA, J. & LINKO, R. (1980) Environmental toxins in Finnish
wildlife. A study on time trends of residue contents in fish during
1973-1978. Chemosphere, 9: 643-661.
PAL, D., WEBER, J.B., & OVERCASH, M.R. (1980) Fate of
polychlorinated biphenyls (PCBs) in soil-plant systems. Residue
Rev., 74: 45-98.
PANTALEONI, G.C., FANINI, D., SPONTA, A.M., PALUMRO, G., GIORGI,
R., & ADAMS, P.M. (1988) Effects of maternal exposure to
polychlorobiphenyls (PCBs) on F1 generation behavior in the rat.
Fundam. appl. Toxicol., 11(3): 440-449.
PARKES, K. (1982) Occupational lung disorders, London, Butterworth.
PARKINSON, A. & SAFE, S. (1981) Aryl hydrocarbon hydroxylase
induction and its relationship to the toxicity of halogenated aryl
hydrocarbons. Toxicol. environ. Chem. Rev., 4: 1-46.
PARKINSON, A., ROBERTSON, L.W., & SAFE, S. (1980) Reconstituted
human breast milk. PCBs as potent inducers of aryl hydrocarbon
hydroxylase. Biochem. biophys. Res. Commun., 96: 882-889.
PARKINSON, A., THOMAS, P.E., RYAN, D.E., REIK, L.M., SAFE, S.H.,
ROBERTSON, L.W., & LEVIN, W. (1983) Immunochemical quantitation of
cytochrome P-450 isoenzymes and epoxidehydrolase in liver
microsomes from polychlorinated and polybrominated biphenyls. A
study of structure activity relationships. J. biol. Chem.,
285: 5967-5976.
PARRISH, P.R. (1973) Aroclor 1254, DDT and DDD, and dieldrin:
Accumulation and loss by American oysters (Crassostrea virginica)
exposed continuously for 56 weeks. Proc. Natl Shellfish Assoc.,
64: 7.
PARSONS, J.R. & SIJM, D.T.H.M. (1988) Biodegradation kinetics of
polychlorinated biphenyls in continuous cultures of a Pseudomonas
strain. Chemosphere, 17: 1755-1766.
PASSINO, D.R.M. & KRAMER, J.M. (1980) Toxicity of arsenic and PCBs
to fry of deepwater ciscoes (Coregonus). Bull. environ. Contam.
Toxicol., 24: 527-534.
PEAKALL, D.B. (1971) Effect of polychlorinated biphenyls (PCBs) on
the eggshells of ring doves. Bull. environ. Contam. Toxicol.,
6: 100-101.
PEAKALL, D.B. (1975) PCB's and their environmental effects. CRC
crit. Rev. environ. Control, 5: 469-508.
PEAKALL, D.B. & PEAKALL, M.L. (1973) Effect of a polychlorinated
biphenyl on the reproduction of artificially and naturally
incubated dove eggs. J. appl. Ecol., 10: 863-868.
PEAKALL, D.B., LINCER, J.L., & BLOOM, S.E. (1972) Embryonic
mortality and chromosomal alterations caused by Aroclor 1254 in
ring doves. Environ. health Perspect., 1: 103-104.
PEARCE, P.A., ELLIOT, J.E., PEAKALL, D.B., & NORSTRÖM, R.J. (1989)
Organochlorine contaminants in eggs of seabirds in the Northwest
Atlantic, 1968-1984. Environ. Pollut., 56: 217-235.
PEREIRA, M.A., HERREN, S.L., BRITT, A.L., & KHOURY, M.M. (1982)
Promotion by polychlorinated biphenyls of enzyme-altered foci in
rat liver. Cancer Lett., 15: 185-190.
PESENDORFER, H., VON, EICHLER, I., & GLOFKE, E. (1973) [Informative
analyses or organochlorine pesticide and PCB residues in human
adipose tissue (from the area of Vienna).] Wiener Klin.
Wochenschr., 85: 218-222 (in German).
PESENDORFER, H. (1975) [Residues of organochlorine pesticides (DDT,
etc.) and chlorinated biphenyls (PCBs) in breastmilk (from the
Vienna and Lower Austria region).] Wiener Kiln. Wochenschr.,
87(21): 732-736 (in German).
PETERSON, R.H. (1973) Temperature selection of atlantic salmon
(Salmo salar) and brook trout (Salvelinus fontinalis) as
influenced by various chlorinated hydrocarbons. J. Fish Res. Board
Can., 30: 1091-1097.
PHILLIPS, W.E.J., HATINA, G., VILLENEUVE, D.C., & GRANT, D.L.
(1972) Effect of parathion administration in rats following
long-term feeding with PCBs. Environ. Physiol. Biochem.,
2: 165-169.
PIENTA, R.J. (1980) Transformation of Syrian hamster embryo cells
by diverse chemicals and correlation with their reported
carcinogenic and mutagenic activities. In: de Serres, F.J. &
Hollaender, A., ed. Chemical mutagens: principles and methods for
their detection, Plenum Press, New York, London, Vol. 6,
pp. 175-202.
PINES, A., CUCOS, S., GRAFSTEIN, O., & LEMESCH, C. (1988) Changing
patterns of cow's milk contamination with organochlorine compounds
(1976-1986). Bull. environ. Contam. Toxicol., 40: 94-101.
PINKNEY, A.E., POJE, G.V., SANSUR, R.M., LEE, C.C., & O'CONNOR,
C.J.M. (1985) Uptake and retention of 14C-Aroclor 1254 in the
amphipod, Gammarus tigrinus, fed contaminated fungus, Fusarium
oxysporum. Arch. environ. Contam. Toxicol., 14: 59-64.
PLAPP, F.W. (1972) Polychlorinated biphenyl: An environmental
contaminant acts as an insecticide synergist. Environ. Entomol.,
1: 580-582.
PLATONOW, N.S. & CHEN, N.Y. (1973) Transplacental transfer of
polychlorinated biphenyls (Aroclor 1254) in a cow. Vet. Rec.,
92: 69-70.
PLATONOW, N.S. & FUNNELL, H.S. (1971) Anti-androgenic-like effect
of polychlorinated biphenyls in cockerels. Vet. Rec., 88: 109-110.
PLATONOW, N.S. & FUNNELL, H.S. (1972) The distribution and some
effects of polychlorinated biphenyls (Aroclor 1254) in cockerels
during prolonged feeding trial. Arch. environ. Contam. Toxicol.,
36: 89-93.
PLATONOW, N.S. & REINHART, B.S. (1973) The effects of
polychlorinated biphenyls (Aroclor 1254) on chicken egg production,
fertility, and hatchability. Can. J. comp. Med., 37: 341-346.
PLATONOW, N.S., LIPTRAP, R.M., & GEISSINGER, H.D. (1972) The
distribution and excretion of polychlorinated biphenyls (Aroclor
1254) and their effect on urinary gonadal steroid levels in the
boar. Bull. environ. Contam. Toxicol., 7: 358-365.
PLATONOW, N.S., MEADS, E.B., LIPTRAP, R.M., & LOTZ, F. (1976)
Effects of some commercial preparations of polychlorinated
biphenyls in growing piglets. Can. J. comp. Med., 40: 421-428.
POLAND, A. & GLOVER, E. (1977) Chlorinated biphenyl induction of
aryl hydrocarbon hydroxylase activity: a study of the
structure-activity relationship. Mol. Pharmacol., 13: 924-938.
POLAND, A., PALEN, D., & GLOVER, E. (1982) Tumour promotion by TCDD
in skin of HRS/J hairless mice. Nature (Lond.) 300: 271-273.
POMERANTZ, I., BURKE, J., FIRESTONE, D., MCKINNEY, J., ROACH, J.,
& TROTTER, W. (1978) Chemistry of PCBs and PBBs. Environ. health
Perspect., 24: 133-146.
PORTER, M.L., YOUNG, S.L.V., & BURKE, J.A. (1970) A method for the
analysis of fish, poultry and animal tissue for chlorinated
pesticide residues. J. Assoc. Off. Anal. Chem., 53: 1300-1303.
PORTMANN, J.E. (1970) Monitoring of organochlorine residues in fish
from around England and Wales, with special reference to
polychlorinated biphenyls, Charlottenlund, Denmark, International
Council for the Exploration of the Sea (Report CM 1970/E:9).
PORTMANN, J.E. & WILSON, K.W. (1971) The toxicity of 140 substances
to the brown shrimp and other marine animals. Shellfish lnf. Leafl.
MAFF, 22: 1-11.
POWERS, R.H., GILBERT, L.C., & AUST, S.D. (1987) The effect of
3,4,3',4'-tetrachlorobiphenyl on plasma retinol and hepatic retinyl
palmitate hydrolase activity in female Sprague-Dawley rats.
Toxicol. appl. Pharmacol., 89: 370-377.
PRECHTL, H.F.R. (1982) Assessment methods for the newborn infant;
a critical evaluation. In: Stratton, P., ed. Psychobiology of the
human new born, New York, Chichester, Brisbane, Toronto, John Wiley
and Sons, pp. 21-520.
PRESTON, B.D., VAN MILLER, J.P., MOORE, R.W., & ALLEN, J.R. (1981)
Promoting effects of polychlorinated biphenyls (Aroclor 1254) and
polychlorinated dibenzofuran-free Aroclor 1254 on diethyl-
nitrosamine-induced tumorigenesis in the rat and mouse. J. Natl
Cancer Inst. 66: 509-515.
PRESTON, B.D., MILLER, J.A., & MILLER, E.C. (1984) Reactions of
2,2',5,5',-tetrachlorobiphenyl 3,4-oxide with methionine, cysteine
and glutathione in relation to the formation of methylthio-
metabolites of 2,2',5,5'-tetrachlorobiphenyl in the rat and mouse.
Chem.-biol. Interact., 50: 289-312.
PRESTON, B.D., MILLER, E.C., & MILLER, J.A. (1985) The activities
of 2,2',5,5'-tetrachlorobiphenyl, its 3,4-oxide metabolite, and
2,2',4,4'-tetrachlorobiphenyl in tumour induction and promotion
assays. Carcinogenesis, 6: 451-453.
PRESTT, I., JEFFERIES, D.J., & MOORE, N.W. (1970) Polychlorinated
biphenyls in wild birds in Britain and their avian toxicity.
Environ. Pollut., 1: 3-26.
PRICE, H.A. & WELCH, R.L. (1972) Occurrence of polychlorinated
biphenyls in humans. Environ. Health Perspect., 1: 73-78.
PROBST, G.S., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K.,
& NEAL, S.B. (1981) Chemically-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: a comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutagen., 3: 11-32.
PUHVEL, S.M., SAKAMOTO, M., ERTL, D.C., & REISNER, R.M. (1982)
Hairless mice as models for chloracne: a study of cutaneous changes
induced by topical application of established chloracnegens.
Toxicol. appl. Pharmacol., 64: 492-503.
PUTTMANN, M., ARAND, M., OESCH, F., MANNSCHRECK, A., & ROBERTSON,
L. (1988) Chirality and the induction of xenobiotic-metabolizing
enzymes: Effects of the atropisomers of the polychlorinated
biphenyl 2,2',3,4,5',6-hexachlorobiphenyl. In: Holmstedt, B.,
Frank, H., & Testa, B., ed. Chirality and biological activity.
Proceedings of an International Symposium held at Tubingen, Federal
Republic of Germany, 5-8 April 1988, New York, Alan R. Liss, Inc.,
pp. 177-184.
QUAZI, S., TAKAHATA, M., HORIO, F., & YOSHIDA, A. (1984) Hepatic
3-hydroxy-3-methylglutaryl coenzyme A reductase and cholesterol
7-alpha-hydroxylase activities in rats fed PCB. Nutr. Rep. Int.,
30: 617-627.
QUENSEN, J.F., TIEDJE, J.M., & BOYD, S.A. (1988) Reductive
dechlorination of polychlorinated biphenyls by anaerobic
microorganisms from sediments. Science, 242: 752-754.
QURESHI, A.M. & ROBERTSON, H.E. (1987) Polychlorinated biphenyls
(PCB) in breast milk from Regina nursing mothers. Can. J. public
Health, 78: 389-392.
RANDS, P.L., WHITE, R.D., CARTER, M.W., ALLEN, S.D., & BRADSHAW,
W.S. (1982) Indicators of developmental toxicity following prenatal
administration of hormonally active compounds in the rat I.
Gestational length. Teratology, 25: 37-43.
RAO, C.V. & BANERJI, A.S. (1988a) Polychlorinated biphenyls in
human amniotic fluid. Bull. environ. Contam. Toxicol., 41: 798-801.
RAO, C.V. & BANERJI, A.S. (1988b) Induction of liver tumors in male
Wistar rats by feeding polychlorinated biphenyls (Aroclor 1260).
Cancer Lett., 39(1): 59-67.
RAPPE, C. (1985) PCB accident in France. In: Komai, R.Y. & Addis,
G., ed. Proceedings of a Seminar on Polychlorinated biphenyls, Palo
Alto, California, Electric Power Research Institute.
RAPPE, C., MARKLUND, S., BERGQVIST, P.A., & HANSSON, M. (1982)
Polychlorinated dioxins (PCDDs), dibenzofurans (PCDFs) and other
polynuclear aromatics (PCPNAs) found during PCB fires. Chem.
Scripta, 20: 56-61.
RAPPE, C., KJELLER L.-O., & MARKLUND, S. (1985a) PCDF isomers and
isomer levels found in PCBs. In: Komai, R.Y. & Addis, G., ed.
Proceedings of a Workshop on PCB By-Product Formation, 4-6 December
1984, Palo Alto, California, Electric Power Research Institute
(CS/EL-4101).
RAPPE, C., MARKLUND, S., KJELLER, L.-O., BERGQVIST, P.A., &
HANSSON, M. (1985b) Composition of PCDFs formed in PCB fires. In:
Keith, L.H., Rappe, C., & Choudary, G., ed. Symposium on
chlorinated dioxins and dibenzofurans in the total environment II,
Boston, Massachusetts, Butterworth Publishers, pp. 401-424.
RAPPE, C., MYGREN, M., MARKLUND, S., KJELLER, L.O., BERGQVIST,
P.A., & HANSSON, M. (1985c) Assessment of human exposure to
polychlorinated dibenzofurans and dioxins. Environ. health
Perspect., 60: 303-304.
RAPPE, C., NYGREN, M., LINDSTROM, G., BUSER, H.R., BLASER, O., &
WUTHRICH, C. (1987) Polychlorinated dibenzofurans and
dibenzo- p-dioxins and other chlorinated contaminants in cow milk
from various locations in Switzerland. Environ. Sci. Technol.,
21(10): 964-970.
REIJNDERS, P.J.H. (1986) Reproductive failure in common seals
feeding on fish from polluted coastal waters. Nature (Lond.),
324: 456-457.
REILLY, T.R., SUNDARESAN, S., & HIGHLAND, J.H. (1986). Cleanup of
PCB contaminated soils and sludges by a solvent extraction process:
A case study. Stud. Environ. Sci., 29: 125-139.
REINKE, J., UTHE, J.F., & O'BRODOVICH, H. (1973) Determination of
polychlorinated biphenyls in the presence of organochlorine
pesticides by thin-layer chromatography. Environ. Lett., 4:
201-210.
RENBERG, L., SUNDSTRÖM, G., & REUTHERGAARDH, L. (1981)
Polychlorinated terphenyls (PCT) in Swedish white-tailed eagles and
in grey seals. A preliminary study. Chemosphere, 6: 477-482.
REYNOLDS, L.M. (1971) Pesticide residue analysis in the presence of
polychlorobiphenyls (PCBs). Residue Rev., 34: 27-45.
REZNICEK, J. (1987) [Rapid determination of chlorinated pesticides
and polychlorinated biphenyls in the blood.] Prac. Lek.,
39: 185-189 (in Czech).
RICE, C.P. & WHITE, D.S. (1987) PCB availability assessment of
river dredging using caged clams and fish. Environ. Toxicol. Chem.,
6: 259-274.
RISEBROUGH, R.W. & ANDERSON, D.W. (1975) Some effects of DDE and
PCB on mallards and their eggs. J. Wildl. Manage., 39: 508-513.
RISEBROUGH, R.W. & DE LAPPE, B. (1972) Accumulation of
polychlorinated biphenyls in ecosystems. Environ. health Perspect.,
1: 39-45.
RISEBROUGH, R.W., RIECHE, P., PEAKALL, D.B., HERMAN, S.G., &
KIRVEN, M.N. (1968) Polychlorinated biphenyls in the global
ecosystem. Nature (Lond.), 220: 1098-1102.
ROBBIANO, L. & PINO. A. (1981) Induction in rats of liver DNA
single-strand breaks by the polychlorinated biphenyl Aroclor 1254.
Boll. Soc. Ital. Biol. Sper., 57: 407-413.
ROBERTS, D. (1975) The effect of pesticides on byssus formation in
the common mussel, Mytilus edulis. Environ. Pollut., 8: 241-254.
ROBERTSON, L.W., PARKINSON, A., BANDIERA, S., LAMBERT, I., MERRILL,
J., & SAFE, S. (1984) PCBs and PBBs: biologic and toxic effects on
C57BL/6J and DBA/2J inbred mice. Toxicology, 31: 191-206.
ROESIJADI, G., ANDERSON, J.W., GIAM, C.S., & PETROCELLI, S.R.
(1976a) Osmoregulation of the grass shrimp Palaemonetes pugio
exposed to polychlorinated biphenyls (PCBs). I. Effect on chloride
and osmotic concentrations and chloride and water-exchange
kinetics. Mar. Biol., 38: 343-355.
ROESIJADI, G., ANDERSON, J.W., & GIAM, C.S. (1976b) Osmoregulation
of the grass shrimp Palaemonetes pugio exposed to polychlorinated
biphenyls (PCBs). II. Effect on free amino acids of muscle tissue.
Mar. Biol., 38: 357-363.
ROGAN, W.J., GLADEN, B.C., MCKINNEY, J.D., CARRERAS, N., HARDY, P.,
THULLEN, J., TINGELSTAD, J., & TULLY, M. (1986) Polychlorinated
biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human
milk: Effects of maternal factors and previous lactation. Am. J.
public Health, 76(2): 172-177.
ROGAN, W.J., GLADEN, B.C., MCKINNEY, J.D., CARRERAS, N., HARDY, P.,
THULLEN, J., TINGELSTAD, J., & TULLY, M. (1987) Polychlorinated
biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human
milk: Effects on growth, morbidity and duration of lactation. Am.
J. public Health, 77: 1294-1297.
ROGAN, W.J., GLADEN, B.C., HUNG, K.-L., KOONG, S.-L., SHIH, L.-Y.,
TAYLOR, J.S., WU, Y.-C., YANG, D., RAGAN, N.B., & HSU, C.-C. (1988)
Congenital poisoning by polychlorinated biphenyls and their
contaminants in Taiwan. Science, 241: 334-336.
ROSIN, D.L. & MARTIN, B.R. (1981) Neurochemical and behavioural
effects of polychlorinated biphenyls in mice. Neurotoxicology,
2: 749-764.
ROSIN, D.L. & MARTIN, B.R. (1983) Comparison of the effects of
acute and sub-chronic administration of Aroclor 1254, a commercial
mixture of polychlorinated biphenyls on pentobarbital-induced sleep
time and [14C]pentobarbital disposition in mice. J. Toxicol.
environ. Health, 11: 917-931.
ROTE, J.W. & MURPHY, P.G. (1971) A method for the quantitation of
polychlorinated biphenyl (PCB) isomers. Bull. environ. Contam.
Toxicol., 6: 377-384.
RUBINSTEIN, N.I., LORES, E., & GREGORY, N.R. (1983) Accumulation of
PCBs, mercury and cadmium by Nereis virens, Mercenaria mercenaria
and Palaemonetes pugio from contaminated harbor sediments. Aquat.
Toxicol., 3: 249-260.
RUOFF, U., HEESCHEN, W., NIJHUIS, H., & BLÜTHGEN, A. (1988)
[Investigations on the significance of drinking water used by
cattle for the contamination of milk with polychlorinated biphenyls
(PCBs).] Kieler Milchwirtsch. Forschungsber., 40(2): 71-80
(in German).
RUZO, L.O., ZABIK, M.J., & SCHUETZ, R.D. (1972) Polychlorinated
biphenyls: Photolysis of 3,4,3',4'-tetrachlorobiphenyl and
4,4'-dichlorobiphenyl in solution. Bull. environ. Contam. Toxicol.,
8: 217-218.
RUZO, L.O., ZABIK, M.J., & SCHUETZ, R.D. (1974) Photochemistry of
bioactive compounds. Photochemical processes of polychlorinated
biphenyls. J. Am. Chem. Soc., 96: 3809-3813.
RUZO, L.O., SAFE, S., & ZABIK, M.J. (1975) Photodecomposition of
unsymmetrical polychlorobiphenyls. J. agric. food Chem.,
23: 594-595.
RYAN, D.E., THOMAS, P.E., KORZENIOWSKI, D., & LEVIN, W. (1979a)
Separation and characterization of highly purified forms of liver
microsomal cytochrome P-450 from rats treated with polychlorinated
biphenyls, phenobarbital and 3-methylcholanthrene. J. biol. Chem.,
254: 1365-1374.
RYAN, D.E., THOMAS, P.E., REIK, L.M., & LEVIN, W. (1979b)
Purification, characterization and regulation of five rat hepatic
cytochrome P-450 isoenzymes. Xenobiotica, 12: 727-744.
RYAN, D.E., THOMAS, P.E., & LEVIN, W. (1982) Purification and
characterization of a minor form of hepatic microsomal cytochrome
P-450 from rats treated with polychlorinated biphenyls. Arch.
Biochem. Biophys., 216: 272-288.
SAFE, S. (1982) Halogenated hydrocarbons and arylhydrocarbons
identified in human tissues. Toxicol. environ. Chem., 5: 153-165.
SAFE, S. (1984) Polychlorinated biphenyls (PCBs) and polybrominated
biphenyls (PBBs): biochemistry, toxicology, and mechanism of
action. CRC crit. Rev. Toxicol., 13: 319-395.
SAFE S. (in press) Polychlorinated biphenyls (PCBs),
dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs) and related
compounds: Environmental and mechanistic considerations which
support the development of toxic equivalency factors (TEfs). CRC
crit. Rev. Toxicol.
SAFE, S. & HUTZINGER, O. (1971) Polychlorinated biphenyls:
photolysis of 2,4,6,2'4',6'-hexachlorobiphenyl. Nature (Lond.),
232: 641-642.
SAFE, S., HUTZINGER, O., & ECOBICHON, D. (1974) Identification of
a 4-chloro-4'-hydroxybiphenyl and 4-4'-dichloro-3-hydroxybiphenyl
as metabolites of 4-chloro-and 4,4'-dichlorobiphenyl fed to rats.
Experientia (Basel), 30: 720-721.
SAFE, S., HUTZINGER, O., ECOBICHON, D., & GREY, A.A. (1975) The
metabolism of 4'-chloro-4-biphenylol in the rat. Can. J. Biochem.,
53: 415-20.
SAFE, S., SAFE, L., & MULLIN, M. (1985a) Polychlorinated biphenyls:
Congener-specific analysis of a commercial mixture and human milk.
J. agric. food Chem., 33: 24-29.
SAFE, S., BANDIERA, S., SAWYER, T., ROBERTSON, L., SAFE, L.,
PARKINSON, A., THOMAS, P.E., RYAN, D.E., REIK, L.M., LEVIN, W.,
DENOMME, M.A., & FUJITA, T. (1985b) PCBs: structure-function
relationships and mechanism of action. Environ. health Perspect.,
60: 47-56.
SAGER, D.B. (1983) Effect of postnatal exposure to polychlorinated
biphenyls on adult male reproductive function. Environ. Res.,
31: 76-94.
SAGER, D.B., SHIH-SCHROEDER, W., & GIRARD, D. (1987) Effect of
early postnatal exposure to polychlorinated biphenyls PCBs) on
fertility in male rats. Bull. environ. Contam. Toxicol.,
38: 946-953.
ST. AMANT, J.R., PARISO, M.E., & SHEFFY, T.B. (1984)
Polychlorinated biphenyls in seven species of Lake Michigan fish,
1971-1981. In: Nriagu, J.O., Nriagu, J.O. & Simmons, M.S., ed.
Toxic contaminants in the Great Lakes; New York, John Wiley and
Sons, pp. 311-319.
SAITO, R., SHIGEMATSU, N., & ISHIMARU, S. (1972) [Immunoglobulin
levels in serum and sputum of patients with PCB poisoning.] Fukuoka
Acta med., 63: 408-411 (in Japanese).
SAITO, M., IKEGAMI, S., ITO, Y., & INNAMI, S. (1982) Influence of
dietary anti-oxidants on polychlorinated biphenyls (PCBs)- induced
hepatic lipid peroxide formation and vitamin A reduction in rats.
J. nutr. Sci. Vitaminol., 28: 455-466.
SAITO, M., IKEGAMI, S., NISHIDE, E., & INNAMI, S. (1983) Relevances
of mixed function oxidase system and ascorbic acid to the lipid
peroxide formation in the liver of rats given polychlorinated
biphenyls (PCB). Fukuoka Acta med., 74: 222-233.
SANDBERG, P.-O. & GLAUMANN, H. (1980) Studies on the cellular
toxicity of polychlorinated biphenyls (PCBs). Partial block and
alteration of intracellular migration of lipoprotein particles in
rat liver. Exp. mol. Pathol., 32: 1-22.
SANDERS, H.O. & CHANDLER, J.H. (1972) Biological magnification of
a polychlorinated biphenyl (Aroclor 1254) from water by aquatic
invertebrates. Bull. environ. Contam. Toxicol., 7: 257-263.
SANDERS, O.T. & KIRKPATRICK, R.L. (1975) Effects of a
polychlorinated biphenyl (PCB) on sleeping times, plasma
corticosteroids, and testicular activity of White-footed mice.
Environ. Physiol. Biochem., 5: 308-313.
SANDERS, O.T., ZEPP, R.L., & KIRKPATRICK, R.L. (1974) Effect of PCB
ingestion on sleeping time, organ weights, food consumption, serum
corticosterone and survival of albino mice. Bull. environ. Contam.
Toxicol., 12(4): 394-399.
SANDERS, O.T., KIRKPATRICK, R.L., & SCANLON, P.E. (1977)
Polychlorinated biphenyls and nutritional restriction: their
effects and interactions on endocrine and reproductive
characteristics of male white mice. Toxicol. appl. Pharmacol.,
40: 91-98.
SANGALANG, G.B., FREEMAN, H.C., & CROWELL, R. (1981) Testicular
abnormalities in cod (Gadus morhua) fed Aroclor 1254. Arch.
environ. Contam. Toxicol., 10: 617-626.
SANO, S., KAWANISHI, S., & SEKI, Y. (1985) Toxicity of
polychlorinated biphenyl with special reference to porphyrin
metabolism. Environ. health Perspect., 59: 137-143.
SARGENT, L., ROLOFF, B., & MEISNER, L. (1989) In vitro chromosome
damage due to PCB interactions. Mutat. Res., 224: 79-88.
SASCHENBRECKER, P.W., FUNNELL, H.S., & PLATONOW, N.S. (1972)
Persistence of polychlorinated biphenyls in the milk of exposed
cows. Vet. Rec., 90: 100-102.
SAWAI, T. & SAWAI, T. (1973) [Photosensitized chain dechlorination
reaction of polychlorinated biphenyls (PCB) in alkaline 2-propanol
solutions.] Kogai, 8: 97-105 (in Japanese).
SAWHNEY, B.L. & HANKIN, L. (1984) Plant contamination by PCBs from
amended soils. J. food Prot., 47: 232-236.
SAWHNEY, B.L. & HANKIN, L. (1985) Polychlorinated biphenyls in
food: A review. J. food Prot., 48(5): 442-448.
SAWYER, T. & SAFE, S. (1982) PCB isomers and congeners: induction
of aryl hydrocarbon hydroxylase and ethoxyresorufin O-deethylase
enzyme activities in rat hepatoma cells. Toxicol. Lett., 13: 87-94.
SAWYER, T.W., VATCHER, A.D., & SAFE, S. (1984) Comparative aryl
hydrocarbon hydroxylase induction activities of commercial PCBs in
Wistar rats and rat hepatoma H-4-II E cells in culture.
Chemosphere, 13: 695-701.
SAYLER, G.S., SHON, M., & COLWELL, R.R. (1977) Growth of an
estuarine Pseudomonas sp. on polychlorinated biphenyl. Microb.
Ecol., 3: 241-255.
SCHAEFFER, E., GREIM, H., & GOESSNER, W. (1984) Pathology of
chronic polychlorinated biphenyl (PCB) feeding in rats. Toxicol.
appl. Pharmacol., 75: 278-288.
SCHECTER, A. (1987) Transient liver pathology in patients consuming
water from a private well contaminated by PCBs from a submersible
water pump. Chemosphere, 16(1): 37-42.
SCHECTER, A. & TIERNAN, T. (1985) Occupational exposure to
polychlorinated dioxins, polychlorinated furans, polychlorinated
biphenyls and biphenylenes after an electrical panel and
transformer accident in an office in Binghamton, N Y. Environ.
health Perspect., 60: 305-313.
SCHECTER, A., TIERNAN, T., SCHAFFNRER, F., TAYLOR, M., GITLITZ, G.,
VAN NESS, G.F., GARRETT, J.H., & WAGEL, D.J. (1985) Patient fat
biopsies for chemical analysis and liver biopsies for
ultrastructural characterization after exposure to polychlorinated
dioxins, furans, and PCBs. Environ. health Perspect., 60: 241-254.
SCHECTER, A., FURST, P., KRÜGER, C., MEEMKEN, H.A., GROEBEL, W., &
CONSTABLE, J.D. (1989a) Levels of polychlorinated dibenzofurans,
dibenzodioxins, PCBs, DDT and DDE, hexachlorobenzene (HCB),
dieldrin, hexachlorocyclohexane and oxychlordane in human breast
milk from the United States, Thailand, Vietnam, and Germany.
Chemosphere, 18(1/6): 445-454.
SCHECTER, A., MES, J., & DAVIES, D. (1989b) Polychlorinated
biphenyl (PCB), DDT, DDE and hexachlorobenzene (HCB) and PCDD/F
isomer levels in various organs in autopsy tissue from North
American patients. Chemosphere, 18(1/6): 811-818.
SCHIMMEL, S.C., HANSEN, D.J., & FORESTER, J. (1974) Effects of
Aroclor 1254 on laboratory-reared embryos and fry of sheepshead
minnows (Cyprinodon variegatus). Trans Am. Fish Soc.,
103: 582-586.
SCHMIDT, T.T., RISEBROUGH, R.W., & GRESS, F. (1971) Input of
polychlorinated biphenyls into California coastal waters from urban
sewage outfalls. Bull. environ. Contam. Toxicol., 6: 235-243.
SCHMITT, C.J., RIBICK, M.A., LUDKE, J.L., & MAY, T.W. (1983)
Organochlorine residues in freshwater fish, 1976-1979: National
pesticide monitoring program, Washington DC, US Department of the
Interior, Fish and Wildlife Service, 62 pp (Resource Publication
No. 152).
SCHMITT, C.J., ZAJICEK, J.L., & RIBICK, M.A. (1985) National
pesticide monitoring program: residues of organochlorine chemicals
in freshwater fish, 1980-81. Arch. environ. Contam. Toxicol.,
14: 225-260.
SCHMOLDT, A., BENTHE, H.F., & FRUEHLING, R. (1974) Induction of rat
liver enzymes by polychlorinated biphenyls (PCBs) in dependence on
the dose and chlorine content. Arch. Toxicol., 32: 69-81.
SCHNELLMAN, R.G., VOLP, R.F., PUTNAM, C.W., & SIPES, I.G. (1984a)
The hydroxylation, dechlorination and glucuronidation of
4,4',-dichlorobiphenyl by human hepatic microsomes. Biochem.
Pharmacol., 33: 3503-3509.
SCHNELLMAN, R.G., PUTNAM, C.W., & SIPES, I.G. (1984b) Metabolism of
2,2',3,3',6,6'-hexachlorobiphenyl and 2,2',4,4',5,5',-hexachloro-
biphenyl by human hepatic microsomes. Biochem. Pharmacol.,
32: 3233-3239.
SCHOENY, R. (1982) Mutagenicity testing of chlorinated biphenyl and
chlorinated dibenzofurans. Mutat. Res., 101: 45-56.
SCHOENY, R., SMITH, C.C., & LOPER J.C. (1979) Non-mutagenicity for
Salmonella of the chlorinated hydrocarbons Aroclor 1254,
1,2,4-trichlorobenzene, Mirex and kepone. Mutat. Res., 68: 125-132.
SCHULTE, E. & MALISCH, R. (1984) Calculation of the real PCB
content in environmental samples. II. Gas chromatographic
determination of the PCB concentration in human milk and butter.
Fresenius Arch. anal. Chem., 319: 54-59.
SCHWARTZ, L. (1943) An outbreak of halowax acne ("cable rash")
among electricians. J. Am. Med. Assoc., 122: 158-161.
SCHWARTZ, P.M., JACOBSON, S.W., FEIN, G.G., JACOBSON, J.L., &
PRICE, H.A. (1983) Lake Michigan fish consumption as a source of
polychlorinated biphenyls in human cord serum, maternal serum and
milk. Am. J. public Health, 73(3): 293-296.
SCHWARTZ, T.R., STALLING, D.L., & RICE, C.L. (1987) Are
polychlorinated biphenyl residues adequately described by Aroclor
mixture equivalents? Isomer-specific principal components analysis
of such residues in fish and turtles. Environ. Sci. Technol.,
21: 72-76.
SCOTT, M.L. (1977) Effects of PCBs, DDT and mercury compounds in
chickens and Japanese quail. Fed. Proc. Am. Soc. Exp. Biol.,
36: 1888-1893.
SCOTT, M.L., VADEHRA, D.V., MULLENHOFF, P.A., RUMSEY, G.L., & RICE,
R.W. (1971) Results of experiments on the effects of PCBs on laying
hen performance. Proceedings of the Cornell Nutrition Conference,
pp. 56.
SCOTT, M.L., ZIMMERMAN, J.R., MARINSKY, S., MULLENHOFF, P.A.,
RUMSEY, G.L., & RICE, R.W. (1975) Effects of PCBs, DDT and mercury
compounds upon egg production, hatchability and shell quality in
chickens and Japanese quail. Poult. Sci., 54: 350-368.
SEEGAL, R.F., BUSH, B., & BROSCH, K.O. (1985) Polychlorinated
biphenyls induce regional changes in brain norepinephrine
concentrations in adult rats. Neurotoxicology, 6: 13-24.
SEKI, Y., KAWANISHI, S., & SANO, S. (1987) Role of inhibition of
uroporphyrinogen decarboxylase in PCB-induced porphyria in mice.
Toxicol. appl. Pharmacol., 90: 116-125.
SEPPALAINEN, A.M., VUOJOLAHTI, P., & ELO, O. (1985) Reversible
nerve lesions after accidental polychlorinated biphenyl exposure.
Scand. J. Work Environ. Health, 11: 91-95.
SEYMOUR, M.P., DUNCAN, I.W., JEFFERIES, T.M., & NOTARIANNI, L.J.
(1986a) Clean-up and separation of chlorobiphenyl isomers after
synthesis by Cadogan coupling using preparative high-performance
liquid chromatography. J. Chromatogr., 368: 174-179.
SEYMOUR, M.P., JEFFERIES, T.M., & NOTARIANNI, L.J. (1986b)
Large-scale separation of lipids from organochlorine pesticides and
polychlorinated biphenyls using a polymeric high-performance liquid
chromatographic column. Analyst, 111: 1203-1205.
SEYMOUR, M.P., JEFFERIES, T.M., & NOTARIANNI, L.J. (1986c)
Limitations in the use of nickel boride dechlorination for the
analysis of polychlorinated biphenyls. Bull. environ. Contam.
Toxicol., 37: 199-206.
SEYMOUR, M.P., JEFFERIES, T.M., FLOYD, A.J., & NOTARIANNI, L.J.
(1987) Routine determination of organochlorine pesticides and
polychlorinated biphenyls in human milk using capillary gas
chromatography - mass spectrometry. Analyst, 112: 427-431.
SHAHIN, M.M., ANDRILLON, P., GOETZ, N., BORE, P., BUGAUT, A., &
KALOPISSIS, G. (1979) Studies on the mutagenicity of
p-phenylenediamine in Salmonella typhimurium: Presence of PCBs
in rat-liver microsomal fraction induced by Aroclor. Mutat. Res.,
68: 327-336.
SHALAT, S.L., TRUE, L.D., FLEMING, L.E., & PACE, P.E. (1989) Kidney
cancer in utility workers exposed to polychlorinated biphenyls
(PCBs). Br. J. ind. Med., 46: 823-824.
SHAW, G.R. & CONNELL, D.W. (1982) Factors influencing
polychlorinated biphenyls in organisms from an estuarine ecosystem.
Aust. J. mar. freshwater Res., 33: 1057-1070.
SHELTON, D.W., COULOMBE, R.A., PEREIRA, C.B., CASTEEL, J.L., &
HENDRICKS, J.D. (1984a) Inhibitory effect of Aroclor 1254 on
aflatoxin-initiated carcinogenesis in rainbow trout and mutagenesis
using Salmonella-trout hepatic activation system. Aquat. Toxicol.,
3: 229-238.
SHELTON, D.W., HENDRICKS, J.D., COULOMBE, R.A., & BAILEY, G.S.
(1984b) Effects of dose on the inhibition of
carcinogenesis/mutagenesis by Aroclor 1254 in rainbow trout fed
aflatoxin B1. J. Toxicol. environ. Health, 13: 659-667.
SHELTON. D.W., GOEGER, D.E., HENDRICKS, J.D., & BAILEY, G.S. (1986)
Mechanisms of anticarcinogenesis: the distribution and metabolism
of aflatoxin B1 in rainbow trout fed Aroclor 1254. Carcinogenesis,
7: 1065-1071.
SHIGEMATSU, N., NORIMATSU, Y., ISHIBASHI, T., YOSHIDA, M.,
SUETSUGU, S., KAWATSU, T., IKEDA, T., SAITO, R., ISHIMURA, S.,
SHIRAKISA, T., KIDO M., EMORI, K., & TOSHIMITSU, H. (1971)
[Clinical and experimental studies on respiratory involvement in
chlorobiphenyls poisoning.] Fukuoka Acta med., 62: 150-156
(in Japanese).
SHIGEMATSU, N., ISHIMANU, S., SAITO, R., IKEDA, T., MATSUBA, K.,
SUGIYAMA, K., & MASUDA, Y. (1978) Respiratory involvement in
polychlorinated biphenyls poisoning. Environ. Res., 16: 92-100.
SHIMADA, T. & UGAWA, M. (1978) Induction of liver microsomal drug
metabolism by polychlorinated biphenyls whose gas chromatographic
profile having much in common with that in human milk. Bull.
environ. Contam. Toxicol., 19: 198-205.
SHIMADA, T. & SAWABE, Y. (1984) Comparative studies on distribution
and covalent tissue binding of 2,4,2',4'- and 3,4,3',4'-tetra-
chlorobiphenyl isomers in the rat. Arch. Toxicol., 55: 182-185.
SHIOTA, K. (1976a) Embryotoxic effects of polychlorinated biphenyls
(Kanechlors 300 and 500) in rats. Okajimas Folia anat. Jpn.,
53: 93-104.
SHIOTA, K. (1976b) Postnatal behavioural effects of prenatal
treatment with PCBs (Polychlorinated biphenyls) in rats. Okajimas
Folia anat. Jpn., 53: 105-114.
SHIRAI, T., MIYATA, Y., NAKANISHI, K., MURASAKI, G., & ITO, N.
(1978) Hepatocarcinogenicity of polychlorinated terphenyl (PCT) in
ICR mice and its enhancement by hexachlorobenzene (HCB). Cancer
Lett., 4: 271-275.
SHIREMAN, R.B. (1988) Lipoprotein-mediated transfer of
2,4,5,2',4',5'-hexachlorobiphenyl into cultured human cells.
Xenobiotica, 18(4): 449-457.
SHIU, W.Y. & MACKAY, D. (1986) A critical review of aqueous
solubilities, vapor pressures, Henry's law constants and
octanol-water partition coefficients of the polychlorinated
biphenyls. J. Phys. Chem. Ref. Data, 15(2): 911-929.
SHULL, L.R., BLEAVINS, M.R., OLSON, B., & AULERICH, R.J. (1982)
Polychlorinated biphenyls (Aroclors 1016 and 1242): effect on
hepatic microsomal mixed function oxidases in mink and ferrets.
Arch. environ. Contam. Toxicol., 11: 313-321.
SILBERGELD, E.K. (1983) Health effects of PCBs: Occupational
exposure. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of the PCB Seminar, The Hague, 28-30 September 1983,
The Hague, Ministry of the Environment, pp. 136-151.
SILKWORTH, J.B. & GRABSTEIN, E.M. (1982) Polychlorinated biphenyl
immunotoxicity: dependence on isomer planarity and the Ah gene
complex. Toxicol. appl. Pharmacol., 65: 109-115.
SILKWORTH, J.B. & LOOSE, L.D. (1979) Environmental chemical-induced
modification of cell-mediated immune response. Adv. exp. Med.
Biol., 121A: 499-522.
SILKWORTH, J.B., ANTRIM, L., & KAMINSKY, L.S. (1984) Correlations
between polychlorinated biphenyl immunotoxicity, the aromatic
hydrocarbon locus and liver microsomal enzyme induction in C57BL/6
and DBA/2 mice. Toxicol. appl. Pharmacol., 75: 156-165.
SINA, J.F., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY, M.O.
(1983) Evaluation of the alkaline elution/rat hepatocyte assay as
a predictor of carcinogenic/mutagenic potential. Mutat. Res.,
113: 357-391.
SINCLAIR, J., GARLAND, S., ARNASON, T., HOPE, P., & GRANVILLE, M.
(1977) Polychlorinated biphenyls and their effect on photosynthesis
and respiration. Can. J. Bot., 55: 2679-2684.
SIPES, I.G., SLOCUMB, M.L., PERRY, D.F., & CARTER, D.E. (1980)
4,4'-Dichlorobiphenyl: distribution, metabolism and excretion in
the dog and monkey. Toxicol. appl. Pharmacol., 55: 554-563.
SIPES, I.G., SLOCUMB, M.L., CHEN, H.S., & CARTER, D.E. (1982a)
2,3,6,2',3',6'-Hexachlorobiphenyl: distribution, metabolism and
excretion in the dog and monkey. Toxicol. appl. Pharmacol.,
62: 317-324.
SIPES, I.G., SLOCUMB, M.L., PERRY, D.F., & CARTER, D.E. (1982b)
2,4,5,2',4',5'-Hexachlorobiphenyl: distribution, metabolism and
excretion in the dog and monkey. Toxicol. appl. Pharmacol.,
65: 264-272.
SIVALINGAN, P.M., YOSHIDA, T., & INADA, Y. (1973) The modes of
inhibitory effect of PCBs on oxidative phosphorylation of
mitochondria. Bull. environ. Contam. Toxicol., 10: 242-247.
SKAARE, J.U., TUVENG, J.M., & SANDE, H.A. (1988) Organochlorine
pesticides and polychlorinated biphenyls in maternal adipose
tissue, blood, milk and cord blood from mothers and their infants
living in Norway. Arch. environ. Contam. Toxicol., 17: 55-63.
SLOAN, R.J., SIMPSON, K.W., SCHROEDER, R.A., & BARNES, C.R. (1983)
Temporal trends toward stability of Hudson River PCB contamination.
Bull. environ. Contam. Toxicol., 31: 377-385.
SLORACH, S.A. (1984) Biological monitoring and analytical quality
assurance for organohalogen compounds. In: Report on the WHO
Consultation on Organohalogen compounds in human milk and related
hazards. Report on a WHO consultation, Bilthoven, 9-11 January
1985, Copenhagen, World Health Organization, Regional Office for
Europe, Annex 6.
SLORACH, S.A. & VAZ, R. (1983) Global Environmental Monitoring
System (GEMS). Assessment of human exposure to selected
organochlorine compounds through biological monitoring, Uppsala,
Sweden, National Food Administration (Prepared for the United
Nations Environmental Programme and the World Health Organization).
SLORACH, S.A. & VAZ, R. (1985) PCB levels in breast milk: Data from
the UNEP/WHO Pilot project on biological monitoring and some other
recent studies. Environ. health Perspect., 60: 121-126.
SLORACH, S.A., JELINEK, C.F., & STILES, A.R. (1982) Global
Environmental Monitoring System (GEMS). Summary and assessment of
data received from the FAO/WHO Collaborating Centres for Food
Contamination Monitoring, Uppsala, Sweden, National Food
Administration.
SMILLIE, R.H. & WAID, J.S. (1987) Polychlorinated biphenyls and
organochlorine pesticides in the Australian fur seal,
Arctocephalus pusillus doriferus. Bull. environ. Contam.
Toxicol., 39: 358-364.
SMITH, B.J. (1984) PCB levels in human fluids: Sheboygan case
study, Madison, Wisconsin, University of Wisconsin, Sea Grant
Institute (Technical Report No. WIS-SG-83-240).
SMITH, A.B., SCHLÖMER, J., LOWRY, L.K., SMALLWOOD, A.W., LIGO,
R.N., TANAKA, S., STRINGER, W., JONES, M., HERVIN, R., & GLUECK,
C.J. (1982) Metabolic and health consequences of occupational
exposure to polychlorinated biphenyls. Br. J. ind. Med.,
39: 361-369.
SNARSKI, V.M. & PUGLISI, F.A. (1976) Effects of Aroclor (trade
mark) 1254 on brook trout Salvelinus fontinalis, Washington, DC,
US Environmental Protection Agency, 34 pp (EPA 600/3-76-112).
SODERGREN, A. (1971) Accumulation and distribution of chlorinated
hydrocarbons in cultures of Chlorella pyrenoidosa
(Chlorophyceae). Oikos, 22: 215-220.
SODERGREN, A. (1972) Chlorinated hydrocarbon residues in airborne
fallout. Nature (Lond.), 236: 395-397.
SODERGREN, A. (1973) Transport, distribution, and degradation of
DDT and PCB in a South Swedish lake ecosystem. Vatten, 2: 90-108.
SODERGREN, A. & GELIN, C. (1983) Effect of PCBs on the rate of
carbon-14 uptake in phytoplankton isolates from oligotrophic and
eutrophic lakes. Bull. environ. Contam. Toxicol., 30: 191-198.
SODERGREN, A. & LARSSON, P. (1982) Transport of PCBs in aquatic
laboratory model ecosystems from sediment to the atmosphere via the
surface microlayer. Ambio, 11: 41-45.
SODERGREN, A. & SVENSSON, B. (1973) Uptake and accumulation of DDT
and PCB by Ephemera danica (Ephemeroptera) in continuous-flow
systems. Bull. environ. Contam. Toxicol., 9: 345-350.
SODERGREN, A. & ULFSTRAND, S. (1972) DDT and PCB relocate when
caged robins use fat reserves. Ambio, 1: 36-40.
SOLLY, S.R.B. & SHANKS, V. (1974) Polychlorinated biphenyls and
organochlorine pesticides in human fat in New Zealand. N. Z. J.
Sci., 17: 535-544.
SOLOMON, K.E., DAHLGREN, R.B., & LINDER, R.L. (1973) Abnormal
embryos in eggs of pheasants given 2,4-D or PCB. Proc. South
Dakota, Acad. Sci., 52: 95-99.
SOLT, D.B. & FARBER, E. (1976) New principle for the analysis of
chemical carcinogenesis. Nature (Lond.), 263: 701-703.
SOSA-LUCERO, J.C., DE LA IGLESIA, F.A., & THOMAS, G.H. (1973)
Distribution of a polychlorinated terphenyl (PCT) (Aroclor 5460) in
rat tissues and effect on hepatic microsomal mixed function
oxidases. Bull. environ. Contam. Toxicol., 10: 248-256.
SPARLING, J., FUNG, D., & SAFE, S. (1980) Bromo- and chlorobiphenyl
metabolism: GC/MS identification of urinary metabolites and the
effects of structure on their rates of excretion. Biomed. mass
Spectrom., 7: 13-20.
SPEAR, P.A. & MOON, T.W. (1985) Low dietary iodine and thyroid
anomalies in ring doves, Streptopelia risoria, exposed to
3,4,3',4'-tetrachlorobiphenyl. Arch. environ. Contam. Toxicol.,
14: 547-553.
SPENCER, F. (1982) An assessment of the reproductive toxic
potential of Aroclor 1254 in female Sprague-Dawley rats. Bull.
environ. Contam. Toxicol., 28: 290-297.
SPITZER, P.R., RISEBROUGH, R.W., WALKER, W., HERNANDEZ, R., POOLE,
A., PULESTON, D., & NISBET, I.C.T. (1978) Productivity of ospreys
in Connecticut-Long Island increases as DDE residues decline.
Science, 202: 333-335.
STADNICKI, S.S., LIN, F.S.D., & ALLEN, J.R. (1979) DNA single
strand breaks caused by 2,2',5,5'-tetrachlorobiphenyl and its
metabolites. Res. Commun. chem. Pathol. Pharmacol., 24: 313-327.
STAHL, R.G. (1979) Effect of a PCB (Aroclor 1254) on the striped
hermit crab, Clibanarius vittatus (Anomura: Diogenidae) in static
bioassays. Bull. environ. Contain. Toxicol., 23: 91-94.
STALLING, D.L. & MAYER, F.L., Jr, (1972) Toxicities of PCBs to fish
and environmental residues. Environ. health Perspect., 1: 159-164.
STALLING, D.L., TRINDLE, R.C., & JOHNSON, J.L. (1972) Clean up of
pesticides and polychlorobiphenyl residues in fish extracts by gel
permeation chromatography. J. Assoc. Off. Anal. Chem., 55: 32-45.
STATE FOOD INSTITUTE (undated) [Pesticide residues in Danish
foodstuffs 1980-1981], Copenhagen, State Food Institute, Central
Laboratory, Department B: Pesticides and Pollution, pp. 7-8, 65-82
(in Danish).
STEELE, G., STEHR-GREEN, P., & WELTY, E. (1986) Estimates of the
biologic half-life of polychlorinated biphenyls in human serum. New
Engl. J. Med., 314(14): 926-927.
STEEN, W.C., PARIS, D.F., & BAUGHMAN, G.L. (1978) Partitioning of
selected polychlorinated biphenyls to natural sediments. Water
Res., 12: 655-657.
STEHR, P.A., FORNEY, D.L., & LIDDLE, J.A. (1985) Amateur radio
operations and exposure to polychlorinated biphenyls. Arch.
environ. Health, 40(1): 18-19.
STEIN, J.E., HOM, T., & VARANASI, U. (1984) Simultaneous exposure
of English sole (Parophrys vetulus) to sediment-associated
xenobiotics: Part 1 - Uptake and disposition of 14C-polychlorinated
biphenyls and 3H-benzo [a]-pyrene. Mar. environ. Res., 13: 97-119.
STEIN, J.E., HOM, T., CASILLAS, E., FRIEDMAN, A., & VARANASI, U.
(1987) Simultaneous exposure of English sole (Parophrys vetulus)
to sediment-associated xenobiotics: Part 2 - Chronic exposure to an
urban estuarine sediment with added 3H-benzo[a]pyrene and
14C-polychlorinated biphenyls. Mar. environ. Res., 22: 123-149.
STENDALL, R.C. (1976) Summary of recent information regarding
effects of PCBs on birds and mammals. In: Ayer, A.F., ed.
Proceedings of the National Conference on Polychlorinated
Biphenyls, Chicago, Illinois, 19-21 November 1975, Washington, DC,
US Environmental Protection Agency, pp. 262-267 (EPA-560/6-75-004,
PB-253-248).
STICKEL, W.H., STICKEL, L.F., DYRLAND, R.A., & HUGHES, D.L. (1984)
Aroclor 1254 residues in birds: Lethal levels and loss rates. Arch.
environ. Contam. Toxicol., 13: 7-13.
STORM, J.E., HART, J.L., & SMITH, R.F. (1981) Behaviour of mice
after pre- and postnatal exposure to Aroclor 1254. Neurobehav.
Toxicol. Teratol., 3: 5-9.
STRACHAN, W.M.J. (1988) Toxic contaminants in rainfall in Canada:
1984. Environ. Toxicol. Chem., 7: 871-877.
STRACHAN, W.M.J. & HUNEAULT, H. (1979) Polychlorinated biphenyls
and organochlorine pesticides in Great Lakes precipitation. J.
Great Lakes Res., 5: 61-68.
STREET, J.C. & SHARMA, R.P. (1975) Alteration of induced cellular
and humoral immune responses by pesticides and chemicals of
environmental concern: quantitative studies of immunosuppression by
DDT, Aroclor 1254, Carbaryl, Carbofuran, and Methylparathion.
Toxicol. appl. Pharmacol., 32: 587-602.
STREK, H.J. & WEBER, J.B. (1982) Behaviour of polychlorinated
biphenyls (PCBs) in soils and plants. Environ. Pollut.,
28: 291-312.
STREK, H.J., WEBER, J.B., SHEA, P.J., MROZEK, E., & OVERCASH, M.R.
(1981) Reduction of polychlorinated biphenyl toxicity and uptake of
carbon-14 activity by plants through the use of activated carbon.
J. agric. food Chem., 29: 288-293.
STRIK, J.J.T.W.A. (1973) Species differences in experimental
porphyria caused by polyhalogenated aromatic compounds. Enzyme,
16: 224-230.
SUBRAMANIAN, A., TANABE, S., HIDAKA, H., & TATSUKAWA, R. (1986)
Bioaccumulation of organochlorines (PCBs and p,p'-DDE) in
Antarctic Adelie penguins Pygoscelis adeliae collected during a
breeding season. Environ. Pollut., 40: 173-189.
SUBRAMANIAN, A.N., TANABE, S., TATSUKAWA, R., SAITO, S., &
MIYAZAKI, N. (1987) Reduction in the testosterone levels by PCBs
and DDE in Dall's porpoises of Northwestern North Pacific. Mar.
Pollut. Bull., 18: 643-646.
SUBRAMANIAN, A., TANABE, S., & TATSUKAWA, R. (1988) Use of
organochlorines as chemical tracers in determining some
reproductive parameters in Dalli-type Dall's porpoise Phocoenoides
dalli. Mar. environ. Res., 25: 161-174.
SUBRAMANIAN, B.R., TANABE, S., HIDAKA, H., & TATSUKAWA, R. (1983)
DDTs and PCB isomers and congeners in Antarctic fish. Arch.
environ. Contam. Toxicol., 12: 621-626.
SUMMERMAN, W., ROHLEDER, H., & KORTE, F. (1978) [Polychlorinated
biphenyls in food.] Z. Lebensmittelunters. Forsch, 166: 137-144
(in German).
SUNDSTROM, G. & JANSSON, B. (1975) The metabolism of
2,2',3,5',6-pentachlorobiphenyl in rats, mice, and quails.
Chemosphere, 4: 361-370.
SUNDSTROM, G. & WACHTMEISTER, C.A. (1975) Structure of a major
metabolite of 2,2',4,5,5'-pentachlorobiphenyl in mice. Chemosphere,
4: 7-11.
SUNDSTROM, G., HUTZINGER, O., & SAFE, S. (1976a) The metabolism of
chlorobiphenyls. A review. Chemosphere, 5: 267-298.
SUNDSTROM, G., HUTZINGER, O., & SAFE, S. (1976b) The metabolism of
2,2',4,4',5,5'-hexachlorobiphenyl by rabbits, rats, and mice.
Chemosphere, 5: 249-253.
SUZUKI, M., AIZAWA, N., OKANO, G., & TAKAHASHI, T. (1977)
Translocation of polychlorobiphenyls in soil into plants: a study
by a method of culture of soybean sprouts. Arch. environ. Contam.
Toxicol., 5: 343-352.
SWAIN, W.R. (1978) Chlorinated organic residues in fish, water and
precipitation from the vicinity of Isle Royale, Lake Superior. J.
Great Lakes Res., 4: 398-407.
TAKAGI, Y., ABURADA, S., OTAKE, T., &. IKEGAMI, N. (1989) Effect of
dam's accumulated tissue PCBs on mouse filial T-cell population and
T-cell subpopulations. Bull. environ. Contam. Toxicol.,
42: 443-450.
TAKAI, T., OHNO, S., & ISHIZUKI, Y. (1979) [Changes in the
concentration of PCB analogs in fish in Japan Sea.] Niigata
Rikagaku, 5: 54-56 (in Japanese).
TAKAMATSU, M., INOUE, Y., & ABE, S. (1974) [Diagnostic meaning of
the blood PCB.] Fukuoka Acta med., 65: 28-31 (in Japanese).
TAKAMATSU, M., OKI, M., MAEDA, K., INOUE, Y., HIRAYAMA, H., &
YOSHIZUKA, K. (1984) PCBs in blood of workers exposed to PCBs and
their health status. Am. J. ind. Med., 5: 59-68.
TAKAMATSU, M., OKI, M., MAEDA, K., INOUE, Y., HIRAYAMA, H., &
YOSHIZUKA, K. (1985) Surveys of workers occupationally exposed to
PCBs and of Yusho patients. Environ. health Perspect., 59: 91-97.
TAKEI, G.H., KAUAHIKAUA, S.M., & LEONG, G.H. (1983) Analyses of
human milk samples collected in Hawaii for residues of
organochlorine pesticides and polychlorobiphenyls. Bull. environ.
Contam. Toxicol., 30: 606-613.
TAKI, I., HISANAGA, S., & AMAGESE, Y. (1969) [Report on Yusho
(chlorobiphenyls poisoning). Especially further study of its
dermatological findings.] Fukuoka Acta med., 62: 132-138
(in Japanese).
TALCOTT, P.A. & KOLLER, L.D. (1983) The effect of inorganic lead
and/or a polychlorinated biphenyl on the developing immune system
of mice. J. Toxicol. environ. Health, 12: 337-352.
TANABE, S. (1985) [Distribution, behavior and fate of PCBs in the
marine environment.] J. Oceanogr. Soc. Jpn, 41: 358-370
(in Japanese).
TANABE, S. (1988) PCB problems in the future: Foresight from
current knowledge. Environ. Pollut., 50: 5-28.
TANABE, S., NAKAGAWA, Y., & TATSUKAWA, R. (1981) Absorption
efficiency and biological half-life of individual chlorobiphenyls
in rats treated with chlorobiphenyl products. Agric. biol. Chem.,
45: 717-726.
TANABE, S., TATSUKAWA, R., MARUYAMA, K., & MIYASAKI, N. (1982)
Transplacental transfer of PCBs and chlorinated hydrocarbon
pesticides from the pregnant striped dolphin (Stenella
coeruleoalba) to her fetus. Agric. biol. Chem., 46: 1249-1254.
TANABE, S., MORI, T., TATSUKAWA, R., & MIYAZAKI, N. (1983) Global
pollution of marine mammals by PCBs, DDTs and HCHs (BHCs).
Chemosphere, 12: 1269-1275.
TANABE, S., TANAKA, H., & TATSUKAWA, R. (1984) Polychlorobiphenyls,
DDT, and hexachlorocyclohexane isomers in the western North Pacific
ecosystem. Arch. environ. Contam. Toxicol., 13: 731-738.
TANABE, S., MIURA, S., & TATSUKAWA, R. (1986a) Variations of
organochlorine residues with age and sex in Antarctic minke whale.
Mem. Natl Inst. polar Res., 44: 174-181.
TANABE, S., SUBRAMANIAN, A., HIDAKA, H., & TATSUKAWA, R. (1986b)
Transfer rates and pattern of PCB isomers and congeners and
p,p'-DDE from mother to egg in Adelie penguin (Pygoscelis
adeliae). Chemosphere, 15: 343-351.
TANABE, S., KANNAN, N., WAKIMOTO, T., & TATSUKAWA, R. (1987a)
Method for the determination of three toxic non-orthochlorine
substituted coplanar PCBs in environmental samples at
part-per-trillion levels. Int. J. environ. anal. Chem.,
29: 199-213.
TANABE, S., KANNAN, N., SUBRAMANIAN, A., WATANABE, S., & TATSUKAWA,
R. (1987b) Highly toxic coplanar PCBs: Occurrence, source,
persistency and toxic implications to wildlife and humans. Environ.
Pollut., 47: 147-163.
TANABE, S., WATANABE, S., KAN, H., & TATSUKAWA, R. (1988) Capacity
and mode of PCB metabolism in small cetaceans. Mar. Mammal Sci.,
4: 103-124.
TANAKA, K. & KOMATSU, F. (1972) [Shortening of hexobarbital
sleeping time after small doses of PCB in rats.] Fukuoka Acta med.,
63: 360-366 (in Japanese).
TANAKA, H. & OGI, H. (1984) [Bioaccumulation of organochlorine
compounds by pelagic sea birds.] Mar. Sci. Mon., 16: 221-225
(in Japanese).
TATEM, H.E. (1986) Bioaccumulation of polychlorinated biphenyls and
metals from contaminated sediment by freshwater prawns,
Macrobrachium rosenbergii and clams, Corbicula fluminea. Arch.
environ. Contam. Toxicol., 15: 171-183.
TATEMATSU, M., NAKANISHI, K., MURASAKI, G., MIYATA, Y., HIROSE, M.,
& ITO, N. (1979) Enhancing effect of inducers of liver microsomal
enzymes on induction of hyperplastic liver nodules by
N-2-fluorenylacetamide in rats. J. Natl Cancer Inst.,
63(6): 1411-1416.
TATSUKAWA, K. & TANABE, S. (1983) Environmental monitoring:
geochemical and biochemical behaviour of PCBs in the open ocean
environment. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of the PCB Seminar, The Hague, 28-30 September 1983,
The Hague, Ministry of the Environment, pp: 99-118.
TATSUKAWA, K. & WATANABE, I. (1972) Air pollution by PCBs. Shoku No
Kagaku, 8: 55-63.
TAYLOR, P.R., LAWRENCE, C.E., HWANG, H.L., & PAULSON, A.S. (1984)
Polychlorinated biphenyls: Influence on birth weight and gestation.
Am. J. public Health, 74(10): 1153-1154.
TAYLOR, P.R., STELMA, J.M., & LAWRENCE, C.E. (1989) The relation of
polychlorinated biphenyls to birth weight and gestational age in
the offspring of occupationally exposed mothers. Am. J. Epidemiol.,
129(2): 395-406.
THOMAS, G.H. & REYNOLDS, L.M. (1973) Polychlorinated terphenyls in
paperboard samples. Bull. environ. Contam. Toxicol., 10: 37-41.
THOMAS, P.T. & HINSDILL, R.D. (1978) Effect of polychlorinated
biphenyls on the immune responses of Rhesus monkeys and mice.
Toxicol. appl. Pharmacol., 44: 41-51.
THOMAS, P.T. & HINSDILL, R.D. (1980) Perinatal PCB exposure and its
effect on the immune system of young rabbits. Drug chem. Toxicol.,
3: 173-184.
TILSON, H.A., DAVIS, G.J., MCLACHLAN, J.A., & LUCIER, G.W. (1979)
The effects of polychlorinated biphenyls given prenatally on the
neurobehavioural development of mice. Environ. Res., 18: 466-474.
TOFTGARD, R., NILSEN, O.G., & GLAUMANN, H. (1980) Polychlorinated
terphenyls are mixed type of inducers of rat liver microsomal
cytochrome P-450. Dev. Biochem., 13: 227-230.
TOOBY, T.E., HURSEY, P.A., & ALABASTER, J.S. (1975) The acute
toxicity of 102 pesticides and miscellaneous substances to fish.
Chem. Ind., 12: 523-526.
TORI, G.M. & PETERLE, T.J. (1983) Effects of PCBs on mourning dove
courtship behavior. Bull. environ. Contam. Toxicol., 30: 44-49.
TRUELOVE, J., GRANT, D., MES, J., TRYPHONAS, H., TRYPHONAS, L., &
ZAWIDZKA, Z. (1982) Polychlorinated biphenyl toxicity in the
pregnant Cynomolgus monkey: A pilot study. Arch. environ. Contam.
Toxicol., 11: 583-588.
TRUELOVE, J.F., TANNER, J.R., LANGLOIS, I.A., STAPLEY, R.A., MES,
J.C., & ARNOLD, D.L. (in press) Effect of polychlorinated biphenyl
on several endocrine reproductive parameters in the female Rhesus
monkey. Fundam. appl. Toxicol.
TRYPHONAS, L., TRUELOVE, J., ZAWIDZKA, Z., WONG, J., MES, J.,
CHARBONNEAU, S., GRANT, D.L., & CAMPBELL, J.S. (1984)
Polychlorinated biphenyl (PCB) toxicity in adult Cynomolgus monkeys
( M. fascicularis): a pilot study. Toxicol. Pathol., 12: 10-25.
TRYPHONAS, L., ARNOLD, D.L., ZAWIDZKA, Z., MES, J., CHARBONNEAU,
S., & WONG, J. (1986a) A pilot study in adult Rhesus monkeys
(M. mullata) treated with Aroclor 1254 for two years. Toxicol.
Pathol., 14(1): 1-10.
TRYPHONAS, L., CHARBONNEAU, S., TRYPHONAS, H., ZAWIDZKA, Z., MES,
J., WONG, J., & ARNOLD, D.L., (1986b) Comparative aspects of
Aroclor 1254 toxicity in adult Cynomolgus and Rhesus monkeys: a
pilot study. Arch. environ. Contam. Toxicol., 15: 159-169.
TSUDA, H., LEE, G., & FARBER, E. (1980) Induction of resistant
hepatocytes as a new principle for a possible short-term in vivo
test for carcinogens. Cancer Res., 40: 1157-1164.
TSUSHIMOTO, G., ASANO, S., TROSKO, J.E., & CHANG, C.-C. (1983)
Inhibition of intercellular communication by various congeners of
polybrominated biphenyl and polychlorinated biphenyl. In: PCBs:
Human and environmental hazards, Woburn, Massachusetts,
Butterworth, pp. 241-251.
TUCKER, E.S., LITSCHGI, W.J., & MEES, W.M. (1975a) Migration of
polychlorinated biphenyls in soil induced by percolating water.
Bull. environ. Contam. Toxicol., 13: 86-93.
TUCKER, E.S., SAEGER, V.W., & HICKS, O. (1975b) Activated sludge
primary biodegradation of polychlorinated biphenyls. Bull. environ.
Contam. Toxicol., 14: 705-712.
TUEY, D.B. & MATTHEWS, H.B. (1977) Pharmacokinetics of
3,3',5,5'-tetrachlorobiphenyl in the male rat. Drug Metab. Dispos.,
5: 444-450.
TUINSTRA, L.M.G.Th. (1983) Quantification of PCB residues in
environmental monitoring. Techniques and results. In: Barros, M.C.,
Könemann, H., & Visser, R., ed. Proceedings of the PCB Seminar, The
Hague, 28-30 September 1983, The Hague, Ministry of the
Environment, pp. 39-53.
TUINSTRA, L.G.M.Th., ERNST, G.F., ROOS, A.H., VAN MAZIJK, R.Y., &
SPANJERSBERG, F. (1985a) [Content of individual chlorophenyls in
complete infant food], Wageningen, RIKILT Institute (Unpublished
report No. 85.102) (in Dutch).
TUINSTRA, L.G.M.Th., ROOS, A.H., & WERDMULLER, G.A. (1985b)
Capillary gas chromatographic determination of some chlorobiphenyls
in eel fat: Interlaboratory study. J. Assoc. Off. Anal. Chem.,
68(4): 756-759.
TUINSTRA, L.G.M.Th., ROOS, A.H., GRIEPINK, B., & WELLS, D.E.
(1985c) Interlaboratory studies of the determination of selected
chlorobiphenyl congeners with capillary gas chromatography using
splitless- and on-column injection techniques. J. high. Res.
Chromatogr. Chromatogr. Commun., 8: 475-480.
TUMASONIS, C.F., BUSH, B., & BAKER, F.D. (1973) PCB levels in egg
yolks associated with embryonic mortality and deformity of hatched
chicks. Arch. environ. Contam. Toxicol., 1: 312-324.
TURNER, J.C. (1979) Transplacental movement of organochlorine
pesticide residues in desert bighorn sheep. Bull. environ. Contam.
Toxicol., 21: 116-124.
TURNER, J.C. & GREEN, R.S. (1974) Effect of polychlorinated
biphenyl (Aroclor 1254) on liver microsomal enzymes in the male
rat. Bull. environ. Contam. Toxicol., 12: 687-693.
TUTELYAN, V.A., KHAN, A.V., LASHNEVA, N.V., SOROKOVAYA, G.K., &
GADZHIEVA, Z.M. (1986) [Monooxygenase system activity and rat liver
microsome peroxidation rate in reinduction with polychlorinated
diphenyls.] Meditsina, 1(1): 38-40 (in Russian).
UENG, T.-H. & ALVARES, A.P. (1985) Selective induction and
inhibition of liver and lung cytochrome P-450-dependent
monooxygenase by the PCBs mixture, Aroclor 1016. Toxicology,
35: 83-94.
ULFSTRAND, S., SODERGREN, A., & RABOL, J. (1971) Effects of PCB on
nocturnal activity in caged robins, Erithacus rubecula L. Nature
(Lond.), 231: 467-468.
UNGER, M., OLSEN, J., & CLAUSEN, J. (1982) Organochlorine compounds
in the adipose tissue of deceased persons with and without cancer:
A statistical survey of some potential confounders. Environ. Res.,
29: 371-376.
URABE, H. (1974) [Foreword.] Fukuoka Acta med., 65: 1-4
(in Japanese).
URABE, H. & ASAHI, M. (1985) Past and current dermatological status
of Yusho patients. Environ. health Perspect., 59: 11-15.
UREY, J.C., KRICHER, J.C., & BOYLAN, J.M. (1976) Bioconcentration
of four pure PCB isomers by Chlorella pyrenoidosa. Bull. environ.
Contam. Toxicol., 16: 81-85.
US DHEW (1978) Subcommittee on health effects of PCBs and PBBs.
Environ. health Perspect., 24: 131-196.
US EPA (1980) Ambient water quality criteria for polychlorinated
biphenyls, Washington, DC, US Environmental Protection Agency,
211 pp (EPA 440/5-80-068).
US EPA (1983) Exposure assessment for polychlorinated biphenyls
(PCBs), Washington, DC, US Environmental Protection Agency.
US EPA (1985) Polychlorinated biphenyls in electrical transformers;
final rule (part IV). Fed. Reg., 50(137): 29170-29201.
US EPA (1987) Drinking-water criteria document for polychlorinated
biphenyls (PCBs): Final, Cincinnati, Ohio, US Environmental
Protection Agency, Environmental Criteria and Assessment Office
(ECAO-CIN-414).
USHIO, F. & DOGUCHI, M. (1977) Dietary intakes of some chlorinated
hydrocarbons and heavy metals estimated in experimentally prepared
diets. Bull. environ. Contam. Toxicol., 17(6): 707-711.
USHIO, F., FUKANO, S., NISHIDA, K., KANI, T., & DOGUCHI, M. (1974)
Some attempt to estimate the total daily intake of pesticides and
PCB residues and trace heavy metals. Annu. Rep. Tokyo Metrop. Res.
Lab., 25: 307-312 (in Japanese).
UZAWA, H., ITO, Y., NOTOMI, A., & KATSUKI, S. (1969)
[Hyperglyceridemia resulting from intake of rice oil contaminated
with chlorinated biphenyls.] Fukuoka Acta med., 60: 449-454
(in Japanese).
UZAWA, H., NOTOMI, A., NAKAMUTA, S., & IKEURA, Y. (1972)
[Consecutive three year follow-up study of serum triglyceride
concentrations of 82 subjects with PCB poisoning.] Fukuoka Acta
med., 63: 401-404 (in Japanese).
VAN DER KOLK, J. (1984) Consideration of a Codex approach to
contamination of foodstuffs with PCBs. Joint FAO/WHO Food Standards
Programme Codex Committee on Pesticide Residues, Sixteenth session,
The Hague, 28 May-4 June 1984, Codex Alimentarius Commission
(CX/PR 84/10).
VAN DER KOLK, J. (1985) Intake by man of organohalogen compounds
through food: the main route of exposure. Report on a WHO
Consultation on: Organohalogen Compounds in Human Milk and Related
Hazards, Bilthoven, Copenhagen, World Health Organization, Regional
Office for Europe, (ICP/CEH 501/m05).
VAN DYK, L.P., LÖTTER, L.H., MULLEN, J.E.C., & KOCK, A., DE (1987)
Organochlorine insecticide residues in human fat and milk samples
in South Africa. Chemosphere, 16(4): 705-711.
VAN HOVE HOLDRINET, M. (in press) Preliminary results of an
inter-laboratory PCB check sample programme. J. environ. Qual.
VAN MILLER, J.P., HSU, I.C., & ALLEN. J.R. (1975) Distribution and
metabolism of 3H-2,5,2',5'-tetrachlorobiphenyl in rats. Proc. Soc.
Exp. Biol. Med., 148: 682-687.
VANNUCCHI, C., SIVIERI, S., & CECCANTI, M. (1978) Residues of
chlorinated naphthalenes, other hydrocarbons and toxic metals (Hg,
Pb, Cd) in tissues of Mediterranean seagulls. Chemosphere,
6: 483-490.
VAN VLIET, T. (1990) Polychlorinated biphenylen (PCBs).
Interactions between different congeners in in vitro enzyme
induction assays, Stockholm, Institute of Environmental Medicine,
Karolinska Institute (IMM Report No. 3/90).
VAZ, R., LINDER, C.E., & NOREN, K. (1982) Levels of organochlorine
pesticides and PCB in Swedish and imported meat, 1972-1977. Var
Föda, 34(Suppl.1): 23-32.
VEITH, G.D., KUEHL, D.W., PUGLISI, F.A., GLASS, G.E., & EATON, J.G.
(1977) Residues of PCB's and DDT in the Western Lake Superior
ecosystem. Arch. environ. Contam. Toxicol., 5: 487-499.
VEITH, G.D., KUEHL, D.W., LEONARD, E.N., PUGLISI, F.A., & LEMKE,
A.E. (1979) Fish, wildlife and estuaries. Polychlorinated biphenyls
and other organic chemical residues in fish from major watersheds
of the United States, 1976. Pestic. monit. J., 13(1): 1-8.
VERNBERG, F.J., GURAM, M.S., & SAVORY, A. (1977) Survival of larval
and adult fiddler crabs exposed to Aroclor 1016 and 1254 and
different temperature-salinity combinations. In: Vernberg, F.J.,
Calabrese, A., Thurberg, F.P., & Vernberg, W.B., ed. Physiological
responses of marine biota to pollutants, New York, London, Academic
Press, pp. 37-50.
VERSAR, INC. (1984) Exposure assessment for polychlorinated
biphenyls (PCBs): Incidental production, recycling and selected
authorized uses. Hypothetical occupational and consumer exposure to
PCBs in natural gas, Springfield, Virginia, Versar, Inc., Vol. II
(Report to US Environmental Protection Agency, Office of Toxic
Substances, Washington).
VILLENEUVE, D.C., GRANT, D.L., PHILLIPS, W.E.J., CLARK, M.L., &
CLEGG, D.J. (1971a) Effects of PCB administration on microsomal
enzyme activity in pregnant rabbits. Bull. environ. Contam.
Toxicol., 6: 120-128.
VILLENEUVE, D.C., GRANT, D.L., KHERA, K., CLEGG, D.J., BAER, H., &
PHILLIPS, W.E.J. (1971b) The fetotoxicity of a polychlorinated
biphenyl mixture (Aroclor 1254) in the rabbit and in the rat.
Environ. Physiol., 1: 67-71.
VILLENEUVE, D.C., GRANT, D.L., & PHILLIPS, W.E.J. (1972)
Modification of pentobarbital sleeping times in rats following
chronic PCB ingestion. Bull. environ. Contam. Toxicol., 7: 264-269.
VILLENEUVE, D.C., REYNOLDS, L.M., THOMAS, G.H., & PHILLIPS, W.E.J.
(1973a). Polychlorinated biphenyls and polychlorinated terphenyls
in Canadian food packaging materials. J. Assoc. Off. Anal. Chem.,
56: 999-1001.
VILLENEUVE, D.C., REYNOLDS, L.M., & PHILLIPS, W.E.J. (1973b)
Residues of PCBs and PCTs in Canadian and imported European cheeses
- 1972. Pestic. monit. J., 7: 95-96.
VODICNIK, M.J., (1986) The effect of pregnancy and lactation on the
disposition of [2,4,2'4-14C] tetrachlorobiphenyl in the mouse.
Fundam. appl. Toxicol., 6: 53-61.
VODICNIK, J.J. & LECH, J.J. (1980) The transfer of 2,4,5,2',4',5'-
hexachlorobiphenyl to fetuses and nursing offspring. I. Disposition
in pregnant and lactating mice and accumulation in young. Toxicol.
appl. Pharmacol., 54: 293-300.
VODICNIK, M.J. & PETERSON, R.E. (1985) The enhancing effect of
spawning on elimination of a persistent polychlorinated biphenyl
from female yellow perch. Fundam. appl. Toxicol., 5: 770-776.
VODICNIK, M.J., ELCOMBE, C.R., & LECH, J.J. (1980) The transfer of
2,4,5,2',4',5'-hexachlorobiphenyl to fetuses and nursing offspring
II. Induction of hepatic microsomal monooxygenase activity in
pregnant and lactating mice and their young. Toxicol. appl.
Pharmacol., 54: 301-310.
VOS, J.G. & KOEMAN, J.H. (1970) Comparative toxicologic study with
polychlorinated biphenyls in chickens with special reference to
porphyria, oedema formation, liver necrosis and tissue residues.
Toxicol. appl. Pharmacol., 17: 656-668.
VOS, J.G., KOEMAN, J.H., VAN DER MAAS, H.J., TEN NOEVER DE BRAUW,
M.C., & DE VOS, R.H. (1970) Identification and toxicological
evaluation of chlorinated dibenzofuran and chlorinated naphthalene
in two commercial polychlorinated biphenyls. Food Cosmet. Toxicol.,
8: 625-633.
VOS, J.G. & BEEMS, R.B. (1971) Dermal toxicity studies of technical
polychlorinated biphenyls and fractions thereof in rabbits.
Toxicol. appl. Pharmacol., 19: 617-633.
VOS, J.G. & NOTENBOOM-RAM, E. (1972) Comparative toxicity study of
2,4,5,2',4',5',-hexachlorobiphenyl and a polychlorinated biphenyl
mixture in rabbits. Toxicol. appl. Pharmacol., 23: 562-578.
VOS, J.G. & DE ROIJ, T. (1972) Immunosuppressive activity of a
polychlorinated biphenyl preparation on the humoral immune response
in guinea-pigs. Toxicol. appl. Pharmacol., 21: 549-555.
VOS, J.G. & VAN DRIEL-GROOTENHUIS, L. (1972) PCB-induced
suppression of the humoral and cell-mediated immunity in
guinea-pigs. Sci. total Environ., 1: 289-300.
VOS, J.G. & VAN GENDEREN, H. (1973) Toxicological aspects of
immunosuppression. In: Deichman, W.B., ed. Pesticides in the
environment, a continuity controversy. 8th International Conference
on Toxicology and Occupational Medicine, New York, Intercontinental
Medical Company.
VREELAND, V. (1974) Uptake of chlorobiphenyls by oysters, Environ.
Pollut., 6: 135-140.
WAKIMOTO, T., KANNAN, N., ONO, M., TATSIUKAWA, R., & MASUDA, Y.
(1988) Isomer-specific determination of polychlorinated
dibenzofurans in Japanese and American polychlorinated biphenyls.
Chemosphere, 17(4): 743-750.
WALLNOFER, P.R. & KONIGER, M. (1974) [Experiments on the uptake of
hexachlorobenzene and polychlorinated biphenyls by cultivated
plants from different substrates.] Z. Pflanzenkr. Pflanzenschutz,
26: 54-57 (in German).
WALLNOFER, P.R., ENGELHARDT, G., SAFE, S., & HUTZINGER, O. (1973)
Microbial hydroxylation of 4-chlorobiphenyl and 4,4'-
dichlorobiphenyl. Chemosphere, 2: 69-72.
WALLNOFER, P., KONIGER, M., & ENGELHARDT, G. (1975) [Behaviour of
xenobiotic chlorinated hydrocarbons (HCBs and PCBs) in cultivated
plants and soil.] Z. Pflanzenkr. Pflanzenschutz, 82: 91-100
(in German).
WALSH, G.E., HOLLISTER, T.A., & FORESTER, J. (1974) Translocation
of four organochlorine compounds by red mangrove (Rhizophora
mangle L.) seedlings. Bull. environ. Contam. Toxicol.,
12: 129-135.
WARD, J.M. (1985) Proliferative lesions of the glandular stomach
and liver in F344 rats fed diets containing Aroclor 1254. Environ.
health Perspect., 60: 89-95.
WARDELL, R.E., SEEGMILLER, R.E., & BRADSHAW, W.S. (1982) Induction
of prenatal toxicity in the rat by diethylstilbestrol, Zeranol,
3,4,3',4'-tetrachlorobiphenyl, cadmium, and lead. Teratology,
26: 229-237.
WARSHAW, R., FISCHBEIN, A., THORNTON, I., MILLER, A., & SELIKOFF,
I.J. (1979) Decrease in vital capacity in PCB-exposed workers in a
capacitor manufacturing facility. Ann. NY Acad. Sci., 320: 277-283.
WASILEWSKA, L., OLOFFS, P.C., & WEBSTER, J.M. (1975) Effects of
carbofuran and a PCB on development of a bacteriophagous nematode
Acrobeloides nanus. Can. J. Zool., 53: 1709-1715.
WASSERMANN, D., WASSERMANN, M., CUCOS, S., & DJAVAHERIAN, M. (1973)
Function of the adrenal gland-zona fasciculata in rats receiving
polychlorinated biphenyls. Environ. Res., 6: 334-338.
WASSERMANN, D., WASSERMANN, M., & LEMESCH, C. (1975) Ultrastructure
of beta-cell of the endocrine pancreas in rats receiving
polychlorinated biphenyls. Environ. Physiol. Biochem., 5: 332-340.
WASSERMANN, M., WASSERMANN, D., CUCOS, S., & MILLER, M.J. (1979)
World PCBs map - storage and effects in man and his biologic
environment in the 1970s. Ann. NY Acad. Sci., 320: 69-124.
WASSERMANN, M., RON, M., BERCOVICI, B., WASSERMANN, D., CUCOS, S.,
& PINES, A. (1982) Premature delivery and organochlorine compounds:
PCB and some organochlorine insecticides. Environ. Res.,
28(1): 106-112.
WATANABE, I., YAKUSHIJI, T., KUWABARA, K., YOSHIDA, S., MAEDA, K.,
KASHIMOTO, T., KOYAMA, K., & KUNITA N. (1979) Surveillance of the
daily PCB intake from diet of Japanese women from 1972 through
1976. Arch. environ. Contam. Toxicol., 8: 67-75.
WATANABE, M. & SUGAHARA, T. (1981) Experimental formation of cleft
palate in mice with polychlorinated biphenyls (PCB). Toxicology,
19: 49-53.
WATANABE, M., HONDA, S., HAYASHI, M., & MATSUDA, T. (1982)
Mutagenic effects of combinations of chemical carcinogens and
environmental pollutants in mice as shown by the micronucleus test.
Mutat. Res., 97: 43-48.
WEBBER, M.D., MONTEITH, H.D., & CORNEAU, D.G.M. (1983) Assessment
of heavy metals and PCBs at sludge application sites. J. WPCF,
55(2): 187-195.
WEBER, J.B. & MROZEK, E. (1979) Polychlorinated biphenyls:
phytotoxicity, absorption and translocation by plants, and
inactivation by activated carbon. Bull. environ. Contam. Toxicol.,
23: 412-417.
WEGMAN, R.C.C. & BERKHOFF, C.J. (1986) [Chemical contaminants in
breastmilk. Report section 5: Polychlorinated biphenyls (PCB
congeners)], Bilthoven, The Netherlands, National Institute of
Public Health and Environmental Hygiene (Unpublished report
No. 638307006) (in Dutch).
WEGMAN, R.C.C. & GREVE, P.A. (1980) Halogenated hydrocarbons in
Dutch water samples over the years 1969-1977. In: Hydrocarbons and
halogenated hydrocarbons in the aquatic environment, New York,
London, Plenum Press, pp. 405-415.
WEIS, P. & WEIS, J.S. (1982) Toxicity of the PCBs Aroclor 1254 and
1242 to embryos and larvae of the mummichog, Fundulus
heteroclitus. Bull. environ. Contam. Toxicol., 28: 298-304.
WESELOH, D.V., MINEAU, P., & HALLETT, D.J. (1979) Organochlorine
contaminants and trends in reproduction in Great Lakes herring
gulls, 1974-1978. Trans. North Am. Wildl. Nat. Resour. Conf.,
44: 543-557.
WESELOH, D.V., TEEPLE, S.M., & GILBERTSON, M. (1983) Double-crested
cormorants of the Great Lakes: egg laying parameters, reproductive
failure, and contaminant residues in eggs, Lake Huron 1972-1973.
Can. J. Zool., 61: 427-436.
WESTCOTT, J.W., SIMON, C.G., & BIDLEMAN, T.F. (1981) Determination
of polychlorinated biphenyl vapour pressures by a semimicro gas
saturation method. Environ. Sci. Technol., 15(11): 1375-1378.
WESTIN, D.T., OLNEY, C.E., & ROGERS, B.A. (1983) Effects of
parental and dietary PCBs on survival, growth, and body burdens of
larval striped bass. Bull. environ. Contam. Toxicol., 30: 50-57.
WESTOO, G. & NOREN, K. (1970a) [Levels of organochlorine pesticides
and polychlorinated biphenyls in fish caught in Swedish water areas
or kept for sale in Sweden, 1967-1970.] Var Föda, 3: 93-146
(in Swedish with English summary).
WESTOO, G. & NOREN, K. (1970b) Determination of organochlorine
pesticides and polychlorinated biphenyls in animal foods. Acta
chem. Scand., 24: 1639-1644.
WESTOO, G., NOREN, K., & ANDERSSON, M. (1971) [Levels of
organochlorine pesticides and polychlorinated biphenyls in some
cereal products.] Var Föda, 10: 341-360 (in Swedish with English
summary).
WHITE, D.H. (1979) Nationwide residues of organochlorine compounds
in wings of adult mallards and black ducks, 1976-77. Pestic. monit.
J., 13: 12-16.
WHITE, R.D., ALLEN, S.D., & BRADSHAW, W.S. (1983) Delay in the
onset of parturition in the rat following prenatal administration
of developmental toxicants. Toxicol. Lett., 18: 185-192.
WHO (1976) Environmental Health Criteria 2: Polychlorinated
biphenyls and terphenyls, Geneva, World Health Organization, 85 pp.
WHO (1985) A review of the 1980-1983 data received from the FAO/WHO
Collaborating Centres for Food Contamination Monitoring. Joint
FAO/WHO Food Contamination Monitoring Programme: PCBs residues in
food, Geneva, World Health Organization, Geneva (Paper prepared for
Codex Committee on Pesticide Residues).
WHO (1986a) Joint FAO/WHO Food Contamination Monitoring Programme.
Summary of 1980-1983 monitoring data, Geneva, World Health
Organization (WHO/EHE/FOS/86.2.).
WHO (1986b) Joint FAO/WHO Food Contamination Monitoring Programme.
Chemical contaminants in foods: 1980-1983, Geneva, World Health
Organization (WHO/EHE/FOS/86.5).
WHO (1989) Environmental Health Criteria 88: Polychlorinated
dibenzo-para-dioxins and dibenzofurans, Geneva, World Health
Organization, 409 pp.
WHO (1990) Evaluation of certain food additives and contaminants.
Thirty-fifth report of the Joint FAO/WHO Expert Committee on Food
Additives, Geneva, World Health Organization (WHO Technical Report
Series 789).
WHO/EURO (1985) Organohalogen compounds in human milk and related
hazards. Report on a WHO consultation, Bilthoven, 9-11 January
1985, Copenhagen, World Health Organization, Regional Office for
Europe (IPC/CEH 501/m05).
WHO/EURO (1987) PCBs, PCDDs, and PCDFs: Prevention and control of
accidental and environmental exposures, Copenhagen, World Health
Organization, Regional Office for Europe (Environmental Health
Series 23).
WHO/EURO (1988) PCBs, PCDDs and PCDFs in breast milk: Assessment of
health risks, Copenhagen, World Health Organization, Regional
Office for Europe (Environmental Health Series 29).
WHO/EURO (1989) Levels of PCBs, PCDDs and PCDFs in breastmilk.
Results on WHO coordinated interlaboratory quality control studies
and analytical field studies, Copenhagen, World Health
Organization, Regional Office for Europe (Environmental Health
Series 34).
WICKIZER, T.M. & BRILLIANT, L.B. (1981) Testing for polychlorinated
biphenyls in human milk. Pediatrics, 68(3): 411-415.
WICKIZER, T.M., BRILLIANT, L.B., COPELAND, R., & TILDEN, R. (1981)
Polychlorinated biphenyl contamination of nursing mother's milk in
Michigan. Am. J. public Health, 71(2): 132-137.
WIEMEYER, S.N., SPITZER, P.R., KRANTZ, W.C., LAMONT, T.G., &
CROMARTIE, E. (1975) Effects of environmental pollutants on
Connecticut and Maryland ospreys. J. Wildl. Manage., 39: 124-139.
WIEMEYER, S.N., LAMONT, T.G., BUNCK, C.M., SINDELAR, C.R.,
GRAMLICH, F.J., FRASER, J.D., & BYRD, M.A. (1984) Organochlorine
pesticide, polychlorobiphenyl, and mercury residues in bald eagle
eggs - 1969 to 1979 - and their relationships to shell thinning and
reproduction. Arch. environ. Contam. Toxicol., 13: 529-549.
WIEMEYER, S.N., SCHMELING, S.K., & ANDERSON, A. (1987)
Environmental pollutant and necropsy data for ospreys from the
eastern United States, 1975-1982. J. Wildl. Dis., 23: 279-291.
WILDISH, D.J. (1970) The toxicity of polychlorinated biphenyls
(PCB) in sea water to Gammarus oceanicus. Bull. environ. Contam.
Toxicol., 5: 202-204.
WILDISH, D.J. & ZITKO, V. (1971) Uptake of polychlorinated
biphenyls from sea water by Gammarus oceanicus. Mar. Biol.,
9: 213-218.
WILDISH, D.J., METCALFE, C.D., AKAGI, H.M., & MCLEESE, D.W. (1980)
Flux of Aroclor 1254 between estuarine sediments and water. Bull.
environ. Contam. Toxicol., 24: 20-26.
WILLETT, L.B. (1980) Polychlorinated biphenyls from dairy farms:
Consequence in market milk. J. dairy Sci., 63: 1961-1965.
WILLETT, L.B., LIU, T.-T.Y, DURST, H.I., SMITH, K.L., & REDMAN,
D.R. (1987) Health and productivity of dairy cows fed
polychlorinated biphenyls. Fundam. appl. Toxicol., 9: 60-68.
WILLFORD, W.A. (1980) Chlorinated hydrocarbons as a limiting factor
in the reproduction of lake trout in Lake Michigan. In: Swain, W.R.
& Shannon, V.R., ed. Proceedings of the 3rd USA-USSR Symposium on
Effluent Pollution and Aquatic Ecosystems, Washington, DC, US
Environmental Protection Agency, pp. 75-83 (PEA-660/9-80-034).
WOLFF, M. (1983) Occupationally derived chemicals in breast milk.
Am. J. ind. Med., 4: 259-281.
WOLFF, M.S. (1984) Analysis of skin lipids for halogenated
hydrocarbons. Anal. Chem., 56: 1492-1496.
WOLFF, M.S. (1985) Occupational exposure to polychlorinated
biphenyls (PCBs). Environ. health Perspect., 60: 133-138.
WOLFF, M.S., ANDERSON, H.A., & SELIKOFF, I.J. (1982) Human tissue
burdens of halogenated aromatic chemicals in Michigan. J. Am. Med.
Assoc., 247(15): 2112-2116.
WÖLFLE, D., MÜNZEL, P., FISCHER, G., & BOCK, K.W. (1988) Altered
growth control of rat hepatocytes after treatment with
3,4,3',4'-tetrachlorobiphenyl in vivo and in vitro.
Carcinogenesis, 9(6): 919-924.
WONG, P.T.S. & KAISER, K.L.E. (1975) Bacterial degradation of
polychlorinated biphenyls II. Rate studies. Bull. environ. Contam.
Toxicol., 13: 249-255.
WONG, A., BASRUR, P.K., & SAFE, S. (1979) The metabolically
mediated DNA damage and subsequent repair by 4-chlorobiphenyl in
Chinese hamster ovary cells. Res. Commun. chem. Pathol. Pharmacol.,
24: 543-548.
WONG, T.K., EVERSON, R.B., & HSU SHU-TAO (1985) Potent induction of
human placental mono-oxygenase activity by previous dietary
exposure to polychlorinated biphenyls and their thermal degradation
products. Lancet, March 30: 721-724.
WREN, C.D., HUNTER, D.B., LEATHERLAND, J.F., & STOKES, P.M. (1987a)
The effects of polychlorinated biphenyls and methylmercury, singly
and in combination, on Mink. I: Uptake and toxic responses. Arch.
environ. Contam. Toxicol., 16: 441-447.
WREN, C.D., HUNTER, D.B., LEATHERLAND, J.F., & STOKES, P.M. (1987a)
The effects of polychlorinated biphenyls and methylmercury, singly
and in combination on mink. II: Reproduction and kit development.
Arch. environ. Contam. Toxicol., 16: 449-454.
WYMAN, K.D. & O'CONNORS, C.H.B. (1980) Implications of short-term
PCB uptake by small estuarine copepods (Genus Acartia) from
PCB-contaminated water, inorganic sediments and phytoplankton.
Estuarine coastal Mar. Sci., 11: 121-131.
WYNDHAM, C., DEVENISH, J., & SAFE, S. (1976) The in vitro
metabolism, macromolecular binding and bacterial mutagenicity of
4-chlorobiphenyl, a model PCB substrate. Res. Commun. chem. Pathol.
Pharmacol., 15: 563-570.
WYSS, P.A., MUHLEBACK, S., & BICKEL, M.H. (1986) Long-term
pharmacokinetics of 2,2',4,4',5,5'-hexachlorobiphenyl (6-CB) in
rats with constant adipose tissue mass. Drug. Metab. Dispos.,
14: 361-365.
YAGAMUCHI, A., YOSHIMURA, T., & KURATSUNE, M. (1971) [A survey on
pregnant women having consumed rice oil contaminated with
chlorobiphenyls and their babies.] Fukuoka Acta med., 62: 112-117
(in Japanese).
YAGI, N. (1980) [Lipid metabolism in PCB poisoned rats.] Jpn. J.
Hyg., 35: 659-664 (in Japanese).
YAGI, N., KAMOHARA, K., & ITOKAWA, Y. (1979) Thiamine deficiency
induced by polychlorinated biphenyls (PCBs) and
dichlorodiphenyltrichloroethane (DDT) administration to rats. J.
environ. Pathol. Toxicol., 2: 1119-1125.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., YOSHIDA, S., KOYAMA, K.,
& KUNITA, N. (1977) Residues of polychlorinated biphenyls and
organochlorine pesticides in human milk, blood, and diet (V). Proc.
Osaka Prefect Inst. Public Health, 8: 35-44.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., YOSHIDA, S., KOYAMA, K.,
HARA, I., & KUNITA, N. (1978) Long-term studies of the excretion of
polychlorinated biphenyls (PCBs) through the mother's milk of an
occupationally exposed worker. Arch. environ. Contam. Toxicol.,
7: 493-504.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., YOSHIDA, S., HORI, S.,
FUKUSHIMA, S., KASHIMOTO, T., KOYAMA, K., & KUNITA, N. (1979)
Levels of organochlorine pesticides and polychlorinated biphenyls
(PCBs) in mothers' milk collected in Osaka Prefecture from 1969 to
1976. Arch. environ. Contam. Toxicol., 8: 59-66.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., TANAKA, R., KASHIMOTO,
T., KUNITA, N., & HARA I. (1984a) Rate of decrease and half-life of
polychlorinated biphenyls (PCBs) in the blood of mothers and their
children occupationally exposed to PCBs. Arch. environ. Contam.
Toxicol., 13: 341-345.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., TANAKA, R., KASHIMOTO,
T., KUNITA, N., & HARA I. (1984b) Postnatal transfer of PCBs from
exposed mothers to their babies: influence of breast-feeding. Arch.
environ. Health, 39(5): 368-375.
YAMAMOTO, H.A. & YOSHIMURA, H. (1973) Metabolic studies on
polychlorinated biphenyls. III. Complete structure and acute
toxicity of the metabolites of 2,4,3',4',-tetrachlorobiphenyl.
Chem. pharm. Bull. (Tokyo), 21: 2237-2242.
YAMASHITA, F. & HAYASHI, M. (1985) Fetal PCB Syndrome: Clinical
features intrauterine growth retardation and possible alteration in
calcium metabolism. Environ. health Perspect., 59: 41-46.
YOBS, A.R. (1972) Levels of polychlorinated biphenyls in adipose
tissue of the general population of the nation. Environ. health
Perspect., 1: 79-81.
YOSHIDA, S. & NAKAMURA, A. (1979) Residual status after
participation of methylsulfone metabolites of polychlorinated
biphenyls in the breast milk of a former employee in a capacitor
factory. Bull. environ. Contam. Toxicol., 21: 111-115.
YOSHIDA, T., TAKASHIMA, F., & WATANABE, T. (1973) Distribution of
[14C] PCBs in Carp. Ambio, 2: 111-113.
YOSHIHARA, S., KAWANO, K., YOSHIMURA, H., KUROKI, H., & MASUDA, Y.
(1979) Toxicological assessment of highly chlorinated biphenyl
congeners retained in the Yusho patients. Chemosphere, 8: 531-538.
YOSHIHARA, S., NAGATA, K., WADA, I., YOSHIMURA, H., KUROKI, H., &
MASUDA, Y. (1982) A unique change of steroid metabolism in rat
liver microsomes induced with highly toxic polychlorinated biphenyl
(PCB) and polychlorinated dibenzofuran (PCDF). J. pharmacobiol.
Dyn., 5: 994-1004.
YOSHIMURA, T. (1971) [Epidemiological analysis of "Yusho" patients
with special reference to sex, age, clinical grades and oil
consumption.] Fukuoka Acta med., 62: 109-116 (in Japanese).
YOSHIMURA, T. (1974) Epidemiological study on Yusho babies born to
mothers who had consumed oil contaminated by PCBs. Fukuoka Igahu
Zushi, 65(1): 74-80.
YOSHIMURA, H. & YAMAMOTO, H. (1975) A novel route of excretion of
2,4,3',4',-tetrachlorobiphenyl in rats. Bull. environ. Contam.
Toxicol., 13: 681-687.
YOSHIMURA, H., YAMAMOTO, H., NAGAI, J., YAE, Y., UZAWA, H., ITO,
Y., NOTOMI, A., MIMAKAMI, S., ITO, A., KATO, K., & TSUJI, H. (1971)
[Studies on the tissue distribution and the urinary and faecal
excretion of 3H-Kanechlor (chlorobiphenyls) in rats.] Fukuoka Acta
med., 62: 11-19 (in Japanese).
YOSHIMURA, H., YAMAMOTO, H., & SAEKI, S. (1973) Metabolic studies
on polychlorinated biphenyls II. Metabolic fate of
2,4,3',4'-tetrachlorobiphenyl in rats. Chem. pharm. Bull. (Tokyo),
21: 2231-2236.
YOSHIMURA, H., YAMAMOTO, H., & KINOSHITA, H. (1974) [Metabolic
studies on polychlorinated biphenyls. V. biliary excretion of
5-hydroxy-2,4,3',4'-tetrachlorobiphenyl, a major metabolite of
2,4,3',4'-tetrachlorobiphenyl.] Fukuoka Acta med., 65: 12-16
(in Japanese).
YOSHIMURA, H., OZAWA, N., & SAEKI, S. (1978) Inductive effect of
polychlorinated biphenyl, biphenyls mixture and individual isomers
on the hepatic microsomal enzymes. Chem. pharm. Bull. (Tokyo),
26: 1215-1221.
YOSHIMURA, H., YOSHIHARA, S., KOGA, N., NAGATA, K., WADA, I.,
KUROKI, J., & HOKAMA, Y. (1985) Inductive effect on hepatic enzymes
and toxicity of congeners of PCBs and PCDFs. Environ. health
Perspect., 59: 113-119.
YOUSSEF, N.N., BRINDLEY, W.A., & STREET, J.C. (1974) Fine
structural alterations in the developing spermatids of Musca
domestica induced by the PCB, Aroclor 1254. Cytobios,
11: 167-183.
ZACK, T.A. & MUSCH, D.C. (1979) Mortality of PCB workers at the
Monsanto Plant in Sanget, Illinois, St. Louis, Illinois, Monsanto
Chemical Co. (Unpublished Report).
ZELL, M., NEU, H.J., & BALLSCHMITTER, K. (1977) [Identification of
PCB components by the retention index. Comparison by capillary gas
chromatography.] Chemosphere, 7: 69-76 (in German).
ZEPP, R.L., Jr, & KIRKPATRICK, R.L. (1976) Reproduction in
cottontails fed diets containing a PCB. J. Wildl. Manage.,
40: 491-495.
ZHANG, Y., ROTT, B., & FREITAG, D. (1983) Accumulation and
elimination of 14C-PCBs by Daphnia magna Straus 1820.
Chemosphere, 12: 1645-1651.
ZIMMERLI, B. & MAREK, B. (1973) [The pesticide load of the Swiss
population.] Mitt. Lebensmittelunters. Hyg., 64(4): 459-479
(in German).
ZINCK, M.E. & ADDISON, R.F. (1974) The fate of 2-, 3-, and
4-chlorobiphenyl following intravenous administration to thorny
skate (Raja radiata) and the winter skate (Raja ocellata).
Arch. environ. Contam. Toxicol., 2: 52-61.
ZINKL, J.G. (1977) Skin and liver lesions in rats fed a
polychlorinated biphenyl mixture. Arch. environ. Contam. Toxicol.,
5(2): 217-228.
ZITKO, V. (1974) Uptake of chlorinated paraffins and PCB from
suspended solids and food by juvenile Atlantic salmon. Bull.
environ. Contam. Toxicol., 12: 406-412.
ZITKO, V. & SAUNDERS, R.L. (1979) Effect of PCBs and other
organochlorine compounds on the hatchability of Atlantic salmon
(Salmo salar) eggs. Bull. environ. Contam. Toxicol., 21: 125-130.
ZITKO, V., HUTZINGER, O., & SAFE, S. (1971) Retention times and
electron-capture detector responses of some individual
chlorobiphenyls. Bull. environ. Contam. Toxicol., 6: 160-163.
ZITKO, V., HUTZINGER, O., & CHOI, P.M.K. (1972) Contamination of
the Bay of Fundy - Gulf of Maine area with polychlorinated
biphenyls, polychlorinated terphenyls, chlorinated dibenzodioxins
and dibenzofurans. Environ. health Perspect., 1: 47-50.
ZULLEI, N. & BENECKE, G. (1978) Application of a new bioassay to
screen the toxicity of polychlorinated biphenyls on blue-green
algae. Bull. environ. Contam. Toxicol., 20: 786-792.
ANNEX 1.
NUMBERING OF PCB CONGENERS
No. Structure No. Structure No. Structure
Monochlorobiphenyls Tetrachlorobiphenyls Pentachlorobiphenyls
1 2 40 2,2',3,3' 82 2,2',3,3',4
2 3 41 2,2',3,4 83 2,2',3,3',5
3 4 42 2,2',3,4' 84 2,2',3,3',6
43 2,2',3,5 85 2,2',3,4,4'
Dichlorobiphenyls 44 2,2',3,5' 86 2,2',3,4,5
4 2,2' 45 2,2',3,6 87 2,2',3,4,5'
5 2,3 46 2,2',3,6 88 2,2',3,4,6
6 2,3' 47 2,2',4,4' 89 2,2',3,4,6'
7 2,4 48 2,2',4,5 90 2,2',3,4',5
8 2,4' 49 2,2',4,5' 91 2,2',3,4',6
9 2,5 50 2,2',4,6 92 2,2',3,5,5'
10 2,6 51 2,2',4,6' 93 2,2',3,5,6
11 3,3' 52 2,2',5,5' 94 2,2',3,5,6'
12 3'4 53 2,2',5,6' 95 2,2',3,5',6
13 3,4' 54 2,2',6,6'
15 4,4' 55 2,3,3',4 Pentachlorobiphenyls
56 2,3,3',4' 96 2,2',3,6,6'
Trichlorobiphenyls 57 2,3,3',5 97 2,2',3',4,5
16 2,2',3 58 2,3,3',5' 98 2,2',3',4,6
17 2,2',4 59 2,3,3',6 99 2,2',4,4',5
18 2,2',5 60 2,3,4,4' 100 2,2',4,4',6
19 2,2',6 61 2,3,4,5 101 2,2',4,5,5'
20 2,3,3' 62 2,3,4,6 102 2,2',4,5,6'
21 2,3,4 63 2,3,4',5 103 2,2',4,5',6
22 2,3,4' 64 2,3,4',6 104 2,2',4,6,6'
23 2,3,5 65 2,3,5,6 105 2,3,3',4,4'
24 2,3,6 66 2,3',4,4' 106 2,3,3',4,5
25 2,3',4 67 2,3',4,5 107 2,3,3',4',5
26 2,3',5 68 2,3',4,5' 108 2,3,3',4,5'
27 2,3',6 69 2,3',4,6 109 2,3,3',4,6
28 2,4,4' 70 2,3',4',5 110 2,3,3',4',6
29 2,4,5 71 2,3',4',6 111 2,3,3',5,5'
30 2,4,6 72 2,3',5,5' 112 2,3,3',5,6
31 2,4',5 73 2,3',5',6 113 2,3,3',5',6
32 2,4',6 74 2,4,4',5 114 2,3,4,4',5
33 2',3,4 75 2,4,4',6 115 2,3,4,4',6
34 2',3,5 76 2',3,4,5 116 2,3,4,5,6
35 3,3',4 77 3,3',4,4' 117 2,3,4',5,6
36 3,3',5 78 3,3',4,5 118 2,3',4,4',5
37 3,4,4' 79 3,3',4,5' 119 2,3',4,4',6
38 3,4,5' 80 3,3',5,5' 120 2,3',4,5,5'
39 3,4',5 81 3,4,4',5 121 2,3',4,5',6
No. Structure No. Structure No. Structure
Pentachlorobiphenyls Hexachlorobiphenyls Octachlorobiphenyls
122 2,3,3',4,5 162 2,3,3',4',5,5' 202 2,2',3,3',5,5',6,6'
123 2',3,4,4',5 163 2,3,3',4',5,6 203 2,2',3,4,4',5,5',6
124 2',3,4,5,5' 164 2,3,3',4',5',6 204 2,2',3,4,4',5,6,6'
125 2',3,4,5,6' 165 2,3,3',5,5',6 205 2,3,3',4,4',5,5',6
126 3,3',4,4',5 166 2,3,4,4',5,6
127 3,3',4,5,5' 167 2,3',4,4',5,5' Nonachlorobiphenyls
168 2,3',4,4',5',6 206 2,2',3,3',4,4',5,5',6
Hexachlorobiphenyls 169 3,3',4,4',5,5' 207 2,2',3,3',4,4',5,6,6'
128 2,2',3,3',4,4' 208 2,2',3,3',4,5,5',6,6'
129 2,2',3,3',4,5 Heptachlorobiphenyls
130 2,2',3,3',4,5' 170 2,2',3,3',4,4',5 Decachlorobiphenyls
131 2,2',3,3',4,6 171 2,2',3,3',4,4',6 209 2,2',3,3',4,4',5,5',6,6'
132 2,2',3,3',4,6' 172 2,2',3,3',4,5,5'
133 2,2',3,3',5,5' 173 2,2',3,3',4,5,6
134 2,2',3,3',5,6 174 2,2',3,3',4,5,6'
135 2,2',3,3',5,6' 175 2,2',3,3',4,5',6
136 2,2',3,3',6,6' 176 2,2',3,3',4,6,6'
137 2,2',3,4,4',5 177 2,2',3,3',4',5,6
138 2,2',3,4,4',5' 178 2,2',3,3',5,5',6
139 2,2',3,4,4',6 179 2,2',3,3',5,6,6'
140 2,2',3,4,4',6' 180 2,2',3,4,4',5,5'
141 2,2',3,4,5,5' 181 2,2',3,4,4',5,6
142 2,2',3,4,5,6 182 2,2',3,4,4',5,6'
143 2,2',3,4,5,6' 183 2,2',3,4,4',5',6
144 2,2',3,4,5',6 184 2,2',3,4,4',6,6'
185 2,2',3,4,5,5',6
Hexachlorobiphenyls 186 2,2',3,4,5,6,6'
145 2,2',3,4,6,6' 187 2,2',3,4',5,5',6
146 2,2',3,4',5,5' 188 2,2',3,4',5,6,6'
147 2,2',3,4',5,6 189 2,3,3',4,4',5,5'
148 2,2',3,4',5,6' 190 2,3,3',4,4',5,6
149 2,2',3,4',5',6 191 2,3,3',4,4',5',6
150 2,2',3,4',6,6' 192 2,3,3',4,5,5',6
151 2,2',3,5,5',6 193 2,3,3',4',5,5',6
152 2,2',3,5,6,6'
153 2,2',4,4',5,5' Octachlorobiphenyls
154 2,2',4,4',5,6' 194 2,2',3,3',4,4',5,5'
155 2,2',4,4',6,6' 195 2,2',3,3',4,4',5,6
156 2,3,3',4,4',5 196 2,2',3,3',4,4',5,6'
157 2,3,3',4,4',5' 197 2,2',3,3',4,4',6,6'
158 2,3,3',4,4',6 198 2,2',3,3',4,5,5',6
159 2,3,3',4,5,5' 199 2,2',3,3',4,5,6,6'
160 2,3,3',4,5,6 200 2,2',3,3',4,5',6,6'
161 2,3,3',4,5',6 201 2,2',3,3',4,5,5',6'
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMENDATIONS
1. Résumé et évaluation
1.1 Introduction
Découverts vers la fin du siècle dernier, les biphényles polychlorés ou
polychlorobiphényles (PCB) ont vu leur intérêt pour l'industrie
rapidement reconnu en raison de leurs propriétés physiques. On les
utilise dans le commerce depuis 1930 comme fluides diélectriques ou
caloporteurs ainsi que pour diverses autres applications. Largement
répartis dans l'environnement un peu par- tout dans le monde, ce sont
des composés persistants qui s'accumulent dans les différentes chaînes
alimentaires. L'exposition humaine aux PCB résulte en grande partie de
la consommation d'aliments contaminés mais peut également résulter d'une
inhalation ou d'une absorption percutanée sur les lieux de travail. Les
PCB s'accumulent dans les tissus adipeux de l'homme et des animaux et
peuvent déterminer des effets toxiques chez les uns et les autres,
notamment en cas d'exposition répétée. Les effets pathologiques
s'exercent principalement au niveau de la peau et du foie mais les voies
digestives, le système immunitaire et le système nerveux peuvent
également être atteints. Les polychlorodibenzofuranes (PCDF) qui
constituent des contaminants des mélanges de PCB du commerce, ont une
part importante dans la toxicité de ces composés. D'après les études
effectuées sur des rongeurs, il semblerait que certains PCB soient
cancérogènes et qu'ils puissent en outre agir comme promoteurs de la
cancérogénicité d'autres produits chimiques.
Il est clair, d'après les données dont on dispose au sujet des
polychlorobiphényles et des polychloroterphényles (PCT), qu'il vaudrait
mieux que les denrées alimentaires soient totalement exemptes de ces
composés. Il est cependant également clair que ramener à "zéro" ou
presque l'exposition aux PCT ou aux PCB résultant de l'alimentation,
conduirait à éliminer (par interdiction de la consommation) de grandes
quantités d'aliments très importants comme le poisson et plus encore,
comme le lait maternel. C'est aux commissions scientifiques nationales
et internationales qu'il appartient de décider du meilleur compromis
entre une protection suffisante de la santé publique et la nécessité
d'éviter de trop grandes pertes de denrées alimentaires.
Les données disponibles ne permettent pas de déterminer le niveau
d'exposition à ces substances qui constituerait une garantie absolue de
sécurité.
1.2 Identité et propiétés physiques et chimiques
Les PCB sont constitués de mélanges de dérivés aromatiques produits par
chloration du biphényle en présence d'un catalyseur convenable. Ils
répondent à la formule brute C12H10-nCln, le nombre n d'atomes de
carbone variant de 1 à 10.
Il y a théoriquement 209 homologues possibles mais seuls 130 d'entre eux
sont probablement utilisés dans des produits commerciaux. En outre, les
PCB peuvent contenir des impuretés consistant en polychlorodibenzofuranes
(PCDF) et en quaterpényles chlorés. Ces impuretés sont assez stables et
résistantes aux réactions chimiques dans les conditions normales. Tous
les PCB sont lipophiles et très peu solubles dans l'eau. Il en résulte
qu'ils pénètrent facilement dans la chaîne alimentaire et s'accumulent
dans les tissus adipeux.
Les mélanges de PCB utilisés dans le commerce contiennent des
polychlorodibenzofuranes à des concentrations qui vont de quelques mg/kg
à 40 mg/kg. Il n'y a pas de dibenzo- p-dioxines polychlorées (PCDD)
dans les PCB du commerce. Toutefois, en cas de mélange de PCB avec
d'autres dérivés chlorés comme les chlorobenzènes utilisés dans les
transformateurs, il arrive que l'on retrouve des PCDD à la suite
d'incendies accidentels ou après incinération.
Les mélanges de PCB du commerce sont d'une couleur qui va du jaune clair
au jaune foncé. Ils ne cristallisent pas, même à basse température, mais
se transforment en résines solides. Dans la pratique les PCB sont plutôt
ininflammables avec des points d'éclair assez élevés. Leur vapeur est
plus lourde que l'air, avec lequel et ils ne forment pas de mélanges
explosifs. Leur conductivité électrique est très faible mais leur
conductivité thermique assez élevée et ils sont extrêmement résistants
à la décomposition thermique. Les PCB sont chimiquement très stables
dans les conditions normales, toutefois, lorsqu'on les chauffe, ils
peuvent donner naissance à d'autres composés toxiques comme les
polychlorodibenzofuranes.
1.3 Méthodes d'analyse
Par suite de la découverte en 1966 de la présence de PCB dans des
échantillons prélevés dans l'environnement, on s'est intéressé à leur
analyse et à leur toxicité pour l'homme et son environnement.
En raison de la diversité des méthodes d'analyse utilisées, les données
disponibles ne sont pas directement comparables; on peut néamoins les
utiliser lorsqu'on se propose de prendre des mesures de contrôle et de
prévention ainsi que pour une évaluation préliminaire des risques pour
la santé et l'environnement imputables à ces produits.
Le dosage des PCB s'effectue par chromatographie en phase gazeuse avec
détection par capture d'électrons, souvent sur colonne garnie, encore
que l'on puisse recourir à des méthodes plus élaborées telles que la
chromatographie sur colonne capillaire et la chromatographie en phase
gazeuse couplée à la spectrométrie de masse, comme on l'a fait récemment
pour identifier les différents homologues, améliorer la comparabilité
des données analytiques issues de différentes sources et établir les
bases d'une évaluation toxicologique.
Ces analyses nécessitent un programme important d'assurance de la
qualité et, comme cela avait été recommandé, on a procédé à des
étalonnages inter-laboratoires. La qualité et l'intérêt des données
analytiques sont tributaires de la validité de l'échantillon et de la
méthode d'échantillonnage. En outre, il est essentiel que le programme
d'échantillonnage soit dûment planifié et documenté; on trouvera la
description d'une technique détaillée d'échantillonnage dans le document
WHO/EURO (1987).
1.4 Production et emplois
La production commerciale des PCB a commencé en 1930. Depuis, on les
utilise largement dans le matériel électrique et également, en petites
quantités, comme liquide ignifuge dans certains systèmes fonctionnant en
circuit fermé.
A la fin de 1980, la production mondiale totale de PCB dépassait un
million de tonnes et depuis, elle s'est poursuivie dans certains pays.
Bien qu'on renonce de pins en pins à leur emploi et que la production
soit soumise à des restrictions croissantes, de grandes quantités
demeurent dans l'environnement, soit du fait de leur utilisation, soit
sous la forme de déchets.
Ces dernières années, de nombreux pays industrialisés ont pris des
mesures pour contrôler et limiter les rejets de PCB dans
l'environnement. C'est probablement une recommandation émise par
l'Organisation de Coopération et de Développement économiques (OCDE) en
1973 qui a joué un rôle prépondérant dans la promulgation de ces
restrictions (OMS 1976; CIRC 1978; OCDE 1982). Depuis, les 24 pays
membres de l'OCDE ont imposé des restrictions à la production, la vente,
l'exportation et l'emploi des PCB et défini un système d'étiquetage de
ces composés.
Actuellement, les émissions de PCB sont imputables à leur volatilisation
à partir des décharges où sont enfouis des éléments de transformateurs,
de condensateurs et autres déchets de ce genre, des boues d'égouts, des
déversements accidentels ou non, des déchets de dragage et au rejet,
dans des conditions défectueuses ou illégales, de ces produits sur
des terrain à ciel ouvert. L'incinération des déchets industriels ou
municipaux peut produire une pollution. La plupart des incinérateurs
utilisés par les municipalités ne sont pas capables de détruire
efficacement les PCB. L'explosion ou la surchauffe de transformateurs ou
de condensateurs peut entraîner la libération de quantités importantes
de PCB à proximité du lieu de l'incident.
Les PCB peuvent être transformés en polychlorodibenzofuranes par
pyrolyse. Au laboratoire, c'est à des températures comprises entre 550
et 700°C qu'on obtient le meilleur rendement en polychloro-
dibenzofuranes. Ainsi, l'incinération incontrôlée des PCB peut
constituer une source importante de polychlorodibenzofuranes dangereux.
Il est donc recommandé que la destruction des déchets contaminés par des
PCB s'effectue dans des conditions soigneusement contrôlées, notamment
en ce qui concerne la température d'incinération (supérieure à 1000°C),
le temps de séjour et la turbulence.
1.5 Transport, distribution et transformation dans l'environnement
Dans l'atmosphère, les PCB sont principalement présents en phase vapeur;
la tendance a s'adsorber sur les particules augmente avec le degré de
chloration. La présence quasi universelle des PCB donne à penser qu'ils
sont transportés par l'atmosphère.
A l'heure actuelle, la principale source d'exposition aux PCB dans
l'environnement général trouve son origine dans la redistribution de ces
produits après leur passage dans le milieu. Cette redistribution
s'effectue par volatilisation à partir du sol et de l'eau puis passage
et transport dans l'atmosphère suivi d'un dépôt à sec ou en milieu
humide (des PCB liés aux particules), les produits se revolatilisant
ensuite pour continuer le cycle. Dans les précipitations, la
concentration en PCB varie de 0,001 à 0,25 µg/litre. Comme la vitesse de
volatilisation et de décomposition des PCB varient d'un homologue à
l'autre, ce processus de redistribution entraîne une modification dans
la composition des mélanges de PCB présents dans le milieu.
Dans l'eau, les PCB sont adsorbés sur les sédiments et autres matières
organiques; les données d'expérience et de surveillance montrent que
leur concentration dans les sédiments et les matières en suspension est
plus élevée que dans la couche d'eau qui les surmonte. La forte
adsorption des PCB sur les sédiments, notamment dans le cas des dérivés
les plus chlorés, réduit leur vitesse de volatilisation. En se basant
sur la solubilité dans l'eau et le coefficient de partage entre le
n-octanol et l'eau, on peut estimer que les PCB les moins chlorés
seront moins fortement sorbés que les homologues plus substitués. Bien
que l'adsorption puisse immobiliser les PCB pendant des périodes
relativement longues dans le milieu aquatique, on a montré de la
désorption dans la couche d'eau environnante s'effectuait par voie
abiotique ou biotique. Les sédiments aquatiques, qui contiennent de
notables quantités de PCB, jouent donc le rôle à la fois de piège et de
réservoir pour les organismes qui vivent dans ce milieu. On pense que
l'essentiel de la charge en PCB du milieu est adsorbé sur les sédiments
aquatiques.
La faible solubilité et la forte adsorption des PCB sur les particules
de sol en limitent le lessivage; le lessivage est d'autant plus
important que la substitution par le chlore est plus faible.
La décomposition des PCB dans l'environnement dépend de leur degré de
substitution. En général, la persistance s'accroit parallèlement au
degré de substitution. Dans l'atmosphère, la réaction en phase vapeur
des PCB avec les radicaux hydroxyles (qui se forment par voie
photochimique sous l'action du rayonnement solaire) pourrait constituer
le principal processus de transformation. On estime que le temps de
demi-réaction dans l'atmosphère varie de 10 jours pour un
monochlorobiphényle à 1,5 année pour un heptachlorobiphényle.
Dans le milieu aquatique, l'hydrolyse et l'oxydation ne jouent pas un
rôle important dans la décomposition des PCB. Dans le milieu, il semble
que le seul processus viable de décomposition soit le photolyse.
Cependant, les données expérimentales sont insuffisantes pour que l'on
puisse en établir la vitesse et la degré dans l'environnement.
Les microorganismes décomposent assez rapidement le mono-, le di- et le
trichlorobiphényle; cette dégradation étant plus grande dans le cas de
tétrachlorobiphényles. Les biphényles plus substitués résistent à la
biodégradation. La position des atomes de chlore sur le noyau biphényle
influe de manière importante sur la vitesse de biodégradation. Les PCB
qui contiennent des atomes de chlore en para sont plus facilement
biodégradés. Les homologues les plus substitués subissent une
biotransformation anaérobie par déchloration réductrice qui abaisse leur
degré de substitution et les transforme en homologues plus facilement
biodégradable par voie aérobie.
Le degré de bioaccumulation dans les tissus adipeux dépend de plusieurs
facteurs: la durée et le niveau de l'exposition, la structure chimique
du composé et notamment le nombre et la position des substituants. En
général, ce sont les dérivés les plus substitués qui s'accumulent le
plus facilement.
Les facteurs de bioconcentration des différents PCB qui ont été mesurés
expérimentalement chez différentes espèces aquatiques (poisson,
crevette, huitre) vont de 200 à 70 000 ou davantage. En haute mer, les
PCB s'accumulent à des niveaux trophiques plus élevés et l'on trouve
davantage de biphényles fortement substitué chez les prédateurs qui se
situent en fin de chaîne alimentaire.
Le passage des PCB du sol à la végétation se produit principalement par
adsorption sur les surfaces externes des plantes terrestres; il n'y a
guère de déplacement à l'intérieur de la plante.
1.6 Concentrations dans l'environnement et exposition humaine
Du fait de leur forte persistance et d'autres propriétés physiques et
chimiques, les PCB sont présent dans tout l'environnement de la planète.
D'une façon générale, les concentrations dans l'air vont de 0,002 à
15 ng/m3. Dans les zones industrielles, les valeurs sont plus élevées
puisqu'elles peuvent être de l'ordre du µg/m3. Dans les précipitations,
elles vont de 1 ng à 250 ng/litre.
Dans les ambiances de travail, les concentrations dans l'air peuvent
être beaucoup plus élevées. Dans certaines conditions, par exemple, dans
le cas de la fabrication des transformateurs ou des condensateurs, on a
pu observer des concentrations allant jusqu'à 1000 µg/m3. En situation
d'urgence, des concentrations atteignant même 16 mg/m3 ont été
mesurées. Après des incendies ou des explosions, la suie qui en résulte
peut contenir de fortes concentrations de PCB. On en a ainsi trouvé
jusqu'à 8000 mg/kg de suie. Dans ce cas d'ailleurs, ils s'accompagnent
de polychlorodibenzofuranes. Dans les accidents impliquant des
transformateurs contenant du chlorobenzène ainsi que des PCB, on trouve
également des dioxines polychlorées.
Dans ces situations d'urgence, des particules de suie peuvent être
ingérées ou inhalées ou encore contaminer la peau et entraîner une grave
exposition du personnel. Quoiqu'il en soit, l'exposition de la
population générale par la voie atmosphérique est très faible.
Les eaux de surface peuvent être contaminées par des PCB provenant de
retombées atmosphériques, d'émissions directes à partir de sources
ponctuelles ou de décharges. Dans certaines conditions, on a mesuré dans
l'eau des concentrations allant jusqu'à 100-500 ng/litre. Dans les
océans, on a observé des concentrations de 0,05 à 0,6 ng/litre.
Dans les régions non contaminées, l'eau de boisson confient moins de
0,01 ng de PCB/litre mais on a fait état de concentrations allant
jusqu'à 5 ng/litre. Selon les régions et en fonction des conditions
locales, le sol et les sédiments peuvent contenir des PCB à des
concentrations allant de <0,01 à 2,0 mg/kg. Dans les régions polluées,
les teneurs sont beaucoup plus fortes puisqu'elles peuvent atteindre
500 mg/kg.
Plusieurs milliers d'échantillons d'aliments divers ont été analysés au
cours des années dans plusieurs pays à la recherche de contaminants et
en particulier de PCB. La plupart des échantillons provenaient de
produits déterminés, notamment du poisson ou d'autres aliments d'origine
animale comme la viande et le lait. Il y a trois voies principales de
contamination de la nourriture humaine:
a) passage de l'environnement aux poissons, oiseaux, bétail (par
l'intermédiaire de la chaîne alimentaire) et récoltes;
b) migration dans les aliments à partir des matériaux de
conditionnement (essentiellement au-dessous de 1 mg/kg, mais dans
certains cas pouvant atteindre 10 mg/kg);
c) contamination directe des aliments destinés à l'homme ou aux
animaux par suite d'un accident industriel.
La contamination des plus importantes denrées alimentaires par des PCB
s'est située dans les limites suivantes: graisses animales
20-240 µg/kg; lait de vache 5-200 µg/kg; beurre 30-80 µg/kg; poisson
10-500 µg/kg -- valeurs rapportées à la teneur en graisse. Certaines
espèces de poissons (anguilles) ou produits tirés du poisson (foie de
poisson ou huile de poisson) en contiennent des quantités beaucoup plus
élevées, pouvant aller jusqu'à 10 mg/kg. Les concentrations relevées
dans les légumes, les céréales, les fruits ainsi qu'un certain nombre
d'autres produits sont inférieures à 10 µg/kg. Les principaux produits
alimentaires dont il faut surveiller la contamination par les PCB sont
le poisson, les fruits de mer, la viande, le lait et les produits
laitiers. Les concentrations médianes dans le poisson observées dans
divers pays sont de l'ordre de 100 µg/kg (par rapport aux graisses). Si
l'on procède à des comparaisons, on constate que la teneur du poisson en
PCB diminue lentement.
Les PCB s'accumulent dans les tissus adipeux et le lait maternel. Leur
concentration dans les différents organes et tissus dépend de la teneur
de ceux-ci en lipides, sauf dans le cas du cerveau. Les résidus de PCB
présents dans les tissus adipeux de la population générale des pays
industrialisés vont de - 1 à 5 mg/kg de graisses.
Dans les lipides du lait humain, la concentration moyenne en PCB totaux
est de l'ordre de 0,5-1,5 mg/kg de lipides selon le lieu de résidence du
sujet, son mode de vie et la méthode d'analyse utilisée. Les femmes qui
habitent des zones urbaines fortement industrialisées et qui consomment
beaucoup de poisson, surtout pêché dans des eaux très contaminées,
peuvent avoir un lait contenant davantage de PCB.
Dans la plupart des cas; les extraits de PCB provenant d'échantillons
prélevés dans l'environnement n'ont pas une composition analogue à celle
des mélanges du commerce. On a également montré, en procédant par
chromatographie en phase gazeuse à haute résolution, que la composition
en homologues et la concentration relative des différents constituants
présents dans les tissus adipeux et le lait maternel étaient très
éloignées de celles des mélanges de PCB du commerce. L'analyse
chromatographique des PCB présents dans les tissus adipeux humains et
dans le lait maternel fait resortir une forte concentration de PCB
fortement substitués tels que le 2,4,5,3',4'-pentachlorobiphényle,
le 2,4,5,2',4',5'- hexachlorobiphényle, le 2,3,4,2',4',5'-
hexochlorobiphényle, le 2,3,4,5,2',4',5'-hepta-chiorobiphényle et le
2,3,4,5,2',3',4'- heptachlorobiphényle. Quelques autres homologues sont
présents en quantités beaucoup plus faibles; c'est le cas de la plupart
des PCB coplanaires toxiques: le 3,4,3',4'-tétra-, le 3,4,5,3',4'-penta
et le 3,4,5,3',4,',5'-hexa-chlorobiphényle.
On a calculé que la dose quotidienne de PCB ingérée par les nourrissons
à partir du lait maternel était de l'ordre de 4,2 µg/kg de poids
corporel (5,2 µg/100 KCal consommées) (WHO/EURO, 1988). La quantité
moyenne totale de PCB ingérée avec le lait maternel au cours des six
premiers mois de la vie est de 4,5 mg contre 357 mg pour le reste de
l'existence (0,2 µg/kg et par jour ingéré avec la nourriture par une
personne de 70 kg au cours d'une vie de 70 ans). La période
d'allaitement correspond donc à 1,3% de la dose totale ingérée au cours
de l'existence, ce qui n'est pas très élevé compte tenu de l'intérêt que
présente l'allaitement au sein (WHO/EURO, 1988).
En s'appuyant sur les données de base ayant fait l'objet d'une
évaluation, on peut calculer que l'apport de PCB par voie alimentaire ne
dépasse pas 100 µg en moyenne par semaine, c'est-à-dire environ
14 µg/personne et par jour. Pour un individu de 70 kg, cela correspond
à un apport quotidien maximum de l'ordre de de 0,2 µg/kg de poids
corporel (WHO/EURO, 1988).
1.7 Cinétique et métabolisme
L'expérimentation animale rapportée dans la littérature comporte
essentiellement l'exposition par voies orale, respiratoire et percutanée
à des mélanges de PCB ou aux différents homologues. En général, les PCB
sont rapidement absorbés, notamment pas la voie digestive après
ingestion. Cette absorption se produit indiscutablement aussi chez
l'homme mais les données concernant les taux d'absorption sont limitées.
D'après les résultats dont on dispose, il semble que la distribution des
PCB dans l'organisme s'effectue selon un processus cinétique biphasé,
les composés étant rapidement éliminés du sang et s'accumulant dans le
foie et les tissus adipeux des divers organes. On est également fondé à
penser que les PCB franchissent la barrière placentaire, s'accumulent
dans le foetus et passent dans le lait maternel. Certaines études
effectuées sur des sujets humains ont révélé une forte concentration de
PCB dans l'épiderme mais la concentration dans l'encéphale était plus
faible que ce que l'on aurait pu penser en s'appuyant sur la teneur en
lipide de cet organe.
La mobilisation des PCB à partir des graisses dépend largement de la
vitesse de métabolisation des différents homologues. L'excrétion est
tributaire de la transformation des PCB en composés plus polaires:
phénols, thiolo-conjugués et autres dérivés hydrosolubles. Les
différentes voies métaboliques observées comportent une hydroxylation,
une conjugaison avec des thiols et d'autres dérivés hydrosolubles avec
parfois intervention d'intermédiaires réactifs comme les oxydes d'arène.
On a montré que la vitesse de métabolisation dépendait de la structure
des différents PCB et qu'elle était tributaire à la fois du degré de
substitution et de la position de substituants. Les métabolites polaires
des PCB les plus chlorés sont éliminés principalement par la voie fécale
mais l'excrétion urinaire n'est pas négligeable. Le lait maternel
constitue une importante voie d'élimination. Certains PCB peuvent
également être éliminés en passant dans le système pileux.
Les données cinétiques disponibles montrent que le demie-vie des divers
PCB est très variable, ce qui peut s'expliquer par la variabilité du
métabolisme en fonction de la structure, le tropisme tissulaire et
d'autres facteurs qui influent sur la mobilisation à partir des sites
d'accumulation.
Il n'y a pas toujours corrélation entre la persistance dans les tissus
et une forte toxicité, et les différences de toxicité d'un homologue à
l'autre peuvent être liées à des métabolites ou à des intermédiaires
particuliers.
1.8 Effets sur les êtres vivants dans leur milieu naturel
Les PCB sont des contaminants universels du milieu et on les rencontre
dans la plupart des compartiments de l'environnement -- biotiques ou
abiotiques -- dans le monde entier. Etant donné que de nombreux pays en
réglementent l'utilisation et la libération dans l'environnement, les
décharges qui peuvent survenir sont beaucoup moins importantes que par
le passé. Toutefois il semble, à la lumière des données disponibles, que
le cycle des PCB dans le milieu entraîne une redistribution progressive
de certains homologues en direction du milieu marin. Les dérivés les
plus substitués sont ceux qui ont tendance à s'accumuler. Les PCB sont
en grande partie adsorbés à la surface des particules de sédiments mais
ils demeurent biodisponibles pour les divers organismes et leur
accumulation se produit à des niveaux de plus en plus élevés de la
chaîne alimentaire.
1.8.1 Etudes en laboratoire
Les mélanges de PCB exercent sur les microorganismes des effets qui
varient énormément d'une espèce à l'autre puisque certaines sont
affectées dès 0,1 mg/litre alors que d'autres supportent sans dommage
des concentrations de 100 mg/litre; ces effets ne varient pas de façon
régulière avec le degré de chloration des différents mélanges. La
presque totalité des études consacrées aux effets des PCB sur les
organismes aquatiques portent sur des mélanges de type Aroclor. Les
résultats en sont très variables et l'on ne peut pas établir de relation
systématique entre le pourcentage de chloration ou les conditions
écologiques et la toxicité, même dans le cas d'organismes très proches.
Sur 96 heures dans des conditions statiques, les valeurs de CL50 varient
de 12 µg/litre à > 10 mg/litre pour différentes es d'invertébrés
aquatiques et divers mélanges de type Aroclor. Dans des conditions
dynamiques, la toxicité des PCB augmente. En général, les mélanges les
plus toxiques sont des Aroclors moyennement chlorés; en revanche,
lorsque le degré de chloration est faible ou élevé, les mélanges sont
moins toxiques. On le constate également dans le cas des effets
sub-létaux, par example sur la reproduction de la daphnie. Les crustacés
paraissent être plus sensibles aux PCB en période de mue. L'exposition
de populations modèles à de l'Aroclor 1254 a permis d'observer une
modification dans la structure de la communauté des espèces
estuariennes, avec diminution du nombre d'amphipodes, de bryozoaires, de
crabes et de mollusques, le nombre d'annélidés, de brachyopodes, de
coelentérés, d'échinodermes et de némertiens restant inchangé. Les
épreuves de toxicité aiguë portaient sur trop peu de ces groupes pour
qu'on puisse en déduire si les résultats obtenus correspondent à des
variations dans la sensibilité aux PCB ou à des différentes dans les
interactions entre espèces.
On constate des variations analogues dans la toxicité de ces mélanges
chez les poissons pour lesquels la CL50 à 96 heures vade de 0,008 à
100 mg/litre. Des épreuves à long terme ont montré que, en cas
d'exposition aiguë, notamment dans des conditions statiques, les données
obtenues ne donnent qu'une valeur très sous-estimée de la toxicité des
mélanges. La truite arc-en-ciel se révèle particulièrement sensible, les
stades embryo-lavaires présentant une CL50 à 22 jours de 0,32 µg/litre
dans le cas de l'Aroclor 1254, et la dose sans effet observable sur
22jours étant de 0,01 µg/litre dans le cas des Aroclors 1016, 1242 et
1254.
Pour l'espèce Pimephales promelas on a obtenu pour la dose sans effet
observable des valeurs respectivement égales à 5,4, 0,1, 1,8 et
1,3 kg/litre pour les Aroclors 1242, 1248, 1254 et 1260; dans le cas
de Pimelometopon pulcher, on a obtenu une dose sans effet observable
de 3,4 et 0,06 µg/litre respectivement pour les Aroclors 1016 et 1254.
On a pu confirmer expérimentalement des observations effectuées en
milieu naturel et qui tendaient à montrer que des phoques se nourrissant
de poissons ayant accumulé des PCB dans leur chair présentaient des
troubles de la reproduction. Cet effet s'observe au cours des dernières
phases du processus et se traduit par l'impossibilité pour l'embryon de
s'implanter dans la paroi utérine.
Lors d'études à court terme, on a constaté que la toxicité de l'Aroclor
pour les oiseaux augmentait avec le pourcentage de chloration; les CL50
par voie alimentaire à cinq jours allaient de 604 à > 6000 mg/kg de
nourriture. Les principaux effets sur la reproduction des oiseaux
consistaient en une plus grande difficulté d'éclosion pour les oeufs et
une certaine embryotoxicité. Ces effets ont continué malgré l'arrêt du
traitement par les PCB, la concentration de PCB chez les poules
diminuant par passage dans les oeufs. Rien n'indique que les Aroclors
provoquent une amincissement de la coquille, tout du moins directement;
toutefois l'effet qu'ils exercent sur la consommation de nourriture et
le poids des poules agit indirectement sur l'épaisseur de la coquille
des oeufs. des effets sub-létaux ont été signalés sur le comportement et
les sécrétions hormonales.
Chez le vison, la toxicité aiguë de l'Aroclor diminue à mesure
qu'augmente le pourcentage de chloration; la DL50 aiguë par voie orale
se situant entre > 750 et 4000 mg/kg de poids corporel; le furet est
moins sensible. L'Aroclor réduit la consommation de nourriture et par
conséquent le taux de croissance des jeunes visons. L'administration
d'Aroclor diminue et va même jusqu'à arrêter la reproduction des visons,
qu'il soit administré directement ou par suite de l'ingestion de poisson
contaminé dans la nature. Les Aroclors à forte teneur en chlore
(notamment le 1254) ont un effet pins marqué. Lorsque cesse
l'administration d'Aroclor par voie alimentaire, le taux de reproduction
revient à la normale.
Les chauves-souris sont affectées par l'Aroclor libéré dans leur
organisme à partir des graisses au cours de la migration.
Etant donné que la grande majorité des épreuves de laboratoire sur les
organismes aquatiques et terrestres ont été effectuées avec des mélanges
de PCB, il n'est pas possible d'attribuer à tel ou tel constituant en
particulier tel ou tel type d'effets. De même, du fait que ces épreuves
ont été exécutées dans des conditions qui ne correspondent pas aux
conditions écologiques réelles (c'est-à-dire à des concentrations
supérieures à la solubilité des différents constituants et sans la
présence de sédiment), il est difficile d'extrapoler les résultats de
laboratoire à la situation réelle. Toutefois, on peut raisonnablement
penser que tout effet sur les différentes populations d'organismes
aquatiques ou terrestres qui pourrait s'observer à l'avenir, aura déjà
été observé sur des populations locales antérieurement exposées à de
fortes concentrations de PCB.
1.8.2 Etudes dans le milieu naturel
Les résultats qui tendraient à accréditer l'idée d'effets des PCB sur
les populations de poissons dans leur milieu naturel ne sont pas
concluants. L'interprétation des données recueillies sur les oiseaux au
sein de leur milieu naturel est difficile, du fait de la présence de
nombreux résidus provenant de divers organochlorés. La plupart des
auteurs ont montré l'existence d'une corrélation entre les effets
(embryotoxicité) observés et les résidus d'organochlorés totaux. Parmi
tous les composés organochlorés présents ce sont les PCB qui offrent la
meilleure corrélation avec les effets observés sur les embryons mais les
résultats ne peuvent pas être considérés comme démontrant l'existence
d'effets des PCB au sein du milieu naturel.
Un certain nombre de faits (confirmés en laboratoire) montrent que les
PCB réduisent la capacité de reproduction des mammifères marins. Il
s'agit d'une effet sur la nidation de l'embryon, mais qui peut également
s'accompagner de modifications physiques au niveau des voies génitales
des femelles.
Il n'est pas possible d'extrapoler les données obtenues en laboratoire
lors d'études de toxicité aiguë et de toxicité à court terme, pour en
tirer des conclusions relatives aux populations vivant dans le milieu
naturel. L'incertitude qui règne quant aux effets attribués à tel ou tel
constituant des mélanges de PCB, la méconnaissance de la nature exacte
des homologues présents dans l'environnement et le caractère aléatoire
de la biodisponibilité des PCB pour les divers organismes, sont autant
de facteurs qui rendent difficile une estimation des l'exposition et des
effets qui en découlent dans l'environnement. On peut considérer comme
démontrés les effets observés sur les populations de mammifères marins
mais on ne sait pas encore à quels constituants des mélanges de PCB les
attribuer.
Du fait de la tendance à la contamination croissante du milieu matin, il
convient de rester très attentif aux effets exercés sur les organismes
marins. Les observations effectuées en laboratoire ou dans le milieu
naturel montrent clairement que la reproduction des populations de
mammifères marins est affectée dans les zones fortement polluées.
Dans les autres secteurs, il est probable que les résidus vont
s'accroître, entraînant par voie de conséquence une augmentation des
effets sur ces mammifères. On a moins de certitudes quant à la question
de savoir si ces effets s'observeront chez d'autres organismes,
notamment les oiseaux qui se nourrissent d'organismes marins.
A en juger par l'expérimentation en laboratoire, on pourrait s'attendre
à des effets sur les populations et les communautés d'organismes
inférieurs tel que le phytoplancton et le zooplancton. Il est difficile
d'en apprécier l'ampleur et la portée. Selon les données actuellement
disponibles, il ne semble pas que les poissons aient à souffrir des
effets des PCB, encore qu'ils constituent une voie de contamination pour
les mammifères et oiseaux piscivores.
Par exemple, les effets sur les espèces terrestres, les mammifères d'eau
douce piscivores et chauves-souris migratrices qui avaient été signalés
antérieurement, devraient être moins visibles à mesure que les résidus
de PCB se redistribuent dans l'environnement. Les résidus présents dans
les biotes terrestres ne semblent généralement guère être en recul à
l'heure actuelle, mais on ne possède que peu ou pas de données sur les
modifications affectant les différents homologues. La diminution des
résidus de PCB fortement chlorés devrait être lente.
1.9 Effets sur les animaux d'expérience et les systèmes in vitro
1.9.1 Après une unique exposition
Après une unique exposition par voie orale, la toxicité aiguë des
Aroclors est généralement faible chez le rat. Les jeunes animaux se
révèlent plus sensibles (DL50: 1,3-2,5 g/kg de poids corporel) que les
adultes (DL50: 4-11 g/kg de poids corporel). La DL50 la plus faible
observées pour l'Aroclor 1254 chez le rat adulte a été de 1,0 g/kg de
poids corporel. Aucune différence n'a été observée entra les sexes.
Chez les lapins, les valeurs de la DL50 dermique variaient de > 1,26
à < 2 g/kg de poids corporel en ce qui concerne l'Aroclor 1260 (dans
l'huile de maïs) et de 0,79 à < 3,17 g/kg de poids corporel pour
certains autres mélanges de PCB non dilués. Dans le cas d'une
administration par voie intraveineuse, on a observé une DL50 de
0,4 g/kg de poids corporel pour l'Aroclor 1254 chez le rat; après
injection intrapéritonéale, la DL50 chez la souris allait de 0,9 à
1,2 g/kg de poids corporel.
1.9.2 Après une exposition de brève durée
Après une exposition de brève durée par voie orale à des PCB purs ou en
mélange, on a constaté que les principaux organes cibles chez les
mammifères étaient le foie, la peau, le système immunitaire et le
système reproducteur. Parmi les espèces étudiées c'est le singe Rhésus
qui s'est révélé le plus sensible, les femelles l'étant davantage que
les mâles. Des guenons adultes Rhésus exposées à un régime alimentaire
contenant de l'Aroclor 1248 à raison de 2,5 mg/kg ou de 0,09 mg/kg
d'Aroclor/kg de poids corporel et par jour, pendant six mois, ont
présenté un accroissement du taux de mortalité, un retard de croissance,
une alopécie, de l'acné, une hypertrophie des glandes de Meibom et
peut-être une immunodépression. L'examen microscopique a révélé une
infiltration graisseuse du foie avec des foyers de nécrose, une
hyperplasie épithéliale et une kératinisation des follicules pileux. A
plus fortes doses, des altérations histopathologiques ont également été
observées dans d'autres tissus épithéliaux tels que les glandes sébacées
et les glandes de Meibom, la muqueuse gastrique, la vésicule biliaire et
le canal cholédoque, le lit inguéal et les améloblastes. Il y avait
réduction des taux sériques de lipides totaux, de triglycérides et de
cholestérol. L'exposition à des mélanges de PCB du commerce a entrainé
l'augmentation de la concentration en lidipes totaux, en triglycérides
et en cholestérol et/ou en phospholipides dans le foie. Parmi les
différents PCB, ce sont le 3,4,3',4'-tétrachlorobiphényle, le
3,4,5,3',4',5'- ainsi que le 2,4,6,2' 4' 6'-hexachlorobiphényle qui se
sont révélés les plus actifs. A la dose quotidienne de 0,2 mg/kg de
poids corporel, l'Aroclor 1254 a également produit différents autres
effets: lésions lymphoréticulaires, chute de ongles, lésions gingivales,
mais ni acné et ni alopécie. La dose sans effet observable en ce qui
concerne la toxicité générale de l'Aroclor 1242 a été évaluée chez le
singe Rhésus à 0,04 mg/kg de poids corporel par jour. Chez des singes
Rhésus à la mamelle on a observé des effets relativement bénins après
exposition à une dose beaucoup plus forte d'Aroclor 1248 (35 mg/kg de
poids corporel par jour). C'est chez le rat qu'on a le mieux étudié les
effets exercés au niveau du foie: il s'agit d'hypertrophie, de
dégénérescence graisseuse, de prolifération du réticulum endoplasmique,
de porphyrie, d'adénofibrose, d'hyperplasie des canaux biliaires, de
kystes et des lésions précancéreuses et cancéreuses. Chez le rat et la
souris, les effets des différents PCB ont été observés au niveau du
foie, de la rate et du thymus, les homologues coplanaires étant les plus
toxiques. Chez le singe, ces homologues ont produit, à des doses de
1-3 mg/kg de nourriture, des effets de nature et de gravité analogues à
ceux que l'on avait observés après administration d'Aroclor 1242 à la
dose de 100 mg/kg de nourriture et d'Aroclor 1248 à raison de 25 mg/kg
de nourriture.
Après avoir été exposés par la voie dermique à certains PCB seuls ou en
mélanges, des lapins et des souris ont présenté des effets au niveau de
la peau et du foie, effets qui étaient analogues à ceux que l'on observe
après administration par voie orale. Chez les lapins, on a également
observé une atrophie du thymus, une réduction des centres germinaux des
glanglions lymphatiques ainsi qu'une leucopénie.
1.10 Reproduction, embryotoxicité et tératogénicité
1.10.1 Reproduction et embryotoxicité
On n'a pas procédé à des études très complètes sur les effets génésiques
ni sur la tératogénicité des PCB. Lors d'une étude de reproduction
portant sur deux générations de rats, on a pu, en se basant sur des
paramètres génésiques, établir dans le cas de l'Aroclor 1254 une dose
sans effet observable de 0,32 mg/kg de poids corporel et de 7,5 mg/kg de
poids corporel dans le cas de l'Aroclor 1260. Toutefois la dose la plus
faible étudiée, qui était de 0,06 mg/kg de poids corporel, a entrainé
une augmentation du poids relatif du foie chez les ratons juste sevrés.
Chez des singes Rhésus exposés à de l'Aroclor 1016, on a estimé à
0,03 mg/kg de poids corporel la dose sans effet observable en s'appuyant
sur des paramètres génésiques. Toutefois, à cette dose, on constatait
une réduction du poids de naissance et la dose la plus faible étudiée,
soit 0,01 mg/kg de poids corporel, produisait une hyperpigmentation
cutanée.
Pour l'Arcolor 1248 (contaminé par des polychlorodibenzofuranes), on a
estimé à 0,09 mg/kg de poids corporel la dose sans effet observable chez
le singe Rhésus, une année après l'arrêt de l'exposition.
1.10.2 Tératogénicité
Les études sur le rat et le singe dont on connaît les résultats ne font
ressortir aucun effet tératogène après administration de PCB par voie
orale aux animaux au cours de l'organogénèse. Chez le rat, on a estimé
à 50 mg/kg de poids corporel la dose d'Aroclor 1254 sans effet
observable relativement au poids des ratons, la dose la plus faible qui
ait produit un effet étant de 2,5 mg/kg de poids corporel. L'effet
retenu était la foetotoxicité (lésions au niveau des cellules
folliculaires de la thyroïde).
Les épreuves de tératogénicité pratiquées sur des singes Rhésus, des
souris et des rats au moyen de divers PCB n'ont pas permis de mettre en
évidence une dose sans effet observable. Chez les singes Rhésus, une
dose de 0,07 mg/k de poids corporel a entrainé des effets toxiques sur
les mères (3,4,3',4'-tétrachlorobiphényle).
1.11 Mutagénicité
Les mélanges de PCB ne provoquent ni mutation ni lésion chromosomique
dans divers systèmes d'épreuve. En revanche le 3,4,3',4'-tétrachloro-
biphényle provoque des ruptures de chromosomes dans les lymphocytes
humains in vitro. A fortes concentrations, les mélanges de PCB peuvent
endommager la structure primaire de l'ADN, comme le montrent les
ruptures constatées sur l'un des brin de l'ADN lors d'épreuves d'élution
en milieu alcalin.
1.12 Cancérogénicité
L'interprétation des données relatives aux effets des mélanges de PCB du
commerce sur l'animal est souvent compliquée du fait l'on manque de
renseignements sur la présence ou la part relative des impuretés que
constituent les chlorodibenzofuranes ainsi que sur la proportion des
divers homologues dans le mélange.
Un certain nombre d'éludes de cancérogénicité à long terme ont été
effectuées sur des rats et des souris à l'aide de mélanges tels que les
Kanéchlors 300, 400 et 500, les Aroclors 1254 et 1260 ainsi que les
Clophènes A30 et A60. Les Clophènes étaient exempts de
chlorodibenzofuranes mais on ne possède aucune donnée sur la pureté des
autres mélanges de PCB.
Chez des souris recevant une alimentation qui contenait du Kanéchlor 500
et de l'Aroclor 1254 à des doses d'environ 15 à 25 mg/kg de poids
corporel, on a constaté une augmentation sensible des adénomes et/ou des
carcinomes hépatocellulaires. Aucune tumeur maligne n'a pu être observée
chez des souris traitées par du Kanéchlor 300 et du Kanéchlor 400.
Chez des rats exposés pendant plus d'une année à de l'Arcolor 1254 et
1260 ainsi qu'à du Clophène A30, on a observé une augmentation de la
fréquence des adénomes et/ou des carcinomes hépatocellulaires. Le nombre
plus élevé d'animaux porteurs de tumeurs observé dans ces études n'a pu
cependant être considéré comme statistiquement significatif, à l'inverse
de deux autres études. Ainsi, un accroissement de l'incidence des
carcinomes hépatocellulaires (trabéculaires) et des adénocarcinomes a
été mis en évidence après administration d'Arcolor 1260 et de Clophène
A30 à la dose d'environ 5 mg/kg de poids corporel.
Les tumeurs hépatiques observées n'étaient pas de type invasif (il
s'agissait de tumeurs bénignes ou de faible malignité, sans métastases)
et elles n'abrégeaient pas la vie des animaux.
Dans certaines de ces études, on a observé une adénofibrose, des lésions
prénéoplasiques et/ou des nodules néoplasiques dans le foie. Une épreuve
portant sur l'Arcolor 1254 a permis de mettre en évidence un
accroissement des lésions métaplasiques intestinales ainsi que des
adénocarcinomes dans la région glandulaire de l'estomac chez le rat.
L'hypothèse selon laquelle les PCB augmenteraient la cancérogénèse
hépatique chez des rongeurs prétraités par des hépatocancérogènes est
étayée par de nombreux faits. Toutefois l'activité initiatrice des
mélanges de PCB chez les rongeurs n'est guère attestée. Sur la base des
études de génotoxicité publiées, on peut conclure que les mélanges de
PCB ne sont pas génotoxiques. Il s'ensuit que le lien entre la présence
de rumeurs hépatiques et l'administration de PCB chez des rongeurs peut
être attribué à des mécanismes épigénétiques entraînant une
prolifération des cellules hépatiques et autres manifestations
d'hépatotoxicité-autrement dit, il serait possible d'évaluer la toxicité
des PCB en envisageant l'existence d'un seuil de toxicité. Il faut donc
étudier la possibilité, pour les PCB, de favoriser la cancérogénèse dans
des tissus autres que les tissus hépatiques, chez les animaux préexposés
à divers cancérogènes spécifiques de tel ou tel tissu. Il est possible
que l'activité anticancérogène des PCB, observée dans certaines études
au cours desquelles on les avait administrés à des animaux pendant ou
avant l'administration de cancérogènes, soit liée au fait que les PCB
sont capables d'induire les enzymes microsomiques, d'où une stimulation
du processus de détoxication.
Globalement, il est justifié d'être prudent dans l'extrapolation à
l'homme des données obtenues sur l'animal en ce qui concerne le pouvoir
cancérogène des PCB.
1.13 Etudes spéciales
Les lésions induites après exposition à divers PCB purs ou en mélange,
s'observent au niveau du foie, de la peau, du système immunitaire, de
l'appareil reproducteur, et alles s'accompagnent d'oedème et de troubles
fonctionnels des voies digestives et de la glande thyroïde.
Les PCB sont capables d'induire diverses enzymes hépatiques. On a pu le
mettre en évidence chez des rats, des souris, des cobayes, des lapins,
des chiens et des singes en ce qui concerne les Aroclors 1248, 1254 et
1260 ainsi que le Kanéclor 400 (induction du cytochrome P450 et P448).
Le pouvoir enzymo-inducteur des PCB augmente avec la teneur en chlore de
la molécule. Il dépend également de la composition du mélange, les PCB
dans lesquels le chlore se trouve en para- et en meta- provoquant
l'induction du P450. En ce qui concerne l'induction de l'AHH, la
position des atomes de chlore semble plus importante que le degré de
chloration. Les inducteurs les plus actifs de l'AHH sont les PCB dont
les deux positions para- et au moins deux positions meta- sont
substituées par du chlore. Des variations interspécifiques distinctes
ont été mises en évidence. C'est avec l'Aroclor 1260 administré à des
rats Osborn-Mendel que l'on a obtenu la dose sans effet observable la
plus faible (0,025 mg/g de poids corporel).
En ce qui concerne les effets sur le système endocrinien, il s'agit de
modifications touchant la liaison aux récepteurs hormonaux et
l'équilibre des hormones stéroïdiennes. On également des preuves
directes et indirectes d'une faible activité oestrogénique exercée par
les divers Arcolors. Chez des rats exposés pendant 36 semaines à une
régime alimentaire contenant 75 mg d'Aroclor 1242/kg de nourriture, on
a constaté une diminution du taux d'hormones gonadiques et une
augmentation du poids relatif des testicules. Chez des souris femelles
exposées pendant trois semaines à de l'Aroclor 1254 administré dans leur
nourriture à raison de 25 mg/kg, on a observé une diminution des taux de
corticostéroïdes plasmatiques sans augmentation concomitante du poids
des surrénales. En revanche, chez une autre souche qui avait reçu
pendant deux semaines une nourriture contenant 200 mg de ce mélange par
kg, on a observé un accroissement du poids des surrénales.
On a constaté chez diverses espèces animales, que les mélanges de PCB
exerçaient un effet immunodépresseur, les espèces les plus sensibles à
cet égard étant les singes et les lapins. La dose sans effet observable
la plus faible était de 0,1 mg/kg de poids corporel chez le singe et de
0,18 mg/kg de poids corporel chez le lapin.
Des souris ayant reçu une seule dose orale de 500 mg d'Aroclor 1254 par
kg de poids corporel ont présenté une dépression de l'activité motrice.
Cet effet s'explique probablement par une inhibition du captage et de la
libération des neurotransmetteurs.
On a constaté que les mélanges de PCB diminuaient la concentration en
vitamines A et B1 dans le sang et le foie de rats. Chez des rats et des
souris exposés à des mélanges de PCB on a observé une diminution des
taux de vitamines A, B1, B2 et B6.
1.14 Facteurs qui modifient la toxicité et le mode d'action
Les PCB du commerce suscitent toute une série de réactions toxiques qui
ressemblent en partie à celles qu'entraînent les polychlorodioxines et
les polychlorodibenzofuranes. En outre, les relations structure-activité
analogues observées parmi les divers PCB homologues, pour ce qui
concerne les réactions toxiques qu'ils suscitent et leur aptitude à
induire l'AHH dépendante du cytochrome P448, indiquent que les PCB
ressemblent plus ou moins à des stéréoisomères de la 2,3,7,8,-TCDD sont
les plus actifs. Ces observations laissent penser qu'il existe un
mécanisme commun à la base de l'affinité de ces composés pour la
protéine réceptrice de l'AH du cytosol. On a proposé des facteurs
d'équivalence toxique pour la 2,3,7,8,-TCDD et ces PCB coplanaires. On
n'a pas suffisamment étudier la nature des interactions probables entre
les PCB et les polychlorodibenzofuranes ou les polychlorodibenzodioxines.
Etant donné que les PCB sont capables de stimuler l'activité des enzymes
microsomiques, ils peuvent avoir une influence sur l'action d'autres
substances chimiques dont le métabolisme est sous la dépendance de ces
enzymes. D'autres PCB, qualifiés de coplanaires, peuvent entraîner des
manifestations toxiques plus subtiles. En outre certains PCB, en
particulier ceux qui sont les moins substitué, peuvent être métabolisés
sous forme d'intermédiaires de type oxyde d'arène et de métabolites
méthylsulfonylés.
1.15 Effets sur l'homme
L'évaluation toxicologique des PCB pose de nombreux problèmes. Les PCB
se présentent en général sous la forme de mélanges de nombreux composés
et nombre des données relatives à la toxicité des PCB reposent sur
l'étude de ces mélanges. Certains constituants des mélanges se
décomposent plus facilement dans l'environnement que d'autres. Ainsi, la
population générale peut-elle être exposée à des mélanges qui diffèrent
de ceux auxquels sont exposés les travailleurs qui manipulent des PCB.
C'est principalement par contamination de la nourriture (organismes
aquatiques, produits carnés et laitiers) que la population générale est
exposée aux PCB. La dose journalière ingérée de PCB est de l'ordre de
quelques microgrammes par personne dans la plupart des pays
industrialisés. Ce type d'exposition n'entraîne pas de manifestations
toxiques. Les nourrissons sont exposés aux PCB par l'intermédiaire du
lait maternel. Par cette voie, la dose ingérée peut atteindre quelques
microgrammes par kg de poids corporel et par jour.
On éprouve beaucoup de difficulté à évaluer les effets qu'exercent
séparément sur la santé humaine les PCB, les polychlorodibenzofuranes et
les polychlorodibenzodioxines étant donné que les polychloro-
dibenzofuranes sont de fréquents contaminants des mélanges de PCB et
qu'occasionnellement, on a mis en cause la présence de polychloro-
dibenzodioxines dans les accidents survenus avec certains mélanges de
PCB. On a montré que les PCB du commerce étaient contaminés par des
polychlorodibenzofuranes et que, par conséquent, il était délicat dans
bien des cas de savoir si les effets constatés sont attribuables aux PCB
eux-mêmes ou aux polychlorodibenzofuranes qui sont beaucoup plus
toxiques. Par conséquent, nombre de données tirés d'épisodes importants
d'intoxication humaine, par exemple ceux de Yusho, de Yu-Cheng, etc.
correspondent probablement à une exposition aux PCB et aux
polychlorodibenzofuranes.
Les signes d'intoxication observés chez les malades de Yusho et de
Yu-Cheng consistaient en hypersécrétion des glandes de Meibom au niveau
des yeux, en oedèmes palpébraux et en pigmentation des ongles et des
muqueuses, parfois associés à de la fatigue, des nausées et des
vomissements. On observait généralement ensuite une hyper-keratose et un
brunissement de la peau avec une hypertrophie folliculaire et des
éruptions acnéiformes. Par ailleurs, on a également observé des oedèmes
des bras et des jambes, une hypertrophie du foie avec troubles
hépatiques, des troubles du système nerveux central et des troubles
respiratoires évoquant la bronchite ainsi que des modifications dans
l'état immunitaire des patients. Chez les enfants des malades de Yusho
et de Yu-Cheng, on a observé une réduction de la croissance, une
hyperpigmentation de la peau et des muqueuses, une hyperplasie
gingivale, des paupières oedématiées avec xérophthalmie, la présence de
dents dès la naissance, une calcification anormale du crâne, des pieds
bots en piolet et une forte incidence de faibles poids de naissance. Il
n'a pas été possible de se prononcer de façon définitive quant à
l'existence d'une corrélation entre l'exposition et l'apparition de
tumeurs malignes chez ces malades car le nombre de décès était trop
faible. Toutefois, on a observé une augmentation statistiquement
significative, chez les hommes, de h mortalité par cancer et plus
spécialement cancer du foie et du poumon.
En cas d'exposition sur les lieux de travail, on observe quelques heures
plus tard des éruptions cutanées. En outre il est arrivé qu'après
l'exposition à de fortes concentrations de PCB, se produisent des
démangeaisons, des sensations de brûlure, une irritation de la
conjonctive, une pigmentation des doigts et des ongles et une chloracné.
La chloracné est une des manifestations qui reviennent le plus
fréquemment chez les travailleurs exposés aux PCB. Outre ces signes
cutanés d'intoxication, divers auteurs ont observé des troubles
hépatiques, une immunodépression, une irritation passagère des muqueuses
respiratoires, des effets neurologiques, psychologiques ou
psychosomatiques aspécifiques tels que céphalées, vertiges, dépression,
troubles du sommeil et de la mémoire, nervosité, fatigue et impuissance.
Ce qu'on peut conclure de tout cela c'est qu'une exposition
professionnelle permanente à de fortes concentrations de PCB et de
polychlorodibenzofuranes peut entraîner des effets sur la peau et le
foie.
Deux importantes études de mortalité ont été effectuées sur des cohortes
de travailleurs. Après exposition à de l'Aroclor 1254, 1242 et 1016, on
a observé une augmentation de la mortalité par cancer du foie et de la
vésicule biliaire dans le cas d'une étude ou par cancer en général et
plus particulièrement cancer des voies digestives dans le cas d'une
autre étude. Aucune des études épidémiologiques disponibles ne donne de
preuves concluantes d'une association entre l'exposition aux PCB et
l'accroissement de la mortalité par cancer, du fait du trop petit nombre
de décès dans la population exposée, de l'absence de relation
dose-réponse et des impuretésprésents dans les mélanges de PCB.
2. Conclusions
2.1 Distribution
Du fait de leurs propriétés physiques et chimiques, les PCB sont
dispersés dans tout l'environnement à l'échelle planétaire.
Les PCB sont presque universellement présents chez tous les êtres
vivants dans leur milieu naturel et s'y accumulent facilement. On a
également mis en évidence une bioconcentration le long de la chaîne
alimentaire.
Les PCB les plus fortement chlorés sont ceux qui s'accumulent le plus.
2.2 Effets sur les animaux d'expérience
Les résultats tirés de l'expérimentation animale incitent à penser que
les PCB ont un effet immunodépresseur comme le montre l'étude de leurs
effets macroscopiques sur la fonction immunitaire (poids de la rate,
poids du thymus et numération lymphocytaire). En ce qui concerne
l'Aroclor 1248, la dose sans effet observable pour le singe a été
évaluée à 100 µg/kg et à < 100 µg/kg de poids corporel dans le cas de
l'Aroclor 1254. Il semble que l'effet immunosuppresseur soit spécifique
de tel ou tel PCB en particulier.
On n'observe en général d'effets toxiques sur la reproduction qu'aux
doses qui produisent une intoxication de la mère. Les animaux
nouveau-nés nourris avec le lait contaminé de leur mère (notamment chez
le singe et les autres animaux utilisés comme modèles) semblent être
particulièrement sensibles aux PCB et présentent, à côté d'autres
symptômes toxiques, une réduction de la croissance. La dose d'Aroclor
1016 sans effet observable sur la reproduction est de 30 µg/kg de poids
corporel chez le singe. Il n'a pas été possible d'en établir une dans le
cas de l'Aroclor 1248.
Les PCB ne sont pas génotoxiques et rien n'indique qu'ils jouent le rôle
d'initiateurs tumoraux. Ils n'ont pas non plus d'activité
tumoropromotrice. On peut en conclure que pour évaluer la toxicité des
des mélanges de PCB, il est possible d'envisager l'existence d'un effet
de seuil.
2.3 Effets sur l'homme
L'exposition de la population générale aux PCB s'effectue principalement
par l'intermédiaire des aliments. Les nourrissons sont exposés par
l'intermédiaire du lait maternel.
Deux importants épisodes d'intoxication ont été observés au Japon
(Yusho) et à Taïwan (Yu-Cheng). Les principaux symptômes observés ont
été fréquemment attribués à la présence de contaminants dans les
mélanges de PCB et notamment de polychlorodibenzofuranes. Le Groupe de
travail en a conclu que ces symptômes pouvaient être dus à une
exposition concomitante aux PCB et aux polychlorodibenzofuranes.
Toutefois, certain, symptômes notamment des effets respiratoires
chroniques, pourraient être dus plus particulièrement aux métabolites
méthylsulfoniques de certains PCB.
2.4 Effets sur l'environnement
Si des effets ont été signalés sur des populations locales d'oiseaux,
l'effet le plus important des PCB sur les êtres vivants dans leur milieu
naturel consiste principalement en une réduction de la capacité de
reproduction des mammifères marins. On a observé cet effet
principalement dans des mers semi-fermées et constaté qu'il conduisait,
localement du moins, à une réduction du nombre de ces mammifères. Comme
on peut s'attendre à ce que les résidus de PCB présents dans
l'environnement se redistribuent progressivement par l'intermédiaire du
milieu marin, on peut penser qu'à l'avenir, les mammifères marins seront
encore plus menacés.
3. Recommandations
* Il est recommandé de parvenir à un accord international sur les
méthodes d'analyse afin d'améliorer la comparabilité des résultats
des programmes de surveillance. Il faudrait continuer à mettre au
point les méthodes d'analyse spécifiques de tel ou tel PCB, sans
toutefois méconnaître la valeur des analyses basées sur les
mélanges.
* Afin d'assurer la fiabilité des données d'analyse, il est fortement
recommandé de procéder à un contrôle de qualité interlaboratoires.
Il est également recommandé de créer un réseau international
d'encadrement et de soutien technique destiné à aider les pays en
développement à participer aux activités de contrôle.
* Afin d'améliorer la précision dans l'évaluation du risque que
représentent les PCB, il est recommandé d'effectuer des études à
long terme sur des homologues déterminés ainsi que sur le mode
d'action des divers constituants des mélanges, plus
particulièrement en ce qui concerne leur activité tumoropromotrice.
* Des études épidémiologiques visant à mieux évaluer le risque pour
les nouveau-nés sont nécessaires, car ces derniers constituent le
groupe le plus vulnérable de la population générale du fait qu'ils
sont fortement exposés aux PCB par l'intermédiaire du lait
maternel.
* Il conviendrait de mettre au point, pour les futures études
épidémiologiques, des marqueurs biologiques sensibles et
spécifiques concernant certaines des manifestations les plus
subtiles de la toxicité des PCB (effets sur la reproduction, effets
immunologiques et effets neurologiques).
* L'élimination des PCB doit s'effectuer par incinération dans des
installations convenablement conçues et exploitées qui garantissent
le maintien de températures élevées (plus de 1000°C), du temps de
séjour et de la turbulence nécessaires pour que la décomposition
des molécules soit complète.
* Il faudrait étudier les moyen d'éliminer les PCB déjà présents dans
les décharges contrôlées,
* Il convient d'inciter les responsables à assurer la surveillance
des PCB dans l'environnement, la faune et la flore à l'échelle
mondiale, afin de suivre la redistribution prévisible des résidus
qui s'y trouvent.
* La contamination par les PCB peut réduire la capacité de
reproduction des mammifères marins. Il faudrait inciter les
responsables à entreprendre des études sur les effectifs de cétacés
et leur capacité à se reproduire, tout en poursuivant les
recherches visant à établir quels sont les PCB qui sont
responsables de ces effets.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMMENDACIONES
1. Resumen y evaluación
1.1 Introducción
Los bifenilos policlorados (BPCs) se descubrieron a finales del siglo
pasado y se reconoció pronto su utilidad para la industria, debido a sus
propiedades físicas. Se utilizan comercialmente desde 1930 como fluidos
dieléctricos e intercambiadores de calor y en otras aplicaciones. Se
encuentran ampliamente distribuidos en el medio ambiente de todo el
mundo, son persistentes y se acumulan en la cadena alimentaria. La
exposición humana a los BPCs se debe fundamentalmente al consumo de
alimentos contaminados, pero también a la inhalación y a la absorción
cutánea en los lugares de trabajo. Los BPCs se acumulan en el tejido
adiposo de los seres humanos y de los animales, causando efectos tóxicos
a ambos, particularmente en el caso de exposiciones repetidas. La
patología se manifiesta sobre todo en la piel y el hígado, aunque
también están expuestos el tracto gastrointestinal, el sistema
inmunitario y el sistema nervioso. Los dibenzofuranos policlorados
(BFPCs), que se encuentran como contaminantes en mezclas comerciales de
BPCs, contribuyen de manera significativa a su toxicidad. Los resultados
de los estudios realizados en roedores indican que algunos compuestos
parecidos a los BPCs pueden ser carcinógenos y fomentar la
carcinogenicidad de otros compuestos químicos.
De los datos disponibles de los bifenilos policlorados (BPCs) y los
terfenilos policlorados (TPCs) es evidente que, en una situación ideal,
sería preferible no tener en absoluto estos compuestos en los alimentos.
Sin embargo, es igualmente claro que la reducción a cero o a un nivel
próximo de la exposición a los BPCs o los TPCs en fuentes alimentarias
significaría la eliminación (prohibición del consumo) de grandes
cantidades de alimentos importantes, como el pescado, pero sobre todo la
leche materna. Son los comités científicos nacionales e internacionales
los que deben establecer el debido equilibrio entre lo que se ha de
hacer para conseguir un grado apropiado de protección de la salud
pública y evitar pérdidas excesivas de alimentos.
A partir de los datos disponibles, no se pueden establecer niveles de
exposición a los BPCs o los TPCs que puedan considerarse de garantía
absoluta de inocuidad.
1.2 Identidad y propiedades físicas y químicas
Los BPCs son mezclas de productos químicos aromáticos, que se obtienen
por cloración del bifenilo en presencia de un catalizador adecuado. La
fórmula química de estos compuestos se representa como C12 H10-n Cln,
donde n es un número de átomos de cloro comprendido entre 1 y 10.
En teoría existen 209 compuestos análogos, pero sólo 130 tienen
probabilidad de aparecer en productos comerciales. Además, los BPCs
pueden contener dibenzofuranos policlorados (DFPCs) y cuarterfenilos
clorados como impurezas. En condiciones normales, estas impurezas son
relativamente estables y resistentes a las reacciones químicas. Todos
los compuestos afines a los BPCs son lipófilos y tienen una solubilidad
en agua muy baja. En consecuencia, se introducen fácilmente en la cadena
alimentaria y se acumulan en el tejido adiposo.
Las mezclas comerciales de BPCs contienen DFPCs en concentraciones que
oscilan entre unos pocos mg/kg y 40 mg/kg. En los BPCs comerciales no se
encuentran dibenzo- p-dioxinas policloradas (DDPCs). Sin embargo, en
casos de incendios accidentales y durante la incineración se pueden
encontrar DDPCs cuando están mezcladas con otros compuestos clorados,
como los clorobencenos utilizados en los transformadores.
Las mezclas comerciales de BPC tienen un color que va del amarillo claro
al oscuro. No cristalizan, ni siquiera a baja temperatura, sino que se
convierten en resinas sólidas. Los BPCs son prácticamente
pirorresistentes, con una temperatura de inflamabilidad bastante
elevada. Forman vapores más densos que el aire, pero no dan lugar a
mezclas explosivas con éste. Su conductividad eléctrica es muy baja, la
térmica es bastante alta y tienen una resistencia muy elevada a la
degradación térmica. En condiciones normales, los BPCs son químicamente
muy estables, pero cuando se calientan pueden producir otros compuestos
tóxicos, como los DFPCs.
1.3 Métodos analíticos
En 1966, a partir del descubrimiento de BPCs en muestras obtenidas del
medio ambiente, aumentó el interés por el análisis de estos compuestos
y por su toxicidad para la especie humana y su medio ambiente.
Los datos disponibles no son directamente comparables debido, a
diferencias en la metodología analítica; no obstante, se pueden utilizar
para establecer medidas de control y prevención y para la evaluación
preliminar de los riesgos para la salud y el medio ambiente asociados a
estos compuestos.
Los BPCs se han determinado mediante técnicas de cromatografía de gases
con captura electrónica, a menudo utilizando columnas de relleno, aunque
en estudios recientes se han empleado métodos más complejos, como la
cromatografía en columna capilar y la de gases combinada con la
espectrometría de masas, para identificar por separado los distintos
compuestos análogos, mejorar la comparabilidad de los datos analíticos
de fuentes diferentes y establecer una base para la evaluación de la
toxicidad.
Para realizar estos análisis es necesario un amplio programa de garantía
de la calidad, y se han realizado y recomendado estudios de
intercalibración. La calidad y utilidad de los datos analíticos dependen
decisivamente de la validez de la muestra y de que el muestreo sea
adecuado. Por otra parte, es imprescindible contar con un programa de
muestreo planificado y bien documentado. En la publicación WHO/EURO
(1987) se describe con detalle un procedimiento de muestreo.
1.4 Producción y usos
La producción comercial de los BPCs comenzó en 1930. Se han utilizado
ampliamente en equipo eléctrico, y en volúmenes más pequenos como
líquido pirorresistente en sistemas de régimen cerrado.
Al final de 1980, la producción mundial total de BPCs era superior a un
millón de toneladas y, desde entonces, la producción ha continuado en
algunos países. A pesar de la creciente retirada del uso y de las
restricciones sobre la producción, en el medio ambiente sigue habiendo
cantidades muy elevades de estos compuestos, bien en uso o como desecho.
En los ultimos años, muchos países industrializados han adoptado medidas
para controlar y limitar el flujo de BPCs hacia el medio ambiente. El
factor decisivo que ha llevado a estas restricciones ha sido
probablemente una recomendación de 1973 de la Organización de
Cooperación y Desarrollo Económicos (OCDE) (OMS, 1976; CIIC, 1978; OCDE,
1982). Desde entonces, los 24 países miembros de la OCDE han limitado la
fabricación, la venta, la importación, la exportación y el uso de BPCs,
además de establecer un sistema de etiquetado de estos productos.
Entre las fuentes actuales de liberación de BPCs figuran la
volatilización de vertederos que contienen transformadores,
condensadores y otros residuos con BPCs, aguas residuales, fangos
cloacales, derrames y desechos de dragado, y la eliminación inadecuada
(o ilegal) en zonas abiertas. Se puede producir contaminación durante la
incineración de desechos industriales y municipales. La mayoría de los
incineradores municipales no son eficaces en la destrucción de los BPCs.
La explosión o el sobrecalentamiento de transformadores y condensadores
pueden liberar cantidades significativas de BPCs al entorno local.
Los BPCs se pueden convertir en DFPCs en condiciones pirolíticas. En las
condiciones de laboratorio, la máxima producción de DFPCs se obtuvo a
temperaturas entre 550°C y 700°C. Así pues, la combustión incontrolada
de BPCs puede ser una importante fuente de los peligrosos DFPCs. Por lo
tanto, se recomienda un cuidadoso control de la destrucción de desechos
contaminados con BPCs, especialmente en relación con la temperatura de
combustión (por encima de los 1000°C), el tiempo de permanencia y la
turbulencia.
1.5 Transporte, distribución y transformación en el medio ambiente
Los BPCs se encuentran en la atmósfera principalmente en fase de vapor;
la tendencia a adsorberse sobre partículas aumenta con el grado de
cloración. La distribución prácticamente universal de los BPCs parece
indicar que los transporta el aire.
En la actualidad, la principal fuente de exposición en el medio ambiente
general parece ser la redistribución de los BPCs que previamente se han
introducido en él. Dicha redistribución se deriva de su volatilización
del suelo y el agua para pasar a la atmósfera, con el posterior
transporte por el aire y la eliminación de la atmósfera mediante
sedimentación húmeda o seca (de los BPCs unidos a partículas), para
luego volver a volatilizarse. Su concentración en las precipitaciones
oscila entre 0,001 y 0,25 µg/litro. Dado que los ritmos de
volatilización y degradación de los BPCs varían según los compuestos,
esta redistribución produce una alteración en la composición de las
mezclas de BPC presentes en el medio ambiente.
En el agua, los BPCs se adsorben en los sedimentos y otra materia
orgánica; los datos experimentales y de supervisión han puesto de
manifiesto que las concentraciones de BPCs en los sedimentos y en la
materia en suspensión son más elevadas que en las masas de agua
correspondientes. Una fuerte adsorción en el sedimento, especialmente en
el caso de BPCs con un grado elevado de cloración, disminuye la tasa de
volatilización. Sobre la base de su solubilidad en agua y los
coeficientes de reparto n-octanol-agua, los compuestos del grupo del
BPC menos clorados se adsorberán con menos fuerza que los isómeros con
más átomos de cloro. Aunque la adsorción puede inmovilizar los BPCs en
el medio acuático durante períodos relativamente largos, se ha
demostrado que la liberación a la masa del agua se produce tanto por vía
abiótica como biótica. Por consiguiente, las importantes cantidades de
BPCs en los sedimentos acuáticos pueden actuar como sumideros del medio
ambiente y como depósito de estos compuestos para los organismos. Se ha
estimado que la mayor parte de los BPCs presentes en el medio ambiente
está en el sedimento acuático.
La baja solubilidad y la fuerte adsorción de los BPCs en las partículas
del suelo limitan la lixiviación; los compuestos con menor grado de
cloración tienen una tendencia mayor a la lixiviación que los más
clorados.
La degradación de los BPCs en el medio ambiente depende del grado de
cloración del bifenilo. En general, la persistencia de los isómeros de
BPC aumenta con el grado de cloración. En la atmósfera, el proceso de
transformación predominante puede ser la reacción en fase de vapor de
los BPCs con radicales hidroxilos (formados fotoquímicamente por la luz
solar). La semivida estimada de esta reacción en la atmósfera oscila
entre unos 10 días para el monoclorobifenilo y año y medio para el
heptaclorobifenilo.
En el medio acuático, la hidrólisis y la oxidación no degradan de manera
significativa los BPCs. La fotólisis parece ser el único proceso
abiótico de degradación viable en el agua; sin embargo, los datos
experimentales disponibles no son suficientes para establecer su
proporción o importancia en el medio ambiente.
Los microorganismos degradan los bifenilos monoclorados, diclorados y
triclorados de manera relativamente rápida, y más lentamente los
bifenilos tetraclorados, mientras que los bifenilos con mayor grado de
cloración son resistentes a la biodegradación. La posición de los átomos
de cloro en el anillo bifenilo parece ser importante para determinar la
tasa de biodegradación. Esta se da con preferencia en los compuestos que
contienen átomos de cloro en posiciones -para. Los compuestos más
clorados experimentan una transformación anaerobia, mediante un
decloración reductora, para dar BPCs con menos átomos de cloro, que
pueden luego continuar la biodegradación mediante procesos aerobios.
El grado de bioacumulación en el tejido adiposo depende de varios
factores: la duración y el nivel de la exposición, la estructura química
del compuesto y la posición y modelo de la sustitución. En general, se
acumulan más fácilmente los compuestos con mayor número de sustituyentes
de cloro.
Los factores de bioconcentración de distintos BPCs determinados
experimentalmente en las especies acuáticas (peces, camarones, ostras)
varía entre 200 y 70 000 o más. En mar abierto, hay bioacumulación de
BPCs en los niveles tróficos más elevados, con una mayor proporción de
los bifenilos más clorados en los depredadores que ocupan un lugar más
alto en la escala.
La transferencia de los BPCs del suelo a la vegetación tiene lugar
principalmente por adsorción en la superficie externa de las plantas
terrestres; los desplazamientos que tienen lugar son escasos.
1.6 Niveles medioambientales y exposición humana
Debido a su elevada persistencia y sus demás propiedades físicas y
químicas, los BPCs están presentes en el medio ambiente en todo el
mundo.
En general, sus concentraciones en el aire son de 0,002 a 15 ng/m3.
En zonas industriales los niveles son más altos (hasta del orden de
µg/m3). En el agua de lluvia y la nieve alcanzan valores entre no
detectables (1 ng) y 250 ng/litro.
En el medio de trabajo, los niveles en el aire pueden ser mucho más
elevados. En ciertas condiciones, como por ejemplo en la fabricación de
transformadores y condensadores, se han observado concentraciones de
hasta 1000 µg/m3. En casos de emergencia grave se han medido niveles de
hasta 16 mg/m3. En casos de incendios o explosiones se puede producir
hollín que contiene niveles altos de BPCs. Se han encontrado niveles de
8000 mg de BPCs/kg de hollín. En este caso también hay DFPCs. En
accidentes con transformadores que contienen bencenos clorados aparecen
también dioxinas policloradas (DDPCs), además de BPCs.
En tales situaciones de emergencia se pueden producir ingestión,
contaminación de la piel o inhalación de partículas de hollín, con una
exposición grave del personal. Sin embargo, la exposición de la
población general a través del aire es muy baja.
Las aguas superficiales se pueden contaminar con BPCs procedentes de la
atmósfera, de emisiones directas de fuentes puntuales o de la
eliminación de desechos. En ciertas condiciones se han medido
concentraciones de 100-500 ng/litro de agua. En los océanos se han
detectado niveles de 0,05 a 0,6 ng/litro.
En zonas no contaminadas, el agua potable contiene cantidades de BPCs
inferiores a 1 ng/litro, pero se han notificado valores de hasta
5 ng/litro. El suelo y los sedimentos de diferentes zonas, dependiendo
de las condiciones locales, contienen concentraciones que oscilan entre
<0,01 hasta 2,0 mg/kg. En las zonas contaminadas los niveles han sido
mucho mayores, es decir, de hasta 500 mg/kg.
En los últimos años se han analizado muchos miles de muestras de
productos alimenticios en varios países para detectar contaminantes,
BPCs inclusive. La mayor parte de las muestras se tomaron de artículos
alimenticios individuales, especialmente pescado y otros alimentos de
origen animal, como carne y leche. Los alimentos humanos se contaminan
con BPCs por tres vías principales:
a) absorción del medio ambiente por los peces, las aves, el ganado (a
través de la cadena alimentaria) y los cultivos;
b) migración de los materiales de envasado a los alimentos
(principalmente por debajo de 1 mg/kg, pero, en algunos casos, hasta
10 mg/kg);
c) contaminación directa del alimento o de los piensos por accidentes
industriales.
Los niveles en los artículos alimenticios más importantes que contenían
BPCs fueron: grasa animal, 20-240 µg/kg; leche de vaca,
5-200 µg/kg; mantequilla, 30-80 µg/kg; pescado, 10-500 µg/kg de grasa.
Ciertas especies de peces (anguila) o productos derivados del pescado
(hígado y aceites de pescado) contienen niveles mucho más altos, de
hasta 10 mg/kg. En hortalizas, cereales, frutas y algunos otros
productos la concentración observadas es de <10 µg/kg. Los principales
alimentos cuya contaminación con BPCs requiere atención son el pescado,
el marisco, la carne, la leche y otros productos lácteos. En diversos
países se han notificado niveles medios en el pescado del orden de 100
µg/kg (de grasa). Las comparaciones realizadas parecen indicar que la
concentración en el pescado está disminuyendo lentamente.
Los BPCs se acumulan en el tejido adiposo humano y en la leche materna.
Su concentración en los distintos órganos y tejidos depende del
contenido en lípidos, con la excepción del cerebro. Los residuos en el
tejido adiposo de la población general de los países industrializados
varía entre menos de 1 y 5 mg/kg de grasa, en función de la residencia
del donante, su tipo de vida y el método analítico utilizado. Las
mujeres que viven en zonas urbanas muy industrializadas, o que consumen
una gran cantidad de pescado, especialmente si procede de aguas con una
contaminación intensa, pueden acumular en la leche concentraciones
superiores de BPCs.
La composición de la mayoría de los extractos de BPCs procedentes de
muestras del medio ambiente no se parecen a las mezclas comerciales.
Utilizando el análisis de cromatografía de gases de alta resolución se
ha demostrado también que la composición del conjunto de los productos
afines y la concentración relativa de cada componente en el tejido
adiposo y la leche materna son notablemente diferentes de las que se
observan en los comerciales. Los BPCs detectados por cromatografía de
gases en el tejido adiposo humano y la leche materna contienen sobre
todo concentraciones relativamente altas de los compuestos más clorados,
como: 2,4,5,3',4'-pentaclorobifenilo; 2,4,5,2',4',5'-hexa-
clorobifenilo y 2,3,4,2',4',5'-hexaclorobifenilo; 2,3,4,5,2',4',5'-
heptaclorobifenilo; 2,3,4,5,2',3',4'-heptaclorobifenilo. Algunos otros
compuestos del grupo de los BPCs están presentes en cantidades mucho más
bajas, como los BPCs coplanares, muy tóxicos: 3,4,3',4'-tetra-
clorobifenilo, 3,4,5,3',4'-pentaclorobifenilo y 3,4,5,3',4',5'-hexa-
clorobifenilo.
Se ha calculado que la ingesta diaria de BPCs de los lactantes con la
leche materna es del orden de 4,2 µg/kg de peso corporal (5,2 µg/
100 kcal consumida) (OMS/EURO, 1988). La cantidad media total de BPCs
ingeridos con la leche materna durante los seis primeros meses de vida
es de 4,5 mg, mientras que la calculada para el resto de su vida es de
357 mg (0,2 µg/kg por día, en la dieta de una persona de 70 kg durante
70 años de vida). Por consiguiente, el período de la lactancia aporta
alrededor del 1,3% a la ingesta de toda la vida, cantidad no muy grande
si se tiene en cuenta los beneficios de la lactancia natural (OMS/EURO,
1988).
De acuerdo con los datos básicos evaluados, el promedio de BPCs en la
ingesta alimentaria de los adultos alcanza un máximo de 100 g por
semana, o alrededor de 14 µg/por persona al día. Para una persona de
70 kg, esto equivale a un máximo de 0,2 µg/k/ de peso corporal al día
(OMS/EURO, 1988).
1.7 Cinética y metabolismo
Se han descrito estudios en animales relativos fundamentalmente a las
exposiciones oral, respiratoria y cutánea a mezclas de BPCs y a
compuestos por separado. En general, los BPCs parece que se absorben con
rapidez, particularmente en el tracto gastrointestinal tras la
exposición oral. Es evidente que se produce absorción en los seres
humanos, pero la información sobre las tasas de absorción de los BPCs en
ellos es limitada.
Los datos de los estudios disponibles sobre su distribución parecen
indicar un proceso cinético bifásico, con eliminación rápida de la
sangre y acumulación en el hígado y en el tejido adiposo de diversos
órganos. También hay pruebas de su transporte a través de la placenta,
su acumulación fetal y su distribución en la leche. En algunos estudios
realizados en la especie humana, la piel contenía una concentración
elevada de BPCs, pero la concentración en el cerebro era inferior a la
prevista en función de su contenido en lípidos.
La movilización de los BPCs de la grasa parece depender en gran medida
de la tasa de metabolismo de cada uno de los BPCs. La excreción depende
de su transformación en compuestos más polares, como fenoles, sistemas
conjugados de compuestos de tiol y otros derivados solubles en agua.
Entre las vías metabólicas están la hidroxilación y la conjugación con
tioles y otros derivados solubles en agua, en algunos casos con la
intervención de productos intermedios reactivos, como los óxidos de
areno. Se ha demostrado que la tasa de metabolismo depende de la
estructura del BPC y está en función del número de átomos de cloro y de
su posición. Los metabolitos polares de los BPCs más clorados parece que
se eliminan sobre todo por las heces, aunque también puede ser
significativa la excreción en la orina. Una importante vía de
eliminación es a través de la leche (materna). Algunos compuestos
también se pueden eliminar por el pelo.
Los estudios cinéticos disponibles indican que hay una amplia
divergencia en la semivida biológica entre los distintos compuestos del
grupo, y esto puede ser debido a diferencias en el metabolismo
dependientes de la estructura, las afinidades tisulares y otros factores
que afectan a la movilización de los lugares de almacenamiento.
No siempre hay correlación entre la persistencia en los tejidos y una
toxicidad elevada, y las diferencias de toxicidad entre los distintos
compuestos pueden estar asociadas a metabolitos concretos o a sus
productos intermedios.
1.8 Efectos sobre los seres vivos del medio ambiente
Los BPCs son contaminantes universales de la naturaleza, y están
presentes en la mayoría de los compartimentos del medio ambiente,
abióticos y bióticos, de todo el mundo. Desde que en numerosos países se
comenzó a controlar el uso y la liberación, su incorporación al ambiente
se ha reducido en comparación con la del pasado. Sin embargo, las
pruebas obtenidas hasta ahora indican que el ciclo que siguen los BPCs
está produciendo una redistribución gradual de algunos de los compuestos
hacia el entorno marino. Existe una tendencia de los compuestos más
clorados a una acumulación preferencial. Aunque gran parte de los BPCs
se adsorben sobre las partículas del sedimento, mantienen la
biodisponibilidad para los organismos, por lo que continuarán
acumulándose en los niveles más altos de la cadena trófica.
1.8.1 Estudios de laboratorio
Los efectos de las mezclas de BPCs en los microorganismos son muy
variables, y mientras que algunas especies presentan efectos adversos
con concentraciones de 0,1 mg/litro, otras no se ven afectadas por
concentraciones de 100 mg/litro; los efectos en las diferentes especies
no dependen de manera sustancial del grado de cloración de las mezclas.
Casi todos los estudios sobre los efectos de los BPCs en los organismos
acuáticos se han realizado con mezclas de Aroclor. Los resultados
obtenidos han sido enormemente variables, sin una relación clara entre
el grado de cloración o las condiciones medio-ambientales y la
toxicidad, incluso en organismos estrechamente relacionados. Los valores
de la CL50 para un período de 96 h en condiciones fijas han variado
entre 12 µg/litro y >10 mg/litro para las distintas especies de
invertebrados acuáticos y las diferentes mezclas de Aroclor. Las
condiciones de flujo aumentaron la toxicidad de los BPCs. En general, la
mezclas más tóxicas fueron las de Aroclor con un grado intermedio
de cloración; las mezclas con un porcentaje de cloro bajo o alto
resultaron menos tóxicas. Esto ocurrió también en los efectos
subletales, como los efectos sobre la reproducción en Daphnia. Los
crustáceos parecen ser más sensibles a los BPCs durante la muda. En
poblaciones utilizadas como modelo, la estructura comunitaria de las
especies de estuario cambió tras la exposición a Aroclor 1254, y
mientras que el número de anfípodos, briozoos, crustáceos y moluscos
disminuyó, el de anélidos, braquiópodos, celentéreos, equinodermos y
nemertinos se mantuvo inalterado. Se ha considerado un número
excesivamente escaso de grupos en las pruebas de toxicidad aguda para
determinar si los resultados reflejan cambios en la susceptibilidad a
los BPCs o diferencias de interacción entre las especies.
La variación de la toxicidad de estos compuestos para los peces es
similar, con una CL50 en 96 horas que oscila entre 0,008 y
> 100 mg/litro. En las pruebas de larga duración se ha puesto de
manifiesto que en la exposición aguda, particularmente en condiciones
fijas, se subestima considerablemente la toxicidad de los BPCs. La
trucha arco iris fue particularmente sensible, con CL50 de
0,32 µg/litro de Aroclor 1254 en 22 días durante las fases
embrionario-larvarias, y un nivel sin efectos observados (NOEL) en 22
días de 0,001 µg/litro de Aroclor 1016, 1242 y 1254.
El pez de agua dulce Pimephales promelas mostró valores del NOEL de
5,4, 0,1, 1,8 y 1,3 µg/litro para los tipos de Aroclor 1242, 1248 1254
y 1260, respectivamente; el NOEL para el pez de estuario Aplodinotus
grunniens fue de 3,4 y 0,06 µg/litro de Aroclor 1016 y 1254,
respectivamente.
Las pruebas experimentales han confirmado las observaciones sobre el
terreno que demostraban la presencia de trastornos de la reproducción en
focas alimentadas con peces que contenían BPCs acumulados en el medio.
El efecto se produce en una fase avanzada de la reproducción, impidiendo
la implantación del embrión en la pared uterina.
En pruebas de corta duración, la toxicidad del Aroclor en las aves
aumentó al hacerlo el porcentaje de cloración; las CL50 con cinco días
de alimentación oscilaban entre 604 y 6000 mg/kg de alimentos. Los
principales efectos de los BPCs sobre la reproducción de las aves fueron
una reducción de la capacidad de eclosión de los huevos y
embriotoxicidad. Estos efectos se mantuvieron tras finalizar la
administración, puesto que las gallinas reducían la cantidad de BPCs por
medio de los huevos. No hay pruebas de que el Aroclor induzca
directamente la formación de cáscaras de los huevos más finas; los
efectos sobre el consumo de alimentos y el peso corporal de las gallinas
influyen indirectamente en el espesor de la cáscara. Se han notificado
efectos subletales en el comportamiento y en la secreción de hormonas.
La toxicidad aguda de los Aroclor en el visón disminuye al hacerlo el
porcentaje de cloración, variando la DL50 de la toxicidad aguda varía
entre >750 y 4000 mg/kg de peso corporal; el hurón es menos sensible.
El Aroclor reduce el consumo de alimentos y, por consiguiente, el ritmo
de crecimiento de los visones jóvenes. También reduce o impide la
reproducción del visón, tanto si se le suministra directamente como si
ingiere pescado contaminado. Cuanto mayor es el porcentaje de cloración
de los Aroclor (sobre todo el 1254), mayores son sus efectos. El índice
de reproducción vuelve a la normalidad tras el cese de la alimentación
con Aroclor.
Los murciélagos son susceptibles al Aroclor que se libera de la grasa
durante la migración.
La gran mayoría de las pruebas de laboratorio sobre animales acuáticos
y terrestres se llevaron a cabo utilizando mezclas de BPCs, por lo que
no es posible identificar qué componentes específicos de la mezclas
fueron los causantes de los efectos. De manera análoga, las pruebas se
realizaron en condiciones ambientales no reales (por ejemplo,
sobrepasando la solubilidad y sin sedimento presente en las pruebas
acuáticas), por lo que es difícil extrapolar los resultados del
laboratorio al campo. Sin embargo, hay motivos para suponer que
cualquier efecto sobre las poblaciones de organismos, que probablemente
se podrán presentar de manera más generalizada en el futuro, ya se
habrán observado en el pasado en poblaciones locales expuestas a altos
niveles de BPCs.
1.8.2 Estudios sobre el terreno
Los resultados que indican efectos de los BPCs en poblaciones de peces
sobre el terreno son poco concluyentes. La interpretación de los datos
de campo en aves es difícil, puesto que también hay presentes residuos
de muchos compuestos organoclorados diferentes.
La mayoría de los autores han señalado una correlación entre los efectos
(embriotoxicidad) y la concentración total de residuos organoclorados.
Del conjunto de los compuestos organoclorados presentes, los residuos de
BPCs son los que tienen mayor correlación con la embriotoxicidad, pero
los resultados no se pueden considerar como efectos de estos residuos
demostrados sobre el terreno.
Hay pruebas (confirmadas en estudios de laboratorio) de que los BPCs
reducen la capacidad reproductiva de los mamíferos acuáticos. Aunque
ejercen su efecto en la implantación del embrión, también pueden
ocasionar cambios físicos en el tracto reproductor de las hembras.
No es posible extrapolar las pruebas de laboratorio de toxicidad aguda
durante un período corto a los efectos sobre el terreno en las
poblaciones. La incertidumbre sobre qué componentes de las mezclas de
BPCs causan los efectos, cuáles son los compuestos específicos presentes
en el medio ambiente y cuál es la biodisponibilidad de los componentes
de los BPCs para el organismo, en conjunto dificultan las estimaciones
de las probables exposiciones en el medio ambiente y sus efectos. Los
efectos sobre las poblaciones de mamíferos marinos se pueden considerar
demostrados, pero todavía no se conoce qué componente o componentes de
las mezclas de BPCs los producen.
Dada la tendencia hacia el aumento de contaminación del medio ambiente
marino, se debería prestar más atención a los efectos sobre los
organismos marinos. Hay pruebas claras de laboratorio y sobre el terreno
de los efectos sobre la reproducción en poblaciones de mamíferos marinos
de zonas intensamente contaminadas. Es probable que en el futuro
aumenten los residuos y los efectos de los BPCs en otras poblaciones de
mamíferos marinos. Es menos claro si se verán los efectos en otros
organismos, como las aves que se alimentan de presas marinas.
Sería de esperar que, de acuerdo con los experimentos de laboratorio, se
produjeran efectos en poblaciones y comunidades de organismos
inferiores, como el fitoplancton y el zooplancton. Es difícil evaluar
tanto la amplitud como la importancia de tales cambios. Con la
información actualmente disponible, no cabe esperar efectos sobre las
poblaciones de peces, aunque éstos sean una vía de exposición para los
mamíferos y las aves que se alimentan de peces.
Los efectos anteriormente descritos sobre especies terrestres, mamíferos
de agua dulce que se alimentan de peces y murciélagos migratorios, por
ejemplo, deberían ser menos evidentes a medida que se redistribuyan los
residuos de BPCs. Los residuos en la biota terrestre muestran en la
actualidad una pequeña disminución general, pero la información acerca
de los cambios de los compuestos del grupo es escasa o nula. Se
considera que la reducción de los compuestos más clorados será lenta.
1.9 Efectos en los animales de experimentación y en sistemas de
prueba in vitro
1.9.1 Exposición única
La toxicidad aguda de los Aroclor, tras una exposición oral única,
generalmente es baja en las ratas. Los animales jóvenes parecen ser más
sensibles (DL50: 1,3-2,5 g/kg de peso corporal) que los adultos (DL50:
4-11 g/kg de peso corporal). La DL50 más baja de Aroclor 1254 de la que
se tiene noticia en ratas adultas fue de 1,0 g/kg de peso corporal. No
se observaron diferencias entre ambos sexos.
La DL50 cutánea en conejos osciló entre >1,26 y <2 g/kg de peso
corporal para el Aroclor 1260 (en aceite de maíz) y de 0,79 a
< 3,17 g/kg de peso corporal para algunas otras mezclas no diluidas de
BPC. Por vía intravenosa, las ratas mostraron para el Aroclor 1254 una
DL50 de 0,4 g/kg de peso corporal; la DL50 en ratones tras la inyección
intraperitoneal varió entre 0,9 y 1,2 g/kg de peso corporal.
1.9.2 Exposición de corta duración
Los principales objetivos a los que llegan las mezclas de BPCs o sus
compuestos por separado en mamíferos con exposición oral de corta
duración son el hígado, la piel y los sistemas inmunitario y
reproductor. La especie más sensible de las probadas fue el mono Rhesus,
siendo la hembra más susceptible que el macho. Las hembras adultas de
mono Rhesus sometidas durante seis meses a una dieta con concentraciones
de 2,5 mg/kg ó 0,09 mg/kg de peso corporal al día de Aroclor 1248
mostraron un aumento de la tasa de mortalidad, retraso del crecimiento,
alopecia, acné, inflamación de las glándulas de Meibomio y posiblemente
inmunosupresión. En el análisis microscópico, se encontró un hígado
adiposo agrandado, con necrosis focal, hiperplasia epitelial y
queratinización de los folículos pilosos. Con niveles de exposición más
elevados, también se han observado cambios en otros tejidos epiteliales,
como las glándulas sebáceas y de Meibomio, la mucosa gástrica, la
vesícula biliar, el conducto biliar, los lechos de las uñas y el
ameloblasto. Los niveles totales de lípidos, triglicéridos y colesterol
en el suero disminuyeron. La exposición breve a mezclas comerciales de
BPCs indujeron un aumento de la concentración de lípidos, triglicéridos,
colesterol y fosfolípidos totales en el hígado. Entre los distintos
compuestos de los BPCs, los más potentes fueron el 3,4,3',4'-
tetraclorobifenilo, el 3,4,5,3',4',5'-hexaclorobifenilo y el
2,4,6,2',4',6'-hexaclorobifenilo. Las concentraciones de 0,2 mg/kg de
peso corporal al día de Aroclor 1254 mostraron también algunos otros
efectos, como lesiones linforreticulares, desprendimiento de las uñas y
efectos gingivales, pero no se produjeron ni acné ni alopecia. En los
monos Rhesus se estableció un NOEL para la toxicidad general del Aroclor
1242 de 0,04 mg/kg de peso corporal al día. En monos Rhesus lactantes
expuestos a dosis mucho más elevadas, de 35 mg/kg de peso corporal al
día de Aroclor 1248, se observaron efectos relativamente ligeros. Donde
mejor se han investigado los efectos sobre el hígado es en ratas, y
entre ellos figuran hipertrofia, degeneración adiposa, proliferación del
retículo endoplásmico, porfiria, adenofibrosis, hiperplasia del conducto
biliar, quistes y cambios preneoplásicos y neoplásicos. En estudios
sobre ratas y ratones, los distintos compuestos de los BPCs causaron
efectos en el hígado, el bazo y el timo, siendo mayor la toxicidad de
los compuestos planares. En los monos, dichos compuestos planares, en
dosis de 1 a 3 mg/kg de dieta, indujeron efectos de carácter y gravedad
análogos a los producidos por dosis de 100 mg/kg de dieta de Aroclor
1242 y dosis de 25 mg/kg de dieta de Aroclor 1248.
Las mezclas de BPCs y algunos de los compuestos causaron a conejos y
ratones, tras una exposición cutánea, efectos en la piel y el hígado
similares a los presentes después de la exposición oral. En los conejos
se observaron también atrofia del timo, reducción de los centros
germinales de los nódulos linfáticos y leucopenia.
1.10 Reproducción, embriotoxicidad y teratogenicidad
1.10.1 Reproducción y embriotoxicidad
No se han realizado estudios completos de la reproducción y la
teratogenicidad. En un estudio de reproducción de dos generaciones en
ratas, se estableció un NOEL de 0,32 mg/kg de peso corporal, basado en
parámetros de la reproducción (Aroclor 1254) y un NOEL de 7,5 mg/kg de
peso corporal (Aroclor 1260). Sin embargo, la dosis más baja de las
probadas, de 0,06 mg/kg de peso corporal, produjo en animales destetados
un aumento del peso relativo del hígado.
En los monos Rhesus expuestos a Aroclor 1016, se estableció un NOEL de
0,03 mg/kg de peso corporal, utilizando como base los parámetros de la
reproducción. Sin embargo, con esta concentración se observó una
disminución del peso al nacer, y la dosis más baja de las probadas, de
0,01 mg/kg de peso corporal, produjo una hiperpigmentación de la piel.
Un año después de cesar la exposición, se detectó en los monos Rhesus un
NOEL de 0,09 mg/kg de peso corporal para el Aroclor 1248 (con DFPCs).
1.10.2 Teratogenicidad
En los estudios disponibles en ratas y monos no hay indicación de ningún
efecto teratogénico después de su exposición oral durante la
organogénesis. En ratas, se apreció para el Aroclor 1254 un NOEL de
50 mg/kg de peso corporal en relación con el peso de las crías, y se
podría suponer un NOEL de 2,5 mg/kg de peso corporal, tomando como base
la fetotoxicidad (lesión en las células foliculares del tiroides).
En las pruebas de teratogenicidad con los compuestos por separado en
ratones, ratas y monos Rhesus, no se estableció el NOEL. Una dosis de
0,07 mg/kg de peso corporal produjo en los monos Rhesus efectos tóxicos
matemos (3,4,3',4'-tetraclorobifenilo).
1.11 Mutagenicidad
Las mezclas de BPCs no causaron mutaciones ni lesiones cromosómicas en
distintos sistemas de prueba. El 3,4,3',4'-tetraclorobifenilo produjo
fragmentación cromosómica de linfocitos humanos in vitro.
Concentraciones elevadas de mezclas de BPCs pueden dar lugar a lesiones
primarias en el ADN, como puso de manifiesto la rotura de cadenas
sencillas de ADN en ensayos con soluciones alcalinas.
1.12 Carcinogenicidad
La interpretación de los datos disponibles sobre animales en relación
con mezclas comerciales de BPCs se ve con frecuencia complicada por la
escasez de información en cuanto a la presencia, o contribución, de las
impurezas de dibenzofuranos clorados, así como a variaciones en la
composición de los compuestos.
Se han llevado a cabo diversos estudios de carcinogenicidad de larga
duración en ratones y ratas. Las mezclas que se utilizaron fueron:
Kanechlor 300, 400 y 500, Aroclor 1254 y 1260 y Clophen A30 y A60. Se
notificó que el Clophen no contenía DFPCs, pero no se aportaron datos
sobre la pureza de los demás mezclas de BPCs.
En ratones alimentados con una dieta que contenía Kanechlor 500 y
Aroclor 1254 en dosis de unos 15 a 25 mg/kg de peso corporal se observó
un aumento significativo de adenomas hepatocelulares y/o carcinomas. En
ratones tratados con Kanechlor 300 y 400 no se pudieron detectar
neoplasmas.
En estudios de exposición de ratas a Aroclor 1254 y 1260 y Clophen A30
durante un período superior a un año se detectó un aumento de adenomas
hepatocelulares y/o carcinomas. No se consideró estadísticamente
significativo en estos estudios el aumento de la frecuencia de animales
con cáncer, pero sí en otros dos estudios. Con Aroclor 1260 y Clophen
A60 administrados a dosis de unos 5 mg/kg de peso corporal se observó un
aumento de la frecuencia de carcinomas hepatocelulares (trabeculares) y
adenocarcinomas.
Se consideró que los tumores hepáticos producidos no eran agresivos
(benignos o de escasa malignidad, sin metástasis) y no acortaban la
vida. En algunos estudios se notificaron casos de adenofibrosis, una
lesión preneoplásica, y/o nódulos neoplásicos. En una prueba en ratas
con Aroclor 1254 se demostró un aumento relacionado con la dosis de
metaplasia intestinal y adenocarcinomas de la parte glandular del
estómago.
Hay pruebas claras que demuestran los efectos potenciadores de los BPCs
en la carcinogénesis del hígado en roedores pretratados con
hepatocarcinógenos. Existen algunos indicios de actividad iniciadora de
las mezclas de BPCs en roedores. De los informes sobre estudios de
genotoxicidad se puede concluir que las mezclas de estos compuestos
carecen de genotoxicidad. De estos resultados se deduce que la
asociación de los tumores hepáticos con la administración de BPCs a
roedores se puede atribuir a algunos mecanismos epigenésicos que inducen
la proliferación celular en el hígado y otras manifestaciones de
hepatotoxicidad, por lo que en la evaluación de la toxicidad de los BPCs
se puede seguir un método de determinación del umbral. Es necesario
tener en cuenta la posibilidad de que los BPCs potencien la
carcinogénesis en otros tejidos distintos del hígado en animales con
exposición previa a diversos carcinógenos específicos de los tejidos.
La actividad anticarcinógena que los BPCs han mostrado en algunos
estudios, al tratar animales con estos compuestos durante la
administración de carcinógenos y antes de ella, puede estar relacionada
con las propiedades inductoras de enzimas microsomales de los BPCs,
dando lugar a un aumento de la destoxificación.
En general, hay que ser prudentes a la hora de extrapolar a los seres
humanos los datos disponibles sobre el potencial carcinógeno de los BPCs
en animales.
1.13 Estudios especiales
Tras la exposición a mezclas de BPCs o a compuestos individuales, se
observaron lesiones en el hígado, la piel, el sistema inmunitario, el
sistema reproductor, edemas y alteraciones del tracto gastrointestinal
y de la glándula tiroides.
Los BPCs pueden inducir la formación de diversas enzimas en el hígado.
Esto se ha demostrado en ratas, ratones, cobayos, conejos, perros y
monos utilizando Aroclor 1248, 1254 y 1260 y Kanechlor 400 (inducción
del citocromo P450 y P448). La capacidad de inducción aumenta con el
contenido de cloro de la molécula. Depende también de la composición de
congéneres: los que tienen el cloro en posición para- y meta- inducen
la enzima P450. Para la inducción de la AHH, la posición del cloro
parece ser más importante que el grado de cloración. Los inductores más
potentes de la AHH son los compuestos con cloro en posición para- y
por los menos dos en posición meta-. Se han observado diferencias
claras entre especies. El NOEL más bajo (0,025 mg/kg de peso corporal)
se encontró para el Aroclor 1260 en ratas Osborn-Mendel.
Se considera que los efectos sobre el sistema endocrino se manifiestan
como alteraciones de la unión al receptor hormonal y del equilibrio
hormonal esteroideo. Hay pruebas directas e indirectas de que diversos
Aroclor producen una débil actividad estrógena. Se observó que en ratas
expuestas a 75 mg de Aroclor 1242/kg de dieta durante 36 semanas se
producía una disminución de los niveles de hormonas gonadales y un
aumento del peso relativo de los testículos. En ratones hembra expuestos
a Aroclor 1254 (25 mg/kg de dieta) durante tres semanas se detectó la
reducción de los niveles de corticosteroides en el plasma, sin aumento
del peso adrenal. En otra raza a la que se suministró una dieta con
200 mg/kg durante dos semanas se observó un aumento del peso adrenal.
Las mezclas de BPC han mostrado un efecto inmunosupresor en varias
especies animales, siendo monos y conejos los más sensibles. Los NOEL
más bajos fueron de 0,1 mg/kg de peso corporal en monos y de
0,18 mg/kg de peso corporal en conejos.
En ratones a los que se suministró una dosis oral única de 500 mg/kg
de peso corporal de Aroclor 1254 se observó una disminución de la
actividad motora. Esto probablemente se debió a una inhibición de la
absorción y liberación de neurotransmisores.
Se ha encontrado que las mezclas de BPCs hacen disminuir en las ratas el
nivel sanguíneo y hepático de las vitaminas A y B1. En ratas y ratones
expuestos a mezclas de BPCs se produjo una reducción en la concentración
de las vitaminas A, B1, B2 y B6.
1.14 Factores modificadores de la toxicidad, mecanismo de acción
Los productos comerciales de BPCs muestran un espectro de respuesta
tóxica en parte parecido al de los DDPCs y DFPCs. Además, los distintos
BPCs tienen unas relaciones análogas entre estructura y actividad con
respecto a la mayor parte de sus respuestas tóxicas y a su capacidad de
inducción de AHH dependiente del P448, lo cual indica que los BPCs que
son aproximadamente esteroisómeros del 2,3,7,8-DDTC son los más activos.
Estos resultados parecen indicar que hay un mecanismo común de acción
basado en la afinidad de estos compuestos por la proteína citosólica
receptora de AH. Se han propuesto factores de equivalencia tóxica para
estos compuestos coplanares en relación con el 2,3,7,8-DDTC. No se ha
investigado adecuadamente la naturaleza de las probables interacciones
entre BPCs, DFPCs y DDPCs. Como los BPCs estimulan la actividad de las
enzimas microsomales, pueden influir en la acción de otros productos
químicos que se ven sometidos al metabolismo microsomal. Otros
compuestos, llamados no planares, pueden producir otras toxicidades más
sutiles. Además, los distintos BPCs, especialmente los menos clorados,
se pueden metabolizar a través de óxidos de areno intermedios y
metabolitos de metilsulfonilo.
1.15 Efectos en el ser humano
La evaluación toxicológica de los BPCs presenta muchos problemas. Los
BPCs normalmente se encuentran como mezclas de numerosos compuestos
distintos, y muchos de los datos sobre su toxicidad se basan en las
pruebas de estas mezclas. Algunos de los componentes de la mezcla se
degradan más fácilmente que otros en el medio ambiente. Así, la
población general puede estar expuesta a mezclas que son diferentes de
las que soportan las personas que trabajan con BPCs.
La población general está expuesta a BPCs fundamentalmente a través de
alimentos contaminados (organismos acuáticos, carne y productos
lácteos). La ingesta diaria de BPCs en la mayoría de los países
industrializados es del orden de unos microgramos por persona. Tales
exposiciones no se han asociado con enfermedades. Los lactantes están
expuestos a través de la leche materna. La ingesta diaria de BPCs puede
ser de unos microgramos/kg de peso corporal.
Es muy difícil evaluar por separado los efectos para la salud humana de
los BPCs, DFPCs o DDPCs, puesto que con mucha frecuencia las mezclas de
BPCs contienen DFPCs. Ocasionalmente se ha detectado también la
presencia de DDPCs en accidentes con ciertas mezclas. Se ha demostrado
que los BPCs comerciales están contaminados con DFPCs y, por
consiguiente, en muchos casos no está claro qué efectos son atribuibles
a los BPCs y cuáles a los DFPCs, mucho más tóxicos. Así pues, muchos de
los datos procedentes de casos importantes de intoxicaciones en el ser
humano, por ejemplo las de Yusho, Yu-Cheng y otras, probablemente
reflejan los efectos de la exposición tanto a los DFPCs como a los BPCs.
Los síntomas de la intoxicación en los pacientes de Yusho y de Yu-Cheng
fueron hipersecreción de las glándulas meibomianas de los ojos,
inflamación de los párpados y pigmentación de las uñas y de las
membranas mucosas, ocasionalmente acompañados de cansancio, náuseas y
vómitos. Estos efectos normalmente iban seguidos de hiperqueratosis y
oscurecimiento de la piel, con agrandamiento folicular y erupción
acneiforme. Además, se observaron edemas en brazos y piernas, aumento
del tamaño del hígado y trastornos hepáticos, alteraciones del sistema
nervioso central, problemas respiratorios, por ejemplo alteraciones del
tipo de la bronquitis, y cambios en el estado inmunitario de los
pacientes. En los hijos de pacientes de Yusho y Yu-Cheng se detectó
disminución del crecimiento, pigmentación oscura de la piel y las
membranas mucosas, hiperplasia gingival, edema xeroftálmico ocular,
dentición al nacer, calcificación anormal del cráneo, curva del talón
más baja y una alta frecuencia de escasez de peso al nacer. No se pudo
concluir de manera definitiva si existía o no correlación entre la
exposición y la formación de neoplasmas malignos en esos pacientes,
porque el número de muertes fue demasiado pequeño. Sin embargo, en
pacientes varones se observó un aumento estadísticamente significativo
de la mortalidad producida por todos los neoplasmas, el cáncer de hígado
y el de pulmón.
En condiciones profesionales, tras unas horas de exposición aguda se
produjo una erupción cutánea. Además, después de una exposición a altas
concentraciones de BPC se observó prurito, escozor, irritación
conjuntival, pigmentación de dedos y uñas y cloracné. La cloracné es uno
de los resultados predominantes entre los trabajadores expuestos a BPCs.
Además de estos signos cutáneos de intoxicación, diferentes autores han
encontrado trastornos hepáticos, cambios en la inmunosupresión,
irritación transitoria de las membranas mucosas del tracto respiratorio
y efectos neurológicos y psicológicos o psicosomáticos inespecíficos,
como dolor de cabeza, mareos, depresión, trastornos del sueño y de la
memoria, nerviosismo, cansancio e impotencia. La conclusión general es
que la exposición profesional constante a altas concentraciones de BPCs
y DFPCs puede tener consecuencias en el hígado y la piel.
Se han llevado a cabo dos amplios estudios de mortalidad en cohortes de
trabajadores. Tras la exposición a Aroclor 1254, 1242 y 1016, en un
estudio se observó un aumento de la mortalidad por cáncer de hígado y de
vesícula biliar, y en el otro por neoplasmas y cáncer del tracto
gastrointestinal. Ninguno de los estudios epidemiológicos disponibles
aporta pruebas concluyentes de una asociación entre la exposición a BPCs
y el aumento de la mortalidad por cáncer, debido al pequeño número de
muertes en las poblaciones expuestas, la falta de relación
dosis-respuesta y el problema de los contaminantes en las mezclas de
BPCs.
2. Conclusiones
2.1 Distribución
Debido a sus propiedades físicas y químicas, los BPCs se han dispersado
en el medio ambiente de todo el mundo.
Los BPCs están casi universalmente presentes en los organismos del medio
ambiente y se bioacumulan fácilmente. También se ha demostrado una
bioamplificación en las cadenas alimentarias.
Se acumulan preferentemente los compuestos más clorados.
2.2 Efectos en animales de experimentación
Los resultados de los estudios en animales indican que los BPCs tienen
una actividad inmunosupresora, evaluada por alteraciones importantes de
la función inmunitaria (peso del bazo, peso del timo y recuento de
linfocitos). En monos, se han estimado unos NOELs de 100 µg/kg para el
Aroclor 1248 y < 100 g/kg de peso corporal para el Aroclor 1254. La
inmunosupresión parece ser un efecto específico de cada compuesto.
En general, sólo se observa toxicidad en la reproducción con dosis que
producen toxicidad sistémica en la madre. Los neonatos que se alimentan
de leche materna contaminada (en monos y otras especies animales
utilizadas como modelo) parecen ser particularmente sensibles a los
BPCs, y muestran una disminución del crecimiento y otros síntomas
tóxicos. El NOEL para los efectos del Aroclor 1016 en la reproducción es
de 30 µg/kg de peso corporal en monos; no se pudo establecer el NOEL
para los efectos en la reproducción del Aroclor 1248.
Los BPCs no son genotóxicos y no hay pruebas definitivas de su acción
como desencadenantes de tumores. Los BPCs sí actúan como estimulantes de
tumores. Se puede concluir que la toxicidad de las mezclas de BPCs se
pueden evaluar sólo en función de su umbral.
2.3 Efectos en el ser humano
La exposición de la población general a los BPCs se produce sobre todo
por los artículos alimenticios. Los lactantes están expuestos a través
de la leche materna.
Se han registrado dos importantes casos de intoxicación humana en el
Japón (Yusho) y en la provincia de Taiwán (Yu-Cheng). Los principales
síntomas de los pacientes de Yusho y Yu-Cheng se han atribuido con
frecuencia a contaminantes de las mezclas de BPCs, en particular a los
DFPCs. Sin embargo, los causantes de algunos de los síntomas,
principalmente los efectos respiratorios crónicos, pueden haber sido los
metabolitos de metilsulfona de algunos compuestos del grupo de los BPCs.
2.4 Efectos en el medio ambiente
Aunque se han notificado efectos en poblaciones locales de aves, el
efecto más importante de los BPCs en organismos del medio ambiente ha
sido sobre la insuficiencia reproductora de los mamíferos marinos. Este
efecto se ha observado principalmente en mares semicerrados, y se ha
traducido en la reducción de las poblaciones locales. El pronóstico de
que los residuos de BPCs en el medio ambiente se redistribuirán
gradualmente hacia el entorno marino indica que hay un peligro creciente
en el futuro para los mamíferos marinos.
3. Recomendaciones
* Se recomienda un acuerdo internacional sobre los procedimientos
analíticos, para mejorar la comparabilidad de los resultados de los
programas de vigilancia. Se debe continuar perfeccionando la
metodología del análisis de los distintos compuestos, aunque se
reconoce el valor de los análisis de mezclas.
* Para asegurar que los datos analíticos sean fidedignos, se
recomiendan firmemente estudios de control de calidad entre
laboratorios. Se recomienda asimismo el establecimiento de una red
internacional de asistencia y supervisión técnica, para permitir la
participación de los países en desarrollo en la vigilancia.
* Se recomiendan estudios de larga duración utilizando distintos
compuestos, y estudios sobre el mecanismo de acción de los
componentes de las mezclas de BPCs, prestando particular atención
al estímulo de los tumores, a fin de mejorar la precisión de la
evaluación del riesgo de los BPCs.
* Son necesarios estudios epidemiológicos que permitan evaluar mejor
los riesgos para los neonatos, dado que los recién nacidos parecen
ser el sector más vulnerable de la población general, debido a su
elevada exposición a través de la leche.
* Se deben poner a punto biomarcadores sensibles y específicos para
algunos de los tipos más sutiles de toxicidad de los BPCs (como la
toxicidad sobre los sistemas reproductor, inmunitario y nervioso),
a fin de utilizarlos en futuros estudios epidemiológicos.
* La eliminación de los BPCs se debería llevar a cabo mediante
incineración en instalaciones con un diseño y un funcionamiento
apropiados que puedan garantizar la temperatura alta constante
(superior a 1000°C), el tiempo de permanencia y la turbulencia que
se necesitan para asegurar su completa descomposición.
* Hay que investigar sistemas de eliminación de los BPCs que se
encuentran ya en vertederos.
* Se ha de promover una vigilancia mundial de los BPCs en el medio
ambiente y en la fauna y flora silvestres, para seguir de cerca la
redistribución prevista de los residuos ya existentes.
* Los mamíferos marinos son susceptibles a una insuficiencia
reproductora a causa de la contaminación con BPCs. Se deben
promover estudios sobre el tamaño de las poblaciones y la eficacia
reproductora de los cetáceos, además de otros estudios para
identificar los compuestos causantes de estos efectos.
The number of different PCTs theoretically possible is orders of
magnitude higher than that for PCBs, but, in practice, as for PCBs,
PCTs are not sold on a composition specification, but on their
physical properties, which depend on the degree of chlorination.
Common Polychlorinated terphenyls - PCTs
name:
Major trade The trade names are generally similar to those
names: given for PCBs. In the Aroclor series, terphenyls are
indicated by 54 in the first two places of the four
digit code. In Japan, the PCTs are coded Kanechlor
KC-C.
1.2 Physical and chemical properties
The physical and chemical properties of PCTs are very close to those
of PCBs, and depend on the degree of chlorination.
1.3 Analytical methods
Extraction and clean-up procedures are similar to those used for PCBs
(sections 2.3.1.1 and 2.3.1.2).
The gas-liquid chromatographic details are different from those of
PCBs, because of the lower volatility of the PCTs. Zitko et al. (1972)
used 3% OV 210 as the stationary phase with a column temperature of
200°C. Thomas & Reynolds (1973) also used OV 210 with a column
temperature of 250°C and another system with 3% Dexsil as a stationary
phase at 300°C with a 63Ni electron capture detector; this was also
used by Addison et al. (1972). Sosa-Lucero et al. (1973) used OV 210
and SE 30 at 255°C and Freudenthal & Greve (1973) used OV 17 with a
temperature programmed from 200°C to 285°C. Thomas & Reynolds (1973)
confirmed the identity by chlorination to tetradecachloroterphenyl
with antimony pentachloride.
2. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
No specific information available. See PCBs.
3. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
Atmospheric input into the Great Lakes has been studied, because the
lakes, as a whole, represent the largest surface area of any
freshwater body in the world. Wingender & Williams (1984) found that
atmospheric transport was a major pathway for the deposition of
polychlorinated terphenyls into the Great Lakes (see section 4.1.1.1).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 Residues in the environment
Relatively few studies have been carried out to determine
polychlorinated terphenyls in biota. Freudenthal & Greve (1973) found
levels of 0.12 mg/kg (wet weight) in oysters and 0.4 mg/kg (fat basis)
in eels from the Netherlands. Levels of PCTs were generally lower than
PCBs in the same samples. Renberg et al. (1981) analysed biota from
the Baltic Sea and found levels of 2.8-17.2 mg/kg (wet weight) in
white-tailed eagles, 0.5-1 mg/kg in grey seals, and 0.08 mg/kg in
eels. In fish, Jan & Malnersic (1978) found PCT levels of
0.003-0.005 mg/kg in trout from the Soca River, Yugoslavia. Mean PCT
levels of 0.0025 mg/kg (Doguchi, 1977) and 0.01 mg/kg (Takai et al.,
1979) have been found in the freshwater and marine environment of
Japan.
Several bird species have been monitored for PCTs; levels of
0.03-2.2 mg/kg have been found in Japanese birds (Doguchi, 1977).
Zitko et al. (1972) found levels of 1.4 mg/kg fat (wet weight) and
0.1 mg/kg in eggs of herring gulls from the Bay of Fundy, Canada.
Hassell & Holmes (1977) analysed the livers of various birds of prey
in the United Kingdom; residues ranged from <0.05 to 1.2 mg/kg. PCT
levels of between 0.61 and 10.51 mg/kg were found in the fat of gulls
from Italy, (Vannucchi et al., 1978) and black-headed gulls from the
Baltic contained mean residues of 1.8 mg/kg in adipose tissue
(Falandysz, 1980).
PCTs were also measured in the monitoring programme carried out all
over Japan in the period 1974-81. In 1974, 1976, and 1978 no PCTs were
found in 60, 156, and 75 samples of water (limit of determination
0.0001 mg/litre). No PCTs were found in sediment samples in 1974, but,
in 1976 and 1978, 21/151 and 37/75 samples were positive, with PCT
levels of 0.001-0.2 and 0.001-1.0 mg/kg, respectively. In fish in
1974, 1976, and 1978, PCTs were found in 3/11, 0/39, and 3/66 samples,
in concentrations of 0.0002-0.2 mg/kg (Environment Agency Japan,
1983).
4.2 Residues in food
Ushio & Doguchi (1977) analysed cereal products, vegetable products
including vegetable oils, seasonings, and seaweed, marine animal
products, and terrestrial animal products including milk and eggs, for
the presence of PCTs. Only the vegetable products contained average
concentration of 0.05 µg/kg. Other authors referred to by Ushio &
Doguchi failed to detect PCTs in edible oil, vegetables, meat, or
fish.
No PCTs could be detected in a Canadian survey on eggs, domestic and
imported cheese (Villeneuve et al., 1973b).
In Japan, the PCT contents of a number of foods were determined. The
PCT contents of fish were lower than the PCB contents (Fukano et al.,
1974). Villeneuve et al. (1973a) analysed packaged food in Canada and
found that 94.5% of the samples contained less than 0.01 mg PCTs/kg
and 5.5% contained 0.01-0.05 mg PCTs/kg.
4.3 Concentrations in adipose tissue
In Japan, Doguchi et al. (1974) found an average PCT level of
0.6 mg/kg in human fat, with a range of 0.1-2.1 mg/kg. In the same
country, Takizawa & Minagawa (1974) found PCT levels of 0.02 mg/kg in
the human liver (n = 6), 0.01 mg/kg in the kidney (n = 2), 0.02 mg/kg
in the brain (n = 3), and 0.04 mg/kg in the pancreas (n = 1). Thirty
samples of adipose tissue (from 18 males and 12 females), obtained in
Tokyo in 1974, were analysed for PCTs. The average level of PCTs was
1.11 mg/kg (range 0.04-9.20 mg/kg), on a fat basis (Fukano & Doguchi,
1977). In the Netherlands, PCTs were found in human fat at levels of
0-1 mg/kg (Freudenthal & Greve, 1973).
4.4 Concentrations in blood
An average PCT level of 5.0 µg/litre was recorded in the blood of
non-occupationally exposed volunteers in Japan (Doguchi & Fukano,
1975). Human blood samples were collected from 10 subjects in Tokyo in
1975 out of 27 subjects from whom blood had been obtained in 1973. The
average concentration of PCTs in whole blood was 6.45 µg/litre
(0.7-19.6 µg/litre) in 1973, and 5.32 µg/litre (1.1-9.4 µg/litre) in
1975 (Fukano & Doguchi, 1977).
5. KINETICS AND METABOLISM
5.1 Absorption
PCTs have been shown to be absorbed from the intestinal tract
(Sosa-Lucero et al., 1973), but very little information is available
on the rate of absorption.
5.2 Distribution
Diets containing Aroclor 5460 at levels of 10, 100, or 1000 mg/kg were
administered to rats for 7 days. The greatest concentration (611 mg/kg
at 1000 mg/kg diet) was in the liver, while the blood level was
5.85 mg/litre at 1000 mg/kg diet. PCT administration did not affect
body weight, but a significant increase in liver weight occurred in
the rats fed 1000 mg/kg diet (Sosa-Lucero et al., 1973). Table 54
shows the tissue distribution obtained in this study in rats fed with
Aroclor 5460 and in another study using Aroclor 1254 (Curley et al.,
1971).
Addison et al. (1972) dosed cod Gadus morhua by gavage with the
polychlorinated terphenyl (PCT) Aroclor 5460 (in herring oil) at
0.5 g/ml. After one week of starvation, the PCTs were present in all
the tissues analysed. Uptake efficiency appeared to be low with a
total of 1-10 mg of Aroclor 5460 being distributed through all tissues
out of 1 g administered. Liver was found to be the organ richest in
PCTs, and probably contained most of the absorbed material. In a
separate group of fish, analysed 70 days later (fish were fed during
this period), PCT residues were not significantly lower.
Table 54. Tissue distribution (mg/kg wet weight) of PCTs
(Aroclor 5460) in rats fed dietary levels of 100 mg/kg
for 7 days and fed PCBs (Aroclor 1254) at 100 mg/kg
for 9 daysa
Tissue Aroclor 5460 Aroclor 1254
Blood 1.32 0.1
Liver 47 6
Brain 5.1 4
Kidney 15.1 5
Heart 21.5 -
Fat - 180
a From: Curley et al. (1971); Sosa-Lucero et al. (1973).
5.3 Biotransformation
There is little information on the biotransformation of PCTs. Addison
et al. (1972), using gas-liquid chromatography, noted a loss of PCTs
with a shorter retention time in the excreta of a cod dosed orally
with Aroclor 5460; the same loss was observed in rat faeces after the
administration of a diet containing Aroclor 5460 (Sosa-Lucero et al.,
1973).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Marine and estuarine organisms
PCTs, Aroclor 5460, did not show any toxic effects at either 1 or
100 mg/litre on Dunaliella, Olisthodiscus, or Thalassiorsira, the
only 3 species tested with this mixture (Craigie & Hutzinger, 1975).
6.2 Terrestrial invertebrates
Lichtenstein et al. (1969) exposed Drosophila melanogaster to the
dry residues of various PCTs. No mortality was observed after a 48-h
exposure to 2999 µg of Aroclor 4465, 5442, or 5460.
6.3 Birds
A single study on the toxicity of Aroclor 5442, produced a 5-day LC50
of 4477 mg/kg (1301-15402 mg/kg) in Japanese quail (aged 14 days)
(Coturnix coturnix) (Hill & Camardese, 1986).
Cecil et al. (1974) found that Aroclor 5442 at a dose level of
20 mg/kg diet did not change the hatchability of chicken eggs.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single oral exposure
Early data, reported in abstract, indicated that the approximate oral
LD50 values of the PCT-mixtures Aroclor 5442 and 5460, in corn oil,
in rats were 10.6 and 19.2 g/kg body weight, respectively. For 3:1
mixtures of PCBs and PCTs, Aroclor 4465 and 2562, in corn oil, the
LD50 values in rats were 16 and 6.3 g/kg body weight, respectively
(US FDA, 1970).
7.2 Short-term oral exposure
7.2.1 Rat
Modifications in the liver were studied in groups of male
Sprague-Dawley rats fed a diet containing Aroclor 5460 at a level of 0
or 10 000 mg/kg diet (equivalent to 0 or 400 mg/kg body weight). Body
weights were slightly decreased after 3 weeks of exposure. The
enlarged livers showed proliferation of the endoplasmic reticulum and
formation of large concentric membrane arrays. Evidence of fatty
degeneration was observed by Toftgard et al. (1980). Biochemical
changes included an increase in microsomal protein and phospholipid,
and a decrease in RNA and cholesterol. The specific esterase
activities, N-demethylase and nitroreductase, were increased and
those of glucose-6-phosphatase and aryl hydrocarbon hydroxylase
decreased (Norback & Allen, 1972).
Sosa-Lucero et al. (1973) did not observe any signs of toxicity in
groups of male Wistar rats exposed to a diet containing Aroclor 5460
at levels of up to 1000 mg/kg diet (equivalent to 50 mg/kg body
weight) for 7 days. At 1000 mg/kg diet, relative liver weights were
increased as well as microsomal protein, cytochrome P-450, and the
specific activities of aniline hydroxylase and aminopyrine-
N-demethylase. Mixed type induction of hepatic microsomal enzymes in
rats exposed to PCTs has been observed by several investigators
(Ahotupa & Aitio, 1980; Toftgard et al., 1980; Nilsen & Toftgard,
1981).
Kiriyama et al. (1974) fed groups of male Wistar rats a control diet
or diets with ortho-, meta-, or para-PCTs at a level of 2000 mg/kg
diet (equivalent to 100 mg/kg body weight) for 2 weeks. Ortho- and
meta-PCTs reduced growth and increased relative kidney weights,
while only meta-PCTs decreased food intake and increased the
relative liver weights. All mixtures increased plasma, but not liver,
cholesterol levels. There was evidence of adrenal hypertrophy.
7.2.2 Monkey
A dietary level of Aroclor 5460 of 5000 mg/kg (equivalent to 200 mg/kg
body weight) over 3 months caused growth retardation and increased
relative liver weights in 6 Rhesus monkeys compared with 3 controls.
After 6 weeks of exposure, the toxic signs observed were similar to
those found within 1 month in a group of monkeys exposed to 300 mg of
Aroclor 1248/kg diet (equivalent to 12 mg/kg body weight), i.e.,
alopecia, facial oedema, swollen eyelids and lips, and purulent eye
discharge. After exposure of both groups for 3 months, proliferation
of the smooth endoplasmic reticulum was observed as well as
hypertrophy and hyperplasia of the gastric mucosa (Allen & Norback,
1973).
7.3 Teratogenicity
Groups of 15 or 16 pregnant ddY mice were fed diets containing 0, 100,
500, or 2500 mg PCTs/kg (not specified) during gestation. The animals
were sacrificed on day 18 and examined for embryonic effects. The
fetuses of dams receiving the 500 and 2500 mg/kg diet showed a higher
incidence of cleft palate in comparison with the controls. Pregnant
ddY mice were administered 0, 50, or 100 mg PCTs/kg with
corticosterone administered subcutaneously on days 11, 12 and 13. A
significant increase was seen in corticosterone levels in the plasma
in the PCT-treated animals on day 14. Furthermore, when pregnant ddY
mice were adrenalectomized on day 10, it did not suppress the
development of cleft palate, but metapyrone, an inhibitor of
corticosterone synthesis, significantly reduced the incidence of cleft
palate in the fetuses. The results suggest that cleft palate induced
by PCTs is not due to a direct effect, but that an increase in the
corticosterone level in the maternal plasma is involved in the
mechanism of its development (Kaneko, 1988).
Pregnant Wistar rats were fed PCTs at levels of 0, 500, or 2500 mg/kg
diet during gestation and the animals were sacrificed on day 20.
Systemic oedema was observed in the fetuses of the animals fed 500 and
2500 mg PCTs/kg diet, but no cleft palate was found (Kaneko, 1988).
7.4 Carcinogenicity
Groups of 35 male ICR mice received a diet containing Kanechlor C
(a mixture of 95% PCTs and 5% PCBs) at levels of 0, 250, or 500 mg/kg
(equivalent to 0, 36, and 70 mg/kg body weight), for 24 weeks. The
mice were sacrificed following 16 exposure-free weeks. Survivors
numbering 28, 28, and 21 mice at 0, 250, and 500 mg/kg diet,
respectively, were autopsied. A dose-related reduction in body weight
gain and a dose-related increase in absolute liver weights were
observed. Neoplastic nodules (nodular hyperplasia) were found in the
livers of 3/28 mice at 250 mg/kg diet and 6/21 mice at 500 mg/kg diet.
Hepatocellular carcinomas were observed in 3/21 mice at 500 mg/kg
diet. No neoplastic nodules were noted in the controls. The increases
at the higher dose level were statistically significant (Shirai et
al., 1978).
7.5 Miscellaneous effects
Evidence for the estrogenic activity of Aroclor 5442 was found using
the glycogen response of the immature rat uterus. Aroclor 5460 was
inactive in this test (Bitman & Cecil, 1970; Bitman et al., 1972).
The mixed type inducer, Aroclor 5460, increased the metabolism of
4-androstene-3,17-dione in male Sprague-Dawley rats intraperitoneally
injected with 4 doses of 300 mg/kg body weight in 4 days (Nilsen &
Toftgard, 1981). Reproductive effects have not been investigated.
Groups of pregnant ddY mice received a control diet or diets
containing PCTs (not specified) at levels of 50 or 500 mg/kg diet
(equivalent to 7 or 70 mg/kg body weight). Increased incidence of
cleft palate and other malformations was reported in the fetuses. In
neonates, reduced growth and survival as well as hyperactivity were
observed (Kimura & Miyake, 1976). (No details available).
REFERENCES
ABBOTT, D.C., COLLIN, G.B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom, 1969-71. Br.
med. J., 2: 553-556.
ABE, S., INOU, Y., & TAKAMATSU, M. (1975) [Polychlorinated biphenyl
residues in plasma of Yusho children born to mothers who had
consumed oil contaminated by PCBs.] Fukuoka Acta med., 66: 605-609
(in Japanese).
ABRAHAMSON, L.J. & ALLEN, J.R. (1973) The biological response of
infant nonhuman primates to a polychlorinated biphenyl. Environ.
health Perspect., 4: 81-86.
ACHILLES, A. (1983) Fire hazard of polychlorinated biphenyls. In:
Barros, M.C., Könemann H., & Visser, R., ed. Proceedings of the PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 187-191.
ACKER, L. & SCHULTE, E. (1970) [The occurrence of chlorinated
biphenyls and hexachlorobenzene alongside chlorinated insecticides
in breastmilk and human fatty tissue.] Naturwissenschaften, 57: 497
(in German).
ACKER, L. & SCHULTE, E. (1974) [Chlorinated hydrocarbons in human
fatty tissue.] Naturwissenschaften, 61: 1-4 (in German).
ACKER, L., BARKE, E., HAPKE, H.-J., HEESCHEN, W., KORANSKY, W.,
KUBLER, W., & REINHARDT, D. (1984) [Residues and impurities in
breast milk. German Association for the Encouragement of Research:
Communication XII of the Committee on Testing of Residues in
Foods], Weinheim, Verlag Chemie, pp. 1-97.
ACQUAVELLA, J.F., HANIS, N.M., NICOLIC, M.J., & PHILLIPS, S.C.
(1986) Assessment of clinical, metabolic, dietary, and occupational
correlations with serum polychlorinated biphenyl levels among
employees at an electrical capacitor manufacturing plant. J. occup.
Med., 28(11): 1177-1180.
ADAMS, J.A. (1983) Effect of PCB (Aroclor 1254) on early
development and mortality in Arbacia eggs. Water air soil
Pollut., 20: 1-5.
ADDISON, R.F. & BRODIE, P.F. (1977) Organochlorine residues in
maternal blubber, milk, and pup blubber from grey seals
(Halichoerus grypus) from Sable Island, Nova Scotia. J. Fish Res.
Board Can., 34: 937-941.
ADDISON, R.F. & SMITH, T.G. (1974) Organochlorine residue levels in
Arctic ringed seals: variation with age and sex. Oikos,
25: 335-337.
ADDISON, R.F., FLETCHER, G.L., RAY, S., & DOANE, J. (1972) Analysis
of a chlorinated terphenyl (Aroclor 5460) and its deposition in
tissues of cod (Gadus morhua). Bull. environ. Contam. Toxicol.,
8: 52-60.
ADDISON, R.F., KERR, S.R., DALE, J., & SERGEANT, D.E. (1973)
Variation of organochlorine residue levels with age in Gulf of St.
Lawrence harp seals (Pagophilus groenlandicus). J. Fish. Res.
Board Can., 30: 595-600.
ADDISON, R.F., ZINCK, M.E., & SMITH, T.G. (1986) PCBs have declined
more than DDT-group residues in Arctic ringed seals (Phoca
hispida) between 1972 and 1981. Environ. Sci. Technol.,
20: 253-256.
AGRAWAL, A.K., TILSON, H.A., & BONDY, S.C. (1981)
3,4,3',4'-tetrachlorobiphenyl given to mice prenatally produces
long-term decreases in striatal dopamine and receptor binding sites
in the caudate nucleus. Toxicol. Lett., 7: 417-424.
AGUILAR, A. & BORRELL, A. (1988) Age- and sex-related changes in
organochlorine compound levels in fin whales (Balaenoptera
physalus) from the Eastern North Atlantic. Mar. environ. Res.,
25: 195-211.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of polychlorinated biphenyl (PCB) and chlorinated
pesticides in water. Anal. Chem., 42: 1483-1486.
AHMED, M. & FOCHT, D.D. (1973a) Degradation of polychlorinated
biphenyls by two species of Achromobacter. Can. J. Microbiol.,
19: 47-52.
AHMED, M. & FOCHT, D.D. (1973b) Oxidation of polychlorinated
biphenyls by Achromobacter PCB. Bull. environ. Contam. Toxicol.,
10: 70-72.
AHNOFF, M. & JOSEFSSON, B. (1973) Confirmation studies on
polychlorinated biphenyls (PCBs) from river waters using mass
fragmentography. Anal. Lett., 6: 1083-1093.
AHNOFF, M. & JOSEFSSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AHNOFF, M. & JOSEFSSON, B. (1975) Clean-up procedures for PCB
analysis on river water extracts. Bull. environ. Contam. Toxicol.,
13: 159-166.
AHOTUPA, M. & AITIO, A. (1980) Effect of chlorinated naphthalenes
and terphenyl on the activities of drug metabolizing enzymes in rat
liver. Biochem. biophys. Res. Commun., 93: 250-257.
AKAGI, K. & OKUMURA, M. (1985) Association of blood pressure and
PCB level in Yusho patients. Environ. health Perspect., 59: 37-39.
AKIYAMA, K., OHI, G., FUJITANI, K., & YAGYU, H. (1975)
Polychlorinated biphenyl residues in maternal and cord blood in
Tokyo metropolitan area. Bull. environ. Contam. Toxicol.,
14: 588-592.
ALBAIGES, J., FARRAN, A., SOLER, M., GALLIFA, A., & MARTIN, P.
(1987) Accumulation and distribution of biogenic and pollutant
hydrocarbons, PCBs and DDT in tissues of Western Mediterranean
fishes. Mar. environ. Res., 22: 1-18.
ALBRO, P.W. & FISHBEIN, L. (1972) Intestinal absorption of
polychlorinated biphenyls in rats. Bull. environ. Contam. Toxicol.,
8: 26-31.
ALBRO, P.W. & PARKER, C.E. (1979) Comparison of the compositions of
Aroclor 1242 and Aroclor 1016. J. Chromatogr., 169: 161-166.
ALBRO, P.W., CORBETT, J.T., & SCHROEDER, J.L. (1981) Quantitative
characterization of polychlorinated biphenyl mixtures (Aroclors
1248, 1254 and 1260) by gas chromatography using capillary columns.
J. Chromatogr., 205: 103-111.
ALENCASTRO, L.F. DE, PRELAZ, V., & TARRADELLAS, J. (1984)
Contamination of silos in Switzerland by PCB residues in coatings.
Bull. environ. Contam. Toxicol., 33(3): 270-276.
ALFORD-STEVENS, A.L. (1986) Analyzing PCBs. Environ. Sci. Technol.,
20(12): 1194-1199.
ALLEN, J.R. (1975) Response of the non-human primate to
polychlorinated biphenyl exposure. Fed. Proc., 34: 1675-1679.
ALLEN, J.R. & ABRAHAMSON, L.J. (1973) Morphological and biochemical
changes in the liver of rats fed polychlorinated biphenyls. Arch.
environ. Contam. Toxicol., 1: 265-280.
ALLEN, J.R. & BARSOTTI, D.A. (1976) The effects of transplacental
and mammary movement of PCBs on infant rhesus monkeys. Toxicology,
6: 331-340.
ALLEN, J.R. & NORBACK, D.H. (1973) Polychlorinated biphenyl- and
triphenyl-induced gastric mucosal hyperplasia in primates. Science,
179: 498-499.
ALLEN, J.R. & NORBACK, D.H. (1976) Pathobiological responses of
primates to polychlorinated biphenyl exposure. In: Proceedings of
the National Conference on Polychlorinated Biphenyls, Chicago,
19-21 November 1975, Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances, pp. 43-49 (EPA-560/6-75-004).
ALLEN, J.R., CARSTENS, L.A., & BARSOTTI, D.A. (1974) Residual
effects of short-term low level exposures of nonhuman primates to
polychlorinated biphenyls. Toxicol. appl. Pharmacol., 30: 440-451.
ALLEN, J.R., CARSTENS, L.A., ABRAHAMSON, L.J., & MARLAR, R.J.
(1975) Responses of rats and non-human primates to
2,5,2',5',-tetrachlorobiphenyl. Environ. Res., 9: 265-273.
ALLEN, J.R., CARSTENS, L.A., & ABRAHAMSON, L.J. (1976) Responses of
rats to polychlorinated biphenyls for fifty-two weeks. Arch.
environ. Contam., 4: 404-419.
ALLEN, J.R., BARSOTTI, D.A., LAMBRECHT, L.K., & VAN MILLER, J.P.
(1979) Reproduction effects of halogenated aromatic hydrocarbons on
nonhuman primates. Ann. NY Acad. Sci., 320: 419-425.
ALLEN, J.R., BARSOTTI, D.A., & CARSTENS, L.A. (1980) Residual
effects of polychlorinated biphenyls on adult nonhuman primates and
their offspring. J. Toxicol. environ. Health, 6: 55-66.
ALTMAN, N.H., NEW, A.E., MCCONNELL, E.E., & FERRELL, T.L. (1979) A
spontaneous outbreak of polychlorinated biphenyl (PCB) toxicity in
Rhesus monkeys (Macaca mulatta): Clinical observations. Lab.
anim. Sci., 29: 661-665.
ALVARES, A.P. (1977) Stimulatory effects of polychlorinated
biphenyls (PCB) on cytochromes P-450 and P-448 mediated microsomal
oxidations. In: Microsomes and drug oxidations, Oxford, New York,
Pergamon Press, pp. 476-483.
ALVARES, A.P. & KAPPAS, A. (1975) Induction of aryl hydrocarbon
hydroxylase by polychlorinated biphenyls in the foeto-placental
unit and neonatal livers during lactation. FEBS Lett., 50: 172-174.
ALVARES, A.P. & KAPPAS, A. (1977) Heterogenicity of cytochrome
P-450s induced by polychlorinated biphenyls. J. biol. Chem.,
252: 6373-6378.
ALVARES, A.P., FISCHBEIN, A., ANDERSON, K.E., & KAPPAS, A. (1977)
Alterations in drug metabolism in workers exposed to
polychlorinated biphenyls. Clin. Pharmacol. Ther., 22(2): 140-145.
ALVARES, A.P., EISEMAN, J.L., UENG, T.-H., & KAPPAS, A. (1982)
Polychlorinated biphenyls: species and tissue specifications of the
induction of monooxygenases in rats, mice, rabbits, and humans. In:
Proceedings of the Workshop on the Combined Effects of Xenobiotics,
Ottawa, 22-23 June 1981, Ottawa, National Research Council of
Canada, pp. 127-149 (Publication No. NRCC 18978).
ANDERSON, M.R. & PANKOW, J.F. (1986) A case study of a chemical
spill: Polychlorinated biphenyls (PCBs). 3. PCB sorption and
retardation in soil underlying the site. Water Resour. Res.,
22(7): 1051-1057.
ANDERSON, L.M., VAN HAVERE, K., & BUDINGER, J.M. (1983) Effects of
polychlorinated biphenyls on lung and liver tumours initiated in
suckling mice by N-nitrosodimethylamine. J. Natl Cancer Inst.,
71: 157-163.
ANDO, M. (1978) Transfer of 2,4,5,2',4',5'-hexachlorobiphenyl and
2,2-bis(p-chlorophenyl), 1,1,1 -trichloroethane ( p-p'DDT) from
maternal to newborn and suckling rats. Arch. Toxicol., 41: 179-186.
ANDO, M., SAITO, H., & WAKISAKA, I. (1984) [Transfer of PCBs from
mother to newborn baby through placenta and milk.] Res. Rep. Natl
Inst. Environ. Stud. Jpn, 67: 333-345 (in Japanese).
ANDO, M., SAITO, H., & WAKISAKA, I. (1985) Transfer of
polychlorinated biphenyls to newborn infants through the placenta
and mother's milk. Arch. environ. Contam. Toxicol., 14(1): 51-57.
ANDREN, A.W. (1982) Processes determining the flux of PCBs across
air/water interfaces. In: Mackay, D., ed. Physical behavior of PCBs
in the Great Lakes; Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., pp. 127-140.
ANDRES, J., LAMBERT, I., ROBERTSON, L., BANDIERA, S., SAWYER, T.,
LOVERING, S., & SAFE, S. (1983) The comparative biologic and toxic
potencies of polychlorinated biphenyls and polybrominated
biphenyls. Toxicol. appl. Pharmacol., 70: 204-215.
ANDREWS, J.E. (1989) Polychlorinated biphenyl (Aroclor 1254)
induced changes in femur morphometry calcium metabolism and
nephrotoxicity. Toxicology, 57: 83-96.
ANON. (1983a) PCB found in Czechoslovakian canned hams during each
of last four years. Food Chem. News, 18 April: 32.
ANON. (1983b) USDA blocks all Czechoslovakian meat imports because
of PCB residues. Food Chem. News, 2 May: 30-31.
ANON. (1985a) Summary of results of the first test to determine
residual contaminants in the air and on surface in floors 2 through
18 of the Binghamton State Office Building. Test report,
Springfield, Virginia, Versar Inc.
ANON. (1985b) Polychlorinated biphenyl transformer incident New
Mexico. Morb. Mortal. wkly Rep., 34(36): 557-559.
ANON. (1987) Polychlorinated biphenyl in fluorescent lighting.
Environ. Health Saf. News, 33(1): 3-18.
ARMOUR, J.A. & BURKE, J.A. (1970) Method for separating
polychlorinated biphenyls from DDT and its analogs. J. Assoc. Off.
Anal. Chem., 53: 761-768.
ARMBRUSTER, G., GEROW, K.G., GUTENMANN, W.H., LITTMAN, C.B., &
LISK, D.J. (1987) The effects of several methods of fish
preparation on residues of polychlorinated biphenyls and sensory
characteristics in striped bass. J. Food Saf., 8: 235-243.
ARNOLD, D.L., MES, J., ZAWIDZKA, Z.Z., & KARPINSKI, K. (1984)
Toxicity of PCB (Aroclor 1254) as a consequence of continuous
exposure during pregnancy and nursing (PA-24). Presented at the
27th Annual Meeting of the Canadian Federation of Biological
Societies, University of Saskatchewan, Canada, 18-22 June 1984.
ATKINSON, S.A. (1979) Chemical contamination of human milk: A
review of current knowledge. J. Can. Diet. Assoc., 40(3): 223-226.
ATLAS, E. & GIAM, C.S. (1981) Global transport of organic
pollutants: Ambient concentrations in the marine atmosphere.
Science, 211: 163-165.
ATSDR (1989) Toxicological profile for selected PCBs (Aroclor 1260,
1254, 1248, 1242, 1232, 1221 and 1016), Atlanta, Georgia, Agency
for Toxic Substances and Disease Registry (ATSDR/TP-88/21).
AULERICH, R.J. & RINGER, R.K. (1977) Current status of PCB toxicity
to mink and effect on their reproduction. Arch. environ. Contam.
Toxicol., 6: 279-292.
AULERICH, R.J., BURSIAN, S.J., BRESLIN, W.J., OLSON, B.A., &
RINGER, R.K. (1985) Toxicological manifestations of
2,4,5,2',4',5',-; 2,3,6,2',3',6',-; and 3,4,5,3',4',5',-
hexachlorobiphenyl and Aroclor 1254 in mink. J. Toxicol. environ.
Health., 15: 63-79.
AX, R.L. & HANSEN, L.G. (1975) Effects of purified polychlorinated
biphenyl analogs on chicken reproduction. Poult. Sci., 54: 895-900.
BACCI, E. & GAGGI, C. (1985) Polychlorinated biphenyls in plant
foliage: Translocation or volatilization from contaminated soils?
Bull. environ. Contam. Toxicol., 35: 673-681.
BACHE, C.A., SERUM, J.W., YOUNGS, W.D., & LISK, D.J. (1972)
Polychlorinated biphenyl residues: Accumulation in Cayuga lake
trout with age. Science, 177: 1191-1192.
BADSHA, K. & EDULJEE, G. (1986) PCB in the UK environment - A
preliminary survey. Chemosphere, 15(2): 211-215.
BADSHA, K., EDULJEE, G., & SCUDAMORE, N. (1986) Environmental
monitoring for PCB and trace metals in the vicinity of a chemical
waste disposal facility - III. Chemosphere, 15(7): 947-957.
BAGLEY, G.E., REICHEL, W.L., & CROMARTIE, E. (1970) Identification
of polychlorinated biphenyls in two bald eagles by combined
gas-liquid chromatography-mass spectroscopy. J. Assoc. Off. Anal.
Chem., 53: 251-261.
BAHN, A.K., ROSENWAIKE, I., HERRMAN, N., GROVER, P., STELLMAN, J.,
& O'LEARY, K. (1976) Melanomas after exposure to PCBs. New Engl. J.
Med., 295: 450.
BAILEY, S. & BUNYAN, P.J. (1972) Interpretation of persistence and
effects of polychlorinated biphenyls in birds. Nature (Lond.),
236: 34-36.
BAILEY, J., KNAUF, V., MUELLER, W., & HOBSON, W. (1980) Transfer of
hexachlorobenzene and polychlorinated biphenyls to nursing infant
Rhesus monkeys: enhanced toxicity. Environ. Res., 21: 190-196.
BAKER, F.D., BUSH, B., TUMASONIS, C.F., & LO, F.-C. (1977) Toxicity
and persistence of low-level PCB in adult Wistar rats, fetuses, and
young. Arch. environ. Contam. Toxicol., 5: 143-156.
BAKER, E.L., Jr., LANDRIGUN, P.J., GLUECK, C.J., ZACK, M.M., Jr.,
LIDDLE, J.A., BURSE, V.W., HOUSEWORTH, W.J., & NEEDHAM, L.L. (1980)
Metabolic consequences of exposure to polychlorinated biphenyls
(PCBs) in sewage sludge. Am. J. Epidemiol., 112(4): 553-563.
BALLSCHMITER, K. & ZELL, M. (1980) Analysis of polychlorinated
biphenyls (PCB) by glass capillary gas chromatography. Composition
of technical Aroclor- and Clophen-PCB mixtures. Fresenius' Z. anal.
Chem., 302: 20-31.
BALLSCHMITER, K., BUCHERT, H., BIHLER, S., SCHOTT, P., RÖPER, H.P.,
& PACHUR, H.J. (1981) Organochlorine pollutant analysis of
contaminated and uncontaminated lake sediments by high resolution
gas chromatography. Chemosphere, 10: 945-956.
BANDIERA, S., SAFE, S., & OKEY, A.B. (1982) Binding of
polychlorinated biphenyls classified as either phenobarbitone-,
3-methylcholanthrene-, or mixed-type inducers to cytosolic Ah
receptor. Chem. biol. Interact., 39: 259-277.
BANDIERA, S., FARRELL, K., MASON, G., KELLEY, M., ROMKES, M.,
BANNISTER, R., & SAFE, S. (1984) Comparative toxicities of the
polychlorinated dibenzofuran (PCDF) and biphenyl (PCB) mixtures
which persist in Yusho victims. Chemosphere, 13(4): 507-512.
BARSOTTI, D.A. & VAN MILLER, J.P. (1984) Accumulation of a
commercial polychlorinated biphenyl mixture (Aroclor 1016) in adult
Rhesus monkeys and their nursing infants. Toxicology, 30: 31-44.
BARSOTTI, D.A., MARLAR, R.J., & ALLEN, J.R. (1976) Reproductive
dysfunction in Rhesus monkeys exposed to low levels of
polychlorinated biphenyls (Aroclor 1248). Food Cosmet. Toxicol.,
14: 99-103.
BASTOMSKY, C.H. (1974) Effects of polychlorinated biphenyl mixture
(Aroclor 1254) and DDT on biliary excretion in rats. Endocrinology,
95: 1150-1155.
BASTOMSKY, C.H. (1977) Goitres in rats fed polychlorinated
biphenyls. Can. J. Physiol. Pharmacol., 55: 288-292.
BASTOMSKY, C.H. & MURTHY, P.V.N. (1976) Enhanced in vitro hepatic
glucuronidation of thyroxine in rats following cutaneous
application or ingestion of polychlorinated biphenyls. Can. J.
Physiol. Pharmacol., 54: 23-26.
BASTOMSKY, C.H., SOLYMOSS, B., ZSIGMOND, G., & WYSE, J.M. (1975) On
the mechanism of polychlorinated biphenyl-induced hypobili-
rubinaemia. Clin. chim. Acta, 61: 171-174.
BAUMANN, M., DEML, E., SCHAEFFER, E., & GREIM, H. (1983) Effects of
polychlorinated biphenyls at low dose levels in rats. Arch.
environ. Contam. Toxicol., 12: 509-515.
BAXTER, R.A., GILBERT, P.E., LIDGETT, R.A., MAINPRIZE, J.H., &
VODDEN, H.A. (1975) The degradation of polychlorinated biphenyls by
microorganisms. Sci. total Environ., 4: 53-61.
BECK, H. & MATHAR, W. (1985) [Analytical procedures for the
determination of selected components of PCBs in foodstuffs.]
Bundesggesundheitsblatt, 28(1): 1-12 (in German).
BECKER, G.M., MCNULTY, W.P., & BELL, M. (1979) Polychlorinated
biphenyl-induced morphologic changes in the gastric mucosa of the
Rhesus monkey. Lab. Invest., 40: 373-383.
BENGTSON, S.A. & SÖDERGREN, A. (1974) DDT and PCB residues in
airborne fallout and animals in Iceland. Ambio, 3: 84-86.
BENGTSSON, B.E. (1979) Increased growth in minnows exposed to PCBs.
Ambio, 8: 169-170.
BENGTSSON, B.E. (1980) Long-term effects of PCB (Clophen A50) on
growth, reproduction and swimming performance in the minnow,
Phoxinus phoxinus. Water Res., 14: 681-687.
BENTHE, H.F., KNOP, J., & SCHMOLDT, A. (1972) [Absorption and
distribution of polychlorinated biphenyls (PCB) after inhalatory
application.] Arch. Toxikol., 29: 85-95 (in German).
BENTLEY, J. (1983) Incineration of PCBs. In: Barros, M.C.,
Könemann, H., & Visser, R., ed. Proceedings of the PCB Seminar, The
Hague, 28-30 September 1983, The Hague, Ministry of the
Environment, pp. 281-288.
BERAN, M., BRANDT, I., & SLANINA, P. (1983) Distribution and effect
of some polychlorinated biphenyls in the hemopoietic tissues. J.
Toxicol. environ. Health., 12: 521-532.
BERCOVICI, B., WASSERMANN, M., CUCOS, S., RON, M., WASSERMAN, D.,
& PINES, A. (1983) Serum levels of polychlorinated biphenyls and
some organochlorine insecticides in women with recent and former
missed abortions. Environ. Res., 30(1): 169-174.
BERG, O.W., DIOSADY, P.L., & REES, G.A.V. (1972) Column
chromatographic separation of polychlorinated biphenyls from
chlorinated hydrocarbon pesticides and their subsequent gas
chromatographic quantitation in terms of derivatives. Bull.
environ. Contam. Toxicol., 7: 338-347.
BERGH, A.K. & PEOPLE, R.S. (1977) PCB distribution in sewage wastes
and their environmental and community effects. Proceedings of the
1977 National Conference on Treatment and Disposal of Industrial
Wastewaters and Residues, Houston, Texas, pp. 4-6.
BERGLUND, F. (1972) Levels of polychlorinated biphenyls in foods in
Sweden. Environ. health Perspect., 1: 67-69.
BERLIN, M., GAGE, J.C., & HOLM, S. (1973) The metabolism and
distribution of 2,4,5,2',5'-pentachlorobiphenyl in the mouse. In:
Proceedings of the Polychlorinated Biphenyls II Conference,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 101-108 (Publication No. 4E).
BERLIN, M., GAGE, J.C., & HOLM, S. (1975) Distribution and
metabolism of polychlorobiphenyls. In: Proceedings of an
International Symposium on Recent Advances in the Assessment of the
Health Effects of Environmental Pollution, Paris, 24-26 June 1974,
Luxembourg, Commission of the European Communities, Vol. 2,
pp. 895-902.
BERRY, D.L., SLAGA, T.J., DIGIOVANNI, J., & JUCHAU, M.R. (1979)
Studies with chlorinated dibenzo- p-dioxins, polybrominated
biphenyls, and polychlorinated biphenyls in a two-stage system of
mouse skin tumorigenesis: potent anticarcinogenic effects. Ann. NY
Acad. Sci., 320: 405-414.
BERRY, D.L., DIGIOVANNI, J., JUCHAU, M.R., BRACKEN, W.M., GLAESON,
G.L., & SLAGA, T.J. (1978) Lack of tumour promoting ability of
certain environmental chemicals in a two-stage mouse skin
tumorigenesis assay. Res. Commun. chem. Pathol. Pharmacol.,
20: 101-108.
BERTAZZI, P.A., ZOCHETTI, C., GUERCILENA, S., FOGLIA, M.D.,
PESATORI, A., & RIBOLDI, L. (1982) Mortality study of male and
female workers exposed to PCBs. In: Prevention of Occupational
Cancer: International Symposium, Geneva, International Labour
Office, pp. 242-248 (Occupational Safety and Health Series No. 46).
BERTAZZI, P.A., RIBOLDI, L., PESATORI, A., RADICE, L., & ZOCHETTI,
C. (1987) Cancer mortality of capacitor manufacturing workers. Am.
J. ind. Med., 11(2): 165-176.
BICKERS, D.R., HARBER, L.C., KAPPAS, A., & ALVARES, A.P. (1972)
Polychlorinated biphenyls: Comparative effects of high and low
chlorine containing Aroclors on hepatic mixed function oxidase.
Res. Commun. Chem. Pathol. Pharmacol., 3: 505-512.
BIDLEMAN, T.F. & OLNEY, C.E. (1974) Chlorinated hydrocarbons in the
Sargasso Sea atmosphere and surface water. Science, 183: 516-518.
BIDLEMAN, T.F., RICE, C.P., & OLNEY, C.E. (1978) High molecular
weight chlorinated hydrocarbons in the air and sea: Rates and
mechanisms of air/sea transfer. In: Windom, H. & Duce, R.A., ed.
Marine pollutant transfer, Lexington, D.C. Heath and Co.,
pp. 323-351.
BIESSMANN, A. (1982) Effects of PCBs on gonads, sex hormone balance
and reproduction processes of Japanese quail Coturnix coturnix
japonica after ingestion during sexual maturation. Environ.
Pollut., 27: 15-30.
BIGGS, D.C., ROWLAND, R.G., POWERS, C.D., O'CONNERS, H., & WURSTER,
C. (1978) A comparison of the effects of chlordane and PCB on the
growth, photosynthesis, and cell size of estuarine phytoplankton.
Environ. Pollut., 15: 253-263.
BIGGS, D.C., ROWLAND, R.G., & WURSTER, C.F. (1979) Effects of
trichloroethylene, hexachlorobenzene and polychlorinated biphenyls
on the growth and cell size of marine phytoplankton. Bull. environ.
Contam. Toxicol., 21: 196-201.
BIGGS, D.C., POWERS, C.D., ROWLAND, R.G., O'CONNORS, H.B., &
WURSTER, C.F. (1980) Uptake of polychlorinated biphenyls by natural
phytoplankton assemblages: field and laboratory determinations of
14C-PCB particle-water index of sorption. Environ. Pollut.,
22: 101-110.
BILLINGS, W.N., BIDLEMAN, T.F., & VERNBERG, W.B. (1978) Movement of
PCB from a contaminated reservoir into a drinking water supply.
Bull. environ. Contam. Toxicol., 19: 215-222.
BIOCCA, M., GUPTA, B.N., CHAE, K., MCKINNEY, J.D., & MOORE, J.A.
(1981) Toxicity of selected symmetrical hexachlorobiphenyl isomers
in the mouse. Toxicol. appl. Pharmacol., 58: 461-474.
BIRGE, W.J., BLACK, J.A., & WESTERMAN, A.G. (1978) Effects of
polychlorinated biphenyl compounds and proposed PCB-replacement
products on embryo-larval stages of fish and amphibians,
Washington, DC, US Department of the Interior, p. 33 (Research
Report No. 118).
BIRNBAUM, L.S. (1983) Distribution and excretion of 2,3,6,2',3',6'-
and 2,4,5,2',4',5'-hexachlorobiphenyl in senescent rats. Toxicol.
appl. Pharmacol., 70: 262-272.
BIRNBAUM, L. & BAIRD, M.B. (1978) Induction of hepatic mixed
function oxidases in senescent rodents-II. Effect of
polychlorinated biphenyls. Exp. Gerontol., 13: 469-477.
BIRNBAUM, L.S., WEBER, H., HARRIS, M.W., LAMB, J.C., IV, &
MCKINNEY, J.D. (1985) Toxic interaction of specific polychlorinated
biphenyls and 2,3,7,8-tetrachlorodibenzo- p-dioxin: increased
incidence of cleft palate in mice. Toxicol. appl. Pharmacol.,
77: 292-302.
BITMAN, J. & CECIL, H.C. (1970) Estrogenic activity of DDT analogs
and polychlorinated biphenyls. J. agric. food Chem., 18: 1108-1112.
BITMAN, J., CECIL, H.C., & HARRIS, S.J. (1972) Biological effects
of polychlorinated biphenyl in rats and quail. Environ. health
Perspect., 1: 145-149.
BJERK, J.E. (1972) [Residues of DDT and polychlorinated biphenyls
in Norwegian human material.] Tidsskr. Nor. Laegeforen., 92: 15-19
(in Norwegian).
BLEAVINS, M.R., AULERICH, R.J., & RINGER, R.K. (1980)
Polychlorinated biphenyls (Aroclors 1016 and 1242): Effects on
survival and reproduction in mink and ferrets. Arch. environ.
Contam. Toxicol., 9: 627-635.
BLEAVINS, M.R., AULERICH, R.J., RINGER, R.K., & BELL, T.G. (1982)
Excessive nail growth in the European ferret induced by Aroclor
1242. Arch. environ. Contam. Toxicol., 11: 305-312.
BLEAVINS, M.R., AULERICH, R.J., & RINGER, R.K. (1981) Placental and
mammary transfer of polychlorinated and polybrominated biphenyls in
the mink and ferret. In: Proceedings of the 2nd Conference on Avian
and Mammalian Wildlife Toxicology, Philadelphia, Pennsylvania,
American Society for Testing and Material, pp. 121-131
(ASTM STP 757).
BLEAVINS, M.R., BRESLIN, W.J., AULERICH, R.J., & RINGER, R.K.
(1984) Placental and mammary transfer of a polychlorinated biphenyl
mixture (Aroclor 1254) in the European ferret (Mustela putorius
furo). Environ. Toxicol. Chem., 3: 637-644.
BLETCHLY, J.D. (1983) Polychlorinated biphenyls. Production,
current use, and possible rates of future disposal in OECD
countries. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of PCB Seminar, The Hague, 28-30 September 1983, The
Hague, Ministry of the Environment, pp. 343-365.
BLETCHLY, J.D. (1985) Report to the Commission of the European
Communities on a study of measures to avoid dispersion into the
environment of polychlorinated biphenyls (PCBs) and polychlorinated
terphenyls (PCTs) from existing installations, Luxembourg,
Commission of the European Communities, pp. 22-33.
BLOK, S.M.G., GREVE, P.A., SANGSTER, B., SAVELKOUL, T.J.F., &
WEGMAN, R.C.C. (1984) [Study of normally occurring values of a
number of organochlorine pesticides and related compounds and their
metabolites, of polychlorine biphenyls and of chlorophenols in the
blood or plasma of healthy volunteers.] Bilthoven, The Netherlands,
National Institute of Public Health and Environmental Hygiene
(Unpublished report No. 638101001) (in Dutch).
BLUS, L.J., LAMONT, T.G., & NEELY, B.S. (1979) Fish, wildlife, and
estuaries: Effects of organochlorine residues on eggshell
thickness, reproduction, and population status of Brown Pelicans
(Pelecanus occidentalis) in South Carolina and Florida, 1969-76.
Pestic. monit. J., 12: 172-184.
BONNYNS, M. & BASTOMSKY, C.H. (1976) Polychlorinated
biphenyl-induced modification of lymphocyte response to plant
mitogens in rats. Experientia (Basel), 32: 522-523.
BORN, E.W., KRAUL, I., & KRISTENSEN, T. (1981) Mercury, DDT, and
PCB in the Atlantic Walrus (Odobenus rosmarus rosmarus) from the
Thule District, North Greenland. Arctic, 34: 255-260.
BOURQUIN, A.W. & CASSIDY, S. (1975) Effect of polychlorinated
biphenyl formulations on the growth of estuarine bacteria. Appl.
microbiol., 29: 125-127.
BOURQUIN, A.W. & KIEFER, L.A. (1975) Inhibition of estuarine
microorganisms by polychlorinated biphenyls. Dev. ind. Microbiol.,
16: 256-261.
BOWES, G.W. & JONKEL, C.J. (1975) Presence and distribution of
polychlorinated biphenyls (PCBs) in arctic and subarctic marine
food chains. J. Fish Res. Board Can., 32: 2111-2123.
BOWES, G.W., MULVIHILL, M.J., SIMONEIT, B.R.T., BURLINGAME, A.L.,
& RISEBROUGH, R.W. (1975) Identification of chlorinated
dibenzofurans in American polychlorinated biphenyls. Nature
(Lond.), 256: 305-307.
BOWMAN, R.E. & HEIRONIMUS, M.P. (1981) Hypoactivity in adolescent
monkeys perinatally exposed to PCBs and hyperactive as juveniles.
Neurobehav. Toxicol. Teratol., 3: 15-18.
BOWMAN, R.E., HEIRONIMUS, M.P., & ALLEN, J.R. (1978) Correlation of
PCB body burden with behavioural toxicology in monkeys. Pharmacol.
Biochem. Behav., 9: 49-56.
BOWMAN, R.E., HEIRONIMUS, M.P., & BARSOTTI, D.A. (1982) Locomotor
hyperactivity in PCB-exposed monkeys. Neurotoxicology, 2: 251-268.
BRANDT-RAUF, P.W. & NIMAN, H.L. (1988) Serum screening for oncogene
proteins in workers exposed to PCBs. Br. J. ind. Med.,
45(10): 689-693.
BRAUN, F. & MEYHOFER, B. (1977) [Investigations on the
concentration of polychlorinated biphenyls (Clophen C) in fish
organs under laboratory conditions.] Fisch Umwelt., 3: 1-11
(in German).
BRAZELTON, T.B. (1973) Neonatal behavioural assessment scale,
Philadelphia, Pennsylvania, J.B. Lippincott.
BREZNER, E., TERKEL, J., & PERRY, A.S. (1984) The effect of Aroclor
1254 (PCB) on the physiology of reproduction in the female rat-I.
Comp. Biochem. Physiol., 77C: 65-70.
BRIGGS, D.M. & HARRIS, J.R. (1973) Polychlorinated biphenyls
influence on hatchability. Poult. Sci., 52: 1291-1294.
BRINKMAN, U.A. & DE KOK, A. (1980) Production properties and usage.
In: Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins
and related products, Amsterdam, Elsevier Biomedical Press,
pp. 1-40.
BRINKMAN, M., FOGELMAN, K., HOEFLEIN, J., LINDH, T., PATEL, M.,
TRENCH, W.C., & AIKENS, D.A. (1980) Distribution of polychlorinated
biphenyls in the Fort Edward, New York, water system. Environ.
Manage., 4(6): 511-520.
BRINKMAN, M., FOGELMAN, K., HOEFLEIN, J., LINDH, T., PATEL, M.,
TRENCH, W.C., & AIKENS, D.A. (1981) Levels of polychlorinated
biphenyls in the Fort Edward, New York, water system. Adv. Identif.
Anal. Org. Pollut. Water, 2(50): 1001-1015.
BRITTON, W.M. & HUSTON, T.M. (1972) Yolk content and hatchability
of egg from hens fed Aroclor 1242. Poult. Sci., 51: 1869.
BROADHURST, M.G. (1972) Use and replaceability of polychlorinated
biphenyls. Environ. health Perspect., 2: 81-102.
BROUWER, A., VAN DEN BERG, K.J., & KUKLER, A. (1985) Time and dose
responses of the reduction in retinoid concentrations in C57BL/Rij
and DBA/2 mice induced by 3,4,3',4'-tetrachlorobiphenyl. Toxico.
appl. Pharmacol., 78: 180-189.
BROUWER, A., KUKLER, A., & VAN DEN BERG, K.J. (1988) Alterations in
retinoid concentrations in several extrahepatic organs of rats by
3,4,3',4'-tetrachlorobiphenyl. Toxicology, 50: 317-330.
BROUWER, A., REIJNDERS, P.J.H., & KOEMAN, J.H. (1989)
Polychlorinated biphenyl (PCB)-contaminated fish induces vitamin A
and thyroid hormone deficiency in the common seal (Phoca
vitulina). Aquat. Toxicol., 15: 99-106.
BROWN, D.P. (1987) Mortality of workers exposed to polychlorinated
biphenyls - An update. Arch. environ. Health, 42(6): 333-339.
BROWN, D.P. & JONES, M. (1981) Mortality and industrial hygiene
study of workers exposed to polychlorinated biphenyls. Arch.
environ. Health, 36: 120-129.
BROWN, J.F. & LAWTON, R.W. (1984) Polychlorinated biphenyl (PCB)
partitioning between adipose tissue and serum. Bull. environ.
Contam. Toxicol., 33: 277-280.
BROWN, J.F., BEDARD, D.L., BRENNAN, M.J., CARNAHAN, J.C., FENG, H.,
& WAGNER, R.E. (1987a) Polychlorinated biphenyl dechlorination in
aquatic sediments. Science, 236: 709-712.
BROWN, J.F., WAGNER, R.E., FENG, H., BEDARD, D.L., BRENNAN, M.J.,
CARNAHAN, J.C., & MAY, R.J. (1987b) Environmental dechlorination of
PCBs. Environ. Toxicol. Chem., 6: 579-593.
BROWN, J.F., CARNAHAN, J.C., DORN, S.B., GROVES, J.T., LIGON, W.V.,
MAY, R.J., WAGNER, R.E., & HAMILTON, S.B. (1988) Levels of
bioactive PCDF congeners in PCB dieletric fluids from capacitors
and transformers. Chemosphere, 17(9): 1697-1702.
BRUCE, R.W. & HEDDLE, J.A. (1979) The mutagenic activity of 61
agents as determined by the micronucleus, Salmonella, and sperm
abnormality assays. Can. J. Genet. Cytol., 21: 319-334.
BRUCKNER, J.V., KHANNA, K.L., & CORNISH, H.H. (1973) Biological
responses of the rat to polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 24: 434-448.
BRUCKNER, J.V., KHANNA, K.L., & CORNISH, H.H. (1974) Effect of
prolonged ingestion of polychlorinated biphenyls on the rat. Food
Cosmet. Toxicol., 12: 323-330.
BRUCKNER, J.V., JIANG, W.-D., BROWN, J.M., PUTCHA, L., CHU, C.K.,
& STELLA, V.J. (1977) The influence of ingestion of environmentally
encountered levels of a commercial polychlorinated biphenyl mixture
(Aroclor 1254) on drug metabolism in the rat. J. Pharmacol. exp.
Ther., 202: 22-31.
BRUNSTROM, B. & DARNERUD, P.O. (1983) Toxicity and distribution in
chick embryos of 3,3,4,4-tetrachlorobiphenyl injected into the
eggs. Toxicology, 27: 103-110.
BRUNSTROM, B. & REUTERGARDH, L. (1986) Differences in sensitivity
of some avian species to the embryotoxicity of a PCB,
3,3',4,4'-tetrachlorobiphenyl, injected into the eggs. Environ.
Pollut., 42: 37-45.
BRUNSTROM, B., KIHLSTROM, I., & LUNDKVIST, U. (1982) Studies of
foetal death and foetal weight in guinea-pigs fed polychlorinated
biphenyls (PCB). Acta pharmacol. toxicol., 50: 100-103.
BRUNSTRÖM, B., DARNERUD, P.O., BRANDT, I., & ORBERG, J. (1982a)
Distribution, metabolism and toxicity of 2,2,4,5-tetrachloro-
biphenyl after injection into the yolk of embryonated hens' eggs.
Ambio, 11: 212-214.
BRUNSTRÖM, B., KIHLSTRÖM, I., & LUNDKVIST, U. (1982b) Studies of
foetal death and foetal weight in guinea-pigs fed polychlorinated
biphenyls (PCB). Acta pharmacol. toxicol., 50: 100-103.
BUCKLEY, E.H. (1982) Accumulation of airborne polychlorinated
biphenyls in foliage. Science, 216: 520-522.
BUCKLEY, E.H. (1983) Decline of background PCB concentrations in
vegetation in New York state. Northeast. environ. Sci., 2: 181-187.
BUHLER, F., SCHMID, P., & SCHLATTER, Ch. (1988) Kinetics of PCB
elimination in man. Chemosphere, 17(9): 1717-1726.
BUNCE, N.J. (1978) Photolysis of 2-chlorobiphenyl in aqueous
acetonitrile. Chemosphere, 7: 653-656.
BUNCE, N.J., KUMAR, Y., & BROWNLEE, B.G. (1978) An assessment of
the impact of solar degradation of polychlorinated biphenyls in the
aquatic environment. Chemosphere, 7: 155-164.
BURKHARD, L.P., ARMSTRONG, D.E., & ANDREN, A.W. (1985) Henry's Law
Constants for the polychlorinated biphenyls. Environ. Sci.
Technol., 19(7): 590-596.
BURNS, J.E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue-Texas 1969-72.
Pestic. monit. J., 7(3/4): 122-126.
BURSE, V.W., KIMBROUGH, R.D., VILLANUEVA, E.C., JENNINGS, R.W.,
LINDER, R.E., & SOVOCOOL, G.W. (1974) Polychlorinated biphenyls;
storage, distribution, excretion, and recovery: liver morphology
after prolonged dietary ingestion. Arch. environ. Health,
29: 301-307.
BUSER, H.R. (1979) Formation of polychlorinated dibenzofurans
(PCDFs) and dibenzo- p-dioxins (PCDDs) from the pyrolysis of
chlorobenzenes. Chemosphere, 8: 415-424.
BUSER, H.R. (1985) Formation, occurrence, and analysis of
polychlorinated dibenzofurans, dioxins and related compounds.
Environ. health Perspect., 60: 259-267.
BUSER, H.R. & BOSSHARDT, H.P. (1978) [Polychlorinated
dibenzo- p-dioxins, dibenzofurans and benzenes in the ash of
community and industrial incineration plants.] Mitt. Geb. Lebensm.
Hyg., 69: 191-199 (in German).
BUSER, H.R. & RAPPE, C. (1979) Formation of polychlorinated
dibenzofurans (PCDFs) from the pyrolysis of individual PCB isomers.
Chemosphere, 3: 157-174.
BUSER, H.R., BOSSHARDT, H.P., & RAPPE, C. (1978a) Formation of
polychlorinated dibenzofurans (PCDF's) from the pyrolysis of PCBs.
Chemosphere, 7: 109-119.
BUSER, H.R., BOSSHARDT, H.P., RAPPE, C., & LINDAHL, R. (1978b)
Identification of polychlorinated dibenzofuran isomers in fly ash
and PCB pyrolysis. Chemosphere, 7: 419-429.
BUSH, B., TUMASONIS, C.F., & BAKER, F.D. (1974) Toxicity and
persistence of PCB homologs and isomers in the avian system. Arch.
environ. Contam. Toxicol., 2: 195-212.
BUSH, B., SNOW, J., & KOBLINTZ, R. (1984) Polychlorobiphenyl (PCB)
congeners, p,p'-DDE, and hexachlorobenzene in maternal and fetal
cord blood from mothers in upstate New York. Arch. environ. Contam.
Toxicol., 13(5): 517-527.
BUSH, B., SNOW, J., CONNOR, S., & KOBLINTZ, R. (1985)
Polychlorinated biphenyl congeners (PCBs), p,p'-DDE and
hexachlorobenzene in human milk in three areas of upstate New York.
Arch. environ. Contam. Toxicol., 14: 443-450.
BUSH, B., SIMPSON, K.W., SHANE, L., & KOBLINTZ, R.R. (1985) PCB
congener analysis of water and caddisfly larvae (Insecta:
Trichoptera) in the Upper Hudson river by glass capillary
chromatography. Bull. environ. Contam. Toxicol., 34: 96-105.
BYRNE, J.J. & SEPKOVIC, D.W. (1987) Inhibition of monovalent cation
transport across the cell membrane by polychlorinated biphenyl but
not by polybrominated biphenyl. Arch. environ. Contam. Toxicol.,
16: 573-577.
BYRNE, J.J., CARBONE, J.P., & PEPE, M.G. (1988) Suppression of
serum adrenal cortex hormones by chronic low-dose polychloro-
biphenyl of polybromobiphenyl treatments. Arch. environ. Contam.
Toxicol., 17: 47-53.
CAIN, B.W. (1981) Nationwide residues of organochlorine compounds
in wings of adult mallards and black ducks, 1979-80. Pestic. monit.
J., 15: 128-134.
CAIN, B.W. & BUNCK, C.M. (1983) Residues of organochlorine
compounds in starlings (Sturnus vulgaris), 1979. Environ. monit.
Assess., 3: 161-172.
CALANDRA, J.C. (1976) Summary of toxicological studies on
commercial PCBs. In: Proceedings of the National Conference of
polychlorinated biphenyls, Washington, DC, US Environmental
Protection Agency (560/6-75-004).
CALIFANO, R.J., O'CONNOR, J.M., & PETERS, L.S. (1980) Uptake,
retention, and elimination of PCB (Aroclor 1254) by larval striped
bass (Morone saxatilis). Bull. environ. Contam. Toxicol.,
24: 467-472.
CALL, D.J. & HARRELL, B.E. (1974) Effects of dieldrin and PCBs upon
the production and morphology of Japanese quail eggs. Bull.
environ. Contam. Toxicol., 11: 70-77.
CALLAHAN, M.A.A., HAMMERSTROM, K.A., & SCHWEER, G. (1983) Present
PCB uses and their potential for release to the environment. In:
Barros, M.C., Könemann, H., & Visser, R., ed. Proceedings of PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 152-172.
CAMP, B.J., HEJTMANCIK, E., ARMOUR, C., & LEWIS, D.H. (1974) Acute
effects of Aroclor 1254 (PCB) on Ictalurus punctatus (catfish).
Bull. environ. Contam. Toxicol., 12: 204-208.
CAMPS, M., PLANAS, J., GOMEZ-CATALAN, J., SABROSO, M., TO-FIGUERAS,
J., & CORBELLA, J. (1989) Organochlorine residues in human adipose
tissue in Spain: Study of an agrarian area. Bull. environ. Contam.
Toxicol., 42: 195-201.
CANTONI, C., FABBRIS, F., ROGLEDI, R., & CAMPAGNARI, A. (1988)
[Organochlorine pesticides found in foods of animal origin in the
1985-1987 biennium.] Ind. Aliment., XXVII(1): 6-8 (in Italian).
CARLSON, R.W. & DUBY, R.T. (1973) Embryotoxic effects of three
PCB's in the chicken. Bull. environ. Contam. Toxicol., 9: 261-266.
CARNES, R.A., DOERGER, J.U., & SPARKS, H.L. (1973) Polychlorinated
biphenyls in solid waste and solid-waste-related materials. Arch.
environ. Contam. Toxicol., 1: 27-35.
CAREY, A.E. & HARVEY, G.R. (1978) Metabolism of polychlorinated
biphenyls by marine bacteria. Bull. environ. Contam. Toxicol.,
20: 527-534.
CARTER, J.W. (1985) Effects of dietary in PCBs (Aroclor 1254) on
serum levels of lipoprotein cholesterol in Fischer rats. Bull.
environ. Contam. Toxicol., 34: 427-431.
CARTER, J.W. & CLANCY, J. (1980) Acutely administered
polychlorinated biphenyls (PCBs) decrease splenic cellularity but
increase its ability to cause graft-versus host reactions in BALB/c
mice. Immunopharmacology, 2: 341-347.
CATELANI, D., SORLINI, C., & TRECCANI, V. (1971) The metabolism of
biphenyl by Pseudomonas putida. Experientia (Basel),
27: 1173-1174.
CECIL, H.C., BITMAN, J., LILLIE, R.J., FRIES, G.F., & VERRETT, J.
(1974) Embryotoxic and teratogenic effects in unhatched fertile
eggs from hens fed polychlorinated biphenyls (PCBs). Bull. environ.
Contam. Toxicol., 11: 489-495.
CETINKAYA, M., GABEL, B., PODBIELSKI, A., & THIEMANN, W. (1984)
[Investigation on the association between nutrition and living
conditions of breastfeeding mothers and the contamination of
breastmilk with organochlorine compounds of low volatility.] Akt.
Ernähr., 9: 157-162 (in German).
CHAKRABORTY, D., BHATTACHARYYA, A., CHATTERJEE, J., CHATTERJEE, K.,
SEN, A., CHATTERJEE, S., MAJUMDAR, K., & CHATTERJEE, G.C. (1978)
Biochemical studies on polychlorinated biphenyl toxicity in rats:
manipulation by vitamin C. Int. J. Vitam. Nutr. Res., 48: 22-31.
CHANG, K.-T., CHENG, J.-S., HUANG, P.-C., & TUNG, T.-C. (1980a)
Study of patients with PCB poisoning. J. Formosan Med. Assoc.,
79: 304-313.
CHANG, K.-J., LU, F.-J., TUNG, T.-C., & LEE, T.-P. (1980b) Studies
on patients with PCB poisoning. Determination of urinary
coproporphyrin, uroporphyrin, delta-aminolaevulinic acid and
prophobilinogen. Res. Commun. chem. Pathol. Pharmacol.,
30(3): 547-554.
CHANG, K.-J., HSIEH, K.-H., LEE, T.-P., TANG, S.-Y., & TUNG, T.-C.
(1981) Immunologic evaluation of patients with polychlorinated
biphenyls poisoning. Determination of lymphocyte subpopulations.
Toxicol. appl. Pharmacol., 61: 58-63.
CHASE, K.H., WONG, O., THOMAS, D., BERNEY, B.W., & SIMON, R.K.
(1982) Clinical and metabolic abnormalities associated with
occupational exposure to polychlorinated biphenyls (PCBs). J.
occup. Med., 24(2): 109-114.
CHEN, P.H., GRAW, J.M., WONG, C.K., & CHEN, C.J. (1980) Levels and
gas chromatography patterns of PCBs in blood of patients after PCB
poisoning in Taiwan. Bull. environ. Contam. Toxicol., 25: 325-329.
CHEN, P.H., CHANG, K.T., & LU, Y.D. (1981) Polychlorinated
biphenyls and polychlorinated benzofurans in the toxic rice-bran
oil caused PCB poisoning in Taichyung. Bull. environ. Contam.
Toxicol., 26(4): 489-495.
CHEN, P.H., LUO, M.L., WONG, C.K., & CHEN, C.J. (1982) Comparative
rates of elimination of some individual polychlorinated biphenyls
from the blood of PCB-poisoned patients in Taiwan. Food chem.
Toxicol., 20(4): 417-425.
CHEN, P.H., WONG, C.-K., RAPPE, C., & NYGREN, M. (1985)
Polychlorinated biphenyls, dibenzofurans and quaterphenyls in toxic
rice-bran oil and in the blood and tissues of patients with PCB
poisoning (Yu-Cheng) in Taiwan. Environ. Health Perspect.,
59: 59-65.
CHEN, P.R., MCKINNEY, J.D., & MATTHEWS, H.B. (1976) Metabolism of
2,4,5,2',5'-pentachlorobiphenyl in the rat. Drug. Metab. Dispos.,
4: 362-367.
CHEN, R.-C., TANG, S.-Y., MIYATA, H., KASHIMOTO, T., CHANG, Y.-C.,
CHANG, K.-J., & TUNG, T.-C. (1985) Polychlorinated biphenyl
poisoning: Correlation of sensory and motor nerve conduction,
neurologic symptoms and blood levels of polychlorinated biphenyls,
quaterphenyls, and dibenzofurans. Environ. Res., 37: 340-348.
CHEN, T.S. & DUBOIS, K.P. (1973) Studies on the enzyme inducing
effects of polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
26: 504-512.
CHEN HSI-SUNG, P., LUO, M.-L., WONG, C.-K., & CHEN, C.-J. (1984)
Polychlorinated biphenyls, dibenzofurans, and quaterphenyls in the
toxic rice-bran oil and PCBs in the blood of patients with PCB
poisoning in Taiwan. Am. J. ind. Med., 5: 133-145.
CHESNEY, C.F. & ALLEN, J.R. (1974) Oxidative phosphorylation and
respiration by liver mitochondria from polychlorinated
biphenyl-intoxicated rats. Biochem. Pharmacol., 23: 1577-1582.
CHIA, L.-G. & CHU, F.-L. (1984) Neurological studies on
polychlorinated biphenyl (PCB)-poisoned patients. Am. J. ind. Med.,
5: 117-126.
CHOU, S.M., MIKE, T., PAYNE, W.M., & DAVIS, G.J. (1979)
Neuropathology of "spinning syndrome" induced by prenatal
intoxication with a PCB in mice. Ann. NY Acad. Sci., 320: 373-395.
CHOW, C.K., THACKER, R., & GAIROLA, C.C. (1979) Increased level of
L-ascorbic acid in the plasma of polychlorobiphenyls-treated rats
and its inhibition by dietary vitamin E. Res. Commun. chem. Pathol.
Pharmacol., 26: 605-608.
CHRISTIANI, D.C., KRIEBEL, D., FOX, N.J., & BAKER, E.L. (1986)
Persistently elevated polychlorinated biphenyl levels from residual
contamination of workplace surfaces. Am. J. ind. Med., 10: 143-151.
CHU, C.K., STELLA, V.J., BRUCKNER, J.V., & JIANG, W.D. (1977)
Effects of long-term exposure to environmental levels of
polychlorinated biphenyls on pharmacokinetics of pentobarbital in
rats. J. pharm. Sci., 66(2): 238-241.
CLARK, D.R. (1978) Uptake of dietary PCB by pregnant Big Brown Bats
(Eptesicus fuscus) and their fetuses. Bull. environ. Contam.
Toxicol., 19: 707-714.
CLARK, D.R. & LAMONT, T.G. (1976) Organochlorine residues and
reproduction in the big brown bat. J. Wildl. Manage., 40: 249-254.
CLARK, D.R. & PROUTY, R.M. (1977) Experimental feeding of DDT and
PCB to female Big Brown Bats (Eptesicus fuscus). J. Toxicol.
environ. Health, 2: 917-928.
CLARK, R.R., CHIAN, E.S.K., & GRIFFIN, R.A. (1979) Degradation of
polychlorinated biphenyls by mixed microbial cultures. Appl.
environ. Microbiol., 37: 680-685.
CLARK, J.R., PATRICK, J.M., MOORE, J.C., & FORESTER, J. (1986)
Accumulation of sediment-bound PCBs by Fiddler crabs. Bull.
environ. Contam. Toxicol., 36: 571-578.
CLAUS, B. & ACKER, L. (1975) [Contamination of milk and milk
products with chlorinated hydrocarbons in Westphalia. II. Results
and discussion.] Z. Lebensmittelunters. Forsch., 159(3): 129-137
(in German).
CLEVENGER, M.A., ROBERTS, S.M., LATTIN, D.L., HARBINSON, R.D., &
JAMES, R.C. (1989) The pharmacokinetics of 2,2,5,5'-tetrachloro-
biphenyl and 3,3',4,4'-tetrachlorobiphenyl and its relationship to
toxicity. Toxicol. appl. Pharmacol., 100: 315-327.
COLE, D.R. & PLAPP, F.W. (1974) Inhibition of growth and
photosynthesis in Chlorella pyrenoidosa by a polychlorinated
biphenyl and several insecticides. Environ. Entomol., 3: 217-220.
COLLINS, W.T. & CAPEN, C.C. (1980a) Ultrastructural and functional
alterations of the rat's thyroid gland produced by polychlorinated
biphenyls compared with iodide excess and deficiency, and
thyrotropin and thyroxine administration. Virchows Arch. cell.
Pathol., B33: 213-231.
COLLINS, W.T. & CAPEN, C.C. (1980b) Biliary excretion of 125
I-thyroxine and fine structural alterations in the thyroid glands
of Gunn rats fed polychlorinated biphenyls (PCB). Lab. Invest.,
43: 158-164.
COLLINS, G.B., HOLMES, D.C., & JACKSON, F.J. (1972) The estimation
of polychlorobiphenyls. J. Chromatogr., 71: 443-449.
COLLINS, W.T., CAPEN, C.C., KASZA, L., CARTER, C., & DAILEY, R.E.
(1977) Effect of polychlorinated biphenyl (PCB) on the thyroid
gland of rats. Am. J. Pathol., 89: 119-136.
CONNELL, D.W. (1987) Age to PCB concentration relationship with the
striped bass (Morone saxatilis) in the Hudson River and Long
Island Sound. Chemosphere, 16: 1469-1474.
COOKE, A.S. (1973) Shell thinning in avian eggs by environmental
pollutants. Environ. Pollut., 4: 85-152.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides and pollution, Huntingdon, United Kingdom, Natural
Environment Research Council, Institute of Terrestrial Ecology,
74 pp.
COOLEY, N.R., KELTNER, J.M., & FORESTER, J. (1972) Mirex and
Aroclor 1254: Effect on accumulation by Tetrahymena pyriformis
strain W. J. Protozool., 19: 636-638.
COOLEY, N.R., KELTNER, J.M., & FORESTER, J. (1973) The
polychlorinated biphenyls, Aroclors 1248 and 1260: Effect on and
accumulation by Tetrahymena pyriformis. J. Protozool.,
20: 443-445.
COSPER, E.M., WURSTER, C.F., & ROWLAND, R.G. (1984) PCB resistance
within phytoplankton populations in polluted and unpolluted marine
environments. Mar. environ. Res., 12: 209-223.
COTE, M.G., PLAA, G.L., VALLI, V.E., & VILLENEUVE, D.C. (1985)
Subchronic effects of a mixture of "persistent" chemicals found in
the Great Lakes. Bull. environ. Contam. Toxicol., 34: 285-290.
COURTNEY, W.A.M. & DENTON, G.R.W. (1976) Persistence of
polychlorinated biphenyls in the hard-clam (Mercenaria mercenaria)
and the effect upon the distribution of these pollutants in the
estuarine environment. Environ. Pollut., 10: 55-64.
COURTNEY, W.A.M. & LANGSTON, W.J. (1978) Uptake of polychlorinated
biphenyl (Aroclor 1254) from sediment and seawater in two
intertidal polychaetes. Environ. Pollut., 15: 303-309.
CRAIGIE, J.S. & HUTZINGER, O. (1975) Effects of commercial
chlorinated hydrocarbons and specific chlorobiphenyls on the growth
of seven species of marine phytoplankton. Chemosphere, 3: 139-144.
CROSBY, D.G. & MOILANEN, K.W. (1973) Photodecomposition of
chlorinated biphenyls and dibenzofurans. Bull. environ. Contam.
Toxicol., 10: 372-377.
CURLEY, A., BURSE, V.W., GRIM, M.E., JENNINGS, R.W., & LINDER, R.E.
(1971) Polychlorinated biphenyls: Distribution and storage in body
fluids and tissues of Sherman rats. Environ. Res., 4: 481-495.
CURLEY, A., BURSE, V.W., & GRIM, M.E. (1973a) Polychlorinated
biphenyls: Evidence of transplacental passage in the Sherman rats.
Food Cosmet. Toxicol., 11: 471-476.
CURLEY, A., BURSE, V.W., JENNINGS, R.W., VILLANUEVA, E.C., TOMATIS,
L., & AKAZAKI, K. (1973b) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond.), 242: 338-340.
DAHLGREN, R.B. & LINDER, R.L. (1971) Effects of polychlorinated
biphenyls on pheasant reproduction, behavior, and survival. J.
Wildl. Manage., 35: 315-319.
DAHLGREN, R.B., LINDER, R.L., & CARLSON, C.W. (1972)
Polychlorinated biphenyls: Their effects on penned pheasants.
Environ. health Perspect., 1: 89-101.
DAHLGREN, R.B., BURY, R.J., LINDER, R.L., & REIDINGER, R.F. (1972)
Residue levels and histopathology in Pheasants given
polychlorinated biphenyls. J. Wildl. Manage., 36: 524-533.
DAVIDORF, F.H. & KNUPP, J.A. (1979) Epidemiology of ocular
melanoma. Incidence and geographic relationship in Ohio
(1967-1977). Ohio State med. J., 75: 561-564.
DAVIES, K. (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17(2): 263-276.
DAVIES, D. & MES, J. (1987) Comparison of the residue levels of
some organochlorine compounds in breast milk of the general and
indigenous Canadian populations. Bull. environ. Contam. Toxicol.,
39: 743-749.
DAVIS, D. & SAFE, S. (1989) Dose-response immunotoxicities of
commercial polychlorinated biphenyls (PCBs) and their interaction
with 2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol. Lett.,
48: 35-43.
DEFOE, D.L., VEITH, G.D., & CARLSON, R.W. (1978) Effects of Aroclor
1248 and 1260 on the Fathead Minnow (Pimephales promelas). J.
Fish Res. Board Can., 35: 997-1002.
DEFREITAS, A.S.W., NORSTRÖM, R.J., & HUTZINGER, O. (1972) Dynamics
and metabolism of polychlorinated biphenyls (PCBs) in cold-exposed
pigeons. Am. Chem. Soc., 12: 118-123.
DE KOCK, A.C. & LORD, D.A. (1988) Kinetics of the uptake and
elimination of polychlorinated biphenyls by an estuarine fish
species (Rhabdosargus holubi) after aqueous exposure.
Chemosphere, 17: 2381-2390.
DELFINO, J.J. (1979) Toxic substances in the Great Lakes. Environ.
Sci. Technol., 13: 1462-1468.
DELONG, R.L., GILMARTIN, W.G., & SIMPSON, J.G. (1973) Premature
births in California sea lions: Association with high
organochlorine pollutant residue levels. Science, 181: 1168-1169.
DEML, E. & OESTERLE, D. (1982) Sex-dependent promoting effect of
polychlorinated biphenyls on enzyme-altered islands induced by
diethylnitrosamine in rat liver. Carcinogenesis, 3: 1449-1453.
DEML, E. & OESTERLE, D. (1987) Dose-response of promotion by
polychlorinated biphenyls and chloroform in rat liver foci
bioassay. Arch. Toxicol., 60: 209-211.
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels of
organochlorine pesticides on microsomal liver enzymes in short-term
toxicity experiments. Toxicology, 2: 371-380.
DE VOS, R.H. & PEET, E.W. (1971) Thin-layer chromatography of
polychlorinated biphenyls. Bull. environ. Contam. Toxicol.,
6: 164-170.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRIJNS, J.K., MUYS,
T., & VAN DER POLL, J.M. (1984) Pesticides and other chemical
residues in Dutch total diet samples (June 1976-July 1978). Food
Chem. Toxicol., 22(1): 11-21.
DFG (1988) [Polychlorinated biphenyls. Stocktaking on analysis,
occurrence, kinetics and toxicology.] German Association for the
Encouragement of Research: Communication XIII of the Government
Committee on the Testing of Residues in Foods], Weinheim, Verlag
Chemie (in German).
DICKHUT, R.M., ANDREN, A.W., & ARMSTRONG, D.E. (1986) Aqueous
solubilities of six polychlorinated biphenyl congeners at four
temperatures. Environ. Sci. Technol., 20(8): 807-810.
DICKHUT, R.M., ANDREN, A.W., & ARMSTRONG, D.E. (1987) Comment on
"Aqueous solubilities of six polychlorinated biphenyl congeners at
four temperatures". Environ. Sci. Technol., 21(9): 926-928.
DIERINGER, C.S., LAMARTINIERE, C.A., SCHILLER, C.M., & LUCIER, G.W.
(1979) Altered ontogeny of hepatic steroid-metabolizing enzymes by
pure polychlorinated biphenyl congeners. Biochem. Pharmacol.,
28: 2511-2514.
DIETER, M.P. (1974) Plasma enzyme activities in Coturnix quail fed
graded doses of DDE, polychlorinated biphenyls, malathion, and
mercuric chloride. Toxicol. appl. Pharmacol., 27: 86-98.
DIGERNES, V. & ASTRUP, E.G. (1982) Are datascreen terminals a
source of increased PCB-concentrations in the working atmosphere?
Int. Arch. occup. Health, 49: 193-197.
DIGIOVANNI, J., BERRY, D.L., SLAGA, T.J., & JUCHAU, M.R. (1979)
Studies on the relationship between induction of biotransformation
and tumour-initiating activity of 7,12-dimethylbenz(a)anthracene in
mouse skin. In: Jones, P.W. & Leber, P., ed. Polynuclear aromatic
hydrocarbons, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 553-568.
DILLON, J.C., MARTIN, G.B., & O'BRIEN, H.T. (1981) Pesticide
residues in human milk. Food Cosmet. Toxicol., 19: 437-442.
DIVE, D., ERB, F., LECLERC, H., PRIEM, M.N., & COLEIN, M.P. (1976)
Toxicité et bioaccumulation d'isomers de polychlorobiphenyles par
le protozoaire cilie Colpidium campylum. Eur. J. Toxicol.
environ. Hyg., 9: 105-111.
DOBSON, S. (1981) Physiological and behavioural effects of
organochlorines on pigeons. Oekol. Voegel, 3: 39-43.
DOGRA, S., FILSER, J.G., COJOCEL, C., GREIM, H., REGEL, U., OESCH,
F., & ROBERTSON, L.W. (1988) Long-term effects of commercial and
congeneric polychlorinated biphenyls on ethane production and
malondialdehyde levels, indicators of in vivo lipid peroxidation.
Arch. Toxicol., 62: 369-374.
DOGUCHI, M. (1977) Polychlorinated terphenyls as an environmental
pollutant in Japan. Ecotoxicol. environ. Saf., 1: 239-248.
DOGUCHI, M. & FUKANO, S. (1975) Residue levels of polychlorinated
terphenyls, polychlorinated biphenyls and DDT in human blood. Bull.
environ. Contam. Toxicol., 13: 57-63.
DOGUCHI, M., FUKANO, S., & USHIO, F. (1974) Polychlorinated
terphenyls in the human fat. Bull. environ. Contam. Toxicol.,
11(2): 157-158.
DOMMARCO, R., DI MUCCIO, A., COMONI, I., & GIGLI, B. (1987)
Organochlorine pesticide and polychlorinated biphenyl residues in
human milk from Rome (Italy) and surroundings. Bull. environ.
Contam. Toxicol., 39: 919-925.
DONKIN, P., MANN, S., & HAMILTON, E.I. (1981) Polychlorinated
biphenyl, DDT, and dieldrin residues in grey seal (Halichoerus
grypus) males, females and mother-foetus pairs sampled at the
Farne islands, England, during the breeding season. Sci. total
Environ., 19: 121-142.
DOUCETTE, W.J. & ANDREN, A.W. (1988) Aqueous solubility of selected
biphenyl, furan and dioxin congeners. Chemosphere, 17(2): 243-252.
DRIJVER, M., DUIJKERS, T.J., KROMHOUT, D., VISSER, T.J., MULDER,
P., & LOUW, R. (1988) Determinations of polychlorinated biphenyls
(PCBs) in human milk. Acta paediatr. Scand., 77: 30-36.
DRILL, V.A., FREISS, S.L., HAYS, H.W., LOOMIS, T.A., & SCHAFFER,
C.B. (1981) Potential health effects in the human from exposure to
polychlorinated biphenyls (PCBs) and related impurities, Arlington,
Virginia, Drill, Freiss, Hays, Loomis and Schaffer, Inc.
(Unpublished report).
DUINKER, J.C. & BOUCHERTALL, F. (1989) On the distribution of
atmospheric polychlorinated biphenyl congeners between vapor phase,
aerosols and rain. Environ. Sci. Technol., 23: 57-62.
DUINKER, J.C. & HILLEBRAND, M.T.J. (1979) Mobilization of
organochlorines from female lipid tissue and transplacental
transfer to fetus in a harbour porpoise (Phocoena phocoena) in a
contaminated area. Bull. environ. Contam. Toxicol., 23: 728-732.
DUINKER, J.C. & HILLEBRAND, M.T.J. (1983) Characterization of PCB
components in Clophen formulations by capillary GC-MS and GC-ECD
techniques. Environ. Sci. Technol., 17: 449-456.
DUINKER, J.C., SCHULTZ, D.E., & PETRICK, G. (1988) Selection of
chlorinated biphenyl congeners for analysis in environmental
samples. Mar. Pollut. Bull., 19(1): 19-25.
DUKE, T.W., LOWE, J.I., & WILSON, A.J. (1970) A polychlorinated
biphenyl (Aroclor 1254) in the water, sediment, and biota of
Escambia Bay, Florida. Bull. environ. Contam. Toxicol., 5: 171-180.
DUNN, W.I., III, STELLING, D.L., SCHWARTZ, T.R., HOGAN, J.W.,
PETTY, J.D., JOHANSSON, E., & WOLD, S. (1984) Pattern recognition
for classification and determination of polychlorinated biphenyls
in environmental samples. Anal. Chem., 56: 1308-1313.
DUNNIVANT, F.M. & ELZERMAN, A.W. (1988) Aqueous solubility and
Henry's Law Constant data for PCB congeners for evaluation of
quantitative structure-property relationships (QSPRs). Chemosphere,
17(3): 525-541.
DURHAM, S.K. & BROUWER, A. (1989) 3,4,3',4'-tetrachloro-
biphenyl-induced effects in the rat liver. I. Serum and hepatic
retinoid reduction and morphologic changes. Toxicol. Pathol.,
17(3): 536-544.
DUTCH AGRICULTURAL ADVISORY COMMISSION ON ENVIRONMENTAL POLLUTANTS
(1983) Annual report, The Hague, Ministry of Agriculture,
Management of Nature, and Fisheries (Unpublished).
DZOGBEFIA, V.P., KLING, D., & GAMBLE, W. (1978) Polychlorinated
biphenyls: in vivo and in vitro modifications of phospholipid
and glyceride biosynthesis. J. environ. Pathol. Toxicol.,
1: 841-856.
ECOBICHON, D.J. & COMEAU, A.M. (1974) Comparative effects of
commercial Aroclor on rat liver enzyme activities. Chem. biol.
Interact., 9: 341-350.
ECOBICHON, D.J. & COMEAU, A.M. (1975) Isomerically pure
chlorobiphenyl congeners and hepatic function in the rat. Influence
of position and degree of chlorination. Toxicol. appl. Pharmacol.,
33: 94-105.
ECOBICHON, D.J. & MACKENZIE, D.O. (1974) The uterotropic activity
of commercial and isomerically-pure chlorobiphenyls in the rat.
Res. Commun. chem. Pathol. Pharmacol., 9: 85-95.
EDULJEE, G., BADSHA, K., & SCUDAMORE, N. (1986) Environmental
monitoring for PCB and trace metals in the vicinity of a chemical
waste disposal facility - II. Chemosphere, 15(1): 81-93.
EISENREICH, S.J., LOONEY, B.B., & THORNTON, J.D. (1981) Airborne
organic contaminants in the Great Lakes ecosystem. Environ. Sci.
Technol., 15: 30-38.
EISLER, R. (1986) Polychlorinated biphenyl hazards to fish,
wildlife, and invertebrates: A synoptic review, Washington, DC, US
Department of Interior, Fish & Wildlife Service, 72 pp (Biology
Report No. 85).
EKSTEDT, J. & ODEN, S. (1974) Chlorinated hydrocarbons in the lower
atmosphere in Sweden, Uppsala, Sweden, Royal Agricultural College,
Department of Soil Science, pp. 1-16.
ELDER, D.L., FOWLER, S.W., & POLIKARPOV, G.G. (1979) Remobilization
of sediment-associated PCBs by the worm Nereis diversicolor.
Bull. environ. Contam. Toxicol., 21: 448-452.
ELLIOTT, J.E., NORSTRÖM, R.J., & KEITH, J.A. (1988) Organochlorines
and eggshell thinning in Northern Gannets (Sula bassanus) from
Eastern Canada, 1968-1984. Environ. Pollut., 52: 81-102.
ELO, O., VUOJOLAHTI, P., JANHUNEN, H., & RANTANEN, J. (1985) Recent
PCB accidents in Finland. Environ. health Perspect., 60: 315-319.
EMMETT, E.A. (1985) Polychlorinated biphenyl exposure and effects
in transformer repair workers. Environ. health Perspect.,
60: 185-192.
EMMETT, E.A., MARONI, M., SCHMITH, J.M., LEVIN, B.K., & JEFFERYS,
J. (1988a) Studies of transformer repair workers exposed to PCBs:
I. Study design, PCB concentrations, Questionnaire, and clinical
examination results. Am. J. ind. Med., 13: 415-427.
EMMETT, E.A., MARONI, M., JEFFERYS, J., SCHMITH, J., LEVIN, B.K.,
& ALVARES, A. (1988b) Studies of transformer repair workers exposed
to PCBs: II. Results of clinical laboratory investigations. Am. J.
ind. Med., 14: 47-62.
ERICKSON, M.D. (1985) The analytical chemistry of PCBs, Boston,
Massachusetts, Butterworth.
ERICKSON, M.D., STANLEY, J.S., TURMAN, J.K., GOING, J.E., REDFORD,
D.P., & HEGGEM, D.T. (1988) Determination of byproduct
polychlorobiphenyls in commercial products and wastes by
high-resolution gas chromatography/electron impact mass
spectrometry. Environ. Sci. Technol., 22(1): 71-76.
ESCHENROEDER, A.Q., DOYLE, C.P., & FAEDER, E.J. (1986) Health risks
of PCB spills from electrical equipment. Risk Anal., 6(2): 213-221.
EWALD, W.G., FRENCH, J.E., & CHAMP, M.A. (1976) Toxicity of
polychlorinated biphenyls (PCBs) to Euglena gracilis: Cell
population growth, carbon fixation, chlorophyll level, oxygen
consumption, and protein and nucleic acid synthesis. Bull. environ.
Contam. Toxicol., 16: 71-80.
EXON, J.H., TALCOTT, P.A., & KOLLER, L.D. (1985) Effect of lead,
polychlorinated biphenyls, and cyclophosphamide on rat natural
killer cells, interleukine 2, and antibody synthesis. Fundam. appl.
Toxicol., 5: 158-164.
FAIT, A., GROSSMAN, E., SELF, S., FEFFRIES, J., PELLIZZARI, E.D.,
& EMMETT, E.A. (1989) Polychlorinated biphenyl congeners in adipose
tissue lipid and serum of past and present transformer repair
workers and a comparison group. Fundam. appl. Toxicol., 12: 42-55.
FALANDYSZ, J. (1980) Chlorinated hydrocarbons in gulls from the
Baltic south coast. Mar. Pollut. Bull., 11: 75-80.
FARBER, E. (1984a) Perspectives in cancer research. The multistep
nature of cancer development. Cancer Res., 44: 4217-4223.
FARBER, E. (1984b) Cellular biochemistry of the step-wise
development of cancer with chemicals. G.H. Clowes Memorial Lecture.
Cancer Res., 44: 5463-5474.
FARBER, E. (1986) Some emerging general principles in the
pathogenesis of hepatocellular carcinoma. Cancer Surv., 5: 695-718.
FDA (1970) Status report on the chemistry and toxicology of
polychlorinated biphenyls (PCBs) or Aroclors as of 1 June 1970,
Washington, DC, Food and Drug Administration, 16 pp.
FDA (1979) Polychlorinated biphenyls (PCBs): Reduction of
tolerances, Fed. Reg., 44(127): 126-136.
FEIN, G.G. (1984) Intrauterine exposure of humans to PCBs: newborn
effects, Duluth, Minnesota, US Environmental Protection Agency
(EPA 600/53-84-060).
FEIN, G.G., JACOBSON, J.L., JACOBSON, S.W., SCHWARTZ, P.M., &
DOWLER, J.K. (1984) Prenatal exposure to polychlorinated biphenyls:
Effects on birth size and gestational age. J. Pediatr.,
105(2): 315-320.
FELT, G.R., MUELLER, W.F., IATROPOULOS, M.J., COULSTON, F., &
KORTE, F. (1977) Chronic toxicity of 2,5,4'-trichlorobiphenyl in
young rhesus monkeys. I. Body distribution, elimination and
metabolism. Toxicol. appl. Pharmacol., 41(3): 619-627.
FELT, G.R., MUELLER, W.F., COULSTON, F., & KORTE, F. (1979)
Distribution and excretion of 2,2',4,4',6-pentachlorobiphenyl in
the rat. Bull. environ. Contam. Toxicol., 22: 582-585.
FINGERMAN, S.W. (1980) Differences in the effects of fuel oil, an
oil dispersant, and three polychlorinated biphenyls on fin
regeneration in the Gulf Coast kill fish, Fundulus grandis. Bull.
environ. Contam. Toxicol., 25: 234-240.
FINGERMAN, S.W. & FINGERMAN, M. (1977) Effects of a polychlorinated
biphenyl and a polychlorinated dibenzofuran on molting of the
fiddler crab, Uca pugilator. Bull. environ. Contam. Toxicol.,
18: 138-142.
FINGERMAN, S.W. & FINGERMAN, M. (1979) Comparison of the effects of
fourteen-day and chronic exposures to a polychlorinated biphenyl,
Aroclor 1242, on molting of the fiddler crab, Uca pugilator.
Bull. environ. Contam. Toxicol., 21: 352-357.
FINGERMAN, S.W. & RUSSELL, L.C. (1980) Effects of the
polychlorinated biphenyl Aroclor 1242 on locomotor activity and on
the neurotransmitters dopamine and norepinephrine in the brain of
the gulf killifish, Fundulus grandis. Bull. environ. Contam.
Toxicol., 25: 682-687.
FINKLEA, J., PRIESTER, L.E., CREASON, J.P., HAUSER, T., HINNERS,
T., & HAMMER, D.I. (1972) Polychlorinated biphenyl residues in
human plasma expose a major urban pollution problem. Am. J. public
Health, 62(5): 645-651.
FIORE, B.J., ANDERSON, H.A., HANRAHAN, L.P., OLSON, L.J., &
SONZOGNI, W.C. (1989) Sport fish consumption and body burden levels
of chlorinated hydrocarbons: A study of Wisconsin anglers. Arch.
environ. Health, 44(2): 82-88.
FISCHBEIN, A. (1985) Liver function tests in workers with
occupational exposure to polychlorinated biphenyls (PCBs):
Comparison with Yusho and Yu-Cheng. Environ. health Perspect.,
60: 145-150.
FISCHBEIN, A. & WOLFF, M.S. (1987) Conjugal exposure to
polychlorinated biphenyls (PCBs). Br. J. ind. Med., 44: 284-286.
FISCHBEIN, A., WOLFF, M.S., LILIS, R., THORNTON, J., & SELIKOFF,
I.J. (1979) Clinical findings among PCB-exposed workers in a
capacitor manufacturing facility. Ann. NY Acad. Sci., 320: 703-715.
FISCHBEIN, A., WOLFF, M.S., BERNSTEIN, J., SELIKOFF, I.J., &
THORNTON, J. (1982) Dermatological findings in capacitor
manufacturing workers exposed to dielectric fluids containing
polychlorinated biphenyls (PCBs). Arch. environ. Health,
37(2): 69-74.
FISCHBEIN, A., RIZZO, J.N., SOLOMON, S.J., & WOLFF, M.S. (1985)
Oculodermatological findings in workers with occupational exposure
to polychlorinated biphenyls (PCBs). Br. J. ind. Med., 42: 426-430.
FISHBEIN, L. (1974) Toxicity of chlorinated biphenyls. Annu. Rev.
Pharmacol., 14: 139-156.
FISHER, N.S. (1975) Chlorinated hydrocarbon pollutants and
photosynthesis of marine phytoplankton: A reassessment. Science,
189: 463-464.
FISHER, N.S. & WURSTER, C.F. (1973) Individual and combined effects
of temperature and polychlorinated biphenyls on the growth of three
species of phytoplankton. Environ. Pollut., 5: 205-212.
FISHER, N.S., GRAHAM, L.B., & CARPENTER, E.J. (1973) Geographic
differences in phytoplankton sensitivity to PCBs. Nature (Lond.),
241: 548-549.
FISHER, N.S., REMSEN, C.C., WURSTER, C.R., & CARPENTER, E.J. (1974)
Effects of PCB on interspecific competition in natural and
gnotobiotic phytoplankton communities in continuous and batch
cultures. Microbiol. Ecol., 1: 39-50.
FISHER, N.S., GUILLARD, R.R., & WURSTER, C.R. (1976) Effects of a
chlorinated hydrocarbon pollutant on the growth kinetics of a
marine diatom. In: Modeling biochemical processes in aquatic
ecosystems, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 305-317.
FISHER, J.B., PETTY, R.L., & LUCK, W. (1983) Release of
polychlorinated biphenyls from contaminated lake sediments: Flux
and apparent diffusivities of four individual PCBs. Environ.
Pollut., B5: 121-132.
FITZGERALD, E.F., WEINSTEIN, A.L., YOUNGBLOOD, L.G., STANDFAST,
S.J., & MELIUS, J.M. (1989) Health effects three years after
potential exposure to the toxic contaminants of an electrical
transformer fire. Arch. environ. Health, 44(4): 214-221.
FLICK, D.F., O'DELL, R.G., & CHILDS, V.A. (1965) Studies of the
chick oedema disease. 3. Similarity of symptoms produced by feeding
chlorinated biphenyl. Poult. Sci., 44: 1460-1465.
FOCARDI, S. & ROMEI, R. (1987) Fingerprint of polychlorinated
biphenyl congeners in samples of human subcutaneous adipose tissue.
Chemosphere, 16(10/12): 2315-2320.
FOCARDI, S., FOSSI, C., LEONZIO, C., & ROMEI, R. (1986) PCB
congeners, hexachlorobenzene, and organochlorine insecticides in
human fat in Italy. Bull. environ. Contam. Toxicol., 36: 644-650.
FOCARDI, S., LEONZIO, C., & FOSSI, C. (1988) Variations in
polychlorinated biphenyl congener composition in eggs of
Mediterranean water birds in relation to their position in the food
chain. Environ. Pollut., 52: 243-255.
FOLMAR, L.C., DICKHOFF, W.W., ZAUGG, W.S., & HODGKINS, H.O. (1982)
The effects of Aroclor 1254 and No. 2 fuel oil on smoltification
and sea-water adaptation of Coho salmon (Oncorhynchus kisutch).
Aquat. Toxicol., 2: 291-299.
FOOKEN, C. & BUTTE, W. (1987) Organochlorine pesticides and
polychlorinated biphenyls in human milk during lactation.
Chemosphere, 16(6): 1301-1309.
FOREMAN, W.T. & BIDLEMAN, T.F. (1985) Vapor pressure estimates of
individual polychlorinated biphenyls and commercial fluids using
gas chromatographic retention data. J. Chromatogr., 330: 203-216.
FORGUE, S.T., PRESTON, B.D., HARGRAVES, W.A., REICH, I.L. & ALLEN,
J.R. (1980) Direct evidence that an arene oxide is a metabolic
intermediate of 2,2',5,5'-tetrachlorobiphenyl. In: Abstracts of the
Nineteenth Annual Meeting of the Society of Toxicology (Abstract
No. 383).
FOWLER, S.W., POLIKARPOV, G.G., ELDER, D.L., PARSI, P., &
VILLENEUVE, J.P. (1978) Polychlorinated biphenyls: Accumulation
from contaminated sediments and water by the polychaete Nereis
diversicolor. Mar. Biol., 48: 303-309.
FRANK, R., RONALD, K., & BRAUN, H.E. (1973) Organochlorine residues
in harp seals (Pagophilus groenlandicus) caught in Eastern
Canadian waters. J. Fish Res. Board Can., 30: 1053-1063.
FRANK, R., HOLDRINET, M.V.H., & RAPLEY, W.A. (1975) Residue of
organochlorine compounds and mercury in birds' eggs from the
Niagara Peninsula, Ontario. Arch. environ. Contam. Toxicol.,
3: 205-218.
FRANK, R., HOLDRINET, M., BRAUN, H.E., DODGE, D.P., & SPRANGLER,
G.E. (1978) Residues of organochlorine insecticides and
polychlorinated biphenyls in fish from Lakes Huron and Superior,
Canada, 1968-76. Pestic. monit. J., 12: 60-68.
FRANK, R., RASPER, J., SMOUT, M.S., & BRAUN, H.E. (1988)
Organochlorine residues in adipose tissues, blood and milk from
Ontario residents, 1976-1985. Can. J. public Health, 79: 150-158.
FRANKLIN, A. (1987) The concentration of metals, organochlorine
pesticide and PCB residues in marine fish and shellfish: Results
from MAFF fish and shellfish monitoring programmes, 1977-1984,
London, Ministry of Agriculture, Fisheries and Food, Directorate of
Fisheries Research (Aquatic Environment Monitoring Report No. 16).
FREEMAN, H.C., SANGALANG, G., & FLEMMING, B. (1982) The sublethal
effects of a polychlorinated biphenyl (Aroclor 1254) diet on the
Atlantic cod (Gadus morhua). Sci. total Environ., 24: 1-11.
FRENCH, J.E. (1976) The effect of the chlorinated polycyclic
hydrocarbons, p,p'-DDT and polychlorinated biphenyls (PCBs) on the
flagellated protozoan, Crithidia fasciculata. Diss. Abstr. Int.
Sci. Eng., B37: 96-97.
FREUDENTHAL, J. & GREVE, P.A. (1973) Polychlorinated terphenyls in
the environment. Bull. environ. Contam. Toxicol., 10: 108-111.
FRIEND M. & TRAINER, D.O. (1970) Polychlorinated biphenyl:
interaction with duck hepatitis virus. Science, 170: 1314-1316.
FRIES, G.F. (1972) Degradation of chlorinated hydrocarbons under
anaerobic conditions. Adv. Chem. Ser., 111: 256-270.
FRIES, G.F. & MARROW, G.S. (1981) Chlorobiphenyl movement from soil
to soybean plants. J. agric. food Chem., 29: 757-759.
FRIES, G.F., MARROW, G.S., Jr, & GORDON, C.H. (1973) Long-term
studies of residue retention and excretion by cows fed a
polychlorinated biphenyl (Aroclor 1254). J. agric. food Chem.,
21: 117-121.
FU, Y.A. (1984) Ocular manifestation of polychlorinated biphenyls
intoxication. Am. J. ind. Med., 5: 127-132.
FUJIWARA, K. (1975) Environmental and food contamination with PCB's
in Japan. Sci. total Environ., 4: 219-247.
FUJIWARA, M. & KURIYAMA, K. (1977) Effect of PCB (polychloro-
biphenyls) on L-ascorbic acid, pyridoxal phosphate and riboflavin
contents in various organs and on hepatic metabolism of L-ascorbic
acid in the rat. Jpn. J. Pharmacol., 27: 621-627.
FUKADA, K., INUYAMA, Y., TAKESHITA, T., & YAMAMOTO, S. (1973)
[Present state of environmental pollution by PCB in the Shimane
Prefecture.] Shimare Igaku, 5: 1-25 (in Japanese).
FUKANO, S. & DOGUCHI, M. (1977) PCT, PCB and pesticide residues in
human fat and blood. Bull. environ. Contam. Toxicol.,
17(5): 613-617.
FUKANO, S., USHIO, F., & DOGUCHI, M. (1974) [PCB, PCT and pesticide
residues in fish collected from the Tama river.] Annu. Rep. Tokyo
Metrop. Res. Lab. P.H., 25: 297-305 (in Japanese).
FUKUSHIMA, M. & KAWAI, S. (1981) Variation of organochlorine
concentration and burden in striped dolphin (Stenella
coeruleoalba) with growth. In: Fujiyama, T., ed. Studies on the
levels of organochlorine compounds and heavy metals in the marine
organisms, Ryukyus, University of Ryukyus, pp. 97-114.
FULLER, G.B., KNAUF, V., MUELLER, W., & HOBSON, W.C. (1980) PCB
augments LH induced progesteron synthesis. Bull. environ. Contam.
Toxicol., 25: 65-68.
FUNATSU, I., YAMASHITA, F., ITO, Y., TZUGAWA, S., FUNATSU, T.,
YOSHIKANE, T., HAYASHI, M., KATO, T., YAKUSHIJI, M., OKAMOTO, G.,
YAMASAKI, S., ARIMA, T., KUNO, T., IDE, H., & IBE, I. (1972) PCB
induced fetopathy. I. Clinical observation. Kurume med. J.,
919: 43-51.
FURUKAWA, K., TONOMURA, K., & KAMIBAYASHI, A. (1978a) Effect of
chlorine substitution on the biodegradability of polychlorinated
biphenyls. Appl. environ. Microbiol., 35: 223-227.
FURUKAWA, K., TOMIZUKA, N., & KAMIBAYASHI, A. (1978b) Effect of
chlorine substitution on the bacterial metabolism of various
polychlorinated biphenyls. Appl. environ. Microbiol., 38: 301-310.
GAGE, J.C. & HOLM, S. (1976) The influence of molecular structure
on the retention and excretion of polychlorinated biphenyls by the
mouse. Toxicol. appl. Pharmacol., 36: 555-560.
GALLENBERG, L.A. & VODICNIK, M.J. (1987) Potential mechanisms for
redistribution of polychlorinated biphenyls during pregnancy and
lactation. Xenobiotica, 17(3): 299-310.
GALLENBERG, L.A., RING, B.J., & VODICNIK, M.J. (1987) Influence of
lipolysis on the mobilization of 2,4,5,2',4',5'-hexachlorobiphenyl
from adipocytes in vitro. J. Toxicol. environ. Health,
20: 163-171.
GARDNER, A.M., RICHTER, H.F., & ROACH, J.A.G. (1976) Excretion of
hydroxylated polychlorinated biphenyl metabolites in cow's milk. J.
Assoc. Off. Anal. Chem., 59: 273-277.
GARTHOFF, L.H., FRIEDMAN, L., FARBER, T.M., LOCKE, K.K., SOBOTKA,
T.J., GREEN, S., HURLEY, N.E., PETERS, E.L., STORY, G.E., MORELAND,
F.M., GRAHAM, C.H., KEYS, J.E., TAYLOR, M.J., SCALERA, J.V.,
ROTHLEIN, J.E., MARKS, E.M., CERRA, F.E., RODI, S.B., & SPORN, E.M.
(1977) Biochemical and cytogenetic effects in rats caused by
short-term ingestion of Aroclor 1254 or Firemaster BP6. J. Toxicol.
environ. Health, 3: 769-796.
GARTHOFF, L.H., CERRA, F.E., & MARKS, E.M. (1981) Blood chemistry
alterations in rats after single and multiple gavage administration
of polychlorinated biphenyl. Toxicol. appl. Pharmacol., 60: 33-44.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985) Pesticides, selected elements and other chemicals in adult
total diet samples, October 1979-September 1980. J. Assoc. Off.
Anal. Chem., 68(6): 1184-1195.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements and other chemicals in adult
total diet samples, October 1980-March 1982. J. Assoc. Off. Anal.
Chem., 69(1): 146-161.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements and other chemicals in infant
and toddler total diet samples, October 1980-March 1982. J. Assoc.
Off. Anal. Chem., 69(1): 123-145.
GASKIN, D.E., FRANK, R., & HOLDRINET, M. (1983) Polychlorinated
biphenyls in harbor porpoises Phocoena phocoena (L.) from the Bay
of Fundy, Canada and adjacent waters, with some information on
chlordane and hexachlorobenzene levels. Arch. environ. Contam.
Toxicol., 12: 211-219.
GELLERT, R.J. (1978) Uterotropic activity of polychlorinated
biphenyls (PCB) and induction of precocious reproductive aging in
neonatally treated female rats. Environ. Res., 16: 123-130.
GELLERT, R.J. & WILSON, C. (1979) Reproductive function in rats
exposed prenatally to pesticides and polychlorinated biphenyls
(PCBs). Environ. Res., 18: 437-443.
GEZONDHEIDSRAAD (1985) [First recommendation on the quality of
breastmilk. Contamination of breastmilk with polychlorinated
biphenyls (PCBs). Recommendation to the Minister and the Secretary
of State for Welfare, Public Health and Culture], Rijswijk, Public
Health Council of the Netherlands (in Dutch).
GIAM, C.S., CHAN, H.S., NEFF, G.S., & ATLAS, E.L. (1978) Phthalate
ester plasticizers: A new class of marine pollutant. Science,
199: 419-420.
GIAM, C.S., ATLAS, E., CHAN, H.S., & NEFF, G. (1980) Phthalate
esters, PCB and DDT residues in the Gulf of Mexico atmosphere.
Atmos. Environ., 14: 65-69.
GIBSON, D.T., ROBERTS, R.L., WELLS, M.C., & KOBAL, V.M. (1973)
Oxidation of biphenyl by a Beijerinckia species. Biochem.
biophys. Res. Commun., 50: 211-219.
GILBERTSON, M. & FOX, G.A. (1977) Pollutant-associated embryonic
mortality of Great Lakes herring gulls. Environ. Pollut.,
12: 211-216.
GILBERTSON, M. & HALE, R. (1974) Characteristics of the breeding
failure of a colony of herring gulls in Lake Ontario. Can.
Field-Nat., 88: 356-358.
GILBERTSON, M., MORRIS, R.D., & HUNTER, R.A. (1976) Abnormal chicks
and PCB residue levels in eggs of colonial birds on the lower Great
Lakes (1971-73). Auk, 93: 434-442.
GLADEN, B.C., ROGAN, W.J., RAGAN, N.B., & SPIERTO, F.W. (1988a)
Urinary porphyrins in children exposed transplacentally to
polyhalogenated aromatics in Taiwan. Arch. environ. Health,
43(1): 54-58.
GLADEN, B.C., ROGAN, W.J., HARDY, P., THULLEN, J., TINGELSTAD, J.,
& TULLY, M. (1988b) Development after exposure to polychlorinated
biphenyls and dichlorodiphenyl dichloroethane transplacentally and
through human milk. J. Pediatr., 113(6): 991-995.
GLOOSCHENKO, V. & GLOOSCHENKO, W. (1975) Effect of polychlorinated
biphenyl compounds on growth of great lakes phytoplankton. Can. J.
Bot., 53: 653-659.
GLOOSHENKO, W.A., STRACHAN, W.M., & SAMPSON, R.C.J. (1976) Residues
in water distribution of pesticides and polychlorinated biphenyls
in water, sediments and session of the Upper Great Lakes. Pestic.
monit. J., 10: 61-67.
GOLDSTEIN, J.A. (1980) Structure-activity relationships for the
biochemical effects and the relationships to toxicity. In:
Kimbrough, R.D., ed. Halogenated biphenyls, terphenyls,
naphthalenes, dibenzodioxins and related products, Amsterdam,
Elsevier/North-Holland Biomedical Press, pp. 151-190 (Topics in
Environmental Health).
GOLDSTEIN, J.A., HICKMAN, P., & JUE, D.L. (1974) Experimental
hepatic porphyria induced by polychlorinated biphenyls. Toxicol.
appl. Pharmacol., 27: 437-448.
GOLDSTEIN, J.A., HICKMAN, P., BURSE, V.W., & BERGMAN, H. (1975) A
comparative study of two polychlorinated biphenyl mixtures
(Aroclors 1242 and 1016) containing 42% chlorine on induction of
hepatic porphyria and drug metabolizing enzymes. Toxicol. appl.
Pharmacol., 32: 461-473.
GOLDSTEIN, J.A., HICKMAN, P., BERGMAN, H., MCKINNEY, J.D., &
WALKER, M.P. (1977) Separation of pure polychlorinated biphenyl
isomers into two types of inducers on the basis of induction of
cytochrome P-450 or P-448. Chem.-biol. Interact., 17: 69-87.
GORCHEV, H.G. & JELINEK, C.F. (1985) A review of the dietary
intakes of chemical contaminants. Bull. World Health Organ.,
63(5): 945-962.
GOTO, M. & HIGUCHI, K. (1969) The symptomatology of Yusho
(chlorobiphenyls poisoning), in dermatology. Fuoka Act. Med.,
60: 409-431.
GOTO, M., SUGIURA, K., HATTORI, M., MIYAGAWA, T., & OKAMURA, M.
(1973) Hydroxylation of dichlorobiphenyls in rats. In: New
collection of papers presented at the Research Conference on New
Methodology in Ecological Chemistry, Susono, Japan, 23-25 November,
Tokyo, International Academic Printing Co. Ltd, pp. 299-302.
GOTO, M., SUGIURA, K., HATTORI, M., MIYAGAWA, T., & OKAMURA, M.
(1974) Metabolism of 2,3-dichlorobiphenyl-14C and
2,3,6-trichlorobiphenyl-14C in the rat. Chemosphere, 3: 227-232.
GOTO, M., HATTORI, M., & SUGIURA, K. (1975) Metabolism of
pentachloro- and hexachlorobiphenyls in the rat. Chemosphere,
4: 177-180.
GRANT, D.L. & PHILLIPS, W.E.J. (1974) The effect of age and sex on
the toxicity of Aroclor 1254, a polychlorinated biphenyl, in the
rat. Bull. environ. Contam. Toxicol., 12(2): 145-152.
GRANT, D.L., PHILLIPS, W.E.J., & VILLENEUVE, D.C. (1971a)
Metabolism of polychlorinated biphenyl (Aroclor 1254) mixture in
the rat. Bull. environ. Contam. Toxicol., 6: 102-112.
GRANT, D.L., VILLENEUVE, D.C., MCCULLY, K.A., & PHILLIPS, W.E.J.
(1971b) Placental transfer of polychlorinated biphenyls in the
rabbit. Environ. Physiol., 1: 61-66.
GRANT, D.L., MOODIE, C.A., & PHILLIPS, W.E.J. (1974) Toxicodynamics
of Aroclor 1254 in the male rat. Environ. Physiol. Biochem.,
4: 214-225.
GREB, W., KLEIN, W., COULSTON, F., GOLBERG, L., & KORTE, F. (1975)
Metabolism of lower polychlorinated biphenyls-C-14 in the Rhesus
monkey. Bull. environ. Contam. Toxicol., 13: 471-76.
GREEN, S., CARR, J.V., PALMER, K.A., & OSWALD, E.J. (1975a) Lack of
cytogenetic effects in bone marrow and spermatogonial cells in rats
treated with polychlorinated biphenyls (Aroclors 1242 and 1254).
Bull. environ. Contam. Toxicol., 13: 14-22.
GREEN, S., SAURO, F.M., & FRIEDMAN, L. (1975b) Lack of dominant
lethality in rats treated with polychlorinated biphenyls (Aroclors
1242 and 1254). Food Cosmet. Toxicol., 13: 507-510.
GREICHUS, Y.A., CALL, D.J., & AMMANN, B.M. (1975) Physiological
effects of polychlorinated biphenyls or a combination of DDT, DDD,
and DDE in penned white Pelicans. Arch. environ. Contam. Toxicol.,
3: 330-343.
GREIG, R.A. & SENNEFELDER, G. (1987) PCB concentration in winter
flounder from Long Island Sound, 1984-1986. Bull. environ. Contam.
Toxicol., 39(5): 863-868.
GREVE, P.A. & VAN HULST, S.J. (1977) [Organochlorine pesticides and
PCBs in total diets], Bilthoven, The Netherlands, National
Institute of Public Health (Report No. 192/77 Tox-Rob) (in Dutch).
GREVE, P.A. & VAN HARTEN, D.C. (1983a) [Organochlorine pesticides
and polychlorobiphenyls in the fatty tissue of the Dutch population
(period 1980).] Bilthoven, The Netherlands, National Institute of
Public Health (Report No. 638205001) (in Dutch).
GREVE, P.A. & VAN HARTEN, D.C. (1983b) [Association between
organochlorine pesticide and PCB contents in fat and blood in man],
Bilthoven, The Netherlands, National Institute of Public Health
(Report No. 638219001) (in Dutch).
GREVE, P.A. & WEGMAN, R.C.C. (1983) PCB residues in animal fats,
human tissues, duplicate 24-hours' diets, eel and sediments. In:
Barros, M.C., Könemann, H., & Visser, R., ed. Proceedings of the
PCB Seminar, The Hague, 28-30 September 1983, The Hague, Ministry
of the Environment, pp. 54-66.
GREVE, P.A. & WEGMAN, R.C.C. (1984) Organochlorine compounds in
human milk: data from a recent investigation in the Netherlands.
In: Report on the WHO Consultation on Organochlorine Compounds in
Human Milk and Related Hazards, Bilthoven, 9-11 January 1985,
Copenhagen World Health Organization, Regional Office for Europe,
Annex 8.
GREVE, P.A., VAN HARTEN, D.C., HEUSINKVELD, H.A.G., LEUSSINK, A.B.,
& VERSCHRAAGEN, C. (1985) [Chemical contaminants in breastmilk.
Section 2: PCBs (determination of total)], Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Hygiene (Report No. 638307002) (in Dutch).
GROLIER, P., CASSAND, P., ANTIGNAC, E., NARBONNE, J.F., ALBRECHT,
R., AZAIS, V., ROBERTSON, L.W., & OESCH, F. (1989) Effects of
prototypic PCBs on benzo(a)pyrene mutagenic activity related to
vitamin A intake. Mutat. Res., 211: 139-145.
GROTE, W., SCHMOLDT, A., & BENTHE, H.F. (1975) Hepatic porphyrin
synthesis in rat after pretreatment with polychlorinated biphenyls.
Acta. pharmacol. toxicol., 36: 215-224.
GRUGER, E.H., KARRICK, N.L., DAVIDSON, A.I., & HRUBY, T. (1975)
Accumulation of 3,4,3',4'-tetrachlorobiphenyl and 2,4,5,2',4',5'-,
and 2,4,6,2',4',6'-hexachlorobiphenyl in juvenile coho salmon.
Environ. Sci. Technol., 9: 121-127.
GRUGER, E.H., HRUBY, T., & KARRICK, N.L. (1976) Sublethal effects
of structurally related tetrachloro-, pentachloro-, and
hexachlorobiphenyl on juvenile Coho Salmon. Environ. Sci. Technol.,
10: 1033-1037.
GUINEY, P.D. & PETERSON, R.E. (1980) Distribution and elimination
of a polychlorinated biphenyl after dietary exposure in yellow
perch and rainbow trout. Arch. environ. Contam. Toxicol.,
9: 667-674.
GUINEY, P.D., PETERSON, R.E., MELANCON, M.J., & LECH, J.J. (1977)
The distribution and elimination of 2,5,2,5-[14C]Tetrachloro-
biphenyl in Rainbow Trout (Salmo gairdneri). Toxicol. appl.
Pharmacol., 39: 329-338.
GUINEY, P.D., MELANCON, M.J., LECH, J.J., & PETERSON, R.E. (1979)
Effects of egg and sperm maturation and spawning on the
distribution and elimination of a polychlorinated biphenyl in
rainbow Trout (Salmo gairdneri). Toxicol. appl. Pharmacol.,
47: 261-272.
GUNDERSON, E.L. (1988b) FDA total diet study, April 1982-April
1984, dietary intakes of pesticides, selected elements and other
chemicals. J. Assoc. Off. Anal. Chem., 71(6): 1200-1209.
GUO, Y.L., EMMETT, E.A., PELLIZZARI, E.D., & ROHDE, C.A. (1987)
Influence of serum cholesterol and albumine on partitioning of PCB
congeners between human serum and adipose tissue. Toxicol. appl.
Pharmacol., 87: 48-56.
GUOTH, J., KACHMAR, P., TELEHA, M., & VASIL, M. (1984) [The
influence of polychlorinated biphenyls (PCB) on the activity of
liver aniline hydroxylase and on some metabolic parameters in pig
blood.] Vet. Med. (Prague), 29: 29-38 (in Czech).
GUSTAVSSON, P., HOGSTEDT, C., & RAPPE, C. (1986) Short-term
mortality and cancer incidence in capacitor manufacturing workers
exposed to polychlorinated biphenyls (PCBs). Am. J. ind. Med.,
10: 341-344.
GYORKOS, J., DENOMME, M.A., LEECE, B., HOMONKO, K., VALLI, V.E., &
SAFE, S. (1985) Reconstituted halogenated hydrocarbon pesticide and
pollutant mixtures found in human tissues: effects on the immature
male Wistar rat after short-term exposure. Can. J. Physiol.
Pharmacol., 63(1): 36-43.
HAAHTI, H. & PERTTILA, M. (1988) Levels and trends of
organochlorines in Cod and Herring in the Northern Baltic. Mar.
Pollut. Bull., 19: 29-32.
HAAKE, J.M., SAFE, S., MAYURA, K., & PHILLIPS, T.D. (1987) Aroclor
1254 as an antagonist of the teratogenicity of 2,3,7,8-tetrachloro-
dibenzo- p-dioxin. Toxicol. Lett., 38: 299-306.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
Coturnix. Bull. environ. Contam. Toxicol., 11: 98-101.
HALTER, M.T. & JOHNSON, H.E. (1974) Acute toxicities of a
polychlorinated biphenyl (PCB) and DDT alone and in combination to
early life stages of Coho salmon (Oncorhynchus kisutch). J. Fish
Res. Board Can., 31: 1543-1547.
HALVERSON, M.R., PHILLIPS, T.D., SAFE, S.H., & ROBERTSON, L.W.
(1985) Metabolism of aflatoxin B1 by rat hepatic microsomes induced
by polyhalogenated biphenyl congeners. Appl. environ. Microbiol.,
49: 882-886.
HAMMOND, A.L. (1972) Chemical pollution: Polychlorinated biphenyls.
Science, 175: 155-156.
HANSEN, D.J., PARRISH, P.R., LOWE, J.I., & WILSON, A.J. (1971)
Chronic toxicity, uptake, and retention of Aroclor 1254 in two
estuarine fishes. Bull. environ. Contam. Toxicol., 6: 113-119.
HANSEN, D.J. (1974) Aroclor 1254: Effect on composition of
developing estuarine animal communities in the laboratory. Contrib.
mar. Sci., 18: 19-33.
HANSEN, D.J., SCHIMMEL, S.C., & MATTHEWS, E. (1974a) Avoidance of
Aroclor 1254 by shrimp and fishes. Bull. environ. Contam. Toxicol.,
12: 253-256.
HANSEN, D.J., PARRISH, P.R., & FORESTER, J. (1974b) Aroclor 1016:
Toxicity to and uptake by estuarine animals. Environ. Res.,
7: 363-373.
HANSEN, D.J., SCHIMMEL, S.C., & FORESTER, J. (1975) Effects of
Aroclor 1016 on embryos, fry, juveniles, and adults of sheepshead
minnow (Cyprinodon variegatus). Trans. Am. Fish Soc.,
104: 584-588.
HANSEN, L.G., WIEKHORST, W.B., & SIMON, J. (1976a) Effects of
dietary Aroclor 1242 on channel catfish (Ictalurus punctatus) and
the selective accumulation of PCB components. J. Fish Res. Board
Can., 33: 1343-1352.
HANSEN, L.G., WILSON, D.W., & BYERLY, C.S. (1976b) Effects on
growing swine and sheep of two polychlorinated biphenyls. Am. J.
vet. Res., 37: 1021-1024.
HANSEN, L.G., WASHKO, P.W., TUINSTRA, L.G.M.TH., DORN, S.B., &
HINESLY, T.D. (1981) Polychlorinated biphenyl, pesticide, and heavy
metal residues in swine foraging on sewage sludge amended soils. J.
agric. food Chem., 29: 1012-1017.
HAQUE, R. & SCHMEDDING, D.W. (1975) A method of measuring the water
solubility of hydrophobic chemicals: Solubility of five
polychlorinated biphenyls. Bull. environ. Contam. Toxicol.,
14: 13-18.
HAQUE, R., SCHMEDDING, D.W., & FREED, V.H. (1974) Aqueous
solubility adsorption and vapor behaviour of polychlorinated
biphenyl Aroclor 1254. Environ. Sci. Technol., 8: 139-142.
HARA, I. (1985) Health status and PCBs in blood of workers exposed
to PCBs and their children. Environ. health Perspect., 59: 85-90.
HARA, I., HARADA, H., KIMURA, S., ENDO, T., & KAWANO, K. (1974)
[Follow-up health examination in an electric condenser factory
after cessation of PCBs usage (1st report).] Jpn. J. ind. Health,
16: 365-366 (in Japanese).
HARBISON, R.D., JAMES, R.C., & ROBERTS, S.M. (1987) Biological data
relevant to the evaluation of carcinogenic risk to humans, Little
Rock, Arkansas, University of Arkansas, Division of
Interdisciplinary Toxicology, (Prepared for Scientific Advisory
Panel, Safe Drinking Water and Toxic Enforcement Act, State of
California).
HARDING, L.W. & PHILLIPS, J.H. (1978a) Polychlorinated biphenyl
(PCB) effects on marine phytoplankton photosynthesis and cell
division. Mar. Biol., 49: 93-101.
HARDING, L.W. & PHILLIPS, J.H. (1978b) Polychlorinated biphenyl
(PCB) uptake by marine phytoplankton. Mar. Biol., 49: 103-111.
HARDWICK, J.P., LINKO, P., & GOLDSTEIN, J.A. (1985) Dose response
for induction of two cytochrome P-450 isoenzymes and their mRNAs by
3,4,5,3',4',5'-hexachlorobiphenyl indicating coordinate regulation
in rat liver. Mol. Pharmacol., 27: 676-682.
HARRIS, M.P. & OSBORN, D. (1981) Effect of a polychlorinated
biphenyl on the survival and breeding of puffins. J. appl. Ecol.,
18: 471-479.
HARRIS, J.R. & ROSE, L. (1972) Toxicity of polychlorinated
biphenyls in poultry. J. Am. Vet. Med. Assoc., 161: 1584-1586.
HARVEY, G.R. & STEINHAUER, W.G. (1974) Atmospheric transport of
polychlorobiphenyls to the North Atlantic. Atmos. Environ.,
8: 777-782.
HARVEY, G.R., STEINHAUER, W.G., & TEAL, J.M. (1973)
Polychlorobiphenyls in North Atlantic Ocean water. Science,
180: 643-644.
HASEGAWA, M. (1973) [Studies on the wild animals as
"Contamination-index". Part 1 - The effects of PCB on frog tadpoles
(Rana chensinensis).] Hokkaidoritsu Eisei Kenkyushoho, 23: 6-9
(in Japanese).
HASEGAWA, H., SATO, M., & TSURUTA, H. (1972a) [PCB concentration in
the blood of workers handling PCB.] Occup. Health, 13(10): 50-55
(in Japanese).
HASEGAWA, H., SATO, M., & TSURUTA, H. (1972b) [PCB concentration in
air of PCB-using plants and health examination of workers.] In:
[Report on special research on prevention of environmental
pollution by PCB-like substances], Tokyo, Science and Technology
Agency, Research Co-ordination Bureau, pp. 141-149 (in Japanese).
HASELTINE, S.D. & PROUTY, R.M. (1980) Aroclor 1242 and reproductive
success of adult mallards (Anas platyrhynchos). Environ. Res.,
23: 29-34.
HASELTINE, S.D., HEINZ, G.H., REICHEL, W.L., & MOORE, J.F. (1981)
Organochlorine and metal residues in eggs of wildfowl nesting on
islands in Lake Michigan off Door county, Wisconsin, 1977-78.
Pestic. monit. J., 15: 90-97.
HASHIMOTO, K., AKASAKA, S., TAKAGI, Y., KATAOKA, M., OTAKA, T.,
MURATA, Y., ABURADA, S., KITAURA, T., & UDA, H. (1976) Distribution
and excretion of (14C)polychlorinated biphenyls after their
prolonged administration to male rats. Toxicol. appl. Pharmacol.,
37: 415-423.
HASSELL, K.D. & HOLMES, D.C. (1977) Polychlorinated terphenyls
(PCT) in some British birds. Bull. environ. Contam. Toxicol.,
17: 618-621.
HATCH, W.I. & ALLEN, D.W. (1979) Alterations in calcium
accumulation behavior in response to calcium availability and
polychlorinated biphenyl administration. Bull. environ. Contam.
Toxicol., 22: 172-174.
HATTULA, M.L. (1985) Mutagenicity of PCBs and their pyrosynthetic
derivatives in cell-mediated assay. Environ. health Perspect.,
60: 255-257.
HATTULA, M.L. & KARLOG, O. (1973) Absorption and elimination of
polychlorinated biphenyls (PCB) in goldfish. Acta pharmacol.
toxicol., 32: 237-245.
HAWES, M.L., KRICHER, J.C., & UREY, J.C. (1976a) The effects of
various Aroclor fractions on the population growth of Chlorella
pyrenoidosa. Bull. environ. Contam. Toxicol., 15: 14-18.
HAWES, M.L., KRICHER, J.C., & UREY, J.C. (1976b) The effects of
various Aroclor fractions on the productivity of Chlorella
pyrenoidosa. Bull. environ. Contam. Toxicol., 15: 588-590.
HAWKER, D.W. (1989) Vapour pressures and Henry's Law Constants of
polychlorinated biphenyls. Environ. Sci. Technol., 23: 1250-1253.
HAWKER, D.W. & CONNELL, D.W. (1988) Octanol-water partition
coefficients of polychlorinated biphenyl congeners. Environ. Sci.
Technol., 22: 382-387.
HAYES, M.A., SAFE, S.H., ARMSTRONG, D., & CAMERON, R.G. (1985)
Influence of cell proliferation on initiating activity of pure
polychlorinated biphenyls and complex mixtures in resistant
hepatocyte in vivo assays for carcinogenicity. J. Natl Cancer
Inst., 74: 1037-1041.
HAYES, M.A., ROBERTS, E., SAFE, S.H., FARBER, E., & CAMERON, R.G.
(1986) Influences of different polychlorinated biphenyls on
cytocidal, mitiinhibitory and nodule-selecting activities of
N-2-fluorenylacetamide in rat liver. J. Natl Cancer Inst.,
76: 683-691.
HAYES, M.A. (1987) Carcinogenic and mutagenic effects of PCBs. In:
Safe, S., ed. Polychlorinated biphenyls (PCBs): Mammalian and
environmental toxicology, Berlin, Heidelberg, New York,
Springer-Verlag, pp. 77-95 (Environmental Toxin Series, Vol. I).
HAYS, H. & RISEBROUGH, R.W. (1972) Pollutant concentrations in
abnormal young terns from Long Island Sound. Auk, 89: 19-35.
HEATH, R.G., SPANN, J.W., KREITZER, J.F., & VANCE, C. (1972)
Effects of polychlorinated biphenyls on birds. In: Proceedings of
the XVth International Ornithological Congress, The Hague, 30
August-5 September 1970, pp. 475-485.
HEDDLE, J.A. & BRUCE, W.R. (1977) Comparison of tests for
mutagenicity or carcinogenicity using assays for sperm
abnormalities, formation of micronuclei and mutation in Salmonella.
In: Origins of human cancer, Book C: Human risk assessment, Section
16. Short-term assays - Predictive value, Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp. 1549-1557.
HEESCHEN, W., BLUTHGEN, A., & NIJHUIS, H. (1986) [Milk hygiene:
Trends in the residue situation.] Kieler Milchwirtsch
Forschungsber., 38(2): 131-145 (in German).
HEINZ, G.H., HILL, E.F., & CONTERA, J.F. (1980) Dopamine and
norepinephrine depletion in ring doves fed DDE, dieldrin, and
Aroclor 1254. Toxicol. appl. Pharmacol., 53: 75-82.
HEINZ, G.H., HASELTINE, S.D., REICHEL, W.L., & HENSLER, G.L. (1983)
Relationships of environmental contaminants to reproductive success
in red-breasted mergansers Mergus serrator from Lake Michigan.
Environ. Pollut., 32: 211-232.
HEINZOW, B.G.J., TINNEBERG, H.R., & BOIE, C. (1988) Effects of some
lipophilic xenobiotics on T-lymphocytes using a modified
erythrocyte rosette inhibition test. Res. Commun. chem. Patho.
Pharmacol., 61(2): 277-280.
HEIRONIMUS, M.P., LAUGHLIN, N.K., & BOWMAN, R.E. (1981) Effects of
early exposure to PCBs on learned irrelevancy of cues in Rhesus
monkeys. An incidental learning paradigm. Teratology, 24: 55A.
HELLE, E., OLSSON, M., & JENSEN, S. (1976a) DDT and PCB levels and
reproduction in ringed seals from the Bothnian bay. Ambio,
5: 188-189.
HELLE, E., OLSSON, M., & JENSEN, S. (1976b) PCB levels correlated
with pathological changes in seal uteri. Ambio, 5: 261-263.
HELLE, E., HYVARINEN, H., PYYSALO, H., & WICKSTROM, K. (1983)
Levels of organochlorine compounds in an inland seal population in
Eastern Finland. Mar. Pollut. Bull., 14: 256-260.
HENDRICKS, D., PUTNAM, T.P., BILLS, D.D., & SINNHUBER, R.O. (1977)
Inhibitory effect of a polychlorinated biphenyl (Aroclor 1254) on
aflatoxin B1 carcinogenesis in rainbow trout (Salmo gairdneri).
J. Natl Cancer Inst., 59: 1545-1551.
HERRING, J.L., HANNAN, E.J., & BILLS, D.D. (1972) UV irradiation of
Aroclor 1254. Bull. environ. Contam. Toxicol., 8: 153-157.
HESSELBERG, R.J. & SCHERR, D.D. (1974) PCBs and p,p' DDE in the
blood of cachectic patients. Bull. environ. Contam. Toxicol.,
11: 202-205.
HILL, H.R., Jr, (1985) Effects of polyhalogenated aromatic
compounds on porphyrin metabolism. Environ. health Perspect.,
60: 139-143.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington,
DC, US Department of the Interior, Fish and Wildlife Service, 147
pp (Fish and Wildlife Technical Report No. 2).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1974)
Polychlorinated biphenyl toxicity to Japanese quail as related to
degree of chlorination. Poult. Sci., 53: 597-604.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 61 pp (Special Scientific Report No. 191).
HILL, E.F., HEATH, R.G., & WILLIAMS, J.D. (1976) Effect of dieldrin
and Aroclor 1242 on Japanese quail eggshell thickness. Bull.
environ. Contam. Toxicol., 16: 445-453.
HINTON, D.E., GLAUMANN, H., & TRUMP, B.F. (1978) Studies on the
cellular toxicity of polychlorinated biphenyls (PCBs) I. Effect of
PCBs on microsomal enzymes and on synthesis and turnover of
microsomal and cytoplasmic lipids of rat liver - A morphological
and biochemical study. Virchows Arch. cell. Pathol., B27: 279-306.
HIRAYAMA, C., IRISA, T., & YAMAMOTO, T. (1969) Fine structural
changes of the liver in a patient with chlorobiphenyls
intoxication. Fukuoka Acta med., 60: 445-461.
HIRAYAMA, C., OKUMURA, M., NAGAI, J., & MASUDA, Y. (1974)
Hypobilirubinemia in patients with polychlorinated biphenyls
poisoning. Clin. Chim. Acta., 55: 97-100.
HIROSE, M., SHIRAI, T., TSUCA, H., FUKUSHIMA, S., OGISO, T., & ITO,
N. (1981) Effect of phenobarbital, polychlorinated biphenyl and
sodium saccharin on hepatic and renal carcinogenesis in
unilaterally nephrectomized rats given n-ethyl- N-hydroxy-
ethylnitrosamine orally. Carcinogenesis, 2: 1299-1302.
HLADKA, A., TAKACHOVA, T., & LISHKA, D. (1983) Exposure to
polychlorinated biphenyls and its effect on selected biochemical
functions. Czech. Med., 5: 8-14.
HODSON, K. (1975) Some aspects of the nesting ecology of
Richardson's merlin (Falco columbarius richardsonii) on the
Canadian prairies, Columbia, Vancouver, University of British
Columbia (MSc Thesis).
HOFFMAN, D.J., RATTNER, B.A., BUNCK, C.M., & KRYNITSKY, A. (1986)
Association between PCBs and lower embryonic weight in
black-crowned night herons in San Francisco Bay. J. Toxicol.
environ. Health, 19: 383-391.
HOGAN, J.W. & BRAUHN, J.L. (1975) Abnormal rainbow trout fry from
eggs containing high residues of a PCB (Aroclor 1242). Prog.
Fish-Cult., 37: 229-230.
HOLA, N. & REZNICEK, J. (1985) [The genetic hazard involved in
occupational exposure to high concentrations of polychlorinated
biphenyls.] Prac. Lek., 37: 386-391 (in Czech).
HOLDEN, A.V. (1970) Source of polychlorinated contamination in the
marine environment. Nature (Lond.), 228: 1220-1221.
HOLDEN, A.V. (1973) Monitoring PCBs in water and wildlife. In:
Proceedings of the Polychlorinated Biphenyl II Conference,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 23-33 (Publication No. 4E).
HOLDEN, A.V. & MARSDEN, K. (1969) Single-stage clean-up of animal
tissue extracts for organochlorine residue analysis. J.
Chromatogr., 44: 481-492.
HOLDGATE, M.W. (1971) The sea bird wreck in the Irish Sea. Autumn
1969, London, Natural Environment Research Council (Publication
Series C4).
HOLDRINET, M.V., BRAUN, H.E., FRANK, R., STOPPS, G.J., SMOUT, M.S.,
& MCWADE, J.W. (1977) Organochlorine residues in human adipose
tissue and milk from Ontario residents 1969-1974. Can. J. public
Health, 68: 74-80.
HOLLEMAN, K.A., BARNETT, B.D., & WICKER, G.W. (1976) Response of
chicks and turkey poults to Aroclor 1242. Poult. Sci.,
55: 2354-2356.
HOLT, R.L., CRUSE, S., & DREER, E.S. (1986) Pesticide and
polychlorinated biphenyl residues in human adipose tissue from
Northeast Louisiana. Bull. environ. Contam. Toxicol., 36: 651-655.
HOM, W., RISEBROUGH, R.W., SOUTAR, A., & YOUNG, D.R. (1974)
Deposition of DDE and polychlorinated biphenyls in dated sediments
of the Santa Barbara Basin. Science, 184: 1197-1199.
HONDA, T., NONAKA, S., MURAYAMA, F., OHGAMI, T., SHIMOYAMA, T., &
YOSHIDA, H. (1983) Effects of KC-400 (polychlorinated biphenyls) on
porphyrin metabolism. J. Dermatol., 10: 259-265.
HOOPINGARNER, R., SAMUEL, A., & KRAUSE, D. (1972) Polychlorinated
biphenyl interactions with tissue culture cells. Environ. health
Perspect., 1: 155-158.
HORI, S., OBANA, H., KASHIMOTO, T., OTAKE, T., NISHIMURA, H.,
IKEGAMI, N., KUNITA, N., & UDA, H. (1982) Effect of polychlorinated
biphenyls and polychlorinated quarterphenyls in Cynomolgus monkey
(Macaca fascicularis). Toxicology, 24: 123-139.
HORNSHAW, T.C., AULERICH, R.J., & JOHNSON, H.E. (1983) Feeding
great lakes fish to mink: Effects on mink and accumulation and
elimination of PCBs by mink. J. Toxicol. environ. Health,
11: 933-946.
HORNSHAW, T.C., SAFRONOFF, J., RINGER, R.K., & AULERICH, R.J.
(1986) LC50 test results in polychlorinated biphenyl-fed mink: Age,
season, and diet comparisons. Arch. Environ. Contam. Toxicol.,
15: 717-723.
HORZEMPA, L.M. & DI TORO, D.M. (1983) The extent of reversibility
of polychlorinated biphenyl adsorption. Water Res., 17: 851-859.
HSIA, M.T.S., LIN, F.S.D., & ALLEN, J.R. (1978) Comparative
mutagenicity and toxic effects of 2,2',5,5'-tetrachlorobiphenyl and
its metabolites in bacterial and mammalian test systems. Res.
Commun. chem. Pathol. Pharmacol., 21: 485-496.
HSU, I.C., VAN MILLER, J.P., SEYMOUR, J.L., & ALLEN, J.R. (1975a)
Urinary metabolites of 2,5,2',5'-tetrachlorobiphenyl in the
non-human primate. Proc. Soc. Exp. Biol. Med., 150: 185-188.
HSU, I.C., VAN MILLER, J.P., & ALLEN, J.R. (1975b) Metabolic fate
of 3H 2,5,2',5'-tetrachlorobiphenyls in infant non-human primates.
Bull. environ. Contam. Toxicol., 14: 233-240.
HSU, S.-T., MA, C.-I., HSU, S.K.-H., WU, S.-S., HSU, N.H.-M., YEH,
C.-C., & WU, S.-B. (1985) Discovery and epidemiology of PCB
poisoning in Taiwan: A four-year follow-up. Environ. health
Perspect., 59: 5-10.
HUCKINS, J.N., SCHWARTZ, T.R., PETTY, J.D. & SMITH, L.M. (1988)
Determination, fate and potential significance of PCBs in fish and
sediment samples with emphasis on selected AHH-inducing congeners.
Chemosphere, 17(10): 1995-2016.
HUDECOVA, A., KOSHINOVA, A., & MADARICH, A. (1979) [Investigations
of the effect of polychlorinated biphenyls on the dynamics of the
changes of the vitamin A and E content in rat liver.] Czech. Hyg.,
24: 59-64 (in Czech).
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife, Washington, DC, US Department
of the Interior, Fish and Wildlife Service, 90 pp (Resource
Publication No. 153).
HURST, J.G., NEWCOMER, W.S., & MORRISON, J.A. (1973) The effects of
polychlorinated biphenyl on longevity of bobwhite quail (Collinus
virginianus): A sex differential. Proc. Soc. Exp. Biol. Med.,
144: 431-435.
HURST, J.G., NEWCOMER, W.S., & MORRISON, J.A. (1974) Some effects
of DDT, toxaphene and polychlorinated biphenyl on thyroid function
in bobwhite quail. Poult. Sci., 53: 125-133.
HUSTERT, K. & KORTE, F. (1972) [Contributions to ecological
chemistry. XXXVIII: Synthesis of polychlorinated biphenyls and
their reactions to UV irradiation.] Chemosphere, 1: 7-10
(in German).
HUTZINGER, O., SAFE, S., & ZITKO, V. (1971) Polychlorinated
biphenyls: photolysis of 2,4,6,2',4',6'-hexachlorobiphenyl,
chlorobiphenyl. Nature (Lond.), 232: 15.
HUTZINGER, O., NASH, D.M., SAFE, S., DEFREITAS, A.S.W., NORSTRÖM,
R.J., WILDISH, D.J., & ZITKO, V. (1972) Polychlorinated
biphenyls-metabolic behaviour of pure isomers in pigeons, rats, and
brook trout. Science, 178: 312-314.
HUTZINGER, O., SAFE, S., & ZITKO, V. (1974) The chemistry of PCBs,
Cleveland, Ohio, CRC Press.
HUTZINGER, O., CHOUDHRY, G.G., CHITTIM, B.G., & JOHNSTON, L.E.
(1985) Formation of polychlorinated dibenzofurans and dioxins
during combustion, electrical equipment fires, and PCB
incineration. Environ. health Perspect., 60: 3-9.
IARC (1978) Polychlorinated biphenyls and polybrominated biphenyls,
Lyon, International Agency for Research on Cancer, pp. 43-103 (IARC
Monographs on the Evaluation of the Carcinogenic Risks of Chemicals
to Humans, Volume 18).
IARC (1987) Overall evaluation of carcinogenicity: An updating of
IARC monographs Volumes 1 to 42, Lyon, International Agency for
Research on Cancer, (IARC Monographs on the Evaluation of the
Carcinogenic Risks of Chemicals to Humans, Supplement 7).
IATROPOULOS, M.J., FELT, G.R., ADAMS, H.P., KORTE, F., & COULSTON,
F. (1977) Chronic toxicity of 2,5,4'-trichlorobiphenyl in young
Rhesus monkeys. II. Histopathology. Toxicol. appl. Pharmacol., 41:
629-638.
IATROPOULOS, M.J., BAILEY, J., ADAMS, H.P., COULSTON, F., & HOBSON,
W. (1978) Response of nursing infant Rhesus to Clophen A30 or
hexachlorobenzene given to lactating mothers. Environ. Res.,
16: 38-47.
IKEDA, M., KURATSUNE, M., NAKAMURA, Y., & HIROHATA, T. (1987) A
cohort study on mortality of Yusho patients - a preliminary report.
Fukuoka Acta med., 78: 297-300.
IMANISHI, J., NOMURA, H., MATSUBARA, M., KITA, M., WON, S.-J.,
MIZUTANI, T., & KISHIDA, T. (1980) Effect of polychlorinated
biphenyl on viral infections in mice. Infect. Immun., 29: 275-277.
IMANISHI, J., OKU, T., OISHI, K., NOMURA, H., & MIZUTANI, T. (1984)
Reduced resistance to experimental viral and bacterial infections
of mice treated with polychlorinated biphenyl. Biken J.,
27: 195-198.
INNAMI, S., NAKAMURA, A., MIYAZAKI, M., NAGAYAMA, S., & NISHIDE, E.
(1976) Further studies on the reduction of vitamin A content in the
livers of rats given polychlorinated biphenyls. J. nutr. Sci.
Vitaminol., 22: 409-418.
INOUE, K., TAKANAKA, A., MIZOKAMI, K., FUJIMORI, K., SUNOUCHI, M.,
KASUYA, Y., & OMORI, Y. (1981) Effects of polychlorinated biphenyls
on the monooxygenase system in fetal livers of rats. Toxicol. appl.
Pharmacol., 59: 540-547.
INTERNATIONAL COUNCIL FOR THE EXPLORATION OF THE SEA (1974) Report
of a working group for the international study of the pollution of
the North Sea and its effects on living resources and their
exploitation, Charlottenlund, Denmark, International Council for
the Exploration of the Sea (Co-operative Research Report No. 39).
IRPTC (1986) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme.
ISEKI, K., TAKAHASHI, M., BAUERFEIND, E., & WONG, C.S. (1981)
Effects of polychlorinated biphenyls (PCBs) on a marine plankton
population and sedimentation in controlled ecosystem enclosures.
Mar. Ecol. Prog. Ser., 5: 207-214.
ISHI, H. (1972) PCB pollution in Japan, Tokyo, Japanese Public
Health Association, pp. 13-28 (Environmental Health Report No. 14).
ISHIDATE, K., YOSHIDA, M., & NAKAZAWA, Y. (1978) Effect of typical
inducers of microsomal drug-metabolizing enzymes on phospholipid
metabolism in rat liver. Biochem. Pharmacol., 27: 2595-2603.
ISHIKAWA, T.T., MCNEELY, S., STEINER, P.M., GLUECK, C.J., MELLIES,
M., GARTSIDE, P.S., & MCMILLIN, C. (1978) Effects of chlorinated
hydrocarbons on plasma alpha-lipoprotein cholesterol in rats.
Metabolism, 27: 89-96.
ITO, N., NAGASAKI, H., ARAI, M., MAKIURA, S., SUGIHARA, S., &
HIRAO, K. (1973) Histopathologic studies on liver tumorigenesis
induced in mice by technical polychlorinated biphenyl and its
promoting effect on liver tumours induced by benzenehexachloride.
J. Natl Cancer Inst., 51(5): 1637-1646.
ITO, N., NAGASAKI, H., MAKIURA, S., & ARAI, M. (1974) Histological
studies on liver tumorigenesis in rats treated with polychlorinated
biphenyls. Gann., 65: 545-549.
ITOKAWA, Y., YAGI, N., KAITO, H., KAMOHARA, K., & FUJIWARA, K.
(1976) Influence of diet on the induction of hepatic ceroid pigment
in rats by polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
36: 131-141.
IVERSON, F., VILLENEUVE, D.C., GRANT, D.L., & HATINA, G.V. (1975)
Effect of Aroclor 1016 and 1242 on selected enzyme systems in the
rat. Bull. environ. Contam. Toxicol., 13: 456-463.
IVERSON, F., TRUELOVE, J., & HIERLIHY, S.L. (1982) Hepatic
microsomal enzyme induction by Aroclors 1248 and 1254 in Cynomolgus
monkeys. Food chem. Toxicol., 20: 307-310.
IWATA, Y. & GUNTHER, F.A. (1976) Translocation of the
polychlorinated biphenyl Aroclor 1254 from soil into carrots under
field conditions. Arch. environ. Contam. Toxicol., 4: 44-59.
IWATA, Y., WESTLAKE, W.E., & GUNTHER, F.A. (1973) Varying
persistence of polychlorinated biphenyls in six California soils
under laboratory conditions. Bull. environ. Contam. Toxicol.,
9: 204-211.
IWATA, Y., GUNTHER, F.A., & WESTLAKE, W.E. (1974) Uptake of a PCB
(Aroclor 1254) from soil by carrots under field conditions. Bull.
environ. Contam. Toxicol., 11: 523-528.
JACOBSON, J.L., JACOBSON, S.W., SCHWARTZ, P.M., FEIN, G.G., &
DOWLER, J.K. (1984a) Prenatal exposure to an environmental toxic:
A test of the multiple effects model. Dev. Psychol., 20: 523-532.
JACOBSON, J.L., FEIN, G.G., JACOBSON, S.W., SCHWARTZ, P.M., &
DOWLER, J.K. (1984b) The transfer of polychlorinated biphenyls
(PCBs) and polybrominated biphenyls-(PBBs) across the human
placenta and into maternal milk. Am. J. public Health,
74(4): 378-379.
JACOBSON, S.W., FEIN, G.G., JACOBSON, J.L., SCHWARTZ, P.M., &
DOWLER, J.K. (1985) The effect of intrauterine PCB exposure on
visual recognition memory. Child Dev., 56: 856-860.
JAN, J. & MALNERSIC, S. (1978) Determination of PCB and PCT
residues in fish by tissue acid hydrolysis and destructive clean-up
of the extract. Bull. environ. Contam. Toxicol., 19: 772-780.
JAN, J. & TRATNIK, M. (1988a) Polychlorinated biphenyls in
residents around the River Krupa, Slovenia, Yugoslavia. Bull.
environ. Contam. Toxicol., 41: 809-814.
JAN, J., TRATNIK, M., & KENDA, A. (1988b) Atmospheric contamination
with polychlorinated biphenyls in Bela Krajina (Yugoslavia);
Emissions from industrial plant, landfill and river areas.
Chemosphere, 17(4): 809-813.
JANI, J.P., PATEL, J.S., SHAH, M.P., GUPTA, S.K., & KASHYAP, S.K.
(1988) Levels of organochlorine pesticides in human milk in
Ahmedabad, India. Int. Arch. occup. environ. Health., 60: 111-113.
JANNETTI, R.A. & ANDERSON, L.M. (1981) Dimethylnitrosamine
demethylase activity in fetal, suckling, and maternal mouse liver
and its transplacental and transmammary induction by
polychlorinated biphenyls. J. Natl Cancer Inst., 67: 461-466.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTRÖM, G., &
VAZ, R. (1975) Identification by GC-MS of phenolic metabolites of
PCB and p,p'-DDE isolated from Baltic guillemot and seal. Ambio,
4: 93-97.
JAPAN ENVIRONMENT AGENCY (1983) Environmental monitoring of
chemicals; Environmental survey report of F.Y. 1980 and 1981.
Tokyo, Japan Environment Agency, Department of Environmental
Health, Offices of Health Studies.
JAPAN ENVIRONMENTAL SANITATION BUREAU (1973) [Survey on food
contamination: Report of a comprehensive investigation on the
prevention of pollution by PCBs], Tokyo, Ministry of Health &
Welfare, Environmental Sanitation Bureau, pp. 191-210 (in
Japanese).
JEFFERIES, D.J. & PARSLOW, J.L.F. (1972) Effect of one
polychlorinated biphenyl on size and activity of the gull thyroid.
Bull. environ. Contam. Toxicol., 8: 306-310.
JEFFERIES, D.J. & PARSLOW, J.L.F. (1976) Thyroid changes in
PCB-dosed guillemots and their indication of one of the mechanisms
of action of these materials. Environ. Pollut., 10: 293-311.
JENSEN, A.A. (1983a) Chemical contaminants in human milk. Res.
Rev., 89: 1-128.
JENSEN, A.A. (1983b) PCB in human milk: An Overview. In: Barros,
M.C., Könemann, H., & Visser, R., ed. Proceedings of the PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 81-98.
JENSEN, A.A. (1984a) Current data of levels of organohalogen
compounds in human milk in countries outside CEC. In: Report on the
WHO Consultation on Organohalogen Compounds in Human Milk and
Related Hazards, Bilthoven, 9-11 January 1985, Copenhagen, World
Health Organization, Regional Office for Europe, Annex 3.
JENSEN, A.A. (1984b) Data extracted from document on "Assessment of
the presence of potentially toxic substances in breast milk with
special emphasis on the European Community" submitted by the
Commission of the European Communities (CEC Study No. 830465,
CEC/V/E/2/lux/35/84). In: Report on the WHO Consultation on
Organohalogen Compounds in Human Milk and Related Hazards,
Bilthoven, 9-11 January 1985, Copenhagen, World Health
Organization, Regional Office for Europe, Annex 7.
JENSEN, A.A. (1987) Polychlorobiphenyls (PCBs),
polychlorodibenzo- p-dioxins (PCDDs) and polychlorodibenzofurans
(PCDFs) in human milk, blood, and adipose tissue. Sci. total
Environ., 64: 259-293.
JENSEN, S. & JANSSON, B. (1976) Methylsulfone metabolites of PCB
and DDE in seals from the Baltic. Ambio, 5: 257-260.
JENSEN, S. & SUNDSTROM, G. (1974a) Structures and levels of most
chlorobiphenyls in two technical PCB products and in human adipose
tissue. Ambio, 3: 70-76.
JENSEN, S. & SUNDSTROM, G. (1974b) Metabolic hydroxylation of a
chlorobiphenyl containing only isolated unsubstituted
positions-2,2',4,4',5,5'-hexachlorobiphenyl. Nature (Lond.),
251: 219-220.
JENSEN, S., JOHNELS, A.G., OLSSON, M., & OTTERLIND, G. (1969) DDT
and PCB in marine animals from Swedish waters. Nature (Lond.),
224: 247-250.
JENSEN, S., JOHNELS, A.G., OLSSON, M., & OTTERLIND, G. (1972a) DDT
and PCB in herring and cod from the Baltic, the Kattegat and the
Skaggerrak. Ambio, 1(Spec. Rep.): 71-85.
JENSEN, S., JOHNELS, A.G., OLSSEN, M., & WESTERMARK, T. (1972b) The
avifauna of Sweden as indicators of environmental contamination
with mercury and chlorinated hydrocarbons. In: Proceedings of the
15th International Ornithology Congress, Leiden, pp. 455-465.
JENSEN, S., RENBERG, L., & VAZ, R. (1973) Problems in
quantification of PCB in biological material. In: Proceedings of
the Polychlorinated Biphenyls Conference II, Stockholm, 1972,
Solna, Sweden, National Environmental Protection Board, pp. 7-13
(Publication No. 4E).
JENSEN, S., ORBERG, J., KIHLSTROM, J.E., OLSSON, M., & LUNDBERG, C.
(1977) Effects of PCB and DDT on mink (Mustela vision) during the
reproductive season. Ambio, 6: 239.
JFCMP (1985) FAO/WHO Collaborating Centres for Food Contamination
Monitoring. PCB's residues in food, Rome, Food and Agriculture
Organization of the United Nations, FAO/WHO Food Contamination
Monitoring Programme (Document EFP/85,3 prepared for the 17th
Session of the Codex Committee on Pesticide Residues).
JOHANSSON, N., LARSSON, A., & LEWANDER, K. (1972) Metabolic effects
of PCB (Polychlorinated biphenyls) on the brown trout (Salmo
trutta). Comp. gen. Pharmacol., 3: 310-314.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1979)
Pesticides and other chemical residues in infant and toddler total
diet samples (I), August 1974-July 1975. Pestic. monit. J.,
13(3): 87-98.
JOHNSTONE, G.J., ECOBICHON, D.J., & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, D.C.L., DAVIS, W.E., Jr, NEWELL, G.W., SASMORE, D.P., &
ROSEN, V.J. (1974) Modification of hexachlorophene toxicity by
dieldrin and Aroclor 1254. Toxicology, 2: 309-318.
JONES, K.C. (1988) Determination of polychlorinated biphenyls in
human foodstuffs and tissues: suggestions for a selective congener
analytical approach. Sci. total Environ., 68: 141-159.
JONES, K.C. (1989) Polychlorinated biphenyls in Welsh soils: a
survey of typical levels. Chemosphere, 18(7-8): 1665-1672.
JONES, J.W. & ALDEN, H.S. (1936) An acneform dermatergosis. Arch.
Dermato. Syphilol., 33: 1022-1034.
JONSSON, H.T., KEIL, J.E., GADDY, R.G., LOADHOLT, C.B., HENNIGAR,
G.R., & WALKER, E.M. (1976) Prolonged ingestion of commercial DDT
and PCB; effects on progesterone levels and reproduction in the
mature female rat. Arch. environ. Contam. Toxicol., 3: 479-490.
JONSSON, H.T., Jr, WALKER, E.M., Jr, GREENE, W.B., HUGHSON, M.D.,
& HENNIGAR, G.R. (1981) Effects of prolonged exposure to dietary
DDT and PCB on rat liver morphology. Arch. environ. Contam.
Toxicol., 10: 171-183.
JURY, W.A., WINER, A.M., SPENCER, W.F., & FOCHT, D.D. (1987)
Transport and transformations of organic chemicals in the
soil-air-water ecosystem. Rev. environ. Contam. Toxicol.,
99: 119-164.
KAISER, K.L.E. (1974) On the optical activity of polychlorinated
biphenyls. Environ. Pollut., 7: 93-101.
KAISER, K.L.E. & WONG, P.T.S. (1974) Bacterial degradation of
polychlorinated biphenyls. I. Identification of some metabolic
products from Aroclor 1242. Bull. environ. Contam. Toxicol.,
11: 291-296.
KAMAL, M., KLEIN, M., & KORTE, F. (1976) Isolation and
identification of metabolites after long-term feeding of
2,2'-dichlorobiphenyl-C-14. Chemosphere, 5: 349-356.
KAMOHARA, T., YAGI, N., & ITOKAWA, Y. (1984) Mechanism of lipid
peroxide formation in polychlorinated biphenyls (PCB) and
dichlorodiphenyltrichloroethane (DDT)-poisoned rats. Environ. Res.,
34: 18-23.
KANEKO, T. (1988) [Polychlorinated terphenyls (PCTs). A study on
the induction of cleft palate by polychlorinated terphenyls (PCTs)
administered maternally with special reference to the role of
corticosterone.] Pharmacometrics, 36(4): 309-327 (in Japanese).
KANNAN, N., TANABE, S., WAKIMOTO, T., & TATSUKAWA, R. (1987)
Coplanar polychlorinated biphenyls in Aroclor and Kanechlor
mixtures. J. Assoc. Off. Anal. Chem., 70(3): 451-454.
KANNAN, N., TANABE, S., & TATSUKAWA, R. (1988) Potentially
hazardous residues of non- ortho chlorine substituted coplanar
PCBs in human adipose tissue. Arch. environ. Health, 43(1): 11-14.
KANNAN, N., WAKIMOTO, T., & TATSUKAWA, R. (1989) Possible
involvement of frontier (n) electrons in the metabolism of
polychlorinated biphenyls (PCBs). Chemosphere, 18(9/10): 1955-1963.
KARLSSON, B., PERSSON, B., SÖDERGREN, A., & ULFSTRAND, S. (1974)
Locomotory and dehydrogenase activities of redstarts Phoenicurus
phoenicurus L. (Aves) given PCB and DDT. Environ. Pollut.,
7: 53-63.
KARPPANEN, E. & KOLHO, L. (1973) The concentration of PCB in human
blood and adipose tissue in three different research groups.
Presented at the Polychlorinated Biphenyls Conference II,
Stockholm, 1972 (Unpublished document).
KASHIMOTO, T., MIYATA, H., FUKUSHIMA, S., KUNITA, N., OHI, G., &
TUNG, T.-C. (1985) PCBs, PCQs, and PCDFs in blood of Yusho and
Yu-Cheng patients. Environ. health Perspect., 59: 73-78.
KASZA, L., WEINBERGER, M.A., HINTON, D.E., TRUMP, B.F., PATEL, C.,
FRIEDMAN, L., & GARTHOFF, L.H. (1978a) Comparative toxicity of
polychlorinated biphenyl and polybrominated biphenyl in the rat
liver: light and electron microscopic alterations after subacute
dietary exposure. J. environ. Pathol. Toxicol., 1: 241-257.
KASZA, L., COLLINS, W.T., CAPEN, C.C., GARTHOFF, L.H., & FRIEDMAN,
L. (1978b) Comparative toxicity of polychlorinated biphenyl and
polybrominated biphenyl in the rat thyroid gland: light and
electron microscopic alterations after subacute dietary exposure.
J. environ. Pathol. Toxicol., 1: 587-599.
KATO, N. & YOSHIDA, A. (1980) Effect of dietary PCB on hepatic
cholesterogenesis in rats. Nutr. Rep. Int., 21: 107-112.
KATO, N. & YOSHIDA, A. (1981) Effect of various dietary xenobiotics
on serum total cholesterol and high density lipoprotein cholesterol
in rats. Nutr. Rep. Int., 23: 825-831.
KATO, N., KATO, M., KIMURA, T., & YOSHIDA, A. (1978) Effect of
dietary addition of PCB, DDT, or BHT and dietary protein on vitamin
A and cholesterol metabolism. Nutr. Rep. Int., 18: 437-446.
KATO, N., KAWAI, K., & YOSHIDA, A. (1982) Effects of dietary
polychlorinated biphenyls and protein level on liver and serum
lipid metabolism of rats. Agric. biol. Chem., 46: 703-707.
KATO, S., MCKINNEY, J.D., & MATTHEWS, H.B. (1980) Metabolism of
symmetrical hexachlorobiphenyl isomers in the rat. Toxicol. appl.
Pharmacol., 53: 389-398.
KATSUKI, S. (1969) Foreword. Fukuoka Acta med., 60: 403-407.
KAWAI, S., FUKUSHIMA, M., MIYAZAKI, N., & TATSUKAWA, R. (1988)
Relationship between lipid composition and organochlorine levels in
the tissues of striped Dolphin. Mar. Pollut. Bull., 19: 129-133.
KAWANISHI, S., SANO, S., MIZUTANI, T., & MATSUMOTO M. (1973)
[Experimental porphyria induced by polychlorinated biphenyls.] Jpn.
J. Hyg., 28: 84 (in Japanese).
KAWANISHI, S., SANO, S., MIZUTANI, T., & MATSUMOTO M. (1974)
[Experimental studies on toxicity of synthetic tetrachlorobiphenyl
isomers.] Jpn. J. Hyg., 29: 81 (in Japanese).
KAWANISHI, S., SANO, S., & MIZUTANI, T. (1975) [A toxicological
study on synthetic hexachlorobiphenyl isomers.] Jpn. J. Hyg.,
30: 124 (in Japanese).
KAWANISHI, S., SEKI, Y., & SANO, S. (1983) Uroporphyrinogen
decarboxylase. Purification, properties, and inhibition by
polychlorinated biphenyl isomers. J. biol. Chem., 258: 4285-4292.
KECK, G. (1981) Effets de la contamination par les
polychlorobiphényles (PCBs) sur le développement de la tumeur
d'Ehrlich chez la souris Swiss. Toxicol. Eur. Res., 3(5): 229-236.
KERKVLIET, N.I. & BAECHER-STEPPAN, L. (1988a) Suppression of
allograft immunity by 3,4,5,3',4',5-hexachlorobiphenyl. I. Effects
of exposure on tumor rejection and cytotoxic T-cell activity
in vivo. Immunopharmacology, 16: 1-12.
KERKVLIET, N.I. & BAECHER-STEPPAN, L. (1988b) Suppression of
allograft immunity by 3,4,5,3',4',5-hexachlorobiphenyl. II. Effects
of exposure on mixed lymphocyte reactivity and induction of
suppressor cell activity in vitro. Immunopharmacology, 16: 13-23.
KERKVLIET, N.I. & KIMELDORF, D.J. (1977) Anti-tumour activity of a
polychlorinate biphenyl mixture, Aroclor 1254, in rats inoculated
with Walker 256 carcinosarcoma cells. J. Natl Cancer Inst.,
59: 951-955.
KHAN, M.A., RAO, R.M., & NOVAK, A.F. (1976) Polychlorinated
biphenyls (PCBs) in food. January 1976. Crit. Rev. food Sci. Nutr.,
January: 103-145.
KHAN, A.V., LASHNEVA, N.V., KUMOK, S.T., GURVICH, YA.A., &
TUTELYAN, V.A. (1985) Study into the effect of ionol on cytochrome
P-450 induction in rat liver by polychlorinated diphenyls. Vopr.
pitan., 4(1): 66-69.
KIESSLING, A., PART, P., RING, O., & LINDAHL-KIESSLING, K. (1983)
Effects of PCB on the adrenergic response in perfused gills and on
levels of muscle glycogen in rainbow trout (Salmo gairdneri
Rich.). Bull. environ. Contam. Toxicol., 31: 712-718.
KIHLSTROM, J.E., LUNDBERG, C., ORBERG, J., DANIELSSON, P.O., &
SYDHOFF, J. (1975) Sexual functions of mice neonatally exposed to
DDT or PCB. Environ. Physiol. Biochem., 5: 54-57.
KIKUCHI, M. (1984) Autopsy of patients with Yusho. Am. J. ind.
Med., 5: 19-30.
KIMBROUGH, R.D. (1980) Occupational exposure. In: Halogenated
biphenyls, terphenyls, naphthalenes, dibenzodioxins and related
products, Amsterdam, Elsevier, North-Holland Biomedical Press,
pp. 373-395 (Topics in Environmental Health, Vol. 4).
KIMBROUGH, R.D. (1987) Human health effects of polychlorinated
biphenyls (PCBs) and polybrominated biphenyls (PBBs). Annu. Rev.
Pharmacol. Toxicol., 27: 87-111.
KIMBROUGH, R.D., LINDER, R.E., & GAINES, T.B. (1972) Morphological
changes in livers of rats fed polychlorinated biphenyls. Light
microscopy and ultrastructure. Arch. environ. Health, 25: 354-364.
KIMBROUGH, R.D. & LINDER, R.E. (1974) Induction of adenofibrosis
and hepatomas of the liver in BALB/cJ mice by polychlorinated
biphenyls (Aroclor 1254). J. Natl Cancer Inst., 53: 547-552.
KIMBROUGH, R.D., SQUIRE, R.A., LINDER, R.E., STRANBERG, J.D.,
MONTALI, R.J., & BURSE, V.W. (1975) Induction of liver tumours in
Sherman strain female rats by polychlorinated biphenyl Aroclor
1260. J. Natl Cancer Inst., 55: 1453-1459.
KIMURA, N.T. & BABA, T. (1973) Neoplastic changes in the rat liver
induced by polychlorinated biphenyls. Gann, 64: 105-108.
KIMURA, I. & MIYAKE, T. (1976) Teratogenic and postnatal growth-
suppressive effects of polychloro-triphenyl (PCT) in dd/Y mice.
Teratology, 14: 243-244 (Abstract).
KIMURA, S., KUMADA, H., & MATIDA, Y. (1974) [Acute toxicity and
accumulation of PCB (KC 300) in freshwater fish. Bull. freshwater
Fish Res. Lab. (Tokyo), 23: 115-123 (in Japanese).
KIMURA, N.T., KANEMATSU, T., & BABA, T. (1976) Polychlorinated
biphenyl(s) as a promoter in experimental hepatocarcinogenesis in
rats. Z. Krebsforsch., 87: 257-266.
KIRIYAMA, S., BANJO, M., & MATSUSHIMA, H. (1974) Effect of
polychlorinated biphenyls (PCB) and related compounds on the weight
of various organs and plasma and liver cholesterol in the rat.
Nutr. Rep. Int., 10: 79-88.
KITAMURA, M., TSUKAMOTO, T., SUMINO, K., HAYAKAWA, K., SHIBITA, T.,
& HIRANO, I. (1973) [The PCB levels in the blood of workers
employed in a condenser factory.] Jpn. J. ind. Health, 47: 354-355
(in Japanese).
KLAAS, E.E. & SWINEFORD, D.M. (1976) Chemical residue content and
hatchability of screech owl eggs. Wilson Bull., 88: 421-426.
KLASSON-WEHLER, E., BERGMAN, A., KOWALSKI, B., & BRANDT, I. (1987)
Metabolism of 2,3,4'6,-tetrachlorobiphenyl: Formation and tissue
localization of mercapturic acid pathway metabolites in mice.
Xenobiotica, 17(4): 477-486.
KLEIN, H. (1983) The PCB situation in the Federal Republic of
Germany. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of PCB Seminar, The Hague, 28-30 September 1983, The
Hague, Ministry of the Environment, pp. 66-79.
KLEKOWSKI, E.J. (1982) Mutation in ferns growing in an environment
contaminated with polychlorinated biphenyls, Amherst,
Massachusetts, University of Massachusetts, Water Resources
Research Center, 23 pp ((NTIS) PB83-150011).
KLEPPEL, G.S. & MCLAUGHLIN, J.J.A. (1980) PCB toxicity to
phytoplankton: Effects of dose and density-dependent recovery
responses. Bull. environ. Contam. Toxicol., 24: 696-703.
KLING, D. & GAMBLE, W. (1982) Cholesterol biosynthesis in
polychlorinated biphenyl-treated rats. Environ. Res., 27: 10-15.
KOBAYASHI, Y. (1972) [Answer report to the questionary paper on
"Regulation on residual levels in foods".] Biol. Pollut., 4: 93-116
(in Japanese).
KODAMA, H. & OTA, H. (1977) Studies on the transfer of PCB to
infants from their mothers (1). Jpn. J. Hyg., 32: 567-573.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated biphenyls
to infants from mothers. Arch. environ. Health, 35: 95-100.
KOEMAN, J.H., TEN HOEVER DE BRAUW, M.C., & DE VOS, R.H. (1969)
Chlorinated biphenyls in fish, mussels and birds from the River
Rhine and the Netherlands coastal area. Nature (Lond.),
221: 1126-1128.
KOEMAN, J.H., PEETERS, W.H.M., SMIT, C.J., TJIOE, P.S., & DE GOEIJ,
J.J.M. (1972) Persistent chemicals in marine mammals. TNO-nieuws,
27: 570-578.
KOEMAN, J.H., VAN VELZEN-BLAD, H.C.W., DE VRIES, R., & VOS, J.G.
(1973) Effects of PCB and DDE in cormorants and evaluation of PCB
residues from an experimental study. J. Reprod. Fertil.,
19(Suppl.): 353-364.
KOHLI, K.K., GUPTA, B.N., ALBRO, P.W., MUKHTAR, H., & MCKINNEY,
J.D. (1979) Biochemical effects of pure isomers of hexachloro-
biphenyl: fatty livers and cell structure. Chem. biol. Interact.,
25: 139-156.
KOHLI, K.K., PHILPOT, R.M., ALBRO, P.W., & MCKINNEY, J.D. (1980)
Induction of different species of cytochrome P-450 by coplanar and
noncoplanar isomers of hexachlorobiphenyl. Life Sci., 26: 945-952.
KOLBYE, A.C., Jr (1972) Food exposure to polychlorinated biphenyls.
Environ. health Perspect., 1: 85-88.
KOLLER, L.D. (1977) Enhanced polychlorinated biphenyls lesions in
Moloney leukemia virus-infected mice. Clin. Toxicol.,
11(1): 107-116.
KOLLER, L.D. & THIGPEN, J.E. (1973) Biphenyl-exposed rabbits. Am.
J. vet. Res., 34: 1605-1606.
KOLLER, L.D. & ZINKL, J.G. (1973) Pathology of polychlorinated
biphenyls in rabbits. Am. J. Pathol., 70: 363-378.
KOMATSU, F. & KIKUCHI, M. (1972) [Skin lesion by 3,4,3',4'-
tetrachlorobiphenyl in rabbits.] Fukuoka Acta med., 63: 384-386
(in Japanese).
KORACH, K.S., SARVER, P., CHAE, K., MCLACHLAN, J.A., & MCKINNEY,
J.D. (1987) Estrogen receptor-binding activity of polychlorinated
hydroxybiphenyls: Conformationally restricted structural probes.
Mol. Pharmacol., 33: 120-126.
KOSHIOKA, M., KANAZAWA, J., IIZUKA, H., & MURAI, T. (1987)
Photodegradation of decachlorobiphenyl. Bull. environ. Contam.
Toxicol., 38: 409-415.
KOSS, G., SEUBERT, S., SEUBERT, A., HERBERT, M., KORANSKY, W., &
IPPEN, H. (1980) Hexachlorobenzene and 2,4,5,2',4',5'-hexachloro-
biphenyl - A comparison of their distribution, biotransformation
and prophyrinogenic action in female rats. In: Holmstedt, B.,
Lauwerys, R., Mercier, M., & Roberfroid, M., ed. Mechanisms of
toxicity and hazard evaluation, Amsterdam, Elsevier/North-Holland
Biomedical Press, pp. 517-520.
KOSUTZKY, J., ADAMEC, O., & BOBAKOVA, E. (1979) Effects of
polychlorinated biphenyls on poultry reproduction. Bull. environ.
Contam. Toxicol., 21: 737-742.
KREISS, K. (1985) Studies on populations exposed to polychlorinated
biphenyls. Environ. health Perspect., 60: 193-199.
KREISS, K., ZACK, M.M., KIMBROUGH, R.D., NEEDHAM, L.L., SMRED,
A.L., & JONES, B.T. (1981) Association of blood pressure and
polychlorinated biphenyl levels. J. Am. Med. Assoc.,
245(24): 2505-2509.
KREITZER, J.F. & HEINZ, G.H. (1974) The effect of sublethal dosages
of five pesticides and a polychlorinated biphenyl on the avoidance
response of Coturnix quail chicks. Environ. Pollut., 6: 21-29.
KREITZER, J.F. & SPANN, J.W. (1973) Tests of pesticidal synergism
with young pheasants and Japanese quail. Bull. environ. Contam.
Toxicol., 9: 250-256.
KRICHER, J.C., BAYER, C.L., & MARTIN, D.A. (1979) Effects of two
Aroclor fractions on the productivity and diversity of algae from
two lentic ecosystems. Int. J. environ. Stud., 13: 159-167.
KROGH DERR, S. (1978) In vivo metabolism of exogenous
progesterone by PCB treated female rats. Bull. environ. Contam.
Toxicol., 19: 729-732.
KUNITA, N., HORI, S., OBANA, H., OTAKE, T., NISHIMURA, H.,
KASHIMOTO, T., & IKEGAMI, N. (1985) Biological effects of PCBs PCQs
and PCDFs present in the oil causing Yusho and Yu-Cheng. Environ.
Health Perspect., 59: 79-84.
KURACHI, M. (1983) A new sulfur-containing derivative and
possibility of conjugate formation of PCBs in mice or rats. Agric.
biol. Med., 47(6): 1183-1191.
KURACHI, M. & MIO, T. (1983) On fluctuation of PCBs under various
unnatural conditions in mice. Agric. biol. Med., 47(6): 1173-1181.
KURATSUNE, M. & MASUDA, Y. (1972) Polychlorinated biphenyls in
non-carbon copypaper. Environ. health Perspect., 1: 61-62.
KURATSUNE, M., YOSHIMURA, T., MATSUZAKA, J., & YAMAGUCHI, A. (1972)
Epidemiologic study on Yusho, a poisoning caused by ingestion of
rice-oil contaminated with a commercial brand of polychlorinated
biphenyls. Environ. health Perspect., 1: 119-128.
KURATSUNE, M., MASUDA, Y., & NAGAYAMA, J. (1976) Some of the recent
findings concerning Yusho. In: Proceedings of the National
Conference on Polychlorinated Biphenyls, Chicago, 19-21 November
1975, Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances, pp. 14-29 (EPA-560/6-75-004).
KUROKI, H. & MASUDA, Y. (1977) Structures and concentrations of the
main components of polychlorinated biphenyls retained in patients
with Yusho. Chemosphere, 6: 469-474.
KUSUDA, M. (1971) [Study on the female sexual function suffering
from the chlorobiphenyls poisoning.] Sanka Fujinka, 4: 1063-1072
(in Japanese).
KUWABARA, K., YAKUSHIJI, T., WATANABE, I., YOSHIDA, S., KOYAMA, K.,
KUNITA, N., & HARA, I. (1978) Relationship between breast feeding
and PCB residues in blood of the children whose mothers were
occupationally exposed to PCBs. Int. Arch. occup. environ. Health,
41: 189-197.
KUWABARA, K., YAKUSHIJI, T., WATANABE, I., YOSHIDA, S., KOYAMA, K.,
& KUNITA, N. (1979) Levels of polychlorinated biphenyls in blood of
breast-fed children whose mothers are non-occupationally exposed to
PCBs. Bull. environ. Contam. Toxicol., 21: 458-462.
LAKE, B.G., COLLINS, M.A., HARRIS, R.A., & GANGOLLI, S.D. (1979)
The induction of hepatic and extrahepatic xenobiotic metabolism in
the rat and ferret by a polychlorinated biphenyl mixture (Aroclor
1254). Xenobiotica, 9: 723-731.
LAN, S.-J., YEN, Y.-Y., KO, Y.-C., & CHEN, E.-R. (1989) Growth and
development of permanent teeth germ of transplacental Yu-Cheng
babies in Taiwan. Bull. environ. Contam. Toxicol., 42: 931-934.
LA ROCCA, P. & CARLSON, G.P. (1979) The effect of polychlorinated
biphenyls on adenosine triphosphatase activity. Toxicol. appl.
Pharmacol., 48: 185-192.
LARSEN, P.F., GADBOIS, D.F., & JOHNSON, A.C. (1985) Observations on
the distribution of PCBs in the deepwater sediments of the Gulf of
Maine. Mar. Pollut. Bull., 16: 439-442.
LARSSON, R. (1984) Transport of PCBs from aquatic to terrestrial
environments by emerging chironomids. Environ. Pollut.,
34: 283-289.
LARSSON, P. (1985a) Contaminated sediments of lakes and oceans act
as sources of chlorinated hydrocarbons for release to water and
atmosphere. Nature (Lond.), 317: 347-349.
LARSSON, P. (1985b) Change in PCB (Clophen A 50) composition when
transported from sediment to air in aquatic model systems. Environ.
Pollut., B9: 81-94.
LARSSON, P. (1987) Uptake of polychlorinated biphenyls (PCBs) by
the macroalga, Cladophora glomerata. Bull. environ. Contam.
Toxicol., 38: 58-62.
LARSSON, P. & OKLA, L. (1987) An attempt to measure the flow of
chlorinated hydrocarbons, such as PCBs, from water to air in the
field. Environ. Pollut., 44: 219-225.
LARSSON, C.M. & TILLBERG, J.E. (1975) Effects of the commercial
polychlorinated biphenyl mixture Aroclor 1242 on growth, viability,
phosphate uptake, respiration and oxygen evolution in Scenedesmus.
Physiol. Plant., 33: 256-260.
LASHNEVA, N.V. & TUTELYAN, V.A. (1984) Induction of cytochrome
P-450 in rat liver by polychlorinated diphenyls. Farmakol.
Toksikol., 6: 77-80.
LASHNEVA, N.V., KHAN, A.V., & TUTELYAN, V.A. (1985) The functional
state of monooxygenase system in rat liver tissue after effect of
ionol and polychlorinated diphenyls. Vopr. med. khim. 5: 7-22.
LASHNEVA, N.V., CHICHILANOVA, G.V., SOROKOVAYA, G.K., & TUTELYAN,
V.A. (1987) Effects of polychlorinated biphenyls on rat liver
cytochrome P-450 system formation at early postnatal period. In:
Abstracts of the USSR-All-Union Conference on Cytochrome P-450 and
Environment, Novosibirsk, 27-31 July 1987, Novosibirsk, USSR
Academy of Medical Sciences, Siberian Division.
LAWRENCE, J. & TOSINE, H.M. (1977) Polychlorinated biphenyl
concentrations in sewage and sludges of some waste treatment plants
in Southern Ontario. Bull. environ. Contam. Toxicol., 17: 49-56.
LAWTON, R.W., ROSS, M.R., FEINGOLD, J., & BROWN, J.F., Jr (1985)
Effects of PCB exposure on biochemical and hematological findings
in capacitor workers. Environ. health Perspect., 60: 165-184.
LAY, J.P., KLEIN, W., & KORTE, F. (1975) Excretion, storage, and
metabolism of 2,3,6,2',4'-pentachlorobiphenyl-14-C after a
long-term feeding experiment on rats. Chemosphere, 4: 161-68.
LAY, J.P., KAMAL, M., KLEIN, W., & KORTE, F. (1979) Fate of
2.5.4'-trichlorobiphenyl in rats. Xenobiotica, 12: 713-721.
LEATHERLAND, J.F. & SONSTEGARD, R.A. (1978) Lowering of serum
thyroxine and triiodothyronine levels in yearling coho salmon,
Oncorhynchus kisutch, by dietary mirex and PCBs. J. Fish Res.
Board Can., 35: 1285-1289.
LEATHERLAND, J.F. & SONSTEGARD, R.A. (1979) Effect of dietary mirex
and PCB (Aroclor 1254) on thyroid activity and lipid reserves in
rainbow trout Salmo gairdneri Richardson. J. Fish Dis., 2: 43-48.
LEDERMAN, T.C. & RHEE, G.-Y. (1982) Bioconcentration of a
hexachlorobiphenyl in Great Lakes planktonic algae. Can. J. Fish.
aquat. Sci., 39: 380-387.
LEECE, B., DENOMME, M.A., TOWNER, R., & LI, S.M.A. (1985)
Polychlorinated biphenyls: correlation between in vivo and
in vitro quantitative structure-activity relationships (QSARs).
J. Toxicol. environ. Health, 16: 379-388.
LEES, P.S.J., CORN, M., & BREYSSE, P.N. (1987) Evidence for dermal
absorption as the major route of body entry during exposure of
transformer maintenance and repairmen to PCBs. Am. Ind. Hyg. Assoc.
J., 48(3): 257-264.
LEMMETYINEN, R., RANTAMAKI, P., & KARLIN, A. (1982) Levels of DDT
and PCB's in different stages of life cycle of the Arctic tern
Sterna paradisaea and the herring gull Larus argentatus.
Chemosphere, 11: 1059-1068.
LEONI, V., FABIANI, L., MARINELLI, G., PUCCETTI, G., TARSITANI,
G.F., DE CAROLIS, A., VESCIA, N., MORINI, A., ALEANDRI, V., POZZI,
V., CAPPA, F., & BARBATI, D. (1989) PCB and other organochlorine
compounds in blood of women with or without miscarriage: A
hypothesis of correlation. Ecotoxicol. environ. Saf., 17: 1-11.
LEVIN, B.D. & BOWMAN, R.E. (1983) Polychlorinated biphenyl effects
on delayed spatial alternation in monkeys. Toxicologist, 3: 68.
LEVIN, E.D., SCHANTZ, S.L., & BOWMAN, R.E. (1988) Delayed spatial
alteration deficits resulting from perinatal PCB exposure in
monkeys. Arch. Toxicol., 62: 267-273.
LEWIN, V., MCBLAIN, W.A., & WOLFE, F.H. (1972) Acute
intraperitoneal toxicity of DDT and PCBs in mice using two
solvents. Bull. environ. Contam. Toxicol., 8(4): 245-250.
LICHTENSTEIN, E.P., SCHULZ, K.R., FUHREMANN, T.W., & LIANG, T.T.
(1969) Biological interaction between plasticizers and
insecticides. J. econ. Entomol., 62: 761-765.
LICHTENSTEIN, E.P. (1972) PCBs and interactions with insecticides.
Environ. health Perspect., 1: 151-153.
LIEB, A.J., BILLS, D.D., & SINNHUBER, R.O. (1974) Accumulation of
dietary polychlorinated biphenyls (Aroclor 1254) by rainbow trout
(Salmo gairdneri). J. agric. food Chem., 22: 638-642.
LILLIE, R.J., HARRIS, S.J., CECIL, H.C., & BITMAN, J. (1974) Normal
reproductive performance of mature cockerels fed Aroclor 1248.
Poult. Sci., 53: 1604-1607.
LIN, F.S., HSIA, M.T., & ALLEN, J.R. (1979) Acute hepatotoxicity of
a tetrachlorobiphenyl-changes in the hepatocyte ultrastructure and
plasma membrane-bound enzymes. Arch. environ. Contam. Toxicol.,
8: 321-333.
LIN, P.S., NYQUIST, S.E., & HACLERODE, J. (1982) Interactions of
polychlorinated biphenyls and delta-9-tetrahydrocannabinol with
testosterone hydroxylation and the hepatic microsomal system in the
rat. Proc. Pa. Acad. Sci., 56: 31-35.
LINDER, R.E., GAINES, T.B., & KIMBROUGH, R.D. (1974) The effect of
polychlorinated biphenyls on rat reproduction. Food Cosmet.
Toxicol., 12: 63-76.
LINDSEY, A.S. & WAGSTAFFE, P.J. (1989) Production and certification
of ten high-purity polychlorinated biphenyls as reference
materials. Analyst, 114(5): 553-557.
LINZEY, A.V. (1987a) Effects of chronic polychlorinated biphenyls
exposure on the reproductive success of white-footed mice
(Peromyscus leucopus). Arch. environ. Contam. Toxicol.,
16: 455-460.
LINZEY, A.V. (1987b) Effects of chronic polychlorinated biphenyls
exposure on growth and reproduction of second generation
white-footed mice (Peromyscus leucopus). Arch. environ. Contam.
Toxicol., 17: 39-45.
LITTERST, C.L. & VAN LOON, E.J. (1974) Time-course of induction of
microsomal enzymes following treatment with polychlorinated
biphenyl. Bull. environ. Contam. Toxicol., 11: 206-212.
LITTERST, C.L., FARBER, T.M., BAKER, A.M., & VAN LOON, E.J. (1972)
Effect of polychlorinated biphenyls on hepatic microsomal enzymes
in the rat. Toxicol. appl. Pharmacol., 23: 112-122.
LIU, D. (1980) Enhancement of PCBs biodegradation by sodium
ligninsulfonate. Water Res., 14: 1467-1475.
LIU, D. (1981) Biodegradation of Aroclor 1221 type PCBs in sewage
wastewater. Bull. environ. Contam. Toxicol., 27: 695-703.
LIU, D. (1982) Assessment of continuous biodegradation of
commercial PCB formulations. Bull. environ. Contam. Toxicol.,
29: 200-207.
LLORENTE, G.A., FARRAN, A., RUIZ, X., & ALBAIGES, J. (1987)
Accumulation and distribution of hydrocarbons, polychlorobiphenyls,
and DDT in tissues of three species of Anatidae from the Ebro Delta
(Spain). Arch. environ. Contam. Toxicol., 16: 563-572.
LOOSE, L.D., PITTMAN, K.A., BENITZ, K.-F., & SILKWORTH, J.B. (1977)
Polychlorinated biphenyl and hexachlorobenzene induced humoral
immunosuppression. J. Reticulendothel. Soc., 22: 253-271.
LOOSE, L.D., SILKWORTH, J.B., PITTMAN, K.A., BENITZ, K.-F., &
MUELLER, W. (1978) Impaired host resistance to endotoxin and
malaria in polychlorinated biphenyl- and hexachlorobenzene-treated
mice. Infect. Immun., 20: 30-35.
LORENZ, H. & NEUMEIER, G. (1983) [Polychlorinated biphenyls (PCBs).
A joint report of the Federal Health Office and the Federal
Environment Office.] Munich, MMV Medizin Press (BGA Publication
No. 4/83) (in German).
LOWE, J.I., PARRISH, P.R., PATRICK, J.M., & FORESTER, J. (1972)
Effects of the polychlorinated biphenyl Aroclor 1254 on the
American oyster Crassostrea virginica. Mar. Biol., 17: 209-214.
LU, Y.-C & WONG P.-N (1984) Dermatological, medical and laboratory
findings of patients in Taiwan and their treatments. Am. J. ind.
Med., 5: 81-115.
LU, Y.-C. & WU, Y.-C. (1985) Clinical findings and immunological
abnormalities in Yu-Cheng patients. Environ. health Perspect.,
59: 17-29.
LUARD, E.J. (1973) Sensitivity of Dunaliella and Scenedesmus
(Chlorophyceae) to chlorinated hydrocarbons. Phycologia,
12: 29-33.
LUBET, R.A., LEMAIRE, B.N., AVERY, D., & KOURI, R.E. (1986)
Induction of immunotoxicity in mice by polychlorinated biphenyls.
Arch. Toxicol., 59: 71-77.
LUND, J., ANDERSSON, O., POELLINGER, L., & GUSTAFSSON, J.A. (1986)
In vitro characterization of possible mechanisms underlying the
selective in vivo accumulation of the PCB metabolite
4,4'-bis(methyl-sulphonyl)-2,5,2',5'-tetrachlorobiphenyl in the
lung. Food chem. Toxicol., 24(6/7): 563-566.
LUNDY, P., WURSTER, C.F., & ROWLAND, R.G. (1984) A two-species
marine algal bioassay for detecting aquatic toxicity of chemical
pollutants. Water Res., 18: 187-194.
LUNT, D. & EVANS, W.C. (1970) Microbial metabolism of biphenyl.
Biochem. J., 118: 54-55.
LUOTAMO, M., JÄRVISALO, J., & AITIO, A. (1985) Analysis of
polychlorinated biphenyls (PCBs) in human serum. Environ. health
Perspect., 60: 327-332.
LUTZ, R.J., DEDRICK, R.L., MATTHEWS, H.B., ELING, T., & ANDERSON,
M.W. (1977) A preliminary pharmacokinetic model for several
chlorinated biphenyls in rat. Drug Metab. Dispos., 5: 386-396.
LYNCH, T.R. & JOHNSON, H.E. (1982) Availability of a
hexachlorobiphenyl isomer to benthic amphipods from experimentally
contaminated natural sediments, Philadelphia, Pennsylvania,
American Society for Testing Materials, Aquatic Toxicology and
Hazard Assessment, pp. 273-287 (STP No. 76).
MAACK, L. & SONZOGNI, W.C. (1988) Analysis of polychlorobiphenyl
congeners in Wisconsin fish. Arch. environ. Contam. Toxicol.,
17: 711-719.
MAC, M.J. & SEELYE, J.G. (1981) Patterns of PCB accumulation by fry
of Lake Trout. Bull. environ. Contam. Toxicol., 27: 368-375.
MCCLURE, V.E. (1976) Transport of heavy chlorinated hydrocarbons in
the atmosphere. Environ. Sci. Technol., 10: 1223-1228.
MCCLURG, T.P. (1984) Trace metals and chlorinated hydrocarbons in
Ross seals from Antarctica. Mar. Pollut. Bull., 15: 384-389.
MCCONNELL, E.E., HASS, J.R., ALTMAN, N., & MOORE, J.A. (1979) A
spontaneous outbreak of polychlorinated biphenyl (PCB) toxicity in
Rhesus monkeys (Macaca mulatta): toxicopathology. Lab. anim.
Sci., 29: 666-673.
MCFARLAND, V.A. & CLARKE, J.U. (1989) Environmental occurrence,
abundance and potential toxicity of polychlorinated biphenyl
congeners: Considerations for a congener-specific analysis.
Environ. health Perspect., 81: 225-239.
MACKAY, D. (1989) Modeling the long-term behavior of an organic
contaminant in a large lake: Application to PCBs in Lake Ontario.
J. Great Lakes Res., 15(2): 283-297.
MCKINNEY, J.D. & SINGH, P. (1981) Structure-activity relationships
in halogenated biphenyls: unifying hypothesis for structural
specificity. Chem. biol. Interact., 33: 271-283.
MCKINNEY, J.D., CHAE, K., MCCONNELL, E.E., & BIRNBAUM, L.S. (1985)
Structure-induction versus structure-toxicity relationships for
polychlorinated biphenyls and related aromatic hydrocarbons.
Environ. Health Perspect., 60: 57-68.
MCKINNEY, J.D., KORACH, K.S., & MCLACHLAN, J.A. (1990)
Detoxification of polychlorinated biphenyls. January 27. Lancet,
335: 222-223.
MCLANE, M.A.R. & HUGHES, D.L. (1980) Reproductive success of
screech owls fed Aroclor 1248. Arch. environ. Contam. Toxicol.,
9: 661-665.
MCLEESE, D.W., METCALFE, C.D., & PEZZACK, D.S. (1980) Uptake of
PCBs from sediment by Nereis virens and Crangon septemspinosa.
Arch. environ. Contam. Toxicol., 9: 507-518.
MACLEOD, H.A., SMITH, D.C., & BLUMAN, N. (1980) Pesticide residues
in the total diet in Canada, V: 1976 to 1978. J. Food Saf.,
2: 141-164.
MCMANUS, G.B., WYMAN, K.D., PETERSON, W.T., & WURSTER, C.F. (1983)
Factors affecting the elimination of PCBs in the marine copepod
Acartia tonsa. Estuarine coastal Shelf Sci., 17: 421-430.
MCNULTY, W.P. (1985) Toxicity and fetotoxicity of TCDD, TCDF and
PCB isomers in Rhesus macaques (Macaca mulatta). Environ. Health
Perspect., 60: 77-88.
MCNULTY, W.P., BECKER, G.M., & CORY, H.T. (1980) Chronic toxicity
of 3,4,3',4'- and 2,5,2',5'-tetrachlorobiphenyls in Rhesus
macaques. Toxicol. appl. Pharmacol., 56: 182-190.
MACLEOD, K.E. (1981) Polychlorinated biphenyls in indoor air.
Environ. Sci. Technol., 15(8): 926-928.
MAFF (1986) Report of file working party on pesticide residues
(1982-1985): 16th report of the Steering Group on Food
Surveillance, London, Ministry of Agriculture, Fisheries, and Food,
Her Majesty's Stationery Office, (Food Surveillance Paper No. 16).
MAFF (1989) Migration of substances from food contact materials
into food: 26th report of the Steering Group on Food Surveillance.
Progress report of the Working Party on Chemical Contaminants from
Food, London, Ministry of Agriculture, Fisheries, and Food, Her
Majesty's Stationery Office, 58 pp (Food Surveillance Paper
No. 26).
MAHANTY, H.K. (1975) A study of the effects of polychlorinated
biphenyl (Aroclor 1242) on an aquatic plant Spirodela oligorrhiza
(Kurz) Hegelm. Bull. environ. Contam. Toxicol., 14: 558-565.
MAHANTY, H.K. & FINERAN, B.A. (1976) Effects of a polychlorinated
biphenyl (Aroclor 1242) on the ultrastructure of frond cells in the
aquatic plant Spirodela oligorrhiza (Kurz) Hegelm. N. Z. J. Bot.,
14: 13-18.
MAHANTY, H.K. & MCWHA, J.A. (1976) Sensitivity of Spirodela
oligorrhiza (Kurz) Hegelm. to a polychlorinated biphenyl (Aroclor
1242). N. Z. J. Bot., 14: 9-12.
MAKI, A.W. & JOHNSON, H.E. (1975) Effects of PCB (Aroclor 1254) and
p,p'DDT on production and survival of Daphnia magna Strauss.
Bull. environ. Contam. Toxicol., 13: 412-416.
MAKIURA, S., AOE, H., SUGIHARA, S., HIARO, K., ARAI, M., & ITO, N.
(1974) Inhibitory effect of polychlorinated biphenyls on liver
tumorigenesis in rats treated with 3'-methyl-4-dimethyl-
aminobenzene, N-2-fluorenylacetamide and diethylnitrosamine. J.
Natl Cancer Inst., 53: 1253-1257.
MALINS, D.C., MCCAIN, B.B., BROWN, D.W., VARANASI, U., KRAHN, M.M.,
MYERS, M.S., & CHAN, S.-L. (1987) Sediment-associated contaminants
and liver diseases in bottom-dwelling fish. Hydrobiologia,
149(1): 64-74.
MANCHESTER-NEESVIG, J.B. & ANDREN, A.W. (1989) Seasonal variation
in the atmospheric concentration of polychlorinated biphenyl
congeners. Environ. Sci. Technol., 23: 1138-1148.
MANSKE, D.D. & JOHNSON, R.D. (1975) Pesticide residues in total
diet samples (VIII). Pestic. monit. J., 9(2): 94-105.
MANSKE, D.D. & JOHNSON, R.D. (1977) Pesticide and other chemical
residues in total diet samples (X). Pestic. monit. J.,
10(4): 134-148.
MARCUS, J.M. & MATHEWS, T.D. (1987) Polychlorinated biphenyls in
blue crabs from South Carolina. Bull. environ. Contam. Toxicol.,
39(5): 857-862.
MARINUCCI, A.C. & BARTHA, R. (1982) Accumulation of the
polychlorinated biphenyl Aroclor 1242 from contaminated detritus
and water by the saltmarsh detritivore, Uca pugnax. Bull.
environ. Contam. Toxicol., 29: 326-333.
MARKARD, C. (1988) [Organic substances in sewage sludge - a danger
to the food chain?] Korresp. Abwasser, 35(5): 449-455 (in German).
MARKS, T.A., KIMMEL, G.L., & STAPLES, R.E. (1981) Influence of
symmetrical polychlorinated biphenyl isomers on embryo and fetal
development in mice I. Teratogenicity of 3,3',4,4',5,5'-
hexachlorobiphenyl. Toxicol. appl. Pharmacol., 61: 269-276.
MARONI, M., COLUMBI, A., CANTONI, S., FERIOLI, E., & FOA, V.
(1981a) Occupational exposure to polychlorinated biphenyls in
electrical workers. I. Environmental and blood polychlorinated
biphenyl concentrations. Br. J. ind. Med., 38(1): 49-54.
MARONI, M., COLUMBI, A., ARBOSTI, G., CANTONI, S., & FOA, V.
(1981b) Occupational exposure to polychlorinated biphenyls in
electrical workers II. Health effects. Br. J. ind. Med.,
38(1): 55-60.
MARONI, M., COLOMBI, A., FERIOLI, A., & FOA, V. (1984) Evaluation
of porphyrinogenesis and enzyme induction in workers exposed to
PCB. Med. Lav., 75(3): 188-199.
MARTINEAU, D., BELAND, P., DESJARDINS, C., & LAGACE, A. (1987)
Levels of organochlorine chemicals in tissues of beluga whales
(Delphinapterus leucas) from the St. Lawrence Estuary, Quebec,
Canada. Arch. environ. Contam. Toxicol., 16: 137-147.
MASSACHUSETTS DEPARTMENT OF PUBLIC HEALTH (1987) The greater New
Bedford PCB health effects study 1984-1987, Boston, Massachusetts,
Massachusetts Department of Public Health.
MASUDA, Y., KAGAWA, R., & KURATSUNE, M. (1972) Polychlorinated
biphenyls in carbonless copying paper. Nature (Lond.), 237: 41-42.
MASUDA, Y., KAGAWA, R., & KURATSUNE, M. (1974a) [Polychlorinated
biphenyls in Yusho patients and ordinary persons.] Fukuoka Acta
med., 65: 17-24 (in Japanese).
MASUDA, Y., KAGAWA, R., SHIMAMURA, K., TAKADA, M., & KURATSUNE, M.
(1974b) [Polychlorinated biphenyls in the blood of Yusho patients
and ordinary persons.] Fukuoka Acta. med., 65: 25-27 (in Japanese).
MASUDA, Y., KAGAWA, R., KUROKI, H., KURATSUNE, M., YOSHIMURA, T.,
TAKI, I., KUSUDA, M., YAMASHITA, F., & HAYASHI, M. (1978a) Transfer
of polychlorinated biphenyls from mothers to foetuses and infants.
Bull. environ. Contam. Toxicol., 16: 543-546.
MASUDA, Y., KAGAWA, R., TOKUDOME, S., & KURATSUNE, M. (1978b)
Transfer of polychlorinated biphenyls to the foetuses and offspring
of mice. Toxicology, 6: 331-340.
MASUDA, Y., KAGAWA, R., KUROKI, H., TOKUDOME, S. & KURATSUNE, M.
(1979) Transfer of various polychlorinated biphenyls to the fetuses
and offspring of mice. Food Cosmet. Toxicol., 17(6): 623-627.
MASUDA, Y., KUROKI, H., HARAGUCHI, K., & NAGAYAMA, J. (1985) PCB
and PCDF congeners in the blood and tissues of Yusho and Yu-Cheng
patients. Environ. health Perspect., 59: 53-58.
MATTHEWS, H.B. & ANDERSON, M.W. (1975a) Effect of chlorination on
the distribution and excretion of polychlorinated biphenyls. Drug
Metab. Dispos., 3: 371-380.
MATTHEWS, H.B. & ANDERSON, M.W. (1975b) The distribution and
excretion of 2,4,5,2',5'-pentachlorobiphenyl in the rat. Drug
Metab. Dispos., 3: 211-219.
MATTHEWS, H.B. & ANDERSON, M.W. (1976) PCB chlorination versus PCB
distribution and excretion. In: Proceedings of the National
Conference on Polychlorinated Biphenyls, Chicago, 19-21 November
1975, Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances, pp. 50-56 (EPA-560/6-75-004).
MATTHEWS, H.B. & DEDRICK, R.L. (1984) Pharmacokinetics of PCBs.
Annu. Rev. Pharmacol. Toxicol., 24: 85-103.
MATTHEWS, H.B. & TUEY, D.B. (1980) The effect of chlorine position
on the distribution and excretion of four hexachlorobiphenyl
isomers. Toxicol. appl. Pharmacol., 53: 377-388.
MATTSSON, R., MATTSSON, A., KIHLSTRÖM, J.E., & LINDAHL-KIESSLING,
K. (1981) Effects of a hexachlorinated biphenyl on lymphoid organs
and resorption of foetuses in pregnant mice. Arch. environ. Contam.
Toxicol., 10: 281-288.
MAUCK, W.L., MEHRLE, P.M., & MAYER, F.L. (1978) Effects of the
polychlorinated biphenyl Aroclor 1254 on growth, survival, and bone
development in brook trout (Salvelinus fontinalis). J. Fish Res.
Board Can., 35: 1084-1088.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Washington, DC, US Department of Commerce,
National Technical Information Service, 274 pp ((NTIS)
PB87-188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior,
Fish & Wildlife Service, pp. 506-553 (Resource Publication
No. 160).
MAYER, F.L, MEHRLE, P.M., & SANDERS, H.O. (1977) Residue dynamics
and biological effects of polychlorinated biphenyls in aquatic
organisms. Arch. environ. Contam. Toxicol., 5: 501-511.
MEHLMAN, M.A., YIN, L., & NIELSEN, R.C. (1974) Metabolic changes in
"frozen-clamped" livers of rats caused by ingestion of
polychlorinated biphenyls. Toxicol. appl. Pharmacol., 27: 300-307.
MEIER, P.G. & REDISKE, R.R. (1984) Oil and PCB interactions on the
uptake and excretion in midges. Bull. environ. Contam. Toxicol.,
33: 223-232.
MEIGS, J.W., ALBOM, J.J., & KARTIN, B.L. (1954) Chloracne from an
unusual exposure to Aroclor. J. Am. Med. Assoc., 154: 1417-1418.
MELVÅS, B. & BRANDT, I. (1973) The distribution and metabolism of
labelled polychlorinated biphenyls in mice and quails. In:
Proceedings of the Polychlorinated Biphenyls Conference II,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 87-90 (Publication No. 4E).
MENDOZA-FIGUEROA, T., LOPEZ-REVILLA, R., & VILLA-TREVINO, S. (1985)
Aroclor 1254 increases the genotoxicity of several carcinogens to
liver primary cell cultures. J. Toxicol. environ. Health,
15: 245-254.
MERKENS, L.S. & KINTER, W.B. (1971) Acute toxicity of a mixture of
polychlorinated biphenyls (Aroclor 1221) and DDT in a marine
teleost (Fundulus heteroclitus) and effect on serum osmolality,
Na+ and K+. Bull. Mt Desert Isl. biol. Lab., 11: 64-68.
MERSON, M.H. & KIRKPATRICK, R.L. (1976) Reproductive performance of
Captive White-footed mice fed a PCB. Bull. environ. Contam.
Toxicol., 16: 392-398.
MES, J. (1987) Polychlorobiphenyl in children's blood. Environ.
Res., 44: 213-220.
MES, J. & MARCHAND, L. (1987) Comparison of some specific
polychlorinated biphenyl isomers in human and monkey milk. Bull.
environ. Contam. Toxicol., 39: 736-742.
MES, J., COFFIN, D.E., & CAMPBELL, D. (1974) Polychlorinated
biphenyl and organochlorine pesticide residues in Canadian chicken
eggs. Pestic. monit. J., 8: 8-11.
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 28: 97-104.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine pesticides in
milk and blood of Canadian women during lactation. Arch. environ.
Contam. Toxicol., 13: 217-223.
MES, J., DAVIES, D.J., TURTON, D., & SUN, W.-F. (1986) Levels and
trends of chlorinated hydrocarbon contaminants in the breast milk
of Canadian women. Food Addit. Contam., 3(4): 313-322.
MES, J., ARNOLD, D.L., BRYCE, F., DAVIES, D.J., & KARPINSKI, K.
(1989a) The effect of long-term feeding of Aroclor 1254 to female
Rhesus monkeys on their polychlorinated biphenyl tissue levels.
Arch. environ. Contam. Toxicol., 18: 858-865.
MES, J., NEWSOME, W.H., & CONACHER, H.B.S. (1989b) Determination of
some specific isomers of polychlorinated biphenyl congeners in
fatty foods of the Canadian diet. Food Addit. Contam.,
6(3): 365-375.
MICHAELS, R.A., ROWLAND, G., & WURSTER, C.F. (1982) Polychlorinated
biphenyls (PCB) inhibit photosynthesis per cell in the marine
diatom Thalassiosira pseudonana. Environ. Pollut., 27: 9-14.
MILLER, D.L. & OGILVIE, D.M. (1975) Temperature selection in brook
trout (Salvelinus fontinalis) following exposure to DDT, PCB, or
phenol. Bull. environ. Contam. Toxicol., 14: 545-551.
MILLER, M.M., GHODBANE, S., WASIK, S.P., TEWARI, Y.B., & MARTIRE,
D.E. (1984) Aqueous solubilities, octanol/water partition
coefficients, and entropies of melting of chlorinated benzenes and
biphenyls. J. chem. Eng. Data, 29: 184-190.
MILLING, A., MULLER, W.F., COULSTON, F., & KORTE, F. (1979)
Comparative metabolism of 2,2'-dichlorobiphenyl-14C in mice, rats
and Rhesus monkeys after a single oral application. Chemosphere,
1: 15-19.
MINEAU, P., FOX, G.A., NORSTROM, R.J., WESELOH, D.V., HALLETT,
D.J., & ELLENTON, J.A. (1984) Using the herring gull to monitor
levels and effects of organochlorine contamination in the Canadian
Great Lakes. In: Nriagu, J.O., Nriagu, J.O., & Simmons, M.S., ed.
Toxic contaminants in the Great Lakes, New York, John Wiley and
Sons, pp. 425-452.
MIO, T. & SUMINO, K. (1985) Mechanism of biosynthesis of
methylsulfones from PCBs and related compounds. Environ. Health
Perspect., 59: 129-135.
MIO, T., SUMINO K., & MIZUTANI, T. (1976) Sulfur-containing
metabolites of 2,5,2',5'-tetrachlorobiphenyl, a major component of
commercial PCBs. Chem. pharm. Bull., 24: 1958-1960.
MIRANDA, C.L., HENDERSON, M.C., WANG, J.L., NAKAUE, H.S., & BUHLER,
D.R. (1987) Effects of polychlorinated biphenyls on porphyrin
synthesis and cytochrome P-450-dependent mono-oxygenase in small
intestine and liver of Japanese quail. J. Toxicol. environ. Health,
20: 27-35.
MIYATA, H., FUKUSHIMA, S., KASHIMOTO, T., & HUNITA, N. (1985) PCBs,
PCQs, and PCDFs in tissues of Yusho and Yu-Cheng patients. Environ.
Health Perspect., 59: 67-72.
MIYATA, H., TAKAYAMA, K., OGAKI, J., KASHIMOTO, T., & FUKUSHIMA, S.
(1987) Polychlorinated dibenzo- p-dioxins in blue mussel from
marine coastal water in Japan. Bull. environ. Contam. Toxicol.,
39(5): 877-883.
MIYAZAKI, A., HOTTA, T., KATAYAMA, J., & KIMURA, Y. (1975)
Absorption and translocation of PCB into crops. Bull. Osaka agric.
Res. Cent., 12: 135-142.
MIZUNOYA, Y., TANIGUCHI, S., KUSUMOTO, K., MORITA, S., YAMADA, A.,
BABA, T., & OGAKI, S. (1974) [Effects of polychlorinated biphenyls
on fetuses and offspring in rats.] J. Food Hyg. Soc. Jpn,
15: 252-260 (in Japanese).
MIZUTANI, T., HIDAKA, K., OHE, T., & MATSUMOTO, M. (1977) A
comparative study on accumulation and elimination of
tetrachlorobiphenyl isomers in mice. Bull. environ. Contam.
Toxicol., 18: 452-461.
MOILANEN, R., PYYSALO, H., WICKSTROM, K., & LINKO, R. (1982) Time
trends of chlordane, DDT, and PCB concentrations in pike (Esox
lucius) and Baltic herring (Clupea harengus) in the Turku
Archipelago, Northern Baltic Sea for the period 1971-1982. Bull.
environ. Contam. Toxicol., 29: 334-340.
MOKSNES, M.T. & NORHEIM, G. (1986) Levels of chlorinated
hydrocarbons and composition of PCB in herring gull Larus
argentatus eggs collected in Norway in 1969 compared to 1979-81.
Environ. Pollut., B11: 109-116.
MOORE, S.A. & HARRISS, R.C. (1972) Effects of polychlorinated
biphenyl on marine phytoplankton communities. Nature (Lond.),
240: 356-357.
MOORE, S.A. & HARRISS, R.C. (1974) Differential sensitivity to PCB
by phytoplankton. Mar. Pollut. Bull., 5: 174-176.
MORGAN, R.W., WARD, J.M., & HARTMAN, P.E. (1981) Aroclor
1254-induced intestinal metaplasia and adenocarcinoma in the
glandular stomach of F344 rats. Cancer Res., 41: 5052-5059.
MORIARTY, F. (1969) The effects of polychlorobiphenyls on
Chorthippus brunneus (Saltatoria: Acrididae). Entomol. Exp.
Appl., 12: 206-210.
MORITA, M., NAKAGAVA J., & RAPPE, C. (1978) Polychlorinated
dibenzofuran (PCDF) formation from PCB mixture by heat and oxygen.
Bull. environ. Contam. Toxicol., 19: 665-670.
MORSE, R.A., CULLINEY, T.W., GUTENMANN, W.H., LITTMAN, C.B., &
LISK, D.J. (1987) Polychlorinated biphenyls in honey bees. Bull.
environ. Contam. Toxicol., 38: 271-276.
MOSELEY, C.L., GERACI, C.L., & BURG, J. (1982) Polychlorinated
biphenyl exposure in transformer maintenance operations. Am. Ind.
Hyg. Assoc. J., 43: 170-174.
MOSSER, J.L., TENG, T., FISHERR, N.S., & WURSTER, C.F. (1972)
Polychlorinated biphenyls: Toxicity to certain phytoplankters.
Science, 175: 191-192.
MOSSER, J.L., TENG, T., WALTHER, W.G., & WURSTER, C.F. (1974)
Interactions of PCBs, DDT, and DDE in a marine diatom. Bull.
environ. Contam. Toxicol., 12: 665-668.
MOZA, P., WEISBERGER, I., KLEIN, W., & KORTE, F. (1974) Metabolism
of 2,2'-dichlorobiphenyl-14C in two plant-water-soil-systems. Bull.
environ. Contam. Toxicol., 12: 541-546.
MOZA, P., WEISBERGER, I., & KLEIN, W. (1976a) Fate of
2,2'-dichlorobiphenyl-14C in carrots, sugar beet, and soil under
outdoor conditions. J. agric. food Chem., 24: 881-885.
MOZA, P., KILZER, L., WEISGERBER, I., & KLEIN, W. (1976b)
Contribution to ecological chemistry CXV. Metabolism of
2,5,4'-trichlorobiphenyl-14C and 2,4,6,2',4'-pentachloro-
biphenyl-14C in the marsh plant Veronica beccabunga. Bull.
environ. Contam. Toxicol., 16: 454-463.
MOZA, P., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1979a) Studies
with 2,4,5-trichlorobiphenyl-14C and 2,2,4,4,6-penta-
chlorobiphenyl-14C in carrots, sugar, beet, and soil. J. agric.
food Chem., 27: 1120-1124.
MOZA, P.N., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1979b) Long-term
uptake of lower chlorinated biphenyls and their conversion products
by spruce trees (Picea abies) from soil treated with sewage
sludge. Chemosphere, 8: 373-375.
MROZEK, E. & LEIDY, R.B. (1981) Investigation of selective uptake
of polychlorinated biphenyls by Spartina alterniflora Loisel.
Bull. environ. Contam. Toxicol., 27: 481-488.
MROZEK, E., SENECA, E.D., & HOBBS, L.L. (1982) Polychlorinated
biphenyl uptake and translation by Spartina alterniflora Loisel.
Water Air Soil Pollut., 17: 3-15.
MROZEK, E., QUEEN, W.H., & HOBBS, L.L. (1983) Effects of
polychlorinated biphenyls on growth of Spartina alterniflora
Loisel. Environ. exp. Bot., 23: 285-292.
MUEHLEBACK, S. & BICKEL, M.H. (1981) Pharmacokinetics in rats of
2,4,5,2',4',5'-hexachlorobiphenyl an unmetabolisable lipophilic
model compound. Xenobiotica, 11: 249-259.
MUIR, D.C.G., NORSTROM, R.J., & SIMON, M. (1988) Organochlorine
contaminants in Arctic marine food-chains: Accumulation of specific
polychlorinated biphenyls and chlordane-related compounds. Environ.
Sci. Technol., 22: 1071-1077.
MULHERN, B.M., CROMARTIE, E., REICHEL, W.L., & BELISLE, A.A. (1971)
Semiquantitative determination of polychlorinated biphenyls in
tissue samples by thin layer chromatography. J. Assoc. Off. Agric.
Chem., 54: 548-550.
MULLER, W.F., HOBSON, W., FULLER, G.B., KNAUF, W., COULSTON, F., &
KORTE, F. (1978) Endocrine effects of chlorinated hydrocarbons in
Rhesus monkeys. Ecotoxicol. Environ. Saf., 2: 161-172.
MULLIN, M.D., POCHINI, C.M., MCCRINDLE, S., ROMKES, M., SAFE, S.H.,
& SAFE, L.M. (1984) High-resolution PCB analysis: Synthesis and
chromatographic properties of all 209 PCB congeners. Environ. Sci.
Technol., 18: 468-476.
MURADO, M.A., TEJEDOR, M.C., & BALUJA, G. (1976) Interactions
between polychlorinated biphenyls (PCBs) and soil microfungi.
Effects of Aroclor 1254 and other PCBs on Aspergillus flavus
cultures. Bull. environ. Contam. Toxicol., 15: 768-774.
MURAI, Y. & KUROIWA, Y. (1971) Peripheral neuropathy in
chlorobiphenyl poisoning. Neurology, 21: 1173-1176.
MURPHY, T.J. (1984) Atmospheric inputs of chlorinated hydrocarbons
to the Great Lakes. In: Nriagu, J.O., Nriagu, J.O., & Simmons,
M.S., ed. Toxic contaminants in the Great Lakes, New York, John
Wiley and Sons, pp. 53-79.
MURPHY, T.J., FORMANSKI, L.J., BROWNAWELL, B., & MEYER, J.A. (1985)
Polychlorinated biphenyl emissions to the atmosphere in the Great
Lakes region. Municipal landfills and incinerators. Environ. Sci.
Technol., 19(10): 942-946.
MUSSALO-RAUHAMAA, H., PYYSALO, H., & MOILANEN, R. (1984) Influence
of diet and other factors on the levels of organochlorine compounds
in human adipose tissue in Finland. J. Toxicol. environ. Health,
13: 689-704.
MUSSALO-RAUHAMAA, H., PYYSALO, H., & ANTERVO, K. (1988) Relation
between the content of organochlorine compounds in Finnish human
milk and characteristics of the mothers. J. Toxicol. environ.
Health, 25: 1-19.
NAGAI, J., FURUKAWA, M., YAE, Y., & IKEDA, Y. (1969)
[Clinicochemical investigation of chlorobiphenyls poisoning.
Especially on the serum lipid analysis of the patients.] Fukuoka
Acta med., 60: 475-488 (in Japanese).
NAGASAKI, H., TOMII, S., MEGA, T., SUGIHARA, S., MIYATA, Y., & ITO,
N. (1974) [Analysis of various factors on liver carcinogenesis in
mice induced by benzene hexachloride (BHC) and technical
polychlorinated biphenyls (PCBs).] J. Nara Med. Assoc., 25: 635-648
(in Japanese).
NAGASAKI, H., KAWABATA, H., MIYATA, Y., INOUE, K., AOE, H., & ITO,
N. (1975) Effects of various factors on induction of liver tumours
in animals by the alpha-isomer of benzenehexachloride. Gann,
66: 185-191.
NAGAYAMA, J. (1975) Chlorinated dibenzofurans in Kanechlors and
rice oils used by patients with Yusho. Fukuoka Acta med., 66: 593.
NAGAYAMA, L., KURATSUNE, M., & MASUDA, Y. (1976) Determination of
chlorinated dibenzofurans in Kanechlors and "Yusho oil". Bull.
environ. Contam. Toxicol., 15(1): 9-13.
NAGAYAMA, J., KURATSUNE, M., & MASUDA, Y. (1981) Formation of
polychlorinated dibenzofurans by heating polychlorinated biphenyls.
Fukuoka Acta med., 72: 136-141.
NAKANISHI, Y., SHIGEMATSU, N., KURITA, Y., MATSUBA, K., KANEGAE,
H., ISHIMURA, S., & KAWAZOE, Y. (1985) Respiratory involvement and
immune status in Yusho patients. Environ. health Perspect.,
59: 31-36.
NARBONNE, J.F. (1979) Determination of the "no effect level" of
Phenoclor DP6 on five microsomal parameters in rat livers.
Toxicology, 14: 91-93.
NARBONNE, J.F. (1980) Time course of induction of microsomal
enzymes following dietary administration of a polychlorinated
biphenyl (Phenoclor DP6). Toxicol. appl. Pharmacol., 56: 1-7.
NARBONNE, J.F., BOURDICHON, M., GALLIS, J.L., & DAUBEZE, M. (1978)
Polychlorinated biphenyls: effect of diet level on ATPase activity
in rats. Bull. environ. Contam. Toxicol., 20: 184-190.
NAS (1979) Polychlorinated biphenyls, Washington, DC, National
Academy of Sciences, 182 pp.
NAU-RITTER, G.M., WURSTER, C.F., & ROWLAND, R.G. (1982)
Partitioning of [14C]PCB between water and particulates with
various organic contents. Water Res., 16: 1615-1618.
NCI (1975) Third national cancer survey, Bethesda, Maryland,
National Cancer Institute.
NCI (1978) Bioassay of Aroclor (trademark) 1254 for possible
carcinogenicity (CAS 27323-18-8). National Cancer Institute
(Carcinogenesis Technical Report Series No. 38 - DHEW Publication
(NIH) 78-838).
NEBEKER, A.V. & PUGLISI, F.A. (1974) Effect of polychlorinated
biphenyls (PCBs) on survival and reproduction of Daphnia,
Gammarus, and Tanytarsus. Trans Am. Fish. Soc., 103: 722-728.
NEBEKER, A.V., PUGLISI, F.A., & DEFOE, D.L. (1974) Effect of
polychlorinated biphenyl compounds on survival and reproduction of
the fathead minnow and flagfish. Trans Am. Fish. Soc.,
103: 562-568.
NEFF, J.M. & GIAM, C.S. (1977) Effects of Aroclor 1016 and Halowax
1099 on juvenile horseshoe crabs Limulus polyphemus. In:
Vernberg, F.J., Calabrese, A., Thurberg, F.P., & Vernberg, W.B.,
ed. Physiological responses of marine biota to pollution, New York,
London, Academic Press, pp. 21-35.
NELSON, J.A. (1974) Effects of dichlorodiphenyltrichloroethane
(DDT) analogs and polychlorinated biphenyl (PCB) on 17
beta-[3H]estradiol binding to rat uterine receptor. Biochem.
Pharmacol., 23: 447-451.
NELSON, N.N., HAMMON, P.B., NISBET, I.C.T, SAROFIM, A.F., & DRURY,
W.H. (1972) Polychlorinated biphenyls - environmental impact.
Environ. Res., 5: 249-362.
NESNOW, S., LEAVITT, S., GARLAND, H., VAUGHAN, T.O., HYATT, B.,
MONTGOMERY, L., & CUDAK, C. (1981) Identification of cocarcinogens
and their potential mechanisms of action using C3H10T1/2CL8 mouse
embryo fibroblasts. Can. Res., 41: 3071-3076.
NESTEL, H. & BUDD, J. (1975) Chronic oral exposure of rainbow trout
(Salmo gairdneri) to a polychlorinated biphenyl (Aroclor 1254):
Pathological effects. Can. J. comp. Med., 39: 208-215.
NEWTON, I. (1979) The ecology of raptors, Berkhamsted, United
Kingdom, T. & A.D. Poyser, 399 pp.
NEWTON, I. & BOGAN, J. (1978) The role of different organo-chlorine
compounds in the breeding of British sparrowhawks. J. appl. Ecol.,
15: 105-116.
NEWTON, I. & HAAS, M.B. (in press) Long-term trends in
organochlorine and mercury residues in some raptorial and
fish-eating birds in Britain. Environ. Pollut.
NEWTON, I., BOGAN, J., MEEK, E., & LITTLE, B. (1982) Organochlorine
compounds and shell-thinning in British merlins Falco columbarius.
Ibis, 124: 328-335.
NEWTON, I., BOGAN, J.A., & ROTHERY, P. (1986) Trends and effects of
organochlorine compounds in sparrowhawk eggs. J. appl. Ecol.,
23: 461-478.
NEWTON, I., BOGAN, J.A., & HAAS, M.B. (1988) Organochlorine and
mercury in the eggs of British peregrine Falco peregrinus. Ibis,
131: 355-376.
NIESSEN, K.H., RAMOLLA, J., BINDER, M., BRÜGMANN, G., & HOFMANN, U.
(1984) Chlorinated hydrocarbons in adipose tissue of infants and
toddlers: Inventory and studies on their association with intake of
mother's milk. Eur. J. Pediatr., 142: 238-243.
NIEHS (NATIONAL INSTITUTE OF ENVIRONMENTAL HEALTH SCIENCES) (1985)
Potential health effects of polychlorinated biphenyls and related
persistent halogenated hydrocarbons: US Symposium, 12-14 September
1983. Environ. health Perspect., 60: 1-431.
NIIMI, A.J. & OLIVER, B.G. (1983) Biological half-lives of
polychlorinated biphenyl (PCB) congeners in whole fish and muscle
of rainbow trout (Salmo gairdneri). Can. J. Fish Aquat. Sci.,
40: 1388-1394.
NIIMI, A.J. & OLIVER, B.G. (1989a) Assessment of relative toxicity
of chlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in
Lake Ontario salmonids to mammalian systems using toxic equivalent
factors (TEF). Chemosphere, 18(7-8): 1413-1423.
NIIMI, A.J. & OLIVER, B.G. (1989b) Distribution of polychlorinated
biphenyl congeners and other halocarbons in whole fish and muscle
among Lake Ontario salmonids. Environ. Sci. Technol., 23: 83-88.
NILSEN, O.G. & TOFTGARD, R. (1981) Effects of polychlorinated
terphenyls and paraffins on rat liver microsomal cytochrome P-450
and in vitro metabolic activities. Arch. Toxicol., 47: 1-11.
NILSSON, B. & RAMEL, C. (1974) Genetic test on Drosophila
melanogaster with polychlorinated biphenyls (PCB). Hereditas,
77: 319-322.
NIMMO, D.R. & BAHNER, L.H. (1974) Some physiological consequences
of polychlorinated biphenyl- and salinity-stress in penaeid shrimp.
In: Vernberg, F.J. & Vernberg, W.B., ed. Pollution and physiology
of marine organisms, New York, London, Academic Press, pp. 427-443.
NIMMO, D.W.R. & BAHNER, L.H. (1976) Metals, pesticides and PCBs:
Toxicities to shrimp singly and in combination. In: Estuarine
processes. Proceedings of the 3rd International Estuarine Research
Conference, Galveston, Texas, October 1975, Estuarine Research
Federation, Vol. 1., pp. 523-532.
NIMMO, D.R., WILSON, P.D., BLACKMAN, R.R., & WILSON, A.J. (1971a)
Polychlorinated biphenyl absorbed from sediments by fiddler crabs
and pink shrimp. Nature, (Lond.), 231: 50-52.
NIMMO, D.R., BLACKMAN, R.R., WILSON, A.J., & FORESTER, J. (1971b)
Toxicity and distribution of Aroclor 1254 in the pink shrimp
Penaeus duorarum. Mar. Biol., 11: 191-197.
NIMMO, D.R., FORESTER, J., HEITMULLER, P.T., & COOK, G.H. (1974)
Accumulation of Aroclor 1254 in grass shrimp (Palaemonetes pugio)
in laboratory and field exposures. Bull. environ. Contam. Toxicol.,
11: 303-308.
NIMMO, D.R., HANSEN, D.J., COUCH, J.A., COOLEY, N.R., PARRISH,
P.R., & LOWE, J.I. (1975) Toxicity of Aroclor 1254 and its
physiological activity in several estuarine organisms. Arch.
environ. Contam. Toxicol., 3: 22-39.
NIOSH (1977) Criteria for a recommended standard. Occupational
exposure to polychlorinated biphenyls (PCBs), Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 224 pp
(NIOSH Publication No. 77-225).
NIOSH (1987) NIOSH manual of analytical methods: Polychlorinated
biphenyls - Method 5503, Cincinnati, Ohio, National Institute for
Occupational Safety and Health, pp. 5503-5503/4.
NISBET, I.C.T. & SAROFIM, A.F. (1972) Rates and routes of transport
of PCBs in the environment. Environ. health Perspect., 1: 21-38.
NISHIHARA, Y. (1983) Effects of polychlorinated biphenyls
(Kanechlor-400) on isolated rat liver mitochondria. Arch. environ.
Contam. Toxicol., 12: 517-522.
NISHIHARA, Y. (1985) Comparative study of the effects of biphenyl
and Kanechlor 400 on the respiratory and energy linked activities
of rat liver mitochondria. Br. J. ind. Med., 42: 128-132.
NISHIMOTO, T., UEDAM, M., TAUL, S., & CHIKAZAWA, K. (1972a)
[Organochlorine pesticide residues and PCB in breast milk.] Igaku
Ayumi, 82: 574-575 (in Japanese).
NISHIMOTO, T., UETA, M., TAUL, S., CHIKAZAURA, K., NISHIUCHI, I.,
& KONDO, K. (1972b) [Deposition of organochlorine pesticide
residues and PCB in human body fat.] Igaku Ayumi, 82: 515-516
(in Japanese).
NISHIZUMI, M. (1970) Light and electron microscope study of
chlorobiphenyl poisoning in mouse and monkey liver. Arch. environ.
Health, 21: 620-632.
NISHIZUMI, M. (1976) Enhancement of diethylnitrosomine
hepatocarcinogenesis in rats by exposure to polychlorinated
biphenyls or phenobarbital. Can. Lett., 2: 11-16.
NISHIZUMI, M. (1980) Reduction of diethylnitrosamine-induced
hepatoma in rats exposed to polychlorinated biphenyls through their
dams. Gann, 71(6): 910-912.
NISHIZUMI, M. (1985) Effect of PCBs on DMH-induced colon
tumorigenesis in rats. Fukuoka Acta med., 76: 204-207.
NISSEN, T.V. (1973) Stability of PCB in soil. Tidsskr. Planteavl,
77: 533-539.
NORBACK, D.H., & WELTMAN, R.H. (1985) Polychlorinated biphenyl
induction of hepatocellular carcinoma in the Sprague-Dawley rat.
Environ. health Perspect., 60: 97-105.
NORBACK, D.H., & ALLEN, J.R. (1972) Chlorinated aromatic
hydrocarbon induced modifications of the hepatic endoplasmic
reticulum: concentric membrane arrays. Environ. health Perspect.,
1: 137-143.
NORBACK, D.H., SEYMOUR, J.L., KNIERIEM, K.M., PETERSON, R.E., &
ALLEN, J.R. (1976) Biliary metabolites of 2,5,2',5'-tetrachloro-
biphenyl in rat. Res. Commun. chem. Pathol. Pharmacol., 14: 527-33.
NORBACK, D.H. MACK, E., BLOMQUIST, K.A., & ALLEN, J.R. (1978)
Metabolic study of 2,4,5,2',4',5'-hexachlorobiphenyl in rhesus
monkeys. Toxicol. appl. Pharmacol., 45: 331 (Abstract).
NOREN, K. (1983) Levels of organochlorine contaminants in human
milk from different parts of Sweden. Ambio, 12(1): 44-46.
NOREN, K. (1988) Changes in the levels of organochlorine
pesticides, polychlorinated biphenyls, dibenzo- p-dioxins and
dibenzofurans in human milk from Stockholm, 1972-1985. Chemosphere,
17(1): 39-49.
NOREN, K. & WESTOO, G. (1968) Determination of some chlorinated
pesticides in vegetable oils, margarine, butter, milk, eggs, meat
and fish by gas chromatography and thin-layer chromatography. Acta
chem. Scand., 22: 2289-2293.
NOREN, K., LUNDEN, A., SJOVALL, J., & BERGMAN, A. (in press)
Coplanar polychlorinated biphenyls in Swedish human milk.
Chemosphere.
NORSTROM, R.J., SIMON, M., MUIR, D.C.G., & SCHWEINSBURG, R.E.
(1988) Organochlorine contaminants in arctic marine food chains:
Identification, geographical distribution, and temporal trends in
polar bears. Environ. Sci. Technol., 22: 1063-1071.
OATMAN, L. & ROY, R. (1986) Surface and indoor air levels of
polychlorinated biphenyls in public buildings. Bull. environ.
Contam. Toxicol., 37: 461-466.
O'CONNORS, H.B., WURSTER, C.F., POWERS, C.D., BIGGS, D.C., &
ROWLAND, R.G. (1978) Polychlorinated biphenyls may alter trophic
pathways by reducing phytoplankton size and production. Science,
201: 737-740.
OECD (1982) Report on the implementation by member countries of the
decision by the Council on the Protection of the Environment by
control of polychlorinated biphenyls, Paris, Organisation of
Economic Co-operation and Development (ENV/CHEM/81.2).
OEHME, M., MANO, S., & MIKALSEN, A. (1987) Formation and presence
of polychlorinated and polycyclic compounds in the emissions of
small and large scale municipal waste incinerators. Chemosphere,
16(1): 143-153.
OESTERLE, D. & DEML, E. (1983) Promoting effects of polychlorinated
biphenyls on development of enzyme-altered islands in livers of
adult and weaning rats. J. Cancer Res. clin. Oncol., 105: 141-147.
OGISO, M., TOYOTA, I., IDO, Y., & INAGAKI, I. (1976) [Behavior of
14C-PCB in flooded soils II.] Aichi-Ken Nogyo Sogo Shikenjo Kenkyu
Hokoku, 8: 109-111 (in Japanese).
OHNISHI, Y. & KOHNO, T. (1979) Polychlorinated biphenyls poisoning
in monkey eye. Invest. Ophthalmol. visual Sci., 18: 981-984.
OISHI, S. & HIRAGA, K. (1980) Effect of polychlorinated biphenyl,
dibenzofuran and dibenzo- p-dioxin in the susceptibility of male
mice to endotoxin. J. environ. Sci. Health, B15(1): 77-85.
O'KEEFE, P.W., SILKWORTH, J.B., GIERTHY, J.F., SMITH, R.M.,
DECAPRIO, A.P., TURNER, J.N., EADON, G., HILKER, D.R., ALDOUS,
K.M., KAMINSKY, L.S., & COLLINS, D.N. (1985) Chemical and
biological investigation of a transformer accident at Binghamton,
NY. Environ. health Perspect., 60: 201-209.
OKLA, L. & LARSSON, P. (1987) Day-night differences in
volatilization rates of polychlorinated biphenyls from water to
air. Environ. Toxicol. Chem., 6: 659-662.
OKUMURA, M. (1984) Past and current medical states of Yusho
patients. Am. J. ind. Med., 5: 13-18.
OKUMURA, M. & KATSUKI, S. (1969) [Clinical observation on Yusho
(chlorobiphenyls poisoning).] Fukuoka Acta med., 60: 440-446
(in Japanese).
OKUMURA, M., MASUDA, Y., & NAKAMUTA, S. (1974) [Correlation between
blood PCB and serum glyceride levels in patients with PCB
poisoning.] Fukuoka Acta med., 65: 84-87 (in Japanese).
OLLING, C.C.Y. (1984) [PCB's in infant foods and other dairy
products.] Jeugdgezondheidszorg, 16(6): 91-93 (in Dutch).
OLOFFS, P.C., ALBRIGHT, L.J., SZETO, S.Y., & LAU, J. (1973) Factors
affecting the behavior of five chlorinated hydrocarbons in two
natural waters and their sediments. J. Fish Res. Board Can.,
30: 1619-1923.
OLOFSSON, S. & LINDAHL, P.E. (1979) Decreased fitness of cod
(Gadus morrhua L.) from polluted waters. Mar. environ. Res.,
2: 33-45.
OLSSON, M., KIHLSTROM, J.E., JENSEN, S., & ORBERG, J. (1979)
Cadmium and mercury concentrations in mink (Mustela vison) after
exposure to PCBs. Ambio, 8: 25.
OPPERHUIZEN, A., BENECKE, J.I., & PARSONS, J.R. (1987) Comment on
"Aqueous solubilities of six polychlorinated biphenyl congeners at
four temperatures". Environ. Sci. Technol., 21: 925-926.
OPPERHUIZEN, A., GOBAS, F.A.P.C., & VAN DER STEEN, J.M.D. (1988)
Aqueous solubility of polychlorinated biphenyls related to
molecular structure. Environ. Sci. Technol., 22: 638-646.
ORBERG, J. (1978) Effects of pure chlorobiphenyls (2,4',5-
trichlorobiphenyl and 2,2',4,4',5,5'-hexachlorobiphenyl) on the
reproductive capacity in female mice. Acta pharmacol. toxicol.,
42: 323-327.
ORBERG, J. & INGVAST, C. (1977) Effects of pure chlorobiphenyls
(2,4',5-trichlorobiphenyl or 2,2',4,4',5,5'-hexachlorobiphenyl) on
the disappearance of 14C from the blood plasma after intravenous
injection of 4-14C-progesterone and on the hepatic drug
metabolizing system in the female rat. Acta pharmacol. toxicol.,
41: 11-17.
ORBERG, J. & KIHLSTROM, J.E. (1973) Effects of long-term feeding of
polychlorinated biphenyls (PCB, Clophen A60) on the length of the
oestrus cycle and the frequency of implanted ova in the mouse.
Environ. Res., 6: 176-179.
ORBERG, J. & LUNDBERG, C. (1974) Some effects of DDT and PCB on the
hormonal system in the male mouse. Environ. Physiol. Biochem.,
4: 116-120.
O'SHEA, S.T.J., BROWNELL, R.L., CLARK, D.R., WALKER, W.A., GAY,
M.L., & LAMONT, T.G. (1980) Fish, wildlife, and estuaries.
Organochlorine pollutants in small cetaceans from the Pacific and
South Atlantic Oceans, November 1968-June 1976. Pestic. monit. J.,
14: 35-46.
OTA (1979) Environmental contaminants in food, Washington, DC,
Office of Technology Assessment (OTA/F-103).
OUW, H.K., SIMPSON, G.R., & SIYALI, D.S. (1976) Use and health
effects of Aroclor 1242, a PCB in an electrical industry. Arch.
environ. Health, 31: 189-196.
PAASIVIRTA, J. & LINKO, R. (1980) Environmental toxins in Finnish
wildlife. A study on time trends of residue contents in fish during
1973-1978. Chemosphere, 9: 643-661.
PAL, D., WEBER, J.B., & OVERCASH, M.R. (1980) Fate of
polychlorinated biphenyls (PCBs) in soil-plant systems. Residue
Rev., 74: 45-98.
PANTALEONI, G.C., FANINI, D., SPONTA, A.M., PALUMRO, G., GIORGI,
R., & ADAMS, P.M. (1988) Effects of maternal exposure to
polychlorobiphenyls (PCBs) on F1 generation behavior in the rat.
Fundam. appl. Toxicol., 11(3): 440-449.
PARKES, K. (1982) Occupational lung disorders, London, Butterworth.
PARKINSON, A. & SAFE, S. (1981) Aryl hydrocarbon hydroxylase
induction and its relationship to the toxicity of halogenated aryl
hydrocarbons. Toxicol. environ. Chem. Rev., 4: 1-46.
PARKINSON, A., ROBERTSON, L.W., & SAFE, S. (1980) Reconstituted
human breast milk. PCBs as potent inducers of aryl hydrocarbon
hydroxylase. Biochem. biophys. Res. Commun., 96: 882-889.
PARKINSON, A., THOMAS, P.E., RYAN, D.E., REIK, L.M., SAFE, S.H.,
ROBERTSON, L.W., & LEVIN, W. (1983) Immunochemical quantitation of
cytochrome P-450 isoenzymes and epoxidehydrolase in liver
microsomes from polychlorinated and polybrominated biphenyls. A
study of structure activity relationships. J. biol. Chem.,
285: 5967-5976.
PARRISH, P.R. (1973) Aroclor 1254, DDT and DDD, and dieldrin:
Accumulation and loss by American oysters (Crassostrea virginica)
exposed continuously for 56 weeks. Proc. Natl Shellfish Assoc.,
64: 7.
PARSONS, J.R. & SIJM, D.T.H.M. (1988) Biodegradation kinetics of
polychlorinated biphenyls in continuous cultures of a Pseudomonas
strain. Chemosphere, 17: 1755-1766.
PASSINO, D.R.M. & KRAMER, J.M. (1980) Toxicity of arsenic and PCBs
to fry of deepwater ciscoes (Coregonus). Bull. environ. Contam.
Toxicol., 24: 527-534.
PEAKALL, D.B. (1971) Effect of polychlorinated biphenyls (PCBs) on
the eggshells of ring doves. Bull. environ. Contam. Toxicol.,
6: 100-101.
PEAKALL, D.B. (1975) PCB's and their environmental effects. CRC
crit. Rev. environ. Control, 5: 469-508.
PEAKALL, D.B. & PEAKALL, M.L. (1973) Effect of a polychlorinated
biphenyl on the reproduction of artificially and naturally
incubated dove eggs. J. appl. Ecol., 10: 863-868.
PEAKALL, D.B., LINCER, J.L., & BLOOM, S.E. (1972) Embryonic
mortality and chromosomal alterations caused by Aroclor 1254 in
ring doves. Environ. health Perspect., 1: 103-104.
PEARCE, P.A., ELLIOT, J.E., PEAKALL, D.B., & NORSTRÖM, R.J. (1989)
Organochlorine contaminants in eggs of seabirds in the Northwest
Atlantic, 1968-1984. Environ. Pollut., 56: 217-235.
PEREIRA, M.A., HERREN, S.L., BRITT, A.L., & KHOURY, M.M. (1982)
Promotion by polychlorinated biphenyls of enzyme-altered foci in
rat liver. Cancer Lett., 15: 185-190.
PESENDORFER, H., VON, EICHLER, I., & GLOFKE, E. (1973) [Informative
analyses or organochlorine pesticide and PCB residues in human
adipose tissue (from the area of Vienna).] Wiener Klin.
Wochenschr., 85: 218-222 (in German).
PESENDORFER, H. (1975) [Residues of organochlorine pesticides (DDT,
etc.) and chlorinated biphenyls (PCBs) in breastmilk (from the
Vienna and Lower Austria region).] Wiener Kiln. Wochenschr.,
87(21): 732-736 (in German).
PETERSON, R.H. (1973) Temperature selection of atlantic salmon
(Salmo salar) and brook trout (Salvelinus fontinalis) as
influenced by various chlorinated hydrocarbons. J. Fish Res. Board
Can., 30: 1091-1097.
PHILLIPS, W.E.J., HATINA, G., VILLENEUVE, D.C., & GRANT, D.L.
(1972) Effect of parathion administration in rats following
long-term feeding with PCBs. Environ. Physiol. Biochem.,
2: 165-169.
PIENTA, R.J. (1980) Transformation of Syrian hamster embryo cells
by diverse chemicals and correlation with their reported
carcinogenic and mutagenic activities. In: de Serres, F.J. &
Hollaender, A., ed. Chemical mutagens: principles and methods for
their detection, Plenum Press, New York, London, Vol. 6,
pp. 175-202.
PINES, A., CUCOS, S., GRAFSTEIN, O., & LEMESCH, C. (1988) Changing
patterns of cow's milk contamination with organochlorine compounds
(1976-1986). Bull. environ. Contam. Toxicol., 40: 94-101.
PINKNEY, A.E., POJE, G.V., SANSUR, R.M., LEE, C.C., & O'CONNOR,
C.J.M. (1985) Uptake and retention of 14C-Aroclor 1254 in the
amphipod, Gammarus tigrinus, fed contaminated fungus, Fusarium
oxysporum. Arch. environ. Contam. Toxicol., 14: 59-64.
PLAPP, F.W. (1972) Polychlorinated biphenyl: An environmental
contaminant acts as an insecticide synergist. Environ. Entomol.,
1: 580-582.
PLATONOW, N.S. & CHEN, N.Y. (1973) Transplacental transfer of
polychlorinated biphenyls (Aroclor 1254) in a cow. Vet. Rec.,
92: 69-70.
PLATONOW, N.S. & FUNNELL, H.S. (1971) Anti-androgenic-like effect
of polychlorinated biphenyls in cockerels. Vet. Rec., 88: 109-110.
PLATONOW, N.S. & FUNNELL, H.S. (1972) The distribution and some
effects of polychlorinated biphenyls (Aroclor 1254) in cockerels
during prolonged feeding trial. Arch. environ. Contam. Toxicol.,
36: 89-93.
PLATONOW, N.S. & REINHART, B.S. (1973) The effects of
polychlorinated biphenyls (Aroclor 1254) on chicken egg production,
fertility, and hatchability. Can. J. comp. Med., 37: 341-346.
PLATONOW, N.S., LIPTRAP, R.M., & GEISSINGER, H.D. (1972) The
distribution and excretion of polychlorinated biphenyls (Aroclor
1254) and their effect on urinary gonadal steroid levels in the
boar. Bull. environ. Contam. Toxicol., 7: 358-365.
PLATONOW, N.S., MEADS, E.B., LIPTRAP, R.M., & LOTZ, F. (1976)
Effects of some commercial preparations of polychlorinated
biphenyls in growing piglets. Can. J. comp. Med., 40: 421-428.
POLAND, A. & GLOVER, E. (1977) Chlorinated biphenyl induction of
aryl hydrocarbon hydroxylase activity: a study of the
structure-activity relationship. Mol. Pharmacol., 13: 924-938.
POLAND, A., PALEN, D., & GLOVER, E. (1982) Tumour promotion by TCDD
in skin of HRS/J hairless mice. Nature (Lond.) 300: 271-273.
POMERANTZ, I., BURKE, J., FIRESTONE, D., MCKINNEY, J., ROACH, J.,
& TROTTER, W. (1978) Chemistry of PCBs and PBBs. Environ. health
Perspect., 24: 133-146.
PORTER, M.L., YOUNG, S.L.V., & BURKE, J.A. (1970) A method for the
analysis of fish, poultry and animal tissue for chlorinated
pesticide residues. J. Assoc. Off. Anal. Chem., 53: 1300-1303.
PORTMANN, J.E. (1970) Monitoring of organochlorine residues in fish
from around England and Wales, with special reference to
polychlorinated biphenyls, Charlottenlund, Denmark, International
Council for the Exploration of the Sea (Report CM 1970/E:9).
PORTMANN, J.E. & WILSON, K.W. (1971) The toxicity of 140 substances
to the brown shrimp and other marine animals. Shellfish lnf. Leafl.
MAFF, 22: 1-11.
POWERS, R.H., GILBERT, L.C., & AUST, S.D. (1987) The effect of
3,4,3',4'-tetrachlorobiphenyl on plasma retinol and hepatic retinyl
palmitate hydrolase activity in female Sprague-Dawley rats.
Toxicol. appl. Pharmacol., 89: 370-377.
PRECHTL, H.F.R. (1982) Assessment methods for the newborn infant;
a critical evaluation. In: Stratton, P., ed. Psychobiology of the
human new born, New York, Chichester, Brisbane, Toronto, John Wiley
and Sons, pp. 21-520.
PRESTON, B.D., VAN MILLER, J.P., MOORE, R.W., & ALLEN, J.R. (1981)
Promoting effects of polychlorinated biphenyls (Aroclor 1254) and
polychlorinated dibenzofuran-free Aroclor 1254 on diethyl-
nitrosamine-induced tumorigenesis in the rat and mouse. J. Natl
Cancer Inst. 66: 509-515.
PRESTON, B.D., MILLER, J.A., & MILLER, E.C. (1984) Reactions of
2,2',5,5',-tetrachlorobiphenyl 3,4-oxide with methionine, cysteine
and glutathione in relation to the formation of methylthio-
metabolites of 2,2',5,5'-tetrachlorobiphenyl in the rat and mouse.
Chem.-biol. Interact., 50: 289-312.
PRESTON, B.D., MILLER, E.C., & MILLER, J.A. (1985) The activities
of 2,2',5,5'-tetrachlorobiphenyl, its 3,4-oxide metabolite, and
2,2',4,4'-tetrachlorobiphenyl in tumour induction and promotion
assays. Carcinogenesis, 6: 451-453.
PRESTT, I., JEFFERIES, D.J., & MOORE, N.W. (1970) Polychlorinated
biphenyls in wild birds in Britain and their avian toxicity.
Environ. Pollut., 1: 3-26.
PRICE, H.A. & WELCH, R.L. (1972) Occurrence of polychlorinated
biphenyls in humans. Environ. Health Perspect., 1: 73-78.
PROBST, G.S., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K.,
& NEAL, S.B. (1981) Chemically-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: a comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutagen., 3: 11-32.
PUHVEL, S.M., SAKAMOTO, M., ERTL, D.C., & REISNER, R.M. (1982)
Hairless mice as models for chloracne: a study of cutaneous changes
induced by topical application of established chloracnegens.
Toxicol. appl. Pharmacol., 64: 492-503.
PUTTMANN, M., ARAND, M., OESCH, F., MANNSCHRECK, A., & ROBERTSON,
L. (1988) Chirality and the induction of xenobiotic-metabolizing
enzymes: Effects of the atropisomers of the polychlorinated
biphenyl 2,2',3,4,5',6-hexachlorobiphenyl. In: Holmstedt, B.,
Frank, H., & Testa, B., ed. Chirality and biological activity.
Proceedings of an International Symposium held at Tubingen, Federal
Republic of Germany, 5-8 April 1988, New York, Alan R. Liss, Inc.,
pp. 177-184.
QUAZI, S., TAKAHATA, M., HORIO, F., & YOSHIDA, A. (1984) Hepatic
3-hydroxy-3-methylglutaryl coenzyme A reductase and cholesterol
7-alpha-hydroxylase activities in rats fed PCB. Nutr. Rep. Int.,
30: 617-627.
QUENSEN, J.F., TIEDJE, J.M., & BOYD, S.A. (1988) Reductive
dechlorination of polychlorinated biphenyls by anaerobic
microorganisms from sediments. Science, 242: 752-754.
QURESHI, A.M. & ROBERTSON, H.E. (1987) Polychlorinated biphenyls
(PCB) in breast milk from Regina nursing mothers. Can. J. public
Health, 78: 389-392.
RANDS, P.L., WHITE, R.D., CARTER, M.W., ALLEN, S.D., & BRADSHAW,
W.S. (1982) Indicators of developmental toxicity following prenatal
administration of hormonally active compounds in the rat I.
Gestational length. Teratology, 25: 37-43.
RAO, C.V. & BANERJI, A.S. (1988a) Polychlorinated biphenyls in
human amniotic fluid. Bull. environ. Contam. Toxicol., 41: 798-801.
RAO, C.V. & BANERJI, A.S. (1988b) Induction of liver tumors in male
Wistar rats by feeding polychlorinated biphenyls (Aroclor 1260).
Cancer Lett., 39(1): 59-67.
RAPPE, C. (1985) PCB accident in France. In: Komai, R.Y. & Addis,
G., ed. Proceedings of a Seminar on Polychlorinated biphenyls, Palo
Alto, California, Electric Power Research Institute.
RAPPE, C., MARKLUND, S., BERGQVIST, P.A., & HANSSON, M. (1982)
Polychlorinated dioxins (PCDDs), dibenzofurans (PCDFs) and other
polynuclear aromatics (PCPNAs) found during PCB fires. Chem.
Scripta, 20: 56-61.
RAPPE, C., KJELLER L.-O., & MARKLUND, S. (1985a) PCDF isomers and
isomer levels found in PCBs. In: Komai, R.Y. & Addis, G., ed.
Proceedings of a Workshop on PCB By-Product Formation, 4-6 December
1984, Palo Alto, California, Electric Power Research Institute
(CS/EL-4101).
RAPPE, C., MARKLUND, S., KJELLER, L.-O., BERGQVIST, P.A., &
HANSSON, M. (1985b) Composition of PCDFs formed in PCB fires. In:
Keith, L.H., Rappe, C., & Choudary, G., ed. Symposium on
chlorinated dioxins and dibenzofurans in the total environment II,
Boston, Massachusetts, Butterworth Publishers, pp. 401-424.
RAPPE, C., MYGREN, M., MARKLUND, S., KJELLER, L.O., BERGQVIST,
P.A., & HANSSON, M. (1985c) Assessment of human exposure to
polychlorinated dibenzofurans and dioxins. Environ. health
Perspect., 60: 303-304.
RAPPE, C., NYGREN, M., LINDSTROM, G., BUSER, H.R., BLASER, O., &
WUTHRICH, C. (1987) Polychlorinated dibenzofurans and
dibenzo- p-dioxins and other chlorinated contaminants in cow milk
from various locations in Switzerland. Environ. Sci. Technol.,
21(10): 964-970.
REIJNDERS, P.J.H. (1986) Reproductive failure in common seals
feeding on fish from polluted coastal waters. Nature (Lond.),
324: 456-457.
REILLY, T.R., SUNDARESAN, S., & HIGHLAND, J.H. (1986). Cleanup of
PCB contaminated soils and sludges by a solvent extraction process:
A case study. Stud. Environ. Sci., 29: 125-139.
REINKE, J., UTHE, J.F., & O'BRODOVICH, H. (1973) Determination of
polychlorinated biphenyls in the presence of organochlorine
pesticides by thin-layer chromatography. Environ. Lett., 4:
201-210.
RENBERG, L., SUNDSTRÖM, G., & REUTHERGAARDH, L. (1981)
Polychlorinated terphenyls (PCT) in Swedish white-tailed eagles and
in grey seals. A preliminary study. Chemosphere, 6: 477-482.
REYNOLDS, L.M. (1971) Pesticide residue analysis in the presence of
polychlorobiphenyls (PCBs). Residue Rev., 34: 27-45.
REZNICEK, J. (1987) [Rapid determination of chlorinated pesticides
and polychlorinated biphenyls in the blood.] Prac. Lek.,
39: 185-189 (in Czech).
RICE, C.P. & WHITE, D.S. (1987) PCB availability assessment of
river dredging using caged clams and fish. Environ. Toxicol. Chem.,
6: 259-274.
RISEBROUGH, R.W. & ANDERSON, D.W. (1975) Some effects of DDE and
PCB on mallards and their eggs. J. Wildl. Manage., 39: 508-513.
RISEBROUGH, R.W. & DE LAPPE, B. (1972) Accumulation of
polychlorinated biphenyls in ecosystems. Environ. health Perspect.,
1: 39-45.
RISEBROUGH, R.W., RIECHE, P., PEAKALL, D.B., HERMAN, S.G., &
KIRVEN, M.N. (1968) Polychlorinated biphenyls in the global
ecosystem. Nature (Lond.), 220: 1098-1102.
ROBBIANO, L. & PINO. A. (1981) Induction in rats of liver DNA
single-strand breaks by the polychlorinated biphenyl Aroclor 1254.
Boll. Soc. Ital. Biol. Sper., 57: 407-413.
ROBERTS, D. (1975) The effect of pesticides on byssus formation in
the common mussel, Mytilus edulis. Environ. Pollut., 8: 241-254.
ROBERTSON, L.W., PARKINSON, A., BANDIERA, S., LAMBERT, I., MERRILL,
J., & SAFE, S. (1984) PCBs and PBBs: biologic and toxic effects on
C57BL/6J and DBA/2J inbred mice. Toxicology, 31: 191-206.
ROESIJADI, G., ANDERSON, J.W., GIAM, C.S., & PETROCELLI, S.R.
(1976a) Osmoregulation of the grass shrimp Palaemonetes pugio
exposed to polychlorinated biphenyls (PCBs). I. Effect on chloride
and osmotic concentrations and chloride and water-exchange
kinetics. Mar. Biol., 38: 343-355.
ROESIJADI, G., ANDERSON, J.W., & GIAM, C.S. (1976b) Osmoregulation
of the grass shrimp Palaemonetes pugio exposed to polychlorinated
biphenyls (PCBs). II. Effect on free amino acids of muscle tissue.
Mar. Biol., 38: 357-363.
ROGAN, W.J., GLADEN, B.C., MCKINNEY, J.D., CARRERAS, N., HARDY, P.,
THULLEN, J., TINGELSTAD, J., & TULLY, M. (1986) Polychlorinated
biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human
milk: Effects of maternal factors and previous lactation. Am. J.
public Health, 76(2): 172-177.
ROGAN, W.J., GLADEN, B.C., MCKINNEY, J.D., CARRERAS, N., HARDY, P.,
THULLEN, J., TINGELSTAD, J., & TULLY, M. (1987) Polychlorinated
biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human
milk: Effects on growth, morbidity and duration of lactation. Am.
J. public Health, 77: 1294-1297.
ROGAN, W.J., GLADEN, B.C., HUNG, K.-L., KOONG, S.-L., SHIH, L.-Y.,
TAYLOR, J.S., WU, Y.-C., YANG, D., RAGAN, N.B., & HSU, C.-C. (1988)
Congenital poisoning by polychlorinated biphenyls and their
contaminants in Taiwan. Science, 241: 334-336.
ROSIN, D.L. & MARTIN, B.R. (1981) Neurochemical and behavioural
effects of polychlorinated biphenyls in mice. Neurotoxicology,
2: 749-764.
ROSIN, D.L. & MARTIN, B.R. (1983) Comparison of the effects of
acute and sub-chronic administration of Aroclor 1254, a commercial
mixture of polychlorinated biphenyls on pentobarbital-induced sleep
time and [14C]pentobarbital disposition in mice. J. Toxicol.
environ. Health, 11: 917-931.
ROTE, J.W. & MURPHY, P.G. (1971) A method for the quantitation of
polychlorinated biphenyl (PCB) isomers. Bull. environ. Contam.
Toxicol., 6: 377-384.
RUBINSTEIN, N.I., LORES, E., & GREGORY, N.R. (1983) Accumulation of
PCBs, mercury and cadmium by Nereis virens, Mercenaria mercenaria
and Palaemonetes pugio from contaminated harbor sediments. Aquat.
Toxicol., 3: 249-260.
RUOFF, U., HEESCHEN, W., NIJHUIS, H., & BLÜTHGEN, A. (1988)
[Investigations on the significance of drinking water used by
cattle for the contamination of milk with polychlorinated biphenyls
(PCBs).] Kieler Milchwirtsch. Forschungsber., 40(2): 71-80
(in German).
RUZO, L.O., ZABIK, M.J., & SCHUETZ, R.D. (1972) Polychlorinated
biphenyls: Photolysis of 3,4,3',4'-tetrachlorobiphenyl and
4,4'-dichlorobiphenyl in solution. Bull. environ. Contam. Toxicol.,
8: 217-218.
RUZO, L.O., ZABIK, M.J., & SCHUETZ, R.D. (1974) Photochemistry of
bioactive compounds. Photochemical processes of polychlorinated
biphenyls. J. Am. Chem. Soc., 96: 3809-3813.
RUZO, L.O., SAFE, S., & ZABIK, M.J. (1975) Photodecomposition of
unsymmetrical polychlorobiphenyls. J. agric. food Chem.,
23: 594-595.
RYAN, D.E., THOMAS, P.E., KORZENIOWSKI, D., & LEVIN, W. (1979a)
Separation and characterization of highly purified forms of liver
microsomal cytochrome P-450 from rats treated with polychlorinated
biphenyls, phenobarbital and 3-methylcholanthrene. J. biol. Chem.,
254: 1365-1374.
RYAN, D.E., THOMAS, P.E., REIK, L.M., & LEVIN, W. (1979b)
Purification, characterization and regulation of five rat hepatic
cytochrome P-450 isoenzymes. Xenobiotica, 12: 727-744.
RYAN, D.E., THOMAS, P.E., & LEVIN, W. (1982) Purification and
characterization of a minor form of hepatic microsomal cytochrome
P-450 from rats treated with polychlorinated biphenyls. Arch.
Biochem. Biophys., 216: 272-288.
SAFE, S. (1982) Halogenated hydrocarbons and arylhydrocarbons
identified in human tissues. Toxicol. environ. Chem., 5: 153-165.
SAFE, S. (1984) Polychlorinated biphenyls (PCBs) and polybrominated
biphenyls (PBBs): biochemistry, toxicology, and mechanism of
action. CRC crit. Rev. Toxicol., 13: 319-395.
SAFE S. (in press) Polychlorinated biphenyls (PCBs),
dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs) and related
compounds: Environmental and mechanistic considerations which
support the development of toxic equivalency factors (TEfs). CRC
crit. Rev. Toxicol.
SAFE, S. & HUTZINGER, O. (1971) Polychlorinated biphenyls:
photolysis of 2,4,6,2'4',6'-hexachlorobiphenyl. Nature (Lond.),
232: 641-642.
SAFE, S., HUTZINGER, O., & ECOBICHON, D. (1974) Identification of
a 4-chloro-4'-hydroxybiphenyl and 4-4'-dichloro-3-hydroxybiphenyl
as metabolites of 4-chloro-and 4,4'-dichlorobiphenyl fed to rats.
Experientia (Basel), 30: 720-721.
SAFE, S., HUTZINGER, O., ECOBICHON, D., & GREY, A.A. (1975) The
metabolism of 4'-chloro-4-biphenylol in the rat. Can. J. Biochem.,
53: 415-20.
SAFE, S., SAFE, L., & MULLIN, M. (1985a) Polychlorinated biphenyls:
Congener-specific analysis of a commercial mixture and human milk.
J. agric. food Chem., 33: 24-29.
SAFE, S., BANDIERA, S., SAWYER, T., ROBERTSON, L., SAFE, L.,
PARKINSON, A., THOMAS, P.E., RYAN, D.E., REIK, L.M., LEVIN, W.,
DENOMME, M.A., & FUJITA, T. (1985b) PCBs: structure-function
relationships and mechanism of action. Environ. health Perspect.,
60: 47-56.
SAGER, D.B. (1983) Effect of postnatal exposure to polychlorinated
biphenyls on adult male reproductive function. Environ. Res.,
31: 76-94.
SAGER, D.B., SHIH-SCHROEDER, W., & GIRARD, D. (1987) Effect of
early postnatal exposure to polychlorinated biphenyls PCBs) on
fertility in male rats. Bull. environ. Contam. Toxicol.,
38: 946-953.
ST. AMANT, J.R., PARISO, M.E., & SHEFFY, T.B. (1984)
Polychlorinated biphenyls in seven species of Lake Michigan fish,
1971-1981. In: Nriagu, J.O., Nriagu, J.O. & Simmons, M.S., ed.
Toxic contaminants in the Great Lakes; New York, John Wiley and
Sons, pp. 311-319.
SAITO, R., SHIGEMATSU, N., & ISHIMARU, S. (1972) [Immunoglobulin
levels in serum and sputum of patients with PCB poisoning.] Fukuoka
Acta med., 63: 408-411 (in Japanese).
SAITO, M., IKEGAMI, S., ITO, Y., & INNAMI, S. (1982) Influence of
dietary anti-oxidants on polychlorinated biphenyls (PCBs)- induced
hepatic lipid peroxide formation and vitamin A reduction in rats.
J. nutr. Sci. Vitaminol., 28: 455-466.
SAITO, M., IKEGAMI, S., NISHIDE, E., & INNAMI, S. (1983) Relevances
of mixed function oxidase system and ascorbic acid to the lipid
peroxide formation in the liver of rats given polychlorinated
biphenyls (PCB). Fukuoka Acta med., 74: 222-233.
SANDBERG, P.-O. & GLAUMANN, H. (1980) Studies on the cellular
toxicity of polychlorinated biphenyls (PCBs). Partial block and
alteration of intracellular migration of lipoprotein particles in
rat liver. Exp. mol. Pathol., 32: 1-22.
SANDERS, H.O. & CHANDLER, J.H. (1972) Biological magnification of
a polychlorinated biphenyl (Aroclor 1254) from water by aquatic
invertebrates. Bull. environ. Contam. Toxicol., 7: 257-263.
SANDERS, O.T. & KIRKPATRICK, R.L. (1975) Effects of a
polychlorinated biphenyl (PCB) on sleeping times, plasma
corticosteroids, and testicular activity of White-footed mice.
Environ. Physiol. Biochem., 5: 308-313.
SANDERS, O.T., ZEPP, R.L., & KIRKPATRICK, R.L. (1974) Effect of PCB
ingestion on sleeping time, organ weights, food consumption, serum
corticosterone and survival of albino mice. Bull. environ. Contam.
Toxicol., 12(4): 394-399.
SANDERS, O.T., KIRKPATRICK, R.L., & SCANLON, P.E. (1977)
Polychlorinated biphenyls and nutritional restriction: their
effects and interactions on endocrine and reproductive
characteristics of male white mice. Toxicol. appl. Pharmacol.,
40: 91-98.
SANGALANG, G.B., FREEMAN, H.C., & CROWELL, R. (1981) Testicular
abnormalities in cod (Gadus morhua) fed Aroclor 1254. Arch.
environ. Contam. Toxicol., 10: 617-626.
SANO, S., KAWANISHI, S., & SEKI, Y. (1985) Toxicity of
polychlorinated biphenyl with special reference to porphyrin
metabolism. Environ. health Perspect., 59: 137-143.
SARGENT, L., ROLOFF, B., & MEISNER, L. (1989) In vitro chromosome
damage due to PCB interactions. Mutat. Res., 224: 79-88.
SASCHENBRECKER, P.W., FUNNELL, H.S., & PLATONOW, N.S. (1972)
Persistence of polychlorinated biphenyls in the milk of exposed
cows. Vet. Rec., 90: 100-102.
SAWAI, T. & SAWAI, T. (1973) [Photosensitized chain dechlorination
reaction of polychlorinated biphenyls (PCB) in alkaline 2-propanol
solutions.] Kogai, 8: 97-105 (in Japanese).
SAWHNEY, B.L. & HANKIN, L. (1984) Plant contamination by PCBs from
amended soils. J. food Prot., 47: 232-236.
SAWHNEY, B.L. & HANKIN, L. (1985) Polychlorinated biphenyls in
food: A review. J. food Prot., 48(5): 442-448.
SAWYER, T. & SAFE, S. (1982) PCB isomers and congeners: induction
of aryl hydrocarbon hydroxylase and ethoxyresorufin O-deethylase
enzyme activities in rat hepatoma cells. Toxicol. Lett., 13: 87-94.
SAWYER, T.W., VATCHER, A.D., & SAFE, S. (1984) Comparative aryl
hydrocarbon hydroxylase induction activities of commercial PCBs in
Wistar rats and rat hepatoma H-4-II E cells in culture.
Chemosphere, 13: 695-701.
SAYLER, G.S., SHON, M., & COLWELL, R.R. (1977) Growth of an
estuarine Pseudomonas sp. on polychlorinated biphenyl. Microb.
Ecol., 3: 241-255.
SCHAEFFER, E., GREIM, H., & GOESSNER, W. (1984) Pathology of
chronic polychlorinated biphenyl (PCB) feeding in rats. Toxicol.
appl. Pharmacol., 75: 278-288.
SCHECTER, A. (1987) Transient liver pathology in patients consuming
water from a private well contaminated by PCBs from a submersible
water pump. Chemosphere, 16(1): 37-42.
SCHECTER, A. & TIERNAN, T. (1985) Occupational exposure to
polychlorinated dioxins, polychlorinated furans, polychlorinated
biphenyls and biphenylenes after an electrical panel and
transformer accident in an office in Binghamton, N Y. Environ.
health Perspect., 60: 305-313.
SCHECTER, A., TIERNAN, T., SCHAFFNRER, F., TAYLOR, M., GITLITZ, G.,
VAN NESS, G.F., GARRETT, J.H., & WAGEL, D.J. (1985) Patient fat
biopsies for chemical analysis and liver biopsies for
ultrastructural characterization after exposure to polychlorinated
dioxins, furans, and PCBs. Environ. health Perspect., 60: 241-254.
SCHECTER, A., FURST, P., KRÜGER, C., MEEMKEN, H.A., GROEBEL, W., &
CONSTABLE, J.D. (1989a) Levels of polychlorinated dibenzofurans,
dibenzodioxins, PCBs, DDT and DDE, hexachlorobenzene (HCB),
dieldrin, hexachlorocyclohexane and oxychlordane in human breast
milk from the United States, Thailand, Vietnam, and Germany.
Chemosphere, 18(1/6): 445-454.
SCHECTER, A., MES, J., & DAVIES, D. (1989b) Polychlorinated
biphenyl (PCB), DDT, DDE and hexachlorobenzene (HCB) and PCDD/F
isomer levels in various organs in autopsy tissue from North
American patients. Chemosphere, 18(1/6): 811-818.
SCHIMMEL, S.C., HANSEN, D.J., & FORESTER, J. (1974) Effects of
Aroclor 1254 on laboratory-reared embryos and fry of sheepshead
minnows (Cyprinodon variegatus). Trans Am. Fish Soc.,
103: 582-586.
SCHMIDT, T.T., RISEBROUGH, R.W., & GRESS, F. (1971) Input of
polychlorinated biphenyls into California coastal waters from urban
sewage outfalls. Bull. environ. Contam. Toxicol., 6: 235-243.
SCHMITT, C.J., RIBICK, M.A., LUDKE, J.L., & MAY, T.W. (1983)
Organochlorine residues in freshwater fish, 1976-1979: National
pesticide monitoring program, Washington DC, US Department of the
Interior, Fish and Wildlife Service, 62 pp (Resource Publication
No. 152).
SCHMITT, C.J., ZAJICEK, J.L., & RIBICK, M.A. (1985) National
pesticide monitoring program: residues of organochlorine chemicals
in freshwater fish, 1980-81. Arch. environ. Contam. Toxicol.,
14: 225-260.
SCHMOLDT, A., BENTHE, H.F., & FRUEHLING, R. (1974) Induction of rat
liver enzymes by polychlorinated biphenyls (PCBs) in dependence on
the dose and chlorine content. Arch. Toxicol., 32: 69-81.
SCHNELLMAN, R.G., VOLP, R.F., PUTNAM, C.W., & SIPES, I.G. (1984a)
The hydroxylation, dechlorination and glucuronidation of
4,4',-dichlorobiphenyl by human hepatic microsomes. Biochem.
Pharmacol., 33: 3503-3509.
SCHNELLMAN, R.G., PUTNAM, C.W., & SIPES, I.G. (1984b) Metabolism of
2,2',3,3',6,6'-hexachlorobiphenyl and 2,2',4,4',5,5',-hexachloro-
biphenyl by human hepatic microsomes. Biochem. Pharmacol.,
32: 3233-3239.
SCHOENY, R. (1982) Mutagenicity testing of chlorinated biphenyl and
chlorinated dibenzofurans. Mutat. Res., 101: 45-56.
SCHOENY, R., SMITH, C.C., & LOPER J.C. (1979) Non-mutagenicity for
Salmonella of the chlorinated hydrocarbons Aroclor 1254,
1,2,4-trichlorobenzene, Mirex and kepone. Mutat. Res., 68: 125-132.
SCHULTE, E. & MALISCH, R. (1984) Calculation of the real PCB
content in environmental samples. II. Gas chromatographic
determination of the PCB concentration in human milk and butter.
Fresenius Arch. anal. Chem., 319: 54-59.
SCHWARTZ, L. (1943) An outbreak of halowax acne ("cable rash")
among electricians. J. Am. Med. Assoc., 122: 158-161.
SCHWARTZ, P.M., JACOBSON, S.W., FEIN, G.G., JACOBSON, J.L., &
PRICE, H.A. (1983) Lake Michigan fish consumption as a source of
polychlorinated biphenyls in human cord serum, maternal serum and
milk. Am. J. public Health, 73(3): 293-296.
SCHWARTZ, T.R., STALLING, D.L., & RICE, C.L. (1987) Are
polychlorinated biphenyl residues adequately described by Aroclor
mixture equivalents? Isomer-specific principal components analysis
of such residues in fish and turtles. Environ. Sci. Technol.,
21: 72-76.
SCOTT, M.L. (1977) Effects of PCBs, DDT and mercury compounds in
chickens and Japanese quail. Fed. Proc. Am. Soc. Exp. Biol.,
36: 1888-1893.
SCOTT, M.L., VADEHRA, D.V., MULLENHOFF, P.A., RUMSEY, G.L., & RICE,
R.W. (1971) Results of experiments on the effects of PCBs on laying
hen performance. Proceedings of the Cornell Nutrition Conference,
pp. 56.
SCOTT, M.L., ZIMMERMAN, J.R., MARINSKY, S., MULLENHOFF, P.A.,
RUMSEY, G.L., & RICE, R.W. (1975) Effects of PCBs, DDT and mercury
compounds upon egg production, hatchability and shell quality in
chickens and Japanese quail. Poult. Sci., 54: 350-368.
SEEGAL, R.F., BUSH, B., & BROSCH, K.O. (1985) Polychlorinated
biphenyls induce regional changes in brain norepinephrine
concentrations in adult rats. Neurotoxicology, 6: 13-24.
SEKI, Y., KAWANISHI, S., & SANO, S. (1987) Role of inhibition of
uroporphyrinogen decarboxylase in PCB-induced porphyria in mice.
Toxicol. appl. Pharmacol., 90: 116-125.
SEPPALAINEN, A.M., VUOJOLAHTI, P., & ELO, O. (1985) Reversible
nerve lesions after accidental polychlorinated biphenyl exposure.
Scand. J. Work Environ. Health, 11: 91-95.
SEYMOUR, M.P., DUNCAN, I.W., JEFFERIES, T.M., & NOTARIANNI, L.J.
(1986a) Clean-up and separation of chlorobiphenyl isomers after
synthesis by Cadogan coupling using preparative high-performance
liquid chromatography. J. Chromatogr., 368: 174-179.
SEYMOUR, M.P., JEFFERIES, T.M., & NOTARIANNI, L.J. (1986b)
Large-scale separation of lipids from organochlorine pesticides and
polychlorinated biphenyls using a polymeric high-performance liquid
chromatographic column. Analyst, 111: 1203-1205.
SEYMOUR, M.P., JEFFERIES, T.M., & NOTARIANNI, L.J. (1986c)
Limitations in the use of nickel boride dechlorination for the
analysis of polychlorinated biphenyls. Bull. environ. Contam.
Toxicol., 37: 199-206.
SEYMOUR, M.P., JEFFERIES, T.M., FLOYD, A.J., & NOTARIANNI, L.J.
(1987) Routine determination of organochlorine pesticides and
polychlorinated biphenyls in human milk using capillary gas
chromatography - mass spectrometry. Analyst, 112: 427-431.
SHAHIN, M.M., ANDRILLON, P., GOETZ, N., BORE, P., BUGAUT, A., &
KALOPISSIS, G. (1979) Studies on the mutagenicity of
p-phenylenediamine in Salmonella typhimurium: Presence of PCBs
in rat-liver microsomal fraction induced by Aroclor. Mutat. Res.,
68: 327-336.
SHALAT, S.L., TRUE, L.D., FLEMING, L.E., & PACE, P.E. (1989) Kidney
cancer in utility workers exposed to polychlorinated biphenyls
(PCBs). Br. J. ind. Med., 46: 823-824.
SHAW, G.R. & CONNELL, D.W. (1982) Factors influencing
polychlorinated biphenyls in organisms from an estuarine ecosystem.
Aust. J. mar. freshwater Res., 33: 1057-1070.
SHELTON, D.W., COULOMBE, R.A., PEREIRA, C.B., CASTEEL, J.L., &
HENDRICKS, J.D. (1984a) Inhibitory effect of Aroclor 1254 on
aflatoxin-initiated carcinogenesis in rainbow trout and mutagenesis
using Salmonella-trout hepatic activation system. Aquat. Toxicol.,
3: 229-238.
SHELTON, D.W., HENDRICKS, J.D., COULOMBE, R.A., & BAILEY, G.S.
(1984b) Effects of dose on the inhibition of
carcinogenesis/mutagenesis by Aroclor 1254 in rainbow trout fed
aflatoxin B1. J. Toxicol. environ. Health, 13: 659-667.
SHELTON. D.W., GOEGER, D.E., HENDRICKS, J.D., & BAILEY, G.S. (1986)
Mechanisms of anticarcinogenesis: the distribution and metabolism
of aflatoxin B1 in rainbow trout fed Aroclor 1254. Carcinogenesis,
7: 1065-1071.
SHIGEMATSU, N., NORIMATSU, Y., ISHIBASHI, T., YOSHIDA, M.,
SUETSUGU, S., KAWATSU, T., IKEDA, T., SAITO, R., ISHIMURA, S.,
SHIRAKISA, T., KIDO M., EMORI, K., & TOSHIMITSU, H. (1971)
[Clinical and experimental studies on respiratory involvement in
chlorobiphenyls poisoning.] Fukuoka Acta med., 62: 150-156
(in Japanese).
SHIGEMATSU, N., ISHIMANU, S., SAITO, R., IKEDA, T., MATSUBA, K.,
SUGIYAMA, K., & MASUDA, Y. (1978) Respiratory involvement in
polychlorinated biphenyls poisoning. Environ. Res., 16: 92-100.
SHIMADA, T. & UGAWA, M. (1978) Induction of liver microsomal drug
metabolism by polychlorinated biphenyls whose gas chromatographic
profile having much in common with that in human milk. Bull.
environ. Contam. Toxicol., 19: 198-205.
SHIMADA, T. & SAWABE, Y. (1984) Comparative studies on distribution
and covalent tissue binding of 2,4,2',4'- and 3,4,3',4'-tetra-
chlorobiphenyl isomers in the rat. Arch. Toxicol., 55: 182-185.
SHIOTA, K. (1976a) Embryotoxic effects of polychlorinated biphenyls
(Kanechlors 300 and 500) in rats. Okajimas Folia anat. Jpn.,
53: 93-104.
SHIOTA, K. (1976b) Postnatal behavioural effects of prenatal
treatment with PCBs (Polychlorinated biphenyls) in rats. Okajimas
Folia anat. Jpn., 53: 105-114.
SHIRAI, T., MIYATA, Y., NAKANISHI, K., MURASAKI, G., & ITO, N.
(1978) Hepatocarcinogenicity of polychlorinated terphenyl (PCT) in
ICR mice and its enhancement by hexachlorobenzene (HCB). Cancer
Lett., 4: 271-275.
SHIREMAN, R.B. (1988) Lipoprotein-mediated transfer of
2,4,5,2',4',5'-hexachlorobiphenyl into cultured human cells.
Xenobiotica, 18(4): 449-457.
SHIU, W.Y. & MACKAY, D. (1986) A critical review of aqueous
solubilities, vapor pressures, Henry's law constants and
octanol-water partition coefficients of the polychlorinated
biphenyls. J. Phys. Chem. Ref. Data, 15(2): 911-929.
SHULL, L.R., BLEAVINS, M.R., OLSON, B., & AULERICH, R.J. (1982)
Polychlorinated biphenyls (Aroclors 1016 and 1242): effect on
hepatic microsomal mixed function oxidases in mink and ferrets.
Arch. environ. Contam. Toxicol., 11: 313-321.
SILBERGELD, E.K. (1983) Health effects of PCBs: Occupational
exposure. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of the PCB Seminar, The Hague, 28-30 September 1983,
The Hague, Ministry of the Environment, pp. 136-151.
SILKWORTH, J.B. & GRABSTEIN, E.M. (1982) Polychlorinated biphenyl
immunotoxicity: dependence on isomer planarity and the Ah gene
complex. Toxicol. appl. Pharmacol., 65: 109-115.
SILKWORTH, J.B. & LOOSE, L.D. (1979) Environmental chemical-induced
modification of cell-mediated immune response. Adv. exp. Med.
Biol., 121A: 499-522.
SILKWORTH, J.B., ANTRIM, L., & KAMINSKY, L.S. (1984) Correlations
between polychlorinated biphenyl immunotoxicity, the aromatic
hydrocarbon locus and liver microsomal enzyme induction in C57BL/6
and DBA/2 mice. Toxicol. appl. Pharmacol., 75: 156-165.
SINA, J.F., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY, M.O.
(1983) Evaluation of the alkaline elution/rat hepatocyte assay as
a predictor of carcinogenic/mutagenic potential. Mutat. Res.,
113: 357-391.
SINCLAIR, J., GARLAND, S., ARNASON, T., HOPE, P., & GRANVILLE, M.
(1977) Polychlorinated biphenyls and their effect on photosynthesis
and respiration. Can. J. Bot., 55: 2679-2684.
SIPES, I.G., SLOCUMB, M.L., PERRY, D.F., & CARTER, D.E. (1980)
4,4'-Dichlorobiphenyl: distribution, metabolism and excretion in
the dog and monkey. Toxicol. appl. Pharmacol., 55: 554-563.
SIPES, I.G., SLOCUMB, M.L., CHEN, H.S., & CARTER, D.E. (1982a)
2,3,6,2',3',6'-Hexachlorobiphenyl: distribution, metabolism and
excretion in the dog and monkey. Toxicol. appl. Pharmacol.,
62: 317-324.
SIPES, I.G., SLOCUMB, M.L., PERRY, D.F., & CARTER, D.E. (1982b)
2,4,5,2',4',5'-Hexachlorobiphenyl: distribution, metabolism and
excretion in the dog and monkey. Toxicol. appl. Pharmacol.,
65: 264-272.
SIVALINGAN, P.M., YOSHIDA, T., & INADA, Y. (1973) The modes of
inhibitory effect of PCBs on oxidative phosphorylation of
mitochondria. Bull. environ. Contam. Toxicol., 10: 242-247.
SKAARE, J.U., TUVENG, J.M., & SANDE, H.A. (1988) Organochlorine
pesticides and polychlorinated biphenyls in maternal adipose
tissue, blood, milk and cord blood from mothers and their infants
living in Norway. Arch. environ. Contam. Toxicol., 17: 55-63.
SLOAN, R.J., SIMPSON, K.W., SCHROEDER, R.A., & BARNES, C.R. (1983)
Temporal trends toward stability of Hudson River PCB contamination.
Bull. environ. Contam. Toxicol., 31: 377-385.
SLORACH, S.A. (1984) Biological monitoring and analytical quality
assurance for organohalogen compounds. In: Report on the WHO
Consultation on Organohalogen compounds in human milk and related
hazards. Report on a WHO consultation, Bilthoven, 9-11 January
1985, Copenhagen, World Health Organization, Regional Office for
Europe, Annex 6.
SLORACH, S.A. & VAZ, R. (1983) Global Environmental Monitoring
System (GEMS). Assessment of human exposure to selected
organochlorine compounds through biological monitoring, Uppsala,
Sweden, National Food Administration (Prepared for the United
Nations Environmental Programme and the World Health Organization).
SLORACH, S.A. & VAZ, R. (1985) PCB levels in breast milk: Data from
the UNEP/WHO Pilot project on biological monitoring and some other
recent studies. Environ. health Perspect., 60: 121-126.
SLORACH, S.A., JELINEK, C.F., & STILES, A.R. (1982) Global
Environmental Monitoring System (GEMS). Summary and assessment of
data received from the FAO/WHO Collaborating Centres for Food
Contamination Monitoring, Uppsala, Sweden, National Food
Administration.
SMILLIE, R.H. & WAID, J.S. (1987) Polychlorinated biphenyls and
organochlorine pesticides in the Australian fur seal,
Arctocephalus pusillus doriferus. Bull. environ. Contam.
Toxicol., 39: 358-364.
SMITH, B.J. (1984) PCB levels in human fluids: Sheboygan case
study, Madison, Wisconsin, University of Wisconsin, Sea Grant
Institute (Technical Report No. WIS-SG-83-240).
SMITH, A.B., SCHLÖMER, J., LOWRY, L.K., SMALLWOOD, A.W., LIGO,
R.N., TANAKA, S., STRINGER, W., JONES, M., HERVIN, R., & GLUECK,
C.J. (1982) Metabolic and health consequences of occupational
exposure to polychlorinated biphenyls. Br. J. ind. Med.,
39: 361-369.
SNARSKI, V.M. & PUGLISI, F.A. (1976) Effects of Aroclor (trade
mark) 1254 on brook trout Salvelinus fontinalis, Washington, DC,
US Environmental Protection Agency, 34 pp (EPA 600/3-76-112).
SODERGREN, A. (1971) Accumulation and distribution of chlorinated
hydrocarbons in cultures of Chlorella pyrenoidosa
(Chlorophyceae). Oikos, 22: 215-220.
SODERGREN, A. (1972) Chlorinated hydrocarbon residues in airborne
fallout. Nature (Lond.), 236: 395-397.
SODERGREN, A. (1973) Transport, distribution, and degradation of
DDT and PCB in a South Swedish lake ecosystem. Vatten, 2: 90-108.
SODERGREN, A. & GELIN, C. (1983) Effect of PCBs on the rate of
carbon-14 uptake in phytoplankton isolates from oligotrophic and
eutrophic lakes. Bull. environ. Contam. Toxicol., 30: 191-198.
SODERGREN, A. & LARSSON, P. (1982) Transport of PCBs in aquatic
laboratory model ecosystems from sediment to the atmosphere via the
surface microlayer. Ambio, 11: 41-45.
SODERGREN, A. & SVENSSON, B. (1973) Uptake and accumulation of DDT
and PCB by Ephemera danica (Ephemeroptera) in continuous-flow
systems. Bull. environ. Contam. Toxicol., 9: 345-350.
SODERGREN, A. & ULFSTRAND, S. (1972) DDT and PCB relocate when
caged robins use fat reserves. Ambio, 1: 36-40.
SOLLY, S.R.B. & SHANKS, V. (1974) Polychlorinated biphenyls and
organochlorine pesticides in human fat in New Zealand. N. Z. J.
Sci., 17: 535-544.
SOLOMON, K.E., DAHLGREN, R.B., & LINDER, R.L. (1973) Abnormal
embryos in eggs of pheasants given 2,4-D or PCB. Proc. South
Dakota, Acad. Sci., 52: 95-99.
SOLT, D.B. & FARBER, E. (1976) New principle for the analysis of
chemical carcinogenesis. Nature (Lond.), 263: 701-703.
SOSA-LUCERO, J.C., DE LA IGLESIA, F.A., & THOMAS, G.H. (1973)
Distribution of a polychlorinated terphenyl (PCT) (Aroclor 5460) in
rat tissues and effect on hepatic microsomal mixed function
oxidases. Bull. environ. Contam. Toxicol., 10: 248-256.
SPARLING, J., FUNG, D., & SAFE, S. (1980) Bromo- and chlorobiphenyl
metabolism: GC/MS identification of urinary metabolites and the
effects of structure on their rates of excretion. Biomed. mass
Spectrom., 7: 13-20.
SPEAR, P.A. & MOON, T.W. (1985) Low dietary iodine and thyroid
anomalies in ring doves, Streptopelia risoria, exposed to
3,4,3',4'-tetrachlorobiphenyl. Arch. environ. Contam. Toxicol.,
14: 547-553.
SPENCER, F. (1982) An assessment of the reproductive toxic
potential of Aroclor 1254 in female Sprague-Dawley rats. Bull.
environ. Contam. Toxicol., 28: 290-297.
SPITZER, P.R., RISEBROUGH, R.W., WALKER, W., HERNANDEZ, R., POOLE,
A., PULESTON, D., & NISBET, I.C.T. (1978) Productivity of ospreys
in Connecticut-Long Island increases as DDE residues decline.
Science, 202: 333-335.
STADNICKI, S.S., LIN, F.S.D., & ALLEN, J.R. (1979) DNA single
strand breaks caused by 2,2',5,5'-tetrachlorobiphenyl and its
metabolites. Res. Commun. chem. Pathol. Pharmacol., 24: 313-327.
STAHL, R.G. (1979) Effect of a PCB (Aroclor 1254) on the striped
hermit crab, Clibanarius vittatus (Anomura: Diogenidae) in static
bioassays. Bull. environ. Contain. Toxicol., 23: 91-94.
STALLING, D.L. & MAYER, F.L., Jr, (1972) Toxicities of PCBs to fish
and environmental residues. Environ. health Perspect., 1: 159-164.
STALLING, D.L., TRINDLE, R.C., & JOHNSON, J.L. (1972) Clean up of
pesticides and polychlorobiphenyl residues in fish extracts by gel
permeation chromatography. J. Assoc. Off. Anal. Chem., 55: 32-45.
STATE FOOD INSTITUTE (undated) [Pesticide residues in Danish
foodstuffs 1980-1981], Copenhagen, State Food Institute, Central
Laboratory, Department B: Pesticides and Pollution, pp. 7-8, 65-82
(in Danish).
STEELE, G., STEHR-GREEN, P., & WELTY, E. (1986) Estimates of the
biologic half-life of polychlorinated biphenyls in human serum. New
Engl. J. Med., 314(14): 926-927.
STEEN, W.C., PARIS, D.F., & BAUGHMAN, G.L. (1978) Partitioning of
selected polychlorinated biphenyls to natural sediments. Water
Res., 12: 655-657.
STEHR, P.A., FORNEY, D.L., & LIDDLE, J.A. (1985) Amateur radio
operations and exposure to polychlorinated biphenyls. Arch.
environ. Health, 40(1): 18-19.
STEIN, J.E., HOM, T., & VARANASI, U. (1984) Simultaneous exposure
of English sole (Parophrys vetulus) to sediment-associated
xenobiotics: Part 1 - Uptake and disposition of 14C-polychlorinated
biphenyls and 3H-benzo [a]-pyrene. Mar. environ. Res., 13: 97-119.
STEIN, J.E., HOM, T., CASILLAS, E., FRIEDMAN, A., & VARANASI, U.
(1987) Simultaneous exposure of English sole (Parophrys vetulus)
to sediment-associated xenobiotics: Part 2 - Chronic exposure to an
urban estuarine sediment with added 3H-benzo[a]pyrene and
14C-polychlorinated biphenyls. Mar. environ. Res., 22: 123-149.
STENDALL, R.C. (1976) Summary of recent information regarding
effects of PCBs on birds and mammals. In: Ayer, A.F., ed.
Proceedings of the National Conference on Polychlorinated
Biphenyls, Chicago, Illinois, 19-21 November 1975, Washington, DC,
US Environmental Protection Agency, pp. 262-267 (EPA-560/6-75-004,
PB-253-248).
STICKEL, W.H., STICKEL, L.F., DYRLAND, R.A., & HUGHES, D.L. (1984)
Aroclor 1254 residues in birds: Lethal levels and loss rates. Arch.
environ. Contam. Toxicol., 13: 7-13.
STORM, J.E., HART, J.L., & SMITH, R.F. (1981) Behaviour of mice
after pre- and postnatal exposure to Aroclor 1254. Neurobehav.
Toxicol. Teratol., 3: 5-9.
STRACHAN, W.M.J. (1988) Toxic contaminants in rainfall in Canada:
1984. Environ. Toxicol. Chem., 7: 871-877.
STRACHAN, W.M.J. & HUNEAULT, H. (1979) Polychlorinated biphenyls
and organochlorine pesticides in Great Lakes precipitation. J.
Great Lakes Res., 5: 61-68.
STREET, J.C. & SHARMA, R.P. (1975) Alteration of induced cellular
and humoral immune responses by pesticides and chemicals of
environmental concern: quantitative studies of immunosuppression by
DDT, Aroclor 1254, Carbaryl, Carbofuran, and Methylparathion.
Toxicol. appl. Pharmacol., 32: 587-602.
STREK, H.J. & WEBER, J.B. (1982) Behaviour of polychlorinated
biphenyls (PCBs) in soils and plants. Environ. Pollut.,
28: 291-312.
STREK, H.J., WEBER, J.B., SHEA, P.J., MROZEK, E., & OVERCASH, M.R.
(1981) Reduction of polychlorinated biphenyl toxicity and uptake of
carbon-14 activity by plants through the use of activated carbon.
J. agric. food Chem., 29: 288-293.
STRIK, J.J.T.W.A. (1973) Species differences in experimental
porphyria caused by polyhalogenated aromatic compounds. Enzyme,
16: 224-230.
SUBRAMANIAN, A., TANABE, S., HIDAKA, H., & TATSUKAWA, R. (1986)
Bioaccumulation of organochlorines (PCBs and p,p'-DDE) in
Antarctic Adelie penguins Pygoscelis adeliae collected during a
breeding season. Environ. Pollut., 40: 173-189.
SUBRAMANIAN, A.N., TANABE, S., TATSUKAWA, R., SAITO, S., &
MIYAZAKI, N. (1987) Reduction in the testosterone levels by PCBs
and DDE in Dall's porpoises of Northwestern North Pacific. Mar.
Pollut. Bull., 18: 643-646.
SUBRAMANIAN, A., TANABE, S., & TATSUKAWA, R. (1988) Use of
organochlorines as chemical tracers in determining some
reproductive parameters in Dalli-type Dall's porpoise Phocoenoides
dalli. Mar. environ. Res., 25: 161-174.
SUBRAMANIAN, B.R., TANABE, S., HIDAKA, H., & TATSUKAWA, R. (1983)
DDTs and PCB isomers and congeners in Antarctic fish. Arch.
environ. Contam. Toxicol., 12: 621-626.
SUMMERMAN, W., ROHLEDER, H., & KORTE, F. (1978) [Polychlorinated
biphenyls in food.] Z. Lebensmittelunters. Forsch, 166: 137-144
(in German).
SUNDSTROM, G. & JANSSON, B. (1975) The metabolism of
2,2',3,5',6-pentachlorobiphenyl in rats, mice, and quails.
Chemosphere, 4: 361-370.
SUNDSTROM, G. & WACHTMEISTER, C.A. (1975) Structure of a major
metabolite of 2,2',4,5,5'-pentachlorobiphenyl in mice. Chemosphere,
4: 7-11.
SUNDSTROM, G., HUTZINGER, O., & SAFE, S. (1976a) The metabolism of
chlorobiphenyls. A review. Chemosphere, 5: 267-298.
SUNDSTROM, G., HUTZINGER, O., & SAFE, S. (1976b) The metabolism of
2,2',4,4',5,5'-hexachlorobiphenyl by rabbits, rats, and mice.
Chemosphere, 5: 249-253.
SUZUKI, M., AIZAWA, N., OKANO, G., & TAKAHASHI, T. (1977)
Translocation of polychlorobiphenyls in soil into plants: a study
by a method of culture of soybean sprouts. Arch. environ. Contam.
Toxicol., 5: 343-352.
SWAIN, W.R. (1978) Chlorinated organic residues in fish, water and
precipitation from the vicinity of Isle Royale, Lake Superior. J.
Great Lakes Res., 4: 398-407.
TAKAGI, Y., ABURADA, S., OTAKE, T., &. IKEGAMI, N. (1989) Effect of
dam's accumulated tissue PCBs on mouse filial T-cell population and
T-cell subpopulations. Bull. environ. Contam. Toxicol.,
42: 443-450.
TAKAI, T., OHNO, S., & ISHIZUKI, Y. (1979) [Changes in the
concentration of PCB analogs in fish in Japan Sea.] Niigata
Rikagaku, 5: 54-56 (in Japanese).
TAKAMATSU, M., INOUE, Y., & ABE, S. (1974) [Diagnostic meaning of
the blood PCB.] Fukuoka Acta med., 65: 28-31 (in Japanese).
TAKAMATSU, M., OKI, M., MAEDA, K., INOUE, Y., HIRAYAMA, H., &
YOSHIZUKA, K. (1984) PCBs in blood of workers exposed to PCBs and
their health status. Am. J. ind. Med., 5: 59-68.
TAKAMATSU, M., OKI, M., MAEDA, K., INOUE, Y., HIRAYAMA, H., &
YOSHIZUKA, K. (1985) Surveys of workers occupationally exposed to
PCBs and of Yusho patients. Environ. health Perspect., 59: 91-97.
TAKEI, G.H., KAUAHIKAUA, S.M., & LEONG, G.H. (1983) Analyses of
human milk samples collected in Hawaii for residues of
organochlorine pesticides and polychlorobiphenyls. Bull. environ.
Contam. Toxicol., 30: 606-613.
TAKI, I., HISANAGA, S., & AMAGESE, Y. (1969) [Report on Yusho
(chlorobiphenyls poisoning). Especially further study of its
dermatological findings.] Fukuoka Acta med., 62: 132-138
(in Japanese).
TALCOTT, P.A. & KOLLER, L.D. (1983) The effect of inorganic lead
and/or a polychlorinated biphenyl on the developing immune system
of mice. J. Toxicol. environ. Health, 12: 337-352.
TANABE, S. (1985) [Distribution, behavior and fate of PCBs in the
marine environment.] J. Oceanogr. Soc. Jpn, 41: 358-370
(in Japanese).
TANABE, S. (1988) PCB problems in the future: Foresight from
current knowledge. Environ. Pollut., 50: 5-28.
TANABE, S., NAKAGAWA, Y., & TATSUKAWA, R. (1981) Absorption
efficiency and biological half-life of individual chlorobiphenyls
in rats treated with chlorobiphenyl products. Agric. biol. Chem.,
45: 717-726.
TANABE, S., TATSUKAWA, R., MARUYAMA, K., & MIYASAKI, N. (1982)
Transplacental transfer of PCBs and chlorinated hydrocarbon
pesticides from the pregnant striped dolphin (Stenella
coeruleoalba) to her fetus. Agric. biol. Chem., 46: 1249-1254.
TANABE, S., MORI, T., TATSUKAWA, R., & MIYAZAKI, N. (1983) Global
pollution of marine mammals by PCBs, DDTs and HCHs (BHCs).
Chemosphere, 12: 1269-1275.
TANABE, S., TANAKA, H., & TATSUKAWA, R. (1984) Polychlorobiphenyls,
DDT, and hexachlorocyclohexane isomers in the western North Pacific
ecosystem. Arch. environ. Contam. Toxicol., 13: 731-738.
TANABE, S., MIURA, S., & TATSUKAWA, R. (1986a) Variations of
organochlorine residues with age and sex in Antarctic minke whale.
Mem. Natl Inst. polar Res., 44: 174-181.
TANABE, S., SUBRAMANIAN, A., HIDAKA, H., & TATSUKAWA, R. (1986b)
Transfer rates and pattern of PCB isomers and congeners and
p,p'-DDE from mother to egg in Adelie penguin (Pygoscelis
adeliae). Chemosphere, 15: 343-351.
TANABE, S., KANNAN, N., WAKIMOTO, T., & TATSUKAWA, R. (1987a)
Method for the determination of three toxic non-orthochlorine
substituted coplanar PCBs in environmental samples at
part-per-trillion levels. Int. J. environ. anal. Chem.,
29: 199-213.
TANABE, S., KANNAN, N., SUBRAMANIAN, A., WATANABE, S., & TATSUKAWA,
R. (1987b) Highly toxic coplanar PCBs: Occurrence, source,
persistency and toxic implications to wildlife and humans. Environ.
Pollut., 47: 147-163.
TANABE, S., WATANABE, S., KAN, H., & TATSUKAWA, R. (1988) Capacity
and mode of PCB metabolism in small cetaceans. Mar. Mammal Sci.,
4: 103-124.
TANAKA, K. & KOMATSU, F. (1972) [Shortening of hexobarbital
sleeping time after small doses of PCB in rats.] Fukuoka Acta med.,
63: 360-366 (in Japanese).
TANAKA, H. & OGI, H. (1984) [Bioaccumulation of organochlorine
compounds by pelagic sea birds.] Mar. Sci. Mon., 16: 221-225
(in Japanese).
TATEM, H.E. (1986) Bioaccumulation of polychlorinated biphenyls and
metals from contaminated sediment by freshwater prawns,
Macrobrachium rosenbergii and clams, Corbicula fluminea. Arch.
environ. Contam. Toxicol., 15: 171-183.
TATEMATSU, M., NAKANISHI, K., MURASAKI, G., MIYATA, Y., HIROSE, M.,
& ITO, N. (1979) Enhancing effect of inducers of liver microsomal
enzymes on induction of hyperplastic liver nodules by
N-2-fluorenylacetamide in rats. J. Natl Cancer Inst.,
63(6): 1411-1416.
TATSUKAWA, K. & TANABE, S. (1983) Environmental monitoring:
geochemical and biochemical behaviour of PCBs in the open ocean
environment. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of the PCB Seminar, The Hague, 28-30 September 1983,
The Hague, Ministry of the Environment, pp: 99-118.
TATSUKAWA, K. & WATANABE, I. (1972) Air pollution by PCBs. Shoku No
Kagaku, 8: 55-63.
TAYLOR, P.R., LAWRENCE, C.E., HWANG, H.L., & PAULSON, A.S. (1984)
Polychlorinated biphenyls: Influence on birth weight and gestation.
Am. J. public Health, 74(10): 1153-1154.
TAYLOR, P.R., STELMA, J.M., & LAWRENCE, C.E. (1989) The relation of
polychlorinated biphenyls to birth weight and gestational age in
the offspring of occupationally exposed mothers. Am. J. Epidemiol.,
129(2): 395-406.
THOMAS, G.H. & REYNOLDS, L.M. (1973) Polychlorinated terphenyls in
paperboard samples. Bull. environ. Contam. Toxicol., 10: 37-41.
THOMAS, P.T. & HINSDILL, R.D. (1978) Effect of polychlorinated
biphenyls on the immune responses of Rhesus monkeys and mice.
Toxicol. appl. Pharmacol., 44: 41-51.
THOMAS, P.T. & HINSDILL, R.D. (1980) Perinatal PCB exposure and its
effect on the immune system of young rabbits. Drug chem. Toxicol.,
3: 173-184.
TILSON, H.A., DAVIS, G.J., MCLACHLAN, J.A., & LUCIER, G.W. (1979)
The effects of polychlorinated biphenyls given prenatally on the
neurobehavioural development of mice. Environ. Res., 18: 466-474.
TOFTGARD, R., NILSEN, O.G., & GLAUMANN, H. (1980) Polychlorinated
terphenyls are mixed type of inducers of rat liver microsomal
cytochrome P-450. Dev. Biochem., 13: 227-230.
TOOBY, T.E., HURSEY, P.A., & ALABASTER, J.S. (1975) The acute
toxicity of 102 pesticides and miscellaneous substances to fish.
Chem. Ind., 12: 523-526.
TORI, G.M. & PETERLE, T.J. (1983) Effects of PCBs on mourning dove
courtship behavior. Bull. environ. Contam. Toxicol., 30: 44-49.
TRUELOVE, J., GRANT, D., MES, J., TRYPHONAS, H., TRYPHONAS, L., &
ZAWIDZKA, Z. (1982) Polychlorinated biphenyl toxicity in the
pregnant Cynomolgus monkey: A pilot study. Arch. environ. Contam.
Toxicol., 11: 583-588.
TRUELOVE, J.F., TANNER, J.R., LANGLOIS, I.A., STAPLEY, R.A., MES,
J.C., & ARNOLD, D.L. (in press) Effect of polychlorinated biphenyl
on several endocrine reproductive parameters in the female Rhesus
monkey. Fundam. appl. Toxicol.
TRYPHONAS, L., TRUELOVE, J., ZAWIDZKA, Z., WONG, J., MES, J.,
CHARBONNEAU, S., GRANT, D.L., & CAMPBELL, J.S. (1984)
Polychlorinated biphenyl (PCB) toxicity in adult Cynomolgus monkeys
( M. fascicularis): a pilot study. Toxicol. Pathol., 12: 10-25.
TRYPHONAS, L., ARNOLD, D.L., ZAWIDZKA, Z., MES, J., CHARBONNEAU,
S., & WONG, J. (1986a) A pilot study in adult Rhesus monkeys
(M. mullata) treated with Aroclor 1254 for two years. Toxicol.
Pathol., 14(1): 1-10.
TRYPHONAS, L., CHARBONNEAU, S., TRYPHONAS, H., ZAWIDZKA, Z., MES,
J., WONG, J., & ARNOLD, D.L., (1986b) Comparative aspects of
Aroclor 1254 toxicity in adult Cynomolgus and Rhesus monkeys: a
pilot study. Arch. environ. Contam. Toxicol., 15: 159-169.
TSUDA, H., LEE, G., & FARBER, E. (1980) Induction of resistant
hepatocytes as a new principle for a possible short-term in vivo
test for carcinogens. Cancer Res., 40: 1157-1164.
TSUSHIMOTO, G., ASANO, S., TROSKO, J.E., & CHANG, C.-C. (1983)
Inhibition of intercellular communication by various congeners of
polybrominated biphenyl and polychlorinated biphenyl. In: PCBs:
Human and environmental hazards, Woburn, Massachusetts,
Butterworth, pp. 241-251.
TUCKER, E.S., LITSCHGI, W.J., & MEES, W.M. (1975a) Migration of
polychlorinated biphenyls in soil induced by percolating water.
Bull. environ. Contam. Toxicol., 13: 86-93.
TUCKER, E.S., SAEGER, V.W., & HICKS, O. (1975b) Activated sludge
primary biodegradation of polychlorinated biphenyls. Bull. environ.
Contam. Toxicol., 14: 705-712.
TUEY, D.B. & MATTHEWS, H.B. (1977) Pharmacokinetics of
3,3',5,5'-tetrachlorobiphenyl in the male rat. Drug Metab. Dispos.,
5: 444-450.
TUINSTRA, L.M.G.Th. (1983) Quantification of PCB residues in
environmental monitoring. Techniques and results. In: Barros, M.C.,
Könemann, H., & Visser, R., ed. Proceedings of the PCB Seminar, The
Hague, 28-30 September 1983, The Hague, Ministry of the
Environment, pp. 39-53.
TUINSTRA, L.G.M.Th., ERNST, G.F., ROOS, A.H., VAN MAZIJK, R.Y., &
SPANJERSBERG, F. (1985a) [Content of individual chlorophenyls in
complete infant food], Wageningen, RIKILT Institute (Unpublished
report No. 85.102) (in Dutch).
TUINSTRA, L.G.M.Th., ROOS, A.H., & WERDMULLER, G.A. (1985b)
Capillary gas chromatographic determination of some chlorobiphenyls
in eel fat: Interlaboratory study. J. Assoc. Off. Anal. Chem.,
68(4): 756-759.
TUINSTRA, L.G.M.Th., ROOS, A.H., GRIEPINK, B., & WELLS, D.E.
(1985c) Interlaboratory studies of the determination of selected
chlorobiphenyl congeners with capillary gas chromatography using
splitless- and on-column injection techniques. J. high. Res.
Chromatogr. Chromatogr. Commun., 8: 475-480.
TUMASONIS, C.F., BUSH, B., & BAKER, F.D. (1973) PCB levels in egg
yolks associated with embryonic mortality and deformity of hatched
chicks. Arch. environ. Contam. Toxicol., 1: 312-324.
TURNER, J.C. (1979) Transplacental movement of organochlorine
pesticide residues in desert bighorn sheep. Bull. environ. Contam.
Toxicol., 21: 116-124.
TURNER, J.C. & GREEN, R.S. (1974) Effect of polychlorinated
biphenyl (Aroclor 1254) on liver microsomal enzymes in the male
rat. Bull. environ. Contam. Toxicol., 12: 687-693.
TUTELYAN, V.A., KHAN, A.V., LASHNEVA, N.V., SOROKOVAYA, G.K., &
GADZHIEVA, Z.M. (1986) [Monooxygenase system activity and rat liver
microsome peroxidation rate in reinduction with polychlorinated
diphenyls.] Meditsina, 1(1): 38-40 (in Russian).
UENG, T.-H. & ALVARES, A.P. (1985) Selective induction and
inhibition of liver and lung cytochrome P-450-dependent
monooxygenase by the PCBs mixture, Aroclor 1016. Toxicology,
35: 83-94.
ULFSTRAND, S., SODERGREN, A., & RABOL, J. (1971) Effects of PCB on
nocturnal activity in caged robins, Erithacus rubecula L. Nature
(Lond.), 231: 467-468.
UNGER, M., OLSEN, J., & CLAUSEN, J. (1982) Organochlorine compounds
in the adipose tissue of deceased persons with and without cancer:
A statistical survey of some potential confounders. Environ. Res.,
29: 371-376.
URABE, H. (1974) [Foreword.] Fukuoka Acta med., 65: 1-4
(in Japanese).
URABE, H. & ASAHI, M. (1985) Past and current dermatological status
of Yusho patients. Environ. health Perspect., 59: 11-15.
UREY, J.C., KRICHER, J.C., & BOYLAN, J.M. (1976) Bioconcentration
of four pure PCB isomers by Chlorella pyrenoidosa. Bull. environ.
Contam. Toxicol., 16: 81-85.
US DHEW (1978) Subcommittee on health effects of PCBs and PBBs.
Environ. health Perspect., 24: 131-196.
US EPA (1980) Ambient water quality criteria for polychlorinated
biphenyls, Washington, DC, US Environmental Protection Agency,
211 pp (EPA 440/5-80-068).
US EPA (1983) Exposure assessment for polychlorinated biphenyls
(PCBs), Washington, DC, US Environmental Protection Agency.
US EPA (1985) Polychlorinated biphenyls in electrical transformers;
final rule (part IV). Fed. Reg., 50(137): 29170-29201.
US EPA (1987) Drinking-water criteria document for polychlorinated
biphenyls (PCBs): Final, Cincinnati, Ohio, US Environmental
Protection Agency, Environmental Criteria and Assessment Office
(ECAO-CIN-414).
USHIO, F. & DOGUCHI, M. (1977) Dietary intakes of some chlorinated
hydrocarbons and heavy metals estimated in experimentally prepared
diets. Bull. environ. Contam. Toxicol., 17(6): 707-711.
USHIO, F., FUKANO, S., NISHIDA, K., KANI, T., & DOGUCHI, M. (1974)
Some attempt to estimate the total daily intake of pesticides and
PCB residues and trace heavy metals. Annu. Rep. Tokyo Metrop. Res.
Lab., 25: 307-312 (in Japanese).
UZAWA, H., ITO, Y., NOTOMI, A., & KATSUKI, S. (1969)
[Hyperglyceridemia resulting from intake of rice oil contaminated
with chlorinated biphenyls.] Fukuoka Acta med., 60: 449-454
(in Japanese).
UZAWA, H., NOTOMI, A., NAKAMUTA, S., & IKEURA, Y. (1972)
[Consecutive three year follow-up study of serum triglyceride
concentrations of 82 subjects with PCB poisoning.] Fukuoka Acta
med., 63: 401-404 (in Japanese).
VAN DER KOLK, J. (1984) Consideration of a Codex approach to
contamination of foodstuffs with PCBs. Joint FAO/WHO Food Standards
Programme Codex Committee on Pesticide Residues, Sixteenth session,
The Hague, 28 May-4 June 1984, Codex Alimentarius Commission
(CX/PR 84/10).
VAN DER KOLK, J. (1985) Intake by man of organohalogen compounds
through food: the main route of exposure. Report on a WHO
Consultation on: Organohalogen Compounds in Human Milk and Related
Hazards, Bilthoven, Copenhagen, World Health Organization, Regional
Office for Europe, (ICP/CEH 501/m05).
VAN DYK, L.P., LÖTTER, L.H., MULLEN, J.E.C., & KOCK, A., DE (1987)
Organochlorine insecticide residues in human fat and milk samples
in South Africa. Chemosphere, 16(4): 705-711.
VAN HOVE HOLDRINET, M. (in press) Preliminary results of an
inter-laboratory PCB check sample programme. J. environ. Qual.
VAN MILLER, J.P., HSU, I.C., & ALLEN. J.R. (1975) Distribution and
metabolism of 3H-2,5,2',5'-tetrachlorobiphenyl in rats. Proc. Soc.
Exp. Biol. Med., 148: 682-687.
VANNUCCHI, C., SIVIERI, S., & CECCANTI, M. (1978) Residues of
chlorinated naphthalenes, other hydrocarbons and toxic metals (Hg,
Pb, Cd) in tissues of Mediterranean seagulls. Chemosphere,
6: 483-490.
VAN VLIET, T. (1990) Polychlorinated biphenylen (PCBs).
Interactions between different congeners in in vitro enzyme
induction assays, Stockholm, Institute of Environmental Medicine,
Karolinska Institute (IMM Report No. 3/90).
VAZ, R., LINDER, C.E., & NOREN, K. (1982) Levels of organochlorine
pesticides and PCB in Swedish and imported meat, 1972-1977. Var
Föda, 34(Suppl.1): 23-32.
VEITH, G.D., KUEHL, D.W., PUGLISI, F.A., GLASS, G.E., & EATON, J.G.
(1977) Residues of PCB's and DDT in the Western Lake Superior
ecosystem. Arch. environ. Contam. Toxicol., 5: 487-499.
VEITH, G.D., KUEHL, D.W., LEONARD, E.N., PUGLISI, F.A., & LEMKE,
A.E. (1979) Fish, wildlife and estuaries. Polychlorinated biphenyls
and other organic chemical residues in fish from major watersheds
of the United States, 1976. Pestic. monit. J., 13(1): 1-8.
VERNBERG, F.J., GURAM, M.S., & SAVORY, A. (1977) Survival of larval
and adult fiddler crabs exposed to Aroclor 1016 and 1254 and
different temperature-salinity combinations. In: Vernberg, F.J.,
Calabrese, A., Thurberg, F.P., & Vernberg, W.B., ed. Physiological
responses of marine biota to pollutants, New York, London, Academic
Press, pp. 37-50.
VERSAR, INC. (1984) Exposure assessment for polychlorinated
biphenyls (PCBs): Incidental production, recycling and selected
authorized uses. Hypothetical occupational and consumer exposure to
PCBs in natural gas, Springfield, Virginia, Versar, Inc., Vol. II
(Report to US Environmental Protection Agency, Office of Toxic
Substances, Washington).
VILLENEUVE, D.C., GRANT, D.L., PHILLIPS, W.E.J., CLARK, M.L., &
CLEGG, D.J. (1971a) Effects of PCB administration on microsomal
enzyme activity in pregnant rabbits. Bull. environ. Contam.
Toxicol., 6: 120-128.
VILLENEUVE, D.C., GRANT, D.L., KHERA, K., CLEGG, D.J., BAER, H., &
PHILLIPS, W.E.J. (1971b) The fetotoxicity of a polychlorinated
biphenyl mixture (Aroclor 1254) in the rabbit and in the rat.
Environ. Physiol., 1: 67-71.
VILLENEUVE, D.C., GRANT, D.L., & PHILLIPS, W.E.J. (1972)
Modification of pentobarbital sleeping times in rats following
chronic PCB ingestion. Bull. environ. Contam. Toxicol., 7: 264-269.
VILLENEUVE, D.C., REYNOLDS, L.M., THOMAS, G.H., & PHILLIPS, W.E.J.
(1973a). Polychlorinated biphenyls and polychlorinated terphenyls
in Canadian food packaging materials. J. Assoc. Off. Anal. Chem.,
56: 999-1001.
VILLENEUVE, D.C., REYNOLDS, L.M., & PHILLIPS, W.E.J. (1973b)
Residues of PCBs and PCTs in Canadian and imported European cheeses
- 1972. Pestic. monit. J., 7: 95-96.
VODICNIK, M.J., (1986) The effect of pregnancy and lactation on the
disposition of [2,4,2'4-14C] tetrachlorobiphenyl in the mouse.
Fundam. appl. Toxicol., 6: 53-61.
VODICNIK, J.J. & LECH, J.J. (1980) The transfer of 2,4,5,2',4',5'-
hexachlorobiphenyl to fetuses and nursing offspring. I. Disposition
in pregnant and lactating mice and accumulation in young. Toxicol.
appl. Pharmacol., 54: 293-300.
VODICNIK, M.J. & PETERSON, R.E. (1985) The enhancing effect of
spawning on elimination of a persistent polychlorinated biphenyl
from female yellow perch. Fundam. appl. Toxicol., 5: 770-776.
VODICNIK, M.J., ELCOMBE, C.R., & LECH, J.J. (1980) The transfer of
2,4,5,2',4',5'-hexachlorobiphenyl to fetuses and nursing offspring
II. Induction of hepatic microsomal monooxygenase activity in
pregnant and lactating mice and their young. Toxicol. appl.
Pharmacol., 54: 301-310.
VOS, J.G. & KOEMAN, J.H. (1970) Comparative toxicologic study with
polychlorinated biphenyls in chickens with special reference to
porphyria, oedema formation, liver necrosis and tissue residues.
Toxicol. appl. Pharmacol., 17: 656-668.
VOS, J.G., KOEMAN, J.H., VAN DER MAAS, H.J., TEN NOEVER DE BRAUW,
M.C., & DE VOS, R.H. (1970) Identification and toxicological
evaluation of chlorinated dibenzofuran and chlorinated naphthalene
in two commercial polychlorinated biphenyls. Food Cosmet. Toxicol.,
8: 625-633.
VOS, J.G. & BEEMS, R.B. (1971) Dermal toxicity studies of technical
polychlorinated biphenyls and fractions thereof in rabbits.
Toxicol. appl. Pharmacol., 19: 617-633.
VOS, J.G. & NOTENBOOM-RAM, E. (1972) Comparative toxicity study of
2,4,5,2',4',5',-hexachlorobiphenyl and a polychlorinated biphenyl
mixture in rabbits. Toxicol. appl. Pharmacol., 23: 562-578.
VOS, J.G. & DE ROIJ, T. (1972) Immunosuppressive activity of a
polychlorinated biphenyl preparation on the humoral immune response
in guinea-pigs. Toxicol. appl. Pharmacol., 21: 549-555.
VOS, J.G. & VAN DRIEL-GROOTENHUIS, L. (1972) PCB-induced
suppression of the humoral and cell-mediated immunity in
guinea-pigs. Sci. total Environ., 1: 289-300.
VOS, J.G. & VAN GENDEREN, H. (1973) Toxicological aspects of
immunosuppression. In: Deichman, W.B., ed. Pesticides in the
environment, a continuity controversy. 8th International Conference
on Toxicology and Occupational Medicine, New York, Intercontinental
Medical Company.
VREELAND, V. (1974) Uptake of chlorobiphenyls by oysters, Environ.
Pollut., 6: 135-140.
WAKIMOTO, T., KANNAN, N., ONO, M., TATSIUKAWA, R., & MASUDA, Y.
(1988) Isomer-specific determination of polychlorinated
dibenzofurans in Japanese and American polychlorinated biphenyls.
Chemosphere, 17(4): 743-750.
WALLNOFER, P.R. & KONIGER, M. (1974) [Experiments on the uptake of
hexachlorobenzene and polychlorinated biphenyls by cultivated
plants from different substrates.] Z. Pflanzenkr. Pflanzenschutz,
26: 54-57 (in German).
WALLNOFER, P.R., ENGELHARDT, G., SAFE, S., & HUTZINGER, O. (1973)
Microbial hydroxylation of 4-chlorobiphenyl and 4,4'-
dichlorobiphenyl. Chemosphere, 2: 69-72.
WALLNOFER, P., KONIGER, M., & ENGELHARDT, G. (1975) [Behaviour of
xenobiotic chlorinated hydrocarbons (HCBs and PCBs) in cultivated
plants and soil.] Z. Pflanzenkr. Pflanzenschutz, 82: 91-100
(in German).
WALSH, G.E., HOLLISTER, T.A., & FORESTER, J. (1974) Translocation
of four organochlorine compounds by red mangrove (Rhizophora
mangle L.) seedlings. Bull. environ. Contam. Toxicol.,
12: 129-135.
WARD, J.M. (1985) Proliferative lesions of the glandular stomach
and liver in F344 rats fed diets containing Aroclor 1254. Environ.
health Perspect., 60: 89-95.
WARDELL, R.E., SEEGMILLER, R.E., & BRADSHAW, W.S. (1982) Induction
of prenatal toxicity in the rat by diethylstilbestrol, Zeranol,
3,4,3',4'-tetrachlorobiphenyl, cadmium, and lead. Teratology,
26: 229-237.
WARSHAW, R., FISCHBEIN, A., THORNTON, I., MILLER, A., & SELIKOFF,
I.J. (1979) Decrease in vital capacity in PCB-exposed workers in a
capacitor manufacturing facility. Ann. NY Acad. Sci., 320: 277-283.
WASILEWSKA, L., OLOFFS, P.C., & WEBSTER, J.M. (1975) Effects of
carbofuran and a PCB on development of a bacteriophagous nematode
Acrobeloides nanus. Can. J. Zool., 53: 1709-1715.
WASSERMANN, D., WASSERMANN, M., CUCOS, S., & DJAVAHERIAN, M. (1973)
Function of the adrenal gland-zona fasciculata in rats receiving
polychlorinated biphenyls. Environ. Res., 6: 334-338.
WASSERMANN, D., WASSERMANN, M., & LEMESCH, C. (1975) Ultrastructure
of beta-cell of the endocrine pancreas in rats receiving
polychlorinated biphenyls. Environ. Physiol. Biochem., 5: 332-340.
WASSERMANN, M., WASSERMANN, D., CUCOS, S., & MILLER, M.J. (1979)
World PCBs map - storage and effects in man and his biologic
environment in the 1970s. Ann. NY Acad. Sci., 320: 69-124.
WASSERMANN, M., RON, M., BERCOVICI, B., WASSERMANN, D., CUCOS, S.,
& PINES, A. (1982) Premature delivery and organochlorine compounds:
PCB and some organochlorine insecticides. Environ. Res.,
28(1): 106-112.
WATANABE, I., YAKUSHIJI, T., KUWABARA, K., YOSHIDA, S., MAEDA, K.,
KASHIMOTO, T., KOYAMA, K., & KUNITA N. (1979) Surveillance of the
daily PCB intake from diet of Japanese women from 1972 through
1976. Arch. environ. Contam. Toxicol., 8: 67-75.
WATANABE, M. & SUGAHARA, T. (1981) Experimental formation of cleft
palate in mice with polychlorinated biphenyls (PCB). Toxicology,
19: 49-53.
WATANABE, M., HONDA, S., HAYASHI, M., & MATSUDA, T. (1982)
Mutagenic effects of combinations of chemical carcinogens and
environmental pollutants in mice as shown by the micronucleus test.
Mutat. Res., 97: 43-48.
WEBBER, M.D., MONTEITH, H.D., & CORNEAU, D.G.M. (1983) Assessment
of heavy metals and PCBs at sludge application sites. J. WPCF,
55(2): 187-195.
WEBER, J.B. & MROZEK, E. (1979) Polychlorinated biphenyls:
phytotoxicity, absorption and translocation by plants, and
inactivation by activated carbon. Bull. environ. Contam. Toxicol.,
23: 412-417.
WEGMAN, R.C.C. & BERKHOFF, C.J. (1986) [Chemical contaminants in
breastmilk. Report section 5: Polychlorinated biphenyls (PCB
congeners)], Bilthoven, The Netherlands, National Institute of
Public Health and Environmental Hygiene (Unpublished report
No. 638307006) (in Dutch).
WEGMAN, R.C.C. & GREVE, P.A. (1980) Halogenated hydrocarbons in
Dutch water samples over the years 1969-1977. In: Hydrocarbons and
halogenated hydrocarbons in the aquatic environment, New York,
London, Plenum Press, pp. 405-415.
WEIS, P. & WEIS, J.S. (1982) Toxicity of the PCBs Aroclor 1254 and
1242 to embryos and larvae of the mummichog, Fundulus
heteroclitus. Bull. environ. Contam. Toxicol., 28: 298-304.
WESELOH, D.V., MINEAU, P., & HALLETT, D.J. (1979) Organochlorine
contaminants and trends in reproduction in Great Lakes herring
gulls, 1974-1978. Trans. North Am. Wildl. Nat. Resour. Conf.,
44: 543-557.
WESELOH, D.V., TEEPLE, S.M., & GILBERTSON, M. (1983) Double-crested
cormorants of the Great Lakes: egg laying parameters, reproductive
failure, and contaminant residues in eggs, Lake Huron 1972-1973.
Can. J. Zool., 61: 427-436.
WESTCOTT, J.W., SIMON, C.G., & BIDLEMAN, T.F. (1981) Determination
of polychlorinated biphenyl vapour pressures by a semimicro gas
saturation method. Environ. Sci. Technol., 15(11): 1375-1378.
WESTIN, D.T., OLNEY, C.E., & ROGERS, B.A. (1983) Effects of
parental and dietary PCBs on survival, growth, and body burdens of
larval striped bass. Bull. environ. Contam. Toxicol., 30: 50-57.
WESTOO, G. & NOREN, K. (1970a) [Levels of organochlorine pesticides
and polychlorinated biphenyls in fish caught in Swedish water areas
or kept for sale in Sweden, 1967-1970.] Var Föda, 3: 93-146
(in Swedish with English summary).
WESTOO, G. & NOREN, K. (1970b) Determination of organochlorine
pesticides and polychlorinated biphenyls in animal foods. Acta
chem. Scand., 24: 1639-1644.
WESTOO, G., NOREN, K., & ANDERSSON, M. (1971) [Levels of
organochlorine pesticides and polychlorinated biphenyls in some
cereal products.] Var Föda, 10: 341-360 (in Swedish with English
summary).
WHITE, D.H. (1979) Nationwide residues of organochlorine compounds
in wings of adult mallards and black ducks, 1976-77. Pestic. monit.
J., 13: 12-16.
WHITE, R.D., ALLEN, S.D., & BRADSHAW, W.S. (1983) Delay in the
onset of parturition in the rat following prenatal administration
of developmental toxicants. Toxicol. Lett., 18: 185-192.
WHO (1976) Environmental Health Criteria 2: Polychlorinated
biphenyls and terphenyls, Geneva, World Health Organization, 85 pp.
WHO (1985) A review of the 1980-1983 data received from the FAO/WHO
Collaborating Centres for Food Contamination Monitoring. Joint
FAO/WHO Food Contamination Monitoring Programme: PCBs residues in
food, Geneva, World Health Organization, Geneva (Paper prepared for
Codex Committee on Pesticide Residues).
WHO (1986a) Joint FAO/WHO Food Contamination Monitoring Programme.
Summary of 1980-1983 monitoring data, Geneva, World Health
Organization (WHO/EHE/FOS/86.2.).
WHO (1986b) Joint FAO/WHO Food Contamination Monitoring Programme.
Chemical contaminants in foods: 1980-1983, Geneva, World Health
Organization (WHO/EHE/FOS/86.5).
WHO (1989) Environmental Health Criteria 88: Polychlorinated
dibenzo-para-dioxins and dibenzofurans, Geneva, World Health
Organization, 409 pp.
WHO (1990) Evaluation of certain food additives and contaminants.
Thirty-fifth report of the Joint FAO/WHO Expert Committee on Food
Additives, Geneva, World Health Organization (WHO Technical Report
Series 789).
WHO/EURO (1985) Organohalogen compounds in human milk and related
hazards. Report on a WHO consultation, Bilthoven, 9-11 January
1985, Copenhagen, World Health Organization, Regional Office for
Europe (IPC/CEH 501/m05).
WHO/EURO (1987) PCBs, PCDDs, and PCDFs: Prevention and control of
accidental and environmental exposures, Copenhagen, World Health
Organization, Regional Office for Europe (Environmental Health
Series 23).
WHO/EURO (1988) PCBs, PCDDs and PCDFs in breast milk: Assessment of
health risks, Copenhagen, World Health Organization, Regional
Office for Europe (Environmental Health Series 29).
WHO/EURO (1989) Levels of PCBs, PCDDs and PCDFs in breastmilk.
Results on WHO coordinated interlaboratory quality control studies
and analytical field studies, Copenhagen, World Health
Organization, Regional Office for Europe (Environmental Health
Series 34).
WICKIZER, T.M. & BRILLIANT, L.B. (1981) Testing for polychlorinated
biphenyls in human milk. Pediatrics, 68(3): 411-415.
WICKIZER, T.M., BRILLIANT, L.B., COPELAND, R., & TILDEN, R. (1981)
Polychlorinated biphenyl contamination of nursing mother's milk in
Michigan. Am. J. public Health, 71(2): 132-137.
WIEMEYER, S.N., SPITZER, P.R., KRANTZ, W.C., LAMONT, T.G., &
CROMARTIE, E. (1975) Effects of environmental pollutants on
Connecticut and Maryland ospreys. J. Wildl. Manage., 39: 124-139.
WIEMEYER, S.N., LAMONT, T.G., BUNCK, C.M., SINDELAR, C.R.,
GRAMLICH, F.J., FRASER, J.D., & BYRD, M.A. (1984) Organochlorine
pesticide, polychlorobiphenyl, and mercury residues in bald eagle
eggs - 1969 to 1979 - and their relationships to shell thinning and
reproduction. Arch. environ. Contam. Toxicol., 13: 529-549.
WIEMEYER, S.N., SCHMELING, S.K., & ANDERSON, A. (1987)
Environmental pollutant and necropsy data for ospreys from the
eastern United States, 1975-1982. J. Wildl. Dis., 23: 279-291.
WILDISH, D.J. (1970) The toxicity of polychlorinated biphenyls
(PCB) in sea water to Gammarus oceanicus. Bull. environ. Contam.
Toxicol., 5: 202-204.
WILDISH, D.J. & ZITKO, V. (1971) Uptake of polychlorinated
biphenyls from sea water by Gammarus oceanicus. Mar. Biol.,
9: 213-218.
WILDISH, D.J., METCALFE, C.D., AKAGI, H.M., & MCLEESE, D.W. (1980)
Flux of Aroclor 1254 between estuarine sediments and water. Bull.
environ. Contam. Toxicol., 24: 20-26.
WILLETT, L.B. (1980) Polychlorinated biphenyls from dairy farms:
Consequence in market milk. J. dairy Sci., 63: 1961-1965.
WILLETT, L.B., LIU, T.-T.Y, DURST, H.I., SMITH, K.L., & REDMAN,
D.R. (1987) Health and productivity of dairy cows fed
polychlorinated biphenyls. Fundam. appl. Toxicol., 9: 60-68.
WILLFORD, W.A. (1980) Chlorinated hydrocarbons as a limiting factor
in the reproduction of lake trout in Lake Michigan. In: Swain, W.R.
& Shannon, V.R., ed. Proceedings of the 3rd USA-USSR Symposium on
Effluent Pollution and Aquatic Ecosystems, Washington, DC, US
Environmental Protection Agency, pp. 75-83 (PEA-660/9-80-034).
WOLFF, M. (1983) Occupationally derived chemicals in breast milk.
Am. J. ind. Med., 4: 259-281.
WOLFF, M.S. (1984) Analysis of skin lipids for halogenated
hydrocarbons. Anal. Chem., 56: 1492-1496.
WOLFF, M.S. (1985) Occupational exposure to polychlorinated
biphenyls (PCBs). Environ. health Perspect., 60: 133-138.
WOLFF, M.S., ANDERSON, H.A., & SELIKOFF, I.J. (1982) Human tissue
burdens of halogenated aromatic chemicals in Michigan. J. Am. Med.
Assoc., 247(15): 2112-2116.
WÖLFLE, D., MÜNZEL, P., FISCHER, G., & BOCK, K.W. (1988) Altered
growth control of rat hepatocytes after treatment with
3,4,3',4'-tetrachlorobiphenyl in vivo and in vitro.
Carcinogenesis, 9(6): 919-924.
WONG, P.T.S. & KAISER, K.L.E. (1975) Bacterial degradation of
polychlorinated biphenyls II. Rate studies. Bull. environ. Contam.
Toxicol., 13: 249-255.
WONG, A., BASRUR, P.K., & SAFE, S. (1979) The metabolically
mediated DNA damage and subsequent repair by 4-chlorobiphenyl in
Chinese hamster ovary cells. Res. Commun. chem. Pathol. Pharmacol.,
24: 543-548.
WONG, T.K., EVERSON, R.B., & HSU SHU-TAO (1985) Potent induction of
human placental mono-oxygenase activity by previous dietary
exposure to polychlorinated biphenyls and their thermal degradation
products. Lancet, March 30: 721-724.
WREN, C.D., HUNTER, D.B., LEATHERLAND, J.F., & STOKES, P.M. (1987a)
The effects of polychlorinated biphenyls and methylmercury, singly
and in combination, on Mink. I: Uptake and toxic responses. Arch.
environ. Contam. Toxicol., 16: 441-447.
WREN, C.D., HUNTER, D.B., LEATHERLAND, J.F., & STOKES, P.M. (1987a)
The effects of polychlorinated biphenyls and methylmercury, singly
and in combination on mink. II: Reproduction and kit development.
Arch. environ. Contam. Toxicol., 16: 449-454.
WYMAN, K.D. & O'CONNORS, C.H.B. (1980) Implications of short-term
PCB uptake by small estuarine copepods (Genus Acartia) from
PCB-contaminated water, inorganic sediments and phytoplankton.
Estuarine coastal Mar. Sci., 11: 121-131.
WYNDHAM, C., DEVENISH, J., & SAFE, S. (1976) The in vitro
metabolism, macromolecular binding and bacterial mutagenicity of
4-chlorobiphenyl, a model PCB substrate. Res. Commun. chem. Pathol.
Pharmacol., 15: 563-570.
WYSS, P.A., MUHLEBACK, S., & BICKEL, M.H. (1986) Long-term
pharmacokinetics of 2,2',4,4',5,5'-hexachlorobiphenyl (6-CB) in
rats with constant adipose tissue mass. Drug. Metab. Dispos.,
14: 361-365.
YAGAMUCHI, A., YOSHIMURA, T., & KURATSUNE, M. (1971) [A survey on
pregnant women having consumed rice oil contaminated with
chlorobiphenyls and their babies.] Fukuoka Acta med., 62: 112-117
(in Japanese).
YAGI, N. (1980) [Lipid metabolism in PCB poisoned rats.] Jpn. J.
Hyg., 35: 659-664 (in Japanese).
YAGI, N., KAMOHARA, K., & ITOKAWA, Y. (1979) Thiamine deficiency
induced by polychlorinated biphenyls (PCBs) and
dichlorodiphenyltrichloroethane (DDT) administration to rats. J.
environ. Pathol. Toxicol., 2: 1119-1125.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., YOSHIDA, S., KOYAMA, K.,
& KUNITA, N. (1977) Residues of polychlorinated biphenyls and
organochlorine pesticides in human milk, blood, and diet (V). Proc.
Osaka Prefect Inst. Public Health, 8: 35-44.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., YOSHIDA, S., KOYAMA, K.,
HARA, I., & KUNITA, N. (1978) Long-term studies of the excretion of
polychlorinated biphenyls (PCBs) through the mother's milk of an
occupationally exposed worker. Arch. environ. Contam. Toxicol.,
7: 493-504.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., YOSHIDA, S., HORI, S.,
FUKUSHIMA, S., KASHIMOTO, T., KOYAMA, K., & KUNITA, N. (1979)
Levels of organochlorine pesticides and polychlorinated biphenyls
(PCBs) in mothers' milk collected in Osaka Prefecture from 1969 to
1976. Arch. environ. Contam. Toxicol., 8: 59-66.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., TANAKA, R., KASHIMOTO,
T., KUNITA, N., & HARA I. (1984a) Rate of decrease and half-life of
polychlorinated biphenyls (PCBs) in the blood of mothers and their
children occupationally exposed to PCBs. Arch. environ. Contam.
Toxicol., 13: 341-345.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., TANAKA, R., KASHIMOTO,
T., KUNITA, N., & HARA I. (1984b) Postnatal transfer of PCBs from
exposed mothers to their babies: influence of breast-feeding. Arch.
environ. Health, 39(5): 368-375.
YAMAMOTO, H.A. & YOSHIMURA, H. (1973) Metabolic studies on
polychlorinated biphenyls. III. Complete structure and acute
toxicity of the metabolites of 2,4,3',4',-tetrachlorobiphenyl.
Chem. pharm. Bull. (Tokyo), 21: 2237-2242.
YAMASHITA, F. & HAYASHI, M. (1985) Fetal PCB Syndrome: Clinical
features intrauterine growth retardation and possible alteration in
calcium metabolism. Environ. health Perspect., 59: 41-46.
YOBS, A.R. (1972) Levels of polychlorinated biphenyls in adipose
tissue of the general population of the nation. Environ. health
Perspect., 1: 79-81.
YOSHIDA, S. & NAKAMURA, A. (1979) Residual status after
participation of methylsulfone metabolites of polychlorinated
biphenyls in the breast milk of a former employee in a capacitor
factory. Bull. environ. Contam. Toxicol., 21: 111-115.
YOSHIDA, T., TAKASHIMA, F., & WATANABE, T. (1973) Distribution of
[14C] PCBs in Carp. Ambio, 2: 111-113.
YOSHIHARA, S., KAWANO, K., YOSHIMURA, H., KUROKI, H., & MASUDA, Y.
(1979) Toxicological assessment of highly chlorinated biphenyl
congeners retained in the Yusho patients. Chemosphere, 8: 531-538.
YOSHIHARA, S., NAGATA, K., WADA, I., YOSHIMURA, H., KUROKI, H., &
MASUDA, Y. (1982) A unique change of steroid metabolism in rat
liver microsomes induced with highly toxic polychlorinated biphenyl
(PCB) and polychlorinated dibenzofuran (PCDF). J. pharmacobiol.
Dyn., 5: 994-1004.
YOSHIMURA, T. (1971) [Epidemiological analysis of "Yusho" patients
with special reference to sex, age, clinical grades and oil
consumption.] Fukuoka Acta med., 62: 109-116 (in Japanese).
YOSHIMURA, T. (1974) Epidemiological study on Yusho babies born to
mothers who had consumed oil contaminated by PCBs. Fukuoka Igahu
Zushi, 65(1): 74-80.
YOSHIMURA, H. & YAMAMOTO, H. (1975) A novel route of excretion of
2,4,3',4',-tetrachlorobiphenyl in rats. Bull. environ. Contam.
Toxicol., 13: 681-687.
YOSHIMURA, H., YAMAMOTO, H., NAGAI, J., YAE, Y., UZAWA, H., ITO,
Y., NOTOMI, A., MIMAKAMI, S., ITO, A., KATO, K., & TSUJI, H. (1971)
[Studies on the tissue distribution and the urinary and faecal
excretion of 3H-Kanechlor (chlorobiphenyls) in rats.] Fukuoka Acta
med., 62: 11-19 (in Japanese).
YOSHIMURA, H., YAMAMOTO, H., & SAEKI, S. (1973) Metabolic studies
on polychlorinated biphenyls II. Metabolic fate of
2,4,3',4'-tetrachlorobiphenyl in rats. Chem. pharm. Bull. (Tokyo),
21: 2231-2236.
YOSHIMURA, H., YAMAMOTO, H., & KINOSHITA, H. (1974) [Metabolic
studies on polychlorinated biphenyls. V. biliary excretion of
5-hydroxy-2,4,3',4'-tetrachlorobiphenyl, a major metabolite of
2,4,3',4'-tetrachlorobiphenyl.] Fukuoka Acta med., 65: 12-16
(in Japanese).
YOSHIMURA, H., OZAWA, N., & SAEKI, S. (1978) Inductive effect of
polychlorinated biphenyl, biphenyls mixture and individual isomers
on the hepatic microsomal enzymes. Chem. pharm. Bull. (Tokyo),
26: 1215-1221.
YOSHIMURA, H., YOSHIHARA, S., KOGA, N., NAGATA, K., WADA, I.,
KUROKI, J., & HOKAMA, Y. (1985) Inductive effect on hepatic enzymes
and toxicity of congeners of PCBs and PCDFs. Environ. health
Perspect., 59: 113-119.
YOUSSEF, N.N., BRINDLEY, W.A., & STREET, J.C. (1974) Fine
structural alterations in the developing spermatids of Musca
domestica induced by the PCB, Aroclor 1254. Cytobios,
11: 167-183.
ZACK, T.A. & MUSCH, D.C. (1979) Mortality of PCB workers at the
Monsanto Plant in Sanget, Illinois, St. Louis, Illinois, Monsanto
Chemical Co. (Unpublished Report).
ZELL, M., NEU, H.J., & BALLSCHMITTER, K. (1977) [Identification of
PCB components by the retention index. Comparison by capillary gas
chromatography.] Chemosphere, 7: 69-76 (in German).
ZEPP, R.L., Jr, & KIRKPATRICK, R.L. (1976) Reproduction in
cottontails fed diets containing a PCB. J. Wildl. Manage.,
40: 491-495.
ZHANG, Y., ROTT, B., & FREITAG, D. (1983) Accumulation and
elimination of 14C-PCBs by Daphnia magna Straus 1820.
Chemosphere, 12: 1645-1651.
ZIMMERLI, B. & MAREK, B. (1973) [The pesticide load of the Swiss
population.] Mitt. Lebensmittelunters. Hyg., 64(4): 459-479
(in German).
ZINCK, M.E. & ADDISON, R.F. (1974) The fate of 2-, 3-, and
4-chlorobiphenyl following intravenous administration to thorny
skate (Raja radiata) and the winter skate (Raja ocellata).
Arch. environ. Contam. Toxicol., 2: 52-61.
ZINKL, J.G. (1977) Skin and liver lesions in rats fed a
polychlorinated biphenyl mixture. Arch. environ. Contam. Toxicol.,
5(2): 217-228.
ZITKO, V. (1974) Uptake of chlorinated paraffins and PCB from
suspended solids and food by juvenile Atlantic salmon. Bull.
environ. Contam. Toxicol., 12: 406-412.
ZITKO, V. & SAUNDERS, R.L. (1979) Effect of PCBs and other
organochlorine compounds on the hatchability of Atlantic salmon
(Salmo salar) eggs. Bull. environ. Contam. Toxicol., 21: 125-130.
ZITKO, V., HUTZINGER, O., & SAFE, S. (1971) Retention times and
electron-capture detector responses of some individual
chlorobiphenyls. Bull. environ. Contam. Toxicol., 6: 160-163.
ZITKO, V., HUTZINGER, O., & CHOI, P.M.K. (1972) Contamination of
the Bay of Fundy - Gulf of Maine area with polychlorinated
biphenyls, polychlorinated terphenyls, chlorinated dibenzodioxins
and dibenzofurans. Environ. health Perspect., 1: 47-50.
ZULLEI, N. & BENECKE, G. (1978) Application of a new bioassay to
screen the toxicity of polychlorinated biphenyls on blue-green
algae. Bull. environ. Contam. Toxicol., 20: 786-792.
ANNEX 1.
NUMBERING OF PCB CONGENERS
No. Structure No. Structure No. Structure
Monochlorobiphenyls Tetrachlorobiphenyls Pentachlorobiphenyls
1 2 40 2,2',3,3' 82 2,2',3,3',4
2 3 41 2,2',3,4 83 2,2',3,3',5
3 4 42 2,2',3,4' 84 2,2',3,3',6
43 2,2',3,5 85 2,2',3,4,4'
Dichlorobiphenyls 44 2,2',3,5' 86 2,2',3,4,5
4 2,2' 45 2,2',3,6 87 2,2',3,4,5'
5 2,3 46 2,2',3,6 88 2,2',3,4,6
6 2,3' 47 2,2',4,4' 89 2,2',3,4,6'
7 2,4 48 2,2',4,5 90 2,2',3,4',5
8 2,4' 49 2,2',4,5' 91 2,2',3,4',6
9 2,5 50 2,2',4,6 92 2,2',3,5,5'
10 2,6 51 2,2',4,6' 93 2,2',3,5,6
11 3,3' 52 2,2',5,5' 94 2,2',3,5,6'
12 3'4 53 2,2',5,6' 95 2,2',3,5',6
13 3,4' 54 2,2',6,6'
15 4,4' 55 2,3,3',4 Pentachlorobiphenyls
56 2,3,3',4' 96 2,2',3,6,6'
Trichlorobiphenyls 57 2,3,3',5 97 2,2',3',4,5
16 2,2',3 58 2,3,3',5' 98 2,2',3',4,6
17 2,2',4 59 2,3,3',6 99 2,2',4,4',5
18 2,2',5 60 2,3,4,4' 100 2,2',4,4',6
19 2,2',6 61 2,3,4,5 101 2,2',4,5,5'
20 2,3,3' 62 2,3,4,6 102 2,2',4,5,6'
21 2,3,4 63 2,3,4',5 103 2,2',4,5',6
22 2,3,4' 64 2,3,4',6 104 2,2',4,6,6'
23 2,3,5 65 2,3,5,6 105 2,3,3',4,4'
24 2,3,6 66 2,3',4,4' 106 2,3,3',4,5
25 2,3',4 67 2,3',4,5 107 2,3,3',4',5
26 2,3',5 68 2,3',4,5' 108 2,3,3',4,5'
27 2,3',6 69 2,3',4,6 109 2,3,3',4,6
28 2,4,4' 70 2,3',4',5 110 2,3,3',4',6
29 2,4,5 71 2,3',4',6 111 2,3,3',5,5'
30 2,4,6 72 2,3',5,5' 112 2,3,3',5,6
31 2,4',5 73 2,3',5',6 113 2,3,3',5',6
32 2,4',6 74 2,4,4',5 114 2,3,4,4',5
33 2',3,4 75 2,4,4',6 115 2,3,4,4',6
34 2',3,5 76 2',3,4,5 116 2,3,4,5,6
35 3,3',4 77 3,3',4,4' 117 2,3,4',5,6
36 3,3',5 78 3,3',4,5 118 2,3',4,4',5
37 3,4,4' 79 3,3',4,5' 119 2,3',4,4',6
38 3,4,5' 80 3,3',5,5' 120 2,3',4,5,5'
39 3,4',5 81 3,4,4',5 121 2,3',4,5',6
No. Structure No. Structure No. Structure
Pentachlorobiphenyls Hexachlorobiphenyls Octachlorobiphenyls
122 2,3,3',4,5 162 2,3,3',4',5,5' 202 2,2',3,3',5,5',6,6'
123 2',3,4,4',5 163 2,3,3',4',5,6 203 2,2',3,4,4',5,5',6
124 2',3,4,5,5' 164 2,3,3',4',5',6 204 2,2',3,4,4',5,6,6'
125 2',3,4,5,6' 165 2,3,3',5,5',6 205 2,3,3',4,4',5,5',6
126 3,3',4,4',5 166 2,3,4,4',5,6
127 3,3',4,5,5' 167 2,3',4,4',5,5' Nonachlorobiphenyls
168 2,3',4,4',5',6 206 2,2',3,3',4,4',5,5',6
Hexachlorobiphenyls 169 3,3',4,4',5,5' 207 2,2',3,3',4,4',5,6,6'
128 2,2',3,3',4,4' 208 2,2',3,3',4,5,5',6,6'
129 2,2',3,3',4,5 Heptachlorobiphenyls
130 2,2',3,3',4,5' 170 2,2',3,3',4,4',5 Decachlorobiphenyls
131 2,2',3,3',4,6 171 2,2',3,3',4,4',6 209 2,2',3,3',4,4',5,5',6,6'
132 2,2',3,3',4,6' 172 2,2',3,3',4,5,5'
133 2,2',3,3',5,5' 173 2,2',3,3',4,5,6
134 2,2',3,3',5,6 174 2,2',3,3',4,5,6'
135 2,2',3,3',5,6' 175 2,2',3,3',4,5',6
136 2,2',3,3',6,6' 176 2,2',3,3',4,6,6'
137 2,2',3,4,4',5 177 2,2',3,3',4',5,6
138 2,2',3,4,4',5' 178 2,2',3,3',5,5',6
139 2,2',3,4,4',6 179 2,2',3,3',5,6,6'
140 2,2',3,4,4',6' 180 2,2',3,4,4',5,5'
141 2,2',3,4,5,5' 181 2,2',3,4,4',5,6
142 2,2',3,4,5,6 182 2,2',3,4,4',5,6'
143 2,2',3,4,5,6' 183 2,2',3,4,4',5',6
144 2,2',3,4,5',6 184 2,2',3,4,4',6,6'
185 2,2',3,4,5,5',6
Hexachlorobiphenyls 186 2,2',3,4,5,6,6'
145 2,2',3,4,6,6' 187 2,2',3,4',5,5',6
146 2,2',3,4',5,5' 188 2,2',3,4',5,6,6'
147 2,2',3,4',5,6 189 2,3,3',4,4',5,5'
148 2,2',3,4',5,6' 190 2,3,3',4,4',5,6
149 2,2',3,4',5',6 191 2,3,3',4,4',5',6
150 2,2',3,4',6,6' 192 2,3,3',4,5,5',6
151 2,2',3,5,5',6 193 2,3,3',4',5,5',6
152 2,2',3,5,6,6'
153 2,2',4,4',5,5' Octachlorobiphenyls
154 2,2',4,4',5,6' 194 2,2',3,3',4,4',5,5'
155 2,2',4,4',6,6' 195 2,2',3,3',4,4',5,6
156 2,3,3',4,4',5 196 2,2',3,3',4,4',5,6'
157 2,3,3',4,4',5' 197 2,2',3,3',4,4',6,6'
158 2,3,3',4,4',6 198 2,2',3,3',4,5,5',6
159 2,3,3',4,5,5' 199 2,2',3,3',4,5,6,6'
160 2,3,3',4,5,6 200 2,2',3,3',4,5',6,6'
161 2,3,3',4,5',6 201 2,2',3,3',4,5,5',6'
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMENDATIONS
1. Résumé et évaluation
1.1 Introduction
Découverts vers la fin du siècle dernier, les biphényles polychlorés ou
polychlorobiphényles (PCB) ont vu leur intérêt pour l'industrie
rapidement reconnu en raison de leurs propriétés physiques. On les
utilise dans le commerce depuis 1930 comme fluides diélectriques ou
caloporteurs ainsi que pour diverses autres applications. Largement
répartis dans l'environnement un peu par- tout dans le monde, ce sont
des composés persistants qui s'accumulent dans les différentes chaînes
alimentaires. L'exposition humaine aux PCB résulte en grande partie de
la consommation d'aliments contaminés mais peut également résulter d'une
inhalation ou d'une absorption percutanée sur les lieux de travail. Les
PCB s'accumulent dans les tissus adipeux de l'homme et des animaux et
peuvent déterminer des effets toxiques chez les uns et les autres,
notamment en cas d'exposition répétée. Les effets pathologiques
s'exercent principalement au niveau de la peau et du foie mais les voies
digestives, le système immunitaire et le système nerveux peuvent
également être atteints. Les polychlorodibenzofuranes (PCDF) qui
constituent des contaminants des mélanges de PCB du commerce, ont une
part importante dans la toxicité de ces composés. D'après les études
effectuées sur des rongeurs, il semblerait que certains PCB soient
cancérogènes et qu'ils puissent en outre agir comme promoteurs de la
cancérogénicité d'autres produits chimiques.
Il est clair, d'après les données dont on dispose au sujet des
polychlorobiphényles et des polychloroterphényles (PCT), qu'il vaudrait
mieux que les denrées alimentaires soient totalement exemptes de ces
composés. Il est cependant également clair que ramener à "zéro" ou
presque l'exposition aux PCT ou aux PCB résultant de l'alimentation,
conduirait à éliminer (par interdiction de la consommation) de grandes
quantités d'aliments très importants comme le poisson et plus encore,
comme le lait maternel. C'est aux commissions scientifiques nationales
et internationales qu'il appartient de décider du meilleur compromis
entre une protection suffisante de la santé publique et la nécessité
d'éviter de trop grandes pertes de denrées alimentaires.
Les données disponibles ne permettent pas de déterminer le niveau
d'exposition à ces substances qui constituerait une garantie absolue de
sécurité.
1.2 Identité et propiétés physiques et chimiques
Les PCB sont constitués de mélanges de dérivés aromatiques produits par
chloration du biphényle en présence d'un catalyseur convenable. Ils
répondent à la formule brute C12H10-nCln, le nombre n d'atomes de
carbone variant de 1 à 10.
Il y a théoriquement 209 homologues possibles mais seuls 130 d'entre eux
sont probablement utilisés dans des produits commerciaux. En outre, les
PCB peuvent contenir des impuretés consistant en polychlorodibenzofuranes
(PCDF) et en quaterpényles chlorés. Ces impuretés sont assez stables et
résistantes aux réactions chimiques dans les conditions normales. Tous
les PCB sont lipophiles et très peu solubles dans l'eau. Il en résulte
qu'ils pénètrent facilement dans la chaîne alimentaire et s'accumulent
dans les tissus adipeux.
Les mélanges de PCB utilisés dans le commerce contiennent des
polychlorodibenzofuranes à des concentrations qui vont de quelques mg/kg
à 40 mg/kg. Il n'y a pas de dibenzo- p-dioxines polychlorées (PCDD)
dans les PCB du commerce. Toutefois, en cas de mélange de PCB avec
d'autres dérivés chlorés comme les chlorobenzènes utilisés dans les
transformateurs, il arrive que l'on retrouve des PCDD à la suite
d'incendies accidentels ou après incinération.
Les mélanges de PCB du commerce sont d'une couleur qui va du jaune clair
au jaune foncé. Ils ne cristallisent pas, même à basse température, mais
se transforment en résines solides. Dans la pratique les PCB sont plutôt
ininflammables avec des points d'éclair assez élevés. Leur vapeur est
plus lourde que l'air, avec lequel et ils ne forment pas de mélanges
explosifs. Leur conductivité électrique est très faible mais leur
conductivité thermique assez élevée et ils sont extrêmement résistants
à la décomposition thermique. Les PCB sont chimiquement très stables
dans les conditions normales, toutefois, lorsqu'on les chauffe, ils
peuvent donner naissance à d'autres composés toxiques comme les
polychlorodibenzofuranes.
1.3 Méthodes d'analyse
Par suite de la découverte en 1966 de la présence de PCB dans des
échantillons prélevés dans l'environnement, on s'est intéressé à leur
analyse et à leur toxicité pour l'homme et son environnement.
En raison de la diversité des méthodes d'analyse utilisées, les données
disponibles ne sont pas directement comparables; on peut néamoins les
utiliser lorsqu'on se propose de prendre des mesures de contrôle et de
prévention ainsi que pour une évaluation préliminaire des risques pour
la santé et l'environnement imputables à ces produits.
Le dosage des PCB s'effectue par chromatographie en phase gazeuse avec
détection par capture d'électrons, souvent sur colonne garnie, encore
que l'on puisse recourir à des méthodes plus élaborées telles que la
chromatographie sur colonne capillaire et la chromatographie en phase
gazeuse couplée à la spectrométrie de masse, comme on l'a fait récemment
pour identifier les différents homologues, améliorer la comparabilité
des données analytiques issues de différentes sources et établir les
bases d'une évaluation toxicologique.
Ces analyses nécessitent un programme important d'assurance de la
qualité et, comme cela avait été recommandé, on a procédé à des
étalonnages inter-laboratoires. La qualité et l'intérêt des données
analytiques sont tributaires de la validité de l'échantillon et de la
méthode d'échantillonnage. En outre, il est essentiel que le programme
d'échantillonnage soit dûment planifié et documenté; on trouvera la
description d'une technique détaillée d'échantillonnage dans le document
WHO/EURO (1987).
1.4 Production et emplois
La production commerciale des PCB a commencé en 1930. Depuis, on les
utilise largement dans le matériel électrique et également, en petites
quantités, comme liquide ignifuge dans certains systèmes fonctionnant en
circuit fermé.
A la fin de 1980, la production mondiale totale de PCB dépassait un
million de tonnes et depuis, elle s'est poursuivie dans certains pays.
Bien qu'on renonce de pins en pins à leur emploi et que la production
soit soumise à des restrictions croissantes, de grandes quantités
demeurent dans l'environnement, soit du fait de leur utilisation, soit
sous la forme de déchets.
Ces dernières années, de nombreux pays industrialisés ont pris des
mesures pour contrôler et limiter les rejets de PCB dans
l'environnement. C'est probablement une recommandation émise par
l'Organisation de Coopération et de Développement économiques (OCDE) en
1973 qui a joué un rôle prépondérant dans la promulgation de ces
restrictions (OMS 1976; CIRC 1978; OCDE 1982). Depuis, les 24 pays
membres de l'OCDE ont imposé des restrictions à la production, la vente,
l'exportation et l'emploi des PCB et défini un système d'étiquetage de
ces composés.
Actuellement, les émissions de PCB sont imputables à leur volatilisation
à partir des décharges où sont enfouis des éléments de transformateurs,
de condensateurs et autres déchets de ce genre, des boues d'égouts, des
déversements accidentels ou non, des déchets de dragage et au rejet,
dans des conditions défectueuses ou illégales, de ces produits sur
des terrain à ciel ouvert. L'incinération des déchets industriels ou
municipaux peut produire une pollution. La plupart des incinérateurs
utilisés par les municipalités ne sont pas capables de détruire
efficacement les PCB. L'explosion ou la surchauffe de transformateurs ou
de condensateurs peut entraîner la libération de quantités importantes
de PCB à proximité du lieu de l'incident.
Les PCB peuvent être transformés en polychlorodibenzofuranes par
pyrolyse. Au laboratoire, c'est à des températures comprises entre 550
et 700°C qu'on obtient le meilleur rendement en polychloro-
dibenzofuranes. Ainsi, l'incinération incontrôlée des PCB peut
constituer une source importante de polychlorodibenzofuranes dangereux.
Il est donc recommandé que la destruction des déchets contaminés par des
PCB s'effectue dans des conditions soigneusement contrôlées, notamment
en ce qui concerne la température d'incinération (supérieure à 1000°C),
le temps de séjour et la turbulence.
1.5 Transport, distribution et transformation dans l'environnement
Dans l'atmosphère, les PCB sont principalement présents en phase vapeur;
la tendance a s'adsorber sur les particules augmente avec le degré de
chloration. La présence quasi universelle des PCB donne à penser qu'ils
sont transportés par l'atmosphère.
A l'heure actuelle, la principale source d'exposition aux PCB dans
l'environnement général trouve son origine dans la redistribution de ces
produits après leur passage dans le milieu. Cette redistribution
s'effectue par volatilisation à partir du sol et de l'eau puis passage
et transport dans l'atmosphère suivi d'un dépôt à sec ou en milieu
humide (des PCB liés aux particules), les produits se revolatilisant
ensuite pour continuer le cycle. Dans les précipitations, la
concentration en PCB varie de 0,001 à 0,25 µg/litre. Comme la vitesse de
volatilisation et de décomposition des PCB varient d'un homologue à
l'autre, ce processus de redistribution entraîne une modification dans
la composition des mélanges de PCB présents dans le milieu.
Dans l'eau, les PCB sont adsorbés sur les sédiments et autres matières
organiques; les données d'expérience et de surveillance montrent que
leur concentration dans les sédiments et les matières en suspension est
plus élevée que dans la couche d'eau qui les surmonte. La forte
adsorption des PCB sur les sédiments, notamment dans le cas des dérivés
les plus chlorés, réduit leur vitesse de volatilisation. En se basant
sur la solubilité dans l'eau et le coefficient de partage entre le
n-octanol et l'eau, on peut estimer que les PCB les moins chlorés
seront moins fortement sorbés que les homologues plus substitués. Bien
que l'adsorption puisse immobiliser les PCB pendant des périodes
relativement longues dans le milieu aquatique, on a montré de la
désorption dans la couche d'eau environnante s'effectuait par voie
abiotique ou biotique. Les sédiments aquatiques, qui contiennent de
notables quantités de PCB, jouent donc le rôle à la fois de piège et de
réservoir pour les organismes qui vivent dans ce milieu. On pense que
l'essentiel de la charge en PCB du milieu est adsorbé sur les sédiments
aquatiques.
La faible solubilité et la forte adsorption des PCB sur les particules
de sol en limitent le lessivage; le lessivage est d'autant plus
important que la substitution par le chlore est plus faible.
La décomposition des PCB dans l'environnement dépend de leur degré de
substitution. En général, la persistance s'accroit parallèlement au
degré de substitution. Dans l'atmosphère, la réaction en phase vapeur
des PCB avec les radicaux hydroxyles (qui se forment par voie
photochimique sous l'action du rayonnement solaire) pourrait constituer
le principal processus de transformation. On estime que le temps de
demi-réaction dans l'atmosphère varie de 10 jours pour un
monochlorobiphényle à 1,5 année pour un heptachlorobiphényle.
Dans le milieu aquatique, l'hydrolyse et l'oxydation ne jouent pas un
rôle important dans la décomposition des PCB. Dans le milieu, il semble
que le seul processus viable de décomposition soit le photolyse.
Cependant, les données expérimentales sont insuffisantes pour que l'on
puisse en établir la vitesse et la degré dans l'environnement.
Les microorganismes décomposent assez rapidement le mono-, le di- et le
trichlorobiphényle; cette dégradation étant plus grande dans le cas de
tétrachlorobiphényles. Les biphényles plus substitués résistent à la
biodégradation. La position des atomes de chlore sur le noyau biphényle
influe de manière importante sur la vitesse de biodégradation. Les PCB
qui contiennent des atomes de chlore en para sont plus facilement
biodégradés. Les homologues les plus substitués subissent une
biotransformation anaérobie par déchloration réductrice qui abaisse leur
degré de substitution et les transforme en homologues plus facilement
biodégradable par voie aérobie.
Le degré de bioaccumulation dans les tissus adipeux dépend de plusieurs
facteurs: la durée et le niveau de l'exposition, la structure chimique
du composé et notamment le nombre et la position des substituants. En
général, ce sont les dérivés les plus substitués qui s'accumulent le
plus facilement.
Les facteurs de bioconcentration des différents PCB qui ont été mesurés
expérimentalement chez différentes espèces aquatiques (poisson,
crevette, huitre) vont de 200 à 70 000 ou davantage. En haute mer, les
PCB s'accumulent à des niveaux trophiques plus élevés et l'on trouve
davantage de biphényles fortement substitué chez les prédateurs qui se
situent en fin de chaîne alimentaire.
Le passage des PCB du sol à la végétation se produit principalement par
adsorption sur les surfaces externes des plantes terrestres; il n'y a
guère de déplacement à l'intérieur de la plante.
1.6 Concentrations dans l'environnement et exposition humaine
Du fait de leur forte persistance et d'autres propriétés physiques et
chimiques, les PCB sont présent dans tout l'environnement de la planète.
D'une façon générale, les concentrations dans l'air vont de 0,002 à
15 ng/m3. Dans les zones industrielles, les valeurs sont plus élevées
puisqu'elles peuvent être de l'ordre du µg/m3. Dans les précipitations,
elles vont de 1 ng à 250 ng/litre.
Dans les ambiances de travail, les concentrations dans l'air peuvent
être beaucoup plus élevées. Dans certaines conditions, par exemple, dans
le cas de la fabrication des transformateurs ou des condensateurs, on a
pu observer des concentrations allant jusqu'à 1000 µg/m3. En situation
d'urgence, des concentrations atteignant même 16 mg/m3 ont été
mesurées. Après des incendies ou des explosions, la suie qui en résulte
peut contenir de fortes concentrations de PCB. On en a ainsi trouvé
jusqu'à 8000 mg/kg de suie. Dans ce cas d'ailleurs, ils s'accompagnent
de polychlorodibenzofuranes. Dans les accidents impliquant des
transformateurs contenant du chlorobenzène ainsi que des PCB, on trouve
également des dioxines polychlorées.
Dans ces situations d'urgence, des particules de suie peuvent être
ingérées ou inhalées ou encore contaminer la peau et entraîner une grave
exposition du personnel. Quoiqu'il en soit, l'exposition de la
population générale par la voie atmosphérique est très faible.
Les eaux de surface peuvent être contaminées par des PCB provenant de
retombées atmosphériques, d'émissions directes à partir de sources
ponctuelles ou de décharges. Dans certaines conditions, on a mesuré dans
l'eau des concentrations allant jusqu'à 100-500 ng/litre. Dans les
océans, on a observé des concentrations de 0,05 à 0,6 ng/litre.
Dans les régions non contaminées, l'eau de boisson confient moins de
0,01 ng de PCB/litre mais on a fait état de concentrations allant
jusqu'à 5 ng/litre. Selon les régions et en fonction des conditions
locales, le sol et les sédiments peuvent contenir des PCB à des
concentrations allant de <0,01 à 2,0 mg/kg. Dans les régions polluées,
les teneurs sont beaucoup plus fortes puisqu'elles peuvent atteindre
500 mg/kg.
Plusieurs milliers d'échantillons d'aliments divers ont été analysés au
cours des années dans plusieurs pays à la recherche de contaminants et
en particulier de PCB. La plupart des échantillons provenaient de
produits déterminés, notamment du poisson ou d'autres aliments d'origine
animale comme la viande et le lait. Il y a trois voies principales de
contamination de la nourriture humaine:
a) passage de l'environnement aux poissons, oiseaux, bétail (par
l'intermédiaire de la chaîne alimentaire) et récoltes;
b) migration dans les aliments à partir des matériaux de
conditionnement (essentiellement au-dessous de 1 mg/kg, mais dans
certains cas pouvant atteindre 10 mg/kg);
c) contamination directe des aliments destinés à l'homme ou aux
animaux par suite d'un accident industriel.
La contamination des plus importantes denrées alimentaires par des PCB
s'est située dans les limites suivantes: graisses animales
20-240 µg/kg; lait de vache 5-200 µg/kg; beurre 30-80 µg/kg; poisson
10-500 µg/kg -- valeurs rapportées à la teneur en graisse. Certaines
espèces de poissons (anguilles) ou produits tirés du poisson (foie de
poisson ou huile de poisson) en contiennent des quantités beaucoup plus
élevées, pouvant aller jusqu'à 10 mg/kg. Les concentrations relevées
dans les légumes, les céréales, les fruits ainsi qu'un certain nombre
d'autres produits sont inférieures à 10 µg/kg. Les principaux produits
alimentaires dont il faut surveiller la contamination par les PCB sont
le poisson, les fruits de mer, la viande, le lait et les produits
laitiers. Les concentrations médianes dans le poisson observées dans
divers pays sont de l'ordre de 100 µg/kg (par rapport aux graisses). Si
l'on procède à des comparaisons, on constate que la teneur du poisson en
PCB diminue lentement.
Les PCB s'accumulent dans les tissus adipeux et le lait maternel. Leur
concentration dans les différents organes et tissus dépend de la teneur
de ceux-ci en lipides, sauf dans le cas du cerveau. Les résidus de PCB
présents dans les tissus adipeux de la population générale des pays
industrialisés vont de - 1 à 5 mg/kg de graisses.
Dans les lipides du lait humain, la concentration moyenne en PCB totaux
est de l'ordre de 0,5-1,5 mg/kg de lipides selon le lieu de résidence du
sujet, son mode de vie et la méthode d'analyse utilisée. Les femmes qui
habitent des zones urbaines fortement industrialisées et qui consomment
beaucoup de poisson, surtout pêché dans des eaux très contaminées,
peuvent avoir un lait contenant davantage de PCB.
Dans la plupart des cas; les extraits de PCB provenant d'échantillons
prélevés dans l'environnement n'ont pas une composition analogue à celle
des mélanges du commerce. On a également montré, en procédant par
chromatographie en phase gazeuse à haute résolution, que la composition
en homologues et la concentration relative des différents constituants
présents dans les tissus adipeux et le lait maternel étaient très
éloignées de celles des mélanges de PCB du commerce. L'analyse
chromatographique des PCB présents dans les tissus adipeux humains et
dans le lait maternel fait resortir une forte concentration de PCB
fortement substitués tels que le 2,4,5,3',4'-pentachlorobiphényle,
le 2,4,5,2',4',5'- hexachlorobiphényle, le 2,3,4,2',4',5'-
hexochlorobiphényle, le 2,3,4,5,2',4',5'-hepta-chiorobiphényle et le
2,3,4,5,2',3',4'- heptachlorobiphényle. Quelques autres homologues sont
présents en quantités beaucoup plus faibles; c'est le cas de la plupart
des PCB coplanaires toxiques: le 3,4,3',4'-tétra-, le 3,4,5,3',4'-penta
et le 3,4,5,3',4,',5'-hexa-chlorobiphényle.
On a calculé que la dose quotidienne de PCB ingérée par les nourrissons
à partir du lait maternel était de l'ordre de 4,2 µg/kg de poids
corporel (5,2 µg/100 KCal consommées) (WHO/EURO, 1988). La quantité
moyenne totale de PCB ingérée avec le lait maternel au cours des six
premiers mois de la vie est de 4,5 mg contre 357 mg pour le reste de
l'existence (0,2 µg/kg et par jour ingéré avec la nourriture par une
personne de 70 kg au cours d'une vie de 70 ans). La période
d'allaitement correspond donc à 1,3% de la dose totale ingérée au cours
de l'existence, ce qui n'est pas très élevé compte tenu de l'intérêt que
présente l'allaitement au sein (WHO/EURO, 1988).
En s'appuyant sur les données de base ayant fait l'objet d'une
évaluation, on peut calculer que l'apport de PCB par voie alimentaire ne
dépasse pas 100 µg en moyenne par semaine, c'est-à-dire environ
14 µg/personne et par jour. Pour un individu de 70 kg, cela correspond
à un apport quotidien maximum de l'ordre de de 0,2 µg/kg de poids
corporel (WHO/EURO, 1988).
1.7 Cinétique et métabolisme
L'expérimentation animale rapportée dans la littérature comporte
essentiellement l'exposition par voies orale, respiratoire et percutanée
à des mélanges de PCB ou aux différents homologues. En général, les PCB
sont rapidement absorbés, notamment pas la voie digestive après
ingestion. Cette absorption se produit indiscutablement aussi chez
l'homme mais les données concernant les taux d'absorption sont limitées.
D'après les résultats dont on dispose, il semble que la distribution des
PCB dans l'organisme s'effectue selon un processus cinétique biphasé,
les composés étant rapidement éliminés du sang et s'accumulant dans le
foie et les tissus adipeux des divers organes. On est également fondé à
penser que les PCB franchissent la barrière placentaire, s'accumulent
dans le foetus et passent dans le lait maternel. Certaines études
effectuées sur des sujets humains ont révélé une forte concentration de
PCB dans l'épiderme mais la concentration dans l'encéphale était plus
faible que ce que l'on aurait pu penser en s'appuyant sur la teneur en
lipide de cet organe.
La mobilisation des PCB à partir des graisses dépend largement de la
vitesse de métabolisation des différents homologues. L'excrétion est
tributaire de la transformation des PCB en composés plus polaires:
phénols, thiolo-conjugués et autres dérivés hydrosolubles. Les
différentes voies métaboliques observées comportent une hydroxylation,
une conjugaison avec des thiols et d'autres dérivés hydrosolubles avec
parfois intervention d'intermédiaires réactifs comme les oxydes d'arène.
On a montré que la vitesse de métabolisation dépendait de la structure
des différents PCB et qu'elle était tributaire à la fois du degré de
substitution et de la position de substituants. Les métabolites polaires
des PCB les plus chlorés sont éliminés principalement par la voie fécale
mais l'excrétion urinaire n'est pas négligeable. Le lait maternel
constitue une importante voie d'élimination. Certains PCB peuvent
également être éliminés en passant dans le système pileux.
Les données cinétiques disponibles montrent que le demie-vie des divers
PCB est très variable, ce qui peut s'expliquer par la variabilité du
métabolisme en fonction de la structure, le tropisme tissulaire et
d'autres facteurs qui influent sur la mobilisation à partir des sites
d'accumulation.
Il n'y a pas toujours corrélation entre la persistance dans les tissus
et une forte toxicité, et les différences de toxicité d'un homologue à
l'autre peuvent être liées à des métabolites ou à des intermédiaires
particuliers.
1.8 Effets sur les êtres vivants dans leur milieu naturel
Les PCB sont des contaminants universels du milieu et on les rencontre
dans la plupart des compartiments de l'environnement -- biotiques ou
abiotiques -- dans le monde entier. Etant donné que de nombreux pays en
réglementent l'utilisation et la libération dans l'environnement, les
décharges qui peuvent survenir sont beaucoup moins importantes que par
le passé. Toutefois il semble, à la lumière des données disponibles, que
le cycle des PCB dans le milieu entraîne une redistribution progressive
de certains homologues en direction du milieu marin. Les dérivés les
plus substitués sont ceux qui ont tendance à s'accumuler. Les PCB sont
en grande partie adsorbés à la surface des particules de sédiments mais
ils demeurent biodisponibles pour les divers organismes et leur
accumulation se produit à des niveaux de plus en plus élevés de la
chaîne alimentaire.
1.8.1 Etudes en laboratoire
Les mélanges de PCB exercent sur les microorganismes des effets qui
varient énormément d'une espèce à l'autre puisque certaines sont
affectées dès 0,1 mg/litre alors que d'autres supportent sans dommage
des concentrations de 100 mg/litre; ces effets ne varient pas de façon
régulière avec le degré de chloration des différents mélanges. La
presque totalité des études consacrées aux effets des PCB sur les
organismes aquatiques portent sur des mélanges de type Aroclor. Les
résultats en sont très variables et l'on ne peut pas établir de relation
systématique entre le pourcentage de chloration ou les conditions
écologiques et la toxicité, même dans le cas d'organismes très proches.
Sur 96 heures dans des conditions statiques, les valeurs de CL50 varient
de 12 µg/litre à > 10 mg/litre pour différentes es d'invertébrés
aquatiques et divers mélanges de type Aroclor. Dans des conditions
dynamiques, la toxicité des PCB augmente. En général, les mélanges les
plus toxiques sont des Aroclors moyennement chlorés; en revanche,
lorsque le degré de chloration est faible ou élevé, les mélanges sont
moins toxiques. On le constate également dans le cas des effets
sub-létaux, par example sur la reproduction de la daphnie. Les crustacés
paraissent être plus sensibles aux PCB en période de mue. L'exposition
de populations modèles à de l'Aroclor 1254 a permis d'observer une
modification dans la structure de la communauté des espèces
estuariennes, avec diminution du nombre d'amphipodes, de bryozoaires, de
crabes et de mollusques, le nombre d'annélidés, de brachyopodes, de
coelentérés, d'échinodermes et de némertiens restant inchangé. Les
épreuves de toxicité aiguë portaient sur trop peu de ces groupes pour
qu'on puisse en déduire si les résultats obtenus correspondent à des
variations dans la sensibilité aux PCB ou à des différentes dans les
interactions entre espèces.
On constate des variations analogues dans la toxicité de ces mélanges
chez les poissons pour lesquels la CL50 à 96 heures vade de 0,008 à
100 mg/litre. Des épreuves à long terme ont montré que, en cas
d'exposition aiguë, notamment dans des conditions statiques, les données
obtenues ne donnent qu'une valeur très sous-estimée de la toxicité des
mélanges. La truite arc-en-ciel se révèle particulièrement sensible, les
stades embryo-lavaires présentant une CL50 à 22 jours de 0,32 µg/litre
dans le cas de l'Aroclor 1254, et la dose sans effet observable sur
22jours étant de 0,01 µg/litre dans le cas des Aroclors 1016, 1242 et
1254.
Pour l'espèce Pimephales promelas on a obtenu pour la dose sans effet
observable des valeurs respectivement égales à 5,4, 0,1, 1,8 et
1,3 kg/litre pour les Aroclors 1242, 1248, 1254 et 1260; dans le cas
de Pimelometopon pulcher, on a obtenu une dose sans effet observable
de 3,4 et 0,06 µg/litre respectivement pour les Aroclors 1016 et 1254.
On a pu confirmer expérimentalement des observations effectuées en
milieu naturel et qui tendaient à montrer que des phoques se nourrissant
de poissons ayant accumulé des PCB dans leur chair présentaient des
troubles de la reproduction. Cet effet s'observe au cours des dernières
phases du processus et se traduit par l'impossibilité pour l'embryon de
s'implanter dans la paroi utérine.
Lors d'études à court terme, on a constaté que la toxicité de l'Aroclor
pour les oiseaux augmentait avec le pourcentage de chloration; les CL50
par voie alimentaire à cinq jours allaient de 604 à > 6000 mg/kg de
nourriture. Les principaux effets sur la reproduction des oiseaux
consistaient en une plus grande difficulté d'éclosion pour les oeufs et
une certaine embryotoxicité. Ces effets ont continué malgré l'arrêt du
traitement par les PCB, la concentration de PCB chez les poules
diminuant par passage dans les oeufs. Rien n'indique que les Aroclors
provoquent une amincissement de la coquille, tout du moins directement;
toutefois l'effet qu'ils exercent sur la consommation de nourriture et
le poids des poules agit indirectement sur l'épaisseur de la coquille
des oeufs. des effets sub-létaux ont été signalés sur le comportement et
les sécrétions hormonales.
Chez le vison, la toxicité aiguë de l'Aroclor diminue à mesure
qu'augmente le pourcentage de chloration; la DL50 aiguë par voie orale
se situant entre > 750 et 4000 mg/kg de poids corporel; le furet est
moins sensible. L'Aroclor réduit la consommation de nourriture et par
conséquent le taux de croissance des jeunes visons. L'administration
d'Aroclor diminue et va même jusqu'à arrêter la reproduction des visons,
qu'il soit administré directement ou par suite de l'ingestion de poisson
contaminé dans la nature. Les Aroclors à forte teneur en chlore
(notamment le 1254) ont un effet pins marqué. Lorsque cesse
l'administration d'Aroclor par voie alimentaire, le taux de reproduction
revient à la normale.
Les chauves-souris sont affectées par l'Aroclor libéré dans leur
organisme à partir des graisses au cours de la migration.
Etant donné que la grande majorité des épreuves de laboratoire sur les
organismes aquatiques et terrestres ont été effectuées avec des mélanges
de PCB, il n'est pas possible d'attribuer à tel ou tel constituant en
particulier tel ou tel type d'effets. De même, du fait que ces épreuves
ont été exécutées dans des conditions qui ne correspondent pas aux
conditions écologiques réelles (c'est-à-dire à des concentrations
supérieures à la solubilité des différents constituants et sans la
présence de sédiment), il est difficile d'extrapoler les résultats de
laboratoire à la situation réelle. Toutefois, on peut raisonnablement
penser que tout effet sur les différentes populations d'organismes
aquatiques ou terrestres qui pourrait s'observer à l'avenir, aura déjà
été observé sur des populations locales antérieurement exposées à de
fortes concentrations de PCB.
1.8.2 Etudes dans le milieu naturel
Les résultats qui tendraient à accréditer l'idée d'effets des PCB sur
les populations de poissons dans leur milieu naturel ne sont pas
concluants. L'interprétation des données recueillies sur les oiseaux au
sein de leur milieu naturel est difficile, du fait de la présence de
nombreux résidus provenant de divers organochlorés. La plupart des
auteurs ont montré l'existence d'une corrélation entre les effets
(embryotoxicité) observés et les résidus d'organochlorés totaux. Parmi
tous les composés organochlorés présents ce sont les PCB qui offrent la
meilleure corrélation avec les effets observés sur les embryons mais les
résultats ne peuvent pas être considérés comme démontrant l'existence
d'effets des PCB au sein du milieu naturel.
Un certain nombre de faits (confirmés en laboratoire) montrent que les
PCB réduisent la capacité de reproduction des mammifères marins. Il
s'agit d'une effet sur la nidation de l'embryon, mais qui peut également
s'accompagner de modifications physiques au niveau des voies génitales
des femelles.
Il n'est pas possible d'extrapoler les données obtenues en laboratoire
lors d'études de toxicité aiguë et de toxicité à court terme, pour en
tirer des conclusions relatives aux populations vivant dans le milieu
naturel. L'incertitude qui règne quant aux effets attribués à tel ou tel
constituant des mélanges de PCB, la méconnaissance de la nature exacte
des homologues présents dans l'environnement et le caractère aléatoire
de la biodisponibilité des PCB pour les divers organismes, sont autant
de facteurs qui rendent difficile une estimation des l'exposition et des
effets qui en découlent dans l'environnement. On peut considérer comme
démontrés les effets observés sur les populations de mammifères marins
mais on ne sait pas encore à quels constituants des mélanges de PCB les
attribuer.
Du fait de la tendance à la contamination croissante du milieu matin, il
convient de rester très attentif aux effets exercés sur les organismes
marins. Les observations effectuées en laboratoire ou dans le milieu
naturel montrent clairement que la reproduction des populations de
mammifères marins est affectée dans les zones fortement polluées.
Dans les autres secteurs, il est probable que les résidus vont
s'accroître, entraînant par voie de conséquence une augmentation des
effets sur ces mammifères. On a moins de certitudes quant à la question
de savoir si ces effets s'observeront chez d'autres organismes,
notamment les oiseaux qui se nourrissent d'organismes marins.
A en juger par l'expérimentation en laboratoire, on pourrait s'attendre
à des effets sur les populations et les communautés d'organismes
inférieurs tel que le phytoplancton et le zooplancton. Il est difficile
d'en apprécier l'ampleur et la portée. Selon les données actuellement
disponibles, il ne semble pas que les poissons aient à souffrir des
effets des PCB, encore qu'ils constituent une voie de contamination pour
les mammifères et oiseaux piscivores.
Par exemple, les effets sur les espèces terrestres, les mammifères d'eau
douce piscivores et chauves-souris migratrices qui avaient été signalés
antérieurement, devraient être moins visibles à mesure que les résidus
de PCB se redistribuent dans l'environnement. Les résidus présents dans
les biotes terrestres ne semblent généralement guère être en recul à
l'heure actuelle, mais on ne possède que peu ou pas de données sur les
modifications affectant les différents homologues. La diminution des
résidus de PCB fortement chlorés devrait être lente.
1.9 Effets sur les animaux d'expérience et les systèmes in vitro
1.9.1 Après une unique exposition
Après une unique exposition par voie orale, la toxicité aiguë des
Aroclors est généralement faible chez le rat. Les jeunes animaux se
révèlent plus sensibles (DL50: 1,3-2,5 g/kg de poids corporel) que les
adultes (DL50: 4-11 g/kg de poids corporel). La DL50 la plus faible
observées pour l'Aroclor 1254 chez le rat adulte a été de 1,0 g/kg de
poids corporel. Aucune différence n'a été observée entra les sexes.
Chez les lapins, les valeurs de la DL50 dermique variaient de > 1,26
à < 2 g/kg de poids corporel en ce qui concerne l'Aroclor 1260 (dans
l'huile de maïs) et de 0,79 à < 3,17 g/kg de poids corporel pour
certains autres mélanges de PCB non dilués. Dans le cas d'une
administration par voie intraveineuse, on a observé une DL50 de
0,4 g/kg de poids corporel pour l'Aroclor 1254 chez le rat; après
injection intrapéritonéale, la DL50 chez la souris allait de 0,9 à
1,2 g/kg de poids corporel.
1.9.2 Après une exposition de brève durée
Après une exposition de brève durée par voie orale à des PCB purs ou en
mélange, on a constaté que les principaux organes cibles chez les
mammifères étaient le foie, la peau, le système immunitaire et le
système reproducteur. Parmi les espèces étudiées c'est le singe Rhésus
qui s'est révélé le plus sensible, les femelles l'étant davantage que
les mâles. Des guenons adultes Rhésus exposées à un régime alimentaire
contenant de l'Aroclor 1248 à raison de 2,5 mg/kg ou de 0,09 mg/kg
d'Aroclor/kg de poids corporel et par jour, pendant six mois, ont
présenté un accroissement du taux de mortalité, un retard de croissance,
une alopécie, de l'acné, une hypertrophie des glandes de Meibom et
peut-être une immunodépression. L'examen microscopique a révélé une
infiltration graisseuse du foie avec des foyers de nécrose, une
hyperplasie épithéliale et une kératinisation des follicules pileux. A
plus fortes doses, des altérations histopathologiques ont également été
observées dans d'autres tissus épithéliaux tels que les glandes sébacées
et les glandes de Meibom, la muqueuse gastrique, la vésicule biliaire et
le canal cholédoque, le lit inguéal et les améloblastes. Il y avait
réduction des taux sériques de lipides totaux, de triglycérides et de
cholestérol. L'exposition à des mélanges de PCB du commerce a entrainé
l'augmentation de la concentration en lidipes totaux, en triglycérides
et en cholestérol et/ou en phospholipides dans le foie. Parmi les
différents PCB, ce sont le 3,4,3',4'-tétrachlorobiphényle, le
3,4,5,3',4',5'- ainsi que le 2,4,6,2' 4' 6'-hexachlorobiphényle qui se
sont révélés les plus actifs. A la dose quotidienne de 0,2 mg/kg de
poids corporel, l'Aroclor 1254 a également produit différents autres
effets: lésions lymphoréticulaires, chute de ongles, lésions gingivales,
mais ni acné et ni alopécie. La dose sans effet observable en ce qui
concerne la toxicité générale de l'Aroclor 1242 a été évaluée chez le
singe Rhésus à 0,04 mg/kg de poids corporel par jour. Chez des singes
Rhésus à la mamelle on a observé des effets relativement bénins après
exposition à une dose beaucoup plus forte d'Aroclor 1248 (35 mg/kg de
poids corporel par jour). C'est chez le rat qu'on a le mieux étudié les
effets exercés au niveau du foie: il s'agit d'hypertrophie, de
dégénérescence graisseuse, de prolifération du réticulum endoplasmique,
de porphyrie, d'adénofibrose, d'hyperplasie des canaux biliaires, de
kystes et des lésions précancéreuses et cancéreuses. Chez le rat et la
souris, les effets des différents PCB ont été observés au niveau du
foie, de la rate et du thymus, les homologues coplanaires étant les plus
toxiques. Chez le singe, ces homologues ont produit, à des doses de
1-3 mg/kg de nourriture, des effets de nature et de gravité analogues à
ceux que l'on avait observés après administration d'Aroclor 1242 à la
dose de 100 mg/kg de nourriture et d'Aroclor 1248 à raison de 25 mg/kg
de nourriture.
Après avoir été exposés par la voie dermique à certains PCB seuls ou en
mélanges, des lapins et des souris ont présenté des effets au niveau de
la peau et du foie, effets qui étaient analogues à ceux que l'on observe
après administration par voie orale. Chez les lapins, on a également
observé une atrophie du thymus, une réduction des centres germinaux des
glanglions lymphatiques ainsi qu'une leucopénie.
1.10 Reproduction, embryotoxicité et tératogénicité
1.10.1 Reproduction et embryotoxicité
On n'a pas procédé à des études très complètes sur les effets génésiques
ni sur la tératogénicité des PCB. Lors d'une étude de reproduction
portant sur deux générations de rats, on a pu, en se basant sur des
paramètres génésiques, établir dans le cas de l'Aroclor 1254 une dose
sans effet observable de 0,32 mg/kg de poids corporel et de 7,5 mg/kg de
poids corporel dans le cas de l'Aroclor 1260. Toutefois la dose la plus
faible étudiée, qui était de 0,06 mg/kg de poids corporel, a entrainé
une augmentation du poids relatif du foie chez les ratons juste sevrés.
Chez des singes Rhésus exposés à de l'Aroclor 1016, on a estimé à
0,03 mg/kg de poids corporel la dose sans effet observable en s'appuyant
sur des paramètres génésiques. Toutefois, à cette dose, on constatait
une réduction du poids de naissance et la dose la plus faible étudiée,
soit 0,01 mg/kg de poids corporel, produisait une hyperpigmentation
cutanée.
Pour l'Arcolor 1248 (contaminé par des polychlorodibenzofuranes), on a
estimé à 0,09 mg/kg de poids corporel la dose sans effet observable chez
le singe Rhésus, une année après l'arrêt de l'exposition.
1.10.2 Tératogénicité
Les études sur le rat et le singe dont on connaît les résultats ne font
ressortir aucun effet tératogène après administration de PCB par voie
orale aux animaux au cours de l'organogénèse. Chez le rat, on a estimé
à 50 mg/kg de poids corporel la dose d'Aroclor 1254 sans effet
observable relativement au poids des ratons, la dose la plus faible qui
ait produit un effet étant de 2,5 mg/kg de poids corporel. L'effet
retenu était la foetotoxicité (lésions au niveau des cellules
folliculaires de la thyroïde).
Les épreuves de tératogénicité pratiquées sur des singes Rhésus, des
souris et des rats au moyen de divers PCB n'ont pas permis de mettre en
évidence une dose sans effet observable. Chez les singes Rhésus, une
dose de 0,07 mg/k de poids corporel a entrainé des effets toxiques sur
les mères (3,4,3',4'-tétrachlorobiphényle).
1.11 Mutagénicité
Les mélanges de PCB ne provoquent ni mutation ni lésion chromosomique
dans divers systèmes d'épreuve. En revanche le 3,4,3',4'-tétrachloro-
biphényle provoque des ruptures de chromosomes dans les lymphocytes
humains in vitro. A fortes concentrations, les mélanges de PCB peuvent
endommager la structure primaire de l'ADN, comme le montrent les
ruptures constatées sur l'un des brin de l'ADN lors d'épreuves d'élution
en milieu alcalin.
1.12 Cancérogénicité
L'interprétation des données relatives aux effets des mélanges de PCB du
commerce sur l'animal est souvent compliquée du fait l'on manque de
renseignements sur la présence ou la part relative des impuretés que
constituent les chlorodibenzofuranes ainsi que sur la proportion des
divers homologues dans le mélange.
Un certain nombre d'éludes de cancérogénicité à long terme ont été
effectuées sur des rats et des souris à l'aide de mélanges tels que les
Kanéchlors 300, 400 et 500, les Aroclors 1254 et 1260 ainsi que les
Clophènes A30 et A60. Les Clophènes étaient exempts de
chlorodibenzofuranes mais on ne possède aucune donnée sur la pureté des
autres mélanges de PCB.
Chez des souris recevant une alimentation qui contenait du Kanéchlor 500
et de l'Aroclor 1254 à des doses d'environ 15 à 25 mg/kg de poids
corporel, on a constaté une augmentation sensible des adénomes et/ou des
carcinomes hépatocellulaires. Aucune tumeur maligne n'a pu être observée
chez des souris traitées par du Kanéchlor 300 et du Kanéchlor 400.
Chez des rats exposés pendant plus d'une année à de l'Arcolor 1254 et
1260 ainsi qu'à du Clophène A30, on a observé une augmentation de la
fréquence des adénomes et/ou des carcinomes hépatocellulaires. Le nombre
plus élevé d'animaux porteurs de tumeurs observé dans ces études n'a pu
cependant être considéré comme statistiquement significatif, à l'inverse
de deux autres études. Ainsi, un accroissement de l'incidence des
carcinomes hépatocellulaires (trabéculaires) et des adénocarcinomes a
été mis en évidence après administration d'Arcolor 1260 et de Clophène
A30 à la dose d'environ 5 mg/kg de poids corporel.
Les tumeurs hépatiques observées n'étaient pas de type invasif (il
s'agissait de tumeurs bénignes ou de faible malignité, sans métastases)
et elles n'abrégeaient pas la vie des animaux.
Dans certaines de ces études, on a observé une adénofibrose, des lésions
prénéoplasiques et/ou des nodules néoplasiques dans le foie. Une épreuve
portant sur l'Arcolor 1254 a permis de mettre en évidence un
accroissement des lésions métaplasiques intestinales ainsi que des
adénocarcinomes dans la région glandulaire de l'estomac chez le rat.
L'hypothèse selon laquelle les PCB augmenteraient la cancérogénèse
hépatique chez des rongeurs prétraités par des hépatocancérogènes est
étayée par de nombreux faits. Toutefois l'activité initiatrice des
mélanges de PCB chez les rongeurs n'est guère attestée. Sur la base des
études de génotoxicité publiées, on peut conclure que les mélanges de
PCB ne sont pas génotoxiques. Il s'ensuit que le lien entre la présence
de rumeurs hépatiques et l'administration de PCB chez des rongeurs peut
être attribué à des mécanismes épigénétiques entraînant une
prolifération des cellules hépatiques et autres manifestations
d'hépatotoxicité-autrement dit, il serait possible d'évaluer la toxicité
des PCB en envisageant l'existence d'un seuil de toxicité. Il faut donc
étudier la possibilité, pour les PCB, de favoriser la cancérogénèse dans
des tissus autres que les tissus hépatiques, chez les animaux préexposés
à divers cancérogènes spécifiques de tel ou tel tissu. Il est possible
que l'activité anticancérogène des PCB, observée dans certaines études
au cours desquelles on les avait administrés à des animaux pendant ou
avant l'administration de cancérogènes, soit liée au fait que les PCB
sont capables d'induire les enzymes microsomiques, d'où une stimulation
du processus de détoxication.
Globalement, il est justifié d'être prudent dans l'extrapolation à
l'homme des données obtenues sur l'animal en ce qui concerne le pouvoir
cancérogène des PCB.
1.13 Etudes spéciales
Les lésions induites après exposition à divers PCB purs ou en mélange,
s'observent au niveau du foie, de la peau, du système immunitaire, de
l'appareil reproducteur, et alles s'accompagnent d'oedème et de troubles
fonctionnels des voies digestives et de la glande thyroïde.
Les PCB sont capables d'induire diverses enzymes hépatiques. On a pu le
mettre en évidence chez des rats, des souris, des cobayes, des lapins,
des chiens et des singes en ce qui concerne les Aroclors 1248, 1254 et
1260 ainsi que le Kanéclor 400 (induction du cytochrome P450 et P448).
Le pouvoir enzymo-inducteur des PCB augmente avec la teneur en chlore de
la molécule. Il dépend également de la composition du mélange, les PCB
dans lesquels le chlore se trouve en para- et en meta- provoquant
l'induction du P450. En ce qui concerne l'induction de l'AHH, la
position des atomes de chlore semble plus importante que le degré de
chloration. Les inducteurs les plus actifs de l'AHH sont les PCB dont
les deux positions para- et au moins deux positions meta- sont
substituées par du chlore. Des variations interspécifiques distinctes
ont été mises en évidence. C'est avec l'Aroclor 1260 administré à des
rats Osborn-Mendel que l'on a obtenu la dose sans effet observable la
plus faible (0,025 mg/g de poids corporel).
En ce qui concerne les effets sur le système endocrinien, il s'agit de
modifications touchant la liaison aux récepteurs hormonaux et
l'équilibre des hormones stéroïdiennes. On également des preuves
directes et indirectes d'une faible activité oestrogénique exercée par
les divers Arcolors. Chez des rats exposés pendant 36 semaines à une
régime alimentaire contenant 75 mg d'Aroclor 1242/kg de nourriture, on
a constaté une diminution du taux d'hormones gonadiques et une
augmentation du poids relatif des testicules. Chez des souris femelles
exposées pendant trois semaines à de l'Aroclor 1254 administré dans leur
nourriture à raison de 25 mg/kg, on a observé une diminution des taux de
corticostéroïdes plasmatiques sans augmentation concomitante du poids
des surrénales. En revanche, chez une autre souche qui avait reçu
pendant deux semaines une nourriture contenant 200 mg de ce mélange par
kg, on a observé un accroissement du poids des surrénales.
On a constaté chez diverses espèces animales, que les mélanges de PCB
exerçaient un effet immunodépresseur, les espèces les plus sensibles à
cet égard étant les singes et les lapins. La dose sans effet observable
la plus faible était de 0,1 mg/kg de poids corporel chez le singe et de
0,18 mg/kg de poids corporel chez le lapin.
Des souris ayant reçu une seule dose orale de 500 mg d'Aroclor 1254 par
kg de poids corporel ont présenté une dépression de l'activité motrice.
Cet effet s'explique probablement par une inhibition du captage et de la
libération des neurotransmetteurs.
On a constaté que les mélanges de PCB diminuaient la concentration en
vitamines A et B1 dans le sang et le foie de rats. Chez des rats et des
souris exposés à des mélanges de PCB on a observé une diminution des
taux de vitamines A, B1, B2 et B6.
1.14 Facteurs qui modifient la toxicité et le mode d'action
Les PCB du commerce suscitent toute une série de réactions toxiques qui
ressemblent en partie à celles qu'entraînent les polychlorodioxines et
les polychlorodibenzofuranes. En outre, les relations structure-activité
analogues observées parmi les divers PCB homologues, pour ce qui
concerne les réactions toxiques qu'ils suscitent et leur aptitude à
induire l'AHH dépendante du cytochrome P448, indiquent que les PCB
ressemblent plus ou moins à des stéréoisomères de la 2,3,7,8,-TCDD sont
les plus actifs. Ces observations laissent penser qu'il existe un
mécanisme commun à la base de l'affinité de ces composés pour la
protéine réceptrice de l'AH du cytosol. On a proposé des facteurs
d'équivalence toxique pour la 2,3,7,8,-TCDD et ces PCB coplanaires. On
n'a pas suffisamment étudier la nature des interactions probables entre
les PCB et les polychlorodibenzofuranes ou les polychlorodibenzodioxines.
Etant donné que les PCB sont capables de stimuler l'activité des enzymes
microsomiques, ils peuvent avoir une influence sur l'action d'autres
substances chimiques dont le métabolisme est sous la dépendance de ces
enzymes. D'autres PCB, qualifiés de coplanaires, peuvent entraîner des
manifestations toxiques plus subtiles. En outre certains PCB, en
particulier ceux qui sont les moins substitué, peuvent être métabolisés
sous forme d'intermédiaires de type oxyde d'arène et de métabolites
méthylsulfonylés.
1.15 Effets sur l'homme
L'évaluation toxicologique des PCB pose de nombreux problèmes. Les PCB
se présentent en général sous la forme de mélanges de nombreux composés
et nombre des données relatives à la toxicité des PCB reposent sur
l'étude de ces mélanges. Certains constituants des mélanges se
décomposent plus facilement dans l'environnement que d'autres. Ainsi, la
population générale peut-elle être exposée à des mélanges qui diffèrent
de ceux auxquels sont exposés les travailleurs qui manipulent des PCB.
C'est principalement par contamination de la nourriture (organismes
aquatiques, produits carnés et laitiers) que la population générale est
exposée aux PCB. La dose journalière ingérée de PCB est de l'ordre de
quelques microgrammes par personne dans la plupart des pays
industrialisés. Ce type d'exposition n'entraîne pas de manifestations
toxiques. Les nourrissons sont exposés aux PCB par l'intermédiaire du
lait maternel. Par cette voie, la dose ingérée peut atteindre quelques
microgrammes par kg de poids corporel et par jour.
On éprouve beaucoup de difficulté à évaluer les effets qu'exercent
séparément sur la santé humaine les PCB, les polychlorodibenzofuranes et
les polychlorodibenzodioxines étant donné que les polychloro-
dibenzofuranes sont de fréquents contaminants des mélanges de PCB et
qu'occasionnellement, on a mis en cause la présence de polychloro-
dibenzodioxines dans les accidents survenus avec certains mélanges de
PCB. On a montré que les PCB du commerce étaient contaminés par des
polychlorodibenzofuranes et que, par conséquent, il était délicat dans
bien des cas de savoir si les effets constatés sont attribuables aux PCB
eux-mêmes ou aux polychlorodibenzofuranes qui sont beaucoup plus
toxiques. Par conséquent, nombre de données tirés d'épisodes importants
d'intoxication humaine, par exemple ceux de Yusho, de Yu-Cheng, etc.
correspondent probablement à une exposition aux PCB et aux
polychlorodibenzofuranes.
Les signes d'intoxication observés chez les malades de Yusho et de
Yu-Cheng consistaient en hypersécrétion des glandes de Meibom au niveau
des yeux, en oedèmes palpébraux et en pigmentation des ongles et des
muqueuses, parfois associés à de la fatigue, des nausées et des
vomissements. On observait généralement ensuite une hyper-keratose et un
brunissement de la peau avec une hypertrophie folliculaire et des
éruptions acnéiformes. Par ailleurs, on a également observé des oedèmes
des bras et des jambes, une hypertrophie du foie avec troubles
hépatiques, des troubles du système nerveux central et des troubles
respiratoires évoquant la bronchite ainsi que des modifications dans
l'état immunitaire des patients. Chez les enfants des malades de Yusho
et de Yu-Cheng, on a observé une réduction de la croissance, une
hyperpigmentation de la peau et des muqueuses, une hyperplasie
gingivale, des paupières oedématiées avec xérophthalmie, la présence de
dents dès la naissance, une calcification anormale du crâne, des pieds
bots en piolet et une forte incidence de faibles poids de naissance. Il
n'a pas été possible de se prononcer de façon définitive quant à
l'existence d'une corrélation entre l'exposition et l'apparition de
tumeurs malignes chez ces malades car le nombre de décès était trop
faible. Toutefois, on a observé une augmentation statistiquement
significative, chez les hommes, de h mortalité par cancer et plus
spécialement cancer du foie et du poumon.
En cas d'exposition sur les lieux de travail, on observe quelques heures
plus tard des éruptions cutanées. En outre il est arrivé qu'après
l'exposition à de fortes concentrations de PCB, se produisent des
démangeaisons, des sensations de brûlure, une irritation de la
conjonctive, une pigmentation des doigts et des ongles et une chloracné.
La chloracné est une des manifestations qui reviennent le plus
fréquemment chez les travailleurs exposés aux PCB. Outre ces signes
cutanés d'intoxication, divers auteurs ont observé des troubles
hépatiques, une immunodépression, une irritation passagère des muqueuses
respiratoires, des effets neurologiques, psychologiques ou
psychosomatiques aspécifiques tels que céphalées, vertiges, dépression,
troubles du sommeil et de la mémoire, nervosité, fatigue et impuissance.
Ce qu'on peut conclure de tout cela c'est qu'une exposition
professionnelle permanente à de fortes concentrations de PCB et de
polychlorodibenzofuranes peut entraîner des effets sur la peau et le
foie.
Deux importantes études de mortalité ont été effectuées sur des cohortes
de travailleurs. Après exposition à de l'Aroclor 1254, 1242 et 1016, on
a observé une augmentation de la mortalité par cancer du foie et de la
vésicule biliaire dans le cas d'une étude ou par cancer en général et
plus particulièrement cancer des voies digestives dans le cas d'une
autre étude. Aucune des études épidémiologiques disponibles ne donne de
preuves concluantes d'une association entre l'exposition aux PCB et
l'accroissement de la mortalité par cancer, du fait du trop petit nombre
de décès dans la population exposée, de l'absence de relation
dose-réponse et des impuretésprésents dans les mélanges de PCB.
2. Conclusions
2.1 Distribution
Du fait de leurs propriétés physiques et chimiques, les PCB sont
dispersés dans tout l'environnement à l'échelle planétaire.
Les PCB sont presque universellement présents chez tous les êtres
vivants dans leur milieu naturel et s'y accumulent facilement. On a
également mis en évidence une bioconcentration le long de la chaîne
alimentaire.
Les PCB les plus fortement chlorés sont ceux qui s'accumulent le plus.
2.2 Effets sur les animaux d'expérience
Les résultats tirés de l'expérimentation animale incitent à penser que
les PCB ont un effet immunodépresseur comme le montre l'étude de leurs
effets macroscopiques sur la fonction immunitaire (poids de la rate,
poids du thymus et numération lymphocytaire). En ce qui concerne
l'Aroclor 1248, la dose sans effet observable pour le singe a été
évaluée à 100 µg/kg et à < 100 µg/kg de poids corporel dans le cas de
l'Aroclor 1254. Il semble que l'effet immunosuppresseur soit spécifique
de tel ou tel PCB en particulier.
On n'observe en général d'effets toxiques sur la reproduction qu'aux
doses qui produisent une intoxication de la mère. Les animaux
nouveau-nés nourris avec le lait contaminé de leur mère (notamment chez
le singe et les autres animaux utilisés comme modèles) semblent être
particulièrement sensibles aux PCB et présentent, à côté d'autres
symptômes toxiques, une réduction de la croissance. La dose d'Aroclor
1016 sans effet observable sur la reproduction est de 30 µg/kg de poids
corporel chez le singe. Il n'a pas été possible d'en établir une dans le
cas de l'Aroclor 1248.
Les PCB ne sont pas génotoxiques et rien n'indique qu'ils jouent le rôle
d'initiateurs tumoraux. Ils n'ont pas non plus d'activité
tumoropromotrice. On peut en conclure que pour évaluer la toxicité des
des mélanges de PCB, il est possible d'envisager l'existence d'un effet
de seuil.
2.3 Effets sur l'homme
L'exposition de la population générale aux PCB s'effectue principalement
par l'intermédiaire des aliments. Les nourrissons sont exposés par
l'intermédiaire du lait maternel.
Deux importants épisodes d'intoxication ont été observés au Japon
(Yusho) et à Taïwan (Yu-Cheng). Les principaux symptômes observés ont
été fréquemment attribués à la présence de contaminants dans les
mélanges de PCB et notamment de polychlorodibenzofuranes. Le Groupe de
travail en a conclu que ces symptômes pouvaient être dus à une
exposition concomitante aux PCB et aux polychlorodibenzofuranes.
Toutefois, certain, symptômes notamment des effets respiratoires
chroniques, pourraient être dus plus particulièrement aux métabolites
méthylsulfoniques de certains PCB.
2.4 Effets sur l'environnement
Si des effets ont été signalés sur des populations locales d'oiseaux,
l'effet le plus important des PCB sur les êtres vivants dans leur milieu
naturel consiste principalement en une réduction de la capacité de
reproduction des mammifères marins. On a observé cet effet
principalement dans des mers semi-fermées et constaté qu'il conduisait,
localement du moins, à une réduction du nombre de ces mammifères. Comme
on peut s'attendre à ce que les résidus de PCB présents dans
l'environnement se redistribuent progressivement par l'intermédiaire du
milieu marin, on peut penser qu'à l'avenir, les mammifères marins seront
encore plus menacés.
3. Recommandations
* Il est recommandé de parvenir à un accord international sur les
méthodes d'analyse afin d'améliorer la comparabilité des résultats
des programmes de surveillance. Il faudrait continuer à mettre au
point les méthodes d'analyse spécifiques de tel ou tel PCB, sans
toutefois méconnaître la valeur des analyses basées sur les
mélanges.
* Afin d'assurer la fiabilité des données d'analyse, il est fortement
recommandé de procéder à un contrôle de qualité interlaboratoires.
Il est également recommandé de créer un réseau international
d'encadrement et de soutien technique destiné à aider les pays en
développement à participer aux activités de contrôle.
* Afin d'améliorer la précision dans l'évaluation du risque que
représentent les PCB, il est recommandé d'effectuer des études à
long terme sur des homologues déterminés ainsi que sur le mode
d'action des divers constituants des mélanges, plus
particulièrement en ce qui concerne leur activité tumoropromotrice.
* Des études épidémiologiques visant à mieux évaluer le risque pour
les nouveau-nés sont nécessaires, car ces derniers constituent le
groupe le plus vulnérable de la population générale du fait qu'ils
sont fortement exposés aux PCB par l'intermédiaire du lait
maternel.
* Il conviendrait de mettre au point, pour les futures études
épidémiologiques, des marqueurs biologiques sensibles et
spécifiques concernant certaines des manifestations les plus
subtiles de la toxicité des PCB (effets sur la reproduction, effets
immunologiques et effets neurologiques).
* L'élimination des PCB doit s'effectuer par incinération dans des
installations convenablement conçues et exploitées qui garantissent
le maintien de températures élevées (plus de 1000°C), du temps de
séjour et de la turbulence nécessaires pour que la décomposition
des molécules soit complète.
* Il faudrait étudier les moyen d'éliminer les PCB déjà présents dans
les décharges contrôlées,
* Il convient d'inciter les responsables à assurer la surveillance
des PCB dans l'environnement, la faune et la flore à l'échelle
mondiale, afin de suivre la redistribution prévisible des résidus
qui s'y trouvent.
* La contamination par les PCB peut réduire la capacité de
reproduction des mammifères marins. Il faudrait inciter les
responsables à entreprendre des études sur les effectifs de cétacés
et leur capacité à se reproduire, tout en poursuivant les
recherches visant à établir quels sont les PCB qui sont
responsables de ces effets.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMMENDACIONES
1. Resumen y evaluación
1.1 Introducción
Los bifenilos policlorados (BPCs) se descubrieron a finales del siglo
pasado y se reconoció pronto su utilidad para la industria, debido a sus
propiedades físicas. Se utilizan comercialmente desde 1930 como fluidos
dieléctricos e intercambiadores de calor y en otras aplicaciones. Se
encuentran ampliamente distribuidos en el medio ambiente de todo el
mundo, son persistentes y se acumulan en la cadena alimentaria. La
exposición humana a los BPCs se debe fundamentalmente al consumo de
alimentos contaminados, pero también a la inhalación y a la absorción
cutánea en los lugares de trabajo. Los BPCs se acumulan en el tejido
adiposo de los seres humanos y de los animales, causando efectos tóxicos
a ambos, particularmente en el caso de exposiciones repetidas. La
patología se manifiesta sobre todo en la piel y el hígado, aunque
también están expuestos el tracto gastrointestinal, el sistema
inmunitario y el sistema nervioso. Los dibenzofuranos policlorados
(BFPCs), que se encuentran como contaminantes en mezclas comerciales de
BPCs, contribuyen de manera significativa a su toxicidad. Los resultados
de los estudios realizados en roedores indican que algunos compuestos
parecidos a los BPCs pueden ser carcinógenos y fomentar la
carcinogenicidad de otros compuestos químicos.
De los datos disponibles de los bifenilos policlorados (BPCs) y los
terfenilos policlorados (TPCs) es evidente que, en una situación ideal,
sería preferible no tener en absoluto estos compuestos en los alimentos.
Sin embargo, es igualmente claro que la reducción a cero o a un nivel
próximo de la exposición a los BPCs o los TPCs en fuentes alimentarias
significaría la eliminación (prohibición del consumo) de grandes
cantidades de alimentos importantes, como el pescado, pero sobre todo la
leche materna. Son los comités científicos nacionales e internacionales
los que deben establecer el debido equilibrio entre lo que se ha de
hacer para conseguir un grado apropiado de protección de la salud
pública y evitar pérdidas excesivas de alimentos.
A partir de los datos disponibles, no se pueden establecer niveles de
exposición a los BPCs o los TPCs que puedan considerarse de garantía
absoluta de inocuidad.
1.2 Identidad y propiedades físicas y químicas
Los BPCs son mezclas de productos químicos aromáticos, que se obtienen
por cloración del bifenilo en presencia de un catalizador adecuado. La
fórmula química de estos compuestos se representa como C12 H10-n Cln,
donde n es un número de átomos de cloro comprendido entre 1 y 10.
En teoría existen 209 compuestos análogos, pero sólo 130 tienen
probabilidad de aparecer en productos comerciales. Además, los BPCs
pueden contener dibenzofuranos policlorados (DFPCs) y cuarterfenilos
clorados como impurezas. En condiciones normales, estas impurezas son
relativamente estables y resistentes a las reacciones químicas. Todos
los compuestos afines a los BPCs son lipófilos y tienen una solubilidad
en agua muy baja. En consecuencia, se introducen fácilmente en la cadena
alimentaria y se acumulan en el tejido adiposo.
Las mezclas comerciales de BPCs contienen DFPCs en concentraciones que
oscilan entre unos pocos mg/kg y 40 mg/kg. En los BPCs comerciales no se
encuentran dibenzo- p-dioxinas policloradas (DDPCs). Sin embargo, en
casos de incendios accidentales y durante la incineración se pueden
encontrar DDPCs cuando están mezcladas con otros compuestos clorados,
como los clorobencenos utilizados en los transformadores.
Las mezclas comerciales de BPC tienen un color que va del amarillo claro
al oscuro. No cristalizan, ni siquiera a baja temperatura, sino que se
convierten en resinas sólidas. Los BPCs son prácticamente
pirorresistentes, con una temperatura de inflamabilidad bastante
elevada. Forman vapores más densos que el aire, pero no dan lugar a
mezclas explosivas con éste. Su conductividad eléctrica es muy baja, la
térmica es bastante alta y tienen una resistencia muy elevada a la
degradación térmica. En condiciones normales, los BPCs son químicamente
muy estables, pero cuando se calientan pueden producir otros compuestos
tóxicos, como los DFPCs.
1.3 Métodos analíticos
En 1966, a partir del descubrimiento de BPCs en muestras obtenidas del
medio ambiente, aumentó el interés por el análisis de estos compuestos
y por su toxicidad para la especie humana y su medio ambiente.
Los datos disponibles no son directamente comparables debido, a
diferencias en la metodología analítica; no obstante, se pueden utilizar
para establecer medidas de control y prevención y para la evaluación
preliminar de los riesgos para la salud y el medio ambiente asociados a
estos compuestos.
Los BPCs se han determinado mediante técnicas de cromatografía de gases
con captura electrónica, a menudo utilizando columnas de relleno, aunque
en estudios recientes se han empleado métodos más complejos, como la
cromatografía en columna capilar y la de gases combinada con la
espectrometría de masas, para identificar por separado los distintos
compuestos análogos, mejorar la comparabilidad de los datos analíticos
de fuentes diferentes y establecer una base para la evaluación de la
toxicidad.
Para realizar estos análisis es necesario un amplio programa de garantía
de la calidad, y se han realizado y recomendado estudios de
intercalibración. La calidad y utilidad de los datos analíticos dependen
decisivamente de la validez de la muestra y de que el muestreo sea
adecuado. Por otra parte, es imprescindible contar con un programa de
muestreo planificado y bien documentado. En la publicación WHO/EURO
(1987) se describe con detalle un procedimiento de muestreo.
1.4 Producción y usos
La producción comercial de los BPCs comenzó en 1930. Se han utilizado
ampliamente en equipo eléctrico, y en volúmenes más pequenos como
líquido pirorresistente en sistemas de régimen cerrado.
Al final de 1980, la producción mundial total de BPCs era superior a un
millón de toneladas y, desde entonces, la producción ha continuado en
algunos países. A pesar de la creciente retirada del uso y de las
restricciones sobre la producción, en el medio ambiente sigue habiendo
cantidades muy elevades de estos compuestos, bien en uso o como desecho.
En los ultimos años, muchos países industrializados han adoptado medidas
para controlar y limitar el flujo de BPCs hacia el medio ambiente. El
factor decisivo que ha llevado a estas restricciones ha sido
probablemente una recomendación de 1973 de la Organización de
Cooperación y Desarrollo Económicos (OCDE) (OMS, 1976; CIIC, 1978; OCDE,
1982). Desde entonces, los 24 países miembros de la OCDE han limitado la
fabricación, la venta, la importación, la exportación y el uso de BPCs,
además de establecer un sistema de etiquetado de estos productos.
Entre las fuentes actuales de liberación de BPCs figuran la
volatilización de vertederos que contienen transformadores,
condensadores y otros residuos con BPCs, aguas residuales, fangos
cloacales, derrames y desechos de dragado, y la eliminación inadecuada
(o ilegal) en zonas abiertas. Se puede producir contaminación durante la
incineración de desechos industriales y municipales. La mayoría de los
incineradores municipales no son eficaces en la destrucción de los BPCs.
La explosión o el sobrecalentamiento de transformadores y condensadores
pueden liberar cantidades significativas de BPCs al entorno local.
Los BPCs se pueden convertir en DFPCs en condiciones pirolíticas. En las
condiciones de laboratorio, la máxima producción de DFPCs se obtuvo a
temperaturas entre 550°C y 700°C. Así pues, la combustión incontrolada
de BPCs puede ser una importante fuente de los peligrosos DFPCs. Por lo
tanto, se recomienda un cuidadoso control de la destrucción de desechos
contaminados con BPCs, especialmente en relación con la temperatura de
combustión (por encima de los 1000°C), el tiempo de permanencia y la
turbulencia.
1.5 Transporte, distribución y transformación en el medio ambiente
Los BPCs se encuentran en la atmósfera principalmente en fase de vapor;
la tendencia a adsorberse sobre partículas aumenta con el grado de
cloración. La distribución prácticamente universal de los BPCs parece
indicar que los transporta el aire.
En la actualidad, la principal fuente de exposición en el medio ambiente
general parece ser la redistribución de los BPCs que previamente se han
introducido en él. Dicha redistribución se deriva de su volatilización
del suelo y el agua para pasar a la atmósfera, con el posterior
transporte por el aire y la eliminación de la atmósfera mediante
sedimentación húmeda o seca (de los BPCs unidos a partículas), para
luego volver a volatilizarse. Su concentración en las precipitaciones
oscila entre 0,001 y 0,25 µg/litro. Dado que los ritmos de
volatilización y degradación de los BPCs varían según los compuestos,
esta redistribución produce una alteración en la composición de las
mezclas de BPC presentes en el medio ambiente.
En el agua, los BPCs se adsorben en los sedimentos y otra materia
orgánica; los datos experimentales y de supervisión han puesto de
manifiesto que las concentraciones de BPCs en los sedimentos y en la
materia en suspensión son más elevadas que en las masas de agua
correspondientes. Una fuerte adsorción en el sedimento, especialmente en
el caso de BPCs con un grado elevado de cloración, disminuye la tasa de
volatilización. Sobre la base de su solubilidad en agua y los
coeficientes de reparto n-octanol-agua, los compuestos del grupo del
BPC menos clorados se adsorberán con menos fuerza que los isómeros con
más átomos de cloro. Aunque la adsorción puede inmovilizar los BPCs en
el medio acuático durante períodos relativamente largos, se ha
demostrado que la liberación a la masa del agua se produce tanto por vía
abiótica como biótica. Por consiguiente, las importantes cantidades de
BPCs en los sedimentos acuáticos pueden actuar como sumideros del medio
ambiente y como depósito de estos compuestos para los organismos. Se ha
estimado que la mayor parte de los BPCs presentes en el medio ambiente
está en el sedimento acuático.
La baja solubilidad y la fuerte adsorción de los BPCs en las partículas
del suelo limitan la lixiviación; los compuestos con menor grado de
cloración tienen una tendencia mayor a la lixiviación que los más
clorados.
La degradación de los BPCs en el medio ambiente depende del grado de
cloración del bifenilo. En general, la persistencia de los isómeros de
BPC aumenta con el grado de cloración. En la atmósfera, el proceso de
transformación predominante puede ser la reacción en fase de vapor de
los BPCs con radicales hidroxilos (formados fotoquímicamente por la luz
solar). La semivida estimada de esta reacción en la atmósfera oscila
entre unos 10 días para el monoclorobifenilo y año y medio para el
heptaclorobifenilo.
En el medio acuático, la hidrólisis y la oxidación no degradan de manera
significativa los BPCs. La fotólisis parece ser el único proceso
abiótico de degradación viable en el agua; sin embargo, los datos
experimentales disponibles no son suficientes para establecer su
proporción o importancia en el medio ambiente.
Los microorganismos degradan los bifenilos monoclorados, diclorados y
triclorados de manera relativamente rápida, y más lentamente los
bifenilos tetraclorados, mientras que los bifenilos con mayor grado de
cloración son resistentes a la biodegradación. La posición de los átomos
de cloro en el anillo bifenilo parece ser importante para determinar la
tasa de biodegradación. Esta se da con preferencia en los compuestos que
contienen átomos de cloro en posiciones -para. Los compuestos más
clorados experimentan una transformación anaerobia, mediante un
decloración reductora, para dar BPCs con menos átomos de cloro, que
pueden luego continuar la biodegradación mediante procesos aerobios.
El grado de bioacumulación en el tejido adiposo depende de varios
factores: la duración y el nivel de la exposición, la estructura química
del compuesto y la posición y modelo de la sustitución. En general, se
acumulan más fácilmente los compuestos con mayor número de sustituyentes
de cloro.
Los factores de bioconcentración de distintos BPCs determinados
experimentalmente en las especies acuáticas (peces, camarones, ostras)
varía entre 200 y 70 000 o más. En mar abierto, hay bioacumulación de
BPCs en los niveles tróficos más elevados, con una mayor proporción de
los bifenilos más clorados en los depredadores que ocupan un lugar más
alto en la escala.
La transferencia de los BPCs del suelo a la vegetación tiene lugar
principalmente por adsorción en la superficie externa de las plantas
terrestres; los desplazamientos que tienen lugar son escasos.
1.6 Niveles medioambientales y exposición humana
Debido a su elevada persistencia y sus demás propiedades físicas y
químicas, los BPCs están presentes en el medio ambiente en todo el
mundo.
En general, sus concentraciones en el aire son de 0,002 a 15 ng/m3.
En zonas industriales los niveles son más altos (hasta del orden de
µg/m3). En el agua de lluvia y la nieve alcanzan valores entre no
detectables (1 ng) y 250 ng/litro.
En el medio de trabajo, los niveles en el aire pueden ser mucho más
elevados. En ciertas condiciones, como por ejemplo en la fabricación de
transformadores y condensadores, se han observado concentraciones de
hasta 1000 µg/m3. En casos de emergencia grave se han medido niveles de
hasta 16 mg/m3. En casos de incendios o explosiones se puede producir
hollín que contiene niveles altos de BPCs. Se han encontrado niveles de
8000 mg de BPCs/kg de hollín. En este caso también hay DFPCs. En
accidentes con transformadores que contienen bencenos clorados aparecen
también dioxinas policloradas (DDPCs), además de BPCs.
En tales situaciones de emergencia se pueden producir ingestión,
contaminación de la piel o inhalación de partículas de hollín, con una
exposición grave del personal. Sin embargo, la exposición de la
población general a través del aire es muy baja.
Las aguas superficiales se pueden contaminar con BPCs procedentes de la
atmósfera, de emisiones directas de fuentes puntuales o de la
eliminación de desechos. En ciertas condiciones se han medido
concentraciones de 100-500 ng/litro de agua. En los océanos se han
detectado niveles de 0,05 a 0,6 ng/litro.
En zonas no contaminadas, el agua potable contiene cantidades de BPCs
inferiores a 1 ng/litro, pero se han notificado valores de hasta
5 ng/litro. El suelo y los sedimentos de diferentes zonas, dependiendo
de las condiciones locales, contienen concentraciones que oscilan entre
<0,01 hasta 2,0 mg/kg. En las zonas contaminadas los niveles han sido
mucho mayores, es decir, de hasta 500 mg/kg.
En los últimos años se han analizado muchos miles de muestras de
productos alimenticios en varios países para detectar contaminantes,
BPCs inclusive. La mayor parte de las muestras se tomaron de artículos
alimenticios individuales, especialmente pescado y otros alimentos de
origen animal, como carne y leche. Los alimentos humanos se contaminan
con BPCs por tres vías principales:
a) absorción del medio ambiente por los peces, las aves, el ganado (a
través de la cadena alimentaria) y los cultivos;
b) migración de los materiales de envasado a los alimentos
(principalmente por debajo de 1 mg/kg, pero, en algunos casos, hasta
10 mg/kg);
c) contaminación directa del alimento o de los piensos por accidentes
industriales.
Los niveles en los artículos alimenticios más importantes que contenían
BPCs fueron: grasa animal, 20-240 µg/kg; leche de vaca,
5-200 µg/kg; mantequilla, 30-80 µg/kg; pescado, 10-500 µg/kg de grasa.
Ciertas especies de peces (anguila) o productos derivados del pescado
(hígado y aceites de pescado) contienen niveles mucho más altos, de
hasta 10 mg/kg. En hortalizas, cereales, frutas y algunos otros
productos la concentración observadas es de <10 µg/kg. Los principales
alimentos cuya contaminación con BPCs requiere atención son el pescado,
el marisco, la carne, la leche y otros productos lácteos. En diversos
países se han notificado niveles medios en el pescado del orden de 100
µg/kg (de grasa). Las comparaciones realizadas parecen indicar que la
concentración en el pescado está disminuyendo lentamente.
Los BPCs se acumulan en el tejido adiposo humano y en la leche materna.
Su concentración en los distintos órganos y tejidos depende del
contenido en lípidos, con la excepción del cerebro. Los residuos en el
tejido adiposo de la población general de los países industrializados
varía entre menos de 1 y 5 mg/kg de grasa, en función de la residencia
del donante, su tipo de vida y el método analítico utilizado. Las
mujeres que viven en zonas urbanas muy industrializadas, o que consumen
una gran cantidad de pescado, especialmente si procede de aguas con una
contaminación intensa, pueden acumular en la leche concentraciones
superiores de BPCs.
La composición de la mayoría de los extractos de BPCs procedentes de
muestras del medio ambiente no se parecen a las mezclas comerciales.
Utilizando el análisis de cromatografía de gases de alta resolución se
ha demostrado también que la composición del conjunto de los productos
afines y la concentración relativa de cada componente en el tejido
adiposo y la leche materna son notablemente diferentes de las que se
observan en los comerciales. Los BPCs detectados por cromatografía de
gases en el tejido adiposo humano y la leche materna contienen sobre
todo concentraciones relativamente altas de los compuestos más clorados,
como: 2,4,5,3',4'-pentaclorobifenilo; 2,4,5,2',4',5'-hexa-
clorobifenilo y 2,3,4,2',4',5'-hexaclorobifenilo; 2,3,4,5,2',4',5'-
heptaclorobifenilo; 2,3,4,5,2',3',4'-heptaclorobifenilo. Algunos otros
compuestos del grupo de los BPCs están presentes en cantidades mucho más
bajas, como los BPCs coplanares, muy tóxicos: 3,4,3',4'-tetra-
clorobifenilo, 3,4,5,3',4'-pentaclorobifenilo y 3,4,5,3',4',5'-hexa-
clorobifenilo.
Se ha calculado que la ingesta diaria de BPCs de los lactantes con la
leche materna es del orden de 4,2 µg/kg de peso corporal (5,2 µg/
100 kcal consumida) (OMS/EURO, 1988). La cantidad media total de BPCs
ingeridos con la leche materna durante los seis primeros meses de vida
es de 4,5 mg, mientras que la calculada para el resto de su vida es de
357 mg (0,2 µg/kg por día, en la dieta de una persona de 70 kg durante
70 años de vida). Por consiguiente, el período de la lactancia aporta
alrededor del 1,3% a la ingesta de toda la vida, cantidad no muy grande
si se tiene en cuenta los beneficios de la lactancia natural (OMS/EURO,
1988).
De acuerdo con los datos básicos evaluados, el promedio de BPCs en la
ingesta alimentaria de los adultos alcanza un máximo de 100 g por
semana, o alrededor de 14 µg/por persona al día. Para una persona de
70 kg, esto equivale a un máximo de 0,2 µg/k/ de peso corporal al día
(OMS/EURO, 1988).
1.7 Cinética y metabolismo
Se han descrito estudios en animales relativos fundamentalmente a las
exposiciones oral, respiratoria y cutánea a mezclas de BPCs y a
compuestos por separado. En general, los BPCs parece que se absorben con
rapidez, particularmente en el tracto gastrointestinal tras la
exposición oral. Es evidente que se produce absorción en los seres
humanos, pero la información sobre las tasas de absorción de los BPCs en
ellos es limitada.
Los datos de los estudios disponibles sobre su distribución parecen
indicar un proceso cinético bifásico, con eliminación rápida de la
sangre y acumulación en el hígado y en el tejido adiposo de diversos
órganos. También hay pruebas de su transporte a través de la placenta,
su acumulación fetal y su distribución en la leche. En algunos estudios
realizados en la especie humana, la piel contenía una concentración
elevada de BPCs, pero la concentración en el cerebro era inferior a la
prevista en función de su contenido en lípidos.
La movilización de los BPCs de la grasa parece depender en gran medida
de la tasa de metabolismo de cada uno de los BPCs. La excreción depende
de su transformación en compuestos más polares, como fenoles, sistemas
conjugados de compuestos de tiol y otros derivados solubles en agua.
Entre las vías metabólicas están la hidroxilación y la conjugación con
tioles y otros derivados solubles en agua, en algunos casos con la
intervención de productos intermedios reactivos, como los óxidos de
areno. Se ha demostrado que la tasa de metabolismo depende de la
estructura del BPC y está en función del número de átomos de cloro y de
su posición. Los metabolitos polares de los BPCs más clorados parece que
se eliminan sobre todo por las heces, aunque también puede ser
significativa la excreción en la orina. Una importante vía de
eliminación es a través de la leche (materna). Algunos compuestos
también se pueden eliminar por el pelo.
Los estudios cinéticos disponibles indican que hay una amplia
divergencia en la semivida biológica entre los distintos compuestos del
grupo, y esto puede ser debido a diferencias en el metabolismo
dependientes de la estructura, las afinidades tisulares y otros factores
que afectan a la movilización de los lugares de almacenamiento.
No siempre hay correlación entre la persistencia en los tejidos y una
toxicidad elevada, y las diferencias de toxicidad entre los distintos
compuestos pueden estar asociadas a metabolitos concretos o a sus
productos intermedios.
1.8 Efectos sobre los seres vivos del medio ambiente
Los BPCs son contaminantes universales de la naturaleza, y están
presentes en la mayoría de los compartimentos del medio ambiente,
abióticos y bióticos, de todo el mundo. Desde que en numerosos países se
comenzó a controlar el uso y la liberación, su incorporación al ambiente
se ha reducido en comparación con la del pasado. Sin embargo, las
pruebas obtenidas hasta ahora indican que el ciclo que siguen los BPCs
está produciendo una redistribución gradual de algunos de los compuestos
hacia el entorno marino. Existe una tendencia de los compuestos más
clorados a una acumulación preferencial. Aunque gran parte de los BPCs
se adsorben sobre las partículas del sedimento, mantienen la
biodisponibilidad para los organismos, por lo que continuarán
acumulándose en los niveles más altos de la cadena trófica.
1.8.1 Estudios de laboratorio
Los efectos de las mezclas de BPCs en los microorganismos son muy
variables, y mientras que algunas especies presentan efectos adversos
con concentraciones de 0,1 mg/litro, otras no se ven afectadas por
concentraciones de 100 mg/litro; los efectos en las diferentes especies
no dependen de manera sustancial del grado de cloración de las mezclas.
Casi todos los estudios sobre los efectos de los BPCs en los organismos
acuáticos se han realizado con mezclas de Aroclor. Los resultados
obtenidos han sido enormemente variables, sin una relación clara entre
el grado de cloración o las condiciones medio-ambientales y la
toxicidad, incluso en organismos estrechamente relacionados. Los valores
de la CL50 para un período de 96 h en condiciones fijas han variado
entre 12 µg/litro y >10 mg/litro para las distintas especies de
invertebrados acuáticos y las diferentes mezclas de Aroclor. Las
condiciones de flujo aumentaron la toxicidad de los BPCs. En general, la
mezclas más tóxicas fueron las de Aroclor con un grado intermedio
de cloración; las mezclas con un porcentaje de cloro bajo o alto
resultaron menos tóxicas. Esto ocurrió también en los efectos
subletales, como los efectos sobre la reproducción en Daphnia. Los
crustáceos parecen ser más sensibles a los BPCs durante la muda. En
poblaciones utilizadas como modelo, la estructura comunitaria de las
especies de estuario cambió tras la exposición a Aroclor 1254, y
mientras que el número de anfípodos, briozoos, crustáceos y moluscos
disminuyó, el de anélidos, braquiópodos, celentéreos, equinodermos y
nemertinos se mantuvo inalterado. Se ha considerado un número
excesivamente escaso de grupos en las pruebas de toxicidad aguda para
determinar si los resultados reflejan cambios en la susceptibilidad a
los BPCs o diferencias de interacción entre las especies.
La variación de la toxicidad de estos compuestos para los peces es
similar, con una CL50 en 96 horas que oscila entre 0,008 y
> 100 mg/litro. En las pruebas de larga duración se ha puesto de
manifiesto que en la exposición aguda, particularmente en condiciones
fijas, se subestima considerablemente la toxicidad de los BPCs. La
trucha arco iris fue particularmente sensible, con CL50 de
0,32 µg/litro de Aroclor 1254 en 22 días durante las fases
embrionario-larvarias, y un nivel sin efectos observados (NOEL) en 22
días de 0,001 µg/litro de Aroclor 1016, 1242 y 1254.
El pez de agua dulce Pimephales promelas mostró valores del NOEL de
5,4, 0,1, 1,8 y 1,3 µg/litro para los tipos de Aroclor 1242, 1248 1254
y 1260, respectivamente; el NOEL para el pez de estuario Aplodinotus
grunniens fue de 3,4 y 0,06 µg/litro de Aroclor 1016 y 1254,
respectivamente.
Las pruebas experimentales han confirmado las observaciones sobre el
terreno que demostraban la presencia de trastornos de la reproducción en
focas alimentadas con peces que contenían BPCs acumulados en el medio.
El efecto se produce en una fase avanzada de la reproducción, impidiendo
la implantación del embrión en la pared uterina.
En pruebas de corta duración, la toxicidad del Aroclor en las aves
aumentó al hacerlo el porcentaje de cloración; las CL50 con cinco días
de alimentación oscilaban entre 604 y 6000 mg/kg de alimentos. Los
principales efectos de los BPCs sobre la reproducción de las aves fueron
una reducción de la capacidad de eclosión de los huevos y
embriotoxicidad. Estos efectos se mantuvieron tras finalizar la
administración, puesto que las gallinas reducían la cantidad de BPCs por
medio de los huevos. No hay pruebas de que el Aroclor induzca
directamente la formación de cáscaras de los huevos más finas; los
efectos sobre el consumo de alimentos y el peso corporal de las gallinas
influyen indirectamente en el espesor de la cáscara. Se han notificado
efectos subletales en el comportamiento y en la secreción de hormonas.
La toxicidad aguda de los Aroclor en el visón disminuye al hacerlo el
porcentaje de cloración, variando la DL50 de la toxicidad aguda varía
entre >750 y 4000 mg/kg de peso corporal; el hurón es menos sensible.
El Aroclor reduce el consumo de alimentos y, por consiguiente, el ritmo
de crecimiento de los visones jóvenes. También reduce o impide la
reproducción del visón, tanto si se le suministra directamente como si
ingiere pescado contaminado. Cuanto mayor es el porcentaje de cloración
de los Aroclor (sobre todo el 1254), mayores son sus efectos. El índice
de reproducción vuelve a la normalidad tras el cese de la alimentación
con Aroclor.
Los murciélagos son susceptibles al Aroclor que se libera de la grasa
durante la migración.
La gran mayoría de las pruebas de laboratorio sobre animales acuáticos
y terrestres se llevaron a cabo utilizando mezclas de BPCs, por lo que
no es posible identificar qué componentes específicos de la mezclas
fueron los causantes de los efectos. De manera análoga, las pruebas se
realizaron en condiciones ambientales no reales (por ejemplo,
sobrepasando la solubilidad y sin sedimento presente en las pruebas
acuáticas), por lo que es difícil extrapolar los resultados del
laboratorio al campo. Sin embargo, hay motivos para suponer que
cualquier efecto sobre las poblaciones de organismos, que probablemente
se podrán presentar de manera más generalizada en el futuro, ya se
habrán observado en el pasado en poblaciones locales expuestas a altos
niveles de BPCs.
1.8.2 Estudios sobre el terreno
Los resultados que indican efectos de los BPCs en poblaciones de peces
sobre el terreno son poco concluyentes. La interpretación de los datos
de campo en aves es difícil, puesto que también hay presentes residuos
de muchos compuestos organoclorados diferentes.
La mayoría de los autores han señalado una correlación entre los efectos
(embriotoxicidad) y la concentración total de residuos organoclorados.
Del conjunto de los compuestos organoclorados presentes, los residuos de
BPCs son los que tienen mayor correlación con la embriotoxicidad, pero
los resultados no se pueden considerar como efectos de estos residuos
demostrados sobre el terreno.
Hay pruebas (confirmadas en estudios de laboratorio) de que los BPCs
reducen la capacidad reproductiva de los mamíferos acuáticos. Aunque
ejercen su efecto en la implantación del embrión, también pueden
ocasionar cambios físicos en el tracto reproductor de las hembras.
No es posible extrapolar las pruebas de laboratorio de toxicidad aguda
durante un período corto a los efectos sobre el terreno en las
poblaciones. La incertidumbre sobre qué componentes de las mezclas de
BPCs causan los efectos, cuáles son los compuestos específicos presentes
en el medio ambiente y cuál es la biodisponibilidad de los componentes
de los BPCs para el organismo, en conjunto dificultan las estimaciones
de las probables exposiciones en el medio ambiente y sus efectos. Los
efectos sobre las poblaciones de mamíferos marinos se pueden considerar
demostrados, pero todavía no se conoce qué componente o componentes de
las mezclas de BPCs los producen.
Dada la tendencia hacia el aumento de contaminación del medio ambiente
marino, se debería prestar más atención a los efectos sobre los
organismos marinos. Hay pruebas claras de laboratorio y sobre el terreno
de los efectos sobre la reproducción en poblaciones de mamíferos marinos
de zonas intensamente contaminadas. Es probable que en el futuro
aumenten los residuos y los efectos de los BPCs en otras poblaciones de
mamíferos marinos. Es menos claro si se verán los efectos en otros
organismos, como las aves que se alimentan de presas marinas.
Sería de esperar que, de acuerdo con los experimentos de laboratorio, se
produjeran efectos en poblaciones y comunidades de organismos
inferiores, como el fitoplancton y el zooplancton. Es difícil evaluar
tanto la amplitud como la importancia de tales cambios. Con la
información actualmente disponible, no cabe esperar efectos sobre las
poblaciones de peces, aunque éstos sean una vía de exposición para los
mamíferos y las aves que se alimentan de peces.
Los efectos anteriormente descritos sobre especies terrestres, mamíferos
de agua dulce que se alimentan de peces y murciélagos migratorios, por
ejemplo, deberían ser menos evidentes a medida que se redistribuyan los
residuos de BPCs. Los residuos en la biota terrestre muestran en la
actualidad una pequeña disminución general, pero la información acerca
de los cambios de los compuestos del grupo es escasa o nula. Se
considera que la reducción de los compuestos más clorados será lenta.
1.9 Efectos en los animales de experimentación y en sistemas de
prueba in vitro
1.9.1 Exposición única
La toxicidad aguda de los Aroclor, tras una exposición oral única,
generalmente es baja en las ratas. Los animales jóvenes parecen ser más
sensibles (DL50: 1,3-2,5 g/kg de peso corporal) que los adultos (DL50:
4-11 g/kg de peso corporal). La DL50 más baja de Aroclor 1254 de la que
se tiene noticia en ratas adultas fue de 1,0 g/kg de peso corporal. No
se observaron diferencias entre ambos sexos.
La DL50 cutánea en conejos osciló entre >1,26 y <2 g/kg de peso
corporal para el Aroclor 1260 (en aceite de maíz) y de 0,79 a
< 3,17 g/kg de peso corporal para algunas otras mezclas no diluidas de
BPC. Por vía intravenosa, las ratas mostraron para el Aroclor 1254 una
DL50 de 0,4 g/kg de peso corporal; la DL50 en ratones tras la inyección
intraperitoneal varió entre 0,9 y 1,2 g/kg de peso corporal.
1.9.2 Exposición de corta duración
Los principales objetivos a los que llegan las mezclas de BPCs o sus
compuestos por separado en mamíferos con exposición oral de corta
duración son el hígado, la piel y los sistemas inmunitario y
reproductor. La especie más sensible de las probadas fue el mono Rhesus,
siendo la hembra más susceptible que el macho. Las hembras adultas de
mono Rhesus sometidas durante seis meses a una dieta con concentraciones
de 2,5 mg/kg ó 0,09 mg/kg de peso corporal al día de Aroclor 1248
mostraron un aumento de la tasa de mortalidad, retraso del crecimiento,
alopecia, acné, inflamación de las glándulas de Meibomio y posiblemente
inmunosupresión. En el análisis microscópico, se encontró un hígado
adiposo agrandado, con necrosis focal, hiperplasia epitelial y
queratinización de los folículos pilosos. Con niveles de exposición más
elevados, también se han observado cambios en otros tejidos epiteliales,
como las glándulas sebáceas y de Meibomio, la mucosa gástrica, la
vesícula biliar, el conducto biliar, los lechos de las uñas y el
ameloblasto. Los niveles totales de lípidos, triglicéridos y colesterol
en el suero disminuyeron. La exposición breve a mezclas comerciales de
BPCs indujeron un aumento de la concentración de lípidos, triglicéridos,
colesterol y fosfolípidos totales en el hígado. Entre los distintos
compuestos de los BPCs, los más potentes fueron el 3,4,3',4'-
tetraclorobifenilo, el 3,4,5,3',4',5'-hexaclorobifenilo y el
2,4,6,2',4',6'-hexaclorobifenilo. Las concentraciones de 0,2 mg/kg de
peso corporal al día de Aroclor 1254 mostraron también algunos otros
efectos, como lesiones linforreticulares, desprendimiento de las uñas y
efectos gingivales, pero no se produjeron ni acné ni alopecia. En los
monos Rhesus se estableció un NOEL para la toxicidad general del Aroclor
1242 de 0,04 mg/kg de peso corporal al día. En monos Rhesus lactantes
expuestos a dosis mucho más elevadas, de 35 mg/kg de peso corporal al
día de Aroclor 1248, se observaron efectos relativamente ligeros. Donde
mejor se han investigado los efectos sobre el hígado es en ratas, y
entre ellos figuran hipertrofia, degeneración adiposa, proliferación del
retículo endoplásmico, porfiria, adenofibrosis, hiperplasia del conducto
biliar, quistes y cambios preneoplásicos y neoplásicos. En estudios
sobre ratas y ratones, los distintos compuestos de los BPCs causaron
efectos en el hígado, el bazo y el timo, siendo mayor la toxicidad de
los compuestos planares. En los monos, dichos compuestos planares, en
dosis de 1 a 3 mg/kg de dieta, indujeron efectos de carácter y gravedad
análogos a los producidos por dosis de 100 mg/kg de dieta de Aroclor
1242 y dosis de 25 mg/kg de dieta de Aroclor 1248.
Las mezclas de BPCs y algunos de los compuestos causaron a conejos y
ratones, tras una exposición cutánea, efectos en la piel y el hígado
similares a los presentes después de la exposición oral. En los conejos
se observaron también atrofia del timo, reducción de los centros
germinales de los nódulos linfáticos y leucopenia.
1.10 Reproducción, embriotoxicidad y teratogenicidad
1.10.1 Reproducción y embriotoxicidad
No se han realizado estudios completos de la reproducción y la
teratogenicidad. En un estudio de reproducción de dos generaciones en
ratas, se estableció un NOEL de 0,32 mg/kg de peso corporal, basado en
parámetros de la reproducción (Aroclor 1254) y un NOEL de 7,5 mg/kg de
peso corporal (Aroclor 1260). Sin embargo, la dosis más baja de las
probadas, de 0,06 mg/kg de peso corporal, produjo en animales destetados
un aumento del peso relativo del hígado.
En los monos Rhesus expuestos a Aroclor 1016, se estableció un NOEL de
0,03 mg/kg de peso corporal, utilizando como base los parámetros de la
reproducción. Sin embargo, con esta concentración se observó una
disminución del peso al nacer, y la dosis más baja de las probadas, de
0,01 mg/kg de peso corporal, produjo una hiperpigmentación de la piel.
Un año después de cesar la exposición, se detectó en los monos Rhesus un
NOEL de 0,09 mg/kg de peso corporal para el Aroclor 1248 (con DFPCs).
1.10.2 Teratogenicidad
En los estudios disponibles en ratas y monos no hay indicación de ningún
efecto teratogénico después de su exposición oral durante la
organogénesis. En ratas, se apreció para el Aroclor 1254 un NOEL de
50 mg/kg de peso corporal en relación con el peso de las crías, y se
podría suponer un NOEL de 2,5 mg/kg de peso corporal, tomando como base
la fetotoxicidad (lesión en las células foliculares del tiroides).
En las pruebas de teratogenicidad con los compuestos por separado en
ratones, ratas y monos Rhesus, no se estableció el NOEL. Una dosis de
0,07 mg/kg de peso corporal produjo en los monos Rhesus efectos tóxicos
matemos (3,4,3',4'-tetraclorobifenilo).
1.11 Mutagenicidad
Las mezclas de BPCs no causaron mutaciones ni lesiones cromosómicas en
distintos sistemas de prueba. El 3,4,3',4'-tetraclorobifenilo produjo
fragmentación cromosómica de linfocitos humanos in vitro.
Concentraciones elevadas de mezclas de BPCs pueden dar lugar a lesiones
primarias en el ADN, como puso de manifiesto la rotura de cadenas
sencillas de ADN en ensayos con soluciones alcalinas.
1.12 Carcinogenicidad
La interpretación de los datos disponibles sobre animales en relación
con mezclas comerciales de BPCs se ve con frecuencia complicada por la
escasez de información en cuanto a la presencia, o contribución, de las
impurezas de dibenzofuranos clorados, así como a variaciones en la
composición de los compuestos.
Se han llevado a cabo diversos estudios de carcinogenicidad de larga
duración en ratones y ratas. Las mezclas que se utilizaron fueron:
Kanechlor 300, 400 y 500, Aroclor 1254 y 1260 y Clophen A30 y A60. Se
notificó que el Clophen no contenía DFPCs, pero no se aportaron datos
sobre la pureza de los demás mezclas de BPCs.
En ratones alimentados con una dieta que contenía Kanechlor 500 y
Aroclor 1254 en dosis de unos 15 a 25 mg/kg de peso corporal se observó
un aumento significativo de adenomas hepatocelulares y/o carcinomas. En
ratones tratados con Kanechlor 300 y 400 no se pudieron detectar
neoplasmas.
En estudios de exposición de ratas a Aroclor 1254 y 1260 y Clophen A30
durante un período superior a un año se detectó un aumento de adenomas
hepatocelulares y/o carcinomas. No se consideró estadísticamente
significativo en estos estudios el aumento de la frecuencia de animales
con cáncer, pero sí en otros dos estudios. Con Aroclor 1260 y Clophen
A60 administrados a dosis de unos 5 mg/kg de peso corporal se observó un
aumento de la frecuencia de carcinomas hepatocelulares (trabeculares) y
adenocarcinomas.
Se consideró que los tumores hepáticos producidos no eran agresivos
(benignos o de escasa malignidad, sin metástasis) y no acortaban la
vida. En algunos estudios se notificaron casos de adenofibrosis, una
lesión preneoplásica, y/o nódulos neoplásicos. En una prueba en ratas
con Aroclor 1254 se demostró un aumento relacionado con la dosis de
metaplasia intestinal y adenocarcinomas de la parte glandular del
estómago.
Hay pruebas claras que demuestran los efectos potenciadores de los BPCs
en la carcinogénesis del hígado en roedores pretratados con
hepatocarcinógenos. Existen algunos indicios de actividad iniciadora de
las mezclas de BPCs en roedores. De los informes sobre estudios de
genotoxicidad se puede concluir que las mezclas de estos compuestos
carecen de genotoxicidad. De estos resultados se deduce que la
asociación de los tumores hepáticos con la administración de BPCs a
roedores se puede atribuir a algunos mecanismos epigenésicos que inducen
la proliferación celular en el hígado y otras manifestaciones de
hepatotoxicidad, por lo que en la evaluación de la toxicidad de los BPCs
se puede seguir un método de determinación del umbral. Es necesario
tener en cuenta la posibilidad de que los BPCs potencien la
carcinogénesis en otros tejidos distintos del hígado en animales con
exposición previa a diversos carcinógenos específicos de los tejidos.
La actividad anticarcinógena que los BPCs han mostrado en algunos
estudios, al tratar animales con estos compuestos durante la
administración de carcinógenos y antes de ella, puede estar relacionada
con las propiedades inductoras de enzimas microsomales de los BPCs,
dando lugar a un aumento de la destoxificación.
En general, hay que ser prudentes a la hora de extrapolar a los seres
humanos los datos disponibles sobre el potencial carcinógeno de los BPCs
en animales.
1.13 Estudios especiales
Tras la exposición a mezclas de BPCs o a compuestos individuales, se
observaron lesiones en el hígado, la piel, el sistema inmunitario, el
sistema reproductor, edemas y alteraciones del tracto gastrointestinal
y de la glándula tiroides.
Los BPCs pueden inducir la formación de diversas enzimas en el hígado.
Esto se ha demostrado en ratas, ratones, cobayos, conejos, perros y
monos utilizando Aroclor 1248, 1254 y 1260 y Kanechlor 400 (inducción
del citocromo P450 y P448). La capacidad de inducción aumenta con el
contenido de cloro de la molécula. Depende también de la composición de
congéneres: los que tienen el cloro en posición para- y meta- inducen
la enzima P450. Para la inducción de la AHH, la posición del cloro
parece ser más importante que el grado de cloración. Los inductores más
potentes de la AHH son los compuestos con cloro en posición para- y
por los menos dos en posición meta-. Se han observado diferencias
claras entre especies. El NOEL más bajo (0,025 mg/kg de peso corporal)
se encontró para el Aroclor 1260 en ratas Osborn-Mendel.
Se considera que los efectos sobre el sistema endocrino se manifiestan
como alteraciones de la unión al receptor hormonal y del equilibrio
hormonal esteroideo. Hay pruebas directas e indirectas de que diversos
Aroclor producen una débil actividad estrógena. Se observó que en ratas
expuestas a 75 mg de Aroclor 1242/kg de dieta durante 36 semanas se
producía una disminución de los niveles de hormonas gonadales y un
aumento del peso relativo de los testículos. En ratones hembra expuestos
a Aroclor 1254 (25 mg/kg de dieta) durante tres semanas se detectó la
reducción de los niveles de corticosteroides en el plasma, sin aumento
del peso adrenal. En otra raza a la que se suministró una dieta con
200 mg/kg durante dos semanas se observó un aumento del peso adrenal.
Las mezclas de BPC han mostrado un efecto inmunosupresor en varias
especies animales, siendo monos y conejos los más sensibles. Los NOEL
más bajos fueron de 0,1 mg/kg de peso corporal en monos y de
0,18 mg/kg de peso corporal en conejos.
En ratones a los que se suministró una dosis oral única de 500 mg/kg
de peso corporal de Aroclor 1254 se observó una disminución de la
actividad motora. Esto probablemente se debió a una inhibición de la
absorción y liberación de neurotransmisores.
Se ha encontrado que las mezclas de BPCs hacen disminuir en las ratas el
nivel sanguíneo y hepático de las vitaminas A y B1. En ratas y ratones
expuestos a mezclas de BPCs se produjo una reducción en la concentración
de las vitaminas A, B1, B2 y B6.
1.14 Factores modificadores de la toxicidad, mecanismo de acción
Los productos comerciales de BPCs muestran un espectro de respuesta
tóxica en parte parecido al de los DDPCs y DFPCs. Además, los distintos
BPCs tienen unas relaciones análogas entre estructura y actividad con
respecto a la mayor parte de sus respuestas tóxicas y a su capacidad de
inducción de AHH dependiente del P448, lo cual indica que los BPCs que
son aproximadamente esteroisómeros del 2,3,7,8-DDTC son los más activos.
Estos resultados parecen indicar que hay un mecanismo común de acción
basado en la afinidad de estos compuestos por la proteína citosólica
receptora de AH. Se han propuesto factores de equivalencia tóxica para
estos compuestos coplanares en relación con el 2,3,7,8-DDTC. No se ha
investigado adecuadamente la naturaleza de las probables interacciones
entre BPCs, DFPCs y DDPCs. Como los BPCs estimulan la actividad de las
enzimas microsomales, pueden influir en la acción de otros productos
químicos que se ven sometidos al metabolismo microsomal. Otros
compuestos, llamados no planares, pueden producir otras toxicidades más
sutiles. Además, los distintos BPCs, especialmente los menos clorados,
se pueden metabolizar a través de óxidos de areno intermedios y
metabolitos de metilsulfonilo.
1.15 Efectos en el ser humano
La evaluación toxicológica de los BPCs presenta muchos problemas. Los
BPCs normalmente se encuentran como mezclas de numerosos compuestos
distintos, y muchos de los datos sobre su toxicidad se basan en las
pruebas de estas mezclas. Algunos de los componentes de la mezcla se
degradan más fácilmente que otros en el medio ambiente. Así, la
población general puede estar expuesta a mezclas que son diferentes de
las que soportan las personas que trabajan con BPCs.
La población general está expuesta a BPCs fundamentalmente a través de
alimentos contaminados (organismos acuáticos, carne y productos
lácteos). La ingesta diaria de BPCs en la mayoría de los países
industrializados es del orden de unos microgramos por persona. Tales
exposiciones no se han asociado con enfermedades. Los lactantes están
expuestos a través de la leche materna. La ingesta diaria de BPCs puede
ser de unos microgramos/kg de peso corporal.
Es muy difícil evaluar por separado los efectos para la salud humana de
los BPCs, DFPCs o DDPCs, puesto que con mucha frecuencia las mezclas de
BPCs contienen DFPCs. Ocasionalmente se ha detectado también la
presencia de DDPCs en accidentes con ciertas mezclas. Se ha demostrado
que los BPCs comerciales están contaminados con DFPCs y, por
consiguiente, en muchos casos no está claro qué efectos son atribuibles
a los BPCs y cuáles a los DFPCs, mucho más tóxicos. Así pues, muchos de
los datos procedentes de casos importantes de intoxicaciones en el ser
humano, por ejemplo las de Yusho, Yu-Cheng y otras, probablemente
reflejan los efectos de la exposición tanto a los DFPCs como a los BPCs.
Los síntomas de la intoxicación en los pacientes de Yusho y de Yu-Cheng
fueron hipersecreción de las glándulas meibomianas de los ojos,
inflamación de los párpados y pigmentación de las uñas y de las
membranas mucosas, ocasionalmente acompañados de cansancio, náuseas y
vómitos. Estos efectos normalmente iban seguidos de hiperqueratosis y
oscurecimiento de la piel, con agrandamiento folicular y erupción
acneiforme. Además, se observaron edemas en brazos y piernas, aumento
del tamaño del hígado y trastornos hepáticos, alteraciones del sistema
nervioso central, problemas respiratorios, por ejemplo alteraciones del
tipo de la bronquitis, y cambios en el estado inmunitario de los
pacientes. En los hijos de pacientes de Yusho y Yu-Cheng se detectó
disminución del crecimiento, pigmentación oscura de la piel y las
membranas mucosas, hiperplasia gingival, edema xeroftálmico ocular,
dentición al nacer, calcificación anormal del cráneo, curva del talón
más baja y una alta frecuencia de escasez de peso al nacer. No se pudo
concluir de manera definitiva si existía o no correlación entre la
exposición y la formación de neoplasmas malignos en esos pacientes,
porque el número de muertes fue demasiado pequeño. Sin embargo, en
pacientes varones se observó un aumento estadísticamente significativo
de la mortalidad producida por todos los neoplasmas, el cáncer de hígado
y el de pulmón.
En condiciones profesionales, tras unas horas de exposición aguda se
produjo una erupción cutánea. Además, después de una exposición a altas
concentraciones de BPC se observó prurito, escozor, irritación
conjuntival, pigmentación de dedos y uñas y cloracné. La cloracné es uno
de los resultados predominantes entre los trabajadores expuestos a BPCs.
Además de estos signos cutáneos de intoxicación, diferentes autores han
encontrado trastornos hepáticos, cambios en la inmunosupresión,
irritación transitoria de las membranas mucosas del tracto respiratorio
y efectos neurológicos y psicológicos o psicosomáticos inespecíficos,
como dolor de cabeza, mareos, depresión, trastornos del sueño y de la
memoria, nerviosismo, cansancio e impotencia. La conclusión general es
que la exposición profesional constante a altas concentraciones de BPCs
y DFPCs puede tener consecuencias en el hígado y la piel.
Se han llevado a cabo dos amplios estudios de mortalidad en cohortes de
trabajadores. Tras la exposición a Aroclor 1254, 1242 y 1016, en un
estudio se observó un aumento de la mortalidad por cáncer de hígado y de
vesícula biliar, y en el otro por neoplasmas y cáncer del tracto
gastrointestinal. Ninguno de los estudios epidemiológicos disponibles
aporta pruebas concluyentes de una asociación entre la exposición a BPCs
y el aumento de la mortalidad por cáncer, debido al pequeño número de
muertes en las poblaciones expuestas, la falta de relación
dosis-respuesta y el problema de los contaminantes en las mezclas de
BPCs.
2. Conclusiones
2.1 Distribución
Debido a sus propiedades físicas y químicas, los BPCs se han dispersado
en el medio ambiente de todo el mundo.
Los BPCs están casi universalmente presentes en los organismos del medio
ambiente y se bioacumulan fácilmente. También se ha demostrado una
bioamplificación en las cadenas alimentarias.
Se acumulan preferentemente los compuestos más clorados.
2.2 Efectos en animales de experimentación
Los resultados de los estudios en animales indican que los BPCs tienen
una actividad inmunosupresora, evaluada por alteraciones importantes de
la función inmunitaria (peso del bazo, peso del timo y recuento de
linfocitos). En monos, se han estimado unos NOELs de 100 µg/kg para el
Aroclor 1248 y < 100 g/kg de peso corporal para el Aroclor 1254. La
inmunosupresión parece ser un efecto específico de cada compuesto.
En general, sólo se observa toxicidad en la reproducción con dosis que
producen toxicidad sistémica en la madre. Los neonatos que se alimentan
de leche materna contaminada (en monos y otras especies animales
utilizadas como modelo) parecen ser particularmente sensibles a los
BPCs, y muestran una disminución del crecimiento y otros síntomas
tóxicos. El NOEL para los efectos del Aroclor 1016 en la reproducción es
de 30 µg/kg de peso corporal en monos; no se pudo establecer el NOEL
para los efectos en la reproducción del Aroclor 1248.
Los BPCs no son genotóxicos y no hay pruebas definitivas de su acción
como desencadenantes de tumores. Los BPCs sí actúan como estimulantes de
tumores. Se puede concluir que la toxicidad de las mezclas de BPCs se
pueden evaluar sólo en función de su umbral.
2.3 Efectos en el ser humano
La exposición de la población general a los BPCs se produce sobre todo
por los artículos alimenticios. Los lactantes están expuestos a través
de la leche materna.
Se han registrado dos importantes casos de intoxicación humana en el
Japón (Yusho) y en la provincia de Taiwán (Yu-Cheng). Los principales
síntomas de los pacientes de Yusho y Yu-Cheng se han atribuido con
frecuencia a contaminantes de las mezclas de BPCs, en particular a los
DFPCs. Sin embargo, los causantes de algunos de los síntomas,
principalmente los efectos respiratorios crónicos, pueden haber sido los
metabolitos de metilsulfona de algunos compuestos del grupo de los BPCs.
2.4 Efectos en el medio ambiente
Aunque se han notificado efectos en poblaciones locales de aves, el
efecto más importante de los BPCs en organismos del medio ambiente ha
sido sobre la insuficiencia reproductora de los mamíferos marinos. Este
efecto se ha observado principalmente en mares semicerrados, y se ha
traducido en la reducción de las poblaciones locales. El pronóstico de
que los residuos de BPCs en el medio ambiente se redistribuirán
gradualmente hacia el entorno marino indica que hay un peligro creciente
en el futuro para los mamíferos marinos.
3. Recomendaciones
* Se recomienda un acuerdo internacional sobre los procedimientos
analíticos, para mejorar la comparabilidad de los resultados de los
programas de vigilancia. Se debe continuar perfeccionando la
metodología del análisis de los distintos compuestos, aunque se
reconoce el valor de los análisis de mezclas.
* Para asegurar que los datos analíticos sean fidedignos, se
recomiendan firmemente estudios de control de calidad entre
laboratorios. Se recomienda asimismo el establecimiento de una red
internacional de asistencia y supervisión técnica, para permitir la
participación de los países en desarrollo en la vigilancia.
* Se recomiendan estudios de larga duración utilizando distintos
compuestos, y estudios sobre el mecanismo de acción de los
componentes de las mezclas de BPCs, prestando particular atención
al estímulo de los tumores, a fin de mejorar la precisión de la
evaluación del riesgo de los BPCs.
* Son necesarios estudios epidemiológicos que permitan evaluar mejor
los riesgos para los neonatos, dado que los recién nacidos parecen
ser el sector más vulnerable de la población general, debido a su
elevada exposición a través de la leche.
* Se deben poner a punto biomarcadores sensibles y específicos para
algunos de los tipos más sutiles de toxicidad de los BPCs (como la
toxicidad sobre los sistemas reproductor, inmunitario y nervioso),
a fin de utilizarlos en futuros estudios epidemiológicos.
* La eliminación de los BPCs se debería llevar a cabo mediante
incineración en instalaciones con un diseño y un funcionamiento
apropiados que puedan garantizar la temperatura alta constante
(superior a 1000°C), el tiempo de permanencia y la turbulencia que
se necesitan para asegurar su completa descomposición.
* Hay que investigar sistemas de eliminación de los BPCs que se
encuentran ya en vertederos.
* Se ha de promover una vigilancia mundial de los BPCs en el medio
ambiente y en la fauna y flora silvestres, para seguir de cerca la
redistribución prevista de los residuos ya existentes.
* Los mamíferos marinos son susceptibles a una insuficiencia
reproductora a causa de la contaminación con BPCs. Se deben
promover estudios sobre el tamaño de las poblaciones y la eficacia
reproductora de los cetáceos, además de otros estudios para
identificar los compuestos causantes de estos efectos.
 The chemical formula can be presented as C12H10-nCln, where n, the
number of chlorine atoms in the molecule, can range from 1 to 10.
2.1.2 Relative molecular mass
The relative molecular mass depends on the degree of substitution.
Monochlorobiphenyl has a relative molecular mass of 188, while
completely chlorinated biphenyl (C12Cl10) has a relative molecular
mass of 494 (US EPA, 1980).
2.1.3 Common name
Common name: polychlorinated biphenyls (PCBs)
CAS Registry number:
The chemical formula can be presented as C12H10-nCln, where n, the
number of chlorine atoms in the molecule, can range from 1 to 10.
2.1.2 Relative molecular mass
The relative molecular mass depends on the degree of substitution.
Monochlorobiphenyl has a relative molecular mass of 188, while
completely chlorinated biphenyl (C12Cl10) has a relative molecular
mass of 494 (US EPA, 1980).
2.1.3 Common name
Common name: polychlorinated biphenyls (PCBs)
CAS Registry number:  The maximum yield of PCDFs was about 10%, calculated on the amount of
PCBs decomposed, and the optimal temperature was between 550 and
650/700°C (Bentley, 1983). Thus, the uncontrolled burning of PCBs can
be an important occupational and environmental source of toxic and
hazardous PCDFs and it is recommended that all destruction of
PCB-contaminated waste should be carefully controlled, especially with
regard to the burning temperature (above 1000°C), residence time, and
turbulence (Bentley, 1983; WHO/EURO, 1987).
In the temperature range 300-400°C, Morita et al. (1978) reported that
the yield of conversion seemed to be in the mg/kg range. However,
Nagayama et al. (1981) reported a dramatic increase in the levels of
PCDFs at these rather low temperatures, in the presence of stainless
steel or nickel.
No, or very low levels of, PCDDs have been reported from the pyrolysis
of PCBs. However, pyrolysis of a mixture of PCBs and chlorobenzenes
(product Askarel) can yield both PCDFs and PCDDs (Buser, 1979).
Rappe et al. (1985b) found that various types of industrial
incinerators, such as copper smelters and steel mills generate PCDFs
and PCDDs. Pyrolysis of chlorinated polymers like polyvinylchloride
(PVC) and Saran also generate these compounds and exhaust gases of
motor cars and their motor oil may contain PCDDs and PCDFs (WHO/EURO,
1987).
In a State Office Building in the centre of Binghamton, New York, a
fire, in conjunction with several explosions, occurred in the basement
mechanical room, in 1981. Approximately 750 litres of Askarel, a
dielectric fluid composed of 65% PCBs (Aroclor 1254) and 35%
polychlorinated benzenes, leaked from a transformer and caught fire.
Pyrolysis of the Askarel led to the formation of a fine oily soot
that spread throughout the building via 2 ventilation shafts. Samples
taken several days after the fire showed average concentrations of
PCBs in the air of the building of 1.5 µg/m3. The average result for
surfaces ranged from 4.6 to 162.2 µg/m2. TCDFs and PCDDs were also
present. The soot samples were analysed for pyrolysis products. They
contained average levels of 3 mg TCDD/kg and 199 mg 2,3,7,8-TCDF/kg
(Fitzgerald et al., 1989). Achilles (1983) reported the following
levels in the deposited smut; 2160 mg PCDFs/kg and 20 mg PCDDs/kg
(including 0.6 mg 2,3,7,8-TCDD/kg).
In the soot from the Binghamton, Reims, and Stockholm accidents, high
levels of polychlorinated biphenylenes (PCBPs) were identified as well
as the PCDFs (Fig. 2) (Rappe et al., 1982, 1985).
Between 1981 and 1985, a number of accidents in electrical equipment
were reported from different countries; 28 accidents were mentioned in
WHO/EURO (1987) including actual capacitor explosions, capacitor
fires, and transformer accidents. In all eases, the accident site was
contaminated by PCDFs, average levels of total PCDFs being in the
range of 1-5 µg/m2.
The maximum yield of PCDFs was about 10%, calculated on the amount of
PCBs decomposed, and the optimal temperature was between 550 and
650/700°C (Bentley, 1983). Thus, the uncontrolled burning of PCBs can
be an important occupational and environmental source of toxic and
hazardous PCDFs and it is recommended that all destruction of
PCB-contaminated waste should be carefully controlled, especially with
regard to the burning temperature (above 1000°C), residence time, and
turbulence (Bentley, 1983; WHO/EURO, 1987).
In the temperature range 300-400°C, Morita et al. (1978) reported that
the yield of conversion seemed to be in the mg/kg range. However,
Nagayama et al. (1981) reported a dramatic increase in the levels of
PCDFs at these rather low temperatures, in the presence of stainless
steel or nickel.
No, or very low levels of, PCDDs have been reported from the pyrolysis
of PCBs. However, pyrolysis of a mixture of PCBs and chlorobenzenes
(product Askarel) can yield both PCDFs and PCDDs (Buser, 1979).
Rappe et al. (1985b) found that various types of industrial
incinerators, such as copper smelters and steel mills generate PCDFs
and PCDDs. Pyrolysis of chlorinated polymers like polyvinylchloride
(PVC) and Saran also generate these compounds and exhaust gases of
motor cars and their motor oil may contain PCDDs and PCDFs (WHO/EURO,
1987).
In a State Office Building in the centre of Binghamton, New York, a
fire, in conjunction with several explosions, occurred in the basement
mechanical room, in 1981. Approximately 750 litres of Askarel, a
dielectric fluid composed of 65% PCBs (Aroclor 1254) and 35%
polychlorinated benzenes, leaked from a transformer and caught fire.
Pyrolysis of the Askarel led to the formation of a fine oily soot
that spread throughout the building via 2 ventilation shafts. Samples
taken several days after the fire showed average concentrations of
PCBs in the air of the building of 1.5 µg/m3. The average result for
surfaces ranged from 4.6 to 162.2 µg/m2. TCDFs and PCDDs were also
present. The soot samples were analysed for pyrolysis products. They
contained average levels of 3 mg TCDD/kg and 199 mg 2,3,7,8-TCDF/kg
(Fitzgerald et al., 1989). Achilles (1983) reported the following
levels in the deposited smut; 2160 mg PCDFs/kg and 20 mg PCDDs/kg
(including 0.6 mg 2,3,7,8-TCDD/kg).
In the soot from the Binghamton, Reims, and Stockholm accidents, high
levels of polychlorinated biphenylenes (PCBPs) were identified as well
as the PCDFs (Fig. 2) (Rappe et al., 1982, 1985).
Between 1981 and 1985, a number of accidents in electrical equipment
were reported from different countries; 28 accidents were mentioned in
WHO/EURO (1987) including actual capacitor explosions, capacitor
fires, and transformer accidents. In all eases, the accident site was
contaminated by PCDFs, average levels of total PCDFs being in the
range of 1-5 µg/m2.
 Hutzinger et al. (1985) also mentioned the presence of polychlorinated
pyrenes (PCPYs).
In the period 1977-85, particulates and flue gas from municipal
incinerators and hazardous waste incinerators in Canada, Denmark,
Netherlands, Sweden, and Switzerland were investigated. It was found
that emissions from incinerators contained many different PCDF and
PCDD isomers. The total levels ranged from ng/m3 to µg/m3. Fly-ash
contained levels of 0.1-0.6 mg/kg (Buser & Bosshardt, 1978; Rappe et
al., 1985c; WHO/EURO, 1987).
Rappe et al. (1985b) studied the emissions of the municipal solid
waste incinerator in Umea, Sweden. The levels of PCDDs and PCDFs
varied under different burning conditions. The amount of dioxins
formed seems to be dependent on the chlorine content in the waste, as
well as the construction of the incinerator. The critical parameters
seem to be temperature, residence time, turbulence, and excess air
(oxygen).
The 2,3,7,8-tetra-CDD was always found to be a very minor constituent,
whereas the 1,2,3,7,8-penta-CDD in all samples gave a medium-sized
peak. The 2,3,7,8-substituted PCDFs were always middle or major
components (WHO/EURO, 1987).
The fact that PCBs may be thermally converted to PCDFs has raised
concern that similar conversions might occur in electrical equipment,
such as capacitors and transformers, in which the dielectric fluids
used are subjected to modest temperature rises accompanied by
electrical stress. Brown et al. (1988) investigated the presence of
PCDFs in both used and unused capacitors and transformers and did not
find any evidence of an increase in PCDFs levels in the heavily used
capacitor or the transformer PCBs compared with levels in unused
samples.
For a number of years, concern has been expressed regarding the
release of PCBs and other dangerous compounds when fluorescent light
ballasts "burn out". The breakdown products may contain vapours and
condensed particles of PCBs and asphalt. In response to concern at a
school, the US EPA met with officials of Blaine Elementary School,
because of material leaking from some fluorescent light fixtures. It
was determined that the leaking material ("oil") contained PCBs
(Aroclor 1242 or 1260). Air samples collected following the burn out
of such lights, at different distances from the light fixture, gave
concentrations of 0.166 and 0.012 mg/m3, respectively, 1 and 6 m from
the light. Three days later, levels of 0.004-0.001 mg/m3 were still
found. In a second series of tests, both burn-out and non-burn-out
ballasts were heated to 150°C, 300°C, and 400°C, in a chamber. No PCBs
were detected at 150°C. At 300°C, concentrations ranged from 0.55 to
1.70 mg/m3 and, at 400°C, 2.54 to 28.2 mg/m3. Wipe samples were
taken in schoolrooms after burn-outs; average concentrations of
Aroclor of 0.34 and 1.22 µg/cm2 were found. It is obvious that PCBs
and asphalt contamination, both surface and atmospheric, can occur
when fluorescent lamp ballasts burn out.
The most serious potential contamination results when thermal runaway
takes place. Thermal runaway volatilizes the asphalt potting compound
and may rupture the capacitor. When the potting compound and the PCBs
are exposed to high temperatures, some of both materials vapourizes.
As the vapours pass through the atmosphere they condense into freely
divided aerosols, less than 1 µm in diameter. Much of the visible
fumes results from volatilization of the asphalt (Anon., 1987).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
A more detailed review of transport mechanisms can be found in Jury et
al. (1987).
4.1.1 Transport in air
The virtually universal distribution of PCBs throughout the world,
including the arctic and other remote areas, suggests that PCBs are
transported in air (Risebrough & de Lappe, 1972). The ability of PCBs
to co-distill, volatilize from landfills into the atmosphere
(adsorption to aerosols with particle size of less than 0.05-20 µm),
and resist degradation at low incinerating temperatures, makes
atmospheric transport the primary mode of global distribution within
the troposphere and stratosphere (Nisbet & Sarofim, 1972; Eisenreich
et al., 1981). PCBs have been measured in air samples at Eniwetok
Atoll in the North Pacific Ocean (Atlas & Giam, 1981), over the North
Atlantic (Giam et al., 1978), and in the Gulf of Mexico (Giam et al.,
1978, 1980). Murphy et al. (1985) estimated that approximately
18 000 kg of PCBs are present in the atmosphere over the USA, at any
given time. The authors also estimated that, if these PCBs had an
atmospheric residence time of one week, then about 900 000 kg/year of
PCBs cycle through the atmosphere of the USA.
Nisbet & Sarofim (1972) suggested that most of the airborne PCBs will
be adsorbed on any particles present. The half-life of particles in
the air will depend greatly on the size of the particles and the
extent of atmospheric precipitation. Most will be deposited within 2-3
days in their areas of origin (usually urban), the small amount
attached to fine particles will last in the atmosphere for longer
periods and can be transported to more remote regions.
Södergren (1972) collected airborne fallout in southern Sweden and
found regional differences in PCB levels, with mean monthly levels
ranging from 620 ng/m2 per month to 10 510 ng/m2 per month. The
lowest level was in a remote forest area. Industrialized areas had
high levels but so too did some agricultural regions. Higher levels
were generally found in the western part of the study region,
suggesting that some PCB fallout may have originated from further
afield and be dependent on the prevailing winds. Seasonal variations
in fallout correlated well with precipitation. Lower levels of PCB
precipitation were found in Iceland by Bengtson & Södergren (1974).
The highest level was found in Northern Iceland at 1050 ng/m2 per
month and, like other sites sampled, showed a seasonal trend with
highest levels in the summer.
Harvey & Steinhauer (1974) measured PCBs in the atmosphere over the
western North Atlantic. They found that concentrations decreased
exponentially with distance from land and concluded that wind
transport is the major method of transport over the oceans. They also
suggested that PCBs are transported primarily in the vapour phase.
4.1.1.1 Dry deposition
Atmospheric input into the Great Lakes has been studied extensively,
because the lakes, as a whole, represent the largest surface area of
any freshwater body in the world, with the lake surface area
comprising from 27% (Ontario) to 64% (Superior) of the total basin
area, and ranging from 19 000 km2 (Ontario) to 82 100 km2
(Superior). Eisenreich et al. (1981) estimated that more than 80% of
the annual mean total input of PCBs in Lake Michigan originated from
the atmosphere. They estimated that approximately 56% of the
9000 kg/year of PCB input in Lake Michigan was in the form of wet
deposition and that 30% of the 6600-8300 kg/year input in Lake
Superior was also in this form. However, Andren (1982) calculated a
precipitation input of 650 kg/year for Lake Michigan, again assuming
that all PCBs were on 0.5 µm airborne particles. Even assuming the
lowest estimate for the annual input of PCBs into the lake,
approximately 60% of the total input might be atmospheric deposition.
Andren (1982) also measured the input of PCB into an isolated lake
(Crystal Lake, Wisconsin), to calibrate a dry deposition model. The
model was then applied to Lake Michigan and the author concluded that,
assuming all particulate inputs of PCB are associated with 0.5 mm
particles, dry deposition inputs were significantly less than wet
inputs.
Manchester-Neesvig & Andren (1989) collected and analysed air samples
from a remote site in the Great Lakes watershed during 1984 and 1985.
Total PCB concentrations varied from 1.82 ng/m3 in the summer to
0.135 ng/m3 in the winter. They found that, on average, 92% of the
PCBs detected were in the vapour phase. When these data were compared
with data collected over the previous 7 years, no significant changes
in PCB concentrations were found. The authors concluded that, on the
basis of the short residence time and the relatively constant annual
average levels of PCBs, repeated cycling between earth and atmosphere
takes place.
Murphy (1984) reviewing data from the Great Lakes region on the
relative distribution of airborne PCBs between particulate matter and
vapour, concluded that they are transported predominantly in vapour.
He stated that there was reasonable evidence to suggest that the
atmosphere is the major source of the PCBs found in Lakes Michigan,
Superior, and Huron, Siskiwit Lake on Isle Royale, and probably in the
upper Great Lakes too.
Using liquid-coated collecting plates in near-shore areas of Lakes
Huron and Michigan, close to urban centres, more PCBs were found on
the upper plates suggesting that much of the dry deposit of PCBs was
associated with large particles (20 µm). This sampling technique also
indicated that, for the areas studied, dry deposition inputs were
higher than wet inputs (Murphy, 1984).
Duinker & Bouchertall (1989) analysed filtered air, particulates, and
rain, in the city of Kiel, Federal Republic of Germany for 14
different PCB congeners. They found that congeners with a low degree
of chlorination were dominant in filtered air, whereas, congeners with
a high degree of chlorination dominated in aerosols and rainfall. The
vapour phase represented up to 99% of the more volatile congeners
(i.e., those with a lower degree of chlorination). The particulates
were found to carry relatively more of the less volatile congeners.
Particle scavenging was the dominant source of PCBs in rain water
despite the small contribution of particulate PCBs to the overall
atmospheric concentration of PCBs (only 1 or 2%).
In a study by Södergren (1973), most of the PCB deposited on a south
Swedish lake was in the form of dry deposit, with 11% as particulate
matter in the precipitation and 2% from precipitation water. McClure
(1976) stated that, on the basis of flux measurements and model
calculations, most of the PCB fallout is in the form of dry deposition
and that most of the dry deposition of aerosol PCB introduced into the
troposphere falls within 100 km of its source.
4.1.1.2 Precipitation deposition
Precipitation scavenging of chlorinated hydrocarbons in the atmosphere
is complex. Scavenging of particles by cloud droplets and by rain
drops in, and below, clouds, and the scavenging of the vapour phase by
rain occurs (Murphy 1984). Thus chlorinated hydrocarbons are
concentrated in precipitation rather than in the atmosphere, resulting
in rainfall levels of many ng/litre. Swain (1978) and Strachan &
Huneault (1979) measured levels in rainfall ranging between 0 (not
detectable) and 230 ng/litre in the Great lakes area.
Murphy (1984) pointed out that variables, such as the amount of
particulate material and PCBs in the atmosphere, the type of rain, and
the rate of rainfall, will affect the precision of precipitation
estimates.
Levels of PCBs in the rainfall throughout Canada during 1984 were
monitored by Strachan (1988). Levels ranged from nd to 17 ng/litre, no
geographical trends were apparent.
4.1.2 Transport in soil
PCBs in soil, derive from particulate deposition (often concentrated
in urban areas), wet deposition, the use of sewage sludge as a
fertilizer, and leaching from landfill sites.
Significant amounts of PCBs are deposited on soil by particulate
deposition (see previous section). Fujiwara (1975) analysed soil
samples in Japan, and found that the main sources of PCB contamination
of agricultural soils are the industries using PCBs. Other sources
include treatment of soil with sewage sludge and accidental spills.
The 15% of soil samples in Indiana (USA) that contained more than
50 mg/kg had been treated with PCB-contaminated dried sludge (Bergh &
People, 1977).
Tucker et al. (1975a) found that, during a 4-month period following
the addition of Aroclor 1016 to soil, the PCBs were not readily
leached by percolating water and that only the lower chlorinated
isomers were leached. The ease of leaching from different soils was in
the order sandy loam > silty loam > silty clay loam.
The behaviour of 14C-labelled PCB in flooded soils was studied by
Ogiso et al. (1976). The amounts of PCB volatilized occurred in the
following order: water > subsoil > soil. The addition of compost
powder to soil reduced the amount that volatilized.
Haque et al. (1974) studied the adsorption of Aroclor 1254 on various
soil particle types in an aqueous solution of 56 µg PCB/litre.
Delmonte sand and silica gel did not adsorb any PCB. Woodburn soil
adsorbed the highest amount followed by illite, montmorillonite, and
kaolinite clays, in decreasing order. The high adsorptive capacity of
Woodburn soil was attributed to the presence of organic matter and
lipophilic or hydrophobic materials. Moza et al. (1976a) found that, 2
years after the application of 14C-labelled dichlorobiphenyl to a
loamy sand soil at 1 mg/kg, most of the detectable PCB was in the top
10 cm of the soil and only 0.2% had reached a depth of 40 cm. In
another study, Suzuki et al. (1977) found that Aroclors 1242 and 1254
did not move upwards through uncontaminated sand deposited over
contaminated soil. The leaching of water from soil may lead to a
downward movement of PCBs, depending on the soil type and clay content
(Pal et al., 1980).
A large spill of Askarel (containing 70% Aroclor 1254 and 30% tri-
and tetrachlorobenzenes) occurred at a transformer-manufacturing
facility in Canada, in 1976. Condie silt from near the site of the
spill was studied with respect to the sorption partition coefficients
and the transport retardation factors. The sorption partition
coefficient values for 2,5,2',5'-tetrachloro-, 2,4,5,2',5'-penta-
chloro-, and 2,4,5,2',4',5'-hexachlorobiphenyl were 5000, 9400, and
26 000, respectively. The mean transport retardation factors for these
3 congeners were 2.7 E + 04, 5.0 E + 04, and 1.4 E + 05, respectively.
This implies that dissolved PCBs will move only very slowly through
unfractured Condie silt (Anderson & Pankow, 1986).
4.1.3 Transport in water
PCBs enter water mainly from discharge points of industrial and urban
wastes into rivers, lakes, and coastal waters. In static water, PCBs
are more concentrated in the surface micro-layer than in subsurface
samples (Bidleman & Olney, 1974). This is probably due to deposition
from the air rather than redistribution in the water. On account of
their low water solubility and high specific activity, it is expected
that most of the PCBs discharged will be adsorbed by sediment at the
bottom of rivers or lakes and transport will be mainly via waterborne
particles (Nisbet & Sarofim, 1972). The bulk of the PCBs will sink to
the bottom sediments. The sinking rate of PCBs from the surface to
deeper layers in the open ocean is relatively slower in tropical
waters than in high-latitude waters (Tanabe, 1985).
Oloffs et al. (1973) added 0.1 mg Aroclor 1260/litre to water samples
in the presence of sediment. After 6 weeks, all of the PCBs had been
adsorbed by the sediment, none being given off to the atmosphere. The
degree of PCBs sorption is inversely related to the size of the
particles (Haque et al., 1974) and the solubility of PCBs in water
(Haque & Schmedding, 1975). Smaller particles have a relatively larger
surface area and so adsorb more PCBs (Steen et al., 1978). Nau-Ritter
et al. (1982) found the adsorption and retention of PCBs to be
directly related to the particle organic content. A significant
correlation was found by Larsen et al. (1985) between PCB levels and
total organic carbon in the deepwater sediments of the Gulf of Maine,
PCBs were concentrated on finer grain particles. Organic carbon and,
therefore, the PCB concentration were also correlated with depth.
Wildish et al. (1980) found that estuarine sediments, especially those
containing higher levels of organic matter, readily adsorbed Aroclor
1254. The PCBs were found to be tightly bound to the sediment with
virtually no desorption. Horzempa & Di Toro (1983) found that the
adsorption of hexachlorobiphenyl was correlated with both sediment
surface area and organic content. Adsorption was found to be
significantly greater at 40°C than at 1°C. Hexachlorobiphenyl is
strongly adsorbed on sediment and weakly desorbed. There is no simple
reversible reaction.
Fisher et al. (1983) found that the rate of release of PCBs from
contaminated sediment was a function of sediment PCB concentration,
chlorine substitution pattern, and degree of chlorination. In the
absence of disturbance, even very low deposition rates of new sediment
will quickly remove PCB-contaminated sediments from diffusional
communication with overlying water. Little change was found (Nimmo et
al., 1971a) in the PCB concentration in sediment at a point downstream
of a contamination source over a period of 9 months. The very small
amounts of PCBs leached from sediment into overlying water may be
taken up by organisms.
Hom et al. (1974) stated that the annual inputs of PCBs into the
southern California bight from waste water and from surface runoff in
1970-71 were estimated to be 10 and 0.25 tonnes, respectively.
Sewage treatment appears to remove PCBs from waste water,
concentrating them in the sludge. However, often, the sludge is then
discharged into open water (Ahling & Jensen, 1970). Holden (1970)
found an average of 3 mg PCBs/kg in wet sewage sludge dumped in the
Clyde estuary, in the United Kingdom, and calculated that this would
be equivalent to approximately one tonne per year. A similar annual
discharge of PCBs in the sludge on the Californian coast was
calculated by Schmidt et al., (1971).
Dredging of inland rivers and harbours may lead to a significant
transfer of PCBs from contaminated sediments, especially when dumped
at sea (Nisbet & Sarofim, 1972). Rice & White (1987) found that there
was an increase in water concentrations of PCBs immediately following
the dredging of sediment in the Shiawassee River, Michigan. The
availability of PCBs for clams and fish, as measured by an increase in
uptake, was found for up to 6 months following dredging.
4.1.4 Transport between media
In a model ecosystem, Södergren & Larsson (1982) found that the
presence of bottom-living organisms, such as Chironomus and
Tubifex, resulted not only in the uptake of PCBs from the sediment
but also in the release of PCBs into the water and to the surface
microlayer, compared with a system without organisms. PCBs were
transported to the air via jet drops from bursting bubbles in the
surface microlayer.
A similar pattern was found using large outdoor artificial ponds
(Larsson, 1985a). Following the addition of Clophen A50 to sediment,
the transport of PCBs from sediment to water followed a seasonal
cycle, with higher levels in the summer than in the winter. The
processes that transfer PCBs across the sediment/water interface
(bioturbation, desorption, and gas convection) are positively related
to temperature. Transfer from water to air was probably dominated by
volatilization with maximum concentrations of PCBs in air at the
highest water concentrations, lower chlorinated biphenyls achieving
the highest concentrations in air. The majority of the airborne phase
was presumed to be in the gaseous phase as it passed through particle
filters. In the same ponds, Larsson & Okla (1987) measured the rate at
which PCBs volatilized from water to air. PCB compounds volatilized at
a rate of 0.9 to 9.6 ng/m2 per h, the rate increasing with the
temperature of the water and the concentration of PCBs. The transport
rate during the day exceeded the rate at night and was positively
correlated with the air temperature (Okla & Larsson, 1987).
Larsson (1985b) added Clophen A50 to the sediment in a model ecosystem
comprising sediment, water, benthic macroinvertebrates, and fish. PCBs
were detected in the water. The transport of PCBs from the water to
air included at least 2 routes, volatilization and jet drop transport.
Both routes were of the same magnitude (0.2-1.0 µg/week). However,
though the PCBs transported by volatilization consisted of lower
chlorinated isomers, those transported by jet drops were identical to
those in the sediment and water.
In an earlier study, Larsson (1984) measured the uptake of PCBs from
sediment by chironomid midge larvae and the concentrations of PCBs
from larva to adult. In the field, chironomid larvae contained
114 µg/kg fresh weight at a sediment concentration of 39 µg/kg wet
weight. Different sediments affected the amount of PCBs available to
the organisms. Adult chironomids sampled near a sewage plant contained
251 µg/kg fresh weight. The chironomid larval population was estimated
to be 9900 per m2 and the authors calculated that these would move
20 µg PCB/m2 per year into the terrestrial compartment of the
environment.
A model, based on the fugacity concept, was described and illustrated
by applying it to the time-varying fate of PCBs in Lake Ontario over
the period 1940-2000. Expressions are included for a great number of
variables, such as loadings and the partitioning of the contaminant
between the phases of air, aerosols, water, suspended and bottom
sediments, various trophic levels of aquatic organisms, and gull eggs.
Also included are expressions for transformation rates, and transport
rates for diffusion between water and sediment, and water and air wet
and dry atmospheric deposition, sediment deposition, burial, and
resuspension, and water and the inflow and outflow of suspended
matter. The results obtained by numerical integration and by assuming
reasonable loading and air concentrations were in accordance with
data. It was shown that PCBs cycle appreciably between the atmosphere
and water by wet and dry deposition and volatilization, and between
water and sediment by deposition, resuspension, and diffusion.
Biomonitors were shown to be particularly valuable indicators of
contamination levels in the ecosystem (MacKay, 1989).
4.2 Biotransformation
4.2.1 Biodegradation
Nissen (1973) did not find any alteration in Aroclor 1254 after a
9-week incubation period in soil. Iwata et al. (1973) added Aroclor
1254 to various soil types. They did not find any change after one
year in soils containing high amounts of organic matter (10.8-19.5%).
Biotransformation had occurred, causing the disappearance of the lower
chlorinated biphenyls, in soils with a low organic matter content
(0.1-3.3%), as diverse as loamy sand and clay. The authors concluded
that, after one year, the material remaining in loamy sand (0.1%
organic matter) consisted of mainly penta- and hexachlorobiphenyl
isomers.
4.2.1.1 Bacteria
The biodegradation of PCB isomers, which is possible with some aerobic
bacteria, depends on the degree of chlorination and the position of
chlorine substitution. Degradation decreases with increasing
chlorination. Dechlorination of PCBs occurs in anaerobic sediments.
Here bacterial activity is preferentially targeted towards PCB
congeners with higher levels of chlorination. Products of
dechlorination are, therefore, more readily degraded by aerobic
systems.
Early experiments were carried out to study the biodegradation of PCBs
using activated sludge inocula; some degradation was found (Baxter et
al., 1975). However, the presence of PCBs in sewage sludge shows that
they are not all readily transformed by microorganisms. Fries (1972)
analysed silage containing PCBs (Aroclor 1254) that had undergone
normal fermentation. The gas chromatogram of the standard was
identical to that of the silage sample. The authors suggested that, if
anaerobic degradation had taken place, it would have been unlikely to
have been uniform for all components. They stated, however, that this
test may not have been a good indication of possible anaerobic
degradation because DDT showed much less degradation, under the same
conditions, compared with other degradation test systems.
Lunt & Evans (1970) postulated a metabolic pathway, used by
microorganisms, for biphenyl oxidation, which was later confirmed by
the findings of Gibson et al. (1973) using a bacterium isolated from a
polluted stream. Lunt & Evans (1970) found that a Gram-negative
bacterium oxidized biphenyl to phenylpyruvic acid with the
intermediary formation of 2,3-dihydroxybiphenyl and
alpha-hydroxy-ß-phenylmuconic semialdehyde. Catelani et al. (1971)
found that the metabolism of biphenyl by Pseudomonas putida was
different, in that, though the intermediate products were the same,
benzoic acid was isolated, not phenylpyruvic acid. Ahmed & Focht
(1973a) isolated 2 species of Achromobacter from sewage effluent
using biphenyl and p-chlorobiphenyl as the sole carbon source. They
found that both sources were rapidly degraded, biphenyl being oxidized
to benzoic acid and both mono and dichlorinated biphenyls to
p-chlorobenzoic acid. In a second study, Ahmed & Focht (1973b)
investigated the biodegradation of other isomers of PCBs, with 2-5
chlorine atoms. The extent of oxidation seemed to be somewhat
dependent on the presence of unsubstituted biphenyl rings. Because of
the absence of chloride in all the supernatants, they concluded that
the bacterium was unable to dechlorinate the PCBs. The fact that
increasing chlorine substitution rendered the molecule more resistant
to microbial attack was used to support this argument. However, Kaiser
& Wong (1974), studying the degradation of Aroclor 1242 by a bacterial
culture, isolated from lake water, showed that the PCBs were degraded
into several metabolites (aliphatic and aromatic hydrocarbons), none
of which contained chlorine. Dechlorination had already taken place at
an early stage of metabolism.
Wong & Kaiser (1975) found that lake water bacteria could use both
Aroclor 1221 and 1242, but not 1254, as a sole carbon source for
growth, but that only 1% of the bacterial culture had this ability.
The authors then followed the degradation of Aroclor 1221. After one
month, the mixture had been totally degraded to several compounds of
low relative molecular mass. Unchlorinated biphenyls were degraded
faster than chlorinated forms.
Tucker et al. (1975b) observed the degradation rates of Aroclors 1221,
1016, 1242, and 1254, and MCS 1043 (a non-commercial mixture). They
found a clear relationship between the level of chlorination and the
relative degradability, when degradation rate was plotted against
percentage chlorine by weight. Volatilization rates fell within the
95% confidence limits of overall disappearance rates and so could be
ruled out. Analysis of the Aroclors, following exposure to the
activated sludge, revealed a redistribution of the dominant PCBs. For
example, the chromatograms for Aroclor 1221 and 1242 were very similar
showing that the lower chlorinated biphenyls were more rapidly
degraded. Furthermore, since Aroclor 1221 was found to be rapidly
degraded, a closer study was performed that showed that most of the
degradation occurred within 24 h.
The degradation of polychlorinated biphenyls by either Nocardia spp.
or Pseudomonas spp. was studied by Baxter et al. (1975). They found
that, under experimental conditions, many of the lower chlorinated
biphenyls (<3 chlorine atoms/molecule) were degraded very readily
and some biphenyls containing as many as 6 chlorine atoms could be
degraded, if the conditions were suitable. When PCB mixtures Aroclor
1016 and 1242 were used, a different pattern of degradation was
observed with an enhanced ability of the microorganisms to degrade.
For example, 4,4'-dichlorobiphenyl degraded to 50% in about 2 days,
when presented to Nocardia spp. as a component of Aroclor 1242, but
it was virtually unaffected after 12 days exposure as the pure isomer.
The authors suggested that mutual solubilization might play some part.
Sayler et al. (1977) found that an estuarine Pseudomonas sp. was
able to degrade both mixtures of PCBs (Aroclor 1254) and pure isomers
of hexachlorobiphenyl. Degradation was dependent on incubation time
and the purity and degree of chlorination of the biphenyl. Appreciable
degradation occurred at all substrate concentrations of the Aroclor
(10, 100, and 1000 µg/litre) within 22 days. Although, over this
22-day period, only 9% had been degraded at the lowest concentration
compared with 30-40% for the other concentrations, after 60 days, this
was reversed with 84% being degraded at 10 µg/litre, 70% at
100 µg/litre, and 63% at 1000 µg/litre. When compared with the pure
isomer, degradation of the Aroclor mixture proceeded at a slower rate.
Even though average chlorination was less, the authors speculated that
this could be owing to the substitution positions of the chlorines.
Chromatographic tracings showed that degradation of the lower
chlorinated components of the Aroclor occurs before degradation of the
more highly chlorinated biphenyls.
Furukawa et al. (1978a,b) examined 31 PCB isomers (mono to
pentachlorobiphenyl) for biodegradability by 2 bacterial species,
Alcaligenes and Acinetobacter. They found the following
relationship between chlorine substitution and biodegradability.
i. Degradation decreased as chlorine substitution increased.
ii. Isomers containing two chlorines at the ortho position of
either a single ring or on both rings showed very poor
degradability.
iii. Isomers, in which all the chlorines were on one ring, were
generally degraded faster.
iv. Molecules with non-chlorinated rings or rings with few chlorines
underwent preferential ring fission.
v. The 4'-chloro-substituted PCBs formed and accumulated a yellow
intermediate during degradation.
vi. Only with respect to 2,4,6-trichlorobiphenyl was there a
significant difference in ability to degrade between the 2
bacteria. This compound was mostly metabolized within 1 h by
Acinetobacter, but was degraded very slowly by Alcaligenes.
It was demonstrated by Carey & Harvey (1978) that mixed cultures of
marine bacteria were capable of metabolizing both pure isomers (tri-
and tetrachlorobiphenyl) and mixtures (Aroclor 1254). They isolated
and partially characterized an acid lactone metabolite. They did not
find any change in the chromatogram trace for the Aroclor but
suggested that this might be related to the insensitivity of the
method, since even if each of the isomers in the mixture had been
metabolized to the same extent as pure isomers, this would still not
have been detectable on the trace. The authors also found that no
metabolism occurred when a chlorobiphenyl isomer in an anaerobic
marine mud was incubated for 6 weeks. Degradation of Aroclor 1242 by
mixed microbial cultures, isolated from soil and river water samples,
was demonstrated by Clark et al. (1979). The predominant organisms in
the cultures were Alcaligenes odorans, Alcaligenes denitrificans,
and an unidentified bacterium. The lower chlorinated isomers were not
only degraded at a faster rate but were also more completely utilized
by the bacteria. In general, the rate of degradation was much faster
than in previous studies. Co-metabolism in the presence of sodium
acetate was studied; greatly enhanced degradation was found for the
more highly chlorinated isomers. Liu (1980) found that sodium
ligninsulfonate also greatly enhanced the biodegradation of commercial
PCB mixtures.
The same author found that a Pseudomonas sp. could oxidize Aroclors
1221, 1016, 1242, and 1254, at a rapid rate. A kinetic study using
resting cells revealed that Aroclor 1221 was degraded much faster
(980 µg/h per mg cell dry weight) than Aroclor 1254 (43 µg/h per mg
cell dry weight). The degradation of the higher chlorinated PCB
(Aroclor 1254) could be enhanced by the addition of Aroclor 1221. Liu
(1981) observed that the oxidation of Aroclor 1221 by the bacteria was
10 times faster than with sewage. Two possible explanations for this
difference were that the sewage contained toxic chemicals that
inhibited the bacteria, but this was found not to be the case, or, the
bacteria preferred Aroclor 1221 to the other substrates. This second
explanation is a possibility, for glucose, a substrate used readily by
most bacteria was poorly oxidized by this bacterium. Pseudomonas
oxidized Aroclor 1221 readily between 15 and 35°C, the rate increasing
with temperature. Reducing the temperature to 4 and 10°C drastically
retarded, but did not halt, degradation. Adjusting the concentrations
of phosphorus and nitrogen from 2 mg to 20 mg/litre (the lower
concentration being that found normally in sewage) did not alter the
rate of degradation by Pseudomonas spp. in raw sewage. But
increasing nitrogen and phosphorus gave more reproducible results,
suggesting that the compounds are on the border of limiting
degradation rates in raw sewage. The oxygen content was found not to
affect degradation at concentrations over 1 mg/litre (oxygen levels
are generally maintained at between 2 and 3 mg/litre in activated
sludge reactors, under the operational conditions of sewage-treatment
plants). Liu (1982) found that, under a limited substrate supply,
Pseudomonas spp. degraded all 7 of the major components of Aroclor
1221. However, with excessive amounts of nutrient, preferential
degradation of certain components was observed. The author stated that
one of the main factors influencing this selective biodegradability
was the position of chlorine substitution on the biphenyl.
4.2.2 Biodegradation; individual congeners
4.2.2.1 Bacteria
In a study by Parsons & Sijm (1988), the co-metabolism was
investigated of several different mono-, di- and tetrachlorobiphenyls
in chemostat continuous cultures of a Pseudomonas strain (JB1). They
found that chemostat conditions favoured degradation compared with
exposure of the Pseudomonos in batch culture, where little or no
degradation was recorded. Using benzoate as the carbon source, results
varied widely, with repeat incubations showing different degrees of
degradation of chlorobiphenyls and, sometimes, no breakdown at all. In
cultures that did degrade the materials, the monosubstituted
4-chlorobiphenyl was rapidly degraded. Of the disubstituted
dibiphenyls, 3,5-dichlorobiphenyl was more readily broken down than
2,5-dichlorobiphenyl. Changing the carbon source available to the
Pseudomonas sp. improved the reproducibility of the results. The
authors reviewed the literature relative to their own findings and
concluded that repeated culture on benzoate leads to the loss of the
ability of the Pseudomonas sp. to degrade biphenyl by meta
cleavage; ortho cleavage is retained. Coding for the meta cleavage
resides on plasmids, which can be lost, whereas coding for the ortho
cleavage is chromosomal. Growth of the Pseudomonas sp. on a
3-methylbenzoate substrate improved degradation of the biphenyls.
3-Methylbenzoate can only be degraded by a meta cleavage favouring
retention of the plasmid. Comparison of degradation of 4
tetrachlorobiphenyls showed the influence of the positions of the
chlorine substitutions. The relative degradability of the 5 compounds,
shown in Fig. 3, was: 2,3,2',3'-tetrachloro- >2,5,3',4'-tetrachloro-
> 2,5,2',5'-tetrachloro- approx. 2,6,2',6'-tetrachloro- approx.
3,4,3',4'-tetrachlorobiphenyl. The authors stated, from the
literature, that the first reaction in the degradation of
chlorobiphenyls is, in most cases, 2,3-dioxygenation, eventually
leading to the formation of chlorobenzoates. Chlorines in the ortho
and meta positions will, therefore, offer steric hindrance to this
reaction.
The low degradation rate of 3,4,3',4'-tetrachlorobiphenyl is not
explained by this mechanism, since it has 2 adjacent unoccupied 2,3
positions, but is more likely explained by its toxicity. Steric
influence on enzyme binding is offered as an explanation in this case.
Similarly, Furukawa et al. (1978a) did not find any degradation of
this compound in initial studies, though they did find degradation to
a dichlorobenzoic acid by Acinetobacter in a later study (Furukawa
et al., 1978b; Rogers, undated(a)).
Hutzinger et al. (1985) also mentioned the presence of polychlorinated
pyrenes (PCPYs).
In the period 1977-85, particulates and flue gas from municipal
incinerators and hazardous waste incinerators in Canada, Denmark,
Netherlands, Sweden, and Switzerland were investigated. It was found
that emissions from incinerators contained many different PCDF and
PCDD isomers. The total levels ranged from ng/m3 to µg/m3. Fly-ash
contained levels of 0.1-0.6 mg/kg (Buser & Bosshardt, 1978; Rappe et
al., 1985c; WHO/EURO, 1987).
Rappe et al. (1985b) studied the emissions of the municipal solid
waste incinerator in Umea, Sweden. The levels of PCDDs and PCDFs
varied under different burning conditions. The amount of dioxins
formed seems to be dependent on the chlorine content in the waste, as
well as the construction of the incinerator. The critical parameters
seem to be temperature, residence time, turbulence, and excess air
(oxygen).
The 2,3,7,8-tetra-CDD was always found to be a very minor constituent,
whereas the 1,2,3,7,8-penta-CDD in all samples gave a medium-sized
peak. The 2,3,7,8-substituted PCDFs were always middle or major
components (WHO/EURO, 1987).
The fact that PCBs may be thermally converted to PCDFs has raised
concern that similar conversions might occur in electrical equipment,
such as capacitors and transformers, in which the dielectric fluids
used are subjected to modest temperature rises accompanied by
electrical stress. Brown et al. (1988) investigated the presence of
PCDFs in both used and unused capacitors and transformers and did not
find any evidence of an increase in PCDFs levels in the heavily used
capacitor or the transformer PCBs compared with levels in unused
samples.
For a number of years, concern has been expressed regarding the
release of PCBs and other dangerous compounds when fluorescent light
ballasts "burn out". The breakdown products may contain vapours and
condensed particles of PCBs and asphalt. In response to concern at a
school, the US EPA met with officials of Blaine Elementary School,
because of material leaking from some fluorescent light fixtures. It
was determined that the leaking material ("oil") contained PCBs
(Aroclor 1242 or 1260). Air samples collected following the burn out
of such lights, at different distances from the light fixture, gave
concentrations of 0.166 and 0.012 mg/m3, respectively, 1 and 6 m from
the light. Three days later, levels of 0.004-0.001 mg/m3 were still
found. In a second series of tests, both burn-out and non-burn-out
ballasts were heated to 150°C, 300°C, and 400°C, in a chamber. No PCBs
were detected at 150°C. At 300°C, concentrations ranged from 0.55 to
1.70 mg/m3 and, at 400°C, 2.54 to 28.2 mg/m3. Wipe samples were
taken in schoolrooms after burn-outs; average concentrations of
Aroclor of 0.34 and 1.22 µg/cm2 were found. It is obvious that PCBs
and asphalt contamination, both surface and atmospheric, can occur
when fluorescent lamp ballasts burn out.
The most serious potential contamination results when thermal runaway
takes place. Thermal runaway volatilizes the asphalt potting compound
and may rupture the capacitor. When the potting compound and the PCBs
are exposed to high temperatures, some of both materials vapourizes.
As the vapours pass through the atmosphere they condense into freely
divided aerosols, less than 1 µm in diameter. Much of the visible
fumes results from volatilization of the asphalt (Anon., 1987).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
A more detailed review of transport mechanisms can be found in Jury et
al. (1987).
4.1.1 Transport in air
The virtually universal distribution of PCBs throughout the world,
including the arctic and other remote areas, suggests that PCBs are
transported in air (Risebrough & de Lappe, 1972). The ability of PCBs
to co-distill, volatilize from landfills into the atmosphere
(adsorption to aerosols with particle size of less than 0.05-20 µm),
and resist degradation at low incinerating temperatures, makes
atmospheric transport the primary mode of global distribution within
the troposphere and stratosphere (Nisbet & Sarofim, 1972; Eisenreich
et al., 1981). PCBs have been measured in air samples at Eniwetok
Atoll in the North Pacific Ocean (Atlas & Giam, 1981), over the North
Atlantic (Giam et al., 1978), and in the Gulf of Mexico (Giam et al.,
1978, 1980). Murphy et al. (1985) estimated that approximately
18 000 kg of PCBs are present in the atmosphere over the USA, at any
given time. The authors also estimated that, if these PCBs had an
atmospheric residence time of one week, then about 900 000 kg/year of
PCBs cycle through the atmosphere of the USA.
Nisbet & Sarofim (1972) suggested that most of the airborne PCBs will
be adsorbed on any particles present. The half-life of particles in
the air will depend greatly on the size of the particles and the
extent of atmospheric precipitation. Most will be deposited within 2-3
days in their areas of origin (usually urban), the small amount
attached to fine particles will last in the atmosphere for longer
periods and can be transported to more remote regions.
Södergren (1972) collected airborne fallout in southern Sweden and
found regional differences in PCB levels, with mean monthly levels
ranging from 620 ng/m2 per month to 10 510 ng/m2 per month. The
lowest level was in a remote forest area. Industrialized areas had
high levels but so too did some agricultural regions. Higher levels
were generally found in the western part of the study region,
suggesting that some PCB fallout may have originated from further
afield and be dependent on the prevailing winds. Seasonal variations
in fallout correlated well with precipitation. Lower levels of PCB
precipitation were found in Iceland by Bengtson & Södergren (1974).
The highest level was found in Northern Iceland at 1050 ng/m2 per
month and, like other sites sampled, showed a seasonal trend with
highest levels in the summer.
Harvey & Steinhauer (1974) measured PCBs in the atmosphere over the
western North Atlantic. They found that concentrations decreased
exponentially with distance from land and concluded that wind
transport is the major method of transport over the oceans. They also
suggested that PCBs are transported primarily in the vapour phase.
4.1.1.1 Dry deposition
Atmospheric input into the Great Lakes has been studied extensively,
because the lakes, as a whole, represent the largest surface area of
any freshwater body in the world, with the lake surface area
comprising from 27% (Ontario) to 64% (Superior) of the total basin
area, and ranging from 19 000 km2 (Ontario) to 82 100 km2
(Superior). Eisenreich et al. (1981) estimated that more than 80% of
the annual mean total input of PCBs in Lake Michigan originated from
the atmosphere. They estimated that approximately 56% of the
9000 kg/year of PCB input in Lake Michigan was in the form of wet
deposition and that 30% of the 6600-8300 kg/year input in Lake
Superior was also in this form. However, Andren (1982) calculated a
precipitation input of 650 kg/year for Lake Michigan, again assuming
that all PCBs were on 0.5 µm airborne particles. Even assuming the
lowest estimate for the annual input of PCBs into the lake,
approximately 60% of the total input might be atmospheric deposition.
Andren (1982) also measured the input of PCB into an isolated lake
(Crystal Lake, Wisconsin), to calibrate a dry deposition model. The
model was then applied to Lake Michigan and the author concluded that,
assuming all particulate inputs of PCB are associated with 0.5 mm
particles, dry deposition inputs were significantly less than wet
inputs.
Manchester-Neesvig & Andren (1989) collected and analysed air samples
from a remote site in the Great Lakes watershed during 1984 and 1985.
Total PCB concentrations varied from 1.82 ng/m3 in the summer to
0.135 ng/m3 in the winter. They found that, on average, 92% of the
PCBs detected were in the vapour phase. When these data were compared
with data collected over the previous 7 years, no significant changes
in PCB concentrations were found. The authors concluded that, on the
basis of the short residence time and the relatively constant annual
average levels of PCBs, repeated cycling between earth and atmosphere
takes place.
Murphy (1984) reviewing data from the Great Lakes region on the
relative distribution of airborne PCBs between particulate matter and
vapour, concluded that they are transported predominantly in vapour.
He stated that there was reasonable evidence to suggest that the
atmosphere is the major source of the PCBs found in Lakes Michigan,
Superior, and Huron, Siskiwit Lake on Isle Royale, and probably in the
upper Great Lakes too.
Using liquid-coated collecting plates in near-shore areas of Lakes
Huron and Michigan, close to urban centres, more PCBs were found on
the upper plates suggesting that much of the dry deposit of PCBs was
associated with large particles (20 µm). This sampling technique also
indicated that, for the areas studied, dry deposition inputs were
higher than wet inputs (Murphy, 1984).
Duinker & Bouchertall (1989) analysed filtered air, particulates, and
rain, in the city of Kiel, Federal Republic of Germany for 14
different PCB congeners. They found that congeners with a low degree
of chlorination were dominant in filtered air, whereas, congeners with
a high degree of chlorination dominated in aerosols and rainfall. The
vapour phase represented up to 99% of the more volatile congeners
(i.e., those with a lower degree of chlorination). The particulates
were found to carry relatively more of the less volatile congeners.
Particle scavenging was the dominant source of PCBs in rain water
despite the small contribution of particulate PCBs to the overall
atmospheric concentration of PCBs (only 1 or 2%).
In a study by Södergren (1973), most of the PCB deposited on a south
Swedish lake was in the form of dry deposit, with 11% as particulate
matter in the precipitation and 2% from precipitation water. McClure
(1976) stated that, on the basis of flux measurements and model
calculations, most of the PCB fallout is in the form of dry deposition
and that most of the dry deposition of aerosol PCB introduced into the
troposphere falls within 100 km of its source.
4.1.1.2 Precipitation deposition
Precipitation scavenging of chlorinated hydrocarbons in the atmosphere
is complex. Scavenging of particles by cloud droplets and by rain
drops in, and below, clouds, and the scavenging of the vapour phase by
rain occurs (Murphy 1984). Thus chlorinated hydrocarbons are
concentrated in precipitation rather than in the atmosphere, resulting
in rainfall levels of many ng/litre. Swain (1978) and Strachan &
Huneault (1979) measured levels in rainfall ranging between 0 (not
detectable) and 230 ng/litre in the Great lakes area.
Murphy (1984) pointed out that variables, such as the amount of
particulate material and PCBs in the atmosphere, the type of rain, and
the rate of rainfall, will affect the precision of precipitation
estimates.
Levels of PCBs in the rainfall throughout Canada during 1984 were
monitored by Strachan (1988). Levels ranged from nd to 17 ng/litre, no
geographical trends were apparent.
4.1.2 Transport in soil
PCBs in soil, derive from particulate deposition (often concentrated
in urban areas), wet deposition, the use of sewage sludge as a
fertilizer, and leaching from landfill sites.
Significant amounts of PCBs are deposited on soil by particulate
deposition (see previous section). Fujiwara (1975) analysed soil
samples in Japan, and found that the main sources of PCB contamination
of agricultural soils are the industries using PCBs. Other sources
include treatment of soil with sewage sludge and accidental spills.
The 15% of soil samples in Indiana (USA) that contained more than
50 mg/kg had been treated with PCB-contaminated dried sludge (Bergh &
People, 1977).
Tucker et al. (1975a) found that, during a 4-month period following
the addition of Aroclor 1016 to soil, the PCBs were not readily
leached by percolating water and that only the lower chlorinated
isomers were leached. The ease of leaching from different soils was in
the order sandy loam > silty loam > silty clay loam.
The behaviour of 14C-labelled PCB in flooded soils was studied by
Ogiso et al. (1976). The amounts of PCB volatilized occurred in the
following order: water > subsoil > soil. The addition of compost
powder to soil reduced the amount that volatilized.
Haque et al. (1974) studied the adsorption of Aroclor 1254 on various
soil particle types in an aqueous solution of 56 µg PCB/litre.
Delmonte sand and silica gel did not adsorb any PCB. Woodburn soil
adsorbed the highest amount followed by illite, montmorillonite, and
kaolinite clays, in decreasing order. The high adsorptive capacity of
Woodburn soil was attributed to the presence of organic matter and
lipophilic or hydrophobic materials. Moza et al. (1976a) found that, 2
years after the application of 14C-labelled dichlorobiphenyl to a
loamy sand soil at 1 mg/kg, most of the detectable PCB was in the top
10 cm of the soil and only 0.2% had reached a depth of 40 cm. In
another study, Suzuki et al. (1977) found that Aroclors 1242 and 1254
did not move upwards through uncontaminated sand deposited over
contaminated soil. The leaching of water from soil may lead to a
downward movement of PCBs, depending on the soil type and clay content
(Pal et al., 1980).
A large spill of Askarel (containing 70% Aroclor 1254 and 30% tri-
and tetrachlorobenzenes) occurred at a transformer-manufacturing
facility in Canada, in 1976. Condie silt from near the site of the
spill was studied with respect to the sorption partition coefficients
and the transport retardation factors. The sorption partition
coefficient values for 2,5,2',5'-tetrachloro-, 2,4,5,2',5'-penta-
chloro-, and 2,4,5,2',4',5'-hexachlorobiphenyl were 5000, 9400, and
26 000, respectively. The mean transport retardation factors for these
3 congeners were 2.7 E + 04, 5.0 E + 04, and 1.4 E + 05, respectively.
This implies that dissolved PCBs will move only very slowly through
unfractured Condie silt (Anderson & Pankow, 1986).
4.1.3 Transport in water
PCBs enter water mainly from discharge points of industrial and urban
wastes into rivers, lakes, and coastal waters. In static water, PCBs
are more concentrated in the surface micro-layer than in subsurface
samples (Bidleman & Olney, 1974). This is probably due to deposition
from the air rather than redistribution in the water. On account of
their low water solubility and high specific activity, it is expected
that most of the PCBs discharged will be adsorbed by sediment at the
bottom of rivers or lakes and transport will be mainly via waterborne
particles (Nisbet & Sarofim, 1972). The bulk of the PCBs will sink to
the bottom sediments. The sinking rate of PCBs from the surface to
deeper layers in the open ocean is relatively slower in tropical
waters than in high-latitude waters (Tanabe, 1985).
Oloffs et al. (1973) added 0.1 mg Aroclor 1260/litre to water samples
in the presence of sediment. After 6 weeks, all of the PCBs had been
adsorbed by the sediment, none being given off to the atmosphere. The
degree of PCBs sorption is inversely related to the size of the
particles (Haque et al., 1974) and the solubility of PCBs in water
(Haque & Schmedding, 1975). Smaller particles have a relatively larger
surface area and so adsorb more PCBs (Steen et al., 1978). Nau-Ritter
et al. (1982) found the adsorption and retention of PCBs to be
directly related to the particle organic content. A significant
correlation was found by Larsen et al. (1985) between PCB levels and
total organic carbon in the deepwater sediments of the Gulf of Maine,
PCBs were concentrated on finer grain particles. Organic carbon and,
therefore, the PCB concentration were also correlated with depth.
Wildish et al. (1980) found that estuarine sediments, especially those
containing higher levels of organic matter, readily adsorbed Aroclor
1254. The PCBs were found to be tightly bound to the sediment with
virtually no desorption. Horzempa & Di Toro (1983) found that the
adsorption of hexachlorobiphenyl was correlated with both sediment
surface area and organic content. Adsorption was found to be
significantly greater at 40°C than at 1°C. Hexachlorobiphenyl is
strongly adsorbed on sediment and weakly desorbed. There is no simple
reversible reaction.
Fisher et al. (1983) found that the rate of release of PCBs from
contaminated sediment was a function of sediment PCB concentration,
chlorine substitution pattern, and degree of chlorination. In the
absence of disturbance, even very low deposition rates of new sediment
will quickly remove PCB-contaminated sediments from diffusional
communication with overlying water. Little change was found (Nimmo et
al., 1971a) in the PCB concentration in sediment at a point downstream
of a contamination source over a period of 9 months. The very small
amounts of PCBs leached from sediment into overlying water may be
taken up by organisms.
Hom et al. (1974) stated that the annual inputs of PCBs into the
southern California bight from waste water and from surface runoff in
1970-71 were estimated to be 10 and 0.25 tonnes, respectively.
Sewage treatment appears to remove PCBs from waste water,
concentrating them in the sludge. However, often, the sludge is then
discharged into open water (Ahling & Jensen, 1970). Holden (1970)
found an average of 3 mg PCBs/kg in wet sewage sludge dumped in the
Clyde estuary, in the United Kingdom, and calculated that this would
be equivalent to approximately one tonne per year. A similar annual
discharge of PCBs in the sludge on the Californian coast was
calculated by Schmidt et al., (1971).
Dredging of inland rivers and harbours may lead to a significant
transfer of PCBs from contaminated sediments, especially when dumped
at sea (Nisbet & Sarofim, 1972). Rice & White (1987) found that there
was an increase in water concentrations of PCBs immediately following
the dredging of sediment in the Shiawassee River, Michigan. The
availability of PCBs for clams and fish, as measured by an increase in
uptake, was found for up to 6 months following dredging.
4.1.4 Transport between media
In a model ecosystem, Södergren & Larsson (1982) found that the
presence of bottom-living organisms, such as Chironomus and
Tubifex, resulted not only in the uptake of PCBs from the sediment
but also in the release of PCBs into the water and to the surface
microlayer, compared with a system without organisms. PCBs were
transported to the air via jet drops from bursting bubbles in the
surface microlayer.
A similar pattern was found using large outdoor artificial ponds
(Larsson, 1985a). Following the addition of Clophen A50 to sediment,
the transport of PCBs from sediment to water followed a seasonal
cycle, with higher levels in the summer than in the winter. The
processes that transfer PCBs across the sediment/water interface
(bioturbation, desorption, and gas convection) are positively related
to temperature. Transfer from water to air was probably dominated by
volatilization with maximum concentrations of PCBs in air at the
highest water concentrations, lower chlorinated biphenyls achieving
the highest concentrations in air. The majority of the airborne phase
was presumed to be in the gaseous phase as it passed through particle
filters. In the same ponds, Larsson & Okla (1987) measured the rate at
which PCBs volatilized from water to air. PCB compounds volatilized at
a rate of 0.9 to 9.6 ng/m2 per h, the rate increasing with the
temperature of the water and the concentration of PCBs. The transport
rate during the day exceeded the rate at night and was positively
correlated with the air temperature (Okla & Larsson, 1987).
Larsson (1985b) added Clophen A50 to the sediment in a model ecosystem
comprising sediment, water, benthic macroinvertebrates, and fish. PCBs
were detected in the water. The transport of PCBs from the water to
air included at least 2 routes, volatilization and jet drop transport.
Both routes were of the same magnitude (0.2-1.0 µg/week). However,
though the PCBs transported by volatilization consisted of lower
chlorinated isomers, those transported by jet drops were identical to
those in the sediment and water.
In an earlier study, Larsson (1984) measured the uptake of PCBs from
sediment by chironomid midge larvae and the concentrations of PCBs
from larva to adult. In the field, chironomid larvae contained
114 µg/kg fresh weight at a sediment concentration of 39 µg/kg wet
weight. Different sediments affected the amount of PCBs available to
the organisms. Adult chironomids sampled near a sewage plant contained
251 µg/kg fresh weight. The chironomid larval population was estimated
to be 9900 per m2 and the authors calculated that these would move
20 µg PCB/m2 per year into the terrestrial compartment of the
environment.
A model, based on the fugacity concept, was described and illustrated
by applying it to the time-varying fate of PCBs in Lake Ontario over
the period 1940-2000. Expressions are included for a great number of
variables, such as loadings and the partitioning of the contaminant
between the phases of air, aerosols, water, suspended and bottom
sediments, various trophic levels of aquatic organisms, and gull eggs.
Also included are expressions for transformation rates, and transport
rates for diffusion between water and sediment, and water and air wet
and dry atmospheric deposition, sediment deposition, burial, and
resuspension, and water and the inflow and outflow of suspended
matter. The results obtained by numerical integration and by assuming
reasonable loading and air concentrations were in accordance with
data. It was shown that PCBs cycle appreciably between the atmosphere
and water by wet and dry deposition and volatilization, and between
water and sediment by deposition, resuspension, and diffusion.
Biomonitors were shown to be particularly valuable indicators of
contamination levels in the ecosystem (MacKay, 1989).
4.2 Biotransformation
4.2.1 Biodegradation
Nissen (1973) did not find any alteration in Aroclor 1254 after a
9-week incubation period in soil. Iwata et al. (1973) added Aroclor
1254 to various soil types. They did not find any change after one
year in soils containing high amounts of organic matter (10.8-19.5%).
Biotransformation had occurred, causing the disappearance of the lower
chlorinated biphenyls, in soils with a low organic matter content
(0.1-3.3%), as diverse as loamy sand and clay. The authors concluded
that, after one year, the material remaining in loamy sand (0.1%
organic matter) consisted of mainly penta- and hexachlorobiphenyl
isomers.
4.2.1.1 Bacteria
The biodegradation of PCB isomers, which is possible with some aerobic
bacteria, depends on the degree of chlorination and the position of
chlorine substitution. Degradation decreases with increasing
chlorination. Dechlorination of PCBs occurs in anaerobic sediments.
Here bacterial activity is preferentially targeted towards PCB
congeners with higher levels of chlorination. Products of
dechlorination are, therefore, more readily degraded by aerobic
systems.
Early experiments were carried out to study the biodegradation of PCBs
using activated sludge inocula; some degradation was found (Baxter et
al., 1975). However, the presence of PCBs in sewage sludge shows that
they are not all readily transformed by microorganisms. Fries (1972)
analysed silage containing PCBs (Aroclor 1254) that had undergone
normal fermentation. The gas chromatogram of the standard was
identical to that of the silage sample. The authors suggested that, if
anaerobic degradation had taken place, it would have been unlikely to
have been uniform for all components. They stated, however, that this
test may not have been a good indication of possible anaerobic
degradation because DDT showed much less degradation, under the same
conditions, compared with other degradation test systems.
Lunt & Evans (1970) postulated a metabolic pathway, used by
microorganisms, for biphenyl oxidation, which was later confirmed by
the findings of Gibson et al. (1973) using a bacterium isolated from a
polluted stream. Lunt & Evans (1970) found that a Gram-negative
bacterium oxidized biphenyl to phenylpyruvic acid with the
intermediary formation of 2,3-dihydroxybiphenyl and
alpha-hydroxy-ß-phenylmuconic semialdehyde. Catelani et al. (1971)
found that the metabolism of biphenyl by Pseudomonas putida was
different, in that, though the intermediate products were the same,
benzoic acid was isolated, not phenylpyruvic acid. Ahmed & Focht
(1973a) isolated 2 species of Achromobacter from sewage effluent
using biphenyl and p-chlorobiphenyl as the sole carbon source. They
found that both sources were rapidly degraded, biphenyl being oxidized
to benzoic acid and both mono and dichlorinated biphenyls to
p-chlorobenzoic acid. In a second study, Ahmed & Focht (1973b)
investigated the biodegradation of other isomers of PCBs, with 2-5
chlorine atoms. The extent of oxidation seemed to be somewhat
dependent on the presence of unsubstituted biphenyl rings. Because of
the absence of chloride in all the supernatants, they concluded that
the bacterium was unable to dechlorinate the PCBs. The fact that
increasing chlorine substitution rendered the molecule more resistant
to microbial attack was used to support this argument. However, Kaiser
& Wong (1974), studying the degradation of Aroclor 1242 by a bacterial
culture, isolated from lake water, showed that the PCBs were degraded
into several metabolites (aliphatic and aromatic hydrocarbons), none
of which contained chlorine. Dechlorination had already taken place at
an early stage of metabolism.
Wong & Kaiser (1975) found that lake water bacteria could use both
Aroclor 1221 and 1242, but not 1254, as a sole carbon source for
growth, but that only 1% of the bacterial culture had this ability.
The authors then followed the degradation of Aroclor 1221. After one
month, the mixture had been totally degraded to several compounds of
low relative molecular mass. Unchlorinated biphenyls were degraded
faster than chlorinated forms.
Tucker et al. (1975b) observed the degradation rates of Aroclors 1221,
1016, 1242, and 1254, and MCS 1043 (a non-commercial mixture). They
found a clear relationship between the level of chlorination and the
relative degradability, when degradation rate was plotted against
percentage chlorine by weight. Volatilization rates fell within the
95% confidence limits of overall disappearance rates and so could be
ruled out. Analysis of the Aroclors, following exposure to the
activated sludge, revealed a redistribution of the dominant PCBs. For
example, the chromatograms for Aroclor 1221 and 1242 were very similar
showing that the lower chlorinated biphenyls were more rapidly
degraded. Furthermore, since Aroclor 1221 was found to be rapidly
degraded, a closer study was performed that showed that most of the
degradation occurred within 24 h.
The degradation of polychlorinated biphenyls by either Nocardia spp.
or Pseudomonas spp. was studied by Baxter et al. (1975). They found
that, under experimental conditions, many of the lower chlorinated
biphenyls (<3 chlorine atoms/molecule) were degraded very readily
and some biphenyls containing as many as 6 chlorine atoms could be
degraded, if the conditions were suitable. When PCB mixtures Aroclor
1016 and 1242 were used, a different pattern of degradation was
observed with an enhanced ability of the microorganisms to degrade.
For example, 4,4'-dichlorobiphenyl degraded to 50% in about 2 days,
when presented to Nocardia spp. as a component of Aroclor 1242, but
it was virtually unaffected after 12 days exposure as the pure isomer.
The authors suggested that mutual solubilization might play some part.
Sayler et al. (1977) found that an estuarine Pseudomonas sp. was
able to degrade both mixtures of PCBs (Aroclor 1254) and pure isomers
of hexachlorobiphenyl. Degradation was dependent on incubation time
and the purity and degree of chlorination of the biphenyl. Appreciable
degradation occurred at all substrate concentrations of the Aroclor
(10, 100, and 1000 µg/litre) within 22 days. Although, over this
22-day period, only 9% had been degraded at the lowest concentration
compared with 30-40% for the other concentrations, after 60 days, this
was reversed with 84% being degraded at 10 µg/litre, 70% at
100 µg/litre, and 63% at 1000 µg/litre. When compared with the pure
isomer, degradation of the Aroclor mixture proceeded at a slower rate.
Even though average chlorination was less, the authors speculated that
this could be owing to the substitution positions of the chlorines.
Chromatographic tracings showed that degradation of the lower
chlorinated components of the Aroclor occurs before degradation of the
more highly chlorinated biphenyls.
Furukawa et al. (1978a,b) examined 31 PCB isomers (mono to
pentachlorobiphenyl) for biodegradability by 2 bacterial species,
Alcaligenes and Acinetobacter. They found the following
relationship between chlorine substitution and biodegradability.
i. Degradation decreased as chlorine substitution increased.
ii. Isomers containing two chlorines at the ortho position of
either a single ring or on both rings showed very poor
degradability.
iii. Isomers, in which all the chlorines were on one ring, were
generally degraded faster.
iv. Molecules with non-chlorinated rings or rings with few chlorines
underwent preferential ring fission.
v. The 4'-chloro-substituted PCBs formed and accumulated a yellow
intermediate during degradation.
vi. Only with respect to 2,4,6-trichlorobiphenyl was there a
significant difference in ability to degrade between the 2
bacteria. This compound was mostly metabolized within 1 h by
Acinetobacter, but was degraded very slowly by Alcaligenes.
It was demonstrated by Carey & Harvey (1978) that mixed cultures of
marine bacteria were capable of metabolizing both pure isomers (tri-
and tetrachlorobiphenyl) and mixtures (Aroclor 1254). They isolated
and partially characterized an acid lactone metabolite. They did not
find any change in the chromatogram trace for the Aroclor but
suggested that this might be related to the insensitivity of the
method, since even if each of the isomers in the mixture had been
metabolized to the same extent as pure isomers, this would still not
have been detectable on the trace. The authors also found that no
metabolism occurred when a chlorobiphenyl isomer in an anaerobic
marine mud was incubated for 6 weeks. Degradation of Aroclor 1242 by
mixed microbial cultures, isolated from soil and river water samples,
was demonstrated by Clark et al. (1979). The predominant organisms in
the cultures were Alcaligenes odorans, Alcaligenes denitrificans,
and an unidentified bacterium. The lower chlorinated isomers were not
only degraded at a faster rate but were also more completely utilized
by the bacteria. In general, the rate of degradation was much faster
than in previous studies. Co-metabolism in the presence of sodium
acetate was studied; greatly enhanced degradation was found for the
more highly chlorinated isomers. Liu (1980) found that sodium
ligninsulfonate also greatly enhanced the biodegradation of commercial
PCB mixtures.
The same author found that a Pseudomonas sp. could oxidize Aroclors
1221, 1016, 1242, and 1254, at a rapid rate. A kinetic study using
resting cells revealed that Aroclor 1221 was degraded much faster
(980 µg/h per mg cell dry weight) than Aroclor 1254 (43 µg/h per mg
cell dry weight). The degradation of the higher chlorinated PCB
(Aroclor 1254) could be enhanced by the addition of Aroclor 1221. Liu
(1981) observed that the oxidation of Aroclor 1221 by the bacteria was
10 times faster than with sewage. Two possible explanations for this
difference were that the sewage contained toxic chemicals that
inhibited the bacteria, but this was found not to be the case, or, the
bacteria preferred Aroclor 1221 to the other substrates. This second
explanation is a possibility, for glucose, a substrate used readily by
most bacteria was poorly oxidized by this bacterium. Pseudomonas
oxidized Aroclor 1221 readily between 15 and 35°C, the rate increasing
with temperature. Reducing the temperature to 4 and 10°C drastically
retarded, but did not halt, degradation. Adjusting the concentrations
of phosphorus and nitrogen from 2 mg to 20 mg/litre (the lower
concentration being that found normally in sewage) did not alter the
rate of degradation by Pseudomonas spp. in raw sewage. But
increasing nitrogen and phosphorus gave more reproducible results,
suggesting that the compounds are on the border of limiting
degradation rates in raw sewage. The oxygen content was found not to
affect degradation at concentrations over 1 mg/litre (oxygen levels
are generally maintained at between 2 and 3 mg/litre in activated
sludge reactors, under the operational conditions of sewage-treatment
plants). Liu (1982) found that, under a limited substrate supply,
Pseudomonas spp. degraded all 7 of the major components of Aroclor
1221. However, with excessive amounts of nutrient, preferential
degradation of certain components was observed. The author stated that
one of the main factors influencing this selective biodegradability
was the position of chlorine substitution on the biphenyl.
4.2.2 Biodegradation; individual congeners
4.2.2.1 Bacteria
In a study by Parsons & Sijm (1988), the co-metabolism was
investigated of several different mono-, di- and tetrachlorobiphenyls
in chemostat continuous cultures of a Pseudomonas strain (JB1). They
found that chemostat conditions favoured degradation compared with
exposure of the Pseudomonos in batch culture, where little or no
degradation was recorded. Using benzoate as the carbon source, results
varied widely, with repeat incubations showing different degrees of
degradation of chlorobiphenyls and, sometimes, no breakdown at all. In
cultures that did degrade the materials, the monosubstituted
4-chlorobiphenyl was rapidly degraded. Of the disubstituted
dibiphenyls, 3,5-dichlorobiphenyl was more readily broken down than
2,5-dichlorobiphenyl. Changing the carbon source available to the
Pseudomonas sp. improved the reproducibility of the results. The
authors reviewed the literature relative to their own findings and
concluded that repeated culture on benzoate leads to the loss of the
ability of the Pseudomonas sp. to degrade biphenyl by meta
cleavage; ortho cleavage is retained. Coding for the meta cleavage
resides on plasmids, which can be lost, whereas coding for the ortho
cleavage is chromosomal. Growth of the Pseudomonas sp. on a
3-methylbenzoate substrate improved degradation of the biphenyls.
3-Methylbenzoate can only be degraded by a meta cleavage favouring
retention of the plasmid. Comparison of degradation of 4
tetrachlorobiphenyls showed the influence of the positions of the
chlorine substitutions. The relative degradability of the 5 compounds,
shown in Fig. 3, was: 2,3,2',3'-tetrachloro- >2,5,3',4'-tetrachloro-
> 2,5,2',5'-tetrachloro- approx. 2,6,2',6'-tetrachloro- approx.
3,4,3',4'-tetrachlorobiphenyl. The authors stated, from the
literature, that the first reaction in the degradation of
chlorobiphenyls is, in most cases, 2,3-dioxygenation, eventually
leading to the formation of chlorobenzoates. Chlorines in the ortho
and meta positions will, therefore, offer steric hindrance to this
reaction.
The low degradation rate of 3,4,3',4'-tetrachlorobiphenyl is not
explained by this mechanism, since it has 2 adjacent unoccupied 2,3
positions, but is more likely explained by its toxicity. Steric
influence on enzyme binding is offered as an explanation in this case.
Similarly, Furukawa et al. (1978a) did not find any degradation of
this compound in initial studies, though they did find degradation to
a dichlorobenzoic acid by Acinetobacter in a later study (Furukawa
et al., 1978b; Rogers, undated(a)).
 Brown et al. (1987a,b) examined patterns of PCB congeners remaining in
sediments after spills of commercial mixtures of Aroclor. Sediment
from 5 different sites was examined. Shifts in gas chromatographic
peak distribution were indicative of dechlorination of congeners by
anaerobic bacteria in the sediment. Analysis of sediment from
different depths indicated less difference from the original traces in
superficial layers and the greatest shift in deeper layers of the
sediment cores. They concluded that dechlorination had taken place and
deduced several different processes involved by comparison between
sites. Six of these processes have been characterized in detail, each
presumed to be mediated by different populations of anaerobic
bacteria, with different selectivity for different congeners in the
PCB mixture. The point of most interest was that congeners with high
degrees of chlorination were selectively dechlorinated by these
anaerobic organisms. Whilst dechlorination still leaves the mass of
PCB intact, congeners with lower chlorination can be more readily
degraded by aerobic bacteria. This anaerobic dechlorination,
therefore, enables further degradation to take place elsewhere and
contributes significantly to the detoxification of the PCBs. While the
combined meta- para selective dechlorinating/oxidizing action of
sediment microbes for PCB residues is likely to be detoxifying, with
respect to dioxin-like effects, there are reservations about whether
this action would be detoxifying in respect of other, more subtle
toxic effects of PCBs and their degradation products, known (such as
the potential reproductive toxicity of the hydroxylated,
ortho-enriched PCBs from sediment microbe action) and unknown. This
is why it is important to study not only the disappearance of PCBs,
but also the exact nature and amounts of the degradation products
(McKinney et al., 1990). Two broad categories of transformation have
been observed: the first dechlorinates in the ortho, meta, and para
positions and the potential for the dechlorination of biphenyls is
related to the reduction potential of the compound, the second
dechlorinates only in the meta and para positions, and the
reactivities of the congeners relate to the molecular shape. The
second category suggested to the authors an active site on a
dechlorinating agent that would be roughly conical with a reducing or
hydrogenating site at the apex. In this schema, para-substituted
molecules could enter the site directly, enough rotation of the
molecule would be possible for the accommodation of meta, but not
ortho, substitution. Quensen et al. (1988) demonstrated this
dechlorinating capacity of anaerobic bacteria from Hudson River
sediments in the laboratory. Dechlorination occurred primarily from
the meta and para positions; ortho-substituted congeners
accumulated selectively. The fastest rate of dechlorination occurred
at the highest exposure used (700 mg Aroclor 1242/kg); 53% of the
total chlorine was removed over a 16-week incubation period. During
incubation, the proportion of mono- and dichlorobiphenyls increased
from 9 to 88%. The authors believed that a sequential anaerobic to
aerobic system could be devised for the biological degradation of
PCBs.
4.2.2.2 Fungi
Wallnofer et al. (1973) incubated a soil fungus Rhizopus japonicus
in a medium containing 3H-labelled 4-chlorobiphenyl or
4,4'-dichlorobiphenyl. After incubation for 1 week, the fungal
mycelium was filtered out. Scans of TLC plates indicated a
hydroxybiphenyl derivative present in the filtrate of both cultures.
To further identify the metabolite, larger amounts of unlabelled
4-chlorobiphenyl were added to a similar culture. The NMR and mass
spectra were identical to a synthetic sample of 4- chloro-4'-hydroxy-
biphenyl; mixed melting point determination showed no depression.
Further positive identification of the product was not possible,
because of limited material, but the experiment indicates the
probability of degradation of biphenyl to a hydroxy derivative by a
fungus.
4.2.3 Photodegradation
Several authors have reported that simple chlorinated biphenyls, as
well as complex commercial PCB mixtures, undergo photoreduction in
organic solvents (Safe & Hutzinger, 1971; Hustert & Korte, 1972; Ruzo
et al., 1972, 1974, 1975; Sawai & Sawai, 1973; Koshioka et al., 1987)
and aqueous systems (Crosby & Moilanen, 1973; Bunce, 1978) in the
laboratory. Herring et al. (1972) found that PCBs degraded faster in
hexane solution than in aqueous solution and slower in benzene
solution.
Bunce et al. (1978) posed the question of the environmental
significance of the photodegradation of PCBs and tried to estimate the
likely degree of photolysis under real environmental conditions,
rather than in solution in organic solvents at high concentrations.
The current best estimate suggests that significant amounts,
particularly of higher chlorinated PCB congeners, might be degraded in
water by the action of sunlight.
4.2.4 Bioaccumulation, distribution in organisms, and elimination
Polychlorinated biphenyls accumulate in almost all organisms, because
of their high lipid solubility and slow rate of metabolism and
elimination. They accumulate preferentially in fat-rich tissues.
Bioconcentration factors (BCFs) should be interpreted with caution,
since they are simple ratios. The exposure concentration, therefore,
makes a marked difference to the BCF obtained; very low exposure
concentrations are likely to lead to high BCFs, since all the PCBs are
absorbed, whilst high exposure concentration will tend to minimize the
BCFs.
Experimental data on the bioconcentration of PCB mixtures and pure
chlorinated biphenyls are presented in Table 9 for microorganisms,
Table 10 for aquatic organisms, and Table 11 for plants, birds, and
mammals.
4.2.4.1 Microorganisms
Uptake of both pure chlorinated biphenyl isomers and commercial PCB
mixtures by microorganisms is rapid, and high bioconcentration factors
are achieved. While there is a suggestion in studies on some species
that PCB congeners with higher levels of chlorination are taken up
preferentially, in the majority of studies, all PCBs appear to be
taken up equally. Uptake is true absorption; adsorption onto the
surface of the organisms represents little of the uptake. Since
resistant forms of microorganisms take up less PCBs than sensitive
forms and dead cells accumulate more PCBs than live ones, there is
some capacity to exclude the compounds.
Harding & Phillips (1978b) studied the uptake of 14C-labelled
2,4,5,2',5'-pentachlorobiphenyl, at concentrations of 0.31 or
9.86 µg/litre water, by 11 marine phytoplankton species including:
diatoms, green algae, chrysophytes, haptophytes, and dinoflagellates.
The cell density of each culture was maintained at 106-109 cells/
litre. Equilibrium between water and cell concentrations of biphenyl
was reached very rapidly after 0.5-2 h; small motile forms reached
equilibrium within 1 h and large centric diatoms after approximately
2 h. Exposure concentration and cell density, within the range given
above, had little effect on the time-course of uptake. Substantial
interspecies differences in adsorptive capacity were shown by
differences in the Freundlich adsorption constant (log K). A large
centric diatom, Coscinodiscus sp., had the highest log K. Nitzschia
longissima, a penate diatom that has been shown to be resistant to
PCBs (Harding & Phillips, 1978a), had the lowest log K value. The
flagellates, with the exception of Monochrysis lutheri, which has
been shown to be very sensitive to the effects of PCBs, had much lower
log K values than diatoms. Concentration factors, calculated from the
Freundlich adsorption isotherms, ranged between 12 300 and 2 410 000.
Biggs et al. (1980) exposed mixed species of estuarine phytoplankton
(numerically dominated by the diatom Skeletonema costatum) to
14C-labelled PCB (approximately 54% chlorine by weight) at
concentrations of 5.8 or 11.6 µg/litre. At a particle concentration of
25 mg/litre, 19-22% of the labelled-PCB was sorbed on the particles
after a 1-h exposure, with 70-72% in the water. At 4 times the above
particle concentration, 66-69% was sorbed on particles and only 22-23%
was retained in the water. Doubling the amount of 14C-PCB doubled the
mean amount of labelled-PCB in both the particles and the water. The
authors calculated an index of sorption (the ratio of 14C-PCB sorbed
on particles to that in an equal volume of water) at an average of
2 ± 1 × 104. The authors suggested that the higher uptake (88%) of
PCBs found by Södergren (1971) was probably the result of an
unnaturally high cell concentration. Phytoplankton sampled in the
surface waters of Long Island Sound, USA, varied seasonally in
concentration from about 0.5 to 30 mg/litre.
Lederman & Rhee (1982) calculated bioconcentration factors for 3
species of Great Lakes planktonic algae (Table 9). In the case of
Fragilaria crotonensis, the uptake of hexachlorobiphenyl into the
frustule (the siliceous wall of the diatom) was investigated. The
bioconcentration factors for frustules were lower by an order of
magnitude than the factors for live and dead cells. It appears,
therefore, that adsorption on the cell surface contributes only a
little to the bioaccumulation of hexachlorobiphenyl.
Table 9. Bioaccumulation of PCBs: Microorganisms
Organism Biomass Temperature PCB type Duration Exposure Bioconcentration Reference
(cells/ml) (°C) (µg/litre) factora
Green alga 2 × 106 20-25 TeCB 1 h 10 3200 Urey et al. (1976)
Chlorella 2 × 106 20-25 HeCB 1 h 10 7000 Urey et al. (1976)
pyrenoidosa 2 × 106 20-25 OcCB 1 h 10 1600 Urey et al. (1976)
2 × 106 20-25 DeCB 1 h 10 5200 Urey et al. (1976)
Algae 3.2 × 105 HeCB 19 h 1 117 000b Lederman & Rhee
Fragilaria 1.6 × 105 HeCB 19 h 1 313 000b (1982)
crotonensis Lederman & Rhee
(1982)
Algae 3.4 × 105 HeCB 6 h 1 619 000b Lederman & Rhee
Ankistrodesmus 1.7 × 105 HeCB 6 h 1 959 000b (1982)
falcatus 8.5 × 104 HeCB 6 h 1 1 207 000b Lederman & Rhee
(1982)
Lederman & Rhee
(1982)
Algae 1.1 × 106 HeCB 6 h 1 129 000b Lederman & Rhee
Mycrocystis sp. 5.5 × 105 HeCB 6 h 1 170 000b (1982)
2.8 × 105 HeCB 6 h 1 264 000b Lederman & Rhee
(1982)
Lederman & Rhee
(1982)
Table 9. (cont'd).
Organism Biomass Temperature PCB type Duration Exposure Bioconcentration Reference
(cells/ml) (°C) (µg/litre) factora
Fungus 22-25 Aroclor 1254 24 h 0.007 mg/kg 1327b,c Pinkney et al. (1985)
Fusarium 22-25 Aroclor 1254 48 h 0.007 mg/kg 1144b,c Pinkney et al. (1985)
oxysporum
a Concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration factors calculated on a wet weight
basis unless otherwise stated.
b Calculated on a dry weight basis.
c Radioactive isotope used to calculate bioconcentration factor.
Table 10. Bioaccumulation of PCBs: Aquatic organisms
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
American oyster flow WB Aroclor 1016 96 h 0.6 6666 Hansen et al. (1974b)
Crassostrea WB Aroclor 1254 56 d 0.01 165 000 Parrish (1973)
virginica WB Aroclor 1254 392 d 0.01 89 000 Parrish (1973)
Polychaete stat WB Aroclor 1254 5 d 1.1 236 Courtney & Langston
Arenicola marina stat WB Aroclor 1254 5 d 1 mg/kgd 0.24 (1978)
Polychaete stat WB Aroclor 1254 5 d 1.1 373 Courtney & Langston
Nereis stat WB Aroclor 1254 5 d 1 mg/kgd 0.36 (1978)
diversicolor
Water flea flow WB 20-22 Aroclor 1254 96 h 1.1 47 000e* Sanders & Chandler
Daphnia magna (1972)
Amphipod (M) statf WB Aroclor 1254 24 h 0.03 8700 Pinkney et al. (1985)
Gammarus statf WB Aroclor 1254 24 h 195.8 mg/kg 0.118 Pinkney et al. (1985)
tigrinus
Scud flow WB 20-22 Aroclor 1254 96 h 1.6 24 000e* Sanders & Chandler
Gammarus flow WB 20-22 Aroclor 1254 21 d 1.6 27 000e (1972)
pseudolimnaeus
Glass shrimp flow WB 20-22 Aroclor 1254 96 h 1.3 12 300e* Sanders & Chandler
Palaemonetes flow WB 20-22 Aroclor 1254 21 d 1.3 16 600e* (1972)
kadiekensis
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Brown shrimp flow WB Aroclor 1016 96 h 0.9 4222 Hansen et al. (1974b)
Penaeus aztecus
Grass shrimp flow WB 17-28 Aroclor 1254 7 d 2.3 11 000 Nimmo et al. (1974)
Palaemonetes flow WB 17-28 Aroclor 1254 16 d 1.3 14 000 Nimmo et al. (1974)
pugio flow WB 17-28 Aroclor 1254 28 d 0.62 17 450 Nimmo et al. (1974)
flow WB 17-28 Aroclor 1254 35 d 0.62 26 580 Nimmo et al. (1974)
flow WB Aroclor 1016 96 h 0.4 2750 Hansen et al. (1974b)
Crayfish flow WB 20-22 Aroclor 1254 96 h 1.2 1700e* Sanders & Chandler
Orconectes nais flow WB 20-22 Aroclor 1254 21 d 1.2 5100e* (1972)
Stonefly flow WB 20-22 Aroclor 1254 96 h 2.8 2500e* Sanders & Chandler
Pteronarcys flow WB 20-22 Aroclor 1254 21 d 2.8 2800e* (1972)
dorsata
Dobsonfly flow WB 20-22 Aroclor 1254 96 h 1.1 4600e* Sanders & Chandler
Corydalus flow WB 20-22 Aroclor 1254 21 d 1.1 6800e* (1972)
cornutus
Phantom midge flow WB 20-22 Aroclor 1254 96 h 1.3 23 600e* Sanders & Chandler
Chaoboruspuncti (1972)
pennis
Mosquito larvae flow WB 20-22 Aroclor 1254 96 h 1.5 18 000e* Sanders & Chandler
Culex tarsalis (1972)
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Mayfly flow WB 8 Clophen A50 6 d 0.526 2940 Södergren &
Ephemera danica Svensson (1973)
Pinfish flow WB Aroclor 1016 96 h 0.8 2750 Hansen et al. (1974b)
Lagodon flow WB Aroclor 1016 28 d 1 n 25 000 Hansen et al. (1974b)
rhomboides flow WB Aroclor 1016 56 d 1 n 17 000 Hansen et al. (1974b)
Sheepshead flowg WB Aroclor 1016 33 d 1 n 26 000 Hansen et al. (1975)
minnow flowg WB Aroclor 1016 28 d 1 n 54 000 Hansen et al. (1975)
Cyprinodon flowg WB Aroclor 1016 28 d 1 n 22 000 Hansen et al. (1975)
variegatus
Spot flow WB Aroclor 1254 7 d 1 n 7200 Hansen et al. (1971)
Leiostomus flow WB Aroclor 1254 14 d 1 n 17 000 Hansen et al. (1971)
xanthurus flow WB Aroclor 1254 28 d 1 n 37 000 Hansen et al. (1971)
flow WB Aroclor 1254 56 d 1 n 27 000 Hansen et al. (1971)
Atlantic salmon flow WB 10-15 Aroclor 1254 33 d 10 mg/kg 0.39 Zitko (1974)
Salmo salar
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Coho salmon flow WB 17 Aroclor 1254 112 d 0.048 mg/kg 9.79 Mayer et al. (1977)
Oncorhynchus flow WB 17 Aroclor 1254 112 d 4.8 mg/kg 0.79 Mayer et al. (1977)
kisutch WB TeCB 17 d 1 mg/kg 0.144 Gruger et al. (1976)
WB TeCB 35 d 1 mg/kg 0.139 Gruger et al. (1976)
WB PeCB 35 d 1 mg/kg 0.162 Gruger et al. (1976)
WB HeCB 35 d 1 mg/kg 0.151 Gruger et al. (1976)
Channel catfish flow WB 26 Aroclor 1232 150 d 2.4 mg/kg 1.875 Mayer et al. (1977)
Ictalurus flow WB 26 Aroclor 1232 193 d 2.4 mg/kg 1.3 Mayer et al. (1977)
punctatus flow WB 26 Aroclor 1248 193 d 2.4 mg/kg 0.79 Mayer et al. (1977)
flow WB 26 Aroclor 1254 193 d 2.4 mg/kg 2 Mayer et al. (1977)
flow WB 26 Aroclor 1260 193 d 2.4 mg/kg 1.46 Mayer et al. (1977)
flow WBh 24-26 Aroclor 1242 130 d 20 mg/kg 0.72 Hansen et al. (1976a)
flow WB Aroclor 1248 77 d 5.8 56 370* Mayer et al. (1977)
flow WB Aroclor 1254 77 d 2.4 61 190* Mayer et al. (1977)
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Fathead (M) flow WB 25 Aroclor 1248 250 d 3 approx. 60 000 DeFoe et al. (1978)
minnow (M) flow WB 25 Aroclor 1260 250 d 2.1 approx. 160 000 DeFoe et al. (1978)
Pimephales (F) flow WB 25 Aroclor 1248 250 d 3 approx. 120 000 DeFoe et al. (1978)
promelas (F) flow WB 25 Aroclor 1260 250 d 2.1 approx. 270 000 DeFoe et al. (1978)
d = Days; M = Male; F = Female; DiCB = dichlorobiphenyl; TeCB = tetrachlorobiphenyl; PeCB = pentachlorobiphenyl;
HeCB = hexachlorobiphenyl; OcCB = octachlorobiphenyl; DeCB = decachlorobiphenyl.
a Stat = static conditions (water unchanged for duration of experiment); flow = flow-through conditions (PCB concentration
in water continously maintained).
b WB = whole body.
c Bioconcentration factor = concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration
factors calculated on a wet weight basis unless otherwise stated. * Radioactive isotope used to calculate
bioconcentration factor.
d Sediment.
e Calculated on a dry weight basis.
f Static conditions, but test solution changed at intervals.
g Intermittent flow-through conditions.
h Not including stomach.
Södergren (1971) maintained the unicellular freshwater green alga
Chlorella pyrenoidosa in water (at a cell concentration of
approximately 900 mg/litre) with added nutrient medium containing
3.7 µg Clophen A50/litre, over a period of 7 days. By the end of the
experiment, 88% of the PCBs had been taken up by the alga. The
remaining PCBs were detected in the water, none being found in the air
samples taken. In another study, Urey et al. (1976) found that both
tetrachloro- and hexachlorobiphenyl isomers, at 10 µg/litre, were
concentrated by dead Chlorella pyrenoidosa cells by 6000 and 15 000
times, respectively, after a 1-h exposure. These concentration factors
are approximately twice those for living cells (Table 9). Similar
findings have been noted with other species of algae (Biggs et al.,
1980; Lederman & Rhee, 1982).
The ciliate Tetrahymena pyriformis was exposed to Aroclors 1248
(0.01, 0.1, and 1 mg/litre) and 1260 (0.001, 0.01, 0.1, and
1 mg/litre) for 7 days (Cooley et al., 1973). Uptake of the toxicant
increased linearly with increasing concentration. Concentration
factors ranged from 14.8 to 40.6 for Aroclor 1248 and from 21 to 79
for Aroclor 1260. Approximately 15-20% of Aroclor 1248 was absorbed at
each concentration compared with means of 37-53%, with increasing
concentration, for Aroclor 1260. If the data from Cooley et al. (1972)
on the uptake from Aroclor 1254 is included, it is clear that
T. pyriformis accumulates more PCBs with increasing degree of
chlorination.
Dive et al. (1976) studied the accumulation of 16 pure isomers of PCB
and one commercial product, Pyralene 3010, by the ciliate protozoan
Colpidium campylum, at concentrations of 0.1, 1, or 10 mg/litre for
43 h. The amount of PCBs taken up at 0.1 mg/litre was very similar for
each of the PCB isomers and the commercial product, ranging from 29.4
to 49%. The percentage uptake did not change greatly for the higher
exposures.
4.2.4.2 Plants
Uptake of PCBs into plants from soil is positively correlated with the
soil concentration of the PCBs. Roots accumulate more than stems and
foliage. Bioconcentration factors are low. More lower chlorinated
congeners of the PCBs are taken up, probably because of their greater
mobility in the soil. Much of the uptake is adsorption on the surfaces
of roots and there is little translocation. PCBs found in leaves have
volatilized from the soil. Uptake on root surfaces can be reduced or
eliminated by adding activated charcoal to the soil.
Lawrence & Tosine (1977) found that plants took up significant amounts
of PCBs (30-140% of the applied PCB concentration) from soil treated
with sewage sludge. In a waste PCB spill besides a North Carolina
highway, levels as high as 4700 mg/kg were recorded in the top 3 cm of
soil. Seven months later, the PCB concentrations were unchanged; the
authors believed that this was because the PCBs were bound to
activated carbon that had been used to treat the spill (Pal et al.,
1980).
Strek & Weber (1982) analysed statistically the data from several
literature sources (Iwata et al., 1974; Wallnofer & Koniger, 1974;
Wallnofer et al., 1975; Iwata & Gunther, 1976; Moza et al., 1976a,
1979a,b; Weber & Mrozek, 1979) on PCB uptake by plants, with the
following conclusions.
i. The PCB content of the plant is significantly dependent on the
soil PCB concentration.
ii. There is a significant difference between plant species,
carrots taking up more PCBs than other plants.
iii. There appears to be a limit of the PCB concentration in the
soil at which no detectable PCBs are taken up by the plants.
iv. Roots take up more PCBs than tops.
v. most of the PCBs in roots may, in fact, be adsorbed on the
surface and not actually taken up.
vi. There is a general trend of increasing PCB content with
decreasing chlorination, for pure PCB congeners.
vii. The amount of chlorination seems to have an effect on the
mobility of PCBs within plant parts. Since lower chlorinated
PCBs have been reported to be more mobile in soils than highly
chlorinated PCBs, they may be more readily transported and
available for plant uptake.
Larsson (1987) maintained the macroalga Cladophora glomerata in a
flowing-water, outdoor pool. Sediment contaminated with Clophen A50 at
2.7 mg/kg dry weight was added and PCB residues in the alga were
monitored. The algal concentration was 3.55 mg/kg dry weight within 3
months. Residues had fallen a year later to 0.2 mg/kg, reflecting the
water levels of PCBs. The authors concluded that a partitioning
process governed the uptake of PCBs by C. glomerata in this
experiment, because the alga accumulated the same PCBs and the same
proportion of PCBs that were present in the water.
Table 11. Bioaccumulation, of PCBs: Plants, birds, and mammals
Organism Organ PCB type Duration Exposure Bioconcentration Reference
(mg/kg) factora
Soilb
Beet (Beta vulgaris) plant top Aroclor 1254 39 days 20 0.041c Strek et al. (1981)
Sorghum (Sorghum bicolor) plant top Aroclor 1254 39 days 20 0.003c Strek et al. (1981)
Peanut (Arachis hypogaea) plant top Aroclor 1254 78 days 20 0.024c Strek et al. (1981)
Corn (Zea mays) plant top Aroclor 1254 13 days 20 0.001c Strek et al. (1981)
Carrot root DiCBd 112 days 0.118 2c Moza et al. (1976a)
leaves DiCBd 112 days 0.118 0.92c Moza et al. (1976a)
Food
White pelican carcase Aroclor 1254 70 days 144 14.8 Greichus et al. (1975)
(Pelecanus erythrorhynchos)
Table 11. (cont'd).
Organism Organ PCB type Duration Exposure Bioconcentration Reference
(mg/kg) factora
Chicken fat Aroclor 1242 28 days 100 2.83 Harris & Rose (1972)
fat Aroclor 1254 28 days 100 5.15 Harris & Rose (1972)
fat Aroclor 1260 28 days 100 4.82 Harris & Rose (1972)
Big brown bat carcase Aroclor 1254 37 days 9.4 6.6 Clark & Prouty
(Eptesicus fuscus) (1977)
Mink fat Aroclor 1254 approx. 56 days 1.5 16.5 Hornshaw et al.
(Mustela vison) fat Aroclor 1254 approx. 126 days 1.5 28.5 (1983)
Hornshaw et al.
(1983)
a Bioconcentration factor = concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration
factors calculated on a wet weight basis, unless otherwise stated.
b Calculated on a dry weight basis.
c Radioactive isotope used to calculate bioconcentration factor.
d DiCB = dichlorobiphenyl.
Red mangrove (Rhizophora mangle) seedlings were grown for 6 weeks in
soil treated with Aroclor 1242 at concentrations of between 0.038 and
6 mg/kg (Walsh et al., 1974). Low levels (detection limit was
0.1 mg/kg) of the PCBs were detected in the roots at exposure
concentrations of 3 or 6 mg/kg, during the exposure period, but no
residues were found in the stems. Residues were detected in both the
hypocotyls and leaves at application rates of 0.3 mg/kg or more. Leaf
residues did not change with time, but PCB concentrations in the
hypocotyls showed an increase. The highest mean residues of 1.5 mg/kg
were found in the hypocotyl in the highest exposure group.
Iwata et al. (1974) treated soil with Aroclor 1254, at a concentration
of 100 mg/kg, and sowed carrots in the plot 7 months later. The
carrots were harvested 3 or 4 months after seeding. The authors found
that the lower chlorinated biphenyls were more readily taken up from
the soil into the carrot root. Analysis of the carrot peel revealed
approximately 97% of the PCB residue, showing that there is little
translocation within the plant; 23 months after sowing carrots in soil
contaminated with 100 mg/kg Aroclor 1254, dissipation from soil
appeared to parallel the degree of chlorination (Iwata & Gunther,
1976). Analysis of the soil revealed that the lower chlorinated
biphenyls were slowly dissipated while the more highly chlorinated
biphenyls appeared to be unaffected. Small amounts of PCBs were found
in carrot foliage and the authors suggested that the PCB composition
indicated that they came from soil dust. Suzuki et al. (1977) also
found that lower chlorinated biphenyls were preferentially taken up by
plants, following exposure of soybean sprouts to soil contaminated
with Aroclor 1254 or 1242 at 100 mg/kg.
Moza et al. (1976a) found that carrot bioconcentrated
2,2'-dichlorobiphenyl (0.118 mg/kg soil) from soil by a factor of 2
(Table 11). No bioaccumulation was found in sugar beet, but the soil
residue was only 0.029 mg/kg. Carrots were grown in soil amended with
either 14C-labelled 2,5,4'-trichlorobiphenyl at 1.28 kg/ha or
2,4,2',4',6-pentachlorobiphenyl at 1.12 kg/ha for one season (Moza et
al., (1979a). Only 32.5% of the trichlorobiphenyl was recovered, the
rest being lost through volatilization. The carrots had taken up 3.1%
of the applied 14C, representing a concentration factor of 2.8. For
the pentachlorobiphenyl, 58.5% was recovered, 1.4% of which had been
taken up by the carrots. Sugar beet grown in the soil the following
year accumulated only 0.4% of the applied 14C.
In a study by Weber & Mrozek (1979), 14C-labelled Aroclor 1254 was
applied to Lakeland soil at a rate of 20 mg/kg. Activated carbon was
mixed with half the pots at a rate of 3.7 t/ha (3333 mg/kg). The pots
were seeded with either soybean or fescue. After harvesting at 16 days
for soybean and 50 days for fescue, the amounts of labelled-PCBs,
recovered from the plant tops, were 0.016% and 0.17% for the 2
species, respectively. The addition of activated carbon to the soil
reduced the uptake of 14C-PCBs, the recovery of labelled-PCBs being
0.001% and 0% for soybean and fescue, respectively. Strek et al.
(1981) also applied 14C-labelled Aroclor 1254, at the same rate, to
Lakeland soil; several species of crop plants were grown in the soil
and bioaccumulation factors, calculated (Table 11). Addition of
activated carbon (3.7 t/ha), equivalent to 3333 mg/kg to replicate
pots, reduced the uptake of the labelled-PCBs by 80-100%.
When approximately 1 mg 14C-labelled Aroclor 1254/kg was applied to
the centre leaflet of the first trifoliate leaf of 18-day-old soybean
plants, only 6.7% was recovered from the plant after 12 days, 76% of
which was still present in the treated leaf (Weber & Mrozek, 1979).
Mrozek & Leidy (1981) transferred the marsh plant Spartina
alterniflora from the field into soil containing 1 mg Aroclor
1254/kg (dry weight) and harvested the plants after a growth period of
90 days. The plants were found to take up selectively the lesser
chlorinated biphenyls. The authors stated that a further shifting of
the chromatographic pattern of PCBs towards the lesser chlorinated
components in aerial tissues suggested that some alteration of the PCB
mixture occurs in the plant. Mrozek et al. (1982) also found that
Spartina accumulates PCBs from both contaminated sand and mud-soil
systems. The total 14C-radioactivity accumulated in plants grown in
sand systems was higher than that in plants grown in mud. The level of
radioactivity accumulated in the green parts of the plants was similar
in both soil systems.
Moza et al. (1976b) applied 76 mg/kg of 14C-labelled 2,5,4'-tri-
chlorobiphenyl or 133 mg/kg of labelled 2,4,6,2',4'-pentachloro-
biphenyl to the leaves of the marsh plant Veronica beccabunga. Six
weeks later, the total recovery from plant, water, and soil was 3.7
and 18.3%, respectively, 86 and 95% of which was recovered from the
plant. In an earlier study, Moza et al. (1974) applied 14C-labelled
2,2'-dichlorobiphenyl in water or soil to 2 higher water plant species
( Ranunculus fluitans and Callitriche sp.) at concentrations of
13.7 and 14.5 mg/kg, respectively. Four weeks after application, the
results showed that the dichlorobiphenyl was metabolized more readily
after addition to water; the authors suggested the involvement of
aquatic bacteria. When applied in soil, 1.2% of the dichlorobiphenyl
was metabolized. This was contributed to the plant.
Moza et al. (1979b) grew 3-year-old spruce trees (Picea abies) in
soil containing 14C-labelled PCBs at approximately 4.2 mg/litre in
sewage sludge. When analysed 4 years later, only 0.8% (0.5% in
needles, 0.3% in stems) of the applied radioactivity was found in the
trees. Leaching of radioactive substances from the soil was less than
0.1% in the first 2 years and undetectable for the remainder of the
study.
In another study, Fries & Marrow (1981) grew soybean (Glycine max)
in pots, to determine residue contamination in plant tops from
14C-labelled 2,5,2'-trichlorobiphenyl, 2,5,2',5'-tetrachlorobiphenyl
or 2,4,5,2',5'-pentachlorobiphenyl, applied to the surface or
subsurface soil. Each compound was added to the soil at a rate of
2-3 mg/kg and the plants were harvested after a period of 52 days.
Detectable residues were only found in plants grown in surface-treated
soil, and concentrations in the plants increased with increasing
chlorination. Little of the labelled PCBs was lost from
subsurface-treated soil, but 20-30% of the surface-treated PCBs were
lost through volatilization. The authors concluded that the PCB
residues in the plant tops were, therefore, due to foliar
contamination from vapour rather than the uptake from the soil via the
roots. Miyazaki et al. (1975) came to the same conclusion when they
found no absorption or translocation of PCBs in sesame or rice seeds,
following the application of 4 types of Kanechlor (KC300, 400, 500,
and 600) at rates of between 0.1 and 100 mg/kg. But the rice straws
contained PCB levels of 0.02-0.08 mg/kg, which were the same as levels
found in plants from untreated soils.
Beets ( Beta vulgaris L.), turnips ( Brassica rapa L.), and beans
( Phaseolus vulgaris L.) were grown (Sawhney & Hankin, 1984) in soil
to which lake sediment contaminated with PCBs had been added. The
plants were exposed to Aroclor 1248 at a concentration of 80 µg/kg,
Aroclor 1254 at 1880 µg/kg, and Aroclor 1260 at 14 440 µg/kg. When
beets and turnips were grown in the soil for 6 months, the plants
showed greater uptake in the leaves than in the roots. For example,
beet roots contained 15, 16, and 35 µg/kg of Aroclors 1248, 1254, and
1260, respectively, while beet leaves contained 22, 94, and 52 µg/kg,
respectively. Total concentrations of the 3 Aroclors in beet roots and
leaves and in turnip roots and leaves were 66 and 168 µg/kg,
respectively, and 66 and 99 µg/kg, respectively. During a second
growing season, turnips and beans were grown for 6 months without any
additional PCB-contaminated sediment. Comparing PCB levels in turnips
between the 2 growing seasons showed a decrease in Aroclor 1248 uptake
relative to Aroclors 1254 and 1260. This was primarily because of a
large reduction in the amount of Aroclor 1248 in the soil after 1
year, due to degradation and volatilization. In beans, higher PCB
levels were found in the leaves and pods than in the stems and seeds.
Ten sludge application sites were selected within the Ontario area to
determine background heavy metal and PCB concentrations in the soils
and crops. Control sites (without sludge application) were adjacent to
the sludge application sites. Grab samples of liquid sludges applied
at each of the sites were taken for analysis. The soil samples were
taken at a depth of 15 cm. Twenty core samples were taken at 20-m
intervals and combined to form 1 sample. Eight of the application
sites were cropped with corn, one with oats, and one was left without
a crop. At the control sites, 7 were cropped with corn, 1 with oats,
and 2 left without a crop. PCB concentrations in the sludges ranged
from 0.13 to 1.61 mg/kg dry solids. PCB concentrations were in the
range of 0.007-0.025 mg/kg in the soil without sludges, and in the
range of 0.018-0.453 mg/kg air-dry weight in the soil with sludges.
The PCB levels in the crops were close to the control values (Webber
et al., 1983).
Bacci & Gaggi (1985) assessed the influence of translocation on the
concentrations of PCBs in the foliage of different plant species.
Beans, broad beans, tomatoes, and cucumbers were grown, either in soil
with a nominal added concentration of 500 mg/kg Fenclor 64 (similar to
Aroclor 1260), or in clean sand, for 28 days, enclosed in a glass box
with a constant turnover of air. The plants grown in clean sands were
exposed to PCBs by volatilization from other pots containing PCBs,
which were in the same growing box. The PCB peak pattern of both sand
and roots was similar to that of Fenclor 64, whereas the peak pattern
for foliage and air had moved towards lesser chlorinated congeners.
The concentrations of PCBs in the roots of tomatoes grown in
contaminated soil ranged from 105 to 168 mg/kg dry weight. But
translocation through the plants does not seem to be very likely since
there was no significant difference in foliage uptake of PCBs between
plants grown in contaminated soil and plants grown in clean soil.
Foliar uptake ranged from 13.8 to 42.6 mg PCB/kg (dry weight) for the
different species in PCB-fortified soil and from 11.8 to 47.1 mg/kg
for plants grown in clean soil.
4.2.4.3 Aquatic invertebrates
Bioconcentration factors are high for PCBs taken up by aquatic
invertebrates exposed to either pure chlorinated biphenyl isomers or
commercial mixtures in the water. Since PCBs are strongly bound to
sediments, this method of exposure is unrealistic. Addition of
sediment to test tanks decreases the uptake of PCBs, particularly by
organisms living in the upper water. However, there is clear evidence
that PCBs can also be readily absorbed into invertebrates from both
sediment and food. For organisms living on or in, sediment, uptake can
take place from the sediment, via food organisms that have absorbed
the PCBs, and from interstitial water or water immediately above the
sediment layer. A high content of organic matter in sediment decreases
the availability of PCBs for organisms. Uptake is rapid in most cases
and equilibrium is often reached in hours, though it may take weeks in
other examples. Uptake increases with increasing temperature. The
route of uptake is often via the gills, but varies among species. Loss
of PCBs is slow, but residues do decrease on cessation of exposure.
PCB uptake by aquatic invertebrates is transferred to predators and
can also be transferred to the terrestrial environment.
(a) Uptake from water
Vreeland (1974) exposed American oysters (Crassostrea virginica) to
various PCB isomers at concentrations of 5.5, 17, or 60 ng/litre
(which is within the range found in coastal waters) for 65 days.
Equilibrium was reached after approximately 1 month of exposure, with
concentration factors ranging from 1200 to 48 000 for PCB isomers with
2-6 chlorine atoms/molecule. The PCB concentration, after equilibrium
had been reached, was directly proportional to the amount of PCBs
added to the water. Lowe et al. (1972) found a linear pattern of
uptake in young American oysters exposed to Aroclor 1254 at 5 µg/litre
for 24 weeks, followed by a further 32 weeks in clean water. The
oysters already contained 17 mg/kg from a previous exposure and, by
the end of the 24-week exposure period, had accumulated 425 mg/kg (a
steady state was not established). By the end of the 32-week period in
clean water, no PCB residues could be detected. In another study on
uncontaminated young oysters, concentration factors of up to 101 000
were achieved after a 25-week exposure to 1 µg Aroclor 1254/litre.
After 12 weeks in clean water, whole-body residues were reduced to
0.2 mg/kg.
Courtney & Denton (1976) fed hard clams (Mercenaria mercenaria)
Aroclor 1254 adsorbed on the surface of alumina particles, at 1.25 and
12.5 µg/litre, for 21 days. The maximum concentration factor was 1800
for visceral mass, when the clams had been exposed to 1.25 µg/litre
for 18 days. The visceral mass accumulated a 1.4-5.3 times greater
concentration of PCBs per unit time than the muscular foot. Tissue
samples contained relatively more lower chlorinated isomers than the
Aroclor 1254 standard and, faeces and mud samples contained more
higher chlorinated isomers. Following exposure, clams from the lowest
dose group showed little change in PCB content after 3 months in clean
seawater. However, at the higher dose level, there was a significant
reduction in the PCB levels found in the foot after 1 month, but PCB
residues in the visceral mass remained unchanged for 6 months.
Pink shrimp (Penaeus duorarum) were exposed to Aroclor 1254 at a
concentration of 2.5 µg/litre, in flowing water, for 22 days (Nimmo et
al., 1971b). Accumulation was linear for the hepatopancreas and whole
body, but a plateau was reached after 2 days in muscle. Residues in
the hepatopancreas reached 510 mg/kg over the exposure period,
representing a concentration factor of 204 000; over the same period,
50% of the shrimps died. In a separate study, the shrimps were exposed
to 7.5 µg/litre for 16 days followed by an elimination period of 5
weeks in clean water. When calculated on the basis of the total tissue
burden of PCBs, an 80% reduction in the hepatopancreas was found,
concomitant with a doubling of the PCB levels in remaining tissues.
However, when data were presented as a concentration, a linear loss
from the hepatopancreas was seen, with the concentration in other
tissues remaining constant. The authors calculated a half-life for
loss of PCBs from the hepatopancreas of 17 days.
Nimmo et al. (1975) sampled shrimp from Pensacola estuary, USA, and
measured the relative concentration of PCBs in the various tissues.
The hepatopancreas contained the greatest amounts (50-75%) followed by
the ventral nerve. The authors studied the uptake of PCBs by pink
shrimp, experimentally, using various regimes with dosed food or dosed
water. They found the same tissue distribution in pink shrimp that had
been exposed to 0.2 µg Aroclor 1254/litre, in water, for 50 days. They
concluded that most of the PCBs were taken up directly from the water
in both the "wild" and laboratory situation. However, they did not
exclude the possibility of some PCBs being taken up from food, which
was found under some of the laboratory regimes.
To determine whether there was a concentration below which shrimps
would be unable to accumulate PCBs, grass shrimp (Palaemonetes pugio)
were exposed to flowing water concentrations of 0.04, 0.09, or
0.62 µg/litre. Whole-body residues of 0.2, 1.0, and 10 mg/kg,
respectively, were accumulated within 3-5 weeks. Even at the lowest
dose, shrimps accumulated more PCBs than the residues found in control
shrimp. Concentrations in the shrimp did not reach equilibrium during
the 5-week exposure, but the rate of accumulation decreased towards
the end of the exposure. When transferred to clean water, the shrimps
lost most of the PCBs within 4 weeks (Nimmo et al., 1975).
Gammarus oceanus were exposed by Wildish & Zitko (1971) to Aroclor
1254 concentrations of 2.5, 10, or 20 mg/litre for up to 6 h. Uptake
increased with increasing PCB concentration. Uptake decreased to half
of the initial rate after 4-6 h exposure at 20 mg/litre. There was
little or no uptake by dead animals. Although uptake was related to
branchial surface area, branchiae were not necessary sites of uptake,
since uptake could occur at an unchanged rate following branchial
removal. The authors did not find any change in the rate of uptake
during the intermoult stage.
Zhang et al. (1983) exposed Daphnia magna to 14C-labelled
2,2'-dichlorobiphenyl, 2,5,4'-trichlorobiphenyl, 2,4,6,2'-tetra-
chlorobiphenyl, or 2,4,6,2',4'-pentachlorobiphenyl at 50 µg/litre.
Equilibrium was reached after 20 h for all except the pentachloro-
biphenyl, which had not reached equilibrium within 24 h.
Bioaccumulation factors at equilibrium ranged from 3741, for the
dichlorobiphenyl, to 18 144, for the trichlorobiphenyl. Concentration
factors were not significantly related to the water solubility or
chlorine content of the biphenyl, but there was a tendency for the
bioaccumulation factor to increase with chlorine content and
decreasing water solubility. The authors studied the rate of
depuration and found it to increase with increasing water temperature
between 2 and 22°C. The rate of depuration was also faster for the
dichlorobiphenyl than for the pentachlorobiphenyl; after 48 h, the
amount of PCBs remaining in Daphnia was 22% and 77% (at 10-11°C) for
the 2 chlorobiphenyls, respectively.
(b) Uptake from sediment
Sediment was collected from the field and spiked with Phenochlor DP-5
to achieve a final PCB concentration of 0.65 mg/kg dry weight,
compared with 0.2 mg/kg in unspiked sediment (Elder et al., 1979).
Worms (Nereis diversicolor) were then added to aquaria containing
the sediment under flowing seawater. Equilibrium was reached within
40-60 days, by which time both groups had concentrated the PCBs by 3.5
times. Upon transfer from spiked to unspiked sediment, the worms took
2 months to attain body levels of PCBs comparable with those of the
unspiked group. A half-life of approximately 27 days was calculated
for incorporated PCBs.
Fowler et al. (1978) exposed Nereis diversicolor to spiked sediment
containing 9.3 or 80 mg Phenochlor DP-5/kg (dry weight), for 120 days,
compared with 0.11 mg PCB/kg in unspiked sediment. At the beginning of
the study, worms in the unspiked sediment had body residues of
0.59 mg/kg dry weight and reached a steady state at 1.2 mg/kg. Those
exposed to spiked sediment reached a steady state after a period of
approximately 2 months, with concentration factors ranging from 3 to
4. The worms maintained at the highest level of PCBs all died within a
90-day exposure period. When transferred to unspiked sediment for a
2-month period, the worms that had taken up PCBs from the unspiked
sediment lost PCBs exponentially. In a separate study, worms were
exposed to PCBs in water alone at a concentration of 0.57 µg/litre. A
steady state was reached much more quickly (2 weeks) than it was in
the presence of sediment, with a concentration factor of approximately
800. By comparing these results with field monitoring, the authors
calculated the relative importance of the 2 media. They stated that
approximately 99% of the PCBs in these studies was taken up from the
sediment. When the water overlying the spiked sediment was monitored,
28 ng PCBs/litre was measured (not leached, but a contaminant in the
experimental system) reducing the figure of uptake from sediment to
89%.
In a study by Courtney & Langston (1978), 1.1 mg Aroclor 1254/kg was
incorporated into intertidal sand. Specimens of 2 intertidal
polychaetes (Arenicola marina and Nereis diversicolor) containing
mean residues of 0.017 and 0.11 mg PCBs/kg (wet weight), respectively,
were collected. After 5 days in the spiked sediment, they contained
0.24 and 0.36 mg/kg, and, after a further 5 days, 0.39 and 0.49 mg/kg,
respectively. During a 3-week post-exposure period, there was no
significant loss of these PCB residues. The authors achieved
comparable PCB residues in these polychaetes after exposure to
1 µg/litre water or 1 mg/kg sediment.
McLeese et al. (1980) exposed the polychaete worm (Nereis virens)
and the shrimp (Crangon septemspinosa) to sediment containing
0.016-0.58 mg Aroclor 1254/kg (dry weight) for 32 days. Uptake was
found to be dependent on the exposure concentration and, in the case
of the worms, on the exposure period. The accumulation of PCBs was
inversely related to animal size; at 32 days, concentration factors
for worms ranged from 10.8 for 0.6-g worms to 3.8 for 4.7-g worms
following exposure to 0.17 mg PCB/kg. Factors of 3.5 and 1.9 were
found for shrimps weighing 0.1 and 2.9 g, respectively, after exposure
to 0.13 mg Aroclor/kg. Shrimps were found to accumulate, on average,
60% less PCBs than worms per unit weight. During the 26 days following
exposure, there was not any obvious loss of PCBs from the worms.
Rubinstein et al. (1983) collected sediments containing various levels
of pollutants (PCBs, 0.46-7.28 mg/kg dry weight; Cd; Hg) and organic
matter (5.5-22.3%). During a 100-day exposure period, only small
increases in PCB concentrations were detected in hard clam
(Mercenaria mercenaria) and grass shrimp (Palaemonetes pugio).
Higher concentrations of PCBs were accumulated by Nereis virens.
Uptake was found to be more dependent on the organic content of the
sediment than on the exposure concentration. Concentration factors
ranged from 1.59 in a low organic sediment to 0.15 in a high organic
sediment. The authors also calculated the maximum water exposure
concentration eluted from each of the sediments. On the basis of a
concentration factor of 800, calculated by Fowler et al. (1978) for
the uptake from water of Nereis sp., body residues of between 0.007
and 0.034 mg PCBs/kg (wet weight) would have been expected if
accumulation were dependent purely on direct partitioning from water.
However, whole-body residues of PCBs were found to be 0.4-0.63 mg/kg,
suggesting that pathways other than direct uptake from water (e.g.,
ingestion and sorption) contributed significantly to the accumulation
of PCBs by the polychaete.
Freshwater prawns (Macrobrachium rosenbergii) and clams (Corbicula
fluminea) were exposed to contaminated sediments for 48-50 days
(Tatem, 1986). Prawns were exposed to sediment containing
approximately 62 mg PCBs/kg (dry weight) and to the same sediment
diluted with sand to 50 and 10% of the original. Clams were exposed to
100, 50, or 10% of another sediment containing approximately 2 mg
PCBs/kg at 100%. The amount of PCBs accumulated was related to the
exposure concentration, with the highest concentration factors at the
lowest exposure (10%) level. Bioaccumulation factors for prawns ranged
from 0.1 to 0.9 for Aroclor 1242 and from 0.2 to 2.4 for Aroclor 1254,
relative to sediment concentrations. Exposed clams accumulated PCBs
(Aroclors 1242 and 1254) at concentration factors of 0.54-12.52,
relative to sediment. When tissues were analysed for Aroclor 1242 and
1254, maximum concentrations in prawns were attained at 7 and 40 days
for the 2 Aroclors, respectively. Exposure of prawns at 100 and 50%
dilution of sediment killed all the animals after 62 and 70 days,
respectively. Clams survived exposure.
Clark et al. (1986) investigated the accumulation of sediment-bound
PCBs by fiddler crabs (Uca pugilator) and (Uca minax). Mud and
mud/sand sediments were used; both were naturally contaminated with
PCBs and no further PCBs were added. Both species were exposed to a
mud sediment containing 1.04 mg PCBs/kg and to a mud/sand sediment
containing 0.37 mg/kg (dry weight). Concentration factors, after a
28-day exposure, were 0.19 and 0.79, for U. minax, and 0.2 and 0.59,
for U. pugilator, for the 2 sediments, respectively. In a second
study, using mud with 0.97 mg PCBs/kg and mud/sand with 0.55 mg
PCBs/kg, U. pugilator showed concentration factors of 0.58 and 0.71,
respectively, after 28 days. The authors did not find any detectable
PCBs in the overlying water, suggesting that the PCBs are tightly
bound to the sediment and leach out only very slowly. Following
transfer to uncontaminated sediment on day 42, no PCB residues were
detected in U. pugilator on day 56, or in U. minax on day 63.
Lynch & Johnson (1982) exposed the amphipod (Gammarus pseudolimnaeus)
to 2,4,5,2',4',5'-hexachlorobiphenyl added to sediment in flow-through
bioassays. Water overflowing from the tank containing the contaminated
sediment was directed into a second tank where further amphipods were
exposed without sediment. The hexachlorobiphenyl was labelled with
14C and added to the sediment at 1 mg/kg; the system was allowed to
equilibrate for 7-15 days prior to addition of amphipods, which were
sampled from the tanks after 24, 48, 96, and 192 h. In the initial
studies, the specific activity of the labelled hexachlorobiphenyl was
insufficient to detect the hexachlorobiphenyl concentrations in water.
However, it was clear that amphipods in the tank with the sediment
accumulated more hexachlorobiphenyl than animals exposed only to the
water overflow (8.8-10.5 times more PCBs). Removal of organic matter
from the sediment, by combustion, before addition of the PCB,
increased uptake of the hexachlorobiphenyl by increasing the
availability of the material to the Gammarus. In later studies,
specific activity was increased and water concentrations could be
measured. These were very low, ranging between 11 and 35 ng/litre in
the upper tank and 9 and 25 ng/litre in the lower tank. The lower end
of this range was found later in the exposure period suggesting that
less hexachlorobiphenyl was released over time. There was little
difference in concentration between water taken from the surface and
that sampled close to the sediment suggesting rapid mixing of the
overlying water. In this later series of studies, the authors
demonstrated that both the organic matter content of the sediment and
the presence of smaller particle sizes (silt and clay) reduced uptake
of hexachlorobiphenyl by the amphipods. Organic matter was the more
important factor. Adding maple leaves, to give about 70% organic
content in the sediment, reduced hexachlorobiphenyl uptake to between
10 and 20% of that in sediment without organic matter. Very high
bioconcentration factors were calculated relative to the very low
water concentrations of hexachlorobiphenyl (ranging between 27 000 and
1 000 000 in the upper tank and 2000 and 460 000 in the lower tank,
increasing with exposure period). These factors would be very low
relative to sediment concentrations of the PCB. However, it is clear
that the amphipod can accumulate hexachlorobiphenyl, leaching in very
small amounts from contaminated sediment.
Cores of lake sediment complete with overlying water were taken by
Larsson (1984) and transported back to the laboratory, still in the
sampling tube. PCBs were introduced at different dose levels by
injection through silicon septa in the walls of the tubes and spread
evenly 10 mm below the surface. The cores were allowed to stabilize in
the dark for 1 week at which time 80-100 chironomid larvae were
introduced. After 8 weeks, the systems were moved and kept at 20°C in
the light. After 2 days, the chironomid larvae began to pupate and
emerge. The study was terminated after 10 weeks. PCBs were measured in
sediment, larvae, adults, and exuviae (discarded skins after
emergence). Ranges of PCBs in sediment were between 0.5 and 14 mg/kg
giving rise to residues in larvae, exuviae, and adults directly
related to sediment concentrations. There was "biomagnification"
between larvae and adult. There was loss of body weight between the
final larval stage and the adult, but little loss of PCBs (only 17%
was retained in the exuviae). The author stated that the low variation
in uptake between animals is an indication of passive physicochemical
factors being involved in the handling of PCBs by chironomids. Active
uptake via ingestion would be expected to lead to more variation in
results. Meier & Rediske (1984) also monitored the uptake of PCBs from
contaminated sediment into chironomid larvae (Glyptotendipes
barbipes). Concentration factors for Aroclor 1242 from sediment
ranged between 20 and 130 for exposures of between 0.01 and 1.0 mg/kg,
considerably lower than concentration factors relative to water
(10 000 for these organisms) (Sanders & Chandler, 1972). Addition of
oil, commonly found in polluted areas where PCBs spills are likely,
reduced the uptake of PCBs from the sediment.
(c) Uptake from food
A detritus diet containing 17 µg Aroclor 1242/kg (wet weight) was fed
to male fiddler crabs (Uca pugnax) for 34 days (Marinucci & Bartha,
1982). The Spartina detritus was placed in the culture system at the
start of the study and, because of rapid depletion, was renewed after
19 days of exposure. Since PCBs leached continually from the food
source into the water, a second study was carried out to examine the
uptake of PCBs from water alone. Contaminated detritus was mixed
thoroughly with water and allowed to equilibrate for 24 h. Water
levels were found to be 14-15 µg/litre. Aroclor 1242 was accumulated
at a more rapid rate from PCB-laden detritus than from water alone.
The linear accumulation rate from litter was calculated to be 1 µg
PCBs/day per animal whereas, from water alone, the uptake was 0.1 µg
PCBs/day per animal. Aroclor 1242 was highly concentrated in the
hepatopancreatic tissue. It was found that the PCB residue in the
crabs was inversely related to their weight. Comparison of the
concentrations of PCBs in animals of the same weight shows that, at
the end of the 34 day exposure, those exposed to water alone had taken
up approximately half of the PCBs of those exposed to detritus. The
authors concluded that the crabs in the study accumulated a similar
amount of PCBs from both the food and the water.
Pinkney et al. (1985) exposed the amphipod Gammarus tigrinus to
Aroclor 1254 (14C-labelled) in fungus (Fusarium oxysporum) as a
food item. The fungus contained 195.8 mg Aroclor/kg dry weight.
Accumulation of PCBs was rapid, reaching a constant level in the
amphipods of 23 mg/kg after 9-24 h. Similar exposure of the amphipods,
but with exclusion from direct contact with the fungus by Teflon mesh
(to monitor uptake of PCBs leached into the water), resulted in
residues of between 0.16 and 3.3 mg/kg (from concentrations in the
water at 0.03 µg/litre), representing between 0.6 and 13.9% of uptake
from water and food combined. The PCB residues in the amphipods were
also monitored over 144 h on uncontaminated food to measure the
elimination rate. The water was changed every 24 h. Within this
period, 57% of the accumulated PCBs was eliminated.
(d) Comparison of different routes of uptake
In a study by Wyman & O'Connors (1980), the uptake by the marine
copepods Acartia tonsa and Acartia clausi of 14C-labelled Aroclor
1254 from water, inorganic sediment, and food, was monitored over a
period of 48 h. Acartia were exposed to water concentrations of
10 µg PCBs/litre. An asymptotic uptake curve was observed; equilibrium
was reached after 36 h, corresponding to whole-body residues of 248 mg
PCBs/kg (dry weight) for A. tonsa and 223 mg/kg for A. clausi.
During exposure, water concentrations fell rapidly to 5 or 6 µg/litre.
A similar pattern of uptake was found after exposure to sediment
contaminated with 20 mg PCBs/kg with maximum levels of PCBs in
A. tonsa of 22 mg/kg after 30 h. As in the water exposure, levels of
PCBs in sediment fell rapidly from 20 mg/kg to 14 mg/kg and then
slowly to 7 mg/kg at the end of the study. Water levels were initially
0.62 µg/litre and fell to 0.15 µg/litre. Uptake of PCBs by A. tonsa
from phytoplankton contaminated with 80 mg PCBs/kg (wet weight) was
very rapid and reached a maximum after 5 h at 61 mg/kg, but
subsequently declined after exhaustion of the food supply. PCB
concentrations in water were similar to those found when copepods were
exposed to contaminated sediment, copepods exposed to these water
concentrations alone accumulated significantly less PCBs than those
fed PCB-dosed phytoplankton.
McManus et al. (1983) exposed the marine copepod Acartia tonsa to
14C-Aroclor 1254 either in the food, as phytoplankton containing
approximately 1.3 mg PCBs, or in water at 1.5 µg/litre, for a period
of 30 h. For copepods exposed to contaminated phytoplankton, PCB
levels ranged from 117 to 163 mg/kg dry weight. For copepods exposed
to contaminated water alone, levels ranged from 82 to 104 mg/kg. When
transferred to clean water, the authors found that copepods lost PCBs
at a significantly faster rate if they were fed during depuration;
after 36 h, PCB concentrations in copepods fed during deputation were
10 mg/copepod whereas those starved contained 30 mg/copepod. No
significant difference in depuration rate was found between those
exposed via food and those exposed via water. In a second study,
elimination in males and females was compared. Although both sexes
contained similar residues at the start of depuration (117 mg/kg and
95 mg/kg, respectively), after 36 h, females contained significantly
lower levels of PCBs than males. During depuration, faecal pellets and
eggs were analysed; similar levels of PCBs were found in both male and
female faecal pellets during this period, but levels of PCBs more than
four times that in the females were found in eggs (407.5 mg/kg dry
weight after 4 h), indicating that egg production is an important
route for PCB elimination.
4.2.4.4 Fish
Fish of all life stages have been shown to take up PCBs readily from
water; bioconcentration factors are high. Time taken to reach
equilibrium is variable, but often long, in excess of 100 days. PCBs
with greater chlorination are more readily taken up and retained. PCB
body burden tends to increase with age and levels are higher in fish
with a greater lipid content. The accumulated PCBs are concentrated in
lipid-rich tissues. PCBs of lower chlorination are eliminated more
rapidly. Loss of PCBs is evident when exposure ends; an initial rapid
loss is followed by a slower rate of loss. Half-life estimates,
therefore, vary greatly, from a few weeks to several years.
Reproduction, with the production of a large mass of eggs or sperm,
allows loss of substantial amounts of the PCB residue. Depending on
the species, habitat, and behaviour, PCBs can be taken up from water,
sediment, or food to different degrees.
(a) Uptake from water
Califano et al. (1980) maintained larval striped bass (Morone
saxatilis) in Hudson river water (filtered and unfiltered)
contaminated with 14C-Aroclor 1254 at 1.36 µg/litre for a period of
48 h. Whole-body residues for filtered and unfiltered water were not
significantly different at 5 mg/kg and 5.9 mg/kg, respectively. Uptake
between 34 and 48 h was very slow, suggesting a steady state had
already been reached. Exposure of fish for a further 72 h in
unfiltered water, supported this theory. Elimination was slow, only
18% being lost in 48 h following a 24-h exposure.
The PCB uptake pattern in lake trout (Salvelinus namaycush) sac fry
was studied by Mac & Seelye (1981) by exposing them to a nominal
concentration of 50 ng Aroclor 1254/litre for 48 days. Patterns of
accumulation were similar, regardless of how the data were expressed
(wet weight, dry weight, or body burden). PCBs levels increased
slowly, reaching a peak after 32 days (just before completion of yolk
absorption), and then decreased by day 48.
Hansen et al. (1975) exposed different life-stages of sheepshead
minnow (Cyprinodon variegatus) to Aroclor 1016 (Table 10). After a
4-week exposure to nominal concentrations of 1, 3.2, or 10 µg/litre,
adult fish laid eggs containing on average 4.2, 17, and 66 mg/kg,
respectively. DeFoe et al. (1978) exposed fathead minnow (Pimephales
promelas) to Aroclor 1248 or 1260 at concentrations of
0.1-3 µg/litre, for 240 days (life cycle). Bioconcentration factors for
the uptake of PCBs were independent of the PCB concentration in the
water. Residues in the fish reached an apparent steady state within
about 100 days of exposure and growth. Females accumulated about twice
as much PCBs as males, because of their higher body lipid content. The
variability of residues in females reflected the variability of their
lipid content. Although mechanisms for uptake were similar for both
Aroclors, greater body burdens were always achieved with exposure to
Aroclor 1260. Bioconcentration factors ranged from 60 000 to 160 000
for males and from 120 000 to 270 000 for females. After transfer to
clean water, 18% of Aroclor 1248 was lost within 28 days and 15% of
Aroclor 1260 in 42 days. The authors stated that, because of
variations between fish, this 10-20% decline in total body burden of
PCBs was insufficient to indicate definite PCB elimination over this
period.
De Kock & Lord (1988) exposed an estuarine fish, the Cape stumpnose
(Rhabdosargus holubi) to a flowing water concentration of 1 µg
Aroclor 1260/litre for 90 days followed by a 90-day period in clean
water. Equilibrium was reached at 90 days with a concentration factor
of 24 000. The depuration rate was calculated to be 0.014 days,
producing a half-life of 50 days.
Goldfish (Carassius auratus) were exposed to Clophen A50 at levels
of 0.01, 0.05, 0.1, or 0.5 mg/litre for 18 days (Hattula & Karlog,
1973). Rapid uptake was observed with concentration factors of over
1000 at 18 days, but equilibrium was not achieved within this period.
Nearly all the fish exposed to 0.5 mg/litre died within 7 days. After
transfer to clean water, fish that had been exposed to 0.1 mg/litre
for 13 days and had attained body residues of 70 mg/kg lost half of
the PCBs within 3 weeks, but still retained levels of approximately
15 mg/kg, after 70 days.
Yoshida et al. (1973) exposed carp (Cyprinus carpio) to 14C-PCBs
(equivalent to Aroclor 1254) in water or in food. By measuring the
radioactivity, they found similar tissue patterns of uptake from both
water and diet. PCBs were localized in the gall bladder, adipose
tissue, and hepatopancreas and, in particular, the adipose tissue of
the skull.
Hansen et al. (1971) exposed spot (Leiostomus xanthurus) to Aroclor
1254 at 1 µg/litre, for 56 days. Maximum tissue levels of PCBs were
achieved between days 14 and 28. Highest levels were found in the
liver (210 mg/kg, after 28 days) followed by the gills, whole fish,
heart, brain, and muscle. Aroclor 1254 was slowly lost from tissues;
after 84 days in clean water, levels of PCBs had dropped by 73%.
In a study by Braun & Meyhofer (1977), rainbow trout (Salmo
gairdneri) fingerlings were exposed to water concentrations of 2 or
20 µg Clophen C/litre, for 8 weeks. Tissue PCB concentrations for
gills, muscle, and liver were found to be 0.62, 0.82, and 3.47 mg/kg,
respectively, for the lower dose and 12.3, 7.6, and 10.6 mg/kg, for
the higher dose. When fish were held in clean water for 10 weeks,
following exposure to 2 µg/litre for 8 weeks, residues decreased by
half in the liver and had disappeared completely from the gills, but
there was no change in the PCB levels in muscle.
Rainbow trout (Salmo gairdneri) were exposed by Guiney et al. (1977)
to 14C-labelled 2,5,2',5'-tetrachlorobiphenyl at 0.5 mg/litre for
36 h. The tissue distribution of 14C was measured at regular
intervals after transfer to clean water. Carcase, muscle, skin, lower
gastrointestinal tract, and fat contained most of the radioactivity
(88%). During the first 14 days after exposure, radioactivity
increased in adipose tissue, carcase, and eyes. Elimination from most
tissues appeared to be biphasic with a 30% loss within 2 weeks
followed by a loss of only 6% in the following 126 days. Losses from
the bile and blood were very rapid and nearly complete within 14 days.
Based on the initial rate of loss, the authors calculated a half-life
of 1.55 days, however, the second phase of eliminated PCBs suggested a
half-life at 2.66 years. In a similar study, Guiney et al. (1979)
calculated half-lives of 1.76 and 1.43 years for female and male
rainbow trout, respectively, based on fish sampled 2-34 weeks after
exposure. For both sexes the half-life of elimination was recalculated
to 0.52 and 0.54 years between weeks 38 and 52 after exposure (the
spawning season). The increased elimination appeared to be because of
loss via eggs and sperm. Vodicnik & Peterson (1985) found a similar
result after dosing yellow perch (Perca flavescens); an elimination
half-life of 22 weeks was calculated. This was later recalculated to
be <0.7 weeks during spawning, returning to 16.3 weeks after the
completion of spawning.
(b) Uptake from sediment
The uptake of Aroclor 1254 from suspended solids by juvenile Atlantic
salmon (Salmo salar) was studied by Zitko (1974). Aroclor 1254 was
mixed with suspended solids (simulated by SilicAR CC7) in hexane at
5 mg/ml. Fish were exposed to contaminated solids at 1 g/litre for up
to 144 days. Over this exposure period, the salmon accumulated 134 mg
Aroclor 1254/kg.
Stein et al. (1984) exposed English sole (Parophrys vetulus) to a
sediment concentration of 1 mg 14C-Aroclor 1254/kg (dry weight).
Seawater was allowed to flow over the sediment for 6 days before the
fish were added. A steady state of PCBs accumulated in the tissues of
the fish was achieved after 10 days of exposure. Highest residue
concentrations were found in the bile and the liver. Concentration
factors were 10 for the bile and 4 for the liver, with other tissues
individually concentrating PCBs by factors of 3 or less. Simultaneous
exposure of sole to PCBs and 3H-benzo[ a]pyrene (3 mg/kg, dry
weight) reduced the amount of PCBs accumulated. Stein et al. (1987)
collected urban sediment containing aromatic hydrocarbons and PCBs at
32 mg/kg and 2.2 mg/kg dry weight, respectively. English sole
accumulated hepatic concentrations of 1.4 mg PCBs/kg (wet weight) over
a period of 108 days exposure to the urban sediment. This was 8 times
the PCBs accumulated by sole exposed to the control sediment, which
did not contain any detectable PCBs. In another study, the same
authors added a 14C-labelled PCBs tracer to the urban sediment. The
concentration of PCB-derived radioactivity in the liver reached a
steady state after 14 days of exposure; the steady state concentration
in the carcase was found to be significantly lower.
(c) Uptake from food
Lieb et al. (1974) fed rainbow trout Salmo gairdneri on a diet
containing 15 mg Aroclor 1254/kg for 16 or 32 weeks. PCB levels in the
lipid fraction increased rapidly for the first 8 weeks, reaching
equilibrium at about 95 mg/kg. The absolute quantity of PCBs continued
to increase as the fish grew. The trout had retained 68% of the total
PCBs ingested at equilibrium. No elimination was found after transfer
to uncontaminated food at 16 weeks (for a period of 16 weeks), or
after starving the fish for 8 weeks following exposure for 32 weeks.
Reductions in PCB levels were found, but these were cancelled out by
concomitant reductions in lipid content.
Coho salmon (Oncorhynchus kisutch) parr were fed 10 mg chloro-
biphenyls/kg (containing equal parts by weight of 3,4,3',4'-tetra-
chlorobiphenyl, 2,4,5,2',4',5'-hexachlorobiphenyl, and
2,4,6,2',4',6'-hexachlorobiphenyl) for up to 165 days (Gruger et al.,
1975). Most of the PCBs were accumulated in the adipose tissue of the
salmon (51.1 mg/kg total chlorobiphenyls after 165 days). Tissue
levels of tetrachlorobiphenyl were about half those of either of the
two hexachlorobiphenyls throughout the exposure period. When fish were
starved for 48 days, the data indicate mobilization or transformation,
with, for example, chlorobiphenyls in the spleens lowered by half and
in adipose tissue increased 5-fold. Most tissues showed an increase in
PCB levels, especially blood levels. In contrast, when a second group
of salmon were fed on a clean diet, chlorobiphenyls were released from
adipose tissue and levels increased in some other tissues, such as the
lateral line dark muscle tissue. The ratio of the different
chlorobiphenyls remained unchanged during both of these post-exposure
treatments. Gruger et al. (1976) fed juvenile coho salmon diets
containing a mixture of 2,5,2',5'-tetrachlorobiphenyl, 2,4,5,2',5'-
pentachlorobiphenyl, and 2,4,5,2',4',5'-hexachlorobiphenyl
at 1, 2, and 12 mg/kg, for up to 72 days. A steady state appeared to
have been reached between 17 and 35 days at the lowest dose (a whole
body concentration of approximately 0.45 µg/kg (wet weight)); steady
state was not achieved at the other 2 dose levels. All 3
chlorobiphenyls were accumulated to similar levels. Comparing these
data with the study by Gruger et al. (1975), suggests that the
position of the chlorine substitution is an important factor.
Hansen et al. (1976a) fed channel catfish (Ictalurus punctatus) on a
diet contaminated with 20 mg Aroclor 1242/kg. The total burden of PCBs
(µg PCB/fish) increased exponentially with exposure time. When fish
were placed on a clean diet (from day 84 for 56 days) a slight net
decrease in body burden was observed, but levels remained constant
when fish were placed on a clean diet for 56 days after 140 days
exposure. On return to a PCB-contaminated diet, accumulation rates
returned to those previously observed. The authors noted that, during
PCB-free periods, there was a shift in residues from edible carcase to
offal.
Mayer et al. (1977) fed fingerling coho salmon with Aroclor 1254 at
concentrations ranging between 1.45 and 14 500 µg/kg body weight.
Equilibrium was reached after 112 days at concentrations of 1.45,
14.5, and 145 µg/kg, with whole body residues of 0.47, 0.5, and
3.8 mg/kg, respectively. A steady state was reached at the 2 highest
dose levels of 1450 and 14 500 µg/kg after 200 days, with
corresponding residues of 57 and 659 mg/kg. In another study, channel
catfish (Ictalurus punctatus) were exposed to Aroclors 1232, 1248,
1254, and 1260 in the diet at concentrations of 48 or 480 µg/kg body
weight, for 193 days. Equilibrium was only achieved at the lowest
exposure dose of Aroclor 1232, within 150 days, with a whole-body
burden of 4.5 mg/kg. Similar whole-body residues were achieved at the
lowest dose of the other Aroclors, but no steady state was reached. At
the higher dose, accumulation increased in the order Aroclor 1232 =
1248 < 1254 < 1260, with residues ranging from 13 to 32 mg/kg after
193 days.
When Zitko (1974) fed juvenile Atlantic salmon (Salmo salar) diets
containing 10 or 100 mg Aroclor 1254/kg, accumulation reached
equilibrium within 30 days at the lower dose, with a whole-body
residue of approximately 3.8 mg/kg. Equilibrium was not reached within
200 days at 100 mg PCBs/kg. A whole-body residue of 30 mg/kg was
recorded at 181 days.
Zinck & Addison (1974) administered a mixture of 2-, 3-, and
4-chlorobiphenyl to thorny skate (Raja radiata) and winter skate
(Raja ocellata) by intravenous injection. All three congeners were
cleared rapidly from blood plasma, 3-chlorobiphenyl consistently being
cleared more rapidly than the other two. Less than 6% of
3-chlorobiphenyl remained in the plasma after 15 min compared with 30%
for the other chlorobiphenyls. All three accumulated in the other
tissues of R. radiata, principally in the liver and muscle. Tissue
levels of 3-chlorobiphenyl were consistently less than the others
during the 53-h sampling period.
In a study by Guiney & Peterson (1980), both yellow perch (a non-fatty
fish) and rainbow trout (a fatty fish) were dosed with 0.8 µg of
14C-labelled 2,5,2',5'-tetrachlorobiphenyl, either orally or by
intraperitoneal injection. Whole-body elimination was found to be
similar for both species and routes. A 20-30% elimination was observed
after 3-4 days with virtually no more PCBs being eliminated during the
rest of the 32-day sampling period. Tissue distribution varied between
the 2 species; uptake in the perch was mainly concentrated in the
viscera and carcase, whereas, in the trout, skeletal muscle and
carcase were the major sites of uptake.
Niimi & Oliver (1983) calculated the biological half-life of 31
dichloro- to decachlorobiphenyl congeners, 105 days after a single
oral dose of 46-261 mg/kg was administered to rainbow trout (Salmo
gairdneri). Whole-body half-lives increased from 5 days to >1000
days as the number of chlorines on the biphenyl increased. From
structure-activity analysis of half-lives in whole fish, the authors
concluded that elimination was enhanced for congeners with a lower
chlorine content and no chlorine substitutions in the ortho
positions, and for those with 2 unsubstituted carbons adjacent on the
biphenyl.
4.2.4.5 Birds
PCBs are taken up from contaminated food or water and concentrated in
the fatty tissues of birds. PCBs of higher chlorination levels are
accumulated to a greater extent. Egg-laying females can lose
substantial amounts of PCBs from body tissues by transferring the PCBs
to the eggs. Redistribution of residues occurs on starvation (of
significance during the migration of birds in the wild). Expressed as
a whole-body concentration, PCB residues fall during starvation.
However, expressed as a concentration in fat, residues rise. Most
critically, PCB residues in the brain increase during starvation and
this may kill the birds without further intake of PCBs.
Brunström et al. (1982a) injected the yolk of developing hens' eggs,
on day 4 of incubation, with 14C-labelled 2,4,2',5'-tetra-
chlorobiphenyl at a concentration of 5 mg/kg. One hour after
injection, radioactivity was found in the sub-blastodermic fluid, the
highest concentrations being in amniotic membranes. None was present
in the yolk, albumen, or embryonic tissues. Uptake was uniform
throughout the embryo, after one day, and, as tissues developed,
became concentrated in certain of them, such as the liver, kidney, and
fluid brain vesicles, by day 7. 14C was found uniformly in the yolk
after 11-14 days and was highly concentrated in the first bile
produced on day 11. The labelled PCBs accumulated in fatty tissue as
it developed from day 14 onwards. In the hatched chick, large amounts
of radioactivity were found to be concentrated in the gall bladder,
intestine, cloaca, and the coiling of the gizzard. When either
3,4,3',4'-tetrachlorobiphenyl or 2,4,2',5'-tetrachlorobiphenyl was
injected into the air sac of hens' eggs on day 14 of incubation at
0.4 mg/kg, no difference in distribution pattern was observed 1-5 days
later (Brunström & Darnerud, 1983). The highest amounts of
radioactivity were found in the fatty tissue, liver, kidneys, and the
gall bladder, 14C was also found in the bone marrow, the adrenals,
and the gonads, but to a lesser extent. The yolk contained less
radioactivity than the yolk analysed in the previous study by
Brunström et al. (1982a), because the PCBs were administered via the
air sac.
White leghorn hens were exposed to 50 mg Aroclor 1254/litre in their
water for 6 weeks (Tumasonis et al., 1973). PCB residues in the yolks
of eggs laid increased during the exposure period to a peak, after 6
weeks, of approximately 205 mg/kg. When hens were given clean water,
the yolk levels of PCBs quickly dropped within 5 weeks to
approximately 100 mg/kg, and then more slowly until, after 20 weeks
without Aroclor 1254 in their water, the hens laid eggs containing
0.7 mg/kg.
During a 4-week exposure to Aroclor 1242, 1254, or 1260, in the feed
of one-day-old chicks, Harris & Rose (1972) found that PCBs
accumulated in the fat and that this accumulation increased with
increasing exposure concentrations of 100, 200, and 400 mg/kg. At the
2 highest dose levels, the hens accumulated more of Aroclor 1260 than
of the other 2 Aroclors (i.e., 482, 1427, and 2151 mg Aroclor 1260/kg
at the 3 exposure concentrations, respectively). At the highest dose,
there was high mortality during exposure to Aroclor 1242 and 1254 and
this might have affected the residues found.
Greichus et al. (1975) fed white pelicans (Pelecanus erythrorhynchos)
on a fish diet containing 100 mg Aroclor 1254/day, for 10 weeks. PCB
residues were measured in the carcase, liver, feathers, and brain;
mean residues found were 2130, 290, 120, and 110 mg/kg wet weight,
respectively.
In a study by Dahlgren et al. (1972), 11-week-old pheasant (Phasianus
colchicus) were dosed with one capsule per day containing 210 mg of
Aroclor 1254. Birds that died between days 1 and 5 contained, on
average, PCB residues of 520 mg/kg in the brain, 2500 mg/kg in the
liver, and 140 mg/kg in muscle. Birds that were sacrificed over the
same period had mean brain, liver, and muscle PCB levels of 370, 1900,
and 83 mg/kg, respectively. All birds dosed with only 10 mg of Aroclor
1254 per day died within 180 days and contained average brain and
liver residues of 360 and 1200 mg/kg, respectively.
Södergren & Ulfstrand (1972) fed robins (Erithacus rubecula)
mealworms containing 1 µg of Clophen A50/day for 15 days. Brain,
breast muscle, and carcase were analysed and contained mean PCB
residues of 0.35, 0.55, and 4.5 mg/kg fresh weight, respectively. A
second group of robins was starved following dosing and all died
within 48 h. PCB levels were higher in the brain and breast muscle at
1.1 and 1.3 mg/kg, respectively, but carcase PCB levels were lower on
a fresh weight basis at 2.6 mg/kg. When the carcase lost some of its
fat content during starvation, PCB levels in terms of fresh weight
decreased. Consequently, because of the low remaining fat content,
residue levels in terms of fat weight increased. Another group of
birds were fed both PCBs and DDT (10.5 µg/day) for 15 days and then
starved. PCB levels in all 3 tissues analysed were higher than those
in birds administered PCBs alone followed by starvation; residues
were: brain, 9.3 mg/kg fresh weight, breast muscle, 8.8 mg/kg, and
carcase, 4.5 mg/kg.
Cormorants (Phalacrocorax carbosinensis) were kept on a fish diet
contaminated with PCBs for one month, followed by gelatin capsules of
PCBs administered daily for the remainder of the exposure (Koeman et
al., 1973). After 14 weeks, the dose rate of Clophen A60 was increased
periodically during the exposure period from 200 to 500 mg/kg. The
birds died between days 55 and 124, and overall residues of PCBs
increased in the tissues, the longer the birds survived. Total-body
residues ranged from 850 to 2750 mg PCBs/kg (wet weight) at death.
Brain and liver residues ranged from 76 to 180 mg/kg and from 210 to
290 mg/kg, respectively. The fat of 2 birds was analysed for PCBs and
was found to contain 10 300 and 20 500 mg/kg.
Harris & Osborn (1981) dosed wild puffins (Fratercula arctica) by
implantation with 30-35 mg of Aroclor 1254. PCBs were quickly taken up
in fat, with concentrations rising to 10-14 times that in control
birds (highest fat residue 654 mg/kg wet weight), and remaining at
this level for up to 10 months. Levels slowly declined, but were still
twice those of controls after 34 months. PCB concentrations in the
liver and muscle tissue were highest shortly after dosing (48.4 and
25.2 mg/kg, respectively) and declined until, after 16 months, no PCBs
were detectable. Levels of PCBs in the kidneys and brain were variable
with no consistent trends.
Common grackles (Quiscalus quiscula), starlings (Sturnus vulgaris),
red-winged blackbirds (Agelaius phoeniceus), and brown-headed
cowbirds (Molothrus ater), were fed diets containing 1500 mg Aroclor
1254/kg over an 8-day period (Stickel et al., 1984). PCB residues in
the brains of birds that died were found to be higher than those in
birds that were sacrificed over a similar period. PCB residues ranged
from 349 to 763 mg/kg in birds that died and from 54 to 301 mg/kg in
birds sacrificed. Liver and whole-body residues tended to be higher in
birds that died, but they overlapped to a large extent. PCB residues
in whole bodies on a lipid basis showed the most clear-cut difference,
ranging from 22 600 to 98 600 mg/kg for birds that died and from 6690
to 22 500 mg/kg for those sacrificed. PCB residues in grackles
declined slowly, when the birds were placed on a clean diet. From a
whole-body level of 1300 mg/kg, residues declined to 169 mg/kg, 224
days later. The rate of decline was irregular, but a half-life was
estimated at 89 days over this period of loss.
4.2.4.6 Mammals
Olsson et al. (1979) fed mink (Mustela vison) on a diet containing
11 mg PCBs/kg for 66 days. Mink accumulated 310 mg PCBs/kg in
extractable fat over the exposure period. Control mink were found to
contain 14 mg PCBs/kg, and, when the control feed was analysed, it was
found to contain 0.05 mg PCBs/kg. The authors also found a significant
increase in cadmium uptake in the kidneys of PCB-treated animals
compared with controls. In another study on mink (Mustela vison),
Hornshaw et al. (1983) administered various PCB-contaminated fish
diets containing between 0.21 and 1.5 mg PCBs/kg. Adipose tissue
samples were taken after 6-8 weeks and after 18 weeks exposure (Table
11). The amount of PCBs accumulated was directly related to the amount
of PCBs in the diet; mean PCB residues ranging from 4 to 24.8 mg/kg
after 6-8 weeks and from 8.1 to 42.8 mg/kg after 18 weeks. When
expressed as individual congeners, it can be seen that the mink showed
the highest accumulation of the PCBs with the chromatographic peak
corresponding to 2,4,5,2',4',5'-hexachlorobiphenyl. To determine the
rate of PCB elimination, male mink that had been on a fish diet
containing 1.5 mg PCBs/kg for 10 weeks were transferred to a control
diet. Over this period, adipose tissue residues of 32 mg PCBs/kg had
accumulated. Over the 16-week elimination period, 60.3% of the total
PCB burden of the adipose tissue was eliminated. This consisted of a
loss of 87.2% of 2,5,2',5'-tetrachlorobiphenyl, 88.9% of
2,3,6,2',5'-pentachlorobiphenyl, and 55.4% of the hexachlorobiphenyl.
The half-life for total PCBs in mink adipose tissue was calculated to
be 98 days.
Wren et al. (1987a,b) fed mink on a commercial mink food supplemented
with 1 mg Aroclor 1254/kg for a period of 6 months. Male mink had
liver residues of 1.98 mg PCBs/kg after 118 days and 2.8 mg/kg after
183 days exposure. The liver of a female, analysed on day 161
contained a residue of 3.1 mg PCBs/kg. Liver PCB levels in 5-week-old
kits were similar to those in adult mink fed the experimental diet for
several months. Bleavins et al. (1981) measured the relative
importance of placental transfer and milk in the transfer of PCB
residues from mother mink to offspring. Newborn kits contained less
than 0.1% of a dose of PCBs injected into the mother mink. At 2 weeks
of age, the kits contained 1.2% of the dose given to the mother,
suggesting that lactation is a major route of exposing the young to
PCBs and a major route for the loss of PCBs from the mother. Placental
transfer of PCBs was greater in the ferret than in the mink (Bleavins
et al., 1984). The ratio of placental to mammary transfer was 1:15 for
offspring whose mothers were dosed during the first trimester of
pregnancy and 1:7 for mothers exposed during the last trimester.
Big brown bats (Eptesicus fuscus) were fed on mealworm diets
containing 9.4 mg Aroclor 1254/kg for up to 37 days (Clark & Prouty,
1977). In bats sacrificed on day 37, residues ranged from 29 to 121 mg
PCBs/kg (wet weight) for the carcase and from not detectable to
4.2 mg/kg in the brain. Bats that were starved following exposure
showed a significant correlation between increasing brain PCB
concentrations and carcase lipid concentrations. The authors stated
that PCBs increased in brain tissue as carcase fat was metabolized.
Clark (1978) exposed pregnant big brown bats to a mealworm diet
containing 6.36 mg Aroclor 1260/kg for approximately 18-28 days, until
the young were born. Mean carcase levels of PCBs were 20.34 mg/kg in
parent females and 4.38 mg/kg in litters. Levels of PCBs in both
adults and young continued to rise throughout the sampling period; the
longer the gestation time, the higher the PCB level in the sample.
4.2.5 Appraisal
Experimental work on mammals has been concentrated on terrestrial
species. Problems with PCB toxicity are important for marine mammals,
but these are less convenient for experimental study. Results in this
section, therefore, have to be related to field observations on marine
species.
Mink take up more chlorinated components of PCB mixtures and can
accumulate large residues of PCBs. On cessation of exposure, more
tetrachloro- and pentachlorobiphenyls were eliminated than
hexachlorobiphenyl. The half-life for total PCBs was calculated to be
98 days. PCB residues are transferred from mother to offspring. The
relative importance of transplacental transfer and transfer in milk
varies between species. Redistribution of residues takes place on
starvation, which is of significance for migratory species; brain
residues, which may be fatal with no further intake of PCBs, increase
as animals are starved.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Levels in the environment
PCBs were detected in the environment in the late 1960s (Risebrough et
al., 1968; Jensen et al., 1969) and, within a short time, were
reported as contaminants in almost every component of the global
ecosystem including air, water, soil, fish, wildlife, human blood,
adipose tissues, and milk (Holdrinet et al., 1977; Wassermann et al.,
1979; Ballschmitter et al., 1981; Buckley, 1982; Safe, 1982b; Bush et
al., 1985; Kannan et al., 1988; Tanabe, 1988).
The lipophilic properties of PCBs are the basis of the bioaccumulation
and biomagnification that has been demonstrated and, thus, numerous
sources within the environment can lead to human exposure.
High-resolution, gas-chromatographic analysis has shown that the
congener composition and relative concentrations of the individual
components in many PCB extracts from environmental samples differ
markedly from those in the commercial PCBs (Jensen & Sundström, 1974a;
Wolff et al., 1982a; Safe et al., 1985a; Brown et al., 1987a,b).
A major problem with data concerning PCB levels in environmental
samples is that they normally are only available for "total PCBs" and
that there are much fewer data on actual "PCB patterns". Moreover,
when comparing results produced from different laboratories or from
the same laboratory at different times, an additional difficulty may
arise from differences in the sampling and analytical techniques used.
It is difficult, if not impossible, to compare data obtained with
different analytical methods, from different laboratories, and
countries. Nowadays, the older data seem less reliable, especially in
the light of the use of improved analytical methods and better
sampling techniques (WHO/EURO, 1987). A comprehensive review of world
PCB levels was published by Wassermann et al. (1979).
5.1.1 Air
PCB concentrations in air differ markedly from location to location,
with the lower levels found over the oceans or over non-industrialized
regions, such as the Canadian Northwest territories. In general,
levels over industrialized areas or over landfills are the highest.
Apparently, these levels influence PCB levels in rainwater and there
is a gradient of values in air from industrial to rural areas. Some
typical values can be found in Table 12.
MacLeod (1981) described a method for the analysis of PCBs using
low-volume, indoor air sampling to estimate the presence of PCBs in
indoor air in work-places and homes in the USA. Three facilities, an
industrial research facility, an academic facility, and a shopping
complex were sampled. The periods of sampling ranged from 2 days up to
6 months. The average concentrations (calculated as Aroclor 1242 plus
Aroclor 1254) ranged from 44 up to 240 ng/m3. Outdoor levels of up to
18 ng/mg3 were found. In the homes, air samples from 14 areas (of
which 9 were kitchens) were also analysed. The average concentrations
in the kitchens ranged from 150 up to 500 ng/m3 and, in the other
rooms, from 39 to 170 ng/m3. In a library, a level of 400 ng/m3 was
found.
The levels of PCB exposure that may occur in buildings in the USA were
determined by Oatman & Roy (1986). Air samples and surface wipe
samples were taken in 5 state-owned, office buildings and 2 elementary
schools. The average levels of airborne PCBs in buildings with PCB
transformers were nearly twice the levels in buildings without
transformers, i.e., 457 ± 223 and 229 ± 106 ng/m3, respectively. The
mean of the surface wipes taken in buildings without PCB transformers
was 0.17 and that in the buildings with transformers 0.23 µg/100 cm2.
There was a wide variation between the different buildings and, as
shown above, the presence of transformers influenced the indoor PCB
concentrations.
5.1.1.1 Rain and snow
In the Netherlands, at Bilthoven, the PCB-concentrations in rainwater
ranged from 0.01 to 1.5 µg/litre (van Zorge, cf. WHO/EURO, 1985). In
the Federal Republic of Germany, concentrations of 0-4 ng/litre were
found (DFG, 1988).
Table 12. PCB levels in air in several countries
Country Location and/or type of sample PCB levels References
average and/or range
Canada Northwestern territories 0.002-0.07 ng/m3 Bidleman et al. (1978)
Germany Industrial area (Ruhr area) 3.3 ng/m3 DFG (1988)
Non-contaminated area 0.003 ng/m3
Japan Within industrial plants: - PCB vapours 13-540 µg/m3 Tatsukawa & Watanabe
- PCBs on airborne particulates 4-650 µg/m3 (1972)
North Pacific, South Pacific, Indian, 0.1-0.3 ng/m3 Tatsukawa & Tanabe
Antarctic and South Atlantic Oceans (1983)
North Atlantic Ocean 0.5 ng/m3 Tatsukawa & Tanabe
(1983)
Sweden Several locations 0.8a-3.9 ng/m3 Ekstedt & Odén (1974)
USA Near the North-East Coast 5 ng/m3 Harvey & Steinhauer
Over the Atlantic Ocean, 2000 km away 0.05 ng/m3 (1974)
from the industrial complex
several locations 1-50 ng/m3 Panel on Hazardous
Substances (1972)
cf WHO/EURO (1988)
Yugoslavia Bela Krajina: - 300 m from an industrial plant 4-7 µg/m3 Jan et al. (1988b)
- air near a waste landfill 45 µg/m3
- over the River Kruga 2-5 µg/m3
a Limit of determination.
5.1.1.2 Natural gas
PCBs were first identified in gas pipelines in January 1981, when a
PCB-containing oil condensate was found in the gas meters of some
residential customers in Long Island, New York. Voluntary monitoring
of condensate and natural gas by 33 transmission companies, showed the
presence of PCBs in 12 companies. PCBs were also found in gas
pipelines. Condensate is a mixture of heavier hydrocarbons and other
liquids, such as water, that condenses, because the gas is transmitted
under pressure. This condensate tends to collect in pools in the
pipes. In the period 1981-83, 1841 samples of condensate from gas
pipelines were analysed: 659 (35.8%) of the samples contained
< 25 mg/kg; 65.8% of the samples contained <1000 mg/kg, and 0.4%,
> 10 000 mg/kg. The maximum level that was found was 42 394 mg/kg
(Versar Inc., 1984).
In the period 1981-83, 138 samples of natural gas in transmission were
analysed. In 29 samples, PCBs were found with a minimum concentration
of <0.004 µg/m3 and a maximum concentration of 1050 µg/m3. Natural
gas in distribution lines was also analysed in the same period. Out of
528 samples, 224 did not contain any PCBs. The levels ranged from
<0.02 to 51 µg/m3.
Indoor concentrations (kitchens, etc.) were measured in 419 samples in
the period 1981-83. No PCBs could be detected in 49 samples, but, in
the others, levels ranged from <0.01 to 1.08 µg/m3 (Versar Inc.,
1984).
5.1.2 Water
Surface water may become contaminated with PCBs from atmospheric
fall-out or from direct emissions from point sources. Because of
adsorption on suspended particles, PCB concentrations in heavily
contaminated waters may be several times greater than their
solubility. Södergren (1973) reported a seasonal variation, which was
attributed to aerial fall-out.
It has been shown that polluted rivers, lakes, and estuaries have
higher PCB values than non-polluted waters (Table 13). On the basis of
scanty information on PCBs and reinforced by extensive analogue
information on DDT, it has been estimated that, for the Great Lakes of
North America, non-polluted freshwaters might contain less than
5 ng/litre, moderately polluted rivers and estuaries, 50 ng/litre, and
highly polluted rivers, 500 ng/litre. These values can be used to
evaluate those reported by several authors and presented in Table 13.
Table 13. PCB levels in water in several countries
Country Location and/or type of PCB levels References
sample average and/or range
Germany Several rivers 5-103 ng/litre Lorenz & Neumeier (1983)
Netherlands River Rhine (1976/1977) 100-500 ng/litre Wegman & Greve (1980)
Sweden Water entering a treatment 0.5 ng/litre Ahling & Jensen (1970)
plant
Tap water produced at the plant 0.33 ng/litre Ahling & Jensen (1970)
Several rivers 0.1-0.3 ng/litre Ahnoff & Josefsson (1974)
USA Polluted coastal area 100-450 ng/litre Panel on Trace Hazardous
Lake Michigan (1970)a Substances (1972) (cf.
WHO/EURO, 1988)
Distribution system feeding the Fort Edwards reservoirs < 12-160 ng/litre Brinkman et al. (1980, 1981)
in New York (1978)
Hudson River at Fort Edward up to 530 ng/litre Brinkman et al. (1980, 1981)
a Followed by a marked decrease in 1971.
5.1.3 Soil
Soil may become contaminated with PCBs from atmospheric fall-out or
from direct emissions from point sources. The presence and behaviour
of these compounds in the soil depend on substance (congener)-specific
characteristics and on a number of soil parameters. Sorption and
condensation processes in the soil also play a role in the removal of
PCBs. Some values of PCB levels in soil can be found in Table 14.
Klein (1983) found that PCBs accumulate in the sediments of rivers and
lakes in the Federal Republic of Germany and that these levels
indirectly reflect the contamination of water by PCBs. Some values for
PCBs in sediments can also be found in Table 14.
An important, though localized, source of PCB contamination of soil,
can be the use of sewage sludge as a fertilizer in agriculture. PCB
levels varying from 0.1 to 765 mg/kg (dry weight) have been reported
in sewage sludge from different countries, the usual range being 0.1
to 9.0 mg/kg (WHO/EURO, 1987). In the USA, 16 sewage sludge samples
from cities contained a mean Aroclor 1254 concentration of 5.2 mg/kg
dry weight (range 0.01-23.1 mg/kg). Other authors reported a range of
1.5-27.3 µg/litre in 36 raw sewage sludges. Some levels that have been
found for PCBs in sludges are presented in Table 14 (WHO/EURO, 1987).
Five sediment samples were collected from the Waukegan Harbour of Lake
Michigan, Illinois, in 1978. Residues of 3,4,3',4'-tetrachlorobiphenyl
ranged from 0.005 to 27.5 mg/kg and residues of 2,3,4,3',4'-penta-
chlorobiphenyl, from 0.102-131 mg/kg. The total PCB contents of the
sediment ranged from 10.6 to 13 360 mg/kg (Huckins et al., 1988).
5.1.4 Aquatic and terrestrial organisms
PCBs have been measured in a wide variety of biota from many different
locations throughout the world. Only a few illustrative examples are
given here, more comprehensive lists of PCB residues can be found in
reviews by Risebrough et al. (1968); Peakall (1975); and Eisler
(1986). Tanabe et al. (1987) reported that the highly toxic, coplanar
PCBs are as widely spread as general PCB pollution.
In the biota of a small upstate New York public water supply system,
which is near the polluted section of the Hudson River and a disposal
site of PCB-containing waste, PCBs were found in detectable
concentrations (Table 13). Five samples of algae showed Aroclor 1254
levels of <25 (nd)-120 µg/kg dry weight, macro-invertebrates showed
levels between <200 and 3800 µg/kg and vertebrates, between <25 and
1100 µg/kg dry weight (Brinkman et al., 1980, 1981).
Table 14. PCB levels in soils, sediments, and sewage sludge in several countries
Country Location and/or type of sample PCB levels References
average and/or range
Germany Soil without sewage sludge 0.02-0.08 mg/kga Markard (1988)
Soil with sewage sludge 0.05-3.0 mg/kga
Sewage sludge ndb-19 mg/kg
Sediments of contaminated waters 0.1-1.0 mg/kga Klein (1983)
Sediments of several rivers 0.16-0.59 mg/kg DFG (1988)
Agricultural soil 0.03 mg/kg DFG (1988)
Japan Agricultural soil < 1 mg/kg Fukada et al. (1973)
Soil near a factory making electrical components 510 mg/kg Fukada et al. (1973)
Netherlands Sediments from several surface waters < 0.01-1.2 mg/kga Greve & Wegman (1983)
United Soil from a waste disposal area with chemical treatment 4.5-44.8 µg/kg Eduljee et al. (1986);
Kingdom and incineration facilities Badsha et al. (1986);
(Scotland) Grass samples from the same area (foliage) 2.9-64.7 µg/kg Badsha & Eduljee (1986)
Soil of rural areas 8 µg/kga (1-23)
Grass of rural areas 9 µg/kga (7-16)
Soil of urban areas 52 µg/kg (11-141)
Soil of industrial locations 41 µg/kg (20-67)
United Surface soil 2.5 µg/kg Jones (1989)
Kingdom (0.2-12.2)
(Wales)
Table 14. (cont'd).
Country Location and/or type of sample PCB levels References
average and/or range
USA Sediments near a point of accidental release of PCBs 1.4-61 mg/kg Nimmo et al. (1971a)
(Florida)
Escambia Sediments 16 km downstream of this point 0.6 mg/kg
river
Escambia Soil samples from the bank, 6.5 km downstream from the 1.4-1.7 mg/kg
Bay point
a Dry weight
b Not detectable
Serious environmental contamination has been documented in enclosed
water bodies close to urban and industrialized areas, such as the
Great Lakes, the Baltic Sea, and Tokyo Bay. PCB levels in aquatic
organisms reflect these localized high concentrations.
Nimmo et al. (1971a) reported that PCB levels in shrimp from Escambia
Bay, Florida (contaminated by an industrial plant on the Escambia
River) contained between 0.6 and 120 mg Aroclor 1254/kg in 1969 and
fiddler crabs, collected in 1970, contained 0.45-1.5 mg/kg.
When fish, sampled throughout the USA, were analysed by Schmitt et al.
(1983, 1985), the highest levels of PCBs were found in the
North-eastern industrialized areas. Delfino (1979) reported
concentrations ranging from 26 to almost 1000 mg PCBs/kg in fish
collected from the Sheboygan River, Wisconsin, contaminated by a
die-casting plant.
Wiemeyer et al. (1975) analysed osprey eggs in 1968-69 and found
average levels of 2.6 mg/kg in Maryland compared with an average level
of 15 mg/kg in eggs from Connecticut. PCB residues in Connecticut eggs
had not changed significantly compared with those collected in 1964.
Buckley (1982) analysed aspen, sumac, and golden rod plants growing at
various distances (< 1200 m) and in different directions from a PCB
dump in New York State, USA. All the plants were growing beyond a
natural drainage ravine, which prevented contamination of soil and
water by PCBs. Downwind of the site, PCB levels in the plants were
found to be approximately 100 mg/kg dry weight (over 600 times
background levels in plants). Levels above background concentrations
were also found in directions from the site less obviously
contaminated by airborne dust.
Eggs of terrestrial birds collected in a rural environment in Canada
contained lower PCB levels than those sampled from urban areas (Frank
et al., 1975).
In the Great Lakes, the highest levels of PCBs were found in Lakes
Michigan and Ontario for fish (Delfino, 1979) and Lake Ontario for
birds (Weseloh et al., 1979); both lakes receive input from industrial
and urban sites. Glooschenko et al. (1976) found concentrations of up
to 8.1 mg/kg in microorganisms from the middle of Lake Huron.
Weseloh et al. (1983) found that the PCB levels in double-crested
cormorant eggs, collected from Lake Superior during 1972 (average of
23.8 mg/kg fresh weight), were higher than those in cormorant eggs
analysed in other Canadian colonies. Mineau et al. (1984) found that
the locations of herring gull colonies with the greatest mean levels
of PCBs, in each of the Great Lakes, corresponded with the locations
of major sources of the contaminant, as indicated by elevated residues
in sediment.
Muir et al. (1988) determined PCB levels in pooled Arctic cod muscle
(Boreogadus saida) and polar bear fat (Ursus maritimus), and in
the blubber and liver of ringed seals (Phoca hispida) from 3
locations in the East/Central Canadian Arctic. The mean arithmetic
concentrations of total-PCBs in the muscle of Arctic cod of 2
locations were 3 and 5 µg/kg wet weight. The mean concentrations shown
in the tabulation below were found in the blubber and liver of ringed
seals.
Year Number of Sex Arithmetic mean ± SD
samples (µg/kg wet weight)
(blubber)
1972 3 female 639 ± 249
1975/76 5 female 600 ± 99
1983 10 male 794 ± 879
16 female 308 ± 138
1984 19 male 568 ± 287
14 female 375 ± 172
(liver)
1984 19 male 6 ± 4
14 female 4 ± 3
The presence of PCBs in polar bears (Ursus maritimus) was studied by
Norström et al. (1988) in the Northwest territories of Canada. Liver
and adipose tissue specimens were obtained by Inuit hunters from 12
zones over the period 1982-84. A total of 121 samples was obtained.
The mean concentrations of total PCBs in pooled samples ranged from
3.24 to 8.25 mg/kg, on a lipid weight basis. The adipose tissue of
polar bear (10 pooled samples collected in 1982 and 10 samples, in
1984) contained 4.42 and 4.57 mg/kg wet weight, respectively. From
these results, biomagnification factors for the food-chain of the
Arctic cod/ringed seal/polar bears were calculated. For total PCBs,
these factors ranged from 3.7 to 8.8 for fish to seal; from 7.4 to
13.9 for seal to bear, and 49.2 for fish to bear. For individual PCB
homologues, for instance, for fish to bear, these factors ranged from
<0.5 (tetrachlorinated PCBs) to 263.4 for heptachlorinated PCBs.
Niimi & Oliver (1989b) monitored the presence of 92 monochloro- to
decachlorobiphenyl congeners in brown and lake trout, small and large
rainbow trout, and small and large coho salmon from Lake Ontario. Each
sample consisted of 8-12 fish. The highest concentrations were among
the penta- and hexachlorobiphenyl homologues, with 2,4,5,2',4',5'-
hexachlorobiphenyl the most common congener.
Total congener concentrations ranged from 1 to 10 mg/kg in whole fish
and from 0.3 to 4 mg/kg in muscle. The 10 most common PCB isomers were
84, 87/97, 101, 110, 118, 138, 149, 153, and 180, and represented 52%
of the total content. This value did not appear to be influenced by
species or by total concentration.
Huckins et al. (1988) collected fish (1-6 fish of 7 species) from the
Waukegan Harbour of Lake Michigan, Illinois in 1978. The fish samples
were analysed for the presence of 3,4,3',4'-tetrachloro- and
2,3,4,3',4'-pentachlorobiphenyl. Total PCB congener residues averaged
33.4 (2.4-56.6) mg/kg. The concentrations of 3,4,3',4'-tetra-
chlorobiphenyl averaged 45.3 µg/kg (2-89 µg/kg) in the whole body. The
concentrations for 2,3,4,3',4'-pentachlorobiphenyl averaged 229 µg/kg
(80-483 µg/kg).
Five times as much PCBs were found in herrings caught in
industrialized areas of Sweden (near Stockholm) compared with those
caught in the cleaner waters off the Swedish west coast. Levels in
plankton fell progressively with increasing distance from
industrialized areas (Jensen et al., 1972a).
Holden (1973) found levels of up to 235 mg/kg in the blubber of seals
sampled in the polluted coastal areas of the United Kingdom compared
with lower levels (2 mg/kg) from unpolluted areas. Higher levels, (up
to 88 mg/kg) were found in the blubber of toothed whales sampled in
the North Sea, but none was detectable in similar species sampled off
New Zealand and Surinam (Koeman et al., 1972).
Peakall (1975) mapped out the global distribution of PCB levels in
marine plankton. The values for the open North Atlantic (300-450 mg/kg
lipid) were found to be very similar to those collected from polluted
areas, such as the Baltic sea and the Firth of Clyde, in the United
Kingdom. Values in the South Atlantic (12-64 mg/kg) were considerably
lower. The highest values shown were for the Eastern coast of the USA
(up to 3050 mg/kg). There were no values for the Pacific Ocean.
When monitoring PCB levels in fish from the Mediterranean, Albaiges et
al. (1987) found that territorial species reflected local inputs of
the pollutant, but migratory species had baseline levels.
Risebrough & de Lappe (1972) reported PCB levels higher than 3 mg/kg
in fish from the industrialized areas of Tokyo Bay and New York Sound.
Tanabe et al. (1986a) analysed Antarctic minke whales and found that
they contained lower PCB levels than those caught in the Northern
hemisphere (Tanabe et al., 1983). McClurg (1984) also found low levels
of PCB in the Antarctic; Ross seals contained 0.09 mg/kg (in blubber).
Mean levels of 0.69 mg PCB/kg (wet weight), found by Smillie & Waid
(1987) in Australian fur seal blubber, were much lower than levels
found in seals from the temperate Northern hemisphere. Similarly,
Antarctic fish had very low PCB residues, ranging from 0.08 to
0.77 µg/kg wet weight (Subramanian et al., 1983).
PCB residues in biota are usually highest near industrial sources, but
this geographical distribution is becoming less pronounced. In fact,
O'Shea et al. (1980) and Tanabe et al. (1988) found PCB levels in
small oceanic cetaceans to be higher than those reported for
terrestrial mammals and birds. For example, Tanabe et al. (1988) found
the mean level of PCBs in the fatty tissue of the striped dolphin to
be 36 mg/kg wet weight.
Subramanian et al. (1986) analysed subcutaneous fat from Adelie
penguins from the Antarctic and found PCB levels of 0.05 mg/kg fat
weight. This is a factor of 100 lower than that in auks caught in the
northern North Pacific (Tanaka & Ogi, 1984) and a factor of 10 000
lower than residues found in the pectoral muscle (on a lipid weight
basis) of herring gulls in the Baltic (Lemmetyinen et al., 1982).
5.1.4.1 Effect of dredging-contaminated sediment on organisms
Dredging to remove contaminated sediments from the Shiawassee River,
Michigan, increased the availability of PCBs, and, thus, residue
levels, in freshwater clams (64.5-88 mg/kg dry weight) and in fish
(fathead minnow; 13.8-18.3 mg/kg), both during dredging and up to 6
months afterwards (Rice & White, 1987).
5.1.4.2 Relationship to lipid content of organisms
PCBs are accumulated in lipid-rich tissues and care must be taken when
interpreting results between species with different amounts of body
fat. Jensen et al. (1969) found that PCB levels in herring and cod,
from the same area of the Baltic Sea, were 0.27 and 0.033 mg/kg, on a
wet weight basis, respectively, even though the cod is at a higher
trophic level. The 2 species were found to have body fat contents of
4.4 and 0.32%, respectively, and when the PCB residues were
recalculated on a lipid weight basis, herring contained 6.8 mg/kg and
cod, 11 mg/kg.
PCBs are particularly accumulated in animals with large amounts of
fat, such as seals, dolphins, porpoises, and whales (Tanabe, 1988) and
in Arctic and Antarctic birds and mammals. Subramanian et al. (1986)
found PCBs in all Adelie penguins sampled in the Antarctic, an area
known to be relatively low in PCBs; the PCBs were mainly concentrated
in fat-rich tissues. Kawai et al. (1988) measured PCBs in striped
dolphins and found that the tissue level of PCBs depended entirely on
their lipid content and, especially, on the amount of triglycerides in
tissues.
Redistribution of PCBs, from fat to other tissues, occurs in animals
during periods of enforced starvation, such as seasonal food shortage,
hibernation, migration, incubation, and the feeding of offspring.
Subramanian et al. (1986) found that, as individuals Adelie penguins
starved during incubation, residues of PCBs increased with declining
fat reserves concomitant with tissue redistribution. Llorente et al.
(1987) found that migratory duck species had a smaller percentage of
the body burden of PCBs in adipose tissue than a resident species. A
similar redistribution during starvation has been shown in the
laboratory in European robins (Södergren & Ulfstrand, 1972) and big
brown bats (Clark & Prouty, 1977) (see sections 4.2.4.5 and 4.2.4.6).
5.1.4.3 Residues in different trophic levels and effects of diets
In a study by Shaw & Connell (1982), bioaccumulation was increasingly
evident in upper trophic level organisms, such as gulls and pelicans,
in an Australian estuary compared with organisms from lower trophic
levels. Veith et al. (1977) found typical PCB concentrations in Lake
Superior biota to be 0.1 mg/kg for large zooplankton, 0.3 mg/kg for
bottom fish, such as sculpins, and 1 mg/kg for pelagic fish.
When various insects were sampled for PCB residues (Morse et al.,
1987), levels in honey bees ranged from <0.1 to 1.5 mg/kg dry weight.
PCB residues in other species ranged from <0.1 to 2.6 mg/kg, with
predatory wasps containing the highest residues.
Prestt et al. (1970) analysed the livers from various bird species in
the United Kingdom. The highest PCB residues were found in freshwater,
fish-eating species (up to approximately 900 mg/kg). The authors did
not find any geographical pattern of distribution of PCBs in the
species studied.
Frank et al. (1975) collected birds' eggs from the Niagara peninsula
in 1971. Eggs from carnivorous species of birds at the top of the
aquatic food chain contained the highest levels of PCBs
(3.5- 74 mg/kg). Terrestrial carnivores contained lower, but still
relatively high, residues (0.2-1 mg/kg). Eggs from herbivorous and
insectivorous birds contained much lower residues of PCBs. Again, eggs
from terrestrial birds tended to contain lower levels (0.05-2 mg/kg)
than those feeding on aquatic prey (0.14-4 mg/kg). Focardi et al.
(1988) compared the PCB residues in the eggs of 8 species of water
bird. The residues were found to be higher in fish-eating birds than
in invertebrate feeders. The invertebrate feeders tended to contain
higher percentages of the lower chlorinated congeners. Bird species
that fed on other birds or fish had higher liver residues of PCBs than
those feeding on mammals (Cooke et al., 1982). Peregrine falcons,
herons, sparrowhawks, kingfishers, and great crested grebes had
relatively high residues of PCBs. By contrast, golden eagles were only
very lightly contaminated with PCBs.
Bowes & Jonkel (1975) found a similar pattern in Arctic and subarctic
food chains with PCB levels following the pattern: Arctic charfish
< seals < adult polar bears < polar bear cubs.
Mean PCB concentrations of 0.0018 mg/kg were found by Tanabe et al.
(1984) in zooplankton, 0.048 mg/kg in myctophid, 0.068 mg/kg in squid,
and 3.7 mg/kg in striped dolphin (all based on a whole-body, wet
weight basis) sampled from the western North Pacific. The authors
concluded that the bioaccumulation of chlorinated hydrocarbons was
dependent on physical and chemical factors, such as water solubility
and lipophilicity, in the lower trophic levels, whereas, in higher
trophic levels, accumulation was affected by biochemical factors, such
as the biodegradability of pollutants and the metabolizing capability
of the organism.
5.1.4.4 Effects of age, sex, and reproductive status on uptake and
elimination
Bache et al. (1972) found that the burden of PCBs increased with age
in lake trout from Cayuga lake, Ithaca, New York, sampled in 1970
(residues ranged from 0.6 to 30.4 mg PCBs/kg). An age- and
length-related increase in PCBs was found in striped bass from the
Hudson River and Long Island Sound; the author (Connell, 1987) stated
that this observed relationship was due to the slow rate of
bioaccumulation of the PCBs, particularly the higher chlorinated
congeners.
PCBs have been shown to accumulate with age in marine mammals, such as
pinnipeds (Addison et al., 1973; Frank et al., 1973; Helle et al.,
1983) and cetaceans (Gaskin et al., 1983; Aguilar & Borrell 1988;
Subramanian et al., 1988). Helle et al. (1983) found mean levels of
5.1 mg PCBs/kg (in extractable fat of blubber) in newly-born ringed
seal pups, 17.3 mg/kg in seals of 2-4 months of age, and 65.3 mg/kg in
sexually mature adults (4-12 years). However, lower levels of PCBs
have been found in females compared with males (Martineau et al.,
1987) and the age-related increase has often not been found in females
(Addison & Smith, 1974). In many studies, while levels of PCBs in
males have increased with age, those measured in females have fallen
(Born et al., 1981; Gaskin et al., 1983; Aguilar & Borrell, 1988).
Gaskin et al. (1983) found that PCB levels in the blubber of male
harbour porpoises increased from 48.4 mg/kg at birth to 161 mg/kg
after 8 years, whereas, in females, levels fell from 51 to 14.7 mg/kg.
A significant decrease in the PCB levels was found by Subramanian et
al. (1988) in female Dall's porpoises from 2 years of age onwards; 2
years is required for the animals to reach sexual maturity. Excretion
of PCBs during reproduction is known, from the laboratory, to be an
important means of females losing residues. This PCB loss has been
shown to be because of the transfer of PCBs to offspring via milk
during lactation (Addison & Brodie, 1977). Addison & Brodie (1977)
calculated that female grey seals excreted about 15% of their body
burden of PCBs via lactation. In striped dolphins, females transferred
between 72 and 98% of their body burden to the offspring (Fukushima &
Kawai, 1981; Tanabe et al., 1982). It was suggested by Tanabe (1988)
that such large transfer was because of the very high lipid content of
the milk. Relocation of the PCB burden during pregnancy is generally
thought not to be as important; in grey seals, the mother transfers
only about 1% of her body burden to her offspring (Donkin et al.,
1981) and in striped dolphins, only 4-9% (Fukushima & Kawai, 1981;
Tanabe et al., 1982). However, Duinker & Hillebrand (1979) suggested
that a much bigger percentage of female body burden (up to 15%) could
be transferred to the fetus across the placenta of Harbour porpoise.
Clark & Lamont (1976) calculated that female big brown bats
transferred between 17 and 32% of their body burden of PCBs to their
young, during gestation. The concentration of PCBs in adult females
plus their litters declined with increasing age of the female. PCB
levels were 0.83-3.6 mg Aroclor 1260/kg (wet weight) in adults and
0.22-3.3 mg/kg in litters.
When Passino & Kramer (1980) measured PCBs in deepwater ciscoes from
Lake Superior, male fish contained significantly higher levels of PCBs
(2.3 mg/kg wet weight) than females (1.2 mg/kg), eggs containing
0.51 mg/kg. Lemmetyinen et al. (1982) found annual rates of
elimination via egg production of 45% in the female Arctic tern and
24% in the herring gull. Adelie penguins eliminated only 4% of their
PCB body burden after laying their annual clutch of 2 eggs (Tanabe et
al., 1986b). Elimination was thought to be dependent on the relative
weights of the egg and mother.
5.1.4.5 Time trends in residues
Buckley (1983) analysed various species of terrestrial plants from New
York state. Total decreases of 42% in PCB residues were found between
1978 and 1980.
PCB levels in fish in the Hudson River, New York declined between 1977
and 1981. The PCB levels were much higher in the Upper Hudson River
(4217-1431 mg/kg of lipid), near to a major discharge of PCBs, than in
the Lower Hudson River (1604-319 mg/kg) (Sloan et al., 1983).
Frank et al. (1978) measured PCB levels in various fish species from
Lakes Huron and Superior during the period 1968-76. PCB residues
declined in lake trout and lake whitefish in Lake Superior between
1971 and 1975, but increased slightly over the same period in bloaters
and white sucker. In Lake Huron, PCB levels decreased between 1968 and
1971, and, in alewife, rainbow smelt, and walleye, between 1975 and
1976. In some of the study areas, residues increased in cisco, yellow
perch, coho salmon, and splake but, at most locations, and, for other
species analysed, no trends in PCB levels were found. St Amant et al.
(1984) analysed fish from Lake Michigan between 1971 and 1981. An
overall decrease in PCB levels was found for all species monitored
except the walleye. Levels decreased from a maximum of 22.4 mg/kg at
the beginning of the study to 3.8 mg/kg or less in 1981.
Fish from all over the USA were analysed in 1980-81 by Schmitt et al.
(1985) who found a significant downward trend (0.88-0.53 mg/kg PCB;
wet weight) when mean residues were compared with fish collected
between 1976 and 1977 (Schmitt et al., 1983). A similar downward
pattern in residues was found in the Baltic when Moilanen et al.
(1982) compared residues found in pike and herring caught between 1978
and 1982 with those in fish sampled between 1972 and 1978 (Paasivirta
& Linko, 1980). Haahti & Perttila (1988) found a continued decline in
PCB residues between 1979 and 1986, when residues in herring muscle
tissue decreased from 2.7-3.7 mg/kg to 0.3-1.1 mg/kg.
An overall fall in PCB levels was found by Newton & Bogan (1978) in
sparrowhawk eggs during the period 1971-74. Cooke et al. (1982)
analysed liver samples from grey herons, kestrels, and barn owls for
PCB residues during the period 1967-77. They found a significant
decline in PCB residues over the sampling period in all 3 species. The
mean residues in heron, kestrel, and barn owl for the period 1967-71
were 5.77, 1.57, and 0.44 mg/kg, respectively, and for 1977, 0.56,
0.6, and 0.15 mg/kg, respectively. However, Newton et al. (1986), when
analysing sparrowhawk eggs from 1971-80, found that, although levels
had fallen in the early 1970s, they had risen again in the late 1970s
(mean PCB residues in eggs ranged from 16 to 293 mg/kg in lipid). Data
on PCB residues in the livers of kestrel, sparrowhawk, heron,
kingfisher, and the great crested grebe, collected from the late 1960s
up to 1987, were analysed statistically by Newton & Haas (1989). For
the great crested grebe, a significant overall decline in PCB residues
was found when comparing data from 1987 with that from the 1960s. For
the other species, there was no significant difference. Spitzer et al.
(1978) reported that there was no significant change in PCB levels in
osprey eggs collected from the Connecticut-New York area during the
period 1969-76. Similarly, Wiemeyer et al. (1987) did not find any
change in the carcase levels of PCBs in ospreys from the Eastern
United States when comparing the 1971-73 and 1975-82 periods. They did
find that adults contained significantly higher concentrations of PCBs
than immature ospreys.
Blus et al. (1979) analysed brown pelican eggs from South Carolina and
Florida between 1969 and 1976. The highest levels of PCBs were found
in South Carolina (means ranged from 5.25 to 7.63 mg/kg wet weight),
but no significant trend was found during the study period. In
Florida, the authors did not find any significant change in eggs
collected from colonies in Florida Bay and on the Gulf Coast over the
study period (means ranged from 0.62 to 1.18 mg/kg), but the Atlantic
coastal colony showed a significant increase in PCB residues (from a
mean of 2.68 to 6.12 mg/kg) between 1969 and 1976.
In analysing herring gull eggs from the Great Lakes between 1974 and
1978, Weseloh et al. (1979) found a significant decline in PCB
residues from colonies on all the lakes. Lake Ontario, the most
contaminated, showed the biggest decline from 170 to 75 mg PCBs/kg at
one of the colonies, with other less contaminated Lakes, Huron,
Superior, and Erie, showing levels in the range of 50-86 mg/kg in 1974
and 32-46 mg/kg in 1978.
Moksnes & Norheim (1986) analysed herring gull eggs collected from the
Norwegian Coast between 1979 and 1981 and found that the PCB levels
were not significantly different from those in eggs collected in 1969;
mean PCB residues ranged from 1.2 to 6.7 mg/kg wet weight. They found
a small but significant increase in the most persistent congeners and
a significant decrease in DDE and the DDE/PCB ratio, but not in total
PCB levels.
An analysis of the eggs of double-crested cormorant (an inshore-
subsurface feeder), Leach's storm petrel (an offshore-surface
feeder) and Atlantic puffin (an offshore-subsurface feeder) was
carried by Pearce et al. (1989), every 4 years, between 1968 and 1984.
In the Bay of Fundy, Canada, PCB levels declined significantly during
this period in all 3 species. PCB levels in the cormorant were
consistently higher throughout than those in the other 2 species,
ranging from 4 to 29.5 mg/kg (wet weight). Petrel and puffin eggs
collected from the Atlantic Coast of Newfoundland showed lower levels
than those in eggs from both the Bay of Fundy and the St Lawrence
River estuary; as in the St Lawrence River, no significant trend in
PCB levels was observed. A significant decline in PCB residues was
found in gannet eggs collected during the same period from the gulf of
St Lawrence (Elliott et al., 1988).
The frequency of occurrence of measurable PCB residues has increased
in large-scale sampling exercises; PCBs in mallard wings increased
from 39% in 1976-77 (White, 1979) to 95% in 1979-80 (Cain, 1981). Cain
& Bunck (1983) found that, in 1976, 21% of European starlings
collected in the USA contained PCBs compared with 83% in 1979.
Addison et al. (1986) analysed the blubber of Arctic ringed seals
(Phoca hispida) from Holman Island, NWT, Canada, in 1981. They found
mean PCB levels of 0.58 mg/kg (wet weight) in the females and
1.28 mg/kg in the males. These concentrations were significantly lower
than those detected in the same species from this area in 1972. Over
this same period, pp'-DDE levels, although at lower levels, also
fell significantly, but it should be noted that total DDT levels in
blubber are much lower than PCB levels and have not changed
significantly.
5.1.4.6 Seasonal patterns in residues
Jensen et al. (1969) observed that there was considerable seasonal
variation in the fat content of herring caught in the Baltic Sea,
ranging from 1% in the spring to 10% in the autumn and that this
seasonal change in fat content led to seasonal changes in the tissue
levels of PCBs.
Cooke et al. (1982) found a seasonal pattern of PCB levels in European
kestrels. Residues in both fat and liver were low in the autumn, but
increased from about January, with a peak almost invariably occurring
during the second quarter of the year (April, May, or June). Seasonal
patterns were based on samples collected over a 10-year period.
Similar trends were found in sparrowhawks and barn owls, but fewer
samples were available.
5.1.5 Appraisal
PCB contamination is widespread and has been measured in a wide
variety of biota between the 1960s and the present day. They are
present throughout the world and, although initially concentrated in
areas of high industrial activity, are now found in organisms living
in remote areas, such as the oceans and the polar regions. In the
past, PCB levels were positively correlated with areas of heavy
industry and consequent discharge but, with the implementation of PCB
controls, in some countries, these geographical differences are
becoming less clear. Generally, levels of PCBs are declining in areas
previously high in PCBs. However, time-trend analysis for the general
environment shows little change in total PCBs since the late 1960s.
The ratio of congeners is, as would be expected, changing, with lower
chlorinated isomers disappearing and the more highly chlorinated ones
becoming more dominant in environmental samples.
PCBs are persistent and bioaccumulate in many organisms, because of
their high lipid solubility and low biodegradability, and usually
enter food-chains from water containing industrial discharge and by
precipitation.
Because of their hydrophobic nature, PCBs are associated with both
oildrop-like aggregates in the surface microlayer of water and with
sediment on the bottom.
They are accumulated by micro- and macroplankton organisms that live
in the surface microlayer and by bottom-living organisms.
5.2 Levels in animal feed
The effects of pollution are seen in the use of fish-meal in poultry
and fish farming. Kolbye (1972) sated that this may contain PCB levels
of 0.6-4.5 mg/kg.
Hansen et al. (1981) studied the transfer of PCBs in swine foraging on
sewage sludge amended soils in 1975-76. Sixteen Berkshire sows were
overwintered for 2 seasons on 4 experimental plots that had been
treated with 0, 126, 252, or 504 tonnes/hectare (on a dry solids
basis) of Chicago sewage sludge for the 8 preceding years. The
estimated PCB residues in the soils of the 4 plots (average of 3-4
samples) were 1.62, 1.88, 2.13, and 2.81 mg/kg dry weight (mean values
of 3-4 samples/plot). The mean concentrations in fat of 3-4 sows per
plot were 36 ± 9, 106 ± 64, 191 ± 97 and 389 ± 118 µg/kg fat basis. Of
the 12 individual congeners that were present in the fat, 3 accounted
for more than 50% of the congeners, e.g., 2,3,4,2',4',5'-,
2,4,5,2',4',5'-hexachlorobiphenyl and 2,3,4,5,2',4',5'-
heptachlorobiphenyl.
In vegetable animal feed (155 samples) originating from 5 areas of the
world, samples, collected in 1984/85, contained PCB levels of 0.0009
(Africa) up to 0.0093 mg/kg dry weight (Europe). In feed from North
and South America and the Far-East, the levels were between 0.0024 and
0.0066 mg/kg. Different types of feed originating from agriculture in
the Federal Republic in Germany, collected in 1985, contained PCB
levels of the order of 0.02 mg/kg dry weight. In feed (301 samples)
originating from animals (exclusive fish meals), collected in 1985,
0.021-0.036 mg/kg dry weight was found (DFG, 1988).
Levels of 10-100 µg/kg are given for groats, soybeans, and cotton
seed, and a mean value of 18 µg/kg is given for mixed feedstuffs. Fish
meal contained levels of 110-330 µg/kg (Klein, 1983).
Samples of fish meal from different areas of the world, collected in
1985, were analysed for the presence of PCBs. In 323 samples, the PCB
contents varied between 0.006 and 0.055 mg/kg dry weight. The PCB
congeners numbers 28, 138, and 153 were present in the highest
quantities (DFG, 1988).
Samples of fish meal from different areas of the world, collected in
1985, were analysed for the presence of PCBs. In 323 samples, the PCB
contents varied between 0.006 and 0.055 mg/kg dry weight. The PCB
congeners numbers 28, 138, and 153 were present in the highest
quantities (DFG, 1988).
5.3 Levels in human food
5.3.1 General
Two general reviews of PCB residues in food, animal feed, human milk,
plants, soils, and packaging materials have been published by Khan et
al. (1976) and Sawhney & Hankin (1985).
The PCB contents of a variety of foods on the Swedish market has been
measured by Westöö & Norén (1970a) and Westöö et al. (1971). Less than
0.1 mg/kg was found in samples of butter, margarine, vegetable oils,
eggs, beef, lamb, chicken, bread, biscuits, and baby food; one sample
of pork out of more than 100 had a PCB content of <0.5 mg/kg.
In the period 1980-81, 5270 food samples were drawn at wholesale or
production levels or at the site of importation including: butter,
cheese, eggs, kidneys from pigs and cattle, and fat of poultry. Levels
in Danish butter (99.4% of the samples) were below 0.05 mg/kg and
those in imported butter (100%), below 0.125 mg/kg; Danish cheese
(100% of the samples) levels were below 0.05 mg/kg and, in imported
cheese, 82.4% of samples had levels below 0.125 mg/kg and the other
17.6%, below 0.2 mg/kg; 100% of eggs had levels below 0.05 mg/kg, and
100% of kidneys of pigs and cattle were below 0.15 mg/kg; 96% of
poultry fat samples had levels below 0.15, and 4%, below 0.20 mg/kg,
on a fat basis (not stated) (Statens Levnedsmiddelinstitut, Danmark,
undated).
Mes et al. (1989b) studied the presence of specific isomers of PCB
congeners in fatty foods of the Canadian diet. A total of 93 food
composites from the cities of Ottawa and Halifax were analysed for 34
PCB isomers, as part of a revised total diet programme. Each market
basket comprised approximately 200 different food types collected from
each of 4 major supermarkets in Ottawa during September 1985 and
January 1986, and, in Halifax, in September 1986. Foods were used
per se, or prepared and cooked in a manner ready for consumption,
then composited to give 112 composites from each market basket.
Thirty-one selected composites, representing the fatty foods were
analysed from each market basket.
PCB isomers 118, 138, 153, and 180 were found in all dairy products,
except skimmed milk. Cheese and butter contained the highest levels of
PCB residues. The residue level of isomer 118 (2,4,5,3',4'-
pentachlorobiphenyl) in butter was the highest e.g., 0.7 µg/kg, of all
PCB isomers found in dairy products. Almost all meat, fish, and
poultry contained PCB isomers 183 and 187. Occasionally, isomers 49,
87, 185, and 189 were also present, but isomer 105 (2,3,4,3'4'-
pentachlorobiphenyl), present in most dairy products, was only found
in some beef samples. Fresh water fish contained most PCB isomers (28
out of 34 selected PCB isomers), at levels considerably higher than
those in any other meat, fish, or poultry samples. The level of isomer
110 in fresh water fish was 3.05 µg/kg. PCB isomers 138, 153, 180, and
187 were present in almost all samples of meat and fish products,
fats, oils, and soups. Cooking fats, salad oils, and margarine
contained relatively low levels of PCB residues. PCB isomers 37, 49,
87, 105, and 185 were not detected in meat and fish products, fats,
oils, or soups.
The calculated sum of all PCB isomer residues found in selected food
commodities (except fish) ranged from 0.03 to 1.98 µg/kg on a wet
basis, and from 0.07 to 10.71 µg/kg on a lipid basis, with mean values
of 0.60 and 3.91 µg/kg, respectively. However, the mean residue levels
of fish and fish products were considerably higher, i.e., 10 and
194 µg/kg on a wet and lipid basis, respectively.
The major PCB isomers in fatty foods were isomers 37, 52, 99, 110,
118, 138, 153, 180, and 187.
The PCB levels obtained in an extensive study by the US Food and Drug
Administration are shown in Table 15. These values are considerably
higher than those reported from Sweden, but they are probably biased,
as they include samples originating from areas previously suspected of
having been subject to local pollution.
In a Canadian survey, PCB levels of less than 0.01 mg/kg were found in
eggs (Mes et al., 1974) and a mean of 0.042 mg/kg was found in
domestic and imported cheese with a maximum of 0.27 mg/kg (Villeneuve
et al., 1973b).
A preliminary study was carried out to estimate the dietary intake of
PCBs in fresh food composites grown in Ontario in 1985. The following
5 food composites: fresh meat and eggs, root vegetables (including
potatoes), fresh fruit, leafy and other above-ground vegetables, and
cow's milk were analysed. The concentrations in the different food
composites were below 0.0005 mg/kg. The annual dietary intake of PCBs
was estimated to be 32.6 µg (Davies, 1988).
In Japan, a similar range of PCB contents has been reported for most
foods; however, some high levels have been reported for rice and
vegetables harvested in fields polluted with PCBs (Environmental
Sanitation Bureau, 1973). The PCB content of most fish on the market
was less than 3 mg/kg.
Table 15. PCB levels in food in the USAa
Food % Positive Level in positive samples (mg/kg)
(0.1 mg/kg)
Mean Maximum
Cheese 6 0.25 1.0
Milk 7 2.3 27.8
Eggs 29 0.55 3.7
Fish 54 1.87 35.3
a From: Kolbye (1972).
Cantoni et al. (1988) analysed different food items, in 1985-87, in
Italy, taking 20-60 samples per item. Different types of meat were
analysed and the median concentrations were 0.25-0.50 mg/kg, on a fat
basis. Twenty to 50% of the samples were positive. Poultry contained
0.028 mg/kg, cow's milk 0.05 mg/kg, cream 0.027 mg/kg, butter
0.065 mg/kg and fish 1.105 mg/kg, on a fat basis; 71% of fish samples
contained PCBs.
When the fat of poultry (42 samples) and 44 eggs was analysed, PCB
values were below 0.3 mg/kg (Dutch Agricultural Advisory Commission,
1983).
In the Federal Republic of Germany, wheat was analysed during the
period 1972-82. The mean concentrations for 1972-78 ranged from 10 to
30 µg/kg; in the period 1980-82, the range was < 2.0-18 µg/kg (Klein,
1983). In wheat and rye (total 850 samples), median levels of
0.4-1 µg/kg product were found in 1984 (Codex Alimentarius, 1986). The
concentrations found in other food items are summarized in Table 16.
Samples of canned ham exported from Czechoslovakia to the USA in 1983
contained PCBs levels of up to 4.8 mg/kg (Anon., 1983a,b).
Table 16. PCBs in food (1982) in the Federal Republic of Germanya
Food Total no. Number of Variation Mean
of samples min-max (µg/kg)
samples below (µg/kg)
detection
limita
Milk 854 234 < 2-3000 126.7 (FB)
Beef 76 43 < 10-687 72.4 (FB)
Pork 58 36 < 10-458 58.1 (FB)
Poultry 64 61 < 10-85 7.3 (FB)c
Meat products 185 86 < 4-2700 114.2 (FB)
Eggs 82 67 < 5-230 9.1 (FW)
Fish (only 70 - 40-87 41.1 (FW)
cod, herring,
plaice)
Food of plant origin
Oil 167 139 < 5-65 7.1 (FB)
Cereals 345 44 < 2-30 6.7 (FW)
Potatoes 106 106 < 2 -
a From: Klein (1983).
b Not stated.
c Only 3 samples.
FB = fat basis
FW = fresh weight.
5.3.2 Drinking-water
Ruoff et al. (1988) examined 83 drinking-water samples from the
Federal Republic of Germany and from 5 other European countries for
their contents of the PCB congeners 28, 52, 101, 138, 153, and 180.
The average total content of the 6 congeners was 0.002 µg/litre water.
The average concentrations of the above-mentioned PCB congeners in the
drinking-water of 6 countries were 0.0001, 0.001, 0.00018, 0.00035,
0.00037, and 0.00042 µg/litre. The variation between the 6 countries
was quite small.
The highest concentration of PCBs reported in domestic tap water was
0.1 µg/litre in the Kyoto area of Japan (Panel on Hazardous Trace
Substances, 1972 cf. WHO/EURO, 1988), but, levels, more likely to be
encountered, should not exceed 0.001 µg/litre.
In the FAO/WHO collaborating centres for the food contamination
monitoring programme, the median levels were:
Cereals below 10 µg/kg
Vegetable fat/oils below 5 µg/kg
Fresh fruit and vegetables 0.5-5 µg/kg
Animal fat (depending on type of
animal and origin) 20-240 µg/kg
Whole fluid cow's milk (depending
on country) 10-200 µg/kg
(on fat basis)
Butter 30-80 µg/kg
Whole dried cow's milk 20-50 µg/kg
Hen eggs < 10 µg/kg
Fresh finfish 10-200 µg/kg
(WHO, 1985b).
The contamination of a drinking-water system in Pickens County, South
Carolina by PCBs discharged from a manufacturing facility was
described by Billings et al. (1978). They observed that PCBs
discharged by a capacitor manufacturing plant resulted in levels as
high as 0.818 µg/litre in finished potable water.
5.3.3 Dairy products
A number of data on food-producing animals have recently become
available within the framework of the Joint FAO/WHO Food Contamination
Monitoring Programme (JFCMP, 1985). All reported median values of PCBs
in animal fat (excluding milk fat) were below the respective limits of
detection, which varied from 0.001 mg/kg in the United Kingdom to a
high of 0.5 mg/kg in Thailand and the USA. Data on PCB levels in cow's
milk fat were supplied by the Federal Republic of Germany, Japan, the
Netherlands, the United Kingdom, and the USA. The United Kingdom and
the USA reported that median concentrations in cow's milk were below
the detection limits of 0.5 µg/kg and 0.5 mg/kg, respectively.
The available data are summarized in Table 17.
From the end of 1982 to the beginning of 1983, high levels of PCBs
were detected in milk from several dairy farms in Switzerland. The
investigations showed that the silo coatings and consequently the
silage from the silos were the origin of the contamination of the
milk. The PCB levels were between 0.80 and 3.80 mg/kg fat. PCB
dissolution in acid juice, mechanical erosion of the coatings, and
volatilization of the coating surface seemed to be the principal
mechanisms explaining the migration of PCBs into the silage
(Alencastro et al., 1984).
Forty-two samples of cow's milk (14 samples in 1976, 14 in 1983, and
14 in 1986) and 41 samples of market milk (10 in 1976, 16 in 1983, and
15 in 1986) were analysed for PCBs, in Israel. During this period, a
change was observed in the PCB distribution in the milk samples. The
percentage of hexachlorobiphenyl decreased with time and the
pentachlorobiphenyl increased (Pines et al., 1988).
The monitoring data for dairy products from all over the world for
1980-83 have been summarized by the Joint FAO/WHO Food Contamination
Monitoring Programme (WHO, 1986a,b).
5.3.4 Fish and shellfish
A summary of the monitoring data on fish from all over the world for
1980-83 has been published by the Joint FAO/WHO Food Contamination
Monitoring Programme (WHO, 1986a,b).
As might be expected, the PCB values found in fish depended on the fat
content and the pollution of the fishing area (Westöö & Norén, 1970a;
Berglund, 1972).
In a collaborative study by 7 national laboratories (International
Council for the Exploration of the Sea, 1974), the PCB contents in the
muscle tissue of fish taken from the North Sea were measured. A mean
of 0.01 mg/kg was found in cod, while herring contained up to
0.48 mg/kg, with most samples in the range of 0.1-0.2 mg/kg; plaice
contained 0.1 mg/kg or less. Similar values were reported by Zitko
(1974) for fish taken from the North Atlantic.
Risebrough & de Lappe (1972) reported levels higher than 3 mg/kg in
fish from New York Sound and Tokyo Bay, both very polluted areas. Even
higher levels of PCBs were found in fish from polluted lakes and
inland waterways, a level of 20 mg/kg being found in fish from Lake
Ontario, and levels over 200 mg/kg in fish from the Hudson River
(Stalling & Mayer, 1972). Similar correlations between pollution and
PCB levels have been reported from the United Kingdom in fish
(Portmann, 1970), and in mussels (Holdgate, 1971).
Table 17. Occurrence of PCBs in dairy products
Country Year Product Number of Mean concentrations Reference
samples in mg/kg on fat basis
(range)
North America
USA 1973-1974 milk (bulk) 198 (9 positive) 1.91 (0.32-4.99) Willett (1980)
Europe
Germany 1982-1986 milk 3279 0.09-0.14a DFG (1988)
(3 areas) 1983-1986 butter/cheese 2088 0.05-0.11
Westphalian area 1972-1974 butter - 0.38 (0.25-0.54) Claus & Acker (1975)
Northern part 1978-1980 milk - 0.17-0.20 Codex Alimentarius
1984 milk 3510 0.013 (1986)
Northern part - butter 1836 0.0077c Codex Alimentarius
(1986)
- meat and fat 957 (about 3/4 0.01b DFG (1988)
positive)
cows entrails 51 0.149b
Sweden 1972-1977 beef, pork and meat 232 (217 < 0.001-0.01 Vaz et al. (1982)
products (domestic negative) (whole product)
and imported)
Denmark 1981-1982 milk - 0.10-0.13 Jensen (1983b)
Table 17. (cont'd).
Country Year Product Number of Mean concentrations Reference
samples in mg/kg on fat basis
(range)
Netherlands 1975-1977 milk 315 0.16 (0.06-0.33) Gezondheidsraad
1980-1983 milk - 0.07-0.13 (1985)
1978-1984 milk 2319 < 0.1-0.2 Olling (1984)
1977-1981 cattle fat - 0.11b (< 0.05-0.55) Greve & Wegman
pork - 0.07 (< 0.05-0.66) (1983)
1983 fat of cattle, pork, 40-45 < 0.03b Dutch Agric. Adv.
calves Comm. (1983)
sheep 22 < 0.03b
Switzerland - milk 6 0.034-0.144 Rappe et al. (1987)
(6 locations)
a Major congeners were Nos. 138 and 153.
b Median value.
c Arithmetic mean.
Jensen et al. (1969) found PCB levels of 0.27 mg/kg and 0.33 mg/kg,
respectively, in the muscle tissue of herring and cod from the same
area of the Baltic, though the cod is at a higher trophic stage. The 2
species had 4.4 and 0.32% of extractable fat, respectively, and, when
the PCB level was calculated on the fat content, values of 6.8 mg/kg
for the herring and 11 mg/kg for the cod were obtained. Cod liver has
a much higher fat content than cod muscle, and Jensen (1973) reported
the ratio of PCB concentrations in cod liver and muscle to be over
100, the maximum in liver being 59 mg/kg. Jensen et al. (1969)
remarked that the considerable seasonal variation in the fat content
of the herring, rising from 1% in spring to 10% in autumn, influenced
the tissue level of PCBs.
There are many examples of different PCB levels in similar species
collected from areas of high and low pollution. Jensen et al. (1972b)
found 5 times as much PCBs in herrings caught in waters off
industrialized areas near Stockholm, as in herrings from the cleaner
waters of the west coast of Sweden.
Different freshwater and seawater fish were analysed for PCB contents,
during the period 1981-83, in the Netherlands. Eel from different
places over the period 1971-81 contained 0.2-13 mg/kg on a product
basis (in the edible part). The median value was between 1 and
2 mg/kg. Sea fish from the North Sea, such as herring and mackerel,
contained 0.1-0.2 mg/kg, on a fat basis. The same level was found in
shrimps and mussels (Freudenthal & Greve, 1973; Greve & Wegman, 1983;
van der Kolk, personal communication, 1984a).
The mean PCB contents in the liver of cod from the North Sea, North
Atlantic, and Baltic Sea, were 2.1-5.7, 0.48, and 10.4-12.8 mg/kg,
respectively (Klein, 1983).
When fish from the North Atlantic, North Sea, and Baltic Sea, were
collected in 1985, PCB concentrations of 0.098-0.123 mg/kg fillet
weight were found in fish from the North Atlantic and North Sea and
0.338 mg/kg fillet weight in fish from the Baltic Sea. In total, 60
samples were analysed. The PCBs 101, 138, and 153 were the major
congeners (DFG, 1988).
The PCB concentration in freshwater fish of the River Rhine was found
to be more than 2 mg/kg. The mean PCBs levels decreased, however, over
the period 1976-81 from 1.92 to 0.38 mg/kg (fresh weight) (Klein,
1983).
In 1984, PCB concentrations in freshwater fish (59 samples) collected
in the River Rhine ranged from 0.742 to 1.017 mg/kg fillet weight. In
this case, the major congeners were 138 and 153, but numbers 28, 52,
101, 180 were also present. In total, 199 samples of eel were
collected in a number of surface waters and analysed for the presence
of PCBs. The levels ranged from 1.42 to 6.51 mg/kg fresh weight. In
studies reported by DFG (1988), the highest levels of PCBs were found
in the River Rhine.
In the United Kingdom, fish and shellfish were analysed for PCBs
during the period 1982-84 (HMSO, 1986). The results are summarized in
Table 18.
Table 18. PCB levels in marine fish and shellfisha
Year Product Tissue No. of Range (mg/kg)
samples
1982 Marine fish (from England) liver 381 0.3-4.1
(7 types of fish)
1982 Marine fish (from England) muscle 326 0.03-0.13
(7 types of fish)
1983 Marine fish (imported) muscle 102 nd-0.06
(5 types of fish)
1983 Shellfish (imported) muscle 53 nd-0.06
(4 types of shellfish)
1984 Fish oils 16 0.11-2.3
a From: HMSO (1986).
Different types of marine fish and shellfish from different areas in
the United Kingdom were analysed during the period 1977-84. Those from
the North Sea coast contained concentrations in the range of 0.04-5.7
and < 0.001-0.058 mg/kg, respectively, while those from the English
channel contained < 0.05-6.9 and < 0.006-0.1 mg/kg, respectively,
and those from the West coast, < 0.002-8.4 and < 0.001-0.25 mg/kg
wet weight. PCB concentrations in fish livers of 0.2 up to 12.9 mg/kg
wet weight were found during this period (Franklin, 1987).
When samples of fish of different species, collected from major USA
watersheds in 1976, were analysed, PCBs were found in 93% of the
samples. Fifty-eight of the samples had levels exceeding 5 mg/kg, on a
whole fish basis. The PCB concentrations ranged from less than 0.3 to
140 mg/kg, on a whole fish basis (Veith et al., 1979).
Maack & Sonzogni (1988) analysed 98 fish (14 species) of different
sizes from Wisconsin waters, for the presence of PCB congeners. Among
the most prominent congeners were numbers 153/132, 138, 66/95, 110,
180, 70/76, 146, 28/31, 149, 118, and 105. The total PCBs (determined
by adding individual congener concentrations) ranged from 0.070 to
7.0 mg/kg. The mean concentration was 1.3 mg/kg.
Blue crabs ( Callinectes sapidus, an important member of the
estuarine food web), collected from Campbell Creek and surroundings in
South Carolina, were analysed for PCBs in 1985. The highest mean total
concentration was 0.861 mg/kg muscle tissue. In 1986, the mean
concentrations in blue crab collected by 8 stations in the same area
ranged from 0.026 to 0.361 mg/kg muscle tissue. Blue crab (15 samples)
collected from the coast of South Carolina, contained concentrations
of < 0.020-0.372 mg/kg tissue (Marcus & Mathews, 1987).
PCBs concentrations in sea fish were determined in 1971-77 in Japan.
In-shore fish (90 samples) showed concentrations of 0.2-0.72 mg/kg
fresh weight and pelagic fish (112 samples), 0.005-0.265 mg/kg fresh
weight (Watanabe et al., 1979).
Data on individual species of fish, submitted by Japan, showed the
following median levels: barracuda, 70 µg/kg; conger eel, 290 µg/kg;
croaker, 200 µg/kg; flounder (yellow-tail), 90 µg/kg; hair-tail,
100 µg/kg; mullet, 84 µg/kg; and seabass, 110 µg/kg. Median levels for
other species of fish, such as cod, mackerel, pacific saury, rockfish,
salmon, and sardines, were below 100 µg/kg (WHO, 1986b).
Using a very sensitive analytical method, Tanabe et al. (1987) found
the toxic non- ortho-substituted coplanar 3,4,3',4'-tetrachloro-,
3,4,5,3',4'-pentachloro-, and 3,4,5,3',4',5'-hexachlorobiphenyl in
finless porpoise, at concentrations of 13.5, 0.89, and 0.64 µg/kg,
respectively.
Blue mussel (Mytilus edulis) was collected from coastal areas near
Osaka and Hokkaido, Japan, in 1984-86. Depending on the site of
collection, the average PCB concentrations (11-13 samples) ranged from
0.56 to 65.0 µg/kg (Miyata et al., 1987).
5.3.5 Influence of food processing
Fifty striped bass (Morone saxatilis) were analysed for the presence
of PCBs in the fish fillets before, and after, boiling, steaming,
baking, frying, microwaving, or poaching, to study the possible
reduction of the PCB residues by these cooking procedures. PCB
contents were reduced by approximately 10%, by all 6 methods of
cooking. No significant reductions were observed with the other
cooking methods (Armbruster et al., 1987).
5.3.6 Food contamination by packaging materials
When Villeneuve et al. (1973a) analysed packaged food in Canada, they
found that 66.7% of the samples contained PCB levels of less than
0.01 mg/kg, 30.7% contained between 0.01 and 1 mg/kg, and 2.6%
contained more than 1 mg/kg. The highest PCB levels were in a rice
sample (2.1 mg/kg), where the packaging material contained 31 mg/kg,
and in a dried fruit sample (4.5 mg/kg), in a container containing
76 mg/kg. In a survey of packaging containers, approximately 80% were
found to contain PCB levels of less than 1 mg/kg, while about 4%
contained levels higher than 10 mg/kg. The most likely source of PCBs
in packaging materials was the recycling of waste paper containing
pressure-sensitive duplicating paper (carbonless copying paper)
(Masuda et al., 1972).
Relatively high PCB levels in some packaged foods in Sweden, mainly of
imported origin, could be attributed to migration from the packaging
material (Westöö et al., 1971). The highest level encountered was
11 mg/kg in a childrens' breakfast cereal; PCB levels of 70 mg/kg and
700 mg/kg were found in the material of the inner bag containing this
product and in the outer cardboard container, respectively. Up to
2000 mg/kg was found in cartons of other samples.
In the United Kingdom, levels in imported waste-paper, which could be
contaminated with PCBs from carbonless copying paper and subsequently
used to manufacture food contact paper and board materials, were found
to be low, compared with the 10 mg/kg limit for PCBs recommended by
the British Paper and Board Industry Federation for food contact
materials (HMSO, 1989).
5.3.7 Appraisal
Foods have become contaminated with PCBs by 3 main routes:
* accumulation of PCBs in the different food-chains in the
environment and consumption of fish, birds, or other animals and
crops;
* direct contamination of food or animal feed by an industrial
accident;
* migration from packaging materials into food.
During the past years, many thousands of samples of different
foodstuffs have been analysed for PCB contamination. The most common
foodstuffs analysed have been fish, meat, and milk. Many fish samples
have been taken in an effort to monitor aquatic pollution. In
addition, samples have been taken, for regulatory or similar purposes,
from sources suspected of being relatively highly contaminated. The
fact that most samples have not been taken at random, jeopardizes the
proper assessment of the exposure of the general population.
5.4 General population exposure
5.4.1 Air
Relatively high levels of PCBs have been detected in indoor air,
especially in kitchens and offices with electric installations
(Jensen, 1983a) (section 3.2.4 and 5.1.1).
Results from the US EPA indicate PCB concentrations in the air ranging
from 1 up to 50 ng/m3; similar results have been reported from Japan
(WHO, 1976). Assuming a level of 5 ng PCBs/m3 in urban air, a
breathing rate of 22 m3/day, retention and absorption of inhaled
particles/vapour of 50%, and a mean residence time of PCBs in the body
of 3 years, air would contribute 0.8 µg/kg to the PCB concentration in
the body. Higher concentrations of PCBs in indoor air could increase
this estimate (WHO/EURO, 1988).
Van der Kolk (1985) calculated air intake through inhalation for the
Dutch population of about 36 ng/day, a quantity approximately 1000
times lower than the intake with food.
During the manufacture, formulation, or use of PCBs, where levels in
the workroom air correspond to exposure limit values, varying between
0.1 mg/m3 and 1 mg/m3, the calculated mean intakes would range
between 1 and 10 mg during an 8-h workshift. In some occupational
situations, much higher concentrations have been measured and the
estimates of intakes would be higher (WHO/EURO, 1987).
5.4.2 Drinking-water
Levels reported in drinking-water are typically between 0.1 and
0.5 ng/litre. Even assuming a PCB level of 2 ng/litre in
drinking-water, consumption of 2 litre/day contributes 0.04 µg/kg body
weight to the PCB concentration in the body. This additional quantity
is negligible in comparison with the intake via food (WHO/EURO, 1988).
5.4.3 Intake by infants through mother's milk
The daily intake of PCBs was calculated in breast-fed infants in the
countries participating in a monitoring study by Slorach & Vaz (1983,
1985) (Table 19).
The intakes in EEC countries were calculated to range from 3 to
11 µg/kg body weight per day, compared with 0.12-0.3 µg/kg body weight
for bottle-fed infants in Denmark (WHO/EURO, 1985).
In Yusho infants with clinical symptoms of poisoning, the daily intake
of PCBs with breast milk was calculated to be 70 µg/kg body weight
(Jensen, 1983b) (see section 9.1.2.2).
5.4.4 Infant and toddler total diet
Johnson et al. (1979) analysed the average diet of 6-month-old infants
and 2-year-old toddlers for the presence of PCBs. Ten market baskets
were collected in 10 cities in the USA. The foods were prepared in the
manner in which they would be prepared and served in the home. Trace
amounts of PCBs were detected in only one infant and one toddler diet.
In the USA, Gartrell et al. (1986b) found a daily intake of 0.011 µg
PCBs/kg body weight in infants consuming infant diets in 1978. In the
years 1979, 1980, and 1981/82, the intake was below the detection
level. The intake by toddlers was 0.099 µg/kg body weight in 1978 and
not detectable in the following 3 years.
Tuinstra et al. (1985a) analysed samples of infant food from the Dutch
market and found average PCB levels of 0.1-0.2 µg/kg food (the maximum
level found was 1.1 µg/kg).
5.4.5 Total intake by adults via food
The oral consumption of contaminated products is presumed to be the
main route of exposure to the PCBs.
Table 19. Calculated daily intakes of PCBs by breast-fed infants (µg/kg body weight)a
Country/area Year(s) Calculation according Calculation according
to US FDA methodb to national methodc
median maximum median maximum
Belgium,
Brussels 1982 3.6 10.4 NR NR
China,
Beijing 1982 NR NR 0.45d 0.45d
Israel,
Jerusalem 1981/82 2.0 9.5 NR NR
Germany,
Hanau 1981 NR NR 9.5 45
Japan,
Osaka 1980/81 1.6 4.4 2.3 6.3
Sweden,
Uppsala 1981 4.4 8.1 5.9 11
USA
22 states 1979 4.5e 13.5 4.5e 22.5
Yugoslavia,
Zagreb 1981/82 2.8 7.2 2.8 7.7
a Assuming a milk consumption of ca 130 g/kg body weight and a milk fat content of
3.5% (w/w). Calculations based on data for all mothers studied. Results for
different methods of PCB analysis shown separately.
From: Slorach & Vaz (1983, 1985); Van der Kolk (1984b).
b Sawyer method.
c "Own method".
d PCB level below limit of detection (0.1 mg/kg fat) in milk samples.
e PCB level below limit of detection (1 mg/kg fat) in milk samples.
NR = No data on levels in milk reported.
It has been stated that the major part of the human dietary intake of
PCBs is from fish (Berglund, 1972; Hammond, 1972). This may well be
true in areas such as Japan or certain localities near the North
American Great Lakes, where fish from polluted waters may form a
relatively large part of the diet. Several investigators from Japan
have measured the daily intake of PCBs in food; the highest mean value
recorded was 48 µg/day, of which 90% was from fish (Kobayashi, 1972);
the lowest was 8 µg/day (Ushio et al., 1974).
In much of Europe and North America, however, the daily intake of fish
is in the region of 30-40 g, and most of the fish is taken from waters
of low pollution with PCB levels in the fish not exceeding 0.1 mg/kg.
Berglund (1972) has estimated that the daily intake of PCBs from fish
in Sweden is in the region of 1 µg, though if the fish consumed were
solely Baltic herring, the intake would be about 10 µg/person. It is
difficult to make an assessment of the PCB intake from foods other
than fish. Westöö et al. (1971) in their extensive study of the
Swedish diet, reported that most foods contained PCB levels of less
than 0.1 mg/kg; and concluded that this corresponds to a daily intake
of less than 100 µg.
Weekly intakes in the range of 23-889 µg/person have been reported
from the USA (OTA, 1979). The higher range concerns people consuming
more than 12 kg/year of Lake Michigan fish.
The intake of total PCBs by the general adult population depends
greatly on the geographical area and food habits.
5.4.6 Total diet/market-basket studies
Data on total-diet studies of PCBs have been reported from a few
countries. These reported intakes show a wide variation, which can
partially be explained by methodological factors, such as the ways in
which samples below the limits of determination are considered,
especially when noting the different limits of determination.
Considering the available data, an average intake of 5-15 µg/day for
the non-occupationally exposed population in industrialized countries
may be the best available estimation.
These estimates apply to the average diet of an average adult citizen.
In practice, few people are really "average" in their consumption
pattern. Given the widespread nature of the contamination, however, a
higher intake in one food group is more or less balanced by a lower
intake in another food group with an equal calorie intake. Total
intake will certainly be higher for diets with a more than average
calorie content (van der Kolk, 1985).
Gartrell et al. (1985) determined the total intake of PCBs by 16- to
19-year-old males in the USA. The samples represent a typical 14-day
diet. Approximately 120 individual food items (of 12 food groups),
including drinking-water, were collected for each market-basket sample
in 20 cities in the period 1979-80. Only 2 samples of meat, fish, and
poultry contained PCBs with an average concentration of 0.002 mg/kg.
Gartrell et al. (1985, 1986a) reported a daily intake in the USA of
0.016, 0.027, 0.014, 0.008, and 0.003 µg PCBs/kg body weight during
the years 1977, 1978, 1979, 1980, and 1981/82, respectively.
Manske & Johnson (1975) collected 35 market baskets in 32 cities over
the period 1971-72. PCB residues were found in the range of
0.035-0.15 mg/kg in 51 composites. Fish and oils, fats, and
shortenings contained the highest levels. The same authors (Manske &
Johnson, 1977) carried out a market-basket study representing the
basic 2-week diet of a 16- to 19-year-old male. The various foods were
prepared in the manner in which they would normally be served and
eaten. Thirty market-baskets, containing 12 classes of foods (in total
360 composites) were collected in 30 cities in the period 1973-74. A
trace of PCB was found once in whole milk, ground beef, and fish
fillet.
The FDA revised the concept of the Total Diet Study in 1982. As
discussed by Gunderson (1988b), the Total Diet Study conducted before
1982 was based on a "composite sample approach", regardless of the
diet involved. The revised study is based on updated dietary survey
information and allows the "total diet" of the US population to be
represented by a relatively small number of food items for a greater
number of age/sex groups. The daily intake expressed in ng/kg body
weight per day for PCBs (Aroclor 1221, 1242, and 1254) in 1982-84 for
the age groups 6-11 months, 2 years, 14-16-year females, 14-16-year
males, 25-30-year females, 25-30-year males, 60-65-year females and
60-65-year males were: 0.8, 1.2, 0.4, 0.5, 0.5, 0.6, 0.4, and
0.5 ng/kg body weight per day, respectively (Gunderson, 1988b).
Foods, representative of Canadian eating habits, as determined by a
national nutritional survey, were prepared for eating, categorized,
and blended into 11 different composites representing the dietary
intake for 5 cities over the period 1976-78. It concerned 194 samples,
collected in winter and in summer. The average dietary intake was
0.001 µg PCB/kg body weight (McLeod et al., 1980).
Over a period of 2 years, 126 different food items of a market-basket
of 16- to 18-year-old males were purchased every 2 months in the
period 1976-78, in the Netherlands. The foodstuffs were prepared for
eating and were combined in 12 commodity groups. The mean
concentration and range of PCBs in 5 food classes was:
Class Mean concentration Range
(mg/kg on fat basis)
Meat, poultry, and eggs - 0.13-0.17 (2)a
Fish 0.07 0.04-0.24 (7)
Dairy products - 0.04-0.06 (2)
Sugar and sweets - 0.08 (1)
Drinks, drinking-water - 0.035 (1)
a In parentheses: number of positive composites.
The authors calculated a daily intake of PCBs of 15 µg/person (a
maximum level was 90 µg/person (de Vos et al., 1984). In the period
May-July 1976, 100 total diets (summer meals) were collected and
besides organochlorine pesticides, PCBs were determined as
decachlorobiphenyl, after perchloration, and calculated as Aroclor
1260. The mean intake of PCBs/person per day was 11.6 µg with a range
of 3-71 µg (Greve & van Hulst, 1977; Greve & Wegman, 1983; van der
Kolk, 1985).
In 1978, another survey was carried out with 100 total diets during
the winter (winter meals). It was estimated that the daily intake was
6 µg/person (range 1-19 µg).
Zimmerli & Marek (1973) studied the total human intake of PCBs from
prepared meals in 1971-72 in Bern, Switzerland. Five typical total
diets were composed and analysed. The intake of PCBs, especially with
daily diets containing cheese, meat, fish, or fat, ranged from 6 to
84 µg.
According to a calculation by Summerman et al. (1978), the average
weekly intake of PCBs in the Federal Republic of Germany was about 215
and 268 µg/week for females and males, respectively. Much lower
figures, 36-44 µg/week, were calculated by Klein (1983).
A survey of the daily PCB intake from the total diet of Japanese women
(number of samples varied from 18 to 60) was performed for the years
1972-76. The daily intake of PCBs averaged approximately 10 µg/person
(range 2.8-21.2 µg). The main source of PCBs in the diet of Japan was
in-shore fish. There was no clear change in daily intake over the
5-year period studied (Watanabe et al., 1979).
Ushio & Doguchi (1977) studied the dietary intake of PCBs in Tokyo.
They found an average daily intake of PCBs of 6.3 µg/person (range,
trace-17 µg/person). It was concluded that the dietary daily intake of
PCBs for the majority of the population of Tokyo rarely exceeded
20 µg/person, when no heavily contaminated fish were consumed.
Yakushiji et al. (1977) found that the PCB daily intake through meals
of unexposed adults living in Osaka prefecture, was 3-20 µg/day.
Data for PCBs in the diets of Canada, Guatamala, Japan, the United
Kingdom, and the USA over the period 1972-83 were summarized by
Gorchev & Jelinek (1985). The mean dietary intake reported was at, or
below, 0.06 µg/kg body weight, the mean intake per person ranged from
< 0.01 to 0.12 µg/kg body weight (Slorach et al., 1982; WHO, 1986b).
5.4.7 Total intake of major congeners by adults via food
In the Federal Republic of Germany, the daily intake of the 3 PCB
congeners numbers 138, 153, and 180, together with the different food
items, was calculated. The intake (µg/day) with meat and meat products
was 0.30; with fish and fish products 0.36; eggs and egg products
0.008; milk and milk products 0.40; cheese 0.11; butter 0.39; fats and
oil 0.098; bread and pastries 0.17; potatoes 0.081; vegetables 0.11
and fruits 0.082 (DFG, 1988).
5.4.8 Time trends in different matrices
Although many countries introduced severe restrictions on the
manufacture, use, and disposal of PCBs many years ago, it is difficult
to discern any marked decline in the levels in human milk fat, from
the published data.
Levels of PCBs were estimated in 1085 samples of different cereals,
collected in the Federal Republic of Germany over the period
1972/74-1984. The levels, which were the highest in 1972/74 0.04 mg/kg
(0.005-0.12 mg/kg), decreased during the years to 0.004-0.005 mg/kg
dry weight in 1984 (DFG, 1988).
Data from the Federal Republic of Germany showed no clear trend in PCB
levels in human milk during 1975-79 (Slorach et al., 1982). The same
was found in the Netherlands over the period 1974-83 (Greve & Wegman,
1984).
Japanese data showed a decline in PCB levels in the fat of whole cow's
milk during the period 1972-79. A decline was also found in PCB levels
in finfish from coastal waters and in total marine fish (Slorach et
al., 1982).
A downward trend was found in human milk from Japan over the period
1972-80. Each year, a large number of samples (361-877 samples/year)
were analysed. In 1972, the median level was about 0.8 mg/kg and, in
1980, 0.5 mg/kg, on a fat basis. A gradual decline was observed
(Slorach & Vaz, 1983).
In Canada, human milk and adipose tissue from Ontario residents were
analysed over the period 1969-74. The values found did not indicate a
trend in this period.
The mean total PCB intakes determined in the FDA Total Diet Study, for
the period 1971-87, for a typical "adult" diet, represented in Fig. 4,
reflect that of a 14- to 16-year-old male during 1982-87. A clear
decline was shown from approximately 7 µg/person per day to less than
0.1 µg/person per day (Gunderson, 1988a).
The daily intake of PCBs, expressed as ng/kg body weight per day, by
6-month-old and 2-year-old children in the years 1980, 1981/82, and
1982/84 did not show a trend, while, in adults, a decrease from 8 to
0.5 ng/kg body weight per day was observed over the same years
(Gunderson, 1988b).
5.5 Concentrations in the body tissues of the general population
The PCB levels in body tissues are a good indication of the overall
and total exposure of the body to PCBs.
Several factors may influence the concentrations of PCBs in body
tissues, including duration and level of exposure, the route and
pattern of exposure, the chemical structure of the PCB (degree and
position of chlorination in the molecule), the amount of adipose
tissue, other simultaneous exposures, as well as other biological
parameters.
5.5.1 Adipose tissue
In general, while highly chlorinated congeners accumulate more easily,
a lower degree of substitution provides more possibilities for
hydroxylation and facilitates excretion. Factors other than the degree
of substitution also affect accumulation, particularly the position
and pattern of substitution (WHO/EURO, 1987).
The available information on the occurrence of PCBs in the body fat of
the general population is summarized in Table 20.
Brown et al. (1987a,b) examined patterns of PCB congeners remaining in
sediments after spills of commercial mixtures of Aroclor. Sediment
from 5 different sites was examined. Shifts in gas chromatographic
peak distribution were indicative of dechlorination of congeners by
anaerobic bacteria in the sediment. Analysis of sediment from
different depths indicated less difference from the original traces in
superficial layers and the greatest shift in deeper layers of the
sediment cores. They concluded that dechlorination had taken place and
deduced several different processes involved by comparison between
sites. Six of these processes have been characterized in detail, each
presumed to be mediated by different populations of anaerobic
bacteria, with different selectivity for different congeners in the
PCB mixture. The point of most interest was that congeners with high
degrees of chlorination were selectively dechlorinated by these
anaerobic organisms. Whilst dechlorination still leaves the mass of
PCB intact, congeners with lower chlorination can be more readily
degraded by aerobic bacteria. This anaerobic dechlorination,
therefore, enables further degradation to take place elsewhere and
contributes significantly to the detoxification of the PCBs. While the
combined meta- para selective dechlorinating/oxidizing action of
sediment microbes for PCB residues is likely to be detoxifying, with
respect to dioxin-like effects, there are reservations about whether
this action would be detoxifying in respect of other, more subtle
toxic effects of PCBs and their degradation products, known (such as
the potential reproductive toxicity of the hydroxylated,
ortho-enriched PCBs from sediment microbe action) and unknown. This
is why it is important to study not only the disappearance of PCBs,
but also the exact nature and amounts of the degradation products
(McKinney et al., 1990). Two broad categories of transformation have
been observed: the first dechlorinates in the ortho, meta, and para
positions and the potential for the dechlorination of biphenyls is
related to the reduction potential of the compound, the second
dechlorinates only in the meta and para positions, and the
reactivities of the congeners relate to the molecular shape. The
second category suggested to the authors an active site on a
dechlorinating agent that would be roughly conical with a reducing or
hydrogenating site at the apex. In this schema, para-substituted
molecules could enter the site directly, enough rotation of the
molecule would be possible for the accommodation of meta, but not
ortho, substitution. Quensen et al. (1988) demonstrated this
dechlorinating capacity of anaerobic bacteria from Hudson River
sediments in the laboratory. Dechlorination occurred primarily from
the meta and para positions; ortho-substituted congeners
accumulated selectively. The fastest rate of dechlorination occurred
at the highest exposure used (700 mg Aroclor 1242/kg); 53% of the
total chlorine was removed over a 16-week incubation period. During
incubation, the proportion of mono- and dichlorobiphenyls increased
from 9 to 88%. The authors believed that a sequential anaerobic to
aerobic system could be devised for the biological degradation of
PCBs.
4.2.2.2 Fungi
Wallnofer et al. (1973) incubated a soil fungus Rhizopus japonicus
in a medium containing 3H-labelled 4-chlorobiphenyl or
4,4'-dichlorobiphenyl. After incubation for 1 week, the fungal
mycelium was filtered out. Scans of TLC plates indicated a
hydroxybiphenyl derivative present in the filtrate of both cultures.
To further identify the metabolite, larger amounts of unlabelled
4-chlorobiphenyl were added to a similar culture. The NMR and mass
spectra were identical to a synthetic sample of 4- chloro-4'-hydroxy-
biphenyl; mixed melting point determination showed no depression.
Further positive identification of the product was not possible,
because of limited material, but the experiment indicates the
probability of degradation of biphenyl to a hydroxy derivative by a
fungus.
4.2.3 Photodegradation
Several authors have reported that simple chlorinated biphenyls, as
well as complex commercial PCB mixtures, undergo photoreduction in
organic solvents (Safe & Hutzinger, 1971; Hustert & Korte, 1972; Ruzo
et al., 1972, 1974, 1975; Sawai & Sawai, 1973; Koshioka et al., 1987)
and aqueous systems (Crosby & Moilanen, 1973; Bunce, 1978) in the
laboratory. Herring et al. (1972) found that PCBs degraded faster in
hexane solution than in aqueous solution and slower in benzene
solution.
Bunce et al. (1978) posed the question of the environmental
significance of the photodegradation of PCBs and tried to estimate the
likely degree of photolysis under real environmental conditions,
rather than in solution in organic solvents at high concentrations.
The current best estimate suggests that significant amounts,
particularly of higher chlorinated PCB congeners, might be degraded in
water by the action of sunlight.
4.2.4 Bioaccumulation, distribution in organisms, and elimination
Polychlorinated biphenyls accumulate in almost all organisms, because
of their high lipid solubility and slow rate of metabolism and
elimination. They accumulate preferentially in fat-rich tissues.
Bioconcentration factors (BCFs) should be interpreted with caution,
since they are simple ratios. The exposure concentration, therefore,
makes a marked difference to the BCF obtained; very low exposure
concentrations are likely to lead to high BCFs, since all the PCBs are
absorbed, whilst high exposure concentration will tend to minimize the
BCFs.
Experimental data on the bioconcentration of PCB mixtures and pure
chlorinated biphenyls are presented in Table 9 for microorganisms,
Table 10 for aquatic organisms, and Table 11 for plants, birds, and
mammals.
4.2.4.1 Microorganisms
Uptake of both pure chlorinated biphenyl isomers and commercial PCB
mixtures by microorganisms is rapid, and high bioconcentration factors
are achieved. While there is a suggestion in studies on some species
that PCB congeners with higher levels of chlorination are taken up
preferentially, in the majority of studies, all PCBs appear to be
taken up equally. Uptake is true absorption; adsorption onto the
surface of the organisms represents little of the uptake. Since
resistant forms of microorganisms take up less PCBs than sensitive
forms and dead cells accumulate more PCBs than live ones, there is
some capacity to exclude the compounds.
Harding & Phillips (1978b) studied the uptake of 14C-labelled
2,4,5,2',5'-pentachlorobiphenyl, at concentrations of 0.31 or
9.86 µg/litre water, by 11 marine phytoplankton species including:
diatoms, green algae, chrysophytes, haptophytes, and dinoflagellates.
The cell density of each culture was maintained at 106-109 cells/
litre. Equilibrium between water and cell concentrations of biphenyl
was reached very rapidly after 0.5-2 h; small motile forms reached
equilibrium within 1 h and large centric diatoms after approximately
2 h. Exposure concentration and cell density, within the range given
above, had little effect on the time-course of uptake. Substantial
interspecies differences in adsorptive capacity were shown by
differences in the Freundlich adsorption constant (log K). A large
centric diatom, Coscinodiscus sp., had the highest log K. Nitzschia
longissima, a penate diatom that has been shown to be resistant to
PCBs (Harding & Phillips, 1978a), had the lowest log K value. The
flagellates, with the exception of Monochrysis lutheri, which has
been shown to be very sensitive to the effects of PCBs, had much lower
log K values than diatoms. Concentration factors, calculated from the
Freundlich adsorption isotherms, ranged between 12 300 and 2 410 000.
Biggs et al. (1980) exposed mixed species of estuarine phytoplankton
(numerically dominated by the diatom Skeletonema costatum) to
14C-labelled PCB (approximately 54% chlorine by weight) at
concentrations of 5.8 or 11.6 µg/litre. At a particle concentration of
25 mg/litre, 19-22% of the labelled-PCB was sorbed on the particles
after a 1-h exposure, with 70-72% in the water. At 4 times the above
particle concentration, 66-69% was sorbed on particles and only 22-23%
was retained in the water. Doubling the amount of 14C-PCB doubled the
mean amount of labelled-PCB in both the particles and the water. The
authors calculated an index of sorption (the ratio of 14C-PCB sorbed
on particles to that in an equal volume of water) at an average of
2 ± 1 × 104. The authors suggested that the higher uptake (88%) of
PCBs found by Södergren (1971) was probably the result of an
unnaturally high cell concentration. Phytoplankton sampled in the
surface waters of Long Island Sound, USA, varied seasonally in
concentration from about 0.5 to 30 mg/litre.
Lederman & Rhee (1982) calculated bioconcentration factors for 3
species of Great Lakes planktonic algae (Table 9). In the case of
Fragilaria crotonensis, the uptake of hexachlorobiphenyl into the
frustule (the siliceous wall of the diatom) was investigated. The
bioconcentration factors for frustules were lower by an order of
magnitude than the factors for live and dead cells. It appears,
therefore, that adsorption on the cell surface contributes only a
little to the bioaccumulation of hexachlorobiphenyl.
Table 9. Bioaccumulation of PCBs: Microorganisms
Organism Biomass Temperature PCB type Duration Exposure Bioconcentration Reference
(cells/ml) (°C) (µg/litre) factora
Green alga 2 × 106 20-25 TeCB 1 h 10 3200 Urey et al. (1976)
Chlorella 2 × 106 20-25 HeCB 1 h 10 7000 Urey et al. (1976)
pyrenoidosa 2 × 106 20-25 OcCB 1 h 10 1600 Urey et al. (1976)
2 × 106 20-25 DeCB 1 h 10 5200 Urey et al. (1976)
Algae 3.2 × 105 HeCB 19 h 1 117 000b Lederman & Rhee
Fragilaria 1.6 × 105 HeCB 19 h 1 313 000b (1982)
crotonensis Lederman & Rhee
(1982)
Algae 3.4 × 105 HeCB 6 h 1 619 000b Lederman & Rhee
Ankistrodesmus 1.7 × 105 HeCB 6 h 1 959 000b (1982)
falcatus 8.5 × 104 HeCB 6 h 1 1 207 000b Lederman & Rhee
(1982)
Lederman & Rhee
(1982)
Algae 1.1 × 106 HeCB 6 h 1 129 000b Lederman & Rhee
Mycrocystis sp. 5.5 × 105 HeCB 6 h 1 170 000b (1982)
2.8 × 105 HeCB 6 h 1 264 000b Lederman & Rhee
(1982)
Lederman & Rhee
(1982)
Table 9. (cont'd).
Organism Biomass Temperature PCB type Duration Exposure Bioconcentration Reference
(cells/ml) (°C) (µg/litre) factora
Fungus 22-25 Aroclor 1254 24 h 0.007 mg/kg 1327b,c Pinkney et al. (1985)
Fusarium 22-25 Aroclor 1254 48 h 0.007 mg/kg 1144b,c Pinkney et al. (1985)
oxysporum
a Concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration factors calculated on a wet weight
basis unless otherwise stated.
b Calculated on a dry weight basis.
c Radioactive isotope used to calculate bioconcentration factor.
Table 10. Bioaccumulation of PCBs: Aquatic organisms
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
American oyster flow WB Aroclor 1016 96 h 0.6 6666 Hansen et al. (1974b)
Crassostrea WB Aroclor 1254 56 d 0.01 165 000 Parrish (1973)
virginica WB Aroclor 1254 392 d 0.01 89 000 Parrish (1973)
Polychaete stat WB Aroclor 1254 5 d 1.1 236 Courtney & Langston
Arenicola marina stat WB Aroclor 1254 5 d 1 mg/kgd 0.24 (1978)
Polychaete stat WB Aroclor 1254 5 d 1.1 373 Courtney & Langston
Nereis stat WB Aroclor 1254 5 d 1 mg/kgd 0.36 (1978)
diversicolor
Water flea flow WB 20-22 Aroclor 1254 96 h 1.1 47 000e* Sanders & Chandler
Daphnia magna (1972)
Amphipod (M) statf WB Aroclor 1254 24 h 0.03 8700 Pinkney et al. (1985)
Gammarus statf WB Aroclor 1254 24 h 195.8 mg/kg 0.118 Pinkney et al. (1985)
tigrinus
Scud flow WB 20-22 Aroclor 1254 96 h 1.6 24 000e* Sanders & Chandler
Gammarus flow WB 20-22 Aroclor 1254 21 d 1.6 27 000e (1972)
pseudolimnaeus
Glass shrimp flow WB 20-22 Aroclor 1254 96 h 1.3 12 300e* Sanders & Chandler
Palaemonetes flow WB 20-22 Aroclor 1254 21 d 1.3 16 600e* (1972)
kadiekensis
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Brown shrimp flow WB Aroclor 1016 96 h 0.9 4222 Hansen et al. (1974b)
Penaeus aztecus
Grass shrimp flow WB 17-28 Aroclor 1254 7 d 2.3 11 000 Nimmo et al. (1974)
Palaemonetes flow WB 17-28 Aroclor 1254 16 d 1.3 14 000 Nimmo et al. (1974)
pugio flow WB 17-28 Aroclor 1254 28 d 0.62 17 450 Nimmo et al. (1974)
flow WB 17-28 Aroclor 1254 35 d 0.62 26 580 Nimmo et al. (1974)
flow WB Aroclor 1016 96 h 0.4 2750 Hansen et al. (1974b)
Crayfish flow WB 20-22 Aroclor 1254 96 h 1.2 1700e* Sanders & Chandler
Orconectes nais flow WB 20-22 Aroclor 1254 21 d 1.2 5100e* (1972)
Stonefly flow WB 20-22 Aroclor 1254 96 h 2.8 2500e* Sanders & Chandler
Pteronarcys flow WB 20-22 Aroclor 1254 21 d 2.8 2800e* (1972)
dorsata
Dobsonfly flow WB 20-22 Aroclor 1254 96 h 1.1 4600e* Sanders & Chandler
Corydalus flow WB 20-22 Aroclor 1254 21 d 1.1 6800e* (1972)
cornutus
Phantom midge flow WB 20-22 Aroclor 1254 96 h 1.3 23 600e* Sanders & Chandler
Chaoboruspuncti (1972)
pennis
Mosquito larvae flow WB 20-22 Aroclor 1254 96 h 1.5 18 000e* Sanders & Chandler
Culex tarsalis (1972)
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Mayfly flow WB 8 Clophen A50 6 d 0.526 2940 Södergren &
Ephemera danica Svensson (1973)
Pinfish flow WB Aroclor 1016 96 h 0.8 2750 Hansen et al. (1974b)
Lagodon flow WB Aroclor 1016 28 d 1 n 25 000 Hansen et al. (1974b)
rhomboides flow WB Aroclor 1016 56 d 1 n 17 000 Hansen et al. (1974b)
Sheepshead flowg WB Aroclor 1016 33 d 1 n 26 000 Hansen et al. (1975)
minnow flowg WB Aroclor 1016 28 d 1 n 54 000 Hansen et al. (1975)
Cyprinodon flowg WB Aroclor 1016 28 d 1 n 22 000 Hansen et al. (1975)
variegatus
Spot flow WB Aroclor 1254 7 d 1 n 7200 Hansen et al. (1971)
Leiostomus flow WB Aroclor 1254 14 d 1 n 17 000 Hansen et al. (1971)
xanthurus flow WB Aroclor 1254 28 d 1 n 37 000 Hansen et al. (1971)
flow WB Aroclor 1254 56 d 1 n 27 000 Hansen et al. (1971)
Atlantic salmon flow WB 10-15 Aroclor 1254 33 d 10 mg/kg 0.39 Zitko (1974)
Salmo salar
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Coho salmon flow WB 17 Aroclor 1254 112 d 0.048 mg/kg 9.79 Mayer et al. (1977)
Oncorhynchus flow WB 17 Aroclor 1254 112 d 4.8 mg/kg 0.79 Mayer et al. (1977)
kisutch WB TeCB 17 d 1 mg/kg 0.144 Gruger et al. (1976)
WB TeCB 35 d 1 mg/kg 0.139 Gruger et al. (1976)
WB PeCB 35 d 1 mg/kg 0.162 Gruger et al. (1976)
WB HeCB 35 d 1 mg/kg 0.151 Gruger et al. (1976)
Channel catfish flow WB 26 Aroclor 1232 150 d 2.4 mg/kg 1.875 Mayer et al. (1977)
Ictalurus flow WB 26 Aroclor 1232 193 d 2.4 mg/kg 1.3 Mayer et al. (1977)
punctatus flow WB 26 Aroclor 1248 193 d 2.4 mg/kg 0.79 Mayer et al. (1977)
flow WB 26 Aroclor 1254 193 d 2.4 mg/kg 2 Mayer et al. (1977)
flow WB 26 Aroclor 1260 193 d 2.4 mg/kg 1.46 Mayer et al. (1977)
flow WBh 24-26 Aroclor 1242 130 d 20 mg/kg 0.72 Hansen et al. (1976a)
flow WB Aroclor 1248 77 d 5.8 56 370* Mayer et al. (1977)
flow WB Aroclor 1254 77 d 2.4 61 190* Mayer et al. (1977)
Table 10. (cont'd).
Organism Stat/ Organb Temperature PCB Type Duration Exposure Bioconcentration Reference
flowa (°C) (µg/litre) factorc
Fathead (M) flow WB 25 Aroclor 1248 250 d 3 approx. 60 000 DeFoe et al. (1978)
minnow (M) flow WB 25 Aroclor 1260 250 d 2.1 approx. 160 000 DeFoe et al. (1978)
Pimephales (F) flow WB 25 Aroclor 1248 250 d 3 approx. 120 000 DeFoe et al. (1978)
promelas (F) flow WB 25 Aroclor 1260 250 d 2.1 approx. 270 000 DeFoe et al. (1978)
d = Days; M = Male; F = Female; DiCB = dichlorobiphenyl; TeCB = tetrachlorobiphenyl; PeCB = pentachlorobiphenyl;
HeCB = hexachlorobiphenyl; OcCB = octachlorobiphenyl; DeCB = decachlorobiphenyl.
a Stat = static conditions (water unchanged for duration of experiment); flow = flow-through conditions (PCB concentration
in water continously maintained).
b WB = whole body.
c Bioconcentration factor = concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration
factors calculated on a wet weight basis unless otherwise stated. * Radioactive isotope used to calculate
bioconcentration factor.
d Sediment.
e Calculated on a dry weight basis.
f Static conditions, but test solution changed at intervals.
g Intermittent flow-through conditions.
h Not including stomach.
Södergren (1971) maintained the unicellular freshwater green alga
Chlorella pyrenoidosa in water (at a cell concentration of
approximately 900 mg/litre) with added nutrient medium containing
3.7 µg Clophen A50/litre, over a period of 7 days. By the end of the
experiment, 88% of the PCBs had been taken up by the alga. The
remaining PCBs were detected in the water, none being found in the air
samples taken. In another study, Urey et al. (1976) found that both
tetrachloro- and hexachlorobiphenyl isomers, at 10 µg/litre, were
concentrated by dead Chlorella pyrenoidosa cells by 6000 and 15 000
times, respectively, after a 1-h exposure. These concentration factors
are approximately twice those for living cells (Table 9). Similar
findings have been noted with other species of algae (Biggs et al.,
1980; Lederman & Rhee, 1982).
The ciliate Tetrahymena pyriformis was exposed to Aroclors 1248
(0.01, 0.1, and 1 mg/litre) and 1260 (0.001, 0.01, 0.1, and
1 mg/litre) for 7 days (Cooley et al., 1973). Uptake of the toxicant
increased linearly with increasing concentration. Concentration
factors ranged from 14.8 to 40.6 for Aroclor 1248 and from 21 to 79
for Aroclor 1260. Approximately 15-20% of Aroclor 1248 was absorbed at
each concentration compared with means of 37-53%, with increasing
concentration, for Aroclor 1260. If the data from Cooley et al. (1972)
on the uptake from Aroclor 1254 is included, it is clear that
T. pyriformis accumulates more PCBs with increasing degree of
chlorination.
Dive et al. (1976) studied the accumulation of 16 pure isomers of PCB
and one commercial product, Pyralene 3010, by the ciliate protozoan
Colpidium campylum, at concentrations of 0.1, 1, or 10 mg/litre for
43 h. The amount of PCBs taken up at 0.1 mg/litre was very similar for
each of the PCB isomers and the commercial product, ranging from 29.4
to 49%. The percentage uptake did not change greatly for the higher
exposures.
4.2.4.2 Plants
Uptake of PCBs into plants from soil is positively correlated with the
soil concentration of the PCBs. Roots accumulate more than stems and
foliage. Bioconcentration factors are low. More lower chlorinated
congeners of the PCBs are taken up, probably because of their greater
mobility in the soil. Much of the uptake is adsorption on the surfaces
of roots and there is little translocation. PCBs found in leaves have
volatilized from the soil. Uptake on root surfaces can be reduced or
eliminated by adding activated charcoal to the soil.
Lawrence & Tosine (1977) found that plants took up significant amounts
of PCBs (30-140% of the applied PCB concentration) from soil treated
with sewage sludge. In a waste PCB spill besides a North Carolina
highway, levels as high as 4700 mg/kg were recorded in the top 3 cm of
soil. Seven months later, the PCB concentrations were unchanged; the
authors believed that this was because the PCBs were bound to
activated carbon that had been used to treat the spill (Pal et al.,
1980).
Strek & Weber (1982) analysed statistically the data from several
literature sources (Iwata et al., 1974; Wallnofer & Koniger, 1974;
Wallnofer et al., 1975; Iwata & Gunther, 1976; Moza et al., 1976a,
1979a,b; Weber & Mrozek, 1979) on PCB uptake by plants, with the
following conclusions.
i. The PCB content of the plant is significantly dependent on the
soil PCB concentration.
ii. There is a significant difference between plant species,
carrots taking up more PCBs than other plants.
iii. There appears to be a limit of the PCB concentration in the
soil at which no detectable PCBs are taken up by the plants.
iv. Roots take up more PCBs than tops.
v. most of the PCBs in roots may, in fact, be adsorbed on the
surface and not actually taken up.
vi. There is a general trend of increasing PCB content with
decreasing chlorination, for pure PCB congeners.
vii. The amount of chlorination seems to have an effect on the
mobility of PCBs within plant parts. Since lower chlorinated
PCBs have been reported to be more mobile in soils than highly
chlorinated PCBs, they may be more readily transported and
available for plant uptake.
Larsson (1987) maintained the macroalga Cladophora glomerata in a
flowing-water, outdoor pool. Sediment contaminated with Clophen A50 at
2.7 mg/kg dry weight was added and PCB residues in the alga were
monitored. The algal concentration was 3.55 mg/kg dry weight within 3
months. Residues had fallen a year later to 0.2 mg/kg, reflecting the
water levels of PCBs. The authors concluded that a partitioning
process governed the uptake of PCBs by C. glomerata in this
experiment, because the alga accumulated the same PCBs and the same
proportion of PCBs that were present in the water.
Table 11. Bioaccumulation, of PCBs: Plants, birds, and mammals
Organism Organ PCB type Duration Exposure Bioconcentration Reference
(mg/kg) factora
Soilb
Beet (Beta vulgaris) plant top Aroclor 1254 39 days 20 0.041c Strek et al. (1981)
Sorghum (Sorghum bicolor) plant top Aroclor 1254 39 days 20 0.003c Strek et al. (1981)
Peanut (Arachis hypogaea) plant top Aroclor 1254 78 days 20 0.024c Strek et al. (1981)
Corn (Zea mays) plant top Aroclor 1254 13 days 20 0.001c Strek et al. (1981)
Carrot root DiCBd 112 days 0.118 2c Moza et al. (1976a)
leaves DiCBd 112 days 0.118 0.92c Moza et al. (1976a)
Food
White pelican carcase Aroclor 1254 70 days 144 14.8 Greichus et al. (1975)
(Pelecanus erythrorhynchos)
Table 11. (cont'd).
Organism Organ PCB type Duration Exposure Bioconcentration Reference
(mg/kg) factora
Chicken fat Aroclor 1242 28 days 100 2.83 Harris & Rose (1972)
fat Aroclor 1254 28 days 100 5.15 Harris & Rose (1972)
fat Aroclor 1260 28 days 100 4.82 Harris & Rose (1972)
Big brown bat carcase Aroclor 1254 37 days 9.4 6.6 Clark & Prouty
(Eptesicus fuscus) (1977)
Mink fat Aroclor 1254 approx. 56 days 1.5 16.5 Hornshaw et al.
(Mustela vison) fat Aroclor 1254 approx. 126 days 1.5 28.5 (1983)
Hornshaw et al.
(1983)
a Bioconcentration factor = concentration of PCBs in organism/concentration of PCBs in medium or food; bioconcentration
factors calculated on a wet weight basis, unless otherwise stated.
b Calculated on a dry weight basis.
c Radioactive isotope used to calculate bioconcentration factor.
d DiCB = dichlorobiphenyl.
Red mangrove (Rhizophora mangle) seedlings were grown for 6 weeks in
soil treated with Aroclor 1242 at concentrations of between 0.038 and
6 mg/kg (Walsh et al., 1974). Low levels (detection limit was
0.1 mg/kg) of the PCBs were detected in the roots at exposure
concentrations of 3 or 6 mg/kg, during the exposure period, but no
residues were found in the stems. Residues were detected in both the
hypocotyls and leaves at application rates of 0.3 mg/kg or more. Leaf
residues did not change with time, but PCB concentrations in the
hypocotyls showed an increase. The highest mean residues of 1.5 mg/kg
were found in the hypocotyl in the highest exposure group.
Iwata et al. (1974) treated soil with Aroclor 1254, at a concentration
of 100 mg/kg, and sowed carrots in the plot 7 months later. The
carrots were harvested 3 or 4 months after seeding. The authors found
that the lower chlorinated biphenyls were more readily taken up from
the soil into the carrot root. Analysis of the carrot peel revealed
approximately 97% of the PCB residue, showing that there is little
translocation within the plant; 23 months after sowing carrots in soil
contaminated with 100 mg/kg Aroclor 1254, dissipation from soil
appeared to parallel the degree of chlorination (Iwata & Gunther,
1976). Analysis of the soil revealed that the lower chlorinated
biphenyls were slowly dissipated while the more highly chlorinated
biphenyls appeared to be unaffected. Small amounts of PCBs were found
in carrot foliage and the authors suggested that the PCB composition
indicated that they came from soil dust. Suzuki et al. (1977) also
found that lower chlorinated biphenyls were preferentially taken up by
plants, following exposure of soybean sprouts to soil contaminated
with Aroclor 1254 or 1242 at 100 mg/kg.
Moza et al. (1976a) found that carrot bioconcentrated
2,2'-dichlorobiphenyl (0.118 mg/kg soil) from soil by a factor of 2
(Table 11). No bioaccumulation was found in sugar beet, but the soil
residue was only 0.029 mg/kg. Carrots were grown in soil amended with
either 14C-labelled 2,5,4'-trichlorobiphenyl at 1.28 kg/ha or
2,4,2',4',6-pentachlorobiphenyl at 1.12 kg/ha for one season (Moza et
al., (1979a). Only 32.5% of the trichlorobiphenyl was recovered, the
rest being lost through volatilization. The carrots had taken up 3.1%
of the applied 14C, representing a concentration factor of 2.8. For
the pentachlorobiphenyl, 58.5% was recovered, 1.4% of which had been
taken up by the carrots. Sugar beet grown in the soil the following
year accumulated only 0.4% of the applied 14C.
In a study by Weber & Mrozek (1979), 14C-labelled Aroclor 1254 was
applied to Lakeland soil at a rate of 20 mg/kg. Activated carbon was
mixed with half the pots at a rate of 3.7 t/ha (3333 mg/kg). The pots
were seeded with either soybean or fescue. After harvesting at 16 days
for soybean and 50 days for fescue, the amounts of labelled-PCBs,
recovered from the plant tops, were 0.016% and 0.17% for the 2
species, respectively. The addition of activated carbon to the soil
reduced the uptake of 14C-PCBs, the recovery of labelled-PCBs being
0.001% and 0% for soybean and fescue, respectively. Strek et al.
(1981) also applied 14C-labelled Aroclor 1254, at the same rate, to
Lakeland soil; several species of crop plants were grown in the soil
and bioaccumulation factors, calculated (Table 11). Addition of
activated carbon (3.7 t/ha), equivalent to 3333 mg/kg to replicate
pots, reduced the uptake of the labelled-PCBs by 80-100%.
When approximately 1 mg 14C-labelled Aroclor 1254/kg was applied to
the centre leaflet of the first trifoliate leaf of 18-day-old soybean
plants, only 6.7% was recovered from the plant after 12 days, 76% of
which was still present in the treated leaf (Weber & Mrozek, 1979).
Mrozek & Leidy (1981) transferred the marsh plant Spartina
alterniflora from the field into soil containing 1 mg Aroclor
1254/kg (dry weight) and harvested the plants after a growth period of
90 days. The plants were found to take up selectively the lesser
chlorinated biphenyls. The authors stated that a further shifting of
the chromatographic pattern of PCBs towards the lesser chlorinated
components in aerial tissues suggested that some alteration of the PCB
mixture occurs in the plant. Mrozek et al. (1982) also found that
Spartina accumulates PCBs from both contaminated sand and mud-soil
systems. The total 14C-radioactivity accumulated in plants grown in
sand systems was higher than that in plants grown in mud. The level of
radioactivity accumulated in the green parts of the plants was similar
in both soil systems.
Moza et al. (1976b) applied 76 mg/kg of 14C-labelled 2,5,4'-tri-
chlorobiphenyl or 133 mg/kg of labelled 2,4,6,2',4'-pentachloro-
biphenyl to the leaves of the marsh plant Veronica beccabunga. Six
weeks later, the total recovery from plant, water, and soil was 3.7
and 18.3%, respectively, 86 and 95% of which was recovered from the
plant. In an earlier study, Moza et al. (1974) applied 14C-labelled
2,2'-dichlorobiphenyl in water or soil to 2 higher water plant species
( Ranunculus fluitans and Callitriche sp.) at concentrations of
13.7 and 14.5 mg/kg, respectively. Four weeks after application, the
results showed that the dichlorobiphenyl was metabolized more readily
after addition to water; the authors suggested the involvement of
aquatic bacteria. When applied in soil, 1.2% of the dichlorobiphenyl
was metabolized. This was contributed to the plant.
Moza et al. (1979b) grew 3-year-old spruce trees (Picea abies) in
soil containing 14C-labelled PCBs at approximately 4.2 mg/litre in
sewage sludge. When analysed 4 years later, only 0.8% (0.5% in
needles, 0.3% in stems) of the applied radioactivity was found in the
trees. Leaching of radioactive substances from the soil was less than
0.1% in the first 2 years and undetectable for the remainder of the
study.
In another study, Fries & Marrow (1981) grew soybean (Glycine max)
in pots, to determine residue contamination in plant tops from
14C-labelled 2,5,2'-trichlorobiphenyl, 2,5,2',5'-tetrachlorobiphenyl
or 2,4,5,2',5'-pentachlorobiphenyl, applied to the surface or
subsurface soil. Each compound was added to the soil at a rate of
2-3 mg/kg and the plants were harvested after a period of 52 days.
Detectable residues were only found in plants grown in surface-treated
soil, and concentrations in the plants increased with increasing
chlorination. Little of the labelled PCBs was lost from
subsurface-treated soil, but 20-30% of the surface-treated PCBs were
lost through volatilization. The authors concluded that the PCB
residues in the plant tops were, therefore, due to foliar
contamination from vapour rather than the uptake from the soil via the
roots. Miyazaki et al. (1975) came to the same conclusion when they
found no absorption or translocation of PCBs in sesame or rice seeds,
following the application of 4 types of Kanechlor (KC300, 400, 500,
and 600) at rates of between 0.1 and 100 mg/kg. But the rice straws
contained PCB levels of 0.02-0.08 mg/kg, which were the same as levels
found in plants from untreated soils.
Beets ( Beta vulgaris L.), turnips ( Brassica rapa L.), and beans
( Phaseolus vulgaris L.) were grown (Sawhney & Hankin, 1984) in soil
to which lake sediment contaminated with PCBs had been added. The
plants were exposed to Aroclor 1248 at a concentration of 80 µg/kg,
Aroclor 1254 at 1880 µg/kg, and Aroclor 1260 at 14 440 µg/kg. When
beets and turnips were grown in the soil for 6 months, the plants
showed greater uptake in the leaves than in the roots. For example,
beet roots contained 15, 16, and 35 µg/kg of Aroclors 1248, 1254, and
1260, respectively, while beet leaves contained 22, 94, and 52 µg/kg,
respectively. Total concentrations of the 3 Aroclors in beet roots and
leaves and in turnip roots and leaves were 66 and 168 µg/kg,
respectively, and 66 and 99 µg/kg, respectively. During a second
growing season, turnips and beans were grown for 6 months without any
additional PCB-contaminated sediment. Comparing PCB levels in turnips
between the 2 growing seasons showed a decrease in Aroclor 1248 uptake
relative to Aroclors 1254 and 1260. This was primarily because of a
large reduction in the amount of Aroclor 1248 in the soil after 1
year, due to degradation and volatilization. In beans, higher PCB
levels were found in the leaves and pods than in the stems and seeds.
Ten sludge application sites were selected within the Ontario area to
determine background heavy metal and PCB concentrations in the soils
and crops. Control sites (without sludge application) were adjacent to
the sludge application sites. Grab samples of liquid sludges applied
at each of the sites were taken for analysis. The soil samples were
taken at a depth of 15 cm. Twenty core samples were taken at 20-m
intervals and combined to form 1 sample. Eight of the application
sites were cropped with corn, one with oats, and one was left without
a crop. At the control sites, 7 were cropped with corn, 1 with oats,
and 2 left without a crop. PCB concentrations in the sludges ranged
from 0.13 to 1.61 mg/kg dry solids. PCB concentrations were in the
range of 0.007-0.025 mg/kg in the soil without sludges, and in the
range of 0.018-0.453 mg/kg air-dry weight in the soil with sludges.
The PCB levels in the crops were close to the control values (Webber
et al., 1983).
Bacci & Gaggi (1985) assessed the influence of translocation on the
concentrations of PCBs in the foliage of different plant species.
Beans, broad beans, tomatoes, and cucumbers were grown, either in soil
with a nominal added concentration of 500 mg/kg Fenclor 64 (similar to
Aroclor 1260), or in clean sand, for 28 days, enclosed in a glass box
with a constant turnover of air. The plants grown in clean sands were
exposed to PCBs by volatilization from other pots containing PCBs,
which were in the same growing box. The PCB peak pattern of both sand
and roots was similar to that of Fenclor 64, whereas the peak pattern
for foliage and air had moved towards lesser chlorinated congeners.
The concentrations of PCBs in the roots of tomatoes grown in
contaminated soil ranged from 105 to 168 mg/kg dry weight. But
translocation through the plants does not seem to be very likely since
there was no significant difference in foliage uptake of PCBs between
plants grown in contaminated soil and plants grown in clean soil.
Foliar uptake ranged from 13.8 to 42.6 mg PCB/kg (dry weight) for the
different species in PCB-fortified soil and from 11.8 to 47.1 mg/kg
for plants grown in clean soil.
4.2.4.3 Aquatic invertebrates
Bioconcentration factors are high for PCBs taken up by aquatic
invertebrates exposed to either pure chlorinated biphenyl isomers or
commercial mixtures in the water. Since PCBs are strongly bound to
sediments, this method of exposure is unrealistic. Addition of
sediment to test tanks decreases the uptake of PCBs, particularly by
organisms living in the upper water. However, there is clear evidence
that PCBs can also be readily absorbed into invertebrates from both
sediment and food. For organisms living on or in, sediment, uptake can
take place from the sediment, via food organisms that have absorbed
the PCBs, and from interstitial water or water immediately above the
sediment layer. A high content of organic matter in sediment decreases
the availability of PCBs for organisms. Uptake is rapid in most cases
and equilibrium is often reached in hours, though it may take weeks in
other examples. Uptake increases with increasing temperature. The
route of uptake is often via the gills, but varies among species. Loss
of PCBs is slow, but residues do decrease on cessation of exposure.
PCB uptake by aquatic invertebrates is transferred to predators and
can also be transferred to the terrestrial environment.
(a) Uptake from water
Vreeland (1974) exposed American oysters (Crassostrea virginica) to
various PCB isomers at concentrations of 5.5, 17, or 60 ng/litre
(which is within the range found in coastal waters) for 65 days.
Equilibrium was reached after approximately 1 month of exposure, with
concentration factors ranging from 1200 to 48 000 for PCB isomers with
2-6 chlorine atoms/molecule. The PCB concentration, after equilibrium
had been reached, was directly proportional to the amount of PCBs
added to the water. Lowe et al. (1972) found a linear pattern of
uptake in young American oysters exposed to Aroclor 1254 at 5 µg/litre
for 24 weeks, followed by a further 32 weeks in clean water. The
oysters already contained 17 mg/kg from a previous exposure and, by
the end of the 24-week exposure period, had accumulated 425 mg/kg (a
steady state was not established). By the end of the 32-week period in
clean water, no PCB residues could be detected. In another study on
uncontaminated young oysters, concentration factors of up to 101 000
were achieved after a 25-week exposure to 1 µg Aroclor 1254/litre.
After 12 weeks in clean water, whole-body residues were reduced to
0.2 mg/kg.
Courtney & Denton (1976) fed hard clams (Mercenaria mercenaria)
Aroclor 1254 adsorbed on the surface of alumina particles, at 1.25 and
12.5 µg/litre, for 21 days. The maximum concentration factor was 1800
for visceral mass, when the clams had been exposed to 1.25 µg/litre
for 18 days. The visceral mass accumulated a 1.4-5.3 times greater
concentration of PCBs per unit time than the muscular foot. Tissue
samples contained relatively more lower chlorinated isomers than the
Aroclor 1254 standard and, faeces and mud samples contained more
higher chlorinated isomers. Following exposure, clams from the lowest
dose group showed little change in PCB content after 3 months in clean
seawater. However, at the higher dose level, there was a significant
reduction in the PCB levels found in the foot after 1 month, but PCB
residues in the visceral mass remained unchanged for 6 months.
Pink shrimp (Penaeus duorarum) were exposed to Aroclor 1254 at a
concentration of 2.5 µg/litre, in flowing water, for 22 days (Nimmo et
al., 1971b). Accumulation was linear for the hepatopancreas and whole
body, but a plateau was reached after 2 days in muscle. Residues in
the hepatopancreas reached 510 mg/kg over the exposure period,
representing a concentration factor of 204 000; over the same period,
50% of the shrimps died. In a separate study, the shrimps were exposed
to 7.5 µg/litre for 16 days followed by an elimination period of 5
weeks in clean water. When calculated on the basis of the total tissue
burden of PCBs, an 80% reduction in the hepatopancreas was found,
concomitant with a doubling of the PCB levels in remaining tissues.
However, when data were presented as a concentration, a linear loss
from the hepatopancreas was seen, with the concentration in other
tissues remaining constant. The authors calculated a half-life for
loss of PCBs from the hepatopancreas of 17 days.
Nimmo et al. (1975) sampled shrimp from Pensacola estuary, USA, and
measured the relative concentration of PCBs in the various tissues.
The hepatopancreas contained the greatest amounts (50-75%) followed by
the ventral nerve. The authors studied the uptake of PCBs by pink
shrimp, experimentally, using various regimes with dosed food or dosed
water. They found the same tissue distribution in pink shrimp that had
been exposed to 0.2 µg Aroclor 1254/litre, in water, for 50 days. They
concluded that most of the PCBs were taken up directly from the water
in both the "wild" and laboratory situation. However, they did not
exclude the possibility of some PCBs being taken up from food, which
was found under some of the laboratory regimes.
To determine whether there was a concentration below which shrimps
would be unable to accumulate PCBs, grass shrimp (Palaemonetes pugio)
were exposed to flowing water concentrations of 0.04, 0.09, or
0.62 µg/litre. Whole-body residues of 0.2, 1.0, and 10 mg/kg,
respectively, were accumulated within 3-5 weeks. Even at the lowest
dose, shrimps accumulated more PCBs than the residues found in control
shrimp. Concentrations in the shrimp did not reach equilibrium during
the 5-week exposure, but the rate of accumulation decreased towards
the end of the exposure. When transferred to clean water, the shrimps
lost most of the PCBs within 4 weeks (Nimmo et al., 1975).
Gammarus oceanus were exposed by Wildish & Zitko (1971) to Aroclor
1254 concentrations of 2.5, 10, or 20 mg/litre for up to 6 h. Uptake
increased with increasing PCB concentration. Uptake decreased to half
of the initial rate after 4-6 h exposure at 20 mg/litre. There was
little or no uptake by dead animals. Although uptake was related to
branchial surface area, branchiae were not necessary sites of uptake,
since uptake could occur at an unchanged rate following branchial
removal. The authors did not find any change in the rate of uptake
during the intermoult stage.
Zhang et al. (1983) exposed Daphnia magna to 14C-labelled
2,2'-dichlorobiphenyl, 2,5,4'-trichlorobiphenyl, 2,4,6,2'-tetra-
chlorobiphenyl, or 2,4,6,2',4'-pentachlorobiphenyl at 50 µg/litre.
Equilibrium was reached after 20 h for all except the pentachloro-
biphenyl, which had not reached equilibrium within 24 h.
Bioaccumulation factors at equilibrium ranged from 3741, for the
dichlorobiphenyl, to 18 144, for the trichlorobiphenyl. Concentration
factors were not significantly related to the water solubility or
chlorine content of the biphenyl, but there was a tendency for the
bioaccumulation factor to increase with chlorine content and
decreasing water solubility. The authors studied the rate of
depuration and found it to increase with increasing water temperature
between 2 and 22°C. The rate of depuration was also faster for the
dichlorobiphenyl than for the pentachlorobiphenyl; after 48 h, the
amount of PCBs remaining in Daphnia was 22% and 77% (at 10-11°C) for
the 2 chlorobiphenyls, respectively.
(b) Uptake from sediment
Sediment was collected from the field and spiked with Phenochlor DP-5
to achieve a final PCB concentration of 0.65 mg/kg dry weight,
compared with 0.2 mg/kg in unspiked sediment (Elder et al., 1979).
Worms (Nereis diversicolor) were then added to aquaria containing
the sediment under flowing seawater. Equilibrium was reached within
40-60 days, by which time both groups had concentrated the PCBs by 3.5
times. Upon transfer from spiked to unspiked sediment, the worms took
2 months to attain body levels of PCBs comparable with those of the
unspiked group. A half-life of approximately 27 days was calculated
for incorporated PCBs.
Fowler et al. (1978) exposed Nereis diversicolor to spiked sediment
containing 9.3 or 80 mg Phenochlor DP-5/kg (dry weight), for 120 days,
compared with 0.11 mg PCB/kg in unspiked sediment. At the beginning of
the study, worms in the unspiked sediment had body residues of
0.59 mg/kg dry weight and reached a steady state at 1.2 mg/kg. Those
exposed to spiked sediment reached a steady state after a period of
approximately 2 months, with concentration factors ranging from 3 to
4. The worms maintained at the highest level of PCBs all died within a
90-day exposure period. When transferred to unspiked sediment for a
2-month period, the worms that had taken up PCBs from the unspiked
sediment lost PCBs exponentially. In a separate study, worms were
exposed to PCBs in water alone at a concentration of 0.57 µg/litre. A
steady state was reached much more quickly (2 weeks) than it was in
the presence of sediment, with a concentration factor of approximately
800. By comparing these results with field monitoring, the authors
calculated the relative importance of the 2 media. They stated that
approximately 99% of the PCBs in these studies was taken up from the
sediment. When the water overlying the spiked sediment was monitored,
28 ng PCBs/litre was measured (not leached, but a contaminant in the
experimental system) reducing the figure of uptake from sediment to
89%.
In a study by Courtney & Langston (1978), 1.1 mg Aroclor 1254/kg was
incorporated into intertidal sand. Specimens of 2 intertidal
polychaetes (Arenicola marina and Nereis diversicolor) containing
mean residues of 0.017 and 0.11 mg PCBs/kg (wet weight), respectively,
were collected. After 5 days in the spiked sediment, they contained
0.24 and 0.36 mg/kg, and, after a further 5 days, 0.39 and 0.49 mg/kg,
respectively. During a 3-week post-exposure period, there was no
significant loss of these PCB residues. The authors achieved
comparable PCB residues in these polychaetes after exposure to
1 µg/litre water or 1 mg/kg sediment.
McLeese et al. (1980) exposed the polychaete worm (Nereis virens)
and the shrimp (Crangon septemspinosa) to sediment containing
0.016-0.58 mg Aroclor 1254/kg (dry weight) for 32 days. Uptake was
found to be dependent on the exposure concentration and, in the case
of the worms, on the exposure period. The accumulation of PCBs was
inversely related to animal size; at 32 days, concentration factors
for worms ranged from 10.8 for 0.6-g worms to 3.8 for 4.7-g worms
following exposure to 0.17 mg PCB/kg. Factors of 3.5 and 1.9 were
found for shrimps weighing 0.1 and 2.9 g, respectively, after exposure
to 0.13 mg Aroclor/kg. Shrimps were found to accumulate, on average,
60% less PCBs than worms per unit weight. During the 26 days following
exposure, there was not any obvious loss of PCBs from the worms.
Rubinstein et al. (1983) collected sediments containing various levels
of pollutants (PCBs, 0.46-7.28 mg/kg dry weight; Cd; Hg) and organic
matter (5.5-22.3%). During a 100-day exposure period, only small
increases in PCB concentrations were detected in hard clam
(Mercenaria mercenaria) and grass shrimp (Palaemonetes pugio).
Higher concentrations of PCBs were accumulated by Nereis virens.
Uptake was found to be more dependent on the organic content of the
sediment than on the exposure concentration. Concentration factors
ranged from 1.59 in a low organic sediment to 0.15 in a high organic
sediment. The authors also calculated the maximum water exposure
concentration eluted from each of the sediments. On the basis of a
concentration factor of 800, calculated by Fowler et al. (1978) for
the uptake from water of Nereis sp., body residues of between 0.007
and 0.034 mg PCBs/kg (wet weight) would have been expected if
accumulation were dependent purely on direct partitioning from water.
However, whole-body residues of PCBs were found to be 0.4-0.63 mg/kg,
suggesting that pathways other than direct uptake from water (e.g.,
ingestion and sorption) contributed significantly to the accumulation
of PCBs by the polychaete.
Freshwater prawns (Macrobrachium rosenbergii) and clams (Corbicula
fluminea) were exposed to contaminated sediments for 48-50 days
(Tatem, 1986). Prawns were exposed to sediment containing
approximately 62 mg PCBs/kg (dry weight) and to the same sediment
diluted with sand to 50 and 10% of the original. Clams were exposed to
100, 50, or 10% of another sediment containing approximately 2 mg
PCBs/kg at 100%. The amount of PCBs accumulated was related to the
exposure concentration, with the highest concentration factors at the
lowest exposure (10%) level. Bioaccumulation factors for prawns ranged
from 0.1 to 0.9 for Aroclor 1242 and from 0.2 to 2.4 for Aroclor 1254,
relative to sediment concentrations. Exposed clams accumulated PCBs
(Aroclors 1242 and 1254) at concentration factors of 0.54-12.52,
relative to sediment. When tissues were analysed for Aroclor 1242 and
1254, maximum concentrations in prawns were attained at 7 and 40 days
for the 2 Aroclors, respectively. Exposure of prawns at 100 and 50%
dilution of sediment killed all the animals after 62 and 70 days,
respectively. Clams survived exposure.
Clark et al. (1986) investigated the accumulation of sediment-bound
PCBs by fiddler crabs (Uca pugilator) and (Uca minax). Mud and
mud/sand sediments were used; both were naturally contaminated with
PCBs and no further PCBs were added. Both species were exposed to a
mud sediment containing 1.04 mg PCBs/kg and to a mud/sand sediment
containing 0.37 mg/kg (dry weight). Concentration factors, after a
28-day exposure, were 0.19 and 0.79, for U. minax, and 0.2 and 0.59,
for U. pugilator, for the 2 sediments, respectively. In a second
study, using mud with 0.97 mg PCBs/kg and mud/sand with 0.55 mg
PCBs/kg, U. pugilator showed concentration factors of 0.58 and 0.71,
respectively, after 28 days. The authors did not find any detectable
PCBs in the overlying water, suggesting that the PCBs are tightly
bound to the sediment and leach out only very slowly. Following
transfer to uncontaminated sediment on day 42, no PCB residues were
detected in U. pugilator on day 56, or in U. minax on day 63.
Lynch & Johnson (1982) exposed the amphipod (Gammarus pseudolimnaeus)
to 2,4,5,2',4',5'-hexachlorobiphenyl added to sediment in flow-through
bioassays. Water overflowing from the tank containing the contaminated
sediment was directed into a second tank where further amphipods were
exposed without sediment. The hexachlorobiphenyl was labelled with
14C and added to the sediment at 1 mg/kg; the system was allowed to
equilibrate for 7-15 days prior to addition of amphipods, which were
sampled from the tanks after 24, 48, 96, and 192 h. In the initial
studies, the specific activity of the labelled hexachlorobiphenyl was
insufficient to detect the hexachlorobiphenyl concentrations in water.
However, it was clear that amphipods in the tank with the sediment
accumulated more hexachlorobiphenyl than animals exposed only to the
water overflow (8.8-10.5 times more PCBs). Removal of organic matter
from the sediment, by combustion, before addition of the PCB,
increased uptake of the hexachlorobiphenyl by increasing the
availability of the material to the Gammarus. In later studies,
specific activity was increased and water concentrations could be
measured. These were very low, ranging between 11 and 35 ng/litre in
the upper tank and 9 and 25 ng/litre in the lower tank. The lower end
of this range was found later in the exposure period suggesting that
less hexachlorobiphenyl was released over time. There was little
difference in concentration between water taken from the surface and
that sampled close to the sediment suggesting rapid mixing of the
overlying water. In this later series of studies, the authors
demonstrated that both the organic matter content of the sediment and
the presence of smaller particle sizes (silt and clay) reduced uptake
of hexachlorobiphenyl by the amphipods. Organic matter was the more
important factor. Adding maple leaves, to give about 70% organic
content in the sediment, reduced hexachlorobiphenyl uptake to between
10 and 20% of that in sediment without organic matter. Very high
bioconcentration factors were calculated relative to the very low
water concentrations of hexachlorobiphenyl (ranging between 27 000 and
1 000 000 in the upper tank and 2000 and 460 000 in the lower tank,
increasing with exposure period). These factors would be very low
relative to sediment concentrations of the PCB. However, it is clear
that the amphipod can accumulate hexachlorobiphenyl, leaching in very
small amounts from contaminated sediment.
Cores of lake sediment complete with overlying water were taken by
Larsson (1984) and transported back to the laboratory, still in the
sampling tube. PCBs were introduced at different dose levels by
injection through silicon septa in the walls of the tubes and spread
evenly 10 mm below the surface. The cores were allowed to stabilize in
the dark for 1 week at which time 80-100 chironomid larvae were
introduced. After 8 weeks, the systems were moved and kept at 20°C in
the light. After 2 days, the chironomid larvae began to pupate and
emerge. The study was terminated after 10 weeks. PCBs were measured in
sediment, larvae, adults, and exuviae (discarded skins after
emergence). Ranges of PCBs in sediment were between 0.5 and 14 mg/kg
giving rise to residues in larvae, exuviae, and adults directly
related to sediment concentrations. There was "biomagnification"
between larvae and adult. There was loss of body weight between the
final larval stage and the adult, but little loss of PCBs (only 17%
was retained in the exuviae). The author stated that the low variation
in uptake between animals is an indication of passive physicochemical
factors being involved in the handling of PCBs by chironomids. Active
uptake via ingestion would be expected to lead to more variation in
results. Meier & Rediske (1984) also monitored the uptake of PCBs from
contaminated sediment into chironomid larvae (Glyptotendipes
barbipes). Concentration factors for Aroclor 1242 from sediment
ranged between 20 and 130 for exposures of between 0.01 and 1.0 mg/kg,
considerably lower than concentration factors relative to water
(10 000 for these organisms) (Sanders & Chandler, 1972). Addition of
oil, commonly found in polluted areas where PCBs spills are likely,
reduced the uptake of PCBs from the sediment.
(c) Uptake from food
A detritus diet containing 17 µg Aroclor 1242/kg (wet weight) was fed
to male fiddler crabs (Uca pugnax) for 34 days (Marinucci & Bartha,
1982). The Spartina detritus was placed in the culture system at the
start of the study and, because of rapid depletion, was renewed after
19 days of exposure. Since PCBs leached continually from the food
source into the water, a second study was carried out to examine the
uptake of PCBs from water alone. Contaminated detritus was mixed
thoroughly with water and allowed to equilibrate for 24 h. Water
levels were found to be 14-15 µg/litre. Aroclor 1242 was accumulated
at a more rapid rate from PCB-laden detritus than from water alone.
The linear accumulation rate from litter was calculated to be 1 µg
PCBs/day per animal whereas, from water alone, the uptake was 0.1 µg
PCBs/day per animal. Aroclor 1242 was highly concentrated in the
hepatopancreatic tissue. It was found that the PCB residue in the
crabs was inversely related to their weight. Comparison of the
concentrations of PCBs in animals of the same weight shows that, at
the end of the 34 day exposure, those exposed to water alone had taken
up approximately half of the PCBs of those exposed to detritus. The
authors concluded that the crabs in the study accumulated a similar
amount of PCBs from both the food and the water.
Pinkney et al. (1985) exposed the amphipod Gammarus tigrinus to
Aroclor 1254 (14C-labelled) in fungus (Fusarium oxysporum) as a
food item. The fungus contained 195.8 mg Aroclor/kg dry weight.
Accumulation of PCBs was rapid, reaching a constant level in the
amphipods of 23 mg/kg after 9-24 h. Similar exposure of the amphipods,
but with exclusion from direct contact with the fungus by Teflon mesh
(to monitor uptake of PCBs leached into the water), resulted in
residues of between 0.16 and 3.3 mg/kg (from concentrations in the
water at 0.03 µg/litre), representing between 0.6 and 13.9% of uptake
from water and food combined. The PCB residues in the amphipods were
also monitored over 144 h on uncontaminated food to measure the
elimination rate. The water was changed every 24 h. Within this
period, 57% of the accumulated PCBs was eliminated.
(d) Comparison of different routes of uptake
In a study by Wyman & O'Connors (1980), the uptake by the marine
copepods Acartia tonsa and Acartia clausi of 14C-labelled Aroclor
1254 from water, inorganic sediment, and food, was monitored over a
period of 48 h. Acartia were exposed to water concentrations of
10 µg PCBs/litre. An asymptotic uptake curve was observed; equilibrium
was reached after 36 h, corresponding to whole-body residues of 248 mg
PCBs/kg (dry weight) for A. tonsa and 223 mg/kg for A. clausi.
During exposure, water concentrations fell rapidly to 5 or 6 µg/litre.
A similar pattern of uptake was found after exposure to sediment
contaminated with 20 mg PCBs/kg with maximum levels of PCBs in
A. tonsa of 22 mg/kg after 30 h. As in the water exposure, levels of
PCBs in sediment fell rapidly from 20 mg/kg to 14 mg/kg and then
slowly to 7 mg/kg at the end of the study. Water levels were initially
0.62 µg/litre and fell to 0.15 µg/litre. Uptake of PCBs by A. tonsa
from phytoplankton contaminated with 80 mg PCBs/kg (wet weight) was
very rapid and reached a maximum after 5 h at 61 mg/kg, but
subsequently declined after exhaustion of the food supply. PCB
concentrations in water were similar to those found when copepods were
exposed to contaminated sediment, copepods exposed to these water
concentrations alone accumulated significantly less PCBs than those
fed PCB-dosed phytoplankton.
McManus et al. (1983) exposed the marine copepod Acartia tonsa to
14C-Aroclor 1254 either in the food, as phytoplankton containing
approximately 1.3 mg PCBs, or in water at 1.5 µg/litre, for a period
of 30 h. For copepods exposed to contaminated phytoplankton, PCB
levels ranged from 117 to 163 mg/kg dry weight. For copepods exposed
to contaminated water alone, levels ranged from 82 to 104 mg/kg. When
transferred to clean water, the authors found that copepods lost PCBs
at a significantly faster rate if they were fed during depuration;
after 36 h, PCB concentrations in copepods fed during deputation were
10 mg/copepod whereas those starved contained 30 mg/copepod. No
significant difference in depuration rate was found between those
exposed via food and those exposed via water. In a second study,
elimination in males and females was compared. Although both sexes
contained similar residues at the start of depuration (117 mg/kg and
95 mg/kg, respectively), after 36 h, females contained significantly
lower levels of PCBs than males. During depuration, faecal pellets and
eggs were analysed; similar levels of PCBs were found in both male and
female faecal pellets during this period, but levels of PCBs more than
four times that in the females were found in eggs (407.5 mg/kg dry
weight after 4 h), indicating that egg production is an important
route for PCB elimination.
4.2.4.4 Fish
Fish of all life stages have been shown to take up PCBs readily from
water; bioconcentration factors are high. Time taken to reach
equilibrium is variable, but often long, in excess of 100 days. PCBs
with greater chlorination are more readily taken up and retained. PCB
body burden tends to increase with age and levels are higher in fish
with a greater lipid content. The accumulated PCBs are concentrated in
lipid-rich tissues. PCBs of lower chlorination are eliminated more
rapidly. Loss of PCBs is evident when exposure ends; an initial rapid
loss is followed by a slower rate of loss. Half-life estimates,
therefore, vary greatly, from a few weeks to several years.
Reproduction, with the production of a large mass of eggs or sperm,
allows loss of substantial amounts of the PCB residue. Depending on
the species, habitat, and behaviour, PCBs can be taken up from water,
sediment, or food to different degrees.
(a) Uptake from water
Califano et al. (1980) maintained larval striped bass (Morone
saxatilis) in Hudson river water (filtered and unfiltered)
contaminated with 14C-Aroclor 1254 at 1.36 µg/litre for a period of
48 h. Whole-body residues for filtered and unfiltered water were not
significantly different at 5 mg/kg and 5.9 mg/kg, respectively. Uptake
between 34 and 48 h was very slow, suggesting a steady state had
already been reached. Exposure of fish for a further 72 h in
unfiltered water, supported this theory. Elimination was slow, only
18% being lost in 48 h following a 24-h exposure.
The PCB uptake pattern in lake trout (Salvelinus namaycush) sac fry
was studied by Mac & Seelye (1981) by exposing them to a nominal
concentration of 50 ng Aroclor 1254/litre for 48 days. Patterns of
accumulation were similar, regardless of how the data were expressed
(wet weight, dry weight, or body burden). PCBs levels increased
slowly, reaching a peak after 32 days (just before completion of yolk
absorption), and then decreased by day 48.
Hansen et al. (1975) exposed different life-stages of sheepshead
minnow (Cyprinodon variegatus) to Aroclor 1016 (Table 10). After a
4-week exposure to nominal concentrations of 1, 3.2, or 10 µg/litre,
adult fish laid eggs containing on average 4.2, 17, and 66 mg/kg,
respectively. DeFoe et al. (1978) exposed fathead minnow (Pimephales
promelas) to Aroclor 1248 or 1260 at concentrations of
0.1-3 µg/litre, for 240 days (life cycle). Bioconcentration factors for
the uptake of PCBs were independent of the PCB concentration in the
water. Residues in the fish reached an apparent steady state within
about 100 days of exposure and growth. Females accumulated about twice
as much PCBs as males, because of their higher body lipid content. The
variability of residues in females reflected the variability of their
lipid content. Although mechanisms for uptake were similar for both
Aroclors, greater body burdens were always achieved with exposure to
Aroclor 1260. Bioconcentration factors ranged from 60 000 to 160 000
for males and from 120 000 to 270 000 for females. After transfer to
clean water, 18% of Aroclor 1248 was lost within 28 days and 15% of
Aroclor 1260 in 42 days. The authors stated that, because of
variations between fish, this 10-20% decline in total body burden of
PCBs was insufficient to indicate definite PCB elimination over this
period.
De Kock & Lord (1988) exposed an estuarine fish, the Cape stumpnose
(Rhabdosargus holubi) to a flowing water concentration of 1 µg
Aroclor 1260/litre for 90 days followed by a 90-day period in clean
water. Equilibrium was reached at 90 days with a concentration factor
of 24 000. The depuration rate was calculated to be 0.014 days,
producing a half-life of 50 days.
Goldfish (Carassius auratus) were exposed to Clophen A50 at levels
of 0.01, 0.05, 0.1, or 0.5 mg/litre for 18 days (Hattula & Karlog,
1973). Rapid uptake was observed with concentration factors of over
1000 at 18 days, but equilibrium was not achieved within this period.
Nearly all the fish exposed to 0.5 mg/litre died within 7 days. After
transfer to clean water, fish that had been exposed to 0.1 mg/litre
for 13 days and had attained body residues of 70 mg/kg lost half of
the PCBs within 3 weeks, but still retained levels of approximately
15 mg/kg, after 70 days.
Yoshida et al. (1973) exposed carp (Cyprinus carpio) to 14C-PCBs
(equivalent to Aroclor 1254) in water or in food. By measuring the
radioactivity, they found similar tissue patterns of uptake from both
water and diet. PCBs were localized in the gall bladder, adipose
tissue, and hepatopancreas and, in particular, the adipose tissue of
the skull.
Hansen et al. (1971) exposed spot (Leiostomus xanthurus) to Aroclor
1254 at 1 µg/litre, for 56 days. Maximum tissue levels of PCBs were
achieved between days 14 and 28. Highest levels were found in the
liver (210 mg/kg, after 28 days) followed by the gills, whole fish,
heart, brain, and muscle. Aroclor 1254 was slowly lost from tissues;
after 84 days in clean water, levels of PCBs had dropped by 73%.
In a study by Braun & Meyhofer (1977), rainbow trout (Salmo
gairdneri) fingerlings were exposed to water concentrations of 2 or
20 µg Clophen C/litre, for 8 weeks. Tissue PCB concentrations for
gills, muscle, and liver were found to be 0.62, 0.82, and 3.47 mg/kg,
respectively, for the lower dose and 12.3, 7.6, and 10.6 mg/kg, for
the higher dose. When fish were held in clean water for 10 weeks,
following exposure to 2 µg/litre for 8 weeks, residues decreased by
half in the liver and had disappeared completely from the gills, but
there was no change in the PCB levels in muscle.
Rainbow trout (Salmo gairdneri) were exposed by Guiney et al. (1977)
to 14C-labelled 2,5,2',5'-tetrachlorobiphenyl at 0.5 mg/litre for
36 h. The tissue distribution of 14C was measured at regular
intervals after transfer to clean water. Carcase, muscle, skin, lower
gastrointestinal tract, and fat contained most of the radioactivity
(88%). During the first 14 days after exposure, radioactivity
increased in adipose tissue, carcase, and eyes. Elimination from most
tissues appeared to be biphasic with a 30% loss within 2 weeks
followed by a loss of only 6% in the following 126 days. Losses from
the bile and blood were very rapid and nearly complete within 14 days.
Based on the initial rate of loss, the authors calculated a half-life
of 1.55 days, however, the second phase of eliminated PCBs suggested a
half-life at 2.66 years. In a similar study, Guiney et al. (1979)
calculated half-lives of 1.76 and 1.43 years for female and male
rainbow trout, respectively, based on fish sampled 2-34 weeks after
exposure. For both sexes the half-life of elimination was recalculated
to 0.52 and 0.54 years between weeks 38 and 52 after exposure (the
spawning season). The increased elimination appeared to be because of
loss via eggs and sperm. Vodicnik & Peterson (1985) found a similar
result after dosing yellow perch (Perca flavescens); an elimination
half-life of 22 weeks was calculated. This was later recalculated to
be <0.7 weeks during spawning, returning to 16.3 weeks after the
completion of spawning.
(b) Uptake from sediment
The uptake of Aroclor 1254 from suspended solids by juvenile Atlantic
salmon (Salmo salar) was studied by Zitko (1974). Aroclor 1254 was
mixed with suspended solids (simulated by SilicAR CC7) in hexane at
5 mg/ml. Fish were exposed to contaminated solids at 1 g/litre for up
to 144 days. Over this exposure period, the salmon accumulated 134 mg
Aroclor 1254/kg.
Stein et al. (1984) exposed English sole (Parophrys vetulus) to a
sediment concentration of 1 mg 14C-Aroclor 1254/kg (dry weight).
Seawater was allowed to flow over the sediment for 6 days before the
fish were added. A steady state of PCBs accumulated in the tissues of
the fish was achieved after 10 days of exposure. Highest residue
concentrations were found in the bile and the liver. Concentration
factors were 10 for the bile and 4 for the liver, with other tissues
individually concentrating PCBs by factors of 3 or less. Simultaneous
exposure of sole to PCBs and 3H-benzo[ a]pyrene (3 mg/kg, dry
weight) reduced the amount of PCBs accumulated. Stein et al. (1987)
collected urban sediment containing aromatic hydrocarbons and PCBs at
32 mg/kg and 2.2 mg/kg dry weight, respectively. English sole
accumulated hepatic concentrations of 1.4 mg PCBs/kg (wet weight) over
a period of 108 days exposure to the urban sediment. This was 8 times
the PCBs accumulated by sole exposed to the control sediment, which
did not contain any detectable PCBs. In another study, the same
authors added a 14C-labelled PCBs tracer to the urban sediment. The
concentration of PCB-derived radioactivity in the liver reached a
steady state after 14 days of exposure; the steady state concentration
in the carcase was found to be significantly lower.
(c) Uptake from food
Lieb et al. (1974) fed rainbow trout Salmo gairdneri on a diet
containing 15 mg Aroclor 1254/kg for 16 or 32 weeks. PCB levels in the
lipid fraction increased rapidly for the first 8 weeks, reaching
equilibrium at about 95 mg/kg. The absolute quantity of PCBs continued
to increase as the fish grew. The trout had retained 68% of the total
PCBs ingested at equilibrium. No elimination was found after transfer
to uncontaminated food at 16 weeks (for a period of 16 weeks), or
after starving the fish for 8 weeks following exposure for 32 weeks.
Reductions in PCB levels were found, but these were cancelled out by
concomitant reductions in lipid content.
Coho salmon (Oncorhynchus kisutch) parr were fed 10 mg chloro-
biphenyls/kg (containing equal parts by weight of 3,4,3',4'-tetra-
chlorobiphenyl, 2,4,5,2',4',5'-hexachlorobiphenyl, and
2,4,6,2',4',6'-hexachlorobiphenyl) for up to 165 days (Gruger et al.,
1975). Most of the PCBs were accumulated in the adipose tissue of the
salmon (51.1 mg/kg total chlorobiphenyls after 165 days). Tissue
levels of tetrachlorobiphenyl were about half those of either of the
two hexachlorobiphenyls throughout the exposure period. When fish were
starved for 48 days, the data indicate mobilization or transformation,
with, for example, chlorobiphenyls in the spleens lowered by half and
in adipose tissue increased 5-fold. Most tissues showed an increase in
PCB levels, especially blood levels. In contrast, when a second group
of salmon were fed on a clean diet, chlorobiphenyls were released from
adipose tissue and levels increased in some other tissues, such as the
lateral line dark muscle tissue. The ratio of the different
chlorobiphenyls remained unchanged during both of these post-exposure
treatments. Gruger et al. (1976) fed juvenile coho salmon diets
containing a mixture of 2,5,2',5'-tetrachlorobiphenyl, 2,4,5,2',5'-
pentachlorobiphenyl, and 2,4,5,2',4',5'-hexachlorobiphenyl
at 1, 2, and 12 mg/kg, for up to 72 days. A steady state appeared to
have been reached between 17 and 35 days at the lowest dose (a whole
body concentration of approximately 0.45 µg/kg (wet weight)); steady
state was not achieved at the other 2 dose levels. All 3
chlorobiphenyls were accumulated to similar levels. Comparing these
data with the study by Gruger et al. (1975), suggests that the
position of the chlorine substitution is an important factor.
Hansen et al. (1976a) fed channel catfish (Ictalurus punctatus) on a
diet contaminated with 20 mg Aroclor 1242/kg. The total burden of PCBs
(µg PCB/fish) increased exponentially with exposure time. When fish
were placed on a clean diet (from day 84 for 56 days) a slight net
decrease in body burden was observed, but levels remained constant
when fish were placed on a clean diet for 56 days after 140 days
exposure. On return to a PCB-contaminated diet, accumulation rates
returned to those previously observed. The authors noted that, during
PCB-free periods, there was a shift in residues from edible carcase to
offal.
Mayer et al. (1977) fed fingerling coho salmon with Aroclor 1254 at
concentrations ranging between 1.45 and 14 500 µg/kg body weight.
Equilibrium was reached after 112 days at concentrations of 1.45,
14.5, and 145 µg/kg, with whole body residues of 0.47, 0.5, and
3.8 mg/kg, respectively. A steady state was reached at the 2 highest
dose levels of 1450 and 14 500 µg/kg after 200 days, with
corresponding residues of 57 and 659 mg/kg. In another study, channel
catfish (Ictalurus punctatus) were exposed to Aroclors 1232, 1248,
1254, and 1260 in the diet at concentrations of 48 or 480 µg/kg body
weight, for 193 days. Equilibrium was only achieved at the lowest
exposure dose of Aroclor 1232, within 150 days, with a whole-body
burden of 4.5 mg/kg. Similar whole-body residues were achieved at the
lowest dose of the other Aroclors, but no steady state was reached. At
the higher dose, accumulation increased in the order Aroclor 1232 =
1248 < 1254 < 1260, with residues ranging from 13 to 32 mg/kg after
193 days.
When Zitko (1974) fed juvenile Atlantic salmon (Salmo salar) diets
containing 10 or 100 mg Aroclor 1254/kg, accumulation reached
equilibrium within 30 days at the lower dose, with a whole-body
residue of approximately 3.8 mg/kg. Equilibrium was not reached within
200 days at 100 mg PCBs/kg. A whole-body residue of 30 mg/kg was
recorded at 181 days.
Zinck & Addison (1974) administered a mixture of 2-, 3-, and
4-chlorobiphenyl to thorny skate (Raja radiata) and winter skate
(Raja ocellata) by intravenous injection. All three congeners were
cleared rapidly from blood plasma, 3-chlorobiphenyl consistently being
cleared more rapidly than the other two. Less than 6% of
3-chlorobiphenyl remained in the plasma after 15 min compared with 30%
for the other chlorobiphenyls. All three accumulated in the other
tissues of R. radiata, principally in the liver and muscle. Tissue
levels of 3-chlorobiphenyl were consistently less than the others
during the 53-h sampling period.
In a study by Guiney & Peterson (1980), both yellow perch (a non-fatty
fish) and rainbow trout (a fatty fish) were dosed with 0.8 µg of
14C-labelled 2,5,2',5'-tetrachlorobiphenyl, either orally or by
intraperitoneal injection. Whole-body elimination was found to be
similar for both species and routes. A 20-30% elimination was observed
after 3-4 days with virtually no more PCBs being eliminated during the
rest of the 32-day sampling period. Tissue distribution varied between
the 2 species; uptake in the perch was mainly concentrated in the
viscera and carcase, whereas, in the trout, skeletal muscle and
carcase were the major sites of uptake.
Niimi & Oliver (1983) calculated the biological half-life of 31
dichloro- to decachlorobiphenyl congeners, 105 days after a single
oral dose of 46-261 mg/kg was administered to rainbow trout (Salmo
gairdneri). Whole-body half-lives increased from 5 days to >1000
days as the number of chlorines on the biphenyl increased. From
structure-activity analysis of half-lives in whole fish, the authors
concluded that elimination was enhanced for congeners with a lower
chlorine content and no chlorine substitutions in the ortho
positions, and for those with 2 unsubstituted carbons adjacent on the
biphenyl.
4.2.4.5 Birds
PCBs are taken up from contaminated food or water and concentrated in
the fatty tissues of birds. PCBs of higher chlorination levels are
accumulated to a greater extent. Egg-laying females can lose
substantial amounts of PCBs from body tissues by transferring the PCBs
to the eggs. Redistribution of residues occurs on starvation (of
significance during the migration of birds in the wild). Expressed as
a whole-body concentration, PCB residues fall during starvation.
However, expressed as a concentration in fat, residues rise. Most
critically, PCB residues in the brain increase during starvation and
this may kill the birds without further intake of PCBs.
Brunström et al. (1982a) injected the yolk of developing hens' eggs,
on day 4 of incubation, with 14C-labelled 2,4,2',5'-tetra-
chlorobiphenyl at a concentration of 5 mg/kg. One hour after
injection, radioactivity was found in the sub-blastodermic fluid, the
highest concentrations being in amniotic membranes. None was present
in the yolk, albumen, or embryonic tissues. Uptake was uniform
throughout the embryo, after one day, and, as tissues developed,
became concentrated in certain of them, such as the liver, kidney, and
fluid brain vesicles, by day 7. 14C was found uniformly in the yolk
after 11-14 days and was highly concentrated in the first bile
produced on day 11. The labelled PCBs accumulated in fatty tissue as
it developed from day 14 onwards. In the hatched chick, large amounts
of radioactivity were found to be concentrated in the gall bladder,
intestine, cloaca, and the coiling of the gizzard. When either
3,4,3',4'-tetrachlorobiphenyl or 2,4,2',5'-tetrachlorobiphenyl was
injected into the air sac of hens' eggs on day 14 of incubation at
0.4 mg/kg, no difference in distribution pattern was observed 1-5 days
later (Brunström & Darnerud, 1983). The highest amounts of
radioactivity were found in the fatty tissue, liver, kidneys, and the
gall bladder, 14C was also found in the bone marrow, the adrenals,
and the gonads, but to a lesser extent. The yolk contained less
radioactivity than the yolk analysed in the previous study by
Brunström et al. (1982a), because the PCBs were administered via the
air sac.
White leghorn hens were exposed to 50 mg Aroclor 1254/litre in their
water for 6 weeks (Tumasonis et al., 1973). PCB residues in the yolks
of eggs laid increased during the exposure period to a peak, after 6
weeks, of approximately 205 mg/kg. When hens were given clean water,
the yolk levels of PCBs quickly dropped within 5 weeks to
approximately 100 mg/kg, and then more slowly until, after 20 weeks
without Aroclor 1254 in their water, the hens laid eggs containing
0.7 mg/kg.
During a 4-week exposure to Aroclor 1242, 1254, or 1260, in the feed
of one-day-old chicks, Harris & Rose (1972) found that PCBs
accumulated in the fat and that this accumulation increased with
increasing exposure concentrations of 100, 200, and 400 mg/kg. At the
2 highest dose levels, the hens accumulated more of Aroclor 1260 than
of the other 2 Aroclors (i.e., 482, 1427, and 2151 mg Aroclor 1260/kg
at the 3 exposure concentrations, respectively). At the highest dose,
there was high mortality during exposure to Aroclor 1242 and 1254 and
this might have affected the residues found.
Greichus et al. (1975) fed white pelicans (Pelecanus erythrorhynchos)
on a fish diet containing 100 mg Aroclor 1254/day, for 10 weeks. PCB
residues were measured in the carcase, liver, feathers, and brain;
mean residues found were 2130, 290, 120, and 110 mg/kg wet weight,
respectively.
In a study by Dahlgren et al. (1972), 11-week-old pheasant (Phasianus
colchicus) were dosed with one capsule per day containing 210 mg of
Aroclor 1254. Birds that died between days 1 and 5 contained, on
average, PCB residues of 520 mg/kg in the brain, 2500 mg/kg in the
liver, and 140 mg/kg in muscle. Birds that were sacrificed over the
same period had mean brain, liver, and muscle PCB levels of 370, 1900,
and 83 mg/kg, respectively. All birds dosed with only 10 mg of Aroclor
1254 per day died within 180 days and contained average brain and
liver residues of 360 and 1200 mg/kg, respectively.
Södergren & Ulfstrand (1972) fed robins (Erithacus rubecula)
mealworms containing 1 µg of Clophen A50/day for 15 days. Brain,
breast muscle, and carcase were analysed and contained mean PCB
residues of 0.35, 0.55, and 4.5 mg/kg fresh weight, respectively. A
second group of robins was starved following dosing and all died
within 48 h. PCB levels were higher in the brain and breast muscle at
1.1 and 1.3 mg/kg, respectively, but carcase PCB levels were lower on
a fresh weight basis at 2.6 mg/kg. When the carcase lost some of its
fat content during starvation, PCB levels in terms of fresh weight
decreased. Consequently, because of the low remaining fat content,
residue levels in terms of fat weight increased. Another group of
birds were fed both PCBs and DDT (10.5 µg/day) for 15 days and then
starved. PCB levels in all 3 tissues analysed were higher than those
in birds administered PCBs alone followed by starvation; residues
were: brain, 9.3 mg/kg fresh weight, breast muscle, 8.8 mg/kg, and
carcase, 4.5 mg/kg.
Cormorants (Phalacrocorax carbosinensis) were kept on a fish diet
contaminated with PCBs for one month, followed by gelatin capsules of
PCBs administered daily for the remainder of the exposure (Koeman et
al., 1973). After 14 weeks, the dose rate of Clophen A60 was increased
periodically during the exposure period from 200 to 500 mg/kg. The
birds died between days 55 and 124, and overall residues of PCBs
increased in the tissues, the longer the birds survived. Total-body
residues ranged from 850 to 2750 mg PCBs/kg (wet weight) at death.
Brain and liver residues ranged from 76 to 180 mg/kg and from 210 to
290 mg/kg, respectively. The fat of 2 birds was analysed for PCBs and
was found to contain 10 300 and 20 500 mg/kg.
Harris & Osborn (1981) dosed wild puffins (Fratercula arctica) by
implantation with 30-35 mg of Aroclor 1254. PCBs were quickly taken up
in fat, with concentrations rising to 10-14 times that in control
birds (highest fat residue 654 mg/kg wet weight), and remaining at
this level for up to 10 months. Levels slowly declined, but were still
twice those of controls after 34 months. PCB concentrations in the
liver and muscle tissue were highest shortly after dosing (48.4 and
25.2 mg/kg, respectively) and declined until, after 16 months, no PCBs
were detectable. Levels of PCBs in the kidneys and brain were variable
with no consistent trends.
Common grackles (Quiscalus quiscula), starlings (Sturnus vulgaris),
red-winged blackbirds (Agelaius phoeniceus), and brown-headed
cowbirds (Molothrus ater), were fed diets containing 1500 mg Aroclor
1254/kg over an 8-day period (Stickel et al., 1984). PCB residues in
the brains of birds that died were found to be higher than those in
birds that were sacrificed over a similar period. PCB residues ranged
from 349 to 763 mg/kg in birds that died and from 54 to 301 mg/kg in
birds sacrificed. Liver and whole-body residues tended to be higher in
birds that died, but they overlapped to a large extent. PCB residues
in whole bodies on a lipid basis showed the most clear-cut difference,
ranging from 22 600 to 98 600 mg/kg for birds that died and from 6690
to 22 500 mg/kg for those sacrificed. PCB residues in grackles
declined slowly, when the birds were placed on a clean diet. From a
whole-body level of 1300 mg/kg, residues declined to 169 mg/kg, 224
days later. The rate of decline was irregular, but a half-life was
estimated at 89 days over this period of loss.
4.2.4.6 Mammals
Olsson et al. (1979) fed mink (Mustela vison) on a diet containing
11 mg PCBs/kg for 66 days. Mink accumulated 310 mg PCBs/kg in
extractable fat over the exposure period. Control mink were found to
contain 14 mg PCBs/kg, and, when the control feed was analysed, it was
found to contain 0.05 mg PCBs/kg. The authors also found a significant
increase in cadmium uptake in the kidneys of PCB-treated animals
compared with controls. In another study on mink (Mustela vison),
Hornshaw et al. (1983) administered various PCB-contaminated fish
diets containing between 0.21 and 1.5 mg PCBs/kg. Adipose tissue
samples were taken after 6-8 weeks and after 18 weeks exposure (Table
11). The amount of PCBs accumulated was directly related to the amount
of PCBs in the diet; mean PCB residues ranging from 4 to 24.8 mg/kg
after 6-8 weeks and from 8.1 to 42.8 mg/kg after 18 weeks. When
expressed as individual congeners, it can be seen that the mink showed
the highest accumulation of the PCBs with the chromatographic peak
corresponding to 2,4,5,2',4',5'-hexachlorobiphenyl. To determine the
rate of PCB elimination, male mink that had been on a fish diet
containing 1.5 mg PCBs/kg for 10 weeks were transferred to a control
diet. Over this period, adipose tissue residues of 32 mg PCBs/kg had
accumulated. Over the 16-week elimination period, 60.3% of the total
PCB burden of the adipose tissue was eliminated. This consisted of a
loss of 87.2% of 2,5,2',5'-tetrachlorobiphenyl, 88.9% of
2,3,6,2',5'-pentachlorobiphenyl, and 55.4% of the hexachlorobiphenyl.
The half-life for total PCBs in mink adipose tissue was calculated to
be 98 days.
Wren et al. (1987a,b) fed mink on a commercial mink food supplemented
with 1 mg Aroclor 1254/kg for a period of 6 months. Male mink had
liver residues of 1.98 mg PCBs/kg after 118 days and 2.8 mg/kg after
183 days exposure. The liver of a female, analysed on day 161
contained a residue of 3.1 mg PCBs/kg. Liver PCB levels in 5-week-old
kits were similar to those in adult mink fed the experimental diet for
several months. Bleavins et al. (1981) measured the relative
importance of placental transfer and milk in the transfer of PCB
residues from mother mink to offspring. Newborn kits contained less
than 0.1% of a dose of PCBs injected into the mother mink. At 2 weeks
of age, the kits contained 1.2% of the dose given to the mother,
suggesting that lactation is a major route of exposing the young to
PCBs and a major route for the loss of PCBs from the mother. Placental
transfer of PCBs was greater in the ferret than in the mink (Bleavins
et al., 1984). The ratio of placental to mammary transfer was 1:15 for
offspring whose mothers were dosed during the first trimester of
pregnancy and 1:7 for mothers exposed during the last trimester.
Big brown bats (Eptesicus fuscus) were fed on mealworm diets
containing 9.4 mg Aroclor 1254/kg for up to 37 days (Clark & Prouty,
1977). In bats sacrificed on day 37, residues ranged from 29 to 121 mg
PCBs/kg (wet weight) for the carcase and from not detectable to
4.2 mg/kg in the brain. Bats that were starved following exposure
showed a significant correlation between increasing brain PCB
concentrations and carcase lipid concentrations. The authors stated
that PCBs increased in brain tissue as carcase fat was metabolized.
Clark (1978) exposed pregnant big brown bats to a mealworm diet
containing 6.36 mg Aroclor 1260/kg for approximately 18-28 days, until
the young were born. Mean carcase levels of PCBs were 20.34 mg/kg in
parent females and 4.38 mg/kg in litters. Levels of PCBs in both
adults and young continued to rise throughout the sampling period; the
longer the gestation time, the higher the PCB level in the sample.
4.2.5 Appraisal
Experimental work on mammals has been concentrated on terrestrial
species. Problems with PCB toxicity are important for marine mammals,
but these are less convenient for experimental study. Results in this
section, therefore, have to be related to field observations on marine
species.
Mink take up more chlorinated components of PCB mixtures and can
accumulate large residues of PCBs. On cessation of exposure, more
tetrachloro- and pentachlorobiphenyls were eliminated than
hexachlorobiphenyl. The half-life for total PCBs was calculated to be
98 days. PCB residues are transferred from mother to offspring. The
relative importance of transplacental transfer and transfer in milk
varies between species. Redistribution of residues takes place on
starvation, which is of significance for migratory species; brain
residues, which may be fatal with no further intake of PCBs, increase
as animals are starved.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Levels in the environment
PCBs were detected in the environment in the late 1960s (Risebrough et
al., 1968; Jensen et al., 1969) and, within a short time, were
reported as contaminants in almost every component of the global
ecosystem including air, water, soil, fish, wildlife, human blood,
adipose tissues, and milk (Holdrinet et al., 1977; Wassermann et al.,
1979; Ballschmitter et al., 1981; Buckley, 1982; Safe, 1982b; Bush et
al., 1985; Kannan et al., 1988; Tanabe, 1988).
The lipophilic properties of PCBs are the basis of the bioaccumulation
and biomagnification that has been demonstrated and, thus, numerous
sources within the environment can lead to human exposure.
High-resolution, gas-chromatographic analysis has shown that the
congener composition and relative concentrations of the individual
components in many PCB extracts from environmental samples differ
markedly from those in the commercial PCBs (Jensen & Sundström, 1974a;
Wolff et al., 1982a; Safe et al., 1985a; Brown et al., 1987a,b).
A major problem with data concerning PCB levels in environmental
samples is that they normally are only available for "total PCBs" and
that there are much fewer data on actual "PCB patterns". Moreover,
when comparing results produced from different laboratories or from
the same laboratory at different times, an additional difficulty may
arise from differences in the sampling and analytical techniques used.
It is difficult, if not impossible, to compare data obtained with
different analytical methods, from different laboratories, and
countries. Nowadays, the older data seem less reliable, especially in
the light of the use of improved analytical methods and better
sampling techniques (WHO/EURO, 1987). A comprehensive review of world
PCB levels was published by Wassermann et al. (1979).
5.1.1 Air
PCB concentrations in air differ markedly from location to location,
with the lower levels found over the oceans or over non-industrialized
regions, such as the Canadian Northwest territories. In general,
levels over industrialized areas or over landfills are the highest.
Apparently, these levels influence PCB levels in rainwater and there
is a gradient of values in air from industrial to rural areas. Some
typical values can be found in Table 12.
MacLeod (1981) described a method for the analysis of PCBs using
low-volume, indoor air sampling to estimate the presence of PCBs in
indoor air in work-places and homes in the USA. Three facilities, an
industrial research facility, an academic facility, and a shopping
complex were sampled. The periods of sampling ranged from 2 days up to
6 months. The average concentrations (calculated as Aroclor 1242 plus
Aroclor 1254) ranged from 44 up to 240 ng/m3. Outdoor levels of up to
18 ng/mg3 were found. In the homes, air samples from 14 areas (of
which 9 were kitchens) were also analysed. The average concentrations
in the kitchens ranged from 150 up to 500 ng/m3 and, in the other
rooms, from 39 to 170 ng/m3. In a library, a level of 400 ng/m3 was
found.
The levels of PCB exposure that may occur in buildings in the USA were
determined by Oatman & Roy (1986). Air samples and surface wipe
samples were taken in 5 state-owned, office buildings and 2 elementary
schools. The average levels of airborne PCBs in buildings with PCB
transformers were nearly twice the levels in buildings without
transformers, i.e., 457 ± 223 and 229 ± 106 ng/m3, respectively. The
mean of the surface wipes taken in buildings without PCB transformers
was 0.17 and that in the buildings with transformers 0.23 µg/100 cm2.
There was a wide variation between the different buildings and, as
shown above, the presence of transformers influenced the indoor PCB
concentrations.
5.1.1.1 Rain and snow
In the Netherlands, at Bilthoven, the PCB-concentrations in rainwater
ranged from 0.01 to 1.5 µg/litre (van Zorge, cf. WHO/EURO, 1985). In
the Federal Republic of Germany, concentrations of 0-4 ng/litre were
found (DFG, 1988).
Table 12. PCB levels in air in several countries
Country Location and/or type of sample PCB levels References
average and/or range
Canada Northwestern territories 0.002-0.07 ng/m3 Bidleman et al. (1978)
Germany Industrial area (Ruhr area) 3.3 ng/m3 DFG (1988)
Non-contaminated area 0.003 ng/m3
Japan Within industrial plants: - PCB vapours 13-540 µg/m3 Tatsukawa & Watanabe
- PCBs on airborne particulates 4-650 µg/m3 (1972)
North Pacific, South Pacific, Indian, 0.1-0.3 ng/m3 Tatsukawa & Tanabe
Antarctic and South Atlantic Oceans (1983)
North Atlantic Ocean 0.5 ng/m3 Tatsukawa & Tanabe
(1983)
Sweden Several locations 0.8a-3.9 ng/m3 Ekstedt & Odén (1974)
USA Near the North-East Coast 5 ng/m3 Harvey & Steinhauer
Over the Atlantic Ocean, 2000 km away 0.05 ng/m3 (1974)
from the industrial complex
several locations 1-50 ng/m3 Panel on Hazardous
Substances (1972)
cf WHO/EURO (1988)
Yugoslavia Bela Krajina: - 300 m from an industrial plant 4-7 µg/m3 Jan et al. (1988b)
- air near a waste landfill 45 µg/m3
- over the River Kruga 2-5 µg/m3
a Limit of determination.
5.1.1.2 Natural gas
PCBs were first identified in gas pipelines in January 1981, when a
PCB-containing oil condensate was found in the gas meters of some
residential customers in Long Island, New York. Voluntary monitoring
of condensate and natural gas by 33 transmission companies, showed the
presence of PCBs in 12 companies. PCBs were also found in gas
pipelines. Condensate is a mixture of heavier hydrocarbons and other
liquids, such as water, that condenses, because the gas is transmitted
under pressure. This condensate tends to collect in pools in the
pipes. In the period 1981-83, 1841 samples of condensate from gas
pipelines were analysed: 659 (35.8%) of the samples contained
< 25 mg/kg; 65.8% of the samples contained <1000 mg/kg, and 0.4%,
> 10 000 mg/kg. The maximum level that was found was 42 394 mg/kg
(Versar Inc., 1984).
In the period 1981-83, 138 samples of natural gas in transmission were
analysed. In 29 samples, PCBs were found with a minimum concentration
of <0.004 µg/m3 and a maximum concentration of 1050 µg/m3. Natural
gas in distribution lines was also analysed in the same period. Out of
528 samples, 224 did not contain any PCBs. The levels ranged from
<0.02 to 51 µg/m3.
Indoor concentrations (kitchens, etc.) were measured in 419 samples in
the period 1981-83. No PCBs could be detected in 49 samples, but, in
the others, levels ranged from <0.01 to 1.08 µg/m3 (Versar Inc.,
1984).
5.1.2 Water
Surface water may become contaminated with PCBs from atmospheric
fall-out or from direct emissions from point sources. Because of
adsorption on suspended particles, PCB concentrations in heavily
contaminated waters may be several times greater than their
solubility. Södergren (1973) reported a seasonal variation, which was
attributed to aerial fall-out.
It has been shown that polluted rivers, lakes, and estuaries have
higher PCB values than non-polluted waters (Table 13). On the basis of
scanty information on PCBs and reinforced by extensive analogue
information on DDT, it has been estimated that, for the Great Lakes of
North America, non-polluted freshwaters might contain less than
5 ng/litre, moderately polluted rivers and estuaries, 50 ng/litre, and
highly polluted rivers, 500 ng/litre. These values can be used to
evaluate those reported by several authors and presented in Table 13.
Table 13. PCB levels in water in several countries
Country Location and/or type of PCB levels References
sample average and/or range
Germany Several rivers 5-103 ng/litre Lorenz & Neumeier (1983)
Netherlands River Rhine (1976/1977) 100-500 ng/litre Wegman & Greve (1980)
Sweden Water entering a treatment 0.5 ng/litre Ahling & Jensen (1970)
plant
Tap water produced at the plant 0.33 ng/litre Ahling & Jensen (1970)
Several rivers 0.1-0.3 ng/litre Ahnoff & Josefsson (1974)
USA Polluted coastal area 100-450 ng/litre Panel on Trace Hazardous
Lake Michigan (1970)a Substances (1972) (cf.
WHO/EURO, 1988)
Distribution system feeding the Fort Edwards reservoirs < 12-160 ng/litre Brinkman et al. (1980, 1981)
in New York (1978)
Hudson River at Fort Edward up to 530 ng/litre Brinkman et al. (1980, 1981)
a Followed by a marked decrease in 1971.
5.1.3 Soil
Soil may become contaminated with PCBs from atmospheric fall-out or
from direct emissions from point sources. The presence and behaviour
of these compounds in the soil depend on substance (congener)-specific
characteristics and on a number of soil parameters. Sorption and
condensation processes in the soil also play a role in the removal of
PCBs. Some values of PCB levels in soil can be found in Table 14.
Klein (1983) found that PCBs accumulate in the sediments of rivers and
lakes in the Federal Republic of Germany and that these levels
indirectly reflect the contamination of water by PCBs. Some values for
PCBs in sediments can also be found in Table 14.
An important, though localized, source of PCB contamination of soil,
can be the use of sewage sludge as a fertilizer in agriculture. PCB
levels varying from 0.1 to 765 mg/kg (dry weight) have been reported
in sewage sludge from different countries, the usual range being 0.1
to 9.0 mg/kg (WHO/EURO, 1987). In the USA, 16 sewage sludge samples
from cities contained a mean Aroclor 1254 concentration of 5.2 mg/kg
dry weight (range 0.01-23.1 mg/kg). Other authors reported a range of
1.5-27.3 µg/litre in 36 raw sewage sludges. Some levels that have been
found for PCBs in sludges are presented in Table 14 (WHO/EURO, 1987).
Five sediment samples were collected from the Waukegan Harbour of Lake
Michigan, Illinois, in 1978. Residues of 3,4,3',4'-tetrachlorobiphenyl
ranged from 0.005 to 27.5 mg/kg and residues of 2,3,4,3',4'-penta-
chlorobiphenyl, from 0.102-131 mg/kg. The total PCB contents of the
sediment ranged from 10.6 to 13 360 mg/kg (Huckins et al., 1988).
5.1.4 Aquatic and terrestrial organisms
PCBs have been measured in a wide variety of biota from many different
locations throughout the world. Only a few illustrative examples are
given here, more comprehensive lists of PCB residues can be found in
reviews by Risebrough et al. (1968); Peakall (1975); and Eisler
(1986). Tanabe et al. (1987) reported that the highly toxic, coplanar
PCBs are as widely spread as general PCB pollution.
In the biota of a small upstate New York public water supply system,
which is near the polluted section of the Hudson River and a disposal
site of PCB-containing waste, PCBs were found in detectable
concentrations (Table 13). Five samples of algae showed Aroclor 1254
levels of <25 (nd)-120 µg/kg dry weight, macro-invertebrates showed
levels between <200 and 3800 µg/kg and vertebrates, between <25 and
1100 µg/kg dry weight (Brinkman et al., 1980, 1981).
Table 14. PCB levels in soils, sediments, and sewage sludge in several countries
Country Location and/or type of sample PCB levels References
average and/or range
Germany Soil without sewage sludge 0.02-0.08 mg/kga Markard (1988)
Soil with sewage sludge 0.05-3.0 mg/kga
Sewage sludge ndb-19 mg/kg
Sediments of contaminated waters 0.1-1.0 mg/kga Klein (1983)
Sediments of several rivers 0.16-0.59 mg/kg DFG (1988)
Agricultural soil 0.03 mg/kg DFG (1988)
Japan Agricultural soil < 1 mg/kg Fukada et al. (1973)
Soil near a factory making electrical components 510 mg/kg Fukada et al. (1973)
Netherlands Sediments from several surface waters < 0.01-1.2 mg/kga Greve & Wegman (1983)
United Soil from a waste disposal area with chemical treatment 4.5-44.8 µg/kg Eduljee et al. (1986);
Kingdom and incineration facilities Badsha et al. (1986);
(Scotland) Grass samples from the same area (foliage) 2.9-64.7 µg/kg Badsha & Eduljee (1986)
Soil of rural areas 8 µg/kga (1-23)
Grass of rural areas 9 µg/kga (7-16)
Soil of urban areas 52 µg/kg (11-141)
Soil of industrial locations 41 µg/kg (20-67)
United Surface soil 2.5 µg/kg Jones (1989)
Kingdom (0.2-12.2)
(Wales)
Table 14. (cont'd).
Country Location and/or type of sample PCB levels References
average and/or range
USA Sediments near a point of accidental release of PCBs 1.4-61 mg/kg Nimmo et al. (1971a)
(Florida)
Escambia Sediments 16 km downstream of this point 0.6 mg/kg
river
Escambia Soil samples from the bank, 6.5 km downstream from the 1.4-1.7 mg/kg
Bay point
a Dry weight
b Not detectable
Serious environmental contamination has been documented in enclosed
water bodies close to urban and industrialized areas, such as the
Great Lakes, the Baltic Sea, and Tokyo Bay. PCB levels in aquatic
organisms reflect these localized high concentrations.
Nimmo et al. (1971a) reported that PCB levels in shrimp from Escambia
Bay, Florida (contaminated by an industrial plant on the Escambia
River) contained between 0.6 and 120 mg Aroclor 1254/kg in 1969 and
fiddler crabs, collected in 1970, contained 0.45-1.5 mg/kg.
When fish, sampled throughout the USA, were analysed by Schmitt et al.
(1983, 1985), the highest levels of PCBs were found in the
North-eastern industrialized areas. Delfino (1979) reported
concentrations ranging from 26 to almost 1000 mg PCBs/kg in fish
collected from the Sheboygan River, Wisconsin, contaminated by a
die-casting plant.
Wiemeyer et al. (1975) analysed osprey eggs in 1968-69 and found
average levels of 2.6 mg/kg in Maryland compared with an average level
of 15 mg/kg in eggs from Connecticut. PCB residues in Connecticut eggs
had not changed significantly compared with those collected in 1964.
Buckley (1982) analysed aspen, sumac, and golden rod plants growing at
various distances (< 1200 m) and in different directions from a PCB
dump in New York State, USA. All the plants were growing beyond a
natural drainage ravine, which prevented contamination of soil and
water by PCBs. Downwind of the site, PCB levels in the plants were
found to be approximately 100 mg/kg dry weight (over 600 times
background levels in plants). Levels above background concentrations
were also found in directions from the site less obviously
contaminated by airborne dust.
Eggs of terrestrial birds collected in a rural environment in Canada
contained lower PCB levels than those sampled from urban areas (Frank
et al., 1975).
In the Great Lakes, the highest levels of PCBs were found in Lakes
Michigan and Ontario for fish (Delfino, 1979) and Lake Ontario for
birds (Weseloh et al., 1979); both lakes receive input from industrial
and urban sites. Glooschenko et al. (1976) found concentrations of up
to 8.1 mg/kg in microorganisms from the middle of Lake Huron.
Weseloh et al. (1983) found that the PCB levels in double-crested
cormorant eggs, collected from Lake Superior during 1972 (average of
23.8 mg/kg fresh weight), were higher than those in cormorant eggs
analysed in other Canadian colonies. Mineau et al. (1984) found that
the locations of herring gull colonies with the greatest mean levels
of PCBs, in each of the Great Lakes, corresponded with the locations
of major sources of the contaminant, as indicated by elevated residues
in sediment.
Muir et al. (1988) determined PCB levels in pooled Arctic cod muscle
(Boreogadus saida) and polar bear fat (Ursus maritimus), and in
the blubber and liver of ringed seals (Phoca hispida) from 3
locations in the East/Central Canadian Arctic. The mean arithmetic
concentrations of total-PCBs in the muscle of Arctic cod of 2
locations were 3 and 5 µg/kg wet weight. The mean concentrations shown
in the tabulation below were found in the blubber and liver of ringed
seals.
Year Number of Sex Arithmetic mean ± SD
samples (µg/kg wet weight)
(blubber)
1972 3 female 639 ± 249
1975/76 5 female 600 ± 99
1983 10 male 794 ± 879
16 female 308 ± 138
1984 19 male 568 ± 287
14 female 375 ± 172
(liver)
1984 19 male 6 ± 4
14 female 4 ± 3
The presence of PCBs in polar bears (Ursus maritimus) was studied by
Norström et al. (1988) in the Northwest territories of Canada. Liver
and adipose tissue specimens were obtained by Inuit hunters from 12
zones over the period 1982-84. A total of 121 samples was obtained.
The mean concentrations of total PCBs in pooled samples ranged from
3.24 to 8.25 mg/kg, on a lipid weight basis. The adipose tissue of
polar bear (10 pooled samples collected in 1982 and 10 samples, in
1984) contained 4.42 and 4.57 mg/kg wet weight, respectively. From
these results, biomagnification factors for the food-chain of the
Arctic cod/ringed seal/polar bears were calculated. For total PCBs,
these factors ranged from 3.7 to 8.8 for fish to seal; from 7.4 to
13.9 for seal to bear, and 49.2 for fish to bear. For individual PCB
homologues, for instance, for fish to bear, these factors ranged from
<0.5 (tetrachlorinated PCBs) to 263.4 for heptachlorinated PCBs.
Niimi & Oliver (1989b) monitored the presence of 92 monochloro- to
decachlorobiphenyl congeners in brown and lake trout, small and large
rainbow trout, and small and large coho salmon from Lake Ontario. Each
sample consisted of 8-12 fish. The highest concentrations were among
the penta- and hexachlorobiphenyl homologues, with 2,4,5,2',4',5'-
hexachlorobiphenyl the most common congener.
Total congener concentrations ranged from 1 to 10 mg/kg in whole fish
and from 0.3 to 4 mg/kg in muscle. The 10 most common PCB isomers were
84, 87/97, 101, 110, 118, 138, 149, 153, and 180, and represented 52%
of the total content. This value did not appear to be influenced by
species or by total concentration.
Huckins et al. (1988) collected fish (1-6 fish of 7 species) from the
Waukegan Harbour of Lake Michigan, Illinois in 1978. The fish samples
were analysed for the presence of 3,4,3',4'-tetrachloro- and
2,3,4,3',4'-pentachlorobiphenyl. Total PCB congener residues averaged
33.4 (2.4-56.6) mg/kg. The concentrations of 3,4,3',4'-tetra-
chlorobiphenyl averaged 45.3 µg/kg (2-89 µg/kg) in the whole body. The
concentrations for 2,3,4,3',4'-pentachlorobiphenyl averaged 229 µg/kg
(80-483 µg/kg).
Five times as much PCBs were found in herrings caught in
industrialized areas of Sweden (near Stockholm) compared with those
caught in the cleaner waters off the Swedish west coast. Levels in
plankton fell progressively with increasing distance from
industrialized areas (Jensen et al., 1972a).
Holden (1973) found levels of up to 235 mg/kg in the blubber of seals
sampled in the polluted coastal areas of the United Kingdom compared
with lower levels (2 mg/kg) from unpolluted areas. Higher levels, (up
to 88 mg/kg) were found in the blubber of toothed whales sampled in
the North Sea, but none was detectable in similar species sampled off
New Zealand and Surinam (Koeman et al., 1972).
Peakall (1975) mapped out the global distribution of PCB levels in
marine plankton. The values for the open North Atlantic (300-450 mg/kg
lipid) were found to be very similar to those collected from polluted
areas, such as the Baltic sea and the Firth of Clyde, in the United
Kingdom. Values in the South Atlantic (12-64 mg/kg) were considerably
lower. The highest values shown were for the Eastern coast of the USA
(up to 3050 mg/kg). There were no values for the Pacific Ocean.
When monitoring PCB levels in fish from the Mediterranean, Albaiges et
al. (1987) found that territorial species reflected local inputs of
the pollutant, but migratory species had baseline levels.
Risebrough & de Lappe (1972) reported PCB levels higher than 3 mg/kg
in fish from the industrialized areas of Tokyo Bay and New York Sound.
Tanabe et al. (1986a) analysed Antarctic minke whales and found that
they contained lower PCB levels than those caught in the Northern
hemisphere (Tanabe et al., 1983). McClurg (1984) also found low levels
of PCB in the Antarctic; Ross seals contained 0.09 mg/kg (in blubber).
Mean levels of 0.69 mg PCB/kg (wet weight), found by Smillie & Waid
(1987) in Australian fur seal blubber, were much lower than levels
found in seals from the temperate Northern hemisphere. Similarly,
Antarctic fish had very low PCB residues, ranging from 0.08 to
0.77 µg/kg wet weight (Subramanian et al., 1983).
PCB residues in biota are usually highest near industrial sources, but
this geographical distribution is becoming less pronounced. In fact,
O'Shea et al. (1980) and Tanabe et al. (1988) found PCB levels in
small oceanic cetaceans to be higher than those reported for
terrestrial mammals and birds. For example, Tanabe et al. (1988) found
the mean level of PCBs in the fatty tissue of the striped dolphin to
be 36 mg/kg wet weight.
Subramanian et al. (1986) analysed subcutaneous fat from Adelie
penguins from the Antarctic and found PCB levels of 0.05 mg/kg fat
weight. This is a factor of 100 lower than that in auks caught in the
northern North Pacific (Tanaka & Ogi, 1984) and a factor of 10 000
lower than residues found in the pectoral muscle (on a lipid weight
basis) of herring gulls in the Baltic (Lemmetyinen et al., 1982).
5.1.4.1 Effect of dredging-contaminated sediment on organisms
Dredging to remove contaminated sediments from the Shiawassee River,
Michigan, increased the availability of PCBs, and, thus, residue
levels, in freshwater clams (64.5-88 mg/kg dry weight) and in fish
(fathead minnow; 13.8-18.3 mg/kg), both during dredging and up to 6
months afterwards (Rice & White, 1987).
5.1.4.2 Relationship to lipid content of organisms
PCBs are accumulated in lipid-rich tissues and care must be taken when
interpreting results between species with different amounts of body
fat. Jensen et al. (1969) found that PCB levels in herring and cod,
from the same area of the Baltic Sea, were 0.27 and 0.033 mg/kg, on a
wet weight basis, respectively, even though the cod is at a higher
trophic level. The 2 species were found to have body fat contents of
4.4 and 0.32%, respectively, and when the PCB residues were
recalculated on a lipid weight basis, herring contained 6.8 mg/kg and
cod, 11 mg/kg.
PCBs are particularly accumulated in animals with large amounts of
fat, such as seals, dolphins, porpoises, and whales (Tanabe, 1988) and
in Arctic and Antarctic birds and mammals. Subramanian et al. (1986)
found PCBs in all Adelie penguins sampled in the Antarctic, an area
known to be relatively low in PCBs; the PCBs were mainly concentrated
in fat-rich tissues. Kawai et al. (1988) measured PCBs in striped
dolphins and found that the tissue level of PCBs depended entirely on
their lipid content and, especially, on the amount of triglycerides in
tissues.
Redistribution of PCBs, from fat to other tissues, occurs in animals
during periods of enforced starvation, such as seasonal food shortage,
hibernation, migration, incubation, and the feeding of offspring.
Subramanian et al. (1986) found that, as individuals Adelie penguins
starved during incubation, residues of PCBs increased with declining
fat reserves concomitant with tissue redistribution. Llorente et al.
(1987) found that migratory duck species had a smaller percentage of
the body burden of PCBs in adipose tissue than a resident species. A
similar redistribution during starvation has been shown in the
laboratory in European robins (Södergren & Ulfstrand, 1972) and big
brown bats (Clark & Prouty, 1977) (see sections 4.2.4.5 and 4.2.4.6).
5.1.4.3 Residues in different trophic levels and effects of diets
In a study by Shaw & Connell (1982), bioaccumulation was increasingly
evident in upper trophic level organisms, such as gulls and pelicans,
in an Australian estuary compared with organisms from lower trophic
levels. Veith et al. (1977) found typical PCB concentrations in Lake
Superior biota to be 0.1 mg/kg for large zooplankton, 0.3 mg/kg for
bottom fish, such as sculpins, and 1 mg/kg for pelagic fish.
When various insects were sampled for PCB residues (Morse et al.,
1987), levels in honey bees ranged from <0.1 to 1.5 mg/kg dry weight.
PCB residues in other species ranged from <0.1 to 2.6 mg/kg, with
predatory wasps containing the highest residues.
Prestt et al. (1970) analysed the livers from various bird species in
the United Kingdom. The highest PCB residues were found in freshwater,
fish-eating species (up to approximately 900 mg/kg). The authors did
not find any geographical pattern of distribution of PCBs in the
species studied.
Frank et al. (1975) collected birds' eggs from the Niagara peninsula
in 1971. Eggs from carnivorous species of birds at the top of the
aquatic food chain contained the highest levels of PCBs
(3.5- 74 mg/kg). Terrestrial carnivores contained lower, but still
relatively high, residues (0.2-1 mg/kg). Eggs from herbivorous and
insectivorous birds contained much lower residues of PCBs. Again, eggs
from terrestrial birds tended to contain lower levels (0.05-2 mg/kg)
than those feeding on aquatic prey (0.14-4 mg/kg). Focardi et al.
(1988) compared the PCB residues in the eggs of 8 species of water
bird. The residues were found to be higher in fish-eating birds than
in invertebrate feeders. The invertebrate feeders tended to contain
higher percentages of the lower chlorinated congeners. Bird species
that fed on other birds or fish had higher liver residues of PCBs than
those feeding on mammals (Cooke et al., 1982). Peregrine falcons,
herons, sparrowhawks, kingfishers, and great crested grebes had
relatively high residues of PCBs. By contrast, golden eagles were only
very lightly contaminated with PCBs.
Bowes & Jonkel (1975) found a similar pattern in Arctic and subarctic
food chains with PCB levels following the pattern: Arctic charfish
< seals < adult polar bears < polar bear cubs.
Mean PCB concentrations of 0.0018 mg/kg were found by Tanabe et al.
(1984) in zooplankton, 0.048 mg/kg in myctophid, 0.068 mg/kg in squid,
and 3.7 mg/kg in striped dolphin (all based on a whole-body, wet
weight basis) sampled from the western North Pacific. The authors
concluded that the bioaccumulation of chlorinated hydrocarbons was
dependent on physical and chemical factors, such as water solubility
and lipophilicity, in the lower trophic levels, whereas, in higher
trophic levels, accumulation was affected by biochemical factors, such
as the biodegradability of pollutants and the metabolizing capability
of the organism.
5.1.4.4 Effects of age, sex, and reproductive status on uptake and
elimination
Bache et al. (1972) found that the burden of PCBs increased with age
in lake trout from Cayuga lake, Ithaca, New York, sampled in 1970
(residues ranged from 0.6 to 30.4 mg PCBs/kg). An age- and
length-related increase in PCBs was found in striped bass from the
Hudson River and Long Island Sound; the author (Connell, 1987) stated
that this observed relationship was due to the slow rate of
bioaccumulation of the PCBs, particularly the higher chlorinated
congeners.
PCBs have been shown to accumulate with age in marine mammals, such as
pinnipeds (Addison et al., 1973; Frank et al., 1973; Helle et al.,
1983) and cetaceans (Gaskin et al., 1983; Aguilar & Borrell 1988;
Subramanian et al., 1988). Helle et al. (1983) found mean levels of
5.1 mg PCBs/kg (in extractable fat of blubber) in newly-born ringed
seal pups, 17.3 mg/kg in seals of 2-4 months of age, and 65.3 mg/kg in
sexually mature adults (4-12 years). However, lower levels of PCBs
have been found in females compared with males (Martineau et al.,
1987) and the age-related increase has often not been found in females
(Addison & Smith, 1974). In many studies, while levels of PCBs in
males have increased with age, those measured in females have fallen
(Born et al., 1981; Gaskin et al., 1983; Aguilar & Borrell, 1988).
Gaskin et al. (1983) found that PCB levels in the blubber of male
harbour porpoises increased from 48.4 mg/kg at birth to 161 mg/kg
after 8 years, whereas, in females, levels fell from 51 to 14.7 mg/kg.
A significant decrease in the PCB levels was found by Subramanian et
al. (1988) in female Dall's porpoises from 2 years of age onwards; 2
years is required for the animals to reach sexual maturity. Excretion
of PCBs during reproduction is known, from the laboratory, to be an
important means of females losing residues. This PCB loss has been
shown to be because of the transfer of PCBs to offspring via milk
during lactation (Addison & Brodie, 1977). Addison & Brodie (1977)
calculated that female grey seals excreted about 15% of their body
burden of PCBs via lactation. In striped dolphins, females transferred
between 72 and 98% of their body burden to the offspring (Fukushima &
Kawai, 1981; Tanabe et al., 1982). It was suggested by Tanabe (1988)
that such large transfer was because of the very high lipid content of
the milk. Relocation of the PCB burden during pregnancy is generally
thought not to be as important; in grey seals, the mother transfers
only about 1% of her body burden to her offspring (Donkin et al.,
1981) and in striped dolphins, only 4-9% (Fukushima & Kawai, 1981;
Tanabe et al., 1982). However, Duinker & Hillebrand (1979) suggested
that a much bigger percentage of female body burden (up to 15%) could
be transferred to the fetus across the placenta of Harbour porpoise.
Clark & Lamont (1976) calculated that female big brown bats
transferred between 17 and 32% of their body burden of PCBs to their
young, during gestation. The concentration of PCBs in adult females
plus their litters declined with increasing age of the female. PCB
levels were 0.83-3.6 mg Aroclor 1260/kg (wet weight) in adults and
0.22-3.3 mg/kg in litters.
When Passino & Kramer (1980) measured PCBs in deepwater ciscoes from
Lake Superior, male fish contained significantly higher levels of PCBs
(2.3 mg/kg wet weight) than females (1.2 mg/kg), eggs containing
0.51 mg/kg. Lemmetyinen et al. (1982) found annual rates of
elimination via egg production of 45% in the female Arctic tern and
24% in the herring gull. Adelie penguins eliminated only 4% of their
PCB body burden after laying their annual clutch of 2 eggs (Tanabe et
al., 1986b). Elimination was thought to be dependent on the relative
weights of the egg and mother.
5.1.4.5 Time trends in residues
Buckley (1983) analysed various species of terrestrial plants from New
York state. Total decreases of 42% in PCB residues were found between
1978 and 1980.
PCB levels in fish in the Hudson River, New York declined between 1977
and 1981. The PCB levels were much higher in the Upper Hudson River
(4217-1431 mg/kg of lipid), near to a major discharge of PCBs, than in
the Lower Hudson River (1604-319 mg/kg) (Sloan et al., 1983).
Frank et al. (1978) measured PCB levels in various fish species from
Lakes Huron and Superior during the period 1968-76. PCB residues
declined in lake trout and lake whitefish in Lake Superior between
1971 and 1975, but increased slightly over the same period in bloaters
and white sucker. In Lake Huron, PCB levels decreased between 1968 and
1971, and, in alewife, rainbow smelt, and walleye, between 1975 and
1976. In some of the study areas, residues increased in cisco, yellow
perch, coho salmon, and splake but, at most locations, and, for other
species analysed, no trends in PCB levels were found. St Amant et al.
(1984) analysed fish from Lake Michigan between 1971 and 1981. An
overall decrease in PCB levels was found for all species monitored
except the walleye. Levels decreased from a maximum of 22.4 mg/kg at
the beginning of the study to 3.8 mg/kg or less in 1981.
Fish from all over the USA were analysed in 1980-81 by Schmitt et al.
(1985) who found a significant downward trend (0.88-0.53 mg/kg PCB;
wet weight) when mean residues were compared with fish collected
between 1976 and 1977 (Schmitt et al., 1983). A similar downward
pattern in residues was found in the Baltic when Moilanen et al.
(1982) compared residues found in pike and herring caught between 1978
and 1982 with those in fish sampled between 1972 and 1978 (Paasivirta
& Linko, 1980). Haahti & Perttila (1988) found a continued decline in
PCB residues between 1979 and 1986, when residues in herring muscle
tissue decreased from 2.7-3.7 mg/kg to 0.3-1.1 mg/kg.
An overall fall in PCB levels was found by Newton & Bogan (1978) in
sparrowhawk eggs during the period 1971-74. Cooke et al. (1982)
analysed liver samples from grey herons, kestrels, and barn owls for
PCB residues during the period 1967-77. They found a significant
decline in PCB residues over the sampling period in all 3 species. The
mean residues in heron, kestrel, and barn owl for the period 1967-71
were 5.77, 1.57, and 0.44 mg/kg, respectively, and for 1977, 0.56,
0.6, and 0.15 mg/kg, respectively. However, Newton et al. (1986), when
analysing sparrowhawk eggs from 1971-80, found that, although levels
had fallen in the early 1970s, they had risen again in the late 1970s
(mean PCB residues in eggs ranged from 16 to 293 mg/kg in lipid). Data
on PCB residues in the livers of kestrel, sparrowhawk, heron,
kingfisher, and the great crested grebe, collected from the late 1960s
up to 1987, were analysed statistically by Newton & Haas (1989). For
the great crested grebe, a significant overall decline in PCB residues
was found when comparing data from 1987 with that from the 1960s. For
the other species, there was no significant difference. Spitzer et al.
(1978) reported that there was no significant change in PCB levels in
osprey eggs collected from the Connecticut-New York area during the
period 1969-76. Similarly, Wiemeyer et al. (1987) did not find any
change in the carcase levels of PCBs in ospreys from the Eastern
United States when comparing the 1971-73 and 1975-82 periods. They did
find that adults contained significantly higher concentrations of PCBs
than immature ospreys.
Blus et al. (1979) analysed brown pelican eggs from South Carolina and
Florida between 1969 and 1976. The highest levels of PCBs were found
in South Carolina (means ranged from 5.25 to 7.63 mg/kg wet weight),
but no significant trend was found during the study period. In
Florida, the authors did not find any significant change in eggs
collected from colonies in Florida Bay and on the Gulf Coast over the
study period (means ranged from 0.62 to 1.18 mg/kg), but the Atlantic
coastal colony showed a significant increase in PCB residues (from a
mean of 2.68 to 6.12 mg/kg) between 1969 and 1976.
In analysing herring gull eggs from the Great Lakes between 1974 and
1978, Weseloh et al. (1979) found a significant decline in PCB
residues from colonies on all the lakes. Lake Ontario, the most
contaminated, showed the biggest decline from 170 to 75 mg PCBs/kg at
one of the colonies, with other less contaminated Lakes, Huron,
Superior, and Erie, showing levels in the range of 50-86 mg/kg in 1974
and 32-46 mg/kg in 1978.
Moksnes & Norheim (1986) analysed herring gull eggs collected from the
Norwegian Coast between 1979 and 1981 and found that the PCB levels
were not significantly different from those in eggs collected in 1969;
mean PCB residues ranged from 1.2 to 6.7 mg/kg wet weight. They found
a small but significant increase in the most persistent congeners and
a significant decrease in DDE and the DDE/PCB ratio, but not in total
PCB levels.
An analysis of the eggs of double-crested cormorant (an inshore-
subsurface feeder), Leach's storm petrel (an offshore-surface
feeder) and Atlantic puffin (an offshore-subsurface feeder) was
carried by Pearce et al. (1989), every 4 years, between 1968 and 1984.
In the Bay of Fundy, Canada, PCB levels declined significantly during
this period in all 3 species. PCB levels in the cormorant were
consistently higher throughout than those in the other 2 species,
ranging from 4 to 29.5 mg/kg (wet weight). Petrel and puffin eggs
collected from the Atlantic Coast of Newfoundland showed lower levels
than those in eggs from both the Bay of Fundy and the St Lawrence
River estuary; as in the St Lawrence River, no significant trend in
PCB levels was observed. A significant decline in PCB residues was
found in gannet eggs collected during the same period from the gulf of
St Lawrence (Elliott et al., 1988).
The frequency of occurrence of measurable PCB residues has increased
in large-scale sampling exercises; PCBs in mallard wings increased
from 39% in 1976-77 (White, 1979) to 95% in 1979-80 (Cain, 1981). Cain
& Bunck (1983) found that, in 1976, 21% of European starlings
collected in the USA contained PCBs compared with 83% in 1979.
Addison et al. (1986) analysed the blubber of Arctic ringed seals
(Phoca hispida) from Holman Island, NWT, Canada, in 1981. They found
mean PCB levels of 0.58 mg/kg (wet weight) in the females and
1.28 mg/kg in the males. These concentrations were significantly lower
than those detected in the same species from this area in 1972. Over
this same period, pp'-DDE levels, although at lower levels, also
fell significantly, but it should be noted that total DDT levels in
blubber are much lower than PCB levels and have not changed
significantly.
5.1.4.6 Seasonal patterns in residues
Jensen et al. (1969) observed that there was considerable seasonal
variation in the fat content of herring caught in the Baltic Sea,
ranging from 1% in the spring to 10% in the autumn and that this
seasonal change in fat content led to seasonal changes in the tissue
levels of PCBs.
Cooke et al. (1982) found a seasonal pattern of PCB levels in European
kestrels. Residues in both fat and liver were low in the autumn, but
increased from about January, with a peak almost invariably occurring
during the second quarter of the year (April, May, or June). Seasonal
patterns were based on samples collected over a 10-year period.
Similar trends were found in sparrowhawks and barn owls, but fewer
samples were available.
5.1.5 Appraisal
PCB contamination is widespread and has been measured in a wide
variety of biota between the 1960s and the present day. They are
present throughout the world and, although initially concentrated in
areas of high industrial activity, are now found in organisms living
in remote areas, such as the oceans and the polar regions. In the
past, PCB levels were positively correlated with areas of heavy
industry and consequent discharge but, with the implementation of PCB
controls, in some countries, these geographical differences are
becoming less clear. Generally, levels of PCBs are declining in areas
previously high in PCBs. However, time-trend analysis for the general
environment shows little change in total PCBs since the late 1960s.
The ratio of congeners is, as would be expected, changing, with lower
chlorinated isomers disappearing and the more highly chlorinated ones
becoming more dominant in environmental samples.
PCBs are persistent and bioaccumulate in many organisms, because of
their high lipid solubility and low biodegradability, and usually
enter food-chains from water containing industrial discharge and by
precipitation.
Because of their hydrophobic nature, PCBs are associated with both
oildrop-like aggregates in the surface microlayer of water and with
sediment on the bottom.
They are accumulated by micro- and macroplankton organisms that live
in the surface microlayer and by bottom-living organisms.
5.2 Levels in animal feed
The effects of pollution are seen in the use of fish-meal in poultry
and fish farming. Kolbye (1972) sated that this may contain PCB levels
of 0.6-4.5 mg/kg.
Hansen et al. (1981) studied the transfer of PCBs in swine foraging on
sewage sludge amended soils in 1975-76. Sixteen Berkshire sows were
overwintered for 2 seasons on 4 experimental plots that had been
treated with 0, 126, 252, or 504 tonnes/hectare (on a dry solids
basis) of Chicago sewage sludge for the 8 preceding years. The
estimated PCB residues in the soils of the 4 plots (average of 3-4
samples) were 1.62, 1.88, 2.13, and 2.81 mg/kg dry weight (mean values
of 3-4 samples/plot). The mean concentrations in fat of 3-4 sows per
plot were 36 ± 9, 106 ± 64, 191 ± 97 and 389 ± 118 µg/kg fat basis. Of
the 12 individual congeners that were present in the fat, 3 accounted
for more than 50% of the congeners, e.g., 2,3,4,2',4',5'-,
2,4,5,2',4',5'-hexachlorobiphenyl and 2,3,4,5,2',4',5'-
heptachlorobiphenyl.
In vegetable animal feed (155 samples) originating from 5 areas of the
world, samples, collected in 1984/85, contained PCB levels of 0.0009
(Africa) up to 0.0093 mg/kg dry weight (Europe). In feed from North
and South America and the Far-East, the levels were between 0.0024 and
0.0066 mg/kg. Different types of feed originating from agriculture in
the Federal Republic in Germany, collected in 1985, contained PCB
levels of the order of 0.02 mg/kg dry weight. In feed (301 samples)
originating from animals (exclusive fish meals), collected in 1985,
0.021-0.036 mg/kg dry weight was found (DFG, 1988).
Levels of 10-100 µg/kg are given for groats, soybeans, and cotton
seed, and a mean value of 18 µg/kg is given for mixed feedstuffs. Fish
meal contained levels of 110-330 µg/kg (Klein, 1983).
Samples of fish meal from different areas of the world, collected in
1985, were analysed for the presence of PCBs. In 323 samples, the PCB
contents varied between 0.006 and 0.055 mg/kg dry weight. The PCB
congeners numbers 28, 138, and 153 were present in the highest
quantities (DFG, 1988).
Samples of fish meal from different areas of the world, collected in
1985, were analysed for the presence of PCBs. In 323 samples, the PCB
contents varied between 0.006 and 0.055 mg/kg dry weight. The PCB
congeners numbers 28, 138, and 153 were present in the highest
quantities (DFG, 1988).
5.3 Levels in human food
5.3.1 General
Two general reviews of PCB residues in food, animal feed, human milk,
plants, soils, and packaging materials have been published by Khan et
al. (1976) and Sawhney & Hankin (1985).
The PCB contents of a variety of foods on the Swedish market has been
measured by Westöö & Norén (1970a) and Westöö et al. (1971). Less than
0.1 mg/kg was found in samples of butter, margarine, vegetable oils,
eggs, beef, lamb, chicken, bread, biscuits, and baby food; one sample
of pork out of more than 100 had a PCB content of <0.5 mg/kg.
In the period 1980-81, 5270 food samples were drawn at wholesale or
production levels or at the site of importation including: butter,
cheese, eggs, kidneys from pigs and cattle, and fat of poultry. Levels
in Danish butter (99.4% of the samples) were below 0.05 mg/kg and
those in imported butter (100%), below 0.125 mg/kg; Danish cheese
(100% of the samples) levels were below 0.05 mg/kg and, in imported
cheese, 82.4% of samples had levels below 0.125 mg/kg and the other
17.6%, below 0.2 mg/kg; 100% of eggs had levels below 0.05 mg/kg, and
100% of kidneys of pigs and cattle were below 0.15 mg/kg; 96% of
poultry fat samples had levels below 0.15, and 4%, below 0.20 mg/kg,
on a fat basis (not stated) (Statens Levnedsmiddelinstitut, Danmark,
undated).
Mes et al. (1989b) studied the presence of specific isomers of PCB
congeners in fatty foods of the Canadian diet. A total of 93 food
composites from the cities of Ottawa and Halifax were analysed for 34
PCB isomers, as part of a revised total diet programme. Each market
basket comprised approximately 200 different food types collected from
each of 4 major supermarkets in Ottawa during September 1985 and
January 1986, and, in Halifax, in September 1986. Foods were used
per se, or prepared and cooked in a manner ready for consumption,
then composited to give 112 composites from each market basket.
Thirty-one selected composites, representing the fatty foods were
analysed from each market basket.
PCB isomers 118, 138, 153, and 180 were found in all dairy products,
except skimmed milk. Cheese and butter contained the highest levels of
PCB residues. The residue level of isomer 118 (2,4,5,3',4'-
pentachlorobiphenyl) in butter was the highest e.g., 0.7 µg/kg, of all
PCB isomers found in dairy products. Almost all meat, fish, and
poultry contained PCB isomers 183 and 187. Occasionally, isomers 49,
87, 185, and 189 were also present, but isomer 105 (2,3,4,3'4'-
pentachlorobiphenyl), present in most dairy products, was only found
in some beef samples. Fresh water fish contained most PCB isomers (28
out of 34 selected PCB isomers), at levels considerably higher than
those in any other meat, fish, or poultry samples. The level of isomer
110 in fresh water fish was 3.05 µg/kg. PCB isomers 138, 153, 180, and
187 were present in almost all samples of meat and fish products,
fats, oils, and soups. Cooking fats, salad oils, and margarine
contained relatively low levels of PCB residues. PCB isomers 37, 49,
87, 105, and 185 were not detected in meat and fish products, fats,
oils, or soups.
The calculated sum of all PCB isomer residues found in selected food
commodities (except fish) ranged from 0.03 to 1.98 µg/kg on a wet
basis, and from 0.07 to 10.71 µg/kg on a lipid basis, with mean values
of 0.60 and 3.91 µg/kg, respectively. However, the mean residue levels
of fish and fish products were considerably higher, i.e., 10 and
194 µg/kg on a wet and lipid basis, respectively.
The major PCB isomers in fatty foods were isomers 37, 52, 99, 110,
118, 138, 153, 180, and 187.
The PCB levels obtained in an extensive study by the US Food and Drug
Administration are shown in Table 15. These values are considerably
higher than those reported from Sweden, but they are probably biased,
as they include samples originating from areas previously suspected of
having been subject to local pollution.
In a Canadian survey, PCB levels of less than 0.01 mg/kg were found in
eggs (Mes et al., 1974) and a mean of 0.042 mg/kg was found in
domestic and imported cheese with a maximum of 0.27 mg/kg (Villeneuve
et al., 1973b).
A preliminary study was carried out to estimate the dietary intake of
PCBs in fresh food composites grown in Ontario in 1985. The following
5 food composites: fresh meat and eggs, root vegetables (including
potatoes), fresh fruit, leafy and other above-ground vegetables, and
cow's milk were analysed. The concentrations in the different food
composites were below 0.0005 mg/kg. The annual dietary intake of PCBs
was estimated to be 32.6 µg (Davies, 1988).
In Japan, a similar range of PCB contents has been reported for most
foods; however, some high levels have been reported for rice and
vegetables harvested in fields polluted with PCBs (Environmental
Sanitation Bureau, 1973). The PCB content of most fish on the market
was less than 3 mg/kg.
Table 15. PCB levels in food in the USAa
Food % Positive Level in positive samples (mg/kg)
(0.1 mg/kg)
Mean Maximum
Cheese 6 0.25 1.0
Milk 7 2.3 27.8
Eggs 29 0.55 3.7
Fish 54 1.87 35.3
a From: Kolbye (1972).
Cantoni et al. (1988) analysed different food items, in 1985-87, in
Italy, taking 20-60 samples per item. Different types of meat were
analysed and the median concentrations were 0.25-0.50 mg/kg, on a fat
basis. Twenty to 50% of the samples were positive. Poultry contained
0.028 mg/kg, cow's milk 0.05 mg/kg, cream 0.027 mg/kg, butter
0.065 mg/kg and fish 1.105 mg/kg, on a fat basis; 71% of fish samples
contained PCBs.
When the fat of poultry (42 samples) and 44 eggs was analysed, PCB
values were below 0.3 mg/kg (Dutch Agricultural Advisory Commission,
1983).
In the Federal Republic of Germany, wheat was analysed during the
period 1972-82. The mean concentrations for 1972-78 ranged from 10 to
30 µg/kg; in the period 1980-82, the range was < 2.0-18 µg/kg (Klein,
1983). In wheat and rye (total 850 samples), median levels of
0.4-1 µg/kg product were found in 1984 (Codex Alimentarius, 1986). The
concentrations found in other food items are summarized in Table 16.
Samples of canned ham exported from Czechoslovakia to the USA in 1983
contained PCBs levels of up to 4.8 mg/kg (Anon., 1983a,b).
Table 16. PCBs in food (1982) in the Federal Republic of Germanya
Food Total no. Number of Variation Mean
of samples min-max (µg/kg)
samples below (µg/kg)
detection
limita
Milk 854 234 < 2-3000 126.7 (FB)
Beef 76 43 < 10-687 72.4 (FB)
Pork 58 36 < 10-458 58.1 (FB)
Poultry 64 61 < 10-85 7.3 (FB)c
Meat products 185 86 < 4-2700 114.2 (FB)
Eggs 82 67 < 5-230 9.1 (FW)
Fish (only 70 - 40-87 41.1 (FW)
cod, herring,
plaice)
Food of plant origin
Oil 167 139 < 5-65 7.1 (FB)
Cereals 345 44 < 2-30 6.7 (FW)
Potatoes 106 106 < 2 -
a From: Klein (1983).
b Not stated.
c Only 3 samples.
FB = fat basis
FW = fresh weight.
5.3.2 Drinking-water
Ruoff et al. (1988) examined 83 drinking-water samples from the
Federal Republic of Germany and from 5 other European countries for
their contents of the PCB congeners 28, 52, 101, 138, 153, and 180.
The average total content of the 6 congeners was 0.002 µg/litre water.
The average concentrations of the above-mentioned PCB congeners in the
drinking-water of 6 countries were 0.0001, 0.001, 0.00018, 0.00035,
0.00037, and 0.00042 µg/litre. The variation between the 6 countries
was quite small.
The highest concentration of PCBs reported in domestic tap water was
0.1 µg/litre in the Kyoto area of Japan (Panel on Hazardous Trace
Substances, 1972 cf. WHO/EURO, 1988), but, levels, more likely to be
encountered, should not exceed 0.001 µg/litre.
In the FAO/WHO collaborating centres for the food contamination
monitoring programme, the median levels were:
Cereals below 10 µg/kg
Vegetable fat/oils below 5 µg/kg
Fresh fruit and vegetables 0.5-5 µg/kg
Animal fat (depending on type of
animal and origin) 20-240 µg/kg
Whole fluid cow's milk (depending
on country) 10-200 µg/kg
(on fat basis)
Butter 30-80 µg/kg
Whole dried cow's milk 20-50 µg/kg
Hen eggs < 10 µg/kg
Fresh finfish 10-200 µg/kg
(WHO, 1985b).
The contamination of a drinking-water system in Pickens County, South
Carolina by PCBs discharged from a manufacturing facility was
described by Billings et al. (1978). They observed that PCBs
discharged by a capacitor manufacturing plant resulted in levels as
high as 0.818 µg/litre in finished potable water.
5.3.3 Dairy products
A number of data on food-producing animals have recently become
available within the framework of the Joint FAO/WHO Food Contamination
Monitoring Programme (JFCMP, 1985). All reported median values of PCBs
in animal fat (excluding milk fat) were below the respective limits of
detection, which varied from 0.001 mg/kg in the United Kingdom to a
high of 0.5 mg/kg in Thailand and the USA. Data on PCB levels in cow's
milk fat were supplied by the Federal Republic of Germany, Japan, the
Netherlands, the United Kingdom, and the USA. The United Kingdom and
the USA reported that median concentrations in cow's milk were below
the detection limits of 0.5 µg/kg and 0.5 mg/kg, respectively.
The available data are summarized in Table 17.
From the end of 1982 to the beginning of 1983, high levels of PCBs
were detected in milk from several dairy farms in Switzerland. The
investigations showed that the silo coatings and consequently the
silage from the silos were the origin of the contamination of the
milk. The PCB levels were between 0.80 and 3.80 mg/kg fat. PCB
dissolution in acid juice, mechanical erosion of the coatings, and
volatilization of the coating surface seemed to be the principal
mechanisms explaining the migration of PCBs into the silage
(Alencastro et al., 1984).
Forty-two samples of cow's milk (14 samples in 1976, 14 in 1983, and
14 in 1986) and 41 samples of market milk (10 in 1976, 16 in 1983, and
15 in 1986) were analysed for PCBs, in Israel. During this period, a
change was observed in the PCB distribution in the milk samples. The
percentage of hexachlorobiphenyl decreased with time and the
pentachlorobiphenyl increased (Pines et al., 1988).
The monitoring data for dairy products from all over the world for
1980-83 have been summarized by the Joint FAO/WHO Food Contamination
Monitoring Programme (WHO, 1986a,b).
5.3.4 Fish and shellfish
A summary of the monitoring data on fish from all over the world for
1980-83 has been published by the Joint FAO/WHO Food Contamination
Monitoring Programme (WHO, 1986a,b).
As might be expected, the PCB values found in fish depended on the fat
content and the pollution of the fishing area (Westöö & Norén, 1970a;
Berglund, 1972).
In a collaborative study by 7 national laboratories (International
Council for the Exploration of the Sea, 1974), the PCB contents in the
muscle tissue of fish taken from the North Sea were measured. A mean
of 0.01 mg/kg was found in cod, while herring contained up to
0.48 mg/kg, with most samples in the range of 0.1-0.2 mg/kg; plaice
contained 0.1 mg/kg or less. Similar values were reported by Zitko
(1974) for fish taken from the North Atlantic.
Risebrough & de Lappe (1972) reported levels higher than 3 mg/kg in
fish from New York Sound and Tokyo Bay, both very polluted areas. Even
higher levels of PCBs were found in fish from polluted lakes and
inland waterways, a level of 20 mg/kg being found in fish from Lake
Ontario, and levels over 200 mg/kg in fish from the Hudson River
(Stalling & Mayer, 1972). Similar correlations between pollution and
PCB levels have been reported from the United Kingdom in fish
(Portmann, 1970), and in mussels (Holdgate, 1971).
Table 17. Occurrence of PCBs in dairy products
Country Year Product Number of Mean concentrations Reference
samples in mg/kg on fat basis
(range)
North America
USA 1973-1974 milk (bulk) 198 (9 positive) 1.91 (0.32-4.99) Willett (1980)
Europe
Germany 1982-1986 milk 3279 0.09-0.14a DFG (1988)
(3 areas) 1983-1986 butter/cheese 2088 0.05-0.11
Westphalian area 1972-1974 butter - 0.38 (0.25-0.54) Claus & Acker (1975)
Northern part 1978-1980 milk - 0.17-0.20 Codex Alimentarius
1984 milk 3510 0.013 (1986)
Northern part - butter 1836 0.0077c Codex Alimentarius
(1986)
- meat and fat 957 (about 3/4 0.01b DFG (1988)
positive)
cows entrails 51 0.149b
Sweden 1972-1977 beef, pork and meat 232 (217 < 0.001-0.01 Vaz et al. (1982)
products (domestic negative) (whole product)
and imported)
Denmark 1981-1982 milk - 0.10-0.13 Jensen (1983b)
Table 17. (cont'd).
Country Year Product Number of Mean concentrations Reference
samples in mg/kg on fat basis
(range)
Netherlands 1975-1977 milk 315 0.16 (0.06-0.33) Gezondheidsraad
1980-1983 milk - 0.07-0.13 (1985)
1978-1984 milk 2319 < 0.1-0.2 Olling (1984)
1977-1981 cattle fat - 0.11b (< 0.05-0.55) Greve & Wegman
pork - 0.07 (< 0.05-0.66) (1983)
1983 fat of cattle, pork, 40-45 < 0.03b Dutch Agric. Adv.
calves Comm. (1983)
sheep 22 < 0.03b
Switzerland - milk 6 0.034-0.144 Rappe et al. (1987)
(6 locations)
a Major congeners were Nos. 138 and 153.
b Median value.
c Arithmetic mean.
Jensen et al. (1969) found PCB levels of 0.27 mg/kg and 0.33 mg/kg,
respectively, in the muscle tissue of herring and cod from the same
area of the Baltic, though the cod is at a higher trophic stage. The 2
species had 4.4 and 0.32% of extractable fat, respectively, and, when
the PCB level was calculated on the fat content, values of 6.8 mg/kg
for the herring and 11 mg/kg for the cod were obtained. Cod liver has
a much higher fat content than cod muscle, and Jensen (1973) reported
the ratio of PCB concentrations in cod liver and muscle to be over
100, the maximum in liver being 59 mg/kg. Jensen et al. (1969)
remarked that the considerable seasonal variation in the fat content
of the herring, rising from 1% in spring to 10% in autumn, influenced
the tissue level of PCBs.
There are many examples of different PCB levels in similar species
collected from areas of high and low pollution. Jensen et al. (1972b)
found 5 times as much PCBs in herrings caught in waters off
industrialized areas near Stockholm, as in herrings from the cleaner
waters of the west coast of Sweden.
Different freshwater and seawater fish were analysed for PCB contents,
during the period 1981-83, in the Netherlands. Eel from different
places over the period 1971-81 contained 0.2-13 mg/kg on a product
basis (in the edible part). The median value was between 1 and
2 mg/kg. Sea fish from the North Sea, such as herring and mackerel,
contained 0.1-0.2 mg/kg, on a fat basis. The same level was found in
shrimps and mussels (Freudenthal & Greve, 1973; Greve & Wegman, 1983;
van der Kolk, personal communication, 1984a).
The mean PCB contents in the liver of cod from the North Sea, North
Atlantic, and Baltic Sea, were 2.1-5.7, 0.48, and 10.4-12.8 mg/kg,
respectively (Klein, 1983).
When fish from the North Atlantic, North Sea, and Baltic Sea, were
collected in 1985, PCB concentrations of 0.098-0.123 mg/kg fillet
weight were found in fish from the North Atlantic and North Sea and
0.338 mg/kg fillet weight in fish from the Baltic Sea. In total, 60
samples were analysed. The PCBs 101, 138, and 153 were the major
congeners (DFG, 1988).
The PCB concentration in freshwater fish of the River Rhine was found
to be more than 2 mg/kg. The mean PCBs levels decreased, however, over
the period 1976-81 from 1.92 to 0.38 mg/kg (fresh weight) (Klein,
1983).
In 1984, PCB concentrations in freshwater fish (59 samples) collected
in the River Rhine ranged from 0.742 to 1.017 mg/kg fillet weight. In
this case, the major congeners were 138 and 153, but numbers 28, 52,
101, 180 were also present. In total, 199 samples of eel were
collected in a number of surface waters and analysed for the presence
of PCBs. The levels ranged from 1.42 to 6.51 mg/kg fresh weight. In
studies reported by DFG (1988), the highest levels of PCBs were found
in the River Rhine.
In the United Kingdom, fish and shellfish were analysed for PCBs
during the period 1982-84 (HMSO, 1986). The results are summarized in
Table 18.
Table 18. PCB levels in marine fish and shellfisha
Year Product Tissue No. of Range (mg/kg)
samples
1982 Marine fish (from England) liver 381 0.3-4.1
(7 types of fish)
1982 Marine fish (from England) muscle 326 0.03-0.13
(7 types of fish)
1983 Marine fish (imported) muscle 102 nd-0.06
(5 types of fish)
1983 Shellfish (imported) muscle 53 nd-0.06
(4 types of shellfish)
1984 Fish oils 16 0.11-2.3
a From: HMSO (1986).
Different types of marine fish and shellfish from different areas in
the United Kingdom were analysed during the period 1977-84. Those from
the North Sea coast contained concentrations in the range of 0.04-5.7
and < 0.001-0.058 mg/kg, respectively, while those from the English
channel contained < 0.05-6.9 and < 0.006-0.1 mg/kg, respectively,
and those from the West coast, < 0.002-8.4 and < 0.001-0.25 mg/kg
wet weight. PCB concentrations in fish livers of 0.2 up to 12.9 mg/kg
wet weight were found during this period (Franklin, 1987).
When samples of fish of different species, collected from major USA
watersheds in 1976, were analysed, PCBs were found in 93% of the
samples. Fifty-eight of the samples had levels exceeding 5 mg/kg, on a
whole fish basis. The PCB concentrations ranged from less than 0.3 to
140 mg/kg, on a whole fish basis (Veith et al., 1979).
Maack & Sonzogni (1988) analysed 98 fish (14 species) of different
sizes from Wisconsin waters, for the presence of PCB congeners. Among
the most prominent congeners were numbers 153/132, 138, 66/95, 110,
180, 70/76, 146, 28/31, 149, 118, and 105. The total PCBs (determined
by adding individual congener concentrations) ranged from 0.070 to
7.0 mg/kg. The mean concentration was 1.3 mg/kg.
Blue crabs ( Callinectes sapidus, an important member of the
estuarine food web), collected from Campbell Creek and surroundings in
South Carolina, were analysed for PCBs in 1985. The highest mean total
concentration was 0.861 mg/kg muscle tissue. In 1986, the mean
concentrations in blue crab collected by 8 stations in the same area
ranged from 0.026 to 0.361 mg/kg muscle tissue. Blue crab (15 samples)
collected from the coast of South Carolina, contained concentrations
of < 0.020-0.372 mg/kg tissue (Marcus & Mathews, 1987).
PCBs concentrations in sea fish were determined in 1971-77 in Japan.
In-shore fish (90 samples) showed concentrations of 0.2-0.72 mg/kg
fresh weight and pelagic fish (112 samples), 0.005-0.265 mg/kg fresh
weight (Watanabe et al., 1979).
Data on individual species of fish, submitted by Japan, showed the
following median levels: barracuda, 70 µg/kg; conger eel, 290 µg/kg;
croaker, 200 µg/kg; flounder (yellow-tail), 90 µg/kg; hair-tail,
100 µg/kg; mullet, 84 µg/kg; and seabass, 110 µg/kg. Median levels for
other species of fish, such as cod, mackerel, pacific saury, rockfish,
salmon, and sardines, were below 100 µg/kg (WHO, 1986b).
Using a very sensitive analytical method, Tanabe et al. (1987) found
the toxic non- ortho-substituted coplanar 3,4,3',4'-tetrachloro-,
3,4,5,3',4'-pentachloro-, and 3,4,5,3',4',5'-hexachlorobiphenyl in
finless porpoise, at concentrations of 13.5, 0.89, and 0.64 µg/kg,
respectively.
Blue mussel (Mytilus edulis) was collected from coastal areas near
Osaka and Hokkaido, Japan, in 1984-86. Depending on the site of
collection, the average PCB concentrations (11-13 samples) ranged from
0.56 to 65.0 µg/kg (Miyata et al., 1987).
5.3.5 Influence of food processing
Fifty striped bass (Morone saxatilis) were analysed for the presence
of PCBs in the fish fillets before, and after, boiling, steaming,
baking, frying, microwaving, or poaching, to study the possible
reduction of the PCB residues by these cooking procedures. PCB
contents were reduced by approximately 10%, by all 6 methods of
cooking. No significant reductions were observed with the other
cooking methods (Armbruster et al., 1987).
5.3.6 Food contamination by packaging materials
When Villeneuve et al. (1973a) analysed packaged food in Canada, they
found that 66.7% of the samples contained PCB levels of less than
0.01 mg/kg, 30.7% contained between 0.01 and 1 mg/kg, and 2.6%
contained more than 1 mg/kg. The highest PCB levels were in a rice
sample (2.1 mg/kg), where the packaging material contained 31 mg/kg,
and in a dried fruit sample (4.5 mg/kg), in a container containing
76 mg/kg. In a survey of packaging containers, approximately 80% were
found to contain PCB levels of less than 1 mg/kg, while about 4%
contained levels higher than 10 mg/kg. The most likely source of PCBs
in packaging materials was the recycling of waste paper containing
pressure-sensitive duplicating paper (carbonless copying paper)
(Masuda et al., 1972).
Relatively high PCB levels in some packaged foods in Sweden, mainly of
imported origin, could be attributed to migration from the packaging
material (Westöö et al., 1971). The highest level encountered was
11 mg/kg in a childrens' breakfast cereal; PCB levels of 70 mg/kg and
700 mg/kg were found in the material of the inner bag containing this
product and in the outer cardboard container, respectively. Up to
2000 mg/kg was found in cartons of other samples.
In the United Kingdom, levels in imported waste-paper, which could be
contaminated with PCBs from carbonless copying paper and subsequently
used to manufacture food contact paper and board materials, were found
to be low, compared with the 10 mg/kg limit for PCBs recommended by
the British Paper and Board Industry Federation for food contact
materials (HMSO, 1989).
5.3.7 Appraisal
Foods have become contaminated with PCBs by 3 main routes:
* accumulation of PCBs in the different food-chains in the
environment and consumption of fish, birds, or other animals and
crops;
* direct contamination of food or animal feed by an industrial
accident;
* migration from packaging materials into food.
During the past years, many thousands of samples of different
foodstuffs have been analysed for PCB contamination. The most common
foodstuffs analysed have been fish, meat, and milk. Many fish samples
have been taken in an effort to monitor aquatic pollution. In
addition, samples have been taken, for regulatory or similar purposes,
from sources suspected of being relatively highly contaminated. The
fact that most samples have not been taken at random, jeopardizes the
proper assessment of the exposure of the general population.
5.4 General population exposure
5.4.1 Air
Relatively high levels of PCBs have been detected in indoor air,
especially in kitchens and offices with electric installations
(Jensen, 1983a) (section 3.2.4 and 5.1.1).
Results from the US EPA indicate PCB concentrations in the air ranging
from 1 up to 50 ng/m3; similar results have been reported from Japan
(WHO, 1976). Assuming a level of 5 ng PCBs/m3 in urban air, a
breathing rate of 22 m3/day, retention and absorption of inhaled
particles/vapour of 50%, and a mean residence time of PCBs in the body
of 3 years, air would contribute 0.8 µg/kg to the PCB concentration in
the body. Higher concentrations of PCBs in indoor air could increase
this estimate (WHO/EURO, 1988).
Van der Kolk (1985) calculated air intake through inhalation for the
Dutch population of about 36 ng/day, a quantity approximately 1000
times lower than the intake with food.
During the manufacture, formulation, or use of PCBs, where levels in
the workroom air correspond to exposure limit values, varying between
0.1 mg/m3 and 1 mg/m3, the calculated mean intakes would range
between 1 and 10 mg during an 8-h workshift. In some occupational
situations, much higher concentrations have been measured and the
estimates of intakes would be higher (WHO/EURO, 1987).
5.4.2 Drinking-water
Levels reported in drinking-water are typically between 0.1 and
0.5 ng/litre. Even assuming a PCB level of 2 ng/litre in
drinking-water, consumption of 2 litre/day contributes 0.04 µg/kg body
weight to the PCB concentration in the body. This additional quantity
is negligible in comparison with the intake via food (WHO/EURO, 1988).
5.4.3 Intake by infants through mother's milk
The daily intake of PCBs was calculated in breast-fed infants in the
countries participating in a monitoring study by Slorach & Vaz (1983,
1985) (Table 19).
The intakes in EEC countries were calculated to range from 3 to
11 µg/kg body weight per day, compared with 0.12-0.3 µg/kg body weight
for bottle-fed infants in Denmark (WHO/EURO, 1985).
In Yusho infants with clinical symptoms of poisoning, the daily intake
of PCBs with breast milk was calculated to be 70 µg/kg body weight
(Jensen, 1983b) (see section 9.1.2.2).
5.4.4 Infant and toddler total diet
Johnson et al. (1979) analysed the average diet of 6-month-old infants
and 2-year-old toddlers for the presence of PCBs. Ten market baskets
were collected in 10 cities in the USA. The foods were prepared in the
manner in which they would be prepared and served in the home. Trace
amounts of PCBs were detected in only one infant and one toddler diet.
In the USA, Gartrell et al. (1986b) found a daily intake of 0.011 µg
PCBs/kg body weight in infants consuming infant diets in 1978. In the
years 1979, 1980, and 1981/82, the intake was below the detection
level. The intake by toddlers was 0.099 µg/kg body weight in 1978 and
not detectable in the following 3 years.
Tuinstra et al. (1985a) analysed samples of infant food from the Dutch
market and found average PCB levels of 0.1-0.2 µg/kg food (the maximum
level found was 1.1 µg/kg).
5.4.5 Total intake by adults via food
The oral consumption of contaminated products is presumed to be the
main route of exposure to the PCBs.
Table 19. Calculated daily intakes of PCBs by breast-fed infants (µg/kg body weight)a
Country/area Year(s) Calculation according Calculation according
to US FDA methodb to national methodc
median maximum median maximum
Belgium,
Brussels 1982 3.6 10.4 NR NR
China,
Beijing 1982 NR NR 0.45d 0.45d
Israel,
Jerusalem 1981/82 2.0 9.5 NR NR
Germany,
Hanau 1981 NR NR 9.5 45
Japan,
Osaka 1980/81 1.6 4.4 2.3 6.3
Sweden,
Uppsala 1981 4.4 8.1 5.9 11
USA
22 states 1979 4.5e 13.5 4.5e 22.5
Yugoslavia,
Zagreb 1981/82 2.8 7.2 2.8 7.7
a Assuming a milk consumption of ca 130 g/kg body weight and a milk fat content of
3.5% (w/w). Calculations based on data for all mothers studied. Results for
different methods of PCB analysis shown separately.
From: Slorach & Vaz (1983, 1985); Van der Kolk (1984b).
b Sawyer method.
c "Own method".
d PCB level below limit of detection (0.1 mg/kg fat) in milk samples.
e PCB level below limit of detection (1 mg/kg fat) in milk samples.
NR = No data on levels in milk reported.
It has been stated that the major part of the human dietary intake of
PCBs is from fish (Berglund, 1972; Hammond, 1972). This may well be
true in areas such as Japan or certain localities near the North
American Great Lakes, where fish from polluted waters may form a
relatively large part of the diet. Several investigators from Japan
have measured the daily intake of PCBs in food; the highest mean value
recorded was 48 µg/day, of which 90% was from fish (Kobayashi, 1972);
the lowest was 8 µg/day (Ushio et al., 1974).
In much of Europe and North America, however, the daily intake of fish
is in the region of 30-40 g, and most of the fish is taken from waters
of low pollution with PCB levels in the fish not exceeding 0.1 mg/kg.
Berglund (1972) has estimated that the daily intake of PCBs from fish
in Sweden is in the region of 1 µg, though if the fish consumed were
solely Baltic herring, the intake would be about 10 µg/person. It is
difficult to make an assessment of the PCB intake from foods other
than fish. Westöö et al. (1971) in their extensive study of the
Swedish diet, reported that most foods contained PCB levels of less
than 0.1 mg/kg; and concluded that this corresponds to a daily intake
of less than 100 µg.
Weekly intakes in the range of 23-889 µg/person have been reported
from the USA (OTA, 1979). The higher range concerns people consuming
more than 12 kg/year of Lake Michigan fish.
The intake of total PCBs by the general adult population depends
greatly on the geographical area and food habits.
5.4.6 Total diet/market-basket studies
Data on total-diet studies of PCBs have been reported from a few
countries. These reported intakes show a wide variation, which can
partially be explained by methodological factors, such as the ways in
which samples below the limits of determination are considered,
especially when noting the different limits of determination.
Considering the available data, an average intake of 5-15 µg/day for
the non-occupationally exposed population in industrialized countries
may be the best available estimation.
These estimates apply to the average diet of an average adult citizen.
In practice, few people are really "average" in their consumption
pattern. Given the widespread nature of the contamination, however, a
higher intake in one food group is more or less balanced by a lower
intake in another food group with an equal calorie intake. Total
intake will certainly be higher for diets with a more than average
calorie content (van der Kolk, 1985).
Gartrell et al. (1985) determined the total intake of PCBs by 16- to
19-year-old males in the USA. The samples represent a typical 14-day
diet. Approximately 120 individual food items (of 12 food groups),
including drinking-water, were collected for each market-basket sample
in 20 cities in the period 1979-80. Only 2 samples of meat, fish, and
poultry contained PCBs with an average concentration of 0.002 mg/kg.
Gartrell et al. (1985, 1986a) reported a daily intake in the USA of
0.016, 0.027, 0.014, 0.008, and 0.003 µg PCBs/kg body weight during
the years 1977, 1978, 1979, 1980, and 1981/82, respectively.
Manske & Johnson (1975) collected 35 market baskets in 32 cities over
the period 1971-72. PCB residues were found in the range of
0.035-0.15 mg/kg in 51 composites. Fish and oils, fats, and
shortenings contained the highest levels. The same authors (Manske &
Johnson, 1977) carried out a market-basket study representing the
basic 2-week diet of a 16- to 19-year-old male. The various foods were
prepared in the manner in which they would normally be served and
eaten. Thirty market-baskets, containing 12 classes of foods (in total
360 composites) were collected in 30 cities in the period 1973-74. A
trace of PCB was found once in whole milk, ground beef, and fish
fillet.
The FDA revised the concept of the Total Diet Study in 1982. As
discussed by Gunderson (1988b), the Total Diet Study conducted before
1982 was based on a "composite sample approach", regardless of the
diet involved. The revised study is based on updated dietary survey
information and allows the "total diet" of the US population to be
represented by a relatively small number of food items for a greater
number of age/sex groups. The daily intake expressed in ng/kg body
weight per day for PCBs (Aroclor 1221, 1242, and 1254) in 1982-84 for
the age groups 6-11 months, 2 years, 14-16-year females, 14-16-year
males, 25-30-year females, 25-30-year males, 60-65-year females and
60-65-year males were: 0.8, 1.2, 0.4, 0.5, 0.5, 0.6, 0.4, and
0.5 ng/kg body weight per day, respectively (Gunderson, 1988b).
Foods, representative of Canadian eating habits, as determined by a
national nutritional survey, were prepared for eating, categorized,
and blended into 11 different composites representing the dietary
intake for 5 cities over the period 1976-78. It concerned 194 samples,
collected in winter and in summer. The average dietary intake was
0.001 µg PCB/kg body weight (McLeod et al., 1980).
Over a period of 2 years, 126 different food items of a market-basket
of 16- to 18-year-old males were purchased every 2 months in the
period 1976-78, in the Netherlands. The foodstuffs were prepared for
eating and were combined in 12 commodity groups. The mean
concentration and range of PCBs in 5 food classes was:
Class Mean concentration Range
(mg/kg on fat basis)
Meat, poultry, and eggs - 0.13-0.17 (2)a
Fish 0.07 0.04-0.24 (7)
Dairy products - 0.04-0.06 (2)
Sugar and sweets - 0.08 (1)
Drinks, drinking-water - 0.035 (1)
a In parentheses: number of positive composites.
The authors calculated a daily intake of PCBs of 15 µg/person (a
maximum level was 90 µg/person (de Vos et al., 1984). In the period
May-July 1976, 100 total diets (summer meals) were collected and
besides organochlorine pesticides, PCBs were determined as
decachlorobiphenyl, after perchloration, and calculated as Aroclor
1260. The mean intake of PCBs/person per day was 11.6 µg with a range
of 3-71 µg (Greve & van Hulst, 1977; Greve & Wegman, 1983; van der
Kolk, 1985).
In 1978, another survey was carried out with 100 total diets during
the winter (winter meals). It was estimated that the daily intake was
6 µg/person (range 1-19 µg).
Zimmerli & Marek (1973) studied the total human intake of PCBs from
prepared meals in 1971-72 in Bern, Switzerland. Five typical total
diets were composed and analysed. The intake of PCBs, especially with
daily diets containing cheese, meat, fish, or fat, ranged from 6 to
84 µg.
According to a calculation by Summerman et al. (1978), the average
weekly intake of PCBs in the Federal Republic of Germany was about 215
and 268 µg/week for females and males, respectively. Much lower
figures, 36-44 µg/week, were calculated by Klein (1983).
A survey of the daily PCB intake from the total diet of Japanese women
(number of samples varied from 18 to 60) was performed for the years
1972-76. The daily intake of PCBs averaged approximately 10 µg/person
(range 2.8-21.2 µg). The main source of PCBs in the diet of Japan was
in-shore fish. There was no clear change in daily intake over the
5-year period studied (Watanabe et al., 1979).
Ushio & Doguchi (1977) studied the dietary intake of PCBs in Tokyo.
They found an average daily intake of PCBs of 6.3 µg/person (range,
trace-17 µg/person). It was concluded that the dietary daily intake of
PCBs for the majority of the population of Tokyo rarely exceeded
20 µg/person, when no heavily contaminated fish were consumed.
Yakushiji et al. (1977) found that the PCB daily intake through meals
of unexposed adults living in Osaka prefecture, was 3-20 µg/day.
Data for PCBs in the diets of Canada, Guatamala, Japan, the United
Kingdom, and the USA over the period 1972-83 were summarized by
Gorchev & Jelinek (1985). The mean dietary intake reported was at, or
below, 0.06 µg/kg body weight, the mean intake per person ranged from
< 0.01 to 0.12 µg/kg body weight (Slorach et al., 1982; WHO, 1986b).
5.4.7 Total intake of major congeners by adults via food
In the Federal Republic of Germany, the daily intake of the 3 PCB
congeners numbers 138, 153, and 180, together with the different food
items, was calculated. The intake (µg/day) with meat and meat products
was 0.30; with fish and fish products 0.36; eggs and egg products
0.008; milk and milk products 0.40; cheese 0.11; butter 0.39; fats and
oil 0.098; bread and pastries 0.17; potatoes 0.081; vegetables 0.11
and fruits 0.082 (DFG, 1988).
5.4.8 Time trends in different matrices
Although many countries introduced severe restrictions on the
manufacture, use, and disposal of PCBs many years ago, it is difficult
to discern any marked decline in the levels in human milk fat, from
the published data.
Levels of PCBs were estimated in 1085 samples of different cereals,
collected in the Federal Republic of Germany over the period
1972/74-1984. The levels, which were the highest in 1972/74 0.04 mg/kg
(0.005-0.12 mg/kg), decreased during the years to 0.004-0.005 mg/kg
dry weight in 1984 (DFG, 1988).
Data from the Federal Republic of Germany showed no clear trend in PCB
levels in human milk during 1975-79 (Slorach et al., 1982). The same
was found in the Netherlands over the period 1974-83 (Greve & Wegman,
1984).
Japanese data showed a decline in PCB levels in the fat of whole cow's
milk during the period 1972-79. A decline was also found in PCB levels
in finfish from coastal waters and in total marine fish (Slorach et
al., 1982).
A downward trend was found in human milk from Japan over the period
1972-80. Each year, a large number of samples (361-877 samples/year)
were analysed. In 1972, the median level was about 0.8 mg/kg and, in
1980, 0.5 mg/kg, on a fat basis. A gradual decline was observed
(Slorach & Vaz, 1983).
In Canada, human milk and adipose tissue from Ontario residents were
analysed over the period 1969-74. The values found did not indicate a
trend in this period.
The mean total PCB intakes determined in the FDA Total Diet Study, for
the period 1971-87, for a typical "adult" diet, represented in Fig. 4,
reflect that of a 14- to 16-year-old male during 1982-87. A clear
decline was shown from approximately 7 µg/person per day to less than
0.1 µg/person per day (Gunderson, 1988a).
The daily intake of PCBs, expressed as ng/kg body weight per day, by
6-month-old and 2-year-old children in the years 1980, 1981/82, and
1982/84 did not show a trend, while, in adults, a decrease from 8 to
0.5 ng/kg body weight per day was observed over the same years
(Gunderson, 1988b).
5.5 Concentrations in the body tissues of the general population
The PCB levels in body tissues are a good indication of the overall
and total exposure of the body to PCBs.
Several factors may influence the concentrations of PCBs in body
tissues, including duration and level of exposure, the route and
pattern of exposure, the chemical structure of the PCB (degree and
position of chlorination in the molecule), the amount of adipose
tissue, other simultaneous exposures, as well as other biological
parameters.
5.5.1 Adipose tissue
In general, while highly chlorinated congeners accumulate more easily,
a lower degree of substitution provides more possibilities for
hydroxylation and facilitates excretion. Factors other than the degree
of substitution also affect accumulation, particularly the position
and pattern of substitution (WHO/EURO, 1987).
The available information on the occurrence of PCBs in the body fat of
the general population is summarized in Table 20.
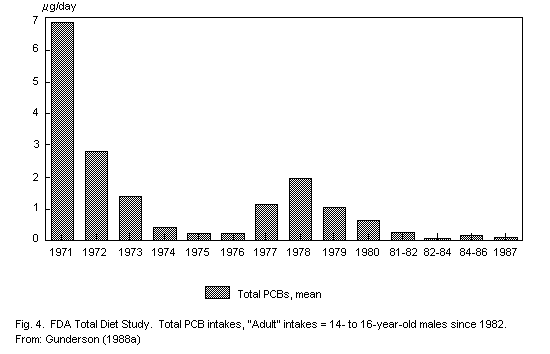 Table 20. Concentrations of PCBs in the body fat of the general population
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
North America
USA (18 states) - 637 < 1 (68.9%)e Yobs (1972)
< 1-2 (25.9%)e Price & Welch (1972)
> 2 (5.2%)f
Northeast Louisiana 1980 8 1.04 (0.38-2.33) Holt et al. (1986)
1984 10 1.23 (0.65-1.44)
Texas 1969-1972 88 (15 positive) 1.7 (0.6-9.9) Burns (1974)
New York - 101 (women) 3.4 ± 1.1 Bush et al. (1984)
(urban and rural
vicinity)
Canada - 99 0.94 (0.04-6.8)a Mes et al. (1982)
Ontario 1976 and 570 2.1-2.2 Frank et al. (1988)
1984
Table 20. (cont'd).
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
Asia
Japan (Kochi area) - - 2.86 (maximum 7.5) Nishimoto et al. (1972a,b)
Japan 1971-1982 - 0.5-6.0a Katsunuma et al. (1985)
Tokyo 1974 30 1.04 (0.38-2.5) Fukano & Doguchi (1977)
Japan - 241 0.30-1.48 Curley et al. (1973b)
New Zealand - 51 0.82 Solly & Shanks (1974)
Africa
South Africa 1982 63 0.15-5.18 van Dijk et al. (1987)
Europe
Austria (Vienna area) - 32 0.3-7.3 Pesendorfer et al. (1973)
Finland - 105 0.2 Mussalo-Rauhamaa et al.
(1984)
Germany, Federal - 20 5.7 Acker & Schulte (1970)
Republic of - 282 8.3 Acker & Schulte (1974)
1982-1983 50b 0.5-1.5 Niessen et al. (1984)
Italy (Siena) 1983-1984 26 1.75c (dry weight) Focardi et al. (1986)
Table 20. (cont'd).
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
Netherlands 1973-1983 24-78 per year 1.6-2.5d Greve & van Harten
(1983a);
Greve & Wegman (1983,
1984)
Norway (Oslo) - 40 1.6 Bjerk (1972)
Spain 1985-1987 14 1.68 Camps et al. (1989)
United Kingdom - 201 < 1.0 Abbott et al. (1972)
1976-1977 236 0.7 (nd-10) HMSO (1986)
1982-1983 187 0.9 (0.1-6.9)
a Wet weight.
b 34 infants, 14 children, and 2 older children.
c About 60% included only five congeners: Nos. 118, 138, 153, 170, 180.
d Median.
e Percentage of samples.
5.5.1.1 PCBs in the fetus
PCBs are also present in serum and all organs of the body in
proportion to their fat content. PCBs pass more, or less (depending on
structure and chlorination), through the placenta into the fetus.
Since the fetus has little adipose tissue until 7 months of age, PCB
concentrations may be higher in vital organs, such as the adrenal
gland, but available data suggest somewhat lower levels in the brain
(Masuda et al., 1978a; Kodama & Ota, 1980).
Masuda et al. (1978a) found PCB levels of 270-960 µg/kg fat in adipose
tissue samples of fetuses beyond 7 months of gestation. Levels in the
adipose tissue of adult females from the same geographical area ranged
from 270 to 1360 µg/kg fat. The mean concentrations were 470 µg/kg for
fetuses and 780 µg/kg for adult females. However, since the ranges
showed an overlap and the number of samples was small, it is not clear
whether this represents a true difference.
5.5.1.2 Congeners in adipose tissue
Wegman & Berkhoff (1986) investigated the presence of the different
congeners in 24 human fat samples, collected in 1984. The following
congeners were present at the highest levels: 2,4,4'-trichloro-,
2,4,5,2',5'-pentachloro-, 2,4,5,3',4'-pentachloro-, 2,3,4,2',3',4'-
hexachloro, 2,3,4,2',4',5'-hexachloro-, 2,4,5,2',4',5'-hexachloro-,
2,3,4,5,2',4',5'-heptachloro, 2,3,4,5,2',3',4',5'-octachloro, and
2,3,5,6,2',3',5',6'-octachlorobiphenyl.
Focardi & Romei (1987) analysed 30 samples of adipose tissue,
obtained from patients in Siena, Italy, in 1986, for the presence of
19 PCB congeners. The results indicate that the mean PCB (as sum of
the congeners) concentration was 1063 µg/kg dry weight (range
391-1918 mg/kg). The major constituents of the PCBs (about 60%) were
the isomers 99, 138, 153, 170, and 180.
Human adipose tissue was analysed for 3 non- ortho chlorine
substituted coplanar congeners: 3,4,3',4'-tetrachloro-, 3,4,5,3',4'-
pentachloro- and 3,4,5,3',4',5'-hexachlorobiphenyl (Kannan et al.,
1988). Twelve samples, from 7 male and 5 female persons were obtained
from hospitals. The average total PCB concentrations were 1.22 and
1.02 mg/kg (wet weight basis), respectively. The concentrations of the
3 congeners were 94-860, 120-730, and 36-200 ng/kg, on a wet weight
basis, respectively.
5.5.2 Blood of the general population
Finklea et al. (1972) studied human plasma of different races of the
population (723 volunteers with ages ranging up to 60 years) of urban
and rural areas of South Carolina. The average concentration was
5 µg/litre (range 0-29 µg/litre). No age effect was found, but ethnic
differences and ethnic residence interactions were significant. Kreiss
(1985) found mean serum concentrations in the non-occupationally
exposed population in the USA, of between 4 and 8 µg/litre, with 95%
of the individuals having serum PCB concentrations of less than
20 µg/litre. More data are summarized in Table 21.
Maternal blood and fetal cord blood were collected from volunteers
from an urban and rural vicinity in upstate New York. Whole blood
samples were taken from 101 women (26 ± 4 years) entering maternity
facilities. Maternal blood contained 3.4 ± 1.1 µg PCBs/kg and fetal
cord blood contained 2.4 ± 1.0 µg/kg whole blood. The PCB congeners
making up these totals were surprisingly few; 38% of the total residue
in the maternal blood and 21% of the fetal cord blood comprised only 4
components, 2,4,4'-trichlorobiphenyl, 2,4,5,2',4',5'-hexachloro-,
2,3,4,2',4',5'-hexachloro-, and 2,3,5,6,2',3',6'-heptachlorobiphenyl.
The congener 2,5,2',5'-tetrachlorobiphenyl crossed the placenta
preferentially (Bush et al., 1984).
The concentrations of PCBs were determined in blood samples from 120
women hospitalized for miscarriages and 120 full-term pregnancy
controls. The average PCB level was higher in women with miscarriages
than in control women (8.65 µg/litre and 6.89 µg/litre, respectively,
as Fenclor 54 and 14.81 and 14.90 µg/litre, respectively, as
decachlorobiphenyl). The reproductive history of each woman was
assessed together with confounding variables and with environmental
exposure and food intake. Food consumption did not indicate diet as
the main source of PCB intake (Leoni et al., 1989).
A cross section of the population of Michigan was studied following an
accidental exposure in 1978. Five years after the accident, PCB and
PBB residues were measured in adipose tissue and serum. Serum levels
of PCB were measured in 1681 adults and 1462 children. Children (430)
were found to have uniform levels throughout the state (mean
concentration 4 ± 2 µg/litre). In adults, the serum PCB levels were
higher in the area with highest PBB levels. The mean serum PCB level
was 21 µg/litre, compared with control levels for the rest of the
state of 9 µg/litre. No sex difference was found (Wolff et al.,
1982a).
Table 21. Concentrations of PCBs in whole blood of the general population
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Canada
Ontario area 1975-1976 118 18 Frank et al. (1988)
(patients suspected 1980-1981
of being exposed 1984
dermally)
Japan
- - 3.2 Doguchi & Fukano (1975)
- 28 (women) 2.6 Kuwabara et al. (1978)
(Osaka area) 1976 16 (women) 2.8 (1.7-4.6) Kuwabara et al. (1979)
1972-1977 - 3-4 Yakushiji et al. (1977)
farmers 1978-1983 - trace-21.4b Katsunuma et al. (1985)
Tokyo 1973 27 3.19 (2.2-5.1) Fukano & Doguchi (1977)
1975 10 2.59 (1.8-3.8)
Table 21. (cont'd).
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Finland - 3.1-12 Karppanen & Kolho (1973)
Netherlands - 34 (women) 4.5 (nd-11.6) Blok et al. (1984)
31 (men) 4.8 (1.0-17.1)
1978 48-127 3.1e Greve & Wegman (1983,
1980 samples/year 3.5 1984)
1981 4.4
1982 4.4
North America
South Carolina 1968 723 5 (4.2-5.5)a Finklea et al. (1972)
(urban and rural
area)
Michigan 1973 1100 56c Kreiss (1985)
(areas of Lake 1979-1981 17.2-23.6c
Michigan)
Lake Michigan 1985 196 5.5 ± 3.7 Schwartz et al. (1983)
(high fish
consumption)
Table 21. (cont'd).
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Yugoslavia 1984-1986 10f 155 (35-480)d Jan & Tratnik (1988a)
(residents around 19g 11 (6-18)
River Krupa; 4h 5 (2-7)
contamination by a
plant using PCBs)
a Plasma.
b Serum.
c Geometric mean.
d Arithmetic mean.
e Median concentration.
f Living close to plant.
g Living 1-3 km from plant.
h Non-exposed other areas.
Specific PCB isomer levels in the blood of 30 children, ages 2-5
years, residing in an area of PCB-contaminated soil in Canada, were
compared with those of 25 children in a non-contaminated area. The sum
of individual PCB isomer levels in the exposed and non-exposed group
were not significantly different, e.g., 0.54 µg/litre (range
0.22-0.99 µg/litre) and 0.88 µg/litre (range 0.28-2.30 µg/litre). The
major component in both groups was 2,4,5,2',4',5'-hexa-chlorobiphenyl
(Mes, 1987).
High levels of PCBs were found in the blood (up to 100 µg/litre) in
patients with severe weight loss (Hesselberg & Scherr, 1974). This was
attributed to the release of PCBs from the mobilization of fat.
Greve & van Harten (1983b) studied the relationship between the levels
of PCBs in the adipose tissue and in the blood of the same persons. A
total of 48 persons were involved in this study. A concentration
factor (concentration in adipose tissue divided by concentration in
blood) of 660 was found.
5.5.3 Human milk
Human milk is the major source of exposure for breast-fed infants. The
amount of human milk secreted varies widely. The composition of the
milk is related to the amount secreted, the stage of lactation, the
timing of withdrawal (early or late in feeding) and to individual
variations among lactating women. The individual variations depend on
maternal age, health, social class, and diet. The concentration of
PCBs depends primarily on the lipid concentration in milk. Wide
variations in published results are caused by inaccuracies inherent in
the analytical methods used for the quantification of lipids, and
whether the milk sample is collected early or late during the feeding
period. The fat content increases during emptying, and the fat content
of milk from the 2 breasts may differ. According to a recent
determination, the fat level in human milk averages 2.6-4.5%
(WHO/EURO, 1988).
Whether the differences in concentration in various countries are
merely a function of the analytical methods used and the type of
samples collected or whether true differences in body burden exist, is
not clear at present. For instance, some countries have reported
levels of PCBs in human milk fat ranging from nondetectable to
14 mg/kg, while, in other countries, the highest levels found have
been around 3 mg/kg. Because of these variations, calculating an
average dose for nursing infants is difficult. The same difficulties
exist when attempts are made to investigate trends over time
(WHO/EURO, 1988).
The results of the older studies have been obtained with a less
sophisticated method using packed column GC. With this method only a
dozen peaks can be separated. The quantitative results are reported as
"total PCB values", though different techniques of quantification and
different types of calculations were used.
In contrast with the situation with many organochlorine insecticides,
the levels of PCBs in human milk fat are higher in European countries,
Japan, and the USA than in China (Slorach & Vaz, 1983, 1985), and are
significant, particularly in the highly industrialized countries.
Results from a large number of countries have been summarized by
Jensen (1983a, 1985, 1987), Acker et al., (1984), Katsunuma et al.
(1985) (especially Japanese data; period 1972-83); and WHO/EURO,
(1987, 1988). The countries concerned are: Argentina, Austria,
Belgium, Canada, Finland, France, Federal Republic of Germany (Klein,
1983), German Democratic Republic, Israel, Japan, the Netherlands,
Norway, Poland, Romania, South Africa, Sweden, Switzerland, Turkey,
United Kingdom, USA, USSR, and Yugoslavia. The average levels of PCBs
in human milk do not appear to differ very much between the
industrialized countries and range between 0.5 and 2 mg/kg milk fat,
except in Czechoslovakia, the Federal Republic of Germany, India,
Denmark and Italy, where levels up to 3 mg/kg milk fat were found
(Jensen, 1983b; Acker et al., 1984) (Table 22).
The variation in residue levels in human milk during lactation was
investigated in 5 women in the Federal Republic of Germany. Month-mix
samples, composed of breast milk samples collected weekly, were
analysed over a lactation period of between 5 and 9 months. The ages
of the women ranged from 23 to 36 years. The PCB concentrations were
between 0.61 and 2.20 mg/kg, on a fat basis. While the concentrations
remained relatively constant, some fluctuations were seen but no trend
was observed over the lactation period investigated (Fooken & Butte,
1987).
Breast milk samples from 16 women in Canada were analysed for PCBs at
8 intervals (7, 14, 28, 42, 56, 70, 84, and 98 days) during the
lactation period. The average PCB concentrations in breast milk varied
between 22.8 and 29.7 µg/kg whole milk. No clear decrease or increase
was observed. The average milk/blood ratio for PCBs was 23 and
remained relatively constant during lactation (Mes et al., 1984).
Wolff (1983) reported the half-life of PCBs (percentage chlorine not
specified) in breast milk to be 5-8 months and found that the
concentration of PCBs in breast milk was 4-10 times that in the
maternal blood. Similar results were reported by Jacobson et al.
(1984b).
Table 22. Concentrations of PCBs in breast milk of the general population
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
North America
USA (Michigan) 1977-1978 1057 1.5 (maximum 5.1) Wickizer et al. (1981);
Wickizer & Brilliant (1981)
Canada (Quebec) - 154 0.84 (nd-4.34) Dillon et al. (1981)
Ontario 1971-1974 - 1.2 (0.1-3.0) Atkinson (1979)
1978 215 0.6 ± 0.3
Ontario 1975-1985 348 0.023 (0.016-0.033)a Frank et al. (1988)
Five regions across Canada 1982 210 0.697 Mes et al. (1986)
Regina, Saskatchewan 1979 80 0.0052 (0.001-0.019)a Qureshi & Robertson (1987)
Table 22. (cont'd).
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
Asia
Japan (Osaka) 1972-1977 - 0.030-0.040a Yakushiji et al. (1977)
1969-1976 19-52 each year 1-2 Yakushiji et al. (1979)
India (Ahmedabad) 1981-1982 50 not present Jani et al. (1988)
Hawaii (different islands) 1979-1980 54 0.80 ± 0.43 (0.13-2.2) Takei et al. (1983)
Europe
Germany, since 1970 several thousands 1.0-2.5 (98% of samples Acker et al. (1984);
Federal Republic of between 0.001-7.2) Cetinkaya et al. (1984);
Heeschen et al. (1986);
Lorenz & Neumeier (1983)
- 2709 1.77 Fooken & Butte (1987)
Netherlands 1983 278 0.72 (0.27-2.20)b Greve et al. (1985);
(11 centres country-wide) Greve & Wegman (1984)
1977-1979, 2649 2.1 Olling (1984)
1981
United Kingdom (Scotland) 1979-1980 30 0.01 (nd-0.04) HMSO (1986)
1983-1984 30 < 0.01 (nd-0.02)
Table 22. (cont'd).
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
Italy (Rome) 1983-1985 65 0.070 (0.007-0.176)a,c Dommarco et al. (1987)
Finland (different parts) 1984-1985 183 (165 of 0.57 (0.05-10.7) Mussalo-Rauhamaa et al.
women) (1988)
Sweden (5 regions) - 300e 1.06-1.18 (four regions) Noren (1983)
1.44 (one region)
1972 227d 1.05 Noren (1988)
1976 245 0.99
1980 340 0.78
1984-1985 102 0.60
Austria (Vienna) - 22 1.54 (0.58-3.78) Pesendorfer (1975)
Other regions 9 1.29 (0.95-1.57) Pesendorfer (1975)
a Whole milk.
b Median concentration.
c Arithmetic mean.
d Number of mothers that provided 4-7 samples each (samples were pooled).
e In each region, 300 mothers gave breast milk 3-5 days after parturition.
In a study by Kuwabara et al. (1978), the relationship was
investigated between breast-feeding and PCB residues in the blood of
children whose mothers were occupationally exposed to PCBs. The
children ingested their mother's milk for periods of < 1 to 3 years.
The age of the children at the time of the study ranged up to 13
years. The data provide evidence that PCBs are retained in the
children's body for many years and that longer intake of mother's milk
tends to increase PCB levels in the blood of the children. The PCB
levels in the blood of the 20 occupationally-exposed women and their
39 children ranged from 8.3 to 84.5 and 0.8 to 93.2 µg/litre,
respectively.
The results suggest that the PCB levels in the blood of children are
much more influenced by the transportation of PCBs through the
mother's milk than through the placenta. Furthermore, it was found
that the gas chromatographic patterns of the blood PCBs of the
children, breast fed for a long time, were different from those of
their mothers. Blood from 16 non-occupationally exposed mothers and
their children (17), showed that, as the length of the breast-feeding
period increased, there was an increase in the PCB levels in the blood
of the children. The mean blood PCB level in mothers was 2.8 ±
0.8 µg/litre; in children, it was 3.8 ± 3.6 µg/litre. In this study,
no clear change in blood PCBs patterns between mothers and children
was observed (Kuwabara et al., 1979).
Samples of maternal blood, milk, and umbilical cord blood were
collected from 43 mothers giving birth to their first or second child;
all the mothers had lived in Oslo during the previous 2 years. Blood
samples were collected immediately after delivery, either by Caesarean
section (16 Norwegians) or normally (20 Norwegians and 7 immigrants).
Subcutaneous fat samples were obtained during the operation. Samples
of colostrum and milk were obtained 3 and 5 days postpartum. PCBs were
found in 135 of the total 168 samples. In the Norwegian women and
infants, PCBs were the major contaminants, whereas only traces of PCBs
were found in the samples of immigrants. The average concentrations in
the maternal serum, cord serum, colostrum, and breast milk of
Norwegian women (Caesarean and normally delivered taken together)
were: 10, 3-5, 18-21, 20-23 µg/kg wet weight (Skaare et al., 1988).
5.5.3.1 Major PCB congeners in human milk
Commercial PCB preparations consist of complex mixtures of
environmentally stable compounds with a wide range of chlorine
contents. PCBs are transferred to breast-fed infants with the fat of
the mother's milk. Thus, infants nurtured on maternal milk are exposed
to relatively high concentrations of the higher chlorinated PCBs in
the short period preceding the full functioning of certain organs,
e.g., the liver (Jensen, 1983b; Slorach & Vaz, 1983; Gezondheidsraad,
1985).
Three major congeners were present in breast milk, e.g., PCB congener
numbers 138, 153, and 180 (DFG, 1988).
Slorach & Vaz (1983) reported that the GC patterns of PCBs in breast
milk samples from different countries were similar. The peaks denoted
146, 174, and 180 were dominant in the gas chromatograms. The total
levels of PCBs and the concentrations of certain congeners in Swedish
human milk, sampled in 1972-89, were studied by Noren et al. (1990).
Minor changes in the distribution of the congeners were found over the
period of study. The most abundant of the non- ortho coplanar PCBs in
Swedish human milk was 3,4,5,3',4'-pentachlorobiphenyl (126), with
levels decreasing from 0.35 µg/kg milk fat (1972) to about 0.10 µg/kg
(1989).
Safe et al. (1985a) analysed a sample of breast milk using the
congener-specific PCB method and found the following major components:
2,4,4'-trichloro-; 2,4,5,4'-tetrachloro-; 2,4,5,2',4'-pentachloro-;
2,4,5,3',4'-pentachloro-; 2,3,4,5,2',5'-hexachloro-;2,4,5,2',4',5'-
hexachloro; 2,3,4,5,2',3',4'-heptachloro-; and 2,3,4,5,2',4',5'-
heptachlorobiphenyls.
The major PCB congeners in the breast milk of Japanese women from the
general population were: 2,4,4'-trichloro-; 2,4,3',4'-tetrachloro-;
2,4,5,3',4'-pentachloro-; 2,3,4,2',3',4'-hexachloro-;2,3,4,5,2',4'-
hexachloro-; and 2,3,4,5,2',4',5'-heptachlorobiphenyls. The congeners
were present in 5% or more samples; a few other congeners were present
in only 1-3% (Gyorkos et al., 1985; Jensen, 1983b).
Sixty-eight breast milk samples collected in the Netherlands were used
to determine the congener distribution. The indicator congeners,
present in the highest concentrations, were: 2,4,4'-trichloro-,
2,4,5,2',5'-pentachloro-, 2,4,5,3',4'-pentachloro-,
2,3,4,2',4',5'-hexachloro-, 2,4,5,2',4',5'-hexachloro-,
2,3,4,5,2',4',5'-heptachlorobiphenyl (Wegman & Berkhoff, 1986).
Schecter et al. (1989a) analysed a total of 17 samples of human milk
from Thailand and Vietnam, for the presence of PCB congeners. The main
congeners that were present were 138, 153, and 180 (each in the range
of 8-31 µg/litre). The other congeners, normally present, were all
below the detection limit of 2 µg/litre.
In a study on pooled human milk samples from a 1982 nation-wide survey
in Canada, Mes & Marchand (1987) compared the relative amounts of 29
selected PCB isomers with amounts in milk samples of unexposed Rhesus
monkeys. In the pooled milk sample, 397 µg PCBs/litre, on a fat basis,
were found and the PCB isomer numbers 74, 99, 118, 138, 153, and 180
were the main contributors. Most of the predominant PCB isomers in
human milk were also observed in monkey's milk, but monkey's milk had
relatively low levels of PCB isomers numbers 74 and 99.
In another study, Davies & Mes (1987) analysed breast milk samples
from Canadian, Indian, and Inuit (Eskimo) mothers in Canada. The 18
samples were received from 5 Indian and Inuit nursing zones. The
combined total PCB isomer level (on a whole-milk basis) of the native
population was comparable with that of the national population. Even
the levels of the 5 largest PCB congeners (Nos. 74, 118, 138, 153, and
180) were comparable.
Individual congeners in the blood of Yusho- and Yu-Cheng patients are
discussed in section 5.6.
5.5.3.2 Factors that influence the intake of PCBs with milk
Present data suggest that the PCB content of human milk varies
considerably from individual to individual.
Many factors affect the level of PCBs and other organochlorine
compounds in breast milk including the fat content of the milk; time
from start of lactation; mother's age; mother's body weight; parity;
number of children previously breast-fed; origin and residence; eating
habits; season; smoking; use of household products; amount of milk;
and exposure at work (WHO/EURO, 1985, 1988).
In a given woman's milk, there are fluctuation in the PCB levels in
whole milk and in milk fat during one nursing session and during the
day (Jensen, 1983b). A decrease of PCB levels in both milk and milk
fat has been found during the lactation period. Furthermore, the PCB
concentration in human whole milk and milk fat increases with the age
of donor. Another confounding factor is that the PCB levels decrease
with increasing numbers of deliveries and lactations (Greve et al.
1985); lactation serves as a period for the biological elimination of
PCBs (Jensen, 1983b). The PCB levels in human milk are higher in
heavily populated and industrialized areas than in rural areas.
Furthermore, in general, the PCB levels in the breast milk of women
from developing countries are lower (Jensen, 1983b).
Cetinkaya et al. (1984) studied the PCB levels in human milk samples
from all over the Federal Republic of Germany. At the same time, data
were collected by means of a detailed questionnaire on residency,
workplace, smoking, drinking and eating habits, and the age of
participating individuals.
The breast milk of 45 women consuming lacto-vegetarian food was
compared with that of 41 women consuming conventional food in the
Federal Republic of Germany in the period 1979-81. The PCB
concentration was comparable, e.g., 2.2 and 2.5 mg/kg, on a fat basis,
respectively (Acker et al., 1984).
Fish consumption was positively correlated with PCB levels in maternal
serum and breast milk. PCB levels in serum increased with age, but
were unrelated to social class, parity, or body weight (Schwartz et
al., 1983).
Eight hundred and one Wisconsin anglers were surveyed for fishing and
consumption habits in 1985. The mean annual number of sport-caught
fish meals was 18 (range 7.1 to 33.3). The mean number of
non-sport-caught fish meals was 24. The median PCB serum congener sum
level for 192 anglers was 1.3 µg/litre (range, nd to 27.1 µg/litre).
Statistically significant positive Spearman correlations were observed
between sport-caught fish meals and PCB levels in serum and between kg
of fish caught and PCB levels in serum (Fiore et al., 1989).
PCBs were measured in maternal serum, cord blood, placenta, and serial
samples of breast milk and colostrum, from 868 women in North Carolina
(USA). Forty-three per cent of the women were primiparous. Breast milk
was collected at 6 weeks, 3 months, and 6 months, and, in a few cases,
up to 18 months postpartum. The median PCB concentration in breast
milk decreased during the sampling period from 1.77 to 1.02 mg/kg, on
a fat basis. The PCB concentration dropped by about 20% over 6 months
and 40% over 18 months. This implies that excretion in milk is a major
factor in lessening the mother's body burden; however, it also implies
substantial exposure of the child. Colostrum contained a median value
of 1.74 mg/kg. PCBs concentrations were higher in milk than in serum
and higher in maternal serum than in the placenta. The levels in cord
blood were almost always below the limit of quantification. Older
women and women who regularly drank alcohol had higher PCB levels in
their milk; blacks had higher levels than whites. In general, women
had higher levels in their first lactation and in the earlier samples
of a given lactation, and levels declined both with time spent
breast-feeding and with number of children nursed (Rogan et al.,
1986a).
Two hundred and forty-two newborn infants of mothers who consumed
moderate quantities of contaminated lake fish and 71 infants whose
mothers did not eat such fish were examined during the immediate post
partum period. PCB exposure was correlated with lower birth weight and
smaller head circumference, and the authors claimed that these effects
were not attributable to any of 37 potential confounding variables,
including socioeconomic status, maternal age, smoking, etc. (Fein et
al., 1984).
The mother's diet may be an important determinant of the PCB levels in
her milk. In some areas of the world, the intake of PCBs from eating
contaminated fish has been claimed to be the most important source of
PCBs in human milk. Dairy products and meat may be contaminated via
natural food or feedstuffs (WHO/EURO, 1988).
In a pilot study on the course of the PCB concentration in human milk
during 6 months of lactation, some PCB determinants were studied in 23
women and their infants. The average PCB concentration in the milk of
14 mothers during a 6-month period amounted to 0.66 ± 0.12 mg/kg, on a
fat basis. In univariate analyses, the PCB concentration on a fat
basis was strongly associated with pre- versus post-pregnancy weight
gain, age, and occupation. After multiple regression analysis, the PCB
concentration on a fat basis remained significantly associated with
changes in weight gain. The pre-pregnancy Quetelet Index of the mother
(height/weight) and the estimated PCB content of the diet (fish) were
correlated with the PCB concentration, on a milk basis (Drijver et
al., 1988).
5.5.4 Other tissues
Schecter et al. (1989b) analysed the tissues of 3 patients from the
North American continent, with no known history of chemical exposure,
for the presence of PCB isomers. The total PCB concentrations in the 9
tissues studied were different. The highest levels were found in
adipose tissue, subcutaneous fat (range 86-423 µg/kg), adrenals
(25-103 µg/kg), liver (3-149 µg/kg), bone marrow (26 µg/kg), kidneys
(2-31 µg/kg); levels in the spleen, lung, and testes were below
12 µg/kg. Congeners present in the highest concentrations were numbers
28, 74, 118, 153, 105, 138, 183, and 180.
5.6 Accidental exposures (Yusho- and Yu-Cheng)
In 1968, a large number of persons in Japan were accidentally poisoned
by the consumption of a batch of rice oil contaminated with Kanechlor
400. A similar accident happened in the Province of Taiwan in 1979,
where the affected persons had also consumed rice-bran oil
contaminated with PCBs. The 2 cases of poisoning were called Yusho and
Yu-Cheng accidents, respectively (see section 9.1.2.1).
The average PCB concentration in the plasma of Yusho children was
6 µg/litre, compared with 3.7 µg/litre in controls. Breast-fed Yusho
children had higher levels than children not breast-fed (Abe et al.,
1975).
The concentrations of PCBs in the adipose tissue, liver, and blood of
Yusho patients, about 5 years after the outbreak, were 1.9 ±
1.4 mg/kg, 0.08 ± 0.06 mg/kg, and 6.7 ± .3 µg/litre, respectively.
These values were only about twice those of controls. The mean blood
PCB level of 278 persons involved in the Yu-Cheng accident was
89.1 µg/litre (range 3-1156 µg/litre). Six months after the exposure,
the concentrations of PCBs in the blood had decreased to
12-50 µg/litre. The mean blood concentration of 165 patients,
9-18 months after the onset of poisoning, was 38 µg/litre (range
10-720 µg/litre) (see section 9.1.2.1). The blood PCB level of some
Yu-Cheng patients (99 ± 163 µg/litre), was much higher than that of
the Taiwanese population (1.2 ± 0.7 µg/litre), one year after the
outbreak of the intoxication.
Chen et al. (1985) analysed the blood of 165 Yu-Cheng patients, 9-18
months after the onset of poisoning, and found 10-720 µg PCBs/litre
with a mean value of 38 µg/litre. The blood of 10 patients, 9-27
months after poisoning, contained 0.02-0.2 µg PCDFs/litre. The
PCDF-congeners found in tissues were the same as those found by Masuda
et al. (1985).
Seven PCB congeners including: 2,4,5,3',4'-pentachloro-; 2,3,4,3',4'-
pentachloro-; 2,4,5,2',4',5'-hexachloro-; 2,3,4,2',4',5'-hexachloro-;
2,3,4,5,3',4'-hexachloro-; 2,3,4,5,2',4',5'-heptachloro-; and
2,3,4,5,2',3',4'-heptachlorobiphenyls, were identified in the blood
and tissues of Yusho, Yu-Cheng patients and controls.
Major PCDF congeners identified in the tissues and blood of Yusho and
Yu-Cheng patients were 2,3,6,8-tetrachloro-; 2,3,7,8-tetrachloro-;
1,2,4,7,8-pentachloro-; 2,3,4,7,8-pentachloro-; and 1,2,3,4,7,8-
hexachlorodibenzofurans. The 2,3,4,7,8-pentachloro-compound was
predominant. The concentrations of PCDFs in the adipose tissue and
liver of Yusho patients were 6-13 µg and 3-25 µg/kg tissue,
respectively. No PCDFs could be detected in the controls. Besides PCBs
and PCDFs, 4-methylthio-2,5,2',5'-tetrachlorobiphenyl (concentrations
ranging from 0.1 to 1.4 µg/kg tissue) and 4-methylsulfone-
2,5,2',5'-tetrachlorobiphenyl (range 0.3-2.5 µg/kg tissue) were also
found (Masuda et al., 1985).
5.7 Occupational exposure
5.7.1 Accidental exposure
Though the volatility of the PCBs is low, they are found in rather
high concentrations in the workroom air in both the long-term open use
of PCBs and in temporary or acute events where evaporation into the
air is possible. The measured air concentrations of PCBs in long-term
exposure situations, such as the manufacturing of transformers or
capacitors, varied from 30 to 1000 µg/m3, depending on the year of
measurement and the factory concerned (Silbergeld, 1983).
In discontinuous work, such as the inspection and repair of
transformers and capacitors, levels of between 0.1 and 60 µg/m3 have
been observed (Wolff, 1985). PCB concentrations in the breathing zone
of workers in transformer repair and maintenance work varied between
0.01 and 24.0 µg/m3 (Moseley et al., 1982).
In the atmosphere of an electroindustrial plant in Bela Krajina,
levels in the manufacturing room, where the autoclave was emptied,
averaged 2000 µg/m3 (range, 1400-3200 µg/m3); an average of
80 µg/m3 (range 40-120 µg/m3) was found in the working environment
in capacitor manufacture (Jan et al., 1988b).
Digernes & Astrup (1982) determined the concentrations of PCBs in the
atmosphere of the workplace of data screen operators, because skin
rashes and eczema had been reported among the workers. The PCB
concentrations in the working atmosphere (3 samples: concentrations
ranging from 0.056 to 0.081 µg/m3) were about 50-80 times higher than
the maximum level of PCBs in 3 samples collected outside the building
(0.0005-0.001 µg/m3). The indoor and outdoor samples also differed
qualitatively. The indoor samples contained only Aroclor 1242, while
outdoor samples contained a mixture of Aroclor 1242 and 1254.
Acute emergency events may cause extremely high concentrations of PCBs
in the air, particularly in cases when PCBs are burnt or heated (fire,
short circuit with electric arcing, burning in welding, etc.). Levels
of up to 10 000-16 000 µg/m3 have been measured. In the case of
extensive leaks of unheated PCBs from capacitors, concentrations of
1900 µg/m3 have been measured in workroom air (Elo et al., 1985;
WHO/EURO, 1987).
In connection with fires and electrical explosions, due to short
circuits, PCBs may be decomposed at elevated temperatures varying from
a few hundred to 2000°C. Soot may be produced in large amounts,
consisting of particles that may contain PCB concentrations up to
5000-8000 mg/kg of soot (Elo et al., 1985; O'Keefe et al., 1985;
WHO/EURO, 1987).
When evaluating PCB exposure, it is important to take into account
skin absorption from surfaces and tools, in addition to exposure via
inhalation. Surface concentrations of PCBs in capacitor factories have
varied between 4 and 60 µg/m2, and, where PCB leaks have occurred,
levels of up to 30 mg/m2 have been measured. Where PCBs have been
used long-term, contamination levels of 1-2 µg/cm2 have been found on
tools and tables.
A transformer was found to have overheated and released an oily mist
containing PCBs and their pyrolysis by-products, in a Department
building in New Mexico. The transformer contained Askarel (87% Aroclor
1260 and 13% of a mixture of tri- and tetrachlorinated benzenes). The
3-storey building was extensively contaminated via the following ways:
* mist entered 2 rooms, adjacent to the basement in which the
transformer was located;
* direct spread of mist and fumes through stairways;
* air drafts created by open windows and exhaust fans, spreading
fumes throughout the building;
* foot traffic by employees and other persons;
* the exhaust vent of the transformer room, located near the intake
vents for the building's air-conditioning system.
Air samples obtained up to 14 h after the incident showed levels of
48 µg/m in the transformer vault and 20 µg/m3 in the room above the
vault. Wipe samples of surfaces showed PCB levels ranging from 30
million µg/m2 for grossly contaminated surfaces to 4700 µg/m2 for
surfaces without visible contamination.
Five to 7 days later, air and surface samples were analysed for
2,3,7,8-tetrachlorodibenzofuran (TCDF), which was found to be present
in the air at an average level of 48 µg/m3 in most contaminated
areas. In wipe samples, the levels ranged from 5 ng/m2 to
41.224 ng/m2. 2,3,7,8-Tetrachlorodibenzo- p-dioxin (TCDD) was not
detectable in either air samples (detection limit, 0.5-5.0 pg/m3 air)
or wipe samples (detection limit 180 ng/m2) (Anon., 1985).
Very high concentrations of these toxic chemicals may be found in soot
emitted in connection with fires and explosions in capacitors.
Thus, skin contamination, and the ingestion and inhalation of soot
particles, may result in serious exposure in PCB accidents and
emergencies.
A short-term, follow-up study was performed on 55 workers in a gear
plant, whose work did not involve the use of PCBs. Exposure was to the
total residual PCB left behind by a capacitor company that had
formerly (3 years before) used the site. Air samples contained
< 10 µg/m3 and mean concentrations in wipe samples ranged from 23 to
161 µg/100 cm2. The 38 workers had a mean PCB concentration in serum
of 14.4 and the 17 office workers, 4.8 µg/litre. When the PCB
determinations were repeated in the 2 following years, no clear
decrease was observed (Christiani et al., 1986).
5.7.2 Occupational exposure during manufacture and use
Occupational exposure occurs during the manufacture of PCBs as well as
during their use by the electrical industry. It may also be widespread
among mechanics in contact with lubricating oils and hydraulic fluids,
among workers exposed to varnishes and paints, and among office
workers who have contact with pressure-sensitive duplicating paper
(carbonless copying paper), some brands of which readily transferred
PCBs to skin (Kuratsune & Masuda, 1972).
5.7.2.1 Adipose tissue
Levels of PCBs in the adipose tissue of occupationally exposed workers
have been found to vary between 26 and 50 mg/kg (range, 2.2-290 mg/kg).
There is a strong correlation between the blood PCB concentration and
PCB levels in adipose tissue, but the distribution of the various
congeners between plasma and adipose tissue is not the same, as
described above.
Emmett (1985) found the following congeners in the adipose tissue of
present and past transformer workers exposed to Aroclor 1242 and 1254:
2,4,3',4',5'-pentachloro-, 2,3,4,3',4'-pentachloro-, 2,3,4,5,2',4'-
hexachloro-, 2,3,4,6,3',4'-hexachloro-, 2,4,5,3',4',5'-hexachloro-,
2,3,4,5,2',3',4'-heptachloro-, and 2,3,4,5,6,3',4'-hepta-
chlorobiphenyl.
5.7.2.2 Blood
Karppanen & Kolho (1973) analysed the blood of 26 persons, 9
non-exposed, 6 persons handling PCBs, and 11 persons employed for 4
years in a capacitor-manufacturing plant in Finland. In the latter
case, Aroclor 1242 was used. The average concentrations in the blood
of the 3 groups were 7.1 µg/kg (3.1-12 µg/kg), 49.5 µg/kg
(36-63 µg/kg), and 440 µg/kg (70-1900 µg/kg), on a wet weight basis.
More recent results of a Finnish control group of workers indicated
serum PCB levels of 1.2 ± 0.6 µg/litre in an industrial area (Luotamo
et al., 1985; WHO/EURO, 1987). With acute exposure to high
concentrations of PCBs in air (8000-16 000 µg/m3), for a short
period, blood PCB concentrations rose to levels of 30 µg/litre; a
return to the normal level of 3 µg/litre was achieved, 4 weeks after
termination of exposure (Elo et al., 1985; WHO/EURO, 1987).
Similar plasma values were found in workers from Japanese capacitor
factories, but, here, skin lesions were noted (Hasegawa et al.,
1972a). In this same study, it was reported that air levels of PCBs of
10-50 µg/m3 were measured in a factory where KC-300 was used in the
manufacture of electric condensers. PCB levels in the serum of workers
ranged from 100 to 650 µg/litre. One month after the use of PCBs had
been suspended, serum levels remained unchanged (90-740 µg/litre).
However, in another factory making electric condensers, serum levels
decreased from an average of 800 to 300 µg/litre, within 3 months of
the use of PCBs being discontinued (Kitamura et al., 1973). According
to Hara et al. (1974), the half-time of PCBs in the blood of workers,
engaged in the manufacture of electric condensers for less than 5
years, was several months, while that of workers employed for more
than 10 years was 2-3 years.
Kuwabara et al. (1978) reported mean PCB levels of 36.8 µg/litre
(range 8.3-84.5 µg/litre) blood in 20 PCB-workers, 39 children had
blood levels of 14.3 µg/litre (0.8-93.2 µg/litre), and 12 Yusho
patients, 4.2 µg/litre (1.8-8.6 µg/litre).
Fact-finding surveys of 63 workers, who were occupationally exposed to
PCBs (Kanechlor 500) in the production of silk thread or of paint,
were carried out in Japan in 1974-75; some of them and their families
were also surveyed again in 1975-82. Nineteen per cent of them showed
PCB levels higher than 50 µg/litre plasma. These persons did not show
the typical clinical findings of Yusho patients. During 7 years, no
clear decline was observed (Takamatsu et al., 1984).
There is clear evidence that relatively high PCB levels persist in the
blood of workers whose "external" exposure ceased several months or
years previously. The blood PCB concentrations in capacitor
manufacturing workers, who had been exposed for 1-24 years, varied
between 24.4 and 192 µg/litre; this was higher than levels in the
blood of a reference population (0.5-33 µg/litre) (Maroni et al.,
1981a).
In Japan, Yakushiji et al. (1984a) studied the rate of decrease and
the half-life of PCBs in the blood of children (aged 1-13 years) and
their mothers, who were occupationally exposed to PCBs, over a 5-year
period (1975-79). The mean concentration of 121 blood samples from
50 children was 17.4 ± 22.9 µg/litre and that in 65 samples from 29
mothers was 32.3 ± 20.6 µg/litre. The concentrations of PCBs in the
blood of the children varied over a wide range, because of differences
in the duration of breast-feeding. The rate of decrease of the PCB
concentration in the blood in both 18 children and 8 mothers was
relatively constant and independent of the PCB concentrations. A
one-compartment model equation was sufficient to represent the
decrease in the concentration of PCBs in the blood. The mean rate
constant of the decrease for the children was 24.2% per year,
approximately 2.6 times higher than that of the mothers (9.2%),
equivalent to half-lives of 2.8 ± 1.1 and 7.1 ± 2.7 years,
respectively. The dilution effect due to the increase in body weight
was the most important factor that affected the reduction of the PCB
concentrations in the children.
A total of 118 blood samples, mainly from employees in industries
using PCBs, were collected in the period 1975-85. In 64 blood samples,
an average level of 17 µg/litre (range nd-110 µg/litre) was found
(Frank et al., 1988).
Brown & Lawton (1984) studied the partitioning of PCBs between adipose
tissue and serum in a population of 173 capacitor workers, who were
occupationally exposed to Aroclors 1254, 1242, and 1016 for various
periods of time. The serum levels of PCBs were significantly dependent
on the level of lipids in the serum, but not on that in the albumin.
The apparent contribution of cholesterol and its esters to PCB
transport is nearly equal to their contribution to the total serum
neutral lipids. The level of serum lipids PCBs must be equal to the
adipose fat PCBs level.
Yakushiji et al. (1984b) studied the relationship between
breast-feeding and the PCB levels in the blood. The blood samples of
50 children (121 samples) and of 29 occupationally exposed mothers (65
samples) were analysed during the period 1975-79. The PCB levels in
the blood of the children were greatly influenced by the duration of
breast-feeding, but showed little relationship to the PCBs levels in
maternal blood.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Inhalation
Studies on rats (6 per group) showed that an aerosol containing a PCB
mixture (Pydraul A200: 42% chlorine), particle size 0.5-3.0 µm, at a
concentration of 30.4 ± 3.4 g/m3 for 30 min, was readily absorbed
through the lungs. The PCB concentration in the liver, 15 min after
cessation of exposure, was 50% of the maximum concentration attained
after 2 h (70 mg/kg tissue) (Benthe et al., 1972).
6.1.2 Dermal
Vos & Beems (1971) and Vos & Notenboom-Ram (1972) applied Aroclor 1260
to the shaved backs of rabbits and found systemic effects in the
kidneys, indicating that PCBs can penetrate the skin (see section
8.2.5).
Nishizumi (1976), using tritium-labelled PCBs (40% chlorine), found
evidence for the dermal absorption of PCBs in rats.
In a study of the occupational exposure of electrical workers to PCBs
(Pyralen 3010 and Apirolio, 42% chlorine content), Maroni et al.
(1981a) concluded that absorption of PCBs occurred through the human
skin. Quantitative data were not available.
6.1.3 Oral
When polychlorobiphenyl isomers were administered orally, by gavage,
to rats, at levels of 5, 50, or 100 mg/kg body weight for the lower
chlorinated compounds and up to 5 mg/kg for the higher chlorinated
compounds, 90% of the compounds were rapidly absorbed by the
gastrointestinal tract (Albro & Fishbein, 1972; Berlin et al., 1973;
Melvås & Brandt, 1973).
Using Rhesus monkeys, Allen et al. (1974a,b) determined that > 90% of
a single oral dose of 1.5 or 3.0 g Aroclor 1248/kg body weight was
absorbed over a period of 2 weeks. Drill et al. (1981) and US EPA
(1985) reviewed a number of studies indicating that PCBs are readily
absorbed from the gastrointestinal tract following oral
administration.
Bleavins et al. (1984) found that, over a period of 5 weeks, European
ferrets absorbed 85.4% of a single dose of 14C-labelled Aroclor 1254
(0.05 mg) given in food.
In contrast to the above studies, Norback et al. (1978) claimed that
59.3-87% of a single oral dose of 2,4,5,2',4',5'-hexachlorobiphenyl
passed unabsorbed through the intestines of monkeys, the first week
after dosing.
6.2 Distribution
6.2.1 Inhalation (rat)
Maximum PCB concentrations in the liver and brain of rats occurred 2
and 24 h, respectively, after a single, 30-min exposure to 30.4 ±
3.4 g/m3 of Pydraul A200 aerosol (42% chlorine content). The
concentrations in these tissues declined, while concentrations in
adipose tissues reached a maximum after 48 h (Benthe et al., 1972).
6.2.2 Oral (rat)
As in the case of other lipophilic substances, the absorption and
distribution of PCBs will, in all probability, take place via the
lymphatic system (by the chylomicrones) (DFG, 1988).
Following absorption, the clearance of PCBs from the blood and tissues
follows a biphasic pattern. The compounds rapidly clear from the blood
and accumulate in the liver and adipose tissue or are metabolized in
the liver to metabolites that are excreted in the urine and/or bile
(Drill et al., 1981).
Kurachi & Mio (1983) exposed mice to Kaneclor 400 at 100 mg/kg diet,
for 5-20 days. High levels were found in the gonads, skin, adipose
tissue, adrenals, and kidneys.
In a study by Grant et al. (1971a), 4 days after an oral dose of
Aroclor 1254 was given to rats at 500 mg/kg, the concentrations of
PCBs in the fat, liver, and brain were 996, 116, and 40 mg/kg,
respectively. Similar results showing that the highest concentration
was in the fat, were obtained in rats given Aroclor 1254 in the diet
(Curley et al., 1971), in boars (Platonow et al., 1972), cows
(Platonow & Chen, 1973), and in pigeons and quail (Bailey & Bunyan,
1972). In the studies of Curley et al. (1971), the tissue
concentrations initially showed a rapid rise and then a slow increase
while the PCB diet was being administered; Grant et al. (1974) fed
diets containing Aroclor 1254 at 0.2, 20, and 100 mg/kg to rats for 8
months, during which period the tissue concentrations reached a steady
state that was dose-dependent (Table 23). Similar tissue distribution
data for Aroclors 1016 and 1242 have been reported by Burse et al.
(1974) and for Kanechlor-400 by Yoshimura et al. (1971).
Table 23. Tissue distribution of PCBs (mg/kg wet weight) in rats fed Aroclor 1254,
Aroclor 1242, or Aroclor 1016 at 100 mg/kg for about 6 months
Tissue Aroclor 1254a Aroclor 1242b Aroclor 1016b
Blood 0.40 0.53 (plasma) 0.38 (plasma)
Liver 16 4.21 7.86
Brain 3.4 1.69 2.98
Kidneys 1.89 3.21
Heart 7.3
Fat 32.0 110 236
Urine 0.03 0.28
a From: Grant et al. (1974).
b From: Burse et al. (1974).
The study by Burse et al. (1974) showed that, with continuous feeding
of 3 types of Aroclor (see Table 23) at 100 mg/kg diet, a steady state
was not reached for 6-8 months and that the decline of stored PCBs in
adipose tissue (when the animals were kept on a PCB-free diet) was
slow and did not reach zero during a recovery period of 5-6 months.
This is surprising because the Aroclor sample used should not have
contained appreciable amounts of hexachlorobiphenyls and higher
isomers. Mizutani et al. (1977), discussing this aspect, came to the
conclusion that mobilization from storage sites rather than metabolism
constitutes the rate-limiting step in the depletion of the body burden
of PCBs.
It was demonstrated that, as the number of chlorine atoms on the
biphenyl rings increased from 1 to 6, the tissue/blood ratio tended to
increase. This increase was also proportional to the amount of lipid
in the tissues with, consequently, a higher degree of bioaccumulation
(Matthews, 1983, cf. WHO/EURO, 1988).
The fat-plasma partition-coefficients for the different PCB congeners
range from 50 up to 310 (DFG, 1988).
6.2.3 Oral (monkey)
Feeding studies were carried out on female rhesus monkeys given doses
of 0, 5, 20, 40, or 80 µg Aroclor 1254/kg body weight per day, for a
period of 37 months (Arnold et al., 1984). Eighty monkeys were divided
into 5 groups, each of 16 animals. The mean body weight of the monkeys
at the start of the study was 6.44 kg. The Aroclor 1254 was dissolved
in corn oil with glycerol as sweetener and fed to the monkeys in
gelatin capsules. Samples of blood, adipose tissue, and faeces were
collected every month and the presence of PCBs, determined. After 27
weeks, levels of 1, 2-3, 9, 18, and 37 mg PCBs/kg fat were found in
the 5 groups; after 47 weeks, blood levels were 1-3, 12, 35, 73, and
129 µg/litre respectively. PCB concentrations in whole blood increased
more rapidly during the first 10 months of the study than in the
remaining 27 months, in all groups. Concentrations in adipose tissue
(fat) increased continuously during the 37 months. The ratio profiles
of PCB levels in blood/adipose tissue, remained relatively static
between the second and twenty-seventh month of feeding. The data in
terms of relative concentrations (concentration/dose) suggest that the
bioaccumulation or retention of PCBs may be dose-dependent,
particularly for adipose tissue. The data available from PCBs in
faeces indicate a dose-dependent PCB absorption.
6.2.4 Oral (humans)
According to the study by Nishimura et al. (1976) cf. Katsunuma et al.
(1985), the PCBs within a human fetus are not evenly distributed. The
concentrations of PCBs were highest in the skin and lowest in the
brain among the 5 major organs (cerebrum, heart, liver, kidneys, and
skin). That the highest level was found in the skin might have been
because of the high solubility of the compounds in adipose tissue. In
other words, PCBs accumulate increasingly as the body fat of a fetus
increases. The authors stated that the low residue levels in the brain
were likely to be because PCBs have a poor affinity for the brain
lipids.
6.2.5 Individual congeners of PCBs
More detailed information on the tissue distribution of PCBs and their
metabolites has been obtained by the administration of pure
14C-labelled compounds, using both whole-body autoradiography and
scintillation counting of tissue samples. Berlin et al. (1975)
demonstrated that, after a single oral dose of 14C-labelled
2,5,2',4',5'-pentachlorobiphenyl, radioactivity rapidly entered the
circulation of mice and was distributed in the tissues, particularly
in the liver, kidneys, lungs, and adrenals. Subsequently, the
radioactivity in the body fat increased, rising to a maximum within
4-24 h. In most other tissues, the radioactivity decreased rapidly
after dosing, but the authors noted a special affinity for the skin,
the bronchiolar epithelium of the lungs, and certain glandular
secreting tissues. Soon after administration of the dose,
radioactivity appeared in the bile and was eliminated in the faeces.
Similar results were obtained by Melvås & Brandt (1973) in mice
treated with 2,4,2',4'-tetrachlorobiphenyl, which possessed a high
affinity for the adrenal cortex, the corpora lutea, and glandular
secreting tissue. In quail treated with 2,4,2',3'- and
2,4,3',4'-tetrachlorobiphenyl, the radioactivity in the egg yolk was
high, exceeding that in the fat. Gage & Holm (1976) determined
concentrations in the abdominal fat of mice, 7 and 21 days after they
were administered a single dose (13-165 µg/mouse) of one of 14 PCB
congeners, by gavage. Relatively low levels (< 10 ng/g per µg dose)
were found at 7 days for 4,4'-dichloro-; 3,2',4',6'-tetrachloro, and
2,3,4,2',4',6'-hexachlorobiphenyl with relatively high levels (>
100 ng/g per µg dose) for 2,4,5,2',4',5'-hexachloro-, and the
4,2',4',6'-, and 2,4,2',4'- tetrachlorobiphenyls.
Muehleback & Bickel (1981) treated rats, by gavage, with a single dose
of 14C-2,4,5,2',4',5'-hexachlorobiphenyl at 0.6 or 3.6 mg/kg body
weight. The rats were examined 1 h, 24 h, 6 weeks, 20 weeks, or 40
weeks after dosing. The highest levels of PCBs were found in the
muscle, liver, adipose tissue, and skin, early in the study. By the
end of the study, the highest PCB levels were found in the adipose
tissue followed by the skin, muscle, and liver. During the 40-week
study period, only 16% of the total dose was excreted.
The pharmacokinetics of individual monochloro-, dichloro-,
tetrachloro-, pentachloro-, and hexachlorobiphenyls were studied by
Matthews & Anderson (1975a,b), Lutz et al. (1977), and Tuey & Matthews
(1977). The mono- and dichlorobiphenyls were largely removed from
adipose tissue within 4-7 days, the 3 higher chlorinated biphenyls
were eliminated much more slowly. The half-life for the
tetrachlorobiphenyl from adipose tissue was 15 days. Skin effects were
more or less comparable.
Beran et al. (1983) studied the distribution of 14C-labelled
2,5,4'-tri-, 2,4,5,2',4',5'-hexa-, and 2,3,4,5,2',3',4',6'-
octachlorobiphenyl in the haematopoietic tissues of squirrel monkeys
(Saimiri scureus) and C67Bl mice using whole-body autoradiography.
An accumulation of radioactivity was observed in the bone marrow of
one monkey after iv injection (substances dissolved in DMSO) of the
tri- or hexachlorobiphenyl. The same was found in 3 normal mice
treated with the octachlorobiphenyl. A study using whole-body
autoradiography and spleen-colony assay in supralethally irradiated
mice, implanted with syngenic bone-marrow cells, indicated that the
major part of the radioactivity was localized outside the bone-marrow
haemic compartment, probably in the fat. Nevertheless, the trichloro-
and octachlorobiphenyls were found to inhibit the in vitro formation
of granulocytic colonies from mouse progenitor cells. Very low uptake
of labelled chlorobiphenyls was observed in the thymus, spleen, and
lymph nodes.
14C-labelled-2,4,2',4'-tetrachloro- and 3,4,3',4'-tetrachlorobiphenyl
were each administered orally to male Sprague-Dawley rats in a single
dose at 0.54 mg/kg and 0.51 mg/kg, body weight, respectively.
Distribution and covalent binding were studied. The accumulation of
2,4,2',4'-tetrachlorobiphenyl in adipose tissue was much higher than
that of 3,4,3',4'-tetrachlorobiphenyl, though the level in the blood
was consistently higher in the 3,4,3',4'-tetrachlorobiphenyl-treated
rats. The radioactivity bound in covalent linkages with cellular
macromolecules in several tissues was determined. The data indicated
that covalent binding was higher in 3,4,3',4'-tetrachloro-
biphenyl-treated rats than in those treated with 2,4,2',4'-
tetrachloro-biphenyl, particularly in the liver and blood components.
These results suggest that the 2 tetrachlorobiphenyl isomers have
different pharmacokinetic properties in rats and that the association
of covalent binding with 3,4,3',4'-tetrachlorobiphenyl induced
toxicities might be important. The microsomal enzyme system is likely
to play an important role in the in vivo covalent binding of
tetrachlorobiphenyls (Shimada & Sawabe, 1984).
In pharmacokinetic studies, 11 groups of 3 male ICR mice/group were
administered daily doses of 100 mg 2,5,2',5'-tetrachlorobiphenyl/kg
body weight dissolved in corn-oil/acetone (9:1), by gavage, for 8
consecutive days. Thirteen groups of 3 mice were administered (by
gavage) 8 mg 3,4,3',4'-tetrachlorobiphenyl/kg in the same vehicle
every other day for 10 doses. One group was sacrificed just before
each of the last 3 doses, the other groups were sacrificed at
intervals of 0.5-336 h after dosing. After dosing to an apparent
steady-state, 2,5,2',5'-tetrachlorobiphenyl was found to have a tissue
elimination half-life of between 39.5 and 70 h. The half-life of
3,4,3',4'-tetrachlorobiphenyl was 26-62.5 h. The 3,4,3',4'-
tetrachlorobiphenyl had a substantially greater partitioning from
serum into adipose tissue, liver, and thymic tissues. Studies were
undertaken to compare the toxic potency of these 2
tetrachlorobiphenyls, when similar tissue concentrations of the 2
isomers were achieved in target and storage tissues. The studies
demonstrated that thymic atrophy occurs at lower doses and tissue
concentrations of 3,4,3'4'-tetrachlorobiphenyl than those required to
produce hepatotoxicity. These two organ toxicities were produced only
by 3,4,3'4'-tetrachlorobiphenyl, despite the fact that equivalent or
higher tissue concentrations of 2,5,2',5'-tetrachlorobiphenyl were
achieved in vivo, in all tissues. The conclusion was that the
in vivo difference in the toxic potency of these tetrachloro-
biphenyl isomers does not result from the differences in their tissue
disposition, elimination, and ultimate bioaccumulation (Clevenger et
al., 1989).
6.2.6 Appraisal
Matthews & Dedrick (1984), in a review, concluded that the
pharmacokinetics of PCBs are complicated by numerous factors, not
least of which is the existence of 209 different chlorinated
biphenyls. While all PCB congeners are highly lipophilic and most are
readily absorbed and rapidly distributed to all tissues, PCBs are
cleared from the tissues at very different rates, and the same
congeners may be cleared at different rates by different species. With
the exception of special situations in which PCBs may be passively
eliminated in lipid sinks, e.g., milk or eggs, clearance is minimal
prior to metabolism to more polar compounds. Rates of PCB metabolism
vary greatly with species and with the degree and positions of
chlorination. Mammals metabolize these compounds most rapidly, but,
even among mammalian species, the rates of metabolism vary greatly. In
all species studied, the more readily metabolized chlorinated
biphenyls have adjacent unsubstituted carbon atoms in the 3-4
positions. Congeners that do not have adjacent unsubstituted carbon
atoms may be metabolized very slowly and therefore cleared very
slowly. PCBs that are not readily cleared concentrate in adipose
tissue.
6.3 Placental transport
6.3.1 Laboratory animals
The results of a number of animal studies have demonstrated that PCBs
and specific congeners can cross the placental barrier and accumulate
in the tissues of fetuses (US EPA, 1987). In studies in which monkeys
were exposed prior to, and during, gestation, signs of PCB
intoxication were observed in nursing, but not in newborn offspring
(Allen & Barsotti, 1976; Iatropoulos et al., 1978). Results such as
these have led to the conclusion that transfer through nursing may
account for higher exposure of the young than placental transfer.
Groups of pregnant ddN mice were fed diets containing Kanechlor 500
(mainly comprising pentachloro- and hexachlorobiphenyls) at 0.01
(controls), 0.94, or 86 mg/kg diet from day 1 to 18 of pregnancy.
Regardless of the dietary level of PCBs, whole-body levels in the
fetuses were only 0.1-0.2% of the total maternal intake, indicating
limited transplacental transfer (Masuda et al., 1978a). Two groups of
ddN mice were fed Kanechlor 500 (mainly containing pentachloro- and
hexachlorobiphenyls at 0 or 0.94 mg/kg diet) from the day of
insemination throughout gestation and for 5 weeks after delivery of
offspring. Total PCBs were 100 times greater in the suckling animal
than in the fetuses at term, from dams fed the same amount, indicating
a considerable transfer of PCBs during lactation (Masuda et al.,
1978a).
Masuda et al. (1979) fed female ddN-mice diets containing
polychlorinated biphenyls: 2,4,4'-trichloro-; 2,5,3',4'-tetrachloro-;
2,4,5,2',5'-pentachloro-; 2,3,4,2',4',5'-hexachloro-;2,4,5,2',4',5'-
hexachloro-; 2,3,4,5,6,2',5'-heptachloro-; and 2,3,4,5,2',3',4',5'-
octachlorobiphenyl at levels of 0.32, 0.42, 0.42, 0.44, 0.44,
0.16, and 0.23 mg/kg diet, respectively, for 18 days prior
to, or after, mating. Animals were either sacrificed on day 18 of
gestation or allowed to deliver and the offspring maintained for 5
weeks on a normal diet. All the PCBs were qualitatively transferred
across the placenta and through the milk. The amount transferred
during lactation was greater than that transferred transplacentally.
The transfer of 2,4,5,2',4',5'-hexachlorobi[14C-]phenyl across the
placenta during the course of pregnancy in Sprague-Dawley mice was
studied by Vodicnik & Lech (1980). The PCB was injected
intraperitoneally at 100 mg/kg body weight, in corn oil, 2 weeks prior
to mating. The concentrations of 14C-PCB in the fetuses from 12- and
18-day pregnant animals were 0.71 and 2.45 mg/kg tissue, respectively.
At birth, the total carcass concentration for all newborn animals was
less than 3 mg/kg tissue, which represents less than 3% of the dose
present in the mothers at birth.
Placental transfer of polychlorinated biphenyls has also been reported
in the mouse by Berlin et al. (1975) and Melvås & Brandt (1973).
Curley et al. (1973a) found some placental transport of Aroclor 1254
in the rat.
Groups of pregnant and non-pregnant Wistar rats received a dose of
14C-2,4,5,2',4',5'-hexachlorobiphenyl (2.1 µC/kg), intraperitoneally.
The amount of radioactivity transferred through the placenta was 2.7%
of the administered dose, whereas 39.2% of the original dose was
transferred through the milk (Ando et al., 1978).
Aroclors 1221 and 1254 were found to cross the placenta of rabbits,
when administered orally to does during gestation. The concentration
in fetal tissues was dose-dependent and much lower with Aroclor 1221
than with Aroclor 1254; the concentration of the latter in the fetal
liver was greater than that in the maternal liver (Grant et al.,
1971b).
Bleavins et al. (1984) fed female European ferrets a single dose of
14C-labelled Aroclor 1254 in the diet (0.05 mg), early (day 14) or
late (day 35) in gestation, and determined the placental transfer of
PCBs. Placental transfer to the kits was 0.01% (per kit) of the
maternal dose, when the dams were exposed early in gestation, and,
0.04%, when the dams were exposed late in gestation. Placental
transfer of PCBs was considerably less than mammary transfer, with a
ratio at 1 week of lactation of 1:15 and 1:7 for offspring of dams
dosed early or late in gestation, respectively.
Groups of lactating mother Rhesus monkeys, between 1 and 3 months post
partum, received 16 mg Clophen A-30/kg per day for 30 days. One
mother/infant pair served as a control. Clophen A-30 concentrations in
the serum of both mother and infant and the milk were determined on
days -14, -7, 0, 1, 2, 4, 8 and at weekly intervals thereafter. One
mother and all infants were killed and tissues taken for PCB analysis.
The concentration of Clophen A-30 in milk was 20 times higher than
maternal serum levels. Infant serum levels were 2-5 times higher than
their mothers. Tissue levels were generally higher in the infants.
Clophen A-30 tended to concentrate in the infant fat, bone marrow, and
adrenals (Bailey et al., 1980).
Groups of 24 Rhesus monkeys were maintained on diets that provided
Aroclor 1016 at doses of 0, 4.5, or 18.1 mg/kg body weight per day
throughout gestation and a 4-month nursing period. At birth, the
concentrations of PCBs in the skin of infants were similar to
concentrations in the subcutaneous fat of the mothers. At weaning, the
PCB content in the mesenteric fat of the infants was 4-7 times greater
than that in the subcutaneous fat of the mothers. Gas chromatographic
patterns showed that the adult adipose tissue did not include the
total spectrum of peaks observed in the Aroclor 1016 standard, and
that all of the peaks in the mesenteric fat of the infants at weaning
and 4 months after weaning were qualitatively similar to those in the
adult adipose tissue. According to the authors, these data suggested
an inability of the fetus to metabolize and excrete certain congeners
that are more readily metabolized and eliminated by adults and older
infants (Barsotti & Van Miller, 1984).
6.3.2 Wildlife
A 6 1/2-year-old desert bighorn (Ovis canadensis cremnobates) ewe
and her term ram fetus were used to study the distribution and
concentrations of PCBs in different organs and tissues. Fourteen
maternal and 13 fetal tissues were analysed for their presence of
organochlorine hydrocarbons. PCBs averaged 85 and 88% of the total
residue loads for maternal and fetal tissues, respectively. It is
remarkable that the "natural" PCB levels in the different organs and
tissues were nearly the same, i.e., in maternal organs and tissues
between 0.37 and 0.44 mg/kg, and, in fetal organs and tissues, between
0.30 and 0.35 mg/kg, on a fat basis (Turner, 1979).
6.3.3 Humans
Four studies of placental passage in humans, based on small samples
drawn from the general Japanese population, have yielded inconsistent
results (Yoshimura, 1974; Akiyama et al., 1975; Kodama & Ota, 1977;
Masuda et al., 1978a).
PCBs were detected in the umbilical tissues, umbilical blood, amniotic
fluid, and baby's blood from a woman who was occupationally exposed to
Kanechlors 300 and 500 in a capacitor factory (Yakushiji et al.,
1978). PCB levels in these tissues and fluids were considerably lower
than that in the mother's blood.
Jacobson et al. (1984b) examined maternal and cord serum (196 or 198
samples each) for the presence of PCBs, in women who resided in the
Michigan area (USA), where, in 1973, a PBB-incident occurred. Mean
concentrations of maternal and cord serum were 4.7 µg/litre (1.1-
14.3 µg/litre) and 2.0 µg/litre (0.1-7.2 µg/litre), respectively.
Placental passage was indicated by a significant maternal to cord
serum correlation for PCBs. The fact that cord serum levels were lower
than those in maternal serum is consistent with the notion that the
placenta may function as a partial barrier. The transfer rate of PCBs
in maternal blood through the placenta to cord blood may vary,
depending on the chemical nature of each PCB isomer (Ando et al.,
1984). Ando et al. (1985) examined the PCB concentrations in the
maternal blood, breast milk, and the placenta of 6 Japanese women.
They found that the congeners present were more typical of Kanechlor
500 than Kanechlor 300, 400, or 600. The results indicated that, as
the chlorine content of the PCB congeners increased, the correlation
between the placental content of congeners and those in the maternal
blood and breast milk also increased. The same was found in laboratory
animals (Allen & Barsotti, 1976; Masuda et al., 1978b).
A study on the transfer of PCBs to infants from their mothers was
carried out in Japan from 1974 to 1976 by Kodama & Ota (1977). When
the cord blood was considered as the infant blood at birth, the level
of PCBs in the blood of breast-fed infants rose gradually with
ingestion of breast milk, exceeded the level in the blood of their
mothers after 3 months, continued to increase up to the age of 1 year
and then significantly decreased, 2 years after birth. The PCB
concentrations in the blood of non-breast-fed infants remained low
(Table 24).
6.4 Excretion and elimination
6.4.1 Following oral dosing
The excretion of PCBs is, to a large extent, dependent on the
metabolism of PCBs to form more polar compounds (US EPA, 1987). At
equilibrium, the elimination of PCBs from all tissues will be
dependent on the structure-dependent metabolism rates of the
individual PCB congeners. For example, the biological half-lives in
the rat range from 1.15 days for 2,2'-dichlorobiphenyl to
approximately 460 days for 2,4,5,2',4',5',-hexachlorobiphenyl (Tanabe
et al., 1981; Wyss et al., 1986). Metabolites of the more highly
chlorinated congeners are eliminated primarily via the faeces (Goto et
al., 1974; WHO/EURO, 1987).
When the analysis of faeces is limited to the determination of
unchanged PCBs, the recovery of the dose administered is incomplete;
in boars receiving single or repeated doses of Aroclor 1254, not more
than 16% of the dose was recovered from the faeces and less than 1% in
the urine (Platonow et al., 1972). Better recoveries have been
obtained with PCB labelled with radioactive isotopes. Yoshimura et al.
(1971) found 70% of the activity from a dose of tritium-labelled
Kanechlor 400 in the faeces and 2% in the urine, over a 4-week period.
Berlin et al. (1973, 1975) found over 75% of the activity from
14C-labelled pentachloro- and hexachlorobiphenyls in the faeces and
less than 2% in the urine; most of the faecal elimination consisted of
PCB metabolites. Similar results were obtained by Melvås & Brandt
(1973) with tetrachlorobiphenyls.
Hashimoto et al. (1976) examined the excretion of 14C-PCB compounds
given to rats by gavage, at a total dose of 6.35-7.85 mg/kg body
weight, over a period of 5-50 days. The PCBs studied were
predominantly tetra- and hexachlorinated isomers. The results
indicated that 1.9-4.9% of the dose of tetrachlorobiphenyls was
excreted in the urine, with higher amounts excreted in rats treated
for longer periods. In rats treated with hexachlorobiphenyls, only
0.3% of the dose was excreted in urine. About 47-68% of the dose of
both tetrachloro- and hexachloro-isomers was eliminated in the faeces.
Table 24. Level of PCBs in mothers' and babies' blood (average over
3 years in µg/litre)a
Maternal blood 4.5 (0.8-15.5)
Cord blood 1.1 (nd-5.6)
Mother's blood (breast-feeding) 2.5 (nd-10.8)
Babies' blood (3 months old) 3.6 (0.2-10.9)
Babies' blood (1 year old) 4.7 (0.8-17.7)
Mother's blood (bottle feeding) 2.7 (0.6-8.7)
Babies' blood (3 months old) 1.6 (nd-7.6)
Babies' blood (1 year old) 0.7 (nd-2.1)
a From: Kodama & Ota (1977).
Bleavins et al. (1984) found 22.1% and 1.8% in the faeces and urine,
respectively, during the first week following dosing of 0.05 mg
14C-labelled Aroclor 1254 to female European ferrets.
A biological half-life of about 200 days was recorded in the fat of
rats after feeding with Aroclor 1254 (Grant et al., 1974). Berlin et
al. (1975) noted that, in mice dosed with a pentachlorobiphenyl, there
was an initial rapid elimination from the liver while liver PCB levels
were high, followed by a slower elimination when most of the PCB was
located in the fat. The author suggested that the mobilization of PCBs
from fat, and, therefore, their half-life in the body, depends upon
their rates of metabolism. Berlin et al. (1973) investigated the
hypothesis that the ability of a PCB to be readily degraded with a
half-life of a few days depended on the presence of 2 adjacent
unsubstituted carbon atoms in the molecule, rather than on the number
of chlorine atoms, though the presence of such unsubstituted pairs
depends to a large extent on the degree of chlorination. They came to
the conclusion that this hypothesis probably applied to unsubstituted
pairs in the 3,4-position, but that in the 2,3-position, their
susceptibility to metabolic degradation was influenced more by the
presence of chlorines in the o-position of the ring bridge.
Sprague-Dawley rats, white Swiss mice, and Rhesus monkeys were
administered a single dose of 14C-2,2'-dichlorobiphenyl, by gavage.
Within 6 days, mice eliminated a total of approximately 46% of the PCB
(urine, 20%; faeces, 26%). There was no clear difference between male
and female mice. In the rat, the total elimination was 51-56% after 9
days, mainly via the biliary/faecal route. The monkeys had the highest
elimination rate, a total of 68.6% (urine, 54%; faeces, 14.6%), within
10 days (Milling et al., 1979).
Male and female Wistar rats were administered a daily dose of
14C-2,5,4'-trichlorobiphenyl for 14 days. The animals were killed 5
days after receiving the last dose. The compound was rapidly
eliminated primarily with the faeces. Most of the trichlorobiphenyl
was metabolized (78.5%) and the major metabolites excreted were
identified as hydroxy-, dichloro-, and conjugated derivatives (Lay et
al., 1979).
The elimination of tetrachlorobiphenyl isomers in mice fed diets
containing a single isomer, at 10 mg/kg diet for 20 days, was studied
by Mizutani et al. (1977). Biological half-lives for the isomers
2,3,2',3'-tetrachloro-, 2,4,2',4'-tetrachloro-, 2,5,2',5'-
tetrachloro-, 3,4,3',4'-tetrachloro-, and 3,5,3',5'-tetrachloro-
biphenyl, were 0.9, 9.2, 3.4, 0.9, and 2.1 days, respectively.
Gage & Holm (1976) studied the influence of molecular structure on the
excretion of 14 PCB congeners in mice. They found that the
4,4'-dichloro-; 3,3',4',6'-tetrachloro-; 2,3,2',4',6'-pentachloro-;
and 2,3,4,2',4',5'-hexachloro-isomers were eliminated most rapidly.
These compounds had at least one pair of unsubstituted ortho-meta,
vicinal carbon atoms, a configuration thought to be important for
rapid metabolism and excretion. The most slowly eliminated compounds
were 2,4,5,2',4',5'- and 2,3,4,2',4',5'-hexachlorobiphenyl.
2,4,5,2',4',5'-Hexachlorobiphenyl was the PCB congener found in the
highest concentration in human adipose tissue, while
2,4,6,2',4',6'-hexachlorobiphenyl was not detected (Jensen &
Sundström, 1974a). As both of these compounds are found in commercial
PCB mixtures and in the environment, the presence of the
2,4,5,2',4',5'-hexachlorobiphenyl in adipose tissue appears to be
related to resistance to metabolism (US EPA, 1987). That this congener
is not, or is only minimally, metabolized is also indicated by the
finding that the blood concentration of this congener decreased only
10% over 300-500 days (Chen et al., 1982) and by the results of
in vitro metabolism studies with human liver microsomes (Schnellman
et al., 1984a,b).
Felt et al. (1977) examined the elimination of 14C-2,5,4'-trichloro-
biphenyl in rhesus monkeys. The monkeys were fed 550 mg of the
compound in fruit, daily, for 84 days. On the basis of total excretion
and recovered radioactivity, the half-life was found to be 4.5-4.8
days.
Male and female Sprague-Dawley rats were administered 14C-labelled
2,4,6,2',4'-pentachlorobiphenyl by gavage and the urine and faeces
were collected. After 8 days, the animals were killed. The elimination
of the PCBs followed a bi-exponential rate expression with a-phase
half-lives of 0.90 and 0.95 days and b-phase half-lives of 4.2 and 3.8
days, for males and females, respectively (Felt et al., 1979).
6.4.2 Following parenteral dosing
The results of injection studies indicate that PCBs can be excreted
unmetabolized into the gastrointestinal tract. Yoshimura & Yamamoto
(1975) recovered unchanged tetrachlorobiphenyl from the duodenal
contents of rats injected intravenously with tetrachlorobiphenyl.
Daily excretion for 4 days ranged from 0.5 to 0.8% of the total
dose/day. Goto et al. (1974) found that 4.7-23.2% of injected PCBs
were excreted unchanged into the gastrointestinal tract by day 10
after dosing, with the excretion of a penta-isomer greater than the
excretion of di-, tri-, or tetra-isomers.
Adult male Sprague-Dawley rats received doses of 4 symmetrical
hexachlorobiphenyl 14C-isomers, i.e., 2,3,5,2',3',5'-hexachloro-,
2,3,6,2',3',6'-hexachloro-, 2,4,5,2',4',5'-hexachloro-, and
2,4,6,2',4',6'-hexachlorobiphenyl, by intravenous injection. Most of
the radioactivity was eliminated in the faeces with less than 1% found
in the urine. The metabolites showed evidence of dechlorination,
chlorine shifts, and possible metabolism by direct insertion of a
hydroxyl group. There was also evidence supporting the intermediate
step of an arene-oxide as a predominant mechanism of PCB metabolism
(Kato et al., 1980).
The disposition of 2 symmetrical 14C-labelled 2,3,6,2',3',6'-
hexachloro- and 2,4,5,2',4',5'-hexachlorobiphenyl was studied in
24-month-old, male, Sprague-Dawley rats, after iv treatment. More than
50% of the 2,3,6,2',3',6'-hexachlorobiphenyl was metabolized and
excreted via the bile into the faeces within 2 days, and only 2% was
excreted in urine. More than 90% was eliminated as metabolites. In
contrast, 2,4,5,2',4',5'-hexachlorobiphenyl was redistributed from the
liver, muscle, and skin to the adipose tissue, where it accumulated
without being metabolized. Only 2% of the total dose was eliminated,
primarily in the faeces, within 21 days. In 2- to 3-month-old rats,
the general pattern of disposition of these hexachlorobiphenyls did
not change with age; however, there were differences in the rates of
elimination and in the tissue levels. There was enhanced metabolic
retention in the muscle, skin, and adipose tissue of older rats, which
suggested an age-related decrease in tissue clearance. The larger
volume of adipose tissue could not explain this observation. In
general, there were few changes in decay rates from tissues or in
biliary excretion, so age had a greater effect on the disposition of
the "persistent" 2,4,5,2',4',5'-hexachlorobiphenyl than on the
metabolizable 2,3,6,2',3',6'-hexachlorobiphenyl (Birnbaum, 1983).
Ethane exhalation was increased in male Sprague-Dawley rats, 30 days
after a single ip injection of Aroclor 1254 (500 mg/kg body weight).
Before day 30, there was no increase in ethane production. Parallel
increases in hepatic malondialdehyde levels were found. A single ip
injection of 3,4,3',4'-tetrachloro-, 2,3,4,5,4'-pentachloro, and
2,4,5,2',4',5'-hexachlorobiphenyl (300 µmol/kg) also increased (after
30 days) the production of malondialdehyde and ethane, indicators of
in vivo lipid peroxidation. These effects were not reflected in
increased diene conjugation (Dogra et al., 1988).
Sipes et al. (1980, 1982a,b) studied the distribution, metabolism, and
excretion of 14C-labelled 4,4-dichloro-, 2,4,5,2'4'5'-hexachloro-, or
2,3,6,2',3',6'-hexachlorobiphenyl in beagle dogs and cynomolgus
monkeys, after a single intravenous dose. The elimination of the test
substances from the blood of both species was shown to be biphasic.
The results for dichlorobiphenyl showed that the dog eliminated 50% of
the dose (urine, 7%; faeces, 43%) within 24 h, while the remainder was
found mainly in the adipose tissue. By 5 days, 90% had been
eliminated. The monkey eliminated less than 15% of the dose within
24 h, with less than 1% in the faeces. The remainder was found in the
adipose tissue. Within 28 days, 59% of the dose had been eliminated,
chiefly in the urine. Biliary excretion after 24 h was shown to be 33%
in the dog and only 0.4% in the monkey.
The data for 2,4,5,2',4',5'-hexachlorobiphenyl showed that the dog
eliminated 66% (urine, 3%; faeces, 63%) within 3 days; the monkey
eliminated 18% of the dose (of which 17% was in the faeces), 90 days
following administration. The remainder was found in the adipose
tissue. In the studies with 2,3,6,2',3',6'-hexachlorobiphenyl, the dog
eliminated 52% of the dose within 24 h (urine, 11%; faeces, 41%) and
70% in 3 days. The monkey eliminated 19% during the first 24 h,
divided equally between urine and faeces. By 15 days, 61% had been
eliminated, primarily in the faeces. The 24-h biliary excretion was
26% and 2.4% in the dog and the monkey, respectively.
6.4.3 Humans
Chen et al. (1982, 1985) studied the presence of PCBs in the blood of
human beings, in the Province of Taiwan, after they had consumed
rice-bran oil contaminated with Kanechlor 500 and PCDFs. Blood samples
from 17 patients were examined, with 2-3 samples taken from each
patient, 2-17 months apart. The results indicated that the
tetrachloro- and some pentachloro- isomers tended to be eliminated
more rapidly than the other pentachloro- and the hexachloro- and
heptachloro- isomers. Half-lives for the 2,4,5,2',4'- and 2,3,4,3',4'-
pentachloro- isomers in the blood were 9.8 and 8.7 months,
respectively. Two adjacent unsubstituted carbon atoms at the meta,
para positions facilitated metabolism and the subsequent elimination
from the blood. PCBs containing adjacent unsubstituted carbon atoms at
the ortho and meta positions of the biphenyl ring are eliminated
very slowly and will accumulate.
Buhler et al. (1988) administered a uniformly 13C-labelled PCB
mixture similar to Aroclor 1254 to a volunteer. A single dose of
329 µg/kg body weight was ingested; blood samples taken over a period
of 260 days were analysed for 13C- and 12C-PCBs using GC/MS and
GC/ECD. Elimination of the isomers followed a first order kinetics.
The half-lives for the isomers 2,3,4,2',4',5'-hexachlorobiphenyl,
2,4,5,2',4',5'-hexachlorobiphenyl, and 2,3,4,5,2',4',5'-hepta-
chlorobiphenyl were 321, 338, and 124 days, respectively.
6.4.4 Elimination via milk (animals)
Vodicnik (1986) studied the disposition of 14C-2,4,2',4'-
tetrachlorobiphenyl (150 mg/kg body weight administered
intraperitoneally) as a function of non-pregnant body weight in
virgin, late pregnant, and early post partum ICR mice and their
offspring. The highest concentrations were observed in adipose tissue
and the mammary glands, regardless of reproductive state. The
concentrations of the tetrachlorobiphenyl equivalents in the tissues
differed among the 3 groups, possibly because of the alterations in
lipid deposition/mobilization associated with pregnancy and lactation.
Approximately 20% of 14C-activity was eliminated from the carcass of
virgin mice, 4 days after administration, but no decrease was seen in
late-pregnant animals. Minimal transplacental transfer of
14C-activity occurred (approximately 1%), but the tetrachlorobiphenyl
was rapidly eliminated in breast milk to nursing offspring. Ninety per
cent of the total-carcass 14C-activity was eliminated from lactating
mice over a 4-day period, approximately 75% of which could be
accounted for in neonatal carcasses.
Saschenbrecker et al. (1972) found that, after oral administration of
doses of Aroclor 1254 of 10 or 100 mg/kg to cows, 6.27 and
74.5 mg/litre, respectively, appeared in the milk after 24 h. These
levels were reduced to less than one-half within 3 days, but traces
still remained at 50 days. Cows receiving 200 mg/day of Aroclor 1254
reached a steady state concentration of 61 mg/kg in the milk fat and
42 mg/kg in the body fat, after 10 days (Fries et al., 1973).
The "carry-over factor" from animal feed into the cow's milk showed
that the lower (tri-, tetra-, and penta-) chlorinated biphenyls have a
lower carry-over factor than the higher (hexa- and hepta-) chlorinated
biphenyls. Thus, it is the latter that are particularly concentrated
in cow's milk fat. From studies in the Federal Republic of Germany, it
was found that the major congeners in cow's milk were numbers 138,
153, and 180 (DFG, 1988).
6.4.4.1 Elimination via breast milk
The composition of common commercial PCB mixtures clearly differs from
the composition of the PCB contents of human fat or human breast milk,
because of the preferential elimination of certain PCB congeners
containing 3 or 4 ortho substituents and the retention of PCBs with
1 or 2 ortho substituents (Kuroki & Masuda, 1977; Watanabe et al.,
1979; Yakushiji et al., 1979).
The major PCB components (and average relative concentrations) that
have been identified in breast milk in the Osaka area in Japan
include: 2,4,4'-trichlorobiphenyl (8.4%); 2,5,2',5'-tetrachloro-
biphenyl (2.0%); 2,4,5,4'-tetrachlorobiphenyl (19%); 2,4,5,2',5'-
pentachlorobiphenyl (2.8%); 2,4,5,3',4'-pentachlorobiphenyl (11.8%);
2,4,5,2',4',5'-hexachlorobiphenyl (15.5%); 2,3,4,2',4',5'-
hexachlorobiphenyl (15.8%); 2,3,4,3',4',5'-hexachlorobiphenyl (2.3%);
2,3,4,6,2',4',5'-heptachlorobiphenyl (1.6%); 2,3,5,6,2',4',5'-
heptachlorobiphenyl (3.2%). These PCB-congeners constituted at least
95% of the PCBs in the breast milk of the women examined in Osaka.
In recent studies, the contents of PCBs in human milk and maternal
blood were compared for US citizens (Bush et al., 1984, 1985). Eight
individual PCB congeners comprised 52% of the total PCB residues in
the milk and 48.5% in the blood. The mean concentrations for total
PCBs were 26.5 µg/kg for whole milk and 3.5 µg/kg for blood. The
percentages of the different congeners are given in Table 25.
6.5 Metabolic transformation
6.5.1 PCBs
The metabolism of PCBs has been investigated in numerous studies on
animals and reviewed by Drill et al. (1981) and the US EPA (1987). The
PCBs were usually administered by the oral or parenteral route.
Phenolic products are the major PCB metabolites, though
sulfur-containing metabolites, trans-dihydrodiols, polyhydroxylated
PCBs, and methyl ether derivatives have also been identified. Although
the effects of the chlorine substitution pattern on sites of oxidation
have not been studied systematically, US EPA (1987) suggested the
following:
* hydroxylation is favoured at the para position in the least
chlorinated phenyl ring, unless this site is sterically hindered
(i.e., 3,5-dichloro-substitution);
* in the lower chlorinated biphenyls the para position of both
biphenyl rings and carbon atoms that are para to the chloro
substituent are all readily hydroxylated (Sparling et al., 1980);
* the availability of 2 vicinal unsubstituted carbon atoms
(particularly C5 and C4 in the biphenyl nucleus) also facilitates
the oxidative metabolism of the PCB substrate, but is not a
necessary requirement for metabolism;
Table 25. Concentrations of most abundant PCB congeners present in whole breast milk and maternal blooda
Congener Milk Maternal blood Ratio
(40 samples) (101 samples) milk/blood
µg/litre % of µg/litre % of
total PCBs total PCBs
2,4,5,2',4',5'-hexachlorobiphenyl 3.2 12 0.31 8.8 10
2,3,5,6,2',3',6-heptachlorobiphenyl 2.5 9.4 0.27 8.0 9.2
2,4,5,2',3',4'-hexachlorobiphenyl 2.1 7.8 0.58 17 3.5
2,5,3'4'-tetrachlorobiphenyl 1.7 6.6 0.01 - 500
2,3,4,5,2'4'5'-heptachlorobiphenyl 1.2 4.5 0.03 3.7 9.4
2,3,4,5,3',4'-hexachlorobiphenyl 1.0 4.0 0.01 - 125
2,4,5,2',4'-pentachlorobiphenyl 1.1 4.0 0.12 3.4 8.9
2,3,4,3',4'-pentachlorobiphenyl 0.97 3.7 0.25 7.6 3.8
Total PCBs 26.5 - 3.5 - 7.5
a Modified from: Bush et al. (1985).
* as the rate of chlorination increases on both phenyl rings, the
rate of metabolism decreases;
* the metabolism of specific PCB isomers by different species can
result in considerable variations in metabolic pattern.
Kannan et al. (1989) studied the possible involvement of frontier
(pi) electrons in the metabolism of polychlorinated biphenyls. The
electron density, at each carbon atom, of the highest occupied pi
orbital of 13 PCB molecules was calculated and the result was compared
with their in vitro and/or in vivo metabolism. It was found that:
* the carbon position at which the frontier electron density was the
highest was most readily hydroxylated or sulfonated;
* if the carbon with the highest frontier (pi) electrons was
occupied by chlorine, either a replacement occurred or the carbon
with the next highest electron density was activated for
metabolism;
* because of steric hindrance, "ortho" carbons were least
preferred for such reactions, in spite of possessing favourable
electron density;
* this was applicable to both phenobarbital (PB)-type and
3-methylcholanthrene (3-MC)-type PCB inducers.
The authors suggested that frontier (pi) electron density could be
an easy guide for understanding the metabolic products of persistent
and toxic environmental pollutants in vitro and in vivo, and for
understanding their environmental fate.
There appears to be little metabolism of PCBs with 6 or more chlorine
substituents (Matthews & Anderson, 1975b). When between 2 and 5
chlorine substituent PCBs are metabolized, the metabolic products are
primarily hydroxylated compounds, frequently found as glucuronide
conjugates (hydroxymethoxy derivatives) and partially dechlorinated
metabolites. In some cases, smaller amounts of dihydrohydroxy
compounds and related substances are also found.
The parent compound is also eliminated in various quantities in
faeces, hair, and maternal milk, but very little unmetabolized
compound is excreted in the urine. This pattern is not unusual for
lipophilic xenobiotics.
PCB metabolism has been examined in primates (monkeys) by Greb et al.
(1975), Hsu et al. (1975a,b), and Allen & Norback (1976); in ungulates
(cows, pigs, and goats) by Platanow & Chen (1973), Safe et al. (1975),
and Gardner et al. (1976); in rats by Grant et al. (1971a), Hutzinger
et al. (1972), Yoshimura et al. (1973), Goto et al. (1973, 1974,
1975), Safe et al. (1974), Matthews & Anderson (1975b), van Miller et
al. (1975), Sundström & Jansson (1975), Sundström et al. (1976a), Lay
et al. (1975, 1979), Chen et al. (1976), Kamal et al. (1976), and
Norback et al. (1976); in mice by Berlin et al. (1973), Yamamoto &
Yoshimura (1973), and Sundström & Jansson (1975); in rabbits by Grant
et al. (1971b), Hutzinger et al. (1974), Sundström & Wachmeister
(1975), and Sundström et al. (1976b); in pigeons, and quails by Koeman
et al. (1969), Hutzinger et al. (1972), Bailey & Bunyan (1972), and
Sundström & Jansson (1975); and in trout by Hutzinger et al. (1972).
The different metabolic products formed from pure isomers in these
various species have been catalogued in an NAS report (1979) and in a
review by Sundström et al. (1976a). Neither of these reports is
complete, but, together, they cover most of the studies up to 1979.
In the rat, monochloro-, dichloro-, trichloro-, tetrachloro-,
pentachloro-, and at least one hexachlorobiphenyl, yielded at least
one hydroxylated metabolite. Some isomers produced as many as 5
different hydroxylated metabolites including both mono- and dihydroxy-
derivatives. Most of the hexachloro-, octachloro-, and
decachlorobiphenyls did not yield detectable levels of hydroxylated
products.
Similar hydroxylated derivatives were also produced in other species,
but the ability to metabolize PCBs is not absolutely uniform in all
species. In the rabbit, dichloro-, tetrachloro-, and
hexachlorobiphenyls were metabolized, while further down the
phylogenetic scale, the pigeon only metabolized monochloro- and
dichlorobiphenyls and the trout failed to metabolize any of the
chlorinated biphenyls tested. Table 26 shows the PCBs tested in
different species and indicates whether or not the organism was able
to metabolize the compound. Although different species may metabolize
a given isomer, the metabolic products are not necessarily identical.
An example of this is found in the simple 4,4'-dichlorobiphenyl which
is metabolized by the rat, rabbit, and goat, but does not give
identical products in these species; all 3 species produce
4,4'-dichloro-3-hydroxybiphenyl as a metabolite, but, in addition, the
rat produces 4,4'-dichloro-, 2,3-dihydroxybiphenyl, and the goat
produces 3,4'-dichloro-4-hydroxybiphenyl as a metabolite.
However, a product such as the 3,4'-dichloro-4-hydroxybiphenyl found
in the goat involves a chlorine shift, which may be indicative of a
more toxic intermediate.
Many different pathways of metabolism have been described as
summarized in Fig. 5 (Safe, 1984; WHO/EURO, 1987).
These pathways include hydroxylation, and conjugation with thiols and
other water-soluble derivatives. The most important pathway seems to
be through hydroxylation and subsequent conjugation. Rats and mice
that were exposed to dichloro-, tetrachloro-, or pentachlorobiphenyls
by intraperitoneal injection or diet, eliminated metabolites as
glutathione conjugates and other sulfur-containing compounds (Kurachi,
1983; Kurachi & Mio, 1983). Mammalian metabolism of many individual
PCBs may proceed via oxide intermediates, which have not been
isolated, but are presumed to be precursors of some of the major
metabolites identified. One type of metabolite is the methylsulfone
PCB metabolite that has been identified in environmental samples by
Jansson et al. (1975) and WHO/EURO (1987), and in human milk by
Yoshida & Nakamura (1979).
The formation of xenobiotic thioether derivatives, including
glutathione, cysteinylglycine, cysteine, and N-acetylcysteine
(mercapturic acid) conjugates, is generally considered a pathway for
the detoxification of reactive intermediates. Mio & Sumino (1985)
detected methylsulfonyl metabolites, by using GC/MS/COM, from the
adipose tissues of mice treated with Kanechlor 300, 400, 500, or
2,5,2',5'-tetrachlorobiphenyl. Metabolites were detected in the faeces
of mice treated with 2,5,2',5'-tetrachlorobiphenyl, e.g., 6
sulfur-containing and 5 non-sulfur-containing metabolites. The
elimination rates for one week were 1.7% and 43%, respectively. The
methylsulfonyl metabolites accumulated in the liver, adipose tissue,
and lungs. Mio & Sumino (1985) proposed the methylsulfonyl metabolic
pathway of 2,5,2',5'-tetrachlorobiphenyl.
Klasson-Wehler et al. (1987) administered a single dose of 2,3,6,4'-
tetrachlorobiphenyl to 3 groups of 5 female C57B1 mice at 0, 10, or
100 mg/kg body weight. 35S-cysteine was administered by ip injections
4 times at 12-h intervals. The animals were sacrificed and the organs
analysed on day 12. Methyl [35S]sulfonyl-tetrachlorobiphenyl was
found in the lungs, kidneys, and fat of the treated mice, as well as
minor amounts of tetrachlorobiphenyl and traces of
methylthiotetrachlorobiphenyl.
The formation of serial methylsulfonyl metabolites can be summarized
as follows: the glutathione conjugate is converted, by cleavage, to a
cysteine or thiol conjugate and translocated into the liver. The thiol
conjugate from the cysteine moiety is transmethylated by
thiol- S-methyltransferase and is oxygenated by cytochromes P-450 and
P-448 oxidase or is glucuronidated by UDP-glucuronyl-transferase in
the liver, resulting in methylsulfonyl derivatives.
Table 26. Metabolism of various PCBs in different organisms
Compound Species
Chlorobiphenyl Trout Pigeon Mouse Rat Rabbit Monkey
4-mono- - + + +
4,4'-di- - + + +
2,2' +
2,4' + +
2,5,2'-tri +
2,4,2',4'-tetra- +
2,5,2',5' - - + + +
3,4,3',4' -
2,3,4,5,6,-penta- +
2,4,5,2',5' + +
2,4,6,2',4' +
2,4,6,2',6' +
2,4,6,3',5' +
2,3,4,6,4' +
2,3,4,3',4' -
2,4,5,2',4',5',-hexa- - - (±)a +
2,4,6,2',4',6' (±)a
2,3,5,6,2',3',5',6'-octa- -
2,3,4,5,6,2',3',4',5',6'-deca -
a These compounds were reported by Sundström et al. (1976a) as failing to produce
hydroxylated derivatives, but were positive in an IARC report referred to in an
NAS report (NAS, 1979).
+ = Compound is metabolized; - = Compound not metabolized: (blank) not tested.
Table 20. Concentrations of PCBs in the body fat of the general population
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
North America
USA (18 states) - 637 < 1 (68.9%)e Yobs (1972)
< 1-2 (25.9%)e Price & Welch (1972)
> 2 (5.2%)f
Northeast Louisiana 1980 8 1.04 (0.38-2.33) Holt et al. (1986)
1984 10 1.23 (0.65-1.44)
Texas 1969-1972 88 (15 positive) 1.7 (0.6-9.9) Burns (1974)
New York - 101 (women) 3.4 ± 1.1 Bush et al. (1984)
(urban and rural
vicinity)
Canada - 99 0.94 (0.04-6.8)a Mes et al. (1982)
Ontario 1976 and 570 2.1-2.2 Frank et al. (1988)
1984
Table 20. (cont'd).
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
Asia
Japan (Kochi area) - - 2.86 (maximum 7.5) Nishimoto et al. (1972a,b)
Japan 1971-1982 - 0.5-6.0a Katsunuma et al. (1985)
Tokyo 1974 30 1.04 (0.38-2.5) Fukano & Doguchi (1977)
Japan - 241 0.30-1.48 Curley et al. (1973b)
New Zealand - 51 0.82 Solly & Shanks (1974)
Africa
South Africa 1982 63 0.15-5.18 van Dijk et al. (1987)
Europe
Austria (Vienna area) - 32 0.3-7.3 Pesendorfer et al. (1973)
Finland - 105 0.2 Mussalo-Rauhamaa et al.
(1984)
Germany, Federal - 20 5.7 Acker & Schulte (1970)
Republic of - 282 8.3 Acker & Schulte (1974)
1982-1983 50b 0.5-1.5 Niessen et al. (1984)
Italy (Siena) 1983-1984 26 1.75c (dry weight) Focardi et al. (1986)
Table 20. (cont'd).
Country Year Number of samples Mean concentration in mg/kg Reference
on fat basis (range)
Netherlands 1973-1983 24-78 per year 1.6-2.5d Greve & van Harten
(1983a);
Greve & Wegman (1983,
1984)
Norway (Oslo) - 40 1.6 Bjerk (1972)
Spain 1985-1987 14 1.68 Camps et al. (1989)
United Kingdom - 201 < 1.0 Abbott et al. (1972)
1976-1977 236 0.7 (nd-10) HMSO (1986)
1982-1983 187 0.9 (0.1-6.9)
a Wet weight.
b 34 infants, 14 children, and 2 older children.
c About 60% included only five congeners: Nos. 118, 138, 153, 170, 180.
d Median.
e Percentage of samples.
5.5.1.1 PCBs in the fetus
PCBs are also present in serum and all organs of the body in
proportion to their fat content. PCBs pass more, or less (depending on
structure and chlorination), through the placenta into the fetus.
Since the fetus has little adipose tissue until 7 months of age, PCB
concentrations may be higher in vital organs, such as the adrenal
gland, but available data suggest somewhat lower levels in the brain
(Masuda et al., 1978a; Kodama & Ota, 1980).
Masuda et al. (1978a) found PCB levels of 270-960 µg/kg fat in adipose
tissue samples of fetuses beyond 7 months of gestation. Levels in the
adipose tissue of adult females from the same geographical area ranged
from 270 to 1360 µg/kg fat. The mean concentrations were 470 µg/kg for
fetuses and 780 µg/kg for adult females. However, since the ranges
showed an overlap and the number of samples was small, it is not clear
whether this represents a true difference.
5.5.1.2 Congeners in adipose tissue
Wegman & Berkhoff (1986) investigated the presence of the different
congeners in 24 human fat samples, collected in 1984. The following
congeners were present at the highest levels: 2,4,4'-trichloro-,
2,4,5,2',5'-pentachloro-, 2,4,5,3',4'-pentachloro-, 2,3,4,2',3',4'-
hexachloro, 2,3,4,2',4',5'-hexachloro-, 2,4,5,2',4',5'-hexachloro-,
2,3,4,5,2',4',5'-heptachloro, 2,3,4,5,2',3',4',5'-octachloro, and
2,3,5,6,2',3',5',6'-octachlorobiphenyl.
Focardi & Romei (1987) analysed 30 samples of adipose tissue,
obtained from patients in Siena, Italy, in 1986, for the presence of
19 PCB congeners. The results indicate that the mean PCB (as sum of
the congeners) concentration was 1063 µg/kg dry weight (range
391-1918 mg/kg). The major constituents of the PCBs (about 60%) were
the isomers 99, 138, 153, 170, and 180.
Human adipose tissue was analysed for 3 non- ortho chlorine
substituted coplanar congeners: 3,4,3',4'-tetrachloro-, 3,4,5,3',4'-
pentachloro- and 3,4,5,3',4',5'-hexachlorobiphenyl (Kannan et al.,
1988). Twelve samples, from 7 male and 5 female persons were obtained
from hospitals. The average total PCB concentrations were 1.22 and
1.02 mg/kg (wet weight basis), respectively. The concentrations of the
3 congeners were 94-860, 120-730, and 36-200 ng/kg, on a wet weight
basis, respectively.
5.5.2 Blood of the general population
Finklea et al. (1972) studied human plasma of different races of the
population (723 volunteers with ages ranging up to 60 years) of urban
and rural areas of South Carolina. The average concentration was
5 µg/litre (range 0-29 µg/litre). No age effect was found, but ethnic
differences and ethnic residence interactions were significant. Kreiss
(1985) found mean serum concentrations in the non-occupationally
exposed population in the USA, of between 4 and 8 µg/litre, with 95%
of the individuals having serum PCB concentrations of less than
20 µg/litre. More data are summarized in Table 21.
Maternal blood and fetal cord blood were collected from volunteers
from an urban and rural vicinity in upstate New York. Whole blood
samples were taken from 101 women (26 ± 4 years) entering maternity
facilities. Maternal blood contained 3.4 ± 1.1 µg PCBs/kg and fetal
cord blood contained 2.4 ± 1.0 µg/kg whole blood. The PCB congeners
making up these totals were surprisingly few; 38% of the total residue
in the maternal blood and 21% of the fetal cord blood comprised only 4
components, 2,4,4'-trichlorobiphenyl, 2,4,5,2',4',5'-hexachloro-,
2,3,4,2',4',5'-hexachloro-, and 2,3,5,6,2',3',6'-heptachlorobiphenyl.
The congener 2,5,2',5'-tetrachlorobiphenyl crossed the placenta
preferentially (Bush et al., 1984).
The concentrations of PCBs were determined in blood samples from 120
women hospitalized for miscarriages and 120 full-term pregnancy
controls. The average PCB level was higher in women with miscarriages
than in control women (8.65 µg/litre and 6.89 µg/litre, respectively,
as Fenclor 54 and 14.81 and 14.90 µg/litre, respectively, as
decachlorobiphenyl). The reproductive history of each woman was
assessed together with confounding variables and with environmental
exposure and food intake. Food consumption did not indicate diet as
the main source of PCB intake (Leoni et al., 1989).
A cross section of the population of Michigan was studied following an
accidental exposure in 1978. Five years after the accident, PCB and
PBB residues were measured in adipose tissue and serum. Serum levels
of PCB were measured in 1681 adults and 1462 children. Children (430)
were found to have uniform levels throughout the state (mean
concentration 4 ± 2 µg/litre). In adults, the serum PCB levels were
higher in the area with highest PBB levels. The mean serum PCB level
was 21 µg/litre, compared with control levels for the rest of the
state of 9 µg/litre. No sex difference was found (Wolff et al.,
1982a).
Table 21. Concentrations of PCBs in whole blood of the general population
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Canada
Ontario area 1975-1976 118 18 Frank et al. (1988)
(patients suspected 1980-1981
of being exposed 1984
dermally)
Japan
- - 3.2 Doguchi & Fukano (1975)
- 28 (women) 2.6 Kuwabara et al. (1978)
(Osaka area) 1976 16 (women) 2.8 (1.7-4.6) Kuwabara et al. (1979)
1972-1977 - 3-4 Yakushiji et al. (1977)
farmers 1978-1983 - trace-21.4b Katsunuma et al. (1985)
Tokyo 1973 27 3.19 (2.2-5.1) Fukano & Doguchi (1977)
1975 10 2.59 (1.8-3.8)
Table 21. (cont'd).
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Finland - 3.1-12 Karppanen & Kolho (1973)
Netherlands - 34 (women) 4.5 (nd-11.6) Blok et al. (1984)
31 (men) 4.8 (1.0-17.1)
1978 48-127 3.1e Greve & Wegman (1983,
1980 samples/year 3.5 1984)
1981 4.4
1982 4.4
North America
South Carolina 1968 723 5 (4.2-5.5)a Finklea et al. (1972)
(urban and rural
area)
Michigan 1973 1100 56c Kreiss (1985)
(areas of Lake 1979-1981 17.2-23.6c
Michigan)
Lake Michigan 1985 196 5.5 ± 3.7 Schwartz et al. (1983)
(high fish
consumption)
Table 21. (cont'd).
Country Year Number of samples Mean concentration in Reference
µg/litre (range)
Yugoslavia 1984-1986 10f 155 (35-480)d Jan & Tratnik (1988a)
(residents around 19g 11 (6-18)
River Krupa; 4h 5 (2-7)
contamination by a
plant using PCBs)
a Plasma.
b Serum.
c Geometric mean.
d Arithmetic mean.
e Median concentration.
f Living close to plant.
g Living 1-3 km from plant.
h Non-exposed other areas.
Specific PCB isomer levels in the blood of 30 children, ages 2-5
years, residing in an area of PCB-contaminated soil in Canada, were
compared with those of 25 children in a non-contaminated area. The sum
of individual PCB isomer levels in the exposed and non-exposed group
were not significantly different, e.g., 0.54 µg/litre (range
0.22-0.99 µg/litre) and 0.88 µg/litre (range 0.28-2.30 µg/litre). The
major component in both groups was 2,4,5,2',4',5'-hexa-chlorobiphenyl
(Mes, 1987).
High levels of PCBs were found in the blood (up to 100 µg/litre) in
patients with severe weight loss (Hesselberg & Scherr, 1974). This was
attributed to the release of PCBs from the mobilization of fat.
Greve & van Harten (1983b) studied the relationship between the levels
of PCBs in the adipose tissue and in the blood of the same persons. A
total of 48 persons were involved in this study. A concentration
factor (concentration in adipose tissue divided by concentration in
blood) of 660 was found.
5.5.3 Human milk
Human milk is the major source of exposure for breast-fed infants. The
amount of human milk secreted varies widely. The composition of the
milk is related to the amount secreted, the stage of lactation, the
timing of withdrawal (early or late in feeding) and to individual
variations among lactating women. The individual variations depend on
maternal age, health, social class, and diet. The concentration of
PCBs depends primarily on the lipid concentration in milk. Wide
variations in published results are caused by inaccuracies inherent in
the analytical methods used for the quantification of lipids, and
whether the milk sample is collected early or late during the feeding
period. The fat content increases during emptying, and the fat content
of milk from the 2 breasts may differ. According to a recent
determination, the fat level in human milk averages 2.6-4.5%
(WHO/EURO, 1988).
Whether the differences in concentration in various countries are
merely a function of the analytical methods used and the type of
samples collected or whether true differences in body burden exist, is
not clear at present. For instance, some countries have reported
levels of PCBs in human milk fat ranging from nondetectable to
14 mg/kg, while, in other countries, the highest levels found have
been around 3 mg/kg. Because of these variations, calculating an
average dose for nursing infants is difficult. The same difficulties
exist when attempts are made to investigate trends over time
(WHO/EURO, 1988).
The results of the older studies have been obtained with a less
sophisticated method using packed column GC. With this method only a
dozen peaks can be separated. The quantitative results are reported as
"total PCB values", though different techniques of quantification and
different types of calculations were used.
In contrast with the situation with many organochlorine insecticides,
the levels of PCBs in human milk fat are higher in European countries,
Japan, and the USA than in China (Slorach & Vaz, 1983, 1985), and are
significant, particularly in the highly industrialized countries.
Results from a large number of countries have been summarized by
Jensen (1983a, 1985, 1987), Acker et al., (1984), Katsunuma et al.
(1985) (especially Japanese data; period 1972-83); and WHO/EURO,
(1987, 1988). The countries concerned are: Argentina, Austria,
Belgium, Canada, Finland, France, Federal Republic of Germany (Klein,
1983), German Democratic Republic, Israel, Japan, the Netherlands,
Norway, Poland, Romania, South Africa, Sweden, Switzerland, Turkey,
United Kingdom, USA, USSR, and Yugoslavia. The average levels of PCBs
in human milk do not appear to differ very much between the
industrialized countries and range between 0.5 and 2 mg/kg milk fat,
except in Czechoslovakia, the Federal Republic of Germany, India,
Denmark and Italy, where levels up to 3 mg/kg milk fat were found
(Jensen, 1983b; Acker et al., 1984) (Table 22).
The variation in residue levels in human milk during lactation was
investigated in 5 women in the Federal Republic of Germany. Month-mix
samples, composed of breast milk samples collected weekly, were
analysed over a lactation period of between 5 and 9 months. The ages
of the women ranged from 23 to 36 years. The PCB concentrations were
between 0.61 and 2.20 mg/kg, on a fat basis. While the concentrations
remained relatively constant, some fluctuations were seen but no trend
was observed over the lactation period investigated (Fooken & Butte,
1987).
Breast milk samples from 16 women in Canada were analysed for PCBs at
8 intervals (7, 14, 28, 42, 56, 70, 84, and 98 days) during the
lactation period. The average PCB concentrations in breast milk varied
between 22.8 and 29.7 µg/kg whole milk. No clear decrease or increase
was observed. The average milk/blood ratio for PCBs was 23 and
remained relatively constant during lactation (Mes et al., 1984).
Wolff (1983) reported the half-life of PCBs (percentage chlorine not
specified) in breast milk to be 5-8 months and found that the
concentration of PCBs in breast milk was 4-10 times that in the
maternal blood. Similar results were reported by Jacobson et al.
(1984b).
Table 22. Concentrations of PCBs in breast milk of the general population
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
North America
USA (Michigan) 1977-1978 1057 1.5 (maximum 5.1) Wickizer et al. (1981);
Wickizer & Brilliant (1981)
Canada (Quebec) - 154 0.84 (nd-4.34) Dillon et al. (1981)
Ontario 1971-1974 - 1.2 (0.1-3.0) Atkinson (1979)
1978 215 0.6 ± 0.3
Ontario 1975-1985 348 0.023 (0.016-0.033)a Frank et al. (1988)
Five regions across Canada 1982 210 0.697 Mes et al. (1986)
Regina, Saskatchewan 1979 80 0.0052 (0.001-0.019)a Qureshi & Robertson (1987)
Table 22. (cont'd).
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
Asia
Japan (Osaka) 1972-1977 - 0.030-0.040a Yakushiji et al. (1977)
1969-1976 19-52 each year 1-2 Yakushiji et al. (1979)
India (Ahmedabad) 1981-1982 50 not present Jani et al. (1988)
Hawaii (different islands) 1979-1980 54 0.80 ± 0.43 (0.13-2.2) Takei et al. (1983)
Europe
Germany, since 1970 several thousands 1.0-2.5 (98% of samples Acker et al. (1984);
Federal Republic of between 0.001-7.2) Cetinkaya et al. (1984);
Heeschen et al. (1986);
Lorenz & Neumeier (1983)
- 2709 1.77 Fooken & Butte (1987)
Netherlands 1983 278 0.72 (0.27-2.20)b Greve et al. (1985);
(11 centres country-wide) Greve & Wegman (1984)
1977-1979, 2649 2.1 Olling (1984)
1981
United Kingdom (Scotland) 1979-1980 30 0.01 (nd-0.04) HMSO (1986)
1983-1984 30 < 0.01 (nd-0.02)
Table 22. (cont'd).
Region Year Number of Mean concentration in Reference
Country samples mg/kg on fat basis (range)
Italy (Rome) 1983-1985 65 0.070 (0.007-0.176)a,c Dommarco et al. (1987)
Finland (different parts) 1984-1985 183 (165 of 0.57 (0.05-10.7) Mussalo-Rauhamaa et al.
women) (1988)
Sweden (5 regions) - 300e 1.06-1.18 (four regions) Noren (1983)
1.44 (one region)
1972 227d 1.05 Noren (1988)
1976 245 0.99
1980 340 0.78
1984-1985 102 0.60
Austria (Vienna) - 22 1.54 (0.58-3.78) Pesendorfer (1975)
Other regions 9 1.29 (0.95-1.57) Pesendorfer (1975)
a Whole milk.
b Median concentration.
c Arithmetic mean.
d Number of mothers that provided 4-7 samples each (samples were pooled).
e In each region, 300 mothers gave breast milk 3-5 days after parturition.
In a study by Kuwabara et al. (1978), the relationship was
investigated between breast-feeding and PCB residues in the blood of
children whose mothers were occupationally exposed to PCBs. The
children ingested their mother's milk for periods of < 1 to 3 years.
The age of the children at the time of the study ranged up to 13
years. The data provide evidence that PCBs are retained in the
children's body for many years and that longer intake of mother's milk
tends to increase PCB levels in the blood of the children. The PCB
levels in the blood of the 20 occupationally-exposed women and their
39 children ranged from 8.3 to 84.5 and 0.8 to 93.2 µg/litre,
respectively.
The results suggest that the PCB levels in the blood of children are
much more influenced by the transportation of PCBs through the
mother's milk than through the placenta. Furthermore, it was found
that the gas chromatographic patterns of the blood PCBs of the
children, breast fed for a long time, were different from those of
their mothers. Blood from 16 non-occupationally exposed mothers and
their children (17), showed that, as the length of the breast-feeding
period increased, there was an increase in the PCB levels in the blood
of the children. The mean blood PCB level in mothers was 2.8 ±
0.8 µg/litre; in children, it was 3.8 ± 3.6 µg/litre. In this study,
no clear change in blood PCBs patterns between mothers and children
was observed (Kuwabara et al., 1979).
Samples of maternal blood, milk, and umbilical cord blood were
collected from 43 mothers giving birth to their first or second child;
all the mothers had lived in Oslo during the previous 2 years. Blood
samples were collected immediately after delivery, either by Caesarean
section (16 Norwegians) or normally (20 Norwegians and 7 immigrants).
Subcutaneous fat samples were obtained during the operation. Samples
of colostrum and milk were obtained 3 and 5 days postpartum. PCBs were
found in 135 of the total 168 samples. In the Norwegian women and
infants, PCBs were the major contaminants, whereas only traces of PCBs
were found in the samples of immigrants. The average concentrations in
the maternal serum, cord serum, colostrum, and breast milk of
Norwegian women (Caesarean and normally delivered taken together)
were: 10, 3-5, 18-21, 20-23 µg/kg wet weight (Skaare et al., 1988).
5.5.3.1 Major PCB congeners in human milk
Commercial PCB preparations consist of complex mixtures of
environmentally stable compounds with a wide range of chlorine
contents. PCBs are transferred to breast-fed infants with the fat of
the mother's milk. Thus, infants nurtured on maternal milk are exposed
to relatively high concentrations of the higher chlorinated PCBs in
the short period preceding the full functioning of certain organs,
e.g., the liver (Jensen, 1983b; Slorach & Vaz, 1983; Gezondheidsraad,
1985).
Three major congeners were present in breast milk, e.g., PCB congener
numbers 138, 153, and 180 (DFG, 1988).
Slorach & Vaz (1983) reported that the GC patterns of PCBs in breast
milk samples from different countries were similar. The peaks denoted
146, 174, and 180 were dominant in the gas chromatograms. The total
levels of PCBs and the concentrations of certain congeners in Swedish
human milk, sampled in 1972-89, were studied by Noren et al. (1990).
Minor changes in the distribution of the congeners were found over the
period of study. The most abundant of the non- ortho coplanar PCBs in
Swedish human milk was 3,4,5,3',4'-pentachlorobiphenyl (126), with
levels decreasing from 0.35 µg/kg milk fat (1972) to about 0.10 µg/kg
(1989).
Safe et al. (1985a) analysed a sample of breast milk using the
congener-specific PCB method and found the following major components:
2,4,4'-trichloro-; 2,4,5,4'-tetrachloro-; 2,4,5,2',4'-pentachloro-;
2,4,5,3',4'-pentachloro-; 2,3,4,5,2',5'-hexachloro-;2,4,5,2',4',5'-
hexachloro; 2,3,4,5,2',3',4'-heptachloro-; and 2,3,4,5,2',4',5'-
heptachlorobiphenyls.
The major PCB congeners in the breast milk of Japanese women from the
general population were: 2,4,4'-trichloro-; 2,4,3',4'-tetrachloro-;
2,4,5,3',4'-pentachloro-; 2,3,4,2',3',4'-hexachloro-;2,3,4,5,2',4'-
hexachloro-; and 2,3,4,5,2',4',5'-heptachlorobiphenyls. The congeners
were present in 5% or more samples; a few other congeners were present
in only 1-3% (Gyorkos et al., 1985; Jensen, 1983b).
Sixty-eight breast milk samples collected in the Netherlands were used
to determine the congener distribution. The indicator congeners,
present in the highest concentrations, were: 2,4,4'-trichloro-,
2,4,5,2',5'-pentachloro-, 2,4,5,3',4'-pentachloro-,
2,3,4,2',4',5'-hexachloro-, 2,4,5,2',4',5'-hexachloro-,
2,3,4,5,2',4',5'-heptachlorobiphenyl (Wegman & Berkhoff, 1986).
Schecter et al. (1989a) analysed a total of 17 samples of human milk
from Thailand and Vietnam, for the presence of PCB congeners. The main
congeners that were present were 138, 153, and 180 (each in the range
of 8-31 µg/litre). The other congeners, normally present, were all
below the detection limit of 2 µg/litre.
In a study on pooled human milk samples from a 1982 nation-wide survey
in Canada, Mes & Marchand (1987) compared the relative amounts of 29
selected PCB isomers with amounts in milk samples of unexposed Rhesus
monkeys. In the pooled milk sample, 397 µg PCBs/litre, on a fat basis,
were found and the PCB isomer numbers 74, 99, 118, 138, 153, and 180
were the main contributors. Most of the predominant PCB isomers in
human milk were also observed in monkey's milk, but monkey's milk had
relatively low levels of PCB isomers numbers 74 and 99.
In another study, Davies & Mes (1987) analysed breast milk samples
from Canadian, Indian, and Inuit (Eskimo) mothers in Canada. The 18
samples were received from 5 Indian and Inuit nursing zones. The
combined total PCB isomer level (on a whole-milk basis) of the native
population was comparable with that of the national population. Even
the levels of the 5 largest PCB congeners (Nos. 74, 118, 138, 153, and
180) were comparable.
Individual congeners in the blood of Yusho- and Yu-Cheng patients are
discussed in section 5.6.
5.5.3.2 Factors that influence the intake of PCBs with milk
Present data suggest that the PCB content of human milk varies
considerably from individual to individual.
Many factors affect the level of PCBs and other organochlorine
compounds in breast milk including the fat content of the milk; time
from start of lactation; mother's age; mother's body weight; parity;
number of children previously breast-fed; origin and residence; eating
habits; season; smoking; use of household products; amount of milk;
and exposure at work (WHO/EURO, 1985, 1988).
In a given woman's milk, there are fluctuation in the PCB levels in
whole milk and in milk fat during one nursing session and during the
day (Jensen, 1983b). A decrease of PCB levels in both milk and milk
fat has been found during the lactation period. Furthermore, the PCB
concentration in human whole milk and milk fat increases with the age
of donor. Another confounding factor is that the PCB levels decrease
with increasing numbers of deliveries and lactations (Greve et al.
1985); lactation serves as a period for the biological elimination of
PCBs (Jensen, 1983b). The PCB levels in human milk are higher in
heavily populated and industrialized areas than in rural areas.
Furthermore, in general, the PCB levels in the breast milk of women
from developing countries are lower (Jensen, 1983b).
Cetinkaya et al. (1984) studied the PCB levels in human milk samples
from all over the Federal Republic of Germany. At the same time, data
were collected by means of a detailed questionnaire on residency,
workplace, smoking, drinking and eating habits, and the age of
participating individuals.
The breast milk of 45 women consuming lacto-vegetarian food was
compared with that of 41 women consuming conventional food in the
Federal Republic of Germany in the period 1979-81. The PCB
concentration was comparable, e.g., 2.2 and 2.5 mg/kg, on a fat basis,
respectively (Acker et al., 1984).
Fish consumption was positively correlated with PCB levels in maternal
serum and breast milk. PCB levels in serum increased with age, but
were unrelated to social class, parity, or body weight (Schwartz et
al., 1983).
Eight hundred and one Wisconsin anglers were surveyed for fishing and
consumption habits in 1985. The mean annual number of sport-caught
fish meals was 18 (range 7.1 to 33.3). The mean number of
non-sport-caught fish meals was 24. The median PCB serum congener sum
level for 192 anglers was 1.3 µg/litre (range, nd to 27.1 µg/litre).
Statistically significant positive Spearman correlations were observed
between sport-caught fish meals and PCB levels in serum and between kg
of fish caught and PCB levels in serum (Fiore et al., 1989).
PCBs were measured in maternal serum, cord blood, placenta, and serial
samples of breast milk and colostrum, from 868 women in North Carolina
(USA). Forty-three per cent of the women were primiparous. Breast milk
was collected at 6 weeks, 3 months, and 6 months, and, in a few cases,
up to 18 months postpartum. The median PCB concentration in breast
milk decreased during the sampling period from 1.77 to 1.02 mg/kg, on
a fat basis. The PCB concentration dropped by about 20% over 6 months
and 40% over 18 months. This implies that excretion in milk is a major
factor in lessening the mother's body burden; however, it also implies
substantial exposure of the child. Colostrum contained a median value
of 1.74 mg/kg. PCBs concentrations were higher in milk than in serum
and higher in maternal serum than in the placenta. The levels in cord
blood were almost always below the limit of quantification. Older
women and women who regularly drank alcohol had higher PCB levels in
their milk; blacks had higher levels than whites. In general, women
had higher levels in their first lactation and in the earlier samples
of a given lactation, and levels declined both with time spent
breast-feeding and with number of children nursed (Rogan et al.,
1986a).
Two hundred and forty-two newborn infants of mothers who consumed
moderate quantities of contaminated lake fish and 71 infants whose
mothers did not eat such fish were examined during the immediate post
partum period. PCB exposure was correlated with lower birth weight and
smaller head circumference, and the authors claimed that these effects
were not attributable to any of 37 potential confounding variables,
including socioeconomic status, maternal age, smoking, etc. (Fein et
al., 1984).
The mother's diet may be an important determinant of the PCB levels in
her milk. In some areas of the world, the intake of PCBs from eating
contaminated fish has been claimed to be the most important source of
PCBs in human milk. Dairy products and meat may be contaminated via
natural food or feedstuffs (WHO/EURO, 1988).
In a pilot study on the course of the PCB concentration in human milk
during 6 months of lactation, some PCB determinants were studied in 23
women and their infants. The average PCB concentration in the milk of
14 mothers during a 6-month period amounted to 0.66 ± 0.12 mg/kg, on a
fat basis. In univariate analyses, the PCB concentration on a fat
basis was strongly associated with pre- versus post-pregnancy weight
gain, age, and occupation. After multiple regression analysis, the PCB
concentration on a fat basis remained significantly associated with
changes in weight gain. The pre-pregnancy Quetelet Index of the mother
(height/weight) and the estimated PCB content of the diet (fish) were
correlated with the PCB concentration, on a milk basis (Drijver et
al., 1988).
5.5.4 Other tissues
Schecter et al. (1989b) analysed the tissues of 3 patients from the
North American continent, with no known history of chemical exposure,
for the presence of PCB isomers. The total PCB concentrations in the 9
tissues studied were different. The highest levels were found in
adipose tissue, subcutaneous fat (range 86-423 µg/kg), adrenals
(25-103 µg/kg), liver (3-149 µg/kg), bone marrow (26 µg/kg), kidneys
(2-31 µg/kg); levels in the spleen, lung, and testes were below
12 µg/kg. Congeners present in the highest concentrations were numbers
28, 74, 118, 153, 105, 138, 183, and 180.
5.6 Accidental exposures (Yusho- and Yu-Cheng)
In 1968, a large number of persons in Japan were accidentally poisoned
by the consumption of a batch of rice oil contaminated with Kanechlor
400. A similar accident happened in the Province of Taiwan in 1979,
where the affected persons had also consumed rice-bran oil
contaminated with PCBs. The 2 cases of poisoning were called Yusho and
Yu-Cheng accidents, respectively (see section 9.1.2.1).
The average PCB concentration in the plasma of Yusho children was
6 µg/litre, compared with 3.7 µg/litre in controls. Breast-fed Yusho
children had higher levels than children not breast-fed (Abe et al.,
1975).
The concentrations of PCBs in the adipose tissue, liver, and blood of
Yusho patients, about 5 years after the outbreak, were 1.9 ±
1.4 mg/kg, 0.08 ± 0.06 mg/kg, and 6.7 ± .3 µg/litre, respectively.
These values were only about twice those of controls. The mean blood
PCB level of 278 persons involved in the Yu-Cheng accident was
89.1 µg/litre (range 3-1156 µg/litre). Six months after the exposure,
the concentrations of PCBs in the blood had decreased to
12-50 µg/litre. The mean blood concentration of 165 patients,
9-18 months after the onset of poisoning, was 38 µg/litre (range
10-720 µg/litre) (see section 9.1.2.1). The blood PCB level of some
Yu-Cheng patients (99 ± 163 µg/litre), was much higher than that of
the Taiwanese population (1.2 ± 0.7 µg/litre), one year after the
outbreak of the intoxication.
Chen et al. (1985) analysed the blood of 165 Yu-Cheng patients, 9-18
months after the onset of poisoning, and found 10-720 µg PCBs/litre
with a mean value of 38 µg/litre. The blood of 10 patients, 9-27
months after poisoning, contained 0.02-0.2 µg PCDFs/litre. The
PCDF-congeners found in tissues were the same as those found by Masuda
et al. (1985).
Seven PCB congeners including: 2,4,5,3',4'-pentachloro-; 2,3,4,3',4'-
pentachloro-; 2,4,5,2',4',5'-hexachloro-; 2,3,4,2',4',5'-hexachloro-;
2,3,4,5,3',4'-hexachloro-; 2,3,4,5,2',4',5'-heptachloro-; and
2,3,4,5,2',3',4'-heptachlorobiphenyls, were identified in the blood
and tissues of Yusho, Yu-Cheng patients and controls.
Major PCDF congeners identified in the tissues and blood of Yusho and
Yu-Cheng patients were 2,3,6,8-tetrachloro-; 2,3,7,8-tetrachloro-;
1,2,4,7,8-pentachloro-; 2,3,4,7,8-pentachloro-; and 1,2,3,4,7,8-
hexachlorodibenzofurans. The 2,3,4,7,8-pentachloro-compound was
predominant. The concentrations of PCDFs in the adipose tissue and
liver of Yusho patients were 6-13 µg and 3-25 µg/kg tissue,
respectively. No PCDFs could be detected in the controls. Besides PCBs
and PCDFs, 4-methylthio-2,5,2',5'-tetrachlorobiphenyl (concentrations
ranging from 0.1 to 1.4 µg/kg tissue) and 4-methylsulfone-
2,5,2',5'-tetrachlorobiphenyl (range 0.3-2.5 µg/kg tissue) were also
found (Masuda et al., 1985).
5.7 Occupational exposure
5.7.1 Accidental exposure
Though the volatility of the PCBs is low, they are found in rather
high concentrations in the workroom air in both the long-term open use
of PCBs and in temporary or acute events where evaporation into the
air is possible. The measured air concentrations of PCBs in long-term
exposure situations, such as the manufacturing of transformers or
capacitors, varied from 30 to 1000 µg/m3, depending on the year of
measurement and the factory concerned (Silbergeld, 1983).
In discontinuous work, such as the inspection and repair of
transformers and capacitors, levels of between 0.1 and 60 µg/m3 have
been observed (Wolff, 1985). PCB concentrations in the breathing zone
of workers in transformer repair and maintenance work varied between
0.01 and 24.0 µg/m3 (Moseley et al., 1982).
In the atmosphere of an electroindustrial plant in Bela Krajina,
levels in the manufacturing room, where the autoclave was emptied,
averaged 2000 µg/m3 (range, 1400-3200 µg/m3); an average of
80 µg/m3 (range 40-120 µg/m3) was found in the working environment
in capacitor manufacture (Jan et al., 1988b).
Digernes & Astrup (1982) determined the concentrations of PCBs in the
atmosphere of the workplace of data screen operators, because skin
rashes and eczema had been reported among the workers. The PCB
concentrations in the working atmosphere (3 samples: concentrations
ranging from 0.056 to 0.081 µg/m3) were about 50-80 times higher than
the maximum level of PCBs in 3 samples collected outside the building
(0.0005-0.001 µg/m3). The indoor and outdoor samples also differed
qualitatively. The indoor samples contained only Aroclor 1242, while
outdoor samples contained a mixture of Aroclor 1242 and 1254.
Acute emergency events may cause extremely high concentrations of PCBs
in the air, particularly in cases when PCBs are burnt or heated (fire,
short circuit with electric arcing, burning in welding, etc.). Levels
of up to 10 000-16 000 µg/m3 have been measured. In the case of
extensive leaks of unheated PCBs from capacitors, concentrations of
1900 µg/m3 have been measured in workroom air (Elo et al., 1985;
WHO/EURO, 1987).
In connection with fires and electrical explosions, due to short
circuits, PCBs may be decomposed at elevated temperatures varying from
a few hundred to 2000°C. Soot may be produced in large amounts,
consisting of particles that may contain PCB concentrations up to
5000-8000 mg/kg of soot (Elo et al., 1985; O'Keefe et al., 1985;
WHO/EURO, 1987).
When evaluating PCB exposure, it is important to take into account
skin absorption from surfaces and tools, in addition to exposure via
inhalation. Surface concentrations of PCBs in capacitor factories have
varied between 4 and 60 µg/m2, and, where PCB leaks have occurred,
levels of up to 30 mg/m2 have been measured. Where PCBs have been
used long-term, contamination levels of 1-2 µg/cm2 have been found on
tools and tables.
A transformer was found to have overheated and released an oily mist
containing PCBs and their pyrolysis by-products, in a Department
building in New Mexico. The transformer contained Askarel (87% Aroclor
1260 and 13% of a mixture of tri- and tetrachlorinated benzenes). The
3-storey building was extensively contaminated via the following ways:
* mist entered 2 rooms, adjacent to the basement in which the
transformer was located;
* direct spread of mist and fumes through stairways;
* air drafts created by open windows and exhaust fans, spreading
fumes throughout the building;
* foot traffic by employees and other persons;
* the exhaust vent of the transformer room, located near the intake
vents for the building's air-conditioning system.
Air samples obtained up to 14 h after the incident showed levels of
48 µg/m in the transformer vault and 20 µg/m3 in the room above the
vault. Wipe samples of surfaces showed PCB levels ranging from 30
million µg/m2 for grossly contaminated surfaces to 4700 µg/m2 for
surfaces without visible contamination.
Five to 7 days later, air and surface samples were analysed for
2,3,7,8-tetrachlorodibenzofuran (TCDF), which was found to be present
in the air at an average level of 48 µg/m3 in most contaminated
areas. In wipe samples, the levels ranged from 5 ng/m2 to
41.224 ng/m2. 2,3,7,8-Tetrachlorodibenzo- p-dioxin (TCDD) was not
detectable in either air samples (detection limit, 0.5-5.0 pg/m3 air)
or wipe samples (detection limit 180 ng/m2) (Anon., 1985).
Very high concentrations of these toxic chemicals may be found in soot
emitted in connection with fires and explosions in capacitors.
Thus, skin contamination, and the ingestion and inhalation of soot
particles, may result in serious exposure in PCB accidents and
emergencies.
A short-term, follow-up study was performed on 55 workers in a gear
plant, whose work did not involve the use of PCBs. Exposure was to the
total residual PCB left behind by a capacitor company that had
formerly (3 years before) used the site. Air samples contained
< 10 µg/m3 and mean concentrations in wipe samples ranged from 23 to
161 µg/100 cm2. The 38 workers had a mean PCB concentration in serum
of 14.4 and the 17 office workers, 4.8 µg/litre. When the PCB
determinations were repeated in the 2 following years, no clear
decrease was observed (Christiani et al., 1986).
5.7.2 Occupational exposure during manufacture and use
Occupational exposure occurs during the manufacture of PCBs as well as
during their use by the electrical industry. It may also be widespread
among mechanics in contact with lubricating oils and hydraulic fluids,
among workers exposed to varnishes and paints, and among office
workers who have contact with pressure-sensitive duplicating paper
(carbonless copying paper), some brands of which readily transferred
PCBs to skin (Kuratsune & Masuda, 1972).
5.7.2.1 Adipose tissue
Levels of PCBs in the adipose tissue of occupationally exposed workers
have been found to vary between 26 and 50 mg/kg (range, 2.2-290 mg/kg).
There is a strong correlation between the blood PCB concentration and
PCB levels in adipose tissue, but the distribution of the various
congeners between plasma and adipose tissue is not the same, as
described above.
Emmett (1985) found the following congeners in the adipose tissue of
present and past transformer workers exposed to Aroclor 1242 and 1254:
2,4,3',4',5'-pentachloro-, 2,3,4,3',4'-pentachloro-, 2,3,4,5,2',4'-
hexachloro-, 2,3,4,6,3',4'-hexachloro-, 2,4,5,3',4',5'-hexachloro-,
2,3,4,5,2',3',4'-heptachloro-, and 2,3,4,5,6,3',4'-hepta-
chlorobiphenyl.
5.7.2.2 Blood
Karppanen & Kolho (1973) analysed the blood of 26 persons, 9
non-exposed, 6 persons handling PCBs, and 11 persons employed for 4
years in a capacitor-manufacturing plant in Finland. In the latter
case, Aroclor 1242 was used. The average concentrations in the blood
of the 3 groups were 7.1 µg/kg (3.1-12 µg/kg), 49.5 µg/kg
(36-63 µg/kg), and 440 µg/kg (70-1900 µg/kg), on a wet weight basis.
More recent results of a Finnish control group of workers indicated
serum PCB levels of 1.2 ± 0.6 µg/litre in an industrial area (Luotamo
et al., 1985; WHO/EURO, 1987). With acute exposure to high
concentrations of PCBs in air (8000-16 000 µg/m3), for a short
period, blood PCB concentrations rose to levels of 30 µg/litre; a
return to the normal level of 3 µg/litre was achieved, 4 weeks after
termination of exposure (Elo et al., 1985; WHO/EURO, 1987).
Similar plasma values were found in workers from Japanese capacitor
factories, but, here, skin lesions were noted (Hasegawa et al.,
1972a). In this same study, it was reported that air levels of PCBs of
10-50 µg/m3 were measured in a factory where KC-300 was used in the
manufacture of electric condensers. PCB levels in the serum of workers
ranged from 100 to 650 µg/litre. One month after the use of PCBs had
been suspended, serum levels remained unchanged (90-740 µg/litre).
However, in another factory making electric condensers, serum levels
decreased from an average of 800 to 300 µg/litre, within 3 months of
the use of PCBs being discontinued (Kitamura et al., 1973). According
to Hara et al. (1974), the half-time of PCBs in the blood of workers,
engaged in the manufacture of electric condensers for less than 5
years, was several months, while that of workers employed for more
than 10 years was 2-3 years.
Kuwabara et al. (1978) reported mean PCB levels of 36.8 µg/litre
(range 8.3-84.5 µg/litre) blood in 20 PCB-workers, 39 children had
blood levels of 14.3 µg/litre (0.8-93.2 µg/litre), and 12 Yusho
patients, 4.2 µg/litre (1.8-8.6 µg/litre).
Fact-finding surveys of 63 workers, who were occupationally exposed to
PCBs (Kanechlor 500) in the production of silk thread or of paint,
were carried out in Japan in 1974-75; some of them and their families
were also surveyed again in 1975-82. Nineteen per cent of them showed
PCB levels higher than 50 µg/litre plasma. These persons did not show
the typical clinical findings of Yusho patients. During 7 years, no
clear decline was observed (Takamatsu et al., 1984).
There is clear evidence that relatively high PCB levels persist in the
blood of workers whose "external" exposure ceased several months or
years previously. The blood PCB concentrations in capacitor
manufacturing workers, who had been exposed for 1-24 years, varied
between 24.4 and 192 µg/litre; this was higher than levels in the
blood of a reference population (0.5-33 µg/litre) (Maroni et al.,
1981a).
In Japan, Yakushiji et al. (1984a) studied the rate of decrease and
the half-life of PCBs in the blood of children (aged 1-13 years) and
their mothers, who were occupationally exposed to PCBs, over a 5-year
period (1975-79). The mean concentration of 121 blood samples from
50 children was 17.4 ± 22.9 µg/litre and that in 65 samples from 29
mothers was 32.3 ± 20.6 µg/litre. The concentrations of PCBs in the
blood of the children varied over a wide range, because of differences
in the duration of breast-feeding. The rate of decrease of the PCB
concentration in the blood in both 18 children and 8 mothers was
relatively constant and independent of the PCB concentrations. A
one-compartment model equation was sufficient to represent the
decrease in the concentration of PCBs in the blood. The mean rate
constant of the decrease for the children was 24.2% per year,
approximately 2.6 times higher than that of the mothers (9.2%),
equivalent to half-lives of 2.8 ± 1.1 and 7.1 ± 2.7 years,
respectively. The dilution effect due to the increase in body weight
was the most important factor that affected the reduction of the PCB
concentrations in the children.
A total of 118 blood samples, mainly from employees in industries
using PCBs, were collected in the period 1975-85. In 64 blood samples,
an average level of 17 µg/litre (range nd-110 µg/litre) was found
(Frank et al., 1988).
Brown & Lawton (1984) studied the partitioning of PCBs between adipose
tissue and serum in a population of 173 capacitor workers, who were
occupationally exposed to Aroclors 1254, 1242, and 1016 for various
periods of time. The serum levels of PCBs were significantly dependent
on the level of lipids in the serum, but not on that in the albumin.
The apparent contribution of cholesterol and its esters to PCB
transport is nearly equal to their contribution to the total serum
neutral lipids. The level of serum lipids PCBs must be equal to the
adipose fat PCBs level.
Yakushiji et al. (1984b) studied the relationship between
breast-feeding and the PCB levels in the blood. The blood samples of
50 children (121 samples) and of 29 occupationally exposed mothers (65
samples) were analysed during the period 1975-79. The PCB levels in
the blood of the children were greatly influenced by the duration of
breast-feeding, but showed little relationship to the PCBs levels in
maternal blood.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Inhalation
Studies on rats (6 per group) showed that an aerosol containing a PCB
mixture (Pydraul A200: 42% chlorine), particle size 0.5-3.0 µm, at a
concentration of 30.4 ± 3.4 g/m3 for 30 min, was readily absorbed
through the lungs. The PCB concentration in the liver, 15 min after
cessation of exposure, was 50% of the maximum concentration attained
after 2 h (70 mg/kg tissue) (Benthe et al., 1972).
6.1.2 Dermal
Vos & Beems (1971) and Vos & Notenboom-Ram (1972) applied Aroclor 1260
to the shaved backs of rabbits and found systemic effects in the
kidneys, indicating that PCBs can penetrate the skin (see section
8.2.5).
Nishizumi (1976), using tritium-labelled PCBs (40% chlorine), found
evidence for the dermal absorption of PCBs in rats.
In a study of the occupational exposure of electrical workers to PCBs
(Pyralen 3010 and Apirolio, 42% chlorine content), Maroni et al.
(1981a) concluded that absorption of PCBs occurred through the human
skin. Quantitative data were not available.
6.1.3 Oral
When polychlorobiphenyl isomers were administered orally, by gavage,
to rats, at levels of 5, 50, or 100 mg/kg body weight for the lower
chlorinated compounds and up to 5 mg/kg for the higher chlorinated
compounds, 90% of the compounds were rapidly absorbed by the
gastrointestinal tract (Albro & Fishbein, 1972; Berlin et al., 1973;
Melvås & Brandt, 1973).
Using Rhesus monkeys, Allen et al. (1974a,b) determined that > 90% of
a single oral dose of 1.5 or 3.0 g Aroclor 1248/kg body weight was
absorbed over a period of 2 weeks. Drill et al. (1981) and US EPA
(1985) reviewed a number of studies indicating that PCBs are readily
absorbed from the gastrointestinal tract following oral
administration.
Bleavins et al. (1984) found that, over a period of 5 weeks, European
ferrets absorbed 85.4% of a single dose of 14C-labelled Aroclor 1254
(0.05 mg) given in food.
In contrast to the above studies, Norback et al. (1978) claimed that
59.3-87% of a single oral dose of 2,4,5,2',4',5'-hexachlorobiphenyl
passed unabsorbed through the intestines of monkeys, the first week
after dosing.
6.2 Distribution
6.2.1 Inhalation (rat)
Maximum PCB concentrations in the liver and brain of rats occurred 2
and 24 h, respectively, after a single, 30-min exposure to 30.4 ±
3.4 g/m3 of Pydraul A200 aerosol (42% chlorine content). The
concentrations in these tissues declined, while concentrations in
adipose tissues reached a maximum after 48 h (Benthe et al., 1972).
6.2.2 Oral (rat)
As in the case of other lipophilic substances, the absorption and
distribution of PCBs will, in all probability, take place via the
lymphatic system (by the chylomicrones) (DFG, 1988).
Following absorption, the clearance of PCBs from the blood and tissues
follows a biphasic pattern. The compounds rapidly clear from the blood
and accumulate in the liver and adipose tissue or are metabolized in
the liver to metabolites that are excreted in the urine and/or bile
(Drill et al., 1981).
Kurachi & Mio (1983) exposed mice to Kaneclor 400 at 100 mg/kg diet,
for 5-20 days. High levels were found in the gonads, skin, adipose
tissue, adrenals, and kidneys.
In a study by Grant et al. (1971a), 4 days after an oral dose of
Aroclor 1254 was given to rats at 500 mg/kg, the concentrations of
PCBs in the fat, liver, and brain were 996, 116, and 40 mg/kg,
respectively. Similar results showing that the highest concentration
was in the fat, were obtained in rats given Aroclor 1254 in the diet
(Curley et al., 1971), in boars (Platonow et al., 1972), cows
(Platonow & Chen, 1973), and in pigeons and quail (Bailey & Bunyan,
1972). In the studies of Curley et al. (1971), the tissue
concentrations initially showed a rapid rise and then a slow increase
while the PCB diet was being administered; Grant et al. (1974) fed
diets containing Aroclor 1254 at 0.2, 20, and 100 mg/kg to rats for 8
months, during which period the tissue concentrations reached a steady
state that was dose-dependent (Table 23). Similar tissue distribution
data for Aroclors 1016 and 1242 have been reported by Burse et al.
(1974) and for Kanechlor-400 by Yoshimura et al. (1971).
Table 23. Tissue distribution of PCBs (mg/kg wet weight) in rats fed Aroclor 1254,
Aroclor 1242, or Aroclor 1016 at 100 mg/kg for about 6 months
Tissue Aroclor 1254a Aroclor 1242b Aroclor 1016b
Blood 0.40 0.53 (plasma) 0.38 (plasma)
Liver 16 4.21 7.86
Brain 3.4 1.69 2.98
Kidneys 1.89 3.21
Heart 7.3
Fat 32.0 110 236
Urine 0.03 0.28
a From: Grant et al. (1974).
b From: Burse et al. (1974).
The study by Burse et al. (1974) showed that, with continuous feeding
of 3 types of Aroclor (see Table 23) at 100 mg/kg diet, a steady state
was not reached for 6-8 months and that the decline of stored PCBs in
adipose tissue (when the animals were kept on a PCB-free diet) was
slow and did not reach zero during a recovery period of 5-6 months.
This is surprising because the Aroclor sample used should not have
contained appreciable amounts of hexachlorobiphenyls and higher
isomers. Mizutani et al. (1977), discussing this aspect, came to the
conclusion that mobilization from storage sites rather than metabolism
constitutes the rate-limiting step in the depletion of the body burden
of PCBs.
It was demonstrated that, as the number of chlorine atoms on the
biphenyl rings increased from 1 to 6, the tissue/blood ratio tended to
increase. This increase was also proportional to the amount of lipid
in the tissues with, consequently, a higher degree of bioaccumulation
(Matthews, 1983, cf. WHO/EURO, 1988).
The fat-plasma partition-coefficients for the different PCB congeners
range from 50 up to 310 (DFG, 1988).
6.2.3 Oral (monkey)
Feeding studies were carried out on female rhesus monkeys given doses
of 0, 5, 20, 40, or 80 µg Aroclor 1254/kg body weight per day, for a
period of 37 months (Arnold et al., 1984). Eighty monkeys were divided
into 5 groups, each of 16 animals. The mean body weight of the monkeys
at the start of the study was 6.44 kg. The Aroclor 1254 was dissolved
in corn oil with glycerol as sweetener and fed to the monkeys in
gelatin capsules. Samples of blood, adipose tissue, and faeces were
collected every month and the presence of PCBs, determined. After 27
weeks, levels of 1, 2-3, 9, 18, and 37 mg PCBs/kg fat were found in
the 5 groups; after 47 weeks, blood levels were 1-3, 12, 35, 73, and
129 µg/litre respectively. PCB concentrations in whole blood increased
more rapidly during the first 10 months of the study than in the
remaining 27 months, in all groups. Concentrations in adipose tissue
(fat) increased continuously during the 37 months. The ratio profiles
of PCB levels in blood/adipose tissue, remained relatively static
between the second and twenty-seventh month of feeding. The data in
terms of relative concentrations (concentration/dose) suggest that the
bioaccumulation or retention of PCBs may be dose-dependent,
particularly for adipose tissue. The data available from PCBs in
faeces indicate a dose-dependent PCB absorption.
6.2.4 Oral (humans)
According to the study by Nishimura et al. (1976) cf. Katsunuma et al.
(1985), the PCBs within a human fetus are not evenly distributed. The
concentrations of PCBs were highest in the skin and lowest in the
brain among the 5 major organs (cerebrum, heart, liver, kidneys, and
skin). That the highest level was found in the skin might have been
because of the high solubility of the compounds in adipose tissue. In
other words, PCBs accumulate increasingly as the body fat of a fetus
increases. The authors stated that the low residue levels in the brain
were likely to be because PCBs have a poor affinity for the brain
lipids.
6.2.5 Individual congeners of PCBs
More detailed information on the tissue distribution of PCBs and their
metabolites has been obtained by the administration of pure
14C-labelled compounds, using both whole-body autoradiography and
scintillation counting of tissue samples. Berlin et al. (1975)
demonstrated that, after a single oral dose of 14C-labelled
2,5,2',4',5'-pentachlorobiphenyl, radioactivity rapidly entered the
circulation of mice and was distributed in the tissues, particularly
in the liver, kidneys, lungs, and adrenals. Subsequently, the
radioactivity in the body fat increased, rising to a maximum within
4-24 h. In most other tissues, the radioactivity decreased rapidly
after dosing, but the authors noted a special affinity for the skin,
the bronchiolar epithelium of the lungs, and certain glandular
secreting tissues. Soon after administration of the dose,
radioactivity appeared in the bile and was eliminated in the faeces.
Similar results were obtained by Melvås & Brandt (1973) in mice
treated with 2,4,2',4'-tetrachlorobiphenyl, which possessed a high
affinity for the adrenal cortex, the corpora lutea, and glandular
secreting tissue. In quail treated with 2,4,2',3'- and
2,4,3',4'-tetrachlorobiphenyl, the radioactivity in the egg yolk was
high, exceeding that in the fat. Gage & Holm (1976) determined
concentrations in the abdominal fat of mice, 7 and 21 days after they
were administered a single dose (13-165 µg/mouse) of one of 14 PCB
congeners, by gavage. Relatively low levels (< 10 ng/g per µg dose)
were found at 7 days for 4,4'-dichloro-; 3,2',4',6'-tetrachloro, and
2,3,4,2',4',6'-hexachlorobiphenyl with relatively high levels (>
100 ng/g per µg dose) for 2,4,5,2',4',5'-hexachloro-, and the
4,2',4',6'-, and 2,4,2',4'- tetrachlorobiphenyls.
Muehleback & Bickel (1981) treated rats, by gavage, with a single dose
of 14C-2,4,5,2',4',5'-hexachlorobiphenyl at 0.6 or 3.6 mg/kg body
weight. The rats were examined 1 h, 24 h, 6 weeks, 20 weeks, or 40
weeks after dosing. The highest levels of PCBs were found in the
muscle, liver, adipose tissue, and skin, early in the study. By the
end of the study, the highest PCB levels were found in the adipose
tissue followed by the skin, muscle, and liver. During the 40-week
study period, only 16% of the total dose was excreted.
The pharmacokinetics of individual monochloro-, dichloro-,
tetrachloro-, pentachloro-, and hexachlorobiphenyls were studied by
Matthews & Anderson (1975a,b), Lutz et al. (1977), and Tuey & Matthews
(1977). The mono- and dichlorobiphenyls were largely removed from
adipose tissue within 4-7 days, the 3 higher chlorinated biphenyls
were eliminated much more slowly. The half-life for the
tetrachlorobiphenyl from adipose tissue was 15 days. Skin effects were
more or less comparable.
Beran et al. (1983) studied the distribution of 14C-labelled
2,5,4'-tri-, 2,4,5,2',4',5'-hexa-, and 2,3,4,5,2',3',4',6'-
octachlorobiphenyl in the haematopoietic tissues of squirrel monkeys
(Saimiri scureus) and C67Bl mice using whole-body autoradiography.
An accumulation of radioactivity was observed in the bone marrow of
one monkey after iv injection (substances dissolved in DMSO) of the
tri- or hexachlorobiphenyl. The same was found in 3 normal mice
treated with the octachlorobiphenyl. A study using whole-body
autoradiography and spleen-colony assay in supralethally irradiated
mice, implanted with syngenic bone-marrow cells, indicated that the
major part of the radioactivity was localized outside the bone-marrow
haemic compartment, probably in the fat. Nevertheless, the trichloro-
and octachlorobiphenyls were found to inhibit the in vitro formation
of granulocytic colonies from mouse progenitor cells. Very low uptake
of labelled chlorobiphenyls was observed in the thymus, spleen, and
lymph nodes.
14C-labelled-2,4,2',4'-tetrachloro- and 3,4,3',4'-tetrachlorobiphenyl
were each administered orally to male Sprague-Dawley rats in a single
dose at 0.54 mg/kg and 0.51 mg/kg, body weight, respectively.
Distribution and covalent binding were studied. The accumulation of
2,4,2',4'-tetrachlorobiphenyl in adipose tissue was much higher than
that of 3,4,3',4'-tetrachlorobiphenyl, though the level in the blood
was consistently higher in the 3,4,3',4'-tetrachlorobiphenyl-treated
rats. The radioactivity bound in covalent linkages with cellular
macromolecules in several tissues was determined. The data indicated
that covalent binding was higher in 3,4,3',4'-tetrachloro-
biphenyl-treated rats than in those treated with 2,4,2',4'-
tetrachloro-biphenyl, particularly in the liver and blood components.
These results suggest that the 2 tetrachlorobiphenyl isomers have
different pharmacokinetic properties in rats and that the association
of covalent binding with 3,4,3',4'-tetrachlorobiphenyl induced
toxicities might be important. The microsomal enzyme system is likely
to play an important role in the in vivo covalent binding of
tetrachlorobiphenyls (Shimada & Sawabe, 1984).
In pharmacokinetic studies, 11 groups of 3 male ICR mice/group were
administered daily doses of 100 mg 2,5,2',5'-tetrachlorobiphenyl/kg
body weight dissolved in corn-oil/acetone (9:1), by gavage, for 8
consecutive days. Thirteen groups of 3 mice were administered (by
gavage) 8 mg 3,4,3',4'-tetrachlorobiphenyl/kg in the same vehicle
every other day for 10 doses. One group was sacrificed just before
each of the last 3 doses, the other groups were sacrificed at
intervals of 0.5-336 h after dosing. After dosing to an apparent
steady-state, 2,5,2',5'-tetrachlorobiphenyl was found to have a tissue
elimination half-life of between 39.5 and 70 h. The half-life of
3,4,3',4'-tetrachlorobiphenyl was 26-62.5 h. The 3,4,3',4'-
tetrachlorobiphenyl had a substantially greater partitioning from
serum into adipose tissue, liver, and thymic tissues. Studies were
undertaken to compare the toxic potency of these 2
tetrachlorobiphenyls, when similar tissue concentrations of the 2
isomers were achieved in target and storage tissues. The studies
demonstrated that thymic atrophy occurs at lower doses and tissue
concentrations of 3,4,3'4'-tetrachlorobiphenyl than those required to
produce hepatotoxicity. These two organ toxicities were produced only
by 3,4,3'4'-tetrachlorobiphenyl, despite the fact that equivalent or
higher tissue concentrations of 2,5,2',5'-tetrachlorobiphenyl were
achieved in vivo, in all tissues. The conclusion was that the
in vivo difference in the toxic potency of these tetrachloro-
biphenyl isomers does not result from the differences in their tissue
disposition, elimination, and ultimate bioaccumulation (Clevenger et
al., 1989).
6.2.6 Appraisal
Matthews & Dedrick (1984), in a review, concluded that the
pharmacokinetics of PCBs are complicated by numerous factors, not
least of which is the existence of 209 different chlorinated
biphenyls. While all PCB congeners are highly lipophilic and most are
readily absorbed and rapidly distributed to all tissues, PCBs are
cleared from the tissues at very different rates, and the same
congeners may be cleared at different rates by different species. With
the exception of special situations in which PCBs may be passively
eliminated in lipid sinks, e.g., milk or eggs, clearance is minimal
prior to metabolism to more polar compounds. Rates of PCB metabolism
vary greatly with species and with the degree and positions of
chlorination. Mammals metabolize these compounds most rapidly, but,
even among mammalian species, the rates of metabolism vary greatly. In
all species studied, the more readily metabolized chlorinated
biphenyls have adjacent unsubstituted carbon atoms in the 3-4
positions. Congeners that do not have adjacent unsubstituted carbon
atoms may be metabolized very slowly and therefore cleared very
slowly. PCBs that are not readily cleared concentrate in adipose
tissue.
6.3 Placental transport
6.3.1 Laboratory animals
The results of a number of animal studies have demonstrated that PCBs
and specific congeners can cross the placental barrier and accumulate
in the tissues of fetuses (US EPA, 1987). In studies in which monkeys
were exposed prior to, and during, gestation, signs of PCB
intoxication were observed in nursing, but not in newborn offspring
(Allen & Barsotti, 1976; Iatropoulos et al., 1978). Results such as
these have led to the conclusion that transfer through nursing may
account for higher exposure of the young than placental transfer.
Groups of pregnant ddN mice were fed diets containing Kanechlor 500
(mainly comprising pentachloro- and hexachlorobiphenyls) at 0.01
(controls), 0.94, or 86 mg/kg diet from day 1 to 18 of pregnancy.
Regardless of the dietary level of PCBs, whole-body levels in the
fetuses were only 0.1-0.2% of the total maternal intake, indicating
limited transplacental transfer (Masuda et al., 1978a). Two groups of
ddN mice were fed Kanechlor 500 (mainly containing pentachloro- and
hexachlorobiphenyls at 0 or 0.94 mg/kg diet) from the day of
insemination throughout gestation and for 5 weeks after delivery of
offspring. Total PCBs were 100 times greater in the suckling animal
than in the fetuses at term, from dams fed the same amount, indicating
a considerable transfer of PCBs during lactation (Masuda et al.,
1978a).
Masuda et al. (1979) fed female ddN-mice diets containing
polychlorinated biphenyls: 2,4,4'-trichloro-; 2,5,3',4'-tetrachloro-;
2,4,5,2',5'-pentachloro-; 2,3,4,2',4',5'-hexachloro-;2,4,5,2',4',5'-
hexachloro-; 2,3,4,5,6,2',5'-heptachloro-; and 2,3,4,5,2',3',4',5'-
octachlorobiphenyl at levels of 0.32, 0.42, 0.42, 0.44, 0.44,
0.16, and 0.23 mg/kg diet, respectively, for 18 days prior
to, or after, mating. Animals were either sacrificed on day 18 of
gestation or allowed to deliver and the offspring maintained for 5
weeks on a normal diet. All the PCBs were qualitatively transferred
across the placenta and through the milk. The amount transferred
during lactation was greater than that transferred transplacentally.
The transfer of 2,4,5,2',4',5'-hexachlorobi[14C-]phenyl across the
placenta during the course of pregnancy in Sprague-Dawley mice was
studied by Vodicnik & Lech (1980). The PCB was injected
intraperitoneally at 100 mg/kg body weight, in corn oil, 2 weeks prior
to mating. The concentrations of 14C-PCB in the fetuses from 12- and
18-day pregnant animals were 0.71 and 2.45 mg/kg tissue, respectively.
At birth, the total carcass concentration for all newborn animals was
less than 3 mg/kg tissue, which represents less than 3% of the dose
present in the mothers at birth.
Placental transfer of polychlorinated biphenyls has also been reported
in the mouse by Berlin et al. (1975) and Melvås & Brandt (1973).
Curley et al. (1973a) found some placental transport of Aroclor 1254
in the rat.
Groups of pregnant and non-pregnant Wistar rats received a dose of
14C-2,4,5,2',4',5'-hexachlorobiphenyl (2.1 µC/kg), intraperitoneally.
The amount of radioactivity transferred through the placenta was 2.7%
of the administered dose, whereas 39.2% of the original dose was
transferred through the milk (Ando et al., 1978).
Aroclors 1221 and 1254 were found to cross the placenta of rabbits,
when administered orally to does during gestation. The concentration
in fetal tissues was dose-dependent and much lower with Aroclor 1221
than with Aroclor 1254; the concentration of the latter in the fetal
liver was greater than that in the maternal liver (Grant et al.,
1971b).
Bleavins et al. (1984) fed female European ferrets a single dose of
14C-labelled Aroclor 1254 in the diet (0.05 mg), early (day 14) or
late (day 35) in gestation, and determined the placental transfer of
PCBs. Placental transfer to the kits was 0.01% (per kit) of the
maternal dose, when the dams were exposed early in gestation, and,
0.04%, when the dams were exposed late in gestation. Placental
transfer of PCBs was considerably less than mammary transfer, with a
ratio at 1 week of lactation of 1:15 and 1:7 for offspring of dams
dosed early or late in gestation, respectively.
Groups of lactating mother Rhesus monkeys, between 1 and 3 months post
partum, received 16 mg Clophen A-30/kg per day for 30 days. One
mother/infant pair served as a control. Clophen A-30 concentrations in
the serum of both mother and infant and the milk were determined on
days -14, -7, 0, 1, 2, 4, 8 and at weekly intervals thereafter. One
mother and all infants were killed and tissues taken for PCB analysis.
The concentration of Clophen A-30 in milk was 20 times higher than
maternal serum levels. Infant serum levels were 2-5 times higher than
their mothers. Tissue levels were generally higher in the infants.
Clophen A-30 tended to concentrate in the infant fat, bone marrow, and
adrenals (Bailey et al., 1980).
Groups of 24 Rhesus monkeys were maintained on diets that provided
Aroclor 1016 at doses of 0, 4.5, or 18.1 mg/kg body weight per day
throughout gestation and a 4-month nursing period. At birth, the
concentrations of PCBs in the skin of infants were similar to
concentrations in the subcutaneous fat of the mothers. At weaning, the
PCB content in the mesenteric fat of the infants was 4-7 times greater
than that in the subcutaneous fat of the mothers. Gas chromatographic
patterns showed that the adult adipose tissue did not include the
total spectrum of peaks observed in the Aroclor 1016 standard, and
that all of the peaks in the mesenteric fat of the infants at weaning
and 4 months after weaning were qualitatively similar to those in the
adult adipose tissue. According to the authors, these data suggested
an inability of the fetus to metabolize and excrete certain congeners
that are more readily metabolized and eliminated by adults and older
infants (Barsotti & Van Miller, 1984).
6.3.2 Wildlife
A 6 1/2-year-old desert bighorn (Ovis canadensis cremnobates) ewe
and her term ram fetus were used to study the distribution and
concentrations of PCBs in different organs and tissues. Fourteen
maternal and 13 fetal tissues were analysed for their presence of
organochlorine hydrocarbons. PCBs averaged 85 and 88% of the total
residue loads for maternal and fetal tissues, respectively. It is
remarkable that the "natural" PCB levels in the different organs and
tissues were nearly the same, i.e., in maternal organs and tissues
between 0.37 and 0.44 mg/kg, and, in fetal organs and tissues, between
0.30 and 0.35 mg/kg, on a fat basis (Turner, 1979).
6.3.3 Humans
Four studies of placental passage in humans, based on small samples
drawn from the general Japanese population, have yielded inconsistent
results (Yoshimura, 1974; Akiyama et al., 1975; Kodama & Ota, 1977;
Masuda et al., 1978a).
PCBs were detected in the umbilical tissues, umbilical blood, amniotic
fluid, and baby's blood from a woman who was occupationally exposed to
Kanechlors 300 and 500 in a capacitor factory (Yakushiji et al.,
1978). PCB levels in these tissues and fluids were considerably lower
than that in the mother's blood.
Jacobson et al. (1984b) examined maternal and cord serum (196 or 198
samples each) for the presence of PCBs, in women who resided in the
Michigan area (USA), where, in 1973, a PBB-incident occurred. Mean
concentrations of maternal and cord serum were 4.7 µg/litre (1.1-
14.3 µg/litre) and 2.0 µg/litre (0.1-7.2 µg/litre), respectively.
Placental passage was indicated by a significant maternal to cord
serum correlation for PCBs. The fact that cord serum levels were lower
than those in maternal serum is consistent with the notion that the
placenta may function as a partial barrier. The transfer rate of PCBs
in maternal blood through the placenta to cord blood may vary,
depending on the chemical nature of each PCB isomer (Ando et al.,
1984). Ando et al. (1985) examined the PCB concentrations in the
maternal blood, breast milk, and the placenta of 6 Japanese women.
They found that the congeners present were more typical of Kanechlor
500 than Kanechlor 300, 400, or 600. The results indicated that, as
the chlorine content of the PCB congeners increased, the correlation
between the placental content of congeners and those in the maternal
blood and breast milk also increased. The same was found in laboratory
animals (Allen & Barsotti, 1976; Masuda et al., 1978b).
A study on the transfer of PCBs to infants from their mothers was
carried out in Japan from 1974 to 1976 by Kodama & Ota (1977). When
the cord blood was considered as the infant blood at birth, the level
of PCBs in the blood of breast-fed infants rose gradually with
ingestion of breast milk, exceeded the level in the blood of their
mothers after 3 months, continued to increase up to the age of 1 year
and then significantly decreased, 2 years after birth. The PCB
concentrations in the blood of non-breast-fed infants remained low
(Table 24).
6.4 Excretion and elimination
6.4.1 Following oral dosing
The excretion of PCBs is, to a large extent, dependent on the
metabolism of PCBs to form more polar compounds (US EPA, 1987). At
equilibrium, the elimination of PCBs from all tissues will be
dependent on the structure-dependent metabolism rates of the
individual PCB congeners. For example, the biological half-lives in
the rat range from 1.15 days for 2,2'-dichlorobiphenyl to
approximately 460 days for 2,4,5,2',4',5',-hexachlorobiphenyl (Tanabe
et al., 1981; Wyss et al., 1986). Metabolites of the more highly
chlorinated congeners are eliminated primarily via the faeces (Goto et
al., 1974; WHO/EURO, 1987).
When the analysis of faeces is limited to the determination of
unchanged PCBs, the recovery of the dose administered is incomplete;
in boars receiving single or repeated doses of Aroclor 1254, not more
than 16% of the dose was recovered from the faeces and less than 1% in
the urine (Platonow et al., 1972). Better recoveries have been
obtained with PCB labelled with radioactive isotopes. Yoshimura et al.
(1971) found 70% of the activity from a dose of tritium-labelled
Kanechlor 400 in the faeces and 2% in the urine, over a 4-week period.
Berlin et al. (1973, 1975) found over 75% of the activity from
14C-labelled pentachloro- and hexachlorobiphenyls in the faeces and
less than 2% in the urine; most of the faecal elimination consisted of
PCB metabolites. Similar results were obtained by Melvås & Brandt
(1973) with tetrachlorobiphenyls.
Hashimoto et al. (1976) examined the excretion of 14C-PCB compounds
given to rats by gavage, at a total dose of 6.35-7.85 mg/kg body
weight, over a period of 5-50 days. The PCBs studied were
predominantly tetra- and hexachlorinated isomers. The results
indicated that 1.9-4.9% of the dose of tetrachlorobiphenyls was
excreted in the urine, with higher amounts excreted in rats treated
for longer periods. In rats treated with hexachlorobiphenyls, only
0.3% of the dose was excreted in urine. About 47-68% of the dose of
both tetrachloro- and hexachloro-isomers was eliminated in the faeces.
Table 24. Level of PCBs in mothers' and babies' blood (average over
3 years in µg/litre)a
Maternal blood 4.5 (0.8-15.5)
Cord blood 1.1 (nd-5.6)
Mother's blood (breast-feeding) 2.5 (nd-10.8)
Babies' blood (3 months old) 3.6 (0.2-10.9)
Babies' blood (1 year old) 4.7 (0.8-17.7)
Mother's blood (bottle feeding) 2.7 (0.6-8.7)
Babies' blood (3 months old) 1.6 (nd-7.6)
Babies' blood (1 year old) 0.7 (nd-2.1)
a From: Kodama & Ota (1977).
Bleavins et al. (1984) found 22.1% and 1.8% in the faeces and urine,
respectively, during the first week following dosing of 0.05 mg
14C-labelled Aroclor 1254 to female European ferrets.
A biological half-life of about 200 days was recorded in the fat of
rats after feeding with Aroclor 1254 (Grant et al., 1974). Berlin et
al. (1975) noted that, in mice dosed with a pentachlorobiphenyl, there
was an initial rapid elimination from the liver while liver PCB levels
were high, followed by a slower elimination when most of the PCB was
located in the fat. The author suggested that the mobilization of PCBs
from fat, and, therefore, their half-life in the body, depends upon
their rates of metabolism. Berlin et al. (1973) investigated the
hypothesis that the ability of a PCB to be readily degraded with a
half-life of a few days depended on the presence of 2 adjacent
unsubstituted carbon atoms in the molecule, rather than on the number
of chlorine atoms, though the presence of such unsubstituted pairs
depends to a large extent on the degree of chlorination. They came to
the conclusion that this hypothesis probably applied to unsubstituted
pairs in the 3,4-position, but that in the 2,3-position, their
susceptibility to metabolic degradation was influenced more by the
presence of chlorines in the o-position of the ring bridge.
Sprague-Dawley rats, white Swiss mice, and Rhesus monkeys were
administered a single dose of 14C-2,2'-dichlorobiphenyl, by gavage.
Within 6 days, mice eliminated a total of approximately 46% of the PCB
(urine, 20%; faeces, 26%). There was no clear difference between male
and female mice. In the rat, the total elimination was 51-56% after 9
days, mainly via the biliary/faecal route. The monkeys had the highest
elimination rate, a total of 68.6% (urine, 54%; faeces, 14.6%), within
10 days (Milling et al., 1979).
Male and female Wistar rats were administered a daily dose of
14C-2,5,4'-trichlorobiphenyl for 14 days. The animals were killed 5
days after receiving the last dose. The compound was rapidly
eliminated primarily with the faeces. Most of the trichlorobiphenyl
was metabolized (78.5%) and the major metabolites excreted were
identified as hydroxy-, dichloro-, and conjugated derivatives (Lay et
al., 1979).
The elimination of tetrachlorobiphenyl isomers in mice fed diets
containing a single isomer, at 10 mg/kg diet for 20 days, was studied
by Mizutani et al. (1977). Biological half-lives for the isomers
2,3,2',3'-tetrachloro-, 2,4,2',4'-tetrachloro-, 2,5,2',5'-
tetrachloro-, 3,4,3',4'-tetrachloro-, and 3,5,3',5'-tetrachloro-
biphenyl, were 0.9, 9.2, 3.4, 0.9, and 2.1 days, respectively.
Gage & Holm (1976) studied the influence of molecular structure on the
excretion of 14 PCB congeners in mice. They found that the
4,4'-dichloro-; 3,3',4',6'-tetrachloro-; 2,3,2',4',6'-pentachloro-;
and 2,3,4,2',4',5'-hexachloro-isomers were eliminated most rapidly.
These compounds had at least one pair of unsubstituted ortho-meta,
vicinal carbon atoms, a configuration thought to be important for
rapid metabolism and excretion. The most slowly eliminated compounds
were 2,4,5,2',4',5'- and 2,3,4,2',4',5'-hexachlorobiphenyl.
2,4,5,2',4',5'-Hexachlorobiphenyl was the PCB congener found in the
highest concentration in human adipose tissue, while
2,4,6,2',4',6'-hexachlorobiphenyl was not detected (Jensen &
Sundström, 1974a). As both of these compounds are found in commercial
PCB mixtures and in the environment, the presence of the
2,4,5,2',4',5'-hexachlorobiphenyl in adipose tissue appears to be
related to resistance to metabolism (US EPA, 1987). That this congener
is not, or is only minimally, metabolized is also indicated by the
finding that the blood concentration of this congener decreased only
10% over 300-500 days (Chen et al., 1982) and by the results of
in vitro metabolism studies with human liver microsomes (Schnellman
et al., 1984a,b).
Felt et al. (1977) examined the elimination of 14C-2,5,4'-trichloro-
biphenyl in rhesus monkeys. The monkeys were fed 550 mg of the
compound in fruit, daily, for 84 days. On the basis of total excretion
and recovered radioactivity, the half-life was found to be 4.5-4.8
days.
Male and female Sprague-Dawley rats were administered 14C-labelled
2,4,6,2',4'-pentachlorobiphenyl by gavage and the urine and faeces
were collected. After 8 days, the animals were killed. The elimination
of the PCBs followed a bi-exponential rate expression with a-phase
half-lives of 0.90 and 0.95 days and b-phase half-lives of 4.2 and 3.8
days, for males and females, respectively (Felt et al., 1979).
6.4.2 Following parenteral dosing
The results of injection studies indicate that PCBs can be excreted
unmetabolized into the gastrointestinal tract. Yoshimura & Yamamoto
(1975) recovered unchanged tetrachlorobiphenyl from the duodenal
contents of rats injected intravenously with tetrachlorobiphenyl.
Daily excretion for 4 days ranged from 0.5 to 0.8% of the total
dose/day. Goto et al. (1974) found that 4.7-23.2% of injected PCBs
were excreted unchanged into the gastrointestinal tract by day 10
after dosing, with the excretion of a penta-isomer greater than the
excretion of di-, tri-, or tetra-isomers.
Adult male Sprague-Dawley rats received doses of 4 symmetrical
hexachlorobiphenyl 14C-isomers, i.e., 2,3,5,2',3',5'-hexachloro-,
2,3,6,2',3',6'-hexachloro-, 2,4,5,2',4',5'-hexachloro-, and
2,4,6,2',4',6'-hexachlorobiphenyl, by intravenous injection. Most of
the radioactivity was eliminated in the faeces with less than 1% found
in the urine. The metabolites showed evidence of dechlorination,
chlorine shifts, and possible metabolism by direct insertion of a
hydroxyl group. There was also evidence supporting the intermediate
step of an arene-oxide as a predominant mechanism of PCB metabolism
(Kato et al., 1980).
The disposition of 2 symmetrical 14C-labelled 2,3,6,2',3',6'-
hexachloro- and 2,4,5,2',4',5'-hexachlorobiphenyl was studied in
24-month-old, male, Sprague-Dawley rats, after iv treatment. More than
50% of the 2,3,6,2',3',6'-hexachlorobiphenyl was metabolized and
excreted via the bile into the faeces within 2 days, and only 2% was
excreted in urine. More than 90% was eliminated as metabolites. In
contrast, 2,4,5,2',4',5'-hexachlorobiphenyl was redistributed from the
liver, muscle, and skin to the adipose tissue, where it accumulated
without being metabolized. Only 2% of the total dose was eliminated,
primarily in the faeces, within 21 days. In 2- to 3-month-old rats,
the general pattern of disposition of these hexachlorobiphenyls did
not change with age; however, there were differences in the rates of
elimination and in the tissue levels. There was enhanced metabolic
retention in the muscle, skin, and adipose tissue of older rats, which
suggested an age-related decrease in tissue clearance. The larger
volume of adipose tissue could not explain this observation. In
general, there were few changes in decay rates from tissues or in
biliary excretion, so age had a greater effect on the disposition of
the "persistent" 2,4,5,2',4',5'-hexachlorobiphenyl than on the
metabolizable 2,3,6,2',3',6'-hexachlorobiphenyl (Birnbaum, 1983).
Ethane exhalation was increased in male Sprague-Dawley rats, 30 days
after a single ip injection of Aroclor 1254 (500 mg/kg body weight).
Before day 30, there was no increase in ethane production. Parallel
increases in hepatic malondialdehyde levels were found. A single ip
injection of 3,4,3',4'-tetrachloro-, 2,3,4,5,4'-pentachloro, and
2,4,5,2',4',5'-hexachlorobiphenyl (300 µmol/kg) also increased (after
30 days) the production of malondialdehyde and ethane, indicators of
in vivo lipid peroxidation. These effects were not reflected in
increased diene conjugation (Dogra et al., 1988).
Sipes et al. (1980, 1982a,b) studied the distribution, metabolism, and
excretion of 14C-labelled 4,4-dichloro-, 2,4,5,2'4'5'-hexachloro-, or
2,3,6,2',3',6'-hexachlorobiphenyl in beagle dogs and cynomolgus
monkeys, after a single intravenous dose. The elimination of the test
substances from the blood of both species was shown to be biphasic.
The results for dichlorobiphenyl showed that the dog eliminated 50% of
the dose (urine, 7%; faeces, 43%) within 24 h, while the remainder was
found mainly in the adipose tissue. By 5 days, 90% had been
eliminated. The monkey eliminated less than 15% of the dose within
24 h, with less than 1% in the faeces. The remainder was found in the
adipose tissue. Within 28 days, 59% of the dose had been eliminated,
chiefly in the urine. Biliary excretion after 24 h was shown to be 33%
in the dog and only 0.4% in the monkey.
The data for 2,4,5,2',4',5'-hexachlorobiphenyl showed that the dog
eliminated 66% (urine, 3%; faeces, 63%) within 3 days; the monkey
eliminated 18% of the dose (of which 17% was in the faeces), 90 days
following administration. The remainder was found in the adipose
tissue. In the studies with 2,3,6,2',3',6'-hexachlorobiphenyl, the dog
eliminated 52% of the dose within 24 h (urine, 11%; faeces, 41%) and
70% in 3 days. The monkey eliminated 19% during the first 24 h,
divided equally between urine and faeces. By 15 days, 61% had been
eliminated, primarily in the faeces. The 24-h biliary excretion was
26% and 2.4% in the dog and the monkey, respectively.
6.4.3 Humans
Chen et al. (1982, 1985) studied the presence of PCBs in the blood of
human beings, in the Province of Taiwan, after they had consumed
rice-bran oil contaminated with Kanechlor 500 and PCDFs. Blood samples
from 17 patients were examined, with 2-3 samples taken from each
patient, 2-17 months apart. The results indicated that the
tetrachloro- and some pentachloro- isomers tended to be eliminated
more rapidly than the other pentachloro- and the hexachloro- and
heptachloro- isomers. Half-lives for the 2,4,5,2',4'- and 2,3,4,3',4'-
pentachloro- isomers in the blood were 9.8 and 8.7 months,
respectively. Two adjacent unsubstituted carbon atoms at the meta,
para positions facilitated metabolism and the subsequent elimination
from the blood. PCBs containing adjacent unsubstituted carbon atoms at
the ortho and meta positions of the biphenyl ring are eliminated
very slowly and will accumulate.
Buhler et al. (1988) administered a uniformly 13C-labelled PCB
mixture similar to Aroclor 1254 to a volunteer. A single dose of
329 µg/kg body weight was ingested; blood samples taken over a period
of 260 days were analysed for 13C- and 12C-PCBs using GC/MS and
GC/ECD. Elimination of the isomers followed a first order kinetics.
The half-lives for the isomers 2,3,4,2',4',5'-hexachlorobiphenyl,
2,4,5,2',4',5'-hexachlorobiphenyl, and 2,3,4,5,2',4',5'-hepta-
chlorobiphenyl were 321, 338, and 124 days, respectively.
6.4.4 Elimination via milk (animals)
Vodicnik (1986) studied the disposition of 14C-2,4,2',4'-
tetrachlorobiphenyl (150 mg/kg body weight administered
intraperitoneally) as a function of non-pregnant body weight in
virgin, late pregnant, and early post partum ICR mice and their
offspring. The highest concentrations were observed in adipose tissue
and the mammary glands, regardless of reproductive state. The
concentrations of the tetrachlorobiphenyl equivalents in the tissues
differed among the 3 groups, possibly because of the alterations in
lipid deposition/mobilization associated with pregnancy and lactation.
Approximately 20% of 14C-activity was eliminated from the carcass of
virgin mice, 4 days after administration, but no decrease was seen in
late-pregnant animals. Minimal transplacental transfer of
14C-activity occurred (approximately 1%), but the tetrachlorobiphenyl
was rapidly eliminated in breast milk to nursing offspring. Ninety per
cent of the total-carcass 14C-activity was eliminated from lactating
mice over a 4-day period, approximately 75% of which could be
accounted for in neonatal carcasses.
Saschenbrecker et al. (1972) found that, after oral administration of
doses of Aroclor 1254 of 10 or 100 mg/kg to cows, 6.27 and
74.5 mg/litre, respectively, appeared in the milk after 24 h. These
levels were reduced to less than one-half within 3 days, but traces
still remained at 50 days. Cows receiving 200 mg/day of Aroclor 1254
reached a steady state concentration of 61 mg/kg in the milk fat and
42 mg/kg in the body fat, after 10 days (Fries et al., 1973).
The "carry-over factor" from animal feed into the cow's milk showed
that the lower (tri-, tetra-, and penta-) chlorinated biphenyls have a
lower carry-over factor than the higher (hexa- and hepta-) chlorinated
biphenyls. Thus, it is the latter that are particularly concentrated
in cow's milk fat. From studies in the Federal Republic of Germany, it
was found that the major congeners in cow's milk were numbers 138,
153, and 180 (DFG, 1988).
6.4.4.1 Elimination via breast milk
The composition of common commercial PCB mixtures clearly differs from
the composition of the PCB contents of human fat or human breast milk,
because of the preferential elimination of certain PCB congeners
containing 3 or 4 ortho substituents and the retention of PCBs with
1 or 2 ortho substituents (Kuroki & Masuda, 1977; Watanabe et al.,
1979; Yakushiji et al., 1979).
The major PCB components (and average relative concentrations) that
have been identified in breast milk in the Osaka area in Japan
include: 2,4,4'-trichlorobiphenyl (8.4%); 2,5,2',5'-tetrachloro-
biphenyl (2.0%); 2,4,5,4'-tetrachlorobiphenyl (19%); 2,4,5,2',5'-
pentachlorobiphenyl (2.8%); 2,4,5,3',4'-pentachlorobiphenyl (11.8%);
2,4,5,2',4',5'-hexachlorobiphenyl (15.5%); 2,3,4,2',4',5'-
hexachlorobiphenyl (15.8%); 2,3,4,3',4',5'-hexachlorobiphenyl (2.3%);
2,3,4,6,2',4',5'-heptachlorobiphenyl (1.6%); 2,3,5,6,2',4',5'-
heptachlorobiphenyl (3.2%). These PCB-congeners constituted at least
95% of the PCBs in the breast milk of the women examined in Osaka.
In recent studies, the contents of PCBs in human milk and maternal
blood were compared for US citizens (Bush et al., 1984, 1985). Eight
individual PCB congeners comprised 52% of the total PCB residues in
the milk and 48.5% in the blood. The mean concentrations for total
PCBs were 26.5 µg/kg for whole milk and 3.5 µg/kg for blood. The
percentages of the different congeners are given in Table 25.
6.5 Metabolic transformation
6.5.1 PCBs
The metabolism of PCBs has been investigated in numerous studies on
animals and reviewed by Drill et al. (1981) and the US EPA (1987). The
PCBs were usually administered by the oral or parenteral route.
Phenolic products are the major PCB metabolites, though
sulfur-containing metabolites, trans-dihydrodiols, polyhydroxylated
PCBs, and methyl ether derivatives have also been identified. Although
the effects of the chlorine substitution pattern on sites of oxidation
have not been studied systematically, US EPA (1987) suggested the
following:
* hydroxylation is favoured at the para position in the least
chlorinated phenyl ring, unless this site is sterically hindered
(i.e., 3,5-dichloro-substitution);
* in the lower chlorinated biphenyls the para position of both
biphenyl rings and carbon atoms that are para to the chloro
substituent are all readily hydroxylated (Sparling et al., 1980);
* the availability of 2 vicinal unsubstituted carbon atoms
(particularly C5 and C4 in the biphenyl nucleus) also facilitates
the oxidative metabolism of the PCB substrate, but is not a
necessary requirement for metabolism;
Table 25. Concentrations of most abundant PCB congeners present in whole breast milk and maternal blooda
Congener Milk Maternal blood Ratio
(40 samples) (101 samples) milk/blood
µg/litre % of µg/litre % of
total PCBs total PCBs
2,4,5,2',4',5'-hexachlorobiphenyl 3.2 12 0.31 8.8 10
2,3,5,6,2',3',6-heptachlorobiphenyl 2.5 9.4 0.27 8.0 9.2
2,4,5,2',3',4'-hexachlorobiphenyl 2.1 7.8 0.58 17 3.5
2,5,3'4'-tetrachlorobiphenyl 1.7 6.6 0.01 - 500
2,3,4,5,2'4'5'-heptachlorobiphenyl 1.2 4.5 0.03 3.7 9.4
2,3,4,5,3',4'-hexachlorobiphenyl 1.0 4.0 0.01 - 125
2,4,5,2',4'-pentachlorobiphenyl 1.1 4.0 0.12 3.4 8.9
2,3,4,3',4'-pentachlorobiphenyl 0.97 3.7 0.25 7.6 3.8
Total PCBs 26.5 - 3.5 - 7.5
a Modified from: Bush et al. (1985).
* as the rate of chlorination increases on both phenyl rings, the
rate of metabolism decreases;
* the metabolism of specific PCB isomers by different species can
result in considerable variations in metabolic pattern.
Kannan et al. (1989) studied the possible involvement of frontier
(pi) electrons in the metabolism of polychlorinated biphenyls. The
electron density, at each carbon atom, of the highest occupied pi
orbital of 13 PCB molecules was calculated and the result was compared
with their in vitro and/or in vivo metabolism. It was found that:
* the carbon position at which the frontier electron density was the
highest was most readily hydroxylated or sulfonated;
* if the carbon with the highest frontier (pi) electrons was
occupied by chlorine, either a replacement occurred or the carbon
with the next highest electron density was activated for
metabolism;
* because of steric hindrance, "ortho" carbons were least
preferred for such reactions, in spite of possessing favourable
electron density;
* this was applicable to both phenobarbital (PB)-type and
3-methylcholanthrene (3-MC)-type PCB inducers.
The authors suggested that frontier (pi) electron density could be
an easy guide for understanding the metabolic products of persistent
and toxic environmental pollutants in vitro and in vivo, and for
understanding their environmental fate.
There appears to be little metabolism of PCBs with 6 or more chlorine
substituents (Matthews & Anderson, 1975b). When between 2 and 5
chlorine substituent PCBs are metabolized, the metabolic products are
primarily hydroxylated compounds, frequently found as glucuronide
conjugates (hydroxymethoxy derivatives) and partially dechlorinated
metabolites. In some cases, smaller amounts of dihydrohydroxy
compounds and related substances are also found.
The parent compound is also eliminated in various quantities in
faeces, hair, and maternal milk, but very little unmetabolized
compound is excreted in the urine. This pattern is not unusual for
lipophilic xenobiotics.
PCB metabolism has been examined in primates (monkeys) by Greb et al.
(1975), Hsu et al. (1975a,b), and Allen & Norback (1976); in ungulates
(cows, pigs, and goats) by Platanow & Chen (1973), Safe et al. (1975),
and Gardner et al. (1976); in rats by Grant et al. (1971a), Hutzinger
et al. (1972), Yoshimura et al. (1973), Goto et al. (1973, 1974,
1975), Safe et al. (1974), Matthews & Anderson (1975b), van Miller et
al. (1975), Sundström & Jansson (1975), Sundström et al. (1976a), Lay
et al. (1975, 1979), Chen et al. (1976), Kamal et al. (1976), and
Norback et al. (1976); in mice by Berlin et al. (1973), Yamamoto &
Yoshimura (1973), and Sundström & Jansson (1975); in rabbits by Grant
et al. (1971b), Hutzinger et al. (1974), Sundström & Wachmeister
(1975), and Sundström et al. (1976b); in pigeons, and quails by Koeman
et al. (1969), Hutzinger et al. (1972), Bailey & Bunyan (1972), and
Sundström & Jansson (1975); and in trout by Hutzinger et al. (1972).
The different metabolic products formed from pure isomers in these
various species have been catalogued in an NAS report (1979) and in a
review by Sundström et al. (1976a). Neither of these reports is
complete, but, together, they cover most of the studies up to 1979.
In the rat, monochloro-, dichloro-, trichloro-, tetrachloro-,
pentachloro-, and at least one hexachlorobiphenyl, yielded at least
one hydroxylated metabolite. Some isomers produced as many as 5
different hydroxylated metabolites including both mono- and dihydroxy-
derivatives. Most of the hexachloro-, octachloro-, and
decachlorobiphenyls did not yield detectable levels of hydroxylated
products.
Similar hydroxylated derivatives were also produced in other species,
but the ability to metabolize PCBs is not absolutely uniform in all
species. In the rabbit, dichloro-, tetrachloro-, and
hexachlorobiphenyls were metabolized, while further down the
phylogenetic scale, the pigeon only metabolized monochloro- and
dichlorobiphenyls and the trout failed to metabolize any of the
chlorinated biphenyls tested. Table 26 shows the PCBs tested in
different species and indicates whether or not the organism was able
to metabolize the compound. Although different species may metabolize
a given isomer, the metabolic products are not necessarily identical.
An example of this is found in the simple 4,4'-dichlorobiphenyl which
is metabolized by the rat, rabbit, and goat, but does not give
identical products in these species; all 3 species produce
4,4'-dichloro-3-hydroxybiphenyl as a metabolite, but, in addition, the
rat produces 4,4'-dichloro-, 2,3-dihydroxybiphenyl, and the goat
produces 3,4'-dichloro-4-hydroxybiphenyl as a metabolite.
However, a product such as the 3,4'-dichloro-4-hydroxybiphenyl found
in the goat involves a chlorine shift, which may be indicative of a
more toxic intermediate.
Many different pathways of metabolism have been described as
summarized in Fig. 5 (Safe, 1984; WHO/EURO, 1987).
These pathways include hydroxylation, and conjugation with thiols and
other water-soluble derivatives. The most important pathway seems to
be through hydroxylation and subsequent conjugation. Rats and mice
that were exposed to dichloro-, tetrachloro-, or pentachlorobiphenyls
by intraperitoneal injection or diet, eliminated metabolites as
glutathione conjugates and other sulfur-containing compounds (Kurachi,
1983; Kurachi & Mio, 1983). Mammalian metabolism of many individual
PCBs may proceed via oxide intermediates, which have not been
isolated, but are presumed to be precursors of some of the major
metabolites identified. One type of metabolite is the methylsulfone
PCB metabolite that has been identified in environmental samples by
Jansson et al. (1975) and WHO/EURO (1987), and in human milk by
Yoshida & Nakamura (1979).
The formation of xenobiotic thioether derivatives, including
glutathione, cysteinylglycine, cysteine, and N-acetylcysteine
(mercapturic acid) conjugates, is generally considered a pathway for
the detoxification of reactive intermediates. Mio & Sumino (1985)
detected methylsulfonyl metabolites, by using GC/MS/COM, from the
adipose tissues of mice treated with Kanechlor 300, 400, 500, or
2,5,2',5'-tetrachlorobiphenyl. Metabolites were detected in the faeces
of mice treated with 2,5,2',5'-tetrachlorobiphenyl, e.g., 6
sulfur-containing and 5 non-sulfur-containing metabolites. The
elimination rates for one week were 1.7% and 43%, respectively. The
methylsulfonyl metabolites accumulated in the liver, adipose tissue,
and lungs. Mio & Sumino (1985) proposed the methylsulfonyl metabolic
pathway of 2,5,2',5'-tetrachlorobiphenyl.
Klasson-Wehler et al. (1987) administered a single dose of 2,3,6,4'-
tetrachlorobiphenyl to 3 groups of 5 female C57B1 mice at 0, 10, or
100 mg/kg body weight. 35S-cysteine was administered by ip injections
4 times at 12-h intervals. The animals were sacrificed and the organs
analysed on day 12. Methyl [35S]sulfonyl-tetrachlorobiphenyl was
found in the lungs, kidneys, and fat of the treated mice, as well as
minor amounts of tetrachlorobiphenyl and traces of
methylthiotetrachlorobiphenyl.
The formation of serial methylsulfonyl metabolites can be summarized
as follows: the glutathione conjugate is converted, by cleavage, to a
cysteine or thiol conjugate and translocated into the liver. The thiol
conjugate from the cysteine moiety is transmethylated by
thiol- S-methyltransferase and is oxygenated by cytochromes P-450 and
P-448 oxidase or is glucuronidated by UDP-glucuronyl-transferase in
the liver, resulting in methylsulfonyl derivatives.
Table 26. Metabolism of various PCBs in different organisms
Compound Species
Chlorobiphenyl Trout Pigeon Mouse Rat Rabbit Monkey
4-mono- - + + +
4,4'-di- - + + +
2,2' +
2,4' + +
2,5,2'-tri +
2,4,2',4'-tetra- +
2,5,2',5' - - + + +
3,4,3',4' -
2,3,4,5,6,-penta- +
2,4,5,2',5' + +
2,4,6,2',4' +
2,4,6,2',6' +
2,4,6,3',5' +
2,3,4,6,4' +
2,3,4,3',4' -
2,4,5,2',4',5',-hexa- - - (±)a +
2,4,6,2',4',6' (±)a
2,3,5,6,2',3',5',6'-octa- -
2,3,4,5,6,2',3',4',5',6'-deca -
a These compounds were reported by Sundström et al. (1976a) as failing to produce
hydroxylated derivatives, but were positive in an IARC report referred to in an
NAS report (NAS, 1979).
+ = Compound is metabolized; - = Compound not metabolized: (blank) not tested.
 The occurrence of trans-dihydrodiol metabolites suggests that the
metabolism of PCBs proceeds through the formation of arene oxide
intermediates (US EPA, 1987). Arene oxides are potential electrophiles
that have been implicated in cellular necrosis, mutagenicity, and
carcinogenicity (Safe et al., 1975; Sundström et al., 1976a).
While the arene oxide pathway is important in carcinogenic
considerations, it may not be the primary pathway for the metabolism
of PCBs, in most cases. So far, most discussions about the metabolism
of PCB isomers have focused on the position and number of the chlorine
substituents.
The metabolic products of dichloro-, trichloro-, tetrachloro-,
pentachloro-, and hexachlorobiphenyl appear to reflect direct
hydroxylation at the meta and/or para positions, relative to the
position of the phenyl-phenyl bond. In a few instances, a methoxy
group is found instead of a second hydroxyl. This direct mechanism
appears to operate, therefore, irrespective of the degree of
chlorination, and, for the most part, irrespective of the position of
the chlorine substituents. In the rabbit, some exceptions have been
found that involve: a chlorine shift and the removal of a chlorine, in
the case of the 4,4'-dichlorobiphenyl, the formation of a dihydrodiol
at the meta and para positions, in the case of 2,5,2',5'-tetra-
chlorobiphenyl, and the removal of a chlorine from one of the rings,
in the case of 2,4,5,2',4',5'-hexachlorobiphenyl (Sundström et al.,
1976a).
Studies carried out by Matthew & Anderson (1975b) and Tuey & Matthews
(1977) showed that monochloro- and dichlorobiphenyls were rapidly
metabolized and excreted and that pentachloro- and hexachlorobiphenyls
were poorly metabolized and retained longer in the adipose tissue and
skin. The situation for the tetrachlorobiphenyls is more complicated.
The following analysis is based on the data on metabolites identified
and reported in the review by Sundström et al. (1976a).
6.5.2 Dichlorobiphenyls
Consideration of the various dichloro- isomers shows that, when
chlorines are only on one ring, hydroxylation occurs on the
nonchlorinated ring. Single hydroxylation occurs para to the
phenyl-phenyl bond; if another hydroxylation occurs, it is always
meta to the phenyl-phenyl bond. This holds true for the 3 different
isomers tested: 2,3-; 2,4-; and 3,4-dichlorobiphenyls. When the
dichloro-compounds are symmetrically chlorinated on each ring, as in
2,2'-; 3,3'-; and 4,4'-dichloro compounds, the same pattern applies
generally, but with a variation on the theme and an exception in the
case of 4,4'-dichlorobiphenyl. In the case of 2,2'- and 3,3'-dichloro
compounds, monohydroxylation occurs meta and para to the
phenyl-phenyl ring, respectively. In both cases, double hydroxylation
involves both meta and para positions on the same ring, (that is,
meta and para to the phenyl-phenyl bond). In the case of
4,4'-dichlorobiphenyl, there appears to be a difference in the rat.
The monohydroxy- derivative is meta, but the dihydroxy derivative is
ortho and meta to the phenyl-phenyl bond. Not only is the rat
metabolism of the 4,4'-compound an exception, but the rabbit also
shows an unusual response to this compound. In the rabbit, the
monohydroxy-derivative is the same meta hydroxy found in the rat,
but, instead of a dihydroxy- compound, the rabbit produces a chlorine
shift and a single hydroxy group in the para position as well as
dechlorination and hydroxylation in the para position. These latter
products have been considered as characteristic of the arene oxide
intermediate pathway. Nevertheless, among the 6 different dichloro-
isomers examined, all produced a monohydroxy- derivative, either meta
or para to the phenyl-phenyl bond, and all but the 4,4' produced
dihydroxy- derivatives were meta and para on the same ring to the
phenyl-phenyl bond.
The absence of a substitution at 4,4'- with vicinal unsubstituted
positions cannot be correlated with rapid metabolism, since this
property is also shared by both a rapid and slowly metabolized isomer.
This is in direct contradiction to the often repeated statement "the
presence of at least two adjacent unsubstituted carbons, particularly
in positions 3,4-, or 5- or 3',4'- or 5'- is required for rapid
metabolism of chloro-biphenyl" (Jensen & Sundström, 1974b; Berlin et
al., 1975; Safe et al., 1975; Matthews & Anderson, 1976; NIOSH, 1977;
Matthews & Tuey, 1980).
6.5.3 Tetrachlorobiphenyls
Examination of the 2 different tetrachloro- isomers, 2,3,5,6,-
tetrachloro- and 2,5,2',5',-tetrachlorobiphenyl, showed that, in the
case of the molecule with all 4 chlorines on one ring, the products
were monohydroxy- derivatives meta or para, dihydroxy- derivatives
meta and para, and a para hydroxy- plus a meta methoxy- group
or a meta hydroxy- and a para methoxy- group, all on the
unsubstituted ring. The symmetrical 2,5,2',5'-tetrachlorobiphenyl in
the rat gave the meta hydroxy-, but in the rabbit a para hydroxy-,
and also in the rabbit a dihydro-dihydroxy-, on the meta and para
positions. The asymmetric 2,4,3',4'-tetrachlorobiphenyl gave
monohydroxy-derivatives, both in the meta position, either in the
three or five position. It seems that an alternative enzyme pathway is
available in the rabbit.
The level of retention was highest for 2,4,2'4'-tetrachlorobiphenyl
descending in the following order; 2,5,2',5'-, 3,5,3',5'-, 3,4,3',4'-,
2,3,2',3'-, and 2,6,2',6'-tetrachlorobiphenyl. Since no PCBs were
detected in the liver, and only small amounts of 2,3,2',3'- and
3,4,3',4'-tetrachlorobiphenyls in the carcass, it can be concluded
that these 2 isomers were readily metabolized and excreted. Both
compounds have unsubstituted vicinal positions. However, the compounds
slowest to be metabolized were 2,4,2',4'- and 2,5,2',5'-tetra-
chlorobiphenyls, which also have unsubstituted vicinal positions.
Metabolic restriction cannot be entirely attributed to substitution at
the 4,4'- positions (Kato et al., 1980), since this also occurred in
3,4,3',4'-tetrachlorobiphenyl, which was removed relatively rapidly.
The excretion of the monohydroxy metabolites of 3,4,3',4'-tetra-
chlorobiphenyl and 2,4,3',4'-tetrachlorobiphenyl (orally administered)
in rats has been demonstrated by Yoshimura et al. (1973); Yamamoto &
Yoshimura (1973); Yoshimura & Yamamoto (1975); and Yoshimura et al.
(1974). They demonstrated that the metabolites of the first isomer
were 2-hydroxy- or 5-hydroxy- compounds, while the metabolites of the
second isomer were 5-hydroxy- and 3-hydroxy- compounds. All hydroxy
metabolites were excreted non-conjugated via the bile and no parent
isomers were found in the bile. Yoshimura & Yamamoto (1975) found that
unchanged 2,4,3',4'-tetrachlorobiphenyl was excreted through the
intestine, when it was intravenously injected in rats with the bile
duct ligated, while no metabolite of this isomer was excreted by this
route.
The results with the 2,5,2',5'- molecule are probably related to an
arene oxide pathway. Direct evidence for this was reported by Forgue
et al. (1980), who showed that 3,3,3,-trichloropropene-1,2-oxide,
which is an inhibitor of epoxide hydrase, blocked the formation of the
suspected arene oxide metabolites. The arene oxide mechanism
supposedly operates in rabbits and monkeys for 2,5,2',5'-tetrachloro-
biphenyl and, possibly, also in rats for 2,4,5,2',4',5'-hexachloro-
biphenyl. Isomers that may utilize the arene oxide pathway, to some
extent, are: 4,4'-dichloro-; 2,5,2',5'-tetrachloro-; and
2,4,5,2',4',5'-hexachlorobiphenyl.
6.5.4 Hexachlorobiphenyls and higher chlorinated compounds
The symmetrical hexachlorobiphenyls were used in a study by Matthews &
Tuey (1980), in which Sprague-Dawley rats were injected intravenously
with the PCBs and killed at increasing time intervals from 15 min to
42 days. The 2,3,6,2',3',6'- isomer was rapidly metabolized and
excreted compared with the other isomers, which were slowly
metabolized and excreted with much longer half-lives. The results
indicated that the metabolism of hexachlorobiphenyls is slow, when the
position of the chlorine atoms is such that arene oxide formation is
inhibited.
2,3,6,2',3',6'-Hexachlorobiphenyl produces only one metabolite in the
rat: 2,3,6,2',3',6'-hexachloro-4'-hydroxybiphenyl, which is believed
to be the result of arene oxide formation. All commercial mixtures of
PCBs will contain congeners that could be metabolized via the arene
oxide pathway. However, it does not seem to be the major pathway of
metabolism for most of the components of the commercial products,
since most of the higher congeners will not have vicinal unsubstituted
carbons.
The metabolic data on individual isomers shows that, at least up to
hexachloro- compounds, ordinary hydroxylation can take place. It is
reasonable to consider that it is not only as a consequence of poor
metabolism that pentachloro- and hexachloro- compounds are persistent
in the tissues, but rather that they are not metabolized as readily,
because they are sequestered from tissues in which the bulk of the
metabolism takes place. In support of this position, it has been shown
by Matthews & Anderson (1975b) that, when animals are caused to lose a
substantial portion of body weight, the stored higher chlorinated
compounds can, indeed, be metabolized. Octachloro- and
decachlorobiphenyls would not be expected to be easily metabolized,
simply because there are few or no sites for hydroxylation to take
place. It was found (Vodicnick & Lech, 1980; Vodicnick et al., 1980)
that almost the entire body burden of 2,4,5,2',4',5'-hexa-
chlorobiphenyl was removed from mothers given this PCB and that it was
transferred to their offspring via the nursing mother's milk. In mice,
the preferential distribution of this PCB in milk reflects the high
fat content of mouse milk.
Virgin, female, Sprague-Dawley mice, injected ip with 50 or 100 mg
[14C] 2,4,5,2',4', 5' -hexachlorobiphenyl/kg body weight in corn oil
for 2 weeks, prior to mating, eliminated virtually their entire body
burden of the compound through milk during one lactation cycle
(Gallenberg & Vodicnick, 1987).
Storage is caused by lack of metabolism and also implies that the
availability of adjacent unsubstituted carbons is the determinant for
metabolism. Direct hydroxylation reactions do not require
unsubstituted adjacent carbons. Rapid storage in fat is the
rate-limiting factor in the removal of most PCBs, with the exception
of isomers that might be very rapidly metabolized by arene oxide
formation, such as 2,3,6,2',3',6'-hexachlorobiphenyl.
Matthews & Anderson (1975b) extended their study to include a fasting
period to reduce the weight of the test rats and showed that severe
fasting mobilized stored PCBs and brought them into the metabolic
pool.
6.5.5 Retention and turnover
Mizutani et al. (1977) studied the pharmacokinetic behaviour of 6
different tetrachlorobiphenyls. They administered mice the 6 isomers,
at 10 mg/kg body weight, for 20 days. The isomer concentrations in the
liver and the remainder of the carcass were determined at various
times during the recovery period. They found that the accumulated body
burden was a function of both storage ratio and biological half-life.
The results of Mizutani et al. (1977) suggest that the correlations
claimed between the position of the chlorine substituents and storage
or metabolic activity (Kato et al., 1980; Matthews & Tuey, 1980) are
not simply explained.
The hypothesis that the position of the chlorine atoms alone
determines the rates of metabolism, accumulation, and excretion does
not appear to be entirely supported. This idea has been used to
support the notion that PCBs with unsubstituted vicinal carbon atoms
favour metabolism by arene oxide formation.
6.5.6 Appraisal
The results of most studies suggest that PCBs are absorbed by the
organ systems (gastrointestinal tract, lung, and liver), representing
the likely routes of entry into the body. This is particularly true
for the gastrointestinal tract where absorption is rapid. PCBs, once
absorbed, are usually distributed in a biphasic manner and are rapidly
cleared from the blood and accumulated in the liver and adipose
tissue, or they can be metabolized in the liver, to form metabolites
that are excreted in the urine and bile. In some studies on humans,
the skin, an organ rich in adipose tissue, had a high PCB content,
whereas the brain content was low. This distribution can also include
the fetus and human milk, an extension of the adipose tissue system in
the body. Mobilization of PCBs from fat appears to depend on their
rates of metabolism. Metabolic pathways include hydroxylation, and
conjugation with thiols and other water-soluble derivatives, some of
which can involve reactive intermediates, such as the arene oxides.
The most important pathway seems to be hydroxylation and subsequent
conjugation. This pathway is facilitated by the presence of at least
one pair of unoccupied vicinal carbon atoms in the PCB structure.
Persistence in tissue is not correlated with high toxicity.
Differences in toxicities among PCBs may be associated with specific
metabolites and/or their associated intermediates.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Toxicity for microorganisms
7.1.1 Freshwater microorganisms
Zullei & Benecke (1978) used the motility of filamentous blue-green
algae (Cyanophyceae) of the genus Phormium as a bioassay for the
rapid determination of the toxicity of various compounds. They tested
the relative toxicity of purified, specific chlorobiphenyls and of PCB
commercial mixtures (Clophen). Inhibition of motility was greatest in
the presence of chlorobiphenyls of low chlorination. All mono- and
dichlorinated isomers were inhibitory at the test concentration of
100 µg per test spot of algae. Tetra- and hexachlorobiphenyls did not
have any effects; the effects of trichlorobiphenyl isomers varied, the
2,5,2' isomer not producing any effects and the 2,4,4' and 3,4,4'
isomers being inhibitory. Tests using the commercial mixtures
confirmed the greater toxicity of low chlorination levels; Clophen A30
was more toxic than Clophen A60, though the presence of more than
expected, low-chlorinated compounds in the Clophen A60 reduced the
difference in toxicity.
Cultures of the green alga Chlorella pyrenoidosa were incubated with
1 mg/litre of Aroclors 1242, 1254, and 1268 (Hawes et al., 1976a).
The initial culture was 5 days old with a cell density of 27 ×
106 cells/ml. After 8 h of incubation with the PCBs, cell densities
in the cultures were 64% lower than controls for Aroclor 1242, 45%
lower for Aroclor 1254, and 36% lower for Aroclor 1268. As the study
progressed, the cell densities in the cultures improved relative to
the controls; cell density in the Aroclor 1254 culture was equal to
that in the control by 129 h and the density in the Aroclor 1268
culture was equal to the control by 59 h. Although the density in the
Aroclor 1242 culture remained much lower than that in the control
throughout the culture period, there was evidence of recovery with
this compound. The toxicity of the Aroclors was inversely proportional
to their degree of chlorination. A concurrent investigation of the
primary productivity of the alga (Hawes et al., 1976b), suggested that
the productivity of individual cells was stimulated by the Aroclors,
with a positive relationship between the level of chlorination and the
effect. However, this did not take into account the differences in
density between cultures (PCBs reduce growth through cell division)
and the authors pointed out that the response of the alga to the PCBs
was not simple. They stated that the density, culture age, and Aroclor
type were all factors that influenced response.
Larsson & Tillberg (1975) cultured the green alga Scenedesmus
obtusiusculus, in a liquid medium, in concentrations of Aroclor 1242
ranging between 10 and 1000 µg/litre. Growth was reduced at
concentrations of 300 µg Aroclor/litre or more; viability was only
affected at the highest concentration of 1000 µg/litre. Reduced
phosphate uptake, which was nearly identical in light or in darkness,
was reduced from 300 µg/litre upwards. The authors regarded this as a
result of an effect on the plasmalemma. At 800 µg PCBs/litre, the
results of some studies suggested an effect of the uncoupling of
oxidative phosphorylation; at 1000 µg/litre, both respiration and
oxygen evolution were inhibited.
The effects of various Aroclors on the respiration and photosynthesis
of the green alga Chlorella vulgaris were examined by Sinclair et
al. (1977). Aroclor 1221, at a final concentration in the medium at
10-4 mol/litre (= 192 mg/litre based on an average relative molecular
mass for the Aroclor at 192), produced a rate of oxygen production in
the light of 43% of control levels and a rate of oxygen uptake in the
dark of 59% of control levels. The Aroclors were dissolved using
dimethylformamide (DMF) as a solubilising agent; whilst this had some
effects on the parameters measured, they were very little compared
with the effects of the Aroclors (94% of control levels). A
dose-response curve of the effect of Aroclor 1221 on the respiratory
uptake of oxygen in the dark, in the presence of glucose, showed that
there was already a marked effect at a concentration of
10-7 mol/litre and that a maximum (at 50% inhibition) was reached at
a concentration of 10-6 mol/litre (= 1.92 mg/litre).
A further series of studies was performed to investigate the
individual processes of oxygen exchange in the alga. Respiration in
the dark was investigated in the absence of added glucose, to monitor
"endogenous" respiration, which was found to be stimulated by Aroclor
1221 at concentrations of 10-4 mol/litre or more. Net oxygen
production in the light (photosynthetic oxygen production minus
respiratory oxygen usage) was reduced at a concentration of
10-4 mol/litre. At this concentration of Aroclor 1221, there was
approximately 50% stimulation of endogenous respiration and
approximately 50% inhibition of net oxygen production. Calculated
photosynthetic rate was unaffected by the Aroclor. Increasing light
intensity reduced the effect of Aroclor 1221 on net oxygen production;
intensities of 8.2 × 104 ergs/cm2 virtually eliminated the effect.
Other measures directly or indirectly associated with photosynthesis
(fluorescence, oxygen evolution in flashing light, the Emerson
enhancement phenomenon) were not affected by the Aroclor. The authors
suggested that the photosynthetic apparatus of Chlorella was
unaffected by Aroclor 1221; the major, and probably the only, effect
of the PCBs being a stimulation of endogenous respiration rate.
Results with Aroclors 1242 and 1268 were consistent with those for
Aroclor 1221; both inhibited net oxygen production in the light and
glucose-driven respiration in the dark, but stimulated endogenous
respiration in the dark (Sinclair et al., 1977).
Luard (1973) reported inhibition of 14C uptake by the green alga
Scenedesmus quadricauda at concentrations of Aroclor 1254 as low as
0.1 µg/litre. At this concentration, the Aroclor caused a 20%
inhibition of 14C uptake, which rose to 65% inhibition at 1 mg/litre.
A marked effect of the initial number of cells in the incubation tube
on the toxicity of Aroclor 1254 for the green alga Chlorella
pyrenoidosa was demonstrated by Cole & Plapp (1974). At a constant
concentration of 1 mg Aroclor 1254/litre, the numbers of algal cells
in the initial incubation medium were as varied as 1, 10, 100, or
1000 µg alga/ml of medium. At the highest inoculation rate, the growth
of the alga was unaffected by the Aroclor. At lower initial
inoculation, the rate of growth was reduced to between 11 and 55% of
control levels. A comparable effect was found using 14C fixation as
the parameter; with inoculation rates of 1000 µg alga/ml medium, the
Aroclor did not have any effects, whereas lower inoculation rates
reduced carbon fixation to between 6 and 13% of control levels.
Mosser et al. (1972) showed that 2 species of freshwater algae
(Euglena gracilis and Chlamydomonas reinhardtii) were unaffected
by 100 µg PCBs/litre (type unspecified). Ewald et al. (1976)
determined the 48-h EC50 on the growth of Euglena gracilis to be
4.4 mg/litre for Aroclor 1221 and 55 mg/litre for Aroclor 1232.
Aroclor 1242 showed no inhibition at 100 mg/litre. Aroclor 1221, at
the EC50 concentration of 4.4 mg/litre, significantly depressed
carbon fixation and chlorophyll levels, but did not affect oxygen
consumption. Uptake of L-leucine was increased 2-fold, but
incorporation was not affected. Uridine uptake was significantly
decreased, but thymidine uptake and incorporation were not affected.
In studies by Glooschenko & Glooschenko (1975), 3 algal species from
the Great Lakes were cultured with Aroclor at concentrations of 1, 5,
10, 20, or 50 µg/litre. Cell numbers of the diatom Synedra acus were
reduced in culture from day 3 of treatment with Aroclor 1242, at 10,
20, or 50 µg/litre, and from day 7, with 1 or 5 µg/litre. The green
alga Scenedesmus quadricauda showed a lag phase of 3 days in all
concentrations of Aroclor 1242. After 3 days, exponential growth
occurred in all treatments, except for the highest dose levels (20 and
50 µg/litre) which showed little or no cell division. A second green
alga (Ankistrodesmus falcatus) was more sensitive, showing
significantly reduced cell numbers at all dose levels of Aroclor 1242.
Ankistrodesmus was used to examine the relative toxicities of
different Aroclors. Cell numbers were 57, 66, 36, and 53% of control
levels for Aroclors 1016, 1221, 1242, and 1248, respectively, after 2
days of culture. Carbon fixation, estimated as uptake of 14C from
solution, was 73, 98, 51, and 59% of control levels for the 4
Aroclors, respectively.
Dive et al. (1976) cultured the ciliate protozoan Colpidium campylum
with purified chlorobiphenyls and with a commercial mixture (Pyralene
3010). None of the 16 isomers affected the ciliate's growth and
reproduction at concentrations of 0.01 or 0.1 mg/litre; similarly,
Pyralene 3010 was not toxic at these concentrations. 2-Mono-
chlorobiphenyl showed little toxicity for the organism at 1 mg/litre
and little or no toxicity was demonstrated by the tetrachloro-,
pentachloro-, and hexachlorobiphenyls at this concentration or at
10 mg/litre (with the exception of 2,5,2',5'-tetrachlorobiphenyl,
which inhibited growth considerably, limiting it to about 10% of
controls at 10 mg/litre). 4,4'-Dichlorobiphenyl was not toxic at any
concentration tested (up to 10 mg/litre) but both 2,3- and
2,5-dichlorobiphenyls were toxic, killing all organisms at both 1 and
10 mg/litre. These results are comparable with other reports that the
lower chlorinated biphenyls are the most toxic for microorganisms;
differences in toxicity could not be explained by the differential
uptake of the different isomers.
It was reported by French (1976) that the EC50 for growth inhibition
of Aroclor 1254 on the flagellated protozoan Crithidia fasciculata
was 10.5 mg/litre. The PCBs slightly inhibited (after 6 h) and then
increased (after 24 h) the carbon dioxide evolution of cultures
utilizing D-glucose. After 6 h exposure, the uptake and incorporation
of thymidine and uridine (but not L-leucine) were inhibited;
inhibition was transient and returned to normal after 12 or 24 h. Fine
structural changes were noted after exposure to PCBs including:
deterioration of the kinetoplast, mitochondrial or cellular swelling,
and the presence of concentric membrane arrays. It was concluded that
cell population growth inhibition was due to disruption of uptake,
incorporation of nucleic acids, and loss of cell regulatory capacity.
7.1.2 Marine and estuarine microorganisms
Bourquin & Cassidy (1975) and Bourquin & Kiefer (1975) investigated
the effects of Aroclors 1016 and 1242 on 85 bacterial isolates from
various estuarine environments near Pensacola, Florida. Twenty six of
the 85 isolates were inhibited to various extents by 0.5 mg of either
of the PCBs, applied to a disc placed on the surface of an agar plate
on which the bacteria were growing. Zones of inhibition ranged from 14
to 20 mm in diameter. Cultures that showed sensitivity to Aroclor 1242
were also inhibited by Aroclor 1016. Sixty five percent of isolates
inhibited by 0.5 mg of Aroclor 1242 were still sensitive at 0.1 mg,
and 58% of those sensitive to Aroclor 1016 at 0.5 mg were still
sensitive to 0.1 mg. Four isolates were examined in a liquid medium.
Inhibition of the cultures was characterized by a greatly extended lag
phase (extended from 2 h to at least 14 h); when growth occurred,
growth curves were parallel with those of the controls. The
physiological activity of sensitive and insensitive isolates were
investigated to try to explain the reasons behind sensitivity. More of
the sensitive isolates were amylase and gelatinase producers (76 and
86%, respectively, compared with 33 and 42%, respectively, for the
whole range of isolates). The significance of this observation is
unclear. Because of the method of exposure in this screening exercise,
it is difficult to relate the results to exposure in natural waters
and to draw conclusions about likely hazards for aquatic bacteria.
Kleppel & McLaughlin (1980) determined the toxic threshold of Aroclor
1254 for the estuarine diatom Skeletonema costatum to be between
3 × 10-9 and 3 × 10-8 µg/cell. When the effect of cell density on the
toxicity of the Aroclor for the organism was examined, maximum
inhibition occurred with the lowest inoculum rates.
Michaels et al. (1982) estimated the effects of Aroclor 1254 on
photosynthesis in the marine diatom Thalassiosira pseudonana
( = Cyclotella nana) by monitoring the uptake of 14C-carbon
dioxide. The numbers of viable cells in the culture were also
estimated. Total cell numbers were estimated at regular intervals
during the 48 h of the experiment and the minimum number of viable
cells required to produce the increase in numbers between periods
calculated. This gave an estimate of the viable numbers,
retrospectively, for each period. Inhibition of 14C-uptake per
culture, per cell, and per viable cell was evident within 1 h of the
start of the incubation. By 48 h, the 14C uptake per culture was
reduced to 0.2% of control levels, per cell, to 13% of control levels,
and per viable cell, to 34% of control levels. The authors concluded
that the effect of Aroclor 1254 on the diatom is a combination of
inhibition of carbon assimilation by individual cells and inhibition
of cell division.
In an earlier study, Fisher & Wurster (1973) exposed 2 estuarine
diatoms (Thalassiosira pseudonana and Rhizosolenia setigera) and
an estuarine green alga (Dunaliella tertiolecta) to Aroclor 1254 at
0.1 or 10 µg/litre, cultured over 100 h. There was no effect on the
growth of the alga. T. pseudonana was unaffected by the PCB at
0.1 µg/litre. The effect of the Aroclor on T. pseudonana at
10 µg/-litre was dependent on temperature; there was a 17% reduction
in growth rate at 25°C, a 44% reduction in growth rate at 18°C, and a
58% reduction in growth rate at 12°C. The growth of control cultures
was greatest at the highest temperature. The growth of R. setigera
was completely stopped by all concentrations of PCB tested, for the
first 48 h of culture. When growth resumed, the degree of inhibition
was greater at 10°C than at 15°C. Fisher et al. (1976) exposed the
marine diatom Thalassiosira pseudonana to 1 µg Aroclor 1254/litre in
cultures containing various levels of nitrate nutrient. The toxic
effects of the PCBs on the growth of the diatom were greatest at low
nitrogen levels. Analysis of variance showed the diatom to be
significantly dependent for growth on nitrogen concentration and on
PCB concentration, and that the PCB effect was significantly nitrogen
dependent. The authors pointed out that marine phytoplankton are often
nitrogen-limited in nature and suggested that the effects of
pollutants, such as PCBs, may, therefore, vary with season. The
greatest effects are likely to occur during bloom conditions, when
competition for nutrients is greatest. Fisher (1975) considered that
the presence of PCBs, even at concentrations far above the maximum
recorded level in sea water, would not affect the overall carbon
fixation by phytoplankton. Although the cell division of some
organisms was adversely affected, enough insensitive species existed
to compensate for the sensitive species. Species diversity and
community structure were likely to be affected.
In a study by Craigie & Hutzinger (1975), 6 marine algae were cultured
for 6 days in the presence of commercial mixtures of PCBs (Aroclors
1221 to 1262; Phenoclors DP3 to DP6), PCTs (Aroclor 5460), and
specific chlorobiphenyls. Each compound or mixture was applied to the
culture medium at 2 concentrations (1 and 100 mg/litre). The algae
were representatives of 6 different classes: Bacillariophyceae -
Skeletonema costatum, Thalassiosira fluviatilis; Chlorophyceae -
Dunaliella tertiolecta; Chrysophyceae - Monochrysis lutheri;
Prainophyceae - Platymonas sp.; Rhodophyceae - Porphyridium sp.;
Xanthophyceae - Olisthodiscus sp. All were cultured at 20°C. Growth
was estimated by turbidity. The response to particular Aroclors was
species dependent; the least sensitive species was Dunaliella and
the most sensitive was Olisthodiscus. At 1 mg/litre of the PCB
mixtures, there was relatively little inhibition of Dunaliella,
Platymonas, Skeletonema, or Thalassiosira. Olisthodiscus was
completely inhibited by Aroclors 1248, 1254, 1260, and Phenoclor DP4.
The Phenoclor series was more toxic for Olisthodiscus. than the
Aroclors. Porphyridium and Monochrysis showed intermediate
sensitivity. Generally, the Aroclors with higher chlorination levels
were less toxic for all species than the Aroclors with lower
chlorination levels. Results with pure, specific chlorinated biphenyls
confirmed that highly chlorinated compounds are less toxic than those
with only 1-4 chlorine atoms per molecule. Experiments with biphenyls
containing identical percentages of chlorine showed that biological
response was highly dependent on the structure of the molecule. The
2,4,2',4'-tetrachlorobiphenyl was more toxic for both Dunaliella and
Olisthodiscus than the 2,5,2',5'- isomer, and both were more toxic
than either the 2,3,4,5- or the 3,4,3',4'- isomers. The last was not
toxic for any of the algae, even when added at 100 mg/litre.
Luard (1973) reported a significant reduction in 14C uptake by the
estuarine green alga Dunaliella tertiolecta in the presence of
Aroclor 1254, at 100 µg/litre. The culture showed 14C uptake at 65%
of control levels. At 1000 µg/litre, uptake was further reduced to 59%
of controls. The 14C uptake was unaffected at 10 µg/litre.
7.1.3 Soil microorganisms
When Murado et al. (1976) added Aroclors to a liquid or solid medium
on which the soil microfungus Aspergillus flavus was cultured,
mycelial growth was reduced progressively as the dose of Aroclor 1254
increased from 5 to 50 mg/litre in liquid culture. At 25 mg/litre, the
dry weight of the mycelium was reduced to 1.4, 3.4, 3.9, 3.3, and
54.6% of control levels by Aroclors 1232, 1242, 1248, 1254, and 1260,
respectively. At the same time, the relative RNA content of the
mycelium increased, rising from a control level of 5.9 µg RNA/mg dry
weight to between 13.2 and 18.6 µg RNA/mg dry weight for Aroclors 1232
to 1254. Aroclor 1260 had no marked effect on RNA. The DNA content was
not affected by any treatment. Cultures on solid medium showed a delay
in sporulation and a decrease in the diameter of colonies at doses up
to 20 µg/cm2.
Glooschenko & Glooschenko (1975) cultured the soil alga Navicula
pelliculosa with Aroclors 1016, 1221, 1242, and 1248 at a
concentration of 20 µg/litre. The numbers of cells in the culture,
after 2 days, were reduced to 66, 53, 46, and 56% of control levels
for the 4 Aroclors, respectively.
7.1.4 Plankton communities
Phytoplankton communities from 2 lakes, one oligotrophic and one
eutrophic, were exposed to PCBs, as Clophen A50, at 26 µg/litre
(Södergren & Gelin, 1983). In the community from the eutrophic lake
with a greater biomass of phytoplankton, measurement of 14C uptake,
monitored immediately after the addition of the PCBs, showed a
reduction of 34% compared with the controls. Monitoring carbon
fixation 16 h later showed the phytoplankton recovering from the
effect of the PCBs, with only 21% inhibition relative to the controls.
The results differed in the community from the oligotrophic lake. 14C
uptake immediately after the addition of the Clophen was 70% less than
that in the controls; 16 h later, the effect was greater, at 84%
inhibition. The authors pointed out that these results parallel
findings in pure culture showing that a high density of organisms
reduces the effects of PCBs.
Mosser et al. (1972) first demonstrated the effects of PCBs on both
single and mixed cultures of marine phytoplankton. Two organisms, a
marine diatom (Thalassiosira pseudonana) and a marine green alga
(Dunaliella tertiolecta) were used. The diatom is sensitive and the
alga insensitive to PCBs. The application of PCBs (not specified) to
mixed cultures changed the usual dominance of the diatom into
dominance of the alga, even at concentrations of the PCBs (1 and
10 µg/litre) that had no discernible effects on the diatom in pure
culture.
The effect of Aroclor 1254 on the relative biomass of 2 species of
marine diatoms Phaeodactylum tricornutum and Cyclotella cryptica
was examined by Lundy et al. (1984). The diatoms were cultured
together in either 10 or 20 µg PCBs/litre for 6 days. At both
concentrations of Aroclor, the ratio between the species was shifted
in favour of Phaeodactylum. After 6 days, the ratio of
Phaeodactylum: Cyclotella was 0.8 in the controls and 2.63 in the
treated cultures (10 µg Aroclor 1254/litre).
Biggs et al. (1979) exposed a mixed culture of 2 marine algae to PCBs
(Aroclor 1254) at 50 µg/litre. In the control culture, Thalassiosira
pseudonana became the dominant organism over Dunaliella
tertiolecta. After exposure to the PCBs, the Thalassiosira was
affected, but the Dunaliella was not. By day 2 of Aroclor 1254
exposure, Dunaliella was the dominant species. Fisher et al. (1974)
showed a similar effect with the greater effects of PCBs on the
sensitive diatom Thalassiosira pseudonana when the organism was in
competition with other organisms. The effect was also demonstrated
using natural communities of phytoplankton, when Thalassiosira was
also selectively affected.
Biggs et al. (1978) exposed a natural community of marine
phytoplankton (from which large detritus and zooplankton had been
filtered) to Aroclor 1254 at either 5 or 10 µg/litre. Cell division,
chlorophyll-a synthesis, and 14C uptake were monitored, as well as
particle size. Treatment with the Aroclor reduced community growth
rates by 20-50%, compared with controls. Growth had not fully
recovered after 10 days. The 14C uptake was reduced for 6 days. The
control cultures became dominated by algae larger than 8 µm in
diameter. The PCB-treated cultures showed strong inhibition of these
larger algal cells and the culture became dominated by small cells.
In a study by Iseki et al. (1981), a natural community of plankton was
exposed, in situ, in a marine environment, to Aroclor 1254 in large
bags holding 68 m3 of seawater. The Aroclor was added to the bag
giving an initial concentration in the upper layers of about
40 µg/litre (15 µg/litre at 10 m depth). Over time, the levels of PCB
fell in the upper layer and increased in the lower layer in the bag.
Six days after addition, the concentrations at all depths were less
than 15 µg/litre. Immediately after addition of the PCBs, the rate of
sedimentation of the particles increased; these particles were thought
to be dead or senescent large cells, such as diatoms, and this
sedimentation was assumed to be responsible for the major part of the
loss of the PCBs from the water. Zooplankters were eliminated from the
bags by this level of Aroclor; no recovery was seen over the 21 days
of the experiment. Large diatoms were selectively eliminated from the
bags and replaced by small flagellates as the dominant organism.
Moore & Harriss (1972, 1974) exposed a natural community of plankton
to PCBs (as Aroclor 1242) at 10 or 25 µg/litre. The population was
collected from natural water, placed in glass bottles suspended
in situ, and monitored for uptake of 14C, added to the bottles as
bicarbonate. After incubation, the community was separated into small
"nannoplankton" and larger "net-plankton" by filtration at a mesh size
of 53 µm. Nannoplankton radiocarbon uptake accounted for 72.6% of the
total community carbon uptake and was not affected by either of the
concentrations of Aroclor 1242. The net-plankton uptake of 14C was
reduced by 56% at 10 µg Aroclor/litre and by 58% at 25 µg/litre. The
authors suggested that PCBs at levels found in natural waters would
alter the species diversity or community structure of microorganisms
and that this might affect higher levels of the food chain with
specialist feeders utilizing one type of prey. O'Connors et al. (1978)
exposed natural communities of phytoplankton, in situ, in dialysis
bags in a salt marsh. Large zooplankton herbivores were removed by
filtration through a mesh. Aroclor 1254 was added to the bags to give
water concentrations of 1-10 µg/litre. Larger diatoms were selectively
inhibited by the Aroclor, even at the lower dose (1 µg/litre). The
authors suggested that, not only would phytoplankton communities be
affected by PCBs in natural waters, but that the effect would be
carried forward through the food chain. Gelatinous predators, such as
jellyfish, could be selected at the expense of fish, since fish tend
to depend directly, or indirectly, on the larger phytoplankton.
Natural communities of phytoplankton from a stream and a reservoir
were cultured with Aroclors 1232 and 1254 by Kricher et al. (1979).
Although the algal communities were different in composition, both
Aroclors exerted similar effects on both communities; primary
productivity was reduced in a dose-dependent manner. Aroclor 1232 was
more toxic than Aroclor 1254 at 1 mg/litre. Algal species within the
populations were differentially affected by the Aroclors; diatoms were
particularly susceptible and treatment produced disproportionate
numbers of blue-green algae, such as Anacystis. The authors pointed
out that the insensitive species still accumulated PCBs and,
therefore, formed the basis for the accumulation of the Aroclors in
aquatic food chains.
7.1.5 Interactions with other chemicals
Mosser et al. (1974) investigated the interactions between Aroclor
1254, DDT, and DDE in a marine diatom Thalassiosira pseudonana. The
diatom was cultured for 4 days with either 10 or 50 µg Aroclor
1254/litre, 100 µg DDE/litre or 500 µg DDT/litre (with each chemical
alone or in combination). The PCBs alone (at 10 µg/litre) and the DDE
alone had little effect on the growth of the diatom; when combined
these treatments were synergistic, growth being less than half that of
the control culture. Higher concentrations of either compound
increased the inhibitory effect. In contrast, DDT reduced the toxic
effects of PCBs at higher concentrations (50 µg/litre). Treatment with
the Aroclor alone at 50 µg/litre almost stopped the growth of the
diatom. Simultaneous treatment with DDT at 500 µg/litre restored
growth to 60-70% of control levels. Lower concentrations of DDT had a
comparable, but reduced, effect. Addition of the DDT to the medium, 12
or 24 h after culture had begun in the presence of PCBs, also reversed
the inhibitory effect.
7.1.6 Tolerance
Fisher et al. (1973) showed that strains of diatoms, isolated from the
Sargasso Sea, were more sensitive to the effects of PCBs than isolates
of the same species, obtained from estuaries or the continental shelf.
It was suggested by the authors that the difference in sensitivity was
derived from the variable environment of the estuarine strains; these
strains are able to cope with wide variations in their living
conditions, not experienced in the open ocean of the Sargasso, and,
therefore, were better able to cope with stress from chemical
pollutants.
When Cosper et al. (1984) compared the sensitivity of clones of 2
species of diatom (Asterionella japonica and Ditylum brightwelii)
from polluted and unpolluted sites, Asterionella was less sensitive
than Ditylum to the action of PCBs (Aroclor 1254); some strains of
the former were tolerant of 25 µg Aroclor/litre whereas no strains of
the latter could tolerate this concentration. There was evidence that
strains of Asterionella from the polluted site were more tolerant
than the same species from unpolluted sites. One strain, from the
polluted site, was tolerant to Aroclor 1254 at 50 µg/litre.
7.2 Toxicity for aquatic organisms
7.2.1 Aquatic plants
Mahanty (1975) grew the aquatic angiosperm Spirodela oligorhiza in a
sterile culture solution, to which had been added Aroclor 1242, at
concentrations of 5-100 mg/litre. The numbers of colonies were counted
throughout the 14-day exposure. The highest dose (100 mg/litre) was
found to be lethal. At both 25 and 50 mg/litre, though there was some
growth, the colonies were small and showed morphological differences
from control colonies including: smaller fronds, in larger numbers
than usual, and a characteristic striped pattern of chlorosis on the
fronds. Even at 5 mg/litre, growth was reduced by 50% (as recorded by
the number of colonies and the fresh weight). Mahanty & McWha (1976),
using only the 5 mg/litre dose, found reduced growth, an unusual
striped pattern of chlorosis, and a reduction in the levels of
chlorophyll and total RNA, but no change in the levels of DNA. When
Mahanty & Fineran (1976) studied treated (5 mg/litre) and untreated
fronds of Spirodela by electron microscopy, they found almost
complete disorganization of the internal structure of the chloroplasts
in chlorotic tissue. Organization of other cell components was largely
unaffected.
7.2.2 Aquatic invertebrates
The acute toxicity of PCBs for aquatic invertebrates is summarized in
Tables 27 and 28. Toxicity is very variable between species, even
closely-related species. For most aquatic invertebrates, there is an
effect of degree of chlorination of the PCBs, but this is not a direct
correlation, either negative or positive, the most toxic PCBs often
being in the mid range of chlorination. Under flow-through conditions,
the toxicity of PCBs appears to be much higher. Over 96 h, under
static conditions, LC50 values ranged between 12 µg/litre and
> 10 mg/litre for different organisms and different PCBs.
MATC (maximum acceptable toxicant concentrations) were set for various
PCBs by the US EPA (1980). These are expressed as a range between
no-observed-effect values and lowest concentration tested that
produced a measurable effect. For Daphnia magna, these values were
1.2 and 3.5 µg/litre for Aroclor 1248, and 2.5 and 7.5 µg/litre for
Aroclor 1254. For the scud (Gammarus pseudolimnaeus) values for
Aroclor 1242 were 2.8 and 8.7 µg/litre and values for Aroclor 1248
were 2.5 and 5.1 µg/litre. Aquatic larvae of the midge (Tanytarsus
dissimilis) had a no-observed-effect level of 0.5 µg/litre and a
lowest effective concentration at 1.2 µg/litre.
7.2.2.1 Short- and long-term toxicity
Roberts (1975) exposed the common mussel (Mytilus edulis) to
Aroclors 1242 and 1254 and studied byssus formation. The 24- and 48-h
EC50s for a reduction in the number of mussels byssally-attaching
were 2.2 and 3.0 mg/litre, respectively, for Aroclor 1254. EC50s
after exposure to Aroclor 1242 were 0.9 mg/litre for 24 h and
1.0 mg/litre for 48 h. Duke et al. (1970) maintained oysters
Table 27. Acute toxicity of PCB mixtures for freshwater invertebrates
Organism Size/age Stat/ Temperature Hardness pH PCB type Parameter Concentration Reference
flowa (°C) (mg/litre)b (Aroclor) (mg/litre)
Scud mature flow 15 272 7.4 1242 96 h - LC50 0.01 Mayer & Ellersieck
(Gammarus (1986)
pseudolimnaeus)
juvenile flow 18 1248 96 h - LC50 0.029 Nebeker & Puglisi
juvenile flow 18 1242 96 h - LC50 0.073 (1974)
Scud mature stat 21 44 7.1 1248 96 h - LC50 0.052 Mayer & Ellersieck
(Gammarus mature stat 21 44 7.1 1254 96 h - LC50 2.4 (1986)
fasciatus)
Glass shrimp mature flow 15 272 7.4 1254 168 h - LC50 0.003 Mayer & Ellersieck
(Palaemonetes (1986)
kadiakensis)
Crayfish early stat 21 44 7.1 1242 168 h - LC50 0.03 Mayer & Ellersieck
(Orconectes nais) instar (1986)
Crayfish early stat 21 44 7.1 1254 168 h - LC50 0.1 Mayer & Ellersieck
(Procambarus sp.) instar (1986)
immature stat 12 44 7.5 1254 96 h - LC50 > 0.55
Table 27. (cont'd)
Organism Size/age Stat/ Temperature Hardness pH PCB type Parameter Concentration Reference
flowa (°C) (mg/litre)b (Aroclor) (mg/litre)
Stonefly first stat 10 170 7.2 1016 96 h - LC50 0.61 Mayer & Ellersieck
(Pteronarcella year (0.42-0.88) (1986)
badia)
Damselfly late flow 15 272 7.4 1242 96 h - LC50 0.4 Mayer & Ellersieck
(Ischnura instar flow 15 272 7.4 1254 96 h - LC50 0.2 (1986)
verticalis)
Dragonfly late stat 21 44 7.1 1242 168 h - LC50 0.8 Mayer & Ellersieck
(Macromia sp.) instar stat 21 44 7.1 1254 168 h - LC50 0.8 (1986)
a Stat = static conditions: water not changed during the exposure; flow = flow-through conditions; concentration of
toxicant continuously maintained.
b Hardness expressed as mg CaCO3/litre, unless otherwise stated.
Table 28. Acute toxicity of PCB mixtures for marine invertebrates
Organism Size/age Stat/ Temperature Salinity PCB type Parameter Concentration Reference
flowa (°C) (%) (mg/litre)
Cockle adult stat 15 Aroclor 1248 48 h - LC50 > 10 Portmann &
(Cardium adult stat 15 Aroclor 1254 48 h - LC50 > 10 Wilson (1971)
edule) adult stat 15 Aroclor 1260 48 h - LC50 > 10
adult stat 15 Aroclor 1262 48 h - LC50 > 10
adult stat 15 Clophen A30 48 h - LC50 3.0
adult stat 15 Clophen A60 48 h - LC50 > 10
Eastern oyster adult flow 28 28 Aroclor 1016 96 h - EC50 0.01 Mayer (1987)
(Crassostrea
virginica)
Brown shrimp adult flow 30 29 Aroclor 1016 96 h - LC50 0.01 Mayer (1987)
(Penaeus
aztecus)
Brown shrimp adult stat 15 Clophen A60 48 h - EC50 > 10 Portmann &
(Crangon adult stat 15 Aroclor 1242 48 h - LC50 1.0 Wilson (1971)
crangon) adult stat 15 Aroclor 1248 48 h - LC50 0.3-1.0
adult stat 15 Aroclor 1254 48 h - LC50 3.0-10.0
adult stat 15 Aroclor 1260 48 h - LC50 > 10
adult stat 15 Aroclor 1262 48 h - LC50 > 10
adult stat 15 Clophen A40 48 h - LC50 0.3-1.0
adult stat 15 Clophen A30 48 h - LC50 1.0-3.3
adult stat 15 Clophen A50 48 h - LC50 3.3-10.0
Table 28. (cont'd).
Organism Size/age Stat/ Temperature Salinity PCB type Parameter Concentration Reference
flowa (°C) (%) (mg/litre)
Grass shrimp 1 day stat 25 25 Aroclor 1016 96 h - LC50 0.15 Mayer (1987)
(Palaemonets 3 days stat 25 25 Aroclor 1016 96 h - LC50 0.021
pugio) 6 days stat 25 25 Aroclor 1016 96 h - LC50 0.017
9 days stat 25 25 Aroclor 1016 96 h - LC50 0.019
12 days stat 25 25 Aroclor 1016 96 h - LC50 0.021
15 days stat 25 25 Aroclor 1016 96 h - LC50 0.024
18 days stat 25 25 Aroclor 1016 96 h - LC50 0.037
30 days stat 25 25 Aroclor 1016 96 h - LC50 0.044
adult stat 25 25 Aroclor 1016 96 h - LC50 0.052 (0.046-0.057)
adult flow 30 28 Aroclor 1016 96 h - LC50 0.012
1 day stat 25 25 Aroclor 1242 96 h - LC50 0.015
3 days stat 25 25 Aroclor 1242 96 h - LC50 0.019
6 days stat 25 25 Aroclor 1242 96 h - LC50 0.015
9 days stat 25 25 Aroclor 1242 96 h - LC50 0.017
12 days stat 25 25 Aroclor 1242 96 h - LC50 0.016
15 days stat 25 25 Aroclor 1242 96 h - LC50 0.024
18 days stat 25 25 Aroclor 1242 96 h - LC50 0.034
30 days stat 25 25 Aroclor 1242 96 h - LC50 0.041
adult stat 25 25 Aroclor 1242 96 h - LC50 0.057 (0.048-0.062)
a stat = static conditions: water not changed during the exposure; flow = flow-through conditions; concentration
of toxicant continuously maintained.
(Crassostrea virginica) at concentrations of 1, 10, and 100 µg
Aroclor 1254/litre and monitored shell growth over a period of 96 h;
the rates of shell growth were decreased by 19, 41, and 100%,
respectively. Lowe et al. (1972) found the growth rate (height and wet
weight) of young oysters (Crassostrea virginica) to be significantly
reduced after exposure, in flowing sea water, to 5 µg Aroclor
1254/litre, for 24 weeks. No effects on growth were observed at
1 µg/litre over a period of 30 weeks. Oysters exposed to 5 µg/litre
showed atrophy of the digestive diverticular epithelium and
degeneration of the vesicular connective tissues of the hepatopancreas
together with leukocytic infiltration. There was complete tissue
recovery after 12 weeks in clean water.
After exposing Daphnia magna to Aroclor 1254, over a period of 14
days under static renewal procedures, Maki & Johnson (1975) calculated
an LC50 of 24 µg/litre.
Nebeker & Puglisi (1974) calculated 3-week LC50 values, under static
conditions, for a range of Aroclors on Daphnia magna. Aroclors were
dissolved in acetone and triton X100 to maintain the PCBs in solution.
Tests were performed in raw Lake Superior water. Results are presented
in Table 29. The Aroclors most toxic for Daphnia had between 48 and
62% chlorination; the most toxic Aroclor was 1248. Under flow-through
conditions, renewing the original test concentration of the Aroclor
continuously, the Aroclors were much more toxic. Two-week LC50 values
for Aroclors 1248 and 1254 were 2.6 and 1.8 µg/litre, respectively,
while the 3-week LC50 for Aroclor 1254 was 1.3 µg/litre. Groups of 40
scud (Gammarus pseudolimnaeus) were exposed to various concentrations
of Aroclor 1242 under flow-through conditions. No animals survived
exposure for 2 months, at Aroclor concentrations of 26 µg/litre or
more.
Survival at lower exposures was 52% at 8.7 µg/litre and 77% at
2.8 µg/litre (control survival was low at 48%).
Duke et al. (1970) conducted acute, flowing-water bioassays on pink
shrimp (Penaeus duorarum). At 0.1 mg/litre, 100% of the shrimps were
killed within 48 h of exposure to Aroclor 1254. There was no mortality
after 48 h of exposure to 0.01 mg/litre Aroclor. In long-term
flowing-water bioassays, Nimmo et al. (1971a) found that Aroclor 1254,
at a concentration of 0.94 µg/litre killed 51% of juvenile pink
shrimps within 15 days. Juveniles were found to be more sensitive than
adults; 50% of adults were killed after exposure to 3.5 µg/litre over
35 days. There were no apparent symptoms of poisoning prior to death.
Table 29. Toxicity of various Aroclors for Daphnia magna in static testsa
Aroclor 3-week Confidence limits (95%)
LC50 (µg/litre)
1221 180 (158.0-205.0)
1232 72 (62.6-82.8)
1242 67 (55.4-81.0)
1248 25 (21.4-29.2)
1254 31 (25.8-37.2)
1260 36 (27.7-46.8)
1262 43 (37.0-49.9)
1268 253 (222.0-288.0)
a From: Nebeker & Puglisi (1974).
Striped hermit crabs (Clibanarius vittatus) were kept in static
seawater solutions containing 3, 5, 10, 15, 20, 25, or 30 µg/litre of
Aroclor 1254 for 96 h (Stahl, 1979). No deaths were reported, though
the crabs exposed to the higher concentrations (20, 25, and
30 µg/litre) were less active. Six crabs already exposed to 30 µg
PCBs/litre were then placed in solutions containing 300 µg/litre for a
further 96 h; there were still no deaths.
Vernberg et al. (1977) exposed fiddler crab (Uca pugilator) larvae
to concentrations of 0.1, 1.0, 5, 10, 50, or 500 µg Aroclor
1254/litre, for 96 h. They did not find any effects on survival at 0.1
or 1.0 µg/litre; Aroclor 1254 at 5 µg/litre increased deaths by 20%,
but the increase was not statistically significant. Exposure to 10 µg
PCBs/litre resulted in a 57% reduction in survival of larvae.
Increasing the PCB concentration to 50 µg/litre did not greatly
increase the effect. The 500 µg/litre concentration killed all larvae.
Increasing exposure time, at 5 µg/litre, produced a significant
reduction in survival after 14 days. Exposure of larvae to Aroclors
1016 or 1254 at 0.1, 1, or 5 µg/litre for periods of up to 168 h, was
then investigated. No significant effects were found on survival with
any concentration of Aroclor 1016, for up to 120 h of exposure. After
168 h, survival rates of larvae exposed to 1 and 5 µg/litre were
reduced to 61 and 66%, respectively. There was no effect at
0.1 µg/litre. Aroclor 1254 did not have any effects on survival at
concentrations of 0.1 or 1 µg/litre. Survival was reduced after
exposure to 5 µg Aroclor 1254/litre for more than 96 h, increasing
from 60-81% up to 168 h. Exposure to 10 µg Aroclor 1254/litre caused
55% deaths after 120 h; there were no further deaths after 168 h.
Fifty per cent of adult male crabs, exposed to Aroclor 1254 or Aroclor
1016 at 50 µg/litre, died after 2 days and after 4-6 days,
respectively. Females (50%) exposed to 50 µg/litre survived for 7 days
after exposure to Aroclor 1016 but for only 4 days after exposure to
Aroclor 1254.
Neff & Giam (1977) exposed juvenile horseshoe crabs (Limulus
polyphemus) to concentrations of Aroclor 1016 of 10, 20, 40, or
80 µg/litre, for up to 96 days. The crabs were divided into 2 groups:
group A consisted of juveniles at the late first tailed stage and
group B, of juveniles at the early second tailed stage. The authors
calculated LT50s (LT50: median survival time for a given exposure
concentration) of 20.8 days at 40 µg/litre and 20.3 days at
80 µg/litre for group A juveniles. The LT50 for group B crabs at
80 µg/litre was 61 days, but less than 50% had died within 96 days at
40 µg/litre.
7.2.2.2 Response to temperature and salinity
In a study by Vernberg et al. (1977), larval fiddler crabs
(Uca pugilator) were exposed to "sub-lethal" concentrations of
Aroclor 1254 and 1016 and the conditions of temperature (15-30°C) and
salinity (15-36%) varied. Exposure to 0.01 µg Aroclor/litre showed no
consistent effects of temperature or salinity, though the organisms
exposed under conditions furthest from the optimum (25°C and 30%) were
more likely to differ significantly from the controls. At the optimum
temperature and salinity, no deaths were recorded in adult crabs
exposed to 0.1, 1, 10, or 100 µg Aroclor 1254/litre for up to 3 weeks.
Lowering the salinity or increasing the temperature did not have any
effects on survival with Aroclor 1254 or 1016 at 5 µg/litre. Lowering
both salinity and temperature (to 5% and 10°C) caused 50% of crabs to
die between 21 and 28 days of exposure to Aroclor 1016 at 5 µg/litre,
but there were no lethal effects of Aroclor 1254 at the same
concentration. Lowering the temperature further (7°C and 5%) reduced
the 50% survival time to between 5 and 8 days for both Aroclors.
Nimmo & Barrier (1974) reported some deaths in adult brown shrimp
(Penaeus aztecus) exposed to a "sub-lethal" concentration of Aroclor
1254 (3 µg/litre), for 7 days, after subjection to salinity shock.
The shrimp normally adapts readily to the wide range of salinity
found in its natural estuarine habitat. Roesijadi et al. (1976a)
exposed the adult grass shrimp (Palaemonetes pugio) to sub-lethal
(6.3-8.8 µg/litre) and lethal (57.6-76.4 µg/litre) concentrations of
Aroclor 1254 for 96 h, at various salinities. They found little effect
on haemolymph chloride concentration or osmolarity, chloride space
(the apparent volume of distribution of chloride ions), or chloride
exchange kinetics. The shrimp showed an adaptive altered permeability
to chloride ions at a salinity of 17%, the isotonic point. PCBs did
not affect this permeability change in adult shrimp. In the juvenile
grass shrimp, there was a reduction in haemolymph chloride levels at
low salinities in non-steady state exposures; the PCBs delayed the
permeability change. This disruption of haemolymph chloride was
associated with high numbers of deaths, even at the "sub-lethal"
exposure concentration. It was concluded that juveniles died from
salinity shock, because of delayed adaptive response. In another study
(Roesijadi et al., 1976b), grass shrimp were exposed to Aroclor 1254
at 29.4 µg/litre for 96 h at various salinities. No appreciable effect
was observed on total free amino acid levels in abdominal muscle,
indicating that intracellular osmoregulation was not a major
consequence of PCB toxicity, though changes in individual amino acid
concentrations suggested an altered metabolic state. The authors found
that blood glycine levels showed large decreases after the transfer of
the shrimps to clean water, a delayed response to the Aroclor
exposure.
7.2.2.3 Reproduction
Sea urchin (Arbacia punctulata) eggs were exposed to concentrations
of Aroclor 1254 of 0.5, 1.0, 5.0, and 10.0 mg/litre (Adams, 1983).
There was no effect on percentage fertilization, percentage pluteus
development, or percentage mortality when eggs were exposed at
fertilization. However, when eggs were exposed 1 h prior to
fertilization, there was a significant reduction in fertilization
efficiency at all doses. At all but the lowest dose, there was a
significant increase in mortality and a significant depression in
successful pluteus development.
Maki & Johnson (1975) calculated EC50s for total young produced,
average brood size, and percentage of days reproducing, during a
14-day exposure of Daphnia magna to Aroclor 1254; the results were
19, 23, and 25 µg/litre, respectively. Daphnia magna were exposed to
a range of Aroclors, by Nebeker & Puglisi (1974) who estimated
reproductive impairment, measured as a percentage of surviving young
relative to controls. The study was conducted over 3 weeks under
static conditions. Results are presented in Table 30. Reproductive
impairment matches lethality of the Aroclors (see Table 29); there was
no indication of reproductive effects of the Aroclors at
concentrations below those leading to the death of adults or young. No
young were produced by scud (Gammarus pseudolimnaeus) exposed to
8.7 µg Aroclor 1242/litre. Scud exposed at 2.8 µg/litre produced fewer
young per surviving adult (4.2), compared with controls (6.8).
Table 30. Reproductive impairment of Daphnia magna exposed to Aroclors under
static conditionsa
Aroclor Concentration (µg/litre) producing reproductive impairment
50% 16%
1221 125 89
1232 66 53
1242 63 48
1248 24 16
1254 28 18
1260 33 22
1262 41 24
1268 206 162
a From: Nebeker & Puglisi (1974).
7.2.2.4 Moulting
Several authors have suggested that crustaceans may be more
susceptible to the toxic effects of PCBs during moult (Duke et al.,
1970; Wildish, 1970; Nimmo et al., 1971a,b). Fingerman & Fingerman
(1979) exposed 2 groups of fiddler crabs (Uca pugilator) at 8 mg
Aroclor 1242/litre, one group for 40 days, and the other for only the
first 14 of the 40 days. Eye-stalks were removed on day 15 to increase
moulting activity. Controls underwent rapid ecdysis, with more than
50% of the population completing moult within 40 days. Crabs exposed
to the Aroclor for the full 40 days showed less moulting, with no more
than 10% of the population completing moult. Crabs exposed for 14 days
showed 20% of the population successfully moulting. Removal of
eye-stalks on day 1 of the study produced similar results, in terms of
numbers of crabs moulting in each treatment, but speeded-up moult in
the controls. No more Aroclor-exposed crabs moulted after eye-stalk
removal. In an earlier study, Fingerman & Fingerman (1977) exposed
fiddler crabs to Aroclor 1242 at 8 mg/litre for 38 days. Either
eye-stalks or 4 walking legs were removed to stimulate moulting. Both
control groups underwent ecdysis rapidly. Treated crabs without
eye-stalks did not undergo any moult. Moulting in those with legs
removed was much slower than in the controls. The authors also exposed
crabs to dibenzofuran (1,2,3,4,5,6,7,8-octachlorodibenzofuran) at
16 ng/litre. This is equivalent to the maximum reported dibenzofuran
contamination of the Aroclor with the dose equivalent to (in terms of
the dibenzofuran) the same concentration (8 mg/litre) in the PCB
mixture. There was only a slight inhibition of moulting caused by the
dibenzofuran.
Neff & Giam (1977) exposed juvenile horseshoe crabs (Limulus
polyphemus) to concentrations of Aroclor 1016 of 10, 20, 40, or
80 µg/litre, for up to 96 days. The crabs were divided into 2 groups:
group A consisted of juveniles at the late first tailed stage and
group B of juveniles at the early second tailed stage. ET50s (median
time for moulting to begin) were calculated between the start of the
study and the first moult (ET50-1) and between subsequent moults
(ET50-2 to -n). No moult occurred within 96 days at concentrations of
Aroclor of 40 or 80 µg/litre in either group of crabs. The ET50-1, in
group A at 10 and 20 µg/litre, was not different from controls; the
ET50-2 was slightly decreased. In group B, 10 and 20 µg/litre did not
affect the ET50-1; 40 and 80 µg/litre delayed the onset of the first
moult by 7 and 9 days, respectively. The ET50-3 were substantially
decreased at 10, 20, and 40 µg Aroclor/litre.
7.2.2.5 Behaviour
Hansen et al. (1974a) studied the avoidance response of the pink
shrimp (Penaeus duorarum) and the grass shrimp (Palaemonetes pugio)
given a choice between clean water and water containing Aroclor 1254,
at concentrations of between 0.001 and 10 mg/litre. Pink shrimp did
not avoid any of the concentrations used; grass shrimp only
significantly avoided the highest dose.
7.2.2.6 Population structure
The composition of communities of estuarine animals, in aquaria, were
studied under different exposures to Aroclor 1254 (0.1, 1.0, and
10.0 µg/litre) for 4 months (Hansen, 1974). The author found that, in
control groups and the group at the lowest concentration, the
community was mainly comprised (>75%) of arthropods, mostly the
amphipod Corophium volutator. At 1 and 10 µg Aroclor 1254/litre, the
numbers of arthropods decreased and the numbers of chordates
increased; at 10 µg/litre, over 75% of the animals were tunicates. The
highest concentration of Aroclor decreased the numbers of phyla,
species, and individuals represented (particularly of amphipods,
bryozoans, crabs, and molluscs), whereas the numbers of annelids,
brachypods, coelenterates, echinoderms, and nemertines were
unaffected.
7.2.2.7 Interactions with other chemicals
Maki & Johnson (1975) exposed Daphnia magna to various combinations
of DDT (0.2-0.75 µg/litre) and Aroclor 1254 (2-24 µg/litre). They
studied adult mortality, total young produced, average brood size, and
percentage days reproducing, during a 14-day exposure period. The
effects of one toxicant significantly enhanced the action of the other
for all test parameters. In the presence of a no-observed-effect level
of PCB (12 µg/litre), the susceptibility of Daphnia to DDT increased
by one third. When combined with 0.5 µg DDT/litre, the toxicity of
Aroclor 1254 was doubled.
In a study by Nimmo & Bahner (1976), the pink shrimp (Penaeus
duorarum) was exposed to various combinations of Aroclor 1254
(0.7-1.1 µg/litre), cadmium (640-829 µg/litre), and methoxychlor
(0.9-1.0 µg/litre) and numbers of shrimps dying were monitored. The
results showed no evidence of synergism or potentiation in any
combination.
7.2.3 Fish
The acute toxicity of PCBs for fish is summarized in Table 31. Values
for 96-h LC50s vary between 0.008 mg/litre, for the fry of the
fathead minnow, to > 100 mg/litre for channel catfish. This
considerable variation is dependent on species and on the PCB mixture
but appears to depend little on test conditions, such as temperature
and water hardness. The toxicity of PCBs appears much greater in
flow-through tests, where the water concentration of the PCBs is
constantly maintained.
MATC (maximum acceptable toxicant concentrations) were set by the US
EPA (1980) and are expressed as a range between the no-observed-effect
level (based on the results of long-term studies and the sub-lethal as
well as the lethal effect) and the lowest concentration showing a
measurable effect. These values, for the fathead minnow, were 5.4 and
15.0 µg/litre for Aroclor 1242; 0.1 and 0.4 µg/litre for Aroclor 1248;
1.8 and 4.6 µg/litre for Aroclor 1254, and 1.3 and 4.0 µg/litre for
Aroclor 1260. The early life stage of the estuarine sheepshead minnow
gave values of 3.4 and 15.0 µg/litre for Aroclor 1016 and 0.06 and
0.16 µg/litre for Aroclor 1254.
7.2.3.1 Short- and long-term toxicity
The toxicity of Aroclors for 3 species of freshwater fish, over
exposure of up to 30 days, was systematically investigated by Mayer et
al. (1977) under flow-through conditions. Results are presented in
Table 32. Short-term tests consistently underestimate the toxicity of
PCBs.
Table 31. Acute toxicity of PCB and PCT mixtures for fish
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Channel catfish 0.60 g stat 20 40 7.4 Aroclor 1016 96-h LC50 > 100
(Ictalurus yolk-sac stat 25 272 7.4 Aroclor 1016 96-h LC50 0.44 (0.34-0.56)
punctatus) 2.80 g flow 17 272 7.4 Aroclor 1242 96-h LC50 >0.10
Mayer & 2.80 g flow 22 272 7.4 Aroclor 1248 96-h LC50 >0.1
Ellersieck 2.80 g flow 22 272 7.4 Aroclor 1254 96-h LC50 >0.20
(1986) 2.80 g flow 22 272 7.4 Aroclor 1260 96-h LC50 >0.40
Atlantic salmon 5.60 g flow 17 314 7.6 Aroclor 1016 96-h LC50 0.13 (0.11-0.16)
(Salmo salar)
Mayer &
Ellersieck (1986)
Brook trout 3.0 g flow 12 314 7.6 Aroclor 1016 96-h LC50 >0.80
(Salvelinus
fontinalis)
Mayer &
Ellersieck (1986)
Brown trout 4.60 g flow 12 314 7.6 Aroclor 1016 96-h LC50 0.14 (0.11-0.18)
(Salmo trutta) 1.10 g stat 13 44 7.4 Aroclor 1260 96-h LC50 > 24.0
Mayer &
Ellersieck (1986)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Cutthroat trout 2.70 g stat 9 159 162 7.4 Aroclor 1221 96-h LC50 1.17 (0.96-1.43)
(Salmo clarki) 2.20 g stat 9 159 162 7.4 Aroclor 1232 96-h LC50 2.5 (1.72-3.08)
Mayer & 2.40 g stat 9 159 162 7.4 Aroclor 1242 96-h LC50 5.42 (3.82-7.68)
Ellersieck 2.50 g stat 9 159 162 7.4 Aroclor 1248 96-h LC50 5.75 (5.1-6.5)
(1986) 2.50 g stat 9 159 162 7.4 Aroclor 1254 96-h LC50 42.5 (38.7-46.7)
2.70 g stat 9 159 162 7.4 Aroclor 1260 96-h LC50 60.9 (55.4-67.0)
2.40 g stat 9 159 162 7.4 Aroclor 1262 96-h LC50 > 50
2.20 g stat 9 159 162 7.4 Aroclor 1268 96-h LC50 > 50
2.70 g stat 9 162 7.4 Aroclor 4465 96-h LC50 > 65
2.10 g stat 9 162 7.4 Aroclor 5442 96-h LC50 > 50
2.90 g stat 9 162 7.4 Aroclor 5460 96-h LC50 > 50
Lake trout fry stat 10 170 7.2 Aroclor 1016 96-h LC50 0.48 (0.39-0.60)
(Salvelinus yolk-sac stat 10 170 7.2 Aroclor 1016 96-h LC50 0.89 (0.69-1.15)
namaycush)
Mayer &
Ellersieck (1986)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Rainbow trout 0.50 g stat 12 44 7.4 Aroclor 1016 96-h LC50 0.14 (0.11-0.16)
(Salmo gairdneri) 2.50 g flow 10 272 7.4 Aroclor 1016 96-h LC50 0.62 (0.42-0.90)
Mayer & fry flow 12 314 7.6 Aroclor 1016 96-h LC50 0.44 (0.37-0.53)
Ellersieck 1.80 g flow 17 272 7.4 Aroclor 1242 120-h LC50 0.07
(1986) 1.80 g flow 17 272 7.4 Aroclor 1248 120-h LC50 0.05
1.80 g flow 17 272 7.4 Aroclor 1254 120-h LC50 0.14
1.80 g flow 17 272 7.4 Aroclor 1260 96-h LC50 >0.23
Harlequin fish 10-30 mm flow 20 20 8.1 Aroclor 1221 96-h LC50 1.05
(Rasbora 10-30 mm flow 20 20 8.1 Aroclor 1232 96-h LC50 0.32
heteromorpha) 10-30 mm flow 20 20 8.1 Aroclor 1242 96-h LC50 0.37
Tooby et al. 10-30 mm flow 20 20 8.1 Aroclor 1254 96-h LC50 1.1
(1975) 10-30 mm flow 20 20 8.1 Aroclor 1262 96-h LC50 >100
Bluegill sunfish 0.90 g stat 12 44 7.4 Aroclor 1016 96-h LC50 0.60
(Lepomis 1.80 g flow 20 272 7.4 Aroclor 1016 96-h LC50 0.46 (0.39-0.54)
macrochirus) 2.20 g flow 17 272 7.4 Aroclor 1242 120-h LC50 0.13
Mayer & 0.80 g stat 18 44 7.1 Aroclor 1248 96-h LC50 0.69 (0.48-0.99)
Ellersieck 2.20 g flow 22 272 7.4 Aroclor 1248 120-h LC50 0.14
(1986) 0.80 g stat 18 44 7.1 Aroclor 1254 96-h LC50 2.74 (1.29-5.81)
2.20 g flow 22 272 7.4 Aroclor 1254 96-h LC50 0.20
2.20 g flow 22 272 7.4 Aroclor 1260 96-h LC50 0.40
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Longnose sucker finger flow 12 314 7.5 Aroclor 1016 96-h LC50 0.33 (0.22-0.49)
(Catostomus
catostomus)
Mayer &
Ellersieck (1986)
Yellow perch 1.20 g flow 17 314 7.6 Aroclor 1242 96-h LC50 >0.15
(Perca flavescens) 1.10 g flow 17 314 7.6 Aroclor 1248 96-h LC50 >0.1
Mayer & 1.00 g flow 17 314 7.6 Aroclor 1254 96-h LC50 >0.15
Ellersieck 1.20 g flow 17 314 7.6 Aroclor 1260 96-h LC50 >0.20
(1986)
Fathead minnow fry flow 24 Aroclor 1254 96-h LC50 0.008
(Pimephales fry flow 24 Aroclor 1242 96-h LC50 0.015
promelas) 3 months flow 24 Aroclor 1242 96-h LC50 0.30
Nebeker
et al. (1974)
Cisco (chub) 22 days flow 7 30-35 40-48 Aroclor 1254 96-h LC50 >10
(Coregonus sp.) 22 days flow 7 30-35 40-48 Aroclor 1254 120-h LC50 3.2 (1.9-5.5)
Passino &
Kramer (1980)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Carp (Cyprinus fry stat 23-25 Kanechlor 300 96-h LC50 1.45
carpio)
Kimura et al.
(1974)
Guppy (Lebistes fry stat 24-25 Kanechlor 300 96-h LC50 0.9
reticulatus) 0.35 g stat 24-25 Kanechlor 300 96-h LC50 3.2
Kimura et al. (1974)
a stat = static conditions: water not changed during the exposure;
flow = flow-through conditions; concentration of toxicant continuously maintained.
b Alkalinity and hardness expressed as mg/litre CaCO3, unless otherwise stated.
Table 32. Toxicity of Aroclors for fish (LC50s in µg/litre) at 17°Ca
Aroclor Exposure (days)
5 10 15 20 25 30
Rainbow trout
1242 67 48 18 10 12 -
1248 54 38 16 6.4 3.4 -
1254 - 160 64 39 27 -
1260 - 326 143 78 49 51
Bluegill sunfish
1242 - - 164 125 120 84
1248 136 115 111 106 100 78
1254 - - 303 260 239 177
1260 - - - - - 400
Channel catfish
1242 - - 219 150 132 87
1248 - 121 121 115 104 75
1254 - 303 286 293 181 139
1260 - 535 482 512 465 433
a From: Mayer et al. (1977).
Duke et al. (1970) kept juvenile pinfish (Lagodon rhomboides) in
seawater containing 1, 10, or 100 µg Aroclor 1254/litre for up to
48 h. There were no deaths at any concentration. It was suggested by
Nimmo et al. (1975) that acute toxicity tests underestimated the true
sensitivity of marine species; in bioassays lasting 1 week or more,
Aroclor proved to be 100 times more toxic than acute exposure
suggested. In tests lasting 2 weeks or longer, Aroclor 1254 was lethal
for longnose killifish (Fundulus similis) at 1 µg/litre and for
pinfish and spot (Leiostomus xanthurus) at 5 µg/litre. Hansen et al.
(1974b) did not find any significant lethal effects in pinfish exposed
to 100 µg Aroclor 1016/litre for 96 h but significant mortality (50%)
was observed after 33 days at 32 µg/litre, and, after 18 days, at
100 µg/litre. Nebeker et al. (1974) exposed the flagfish (Jordanella
floridae) to Aroclor 1248 for 40 days. No fish survived at a
concentration of 18 µg/litre and only 35% survived at 5.1 µg/litre.
The fish at these two concentrations almost completely lost their fins
and tails. Fish survival was not affected at concentrations of
2.2 µg/litre or less. In a study by Defoe et al. (1978), fathead
minnow (Pimephales promelas) larvae were exposed, in flow-through
bioassays, to Aroclors 1248 and 1260 for 30 days; the LC50s were
calculated to be 4.7 and 3.3 µg/litre, respectively.
Hansen et al. (1976a) fed fingerling channel catfish (Ictalurus
punctatus) a diet containing 20 mg Aroclor 1242/kg for 20 weeks. The
fish showed a reduced weight gain and hypertrophy of the liver. When
the treated fish were transferred to a control diet for 8 weeks and
then back to the dosed diet for a further 8 weeks, weight gain and
liver weights returned to normal levels. No histopathological lesions
were observed in any of the fish fed PCBs.
Rainbow trout (Salmo gairdneri) were fed a diet containing 1, 10, or
100 mg Aroclor 1254/kg over a period of 330 days (Nestel & Budd,
1975). No effects on growth rates were seen, but renal lesions were
observed at all doses; however, they were not dose-related. Foci of
renal necrosis, with cellular or granular cast formation were seen. A
significant increase in the number of hepatocytes per unit area in the
liver was observed at all doses and appeared to be dose-related. A
reduction of 'white pulp' (lymphatic elements) in the spleen was
observed at 10 and 100 mg/kg diet. Fish with renal necrosis also had
reduced splenic white pulp and a reduced white cell count.
7.2.3.2 Carcinogenicity
Hendricks et al. (1977) studied the combined effects of Aroclor 1254
(100 mg/kg diet) and aflatoxin B1 (6 mg/kg diet) in rainbow trout. A
significantly reduced incidence of liver tumours was observed in the
combination. There was no retardation of growth in the treated
animals, but they showed glycogen depletion in hepatocytes,
hyperaemia, and white pulp depletion in the spleen.
PCB administration prior to aflatoxin B1 treatment also decreased the
liver tumour incidence, whereas when Aroclor 1254 was fed after
exposure of trout embryo to aflatoxin B1, there was no effect on the
formation of liver tumours (Shelton et al., 1984b).
When rainbow trout were fed 0, 1, 4, or 8 mg aflatoxin B1/kg diet or
aflatoxin B1 at these dose levels plus 50 mg Aroclor 1254/kg diet,
the incidence of hepatic tumours was lower in the groups receiving
aflatoxin combined with PCBs (Shelton et al., 1984a). The inhibition
of aflatoxin B1-mediated carcinogenicity was also correlated with the
decreased bacterial mutagenicity of this compound in the presence of
an Aroclor 1254-induced drug metabolizing enzyme fraction from fish.
The potential mechanisms of this interaction were further investigated
by Shelton et al. (1986) by studying the effects of Aroclor 1254 on
aflatoxin B1 distribution, metabolism, and adduct formation. The
results from the in vivo studies showed that PCB treatment resulted
in a marked increase in the metabolism of aflatoxin B1 to aflatoxin
M1 and their glucuronide conjugates. The DNA-adduct levels in the
PCB-treated fish were 48-96% lower than those in the controls. The
results in the fish model using aflatoxin B1 as the carcinogen were
associated with the activity of Aroclor as an inducer of cytochrome
P-450-dependent monooxygenases (Halverson et al., 1985; Shelton et
al., 1986).
7.2.3.3 Effects on developmental stages and reproduction
Birge et al. (1978) determined LC50s and LC1s for 4 species of fish
from the fertilization of the eggs to 4 days after hatching. Exposure
times varied with species dependent on the time taken to hatching of
the eggs; hatching took 22 days for rainbow trout and 3-4 days for the
other species. In most species, eggs were considerably less sensitive
to the toxic effects of Aroclors than larvae. The major exception was
the rainbow trout for which the duration of exposure of the eggs until
hatching was considerably longer than for other species. LC50s ranged
from 0.32 to 11.16 µg/litre for the 4 species (up to 4 days after
hatching) and 4 different PCB mixtures; LC1s ranged from 0.009 to
0.26 µg/litre. Results are presented in Table 33. The rainbow trout
was the most sensitive species tested.
Adult minnows (Phoxinus phoxinus) were fed Clophen A50 at 20, 200,
or 2000 mg/kg diet (on a dry weight basis), for 40 days (Bengtsson,
1980). Growth was monitored from day 46 to day 79; a significant
increase in growth (relative to controls) was seen in fish fed the
highest dose. Other doses caused increases in growth, but these were
not significant. Stimulated growth had been observed in previous
studies with minnows fed Clophen A50 at 0.88-78 mg/kg diet (Bengtsson,
1979). Between days 127 and 166, the swimming performance of the fish
was tested using a rotary flow technique. Although the PCBs impaired
swimming performance, this was not statistically significant.
Reproduction was monitored from day 235 (first day of spawning) to day
300. Spawning was delayed in the treated groups by 1 day, 1 week, and
3 weeks for the 3 treatments (20, 200, and 2000 mg/kg diet),
respectively. Only the highest dose affected hatchability of eggs,
which was reduced by approximately 80%.
Snarski & Puglisi (1976) did not find any adverse effects on survival,
growth, or reproduction of brook trout (Salvelinus fontinalis)
exposed to concentrations of Aroclor 1254 of up to 0.94 µg/litre, for
71 weeks. Survival and growth of alevin-juveniles from exposed parents
were unaffected for up to 90 days.
Continuous-flow bioassays were conducted over 8 months on fathead
minnow (Pimephales promelas), exposing the fish to either Aroclor
1242 or 1254 (Nebeker et al., 1974). Reproduction occurred at, and
below, 5.4 µg Aroclor 1242/litre, but spawning and egg production
were very variable. With Aroclor 1254, reproduction occurred at, and
below, 1.8 µg/litre. Spawning occurred at 1.8 µg/litre, but was
significantly less than spawning at the lower concentrations of 0.23
and 0.52 µg/litre. Egg hatchability and fry survival were good at
1.8 µg/litre. Eggs were more resistant than fry at 15 and 51 µg
Aroclor 1242/litre; with Aroclor 1254 at a concentration of
15 µg/litre, eggs hatched readily, but all fry were dead within 96 h.
Halter & Johnson (1974) kept coho salmon (Oncorhynchus kisutch) eggs
in solutions containing 4.4-56.4 µg Aroclor 1254/litre. Exposure
continued for 2 weeks before, and 4 weeks after hatching, until the
young were alevins. Hatchability was reduced by 30% at the highest
concentration of 56.4 µg/litre. Survival of alevins was markedly
higher when eggs were transferred to clean water prior to hatching,
but there was still 58% mortality in alevins hatched from eggs at the
highest dose. When the alevins were exposed to the PCBs for 4 weeks
after hatching, survival was inversely related to exposure
concentration. No group survived as well as the controls (for example,
18% died at 4.4 µg/litre and 90% at 26 µg/litre, or more).
In a study by Defoe et al. (1978), fathead minnow (Pimephales
promelas) were maintained in flow-through bioassays in solutions of
Aroclors 1248 or 1260, for 240 days (a full life-cycle test).
Reproduction occurred at all concentrations tested (up to 3 µg/litre
for Aroclor 1248 and up to 2.1 µg/litre for Aroclor 1260). The authors
concluded that PCBs did not produce major effects on reproduction at
concentrations up to the 30-day LC50 and that reduced populations
after long-term exposure are largely due to larval mortality.
Weis & Weis (1982) exposed eggs of the mummichog (Fundulus
heteroclitus) to concentrations of Aroclor 1254 of 0.01-10 mg/litre
(after cleavage had begun). No effect was found on embryonic
development or hatching and embryonic mortality was negligible.
Seven-day larval tests also showed no effect on mortality up to the
highest concentration. However, the authors found approximately 20%
larval mortality, when larvae were exposed to 5 mg/litre for 72 h,
after hatching from eggs at the highest exposure concentration. In a
second group of studies, eggs were exposed to 10 mg Aroclor
1242/litre; no malformations were found, though there was a consistent
retardation of hatching. There was a positive correlation between
hatching rate and female length (length being related to age). Larvae
exposed to 5 mg/litre showed an average of 45% mortality within 72 h.
Pre-exposure as eggs to 10 mg/litre greatly increased larval
mortality; pre-exposure to 1 mg/litre resulted in an intermediate
response. There was no mortality in control larvae, even when they had
been pre-exposed as eggs.
Eggs of brook trout (Salvelinus fontinalis) were exposed to Aroclor
1254 (0.043-13 µg/litre) for 10 days before hatching, and the fry for
118 days after hatching (Mauck et al., 1978). Median hatching time,
egg hatchability, and sac-fry survival were not affected by the PCBs.
Significantly decreased survival (32% survived) was seen after 48 days
at 3 µg/litre. There was significant mortality of fry after 118 days
with exposure to concentrations of 3.1 µg/litre and above. Growth of
the trout, as measured by weight, was significantly decreased after 48
days at concentrations of 1.5 µg/litre or more. By the end of the
study (118 days), no significant differences in weight were seen
between surviving fry on different treatments. Analysis of the
backbone composition at this time showed that hydroxyproline and
phosphorus were significantly decreased by concentrations of
0.43 µg/litre or more and that, at 0.69 µg or more/litre, calcium
levels were significantly increased. Although collagen was
significantly decreased at 0.69, 3.1, and 6.2 µg/litre, it was
unaffected at 1.5 µg/litre; no explanation for the anomaly was
suggested.
Schimmel et al. (1974) exposed sheepshead minnow (Cyprinodon
variegatus) eggs, immediately after fertilization, to Aroclor 1254
at concentrations of between 0.1 and 10 µg/litre. The fertility of
eggs was unaffected. Hatching was significantly reduced (by 30%) only
at the highest exposure. Survival of fry to 2 weeks was significantly
reduced at concentrations of 0.32 µg/litre or above (30% survival at
0.32 µg/litre and 9% survival at 10 µg/litre). The 3-week LC50 for
embryo-fry was calculated to be 0.93 µg/litre for Aroclor 1254. Many
of the dying fish exhibited fin rot. Exposure of juveniles and adults
to the same concentrations of the Aroclor produced 24% mortality in
juveniles at the highest exposure rate and no mortality in adults.
Some fin rot was seen in adults.
Table 33. Toxicity of PCBs for the embryolarval stages of fisha,b
Organism Aroclor 1016 Aroclor 1242 Aroclor 1254
LC1 LC50 LC1 LC50 LC1 LC50
(µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre)
Channel catfish 0.08 11.16 0.14 4.24 0.05 1.76
(Ictalurus punctatus) (9.93-12.97) (0.07-0.23) (3.32-5.34) (0.02-0.09) (1.36-2.24)
Goldfish 0.10 13.21 0.04 2.64 0.02 1.18
(Carassius auratus) (10.63-16.43) (0.01-0.08) (1.89-3.61) (0.01-0.04) (0.84-1.61)
Rainbow trout 0.011 1.08 0.01 1.03 0.009 0.32
(Salmo gairdneri) (0.003-0.027) (0.7-1.56) (0.002-0.025) (0.67-1.51) (0.003-0.02) (0.22-0.45)
Redear sunfish 0.26 7.82 0.19 3.56 0.02 0.53
(Lepomis microlophus) (0.1-0.51) (5.74-10.35) (0.08-0.35) (0.65-4.66) (0.01-0.04) (0.39-0.7)
a From: Birge et al. (1978).
b Exposure under static conditions, but with water renewed every 12 h. Exposure was initiated 2-6 h after spawning (except
for rainbow trout where exposure was initiated 15 min after fertilization) and continued to 4 days post-hatching. Hatching
times varied: 22 days for rainbow trout (13.5-14.3°C); 3 days for catfish (29-31°C); 3-4 days for goldfish and sunfish
(20-24°C); hardness 90-115 mg/litre; pH 7.6-8.1.
In another study, Hansen et al. (1975) exposed embryos, fry,
juveniles, and adults of sheepshead minnow (Cyprinodon variegatus)
to concentrations of Aroclor 1016 of 0.1-10 µg/litre, for 28 days, in
intermittent flow bioassays. No effects on survival were observed
during this period. When exposed to concentrations of 32 or
100 µg/litre, there was high mortality in eggs, juveniles, and adults;
all were killed at 100 µg/litre. The authors calculated that the
28-day LC50s for juveniles and adults were 20 and 19 µg/litre,
respectively.
Freeman et al. (1982) fed Atlantic cod (Gadus morrhua) diets
containing 1-50 mg Aroclor 1254/kg for 5.5 months. Altered steroid
biosynthetic patterns in vitro were observed in the testes and head
kidneys (adrenal equivalent) of dosed fish. Histological examination
revealed abnormalities in the testes, gills, and livers. Testicular
abnormalities included derangement of lobules, hyperplasia of lobule
walls, and disintegration and/or fatty necrosis of spermatogenic
elements. In fish fed at dietary rates of 5-50 mg/kg, hyperplasia of
the epithelial layer of the secondary lamellae of the gills was noted.
Fatty degeneration of the liver was observed in all treated fish.
Similar testicular abnormalities were observed by Sangalang et al.
(1981), but only in sexually mature individuals or at a stage of rapid
spermatogenic proliferation.
7.2.3.4 Physiological and biochemical effects
Coho salmon (Oncorhynchus kisutch) were fed diets containing a
mixture of PCBs (1:4, Aroclor 1242:1254) at a concentration of 50 or
500 mg/kg dry feed (Leatherland & Sonstegard, 1978). Serum
triiodothyronine (T3) levels were significantly reduced after 3
months, in fish fed the highest dose. Thyroxine (T4) levels were not
affected. After 3 months, the T3:T4 ratio was significantly higher in
fish fed the highest dose than in control and low-dose fish. Fish on
500 mg/kg had significantly lower body weights than controls by the
end of the study. A mixture of 50 mg PCBs/kg and 5 mg mirex/kg
significantly reduced serum triiodothyronine and thyroxine levels over
a period of 3 months, but did not affect the T3:T4 ratio. Leatherland
& Sonstegard (1979) fed rainbow trout (Salmo gairdneri) diets
containing Aroclor 1254 at 500 mg/kg dry feed, for up to 2 months. The
PCBs did not have any significant effects on thyroid histology or on
serum thyroid hormone levels. Liver weights, total liver lipid
content, and carcase lipid content were significantly greater in
treated fish. Mayer et al. (1977) fed fingerling coho salmon
(Oncorhynchus kisutch) 1.45-14 500 µg Aroclor 1254/kg body weight
per day, for 260 days. Channel catfish (Ictalurus punctatus) were
fed the same Aroclor at rates of 48 and 480 µg/kg body weight per day
for 193 days. Thyroid activity (as measured by 125I uptake) was
significantly stimulated at dose rates of 14.5 µg/kg per day, and
above, in coho salmon. Stimulation ranged from 52%, at 14.5 µg/kg per
day, to 119%, at 14 500 µg/kg per day, compared with controls. In
catfish, both dose rates of Aroclor 1254 caused significant increases
in thyroid activity, whereas other Aroclors (1232, 1248, and 1260) did
not have any significant effects on the thyroid at the same dose
rates. Folmar et al. (1982) injected yearling coho salmon
(Oncorhynchus kisutch) intraperitoneally with a total of 150 µg
Aroclor 1254/kg body weight (2 injections, 10 days apart), prior to
smoltification. Over a 6-week period, the authors did not find any
significant effects on gill Na-K ATPase activity. However, the PCBs
did alter the normal developmental patterns of thyroxine; there was a
delay in the normal increase in circulating thyroxine levels.
Triiodothyronine levels were significantly elevated after
approximately 3 weeks, but then fell to well below control levels
after 6 weeks. Fish were transferred to seawater; there was no
significant effect on the gill Na-K ATPase activity in the treated
group, but there was a significant increase in mortality (6%). Ten per
cent of fish, dosed with PCBs and placed in sea water containing No 2
fuel oil at 700 µg/litre, died; this was an additive effect.
Fingerman (1980) kept Gulf killifish (Fundulus grandis) in a
seawater solution containing Aroclor 1242, 1254, or 1268 at
8 mg/litre, for up to 28 days. The author removed the lower half of
the caudal fin to study fin regeneration. No significant effects were
observed with either Aroclor 1242 or 1254. With Aroclor 1268, a
significant decrease in regeneration rate was observed after 28 days,
when the study was conducted in the spring, and after 7 days, in the
autumn. No differences were found at other sampling times.
Rainbow trout (Salmo gairdneri) were administered capsules
containing 0.173 g Aroclor 1254 every second day over a 6-day period
(Kiessling et al., 1983). Two to 4 weeks after the last capsule,
isolated gills were perfused. There was no significant difference in
adrenergic response in the gill vascular bed and no significant
difference in the "oxygen transfer factor" (% changes in oxygen in
saline from dorsal aorta before, and after, addition of adrenaline).
Similarly, there was no effect on muscle glycogen content.
Johansson et al. (1972) dosed brown trout (Salmo trutta) twice, 4
days apart, with 5 mg Clophen A50/kg body weight, either by capsule or
by intramuscular injection. The fish were fed for 43 days, starved for
116 days, and fed again for another 87 days. Metabolic analysis of the
fish was undertaken on day 43 and at the end of the study. A
significant increase in body weight was noted at the end of the study,
but not after 43 days. Blood glucose, muscle glycogen, and the
liver-somatic index (liver weight as a ratio of body weight), which
had all increased significantly after 43 days, decreased significantly
by the end of the study (compared with controls). Both haematocrit and
haemoglobin levels had significantly decreased after 43 days but, by
the end of the study, were not significantly different from those of
the controls.
In a study by Camp et al. (1974), fingerling catfish (Ictalurus
punctatus) were kept in water containing Aroclor 1254 at 8 mg/litre.
There was a significant increase in the serum transaminase activity of
the fish after 4 h. The cortisol content of the serum was depressed,
but not significantly so. The sodium:potassium ratio was constant.
Merkins & Kinter (1971) exposed the killifish (Fundulus heteroclitus)
to concentrations of Aroclor 1221 of 7.5, 25, or 75 mg/litre. No fish
died, at the lowest concentration, over a period of 4 days; 50% died
at 25 mg/litre within 24 h and 88% at 75 mg/litre, within the same
period. Serum osmolality, and serum ion levels (Na and K) were then
measured. Within 6 h at 75 mg/litre, blood osmolality significantly
increased, but sodium and potassium ions in the blood were not
affected. After 24 h at 75 mg/litre, there was also a significant
increase in sodium ions, but no effects on potassium ions. None of
these parameters were affected by exposure at 25 mg/litre for 24 h.
7.2.3.5 Behavioural effects
Fingerman & Russell (1980) exposed male Gulf killifish (Fundulus
grandis) to Aroclor 1242 at 4 mg/litre for 24 h. A significant
reduction in whole-brain levels of both noradrenalin and dopamine were
reported over this period. The swimming activity of the fish was
monitored by counting the number of times they crossed lines marked on
the bottom of the tank within a 10-min period, after exposure to the
Aroclor for 24 h. Activity was significantly increased and remained so
for a further 2 days.
The avoidance response was studied by Hansen et al. (1974a) in the
sheepshead minnow (Cyprinodon variegatus), the pinfish (Lagodon
rhomboides), and the mosquito fish (Gambusia affinis), when given
a choice between clean water and water containing Aroclor 1254 at
0.001, 0.01, 0.1, 1.0, or 10 µg/litre. Sheepshead minnow did not avoid
any concentration; pinfish avoided only the highest concentration of
the Aroclor. Mosquitofish significantly avoided concentrations of 0.1,
1.0, and 10 µg/litre.
Peterson (1973) did not find any effect on temperature selection in
Atlantic salmon (Salmo salar) exposed to 2 mg Aroclor 1254/litre,
for 24 h prior to a horizontal temperature gradient test. Similarly,
Miller & Ogilvie (1975) did not find any effect of Aroclor 1254 on
temperature selection when brook trout (Salvelinus fontinalis) were
exposed to a water concentration of 25-100 mg/litre, for 24 h, prior
to temperature gradient tests. Even at a concentration of 100 mg/litre
for 48 h, which was sufficient to cause some mortality, temperature
selection was still unaffected.
7.2.3.6 Interactions with other chemicals
Halter & Johnson (1974) found that the median survival time for coho
salmon (Oncorhynchus kisutch) fry, exposed to Aroclor 1254 at
32.2 µg/litre, was greater than 336 h. When fry were exposed to
mixtures of Aroclor and DDT, for 2 weeks, the survival times were
always similar to the more rapid reaction time found for DDT alone.
The authors suggested that this indicated the lack of an additive
effect.
7.2.4 Amphibians
Tadpoles (Rana chensinenis) were maintained in water containing PCBs
(as Kanechlor 300) at 0.5, 5.0, 50, or 500 µg/litre. At the 2 highest
doses, all individuals died rapidly. The time of onset of lethality
was related to dose level; at 5 µg/litre, death occurred between 15
and 21 days, and, at 0.5 µg/litre, on the thirty-second day after
first exposure. Growth at 0.5 and 5.0 µg/litre did not differ from
that of controls. Tail abnormalities were found, but there was no
correlation with PCB concentrations and a NOEL could not be
established. The mechanism by which PCBs caused tail malformation was
not known (Hasegawa, 1973).
Birge et al. (1978) conducted embryo-larval bioassays on 3 species of
amphibia. Exposure to various PCBs was maintained from 2-6 h after
spawning to 4 days after hatching, using static renewal procedures
(Table 34). Toxicity increased with increasing chlorination, the
leopard frog being the most sensitive species with an LC50 of
1.03 µg/litre after exposure to Aroclor 1254. The authors also
calculated an LC1 value from the same study; the leopard frog and
American toad were equally sensitive at 0.02 µg/litre. The eggs were
much less sensitive to the PCBs than the hatched larvae, with LC50s
ranging from 3.5 to 250 µg/litre, for 3 different Aroclors and
Capacitor 21, and 3 species of tadpole.
Table 34. Toxicity of PCBs for the tadpoles of amphibiansa,b
Organism Aroclor 1016 Aroclor 1242 Aroclor 1254 Capacitor 21
LC1 LC50 LC1 LC50 LC1 LC50 LC1 LC50
(µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre)
American toad 0.35 7.16 0.03 2.71 0.02 2.02 0.21 9.97
(Bufo americanus) (0.15-0.64) (5.39-9.34) (0.01-0.06) (1.91-3.75) (0.01-0.05) (1.44-2.77) (0.08-0.42) (7.21-13.53)
Fowler's toad 0.18 27.72 0.22 12.09 0.07 3.74 0.55 28.02
(Bufo fowleri) (0.09-0.33) (21.77-35.08) (0.12-0.36) (9.74-14.91) (0.04-0.11) (2.98-4.64) (0.3-0.91) (22.59-34.47)
Leopard frog 0.1 6.19 0.04 2.13 0.02 1.03 0.03 2.87
(Rana pipiens) (0.05-0.16) (4.95-7.69) (0.02-0.06) (1.72-2.63) (0.01-0.03) (0.83-1.27) (0.02-0.06) (2.29-3.57)
a From: Birge et al. (1978).
b Exposure under static conditions, but with water renewed every 12 h. Exposure was initiated 2-6 h after spawning and continued to
4 days post-hatching. Hatching times varied between 3 and 4 days, therefore, exposure varied between 7 and 8 days.
Temperature 20-24°C; hardness 90-115 mg/litre; pH 7.6-8.1.
7.2.5 Aquatic mammals
Following-up on field reports of the reproductive effects of PCBs on
seal reproduction (see section 7.4.4), Reijnders (1986) conducted a
study on captive common seals (Phoca vitulina) fed fish contaminated
with PCBs. The contaminated diet produced an average daily intake of
PCBs of 1.5 mg compared with the control level of 0.22 mg/day. Twelve
female seals were used as controls and 12 as the treated group. Blood
samples were taken regularly and assayed for circulating steroid
hormones progesterone and estradiol. Females were mated with undosed
males. Of the 12 females in the control group, 10 became pregnant; all
12 ovulated. Only 4 females became pregnant out of the 12 fed the
PCB-contaminated diet; again all 12 ovulated. Throughout the breeding
cycle, no significant differences were found between the hormonal
profiles of pregnant animals in the treated and control groups. No
significant differences were observed in progesterone levels in the
treated and control groups, despite the fact that many fewer treated
females became pregnant. However, a rise in estradiol levels in
non-pregnant females in the control group was not found in
non-pregnant females in the treated group, suggesting a difference in
non-pregnancy in treated females. The effects of PCBs occurred only
late in the breeding cycle at the time of implantation of the embryo.
Seals, like mink, show delayed implantation of the embryo as a normal
component of the annual reproductive cycle. No conclusions about the
mechanism of action could be drawn.
Brouwer et al. (1989) fed common seals (Phoca vitulina) on a diet of
polychlorinated biphenyl-contaminated fish (average daily intake
1.5 mg PCBs) for almost 2 years. Significant reductions in levels of
plasma total and free thyroxine, triiodothyronin, and retinol were
found compared with those in seals maintained on a "low" contaminated
diet (average daily intake 0.22 mg PCBs). It should be noted that the
diet consisted of fish contaminated in the environment and not dosed.
No attempt was made to analyse levels of retinol in the different fish
diets. The "high" contamination group were caught in the Wadden sea
and the "low" contamination group in the north-east Atlantic. When the
fish were analysed for other likely contaminants, it was found that
pp'-DDE also showed higher levels in the "high" contamination group
than the "low" contamination group; average daily intakes of pp'-DDE
were estimated to be 0.4 mg and 0.13 mg, respectively.
7.3 Toxicity for terrestrial organisms
7.3.1 Plants
Aroclor 1254 was applied to soil at rates of 10, 100, or 1000 mg/kg
(Weber & Mrozek, 1979). Both soybean (Glycine max) and rescue
(Fescue arundinacea) were grown in the soil, from seed, for up to 26
and 42 days, respectively. The height of the soybean plants and the
fresh top weights of both plants were measured. PCBs applied to the
soil significantly reduced height and fresh top weight of soybean
plants, only at the highest rate of application. Low rates were
inhibitory, but not significantly so. Aroclor applied to the soil also
reduced the fresh top weight of rescue at the highest rate of
application (1000 mg/kg); lower application rates of the Aroclor did
not have any effects. The addition of activated carbon to the soil
(3.7 tonnes/ha; approximately 3333 mg/kg) annulled the inhibitory
effect of PCBs. The Aroclor also inhibited the uptake of water by the
soybean in proportion to the dose applied to the soil; water uptake
was monitored between 21 and 25 days after sowing of the seed. The
reduction in water uptake over this 5-day period was 12%, with the
application of 1 mg Aroclor 1254/kg soil, rising to 52% at 1000 mg/kg
soil. Again, the effect on water uptake was eliminated by the addition
of carbon to the soil (1% rising to 4% inhibition over the dose
range).
Continuation of the experiment through a second and third crop of
soybeans on the same soil, without further addition of Aroclor 1254
(Strek et al., 1981), showed similar effects on the height, top fresh
weight, and water uptake of the plants, reflecting the persistence of
the Aroclor. However, there were no significant effects at doses lower
than 1000 mg/kg, with the exception of reduced height in the third
crop, seen at all dose levels. All effects were eliminated by the
addition of activated carbon to the soil. The same authors found that
beet (Beta vulgaris) was significantly affected by 1000 mg Aroclor
1254/kg, using the same parameters of water uptake, height, and fresh
top weight between 14 and 56 days after sowing. Doses of 100 mg/kg or
less did not have any significant effects. Effects at the highest dose
were again eliminated by the addition of activated carbon to the soil.
Growth parameters, taken at harvest, showed no apparent inhibition of
corn (Zea mays) or sorghum (Sorghum bicolor) by Aroclor 1254 over
the same dose range. There was, however, a reduction of plant height
over the first 5 days of growth at 100 and 1000 mg/kg in corn, but the
plants recovered.
Mrozek et al. (1983) grew Spartina alterniflora plants in mud or
sandy soils in the presence of 2.2 µg PCBs/kg (54% chlorine similar to
Aroclor 1254), admixed with the soil, over a 6-week period. Plants
grown in sand showed significantly reduced values for cumulative
change in height (approx. 30%) and the number of live leaves per stem
(approx. 25%), and increased values for the number of stems per plant
(approx. 300%), whereas plants grown in mud showed a significantly
reduced value for cumulative change in the number of stems per plant
(approx. 75%). Mud-grown plants also exhibited an altered biomass
distribution, as indicated by the aerial:below ground biomass ratio,
which increased from 1.2 to 1.5, on a dry weight basis.
7.3.2 Terrestrial invertebrates
Hatch & Allen (1979) observed the behaviour of the snail (Cepeae
(=Helix) nemoralis), with regard to the rasping of conspecifics'
shells to obtain calcium. On a low calcium diet (0.53 mg calcium/kg),
snails showed an increased tendency to rasp the shells of other
snails, in order to obtain calcium. The best indicator of this
behaviour was found to be the counting of holes bored completely
through the shell. This behaviour was not seen with a high calcium
diet (250 mg calcium/kg). The addition of PCBs, as a mixture of
Aroclors 1016 and 1254, to the high calcium diet at a rate of 0.5,
1.0, or 5.0 mg/kg increased the number of snail shells penetrated by
other snails. Penetration increased in a dose-dependent manner with 2,
5, and 7% penetration for the 3 dose rates, respectively. Damage to
shells, without actual penetration, also increased with PCB treatment
from the low level found on the control diet to between 16 and 21% on
the PCB diet. No clear dose-dependent effect was seen using this
method of assessing damage. Fourth instar nymphs of the grasshopper
(Chorthippus brunneus), were dosed topically with the PCB mixture
Aroclor 1254 (Moriarty, 1969). A single dose of either 12.5, 50, or
200 µg/insect was applied in a volume of 1 µl of 1,4-dioxan. No
sublethal effects were detected on either development or reproductive
potential. At the highest dose, there appeared to be a latent toxicity
that could be correlated with the mobilization of lipids at moult;
more moulted males died than unmoulted males over the test period.
Females took longer to moult than males and showed a distinctly
bimodal distribution in time to death, with a similar correlation
between toxicity and moult. Males showed 46% mortality and females,
41%, after treatment with 200 µg Aroclor 1254. Fungal infection
affected insects on the lower doses and mortality figures are,
therefore, unreliable.
Lichtenstein et al. (1969) exposed Drosophila melanogaster to the
dry residue of various PCBs. They exposed flies to Aroclors 1221,
1232, 1242, and 1248 at 200 or 800 µg. No mortality was observed after
48 h at 200 µg. At 800 µg, there was an increase in mortality with
decreasing chlorination (after a 48-h exposure to Aroclor 1221, 92%
had died; only 45% died after exposure to Aroclor 1248 over the same
length of time). No mortality was observed after a 48-h exposure to
2000 µg of Aroclors 1254, 1260, 1262, or 1268. In a separate study,
the authors treated houseflies (Musca domestica) topically with
either 10 or 20 µg (in 2 µl of acetone); mortality was assessed after
24 h. Results were comparable with those from the study on
Drosophila; deaths were dose related and occurred with Aroclors up
to 1254, where mortality was 10% at the higher dose. Aroclors of
higher chlorination than 1254 had no effect. Lower chlorinated
Aroclors had the greatest effect with more deaths at the highest dose
(20 µg) and lowest chlorination (Aroclor 1221) than with any other
treatment (43% killed). Plapp (1972) found that the 24-h LC50 for
Aroclor 1254 in the housefly (Musca domestica) was > 3000 µg/jar
where the Aroclor was added to a container in acetone, which was dried
before the addition of the flies. This was true for both
DDT-susceptible and DDT-tolerant strains. The same author reported a
powerful synergistic effect between carbaryl and Aroclor 1254; the
LC50 with carbaryl alone was calculated to be 1386 µg/jar and that
for carbaryl:PCB in the ratio of 1:5, 96 µg/jar. The Aroclor was as
powerful a synergizing agent as piperonyl butoxide.
Youssef et al. (1974) hatched eggs of the housefly (Musca domestica)
on a medium of paper tissue dosed with 0.808 g of Aroclor 1254 per
200 g of tissue. The adult flies hatching from the eggs were examined
using the electron microscope for effects on the male reproductive
tissue. The PCBs induced nuclear and flagellar abnormalities in
developing spermatids. Spermatid nuclei failed to elongate and
membranes originating from the nuclear envelope formed invaginations
into the nucleus. These resulted in the appearance of cytoplasmic
inclusions in the nucleus. Spermatid flagellae contained an abnormal
number of axonemes and mitochondrial derivatives; abnormal spermatids
did not coil and degenerated.
In a study by Wasilewska et al. (1975), female nematodes
(Acrobeloides nanus) were exposed to Aroclor 1254. Initially, 60 µg
of the Aroclor were added to a petri dish (on the surface of agar) in
which there were 20 nematode worms. The nematodes fed on a culture of
bacteria introduced to the agar at the same time as the worms. After 5
days of exposure, eggs and adult nematodes were counted and adult
weights determined. No significant effects were found. In a second
study, over a longer period (10 days), nematodes were exposed to 15,
30, or 60 µg of the Aroclor. Adverse effects increased with dose, and
even at the lowest dose, the number of adults was reduced from 123 to
76, the number of eggs from 539 to 288, and the weight of adults from
18.9 to 9.4 µg. At the highest dose of 60 µg per dish, the number of
adults was 32, the number of eggs, 37, and the weight of adults,
4.9 µg.
7.3.3 Birds
Five-day dietary LC50s for PCBs in birds ranged from 604 to >
6000 mg/kg diet (Table 35). Generally, the oral single dose LD50 and
the dietary LC50 data are similar to those for mammals. PCBs are less
toxic for birds than other organochlorines, such as DDT and its
metabolites and the chlorinated cyclodienes.
The toxicity of Aroclors in birds increases with the percentage
chlorination (generally reflected in the final 2 digits of the Aroclor
number), according to the data of Hill et al. (1975) and Hill &
Camardese (1986). Hill et al. (1974) noted that the toxicity of
Aroclors is not simply a reflection of the chlorine content of the
different Aroclors. They adjusted the dietary content of the Aroclors
to a constant dietary chlorine level and found the same increased
toxicity with higher Aroclor numbers.
Dahlgren et al. (1972) reported some mortality in sub-adult pheasants
after regular oral doses of Aroclor 1254 ranging from 10 to 210 mg.
Mortality was related to both dose and body weight; heavier birds
lived longer, though they lost a greater proportion of body weight. A
sudden heavy intake of PCBs led to high brain residues. Brain residues
were best correlated with death; residues in the brain of about
300-400 mg/kg were considered by the authors to be diagnostic of acute
poisoning and death (Dahlgren et al., 1972). Stickel et al. (1984)
concluded that similar levels in the brain killed red-winged
blackbirds, starlings, brown-headed cowbirds, and grackles. Intake of
lower doses of PCBs over long periods does not lead to such high brain
residues; the cause of death after long-term exposure appears to be
oedema and related symptoms.
7.3.3.1 Short-term toxicity
Hurst et al. (1973) observed differential toxicity of PCBs between
bobwhite quail hens and cocks. This differential was eliminated when
the tests were conducted on birds not in the breeding condition and
with short daylengths. Females survived better than males, only when
they were laying eggs, and survival was well correlated with the
numbers of eggs produced. The authors concluded that females reduce
their exposure to PCBs by eliminating the compound in the eggs.
When Koeman et al. (1969) fed Japanese quail a diet containing 2000 mg
Phenochlor DP6/kg, all the dosed birds died between 6 and 55 days of
dosing. The quail developed hydropericardia at this dose level. Vos &
Koeman (1970) fed one-day-old cockerels a diet containing PCBs at
400 mg/kg, for 60 days; the PCBs were in one of the following forms,
Phenochlor DP6, Clophen A60, or Aroclor 1260 (all 3 are 60%
chlorinated). The mean survival time was calculated to be 24.3 days
for Phenochlor and 20.5 days for Clophen, only 3 out of 20 birds died
on the diet containing the Aroclor. Microscopically, centrolobular
liver necrosis was found in chicks fed the first 2 compounds. Atrophy
of the spleen and porphyria were observed in all dosed groups.
Table 35. Toxicity of PCBs for birds
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Bobwhite quail 10 days diet Aroclor 1221 5-d LC50 >6000 Hill et al. (1975)
(Colinus virginianus) 10 days diet Aroclor 1232 5-d LC50 3002 (2577-3501) Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 2098 (1706-2610) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 1175 (966-1440) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 604 (410-840) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 747 (577-937) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 871 (702-1069) Hill et al. (1975)
male 1 year oral Aroclor 1268 acute LD50 >2000 Hudson et al. (1984)
Japanese quail 14 days diet Aroclor 1221 5-d LC50 >5000 Hill & Camardese (1986)
(Coturnix coturnix 14 days diet Aroclor 1232 5-d LC50 >5000 Hill & Camardese (1986)
japonica) 14 days diet Aroclor 1242 5-d LC50 >6000 Hill & Camardese (1986)
14 days diet Aroclor 1248 5-d LC50 4819 (4267-5443) Hill & Camardese (1986)
14 days diet Aroclor 1254 5-d LC50 2929 (2516-3409) Hill & Camardese (1986)
14 days diet Aroclor 1260 5-d LC50 2195 (1861-2589) Hill & Camardese (1986)
14 days diet Aroclor 1262 5-d LC50 2304 (1978-2684) Hill & Camardese (1986)
Table 35. (cont'd).
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Mallard 10 days diet Aroclor 1221 5-d LC50 >5000 Hill et al. (1975)
(Anas platyrhynchos) 10 days diet Aroclor 1232 5-d LC50 >6000 Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 3182 (2613-3879) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 2798 (2264-3422) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 2699 (2159-3309) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 1975 (1363-2749) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 3008 (2461-3634) Hill et al. (1975)
male 8-9 months oral Aroclor 1242 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1254 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1260 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1268 acute LD50 >2000 Hudson et al. (1984)
Red-winged blackbird diet Aroclor 1254 6-d LC50 1500 Stickel et al. (1984)
(Agelaius phoeniceus)
Ring-necked pheasant 10 days diet Aroclor 1221 5-d LC50 >5000 Hill et al. (1975)
(Phasianus colchicus) 10 days diet Aroclor 1232 5-d LC50 3146 (2626-3948) Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 2078 (1843-3879) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 1312 (1166-1477) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 1091 (968-1228) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 1260 (1106-1433) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 1234 (1086-1402) Hill et al. (1975)
Table 35. (cont'd).
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Starling diet Aroclor 1254 4-d LC50 1500 Stickel et al. (1984)
(Sturnus vulgaris)
Brown-headed cowbird diet Aroclor 1254 7-d LC50 1500 Stickel et al. (1984)
(Molothrus ater)
Grackle diet Aroclor 1254 8-d LC50 1500 Stickel et al. (1984)
(Quiscalus quiscula)
a oral = acute oral test (result expressed as mg/kg body weight); diet = dietary test (result expressed as mg/kg diet).
Miranda et al. (1987) studied the effects of acute oral exposure of
Japanese quail to Aroclor 1242 (100, 250, or 500 mg/kg), or
2,4,2',4'-tetrachlorobiphenyl and 3,4,3',4'-tetrachlorobiphenyl (both
87.6 mg/kg) in corn oil. Control birds received only the corn oil. The
birds were killed after 48 h. All the PCB compounds caused a
significant increase in porphyrin content and delta- aminolevulinic
acid synthetase (ALA-S) activity in the small intestine and liver. All
the compounds increased the cytochrome P-450 content of the liver. In
the intestine, the P-450 content was only increased by Aroclor 1242
and 2,4,2',4'-tetrachlorobiphenyl. The activity of 7-ethoxyresorufin
O-deethylase was increased by all compounds in both the intestines
and liver. In the liver, 7-ethoxycoumarin O-deethylase (ECOD)
activity was unchanged or decreased, but, in the intestines, ECOD
activity increased with dose. No tissue differences in ECOD activity
were found after treatment with 2,4,2',4'-tetrachlorobiphenyl and
3,4,3',4'-tetrachlorobiphenyl. It was concluded that the small
intestine was more responsive than the liver to the porphyrinogenic
effect of a single oral dose of PCBs, and, that the induction of drug
metabolizing enzymes in the quail was tissue-specific, depending on
the PCB preparation used.
Day-old chicks were fed on a diet containing 500 mg PCBs/kg. All the
birds died between the third and the tenth week of dosing; a reduction
in the dosage to 250 mg/kg delayed the onset of death until the
thirteenth week (Platonow & Funnell, 1971). Mortality did not occur in
chickens dosed at 200 mg/kg over a period of 3 weeks (Flick et al.,
1965). Harris & Rose (1972) fed one-day-old broiler chicks diets
containing 100, 200, or 400 mg PCBs/kg (Aroclors 1242, 1254, and
1260). No mortality occurred at doses of up to 100 mg/kg, over a
period of 4 weeks. Over the same period, all the birds on Aroclor
1242, 60% of the birds on Aroclor 1254, and none of the birds on
Aroclor 1260, died, all at a dosage of 400 mg/kg. Holleman et al.
(1976) fed day-old broiler chicks and turkey poults on diets
containing Aroclor 1242 at 38, 75, or 150 mg/kg for 4 weeks. The
authors found increased mortality at 75 mg/kg (21% mortality) with the
chicks, but this was not significantly different from controls;
however, there was a significant increase in deaths at 150 mg/kg (75%
mortality). Significantly increased mortality was not found in the
turkeys at any dose level, but the mortality rate in the controls was
33%. Both the 75 and 150 mg/kg diets produced oedema and other lesions
attributed to PCB toxicity. Prestt et al. (1970) maintained Bengalese
finches on a diet containing various concentrations of Aroclor 1254.
The estimated dose rate for 50% mortality, over 56 days, was 254 mg/kg
per day.
7.3.3.2 Egg production
Most studies demonstrating the lowering of egg production by PCBs were
conducted on chickens. The most severe effects came from dosing with
Aroclors in the middle of the range of chlorination (Aroclors
1232-1254). The literature has been reviewed by Stendall (1976).
Platonow & Reinhart (1973) fed chickens with Aroclor 1254 at either 5
or 50 mg/kg diet over 39 weeks. Egg production was erratically reduced
with the lower doses and sharply reduced on 50 mg/kg diet. A dietary
dose of 2 mg/kg did not have any reproductive effects on chickens over
9 weeks (Lillie et al., 1974) or after 39 weeks (Platonow & Reinhart,
1973). Scott et al. (1975) showed a 10% reduction in egg production in
chickens related to egg residues of PCBs (Aroclor 1248) of 3 mg/kg.
When egg residues reached 4.5 mg/kg, the production rate was further
reduced. A significant reduction in egg production was demonstrated by
Call & Harrell (1974) after dosing Japanese quail with 3 different
Aroclors at 62.5-5000 mg/kg, over 33-264 days.
7.3.3.3 Hatchability and embryotoxicity
Aroclors reduced the hatchability of chicken eggs. In 2 studies,
Lillie et al. (1974) examined the effects on hatchability of Aroclors
1221, 1232, 1242, 1248, and 1268, all fed at 2 or 20 mg/kg diet, over
9 weeks. Aroclors 1221 and 1268, with low and high chlorination,
respectively, showed no effects at 20 mg/kg diet. Aroclor 1248
produced some adult mortality at 20 mg/kg diet and nearly eliminated
hatching of the eggs produced. Aroclor 1242 showed similar, but
slightly less severe, effects; there was even less effect with Aroclor
1232. Cecil et al. (1974) tested a similar range of PCBs at the same
dosages (2 and 20 mg/kg diet). They also reported no effects for
Aroclors 1221 and 1268. Aroclors 1254, 1232, 1242, and 1248 reduced
hatchability, as in the previous study. PCBs that reduced hatchability
also produced abnormalities in the chicks. The fertility of eggs was
not affected by any of the treatments. Females were artificially
inseminated with semen collected from males fed a similar diet of
PCBs. Scott (1977) dosed chickens with 0.5, 1, 10, or 20 mg PCBs/kg
diet and found no effect at the 2 lowest doses. Hatchability was
reduced at 10-20 mg Aroclor 1248/kg diet. Kosutzky et al. (1979) dosed
chickens with 2 other PCBs (Delor 103 and 105) (42 and 54%
chlorination, respectively), at 5 mg/kg diet, for 6 weeks; there was
little effect on hatchability, which returned to control levels soon
after a return to a clean diet. Solomon et al. (1973) studied the
effects of PCBs (Aroclor 1254) on pheasants. The birds were dosed at
weekly intervals, for 17 weeks, with gelatin capsules containing
50 mg/bird for the hens or 25 mg/bird for the cock birds. No effects
were observed on fertility and there was no increase in the numbers of
abnormal embryos. In another study, Ax & Hansen (1975) maintained
white leghorn pullets on a diet containing Aroclor 1242 or 1254 at
20 mg/kg, or 2,4,5,3',4'-pentachlorobiphenyl, for a period of 10
weeks. Average embryonic mortality was found to be significantly
increased, i.e., 54.7, 59.2, and 74% for the 3 compounds,
respectively. In the same study, the authors found that average
embryonic mortality in eggs laid by birds dosed with
2,5,2'-trichloro-, 2,5,2',5'-tetrachloro-, or 2,4,5,2',4',5'-
hexachlorobiphenyl was not significantly different from that in the
controls. When both broiler breeder hens and leghorn hens were fed
diets containing either 20 or 50 mg Aroclor 1242/kg, for 1 week, the
hatchability of the eggs laid was reduced by 67.3 and 26.8%,
respectively, of control levels, on the 50 mg/kg diet (Briggs &
Harris, 1973). Hatchability also was reduced at 20 mg/kg diet, but the
time required to achieve the same depression as that found with the
higher dose was doubled. Even after dosing had finished,
embryotoxicity continued and, in fact, increased until, after 6 weeks,
hatchability was between 0 and 10% of controls for both birds at both
doses.
Chickens given Aroclor 1254 at 50 mg/litre in the drinking-water, for
6 weeks, showed progressive reduction in egg hatchability. This fell
to zero after 3 weeks (Bush et al., 1974). Hatchability remained at
almost zero for the first 8 weeks of the chickens receiving control
water following dosing, but returned to normal after a further 8
weeks.
Platonow & Reinhart (1973) fed chickens on a diet containing 50 mg
Aroclor 1254/kg for 39 weeks. There was some adult mortality; egg
production and hatchability fell almost to zero. Residues of Aroclor
in the last eggs produced ranged between 25 and 50 mg/kg. After 6
weeks of uncontaminated food, egg residues dropped and hatchability
improved. The authors reported that egg residues of less than 5 mg/kg
had no effect on hatchability, whereas residues greater than
10-15 mg/kg led to embryotoxic effects. Scott et al. (1975) related
hatchability to egg residues. At residue levels of 3 mg/kg,
hatchability was reduced by 44%, and, at residue levels of 4.5 mg/kg,
it was reduced to almost zero.
The yolk-sacs of eggs from pheasant, mallard, goldeneye duck, and
black-headed gull were injected with 3,4,3',4'-tetrachlorobiphenyl at
0.1 mg/kg (pheasant and mallard) or 1.0 mg/kg (pheasant, goldeneye
duck and black-headed gull) after 4 or 5 days of incubation (Brunström
& Reutergardh, 1986). A significant decrease in the hatching rate was
seen only in pheasants, at the highest dose. At a dose of 1 mg/kg, all
the embryos died before hatching, but, at 0.1 mg/kg, no effect on
hatching was observed. No gross abnormalities were noted in either
hatched chicks or dead embryos. A great difference was noted by the
authors between the avian embryos in this study and chicken embryos,
with regard to sensitivity towards tetrachlorobiphenyl. In chicken
embryos, a dose of 0.004 mg/kg, administered on day 4 of incubation,
gave a significant reduction in hatching. At 0.02 mg/kg, no embryos
survived to hatching (Brunström & Darnerud, 1983). Carlson & Duby
(1973) injected Aroclors directly into chicken eggs on the first day
of incubation, or 9 days later. Aroclor 1242 severely limited
hatchability at levels of more than 2.5 mg/kg. Aroclors 1254 and 1260
had no effect at 10 mg/kg. Delaying the injection until day 9 of
incubation reduced the effect of Aroclor 1242. With 5 mg/kg injected
at day zero, hatchability was 8.3%; when the same dose was given at
day 9, 82% of eggs hatched. These results are not compatible with
those of Scott et al. (1971) who reported that most embryonic deaths
occurred late in incubation. However, these authors were not dosing
the eggs directly, but measuring the residues in eggs from dosed
females. PCBs administered directly into the eggs may not produce
effects comparable with those produced by the same material received
from the mother hen, because of different distribution in the egg.
Platonow & Reinhart (1973) dosed hens at 50 mg/kg diet. Early in the
study, the majority of embryo deaths occurred late in incubation. As
the study progressed, the time of embryo death moved to earlier in the
incubation period. Bush et al. (1974) showed that there was greater
mortality for any given egg residue as the period of dosing the mother
hen progressed. Their experimental chickens were dosed for 6 weeks at
50 mg/litre drinking-water and then kept for a further 20 weeks on
clean water. On day 11 of the study, an egg yolk residue of 50 mg/kg
was associated with 50% mortality in the embryos. On day 131, 50%
mortality was associated with an egg residue of only 10 mg/kg. The
greatest toxicity of PCBs for chicken embryos occurred after 11 weeks
of clean water, that is 17 weeks into the study. At this stage, the
eggs would be receiving doses of PCBs from material stored within the
hen. Late in the study, residues of between 6 and 8 mg/kg in egg yolks
(equivalent to 3.6 mg/kg whole egg) were correlated with between 14
and 36% mortality. Platonow & Reinhart (1973) reported that egg
residues greater than 10-15 mg/kg caused embryotoxic effects whereas
low residues of less than 5 mg/kg did not produce any effects.
Abnormalities were reported by Cecil et al. (1974) in 34% of 843
embryos that died during their study. The most common abnormality was
oedema, which was seen in 50% of all chicks showing any abnormality.
Tumasonis et al. (1973) also reported deformities in chicks.
7.3.3.4 Eggshell thinning
Since the 1950s, thin eggshells have been characteristic of many wild
bird populations, though the effect was not noticed until some time
afterwards. Thin shells has been a contributory factor to reduced
reproductive capacity, particularly in birds of prey. The main
chemical causing thin shells appears to be DDE, a metabolite of DDT.
It has been shown to cause thin eggshells in laboratory experiments,
as well as through the correlation of field data. Literature on thin
eggshells has been reviewed by Cooke (1973). Most experimental studies
using PCBs have shown no effect on shell thickness. Peakall (1971), in
the first controlled study, dosed ring doves at 10 mg PCBs (Aroclor
1254)/kg diet or at 25 mg (equivalent to 160 mg/kg body weight)
injected ip. Shells were ashed and weighed. Two separate studies on
dietary dosing showed no effect on shell weight. In the first study, 2
groups of birds were compared, in the second the same birds were used,
comparing their eggs before and after dosing. Injection of PCBs, 1-4
days prior to egg laying, also had no effect. Studies on mallard dosed
with Aroclor 1254 at 25 mg/kg diet and bobwhite quail dosed at
50 mg/kg diet over 2 years and also on mallard dosed at up to
500 mg/kg diet for 5 weeks (Heath et al., 1972) showed no effects on
shell thickness. There was an apparent shell thickening of about 6% at
the highest dose, which could not be statistically confirmed. The same
authors outlined results from a study on white leghorn chickens.
Aroclor 1242 at 10 or 100 mg/kg diet or Aroclor 1254 at 100 mg/kg diet
did significantly reduce shell thickness. There were no measurable
effects of: Aroclor 1242 at 1 mg/kg diet, Aroclor 1254 at 10 mg/kg, or
Aroclor 1260 at 100 mg/kg diet (Heath et al., 1972). Experimental
details and detailed results were not given for the work on chickens.
Lillie et al. (1974) dosed chickens with a range of Aroclors, at 2 or
25 mg/kg diet, for 9 weeks. Aroclors 1248 and 1242 greatly reduced the
hatchability of eggs and caused some adult mortality, but failed to
cause thinning of the eggshells. When Britton & Huston (1972) fed
single comb White Leghorns Aroclor 1242 at 80 mg/kg diet, for 6 weeks,
no effects on shell thickness were observed. Dahlgren & Linder (1971)
failed to demonstrate any deleterious effects on the eggshells of
pheasants, dosed by gelatin capsule, once a week for 17 weeks, with
doses of Aroclor 1254 up to 50 mg. Call & Harrell (1974) fed various
Aroclors in the diet to Japanese quail for 21 days. Very significant
shell thinning was found with Aroclors 1254 and 1260 at doses of 1250
and 1000 mg/kg diet, respectively. At these high doses, egg production
was severely diminished and shell dimensions were based on very few
eggs. Adult mortality might have occurred at these doses; the paper
does not make it clear whether this actually happened. At lower doses
of 78.1 and 62.5 mg/kg diet of Aroclors 1254 and 1260, respectively,
there was also significant egg-shell thinning and reduced egg
production. Aroclor 1242 was tested at 312.5 and 5000 mg/kg diet and
both doses caused shell thinning, though this was to a lesser degree
than with other Aroclors at similar doses. Risebrough & Anderson
(1975) showed that eggshells thinned by dietary DDE were not further
affected by adding PCB (Aroclor 1254) to the experimental DDE diet.
Results on shell thinning are, therefore, not completely clear. It is
generally agreed that PCBs do not affect birds in this way and the few
results suggesting shell effects are regarded as anomalous or
difficult to interpret, because of experimental design. Shell thinning
can occur because of several different direct and indirect factors.
DDE and sulfanilamide have direct effects on the deposition of calcium
in the shell or on its mobilization from the skeleton, which acts as a
calcium store. PCBs are more likely to affect shells indirectly by
reducing food consumption; none of the studies cited above reported
whether individual birds took less food because of the dosing with
Aroclors. Haseltine & Prouty (1980) fed 24 pairs of mallard with
Aroclor 1242 at 0 or 150 mg/kg diet for 12 weeks and reported a
reduction in shell thickness of 8.9%. They pointed out that all
females laying thin-shelled eggs showed a significant depression in
body weight. This, they regarded as sufficient explanation for the
shell thinning. Much of the shell thinning found in Japanese quail
eggs, laid by females given a single oral dose of Aroclor 1254 of
500 mg/kg body weight, was thought to be due to reduced food
consumption (Haegele & Tucker, 1974).
Biessmann (1982) did not find any effects on eggshell thickness on
dosing Japanese quail with Clophen A60 at levels of up to 150 mg/kg
diet. However, the breaking strength of the eggs was reduced.
Hill et al. (1976) fed 6-month-old laying Japanese quail hens a diet
containing 10 mg Aroclor 1242/kg, for 40 days. Eggs were collected and
measured and, after 40 days, were found to have significantly thinner
shells (5.2%) than the controls. The authors stated that the handling
of the birds and the diet had no effect on food consumption or hen
weights during the test. This is the only study showing shell thinning
at moderate dose levels without an effect on food consumption. The
question of whether PCBs can cause shell thinning, therefore, remains
open.
7.3.3.5 Effects on the male
Platonow & Funnell (1971) kept day-old, white leghorn cockerels on a
diet containing 250 mg Aroclor 1254/kg, for up to 13 weeks. They found
a significant reduction in the weight of both combs and testes after 9
and 13 weeks and, a reduction in comb weight, only, after 6 weeks of
dosing. In a later study, Platonow & Funnell (1972) found a more
severe effect at 500 mg/kg; the comb was significantly reduced in
weight after just one week of dosing and the testicular weight
significantly reduced after 4 weeks, relative to the controls. The
control combs and testes increased in weight during the course of the
study; treated birds failed to develop either comb or testes.
Lillie et al. (1974) did not find any effects on weight gain, food
intake, or semen characteristics in leghorn cockerels fed Aroclor 1248
at 10 or 20 mg/kg diet for 8 weeks. They also did not find any effects
on fertility or hatchability of fertile eggs laid by similarly dosed
females. Liver weights were significantly increased at both dose
levels, and heart weights were significantly decreased at the highest
dose.
7.3.3.6 The effects of stress
Stress, imposed in various ways, increases the sensitivity of birds to
PCBs. Stress seems to have its effect by increasing the mobilization
of fat. Lower fat storage decreases the attenuation of PCB toxicity
seen when fat uptake of the material acts as an effective temporary
detoxification mechanism. Dahlgren et al. (1972) showed that brain
residues were higher in pheasants subject to starvation stress than in
unstressed birds dosed at the same rate. deFreitas et al. (1972)
obtained similar results using cold stress or starvation in pigeons.
As a corollary, biochemical adaptation to stress is inhibited by
exposure to PCBs. This is presumed to be due to residues in non-lipid
tissues (Dieter, 1974).
7.3.3.7 Physiological, biochemical, and behavioural effects
Jefferies & Parslow (1972) dosed young lesser blackbacked gulls
(Larus fuscus) with daily gelatin capsules containing Aroclor 1254
at 50, 100, 200, or 400 mg/kg body weight, for 8 weeks. Mean
individual thyroid weights were significantly increased by 32% (taking
all dosed birds as a single group). There was also an increase in the
mean cross-sectional area of the thyroid. There was, however, no
dose-related effect of PCBs on thyroid weight. The same authors
(Jefferies & Parslow, 1976) showed a similar effect of increased
thyroid weight when they dosed guillemots (Uria aalge) for 45 days
with Aroclor 1254 at 12 or 25 mg/kg body weight. Hurst et al. (1974)
also found a significant stimulation of thyroid growth after feeding
bobwhite quail a diet containing 5, 50, or 500 mg Aroclor 1260/kg for
4 months. Spear & Moon (1985) raised ring doves on either a low iodine
or normal diet. Insufficient iodine caused thyroid hyperplasia. This
hyperplasia was reversed within 7 days by a single dose of
3,4,3',4'-tetrachlorobiphenyl at 60 mg/kg body weight. The PCB
treatment also caused a significant decrease in core body temperature
and serum total thyroxine (T4) and triiodothyronine (T3). No effect,
other than decreased serum T3 and T4, was caused by dosing doves with
PCBs on a diet containing normal iodine levels.
Behavioural effects of PCBs have been noted by several authors.
Peakall & Peakall (1973) reported decreased parental attentiveness in
ring doves dosed at 10 mg Aroclor 1254/kg diet. Kreitzer & Heinz
(1974) measured the avoidance response (from a moving silhouette) in
Japanese quail chicks, for 14 days before, and 8 days after, dosing
with Aroclor 1254 at 200 mg/kg diet. After dosing, the avoidance
response was significantly reduced. Normal responsiveness to the
silhouette was not recovered after 6 days on a clean diet. Two
examples of hyperactivity in birds were also noted. European robins
fed one mealworm/day containing 5 µg Clophen A50, for 11-13 days,
showed increased migratory restlessness (Ulfstrand et al., 1971).
There were similar tendencies in redstart fed one mealworm/day,
containing 11µg Clophen A50, for 12 days, it was estimated that the
birds had ingested 132 µg of PCB overall (Karlsson et al., 1974).
A reduction was reported by Dobson (1981)in the nest-building activity
of pigeons (Columba livia), dosed orally, by gelatin capsule, with
15 mg Aroclor 1254/day, throughout a courtship cycle. The birds
produced a nest but the number of twigs used was reduced compared with
the controls. Reproductive and thyroid hormones were measured in blood
plasma samples, taken each day during the courtship cycle. While the
patterns of hormone secretion remained the same in both control and
treated birds (rises and falls of hormone levels occurred at
comparable times in the 2 groups) the absolute circulating levels of
the hormones were changed by the treatment. Both thyroxine and
luteinising hormone levels in the treated birds were elevated relative
to the controls. The levels were significantly higher, except at the
beginning and the end of the cycle. It was concluded that hormone
levels were unaffected, except when they would naturally be changing,
suggesting an interference with the feedback control of hormone
secretion and a central nervous site of action. Tori & Peterle (1983)
kept mourning doves (Zenaida macroura carolinensis) on a diet
containing Aroclor 1254 at 10 or 40 mg/kg for 42 days. The doves were
then paired and observed each day for 30 days. Both treatments
significantly increased the mean number of days in the courtship
phase; only 4 out of 8 pairs on 10 mg PCBs/kg completed this phase and
moved onto the nesting phase; none of the birds on 40 mg/kg had
completed the courtship phase within 30 days. Behaviour was scored to
measure intensity and, at both doses, this was significantly reduced
overall. Although dosed birds formed pair-bonds approximately 4 days
sooner than controls, there was no significant difference in the
length of the pair-bond formation period or in behaviour scores during
this period in doves fed 10 mg/kg. The length of time spent in the
courtship period was extended significantly (by 8.5 days) by PCBs at
10 mg/kg. Behaviour scores were not significantly affected, but dosed
birds averaged 32% lower scores. Of the birds reaching the nesting
phase, there was no significant difference between controls and dosed
birds with regard to length of time spent nesting or behaviour scores
in the nesting phase. PCBs, however, significantly delayed the onset
of nest initiation (by approximately 7 days) and, therefore, egg
laying.
Japanese quail were dosed with Clophen A60 in the feed at 150 mg/kg,
from the first week of life up to 42 days of age, while the birds were
developing sexually and becoming reproductively mature (Biessmann,
1982). In females, progesterone levels were not greatly affected by
the PCBs, but estradiol levels in blood plasma were lower before
sexual maturity and were less stable during egg laying. In males,
levels of testosterone and dihydrotestosterone (the primary
metabolite) were not affected. Quail fed up to 150 mg Clophen A60/kg
diet during the time of sexual maturation (second to fourth weeks of
age) showed delayed onset of egglaying and a diminished capacity to
lay eggs. Hormone levels in both males and females were not
significantly different from those in the controls.
A possible mechanism for central nervous effects was provided from
studies on neurotransmitters. Dopamine and noradrenalin were depleted
in the brain of the ring dove in a dose-related manner with increasing
brain residues of PCBs (Heinz et al., 1980).
7.3.3.8 Interactive effects with other chemicals
The only information on the interaction between PCBs and other
chemicals in birds has shown PCBs to be additive and not synergistic.
Kreitzer & Spann (1973) carried out tests on several pairs of
chemicals to study pesticidal synergism in young pheasants and
Japanese quail. Two PCBs were used in the study. Aroclor 1262 and
malathion showed additive results, when fed in the diet to 16-day-old
Japanese quail. Aroclor 1254 and DDE were also additive, when given in
the diet to 9-day-old quail. Another study on the possible interactive
effects of PCBs was conducted by Heath et al. (1972), who found that
feeding Aroclor 1254 and DDE in the diet to 14-day-old Japanese quail
gave additive results. There was no evidence of mutual potentiation or
antagonism.
7.3.4 Terrestrial mammals
Acute oral LD50 values reported for PCBs in mink ranged from >750 to
4000 mg/kg body weight. Acute LD50 values for 3 Aroclors in mink were
determined by Aulerich & Ringer (1977) after administration orally, by
gavage, or by intraperitoneal injection. Mortality was assessed 4 days
after i.p. administration and 14 days after oral administration. The
lethality of the Aroclors was found to be inversely related to the
chlorine content; Aroclor 1221 was most toxic and Aroclor 1254 least
toxic (Table 36). This is in marked contrast to the situation in
birds, where toxicity was correlated positively with chlorine content
of the PCBs (see section 7.3.3).
Table 36. Acute toxicity of Aroclors for minka
Aroclor LD50 (mg/kg body weight)
Intraperitoneal Oral
Aroclor 1221 >500-<750 >750-<1000
Aroclor 1242 1000 >3000
Aroclor 1254 >1250-<2250 4000
a From: Aulerich & Ringer (1977).
7.3.4.1 Short-term toxicity
Ferrets (Mustela putorius furo), fed a diet containing 20 mg of
Aroclor 1242/kg, for 8 months, developed enlarged, thickened, and
deformed toe-nails with hyperkeratosis at the junction of the skin and
sponchium, and dysplasia of the root of the nail and the matrix. The
same diet containing Aroclor 1016 did not produce these effects
(Bleavins et al., 1982).
Bleavins et al. (1980) showed the ferret to be less sensitive to PCBs
than the mink, though LD50 values were not determined. Aroclor 1242
at 20 mg/kg diet killed all mink (3 males and 12 females) to which it
was fed. The same diet did not kill any ferrets, though it did cause
reproductive failure.
No mortality occurred in mink fed a diet containing 1 mg PCBs/kg over
183 days (Wren et al., 1987a).
Hornshaw et al. (1986) conducted 28-day LC50 tests on mink, using
Aroclor 1254, in a study to investigate the effects of age, season,
and diet on the toxicity of PCBs; no effects were noted on any of
these parameters. In replicate tests, the calculated LC50 values
varied between 79 and 84 mg/kg diet (48-132, range of confidence
limits). The authors noted that the period of observation after dosing
was critical in assessing the results, since mortality continued after
dosing had stopped and the PCBs were persistent in the body. Taking
total mortality over 28 days of dosing and a further 7 days of
observation, the LC50 values fell to between 47 and 58 mg/kg diet.
A consistent finding among various studies is an effect of PCBs on
food consumption and, therefore, on body weight. The most detailed
analysis of food consumption during the feeding of Aroclor 1254 to
mink is presented by Hornshaw et al. (1986). Young mink fed the
Aroclor over 28 days showed a dose-dependent decrease in the amount of
food consumed. The cumulative weight of food consumed over 5 weeks
(1 week predosing and 4 weeks dosing) for controls was 7574 g. This
was reduced progressively with increasing dose of Aroclor 1254 at 10,
18, 32.4, 58.3, and 105 mg/kg diet to 6447, 6153, 4816, 3556, and
2723 g, respectively. This led to loss of original body weight over
the study period rising to more than 40% at the highest dose. Similar
effects were seen in adults of both sexes; females, with a smaller
initial body weight, were more severely affected than males. This
effect has implications for the interpretation of results of dietary
toxicity tests. The effects seen are a combination of the direct toxic
effects of the compound and the indirect effects of progressive
starvation. The doses of PCBs to which the animals are exposed must
also be calculated with reduced food intake in mind; apparent doses
are higher than real exposure as dose rates increase.
Organ weights (expressed as a percentage of brain weight) were
unaffected by Aroclor 1254 at doses up to 105 mg/kg diet, with the
exception of the heart and the adrenal glands. Heart weight was
reduced in both adult and young mink fed Aroclor 1254 at 58 mg/kg diet
or more; adrenal weights were increased by doses of 13 mg/kg diet or
more (Hornshaw et al., 1986).
Female minks received 0.1 or 0.5 mg of 3,4,5,3',4',5'-
hexachlorobiphenyl/kg diet, or 2.5 or 5.0 mg of 2,4,6,2',4',6'- and
2,3,6,2',3',6'-hexachlorobiphenyl/kg diet for 12.5-14.5 weeks. In both
studies, 3,4,5,3',4',5'-hexachlorobiphenyl was the most toxic isomer,
causing high mortality and reduced body weights (Aulerich et al.,
1985).
Clark & Prouty (1977) fed female big brown bats (Eptesicus fuscus)
on a diet of meal-worms containing 10 mg Aroclor 1254/kg. After the
feeding period of 54 days, the bats were starved to simulate loss of
body fat during the period of migration (when the animals do not
feed). Two out of 12 bats died. The brain residues of 20 mg PCBs/kg at
the end of the study were considered to be sub-lethal, since no
neurotoxic symptoms were observed before death.
7.3.4.2 Reproductive effects
Experimental investigations of mink reproduction in relation to
environmental pollution were carried out as a result of the reduced
reproductive success seen after feeding farm mink with fish from the
Great Lakes. Early studies, therefore, involved the analysis of fish
for pollutants and the experimental feeding of both the fish and of
mixtures of chemicals contained in various fish in the Great Lakes.
Aulerich & Ringer (1977) performed a comprehensive series of feeding
studies using coho salmon from 2 of the Great Lakes (Michigan and
Erie), other fish species from the same source, salmon from the west
coast of the USA, and various combinations of organochlorine
contaminants.
In their first study, ocean fish (perch or whiting) were used as
control diets and the reproductive performances were compared of dosed
and undosed female mink mated with undosed males. Lake Michigan coho
salmon, as 30% of the diet, had the most severe effect on
reproduction, i.e., total reproductive failure, as measured by numbers
of live kits surviving 4 weeks after parturition. Coho salmon from
Lake Erie also reduced reproductive success; 12 females produced only
7 kits still alive 4 weeks after birth. Two other species of fish from
Lake Michigan produced less severe effects, 5 kits being produced on a
diet of 30% bloater chub and 15 kits, on a diet of yellow perch.
Controls produced more than 40 kits over the same period. Kits
produced on Lake Michigan or Lake Erie fish, other than salmon, and
surviving to 4 weeks of age showed significantly lower body weights
than the controls. The small numbers of surviving kits from mothers
fed Lake Erie salmon also showed reduced body weight at 4 weeks of
age. No kits were produced after feeding Lake Michigan salmon diets to
females (Aulerich & Ringer, 1977).
In a long-term, low-level feeding study, 4 different Aroclors (1016,
1221, 1242, and 1254) were included in the diet of mink at a rate of
2 mg/kg. Groups of 8 female and 2 male animals were given this, or a
control, diet for 11 months, from August to June. The reproductive
performance of the animals on different diets is summarized, together
with mortality, in Table 37. Body weight, haemoglobin levels, and
haematocrit were monitored at monthly intervals during the study and
no significant effects of PCBs were noted. Only one of the test diets
(containing Aroclor 1254) adversely affected reproduction, with only 1
live birth in the study period. This single kit was considerably
lighter at birth than the controls and failed to survive 4 weeks after
birth (Aulerich & Ringer, 1977).
The reproductive effects of either Lake Michigan coho salmon or
Aroclor 1254 were reversible, when animals were transferred to a
control diet. Eleven females fed salmon as 30% of the diet for a year,
and then given control food for a further year, produced young with an
average litter size of 3.5 kits per mated female, in the second year
of the study. No young had been produced in the first year of the
study, during dosing. Similarly, 3 females given a year of control
food following a year on a diet containing 5 mg Aroclor 1254/kg,
produced an average of 4.3 young per mated female in the second year
of observation (Aulerich & Ringer, 1977).
In a later study, Bleavins et al. (1980) fed 2 different Aroclors at
various dose levels to mink and ferrets. Aroclor 1242 was fed to mink
at doses ranging from 5 to 40 mg/kg diet; Aroclor 1016 was given at
only 20 mg/kg diet. Results for mortality and reproductive effects are
summarized in Table 38, together with some data for Aroclor 1254 taken
from Aulerich & Ringer (1977).
There was a clear reproductive effect of Aroclor 1242 at a dose of
5 mg/kg diet, but no significant mortality. Aroclor 1242 caused 66%
mortality at 10 mg/kg diet and 100% mortality at 20 mg/kg diet.
Aroclor 1016, at 20 mg/kg diet, caused some deaths of adults and
reduced birth-weight and survival of kits, but the reproductive
effects were considerably less severe than those caused by Aroclor
1242 at 5 mg/kg diet. The authors (Bleavins et al., 1980) calculated
dietary LC50 values for Aroclors 1242 and 1254, using their own data
and data from Aulerich & Ringer (1977), to be 8.6 and 6.7 mg/kg diet,
respectively. It is clear that reproductive effects are less marked at
lower levels of chlorination of Aroclors.
Table 37. Effects of Aroclors on mortality and reproduction in minka
Aroclorb Adult females Kits
Number Number Number Number born Whelped/ Alive at Average weight
died (%) mated whelped female 4 (g ± SE) at
live dead mated weeks birth
Control 0 8 8 28 5 4.1 18 9.9 ± 0.32
1016 0 8 8 28 8 4.5 16 9.2 ± 0.33
1221 12 7 7 43 1 6.3 37 9.6 ± 0.22
1242 12 7 7 35 4 5.6 32 9.3 ± 0.27
1254 12 7 2 1 1 0.3 0 5.4
a From: Aulerich & Ringer (1977).
b Aroclors all fed at 2 mg/kg diet.
Reproductive effects on mink were reported by Jensen et al. (1977),
who fed groups of 10 females at 0.05, 3.3, or 11 mg PCBs/kg diet (type
unspecified), for 66 days. The highest dose eliminated successful
reproduction, whereas 3.3 mg/kg severely reduced the number of kits
born per female.
Table 38. Summary of mortality and reproduction in mink fed various dietary
levels of Aroclors
Treatment level Period fed Number dead/ Number of kits/
(mg/kg diet) (days) total number female
Aroclor 1254a
0 280 1/7 5.0
0 297 0/8 4.1
2 297 1/8 0.3
5 280 2/7 0.0
10 280 5/7 0.0
Aroclor 1242b
0 247 3/30 4.9
2 297 1/8 5.6
5 247 1/15 0.0
10 247 10/15 0.0
20 192c 15/15 0.0
40 138d 15/15 0.0
Aroclor 1016b
0 247 3/30 4.9
2 297 0/8 4.5
20 247 3/15 6.3
a From Aulerich & Ringer (1977).
b From: Bleavins et al. (1980).
c All mink died within 192 days on diet.
d All mink died within 138 days on diet: no females survived to whelping.
Wren et al. (1987b) did not find any significant effects on numbers of
kits produced or surviving to weaning age (5 weeks) after feeding mink
with Aroclor 1254 at 1.0 mg/kg diet, over 183 days. The dosing period
covered the seasonal period when the animals came into breeding
condition as well as a period of giving birth to the young. Although
the weights of kits born to dosed females were not significantly
different at 1 week postpartum, the weight gain of kits was then
affected and weights were significantly different from those of the
controls at ages 3 and 5 weeks. At age 5 weeks, when the kits were
weaned, the mean body weight of kits of the controls was 227.8 g,
while the mean body weight of kits fed by dosed mothers was 161.2 g.
Similar effects on the reproduction of female mink fed PCBs (type
unspecified) were observed by Jensen et al. (1977), i.e., a reduction
in the numbers of whelps born per pregnant female. The authors killed
the females after they had given birth and examined the numbers of
implantation sites in the uterus. This did not differ statistically
between groups (on average, 6.6 in control females, 6.1, in females
fed 5 mg/kg diet, and 4.5, in females fed 15 mg/kg diet PCBs).
However, the number of kits born to the same females showed a marked
effect of the PCBs: 5.1 (on average) born to control mothers; 2.9, to
mothers fed 5 mg/kg diet PCBs, and 0, to mothers fed 15 mg PCBs/kg
diet. The authors concluded that the effects of PCBs occur at the time
of implantation or later, causing resorption of implanted embryos.
Male mink seemed unaffected by doses of Aroclor that caused
reproductive effects in females. Males, dosed and mated with undosed
females, fathered normal numbers of kits (Aulerich & Ringer, 1977).
Wren et al. (1987b) did not note any effects of Aroclor 1254, fed to
male mink over 183 days at a rate of 1 mg/kg diet. Testicular size and
testicular histology were unaffected by the PCBs at any stage of the
reproductive cycle.
Treatment of adult, male, white-footed and white mice with Aroclor
1254 in the diet at a level of 400 or 200 mg/kg (equivalent to 57 or
29 mg/kg body weight), for 2 weeks resulted in a reduced testicular
spermatozoan concentration and, in the white-looted mice, a reduced
absolute weight of the seminal vesicles. In both strains, the absolute
weights of the testes and the final body weights were unchanged
(Sanders & Kirkpatrick, 1975; Sanders et al., 1977).
The reproductive performance of 27 pairs of white-looted mice (44-222
days of age), was compared with that of 26 control pairs, within 60
days of exposure to Aroclor 1254 at a dietary level of 200 mg/kg.
One-third of the exposed pairs did not survive the exposure but
produced at least one litter. The number of pairs producing at least
one litter was reduced by 65% and the number producing 2 or more
litters was reduced by 91%. Litter size was not affected. No offspring
survived to weaning in the 7 first litters of PCB-fed pairs (Merson &
Kirkpatrick, 1976).
A group of 10 pairs of wild-caught, white-footed, mice and groups of
18 (12 weeks of age) and 19 (16 weeks of age) pairs of
laboratory-raised, white-looted mice received Aroclor 1254 in the diet
at 10 mg/kg. Control groups comprised 10, 15, and 20 pairs,
respectively. The reproductive performance of the first group was
recorded for 18 months. The duration of the other studies varied from
7 to 15 months. The number of young per litter, 28 days after birth,
was lower in all treated groups. In laboratory-raised mice paired at
12 weeks of age, the birth interval was increased and the number of
young per litter at birth reduced (Linzey, 1987a). The second
generation of mice, maintained on the same diet as their parents, did
not differ in weight at birth, but were significantly smaller at 4, 8,
and 12 weeks of age. A similar trend was observed in the few young of
the third generation. The uterus, ovaries, and accessory glands, but
not the testes, weighed less in exposed groups than in the controls
(Linzey, 1987b). Linzey (1987a) reported similar reproductive effects
of Aroclor 1254 at a much lower dose.
The author suggested that the major consistent effect on the survival
of the young being fed milk was the result of much higher levels of
PCBs being transported via lactation than via the placenta.
Cottontail rabbits (Sylvilagus floridanus) were fed Aroclor 1254 at
10 mg/kg diet for 12 weeks, and then transferred to a clean diet and
allowed to breed. No effects were observed on any reproductive
parameters, and reduction in food availability did not change this
lack of effect (Zepp & Kirkpatrick, 1976).
7.3.4.3 Physiological effects
Wren et al. (1987a) examined histologically various organs in male and
female mink, dosed for 183 days with Aroclor 1254 in the diet. At
autopsy, no effects were seen on the histology of the pituitary and
adrenal glands. Brain histology also appeared normal. Thyroid
follicles gave the general appearance of minimal activity but did not
differ between treatments. Measurement of plasma thyroid hormones
(T3 -triiodothyronine; T4 -thyroxine) did not show any significant
differences between treatments in male mink. Females showed reduced
circulating T3 in a single sample, in January, but no other
differences were seen at other stages of the study. Thyroxine levels
were not affected at any time.
7.4 Effects on organisms in the field
The acute toxicity of PCBs is relatively low for most species and will
not, therefore, kill enough individuals to affect populations.
However, because of the high potential for bioaccumulation sufficient
residues of PCBs may build up to cause direct lethal effects over
time. Although PCBs are almost universally present in the tissues of
organisms in the environment, there are relatively few examples of
proved effects of these residues on populations of the organisms.
Sublethal effects, affecting populations by reducing reproduction or
growth, are possible, but difficult to prove, because PCBs are always
present with other environmental contaminants. Many possible effects
of PCBs in the environment have been suggested in the literature, but
few have actually been investigated in the field. It has proved
difficult, if not impossible, to relate residues of PCBs in tissues to
possible sublethal toxic effects; residues found after laboratory
dosing cannot be directly related to the field situation.
7.4.1 Plants
Klekowski (1982) studied the ostrich fern (Matreuccia struthiopteris)
growing in the flood plain of the Housatonic River, Massachusetts,
USA. This area of the river is contaminated with PCBs from land-fill
sites containing waste materials from the manufacture of transformers
in the nearby city of Pittsfield. Contamination with PCBs (principally
Aroclor 1254) had been a regular feature of the river area for a
period of more than 40 years. The frequency of somatic mutations in
the fern population was compared to a control population from an
uncontaminated area. The levels of PCBs in river sediments ranged from
1.4 to 139 mg/kg dry weight; at the site where the majority of fern
spores were collected, the level of PCBs was 26.3 mg/kg. The somatic
mutation frequency for the contaminated population was 5.2-6.2 times
higher than that for the controls. It is not known whether similar
genetic damage had occurred in other inhabitants of this contaminated
habitat. No other studies seem to have been conducted on the possible
effects of PCBs, from land-fill sites, on plants.
7.4.2 Fish
There have been many suggestions in the literature that PCBs might
affect populations of fish in the wild. Studies attempting to
demonstrate such an effect are few and, generally, inconclusive or
negative.
Olofsson & Lindahl (1979) used the ability of the cod (Gadus morrhua)
to react to different velocities of water under rotary flow, to
examine the effects of water pollution. Cod sampled from polluted
waters off the Swedish Coast were compared with cod sampled from
unpolluted areas. The ability of the fish to react to rotary flow was
significantly reduced in animals from polluted areas. However, the
authors were unable to relate the reduced reaction of the fish to
levels of various pollutants measured in muscle tissue. Experimental
studies showed that PCBs affected the reactions of the fish; the
residue level in muscle that was associated with this effect was
1.8 mg/kg. This was 30 times greater than the actual residues of PCBs
measured in the cod from the polluted area. While the authors stated
that the distribution of the PCBs in experimental fish and fish taken
from the wild would almost certainly have been different, that PCBs
exert the above effect in the field must be regarded as not proved.
Zitko & Saunders (1979) collected eggs of Atlantic salmon (Salmo
salar) from various areas and measured the PCB contents of the eggs
that proved infertile. The hatchability of different batches of eggs
was tested in the laboratory. No correlation was found between
residues of PCBs and the hatchability of the eggs; in fact the batch
of eggs showing the lowest hatchability also showed very low residues
of PCBs. However, it should be stated that hatchability was seldom
affected by PCBs in laboratory experiments; effects were more usually
seen on the developing young. Hogan & Brauhn (1975) related the
survival of fry hatched from rainbow trout (Salmo gairdneri) eggs to
the contamination of the eggs with PCBs. Five batches of eggs hatched
in 1971 had shown percentage mortalities, 30 days after hatching,
ranging from 10 to 28%. The eggs contained total organochlorine
residues of between 0.31 and 1.30 mg/kg, the majority of which was
PCBs. A batch of eggs collected in 1972 showed 75% mortality, 30 days
after hatching. Many of the fish hatched with such deformities as
scoliosis, lordosis, kyphosis, absence of caudal vertebrae, cranial
deformities, and projecting mandibles. This batch of eggs contained
2.7 mg PCBs/kg and 0.09 mg total DDT/kg (metabolites present not
stated).
Westin et al. (1983) investigated the effects of PCBs, passed on to
the eggs of striped bass (Morone saxatilis) by the female fish, and
of PCBs in food organisms fed to the hatched larvae. The hatchlings
were fed on brine shrimp ( Artemia sp.) from 2 sources, one
contaminated with PCBs and the other not. No effects of either
maternal PCBs or PCBs from the food were found. Residues of PCBs in
young fish decreased consistently throughout the study, which was
conducted in water free of PCBs. The authors suggested that PCBs from
the mother and from food are unlikely to affect the offspring in the
wild. A similar conclusion was drawn about the survival of lake trout
(Salvelinus namaycush) fry, hatched from eggs contaminated with PCBs
in the wild (Willford, 1980). Eggs taken from the wild and hatched in
clean water showed good survival of the offspring (suggesting that
residues of PCBs in the eggs were not responsible for the failure of
the species in Lake Michigan). However, experimental exposure of eggs,
and hatched larvae/fry to levels of PCBs in water and food, similar to
those found in the lake, led to high mortality and the increased
occurrence of deformities in fry. The author concluded that the levels
of PCBs in lake water and food items would be sufficient to lead to
the population decline seen in the lake. It should, however, be
pointed out that other factors in Lake Michigan could also have
contributed to the failure of the lake trout. Sea lampreys had
increased in number in the lake and could have caused population
decline by predation.
The relationships between the occurrence of hepatic diseases and
specific chemicals present in sediment were studied by Malins et al.
(1987). The concentrations of PCBs in the sediments from 4 urban and 2
non-urban areas in Puget Sound USA, were determined. In 3 of the
sites, the concentrations were <0.01 and, in the other 3, the
concentrations ranged from 0.11 to 0.53 mg/kg. Over 900 individual
organic compounds were found. To study the fish diseases, English sole
(Parophrys vetulus), rock sole (Lepidopsetta bilineata), and
Pacific staghorn sculpin (Leptocottus armatus) were collected. The
organs of fish containing the greatest number of lesions were the
liver, kidneys, and gills. Liver cell adenoma, hepatocellular
carcinoma, cholangiocellular carcinoma, haemangioma, and fibroma
constituted major types of liver lesions. Statistically significant
correlations between levels of chemicals in sediment and hepatic
neoplasms in the bottom-dwelling fish suggest a general
cause-and-effect relationship, but there is little firm evidence about
the actual cause of these neoplasms, in particular, in this case,
whether PCBs were involved.
7.4.3 Birds
In studies on chickens and different PCB-mixtures, Vos et al. (1970)
and Vos & Koeman (1970) found that the induction of subcutaneous and
abdominal oedema, centrilobular liver necrosis, hydropericardium, and
higher mortality was more or less related to the presence of
tetrachloro- and pentachlorodibenzofurans, as impurities in the PCB
samples.
From the middle of February to the end of March 1968, an epizootic
disease closely resembling chicken oedema disease occurred in Japan.
Two million chickens were involved, of which 400 000 (20%) died. The
clinical signs were laboured breathing, droopiness, ruffled feathers,
high mortality, and decreased egg production. Autopsy revealed marked
subcutaneous oedema, hydropericardium, ascites, pulmonary oedema,
muscular ecchymosis in the thorax or inside of the thigh, and
yellowish mottled appearance of the liver. The cause of the disease
was found to be Kanechlor 400 contamination of the feed. Experimental
reproduction of these symptoms with Kanechlor 400 was successful. The
remaining sample chicken feed contained 1300 mg of Kanechlor 400/kg
feed (Kuratsune et al., 1972).
Administration of PCBs leads to an atrophy of lymphoid tissue in
chickens (Flick et al., 1965; Vos & Koeman, 1970), and in pheasants
(Dahlgren et al., 1972). Vos & de Roij (1972) and Vos & van
Driel-Grootenhuis (1972) came to the conclusion that these effects
could be attributed to an immunosuppressive effect of PCBs. Vos & de
Roij (1972) suggested that the ability of PCBs to increase the
susceptibility of ducklings to duck hepatitis virus (Friend & Trainer,
1970) and of fish to fungal disease (Hansen et al., 1971) could be
attributed to this immunosuppressive effect.
Koeman et al. (1973) analysed 6 adult cormorants (Phalacrocorax carbo
sinenis), found dead in the wild, for PCB residues. Three additional
birds were shot and 6 fully grown nestlings were also taken. The
authors also carried out a study on 5 cormorants that were dosed with
the PCB, Clophen A60 (a 60% chlorinated PCB mixture that seemed to
correspond most closely with the PCBs profile of material found in
dead birds taken from the wild). The birds were initially dosed with
the PCBs in the diet, but, subsequently, the PCBs were administered
orally in gelatin capsules, until they died. PCB levels in the brains
and livers of the dead birds found in the wild were higher, overall,
than the levels obtained by dosing. The authors thought it highly
probable that this was indicative of PCB poisoning of the birds in the
wild. Without further detailed studies, this conclusion can only be
implied. The higher residues in dead birds from the wild suggest that
a large body burden was taken up, relatively safely in fat, and
released quickly on starvation, prior to death.
A field study, to show the effects of a sub-lethal dose of PCBs on
puffin (Fratercula arctica) breeding success and survival, was
conducted by Harris & Osborn (1981). A total of 150 puffins, trapped
on the Isle-of-May National Nature Reserve, Fife, Scotland, were
implanted, 108 with between 30 and 35 mg of PCBs (Aroclor 1254) and 42
with sucrose as controls. The test chemicals were implanted in
open-ended silastic tubes into the peritoneum. All birds were then
marked and released back into the wild. The same birds returned in
successive years to breed on the island. Breeding and survival of the
implanted birds were monitored through the breeding seasons of 1977,
1978, and 1979. Observations on survival were also made in 1980. Some
implanted birds were killed on recapture, and analysed for PCBs, in
each of the years of observation. No effects on survival or breeding
were seen. PCB levels in fat increased by a factor of between 10 and
14 compared with levels in birds that had not been implanted with the
Aroclor.
Herring gull (Larus argentatus) reproductive success in the area of
the Great Lakes declined with increasing residues of organochlorines
in the birds. Breeding success improved as these residues fell in the
1970s (Weseloh et al., 1979). The species was chosen as an indicator
of environmental pollution in the area and has been extensively
studied. Poor nesting success in the species is related to high
embryonic mortality (Gilbertson & Hale, 1974). Abnormal chicks have
been reported for herring gulls and also for other fish-eating species
from the area including: night herons, ring-billed gulls, common
terns, and Caspian terns (Gilbertson et al., 1976). Eggs from these
species contained residues of PCBs, but it was not possible to
directly relate these residues to chick abnormalities. Gilbertson &
Fox (1977) found a correlation between total organochlorine content
and the hatchability of herring gull eggs. While it cannot be shown
that PCBs were directly responsible for the overall effect, PCB
residues in the livers of hatched chicks, were the only contaminant
that was significantly correlated with the presence of pericardial
oedema. Weseloh et al. (1979) concluded that the reproductive success
of the herring gull can only safely be correlated with total
organochlorine residues and that the possible effects of PCBs cannot
be isolated. Shell thinning usually attributed to DDE alone, does not
occur significantly in these gulls. Hays & Risebrough (1972) suggested
that PCB residues in terns were responsible for the high percentage of
abnormal young in a colony from Long Island Sound.
Twenty-four black-crowned night heron (Nycticorax nycticorax) eggs
were collected at the San Francisco Bay National Wildlife Refuge in
1983. Twelve of these were collected from separate nests, when
late-stage embryos were pipping and an additional egg was randomly
collected from each nest for organochlorine analysis. Other anomalies
and skeletal defects were not apparent. Embryonic weights (with
partially absorbed yolk sacs removed) were 15% lower in comparison
with controls collected at Patuxent Wildlife Research Center.
Crown-rump length and femur length were shorter in the San Francisco
Bay embryos. The geometric mean PCB concentration was 4.1 mg/kg wet
weight with a range of 0.8-52.0 mg/kg. A negative correlation existed
between embryonic weight and log-transformed PCB residues in whole
eggs, suggesting a possible impact of PCBs on embryonic growth. DDE
did not show such a correlation (Hoffman et al., 1986).
Klaas & Swineford (1976) found low PCB residues (0.26-3.4 mg/kg wet
weight) in 35 screech owl (Otus asio) eggs (16 of which were known
to be addled) taken from the wild; there was no relationship between
the presence of residues and hatching failure. Dosing captive screech
owls with 3 mg Aroclor 1248/kg (McLane & Hughes, 1980) showed no
effects on eggshell thickness, number of eggs produced, young hatched,
or young fledged. Residues of PCBs measured in the eggs ranged between
3.9 and 17.8 mg/kg. This range covers the egg residue levels that were
clearly associated with effects in experimental chickens and
pheasants.
Eggs from 315 clutches of sparrowhawks (Accipiter nisus) from 9
sites in Scotland were examined by Newton & Bogan (1978) and Newton
(1979). Eggs that failed to hatch were collected and analysed for
PCBs, DDE, and for aldrin and dieldrin. Statistical analysis showed
little variation in residues within a single clutch, but wide
variation between clutches, even clutches from the same female in the
same area in different years. On this basis, analysis of single eggs
was taken as representative of the complete clutch. Analysis of
variance and multiple regression were used to try to relate particular
effects, such as shell thinning, addling of eggs, and late-embryo
mortality, with particular organochlorines. Results showed that
addling of eggs was significantly correlated with levels of both DDE
and PCBs. Because of the close correlation between residues of DDE and
PCBs in eggs of sparrowhawks, it is not possible to tell whether only
one, or both, organochlorines were involved in egg addling. PCBs
showed the strongest relationship with addling. Shell thinning was
more directly linked with DDE. Newton et al. (1982) concluded that the
level of PCB residues in merlin (Falco columbarius) was not
sufficiently high to have exerted any effect on the breeding success
of this species in Britain. Hodson (1975) did not find any effects of
PCB residues in Richardson's merlin in Canada and attributed all
effects to DDE. A relationship between DDE residues and shell thinning
and the breeding success of the bald eagle (Haliaetus leucocephalus)
was demonstrated by Wiemeyer et al. (1984). PCB residues were
correlated with DDE residues. The authors concluded that contaminants
other than DDE contributed no more than a minor role in reproductive
effects on this species in the field. It was considered that PCBs
might have contributed to reproductive problems, but that the evidence
was unclear. Newton et al. (1986) presented a statistical analysis of
residues in sparrowhawks related to reproductive success. Study
populations from many sites throughout the British Isles showed
different reproductive success. Some of this variation could be
related to levels of organochlorine compounds in eggs. When shell
thickness index was related to PCBs alone, a significant negative
correlation was detected. However, once DDE residues were included,
PCBs did not improve the model. It was concluded that DDE alone could
account for all of the reproductive effects recorded and that PCBs, at
the levels of contamination found, probably did not play a role in
reduced breeding performance. Newton et al. (1988) examined new data
on residues of organochlorine compounds in the eggs of the peregrine
falcon (Falco peregrinus) and also reassessed older data. Total
information considered included information on residues and breeding
success covering the period 1963-86. The PCB residues found were not
considered to have had a significant effect on breeding success in
this species. In earlier studies, Wiemeyer et al. (1975, 1987) could
not isolate any single organochlorine compound as responsible for the
reduced breeding success of the fish-eating osprey (Pandion
haliaetus).
Heinz et al. (1983) examined the breeding success of groups of
red-breasted mergansers (Mergus serrator) on islands in northwestern
Lake Michigan. This is a fish-eating species and, since eating fish
from the Great Lakes had been shown to affect captive mink, the
authors investigated the possible effects of contaminants (measured in
blood samples taken from the birds) on reproduction. Other,
non-contaminant effects were also examined. Many organochlorine
contaminants of the environment were found in eggs including 14
different organochlorine compounds; there was a correlation between
the levels of many of these compounds. No relationship could be
established between levels of PCBs, or any other contaminant, and the
breeding success of the birds. The hatching success of dabbling ducks
also seemed to be largely unaffected by feeding in Lake Michigan,
despite the presence of residues of PCBs and other contaminants in the
eggs (Haseltine et al., 1981).
Appraisal
Effects of PCBs have been shown in laboratory studies on many domestic
species of birds, but few wild species. However, there are many
studies showing measurable residues of PCBs in wild birds. PCBs have
been implicated in population decline in several bird species in
different parts of the world, but it is seldom easy to demonstrate
directly the effects of PCBs on populations of birds in the field.
This is largely because PCB residues invariably occur together with
other organochlorine compounds, such as DDE and dieldrin. Separating
out individual effects can only be done completely satisfactorily when
laboratory data are available for the same species and each individual
pollutant. Some idea of likely effects can be obtained from
statistical manipulation of data from the field, though, in this case,
correlation is the best that can be achieved. In practice, few field
studies give the results that have been predicted from laboratory
studies on other species; that is direct extrapolation from laboratory
to field and species to species is not straightforward.
7.4.4 Mammals
Laboratory studies involving the dosing of non-laboratory mammals with
PCBs are limited to a few species. Field studies have been conducted
on a wider range of species.
Norström et al. (1988) studied liver and adipose tissue specimens of
polar bears (121 samples) obtained by Inuit hunters from 12 zones in
the Canadian Arctic Archipelago in 1982-84 (see section 5.1.4). Six
PCB congeners (Nos 99, 153, 138, 180, 170, and 194) constituted
approximately 93% of total PCBs. The major PCBs accumulated belong to
the group formed by combinations of 2,4-dichloro-, 2,4,5-,
2,3,4-trichloro-, and 2,3,4,5-tetrachloro- substitution on each ring.
Congeners with 2,4-, 3,4-dichloro-, 2,3,4-, 2,3,5-trichloro-,
2,3,4,6-, 2,3,5,6-tetrachloro- or 2,3,4,5,6-pentachloro- substitution
on one ring, such as PCB Nos 118, 138, 187, 183 and 196, which usually
bioaccumulate readily in mammals, were all at lower levels compared
with congeners with only 2,4,5-trichloro- and 2,3,4,5-tetrachloro-
substitution in ringed seals, such as PCB Nos 153, 180, and 170. The
last 3 congeners accounted for 71% of total PCBs in polar bears. Thus,
it appears that the polar bear is able to metabolize PCB congeners in
which there are nonchlorinated para positions, adjacent
nonchlorinated ortho-meta positions, or both ortho positions
chlorinated in one ring.
DeLong et al. (1973) measured various organochlorine pollutants (DDT,
DDD, DDE, dieldrin, and PCBs) in sea lions from the Channel Islands
off the Californian coast. Previous observations had recorded a high
incidence of premature births in the population and organochlorine
residues from females showing premature or normally-timed parturition
were compared. Both DDT and PCB residues (measured against a standard
of Aroclor 1254) were significantly higher in the females producing
premature pups. Residues were estimated in the blubber, liver, and
brain, and, for the first 2 tissues, the ranges of values, for the 2
groups of females, did not overlap. Previous studies on both
substances indicated possible reproductive effects in mammals,
corresponding to some extent with those observed in the sea lions.
Cause and effect could not, therefore, be directly established for
each of the pollutants alone. Helle et al. (1976a) collected ringed
seals from Simo, on the northern Bothnian Bay area of the Baltic Sea,
in October/November. This population of seals shows reduced
reproductive capacity and the sampled population showed only 27% of
females pregnant, compared with other reports of 62.5% and 85-90% for
the same species elsewhere. Residues of DDT and PCBs were compared
with those in seals from other areas of the Baltic and found to be
lower (Table 39). The seals sampled from Simo were divided into
pregnant and non-pregnant groups (n = 15 and 26, respectively).
DDT and PCBs levels were both significantly higher in non-pregnant
animals (Table 40). All females from both groups showed a corpus
luteum in one ovary, indicating that all had ovulated. About half of
the non-pregnant females showed indications of an embryo having been
implanted, which had subsequently aborted or resorbed. Again cause and
effect could not be established directly. The authors pointed out that
normally breeding Californian sea lions had DDT levels as high as
those in Baltic seals showing reproductive failure and proposed this
as an indication that PCBs were the causative agent.
Table 39. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable fat of
blubber from ringed seal from the Baltic Seaa
Area Number DDT PCBs
Northern most part 40 110 ± 10b 69 ± 4.4b
of the Bothnian Bay
Gulf of Bothnia 33 200 ± 28 110 ± 15
a Data from: Helle et al. (1976a).
b Value significantly different from those in the Gulf of Bothnia (P < 0.05).
Table 40. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable
fat of blubber in non-pregnant and pregnant ringed seal of
reproductive agea
Number DDT PCBs
Non-pregnant 26 130 ± 13b 77 ± 5.2b
Pregnant 15 75 ± 11 56 ± 6
a Data from: Helle et al. (1976a).
b Values for non-pregnant females significantly higher than
those of pregnant females. DDT P < 0.01; PCBs P < 0.05.
It was further pointed out that the group of non-pregnant females
would include some animals that were not pregnant for reasons other
than the presence of organochlorine compounds. In a later paper (Helle
et al., 1976b), the same authors reported that some of the
non-pregnant females showed abnormal uteri with stenosis or occlusion
of the uterine horns. They, therefore, subdivided the non-pregnant
females in their second sample into those showing the anatomical
abnormality and those not. Both DDT and PCBs were significantly higher
in the occluded group than in pregnant animals. Non-pregnant females,
without occlusions, showed residues of DDT and PCBs not significantly
different from those in pregnant animals, and significantly lower than
those females with occlusions. Residues in males were comparable with
the highest residues found in females (Table 41).
There is some indication of a positive correlation between PCB
residues and age in male seals but not in females (Addison et al.,
1973; Addison & Smith, 1974). These observations are presumed to
indicate that females excrete some of their body burden of PCBs in the
milk.
These field observations have been confirmed in a study in which
captive seals were fed on fish from the Wadden Sea (where reproductive
problems had been found in the wild seal population) and compared with
controls fed fish from the Atlantic Ocean. Seals eating the
contaminated fish, which differed from the control fish only in the
PCB content, showed the same failure to carry the fetus successfully
to term seen in the wild (Reijnders, 1986; see section 7.2.5).
Table 41. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable fat from
blubber of ringed seal from Simo, Bothnian Baya
Group Sample description Number DDT PCBs
I pregnant females 24 88 ± 9.7 73 ± 6.6
II non-pregnant females 29 130 ± 10 110 ± 7.8
with stenoses and
occlusions
III non-pregnant females 8 100 ± 15 89 ± 11
with normal uteri
IV fetuses 24 62 ± 4.3 49 ± 3.0
V males 24 130 ± 18 100 ± 13
probability of similarity between test groups (t-test)
I-II P < 0.01b P < 0.01b
I-III P > 0.05 P > 0.05
I-IV P < 0.05c P < 0.01b
a Data from: Helle et al. (1976b).
b Groups significantly different at the 99% level.
c Groups significantly different at the 95% level.
Subramanian et al. (1987) collected samples of blubber from male
Dall's porpoise (Phocoenoides dalli) in the northwestern Pacific
Ocean and analysed the samples for organochlorine content. Blood
samples taken from the same animals were analysed for testosterone, a
male sex steroid, and for aldosterone, another steroid hormone,
responsible for blood electrolyte balance. Testosterone levels in
blood were correlated with blubber levels of DDT and PCBs; as the
organochlorine content increased, testosterone levels decreased. There
was no relationship between blubber organochlorine levels and blood
levels of aldosterone. The number of samples was small (n = 12) and,
though the relationship between DDT and testosterone was significant,
the apparent relationship with PCBs was not. The animals were sampled
outside the breeding season, when testosterone levels would be
expected to be low.
In a study by Clark & Lamont (1976), 26 pregnant big brown bats
(Eptesicus fuscus) were collected from the field and maintained on a
control diet, in captivity, until they gave birth to young. Levels of
PCBs were measured in both the adults and the offspring. The
concentrations of PCBs in litters with dead young were significantly
greater than in litters where both young were born alive (mean for
litters with dead young was 2.44 mg/kg; mean for all other litters was
0.34 mg/kg). The contents of PCBs ranged between 1.07 and 1.96 mg/kg
wet weight in adults and between 0.28 and 1.69 mg/kg in the young.
7.4.4.1 Appraisal
PCBs have been implicated in population declines of seals and sea
lions. Population decreases and reproductive failure have been
observed in seals from the Baltic Sea, the Wadden Sea (southeastern
North Sea), and the Gulf of St. Lawrence and in sea lions off the
Californian coast. Poor reproduction has been correlated with residues
of PCBs in the affected animals. A major problem in such studies is
the occurrence of more than one chemical pollutant in the animals.
PCBs are found together with other organochlorine compounds and heavy
metals. Conclusions, therefore, have often relied on making the best
correlation between observed effects and residues and checking cause
and effect relationships on animals more amenable to laboratory study.
A study on captive seals confirmed field observations on the effects
of PCBs on these marine mammals (see section 7.2.5).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Evaluation of the toxicity of Aroclors and other commercial PCB
mixtures is complicated by numerous factors, including isomer and
congener composition, differences in species susceptibility,
quantitatively inconsistent data, and various degrees of contamination
with toxic compounds, such as chlorinated dibenzofurans. Because of
these factors and a lack of data for some of the Aroclors (most
studies have been conducted on the higher chlorinated Aroclors), it is
assumed that effects resulting from exposure to a specific Aroclor are
representative of effects that may be produced by the other Aroclors.
Many of the literature sources do not give details of the composition
of the PCB mixture used in the studies.
Reviews on the literature concerning the toxicity of PCBs are given by
ATSDR (1989), Kimbrough (1980, 1987), Safe (1984), NIH (1985), and
Lorenz & Neumeier (1983).
8.1 Single exposures
8.1.1 Oral
(a) Aroclors
The acute oral LD50 values for a number of Aroclors are presented in
Table 42. The lowest oral LD50 in the rat was 1.0 g/kg body weight
for Aroclor 1254 (Garthoff et al., 1981).
In Sherman rats, lethal doses of Aroclor 1254 and 1260 in peanut oil
caused ulceration in the duodenum and glandular stomach (Kimbrough et
al., 1972). Osborne-Mendel rats, receiving a single oral dose of
50 mg/kg body weight of Aroclor 1254 in corn oil, showed an increased
relative liver weight together with the induction of microsomal
enzymes, within 12 h (Litterst et al., 1974).
Monkeys, sacrificed 4 days after gastric intubation of Aroclor 1248 at
1.5 or 3.0 g/kg body weight (no vehicle reported), exhibited enlarged
livers with proliferation of the endoplasmic reticulum and hypertrophy
and hyperplasia of the gastric mucosa (Allen et al., 1974a).
(b) Individual congeners
Estimated oral LD50 values in 30 days for individual congeners in
corn oil in Hartley guinea-pigs (probably single application) were
0.5 mg/kg body weight for 3,4,5,3',4',5'-hexachlorobiphenyl, less than
1 mg/kg body weight for 3,4,3',4',-tetrachlorobiphenyl, more than
3 mg/kg body weight for 2,3,4,5,3',4',5'-heptachlorobiphenyl, and more
than 10 mg/kg body weight for 2,4,5,2',4',5'-hexachlorobiphenyl
(McKinney et al., 1985).
Table 42. Acute oral LD50s of Aroclorsa
Aroclor Species/strain Sex/ageb LD50 (g/kg body weight) References
1254 rat/Wistar male/30 days 1.3 Grant & Phillips (1974)
female/30 days 1.4
male/60 days 1.4
female/60 days 1.4
male/120 days 2.0
female/120 days 2.5
rat/Sherman male/weanling 1.295 Linder et al. (1974);
NR/adult 4-10 Kimbrough et al. (1972);
rat/Osborne-Mendel male/adult 1.01 (single dose) Garthoff et al. (1981)
1.53 (5 doses over 2 1/2 weeks)
1.99 (5 doses, 1 day/week)
1221 rat/Sherman female/NR 4.0 Nelson et al. (1972)
1260 rat/Sherman NR/adult 4-10 Linder et al. (1974)
M/weanling 1.315
1242 rat/Sprague-Dawley male/adult 4.25 Bruckner et al. (1973)
1262 rat NR 11.3 Panel on Hazardous Trace
Substances (1972)
a References: ATSDR (1989); WHO (1976).
b NR = not reported.
Information on solvents used was not available.
8.1.2 Inhalation
No acute data were available.
8.1.3 Dermal
Median lethal doses for a single application of Aroclors to the skin
of rabbits ranged from >0.79 to <1.27 g/kg body weight for Aroclors
1242 and 1248 in 50% corn oil, to >1.0 to <3.17 g/kg body weight for
undiluted Aroclor 1221, and >1.26 to <2.0 g/kg body weight for
Aroclor 1260 (Fishbein, 1974).
8.1.4 Other routes
(a) Aroclors
With a single intravenous dose of Aroclor 1254 in a 1% lecithin-saline
suspension, the LD50 for adult female Sherman rats was 0.4 g/kg body
weight (Linder et al., 1974). The LD50s for Aroclor 1254 in DMSO for
various mouse strains, following a single intraperitoneal injection,
varied between 0.9 and 1.2 g/kg body weight (Lewin et al., 1972).
(b) Individual congeners
The intraperitoneal LD50 for 2,4,3',4'-tetrachlorobiphenyl in CF-1
mice was 2.15 g/kg body weight, while that of its main metabolite,
(5-hydroxy-2,4,3',4'-tetrachlorobiphenyl) was 0.43 g/kg body weight,
which suggests that the acute toxicity of 2,4,3',4'-tetra-
chlorobiphenyl might be attributable to this phenolic metabolite
(Yamamoto & Yoshimura, 1973).
In male Wistar and Charles-River CD rats, a single intraperitoneal
dose of 1 mg 3,4,5,3',4',-pentachlorobiphenyl/kg body weight or a
single oral dose of 50 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body
weight caused significant liver enlargement accompanied by fatty
changes, atrophy of the thymus, and decreased relative spleen weights.
Mono- ortho substituted biphenyls, such as 2,3,4,5,3',4'-hexa-
chlorobiphenyl and 2,4,5,3',4'-pentachlorobiphenyl, induced these
effects in the liver and thymus to a minor degree following an
intraperitoneal dose of 50 mg/kg body weight, while di- ortho
substituted hexachloro- and heptachlorobiphenyls did not cause adverse
effects at this dose level (Kohli et al., 1979; Yoshihara et al.,
1979).
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Aroclors
(a) Mouse
While oral intoxications by PCB mixtures in monkeys are easily
recognizable by their effects on the skin, skin lesions in female ddN
mice were only observed after 2-3 months of daily oral exposure to
1.6 mg of a technical PCB mixture (48% chlorine) per mouse (80 mg/kg
body weight per day) in olive oil. The lesions included alopecia,
erosions, ulcerations, and eczematous changes around the eyelids. No
increase in mortality and only slight growth retardation were seen
after 26 weeks of exposure (Nishizumi, 1970).
Koller (1977) found histological changes in the liver of mice exposed
to 37.5 mg Aroclor 1254/kg diet for 6 months. A dose level of
3.75 mg/kg diet was without effect.
The oral toxicities of Kanechlor 400, 500, and 600 were compared by
feeding male mice diets containing 300 mg PCBs/kg for 14 weeks
(Kawanishi et al., 1975). Fatty degeneration and accumulation of
pigment in the liver were observed in the mice fed Kanechlor 600.
(b) Rat
At lethal, oral doses of undiluted Aroclor 1242 (100 mg/kg body
weight, every 2 days, for 3 weeks), Sprague-Dawley rats showed reduced
body weight, thymus atrophy, chromodacryorrhea, progressive
dehydration, and central nervous system depression with terminal
ataxia and coma. Fatty changes were observed in the liver and kidneys
(Bruckner et al., 1973).
Male and female Sherman rats (10 animals of each sex) were fed diets
containing 0, 20, 100, 500, or 1000 mg of Aroclor 1260 or Aroclor
1254/kg (equivalent to 0, 1.5, 7, 36, or 72 mg/kg body weight,
respectively) for 8 months. The animals receiving the 2 highest dose
levels showed reduced growth. Female rats fed Aroclor 1260 at 500 and
1000 mg/kg diet showed a high mortality rate. However, only 3 rats fed
Aroclor 1254 at 500 mg/kg diet died. Significant increases in relative
liver/body weight ratios were found for both Aroclors at all doses
tested. Microscopically enlarged hepatocytes, cytoplasmic inclusions,
increased lipid levels, and foamy cytoplasm were all found
consistently. Adenofibrosis was found at higher doses (500 and
1000 mg/kg diet) and corresponded to the glistering white areas seen
on gross inspection. These areas also showed cholangiofibrosis
(according to Kimbrough, synonymous for bile duct proliferation, bile
duct adenomatosis, and fibroadenoma) (Kimbrough et al., 1972).
Growth and mortality rates were unaffected in male Sprague-Dawley rats
exposed for 1 year to a diet containing Aroclors 1248, 1254, or 1262
at 100 mg/kg diet (equivalent to 5 mg/kg body weight) compared with
controls (Allen et al., 1976).
Female Charles-River CD rats were fed for 20 weeks on a diet
containing 0, 10, 30, or 100 mg Aroclor 1254/kg (equivalent to 0, 0.5,
1.5, and 5 mg/kg body weight, respectively). No increase in mortality
rates occurred but growth inhibition was seen at 30 mg/kg from month 2
onwards and at 100 mg/kg diet from week 2 onwards. Skin lesions,
initially on the ears, were found after 10-20 weeks of exposure to
Aroclor 1254 at all dose levels. The lesions, including alopecia and
reddened and thickened skin with hyperkeratosis, subcutaneous oedema,
and infiltration by polymorphonuclear leukocytes, also involved the
nose, tail, and feet at the highest exposure level (Zinkl, 1977).
Effects on the liver following exposure to PCB mixtures have mainly
been investigated in rats (see section 8.6.1.1).
(c) Rabbit
Four groups of 5 male and 5 female New Zealand White rabbits were
administered 300 mg Aroclor 1221, 1242, or 1254 in corn oil, via a
stomach tube, once a week, for 14 weeks. The fourth group received
only the vehicle.
The mortality rate was increased and the body weight gain was reduced
after 14 weeks of oral exposure to Aroclor 1254, but not after
exposure to Aroclor 1221 or 1242. Liver/body weight ratio and SGOT and
SGPT activities were increased in the animals treated with Aroclor
1242 or 1254, but not in those treated with Aroclor 1221. In the
animals treated with Aroclor 1254, the smooth endoplasmic reticulum
was condensed in the liver cell and formed hyalin inclusions which
might have been accompanied by a loss of enzyme activity. Lipid
accumulation, pigment deposition, nuclear changes, and necrosis were
also found (Koller & Zinkl, 1973).
(d) Pig
Pigs, fed Aroclor 1242 or 1254 at a dietary level of 20 mg/kg
(equivalent to 0.8 mg/kg body weight) for 91 days, showed gastric
lesions, consisting of erosions and necrosis. Two pigs fed a high-dose
regimen of 100 mg of Aroclor 1254/kg body weight for 11 days, also
showed hypertrophy and hyperplasia of the gastric mucosa; this was
also found in monkeys at low exposure levels (Hansen et al., 1976b).
(e) Cow
Holstein cows were studied throughout a complete lactation period, a
non-lactating period, and 42 days of a subsequent lactation period for
overt responses to Aroclor 1254. Four cows received daily doses of 0,
10, 100, or 1000 mg/cow (1000 mg/cow is equivalent to 1.67 mg/kg body
weight) over 60 days. The mean daily milk production and net energy of
a complete lactation period did not differ between control and
PCB-treated animals. At the end of the study, the concentrations of
PCBs were 0.005, 0.021, 0.14 in blood plasma; 1.9, 10.9, 91.3 in milk
fat, and 1.4, 6.9, 70.0 µg/kg in adipose tissue for the 10, 100, and
1000 mg PCB groups, respectively. No signs of impaired health,
productivity, or changes in blood and urine chemistry were observed
(Willett et al., 1987).
(f) Monkey
Rhesus monkey
(i) Aroclor 1242
Becker et al. (1979) exposed 6, 7-8-month-old, male Rhesus monkeys to
Aroclor 1242 at levels of 0, 3, 10, 30, or 100 mg/kg diet (equivalent
to 0, 0.12, 0.4, 0.4, 1.2, or 4.0 mg/kg body weight, respectively) to
study changes in the stomach mucosa. At 3, 10, 30, and 100 mg/kg diet,
4 monkeys died after 245, 146, 92, and 137 days of exposure,
respectively. All exposed monkeys showed a decreased body weight gain.
At all dose levels, changes were observed in the folds of the
suborbital facial skin, and eyelids became swollen and red. From day
71 at 3 mg/kg diet, days 69 and 77 at 10 mg/kg diet, and day 12 at the
higher exposure levels, stomach biopsies revealed an apparent arrest
of the differentiation of generative cells of the isthmus and neck
regions into parietal and zymogenic cells. Mature parietal and
zymogenic cells, which were found only in the bases of the glands,
showed signs of injury, such as dilatation of the rough endoplasmic
reticulum on the zymogenic cells, irregularity of the mitochondria in
parietal cells, and irregular luminal membranes and an increase in the
number of autophagic vesicles in both type of cells. The severity of
the lesions was directly correlated with both duration and level
exposure. A no-effect level was not obtained in this study.
A group of 5 Rhesus monkeys, 1-2.5 years old, was exposed to a diet
containing 1 mg of Aroclor 1242/kg (equivalent to 0.04 mg/kg body
weight) for 133 days. A control group contained 4 monkeys. No adverse
effects were found (McNulty et al., 1980).
Characteristic lesions, metaplasia in epithelial structures, such as
sebaceous glands, nail beds, gastric mucosa, and ameloblast
surrounding unerupted teeth were reported to have developed in Rhesus
monkeys, 13 months after a 40-day diet containing 400 mg Aroclor
1242/kg (equivalent to 16 mg/kg body weight) (McNulty, 1985).
(ii) Aroclor 1248
A group of 5 (4 males and 1 female), 1-month-old Rhesus monkeys,
without previous exposure, were administered 30 daily doses of 35 mg
of Aroclor 1248/kg body weight, by gavage, in corn oil. Four animals
received only corn oil. No mortality or clinical signs were observed,
except for a decrease in body weight gain, slightly reduced food
consumption, and anaemia. Mild microscopic changes were observed in
the thymus, bone marrow, eye, skin, stomach mucosa, and liver
(Abrahamson & Allen, 1973).
Six male Rhesus monkeys, aged 1 1/2-2 years were fed a diet containing
300 mg Aroclor 1248/kg for 3 months. Three animals served as controls.
A decrease in body weight was observed. Within one month, all the
animals fed PCBs had alopecia, subcutaneous oedema, particularly of
the face, which manifested as swollen eyelids, erythema, and acneiform
lesions involving the areas devoid of hair (Allen & Norback, 1972 cf.
Hayes (1987)).
Six adult, female Rhesus monkeys were fed 25 mg Aroclor 1248/kg diet
for 2 months. Facial oedema, alopecia, and acneiform changes developed
within 1 month and 1 animal died 2 months after removal from the
experimental diet. In addition to the above changes, this animal
showed anaemia, hypoproteinaemia, hypertrophy, and hyperplasia with
invasion through the muscularis mucosa, focal haemorrhages and
ulcerations of the gastric mucosa, and bone marrow hypoplasia. Eight
months later, the 5 surviving animals continued to show clinical signs
of intoxication. The PCB concentration in body fat, which, after 2
months of treatment, averaged 127 mg/kg, had decreased 8 months later
to 34 mg/kg (Allen et al., 1974a).
Groups of 9 adult, female Rhesus monkeys (weight approximately
5.6 kg), were fed a diet containing 0, 2.5, or 5.0 mg Aroclor 1248/kg
(equivalent to 0, 0.1, or 0.2 mg/kg body weight, respectively) for an
average period of 18.2 months. An additional group of 4 males was fed
5.0 mg/kg diet. Control groups contained 12 female and 6 male monkeys.
The Aroclor 1248 contained 4.4-8.7 ng polychlorinated dibenzofurans/kg
diet. The female monkeys were mated with control males after 6 months
of exposure (the reproductive effects are discussed in section
8.4.1.3). Levels of Aroclor 1248 in adipose tissue reached a plateau
after 1 year at 2.5 mg/kg diet and after 6 months at 5 mg/kg diet.
Exposed males showed slight to moderate periorbital oedema and
congestion of the eyes. Females showed an average 15% loss in body
weight over the first 5 months of both exposures, while food
consumption was normal. At 6 months, they all showed loss of hair,
acne of the face and neck, and erythema and swelling of the eyelids.
Skin biopsies revealed keratinization of the affected hair follicles.
One female monkey died after 173 days of exposure to 2.5 mg/kg diet
and one female died after 310 days of exposure to 5.0 mg/kg diet. Both
animals developed terminal enteritis due to Shigellosis, which was
resistant to treatment. At autopsy, the animals showed generalized
alopecia, subcutaneous oedema, and acne. Microscopically, follicular
epithelial hyperplasia with inflammation of the surrounding tissue,
and keratinization of the hair follicles were observed. The livers
showed focal areas of necrosis, enlarged hepatocytes, and fatty
changes (Barsotti et al., 1976).
The surviving females in the former study, 8 per dose level, were
placed on a control diet for approximately 1 year after an average
total intake of 270 and 498 mg Aroclor 1248, respectively. There was a
gradual improvement in their physical condition and, within one year,
their gross appearance was no longer different from that of the
controls. However, their breeding performance was still affected, as
outlined in section 8.4.1.3 (Allen et al., 1980).
(iii) Aroclor 1254
Several pilot studies were carried out before this major study to
characterize the toxicity of Aroclor 1254 and to compare the toxic
findings in the Rhesus and Cynomolgus monkey (Tryphonas et al., 1984,
1986b; see section 8.2.1.6). In these pilot studies, Mes & Marchand
(1987) and Mes et al. (1989a) measured the concentrations of PCBs in
the blood, adipose tissue, and faeces.
A preliminary report describes the results of an ongoing study after
54 weeks of daily oral administration of gelatin capsules containing
0, 5, 20, 40, or 80 µg Aroclor 1254/kg body weight, in corn
oil-glycerol, to groups of 16 adult, female Rhesus monkeys (Macaca
mulatta). At this stage, a slight decrease in body weight gain was
observed in the exposed monkeys together with a decrease in water
consumption, but not in food consumption. In week 52, the incidences
of prominent nail beds, of nails separated from the beds, and of
prominent tarsal glands increased in the animals exposed at 80 µg/kg
body weight (Arnold et al., 1984).
Aroclor 1254, at a dose level equivalent to 280 µg/kg body weight was
given for 5 days per week to Rhesus monkeys (Macaca mulatta) over a
period of 27-28 months. Four animals were treated as described and 4
animals served as controls. The Aroclor 1254 was administered in
apple-juice-gelatin-corn oil emulsion. The weight of the monkeys at
the beginning of the study was approximately 4 kg. Terminal clinical
signs of varying severity included finger nail detachment, exuberant
nail beds, weight loss, stomatitis, and normocytic anaemia. At
necropsy, the bone marrow was hypocellular with cytoplasmic vacuoles
in erythroid precursor cells. Histopathological lesions included
dilatation of the tarsal gland ducts, atrophy, or absence of, splenic
and lymphonodal germinal centres, bone marrow depletion, gingival
erosion and ulceration, moderate mucinous hypertrophic gastropathy
with cystic dilatation of occasional gastric glands, hepatocellular
enlargement and necrosis, hypertrophy of biliary duct epithelium,
hyperplasia of biliary ducts, hypertrophy of the gall bladder
epithelium, and an equivocal increase in the number of lysosomes in
the thyroid follicular epithelial cells. The terminal PCBs
concentrations in a number of organs were as follows: adipose tissue
106.7-2073.2 mg/kg (in control animals 0.26-0.65 mg/kg); brain,
31.2-252.0 mg/kg; kidneys, 85.4-964.1 mg/kg; and liver,
255.1-828.1 mg/kg tissue. The PCB concentrations were expressed on a
mg/kg fat basis. It was concluded that skin appendicular lesions are
good clinical indices of PCB exposure in monkeys and that
lymphoreticular lesions (atrophy and absence of lymphoid follicular
centres) are good indicators of impending or active immunological
crisis (Tryphonas et al., 1986a).
(iv) Miscellaneous studies
Accidental exposure of a colony of 256 Rhesus monkeys to PCBs in a
concrete sealant produced a disease characterized by high mortality,
gradual weight loss, behavioural changes, alopecia, acne, facial
oedema, swollen eyelids, diarrhoea, anaemia, poor breeding
performance, and high incidences of abortions and still births.
Samples of the concrete slabs within several buildings were obtained
and analysed. Significant levels of PCBs (5280 mg PCB/kg sample) were
found (Altman et al., 1979; McConnell et al., 1979).
The effects of exposure to PCBs on the eyes were investigated by
Ohnishi & Kohno (1979), who administered a banana injected with 0.5 mg
of PCBs/kg body weight, daily, to 8 adult Rhesus monkeys of both sexes
for 1-5 months. Two out of this group were fed PCBs with
polychlorinated dibenzofurans (2.5 µg/kg body weight). Four untreated
animals were used as controls. One month after the onset of the
exposures there was a reduction in body weight. When pressure was
applied to the eyelids of treated monkeys, white secretions extruded.
Within 3 months alopecia, swelling of the eyelids, and acne-form
eruptions developed. The retina and choroid were normal. The
histopathological changes in the eyelids were comparable in both
groups of exposed monkeys and included the appearance of keratinous
cysts and atrophy of the Meibomian glands with hyperkeratosis and
hyperplasia of the ductal epithelium.
Cynomolgus monkey
Groups of 5 or 6 adult, female Cynomolgus monkeys (Macaca
fascicularis) were exposed to 3, weekly doses of 4.7 mg of Aroclor
1248/kg body weight (equivalent to 2 mg/kg per day) or to 3-weekly
doses of 11.7 mg of Aroclor 1254/kg body weight (equivalent to 5 mg/kg
per day) in an apple juice-corn oil emulsion. The monkeys were exposed
until necropsy at day 30-164 in dead or moribund condition. A control
group contained 5 monkeys. In both exposed groups, body weight loss,
emaciation, facial oedema, finger-nail loss, and lacrimation were
observed. Common histopathological lesions were: dilated Meibomian
gland ducts, mucinous hyperplasia and hypertrophy of the gastric
mucosa, enlargement, fatty degeneration, and necrosis of hepatocytes,
bile duct and gall bladder epithelial cell hypertrophy and
hyperplasia, and thyroid changes in follicular cell size and the
number of intracytoplasmic lysozomes.
The onset of the signs and lesions of toxicity was not as rapid and
uniform as that in Rhesus monkeys. Aroclor 1248 appeared more toxic
than Aroclor 1254 (Tryphonas et al., 1984).
Groups of 4 adult, Rhesus and 4 adult, Cynomolgus female monkeys,
weighing 3.2-4.5 and 3.2-5.2 kg, respectively, received doses of
Aroclor 1254 at 0 or 280 µg/kg body weight for 5 days/week (equivalent
to 200 µg/kg per day) in an apple-juice-gelatin-corn oil emulsion for
27-28 and 12-13 months, respectively. This study showed that the
Rhesus monkey is more susceptible to PCB toxicity than the Cynomolgus
monkey (Tryphonas et al., 1986b).
Hori et al. (1982) exposed 1 female, Cynomolgus monkey to daily doses
of Kanechlor 400 (without detectable quantities of polychlorinated
dibenzofurans) at 2 mg/kg body weight, in olive oil. They also exposed
3 monkeys to a PCB mixture with a chromatographic pattern similar to
that of the Yusho mixture (2 or 4 mg/kg body weight), and 1 monkey to
2 mg/kg body weight of the same mixture, without detectable quantities
of polychlorinated dibenzofurans (detection limit not given). The
doses were administered 6 times per week in a piece of banana. Two
controls received only the vehicle. The 2 monkeys receiving the Yusho
mixture at 4 mg/kg body weight died within 4 and 8 weeks,
respectively. The other monkeys were kept for 20 weeks. In all monkeys
exposed to the Yusho mixture, toxic effects were similar to those
already described above. In addition, there was cytoplasmic
vacuolation and dilatation of the convoluted tubules with cytoplasmic
casts in the kidneys. The other 2 mixtures induced less severe
reductions in body weight gain, immunosuppression and
histopathological alterations in liver, kidneys, and periorbital skin.
The effects in the animals fed a diet with dibenzofurans yielded
enhanced decreases in body weight, immunosuppression, fatty liver and
histological changes, in addition to hair loss, acne-form eruptions,
oedema of the eyelids and cornification of the skin, compared with the
other test substances.
8.2.1.2 Individual congeners
(a) Monkey
Rhesus monkeys have been exposed to various congeners, as outlined in
Table 43.
Table 43. Toxicity of PCB-congeners in Rhesus monkeysa
Congenerb No. of Exposure Time clinical Deaths
animals/ toxicity
group mg/kg period first noted
diet (days) (day)
2,5,4'-TriCB 4 5 84 - 0
3,4,3',4'-TCB 3 3=>1c 215 14-21 3
5 1 38 27 1
2,5,2',5'-TCB 3 3=>1c 215 - 0
5 1=>5d 200 - 0
3,4,5,3',4',5'-HCB 1 0.1 127 117 1
4 0.5 63 28-30 4
1 1 57 20 1
2,4,5,2',4',5'-HCB 4 15 122 - 0
1 65 63 - 0
2,4,6,2',4',6'-HCB 4 15 122 - 0
1 65 64 - 0
2,3,6,2',3',6'-HCB 4 15 122 - 0
a From: McNulty et al. (1980); McNulty (1985); Iatropoulos et al. (1977).
b TriCB = trichlorobiphenyl; TCB = tetrachlorobiphenyl; HCB = hexachlorobiphenyl.
c Dietary level reduced after 23 days.
d Dietary level raised after 133 days.
No toxicity could be demonstrated either by clinical appearance or by
histopathological examination for isomers with 2 or 4 chlorine atoms
ortho to the biphenyl bridge. The clinical signs observed in monkeys
exposed to 3,4,3',4'-tetrachlorobiphenyl (up to 3 mg/kg diet) and
3,4,5,3',4',5'-hexachlorobiphenyl (at 1 mg/kg diet) were similar in
character and severity to those produced by Aroclor 1242 (at 100 mg/kg
diet) and Aroclor 1248 (at 25 mg/kg diet) in monkeys (Allen et al.,
1974a). The same histopathological lesions were found. The lesions of
the skin and eyes were described as an expression of squamous atrophy
or squamous cyst formation of the sebaceous glands. The epithelial
changes in the skin and nails were found to be reversible. In this
study, 2,5,2',5'-tetrachlorobiphenyl did not produce any clinical or
pathological lesions at 3 mg/kg diet. Aroclor 1242 and 1248 were
reported to contain about 0.24 and 0.34% of 3,4,3',4'-tetra-
chlorobiphenyl, and it was concluded that this congener could account
for some of the toxicity of the commercial mixtures (McNulty et al.,
1980; McNulty, 1985).
Rhesus monkeys exposed to 2,5,4'-trichlorobiphenyl showed a reversible
primary injury of the arterioles, capillaries, and venules in the
adrenal glands, kidneys, liver, brain, and lungs (Iatropoulos et al.,
1977).
(b) Other animal species
In studies on rats and mice, individual PCB-congeners caused adverse
effects on the liver, spleen, and thymus. The most toxic compounds
were the planar congeners 3,4,3',4'-tetrachlorobiphenyl,
3,4,5,3',4'-pentachlorobiphenyl and 3,4,5,3',4',5'-hexachlorobiphenyl.
Biocca et al. (1981) and Aulerich et al. (1985) compared the
toxicities of various hexachlorobiphenyl isomers in mice. Male C57
BL/6 mice were exposed via the diet to 0.3, 1, 3, 10, 30, 100, or
300 mg 3,4,5,3',4',5'-hexachlorobiphenyl; 10, 30, 100, or 300 mg
2,4,5,2',4',5'-hexachlorobiphenyl, 2,3,6,2',3',6'-hexachlorobiphenyl,
or 2,4,6,2',4',6'-hexachlorobiphenyl/kg diet for 28 days. There were
marked differences in dose response and in the severity of the
pathological effects among the isomers. 3,4,5,3',4',5'-Hexa-
chlorobiphenyl was the most toxic isomer causing mortality, and body
and organ weight effects at all dose levels and was the only isomer
that produced excess porphyrin accumulation. It was also the isomer
that occurred in the highest concentration in the fat and the liver.
3,4,5,3',4',5'-Hexachlorobiphenyl caused subcutaneous oedema,
enlargement of the liver with accentuated hepatic lobular markings,
fatty infiltration, hepatocellular swelling and necrosis, and atrophy
of the thymus. The other 2 isomers caused the same lesions, but to a
lesser extent.
In the mice, the overall order of toxicity was 3,4,5,3',4',5'-
hexa-chlorobiphenyl > 2,4,6,2',4',6'-hexachlorobiphenyl >
2,4,5,2',4',5'-hexachlorobiphenyl > 2,3,6,2',3',6'-hexachlorobiphenyl,
based on the effects on mortality and growth, and on histopathology.
8.2.2 Intraperitoneal: reconstituted PCB mixtures
Bandiera et al. (1984) administered reconstituted mixtures of PCDFs
and reconstituted mixtures of PCBs, by intraperitoneal injection, to
immature Wistar rats, to determine the effects on weight loss, thymic
atrophy, and the induction of P-448 dependent monooxygenases. The
mixtures consisted of compounds that persisted in the blood and liver
of Yusho patients. From the results, it was clear that the PCDFs were
600 to 2100 times more toxic than the PCBs.
A group of 4, one-month-old, male Wistar rats received
intraperitoneally a reconstituted PCB mixture containing 13 of the
major congeners that have been identified in human milk, at the
corresponding relative concentrations. The mixture was injected on
days 1 and 3 in corn oil at dose-levels of 0, 0.45, 0.90, 4.5, or
45 mg/kg body weight. The rats were killed on day 6 for
histopathological studies. At the highest exposure level, increased
relative liver weights and enlarged and vacuolated hepatocytes were
observed together with changes in nuclei. In the thyroid, a mild
reduction in follicular size, focal collapse, and changes in nuclei
were found. No changes were seen with 0.9 mg/kg body weight (Gyorkos
et al., 1985) (see section 8.8.1.1).
8.2.3 Dermal exposure
(a) Aroclors
Solutions of Phenoclor DP6, Clophen A60, or Aroclor 1260 in
isopropanol were applied in doses of 118 mg on 50 cm2 of the shaved
back skin of groups of 4 New Zealand rabbits, 5 times per week, for 38
days. A group of 4 rabbits received the vehicle only. After initial
reddening, transverse wrinkling and thickening of the skin developed
with hyperplasia and hyperkeratosis of the epidermal and follicular
epithelium. These effects were more marked with Clophen and Phenoclor
than with Aroclors. During the study, deaths occurred in the Clophen-
and Phenoclor-treated groups. Body weights and relative kidney weights
were decreased in the Aroclor-treated rabbits. Histological liver
changes were least marked in the Aroclor-treated rabbits. Treated
rabbits had fluorescing livers and bone under UV radiation and also
showed other evidence of porphyria. In the kidneys, hydropic
degeneration of the convoluted tubuli, and tubular dilatation were
found. There was atrophy of the thymic cortex and a reduction of
germinal centres of the lymph nodes, as well as leukopenia, and some
animals in all groups showed oedema of the abdominal and thoracic
cavities, subcutaneous tissue, and pericardium. Faecal elimination of
copro- and protoporphyrine was increased by all 3 PCBs, but was lowest
with Aroclor 1260 (Vos & Beems, 1971).
Puhvel et al. (1982) exposed groups of 3 female, hairless mice of 2
strains, (Skh:HR-1 and HRS/J), topically to Aroclor 1254 (4 doses of 1
or 8 mg/week, for 6 weeks) or Phenoclor 54 (5 doses of 0.2 mg/week,
for 10 weeks) in acetone or an acetone-mineral oil-Tween 80 emulsion,
or to the vehicle only. Punch biopsies of the skin were taken
regularly. The skin of treated mice appeared grossly normal after the
exposures, but examination of microscopic skin samples of
Phenoclor-treated mice showed hyperkeratosis of the stratum corneum,
epidermal hyperplasia, disappearance of sebaceous glands, and the
presence of numerous keratinous cysts. No histological changes were
observed in the internal organs.
(b) Individual congeners
In the study of Puhvel et al. (1982), described above, groups of 3
hairless mice of both strains were also exposed topically to 5 doses
of 0.2 mg 3,4,3',4'-tetrachlorobiphenyl/week, for 10 weeks. Grossly
there were no visible changes. The histological changes induced in
HRS/J mice were similar to those found after Phenoclor treatment.
However, identical changes induced in Skh:HR-1 mice were already
observed by 4 weeks. After 8 weeks, these lesions were more marked and
also included hyperkeratosis of the sebaceous follicles and
hyperkeratinization of intradermal pilar cysts. Often these cysts
ruptured into the dermis leading to dense infiltrates of
polymorphonuclear leukocytes. The treated mice showed weight gain,
primarily because of large intra-abdominal fat deposits.
In a dermal toxicity study on rabbits, with a protocol identical to
that of the study of Vos & Beems (1971), skin lesions on animals
treated with 2,4,5,2',4',5'-hexachlorobiphenyl were less severe than
those on Aroclor 1260-treated animals. The liver damage observed
histologically was essentially the same after treatment with either
Aroclor 1260 or 2,4,5,2',4',5'-hexachlorobiphenyl, but the individual
congener was more porphyrogenic (Vos & Nootenboom-Ram, 1972). In
another study, 4 applications of a 25% solution of 3,4,3',4'-
tetrachlorobiphenyl in olive oil to the inner surface of the ears
of rabbits resulted in the same lesions that were found after 2
applications of undiluted Kanechlor 400 or 500. The lesions included
hyperkeratosis, dilatation of hair follicles, and the formation of
keratinous cysts (Komatsu & Kikuchi, 1972).
8.2.4 Appraisal
Rhesus monkey is the most sensitive test species with regard to the
general toxicity of PCBs, both as a mixture and as individual
congeners. The toxicity of mixtures may be confounded by the presence
of impurities, such as PCDFs, which are, or may have been, present in
the mixtures tested. PCBs induce some of the biological and
toxicological effects qualitatively similar to those induced by PCDFs.
Another confounding variable in these studies is the difference in the
composition of the mixtures (Aroclor 1242, 1248, 1254) used.
Beating the above in mind, the available data show that Aroclor 1248,
containing 4.4-8.7 ng of PCDFs/kg, still showed adverse general toxic
effects in Rhesus monkeys at a dose of 0.1 mg/kg diet per day
(0.09 mg/kg body weight per day) administered for an average of 18.2
months (Bowman et al., 1981). A NOEL for general toxicity was not
established for Aroclor 1248. The NOEL for the general toxicity of
Aroclor 1242 was 0.04 mg/kg body weight per day, as established after
dietary exposure for 133 days. The main effects induced by Aroclor
1248 at 0.09 mg/kg body weight per day were an increased mortality
rate, growth retardation, alopecia, acne, swelling of the Meibomian
glands, and, possibly, immunotoxicity. Microscopically, enlarged
hepatocytes, fatty liver with focal necrosis, and epithelial
hyperplasia and keratinization of hair follicles were found. These
effects appeared reversible. Aroclor 1254 at a dose level of
0.200 mg/kg body weight per day showed several effects, not reported
for 1248 (lymforeticular lesions, finger-nail detachment, gingival
effects) and vice-versa (acne, alopecia), which could be related to
the confounding variables noted above. Several effects observed in the
monkeys exposed to Aroclor 1254 (hypertrophic gastropathy, bone marrow
hyperplasia) were also observed in monkeys exposed to Aroclor 1248,
but at a higher dose (4 mg/kg body weight per day). In contrast with
the severe effects observed in adult Rhesus monkeys at low doses,
relatively mild effects were shown by suckling Rhesus monkeys exposed
to much higher doses.
8.3 Skin and eye irritation, sensitization
The injury to skin and eyelids following oral and/or dermal exposure
to PCBs has been discussed in sections 8.2.1 and 8.2.3.
8.4 Reproduction, embryotoxicity, and teratogenicity
8.4.1 Reproduction and embryotoxicity
8.4.1.1 Oral
(a) Mouse
In castrated, mature, male NMRI mice (10-13 per group) that received
28 daily doses of 0.25 mg Aroclor 1254/mouse, in peanut oil, the
weight of the seminal vesicles was decreased, but this was not seen in
intact mice (Orberg & Lundberg, 1974).
When 23 adult female NMRI mice were mated with 22 control males and
orally intubated with 0.025 mg Clophen A60/day, in peanut oil, for 62
days prior to mating and up to days 8-10 of gestation, blastocytes
failed to implant. Twenty-five (14 experimental and 11 control
animals) out of the 45 animals were used to study the effects of PCBs
on the estrous cycle. Effects, such as prolonged estrous cycle and
less frequent periods of sexual receptivity and a decline in the
number of implanted ova, were found (Orberg & Kihlström, 1973).
In order to study the effects of PCBs on the development of sexual
functions in the early postnatal period, these authors also mated mice
that had been suckled by mothers dosed with Clophen A60 during the
lactation period. A decrease in the frequency of implanted ova was
noted, when both parents of the couple had been suckled with milk
containing PCBs. When adult female NMRI mice received 50 mg of Clophen
A60/kg body weight once per week, subcutaneously, during lactation,
the same effect was observed in the offspring after mating with
similarly exposed males (Kihlström et al., 1975).
(b) Rat
In a 2-generation reproduction study, groups of 10 male and 20 female
Sherman weanling rats were fed diets containing 1, 5, 20, or 100 mg of
Aroclor 1254/kg (equivalent to 0.06, 0.32, 1.5, and 7.6 mg/kg body
weight, respectively), or diets containing 5, 20, or 100 mg/kg of
Aroclor 1260 (equivalent to 0.39, 1.5, and 7.5 mg/kg body weight,
respectively). Control groups comprised 20 male and 40 female rats.
The F0 rats were started on the diet at 3-4 weeks of age and the F1
rats, at weaning. The F0 rats were pair-mated when 3 and 7 months old
to produce the F1a and F1b generations, respectively. Breeding-stock
F1b rats were selected at weaning and pair-mated when 3 months old to
produce the F2a, and, when 8 months old, the F2b generation. Rats
exposed to Aroclor 1254 at 20 mg/kg diet or more showed a reduced
litter size at birth, but not when exposed at weaning, in the F1b and
F2 generations. At 100 mg/kg diet, the number of litters in the F2
generation was decreased. In 2 F2a and 2 F2b litters no live offspring
were found at birth, while pup survival at weaning was reduced in the
F2a generation. At weaning, exposed F1a pups weighed less than their
controls. Increased relative liver weights were found in male F1a
weanlings at 1 mg Aroclor 1254/kg diet and in all weanlings at 5 mg/kg
diet or more. Adult rats showed increased relative liver weights at
levels of 20 mg/kg diet or more. No reproductive effects were found
with 5 mg Aroclor 1254/kg diet. In groups treated with Aroclor 1260,
increased liver weights were found in all weanlings at 5 mg/kg diet or
more, but no effects on reproduction were seen, even at 100 mg/kg diet
(Linder et al., 1974).
The only effect observed in Holtzman rats fed Aroclor 1254 at a level
of 500 mg/kg diet, for 3 weeks, was an increase in relative testes
weights (Garthoff et al., 1977). Increased absolute testes weights and
unchanged body weights were found in 6-month-old offspring of
Sprague-Dawley dams exposed to daily doses of 30 mg Aroclor 1260/kg
body weight in ethanol-sesame oil on days 14-20 of gestation. No
effects on testes weight were found in animals treated with Aroclor
1221 or Aroclor 1242 (Gellert & Wilson, 1979).
Sager (1983) evaluated the effects on the reproductive function of
adult male Holtzman rats following exposure of their mothers to doses
of 8, 32, or 64 mg Aroclor 1254/kg body weight in peanut oil, on days
1-3, 5, 7, or 9 of lactation. At all dose levels, 165-day-old males
showed a decreased relative ventral prostate weight, and, at the 2
higher doses, a decreased relative weight of seminal vesicles and
testes as well as decreased body weight. In the ventral prostate,
alveoli were decreased in number and showed folding of the mucosa and
flattened epithelial cells. At 130 days of age, the male offspring at
all 3 dose levels showed a reluctance to mate with control breeders,
leading to a decreased number of pregnancies. Moreover, at the 2
higher dose-levels, the females showed a reduced number of
implantations and an increased resorption rate. The litters showed a
reduced weight gain up to 11 days of age.
This study was repeated with an evaluation of the reproductive
performance of the second generation male rats from 130 days of age,
following mating with normal females. In the first study, autopsy on
pregnant females was carried out on day 11 or 12 of gestation. The
females had fewer implants, fewer embryos, and a reduced proportion of
ovulated eggs that implanted, compared with controls. The effects were
dose-related. In a second study, females mated to the same males were
autopsied on day 2 or 4 after mating. Sperm counts were not affected.
At the 2 highest doses, fewer females had eggs in the expected state
of development, the average number of blastocytes found in one uterine
horn on day 4 was reduced, and an increased incidence of abnormally
developed embryos was observed (Sager et al., 1987).
Female Wistar rats exposed to daily doses of 10 mg Aroclor 1254/kg
body weight, for at least one month, showed a prolonged estrous cycle,
decrease in sexual receptivity, delay in timing of copulation, vaginal
bleeding during gestation, decrease in litter size, and delay in the
time of parturition. After mating of the rats to control males, the
female offspring, exposed in utero and during lactation, showed a
slower rate of body weight gain, higher mortality, earlier vaginal
opening, and a delay in the appearance of the first estrous cycle
(Brezner et al., 1984).
(c) Monkey
Groups of 9 adult female Rhesus monkeys were exposed to a diet
containing Aroclor 1248 (containing polychlorinated dibenzofurans) at
levels of 2.5 or 5.0 mg/kg (Barsotti et al., 1976; Allen et al., 1979,
1980). The study and the maternal toxicity data have already been
described in section 8.2.1.6. Within 4 months, menstrual bleeding and
the duration of the menstrual cycle were increased. Flattening and
prolongation of the serum progesterone peak during the menstrual cycle
was observed. After 6 months of exposure, the females were mated with
control males. Reproductive dysfunction was obvious as shown in Table
44. Following the total exposure period of 18.2 months, the mothers
were put on a control diet. Their menstrual cycles and serum
progesterone levels returned to pre-exposure values. One year after
exposure ceased, the females were again mated with control males and
showed a recovery of their reproductive status (Table 44; Allen et
al., 1980).
Other groups of adult, female Rhesus monkeys were continuously fed
diets with 0, 0.25, and 1.0 mg Aroclor 1016/kg (equivalent to 0, 0.01
and 0.04 mg/kg body weight, respectively), in which no polychlorinated
dibenzofurans were detected (no details). No maternal toxicity was
noted at these levels. In this study, the females were mated with
control males after 7 months of exposure. Reproductive dysfunction
was not observed. Decreased birth weights were found in the offspring
of mothers exposed to Aroclor 1016 at 1.0 mg/kg diet. Skin
hyper-pigmentation occurred in both exposure groups (Barsotti & Van
Miller, 1984). Preliminary reports have indicated possible effects on
learning and behavioural tasks. The milk contained an average of 1.45
and 3.92 mg/kg fat at 0.25 and 1.0 mg/kg, respectively, whereas the
serum of the mothers contained 0.012 and 0.027 mg/litre, respectively
(Heironimus et al., 1981; Levin & Bowman, 1983).
Table 44. Reproductive status of Rhesus monkeys exposed to dietary levels of Aroclor 1016 or 1248
PCB Exposure level Total intake Conceptions Abortions Stillborn Live Reference
mixture at and births
(Aroclor) Diet Body conception resorptions
(mg/kg) weight (mg/kg)
(mg/kg)
1016 0 0 0 8/8 0/8 0/8 8/8 Barsotti &
0.25 0.01 8c 8/8 0/8 0/8 8/8 van Miller
1.0 0.04 30c 8/8 0/8 0/8 8/8 (1984)
1248 0 0 0 12/12 0/12 0/12 12/12 Barsotti et al.
2.5 0.09a 105 8/8 3/8 0/8 5/8 (1976)
5.0 0.2 210 6/8 4/8 1/8 1/8
1248 0 0 0 8/8 0/8 0/8 8/8 Allen et al.
2.5 0.09a 270 8/8b 1/8 0/8 7/8 (1980)
5.0 0.2 498 7/7b 1/7 2/7 4/7
a From: Bowman et al. (1981) amended.
b Breeding 1 year after exposure.
c Calculated assuming a body weight of 5 kg.
In these studies, the monkeys were maintained on the diets during the
gestation and lactation of the first generation. PCBs are known to
cross the placenta and to be excreted via breast milk (see section
6.3). The 6 infants born to monkeys during exposure at 2.5 or 5.0 mg
Aroclor 1248/kg diet showed decreased birth weights, a small stature,
and a decreased body weight gain during nursing. Within 2 months,
signs of intoxication appeared including acne, increased skin
pigmentation, swelling of the eyelids, and loss of eyelashes. In 3
milk samples, values ranging from 0.154 to 0.397 mg PCBs/kg milk were
measured, and, 1 milk sample contained 16.44 mg PCBs/kg fat. Three
infants died. Necropsy and histopathology revealed atrophy of the
thymus and lymph nodes, hypocellular bone marrow, moderate fatty
infiltration of the liver, hypertrophic Meibomian glands, and
keratinization of hair follicles. One dead infant showed hyperplasia
of the gastric mucosa. The 3 surviving infants were weaned and
subsequently showed marked improvements in their physical status
(Allen & Barsotti, 1976; Allen et al., 1979, 1980). At 6 and 12 months
of age, they were found to be hyperactive in a locomotor activity test
and, between 7 and 24 months of age, they did not learn reversal tasks
as readily as the controls. The PCB body burdens of these infants
ranged between 11 and 27 mg/kg body fat, at the age of 8 months, and
dropped to 0-1.6 mg/kg, at the age of 23 months. However, using the
same apparatus, these infants showed hypolocomotor activity at 44
months of age in comparison with the same controls (Bowman et al.,
1978; Bowman & Heironimus, 1981; Bowman et al., 1982; Levin & Bowman,
1983).
The infants delivered by the same adult females, after 1 year on a
control diet, showed a reduced body weight and signs of intoxication
similar to those observed in their siblings of the first breed.
Two infants in each exposed group died. Milk samples contained
0.02-0.19 mg PCBs/kg milk (Allen et al., 1980). At 12 months of age,
when the PCB body burdens were only slightly higher than those of the
controls, the infants showed hyperlocomotor activity (Bowman et al.,
1982).
Groups of 4 or 6 Rhesus monkeys, which had been exposed to 0 or 2.5 mg
Aroclor 1248/kg diet in utero and during nursing until 4 months
after birth, were tested at 4-6 years of age on delayed spatial
alternation (DSA), a spatial learning and memory task. Deficits in
performance accuracy were detected in 2 cohorts of monkeys, whose
mothers had been fed 2.5 mg Aroclor 1248/kg diet for an 18-month
period ending at least 12 months prior to pregnancy. The deficit was
most apparent at the shorter delays, suggesting impairments in
association or attention processes were involved rather than memory
impairment. Such a deficit was also found in monkeys fed 1.0 mg
Aroclor 1016/kg diet, but the effect was less pronounced. The
appearance of a PCB-induced cognitive deficit more than 3 years after
the end of exposure indicated the existence of long-term adverse
consequences of perinatal PCB exposure (Levin et al., 1988).
Clophen A30, which did not contain detectable levels of
polychlorinated dibenzofurans (limit of detection <1 mg/kg), was
given by gavage in a 1% solution of methylcellulose in water, once
daily for 30 days, to 3 lactating Rhesus monkeys and their offspring
at a level of 16 mg/kg body weight. PCB concentrations were measured
in the serum of both mothers and infants and in the milk, on days -14,
-7, 0, 1, 2, 4, 8, and then at weekly intervals until the end of the
study. The mean PCB concentrations in the serum of mothers and infants
were between 0.13 and 1.16 mg/litre and 0.07 and 2.67 mg/litre,
respectively (before treatment days -14 and -7). The mean PCB levels
in milk ranged from 0.63 mg/kg on day 1 of exposure to 18.90 mg/kg. On
days -14 and -7, the concentrations in milk were 0.14 and 0.34 mg/kg
(wet weight). Five control pairs were used. One dam and her offspring
were sacrificed on day 22, exhibiting symptoms of anorexia,
depression, lethargy, and ataxia. Two of 3 infants showed a decreased
body weight gain. At autopsy of the infants, after the exposure
period, slight degenerative changes were seen in the liver and the
kidneys, together with slight demyelination of the central nervous
system, slight to moderate gliosis of the cerebrum, and slight
granular cell layer thinning of the cerebellum. On the basis of
earlier work with adults in which the degenerative changes described
above were considerable, the authors concluded that the nursing
infants seemed to be less susceptible than the adults under the
conditions of the studies (Iatropoulos et al., 1978; Bailey et al.,
1980).
8.4.2 Teratogenicity
8.4.2.1 Aroclors (oral)
(a) Mouse
Haake et al. (1987) reported that treatment of pregnant C57Bl/6 mice
with Aroclor 1254, by gavage, at 224 mg/kg body weight, on day 9 of
gestation, did not result in any fetuses with cleft palate (see
section 8.6.6).
(b) Rat
Wistar rats were exposed to dietary levels of Kanechlor 400 of up to
250 mg/kg from day 1 to day 21 of gestation (Mizunoya et al., 1974).
Fetuses showed decreased body weights from 10 mg/kg diet onward
(equivalent to 0.67 mg/kg body weight), but did not show any increased
incidence of malformations. Maternal toxicity was not observed and
litter size and the number of litters were unaffected. Decreased pup
survival was noted at dietary levels from 50 mg/kg (equivalent to
3.5 mg/kg body weight) upwards. The 28-day-old offspring showed
decreased body weight and increased relative liver weight from
10 mg/kg.
Commercial Kanechlor 300 or 500 was mixed with food and administered
to pregnant Sprague-Dawley rats, throughout gestation, at levels of
20, 100, or 500 mg/kg diet. On day 21, about three-quarters of the
pregnant females were sacrificed; the remainder were allowed to litter
naturally and the postnatal development of the pups was observed.
Kanechlor 500 at a concentration of 500 mg/kg resulted in decreased
maternal weight gain and decreased food consumption. At 20 and 500 mg
Kanechlor 300/kg, and 500 mg Kanechlor 500/kg, the fetal weight
decreased significantly. Resorption and malformations were not
increased by treatment with Kanechlors. The Kanechlors did not show a
teratogenic potential in this study (Shiota, 1976a).
Offspring of Sprague-Dawley rats that received 20 mg of Kanechlor
500/kg body weight on days 15-21 of gestation were slower than
controls in achieving the water maze test at the age of 12-13 weeks,
but did not perform worse in the open field test and in the swimming
test (Shiota, 1976b). Behavioural effects were also observed in the
offspring of ICR dams, 23-27 days of age, exposed to Aroclor 1254 in
the diet at levels of 11 or 82 mg/kg (equivalent to 1.7 and 12 mg/kg
body weight) from 3 days before mating up to weaning. The offspring
were maintained on the same diet. PCB exposure did not have any
effects on the ability to learn an avoidance response, but increased
the latency to make such a response. Moreover, the young mice
exhibited slower habituation to an open field (Storm et al., 1981).
The effects of Fenchlor 42 (trichloro- 63%, tetrachloro- 33%, and
small amounts of dichlorobiphenyl; purity 97.5%) exposure of Fischer
344 male and female rats were studied through assessment of the
behavioural development of their F1 progeny. Female rats were
administered 5 daily ip injections of corn oil or 5-10 mg Fenchlor
42/kg body weight per day, 2 weeks prior to mating. Another group
received 2-4 mg/kg per day during gestation (days 6-15 of pregnancy)
and a third group of 8 previously treated lactating females received
corn oil or 1-2 mg/kg per day on postnatal days 1-21. The total doses
in the 3 groups were 25-50, 20-40 and 20-40 mg/kg body weight.
Dose-dependent differences in behaviour were found in the offspring of
the PCB-treated animals. Differences in the development of cliff
avoidance reflexive behaviour, swimming ability, and open field
activity were evident. The PCB exposure of female animals during
gestation and lactation resulted in impaired acquisition of the active
avoidance behaviour, while preconception PCB exposure affected active
avoidance performance, as reflected in an increased number of
avoidance responses to reach criterion for extinction (Pantaleoni et
al., 1988).
Doses of 0, 6.25, 12.5, 25, 50, or 100 mg/kg body weight per day of
Aroclor 1254 were administered to rats, by gavage, on days 6-15 of
gestation. Average pup weights were reduced at 100 mg/kg, though total
litter weight (average weight times number of fetuses) did not differ
from controls. There were no skeletal or visceral abnormalities or
effects on conception, resorptions, litter size or number, or average
litter weight in any of the treated groups (Villeneuve et al., 1971b).
Spencer (1982) exposed Holtzman rats to a diet containing Aroclor 1254
at levels of 25 up to 900 mg/kg diet from day 6 to day 15 of gestation
and found reduced maternal body weight gain and decreased fetal
survival at birth from 300 mg/kg diet (equivalent to 18 mg/kg body
weight) upwards, and decreased fetal weights at birth from 100 mg/kg
(equivalent to 8 mg/kg body weight) upwards. No visceral or skeletal
data were available.
When Sherman rats were exposed to doses of Aroclor 1254 of up to
100 mg/kg body weight, in peanut oil, from day 7 to day 15 of
gestation, a decrease in the survival of the pups was found. At
weaning, the survival and body weight of the grossly normal pups were
reduced at the 100 mg/kg dose level, but not at 50 mg/kg body weight.
No effects were observed at 100 mg Aroclor 1260/kg body weight (Linder
et al., 1974).
(c) Monkey
Two pregnant Cynomolgus monkeys (Macaca fascicularis) were dosed
with Aroclor 1254 at 100 mg/kg body weight per day and 1 monkey, at
400 mg/kg body weight per day, from day 60 of gestation. One control
animal received the vehicle, corn oil. The 2 monkeys fed 100 mg/kg
delivered dead male infants after 105 and 108 days of dosing, and the
female fed 400 mg/kg delivered a female infant (with no overt clinical
signs of toxicity) that died at 139 days of age with an acute
bronchopneumonia. The breast milk of the monkey fed 400 mg/kg
contained, over a period of 5-75 days after parturition,
concentrations of 73.7 up to 139.4 mg/kg, on a fat basis. No overt
signs of toxicity were observed in the adult animals, with exception
of finger nail loss. All 3 treated monkeys showed impaired
immunological capacity, assessed at approximately 50 days postpartum
(148 days of treatment) (Truelove et al., 1982).
(d) Rabbit
Rabbits were exposed to 0, 1.0, or 10.0 mg Aroclor 1254/kg body weight
and in another study to 0, 12.5, 25.0, or 50 mg/kg body weight (purity
not reported), by gavage, on days 1-28 of pregnancy. Abortions, still
births, and maternal deaths occurred at 12.5 mg/kg body weight or
more, but no teratogenic effects were found (Villeneuve et al.,
1971a,b).
8.4.2.2 Aroclors (subcutaneous)
(a) Mouse
A possible teratogenic effect was observed in ddy mice subcutaneously
exposed to doses of 1-5 mg Kanechlor 500/mouse (equivalent to
40-200 mg/kg body weight) in 95% ethanol from day 6 to day 15 of
gestation. A dose-related increase in maternal mortality was observed
from a dose of 3 mg/mouse onwards. Some dams showed skin lesions,
alopecia, or swelling of the liver, but no effects on body weight
gain. A slight increase was noted in the incidence of dead and
resorbed fetuses. The incidence of cleft palate was increased in a
dose-related manner from the lowest dose (Watanabe & Sugahara, 1981).
8.4.2.3 Individual congeners (oral)
(a) Mouse
Orberg (1978) fed groups of 20-56 pregnant female NMRI-mice 0, 0.05,
or 0.5 mg 2,5,4'-trichloro- or 2,4,5,2',4',5'-hexachlorobiphenyl,
dissolved in peanut oil, per animal, from days 1 to 6 of gestation. A
significant decrease in the pregnancy of implanted ova was found with
the 0.5 mg treatment. No effects on percentage of pregnancies and mean
number of corpora lutea were found. There were no effects at the lower
dose level.
A dose-related increase in embryotoxicity and the incidence of
malformed fetuses, mainly showing cleft palate and hydronephrosis, was
found in pregnant CD-1 mice after exposure to daily doses of 2, 4, 8,
or 16 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body weight, in
cotton-seed oil, on days 6-15 of gestation. Lower dose levels, e.g.,
0.1 and 1 mg/kg body weight were without effects. No dibenzofurans
were detectable (no details) in the test compound. The dams showed a
decreased body weight gain at 8 mg/kg body weight. The authors
reported that 3,4,3',4'-tetrachlorobiphenyl and 2,3,4,2',3',4'-hexa-
chlorobiphenyl also produced the same teratogenic effects, though they
were less potent than those of 3,4,5,3',4',5'-hexachlorobiphenyl
(Marks et al., 1981).
A neurobehavioural "spinning" syndrome (a syndrome characterized by
the fact that the mice rotate or spin in a circular motion when held
by the tail) and hydronephrosis developed in CD-1 mouse weanlings,
whose dams received, by gavage, 32 mg 3,4,3',4'-tetrachlorobiphenyl/kg
body weight, in corn oil, on days 10-16 of gestation. Maternal
neurotoxicity was not observed. Histological and ultrastructural
examination of the CNS of affected mice revealed longitudinal
projections of the cylindrical CNS in the ventral and dorsal roots
and, to a lesser extent, in cranial nerve roots. The effect was
possibly related to an observed altered development of striatal
synapses (Chou et al., 1979; Tilson et al., 1979; Agrawal et al.,
1981).
(b) Rat
The congener 3,4,3',4'-tetrachlorobiphenyl was found to be embyrotoxic
and caused accumulation of blood in the amniotic fluid and the
gastrointestinal tract of fetuses from Sprague-Dawley rats treated on
days 6-18 of gestation with doses of 3 or 10 mg/kg body weight in corn
oil. Decreased fetal growth was also observed (Wardell et al., 1982).
When the rats were allowed to deliver, high perinatal mortality was
observed, which appeared to be related to an increase in gestational
length and to be independent of the smaller total litter size. In
addition, pup weights were found to be lower than those of controls
(Rands et al., 1982; White et al., 1983).
(c) Guinea-pig, mouse
Neither embryotoxicity nor teratogenicity were found in Dunkin Hartley
guinea-pigs, and CBA mice exposed in utero to 2,4,5,2',4',5'-
hexa-chlorobiphenyl during gestation (Mattsson et al., 1981; Brunström
et al., 1982; Aulerich et al., 1985) and in C57BL/6N mice similarly
exposed to 2,4,5,2',4',5'- or 2,3,4,5,3',4'-hexachlorobiphenyl
(Birnbaum et al., 1985).
Pregnant guinea-pigs received a total dose of 100 mg technical grade
Clophen A50 orally, in peanut oil, from day 16 to day 60 of gestation,
25 mg 2,4,5,2',4',5'-hexachlorobiphenyl from day 16 to day 60 of
gestation, or 100 mg from day 22 to day 60 of gestation. The
administration of Clophen A50 resulted in fetal deaths, but no
maternal deaths. In contrast, 2,4,5,2',4',5'-hexachlorobiphenyl did
not cause fetal deaths. Prenatal weight of live fetuses was increased
by a dose of 25 mg, but not by 100 mg of 2,4,5,2',4',5'-hexa-
chlorobiphenyl (Brunström et al., 1982).
(d) Monkey
In a briefly reported study, 6 female Rhesus monkeys received 9 doses
of 70 or 350 µg 3,4,3',4'-tetrachlorobiphenyl/kg body weight by
gavage, in corn oil, from day 20 to day 40 of gestation. A control
group comprised 12 animals. Maternal toxicity (not specified) but no
mortality, was reported in all exposed monkeys from day 31 following
exposure. Between days 17 and 35 following exposure all exposed
fetuses and 3 out of 12 control fetuses aborted (McNulty, 1985).
8.4.3 Appraisal
The Rhesus monkey is the most sensitive species with regard to general
toxicity (see section 8.2) and particularly with regard to
reproductive toxicity. The presence of PCDFs and the variation in PCB
composition may be confounding factors in determining the reproductive
toxicity of PCB mixtures. Aroclor 1248, containing 4.4-8.7 µg
PCDFs/kg, adversely affected the reproductive performance of female
Rhesus monkeys, mated with control males after 6 months of dietary
exposure to a toxic dose of 0.09 mg/kg body weight per day and
continuation of the exposure for an average of up to 10 months. This
effect was reversible after an exposure-free period of 1 year. No
effect on reproduction was found in female Rhesus monkeys exposed to a
non-toxic dose of 0.01 or 0.03 mg Aroclor 1016 (reported not to
contain PCDFs)/kg body weight per day and mated after 7 months with
control males.
Neonates of the nursing mothers exposed to Aroclor 1248 showed adverse
effects similar to those seen in their mothers and, in addition,
persistent behavioural disturbances, atrophy of the thymus and lymph
nodes, bone marrow hypoplasia, and hyperplasia of the gastric mucosa.
Neonates of the mothers after the recovery period still showed adverse
effects, as well as the neonates of the mothers exposed to Aroclor
1016. These effects were caused by PCBs, with or claimed to be
without, PCDFs, transmitted via the placenta during gestation and
later via the breast milk. Neonates have much greater susceptibility
to PCB toxicity when exposed via the mothers compared with suckling
monkeys orally exposed. Reproductive toxicity has also been observed
in the mink, rabbit, and rat. The changes seemed to be related to
alterations in the serum levels of gonadal steroid hormones, as a
result of enzyme induction. PCBs may also bind to the cytoplasmic
estrogen receptor. Effects have also been observed on the estrus cycle
of female rats, minks, and monkeys, on the sex organs of male rats,
and on the implantation rate of fertilized ova following exposure of
female mice or male rats.
Comprehensive teratological examinations have not been conducted;
however, the available studies indicated that the Aroclors were not
teratogenic in rats and nonhuman primates, when tested via the oral
route during the critical periods of organogenesis at doses that
produced fetotoxicity and/or maternal toxicity. Although the
fetotoxicity of Aroclors is documented in several species of animals,
the possibility that contaminants (e.g., PCDFs) might be (partly)
responsible for the effects should be recognized.
The results of the reproduction and teratogenicity studies are
summarized in Tables 45, 46, and 47.
8.4.4 Mutagenicity and related end-points
8.4.4.1 DNA damage
PCBs have been shown to interact with the proteins, RNA and DNA, after
metabolic activation. The potential of readily metabolizable
PCB-congeners to cause primary DNA damage was indicated by the
activity of 2,5,2',5'-tetrachlorobiphenyl and its metabolites in
causing DNA, single-strand breaks in an alkaline elution assay with
L-929 cells in vitro (Stadnicki et al., 1979). Furthermore,
unscheduled DNA synthesis was elicited by 4-chlorobiphenyl in vitro
in Chinese hamster ovary cells (Wong et al., 1979). No unscheduled DNA
synthesis was elicited by Aroclor 1254 in rat hepatocytes in vitro
(Probst et al., 1981).
DNA-breaking activity was found in an alkaline elution assay with
hepatocytes of intact rats treated in vivo with a single, high dose
(500 mg Aroclor 1254/kg body weight, intraperitoneally, or 1295 mg/kg
body weight, orally) with complete repair of the damage within 48 h
(Robbiano & Pino, 1981). Aroclor 1254 was also shown to be a
DNA-breaking agent in an alkaline elution assay in vitro with rat
hepatocytes (Sina et al., 1983). An alkaline sedimentation assay
showed the DNA-breaking activity of Aroclor 1254 in rats treated
in vivo with a single intraperitoneal dose of 500 mg/kg body weight.
In this assay, the same Aroclor 1254 pretreatment of the rats in vivo
elevated the DNA-breaking activity of the direct-acting, alkylating
N-methyl- N'-nitro- N-nitrosoguanidine and the carcinogens,
dimethylnitrosamine and benzo (a)pyrene, in vitro (Mendoza-Figueroa
et al., 1985).
8.4.4.2 Mutagenicity tests
Many mutagenicity tests have been carried out over the years, with
different PCB mixtures. Most of these were commercial mixtures the
composition of which was not described. Only a few studies are
available on specific congeners. Besides studies on microorganisms,
mammalian cell point mutation, dominant lethal assays, micronucleus
tests, chromosome and cytogenicity studies, and DNA repair studies
were carried out. With a few exceptions the results of most of the
studies with PCB mixtures were negative (see Table 48).
Aroclor mixtures and the congener 2,5,2',5'-tetrachlorobiphenyl and
its metabolites did not induce point mutations in Salmonella
typhimurium TA 98, TA 100, TA 1535, TA 1537, and TA 1538 with, and
without, metabolic activation (Wyndham et al., 1976; Hsia et al.,
1978; Bruce & Heddle, 1979; Schoeny et al., 1979; Shahin et al.,
1979), neither did 2,4,2',4'- and 3,4,3',4'-tetrachlorobiphenyl and
2,4,6,2',4',6'-hexachlorobiphenyl in the strains TA 98 and TA 100
(Schoeny, 1982).
However, Wyndham et al. (1976) found a dose-related mutagenic activity
of 4-chlorobiphenyl and, to a lesser extent, of Aroclor 1221 in the
strain TA 1538, after metabolic activation by the S9 liver fraction of
uninduced rabbits. The study was repeated 3 times in the same
laboratory, but the results of Wyndham et al. (1976) could not be
confirmed (Safe, 1980). Schoeny (1982) could not detect any mutagenic
activity of 4-chlorobiphenyl in the same dose range in the strains TA
98, TA 100, TA 1535, and TA 1537, with, or without, the S9 liver
fraction of induced rats.
Table 45. PCBs: reproduction and embryo toxicity
Animal (strain, PCB mixture Exposure period NOAEL LOAEL Parameters, effects Reference
sex) (oral) (mg/kg body (mg/kg body
weight) weight)
Rat (Sherman, Aroclor 1254 continuous up to 0.06 (1254) increased relative liver Linder et al.
10 male, Aroclor 1260 weaning weights in male F1A (1974)
20 female) weanlings
0.32 (1254)
former effect in all weanlings
0.32 (1254)
7.5 (1260) reproduction parameters
reproduction parameters
Rhesus monkey Aroclor 1016 7 months 0.03 (0.04) reproduction parameters Barsotti &
(female) [0.03(0.04): decreased birth van Miller
weight] [0.01: skin (1984)
hyperpigmentation]
Rhesus monkey Aroclor 1248 6 months 0.09 abortions, resorptions, live Barsotti et al.
(female) births (1976)
1 year after 0.09 0.2 Allen et al.
exposure stillborn, live births (1980)
Table 46. PCBs: teratogenicity
Animal (strain) PCB-mixture Exposure period NOAEL LOAEL Parameters, effects Reference
(oral) (mg/kg body (mg/kg body
weight) weight)
Rat Aroclor 1254 days 6 to 15 of 50 100 reduced average pup Villeneuve et al.
gestation weight (1971b)
Rat (Holtzman) Aroclor 1254 days 6 to 15 of < 8 8 decreased fetal weight at Spencer (1982)
gestation birth
Rat (Sherman) Aroclor 1254 days 7 to 15 of 100 reproduction effects Spencer (1982)
gestation
Rabbit Aroclor 1254 days 1 to 28 of 10 12.5 abortions, still births, Villeneuve et al.
gestation maternal deaths (1971 a,b)
Aroclor 1221 days 1 to 28 of 25 fetotoxicity
gestation
Table 47. PCBs: teratogenicity of individual congeners
Animal PCB-mixture Exposure period NOAEL LOAEL Parameters, effects Reference
(strain) (oral) (mg/kg body (mg/kg body
weight) weight)
Mice 2,5,4'-TCB day 1 to 6 of 0.05/animal 0.5/animal decrease in the number Örberg (1978)
(NMRI) 2,4,5,2',4', gestation of implant/dams
5'-HCB
Mice 3,4,5,3',4', day 6 to 15 of 2 embryotoxicity, malformations Marks et al.
(CD-1) 5'-HCB gestation (cleft palate, hydronephrosis) (1981)
3,4,3',4'-TCB 8 maternal toxicity, less potent
2,3,4,2', than that of 3,4,5,3',4',5'-HCB
3'4'-HCB
Rat 3,4,3',4'-TCB day 6 to 18 of 3 embryotoxicity Wardell et al.
(Sprague- gestation (1982)
Dawley)
Rhesus 3,4,3',4'-TCB day 20 to 40 of 0.07 maternal toxicity, total abortions McNulty (1985)
monkey gestation
Table 48. Results of mutagenicity, and related, tests
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
2,5,2',5'- Salmonella TA 1538 200 µg/plate modified - Wyndham et al.
tetrachloro typhimurium 100 µg/plate microsomal - (1976)
biphenyl 50 µg/plate fraction -
10 µg/plate from rabbits -
Aroclor 1268 Salmonella TA 1538 200 µg/plate modified - Wyndham et al.
typhimurium 100 µg/plate microsomal - (1976)
50 µg/plate fraction -
10 µg/plate from rabbits -
Aroclor 1254 Salmonella TA 1535 4 different both with, and - Heddle & Bruce
typhimurium TA 1537 concentrations, without, S-9 in - (1977)
TA 98 figures not all cases -
TA 100 given -
Aroclor 1254 Salmonella TA 1535 8 different both with, and - Schoeny et al.
typhimurium TA 1537 concentrations without, S-9 - (1979)
TA 98 from 0.5 to -
TA 100 500 µg/plate -
Aroclor 1254 Salmonella TA 1535 0.05, 0.5, both with, and - Bruce & Heddle
typhimurium TA 1537 5, 50, and without, S-9 - (1979)
TA 98 500 µg/plate -
TA 100 -
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Aroclor 1254 Salmonella TA 1538 50, 100, 500, both with, and - Shahin et al.
typhimurium TA 98 1000, 2000, without, S-9 - (1979)
5000 µg/plate
Aroclor 1242 V79 50, 100, and without - Hattula (1985)
Clophen A60 hamster cells 150 µg/ml metabolizing -
co-cultivated cells
with lethally
irridatiated
hepatocytes
Aroclor 1254 Micronucleus 4 different - Heddle & Bruce
test concentrations, (1977)
(erythrocytes) figures not
given
Aroclor 1254 Micronucleus (C57B1/6 × (approximately) - Bruce & Heddle
test C3H/He) LD50, 1/2, 1/4, (at all doses) (1979)
F1 mice and 1/8 of the
highest dose
(5 consecutive
days, ip)
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Kanechlor 500 Micronucleus ddY-mice 100 mg/kg in - - Watanabe et al.
test with corn oil orally (1982)
polychromatic and 100 mg/kg
erythrocytes 95% ethanol
subcutaneously
Aroclor 1254 Chromosomal (embryonic 0 or 10 mg/kg inconclusive Peakall et al.
aberrations Ring diet (1972)
Doves)
Aroclor 1254 Chromosomal (human 100 mg/litre - Hoopingarner
aberrations lymphocytes) culture medium et al. (1972)
Aroclor 1254 Chromosomal male 5, 50, 500 mg/kg negative at all Garthoff et al.
abnormalities in Holtzmann diet doses (1977)
bone marrow and rats
spermatogonial
cells
Aroclor 1242 Chromosomal Osborne- 5000 mg/kg × 1a - Green et al.
abnormalities Mendel 2500 mg/kg × 1 - (1975a)
in bone marrow rats 1250 mg/kg × 1 -
cells and 500 mg/kg × 4 -
spermatogonial
cells
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Clophen A30 Drosophila 62.5, 125, 250, -b Nilsson & Ramel
melanogaster and 500 mg/litre (1974)
(adults or larvae) substrate
Clophen A50 Drosophila 25, 50, 100, -b Nilsson & Ramel
melanogaster and 200 mg/litre (1974)
(adults or larvae) substrate
Aroclor 1242 Dominant Lethal Osborne- 2500 mg/kg × 1a - Green et al.
test Mendel 1250 mg/kg × 1 - (1975b)
rats 625 mg/kg × 1 -
250 mg/kg × 5 -
125 mg/kg × 5 -
Aroclor 1254 Dominant Lethal Osborne- 150 mg/kg × 5a - Green et al.
test Mendel 75 mg/kg × 5 - (1975b)
rats
Aroclor 1254 Dominant Lethal Osborne- 25, 100 mg/kg - Green et al.
test Mendel diet for 70 days - (1975b)
rats
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Aroclor 1254 Sperm (C57 B1/6 × (approximately)LD50, negative at all Bruce & Heddle
Abnormality C3H/He)F1 1/2, 1/4, 1/8 top dose doses (1979)
mice on 5 consecutive
days, ip
Aroclor 1254 mitotic index human 100 mg/litre mitotic index Hoopingarner
lymphocytes culture medium equivocal et al. (1972)
4-chloro- DNA repair and Chinese 10-5 mmol/litre covalent- Wong et al.
biphenyl and unscheduled hamster 3H-4-chloro- binding to (1979)
metabolites synthesis ovary cells biphenyl, 24 h protein, RNA,
(hydroxyurea and DNA
addition suppress Increase
DNA synthesis) specific
activity with
DNA
a Means single dose (× 1) or 5 doses in 5 days (× 5).
b No loss or non-disjunction of sex-chromosomes.
SD=Significant decrease.
Dose-related chromosome breakage was found in human lymphocytes
exposed to the planar PCB congener, 3,4,3',4'-tetrachlorobiphenyl, at
0.1-10-4 µg/ml (Sargent et al. (1989). In contrast, the non-planar
2,5,2',5'-tetrachlorobiphenyl did not cause chromosome damage in a
comparable test, even at concentrations as high as 1 µg/ml. However, a
combination of 3,4,3',4'-tetrachlorobiphenyl at a concentration of
10-5 µg/ml with 2,5,2',5'-tetrachlorobiphenyl caused chromosomal
damage that was far in excess of what might be expected from higher
doses of 3,4,3',4'-tetrachlorobiphenyl alone. The results suggest that
some PCB congeners may interact to cause synergistic effects.
Peakall et al. (1972) carried out cytogenic studies on Ring dove
embryos (Streptopelia risoria); 6 embryos were from dove pairs not
fed PCBs (controls) and 17 embryos were from PCB-fed (10 mg/kg diet)
pairs. The frequencies of chromosome aberrations were recorded for
chromosome pairs occurring in metaphase cells of allantoic sac and
limb bud origin. Mean aberration rates were as follows: control 0.8%
(range 0-2.0%) and PCB-treated 1.8% (range 0-9.4%). It was concluded
by the authors that these results were indicative of a possible
clastogenic action of PCBs.
A DNA repair assay was carried out by Wong et al. (1979) using CHO
cells and measuring the effects of 4-chlorobiphenyl (10 mol/litre) on
the unscheduled DNA synthesis (UDS) in the presence of hydroxyurea
(HU), a chemical agent that suppresses normal replicative DNA
synthesis. The quantification of DNA synthesis was determined by the
uptake of [H3]-thymidine into the cellular DNA. A significant
(1.6-fold) enhancement of UDS was found when the cells were incubated
for 2.5 h in the presence of HU, 4-chlorobiphenyl, and thymidine.
8.4.4.3 Cell transformation
Aroclor 1254 did not cause an increase in benzo (a)pyrene-induced
transformation in a test using C3H10 T1/2 CL8 mouse embryo fibroblasts
(Nesnow et al., 1981).
Aroclor 1254 also failed to transform Golden Syrian hamster cells
76-582 in culture at 50 µl/ml (Pienta, 1980).
8.4.4.4 Cell to cell communication
The congener 2,4,5,2',4',5'-hexachlorobiphenyl inhibited one form of
intercellular communication in V79 Chinese hamster cells, i.e.,
metabolic cooperation by mutant rescue at non-cytotoxic levels, while
3,4,5,3',4',5'-hexachlorobiphenyl was inactive (Tsushimoto et al.,
1983).
8.4.4.5 Interaction
Grolier et al. (1989) studied the effects of Vitamin A dietary intake
(2 and 20 IU/g of food) on the mutagenicity of benzo (a)pyrene
(B (a)P) towards Salmonella typhymurium TA 98, either in control
rats or in animals treated with 2,4,5,2',4',5'- hexachlorobiphenyl and
3,4,3',4'-tetrachlorobiphenyl. The planar tetrachlorobiphenyl strongly
increased B (a)P-monooxygenase activity and glutathione transferase,
while the non planar hexachlorobiphenyl was a strong inducer of
epoxide hydrolase and a weak inducer of B (a)P-monooxygenase. Enzyme
induction was not modified by changes in Vitamin A intake. A greater
mutagenic effect was observed in the tetrachlorobiphenyl group than in
the hexachlorobiphenyl group. This could be related to the specific
form of cytochrome P-450 induced by the tetrachlorobiphenyl congener.
In PCB-treated rats, the mutagenic activity of B (a)P was higher in
the 20-IU group than in the 2-IU group.
8.4.4.6 Cell division parameters
Tests on Osborne-Mendel rats gave various results, but may provide the
most important clue to the mechanism of action of PCBs in
carcinogenesis. At high doses (5000 mg Aroclor 1242/kg given once, and
500 mg/kg, given in a series of 4 daily doses), there were significant
decreases in the numbers of spermatogonial cells in mitosis. Single
dose levels of 1250 or 2500 mg/kg gave negative results (Green et al.,
1975a). Garthoff et al. (1977) also found negative results in male
Holtzmann rats treated with 0, 5, 50, or 500 mg Aroclor 1254/kg diet
for 5 weeks with regard to the mitotic indices of bone marrow and
spermatogonial cells. The data of Hoopingarner et al. (1972) showed an
increase in mitotic index in human lymphocytes exposed to Aroclor
1254.
The above-mentioned studies provide evidence that Aroclors can enhance
cell proliferation, and this is of special interest because it
suggests that the Aroclors may act to promote carcinogenesis to a
greater extent than to initiate it. The effect on cell proliferation
requires further examination in a variety of systems.
8.5 Carcinogenicity
Hayes (1987) critically reviewed the available evidence for, and
against, the view that environmental PCBs present a significant
potential carcinogenic hazard for humans.
8.5.1 Long-term toxicity/carcinogenicity
(a) Mouse
Nagasaki et al. (1974) exposed 10 groups of dd mice (6-12 of each sex)
to Kanechlor 300, 400, or 500 in the diet at levels of 0, 100, 250, or
500 mg/kg (equivalent to 0, 5, 12.5, and 25 mg/kg body weight,
respectively) for 32 weeks. Nine nodular hyperplasia and 7
hepatocellular carcinomas were found in 17 male mice, and 4 cases of
liver hypertrophy in 17 female mice, after exposure to 500 mg
Kanechlor 500/kg diet. No neoplasms were found in the other groups.
In another mouse study, groups of male BALB/cJ mice were fed 0 or
300 mg Aroclor 1254/kg diet (equivalent to 50 mg/kg body weight) for 6
or 11 months. Adenofibrosis was observed in the livers of all 22 mice
fed Aroclor 1254 for 11 months, but not in those of the 24 mice
exposed for 6 months. Hepatomas were noted in 9/22 mice exposed for 11
months and in 1/24 mice exposed for 6 months. No tumours were found in
the controls (Kimbrough & Linder, 1974).
(b) Rat
In a preliminary study, liver tumours (multiple adenomatous nodules)
were induced by Kanechlor 400 (containing 2,4,3',4'-; 2,5,3,3'-;
2,3,4,4'-; and 3,4,3',4'-tetrachlorobiphenyls) in 6/10 females, but
not in male Donryu rats, in 400 days. The dietary exposure was
periodically adjusted according to animal weights and ranged from 38.5
to 616 mg/kg diet. The latter dose level was administered for 275
days. The number of animals used was small (10 treated and 5 control
rats of each sex). Increased incidences of pneumonia, and lung and
intracranial abscesses were found in rats on a diet containing
Kanechlor 400, possibly because of lowered resistance to infection
(Kimura & Baba, 1973).
Ito et al. (1974) fed 10 groups of 29 male Wistar rats with Kanechlor
300, 400, or 500 at dose levels of 100, 500, or 1000 mg/kg diet
(equivalent to 5, 25, and 50 mg/kg body weight, respectively) for
28-52 weeks. Another group received the control diet. A number of
animals died in all groups (4 up to 21 animals); within the treated
groups, deaths were more or less dose related. Adenofibrosis was
observed in the livers of rats fed 1000 mg/kg diet of all 3 mixtures.
Kanechlor 500 produced nodular hyperplasia at all dose-levels and at a
higher incidence than the mixtures with a lower chlorine content. No
neoplastic nodules were observed in the controls. Kanechlor 300 and
500 (100 mg/kg diet) did not show significant growth inhibition or
increases in liver weight. No fibrosis or cholangiofibrosis, bile duct
proliferations, fatty changes, or cellular hypertrophy in the liver
were found. The liver nodular hyperplasia, designated as preneoplastic
by the investigators, was found in 3/25 of the Kanechlor 500
(100 mg/kg diet) treated animals, 1/22 of the Kanechlor 300 (100 mg/kg
diet) treated animals, and 0/18 controls. The 1/22 (4.5% incidence in
the Kanechlor 300, 100 mg/kg diet) is not significant and the 2 higher
dose levels of this product did not induce such changes. It can be
concluded that Kanechlor 300 did not induce neoplasia at dietary
levels of up to 1000 mg/kg over a 52-week period, in this study. At
the 1000 mg/kg level, Kanechlor 300 did produce other evidence of
chronic liver toxicity including oval cell and bile-duct
proliferation, fatty changes, and cellular hypertrophy and, possibly,
cholangiofibrosis (2/15). In the case of Kanechlor 500 (100 mg/kg
diet), essentially the same picture was obtained. In this group, 3/25
cases of nodular hyperplasia were found. In the case of Kanechlor 400
with the dietary levels of 100 and 1000 mg/kg, 2/16 and 3/10 of the
animals had nodular hyperplasia in the liver, respectively.
In a preliminary study on Sherman rats (10 animals of each sex/dose),
Aroclor 1254 at dietary levels of 0, 20, 100, or 500 mg/kg, and
Aroclor 1260 at levels of 0, 20, 100, 500, or 1000 mg/kg, for 8
months, did not give neoplastic nodules or hepatocellular carcinoma.
At 500 mg/kg, Aroclor 1254 produced adenofibrosis in 10/10 male
animals and at 100 and 500 mg/kg, in 7/10 and 9/10 females,
respectively. This change was only seen in 2/10 male and 4/10 female
animals with Aroclor 1260. The authors stated that hepatocellular
adenofibrosis is a persistent progressive lesion that consists of a
marked proliferation of fibrous tissue and epithelial glandular cells
that are well differentiated in mice, but appear atypical in rats
(Kimbrough et al., 1972).
A group of 200 female Sherman rats was given Aroclor 1260 at an
average dietary level of 100 mg/kg (range, 70-107 mg/kg), for 21
months. The PCB intake declined from 11.6 mg/kg per day during the
first week to 6.1 mg/kg at 3 months and to 4.3 mg/kg body weight per
day, later on. The control group also comprised 200 rats. The survival
rate and the food intake were not affected and no treatment-related
signs of toxicity were observed. Body weight gain was decreased from 3
months after the onset of the exposures. Hepatocellular carcinomas
were present in the liver of 26/184 (14%) exposed rats and 1/173
(0.58%) control rats. The livers of most of the remaining exposed rats
(144/184) showed hyperplastic nodules, while none were found in
control rats. A total of 182 exposed rats and 28 control rats had
livers with foci or areas of cytoplasmic alteration. A few livers of
exposed rats showed adenofibrosis. No induction of tumours in other
organs and no metastases from the liver tumours were found (Kimbrough
et al., 1975).
Calandra (1976) reported the findings from several long-term studies,
performed by a commercial laboratory for Monsanto. (These studies have
never been published). In these studies, 1000 rats were divided into
10 groups of 100 animals (50 of each sex) and 9 of the groups were
exposed to Aroclors 1242, 1254, or 1260 at dietary levels of 1, 10, or
100 mg/kg diet. Apparently, 5 animals of each sex were sacrificed at
3, 6, and 12 months with about 35 animals killed at the end of the
2-year studies. In the animals sacrificed early, only one nodular
hyperplasia was observed and it was in the group fed 100 mg Aroclor
1260/kg for 12 months. Mortality in these studies was high and
approximately one-third of the 105 animals anticipated to be exposed
for 2 years at each dietary level died. Hepatomas were observed in
7/25 livers from animals fed 100 mg Aroclor 1260/kg, in 4/26 fed
Aroclor 1254, 3/19 fed Aroclor 1242, and only in 1/168 animals
receiving the 1-10 mg/kg diets. Nodular hyperplasia was twice as
prevalent as hepatomas in the high-dose animals, particularly in the
Aroclor 1254 group (Harbison et al., 1987).
In a limited study, groups of 24 Fischer 344 rats of each sex received
a diet containing Aroclor 1254 at 0, 25, 50, or 100 mg/kg diet
(equivalent to 0, 1.2, 2.5, and 5 mg/kg body weight, respectively) for
104-105 weeks. The survival rate decreased with a dose-related trend
in male, but not in female rats (92, 83, 58, and 46%, respectively).
From 10 weeks of exposure onwards, the body weight gain of all rats,
except that of the low-dose males, decreased. In the groups receiving
the 2 highest dose levels, alopecia, facial oedema, exophthalmos, and
cyanosis were observed. Foci of hepatocellular alterations, which were
dose-related, were found at all dose levels, but not in the controls.
The incidence of non-neoplastic hyperplastic nodules in male rats with
25, 50, or 100 mg/kg diet was 5/24, 8/24, and 12/24 and in female rats
6/24, 9/22, and 17/24, respectively (US EPA, 1980). None was found in
the controls. Hepatocellular adenoma and carcinomas were found in 1/24
males and 1/24 females on the 50 mg/kg diet and in 3/24 males and in
2/24 females on the 100 mg/kg diet. Non-neoplastic liver lesions
included degenerative changes and aggregates of macrophages with
crystalline cytoplasmic structures and pigment granules. An apparently
dose-related increase in the incidence of intestinal metaplasia was
observed in both sexes and 0, 1, 3, and 2 adenocarcinomas located in
the pyloric region of the glandular stomach were found at 0, 25, 50,
and 100 mg/kg diet, respectively. Morgan et al. (1981) and Ward (1985)
reexamined the NCI (1978) data with respect to gastric
adenocarcinomas, hepatocellular adenomas, and carcinomas. Morgan et
al. (1981) found incidences of focal stomach lesions, mostly
metaplasia, of 6, 10, 17, and 35% in rats receiving 0, 25, 50, or
100 mg Aroclor 1254/kg diet, respectively. Adenomas were found in 6
treated rats. When compared with the incidences of stomach
adenocarcinomas in historical controls (1/3548), the incidence of
6/144 was statistically significantly increased (NCI, 1978; Morgan et
al., 1981; Ward, 1985).
Groups of 70 Sprague-Dawley rats of each sex received a diet
containing Aroclor 1260 in corn oil at a concentration of 100 mg/kg
diet (equivalent to 5 mg/kg body weight) for 16 months and 50 mg/kg
diet (equivalent to 2.5 mg/kg body weight) for an additional 8 months.
All surviving rats received a basal diet from month 25 to month 29.
The control group comprising 63 rats of each sex, received the basal
diet with corn oil for 18 months and the basal diet alone for an
additional 5 months. All surviving rats received the basal diet from
the 25th month to the 29th month. Data on growth were not available.
Groups of 2 control or 3 PCB-treated rats of each sex were partially
hepatectomized at 1, 3, 6, 9, 12, 15, and 18 months. At 24 months, a
similar group was sacrificed; after 29 months all remaining animals
were sacrificed. The mortality rate was not affected by the exposure.
In the livers of exposed rats, centrolobular hypertrophy was apparent
at 1 month, foci at 3 months, and areas of cellular alteration after 6
months, neoplastic nodules after 12 months, trabecular carcinomas
after 15 months, and adenocarcinomas after 24 months. Metastases in
the lung were not found. In exposed rats that survived 18 months or
longer, hepatocellular carcinomas were present in 43/47 females and in
2/46 males, but were absent in 81 controls. Simple and cystic
cholangioma at 18 and 23 months, respectively, and adenofibrosis at 22
months were present in the treated rats (Norback & Weltman, 1985).
Rao & Banerji (1988b) fed 3 groups of 32 weanling Wistar rats a
protein diet containing 0 (coconut oil), 50, or 100 mg Aroclor 1260/kg
diet, for 120 days. The incidence of neoplastic nodules in the liver
was 0/32, 24/32 and 16/32, respectively. Adenofibrosis was also
observed in the treated animals.
In order to exclude possible effects of dibenzofurans and to
investigate the effect of the degree of biphenyl-chlorination, groups
of 152 and 144 male Wistar rats were exposed to Clophen A30 and
Clophen A60, respectively, not containing detectable quantities
(detection limit not stated) of dibenzofurans, for 800 days at a dose
of 100 mg/kg diet. A group of 139 rats received a control diet. After
800 days, randomly selected rats were killed daily, until all
survivors had been examined by day 832. The survival rate of the
remaining exposed rats was increased by day 800. In rats autopsied by
day 800 and in rats autopsied later, the incidence of hepatocellular
carcinoma was increased by exposure to Clophen A60 (9/129 and 52/85,
respectively) relative to controls (0/131 and 1/53, respectively), but
not by exposure to Clophen A30 (1/138 and 3/87, respectively). There
was a marked trend from foci of hepatocellular alteration to
neoplastic nodule to carcinoma with increasing time and degree of
biphenyl chlorination. Controls mainly showed foci. Non-neoplastic
liver lesions with increased incidences of bile duct hyperplasia were
found in rats receiving Clophen A30 and A60, and cysts in rats
receiving Clophen A60. The incidence of adenofibrosis in the liver was
decreased, compared with that in the controls, in all exposed rats
that were killed after exposure (Schaeffer et al., 1984).
8.5.2 Tumour promotion/anticarcinogenic effects
(a) Mice
Tatematsu et al. (1979) studied the effects of inducers of liver
microsomal enzymes on the induction of hyperplastic liver nodules by
N-2-fluorenylacetamide (2-FAA) in male F344 rats. The rats were fed
a diet containing 200 mg 2-FAA/kg diet for 2 weeks and then given 500
or 1000 mg Kanechlor 500/kg diet for the following 8 weeks. Partial
hepatectomies were performed at the end of the third week of the
study. Kanechlor 500 in the dose levels applied showed a promoting
effect.
Ito et al. (1973) and Nagasaki et al. (1974, 1975) exposed groups of
20-38 male dd mice to diets containing Kanechlor 400 or 500 at 100 or
250 mg/kg for 24 weeks. Control groups consisted of 20 mice. Combined
exposure to Kanechlor 500 at 100 and 250 mg and either 50, 100, or
250 mg alpha- or ß-BHC (hexachlorocyclohexane)/kg diet enhanced the
development of nodular hyperplasia and hepatocellular carcinomas. A
combination of Kanechlor 500 and gamma-hexachlorocyclohexane did not
produce tumours. Dosing with Kanechlor 500 alone at a dietary level of
100 and 250 mg/kg, and ß- or gamma-hexachlorocyclohexane at dietary
levels of up to 500 mg/kg did not produce tumours. However,
alpha-hexachlorocyclohexane (250 mg/kg diet) produced 10/38
hepatocellular carcinomas and 30/38 hyperplastic nodules.
In another study, both inhibition and promotion were observed
following transplacental and transmammary exposure of mice to PCBs
prior to, or simultaneously with, exposure to dimethylnitrosamine
(DMNA). In this study, Aroclor 1254 was administered intraperitoneally
to female Swiss CD-1 mice on the 19th day of gestation, at a dose of
500 mg/kg body weight. Groups of 17-31 sucklings of these mice and of
controls were then treated intraperitoneally with DMNA on postnatal
day 4 or 14, or remained untreated. The progeny were killed at 28
weeks or at 18 months of age. Aroclor 1254 exposure decreased the
incidence of tumours in the liver and lung, induced by DMNA
administered at postnatal day 14. The average numbers of lung tumours
per mouse were also decreased. However, Aroclor 1254 increased the
liver tumour-bearing mice with extensive DMNA-initiated liver tumours
at 18 months of age, especially when DMNA was administered on
postnatal day 4. Mice exposed only to Aroclor 1254 did not show tumour
incidences higher than those of controls (Anderson et al., 1983). The
authors also reported that 2 higher chlorinated biphenyls,
2,4,5,2',4',5'-hexachlorobiphenyl and 2,3,5,2',3',5'-hexa-
chlorobiphenyl, were the dominant congeners persisting in the tissues
of the PCB-treated mice. It should be noted that only the more highly
chlorinated PCBs (>50% by weight) were reported as promoters of
hepatocarcinogenesis in rodents. The promoting activities of the lower
chlorinated PCBs have not been determined so far.
(b) Rat
As shown in Table 49, administration of PCBs to rats after exposure to
several initiating agents promoted the development of neoplastic
lesions of the liver. In these studies, treatment of the rats with a
control diet of PCBs alone did not usually lead to neoplastic changes
in the liver. Preston et al. (1981) compared the promoting effect of
exposure of rats to Aroclor 1254 with that of exposure to Aroclor 1254
from which the polychlorinated dibenzofuran moieties had been removed,
and did not find any significant differences. The results of this
study suggested that the promoting effect of commercial PCBs cannot be
ascribed entirely to the presence of chlorinated dibenzofurans.
Tatematsu et al. (1979) showed a dose-effect relationship in the
observed promoting effect of Kanechlor 500.
Inhibition, rather than promotion, of liver neoplasms was observed
when female Donryu rats were exposed to Kanechlor 400 preceding, or
simultaneously with, exposure to 3'-methyl-4-dimethylaminoazobenzene
(3'-Me-DAB) (Kimura et al., 1976). Simultaneous dietary exposure of
groups of 16-24 male Sprague-Dawley rats to Kanechlor 500, at a
dose-level of 500 mg/kg (25 mg/kg body weight per day), and to
3'-Me-DAB, or N-2-fluorenylacetamide (2-FAA), or diethylnitrosamine
(DENA), or combinations of these carcinogens, for 20 weeks, showed
almost complete inhibition of neoplastic nodules and hepatocarcinomas
(Makiura et al., 1974). It should be noted that the results of this
study may be influenced by the extreme toxicity, as shown by the
weight records, especially when the substances were combined.
The antitumour activity of Aroclor 1254 was demonstrated in male
Sprague-Dawley rats, inoculated with Walker 256 tumour cells. Groups
of 16 rats received PCB doses of 50, 100, or 200 mg/kg body weight for
2 weeks, once in 2 days, starting on the day of tumour cell injection.
A group of 16 rats did not receive PCBs. The inhibition of tumour
growth and transplantability were dose-related (Kerkvliet & Kimeldorf,
1977). The antitumour activity of Phenoclor DP5 was observed in groups
of 20 female Swiss mice inoculated with Ehrlich's tumoral ascites
liquid, after receiving PCBs in the diet at levels of 0, 10, 50, or
250 mg/kg, for 120 days (Keck, 1982).
Nishizumi (1980) showed that placentally transferred PCBs inhibited
diethylnitrosamine (DENA)-induced liver tumours. Groups of ten,
10-week-old female Wistar rats were treated with 40 or 200 mg
Kanechlor 500/kg body weight, by gavage, on days 5, 10, and 15 of
gestation. One F1 offspring from each litter was killed for
quantification of liver PCBs. The remaining F1 offspring were exposed
to 50 mg DENA/litre drinking-water, continuously for 5 weeks. At 16,
20, and 24 weeks after the beginning of the DENA exposure, 6-8 rats of
Table 49. Promotion of liver neoplasms in rats exposed to PCBs after treatment with carcinogenic substances
Strain Sex Group size Carcinogenic PCBs Doseb Exposure Neoplasms Reference
substancesa (mg/kg diet) period promoted by
(weeks) PCBs
Donryu female 25 3'-Me-DAB Kanechlor 400 400 26 carcinoma Kimura et al.
(1976)
Wistar male 20-24 DENA Kanechlor 500 2 × 15 4 nodules Nishizumi (1976)
mg/week carcinoma
F 344 male 15-16 2-FAA Kanechlor 500 500 and 8 hyperplasia Tatematsu et al.
1000 nodules (1979)
F 344 male 20 EHEN Kanechlor 500 32 carcinoma Hirose et al.
(1981)
Sprague- male 40 DENA Aroclor 1254c 100 18 carcinoma Preston et al.
Dawley (1981)
a 3'-Me-DAB = 3'-methyl-4-dimethylaminoazobenzene; DENA = diethylnitrosamine; 2-FAA = N-2-fluorenylacetamide;
EHEN = N-ethyl-N-hydroxyethylnitrosamine.
b Unless otherwise specified.
c With and without PCDFs.
each sex from each treatment group were killed and examined
histologically. A significant reduction in tumour incidence occurred
only in male offspring in the 200-mg group. The liver-PCB values in
28-day-old F1 mice were < 1, 18 ± 7, and 360 ± 30 mg/kg tissue for
the controls, 40, and 200 mg/kg groups, respectively. It was suggested
that placental transfer of PCBs protected the treated rats from
DENA-induced liver tumours.
The inhibitory effect of PCBs on tumour initiators following
simultaneous exposure has been explained by an enhanced metabolism of
the initiator by PCB-induced mixed function oxygenase.
Tests for putatively preneoplastic enzyme-altered foci in the liver of
rats have shown a dose-related increase in the promotion of such foci
by intraperitoneal exposure to Aroclor 1254 in tricaprylin or by oral
exposure to Clophen A50 in olive oil, following oral administration of
diethylnitrosamine (Deml & Oesterle, 1982; Pereira et al., 1982;
Oesterle & Deml, 1983).
The highest oral dose of Clophen A50 not enhancing the number and area
of enzyme-altered loci in female Sprague-Dawley rats, treated for 11
weeks with doses of 0, 0.1, 0.5, 1, 5, or 10 mg/kg body weight after
initiation by a single dose of 8 mg/kg body weight of
diethylnitrosamine, was 0.5 mg/kg body weight (Deml & Oesterle, 1987).
8.5.3 Initiation, promotion, and other special studies on individual
congeners
2,5,2',5'-Tetrachlorobiphenyl and its metabolite 2,5,2',5'-
tetrachlorobiphenyl-3,4-oxide were tested in a pulmonary tumour
induction assay with intraperitoneally injected A/T mice of both
sexes, and in a two-stage skin carcinogenicity assay with
dermally-exposed female SENCAR mice. Pulmonary adenomas and skin
papillomas were not induced (Preston et al., 1985).
Female Harlan-Sprague-Dawley rats received 2,4,2',4'-
tetrachlorobiphenyl or 2,5,2',5'-tetrachlorobiphenyl at 100 mg/kg
diet for 28 weeks, 1 week after oral exposure to diethylnitrosamine
(DENA). Both congeners showed a promoting effect on the development of
foci of hepatocellular alteration. The effect was approximately
10-fold greater in rats receiving 2,4,2',4'-tetrachlorobiphenyl
(Preston et al., 1985).
The effects of PCB mixtures and selected congeners have also been
investigated by Hayes et al. (1985, 1986) using the resistant
hepatocyte model developed by Farber and coworkers (Solt & Farber,
1976; Tsuda et al., 1980; Farber, 1984a,b, 1986). The ability of
2,4,2',4' and 2,5,2',5'-tetrachlorobiphenyl, 2,4,5,2',4',5'-
hexachlorobiphenyl, and a mixture of PCB-congeners (the composition of
which resembled that ascertained in human breast milk) to initiate
enzyme-altered hepatocellular nodules was investigated in
proliferating hepatocytes of neonatal or partially hepatectomized
adult rats (the PCB-congeners did not contain detectable levels of
dibenzofurans or dioxins). Neonatal rats were exposed 3 times in 3
weeks and adult rats once. After several weeks, the rats received a
selection regimen of 2-acetylaminofluorene followed by partial
hepatectomy (neonates) or necrotizing carbon tetrachloride (adults).
None of the PCB exposures generated nodules in contrast to known
initiators (Hayes et al., 1985).
Subsequent studies by Hayes et al. (1986) using the afore-mentioned
compounds and 3,4,3',4'-tetrachlorobiphenyl (a typical MC-type inducer
of cytochrome P-450-dependent monooxygenases) showed that these PCBs
(50 µmol/kg body weight), given 10 days after a dose of the initiator,
DENA, and 7 days before 2-AAF, all reduced the size of the
2-AAF-selected gamma-glutamyltranspeptidase-positive nodules. These
results show that, in contrast to the previous studies, PCBs also
exhibit "anti-promoting" activities in this Farber-model, which
utilizes 2-AAF as a mito-inhibitory toxin.
8.5.4 Skin carcinogenicity
Aroclor 1254 (100 µg/mouse) administered 18 h prior to the initiator,
7,12-dimethylbenz (a)anthracene (DMBA), significantly decreased the
incidence of papilloma formation in female Charles-River CD-1 mice.
2,4,5,2',4',5'-Hexachlorobiphenyl (625 µg/mouse), a PCB congener that
resembles phenobarbital in its mode of induction of drug-metabolizing
enzymes, did not act as an anticarcinogen, whereas 3,4,3',4'-
tetrachlorobiphenyl was more active than Aroclor 1254 as an
inhibitor. 3,4,3',4'-Tetrachlorobiphenyl resembled methylcholanthrene
in their mode of cytochrome P-450 induction (Parkinson et al., 1983)
and decreased the number of DMBA-initiated papillomas/mouse. Although
the PCB treatment did not modulate the incidence of papillomas caused
by benzo (a)pyrene, it was suggested that the anticarcinogenic
effects of PCBs on mouse skin tumours initiated by DMBA were due to
altered metabolism and DNA binding of the carcinogen by the
PCB-induced skin monooxygenases (DiGiovanni et al., 1979).
Berry et al. (1978, 1979) studied the tumour-promoting activity of
Aroclor 1254 in groups of 30 female CD-1 mice initiated with
0.2 mmol/litre (equivalent to 51 µg) DMBA. One week later, a positive
control group received 2 µg tetradecanoylphorbolacetate (TPA) and an
experimental group received 100 µg Aroclor 1254 in acetone. The TPA
and Aroclor applications were made twice weekly for 30 weeks. The TPA
promotion resulted in 92% of the animals developing papillomas, while
none developed in the Aroclor-treated animals. It was concluded that
Aroclor 1254 was not a skin tumour promoter at the dose used in this
study. Inhibition of skin papilloma was observed when Aroclor 1254 was
administered 18 to 72 h prior to DMBA treatment and promotion by TPA.
Poland et al. (1982) used HRS/J hairless mice to study the tumour
promotion activity of Aroclor 1254 in combination with N-methyl-
N'-nitro- N-nitrosoguanidine (MNNG). Twenty female mice per group
received a single administration of MNNG (5 micromol in acetone) or
acetone alone applied on the skin, and were then treated topically,
twice weekly, with 1 mg Aroclor/mouse, dissolved in acetone, for 20
weeks. No tumours were induced in the control group; in the
MNNG-treated mice, 4/19 had papillomas. It was concluded that Aroclor
had a weak promoting effect.
8.5.5 Appraisal
In summarizing the potential carcinogenic activity of PCBs, it is
perhaps more informative to express it in terms of what is known about
the mechanisms of chemical carcinogenicity (Hayes, 1987). In other
words, what the evidence is to support the carcinogenicity of PCBs
through genotoxic/initiating, cocarcinogenic, promotional/
antipromotional, and progressional activities. There is no evidence to
support genotoxic activity for PCBs and, in in vitro studies,
evidence for initiating activity through direct interactions with DNA
is weak. Poor initiating activity is consistent with the demonstrated
lack of mutagenic activity in various short-term tests. There is
evidence to suggest that PCBs can potentiate the activity of known
carcinogens (or act as cocarcinogens) in in vitro systems, but the
opposite result is often seen in in vivo studies, suggesting that
certain protecting enzyme systems present in the intact systems are
absent in the in vitro systems. There is a substantial body of
evidence to support the promotional activity of PCBs, particularly the
more highly chlorinated ones, in rodent liver, and this activity may
depend on the sequence in which the chemicals are administered in
experimental animal studies. In addition, promotional activity is
correlated closely, but not consistently, with the induction of MFO
activity. The hyperplastic effect (stimulation of cell proliferation)
of PCB inducers could promote preneoplastic growth. This type of
activity may possibly involve a threshold, suggesting that it may not
be a factor in low-level exposures to PCBs. The anticarcinogenic
activity of PCBs may also depend on the sequence of events in
experimental animal studies, and it may be related more to the
antipromotional properties of PCBs, possibly functioning as
protectants of mito-inhibitory toxicity. The interpretation of the
available animal data involving the commercial PCB mixtures is often
complicated by lack of information concerning the presence and
contribution of chlorinated dibenzofuran impurities, as well as
variations in congener composition to toxicity. In structure-activity
terms, a key factor in determining promotional activity appears to be
the degree of chlorination, which may reflect increased resistance to
metabolism and elimination and possibly also higher degrees of
ortho-substitution among the congeners present. Ortho-substituted
PCBs (possibly acting as persistent PB type inducers) have been shown
to be effective tumour promoters, and, at least in the case of the
closely related PBBs, it has been shown that a non-toxic and
non-promoting dose of a non- ortho-substituted congener in
combination with a promoting dose of a highly ortho-substituted
congener has a synergistic effect. This result may explain why the
mixtures can have greater promoting ability than the individual
congeners involved. This result might also suggest multiple pathways
for promoting activity, possibly involving the Ah receptor as well as
the putative receptor for phenobarbital. The possibility that PCBs
might promote carcinogenesis in tissues, other than liver, in animals
exposed to various tissue-specific, initiating agents, needs to be
addressed. Nevertheless, the potential for human liver cancer from
exposure to PCBs cannot be reliably predicted from animal studies.
Overall, there is reason to exercise caution in extrapolating the
available animal data on the carcinogenic potential of PCBs for
humans.
8.6 Special studies: target-organ effects
The lesions commonly introduced in animals after acute, short-term, or
long-term administration/application of PCB mixtures and/or individual
congeners concern the liver, skin, immune system, reproductive system,
oedema at various sites, as well as disturbances of the
gastrointestinal tract and the thyroid gland.
8.6.1 Liver
8.6.1.1 PCB mixtures
The toxic effects of PCBs result both directly and indirectly from
their presence in certain organs, such as the liver, where they
induce, in various degrees, a variety of liver enzymes. Some of these
enzymes are active in the metabolism of the PCBs themselves, while
others involve activation, deactivation, detoxication, etc., of other
compounds.
In itself, induction of enzymes by xenobiotics does not represent a
toxic manifestation, rather it is the ordinary response to such
foreign chemicals, which results generally in their detoxication and
ultimate modification, enabling them to be excreted from the organism.
In this sense, the response of the liver to such compounds is a
biological protective mechanism. Since some compounds, including PCBs,
are capable of inducing not only enzymes that result in their own
detoxication, but others as well, and the level of enzyme induction
may be high enough to cause liver pathology, a line cannot be drawn
that clearly separates a normal biological function from a toxic
manifestation. Superimposed on this situation is the direct toxic
action of the compounds on liver tissue, because of the properties of
the parent compound or its metabolites.
An enlarged liver and increased absolute and relative liver weights
are commonly reported as gross effects of PCB administration. The
lowest-observed-effect levels, in studies on different rat strains,
exposed to a diet containing Aroclor 1254, for a (dose-related)
increase in relative liver weights, vary between 20 and 100 mg/kg diet
(equivalent to 1 and 5 mg/kg body weight, respectively) (Kimbrough et
al., 1972; Bruckner et al., 1974; Burse et al., 1974; Grant et al.,
1974; Allen et al., 1976; Zinkl, 1977; Hinton et al., 1978; Kasza et
al., 1978b; Jonsson et al., 1981; Baumann et al., 1983).
The liver hypertrophy is microscopically visible as enlarged,
pleiomorphic hepatocytes, sometimes multinucleated or with enlarged
nuclei. A liver alteration observed by many investigators after
exposure of rats to various PCB-mixtures is fatty degeneration,
characterized by fat vacuolation and/or a foamy appearance of the
cytoplasm. Ultrastructurally, an increase in the number and size of
cytoplasmic lipid droplets and liposomes (membrane-associated lipid
droplets) can be observed. Fatty degeneration of the liver was already
observed after exposure of male Sprague-Dawley rats to a diet
containing 5 mg Aroclor 1242/kg diet (equivalent to 0.25 mg/kg body
weight) for 2-6 months (Bruckner et al., 1974) and after exposure of
male Holtzman rats to a diet containing 5 mg Aroclor 1254/kg for 5
weeks (Kasza et al., 1978b). Chu et al. (1977) found a comparable
effect on the liver with >20 mg Aroclor 1254 or 1260/kg diet for 28
days. Another characteristic change is the appearance of eosinophilic,
lamellar, cytoplasmic inclusions (Kimbrough et al., 1972; Kasza et
al., 1978b) or "hyaline-like material" (Grant et al., 1974). Electron
microscopy revealed that these changes corresponded to concentric
laminated membranes of smooth endoplasmic reticulum ("whorls"; "myelin
figures") (Vos & Beems, 1971; Kimbrough et al., 1972; Allen et al.,
1976; Kasza et al., 1978b; Jonsson et al., 1981). Proliferation of the
smooth endoplasmic reticulum and a decrease in rough endoplasmic
reticulum are commonly observed in rats exposed to dietary levels of
PCB mixtures that also induce fatty degeneration. Kasza et al. (1978b)
further observed a marked proliferation of Golgi condensing vesicles
containing lipoprotein in the livers of male Holtzman rats fed Aroclor
1254, for 5 weeks, at 5 mg/kg diet. A decreased number of these
vesicles was seen at 50 and 500 mg/kg diet (equivalent to 2.5 and
25 mg/kg body weight, respectively), together with a marked increase
in the smooth endoplasmic reticulum and lysosomes. The above decrease
in Golgi vesicles was also observed in Sprague-Dawley rats by Hinton
et al. (1978). The proliferative changes of the endoplasmic reticulum
are closely related to the observed induction of microsomal enzymes,
as discussed in section 8.6.1.2. Atypical mitochondria (Burse et al.,
1974; Kasza et al., 1978a,b), and single cell and focal necrosis
(Grant et al., 1974; Allen et al., 1976; Jonsson et al., 1981; Baumann
et al., 1983) have also been described.
Hypobilirubinaemia was produced in rats by Bastomsky et al. (1975),
who investigated the mechanism by administering daily intraperitoneal
injections of Aroclor 1254 (25 mg/kg body weight, in corn oil) to
female rats for 4 days, before measuring bilirubin glucuronide
formation by hepatic microsomes in vitro. PCB treatment was not
effective in increasing UDP-glucuronosyltransferase (EC 2.4.1.7)
activity. Serum bilirubin levels in Gunn rats were also significantly
decreased by PCB treatment; the rats are genetically deficient in
UDP-glucuronosyltransferase (2.4.1.7) activity.
The fluorescing of livers on exposure to UV radiation, consistent with
the presence of porphyrin, and accumulation of brown pigment, positive
for iron, especially in Kupffer cells and perivascular macrophages
have also been reported (Kimbrough et al., 1972; Burse et al., 1974;
Zinkl, 1977; Jonsson et al., 1981). The changes described in the
livers of mice (Nishizumi, 1970) and rabbits (Koller & Zinkl, 1973),
following exposure to PCB mixtures, are comparable with those in rats.
8.6.1.2 Individual congeners
The effects of chlorination and the chemical composition of PCBs, with
regard to the dose-effects relations in liver toxicity after
short-term exposure, are indicated by the data of Biocca et al.
(1981). In this study, hepatotoxic effects were observed in mice after
5 weeks of maintenance on diets containing 0.3 mg of 3,4,5,3',4',5'-
hexachlorobiphenyl, while similar effects were observed only after
30 mg of 2,4,5,2',4',5'-hexachlorobiphenyl and 100 mg of 2,4,6,2',4',6'-
hexachlorobiphenyl/kg diet. No effects were found with 300 mg of
2,3,6,2',3',6'-hexachlorobiphenyl/kg diet. Similar dependence of liver
toxicity on the chemical composition of the PCB mixture would be
anticipated following long-term exposure in mice and other species.
8.6.2 Enzyme induction
8.6.2.1 Effects on liver enzymes of PCBs
Proliferation of the smooth endoplasmic reticulum is a common
observation in the liver cells of experimental animals following
exposure to PCB mixtures. This effect is accompanied by an increase in
microsomal protein and the induction of cytochrome P-450, cytochrome
P-448, and drug-metabolizing enzymes, including the microsomal
monooxygenases (EC 1.14.14.1), epoxide hydrolases (EC 3.3.2.3),
UDP-glucuronosyltransferases (EC 2.4.1.17), NADPH-cytochrome c
reductase (EC 1.6.2.4) and esterases (EC 3.1.1.1), and the cytosolic
glutathione S-transferase (EC 2.5.1.18). The subject has been
reviewed by Safe (1984).
The spectral, enzymatic, and electrophoretic properties of the
microsomal enzymes, induced by Aroclor 1248, 1254, 1260, and Kanechlor
400, are consistent with the inducing properties of both the
phenobarbital (PB) and 3-methylcholanthrene (MC) classes of inducers.
They induce both cytochromes P-450 and P-448 and associated enzymes
(Alvares & Kappas, 1977; Goldstein et al., 1977; Yoshimura et al.,
1978; Iverson et al., 1982; Lashneva & Tutelyan, 1984; Khan et al.,
1985; Tutelyan et al., 1986). Aroclor 1016, administered to
Sprague-Dawley rats at 50 mg/kg per day for 4 days, intraperitoneally,
elicited a barbiturate type of inducing effect on the hepatic
microsomal oxidative enzyme system. Aroclor 1016 caused increases in
liver cytochrome P-450 content, microsomal protein, and its
ethylmorphine N-demethylase activity. It did not induce cytochrome
P-448 in liver microsomes.
A dose-related induction of hepatic and, in some cases, extrahepatic
microsomal enzymes was observed in several animal species including
the rat, rabbit, mouse, ferret, guinea-pig, hamster (Safe, 1984), mink
(Shull et al., 1982; Aulerich et al., 1985) and monkey (Iverson et
al., 1982). Distinct interspecies variations have been demonstrated.
For example, while 6 daily intraperitoneal doses of 25 mg of Aroclor
1254/kg body weight caused a potent induction of benzo (a)pyrene
hydroxylase in adult, male Sprague-Dawley rats, no, or a minimal,
induction of this monooxygenase was observed in adult, male Swiss mice
after 4 daily intraperitoneal doses of 50 mg/kg body weight and in
male New Zealand rabbits after 2 intraperitoneal doses of 100 mg/kg
body weight on days 1 and 4 (Alvares et al., 1982). Furthermore, when
comparing the inducing potency of Aroclor 1242 in the mink and the
genetically related ferret, Shull et al. (1982) measured a greater
induction of cytochrome P-448 and MC-type monooxygenases and no toxic
effects in the ferret at a dosing regime that resulted in toxic
effects in the mink (100 mg/kg body weight on day 1,200 mg/kg body
weight on day 5, sacrifice on day 10). The authors considered the
observed induction moderate in both species compared with that
observed in the rat. Earlier, it had been found that male
Sprague-Dawley rats were indeed more sensitive than ferrets with
respect to the inducing effect of Aroclor 1254 following a single
intraperitoneal dose of 500 mg/kg body weight, though the responses of
both species were comparable qualitatively (Lake et al., 1979).
Moreover, pretreatment of rats with Aroclor 1254 resulted in the
induction of microsomal cytochromes P-450 c, P-450 d (MC-inducible),
P-450 b and P-450 e (PB-inducible) (Ryan et al., 1979a,b, 1982). In
general, the extent of induction of microsomal enzymes by PCB-mixtures
increased with increasing chlorine content up to 54%. The effect has
also been demonstrated with single pure PCBs administered orally
(Ecobichon & Comeau, 1975). The results are summarized in Table 50 and
show a greater degree of enzyme induction with the higher chlorinated
compounds (see section 8.6.1.2).
Table 50. Stimulation of microsomal enzyme activity by single chlorinated biphenylsa
Hepatic microsomal enzyme activity
Chlorine O-Demethylation N-Demethylation Aniline Nitro-
substituents hydroxylation reduction
4 0 0 0 0
2,2' 0 + + 0
2,4' 0 0 + 0
4,4' + + + + + + +
2,5,2',5' 0 + + + +
2,4,2',4' + + + + + + 0
2,4,5,2',4',5' + + + + + + + +
2,3,5,2',3',5' + + + + + + + +
2,4,6,2',4',6' + + + + + + + +
2,3,4,5,2',3',4',5' + + + + + + + +
a From: Johnstone et al. (1974).
0 = No activity.
+ = Slight activity.
+ + = Marked activity.
Litterst et al. (1972) exposed groups of 6 male Osborn-Mendel rats to
Aroclors 1242, 1248, 1254, or 1260 in the diet, at concentrations of
0, 0.5, 5.0, 50, or 500 mg/kg diet, for 4 weeks. Increased microsomal
nitroreductase and demethylase activities occurred at 0.5 mg/kg or
more, increased pentobarbital hydroxylation and increased relative
liver weight occurred at 5.0 mg/kg or more, and increased liver
triglycerides occurred at 50 mg/kg diet. An inducing activity similar
to, or lower than, that of Aroclor 1254 has often been found for more
chlorinated mixtures (Villeneuve et al., 1971a, 1972; Bickers et al.,
1972; Chen & Dubois, 1973; Ecobichon & Comeau, 1974; Schmoldt et al.,
1974; Sawyer et al., 1984). Aroclor 1016 and 1242, both containing 42%
chlorine but differing in congener composition, showed qualitative and
quantitative differences in inducing effects. For example, Aroclor
1254 enhanced ethylmorphine N-demethylase activity 3-fold, while the
maximum increase produced by Aroclor 1016 was only 40%. The 2 Aroclors
also differed in their induction of the various forms of cytochrome
P-450 (Alvares et al., 1982). Adult, male Sprague-Dawley rats were
administered, intraperitoneally, a dosage of 0 or 50 mg Aroclor 1016
in corn oil/kg body weight, for 4 days. Aroclor 1016 was a potent
inducer of N-methylase but a poor inducer of benzo (a)pyrene
hydroxylase. Administration of 100 mg Aroclor 1016 in corn oil/kg body
weight per day to adult male New Zealand White rabbits, for 4 days,
resulted in an increase in liver cytochrome P-450 activity and
decreases in benzphetamine- N-demethylase and benzo (a)pyrene
hydroxylase activities compared with the controls. 7-Ethoxycoumarine-
O-deethylase and 7-ethoxyresorufin- O-deethylase activities were
comparable with those in the controls (Ueng & Alvares, 1985).
Therefore, it can be concluded that the degree and type of induction
not only depends on the chlorine content of the mixture, but is also a
function of the congener composition, as will be discussed further in
the next section.
Not only species specificity, but also marked tissue specificity has
been observed. While Aroclor 1254 was found to be a potent inducer of
cytochrome P-450 content and benzo (a)pyrene hydroxylase activity in
the rat lung and liver (Alvares & Kappas, 1977), it caused a 46%
decrease in cytochrome P-450 content, a 31% decrease in
benzo (a)pyrene hydroxylase activity, and a 61% decrease in
ethylmorphine N-demethylase activity in the rabbit lung. Aroclor
1254 caused induction of cytochrome P-450 and both enzymes in the
kidneys of these rabbits, but benzo (a)pyrene hydroxylase was not
induced in the liver (Alvares et al., 1982).
The inducing effect of PCB mixtures on the monooxygenase system has
been observed in the livers of both male and female rats (Chen &
Dubois, 1973; Grant & Phillips, 1974), minks and ferrets (Lake et al.,
1979; Shull et al., 1982), in the livers of pregnant rats (Alvares,
1977) and rabbits (Villeneuve et al., 1971a), in the placenta of rats
(Alvares & Kappas, 1975), in fetal and neonatal rat livers (Alvares &
Kappas, 1975; Baker et al., 1977; Inoue et al., 1981; Jannetti &
Anderson, 1981; Lashneva et al., 1987), in immature rat livers (Chen &
Du Bois, 1973; Narbonne, 1980), and in mature and senescent rat livers
(Birnbaum & Baird, 1978).
The lowest-observed-adverse-effect levels and the no-observed-effect
levels for enzyme induction, found in short-term diet studies on rats,
are presented in Table 51.
Bruckner et al. (1977) showed that when Aroclor 1254 was administered
in the diet, at a level equivalent to 0.25 mg/kg body weight,
microsomal enzyme activity was induced after 1 day of exposure.
Narbonne (1980) found a significant induction with 0.1 mg Phenoclor
DP6/kg body weight, given in the diet, after 3-5 days.
After a few weeks of exposure to Aroclor 1254 or 1260, at low dietary
levels of between 0.25 and 1.25 mg/kg body weight, a plateau in
microsomal enzyme activity was reached that was maintained over
several months of exposure (Chen & Du Bois, 1973; Grant et al., 1974;
Bruckner et al., 1977). Following short-term exposure, dietary levels
of between 5 and 25 mg/kg may stimulate microsomal enzymes for up to 4
months (Grant et al., 1974; Bruckner et al., 1977). Levels of 0.05 or
0.1 mg/kg body weight failed to produce any effects or the periods of
induction at these levels were long (Grant et al., 1974; Chen & Du
Bois, 1973).
A marked induction of the liver monooxygenase system was observed in
the offspring of female rats given a single oral dose of a mixture of
PCBs (Sovol) (500 mg/kg body weight) on the 14th day of pregnancy. In
one-day-old rats, an increase in cytochrome P-450 content associated
with an increase in the cytochrome b 5 level, an increase in NADPH-
cytochrome c reductase activity, an increased rate of aminopyrine-
N-demethylation activity in microsomes and also increased
3,4-benzo (a)pyrene hydroxylation, 7-ethoxycoumarin O-deethylation,
and NADPH-dependent lipid peroxidation were found. The activity of the
cytochrome P-450 system in the young rats remained elevated during the
early postnatal period (Lashneva et al., 1987).
8.6.2.2 Effects on liver enzymes of "biologically filtered" PCB
mixtures
Young Sprague-Dawley rats were administered a total of 38 oral doses
of a PCB mixture in olive oil at 0, 0.25, 1.0, 4.0, 16.0, 64.0, 256,
or 1025 µg/kg body weight, twice a day, over 1 month; the mixture
contained 55% chlorine and had a gas-chromatographic profile very
similar to that of the congeners found in the breast milk of Japanese
women. A dose-related induction of liver aminopyrine demethylase and
benzo (a)pyrene hydroxylase (EC 1.14.14.1) was found with doses of
1.0 µg/kg body weight or more. PCB-binding to liver microsomes was
increased at doses of 4.0 µg/kg body weight or more (Shimada & Ugawa,
1978). In another study, 1-month-old, male Wistar rats received
(intraperitoneally) doses of 0, 1, 10, 25, 50, or 100 mg/kg body
weight of a reconstituted PCB-mixture in corn oil, containing average
levels of 13 of the major congeners found in the breast milk of
Japanese women (purity > 98.5%). Each dose was administered in 2
portions on days 1 and 3. The same dose regimen of Kanechlor 500 was
also tested. The rats were killed on day 6. The reconstituted PCB
mixture and Kanechlor 500 caused dose-related increases in liver
Table 51. Microsomal enzyme induction by PCB-mixtures in rats
PCB-mixture Rat strain Sex Exposure No-effect-level Lowest-observed- Reference
male/ period mg/kg body adverse effect level,
female weight mg/kg body weight
Aroclor 1016 Sprague-Dawley male 3 weeksa 0.1 1 Iverson et al. (1975)
Aroclor 1242 Sprague-Dawley male 3 weeksa 0.1 1 Iverson et al. (1975)
Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Sprague-Dawley male 2-6 months < 0.25 0.25 Bruckner et al.
(1974, 1977)
Aroclor 1248 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Aroclor 1254 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Wistar male 2-8 months < 0.1 0.1c Grant et al. (1974)
Wistar male 2 weeks 0.25 0.5 Den Tonkelaar &
Wistar male 12 weeks 0.05 0.5 van Esch (1974)
Sprague-Dawley male 0-20 weeks 0.05 0.25 Turner & Green (1974)
Holtzman male 3 weeks < 0.25 0.25 Bruckner et al. (1977)
Garthoff et al. (1977)
Table 51. (cont'd).
PCB-mixture Rat strain Sex Exposure No-effect-level Lowest-observed- Reference
male/ period mg/kg body adverse effect level,
female weight mg/kg body weight
Aroclor 1260 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Holtzman male 1-13 weeks < 0.05 0.05 Chen & DuBois (1973)
female 1-13 weeks 0.05 0.25
Phenoclor DP6 Sprague- male 3 days < 0.1 0.1 Narbonne (1979, 1980)
Dawleyb
a Daily dosing by gavage; the other studies are all diet studies.
b Immature rats (60-65 g).
c Significant effect after months 4 and 6, but not after months 2 and 8.
benzo (a)pyrene hydroxylase activity that were 3.5 and 2.2 times the
control value, respectively, at 1 mg/kg body weight. The ED50 of the
reconstituted breast milk PCB mixture for the induction of rat hepatic
microsomal aryl hydroxylase (AHH) was 7 times lower than that of
Kanechlor 500. The authors concluded that the increased potency of the
breast milk-PCB mixture reflected the preferential bioconcentration of
the relatively toxic congeners 2,4,5,3',4'-penta-, 2,3,4,3',4'-penta-,
and 2,3,4,5,3',4'-hexachlorobiphenyl (Parkinson, et al., 1980b). When
Gyorkos et al. (1985) repeated the studies, the reconstituted mixture
was inactive at the lowest dose level but exhibited mixed-type
microsomal enzyme induction characteristics at the higher dose levels.
Increases in the activities of several hepatic microsomal
monooxygenases, including dimethylaminoantipyrine N-demethylase,
aldrin epoxidase, benzo (a)pyrene hydroxylase and
ethoxyresorufin- O-deethylase were found.
8.6.2.3 Effects of individual congeners on liver enzymes
The enzyme-inducing potencies of individual PCB congeners have been
studied extensively and reviews have been published (Goldstein, 1980;
Safe, 1984; Safe et al., 1985b), in which the following
structure-activity relationships are proposed. The most active
congeners with respect to the induction of aryl hydrocarbon
hydroxylase (and toxicity), 3,4,5,4'-tetrachloro-, 3,4,3',4'-
tetrachloro-, 3,4,5,3',4'-pentachloro-, and 3,4,5,3',4',5'-
hexachlorobiphenyl, are substituted at both para positions, at 2 or
more meta positions, but not at ortho positions. These congeners
can assume coplanar conformations and are approximate stereoisomers of
2,3,7,8-tetrachlorodibenzo- para-dioxin. They resemble
3-methylcholanthrene and 2,3,7,8-tetrachlorodibenzo- para-dioxin in
their mode of hepatic enzyme induction, inducing hepatic microsomal
benzo (a)pyrene hydroxylase, ethoxyresorufin- O-deethylase, and the
cytochromes P-450 a, P-450 c, and P-450 d.
These congeners are only present as trace compounds in commercial PCB
mixtures, but appear in significant quantities in breast milk (Noren
et al., 1990).
The least active of these 4 coplanar congeners, 3,4,5,4'-
tetrachlorobiphenyl, also shows a phenobarbital type of hepatic
microsomal enzyme induction, inducing dimethylaminoantipyrine,
ethylmorphine and related N-dealkylases, biphenyl-4-hydroxylase,
aldrin epoxidase, several O-dealkylases, and the cytochromes P-450 a,
P-450 b, and P-450 e. This "mixed-type" induction pattern is also
shown by 3,4,4'-trichlorobiphenyl, and by all the mono- ortho, and at
least 7 di- ortho, substituted analogues of the coplanar PCB
congeners. Several of these congeners, e.g., 2,4,5,3',4'-penta,
2,3,3',4,4'-penta, 2,3,4,5,3',4'-hexa-, and 2,3,4,5,2',4'-
hexachlorobiphenyl are components of commercial PCB mixtures and have
been identified in breast milk.
Studies have revealed that 4,4'-dichlorobiphenyl, with no meta
substituents exhibits a PB-type induction pattern in rats. Adding
meta substituents as in 3,4,4'-tri-, and 3,4,5,4'-tetrachloro-
biphenyl will give a mixed PB- and 3-MC-type induction pattern. While
3,4,3',4'-tetrachlorobiphenyl is a potent inducer of microsomal
hepatic aryl hydrocarbon hydroxylase (AHH), it did not significantly
increase the activities of benzo (a)pyrene-hydroxylase, [3H]-4-
chlorobiphenyl-hydroxylase, and ethoxyresofurin- O-deethylase (EROD)
at a dose level of 10 µmol/kg (Andres et al., 1983).
Most other PCB congeners are phenobarbital-type inducers or are
inactive. In general, the more highly chlorinated of these congeners
are more active inducers than the lower chlorinated ones, probably
reflecting the relative half-lives of these compounds. The
non-availability of 2 adjacent unhalogenated carbon atoms and
para-substitution are 2 factors that decrease the degradability of
the congeners and increase their inducing activity.
Various congeners were tested for their inducing activity in
responsive C57BL/6J mice, i.e., mice containing the Ah receptor
protein, and in non-responsive DBA/2J mice, lacking this receptor,
after single intraperitoneal (Robertson et al., 1984; Silkworth et
al., 1984) or oral (Kohli et al., 1980) doses in corn oil and
cottonseed oil, respectively. The coplanar PCBs and their mono-ortho
substituted analogues all induced benzo (a)pyrene hydroxylase or
ethoxyresorufin deethylase in responsive mice, but not, or only to a
minor degree, in non-responsive mice. Most tested mono-ortho
substituted analogues of coplanar PCBs slightly induced aminopyrine
N-demethylase in both strains, while the coplanar congeners did not
induce this enzyme.
The above structure-activity relationships were confirmed in a few
limited studies on monkeys. Hepatic aryl hydrocarbon hydroxylase was
induced in 3 young, male Rhesus monkeys after a single oral dose of
1 mg of 3,4,3',4'-tetrachlorobiphenyl/kg body weight and in 3 young
male monkeys during continued feeding of a diet containing 0.5 mg of
3,4,5,3',4',5'-hexachlorobiphenyl/kg (McNulty, 1985). A single oral
dose of 18 mg of 2,5,2',5',-tetrachlorobiphenyl/kg body weight,
administered to male Rhesus monkeys, in corn oil, elevated hepatic
cytochrome P-450 levels, while no change was noted in the activities
of several microsomal enzymes (Allen et al., 1975b). Mono- ortho or
di- ortho substituted analogues of coplanar PCB congeners have not
been tested in monkeys.
Vodicnik et al. (1980) studied the effect of 2,4,5,2',4',5'-
hexa-chlorobiphenyl on hepatic microsomal monooxygenase activity in
virgin or pregnant and lactating Sprague-Dawley mice and their
offspring. A single intraperitoneal dose of 100 mg/kg body weight, was
administered 14 days before mating. Prior to, and during early
pregnancy, hepatic monooxygenase activity in pretreated mice was
greater than that in the controls. No differences were found between
pregnant and virgin mice. Mothers, pretreated with the
hexachlorobiphenyl and sacrificed on the day of birth, had lower
microsomal monooxygenase activity and cytochrome P-450 content than
PCB-pretreated virgins sacrificed concurrently. No differences were
noted between these groups of animals during lactation. Hepatic enzyme
activities and cytochrome P-450 content were not different between
newborn offspring of corn oil- and PCB-pretreated mothers. However,
these parameters were elevated in 5- to 20-day postpartum nursing
offspring from pretreated mothers, compared with those from corn
oil-pretreated mothers suggesting the transfer of hexachlorobiphenyl
through the breast milk in quantities sufficient to affect hepatic
microsomal monooxygenase activity.
In a study on ICR mice, Vodicnik (1986) administered 150 mg
14C-2,4,2',4'-tetrachlorobiphenyl intraperitoneally, and compared
hepatic microsomal ethoxycoumarin- O-deethylase activity and liver
concentrations of 14C-activity. It was shown that pregnant mice were
less responsive to the inducing effects of the tetrachlorobiphenyl
than virgin or postpartum mice. This diminution in response may be, in
part, responsible for the lack of elimination of the
tetrachlorobiphenyl equivalents from the late pregnant animal during
the 4-day experimental period (see section 6.4.3).
Hardwick et al. (1985) studied both the time course and dose-response
for the induction of the 2 isoenzymes and their respective mRNAs after
administration of 3,4,5,3',4',5'-hexachlorobiphenyl to rats. It was
concluded that the congener under study induced 2 major 3-MC-inducible
isoenzymes of cytochrome P-450 and their mRNAs in a coordinated
manner, probably via a common mechanism. The data are consistent with
the hypothesis that both genes are probably regulated by a single
receptor in the rat. The magnitude of the increase in the isoenzymes
was greater than the increase in the amount of translationally active
mRNA in polysomes, suggesting that other factors may also influence
the relative induction of these P-450 isoenzymes.
In this study, the BP-type inducers, 2,4,6,2',4',6'- and
2,4,5,2',4',5'-hexachlorobiphenyl, both of which have been reported to
increase the hepatic cytosolic receptor level and consequently enhance
enzyme induction in vivo, were not able to enhance EROD or AHH
induction by 3,4,5,3',4'-pentachlorobiphenyl in vitro.
The mixed-type inducer 2,3,4,2',4',5'-hexachlorobiphenyl inhibited
enzyme induction by 3,4,5,3',4'-pentachlorobiphenyl in vitro, when
used in concentrations of at least 400 times higher than that of the
3,4,5,3',4'-pentachlorobiphenyl. Enzyme induction by 3,4,3',4'-
tetrachlorobiphenyl was inhibited by 2,3,4,2',4',5'-hexachlorobiphenyl
at concentrations at least 40 times higher, and enzyme induction by
3,4,5,3',4',5'-hexachlorobiphenyl was inhibited by 2,3,4,2',4',5'-
hexachlorobiphenyl at concentrations at least 8 times higher. Since
the concentration of 2,3,4,2',4',5'-hexachlorobiphenyl found in human
adipose tissue is about 300 times higher than those of the 3 coplanar
PCBs, this inhibition of enzyme induction probably occurs after
natural exposure to PCB mixtures. If enzyme induction by
3,4,3',4'-tetrachlorobiphenyl and 3,4,5,3',4',5'-hexachlorobiphenyl is
also inhibited by various concentrations of 2,3,4,2',3',4'-
hexachlorobiphenyl, inhibition of enzyme induction after natural
exposure to a mixture of PCBs is to be expected.
It was concluded that the in vitro enzyme induction by mixtures of
PCBs cannot be determined by the simple addition of the induction by
the individual PCBs. Possibly, in vivo enzyme induction seems
additive, because some compounds increase the receptor level, while
other compounds inhibit enzyme induction (van Vliet, 1990).
8.6.2.4 Appraisal
The liver is the organ most often implicated in the toxicity of PCBs
in animals. Hepatotoxicity has been observed in numerous studies with
exposed mice, rats, guinea-pigs, rabbits, dogs, and monkeys. The
effects, which appear to be reversible at low doses, are similar among
the species and include enzyme induction, liver enlargement, fat
deposition, and necrosis. Enzyme induction is the most sensitive
indicator of hepatic effects, but few studies have been designed to
define the minimum effective doses of PCB mixtures. The liver
enlargement is associated with hepatocyte enlargement and an increase
in smooth endoplasmic reticulum and/or increased enzymatic activity.
Proliferative lesions in the liver have been attributed to Aroclor
treatment. The hepatic effects of Aroclors in animals appear to be
typical of chlorinated hydrocarbons. Histologically-documented liver
damage is a consistent finding among PCB-exposed animals.
8.6.3 Effects on vitamins and mineral metabolism
8.6.3.1 Effects of PCB mixtures
PCB mixtures have been found to decrease levels of retinol (Vitamin A)
in the liver of rats (Innami et al., 1976; Kato et al., 1978; Hudecova
et al., 1979), rabbits (Villeneuve et al., 1971a), and in the plasma
of pigs (Guoth et al., 1984). Levels of thiamine (Vitamin B1) were
decreased in the blood, liver, and sciatic nerve of rats (Yagi et al.,
1979) and levels of pyridoxal phosphate (Vitamin B6) were decreased in
several tissues of rats, while riboflavin (Vitamin B2) levels remained
unaffected (Fujiwara & Kuriyama, 1977). The changes in the levels of
retinol and thiamine were thought to be secondary to the induction of
metabolizing enzymes (Yagi et al., 1979; Saito et al., 1982). The
induction of these enzymes was also found to be responsible for the
increased de novo synthesis of L-ascorbic acid that was observed in
the plasma, tissues, and urine of PCB-treated rats (Fujiwara &
Kuriyama, 1977; Chakraborty et al., 1978; Chow et al., 1979; Saito et
al., 1983). Lipid peroxidation was increased in the liver of
PCB-treated rats and Saito et al. (1983) suggested that ascorbic acid
may have initiated the peroxidation.
It was shown that induction of NADP-cytochrome c reductase (EC
1.6.2.4) and insufficiency of lipid peroxide scavengers, such as
alpha-tocopherol (Vitamin E) and glutathione peroxidase (EC 1.11.1.9),
could also be involved in the enhancement of lipid peroxidation by
PCBs (Saito et al., 1982, 1983; Kamohara et al., 1984).
PCB mixtures decreased the activity of both sodium/potassium- and
magnesium-dependent adenosinetriphosphatase (EC 3.6.1.3) in the
tissues of rats (Narbonne et al., 1978; La Rocca & Carlson, 1979). The
results of in vitro studies on isolated rat mitochondria showed that
PCB mixtures may act as inhibitors of respiration and uncouplers of
oxidative phosphorylation (Sivalingan et al., 1973; Nishihara, 1983,
1985). However, contradictory results have been obtained in vivo
with respect to the NAD/NADH ratio, the ADP/O ratio, and state 3 and
state 4 respiration rates (Mehlman et al., 1974; Chesney & Allen,
1974; Garthoff et al., 1977).
Byrne & Sepkovic (1987) studied the in vitro incorporation of
monovalent cations into rat erythrocytes as a model for evaluating the
impairment of electrogenic transport by PCBs. Female, Sprague-Dawley
rats were fed 50 mg Aroclor 1242 or 1254/kg diet for 7 months. The
uptake of 86Rb by erythrocytes in the Aroclor 1254 group was
depressed compared with that in the control group in K+ - depleted
culture media. No changes were observed with Aroclor 1242. A reduction
in 86Rb incorporation was also seen in erythrocytes from the Aroclor
1254 group in a Na+ -depleted medium. Ouabain did not have any
effect in the Aroclor 1254 group, because Aroclor 1254 suppressed the
cationic transport maximally. This study provides evidence that PCBs
(Aroclor 1254) can damage the cell sufficiently to decrease the active
transport of monovalent cations.
Male Fischer 344 rats were dosed daily, intragastrically, for 5, 10,
or 15 weeks with 0, 0.1, 1, 10, or 25 mg Aroclor 1254/kg body weight
in corn oil, to investigate the effects on calcium metabolism, femur
morphometry, and nephrotoxicity. The relative liver weights were
increased significantly with doses of 1.0 mg/kg or more after 5 weeks
treatment. The relative kidney weights were increased after 15 weeks
treatment in the 10 and 25 mg/kg groups. Hypercalcaemia was present in
the 25 mg/kg group after 5 and 10 weeks treatment, but not after 15
weeks. Serum triglyceride levels were elevated after 5 weeks
treatment, but decreased after 10 and 15 weeks. Serum cholesterol
levels were increased at the 2 higher dose levels with all 3 lengths
of treatment. Urinary alkaline phosphatase and lactate dehydrogenase
activities were elevated at 5, 10, and 15 weeks of treatment. Femur
density was increased at the 10 mg/kg dose level after 5 weeks, and at
all dose levels after 10 and 15 weeks. Cross-sectional, medullary, and
cortical areas of the midpoint of the femur were significantly
decreased at the higher dose levels after 10 and 15 weeks of exposure.
The per cent medullary area was decreased after 10 and 15 weeks
treatment indicating a decrease in medullary size and also a decrease
relative to the cortical bone area. The result was weaker bones after
15 weeks at the highest dose level. Thus, PCB exposure affects calcium
metabolism and bone morphometry (Andrews, 1989).
8.6.3.2 Effects of individual congeners
3,4,3',4'-Tetrachlorobiphenyl induced a decrease in serum and liver
retinol and retinyl palmitate in C57BL/Rij mice. In "non-responsive"
DBA/2 mice, only serum retinol was decreased. The time and
dose-responses observed suggested that the difference in aryl
hydrocarbon hydroxylase responsiveness was not directly involved in
the effects on retinoid levels (Brouwer et al., 1985).
Powers et al. (1987) administered female, Sprague-Dawley rats single,
intraperitoneal injections of 1, 5, or 15 mg 3,4,3',4'-
tetrachlorobiphenyl/kg body weight and found a dose-related
depression of plasma retinol levels, 24 h after treatment. The loss of
plasma retinol appeared to be a function of depressed levels of the
retinol-binding protein (RBP)-transthyretin ternary complex. No free
retinol was observed in the plasma. Hepatic retinyl palmitate
hydrolase (RPH) activity was depressed and highly and positively
correlated with the plasma retinol levels. Doses of either
2,4,5,2',4',5'- and 3,4,5,3',4',5'-hexachlorobiphenyl, equimolar to
the 15 mg/kg tetrachlorobiphenyl dose, failed to cause a similar
depression in plasma retinol in treated female rats.
A study was carried out to investigate the effects of PCBs on retinoid
homeostasis in Sprague-Dawley rats. Female Sprague-Dawley/Rij rats
were fed a Vitamin A-deficient diet for 12-16 weeks. Serum retinol
concentrations at the end of this period were decreased to
approximately 10% of the normal retinol level. The rats were repleted
with radiolabelled [3H]retinol by feeding a diet containing 18.5 MBq
(8000 IU) of retinol/kg diet for 14 days. Saturation in the blood was
reached after 6 days [3H]retinol repletion. On day 7, the rats were
either treated with an intraperitoneal dose of 3,4,3',4'-
tetrachlorobiphenyl (15 mg/kg) in corn oil, or corn oil alone.
Exposure to tetrachlorobiphenyl resulted in significant reductions in
both retinol and retinyl ester concentrations in the liver and lung to
25% and 44% of the controls, respectively, and a reduction of retinol
in the heart of 35% of the controls. No changes in concentrations were
observed in the skin and kidneys (Brouwer et al., 1988).
Female WAG/Rij rats received a single ip injection of corn oil, or 15
or 200 mg 3,4,3',4'-tetrachlorobiphenyl/kg body weight and were killed
on days 1,3,7, or 14 to study the effects on serum and hepatic
retinoid contents and liver morphology. There was a significant
increase in liver weight at the highest dose level after 3, 7, and 14
days. There was a rapid increase in the 3H-tetrachlorobiphenyl levels
present after 7 days, after which a rapid decline occurred.
Tetrachlorobiphenyl induced a significant decrease in serum retinol
content in the 200 mg tetrachlorobiphenyl group on days 3 and 7. The
same was found for the retinol and retinyl palmitate contents of the
liver. Ultrastructural alterations in the hepatocytes, such as
proliferation and vesiculation of the endoplasmic reticulum and
mitochondrial enlargement with inclusions, were found (Durham &
Brouwer, 1989).
2,2',5,5'-Tetrachlorobiphenyl caused inhibition of Ca/Mg- and
Mg-dependent adenosinetriphosphatase in the liver of rats (Lin et al.,
1979). In vitro, several PCB congeners inhibited Na/K- and
Mg-dependent adenosinetriphosphatase. Although a general trend towards
increased inhibition, paralleling increased chlorination, was
observed, no correlation was evident between chlorine substitution
patterns and inhibitory activity (La Rocca & Carlson, 1979).
8.6.4 Effects on the gastrointestinal tract
Effects on the stomach have been studied or observed by Allen &
Norback (1973); Allen et al. (1974a); Allen (1975); Becker et al.
(1979) and Tryphonas et al. (1986a) in monkeys. Oral administration of
Aroclor 1242, 1248, or 1254 to monkeys produced gastritis, which
progressed to hypertrophy and hyperplasia of the gastric mucosa.
Related effects included mucous-filled cysts that penetrated the
muscularis mucosa. These effects were initiated by exposure as low
and/or short as a single gavage dose of 1.5 g Aroclor 1248/kg body
weight, 25 mg Aroclor 1248/kg diet for up to 1 year, 3 mg of Aroclor
1242/kg diet, for 71 days, or 280 µg/kg body weight for 28 months.
In studies on monkeys, Becker et al. (1979) carried out stomach
biopsies and found microscopically apparent arrest of the
differentiation of generative cells of the isthmus and neck into
parietal and zymogenic cells. Mature parietal and zymogenic cells,
which were found only in the bases of the glands, showed signs of
injury, such as dilatation of the rough endoplasmic reticulum on the
zymogenic cells, irregularity of the mitochondria and irregular
luminal membranes in parietal cells, and an increase in the number of
autophagic vesicles on both types of cell (see 8.2.1.6).
The Aroclor-induced gastric lesions, which occurred mainly along the
greater curvature of the stomach (not in the cardiac or pyloric
regions) and did not occur in other sections of the gastrointestinal
tract, have only been observed in pigs and monkeys (Hansen et al.,
1976b; Becker et al., 1979; Drill et al., 1981). The gastric effects
may therefore be species specific. Aroclor 1254 induced metaplasia and
adenocarcinoma in the glandular stomach of F344 rats (see section
8.7.1.2).
8.6.5 Effects on lipid metabolism
8.6.5.1 Effects of PCB mixtures
Consistent with the histopathological observation of fatty
degeneration in the liver (see section 8.2.1), short-term exposure to
commercial mixtures of PCBs induced increases in the contents and
concentrations of total lipids, triglycerides, cholesterol, and/or
phospholipids in this organ of the rat and rabbit (Litterst et al.,
1972; Bruckner et al., 1974; Itokawa et al., 1976; Garthoff et al.,
1977; Hinton et al., 1978; Ishidate et al., 1978; Dzogbefia et al.,
1978; Yagi, 1980; Kato & Yoshida, 1980, 1981; Kato et al., 1982).
Litterst et al. (1972) exposed male Osborne-Mendel rats, for 4 weeks,
to diets containing Aroclor 1242, 1248, 1254, or 1260 at levels of, or
between, 0.5, 5.0, 50, and 500 mg/kg (equivalent to 0.025, 0.25, 2.5,
and 25 mg/kg body weight). Aroclor 1248 caused the highest
dose-related increase in the triglyceride concentration in the liver,
which was significant at 500 mg/kg diet (equivalent to 25 mg/kg body
weight). The lowest-observed-effect level was reported by Bruckner et
al. (1974), who exposed male Sprague-Dawley rats to 0, 5, or 25 mg of
Aroclor 1242/kg diet (equivalent to 0, 0.3, and 1.5 mg/kg body weight,
respectively), for 2, 4, or 6 months and found a slight increase in
the concentrations of total lipids in the liver at both exposure
levels.
Levels of total lipids, triglycerides, and/or cholesterol in the serum
of rats and rabbits, exposed to PCB mixtures, were found to be
increased (Koller & Zinkl, 1973; Allen et al., 1976; Itokawa et al.,
1976; Garthoff et al., 1977; Zinkl, 1977; Kato & Yoshida, 1980, 1981;
Yagi, 1980; Kato et al., 1982; Baumann et al., 1983; Hladkà et al.,
1983; Carter, 1985). Wistar rats received Clophen A50, twice weekly,
by gavage, at levels of 2, 10, 50, 150, or 250 mg/kg body weight, for
6 weeks. Serum triglyceride and cholesterol levels were increased in a
dose-related manner at 50 and 2 mg/kg body weight, respectively
(Baumann et al., 1983). Decreased serum triglyceride levels were
reported by Kato et al. (1982). Fischer rats exposed for 8 days to
Aroclor 1254 in the diet at levels of 8 mg/kg (0.4 mg/kg body weight)
or more showed a dose-related increase in serum total cholesterol
concentrations. Hypercholesterolaemia was not found at 4 mg/kg diet
(Carter, 1985). Studies on monkeys exposed to Aroclors 1248 or 1254
for 1-2 years revealed lowered serum levels of total lipids,
triglycerides, and cholesterol (Barsotti et al., 1976; Arnold et al.,
1984). The cause of these changes may be an altered synthesis and/or
lipoprotein transport in the liver. No increase in the rate of
synthesis of liver triglycerides was found following intraperitoneal
exposure of rats to 3-8 daily doses of 50 mg of Aroclor 1254/kg body
weight (Hinton et al., 1978; Sandberg & Glaumann, 1980). The observed
increases in the half-lives of liver triglycerides and phospholipids
(Hinton et al., 1978) and the observed increase in the number of Very
Low Density Lipoproteins (VLDL) in the liver, without a change in
lipid composition (Sandberg & Glaumann, 1980), seems to be indicative
of impaired transport of these lipids from the liver to the blood.
This was also demonstrated by the repression in serum VLDL and in the
incorporation of tritiated water in serum total lipids following
tritiated water injection, found in rats exposed for 24 days to a
low-protein diet containing 1000 mg of Aroclor 1248/kg (50 mg/kg body
weight) (Kato et al., 1982). Sandberg & Glaumann (1980) observed an
impaired transport of VLDL from the endoplasmic reticulum to the Golgi
apparatus. This compares well with the observed flattening of the
Golgi apparatus, which also lacks secretory vesicles with lipoprotein
particles (Hinton et al., 1978). No explanation was found in the
available literature for the observed increase in serum triglyceride
levels in rats.
Ishidate et al. (1978) measured a decreased rate of synthesis of
phospholipids, especially of phosphatidyl choline, in the liver of
rats that had received 2 daily doses of a PCB mixture at 100 mg/kg
body weight, composed mainly of tetrachlorobiphenyl isomers. Decreased
phospholipid synthesis was also observed by Hinton et al. (1978). The
accumulation of phospholipids in the proliferated endoplasmic
reticulum was ascribed to the observed depression of the secretion of
lipoproteins into blood (Hinton et al., 1978; Ishidate et al., 1978;
Sandberg & Glaumann, 1980) and to a depressed catabolism of liver
phospholipids (Ishidate et al., 1978).
The synthesis of cholesterol in the rat liver may be increased by PCB
mixtures considering the increased concentration of labelled
cholesterol in the liver following 3H20-injection (Kato et al.,
1982) or 14C-glucose or 14C-acetate administration (Yagi, 1980) in
rats that had been exposed for 3-5 weeks to diets containing 1000 mg
of Aroclor 1248/kg diet or 500 mg Kanechlor 500/kg diet, respectively.
It was also shown that the activity of 3-hydroxy-3-methylglutaryl
Coenzyme A reductase (EC 1.1.1.34) was increased in rats following a
6-day exposure to a diet containing 1000 mg Aroclor 1248/kg diet
(equivalent to 50 mg/kg body weight) (Kato & Yoshida, 1980). The
enhanced synthesis of cholesterol has to compete with an enhanced
degradation, as Aroclor 1248 has been shown to induce cholesterol
7-alpha-hydroxylase (EC 1.14.14.1) in rats (Quazi et al., 1984).
Decreased biosynthesis of liver cholesterol was found in rats exposed
for 30 days to Aroclor 1254 at a dietary level of 500 mg/kg (Kling &
Gamble, 1982). Hypercholesterolaemia in PCB-exposed rats can be
explained partly by an increased synthesis of cholesterol and/or an
increase in serum high density lipoprotein cholesterol, which was
observed in several studies (Ishikawa et al., 1978; Yagi, 1980; Kato &
Yoshida, 1981; Carter, 1985).
Isolated hepatocytes were capable of secreting protein and
triacylglycerol in the form of VLDL into serum-free media. Eighty per
cent of 2,4,5,2',4',5'-hexachlorobiphenyl released from hepatocytes
was in association with VLDL, the remainder being in association with
protein (Gallenberg & Vodicnik, 1987).
8.6.5.2 Effects of individual congeners
Charles-River CD rats received a single oral dose of 3,4,5,3',4',5'-,
2,4,5,2',4',5'-, or 2,3,5,2',3',5'-hexachlorobiphenyl in cotton-seed
oil. After 72 h, all isomers had increased the levels of total lipids
in the liver. 3,4,5,3',4',5'-Hexachlorobiphenyl had the most
pronounced effect. This isomer was the only one that increased the
levels of total cholesterol, cholesterol esters, and triglycerides in
the liver, while the other 2 isomers slightly increased the content of
liver phospholipids (Kohli et al., 1979).
Shireman (1988) studied the lipoprotein-mediated transfer of
2,4,5,2',4',5'-hexachlorobiphenyl into cultured human fibroblasts, and
found that the plasma lipoproteins may play a role in the distribution
of this hexachlorobiphenyl to peripheral cells. Using normal skin
fibroblasts incubated with medium containing serum LDL or high density
lipoproteins (HDL) labelled with the 14C-hexachlorobiphenyl, the
author characterized the cellular incorporation, and efflux from
cells, of this congener and concluded that HDL might be involved in
the delivery of hexachlorobiphenyl to cells and not, as generally
thought, in the transport from cells.
2,4,5,2',4',5'-Hexachlorobiphenyl was shown to be distributed among
rat and human plasma lipoproteins and protein in vitro. It was
readily transferred among plasma constituents and its distribution was
related to the triacylglycerol:protein ratio in the plasma. One h
following intravenous administration of 70 µg labelled
hexachlorobiphenyl to virgin, female Sprague-Dawley rats, the
hexachlorobiphenyl was primarily distributed to low density
lipoprotein (LDL) with the hypertriglyceridemia of late pregnancy;
more than 70% of circulating hexachlorobiphenyl was associated with
very low density lipoproteins (VLDL). VLDL is a major substrate for
mammary gland lipoprotein lipase, which is elevated during lactation.
When hexachlorobiphenyl was complexed with human VLDL and injected
intravenously into late pregnant mice, mammary gland concentrations of
the compound exceeded those in the adipose tissue at all sacrifice
times between 5 min and 6 h (Gallenberg & Vodicnik, 1987; Gallenberg
et al., 1987).
8.6.6 Effects on porphyrin metabolism
8.6.6.1 Effects of PCB mixtures
Hepatic porphyria has been induced by a number of commercial PCB
mixtures (Clophen A60; Phenochlor DP6; Aroclor 1016, 1232, 1242, 1254,
and 1260; Kanechlor 400, 500, and 600) in mice, rats, rabbits,
chickens, and Japanese quail. Young rats, guinea-pigs, and minks seem
to be less sensitive (Strik, 1973). The porphyria was characterized by
the presence of pigment in the liver which fluoresced red under UV
radiation (Vos & Beems, 1971; Kimbrough et al., 1972; Vos &
Notenboom-Ram, 1972; Zinkl, 1977; Honda et al., 1983), an increase in
the concentration of porphyrins in the liver (Goldstein et al., 1974,
1975; Grote et al., 1975; Iverson et al., 1975; Kawanishi et al.,
1975) and an increase in the concentrations of delta-aminolevulinic
acid, porphobilinogen, and porphyrins in the urine or faeces (Vos &
Beems, 1971; Vos & Notenboom-Ram, 1972; Goldstein et al., 1974, 1975;
Baumann et al., 1983; Honda et al., 1983). Vos & Beems (1971) found
increased faecal elimination of coproporphyrin and protoporphyrin in
rabbits dermally treated with 118 mg Aroclor 1260/day (free of PCDFs),
5 days/week, for 36 days and Vos & Notenboom-Ram (1972) found the same
results when female, New Zealand rabbits received a 120 mg application
of Aroclor 1260 on the shaved skin, 5 days/week, for 4 weeks.
When Sherman rats were exposed for up to 13 months to a diet
containing 100 mg Aroclor 1254/kg or for up to 26 weeks to Aroclor
1242 at 100 or 500 mg/kg diet (equivalent to 5 and 25 mg/kg body
weight, respectively) a delayed onset of porphyria was noted after 2-7
months of exposure. The porphyria was mainly characterized by the
excretion and hepatic storage of uroporphyrin and heptacarboxy-
porphyrin, resembling human porphyria cutanea tarda (Goldstein et al.,
1974, 1975). A dose-dependent increase in the concentration of liver
porphyrins was observed in female, Sprague-Dawley rats receiving 21
daily doses of Aroclor 1242 (by gavage) in corn oil at 10 or 100 mg/kg
body weight, but not at 1 mg/kg body weight. Female rats were more
sensitive than male rats and Aroclor 1016 at the same dietary level
had less effect than Aroclor 1242 (Iverson et al., 1975). Others also
noted the greater effect of higher chlorinated PCB mixtures on liver
and urinary levels of porphyrins (Goldstein et al., 1974, 1975;
Kawanishi et al., 1975). Kawanishi et al. (1973, 1974) showed that
administration, in the diet, of Kanechlors KC-300 and KC-500 to rats
at 500 mg/kg produced a marked increase in urinary excretion of copro-
and uroporphyrins, and in faecal elimination of protoporphyrin, but no
increases were observed with Kanechlor KC-400.
Increased urinary coproporphyrin levels were found in male
Sprague-Dawley rats exposed for 2, 4, or 6 months to a diet containing
Aroclor 1242 at 5 or 25 mg/kg (equivalent to 0.25 and 1.25 mg/kg body
weight) (Bruckner et al., 1974).
Porphyria in rats and rabbits has been associated with the observed
stimulation of delta-aminolevulinate synthase (EC 2.3.1.37), the
rate-limiting enzyme in the haem synthesis of porphyrins (Goldstein et
al., 1974, 1975; Grote et al., 1975; Drill et al., 1981; Hill, 1985),
and with the inhibition of uroporphyrin decarboxylase (EC 4.1.1.37),
as measured in chick embryo cells and chicken erythrocytes in vitro
(Kawanishi et al., 1983; Sano et al., 1985). Seki et al. (1987)
observed 80% inhibition of liver uroporphyrin decarboxylase together
with a 15-fold increase in the activity of liver delta-aminolevulinate
synthase and accumulation in the liver of a large amount of
uroporphyrin in C57BL/6 mice exposed for 3 weeks to Kanechlor 500 at a
dietary dose of 500 mg/kg. Liver microsomal cytochrome P-450 was
increased and induction of microsomal enzymes was observed. The
effects were less outstanding in ddY mice whereas liver cytosol levels
of the PCBs were comparable in both strains. The authors postulated
that the development of porphyria is causally related to the
inhibition of uroporphyrin decarboxylase rather than the induction of
drug metabolizing function. Porphyria would develop only when the
ratio of hepatic uroporphyrin decarboxylase and delta-aminolevulinate
synthase decreased to less than 1.0.
8.6.6.2 Effects of individual congeners
The levels of coproporphyrin and protoporphyrin found in the faeces of
rabbits, dermally exposed to 5 doses/week of 120 mg of
2,4,5,2',4',5'-hexachlorobiphenyl (no dibenzofurans detected) in
isopropanol, for 4 weeks, were more elevated than those in the faeces
of rabbits exposed similarly to Aroclor 1260 (Vos & Notenboom-Ram,
1972). Koss et al. (1980) also found 2,4,5,2',4',5'-hexachlorobiphenyl
highly effective in inducing porphyria in female rats receiving,
orally, 64 mg of this PCB-congener/kg body weight in oil, once every 2
days, for 10 weeks. In mice receiving a diet containing 300 mg of one
of various tetrachlorobiphenyls, hexachlorobiphenyls, or
Kanechlors/kg, for 14 weeks, the most pronounced increases in the
levels of coproporphyrin and protoporphyrin in the liver were found in
animals fed 3,4,5,3',4',5'- and 2,4,6,2',4',6'-hexachlorobiphenyl, and
Kanechlor 600, followed by animals fed 3,5,3',5'- and 2,5,2',5'-
tetra-chlorobiphenyls and Kanechlor 500. No porphyrinogenic action was
found in mice fed 3,4,3',4'-, 2,4,2',4'-, 2,3,2',3'-, or 2,6,2',6'-
tetra-chlorobiphenyl, 2,3,4,2',3',4'-hexachlorobiphenyl, or Kanechlor
400 (Kawanishi et al., 1975). Accumulation of uroporphyrins was
observed in the livers of "responsive" C57BL/6 mice treated with
3,4,5,3',4',5'-hexachlorobiphenyl, but not in the livers of
"non-responsive" ddY mice. It was suggested that induction of
apocytochrome P-450 may take part in inducing porphyrin synthesis
(Sano et al., 1985).
Sano et al. (1985) studied the mechanism of the porphyrinogenic
activity of PCBs using cultured chick embryo liver cells to examine
the relationship between the induction of delta-aminolaevulinic acid
(ALA) synthetase and the inhibition of uroporphyrinogen dicarboxylase.
The porphyrinogenic effect of PCBs exhibited a defined
structure-activity relationship in that only 3,4,3',4'-tetrachloro-
and 3,4,5,3',4',5'-hexachlorobiphenyl out of 9 biphenyls produced a
marked accumulation of uroporphyrin in the liver cells. In
ALA-supplemented cultures, these 2 congeners led to the accumulation
of a large amount of uroporphyrin III, whereas with the other PCBs
(which were weak inducers of porphyrin synthesis) the accumulated
porphyrin was mostly protoporphyrin. These results suggested that the
active inducers of porphyrin synthesis also inhibit uroporphyrinogen
decarboxylase, in 2 steps, i.e., first, in the formation of
hexacarboxylic porphyrinogen III from heptacarboxylic porphyrinogen
III, and, second, in the formation of heptacarboxylic porphyrinogen
III from uroporphyrinogen III. The inhibition of uroporphyrinogen
decarboxylase leads to a depletion of haem. In addition, induction of
apocytochrome P-450 by PCBs may contribute to a decrease of haem. As a
result, synthesis of ALA synthetase increases, leading to an
accumulation of uroporphyrin in liver.
8.6.7 Effects on the endocrine system
8.6.7.1 Effects of PCB mixtures
The underlying cause of the reproductive toxicity of PCBs, described
in section 8.4, may be alterations in hormonal receptor binding and/or
alterations in the steroid hormone balance through effects on
metabolism and excretion.
Precocious vaginal opening was observed in neonatal Sprague-Dawley
rats receiving a subcutaneous dose of 10 mg of Aroclor 1221
(2000 mg/kg body weight) in sesame oil on days 2 and 3 of life. At 6
months of age, these females showed persistent vaginal estrus and
anovulation, despite no further exposure to Aroclor 1221. Doses of
Aroclor 1221, 1242, 1254, or 1260 at 1 mg/kg were without effect.
Groups of 22-day-old Sprague-Dawley rats were injected subcutaneously
with Aroclor 1221 or 1242 at 1, 10, 100, or 1000 mg/kg body weight or
Aroclor 1254 or 1260 mixed in sesame oil at 1, 10, or 100 mg/kg body
weight. Uteri were weighed. New-born female pups were injected
subcutaneously on the second and third day postpartum with Aroclor
1221 at 1 or 10 mg/day or Aroclor 1242, 1254, or 1260 in sesame oil or
dimethylsulfoxide at 1 mg/day. Pups were weaned at 21 days and
examined daily from the 25th day of puberty. Animals were sacrificed
at 7 or 8 months, at which time organs were examined. A significant
uterotrophic response was noted with 1000 mg Aroclor 1221/kg, but not
with the other PCBs (Gellert, 1978).
Indirect evidence for a weak estrogenic activity of PCBs was found for
various Aroclors by the glycogen response of immature rat uterus
(Bitman & Cecil, 1970; Bitman et al., 1972; Ecobichon & Mackenzie,
1974) or the less sensitive uterotropic response, observed in immature
rats exposed to Aroclor 1221, 1232, or 1248, but not in immature rats
exposed to Aroclor 1254 or 1260 (Ecobichon & Mackenzie, 1974; Gellert,
1978). More direct evidence is the inhibition in vitro of the
binding of labelled 17-beta-estradiol to the rat uterine receptor by
Aroclors 1221 and 1254 (Nelson, 1974).
Pregnant mares' serum was administered to immature female outbred rats
on day 29 postpartum, and, 60 h later, rats were injected with human
chorionic gonadotrophin. On day 34, the animals were divided into
groups and treated orally with sesame oil (control), or 20 mg PCBs/kg
(Clophen A30). Two days later they were killed and the ovaries removed
and analysed for in vitro synthesis of progesteron (unincubated,
incubated, and incubated with luteinizing hormone). The addition of
luteinizing hormone resulted in an approximately 100% increase in
progesterone synthesis above basal level with tissue exposed to PCBs.
With control tissue there was a 31% increase with luteinizing hormone
(Fuller et al., 1980).
PCBs induced a decrease in gonadal steroid hormone levels in rats,
minks, seals, and monkeys. When, after confirmed ovulation, mature
female Rhesus monkeys were exposed during the following cycle to daily
gavage doses of 4, 16, or 64 mg of Clophen A30/kg body weight, for 28
days, ovulation was blocked in 2 out of 4 treated monkeys. One out of
16 controls was anovulatory. The levels of luteinizing hormone and
follicle-stimulating hormone were not changed by the treatment (Muller
et al., 1978).
Plasma progesterone levels were decreased in female rats exposed for
36 weeks to a dietary level of Aroclor 1242 of 75 mg/kg (equivalent to
3.7 mg/kg body weight) (Jonsson et al., 1976), and in female minks
exposed for 12.5-14.5 months to a dietary level of 2.5 mg Aroclor
1254/kg (equivalent to 0.25 mg/kg body weight) (Aulerich et al.,
1985). The decreased levels of gonadal hormones can be explained by
enhanced metabolism of steroids, which are normal substrates for
microsomal enzymes. Increases in the formation of the metabolites of
progesterone and/or testosterone were measured in rats
intraperitoneally exposed 1-5 times to Aroclor 1260, Aroclor 1254, or
Kanechlor 400 (Krogh Derr, 1978; Lin et al., 1982; Yoshihara et al.,
1982). In contrast with these findings, increased testosterone levels
were found in male piglets exposed for 6-12 weeks to Aroclor 1232,
1242, or 1254 at 250 mg/diet. This increased production of
testosterone was related to increased relative testes weights
(Platonow et al., 1976).
Female rhesus monkeys (Macaca mulatta) were administered gelatin
capsules containing daily doses of 0, 5, 20, 40, or 80 µg Aroclor
1254/kg body weight, dissolved in corn oil plus glycerol. After
approximately 2 years of dosing, when the monkeys were considered to
be in a state approaching adipose-tissue PCBs equilibrium, each dose
group of 16 animals was divided into 2 test groups. Daily blood
samples from both test groups were acquired for estrogen and
progesteron analysis during one menstrual cycle. Serum estrogen and
progesteron concentrations in PCB-dosed monkeys were comparable with
those in the controls, except the luteal phase progesterone levels in
monkeys dosed with 20 and 80 µg/kg. There were no apparent
treatment-related differences in the incidence of anovulatory cycles
or in the temporal relationship between the estrogen peak and mensus
onset, mensus end, or the progesterone peak. Mean PCB concentrations
in the blood and adipose tissue for the different dose levels
administered were as follows: blood, 1, 11, 37, 74, and 125 µg/litre
and for adipose tissue 0.79, 7.88, 22.62, 47.6 and 85.3 mg/kg tissue,
respectively (Truelove et al., 1987).
Effects on plasma corticosteroid levels have also been observed. The
levels were decreased in female mice exposed to a diet containing
25 mg Aroclor 1254/kg (equivalent to 3.7 mg/kg body weight) for 3
weeks, and in male mice exposed to a diet containing 400 mg Aroclor
1254/kg (equivalent to 57 mg/kg body weight) for 2 weeks. No effects
were found on adrenal weight (Sanders & Kirkpatrick, 1975). However,
increased levels of plasma corticosterone and enlarged adrenal glands
were observed in male mice of another strain exposed to a diet
containing 200 mg Aroclor 1254/kg, for 2 weeks (Sanders et al., 1977).
Wasserman et al. (1973) reported increased plasma corticosterone
levels in rats receiving Aroclor 1221 in the drinking-water, at a
concentration of 250 mg/litre, for 10 weeks. This finding complies
with morphological features of hyperfunction of the adrenal zona
fasciculata found in rats that had received 200 mg Aroclor 1221/litre
drinking-water, for 6 weeks.
When female rats were exposed to Aroclor 1254, in their diet at doses
of 0, 1, 5, 10, or 50 mg/kg (equivalent to 0, 0.05, 0.25, 0.5, and
2.5 mg/kg body weight/day, respectively) for 5-7 months, relative
adrenal weights as well as serum levels of corticosterone,
dehydro-epiandrosterone, and dehydro-epiandrosterone sulfate were
decreased in a dose- and time-related manner. In the same studies, the
effects with less chlorinated Aroclors were less pronounced (Byrne et
al., 1988).
In addition, the ultrastructure of beta-cells of the pancreas of rats
was found to be changed after 13 months of exposure to 200 mg Aroclor
1254/litre drinking-water. These changes included marked dilatation
and vesiculation of the rough endoplasmic reticulum, hyperplastic
Golgi complexes with a reduction in the number of secretory granules,
and an increase in the number of beta-acinar and acinar-beta cells.
The changes in the pancreas were suggested to be secondary to the
increase in the level of glucocorticoids (Wasserman et al., 1975). An
increased relative adrenal weight was observed in pigs fed Aroclor
1242 or 1254/kg at 20 mg/kg diet (equivalent to 0.8 mg/kg body weight)
for 91 days (Hansen et al., 1976b).
Thyroid hormone levels were decreased in rats exposed to Aroclor 1254
at levels of 50 mg/kg diet or more for 4-12 weeks (Collins et al.,
1977; Collins & Capen, 1980a). Two explanations were offered. One was
the observed increase in biliary excretion of thyroxine and
triiodothyroxine (Bastomsky, 1974; Collins & Capen, 1980b) and the
larger proportion of biliary thyroxine present as glucuronide
(Bastomsky, 1974), most likely as a result of induction of microsomal
uridine diphosphate-glucuronosyltransferase (EC 2.4.1.17) (Bastomsky &
Murthy, 1976). The other explanation was a direct effect of PCBs on
thyroid follicular cells. When male Holtzman or Osborne-Mendel rats
were fed a diet containing Aroclor 1254 at a level of 0, 5, 50, or
500 mg/kg (equivalent to 0, 0.25, 2.5, and 25 mg/kg body weight),
thyroid follicular cells exhibited a dose-dependent hypertrophy and
hyperplasia. An abnormal accumulation of large colloid droplets and
irregular lysosomes in the follicular cells were observed at 5 mg/kg
diet or more and reduced serum thyroxine occurred at 50 mg/kg diet or
more. A no-observed-effect level could not be established. Microvilli
were decreased in number, shortened, and irregularly branched (Collins
et al., 1977; Kasza et al., 1978a,b; Collins & Capen, 1980a, 1980b).
The hypothalamus-pituitary axis seems not to be affected in view of
the observed increase in the serum level of thyroid-stimulating
hormone and in the iodine uptake by the thyroid following PCB exposure
(Bastomsky, 1974, 1977; Collins & Capen, 1980a).
Collins & Capen (1980a) suggested that the well-documented
PCBs-related disturbances in reproduction, growth, and development may
be related to alterations in thyroid structure and function in the
dam, fetus, or neonate. The lowering of serum thyroxine appears to be
the combined result of a direct effect on thyroid follicular cells
with an interference in hormone secretion plus an enhanced peripheral
metabolism of thyroxine.
8.6.7.2 Effects of individual congeners
Exposure of rats to various congeners produced different responses in
steroid metabolism. The most marked effects were observed after
exposure to 2,4,5,2',4',5'-hexachlorobiphenyl, which was found to
decrease the half-life of progesterone (Örberg & Ingvast, 1977), to
increase hydroxylation of progesterone, testosterone, and
androstenedione, and to decrease the 5-alpha-reduction of progesterone
and testosterone (Dieringer et al., 1979; Yoshihara et al., 1982).
3,4,5,3',4'-Pentachlorobiphenyl was found to depress the total
microsomal metabolism of progesterone and testosterone, though the
7-alpha-hydroxylation of these steroids was markedly stimulated
(Yoshihara et al., 1982). No, or very slight, effects on steroid
metabolism were found in rats exposed to chlorobiphenyls with 4
chlorine atoms or less (Örberg & Ingvast, 1977; Dieringer et al.,
1979).
Yoshimura et al. (1985) described a marked induction of liver
microsomal cytochrome P-450 and cytosolic DT-diaphorase as a cause of
a possible disorder of steroid homeostasis and promotion of
carcinogenicity of 4-nitroquinoline N-oxide (4-NQO) in rats
pretreated with 3,4,5,3',4'-pentachlorobiphenyl. The animals were
sacrificed 5 days after pretreatment. The results of the studies
showed that 7-alpha-hydroxylation of both progesterone and
testosterone in liver microsomes was increased, but hydroxylation at
the 2-alpha-, 6 alpha-, and 16 alpha-positions were depressed,
together with 5 alpha-reduction. The induced isoenzyme P-452 was most
responsible for the 7-alpha-hydroxylation of testosterone.
The major component (32 mol %) of the Aroclor 1221 mixture is
2-chlorobiphenyl. The major metabolite (4,4'-dihydroxy-2-
chlorobiphenyl) of 2-chlorobiphenyl has been shown to have a
significant binding activity with the soluble uterine estrogen
receptor protein in the rat, suggesting a possible explanation for the
unique estrogenic activity of Aroclor 1221 in the rat (Korach et al.,
1987).
8.6.8 Immunotoxicity
Some of the studies described below are summarized in Table 52.
8.6.8.1 Effects of PCB mixtures
(a) Mouse
Relative thymus and spleen weights of C57BL/6 mice were unaffected by
exposure to Aroclor 1016, for 3-41 weeks, at a dietary level of
167 mg/kg (Silkworth & Loose, 1979). Dietary exposure of outbred mice
(Mus musculus) to Aroclor 1248 at 50, 100, 500, or 1000 mg/kg diet
(equivalent to 7.1 up to 143 mg/kg body weight), for 3 or 5 weeks, did
not elicit gross signs of immunotoxicity (Thomas & Hinsdill, 1978).
BALB/c mice fed Aroclor 1242 at a dose-level of 0 or 167 mg/kg diet
(equivalent to 0 and 29 mg/kg body weight) for 3-9 weeks, did not show
adverse effects on the thymus, spleen, and lymph nodes (Loose et al.,
1977, 1978).
In addition, Carter & Clancy (1980) observed an increased graft versus
host response in a decreased number of spleen cells in 4BALB/c mice,
which were exposed to a single intraperitoneal dose of 1000 mg Aroclor
1242/kg body weight in corn oil. Spleen enlargement and lymphocyte
depletion were observed.
Offspring of Swiss-Webster mice, exposed via the dams which were fed
Aroclor 1254 at dietary levels of 10, 100, or 250 mg/kg, did not
exhibit an altered hypersensitivity reaction to oxazoline, an altered
anti-bovine serum albumin antibody titre or an altered degree of
phagocytosis of sheep red blood cells by peritoneal macrophages,
compared with controls (Talcott & Koller, 1983).
Pathogen-free ICR/JCL mice (aged 4 weeks) were intubated orally, once
a week, for 4 weeks, with 0, 10, or 100 µg Kanechlor 500/kg body
weight. Two days after the final treatment, half of the animals of
each group were injected intraperitoneally with 0, 50, 250, or 500 µg
E. coli endotoxin/mouse. Sensitivity to endotoxin was determined by
24-h mortality rate. The oral administration of Kanechlor 500 up to a
dose level of 100 µg/kg body weight did not have any effect on the
sensitivity to the endotoxin (Oishi & Hiraga, 1980).
The relative potencies of PCB mixtures Aroclors 1260, 1254, 1248,
1242, 1016, and 1232 to inhibit the murine, splenic, plaque-forming
cell response to sheep red blood cells was determined for C57Bl/6
mice. The ED50 values for the reduction in the splenic,
plaque-forming cells were 104, 118, 190, 391, 408, and 464 mg/kg body
weight, respectively. It was apparent that the higher PCBs (Aroclors
1260, 1254, and 1248) were more potent than the lower chlorinated
mixtures.
Previous studies have shown that a subeffective dose of Aroclor 1254
(25 mg/kg), interacted with an immunotoxic dose of TCDD (3.7 nmol/kg),
resulting in, a significant antagonism of the toxicity of the latter
compound. Co-treatment of mice with a dose of all these PCB mixtures
at 25 mg/kg and a reconstituted PCB mixture, as occurs in breast milk,
in combination with TCDD (3.7 nmol/kg) showed that all (except Aroclor
1232) significantly antagonized the TCDD-mediated inhibition of the
splenic, plaque-forming cell response in C56Bl/6 mice (Davis & Safe,
1989).
Table 52. The humoral and cell-mediated immunotoxicity of PCBs administered via the diet in short-term studies
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Monkey
Rhesus Aroclor 44 anti-SRBC antibody titre D 5.0 (d) 2.5 (d) Thomas &
1248 anti-tetanus toxicoid Hinsdill
antibody titre NE - 5.0 (d) (1978)
serum gamma-globulin
fraction D 5.0 (d) 2.5 (d)
Cynomolgus Kanechlor 20 anti-SRBC antibody titre D 2 (bw) Hori et al.
400 serum gamma-globulin (1982)
(purified) fraction D 2 (bw)
Cynomolgus Aroclor 21 anti-SRBC antibody titre D 2.5 (d) Truelove
1254 anti-tetanus toxicoid et al. (1982)
antibody titre NE 10 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Rabbit
New Zealand Aroclor 5 anti-SRBC antibody titre NE 6.54 (bw) Street &
1254 serum gamma-globulin Sharma
levels NE 6.54 (bw) (1975)
delayed hypersensitivity
reaction to tuberculin NE 6.54 (bw)
popliteal lymph node
antibody-forming cells D 0.92 (bw) 0.18 (bw)
Guinea-pig
Clophen 4-7 anti-tetanus toxicoid Vos & van
A60 and antibody titre D 50 (d) 10 (d) Driel-
Aroclor delayed hypersensitivity Grootenhuis
1260 reaction to tuberculin D 50 (d) 10 (d) (1972)
anti-tetanus toxicoid
producing cells in
popliteal lymph nodes D 50 (d) 10 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Rat
Sprague- Aroclor 1 mitogen response to Bonnyns &
Dawley 1254 phytohaemagglutinin I 250 (d) Bastomsky
response to poke-weed (1976)
mitogen NE 250 (d)
serum gamma-globulin
fraction D 250 (d)
Sprague- Aroclor 10 interleukin 2 production Exon et al.
Dawley 1254 induction by KLH D 50 (d) (1985)
natural killer cell
cytotoxicity D 50 (d)
anti-KLH antibody titer D 50 (d)
Mouse
ICR Kanechlor 3 host-resistance to herpes Imanishi
500 simplex virus D 33 (bw) 18 (bw) et al. (1980)
host-resistance to
ectomelia virus D 33 (bw) 18 (bw)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
ICR Kanechlor 3 host-resistance to Imanishi
500 influenza virus D 400 (d) 200 (d) et al. (1984)
host-resistance to
Staphylococcus aureus D 100 (d)
ICR/JCL Kanechlor 4 sensitivity to NE - 100 µg/kg Oishi &
500 E. coli endotoxin (bw) Hiraga
(1980)
Swiss-Webster Aroclor 12 hypersensitivity reaction NE - > 250 (d) Talcott &
1254 to oxazoline, anti-bovine Koller
serum albumine antibody (1983)
titre and phagocytosis
of SRBC by macrophages
BALB/c Aroclor 3-6 host-resistance to Loose et al.
1242 endotoxin D 167 (d) (1978)
host-resistance to
malaria D 167 (d)
BALB/c Aroclor 6 spleen cellularity NE 167 (d) Loose et al.
1242 spleen PFC D 167 (d) (1977)
serum immunoglobulins
G1, A, M D 167 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
C57BL/6 Aroclor 3-41 graft versus host response NE 167 (d) Silkworth &
1016 mixed lymphocyte Loose (1979)
response I 167 (d)
mitogen response to
lipopolysaccharide I 167 (d)
mitogen response to
concanavalin A I 167 (d)
Mus musculus Aroclor 5 host-resistance to Thomas &
1248 Salmonella typhimurium D 1000 (d) Hinsdill
host-resistance to (1978)
endotoxin D 1000 (d)
a Up to day of primary immunization.
b SRBC = Sheep red blood cells; KLH = Keyhole limpet haemocyanin; PFC = Plaque forming cells.
c I = Increased; D = Decreased; NE = No effect found.
d LOEL = Lowest-observed-effect-level; NOEL = No-observed-effect level: in mg/kg diet (d)
or mg/kg body weight per day (bw).
A single administration of 500 mg Aroclor 1254/kg, intraperitoneally,
inhibited the plaque-forming (PFC) response to subsequent challenge
with sheep erythrocytes in Ah locus positive (C57Bl/6N or B6C3F1N)
mice. However, Aroclor 1254 did not give induction in the Ah locus
negative DBA/2N mice. When B6C3F1 mice were challenged with sheep red
blood cells, 6 or 16 weeks after Aroclor 1524 treatment, substantial
recovery of a PFC response was observed. In older (76-week-old) B6C3F1
mice severe depression of the PFC response was observed.
In contrast with its profound depression of a PFC response, Aroclor
1254 (up to 1250 mg/kg) caused a slight increase in lymphocyte
proliferation induced by either T or B cell mitogens. A single
500 mg/kg dose of this Aroclor also suppressed the ability of
recipient B6C3F1 animals to reject a challenge with either the
syngenic fibrosarcoma (PYB6) or the gram negative pathogen Listeria
monocytogenes (Lubet et al., 1986).
Heinzow et al. (1988) studied the effect of 2,4,5,2',4',5'-hexa-
chlorobiphenyl in the E rosette formation with sheep red blood cells
(SRBC) as one of the characteristics of human T-lymphocytes. The
minimal concentration eliciting a significant monoclonal CD2 receptor
antibody sparing effect was 1.5 × 10-10 mol/litre.
C3H/HeN mice were treated, twice a week, for 2 or 3 weeks prior to
mating, with olive oil or with Kanechlor 500 at an oral dose of
50 mg/kg body weight. The offspring, some of which were nursed by
unexposed dams, were tested for immunocompetence, 4-15 weeks after
birth. The dams did not show any adverse effects on body weight,
absolute spleen weight, and spleen cellularity. The B-cell activity of
the offspring was comparable with that of controls. The helper T-cell
activity was reduced up to 7-11 weeks after birth: the effect was more
pronounced in prenatally-exposed groups (Takagi et al., 1989).
(b) Rabbit
Rabbits appear most sensitive with respect to the immunotoxicity of
PCBs. Street & Sharma (1975) exposed groups of 5-7 New Zealand rabbits
to Aroclor 1254 at dietary levels of 0, 3.7, 20, 45.8, or 170 mg/kg
(equivalent to 0, 0.18, 0.92, 2.1, or 6.54 mg/kg body weight) for
44-57 days. When compared with control animals, an increased degree of
thymus atrophy was observed at all dose levels except for 20.0 mg/kg.
At the 2 higher dose-levels, relative spleen weights were decreased
and the number of germinal centres reduced. When 8 female New Zealand
rabbits received 118 mg of Phenochlor DP6, Clophen A60, or Aroclor
1260 (free of PCDFs), on the back skin, 5 times per week, for 38 weeks
they showed leukopenia, thymus atrophy, and loss of germinal centres
in the spleen and lymph nodes. No such changes were observed in the 4
controls (Vos & Beems, 1971). Vos & Notenboom-Ram (1972) found the
same effects in rabbits administered 120 mg Aroclor 1260 (free of
PCDFs)/day, 5 days/week, for 4 weeks. No adverse gross immunotoxic
effects were observed in groups of 10 New Zealand rabbits fed various
Aroclors at dose levels of 150 mg/kg body weight, once a week, for
12-14 weeks (Koller & Thigpen, 1973).
When New Zealand rabbits were exposed orally, via intubation, to
Aroclor 1242 at a dose of 150 mg/kg body weight, once a week, for 11
weeks, the anti-pseudo rabies virus antibody titre and serum
gamma-globulin levels were decreased (Koller & Thigpen, 1973). The
overall picture is one of immunosuppression, though in 2 diet studies
an increased activation of the cell-mediated immune response was
observed (Bonnyns & Bastomsky, 1976; Silkworth & Loose, 1979).
New Zealand rabbit offspring, exposed via the dams fed 0, 10, 100, or
250 mg of Aroclor 1248/kg diet, showed a decrease in the delayed
hypersensitivity reaction to dinitrofluorobenzene in the highest dose
group. No effects were observed on the splenic, plaque-forming cell
response, the antibody titre against sheep red blood cells, and the
mitogen responses to Concanavalin A and Phytohaemagglutinin (Thomas &
Hinsdill, 1980).
(c) Guinea-pig
Atrophy of the thymus has been reported in female guinea-pigs exposed
to Clophen A60 or Aroclor 1260 for 4-7 weeks at a dietary level of
50 mg/kg (equivalent to 2 mg/kg body weight). Following stimulation
with tetanus toxoid, the authors found a lower antitoxin titre and a
lower count of antitoxin-producing cells in comparison with control
guinea-pigs, resulting in a significant reduction in immunoglobulins.
The skin reaction after tuberculination in animals immunized with
Freund's complete adjuvant (as a parameter of cell-mediated immunity)
was also depressed at the 50 mg/kg dose level (Vos & van
Driel-Grootenhuis, 1972).
Female guinea-pigs fed diets containing 50 mg Aroclor 1260/kg for 6
weeks had significantly lowered tetanus antitoxin titres, circulating
leukocytes and lymphocytes, and thymus atrophy (Vos & van Genderen,
1973). Also treatment with 10 mg Aroclor 1260/kg diet for 8 weeks
produced splenic atrophy (Vos & de Roij, 1972). The Aroclor 1260 was
free from PCDFs (no details).
(d) Monkey
Offspring of Rhesus monkeys appeared very sensitive to the toxic
effects of PCB exposure during gestation and nursing, as already
discussed in section 8.4.1. Thymic atrophy, loss of germinal centres
and of lymph nodules of the spleen, and bone marrow hypocellularity
were found in 3 out of 6 offspring that died during their first year
of life due to exposure to PCBs via their mothers. The mothers, 9-12
per group, were fed diets containing 0, 2.5, or 5.0 mg of Aroclor
1248/kg (equivalent to 0, 0.09, and 0.2 mg/kg body weight), for 18
months and were bred after 6 months of exposure. One mother in each
exposed group died during exposure, showing an increased
susceptibility to Shigella flexneri. At autopsy, no lesions were
observed in lymphoid organs and tissues (Allen & Barsotti, 1976;
Barsotti et al., 1976). When the surviving mothers were placed on a
control diet for approximately 1 year after exposure and then rebred,
there was a decided improvement in their health, but their infants
were still severely affected by PCBs. In the 2 offspring in each
exposed group that died after weaning at the age of 4 months, the
effects on the thymus, spleen, and bone marrow were similar to those
described for the first generation (Allen et al., 1980).
Groups of mature, female Rhesus monkeys received diets containing 0,
2.5, or 5.0 mg Aroclor 1248/kg. After 11 months, all monkeys received
intravenous injections of sheep red blood cells (SRBC) as well as an
intramuscular injection of tetatus toxin (TT). Booster injections and
a second TT injection were given after a number of weeks. Blood
samples were taken over a period of 20 weeks after immunization.
The anti-sheep red blood cells (SRBC) antibody titres, and antibody
response to TT were not clearly affected. The gamma-globulin-levels
were lower in the PCB-treated animals. After 6 months, the PCB-treated
monkeys developed chloracne, alopecia, and facial oedema (Thomas &
Hinsdill, 1978).
Hori et al. (1982) also found immunosuppression in monkeys exposed to
PCB mixtures (without detectable quantities of PCDFs), compared with 2
control monkeys. A more severe immunosuppression was observed in
another monkey exposed to a comparable PCB mixture with PCDFs.
In a pilot study, one infant Cynomolgus monkey, the mother of which
had been exposed to Aroclor 1254 at a dose-level of 400 µg/kg body
weight, showed a decreased anti-sheep erythrocyte antibody titre
following primary immunization in comparison with one control infant
(Truelove et al., 1982).
Atrophy and loss of germinal centres in the spleen and other lymphoid
tissues were observed in groups of 5-6 female Cynomolgus monkeys
following exposure to Aroclor 1254 or 1248, in an apple juice-corn oil
emulsion, 3 times per week, at dose levels of 5 and 2 mg/kg body
weight, respectively, until death at day 29-164. The monkeys exposed
to Aroclor 1254 showed bone marrow hypocellularity and leukopenia.
Lesions seen in control monkeys were similar to those described as
spontaneous (Tryphonas et al., 1984; see also section 8.2.1).
Limited data exist on the humoral or cell-mediated responses in
infants, exposed to PCBs via their mothers.
8.6.8.2 Effects of individual congeners
Thymus atrophy was observed in monkeys exposed for 1-6 months to
3,4,3',4'-tetrachlorobiphenyl, but not in monkeys exposed to
2,5,2',5'-tetrachlorobiphenyl (McNulty et al., 1980).
Decreased relative weights of the thymus and increased or decreased
relative weights of the spleen were induced by single intraperitoneal
doses of the planar congeners 3,4,3',4'-tetrachlorobiphenyl (at
10 mg/kg body weight) and 3,4,5,3',4'-pentachlorobiphenyl (at
245 mg/kg body weight) in "responsive" C57BL/6 mice, i.e., mice
possessing the cytosolic Ah receptor protein, but not, or only to a
minor degree, in "non-responsive" DBA/2 mice (lacking this receptor).
The tetrachlorobiphenyl further decreased the number of cells per
spleen and the number of splenic, plaque-forming cells at 10 and
100 mg/kg body weight, respectively. Further chlorination at the
ortho-position decreased the toxicity of these congeners. No adverse
effects were induced by 2,4,5,3',4'- and 3,4,5,2',4'-pentachloro-
biphenyl (at 490 mg/kg body weight), 2,3,4,5,3',4',5'-heptachloro-
biphenyl (at 593 mg/kg body weight) and the di- ortho substituted
tetrachlorobiphenyls (at 100 mg/kg body weight (Silkworth & Grabstein,
1982; Robertson et al., 1984; Silkworth et al., 1984).
Similar trends were observed in Wistar rats with respect to the
induction of decreased relative thymus and spleen weights (see section
8.2.1.1). Biocca et al. (1981) exposed C57BL/6J mice to various
hexachlorobiphenyl isomers in the feed for 28 days. The most toxic
isomer tested was 3,4,5,3',4',5'- hexachlorobiphenyl which, among
others, caused a marked thymus atrophy and a moderate depletion of
lymphocytes in the spleen. 2,4,6,2',4',6'-Hexachlorobiphenyl caused
the same lesions, albeit at much higher dose-levels, while the
2,4,5,2',4',5'- and 2,3,6,2',3',6'-hexachlorobiphenyls were virtually
inactive.
The in vivo generation of cytotoxic T-lymphocytes (CTL) in response
to allogeneic tumour challenge is sensitive to suppression by
3,4,5,3',4',5'-hexachlorobiphenyl, a poorly metabolized, Ah
receptor-binding PCB isomer. Groups of 5-8 C57Bl/5 mice treated with a
single oral dose of 0, 10, or 100 mg 3,4,5,3',4',5'-hexachloro-
biphenyl/kg body weight, 2 days prior to the intraperitoneal injection
of allogeneic P815 tumour cells, exhibited a dose-dependent reduction
in peak CTL activity in the spleen. When examined on a kinetic basis,
the TCL response was reduced in magnitude with no evidence for a shift
in the kinetics of the response induced by 3,4,5,3',4',5'-hexachloro-
biphenyl.
3,4,5,3',4',5'-Hexachlorobiphenyl exposure, prior to antigen challenge
(day -14, -7, or -1 relative to P815 injection on day 0), produced
significant suppression of the CTL response. 3,4,5,3',4',5'-
Hexa-chlorobiphenyl treatment (10 mg/kg body weight), 6 weeks prior to
such a challenge, was still significantly suppressive, though the
reduced degree of suppression suggested that recovery was in progress.
When 3,4,5,3',4',5'-hexachlorobiphenyl exposure occurred after antigen
challenge, significant suppression was produced only when exposure
occurred within the first 3 days of the response, suggesting that, as
the CTL matured, their sensitivity to 3,4,5,3',4',5'-hexachloro-
biphenyl diminished. Clearance of the allogeneic tumour cells from the
peritoneal cavity was delayed in 3,4,5,3',4',5'-hexachlorobiphenyl-
treated mice and was associated with an altered composition of the
white blood cell infiltrate in this cavity. Symptoms of overt
toxicity, as well as immunotoxicity, were apparent at lower doses of
3,4,5,3',4',5'-hexachlorobiphenyl in male compared with female mice.
In addition, interactive effects of 3,4,5,3',4',5'-hexachlorobiphenyl
exposure and P815 antigen challenge on body weight and thymic
involution were observed in both male and female mice (Kerkvliet &
Baecher-Steppan, 1988a).
A modest dose-dependent suppression of the proliferative response to
alloantigen in mixed lymphocyte culture (MLC) was observed with
lymphocytes from C57Bl/6 mice (groups of 5-6 mice) exposed to 10 or
100 mg 3,4,5,3',4',5'-hexachlorobiphenyl, while the cytotoxic
T-lymphocytes CTL response generated in MLC was significantly
suppressed only with 100 mg/kg. The amount of time between treatment
with 3,4,5,3',4',5'-hexachlorobiphenyl and sacrifice, which ranged
from 2 to 23 days, did not appear to influence the degree of
immunosuppression produced by 3,4,5,3',4',5'-hexachlorobiphenyl
exposure. Mitomycin C-treated lymphocytes from C57Bl/6 mice treated
with 10 or 100 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body weight,
were not suppressive when added as third party cells to an independent
MLC. However, if the mice were alloimmune, lymphocyte-mediated
suppression of the MLC response was observed and directly correlated
with the magnitude of the CTL response present in the same population.
Thus, 3,4,5,3',4',5'-hexachlorobiphenyl-treated mice that had less CTL
activity compared with vehicle-treated mice also had less suppressor
activity. Avoidance of stimulator cells lysis by using H-2
incompatible MLC stimulator cells revealed the existence of
antigen-nonspecific suppressor activity that was greater with
lymphocytes from vehicle-treated mice than from 3,4,5,3',4',5'-
hexachlorobiphenyl-treated mice, suggesting that both CTL and
suppressor cell activities were suppressed by 3,4,5,3',4',5'-
hexa-chlorobiphenyl exposure. Direct addition of 3,4,5,3',4',5'-
hexachloro-biphenyl to lymphocyte cultures in vitro indicated
a lack of direct toxicity of 3,4,5,3',4',5'-hexachlorobiphenyl on
lymphoproliferative responses to mitogen or alloantigen at
concentrations equal to or less than 1 × 10-6 mol/litre. Thus,
the in vitro functional integrity of lymphocytes obtained from
3,4,5,3',4',5'-hexachlorobiphenyl-treated mice coupled with the lack
of a direct lymphocytic effect in vitro suggest an indirect
mechanism of action for the 3,4,5,3',4',5'-hexachlorobiphenyl-mediated
suppression of CTL activity in vivo. Previous reports implicating
suppressor cell induction and/or activation by Ah-receptor-binding,
halogenated, aromatic hydrocarbons that mediate the inhibition of CTL
generation were not confirmed (Kerkvliet & Baecher-Steppan, 1988b).
8.6.8.3 Appraisal
The alterations in gross measures of immunological function (spleen
and thymus weights, lymphocyte counts, histology of lymphoid organs
and tissues) in mammals are highly suggestive of an immunosuppressive
effect of PCB mixtures and some higher chlorinated congeners. More
direct evidence of an immunodepressive effect has been obtained by
methods that detect functional alterations in the humoral and
cell-mediated immunity in mammals. One study on monkeys demonstrated a
more severe immunosuppression by a PCDF-contaminated PCB mixture
compared with a non-contaminated PCB mixture. Rabbits and monkeys are
the most sensitive species. No-observed-effect levels are 100 µg
Aroclor 1248/kg body weight per day and <100 µg of Aroclor 1254/kg
body weight per day for monkeys and 180 µg of Aroclor 1254/kg body
weight per day for rabbits.
8.6.9 Neurotoxic effects
Depressed spontaneous motor activity was shown by male CD-mice exposed
to a single oral dose of 500 mg Aroclor 1254/kg body weight in
Emulphor:saline. No effects were found in motor coordination tests and
on pentenylene-tetrazol-induced seizures. Neurochemical tests with
isolated mouse brain synaptosomes showed inhibition of the uptake of
neurotransmitters and precursors, and stimulation of the release of
neurotransmitters (Rosin & Martin, 1981).
Male Wistar rats exposed to doses of 500 or 1000 mg Aroclor 1254 and
1260/kg body weight, in corn oil, showed a reduced norepinephrine
concentration in the frontal cortex and hippocampus. No changes were
measured in the hypothalamus and brainstem. The neurochemical effects
appeared to be associated with the actual presence of PCBs in the
tissues (Seegal et al., 1985).
8.6.10 Skin effects
Cutaneous effects occurred in Rhesus monkeys fed diets that contained
Aroclors, for short periods (Allen & Norback, 1973; Allen et al.,
1974a; Allen, 1975; Barsotti et al., 1976; Thomas & Hinsdill, 1978;
Allen et al., 1979; Altman et al., 1979; Becker et al., 1979;
McConnell et al., 1979; McNulty et al., 1980). The effects included
facial (particularly periorbital) oedema, purulent discharge from the
eyes, chloracne, and alopecia. The effects, which appeared to be
reversible, were produced by doses as low as 2.5 mg Aroclor 1248/kg
for 1-6 months, and 1 mg Aroclor 1242/kg (equivalent to 0.04 mg/kg
body weight) for 6 months. Rats exposed to Aroclor 1254 in the diet
developed alopecia and facial oedema after 104 weeks at 50 mg/kg, and
exophthalamos after 72 weeks at 50 mg/kg. These effects did not occur
after 104 weeks at 25 mg/kg (equivalent to 1.25 mg/kg body weight)
(NCI, 1978).
8.6.11 Effects on the lung
Many PCBs are metabolized to methylthio derivatives in mice (Mio et
al., 1976), seals (Jensen & Jansson, 1976) and humans (Yoshida &
Nakamura, 1979). The metabolic pathways leading to the generation of
these metabolites have been shown to involve glutathione conjugation
of an arenoxide intermediate (Preston et al., 1984). Lund et al.
(1986) studied the interactions of these metabolites with the lung. It
was found that the PCB metabolite, 4,4'-bis-(methylsulfonyl)-
2,5,2',5'-tetrachlorobiphenyl ((MeSO2)2TCB), selectively accumulates
in the Clara cells of the bronchiolar epithelium and in the secretory
contents of the bronchiolar lumen. In vitro characterization of this
interaction of tritiated (MeSO2)2TCB with lung suggests that this
selective accumulation is due to the presence of a secreted
(MeSO2)2TCB-binding protein in the respiratory tract of rats, mice,
and humans. The protein appears to be an almost globular,
low-relative-molecular-mass acidic protein that binds with
methylsulfonyl-PCBs (Lund et al., 1986).
In a study on 3 groups of C57Bl mice, administered orally 0, 10, or
100 mg 2,3,6,4'-tetrachlorobiphenyl/kg body weight with repeated
administration of 35S-cysteine, Klasson-Wehler et al. (1987) found
that the major compound present in the lung was 4-methylsulfonyl-
tetrachlorobiphenyl, indicating the presence of specific binding sites
for this metabolite in lung tissue, mainly in the tracheo-bronchial
mucosa.
Groups of 20 male SD rats were given (gastric intubation) 0 or 25 mg
Kanechlor 400 in edible oil once, and, in 2 other groups, the same
doses 4 times per week. Groups of 5 animals were killed 2, 7, 14, and
28 days after the last ingestion, and tissues were studied with light
and electron microscopy. The lungs of the rats particularly showed
peribronchiolar cell infiltrations, and electron microscopy revealed
lipid vacuoles and altered lamellar bodies or lysosomes in type II
alveolar cells and alveolar macrophages. These changes were most
marked 7 days after the last ingestion and were more severe in the
short-term application (Shigematsu et al., 1978).
8.6.12 Miscellaneous
Haake et al. (1987) used mature, male C57Bl/6J mice and virgin, female
C57Bl/6N mice to study the influence of Aroclor 1254 on the
2,3,7,8,-TCDD induction of teratogenic abnormalities. Dams were
treated by oral gavage with either corn oil, Aroclor 1254 (244 mg/kg),
or TCDD (20 µg/kg) or Aroclor followed by TCDD or Aroclor followed by
dexamethasone (90 mg/kg). Aroclor 1254 alone was administered on day
9, corn oil and TCDD on day 10, and dexamethasone on day 13. In the
combinations, the Aroclor was given the day before the TCDD or
dexamethasone. TCDD induced 61.8 ± 23.1% cleft palate per litter;
Aroclor with TCDD, 8.2 ± 1.5%; Aroclor alone, 0%; dexamethasone alone,
69.9 ± 18.2%, and Aroclor followed by dexamethasone, 85.8 ± 29.1%.
Previous studies have shown that Aroclor 1254 can act as a partial
antagonist of the microsomal enzyme induction and immunotoxic effects
of TCDD in the mice strain used and, in this study, it was shown that
Aroclor 1254 also antagonizes TCDD-mediated teratogenicity. It did not
have any effect on the effects mediated by dexamethasone.
Wölfle et al. (1988) found that treatment of male Wistar rats with
200 mg 3,4,3',4'-tetrachlorobiphenyl/kg body weight, injected
intraperitoneally, markedly stimulated growth of enzyme-altered liver
foci and [3H]-thymidine incorporation into nuclear DNA. In the liver,
enlarged hepatocytes, due to hypertrophy, and fine-to-medium
fatty-droplet deposition in hepatocytes were found, but no liver
necrosis. Hence, it was concluded, that post-necrotic regenerative
growth as the cause of the tetrachlorobiphenyl-mediated stimulation of
hepatocytes proliferation, could be excluded. The treatment with
tetrachlorobiphenyl in vivo, markedly increased EGF-stimulated
autophosphorylation of the EGF-receptor (a plasma membrane protein) in
liver plasma membranes. These results suggest that altered growth
control is due to a direct effect of tetrachlorobiphenyl on
hepatocytes.
8.7 Factors modifying toxicity; mode of action
8.7.1 Factors modifying toxicity
As PCBs can stimulate microsomal enzyme activity, it can be expected
that they may potentiate the action of other chemicals that undergo
microsomal activation, and antagonize the action of those that are
detoxified. Antagonism was for example observed in studies on rodents
with drugs like pentobarbital (Villeneuve et al., 1972; Sanders et
al., 1974; Sanders & Kirkpatrick, 1975; Rosin & Martin, 1983),
hexobarbital (Bickers et al., 1972; Tanaka & Komatsu, 1972), and
zoxazolamine (Bickers et al., 1972).
Lashneva et al. (1985) and Khan et al. (1985) found potentiation of
the rate of microsomal enzyme induction in rats with a combination of
50 mg Sovol (mixture of PCBs)/kg body weight and 500 mg 2,6-ditert-
butyl-4-methylphenol (ionol)/kg body weight.
Villeneuve et al. (1972) demonstrated the antagonistic effect by the
reduction of phenobarbital sleeping time in rats receiving Aroclors
1242, 1254, and 1260 in their diet, but not in those receiving Aroclor
1221. This was confirmed by Johnstone et al. (1974) with a series of
single PCBs. Tanaka & Komatsu (1972) found that the hexobarbital-
induced sleeping time in female rats was reduced to 49% of the control
value by daily oral doses of Kanechlor 500 of 2 mg/kg for 3 days
(total 6 mg/kg). When a daily dose of 0.4 mg/kg was given for 15 days
(total 6 mg/kg), no reduction in sleeping time was observed. When this
small dose was continued for 45 and 53 days, the reduction remained at
12-13%. Phillips et al. (1972) did not find any potentiation of the
cholinesterase-inhibitory action of parathion in rats dosed with
Aroclors 1221 and 1254; this does not necessarily imply that there was
no enhanced activation of parathion, as a stimulation of detoxication
may have occurred concurrently. A stimulation of parathion
detoxication, but not of activation, has been demonstrated in rabbit
microsomes (Villeneuve et al., 1971a). Lichtenstein (1972) reported a
potentiation by PCBs of the toxicity of parathion for flies.
Aroclor 1254 at 160 mg/kg diet fed to 5-week-old male and female
Fischer-344 rats, for 8 weeks, reduced mortality due to feeding
hexachlorophene at a concentration of 600 mg/kg diet, from 77% to 7%
and completely prevented the paralysis that was observed in all
animals on the hexachlorophene diet alone. However, histological
changes in the brain characteristic of hexachlorophene exposure were
still apparent in the animals on the combined treatment, and the
possibility of delayed toxicity beyond the 8 weeks of the study could
not be eliminated. The protective effect of Aroclor 1254 was explained
by its capacity to enhance detoxication by means of hepatic microsomal
enzyme induction (Jones et al., 1974).
Coté et al. (1985) studied a mixture of 15 "persistent" chemicals,
including Aroclor 1254, in Sprague-Dawley rats at dose levels of 0, 1,
10, 100, and 1000 times the Canadian water quality objectives (WQO) of
each chemical. The PCB (Aroclor 1254) treatments were 0, 0.001, 0.01,
0.1, or 1.0 µg/kg diet for 90 days. No influence on food intake, body
weight, organ weights, clinical chemistry, haematology, or
histopathology were observed.
As these polychlorinated hydrocarbons seem to have the same mechanism
of action, questions arise on the possible interactions between these
compounds. In one reported teratogenicity study, groups of 18-21
pregnant C57BL/6N mice received (by gavage) the vehicle corn oil, or
daily doses of 3 µg 2,3,7,8-TCDD/kg body weight, 10 or 20 mg
2,3,4,5,3',4'-hexachlorobiphenyl/kg body weight, 25 or 50 mg
2,4,5,2',4',5'-hexachlorobiphenyl/kg body weight, or combinations of
TCDD and hexachlorobiphenyl at these dose-levels in corn oil, from day
10 to day 13 of gestation. All chemicals were more than 98.9% pure and
the hexachlorobiphenyls did not contain detectable levels of
dibenzofurans or TCDD. TCDD alone caused a low incidence of cleft
palate and moderate hydronephrosis, 2,3,4,5,3',4'-hexachlorobiphenyl
only caused mild hydronephrosis, and 2,4,5,2',4',5'-hexachlorobiphenyl
did not produce any effects. However, treatment of pregnant mice with
a combination of TCDD and 2,3,4,5,3',4'-hexachlorobiphenyl caused
5- and 10-fold increases in the incidence of cleft palate at 10 and
20 mg of hexachlorobiphenyl/kg body weight, respectively. No
enhancement of TCDD-induced hydronephrosis was observed, and the
incidence of TCDD-induced cleft palate was not affected by
simultaneous 2,4,5,2',4',5'-HCB treatment (Birnbaum et al., 1985).
Male Sprague-Dawley rats were given a regimen consisting of PCBs,
1 mg/day; polychlorinated quarterphenyls (PCQs), 1 mg/day; PCDFs,
10 µg/day or a mixture of PCBs, PCQs, and PCDFs (1 mg + 1 mg +
10 µg/day) in olive oil, orally, for 22 days. The congeners and ratios
in the PCBs, PCQs, and PCDFs were the same as those in Japanese Yusho
oil (see section 9.1.2.1). The PCB-treated rats showed hepatic
hypertrophy, immunosuppression, and increased drug-metabolizing enzyme
activities in hepatic microsomes. PCQ treatment did not produce any
significant effects. PCDF and the mixture PCBs + PCDFs caused
hypertrophy of the liver, immunosuppression, increased and
drug-metabolizing enzyme activity to a much greater extent than that
found for PCBs (more than 100 times more) and weight loss and thymic
atrophy (Kunita et al., 1985).
Female Cynomolgus monkeys were administered PCBs (5 mg), PCQs (5 mg),
or a mixture containing 5 mg PCBs + 20 µg PCDFs in olive oil injected
in a piece of banana, daily, for 20 weeks. The PCBs and PCDFs
comprised the same congeners as those in Japanese Yusho oil. The
PCB-treated monkeys showed hepatic hypertrophy, immunosuppression, and
increased drug-metabolizing enzyme activities in hepatic microsomes,
but were devoid of the dermal symptoms characteristic of Yusho. PCQs
caused an increase in drug-metabolizing enzyme activities in hepatic
microsomes and immunosuppression, but these effects were much less
severe than those of PCBs. The mixture with PCDFs caused hypertrophy
of the liver, immunosuppression, increase in drug-metabolizing enzyme
activities (more than 100 times that of PCBs) and weight loss and
thymic atrophy. Dermal symptoms characteristic of Yusho patients were
also found, but not with PCBs or PCQs alone (Kunita et al., 1985).
8.7.2 Mechanisms of toxicity
The analogous structure-activity relations of individual PCB congeners
with respect to most of their toxic responses and to their potency in
inducing cytochrome P-448-dependent aryl hydrocarbon hydroxylase,
indicate that the most active PCB congeners (the coplanar congeners)
are those that are approximate stereoisomers of 2,3,7,8,-tetra-
chlorodibenzo- p-dioxin (TCDD). These findings suggest a common
mechanism of action.
As is proposed for 2,3,7,8-TCDD, this mechanism is based on the
binding affinity of PCB congeners to the cytosolic Ah-receptor
protein, a product of the regulator Ah gene (Poland & Glover, 1977;
Parkinson & Safe, 1981; Bandiera et al., 1982). The induction is
dependent on the position and number of chlorine atoms in the molecule
and the congeners that bind most strongly to the Ah-receptor show the
strongest induction of monooxygenases and the highest toxicity
(effects on the liver including increase in liver weight, increase in
liver enzyme activity and lipid content, porphyria, atrophy of the
thymus, and effects on reproduction) (Ecobichon & Comeau, 1975;
Goldstein, 1980). These very active congeners are the non
ortho-substituted PCBs 3,4,3',4'- tetrachloro-, 3,4,5,3',4'-
pentachloro-, and 3,4,5,3',4',5'-hexachlorobiphenyl, which are at
least twice substituted in the meta and para positions.
In this model, the inducer-receptor complex is translocated into the
nucleus, interacts with DNA, and eventually triggers the pleiotropic
responses observed. The role of the receptor protein in the mechanism
of action of PCBs is further substantiated by the differential effects
of the congeners in non-responsive DBA/2J mice and responsive C57Bl/6J
mice. Furthermore, there is a good relationship between the aryl
hydrocarbon hydroxylase and ethoxyresorufin O-deethylase induction
potencies in rat hepatoma H-4-II-E cells in vitro (Sawyer & Safe,
1982) and their relative binding affinities for the male Wistar rat
hepatic cytosol receptor protein (Bandiera et al., 1982; Safe et al.,
1985b).
Thus, the coplanar PCBs have mechanisms of action similar to those of
the polychlorinated dioxins (PCDDs) and dibenzofurans (PCDFs) (see
also WHO, 1989).
On the basis of the comparative toxic and biochemical potencies of
coplanar and mono ortho coplanar PCBs, Safe (1990) suggested toxic
equivalent factors TEFs (relating to 2,3,7,8-tetrachloro-dibenzo-
p-dioxin, TCDD, see WHO/EURO, 1987) for these compounds. See Table
53. Although there are certain limitations and uncertainties
associated with the use of TEFs (WHO/EURO, 1987), they may be useful
for an attempt to assess the risk of the combined exposure to coplanar
PCBs and PCDDs/PCDFs.
Table 53. Proposed Toxic Equivalent Factor (TEF) values for the
coplanar and mono ortho coplanar PCB congenersa
Congener TEF value Relative potency range
1. Coplanar PCBs
3,4,5,3',4'-PeCB 0.1 0.3-0.0006
3,4,5,3',4',5'-HxCB 0.05 0.1-0.0012
3,4,3',4',-TCB 0.01 0.009-0.00008
2. Mono ortho coplanar
2,4,5,3',4'-PeCB 0.001 0.0004-0.000006
2,3,4,3',4'-PeCB 0.001 0.0008-0.00006
3,4,5,2',4'-PeCB 0.001 0.00013-0.000018
2,3,4,5,4'-PeCB 0.001 0.00045-0.000074
2,3,4,3',4',5'-HxCB 0.001 0.0005-0.0000014
2,3,4,5,3',4'-HxCB 0.001 0.0004-0.0000065
2,4,5,3',4',5'-HxCB 0.001 0.0000055
2,3,4,5,3',4',5'-HpCB 0.001 no data available
a From: Safe (1990).
The frequently occurring mixed type, the PB-type, and the
weak/noninducing type congeners with some degree of ortho
substitution may also exert other more subtle toxic effects, either
directly via conversion to hydroxylated derivatives, or indirectly
through environmental transformations involving regiospecific
dechlorination followed by hydroxylation.
In addition, some PCBs, particularly the lower chlorinated ones, can
be more readily metabolized through arene oxide intermediates that may
be directly genotoxic/carcinogenic. Arene oxide intermediates are also
involved in the formation of the methylsulfonyl metabolites of PCBs,
which selectively accumulate in the Clara cells of the bronchiolar
epithelium and in the secretory contents of the bronchiolar lumen of
the lungs. This apparently also involves specific binding proteins
that may be important in the expression of certain types of pulmonary
toxicity.
Finally, there are other forms of toxicity associated with PCBs that
appear to involve certain non-receptor, protein-binding interactions.
8.7.3 Toxicity of impurities in commercial PCBs
In many toxicological studies on the effects of commercial PCB
mixtures, the quantitative contribution of impurities to the toxic
responses found is largely unknown (WHO, 1989; WHO/EURO, 1987).
9. EFFECTS ON HUMANS
The toxicological evaluation of PCBs presents many problems. PCBs
usually occur as mixtures of many congeners, and many of the data on
the toxicity of the PCBs are based on the testing of these mixtures.
Some components of the mixtures are more easily degraded in the
environment than others. Thus, the general population may be exposed
to mixtures that are different from those to which workers, working
with PCBs, are exposed.
There are great difficulties in assessing human health effects
separately for PCBs, since, quite frequently, PCDFs have been present
in the PCB mixtures to which humans have been exposed. The presence of
PCDDs has occasionally been seen in accidents with certain
PCB/chlorobenzene mixtures. Commercial PCBs have been shown to be
contaminated with PCDFs and, therefore, in many cases it is unclear
whether effects were attributable to the PCBs themselves or to the
much more toxic PCDFs.
Because PCBs are ubiquitous and very persistent in the environment,
humans have been, and will continue to be, exposed to them,
particularly in industrialized countries. PCBs may be inhaled in small
amounts through the air or ingested through food. People are primarily
exposed to PCBs by consuming fish from contaminated water, but they
can be also exposed via other food.
Furthermore, exposures have occurred through accidents and
occupational exposure; in the latter case, for example, during the
repair of transformers, capacitors, or during the handling of toxic
wastes.
Since PCBs are lipophilic, they are preferentially stored in adipose
tissue. They are also present, to a smaller extent, in serum, organs
and tissues, and human milk. The concentrations of PCBs in the
different organs depend on the lipid content of such organs, with the
exception of the brain, where the concentration is lower than the
lipid content would indicate. PCBs pass, to a certain extent,
(depending on chlorination and structure) through the placenta. They
are primarily excreted through the bile and milk. In addition to the
lipid content, the ratios between adipose tissue, blood, and organs
are influenced by exposure level, sex, age, duration of exposure, and
also by whether exposure is current (see section 5).
Since human milk is relatively easy to obtain, it has been used to
monitor human exposure. Results of the many monitoring studies carried
out in many countries all over the world have shown that average
levels of total PCBs are below 2 mg/kg milk fat, though women living
in heavily industrialized urban areas or who consume large quantities
of fish from heavily contaminated areas, may have higher levels.
9.1 General population exposure
The general population is exposed to PCBs primarily by the oral route,
e.g., by consumption of fish from contaminated waters. The monitoring
data on adipose tissues, blood, and breast milk indicate that PCBs are
absorbed via the gastrointestinal tract, but do not provide
information regarding the extent of the absorption.
9.1.1 Acute effects -- poisoning incidents
No data available.
9.1.2 Effects of short- and long-term exposure
9.1.2.1 Yusho and Yu-Cheng accidents
(a) Yusho accident
In June 1968, patients appeared at the Dermatology Clinic of Kyushu
University Hospital, Fukuoka, Japan, suffering from chloracne. A group
at the University undertook intensive clinical, chemical, and
epidemiological investigations and found that the disease originated
from the consumption of a batch of rice oil supplied in February 1968;
the disease was called Yusho (rice oil disease) (Katsuki, 1969). This
batch of rice oil was found to be contaminated with Kanechlor 400, a
48% chlorinated biphenyl, at 2000-3000 mg/kg, which entered the oil
through a leak in a heat exchanger (Tsukamoto, 1969). Chlorinated
dibenzofurans at 5 mg/kg were found in 3 samples of the toxic rice oil
that contained PCB levels of about 1000 mg/kg (Nagayama et al., 1976).
The Japanese literature on this incident has been summarized in
English by Kuratsune et al. (1976). The average estimated intake was
633 mg PCBs, 3.4 mg PCDFs, and 596 mg PCQs, roughly equivalent to
157 µg PCBs/kg per day, 0.9 µg PCDFs/kg per day, and 148 µg PCQs/kg
body weight per day (Chen et al., 1985; Masuda et al., 1985).
The symptoms and signs of Yusho were described by Goto & Higuchi
(1969) and by Okumura & Katsuki (1969). The earliest signs were
enlargement and hypersecretion of the Meibomian glands of the eyes,
swelling of the eyelids, and pigmentation of the nails and mucous
membranes, occasionally associated with fatigue, nausea, and vomiting.
This was usually followed by hyperkeratosis and darkening of the skin
with follicular enlargement and acneiform eruptions, frequently with a
secondary staphylococcal infection. These skin changes were most often
seen on the neck and upper chest, but, in severe cases, extended to
the whole body. It was estimated that the mean length of the latency
period between exposure and the onset of clinical illness was 71 days,
with a range of from 20 to 190 days (Kuratsune et al., 1972).
Biopsy skin samples showed hyperkeratosis, dilation of the follicles,
and an accumulation of melanin in the basal cells of the epidermis;
melanin granules have also been observed in biopsy samples of the
conjunctiva. Oedema of the arms and legs was also seen in some
patients. There were no definite signs of liver enlargement or liver
disorders (Okumura & Katsuki, 1969), but slight rises in serum
transaminases and in alkaline phosphatase were detected, and a liver
sample from a Yusho patient showed an increase in the smooth
endoplasmic reticulum (Hirayama et al., 1969). The majority of the
patients were found to have respiratory symptoms, and suffered from a
chronic bronchitis-like disturbance that persisted for several years
(Shigematsu et al., 1971, 1978).
Kikuchi (1984) described the autopsy findings, up to July 1982, of 12
patients with Yusho including 2 stillborn babies. Characteristic
pathological changes were acne-like eruptions and cutaneous
pigmentation with histological features of follicular hyperkeratosis,
dilated hair follicles, and an increase of melanin pigment in the
epidermis. In addition, multiplication of the duct epithelium of the
oesophageal glands was found in 6 patients. Twenty-four Yusho patients
were observed clinically over the period 1968-78. During this decade,
the various clinical symptoms of the Yusho patients gradually
diminished. However, some of the symptoms and signs, such as
pigmentation of the skin, conjunctiva, and gingiva, eye discharge, and
various non-specific symptoms still remained in a number of severely
ill patients (Okumura, 1984).
Nakanishi et al. (1985) carried out clinical and experimental studies
on respiratory involvement and alterations in the immune status. PCBs
were not taken up by the bronchi, but were evenly distributed
throughout the lung parenchyma. However, specific dose dependence and
structural requirements of PCBs were shown to exist for accumulation
in bronchial mucosa. A large amount of expectoration at an early stage
of the disease may be related to this. Pathophysiological findings in
Yusho patients revealed that respiratory involvement was mainly small
airways disease, which may be caused by involvement of the cellular
component (Clara cells) in the bronchioles and/or associated with
viral or bacterial infections.
Changes in the immune status in these Yusho patients were decreases in
IgA and IgM in the serum at an early stage of the disease and then a
return to normal and suppression of cellular immunity. The changes in
immune status in these Japanese patients were comparable with the
findings in the Taiwanese patients (see below).
Hirayama et al. (1974) also reported that the serum bilirubin levels
of patients were significantly lower than the normal level and that it
was negatively correlated with the blood level of PCBs and the serum
triglyceride level.
A considerable number of patients had elevated serum triglyceride
levels, up to 4 times the normal values, though this was not
correlated with the severity of the symptoms; these high values were
maintained for 3 years in many patients (Uzawa et al., 1972). There
were no marked abnormalities in serum cholesterol and phospholipid
levels (Okumura & Katsuki, 1969; Uzawa et al., 1969). Nagai et al.
(1969) reported an increase in urinary 17-ketosteroids excretion.
Kusuda (1971) also observed changes in the menstrual cycle in
approximately 60% of 81 female Yusho patients, when compared with
their cycles prior to exposure. A positive correlation was observed by
Okumura et al. (1974) between the blood levels of triglycerides and
PCBs in 42 patients.
Shigematsu et al. (1971) examined serum immunoglobulin levels in 38
patients, 2 years after the onset of the disease, and observed a
decrease in IgA and IgM and an increase in IgG. Lower IgM levels were
reported in patients showing chloracne (Saito et al., 1972) (see also
section 9.2.4.2).
Yusho patients did not appear to suffer from central nervous effects,
but some complained of numbness of the arms and legs. Murai & Kuroiwa
(1971) found a decrease in the conduction velocity in peripheral
sensory nerves.
Determinations of PCB concentrations in the tissues of Yusho patients
were made several months after the ingestion of the oil, using an
X-ray fluorescence method for organic chlorine (Goto & Higuchi, 1969).
Abdominal fat contained 13.1 mg/kg, subcutaneous fat 75.5 mg/kg, and
nails 59 mg/kg. The mesenteric adipose tissue in 6 Yusho patients,
analysed by gas-liquid chromatography 1-3 years after the occurrence
of intoxication, contained an average PCB level of 2.5 mg/kg, which
was considerably higher than the normal value (Masuda et al., 1974a).
The mean blood level of PCBs in patients was 6 or 7 µg/litre
(3 µg/litre for the general population), 5 years after exposure
(Masuda et al., 1974b; Takamatsu et al., 1974). These authors also
noted a specific gas-liquid chromatographic pattern that was peculiar
to Yusho patients.
Eleven years after exposure, a mean concentrations of 6 µg PCB/litre
and 2 mg PCQs/litre, but no PCDFs, were found (Kashimoto et al.,
1985).
Urabe (1974) reported that the total number of Yusho patients had
reached 1200 by September 1973 and that 22 of them had died. At the
end of 1982, the number of identified patients was 1788 (Urabe &
Asahi, 1985). Mucocutaneous signs had decreased year by year, but
neurological and respiratory signs and symptoms and various
complaints, such as general fatigue, anorexia, abdominal pain, and
headache, had become more prominent among the patients. Over time, the
severity and the extent of the skin lesions decreased considerably in
the exposed population. Fifteen years after the accident, only a few
patients still had extensive chloracne.
By 1979, 31 Yusho patients had died, 11 (35.4%) of these from
malignant neoplasms. Only 21.1% of all deaths in this Japanese
prefecture would be expected from malignant neoplasms, but no clear
correlation between the occurrence of Yusho and increased deaths from
malignant neoplasms could be made, because of the small number of
deaths observed and the unknown latency period.
By the end of 1983, 120 Yusho patients had died, 41 of these from
malignant neoplasms. These included 8 stomach cancers, 11 liver
cancers, and 8 neoplasms of the lung. A statistically significant
excess mortality was seen for malignant neoplasms, cancer of the liver
and cancer of the lung, trachea, and bronchi in males, but no such
excess was noted in females. The excess from liver cancer deaths was
seen mainly in Fukuoka prefecture, while no excess was seen in the
Nagasaki prefecture (Ikeda et al., 1987).
(b) Yu-Cheng accident
In 1979, a similar incident occurred in Taiwan, the number of persons
involved, by the end of 1980, was 1843. In the course of 3.5 years,
2061 persons were determined to be victims of PCB poisoning. The
incident has been referred to as Yu-Cheng (Chang et al. 1980a,b, 1981;
Chen et al., 1980, 1981; Hsu et al., 1985). The affected persons had
consumed rice-bran oil contaminated with PCBs that was used as a heat
transfer medium in the manufacture of the oil. The PCB intake was
estimated to be 0.7-1.84 g and the latent period from the time of
intake to the onset of clinical manifestations was approximately 3-4
months. The average estimated PCDFs intake was 3.8 mg and 586 mg PCQs
(Chen et al., 1985a). Blood PCB levels ranged from 3 to 1156 µg/litre:
44.3% of 613 patients had levels of 51-100 µg/litre, and 27.6%, blood
levels over 100 µg/litre. Six months after the exposure, the
concentrations of PCBs, PCDFs, and PCQs were 12-50, 0.062-0.24, and
1.7-11 µg/litre. These blood levels were much higher than in the Yusho
incident.
The concentrations of PCBs, PCDFs, and PCQs in 6 samples of rice-bran
oil were 53-99 mg/kg, 0.18-0.40 mg/kg, and 25-53 mg/kg oil,
respectively (Chen et al., 1981; Hsu et al., 1985; Chen et al.,
1985a). Miyata et al. (1985) found averages of 62 mg PCB/kg, 140 µg
PCDFs/kg, and 20 mg PCQs/kg in 5 samples of oil. The levels of toxic
compounds in rice-oil samples collected from the factory and school
cafeterias and the families of the intoxicated patients in Taiwan were
in the range of 53-99 mg PCBs/litre (except for one sample with
405 mg/litre), 0.18-0.40 mg PCDFs/litre, and 25-53 mg PCQs/litre,
respectively. The most toxic PCB reported in commercial PCB
preparations was 3,4,3',4'-tetrachlorobiphenyl (Chen et al., 1984).
One hundred and thirty patients (46 males and 84 females), exposed
accidentally to PCBs in Taiwan, were examined for ocular
manifestations in 1979-80. Eye discharge was present in 80.5%,
swelling of the upper lids in 60.4%, pigmentation of conjunctiva in
67.6%, hypersecretion and cystic swelling of the Meibomian glands in
70.7%. Heavy pigmentation of conjunctiva, abnormal cystic formation
and hypersecretion of the Meibomian glands occurred in patients whose
blood PCBs concentration was above 40 µg/litre. There was a
correlation of the ocular effects and the blood concentration of PCBs
(Fu, 1984).
Wong et al. (1985), determined the enzyme activity in placental
tissue, obtained from 4 women who were exposed to contaminated
rice-oil in Yu-Cheng 3-4 years before conception. Placental
homogenates showed increases in monooxygenase enzymes, including aryl
hydrocarbon hydroxylase, 7-ethoxycoumarin O-deethylase, and diol,
quinone and phenolic metabolites of benzo (a)pyrene.
Lu & Wong (1984) described, in detail, the dermatological, medical,
and laboratory findings on 829 patients (half males and half females)
in Taiwan, poisoned with PCBs and related compounds. The ages of the
patients ranged from 7 days to 78 years. A grading of the clinical
severity of these cases was tried, and a possible association with the
PCB concentrations in their blood was examined, but could not be
demonstrated. The mean PCB concentration in 278 patients was 89.1 ±
0.9 µg/litre (median value 55 µg/litre); the maximum level was
1156 µg/litre and the minimum, 3 µg/litre.
One hundred and ten patients were studied within one year of the
exposure. The mean blood PCB level was 39.3 ± 16.6 µg/litre, and the
mean blood PCDFs and PCQs levels were 0.076 ± 0.038 and 8.6 ±
4.8 µg/litre, respectively. Both the sensory and motor nerve
conduction velocities of the patients were significantly lower than
those of the controls (cases studied in the past who did not have
neurological diseases) (Chen et al., 1985b).
Thirty-five patients out of 2000 cases of PCB poisoning in Taiwan were
examined neurologically 2 years after the accident. The neurological
manifestations included clinical, peripheral sensory neuropathy,
headache, and dizziness. There was no relationship between the blood
PCB concentrations in patients with neurological manifestations and
those without. Sensory nerve conduction velocity was reduced and motor
nerve conduction was delayed in about one-third to one-half of the
patients (Chia & Chu, 1984).
The blood samples of 165 patients, collected 9-18 months after the
onset of poisoning, contained 10-720 µg PCBs/litre with a mean value
of 38 µg/litre (Chen et al., 1984).
It is worth noting that the highly toxic 2,3,7,8-tetrachloro-,
2,3,4,7,8-pentachloro-, and 1,2,3,4,7,8-hexachlorodibenzofuran isomers
were present in samples from both the Japanese (Yusho) and the
Taiwanese (Yu-Cheng) incidents.
The most common symptoms noticed were acneiform eruptions and
follicular accentuation, skin and nail pigmentation, swelling of the
eyelids and increased discharge from the eyes; headache, nausea, and
numbness of the limbs. The major blood disorders were decreased
erythrocyte counts, haemoglobin concentration, and gamma-immunoglobin,
and increased white blood cell counts, serum triglyceride levels, and
SGOT, SGPT, and serum alkaline phosphatase activities. Decreased
concentrations of delta-aminolaevulinic acid and uroporphyrin were
also observed (Chang et al., 1980a,b).
9.1.2.2 Effects of PCBs on babies and infants
Yoshimura (1971) reported diminished growth in boys, but not in girls,
who had consumed the oil. Babies born to Yusho mothers were smaller
than normal. Newborn babies showed a dark brown skin pigmentation that
disappeared after a few months (Taki et al., 1969; Yagamuchi et al.,
1971). Funatsu et al. (1972) found spotted and sporadic ossification
of the skull and facial oedema with exophthalmia in 4 babies, but
there was no evidence of any teratogenic action. Pregnant Yusho
mothers delivered babies with a peculiar clinical manifestation, which
was called Fetal PCB Syndrome (FPS). In total, 36 babies showed this
syndrome. It consisted of dark brown pigmentation of the skin and
mucous membranes, gingival hyperplasia, exophthalmic oedematous eye,
dentition at birth, abnormal calcification of the skull (as
demonstrated by X-ray), rocker bottom heel, and a high incidence of
low birth weight babies. It was suggested by the authors that a
possible alteration in calcium metabolism in FPS might be related to
the action of PCBs (PCDFs) on female hormones. There was no evidence
of hypoadrenocorticism which would explain dark pigmentation in FPS
children (Yamashita & Hayashi, 1985).
Jensen (1983b) calculated that the daily intake of PCBs by Yusho
infants with clinical symptoms of poisoning was of the order of
70 µg/kg body weight.
Kuratsune et al. (1972) investigated whether Yusho disturbed
children's growth. The affected school children, 23 boys and 19 girls,
were compared in 1967, 1968, and 1969, with 719 healthy classmates
matched by sex. The gains of the affected boys in both height and
weight decreased significantly after the poisoning, while the affected
girls did not show any changes in these respects.
Studies were carried out by Fein et al. (1984), Fein (1984), and
Jacobson et al. (1984a,b) on 242 newborn infants whose mothers had
consumed moderate quantities of contaminated lake fish and 71 newborn
infants whose mothers had not eaten such fish, during the immediate
postpartum period. PCB exposure (measured by both contaminated fish
consumption and cord serum PCB levels) predicted lower birth weight,
shorter length of gestation, and smaller head circumference. Both
maternal consumption of fish and levels of PCBs in cord serum were
positively correlated with lower birth weight, shorter gestation and
smaller head circumference, and with impaired autonomic maturity and
increased numbers of abnormal reflexes.
In the studies by Schwartz et al. (1983), Fein et al. (1984), and
Jacobson et al. (1984a), the influence of important variables, such as
smoking and alcohol use, were not studied extensively enough. The
Brazelton test (Brazelton, 1973) was used in these studies. However,
this test was never intended to be used to evaluate neurological
conditions (Prechtl, 1982). The value of this test to predict
behavioural abnormalities in infants is small. The Public Health
Council of the Netherlands (1985) concluded, therefore, that the
reported changes could not be interpreted by the Brazelton test. The
important confounding factors "smoking" and "alcohol" were not studied
or not well studied, while it is known that these factors can result
in such changes. Furthermore, there was an indication that women
consuming more fish also consumed more alcohol and coffee and used
more medical drugs than those who were not fish eaters.
Jacobson et al. (1985) examined visual recognition memory in
7-month-old infants of women who had consumed contaminated Lake
Michigan fish. The authors reported a statistically significant
correlation between cord serum PCB levels and impairment of visual
recognition memory. It should be mentioned, however, that
interpretation of these test results is difficult. In view of the
variability associated with the measurements of fixation time (no
standard deviations were reported), it is unclear whether any of the
group means are statistically different. Moreover, the clinical
meaning of the differences noted is not known.
Neonatal effects of transplacental exposure to PCBs (and DDE) were
examined in a study on 912 children born between 1978 and 1982 in
North Carolina. When the infants were born, samples of placenta,
maternal and cord serum, and milk/colostrum were collected. Physical
examination of each infant was performed and the Brazelton test
(Brazelton, 1973) was applied. Fifty-nine per cent of the examinations
were carried out in the first week, 20% in the second week, and 16% in
the third week. The PCB levels in milk fat at birth (866 samples)
ranged from nd to 4.0 mg/kg. There was no association between PCB
levels and birth weight, head circumference, and hyperbilirubinaemia
(neonatal jaundice). For the Brazelton test, the only cluster scores
to be significantly affected by PCBs were the tonicity and reflex
scores, with higher PCB levels (above 3.5 mg/kg milk fat). The results
showed that higher PCB levels were associated with hypotonicity and
hyporeflexia while higher DDE levels (4 mg/kg milk fat) were
associated with hyporeflexia (DDE concentrations in milk fat ranged
from nd to >6 mg/kg milk fat) (Rogan et al., 1986a). As a follow-up
study, Rogan et al. (1987) followed 858 children in the USA, from
birth to one year of age, to determine whether the presence of PCBs in
breast milk affected their growth or health. The PCB concentrations in
breast milk ranged from 0.49 to 15.80 mg/kg, on a fat basis, and the
DDE concentrations from 0.31 to 23.8 mg/kg milk fat. The lactation
period varied from 13 (mothers with 4.00-15.80 mg PCBs/kg in their
milk) to 26 weeks. No adverse effects on body weight or the frequency
of visits for various illnesses were observed. There was no difference
between bottle-fed and breast-fed children. In 1985, about 6 years
after the mass poisoning in Taiwan, 117 children born to affected
women ( in utero exposure during, or after, the period of oil
contamination) and 108 unexposed controls were examined (Rogan et al.,
1988). The exposed children were shorter and lighter than the
controls; they had more frequent abnormalities of the gingiva, skin,
nails, teeth, and lungs than control children. The exposed children
showed delay in developmental milestones, deficits on formal
development testing, and abnormalities in behavioural assessment.
A follow-up study was carried out to determine the relationship
between PCBs in mother's serum and breast milk and the health and
development of the born infants, in Sheboygan, Wisconsin, USA, in the
period 1980-81. Seventy-three mothers gave birth to 62 infants that
were breast-fed and 11 that were bottle-fed. The ages of the mothers
ranged from 18 to 36 years. The mean serum PCB level for the study
population was 5.76 µg/litre (range, 1.29-14.9 µg/litre). Breast milk
contained a mean PCB level of 1.13 mg/kg (range, 0.29-4.02 mg/kg) on a
fat basis. The mother's blood serum PCB level during pregnancy was
positively associated with the number and type of infectious illnesses
the infants suffered later, such as colds, earache, and influenza
during the first 4 months of life. The development and growth of the
infant up to the age of 4 months was normal and was not affected by
PCB levels (Smith, 1984).
Lan et al. (1989) selected 18 exposed children (9 males and 9
females), and a reference group of 44 unexposed children (26 males and
18 females), to study the congenital absence of permanent teeth. Among
9 transplacental Yu-Cheng girls and 9 boys, the permanent teeth germ
was missing due to congenital factors in 4 girls and one boy. In the
control group, one boy showed this phenomenon. Fukuyama et al. cf. Lan
et al. (1989) had already reported this effect in 1979.
Gladen et al. (1988a) investigated whether PCBs, either transplacental
or through breast feeding, affected the scores on the Bayley Scales of
infant development at 6 or 12 months of age, in 802 infants. Higher
transplacental exposure to PCBs was associated with lower psychomotor
scores at both 6 and 12 months of age. Exposure to PCBs through
breast-feeding was apparently unrelated to Bayley scores.
The urine of 75 children born to mothers exposed to contaminated rice
oil in Taiwan (1979), 74 controls, and 12 siblings of the children
exposed between 1978 and 1985, was analysed for the presence of
porphyrins. Total porphyrin excretion was elevated in the exposed
children in comparison with the other 2 groups (exposed group,
95.2 µg/litre, control group, 80.7 µg/litre, and siblings,
72.6 µg/litre. The exposed children did not appear to have symptoms
directly attributable to their porphyria, but the authors concluded
that a mild disturbance in their porphyria metabolism appeared to be
related to their intrauterine exposure (Gladen et al. 1988b).
Thirty-nine babies showing hyperpigmentation were born to PCB-poisoned
mothers. In the orally exposed population of the Yu-Cheng episode, 24
deaths were reported and as many as 12 cases of hepatic disease
including hepatomas, which was more than expected (Hsu et al., 1985).
9.1.3 Appraisal
Several Japanese research groups have concluded that the main signs
and symptoms involved in the Yusho intoxications were caused by
contaminants in the PCB-mixture, i.e., mainly PCDFs (Masuda et al.,
1985; Kunita et al., 1985). This conclusion has mainly been based on
the following observations:
(a) Blood levels of PCBs in the victims were not very different from
those in the general population and several occupationally-exposed
groups had higher PCB blood levels in the absence of any recognizable
adverse health effects.
(b) There was an unusually high level of PCDF-contamination in the
PCBs that contaminated the rice oil.
(c) Signs and symptoms did agree with what could be expected from
exposure to PCBs and/or PCDFs (PCDDs).
However, blood levels in the Yusho victims were determined 5 years
after exposure. Consequently, at the time of the intoxication, blood
levels might have been much higher.
Furthermore, later studies on the biological potency of the
dioxin-like coplanar PCBs indicated that the occurrence of these might
have added significantly to the overall toxicity of the PCDFs (Safe,
1990).
In the case of the Yu-Cheng intoxication, blood levels of PCBs were
determined within 1 year of the accident and were found to be much
higher (i.e., about 70 µg/litre) than in the Yusho intoxication.
However, even at this time, some elimination of PCBs, especially lower
chlorinated PCB congeners, can be assumed to have taken place. Thus,
blood levels in the Yu-Cheng intoxication might also have been higher
initially.
In summary, it can be concluded that the main symptoms of the Yusho
and Yu-Cheng intoxications might have been caused mainly by combined
exposure to PCBs (mainly the coplanar ones) and PCDFs. However, some
of the symptoms, especially the chronic respiratory effects, may have
been caused specifically by the methylsulfone metabolites of certain
PCB congeners.
9.2 Occupational exposure
9.2.1 Acute toxicity -- poisoning incidents
9.2.1.1 Acute dermal effects
Skin rash has occurred within a few hours after acute exposure to
PCBs. Furthermore, itching, burning sensations, smarting, and sweating
have been reported. Irritation of the conjunctiva was a constant
symptom in acute exposure to high concentrations (Elo et al., 1985;
Schecter & Tiernan, 1985). A few weeks or months after acute exposure
(lasting a few hours) to high concentrations of PCBs (10-16 mg/m3),
several skin symptoms were observed in some of the accidentally
exposed population, such as slight pigmentation, ridges on the nails,
and the worsening of ache vulgaris (Elo et al., 1985). Skin wipe tests
on PCB-exposed workers were carded out by Maroni et al. (1981a), Smith
et al. (1982), and Wolff (1984) for capacitor manufacturers,
electrical equipment manufacturers, and transformer inspectors.
Concentrations varying between 2 and 28 000 µg/m2 of skin were
measured. Considerable concentrations of PCBs were also found on the
surfaces of hand tools in the factories (Maroni et al., 1981a;
WHO/EURO, 1987).
9.2.2 Effects of short- and long-term exposure
Exposure through ingestion is possible in the working environment
through the direct ingestion of soot particles or through the
contamination of cigarettes or food by hands. Maroni et al. (1981a)
measured high concentrations of PCBs in the palmar skin of capacitor
workers; this might lead to the oral ingestion of PCBs, in addition to
exposure via the dermal route.
Skin exposure is important in the case of long-term exposure, even
though the ambient concentrations may be low. According to
calculations made by Wolff (1985), in long-term exposure situations,
skin may be responsible for up to 20% of the total body uptake of PCBs
in workers exposed in capacitor manufacturing (WHO/EURO, 1987).
Symptoms similar to those of Yusho have been observed in workers in a
Japanese condenser factory, including pigmentation of the fingers and
nails, and acneiform eruptions on the jaw, hack, and thighs. It was
thought that these effects arose from local contact with PCBs; when
the use of PCBs ceased, the symptoms disappeared (Hasegawa et al.,
1972b). Chloracne is one of the most prevalent findings among
PCB-exposed workers and particularly among those exposed to highly
chlorinated compounds. Hara (1985) found prevalences of comedones and
acne of 40%, and skin irritation and erythema of 13% in workers
exposed for 1-24 years to Kanechlor 300 and 500. The blood PCB levels
were 21-117 µg/litre. The degree of skin pathology was correlated with
the blood PCB concentrations. In the production of capacitors,
(oculo-) dermatological abnormalities were found in 37% of the cases,
but typical PCB-associated changes were less prevalent. Fischbein et
al. (1979) suggested that these signs and symptoms were due to
exposure to PCDFs and/or PCDDs.
Fischbein et al. (1982) evaluated the dermatological effects of
long-term (< 5 - > 20 years) occupational exposure to PCBs, in a
cross-sectional clinical survey of 326 capacitor manufacturing
workers. Air PCB levels varied in the plant from 0.007 to 11.0 mg/m3.
A high prevalence (37%) of a wide spectrum of dermatological
abnormalities was found, such as rashes, burning sensation of the
skin, and chloracne, in most cases associated with typical comedones
(6%), but the occurrence was less than that observed in Yusho
patients, even though the serum PCB levels in the workers were much
higher.
Two persons were reported with dermatological abnormalities suggestive
of, but not specific for, chloracne, after occupational exposure to
PCBs (Fischbein & Wolff, 1987). They had raised serum PCB
concentrations (of the order of 80-100 µg/litre). Their wives also had
increased blood PCB levels, with the same PCB pattern as their
husbands. It was suggested by the authors that it would seem prudent
to take appropriate industrial hygiene measures, to prevent the
transmission of PCBs from the occupational environment into the home.
A cross-sectional study on 120 male workers was conducted to determine
the prevalence of increased PCB absorption, as well as the presence of
potentially-related clinical and metabolic abnormalities. Three groups
were used: an exposed group (86), a nominally exposed (15), and an
unexposed group (19 subjects). The exposed group had direct contact
with PCB-containing transformer fluids, while the nominally exposed
group worked in the same facility, but without direct contact, and the
unexposed group was employed elsewhere. The average length of
employment was 17 years (range, few months-40 years), 3 years and 9
months, and 4 years and 3 months, for the 3 groups, respectively. The
average plasma PCB levels were 33.4, 14.2, and 12.0 µg/litre, and the
average concentrations in adipose tissue were 5.6, 1.4, and 1.3 mg/kg,
respectively. There were no statistically significant differences
among the groups in levels of triglycerides, cholesterol, high-density
lipoproteins, and SGOT. A significant correlation was demonstrated
between plasma PCBs and triglycerides and SGOT values, but not SGPT
and gamma-GTP values (Chase et al., 1982).
To investigate the prevalence of oculo-dermatological findings, such
as hypersecretion of the Meibomian glands, swelling of the upper
eyelids, and hyperpigmentation of the conjunctiva (typical Yusho
symptoms), in a population with long-term occupational exposure to
PCBs, a group of 246 workers employed in 2 capacitor manufacturing
facilities were studied in 1976, and 181 of these workers, again in
1979. The median plasma values of lower chlorinated PCBs were
63 µg/litre in 1976 and 49 µg/litre in 1979. For the higher
chlorinated PCBs, these values were 18 and 17.5 µg/litre,
respectively. The prevalences of oculo-dermatological findings,
potentially related to the effects of PCBs, were 9.4 and 13.3%, at the
two examinations. There was no significant association between such
abnormalities and blood plasma/serum concentrations of PCBs (Fischbein
et al., 1985).
Lees et al. (1987) studied the hypothesis that the dermal route of PCB
exposure is a major contributor to the total body burden of PCBs in
workers. The investigation was conducted simultaneously with a
clinical study on switchgear workers engaged in transformer
maintenance and repair operations. The geometric means in the serum
and adipose tissue of exposed workers, previously exposed workers, and
comparison group were, respectively: (serum) 12.2, 5.9, and
4.6 µg/litre, (adipose tissue) 2.1, 0.83, and 0.6 mg/kg. The geometric
mean 8-h TWA concentrations in the different work areas (55 samples)
ranged from 0.5 to 6.1 µg/m3. The geometric mean surface
concentrations (102 samples) ranged from 0.007 to 1.075 µg/m2. From
the available data, it was calculated that exposure by the dermal
route (i.e., skin absorption) was considerable in comparison with
respiratory exposure. The daily calculated total dose through
inhalation ranged from 4.0 to 48.8 µg in the different work areas and
via the dermal route, from 1.2 to 215.0 µg. It was considered though
not conclusively, that the dermal and dermal/oral routes of exposure
are the predominant contributors to body burden.
Shalat et al. (1989) reported 3 cases of kidney adenocarcinomas among
young male utility workers who were responsible for maintaining
electrical transmission equipment, including transformers. The
duration of exposure ranged from 5 to 35 years.
The occurrence of chloracne and abnormal hepatic function as a result
of occupational exposure to PCBs had already been reported by Jones &
Alden (1936), Schwartz (1943), and Meigs et al. (1954).
Effects, such as chloracne, skin rashes, and burning eyes and skin,
have been associated with occupational exposure to Aroclors and
Kanechlors (Ouw et al., 1976; NIOSH, 1977; Fischbein et al., 1979,
1982, 1985; Baker et al., 1980; Drill et al., 1981; Kimbrough, 1987;
US EPA, 1987). In these studies, monitoring data did not adequately
characterize exposure levels, consequently correlations between the
occurrence of skin lesions and the duration of exposure, or blood
concentrations of the PCBs, are poor or nonexistent. Furthermore, the
contamination of the PCBs with PCDFs and PCQs may be partly the cause
of these skin changes.
Other effects reported in human exposure have been considered in a
criteria document for recommended standards for occupational exposure
to PCBs (NIOSH, 1977) and include several instances of chloracne that
resulted from exposure to PCB vapours in various work situations.
Other symptoms noted were sore throat, gastrointestinal disorders, and
eye disturbances.
Fischbein et al. (1979) examined 168 male and 158 female workers at a
capacitor plant, where they were exposed for <5 up to 25 years to
Aroclors 1254, 1242, 1016, and 1221. TWAs for 8 h with ranges of
0.07-0.40, 0.40-0.60, and 0.60-11.0 mg/m3 were considered low,
medium, and high, respectively. Among work-related symptoms they found
upper respiratory irritation and decreased rectal capacity,
gastrointestinal, neurological, and dermatological symptoms. The
dermatological symptoms occurred among 45% of the males and 55% of the
females and are comparable with the symptoms found in Yusho victims.
There was a significant correlation between plasma PCB levels and SGOT
levels, though changes in most liver tests were not prevalent.
Maroni et al. (1981b) reported blood PCB concentrations of
41-1319 µg/litre in 80 electrical workers (half males, half females)
employed in electric capacitor manufacture and testing plants.
Sixty-seven persons were exposed to Pyralone 3010 and 13, to Apirolio
(both PCBs containing 42% of chlorine). The mean age of the workers
was 37 ± 8 years, and the mean duration of employment was 12 ± 6
years. There were 6 cases of chloracne among the 80 workers. Sixteen
of the males had liver abnormalities, including hepatomegaly and
increased serum enzymatic activities; for 20% of these, the PCB
concentration in the blood was < 200 µg/litre. The females included 2
cases of bleeding haemangioma, one of whom also had chronic myelocytic
leukaemia, but none of the females had liver abnormalities.
Ouw et al. (1976) studied 34 electrical industry workers exposed to
0.32-2.22 mg Aroclor 1242 (free from impurities)/m3 compared with 30
control workers. The electrical workers consisted of 15 males (6
months-23 years employment) and 19 females (1 month-7 years
employment). No clear indications of liver changes were found. Major
complaints were burning of the eyes, face, and skin. One worker had
chloracne without systemic effects and 5 workers had eczematous hand
and leg rashes. These dermatological effects occurred at an air
concentration of <1 mg PCBs/m3. There were no significant health
effects in workers at, or below, a blood PCB level of 200 µg/litre.
Drill et al. (1981) concluded that individuals with blood levels of
>200 µg/litre have an increased risk of chloracne and that
chloracne may occur more frequently in workers exposed to PCBs that
have been heated (presence of PCDFs) and to PCBs that have a >54%
of chlorine.
A study was conducted to determine whether exposure to fumes or oil at
the transformer incident site at New Mexico had caused illness.
Exposed persons of different disciplines and unexposed employees were
asked to complete a questionnaire. Eighty of the 101 persons with
known exposure completed the questionnaire. The most common symptoms
were: nausea (27.5%), eye irritation (22.5%), sore throat (21.2%),
nose irritation (18.8%), chest tightness (15.0%), and headache
(15.0%). The symptoms were transient and usually resolved as soon as
the person left the site. Fifty-six exposed persons submitted sera for
PCB analysis as did 20 controls. All but 4 persons had levels below
10 µg/litre. The medium for exposed persons was 4.1 µg/litre (range,
1.2-41.8 µg/litre compared with 2.4 µg/litre (range 0.9-8.0 µg/litre)
for the controls (Anon., 1985).
A follow-up study on capacitor-manufacturing workers, exposed to PCBs,
and their children, was conducted over the period 1973-79. The PCBs
that were used were Kanechlor 300 and 500. PCB levels in the blood (up
to 120 µg/litre), as well as in breast milk, were 10-100 times higher
than those of non-exposed Japanese persons. The levels in 8 women
ranged from less than 50 µg/litre to about 400 µg/litre in whole milk.
Blood PCB levels were correlated with the duration of PCB handling and
breast milk PCB levels. The blood PCB levels ranged from 18.7 to
117 µg/litre in this population, 1 year after the use of PCBs was
discontinued. The rate of decline of blood PCB levels, as well as the
changes in the gas chromatography of blood PCBs over 7 years varied
with the kind of PCB handled. The blood PCB levels tended to be higher
(<3 up to >10 µg/litre) in children fed PCB-contaminated breast milk
for a long period. The great majority of the workers had
dermatological complaints, but these symptoms gradually disappeared
with discontinuation of contact with PCBs. The blood chemistry of the
workers showed only a correlation between PCB blood levels and serum
triglycerides. Several of the children fed breast milk for a long
period, showed the same medical findings as in Yusho (itchy skin,
eczema, red eye, fever, catching cold, carious teeth). However, they
were not diagnosed as suffering from PCB-poisoning, because the
findings were neither serious nor related to the blood PCB levels
(Hara, 1985).
Workers occupationally exposed to Kanechlor 500 or 600 showed higher
PCB levels in plasma (2-251 µg/litre) than Japanese Yusho patients.
Gas chromatographic patterns of their PCBs corresponded to the
patterns of PCBs to which they were exposed, but, with time, the PCB
pattern in plasma is changing. PCQs could not be detected in the
plasma of the workers (Takamatsu et al., 1985).
Lawton et al. (1985) studied a group of 194 workers in capacitor
manufacturing, exposed to Aroclor 1016, 1242 and/or 1254 before (1976)
and after (1979). The use of PCBs in the operations was discontinued
in 1977. The geometric mean serum levels and 5-95% ranges were: lower
chlorinated PCBs, 363 µg/litre (57-2270 µg/litre) and 68 µg/litre
(12-392 µg/litre), higher chlorinated PCBs, 30 µg/litre
(6-142 µg/litre) and 19 µg/litre (4-108 µg/litre), respectively. The
statistical findings were a depression in serum bilirubin and
elevations in serum gamma-glutamyltranspeptidase (GGTP) and lymphocyte
levels, at the time of the first examination, and only an elevation of
monocytes at the second.
In 1982, a survey was conducted in an electrical capacitor factory in
the USA, using Aroclor 1242 from 1941 to 1971, with a change to
Aroclor 1016 from 1971 to 1977. Of approximately 500 current employees
(with an average of 12.9 years of employment) 205 took part in the
survey. The geometrical mean PCB value for serum was 18.2 µg/litre
(range, nd-424 µg/litre). Only 39% of the workers ever worked in areas
with potential PCB exposure. More than 70% had serum levels below
30 µg/litre. There were no indications of acute PCB-related clinical
effects. The workers' serum PCB levels were a function of duration of
employment, cumulative occupational exposure, cumulative fish
consumption, and cholesterol level (Acquavella et al., 1986).
The blood of women occupationally exposed to PCBs (Kanechlor 300 and
500) was analysed over the period 1975-79. Sixty-five samples were
taken from 29 mothers. The PCB concentrations varied between 6.4 and
52.6 µg/kg (mean concentration 32.3 µg/kg). Clinical symptoms observed
during the use of PCBs were minor in comparison with those of Yusho
patients (Yakushiji et al., 1984b).
Guo et al. (1987) studied the influence of serum cholesterol and
albumin on the partitioning of PCB congeners between human serum and
adipose tissue. Fifty-five repair workers, who were either currently
or had been previously exposed, and 56 comparison workers without
exposure to PCBs were used. Seven PCB congeners, which had been
quantified in both serum and adipose tissue in at least one-third of
the selected populations, were evaluated. The effects of serum
cholesterol in modifying the serum PCB concentrations are likely to be
apparent in groups exposed to PCBs containing higher chlorinated
congeners, such as Aroclors 1254 and 1260, rather than those
containing lower chlorinated congeners, such as Aroclors 1242 and
1221.
A selected group of 51 workers (25 males and 26 females) with a mean
length of exposure of 10 years (range 1-30 years) were compared with 2
groups consisting of 74 subjects (37 males and 37 females) and the
same reference group of 67 workers (30 males and 37 females) used in
another study, residing in the same areas, but without exposure to
PCBs. The PCB concentrations in the blood of 28 out of the 51 subjects
ranged from 88 to 1359 µg/litre. A statistically significant increase
was found in serum GGT activity, urinary D-glucaric acid (GLA), and
porphyrin excretion, when compared with the respective control groups.
Although the PCB workers had an average urinary excretion of
porphyrins almost twice as high as those of the control groups, no
dose-response relationship was found between urinary porphyrin
excretion and blood PCB concentrations (Maroni et al., 1984).
Steele et al. (1986) found a gradual decrease in the body burden of
the more highly chlorinated PCBs, as well as a more rapid decrease of
the less chlorinated congeners over the period 1977-84 in groups of 5
current and retired workers, in comparison with 6 subjects without
current exposure. The authors calculated the half-life for the lower
chlorinated PCBs to be 6-7 months, and, for the higher chlorinated
PCBs, 33-34 months. The evidence of different half-life estimates for
serum PCBs, depending on the degree of chlorination, is consistent
with the present knowledge of the pharmacokinetics.
Hola & Reznicek (1985) carried out a cytogenetic analysis of the
peripheral lymphocytes of 48 employees at a precoated gravel plant
using Delor 103 (a mixture of mainly trichlorobiphenyls) in comparison
with 24 workers not exposed and 13 workers exposed to PCBs during the
impregnation of condensers. The frequency of aberrant cells,
percentage of blastic transformation, mitotic index, and proliferation
index in peripheral lymphocytes, and a number of biochemical
parameters were determined. In the 48 workers at the precoated gravel
plant, there were 2.87% aberrations (PCB concentrations in plasma,
107 ± 104 µg/litre); 3.14% in the 13 PCB-exposed workers (PCB
concentration in plasma 308 ± 253 µg/litre), and 2.04% in 24
non-exposed workers; 1.50% of aberrations were found in 20 controls.
Emmett (1985) studied a total of 55 workers (currently exposed (38)
and 17 past transformer repair workers) in comparison with 56
unexposed workers. PCB exposures occurred from the air and
contaminated surfaces, predominantly to Aroclor 1260, but there was
some exposure to Aroclor 1242. There was widespread PCB contamination
of the workplace surfaces and the 8-h time-weighted average
concentration was between 0.7 and 24.0 µg/m3, depending on the task
of the worker. The bulk oils and air from the transformer were
analysed revealing that PCDFs (13-116 µg/kg) were present as well as
PCBs. In one of the samples, 2,3,7,8-TCDF was found at 31 µg/kg. Eye
irritation and tearing were more prevalent in the exposed group, but
the symptoms were mild and/or transient. Ocular symptoms were also
found, possibly caused by 1,1,1-trichloroethane and/or
trichlorobenzene. Chloracne was not found. Two exposed workers
reported a history of melanoma; none were reported in the control
group. However, the difference was not statistically significant.
Adipose tissue and serum PCB geometrical mean concentrations in
exposed workers were 2.1 mg and 12.2 µg/kg, respectively, those in
unexposed workers were 0.6 mg and 4.6 µg/kg, and those in previously
exposed workers, 0.83 mg and 5.9 µg/kg. No correlations were observed
between liver function tests and either adipose tissue or serum PCB
concentrations. A significant negative correlation was found, after
adjustment for confounding variables, between adipose tissue PCB
levels and 24-h urinary 17-hydroxycorticosteroid excretion and a
positive correlation between serum PCB levels and serum-GGT. No
correlation was found between adipose tissue PCB concentrations and
any serum lipid component (Emmett et al., 1988a,b).
Bercovici et al. (1983) collected blood from 17 women with recent
missed abortions, 7 women who had experienced one or more missed
abortions in the past, and 7 women with normal, second trimester
pregnancies, and estimated the serum levels of PCBs and other
organochlorine pesticides. The range of serum PCB levels in recent
missed abortions was 10.9-416.5 µg/litre, and that of the control
group, 12.2-40.0 µg/litre. Forty-seven per cent of the recent missed
abortion group had PCB levels of the same magnitude as the controls
(range, 10.90-42.8 µg/litre) and 53% had higher levels. In the former
missed abortion group, PCB levels in the range of 45.3-109.1 µg/litre
were found. The number of women examined in the different groups was
small. Furthermore, half of the women with missed abortions had PCB
levels in serum comparable with those of the controls. The authors
also did not control for many variables that could significantly
influence the incidence of abortions. It seems, therefore, that missed
abortions, either recent or in the past, may be associated with PCB
exposure.
PCB concentrations were determined in the blood from 10 women with
normal pregnancies (controls) and from 17 women with premature
deliveries. A significant difference was seen in blood serum
concentrations of PCBs between the women with normal and those with
premature deliveries. When the premature delivery group was split into
a high-serum- (8/17) and a low-serum-PCB group (9/17), the
high-serum-PCB group had a significantly higher serum PCB
concentration than the control group. The values were 128 mg/litre in
the high group, 19.3 µg/litre in the control group, and 21.4 µg/litre
in the low group. In the high-PCB, premature delivery group, the mean
serum concentration of tetrachloro-isomers was lower than that of the
control group (0.6 vs 1.86 µg/litre), while the mean serum
concentrations of pentachloro and hexachloro-isomers were higher
(78.2 vs 15.67 µg/litre and 48.9 vs 1.72 µg/litre, respectively)
(Wassermann et al., 1982). The indications that higher serum PCB
levels may be associated with an increase in incomplete abortions and
premature deliveries (Bercovici et al., 1983; Wassermann et al., 1982)
could not be established as a definitive causal relationship.
Taylor et al. (1984) studied the relation of Aroclor 1254, 1242,
and/or 1016 exposure to birth weight and gestational age in the
offspring of women working in 2 capacitor plants. Fifty-one infants,
born to 39 women employed at the 2 capacitor manufacturing facilities,
had a mean birth weight of 153 g less than 337 infants born to 280
women employed in areas of the facilities without direct exposure.
Mean gestational age in the first group was reduced by 6.6 days
compared with the latter group. It was concluded that the small
decrease in mean birth weight seemed likely to have resulted from a
shortening of the gestation period rather than a retardation of
intrauterine growth. Smoking and alcohol consumption by the mothers
were not considered, and whether the socio-economic status of this
group of women was similar to that of the control group is not clear.
In an update of this study, 200 women with direct exposure and 205
women without direct exposure, were used to study the relation of PCBs
to birth weight and gestational age in the offspring. The authors
concluded that these data indicated that there was a significant
relationship between an increased serum PCB level and decreased birth
weight and gestational age, and that the decrease in birth weight was,
at least partially, related to shortened gestational age (Taylor et
al., 1989).
9.2.3 Appraisal
A discussion on the occupational epidemiological data on the basis of
dose-response considerations needs an acceptable indicator of the
degree of exposure and, in the particular case of PCB mixtures, a
discussion on the nature of the congeners present. For most
epidemiological studies reported, there are some, albeit often
limited, data on levels of total PCBs in blood, which could be used as
indicators for PCB exposure. It is recognized that the analytical
procedures used are different. The blood of continuously exposed
workers will contain absolutely and relatively more of the lower
chlorinated congeners than that of human beings with background
exposure only, or with past exposures (e.g., Yusho and Yu-Cheng
populations). Consequently, the toxicological profile for these 2
types of exposure will differ (see also the appraisal on
Yusho/Yu-Cheng).
In some cases, the epidemiology of the continuously exposed workers
shows a possible association between elevated exposure to PCB mixtures
and the occurrence of liver enzyme alterations, hepatomegaly, and
dermatological abnormalities, such as rashes and acne. In many
studies, no associations were found. In some of these studies, limited
end-points were investigated. Within the positive studies, there is a
poor or non-existent correlation between the incidence and degree of
effects and blood concentrations. Among the positive studies, adverse
effects are predominantly reported in the studies with the higher
blood levels. Possible contamination of used PCBs and PCB mixtures
(particularly in transformers), with PCDFs and PCQs, may contribute
to, or even determine, the toxicity observed. The overall conclusion
is that continuous exposure to high concentrations of PCBs and PCDFs
may result in effects on the skin and liver.
9.2.4 Special studies (target organ effects)
9.2.4.1 Liver
The liver is considered to be one of the most important target organs
for PCB toxicity. Acute exposures to PCBs cause alterations in liver
enzyme activities. Smith et al. (1982) found a statistically
significant correlation between elevated liver enzyme activities and
blood PCB concentrations, in workers exposed to PCBs in electrical
equipment manufacturing or maintenance. A negative correlation was
found in relation to the HDL cholesterol concentration and serum PCBs.
Positive correlations were found between the liver enzyme activities
of serum alanine aminotransferase (S-ALAT), serum aspartate
aminotransferase (S-ASAT), serum gamma-glutamyltranspeptidase
(S-Gamma-GT) and SGOT, and blood PCB concentrations, among workers
exposed in an Italian capacitor factory. Hepatomegaly was also
detected in most of the cases studied by Maroni et al. (1981a,b).
Workers occupationally exposed to PCBs, but mostly also exposed to
PCDDs and/or PCDFs, showed significantly increased S-ASAT, S-ALAT, and
gamma-GT activities. Sometimes elevated serum triglyceride values were
also found. Recovery of these disturbances in liver function and
morphology requires several weeks or months (Elo et al., 1985;
Schecter et al., 1985). Fischbein (1985) reported the results of liver
function tests in a population engaged in the manufacture of
capacitors and transformers. A low prevalence of abnormal liver
function tests was found and mean values for all tests were within
normal ranges in 5 workers occupationally exposed to Aroclor 1016.
Plasma antipyrine half-life was significantly lower than in matched
controls, suggesting induction of hepatic mixed-function oxidases
(Alvares et al., 1977). At the initial examination (in 1976, when PCBs
were still being used), statistically significant correlations were
found between log LDH and plasma levels of log HPCB (higher PCB
congeners) and log TPCB (total PCBs) among female workers, while
log-gamma-GTP was significantly correlated only with log HPCB among
male workers. A significant increase to abnormal levels of gamma-GTP
was noted at the follow-up examination (1979, 2.5 years after the use
of PCBs had been discontinued) in both male and female workers, and
preliminary results indicated significant correlations between
gamma-GTP and serum levels of PCBs among male workers. In
occupationally exposed individuals, the serum or plasma PCB levels
were higher than those found in patients in the Yusho and Yu-Cheng
incidents. An effect transmitted via liver activity was hyperlipaemia,
in which triglycerides and, in some instances, also cholesterol levels
in the blood were elevated (Smith et al., 1982).
Hepatotoxicity is suggested in occupationally-exposed humans (Drill et
al., 1981; US EPA, 1987). Drill et al. (1981) concluded that SGOT
and/or GGPT appear to be the most sensitive indicators of PCB exposure
in humans, and that changes in liver enzymes occur at levels below
those at which chloracne occurs.
9.2.4.2 Immunotoxicity
Reports on the immunological effects of long-term, occupational
exposures are sparse. Fischbein et al. (1979) found a suggestive
increase in the occurrence of trivial infections in exposed workers.
Immunological responses were found to be affected in Yu-Cheng victims
(Lu & Wu, 1985) and in Yusho patients (Nakanishi et al., 1985). Acute
accidental exposures of workers to PCBs and PCDFs have been studied
for immunological responses and immunosuppressive changes have been
found. The most important alterations were decreased numbers of
T-lymphocytes and lowered T-helper/ T-suppressor cell ratios.
Responses of lymphocytes to phytohaemagglutinin, concavalin A, and
pokeweed mitogen were also lowered. The observed changes persisted for
6 months after the acute exposure. No quantitative changes were
observed for immunoglobulins (Elo et al., 1985; WHO/EURO, 1987, 1988).
Lu & Wu (1985) found that PCB patients suffered from various kinds of
infections. Most frequent were those of the respiratory tract and
skin, including pyoderma, tinea versicolor, dermatophytosis and warts.
The low resistance of the patients suggested some degree of
immunosuppression. The function of the immune system was tested in 143
patients. Examination during the first year revealed: decreased
concentrations of IgM and IgA, but not of IgG; decreased percentages
of total T-cells, active T-cells, and helper T-cells, but normal
percentages of B-cells and suppressor T-cells; suppression of delayed
type response to recalling antigens; enhancement of lymphocyte
spontaneous proliferation; and enhancement of lymphocyte proliferation
with phytohaemagglutinin, pokeweed mitogen, and tuberculin
stimulation, but not with concanavalin A. After 3 years, the positive
rate of the tuberculin test recovered somewhat with time. The total
numbers of T-cells and B-cells were normal, the number of suppressor
T-cells (OKT-8) increased, but the number of helper T-cells (OKT 4)
was still lower, so the immuno-regulating index (OKT 4/OKT8) was still
very low. The lymphocyte proliferation stimulated by various mitogens
was also enhanced.
In patients with PCB poisoning, IgA and IgM levels in serum apparently
decreased for 2 years after the onset of the disease, but returned to
normal in most cases, in spite of the persistence of the respiratory
symptoms (Shigematsu et al., 1978, see section 9.2.4.3).
9.2.4.3 Respiratory system
In long-term exposure, up to 0.3-10 mg of PCBs may be inhaled in an
8-h working day. The respiratory tract is certainly the most important
route of exposure in the case of acute emergency situations, where
unprotected personnel working in areas containing such PCB
concentrations may, in theory, inhale a total dose of up to 10 mg/day.
This may imply a considerable cumulation of PCBs during long-term
exposure (WHO/EURO, 1987, 1988).
Transient irritation of the mucous membranes of the respiratory tract
has been reported in emergency situations, as well as difficulty in
breathing at high concentrations. It has not been confirmed that
short-term exposure causes other important respiratory effects, though
increased susceptibility and a high risk of contracting chronic
bronchitis have been suggested (Kimbrough, 1980; Elo et al., 1985;
WHO/EURO, 1987).
In the case of unheated commercial PCBs, the amount of PCDFs inhaled
might be very low, if any at all. The situation is totally different
in the case of acute exposures to heated or decomposed PCBs, in which
the inhaled total concentrations might be several orders of magnitude
higher than above, though the irritative effect may prevent breathing
in such contaminated rooms. Since the soot often contains a
considerable fraction of particles, a few microns in size, it is
partly breathed in and, thus, may lead to alveolar retention of both
soot and adsorbed chemicals. Carbon particles can accumulate in the
lung tissues and regional lymph nodes. Inhalation of soot particles
containing high concentrations of both PCBs and PCDFs would, in
practice, be the most important mechanism of exposure (Parkes, 1982;
WHO/EURO, 1987).
Warshaw et al. (1979) and Smith et al. (1982) found a correlation
between serum PCB concentration and respiratory tract symptoms among
workers exposed long-term to PCBs. An increase in the occurrence of
chronic bronchitis was possibly due to a decrease in the immunological
defence mechanism. No increase in mortality from respiratory system
diseases was found. An indication for acute and chronic irritation of
the respiratory tract was found by Brown & Jones (1981).
Shigematsu et al. (1978) studied the clinical, laboratory, and
pathological findings on respiratory involvement in PCB poisoning in
401 patients. Respiratory symptoms included expectoration in 40% of
the 289 non-smoking patients with PCB poisoning and mild wheezing in
2%. The incidence and severity of the symptoms was well correlated
with the concentrations of PCBs in blood and sputa. The clinical
examinations revealed bronchiolitis and pneumonia or atelectasis in
about one-tenth of the patients with reticulo-linear shadows. The PCB
concentrations in the blood and sputa were 27 and 8 µg/litre,
respectively. The presence of PCBs in sputum may have been associated
with the excretion from bronchial cells and/or with lipid II cells of
the lung, phagocytosed in alveolar macrophages and expectorated.
9.2.4.4 Neurotoxicity
Acute and long-term exposures to PCBs have been reported to cause
neurological and unspecific psychological or psychosomatic effects,
such as headache, dizziness, nausea, depression, sleep and memory
disturbances, nervousness, fatigue, and impotence (Smith et al., 1982;
Elo et al., 1985; Hara, 1985; Schecter et al., 1985; Takamatsu et al.,
1985; WHO/EURO, 1987).
Fischbein et al. (1979) reported the occurrence of these symptoms in
39% of male and 58% of female capacitor-manufacturing workers, exposed
to PCBs for long periods (over 5 years). To what extent these symptoms
were the direct consequences of exposure to PCBs and related compounds
and how much they were dependent on general conditions in an emergency
situation remains unclear.
Seppalainen et al. (1985) examined 16 men who were exposed to fumes
resulting from the explosion of capacitors containing Clophen A30. Air
concentrations of PCBs, measured 5.5 h after the explosion, were
8-16 mg/m3 air (PCDFs and other compounds, such as monochloropyrenes
and dichloropyrenes, were also formed). Most of the men had a
transient sensory neuropathy in their lower extremities (WHO/EURO,
1987, 1988).
9.2.4.5 Blood pressure
Kreiss et al. (1981) examined 458 volunteers (>12 years of age) from
Triana (Alabama) and correlated serum PCB levels (Aroclor 1260) with
blood pressure. This population was excessively exposed to DDT
residues through the consumption of contaminated fish. The residents
of this small rural town also had elevated PCB body burdens that were
positively correlated with fish consumption. The mean serum PCB level
was 17.2 µg/litre. The incidence of borderline (systolic of 140-159 mm
Hg and diastolic of 90-94 mm Hg) and definite hypertension (systolic
of >160 mm Hg and diastolic of >95 mm Hg) was 30% more than
would be expected for a general population of the same age, race, and
sex composition. However, this study did not have a control group in
its design, and there were more confounding factors that make the
study inadequate to conclude an association between blood PCB levels
and hypertension.
A study was carried out to test the association of serum PCB levels
and elevated blood pressure in 840 residents of New Bedford, Acushnet,
Dartmouth and Fairhaven, Canada, in the period 1984-87. The mean PCB
levels (as Aroclor 1254) were 5.9 and 5.8 µg/litre in 391 males and
449 females, respectively. The range in serum PCB levels for the total
group was 0.38-154.2 µg/litre. There was a relationship of serum PCB
level to age among the 840 individuals. In the 5 age groups: 18-24,
25-34, 35-44, 45-54, and 55-64 years, the mean serum PCB levels were
2.59, 3.84, 5.30, 8.18, and 8.96 µg/litre, respectively. Blood
pressure levels did not appear to be correlated with serum PCB levels.
The mean systolic readings, taken at 3 different times, for the 840
individuals were 115.26 ± 18.85, 113.69 ± 17.62, and 114.28 ± 11.41.
The diastolic readings were 72.19 ± 10.94, 73.19 ± 10.95, and 73.17 ±
19.94. There was no between the sexes difference (Massachusetts Dept
Public Health, 1987).
Akagi & Okumura (1985) studied the correlation of blood PCBs levels or
PCB patterns and blood pressure in 59 Yusho patients (more than 40
years old). In spite of the passage of 13 years from the onset of the
disease, 52.5% of the patients still had PCB levels higher than those
of the general population. The frequency of hypertension in these
patients was 16.9%, a value similar to that found in the general
population of the same age and sex. Blood pressure was not associated
with blood PCB levels or PCB pattern, but was associated with the well
known factors influencing blood pressure, such as age, obesity, and
habitual alcohol intake.
9.2.5 Mortality studies
Davidorf & Knupp (1979) conducted an epidemiological study on ocular
melanoma incidence in Ohio from 1967 to 1977, attempting to associate
Ohio counties with known high concentrations of PCBs and those with
industries that might use PCBs with an increased incidence of ocular
melanoma. The authors concluded that there was no causal relationship
between PCB exposure and an increased annual occurrence of ocular
melanoma in Ohio counties in the period 1967-77. Bahn et al. (1976)
reported on 31 research and development employees subjected to "heavy"
Aroclor exposure (quantity not reported) in a US petrochemical plant.
Two had malignant melanomas, and according to the standard of the
Third National Cancer Survey, incidence rates of only 0.04 would be
expected among 31 persons (NCI, 1975). The retrospective cohort
mortality studies of Brown & Jones (1981) and Brown (1987) reported
data on PCB-exposed individuals who had worked in 2 electrical
capacitor plants, one in New York, and the other in Massachusetts.
Both plants had produced this type of capacitor for more than 30
years. The PCBs used were Aroclor 1254, Aroclor 1242, and Aroclor
1916. A combined total of 2588 exposed workers from both factories,
with 3 or more months of exposure, were studied. The overall mortality
(295 deaths) was lower than expected (318) and the mortality for
cancer deaths (62 observed) was also lower than expected (80). A
statistically significant excess in deaths was observed in the disease
category that includes cancer of the liver (primary and unspecified),
gall bladder, and biliary tract (5 observed vs 1.9 expected). Most of
the excess was observed in women employed in one plant. According to
the authors, because of the small number of deaths and the variability
of specific causes of death within this category, it remains difficult
to interpret these findings with regard to PCB exposure.
At the first plant, there were 2 different facilities, a power
capacitor manufacturing facility, and a small capacitor manufacturing
facility. At the power capacitor facility, the TWAs for personal air
samples were between 24 and 393 µg/m3 for various jobs: in the
winding work area and soldering work area, they were as low as
3 µg/m3 and as high as 476 µg/m3, respectively. At the second plant,
where a few cases of rectal and liver cancer were found (see above)
the PCB levels were much higher. Degreasers and solderers, for
example, had TWAs for personal air of 1.260 and 1.060 µg/m3,
respectively, and heat soak operators and tankers had TWAs of 630 and
850 µg/m3, respectively. The work area air samples contained levels
as high as a TWA of 810 µg/m3. The duration of exposure, without
information concerning the level of exposure, when the levels varied
so widely, weakens the statement about lack of correlation between
duration of exposure and cancer mortality. In fact, the observed
cancer cases for both the liver and rectum were markedly increased in
the factory that had, at the time of measurement, the higher PCB
levels. Notwithstanding the absence of detailed information, the data
presented are suggestive of a dose-related increased incidence of
mortality from rectal cancer and possibly liver cancer.
Although there was no correlation between the latent period and cancer
mortality or between the duration of employment and cancer mortality,
most of the cancers occurred in the second plant, which had the higher
levels of PCBs at the time that the measurements were made. PCB levels
were monitored in 1977 for personal air and work area air. Since
procedures and processes were somewhat different during the years in
which most of the workers were exposed, the figures on PCB levels do
not necessarily indicate the exposure levels of the subjects.
Nevertheless, the figures given do indicate the wide variation in PCB
levels in air, with a 15-fold difference between the lowest and
highest levels among the different jobs. Although the different levels
of dust and particulate matter are not known, it would be anticipated
that an equally wide variation would exist.
A medical surveillance programme has been established for 482 persons,
who were potentially exposed to PCBs, PCDFs, and PCDDs from an
electrical transformer fire in Binghamton in 1981. Mean serum PCB
concentrations (98% of the samples) were below 20 µg/litre, a value
typical of a population with no unusual exposure. Mortality,
symptomatology, cancer incidence, and reproduction events were
assessed through 1984. The numbers of deaths, cases of cancer, fetal
deaths, and infants with low birth weight or congenital malformations,
were similar to those expected on the basis of age and sex-specific
rates for upstate New York and other comparison populations. One-third
of the fire-fighters and a number of persons, who were in the building
during the first 24 h (or longer) reported a rash or itching skin, but
no chloracne (Fitzgerald et al., 1989).
Bertazzi et al. (1981) reported the results of a mortality study on
PCB-exposed workers, who were employed in the manufacture of
electrical capacitors in an industrial area near Milan. The PCBs used
over the period from 1946 to 1970 were Aroclor 1254, Pyralene 1476
(54% chlorine), and Pyralene 3010 and 3011 (42% chlorine). In 1954, a
few measurements in the air were performed and the values of Aroclor
1254 were 5200-6800 µg/m3. In 1977, airborne concentrations of
Pyralene 3010 ranged from 48 to 275 µg/m3. The minimum and maximum
values of PCBs recovered from workplace surfaces and worker's hands
were, 0.2-159 and 0.3-9.2 µg/cm2, in 1977, and, in 1982 (2 years
after the ban on production), 0.003-6.3 and 0.09-1.5 µg/cm2,
respectively. The mortality study spanned the 25-year period from 1954
to 1978. The control mortality rates were for subjects from the city
in which the plant was located. There were 1310 workers (1020 females
and 290 males) and the vital status was obtained for 98% of both
sexes.
The study was enlarged and extended to include 2100 workers and to
cover the period 1946-82. Vital status was ascertained for over 99% of
the subjects and death certificates were obtained for all deceased
persons. Expected deaths were calculated using 2 sets of mortality
rates, national and local. Among male workers, cancer deaths (14
observed vs 7.6 expected) were significantly increased as were deaths
owing to cancer of the gastrointestinal tract (6 observed vs 2.2
expected). Also, mortality from haematological neoplasms (3 observed),
and lung cancer (3 observed) was higher than expected. However, the
excess was not statistically significant. Female workers exhibited an
overall mortality that was significantly increased above expectations.
Cancer deaths (12 observed vs 5.3 expected) and haematological
neoplasms (4 observed vs 1.1 expected) were significantly higher than
expected when compared with the local population. Interpretation of
the results is limited by the small number of deaths: however, it is
of interest that the gastrointestinal tract and the lymphatic and
haematopoietic tissue seem to be the most probable human target sites
for PCB carcinogenic activity (Bertazzi et al., 1987).
A cohort study on 142 male Swedish capacitor-manufacturing workers was
performed between 1960 and 1978. The PCB was 42% chlorinated and
contained different PCDFs totalling about 1400 µg/kg. The mean
exposure time of the workers was 6.5 years. In 1973, 0.1 mg PCBs/m3
was found in the air. Mortality was investigated for the period
1965-82 and cancer incidence from 1965 to 1980. Twenty-one deaths and
7 cancers were observed, which was in agreement with the anticipated
numbers calculated from national statistics (Gustavsson et al., 1986).
Zack & Musch (1979) examined a small cohort of workers (89) with
occupational exposure to PCBs. No liver cancer was reported among the
30 deaths that occurred in this study. There were increases for all
malignancies (8 observed vs 4.4 expected, SMR=179) and elevated lung
cancer (4 observed vs 1.44 expected). Adjustment for the confounding
variables due to multiple exposure to other agents was not made. By
1979, 31 Yusho patients had died, 11 (35.4%) of these from malignant
neoplasms. Only 21.1% of all deaths in this Japanese prefecture would
be expected from malignant neoplasms, but no clear correlation between
the occurrence of Yusho and increased deaths from malignant neoplasms
could be made, because of the small number of deaths observed and the
unknown latency period.
By the end of 1983, 120 Yusho patients had died, 41 of these from
malignant neoplasms. These included 8 stomach cancers, 11 liver
cancers, and 8 neoplasms of the lung. A statistically significant
excess mortality was seen for malignant neoplasms, cancer of the
liver, and cancer of the lung, trachea, and bronchus, in males, but no
such excess was noted in females. The excess from liver cancer deaths
was seen mainly in Fukuoka prefecture, while no excess was seen in
Nagasaki prefecture (Ikeda et al., 1987).
9.2.6 Appraisal
Some epidemiological studies on occupationally exposed workers and
Yusho patients indicate an association between PCB exposure and
cancer, especially with regard to hepatobiliary tumours. However, no
definite conclusions can be drawn from available data, because of the
small numbers of deaths in the population studies, the lack of clear
dose-response relationships in the occupational studies, and the
difficulty in evaluating the effects of other compounds present in
PCBs.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The PCBs were evaluated by IARC in 1978 and 1987 (IARC, 1978; 1987).
In 1987, IARC concluded that, because the role of impurities in PCBs
in the carcinogenicity could not be excluded, and, because of the lack
of knowledge on dose-response relationships, the evidence from
epidemiological studies is limited. However, the evidence of
carcinogenicity in laboratory animals is sufficient. Taking the
combined evidence from human and experimental animal studies, the IARC
Group concluded that PCBs are probably carcinogenic for humans (IARC,
1987).
Many countries and intergovernmental organizations have banned or
severely restricted the production, use, handling, transport, and
disposal of PCBs and PCTs. For an overview of these measures and
regulations we refer to the Health and Safety Guide on PCBs and PCTs
(WHO, 1992; IRPTC, 1986b).
At the meeting of the Joint FAO/WHO Expert Committee on Food Additives
(WHO, 1990), particular attention was paid to the possible health
consequences of the intake of PCBs by the suckling infant. It was not
anticipated that adverse health effects would occur as a result of
consuming breast milk. It should also be kept in mind that the infant
consumes breast milk for a short period (1-2% of its total life span).
In addition, other factors need to be considered:
* the benefits of breast milk and breast-feeding, including the
nutritional, immunological, and other properties of the milk, as
well as the psychological advantages, should not be discounted;
* the disadvantages of breast milk substitutes, because of the
potential contamination due to infective agents, incorrect
preparation, inadequate hygiene, etc.
For these reasons, JECFA was of the opinion that the advantages to the
infant of breast-feeding outweigh any potential hazards due to the PCB
content of breast milk, and advises that there is absolutely no
justification for discouraging this practice.
The monitoring data have indicated, up to now, that the occurrence of
PCBs in human milk persists at about the same levels during the years,
with slight decreases or increases in the PCB concentration in breast
milk in certain countries. Since the PCB levels in human milk are
still too high, every effort should be made to prevent the entry of
PCBs into the environment and to control their occurrence in the food
supply. The Committee was reassured by the observation that the
production of PCBs has largely ceased. Thus, it is expected that the
levels of PCBs in the environment and food, and consequently in breast
milk, will decrease with time (WHO, 1986b).
On the basis of the evaluated background data, an average dietary
intake of PCBs for adults was estimated to amount to a maximum of
100 µg/week, or approximately 14 µg/day. For a 70-kg person, this is
an intake equivalent to a maximum quantity of 0.2 µg/kg body weight
per day (WHO/EURO, 1988).
The above data suggest that the main exposure of the general
population to PCBs occurs through food. The daily intake of these
compounds by breast-fed infants is about 1-2 orders of magnitude
higher than for the rest of the population, compared either on the
basis of body weight or energy consumption. However, compared with
lifetime intake, a 6-month, breast-feeding period contributes less
than 5% of the total body burden from lifetime exposure (WHO/EURO,
1988).
POLYCHLORINATED TERPHENYLS
(Data relating specifically to polychlorinated terphenyls are scarce.
Nevertheless, they are presented separately in this section.)
1. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
1.1 Identity
The chemical formula of the polychlorinated terphenyls (PCTs) can be
given as C18H14-nCln, in which n is the number of chlorine atoms,
which can range from 1-14.
The chemical structure is:
The occurrence of trans-dihydrodiol metabolites suggests that the
metabolism of PCBs proceeds through the formation of arene oxide
intermediates (US EPA, 1987). Arene oxides are potential electrophiles
that have been implicated in cellular necrosis, mutagenicity, and
carcinogenicity (Safe et al., 1975; Sundström et al., 1976a).
While the arene oxide pathway is important in carcinogenic
considerations, it may not be the primary pathway for the metabolism
of PCBs, in most cases. So far, most discussions about the metabolism
of PCB isomers have focused on the position and number of the chlorine
substituents.
The metabolic products of dichloro-, trichloro-, tetrachloro-,
pentachloro-, and hexachlorobiphenyl appear to reflect direct
hydroxylation at the meta and/or para positions, relative to the
position of the phenyl-phenyl bond. In a few instances, a methoxy
group is found instead of a second hydroxyl. This direct mechanism
appears to operate, therefore, irrespective of the degree of
chlorination, and, for the most part, irrespective of the position of
the chlorine substituents. In the rabbit, some exceptions have been
found that involve: a chlorine shift and the removal of a chlorine, in
the case of the 4,4'-dichlorobiphenyl, the formation of a dihydrodiol
at the meta and para positions, in the case of 2,5,2',5'-tetra-
chlorobiphenyl, and the removal of a chlorine from one of the rings,
in the case of 2,4,5,2',4',5'-hexachlorobiphenyl (Sundström et al.,
1976a).
Studies carried out by Matthew & Anderson (1975b) and Tuey & Matthews
(1977) showed that monochloro- and dichlorobiphenyls were rapidly
metabolized and excreted and that pentachloro- and hexachlorobiphenyls
were poorly metabolized and retained longer in the adipose tissue and
skin. The situation for the tetrachlorobiphenyls is more complicated.
The following analysis is based on the data on metabolites identified
and reported in the review by Sundström et al. (1976a).
6.5.2 Dichlorobiphenyls
Consideration of the various dichloro- isomers shows that, when
chlorines are only on one ring, hydroxylation occurs on the
nonchlorinated ring. Single hydroxylation occurs para to the
phenyl-phenyl bond; if another hydroxylation occurs, it is always
meta to the phenyl-phenyl bond. This holds true for the 3 different
isomers tested: 2,3-; 2,4-; and 3,4-dichlorobiphenyls. When the
dichloro-compounds are symmetrically chlorinated on each ring, as in
2,2'-; 3,3'-; and 4,4'-dichloro compounds, the same pattern applies
generally, but with a variation on the theme and an exception in the
case of 4,4'-dichlorobiphenyl. In the case of 2,2'- and 3,3'-dichloro
compounds, monohydroxylation occurs meta and para to the
phenyl-phenyl ring, respectively. In both cases, double hydroxylation
involves both meta and para positions on the same ring, (that is,
meta and para to the phenyl-phenyl bond). In the case of
4,4'-dichlorobiphenyl, there appears to be a difference in the rat.
The monohydroxy- derivative is meta, but the dihydroxy derivative is
ortho and meta to the phenyl-phenyl bond. Not only is the rat
metabolism of the 4,4'-compound an exception, but the rabbit also
shows an unusual response to this compound. In the rabbit, the
monohydroxy-derivative is the same meta hydroxy found in the rat,
but, instead of a dihydroxy- compound, the rabbit produces a chlorine
shift and a single hydroxy group in the para position as well as
dechlorination and hydroxylation in the para position. These latter
products have been considered as characteristic of the arene oxide
intermediate pathway. Nevertheless, among the 6 different dichloro-
isomers examined, all produced a monohydroxy- derivative, either meta
or para to the phenyl-phenyl bond, and all but the 4,4' produced
dihydroxy- derivatives were meta and para on the same ring to the
phenyl-phenyl bond.
The absence of a substitution at 4,4'- with vicinal unsubstituted
positions cannot be correlated with rapid metabolism, since this
property is also shared by both a rapid and slowly metabolized isomer.
This is in direct contradiction to the often repeated statement "the
presence of at least two adjacent unsubstituted carbons, particularly
in positions 3,4-, or 5- or 3',4'- or 5'- is required for rapid
metabolism of chloro-biphenyl" (Jensen & Sundström, 1974b; Berlin et
al., 1975; Safe et al., 1975; Matthews & Anderson, 1976; NIOSH, 1977;
Matthews & Tuey, 1980).
6.5.3 Tetrachlorobiphenyls
Examination of the 2 different tetrachloro- isomers, 2,3,5,6,-
tetrachloro- and 2,5,2',5',-tetrachlorobiphenyl, showed that, in the
case of the molecule with all 4 chlorines on one ring, the products
were monohydroxy- derivatives meta or para, dihydroxy- derivatives
meta and para, and a para hydroxy- plus a meta methoxy- group
or a meta hydroxy- and a para methoxy- group, all on the
unsubstituted ring. The symmetrical 2,5,2',5'-tetrachlorobiphenyl in
the rat gave the meta hydroxy-, but in the rabbit a para hydroxy-,
and also in the rabbit a dihydro-dihydroxy-, on the meta and para
positions. The asymmetric 2,4,3',4'-tetrachlorobiphenyl gave
monohydroxy-derivatives, both in the meta position, either in the
three or five position. It seems that an alternative enzyme pathway is
available in the rabbit.
The level of retention was highest for 2,4,2'4'-tetrachlorobiphenyl
descending in the following order; 2,5,2',5'-, 3,5,3',5'-, 3,4,3',4'-,
2,3,2',3'-, and 2,6,2',6'-tetrachlorobiphenyl. Since no PCBs were
detected in the liver, and only small amounts of 2,3,2',3'- and
3,4,3',4'-tetrachlorobiphenyls in the carcass, it can be concluded
that these 2 isomers were readily metabolized and excreted. Both
compounds have unsubstituted vicinal positions. However, the compounds
slowest to be metabolized were 2,4,2',4'- and 2,5,2',5'-tetra-
chlorobiphenyls, which also have unsubstituted vicinal positions.
Metabolic restriction cannot be entirely attributed to substitution at
the 4,4'- positions (Kato et al., 1980), since this also occurred in
3,4,3',4'-tetrachlorobiphenyl, which was removed relatively rapidly.
The excretion of the monohydroxy metabolites of 3,4,3',4'-tetra-
chlorobiphenyl and 2,4,3',4'-tetrachlorobiphenyl (orally administered)
in rats has been demonstrated by Yoshimura et al. (1973); Yamamoto &
Yoshimura (1973); Yoshimura & Yamamoto (1975); and Yoshimura et al.
(1974). They demonstrated that the metabolites of the first isomer
were 2-hydroxy- or 5-hydroxy- compounds, while the metabolites of the
second isomer were 5-hydroxy- and 3-hydroxy- compounds. All hydroxy
metabolites were excreted non-conjugated via the bile and no parent
isomers were found in the bile. Yoshimura & Yamamoto (1975) found that
unchanged 2,4,3',4'-tetrachlorobiphenyl was excreted through the
intestine, when it was intravenously injected in rats with the bile
duct ligated, while no metabolite of this isomer was excreted by this
route.
The results with the 2,5,2',5'- molecule are probably related to an
arene oxide pathway. Direct evidence for this was reported by Forgue
et al. (1980), who showed that 3,3,3,-trichloropropene-1,2-oxide,
which is an inhibitor of epoxide hydrase, blocked the formation of the
suspected arene oxide metabolites. The arene oxide mechanism
supposedly operates in rabbits and monkeys for 2,5,2',5'-tetrachloro-
biphenyl and, possibly, also in rats for 2,4,5,2',4',5'-hexachloro-
biphenyl. Isomers that may utilize the arene oxide pathway, to some
extent, are: 4,4'-dichloro-; 2,5,2',5'-tetrachloro-; and
2,4,5,2',4',5'-hexachlorobiphenyl.
6.5.4 Hexachlorobiphenyls and higher chlorinated compounds
The symmetrical hexachlorobiphenyls were used in a study by Matthews &
Tuey (1980), in which Sprague-Dawley rats were injected intravenously
with the PCBs and killed at increasing time intervals from 15 min to
42 days. The 2,3,6,2',3',6'- isomer was rapidly metabolized and
excreted compared with the other isomers, which were slowly
metabolized and excreted with much longer half-lives. The results
indicated that the metabolism of hexachlorobiphenyls is slow, when the
position of the chlorine atoms is such that arene oxide formation is
inhibited.
2,3,6,2',3',6'-Hexachlorobiphenyl produces only one metabolite in the
rat: 2,3,6,2',3',6'-hexachloro-4'-hydroxybiphenyl, which is believed
to be the result of arene oxide formation. All commercial mixtures of
PCBs will contain congeners that could be metabolized via the arene
oxide pathway. However, it does not seem to be the major pathway of
metabolism for most of the components of the commercial products,
since most of the higher congeners will not have vicinal unsubstituted
carbons.
The metabolic data on individual isomers shows that, at least up to
hexachloro- compounds, ordinary hydroxylation can take place. It is
reasonable to consider that it is not only as a consequence of poor
metabolism that pentachloro- and hexachloro- compounds are persistent
in the tissues, but rather that they are not metabolized as readily,
because they are sequestered from tissues in which the bulk of the
metabolism takes place. In support of this position, it has been shown
by Matthews & Anderson (1975b) that, when animals are caused to lose a
substantial portion of body weight, the stored higher chlorinated
compounds can, indeed, be metabolized. Octachloro- and
decachlorobiphenyls would not be expected to be easily metabolized,
simply because there are few or no sites for hydroxylation to take
place. It was found (Vodicnick & Lech, 1980; Vodicnick et al., 1980)
that almost the entire body burden of 2,4,5,2',4',5'-hexa-
chlorobiphenyl was removed from mothers given this PCB and that it was
transferred to their offspring via the nursing mother's milk. In mice,
the preferential distribution of this PCB in milk reflects the high
fat content of mouse milk.
Virgin, female, Sprague-Dawley mice, injected ip with 50 or 100 mg
[14C] 2,4,5,2',4', 5' -hexachlorobiphenyl/kg body weight in corn oil
for 2 weeks, prior to mating, eliminated virtually their entire body
burden of the compound through milk during one lactation cycle
(Gallenberg & Vodicnick, 1987).
Storage is caused by lack of metabolism and also implies that the
availability of adjacent unsubstituted carbons is the determinant for
metabolism. Direct hydroxylation reactions do not require
unsubstituted adjacent carbons. Rapid storage in fat is the
rate-limiting factor in the removal of most PCBs, with the exception
of isomers that might be very rapidly metabolized by arene oxide
formation, such as 2,3,6,2',3',6'-hexachlorobiphenyl.
Matthews & Anderson (1975b) extended their study to include a fasting
period to reduce the weight of the test rats and showed that severe
fasting mobilized stored PCBs and brought them into the metabolic
pool.
6.5.5 Retention and turnover
Mizutani et al. (1977) studied the pharmacokinetic behaviour of 6
different tetrachlorobiphenyls. They administered mice the 6 isomers,
at 10 mg/kg body weight, for 20 days. The isomer concentrations in the
liver and the remainder of the carcass were determined at various
times during the recovery period. They found that the accumulated body
burden was a function of both storage ratio and biological half-life.
The results of Mizutani et al. (1977) suggest that the correlations
claimed between the position of the chlorine substituents and storage
or metabolic activity (Kato et al., 1980; Matthews & Tuey, 1980) are
not simply explained.
The hypothesis that the position of the chlorine atoms alone
determines the rates of metabolism, accumulation, and excretion does
not appear to be entirely supported. This idea has been used to
support the notion that PCBs with unsubstituted vicinal carbon atoms
favour metabolism by arene oxide formation.
6.5.6 Appraisal
The results of most studies suggest that PCBs are absorbed by the
organ systems (gastrointestinal tract, lung, and liver), representing
the likely routes of entry into the body. This is particularly true
for the gastrointestinal tract where absorption is rapid. PCBs, once
absorbed, are usually distributed in a biphasic manner and are rapidly
cleared from the blood and accumulated in the liver and adipose
tissue, or they can be metabolized in the liver, to form metabolites
that are excreted in the urine and bile. In some studies on humans,
the skin, an organ rich in adipose tissue, had a high PCB content,
whereas the brain content was low. This distribution can also include
the fetus and human milk, an extension of the adipose tissue system in
the body. Mobilization of PCBs from fat appears to depend on their
rates of metabolism. Metabolic pathways include hydroxylation, and
conjugation with thiols and other water-soluble derivatives, some of
which can involve reactive intermediates, such as the arene oxides.
The most important pathway seems to be hydroxylation and subsequent
conjugation. This pathway is facilitated by the presence of at least
one pair of unoccupied vicinal carbon atoms in the PCB structure.
Persistence in tissue is not correlated with high toxicity.
Differences in toxicities among PCBs may be associated with specific
metabolites and/or their associated intermediates.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Toxicity for microorganisms
7.1.1 Freshwater microorganisms
Zullei & Benecke (1978) used the motility of filamentous blue-green
algae (Cyanophyceae) of the genus Phormium as a bioassay for the
rapid determination of the toxicity of various compounds. They tested
the relative toxicity of purified, specific chlorobiphenyls and of PCB
commercial mixtures (Clophen). Inhibition of motility was greatest in
the presence of chlorobiphenyls of low chlorination. All mono- and
dichlorinated isomers were inhibitory at the test concentration of
100 µg per test spot of algae. Tetra- and hexachlorobiphenyls did not
have any effects; the effects of trichlorobiphenyl isomers varied, the
2,5,2' isomer not producing any effects and the 2,4,4' and 3,4,4'
isomers being inhibitory. Tests using the commercial mixtures
confirmed the greater toxicity of low chlorination levels; Clophen A30
was more toxic than Clophen A60, though the presence of more than
expected, low-chlorinated compounds in the Clophen A60 reduced the
difference in toxicity.
Cultures of the green alga Chlorella pyrenoidosa were incubated with
1 mg/litre of Aroclors 1242, 1254, and 1268 (Hawes et al., 1976a).
The initial culture was 5 days old with a cell density of 27 ×
106 cells/ml. After 8 h of incubation with the PCBs, cell densities
in the cultures were 64% lower than controls for Aroclor 1242, 45%
lower for Aroclor 1254, and 36% lower for Aroclor 1268. As the study
progressed, the cell densities in the cultures improved relative to
the controls; cell density in the Aroclor 1254 culture was equal to
that in the control by 129 h and the density in the Aroclor 1268
culture was equal to the control by 59 h. Although the density in the
Aroclor 1242 culture remained much lower than that in the control
throughout the culture period, there was evidence of recovery with
this compound. The toxicity of the Aroclors was inversely proportional
to their degree of chlorination. A concurrent investigation of the
primary productivity of the alga (Hawes et al., 1976b), suggested that
the productivity of individual cells was stimulated by the Aroclors,
with a positive relationship between the level of chlorination and the
effect. However, this did not take into account the differences in
density between cultures (PCBs reduce growth through cell division)
and the authors pointed out that the response of the alga to the PCBs
was not simple. They stated that the density, culture age, and Aroclor
type were all factors that influenced response.
Larsson & Tillberg (1975) cultured the green alga Scenedesmus
obtusiusculus, in a liquid medium, in concentrations of Aroclor 1242
ranging between 10 and 1000 µg/litre. Growth was reduced at
concentrations of 300 µg Aroclor/litre or more; viability was only
affected at the highest concentration of 1000 µg/litre. Reduced
phosphate uptake, which was nearly identical in light or in darkness,
was reduced from 300 µg/litre upwards. The authors regarded this as a
result of an effect on the plasmalemma. At 800 µg PCBs/litre, the
results of some studies suggested an effect of the uncoupling of
oxidative phosphorylation; at 1000 µg/litre, both respiration and
oxygen evolution were inhibited.
The effects of various Aroclors on the respiration and photosynthesis
of the green alga Chlorella vulgaris were examined by Sinclair et
al. (1977). Aroclor 1221, at a final concentration in the medium at
10-4 mol/litre (= 192 mg/litre based on an average relative molecular
mass for the Aroclor at 192), produced a rate of oxygen production in
the light of 43% of control levels and a rate of oxygen uptake in the
dark of 59% of control levels. The Aroclors were dissolved using
dimethylformamide (DMF) as a solubilising agent; whilst this had some
effects on the parameters measured, they were very little compared
with the effects of the Aroclors (94% of control levels). A
dose-response curve of the effect of Aroclor 1221 on the respiratory
uptake of oxygen in the dark, in the presence of glucose, showed that
there was already a marked effect at a concentration of
10-7 mol/litre and that a maximum (at 50% inhibition) was reached at
a concentration of 10-6 mol/litre (= 1.92 mg/litre).
A further series of studies was performed to investigate the
individual processes of oxygen exchange in the alga. Respiration in
the dark was investigated in the absence of added glucose, to monitor
"endogenous" respiration, which was found to be stimulated by Aroclor
1221 at concentrations of 10-4 mol/litre or more. Net oxygen
production in the light (photosynthetic oxygen production minus
respiratory oxygen usage) was reduced at a concentration of
10-4 mol/litre. At this concentration of Aroclor 1221, there was
approximately 50% stimulation of endogenous respiration and
approximately 50% inhibition of net oxygen production. Calculated
photosynthetic rate was unaffected by the Aroclor. Increasing light
intensity reduced the effect of Aroclor 1221 on net oxygen production;
intensities of 8.2 × 104 ergs/cm2 virtually eliminated the effect.
Other measures directly or indirectly associated with photosynthesis
(fluorescence, oxygen evolution in flashing light, the Emerson
enhancement phenomenon) were not affected by the Aroclor. The authors
suggested that the photosynthetic apparatus of Chlorella was
unaffected by Aroclor 1221; the major, and probably the only, effect
of the PCBs being a stimulation of endogenous respiration rate.
Results with Aroclors 1242 and 1268 were consistent with those for
Aroclor 1221; both inhibited net oxygen production in the light and
glucose-driven respiration in the dark, but stimulated endogenous
respiration in the dark (Sinclair et al., 1977).
Luard (1973) reported inhibition of 14C uptake by the green alga
Scenedesmus quadricauda at concentrations of Aroclor 1254 as low as
0.1 µg/litre. At this concentration, the Aroclor caused a 20%
inhibition of 14C uptake, which rose to 65% inhibition at 1 mg/litre.
A marked effect of the initial number of cells in the incubation tube
on the toxicity of Aroclor 1254 for the green alga Chlorella
pyrenoidosa was demonstrated by Cole & Plapp (1974). At a constant
concentration of 1 mg Aroclor 1254/litre, the numbers of algal cells
in the initial incubation medium were as varied as 1, 10, 100, or
1000 µg alga/ml of medium. At the highest inoculation rate, the growth
of the alga was unaffected by the Aroclor. At lower initial
inoculation, the rate of growth was reduced to between 11 and 55% of
control levels. A comparable effect was found using 14C fixation as
the parameter; with inoculation rates of 1000 µg alga/ml medium, the
Aroclor did not have any effects, whereas lower inoculation rates
reduced carbon fixation to between 6 and 13% of control levels.
Mosser et al. (1972) showed that 2 species of freshwater algae
(Euglena gracilis and Chlamydomonas reinhardtii) were unaffected
by 100 µg PCBs/litre (type unspecified). Ewald et al. (1976)
determined the 48-h EC50 on the growth of Euglena gracilis to be
4.4 mg/litre for Aroclor 1221 and 55 mg/litre for Aroclor 1232.
Aroclor 1242 showed no inhibition at 100 mg/litre. Aroclor 1221, at
the EC50 concentration of 4.4 mg/litre, significantly depressed
carbon fixation and chlorophyll levels, but did not affect oxygen
consumption. Uptake of L-leucine was increased 2-fold, but
incorporation was not affected. Uridine uptake was significantly
decreased, but thymidine uptake and incorporation were not affected.
In studies by Glooschenko & Glooschenko (1975), 3 algal species from
the Great Lakes were cultured with Aroclor at concentrations of 1, 5,
10, 20, or 50 µg/litre. Cell numbers of the diatom Synedra acus were
reduced in culture from day 3 of treatment with Aroclor 1242, at 10,
20, or 50 µg/litre, and from day 7, with 1 or 5 µg/litre. The green
alga Scenedesmus quadricauda showed a lag phase of 3 days in all
concentrations of Aroclor 1242. After 3 days, exponential growth
occurred in all treatments, except for the highest dose levels (20 and
50 µg/litre) which showed little or no cell division. A second green
alga (Ankistrodesmus falcatus) was more sensitive, showing
significantly reduced cell numbers at all dose levels of Aroclor 1242.
Ankistrodesmus was used to examine the relative toxicities of
different Aroclors. Cell numbers were 57, 66, 36, and 53% of control
levels for Aroclors 1016, 1221, 1242, and 1248, respectively, after 2
days of culture. Carbon fixation, estimated as uptake of 14C from
solution, was 73, 98, 51, and 59% of control levels for the 4
Aroclors, respectively.
Dive et al. (1976) cultured the ciliate protozoan Colpidium campylum
with purified chlorobiphenyls and with a commercial mixture (Pyralene
3010). None of the 16 isomers affected the ciliate's growth and
reproduction at concentrations of 0.01 or 0.1 mg/litre; similarly,
Pyralene 3010 was not toxic at these concentrations. 2-Mono-
chlorobiphenyl showed little toxicity for the organism at 1 mg/litre
and little or no toxicity was demonstrated by the tetrachloro-,
pentachloro-, and hexachlorobiphenyls at this concentration or at
10 mg/litre (with the exception of 2,5,2',5'-tetrachlorobiphenyl,
which inhibited growth considerably, limiting it to about 10% of
controls at 10 mg/litre). 4,4'-Dichlorobiphenyl was not toxic at any
concentration tested (up to 10 mg/litre) but both 2,3- and
2,5-dichlorobiphenyls were toxic, killing all organisms at both 1 and
10 mg/litre. These results are comparable with other reports that the
lower chlorinated biphenyls are the most toxic for microorganisms;
differences in toxicity could not be explained by the differential
uptake of the different isomers.
It was reported by French (1976) that the EC50 for growth inhibition
of Aroclor 1254 on the flagellated protozoan Crithidia fasciculata
was 10.5 mg/litre. The PCBs slightly inhibited (after 6 h) and then
increased (after 24 h) the carbon dioxide evolution of cultures
utilizing D-glucose. After 6 h exposure, the uptake and incorporation
of thymidine and uridine (but not L-leucine) were inhibited;
inhibition was transient and returned to normal after 12 or 24 h. Fine
structural changes were noted after exposure to PCBs including:
deterioration of the kinetoplast, mitochondrial or cellular swelling,
and the presence of concentric membrane arrays. It was concluded that
cell population growth inhibition was due to disruption of uptake,
incorporation of nucleic acids, and loss of cell regulatory capacity.
7.1.2 Marine and estuarine microorganisms
Bourquin & Cassidy (1975) and Bourquin & Kiefer (1975) investigated
the effects of Aroclors 1016 and 1242 on 85 bacterial isolates from
various estuarine environments near Pensacola, Florida. Twenty six of
the 85 isolates were inhibited to various extents by 0.5 mg of either
of the PCBs, applied to a disc placed on the surface of an agar plate
on which the bacteria were growing. Zones of inhibition ranged from 14
to 20 mm in diameter. Cultures that showed sensitivity to Aroclor 1242
were also inhibited by Aroclor 1016. Sixty five percent of isolates
inhibited by 0.5 mg of Aroclor 1242 were still sensitive at 0.1 mg,
and 58% of those sensitive to Aroclor 1016 at 0.5 mg were still
sensitive to 0.1 mg. Four isolates were examined in a liquid medium.
Inhibition of the cultures was characterized by a greatly extended lag
phase (extended from 2 h to at least 14 h); when growth occurred,
growth curves were parallel with those of the controls. The
physiological activity of sensitive and insensitive isolates were
investigated to try to explain the reasons behind sensitivity. More of
the sensitive isolates were amylase and gelatinase producers (76 and
86%, respectively, compared with 33 and 42%, respectively, for the
whole range of isolates). The significance of this observation is
unclear. Because of the method of exposure in this screening exercise,
it is difficult to relate the results to exposure in natural waters
and to draw conclusions about likely hazards for aquatic bacteria.
Kleppel & McLaughlin (1980) determined the toxic threshold of Aroclor
1254 for the estuarine diatom Skeletonema costatum to be between
3 × 10-9 and 3 × 10-8 µg/cell. When the effect of cell density on the
toxicity of the Aroclor for the organism was examined, maximum
inhibition occurred with the lowest inoculum rates.
Michaels et al. (1982) estimated the effects of Aroclor 1254 on
photosynthesis in the marine diatom Thalassiosira pseudonana
( = Cyclotella nana) by monitoring the uptake of 14C-carbon
dioxide. The numbers of viable cells in the culture were also
estimated. Total cell numbers were estimated at regular intervals
during the 48 h of the experiment and the minimum number of viable
cells required to produce the increase in numbers between periods
calculated. This gave an estimate of the viable numbers,
retrospectively, for each period. Inhibition of 14C-uptake per
culture, per cell, and per viable cell was evident within 1 h of the
start of the incubation. By 48 h, the 14C uptake per culture was
reduced to 0.2% of control levels, per cell, to 13% of control levels,
and per viable cell, to 34% of control levels. The authors concluded
that the effect of Aroclor 1254 on the diatom is a combination of
inhibition of carbon assimilation by individual cells and inhibition
of cell division.
In an earlier study, Fisher & Wurster (1973) exposed 2 estuarine
diatoms (Thalassiosira pseudonana and Rhizosolenia setigera) and
an estuarine green alga (Dunaliella tertiolecta) to Aroclor 1254 at
0.1 or 10 µg/litre, cultured over 100 h. There was no effect on the
growth of the alga. T. pseudonana was unaffected by the PCB at
0.1 µg/litre. The effect of the Aroclor on T. pseudonana at
10 µg/-litre was dependent on temperature; there was a 17% reduction
in growth rate at 25°C, a 44% reduction in growth rate at 18°C, and a
58% reduction in growth rate at 12°C. The growth of control cultures
was greatest at the highest temperature. The growth of R. setigera
was completely stopped by all concentrations of PCB tested, for the
first 48 h of culture. When growth resumed, the degree of inhibition
was greater at 10°C than at 15°C. Fisher et al. (1976) exposed the
marine diatom Thalassiosira pseudonana to 1 µg Aroclor 1254/litre in
cultures containing various levels of nitrate nutrient. The toxic
effects of the PCBs on the growth of the diatom were greatest at low
nitrogen levels. Analysis of variance showed the diatom to be
significantly dependent for growth on nitrogen concentration and on
PCB concentration, and that the PCB effect was significantly nitrogen
dependent. The authors pointed out that marine phytoplankton are often
nitrogen-limited in nature and suggested that the effects of
pollutants, such as PCBs, may, therefore, vary with season. The
greatest effects are likely to occur during bloom conditions, when
competition for nutrients is greatest. Fisher (1975) considered that
the presence of PCBs, even at concentrations far above the maximum
recorded level in sea water, would not affect the overall carbon
fixation by phytoplankton. Although the cell division of some
organisms was adversely affected, enough insensitive species existed
to compensate for the sensitive species. Species diversity and
community structure were likely to be affected.
In a study by Craigie & Hutzinger (1975), 6 marine algae were cultured
for 6 days in the presence of commercial mixtures of PCBs (Aroclors
1221 to 1262; Phenoclors DP3 to DP6), PCTs (Aroclor 5460), and
specific chlorobiphenyls. Each compound or mixture was applied to the
culture medium at 2 concentrations (1 and 100 mg/litre). The algae
were representatives of 6 different classes: Bacillariophyceae -
Skeletonema costatum, Thalassiosira fluviatilis; Chlorophyceae -
Dunaliella tertiolecta; Chrysophyceae - Monochrysis lutheri;
Prainophyceae - Platymonas sp.; Rhodophyceae - Porphyridium sp.;
Xanthophyceae - Olisthodiscus sp. All were cultured at 20°C. Growth
was estimated by turbidity. The response to particular Aroclors was
species dependent; the least sensitive species was Dunaliella and
the most sensitive was Olisthodiscus. At 1 mg/litre of the PCB
mixtures, there was relatively little inhibition of Dunaliella,
Platymonas, Skeletonema, or Thalassiosira. Olisthodiscus was
completely inhibited by Aroclors 1248, 1254, 1260, and Phenoclor DP4.
The Phenoclor series was more toxic for Olisthodiscus. than the
Aroclors. Porphyridium and Monochrysis showed intermediate
sensitivity. Generally, the Aroclors with higher chlorination levels
were less toxic for all species than the Aroclors with lower
chlorination levels. Results with pure, specific chlorinated biphenyls
confirmed that highly chlorinated compounds are less toxic than those
with only 1-4 chlorine atoms per molecule. Experiments with biphenyls
containing identical percentages of chlorine showed that biological
response was highly dependent on the structure of the molecule. The
2,4,2',4'-tetrachlorobiphenyl was more toxic for both Dunaliella and
Olisthodiscus than the 2,5,2',5'- isomer, and both were more toxic
than either the 2,3,4,5- or the 3,4,3',4'- isomers. The last was not
toxic for any of the algae, even when added at 100 mg/litre.
Luard (1973) reported a significant reduction in 14C uptake by the
estuarine green alga Dunaliella tertiolecta in the presence of
Aroclor 1254, at 100 µg/litre. The culture showed 14C uptake at 65%
of control levels. At 1000 µg/litre, uptake was further reduced to 59%
of controls. The 14C uptake was unaffected at 10 µg/litre.
7.1.3 Soil microorganisms
When Murado et al. (1976) added Aroclors to a liquid or solid medium
on which the soil microfungus Aspergillus flavus was cultured,
mycelial growth was reduced progressively as the dose of Aroclor 1254
increased from 5 to 50 mg/litre in liquid culture. At 25 mg/litre, the
dry weight of the mycelium was reduced to 1.4, 3.4, 3.9, 3.3, and
54.6% of control levels by Aroclors 1232, 1242, 1248, 1254, and 1260,
respectively. At the same time, the relative RNA content of the
mycelium increased, rising from a control level of 5.9 µg RNA/mg dry
weight to between 13.2 and 18.6 µg RNA/mg dry weight for Aroclors 1232
to 1254. Aroclor 1260 had no marked effect on RNA. The DNA content was
not affected by any treatment. Cultures on solid medium showed a delay
in sporulation and a decrease in the diameter of colonies at doses up
to 20 µg/cm2.
Glooschenko & Glooschenko (1975) cultured the soil alga Navicula
pelliculosa with Aroclors 1016, 1221, 1242, and 1248 at a
concentration of 20 µg/litre. The numbers of cells in the culture,
after 2 days, were reduced to 66, 53, 46, and 56% of control levels
for the 4 Aroclors, respectively.
7.1.4 Plankton communities
Phytoplankton communities from 2 lakes, one oligotrophic and one
eutrophic, were exposed to PCBs, as Clophen A50, at 26 µg/litre
(Södergren & Gelin, 1983). In the community from the eutrophic lake
with a greater biomass of phytoplankton, measurement of 14C uptake,
monitored immediately after the addition of the PCBs, showed a
reduction of 34% compared with the controls. Monitoring carbon
fixation 16 h later showed the phytoplankton recovering from the
effect of the PCBs, with only 21% inhibition relative to the controls.
The results differed in the community from the oligotrophic lake. 14C
uptake immediately after the addition of the Clophen was 70% less than
that in the controls; 16 h later, the effect was greater, at 84%
inhibition. The authors pointed out that these results parallel
findings in pure culture showing that a high density of organisms
reduces the effects of PCBs.
Mosser et al. (1972) first demonstrated the effects of PCBs on both
single and mixed cultures of marine phytoplankton. Two organisms, a
marine diatom (Thalassiosira pseudonana) and a marine green alga
(Dunaliella tertiolecta) were used. The diatom is sensitive and the
alga insensitive to PCBs. The application of PCBs (not specified) to
mixed cultures changed the usual dominance of the diatom into
dominance of the alga, even at concentrations of the PCBs (1 and
10 µg/litre) that had no discernible effects on the diatom in pure
culture.
The effect of Aroclor 1254 on the relative biomass of 2 species of
marine diatoms Phaeodactylum tricornutum and Cyclotella cryptica
was examined by Lundy et al. (1984). The diatoms were cultured
together in either 10 or 20 µg PCBs/litre for 6 days. At both
concentrations of Aroclor, the ratio between the species was shifted
in favour of Phaeodactylum. After 6 days, the ratio of
Phaeodactylum: Cyclotella was 0.8 in the controls and 2.63 in the
treated cultures (10 µg Aroclor 1254/litre).
Biggs et al. (1979) exposed a mixed culture of 2 marine algae to PCBs
(Aroclor 1254) at 50 µg/litre. In the control culture, Thalassiosira
pseudonana became the dominant organism over Dunaliella
tertiolecta. After exposure to the PCBs, the Thalassiosira was
affected, but the Dunaliella was not. By day 2 of Aroclor 1254
exposure, Dunaliella was the dominant species. Fisher et al. (1974)
showed a similar effect with the greater effects of PCBs on the
sensitive diatom Thalassiosira pseudonana when the organism was in
competition with other organisms. The effect was also demonstrated
using natural communities of phytoplankton, when Thalassiosira was
also selectively affected.
Biggs et al. (1978) exposed a natural community of marine
phytoplankton (from which large detritus and zooplankton had been
filtered) to Aroclor 1254 at either 5 or 10 µg/litre. Cell division,
chlorophyll-a synthesis, and 14C uptake were monitored, as well as
particle size. Treatment with the Aroclor reduced community growth
rates by 20-50%, compared with controls. Growth had not fully
recovered after 10 days. The 14C uptake was reduced for 6 days. The
control cultures became dominated by algae larger than 8 µm in
diameter. The PCB-treated cultures showed strong inhibition of these
larger algal cells and the culture became dominated by small cells.
In a study by Iseki et al. (1981), a natural community of plankton was
exposed, in situ, in a marine environment, to Aroclor 1254 in large
bags holding 68 m3 of seawater. The Aroclor was added to the bag
giving an initial concentration in the upper layers of about
40 µg/litre (15 µg/litre at 10 m depth). Over time, the levels of PCB
fell in the upper layer and increased in the lower layer in the bag.
Six days after addition, the concentrations at all depths were less
than 15 µg/litre. Immediately after addition of the PCBs, the rate of
sedimentation of the particles increased; these particles were thought
to be dead or senescent large cells, such as diatoms, and this
sedimentation was assumed to be responsible for the major part of the
loss of the PCBs from the water. Zooplankters were eliminated from the
bags by this level of Aroclor; no recovery was seen over the 21 days
of the experiment. Large diatoms were selectively eliminated from the
bags and replaced by small flagellates as the dominant organism.
Moore & Harriss (1972, 1974) exposed a natural community of plankton
to PCBs (as Aroclor 1242) at 10 or 25 µg/litre. The population was
collected from natural water, placed in glass bottles suspended
in situ, and monitored for uptake of 14C, added to the bottles as
bicarbonate. After incubation, the community was separated into small
"nannoplankton" and larger "net-plankton" by filtration at a mesh size
of 53 µm. Nannoplankton radiocarbon uptake accounted for 72.6% of the
total community carbon uptake and was not affected by either of the
concentrations of Aroclor 1242. The net-plankton uptake of 14C was
reduced by 56% at 10 µg Aroclor/litre and by 58% at 25 µg/litre. The
authors suggested that PCBs at levels found in natural waters would
alter the species diversity or community structure of microorganisms
and that this might affect higher levels of the food chain with
specialist feeders utilizing one type of prey. O'Connors et al. (1978)
exposed natural communities of phytoplankton, in situ, in dialysis
bags in a salt marsh. Large zooplankton herbivores were removed by
filtration through a mesh. Aroclor 1254 was added to the bags to give
water concentrations of 1-10 µg/litre. Larger diatoms were selectively
inhibited by the Aroclor, even at the lower dose (1 µg/litre). The
authors suggested that, not only would phytoplankton communities be
affected by PCBs in natural waters, but that the effect would be
carried forward through the food chain. Gelatinous predators, such as
jellyfish, could be selected at the expense of fish, since fish tend
to depend directly, or indirectly, on the larger phytoplankton.
Natural communities of phytoplankton from a stream and a reservoir
were cultured with Aroclors 1232 and 1254 by Kricher et al. (1979).
Although the algal communities were different in composition, both
Aroclors exerted similar effects on both communities; primary
productivity was reduced in a dose-dependent manner. Aroclor 1232 was
more toxic than Aroclor 1254 at 1 mg/litre. Algal species within the
populations were differentially affected by the Aroclors; diatoms were
particularly susceptible and treatment produced disproportionate
numbers of blue-green algae, such as Anacystis. The authors pointed
out that the insensitive species still accumulated PCBs and,
therefore, formed the basis for the accumulation of the Aroclors in
aquatic food chains.
7.1.5 Interactions with other chemicals
Mosser et al. (1974) investigated the interactions between Aroclor
1254, DDT, and DDE in a marine diatom Thalassiosira pseudonana. The
diatom was cultured for 4 days with either 10 or 50 µg Aroclor
1254/litre, 100 µg DDE/litre or 500 µg DDT/litre (with each chemical
alone or in combination). The PCBs alone (at 10 µg/litre) and the DDE
alone had little effect on the growth of the diatom; when combined
these treatments were synergistic, growth being less than half that of
the control culture. Higher concentrations of either compound
increased the inhibitory effect. In contrast, DDT reduced the toxic
effects of PCBs at higher concentrations (50 µg/litre). Treatment with
the Aroclor alone at 50 µg/litre almost stopped the growth of the
diatom. Simultaneous treatment with DDT at 500 µg/litre restored
growth to 60-70% of control levels. Lower concentrations of DDT had a
comparable, but reduced, effect. Addition of the DDT to the medium, 12
or 24 h after culture had begun in the presence of PCBs, also reversed
the inhibitory effect.
7.1.6 Tolerance
Fisher et al. (1973) showed that strains of diatoms, isolated from the
Sargasso Sea, were more sensitive to the effects of PCBs than isolates
of the same species, obtained from estuaries or the continental shelf.
It was suggested by the authors that the difference in sensitivity was
derived from the variable environment of the estuarine strains; these
strains are able to cope with wide variations in their living
conditions, not experienced in the open ocean of the Sargasso, and,
therefore, were better able to cope with stress from chemical
pollutants.
When Cosper et al. (1984) compared the sensitivity of clones of 2
species of diatom (Asterionella japonica and Ditylum brightwelii)
from polluted and unpolluted sites, Asterionella was less sensitive
than Ditylum to the action of PCBs (Aroclor 1254); some strains of
the former were tolerant of 25 µg Aroclor/litre whereas no strains of
the latter could tolerate this concentration. There was evidence that
strains of Asterionella from the polluted site were more tolerant
than the same species from unpolluted sites. One strain, from the
polluted site, was tolerant to Aroclor 1254 at 50 µg/litre.
7.2 Toxicity for aquatic organisms
7.2.1 Aquatic plants
Mahanty (1975) grew the aquatic angiosperm Spirodela oligorhiza in a
sterile culture solution, to which had been added Aroclor 1242, at
concentrations of 5-100 mg/litre. The numbers of colonies were counted
throughout the 14-day exposure. The highest dose (100 mg/litre) was
found to be lethal. At both 25 and 50 mg/litre, though there was some
growth, the colonies were small and showed morphological differences
from control colonies including: smaller fronds, in larger numbers
than usual, and a characteristic striped pattern of chlorosis on the
fronds. Even at 5 mg/litre, growth was reduced by 50% (as recorded by
the number of colonies and the fresh weight). Mahanty & McWha (1976),
using only the 5 mg/litre dose, found reduced growth, an unusual
striped pattern of chlorosis, and a reduction in the levels of
chlorophyll and total RNA, but no change in the levels of DNA. When
Mahanty & Fineran (1976) studied treated (5 mg/litre) and untreated
fronds of Spirodela by electron microscopy, they found almost
complete disorganization of the internal structure of the chloroplasts
in chlorotic tissue. Organization of other cell components was largely
unaffected.
7.2.2 Aquatic invertebrates
The acute toxicity of PCBs for aquatic invertebrates is summarized in
Tables 27 and 28. Toxicity is very variable between species, even
closely-related species. For most aquatic invertebrates, there is an
effect of degree of chlorination of the PCBs, but this is not a direct
correlation, either negative or positive, the most toxic PCBs often
being in the mid range of chlorination. Under flow-through conditions,
the toxicity of PCBs appears to be much higher. Over 96 h, under
static conditions, LC50 values ranged between 12 µg/litre and
> 10 mg/litre for different organisms and different PCBs.
MATC (maximum acceptable toxicant concentrations) were set for various
PCBs by the US EPA (1980). These are expressed as a range between
no-observed-effect values and lowest concentration tested that
produced a measurable effect. For Daphnia magna, these values were
1.2 and 3.5 µg/litre for Aroclor 1248, and 2.5 and 7.5 µg/litre for
Aroclor 1254. For the scud (Gammarus pseudolimnaeus) values for
Aroclor 1242 were 2.8 and 8.7 µg/litre and values for Aroclor 1248
were 2.5 and 5.1 µg/litre. Aquatic larvae of the midge (Tanytarsus
dissimilis) had a no-observed-effect level of 0.5 µg/litre and a
lowest effective concentration at 1.2 µg/litre.
7.2.2.1 Short- and long-term toxicity
Roberts (1975) exposed the common mussel (Mytilus edulis) to
Aroclors 1242 and 1254 and studied byssus formation. The 24- and 48-h
EC50s for a reduction in the number of mussels byssally-attaching
were 2.2 and 3.0 mg/litre, respectively, for Aroclor 1254. EC50s
after exposure to Aroclor 1242 were 0.9 mg/litre for 24 h and
1.0 mg/litre for 48 h. Duke et al. (1970) maintained oysters
Table 27. Acute toxicity of PCB mixtures for freshwater invertebrates
Organism Size/age Stat/ Temperature Hardness pH PCB type Parameter Concentration Reference
flowa (°C) (mg/litre)b (Aroclor) (mg/litre)
Scud mature flow 15 272 7.4 1242 96 h - LC50 0.01 Mayer & Ellersieck
(Gammarus (1986)
pseudolimnaeus)
juvenile flow 18 1248 96 h - LC50 0.029 Nebeker & Puglisi
juvenile flow 18 1242 96 h - LC50 0.073 (1974)
Scud mature stat 21 44 7.1 1248 96 h - LC50 0.052 Mayer & Ellersieck
(Gammarus mature stat 21 44 7.1 1254 96 h - LC50 2.4 (1986)
fasciatus)
Glass shrimp mature flow 15 272 7.4 1254 168 h - LC50 0.003 Mayer & Ellersieck
(Palaemonetes (1986)
kadiakensis)
Crayfish early stat 21 44 7.1 1242 168 h - LC50 0.03 Mayer & Ellersieck
(Orconectes nais) instar (1986)
Crayfish early stat 21 44 7.1 1254 168 h - LC50 0.1 Mayer & Ellersieck
(Procambarus sp.) instar (1986)
immature stat 12 44 7.5 1254 96 h - LC50 > 0.55
Table 27. (cont'd)
Organism Size/age Stat/ Temperature Hardness pH PCB type Parameter Concentration Reference
flowa (°C) (mg/litre)b (Aroclor) (mg/litre)
Stonefly first stat 10 170 7.2 1016 96 h - LC50 0.61 Mayer & Ellersieck
(Pteronarcella year (0.42-0.88) (1986)
badia)
Damselfly late flow 15 272 7.4 1242 96 h - LC50 0.4 Mayer & Ellersieck
(Ischnura instar flow 15 272 7.4 1254 96 h - LC50 0.2 (1986)
verticalis)
Dragonfly late stat 21 44 7.1 1242 168 h - LC50 0.8 Mayer & Ellersieck
(Macromia sp.) instar stat 21 44 7.1 1254 168 h - LC50 0.8 (1986)
a Stat = static conditions: water not changed during the exposure; flow = flow-through conditions; concentration of
toxicant continuously maintained.
b Hardness expressed as mg CaCO3/litre, unless otherwise stated.
Table 28. Acute toxicity of PCB mixtures for marine invertebrates
Organism Size/age Stat/ Temperature Salinity PCB type Parameter Concentration Reference
flowa (°C) (%) (mg/litre)
Cockle adult stat 15 Aroclor 1248 48 h - LC50 > 10 Portmann &
(Cardium adult stat 15 Aroclor 1254 48 h - LC50 > 10 Wilson (1971)
edule) adult stat 15 Aroclor 1260 48 h - LC50 > 10
adult stat 15 Aroclor 1262 48 h - LC50 > 10
adult stat 15 Clophen A30 48 h - LC50 3.0
adult stat 15 Clophen A60 48 h - LC50 > 10
Eastern oyster adult flow 28 28 Aroclor 1016 96 h - EC50 0.01 Mayer (1987)
(Crassostrea
virginica)
Brown shrimp adult flow 30 29 Aroclor 1016 96 h - LC50 0.01 Mayer (1987)
(Penaeus
aztecus)
Brown shrimp adult stat 15 Clophen A60 48 h - EC50 > 10 Portmann &
(Crangon adult stat 15 Aroclor 1242 48 h - LC50 1.0 Wilson (1971)
crangon) adult stat 15 Aroclor 1248 48 h - LC50 0.3-1.0
adult stat 15 Aroclor 1254 48 h - LC50 3.0-10.0
adult stat 15 Aroclor 1260 48 h - LC50 > 10
adult stat 15 Aroclor 1262 48 h - LC50 > 10
adult stat 15 Clophen A40 48 h - LC50 0.3-1.0
adult stat 15 Clophen A30 48 h - LC50 1.0-3.3
adult stat 15 Clophen A50 48 h - LC50 3.3-10.0
Table 28. (cont'd).
Organism Size/age Stat/ Temperature Salinity PCB type Parameter Concentration Reference
flowa (°C) (%) (mg/litre)
Grass shrimp 1 day stat 25 25 Aroclor 1016 96 h - LC50 0.15 Mayer (1987)
(Palaemonets 3 days stat 25 25 Aroclor 1016 96 h - LC50 0.021
pugio) 6 days stat 25 25 Aroclor 1016 96 h - LC50 0.017
9 days stat 25 25 Aroclor 1016 96 h - LC50 0.019
12 days stat 25 25 Aroclor 1016 96 h - LC50 0.021
15 days stat 25 25 Aroclor 1016 96 h - LC50 0.024
18 days stat 25 25 Aroclor 1016 96 h - LC50 0.037
30 days stat 25 25 Aroclor 1016 96 h - LC50 0.044
adult stat 25 25 Aroclor 1016 96 h - LC50 0.052 (0.046-0.057)
adult flow 30 28 Aroclor 1016 96 h - LC50 0.012
1 day stat 25 25 Aroclor 1242 96 h - LC50 0.015
3 days stat 25 25 Aroclor 1242 96 h - LC50 0.019
6 days stat 25 25 Aroclor 1242 96 h - LC50 0.015
9 days stat 25 25 Aroclor 1242 96 h - LC50 0.017
12 days stat 25 25 Aroclor 1242 96 h - LC50 0.016
15 days stat 25 25 Aroclor 1242 96 h - LC50 0.024
18 days stat 25 25 Aroclor 1242 96 h - LC50 0.034
30 days stat 25 25 Aroclor 1242 96 h - LC50 0.041
adult stat 25 25 Aroclor 1242 96 h - LC50 0.057 (0.048-0.062)
a stat = static conditions: water not changed during the exposure; flow = flow-through conditions; concentration
of toxicant continuously maintained.
(Crassostrea virginica) at concentrations of 1, 10, and 100 µg
Aroclor 1254/litre and monitored shell growth over a period of 96 h;
the rates of shell growth were decreased by 19, 41, and 100%,
respectively. Lowe et al. (1972) found the growth rate (height and wet
weight) of young oysters (Crassostrea virginica) to be significantly
reduced after exposure, in flowing sea water, to 5 µg Aroclor
1254/litre, for 24 weeks. No effects on growth were observed at
1 µg/litre over a period of 30 weeks. Oysters exposed to 5 µg/litre
showed atrophy of the digestive diverticular epithelium and
degeneration of the vesicular connective tissues of the hepatopancreas
together with leukocytic infiltration. There was complete tissue
recovery after 12 weeks in clean water.
After exposing Daphnia magna to Aroclor 1254, over a period of 14
days under static renewal procedures, Maki & Johnson (1975) calculated
an LC50 of 24 µg/litre.
Nebeker & Puglisi (1974) calculated 3-week LC50 values, under static
conditions, for a range of Aroclors on Daphnia magna. Aroclors were
dissolved in acetone and triton X100 to maintain the PCBs in solution.
Tests were performed in raw Lake Superior water. Results are presented
in Table 29. The Aroclors most toxic for Daphnia had between 48 and
62% chlorination; the most toxic Aroclor was 1248. Under flow-through
conditions, renewing the original test concentration of the Aroclor
continuously, the Aroclors were much more toxic. Two-week LC50 values
for Aroclors 1248 and 1254 were 2.6 and 1.8 µg/litre, respectively,
while the 3-week LC50 for Aroclor 1254 was 1.3 µg/litre. Groups of 40
scud (Gammarus pseudolimnaeus) were exposed to various concentrations
of Aroclor 1242 under flow-through conditions. No animals survived
exposure for 2 months, at Aroclor concentrations of 26 µg/litre or
more.
Survival at lower exposures was 52% at 8.7 µg/litre and 77% at
2.8 µg/litre (control survival was low at 48%).
Duke et al. (1970) conducted acute, flowing-water bioassays on pink
shrimp (Penaeus duorarum). At 0.1 mg/litre, 100% of the shrimps were
killed within 48 h of exposure to Aroclor 1254. There was no mortality
after 48 h of exposure to 0.01 mg/litre Aroclor. In long-term
flowing-water bioassays, Nimmo et al. (1971a) found that Aroclor 1254,
at a concentration of 0.94 µg/litre killed 51% of juvenile pink
shrimps within 15 days. Juveniles were found to be more sensitive than
adults; 50% of adults were killed after exposure to 3.5 µg/litre over
35 days. There were no apparent symptoms of poisoning prior to death.
Table 29. Toxicity of various Aroclors for Daphnia magna in static testsa
Aroclor 3-week Confidence limits (95%)
LC50 (µg/litre)
1221 180 (158.0-205.0)
1232 72 (62.6-82.8)
1242 67 (55.4-81.0)
1248 25 (21.4-29.2)
1254 31 (25.8-37.2)
1260 36 (27.7-46.8)
1262 43 (37.0-49.9)
1268 253 (222.0-288.0)
a From: Nebeker & Puglisi (1974).
Striped hermit crabs (Clibanarius vittatus) were kept in static
seawater solutions containing 3, 5, 10, 15, 20, 25, or 30 µg/litre of
Aroclor 1254 for 96 h (Stahl, 1979). No deaths were reported, though
the crabs exposed to the higher concentrations (20, 25, and
30 µg/litre) were less active. Six crabs already exposed to 30 µg
PCBs/litre were then placed in solutions containing 300 µg/litre for a
further 96 h; there were still no deaths.
Vernberg et al. (1977) exposed fiddler crab (Uca pugilator) larvae
to concentrations of 0.1, 1.0, 5, 10, 50, or 500 µg Aroclor
1254/litre, for 96 h. They did not find any effects on survival at 0.1
or 1.0 µg/litre; Aroclor 1254 at 5 µg/litre increased deaths by 20%,
but the increase was not statistically significant. Exposure to 10 µg
PCBs/litre resulted in a 57% reduction in survival of larvae.
Increasing the PCB concentration to 50 µg/litre did not greatly
increase the effect. The 500 µg/litre concentration killed all larvae.
Increasing exposure time, at 5 µg/litre, produced a significant
reduction in survival after 14 days. Exposure of larvae to Aroclors
1016 or 1254 at 0.1, 1, or 5 µg/litre for periods of up to 168 h, was
then investigated. No significant effects were found on survival with
any concentration of Aroclor 1016, for up to 120 h of exposure. After
168 h, survival rates of larvae exposed to 1 and 5 µg/litre were
reduced to 61 and 66%, respectively. There was no effect at
0.1 µg/litre. Aroclor 1254 did not have any effects on survival at
concentrations of 0.1 or 1 µg/litre. Survival was reduced after
exposure to 5 µg Aroclor 1254/litre for more than 96 h, increasing
from 60-81% up to 168 h. Exposure to 10 µg Aroclor 1254/litre caused
55% deaths after 120 h; there were no further deaths after 168 h.
Fifty per cent of adult male crabs, exposed to Aroclor 1254 or Aroclor
1016 at 50 µg/litre, died after 2 days and after 4-6 days,
respectively. Females (50%) exposed to 50 µg/litre survived for 7 days
after exposure to Aroclor 1016 but for only 4 days after exposure to
Aroclor 1254.
Neff & Giam (1977) exposed juvenile horseshoe crabs (Limulus
polyphemus) to concentrations of Aroclor 1016 of 10, 20, 40, or
80 µg/litre, for up to 96 days. The crabs were divided into 2 groups:
group A consisted of juveniles at the late first tailed stage and
group B, of juveniles at the early second tailed stage. The authors
calculated LT50s (LT50: median survival time for a given exposure
concentration) of 20.8 days at 40 µg/litre and 20.3 days at
80 µg/litre for group A juveniles. The LT50 for group B crabs at
80 µg/litre was 61 days, but less than 50% had died within 96 days at
40 µg/litre.
7.2.2.2 Response to temperature and salinity
In a study by Vernberg et al. (1977), larval fiddler crabs
(Uca pugilator) were exposed to "sub-lethal" concentrations of
Aroclor 1254 and 1016 and the conditions of temperature (15-30°C) and
salinity (15-36%) varied. Exposure to 0.01 µg Aroclor/litre showed no
consistent effects of temperature or salinity, though the organisms
exposed under conditions furthest from the optimum (25°C and 30%) were
more likely to differ significantly from the controls. At the optimum
temperature and salinity, no deaths were recorded in adult crabs
exposed to 0.1, 1, 10, or 100 µg Aroclor 1254/litre for up to 3 weeks.
Lowering the salinity or increasing the temperature did not have any
effects on survival with Aroclor 1254 or 1016 at 5 µg/litre. Lowering
both salinity and temperature (to 5% and 10°C) caused 50% of crabs to
die between 21 and 28 days of exposure to Aroclor 1016 at 5 µg/litre,
but there were no lethal effects of Aroclor 1254 at the same
concentration. Lowering the temperature further (7°C and 5%) reduced
the 50% survival time to between 5 and 8 days for both Aroclors.
Nimmo & Barrier (1974) reported some deaths in adult brown shrimp
(Penaeus aztecus) exposed to a "sub-lethal" concentration of Aroclor
1254 (3 µg/litre), for 7 days, after subjection to salinity shock.
The shrimp normally adapts readily to the wide range of salinity
found in its natural estuarine habitat. Roesijadi et al. (1976a)
exposed the adult grass shrimp (Palaemonetes pugio) to sub-lethal
(6.3-8.8 µg/litre) and lethal (57.6-76.4 µg/litre) concentrations of
Aroclor 1254 for 96 h, at various salinities. They found little effect
on haemolymph chloride concentration or osmolarity, chloride space
(the apparent volume of distribution of chloride ions), or chloride
exchange kinetics. The shrimp showed an adaptive altered permeability
to chloride ions at a salinity of 17%, the isotonic point. PCBs did
not affect this permeability change in adult shrimp. In the juvenile
grass shrimp, there was a reduction in haemolymph chloride levels at
low salinities in non-steady state exposures; the PCBs delayed the
permeability change. This disruption of haemolymph chloride was
associated with high numbers of deaths, even at the "sub-lethal"
exposure concentration. It was concluded that juveniles died from
salinity shock, because of delayed adaptive response. In another study
(Roesijadi et al., 1976b), grass shrimp were exposed to Aroclor 1254
at 29.4 µg/litre for 96 h at various salinities. No appreciable effect
was observed on total free amino acid levels in abdominal muscle,
indicating that intracellular osmoregulation was not a major
consequence of PCB toxicity, though changes in individual amino acid
concentrations suggested an altered metabolic state. The authors found
that blood glycine levels showed large decreases after the transfer of
the shrimps to clean water, a delayed response to the Aroclor
exposure.
7.2.2.3 Reproduction
Sea urchin (Arbacia punctulata) eggs were exposed to concentrations
of Aroclor 1254 of 0.5, 1.0, 5.0, and 10.0 mg/litre (Adams, 1983).
There was no effect on percentage fertilization, percentage pluteus
development, or percentage mortality when eggs were exposed at
fertilization. However, when eggs were exposed 1 h prior to
fertilization, there was a significant reduction in fertilization
efficiency at all doses. At all but the lowest dose, there was a
significant increase in mortality and a significant depression in
successful pluteus development.
Maki & Johnson (1975) calculated EC50s for total young produced,
average brood size, and percentage of days reproducing, during a
14-day exposure of Daphnia magna to Aroclor 1254; the results were
19, 23, and 25 µg/litre, respectively. Daphnia magna were exposed to
a range of Aroclors, by Nebeker & Puglisi (1974) who estimated
reproductive impairment, measured as a percentage of surviving young
relative to controls. The study was conducted over 3 weeks under
static conditions. Results are presented in Table 30. Reproductive
impairment matches lethality of the Aroclors (see Table 29); there was
no indication of reproductive effects of the Aroclors at
concentrations below those leading to the death of adults or young. No
young were produced by scud (Gammarus pseudolimnaeus) exposed to
8.7 µg Aroclor 1242/litre. Scud exposed at 2.8 µg/litre produced fewer
young per surviving adult (4.2), compared with controls (6.8).
Table 30. Reproductive impairment of Daphnia magna exposed to Aroclors under
static conditionsa
Aroclor Concentration (µg/litre) producing reproductive impairment
50% 16%
1221 125 89
1232 66 53
1242 63 48
1248 24 16
1254 28 18
1260 33 22
1262 41 24
1268 206 162
a From: Nebeker & Puglisi (1974).
7.2.2.4 Moulting
Several authors have suggested that crustaceans may be more
susceptible to the toxic effects of PCBs during moult (Duke et al.,
1970; Wildish, 1970; Nimmo et al., 1971a,b). Fingerman & Fingerman
(1979) exposed 2 groups of fiddler crabs (Uca pugilator) at 8 mg
Aroclor 1242/litre, one group for 40 days, and the other for only the
first 14 of the 40 days. Eye-stalks were removed on day 15 to increase
moulting activity. Controls underwent rapid ecdysis, with more than
50% of the population completing moult within 40 days. Crabs exposed
to the Aroclor for the full 40 days showed less moulting, with no more
than 10% of the population completing moult. Crabs exposed for 14 days
showed 20% of the population successfully moulting. Removal of
eye-stalks on day 1 of the study produced similar results, in terms of
numbers of crabs moulting in each treatment, but speeded-up moult in
the controls. No more Aroclor-exposed crabs moulted after eye-stalk
removal. In an earlier study, Fingerman & Fingerman (1977) exposed
fiddler crabs to Aroclor 1242 at 8 mg/litre for 38 days. Either
eye-stalks or 4 walking legs were removed to stimulate moulting. Both
control groups underwent ecdysis rapidly. Treated crabs without
eye-stalks did not undergo any moult. Moulting in those with legs
removed was much slower than in the controls. The authors also exposed
crabs to dibenzofuran (1,2,3,4,5,6,7,8-octachlorodibenzofuran) at
16 ng/litre. This is equivalent to the maximum reported dibenzofuran
contamination of the Aroclor with the dose equivalent to (in terms of
the dibenzofuran) the same concentration (8 mg/litre) in the PCB
mixture. There was only a slight inhibition of moulting caused by the
dibenzofuran.
Neff & Giam (1977) exposed juvenile horseshoe crabs (Limulus
polyphemus) to concentrations of Aroclor 1016 of 10, 20, 40, or
80 µg/litre, for up to 96 days. The crabs were divided into 2 groups:
group A consisted of juveniles at the late first tailed stage and
group B of juveniles at the early second tailed stage. ET50s (median
time for moulting to begin) were calculated between the start of the
study and the first moult (ET50-1) and between subsequent moults
(ET50-2 to -n). No moult occurred within 96 days at concentrations of
Aroclor of 40 or 80 µg/litre in either group of crabs. The ET50-1, in
group A at 10 and 20 µg/litre, was not different from controls; the
ET50-2 was slightly decreased. In group B, 10 and 20 µg/litre did not
affect the ET50-1; 40 and 80 µg/litre delayed the onset of the first
moult by 7 and 9 days, respectively. The ET50-3 were substantially
decreased at 10, 20, and 40 µg Aroclor/litre.
7.2.2.5 Behaviour
Hansen et al. (1974a) studied the avoidance response of the pink
shrimp (Penaeus duorarum) and the grass shrimp (Palaemonetes pugio)
given a choice between clean water and water containing Aroclor 1254,
at concentrations of between 0.001 and 10 mg/litre. Pink shrimp did
not avoid any of the concentrations used; grass shrimp only
significantly avoided the highest dose.
7.2.2.6 Population structure
The composition of communities of estuarine animals, in aquaria, were
studied under different exposures to Aroclor 1254 (0.1, 1.0, and
10.0 µg/litre) for 4 months (Hansen, 1974). The author found that, in
control groups and the group at the lowest concentration, the
community was mainly comprised (>75%) of arthropods, mostly the
amphipod Corophium volutator. At 1 and 10 µg Aroclor 1254/litre, the
numbers of arthropods decreased and the numbers of chordates
increased; at 10 µg/litre, over 75% of the animals were tunicates. The
highest concentration of Aroclor decreased the numbers of phyla,
species, and individuals represented (particularly of amphipods,
bryozoans, crabs, and molluscs), whereas the numbers of annelids,
brachypods, coelenterates, echinoderms, and nemertines were
unaffected.
7.2.2.7 Interactions with other chemicals
Maki & Johnson (1975) exposed Daphnia magna to various combinations
of DDT (0.2-0.75 µg/litre) and Aroclor 1254 (2-24 µg/litre). They
studied adult mortality, total young produced, average brood size, and
percentage days reproducing, during a 14-day exposure period. The
effects of one toxicant significantly enhanced the action of the other
for all test parameters. In the presence of a no-observed-effect level
of PCB (12 µg/litre), the susceptibility of Daphnia to DDT increased
by one third. When combined with 0.5 µg DDT/litre, the toxicity of
Aroclor 1254 was doubled.
In a study by Nimmo & Bahner (1976), the pink shrimp (Penaeus
duorarum) was exposed to various combinations of Aroclor 1254
(0.7-1.1 µg/litre), cadmium (640-829 µg/litre), and methoxychlor
(0.9-1.0 µg/litre) and numbers of shrimps dying were monitored. The
results showed no evidence of synergism or potentiation in any
combination.
7.2.3 Fish
The acute toxicity of PCBs for fish is summarized in Table 31. Values
for 96-h LC50s vary between 0.008 mg/litre, for the fry of the
fathead minnow, to > 100 mg/litre for channel catfish. This
considerable variation is dependent on species and on the PCB mixture
but appears to depend little on test conditions, such as temperature
and water hardness. The toxicity of PCBs appears much greater in
flow-through tests, where the water concentration of the PCBs is
constantly maintained.
MATC (maximum acceptable toxicant concentrations) were set by the US
EPA (1980) and are expressed as a range between the no-observed-effect
level (based on the results of long-term studies and the sub-lethal as
well as the lethal effect) and the lowest concentration showing a
measurable effect. These values, for the fathead minnow, were 5.4 and
15.0 µg/litre for Aroclor 1242; 0.1 and 0.4 µg/litre for Aroclor 1248;
1.8 and 4.6 µg/litre for Aroclor 1254, and 1.3 and 4.0 µg/litre for
Aroclor 1260. The early life stage of the estuarine sheepshead minnow
gave values of 3.4 and 15.0 µg/litre for Aroclor 1016 and 0.06 and
0.16 µg/litre for Aroclor 1254.
7.2.3.1 Short- and long-term toxicity
The toxicity of Aroclors for 3 species of freshwater fish, over
exposure of up to 30 days, was systematically investigated by Mayer et
al. (1977) under flow-through conditions. Results are presented in
Table 32. Short-term tests consistently underestimate the toxicity of
PCBs.
Table 31. Acute toxicity of PCB and PCT mixtures for fish
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Channel catfish 0.60 g stat 20 40 7.4 Aroclor 1016 96-h LC50 > 100
(Ictalurus yolk-sac stat 25 272 7.4 Aroclor 1016 96-h LC50 0.44 (0.34-0.56)
punctatus) 2.80 g flow 17 272 7.4 Aroclor 1242 96-h LC50 >0.10
Mayer & 2.80 g flow 22 272 7.4 Aroclor 1248 96-h LC50 >0.1
Ellersieck 2.80 g flow 22 272 7.4 Aroclor 1254 96-h LC50 >0.20
(1986) 2.80 g flow 22 272 7.4 Aroclor 1260 96-h LC50 >0.40
Atlantic salmon 5.60 g flow 17 314 7.6 Aroclor 1016 96-h LC50 0.13 (0.11-0.16)
(Salmo salar)
Mayer &
Ellersieck (1986)
Brook trout 3.0 g flow 12 314 7.6 Aroclor 1016 96-h LC50 >0.80
(Salvelinus
fontinalis)
Mayer &
Ellersieck (1986)
Brown trout 4.60 g flow 12 314 7.6 Aroclor 1016 96-h LC50 0.14 (0.11-0.18)
(Salmo trutta) 1.10 g stat 13 44 7.4 Aroclor 1260 96-h LC50 > 24.0
Mayer &
Ellersieck (1986)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Cutthroat trout 2.70 g stat 9 159 162 7.4 Aroclor 1221 96-h LC50 1.17 (0.96-1.43)
(Salmo clarki) 2.20 g stat 9 159 162 7.4 Aroclor 1232 96-h LC50 2.5 (1.72-3.08)
Mayer & 2.40 g stat 9 159 162 7.4 Aroclor 1242 96-h LC50 5.42 (3.82-7.68)
Ellersieck 2.50 g stat 9 159 162 7.4 Aroclor 1248 96-h LC50 5.75 (5.1-6.5)
(1986) 2.50 g stat 9 159 162 7.4 Aroclor 1254 96-h LC50 42.5 (38.7-46.7)
2.70 g stat 9 159 162 7.4 Aroclor 1260 96-h LC50 60.9 (55.4-67.0)
2.40 g stat 9 159 162 7.4 Aroclor 1262 96-h LC50 > 50
2.20 g stat 9 159 162 7.4 Aroclor 1268 96-h LC50 > 50
2.70 g stat 9 162 7.4 Aroclor 4465 96-h LC50 > 65
2.10 g stat 9 162 7.4 Aroclor 5442 96-h LC50 > 50
2.90 g stat 9 162 7.4 Aroclor 5460 96-h LC50 > 50
Lake trout fry stat 10 170 7.2 Aroclor 1016 96-h LC50 0.48 (0.39-0.60)
(Salvelinus yolk-sac stat 10 170 7.2 Aroclor 1016 96-h LC50 0.89 (0.69-1.15)
namaycush)
Mayer &
Ellersieck (1986)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Rainbow trout 0.50 g stat 12 44 7.4 Aroclor 1016 96-h LC50 0.14 (0.11-0.16)
(Salmo gairdneri) 2.50 g flow 10 272 7.4 Aroclor 1016 96-h LC50 0.62 (0.42-0.90)
Mayer & fry flow 12 314 7.6 Aroclor 1016 96-h LC50 0.44 (0.37-0.53)
Ellersieck 1.80 g flow 17 272 7.4 Aroclor 1242 120-h LC50 0.07
(1986) 1.80 g flow 17 272 7.4 Aroclor 1248 120-h LC50 0.05
1.80 g flow 17 272 7.4 Aroclor 1254 120-h LC50 0.14
1.80 g flow 17 272 7.4 Aroclor 1260 96-h LC50 >0.23
Harlequin fish 10-30 mm flow 20 20 8.1 Aroclor 1221 96-h LC50 1.05
(Rasbora 10-30 mm flow 20 20 8.1 Aroclor 1232 96-h LC50 0.32
heteromorpha) 10-30 mm flow 20 20 8.1 Aroclor 1242 96-h LC50 0.37
Tooby et al. 10-30 mm flow 20 20 8.1 Aroclor 1254 96-h LC50 1.1
(1975) 10-30 mm flow 20 20 8.1 Aroclor 1262 96-h LC50 >100
Bluegill sunfish 0.90 g stat 12 44 7.4 Aroclor 1016 96-h LC50 0.60
(Lepomis 1.80 g flow 20 272 7.4 Aroclor 1016 96-h LC50 0.46 (0.39-0.54)
macrochirus) 2.20 g flow 17 272 7.4 Aroclor 1242 120-h LC50 0.13
Mayer & 0.80 g stat 18 44 7.1 Aroclor 1248 96-h LC50 0.69 (0.48-0.99)
Ellersieck 2.20 g flow 22 272 7.4 Aroclor 1248 120-h LC50 0.14
(1986) 0.80 g stat 18 44 7.1 Aroclor 1254 96-h LC50 2.74 (1.29-5.81)
2.20 g flow 22 272 7.4 Aroclor 1254 96-h LC50 0.20
2.20 g flow 22 272 7.4 Aroclor 1260 96-h LC50 0.40
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Longnose sucker finger flow 12 314 7.5 Aroclor 1016 96-h LC50 0.33 (0.22-0.49)
(Catostomus
catostomus)
Mayer &
Ellersieck (1986)
Yellow perch 1.20 g flow 17 314 7.6 Aroclor 1242 96-h LC50 >0.15
(Perca flavescens) 1.10 g flow 17 314 7.6 Aroclor 1248 96-h LC50 >0.1
Mayer & 1.00 g flow 17 314 7.6 Aroclor 1254 96-h LC50 >0.15
Ellersieck 1.20 g flow 17 314 7.6 Aroclor 1260 96-h LC50 >0.20
(1986)
Fathead minnow fry flow 24 Aroclor 1254 96-h LC50 0.008
(Pimephales fry flow 24 Aroclor 1242 96-h LC50 0.015
promelas) 3 months flow 24 Aroclor 1242 96-h LC50 0.30
Nebeker
et al. (1974)
Cisco (chub) 22 days flow 7 30-35 40-48 Aroclor 1254 96-h LC50 >10
(Coregonus sp.) 22 days flow 7 30-35 40-48 Aroclor 1254 120-h LC50 3.2 (1.9-5.5)
Passino &
Kramer (1980)
Table 31. (cont'd).
Organism/ Size/ Stat/ Temperature Alkalinityb Hardnessb pH PCB type Parameter Concentration
reference age flowa (°C) (mg/litre)
Carp (Cyprinus fry stat 23-25 Kanechlor 300 96-h LC50 1.45
carpio)
Kimura et al.
(1974)
Guppy (Lebistes fry stat 24-25 Kanechlor 300 96-h LC50 0.9
reticulatus) 0.35 g stat 24-25 Kanechlor 300 96-h LC50 3.2
Kimura et al. (1974)
a stat = static conditions: water not changed during the exposure;
flow = flow-through conditions; concentration of toxicant continuously maintained.
b Alkalinity and hardness expressed as mg/litre CaCO3, unless otherwise stated.
Table 32. Toxicity of Aroclors for fish (LC50s in µg/litre) at 17°Ca
Aroclor Exposure (days)
5 10 15 20 25 30
Rainbow trout
1242 67 48 18 10 12 -
1248 54 38 16 6.4 3.4 -
1254 - 160 64 39 27 -
1260 - 326 143 78 49 51
Bluegill sunfish
1242 - - 164 125 120 84
1248 136 115 111 106 100 78
1254 - - 303 260 239 177
1260 - - - - - 400
Channel catfish
1242 - - 219 150 132 87
1248 - 121 121 115 104 75
1254 - 303 286 293 181 139
1260 - 535 482 512 465 433
a From: Mayer et al. (1977).
Duke et al. (1970) kept juvenile pinfish (Lagodon rhomboides) in
seawater containing 1, 10, or 100 µg Aroclor 1254/litre for up to
48 h. There were no deaths at any concentration. It was suggested by
Nimmo et al. (1975) that acute toxicity tests underestimated the true
sensitivity of marine species; in bioassays lasting 1 week or more,
Aroclor proved to be 100 times more toxic than acute exposure
suggested. In tests lasting 2 weeks or longer, Aroclor 1254 was lethal
for longnose killifish (Fundulus similis) at 1 µg/litre and for
pinfish and spot (Leiostomus xanthurus) at 5 µg/litre. Hansen et al.
(1974b) did not find any significant lethal effects in pinfish exposed
to 100 µg Aroclor 1016/litre for 96 h but significant mortality (50%)
was observed after 33 days at 32 µg/litre, and, after 18 days, at
100 µg/litre. Nebeker et al. (1974) exposed the flagfish (Jordanella
floridae) to Aroclor 1248 for 40 days. No fish survived at a
concentration of 18 µg/litre and only 35% survived at 5.1 µg/litre.
The fish at these two concentrations almost completely lost their fins
and tails. Fish survival was not affected at concentrations of
2.2 µg/litre or less. In a study by Defoe et al. (1978), fathead
minnow (Pimephales promelas) larvae were exposed, in flow-through
bioassays, to Aroclors 1248 and 1260 for 30 days; the LC50s were
calculated to be 4.7 and 3.3 µg/litre, respectively.
Hansen et al. (1976a) fed fingerling channel catfish (Ictalurus
punctatus) a diet containing 20 mg Aroclor 1242/kg for 20 weeks. The
fish showed a reduced weight gain and hypertrophy of the liver. When
the treated fish were transferred to a control diet for 8 weeks and
then back to the dosed diet for a further 8 weeks, weight gain and
liver weights returned to normal levels. No histopathological lesions
were observed in any of the fish fed PCBs.
Rainbow trout (Salmo gairdneri) were fed a diet containing 1, 10, or
100 mg Aroclor 1254/kg over a period of 330 days (Nestel & Budd,
1975). No effects on growth rates were seen, but renal lesions were
observed at all doses; however, they were not dose-related. Foci of
renal necrosis, with cellular or granular cast formation were seen. A
significant increase in the number of hepatocytes per unit area in the
liver was observed at all doses and appeared to be dose-related. A
reduction of 'white pulp' (lymphatic elements) in the spleen was
observed at 10 and 100 mg/kg diet. Fish with renal necrosis also had
reduced splenic white pulp and a reduced white cell count.
7.2.3.2 Carcinogenicity
Hendricks et al. (1977) studied the combined effects of Aroclor 1254
(100 mg/kg diet) and aflatoxin B1 (6 mg/kg diet) in rainbow trout. A
significantly reduced incidence of liver tumours was observed in the
combination. There was no retardation of growth in the treated
animals, but they showed glycogen depletion in hepatocytes,
hyperaemia, and white pulp depletion in the spleen.
PCB administration prior to aflatoxin B1 treatment also decreased the
liver tumour incidence, whereas when Aroclor 1254 was fed after
exposure of trout embryo to aflatoxin B1, there was no effect on the
formation of liver tumours (Shelton et al., 1984b).
When rainbow trout were fed 0, 1, 4, or 8 mg aflatoxin B1/kg diet or
aflatoxin B1 at these dose levels plus 50 mg Aroclor 1254/kg diet,
the incidence of hepatic tumours was lower in the groups receiving
aflatoxin combined with PCBs (Shelton et al., 1984a). The inhibition
of aflatoxin B1-mediated carcinogenicity was also correlated with the
decreased bacterial mutagenicity of this compound in the presence of
an Aroclor 1254-induced drug metabolizing enzyme fraction from fish.
The potential mechanisms of this interaction were further investigated
by Shelton et al. (1986) by studying the effects of Aroclor 1254 on
aflatoxin B1 distribution, metabolism, and adduct formation. The
results from the in vivo studies showed that PCB treatment resulted
in a marked increase in the metabolism of aflatoxin B1 to aflatoxin
M1 and their glucuronide conjugates. The DNA-adduct levels in the
PCB-treated fish were 48-96% lower than those in the controls. The
results in the fish model using aflatoxin B1 as the carcinogen were
associated with the activity of Aroclor as an inducer of cytochrome
P-450-dependent monooxygenases (Halverson et al., 1985; Shelton et
al., 1986).
7.2.3.3 Effects on developmental stages and reproduction
Birge et al. (1978) determined LC50s and LC1s for 4 species of fish
from the fertilization of the eggs to 4 days after hatching. Exposure
times varied with species dependent on the time taken to hatching of
the eggs; hatching took 22 days for rainbow trout and 3-4 days for the
other species. In most species, eggs were considerably less sensitive
to the toxic effects of Aroclors than larvae. The major exception was
the rainbow trout for which the duration of exposure of the eggs until
hatching was considerably longer than for other species. LC50s ranged
from 0.32 to 11.16 µg/litre for the 4 species (up to 4 days after
hatching) and 4 different PCB mixtures; LC1s ranged from 0.009 to
0.26 µg/litre. Results are presented in Table 33. The rainbow trout
was the most sensitive species tested.
Adult minnows (Phoxinus phoxinus) were fed Clophen A50 at 20, 200,
or 2000 mg/kg diet (on a dry weight basis), for 40 days (Bengtsson,
1980). Growth was monitored from day 46 to day 79; a significant
increase in growth (relative to controls) was seen in fish fed the
highest dose. Other doses caused increases in growth, but these were
not significant. Stimulated growth had been observed in previous
studies with minnows fed Clophen A50 at 0.88-78 mg/kg diet (Bengtsson,
1979). Between days 127 and 166, the swimming performance of the fish
was tested using a rotary flow technique. Although the PCBs impaired
swimming performance, this was not statistically significant.
Reproduction was monitored from day 235 (first day of spawning) to day
300. Spawning was delayed in the treated groups by 1 day, 1 week, and
3 weeks for the 3 treatments (20, 200, and 2000 mg/kg diet),
respectively. Only the highest dose affected hatchability of eggs,
which was reduced by approximately 80%.
Snarski & Puglisi (1976) did not find any adverse effects on survival,
growth, or reproduction of brook trout (Salvelinus fontinalis)
exposed to concentrations of Aroclor 1254 of up to 0.94 µg/litre, for
71 weeks. Survival and growth of alevin-juveniles from exposed parents
were unaffected for up to 90 days.
Continuous-flow bioassays were conducted over 8 months on fathead
minnow (Pimephales promelas), exposing the fish to either Aroclor
1242 or 1254 (Nebeker et al., 1974). Reproduction occurred at, and
below, 5.4 µg Aroclor 1242/litre, but spawning and egg production
were very variable. With Aroclor 1254, reproduction occurred at, and
below, 1.8 µg/litre. Spawning occurred at 1.8 µg/litre, but was
significantly less than spawning at the lower concentrations of 0.23
and 0.52 µg/litre. Egg hatchability and fry survival were good at
1.8 µg/litre. Eggs were more resistant than fry at 15 and 51 µg
Aroclor 1242/litre; with Aroclor 1254 at a concentration of
15 µg/litre, eggs hatched readily, but all fry were dead within 96 h.
Halter & Johnson (1974) kept coho salmon (Oncorhynchus kisutch) eggs
in solutions containing 4.4-56.4 µg Aroclor 1254/litre. Exposure
continued for 2 weeks before, and 4 weeks after hatching, until the
young were alevins. Hatchability was reduced by 30% at the highest
concentration of 56.4 µg/litre. Survival of alevins was markedly
higher when eggs were transferred to clean water prior to hatching,
but there was still 58% mortality in alevins hatched from eggs at the
highest dose. When the alevins were exposed to the PCBs for 4 weeks
after hatching, survival was inversely related to exposure
concentration. No group survived as well as the controls (for example,
18% died at 4.4 µg/litre and 90% at 26 µg/litre, or more).
In a study by Defoe et al. (1978), fathead minnow (Pimephales
promelas) were maintained in flow-through bioassays in solutions of
Aroclors 1248 or 1260, for 240 days (a full life-cycle test).
Reproduction occurred at all concentrations tested (up to 3 µg/litre
for Aroclor 1248 and up to 2.1 µg/litre for Aroclor 1260). The authors
concluded that PCBs did not produce major effects on reproduction at
concentrations up to the 30-day LC50 and that reduced populations
after long-term exposure are largely due to larval mortality.
Weis & Weis (1982) exposed eggs of the mummichog (Fundulus
heteroclitus) to concentrations of Aroclor 1254 of 0.01-10 mg/litre
(after cleavage had begun). No effect was found on embryonic
development or hatching and embryonic mortality was negligible.
Seven-day larval tests also showed no effect on mortality up to the
highest concentration. However, the authors found approximately 20%
larval mortality, when larvae were exposed to 5 mg/litre for 72 h,
after hatching from eggs at the highest exposure concentration. In a
second group of studies, eggs were exposed to 10 mg Aroclor
1242/litre; no malformations were found, though there was a consistent
retardation of hatching. There was a positive correlation between
hatching rate and female length (length being related to age). Larvae
exposed to 5 mg/litre showed an average of 45% mortality within 72 h.
Pre-exposure as eggs to 10 mg/litre greatly increased larval
mortality; pre-exposure to 1 mg/litre resulted in an intermediate
response. There was no mortality in control larvae, even when they had
been pre-exposed as eggs.
Eggs of brook trout (Salvelinus fontinalis) were exposed to Aroclor
1254 (0.043-13 µg/litre) for 10 days before hatching, and the fry for
118 days after hatching (Mauck et al., 1978). Median hatching time,
egg hatchability, and sac-fry survival were not affected by the PCBs.
Significantly decreased survival (32% survived) was seen after 48 days
at 3 µg/litre. There was significant mortality of fry after 118 days
with exposure to concentrations of 3.1 µg/litre and above. Growth of
the trout, as measured by weight, was significantly decreased after 48
days at concentrations of 1.5 µg/litre or more. By the end of the
study (118 days), no significant differences in weight were seen
between surviving fry on different treatments. Analysis of the
backbone composition at this time showed that hydroxyproline and
phosphorus were significantly decreased by concentrations of
0.43 µg/litre or more and that, at 0.69 µg or more/litre, calcium
levels were significantly increased. Although collagen was
significantly decreased at 0.69, 3.1, and 6.2 µg/litre, it was
unaffected at 1.5 µg/litre; no explanation for the anomaly was
suggested.
Schimmel et al. (1974) exposed sheepshead minnow (Cyprinodon
variegatus) eggs, immediately after fertilization, to Aroclor 1254
at concentrations of between 0.1 and 10 µg/litre. The fertility of
eggs was unaffected. Hatching was significantly reduced (by 30%) only
at the highest exposure. Survival of fry to 2 weeks was significantly
reduced at concentrations of 0.32 µg/litre or above (30% survival at
0.32 µg/litre and 9% survival at 10 µg/litre). The 3-week LC50 for
embryo-fry was calculated to be 0.93 µg/litre for Aroclor 1254. Many
of the dying fish exhibited fin rot. Exposure of juveniles and adults
to the same concentrations of the Aroclor produced 24% mortality in
juveniles at the highest exposure rate and no mortality in adults.
Some fin rot was seen in adults.
Table 33. Toxicity of PCBs for the embryolarval stages of fisha,b
Organism Aroclor 1016 Aroclor 1242 Aroclor 1254
LC1 LC50 LC1 LC50 LC1 LC50
(µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre)
Channel catfish 0.08 11.16 0.14 4.24 0.05 1.76
(Ictalurus punctatus) (9.93-12.97) (0.07-0.23) (3.32-5.34) (0.02-0.09) (1.36-2.24)
Goldfish 0.10 13.21 0.04 2.64 0.02 1.18
(Carassius auratus) (10.63-16.43) (0.01-0.08) (1.89-3.61) (0.01-0.04) (0.84-1.61)
Rainbow trout 0.011 1.08 0.01 1.03 0.009 0.32
(Salmo gairdneri) (0.003-0.027) (0.7-1.56) (0.002-0.025) (0.67-1.51) (0.003-0.02) (0.22-0.45)
Redear sunfish 0.26 7.82 0.19 3.56 0.02 0.53
(Lepomis microlophus) (0.1-0.51) (5.74-10.35) (0.08-0.35) (0.65-4.66) (0.01-0.04) (0.39-0.7)
a From: Birge et al. (1978).
b Exposure under static conditions, but with water renewed every 12 h. Exposure was initiated 2-6 h after spawning (except
for rainbow trout where exposure was initiated 15 min after fertilization) and continued to 4 days post-hatching. Hatching
times varied: 22 days for rainbow trout (13.5-14.3°C); 3 days for catfish (29-31°C); 3-4 days for goldfish and sunfish
(20-24°C); hardness 90-115 mg/litre; pH 7.6-8.1.
In another study, Hansen et al. (1975) exposed embryos, fry,
juveniles, and adults of sheepshead minnow (Cyprinodon variegatus)
to concentrations of Aroclor 1016 of 0.1-10 µg/litre, for 28 days, in
intermittent flow bioassays. No effects on survival were observed
during this period. When exposed to concentrations of 32 or
100 µg/litre, there was high mortality in eggs, juveniles, and adults;
all were killed at 100 µg/litre. The authors calculated that the
28-day LC50s for juveniles and adults were 20 and 19 µg/litre,
respectively.
Freeman et al. (1982) fed Atlantic cod (Gadus morrhua) diets
containing 1-50 mg Aroclor 1254/kg for 5.5 months. Altered steroid
biosynthetic patterns in vitro were observed in the testes and head
kidneys (adrenal equivalent) of dosed fish. Histological examination
revealed abnormalities in the testes, gills, and livers. Testicular
abnormalities included derangement of lobules, hyperplasia of lobule
walls, and disintegration and/or fatty necrosis of spermatogenic
elements. In fish fed at dietary rates of 5-50 mg/kg, hyperplasia of
the epithelial layer of the secondary lamellae of the gills was noted.
Fatty degeneration of the liver was observed in all treated fish.
Similar testicular abnormalities were observed by Sangalang et al.
(1981), but only in sexually mature individuals or at a stage of rapid
spermatogenic proliferation.
7.2.3.4 Physiological and biochemical effects
Coho salmon (Oncorhynchus kisutch) were fed diets containing a
mixture of PCBs (1:4, Aroclor 1242:1254) at a concentration of 50 or
500 mg/kg dry feed (Leatherland & Sonstegard, 1978). Serum
triiodothyronine (T3) levels were significantly reduced after 3
months, in fish fed the highest dose. Thyroxine (T4) levels were not
affected. After 3 months, the T3:T4 ratio was significantly higher in
fish fed the highest dose than in control and low-dose fish. Fish on
500 mg/kg had significantly lower body weights than controls by the
end of the study. A mixture of 50 mg PCBs/kg and 5 mg mirex/kg
significantly reduced serum triiodothyronine and thyroxine levels over
a period of 3 months, but did not affect the T3:T4 ratio. Leatherland
& Sonstegard (1979) fed rainbow trout (Salmo gairdneri) diets
containing Aroclor 1254 at 500 mg/kg dry feed, for up to 2 months. The
PCBs did not have any significant effects on thyroid histology or on
serum thyroid hormone levels. Liver weights, total liver lipid
content, and carcase lipid content were significantly greater in
treated fish. Mayer et al. (1977) fed fingerling coho salmon
(Oncorhynchus kisutch) 1.45-14 500 µg Aroclor 1254/kg body weight
per day, for 260 days. Channel catfish (Ictalurus punctatus) were
fed the same Aroclor at rates of 48 and 480 µg/kg body weight per day
for 193 days. Thyroid activity (as measured by 125I uptake) was
significantly stimulated at dose rates of 14.5 µg/kg per day, and
above, in coho salmon. Stimulation ranged from 52%, at 14.5 µg/kg per
day, to 119%, at 14 500 µg/kg per day, compared with controls. In
catfish, both dose rates of Aroclor 1254 caused significant increases
in thyroid activity, whereas other Aroclors (1232, 1248, and 1260) did
not have any significant effects on the thyroid at the same dose
rates. Folmar et al. (1982) injected yearling coho salmon
(Oncorhynchus kisutch) intraperitoneally with a total of 150 µg
Aroclor 1254/kg body weight (2 injections, 10 days apart), prior to
smoltification. Over a 6-week period, the authors did not find any
significant effects on gill Na-K ATPase activity. However, the PCBs
did alter the normal developmental patterns of thyroxine; there was a
delay in the normal increase in circulating thyroxine levels.
Triiodothyronine levels were significantly elevated after
approximately 3 weeks, but then fell to well below control levels
after 6 weeks. Fish were transferred to seawater; there was no
significant effect on the gill Na-K ATPase activity in the treated
group, but there was a significant increase in mortality (6%). Ten per
cent of fish, dosed with PCBs and placed in sea water containing No 2
fuel oil at 700 µg/litre, died; this was an additive effect.
Fingerman (1980) kept Gulf killifish (Fundulus grandis) in a
seawater solution containing Aroclor 1242, 1254, or 1268 at
8 mg/litre, for up to 28 days. The author removed the lower half of
the caudal fin to study fin regeneration. No significant effects were
observed with either Aroclor 1242 or 1254. With Aroclor 1268, a
significant decrease in regeneration rate was observed after 28 days,
when the study was conducted in the spring, and after 7 days, in the
autumn. No differences were found at other sampling times.
Rainbow trout (Salmo gairdneri) were administered capsules
containing 0.173 g Aroclor 1254 every second day over a 6-day period
(Kiessling et al., 1983). Two to 4 weeks after the last capsule,
isolated gills were perfused. There was no significant difference in
adrenergic response in the gill vascular bed and no significant
difference in the "oxygen transfer factor" (% changes in oxygen in
saline from dorsal aorta before, and after, addition of adrenaline).
Similarly, there was no effect on muscle glycogen content.
Johansson et al. (1972) dosed brown trout (Salmo trutta) twice, 4
days apart, with 5 mg Clophen A50/kg body weight, either by capsule or
by intramuscular injection. The fish were fed for 43 days, starved for
116 days, and fed again for another 87 days. Metabolic analysis of the
fish was undertaken on day 43 and at the end of the study. A
significant increase in body weight was noted at the end of the study,
but not after 43 days. Blood glucose, muscle glycogen, and the
liver-somatic index (liver weight as a ratio of body weight), which
had all increased significantly after 43 days, decreased significantly
by the end of the study (compared with controls). Both haematocrit and
haemoglobin levels had significantly decreased after 43 days but, by
the end of the study, were not significantly different from those of
the controls.
In a study by Camp et al. (1974), fingerling catfish (Ictalurus
punctatus) were kept in water containing Aroclor 1254 at 8 mg/litre.
There was a significant increase in the serum transaminase activity of
the fish after 4 h. The cortisol content of the serum was depressed,
but not significantly so. The sodium:potassium ratio was constant.
Merkins & Kinter (1971) exposed the killifish (Fundulus heteroclitus)
to concentrations of Aroclor 1221 of 7.5, 25, or 75 mg/litre. No fish
died, at the lowest concentration, over a period of 4 days; 50% died
at 25 mg/litre within 24 h and 88% at 75 mg/litre, within the same
period. Serum osmolality, and serum ion levels (Na and K) were then
measured. Within 6 h at 75 mg/litre, blood osmolality significantly
increased, but sodium and potassium ions in the blood were not
affected. After 24 h at 75 mg/litre, there was also a significant
increase in sodium ions, but no effects on potassium ions. None of
these parameters were affected by exposure at 25 mg/litre for 24 h.
7.2.3.5 Behavioural effects
Fingerman & Russell (1980) exposed male Gulf killifish (Fundulus
grandis) to Aroclor 1242 at 4 mg/litre for 24 h. A significant
reduction in whole-brain levels of both noradrenalin and dopamine were
reported over this period. The swimming activity of the fish was
monitored by counting the number of times they crossed lines marked on
the bottom of the tank within a 10-min period, after exposure to the
Aroclor for 24 h. Activity was significantly increased and remained so
for a further 2 days.
The avoidance response was studied by Hansen et al. (1974a) in the
sheepshead minnow (Cyprinodon variegatus), the pinfish (Lagodon
rhomboides), and the mosquito fish (Gambusia affinis), when given
a choice between clean water and water containing Aroclor 1254 at
0.001, 0.01, 0.1, 1.0, or 10 µg/litre. Sheepshead minnow did not avoid
any concentration; pinfish avoided only the highest concentration of
the Aroclor. Mosquitofish significantly avoided concentrations of 0.1,
1.0, and 10 µg/litre.
Peterson (1973) did not find any effect on temperature selection in
Atlantic salmon (Salmo salar) exposed to 2 mg Aroclor 1254/litre,
for 24 h prior to a horizontal temperature gradient test. Similarly,
Miller & Ogilvie (1975) did not find any effect of Aroclor 1254 on
temperature selection when brook trout (Salvelinus fontinalis) were
exposed to a water concentration of 25-100 mg/litre, for 24 h, prior
to temperature gradient tests. Even at a concentration of 100 mg/litre
for 48 h, which was sufficient to cause some mortality, temperature
selection was still unaffected.
7.2.3.6 Interactions with other chemicals
Halter & Johnson (1974) found that the median survival time for coho
salmon (Oncorhynchus kisutch) fry, exposed to Aroclor 1254 at
32.2 µg/litre, was greater than 336 h. When fry were exposed to
mixtures of Aroclor and DDT, for 2 weeks, the survival times were
always similar to the more rapid reaction time found for DDT alone.
The authors suggested that this indicated the lack of an additive
effect.
7.2.4 Amphibians
Tadpoles (Rana chensinenis) were maintained in water containing PCBs
(as Kanechlor 300) at 0.5, 5.0, 50, or 500 µg/litre. At the 2 highest
doses, all individuals died rapidly. The time of onset of lethality
was related to dose level; at 5 µg/litre, death occurred between 15
and 21 days, and, at 0.5 µg/litre, on the thirty-second day after
first exposure. Growth at 0.5 and 5.0 µg/litre did not differ from
that of controls. Tail abnormalities were found, but there was no
correlation with PCB concentrations and a NOEL could not be
established. The mechanism by which PCBs caused tail malformation was
not known (Hasegawa, 1973).
Birge et al. (1978) conducted embryo-larval bioassays on 3 species of
amphibia. Exposure to various PCBs was maintained from 2-6 h after
spawning to 4 days after hatching, using static renewal procedures
(Table 34). Toxicity increased with increasing chlorination, the
leopard frog being the most sensitive species with an LC50 of
1.03 µg/litre after exposure to Aroclor 1254. The authors also
calculated an LC1 value from the same study; the leopard frog and
American toad were equally sensitive at 0.02 µg/litre. The eggs were
much less sensitive to the PCBs than the hatched larvae, with LC50s
ranging from 3.5 to 250 µg/litre, for 3 different Aroclors and
Capacitor 21, and 3 species of tadpole.
Table 34. Toxicity of PCBs for the tadpoles of amphibiansa,b
Organism Aroclor 1016 Aroclor 1242 Aroclor 1254 Capacitor 21
LC1 LC50 LC1 LC50 LC1 LC50 LC1 LC50
(µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre) (µg/litre)
American toad 0.35 7.16 0.03 2.71 0.02 2.02 0.21 9.97
(Bufo americanus) (0.15-0.64) (5.39-9.34) (0.01-0.06) (1.91-3.75) (0.01-0.05) (1.44-2.77) (0.08-0.42) (7.21-13.53)
Fowler's toad 0.18 27.72 0.22 12.09 0.07 3.74 0.55 28.02
(Bufo fowleri) (0.09-0.33) (21.77-35.08) (0.12-0.36) (9.74-14.91) (0.04-0.11) (2.98-4.64) (0.3-0.91) (22.59-34.47)
Leopard frog 0.1 6.19 0.04 2.13 0.02 1.03 0.03 2.87
(Rana pipiens) (0.05-0.16) (4.95-7.69) (0.02-0.06) (1.72-2.63) (0.01-0.03) (0.83-1.27) (0.02-0.06) (2.29-3.57)
a From: Birge et al. (1978).
b Exposure under static conditions, but with water renewed every 12 h. Exposure was initiated 2-6 h after spawning and continued to
4 days post-hatching. Hatching times varied between 3 and 4 days, therefore, exposure varied between 7 and 8 days.
Temperature 20-24°C; hardness 90-115 mg/litre; pH 7.6-8.1.
7.2.5 Aquatic mammals
Following-up on field reports of the reproductive effects of PCBs on
seal reproduction (see section 7.4.4), Reijnders (1986) conducted a
study on captive common seals (Phoca vitulina) fed fish contaminated
with PCBs. The contaminated diet produced an average daily intake of
PCBs of 1.5 mg compared with the control level of 0.22 mg/day. Twelve
female seals were used as controls and 12 as the treated group. Blood
samples were taken regularly and assayed for circulating steroid
hormones progesterone and estradiol. Females were mated with undosed
males. Of the 12 females in the control group, 10 became pregnant; all
12 ovulated. Only 4 females became pregnant out of the 12 fed the
PCB-contaminated diet; again all 12 ovulated. Throughout the breeding
cycle, no significant differences were found between the hormonal
profiles of pregnant animals in the treated and control groups. No
significant differences were observed in progesterone levels in the
treated and control groups, despite the fact that many fewer treated
females became pregnant. However, a rise in estradiol levels in
non-pregnant females in the control group was not found in
non-pregnant females in the treated group, suggesting a difference in
non-pregnancy in treated females. The effects of PCBs occurred only
late in the breeding cycle at the time of implantation of the embryo.
Seals, like mink, show delayed implantation of the embryo as a normal
component of the annual reproductive cycle. No conclusions about the
mechanism of action could be drawn.
Brouwer et al. (1989) fed common seals (Phoca vitulina) on a diet of
polychlorinated biphenyl-contaminated fish (average daily intake
1.5 mg PCBs) for almost 2 years. Significant reductions in levels of
plasma total and free thyroxine, triiodothyronin, and retinol were
found compared with those in seals maintained on a "low" contaminated
diet (average daily intake 0.22 mg PCBs). It should be noted that the
diet consisted of fish contaminated in the environment and not dosed.
No attempt was made to analyse levels of retinol in the different fish
diets. The "high" contamination group were caught in the Wadden sea
and the "low" contamination group in the north-east Atlantic. When the
fish were analysed for other likely contaminants, it was found that
pp'-DDE also showed higher levels in the "high" contamination group
than the "low" contamination group; average daily intakes of pp'-DDE
were estimated to be 0.4 mg and 0.13 mg, respectively.
7.3 Toxicity for terrestrial organisms
7.3.1 Plants
Aroclor 1254 was applied to soil at rates of 10, 100, or 1000 mg/kg
(Weber & Mrozek, 1979). Both soybean (Glycine max) and rescue
(Fescue arundinacea) were grown in the soil, from seed, for up to 26
and 42 days, respectively. The height of the soybean plants and the
fresh top weights of both plants were measured. PCBs applied to the
soil significantly reduced height and fresh top weight of soybean
plants, only at the highest rate of application. Low rates were
inhibitory, but not significantly so. Aroclor applied to the soil also
reduced the fresh top weight of rescue at the highest rate of
application (1000 mg/kg); lower application rates of the Aroclor did
not have any effects. The addition of activated carbon to the soil
(3.7 tonnes/ha; approximately 3333 mg/kg) annulled the inhibitory
effect of PCBs. The Aroclor also inhibited the uptake of water by the
soybean in proportion to the dose applied to the soil; water uptake
was monitored between 21 and 25 days after sowing of the seed. The
reduction in water uptake over this 5-day period was 12%, with the
application of 1 mg Aroclor 1254/kg soil, rising to 52% at 1000 mg/kg
soil. Again, the effect on water uptake was eliminated by the addition
of carbon to the soil (1% rising to 4% inhibition over the dose
range).
Continuation of the experiment through a second and third crop of
soybeans on the same soil, without further addition of Aroclor 1254
(Strek et al., 1981), showed similar effects on the height, top fresh
weight, and water uptake of the plants, reflecting the persistence of
the Aroclor. However, there were no significant effects at doses lower
than 1000 mg/kg, with the exception of reduced height in the third
crop, seen at all dose levels. All effects were eliminated by the
addition of activated carbon to the soil. The same authors found that
beet (Beta vulgaris) was significantly affected by 1000 mg Aroclor
1254/kg, using the same parameters of water uptake, height, and fresh
top weight between 14 and 56 days after sowing. Doses of 100 mg/kg or
less did not have any significant effects. Effects at the highest dose
were again eliminated by the addition of activated carbon to the soil.
Growth parameters, taken at harvest, showed no apparent inhibition of
corn (Zea mays) or sorghum (Sorghum bicolor) by Aroclor 1254 over
the same dose range. There was, however, a reduction of plant height
over the first 5 days of growth at 100 and 1000 mg/kg in corn, but the
plants recovered.
Mrozek et al. (1983) grew Spartina alterniflora plants in mud or
sandy soils in the presence of 2.2 µg PCBs/kg (54% chlorine similar to
Aroclor 1254), admixed with the soil, over a 6-week period. Plants
grown in sand showed significantly reduced values for cumulative
change in height (approx. 30%) and the number of live leaves per stem
(approx. 25%), and increased values for the number of stems per plant
(approx. 300%), whereas plants grown in mud showed a significantly
reduced value for cumulative change in the number of stems per plant
(approx. 75%). Mud-grown plants also exhibited an altered biomass
distribution, as indicated by the aerial:below ground biomass ratio,
which increased from 1.2 to 1.5, on a dry weight basis.
7.3.2 Terrestrial invertebrates
Hatch & Allen (1979) observed the behaviour of the snail (Cepeae
(=Helix) nemoralis), with regard to the rasping of conspecifics'
shells to obtain calcium. On a low calcium diet (0.53 mg calcium/kg),
snails showed an increased tendency to rasp the shells of other
snails, in order to obtain calcium. The best indicator of this
behaviour was found to be the counting of holes bored completely
through the shell. This behaviour was not seen with a high calcium
diet (250 mg calcium/kg). The addition of PCBs, as a mixture of
Aroclors 1016 and 1254, to the high calcium diet at a rate of 0.5,
1.0, or 5.0 mg/kg increased the number of snail shells penetrated by
other snails. Penetration increased in a dose-dependent manner with 2,
5, and 7% penetration for the 3 dose rates, respectively. Damage to
shells, without actual penetration, also increased with PCB treatment
from the low level found on the control diet to between 16 and 21% on
the PCB diet. No clear dose-dependent effect was seen using this
method of assessing damage. Fourth instar nymphs of the grasshopper
(Chorthippus brunneus), were dosed topically with the PCB mixture
Aroclor 1254 (Moriarty, 1969). A single dose of either 12.5, 50, or
200 µg/insect was applied in a volume of 1 µl of 1,4-dioxan. No
sublethal effects were detected on either development or reproductive
potential. At the highest dose, there appeared to be a latent toxicity
that could be correlated with the mobilization of lipids at moult;
more moulted males died than unmoulted males over the test period.
Females took longer to moult than males and showed a distinctly
bimodal distribution in time to death, with a similar correlation
between toxicity and moult. Males showed 46% mortality and females,
41%, after treatment with 200 µg Aroclor 1254. Fungal infection
affected insects on the lower doses and mortality figures are,
therefore, unreliable.
Lichtenstein et al. (1969) exposed Drosophila melanogaster to the
dry residue of various PCBs. They exposed flies to Aroclors 1221,
1232, 1242, and 1248 at 200 or 800 µg. No mortality was observed after
48 h at 200 µg. At 800 µg, there was an increase in mortality with
decreasing chlorination (after a 48-h exposure to Aroclor 1221, 92%
had died; only 45% died after exposure to Aroclor 1248 over the same
length of time). No mortality was observed after a 48-h exposure to
2000 µg of Aroclors 1254, 1260, 1262, or 1268. In a separate study,
the authors treated houseflies (Musca domestica) topically with
either 10 or 20 µg (in 2 µl of acetone); mortality was assessed after
24 h. Results were comparable with those from the study on
Drosophila; deaths were dose related and occurred with Aroclors up
to 1254, where mortality was 10% at the higher dose. Aroclors of
higher chlorination than 1254 had no effect. Lower chlorinated
Aroclors had the greatest effect with more deaths at the highest dose
(20 µg) and lowest chlorination (Aroclor 1221) than with any other
treatment (43% killed). Plapp (1972) found that the 24-h LC50 for
Aroclor 1254 in the housefly (Musca domestica) was > 3000 µg/jar
where the Aroclor was added to a container in acetone, which was dried
before the addition of the flies. This was true for both
DDT-susceptible and DDT-tolerant strains. The same author reported a
powerful synergistic effect between carbaryl and Aroclor 1254; the
LC50 with carbaryl alone was calculated to be 1386 µg/jar and that
for carbaryl:PCB in the ratio of 1:5, 96 µg/jar. The Aroclor was as
powerful a synergizing agent as piperonyl butoxide.
Youssef et al. (1974) hatched eggs of the housefly (Musca domestica)
on a medium of paper tissue dosed with 0.808 g of Aroclor 1254 per
200 g of tissue. The adult flies hatching from the eggs were examined
using the electron microscope for effects on the male reproductive
tissue. The PCBs induced nuclear and flagellar abnormalities in
developing spermatids. Spermatid nuclei failed to elongate and
membranes originating from the nuclear envelope formed invaginations
into the nucleus. These resulted in the appearance of cytoplasmic
inclusions in the nucleus. Spermatid flagellae contained an abnormal
number of axonemes and mitochondrial derivatives; abnormal spermatids
did not coil and degenerated.
In a study by Wasilewska et al. (1975), female nematodes
(Acrobeloides nanus) were exposed to Aroclor 1254. Initially, 60 µg
of the Aroclor were added to a petri dish (on the surface of agar) in
which there were 20 nematode worms. The nematodes fed on a culture of
bacteria introduced to the agar at the same time as the worms. After 5
days of exposure, eggs and adult nematodes were counted and adult
weights determined. No significant effects were found. In a second
study, over a longer period (10 days), nematodes were exposed to 15,
30, or 60 µg of the Aroclor. Adverse effects increased with dose, and
even at the lowest dose, the number of adults was reduced from 123 to
76, the number of eggs from 539 to 288, and the weight of adults from
18.9 to 9.4 µg. At the highest dose of 60 µg per dish, the number of
adults was 32, the number of eggs, 37, and the weight of adults,
4.9 µg.
7.3.3 Birds
Five-day dietary LC50s for PCBs in birds ranged from 604 to >
6000 mg/kg diet (Table 35). Generally, the oral single dose LD50 and
the dietary LC50 data are similar to those for mammals. PCBs are less
toxic for birds than other organochlorines, such as DDT and its
metabolites and the chlorinated cyclodienes.
The toxicity of Aroclors in birds increases with the percentage
chlorination (generally reflected in the final 2 digits of the Aroclor
number), according to the data of Hill et al. (1975) and Hill &
Camardese (1986). Hill et al. (1974) noted that the toxicity of
Aroclors is not simply a reflection of the chlorine content of the
different Aroclors. They adjusted the dietary content of the Aroclors
to a constant dietary chlorine level and found the same increased
toxicity with higher Aroclor numbers.
Dahlgren et al. (1972) reported some mortality in sub-adult pheasants
after regular oral doses of Aroclor 1254 ranging from 10 to 210 mg.
Mortality was related to both dose and body weight; heavier birds
lived longer, though they lost a greater proportion of body weight. A
sudden heavy intake of PCBs led to high brain residues. Brain residues
were best correlated with death; residues in the brain of about
300-400 mg/kg were considered by the authors to be diagnostic of acute
poisoning and death (Dahlgren et al., 1972). Stickel et al. (1984)
concluded that similar levels in the brain killed red-winged
blackbirds, starlings, brown-headed cowbirds, and grackles. Intake of
lower doses of PCBs over long periods does not lead to such high brain
residues; the cause of death after long-term exposure appears to be
oedema and related symptoms.
7.3.3.1 Short-term toxicity
Hurst et al. (1973) observed differential toxicity of PCBs between
bobwhite quail hens and cocks. This differential was eliminated when
the tests were conducted on birds not in the breeding condition and
with short daylengths. Females survived better than males, only when
they were laying eggs, and survival was well correlated with the
numbers of eggs produced. The authors concluded that females reduce
their exposure to PCBs by eliminating the compound in the eggs.
When Koeman et al. (1969) fed Japanese quail a diet containing 2000 mg
Phenochlor DP6/kg, all the dosed birds died between 6 and 55 days of
dosing. The quail developed hydropericardia at this dose level. Vos &
Koeman (1970) fed one-day-old cockerels a diet containing PCBs at
400 mg/kg, for 60 days; the PCBs were in one of the following forms,
Phenochlor DP6, Clophen A60, or Aroclor 1260 (all 3 are 60%
chlorinated). The mean survival time was calculated to be 24.3 days
for Phenochlor and 20.5 days for Clophen, only 3 out of 20 birds died
on the diet containing the Aroclor. Microscopically, centrolobular
liver necrosis was found in chicks fed the first 2 compounds. Atrophy
of the spleen and porphyria were observed in all dosed groups.
Table 35. Toxicity of PCBs for birds
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Bobwhite quail 10 days diet Aroclor 1221 5-d LC50 >6000 Hill et al. (1975)
(Colinus virginianus) 10 days diet Aroclor 1232 5-d LC50 3002 (2577-3501) Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 2098 (1706-2610) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 1175 (966-1440) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 604 (410-840) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 747 (577-937) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 871 (702-1069) Hill et al. (1975)
male 1 year oral Aroclor 1268 acute LD50 >2000 Hudson et al. (1984)
Japanese quail 14 days diet Aroclor 1221 5-d LC50 >5000 Hill & Camardese (1986)
(Coturnix coturnix 14 days diet Aroclor 1232 5-d LC50 >5000 Hill & Camardese (1986)
japonica) 14 days diet Aroclor 1242 5-d LC50 >6000 Hill & Camardese (1986)
14 days diet Aroclor 1248 5-d LC50 4819 (4267-5443) Hill & Camardese (1986)
14 days diet Aroclor 1254 5-d LC50 2929 (2516-3409) Hill & Camardese (1986)
14 days diet Aroclor 1260 5-d LC50 2195 (1861-2589) Hill & Camardese (1986)
14 days diet Aroclor 1262 5-d LC50 2304 (1978-2684) Hill & Camardese (1986)
Table 35. (cont'd).
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Mallard 10 days diet Aroclor 1221 5-d LC50 >5000 Hill et al. (1975)
(Anas platyrhynchos) 10 days diet Aroclor 1232 5-d LC50 >6000 Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 3182 (2613-3879) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 2798 (2264-3422) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 2699 (2159-3309) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 1975 (1363-2749) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 3008 (2461-3634) Hill et al. (1975)
male 8-9 months oral Aroclor 1242 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1254 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1260 acute LD50 >2000 Hudson et al. (1984)
male 8-9 months oral Aroclor 1268 acute LD50 >2000 Hudson et al. (1984)
Red-winged blackbird diet Aroclor 1254 6-d LC50 1500 Stickel et al. (1984)
(Agelaius phoeniceus)
Ring-necked pheasant 10 days diet Aroclor 1221 5-d LC50 >5000 Hill et al. (1975)
(Phasianus colchicus) 10 days diet Aroclor 1232 5-d LC50 3146 (2626-3948) Hill et al. (1975)
10 days diet Aroclor 1242 5-d LC50 2078 (1843-3879) Hill et al. (1975)
10 days diet Aroclor 1248 5-d LC50 1312 (1166-1477) Hill et al. (1975)
10 days diet Aroclor 1254 5-d LC50 1091 (968-1228) Hill et al. (1975)
10 days diet Aroclor 1260 5-d LC50 1260 (1106-1433) Hill et al. (1975)
10 days diet Aroclor 1262 5-d LC50 1234 (1086-1402) Hill et al. (1975)
Table 35. (cont'd).
Species Sex Age Routea PCB type Parameter Dose/concentration Reference
(mg/kg)
Starling diet Aroclor 1254 4-d LC50 1500 Stickel et al. (1984)
(Sturnus vulgaris)
Brown-headed cowbird diet Aroclor 1254 7-d LC50 1500 Stickel et al. (1984)
(Molothrus ater)
Grackle diet Aroclor 1254 8-d LC50 1500 Stickel et al. (1984)
(Quiscalus quiscula)
a oral = acute oral test (result expressed as mg/kg body weight); diet = dietary test (result expressed as mg/kg diet).
Miranda et al. (1987) studied the effects of acute oral exposure of
Japanese quail to Aroclor 1242 (100, 250, or 500 mg/kg), or
2,4,2',4'-tetrachlorobiphenyl and 3,4,3',4'-tetrachlorobiphenyl (both
87.6 mg/kg) in corn oil. Control birds received only the corn oil. The
birds were killed after 48 h. All the PCB compounds caused a
significant increase in porphyrin content and delta- aminolevulinic
acid synthetase (ALA-S) activity in the small intestine and liver. All
the compounds increased the cytochrome P-450 content of the liver. In
the intestine, the P-450 content was only increased by Aroclor 1242
and 2,4,2',4'-tetrachlorobiphenyl. The activity of 7-ethoxyresorufin
O-deethylase was increased by all compounds in both the intestines
and liver. In the liver, 7-ethoxycoumarin O-deethylase (ECOD)
activity was unchanged or decreased, but, in the intestines, ECOD
activity increased with dose. No tissue differences in ECOD activity
were found after treatment with 2,4,2',4'-tetrachlorobiphenyl and
3,4,3',4'-tetrachlorobiphenyl. It was concluded that the small
intestine was more responsive than the liver to the porphyrinogenic
effect of a single oral dose of PCBs, and, that the induction of drug
metabolizing enzymes in the quail was tissue-specific, depending on
the PCB preparation used.
Day-old chicks were fed on a diet containing 500 mg PCBs/kg. All the
birds died between the third and the tenth week of dosing; a reduction
in the dosage to 250 mg/kg delayed the onset of death until the
thirteenth week (Platonow & Funnell, 1971). Mortality did not occur in
chickens dosed at 200 mg/kg over a period of 3 weeks (Flick et al.,
1965). Harris & Rose (1972) fed one-day-old broiler chicks diets
containing 100, 200, or 400 mg PCBs/kg (Aroclors 1242, 1254, and
1260). No mortality occurred at doses of up to 100 mg/kg, over a
period of 4 weeks. Over the same period, all the birds on Aroclor
1242, 60% of the birds on Aroclor 1254, and none of the birds on
Aroclor 1260, died, all at a dosage of 400 mg/kg. Holleman et al.
(1976) fed day-old broiler chicks and turkey poults on diets
containing Aroclor 1242 at 38, 75, or 150 mg/kg for 4 weeks. The
authors found increased mortality at 75 mg/kg (21% mortality) with the
chicks, but this was not significantly different from controls;
however, there was a significant increase in deaths at 150 mg/kg (75%
mortality). Significantly increased mortality was not found in the
turkeys at any dose level, but the mortality rate in the controls was
33%. Both the 75 and 150 mg/kg diets produced oedema and other lesions
attributed to PCB toxicity. Prestt et al. (1970) maintained Bengalese
finches on a diet containing various concentrations of Aroclor 1254.
The estimated dose rate for 50% mortality, over 56 days, was 254 mg/kg
per day.
7.3.3.2 Egg production
Most studies demonstrating the lowering of egg production by PCBs were
conducted on chickens. The most severe effects came from dosing with
Aroclors in the middle of the range of chlorination (Aroclors
1232-1254). The literature has been reviewed by Stendall (1976).
Platonow & Reinhart (1973) fed chickens with Aroclor 1254 at either 5
or 50 mg/kg diet over 39 weeks. Egg production was erratically reduced
with the lower doses and sharply reduced on 50 mg/kg diet. A dietary
dose of 2 mg/kg did not have any reproductive effects on chickens over
9 weeks (Lillie et al., 1974) or after 39 weeks (Platonow & Reinhart,
1973). Scott et al. (1975) showed a 10% reduction in egg production in
chickens related to egg residues of PCBs (Aroclor 1248) of 3 mg/kg.
When egg residues reached 4.5 mg/kg, the production rate was further
reduced. A significant reduction in egg production was demonstrated by
Call & Harrell (1974) after dosing Japanese quail with 3 different
Aroclors at 62.5-5000 mg/kg, over 33-264 days.
7.3.3.3 Hatchability and embryotoxicity
Aroclors reduced the hatchability of chicken eggs. In 2 studies,
Lillie et al. (1974) examined the effects on hatchability of Aroclors
1221, 1232, 1242, 1248, and 1268, all fed at 2 or 20 mg/kg diet, over
9 weeks. Aroclors 1221 and 1268, with low and high chlorination,
respectively, showed no effects at 20 mg/kg diet. Aroclor 1248
produced some adult mortality at 20 mg/kg diet and nearly eliminated
hatching of the eggs produced. Aroclor 1242 showed similar, but
slightly less severe, effects; there was even less effect with Aroclor
1232. Cecil et al. (1974) tested a similar range of PCBs at the same
dosages (2 and 20 mg/kg diet). They also reported no effects for
Aroclors 1221 and 1268. Aroclors 1254, 1232, 1242, and 1248 reduced
hatchability, as in the previous study. PCBs that reduced hatchability
also produced abnormalities in the chicks. The fertility of eggs was
not affected by any of the treatments. Females were artificially
inseminated with semen collected from males fed a similar diet of
PCBs. Scott (1977) dosed chickens with 0.5, 1, 10, or 20 mg PCBs/kg
diet and found no effect at the 2 lowest doses. Hatchability was
reduced at 10-20 mg Aroclor 1248/kg diet. Kosutzky et al. (1979) dosed
chickens with 2 other PCBs (Delor 103 and 105) (42 and 54%
chlorination, respectively), at 5 mg/kg diet, for 6 weeks; there was
little effect on hatchability, which returned to control levels soon
after a return to a clean diet. Solomon et al. (1973) studied the
effects of PCBs (Aroclor 1254) on pheasants. The birds were dosed at
weekly intervals, for 17 weeks, with gelatin capsules containing
50 mg/bird for the hens or 25 mg/bird for the cock birds. No effects
were observed on fertility and there was no increase in the numbers of
abnormal embryos. In another study, Ax & Hansen (1975) maintained
white leghorn pullets on a diet containing Aroclor 1242 or 1254 at
20 mg/kg, or 2,4,5,3',4'-pentachlorobiphenyl, for a period of 10
weeks. Average embryonic mortality was found to be significantly
increased, i.e., 54.7, 59.2, and 74% for the 3 compounds,
respectively. In the same study, the authors found that average
embryonic mortality in eggs laid by birds dosed with
2,5,2'-trichloro-, 2,5,2',5'-tetrachloro-, or 2,4,5,2',4',5'-
hexachlorobiphenyl was not significantly different from that in the
controls. When both broiler breeder hens and leghorn hens were fed
diets containing either 20 or 50 mg Aroclor 1242/kg, for 1 week, the
hatchability of the eggs laid was reduced by 67.3 and 26.8%,
respectively, of control levels, on the 50 mg/kg diet (Briggs &
Harris, 1973). Hatchability also was reduced at 20 mg/kg diet, but the
time required to achieve the same depression as that found with the
higher dose was doubled. Even after dosing had finished,
embryotoxicity continued and, in fact, increased until, after 6 weeks,
hatchability was between 0 and 10% of controls for both birds at both
doses.
Chickens given Aroclor 1254 at 50 mg/litre in the drinking-water, for
6 weeks, showed progressive reduction in egg hatchability. This fell
to zero after 3 weeks (Bush et al., 1974). Hatchability remained at
almost zero for the first 8 weeks of the chickens receiving control
water following dosing, but returned to normal after a further 8
weeks.
Platonow & Reinhart (1973) fed chickens on a diet containing 50 mg
Aroclor 1254/kg for 39 weeks. There was some adult mortality; egg
production and hatchability fell almost to zero. Residues of Aroclor
in the last eggs produced ranged between 25 and 50 mg/kg. After 6
weeks of uncontaminated food, egg residues dropped and hatchability
improved. The authors reported that egg residues of less than 5 mg/kg
had no effect on hatchability, whereas residues greater than
10-15 mg/kg led to embryotoxic effects. Scott et al. (1975) related
hatchability to egg residues. At residue levels of 3 mg/kg,
hatchability was reduced by 44%, and, at residue levels of 4.5 mg/kg,
it was reduced to almost zero.
The yolk-sacs of eggs from pheasant, mallard, goldeneye duck, and
black-headed gull were injected with 3,4,3',4'-tetrachlorobiphenyl at
0.1 mg/kg (pheasant and mallard) or 1.0 mg/kg (pheasant, goldeneye
duck and black-headed gull) after 4 or 5 days of incubation (Brunström
& Reutergardh, 1986). A significant decrease in the hatching rate was
seen only in pheasants, at the highest dose. At a dose of 1 mg/kg, all
the embryos died before hatching, but, at 0.1 mg/kg, no effect on
hatching was observed. No gross abnormalities were noted in either
hatched chicks or dead embryos. A great difference was noted by the
authors between the avian embryos in this study and chicken embryos,
with regard to sensitivity towards tetrachlorobiphenyl. In chicken
embryos, a dose of 0.004 mg/kg, administered on day 4 of incubation,
gave a significant reduction in hatching. At 0.02 mg/kg, no embryos
survived to hatching (Brunström & Darnerud, 1983). Carlson & Duby
(1973) injected Aroclors directly into chicken eggs on the first day
of incubation, or 9 days later. Aroclor 1242 severely limited
hatchability at levels of more than 2.5 mg/kg. Aroclors 1254 and 1260
had no effect at 10 mg/kg. Delaying the injection until day 9 of
incubation reduced the effect of Aroclor 1242. With 5 mg/kg injected
at day zero, hatchability was 8.3%; when the same dose was given at
day 9, 82% of eggs hatched. These results are not compatible with
those of Scott et al. (1971) who reported that most embryonic deaths
occurred late in incubation. However, these authors were not dosing
the eggs directly, but measuring the residues in eggs from dosed
females. PCBs administered directly into the eggs may not produce
effects comparable with those produced by the same material received
from the mother hen, because of different distribution in the egg.
Platonow & Reinhart (1973) dosed hens at 50 mg/kg diet. Early in the
study, the majority of embryo deaths occurred late in incubation. As
the study progressed, the time of embryo death moved to earlier in the
incubation period. Bush et al. (1974) showed that there was greater
mortality for any given egg residue as the period of dosing the mother
hen progressed. Their experimental chickens were dosed for 6 weeks at
50 mg/litre drinking-water and then kept for a further 20 weeks on
clean water. On day 11 of the study, an egg yolk residue of 50 mg/kg
was associated with 50% mortality in the embryos. On day 131, 50%
mortality was associated with an egg residue of only 10 mg/kg. The
greatest toxicity of PCBs for chicken embryos occurred after 11 weeks
of clean water, that is 17 weeks into the study. At this stage, the
eggs would be receiving doses of PCBs from material stored within the
hen. Late in the study, residues of between 6 and 8 mg/kg in egg yolks
(equivalent to 3.6 mg/kg whole egg) were correlated with between 14
and 36% mortality. Platonow & Reinhart (1973) reported that egg
residues greater than 10-15 mg/kg caused embryotoxic effects whereas
low residues of less than 5 mg/kg did not produce any effects.
Abnormalities were reported by Cecil et al. (1974) in 34% of 843
embryos that died during their study. The most common abnormality was
oedema, which was seen in 50% of all chicks showing any abnormality.
Tumasonis et al. (1973) also reported deformities in chicks.
7.3.3.4 Eggshell thinning
Since the 1950s, thin eggshells have been characteristic of many wild
bird populations, though the effect was not noticed until some time
afterwards. Thin shells has been a contributory factor to reduced
reproductive capacity, particularly in birds of prey. The main
chemical causing thin shells appears to be DDE, a metabolite of DDT.
It has been shown to cause thin eggshells in laboratory experiments,
as well as through the correlation of field data. Literature on thin
eggshells has been reviewed by Cooke (1973). Most experimental studies
using PCBs have shown no effect on shell thickness. Peakall (1971), in
the first controlled study, dosed ring doves at 10 mg PCBs (Aroclor
1254)/kg diet or at 25 mg (equivalent to 160 mg/kg body weight)
injected ip. Shells were ashed and weighed. Two separate studies on
dietary dosing showed no effect on shell weight. In the first study, 2
groups of birds were compared, in the second the same birds were used,
comparing their eggs before and after dosing. Injection of PCBs, 1-4
days prior to egg laying, also had no effect. Studies on mallard dosed
with Aroclor 1254 at 25 mg/kg diet and bobwhite quail dosed at
50 mg/kg diet over 2 years and also on mallard dosed at up to
500 mg/kg diet for 5 weeks (Heath et al., 1972) showed no effects on
shell thickness. There was an apparent shell thickening of about 6% at
the highest dose, which could not be statistically confirmed. The same
authors outlined results from a study on white leghorn chickens.
Aroclor 1242 at 10 or 100 mg/kg diet or Aroclor 1254 at 100 mg/kg diet
did significantly reduce shell thickness. There were no measurable
effects of: Aroclor 1242 at 1 mg/kg diet, Aroclor 1254 at 10 mg/kg, or
Aroclor 1260 at 100 mg/kg diet (Heath et al., 1972). Experimental
details and detailed results were not given for the work on chickens.
Lillie et al. (1974) dosed chickens with a range of Aroclors, at 2 or
25 mg/kg diet, for 9 weeks. Aroclors 1248 and 1242 greatly reduced the
hatchability of eggs and caused some adult mortality, but failed to
cause thinning of the eggshells. When Britton & Huston (1972) fed
single comb White Leghorns Aroclor 1242 at 80 mg/kg diet, for 6 weeks,
no effects on shell thickness were observed. Dahlgren & Linder (1971)
failed to demonstrate any deleterious effects on the eggshells of
pheasants, dosed by gelatin capsule, once a week for 17 weeks, with
doses of Aroclor 1254 up to 50 mg. Call & Harrell (1974) fed various
Aroclors in the diet to Japanese quail for 21 days. Very significant
shell thinning was found with Aroclors 1254 and 1260 at doses of 1250
and 1000 mg/kg diet, respectively. At these high doses, egg production
was severely diminished and shell dimensions were based on very few
eggs. Adult mortality might have occurred at these doses; the paper
does not make it clear whether this actually happened. At lower doses
of 78.1 and 62.5 mg/kg diet of Aroclors 1254 and 1260, respectively,
there was also significant egg-shell thinning and reduced egg
production. Aroclor 1242 was tested at 312.5 and 5000 mg/kg diet and
both doses caused shell thinning, though this was to a lesser degree
than with other Aroclors at similar doses. Risebrough & Anderson
(1975) showed that eggshells thinned by dietary DDE were not further
affected by adding PCB (Aroclor 1254) to the experimental DDE diet.
Results on shell thinning are, therefore, not completely clear. It is
generally agreed that PCBs do not affect birds in this way and the few
results suggesting shell effects are regarded as anomalous or
difficult to interpret, because of experimental design. Shell thinning
can occur because of several different direct and indirect factors.
DDE and sulfanilamide have direct effects on the deposition of calcium
in the shell or on its mobilization from the skeleton, which acts as a
calcium store. PCBs are more likely to affect shells indirectly by
reducing food consumption; none of the studies cited above reported
whether individual birds took less food because of the dosing with
Aroclors. Haseltine & Prouty (1980) fed 24 pairs of mallard with
Aroclor 1242 at 0 or 150 mg/kg diet for 12 weeks and reported a
reduction in shell thickness of 8.9%. They pointed out that all
females laying thin-shelled eggs showed a significant depression in
body weight. This, they regarded as sufficient explanation for the
shell thinning. Much of the shell thinning found in Japanese quail
eggs, laid by females given a single oral dose of Aroclor 1254 of
500 mg/kg body weight, was thought to be due to reduced food
consumption (Haegele & Tucker, 1974).
Biessmann (1982) did not find any effects on eggshell thickness on
dosing Japanese quail with Clophen A60 at levels of up to 150 mg/kg
diet. However, the breaking strength of the eggs was reduced.
Hill et al. (1976) fed 6-month-old laying Japanese quail hens a diet
containing 10 mg Aroclor 1242/kg, for 40 days. Eggs were collected and
measured and, after 40 days, were found to have significantly thinner
shells (5.2%) than the controls. The authors stated that the handling
of the birds and the diet had no effect on food consumption or hen
weights during the test. This is the only study showing shell thinning
at moderate dose levels without an effect on food consumption. The
question of whether PCBs can cause shell thinning, therefore, remains
open.
7.3.3.5 Effects on the male
Platonow & Funnell (1971) kept day-old, white leghorn cockerels on a
diet containing 250 mg Aroclor 1254/kg, for up to 13 weeks. They found
a significant reduction in the weight of both combs and testes after 9
and 13 weeks and, a reduction in comb weight, only, after 6 weeks of
dosing. In a later study, Platonow & Funnell (1972) found a more
severe effect at 500 mg/kg; the comb was significantly reduced in
weight after just one week of dosing and the testicular weight
significantly reduced after 4 weeks, relative to the controls. The
control combs and testes increased in weight during the course of the
study; treated birds failed to develop either comb or testes.
Lillie et al. (1974) did not find any effects on weight gain, food
intake, or semen characteristics in leghorn cockerels fed Aroclor 1248
at 10 or 20 mg/kg diet for 8 weeks. They also did not find any effects
on fertility or hatchability of fertile eggs laid by similarly dosed
females. Liver weights were significantly increased at both dose
levels, and heart weights were significantly decreased at the highest
dose.
7.3.3.6 The effects of stress
Stress, imposed in various ways, increases the sensitivity of birds to
PCBs. Stress seems to have its effect by increasing the mobilization
of fat. Lower fat storage decreases the attenuation of PCB toxicity
seen when fat uptake of the material acts as an effective temporary
detoxification mechanism. Dahlgren et al. (1972) showed that brain
residues were higher in pheasants subject to starvation stress than in
unstressed birds dosed at the same rate. deFreitas et al. (1972)
obtained similar results using cold stress or starvation in pigeons.
As a corollary, biochemical adaptation to stress is inhibited by
exposure to PCBs. This is presumed to be due to residues in non-lipid
tissues (Dieter, 1974).
7.3.3.7 Physiological, biochemical, and behavioural effects
Jefferies & Parslow (1972) dosed young lesser blackbacked gulls
(Larus fuscus) with daily gelatin capsules containing Aroclor 1254
at 50, 100, 200, or 400 mg/kg body weight, for 8 weeks. Mean
individual thyroid weights were significantly increased by 32% (taking
all dosed birds as a single group). There was also an increase in the
mean cross-sectional area of the thyroid. There was, however, no
dose-related effect of PCBs on thyroid weight. The same authors
(Jefferies & Parslow, 1976) showed a similar effect of increased
thyroid weight when they dosed guillemots (Uria aalge) for 45 days
with Aroclor 1254 at 12 or 25 mg/kg body weight. Hurst et al. (1974)
also found a significant stimulation of thyroid growth after feeding
bobwhite quail a diet containing 5, 50, or 500 mg Aroclor 1260/kg for
4 months. Spear & Moon (1985) raised ring doves on either a low iodine
or normal diet. Insufficient iodine caused thyroid hyperplasia. This
hyperplasia was reversed within 7 days by a single dose of
3,4,3',4'-tetrachlorobiphenyl at 60 mg/kg body weight. The PCB
treatment also caused a significant decrease in core body temperature
and serum total thyroxine (T4) and triiodothyronine (T3). No effect,
other than decreased serum T3 and T4, was caused by dosing doves with
PCBs on a diet containing normal iodine levels.
Behavioural effects of PCBs have been noted by several authors.
Peakall & Peakall (1973) reported decreased parental attentiveness in
ring doves dosed at 10 mg Aroclor 1254/kg diet. Kreitzer & Heinz
(1974) measured the avoidance response (from a moving silhouette) in
Japanese quail chicks, for 14 days before, and 8 days after, dosing
with Aroclor 1254 at 200 mg/kg diet. After dosing, the avoidance
response was significantly reduced. Normal responsiveness to the
silhouette was not recovered after 6 days on a clean diet. Two
examples of hyperactivity in birds were also noted. European robins
fed one mealworm/day containing 5 µg Clophen A50, for 11-13 days,
showed increased migratory restlessness (Ulfstrand et al., 1971).
There were similar tendencies in redstart fed one mealworm/day,
containing 11µg Clophen A50, for 12 days, it was estimated that the
birds had ingested 132 µg of PCB overall (Karlsson et al., 1974).
A reduction was reported by Dobson (1981)in the nest-building activity
of pigeons (Columba livia), dosed orally, by gelatin capsule, with
15 mg Aroclor 1254/day, throughout a courtship cycle. The birds
produced a nest but the number of twigs used was reduced compared with
the controls. Reproductive and thyroid hormones were measured in blood
plasma samples, taken each day during the courtship cycle. While the
patterns of hormone secretion remained the same in both control and
treated birds (rises and falls of hormone levels occurred at
comparable times in the 2 groups) the absolute circulating levels of
the hormones were changed by the treatment. Both thyroxine and
luteinising hormone levels in the treated birds were elevated relative
to the controls. The levels were significantly higher, except at the
beginning and the end of the cycle. It was concluded that hormone
levels were unaffected, except when they would naturally be changing,
suggesting an interference with the feedback control of hormone
secretion and a central nervous site of action. Tori & Peterle (1983)
kept mourning doves (Zenaida macroura carolinensis) on a diet
containing Aroclor 1254 at 10 or 40 mg/kg for 42 days. The doves were
then paired and observed each day for 30 days. Both treatments
significantly increased the mean number of days in the courtship
phase; only 4 out of 8 pairs on 10 mg PCBs/kg completed this phase and
moved onto the nesting phase; none of the birds on 40 mg/kg had
completed the courtship phase within 30 days. Behaviour was scored to
measure intensity and, at both doses, this was significantly reduced
overall. Although dosed birds formed pair-bonds approximately 4 days
sooner than controls, there was no significant difference in the
length of the pair-bond formation period or in behaviour scores during
this period in doves fed 10 mg/kg. The length of time spent in the
courtship period was extended significantly (by 8.5 days) by PCBs at
10 mg/kg. Behaviour scores were not significantly affected, but dosed
birds averaged 32% lower scores. Of the birds reaching the nesting
phase, there was no significant difference between controls and dosed
birds with regard to length of time spent nesting or behaviour scores
in the nesting phase. PCBs, however, significantly delayed the onset
of nest initiation (by approximately 7 days) and, therefore, egg
laying.
Japanese quail were dosed with Clophen A60 in the feed at 150 mg/kg,
from the first week of life up to 42 days of age, while the birds were
developing sexually and becoming reproductively mature (Biessmann,
1982). In females, progesterone levels were not greatly affected by
the PCBs, but estradiol levels in blood plasma were lower before
sexual maturity and were less stable during egg laying. In males,
levels of testosterone and dihydrotestosterone (the primary
metabolite) were not affected. Quail fed up to 150 mg Clophen A60/kg
diet during the time of sexual maturation (second to fourth weeks of
age) showed delayed onset of egglaying and a diminished capacity to
lay eggs. Hormone levels in both males and females were not
significantly different from those in the controls.
A possible mechanism for central nervous effects was provided from
studies on neurotransmitters. Dopamine and noradrenalin were depleted
in the brain of the ring dove in a dose-related manner with increasing
brain residues of PCBs (Heinz et al., 1980).
7.3.3.8 Interactive effects with other chemicals
The only information on the interaction between PCBs and other
chemicals in birds has shown PCBs to be additive and not synergistic.
Kreitzer & Spann (1973) carried out tests on several pairs of
chemicals to study pesticidal synergism in young pheasants and
Japanese quail. Two PCBs were used in the study. Aroclor 1262 and
malathion showed additive results, when fed in the diet to 16-day-old
Japanese quail. Aroclor 1254 and DDE were also additive, when given in
the diet to 9-day-old quail. Another study on the possible interactive
effects of PCBs was conducted by Heath et al. (1972), who found that
feeding Aroclor 1254 and DDE in the diet to 14-day-old Japanese quail
gave additive results. There was no evidence of mutual potentiation or
antagonism.
7.3.4 Terrestrial mammals
Acute oral LD50 values reported for PCBs in mink ranged from >750 to
4000 mg/kg body weight. Acute LD50 values for 3 Aroclors in mink were
determined by Aulerich & Ringer (1977) after administration orally, by
gavage, or by intraperitoneal injection. Mortality was assessed 4 days
after i.p. administration and 14 days after oral administration. The
lethality of the Aroclors was found to be inversely related to the
chlorine content; Aroclor 1221 was most toxic and Aroclor 1254 least
toxic (Table 36). This is in marked contrast to the situation in
birds, where toxicity was correlated positively with chlorine content
of the PCBs (see section 7.3.3).
Table 36. Acute toxicity of Aroclors for minka
Aroclor LD50 (mg/kg body weight)
Intraperitoneal Oral
Aroclor 1221 >500-<750 >750-<1000
Aroclor 1242 1000 >3000
Aroclor 1254 >1250-<2250 4000
a From: Aulerich & Ringer (1977).
7.3.4.1 Short-term toxicity
Ferrets (Mustela putorius furo), fed a diet containing 20 mg of
Aroclor 1242/kg, for 8 months, developed enlarged, thickened, and
deformed toe-nails with hyperkeratosis at the junction of the skin and
sponchium, and dysplasia of the root of the nail and the matrix. The
same diet containing Aroclor 1016 did not produce these effects
(Bleavins et al., 1982).
Bleavins et al. (1980) showed the ferret to be less sensitive to PCBs
than the mink, though LD50 values were not determined. Aroclor 1242
at 20 mg/kg diet killed all mink (3 males and 12 females) to which it
was fed. The same diet did not kill any ferrets, though it did cause
reproductive failure.
No mortality occurred in mink fed a diet containing 1 mg PCBs/kg over
183 days (Wren et al., 1987a).
Hornshaw et al. (1986) conducted 28-day LC50 tests on mink, using
Aroclor 1254, in a study to investigate the effects of age, season,
and diet on the toxicity of PCBs; no effects were noted on any of
these parameters. In replicate tests, the calculated LC50 values
varied between 79 and 84 mg/kg diet (48-132, range of confidence
limits). The authors noted that the period of observation after dosing
was critical in assessing the results, since mortality continued after
dosing had stopped and the PCBs were persistent in the body. Taking
total mortality over 28 days of dosing and a further 7 days of
observation, the LC50 values fell to between 47 and 58 mg/kg diet.
A consistent finding among various studies is an effect of PCBs on
food consumption and, therefore, on body weight. The most detailed
analysis of food consumption during the feeding of Aroclor 1254 to
mink is presented by Hornshaw et al. (1986). Young mink fed the
Aroclor over 28 days showed a dose-dependent decrease in the amount of
food consumed. The cumulative weight of food consumed over 5 weeks
(1 week predosing and 4 weeks dosing) for controls was 7574 g. This
was reduced progressively with increasing dose of Aroclor 1254 at 10,
18, 32.4, 58.3, and 105 mg/kg diet to 6447, 6153, 4816, 3556, and
2723 g, respectively. This led to loss of original body weight over
the study period rising to more than 40% at the highest dose. Similar
effects were seen in adults of both sexes; females, with a smaller
initial body weight, were more severely affected than males. This
effect has implications for the interpretation of results of dietary
toxicity tests. The effects seen are a combination of the direct toxic
effects of the compound and the indirect effects of progressive
starvation. The doses of PCBs to which the animals are exposed must
also be calculated with reduced food intake in mind; apparent doses
are higher than real exposure as dose rates increase.
Organ weights (expressed as a percentage of brain weight) were
unaffected by Aroclor 1254 at doses up to 105 mg/kg diet, with the
exception of the heart and the adrenal glands. Heart weight was
reduced in both adult and young mink fed Aroclor 1254 at 58 mg/kg diet
or more; adrenal weights were increased by doses of 13 mg/kg diet or
more (Hornshaw et al., 1986).
Female minks received 0.1 or 0.5 mg of 3,4,5,3',4',5'-
hexachlorobiphenyl/kg diet, or 2.5 or 5.0 mg of 2,4,6,2',4',6'- and
2,3,6,2',3',6'-hexachlorobiphenyl/kg diet for 12.5-14.5 weeks. In both
studies, 3,4,5,3',4',5'-hexachlorobiphenyl was the most toxic isomer,
causing high mortality and reduced body weights (Aulerich et al.,
1985).
Clark & Prouty (1977) fed female big brown bats (Eptesicus fuscus)
on a diet of meal-worms containing 10 mg Aroclor 1254/kg. After the
feeding period of 54 days, the bats were starved to simulate loss of
body fat during the period of migration (when the animals do not
feed). Two out of 12 bats died. The brain residues of 20 mg PCBs/kg at
the end of the study were considered to be sub-lethal, since no
neurotoxic symptoms were observed before death.
7.3.4.2 Reproductive effects
Experimental investigations of mink reproduction in relation to
environmental pollution were carried out as a result of the reduced
reproductive success seen after feeding farm mink with fish from the
Great Lakes. Early studies, therefore, involved the analysis of fish
for pollutants and the experimental feeding of both the fish and of
mixtures of chemicals contained in various fish in the Great Lakes.
Aulerich & Ringer (1977) performed a comprehensive series of feeding
studies using coho salmon from 2 of the Great Lakes (Michigan and
Erie), other fish species from the same source, salmon from the west
coast of the USA, and various combinations of organochlorine
contaminants.
In their first study, ocean fish (perch or whiting) were used as
control diets and the reproductive performances were compared of dosed
and undosed female mink mated with undosed males. Lake Michigan coho
salmon, as 30% of the diet, had the most severe effect on
reproduction, i.e., total reproductive failure, as measured by numbers
of live kits surviving 4 weeks after parturition. Coho salmon from
Lake Erie also reduced reproductive success; 12 females produced only
7 kits still alive 4 weeks after birth. Two other species of fish from
Lake Michigan produced less severe effects, 5 kits being produced on a
diet of 30% bloater chub and 15 kits, on a diet of yellow perch.
Controls produced more than 40 kits over the same period. Kits
produced on Lake Michigan or Lake Erie fish, other than salmon, and
surviving to 4 weeks of age showed significantly lower body weights
than the controls. The small numbers of surviving kits from mothers
fed Lake Erie salmon also showed reduced body weight at 4 weeks of
age. No kits were produced after feeding Lake Michigan salmon diets to
females (Aulerich & Ringer, 1977).
In a long-term, low-level feeding study, 4 different Aroclors (1016,
1221, 1242, and 1254) were included in the diet of mink at a rate of
2 mg/kg. Groups of 8 female and 2 male animals were given this, or a
control, diet for 11 months, from August to June. The reproductive
performance of the animals on different diets is summarized, together
with mortality, in Table 37. Body weight, haemoglobin levels, and
haematocrit were monitored at monthly intervals during the study and
no significant effects of PCBs were noted. Only one of the test diets
(containing Aroclor 1254) adversely affected reproduction, with only 1
live birth in the study period. This single kit was considerably
lighter at birth than the controls and failed to survive 4 weeks after
birth (Aulerich & Ringer, 1977).
The reproductive effects of either Lake Michigan coho salmon or
Aroclor 1254 were reversible, when animals were transferred to a
control diet. Eleven females fed salmon as 30% of the diet for a year,
and then given control food for a further year, produced young with an
average litter size of 3.5 kits per mated female, in the second year
of the study. No young had been produced in the first year of the
study, during dosing. Similarly, 3 females given a year of control
food following a year on a diet containing 5 mg Aroclor 1254/kg,
produced an average of 4.3 young per mated female in the second year
of observation (Aulerich & Ringer, 1977).
In a later study, Bleavins et al. (1980) fed 2 different Aroclors at
various dose levels to mink and ferrets. Aroclor 1242 was fed to mink
at doses ranging from 5 to 40 mg/kg diet; Aroclor 1016 was given at
only 20 mg/kg diet. Results for mortality and reproductive effects are
summarized in Table 38, together with some data for Aroclor 1254 taken
from Aulerich & Ringer (1977).
There was a clear reproductive effect of Aroclor 1242 at a dose of
5 mg/kg diet, but no significant mortality. Aroclor 1242 caused 66%
mortality at 10 mg/kg diet and 100% mortality at 20 mg/kg diet.
Aroclor 1016, at 20 mg/kg diet, caused some deaths of adults and
reduced birth-weight and survival of kits, but the reproductive
effects were considerably less severe than those caused by Aroclor
1242 at 5 mg/kg diet. The authors (Bleavins et al., 1980) calculated
dietary LC50 values for Aroclors 1242 and 1254, using their own data
and data from Aulerich & Ringer (1977), to be 8.6 and 6.7 mg/kg diet,
respectively. It is clear that reproductive effects are less marked at
lower levels of chlorination of Aroclors.
Table 37. Effects of Aroclors on mortality and reproduction in minka
Aroclorb Adult females Kits
Number Number Number Number born Whelped/ Alive at Average weight
died (%) mated whelped female 4 (g ± SE) at
live dead mated weeks birth
Control 0 8 8 28 5 4.1 18 9.9 ± 0.32
1016 0 8 8 28 8 4.5 16 9.2 ± 0.33
1221 12 7 7 43 1 6.3 37 9.6 ± 0.22
1242 12 7 7 35 4 5.6 32 9.3 ± 0.27
1254 12 7 2 1 1 0.3 0 5.4
a From: Aulerich & Ringer (1977).
b Aroclors all fed at 2 mg/kg diet.
Reproductive effects on mink were reported by Jensen et al. (1977),
who fed groups of 10 females at 0.05, 3.3, or 11 mg PCBs/kg diet (type
unspecified), for 66 days. The highest dose eliminated successful
reproduction, whereas 3.3 mg/kg severely reduced the number of kits
born per female.
Table 38. Summary of mortality and reproduction in mink fed various dietary
levels of Aroclors
Treatment level Period fed Number dead/ Number of kits/
(mg/kg diet) (days) total number female
Aroclor 1254a
0 280 1/7 5.0
0 297 0/8 4.1
2 297 1/8 0.3
5 280 2/7 0.0
10 280 5/7 0.0
Aroclor 1242b
0 247 3/30 4.9
2 297 1/8 5.6
5 247 1/15 0.0
10 247 10/15 0.0
20 192c 15/15 0.0
40 138d 15/15 0.0
Aroclor 1016b
0 247 3/30 4.9
2 297 0/8 4.5
20 247 3/15 6.3
a From Aulerich & Ringer (1977).
b From: Bleavins et al. (1980).
c All mink died within 192 days on diet.
d All mink died within 138 days on diet: no females survived to whelping.
Wren et al. (1987b) did not find any significant effects on numbers of
kits produced or surviving to weaning age (5 weeks) after feeding mink
with Aroclor 1254 at 1.0 mg/kg diet, over 183 days. The dosing period
covered the seasonal period when the animals came into breeding
condition as well as a period of giving birth to the young. Although
the weights of kits born to dosed females were not significantly
different at 1 week postpartum, the weight gain of kits was then
affected and weights were significantly different from those of the
controls at ages 3 and 5 weeks. At age 5 weeks, when the kits were
weaned, the mean body weight of kits of the controls was 227.8 g,
while the mean body weight of kits fed by dosed mothers was 161.2 g.
Similar effects on the reproduction of female mink fed PCBs (type
unspecified) were observed by Jensen et al. (1977), i.e., a reduction
in the numbers of whelps born per pregnant female. The authors killed
the females after they had given birth and examined the numbers of
implantation sites in the uterus. This did not differ statistically
between groups (on average, 6.6 in control females, 6.1, in females
fed 5 mg/kg diet, and 4.5, in females fed 15 mg/kg diet PCBs).
However, the number of kits born to the same females showed a marked
effect of the PCBs: 5.1 (on average) born to control mothers; 2.9, to
mothers fed 5 mg/kg diet PCBs, and 0, to mothers fed 15 mg PCBs/kg
diet. The authors concluded that the effects of PCBs occur at the time
of implantation or later, causing resorption of implanted embryos.
Male mink seemed unaffected by doses of Aroclor that caused
reproductive effects in females. Males, dosed and mated with undosed
females, fathered normal numbers of kits (Aulerich & Ringer, 1977).
Wren et al. (1987b) did not note any effects of Aroclor 1254, fed to
male mink over 183 days at a rate of 1 mg/kg diet. Testicular size and
testicular histology were unaffected by the PCBs at any stage of the
reproductive cycle.
Treatment of adult, male, white-footed and white mice with Aroclor
1254 in the diet at a level of 400 or 200 mg/kg (equivalent to 57 or
29 mg/kg body weight), for 2 weeks resulted in a reduced testicular
spermatozoan concentration and, in the white-looted mice, a reduced
absolute weight of the seminal vesicles. In both strains, the absolute
weights of the testes and the final body weights were unchanged
(Sanders & Kirkpatrick, 1975; Sanders et al., 1977).
The reproductive performance of 27 pairs of white-looted mice (44-222
days of age), was compared with that of 26 control pairs, within 60
days of exposure to Aroclor 1254 at a dietary level of 200 mg/kg.
One-third of the exposed pairs did not survive the exposure but
produced at least one litter. The number of pairs producing at least
one litter was reduced by 65% and the number producing 2 or more
litters was reduced by 91%. Litter size was not affected. No offspring
survived to weaning in the 7 first litters of PCB-fed pairs (Merson &
Kirkpatrick, 1976).
A group of 10 pairs of wild-caught, white-footed, mice and groups of
18 (12 weeks of age) and 19 (16 weeks of age) pairs of
laboratory-raised, white-looted mice received Aroclor 1254 in the diet
at 10 mg/kg. Control groups comprised 10, 15, and 20 pairs,
respectively. The reproductive performance of the first group was
recorded for 18 months. The duration of the other studies varied from
7 to 15 months. The number of young per litter, 28 days after birth,
was lower in all treated groups. In laboratory-raised mice paired at
12 weeks of age, the birth interval was increased and the number of
young per litter at birth reduced (Linzey, 1987a). The second
generation of mice, maintained on the same diet as their parents, did
not differ in weight at birth, but were significantly smaller at 4, 8,
and 12 weeks of age. A similar trend was observed in the few young of
the third generation. The uterus, ovaries, and accessory glands, but
not the testes, weighed less in exposed groups than in the controls
(Linzey, 1987b). Linzey (1987a) reported similar reproductive effects
of Aroclor 1254 at a much lower dose.
The author suggested that the major consistent effect on the survival
of the young being fed milk was the result of much higher levels of
PCBs being transported via lactation than via the placenta.
Cottontail rabbits (Sylvilagus floridanus) were fed Aroclor 1254 at
10 mg/kg diet for 12 weeks, and then transferred to a clean diet and
allowed to breed. No effects were observed on any reproductive
parameters, and reduction in food availability did not change this
lack of effect (Zepp & Kirkpatrick, 1976).
7.3.4.3 Physiological effects
Wren et al. (1987a) examined histologically various organs in male and
female mink, dosed for 183 days with Aroclor 1254 in the diet. At
autopsy, no effects were seen on the histology of the pituitary and
adrenal glands. Brain histology also appeared normal. Thyroid
follicles gave the general appearance of minimal activity but did not
differ between treatments. Measurement of plasma thyroid hormones
(T3 -triiodothyronine; T4 -thyroxine) did not show any significant
differences between treatments in male mink. Females showed reduced
circulating T3 in a single sample, in January, but no other
differences were seen at other stages of the study. Thyroxine levels
were not affected at any time.
7.4 Effects on organisms in the field
The acute toxicity of PCBs is relatively low for most species and will
not, therefore, kill enough individuals to affect populations.
However, because of the high potential for bioaccumulation sufficient
residues of PCBs may build up to cause direct lethal effects over
time. Although PCBs are almost universally present in the tissues of
organisms in the environment, there are relatively few examples of
proved effects of these residues on populations of the organisms.
Sublethal effects, affecting populations by reducing reproduction or
growth, are possible, but difficult to prove, because PCBs are always
present with other environmental contaminants. Many possible effects
of PCBs in the environment have been suggested in the literature, but
few have actually been investigated in the field. It has proved
difficult, if not impossible, to relate residues of PCBs in tissues to
possible sublethal toxic effects; residues found after laboratory
dosing cannot be directly related to the field situation.
7.4.1 Plants
Klekowski (1982) studied the ostrich fern (Matreuccia struthiopteris)
growing in the flood plain of the Housatonic River, Massachusetts,
USA. This area of the river is contaminated with PCBs from land-fill
sites containing waste materials from the manufacture of transformers
in the nearby city of Pittsfield. Contamination with PCBs (principally
Aroclor 1254) had been a regular feature of the river area for a
period of more than 40 years. The frequency of somatic mutations in
the fern population was compared to a control population from an
uncontaminated area. The levels of PCBs in river sediments ranged from
1.4 to 139 mg/kg dry weight; at the site where the majority of fern
spores were collected, the level of PCBs was 26.3 mg/kg. The somatic
mutation frequency for the contaminated population was 5.2-6.2 times
higher than that for the controls. It is not known whether similar
genetic damage had occurred in other inhabitants of this contaminated
habitat. No other studies seem to have been conducted on the possible
effects of PCBs, from land-fill sites, on plants.
7.4.2 Fish
There have been many suggestions in the literature that PCBs might
affect populations of fish in the wild. Studies attempting to
demonstrate such an effect are few and, generally, inconclusive or
negative.
Olofsson & Lindahl (1979) used the ability of the cod (Gadus morrhua)
to react to different velocities of water under rotary flow, to
examine the effects of water pollution. Cod sampled from polluted
waters off the Swedish Coast were compared with cod sampled from
unpolluted areas. The ability of the fish to react to rotary flow was
significantly reduced in animals from polluted areas. However, the
authors were unable to relate the reduced reaction of the fish to
levels of various pollutants measured in muscle tissue. Experimental
studies showed that PCBs affected the reactions of the fish; the
residue level in muscle that was associated with this effect was
1.8 mg/kg. This was 30 times greater than the actual residues of PCBs
measured in the cod from the polluted area. While the authors stated
that the distribution of the PCBs in experimental fish and fish taken
from the wild would almost certainly have been different, that PCBs
exert the above effect in the field must be regarded as not proved.
Zitko & Saunders (1979) collected eggs of Atlantic salmon (Salmo
salar) from various areas and measured the PCB contents of the eggs
that proved infertile. The hatchability of different batches of eggs
was tested in the laboratory. No correlation was found between
residues of PCBs and the hatchability of the eggs; in fact the batch
of eggs showing the lowest hatchability also showed very low residues
of PCBs. However, it should be stated that hatchability was seldom
affected by PCBs in laboratory experiments; effects were more usually
seen on the developing young. Hogan & Brauhn (1975) related the
survival of fry hatched from rainbow trout (Salmo gairdneri) eggs to
the contamination of the eggs with PCBs. Five batches of eggs hatched
in 1971 had shown percentage mortalities, 30 days after hatching,
ranging from 10 to 28%. The eggs contained total organochlorine
residues of between 0.31 and 1.30 mg/kg, the majority of which was
PCBs. A batch of eggs collected in 1972 showed 75% mortality, 30 days
after hatching. Many of the fish hatched with such deformities as
scoliosis, lordosis, kyphosis, absence of caudal vertebrae, cranial
deformities, and projecting mandibles. This batch of eggs contained
2.7 mg PCBs/kg and 0.09 mg total DDT/kg (metabolites present not
stated).
Westin et al. (1983) investigated the effects of PCBs, passed on to
the eggs of striped bass (Morone saxatilis) by the female fish, and
of PCBs in food organisms fed to the hatched larvae. The hatchlings
were fed on brine shrimp ( Artemia sp.) from 2 sources, one
contaminated with PCBs and the other not. No effects of either
maternal PCBs or PCBs from the food were found. Residues of PCBs in
young fish decreased consistently throughout the study, which was
conducted in water free of PCBs. The authors suggested that PCBs from
the mother and from food are unlikely to affect the offspring in the
wild. A similar conclusion was drawn about the survival of lake trout
(Salvelinus namaycush) fry, hatched from eggs contaminated with PCBs
in the wild (Willford, 1980). Eggs taken from the wild and hatched in
clean water showed good survival of the offspring (suggesting that
residues of PCBs in the eggs were not responsible for the failure of
the species in Lake Michigan). However, experimental exposure of eggs,
and hatched larvae/fry to levels of PCBs in water and food, similar to
those found in the lake, led to high mortality and the increased
occurrence of deformities in fry. The author concluded that the levels
of PCBs in lake water and food items would be sufficient to lead to
the population decline seen in the lake. It should, however, be
pointed out that other factors in Lake Michigan could also have
contributed to the failure of the lake trout. Sea lampreys had
increased in number in the lake and could have caused population
decline by predation.
The relationships between the occurrence of hepatic diseases and
specific chemicals present in sediment were studied by Malins et al.
(1987). The concentrations of PCBs in the sediments from 4 urban and 2
non-urban areas in Puget Sound USA, were determined. In 3 of the
sites, the concentrations were <0.01 and, in the other 3, the
concentrations ranged from 0.11 to 0.53 mg/kg. Over 900 individual
organic compounds were found. To study the fish diseases, English sole
(Parophrys vetulus), rock sole (Lepidopsetta bilineata), and
Pacific staghorn sculpin (Leptocottus armatus) were collected. The
organs of fish containing the greatest number of lesions were the
liver, kidneys, and gills. Liver cell adenoma, hepatocellular
carcinoma, cholangiocellular carcinoma, haemangioma, and fibroma
constituted major types of liver lesions. Statistically significant
correlations between levels of chemicals in sediment and hepatic
neoplasms in the bottom-dwelling fish suggest a general
cause-and-effect relationship, but there is little firm evidence about
the actual cause of these neoplasms, in particular, in this case,
whether PCBs were involved.
7.4.3 Birds
In studies on chickens and different PCB-mixtures, Vos et al. (1970)
and Vos & Koeman (1970) found that the induction of subcutaneous and
abdominal oedema, centrilobular liver necrosis, hydropericardium, and
higher mortality was more or less related to the presence of
tetrachloro- and pentachlorodibenzofurans, as impurities in the PCB
samples.
From the middle of February to the end of March 1968, an epizootic
disease closely resembling chicken oedema disease occurred in Japan.
Two million chickens were involved, of which 400 000 (20%) died. The
clinical signs were laboured breathing, droopiness, ruffled feathers,
high mortality, and decreased egg production. Autopsy revealed marked
subcutaneous oedema, hydropericardium, ascites, pulmonary oedema,
muscular ecchymosis in the thorax or inside of the thigh, and
yellowish mottled appearance of the liver. The cause of the disease
was found to be Kanechlor 400 contamination of the feed. Experimental
reproduction of these symptoms with Kanechlor 400 was successful. The
remaining sample chicken feed contained 1300 mg of Kanechlor 400/kg
feed (Kuratsune et al., 1972).
Administration of PCBs leads to an atrophy of lymphoid tissue in
chickens (Flick et al., 1965; Vos & Koeman, 1970), and in pheasants
(Dahlgren et al., 1972). Vos & de Roij (1972) and Vos & van
Driel-Grootenhuis (1972) came to the conclusion that these effects
could be attributed to an immunosuppressive effect of PCBs. Vos & de
Roij (1972) suggested that the ability of PCBs to increase the
susceptibility of ducklings to duck hepatitis virus (Friend & Trainer,
1970) and of fish to fungal disease (Hansen et al., 1971) could be
attributed to this immunosuppressive effect.
Koeman et al. (1973) analysed 6 adult cormorants (Phalacrocorax carbo
sinenis), found dead in the wild, for PCB residues. Three additional
birds were shot and 6 fully grown nestlings were also taken. The
authors also carried out a study on 5 cormorants that were dosed with
the PCB, Clophen A60 (a 60% chlorinated PCB mixture that seemed to
correspond most closely with the PCBs profile of material found in
dead birds taken from the wild). The birds were initially dosed with
the PCBs in the diet, but, subsequently, the PCBs were administered
orally in gelatin capsules, until they died. PCB levels in the brains
and livers of the dead birds found in the wild were higher, overall,
than the levels obtained by dosing. The authors thought it highly
probable that this was indicative of PCB poisoning of the birds in the
wild. Without further detailed studies, this conclusion can only be
implied. The higher residues in dead birds from the wild suggest that
a large body burden was taken up, relatively safely in fat, and
released quickly on starvation, prior to death.
A field study, to show the effects of a sub-lethal dose of PCBs on
puffin (Fratercula arctica) breeding success and survival, was
conducted by Harris & Osborn (1981). A total of 150 puffins, trapped
on the Isle-of-May National Nature Reserve, Fife, Scotland, were
implanted, 108 with between 30 and 35 mg of PCBs (Aroclor 1254) and 42
with sucrose as controls. The test chemicals were implanted in
open-ended silastic tubes into the peritoneum. All birds were then
marked and released back into the wild. The same birds returned in
successive years to breed on the island. Breeding and survival of the
implanted birds were monitored through the breeding seasons of 1977,
1978, and 1979. Observations on survival were also made in 1980. Some
implanted birds were killed on recapture, and analysed for PCBs, in
each of the years of observation. No effects on survival or breeding
were seen. PCB levels in fat increased by a factor of between 10 and
14 compared with levels in birds that had not been implanted with the
Aroclor.
Herring gull (Larus argentatus) reproductive success in the area of
the Great Lakes declined with increasing residues of organochlorines
in the birds. Breeding success improved as these residues fell in the
1970s (Weseloh et al., 1979). The species was chosen as an indicator
of environmental pollution in the area and has been extensively
studied. Poor nesting success in the species is related to high
embryonic mortality (Gilbertson & Hale, 1974). Abnormal chicks have
been reported for herring gulls and also for other fish-eating species
from the area including: night herons, ring-billed gulls, common
terns, and Caspian terns (Gilbertson et al., 1976). Eggs from these
species contained residues of PCBs, but it was not possible to
directly relate these residues to chick abnormalities. Gilbertson &
Fox (1977) found a correlation between total organochlorine content
and the hatchability of herring gull eggs. While it cannot be shown
that PCBs were directly responsible for the overall effect, PCB
residues in the livers of hatched chicks, were the only contaminant
that was significantly correlated with the presence of pericardial
oedema. Weseloh et al. (1979) concluded that the reproductive success
of the herring gull can only safely be correlated with total
organochlorine residues and that the possible effects of PCBs cannot
be isolated. Shell thinning usually attributed to DDE alone, does not
occur significantly in these gulls. Hays & Risebrough (1972) suggested
that PCB residues in terns were responsible for the high percentage of
abnormal young in a colony from Long Island Sound.
Twenty-four black-crowned night heron (Nycticorax nycticorax) eggs
were collected at the San Francisco Bay National Wildlife Refuge in
1983. Twelve of these were collected from separate nests, when
late-stage embryos were pipping and an additional egg was randomly
collected from each nest for organochlorine analysis. Other anomalies
and skeletal defects were not apparent. Embryonic weights (with
partially absorbed yolk sacs removed) were 15% lower in comparison
with controls collected at Patuxent Wildlife Research Center.
Crown-rump length and femur length were shorter in the San Francisco
Bay embryos. The geometric mean PCB concentration was 4.1 mg/kg wet
weight with a range of 0.8-52.0 mg/kg. A negative correlation existed
between embryonic weight and log-transformed PCB residues in whole
eggs, suggesting a possible impact of PCBs on embryonic growth. DDE
did not show such a correlation (Hoffman et al., 1986).
Klaas & Swineford (1976) found low PCB residues (0.26-3.4 mg/kg wet
weight) in 35 screech owl (Otus asio) eggs (16 of which were known
to be addled) taken from the wild; there was no relationship between
the presence of residues and hatching failure. Dosing captive screech
owls with 3 mg Aroclor 1248/kg (McLane & Hughes, 1980) showed no
effects on eggshell thickness, number of eggs produced, young hatched,
or young fledged. Residues of PCBs measured in the eggs ranged between
3.9 and 17.8 mg/kg. This range covers the egg residue levels that were
clearly associated with effects in experimental chickens and
pheasants.
Eggs from 315 clutches of sparrowhawks (Accipiter nisus) from 9
sites in Scotland were examined by Newton & Bogan (1978) and Newton
(1979). Eggs that failed to hatch were collected and analysed for
PCBs, DDE, and for aldrin and dieldrin. Statistical analysis showed
little variation in residues within a single clutch, but wide
variation between clutches, even clutches from the same female in the
same area in different years. On this basis, analysis of single eggs
was taken as representative of the complete clutch. Analysis of
variance and multiple regression were used to try to relate particular
effects, such as shell thinning, addling of eggs, and late-embryo
mortality, with particular organochlorines. Results showed that
addling of eggs was significantly correlated with levels of both DDE
and PCBs. Because of the close correlation between residues of DDE and
PCBs in eggs of sparrowhawks, it is not possible to tell whether only
one, or both, organochlorines were involved in egg addling. PCBs
showed the strongest relationship with addling. Shell thinning was
more directly linked with DDE. Newton et al. (1982) concluded that the
level of PCB residues in merlin (Falco columbarius) was not
sufficiently high to have exerted any effect on the breeding success
of this species in Britain. Hodson (1975) did not find any effects of
PCB residues in Richardson's merlin in Canada and attributed all
effects to DDE. A relationship between DDE residues and shell thinning
and the breeding success of the bald eagle (Haliaetus leucocephalus)
was demonstrated by Wiemeyer et al. (1984). PCB residues were
correlated with DDE residues. The authors concluded that contaminants
other than DDE contributed no more than a minor role in reproductive
effects on this species in the field. It was considered that PCBs
might have contributed to reproductive problems, but that the evidence
was unclear. Newton et al. (1986) presented a statistical analysis of
residues in sparrowhawks related to reproductive success. Study
populations from many sites throughout the British Isles showed
different reproductive success. Some of this variation could be
related to levels of organochlorine compounds in eggs. When shell
thickness index was related to PCBs alone, a significant negative
correlation was detected. However, once DDE residues were included,
PCBs did not improve the model. It was concluded that DDE alone could
account for all of the reproductive effects recorded and that PCBs, at
the levels of contamination found, probably did not play a role in
reduced breeding performance. Newton et al. (1988) examined new data
on residues of organochlorine compounds in the eggs of the peregrine
falcon (Falco peregrinus) and also reassessed older data. Total
information considered included information on residues and breeding
success covering the period 1963-86. The PCB residues found were not
considered to have had a significant effect on breeding success in
this species. In earlier studies, Wiemeyer et al. (1975, 1987) could
not isolate any single organochlorine compound as responsible for the
reduced breeding success of the fish-eating osprey (Pandion
haliaetus).
Heinz et al. (1983) examined the breeding success of groups of
red-breasted mergansers (Mergus serrator) on islands in northwestern
Lake Michigan. This is a fish-eating species and, since eating fish
from the Great Lakes had been shown to affect captive mink, the
authors investigated the possible effects of contaminants (measured in
blood samples taken from the birds) on reproduction. Other,
non-contaminant effects were also examined. Many organochlorine
contaminants of the environment were found in eggs including 14
different organochlorine compounds; there was a correlation between
the levels of many of these compounds. No relationship could be
established between levels of PCBs, or any other contaminant, and the
breeding success of the birds. The hatching success of dabbling ducks
also seemed to be largely unaffected by feeding in Lake Michigan,
despite the presence of residues of PCBs and other contaminants in the
eggs (Haseltine et al., 1981).
Appraisal
Effects of PCBs have been shown in laboratory studies on many domestic
species of birds, but few wild species. However, there are many
studies showing measurable residues of PCBs in wild birds. PCBs have
been implicated in population decline in several bird species in
different parts of the world, but it is seldom easy to demonstrate
directly the effects of PCBs on populations of birds in the field.
This is largely because PCB residues invariably occur together with
other organochlorine compounds, such as DDE and dieldrin. Separating
out individual effects can only be done completely satisfactorily when
laboratory data are available for the same species and each individual
pollutant. Some idea of likely effects can be obtained from
statistical manipulation of data from the field, though, in this case,
correlation is the best that can be achieved. In practice, few field
studies give the results that have been predicted from laboratory
studies on other species; that is direct extrapolation from laboratory
to field and species to species is not straightforward.
7.4.4 Mammals
Laboratory studies involving the dosing of non-laboratory mammals with
PCBs are limited to a few species. Field studies have been conducted
on a wider range of species.
Norström et al. (1988) studied liver and adipose tissue specimens of
polar bears (121 samples) obtained by Inuit hunters from 12 zones in
the Canadian Arctic Archipelago in 1982-84 (see section 5.1.4). Six
PCB congeners (Nos 99, 153, 138, 180, 170, and 194) constituted
approximately 93% of total PCBs. The major PCBs accumulated belong to
the group formed by combinations of 2,4-dichloro-, 2,4,5-,
2,3,4-trichloro-, and 2,3,4,5-tetrachloro- substitution on each ring.
Congeners with 2,4-, 3,4-dichloro-, 2,3,4-, 2,3,5-trichloro-,
2,3,4,6-, 2,3,5,6-tetrachloro- or 2,3,4,5,6-pentachloro- substitution
on one ring, such as PCB Nos 118, 138, 187, 183 and 196, which usually
bioaccumulate readily in mammals, were all at lower levels compared
with congeners with only 2,4,5-trichloro- and 2,3,4,5-tetrachloro-
substitution in ringed seals, such as PCB Nos 153, 180, and 170. The
last 3 congeners accounted for 71% of total PCBs in polar bears. Thus,
it appears that the polar bear is able to metabolize PCB congeners in
which there are nonchlorinated para positions, adjacent
nonchlorinated ortho-meta positions, or both ortho positions
chlorinated in one ring.
DeLong et al. (1973) measured various organochlorine pollutants (DDT,
DDD, DDE, dieldrin, and PCBs) in sea lions from the Channel Islands
off the Californian coast. Previous observations had recorded a high
incidence of premature births in the population and organochlorine
residues from females showing premature or normally-timed parturition
were compared. Both DDT and PCB residues (measured against a standard
of Aroclor 1254) were significantly higher in the females producing
premature pups. Residues were estimated in the blubber, liver, and
brain, and, for the first 2 tissues, the ranges of values, for the 2
groups of females, did not overlap. Previous studies on both
substances indicated possible reproductive effects in mammals,
corresponding to some extent with those observed in the sea lions.
Cause and effect could not, therefore, be directly established for
each of the pollutants alone. Helle et al. (1976a) collected ringed
seals from Simo, on the northern Bothnian Bay area of the Baltic Sea,
in October/November. This population of seals shows reduced
reproductive capacity and the sampled population showed only 27% of
females pregnant, compared with other reports of 62.5% and 85-90% for
the same species elsewhere. Residues of DDT and PCBs were compared
with those in seals from other areas of the Baltic and found to be
lower (Table 39). The seals sampled from Simo were divided into
pregnant and non-pregnant groups (n = 15 and 26, respectively).
DDT and PCBs levels were both significantly higher in non-pregnant
animals (Table 40). All females from both groups showed a corpus
luteum in one ovary, indicating that all had ovulated. About half of
the non-pregnant females showed indications of an embryo having been
implanted, which had subsequently aborted or resorbed. Again cause and
effect could not be established directly. The authors pointed out that
normally breeding Californian sea lions had DDT levels as high as
those in Baltic seals showing reproductive failure and proposed this
as an indication that PCBs were the causative agent.
Table 39. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable fat of
blubber from ringed seal from the Baltic Seaa
Area Number DDT PCBs
Northern most part 40 110 ± 10b 69 ± 4.4b
of the Bothnian Bay
Gulf of Bothnia 33 200 ± 28 110 ± 15
a Data from: Helle et al. (1976a).
b Value significantly different from those in the Gulf of Bothnia (P < 0.05).
Table 40. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable
fat of blubber in non-pregnant and pregnant ringed seal of
reproductive agea
Number DDT PCBs
Non-pregnant 26 130 ± 13b 77 ± 5.2b
Pregnant 15 75 ± 11 56 ± 6
a Data from: Helle et al. (1976a).
b Values for non-pregnant females significantly higher than
those of pregnant females. DDT P < 0.01; PCBs P < 0.05.
It was further pointed out that the group of non-pregnant females
would include some animals that were not pregnant for reasons other
than the presence of organochlorine compounds. In a later paper (Helle
et al., 1976b), the same authors reported that some of the
non-pregnant females showed abnormal uteri with stenosis or occlusion
of the uterine horns. They, therefore, subdivided the non-pregnant
females in their second sample into those showing the anatomical
abnormality and those not. Both DDT and PCBs were significantly higher
in the occluded group than in pregnant animals. Non-pregnant females,
without occlusions, showed residues of DDT and PCBs not significantly
different from those in pregnant animals, and significantly lower than
those females with occlusions. Residues in males were comparable with
the highest residues found in females (Table 41).
There is some indication of a positive correlation between PCB
residues and age in male seals but not in females (Addison et al.,
1973; Addison & Smith, 1974). These observations are presumed to
indicate that females excrete some of their body burden of PCBs in the
milk.
These field observations have been confirmed in a study in which
captive seals were fed on fish from the Wadden Sea (where reproductive
problems had been found in the wild seal population) and compared with
controls fed fish from the Atlantic Ocean. Seals eating the
contaminated fish, which differed from the control fish only in the
PCB content, showed the same failure to carry the fetus successfully
to term seen in the wild (Reijnders, 1986; see section 7.2.5).
Table 41. Levels of DDT and PCBs (mean mg/kg ± S.E.) in extractable fat from
blubber of ringed seal from Simo, Bothnian Baya
Group Sample description Number DDT PCBs
I pregnant females 24 88 ± 9.7 73 ± 6.6
II non-pregnant females 29 130 ± 10 110 ± 7.8
with stenoses and
occlusions
III non-pregnant females 8 100 ± 15 89 ± 11
with normal uteri
IV fetuses 24 62 ± 4.3 49 ± 3.0
V males 24 130 ± 18 100 ± 13
probability of similarity between test groups (t-test)
I-II P < 0.01b P < 0.01b
I-III P > 0.05 P > 0.05
I-IV P < 0.05c P < 0.01b
a Data from: Helle et al. (1976b).
b Groups significantly different at the 99% level.
c Groups significantly different at the 95% level.
Subramanian et al. (1987) collected samples of blubber from male
Dall's porpoise (Phocoenoides dalli) in the northwestern Pacific
Ocean and analysed the samples for organochlorine content. Blood
samples taken from the same animals were analysed for testosterone, a
male sex steroid, and for aldosterone, another steroid hormone,
responsible for blood electrolyte balance. Testosterone levels in
blood were correlated with blubber levels of DDT and PCBs; as the
organochlorine content increased, testosterone levels decreased. There
was no relationship between blubber organochlorine levels and blood
levels of aldosterone. The number of samples was small (n = 12) and,
though the relationship between DDT and testosterone was significant,
the apparent relationship with PCBs was not. The animals were sampled
outside the breeding season, when testosterone levels would be
expected to be low.
In a study by Clark & Lamont (1976), 26 pregnant big brown bats
(Eptesicus fuscus) were collected from the field and maintained on a
control diet, in captivity, until they gave birth to young. Levels of
PCBs were measured in both the adults and the offspring. The
concentrations of PCBs in litters with dead young were significantly
greater than in litters where both young were born alive (mean for
litters with dead young was 2.44 mg/kg; mean for all other litters was
0.34 mg/kg). The contents of PCBs ranged between 1.07 and 1.96 mg/kg
wet weight in adults and between 0.28 and 1.69 mg/kg in the young.
7.4.4.1 Appraisal
PCBs have been implicated in population declines of seals and sea
lions. Population decreases and reproductive failure have been
observed in seals from the Baltic Sea, the Wadden Sea (southeastern
North Sea), and the Gulf of St. Lawrence and in sea lions off the
Californian coast. Poor reproduction has been correlated with residues
of PCBs in the affected animals. A major problem in such studies is
the occurrence of more than one chemical pollutant in the animals.
PCBs are found together with other organochlorine compounds and heavy
metals. Conclusions, therefore, have often relied on making the best
correlation between observed effects and residues and checking cause
and effect relationships on animals more amenable to laboratory study.
A study on captive seals confirmed field observations on the effects
of PCBs on these marine mammals (see section 7.2.5).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Evaluation of the toxicity of Aroclors and other commercial PCB
mixtures is complicated by numerous factors, including isomer and
congener composition, differences in species susceptibility,
quantitatively inconsistent data, and various degrees of contamination
with toxic compounds, such as chlorinated dibenzofurans. Because of
these factors and a lack of data for some of the Aroclors (most
studies have been conducted on the higher chlorinated Aroclors), it is
assumed that effects resulting from exposure to a specific Aroclor are
representative of effects that may be produced by the other Aroclors.
Many of the literature sources do not give details of the composition
of the PCB mixture used in the studies.
Reviews on the literature concerning the toxicity of PCBs are given by
ATSDR (1989), Kimbrough (1980, 1987), Safe (1984), NIH (1985), and
Lorenz & Neumeier (1983).
8.1 Single exposures
8.1.1 Oral
(a) Aroclors
The acute oral LD50 values for a number of Aroclors are presented in
Table 42. The lowest oral LD50 in the rat was 1.0 g/kg body weight
for Aroclor 1254 (Garthoff et al., 1981).
In Sherman rats, lethal doses of Aroclor 1254 and 1260 in peanut oil
caused ulceration in the duodenum and glandular stomach (Kimbrough et
al., 1972). Osborne-Mendel rats, receiving a single oral dose of
50 mg/kg body weight of Aroclor 1254 in corn oil, showed an increased
relative liver weight together with the induction of microsomal
enzymes, within 12 h (Litterst et al., 1974).
Monkeys, sacrificed 4 days after gastric intubation of Aroclor 1248 at
1.5 or 3.0 g/kg body weight (no vehicle reported), exhibited enlarged
livers with proliferation of the endoplasmic reticulum and hypertrophy
and hyperplasia of the gastric mucosa (Allen et al., 1974a).
(b) Individual congeners
Estimated oral LD50 values in 30 days for individual congeners in
corn oil in Hartley guinea-pigs (probably single application) were
0.5 mg/kg body weight for 3,4,5,3',4',5'-hexachlorobiphenyl, less than
1 mg/kg body weight for 3,4,3',4',-tetrachlorobiphenyl, more than
3 mg/kg body weight for 2,3,4,5,3',4',5'-heptachlorobiphenyl, and more
than 10 mg/kg body weight for 2,4,5,2',4',5'-hexachlorobiphenyl
(McKinney et al., 1985).
Table 42. Acute oral LD50s of Aroclorsa
Aroclor Species/strain Sex/ageb LD50 (g/kg body weight) References
1254 rat/Wistar male/30 days 1.3 Grant & Phillips (1974)
female/30 days 1.4
male/60 days 1.4
female/60 days 1.4
male/120 days 2.0
female/120 days 2.5
rat/Sherman male/weanling 1.295 Linder et al. (1974);
NR/adult 4-10 Kimbrough et al. (1972);
rat/Osborne-Mendel male/adult 1.01 (single dose) Garthoff et al. (1981)
1.53 (5 doses over 2 1/2 weeks)
1.99 (5 doses, 1 day/week)
1221 rat/Sherman female/NR 4.0 Nelson et al. (1972)
1260 rat/Sherman NR/adult 4-10 Linder et al. (1974)
M/weanling 1.315
1242 rat/Sprague-Dawley male/adult 4.25 Bruckner et al. (1973)
1262 rat NR 11.3 Panel on Hazardous Trace
Substances (1972)
a References: ATSDR (1989); WHO (1976).
b NR = not reported.
Information on solvents used was not available.
8.1.2 Inhalation
No acute data were available.
8.1.3 Dermal
Median lethal doses for a single application of Aroclors to the skin
of rabbits ranged from >0.79 to <1.27 g/kg body weight for Aroclors
1242 and 1248 in 50% corn oil, to >1.0 to <3.17 g/kg body weight for
undiluted Aroclor 1221, and >1.26 to <2.0 g/kg body weight for
Aroclor 1260 (Fishbein, 1974).
8.1.4 Other routes
(a) Aroclors
With a single intravenous dose of Aroclor 1254 in a 1% lecithin-saline
suspension, the LD50 for adult female Sherman rats was 0.4 g/kg body
weight (Linder et al., 1974). The LD50s for Aroclor 1254 in DMSO for
various mouse strains, following a single intraperitoneal injection,
varied between 0.9 and 1.2 g/kg body weight (Lewin et al., 1972).
(b) Individual congeners
The intraperitoneal LD50 for 2,4,3',4'-tetrachlorobiphenyl in CF-1
mice was 2.15 g/kg body weight, while that of its main metabolite,
(5-hydroxy-2,4,3',4'-tetrachlorobiphenyl) was 0.43 g/kg body weight,
which suggests that the acute toxicity of 2,4,3',4'-tetra-
chlorobiphenyl might be attributable to this phenolic metabolite
(Yamamoto & Yoshimura, 1973).
In male Wistar and Charles-River CD rats, a single intraperitoneal
dose of 1 mg 3,4,5,3',4',-pentachlorobiphenyl/kg body weight or a
single oral dose of 50 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body
weight caused significant liver enlargement accompanied by fatty
changes, atrophy of the thymus, and decreased relative spleen weights.
Mono- ortho substituted biphenyls, such as 2,3,4,5,3',4'-hexa-
chlorobiphenyl and 2,4,5,3',4'-pentachlorobiphenyl, induced these
effects in the liver and thymus to a minor degree following an
intraperitoneal dose of 50 mg/kg body weight, while di- ortho
substituted hexachloro- and heptachlorobiphenyls did not cause adverse
effects at this dose level (Kohli et al., 1979; Yoshihara et al.,
1979).
8.2 Short-term exposures
8.2.1 Oral
8.2.1.1 Aroclors
(a) Mouse
While oral intoxications by PCB mixtures in monkeys are easily
recognizable by their effects on the skin, skin lesions in female ddN
mice were only observed after 2-3 months of daily oral exposure to
1.6 mg of a technical PCB mixture (48% chlorine) per mouse (80 mg/kg
body weight per day) in olive oil. The lesions included alopecia,
erosions, ulcerations, and eczematous changes around the eyelids. No
increase in mortality and only slight growth retardation were seen
after 26 weeks of exposure (Nishizumi, 1970).
Koller (1977) found histological changes in the liver of mice exposed
to 37.5 mg Aroclor 1254/kg diet for 6 months. A dose level of
3.75 mg/kg diet was without effect.
The oral toxicities of Kanechlor 400, 500, and 600 were compared by
feeding male mice diets containing 300 mg PCBs/kg for 14 weeks
(Kawanishi et al., 1975). Fatty degeneration and accumulation of
pigment in the liver were observed in the mice fed Kanechlor 600.
(b) Rat
At lethal, oral doses of undiluted Aroclor 1242 (100 mg/kg body
weight, every 2 days, for 3 weeks), Sprague-Dawley rats showed reduced
body weight, thymus atrophy, chromodacryorrhea, progressive
dehydration, and central nervous system depression with terminal
ataxia and coma. Fatty changes were observed in the liver and kidneys
(Bruckner et al., 1973).
Male and female Sherman rats (10 animals of each sex) were fed diets
containing 0, 20, 100, 500, or 1000 mg of Aroclor 1260 or Aroclor
1254/kg (equivalent to 0, 1.5, 7, 36, or 72 mg/kg body weight,
respectively) for 8 months. The animals receiving the 2 highest dose
levels showed reduced growth. Female rats fed Aroclor 1260 at 500 and
1000 mg/kg diet showed a high mortality rate. However, only 3 rats fed
Aroclor 1254 at 500 mg/kg diet died. Significant increases in relative
liver/body weight ratios were found for both Aroclors at all doses
tested. Microscopically enlarged hepatocytes, cytoplasmic inclusions,
increased lipid levels, and foamy cytoplasm were all found
consistently. Adenofibrosis was found at higher doses (500 and
1000 mg/kg diet) and corresponded to the glistering white areas seen
on gross inspection. These areas also showed cholangiofibrosis
(according to Kimbrough, synonymous for bile duct proliferation, bile
duct adenomatosis, and fibroadenoma) (Kimbrough et al., 1972).
Growth and mortality rates were unaffected in male Sprague-Dawley rats
exposed for 1 year to a diet containing Aroclors 1248, 1254, or 1262
at 100 mg/kg diet (equivalent to 5 mg/kg body weight) compared with
controls (Allen et al., 1976).
Female Charles-River CD rats were fed for 20 weeks on a diet
containing 0, 10, 30, or 100 mg Aroclor 1254/kg (equivalent to 0, 0.5,
1.5, and 5 mg/kg body weight, respectively). No increase in mortality
rates occurred but growth inhibition was seen at 30 mg/kg from month 2
onwards and at 100 mg/kg diet from week 2 onwards. Skin lesions,
initially on the ears, were found after 10-20 weeks of exposure to
Aroclor 1254 at all dose levels. The lesions, including alopecia and
reddened and thickened skin with hyperkeratosis, subcutaneous oedema,
and infiltration by polymorphonuclear leukocytes, also involved the
nose, tail, and feet at the highest exposure level (Zinkl, 1977).
Effects on the liver following exposure to PCB mixtures have mainly
been investigated in rats (see section 8.6.1.1).
(c) Rabbit
Four groups of 5 male and 5 female New Zealand White rabbits were
administered 300 mg Aroclor 1221, 1242, or 1254 in corn oil, via a
stomach tube, once a week, for 14 weeks. The fourth group received
only the vehicle.
The mortality rate was increased and the body weight gain was reduced
after 14 weeks of oral exposure to Aroclor 1254, but not after
exposure to Aroclor 1221 or 1242. Liver/body weight ratio and SGOT and
SGPT activities were increased in the animals treated with Aroclor
1242 or 1254, but not in those treated with Aroclor 1221. In the
animals treated with Aroclor 1254, the smooth endoplasmic reticulum
was condensed in the liver cell and formed hyalin inclusions which
might have been accompanied by a loss of enzyme activity. Lipid
accumulation, pigment deposition, nuclear changes, and necrosis were
also found (Koller & Zinkl, 1973).
(d) Pig
Pigs, fed Aroclor 1242 or 1254 at a dietary level of 20 mg/kg
(equivalent to 0.8 mg/kg body weight) for 91 days, showed gastric
lesions, consisting of erosions and necrosis. Two pigs fed a high-dose
regimen of 100 mg of Aroclor 1254/kg body weight for 11 days, also
showed hypertrophy and hyperplasia of the gastric mucosa; this was
also found in monkeys at low exposure levels (Hansen et al., 1976b).
(e) Cow
Holstein cows were studied throughout a complete lactation period, a
non-lactating period, and 42 days of a subsequent lactation period for
overt responses to Aroclor 1254. Four cows received daily doses of 0,
10, 100, or 1000 mg/cow (1000 mg/cow is equivalent to 1.67 mg/kg body
weight) over 60 days. The mean daily milk production and net energy of
a complete lactation period did not differ between control and
PCB-treated animals. At the end of the study, the concentrations of
PCBs were 0.005, 0.021, 0.14 in blood plasma; 1.9, 10.9, 91.3 in milk
fat, and 1.4, 6.9, 70.0 µg/kg in adipose tissue for the 10, 100, and
1000 mg PCB groups, respectively. No signs of impaired health,
productivity, or changes in blood and urine chemistry were observed
(Willett et al., 1987).
(f) Monkey
Rhesus monkey
(i) Aroclor 1242
Becker et al. (1979) exposed 6, 7-8-month-old, male Rhesus monkeys to
Aroclor 1242 at levels of 0, 3, 10, 30, or 100 mg/kg diet (equivalent
to 0, 0.12, 0.4, 0.4, 1.2, or 4.0 mg/kg body weight, respectively) to
study changes in the stomach mucosa. At 3, 10, 30, and 100 mg/kg diet,
4 monkeys died after 245, 146, 92, and 137 days of exposure,
respectively. All exposed monkeys showed a decreased body weight gain.
At all dose levels, changes were observed in the folds of the
suborbital facial skin, and eyelids became swollen and red. From day
71 at 3 mg/kg diet, days 69 and 77 at 10 mg/kg diet, and day 12 at the
higher exposure levels, stomach biopsies revealed an apparent arrest
of the differentiation of generative cells of the isthmus and neck
regions into parietal and zymogenic cells. Mature parietal and
zymogenic cells, which were found only in the bases of the glands,
showed signs of injury, such as dilatation of the rough endoplasmic
reticulum on the zymogenic cells, irregularity of the mitochondria in
parietal cells, and irregular luminal membranes and an increase in the
number of autophagic vesicles in both type of cells. The severity of
the lesions was directly correlated with both duration and level
exposure. A no-effect level was not obtained in this study.
A group of 5 Rhesus monkeys, 1-2.5 years old, was exposed to a diet
containing 1 mg of Aroclor 1242/kg (equivalent to 0.04 mg/kg body
weight) for 133 days. A control group contained 4 monkeys. No adverse
effects were found (McNulty et al., 1980).
Characteristic lesions, metaplasia in epithelial structures, such as
sebaceous glands, nail beds, gastric mucosa, and ameloblast
surrounding unerupted teeth were reported to have developed in Rhesus
monkeys, 13 months after a 40-day diet containing 400 mg Aroclor
1242/kg (equivalent to 16 mg/kg body weight) (McNulty, 1985).
(ii) Aroclor 1248
A group of 5 (4 males and 1 female), 1-month-old Rhesus monkeys,
without previous exposure, were administered 30 daily doses of 35 mg
of Aroclor 1248/kg body weight, by gavage, in corn oil. Four animals
received only corn oil. No mortality or clinical signs were observed,
except for a decrease in body weight gain, slightly reduced food
consumption, and anaemia. Mild microscopic changes were observed in
the thymus, bone marrow, eye, skin, stomach mucosa, and liver
(Abrahamson & Allen, 1973).
Six male Rhesus monkeys, aged 1 1/2-2 years were fed a diet containing
300 mg Aroclor 1248/kg for 3 months. Three animals served as controls.
A decrease in body weight was observed. Within one month, all the
animals fed PCBs had alopecia, subcutaneous oedema, particularly of
the face, which manifested as swollen eyelids, erythema, and acneiform
lesions involving the areas devoid of hair (Allen & Norback, 1972 cf.
Hayes (1987)).
Six adult, female Rhesus monkeys were fed 25 mg Aroclor 1248/kg diet
for 2 months. Facial oedema, alopecia, and acneiform changes developed
within 1 month and 1 animal died 2 months after removal from the
experimental diet. In addition to the above changes, this animal
showed anaemia, hypoproteinaemia, hypertrophy, and hyperplasia with
invasion through the muscularis mucosa, focal haemorrhages and
ulcerations of the gastric mucosa, and bone marrow hypoplasia. Eight
months later, the 5 surviving animals continued to show clinical signs
of intoxication. The PCB concentration in body fat, which, after 2
months of treatment, averaged 127 mg/kg, had decreased 8 months later
to 34 mg/kg (Allen et al., 1974a).
Groups of 9 adult, female Rhesus monkeys (weight approximately
5.6 kg), were fed a diet containing 0, 2.5, or 5.0 mg Aroclor 1248/kg
(equivalent to 0, 0.1, or 0.2 mg/kg body weight, respectively) for an
average period of 18.2 months. An additional group of 4 males was fed
5.0 mg/kg diet. Control groups contained 12 female and 6 male monkeys.
The Aroclor 1248 contained 4.4-8.7 ng polychlorinated dibenzofurans/kg
diet. The female monkeys were mated with control males after 6 months
of exposure (the reproductive effects are discussed in section
8.4.1.3). Levels of Aroclor 1248 in adipose tissue reached a plateau
after 1 year at 2.5 mg/kg diet and after 6 months at 5 mg/kg diet.
Exposed males showed slight to moderate periorbital oedema and
congestion of the eyes. Females showed an average 15% loss in body
weight over the first 5 months of both exposures, while food
consumption was normal. At 6 months, they all showed loss of hair,
acne of the face and neck, and erythema and swelling of the eyelids.
Skin biopsies revealed keratinization of the affected hair follicles.
One female monkey died after 173 days of exposure to 2.5 mg/kg diet
and one female died after 310 days of exposure to 5.0 mg/kg diet. Both
animals developed terminal enteritis due to Shigellosis, which was
resistant to treatment. At autopsy, the animals showed generalized
alopecia, subcutaneous oedema, and acne. Microscopically, follicular
epithelial hyperplasia with inflammation of the surrounding tissue,
and keratinization of the hair follicles were observed. The livers
showed focal areas of necrosis, enlarged hepatocytes, and fatty
changes (Barsotti et al., 1976).
The surviving females in the former study, 8 per dose level, were
placed on a control diet for approximately 1 year after an average
total intake of 270 and 498 mg Aroclor 1248, respectively. There was a
gradual improvement in their physical condition and, within one year,
their gross appearance was no longer different from that of the
controls. However, their breeding performance was still affected, as
outlined in section 8.4.1.3 (Allen et al., 1980).
(iii) Aroclor 1254
Several pilot studies were carried out before this major study to
characterize the toxicity of Aroclor 1254 and to compare the toxic
findings in the Rhesus and Cynomolgus monkey (Tryphonas et al., 1984,
1986b; see section 8.2.1.6). In these pilot studies, Mes & Marchand
(1987) and Mes et al. (1989a) measured the concentrations of PCBs in
the blood, adipose tissue, and faeces.
A preliminary report describes the results of an ongoing study after
54 weeks of daily oral administration of gelatin capsules containing
0, 5, 20, 40, or 80 µg Aroclor 1254/kg body weight, in corn
oil-glycerol, to groups of 16 adult, female Rhesus monkeys (Macaca
mulatta). At this stage, a slight decrease in body weight gain was
observed in the exposed monkeys together with a decrease in water
consumption, but not in food consumption. In week 52, the incidences
of prominent nail beds, of nails separated from the beds, and of
prominent tarsal glands increased in the animals exposed at 80 µg/kg
body weight (Arnold et al., 1984).
Aroclor 1254, at a dose level equivalent to 280 µg/kg body weight was
given for 5 days per week to Rhesus monkeys (Macaca mulatta) over a
period of 27-28 months. Four animals were treated as described and 4
animals served as controls. The Aroclor 1254 was administered in
apple-juice-gelatin-corn oil emulsion. The weight of the monkeys at
the beginning of the study was approximately 4 kg. Terminal clinical
signs of varying severity included finger nail detachment, exuberant
nail beds, weight loss, stomatitis, and normocytic anaemia. At
necropsy, the bone marrow was hypocellular with cytoplasmic vacuoles
in erythroid precursor cells. Histopathological lesions included
dilatation of the tarsal gland ducts, atrophy, or absence of, splenic
and lymphonodal germinal centres, bone marrow depletion, gingival
erosion and ulceration, moderate mucinous hypertrophic gastropathy
with cystic dilatation of occasional gastric glands, hepatocellular
enlargement and necrosis, hypertrophy of biliary duct epithelium,
hyperplasia of biliary ducts, hypertrophy of the gall bladder
epithelium, and an equivocal increase in the number of lysosomes in
the thyroid follicular epithelial cells. The terminal PCBs
concentrations in a number of organs were as follows: adipose tissue
106.7-2073.2 mg/kg (in control animals 0.26-0.65 mg/kg); brain,
31.2-252.0 mg/kg; kidneys, 85.4-964.1 mg/kg; and liver,
255.1-828.1 mg/kg tissue. The PCB concentrations were expressed on a
mg/kg fat basis. It was concluded that skin appendicular lesions are
good clinical indices of PCB exposure in monkeys and that
lymphoreticular lesions (atrophy and absence of lymphoid follicular
centres) are good indicators of impending or active immunological
crisis (Tryphonas et al., 1986a).
(iv) Miscellaneous studies
Accidental exposure of a colony of 256 Rhesus monkeys to PCBs in a
concrete sealant produced a disease characterized by high mortality,
gradual weight loss, behavioural changes, alopecia, acne, facial
oedema, swollen eyelids, diarrhoea, anaemia, poor breeding
performance, and high incidences of abortions and still births.
Samples of the concrete slabs within several buildings were obtained
and analysed. Significant levels of PCBs (5280 mg PCB/kg sample) were
found (Altman et al., 1979; McConnell et al., 1979).
The effects of exposure to PCBs on the eyes were investigated by
Ohnishi & Kohno (1979), who administered a banana injected with 0.5 mg
of PCBs/kg body weight, daily, to 8 adult Rhesus monkeys of both sexes
for 1-5 months. Two out of this group were fed PCBs with
polychlorinated dibenzofurans (2.5 µg/kg body weight). Four untreated
animals were used as controls. One month after the onset of the
exposures there was a reduction in body weight. When pressure was
applied to the eyelids of treated monkeys, white secretions extruded.
Within 3 months alopecia, swelling of the eyelids, and acne-form
eruptions developed. The retina and choroid were normal. The
histopathological changes in the eyelids were comparable in both
groups of exposed monkeys and included the appearance of keratinous
cysts and atrophy of the Meibomian glands with hyperkeratosis and
hyperplasia of the ductal epithelium.
Cynomolgus monkey
Groups of 5 or 6 adult, female Cynomolgus monkeys (Macaca
fascicularis) were exposed to 3, weekly doses of 4.7 mg of Aroclor
1248/kg body weight (equivalent to 2 mg/kg per day) or to 3-weekly
doses of 11.7 mg of Aroclor 1254/kg body weight (equivalent to 5 mg/kg
per day) in an apple juice-corn oil emulsion. The monkeys were exposed
until necropsy at day 30-164 in dead or moribund condition. A control
group contained 5 monkeys. In both exposed groups, body weight loss,
emaciation, facial oedema, finger-nail loss, and lacrimation were
observed. Common histopathological lesions were: dilated Meibomian
gland ducts, mucinous hyperplasia and hypertrophy of the gastric
mucosa, enlargement, fatty degeneration, and necrosis of hepatocytes,
bile duct and gall bladder epithelial cell hypertrophy and
hyperplasia, and thyroid changes in follicular cell size and the
number of intracytoplasmic lysozomes.
The onset of the signs and lesions of toxicity was not as rapid and
uniform as that in Rhesus monkeys. Aroclor 1248 appeared more toxic
than Aroclor 1254 (Tryphonas et al., 1984).
Groups of 4 adult, Rhesus and 4 adult, Cynomolgus female monkeys,
weighing 3.2-4.5 and 3.2-5.2 kg, respectively, received doses of
Aroclor 1254 at 0 or 280 µg/kg body weight for 5 days/week (equivalent
to 200 µg/kg per day) in an apple-juice-gelatin-corn oil emulsion for
27-28 and 12-13 months, respectively. This study showed that the
Rhesus monkey is more susceptible to PCB toxicity than the Cynomolgus
monkey (Tryphonas et al., 1986b).
Hori et al. (1982) exposed 1 female, Cynomolgus monkey to daily doses
of Kanechlor 400 (without detectable quantities of polychlorinated
dibenzofurans) at 2 mg/kg body weight, in olive oil. They also exposed
3 monkeys to a PCB mixture with a chromatographic pattern similar to
that of the Yusho mixture (2 or 4 mg/kg body weight), and 1 monkey to
2 mg/kg body weight of the same mixture, without detectable quantities
of polychlorinated dibenzofurans (detection limit not given). The
doses were administered 6 times per week in a piece of banana. Two
controls received only the vehicle. The 2 monkeys receiving the Yusho
mixture at 4 mg/kg body weight died within 4 and 8 weeks,
respectively. The other monkeys were kept for 20 weeks. In all monkeys
exposed to the Yusho mixture, toxic effects were similar to those
already described above. In addition, there was cytoplasmic
vacuolation and dilatation of the convoluted tubules with cytoplasmic
casts in the kidneys. The other 2 mixtures induced less severe
reductions in body weight gain, immunosuppression and
histopathological alterations in liver, kidneys, and periorbital skin.
The effects in the animals fed a diet with dibenzofurans yielded
enhanced decreases in body weight, immunosuppression, fatty liver and
histological changes, in addition to hair loss, acne-form eruptions,
oedema of the eyelids and cornification of the skin, compared with the
other test substances.
8.2.1.2 Individual congeners
(a) Monkey
Rhesus monkeys have been exposed to various congeners, as outlined in
Table 43.
Table 43. Toxicity of PCB-congeners in Rhesus monkeysa
Congenerb No. of Exposure Time clinical Deaths
animals/ toxicity
group mg/kg period first noted
diet (days) (day)
2,5,4'-TriCB 4 5 84 - 0
3,4,3',4'-TCB 3 3=>1c 215 14-21 3
5 1 38 27 1
2,5,2',5'-TCB 3 3=>1c 215 - 0
5 1=>5d 200 - 0
3,4,5,3',4',5'-HCB 1 0.1 127 117 1
4 0.5 63 28-30 4
1 1 57 20 1
2,4,5,2',4',5'-HCB 4 15 122 - 0
1 65 63 - 0
2,4,6,2',4',6'-HCB 4 15 122 - 0
1 65 64 - 0
2,3,6,2',3',6'-HCB 4 15 122 - 0
a From: McNulty et al. (1980); McNulty (1985); Iatropoulos et al. (1977).
b TriCB = trichlorobiphenyl; TCB = tetrachlorobiphenyl; HCB = hexachlorobiphenyl.
c Dietary level reduced after 23 days.
d Dietary level raised after 133 days.
No toxicity could be demonstrated either by clinical appearance or by
histopathological examination for isomers with 2 or 4 chlorine atoms
ortho to the biphenyl bridge. The clinical signs observed in monkeys
exposed to 3,4,3',4'-tetrachlorobiphenyl (up to 3 mg/kg diet) and
3,4,5,3',4',5'-hexachlorobiphenyl (at 1 mg/kg diet) were similar in
character and severity to those produced by Aroclor 1242 (at 100 mg/kg
diet) and Aroclor 1248 (at 25 mg/kg diet) in monkeys (Allen et al.,
1974a). The same histopathological lesions were found. The lesions of
the skin and eyes were described as an expression of squamous atrophy
or squamous cyst formation of the sebaceous glands. The epithelial
changes in the skin and nails were found to be reversible. In this
study, 2,5,2',5'-tetrachlorobiphenyl did not produce any clinical or
pathological lesions at 3 mg/kg diet. Aroclor 1242 and 1248 were
reported to contain about 0.24 and 0.34% of 3,4,3',4'-tetra-
chlorobiphenyl, and it was concluded that this congener could account
for some of the toxicity of the commercial mixtures (McNulty et al.,
1980; McNulty, 1985).
Rhesus monkeys exposed to 2,5,4'-trichlorobiphenyl showed a reversible
primary injury of the arterioles, capillaries, and venules in the
adrenal glands, kidneys, liver, brain, and lungs (Iatropoulos et al.,
1977).
(b) Other animal species
In studies on rats and mice, individual PCB-congeners caused adverse
effects on the liver, spleen, and thymus. The most toxic compounds
were the planar congeners 3,4,3',4'-tetrachlorobiphenyl,
3,4,5,3',4'-pentachlorobiphenyl and 3,4,5,3',4',5'-hexachlorobiphenyl.
Biocca et al. (1981) and Aulerich et al. (1985) compared the
toxicities of various hexachlorobiphenyl isomers in mice. Male C57
BL/6 mice were exposed via the diet to 0.3, 1, 3, 10, 30, 100, or
300 mg 3,4,5,3',4',5'-hexachlorobiphenyl; 10, 30, 100, or 300 mg
2,4,5,2',4',5'-hexachlorobiphenyl, 2,3,6,2',3',6'-hexachlorobiphenyl,
or 2,4,6,2',4',6'-hexachlorobiphenyl/kg diet for 28 days. There were
marked differences in dose response and in the severity of the
pathological effects among the isomers. 3,4,5,3',4',5'-Hexa-
chlorobiphenyl was the most toxic isomer causing mortality, and body
and organ weight effects at all dose levels and was the only isomer
that produced excess porphyrin accumulation. It was also the isomer
that occurred in the highest concentration in the fat and the liver.
3,4,5,3',4',5'-Hexachlorobiphenyl caused subcutaneous oedema,
enlargement of the liver with accentuated hepatic lobular markings,
fatty infiltration, hepatocellular swelling and necrosis, and atrophy
of the thymus. The other 2 isomers caused the same lesions, but to a
lesser extent.
In the mice, the overall order of toxicity was 3,4,5,3',4',5'-
hexa-chlorobiphenyl > 2,4,6,2',4',6'-hexachlorobiphenyl >
2,4,5,2',4',5'-hexachlorobiphenyl > 2,3,6,2',3',6'-hexachlorobiphenyl,
based on the effects on mortality and growth, and on histopathology.
8.2.2 Intraperitoneal: reconstituted PCB mixtures
Bandiera et al. (1984) administered reconstituted mixtures of PCDFs
and reconstituted mixtures of PCBs, by intraperitoneal injection, to
immature Wistar rats, to determine the effects on weight loss, thymic
atrophy, and the induction of P-448 dependent monooxygenases. The
mixtures consisted of compounds that persisted in the blood and liver
of Yusho patients. From the results, it was clear that the PCDFs were
600 to 2100 times more toxic than the PCBs.
A group of 4, one-month-old, male Wistar rats received
intraperitoneally a reconstituted PCB mixture containing 13 of the
major congeners that have been identified in human milk, at the
corresponding relative concentrations. The mixture was injected on
days 1 and 3 in corn oil at dose-levels of 0, 0.45, 0.90, 4.5, or
45 mg/kg body weight. The rats were killed on day 6 for
histopathological studies. At the highest exposure level, increased
relative liver weights and enlarged and vacuolated hepatocytes were
observed together with changes in nuclei. In the thyroid, a mild
reduction in follicular size, focal collapse, and changes in nuclei
were found. No changes were seen with 0.9 mg/kg body weight (Gyorkos
et al., 1985) (see section 8.8.1.1).
8.2.3 Dermal exposure
(a) Aroclors
Solutions of Phenoclor DP6, Clophen A60, or Aroclor 1260 in
isopropanol were applied in doses of 118 mg on 50 cm2 of the shaved
back skin of groups of 4 New Zealand rabbits, 5 times per week, for 38
days. A group of 4 rabbits received the vehicle only. After initial
reddening, transverse wrinkling and thickening of the skin developed
with hyperplasia and hyperkeratosis of the epidermal and follicular
epithelium. These effects were more marked with Clophen and Phenoclor
than with Aroclors. During the study, deaths occurred in the Clophen-
and Phenoclor-treated groups. Body weights and relative kidney weights
were decreased in the Aroclor-treated rabbits. Histological liver
changes were least marked in the Aroclor-treated rabbits. Treated
rabbits had fluorescing livers and bone under UV radiation and also
showed other evidence of porphyria. In the kidneys, hydropic
degeneration of the convoluted tubuli, and tubular dilatation were
found. There was atrophy of the thymic cortex and a reduction of
germinal centres of the lymph nodes, as well as leukopenia, and some
animals in all groups showed oedema of the abdominal and thoracic
cavities, subcutaneous tissue, and pericardium. Faecal elimination of
copro- and protoporphyrine was increased by all 3 PCBs, but was lowest
with Aroclor 1260 (Vos & Beems, 1971).
Puhvel et al. (1982) exposed groups of 3 female, hairless mice of 2
strains, (Skh:HR-1 and HRS/J), topically to Aroclor 1254 (4 doses of 1
or 8 mg/week, for 6 weeks) or Phenoclor 54 (5 doses of 0.2 mg/week,
for 10 weeks) in acetone or an acetone-mineral oil-Tween 80 emulsion,
or to the vehicle only. Punch biopsies of the skin were taken
regularly. The skin of treated mice appeared grossly normal after the
exposures, but examination of microscopic skin samples of
Phenoclor-treated mice showed hyperkeratosis of the stratum corneum,
epidermal hyperplasia, disappearance of sebaceous glands, and the
presence of numerous keratinous cysts. No histological changes were
observed in the internal organs.
(b) Individual congeners
In the study of Puhvel et al. (1982), described above, groups of 3
hairless mice of both strains were also exposed topically to 5 doses
of 0.2 mg 3,4,3',4'-tetrachlorobiphenyl/week, for 10 weeks. Grossly
there were no visible changes. The histological changes induced in
HRS/J mice were similar to those found after Phenoclor treatment.
However, identical changes induced in Skh:HR-1 mice were already
observed by 4 weeks. After 8 weeks, these lesions were more marked and
also included hyperkeratosis of the sebaceous follicles and
hyperkeratinization of intradermal pilar cysts. Often these cysts
ruptured into the dermis leading to dense infiltrates of
polymorphonuclear leukocytes. The treated mice showed weight gain,
primarily because of large intra-abdominal fat deposits.
In a dermal toxicity study on rabbits, with a protocol identical to
that of the study of Vos & Beems (1971), skin lesions on animals
treated with 2,4,5,2',4',5'-hexachlorobiphenyl were less severe than
those on Aroclor 1260-treated animals. The liver damage observed
histologically was essentially the same after treatment with either
Aroclor 1260 or 2,4,5,2',4',5'-hexachlorobiphenyl, but the individual
congener was more porphyrogenic (Vos & Nootenboom-Ram, 1972). In
another study, 4 applications of a 25% solution of 3,4,3',4'-
tetrachlorobiphenyl in olive oil to the inner surface of the ears
of rabbits resulted in the same lesions that were found after 2
applications of undiluted Kanechlor 400 or 500. The lesions included
hyperkeratosis, dilatation of hair follicles, and the formation of
keratinous cysts (Komatsu & Kikuchi, 1972).
8.2.4 Appraisal
Rhesus monkey is the most sensitive test species with regard to the
general toxicity of PCBs, both as a mixture and as individual
congeners. The toxicity of mixtures may be confounded by the presence
of impurities, such as PCDFs, which are, or may have been, present in
the mixtures tested. PCBs induce some of the biological and
toxicological effects qualitatively similar to those induced by PCDFs.
Another confounding variable in these studies is the difference in the
composition of the mixtures (Aroclor 1242, 1248, 1254) used.
Beating the above in mind, the available data show that Aroclor 1248,
containing 4.4-8.7 ng of PCDFs/kg, still showed adverse general toxic
effects in Rhesus monkeys at a dose of 0.1 mg/kg diet per day
(0.09 mg/kg body weight per day) administered for an average of 18.2
months (Bowman et al., 1981). A NOEL for general toxicity was not
established for Aroclor 1248. The NOEL for the general toxicity of
Aroclor 1242 was 0.04 mg/kg body weight per day, as established after
dietary exposure for 133 days. The main effects induced by Aroclor
1248 at 0.09 mg/kg body weight per day were an increased mortality
rate, growth retardation, alopecia, acne, swelling of the Meibomian
glands, and, possibly, immunotoxicity. Microscopically, enlarged
hepatocytes, fatty liver with focal necrosis, and epithelial
hyperplasia and keratinization of hair follicles were found. These
effects appeared reversible. Aroclor 1254 at a dose level of
0.200 mg/kg body weight per day showed several effects, not reported
for 1248 (lymforeticular lesions, finger-nail detachment, gingival
effects) and vice-versa (acne, alopecia), which could be related to
the confounding variables noted above. Several effects observed in the
monkeys exposed to Aroclor 1254 (hypertrophic gastropathy, bone marrow
hyperplasia) were also observed in monkeys exposed to Aroclor 1248,
but at a higher dose (4 mg/kg body weight per day). In contrast with
the severe effects observed in adult Rhesus monkeys at low doses,
relatively mild effects were shown by suckling Rhesus monkeys exposed
to much higher doses.
8.3 Skin and eye irritation, sensitization
The injury to skin and eyelids following oral and/or dermal exposure
to PCBs has been discussed in sections 8.2.1 and 8.2.3.
8.4 Reproduction, embryotoxicity, and teratogenicity
8.4.1 Reproduction and embryotoxicity
8.4.1.1 Oral
(a) Mouse
In castrated, mature, male NMRI mice (10-13 per group) that received
28 daily doses of 0.25 mg Aroclor 1254/mouse, in peanut oil, the
weight of the seminal vesicles was decreased, but this was not seen in
intact mice (Orberg & Lundberg, 1974).
When 23 adult female NMRI mice were mated with 22 control males and
orally intubated with 0.025 mg Clophen A60/day, in peanut oil, for 62
days prior to mating and up to days 8-10 of gestation, blastocytes
failed to implant. Twenty-five (14 experimental and 11 control
animals) out of the 45 animals were used to study the effects of PCBs
on the estrous cycle. Effects, such as prolonged estrous cycle and
less frequent periods of sexual receptivity and a decline in the
number of implanted ova, were found (Orberg & Kihlström, 1973).
In order to study the effects of PCBs on the development of sexual
functions in the early postnatal period, these authors also mated mice
that had been suckled by mothers dosed with Clophen A60 during the
lactation period. A decrease in the frequency of implanted ova was
noted, when both parents of the couple had been suckled with milk
containing PCBs. When adult female NMRI mice received 50 mg of Clophen
A60/kg body weight once per week, subcutaneously, during lactation,
the same effect was observed in the offspring after mating with
similarly exposed males (Kihlström et al., 1975).
(b) Rat
In a 2-generation reproduction study, groups of 10 male and 20 female
Sherman weanling rats were fed diets containing 1, 5, 20, or 100 mg of
Aroclor 1254/kg (equivalent to 0.06, 0.32, 1.5, and 7.6 mg/kg body
weight, respectively), or diets containing 5, 20, or 100 mg/kg of
Aroclor 1260 (equivalent to 0.39, 1.5, and 7.5 mg/kg body weight,
respectively). Control groups comprised 20 male and 40 female rats.
The F0 rats were started on the diet at 3-4 weeks of age and the F1
rats, at weaning. The F0 rats were pair-mated when 3 and 7 months old
to produce the F1a and F1b generations, respectively. Breeding-stock
F1b rats were selected at weaning and pair-mated when 3 months old to
produce the F2a, and, when 8 months old, the F2b generation. Rats
exposed to Aroclor 1254 at 20 mg/kg diet or more showed a reduced
litter size at birth, but not when exposed at weaning, in the F1b and
F2 generations. At 100 mg/kg diet, the number of litters in the F2
generation was decreased. In 2 F2a and 2 F2b litters no live offspring
were found at birth, while pup survival at weaning was reduced in the
F2a generation. At weaning, exposed F1a pups weighed less than their
controls. Increased relative liver weights were found in male F1a
weanlings at 1 mg Aroclor 1254/kg diet and in all weanlings at 5 mg/kg
diet or more. Adult rats showed increased relative liver weights at
levels of 20 mg/kg diet or more. No reproductive effects were found
with 5 mg Aroclor 1254/kg diet. In groups treated with Aroclor 1260,
increased liver weights were found in all weanlings at 5 mg/kg diet or
more, but no effects on reproduction were seen, even at 100 mg/kg diet
(Linder et al., 1974).
The only effect observed in Holtzman rats fed Aroclor 1254 at a level
of 500 mg/kg diet, for 3 weeks, was an increase in relative testes
weights (Garthoff et al., 1977). Increased absolute testes weights and
unchanged body weights were found in 6-month-old offspring of
Sprague-Dawley dams exposed to daily doses of 30 mg Aroclor 1260/kg
body weight in ethanol-sesame oil on days 14-20 of gestation. No
effects on testes weight were found in animals treated with Aroclor
1221 or Aroclor 1242 (Gellert & Wilson, 1979).
Sager (1983) evaluated the effects on the reproductive function of
adult male Holtzman rats following exposure of their mothers to doses
of 8, 32, or 64 mg Aroclor 1254/kg body weight in peanut oil, on days
1-3, 5, 7, or 9 of lactation. At all dose levels, 165-day-old males
showed a decreased relative ventral prostate weight, and, at the 2
higher doses, a decreased relative weight of seminal vesicles and
testes as well as decreased body weight. In the ventral prostate,
alveoli were decreased in number and showed folding of the mucosa and
flattened epithelial cells. At 130 days of age, the male offspring at
all 3 dose levels showed a reluctance to mate with control breeders,
leading to a decreased number of pregnancies. Moreover, at the 2
higher dose-levels, the females showed a reduced number of
implantations and an increased resorption rate. The litters showed a
reduced weight gain up to 11 days of age.
This study was repeated with an evaluation of the reproductive
performance of the second generation male rats from 130 days of age,
following mating with normal females. In the first study, autopsy on
pregnant females was carried out on day 11 or 12 of gestation. The
females had fewer implants, fewer embryos, and a reduced proportion of
ovulated eggs that implanted, compared with controls. The effects were
dose-related. In a second study, females mated to the same males were
autopsied on day 2 or 4 after mating. Sperm counts were not affected.
At the 2 highest doses, fewer females had eggs in the expected state
of development, the average number of blastocytes found in one uterine
horn on day 4 was reduced, and an increased incidence of abnormally
developed embryos was observed (Sager et al., 1987).
Female Wistar rats exposed to daily doses of 10 mg Aroclor 1254/kg
body weight, for at least one month, showed a prolonged estrous cycle,
decrease in sexual receptivity, delay in timing of copulation, vaginal
bleeding during gestation, decrease in litter size, and delay in the
time of parturition. After mating of the rats to control males, the
female offspring, exposed in utero and during lactation, showed a
slower rate of body weight gain, higher mortality, earlier vaginal
opening, and a delay in the appearance of the first estrous cycle
(Brezner et al., 1984).
(c) Monkey
Groups of 9 adult female Rhesus monkeys were exposed to a diet
containing Aroclor 1248 (containing polychlorinated dibenzofurans) at
levels of 2.5 or 5.0 mg/kg (Barsotti et al., 1976; Allen et al., 1979,
1980). The study and the maternal toxicity data have already been
described in section 8.2.1.6. Within 4 months, menstrual bleeding and
the duration of the menstrual cycle were increased. Flattening and
prolongation of the serum progesterone peak during the menstrual cycle
was observed. After 6 months of exposure, the females were mated with
control males. Reproductive dysfunction was obvious as shown in Table
44. Following the total exposure period of 18.2 months, the mothers
were put on a control diet. Their menstrual cycles and serum
progesterone levels returned to pre-exposure values. One year after
exposure ceased, the females were again mated with control males and
showed a recovery of their reproductive status (Table 44; Allen et
al., 1980).
Other groups of adult, female Rhesus monkeys were continuously fed
diets with 0, 0.25, and 1.0 mg Aroclor 1016/kg (equivalent to 0, 0.01
and 0.04 mg/kg body weight, respectively), in which no polychlorinated
dibenzofurans were detected (no details). No maternal toxicity was
noted at these levels. In this study, the females were mated with
control males after 7 months of exposure. Reproductive dysfunction
was not observed. Decreased birth weights were found in the offspring
of mothers exposed to Aroclor 1016 at 1.0 mg/kg diet. Skin
hyper-pigmentation occurred in both exposure groups (Barsotti & Van
Miller, 1984). Preliminary reports have indicated possible effects on
learning and behavioural tasks. The milk contained an average of 1.45
and 3.92 mg/kg fat at 0.25 and 1.0 mg/kg, respectively, whereas the
serum of the mothers contained 0.012 and 0.027 mg/litre, respectively
(Heironimus et al., 1981; Levin & Bowman, 1983).
Table 44. Reproductive status of Rhesus monkeys exposed to dietary levels of Aroclor 1016 or 1248
PCB Exposure level Total intake Conceptions Abortions Stillborn Live Reference
mixture at and births
(Aroclor) Diet Body conception resorptions
(mg/kg) weight (mg/kg)
(mg/kg)
1016 0 0 0 8/8 0/8 0/8 8/8 Barsotti &
0.25 0.01 8c 8/8 0/8 0/8 8/8 van Miller
1.0 0.04 30c 8/8 0/8 0/8 8/8 (1984)
1248 0 0 0 12/12 0/12 0/12 12/12 Barsotti et al.
2.5 0.09a 105 8/8 3/8 0/8 5/8 (1976)
5.0 0.2 210 6/8 4/8 1/8 1/8
1248 0 0 0 8/8 0/8 0/8 8/8 Allen et al.
2.5 0.09a 270 8/8b 1/8 0/8 7/8 (1980)
5.0 0.2 498 7/7b 1/7 2/7 4/7
a From: Bowman et al. (1981) amended.
b Breeding 1 year after exposure.
c Calculated assuming a body weight of 5 kg.
In these studies, the monkeys were maintained on the diets during the
gestation and lactation of the first generation. PCBs are known to
cross the placenta and to be excreted via breast milk (see section
6.3). The 6 infants born to monkeys during exposure at 2.5 or 5.0 mg
Aroclor 1248/kg diet showed decreased birth weights, a small stature,
and a decreased body weight gain during nursing. Within 2 months,
signs of intoxication appeared including acne, increased skin
pigmentation, swelling of the eyelids, and loss of eyelashes. In 3
milk samples, values ranging from 0.154 to 0.397 mg PCBs/kg milk were
measured, and, 1 milk sample contained 16.44 mg PCBs/kg fat. Three
infants died. Necropsy and histopathology revealed atrophy of the
thymus and lymph nodes, hypocellular bone marrow, moderate fatty
infiltration of the liver, hypertrophic Meibomian glands, and
keratinization of hair follicles. One dead infant showed hyperplasia
of the gastric mucosa. The 3 surviving infants were weaned and
subsequently showed marked improvements in their physical status
(Allen & Barsotti, 1976; Allen et al., 1979, 1980). At 6 and 12 months
of age, they were found to be hyperactive in a locomotor activity test
and, between 7 and 24 months of age, they did not learn reversal tasks
as readily as the controls. The PCB body burdens of these infants
ranged between 11 and 27 mg/kg body fat, at the age of 8 months, and
dropped to 0-1.6 mg/kg, at the age of 23 months. However, using the
same apparatus, these infants showed hypolocomotor activity at 44
months of age in comparison with the same controls (Bowman et al.,
1978; Bowman & Heironimus, 1981; Bowman et al., 1982; Levin & Bowman,
1983).
The infants delivered by the same adult females, after 1 year on a
control diet, showed a reduced body weight and signs of intoxication
similar to those observed in their siblings of the first breed.
Two infants in each exposed group died. Milk samples contained
0.02-0.19 mg PCBs/kg milk (Allen et al., 1980). At 12 months of age,
when the PCB body burdens were only slightly higher than those of the
controls, the infants showed hyperlocomotor activity (Bowman et al.,
1982).
Groups of 4 or 6 Rhesus monkeys, which had been exposed to 0 or 2.5 mg
Aroclor 1248/kg diet in utero and during nursing until 4 months
after birth, were tested at 4-6 years of age on delayed spatial
alternation (DSA), a spatial learning and memory task. Deficits in
performance accuracy were detected in 2 cohorts of monkeys, whose
mothers had been fed 2.5 mg Aroclor 1248/kg diet for an 18-month
period ending at least 12 months prior to pregnancy. The deficit was
most apparent at the shorter delays, suggesting impairments in
association or attention processes were involved rather than memory
impairment. Such a deficit was also found in monkeys fed 1.0 mg
Aroclor 1016/kg diet, but the effect was less pronounced. The
appearance of a PCB-induced cognitive deficit more than 3 years after
the end of exposure indicated the existence of long-term adverse
consequences of perinatal PCB exposure (Levin et al., 1988).
Clophen A30, which did not contain detectable levels of
polychlorinated dibenzofurans (limit of detection <1 mg/kg), was
given by gavage in a 1% solution of methylcellulose in water, once
daily for 30 days, to 3 lactating Rhesus monkeys and their offspring
at a level of 16 mg/kg body weight. PCB concentrations were measured
in the serum of both mothers and infants and in the milk, on days -14,
-7, 0, 1, 2, 4, 8, and then at weekly intervals until the end of the
study. The mean PCB concentrations in the serum of mothers and infants
were between 0.13 and 1.16 mg/litre and 0.07 and 2.67 mg/litre,
respectively (before treatment days -14 and -7). The mean PCB levels
in milk ranged from 0.63 mg/kg on day 1 of exposure to 18.90 mg/kg. On
days -14 and -7, the concentrations in milk were 0.14 and 0.34 mg/kg
(wet weight). Five control pairs were used. One dam and her offspring
were sacrificed on day 22, exhibiting symptoms of anorexia,
depression, lethargy, and ataxia. Two of 3 infants showed a decreased
body weight gain. At autopsy of the infants, after the exposure
period, slight degenerative changes were seen in the liver and the
kidneys, together with slight demyelination of the central nervous
system, slight to moderate gliosis of the cerebrum, and slight
granular cell layer thinning of the cerebellum. On the basis of
earlier work with adults in which the degenerative changes described
above were considerable, the authors concluded that the nursing
infants seemed to be less susceptible than the adults under the
conditions of the studies (Iatropoulos et al., 1978; Bailey et al.,
1980).
8.4.2 Teratogenicity
8.4.2.1 Aroclors (oral)
(a) Mouse
Haake et al. (1987) reported that treatment of pregnant C57Bl/6 mice
with Aroclor 1254, by gavage, at 224 mg/kg body weight, on day 9 of
gestation, did not result in any fetuses with cleft palate (see
section 8.6.6).
(b) Rat
Wistar rats were exposed to dietary levels of Kanechlor 400 of up to
250 mg/kg from day 1 to day 21 of gestation (Mizunoya et al., 1974).
Fetuses showed decreased body weights from 10 mg/kg diet onward
(equivalent to 0.67 mg/kg body weight), but did not show any increased
incidence of malformations. Maternal toxicity was not observed and
litter size and the number of litters were unaffected. Decreased pup
survival was noted at dietary levels from 50 mg/kg (equivalent to
3.5 mg/kg body weight) upwards. The 28-day-old offspring showed
decreased body weight and increased relative liver weight from
10 mg/kg.
Commercial Kanechlor 300 or 500 was mixed with food and administered
to pregnant Sprague-Dawley rats, throughout gestation, at levels of
20, 100, or 500 mg/kg diet. On day 21, about three-quarters of the
pregnant females were sacrificed; the remainder were allowed to litter
naturally and the postnatal development of the pups was observed.
Kanechlor 500 at a concentration of 500 mg/kg resulted in decreased
maternal weight gain and decreased food consumption. At 20 and 500 mg
Kanechlor 300/kg, and 500 mg Kanechlor 500/kg, the fetal weight
decreased significantly. Resorption and malformations were not
increased by treatment with Kanechlors. The Kanechlors did not show a
teratogenic potential in this study (Shiota, 1976a).
Offspring of Sprague-Dawley rats that received 20 mg of Kanechlor
500/kg body weight on days 15-21 of gestation were slower than
controls in achieving the water maze test at the age of 12-13 weeks,
but did not perform worse in the open field test and in the swimming
test (Shiota, 1976b). Behavioural effects were also observed in the
offspring of ICR dams, 23-27 days of age, exposed to Aroclor 1254 in
the diet at levels of 11 or 82 mg/kg (equivalent to 1.7 and 12 mg/kg
body weight) from 3 days before mating up to weaning. The offspring
were maintained on the same diet. PCB exposure did not have any
effects on the ability to learn an avoidance response, but increased
the latency to make such a response. Moreover, the young mice
exhibited slower habituation to an open field (Storm et al., 1981).
The effects of Fenchlor 42 (trichloro- 63%, tetrachloro- 33%, and
small amounts of dichlorobiphenyl; purity 97.5%) exposure of Fischer
344 male and female rats were studied through assessment of the
behavioural development of their F1 progeny. Female rats were
administered 5 daily ip injections of corn oil or 5-10 mg Fenchlor
42/kg body weight per day, 2 weeks prior to mating. Another group
received 2-4 mg/kg per day during gestation (days 6-15 of pregnancy)
and a third group of 8 previously treated lactating females received
corn oil or 1-2 mg/kg per day on postnatal days 1-21. The total doses
in the 3 groups were 25-50, 20-40 and 20-40 mg/kg body weight.
Dose-dependent differences in behaviour were found in the offspring of
the PCB-treated animals. Differences in the development of cliff
avoidance reflexive behaviour, swimming ability, and open field
activity were evident. The PCB exposure of female animals during
gestation and lactation resulted in impaired acquisition of the active
avoidance behaviour, while preconception PCB exposure affected active
avoidance performance, as reflected in an increased number of
avoidance responses to reach criterion for extinction (Pantaleoni et
al., 1988).
Doses of 0, 6.25, 12.5, 25, 50, or 100 mg/kg body weight per day of
Aroclor 1254 were administered to rats, by gavage, on days 6-15 of
gestation. Average pup weights were reduced at 100 mg/kg, though total
litter weight (average weight times number of fetuses) did not differ
from controls. There were no skeletal or visceral abnormalities or
effects on conception, resorptions, litter size or number, or average
litter weight in any of the treated groups (Villeneuve et al., 1971b).
Spencer (1982) exposed Holtzman rats to a diet containing Aroclor 1254
at levels of 25 up to 900 mg/kg diet from day 6 to day 15 of gestation
and found reduced maternal body weight gain and decreased fetal
survival at birth from 300 mg/kg diet (equivalent to 18 mg/kg body
weight) upwards, and decreased fetal weights at birth from 100 mg/kg
(equivalent to 8 mg/kg body weight) upwards. No visceral or skeletal
data were available.
When Sherman rats were exposed to doses of Aroclor 1254 of up to
100 mg/kg body weight, in peanut oil, from day 7 to day 15 of
gestation, a decrease in the survival of the pups was found. At
weaning, the survival and body weight of the grossly normal pups were
reduced at the 100 mg/kg dose level, but not at 50 mg/kg body weight.
No effects were observed at 100 mg Aroclor 1260/kg body weight (Linder
et al., 1974).
(c) Monkey
Two pregnant Cynomolgus monkeys (Macaca fascicularis) were dosed
with Aroclor 1254 at 100 mg/kg body weight per day and 1 monkey, at
400 mg/kg body weight per day, from day 60 of gestation. One control
animal received the vehicle, corn oil. The 2 monkeys fed 100 mg/kg
delivered dead male infants after 105 and 108 days of dosing, and the
female fed 400 mg/kg delivered a female infant (with no overt clinical
signs of toxicity) that died at 139 days of age with an acute
bronchopneumonia. The breast milk of the monkey fed 400 mg/kg
contained, over a period of 5-75 days after parturition,
concentrations of 73.7 up to 139.4 mg/kg, on a fat basis. No overt
signs of toxicity were observed in the adult animals, with exception
of finger nail loss. All 3 treated monkeys showed impaired
immunological capacity, assessed at approximately 50 days postpartum
(148 days of treatment) (Truelove et al., 1982).
(d) Rabbit
Rabbits were exposed to 0, 1.0, or 10.0 mg Aroclor 1254/kg body weight
and in another study to 0, 12.5, 25.0, or 50 mg/kg body weight (purity
not reported), by gavage, on days 1-28 of pregnancy. Abortions, still
births, and maternal deaths occurred at 12.5 mg/kg body weight or
more, but no teratogenic effects were found (Villeneuve et al.,
1971a,b).
8.4.2.2 Aroclors (subcutaneous)
(a) Mouse
A possible teratogenic effect was observed in ddy mice subcutaneously
exposed to doses of 1-5 mg Kanechlor 500/mouse (equivalent to
40-200 mg/kg body weight) in 95% ethanol from day 6 to day 15 of
gestation. A dose-related increase in maternal mortality was observed
from a dose of 3 mg/mouse onwards. Some dams showed skin lesions,
alopecia, or swelling of the liver, but no effects on body weight
gain. A slight increase was noted in the incidence of dead and
resorbed fetuses. The incidence of cleft palate was increased in a
dose-related manner from the lowest dose (Watanabe & Sugahara, 1981).
8.4.2.3 Individual congeners (oral)
(a) Mouse
Orberg (1978) fed groups of 20-56 pregnant female NMRI-mice 0, 0.05,
or 0.5 mg 2,5,4'-trichloro- or 2,4,5,2',4',5'-hexachlorobiphenyl,
dissolved in peanut oil, per animal, from days 1 to 6 of gestation. A
significant decrease in the pregnancy of implanted ova was found with
the 0.5 mg treatment. No effects on percentage of pregnancies and mean
number of corpora lutea were found. There were no effects at the lower
dose level.
A dose-related increase in embryotoxicity and the incidence of
malformed fetuses, mainly showing cleft palate and hydronephrosis, was
found in pregnant CD-1 mice after exposure to daily doses of 2, 4, 8,
or 16 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body weight, in
cotton-seed oil, on days 6-15 of gestation. Lower dose levels, e.g.,
0.1 and 1 mg/kg body weight were without effects. No dibenzofurans
were detectable (no details) in the test compound. The dams showed a
decreased body weight gain at 8 mg/kg body weight. The authors
reported that 3,4,3',4'-tetrachlorobiphenyl and 2,3,4,2',3',4'-hexa-
chlorobiphenyl also produced the same teratogenic effects, though they
were less potent than those of 3,4,5,3',4',5'-hexachlorobiphenyl
(Marks et al., 1981).
A neurobehavioural "spinning" syndrome (a syndrome characterized by
the fact that the mice rotate or spin in a circular motion when held
by the tail) and hydronephrosis developed in CD-1 mouse weanlings,
whose dams received, by gavage, 32 mg 3,4,3',4'-tetrachlorobiphenyl/kg
body weight, in corn oil, on days 10-16 of gestation. Maternal
neurotoxicity was not observed. Histological and ultrastructural
examination of the CNS of affected mice revealed longitudinal
projections of the cylindrical CNS in the ventral and dorsal roots
and, to a lesser extent, in cranial nerve roots. The effect was
possibly related to an observed altered development of striatal
synapses (Chou et al., 1979; Tilson et al., 1979; Agrawal et al.,
1981).
(b) Rat
The congener 3,4,3',4'-tetrachlorobiphenyl was found to be embyrotoxic
and caused accumulation of blood in the amniotic fluid and the
gastrointestinal tract of fetuses from Sprague-Dawley rats treated on
days 6-18 of gestation with doses of 3 or 10 mg/kg body weight in corn
oil. Decreased fetal growth was also observed (Wardell et al., 1982).
When the rats were allowed to deliver, high perinatal mortality was
observed, which appeared to be related to an increase in gestational
length and to be independent of the smaller total litter size. In
addition, pup weights were found to be lower than those of controls
(Rands et al., 1982; White et al., 1983).
(c) Guinea-pig, mouse
Neither embryotoxicity nor teratogenicity were found in Dunkin Hartley
guinea-pigs, and CBA mice exposed in utero to 2,4,5,2',4',5'-
hexa-chlorobiphenyl during gestation (Mattsson et al., 1981; Brunström
et al., 1982; Aulerich et al., 1985) and in C57BL/6N mice similarly
exposed to 2,4,5,2',4',5'- or 2,3,4,5,3',4'-hexachlorobiphenyl
(Birnbaum et al., 1985).
Pregnant guinea-pigs received a total dose of 100 mg technical grade
Clophen A50 orally, in peanut oil, from day 16 to day 60 of gestation,
25 mg 2,4,5,2',4',5'-hexachlorobiphenyl from day 16 to day 60 of
gestation, or 100 mg from day 22 to day 60 of gestation. The
administration of Clophen A50 resulted in fetal deaths, but no
maternal deaths. In contrast, 2,4,5,2',4',5'-hexachlorobiphenyl did
not cause fetal deaths. Prenatal weight of live fetuses was increased
by a dose of 25 mg, but not by 100 mg of 2,4,5,2',4',5'-hexa-
chlorobiphenyl (Brunström et al., 1982).
(d) Monkey
In a briefly reported study, 6 female Rhesus monkeys received 9 doses
of 70 or 350 µg 3,4,3',4'-tetrachlorobiphenyl/kg body weight by
gavage, in corn oil, from day 20 to day 40 of gestation. A control
group comprised 12 animals. Maternal toxicity (not specified) but no
mortality, was reported in all exposed monkeys from day 31 following
exposure. Between days 17 and 35 following exposure all exposed
fetuses and 3 out of 12 control fetuses aborted (McNulty, 1985).
8.4.3 Appraisal
The Rhesus monkey is the most sensitive species with regard to general
toxicity (see section 8.2) and particularly with regard to
reproductive toxicity. The presence of PCDFs and the variation in PCB
composition may be confounding factors in determining the reproductive
toxicity of PCB mixtures. Aroclor 1248, containing 4.4-8.7 µg
PCDFs/kg, adversely affected the reproductive performance of female
Rhesus monkeys, mated with control males after 6 months of dietary
exposure to a toxic dose of 0.09 mg/kg body weight per day and
continuation of the exposure for an average of up to 10 months. This
effect was reversible after an exposure-free period of 1 year. No
effect on reproduction was found in female Rhesus monkeys exposed to a
non-toxic dose of 0.01 or 0.03 mg Aroclor 1016 (reported not to
contain PCDFs)/kg body weight per day and mated after 7 months with
control males.
Neonates of the nursing mothers exposed to Aroclor 1248 showed adverse
effects similar to those seen in their mothers and, in addition,
persistent behavioural disturbances, atrophy of the thymus and lymph
nodes, bone marrow hypoplasia, and hyperplasia of the gastric mucosa.
Neonates of the mothers after the recovery period still showed adverse
effects, as well as the neonates of the mothers exposed to Aroclor
1016. These effects were caused by PCBs, with or claimed to be
without, PCDFs, transmitted via the placenta during gestation and
later via the breast milk. Neonates have much greater susceptibility
to PCB toxicity when exposed via the mothers compared with suckling
monkeys orally exposed. Reproductive toxicity has also been observed
in the mink, rabbit, and rat. The changes seemed to be related to
alterations in the serum levels of gonadal steroid hormones, as a
result of enzyme induction. PCBs may also bind to the cytoplasmic
estrogen receptor. Effects have also been observed on the estrus cycle
of female rats, minks, and monkeys, on the sex organs of male rats,
and on the implantation rate of fertilized ova following exposure of
female mice or male rats.
Comprehensive teratological examinations have not been conducted;
however, the available studies indicated that the Aroclors were not
teratogenic in rats and nonhuman primates, when tested via the oral
route during the critical periods of organogenesis at doses that
produced fetotoxicity and/or maternal toxicity. Although the
fetotoxicity of Aroclors is documented in several species of animals,
the possibility that contaminants (e.g., PCDFs) might be (partly)
responsible for the effects should be recognized.
The results of the reproduction and teratogenicity studies are
summarized in Tables 45, 46, and 47.
8.4.4 Mutagenicity and related end-points
8.4.4.1 DNA damage
PCBs have been shown to interact with the proteins, RNA and DNA, after
metabolic activation. The potential of readily metabolizable
PCB-congeners to cause primary DNA damage was indicated by the
activity of 2,5,2',5'-tetrachlorobiphenyl and its metabolites in
causing DNA, single-strand breaks in an alkaline elution assay with
L-929 cells in vitro (Stadnicki et al., 1979). Furthermore,
unscheduled DNA synthesis was elicited by 4-chlorobiphenyl in vitro
in Chinese hamster ovary cells (Wong et al., 1979). No unscheduled DNA
synthesis was elicited by Aroclor 1254 in rat hepatocytes in vitro
(Probst et al., 1981).
DNA-breaking activity was found in an alkaline elution assay with
hepatocytes of intact rats treated in vivo with a single, high dose
(500 mg Aroclor 1254/kg body weight, intraperitoneally, or 1295 mg/kg
body weight, orally) with complete repair of the damage within 48 h
(Robbiano & Pino, 1981). Aroclor 1254 was also shown to be a
DNA-breaking agent in an alkaline elution assay in vitro with rat
hepatocytes (Sina et al., 1983). An alkaline sedimentation assay
showed the DNA-breaking activity of Aroclor 1254 in rats treated
in vivo with a single intraperitoneal dose of 500 mg/kg body weight.
In this assay, the same Aroclor 1254 pretreatment of the rats in vivo
elevated the DNA-breaking activity of the direct-acting, alkylating
N-methyl- N'-nitro- N-nitrosoguanidine and the carcinogens,
dimethylnitrosamine and benzo (a)pyrene, in vitro (Mendoza-Figueroa
et al., 1985).
8.4.4.2 Mutagenicity tests
Many mutagenicity tests have been carried out over the years, with
different PCB mixtures. Most of these were commercial mixtures the
composition of which was not described. Only a few studies are
available on specific congeners. Besides studies on microorganisms,
mammalian cell point mutation, dominant lethal assays, micronucleus
tests, chromosome and cytogenicity studies, and DNA repair studies
were carried out. With a few exceptions the results of most of the
studies with PCB mixtures were negative (see Table 48).
Aroclor mixtures and the congener 2,5,2',5'-tetrachlorobiphenyl and
its metabolites did not induce point mutations in Salmonella
typhimurium TA 98, TA 100, TA 1535, TA 1537, and TA 1538 with, and
without, metabolic activation (Wyndham et al., 1976; Hsia et al.,
1978; Bruce & Heddle, 1979; Schoeny et al., 1979; Shahin et al.,
1979), neither did 2,4,2',4'- and 3,4,3',4'-tetrachlorobiphenyl and
2,4,6,2',4',6'-hexachlorobiphenyl in the strains TA 98 and TA 100
(Schoeny, 1982).
However, Wyndham et al. (1976) found a dose-related mutagenic activity
of 4-chlorobiphenyl and, to a lesser extent, of Aroclor 1221 in the
strain TA 1538, after metabolic activation by the S9 liver fraction of
uninduced rabbits. The study was repeated 3 times in the same
laboratory, but the results of Wyndham et al. (1976) could not be
confirmed (Safe, 1980). Schoeny (1982) could not detect any mutagenic
activity of 4-chlorobiphenyl in the same dose range in the strains TA
98, TA 100, TA 1535, and TA 1537, with, or without, the S9 liver
fraction of induced rats.
Table 45. PCBs: reproduction and embryo toxicity
Animal (strain, PCB mixture Exposure period NOAEL LOAEL Parameters, effects Reference
sex) (oral) (mg/kg body (mg/kg body
weight) weight)
Rat (Sherman, Aroclor 1254 continuous up to 0.06 (1254) increased relative liver Linder et al.
10 male, Aroclor 1260 weaning weights in male F1A (1974)
20 female) weanlings
0.32 (1254)
former effect in all weanlings
0.32 (1254)
7.5 (1260) reproduction parameters
reproduction parameters
Rhesus monkey Aroclor 1016 7 months 0.03 (0.04) reproduction parameters Barsotti &
(female) [0.03(0.04): decreased birth van Miller
weight] [0.01: skin (1984)
hyperpigmentation]
Rhesus monkey Aroclor 1248 6 months 0.09 abortions, resorptions, live Barsotti et al.
(female) births (1976)
1 year after 0.09 0.2 Allen et al.
exposure stillborn, live births (1980)
Table 46. PCBs: teratogenicity
Animal (strain) PCB-mixture Exposure period NOAEL LOAEL Parameters, effects Reference
(oral) (mg/kg body (mg/kg body
weight) weight)
Rat Aroclor 1254 days 6 to 15 of 50 100 reduced average pup Villeneuve et al.
gestation weight (1971b)
Rat (Holtzman) Aroclor 1254 days 6 to 15 of < 8 8 decreased fetal weight at Spencer (1982)
gestation birth
Rat (Sherman) Aroclor 1254 days 7 to 15 of 100 reproduction effects Spencer (1982)
gestation
Rabbit Aroclor 1254 days 1 to 28 of 10 12.5 abortions, still births, Villeneuve et al.
gestation maternal deaths (1971 a,b)
Aroclor 1221 days 1 to 28 of 25 fetotoxicity
gestation
Table 47. PCBs: teratogenicity of individual congeners
Animal PCB-mixture Exposure period NOAEL LOAEL Parameters, effects Reference
(strain) (oral) (mg/kg body (mg/kg body
weight) weight)
Mice 2,5,4'-TCB day 1 to 6 of 0.05/animal 0.5/animal decrease in the number Örberg (1978)
(NMRI) 2,4,5,2',4', gestation of implant/dams
5'-HCB
Mice 3,4,5,3',4', day 6 to 15 of 2 embryotoxicity, malformations Marks et al.
(CD-1) 5'-HCB gestation (cleft palate, hydronephrosis) (1981)
3,4,3',4'-TCB 8 maternal toxicity, less potent
2,3,4,2', than that of 3,4,5,3',4',5'-HCB
3'4'-HCB
Rat 3,4,3',4'-TCB day 6 to 18 of 3 embryotoxicity Wardell et al.
(Sprague- gestation (1982)
Dawley)
Rhesus 3,4,3',4'-TCB day 20 to 40 of 0.07 maternal toxicity, total abortions McNulty (1985)
monkey gestation
Table 48. Results of mutagenicity, and related, tests
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
2,5,2',5'- Salmonella TA 1538 200 µg/plate modified - Wyndham et al.
tetrachloro typhimurium 100 µg/plate microsomal - (1976)
biphenyl 50 µg/plate fraction -
10 µg/plate from rabbits -
Aroclor 1268 Salmonella TA 1538 200 µg/plate modified - Wyndham et al.
typhimurium 100 µg/plate microsomal - (1976)
50 µg/plate fraction -
10 µg/plate from rabbits -
Aroclor 1254 Salmonella TA 1535 4 different both with, and - Heddle & Bruce
typhimurium TA 1537 concentrations, without, S-9 in - (1977)
TA 98 figures not all cases -
TA 100 given -
Aroclor 1254 Salmonella TA 1535 8 different both with, and - Schoeny et al.
typhimurium TA 1537 concentrations without, S-9 - (1979)
TA 98 from 0.5 to -
TA 100 500 µg/plate -
Aroclor 1254 Salmonella TA 1535 0.05, 0.5, both with, and - Bruce & Heddle
typhimurium TA 1537 5, 50, and without, S-9 - (1979)
TA 98 500 µg/plate -
TA 100 -
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Aroclor 1254 Salmonella TA 1538 50, 100, 500, both with, and - Shahin et al.
typhimurium TA 98 1000, 2000, without, S-9 - (1979)
5000 µg/plate
Aroclor 1242 V79 50, 100, and without - Hattula (1985)
Clophen A60 hamster cells 150 µg/ml metabolizing -
co-cultivated cells
with lethally
irridatiated
hepatocytes
Aroclor 1254 Micronucleus 4 different - Heddle & Bruce
test concentrations, (1977)
(erythrocytes) figures not
given
Aroclor 1254 Micronucleus (C57B1/6 × (approximately) - Bruce & Heddle
test C3H/He) LD50, 1/2, 1/4, (at all doses) (1979)
F1 mice and 1/8 of the
highest dose
(5 consecutive
days, ip)
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Kanechlor 500 Micronucleus ddY-mice 100 mg/kg in - - Watanabe et al.
test with corn oil orally (1982)
polychromatic and 100 mg/kg
erythrocytes 95% ethanol
subcutaneously
Aroclor 1254 Chromosomal (embryonic 0 or 10 mg/kg inconclusive Peakall et al.
aberrations Ring diet (1972)
Doves)
Aroclor 1254 Chromosomal (human 100 mg/litre - Hoopingarner
aberrations lymphocytes) culture medium et al. (1972)
Aroclor 1254 Chromosomal male 5, 50, 500 mg/kg negative at all Garthoff et al.
abnormalities in Holtzmann diet doses (1977)
bone marrow and rats
spermatogonial
cells
Aroclor 1242 Chromosomal Osborne- 5000 mg/kg × 1a - Green et al.
abnormalities Mendel 2500 mg/kg × 1 - (1975a)
in bone marrow rats 1250 mg/kg × 1 -
cells and 500 mg/kg × 4 -
spermatogonial
cells
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Clophen A30 Drosophila 62.5, 125, 250, -b Nilsson & Ramel
melanogaster and 500 mg/litre (1974)
(adults or larvae) substrate
Clophen A50 Drosophila 25, 50, 100, -b Nilsson & Ramel
melanogaster and 200 mg/litre (1974)
(adults or larvae) substrate
Aroclor 1242 Dominant Lethal Osborne- 2500 mg/kg × 1a - Green et al.
test Mendel 1250 mg/kg × 1 - (1975b)
rats 625 mg/kg × 1 -
250 mg/kg × 5 -
125 mg/kg × 5 -
Aroclor 1254 Dominant Lethal Osborne- 150 mg/kg × 5a - Green et al.
test Mendel 75 mg/kg × 5 - (1975b)
rats
Aroclor 1254 Dominant Lethal Osborne- 25, 100 mg/kg - Green et al.
test Mendel diet for 70 days - (1975b)
rats
Table 48. (cont'd).
Chemical Test system Strain Dose Metabolic Result Reference
substance activation
Aroclor 1254 Sperm (C57 B1/6 × (approximately)LD50, negative at all Bruce & Heddle
Abnormality C3H/He)F1 1/2, 1/4, 1/8 top dose doses (1979)
mice on 5 consecutive
days, ip
Aroclor 1254 mitotic index human 100 mg/litre mitotic index Hoopingarner
lymphocytes culture medium equivocal et al. (1972)
4-chloro- DNA repair and Chinese 10-5 mmol/litre covalent- Wong et al.
biphenyl and unscheduled hamster 3H-4-chloro- binding to (1979)
metabolites synthesis ovary cells biphenyl, 24 h protein, RNA,
(hydroxyurea and DNA
addition suppress Increase
DNA synthesis) specific
activity with
DNA
a Means single dose (× 1) or 5 doses in 5 days (× 5).
b No loss or non-disjunction of sex-chromosomes.
SD=Significant decrease.
Dose-related chromosome breakage was found in human lymphocytes
exposed to the planar PCB congener, 3,4,3',4'-tetrachlorobiphenyl, at
0.1-10-4 µg/ml (Sargent et al. (1989). In contrast, the non-planar
2,5,2',5'-tetrachlorobiphenyl did not cause chromosome damage in a
comparable test, even at concentrations as high as 1 µg/ml. However, a
combination of 3,4,3',4'-tetrachlorobiphenyl at a concentration of
10-5 µg/ml with 2,5,2',5'-tetrachlorobiphenyl caused chromosomal
damage that was far in excess of what might be expected from higher
doses of 3,4,3',4'-tetrachlorobiphenyl alone. The results suggest that
some PCB congeners may interact to cause synergistic effects.
Peakall et al. (1972) carried out cytogenic studies on Ring dove
embryos (Streptopelia risoria); 6 embryos were from dove pairs not
fed PCBs (controls) and 17 embryos were from PCB-fed (10 mg/kg diet)
pairs. The frequencies of chromosome aberrations were recorded for
chromosome pairs occurring in metaphase cells of allantoic sac and
limb bud origin. Mean aberration rates were as follows: control 0.8%
(range 0-2.0%) and PCB-treated 1.8% (range 0-9.4%). It was concluded
by the authors that these results were indicative of a possible
clastogenic action of PCBs.
A DNA repair assay was carried out by Wong et al. (1979) using CHO
cells and measuring the effects of 4-chlorobiphenyl (10 mol/litre) on
the unscheduled DNA synthesis (UDS) in the presence of hydroxyurea
(HU), a chemical agent that suppresses normal replicative DNA
synthesis. The quantification of DNA synthesis was determined by the
uptake of [H3]-thymidine into the cellular DNA. A significant
(1.6-fold) enhancement of UDS was found when the cells were incubated
for 2.5 h in the presence of HU, 4-chlorobiphenyl, and thymidine.
8.4.4.3 Cell transformation
Aroclor 1254 did not cause an increase in benzo (a)pyrene-induced
transformation in a test using C3H10 T1/2 CL8 mouse embryo fibroblasts
(Nesnow et al., 1981).
Aroclor 1254 also failed to transform Golden Syrian hamster cells
76-582 in culture at 50 µl/ml (Pienta, 1980).
8.4.4.4 Cell to cell communication
The congener 2,4,5,2',4',5'-hexachlorobiphenyl inhibited one form of
intercellular communication in V79 Chinese hamster cells, i.e.,
metabolic cooperation by mutant rescue at non-cytotoxic levels, while
3,4,5,3',4',5'-hexachlorobiphenyl was inactive (Tsushimoto et al.,
1983).
8.4.4.5 Interaction
Grolier et al. (1989) studied the effects of Vitamin A dietary intake
(2 and 20 IU/g of food) on the mutagenicity of benzo (a)pyrene
(B (a)P) towards Salmonella typhymurium TA 98, either in control
rats or in animals treated with 2,4,5,2',4',5'- hexachlorobiphenyl and
3,4,3',4'-tetrachlorobiphenyl. The planar tetrachlorobiphenyl strongly
increased B (a)P-monooxygenase activity and glutathione transferase,
while the non planar hexachlorobiphenyl was a strong inducer of
epoxide hydrolase and a weak inducer of B (a)P-monooxygenase. Enzyme
induction was not modified by changes in Vitamin A intake. A greater
mutagenic effect was observed in the tetrachlorobiphenyl group than in
the hexachlorobiphenyl group. This could be related to the specific
form of cytochrome P-450 induced by the tetrachlorobiphenyl congener.
In PCB-treated rats, the mutagenic activity of B (a)P was higher in
the 20-IU group than in the 2-IU group.
8.4.4.6 Cell division parameters
Tests on Osborne-Mendel rats gave various results, but may provide the
most important clue to the mechanism of action of PCBs in
carcinogenesis. At high doses (5000 mg Aroclor 1242/kg given once, and
500 mg/kg, given in a series of 4 daily doses), there were significant
decreases in the numbers of spermatogonial cells in mitosis. Single
dose levels of 1250 or 2500 mg/kg gave negative results (Green et al.,
1975a). Garthoff et al. (1977) also found negative results in male
Holtzmann rats treated with 0, 5, 50, or 500 mg Aroclor 1254/kg diet
for 5 weeks with regard to the mitotic indices of bone marrow and
spermatogonial cells. The data of Hoopingarner et al. (1972) showed an
increase in mitotic index in human lymphocytes exposed to Aroclor
1254.
The above-mentioned studies provide evidence that Aroclors can enhance
cell proliferation, and this is of special interest because it
suggests that the Aroclors may act to promote carcinogenesis to a
greater extent than to initiate it. The effect on cell proliferation
requires further examination in a variety of systems.
8.5 Carcinogenicity
Hayes (1987) critically reviewed the available evidence for, and
against, the view that environmental PCBs present a significant
potential carcinogenic hazard for humans.
8.5.1 Long-term toxicity/carcinogenicity
(a) Mouse
Nagasaki et al. (1974) exposed 10 groups of dd mice (6-12 of each sex)
to Kanechlor 300, 400, or 500 in the diet at levels of 0, 100, 250, or
500 mg/kg (equivalent to 0, 5, 12.5, and 25 mg/kg body weight,
respectively) for 32 weeks. Nine nodular hyperplasia and 7
hepatocellular carcinomas were found in 17 male mice, and 4 cases of
liver hypertrophy in 17 female mice, after exposure to 500 mg
Kanechlor 500/kg diet. No neoplasms were found in the other groups.
In another mouse study, groups of male BALB/cJ mice were fed 0 or
300 mg Aroclor 1254/kg diet (equivalent to 50 mg/kg body weight) for 6
or 11 months. Adenofibrosis was observed in the livers of all 22 mice
fed Aroclor 1254 for 11 months, but not in those of the 24 mice
exposed for 6 months. Hepatomas were noted in 9/22 mice exposed for 11
months and in 1/24 mice exposed for 6 months. No tumours were found in
the controls (Kimbrough & Linder, 1974).
(b) Rat
In a preliminary study, liver tumours (multiple adenomatous nodules)
were induced by Kanechlor 400 (containing 2,4,3',4'-; 2,5,3,3'-;
2,3,4,4'-; and 3,4,3',4'-tetrachlorobiphenyls) in 6/10 females, but
not in male Donryu rats, in 400 days. The dietary exposure was
periodically adjusted according to animal weights and ranged from 38.5
to 616 mg/kg diet. The latter dose level was administered for 275
days. The number of animals used was small (10 treated and 5 control
rats of each sex). Increased incidences of pneumonia, and lung and
intracranial abscesses were found in rats on a diet containing
Kanechlor 400, possibly because of lowered resistance to infection
(Kimura & Baba, 1973).
Ito et al. (1974) fed 10 groups of 29 male Wistar rats with Kanechlor
300, 400, or 500 at dose levels of 100, 500, or 1000 mg/kg diet
(equivalent to 5, 25, and 50 mg/kg body weight, respectively) for
28-52 weeks. Another group received the control diet. A number of
animals died in all groups (4 up to 21 animals); within the treated
groups, deaths were more or less dose related. Adenofibrosis was
observed in the livers of rats fed 1000 mg/kg diet of all 3 mixtures.
Kanechlor 500 produced nodular hyperplasia at all dose-levels and at a
higher incidence than the mixtures with a lower chlorine content. No
neoplastic nodules were observed in the controls. Kanechlor 300 and
500 (100 mg/kg diet) did not show significant growth inhibition or
increases in liver weight. No fibrosis or cholangiofibrosis, bile duct
proliferations, fatty changes, or cellular hypertrophy in the liver
were found. The liver nodular hyperplasia, designated as preneoplastic
by the investigators, was found in 3/25 of the Kanechlor 500
(100 mg/kg diet) treated animals, 1/22 of the Kanechlor 300 (100 mg/kg
diet) treated animals, and 0/18 controls. The 1/22 (4.5% incidence in
the Kanechlor 300, 100 mg/kg diet) is not significant and the 2 higher
dose levels of this product did not induce such changes. It can be
concluded that Kanechlor 300 did not induce neoplasia at dietary
levels of up to 1000 mg/kg over a 52-week period, in this study. At
the 1000 mg/kg level, Kanechlor 300 did produce other evidence of
chronic liver toxicity including oval cell and bile-duct
proliferation, fatty changes, and cellular hypertrophy and, possibly,
cholangiofibrosis (2/15). In the case of Kanechlor 500 (100 mg/kg
diet), essentially the same picture was obtained. In this group, 3/25
cases of nodular hyperplasia were found. In the case of Kanechlor 400
with the dietary levels of 100 and 1000 mg/kg, 2/16 and 3/10 of the
animals had nodular hyperplasia in the liver, respectively.
In a preliminary study on Sherman rats (10 animals of each sex/dose),
Aroclor 1254 at dietary levels of 0, 20, 100, or 500 mg/kg, and
Aroclor 1260 at levels of 0, 20, 100, 500, or 1000 mg/kg, for 8
months, did not give neoplastic nodules or hepatocellular carcinoma.
At 500 mg/kg, Aroclor 1254 produced adenofibrosis in 10/10 male
animals and at 100 and 500 mg/kg, in 7/10 and 9/10 females,
respectively. This change was only seen in 2/10 male and 4/10 female
animals with Aroclor 1260. The authors stated that hepatocellular
adenofibrosis is a persistent progressive lesion that consists of a
marked proliferation of fibrous tissue and epithelial glandular cells
that are well differentiated in mice, but appear atypical in rats
(Kimbrough et al., 1972).
A group of 200 female Sherman rats was given Aroclor 1260 at an
average dietary level of 100 mg/kg (range, 70-107 mg/kg), for 21
months. The PCB intake declined from 11.6 mg/kg per day during the
first week to 6.1 mg/kg at 3 months and to 4.3 mg/kg body weight per
day, later on. The control group also comprised 200 rats. The survival
rate and the food intake were not affected and no treatment-related
signs of toxicity were observed. Body weight gain was decreased from 3
months after the onset of the exposures. Hepatocellular carcinomas
were present in the liver of 26/184 (14%) exposed rats and 1/173
(0.58%) control rats. The livers of most of the remaining exposed rats
(144/184) showed hyperplastic nodules, while none were found in
control rats. A total of 182 exposed rats and 28 control rats had
livers with foci or areas of cytoplasmic alteration. A few livers of
exposed rats showed adenofibrosis. No induction of tumours in other
organs and no metastases from the liver tumours were found (Kimbrough
et al., 1975).
Calandra (1976) reported the findings from several long-term studies,
performed by a commercial laboratory for Monsanto. (These studies have
never been published). In these studies, 1000 rats were divided into
10 groups of 100 animals (50 of each sex) and 9 of the groups were
exposed to Aroclors 1242, 1254, or 1260 at dietary levels of 1, 10, or
100 mg/kg diet. Apparently, 5 animals of each sex were sacrificed at
3, 6, and 12 months with about 35 animals killed at the end of the
2-year studies. In the animals sacrificed early, only one nodular
hyperplasia was observed and it was in the group fed 100 mg Aroclor
1260/kg for 12 months. Mortality in these studies was high and
approximately one-third of the 105 animals anticipated to be exposed
for 2 years at each dietary level died. Hepatomas were observed in
7/25 livers from animals fed 100 mg Aroclor 1260/kg, in 4/26 fed
Aroclor 1254, 3/19 fed Aroclor 1242, and only in 1/168 animals
receiving the 1-10 mg/kg diets. Nodular hyperplasia was twice as
prevalent as hepatomas in the high-dose animals, particularly in the
Aroclor 1254 group (Harbison et al., 1987).
In a limited study, groups of 24 Fischer 344 rats of each sex received
a diet containing Aroclor 1254 at 0, 25, 50, or 100 mg/kg diet
(equivalent to 0, 1.2, 2.5, and 5 mg/kg body weight, respectively) for
104-105 weeks. The survival rate decreased with a dose-related trend
in male, but not in female rats (92, 83, 58, and 46%, respectively).
From 10 weeks of exposure onwards, the body weight gain of all rats,
except that of the low-dose males, decreased. In the groups receiving
the 2 highest dose levels, alopecia, facial oedema, exophthalmos, and
cyanosis were observed. Foci of hepatocellular alterations, which were
dose-related, were found at all dose levels, but not in the controls.
The incidence of non-neoplastic hyperplastic nodules in male rats with
25, 50, or 100 mg/kg diet was 5/24, 8/24, and 12/24 and in female rats
6/24, 9/22, and 17/24, respectively (US EPA, 1980). None was found in
the controls. Hepatocellular adenoma and carcinomas were found in 1/24
males and 1/24 females on the 50 mg/kg diet and in 3/24 males and in
2/24 females on the 100 mg/kg diet. Non-neoplastic liver lesions
included degenerative changes and aggregates of macrophages with
crystalline cytoplasmic structures and pigment granules. An apparently
dose-related increase in the incidence of intestinal metaplasia was
observed in both sexes and 0, 1, 3, and 2 adenocarcinomas located in
the pyloric region of the glandular stomach were found at 0, 25, 50,
and 100 mg/kg diet, respectively. Morgan et al. (1981) and Ward (1985)
reexamined the NCI (1978) data with respect to gastric
adenocarcinomas, hepatocellular adenomas, and carcinomas. Morgan et
al. (1981) found incidences of focal stomach lesions, mostly
metaplasia, of 6, 10, 17, and 35% in rats receiving 0, 25, 50, or
100 mg Aroclor 1254/kg diet, respectively. Adenomas were found in 6
treated rats. When compared with the incidences of stomach
adenocarcinomas in historical controls (1/3548), the incidence of
6/144 was statistically significantly increased (NCI, 1978; Morgan et
al., 1981; Ward, 1985).
Groups of 70 Sprague-Dawley rats of each sex received a diet
containing Aroclor 1260 in corn oil at a concentration of 100 mg/kg
diet (equivalent to 5 mg/kg body weight) for 16 months and 50 mg/kg
diet (equivalent to 2.5 mg/kg body weight) for an additional 8 months.
All surviving rats received a basal diet from month 25 to month 29.
The control group comprising 63 rats of each sex, received the basal
diet with corn oil for 18 months and the basal diet alone for an
additional 5 months. All surviving rats received the basal diet from
the 25th month to the 29th month. Data on growth were not available.
Groups of 2 control or 3 PCB-treated rats of each sex were partially
hepatectomized at 1, 3, 6, 9, 12, 15, and 18 months. At 24 months, a
similar group was sacrificed; after 29 months all remaining animals
were sacrificed. The mortality rate was not affected by the exposure.
In the livers of exposed rats, centrolobular hypertrophy was apparent
at 1 month, foci at 3 months, and areas of cellular alteration after 6
months, neoplastic nodules after 12 months, trabecular carcinomas
after 15 months, and adenocarcinomas after 24 months. Metastases in
the lung were not found. In exposed rats that survived 18 months or
longer, hepatocellular carcinomas were present in 43/47 females and in
2/46 males, but were absent in 81 controls. Simple and cystic
cholangioma at 18 and 23 months, respectively, and adenofibrosis at 22
months were present in the treated rats (Norback & Weltman, 1985).
Rao & Banerji (1988b) fed 3 groups of 32 weanling Wistar rats a
protein diet containing 0 (coconut oil), 50, or 100 mg Aroclor 1260/kg
diet, for 120 days. The incidence of neoplastic nodules in the liver
was 0/32, 24/32 and 16/32, respectively. Adenofibrosis was also
observed in the treated animals.
In order to exclude possible effects of dibenzofurans and to
investigate the effect of the degree of biphenyl-chlorination, groups
of 152 and 144 male Wistar rats were exposed to Clophen A30 and
Clophen A60, respectively, not containing detectable quantities
(detection limit not stated) of dibenzofurans, for 800 days at a dose
of 100 mg/kg diet. A group of 139 rats received a control diet. After
800 days, randomly selected rats were killed daily, until all
survivors had been examined by day 832. The survival rate of the
remaining exposed rats was increased by day 800. In rats autopsied by
day 800 and in rats autopsied later, the incidence of hepatocellular
carcinoma was increased by exposure to Clophen A60 (9/129 and 52/85,
respectively) relative to controls (0/131 and 1/53, respectively), but
not by exposure to Clophen A30 (1/138 and 3/87, respectively). There
was a marked trend from foci of hepatocellular alteration to
neoplastic nodule to carcinoma with increasing time and degree of
biphenyl chlorination. Controls mainly showed foci. Non-neoplastic
liver lesions with increased incidences of bile duct hyperplasia were
found in rats receiving Clophen A30 and A60, and cysts in rats
receiving Clophen A60. The incidence of adenofibrosis in the liver was
decreased, compared with that in the controls, in all exposed rats
that were killed after exposure (Schaeffer et al., 1984).
8.5.2 Tumour promotion/anticarcinogenic effects
(a) Mice
Tatematsu et al. (1979) studied the effects of inducers of liver
microsomal enzymes on the induction of hyperplastic liver nodules by
N-2-fluorenylacetamide (2-FAA) in male F344 rats. The rats were fed
a diet containing 200 mg 2-FAA/kg diet for 2 weeks and then given 500
or 1000 mg Kanechlor 500/kg diet for the following 8 weeks. Partial
hepatectomies were performed at the end of the third week of the
study. Kanechlor 500 in the dose levels applied showed a promoting
effect.
Ito et al. (1973) and Nagasaki et al. (1974, 1975) exposed groups of
20-38 male dd mice to diets containing Kanechlor 400 or 500 at 100 or
250 mg/kg for 24 weeks. Control groups consisted of 20 mice. Combined
exposure to Kanechlor 500 at 100 and 250 mg and either 50, 100, or
250 mg alpha- or ß-BHC (hexachlorocyclohexane)/kg diet enhanced the
development of nodular hyperplasia and hepatocellular carcinomas. A
combination of Kanechlor 500 and gamma-hexachlorocyclohexane did not
produce tumours. Dosing with Kanechlor 500 alone at a dietary level of
100 and 250 mg/kg, and ß- or gamma-hexachlorocyclohexane at dietary
levels of up to 500 mg/kg did not produce tumours. However,
alpha-hexachlorocyclohexane (250 mg/kg diet) produced 10/38
hepatocellular carcinomas and 30/38 hyperplastic nodules.
In another study, both inhibition and promotion were observed
following transplacental and transmammary exposure of mice to PCBs
prior to, or simultaneously with, exposure to dimethylnitrosamine
(DMNA). In this study, Aroclor 1254 was administered intraperitoneally
to female Swiss CD-1 mice on the 19th day of gestation, at a dose of
500 mg/kg body weight. Groups of 17-31 sucklings of these mice and of
controls were then treated intraperitoneally with DMNA on postnatal
day 4 or 14, or remained untreated. The progeny were killed at 28
weeks or at 18 months of age. Aroclor 1254 exposure decreased the
incidence of tumours in the liver and lung, induced by DMNA
administered at postnatal day 14. The average numbers of lung tumours
per mouse were also decreased. However, Aroclor 1254 increased the
liver tumour-bearing mice with extensive DMNA-initiated liver tumours
at 18 months of age, especially when DMNA was administered on
postnatal day 4. Mice exposed only to Aroclor 1254 did not show tumour
incidences higher than those of controls (Anderson et al., 1983). The
authors also reported that 2 higher chlorinated biphenyls,
2,4,5,2',4',5'-hexachlorobiphenyl and 2,3,5,2',3',5'-hexa-
chlorobiphenyl, were the dominant congeners persisting in the tissues
of the PCB-treated mice. It should be noted that only the more highly
chlorinated PCBs (>50% by weight) were reported as promoters of
hepatocarcinogenesis in rodents. The promoting activities of the lower
chlorinated PCBs have not been determined so far.
(b) Rat
As shown in Table 49, administration of PCBs to rats after exposure to
several initiating agents promoted the development of neoplastic
lesions of the liver. In these studies, treatment of the rats with a
control diet of PCBs alone did not usually lead to neoplastic changes
in the liver. Preston et al. (1981) compared the promoting effect of
exposure of rats to Aroclor 1254 with that of exposure to Aroclor 1254
from which the polychlorinated dibenzofuran moieties had been removed,
and did not find any significant differences. The results of this
study suggested that the promoting effect of commercial PCBs cannot be
ascribed entirely to the presence of chlorinated dibenzofurans.
Tatematsu et al. (1979) showed a dose-effect relationship in the
observed promoting effect of Kanechlor 500.
Inhibition, rather than promotion, of liver neoplasms was observed
when female Donryu rats were exposed to Kanechlor 400 preceding, or
simultaneously with, exposure to 3'-methyl-4-dimethylaminoazobenzene
(3'-Me-DAB) (Kimura et al., 1976). Simultaneous dietary exposure of
groups of 16-24 male Sprague-Dawley rats to Kanechlor 500, at a
dose-level of 500 mg/kg (25 mg/kg body weight per day), and to
3'-Me-DAB, or N-2-fluorenylacetamide (2-FAA), or diethylnitrosamine
(DENA), or combinations of these carcinogens, for 20 weeks, showed
almost complete inhibition of neoplastic nodules and hepatocarcinomas
(Makiura et al., 1974). It should be noted that the results of this
study may be influenced by the extreme toxicity, as shown by the
weight records, especially when the substances were combined.
The antitumour activity of Aroclor 1254 was demonstrated in male
Sprague-Dawley rats, inoculated with Walker 256 tumour cells. Groups
of 16 rats received PCB doses of 50, 100, or 200 mg/kg body weight for
2 weeks, once in 2 days, starting on the day of tumour cell injection.
A group of 16 rats did not receive PCBs. The inhibition of tumour
growth and transplantability were dose-related (Kerkvliet & Kimeldorf,
1977). The antitumour activity of Phenoclor DP5 was observed in groups
of 20 female Swiss mice inoculated with Ehrlich's tumoral ascites
liquid, after receiving PCBs in the diet at levels of 0, 10, 50, or
250 mg/kg, for 120 days (Keck, 1982).
Nishizumi (1980) showed that placentally transferred PCBs inhibited
diethylnitrosamine (DENA)-induced liver tumours. Groups of ten,
10-week-old female Wistar rats were treated with 40 or 200 mg
Kanechlor 500/kg body weight, by gavage, on days 5, 10, and 15 of
gestation. One F1 offspring from each litter was killed for
quantification of liver PCBs. The remaining F1 offspring were exposed
to 50 mg DENA/litre drinking-water, continuously for 5 weeks. At 16,
20, and 24 weeks after the beginning of the DENA exposure, 6-8 rats of
Table 49. Promotion of liver neoplasms in rats exposed to PCBs after treatment with carcinogenic substances
Strain Sex Group size Carcinogenic PCBs Doseb Exposure Neoplasms Reference
substancesa (mg/kg diet) period promoted by
(weeks) PCBs
Donryu female 25 3'-Me-DAB Kanechlor 400 400 26 carcinoma Kimura et al.
(1976)
Wistar male 20-24 DENA Kanechlor 500 2 × 15 4 nodules Nishizumi (1976)
mg/week carcinoma
F 344 male 15-16 2-FAA Kanechlor 500 500 and 8 hyperplasia Tatematsu et al.
1000 nodules (1979)
F 344 male 20 EHEN Kanechlor 500 32 carcinoma Hirose et al.
(1981)
Sprague- male 40 DENA Aroclor 1254c 100 18 carcinoma Preston et al.
Dawley (1981)
a 3'-Me-DAB = 3'-methyl-4-dimethylaminoazobenzene; DENA = diethylnitrosamine; 2-FAA = N-2-fluorenylacetamide;
EHEN = N-ethyl-N-hydroxyethylnitrosamine.
b Unless otherwise specified.
c With and without PCDFs.
each sex from each treatment group were killed and examined
histologically. A significant reduction in tumour incidence occurred
only in male offspring in the 200-mg group. The liver-PCB values in
28-day-old F1 mice were < 1, 18 ± 7, and 360 ± 30 mg/kg tissue for
the controls, 40, and 200 mg/kg groups, respectively. It was suggested
that placental transfer of PCBs protected the treated rats from
DENA-induced liver tumours.
The inhibitory effect of PCBs on tumour initiators following
simultaneous exposure has been explained by an enhanced metabolism of
the initiator by PCB-induced mixed function oxygenase.
Tests for putatively preneoplastic enzyme-altered foci in the liver of
rats have shown a dose-related increase in the promotion of such foci
by intraperitoneal exposure to Aroclor 1254 in tricaprylin or by oral
exposure to Clophen A50 in olive oil, following oral administration of
diethylnitrosamine (Deml & Oesterle, 1982; Pereira et al., 1982;
Oesterle & Deml, 1983).
The highest oral dose of Clophen A50 not enhancing the number and area
of enzyme-altered loci in female Sprague-Dawley rats, treated for 11
weeks with doses of 0, 0.1, 0.5, 1, 5, or 10 mg/kg body weight after
initiation by a single dose of 8 mg/kg body weight of
diethylnitrosamine, was 0.5 mg/kg body weight (Deml & Oesterle, 1987).
8.5.3 Initiation, promotion, and other special studies on individual
congeners
2,5,2',5'-Tetrachlorobiphenyl and its metabolite 2,5,2',5'-
tetrachlorobiphenyl-3,4-oxide were tested in a pulmonary tumour
induction assay with intraperitoneally injected A/T mice of both
sexes, and in a two-stage skin carcinogenicity assay with
dermally-exposed female SENCAR mice. Pulmonary adenomas and skin
papillomas were not induced (Preston et al., 1985).
Female Harlan-Sprague-Dawley rats received 2,4,2',4'-
tetrachlorobiphenyl or 2,5,2',5'-tetrachlorobiphenyl at 100 mg/kg
diet for 28 weeks, 1 week after oral exposure to diethylnitrosamine
(DENA). Both congeners showed a promoting effect on the development of
foci of hepatocellular alteration. The effect was approximately
10-fold greater in rats receiving 2,4,2',4'-tetrachlorobiphenyl
(Preston et al., 1985).
The effects of PCB mixtures and selected congeners have also been
investigated by Hayes et al. (1985, 1986) using the resistant
hepatocyte model developed by Farber and coworkers (Solt & Farber,
1976; Tsuda et al., 1980; Farber, 1984a,b, 1986). The ability of
2,4,2',4' and 2,5,2',5'-tetrachlorobiphenyl, 2,4,5,2',4',5'-
hexachlorobiphenyl, and a mixture of PCB-congeners (the composition of
which resembled that ascertained in human breast milk) to initiate
enzyme-altered hepatocellular nodules was investigated in
proliferating hepatocytes of neonatal or partially hepatectomized
adult rats (the PCB-congeners did not contain detectable levels of
dibenzofurans or dioxins). Neonatal rats were exposed 3 times in 3
weeks and adult rats once. After several weeks, the rats received a
selection regimen of 2-acetylaminofluorene followed by partial
hepatectomy (neonates) or necrotizing carbon tetrachloride (adults).
None of the PCB exposures generated nodules in contrast to known
initiators (Hayes et al., 1985).
Subsequent studies by Hayes et al. (1986) using the afore-mentioned
compounds and 3,4,3',4'-tetrachlorobiphenyl (a typical MC-type inducer
of cytochrome P-450-dependent monooxygenases) showed that these PCBs
(50 µmol/kg body weight), given 10 days after a dose of the initiator,
DENA, and 7 days before 2-AAF, all reduced the size of the
2-AAF-selected gamma-glutamyltranspeptidase-positive nodules. These
results show that, in contrast to the previous studies, PCBs also
exhibit "anti-promoting" activities in this Farber-model, which
utilizes 2-AAF as a mito-inhibitory toxin.
8.5.4 Skin carcinogenicity
Aroclor 1254 (100 µg/mouse) administered 18 h prior to the initiator,
7,12-dimethylbenz (a)anthracene (DMBA), significantly decreased the
incidence of papilloma formation in female Charles-River CD-1 mice.
2,4,5,2',4',5'-Hexachlorobiphenyl (625 µg/mouse), a PCB congener that
resembles phenobarbital in its mode of induction of drug-metabolizing
enzymes, did not act as an anticarcinogen, whereas 3,4,3',4'-
tetrachlorobiphenyl was more active than Aroclor 1254 as an
inhibitor. 3,4,3',4'-Tetrachlorobiphenyl resembled methylcholanthrene
in their mode of cytochrome P-450 induction (Parkinson et al., 1983)
and decreased the number of DMBA-initiated papillomas/mouse. Although
the PCB treatment did not modulate the incidence of papillomas caused
by benzo (a)pyrene, it was suggested that the anticarcinogenic
effects of PCBs on mouse skin tumours initiated by DMBA were due to
altered metabolism and DNA binding of the carcinogen by the
PCB-induced skin monooxygenases (DiGiovanni et al., 1979).
Berry et al. (1978, 1979) studied the tumour-promoting activity of
Aroclor 1254 in groups of 30 female CD-1 mice initiated with
0.2 mmol/litre (equivalent to 51 µg) DMBA. One week later, a positive
control group received 2 µg tetradecanoylphorbolacetate (TPA) and an
experimental group received 100 µg Aroclor 1254 in acetone. The TPA
and Aroclor applications were made twice weekly for 30 weeks. The TPA
promotion resulted in 92% of the animals developing papillomas, while
none developed in the Aroclor-treated animals. It was concluded that
Aroclor 1254 was not a skin tumour promoter at the dose used in this
study. Inhibition of skin papilloma was observed when Aroclor 1254 was
administered 18 to 72 h prior to DMBA treatment and promotion by TPA.
Poland et al. (1982) used HRS/J hairless mice to study the tumour
promotion activity of Aroclor 1254 in combination with N-methyl-
N'-nitro- N-nitrosoguanidine (MNNG). Twenty female mice per group
received a single administration of MNNG (5 micromol in acetone) or
acetone alone applied on the skin, and were then treated topically,
twice weekly, with 1 mg Aroclor/mouse, dissolved in acetone, for 20
weeks. No tumours were induced in the control group; in the
MNNG-treated mice, 4/19 had papillomas. It was concluded that Aroclor
had a weak promoting effect.
8.5.5 Appraisal
In summarizing the potential carcinogenic activity of PCBs, it is
perhaps more informative to express it in terms of what is known about
the mechanisms of chemical carcinogenicity (Hayes, 1987). In other
words, what the evidence is to support the carcinogenicity of PCBs
through genotoxic/initiating, cocarcinogenic, promotional/
antipromotional, and progressional activities. There is no evidence to
support genotoxic activity for PCBs and, in in vitro studies,
evidence for initiating activity through direct interactions with DNA
is weak. Poor initiating activity is consistent with the demonstrated
lack of mutagenic activity in various short-term tests. There is
evidence to suggest that PCBs can potentiate the activity of known
carcinogens (or act as cocarcinogens) in in vitro systems, but the
opposite result is often seen in in vivo studies, suggesting that
certain protecting enzyme systems present in the intact systems are
absent in the in vitro systems. There is a substantial body of
evidence to support the promotional activity of PCBs, particularly the
more highly chlorinated ones, in rodent liver, and this activity may
depend on the sequence in which the chemicals are administered in
experimental animal studies. In addition, promotional activity is
correlated closely, but not consistently, with the induction of MFO
activity. The hyperplastic effect (stimulation of cell proliferation)
of PCB inducers could promote preneoplastic growth. This type of
activity may possibly involve a threshold, suggesting that it may not
be a factor in low-level exposures to PCBs. The anticarcinogenic
activity of PCBs may also depend on the sequence of events in
experimental animal studies, and it may be related more to the
antipromotional properties of PCBs, possibly functioning as
protectants of mito-inhibitory toxicity. The interpretation of the
available animal data involving the commercial PCB mixtures is often
complicated by lack of information concerning the presence and
contribution of chlorinated dibenzofuran impurities, as well as
variations in congener composition to toxicity. In structure-activity
terms, a key factor in determining promotional activity appears to be
the degree of chlorination, which may reflect increased resistance to
metabolism and elimination and possibly also higher degrees of
ortho-substitution among the congeners present. Ortho-substituted
PCBs (possibly acting as persistent PB type inducers) have been shown
to be effective tumour promoters, and, at least in the case of the
closely related PBBs, it has been shown that a non-toxic and
non-promoting dose of a non- ortho-substituted congener in
combination with a promoting dose of a highly ortho-substituted
congener has a synergistic effect. This result may explain why the
mixtures can have greater promoting ability than the individual
congeners involved. This result might also suggest multiple pathways
for promoting activity, possibly involving the Ah receptor as well as
the putative receptor for phenobarbital. The possibility that PCBs
might promote carcinogenesis in tissues, other than liver, in animals
exposed to various tissue-specific, initiating agents, needs to be
addressed. Nevertheless, the potential for human liver cancer from
exposure to PCBs cannot be reliably predicted from animal studies.
Overall, there is reason to exercise caution in extrapolating the
available animal data on the carcinogenic potential of PCBs for
humans.
8.6 Special studies: target-organ effects
The lesions commonly introduced in animals after acute, short-term, or
long-term administration/application of PCB mixtures and/or individual
congeners concern the liver, skin, immune system, reproductive system,
oedema at various sites, as well as disturbances of the
gastrointestinal tract and the thyroid gland.
8.6.1 Liver
8.6.1.1 PCB mixtures
The toxic effects of PCBs result both directly and indirectly from
their presence in certain organs, such as the liver, where they
induce, in various degrees, a variety of liver enzymes. Some of these
enzymes are active in the metabolism of the PCBs themselves, while
others involve activation, deactivation, detoxication, etc., of other
compounds.
In itself, induction of enzymes by xenobiotics does not represent a
toxic manifestation, rather it is the ordinary response to such
foreign chemicals, which results generally in their detoxication and
ultimate modification, enabling them to be excreted from the organism.
In this sense, the response of the liver to such compounds is a
biological protective mechanism. Since some compounds, including PCBs,
are capable of inducing not only enzymes that result in their own
detoxication, but others as well, and the level of enzyme induction
may be high enough to cause liver pathology, a line cannot be drawn
that clearly separates a normal biological function from a toxic
manifestation. Superimposed on this situation is the direct toxic
action of the compounds on liver tissue, because of the properties of
the parent compound or its metabolites.
An enlarged liver and increased absolute and relative liver weights
are commonly reported as gross effects of PCB administration. The
lowest-observed-effect levels, in studies on different rat strains,
exposed to a diet containing Aroclor 1254, for a (dose-related)
increase in relative liver weights, vary between 20 and 100 mg/kg diet
(equivalent to 1 and 5 mg/kg body weight, respectively) (Kimbrough et
al., 1972; Bruckner et al., 1974; Burse et al., 1974; Grant et al.,
1974; Allen et al., 1976; Zinkl, 1977; Hinton et al., 1978; Kasza et
al., 1978b; Jonsson et al., 1981; Baumann et al., 1983).
The liver hypertrophy is microscopically visible as enlarged,
pleiomorphic hepatocytes, sometimes multinucleated or with enlarged
nuclei. A liver alteration observed by many investigators after
exposure of rats to various PCB-mixtures is fatty degeneration,
characterized by fat vacuolation and/or a foamy appearance of the
cytoplasm. Ultrastructurally, an increase in the number and size of
cytoplasmic lipid droplets and liposomes (membrane-associated lipid
droplets) can be observed. Fatty degeneration of the liver was already
observed after exposure of male Sprague-Dawley rats to a diet
containing 5 mg Aroclor 1242/kg diet (equivalent to 0.25 mg/kg body
weight) for 2-6 months (Bruckner et al., 1974) and after exposure of
male Holtzman rats to a diet containing 5 mg Aroclor 1254/kg for 5
weeks (Kasza et al., 1978b). Chu et al. (1977) found a comparable
effect on the liver with >20 mg Aroclor 1254 or 1260/kg diet for 28
days. Another characteristic change is the appearance of eosinophilic,
lamellar, cytoplasmic inclusions (Kimbrough et al., 1972; Kasza et
al., 1978b) or "hyaline-like material" (Grant et al., 1974). Electron
microscopy revealed that these changes corresponded to concentric
laminated membranes of smooth endoplasmic reticulum ("whorls"; "myelin
figures") (Vos & Beems, 1971; Kimbrough et al., 1972; Allen et al.,
1976; Kasza et al., 1978b; Jonsson et al., 1981). Proliferation of the
smooth endoplasmic reticulum and a decrease in rough endoplasmic
reticulum are commonly observed in rats exposed to dietary levels of
PCB mixtures that also induce fatty degeneration. Kasza et al. (1978b)
further observed a marked proliferation of Golgi condensing vesicles
containing lipoprotein in the livers of male Holtzman rats fed Aroclor
1254, for 5 weeks, at 5 mg/kg diet. A decreased number of these
vesicles was seen at 50 and 500 mg/kg diet (equivalent to 2.5 and
25 mg/kg body weight, respectively), together with a marked increase
in the smooth endoplasmic reticulum and lysosomes. The above decrease
in Golgi vesicles was also observed in Sprague-Dawley rats by Hinton
et al. (1978). The proliferative changes of the endoplasmic reticulum
are closely related to the observed induction of microsomal enzymes,
as discussed in section 8.6.1.2. Atypical mitochondria (Burse et al.,
1974; Kasza et al., 1978a,b), and single cell and focal necrosis
(Grant et al., 1974; Allen et al., 1976; Jonsson et al., 1981; Baumann
et al., 1983) have also been described.
Hypobilirubinaemia was produced in rats by Bastomsky et al. (1975),
who investigated the mechanism by administering daily intraperitoneal
injections of Aroclor 1254 (25 mg/kg body weight, in corn oil) to
female rats for 4 days, before measuring bilirubin glucuronide
formation by hepatic microsomes in vitro. PCB treatment was not
effective in increasing UDP-glucuronosyltransferase (EC 2.4.1.7)
activity. Serum bilirubin levels in Gunn rats were also significantly
decreased by PCB treatment; the rats are genetically deficient in
UDP-glucuronosyltransferase (2.4.1.7) activity.
The fluorescing of livers on exposure to UV radiation, consistent with
the presence of porphyrin, and accumulation of brown pigment, positive
for iron, especially in Kupffer cells and perivascular macrophages
have also been reported (Kimbrough et al., 1972; Burse et al., 1974;
Zinkl, 1977; Jonsson et al., 1981). The changes described in the
livers of mice (Nishizumi, 1970) and rabbits (Koller & Zinkl, 1973),
following exposure to PCB mixtures, are comparable with those in rats.
8.6.1.2 Individual congeners
The effects of chlorination and the chemical composition of PCBs, with
regard to the dose-effects relations in liver toxicity after
short-term exposure, are indicated by the data of Biocca et al.
(1981). In this study, hepatotoxic effects were observed in mice after
5 weeks of maintenance on diets containing 0.3 mg of 3,4,5,3',4',5'-
hexachlorobiphenyl, while similar effects were observed only after
30 mg of 2,4,5,2',4',5'-hexachlorobiphenyl and 100 mg of 2,4,6,2',4',6'-
hexachlorobiphenyl/kg diet. No effects were found with 300 mg of
2,3,6,2',3',6'-hexachlorobiphenyl/kg diet. Similar dependence of liver
toxicity on the chemical composition of the PCB mixture would be
anticipated following long-term exposure in mice and other species.
8.6.2 Enzyme induction
8.6.2.1 Effects on liver enzymes of PCBs
Proliferation of the smooth endoplasmic reticulum is a common
observation in the liver cells of experimental animals following
exposure to PCB mixtures. This effect is accompanied by an increase in
microsomal protein and the induction of cytochrome P-450, cytochrome
P-448, and drug-metabolizing enzymes, including the microsomal
monooxygenases (EC 1.14.14.1), epoxide hydrolases (EC 3.3.2.3),
UDP-glucuronosyltransferases (EC 2.4.1.17), NADPH-cytochrome c
reductase (EC 1.6.2.4) and esterases (EC 3.1.1.1), and the cytosolic
glutathione S-transferase (EC 2.5.1.18). The subject has been
reviewed by Safe (1984).
The spectral, enzymatic, and electrophoretic properties of the
microsomal enzymes, induced by Aroclor 1248, 1254, 1260, and Kanechlor
400, are consistent with the inducing properties of both the
phenobarbital (PB) and 3-methylcholanthrene (MC) classes of inducers.
They induce both cytochromes P-450 and P-448 and associated enzymes
(Alvares & Kappas, 1977; Goldstein et al., 1977; Yoshimura et al.,
1978; Iverson et al., 1982; Lashneva & Tutelyan, 1984; Khan et al.,
1985; Tutelyan et al., 1986). Aroclor 1016, administered to
Sprague-Dawley rats at 50 mg/kg per day for 4 days, intraperitoneally,
elicited a barbiturate type of inducing effect on the hepatic
microsomal oxidative enzyme system. Aroclor 1016 caused increases in
liver cytochrome P-450 content, microsomal protein, and its
ethylmorphine N-demethylase activity. It did not induce cytochrome
P-448 in liver microsomes.
A dose-related induction of hepatic and, in some cases, extrahepatic
microsomal enzymes was observed in several animal species including
the rat, rabbit, mouse, ferret, guinea-pig, hamster (Safe, 1984), mink
(Shull et al., 1982; Aulerich et al., 1985) and monkey (Iverson et
al., 1982). Distinct interspecies variations have been demonstrated.
For example, while 6 daily intraperitoneal doses of 25 mg of Aroclor
1254/kg body weight caused a potent induction of benzo (a)pyrene
hydroxylase in adult, male Sprague-Dawley rats, no, or a minimal,
induction of this monooxygenase was observed in adult, male Swiss mice
after 4 daily intraperitoneal doses of 50 mg/kg body weight and in
male New Zealand rabbits after 2 intraperitoneal doses of 100 mg/kg
body weight on days 1 and 4 (Alvares et al., 1982). Furthermore, when
comparing the inducing potency of Aroclor 1242 in the mink and the
genetically related ferret, Shull et al. (1982) measured a greater
induction of cytochrome P-448 and MC-type monooxygenases and no toxic
effects in the ferret at a dosing regime that resulted in toxic
effects in the mink (100 mg/kg body weight on day 1,200 mg/kg body
weight on day 5, sacrifice on day 10). The authors considered the
observed induction moderate in both species compared with that
observed in the rat. Earlier, it had been found that male
Sprague-Dawley rats were indeed more sensitive than ferrets with
respect to the inducing effect of Aroclor 1254 following a single
intraperitoneal dose of 500 mg/kg body weight, though the responses of
both species were comparable qualitatively (Lake et al., 1979).
Moreover, pretreatment of rats with Aroclor 1254 resulted in the
induction of microsomal cytochromes P-450 c, P-450 d (MC-inducible),
P-450 b and P-450 e (PB-inducible) (Ryan et al., 1979a,b, 1982). In
general, the extent of induction of microsomal enzymes by PCB-mixtures
increased with increasing chlorine content up to 54%. The effect has
also been demonstrated with single pure PCBs administered orally
(Ecobichon & Comeau, 1975). The results are summarized in Table 50 and
show a greater degree of enzyme induction with the higher chlorinated
compounds (see section 8.6.1.2).
Table 50. Stimulation of microsomal enzyme activity by single chlorinated biphenylsa
Hepatic microsomal enzyme activity
Chlorine O-Demethylation N-Demethylation Aniline Nitro-
substituents hydroxylation reduction
4 0 0 0 0
2,2' 0 + + 0
2,4' 0 0 + 0
4,4' + + + + + + +
2,5,2',5' 0 + + + +
2,4,2',4' + + + + + + 0
2,4,5,2',4',5' + + + + + + + +
2,3,5,2',3',5' + + + + + + + +
2,4,6,2',4',6' + + + + + + + +
2,3,4,5,2',3',4',5' + + + + + + + +
a From: Johnstone et al. (1974).
0 = No activity.
+ = Slight activity.
+ + = Marked activity.
Litterst et al. (1972) exposed groups of 6 male Osborn-Mendel rats to
Aroclors 1242, 1248, 1254, or 1260 in the diet, at concentrations of
0, 0.5, 5.0, 50, or 500 mg/kg diet, for 4 weeks. Increased microsomal
nitroreductase and demethylase activities occurred at 0.5 mg/kg or
more, increased pentobarbital hydroxylation and increased relative
liver weight occurred at 5.0 mg/kg or more, and increased liver
triglycerides occurred at 50 mg/kg diet. An inducing activity similar
to, or lower than, that of Aroclor 1254 has often been found for more
chlorinated mixtures (Villeneuve et al., 1971a, 1972; Bickers et al.,
1972; Chen & Dubois, 1973; Ecobichon & Comeau, 1974; Schmoldt et al.,
1974; Sawyer et al., 1984). Aroclor 1016 and 1242, both containing 42%
chlorine but differing in congener composition, showed qualitative and
quantitative differences in inducing effects. For example, Aroclor
1254 enhanced ethylmorphine N-demethylase activity 3-fold, while the
maximum increase produced by Aroclor 1016 was only 40%. The 2 Aroclors
also differed in their induction of the various forms of cytochrome
P-450 (Alvares et al., 1982). Adult, male Sprague-Dawley rats were
administered, intraperitoneally, a dosage of 0 or 50 mg Aroclor 1016
in corn oil/kg body weight, for 4 days. Aroclor 1016 was a potent
inducer of N-methylase but a poor inducer of benzo (a)pyrene
hydroxylase. Administration of 100 mg Aroclor 1016 in corn oil/kg body
weight per day to adult male New Zealand White rabbits, for 4 days,
resulted in an increase in liver cytochrome P-450 activity and
decreases in benzphetamine- N-demethylase and benzo (a)pyrene
hydroxylase activities compared with the controls. 7-Ethoxycoumarine-
O-deethylase and 7-ethoxyresorufin- O-deethylase activities were
comparable with those in the controls (Ueng & Alvares, 1985).
Therefore, it can be concluded that the degree and type of induction
not only depends on the chlorine content of the mixture, but is also a
function of the congener composition, as will be discussed further in
the next section.
Not only species specificity, but also marked tissue specificity has
been observed. While Aroclor 1254 was found to be a potent inducer of
cytochrome P-450 content and benzo (a)pyrene hydroxylase activity in
the rat lung and liver (Alvares & Kappas, 1977), it caused a 46%
decrease in cytochrome P-450 content, a 31% decrease in
benzo (a)pyrene hydroxylase activity, and a 61% decrease in
ethylmorphine N-demethylase activity in the rabbit lung. Aroclor
1254 caused induction of cytochrome P-450 and both enzymes in the
kidneys of these rabbits, but benzo (a)pyrene hydroxylase was not
induced in the liver (Alvares et al., 1982).
The inducing effect of PCB mixtures on the monooxygenase system has
been observed in the livers of both male and female rats (Chen &
Dubois, 1973; Grant & Phillips, 1974), minks and ferrets (Lake et al.,
1979; Shull et al., 1982), in the livers of pregnant rats (Alvares,
1977) and rabbits (Villeneuve et al., 1971a), in the placenta of rats
(Alvares & Kappas, 1975), in fetal and neonatal rat livers (Alvares &
Kappas, 1975; Baker et al., 1977; Inoue et al., 1981; Jannetti &
Anderson, 1981; Lashneva et al., 1987), in immature rat livers (Chen &
Du Bois, 1973; Narbonne, 1980), and in mature and senescent rat livers
(Birnbaum & Baird, 1978).
The lowest-observed-adverse-effect levels and the no-observed-effect
levels for enzyme induction, found in short-term diet studies on rats,
are presented in Table 51.
Bruckner et al. (1977) showed that when Aroclor 1254 was administered
in the diet, at a level equivalent to 0.25 mg/kg body weight,
microsomal enzyme activity was induced after 1 day of exposure.
Narbonne (1980) found a significant induction with 0.1 mg Phenoclor
DP6/kg body weight, given in the diet, after 3-5 days.
After a few weeks of exposure to Aroclor 1254 or 1260, at low dietary
levels of between 0.25 and 1.25 mg/kg body weight, a plateau in
microsomal enzyme activity was reached that was maintained over
several months of exposure (Chen & Du Bois, 1973; Grant et al., 1974;
Bruckner et al., 1977). Following short-term exposure, dietary levels
of between 5 and 25 mg/kg may stimulate microsomal enzymes for up to 4
months (Grant et al., 1974; Bruckner et al., 1977). Levels of 0.05 or
0.1 mg/kg body weight failed to produce any effects or the periods of
induction at these levels were long (Grant et al., 1974; Chen & Du
Bois, 1973).
A marked induction of the liver monooxygenase system was observed in
the offspring of female rats given a single oral dose of a mixture of
PCBs (Sovol) (500 mg/kg body weight) on the 14th day of pregnancy. In
one-day-old rats, an increase in cytochrome P-450 content associated
with an increase in the cytochrome b 5 level, an increase in NADPH-
cytochrome c reductase activity, an increased rate of aminopyrine-
N-demethylation activity in microsomes and also increased
3,4-benzo (a)pyrene hydroxylation, 7-ethoxycoumarin O-deethylation,
and NADPH-dependent lipid peroxidation were found. The activity of the
cytochrome P-450 system in the young rats remained elevated during the
early postnatal period (Lashneva et al., 1987).
8.6.2.2 Effects on liver enzymes of "biologically filtered" PCB
mixtures
Young Sprague-Dawley rats were administered a total of 38 oral doses
of a PCB mixture in olive oil at 0, 0.25, 1.0, 4.0, 16.0, 64.0, 256,
or 1025 µg/kg body weight, twice a day, over 1 month; the mixture
contained 55% chlorine and had a gas-chromatographic profile very
similar to that of the congeners found in the breast milk of Japanese
women. A dose-related induction of liver aminopyrine demethylase and
benzo (a)pyrene hydroxylase (EC 1.14.14.1) was found with doses of
1.0 µg/kg body weight or more. PCB-binding to liver microsomes was
increased at doses of 4.0 µg/kg body weight or more (Shimada & Ugawa,
1978). In another study, 1-month-old, male Wistar rats received
(intraperitoneally) doses of 0, 1, 10, 25, 50, or 100 mg/kg body
weight of a reconstituted PCB-mixture in corn oil, containing average
levels of 13 of the major congeners found in the breast milk of
Japanese women (purity > 98.5%). Each dose was administered in 2
portions on days 1 and 3. The same dose regimen of Kanechlor 500 was
also tested. The rats were killed on day 6. The reconstituted PCB
mixture and Kanechlor 500 caused dose-related increases in liver
Table 51. Microsomal enzyme induction by PCB-mixtures in rats
PCB-mixture Rat strain Sex Exposure No-effect-level Lowest-observed- Reference
male/ period mg/kg body adverse effect level,
female weight mg/kg body weight
Aroclor 1016 Sprague-Dawley male 3 weeksa 0.1 1 Iverson et al. (1975)
Aroclor 1242 Sprague-Dawley male 3 weeksa 0.1 1 Iverson et al. (1975)
Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Sprague-Dawley male 2-6 months < 0.25 0.25 Bruckner et al.
(1974, 1977)
Aroclor 1248 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Aroclor 1254 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Wistar male 2-8 months < 0.1 0.1c Grant et al. (1974)
Wistar male 2 weeks 0.25 0.5 Den Tonkelaar &
Wistar male 12 weeks 0.05 0.5 van Esch (1974)
Sprague-Dawley male 0-20 weeks 0.05 0.25 Turner & Green (1974)
Holtzman male 3 weeks < 0.25 0.25 Bruckner et al. (1977)
Garthoff et al. (1977)
Table 51. (cont'd).
PCB-mixture Rat strain Sex Exposure No-effect-level Lowest-observed- Reference
male/ period mg/kg body adverse effect level,
female weight mg/kg body weight
Aroclor 1260 Osborne-Mendel male 4 weeks < 0.025 0.025 Litterst et al. (1972)
Holtzman male 1-13 weeks < 0.05 0.05 Chen & DuBois (1973)
female 1-13 weeks 0.05 0.25
Phenoclor DP6 Sprague- male 3 days < 0.1 0.1 Narbonne (1979, 1980)
Dawleyb
a Daily dosing by gavage; the other studies are all diet studies.
b Immature rats (60-65 g).
c Significant effect after months 4 and 6, but not after months 2 and 8.
benzo (a)pyrene hydroxylase activity that were 3.5 and 2.2 times the
control value, respectively, at 1 mg/kg body weight. The ED50 of the
reconstituted breast milk PCB mixture for the induction of rat hepatic
microsomal aryl hydroxylase (AHH) was 7 times lower than that of
Kanechlor 500. The authors concluded that the increased potency of the
breast milk-PCB mixture reflected the preferential bioconcentration of
the relatively toxic congeners 2,4,5,3',4'-penta-, 2,3,4,3',4'-penta-,
and 2,3,4,5,3',4'-hexachlorobiphenyl (Parkinson, et al., 1980b). When
Gyorkos et al. (1985) repeated the studies, the reconstituted mixture
was inactive at the lowest dose level but exhibited mixed-type
microsomal enzyme induction characteristics at the higher dose levels.
Increases in the activities of several hepatic microsomal
monooxygenases, including dimethylaminoantipyrine N-demethylase,
aldrin epoxidase, benzo (a)pyrene hydroxylase and
ethoxyresorufin- O-deethylase were found.
8.6.2.3 Effects of individual congeners on liver enzymes
The enzyme-inducing potencies of individual PCB congeners have been
studied extensively and reviews have been published (Goldstein, 1980;
Safe, 1984; Safe et al., 1985b), in which the following
structure-activity relationships are proposed. The most active
congeners with respect to the induction of aryl hydrocarbon
hydroxylase (and toxicity), 3,4,5,4'-tetrachloro-, 3,4,3',4'-
tetrachloro-, 3,4,5,3',4'-pentachloro-, and 3,4,5,3',4',5'-
hexachlorobiphenyl, are substituted at both para positions, at 2 or
more meta positions, but not at ortho positions. These congeners
can assume coplanar conformations and are approximate stereoisomers of
2,3,7,8-tetrachlorodibenzo- para-dioxin. They resemble
3-methylcholanthrene and 2,3,7,8-tetrachlorodibenzo- para-dioxin in
their mode of hepatic enzyme induction, inducing hepatic microsomal
benzo (a)pyrene hydroxylase, ethoxyresorufin- O-deethylase, and the
cytochromes P-450 a, P-450 c, and P-450 d.
These congeners are only present as trace compounds in commercial PCB
mixtures, but appear in significant quantities in breast milk (Noren
et al., 1990).
The least active of these 4 coplanar congeners, 3,4,5,4'-
tetrachlorobiphenyl, also shows a phenobarbital type of hepatic
microsomal enzyme induction, inducing dimethylaminoantipyrine,
ethylmorphine and related N-dealkylases, biphenyl-4-hydroxylase,
aldrin epoxidase, several O-dealkylases, and the cytochromes P-450 a,
P-450 b, and P-450 e. This "mixed-type" induction pattern is also
shown by 3,4,4'-trichlorobiphenyl, and by all the mono- ortho, and at
least 7 di- ortho, substituted analogues of the coplanar PCB
congeners. Several of these congeners, e.g., 2,4,5,3',4'-penta,
2,3,3',4,4'-penta, 2,3,4,5,3',4'-hexa-, and 2,3,4,5,2',4'-
hexachlorobiphenyl are components of commercial PCB mixtures and have
been identified in breast milk.
Studies have revealed that 4,4'-dichlorobiphenyl, with no meta
substituents exhibits a PB-type induction pattern in rats. Adding
meta substituents as in 3,4,4'-tri-, and 3,4,5,4'-tetrachloro-
biphenyl will give a mixed PB- and 3-MC-type induction pattern. While
3,4,3',4'-tetrachlorobiphenyl is a potent inducer of microsomal
hepatic aryl hydrocarbon hydroxylase (AHH), it did not significantly
increase the activities of benzo (a)pyrene-hydroxylase, [3H]-4-
chlorobiphenyl-hydroxylase, and ethoxyresofurin- O-deethylase (EROD)
at a dose level of 10 µmol/kg (Andres et al., 1983).
Most other PCB congeners are phenobarbital-type inducers or are
inactive. In general, the more highly chlorinated of these congeners
are more active inducers than the lower chlorinated ones, probably
reflecting the relative half-lives of these compounds. The
non-availability of 2 adjacent unhalogenated carbon atoms and
para-substitution are 2 factors that decrease the degradability of
the congeners and increase their inducing activity.
Various congeners were tested for their inducing activity in
responsive C57BL/6J mice, i.e., mice containing the Ah receptor
protein, and in non-responsive DBA/2J mice, lacking this receptor,
after single intraperitoneal (Robertson et al., 1984; Silkworth et
al., 1984) or oral (Kohli et al., 1980) doses in corn oil and
cottonseed oil, respectively. The coplanar PCBs and their mono-ortho
substituted analogues all induced benzo (a)pyrene hydroxylase or
ethoxyresorufin deethylase in responsive mice, but not, or only to a
minor degree, in non-responsive mice. Most tested mono-ortho
substituted analogues of coplanar PCBs slightly induced aminopyrine
N-demethylase in both strains, while the coplanar congeners did not
induce this enzyme.
The above structure-activity relationships were confirmed in a few
limited studies on monkeys. Hepatic aryl hydrocarbon hydroxylase was
induced in 3 young, male Rhesus monkeys after a single oral dose of
1 mg of 3,4,3',4'-tetrachlorobiphenyl/kg body weight and in 3 young
male monkeys during continued feeding of a diet containing 0.5 mg of
3,4,5,3',4',5'-hexachlorobiphenyl/kg (McNulty, 1985). A single oral
dose of 18 mg of 2,5,2',5',-tetrachlorobiphenyl/kg body weight,
administered to male Rhesus monkeys, in corn oil, elevated hepatic
cytochrome P-450 levels, while no change was noted in the activities
of several microsomal enzymes (Allen et al., 1975b). Mono- ortho or
di- ortho substituted analogues of coplanar PCB congeners have not
been tested in monkeys.
Vodicnik et al. (1980) studied the effect of 2,4,5,2',4',5'-
hexa-chlorobiphenyl on hepatic microsomal monooxygenase activity in
virgin or pregnant and lactating Sprague-Dawley mice and their
offspring. A single intraperitoneal dose of 100 mg/kg body weight, was
administered 14 days before mating. Prior to, and during early
pregnancy, hepatic monooxygenase activity in pretreated mice was
greater than that in the controls. No differences were found between
pregnant and virgin mice. Mothers, pretreated with the
hexachlorobiphenyl and sacrificed on the day of birth, had lower
microsomal monooxygenase activity and cytochrome P-450 content than
PCB-pretreated virgins sacrificed concurrently. No differences were
noted between these groups of animals during lactation. Hepatic enzyme
activities and cytochrome P-450 content were not different between
newborn offspring of corn oil- and PCB-pretreated mothers. However,
these parameters were elevated in 5- to 20-day postpartum nursing
offspring from pretreated mothers, compared with those from corn
oil-pretreated mothers suggesting the transfer of hexachlorobiphenyl
through the breast milk in quantities sufficient to affect hepatic
microsomal monooxygenase activity.
In a study on ICR mice, Vodicnik (1986) administered 150 mg
14C-2,4,2',4'-tetrachlorobiphenyl intraperitoneally, and compared
hepatic microsomal ethoxycoumarin- O-deethylase activity and liver
concentrations of 14C-activity. It was shown that pregnant mice were
less responsive to the inducing effects of the tetrachlorobiphenyl
than virgin or postpartum mice. This diminution in response may be, in
part, responsible for the lack of elimination of the
tetrachlorobiphenyl equivalents from the late pregnant animal during
the 4-day experimental period (see section 6.4.3).
Hardwick et al. (1985) studied both the time course and dose-response
for the induction of the 2 isoenzymes and their respective mRNAs after
administration of 3,4,5,3',4',5'-hexachlorobiphenyl to rats. It was
concluded that the congener under study induced 2 major 3-MC-inducible
isoenzymes of cytochrome P-450 and their mRNAs in a coordinated
manner, probably via a common mechanism. The data are consistent with
the hypothesis that both genes are probably regulated by a single
receptor in the rat. The magnitude of the increase in the isoenzymes
was greater than the increase in the amount of translationally active
mRNA in polysomes, suggesting that other factors may also influence
the relative induction of these P-450 isoenzymes.
In this study, the BP-type inducers, 2,4,6,2',4',6'- and
2,4,5,2',4',5'-hexachlorobiphenyl, both of which have been reported to
increase the hepatic cytosolic receptor level and consequently enhance
enzyme induction in vivo, were not able to enhance EROD or AHH
induction by 3,4,5,3',4'-pentachlorobiphenyl in vitro.
The mixed-type inducer 2,3,4,2',4',5'-hexachlorobiphenyl inhibited
enzyme induction by 3,4,5,3',4'-pentachlorobiphenyl in vitro, when
used in concentrations of at least 400 times higher than that of the
3,4,5,3',4'-pentachlorobiphenyl. Enzyme induction by 3,4,3',4'-
tetrachlorobiphenyl was inhibited by 2,3,4,2',4',5'-hexachlorobiphenyl
at concentrations at least 40 times higher, and enzyme induction by
3,4,5,3',4',5'-hexachlorobiphenyl was inhibited by 2,3,4,2',4',5'-
hexachlorobiphenyl at concentrations at least 8 times higher. Since
the concentration of 2,3,4,2',4',5'-hexachlorobiphenyl found in human
adipose tissue is about 300 times higher than those of the 3 coplanar
PCBs, this inhibition of enzyme induction probably occurs after
natural exposure to PCB mixtures. If enzyme induction by
3,4,3',4'-tetrachlorobiphenyl and 3,4,5,3',4',5'-hexachlorobiphenyl is
also inhibited by various concentrations of 2,3,4,2',3',4'-
hexachlorobiphenyl, inhibition of enzyme induction after natural
exposure to a mixture of PCBs is to be expected.
It was concluded that the in vitro enzyme induction by mixtures of
PCBs cannot be determined by the simple addition of the induction by
the individual PCBs. Possibly, in vivo enzyme induction seems
additive, because some compounds increase the receptor level, while
other compounds inhibit enzyme induction (van Vliet, 1990).
8.6.2.4 Appraisal
The liver is the organ most often implicated in the toxicity of PCBs
in animals. Hepatotoxicity has been observed in numerous studies with
exposed mice, rats, guinea-pigs, rabbits, dogs, and monkeys. The
effects, which appear to be reversible at low doses, are similar among
the species and include enzyme induction, liver enlargement, fat
deposition, and necrosis. Enzyme induction is the most sensitive
indicator of hepatic effects, but few studies have been designed to
define the minimum effective doses of PCB mixtures. The liver
enlargement is associated with hepatocyte enlargement and an increase
in smooth endoplasmic reticulum and/or increased enzymatic activity.
Proliferative lesions in the liver have been attributed to Aroclor
treatment. The hepatic effects of Aroclors in animals appear to be
typical of chlorinated hydrocarbons. Histologically-documented liver
damage is a consistent finding among PCB-exposed animals.
8.6.3 Effects on vitamins and mineral metabolism
8.6.3.1 Effects of PCB mixtures
PCB mixtures have been found to decrease levels of retinol (Vitamin A)
in the liver of rats (Innami et al., 1976; Kato et al., 1978; Hudecova
et al., 1979), rabbits (Villeneuve et al., 1971a), and in the plasma
of pigs (Guoth et al., 1984). Levels of thiamine (Vitamin B1) were
decreased in the blood, liver, and sciatic nerve of rats (Yagi et al.,
1979) and levels of pyridoxal phosphate (Vitamin B6) were decreased in
several tissues of rats, while riboflavin (Vitamin B2) levels remained
unaffected (Fujiwara & Kuriyama, 1977). The changes in the levels of
retinol and thiamine were thought to be secondary to the induction of
metabolizing enzymes (Yagi et al., 1979; Saito et al., 1982). The
induction of these enzymes was also found to be responsible for the
increased de novo synthesis of L-ascorbic acid that was observed in
the plasma, tissues, and urine of PCB-treated rats (Fujiwara &
Kuriyama, 1977; Chakraborty et al., 1978; Chow et al., 1979; Saito et
al., 1983). Lipid peroxidation was increased in the liver of
PCB-treated rats and Saito et al. (1983) suggested that ascorbic acid
may have initiated the peroxidation.
It was shown that induction of NADP-cytochrome c reductase (EC
1.6.2.4) and insufficiency of lipid peroxide scavengers, such as
alpha-tocopherol (Vitamin E) and glutathione peroxidase (EC 1.11.1.9),
could also be involved in the enhancement of lipid peroxidation by
PCBs (Saito et al., 1982, 1983; Kamohara et al., 1984).
PCB mixtures decreased the activity of both sodium/potassium- and
magnesium-dependent adenosinetriphosphatase (EC 3.6.1.3) in the
tissues of rats (Narbonne et al., 1978; La Rocca & Carlson, 1979). The
results of in vitro studies on isolated rat mitochondria showed that
PCB mixtures may act as inhibitors of respiration and uncouplers of
oxidative phosphorylation (Sivalingan et al., 1973; Nishihara, 1983,
1985). However, contradictory results have been obtained in vivo
with respect to the NAD/NADH ratio, the ADP/O ratio, and state 3 and
state 4 respiration rates (Mehlman et al., 1974; Chesney & Allen,
1974; Garthoff et al., 1977).
Byrne & Sepkovic (1987) studied the in vitro incorporation of
monovalent cations into rat erythrocytes as a model for evaluating the
impairment of electrogenic transport by PCBs. Female, Sprague-Dawley
rats were fed 50 mg Aroclor 1242 or 1254/kg diet for 7 months. The
uptake of 86Rb by erythrocytes in the Aroclor 1254 group was
depressed compared with that in the control group in K+ - depleted
culture media. No changes were observed with Aroclor 1242. A reduction
in 86Rb incorporation was also seen in erythrocytes from the Aroclor
1254 group in a Na+ -depleted medium. Ouabain did not have any
effect in the Aroclor 1254 group, because Aroclor 1254 suppressed the
cationic transport maximally. This study provides evidence that PCBs
(Aroclor 1254) can damage the cell sufficiently to decrease the active
transport of monovalent cations.
Male Fischer 344 rats were dosed daily, intragastrically, for 5, 10,
or 15 weeks with 0, 0.1, 1, 10, or 25 mg Aroclor 1254/kg body weight
in corn oil, to investigate the effects on calcium metabolism, femur
morphometry, and nephrotoxicity. The relative liver weights were
increased significantly with doses of 1.0 mg/kg or more after 5 weeks
treatment. The relative kidney weights were increased after 15 weeks
treatment in the 10 and 25 mg/kg groups. Hypercalcaemia was present in
the 25 mg/kg group after 5 and 10 weeks treatment, but not after 15
weeks. Serum triglyceride levels were elevated after 5 weeks
treatment, but decreased after 10 and 15 weeks. Serum cholesterol
levels were increased at the 2 higher dose levels with all 3 lengths
of treatment. Urinary alkaline phosphatase and lactate dehydrogenase
activities were elevated at 5, 10, and 15 weeks of treatment. Femur
density was increased at the 10 mg/kg dose level after 5 weeks, and at
all dose levels after 10 and 15 weeks. Cross-sectional, medullary, and
cortical areas of the midpoint of the femur were significantly
decreased at the higher dose levels after 10 and 15 weeks of exposure.
The per cent medullary area was decreased after 10 and 15 weeks
treatment indicating a decrease in medullary size and also a decrease
relative to the cortical bone area. The result was weaker bones after
15 weeks at the highest dose level. Thus, PCB exposure affects calcium
metabolism and bone morphometry (Andrews, 1989).
8.6.3.2 Effects of individual congeners
3,4,3',4'-Tetrachlorobiphenyl induced a decrease in serum and liver
retinol and retinyl palmitate in C57BL/Rij mice. In "non-responsive"
DBA/2 mice, only serum retinol was decreased. The time and
dose-responses observed suggested that the difference in aryl
hydrocarbon hydroxylase responsiveness was not directly involved in
the effects on retinoid levels (Brouwer et al., 1985).
Powers et al. (1987) administered female, Sprague-Dawley rats single,
intraperitoneal injections of 1, 5, or 15 mg 3,4,3',4'-
tetrachlorobiphenyl/kg body weight and found a dose-related
depression of plasma retinol levels, 24 h after treatment. The loss of
plasma retinol appeared to be a function of depressed levels of the
retinol-binding protein (RBP)-transthyretin ternary complex. No free
retinol was observed in the plasma. Hepatic retinyl palmitate
hydrolase (RPH) activity was depressed and highly and positively
correlated with the plasma retinol levels. Doses of either
2,4,5,2',4',5'- and 3,4,5,3',4',5'-hexachlorobiphenyl, equimolar to
the 15 mg/kg tetrachlorobiphenyl dose, failed to cause a similar
depression in plasma retinol in treated female rats.
A study was carried out to investigate the effects of PCBs on retinoid
homeostasis in Sprague-Dawley rats. Female Sprague-Dawley/Rij rats
were fed a Vitamin A-deficient diet for 12-16 weeks. Serum retinol
concentrations at the end of this period were decreased to
approximately 10% of the normal retinol level. The rats were repleted
with radiolabelled [3H]retinol by feeding a diet containing 18.5 MBq
(8000 IU) of retinol/kg diet for 14 days. Saturation in the blood was
reached after 6 days [3H]retinol repletion. On day 7, the rats were
either treated with an intraperitoneal dose of 3,4,3',4'-
tetrachlorobiphenyl (15 mg/kg) in corn oil, or corn oil alone.
Exposure to tetrachlorobiphenyl resulted in significant reductions in
both retinol and retinyl ester concentrations in the liver and lung to
25% and 44% of the controls, respectively, and a reduction of retinol
in the heart of 35% of the controls. No changes in concentrations were
observed in the skin and kidneys (Brouwer et al., 1988).
Female WAG/Rij rats received a single ip injection of corn oil, or 15
or 200 mg 3,4,3',4'-tetrachlorobiphenyl/kg body weight and were killed
on days 1,3,7, or 14 to study the effects on serum and hepatic
retinoid contents and liver morphology. There was a significant
increase in liver weight at the highest dose level after 3, 7, and 14
days. There was a rapid increase in the 3H-tetrachlorobiphenyl levels
present after 7 days, after which a rapid decline occurred.
Tetrachlorobiphenyl induced a significant decrease in serum retinol
content in the 200 mg tetrachlorobiphenyl group on days 3 and 7. The
same was found for the retinol and retinyl palmitate contents of the
liver. Ultrastructural alterations in the hepatocytes, such as
proliferation and vesiculation of the endoplasmic reticulum and
mitochondrial enlargement with inclusions, were found (Durham &
Brouwer, 1989).
2,2',5,5'-Tetrachlorobiphenyl caused inhibition of Ca/Mg- and
Mg-dependent adenosinetriphosphatase in the liver of rats (Lin et al.,
1979). In vitro, several PCB congeners inhibited Na/K- and
Mg-dependent adenosinetriphosphatase. Although a general trend towards
increased inhibition, paralleling increased chlorination, was
observed, no correlation was evident between chlorine substitution
patterns and inhibitory activity (La Rocca & Carlson, 1979).
8.6.4 Effects on the gastrointestinal tract
Effects on the stomach have been studied or observed by Allen &
Norback (1973); Allen et al. (1974a); Allen (1975); Becker et al.
(1979) and Tryphonas et al. (1986a) in monkeys. Oral administration of
Aroclor 1242, 1248, or 1254 to monkeys produced gastritis, which
progressed to hypertrophy and hyperplasia of the gastric mucosa.
Related effects included mucous-filled cysts that penetrated the
muscularis mucosa. These effects were initiated by exposure as low
and/or short as a single gavage dose of 1.5 g Aroclor 1248/kg body
weight, 25 mg Aroclor 1248/kg diet for up to 1 year, 3 mg of Aroclor
1242/kg diet, for 71 days, or 280 µg/kg body weight for 28 months.
In studies on monkeys, Becker et al. (1979) carried out stomach
biopsies and found microscopically apparent arrest of the
differentiation of generative cells of the isthmus and neck into
parietal and zymogenic cells. Mature parietal and zymogenic cells,
which were found only in the bases of the glands, showed signs of
injury, such as dilatation of the rough endoplasmic reticulum on the
zymogenic cells, irregularity of the mitochondria and irregular
luminal membranes in parietal cells, and an increase in the number of
autophagic vesicles on both types of cell (see 8.2.1.6).
The Aroclor-induced gastric lesions, which occurred mainly along the
greater curvature of the stomach (not in the cardiac or pyloric
regions) and did not occur in other sections of the gastrointestinal
tract, have only been observed in pigs and monkeys (Hansen et al.,
1976b; Becker et al., 1979; Drill et al., 1981). The gastric effects
may therefore be species specific. Aroclor 1254 induced metaplasia and
adenocarcinoma in the glandular stomach of F344 rats (see section
8.7.1.2).
8.6.5 Effects on lipid metabolism
8.6.5.1 Effects of PCB mixtures
Consistent with the histopathological observation of fatty
degeneration in the liver (see section 8.2.1), short-term exposure to
commercial mixtures of PCBs induced increases in the contents and
concentrations of total lipids, triglycerides, cholesterol, and/or
phospholipids in this organ of the rat and rabbit (Litterst et al.,
1972; Bruckner et al., 1974; Itokawa et al., 1976; Garthoff et al.,
1977; Hinton et al., 1978; Ishidate et al., 1978; Dzogbefia et al.,
1978; Yagi, 1980; Kato & Yoshida, 1980, 1981; Kato et al., 1982).
Litterst et al. (1972) exposed male Osborne-Mendel rats, for 4 weeks,
to diets containing Aroclor 1242, 1248, 1254, or 1260 at levels of, or
between, 0.5, 5.0, 50, and 500 mg/kg (equivalent to 0.025, 0.25, 2.5,
and 25 mg/kg body weight). Aroclor 1248 caused the highest
dose-related increase in the triglyceride concentration in the liver,
which was significant at 500 mg/kg diet (equivalent to 25 mg/kg body
weight). The lowest-observed-effect level was reported by Bruckner et
al. (1974), who exposed male Sprague-Dawley rats to 0, 5, or 25 mg of
Aroclor 1242/kg diet (equivalent to 0, 0.3, and 1.5 mg/kg body weight,
respectively), for 2, 4, or 6 months and found a slight increase in
the concentrations of total lipids in the liver at both exposure
levels.
Levels of total lipids, triglycerides, and/or cholesterol in the serum
of rats and rabbits, exposed to PCB mixtures, were found to be
increased (Koller & Zinkl, 1973; Allen et al., 1976; Itokawa et al.,
1976; Garthoff et al., 1977; Zinkl, 1977; Kato & Yoshida, 1980, 1981;
Yagi, 1980; Kato et al., 1982; Baumann et al., 1983; Hladkà et al.,
1983; Carter, 1985). Wistar rats received Clophen A50, twice weekly,
by gavage, at levels of 2, 10, 50, 150, or 250 mg/kg body weight, for
6 weeks. Serum triglyceride and cholesterol levels were increased in a
dose-related manner at 50 and 2 mg/kg body weight, respectively
(Baumann et al., 1983). Decreased serum triglyceride levels were
reported by Kato et al. (1982). Fischer rats exposed for 8 days to
Aroclor 1254 in the diet at levels of 8 mg/kg (0.4 mg/kg body weight)
or more showed a dose-related increase in serum total cholesterol
concentrations. Hypercholesterolaemia was not found at 4 mg/kg diet
(Carter, 1985). Studies on monkeys exposed to Aroclors 1248 or 1254
for 1-2 years revealed lowered serum levels of total lipids,
triglycerides, and cholesterol (Barsotti et al., 1976; Arnold et al.,
1984). The cause of these changes may be an altered synthesis and/or
lipoprotein transport in the liver. No increase in the rate of
synthesis of liver triglycerides was found following intraperitoneal
exposure of rats to 3-8 daily doses of 50 mg of Aroclor 1254/kg body
weight (Hinton et al., 1978; Sandberg & Glaumann, 1980). The observed
increases in the half-lives of liver triglycerides and phospholipids
(Hinton et al., 1978) and the observed increase in the number of Very
Low Density Lipoproteins (VLDL) in the liver, without a change in
lipid composition (Sandberg & Glaumann, 1980), seems to be indicative
of impaired transport of these lipids from the liver to the blood.
This was also demonstrated by the repression in serum VLDL and in the
incorporation of tritiated water in serum total lipids following
tritiated water injection, found in rats exposed for 24 days to a
low-protein diet containing 1000 mg of Aroclor 1248/kg (50 mg/kg body
weight) (Kato et al., 1982). Sandberg & Glaumann (1980) observed an
impaired transport of VLDL from the endoplasmic reticulum to the Golgi
apparatus. This compares well with the observed flattening of the
Golgi apparatus, which also lacks secretory vesicles with lipoprotein
particles (Hinton et al., 1978). No explanation was found in the
available literature for the observed increase in serum triglyceride
levels in rats.
Ishidate et al. (1978) measured a decreased rate of synthesis of
phospholipids, especially of phosphatidyl choline, in the liver of
rats that had received 2 daily doses of a PCB mixture at 100 mg/kg
body weight, composed mainly of tetrachlorobiphenyl isomers. Decreased
phospholipid synthesis was also observed by Hinton et al. (1978). The
accumulation of phospholipids in the proliferated endoplasmic
reticulum was ascribed to the observed depression of the secretion of
lipoproteins into blood (Hinton et al., 1978; Ishidate et al., 1978;
Sandberg & Glaumann, 1980) and to a depressed catabolism of liver
phospholipids (Ishidate et al., 1978).
The synthesis of cholesterol in the rat liver may be increased by PCB
mixtures considering the increased concentration of labelled
cholesterol in the liver following 3H20-injection (Kato et al.,
1982) or 14C-glucose or 14C-acetate administration (Yagi, 1980) in
rats that had been exposed for 3-5 weeks to diets containing 1000 mg
of Aroclor 1248/kg diet or 500 mg Kanechlor 500/kg diet, respectively.
It was also shown that the activity of 3-hydroxy-3-methylglutaryl
Coenzyme A reductase (EC 1.1.1.34) was increased in rats following a
6-day exposure to a diet containing 1000 mg Aroclor 1248/kg diet
(equivalent to 50 mg/kg body weight) (Kato & Yoshida, 1980). The
enhanced synthesis of cholesterol has to compete with an enhanced
degradation, as Aroclor 1248 has been shown to induce cholesterol
7-alpha-hydroxylase (EC 1.14.14.1) in rats (Quazi et al., 1984).
Decreased biosynthesis of liver cholesterol was found in rats exposed
for 30 days to Aroclor 1254 at a dietary level of 500 mg/kg (Kling &
Gamble, 1982). Hypercholesterolaemia in PCB-exposed rats can be
explained partly by an increased synthesis of cholesterol and/or an
increase in serum high density lipoprotein cholesterol, which was
observed in several studies (Ishikawa et al., 1978; Yagi, 1980; Kato &
Yoshida, 1981; Carter, 1985).
Isolated hepatocytes were capable of secreting protein and
triacylglycerol in the form of VLDL into serum-free media. Eighty per
cent of 2,4,5,2',4',5'-hexachlorobiphenyl released from hepatocytes
was in association with VLDL, the remainder being in association with
protein (Gallenberg & Vodicnik, 1987).
8.6.5.2 Effects of individual congeners
Charles-River CD rats received a single oral dose of 3,4,5,3',4',5'-,
2,4,5,2',4',5'-, or 2,3,5,2',3',5'-hexachlorobiphenyl in cotton-seed
oil. After 72 h, all isomers had increased the levels of total lipids
in the liver. 3,4,5,3',4',5'-Hexachlorobiphenyl had the most
pronounced effect. This isomer was the only one that increased the
levels of total cholesterol, cholesterol esters, and triglycerides in
the liver, while the other 2 isomers slightly increased the content of
liver phospholipids (Kohli et al., 1979).
Shireman (1988) studied the lipoprotein-mediated transfer of
2,4,5,2',4',5'-hexachlorobiphenyl into cultured human fibroblasts, and
found that the plasma lipoproteins may play a role in the distribution
of this hexachlorobiphenyl to peripheral cells. Using normal skin
fibroblasts incubated with medium containing serum LDL or high density
lipoproteins (HDL) labelled with the 14C-hexachlorobiphenyl, the
author characterized the cellular incorporation, and efflux from
cells, of this congener and concluded that HDL might be involved in
the delivery of hexachlorobiphenyl to cells and not, as generally
thought, in the transport from cells.
2,4,5,2',4',5'-Hexachlorobiphenyl was shown to be distributed among
rat and human plasma lipoproteins and protein in vitro. It was
readily transferred among plasma constituents and its distribution was
related to the triacylglycerol:protein ratio in the plasma. One h
following intravenous administration of 70 µg labelled
hexachlorobiphenyl to virgin, female Sprague-Dawley rats, the
hexachlorobiphenyl was primarily distributed to low density
lipoprotein (LDL) with the hypertriglyceridemia of late pregnancy;
more than 70% of circulating hexachlorobiphenyl was associated with
very low density lipoproteins (VLDL). VLDL is a major substrate for
mammary gland lipoprotein lipase, which is elevated during lactation.
When hexachlorobiphenyl was complexed with human VLDL and injected
intravenously into late pregnant mice, mammary gland concentrations of
the compound exceeded those in the adipose tissue at all sacrifice
times between 5 min and 6 h (Gallenberg & Vodicnik, 1987; Gallenberg
et al., 1987).
8.6.6 Effects on porphyrin metabolism
8.6.6.1 Effects of PCB mixtures
Hepatic porphyria has been induced by a number of commercial PCB
mixtures (Clophen A60; Phenochlor DP6; Aroclor 1016, 1232, 1242, 1254,
and 1260; Kanechlor 400, 500, and 600) in mice, rats, rabbits,
chickens, and Japanese quail. Young rats, guinea-pigs, and minks seem
to be less sensitive (Strik, 1973). The porphyria was characterized by
the presence of pigment in the liver which fluoresced red under UV
radiation (Vos & Beems, 1971; Kimbrough et al., 1972; Vos &
Notenboom-Ram, 1972; Zinkl, 1977; Honda et al., 1983), an increase in
the concentration of porphyrins in the liver (Goldstein et al., 1974,
1975; Grote et al., 1975; Iverson et al., 1975; Kawanishi et al.,
1975) and an increase in the concentrations of delta-aminolevulinic
acid, porphobilinogen, and porphyrins in the urine or faeces (Vos &
Beems, 1971; Vos & Notenboom-Ram, 1972; Goldstein et al., 1974, 1975;
Baumann et al., 1983; Honda et al., 1983). Vos & Beems (1971) found
increased faecal elimination of coproporphyrin and protoporphyrin in
rabbits dermally treated with 118 mg Aroclor 1260/day (free of PCDFs),
5 days/week, for 36 days and Vos & Notenboom-Ram (1972) found the same
results when female, New Zealand rabbits received a 120 mg application
of Aroclor 1260 on the shaved skin, 5 days/week, for 4 weeks.
When Sherman rats were exposed for up to 13 months to a diet
containing 100 mg Aroclor 1254/kg or for up to 26 weeks to Aroclor
1242 at 100 or 500 mg/kg diet (equivalent to 5 and 25 mg/kg body
weight, respectively) a delayed onset of porphyria was noted after 2-7
months of exposure. The porphyria was mainly characterized by the
excretion and hepatic storage of uroporphyrin and heptacarboxy-
porphyrin, resembling human porphyria cutanea tarda (Goldstein et al.,
1974, 1975). A dose-dependent increase in the concentration of liver
porphyrins was observed in female, Sprague-Dawley rats receiving 21
daily doses of Aroclor 1242 (by gavage) in corn oil at 10 or 100 mg/kg
body weight, but not at 1 mg/kg body weight. Female rats were more
sensitive than male rats and Aroclor 1016 at the same dietary level
had less effect than Aroclor 1242 (Iverson et al., 1975). Others also
noted the greater effect of higher chlorinated PCB mixtures on liver
and urinary levels of porphyrins (Goldstein et al., 1974, 1975;
Kawanishi et al., 1975). Kawanishi et al. (1973, 1974) showed that
administration, in the diet, of Kanechlors KC-300 and KC-500 to rats
at 500 mg/kg produced a marked increase in urinary excretion of copro-
and uroporphyrins, and in faecal elimination of protoporphyrin, but no
increases were observed with Kanechlor KC-400.
Increased urinary coproporphyrin levels were found in male
Sprague-Dawley rats exposed for 2, 4, or 6 months to a diet containing
Aroclor 1242 at 5 or 25 mg/kg (equivalent to 0.25 and 1.25 mg/kg body
weight) (Bruckner et al., 1974).
Porphyria in rats and rabbits has been associated with the observed
stimulation of delta-aminolevulinate synthase (EC 2.3.1.37), the
rate-limiting enzyme in the haem synthesis of porphyrins (Goldstein et
al., 1974, 1975; Grote et al., 1975; Drill et al., 1981; Hill, 1985),
and with the inhibition of uroporphyrin decarboxylase (EC 4.1.1.37),
as measured in chick embryo cells and chicken erythrocytes in vitro
(Kawanishi et al., 1983; Sano et al., 1985). Seki et al. (1987)
observed 80% inhibition of liver uroporphyrin decarboxylase together
with a 15-fold increase in the activity of liver delta-aminolevulinate
synthase and accumulation in the liver of a large amount of
uroporphyrin in C57BL/6 mice exposed for 3 weeks to Kanechlor 500 at a
dietary dose of 500 mg/kg. Liver microsomal cytochrome P-450 was
increased and induction of microsomal enzymes was observed. The
effects were less outstanding in ddY mice whereas liver cytosol levels
of the PCBs were comparable in both strains. The authors postulated
that the development of porphyria is causally related to the
inhibition of uroporphyrin decarboxylase rather than the induction of
drug metabolizing function. Porphyria would develop only when the
ratio of hepatic uroporphyrin decarboxylase and delta-aminolevulinate
synthase decreased to less than 1.0.
8.6.6.2 Effects of individual congeners
The levels of coproporphyrin and protoporphyrin found in the faeces of
rabbits, dermally exposed to 5 doses/week of 120 mg of
2,4,5,2',4',5'-hexachlorobiphenyl (no dibenzofurans detected) in
isopropanol, for 4 weeks, were more elevated than those in the faeces
of rabbits exposed similarly to Aroclor 1260 (Vos & Notenboom-Ram,
1972). Koss et al. (1980) also found 2,4,5,2',4',5'-hexachlorobiphenyl
highly effective in inducing porphyria in female rats receiving,
orally, 64 mg of this PCB-congener/kg body weight in oil, once every 2
days, for 10 weeks. In mice receiving a diet containing 300 mg of one
of various tetrachlorobiphenyls, hexachlorobiphenyls, or
Kanechlors/kg, for 14 weeks, the most pronounced increases in the
levels of coproporphyrin and protoporphyrin in the liver were found in
animals fed 3,4,5,3',4',5'- and 2,4,6,2',4',6'-hexachlorobiphenyl, and
Kanechlor 600, followed by animals fed 3,5,3',5'- and 2,5,2',5'-
tetra-chlorobiphenyls and Kanechlor 500. No porphyrinogenic action was
found in mice fed 3,4,3',4'-, 2,4,2',4'-, 2,3,2',3'-, or 2,6,2',6'-
tetra-chlorobiphenyl, 2,3,4,2',3',4'-hexachlorobiphenyl, or Kanechlor
400 (Kawanishi et al., 1975). Accumulation of uroporphyrins was
observed in the livers of "responsive" C57BL/6 mice treated with
3,4,5,3',4',5'-hexachlorobiphenyl, but not in the livers of
"non-responsive" ddY mice. It was suggested that induction of
apocytochrome P-450 may take part in inducing porphyrin synthesis
(Sano et al., 1985).
Sano et al. (1985) studied the mechanism of the porphyrinogenic
activity of PCBs using cultured chick embryo liver cells to examine
the relationship between the induction of delta-aminolaevulinic acid
(ALA) synthetase and the inhibition of uroporphyrinogen dicarboxylase.
The porphyrinogenic effect of PCBs exhibited a defined
structure-activity relationship in that only 3,4,3',4'-tetrachloro-
and 3,4,5,3',4',5'-hexachlorobiphenyl out of 9 biphenyls produced a
marked accumulation of uroporphyrin in the liver cells. In
ALA-supplemented cultures, these 2 congeners led to the accumulation
of a large amount of uroporphyrin III, whereas with the other PCBs
(which were weak inducers of porphyrin synthesis) the accumulated
porphyrin was mostly protoporphyrin. These results suggested that the
active inducers of porphyrin synthesis also inhibit uroporphyrinogen
decarboxylase, in 2 steps, i.e., first, in the formation of
hexacarboxylic porphyrinogen III from heptacarboxylic porphyrinogen
III, and, second, in the formation of heptacarboxylic porphyrinogen
III from uroporphyrinogen III. The inhibition of uroporphyrinogen
decarboxylase leads to a depletion of haem. In addition, induction of
apocytochrome P-450 by PCBs may contribute to a decrease of haem. As a
result, synthesis of ALA synthetase increases, leading to an
accumulation of uroporphyrin in liver.
8.6.7 Effects on the endocrine system
8.6.7.1 Effects of PCB mixtures
The underlying cause of the reproductive toxicity of PCBs, described
in section 8.4, may be alterations in hormonal receptor binding and/or
alterations in the steroid hormone balance through effects on
metabolism and excretion.
Precocious vaginal opening was observed in neonatal Sprague-Dawley
rats receiving a subcutaneous dose of 10 mg of Aroclor 1221
(2000 mg/kg body weight) in sesame oil on days 2 and 3 of life. At 6
months of age, these females showed persistent vaginal estrus and
anovulation, despite no further exposure to Aroclor 1221. Doses of
Aroclor 1221, 1242, 1254, or 1260 at 1 mg/kg were without effect.
Groups of 22-day-old Sprague-Dawley rats were injected subcutaneously
with Aroclor 1221 or 1242 at 1, 10, 100, or 1000 mg/kg body weight or
Aroclor 1254 or 1260 mixed in sesame oil at 1, 10, or 100 mg/kg body
weight. Uteri were weighed. New-born female pups were injected
subcutaneously on the second and third day postpartum with Aroclor
1221 at 1 or 10 mg/day or Aroclor 1242, 1254, or 1260 in sesame oil or
dimethylsulfoxide at 1 mg/day. Pups were weaned at 21 days and
examined daily from the 25th day of puberty. Animals were sacrificed
at 7 or 8 months, at which time organs were examined. A significant
uterotrophic response was noted with 1000 mg Aroclor 1221/kg, but not
with the other PCBs (Gellert, 1978).
Indirect evidence for a weak estrogenic activity of PCBs was found for
various Aroclors by the glycogen response of immature rat uterus
(Bitman & Cecil, 1970; Bitman et al., 1972; Ecobichon & Mackenzie,
1974) or the less sensitive uterotropic response, observed in immature
rats exposed to Aroclor 1221, 1232, or 1248, but not in immature rats
exposed to Aroclor 1254 or 1260 (Ecobichon & Mackenzie, 1974; Gellert,
1978). More direct evidence is the inhibition in vitro of the
binding of labelled 17-beta-estradiol to the rat uterine receptor by
Aroclors 1221 and 1254 (Nelson, 1974).
Pregnant mares' serum was administered to immature female outbred rats
on day 29 postpartum, and, 60 h later, rats were injected with human
chorionic gonadotrophin. On day 34, the animals were divided into
groups and treated orally with sesame oil (control), or 20 mg PCBs/kg
(Clophen A30). Two days later they were killed and the ovaries removed
and analysed for in vitro synthesis of progesteron (unincubated,
incubated, and incubated with luteinizing hormone). The addition of
luteinizing hormone resulted in an approximately 100% increase in
progesterone synthesis above basal level with tissue exposed to PCBs.
With control tissue there was a 31% increase with luteinizing hormone
(Fuller et al., 1980).
PCBs induced a decrease in gonadal steroid hormone levels in rats,
minks, seals, and monkeys. When, after confirmed ovulation, mature
female Rhesus monkeys were exposed during the following cycle to daily
gavage doses of 4, 16, or 64 mg of Clophen A30/kg body weight, for 28
days, ovulation was blocked in 2 out of 4 treated monkeys. One out of
16 controls was anovulatory. The levels of luteinizing hormone and
follicle-stimulating hormone were not changed by the treatment (Muller
et al., 1978).
Plasma progesterone levels were decreased in female rats exposed for
36 weeks to a dietary level of Aroclor 1242 of 75 mg/kg (equivalent to
3.7 mg/kg body weight) (Jonsson et al., 1976), and in female minks
exposed for 12.5-14.5 months to a dietary level of 2.5 mg Aroclor
1254/kg (equivalent to 0.25 mg/kg body weight) (Aulerich et al.,
1985). The decreased levels of gonadal hormones can be explained by
enhanced metabolism of steroids, which are normal substrates for
microsomal enzymes. Increases in the formation of the metabolites of
progesterone and/or testosterone were measured in rats
intraperitoneally exposed 1-5 times to Aroclor 1260, Aroclor 1254, or
Kanechlor 400 (Krogh Derr, 1978; Lin et al., 1982; Yoshihara et al.,
1982). In contrast with these findings, increased testosterone levels
were found in male piglets exposed for 6-12 weeks to Aroclor 1232,
1242, or 1254 at 250 mg/diet. This increased production of
testosterone was related to increased relative testes weights
(Platonow et al., 1976).
Female rhesus monkeys (Macaca mulatta) were administered gelatin
capsules containing daily doses of 0, 5, 20, 40, or 80 µg Aroclor
1254/kg body weight, dissolved in corn oil plus glycerol. After
approximately 2 years of dosing, when the monkeys were considered to
be in a state approaching adipose-tissue PCBs equilibrium, each dose
group of 16 animals was divided into 2 test groups. Daily blood
samples from both test groups were acquired for estrogen and
progesteron analysis during one menstrual cycle. Serum estrogen and
progesteron concentrations in PCB-dosed monkeys were comparable with
those in the controls, except the luteal phase progesterone levels in
monkeys dosed with 20 and 80 µg/kg. There were no apparent
treatment-related differences in the incidence of anovulatory cycles
or in the temporal relationship between the estrogen peak and mensus
onset, mensus end, or the progesterone peak. Mean PCB concentrations
in the blood and adipose tissue for the different dose levels
administered were as follows: blood, 1, 11, 37, 74, and 125 µg/litre
and for adipose tissue 0.79, 7.88, 22.62, 47.6 and 85.3 mg/kg tissue,
respectively (Truelove et al., 1987).
Effects on plasma corticosteroid levels have also been observed. The
levels were decreased in female mice exposed to a diet containing
25 mg Aroclor 1254/kg (equivalent to 3.7 mg/kg body weight) for 3
weeks, and in male mice exposed to a diet containing 400 mg Aroclor
1254/kg (equivalent to 57 mg/kg body weight) for 2 weeks. No effects
were found on adrenal weight (Sanders & Kirkpatrick, 1975). However,
increased levels of plasma corticosterone and enlarged adrenal glands
were observed in male mice of another strain exposed to a diet
containing 200 mg Aroclor 1254/kg, for 2 weeks (Sanders et al., 1977).
Wasserman et al. (1973) reported increased plasma corticosterone
levels in rats receiving Aroclor 1221 in the drinking-water, at a
concentration of 250 mg/litre, for 10 weeks. This finding complies
with morphological features of hyperfunction of the adrenal zona
fasciculata found in rats that had received 200 mg Aroclor 1221/litre
drinking-water, for 6 weeks.
When female rats were exposed to Aroclor 1254, in their diet at doses
of 0, 1, 5, 10, or 50 mg/kg (equivalent to 0, 0.05, 0.25, 0.5, and
2.5 mg/kg body weight/day, respectively) for 5-7 months, relative
adrenal weights as well as serum levels of corticosterone,
dehydro-epiandrosterone, and dehydro-epiandrosterone sulfate were
decreased in a dose- and time-related manner. In the same studies, the
effects with less chlorinated Aroclors were less pronounced (Byrne et
al., 1988).
In addition, the ultrastructure of beta-cells of the pancreas of rats
was found to be changed after 13 months of exposure to 200 mg Aroclor
1254/litre drinking-water. These changes included marked dilatation
and vesiculation of the rough endoplasmic reticulum, hyperplastic
Golgi complexes with a reduction in the number of secretory granules,
and an increase in the number of beta-acinar and acinar-beta cells.
The changes in the pancreas were suggested to be secondary to the
increase in the level of glucocorticoids (Wasserman et al., 1975). An
increased relative adrenal weight was observed in pigs fed Aroclor
1242 or 1254/kg at 20 mg/kg diet (equivalent to 0.8 mg/kg body weight)
for 91 days (Hansen et al., 1976b).
Thyroid hormone levels were decreased in rats exposed to Aroclor 1254
at levels of 50 mg/kg diet or more for 4-12 weeks (Collins et al.,
1977; Collins & Capen, 1980a). Two explanations were offered. One was
the observed increase in biliary excretion of thyroxine and
triiodothyroxine (Bastomsky, 1974; Collins & Capen, 1980b) and the
larger proportion of biliary thyroxine present as glucuronide
(Bastomsky, 1974), most likely as a result of induction of microsomal
uridine diphosphate-glucuronosyltransferase (EC 2.4.1.17) (Bastomsky &
Murthy, 1976). The other explanation was a direct effect of PCBs on
thyroid follicular cells. When male Holtzman or Osborne-Mendel rats
were fed a diet containing Aroclor 1254 at a level of 0, 5, 50, or
500 mg/kg (equivalent to 0, 0.25, 2.5, and 25 mg/kg body weight),
thyroid follicular cells exhibited a dose-dependent hypertrophy and
hyperplasia. An abnormal accumulation of large colloid droplets and
irregular lysosomes in the follicular cells were observed at 5 mg/kg
diet or more and reduced serum thyroxine occurred at 50 mg/kg diet or
more. A no-observed-effect level could not be established. Microvilli
were decreased in number, shortened, and irregularly branched (Collins
et al., 1977; Kasza et al., 1978a,b; Collins & Capen, 1980a, 1980b).
The hypothalamus-pituitary axis seems not to be affected in view of
the observed increase in the serum level of thyroid-stimulating
hormone and in the iodine uptake by the thyroid following PCB exposure
(Bastomsky, 1974, 1977; Collins & Capen, 1980a).
Collins & Capen (1980a) suggested that the well-documented
PCBs-related disturbances in reproduction, growth, and development may
be related to alterations in thyroid structure and function in the
dam, fetus, or neonate. The lowering of serum thyroxine appears to be
the combined result of a direct effect on thyroid follicular cells
with an interference in hormone secretion plus an enhanced peripheral
metabolism of thyroxine.
8.6.7.2 Effects of individual congeners
Exposure of rats to various congeners produced different responses in
steroid metabolism. The most marked effects were observed after
exposure to 2,4,5,2',4',5'-hexachlorobiphenyl, which was found to
decrease the half-life of progesterone (Örberg & Ingvast, 1977), to
increase hydroxylation of progesterone, testosterone, and
androstenedione, and to decrease the 5-alpha-reduction of progesterone
and testosterone (Dieringer et al., 1979; Yoshihara et al., 1982).
3,4,5,3',4'-Pentachlorobiphenyl was found to depress the total
microsomal metabolism of progesterone and testosterone, though the
7-alpha-hydroxylation of these steroids was markedly stimulated
(Yoshihara et al., 1982). No, or very slight, effects on steroid
metabolism were found in rats exposed to chlorobiphenyls with 4
chlorine atoms or less (Örberg & Ingvast, 1977; Dieringer et al.,
1979).
Yoshimura et al. (1985) described a marked induction of liver
microsomal cytochrome P-450 and cytosolic DT-diaphorase as a cause of
a possible disorder of steroid homeostasis and promotion of
carcinogenicity of 4-nitroquinoline N-oxide (4-NQO) in rats
pretreated with 3,4,5,3',4'-pentachlorobiphenyl. The animals were
sacrificed 5 days after pretreatment. The results of the studies
showed that 7-alpha-hydroxylation of both progesterone and
testosterone in liver microsomes was increased, but hydroxylation at
the 2-alpha-, 6 alpha-, and 16 alpha-positions were depressed,
together with 5 alpha-reduction. The induced isoenzyme P-452 was most
responsible for the 7-alpha-hydroxylation of testosterone.
The major component (32 mol %) of the Aroclor 1221 mixture is
2-chlorobiphenyl. The major metabolite (4,4'-dihydroxy-2-
chlorobiphenyl) of 2-chlorobiphenyl has been shown to have a
significant binding activity with the soluble uterine estrogen
receptor protein in the rat, suggesting a possible explanation for the
unique estrogenic activity of Aroclor 1221 in the rat (Korach et al.,
1987).
8.6.8 Immunotoxicity
Some of the studies described below are summarized in Table 52.
8.6.8.1 Effects of PCB mixtures
(a) Mouse
Relative thymus and spleen weights of C57BL/6 mice were unaffected by
exposure to Aroclor 1016, for 3-41 weeks, at a dietary level of
167 mg/kg (Silkworth & Loose, 1979). Dietary exposure of outbred mice
(Mus musculus) to Aroclor 1248 at 50, 100, 500, or 1000 mg/kg diet
(equivalent to 7.1 up to 143 mg/kg body weight), for 3 or 5 weeks, did
not elicit gross signs of immunotoxicity (Thomas & Hinsdill, 1978).
BALB/c mice fed Aroclor 1242 at a dose-level of 0 or 167 mg/kg diet
(equivalent to 0 and 29 mg/kg body weight) for 3-9 weeks, did not show
adverse effects on the thymus, spleen, and lymph nodes (Loose et al.,
1977, 1978).
In addition, Carter & Clancy (1980) observed an increased graft versus
host response in a decreased number of spleen cells in 4BALB/c mice,
which were exposed to a single intraperitoneal dose of 1000 mg Aroclor
1242/kg body weight in corn oil. Spleen enlargement and lymphocyte
depletion were observed.
Offspring of Swiss-Webster mice, exposed via the dams which were fed
Aroclor 1254 at dietary levels of 10, 100, or 250 mg/kg, did not
exhibit an altered hypersensitivity reaction to oxazoline, an altered
anti-bovine serum albumin antibody titre or an altered degree of
phagocytosis of sheep red blood cells by peritoneal macrophages,
compared with controls (Talcott & Koller, 1983).
Pathogen-free ICR/JCL mice (aged 4 weeks) were intubated orally, once
a week, for 4 weeks, with 0, 10, or 100 µg Kanechlor 500/kg body
weight. Two days after the final treatment, half of the animals of
each group were injected intraperitoneally with 0, 50, 250, or 500 µg
E. coli endotoxin/mouse. Sensitivity to endotoxin was determined by
24-h mortality rate. The oral administration of Kanechlor 500 up to a
dose level of 100 µg/kg body weight did not have any effect on the
sensitivity to the endotoxin (Oishi & Hiraga, 1980).
The relative potencies of PCB mixtures Aroclors 1260, 1254, 1248,
1242, 1016, and 1232 to inhibit the murine, splenic, plaque-forming
cell response to sheep red blood cells was determined for C57Bl/6
mice. The ED50 values for the reduction in the splenic,
plaque-forming cells were 104, 118, 190, 391, 408, and 464 mg/kg body
weight, respectively. It was apparent that the higher PCBs (Aroclors
1260, 1254, and 1248) were more potent than the lower chlorinated
mixtures.
Previous studies have shown that a subeffective dose of Aroclor 1254
(25 mg/kg), interacted with an immunotoxic dose of TCDD (3.7 nmol/kg),
resulting in, a significant antagonism of the toxicity of the latter
compound. Co-treatment of mice with a dose of all these PCB mixtures
at 25 mg/kg and a reconstituted PCB mixture, as occurs in breast milk,
in combination with TCDD (3.7 nmol/kg) showed that all (except Aroclor
1232) significantly antagonized the TCDD-mediated inhibition of the
splenic, plaque-forming cell response in C56Bl/6 mice (Davis & Safe,
1989).
Table 52. The humoral and cell-mediated immunotoxicity of PCBs administered via the diet in short-term studies
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Monkey
Rhesus Aroclor 44 anti-SRBC antibody titre D 5.0 (d) 2.5 (d) Thomas &
1248 anti-tetanus toxicoid Hinsdill
antibody titre NE - 5.0 (d) (1978)
serum gamma-globulin
fraction D 5.0 (d) 2.5 (d)
Cynomolgus Kanechlor 20 anti-SRBC antibody titre D 2 (bw) Hori et al.
400 serum gamma-globulin (1982)
(purified) fraction D 2 (bw)
Cynomolgus Aroclor 21 anti-SRBC antibody titre D 2.5 (d) Truelove
1254 anti-tetanus toxicoid et al. (1982)
antibody titre NE 10 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Rabbit
New Zealand Aroclor 5 anti-SRBC antibody titre NE 6.54 (bw) Street &
1254 serum gamma-globulin Sharma
levels NE 6.54 (bw) (1975)
delayed hypersensitivity
reaction to tuberculin NE 6.54 (bw)
popliteal lymph node
antibody-forming cells D 0.92 (bw) 0.18 (bw)
Guinea-pig
Clophen 4-7 anti-tetanus toxicoid Vos & van
A60 and antibody titre D 50 (d) 10 (d) Driel-
Aroclor delayed hypersensitivity Grootenhuis
1260 reaction to tuberculin D 50 (d) 10 (d) (1972)
anti-tetanus toxicoid
producing cells in
popliteal lymph nodes D 50 (d) 10 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
Rat
Sprague- Aroclor 1 mitogen response to Bonnyns &
Dawley 1254 phytohaemagglutinin I 250 (d) Bastomsky
response to poke-weed (1976)
mitogen NE 250 (d)
serum gamma-globulin
fraction D 250 (d)
Sprague- Aroclor 10 interleukin 2 production Exon et al.
Dawley 1254 induction by KLH D 50 (d) (1985)
natural killer cell
cytotoxicity D 50 (d)
anti-KLH antibody titer D 50 (d)
Mouse
ICR Kanechlor 3 host-resistance to herpes Imanishi
500 simplex virus D 33 (bw) 18 (bw) et al. (1980)
host-resistance to
ectomelia virus D 33 (bw) 18 (bw)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
ICR Kanechlor 3 host-resistance to Imanishi
500 influenza virus D 400 (d) 200 (d) et al. (1984)
host-resistance to
Staphylococcus aureus D 100 (d)
ICR/JCL Kanechlor 4 sensitivity to NE - 100 µg/kg Oishi &
500 E. coli endotoxin (bw) Hiraga
(1980)
Swiss-Webster Aroclor 12 hypersensitivity reaction NE - > 250 (d) Talcott &
1254 to oxazoline, anti-bovine Koller
serum albumine antibody (1983)
titre and phagocytosis
of SRBC by macrophages
BALB/c Aroclor 3-6 host-resistance to Loose et al.
1242 endotoxin D 167 (d) (1978)
host-resistance to
malaria D 167 (d)
BALB/c Aroclor 6 spleen cellularity NE 167 (d) Loose et al.
1242 spleen PFC D 167 (d) (1977)
serum immunoglobulins
G1, A, M D 167 (d)
Table 52. (cont'd).
Strain PCB- Exposure Parameter testedb Resultc LOELd NOELd Reference
mixture period (mg/kg) (mg/kg)
(weeks)a
C57BL/6 Aroclor 3-41 graft versus host response NE 167 (d) Silkworth &
1016 mixed lymphocyte Loose (1979)
response I 167 (d)
mitogen response to
lipopolysaccharide I 167 (d)
mitogen response to
concanavalin A I 167 (d)
Mus musculus Aroclor 5 host-resistance to Thomas &
1248 Salmonella typhimurium D 1000 (d) Hinsdill
host-resistance to (1978)
endotoxin D 1000 (d)
a Up to day of primary immunization.
b SRBC = Sheep red blood cells; KLH = Keyhole limpet haemocyanin; PFC = Plaque forming cells.
c I = Increased; D = Decreased; NE = No effect found.
d LOEL = Lowest-observed-effect-level; NOEL = No-observed-effect level: in mg/kg diet (d)
or mg/kg body weight per day (bw).
A single administration of 500 mg Aroclor 1254/kg, intraperitoneally,
inhibited the plaque-forming (PFC) response to subsequent challenge
with sheep erythrocytes in Ah locus positive (C57Bl/6N or B6C3F1N)
mice. However, Aroclor 1254 did not give induction in the Ah locus
negative DBA/2N mice. When B6C3F1 mice were challenged with sheep red
blood cells, 6 or 16 weeks after Aroclor 1524 treatment, substantial
recovery of a PFC response was observed. In older (76-week-old) B6C3F1
mice severe depression of the PFC response was observed.
In contrast with its profound depression of a PFC response, Aroclor
1254 (up to 1250 mg/kg) caused a slight increase in lymphocyte
proliferation induced by either T or B cell mitogens. A single
500 mg/kg dose of this Aroclor also suppressed the ability of
recipient B6C3F1 animals to reject a challenge with either the
syngenic fibrosarcoma (PYB6) or the gram negative pathogen Listeria
monocytogenes (Lubet et al., 1986).
Heinzow et al. (1988) studied the effect of 2,4,5,2',4',5'-hexa-
chlorobiphenyl in the E rosette formation with sheep red blood cells
(SRBC) as one of the characteristics of human T-lymphocytes. The
minimal concentration eliciting a significant monoclonal CD2 receptor
antibody sparing effect was 1.5 × 10-10 mol/litre.
C3H/HeN mice were treated, twice a week, for 2 or 3 weeks prior to
mating, with olive oil or with Kanechlor 500 at an oral dose of
50 mg/kg body weight. The offspring, some of which were nursed by
unexposed dams, were tested for immunocompetence, 4-15 weeks after
birth. The dams did not show any adverse effects on body weight,
absolute spleen weight, and spleen cellularity. The B-cell activity of
the offspring was comparable with that of controls. The helper T-cell
activity was reduced up to 7-11 weeks after birth: the effect was more
pronounced in prenatally-exposed groups (Takagi et al., 1989).
(b) Rabbit
Rabbits appear most sensitive with respect to the immunotoxicity of
PCBs. Street & Sharma (1975) exposed groups of 5-7 New Zealand rabbits
to Aroclor 1254 at dietary levels of 0, 3.7, 20, 45.8, or 170 mg/kg
(equivalent to 0, 0.18, 0.92, 2.1, or 6.54 mg/kg body weight) for
44-57 days. When compared with control animals, an increased degree of
thymus atrophy was observed at all dose levels except for 20.0 mg/kg.
At the 2 higher dose-levels, relative spleen weights were decreased
and the number of germinal centres reduced. When 8 female New Zealand
rabbits received 118 mg of Phenochlor DP6, Clophen A60, or Aroclor
1260 (free of PCDFs), on the back skin, 5 times per week, for 38 weeks
they showed leukopenia, thymus atrophy, and loss of germinal centres
in the spleen and lymph nodes. No such changes were observed in the 4
controls (Vos & Beems, 1971). Vos & Notenboom-Ram (1972) found the
same effects in rabbits administered 120 mg Aroclor 1260 (free of
PCDFs)/day, 5 days/week, for 4 weeks. No adverse gross immunotoxic
effects were observed in groups of 10 New Zealand rabbits fed various
Aroclors at dose levels of 150 mg/kg body weight, once a week, for
12-14 weeks (Koller & Thigpen, 1973).
When New Zealand rabbits were exposed orally, via intubation, to
Aroclor 1242 at a dose of 150 mg/kg body weight, once a week, for 11
weeks, the anti-pseudo rabies virus antibody titre and serum
gamma-globulin levels were decreased (Koller & Thigpen, 1973). The
overall picture is one of immunosuppression, though in 2 diet studies
an increased activation of the cell-mediated immune response was
observed (Bonnyns & Bastomsky, 1976; Silkworth & Loose, 1979).
New Zealand rabbit offspring, exposed via the dams fed 0, 10, 100, or
250 mg of Aroclor 1248/kg diet, showed a decrease in the delayed
hypersensitivity reaction to dinitrofluorobenzene in the highest dose
group. No effects were observed on the splenic, plaque-forming cell
response, the antibody titre against sheep red blood cells, and the
mitogen responses to Concanavalin A and Phytohaemagglutinin (Thomas &
Hinsdill, 1980).
(c) Guinea-pig
Atrophy of the thymus has been reported in female guinea-pigs exposed
to Clophen A60 or Aroclor 1260 for 4-7 weeks at a dietary level of
50 mg/kg (equivalent to 2 mg/kg body weight). Following stimulation
with tetanus toxoid, the authors found a lower antitoxin titre and a
lower count of antitoxin-producing cells in comparison with control
guinea-pigs, resulting in a significant reduction in immunoglobulins.
The skin reaction after tuberculination in animals immunized with
Freund's complete adjuvant (as a parameter of cell-mediated immunity)
was also depressed at the 50 mg/kg dose level (Vos & van
Driel-Grootenhuis, 1972).
Female guinea-pigs fed diets containing 50 mg Aroclor 1260/kg for 6
weeks had significantly lowered tetanus antitoxin titres, circulating
leukocytes and lymphocytes, and thymus atrophy (Vos & van Genderen,
1973). Also treatment with 10 mg Aroclor 1260/kg diet for 8 weeks
produced splenic atrophy (Vos & de Roij, 1972). The Aroclor 1260 was
free from PCDFs (no details).
(d) Monkey
Offspring of Rhesus monkeys appeared very sensitive to the toxic
effects of PCB exposure during gestation and nursing, as already
discussed in section 8.4.1. Thymic atrophy, loss of germinal centres
and of lymph nodules of the spleen, and bone marrow hypocellularity
were found in 3 out of 6 offspring that died during their first year
of life due to exposure to PCBs via their mothers. The mothers, 9-12
per group, were fed diets containing 0, 2.5, or 5.0 mg of Aroclor
1248/kg (equivalent to 0, 0.09, and 0.2 mg/kg body weight), for 18
months and were bred after 6 months of exposure. One mother in each
exposed group died during exposure, showing an increased
susceptibility to Shigella flexneri. At autopsy, no lesions were
observed in lymphoid organs and tissues (Allen & Barsotti, 1976;
Barsotti et al., 1976). When the surviving mothers were placed on a
control diet for approximately 1 year after exposure and then rebred,
there was a decided improvement in their health, but their infants
were still severely affected by PCBs. In the 2 offspring in each
exposed group that died after weaning at the age of 4 months, the
effects on the thymus, spleen, and bone marrow were similar to those
described for the first generation (Allen et al., 1980).
Groups of mature, female Rhesus monkeys received diets containing 0,
2.5, or 5.0 mg Aroclor 1248/kg. After 11 months, all monkeys received
intravenous injections of sheep red blood cells (SRBC) as well as an
intramuscular injection of tetatus toxin (TT). Booster injections and
a second TT injection were given after a number of weeks. Blood
samples were taken over a period of 20 weeks after immunization.
The anti-sheep red blood cells (SRBC) antibody titres, and antibody
response to TT were not clearly affected. The gamma-globulin-levels
were lower in the PCB-treated animals. After 6 months, the PCB-treated
monkeys developed chloracne, alopecia, and facial oedema (Thomas &
Hinsdill, 1978).
Hori et al. (1982) also found immunosuppression in monkeys exposed to
PCB mixtures (without detectable quantities of PCDFs), compared with 2
control monkeys. A more severe immunosuppression was observed in
another monkey exposed to a comparable PCB mixture with PCDFs.
In a pilot study, one infant Cynomolgus monkey, the mother of which
had been exposed to Aroclor 1254 at a dose-level of 400 µg/kg body
weight, showed a decreased anti-sheep erythrocyte antibody titre
following primary immunization in comparison with one control infant
(Truelove et al., 1982).
Atrophy and loss of germinal centres in the spleen and other lymphoid
tissues were observed in groups of 5-6 female Cynomolgus monkeys
following exposure to Aroclor 1254 or 1248, in an apple juice-corn oil
emulsion, 3 times per week, at dose levels of 5 and 2 mg/kg body
weight, respectively, until death at day 29-164. The monkeys exposed
to Aroclor 1254 showed bone marrow hypocellularity and leukopenia.
Lesions seen in control monkeys were similar to those described as
spontaneous (Tryphonas et al., 1984; see also section 8.2.1).
Limited data exist on the humoral or cell-mediated responses in
infants, exposed to PCBs via their mothers.
8.6.8.2 Effects of individual congeners
Thymus atrophy was observed in monkeys exposed for 1-6 months to
3,4,3',4'-tetrachlorobiphenyl, but not in monkeys exposed to
2,5,2',5'-tetrachlorobiphenyl (McNulty et al., 1980).
Decreased relative weights of the thymus and increased or decreased
relative weights of the spleen were induced by single intraperitoneal
doses of the planar congeners 3,4,3',4'-tetrachlorobiphenyl (at
10 mg/kg body weight) and 3,4,5,3',4'-pentachlorobiphenyl (at
245 mg/kg body weight) in "responsive" C57BL/6 mice, i.e., mice
possessing the cytosolic Ah receptor protein, but not, or only to a
minor degree, in "non-responsive" DBA/2 mice (lacking this receptor).
The tetrachlorobiphenyl further decreased the number of cells per
spleen and the number of splenic, plaque-forming cells at 10 and
100 mg/kg body weight, respectively. Further chlorination at the
ortho-position decreased the toxicity of these congeners. No adverse
effects were induced by 2,4,5,3',4'- and 3,4,5,2',4'-pentachloro-
biphenyl (at 490 mg/kg body weight), 2,3,4,5,3',4',5'-heptachloro-
biphenyl (at 593 mg/kg body weight) and the di- ortho substituted
tetrachlorobiphenyls (at 100 mg/kg body weight (Silkworth & Grabstein,
1982; Robertson et al., 1984; Silkworth et al., 1984).
Similar trends were observed in Wistar rats with respect to the
induction of decreased relative thymus and spleen weights (see section
8.2.1.1). Biocca et al. (1981) exposed C57BL/6J mice to various
hexachlorobiphenyl isomers in the feed for 28 days. The most toxic
isomer tested was 3,4,5,3',4',5'- hexachlorobiphenyl which, among
others, caused a marked thymus atrophy and a moderate depletion of
lymphocytes in the spleen. 2,4,6,2',4',6'-Hexachlorobiphenyl caused
the same lesions, albeit at much higher dose-levels, while the
2,4,5,2',4',5'- and 2,3,6,2',3',6'-hexachlorobiphenyls were virtually
inactive.
The in vivo generation of cytotoxic T-lymphocytes (CTL) in response
to allogeneic tumour challenge is sensitive to suppression by
3,4,5,3',4',5'-hexachlorobiphenyl, a poorly metabolized, Ah
receptor-binding PCB isomer. Groups of 5-8 C57Bl/5 mice treated with a
single oral dose of 0, 10, or 100 mg 3,4,5,3',4',5'-hexachloro-
biphenyl/kg body weight, 2 days prior to the intraperitoneal injection
of allogeneic P815 tumour cells, exhibited a dose-dependent reduction
in peak CTL activity in the spleen. When examined on a kinetic basis,
the TCL response was reduced in magnitude with no evidence for a shift
in the kinetics of the response induced by 3,4,5,3',4',5'-hexachloro-
biphenyl.
3,4,5,3',4',5'-Hexachlorobiphenyl exposure, prior to antigen challenge
(day -14, -7, or -1 relative to P815 injection on day 0), produced
significant suppression of the CTL response. 3,4,5,3',4',5'-
Hexa-chlorobiphenyl treatment (10 mg/kg body weight), 6 weeks prior to
such a challenge, was still significantly suppressive, though the
reduced degree of suppression suggested that recovery was in progress.
When 3,4,5,3',4',5'-hexachlorobiphenyl exposure occurred after antigen
challenge, significant suppression was produced only when exposure
occurred within the first 3 days of the response, suggesting that, as
the CTL matured, their sensitivity to 3,4,5,3',4',5'-hexachloro-
biphenyl diminished. Clearance of the allogeneic tumour cells from the
peritoneal cavity was delayed in 3,4,5,3',4',5'-hexachlorobiphenyl-
treated mice and was associated with an altered composition of the
white blood cell infiltrate in this cavity. Symptoms of overt
toxicity, as well as immunotoxicity, were apparent at lower doses of
3,4,5,3',4',5'-hexachlorobiphenyl in male compared with female mice.
In addition, interactive effects of 3,4,5,3',4',5'-hexachlorobiphenyl
exposure and P815 antigen challenge on body weight and thymic
involution were observed in both male and female mice (Kerkvliet &
Baecher-Steppan, 1988a).
A modest dose-dependent suppression of the proliferative response to
alloantigen in mixed lymphocyte culture (MLC) was observed with
lymphocytes from C57Bl/6 mice (groups of 5-6 mice) exposed to 10 or
100 mg 3,4,5,3',4',5'-hexachlorobiphenyl, while the cytotoxic
T-lymphocytes CTL response generated in MLC was significantly
suppressed only with 100 mg/kg. The amount of time between treatment
with 3,4,5,3',4',5'-hexachlorobiphenyl and sacrifice, which ranged
from 2 to 23 days, did not appear to influence the degree of
immunosuppression produced by 3,4,5,3',4',5'-hexachlorobiphenyl
exposure. Mitomycin C-treated lymphocytes from C57Bl/6 mice treated
with 10 or 100 mg 3,4,5,3',4',5'-hexachlorobiphenyl/kg body weight,
were not suppressive when added as third party cells to an independent
MLC. However, if the mice were alloimmune, lymphocyte-mediated
suppression of the MLC response was observed and directly correlated
with the magnitude of the CTL response present in the same population.
Thus, 3,4,5,3',4',5'-hexachlorobiphenyl-treated mice that had less CTL
activity compared with vehicle-treated mice also had less suppressor
activity. Avoidance of stimulator cells lysis by using H-2
incompatible MLC stimulator cells revealed the existence of
antigen-nonspecific suppressor activity that was greater with
lymphocytes from vehicle-treated mice than from 3,4,5,3',4',5'-
hexachlorobiphenyl-treated mice, suggesting that both CTL and
suppressor cell activities were suppressed by 3,4,5,3',4',5'-
hexa-chlorobiphenyl exposure. Direct addition of 3,4,5,3',4',5'-
hexachloro-biphenyl to lymphocyte cultures in vitro indicated
a lack of direct toxicity of 3,4,5,3',4',5'-hexachlorobiphenyl on
lymphoproliferative responses to mitogen or alloantigen at
concentrations equal to or less than 1 × 10-6 mol/litre. Thus,
the in vitro functional integrity of lymphocytes obtained from
3,4,5,3',4',5'-hexachlorobiphenyl-treated mice coupled with the lack
of a direct lymphocytic effect in vitro suggest an indirect
mechanism of action for the 3,4,5,3',4',5'-hexachlorobiphenyl-mediated
suppression of CTL activity in vivo. Previous reports implicating
suppressor cell induction and/or activation by Ah-receptor-binding,
halogenated, aromatic hydrocarbons that mediate the inhibition of CTL
generation were not confirmed (Kerkvliet & Baecher-Steppan, 1988b).
8.6.8.3 Appraisal
The alterations in gross measures of immunological function (spleen
and thymus weights, lymphocyte counts, histology of lymphoid organs
and tissues) in mammals are highly suggestive of an immunosuppressive
effect of PCB mixtures and some higher chlorinated congeners. More
direct evidence of an immunodepressive effect has been obtained by
methods that detect functional alterations in the humoral and
cell-mediated immunity in mammals. One study on monkeys demonstrated a
more severe immunosuppression by a PCDF-contaminated PCB mixture
compared with a non-contaminated PCB mixture. Rabbits and monkeys are
the most sensitive species. No-observed-effect levels are 100 µg
Aroclor 1248/kg body weight per day and <100 µg of Aroclor 1254/kg
body weight per day for monkeys and 180 µg of Aroclor 1254/kg body
weight per day for rabbits.
8.6.9 Neurotoxic effects
Depressed spontaneous motor activity was shown by male CD-mice exposed
to a single oral dose of 500 mg Aroclor 1254/kg body weight in
Emulphor:saline. No effects were found in motor coordination tests and
on pentenylene-tetrazol-induced seizures. Neurochemical tests with
isolated mouse brain synaptosomes showed inhibition of the uptake of
neurotransmitters and precursors, and stimulation of the release of
neurotransmitters (Rosin & Martin, 1981).
Male Wistar rats exposed to doses of 500 or 1000 mg Aroclor 1254 and
1260/kg body weight, in corn oil, showed a reduced norepinephrine
concentration in the frontal cortex and hippocampus. No changes were
measured in the hypothalamus and brainstem. The neurochemical effects
appeared to be associated with the actual presence of PCBs in the
tissues (Seegal et al., 1985).
8.6.10 Skin effects
Cutaneous effects occurred in Rhesus monkeys fed diets that contained
Aroclors, for short periods (Allen & Norback, 1973; Allen et al.,
1974a; Allen, 1975; Barsotti et al., 1976; Thomas & Hinsdill, 1978;
Allen et al., 1979; Altman et al., 1979; Becker et al., 1979;
McConnell et al., 1979; McNulty et al., 1980). The effects included
facial (particularly periorbital) oedema, purulent discharge from the
eyes, chloracne, and alopecia. The effects, which appeared to be
reversible, were produced by doses as low as 2.5 mg Aroclor 1248/kg
for 1-6 months, and 1 mg Aroclor 1242/kg (equivalent to 0.04 mg/kg
body weight) for 6 months. Rats exposed to Aroclor 1254 in the diet
developed alopecia and facial oedema after 104 weeks at 50 mg/kg, and
exophthalamos after 72 weeks at 50 mg/kg. These effects did not occur
after 104 weeks at 25 mg/kg (equivalent to 1.25 mg/kg body weight)
(NCI, 1978).
8.6.11 Effects on the lung
Many PCBs are metabolized to methylthio derivatives in mice (Mio et
al., 1976), seals (Jensen & Jansson, 1976) and humans (Yoshida &
Nakamura, 1979). The metabolic pathways leading to the generation of
these metabolites have been shown to involve glutathione conjugation
of an arenoxide intermediate (Preston et al., 1984). Lund et al.
(1986) studied the interactions of these metabolites with the lung. It
was found that the PCB metabolite, 4,4'-bis-(methylsulfonyl)-
2,5,2',5'-tetrachlorobiphenyl ((MeSO2)2TCB), selectively accumulates
in the Clara cells of the bronchiolar epithelium and in the secretory
contents of the bronchiolar lumen. In vitro characterization of this
interaction of tritiated (MeSO2)2TCB with lung suggests that this
selective accumulation is due to the presence of a secreted
(MeSO2)2TCB-binding protein in the respiratory tract of rats, mice,
and humans. The protein appears to be an almost globular,
low-relative-molecular-mass acidic protein that binds with
methylsulfonyl-PCBs (Lund et al., 1986).
In a study on 3 groups of C57Bl mice, administered orally 0, 10, or
100 mg 2,3,6,4'-tetrachlorobiphenyl/kg body weight with repeated
administration of 35S-cysteine, Klasson-Wehler et al. (1987) found
that the major compound present in the lung was 4-methylsulfonyl-
tetrachlorobiphenyl, indicating the presence of specific binding sites
for this metabolite in lung tissue, mainly in the tracheo-bronchial
mucosa.
Groups of 20 male SD rats were given (gastric intubation) 0 or 25 mg
Kanechlor 400 in edible oil once, and, in 2 other groups, the same
doses 4 times per week. Groups of 5 animals were killed 2, 7, 14, and
28 days after the last ingestion, and tissues were studied with light
and electron microscopy. The lungs of the rats particularly showed
peribronchiolar cell infiltrations, and electron microscopy revealed
lipid vacuoles and altered lamellar bodies or lysosomes in type II
alveolar cells and alveolar macrophages. These changes were most
marked 7 days after the last ingestion and were more severe in the
short-term application (Shigematsu et al., 1978).
8.6.12 Miscellaneous
Haake et al. (1987) used mature, male C57Bl/6J mice and virgin, female
C57Bl/6N mice to study the influence of Aroclor 1254 on the
2,3,7,8,-TCDD induction of teratogenic abnormalities. Dams were
treated by oral gavage with either corn oil, Aroclor 1254 (244 mg/kg),
or TCDD (20 µg/kg) or Aroclor followed by TCDD or Aroclor followed by
dexamethasone (90 mg/kg). Aroclor 1254 alone was administered on day
9, corn oil and TCDD on day 10, and dexamethasone on day 13. In the
combinations, the Aroclor was given the day before the TCDD or
dexamethasone. TCDD induced 61.8 ± 23.1% cleft palate per litter;
Aroclor with TCDD, 8.2 ± 1.5%; Aroclor alone, 0%; dexamethasone alone,
69.9 ± 18.2%, and Aroclor followed by dexamethasone, 85.8 ± 29.1%.
Previous studies have shown that Aroclor 1254 can act as a partial
antagonist of the microsomal enzyme induction and immunotoxic effects
of TCDD in the mice strain used and, in this study, it was shown that
Aroclor 1254 also antagonizes TCDD-mediated teratogenicity. It did not
have any effect on the effects mediated by dexamethasone.
Wölfle et al. (1988) found that treatment of male Wistar rats with
200 mg 3,4,3',4'-tetrachlorobiphenyl/kg body weight, injected
intraperitoneally, markedly stimulated growth of enzyme-altered liver
foci and [3H]-thymidine incorporation into nuclear DNA. In the liver,
enlarged hepatocytes, due to hypertrophy, and fine-to-medium
fatty-droplet deposition in hepatocytes were found, but no liver
necrosis. Hence, it was concluded, that post-necrotic regenerative
growth as the cause of the tetrachlorobiphenyl-mediated stimulation of
hepatocytes proliferation, could be excluded. The treatment with
tetrachlorobiphenyl in vivo, markedly increased EGF-stimulated
autophosphorylation of the EGF-receptor (a plasma membrane protein) in
liver plasma membranes. These results suggest that altered growth
control is due to a direct effect of tetrachlorobiphenyl on
hepatocytes.
8.7 Factors modifying toxicity; mode of action
8.7.1 Factors modifying toxicity
As PCBs can stimulate microsomal enzyme activity, it can be expected
that they may potentiate the action of other chemicals that undergo
microsomal activation, and antagonize the action of those that are
detoxified. Antagonism was for example observed in studies on rodents
with drugs like pentobarbital (Villeneuve et al., 1972; Sanders et
al., 1974; Sanders & Kirkpatrick, 1975; Rosin & Martin, 1983),
hexobarbital (Bickers et al., 1972; Tanaka & Komatsu, 1972), and
zoxazolamine (Bickers et al., 1972).
Lashneva et al. (1985) and Khan et al. (1985) found potentiation of
the rate of microsomal enzyme induction in rats with a combination of
50 mg Sovol (mixture of PCBs)/kg body weight and 500 mg 2,6-ditert-
butyl-4-methylphenol (ionol)/kg body weight.
Villeneuve et al. (1972) demonstrated the antagonistic effect by the
reduction of phenobarbital sleeping time in rats receiving Aroclors
1242, 1254, and 1260 in their diet, but not in those receiving Aroclor
1221. This was confirmed by Johnstone et al. (1974) with a series of
single PCBs. Tanaka & Komatsu (1972) found that the hexobarbital-
induced sleeping time in female rats was reduced to 49% of the control
value by daily oral doses of Kanechlor 500 of 2 mg/kg for 3 days
(total 6 mg/kg). When a daily dose of 0.4 mg/kg was given for 15 days
(total 6 mg/kg), no reduction in sleeping time was observed. When this
small dose was continued for 45 and 53 days, the reduction remained at
12-13%. Phillips et al. (1972) did not find any potentiation of the
cholinesterase-inhibitory action of parathion in rats dosed with
Aroclors 1221 and 1254; this does not necessarily imply that there was
no enhanced activation of parathion, as a stimulation of detoxication
may have occurred concurrently. A stimulation of parathion
detoxication, but not of activation, has been demonstrated in rabbit
microsomes (Villeneuve et al., 1971a). Lichtenstein (1972) reported a
potentiation by PCBs of the toxicity of parathion for flies.
Aroclor 1254 at 160 mg/kg diet fed to 5-week-old male and female
Fischer-344 rats, for 8 weeks, reduced mortality due to feeding
hexachlorophene at a concentration of 600 mg/kg diet, from 77% to 7%
and completely prevented the paralysis that was observed in all
animals on the hexachlorophene diet alone. However, histological
changes in the brain characteristic of hexachlorophene exposure were
still apparent in the animals on the combined treatment, and the
possibility of delayed toxicity beyond the 8 weeks of the study could
not be eliminated. The protective effect of Aroclor 1254 was explained
by its capacity to enhance detoxication by means of hepatic microsomal
enzyme induction (Jones et al., 1974).
Coté et al. (1985) studied a mixture of 15 "persistent" chemicals,
including Aroclor 1254, in Sprague-Dawley rats at dose levels of 0, 1,
10, 100, and 1000 times the Canadian water quality objectives (WQO) of
each chemical. The PCB (Aroclor 1254) treatments were 0, 0.001, 0.01,
0.1, or 1.0 µg/kg diet for 90 days. No influence on food intake, body
weight, organ weights, clinical chemistry, haematology, or
histopathology were observed.
As these polychlorinated hydrocarbons seem to have the same mechanism
of action, questions arise on the possible interactions between these
compounds. In one reported teratogenicity study, groups of 18-21
pregnant C57BL/6N mice received (by gavage) the vehicle corn oil, or
daily doses of 3 µg 2,3,7,8-TCDD/kg body weight, 10 or 20 mg
2,3,4,5,3',4'-hexachlorobiphenyl/kg body weight, 25 or 50 mg
2,4,5,2',4',5'-hexachlorobiphenyl/kg body weight, or combinations of
TCDD and hexachlorobiphenyl at these dose-levels in corn oil, from day
10 to day 13 of gestation. All chemicals were more than 98.9% pure and
the hexachlorobiphenyls did not contain detectable levels of
dibenzofurans or TCDD. TCDD alone caused a low incidence of cleft
palate and moderate hydronephrosis, 2,3,4,5,3',4'-hexachlorobiphenyl
only caused mild hydronephrosis, and 2,4,5,2',4',5'-hexachlorobiphenyl
did not produce any effects. However, treatment of pregnant mice with
a combination of TCDD and 2,3,4,5,3',4'-hexachlorobiphenyl caused
5- and 10-fold increases in the incidence of cleft palate at 10 and
20 mg of hexachlorobiphenyl/kg body weight, respectively. No
enhancement of TCDD-induced hydronephrosis was observed, and the
incidence of TCDD-induced cleft palate was not affected by
simultaneous 2,4,5,2',4',5'-HCB treatment (Birnbaum et al., 1985).
Male Sprague-Dawley rats were given a regimen consisting of PCBs,
1 mg/day; polychlorinated quarterphenyls (PCQs), 1 mg/day; PCDFs,
10 µg/day or a mixture of PCBs, PCQs, and PCDFs (1 mg + 1 mg +
10 µg/day) in olive oil, orally, for 22 days. The congeners and ratios
in the PCBs, PCQs, and PCDFs were the same as those in Japanese Yusho
oil (see section 9.1.2.1). The PCB-treated rats showed hepatic
hypertrophy, immunosuppression, and increased drug-metabolizing enzyme
activities in hepatic microsomes. PCQ treatment did not produce any
significant effects. PCDF and the mixture PCBs + PCDFs caused
hypertrophy of the liver, immunosuppression, increased and
drug-metabolizing enzyme activity to a much greater extent than that
found for PCBs (more than 100 times more) and weight loss and thymic
atrophy (Kunita et al., 1985).
Female Cynomolgus monkeys were administered PCBs (5 mg), PCQs (5 mg),
or a mixture containing 5 mg PCBs + 20 µg PCDFs in olive oil injected
in a piece of banana, daily, for 20 weeks. The PCBs and PCDFs
comprised the same congeners as those in Japanese Yusho oil. The
PCB-treated monkeys showed hepatic hypertrophy, immunosuppression, and
increased drug-metabolizing enzyme activities in hepatic microsomes,
but were devoid of the dermal symptoms characteristic of Yusho. PCQs
caused an increase in drug-metabolizing enzyme activities in hepatic
microsomes and immunosuppression, but these effects were much less
severe than those of PCBs. The mixture with PCDFs caused hypertrophy
of the liver, immunosuppression, increase in drug-metabolizing enzyme
activities (more than 100 times that of PCBs) and weight loss and
thymic atrophy. Dermal symptoms characteristic of Yusho patients were
also found, but not with PCBs or PCQs alone (Kunita et al., 1985).
8.7.2 Mechanisms of toxicity
The analogous structure-activity relations of individual PCB congeners
with respect to most of their toxic responses and to their potency in
inducing cytochrome P-448-dependent aryl hydrocarbon hydroxylase,
indicate that the most active PCB congeners (the coplanar congeners)
are those that are approximate stereoisomers of 2,3,7,8,-tetra-
chlorodibenzo- p-dioxin (TCDD). These findings suggest a common
mechanism of action.
As is proposed for 2,3,7,8-TCDD, this mechanism is based on the
binding affinity of PCB congeners to the cytosolic Ah-receptor
protein, a product of the regulator Ah gene (Poland & Glover, 1977;
Parkinson & Safe, 1981; Bandiera et al., 1982). The induction is
dependent on the position and number of chlorine atoms in the molecule
and the congeners that bind most strongly to the Ah-receptor show the
strongest induction of monooxygenases and the highest toxicity
(effects on the liver including increase in liver weight, increase in
liver enzyme activity and lipid content, porphyria, atrophy of the
thymus, and effects on reproduction) (Ecobichon & Comeau, 1975;
Goldstein, 1980). These very active congeners are the non
ortho-substituted PCBs 3,4,3',4'- tetrachloro-, 3,4,5,3',4'-
pentachloro-, and 3,4,5,3',4',5'-hexachlorobiphenyl, which are at
least twice substituted in the meta and para positions.
In this model, the inducer-receptor complex is translocated into the
nucleus, interacts with DNA, and eventually triggers the pleiotropic
responses observed. The role of the receptor protein in the mechanism
of action of PCBs is further substantiated by the differential effects
of the congeners in non-responsive DBA/2J mice and responsive C57Bl/6J
mice. Furthermore, there is a good relationship between the aryl
hydrocarbon hydroxylase and ethoxyresorufin O-deethylase induction
potencies in rat hepatoma H-4-II-E cells in vitro (Sawyer & Safe,
1982) and their relative binding affinities for the male Wistar rat
hepatic cytosol receptor protein (Bandiera et al., 1982; Safe et al.,
1985b).
Thus, the coplanar PCBs have mechanisms of action similar to those of
the polychlorinated dioxins (PCDDs) and dibenzofurans (PCDFs) (see
also WHO, 1989).
On the basis of the comparative toxic and biochemical potencies of
coplanar and mono ortho coplanar PCBs, Safe (1990) suggested toxic
equivalent factors TEFs (relating to 2,3,7,8-tetrachloro-dibenzo-
p-dioxin, TCDD, see WHO/EURO, 1987) for these compounds. See Table
53. Although there are certain limitations and uncertainties
associated with the use of TEFs (WHO/EURO, 1987), they may be useful
for an attempt to assess the risk of the combined exposure to coplanar
PCBs and PCDDs/PCDFs.
Table 53. Proposed Toxic Equivalent Factor (TEF) values for the
coplanar and mono ortho coplanar PCB congenersa
Congener TEF value Relative potency range
1. Coplanar PCBs
3,4,5,3',4'-PeCB 0.1 0.3-0.0006
3,4,5,3',4',5'-HxCB 0.05 0.1-0.0012
3,4,3',4',-TCB 0.01 0.009-0.00008
2. Mono ortho coplanar
2,4,5,3',4'-PeCB 0.001 0.0004-0.000006
2,3,4,3',4'-PeCB 0.001 0.0008-0.00006
3,4,5,2',4'-PeCB 0.001 0.00013-0.000018
2,3,4,5,4'-PeCB 0.001 0.00045-0.000074
2,3,4,3',4',5'-HxCB 0.001 0.0005-0.0000014
2,3,4,5,3',4'-HxCB 0.001 0.0004-0.0000065
2,4,5,3',4',5'-HxCB 0.001 0.0000055
2,3,4,5,3',4',5'-HpCB 0.001 no data available
a From: Safe (1990).
The frequently occurring mixed type, the PB-type, and the
weak/noninducing type congeners with some degree of ortho
substitution may also exert other more subtle toxic effects, either
directly via conversion to hydroxylated derivatives, or indirectly
through environmental transformations involving regiospecific
dechlorination followed by hydroxylation.
In addition, some PCBs, particularly the lower chlorinated ones, can
be more readily metabolized through arene oxide intermediates that may
be directly genotoxic/carcinogenic. Arene oxide intermediates are also
involved in the formation of the methylsulfonyl metabolites of PCBs,
which selectively accumulate in the Clara cells of the bronchiolar
epithelium and in the secretory contents of the bronchiolar lumen of
the lungs. This apparently also involves specific binding proteins
that may be important in the expression of certain types of pulmonary
toxicity.
Finally, there are other forms of toxicity associated with PCBs that
appear to involve certain non-receptor, protein-binding interactions.
8.7.3 Toxicity of impurities in commercial PCBs
In many toxicological studies on the effects of commercial PCB
mixtures, the quantitative contribution of impurities to the toxic
responses found is largely unknown (WHO, 1989; WHO/EURO, 1987).
9. EFFECTS ON HUMANS
The toxicological evaluation of PCBs presents many problems. PCBs
usually occur as mixtures of many congeners, and many of the data on
the toxicity of the PCBs are based on the testing of these mixtures.
Some components of the mixtures are more easily degraded in the
environment than others. Thus, the general population may be exposed
to mixtures that are different from those to which workers, working
with PCBs, are exposed.
There are great difficulties in assessing human health effects
separately for PCBs, since, quite frequently, PCDFs have been present
in the PCB mixtures to which humans have been exposed. The presence of
PCDDs has occasionally been seen in accidents with certain
PCB/chlorobenzene mixtures. Commercial PCBs have been shown to be
contaminated with PCDFs and, therefore, in many cases it is unclear
whether effects were attributable to the PCBs themselves or to the
much more toxic PCDFs.
Because PCBs are ubiquitous and very persistent in the environment,
humans have been, and will continue to be, exposed to them,
particularly in industrialized countries. PCBs may be inhaled in small
amounts through the air or ingested through food. People are primarily
exposed to PCBs by consuming fish from contaminated water, but they
can be also exposed via other food.
Furthermore, exposures have occurred through accidents and
occupational exposure; in the latter case, for example, during the
repair of transformers, capacitors, or during the handling of toxic
wastes.
Since PCBs are lipophilic, they are preferentially stored in adipose
tissue. They are also present, to a smaller extent, in serum, organs
and tissues, and human milk. The concentrations of PCBs in the
different organs depend on the lipid content of such organs, with the
exception of the brain, where the concentration is lower than the
lipid content would indicate. PCBs pass, to a certain extent,
(depending on chlorination and structure) through the placenta. They
are primarily excreted through the bile and milk. In addition to the
lipid content, the ratios between adipose tissue, blood, and organs
are influenced by exposure level, sex, age, duration of exposure, and
also by whether exposure is current (see section 5).
Since human milk is relatively easy to obtain, it has been used to
monitor human exposure. Results of the many monitoring studies carried
out in many countries all over the world have shown that average
levels of total PCBs are below 2 mg/kg milk fat, though women living
in heavily industrialized urban areas or who consume large quantities
of fish from heavily contaminated areas, may have higher levels.
9.1 General population exposure
The general population is exposed to PCBs primarily by the oral route,
e.g., by consumption of fish from contaminated waters. The monitoring
data on adipose tissues, blood, and breast milk indicate that PCBs are
absorbed via the gastrointestinal tract, but do not provide
information regarding the extent of the absorption.
9.1.1 Acute effects -- poisoning incidents
No data available.
9.1.2 Effects of short- and long-term exposure
9.1.2.1 Yusho and Yu-Cheng accidents
(a) Yusho accident
In June 1968, patients appeared at the Dermatology Clinic of Kyushu
University Hospital, Fukuoka, Japan, suffering from chloracne. A group
at the University undertook intensive clinical, chemical, and
epidemiological investigations and found that the disease originated
from the consumption of a batch of rice oil supplied in February 1968;
the disease was called Yusho (rice oil disease) (Katsuki, 1969). This
batch of rice oil was found to be contaminated with Kanechlor 400, a
48% chlorinated biphenyl, at 2000-3000 mg/kg, which entered the oil
through a leak in a heat exchanger (Tsukamoto, 1969). Chlorinated
dibenzofurans at 5 mg/kg were found in 3 samples of the toxic rice oil
that contained PCB levels of about 1000 mg/kg (Nagayama et al., 1976).
The Japanese literature on this incident has been summarized in
English by Kuratsune et al. (1976). The average estimated intake was
633 mg PCBs, 3.4 mg PCDFs, and 596 mg PCQs, roughly equivalent to
157 µg PCBs/kg per day, 0.9 µg PCDFs/kg per day, and 148 µg PCQs/kg
body weight per day (Chen et al., 1985; Masuda et al., 1985).
The symptoms and signs of Yusho were described by Goto & Higuchi
(1969) and by Okumura & Katsuki (1969). The earliest signs were
enlargement and hypersecretion of the Meibomian glands of the eyes,
swelling of the eyelids, and pigmentation of the nails and mucous
membranes, occasionally associated with fatigue, nausea, and vomiting.
This was usually followed by hyperkeratosis and darkening of the skin
with follicular enlargement and acneiform eruptions, frequently with a
secondary staphylococcal infection. These skin changes were most often
seen on the neck and upper chest, but, in severe cases, extended to
the whole body. It was estimated that the mean length of the latency
period between exposure and the onset of clinical illness was 71 days,
with a range of from 20 to 190 days (Kuratsune et al., 1972).
Biopsy skin samples showed hyperkeratosis, dilation of the follicles,
and an accumulation of melanin in the basal cells of the epidermis;
melanin granules have also been observed in biopsy samples of the
conjunctiva. Oedema of the arms and legs was also seen in some
patients. There were no definite signs of liver enlargement or liver
disorders (Okumura & Katsuki, 1969), but slight rises in serum
transaminases and in alkaline phosphatase were detected, and a liver
sample from a Yusho patient showed an increase in the smooth
endoplasmic reticulum (Hirayama et al., 1969). The majority of the
patients were found to have respiratory symptoms, and suffered from a
chronic bronchitis-like disturbance that persisted for several years
(Shigematsu et al., 1971, 1978).
Kikuchi (1984) described the autopsy findings, up to July 1982, of 12
patients with Yusho including 2 stillborn babies. Characteristic
pathological changes were acne-like eruptions and cutaneous
pigmentation with histological features of follicular hyperkeratosis,
dilated hair follicles, and an increase of melanin pigment in the
epidermis. In addition, multiplication of the duct epithelium of the
oesophageal glands was found in 6 patients. Twenty-four Yusho patients
were observed clinically over the period 1968-78. During this decade,
the various clinical symptoms of the Yusho patients gradually
diminished. However, some of the symptoms and signs, such as
pigmentation of the skin, conjunctiva, and gingiva, eye discharge, and
various non-specific symptoms still remained in a number of severely
ill patients (Okumura, 1984).
Nakanishi et al. (1985) carried out clinical and experimental studies
on respiratory involvement and alterations in the immune status. PCBs
were not taken up by the bronchi, but were evenly distributed
throughout the lung parenchyma. However, specific dose dependence and
structural requirements of PCBs were shown to exist for accumulation
in bronchial mucosa. A large amount of expectoration at an early stage
of the disease may be related to this. Pathophysiological findings in
Yusho patients revealed that respiratory involvement was mainly small
airways disease, which may be caused by involvement of the cellular
component (Clara cells) in the bronchioles and/or associated with
viral or bacterial infections.
Changes in the immune status in these Yusho patients were decreases in
IgA and IgM in the serum at an early stage of the disease and then a
return to normal and suppression of cellular immunity. The changes in
immune status in these Japanese patients were comparable with the
findings in the Taiwanese patients (see below).
Hirayama et al. (1974) also reported that the serum bilirubin levels
of patients were significantly lower than the normal level and that it
was negatively correlated with the blood level of PCBs and the serum
triglyceride level.
A considerable number of patients had elevated serum triglyceride
levels, up to 4 times the normal values, though this was not
correlated with the severity of the symptoms; these high values were
maintained for 3 years in many patients (Uzawa et al., 1972). There
were no marked abnormalities in serum cholesterol and phospholipid
levels (Okumura & Katsuki, 1969; Uzawa et al., 1969). Nagai et al.
(1969) reported an increase in urinary 17-ketosteroids excretion.
Kusuda (1971) also observed changes in the menstrual cycle in
approximately 60% of 81 female Yusho patients, when compared with
their cycles prior to exposure. A positive correlation was observed by
Okumura et al. (1974) between the blood levels of triglycerides and
PCBs in 42 patients.
Shigematsu et al. (1971) examined serum immunoglobulin levels in 38
patients, 2 years after the onset of the disease, and observed a
decrease in IgA and IgM and an increase in IgG. Lower IgM levels were
reported in patients showing chloracne (Saito et al., 1972) (see also
section 9.2.4.2).
Yusho patients did not appear to suffer from central nervous effects,
but some complained of numbness of the arms and legs. Murai & Kuroiwa
(1971) found a decrease in the conduction velocity in peripheral
sensory nerves.
Determinations of PCB concentrations in the tissues of Yusho patients
were made several months after the ingestion of the oil, using an
X-ray fluorescence method for organic chlorine (Goto & Higuchi, 1969).
Abdominal fat contained 13.1 mg/kg, subcutaneous fat 75.5 mg/kg, and
nails 59 mg/kg. The mesenteric adipose tissue in 6 Yusho patients,
analysed by gas-liquid chromatography 1-3 years after the occurrence
of intoxication, contained an average PCB level of 2.5 mg/kg, which
was considerably higher than the normal value (Masuda et al., 1974a).
The mean blood level of PCBs in patients was 6 or 7 µg/litre
(3 µg/litre for the general population), 5 years after exposure
(Masuda et al., 1974b; Takamatsu et al., 1974). These authors also
noted a specific gas-liquid chromatographic pattern that was peculiar
to Yusho patients.
Eleven years after exposure, a mean concentrations of 6 µg PCB/litre
and 2 mg PCQs/litre, but no PCDFs, were found (Kashimoto et al.,
1985).
Urabe (1974) reported that the total number of Yusho patients had
reached 1200 by September 1973 and that 22 of them had died. At the
end of 1982, the number of identified patients was 1788 (Urabe &
Asahi, 1985). Mucocutaneous signs had decreased year by year, but
neurological and respiratory signs and symptoms and various
complaints, such as general fatigue, anorexia, abdominal pain, and
headache, had become more prominent among the patients. Over time, the
severity and the extent of the skin lesions decreased considerably in
the exposed population. Fifteen years after the accident, only a few
patients still had extensive chloracne.
By 1979, 31 Yusho patients had died, 11 (35.4%) of these from
malignant neoplasms. Only 21.1% of all deaths in this Japanese
prefecture would be expected from malignant neoplasms, but no clear
correlation between the occurrence of Yusho and increased deaths from
malignant neoplasms could be made, because of the small number of
deaths observed and the unknown latency period.
By the end of 1983, 120 Yusho patients had died, 41 of these from
malignant neoplasms. These included 8 stomach cancers, 11 liver
cancers, and 8 neoplasms of the lung. A statistically significant
excess mortality was seen for malignant neoplasms, cancer of the liver
and cancer of the lung, trachea, and bronchi in males, but no such
excess was noted in females. The excess from liver cancer deaths was
seen mainly in Fukuoka prefecture, while no excess was seen in the
Nagasaki prefecture (Ikeda et al., 1987).
(b) Yu-Cheng accident
In 1979, a similar incident occurred in Taiwan, the number of persons
involved, by the end of 1980, was 1843. In the course of 3.5 years,
2061 persons were determined to be victims of PCB poisoning. The
incident has been referred to as Yu-Cheng (Chang et al. 1980a,b, 1981;
Chen et al., 1980, 1981; Hsu et al., 1985). The affected persons had
consumed rice-bran oil contaminated with PCBs that was used as a heat
transfer medium in the manufacture of the oil. The PCB intake was
estimated to be 0.7-1.84 g and the latent period from the time of
intake to the onset of clinical manifestations was approximately 3-4
months. The average estimated PCDFs intake was 3.8 mg and 586 mg PCQs
(Chen et al., 1985a). Blood PCB levels ranged from 3 to 1156 µg/litre:
44.3% of 613 patients had levels of 51-100 µg/litre, and 27.6%, blood
levels over 100 µg/litre. Six months after the exposure, the
concentrations of PCBs, PCDFs, and PCQs were 12-50, 0.062-0.24, and
1.7-11 µg/litre. These blood levels were much higher than in the Yusho
incident.
The concentrations of PCBs, PCDFs, and PCQs in 6 samples of rice-bran
oil were 53-99 mg/kg, 0.18-0.40 mg/kg, and 25-53 mg/kg oil,
respectively (Chen et al., 1981; Hsu et al., 1985; Chen et al.,
1985a). Miyata et al. (1985) found averages of 62 mg PCB/kg, 140 µg
PCDFs/kg, and 20 mg PCQs/kg in 5 samples of oil. The levels of toxic
compounds in rice-oil samples collected from the factory and school
cafeterias and the families of the intoxicated patients in Taiwan were
in the range of 53-99 mg PCBs/litre (except for one sample with
405 mg/litre), 0.18-0.40 mg PCDFs/litre, and 25-53 mg PCQs/litre,
respectively. The most toxic PCB reported in commercial PCB
preparations was 3,4,3',4'-tetrachlorobiphenyl (Chen et al., 1984).
One hundred and thirty patients (46 males and 84 females), exposed
accidentally to PCBs in Taiwan, were examined for ocular
manifestations in 1979-80. Eye discharge was present in 80.5%,
swelling of the upper lids in 60.4%, pigmentation of conjunctiva in
67.6%, hypersecretion and cystic swelling of the Meibomian glands in
70.7%. Heavy pigmentation of conjunctiva, abnormal cystic formation
and hypersecretion of the Meibomian glands occurred in patients whose
blood PCBs concentration was above 40 µg/litre. There was a
correlation of the ocular effects and the blood concentration of PCBs
(Fu, 1984).
Wong et al. (1985), determined the enzyme activity in placental
tissue, obtained from 4 women who were exposed to contaminated
rice-oil in Yu-Cheng 3-4 years before conception. Placental
homogenates showed increases in monooxygenase enzymes, including aryl
hydrocarbon hydroxylase, 7-ethoxycoumarin O-deethylase, and diol,
quinone and phenolic metabolites of benzo (a)pyrene.
Lu & Wong (1984) described, in detail, the dermatological, medical,
and laboratory findings on 829 patients (half males and half females)
in Taiwan, poisoned with PCBs and related compounds. The ages of the
patients ranged from 7 days to 78 years. A grading of the clinical
severity of these cases was tried, and a possible association with the
PCB concentrations in their blood was examined, but could not be
demonstrated. The mean PCB concentration in 278 patients was 89.1 ±
0.9 µg/litre (median value 55 µg/litre); the maximum level was
1156 µg/litre and the minimum, 3 µg/litre.
One hundred and ten patients were studied within one year of the
exposure. The mean blood PCB level was 39.3 ± 16.6 µg/litre, and the
mean blood PCDFs and PCQs levels were 0.076 ± 0.038 and 8.6 ±
4.8 µg/litre, respectively. Both the sensory and motor nerve
conduction velocities of the patients were significantly lower than
those of the controls (cases studied in the past who did not have
neurological diseases) (Chen et al., 1985b).
Thirty-five patients out of 2000 cases of PCB poisoning in Taiwan were
examined neurologically 2 years after the accident. The neurological
manifestations included clinical, peripheral sensory neuropathy,
headache, and dizziness. There was no relationship between the blood
PCB concentrations in patients with neurological manifestations and
those without. Sensory nerve conduction velocity was reduced and motor
nerve conduction was delayed in about one-third to one-half of the
patients (Chia & Chu, 1984).
The blood samples of 165 patients, collected 9-18 months after the
onset of poisoning, contained 10-720 µg PCBs/litre with a mean value
of 38 µg/litre (Chen et al., 1984).
It is worth noting that the highly toxic 2,3,7,8-tetrachloro-,
2,3,4,7,8-pentachloro-, and 1,2,3,4,7,8-hexachlorodibenzofuran isomers
were present in samples from both the Japanese (Yusho) and the
Taiwanese (Yu-Cheng) incidents.
The most common symptoms noticed were acneiform eruptions and
follicular accentuation, skin and nail pigmentation, swelling of the
eyelids and increased discharge from the eyes; headache, nausea, and
numbness of the limbs. The major blood disorders were decreased
erythrocyte counts, haemoglobin concentration, and gamma-immunoglobin,
and increased white blood cell counts, serum triglyceride levels, and
SGOT, SGPT, and serum alkaline phosphatase activities. Decreased
concentrations of delta-aminolaevulinic acid and uroporphyrin were
also observed (Chang et al., 1980a,b).
9.1.2.2 Effects of PCBs on babies and infants
Yoshimura (1971) reported diminished growth in boys, but not in girls,
who had consumed the oil. Babies born to Yusho mothers were smaller
than normal. Newborn babies showed a dark brown skin pigmentation that
disappeared after a few months (Taki et al., 1969; Yagamuchi et al.,
1971). Funatsu et al. (1972) found spotted and sporadic ossification
of the skull and facial oedema with exophthalmia in 4 babies, but
there was no evidence of any teratogenic action. Pregnant Yusho
mothers delivered babies with a peculiar clinical manifestation, which
was called Fetal PCB Syndrome (FPS). In total, 36 babies showed this
syndrome. It consisted of dark brown pigmentation of the skin and
mucous membranes, gingival hyperplasia, exophthalmic oedematous eye,
dentition at birth, abnormal calcification of the skull (as
demonstrated by X-ray), rocker bottom heel, and a high incidence of
low birth weight babies. It was suggested by the authors that a
possible alteration in calcium metabolism in FPS might be related to
the action of PCBs (PCDFs) on female hormones. There was no evidence
of hypoadrenocorticism which would explain dark pigmentation in FPS
children (Yamashita & Hayashi, 1985).
Jensen (1983b) calculated that the daily intake of PCBs by Yusho
infants with clinical symptoms of poisoning was of the order of
70 µg/kg body weight.
Kuratsune et al. (1972) investigated whether Yusho disturbed
children's growth. The affected school children, 23 boys and 19 girls,
were compared in 1967, 1968, and 1969, with 719 healthy classmates
matched by sex. The gains of the affected boys in both height and
weight decreased significantly after the poisoning, while the affected
girls did not show any changes in these respects.
Studies were carried out by Fein et al. (1984), Fein (1984), and
Jacobson et al. (1984a,b) on 242 newborn infants whose mothers had
consumed moderate quantities of contaminated lake fish and 71 newborn
infants whose mothers had not eaten such fish, during the immediate
postpartum period. PCB exposure (measured by both contaminated fish
consumption and cord serum PCB levels) predicted lower birth weight,
shorter length of gestation, and smaller head circumference. Both
maternal consumption of fish and levels of PCBs in cord serum were
positively correlated with lower birth weight, shorter gestation and
smaller head circumference, and with impaired autonomic maturity and
increased numbers of abnormal reflexes.
In the studies by Schwartz et al. (1983), Fein et al. (1984), and
Jacobson et al. (1984a), the influence of important variables, such as
smoking and alcohol use, were not studied extensively enough. The
Brazelton test (Brazelton, 1973) was used in these studies. However,
this test was never intended to be used to evaluate neurological
conditions (Prechtl, 1982). The value of this test to predict
behavioural abnormalities in infants is small. The Public Health
Council of the Netherlands (1985) concluded, therefore, that the
reported changes could not be interpreted by the Brazelton test. The
important confounding factors "smoking" and "alcohol" were not studied
or not well studied, while it is known that these factors can result
in such changes. Furthermore, there was an indication that women
consuming more fish also consumed more alcohol and coffee and used
more medical drugs than those who were not fish eaters.
Jacobson et al. (1985) examined visual recognition memory in
7-month-old infants of women who had consumed contaminated Lake
Michigan fish. The authors reported a statistically significant
correlation between cord serum PCB levels and impairment of visual
recognition memory. It should be mentioned, however, that
interpretation of these test results is difficult. In view of the
variability associated with the measurements of fixation time (no
standard deviations were reported), it is unclear whether any of the
group means are statistically different. Moreover, the clinical
meaning of the differences noted is not known.
Neonatal effects of transplacental exposure to PCBs (and DDE) were
examined in a study on 912 children born between 1978 and 1982 in
North Carolina. When the infants were born, samples of placenta,
maternal and cord serum, and milk/colostrum were collected. Physical
examination of each infant was performed and the Brazelton test
(Brazelton, 1973) was applied. Fifty-nine per cent of the examinations
were carried out in the first week, 20% in the second week, and 16% in
the third week. The PCB levels in milk fat at birth (866 samples)
ranged from nd to 4.0 mg/kg. There was no association between PCB
levels and birth weight, head circumference, and hyperbilirubinaemia
(neonatal jaundice). For the Brazelton test, the only cluster scores
to be significantly affected by PCBs were the tonicity and reflex
scores, with higher PCB levels (above 3.5 mg/kg milk fat). The results
showed that higher PCB levels were associated with hypotonicity and
hyporeflexia while higher DDE levels (4 mg/kg milk fat) were
associated with hyporeflexia (DDE concentrations in milk fat ranged
from nd to >6 mg/kg milk fat) (Rogan et al., 1986a). As a follow-up
study, Rogan et al. (1987) followed 858 children in the USA, from
birth to one year of age, to determine whether the presence of PCBs in
breast milk affected their growth or health. The PCB concentrations in
breast milk ranged from 0.49 to 15.80 mg/kg, on a fat basis, and the
DDE concentrations from 0.31 to 23.8 mg/kg milk fat. The lactation
period varied from 13 (mothers with 4.00-15.80 mg PCBs/kg in their
milk) to 26 weeks. No adverse effects on body weight or the frequency
of visits for various illnesses were observed. There was no difference
between bottle-fed and breast-fed children. In 1985, about 6 years
after the mass poisoning in Taiwan, 117 children born to affected
women ( in utero exposure during, or after, the period of oil
contamination) and 108 unexposed controls were examined (Rogan et al.,
1988). The exposed children were shorter and lighter than the
controls; they had more frequent abnormalities of the gingiva, skin,
nails, teeth, and lungs than control children. The exposed children
showed delay in developmental milestones, deficits on formal
development testing, and abnormalities in behavioural assessment.
A follow-up study was carried out to determine the relationship
between PCBs in mother's serum and breast milk and the health and
development of the born infants, in Sheboygan, Wisconsin, USA, in the
period 1980-81. Seventy-three mothers gave birth to 62 infants that
were breast-fed and 11 that were bottle-fed. The ages of the mothers
ranged from 18 to 36 years. The mean serum PCB level for the study
population was 5.76 µg/litre (range, 1.29-14.9 µg/litre). Breast milk
contained a mean PCB level of 1.13 mg/kg (range, 0.29-4.02 mg/kg) on a
fat basis. The mother's blood serum PCB level during pregnancy was
positively associated with the number and type of infectious illnesses
the infants suffered later, such as colds, earache, and influenza
during the first 4 months of life. The development and growth of the
infant up to the age of 4 months was normal and was not affected by
PCB levels (Smith, 1984).
Lan et al. (1989) selected 18 exposed children (9 males and 9
females), and a reference group of 44 unexposed children (26 males and
18 females), to study the congenital absence of permanent teeth. Among
9 transplacental Yu-Cheng girls and 9 boys, the permanent teeth germ
was missing due to congenital factors in 4 girls and one boy. In the
control group, one boy showed this phenomenon. Fukuyama et al. cf. Lan
et al. (1989) had already reported this effect in 1979.
Gladen et al. (1988a) investigated whether PCBs, either transplacental
or through breast feeding, affected the scores on the Bayley Scales of
infant development at 6 or 12 months of age, in 802 infants. Higher
transplacental exposure to PCBs was associated with lower psychomotor
scores at both 6 and 12 months of age. Exposure to PCBs through
breast-feeding was apparently unrelated to Bayley scores.
The urine of 75 children born to mothers exposed to contaminated rice
oil in Taiwan (1979), 74 controls, and 12 siblings of the children
exposed between 1978 and 1985, was analysed for the presence of
porphyrins. Total porphyrin excretion was elevated in the exposed
children in comparison with the other 2 groups (exposed group,
95.2 µg/litre, control group, 80.7 µg/litre, and siblings,
72.6 µg/litre. The exposed children did not appear to have symptoms
directly attributable to their porphyria, but the authors concluded
that a mild disturbance in their porphyria metabolism appeared to be
related to their intrauterine exposure (Gladen et al. 1988b).
Thirty-nine babies showing hyperpigmentation were born to PCB-poisoned
mothers. In the orally exposed population of the Yu-Cheng episode, 24
deaths were reported and as many as 12 cases of hepatic disease
including hepatomas, which was more than expected (Hsu et al., 1985).
9.1.3 Appraisal
Several Japanese research groups have concluded that the main signs
and symptoms involved in the Yusho intoxications were caused by
contaminants in the PCB-mixture, i.e., mainly PCDFs (Masuda et al.,
1985; Kunita et al., 1985). This conclusion has mainly been based on
the following observations:
(a) Blood levels of PCBs in the victims were not very different from
those in the general population and several occupationally-exposed
groups had higher PCB blood levels in the absence of any recognizable
adverse health effects.
(b) There was an unusually high level of PCDF-contamination in the
PCBs that contaminated the rice oil.
(c) Signs and symptoms did agree with what could be expected from
exposure to PCBs and/or PCDFs (PCDDs).
However, blood levels in the Yusho victims were determined 5 years
after exposure. Consequently, at the time of the intoxication, blood
levels might have been much higher.
Furthermore, later studies on the biological potency of the
dioxin-like coplanar PCBs indicated that the occurrence of these might
have added significantly to the overall toxicity of the PCDFs (Safe,
1990).
In the case of the Yu-Cheng intoxication, blood levels of PCBs were
determined within 1 year of the accident and were found to be much
higher (i.e., about 70 µg/litre) than in the Yusho intoxication.
However, even at this time, some elimination of PCBs, especially lower
chlorinated PCB congeners, can be assumed to have taken place. Thus,
blood levels in the Yu-Cheng intoxication might also have been higher
initially.
In summary, it can be concluded that the main symptoms of the Yusho
and Yu-Cheng intoxications might have been caused mainly by combined
exposure to PCBs (mainly the coplanar ones) and PCDFs. However, some
of the symptoms, especially the chronic respiratory effects, may have
been caused specifically by the methylsulfone metabolites of certain
PCB congeners.
9.2 Occupational exposure
9.2.1 Acute toxicity -- poisoning incidents
9.2.1.1 Acute dermal effects
Skin rash has occurred within a few hours after acute exposure to
PCBs. Furthermore, itching, burning sensations, smarting, and sweating
have been reported. Irritation of the conjunctiva was a constant
symptom in acute exposure to high concentrations (Elo et al., 1985;
Schecter & Tiernan, 1985). A few weeks or months after acute exposure
(lasting a few hours) to high concentrations of PCBs (10-16 mg/m3),
several skin symptoms were observed in some of the accidentally
exposed population, such as slight pigmentation, ridges on the nails,
and the worsening of ache vulgaris (Elo et al., 1985). Skin wipe tests
on PCB-exposed workers were carded out by Maroni et al. (1981a), Smith
et al. (1982), and Wolff (1984) for capacitor manufacturers,
electrical equipment manufacturers, and transformer inspectors.
Concentrations varying between 2 and 28 000 µg/m2 of skin were
measured. Considerable concentrations of PCBs were also found on the
surfaces of hand tools in the factories (Maroni et al., 1981a;
WHO/EURO, 1987).
9.2.2 Effects of short- and long-term exposure
Exposure through ingestion is possible in the working environment
through the direct ingestion of soot particles or through the
contamination of cigarettes or food by hands. Maroni et al. (1981a)
measured high concentrations of PCBs in the palmar skin of capacitor
workers; this might lead to the oral ingestion of PCBs, in addition to
exposure via the dermal route.
Skin exposure is important in the case of long-term exposure, even
though the ambient concentrations may be low. According to
calculations made by Wolff (1985), in long-term exposure situations,
skin may be responsible for up to 20% of the total body uptake of PCBs
in workers exposed in capacitor manufacturing (WHO/EURO, 1987).
Symptoms similar to those of Yusho have been observed in workers in a
Japanese condenser factory, including pigmentation of the fingers and
nails, and acneiform eruptions on the jaw, hack, and thighs. It was
thought that these effects arose from local contact with PCBs; when
the use of PCBs ceased, the symptoms disappeared (Hasegawa et al.,
1972b). Chloracne is one of the most prevalent findings among
PCB-exposed workers and particularly among those exposed to highly
chlorinated compounds. Hara (1985) found prevalences of comedones and
acne of 40%, and skin irritation and erythema of 13% in workers
exposed for 1-24 years to Kanechlor 300 and 500. The blood PCB levels
were 21-117 µg/litre. The degree of skin pathology was correlated with
the blood PCB concentrations. In the production of capacitors,
(oculo-) dermatological abnormalities were found in 37% of the cases,
but typical PCB-associated changes were less prevalent. Fischbein et
al. (1979) suggested that these signs and symptoms were due to
exposure to PCDFs and/or PCDDs.
Fischbein et al. (1982) evaluated the dermatological effects of
long-term (< 5 - > 20 years) occupational exposure to PCBs, in a
cross-sectional clinical survey of 326 capacitor manufacturing
workers. Air PCB levels varied in the plant from 0.007 to 11.0 mg/m3.
A high prevalence (37%) of a wide spectrum of dermatological
abnormalities was found, such as rashes, burning sensation of the
skin, and chloracne, in most cases associated with typical comedones
(6%), but the occurrence was less than that observed in Yusho
patients, even though the serum PCB levels in the workers were much
higher.
Two persons were reported with dermatological abnormalities suggestive
of, but not specific for, chloracne, after occupational exposure to
PCBs (Fischbein & Wolff, 1987). They had raised serum PCB
concentrations (of the order of 80-100 µg/litre). Their wives also had
increased blood PCB levels, with the same PCB pattern as their
husbands. It was suggested by the authors that it would seem prudent
to take appropriate industrial hygiene measures, to prevent the
transmission of PCBs from the occupational environment into the home.
A cross-sectional study on 120 male workers was conducted to determine
the prevalence of increased PCB absorption, as well as the presence of
potentially-related clinical and metabolic abnormalities. Three groups
were used: an exposed group (86), a nominally exposed (15), and an
unexposed group (19 subjects). The exposed group had direct contact
with PCB-containing transformer fluids, while the nominally exposed
group worked in the same facility, but without direct contact, and the
unexposed group was employed elsewhere. The average length of
employment was 17 years (range, few months-40 years), 3 years and 9
months, and 4 years and 3 months, for the 3 groups, respectively. The
average plasma PCB levels were 33.4, 14.2, and 12.0 µg/litre, and the
average concentrations in adipose tissue were 5.6, 1.4, and 1.3 mg/kg,
respectively. There were no statistically significant differences
among the groups in levels of triglycerides, cholesterol, high-density
lipoproteins, and SGOT. A significant correlation was demonstrated
between plasma PCBs and triglycerides and SGOT values, but not SGPT
and gamma-GTP values (Chase et al., 1982).
To investigate the prevalence of oculo-dermatological findings, such
as hypersecretion of the Meibomian glands, swelling of the upper
eyelids, and hyperpigmentation of the conjunctiva (typical Yusho
symptoms), in a population with long-term occupational exposure to
PCBs, a group of 246 workers employed in 2 capacitor manufacturing
facilities were studied in 1976, and 181 of these workers, again in
1979. The median plasma values of lower chlorinated PCBs were
63 µg/litre in 1976 and 49 µg/litre in 1979. For the higher
chlorinated PCBs, these values were 18 and 17.5 µg/litre,
respectively. The prevalences of oculo-dermatological findings,
potentially related to the effects of PCBs, were 9.4 and 13.3%, at the
two examinations. There was no significant association between such
abnormalities and blood plasma/serum concentrations of PCBs (Fischbein
et al., 1985).
Lees et al. (1987) studied the hypothesis that the dermal route of PCB
exposure is a major contributor to the total body burden of PCBs in
workers. The investigation was conducted simultaneously with a
clinical study on switchgear workers engaged in transformer
maintenance and repair operations. The geometric means in the serum
and adipose tissue of exposed workers, previously exposed workers, and
comparison group were, respectively: (serum) 12.2, 5.9, and
4.6 µg/litre, (adipose tissue) 2.1, 0.83, and 0.6 mg/kg. The geometric
mean 8-h TWA concentrations in the different work areas (55 samples)
ranged from 0.5 to 6.1 µg/m3. The geometric mean surface
concentrations (102 samples) ranged from 0.007 to 1.075 µg/m2. From
the available data, it was calculated that exposure by the dermal
route (i.e., skin absorption) was considerable in comparison with
respiratory exposure. The daily calculated total dose through
inhalation ranged from 4.0 to 48.8 µg in the different work areas and
via the dermal route, from 1.2 to 215.0 µg. It was considered though
not conclusively, that the dermal and dermal/oral routes of exposure
are the predominant contributors to body burden.
Shalat et al. (1989) reported 3 cases of kidney adenocarcinomas among
young male utility workers who were responsible for maintaining
electrical transmission equipment, including transformers. The
duration of exposure ranged from 5 to 35 years.
The occurrence of chloracne and abnormal hepatic function as a result
of occupational exposure to PCBs had already been reported by Jones &
Alden (1936), Schwartz (1943), and Meigs et al. (1954).
Effects, such as chloracne, skin rashes, and burning eyes and skin,
have been associated with occupational exposure to Aroclors and
Kanechlors (Ouw et al., 1976; NIOSH, 1977; Fischbein et al., 1979,
1982, 1985; Baker et al., 1980; Drill et al., 1981; Kimbrough, 1987;
US EPA, 1987). In these studies, monitoring data did not adequately
characterize exposure levels, consequently correlations between the
occurrence of skin lesions and the duration of exposure, or blood
concentrations of the PCBs, are poor or nonexistent. Furthermore, the
contamination of the PCBs with PCDFs and PCQs may be partly the cause
of these skin changes.
Other effects reported in human exposure have been considered in a
criteria document for recommended standards for occupational exposure
to PCBs (NIOSH, 1977) and include several instances of chloracne that
resulted from exposure to PCB vapours in various work situations.
Other symptoms noted were sore throat, gastrointestinal disorders, and
eye disturbances.
Fischbein et al. (1979) examined 168 male and 158 female workers at a
capacitor plant, where they were exposed for <5 up to 25 years to
Aroclors 1254, 1242, 1016, and 1221. TWAs for 8 h with ranges of
0.07-0.40, 0.40-0.60, and 0.60-11.0 mg/m3 were considered low,
medium, and high, respectively. Among work-related symptoms they found
upper respiratory irritation and decreased rectal capacity,
gastrointestinal, neurological, and dermatological symptoms. The
dermatological symptoms occurred among 45% of the males and 55% of the
females and are comparable with the symptoms found in Yusho victims.
There was a significant correlation between plasma PCB levels and SGOT
levels, though changes in most liver tests were not prevalent.
Maroni et al. (1981b) reported blood PCB concentrations of
41-1319 µg/litre in 80 electrical workers (half males, half females)
employed in electric capacitor manufacture and testing plants.
Sixty-seven persons were exposed to Pyralone 3010 and 13, to Apirolio
(both PCBs containing 42% of chlorine). The mean age of the workers
was 37 ± 8 years, and the mean duration of employment was 12 ± 6
years. There were 6 cases of chloracne among the 80 workers. Sixteen
of the males had liver abnormalities, including hepatomegaly and
increased serum enzymatic activities; for 20% of these, the PCB
concentration in the blood was < 200 µg/litre. The females included 2
cases of bleeding haemangioma, one of whom also had chronic myelocytic
leukaemia, but none of the females had liver abnormalities.
Ouw et al. (1976) studied 34 electrical industry workers exposed to
0.32-2.22 mg Aroclor 1242 (free from impurities)/m3 compared with 30
control workers. The electrical workers consisted of 15 males (6
months-23 years employment) and 19 females (1 month-7 years
employment). No clear indications of liver changes were found. Major
complaints were burning of the eyes, face, and skin. One worker had
chloracne without systemic effects and 5 workers had eczematous hand
and leg rashes. These dermatological effects occurred at an air
concentration of <1 mg PCBs/m3. There were no significant health
effects in workers at, or below, a blood PCB level of 200 µg/litre.
Drill et al. (1981) concluded that individuals with blood levels of
>200 µg/litre have an increased risk of chloracne and that
chloracne may occur more frequently in workers exposed to PCBs that
have been heated (presence of PCDFs) and to PCBs that have a >54%
of chlorine.
A study was conducted to determine whether exposure to fumes or oil at
the transformer incident site at New Mexico had caused illness.
Exposed persons of different disciplines and unexposed employees were
asked to complete a questionnaire. Eighty of the 101 persons with
known exposure completed the questionnaire. The most common symptoms
were: nausea (27.5%), eye irritation (22.5%), sore throat (21.2%),
nose irritation (18.8%), chest tightness (15.0%), and headache
(15.0%). The symptoms were transient and usually resolved as soon as
the person left the site. Fifty-six exposed persons submitted sera for
PCB analysis as did 20 controls. All but 4 persons had levels below
10 µg/litre. The medium for exposed persons was 4.1 µg/litre (range,
1.2-41.8 µg/litre compared with 2.4 µg/litre (range 0.9-8.0 µg/litre)
for the controls (Anon., 1985).
A follow-up study on capacitor-manufacturing workers, exposed to PCBs,
and their children, was conducted over the period 1973-79. The PCBs
that were used were Kanechlor 300 and 500. PCB levels in the blood (up
to 120 µg/litre), as well as in breast milk, were 10-100 times higher
than those of non-exposed Japanese persons. The levels in 8 women
ranged from less than 50 µg/litre to about 400 µg/litre in whole milk.
Blood PCB levels were correlated with the duration of PCB handling and
breast milk PCB levels. The blood PCB levels ranged from 18.7 to
117 µg/litre in this population, 1 year after the use of PCBs was
discontinued. The rate of decline of blood PCB levels, as well as the
changes in the gas chromatography of blood PCBs over 7 years varied
with the kind of PCB handled. The blood PCB levels tended to be higher
(<3 up to >10 µg/litre) in children fed PCB-contaminated breast milk
for a long period. The great majority of the workers had
dermatological complaints, but these symptoms gradually disappeared
with discontinuation of contact with PCBs. The blood chemistry of the
workers showed only a correlation between PCB blood levels and serum
triglycerides. Several of the children fed breast milk for a long
period, showed the same medical findings as in Yusho (itchy skin,
eczema, red eye, fever, catching cold, carious teeth). However, they
were not diagnosed as suffering from PCB-poisoning, because the
findings were neither serious nor related to the blood PCB levels
(Hara, 1985).
Workers occupationally exposed to Kanechlor 500 or 600 showed higher
PCB levels in plasma (2-251 µg/litre) than Japanese Yusho patients.
Gas chromatographic patterns of their PCBs corresponded to the
patterns of PCBs to which they were exposed, but, with time, the PCB
pattern in plasma is changing. PCQs could not be detected in the
plasma of the workers (Takamatsu et al., 1985).
Lawton et al. (1985) studied a group of 194 workers in capacitor
manufacturing, exposed to Aroclor 1016, 1242 and/or 1254 before (1976)
and after (1979). The use of PCBs in the operations was discontinued
in 1977. The geometric mean serum levels and 5-95% ranges were: lower
chlorinated PCBs, 363 µg/litre (57-2270 µg/litre) and 68 µg/litre
(12-392 µg/litre), higher chlorinated PCBs, 30 µg/litre
(6-142 µg/litre) and 19 µg/litre (4-108 µg/litre), respectively. The
statistical findings were a depression in serum bilirubin and
elevations in serum gamma-glutamyltranspeptidase (GGTP) and lymphocyte
levels, at the time of the first examination, and only an elevation of
monocytes at the second.
In 1982, a survey was conducted in an electrical capacitor factory in
the USA, using Aroclor 1242 from 1941 to 1971, with a change to
Aroclor 1016 from 1971 to 1977. Of approximately 500 current employees
(with an average of 12.9 years of employment) 205 took part in the
survey. The geometrical mean PCB value for serum was 18.2 µg/litre
(range, nd-424 µg/litre). Only 39% of the workers ever worked in areas
with potential PCB exposure. More than 70% had serum levels below
30 µg/litre. There were no indications of acute PCB-related clinical
effects. The workers' serum PCB levels were a function of duration of
employment, cumulative occupational exposure, cumulative fish
consumption, and cholesterol level (Acquavella et al., 1986).
The blood of women occupationally exposed to PCBs (Kanechlor 300 and
500) was analysed over the period 1975-79. Sixty-five samples were
taken from 29 mothers. The PCB concentrations varied between 6.4 and
52.6 µg/kg (mean concentration 32.3 µg/kg). Clinical symptoms observed
during the use of PCBs were minor in comparison with those of Yusho
patients (Yakushiji et al., 1984b).
Guo et al. (1987) studied the influence of serum cholesterol and
albumin on the partitioning of PCB congeners between human serum and
adipose tissue. Fifty-five repair workers, who were either currently
or had been previously exposed, and 56 comparison workers without
exposure to PCBs were used. Seven PCB congeners, which had been
quantified in both serum and adipose tissue in at least one-third of
the selected populations, were evaluated. The effects of serum
cholesterol in modifying the serum PCB concentrations are likely to be
apparent in groups exposed to PCBs containing higher chlorinated
congeners, such as Aroclors 1254 and 1260, rather than those
containing lower chlorinated congeners, such as Aroclors 1242 and
1221.
A selected group of 51 workers (25 males and 26 females) with a mean
length of exposure of 10 years (range 1-30 years) were compared with 2
groups consisting of 74 subjects (37 males and 37 females) and the
same reference group of 67 workers (30 males and 37 females) used in
another study, residing in the same areas, but without exposure to
PCBs. The PCB concentrations in the blood of 28 out of the 51 subjects
ranged from 88 to 1359 µg/litre. A statistically significant increase
was found in serum GGT activity, urinary D-glucaric acid (GLA), and
porphyrin excretion, when compared with the respective control groups.
Although the PCB workers had an average urinary excretion of
porphyrins almost twice as high as those of the control groups, no
dose-response relationship was found between urinary porphyrin
excretion and blood PCB concentrations (Maroni et al., 1984).
Steele et al. (1986) found a gradual decrease in the body burden of
the more highly chlorinated PCBs, as well as a more rapid decrease of
the less chlorinated congeners over the period 1977-84 in groups of 5
current and retired workers, in comparison with 6 subjects without
current exposure. The authors calculated the half-life for the lower
chlorinated PCBs to be 6-7 months, and, for the higher chlorinated
PCBs, 33-34 months. The evidence of different half-life estimates for
serum PCBs, depending on the degree of chlorination, is consistent
with the present knowledge of the pharmacokinetics.
Hola & Reznicek (1985) carried out a cytogenetic analysis of the
peripheral lymphocytes of 48 employees at a precoated gravel plant
using Delor 103 (a mixture of mainly trichlorobiphenyls) in comparison
with 24 workers not exposed and 13 workers exposed to PCBs during the
impregnation of condensers. The frequency of aberrant cells,
percentage of blastic transformation, mitotic index, and proliferation
index in peripheral lymphocytes, and a number of biochemical
parameters were determined. In the 48 workers at the precoated gravel
plant, there were 2.87% aberrations (PCB concentrations in plasma,
107 ± 104 µg/litre); 3.14% in the 13 PCB-exposed workers (PCB
concentration in plasma 308 ± 253 µg/litre), and 2.04% in 24
non-exposed workers; 1.50% of aberrations were found in 20 controls.
Emmett (1985) studied a total of 55 workers (currently exposed (38)
and 17 past transformer repair workers) in comparison with 56
unexposed workers. PCB exposures occurred from the air and
contaminated surfaces, predominantly to Aroclor 1260, but there was
some exposure to Aroclor 1242. There was widespread PCB contamination
of the workplace surfaces and the 8-h time-weighted average
concentration was between 0.7 and 24.0 µg/m3, depending on the task
of the worker. The bulk oils and air from the transformer were
analysed revealing that PCDFs (13-116 µg/kg) were present as well as
PCBs. In one of the samples, 2,3,7,8-TCDF was found at 31 µg/kg. Eye
irritation and tearing were more prevalent in the exposed group, but
the symptoms were mild and/or transient. Ocular symptoms were also
found, possibly caused by 1,1,1-trichloroethane and/or
trichlorobenzene. Chloracne was not found. Two exposed workers
reported a history of melanoma; none were reported in the control
group. However, the difference was not statistically significant.
Adipose tissue and serum PCB geometrical mean concentrations in
exposed workers were 2.1 mg and 12.2 µg/kg, respectively, those in
unexposed workers were 0.6 mg and 4.6 µg/kg, and those in previously
exposed workers, 0.83 mg and 5.9 µg/kg. No correlations were observed
between liver function tests and either adipose tissue or serum PCB
concentrations. A significant negative correlation was found, after
adjustment for confounding variables, between adipose tissue PCB
levels and 24-h urinary 17-hydroxycorticosteroid excretion and a
positive correlation between serum PCB levels and serum-GGT. No
correlation was found between adipose tissue PCB concentrations and
any serum lipid component (Emmett et al., 1988a,b).
Bercovici et al. (1983) collected blood from 17 women with recent
missed abortions, 7 women who had experienced one or more missed
abortions in the past, and 7 women with normal, second trimester
pregnancies, and estimated the serum levels of PCBs and other
organochlorine pesticides. The range of serum PCB levels in recent
missed abortions was 10.9-416.5 µg/litre, and that of the control
group, 12.2-40.0 µg/litre. Forty-seven per cent of the recent missed
abortion group had PCB levels of the same magnitude as the controls
(range, 10.90-42.8 µg/litre) and 53% had higher levels. In the former
missed abortion group, PCB levels in the range of 45.3-109.1 µg/litre
were found. The number of women examined in the different groups was
small. Furthermore, half of the women with missed abortions had PCB
levels in serum comparable with those of the controls. The authors
also did not control for many variables that could significantly
influence the incidence of abortions. It seems, therefore, that missed
abortions, either recent or in the past, may be associated with PCB
exposure.
PCB concentrations were determined in the blood from 10 women with
normal pregnancies (controls) and from 17 women with premature
deliveries. A significant difference was seen in blood serum
concentrations of PCBs between the women with normal and those with
premature deliveries. When the premature delivery group was split into
a high-serum- (8/17) and a low-serum-PCB group (9/17), the
high-serum-PCB group had a significantly higher serum PCB
concentration than the control group. The values were 128 mg/litre in
the high group, 19.3 µg/litre in the control group, and 21.4 µg/litre
in the low group. In the high-PCB, premature delivery group, the mean
serum concentration of tetrachloro-isomers was lower than that of the
control group (0.6 vs 1.86 µg/litre), while the mean serum
concentrations of pentachloro and hexachloro-isomers were higher
(78.2 vs 15.67 µg/litre and 48.9 vs 1.72 µg/litre, respectively)
(Wassermann et al., 1982). The indications that higher serum PCB
levels may be associated with an increase in incomplete abortions and
premature deliveries (Bercovici et al., 1983; Wassermann et al., 1982)
could not be established as a definitive causal relationship.
Taylor et al. (1984) studied the relation of Aroclor 1254, 1242,
and/or 1016 exposure to birth weight and gestational age in the
offspring of women working in 2 capacitor plants. Fifty-one infants,
born to 39 women employed at the 2 capacitor manufacturing facilities,
had a mean birth weight of 153 g less than 337 infants born to 280
women employed in areas of the facilities without direct exposure.
Mean gestational age in the first group was reduced by 6.6 days
compared with the latter group. It was concluded that the small
decrease in mean birth weight seemed likely to have resulted from a
shortening of the gestation period rather than a retardation of
intrauterine growth. Smoking and alcohol consumption by the mothers
were not considered, and whether the socio-economic status of this
group of women was similar to that of the control group is not clear.
In an update of this study, 200 women with direct exposure and 205
women without direct exposure, were used to study the relation of PCBs
to birth weight and gestational age in the offspring. The authors
concluded that these data indicated that there was a significant
relationship between an increased serum PCB level and decreased birth
weight and gestational age, and that the decrease in birth weight was,
at least partially, related to shortened gestational age (Taylor et
al., 1989).
9.2.3 Appraisal
A discussion on the occupational epidemiological data on the basis of
dose-response considerations needs an acceptable indicator of the
degree of exposure and, in the particular case of PCB mixtures, a
discussion on the nature of the congeners present. For most
epidemiological studies reported, there are some, albeit often
limited, data on levels of total PCBs in blood, which could be used as
indicators for PCB exposure. It is recognized that the analytical
procedures used are different. The blood of continuously exposed
workers will contain absolutely and relatively more of the lower
chlorinated congeners than that of human beings with background
exposure only, or with past exposures (e.g., Yusho and Yu-Cheng
populations). Consequently, the toxicological profile for these 2
types of exposure will differ (see also the appraisal on
Yusho/Yu-Cheng).
In some cases, the epidemiology of the continuously exposed workers
shows a possible association between elevated exposure to PCB mixtures
and the occurrence of liver enzyme alterations, hepatomegaly, and
dermatological abnormalities, such as rashes and acne. In many
studies, no associations were found. In some of these studies, limited
end-points were investigated. Within the positive studies, there is a
poor or non-existent correlation between the incidence and degree of
effects and blood concentrations. Among the positive studies, adverse
effects are predominantly reported in the studies with the higher
blood levels. Possible contamination of used PCBs and PCB mixtures
(particularly in transformers), with PCDFs and PCQs, may contribute
to, or even determine, the toxicity observed. The overall conclusion
is that continuous exposure to high concentrations of PCBs and PCDFs
may result in effects on the skin and liver.
9.2.4 Special studies (target organ effects)
9.2.4.1 Liver
The liver is considered to be one of the most important target organs
for PCB toxicity. Acute exposures to PCBs cause alterations in liver
enzyme activities. Smith et al. (1982) found a statistically
significant correlation between elevated liver enzyme activities and
blood PCB concentrations, in workers exposed to PCBs in electrical
equipment manufacturing or maintenance. A negative correlation was
found in relation to the HDL cholesterol concentration and serum PCBs.
Positive correlations were found between the liver enzyme activities
of serum alanine aminotransferase (S-ALAT), serum aspartate
aminotransferase (S-ASAT), serum gamma-glutamyltranspeptidase
(S-Gamma-GT) and SGOT, and blood PCB concentrations, among workers
exposed in an Italian capacitor factory. Hepatomegaly was also
detected in most of the cases studied by Maroni et al. (1981a,b).
Workers occupationally exposed to PCBs, but mostly also exposed to
PCDDs and/or PCDFs, showed significantly increased S-ASAT, S-ALAT, and
gamma-GT activities. Sometimes elevated serum triglyceride values were
also found. Recovery of these disturbances in liver function and
morphology requires several weeks or months (Elo et al., 1985;
Schecter et al., 1985). Fischbein (1985) reported the results of liver
function tests in a population engaged in the manufacture of
capacitors and transformers. A low prevalence of abnormal liver
function tests was found and mean values for all tests were within
normal ranges in 5 workers occupationally exposed to Aroclor 1016.
Plasma antipyrine half-life was significantly lower than in matched
controls, suggesting induction of hepatic mixed-function oxidases
(Alvares et al., 1977). At the initial examination (in 1976, when PCBs
were still being used), statistically significant correlations were
found between log LDH and plasma levels of log HPCB (higher PCB
congeners) and log TPCB (total PCBs) among female workers, while
log-gamma-GTP was significantly correlated only with log HPCB among
male workers. A significant increase to abnormal levels of gamma-GTP
was noted at the follow-up examination (1979, 2.5 years after the use
of PCBs had been discontinued) in both male and female workers, and
preliminary results indicated significant correlations between
gamma-GTP and serum levels of PCBs among male workers. In
occupationally exposed individuals, the serum or plasma PCB levels
were higher than those found in patients in the Yusho and Yu-Cheng
incidents. An effect transmitted via liver activity was hyperlipaemia,
in which triglycerides and, in some instances, also cholesterol levels
in the blood were elevated (Smith et al., 1982).
Hepatotoxicity is suggested in occupationally-exposed humans (Drill et
al., 1981; US EPA, 1987). Drill et al. (1981) concluded that SGOT
and/or GGPT appear to be the most sensitive indicators of PCB exposure
in humans, and that changes in liver enzymes occur at levels below
those at which chloracne occurs.
9.2.4.2 Immunotoxicity
Reports on the immunological effects of long-term, occupational
exposures are sparse. Fischbein et al. (1979) found a suggestive
increase in the occurrence of trivial infections in exposed workers.
Immunological responses were found to be affected in Yu-Cheng victims
(Lu & Wu, 1985) and in Yusho patients (Nakanishi et al., 1985). Acute
accidental exposures of workers to PCBs and PCDFs have been studied
for immunological responses and immunosuppressive changes have been
found. The most important alterations were decreased numbers of
T-lymphocytes and lowered T-helper/ T-suppressor cell ratios.
Responses of lymphocytes to phytohaemagglutinin, concavalin A, and
pokeweed mitogen were also lowered. The observed changes persisted for
6 months after the acute exposure. No quantitative changes were
observed for immunoglobulins (Elo et al., 1985; WHO/EURO, 1987, 1988).
Lu & Wu (1985) found that PCB patients suffered from various kinds of
infections. Most frequent were those of the respiratory tract and
skin, including pyoderma, tinea versicolor, dermatophytosis and warts.
The low resistance of the patients suggested some degree of
immunosuppression. The function of the immune system was tested in 143
patients. Examination during the first year revealed: decreased
concentrations of IgM and IgA, but not of IgG; decreased percentages
of total T-cells, active T-cells, and helper T-cells, but normal
percentages of B-cells and suppressor T-cells; suppression of delayed
type response to recalling antigens; enhancement of lymphocyte
spontaneous proliferation; and enhancement of lymphocyte proliferation
with phytohaemagglutinin, pokeweed mitogen, and tuberculin
stimulation, but not with concanavalin A. After 3 years, the positive
rate of the tuberculin test recovered somewhat with time. The total
numbers of T-cells and B-cells were normal, the number of suppressor
T-cells (OKT-8) increased, but the number of helper T-cells (OKT 4)
was still lower, so the immuno-regulating index (OKT 4/OKT8) was still
very low. The lymphocyte proliferation stimulated by various mitogens
was also enhanced.
In patients with PCB poisoning, IgA and IgM levels in serum apparently
decreased for 2 years after the onset of the disease, but returned to
normal in most cases, in spite of the persistence of the respiratory
symptoms (Shigematsu et al., 1978, see section 9.2.4.3).
9.2.4.3 Respiratory system
In long-term exposure, up to 0.3-10 mg of PCBs may be inhaled in an
8-h working day. The respiratory tract is certainly the most important
route of exposure in the case of acute emergency situations, where
unprotected personnel working in areas containing such PCB
concentrations may, in theory, inhale a total dose of up to 10 mg/day.
This may imply a considerable cumulation of PCBs during long-term
exposure (WHO/EURO, 1987, 1988).
Transient irritation of the mucous membranes of the respiratory tract
has been reported in emergency situations, as well as difficulty in
breathing at high concentrations. It has not been confirmed that
short-term exposure causes other important respiratory effects, though
increased susceptibility and a high risk of contracting chronic
bronchitis have been suggested (Kimbrough, 1980; Elo et al., 1985;
WHO/EURO, 1987).
In the case of unheated commercial PCBs, the amount of PCDFs inhaled
might be very low, if any at all. The situation is totally different
in the case of acute exposures to heated or decomposed PCBs, in which
the inhaled total concentrations might be several orders of magnitude
higher than above, though the irritative effect may prevent breathing
in such contaminated rooms. Since the soot often contains a
considerable fraction of particles, a few microns in size, it is
partly breathed in and, thus, may lead to alveolar retention of both
soot and adsorbed chemicals. Carbon particles can accumulate in the
lung tissues and regional lymph nodes. Inhalation of soot particles
containing high concentrations of both PCBs and PCDFs would, in
practice, be the most important mechanism of exposure (Parkes, 1982;
WHO/EURO, 1987).
Warshaw et al. (1979) and Smith et al. (1982) found a correlation
between serum PCB concentration and respiratory tract symptoms among
workers exposed long-term to PCBs. An increase in the occurrence of
chronic bronchitis was possibly due to a decrease in the immunological
defence mechanism. No increase in mortality from respiratory system
diseases was found. An indication for acute and chronic irritation of
the respiratory tract was found by Brown & Jones (1981).
Shigematsu et al. (1978) studied the clinical, laboratory, and
pathological findings on respiratory involvement in PCB poisoning in
401 patients. Respiratory symptoms included expectoration in 40% of
the 289 non-smoking patients with PCB poisoning and mild wheezing in
2%. The incidence and severity of the symptoms was well correlated
with the concentrations of PCBs in blood and sputa. The clinical
examinations revealed bronchiolitis and pneumonia or atelectasis in
about one-tenth of the patients with reticulo-linear shadows. The PCB
concentrations in the blood and sputa were 27 and 8 µg/litre,
respectively. The presence of PCBs in sputum may have been associated
with the excretion from bronchial cells and/or with lipid II cells of
the lung, phagocytosed in alveolar macrophages and expectorated.
9.2.4.4 Neurotoxicity
Acute and long-term exposures to PCBs have been reported to cause
neurological and unspecific psychological or psychosomatic effects,
such as headache, dizziness, nausea, depression, sleep and memory
disturbances, nervousness, fatigue, and impotence (Smith et al., 1982;
Elo et al., 1985; Hara, 1985; Schecter et al., 1985; Takamatsu et al.,
1985; WHO/EURO, 1987).
Fischbein et al. (1979) reported the occurrence of these symptoms in
39% of male and 58% of female capacitor-manufacturing workers, exposed
to PCBs for long periods (over 5 years). To what extent these symptoms
were the direct consequences of exposure to PCBs and related compounds
and how much they were dependent on general conditions in an emergency
situation remains unclear.
Seppalainen et al. (1985) examined 16 men who were exposed to fumes
resulting from the explosion of capacitors containing Clophen A30. Air
concentrations of PCBs, measured 5.5 h after the explosion, were
8-16 mg/m3 air (PCDFs and other compounds, such as monochloropyrenes
and dichloropyrenes, were also formed). Most of the men had a
transient sensory neuropathy in their lower extremities (WHO/EURO,
1987, 1988).
9.2.4.5 Blood pressure
Kreiss et al. (1981) examined 458 volunteers (>12 years of age) from
Triana (Alabama) and correlated serum PCB levels (Aroclor 1260) with
blood pressure. This population was excessively exposed to DDT
residues through the consumption of contaminated fish. The residents
of this small rural town also had elevated PCB body burdens that were
positively correlated with fish consumption. The mean serum PCB level
was 17.2 µg/litre. The incidence of borderline (systolic of 140-159 mm
Hg and diastolic of 90-94 mm Hg) and definite hypertension (systolic
of >160 mm Hg and diastolic of >95 mm Hg) was 30% more than
would be expected for a general population of the same age, race, and
sex composition. However, this study did not have a control group in
its design, and there were more confounding factors that make the
study inadequate to conclude an association between blood PCB levels
and hypertension.
A study was carried out to test the association of serum PCB levels
and elevated blood pressure in 840 residents of New Bedford, Acushnet,
Dartmouth and Fairhaven, Canada, in the period 1984-87. The mean PCB
levels (as Aroclor 1254) were 5.9 and 5.8 µg/litre in 391 males and
449 females, respectively. The range in serum PCB levels for the total
group was 0.38-154.2 µg/litre. There was a relationship of serum PCB
level to age among the 840 individuals. In the 5 age groups: 18-24,
25-34, 35-44, 45-54, and 55-64 years, the mean serum PCB levels were
2.59, 3.84, 5.30, 8.18, and 8.96 µg/litre, respectively. Blood
pressure levels did not appear to be correlated with serum PCB levels.
The mean systolic readings, taken at 3 different times, for the 840
individuals were 115.26 ± 18.85, 113.69 ± 17.62, and 114.28 ± 11.41.
The diastolic readings were 72.19 ± 10.94, 73.19 ± 10.95, and 73.17 ±
19.94. There was no between the sexes difference (Massachusetts Dept
Public Health, 1987).
Akagi & Okumura (1985) studied the correlation of blood PCBs levels or
PCB patterns and blood pressure in 59 Yusho patients (more than 40
years old). In spite of the passage of 13 years from the onset of the
disease, 52.5% of the patients still had PCB levels higher than those
of the general population. The frequency of hypertension in these
patients was 16.9%, a value similar to that found in the general
population of the same age and sex. Blood pressure was not associated
with blood PCB levels or PCB pattern, but was associated with the well
known factors influencing blood pressure, such as age, obesity, and
habitual alcohol intake.
9.2.5 Mortality studies
Davidorf & Knupp (1979) conducted an epidemiological study on ocular
melanoma incidence in Ohio from 1967 to 1977, attempting to associate
Ohio counties with known high concentrations of PCBs and those with
industries that might use PCBs with an increased incidence of ocular
melanoma. The authors concluded that there was no causal relationship
between PCB exposure and an increased annual occurrence of ocular
melanoma in Ohio counties in the period 1967-77. Bahn et al. (1976)
reported on 31 research and development employees subjected to "heavy"
Aroclor exposure (quantity not reported) in a US petrochemical plant.
Two had malignant melanomas, and according to the standard of the
Third National Cancer Survey, incidence rates of only 0.04 would be
expected among 31 persons (NCI, 1975). The retrospective cohort
mortality studies of Brown & Jones (1981) and Brown (1987) reported
data on PCB-exposed individuals who had worked in 2 electrical
capacitor plants, one in New York, and the other in Massachusetts.
Both plants had produced this type of capacitor for more than 30
years. The PCBs used were Aroclor 1254, Aroclor 1242, and Aroclor
1916. A combined total of 2588 exposed workers from both factories,
with 3 or more months of exposure, were studied. The overall mortality
(295 deaths) was lower than expected (318) and the mortality for
cancer deaths (62 observed) was also lower than expected (80). A
statistically significant excess in deaths was observed in the disease
category that includes cancer of the liver (primary and unspecified),
gall bladder, and biliary tract (5 observed vs 1.9 expected). Most of
the excess was observed in women employed in one plant. According to
the authors, because of the small number of deaths and the variability
of specific causes of death within this category, it remains difficult
to interpret these findings with regard to PCB exposure.
At the first plant, there were 2 different facilities, a power
capacitor manufacturing facility, and a small capacitor manufacturing
facility. At the power capacitor facility, the TWAs for personal air
samples were between 24 and 393 µg/m3 for various jobs: in the
winding work area and soldering work area, they were as low as
3 µg/m3 and as high as 476 µg/m3, respectively. At the second plant,
where a few cases of rectal and liver cancer were found (see above)
the PCB levels were much higher. Degreasers and solderers, for
example, had TWAs for personal air of 1.260 and 1.060 µg/m3,
respectively, and heat soak operators and tankers had TWAs of 630 and
850 µg/m3, respectively. The work area air samples contained levels
as high as a TWA of 810 µg/m3. The duration of exposure, without
information concerning the level of exposure, when the levels varied
so widely, weakens the statement about lack of correlation between
duration of exposure and cancer mortality. In fact, the observed
cancer cases for both the liver and rectum were markedly increased in
the factory that had, at the time of measurement, the higher PCB
levels. Notwithstanding the absence of detailed information, the data
presented are suggestive of a dose-related increased incidence of
mortality from rectal cancer and possibly liver cancer.
Although there was no correlation between the latent period and cancer
mortality or between the duration of employment and cancer mortality,
most of the cancers occurred in the second plant, which had the higher
levels of PCBs at the time that the measurements were made. PCB levels
were monitored in 1977 for personal air and work area air. Since
procedures and processes were somewhat different during the years in
which most of the workers were exposed, the figures on PCB levels do
not necessarily indicate the exposure levels of the subjects.
Nevertheless, the figures given do indicate the wide variation in PCB
levels in air, with a 15-fold difference between the lowest and
highest levels among the different jobs. Although the different levels
of dust and particulate matter are not known, it would be anticipated
that an equally wide variation would exist.
A medical surveillance programme has been established for 482 persons,
who were potentially exposed to PCBs, PCDFs, and PCDDs from an
electrical transformer fire in Binghamton in 1981. Mean serum PCB
concentrations (98% of the samples) were below 20 µg/litre, a value
typical of a population with no unusual exposure. Mortality,
symptomatology, cancer incidence, and reproduction events were
assessed through 1984. The numbers of deaths, cases of cancer, fetal
deaths, and infants with low birth weight or congenital malformations,
were similar to those expected on the basis of age and sex-specific
rates for upstate New York and other comparison populations. One-third
of the fire-fighters and a number of persons, who were in the building
during the first 24 h (or longer) reported a rash or itching skin, but
no chloracne (Fitzgerald et al., 1989).
Bertazzi et al. (1981) reported the results of a mortality study on
PCB-exposed workers, who were employed in the manufacture of
electrical capacitors in an industrial area near Milan. The PCBs used
over the period from 1946 to 1970 were Aroclor 1254, Pyralene 1476
(54% chlorine), and Pyralene 3010 and 3011 (42% chlorine). In 1954, a
few measurements in the air were performed and the values of Aroclor
1254 were 5200-6800 µg/m3. In 1977, airborne concentrations of
Pyralene 3010 ranged from 48 to 275 µg/m3. The minimum and maximum
values of PCBs recovered from workplace surfaces and worker's hands
were, 0.2-159 and 0.3-9.2 µg/cm2, in 1977, and, in 1982 (2 years
after the ban on production), 0.003-6.3 and 0.09-1.5 µg/cm2,
respectively. The mortality study spanned the 25-year period from 1954
to 1978. The control mortality rates were for subjects from the city
in which the plant was located. There were 1310 workers (1020 females
and 290 males) and the vital status was obtained for 98% of both
sexes.
The study was enlarged and extended to include 2100 workers and to
cover the period 1946-82. Vital status was ascertained for over 99% of
the subjects and death certificates were obtained for all deceased
persons. Expected deaths were calculated using 2 sets of mortality
rates, national and local. Among male workers, cancer deaths (14
observed vs 7.6 expected) were significantly increased as were deaths
owing to cancer of the gastrointestinal tract (6 observed vs 2.2
expected). Also, mortality from haematological neoplasms (3 observed),
and lung cancer (3 observed) was higher than expected. However, the
excess was not statistically significant. Female workers exhibited an
overall mortality that was significantly increased above expectations.
Cancer deaths (12 observed vs 5.3 expected) and haematological
neoplasms (4 observed vs 1.1 expected) were significantly higher than
expected when compared with the local population. Interpretation of
the results is limited by the small number of deaths: however, it is
of interest that the gastrointestinal tract and the lymphatic and
haematopoietic tissue seem to be the most probable human target sites
for PCB carcinogenic activity (Bertazzi et al., 1987).
A cohort study on 142 male Swedish capacitor-manufacturing workers was
performed between 1960 and 1978. The PCB was 42% chlorinated and
contained different PCDFs totalling about 1400 µg/kg. The mean
exposure time of the workers was 6.5 years. In 1973, 0.1 mg PCBs/m3
was found in the air. Mortality was investigated for the period
1965-82 and cancer incidence from 1965 to 1980. Twenty-one deaths and
7 cancers were observed, which was in agreement with the anticipated
numbers calculated from national statistics (Gustavsson et al., 1986).
Zack & Musch (1979) examined a small cohort of workers (89) with
occupational exposure to PCBs. No liver cancer was reported among the
30 deaths that occurred in this study. There were increases for all
malignancies (8 observed vs 4.4 expected, SMR=179) and elevated lung
cancer (4 observed vs 1.44 expected). Adjustment for the confounding
variables due to multiple exposure to other agents was not made. By
1979, 31 Yusho patients had died, 11 (35.4%) of these from malignant
neoplasms. Only 21.1% of all deaths in this Japanese prefecture would
be expected from malignant neoplasms, but no clear correlation between
the occurrence of Yusho and increased deaths from malignant neoplasms
could be made, because of the small number of deaths observed and the
unknown latency period.
By the end of 1983, 120 Yusho patients had died, 41 of these from
malignant neoplasms. These included 8 stomach cancers, 11 liver
cancers, and 8 neoplasms of the lung. A statistically significant
excess mortality was seen for malignant neoplasms, cancer of the
liver, and cancer of the lung, trachea, and bronchus, in males, but no
such excess was noted in females. The excess from liver cancer deaths
was seen mainly in Fukuoka prefecture, while no excess was seen in
Nagasaki prefecture (Ikeda et al., 1987).
9.2.6 Appraisal
Some epidemiological studies on occupationally exposed workers and
Yusho patients indicate an association between PCB exposure and
cancer, especially with regard to hepatobiliary tumours. However, no
definite conclusions can be drawn from available data, because of the
small numbers of deaths in the population studies, the lack of clear
dose-response relationships in the occupational studies, and the
difficulty in evaluating the effects of other compounds present in
PCBs.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The PCBs were evaluated by IARC in 1978 and 1987 (IARC, 1978; 1987).
In 1987, IARC concluded that, because the role of impurities in PCBs
in the carcinogenicity could not be excluded, and, because of the lack
of knowledge on dose-response relationships, the evidence from
epidemiological studies is limited. However, the evidence of
carcinogenicity in laboratory animals is sufficient. Taking the
combined evidence from human and experimental animal studies, the IARC
Group concluded that PCBs are probably carcinogenic for humans (IARC,
1987).
Many countries and intergovernmental organizations have banned or
severely restricted the production, use, handling, transport, and
disposal of PCBs and PCTs. For an overview of these measures and
regulations we refer to the Health and Safety Guide on PCBs and PCTs
(WHO, 1992; IRPTC, 1986b).
At the meeting of the Joint FAO/WHO Expert Committee on Food Additives
(WHO, 1990), particular attention was paid to the possible health
consequences of the intake of PCBs by the suckling infant. It was not
anticipated that adverse health effects would occur as a result of
consuming breast milk. It should also be kept in mind that the infant
consumes breast milk for a short period (1-2% of its total life span).
In addition, other factors need to be considered:
* the benefits of breast milk and breast-feeding, including the
nutritional, immunological, and other properties of the milk, as
well as the psychological advantages, should not be discounted;
* the disadvantages of breast milk substitutes, because of the
potential contamination due to infective agents, incorrect
preparation, inadequate hygiene, etc.
For these reasons, JECFA was of the opinion that the advantages to the
infant of breast-feeding outweigh any potential hazards due to the PCB
content of breast milk, and advises that there is absolutely no
justification for discouraging this practice.
The monitoring data have indicated, up to now, that the occurrence of
PCBs in human milk persists at about the same levels during the years,
with slight decreases or increases in the PCB concentration in breast
milk in certain countries. Since the PCB levels in human milk are
still too high, every effort should be made to prevent the entry of
PCBs into the environment and to control their occurrence in the food
supply. The Committee was reassured by the observation that the
production of PCBs has largely ceased. Thus, it is expected that the
levels of PCBs in the environment and food, and consequently in breast
milk, will decrease with time (WHO, 1986b).
On the basis of the evaluated background data, an average dietary
intake of PCBs for adults was estimated to amount to a maximum of
100 µg/week, or approximately 14 µg/day. For a 70-kg person, this is
an intake equivalent to a maximum quantity of 0.2 µg/kg body weight
per day (WHO/EURO, 1988).
The above data suggest that the main exposure of the general
population to PCBs occurs through food. The daily intake of these
compounds by breast-fed infants is about 1-2 orders of magnitude
higher than for the rest of the population, compared either on the
basis of body weight or energy consumption. However, compared with
lifetime intake, a 6-month, breast-feeding period contributes less
than 5% of the total body burden from lifetime exposure (WHO/EURO,
1988).
POLYCHLORINATED TERPHENYLS
(Data relating specifically to polychlorinated terphenyls are scarce.
Nevertheless, they are presented separately in this section.)
1. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
1.1 Identity
The chemical formula of the polychlorinated terphenyls (PCTs) can be
given as C18H14-nCln, in which n is the number of chlorine atoms,
which can range from 1-14.
The chemical structure is:
 The number of different PCTs theoretically possible is orders of
magnitude higher than that for PCBs, but, in practice, as for PCBs,
PCTs are not sold on a composition specification, but on their
physical properties, which depend on the degree of chlorination.
Common Polychlorinated terphenyls - PCTs
name:
Major trade The trade names are generally similar to those
names: given for PCBs. In the Aroclor series, terphenyls are
indicated by 54 in the first two places of the four
digit code. In Japan, the PCTs are coded Kanechlor
KC-C.
1.2 Physical and chemical properties
The physical and chemical properties of PCTs are very close to those
of PCBs, and depend on the degree of chlorination.
1.3 Analytical methods
Extraction and clean-up procedures are similar to those used for PCBs
(sections 2.3.1.1 and 2.3.1.2).
The gas-liquid chromatographic details are different from those of
PCBs, because of the lower volatility of the PCTs. Zitko et al. (1972)
used 3% OV 210 as the stationary phase with a column temperature of
200°C. Thomas & Reynolds (1973) also used OV 210 with a column
temperature of 250°C and another system with 3% Dexsil as a stationary
phase at 300°C with a 63Ni electron capture detector; this was also
used by Addison et al. (1972). Sosa-Lucero et al. (1973) used OV 210
and SE 30 at 255°C and Freudenthal & Greve (1973) used OV 17 with a
temperature programmed from 200°C to 285°C. Thomas & Reynolds (1973)
confirmed the identity by chlorination to tetradecachloroterphenyl
with antimony pentachloride.
2. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
No specific information available. See PCBs.
3. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
Atmospheric input into the Great Lakes has been studied, because the
lakes, as a whole, represent the largest surface area of any
freshwater body in the world. Wingender & Williams (1984) found that
atmospheric transport was a major pathway for the deposition of
polychlorinated terphenyls into the Great Lakes (see section 4.1.1.1).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 Residues in the environment
Relatively few studies have been carried out to determine
polychlorinated terphenyls in biota. Freudenthal & Greve (1973) found
levels of 0.12 mg/kg (wet weight) in oysters and 0.4 mg/kg (fat basis)
in eels from the Netherlands. Levels of PCTs were generally lower than
PCBs in the same samples. Renberg et al. (1981) analysed biota from
the Baltic Sea and found levels of 2.8-17.2 mg/kg (wet weight) in
white-tailed eagles, 0.5-1 mg/kg in grey seals, and 0.08 mg/kg in
eels. In fish, Jan & Malnersic (1978) found PCT levels of
0.003-0.005 mg/kg in trout from the Soca River, Yugoslavia. Mean PCT
levels of 0.0025 mg/kg (Doguchi, 1977) and 0.01 mg/kg (Takai et al.,
1979) have been found in the freshwater and marine environment of
Japan.
Several bird species have been monitored for PCTs; levels of
0.03-2.2 mg/kg have been found in Japanese birds (Doguchi, 1977).
Zitko et al. (1972) found levels of 1.4 mg/kg fat (wet weight) and
0.1 mg/kg in eggs of herring gulls from the Bay of Fundy, Canada.
Hassell & Holmes (1977) analysed the livers of various birds of prey
in the United Kingdom; residues ranged from <0.05 to 1.2 mg/kg. PCT
levels of between 0.61 and 10.51 mg/kg were found in the fat of gulls
from Italy, (Vannucchi et al., 1978) and black-headed gulls from the
Baltic contained mean residues of 1.8 mg/kg in adipose tissue
(Falandysz, 1980).
PCTs were also measured in the monitoring programme carried out all
over Japan in the period 1974-81. In 1974, 1976, and 1978 no PCTs were
found in 60, 156, and 75 samples of water (limit of determination
0.0001 mg/litre). No PCTs were found in sediment samples in 1974, but,
in 1976 and 1978, 21/151 and 37/75 samples were positive, with PCT
levels of 0.001-0.2 and 0.001-1.0 mg/kg, respectively. In fish in
1974, 1976, and 1978, PCTs were found in 3/11, 0/39, and 3/66 samples,
in concentrations of 0.0002-0.2 mg/kg (Environment Agency Japan,
1983).
4.2 Residues in food
Ushio & Doguchi (1977) analysed cereal products, vegetable products
including vegetable oils, seasonings, and seaweed, marine animal
products, and terrestrial animal products including milk and eggs, for
the presence of PCTs. Only the vegetable products contained average
concentration of 0.05 µg/kg. Other authors referred to by Ushio &
Doguchi failed to detect PCTs in edible oil, vegetables, meat, or
fish.
No PCTs could be detected in a Canadian survey on eggs, domestic and
imported cheese (Villeneuve et al., 1973b).
In Japan, the PCT contents of a number of foods were determined. The
PCT contents of fish were lower than the PCB contents (Fukano et al.,
1974). Villeneuve et al. (1973a) analysed packaged food in Canada and
found that 94.5% of the samples contained less than 0.01 mg PCTs/kg
and 5.5% contained 0.01-0.05 mg PCTs/kg.
4.3 Concentrations in adipose tissue
In Japan, Doguchi et al. (1974) found an average PCT level of
0.6 mg/kg in human fat, with a range of 0.1-2.1 mg/kg. In the same
country, Takizawa & Minagawa (1974) found PCT levels of 0.02 mg/kg in
the human liver (n = 6), 0.01 mg/kg in the kidney (n = 2), 0.02 mg/kg
in the brain (n = 3), and 0.04 mg/kg in the pancreas (n = 1). Thirty
samples of adipose tissue (from 18 males and 12 females), obtained in
Tokyo in 1974, were analysed for PCTs. The average level of PCTs was
1.11 mg/kg (range 0.04-9.20 mg/kg), on a fat basis (Fukano & Doguchi,
1977). In the Netherlands, PCTs were found in human fat at levels of
0-1 mg/kg (Freudenthal & Greve, 1973).
4.4 Concentrations in blood
An average PCT level of 5.0 µg/litre was recorded in the blood of
non-occupationally exposed volunteers in Japan (Doguchi & Fukano,
1975). Human blood samples were collected from 10 subjects in Tokyo in
1975 out of 27 subjects from whom blood had been obtained in 1973. The
average concentration of PCTs in whole blood was 6.45 µg/litre
(0.7-19.6 µg/litre) in 1973, and 5.32 µg/litre (1.1-9.4 µg/litre) in
1975 (Fukano & Doguchi, 1977).
5. KINETICS AND METABOLISM
5.1 Absorption
PCTs have been shown to be absorbed from the intestinal tract
(Sosa-Lucero et al., 1973), but very little information is available
on the rate of absorption.
5.2 Distribution
Diets containing Aroclor 5460 at levels of 10, 100, or 1000 mg/kg were
administered to rats for 7 days. The greatest concentration (611 mg/kg
at 1000 mg/kg diet) was in the liver, while the blood level was
5.85 mg/litre at 1000 mg/kg diet. PCT administration did not affect
body weight, but a significant increase in liver weight occurred in
the rats fed 1000 mg/kg diet (Sosa-Lucero et al., 1973). Table 54
shows the tissue distribution obtained in this study in rats fed with
Aroclor 5460 and in another study using Aroclor 1254 (Curley et al.,
1971).
Addison et al. (1972) dosed cod Gadus morhua by gavage with the
polychlorinated terphenyl (PCT) Aroclor 5460 (in herring oil) at
0.5 g/ml. After one week of starvation, the PCTs were present in all
the tissues analysed. Uptake efficiency appeared to be low with a
total of 1-10 mg of Aroclor 5460 being distributed through all tissues
out of 1 g administered. Liver was found to be the organ richest in
PCTs, and probably contained most of the absorbed material. In a
separate group of fish, analysed 70 days later (fish were fed during
this period), PCT residues were not significantly lower.
Table 54. Tissue distribution (mg/kg wet weight) of PCTs
(Aroclor 5460) in rats fed dietary levels of 100 mg/kg
for 7 days and fed PCBs (Aroclor 1254) at 100 mg/kg
for 9 daysa
Tissue Aroclor 5460 Aroclor 1254
Blood 1.32 0.1
Liver 47 6
Brain 5.1 4
Kidney 15.1 5
Heart 21.5 -
Fat - 180
a From: Curley et al. (1971); Sosa-Lucero et al. (1973).
5.3 Biotransformation
There is little information on the biotransformation of PCTs. Addison
et al. (1972), using gas-liquid chromatography, noted a loss of PCTs
with a shorter retention time in the excreta of a cod dosed orally
with Aroclor 5460; the same loss was observed in rat faeces after the
administration of a diet containing Aroclor 5460 (Sosa-Lucero et al.,
1973).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Marine and estuarine organisms
PCTs, Aroclor 5460, did not show any toxic effects at either 1 or
100 mg/litre on Dunaliella, Olisthodiscus, or Thalassiorsira, the
only 3 species tested with this mixture (Craigie & Hutzinger, 1975).
6.2 Terrestrial invertebrates
Lichtenstein et al. (1969) exposed Drosophila melanogaster to the
dry residues of various PCTs. No mortality was observed after a 48-h
exposure to 2999 µg of Aroclor 4465, 5442, or 5460.
6.3 Birds
A single study on the toxicity of Aroclor 5442, produced a 5-day LC50
of 4477 mg/kg (1301-15402 mg/kg) in Japanese quail (aged 14 days)
(Coturnix coturnix) (Hill & Camardese, 1986).
Cecil et al. (1974) found that Aroclor 5442 at a dose level of
20 mg/kg diet did not change the hatchability of chicken eggs.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single oral exposure
Early data, reported in abstract, indicated that the approximate oral
LD50 values of the PCT-mixtures Aroclor 5442 and 5460, in corn oil,
in rats were 10.6 and 19.2 g/kg body weight, respectively. For 3:1
mixtures of PCBs and PCTs, Aroclor 4465 and 2562, in corn oil, the
LD50 values in rats were 16 and 6.3 g/kg body weight, respectively
(US FDA, 1970).
7.2 Short-term oral exposure
7.2.1 Rat
Modifications in the liver were studied in groups of male
Sprague-Dawley rats fed a diet containing Aroclor 5460 at a level of 0
or 10 000 mg/kg diet (equivalent to 0 or 400 mg/kg body weight). Body
weights were slightly decreased after 3 weeks of exposure. The
enlarged livers showed proliferation of the endoplasmic reticulum and
formation of large concentric membrane arrays. Evidence of fatty
degeneration was observed by Toftgard et al. (1980). Biochemical
changes included an increase in microsomal protein and phospholipid,
and a decrease in RNA and cholesterol. The specific esterase
activities, N-demethylase and nitroreductase, were increased and
those of glucose-6-phosphatase and aryl hydrocarbon hydroxylase
decreased (Norback & Allen, 1972).
Sosa-Lucero et al. (1973) did not observe any signs of toxicity in
groups of male Wistar rats exposed to a diet containing Aroclor 5460
at levels of up to 1000 mg/kg diet (equivalent to 50 mg/kg body
weight) for 7 days. At 1000 mg/kg diet, relative liver weights were
increased as well as microsomal protein, cytochrome P-450, and the
specific activities of aniline hydroxylase and aminopyrine-
N-demethylase. Mixed type induction of hepatic microsomal enzymes in
rats exposed to PCTs has been observed by several investigators
(Ahotupa & Aitio, 1980; Toftgard et al., 1980; Nilsen & Toftgard,
1981).
Kiriyama et al. (1974) fed groups of male Wistar rats a control diet
or diets with ortho-, meta-, or para-PCTs at a level of 2000 mg/kg
diet (equivalent to 100 mg/kg body weight) for 2 weeks. Ortho- and
meta-PCTs reduced growth and increased relative kidney weights,
while only meta-PCTs decreased food intake and increased the
relative liver weights. All mixtures increased plasma, but not liver,
cholesterol levels. There was evidence of adrenal hypertrophy.
7.2.2 Monkey
A dietary level of Aroclor 5460 of 5000 mg/kg (equivalent to 200 mg/kg
body weight) over 3 months caused growth retardation and increased
relative liver weights in 6 Rhesus monkeys compared with 3 controls.
After 6 weeks of exposure, the toxic signs observed were similar to
those found within 1 month in a group of monkeys exposed to 300 mg of
Aroclor 1248/kg diet (equivalent to 12 mg/kg body weight), i.e.,
alopecia, facial oedema, swollen eyelids and lips, and purulent eye
discharge. After exposure of both groups for 3 months, proliferation
of the smooth endoplasmic reticulum was observed as well as
hypertrophy and hyperplasia of the gastric mucosa (Allen & Norback,
1973).
7.3 Teratogenicity
Groups of 15 or 16 pregnant ddY mice were fed diets containing 0, 100,
500, or 2500 mg PCTs/kg (not specified) during gestation. The animals
were sacrificed on day 18 and examined for embryonic effects. The
fetuses of dams receiving the 500 and 2500 mg/kg diet showed a higher
incidence of cleft palate in comparison with the controls. Pregnant
ddY mice were administered 0, 50, or 100 mg PCTs/kg with
corticosterone administered subcutaneously on days 11, 12 and 13. A
significant increase was seen in corticosterone levels in the plasma
in the PCT-treated animals on day 14. Furthermore, when pregnant ddY
mice were adrenalectomized on day 10, it did not suppress the
development of cleft palate, but metapyrone, an inhibitor of
corticosterone synthesis, significantly reduced the incidence of cleft
palate in the fetuses. The results suggest that cleft palate induced
by PCTs is not due to a direct effect, but that an increase in the
corticosterone level in the maternal plasma is involved in the
mechanism of its development (Kaneko, 1988).
Pregnant Wistar rats were fed PCTs at levels of 0, 500, or 2500 mg/kg
diet during gestation and the animals were sacrificed on day 20.
Systemic oedema was observed in the fetuses of the animals fed 500 and
2500 mg PCTs/kg diet, but no cleft palate was found (Kaneko, 1988).
7.4 Carcinogenicity
Groups of 35 male ICR mice received a diet containing Kanechlor C
(a mixture of 95% PCTs and 5% PCBs) at levels of 0, 250, or 500 mg/kg
(equivalent to 0, 36, and 70 mg/kg body weight), for 24 weeks. The
mice were sacrificed following 16 exposure-free weeks. Survivors
numbering 28, 28, and 21 mice at 0, 250, and 500 mg/kg diet,
respectively, were autopsied. A dose-related reduction in body weight
gain and a dose-related increase in absolute liver weights were
observed. Neoplastic nodules (nodular hyperplasia) were found in the
livers of 3/28 mice at 250 mg/kg diet and 6/21 mice at 500 mg/kg diet.
Hepatocellular carcinomas were observed in 3/21 mice at 500 mg/kg
diet. No neoplastic nodules were noted in the controls. The increases
at the higher dose level were statistically significant (Shirai et
al., 1978).
7.5 Miscellaneous effects
Evidence for the estrogenic activity of Aroclor 5442 was found using
the glycogen response of the immature rat uterus. Aroclor 5460 was
inactive in this test (Bitman & Cecil, 1970; Bitman et al., 1972).
The mixed type inducer, Aroclor 5460, increased the metabolism of
4-androstene-3,17-dione in male Sprague-Dawley rats intraperitoneally
injected with 4 doses of 300 mg/kg body weight in 4 days (Nilsen &
Toftgard, 1981). Reproductive effects have not been investigated.
Groups of pregnant ddY mice received a control diet or diets
containing PCTs (not specified) at levels of 50 or 500 mg/kg diet
(equivalent to 7 or 70 mg/kg body weight). Increased incidence of
cleft palate and other malformations was reported in the fetuses. In
neonates, reduced growth and survival as well as hyperactivity were
observed (Kimura & Miyake, 1976). (No details available).
REFERENCES
ABBOTT, D.C., COLLIN, G.B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom, 1969-71. Br.
med. J., 2: 553-556.
ABE, S., INOU, Y., & TAKAMATSU, M. (1975) [Polychlorinated biphenyl
residues in plasma of Yusho children born to mothers who had
consumed oil contaminated by PCBs.] Fukuoka Acta med., 66: 605-609
(in Japanese).
ABRAHAMSON, L.J. & ALLEN, J.R. (1973) The biological response of
infant nonhuman primates to a polychlorinated biphenyl. Environ.
health Perspect., 4: 81-86.
ACHILLES, A. (1983) Fire hazard of polychlorinated biphenyls. In:
Barros, M.C., Könemann H., & Visser, R., ed. Proceedings of the PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 187-191.
ACKER, L. & SCHULTE, E. (1970) [The occurrence of chlorinated
biphenyls and hexachlorobenzene alongside chlorinated insecticides
in breastmilk and human fatty tissue.] Naturwissenschaften, 57: 497
(in German).
ACKER, L. & SCHULTE, E. (1974) [Chlorinated hydrocarbons in human
fatty tissue.] Naturwissenschaften, 61: 1-4 (in German).
ACKER, L., BARKE, E., HAPKE, H.-J., HEESCHEN, W., KORANSKY, W.,
KUBLER, W., & REINHARDT, D. (1984) [Residues and impurities in
breast milk. German Association for the Encouragement of Research:
Communication XII of the Committee on Testing of Residues in
Foods], Weinheim, Verlag Chemie, pp. 1-97.
ACQUAVELLA, J.F., HANIS, N.M., NICOLIC, M.J., & PHILLIPS, S.C.
(1986) Assessment of clinical, metabolic, dietary, and occupational
correlations with serum polychlorinated biphenyl levels among
employees at an electrical capacitor manufacturing plant. J. occup.
Med., 28(11): 1177-1180.
ADAMS, J.A. (1983) Effect of PCB (Aroclor 1254) on early
development and mortality in Arbacia eggs. Water air soil
Pollut., 20: 1-5.
ADDISON, R.F. & BRODIE, P.F. (1977) Organochlorine residues in
maternal blubber, milk, and pup blubber from grey seals
(Halichoerus grypus) from Sable Island, Nova Scotia. J. Fish Res.
Board Can., 34: 937-941.
ADDISON, R.F. & SMITH, T.G. (1974) Organochlorine residue levels in
Arctic ringed seals: variation with age and sex. Oikos,
25: 335-337.
ADDISON, R.F., FLETCHER, G.L., RAY, S., & DOANE, J. (1972) Analysis
of a chlorinated terphenyl (Aroclor 5460) and its deposition in
tissues of cod (Gadus morhua). Bull. environ. Contam. Toxicol.,
8: 52-60.
ADDISON, R.F., KERR, S.R., DALE, J., & SERGEANT, D.E. (1973)
Variation of organochlorine residue levels with age in Gulf of St.
Lawrence harp seals (Pagophilus groenlandicus). J. Fish. Res.
Board Can., 30: 595-600.
ADDISON, R.F., ZINCK, M.E., & SMITH, T.G. (1986) PCBs have declined
more than DDT-group residues in Arctic ringed seals (Phoca
hispida) between 1972 and 1981. Environ. Sci. Technol.,
20: 253-256.
AGRAWAL, A.K., TILSON, H.A., & BONDY, S.C. (1981)
3,4,3',4'-tetrachlorobiphenyl given to mice prenatally produces
long-term decreases in striatal dopamine and receptor binding sites
in the caudate nucleus. Toxicol. Lett., 7: 417-424.
AGUILAR, A. & BORRELL, A. (1988) Age- and sex-related changes in
organochlorine compound levels in fin whales (Balaenoptera
physalus) from the Eastern North Atlantic. Mar. environ. Res.,
25: 195-211.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of polychlorinated biphenyl (PCB) and chlorinated
pesticides in water. Anal. Chem., 42: 1483-1486.
AHMED, M. & FOCHT, D.D. (1973a) Degradation of polychlorinated
biphenyls by two species of Achromobacter. Can. J. Microbiol.,
19: 47-52.
AHMED, M. & FOCHT, D.D. (1973b) Oxidation of polychlorinated
biphenyls by Achromobacter PCB. Bull. environ. Contam. Toxicol.,
10: 70-72.
AHNOFF, M. & JOSEFSSON, B. (1973) Confirmation studies on
polychlorinated biphenyls (PCBs) from river waters using mass
fragmentography. Anal. Lett., 6: 1083-1093.
AHNOFF, M. & JOSEFSSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AHNOFF, M. & JOSEFSSON, B. (1975) Clean-up procedures for PCB
analysis on river water extracts. Bull. environ. Contam. Toxicol.,
13: 159-166.
AHOTUPA, M. & AITIO, A. (1980) Effect of chlorinated naphthalenes
and terphenyl on the activities of drug metabolizing enzymes in rat
liver. Biochem. biophys. Res. Commun., 93: 250-257.
AKAGI, K. & OKUMURA, M. (1985) Association of blood pressure and
PCB level in Yusho patients. Environ. health Perspect., 59: 37-39.
AKIYAMA, K., OHI, G., FUJITANI, K., & YAGYU, H. (1975)
Polychlorinated biphenyl residues in maternal and cord blood in
Tokyo metropolitan area. Bull. environ. Contam. Toxicol.,
14: 588-592.
ALBAIGES, J., FARRAN, A., SOLER, M., GALLIFA, A., & MARTIN, P.
(1987) Accumulation and distribution of biogenic and pollutant
hydrocarbons, PCBs and DDT in tissues of Western Mediterranean
fishes. Mar. environ. Res., 22: 1-18.
ALBRO, P.W. & FISHBEIN, L. (1972) Intestinal absorption of
polychlorinated biphenyls in rats. Bull. environ. Contam. Toxicol.,
8: 26-31.
ALBRO, P.W. & PARKER, C.E. (1979) Comparison of the compositions of
Aroclor 1242 and Aroclor 1016. J. Chromatogr., 169: 161-166.
ALBRO, P.W., CORBETT, J.T., & SCHROEDER, J.L. (1981) Quantitative
characterization of polychlorinated biphenyl mixtures (Aroclors
1248, 1254 and 1260) by gas chromatography using capillary columns.
J. Chromatogr., 205: 103-111.
ALENCASTRO, L.F. DE, PRELAZ, V., & TARRADELLAS, J. (1984)
Contamination of silos in Switzerland by PCB residues in coatings.
Bull. environ. Contam. Toxicol., 33(3): 270-276.
ALFORD-STEVENS, A.L. (1986) Analyzing PCBs. Environ. Sci. Technol.,
20(12): 1194-1199.
ALLEN, J.R. (1975) Response of the non-human primate to
polychlorinated biphenyl exposure. Fed. Proc., 34: 1675-1679.
ALLEN, J.R. & ABRAHAMSON, L.J. (1973) Morphological and biochemical
changes in the liver of rats fed polychlorinated biphenyls. Arch.
environ. Contam. Toxicol., 1: 265-280.
ALLEN, J.R. & BARSOTTI, D.A. (1976) The effects of transplacental
and mammary movement of PCBs on infant rhesus monkeys. Toxicology,
6: 331-340.
ALLEN, J.R. & NORBACK, D.H. (1973) Polychlorinated biphenyl- and
triphenyl-induced gastric mucosal hyperplasia in primates. Science,
179: 498-499.
ALLEN, J.R. & NORBACK, D.H. (1976) Pathobiological responses of
primates to polychlorinated biphenyl exposure. In: Proceedings of
the National Conference on Polychlorinated Biphenyls, Chicago,
19-21 November 1975, Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances, pp. 43-49 (EPA-560/6-75-004).
ALLEN, J.R., CARSTENS, L.A., & BARSOTTI, D.A. (1974) Residual
effects of short-term low level exposures of nonhuman primates to
polychlorinated biphenyls. Toxicol. appl. Pharmacol., 30: 440-451.
ALLEN, J.R., CARSTENS, L.A., ABRAHAMSON, L.J., & MARLAR, R.J.
(1975) Responses of rats and non-human primates to
2,5,2',5',-tetrachlorobiphenyl. Environ. Res., 9: 265-273.
ALLEN, J.R., CARSTENS, L.A., & ABRAHAMSON, L.J. (1976) Responses of
rats to polychlorinated biphenyls for fifty-two weeks. Arch.
environ. Contam., 4: 404-419.
ALLEN, J.R., BARSOTTI, D.A., LAMBRECHT, L.K., & VAN MILLER, J.P.
(1979) Reproduction effects of halogenated aromatic hydrocarbons on
nonhuman primates. Ann. NY Acad. Sci., 320: 419-425.
ALLEN, J.R., BARSOTTI, D.A., & CARSTENS, L.A. (1980) Residual
effects of polychlorinated biphenyls on adult nonhuman primates and
their offspring. J. Toxicol. environ. Health, 6: 55-66.
ALTMAN, N.H., NEW, A.E., MCCONNELL, E.E., & FERRELL, T.L. (1979) A
spontaneous outbreak of polychlorinated biphenyl (PCB) toxicity in
Rhesus monkeys (Macaca mulatta): Clinical observations. Lab.
anim. Sci., 29: 661-665.
ALVARES, A.P. (1977) Stimulatory effects of polychlorinated
biphenyls (PCB) on cytochromes P-450 and P-448 mediated microsomal
oxidations. In: Microsomes and drug oxidations, Oxford, New York,
Pergamon Press, pp. 476-483.
ALVARES, A.P. & KAPPAS, A. (1975) Induction of aryl hydrocarbon
hydroxylase by polychlorinated biphenyls in the foeto-placental
unit and neonatal livers during lactation. FEBS Lett., 50: 172-174.
ALVARES, A.P. & KAPPAS, A. (1977) Heterogenicity of cytochrome
P-450s induced by polychlorinated biphenyls. J. biol. Chem.,
252: 6373-6378.
ALVARES, A.P., FISCHBEIN, A., ANDERSON, K.E., & KAPPAS, A. (1977)
Alterations in drug metabolism in workers exposed to
polychlorinated biphenyls. Clin. Pharmacol. Ther., 22(2): 140-145.
ALVARES, A.P., EISEMAN, J.L., UENG, T.-H., & KAPPAS, A. (1982)
Polychlorinated biphenyls: species and tissue specifications of the
induction of monooxygenases in rats, mice, rabbits, and humans. In:
Proceedings of the Workshop on the Combined Effects of Xenobiotics,
Ottawa, 22-23 June 1981, Ottawa, National Research Council of
Canada, pp. 127-149 (Publication No. NRCC 18978).
ANDERSON, M.R. & PANKOW, J.F. (1986) A case study of a chemical
spill: Polychlorinated biphenyls (PCBs). 3. PCB sorption and
retardation in soil underlying the site. Water Resour. Res.,
22(7): 1051-1057.
ANDERSON, L.M., VAN HAVERE, K., & BUDINGER, J.M. (1983) Effects of
polychlorinated biphenyls on lung and liver tumours initiated in
suckling mice by N-nitrosodimethylamine. J. Natl Cancer Inst.,
71: 157-163.
ANDO, M. (1978) Transfer of 2,4,5,2',4',5'-hexachlorobiphenyl and
2,2-bis(p-chlorophenyl), 1,1,1 -trichloroethane ( p-p'DDT) from
maternal to newborn and suckling rats. Arch. Toxicol., 41: 179-186.
ANDO, M., SAITO, H., & WAKISAKA, I. (1984) [Transfer of PCBs from
mother to newborn baby through placenta and milk.] Res. Rep. Natl
Inst. Environ. Stud. Jpn, 67: 333-345 (in Japanese).
ANDO, M., SAITO, H., & WAKISAKA, I. (1985) Transfer of
polychlorinated biphenyls to newborn infants through the placenta
and mother's milk. Arch. environ. Contam. Toxicol., 14(1): 51-57.
ANDREN, A.W. (1982) Processes determining the flux of PCBs across
air/water interfaces. In: Mackay, D., ed. Physical behavior of PCBs
in the Great Lakes; Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., pp. 127-140.
ANDRES, J., LAMBERT, I., ROBERTSON, L., BANDIERA, S., SAWYER, T.,
LOVERING, S., & SAFE, S. (1983) The comparative biologic and toxic
potencies of polychlorinated biphenyls and polybrominated
biphenyls. Toxicol. appl. Pharmacol., 70: 204-215.
ANDREWS, J.E. (1989) Polychlorinated biphenyl (Aroclor 1254)
induced changes in femur morphometry calcium metabolism and
nephrotoxicity. Toxicology, 57: 83-96.
ANON. (1983a) PCB found in Czechoslovakian canned hams during each
of last four years. Food Chem. News, 18 April: 32.
ANON. (1983b) USDA blocks all Czechoslovakian meat imports because
of PCB residues. Food Chem. News, 2 May: 30-31.
ANON. (1985a) Summary of results of the first test to determine
residual contaminants in the air and on surface in floors 2 through
18 of the Binghamton State Office Building. Test report,
Springfield, Virginia, Versar Inc.
ANON. (1985b) Polychlorinated biphenyl transformer incident New
Mexico. Morb. Mortal. wkly Rep., 34(36): 557-559.
ANON. (1987) Polychlorinated biphenyl in fluorescent lighting.
Environ. Health Saf. News, 33(1): 3-18.
ARMOUR, J.A. & BURKE, J.A. (1970) Method for separating
polychlorinated biphenyls from DDT and its analogs. J. Assoc. Off.
Anal. Chem., 53: 761-768.
ARMBRUSTER, G., GEROW, K.G., GUTENMANN, W.H., LITTMAN, C.B., &
LISK, D.J. (1987) The effects of several methods of fish
preparation on residues of polychlorinated biphenyls and sensory
characteristics in striped bass. J. Food Saf., 8: 235-243.
ARNOLD, D.L., MES, J., ZAWIDZKA, Z.Z., & KARPINSKI, K. (1984)
Toxicity of PCB (Aroclor 1254) as a consequence of continuous
exposure during pregnancy and nursing (PA-24). Presented at the
27th Annual Meeting of the Canadian Federation of Biological
Societies, University of Saskatchewan, Canada, 18-22 June 1984.
ATKINSON, S.A. (1979) Chemical contamination of human milk: A
review of current knowledge. J. Can. Diet. Assoc., 40(3): 223-226.
ATLAS, E. & GIAM, C.S. (1981) Global transport of organic
pollutants: Ambient concentrations in the marine atmosphere.
Science, 211: 163-165.
ATSDR (1989) Toxicological profile for selected PCBs (Aroclor 1260,
1254, 1248, 1242, 1232, 1221 and 1016), Atlanta, Georgia, Agency
for Toxic Substances and Disease Registry (ATSDR/TP-88/21).
AULERICH, R.J. & RINGER, R.K. (1977) Current status of PCB toxicity
to mink and effect on their reproduction. Arch. environ. Contam.
Toxicol., 6: 279-292.
AULERICH, R.J., BURSIAN, S.J., BRESLIN, W.J., OLSON, B.A., &
RINGER, R.K. (1985) Toxicological manifestations of
2,4,5,2',4',5',-; 2,3,6,2',3',6',-; and 3,4,5,3',4',5',-
hexachlorobiphenyl and Aroclor 1254 in mink. J. Toxicol. environ.
Health., 15: 63-79.
AX, R.L. & HANSEN, L.G. (1975) Effects of purified polychlorinated
biphenyl analogs on chicken reproduction. Poult. Sci., 54: 895-900.
BACCI, E. & GAGGI, C. (1985) Polychlorinated biphenyls in plant
foliage: Translocation or volatilization from contaminated soils?
Bull. environ. Contam. Toxicol., 35: 673-681.
BACHE, C.A., SERUM, J.W., YOUNGS, W.D., & LISK, D.J. (1972)
Polychlorinated biphenyl residues: Accumulation in Cayuga lake
trout with age. Science, 177: 1191-1192.
BADSHA, K. & EDULJEE, G. (1986) PCB in the UK environment - A
preliminary survey. Chemosphere, 15(2): 211-215.
BADSHA, K., EDULJEE, G., & SCUDAMORE, N. (1986) Environmental
monitoring for PCB and trace metals in the vicinity of a chemical
waste disposal facility - III. Chemosphere, 15(7): 947-957.
BAGLEY, G.E., REICHEL, W.L., & CROMARTIE, E. (1970) Identification
of polychlorinated biphenyls in two bald eagles by combined
gas-liquid chromatography-mass spectroscopy. J. Assoc. Off. Anal.
Chem., 53: 251-261.
BAHN, A.K., ROSENWAIKE, I., HERRMAN, N., GROVER, P., STELLMAN, J.,
& O'LEARY, K. (1976) Melanomas after exposure to PCBs. New Engl. J.
Med., 295: 450.
BAILEY, S. & BUNYAN, P.J. (1972) Interpretation of persistence and
effects of polychlorinated biphenyls in birds. Nature (Lond.),
236: 34-36.
BAILEY, J., KNAUF, V., MUELLER, W., & HOBSON, W. (1980) Transfer of
hexachlorobenzene and polychlorinated biphenyls to nursing infant
Rhesus monkeys: enhanced toxicity. Environ. Res., 21: 190-196.
BAKER, F.D., BUSH, B., TUMASONIS, C.F., & LO, F.-C. (1977) Toxicity
and persistence of low-level PCB in adult Wistar rats, fetuses, and
young. Arch. environ. Contam. Toxicol., 5: 143-156.
BAKER, E.L., Jr., LANDRIGUN, P.J., GLUECK, C.J., ZACK, M.M., Jr.,
LIDDLE, J.A., BURSE, V.W., HOUSEWORTH, W.J., & NEEDHAM, L.L. (1980)
Metabolic consequences of exposure to polychlorinated biphenyls
(PCBs) in sewage sludge. Am. J. Epidemiol., 112(4): 553-563.
BALLSCHMITER, K. & ZELL, M. (1980) Analysis of polychlorinated
biphenyls (PCB) by glass capillary gas chromatography. Composition
of technical Aroclor- and Clophen-PCB mixtures. Fresenius' Z. anal.
Chem., 302: 20-31.
BALLSCHMITER, K., BUCHERT, H., BIHLER, S., SCHOTT, P., RÖPER, H.P.,
& PACHUR, H.J. (1981) Organochlorine pollutant analysis of
contaminated and uncontaminated lake sediments by high resolution
gas chromatography. Chemosphere, 10: 945-956.
BANDIERA, S., SAFE, S., & OKEY, A.B. (1982) Binding of
polychlorinated biphenyls classified as either phenobarbitone-,
3-methylcholanthrene-, or mixed-type inducers to cytosolic Ah
receptor. Chem. biol. Interact., 39: 259-277.
BANDIERA, S., FARRELL, K., MASON, G., KELLEY, M., ROMKES, M.,
BANNISTER, R., & SAFE, S. (1984) Comparative toxicities of the
polychlorinated dibenzofuran (PCDF) and biphenyl (PCB) mixtures
which persist in Yusho victims. Chemosphere, 13(4): 507-512.
BARSOTTI, D.A. & VAN MILLER, J.P. (1984) Accumulation of a
commercial polychlorinated biphenyl mixture (Aroclor 1016) in adult
Rhesus monkeys and their nursing infants. Toxicology, 30: 31-44.
BARSOTTI, D.A., MARLAR, R.J., & ALLEN, J.R. (1976) Reproductive
dysfunction in Rhesus monkeys exposed to low levels of
polychlorinated biphenyls (Aroclor 1248). Food Cosmet. Toxicol.,
14: 99-103.
BASTOMSKY, C.H. (1974) Effects of polychlorinated biphenyl mixture
(Aroclor 1254) and DDT on biliary excretion in rats. Endocrinology,
95: 1150-1155.
BASTOMSKY, C.H. (1977) Goitres in rats fed polychlorinated
biphenyls. Can. J. Physiol. Pharmacol., 55: 288-292.
BASTOMSKY, C.H. & MURTHY, P.V.N. (1976) Enhanced in vitro hepatic
glucuronidation of thyroxine in rats following cutaneous
application or ingestion of polychlorinated biphenyls. Can. J.
Physiol. Pharmacol., 54: 23-26.
BASTOMSKY, C.H., SOLYMOSS, B., ZSIGMOND, G., & WYSE, J.M. (1975) On
the mechanism of polychlorinated biphenyl-induced hypobili-
rubinaemia. Clin. chim. Acta, 61: 171-174.
BAUMANN, M., DEML, E., SCHAEFFER, E., & GREIM, H. (1983) Effects of
polychlorinated biphenyls at low dose levels in rats. Arch.
environ. Contam. Toxicol., 12: 509-515.
BAXTER, R.A., GILBERT, P.E., LIDGETT, R.A., MAINPRIZE, J.H., &
VODDEN, H.A. (1975) The degradation of polychlorinated biphenyls by
microorganisms. Sci. total Environ., 4: 53-61.
BECK, H. & MATHAR, W. (1985) [Analytical procedures for the
determination of selected components of PCBs in foodstuffs.]
Bundesggesundheitsblatt, 28(1): 1-12 (in German).
BECKER, G.M., MCNULTY, W.P., & BELL, M. (1979) Polychlorinated
biphenyl-induced morphologic changes in the gastric mucosa of the
Rhesus monkey. Lab. Invest., 40: 373-383.
BENGTSON, S.A. & SÖDERGREN, A. (1974) DDT and PCB residues in
airborne fallout and animals in Iceland. Ambio, 3: 84-86.
BENGTSSON, B.E. (1979) Increased growth in minnows exposed to PCBs.
Ambio, 8: 169-170.
BENGTSSON, B.E. (1980) Long-term effects of PCB (Clophen A50) on
growth, reproduction and swimming performance in the minnow,
Phoxinus phoxinus. Water Res., 14: 681-687.
BENTHE, H.F., KNOP, J., & SCHMOLDT, A. (1972) [Absorption and
distribution of polychlorinated biphenyls (PCB) after inhalatory
application.] Arch. Toxikol., 29: 85-95 (in German).
BENTLEY, J. (1983) Incineration of PCBs. In: Barros, M.C.,
Könemann, H., & Visser, R., ed. Proceedings of the PCB Seminar, The
Hague, 28-30 September 1983, The Hague, Ministry of the
Environment, pp. 281-288.
BERAN, M., BRANDT, I., & SLANINA, P. (1983) Distribution and effect
of some polychlorinated biphenyls in the hemopoietic tissues. J.
Toxicol. environ. Health., 12: 521-532.
BERCOVICI, B., WASSERMANN, M., CUCOS, S., RON, M., WASSERMAN, D.,
& PINES, A. (1983) Serum levels of polychlorinated biphenyls and
some organochlorine insecticides in women with recent and former
missed abortions. Environ. Res., 30(1): 169-174.
BERG, O.W., DIOSADY, P.L., & REES, G.A.V. (1972) Column
chromatographic separation of polychlorinated biphenyls from
chlorinated hydrocarbon pesticides and their subsequent gas
chromatographic quantitation in terms of derivatives. Bull.
environ. Contam. Toxicol., 7: 338-347.
BERGH, A.K. & PEOPLE, R.S. (1977) PCB distribution in sewage wastes
and their environmental and community effects. Proceedings of the
1977 National Conference on Treatment and Disposal of Industrial
Wastewaters and Residues, Houston, Texas, pp. 4-6.
BERGLUND, F. (1972) Levels of polychlorinated biphenyls in foods in
Sweden. Environ. health Perspect., 1: 67-69.
BERLIN, M., GAGE, J.C., & HOLM, S. (1973) The metabolism and
distribution of 2,4,5,2',5'-pentachlorobiphenyl in the mouse. In:
Proceedings of the Polychlorinated Biphenyls II Conference,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 101-108 (Publication No. 4E).
BERLIN, M., GAGE, J.C., & HOLM, S. (1975) Distribution and
metabolism of polychlorobiphenyls. In: Proceedings of an
International Symposium on Recent Advances in the Assessment of the
Health Effects of Environmental Pollution, Paris, 24-26 June 1974,
Luxembourg, Commission of the European Communities, Vol. 2,
pp. 895-902.
BERRY, D.L., SLAGA, T.J., DIGIOVANNI, J., & JUCHAU, M.R. (1979)
Studies with chlorinated dibenzo- p-dioxins, polybrominated
biphenyls, and polychlorinated biphenyls in a two-stage system of
mouse skin tumorigenesis: potent anticarcinogenic effects. Ann. NY
Acad. Sci., 320: 405-414.
BERRY, D.L., DIGIOVANNI, J., JUCHAU, M.R., BRACKEN, W.M., GLAESON,
G.L., & SLAGA, T.J. (1978) Lack of tumour promoting ability of
certain environmental chemicals in a two-stage mouse skin
tumorigenesis assay. Res. Commun. chem. Pathol. Pharmacol.,
20: 101-108.
BERTAZZI, P.A., ZOCHETTI, C., GUERCILENA, S., FOGLIA, M.D.,
PESATORI, A., & RIBOLDI, L. (1982) Mortality study of male and
female workers exposed to PCBs. In: Prevention of Occupational
Cancer: International Symposium, Geneva, International Labour
Office, pp. 242-248 (Occupational Safety and Health Series No. 46).
BERTAZZI, P.A., RIBOLDI, L., PESATORI, A., RADICE, L., & ZOCHETTI,
C. (1987) Cancer mortality of capacitor manufacturing workers. Am.
J. ind. Med., 11(2): 165-176.
BICKERS, D.R., HARBER, L.C., KAPPAS, A., & ALVARES, A.P. (1972)
Polychlorinated biphenyls: Comparative effects of high and low
chlorine containing Aroclors on hepatic mixed function oxidase.
Res. Commun. Chem. Pathol. Pharmacol., 3: 505-512.
BIDLEMAN, T.F. & OLNEY, C.E. (1974) Chlorinated hydrocarbons in the
Sargasso Sea atmosphere and surface water. Science, 183: 516-518.
BIDLEMAN, T.F., RICE, C.P., & OLNEY, C.E. (1978) High molecular
weight chlorinated hydrocarbons in the air and sea: Rates and
mechanisms of air/sea transfer. In: Windom, H. & Duce, R.A., ed.
Marine pollutant transfer, Lexington, D.C. Heath and Co.,
pp. 323-351.
BIESSMANN, A. (1982) Effects of PCBs on gonads, sex hormone balance
and reproduction processes of Japanese quail Coturnix coturnix
japonica after ingestion during sexual maturation. Environ.
Pollut., 27: 15-30.
BIGGS, D.C., ROWLAND, R.G., POWERS, C.D., O'CONNERS, H., & WURSTER,
C. (1978) A comparison of the effects of chlordane and PCB on the
growth, photosynthesis, and cell size of estuarine phytoplankton.
Environ. Pollut., 15: 253-263.
BIGGS, D.C., ROWLAND, R.G., & WURSTER, C.F. (1979) Effects of
trichloroethylene, hexachlorobenzene and polychlorinated biphenyls
on the growth and cell size of marine phytoplankton. Bull. environ.
Contam. Toxicol., 21: 196-201.
BIGGS, D.C., POWERS, C.D., ROWLAND, R.G., O'CONNORS, H.B., &
WURSTER, C.F. (1980) Uptake of polychlorinated biphenyls by natural
phytoplankton assemblages: field and laboratory determinations of
14C-PCB particle-water index of sorption. Environ. Pollut.,
22: 101-110.
BILLINGS, W.N., BIDLEMAN, T.F., & VERNBERG, W.B. (1978) Movement of
PCB from a contaminated reservoir into a drinking water supply.
Bull. environ. Contam. Toxicol., 19: 215-222.
BIOCCA, M., GUPTA, B.N., CHAE, K., MCKINNEY, J.D., & MOORE, J.A.
(1981) Toxicity of selected symmetrical hexachlorobiphenyl isomers
in the mouse. Toxicol. appl. Pharmacol., 58: 461-474.
BIRGE, W.J., BLACK, J.A., & WESTERMAN, A.G. (1978) Effects of
polychlorinated biphenyl compounds and proposed PCB-replacement
products on embryo-larval stages of fish and amphibians,
Washington, DC, US Department of the Interior, p. 33 (Research
Report No. 118).
BIRNBAUM, L.S. (1983) Distribution and excretion of 2,3,6,2',3',6'-
and 2,4,5,2',4',5'-hexachlorobiphenyl in senescent rats. Toxicol.
appl. Pharmacol., 70: 262-272.
BIRNBAUM, L. & BAIRD, M.B. (1978) Induction of hepatic mixed
function oxidases in senescent rodents-II. Effect of
polychlorinated biphenyls. Exp. Gerontol., 13: 469-477.
BIRNBAUM, L.S., WEBER, H., HARRIS, M.W., LAMB, J.C., IV, &
MCKINNEY, J.D. (1985) Toxic interaction of specific polychlorinated
biphenyls and 2,3,7,8-tetrachlorodibenzo- p-dioxin: increased
incidence of cleft palate in mice. Toxicol. appl. Pharmacol.,
77: 292-302.
BITMAN, J. & CECIL, H.C. (1970) Estrogenic activity of DDT analogs
and polychlorinated biphenyls. J. agric. food Chem., 18: 1108-1112.
BITMAN, J., CECIL, H.C., & HARRIS, S.J. (1972) Biological effects
of polychlorinated biphenyl in rats and quail. Environ. health
Perspect., 1: 145-149.
BJERK, J.E. (1972) [Residues of DDT and polychlorinated biphenyls
in Norwegian human material.] Tidsskr. Nor. Laegeforen., 92: 15-19
(in Norwegian).
BLEAVINS, M.R., AULERICH, R.J., & RINGER, R.K. (1980)
Polychlorinated biphenyls (Aroclors 1016 and 1242): Effects on
survival and reproduction in mink and ferrets. Arch. environ.
Contam. Toxicol., 9: 627-635.
BLEAVINS, M.R., AULERICH, R.J., RINGER, R.K., & BELL, T.G. (1982)
Excessive nail growth in the European ferret induced by Aroclor
1242. Arch. environ. Contam. Toxicol., 11: 305-312.
BLEAVINS, M.R., AULERICH, R.J., & RINGER, R.K. (1981) Placental and
mammary transfer of polychlorinated and polybrominated biphenyls in
the mink and ferret. In: Proceedings of the 2nd Conference on Avian
and Mammalian Wildlife Toxicology, Philadelphia, Pennsylvania,
American Society for Testing and Material, pp. 121-131
(ASTM STP 757).
BLEAVINS, M.R., BRESLIN, W.J., AULERICH, R.J., & RINGER, R.K.
(1984) Placental and mammary transfer of a polychlorinated biphenyl
mixture (Aroclor 1254) in the European ferret (Mustela putorius
furo). Environ. Toxicol. Chem., 3: 637-644.
BLETCHLY, J.D. (1983) Polychlorinated biphenyls. Production,
current use, and possible rates of future disposal in OECD
countries. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of PCB Seminar, The Hague, 28-30 September 1983, The
Hague, Ministry of the Environment, pp. 343-365.
BLETCHLY, J.D. (1985) Report to the Commission of the European
Communities on a study of measures to avoid dispersion into the
environment of polychlorinated biphenyls (PCBs) and polychlorinated
terphenyls (PCTs) from existing installations, Luxembourg,
Commission of the European Communities, pp. 22-33.
BLOK, S.M.G., GREVE, P.A., SANGSTER, B., SAVELKOUL, T.J.F., &
WEGMAN, R.C.C. (1984) [Study of normally occurring values of a
number of organochlorine pesticides and related compounds and their
metabolites, of polychlorine biphenyls and of chlorophenols in the
blood or plasma of healthy volunteers.] Bilthoven, The Netherlands,
National Institute of Public Health and Environmental Hygiene
(Unpublished report No. 638101001) (in Dutch).
BLUS, L.J., LAMONT, T.G., & NEELY, B.S. (1979) Fish, wildlife, and
estuaries: Effects of organochlorine residues on eggshell
thickness, reproduction, and population status of Brown Pelicans
(Pelecanus occidentalis) in South Carolina and Florida, 1969-76.
Pestic. monit. J., 12: 172-184.
BONNYNS, M. & BASTOMSKY, C.H. (1976) Polychlorinated
biphenyl-induced modification of lymphocyte response to plant
mitogens in rats. Experientia (Basel), 32: 522-523.
BORN, E.W., KRAUL, I., & KRISTENSEN, T. (1981) Mercury, DDT, and
PCB in the Atlantic Walrus (Odobenus rosmarus rosmarus) from the
Thule District, North Greenland. Arctic, 34: 255-260.
BOURQUIN, A.W. & CASSIDY, S. (1975) Effect of polychlorinated
biphenyl formulations on the growth of estuarine bacteria. Appl.
microbiol., 29: 125-127.
BOURQUIN, A.W. & KIEFER, L.A. (1975) Inhibition of estuarine
microorganisms by polychlorinated biphenyls. Dev. ind. Microbiol.,
16: 256-261.
BOWES, G.W. & JONKEL, C.J. (1975) Presence and distribution of
polychlorinated biphenyls (PCBs) in arctic and subarctic marine
food chains. J. Fish Res. Board Can., 32: 2111-2123.
BOWES, G.W., MULVIHILL, M.J., SIMONEIT, B.R.T., BURLINGAME, A.L.,
& RISEBROUGH, R.W. (1975) Identification of chlorinated
dibenzofurans in American polychlorinated biphenyls. Nature
(Lond.), 256: 305-307.
BOWMAN, R.E. & HEIRONIMUS, M.P. (1981) Hypoactivity in adolescent
monkeys perinatally exposed to PCBs and hyperactive as juveniles.
Neurobehav. Toxicol. Teratol., 3: 15-18.
BOWMAN, R.E., HEIRONIMUS, M.P., & ALLEN, J.R. (1978) Correlation of
PCB body burden with behavioural toxicology in monkeys. Pharmacol.
Biochem. Behav., 9: 49-56.
BOWMAN, R.E., HEIRONIMUS, M.P., & BARSOTTI, D.A. (1982) Locomotor
hyperactivity in PCB-exposed monkeys. Neurotoxicology, 2: 251-268.
BRANDT-RAUF, P.W. & NIMAN, H.L. (1988) Serum screening for oncogene
proteins in workers exposed to PCBs. Br. J. ind. Med.,
45(10): 689-693.
BRAUN, F. & MEYHOFER, B. (1977) [Investigations on the
concentration of polychlorinated biphenyls (Clophen C) in fish
organs under laboratory conditions.] Fisch Umwelt., 3: 1-11
(in German).
BRAZELTON, T.B. (1973) Neonatal behavioural assessment scale,
Philadelphia, Pennsylvania, J.B. Lippincott.
BREZNER, E., TERKEL, J., & PERRY, A.S. (1984) The effect of Aroclor
1254 (PCB) on the physiology of reproduction in the female rat-I.
Comp. Biochem. Physiol., 77C: 65-70.
BRIGGS, D.M. & HARRIS, J.R. (1973) Polychlorinated biphenyls
influence on hatchability. Poult. Sci., 52: 1291-1294.
BRINKMAN, U.A. & DE KOK, A. (1980) Production properties and usage.
In: Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins
and related products, Amsterdam, Elsevier Biomedical Press,
pp. 1-40.
BRINKMAN, M., FOGELMAN, K., HOEFLEIN, J., LINDH, T., PATEL, M.,
TRENCH, W.C., & AIKENS, D.A. (1980) Distribution of polychlorinated
biphenyls in the Fort Edward, New York, water system. Environ.
Manage., 4(6): 511-520.
BRINKMAN, M., FOGELMAN, K., HOEFLEIN, J., LINDH, T., PATEL, M.,
TRENCH, W.C., & AIKENS, D.A. (1981) Levels of polychlorinated
biphenyls in the Fort Edward, New York, water system. Adv. Identif.
Anal. Org. Pollut. Water, 2(50): 1001-1015.
BRITTON, W.M. & HUSTON, T.M. (1972) Yolk content and hatchability
of egg from hens fed Aroclor 1242. Poult. Sci., 51: 1869.
BROADHURST, M.G. (1972) Use and replaceability of polychlorinated
biphenyls. Environ. health Perspect., 2: 81-102.
BROUWER, A., VAN DEN BERG, K.J., & KUKLER, A. (1985) Time and dose
responses of the reduction in retinoid concentrations in C57BL/Rij
and DBA/2 mice induced by 3,4,3',4'-tetrachlorobiphenyl. Toxico.
appl. Pharmacol., 78: 180-189.
BROUWER, A., KUKLER, A., & VAN DEN BERG, K.J. (1988) Alterations in
retinoid concentrations in several extrahepatic organs of rats by
3,4,3',4'-tetrachlorobiphenyl. Toxicology, 50: 317-330.
BROUWER, A., REIJNDERS, P.J.H., & KOEMAN, J.H. (1989)
Polychlorinated biphenyl (PCB)-contaminated fish induces vitamin A
and thyroid hormone deficiency in the common seal (Phoca
vitulina). Aquat. Toxicol., 15: 99-106.
BROWN, D.P. (1987) Mortality of workers exposed to polychlorinated
biphenyls - An update. Arch. environ. Health, 42(6): 333-339.
BROWN, D.P. & JONES, M. (1981) Mortality and industrial hygiene
study of workers exposed to polychlorinated biphenyls. Arch.
environ. Health, 36: 120-129.
BROWN, J.F. & LAWTON, R.W. (1984) Polychlorinated biphenyl (PCB)
partitioning between adipose tissue and serum. Bull. environ.
Contam. Toxicol., 33: 277-280.
BROWN, J.F., BEDARD, D.L., BRENNAN, M.J., CARNAHAN, J.C., FENG, H.,
& WAGNER, R.E. (1987a) Polychlorinated biphenyl dechlorination in
aquatic sediments. Science, 236: 709-712.
BROWN, J.F., WAGNER, R.E., FENG, H., BEDARD, D.L., BRENNAN, M.J.,
CARNAHAN, J.C., & MAY, R.J. (1987b) Environmental dechlorination of
PCBs. Environ. Toxicol. Chem., 6: 579-593.
BROWN, J.F., CARNAHAN, J.C., DORN, S.B., GROVES, J.T., LIGON, W.V.,
MAY, R.J., WAGNER, R.E., & HAMILTON, S.B. (1988) Levels of
bioactive PCDF congeners in PCB dieletric fluids from capacitors
and transformers. Chemosphere, 17(9): 1697-1702.
BRUCE, R.W. & HEDDLE, J.A. (1979) The mutagenic activity of 61
agents as determined by the micronucleus, Salmonella, and sperm
abnormality assays. Can. J. Genet. Cytol., 21: 319-334.
BRUCKNER, J.V., KHANNA, K.L., & CORNISH, H.H. (1973) Biological
responses of the rat to polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 24: 434-448.
BRUCKNER, J.V., KHANNA, K.L., & CORNISH, H.H. (1974) Effect of
prolonged ingestion of polychlorinated biphenyls on the rat. Food
Cosmet. Toxicol., 12: 323-330.
BRUCKNER, J.V., JIANG, W.-D., BROWN, J.M., PUTCHA, L., CHU, C.K.,
& STELLA, V.J. (1977) The influence of ingestion of environmentally
encountered levels of a commercial polychlorinated biphenyl mixture
(Aroclor 1254) on drug metabolism in the rat. J. Pharmacol. exp.
Ther., 202: 22-31.
BRUNSTROM, B. & DARNERUD, P.O. (1983) Toxicity and distribution in
chick embryos of 3,3,4,4-tetrachlorobiphenyl injected into the
eggs. Toxicology, 27: 103-110.
BRUNSTROM, B. & REUTERGARDH, L. (1986) Differences in sensitivity
of some avian species to the embryotoxicity of a PCB,
3,3',4,4'-tetrachlorobiphenyl, injected into the eggs. Environ.
Pollut., 42: 37-45.
BRUNSTROM, B., KIHLSTROM, I., & LUNDKVIST, U. (1982) Studies of
foetal death and foetal weight in guinea-pigs fed polychlorinated
biphenyls (PCB). Acta pharmacol. toxicol., 50: 100-103.
BRUNSTRÖM, B., DARNERUD, P.O., BRANDT, I., & ORBERG, J. (1982a)
Distribution, metabolism and toxicity of 2,2,4,5-tetrachloro-
biphenyl after injection into the yolk of embryonated hens' eggs.
Ambio, 11: 212-214.
BRUNSTRÖM, B., KIHLSTRÖM, I., & LUNDKVIST, U. (1982b) Studies of
foetal death and foetal weight in guinea-pigs fed polychlorinated
biphenyls (PCB). Acta pharmacol. toxicol., 50: 100-103.
BUCKLEY, E.H. (1982) Accumulation of airborne polychlorinated
biphenyls in foliage. Science, 216: 520-522.
BUCKLEY, E.H. (1983) Decline of background PCB concentrations in
vegetation in New York state. Northeast. environ. Sci., 2: 181-187.
BUHLER, F., SCHMID, P., & SCHLATTER, Ch. (1988) Kinetics of PCB
elimination in man. Chemosphere, 17(9): 1717-1726.
BUNCE, N.J. (1978) Photolysis of 2-chlorobiphenyl in aqueous
acetonitrile. Chemosphere, 7: 653-656.
BUNCE, N.J., KUMAR, Y., & BROWNLEE, B.G. (1978) An assessment of
the impact of solar degradation of polychlorinated biphenyls in the
aquatic environment. Chemosphere, 7: 155-164.
BURKHARD, L.P., ARMSTRONG, D.E., & ANDREN, A.W. (1985) Henry's Law
Constants for the polychlorinated biphenyls. Environ. Sci.
Technol., 19(7): 590-596.
BURNS, J.E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue-Texas 1969-72.
Pestic. monit. J., 7(3/4): 122-126.
BURSE, V.W., KIMBROUGH, R.D., VILLANUEVA, E.C., JENNINGS, R.W.,
LINDER, R.E., & SOVOCOOL, G.W. (1974) Polychlorinated biphenyls;
storage, distribution, excretion, and recovery: liver morphology
after prolonged dietary ingestion. Arch. environ. Health,
29: 301-307.
BUSER, H.R. (1979) Formation of polychlorinated dibenzofurans
(PCDFs) and dibenzo- p-dioxins (PCDDs) from the pyrolysis of
chlorobenzenes. Chemosphere, 8: 415-424.
BUSER, H.R. (1985) Formation, occurrence, and analysis of
polychlorinated dibenzofurans, dioxins and related compounds.
Environ. health Perspect., 60: 259-267.
BUSER, H.R. & BOSSHARDT, H.P. (1978) [Polychlorinated
dibenzo- p-dioxins, dibenzofurans and benzenes in the ash of
community and industrial incineration plants.] Mitt. Geb. Lebensm.
Hyg., 69: 191-199 (in German).
BUSER, H.R. & RAPPE, C. (1979) Formation of polychlorinated
dibenzofurans (PCDFs) from the pyrolysis of individual PCB isomers.
Chemosphere, 3: 157-174.
BUSER, H.R., BOSSHARDT, H.P., & RAPPE, C. (1978a) Formation of
polychlorinated dibenzofurans (PCDF's) from the pyrolysis of PCBs.
Chemosphere, 7: 109-119.
BUSER, H.R., BOSSHARDT, H.P., RAPPE, C., & LINDAHL, R. (1978b)
Identification of polychlorinated dibenzofuran isomers in fly ash
and PCB pyrolysis. Chemosphere, 7: 419-429.
BUSH, B., TUMASONIS, C.F., & BAKER, F.D. (1974) Toxicity and
persistence of PCB homologs and isomers in the avian system. Arch.
environ. Contam. Toxicol., 2: 195-212.
BUSH, B., SNOW, J., & KOBLINTZ, R. (1984) Polychlorobiphenyl (PCB)
congeners, p,p'-DDE, and hexachlorobenzene in maternal and fetal
cord blood from mothers in upstate New York. Arch. environ. Contam.
Toxicol., 13(5): 517-527.
BUSH, B., SNOW, J., CONNOR, S., & KOBLINTZ, R. (1985)
Polychlorinated biphenyl congeners (PCBs), p,p'-DDE and
hexachlorobenzene in human milk in three areas of upstate New York.
Arch. environ. Contam. Toxicol., 14: 443-450.
BUSH, B., SIMPSON, K.W., SHANE, L., & KOBLINTZ, R.R. (1985) PCB
congener analysis of water and caddisfly larvae (Insecta:
Trichoptera) in the Upper Hudson river by glass capillary
chromatography. Bull. environ. Contam. Toxicol., 34: 96-105.
BYRNE, J.J. & SEPKOVIC, D.W. (1987) Inhibition of monovalent cation
transport across the cell membrane by polychlorinated biphenyl but
not by polybrominated biphenyl. Arch. environ. Contam. Toxicol.,
16: 573-577.
BYRNE, J.J., CARBONE, J.P., & PEPE, M.G. (1988) Suppression of
serum adrenal cortex hormones by chronic low-dose polychloro-
biphenyl of polybromobiphenyl treatments. Arch. environ. Contam.
Toxicol., 17: 47-53.
CAIN, B.W. (1981) Nationwide residues of organochlorine compounds
in wings of adult mallards and black ducks, 1979-80. Pestic. monit.
J., 15: 128-134.
CAIN, B.W. & BUNCK, C.M. (1983) Residues of organochlorine
compounds in starlings (Sturnus vulgaris), 1979. Environ. monit.
Assess., 3: 161-172.
CALANDRA, J.C. (1976) Summary of toxicological studies on
commercial PCBs. In: Proceedings of the National Conference of
polychlorinated biphenyls, Washington, DC, US Environmental
Protection Agency (560/6-75-004).
CALIFANO, R.J., O'CONNOR, J.M., & PETERS, L.S. (1980) Uptake,
retention, and elimination of PCB (Aroclor 1254) by larval striped
bass (Morone saxatilis). Bull. environ. Contam. Toxicol.,
24: 467-472.
CALL, D.J. & HARRELL, B.E. (1974) Effects of dieldrin and PCBs upon
the production and morphology of Japanese quail eggs. Bull.
environ. Contam. Toxicol., 11: 70-77.
CALLAHAN, M.A.A., HAMMERSTROM, K.A., & SCHWEER, G. (1983) Present
PCB uses and their potential for release to the environment. In:
Barros, M.C., Könemann, H., & Visser, R., ed. Proceedings of PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 152-172.
CAMP, B.J., HEJTMANCIK, E., ARMOUR, C., & LEWIS, D.H. (1974) Acute
effects of Aroclor 1254 (PCB) on Ictalurus punctatus (catfish).
Bull. environ. Contam. Toxicol., 12: 204-208.
CAMPS, M., PLANAS, J., GOMEZ-CATALAN, J., SABROSO, M., TO-FIGUERAS,
J., & CORBELLA, J. (1989) Organochlorine residues in human adipose
tissue in Spain: Study of an agrarian area. Bull. environ. Contam.
Toxicol., 42: 195-201.
CANTONI, C., FABBRIS, F., ROGLEDI, R., & CAMPAGNARI, A. (1988)
[Organochlorine pesticides found in foods of animal origin in the
1985-1987 biennium.] Ind. Aliment., XXVII(1): 6-8 (in Italian).
CARLSON, R.W. & DUBY, R.T. (1973) Embryotoxic effects of three
PCB's in the chicken. Bull. environ. Contam. Toxicol., 9: 261-266.
CARNES, R.A., DOERGER, J.U., & SPARKS, H.L. (1973) Polychlorinated
biphenyls in solid waste and solid-waste-related materials. Arch.
environ. Contam. Toxicol., 1: 27-35.
CAREY, A.E. & HARVEY, G.R. (1978) Metabolism of polychlorinated
biphenyls by marine bacteria. Bull. environ. Contam. Toxicol.,
20: 527-534.
CARTER, J.W. (1985) Effects of dietary in PCBs (Aroclor 1254) on
serum levels of lipoprotein cholesterol in Fischer rats. Bull.
environ. Contam. Toxicol., 34: 427-431.
CARTER, J.W. & CLANCY, J. (1980) Acutely administered
polychlorinated biphenyls (PCBs) decrease splenic cellularity but
increase its ability to cause graft-versus host reactions in BALB/c
mice. Immunopharmacology, 2: 341-347.
CATELANI, D., SORLINI, C., & TRECCANI, V. (1971) The metabolism of
biphenyl by Pseudomonas putida. Experientia (Basel),
27: 1173-1174.
CECIL, H.C., BITMAN, J., LILLIE, R.J., FRIES, G.F., & VERRETT, J.
(1974) Embryotoxic and teratogenic effects in unhatched fertile
eggs from hens fed polychlorinated biphenyls (PCBs). Bull. environ.
Contam. Toxicol., 11: 489-495.
CETINKAYA, M., GABEL, B., PODBIELSKI, A., & THIEMANN, W. (1984)
[Investigation on the association between nutrition and living
conditions of breastfeeding mothers and the contamination of
breastmilk with organochlorine compounds of low volatility.] Akt.
Ernähr., 9: 157-162 (in German).
CHAKRABORTY, D., BHATTACHARYYA, A., CHATTERJEE, J., CHATTERJEE, K.,
SEN, A., CHATTERJEE, S., MAJUMDAR, K., & CHATTERJEE, G.C. (1978)
Biochemical studies on polychlorinated biphenyl toxicity in rats:
manipulation by vitamin C. Int. J. Vitam. Nutr. Res., 48: 22-31.
CHANG, K.-T., CHENG, J.-S., HUANG, P.-C., & TUNG, T.-C. (1980a)
Study of patients with PCB poisoning. J. Formosan Med. Assoc.,
79: 304-313.
CHANG, K.-J., LU, F.-J., TUNG, T.-C., & LEE, T.-P. (1980b) Studies
on patients with PCB poisoning. Determination of urinary
coproporphyrin, uroporphyrin, delta-aminolaevulinic acid and
prophobilinogen. Res. Commun. chem. Pathol. Pharmacol.,
30(3): 547-554.
CHANG, K.-J., HSIEH, K.-H., LEE, T.-P., TANG, S.-Y., & TUNG, T.-C.
(1981) Immunologic evaluation of patients with polychlorinated
biphenyls poisoning. Determination of lymphocyte subpopulations.
Toxicol. appl. Pharmacol., 61: 58-63.
CHASE, K.H., WONG, O., THOMAS, D., BERNEY, B.W., & SIMON, R.K.
(1982) Clinical and metabolic abnormalities associated with
occupational exposure to polychlorinated biphenyls (PCBs). J.
occup. Med., 24(2): 109-114.
CHEN, P.H., GRAW, J.M., WONG, C.K., & CHEN, C.J. (1980) Levels and
gas chromatography patterns of PCBs in blood of patients after PCB
poisoning in Taiwan. Bull. environ. Contam. Toxicol., 25: 325-329.
CHEN, P.H., CHANG, K.T., & LU, Y.D. (1981) Polychlorinated
biphenyls and polychlorinated benzofurans in the toxic rice-bran
oil caused PCB poisoning in Taichyung. Bull. environ. Contam.
Toxicol., 26(4): 489-495.
CHEN, P.H., LUO, M.L., WONG, C.K., & CHEN, C.J. (1982) Comparative
rates of elimination of some individual polychlorinated biphenyls
from the blood of PCB-poisoned patients in Taiwan. Food chem.
Toxicol., 20(4): 417-425.
CHEN, P.H., WONG, C.-K., RAPPE, C., & NYGREN, M. (1985)
Polychlorinated biphenyls, dibenzofurans and quaterphenyls in toxic
rice-bran oil and in the blood and tissues of patients with PCB
poisoning (Yu-Cheng) in Taiwan. Environ. Health Perspect.,
59: 59-65.
CHEN, P.R., MCKINNEY, J.D., & MATTHEWS, H.B. (1976) Metabolism of
2,4,5,2',5'-pentachlorobiphenyl in the rat. Drug. Metab. Dispos.,
4: 362-367.
CHEN, R.-C., TANG, S.-Y., MIYATA, H., KASHIMOTO, T., CHANG, Y.-C.,
CHANG, K.-J., & TUNG, T.-C. (1985) Polychlorinated biphenyl
poisoning: Correlation of sensory and motor nerve conduction,
neurologic symptoms and blood levels of polychlorinated biphenyls,
quaterphenyls, and dibenzofurans. Environ. Res., 37: 340-348.
CHEN, T.S. & DUBOIS, K.P. (1973) Studies on the enzyme inducing
effects of polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
26: 504-512.
CHEN HSI-SUNG, P., LUO, M.-L., WONG, C.-K., & CHEN, C.-J. (1984)
Polychlorinated biphenyls, dibenzofurans, and quaterphenyls in the
toxic rice-bran oil and PCBs in the blood of patients with PCB
poisoning in Taiwan. Am. J. ind. Med., 5: 133-145.
CHESNEY, C.F. & ALLEN, J.R. (1974) Oxidative phosphorylation and
respiration by liver mitochondria from polychlorinated
biphenyl-intoxicated rats. Biochem. Pharmacol., 23: 1577-1582.
CHIA, L.-G. & CHU, F.-L. (1984) Neurological studies on
polychlorinated biphenyl (PCB)-poisoned patients. Am. J. ind. Med.,
5: 117-126.
CHOU, S.M., MIKE, T., PAYNE, W.M., & DAVIS, G.J. (1979)
Neuropathology of "spinning syndrome" induced by prenatal
intoxication with a PCB in mice. Ann. NY Acad. Sci., 320: 373-395.
CHOW, C.K., THACKER, R., & GAIROLA, C.C. (1979) Increased level of
L-ascorbic acid in the plasma of polychlorobiphenyls-treated rats
and its inhibition by dietary vitamin E. Res. Commun. chem. Pathol.
Pharmacol., 26: 605-608.
CHRISTIANI, D.C., KRIEBEL, D., FOX, N.J., & BAKER, E.L. (1986)
Persistently elevated polychlorinated biphenyl levels from residual
contamination of workplace surfaces. Am. J. ind. Med., 10: 143-151.
CHU, C.K., STELLA, V.J., BRUCKNER, J.V., & JIANG, W.D. (1977)
Effects of long-term exposure to environmental levels of
polychlorinated biphenyls on pharmacokinetics of pentobarbital in
rats. J. pharm. Sci., 66(2): 238-241.
CLARK, D.R. (1978) Uptake of dietary PCB by pregnant Big Brown Bats
(Eptesicus fuscus) and their fetuses. Bull. environ. Contam.
Toxicol., 19: 707-714.
CLARK, D.R. & LAMONT, T.G. (1976) Organochlorine residues and
reproduction in the big brown bat. J. Wildl. Manage., 40: 249-254.
CLARK, D.R. & PROUTY, R.M. (1977) Experimental feeding of DDT and
PCB to female Big Brown Bats (Eptesicus fuscus). J. Toxicol.
environ. Health, 2: 917-928.
CLARK, R.R., CHIAN, E.S.K., & GRIFFIN, R.A. (1979) Degradation of
polychlorinated biphenyls by mixed microbial cultures. Appl.
environ. Microbiol., 37: 680-685.
CLARK, J.R., PATRICK, J.M., MOORE, J.C., & FORESTER, J. (1986)
Accumulation of sediment-bound PCBs by Fiddler crabs. Bull.
environ. Contam. Toxicol., 36: 571-578.
CLAUS, B. & ACKER, L. (1975) [Contamination of milk and milk
products with chlorinated hydrocarbons in Westphalia. II. Results
and discussion.] Z. Lebensmittelunters. Forsch., 159(3): 129-137
(in German).
CLEVENGER, M.A., ROBERTS, S.M., LATTIN, D.L., HARBINSON, R.D., &
JAMES, R.C. (1989) The pharmacokinetics of 2,2,5,5'-tetrachloro-
biphenyl and 3,3',4,4'-tetrachlorobiphenyl and its relationship to
toxicity. Toxicol. appl. Pharmacol., 100: 315-327.
COLE, D.R. & PLAPP, F.W. (1974) Inhibition of growth and
photosynthesis in Chlorella pyrenoidosa by a polychlorinated
biphenyl and several insecticides. Environ. Entomol., 3: 217-220.
COLLINS, W.T. & CAPEN, C.C. (1980a) Ultrastructural and functional
alterations of the rat's thyroid gland produced by polychlorinated
biphenyls compared with iodide excess and deficiency, and
thyrotropin and thyroxine administration. Virchows Arch. cell.
Pathol., B33: 213-231.
COLLINS, W.T. & CAPEN, C.C. (1980b) Biliary excretion of 125
I-thyroxine and fine structural alterations in the thyroid glands
of Gunn rats fed polychlorinated biphenyls (PCB). Lab. Invest.,
43: 158-164.
COLLINS, G.B., HOLMES, D.C., & JACKSON, F.J. (1972) The estimation
of polychlorobiphenyls. J. Chromatogr., 71: 443-449.
COLLINS, W.T., CAPEN, C.C., KASZA, L., CARTER, C., & DAILEY, R.E.
(1977) Effect of polychlorinated biphenyl (PCB) on the thyroid
gland of rats. Am. J. Pathol., 89: 119-136.
CONNELL, D.W. (1987) Age to PCB concentration relationship with the
striped bass (Morone saxatilis) in the Hudson River and Long
Island Sound. Chemosphere, 16: 1469-1474.
COOKE, A.S. (1973) Shell thinning in avian eggs by environmental
pollutants. Environ. Pollut., 4: 85-152.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides and pollution, Huntingdon, United Kingdom, Natural
Environment Research Council, Institute of Terrestrial Ecology,
74 pp.
COOLEY, N.R., KELTNER, J.M., & FORESTER, J. (1972) Mirex and
Aroclor 1254: Effect on accumulation by Tetrahymena pyriformis
strain W. J. Protozool., 19: 636-638.
COOLEY, N.R., KELTNER, J.M., & FORESTER, J. (1973) The
polychlorinated biphenyls, Aroclors 1248 and 1260: Effect on and
accumulation by Tetrahymena pyriformis. J. Protozool.,
20: 443-445.
COSPER, E.M., WURSTER, C.F., & ROWLAND, R.G. (1984) PCB resistance
within phytoplankton populations in polluted and unpolluted marine
environments. Mar. environ. Res., 12: 209-223.
COTE, M.G., PLAA, G.L., VALLI, V.E., & VILLENEUVE, D.C. (1985)
Subchronic effects of a mixture of "persistent" chemicals found in
the Great Lakes. Bull. environ. Contam. Toxicol., 34: 285-290.
COURTNEY, W.A.M. & DENTON, G.R.W. (1976) Persistence of
polychlorinated biphenyls in the hard-clam (Mercenaria mercenaria)
and the effect upon the distribution of these pollutants in the
estuarine environment. Environ. Pollut., 10: 55-64.
COURTNEY, W.A.M. & LANGSTON, W.J. (1978) Uptake of polychlorinated
biphenyl (Aroclor 1254) from sediment and seawater in two
intertidal polychaetes. Environ. Pollut., 15: 303-309.
CRAIGIE, J.S. & HUTZINGER, O. (1975) Effects of commercial
chlorinated hydrocarbons and specific chlorobiphenyls on the growth
of seven species of marine phytoplankton. Chemosphere, 3: 139-144.
CROSBY, D.G. & MOILANEN, K.W. (1973) Photodecomposition of
chlorinated biphenyls and dibenzofurans. Bull. environ. Contam.
Toxicol., 10: 372-377.
CURLEY, A., BURSE, V.W., GRIM, M.E., JENNINGS, R.W., & LINDER, R.E.
(1971) Polychlorinated biphenyls: Distribution and storage in body
fluids and tissues of Sherman rats. Environ. Res., 4: 481-495.
CURLEY, A., BURSE, V.W., & GRIM, M.E. (1973a) Polychlorinated
biphenyls: Evidence of transplacental passage in the Sherman rats.
Food Cosmet. Toxicol., 11: 471-476.
CURLEY, A., BURSE, V.W., JENNINGS, R.W., VILLANUEVA, E.C., TOMATIS,
L., & AKAZAKI, K. (1973b) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond.), 242: 338-340.
DAHLGREN, R.B. & LINDER, R.L. (1971) Effects of polychlorinated
biphenyls on pheasant reproduction, behavior, and survival. J.
Wildl. Manage., 35: 315-319.
DAHLGREN, R.B., LINDER, R.L., & CARLSON, C.W. (1972)
Polychlorinated biphenyls: Their effects on penned pheasants.
Environ. health Perspect., 1: 89-101.
DAHLGREN, R.B., BURY, R.J., LINDER, R.L., & REIDINGER, R.F. (1972)
Residue levels and histopathology in Pheasants given
polychlorinated biphenyls. J. Wildl. Manage., 36: 524-533.
DAVIDORF, F.H. & KNUPP, J.A. (1979) Epidemiology of ocular
melanoma. Incidence and geographic relationship in Ohio
(1967-1977). Ohio State med. J., 75: 561-564.
DAVIES, K. (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17(2): 263-276.
DAVIES, D. & MES, J. (1987) Comparison of the residue levels of
some organochlorine compounds in breast milk of the general and
indigenous Canadian populations. Bull. environ. Contam. Toxicol.,
39: 743-749.
DAVIS, D. & SAFE, S. (1989) Dose-response immunotoxicities of
commercial polychlorinated biphenyls (PCBs) and their interaction
with 2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol. Lett.,
48: 35-43.
DEFOE, D.L., VEITH, G.D., & CARLSON, R.W. (1978) Effects of Aroclor
1248 and 1260 on the Fathead Minnow (Pimephales promelas). J.
Fish Res. Board Can., 35: 997-1002.
DEFREITAS, A.S.W., NORSTRÖM, R.J., & HUTZINGER, O. (1972) Dynamics
and metabolism of polychlorinated biphenyls (PCBs) in cold-exposed
pigeons. Am. Chem. Soc., 12: 118-123.
DE KOCK, A.C. & LORD, D.A. (1988) Kinetics of the uptake and
elimination of polychlorinated biphenyls by an estuarine fish
species (Rhabdosargus holubi) after aqueous exposure.
Chemosphere, 17: 2381-2390.
DELFINO, J.J. (1979) Toxic substances in the Great Lakes. Environ.
Sci. Technol., 13: 1462-1468.
DELONG, R.L., GILMARTIN, W.G., & SIMPSON, J.G. (1973) Premature
births in California sea lions: Association with high
organochlorine pollutant residue levels. Science, 181: 1168-1169.
DEML, E. & OESTERLE, D. (1982) Sex-dependent promoting effect of
polychlorinated biphenyls on enzyme-altered islands induced by
diethylnitrosamine in rat liver. Carcinogenesis, 3: 1449-1453.
DEML, E. & OESTERLE, D. (1987) Dose-response of promotion by
polychlorinated biphenyls and chloroform in rat liver foci
bioassay. Arch. Toxicol., 60: 209-211.
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels of
organochlorine pesticides on microsomal liver enzymes in short-term
toxicity experiments. Toxicology, 2: 371-380.
DE VOS, R.H. & PEET, E.W. (1971) Thin-layer chromatography of
polychlorinated biphenyls. Bull. environ. Contam. Toxicol.,
6: 164-170.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRIJNS, J.K., MUYS,
T., & VAN DER POLL, J.M. (1984) Pesticides and other chemical
residues in Dutch total diet samples (June 1976-July 1978). Food
Chem. Toxicol., 22(1): 11-21.
DFG (1988) [Polychlorinated biphenyls. Stocktaking on analysis,
occurrence, kinetics and toxicology.] German Association for the
Encouragement of Research: Communication XIII of the Government
Committee on the Testing of Residues in Foods], Weinheim, Verlag
Chemie (in German).
DICKHUT, R.M., ANDREN, A.W., & ARMSTRONG, D.E. (1986) Aqueous
solubilities of six polychlorinated biphenyl congeners at four
temperatures. Environ. Sci. Technol., 20(8): 807-810.
DICKHUT, R.M., ANDREN, A.W., & ARMSTRONG, D.E. (1987) Comment on
"Aqueous solubilities of six polychlorinated biphenyl congeners at
four temperatures". Environ. Sci. Technol., 21(9): 926-928.
DIERINGER, C.S., LAMARTINIERE, C.A., SCHILLER, C.M., & LUCIER, G.W.
(1979) Altered ontogeny of hepatic steroid-metabolizing enzymes by
pure polychlorinated biphenyl congeners. Biochem. Pharmacol.,
28: 2511-2514.
DIETER, M.P. (1974) Plasma enzyme activities in Coturnix quail fed
graded doses of DDE, polychlorinated biphenyls, malathion, and
mercuric chloride. Toxicol. appl. Pharmacol., 27: 86-98.
DIGERNES, V. & ASTRUP, E.G. (1982) Are datascreen terminals a
source of increased PCB-concentrations in the working atmosphere?
Int. Arch. occup. Health, 49: 193-197.
DIGIOVANNI, J., BERRY, D.L., SLAGA, T.J., & JUCHAU, M.R. (1979)
Studies on the relationship between induction of biotransformation
and tumour-initiating activity of 7,12-dimethylbenz(a)anthracene in
mouse skin. In: Jones, P.W. & Leber, P., ed. Polynuclear aromatic
hydrocarbons, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 553-568.
DILLON, J.C., MARTIN, G.B., & O'BRIEN, H.T. (1981) Pesticide
residues in human milk. Food Cosmet. Toxicol., 19: 437-442.
DIVE, D., ERB, F., LECLERC, H., PRIEM, M.N., & COLEIN, M.P. (1976)
Toxicité et bioaccumulation d'isomers de polychlorobiphenyles par
le protozoaire cilie Colpidium campylum. Eur. J. Toxicol.
environ. Hyg., 9: 105-111.
DOBSON, S. (1981) Physiological and behavioural effects of
organochlorines on pigeons. Oekol. Voegel, 3: 39-43.
DOGRA, S., FILSER, J.G., COJOCEL, C., GREIM, H., REGEL, U., OESCH,
F., & ROBERTSON, L.W. (1988) Long-term effects of commercial and
congeneric polychlorinated biphenyls on ethane production and
malondialdehyde levels, indicators of in vivo lipid peroxidation.
Arch. Toxicol., 62: 369-374.
DOGUCHI, M. (1977) Polychlorinated terphenyls as an environmental
pollutant in Japan. Ecotoxicol. environ. Saf., 1: 239-248.
DOGUCHI, M. & FUKANO, S. (1975) Residue levels of polychlorinated
terphenyls, polychlorinated biphenyls and DDT in human blood. Bull.
environ. Contam. Toxicol., 13: 57-63.
DOGUCHI, M., FUKANO, S., & USHIO, F. (1974) Polychlorinated
terphenyls in the human fat. Bull. environ. Contam. Toxicol.,
11(2): 157-158.
DOMMARCO, R., DI MUCCIO, A., COMONI, I., & GIGLI, B. (1987)
Organochlorine pesticide and polychlorinated biphenyl residues in
human milk from Rome (Italy) and surroundings. Bull. environ.
Contam. Toxicol., 39: 919-925.
DONKIN, P., MANN, S., & HAMILTON, E.I. (1981) Polychlorinated
biphenyl, DDT, and dieldrin residues in grey seal (Halichoerus
grypus) males, females and mother-foetus pairs sampled at the
Farne islands, England, during the breeding season. Sci. total
Environ., 19: 121-142.
DOUCETTE, W.J. & ANDREN, A.W. (1988) Aqueous solubility of selected
biphenyl, furan and dioxin congeners. Chemosphere, 17(2): 243-252.
DRIJVER, M., DUIJKERS, T.J., KROMHOUT, D., VISSER, T.J., MULDER,
P., & LOUW, R. (1988) Determinations of polychlorinated biphenyls
(PCBs) in human milk. Acta paediatr. Scand., 77: 30-36.
DRILL, V.A., FREISS, S.L., HAYS, H.W., LOOMIS, T.A., & SCHAFFER,
C.B. (1981) Potential health effects in the human from exposure to
polychlorinated biphenyls (PCBs) and related impurities, Arlington,
Virginia, Drill, Freiss, Hays, Loomis and Schaffer, Inc.
(Unpublished report).
DUINKER, J.C. & BOUCHERTALL, F. (1989) On the distribution of
atmospheric polychlorinated biphenyl congeners between vapor phase,
aerosols and rain. Environ. Sci. Technol., 23: 57-62.
DUINKER, J.C. & HILLEBRAND, M.T.J. (1979) Mobilization of
organochlorines from female lipid tissue and transplacental
transfer to fetus in a harbour porpoise (Phocoena phocoena) in a
contaminated area. Bull. environ. Contam. Toxicol., 23: 728-732.
DUINKER, J.C. & HILLEBRAND, M.T.J. (1983) Characterization of PCB
components in Clophen formulations by capillary GC-MS and GC-ECD
techniques. Environ. Sci. Technol., 17: 449-456.
DUINKER, J.C., SCHULTZ, D.E., & PETRICK, G. (1988) Selection of
chlorinated biphenyl congeners for analysis in environmental
samples. Mar. Pollut. Bull., 19(1): 19-25.
DUKE, T.W., LOWE, J.I., & WILSON, A.J. (1970) A polychlorinated
biphenyl (Aroclor 1254) in the water, sediment, and biota of
Escambia Bay, Florida. Bull. environ. Contam. Toxicol., 5: 171-180.
DUNN, W.I., III, STELLING, D.L., SCHWARTZ, T.R., HOGAN, J.W.,
PETTY, J.D., JOHANSSON, E., & WOLD, S. (1984) Pattern recognition
for classification and determination of polychlorinated biphenyls
in environmental samples. Anal. Chem., 56: 1308-1313.
DUNNIVANT, F.M. & ELZERMAN, A.W. (1988) Aqueous solubility and
Henry's Law Constant data for PCB congeners for evaluation of
quantitative structure-property relationships (QSPRs). Chemosphere,
17(3): 525-541.
DURHAM, S.K. & BROUWER, A. (1989) 3,4,3',4'-tetrachloro-
biphenyl-induced effects in the rat liver. I. Serum and hepatic
retinoid reduction and morphologic changes. Toxicol. Pathol.,
17(3): 536-544.
DUTCH AGRICULTURAL ADVISORY COMMISSION ON ENVIRONMENTAL POLLUTANTS
(1983) Annual report, The Hague, Ministry of Agriculture,
Management of Nature, and Fisheries (Unpublished).
DZOGBEFIA, V.P., KLING, D., & GAMBLE, W. (1978) Polychlorinated
biphenyls: in vivo and in vitro modifications of phospholipid
and glyceride biosynthesis. J. environ. Pathol. Toxicol.,
1: 841-856.
ECOBICHON, D.J. & COMEAU, A.M. (1974) Comparative effects of
commercial Aroclor on rat liver enzyme activities. Chem. biol.
Interact., 9: 341-350.
ECOBICHON, D.J. & COMEAU, A.M. (1975) Isomerically pure
chlorobiphenyl congeners and hepatic function in the rat. Influence
of position and degree of chlorination. Toxicol. appl. Pharmacol.,
33: 94-105.
ECOBICHON, D.J. & MACKENZIE, D.O. (1974) The uterotropic activity
of commercial and isomerically-pure chlorobiphenyls in the rat.
Res. Commun. chem. Pathol. Pharmacol., 9: 85-95.
EDULJEE, G., BADSHA, K., & SCUDAMORE, N. (1986) Environmental
monitoring for PCB and trace metals in the vicinity of a chemical
waste disposal facility - II. Chemosphere, 15(1): 81-93.
EISENREICH, S.J., LOONEY, B.B., & THORNTON, J.D. (1981) Airborne
organic contaminants in the Great Lakes ecosystem. Environ. Sci.
Technol., 15: 30-38.
EISLER, R. (1986) Polychlorinated biphenyl hazards to fish,
wildlife, and invertebrates: A synoptic review, Washington, DC, US
Department of Interior, Fish & Wildlife Service, 72 pp (Biology
Report No. 85).
EKSTEDT, J. & ODEN, S. (1974) Chlorinated hydrocarbons in the lower
atmosphere in Sweden, Uppsala, Sweden, Royal Agricultural College,
Department of Soil Science, pp. 1-16.
ELDER, D.L., FOWLER, S.W., & POLIKARPOV, G.G. (1979) Remobilization
of sediment-associated PCBs by the worm Nereis diversicolor.
Bull. environ. Contam. Toxicol., 21: 448-452.
ELLIOTT, J.E., NORSTRÖM, R.J., & KEITH, J.A. (1988) Organochlorines
and eggshell thinning in Northern Gannets (Sula bassanus) from
Eastern Canada, 1968-1984. Environ. Pollut., 52: 81-102.
ELO, O., VUOJOLAHTI, P., JANHUNEN, H., & RANTANEN, J. (1985) Recent
PCB accidents in Finland. Environ. health Perspect., 60: 315-319.
EMMETT, E.A. (1985) Polychlorinated biphenyl exposure and effects
in transformer repair workers. Environ. health Perspect.,
60: 185-192.
EMMETT, E.A., MARONI, M., SCHMITH, J.M., LEVIN, B.K., & JEFFERYS,
J. (1988a) Studies of transformer repair workers exposed to PCBs:
I. Study design, PCB concentrations, Questionnaire, and clinical
examination results. Am. J. ind. Med., 13: 415-427.
EMMETT, E.A., MARONI, M., JEFFERYS, J., SCHMITH, J., LEVIN, B.K.,
& ALVARES, A. (1988b) Studies of transformer repair workers exposed
to PCBs: II. Results of clinical laboratory investigations. Am. J.
ind. Med., 14: 47-62.
ERICKSON, M.D. (1985) The analytical chemistry of PCBs, Boston,
Massachusetts, Butterworth.
ERICKSON, M.D., STANLEY, J.S., TURMAN, J.K., GOING, J.E., REDFORD,
D.P., & HEGGEM, D.T. (1988) Determination of byproduct
polychlorobiphenyls in commercial products and wastes by
high-resolution gas chromatography/electron impact mass
spectrometry. Environ. Sci. Technol., 22(1): 71-76.
ESCHENROEDER, A.Q., DOYLE, C.P., & FAEDER, E.J. (1986) Health risks
of PCB spills from electrical equipment. Risk Anal., 6(2): 213-221.
EWALD, W.G., FRENCH, J.E., & CHAMP, M.A. (1976) Toxicity of
polychlorinated biphenyls (PCBs) to Euglena gracilis: Cell
population growth, carbon fixation, chlorophyll level, oxygen
consumption, and protein and nucleic acid synthesis. Bull. environ.
Contam. Toxicol., 16: 71-80.
EXON, J.H., TALCOTT, P.A., & KOLLER, L.D. (1985) Effect of lead,
polychlorinated biphenyls, and cyclophosphamide on rat natural
killer cells, interleukine 2, and antibody synthesis. Fundam. appl.
Toxicol., 5: 158-164.
FAIT, A., GROSSMAN, E., SELF, S., FEFFRIES, J., PELLIZZARI, E.D.,
& EMMETT, E.A. (1989) Polychlorinated biphenyl congeners in adipose
tissue lipid and serum of past and present transformer repair
workers and a comparison group. Fundam. appl. Toxicol., 12: 42-55.
FALANDYSZ, J. (1980) Chlorinated hydrocarbons in gulls from the
Baltic south coast. Mar. Pollut. Bull., 11: 75-80.
FARBER, E. (1984a) Perspectives in cancer research. The multistep
nature of cancer development. Cancer Res., 44: 4217-4223.
FARBER, E. (1984b) Cellular biochemistry of the step-wise
development of cancer with chemicals. G.H. Clowes Memorial Lecture.
Cancer Res., 44: 5463-5474.
FARBER, E. (1986) Some emerging general principles in the
pathogenesis of hepatocellular carcinoma. Cancer Surv., 5: 695-718.
FDA (1970) Status report on the chemistry and toxicology of
polychlorinated biphenyls (PCBs) or Aroclors as of 1 June 1970,
Washington, DC, Food and Drug Administration, 16 pp.
FDA (1979) Polychlorinated biphenyls (PCBs): Reduction of
tolerances, Fed. Reg., 44(127): 126-136.
FEIN, G.G. (1984) Intrauterine exposure of humans to PCBs: newborn
effects, Duluth, Minnesota, US Environmental Protection Agency
(EPA 600/53-84-060).
FEIN, G.G., JACOBSON, J.L., JACOBSON, S.W., SCHWARTZ, P.M., &
DOWLER, J.K. (1984) Prenatal exposure to polychlorinated biphenyls:
Effects on birth size and gestational age. J. Pediatr.,
105(2): 315-320.
FELT, G.R., MUELLER, W.F., IATROPOULOS, M.J., COULSTON, F., &
KORTE, F. (1977) Chronic toxicity of 2,5,4'-trichlorobiphenyl in
young rhesus monkeys. I. Body distribution, elimination and
metabolism. Toxicol. appl. Pharmacol., 41(3): 619-627.
FELT, G.R., MUELLER, W.F., COULSTON, F., & KORTE, F. (1979)
Distribution and excretion of 2,2',4,4',6-pentachlorobiphenyl in
the rat. Bull. environ. Contam. Toxicol., 22: 582-585.
FINGERMAN, S.W. (1980) Differences in the effects of fuel oil, an
oil dispersant, and three polychlorinated biphenyls on fin
regeneration in the Gulf Coast kill fish, Fundulus grandis. Bull.
environ. Contam. Toxicol., 25: 234-240.
FINGERMAN, S.W. & FINGERMAN, M. (1977) Effects of a polychlorinated
biphenyl and a polychlorinated dibenzofuran on molting of the
fiddler crab, Uca pugilator. Bull. environ. Contam. Toxicol.,
18: 138-142.
FINGERMAN, S.W. & FINGERMAN, M. (1979) Comparison of the effects of
fourteen-day and chronic exposures to a polychlorinated biphenyl,
Aroclor 1242, on molting of the fiddler crab, Uca pugilator.
Bull. environ. Contam. Toxicol., 21: 352-357.
FINGERMAN, S.W. & RUSSELL, L.C. (1980) Effects of the
polychlorinated biphenyl Aroclor 1242 on locomotor activity and on
the neurotransmitters dopamine and norepinephrine in the brain of
the gulf killifish, Fundulus grandis. Bull. environ. Contam.
Toxicol., 25: 682-687.
FINKLEA, J., PRIESTER, L.E., CREASON, J.P., HAUSER, T., HINNERS,
T., & HAMMER, D.I. (1972) Polychlorinated biphenyl residues in
human plasma expose a major urban pollution problem. Am. J. public
Health, 62(5): 645-651.
FIORE, B.J., ANDERSON, H.A., HANRAHAN, L.P., OLSON, L.J., &
SONZOGNI, W.C. (1989) Sport fish consumption and body burden levels
of chlorinated hydrocarbons: A study of Wisconsin anglers. Arch.
environ. Health, 44(2): 82-88.
FISCHBEIN, A. (1985) Liver function tests in workers with
occupational exposure to polychlorinated biphenyls (PCBs):
Comparison with Yusho and Yu-Cheng. Environ. health Perspect.,
60: 145-150.
FISCHBEIN, A. & WOLFF, M.S. (1987) Conjugal exposure to
polychlorinated biphenyls (PCBs). Br. J. ind. Med., 44: 284-286.
FISCHBEIN, A., WOLFF, M.S., LILIS, R., THORNTON, J., & SELIKOFF,
I.J. (1979) Clinical findings among PCB-exposed workers in a
capacitor manufacturing facility. Ann. NY Acad. Sci., 320: 703-715.
FISCHBEIN, A., WOLFF, M.S., BERNSTEIN, J., SELIKOFF, I.J., &
THORNTON, J. (1982) Dermatological findings in capacitor
manufacturing workers exposed to dielectric fluids containing
polychlorinated biphenyls (PCBs). Arch. environ. Health,
37(2): 69-74.
FISCHBEIN, A., RIZZO, J.N., SOLOMON, S.J., & WOLFF, M.S. (1985)
Oculodermatological findings in workers with occupational exposure
to polychlorinated biphenyls (PCBs). Br. J. ind. Med., 42: 426-430.
FISHBEIN, L. (1974) Toxicity of chlorinated biphenyls. Annu. Rev.
Pharmacol., 14: 139-156.
FISHER, N.S. (1975) Chlorinated hydrocarbon pollutants and
photosynthesis of marine phytoplankton: A reassessment. Science,
189: 463-464.
FISHER, N.S. & WURSTER, C.F. (1973) Individual and combined effects
of temperature and polychlorinated biphenyls on the growth of three
species of phytoplankton. Environ. Pollut., 5: 205-212.
FISHER, N.S., GRAHAM, L.B., & CARPENTER, E.J. (1973) Geographic
differences in phytoplankton sensitivity to PCBs. Nature (Lond.),
241: 548-549.
FISHER, N.S., REMSEN, C.C., WURSTER, C.R., & CARPENTER, E.J. (1974)
Effects of PCB on interspecific competition in natural and
gnotobiotic phytoplankton communities in continuous and batch
cultures. Microbiol. Ecol., 1: 39-50.
FISHER, N.S., GUILLARD, R.R., & WURSTER, C.R. (1976) Effects of a
chlorinated hydrocarbon pollutant on the growth kinetics of a
marine diatom. In: Modeling biochemical processes in aquatic
ecosystems, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 305-317.
FISHER, J.B., PETTY, R.L., & LUCK, W. (1983) Release of
polychlorinated biphenyls from contaminated lake sediments: Flux
and apparent diffusivities of four individual PCBs. Environ.
Pollut., B5: 121-132.
FITZGERALD, E.F., WEINSTEIN, A.L., YOUNGBLOOD, L.G., STANDFAST,
S.J., & MELIUS, J.M. (1989) Health effects three years after
potential exposure to the toxic contaminants of an electrical
transformer fire. Arch. environ. Health, 44(4): 214-221.
FLICK, D.F., O'DELL, R.G., & CHILDS, V.A. (1965) Studies of the
chick oedema disease. 3. Similarity of symptoms produced by feeding
chlorinated biphenyl. Poult. Sci., 44: 1460-1465.
FOCARDI, S. & ROMEI, R. (1987) Fingerprint of polychlorinated
biphenyl congeners in samples of human subcutaneous adipose tissue.
Chemosphere, 16(10/12): 2315-2320.
FOCARDI, S., FOSSI, C., LEONZIO, C., & ROMEI, R. (1986) PCB
congeners, hexachlorobenzene, and organochlorine insecticides in
human fat in Italy. Bull. environ. Contam. Toxicol., 36: 644-650.
FOCARDI, S., LEONZIO, C., & FOSSI, C. (1988) Variations in
polychlorinated biphenyl congener composition in eggs of
Mediterranean water birds in relation to their position in the food
chain. Environ. Pollut., 52: 243-255.
FOLMAR, L.C., DICKHOFF, W.W., ZAUGG, W.S., & HODGKINS, H.O. (1982)
The effects of Aroclor 1254 and No. 2 fuel oil on smoltification
and sea-water adaptation of Coho salmon (Oncorhynchus kisutch).
Aquat. Toxicol., 2: 291-299.
FOOKEN, C. & BUTTE, W. (1987) Organochlorine pesticides and
polychlorinated biphenyls in human milk during lactation.
Chemosphere, 16(6): 1301-1309.
FOREMAN, W.T. & BIDLEMAN, T.F. (1985) Vapor pressure estimates of
individual polychlorinated biphenyls and commercial fluids using
gas chromatographic retention data. J. Chromatogr., 330: 203-216.
FORGUE, S.T., PRESTON, B.D., HARGRAVES, W.A., REICH, I.L. & ALLEN,
J.R. (1980) Direct evidence that an arene oxide is a metabolic
intermediate of 2,2',5,5'-tetrachlorobiphenyl. In: Abstracts of the
Nineteenth Annual Meeting of the Society of Toxicology (Abstract
No. 383).
FOWLER, S.W., POLIKARPOV, G.G., ELDER, D.L., PARSI, P., &
VILLENEUVE, J.P. (1978) Polychlorinated biphenyls: Accumulation
from contaminated sediments and water by the polychaete Nereis
diversicolor. Mar. Biol., 48: 303-309.
FRANK, R., RONALD, K., & BRAUN, H.E. (1973) Organochlorine residues
in harp seals (Pagophilus groenlandicus) caught in Eastern
Canadian waters. J. Fish Res. Board Can., 30: 1053-1063.
FRANK, R., HOLDRINET, M.V.H., & RAPLEY, W.A. (1975) Residue of
organochlorine compounds and mercury in birds' eggs from the
Niagara Peninsula, Ontario. Arch. environ. Contam. Toxicol.,
3: 205-218.
FRANK, R., HOLDRINET, M., BRAUN, H.E., DODGE, D.P., & SPRANGLER,
G.E. (1978) Residues of organochlorine insecticides and
polychlorinated biphenyls in fish from Lakes Huron and Superior,
Canada, 1968-76. Pestic. monit. J., 12: 60-68.
FRANK, R., RASPER, J., SMOUT, M.S., & BRAUN, H.E. (1988)
Organochlorine residues in adipose tissues, blood and milk from
Ontario residents, 1976-1985. Can. J. public Health, 79: 150-158.
FRANKLIN, A. (1987) The concentration of metals, organochlorine
pesticide and PCB residues in marine fish and shellfish: Results
from MAFF fish and shellfish monitoring programmes, 1977-1984,
London, Ministry of Agriculture, Fisheries and Food, Directorate of
Fisheries Research (Aquatic Environment Monitoring Report No. 16).
FREEMAN, H.C., SANGALANG, G., & FLEMMING, B. (1982) The sublethal
effects of a polychlorinated biphenyl (Aroclor 1254) diet on the
Atlantic cod (Gadus morhua). Sci. total Environ., 24: 1-11.
FRENCH, J.E. (1976) The effect of the chlorinated polycyclic
hydrocarbons, p,p'-DDT and polychlorinated biphenyls (PCBs) on the
flagellated protozoan, Crithidia fasciculata. Diss. Abstr. Int.
Sci. Eng., B37: 96-97.
FREUDENTHAL, J. & GREVE, P.A. (1973) Polychlorinated terphenyls in
the environment. Bull. environ. Contam. Toxicol., 10: 108-111.
FRIEND M. & TRAINER, D.O. (1970) Polychlorinated biphenyl:
interaction with duck hepatitis virus. Science, 170: 1314-1316.
FRIES, G.F. (1972) Degradation of chlorinated hydrocarbons under
anaerobic conditions. Adv. Chem. Ser., 111: 256-270.
FRIES, G.F. & MARROW, G.S. (1981) Chlorobiphenyl movement from soil
to soybean plants. J. agric. food Chem., 29: 757-759.
FRIES, G.F., MARROW, G.S., Jr, & GORDON, C.H. (1973) Long-term
studies of residue retention and excretion by cows fed a
polychlorinated biphenyl (Aroclor 1254). J. agric. food Chem.,
21: 117-121.
FU, Y.A. (1984) Ocular manifestation of polychlorinated biphenyls
intoxication. Am. J. ind. Med., 5: 127-132.
FUJIWARA, K. (1975) Environmental and food contamination with PCB's
in Japan. Sci. total Environ., 4: 219-247.
FUJIWARA, M. & KURIYAMA, K. (1977) Effect of PCB (polychloro-
biphenyls) on L-ascorbic acid, pyridoxal phosphate and riboflavin
contents in various organs and on hepatic metabolism of L-ascorbic
acid in the rat. Jpn. J. Pharmacol., 27: 621-627.
FUKADA, K., INUYAMA, Y., TAKESHITA, T., & YAMAMOTO, S. (1973)
[Present state of environmental pollution by PCB in the Shimane
Prefecture.] Shimare Igaku, 5: 1-25 (in Japanese).
FUKANO, S. & DOGUCHI, M. (1977) PCT, PCB and pesticide residues in
human fat and blood. Bull. environ. Contam. Toxicol.,
17(5): 613-617.
FUKANO, S., USHIO, F., & DOGUCHI, M. (1974) [PCB, PCT and pesticide
residues in fish collected from the Tama river.] Annu. Rep. Tokyo
Metrop. Res. Lab. P.H., 25: 297-305 (in Japanese).
FUKUSHIMA, M. & KAWAI, S. (1981) Variation of organochlorine
concentration and burden in striped dolphin (Stenella
coeruleoalba) with growth. In: Fujiyama, T., ed. Studies on the
levels of organochlorine compounds and heavy metals in the marine
organisms, Ryukyus, University of Ryukyus, pp. 97-114.
FULLER, G.B., KNAUF, V., MUELLER, W., & HOBSON, W.C. (1980) PCB
augments LH induced progesteron synthesis. Bull. environ. Contam.
Toxicol., 25: 65-68.
FUNATSU, I., YAMASHITA, F., ITO, Y., TZUGAWA, S., FUNATSU, T.,
YOSHIKANE, T., HAYASHI, M., KATO, T., YAKUSHIJI, M., OKAMOTO, G.,
YAMASAKI, S., ARIMA, T., KUNO, T., IDE, H., & IBE, I. (1972) PCB
induced fetopathy. I. Clinical observation. Kurume med. J.,
919: 43-51.
FURUKAWA, K., TONOMURA, K., & KAMIBAYASHI, A. (1978a) Effect of
chlorine substitution on the biodegradability of polychlorinated
biphenyls. Appl. environ. Microbiol., 35: 223-227.
FURUKAWA, K., TOMIZUKA, N., & KAMIBAYASHI, A. (1978b) Effect of
chlorine substitution on the bacterial metabolism of various
polychlorinated biphenyls. Appl. environ. Microbiol., 38: 301-310.
GAGE, J.C. & HOLM, S. (1976) The influence of molecular structure
on the retention and excretion of polychlorinated biphenyls by the
mouse. Toxicol. appl. Pharmacol., 36: 555-560.
GALLENBERG, L.A. & VODICNIK, M.J. (1987) Potential mechanisms for
redistribution of polychlorinated biphenyls during pregnancy and
lactation. Xenobiotica, 17(3): 299-310.
GALLENBERG, L.A., RING, B.J., & VODICNIK, M.J. (1987) Influence of
lipolysis on the mobilization of 2,4,5,2',4',5'-hexachlorobiphenyl
from adipocytes in vitro. J. Toxicol. environ. Health,
20: 163-171.
GARDNER, A.M., RICHTER, H.F., & ROACH, J.A.G. (1976) Excretion of
hydroxylated polychlorinated biphenyl metabolites in cow's milk. J.
Assoc. Off. Anal. Chem., 59: 273-277.
GARTHOFF, L.H., FRIEDMAN, L., FARBER, T.M., LOCKE, K.K., SOBOTKA,
T.J., GREEN, S., HURLEY, N.E., PETERS, E.L., STORY, G.E., MORELAND,
F.M., GRAHAM, C.H., KEYS, J.E., TAYLOR, M.J., SCALERA, J.V.,
ROTHLEIN, J.E., MARKS, E.M., CERRA, F.E., RODI, S.B., & SPORN, E.M.
(1977) Biochemical and cytogenetic effects in rats caused by
short-term ingestion of Aroclor 1254 or Firemaster BP6. J. Toxicol.
environ. Health, 3: 769-796.
GARTHOFF, L.H., CERRA, F.E., & MARKS, E.M. (1981) Blood chemistry
alterations in rats after single and multiple gavage administration
of polychlorinated biphenyl. Toxicol. appl. Pharmacol., 60: 33-44.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985) Pesticides, selected elements and other chemicals in adult
total diet samples, October 1979-September 1980. J. Assoc. Off.
Anal. Chem., 68(6): 1184-1195.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements and other chemicals in adult
total diet samples, October 1980-March 1982. J. Assoc. Off. Anal.
Chem., 69(1): 146-161.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements and other chemicals in infant
and toddler total diet samples, October 1980-March 1982. J. Assoc.
Off. Anal. Chem., 69(1): 123-145.
GASKIN, D.E., FRANK, R., & HOLDRINET, M. (1983) Polychlorinated
biphenyls in harbor porpoises Phocoena phocoena (L.) from the Bay
of Fundy, Canada and adjacent waters, with some information on
chlordane and hexachlorobenzene levels. Arch. environ. Contam.
Toxicol., 12: 211-219.
GELLERT, R.J. (1978) Uterotropic activity of polychlorinated
biphenyls (PCB) and induction of precocious reproductive aging in
neonatally treated female rats. Environ. Res., 16: 123-130.
GELLERT, R.J. & WILSON, C. (1979) Reproductive function in rats
exposed prenatally to pesticides and polychlorinated biphenyls
(PCBs). Environ. Res., 18: 437-443.
GEZONDHEIDSRAAD (1985) [First recommendation on the quality of
breastmilk. Contamination of breastmilk with polychlorinated
biphenyls (PCBs). Recommendation to the Minister and the Secretary
of State for Welfare, Public Health and Culture], Rijswijk, Public
Health Council of the Netherlands (in Dutch).
GIAM, C.S., CHAN, H.S., NEFF, G.S., & ATLAS, E.L. (1978) Phthalate
ester plasticizers: A new class of marine pollutant. Science,
199: 419-420.
GIAM, C.S., ATLAS, E., CHAN, H.S., & NEFF, G. (1980) Phthalate
esters, PCB and DDT residues in the Gulf of Mexico atmosphere.
Atmos. Environ., 14: 65-69.
GIBSON, D.T., ROBERTS, R.L., WELLS, M.C., & KOBAL, V.M. (1973)
Oxidation of biphenyl by a Beijerinckia species. Biochem.
biophys. Res. Commun., 50: 211-219.
GILBERTSON, M. & FOX, G.A. (1977) Pollutant-associated embryonic
mortality of Great Lakes herring gulls. Environ. Pollut.,
12: 211-216.
GILBERTSON, M. & HALE, R. (1974) Characteristics of the breeding
failure of a colony of herring gulls in Lake Ontario. Can.
Field-Nat., 88: 356-358.
GILBERTSON, M., MORRIS, R.D., & HUNTER, R.A. (1976) Abnormal chicks
and PCB residue levels in eggs of colonial birds on the lower Great
Lakes (1971-73). Auk, 93: 434-442.
GLADEN, B.C., ROGAN, W.J., RAGAN, N.B., & SPIERTO, F.W. (1988a)
Urinary porphyrins in children exposed transplacentally to
polyhalogenated aromatics in Taiwan. Arch. environ. Health,
43(1): 54-58.
GLADEN, B.C., ROGAN, W.J., HARDY, P., THULLEN, J., TINGELSTAD, J.,
& TULLY, M. (1988b) Development after exposure to polychlorinated
biphenyls and dichlorodiphenyl dichloroethane transplacentally and
through human milk. J. Pediatr., 113(6): 991-995.
GLOOSCHENKO, V. & GLOOSCHENKO, W. (1975) Effect of polychlorinated
biphenyl compounds on growth of great lakes phytoplankton. Can. J.
Bot., 53: 653-659.
GLOOSHENKO, W.A., STRACHAN, W.M., & SAMPSON, R.C.J. (1976) Residues
in water distribution of pesticides and polychlorinated biphenyls
in water, sediments and session of the Upper Great Lakes. Pestic.
monit. J., 10: 61-67.
GOLDSTEIN, J.A. (1980) Structure-activity relationships for the
biochemical effects and the relationships to toxicity. In:
Kimbrough, R.D., ed. Halogenated biphenyls, terphenyls,
naphthalenes, dibenzodioxins and related products, Amsterdam,
Elsevier/North-Holland Biomedical Press, pp. 151-190 (Topics in
Environmental Health).
GOLDSTEIN, J.A., HICKMAN, P., & JUE, D.L. (1974) Experimental
hepatic porphyria induced by polychlorinated biphenyls. Toxicol.
appl. Pharmacol., 27: 437-448.
GOLDSTEIN, J.A., HICKMAN, P., BURSE, V.W., & BERGMAN, H. (1975) A
comparative study of two polychlorinated biphenyl mixtures
(Aroclors 1242 and 1016) containing 42% chlorine on induction of
hepatic porphyria and drug metabolizing enzymes. Toxicol. appl.
Pharmacol., 32: 461-473.
GOLDSTEIN, J.A., HICKMAN, P., BERGMAN, H., MCKINNEY, J.D., &
WALKER, M.P. (1977) Separation of pure polychlorinated biphenyl
isomers into two types of inducers on the basis of induction of
cytochrome P-450 or P-448. Chem.-biol. Interact., 17: 69-87.
GORCHEV, H.G. & JELINEK, C.F. (1985) A review of the dietary
intakes of chemical contaminants. Bull. World Health Organ.,
63(5): 945-962.
GOTO, M. & HIGUCHI, K. (1969) The symptomatology of Yusho
(chlorobiphenyls poisoning), in dermatology. Fuoka Act. Med.,
60: 409-431.
GOTO, M., SUGIURA, K., HATTORI, M., MIYAGAWA, T., & OKAMURA, M.
(1973) Hydroxylation of dichlorobiphenyls in rats. In: New
collection of papers presented at the Research Conference on New
Methodology in Ecological Chemistry, Susono, Japan, 23-25 November,
Tokyo, International Academic Printing Co. Ltd, pp. 299-302.
GOTO, M., SUGIURA, K., HATTORI, M., MIYAGAWA, T., & OKAMURA, M.
(1974) Metabolism of 2,3-dichlorobiphenyl-14C and
2,3,6-trichlorobiphenyl-14C in the rat. Chemosphere, 3: 227-232.
GOTO, M., HATTORI, M., & SUGIURA, K. (1975) Metabolism of
pentachloro- and hexachlorobiphenyls in the rat. Chemosphere,
4: 177-180.
GRANT, D.L. & PHILLIPS, W.E.J. (1974) The effect of age and sex on
the toxicity of Aroclor 1254, a polychlorinated biphenyl, in the
rat. Bull. environ. Contam. Toxicol., 12(2): 145-152.
GRANT, D.L., PHILLIPS, W.E.J., & VILLENEUVE, D.C. (1971a)
Metabolism of polychlorinated biphenyl (Aroclor 1254) mixture in
the rat. Bull. environ. Contam. Toxicol., 6: 102-112.
GRANT, D.L., VILLENEUVE, D.C., MCCULLY, K.A., & PHILLIPS, W.E.J.
(1971b) Placental transfer of polychlorinated biphenyls in the
rabbit. Environ. Physiol., 1: 61-66.
GRANT, D.L., MOODIE, C.A., & PHILLIPS, W.E.J. (1974) Toxicodynamics
of Aroclor 1254 in the male rat. Environ. Physiol. Biochem.,
4: 214-225.
GREB, W., KLEIN, W., COULSTON, F., GOLBERG, L., & KORTE, F. (1975)
Metabolism of lower polychlorinated biphenyls-C-14 in the Rhesus
monkey. Bull. environ. Contam. Toxicol., 13: 471-76.
GREEN, S., CARR, J.V., PALMER, K.A., & OSWALD, E.J. (1975a) Lack of
cytogenetic effects in bone marrow and spermatogonial cells in rats
treated with polychlorinated biphenyls (Aroclors 1242 and 1254).
Bull. environ. Contam. Toxicol., 13: 14-22.
GREEN, S., SAURO, F.M., & FRIEDMAN, L. (1975b) Lack of dominant
lethality in rats treated with polychlorinated biphenyls (Aroclors
1242 and 1254). Food Cosmet. Toxicol., 13: 507-510.
GREICHUS, Y.A., CALL, D.J., & AMMANN, B.M. (1975) Physiological
effects of polychlorinated biphenyls or a combination of DDT, DDD,
and DDE in penned white Pelicans. Arch. environ. Contam. Toxicol.,
3: 330-343.
GREIG, R.A. & SENNEFELDER, G. (1987) PCB concentration in winter
flounder from Long Island Sound, 1984-1986. Bull. environ. Contam.
Toxicol., 39(5): 863-868.
GREVE, P.A. & VAN HULST, S.J. (1977) [Organochlorine pesticides and
PCBs in total diets], Bilthoven, The Netherlands, National
Institute of Public Health (Report No. 192/77 Tox-Rob) (in Dutch).
GREVE, P.A. & VAN HARTEN, D.C. (1983a) [Organochlorine pesticides
and polychlorobiphenyls in the fatty tissue of the Dutch population
(period 1980).] Bilthoven, The Netherlands, National Institute of
Public Health (Report No. 638205001) (in Dutch).
GREVE, P.A. & VAN HARTEN, D.C. (1983b) [Association between
organochlorine pesticide and PCB contents in fat and blood in man],
Bilthoven, The Netherlands, National Institute of Public Health
(Report No. 638219001) (in Dutch).
GREVE, P.A. & WEGMAN, R.C.C. (1983) PCB residues in animal fats,
human tissues, duplicate 24-hours' diets, eel and sediments. In:
Barros, M.C., Könemann, H., & Visser, R., ed. Proceedings of the
PCB Seminar, The Hague, 28-30 September 1983, The Hague, Ministry
of the Environment, pp. 54-66.
GREVE, P.A. & WEGMAN, R.C.C. (1984) Organochlorine compounds in
human milk: data from a recent investigation in the Netherlands.
In: Report on the WHO Consultation on Organochlorine Compounds in
Human Milk and Related Hazards, Bilthoven, 9-11 January 1985,
Copenhagen World Health Organization, Regional Office for Europe,
Annex 8.
GREVE, P.A., VAN HARTEN, D.C., HEUSINKVELD, H.A.G., LEUSSINK, A.B.,
& VERSCHRAAGEN, C. (1985) [Chemical contaminants in breastmilk.
Section 2: PCBs (determination of total)], Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Hygiene (Report No. 638307002) (in Dutch).
GROLIER, P., CASSAND, P., ANTIGNAC, E., NARBONNE, J.F., ALBRECHT,
R., AZAIS, V., ROBERTSON, L.W., & OESCH, F. (1989) Effects of
prototypic PCBs on benzo(a)pyrene mutagenic activity related to
vitamin A intake. Mutat. Res., 211: 139-145.
GROTE, W., SCHMOLDT, A., & BENTHE, H.F. (1975) Hepatic porphyrin
synthesis in rat after pretreatment with polychlorinated biphenyls.
Acta. pharmacol. toxicol., 36: 215-224.
GRUGER, E.H., KARRICK, N.L., DAVIDSON, A.I., & HRUBY, T. (1975)
Accumulation of 3,4,3',4'-tetrachlorobiphenyl and 2,4,5,2',4',5'-,
and 2,4,6,2',4',6'-hexachlorobiphenyl in juvenile coho salmon.
Environ. Sci. Technol., 9: 121-127.
GRUGER, E.H., HRUBY, T., & KARRICK, N.L. (1976) Sublethal effects
of structurally related tetrachloro-, pentachloro-, and
hexachlorobiphenyl on juvenile Coho Salmon. Environ. Sci. Technol.,
10: 1033-1037.
GUINEY, P.D. & PETERSON, R.E. (1980) Distribution and elimination
of a polychlorinated biphenyl after dietary exposure in yellow
perch and rainbow trout. Arch. environ. Contam. Toxicol.,
9: 667-674.
GUINEY, P.D., PETERSON, R.E., MELANCON, M.J., & LECH, J.J. (1977)
The distribution and elimination of 2,5,2,5-[14C]Tetrachloro-
biphenyl in Rainbow Trout (Salmo gairdneri). Toxicol. appl.
Pharmacol., 39: 329-338.
GUINEY, P.D., MELANCON, M.J., LECH, J.J., & PETERSON, R.E. (1979)
Effects of egg and sperm maturation and spawning on the
distribution and elimination of a polychlorinated biphenyl in
rainbow Trout (Salmo gairdneri). Toxicol. appl. Pharmacol.,
47: 261-272.
GUNDERSON, E.L. (1988b) FDA total diet study, April 1982-April
1984, dietary intakes of pesticides, selected elements and other
chemicals. J. Assoc. Off. Anal. Chem., 71(6): 1200-1209.
GUO, Y.L., EMMETT, E.A., PELLIZZARI, E.D., & ROHDE, C.A. (1987)
Influence of serum cholesterol and albumine on partitioning of PCB
congeners between human serum and adipose tissue. Toxicol. appl.
Pharmacol., 87: 48-56.
GUOTH, J., KACHMAR, P., TELEHA, M., & VASIL, M. (1984) [The
influence of polychlorinated biphenyls (PCB) on the activity of
liver aniline hydroxylase and on some metabolic parameters in pig
blood.] Vet. Med. (Prague), 29: 29-38 (in Czech).
GUSTAVSSON, P., HOGSTEDT, C., & RAPPE, C. (1986) Short-term
mortality and cancer incidence in capacitor manufacturing workers
exposed to polychlorinated biphenyls (PCBs). Am. J. ind. Med.,
10: 341-344.
GYORKOS, J., DENOMME, M.A., LEECE, B., HOMONKO, K., VALLI, V.E., &
SAFE, S. (1985) Reconstituted halogenated hydrocarbon pesticide and
pollutant mixtures found in human tissues: effects on the immature
male Wistar rat after short-term exposure. Can. J. Physiol.
Pharmacol., 63(1): 36-43.
HAAHTI, H. & PERTTILA, M. (1988) Levels and trends of
organochlorines in Cod and Herring in the Northern Baltic. Mar.
Pollut. Bull., 19: 29-32.
HAAKE, J.M., SAFE, S., MAYURA, K., & PHILLIPS, T.D. (1987) Aroclor
1254 as an antagonist of the teratogenicity of 2,3,7,8-tetrachloro-
dibenzo- p-dioxin. Toxicol. Lett., 38: 299-306.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
Coturnix. Bull. environ. Contam. Toxicol., 11: 98-101.
HALTER, M.T. & JOHNSON, H.E. (1974) Acute toxicities of a
polychlorinated biphenyl (PCB) and DDT alone and in combination to
early life stages of Coho salmon (Oncorhynchus kisutch). J. Fish
Res. Board Can., 31: 1543-1547.
HALVERSON, M.R., PHILLIPS, T.D., SAFE, S.H., & ROBERTSON, L.W.
(1985) Metabolism of aflatoxin B1 by rat hepatic microsomes induced
by polyhalogenated biphenyl congeners. Appl. environ. Microbiol.,
49: 882-886.
HAMMOND, A.L. (1972) Chemical pollution: Polychlorinated biphenyls.
Science, 175: 155-156.
HANSEN, D.J., PARRISH, P.R., LOWE, J.I., & WILSON, A.J. (1971)
Chronic toxicity, uptake, and retention of Aroclor 1254 in two
estuarine fishes. Bull. environ. Contam. Toxicol., 6: 113-119.
HANSEN, D.J. (1974) Aroclor 1254: Effect on composition of
developing estuarine animal communities in the laboratory. Contrib.
mar. Sci., 18: 19-33.
HANSEN, D.J., SCHIMMEL, S.C., & MATTHEWS, E. (1974a) Avoidance of
Aroclor 1254 by shrimp and fishes. Bull. environ. Contam. Toxicol.,
12: 253-256.
HANSEN, D.J., PARRISH, P.R., & FORESTER, J. (1974b) Aroclor 1016:
Toxicity to and uptake by estuarine animals. Environ. Res.,
7: 363-373.
HANSEN, D.J., SCHIMMEL, S.C., & FORESTER, J. (1975) Effects of
Aroclor 1016 on embryos, fry, juveniles, and adults of sheepshead
minnow (Cyprinodon variegatus). Trans. Am. Fish Soc.,
104: 584-588.
HANSEN, L.G., WIEKHORST, W.B., & SIMON, J. (1976a) Effects of
dietary Aroclor 1242 on channel catfish (Ictalurus punctatus) and
the selective accumulation of PCB components. J. Fish Res. Board
Can., 33: 1343-1352.
HANSEN, L.G., WILSON, D.W., & BYERLY, C.S. (1976b) Effects on
growing swine and sheep of two polychlorinated biphenyls. Am. J.
vet. Res., 37: 1021-1024.
HANSEN, L.G., WASHKO, P.W., TUINSTRA, L.G.M.TH., DORN, S.B., &
HINESLY, T.D. (1981) Polychlorinated biphenyl, pesticide, and heavy
metal residues in swine foraging on sewage sludge amended soils. J.
agric. food Chem., 29: 1012-1017.
HAQUE, R. & SCHMEDDING, D.W. (1975) A method of measuring the water
solubility of hydrophobic chemicals: Solubility of five
polychlorinated biphenyls. Bull. environ. Contam. Toxicol.,
14: 13-18.
HAQUE, R., SCHMEDDING, D.W., & FREED, V.H. (1974) Aqueous
solubility adsorption and vapor behaviour of polychlorinated
biphenyl Aroclor 1254. Environ. Sci. Technol., 8: 139-142.
HARA, I. (1985) Health status and PCBs in blood of workers exposed
to PCBs and their children. Environ. health Perspect., 59: 85-90.
HARA, I., HARADA, H., KIMURA, S., ENDO, T., & KAWANO, K. (1974)
[Follow-up health examination in an electric condenser factory
after cessation of PCBs usage (1st report).] Jpn. J. ind. Health,
16: 365-366 (in Japanese).
HARBISON, R.D., JAMES, R.C., & ROBERTS, S.M. (1987) Biological data
relevant to the evaluation of carcinogenic risk to humans, Little
Rock, Arkansas, University of Arkansas, Division of
Interdisciplinary Toxicology, (Prepared for Scientific Advisory
Panel, Safe Drinking Water and Toxic Enforcement Act, State of
California).
HARDING, L.W. & PHILLIPS, J.H. (1978a) Polychlorinated biphenyl
(PCB) effects on marine phytoplankton photosynthesis and cell
division. Mar. Biol., 49: 93-101.
HARDING, L.W. & PHILLIPS, J.H. (1978b) Polychlorinated biphenyl
(PCB) uptake by marine phytoplankton. Mar. Biol., 49: 103-111.
HARDWICK, J.P., LINKO, P., & GOLDSTEIN, J.A. (1985) Dose response
for induction of two cytochrome P-450 isoenzymes and their mRNAs by
3,4,5,3',4',5'-hexachlorobiphenyl indicating coordinate regulation
in rat liver. Mol. Pharmacol., 27: 676-682.
HARRIS, M.P. & OSBORN, D. (1981) Effect of a polychlorinated
biphenyl on the survival and breeding of puffins. J. appl. Ecol.,
18: 471-479.
HARRIS, J.R. & ROSE, L. (1972) Toxicity of polychlorinated
biphenyls in poultry. J. Am. Vet. Med. Assoc., 161: 1584-1586.
HARVEY, G.R. & STEINHAUER, W.G. (1974) Atmospheric transport of
polychlorobiphenyls to the North Atlantic. Atmos. Environ.,
8: 777-782.
HARVEY, G.R., STEINHAUER, W.G., & TEAL, J.M. (1973)
Polychlorobiphenyls in North Atlantic Ocean water. Science,
180: 643-644.
HASEGAWA, M. (1973) [Studies on the wild animals as
"Contamination-index". Part 1 - The effects of PCB on frog tadpoles
(Rana chensinensis).] Hokkaidoritsu Eisei Kenkyushoho, 23: 6-9
(in Japanese).
HASEGAWA, H., SATO, M., & TSURUTA, H. (1972a) [PCB concentration in
the blood of workers handling PCB.] Occup. Health, 13(10): 50-55
(in Japanese).
HASEGAWA, H., SATO, M., & TSURUTA, H. (1972b) [PCB concentration in
air of PCB-using plants and health examination of workers.] In:
[Report on special research on prevention of environmental
pollution by PCB-like substances], Tokyo, Science and Technology
Agency, Research Co-ordination Bureau, pp. 141-149 (in Japanese).
HASELTINE, S.D. & PROUTY, R.M. (1980) Aroclor 1242 and reproductive
success of adult mallards (Anas platyrhynchos). Environ. Res.,
23: 29-34.
HASELTINE, S.D., HEINZ, G.H., REICHEL, W.L., & MOORE, J.F. (1981)
Organochlorine and metal residues in eggs of wildfowl nesting on
islands in Lake Michigan off Door county, Wisconsin, 1977-78.
Pestic. monit. J., 15: 90-97.
HASHIMOTO, K., AKASAKA, S., TAKAGI, Y., KATAOKA, M., OTAKA, T.,
MURATA, Y., ABURADA, S., KITAURA, T., & UDA, H. (1976) Distribution
and excretion of (14C)polychlorinated biphenyls after their
prolonged administration to male rats. Toxicol. appl. Pharmacol.,
37: 415-423.
HASSELL, K.D. & HOLMES, D.C. (1977) Polychlorinated terphenyls
(PCT) in some British birds. Bull. environ. Contam. Toxicol.,
17: 618-621.
HATCH, W.I. & ALLEN, D.W. (1979) Alterations in calcium
accumulation behavior in response to calcium availability and
polychlorinated biphenyl administration. Bull. environ. Contam.
Toxicol., 22: 172-174.
HATTULA, M.L. (1985) Mutagenicity of PCBs and their pyrosynthetic
derivatives in cell-mediated assay. Environ. health Perspect.,
60: 255-257.
HATTULA, M.L. & KARLOG, O. (1973) Absorption and elimination of
polychlorinated biphenyls (PCB) in goldfish. Acta pharmacol.
toxicol., 32: 237-245.
HAWES, M.L., KRICHER, J.C., & UREY, J.C. (1976a) The effects of
various Aroclor fractions on the population growth of Chlorella
pyrenoidosa. Bull. environ. Contam. Toxicol., 15: 14-18.
HAWES, M.L., KRICHER, J.C., & UREY, J.C. (1976b) The effects of
various Aroclor fractions on the productivity of Chlorella
pyrenoidosa. Bull. environ. Contam. Toxicol., 15: 588-590.
HAWKER, D.W. (1989) Vapour pressures and Henry's Law Constants of
polychlorinated biphenyls. Environ. Sci. Technol., 23: 1250-1253.
HAWKER, D.W. & CONNELL, D.W. (1988) Octanol-water partition
coefficients of polychlorinated biphenyl congeners. Environ. Sci.
Technol., 22: 382-387.
HAYES, M.A., SAFE, S.H., ARMSTRONG, D., & CAMERON, R.G. (1985)
Influence of cell proliferation on initiating activity of pure
polychlorinated biphenyls and complex mixtures in resistant
hepatocyte in vivo assays for carcinogenicity. J. Natl Cancer
Inst., 74: 1037-1041.
HAYES, M.A., ROBERTS, E., SAFE, S.H., FARBER, E., & CAMERON, R.G.
(1986) Influences of different polychlorinated biphenyls on
cytocidal, mitiinhibitory and nodule-selecting activities of
N-2-fluorenylacetamide in rat liver. J. Natl Cancer Inst.,
76: 683-691.
HAYES, M.A. (1987) Carcinogenic and mutagenic effects of PCBs. In:
Safe, S., ed. Polychlorinated biphenyls (PCBs): Mammalian and
environmental toxicology, Berlin, Heidelberg, New York,
Springer-Verlag, pp. 77-95 (Environmental Toxin Series, Vol. I).
HAYS, H. & RISEBROUGH, R.W. (1972) Pollutant concentrations in
abnormal young terns from Long Island Sound. Auk, 89: 19-35.
HEATH, R.G., SPANN, J.W., KREITZER, J.F., & VANCE, C. (1972)
Effects of polychlorinated biphenyls on birds. In: Proceedings of
the XVth International Ornithological Congress, The Hague, 30
August-5 September 1970, pp. 475-485.
HEDDLE, J.A. & BRUCE, W.R. (1977) Comparison of tests for
mutagenicity or carcinogenicity using assays for sperm
abnormalities, formation of micronuclei and mutation in Salmonella.
In: Origins of human cancer, Book C: Human risk assessment, Section
16. Short-term assays - Predictive value, Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp. 1549-1557.
HEESCHEN, W., BLUTHGEN, A., & NIJHUIS, H. (1986) [Milk hygiene:
Trends in the residue situation.] Kieler Milchwirtsch
Forschungsber., 38(2): 131-145 (in German).
HEINZ, G.H., HILL, E.F., & CONTERA, J.F. (1980) Dopamine and
norepinephrine depletion in ring doves fed DDE, dieldrin, and
Aroclor 1254. Toxicol. appl. Pharmacol., 53: 75-82.
HEINZ, G.H., HASELTINE, S.D., REICHEL, W.L., & HENSLER, G.L. (1983)
Relationships of environmental contaminants to reproductive success
in red-breasted mergansers Mergus serrator from Lake Michigan.
Environ. Pollut., 32: 211-232.
HEINZOW, B.G.J., TINNEBERG, H.R., & BOIE, C. (1988) Effects of some
lipophilic xenobiotics on T-lymphocytes using a modified
erythrocyte rosette inhibition test. Res. Commun. chem. Patho.
Pharmacol., 61(2): 277-280.
HEIRONIMUS, M.P., LAUGHLIN, N.K., & BOWMAN, R.E. (1981) Effects of
early exposure to PCBs on learned irrelevancy of cues in Rhesus
monkeys. An incidental learning paradigm. Teratology, 24: 55A.
HELLE, E., OLSSON, M., & JENSEN, S. (1976a) DDT and PCB levels and
reproduction in ringed seals from the Bothnian bay. Ambio,
5: 188-189.
HELLE, E., OLSSON, M., & JENSEN, S. (1976b) PCB levels correlated
with pathological changes in seal uteri. Ambio, 5: 261-263.
HELLE, E., HYVARINEN, H., PYYSALO, H., & WICKSTROM, K. (1983)
Levels of organochlorine compounds in an inland seal population in
Eastern Finland. Mar. Pollut. Bull., 14: 256-260.
HENDRICKS, D., PUTNAM, T.P., BILLS, D.D., & SINNHUBER, R.O. (1977)
Inhibitory effect of a polychlorinated biphenyl (Aroclor 1254) on
aflatoxin B1 carcinogenesis in rainbow trout (Salmo gairdneri).
J. Natl Cancer Inst., 59: 1545-1551.
HERRING, J.L., HANNAN, E.J., & BILLS, D.D. (1972) UV irradiation of
Aroclor 1254. Bull. environ. Contam. Toxicol., 8: 153-157.
HESSELBERG, R.J. & SCHERR, D.D. (1974) PCBs and p,p' DDE in the
blood of cachectic patients. Bull. environ. Contam. Toxicol.,
11: 202-205.
HILL, H.R., Jr, (1985) Effects of polyhalogenated aromatic
compounds on porphyrin metabolism. Environ. health Perspect.,
60: 139-143.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington,
DC, US Department of the Interior, Fish and Wildlife Service, 147
pp (Fish and Wildlife Technical Report No. 2).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1974)
Polychlorinated biphenyl toxicity to Japanese quail as related to
degree of chlorination. Poult. Sci., 53: 597-604.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 61 pp (Special Scientific Report No. 191).
HILL, E.F., HEATH, R.G., & WILLIAMS, J.D. (1976) Effect of dieldrin
and Aroclor 1242 on Japanese quail eggshell thickness. Bull.
environ. Contam. Toxicol., 16: 445-453.
HINTON, D.E., GLAUMANN, H., & TRUMP, B.F. (1978) Studies on the
cellular toxicity of polychlorinated biphenyls (PCBs) I. Effect of
PCBs on microsomal enzymes and on synthesis and turnover of
microsomal and cytoplasmic lipids of rat liver - A morphological
and biochemical study. Virchows Arch. cell. Pathol., B27: 279-306.
HIRAYAMA, C., IRISA, T., & YAMAMOTO, T. (1969) Fine structural
changes of the liver in a patient with chlorobiphenyls
intoxication. Fukuoka Acta med., 60: 445-461.
HIRAYAMA, C., OKUMURA, M., NAGAI, J., & MASUDA, Y. (1974)
Hypobilirubinemia in patients with polychlorinated biphenyls
poisoning. Clin. Chim. Acta., 55: 97-100.
HIROSE, M., SHIRAI, T., TSUCA, H., FUKUSHIMA, S., OGISO, T., & ITO,
N. (1981) Effect of phenobarbital, polychlorinated biphenyl and
sodium saccharin on hepatic and renal carcinogenesis in
unilaterally nephrectomized rats given n-ethyl- N-hydroxy-
ethylnitrosamine orally. Carcinogenesis, 2: 1299-1302.
HLADKA, A., TAKACHOVA, T., & LISHKA, D. (1983) Exposure to
polychlorinated biphenyls and its effect on selected biochemical
functions. Czech. Med., 5: 8-14.
HODSON, K. (1975) Some aspects of the nesting ecology of
Richardson's merlin (Falco columbarius richardsonii) on the
Canadian prairies, Columbia, Vancouver, University of British
Columbia (MSc Thesis).
HOFFMAN, D.J., RATTNER, B.A., BUNCK, C.M., & KRYNITSKY, A. (1986)
Association between PCBs and lower embryonic weight in
black-crowned night herons in San Francisco Bay. J. Toxicol.
environ. Health, 19: 383-391.
HOGAN, J.W. & BRAUHN, J.L. (1975) Abnormal rainbow trout fry from
eggs containing high residues of a PCB (Aroclor 1242). Prog.
Fish-Cult., 37: 229-230.
HOLA, N. & REZNICEK, J. (1985) [The genetic hazard involved in
occupational exposure to high concentrations of polychlorinated
biphenyls.] Prac. Lek., 37: 386-391 (in Czech).
HOLDEN, A.V. (1970) Source of polychlorinated contamination in the
marine environment. Nature (Lond.), 228: 1220-1221.
HOLDEN, A.V. (1973) Monitoring PCBs in water and wildlife. In:
Proceedings of the Polychlorinated Biphenyl II Conference,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 23-33 (Publication No. 4E).
HOLDEN, A.V. & MARSDEN, K. (1969) Single-stage clean-up of animal
tissue extracts for organochlorine residue analysis. J.
Chromatogr., 44: 481-492.
HOLDGATE, M.W. (1971) The sea bird wreck in the Irish Sea. Autumn
1969, London, Natural Environment Research Council (Publication
Series C4).
HOLDRINET, M.V., BRAUN, H.E., FRANK, R., STOPPS, G.J., SMOUT, M.S.,
& MCWADE, J.W. (1977) Organochlorine residues in human adipose
tissue and milk from Ontario residents 1969-1974. Can. J. public
Health, 68: 74-80.
HOLLEMAN, K.A., BARNETT, B.D., & WICKER, G.W. (1976) Response of
chicks and turkey poults to Aroclor 1242. Poult. Sci.,
55: 2354-2356.
HOLT, R.L., CRUSE, S., & DREER, E.S. (1986) Pesticide and
polychlorinated biphenyl residues in human adipose tissue from
Northeast Louisiana. Bull. environ. Contam. Toxicol., 36: 651-655.
HOM, W., RISEBROUGH, R.W., SOUTAR, A., & YOUNG, D.R. (1974)
Deposition of DDE and polychlorinated biphenyls in dated sediments
of the Santa Barbara Basin. Science, 184: 1197-1199.
HONDA, T., NONAKA, S., MURAYAMA, F., OHGAMI, T., SHIMOYAMA, T., &
YOSHIDA, H. (1983) Effects of KC-400 (polychlorinated biphenyls) on
porphyrin metabolism. J. Dermatol., 10: 259-265.
HOOPINGARNER, R., SAMUEL, A., & KRAUSE, D. (1972) Polychlorinated
biphenyl interactions with tissue culture cells. Environ. health
Perspect., 1: 155-158.
HORI, S., OBANA, H., KASHIMOTO, T., OTAKE, T., NISHIMURA, H.,
IKEGAMI, N., KUNITA, N., & UDA, H. (1982) Effect of polychlorinated
biphenyls and polychlorinated quarterphenyls in Cynomolgus monkey
(Macaca fascicularis). Toxicology, 24: 123-139.
HORNSHAW, T.C., AULERICH, R.J., & JOHNSON, H.E. (1983) Feeding
great lakes fish to mink: Effects on mink and accumulation and
elimination of PCBs by mink. J. Toxicol. environ. Health,
11: 933-946.
HORNSHAW, T.C., SAFRONOFF, J., RINGER, R.K., & AULERICH, R.J.
(1986) LC50 test results in polychlorinated biphenyl-fed mink: Age,
season, and diet comparisons. Arch. Environ. Contam. Toxicol.,
15: 717-723.
HORZEMPA, L.M. & DI TORO, D.M. (1983) The extent of reversibility
of polychlorinated biphenyl adsorption. Water Res., 17: 851-859.
HSIA, M.T.S., LIN, F.S.D., & ALLEN, J.R. (1978) Comparative
mutagenicity and toxic effects of 2,2',5,5'-tetrachlorobiphenyl and
its metabolites in bacterial and mammalian test systems. Res.
Commun. chem. Pathol. Pharmacol., 21: 485-496.
HSU, I.C., VAN MILLER, J.P., SEYMOUR, J.L., & ALLEN, J.R. (1975a)
Urinary metabolites of 2,5,2',5'-tetrachlorobiphenyl in the
non-human primate. Proc. Soc. Exp. Biol. Med., 150: 185-188.
HSU, I.C., VAN MILLER, J.P., & ALLEN, J.R. (1975b) Metabolic fate
of 3H 2,5,2',5'-tetrachlorobiphenyls in infant non-human primates.
Bull. environ. Contam. Toxicol., 14: 233-240.
HSU, S.-T., MA, C.-I., HSU, S.K.-H., WU, S.-S., HSU, N.H.-M., YEH,
C.-C., & WU, S.-B. (1985) Discovery and epidemiology of PCB
poisoning in Taiwan: A four-year follow-up. Environ. health
Perspect., 59: 5-10.
HUCKINS, J.N., SCHWARTZ, T.R., PETTY, J.D. & SMITH, L.M. (1988)
Determination, fate and potential significance of PCBs in fish and
sediment samples with emphasis on selected AHH-inducing congeners.
Chemosphere, 17(10): 1995-2016.
HUDECOVA, A., KOSHINOVA, A., & MADARICH, A. (1979) [Investigations
of the effect of polychlorinated biphenyls on the dynamics of the
changes of the vitamin A and E content in rat liver.] Czech. Hyg.,
24: 59-64 (in Czech).
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife, Washington, DC, US Department
of the Interior, Fish and Wildlife Service, 90 pp (Resource
Publication No. 153).
HURST, J.G., NEWCOMER, W.S., & MORRISON, J.A. (1973) The effects of
polychlorinated biphenyl on longevity of bobwhite quail (Collinus
virginianus): A sex differential. Proc. Soc. Exp. Biol. Med.,
144: 431-435.
HURST, J.G., NEWCOMER, W.S., & MORRISON, J.A. (1974) Some effects
of DDT, toxaphene and polychlorinated biphenyl on thyroid function
in bobwhite quail. Poult. Sci., 53: 125-133.
HUSTERT, K. & KORTE, F. (1972) [Contributions to ecological
chemistry. XXXVIII: Synthesis of polychlorinated biphenyls and
their reactions to UV irradiation.] Chemosphere, 1: 7-10
(in German).
HUTZINGER, O., SAFE, S., & ZITKO, V. (1971) Polychlorinated
biphenyls: photolysis of 2,4,6,2',4',6'-hexachlorobiphenyl,
chlorobiphenyl. Nature (Lond.), 232: 15.
HUTZINGER, O., NASH, D.M., SAFE, S., DEFREITAS, A.S.W., NORSTRÖM,
R.J., WILDISH, D.J., & ZITKO, V. (1972) Polychlorinated
biphenyls-metabolic behaviour of pure isomers in pigeons, rats, and
brook trout. Science, 178: 312-314.
HUTZINGER, O., SAFE, S., & ZITKO, V. (1974) The chemistry of PCBs,
Cleveland, Ohio, CRC Press.
HUTZINGER, O., CHOUDHRY, G.G., CHITTIM, B.G., & JOHNSTON, L.E.
(1985) Formation of polychlorinated dibenzofurans and dioxins
during combustion, electrical equipment fires, and PCB
incineration. Environ. health Perspect., 60: 3-9.
IARC (1978) Polychlorinated biphenyls and polybrominated biphenyls,
Lyon, International Agency for Research on Cancer, pp. 43-103 (IARC
Monographs on the Evaluation of the Carcinogenic Risks of Chemicals
to Humans, Volume 18).
IARC (1987) Overall evaluation of carcinogenicity: An updating of
IARC monographs Volumes 1 to 42, Lyon, International Agency for
Research on Cancer, (IARC Monographs on the Evaluation of the
Carcinogenic Risks of Chemicals to Humans, Supplement 7).
IATROPOULOS, M.J., FELT, G.R., ADAMS, H.P., KORTE, F., & COULSTON,
F. (1977) Chronic toxicity of 2,5,4'-trichlorobiphenyl in young
Rhesus monkeys. II. Histopathology. Toxicol. appl. Pharmacol., 41:
629-638.
IATROPOULOS, M.J., BAILEY, J., ADAMS, H.P., COULSTON, F., & HOBSON,
W. (1978) Response of nursing infant Rhesus to Clophen A30 or
hexachlorobenzene given to lactating mothers. Environ. Res.,
16: 38-47.
IKEDA, M., KURATSUNE, M., NAKAMURA, Y., & HIROHATA, T. (1987) A
cohort study on mortality of Yusho patients - a preliminary report.
Fukuoka Acta med., 78: 297-300.
IMANISHI, J., NOMURA, H., MATSUBARA, M., KITA, M., WON, S.-J.,
MIZUTANI, T., & KISHIDA, T. (1980) Effect of polychlorinated
biphenyl on viral infections in mice. Infect. Immun., 29: 275-277.
IMANISHI, J., OKU, T., OISHI, K., NOMURA, H., & MIZUTANI, T. (1984)
Reduced resistance to experimental viral and bacterial infections
of mice treated with polychlorinated biphenyl. Biken J.,
27: 195-198.
INNAMI, S., NAKAMURA, A., MIYAZAKI, M., NAGAYAMA, S., & NISHIDE, E.
(1976) Further studies on the reduction of vitamin A content in the
livers of rats given polychlorinated biphenyls. J. nutr. Sci.
Vitaminol., 22: 409-418.
INOUE, K., TAKANAKA, A., MIZOKAMI, K., FUJIMORI, K., SUNOUCHI, M.,
KASUYA, Y., & OMORI, Y. (1981) Effects of polychlorinated biphenyls
on the monooxygenase system in fetal livers of rats. Toxicol. appl.
Pharmacol., 59: 540-547.
INTERNATIONAL COUNCIL FOR THE EXPLORATION OF THE SEA (1974) Report
of a working group for the international study of the pollution of
the North Sea and its effects on living resources and their
exploitation, Charlottenlund, Denmark, International Council for
the Exploration of the Sea (Co-operative Research Report No. 39).
IRPTC (1986) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme.
ISEKI, K., TAKAHASHI, M., BAUERFEIND, E., & WONG, C.S. (1981)
Effects of polychlorinated biphenyls (PCBs) on a marine plankton
population and sedimentation in controlled ecosystem enclosures.
Mar. Ecol. Prog. Ser., 5: 207-214.
ISHI, H. (1972) PCB pollution in Japan, Tokyo, Japanese Public
Health Association, pp. 13-28 (Environmental Health Report No. 14).
ISHIDATE, K., YOSHIDA, M., & NAKAZAWA, Y. (1978) Effect of typical
inducers of microsomal drug-metabolizing enzymes on phospholipid
metabolism in rat liver. Biochem. Pharmacol., 27: 2595-2603.
ISHIKAWA, T.T., MCNEELY, S., STEINER, P.M., GLUECK, C.J., MELLIES,
M., GARTSIDE, P.S., & MCMILLIN, C. (1978) Effects of chlorinated
hydrocarbons on plasma alpha-lipoprotein cholesterol in rats.
Metabolism, 27: 89-96.
ITO, N., NAGASAKI, H., ARAI, M., MAKIURA, S., SUGIHARA, S., &
HIRAO, K. (1973) Histopathologic studies on liver tumorigenesis
induced in mice by technical polychlorinated biphenyl and its
promoting effect on liver tumours induced by benzenehexachloride.
J. Natl Cancer Inst., 51(5): 1637-1646.
ITO, N., NAGASAKI, H., MAKIURA, S., & ARAI, M. (1974) Histological
studies on liver tumorigenesis in rats treated with polychlorinated
biphenyls. Gann., 65: 545-549.
ITOKAWA, Y., YAGI, N., KAITO, H., KAMOHARA, K., & FUJIWARA, K.
(1976) Influence of diet on the induction of hepatic ceroid pigment
in rats by polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
36: 131-141.
IVERSON, F., VILLENEUVE, D.C., GRANT, D.L., & HATINA, G.V. (1975)
Effect of Aroclor 1016 and 1242 on selected enzyme systems in the
rat. Bull. environ. Contam. Toxicol., 13: 456-463.
IVERSON, F., TRUELOVE, J., & HIERLIHY, S.L. (1982) Hepatic
microsomal enzyme induction by Aroclors 1248 and 1254 in Cynomolgus
monkeys. Food chem. Toxicol., 20: 307-310.
IWATA, Y. & GUNTHER, F.A. (1976) Translocation of the
polychlorinated biphenyl Aroclor 1254 from soil into carrots under
field conditions. Arch. environ. Contam. Toxicol., 4: 44-59.
IWATA, Y., WESTLAKE, W.E., & GUNTHER, F.A. (1973) Varying
persistence of polychlorinated biphenyls in six California soils
under laboratory conditions. Bull. environ. Contam. Toxicol.,
9: 204-211.
IWATA, Y., GUNTHER, F.A., & WESTLAKE, W.E. (1974) Uptake of a PCB
(Aroclor 1254) from soil by carrots under field conditions. Bull.
environ. Contam. Toxicol., 11: 523-528.
JACOBSON, J.L., JACOBSON, S.W., SCHWARTZ, P.M., FEIN, G.G., &
DOWLER, J.K. (1984a) Prenatal exposure to an environmental toxic:
A test of the multiple effects model. Dev. Psychol., 20: 523-532.
JACOBSON, J.L., FEIN, G.G., JACOBSON, S.W., SCHWARTZ, P.M., &
DOWLER, J.K. (1984b) The transfer of polychlorinated biphenyls
(PCBs) and polybrominated biphenyls-(PBBs) across the human
placenta and into maternal milk. Am. J. public Health,
74(4): 378-379.
JACOBSON, S.W., FEIN, G.G., JACOBSON, J.L., SCHWARTZ, P.M., &
DOWLER, J.K. (1985) The effect of intrauterine PCB exposure on
visual recognition memory. Child Dev., 56: 856-860.
JAN, J. & MALNERSIC, S. (1978) Determination of PCB and PCT
residues in fish by tissue acid hydrolysis and destructive clean-up
of the extract. Bull. environ. Contam. Toxicol., 19: 772-780.
JAN, J. & TRATNIK, M. (1988a) Polychlorinated biphenyls in
residents around the River Krupa, Slovenia, Yugoslavia. Bull.
environ. Contam. Toxicol., 41: 809-814.
JAN, J., TRATNIK, M., & KENDA, A. (1988b) Atmospheric contamination
with polychlorinated biphenyls in Bela Krajina (Yugoslavia);
Emissions from industrial plant, landfill and river areas.
Chemosphere, 17(4): 809-813.
JANI, J.P., PATEL, J.S., SHAH, M.P., GUPTA, S.K., & KASHYAP, S.K.
(1988) Levels of organochlorine pesticides in human milk in
Ahmedabad, India. Int. Arch. occup. environ. Health., 60: 111-113.
JANNETTI, R.A. & ANDERSON, L.M. (1981) Dimethylnitrosamine
demethylase activity in fetal, suckling, and maternal mouse liver
and its transplacental and transmammary induction by
polychlorinated biphenyls. J. Natl Cancer Inst., 67: 461-466.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTRÖM, G., &
VAZ, R. (1975) Identification by GC-MS of phenolic metabolites of
PCB and p,p'-DDE isolated from Baltic guillemot and seal. Ambio,
4: 93-97.
JAPAN ENVIRONMENT AGENCY (1983) Environmental monitoring of
chemicals; Environmental survey report of F.Y. 1980 and 1981.
Tokyo, Japan Environment Agency, Department of Environmental
Health, Offices of Health Studies.
JAPAN ENVIRONMENTAL SANITATION BUREAU (1973) [Survey on food
contamination: Report of a comprehensive investigation on the
prevention of pollution by PCBs], Tokyo, Ministry of Health &
Welfare, Environmental Sanitation Bureau, pp. 191-210 (in
Japanese).
JEFFERIES, D.J. & PARSLOW, J.L.F. (1972) Effect of one
polychlorinated biphenyl on size and activity of the gull thyroid.
Bull. environ. Contam. Toxicol., 8: 306-310.
JEFFERIES, D.J. & PARSLOW, J.L.F. (1976) Thyroid changes in
PCB-dosed guillemots and their indication of one of the mechanisms
of action of these materials. Environ. Pollut., 10: 293-311.
JENSEN, A.A. (1983a) Chemical contaminants in human milk. Res.
Rev., 89: 1-128.
JENSEN, A.A. (1983b) PCB in human milk: An Overview. In: Barros,
M.C., Könemann, H., & Visser, R., ed. Proceedings of the PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 81-98.
JENSEN, A.A. (1984a) Current data of levels of organohalogen
compounds in human milk in countries outside CEC. In: Report on the
WHO Consultation on Organohalogen Compounds in Human Milk and
Related Hazards, Bilthoven, 9-11 January 1985, Copenhagen, World
Health Organization, Regional Office for Europe, Annex 3.
JENSEN, A.A. (1984b) Data extracted from document on "Assessment of
the presence of potentially toxic substances in breast milk with
special emphasis on the European Community" submitted by the
Commission of the European Communities (CEC Study No. 830465,
CEC/V/E/2/lux/35/84). In: Report on the WHO Consultation on
Organohalogen Compounds in Human Milk and Related Hazards,
Bilthoven, 9-11 January 1985, Copenhagen, World Health
Organization, Regional Office for Europe, Annex 7.
JENSEN, A.A. (1987) Polychlorobiphenyls (PCBs),
polychlorodibenzo- p-dioxins (PCDDs) and polychlorodibenzofurans
(PCDFs) in human milk, blood, and adipose tissue. Sci. total
Environ., 64: 259-293.
JENSEN, S. & JANSSON, B. (1976) Methylsulfone metabolites of PCB
and DDE in seals from the Baltic. Ambio, 5: 257-260.
JENSEN, S. & SUNDSTROM, G. (1974a) Structures and levels of most
chlorobiphenyls in two technical PCB products and in human adipose
tissue. Ambio, 3: 70-76.
JENSEN, S. & SUNDSTROM, G. (1974b) Metabolic hydroxylation of a
chlorobiphenyl containing only isolated unsubstituted
positions-2,2',4,4',5,5'-hexachlorobiphenyl. Nature (Lond.),
251: 219-220.
JENSEN, S., JOHNELS, A.G., OLSSON, M., & OTTERLIND, G. (1969) DDT
and PCB in marine animals from Swedish waters. Nature (Lond.),
224: 247-250.
JENSEN, S., JOHNELS, A.G., OLSSON, M., & OTTERLIND, G. (1972a) DDT
and PCB in herring and cod from the Baltic, the Kattegat and the
Skaggerrak. Ambio, 1(Spec. Rep.): 71-85.
JENSEN, S., JOHNELS, A.G., OLSSEN, M., & WESTERMARK, T. (1972b) The
avifauna of Sweden as indicators of environmental contamination
with mercury and chlorinated hydrocarbons. In: Proceedings of the
15th International Ornithology Congress, Leiden, pp. 455-465.
JENSEN, S., RENBERG, L., & VAZ, R. (1973) Problems in
quantification of PCB in biological material. In: Proceedings of
the Polychlorinated Biphenyls Conference II, Stockholm, 1972,
Solna, Sweden, National Environmental Protection Board, pp. 7-13
(Publication No. 4E).
JENSEN, S., ORBERG, J., KIHLSTROM, J.E., OLSSON, M., & LUNDBERG, C.
(1977) Effects of PCB and DDT on mink (Mustela vision) during the
reproductive season. Ambio, 6: 239.
JFCMP (1985) FAO/WHO Collaborating Centres for Food Contamination
Monitoring. PCB's residues in food, Rome, Food and Agriculture
Organization of the United Nations, FAO/WHO Food Contamination
Monitoring Programme (Document EFP/85,3 prepared for the 17th
Session of the Codex Committee on Pesticide Residues).
JOHANSSON, N., LARSSON, A., & LEWANDER, K. (1972) Metabolic effects
of PCB (Polychlorinated biphenyls) on the brown trout (Salmo
trutta). Comp. gen. Pharmacol., 3: 310-314.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1979)
Pesticides and other chemical residues in infant and toddler total
diet samples (I), August 1974-July 1975. Pestic. monit. J.,
13(3): 87-98.
JOHNSTONE, G.J., ECOBICHON, D.J., & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, D.C.L., DAVIS, W.E., Jr, NEWELL, G.W., SASMORE, D.P., &
ROSEN, V.J. (1974) Modification of hexachlorophene toxicity by
dieldrin and Aroclor 1254. Toxicology, 2: 309-318.
JONES, K.C. (1988) Determination of polychlorinated biphenyls in
human foodstuffs and tissues: suggestions for a selective congener
analytical approach. Sci. total Environ., 68: 141-159.
JONES, K.C. (1989) Polychlorinated biphenyls in Welsh soils: a
survey of typical levels. Chemosphere, 18(7-8): 1665-1672.
JONES, J.W. & ALDEN, H.S. (1936) An acneform dermatergosis. Arch.
Dermato. Syphilol., 33: 1022-1034.
JONSSON, H.T., KEIL, J.E., GADDY, R.G., LOADHOLT, C.B., HENNIGAR,
G.R., & WALKER, E.M. (1976) Prolonged ingestion of commercial DDT
and PCB; effects on progesterone levels and reproduction in the
mature female rat. Arch. environ. Contam. Toxicol., 3: 479-490.
JONSSON, H.T., Jr, WALKER, E.M., Jr, GREENE, W.B., HUGHSON, M.D.,
& HENNIGAR, G.R. (1981) Effects of prolonged exposure to dietary
DDT and PCB on rat liver morphology. Arch. environ. Contam.
Toxicol., 10: 171-183.
JURY, W.A., WINER, A.M., SPENCER, W.F., & FOCHT, D.D. (1987)
Transport and transformations of organic chemicals in the
soil-air-water ecosystem. Rev. environ. Contam. Toxicol.,
99: 119-164.
KAISER, K.L.E. (1974) On the optical activity of polychlorinated
biphenyls. Environ. Pollut., 7: 93-101.
KAISER, K.L.E. & WONG, P.T.S. (1974) Bacterial degradation of
polychlorinated biphenyls. I. Identification of some metabolic
products from Aroclor 1242. Bull. environ. Contam. Toxicol.,
11: 291-296.
KAMAL, M., KLEIN, M., & KORTE, F. (1976) Isolation and
identification of metabolites after long-term feeding of
2,2'-dichlorobiphenyl-C-14. Chemosphere, 5: 349-356.
KAMOHARA, T., YAGI, N., & ITOKAWA, Y. (1984) Mechanism of lipid
peroxide formation in polychlorinated biphenyls (PCB) and
dichlorodiphenyltrichloroethane (DDT)-poisoned rats. Environ. Res.,
34: 18-23.
KANEKO, T. (1988) [Polychlorinated terphenyls (PCTs). A study on
the induction of cleft palate by polychlorinated terphenyls (PCTs)
administered maternally with special reference to the role of
corticosterone.] Pharmacometrics, 36(4): 309-327 (in Japanese).
KANNAN, N., TANABE, S., WAKIMOTO, T., & TATSUKAWA, R. (1987)
Coplanar polychlorinated biphenyls in Aroclor and Kanechlor
mixtures. J. Assoc. Off. Anal. Chem., 70(3): 451-454.
KANNAN, N., TANABE, S., & TATSUKAWA, R. (1988) Potentially
hazardous residues of non- ortho chlorine substituted coplanar
PCBs in human adipose tissue. Arch. environ. Health, 43(1): 11-14.
KANNAN, N., WAKIMOTO, T., & TATSUKAWA, R. (1989) Possible
involvement of frontier (n) electrons in the metabolism of
polychlorinated biphenyls (PCBs). Chemosphere, 18(9/10): 1955-1963.
KARLSSON, B., PERSSON, B., SÖDERGREN, A., & ULFSTRAND, S. (1974)
Locomotory and dehydrogenase activities of redstarts Phoenicurus
phoenicurus L. (Aves) given PCB and DDT. Environ. Pollut.,
7: 53-63.
KARPPANEN, E. & KOLHO, L. (1973) The concentration of PCB in human
blood and adipose tissue in three different research groups.
Presented at the Polychlorinated Biphenyls Conference II,
Stockholm, 1972 (Unpublished document).
KASHIMOTO, T., MIYATA, H., FUKUSHIMA, S., KUNITA, N., OHI, G., &
TUNG, T.-C. (1985) PCBs, PCQs, and PCDFs in blood of Yusho and
Yu-Cheng patients. Environ. health Perspect., 59: 73-78.
KASZA, L., WEINBERGER, M.A., HINTON, D.E., TRUMP, B.F., PATEL, C.,
FRIEDMAN, L., & GARTHOFF, L.H. (1978a) Comparative toxicity of
polychlorinated biphenyl and polybrominated biphenyl in the rat
liver: light and electron microscopic alterations after subacute
dietary exposure. J. environ. Pathol. Toxicol., 1: 241-257.
KASZA, L., COLLINS, W.T., CAPEN, C.C., GARTHOFF, L.H., & FRIEDMAN,
L. (1978b) Comparative toxicity of polychlorinated biphenyl and
polybrominated biphenyl in the rat thyroid gland: light and
electron microscopic alterations after subacute dietary exposure.
J. environ. Pathol. Toxicol., 1: 587-599.
KATO, N. & YOSHIDA, A. (1980) Effect of dietary PCB on hepatic
cholesterogenesis in rats. Nutr. Rep. Int., 21: 107-112.
KATO, N. & YOSHIDA, A. (1981) Effect of various dietary xenobiotics
on serum total cholesterol and high density lipoprotein cholesterol
in rats. Nutr. Rep. Int., 23: 825-831.
KATO, N., KATO, M., KIMURA, T., & YOSHIDA, A. (1978) Effect of
dietary addition of PCB, DDT, or BHT and dietary protein on vitamin
A and cholesterol metabolism. Nutr. Rep. Int., 18: 437-446.
KATO, N., KAWAI, K., & YOSHIDA, A. (1982) Effects of dietary
polychlorinated biphenyls and protein level on liver and serum
lipid metabolism of rats. Agric. biol. Chem., 46: 703-707.
KATO, S., MCKINNEY, J.D., & MATTHEWS, H.B. (1980) Metabolism of
symmetrical hexachlorobiphenyl isomers in the rat. Toxicol. appl.
Pharmacol., 53: 389-398.
KATSUKI, S. (1969) Foreword. Fukuoka Acta med., 60: 403-407.
KAWAI, S., FUKUSHIMA, M., MIYAZAKI, N., & TATSUKAWA, R. (1988)
Relationship between lipid composition and organochlorine levels in
the tissues of striped Dolphin. Mar. Pollut. Bull., 19: 129-133.
KAWANISHI, S., SANO, S., MIZUTANI, T., & MATSUMOTO M. (1973)
[Experimental porphyria induced by polychlorinated biphenyls.] Jpn.
J. Hyg., 28: 84 (in Japanese).
KAWANISHI, S., SANO, S., MIZUTANI, T., & MATSUMOTO M. (1974)
[Experimental studies on toxicity of synthetic tetrachlorobiphenyl
isomers.] Jpn. J. Hyg., 29: 81 (in Japanese).
KAWANISHI, S., SANO, S., & MIZUTANI, T. (1975) [A toxicological
study on synthetic hexachlorobiphenyl isomers.] Jpn. J. Hyg.,
30: 124 (in Japanese).
KAWANISHI, S., SEKI, Y., & SANO, S. (1983) Uroporphyrinogen
decarboxylase. Purification, properties, and inhibition by
polychlorinated biphenyl isomers. J. biol. Chem., 258: 4285-4292.
KECK, G. (1981) Effets de la contamination par les
polychlorobiphényles (PCBs) sur le développement de la tumeur
d'Ehrlich chez la souris Swiss. Toxicol. Eur. Res., 3(5): 229-236.
KERKVLIET, N.I. & BAECHER-STEPPAN, L. (1988a) Suppression of
allograft immunity by 3,4,5,3',4',5-hexachlorobiphenyl. I. Effects
of exposure on tumor rejection and cytotoxic T-cell activity
in vivo. Immunopharmacology, 16: 1-12.
KERKVLIET, N.I. & BAECHER-STEPPAN, L. (1988b) Suppression of
allograft immunity by 3,4,5,3',4',5-hexachlorobiphenyl. II. Effects
of exposure on mixed lymphocyte reactivity and induction of
suppressor cell activity in vitro. Immunopharmacology, 16: 13-23.
KERKVLIET, N.I. & KIMELDORF, D.J. (1977) Anti-tumour activity of a
polychlorinate biphenyl mixture, Aroclor 1254, in rats inoculated
with Walker 256 carcinosarcoma cells. J. Natl Cancer Inst.,
59: 951-955.
KHAN, M.A., RAO, R.M., & NOVAK, A.F. (1976) Polychlorinated
biphenyls (PCBs) in food. January 1976. Crit. Rev. food Sci. Nutr.,
January: 103-145.
KHAN, A.V., LASHNEVA, N.V., KUMOK, S.T., GURVICH, YA.A., &
TUTELYAN, V.A. (1985) Study into the effect of ionol on cytochrome
P-450 induction in rat liver by polychlorinated diphenyls. Vopr.
pitan., 4(1): 66-69.
KIESSLING, A., PART, P., RING, O., & LINDAHL-KIESSLING, K. (1983)
Effects of PCB on the adrenergic response in perfused gills and on
levels of muscle glycogen in rainbow trout (Salmo gairdneri
Rich.). Bull. environ. Contam. Toxicol., 31: 712-718.
KIHLSTROM, J.E., LUNDBERG, C., ORBERG, J., DANIELSSON, P.O., &
SYDHOFF, J. (1975) Sexual functions of mice neonatally exposed to
DDT or PCB. Environ. Physiol. Biochem., 5: 54-57.
KIKUCHI, M. (1984) Autopsy of patients with Yusho. Am. J. ind.
Med., 5: 19-30.
KIMBROUGH, R.D. (1980) Occupational exposure. In: Halogenated
biphenyls, terphenyls, naphthalenes, dibenzodioxins and related
products, Amsterdam, Elsevier, North-Holland Biomedical Press,
pp. 373-395 (Topics in Environmental Health, Vol. 4).
KIMBROUGH, R.D. (1987) Human health effects of polychlorinated
biphenyls (PCBs) and polybrominated biphenyls (PBBs). Annu. Rev.
Pharmacol. Toxicol., 27: 87-111.
KIMBROUGH, R.D., LINDER, R.E., & GAINES, T.B. (1972) Morphological
changes in livers of rats fed polychlorinated biphenyls. Light
microscopy and ultrastructure. Arch. environ. Health, 25: 354-364.
KIMBROUGH, R.D. & LINDER, R.E. (1974) Induction of adenofibrosis
and hepatomas of the liver in BALB/cJ mice by polychlorinated
biphenyls (Aroclor 1254). J. Natl Cancer Inst., 53: 547-552.
KIMBROUGH, R.D., SQUIRE, R.A., LINDER, R.E., STRANBERG, J.D.,
MONTALI, R.J., & BURSE, V.W. (1975) Induction of liver tumours in
Sherman strain female rats by polychlorinated biphenyl Aroclor
1260. J. Natl Cancer Inst., 55: 1453-1459.
KIMURA, N.T. & BABA, T. (1973) Neoplastic changes in the rat liver
induced by polychlorinated biphenyls. Gann, 64: 105-108.
KIMURA, I. & MIYAKE, T. (1976) Teratogenic and postnatal growth-
suppressive effects of polychloro-triphenyl (PCT) in dd/Y mice.
Teratology, 14: 243-244 (Abstract).
KIMURA, S., KUMADA, H., & MATIDA, Y. (1974) [Acute toxicity and
accumulation of PCB (KC 300) in freshwater fish. Bull. freshwater
Fish Res. Lab. (Tokyo), 23: 115-123 (in Japanese).
KIMURA, N.T., KANEMATSU, T., & BABA, T. (1976) Polychlorinated
biphenyl(s) as a promoter in experimental hepatocarcinogenesis in
rats. Z. Krebsforsch., 87: 257-266.
KIRIYAMA, S., BANJO, M., & MATSUSHIMA, H. (1974) Effect of
polychlorinated biphenyls (PCB) and related compounds on the weight
of various organs and plasma and liver cholesterol in the rat.
Nutr. Rep. Int., 10: 79-88.
KITAMURA, M., TSUKAMOTO, T., SUMINO, K., HAYAKAWA, K., SHIBITA, T.,
& HIRANO, I. (1973) [The PCB levels in the blood of workers
employed in a condenser factory.] Jpn. J. ind. Health, 47: 354-355
(in Japanese).
KLAAS, E.E. & SWINEFORD, D.M. (1976) Chemical residue content and
hatchability of screech owl eggs. Wilson Bull., 88: 421-426.
KLASSON-WEHLER, E., BERGMAN, A., KOWALSKI, B., & BRANDT, I. (1987)
Metabolism of 2,3,4'6,-tetrachlorobiphenyl: Formation and tissue
localization of mercapturic acid pathway metabolites in mice.
Xenobiotica, 17(4): 477-486.
KLEIN, H. (1983) The PCB situation in the Federal Republic of
Germany. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of PCB Seminar, The Hague, 28-30 September 1983, The
Hague, Ministry of the Environment, pp. 66-79.
KLEKOWSKI, E.J. (1982) Mutation in ferns growing in an environment
contaminated with polychlorinated biphenyls, Amherst,
Massachusetts, University of Massachusetts, Water Resources
Research Center, 23 pp ((NTIS) PB83-150011).
KLEPPEL, G.S. & MCLAUGHLIN, J.J.A. (1980) PCB toxicity to
phytoplankton: Effects of dose and density-dependent recovery
responses. Bull. environ. Contam. Toxicol., 24: 696-703.
KLING, D. & GAMBLE, W. (1982) Cholesterol biosynthesis in
polychlorinated biphenyl-treated rats. Environ. Res., 27: 10-15.
KOBAYASHI, Y. (1972) [Answer report to the questionary paper on
"Regulation on residual levels in foods".] Biol. Pollut., 4: 93-116
(in Japanese).
KODAMA, H. & OTA, H. (1977) Studies on the transfer of PCB to
infants from their mothers (1). Jpn. J. Hyg., 32: 567-573.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated biphenyls
to infants from mothers. Arch. environ. Health, 35: 95-100.
KOEMAN, J.H., TEN HOEVER DE BRAUW, M.C., & DE VOS, R.H. (1969)
Chlorinated biphenyls in fish, mussels and birds from the River
Rhine and the Netherlands coastal area. Nature (Lond.),
221: 1126-1128.
KOEMAN, J.H., PEETERS, W.H.M., SMIT, C.J., TJIOE, P.S., & DE GOEIJ,
J.J.M. (1972) Persistent chemicals in marine mammals. TNO-nieuws,
27: 570-578.
KOEMAN, J.H., VAN VELZEN-BLAD, H.C.W., DE VRIES, R., & VOS, J.G.
(1973) Effects of PCB and DDE in cormorants and evaluation of PCB
residues from an experimental study. J. Reprod. Fertil.,
19(Suppl.): 353-364.
KOHLI, K.K., GUPTA, B.N., ALBRO, P.W., MUKHTAR, H., & MCKINNEY,
J.D. (1979) Biochemical effects of pure isomers of hexachloro-
biphenyl: fatty livers and cell structure. Chem. biol. Interact.,
25: 139-156.
KOHLI, K.K., PHILPOT, R.M., ALBRO, P.W., & MCKINNEY, J.D. (1980)
Induction of different species of cytochrome P-450 by coplanar and
noncoplanar isomers of hexachlorobiphenyl. Life Sci., 26: 945-952.
KOLBYE, A.C., Jr (1972) Food exposure to polychlorinated biphenyls.
Environ. health Perspect., 1: 85-88.
KOLLER, L.D. (1977) Enhanced polychlorinated biphenyls lesions in
Moloney leukemia virus-infected mice. Clin. Toxicol.,
11(1): 107-116.
KOLLER, L.D. & THIGPEN, J.E. (1973) Biphenyl-exposed rabbits. Am.
J. vet. Res., 34: 1605-1606.
KOLLER, L.D. & ZINKL, J.G. (1973) Pathology of polychlorinated
biphenyls in rabbits. Am. J. Pathol., 70: 363-378.
KOMATSU, F. & KIKUCHI, M. (1972) [Skin lesion by 3,4,3',4'-
tetrachlorobiphenyl in rabbits.] Fukuoka Acta med., 63: 384-386
(in Japanese).
KORACH, K.S., SARVER, P., CHAE, K., MCLACHLAN, J.A., & MCKINNEY,
J.D. (1987) Estrogen receptor-binding activity of polychlorinated
hydroxybiphenyls: Conformationally restricted structural probes.
Mol. Pharmacol., 33: 120-126.
KOSHIOKA, M., KANAZAWA, J., IIZUKA, H., & MURAI, T. (1987)
Photodegradation of decachlorobiphenyl. Bull. environ. Contam.
Toxicol., 38: 409-415.
KOSS, G., SEUBERT, S., SEUBERT, A., HERBERT, M., KORANSKY, W., &
IPPEN, H. (1980) Hexachlorobenzene and 2,4,5,2',4',5'-hexachloro-
biphenyl - A comparison of their distribution, biotransformation
and prophyrinogenic action in female rats. In: Holmstedt, B.,
Lauwerys, R., Mercier, M., & Roberfroid, M., ed. Mechanisms of
toxicity and hazard evaluation, Amsterdam, Elsevier/North-Holland
Biomedical Press, pp. 517-520.
KOSUTZKY, J., ADAMEC, O., & BOBAKOVA, E. (1979) Effects of
polychlorinated biphenyls on poultry reproduction. Bull. environ.
Contam. Toxicol., 21: 737-742.
KREISS, K. (1985) Studies on populations exposed to polychlorinated
biphenyls. Environ. health Perspect., 60: 193-199.
KREISS, K., ZACK, M.M., KIMBROUGH, R.D., NEEDHAM, L.L., SMRED,
A.L., & JONES, B.T. (1981) Association of blood pressure and
polychlorinated biphenyl levels. J. Am. Med. Assoc.,
245(24): 2505-2509.
KREITZER, J.F. & HEINZ, G.H. (1974) The effect of sublethal dosages
of five pesticides and a polychlorinated biphenyl on the avoidance
response of Coturnix quail chicks. Environ. Pollut., 6: 21-29.
KREITZER, J.F. & SPANN, J.W. (1973) Tests of pesticidal synergism
with young pheasants and Japanese quail. Bull. environ. Contam.
Toxicol., 9: 250-256.
KRICHER, J.C., BAYER, C.L., & MARTIN, D.A. (1979) Effects of two
Aroclor fractions on the productivity and diversity of algae from
two lentic ecosystems. Int. J. environ. Stud., 13: 159-167.
KROGH DERR, S. (1978) In vivo metabolism of exogenous
progesterone by PCB treated female rats. Bull. environ. Contam.
Toxicol., 19: 729-732.
KUNITA, N., HORI, S., OBANA, H., OTAKE, T., NISHIMURA, H.,
KASHIMOTO, T., & IKEGAMI, N. (1985) Biological effects of PCBs PCQs
and PCDFs present in the oil causing Yusho and Yu-Cheng. Environ.
Health Perspect., 59: 79-84.
KURACHI, M. (1983) A new sulfur-containing derivative and
possibility of conjugate formation of PCBs in mice or rats. Agric.
biol. Med., 47(6): 1183-1191.
KURACHI, M. & MIO, T. (1983) On fluctuation of PCBs under various
unnatural conditions in mice. Agric. biol. Med., 47(6): 1173-1181.
KURATSUNE, M. & MASUDA, Y. (1972) Polychlorinated biphenyls in
non-carbon copypaper. Environ. health Perspect., 1: 61-62.
KURATSUNE, M., YOSHIMURA, T., MATSUZAKA, J., & YAMAGUCHI, A. (1972)
Epidemiologic study on Yusho, a poisoning caused by ingestion of
rice-oil contaminated with a commercial brand of polychlorinated
biphenyls. Environ. health Perspect., 1: 119-128.
KURATSUNE, M., MASUDA, Y., & NAGAYAMA, J. (1976) Some of the recent
findings concerning Yusho. In: Proceedings of the National
Conference on Polychlorinated Biphenyls, Chicago, 19-21 November
1975, Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances, pp. 14-29 (EPA-560/6-75-004).
KUROKI, H. & MASUDA, Y. (1977) Structures and concentrations of the
main components of polychlorinated biphenyls retained in patients
with Yusho. Chemosphere, 6: 469-474.
KUSUDA, M. (1971) [Study on the female sexual function suffering
from the chlorobiphenyls poisoning.] Sanka Fujinka, 4: 1063-1072
(in Japanese).
KUWABARA, K., YAKUSHIJI, T., WATANABE, I., YOSHIDA, S., KOYAMA, K.,
KUNITA, N., & HARA, I. (1978) Relationship between breast feeding
and PCB residues in blood of the children whose mothers were
occupationally exposed to PCBs. Int. Arch. occup. environ. Health,
41: 189-197.
KUWABARA, K., YAKUSHIJI, T., WATANABE, I., YOSHIDA, S., KOYAMA, K.,
& KUNITA, N. (1979) Levels of polychlorinated biphenyls in blood of
breast-fed children whose mothers are non-occupationally exposed to
PCBs. Bull. environ. Contam. Toxicol., 21: 458-462.
LAKE, B.G., COLLINS, M.A., HARRIS, R.A., & GANGOLLI, S.D. (1979)
The induction of hepatic and extrahepatic xenobiotic metabolism in
the rat and ferret by a polychlorinated biphenyl mixture (Aroclor
1254). Xenobiotica, 9: 723-731.
LAN, S.-J., YEN, Y.-Y., KO, Y.-C., & CHEN, E.-R. (1989) Growth and
development of permanent teeth germ of transplacental Yu-Cheng
babies in Taiwan. Bull. environ. Contam. Toxicol., 42: 931-934.
LA ROCCA, P. & CARLSON, G.P. (1979) The effect of polychlorinated
biphenyls on adenosine triphosphatase activity. Toxicol. appl.
Pharmacol., 48: 185-192.
LARSEN, P.F., GADBOIS, D.F., & JOHNSON, A.C. (1985) Observations on
the distribution of PCBs in the deepwater sediments of the Gulf of
Maine. Mar. Pollut. Bull., 16: 439-442.
LARSSON, R. (1984) Transport of PCBs from aquatic to terrestrial
environments by emerging chironomids. Environ. Pollut.,
34: 283-289.
LARSSON, P. (1985a) Contaminated sediments of lakes and oceans act
as sources of chlorinated hydrocarbons for release to water and
atmosphere. Nature (Lond.), 317: 347-349.
LARSSON, P. (1985b) Change in PCB (Clophen A 50) composition when
transported from sediment to air in aquatic model systems. Environ.
Pollut., B9: 81-94.
LARSSON, P. (1987) Uptake of polychlorinated biphenyls (PCBs) by
the macroalga, Cladophora glomerata. Bull. environ. Contam.
Toxicol., 38: 58-62.
LARSSON, P. & OKLA, L. (1987) An attempt to measure the flow of
chlorinated hydrocarbons, such as PCBs, from water to air in the
field. Environ. Pollut., 44: 219-225.
LARSSON, C.M. & TILLBERG, J.E. (1975) Effects of the commercial
polychlorinated biphenyl mixture Aroclor 1242 on growth, viability,
phosphate uptake, respiration and oxygen evolution in Scenedesmus.
Physiol. Plant., 33: 256-260.
LASHNEVA, N.V. & TUTELYAN, V.A. (1984) Induction of cytochrome
P-450 in rat liver by polychlorinated diphenyls. Farmakol.
Toksikol., 6: 77-80.
LASHNEVA, N.V., KHAN, A.V., & TUTELYAN, V.A. (1985) The functional
state of monooxygenase system in rat liver tissue after effect of
ionol and polychlorinated diphenyls. Vopr. med. khim. 5: 7-22.
LASHNEVA, N.V., CHICHILANOVA, G.V., SOROKOVAYA, G.K., & TUTELYAN,
V.A. (1987) Effects of polychlorinated biphenyls on rat liver
cytochrome P-450 system formation at early postnatal period. In:
Abstracts of the USSR-All-Union Conference on Cytochrome P-450 and
Environment, Novosibirsk, 27-31 July 1987, Novosibirsk, USSR
Academy of Medical Sciences, Siberian Division.
LAWRENCE, J. & TOSINE, H.M. (1977) Polychlorinated biphenyl
concentrations in sewage and sludges of some waste treatment plants
in Southern Ontario. Bull. environ. Contam. Toxicol., 17: 49-56.
LAWTON, R.W., ROSS, M.R., FEINGOLD, J., & BROWN, J.F., Jr (1985)
Effects of PCB exposure on biochemical and hematological findings
in capacitor workers. Environ. health Perspect., 60: 165-184.
LAY, J.P., KLEIN, W., & KORTE, F. (1975) Excretion, storage, and
metabolism of 2,3,6,2',4'-pentachlorobiphenyl-14-C after a
long-term feeding experiment on rats. Chemosphere, 4: 161-68.
LAY, J.P., KAMAL, M., KLEIN, W., & KORTE, F. (1979) Fate of
2.5.4'-trichlorobiphenyl in rats. Xenobiotica, 12: 713-721.
LEATHERLAND, J.F. & SONSTEGARD, R.A. (1978) Lowering of serum
thyroxine and triiodothyronine levels in yearling coho salmon,
Oncorhynchus kisutch, by dietary mirex and PCBs. J. Fish Res.
Board Can., 35: 1285-1289.
LEATHERLAND, J.F. & SONSTEGARD, R.A. (1979) Effect of dietary mirex
and PCB (Aroclor 1254) on thyroid activity and lipid reserves in
rainbow trout Salmo gairdneri Richardson. J. Fish Dis., 2: 43-48.
LEDERMAN, T.C. & RHEE, G.-Y. (1982) Bioconcentration of a
hexachlorobiphenyl in Great Lakes planktonic algae. Can. J. Fish.
aquat. Sci., 39: 380-387.
LEECE, B., DENOMME, M.A., TOWNER, R., & LI, S.M.A. (1985)
Polychlorinated biphenyls: correlation between in vivo and
in vitro quantitative structure-activity relationships (QSARs).
J. Toxicol. environ. Health, 16: 379-388.
LEES, P.S.J., CORN, M., & BREYSSE, P.N. (1987) Evidence for dermal
absorption as the major route of body entry during exposure of
transformer maintenance and repairmen to PCBs. Am. Ind. Hyg. Assoc.
J., 48(3): 257-264.
LEMMETYINEN, R., RANTAMAKI, P., & KARLIN, A. (1982) Levels of DDT
and PCB's in different stages of life cycle of the Arctic tern
Sterna paradisaea and the herring gull Larus argentatus.
Chemosphere, 11: 1059-1068.
LEONI, V., FABIANI, L., MARINELLI, G., PUCCETTI, G., TARSITANI,
G.F., DE CAROLIS, A., VESCIA, N., MORINI, A., ALEANDRI, V., POZZI,
V., CAPPA, F., & BARBATI, D. (1989) PCB and other organochlorine
compounds in blood of women with or without miscarriage: A
hypothesis of correlation. Ecotoxicol. environ. Saf., 17: 1-11.
LEVIN, B.D. & BOWMAN, R.E. (1983) Polychlorinated biphenyl effects
on delayed spatial alternation in monkeys. Toxicologist, 3: 68.
LEVIN, E.D., SCHANTZ, S.L., & BOWMAN, R.E. (1988) Delayed spatial
alteration deficits resulting from perinatal PCB exposure in
monkeys. Arch. Toxicol., 62: 267-273.
LEWIN, V., MCBLAIN, W.A., & WOLFE, F.H. (1972) Acute
intraperitoneal toxicity of DDT and PCBs in mice using two
solvents. Bull. environ. Contam. Toxicol., 8(4): 245-250.
LICHTENSTEIN, E.P., SCHULZ, K.R., FUHREMANN, T.W., & LIANG, T.T.
(1969) Biological interaction between plasticizers and
insecticides. J. econ. Entomol., 62: 761-765.
LICHTENSTEIN, E.P. (1972) PCBs and interactions with insecticides.
Environ. health Perspect., 1: 151-153.
LIEB, A.J., BILLS, D.D., & SINNHUBER, R.O. (1974) Accumulation of
dietary polychlorinated biphenyls (Aroclor 1254) by rainbow trout
(Salmo gairdneri). J. agric. food Chem., 22: 638-642.
LILLIE, R.J., HARRIS, S.J., CECIL, H.C., & BITMAN, J. (1974) Normal
reproductive performance of mature cockerels fed Aroclor 1248.
Poult. Sci., 53: 1604-1607.
LIN, F.S., HSIA, M.T., & ALLEN, J.R. (1979) Acute hepatotoxicity of
a tetrachlorobiphenyl-changes in the hepatocyte ultrastructure and
plasma membrane-bound enzymes. Arch. environ. Contam. Toxicol.,
8: 321-333.
LIN, P.S., NYQUIST, S.E., & HACLERODE, J. (1982) Interactions of
polychlorinated biphenyls and delta-9-tetrahydrocannabinol with
testosterone hydroxylation and the hepatic microsomal system in the
rat. Proc. Pa. Acad. Sci., 56: 31-35.
LINDER, R.E., GAINES, T.B., & KIMBROUGH, R.D. (1974) The effect of
polychlorinated biphenyls on rat reproduction. Food Cosmet.
Toxicol., 12: 63-76.
LINDSEY, A.S. & WAGSTAFFE, P.J. (1989) Production and certification
of ten high-purity polychlorinated biphenyls as reference
materials. Analyst, 114(5): 553-557.
LINZEY, A.V. (1987a) Effects of chronic polychlorinated biphenyls
exposure on the reproductive success of white-footed mice
(Peromyscus leucopus). Arch. environ. Contam. Toxicol.,
16: 455-460.
LINZEY, A.V. (1987b) Effects of chronic polychlorinated biphenyls
exposure on growth and reproduction of second generation
white-footed mice (Peromyscus leucopus). Arch. environ. Contam.
Toxicol., 17: 39-45.
LITTERST, C.L. & VAN LOON, E.J. (1974) Time-course of induction of
microsomal enzymes following treatment with polychlorinated
biphenyl. Bull. environ. Contam. Toxicol., 11: 206-212.
LITTERST, C.L., FARBER, T.M., BAKER, A.M., & VAN LOON, E.J. (1972)
Effect of polychlorinated biphenyls on hepatic microsomal enzymes
in the rat. Toxicol. appl. Pharmacol., 23: 112-122.
LIU, D. (1980) Enhancement of PCBs biodegradation by sodium
ligninsulfonate. Water Res., 14: 1467-1475.
LIU, D. (1981) Biodegradation of Aroclor 1221 type PCBs in sewage
wastewater. Bull. environ. Contam. Toxicol., 27: 695-703.
LIU, D. (1982) Assessment of continuous biodegradation of
commercial PCB formulations. Bull. environ. Contam. Toxicol.,
29: 200-207.
LLORENTE, G.A., FARRAN, A., RUIZ, X., & ALBAIGES, J. (1987)
Accumulation and distribution of hydrocarbons, polychlorobiphenyls,
and DDT in tissues of three species of Anatidae from the Ebro Delta
(Spain). Arch. environ. Contam. Toxicol., 16: 563-572.
LOOSE, L.D., PITTMAN, K.A., BENITZ, K.-F., & SILKWORTH, J.B. (1977)
Polychlorinated biphenyl and hexachlorobenzene induced humoral
immunosuppression. J. Reticulendothel. Soc., 22: 253-271.
LOOSE, L.D., SILKWORTH, J.B., PITTMAN, K.A., BENITZ, K.-F., &
MUELLER, W. (1978) Impaired host resistance to endotoxin and
malaria in polychlorinated biphenyl- and hexachlorobenzene-treated
mice. Infect. Immun., 20: 30-35.
LORENZ, H. & NEUMEIER, G. (1983) [Polychlorinated biphenyls (PCBs).
A joint report of the Federal Health Office and the Federal
Environment Office.] Munich, MMV Medizin Press (BGA Publication
No. 4/83) (in German).
LOWE, J.I., PARRISH, P.R., PATRICK, J.M., & FORESTER, J. (1972)
Effects of the polychlorinated biphenyl Aroclor 1254 on the
American oyster Crassostrea virginica. Mar. Biol., 17: 209-214.
LU, Y.-C & WONG P.-N (1984) Dermatological, medical and laboratory
findings of patients in Taiwan and their treatments. Am. J. ind.
Med., 5: 81-115.
LU, Y.-C. & WU, Y.-C. (1985) Clinical findings and immunological
abnormalities in Yu-Cheng patients. Environ. health Perspect.,
59: 17-29.
LUARD, E.J. (1973) Sensitivity of Dunaliella and Scenedesmus
(Chlorophyceae) to chlorinated hydrocarbons. Phycologia,
12: 29-33.
LUBET, R.A., LEMAIRE, B.N., AVERY, D., & KOURI, R.E. (1986)
Induction of immunotoxicity in mice by polychlorinated biphenyls.
Arch. Toxicol., 59: 71-77.
LUND, J., ANDERSSON, O., POELLINGER, L., & GUSTAFSSON, J.A. (1986)
In vitro characterization of possible mechanisms underlying the
selective in vivo accumulation of the PCB metabolite
4,4'-bis(methyl-sulphonyl)-2,5,2',5'-tetrachlorobiphenyl in the
lung. Food chem. Toxicol., 24(6/7): 563-566.
LUNDY, P., WURSTER, C.F., & ROWLAND, R.G. (1984) A two-species
marine algal bioassay for detecting aquatic toxicity of chemical
pollutants. Water Res., 18: 187-194.
LUNT, D. & EVANS, W.C. (1970) Microbial metabolism of biphenyl.
Biochem. J., 118: 54-55.
LUOTAMO, M., JÄRVISALO, J., & AITIO, A. (1985) Analysis of
polychlorinated biphenyls (PCBs) in human serum. Environ. health
Perspect., 60: 327-332.
LUTZ, R.J., DEDRICK, R.L., MATTHEWS, H.B., ELING, T., & ANDERSON,
M.W. (1977) A preliminary pharmacokinetic model for several
chlorinated biphenyls in rat. Drug Metab. Dispos., 5: 386-396.
LYNCH, T.R. & JOHNSON, H.E. (1982) Availability of a
hexachlorobiphenyl isomer to benthic amphipods from experimentally
contaminated natural sediments, Philadelphia, Pennsylvania,
American Society for Testing Materials, Aquatic Toxicology and
Hazard Assessment, pp. 273-287 (STP No. 76).
MAACK, L. & SONZOGNI, W.C. (1988) Analysis of polychlorobiphenyl
congeners in Wisconsin fish. Arch. environ. Contam. Toxicol.,
17: 711-719.
MAC, M.J. & SEELYE, J.G. (1981) Patterns of PCB accumulation by fry
of Lake Trout. Bull. environ. Contam. Toxicol., 27: 368-375.
MCCLURE, V.E. (1976) Transport of heavy chlorinated hydrocarbons in
the atmosphere. Environ. Sci. Technol., 10: 1223-1228.
MCCLURG, T.P. (1984) Trace metals and chlorinated hydrocarbons in
Ross seals from Antarctica. Mar. Pollut. Bull., 15: 384-389.
MCCONNELL, E.E., HASS, J.R., ALTMAN, N., & MOORE, J.A. (1979) A
spontaneous outbreak of polychlorinated biphenyl (PCB) toxicity in
Rhesus monkeys (Macaca mulatta): toxicopathology. Lab. anim.
Sci., 29: 666-673.
MCFARLAND, V.A. & CLARKE, J.U. (1989) Environmental occurrence,
abundance and potential toxicity of polychlorinated biphenyl
congeners: Considerations for a congener-specific analysis.
Environ. health Perspect., 81: 225-239.
MACKAY, D. (1989) Modeling the long-term behavior of an organic
contaminant in a large lake: Application to PCBs in Lake Ontario.
J. Great Lakes Res., 15(2): 283-297.
MCKINNEY, J.D. & SINGH, P. (1981) Structure-activity relationships
in halogenated biphenyls: unifying hypothesis for structural
specificity. Chem. biol. Interact., 33: 271-283.
MCKINNEY, J.D., CHAE, K., MCCONNELL, E.E., & BIRNBAUM, L.S. (1985)
Structure-induction versus structure-toxicity relationships for
polychlorinated biphenyls and related aromatic hydrocarbons.
Environ. Health Perspect., 60: 57-68.
MCKINNEY, J.D., KORACH, K.S., & MCLACHLAN, J.A. (1990)
Detoxification of polychlorinated biphenyls. January 27. Lancet,
335: 222-223.
MCLANE, M.A.R. & HUGHES, D.L. (1980) Reproductive success of
screech owls fed Aroclor 1248. Arch. environ. Contam. Toxicol.,
9: 661-665.
MCLEESE, D.W., METCALFE, C.D., & PEZZACK, D.S. (1980) Uptake of
PCBs from sediment by Nereis virens and Crangon septemspinosa.
Arch. environ. Contam. Toxicol., 9: 507-518.
MACLEOD, H.A., SMITH, D.C., & BLUMAN, N. (1980) Pesticide residues
in the total diet in Canada, V: 1976 to 1978. J. Food Saf.,
2: 141-164.
MCMANUS, G.B., WYMAN, K.D., PETERSON, W.T., & WURSTER, C.F. (1983)
Factors affecting the elimination of PCBs in the marine copepod
Acartia tonsa. Estuarine coastal Shelf Sci., 17: 421-430.
MCNULTY, W.P. (1985) Toxicity and fetotoxicity of TCDD, TCDF and
PCB isomers in Rhesus macaques (Macaca mulatta). Environ. Health
Perspect., 60: 77-88.
MCNULTY, W.P., BECKER, G.M., & CORY, H.T. (1980) Chronic toxicity
of 3,4,3',4'- and 2,5,2',5'-tetrachlorobiphenyls in Rhesus
macaques. Toxicol. appl. Pharmacol., 56: 182-190.
MACLEOD, K.E. (1981) Polychlorinated biphenyls in indoor air.
Environ. Sci. Technol., 15(8): 926-928.
MAFF (1986) Report of file working party on pesticide residues
(1982-1985): 16th report of the Steering Group on Food
Surveillance, London, Ministry of Agriculture, Fisheries, and Food,
Her Majesty's Stationery Office, (Food Surveillance Paper No. 16).
MAFF (1989) Migration of substances from food contact materials
into food: 26th report of the Steering Group on Food Surveillance.
Progress report of the Working Party on Chemical Contaminants from
Food, London, Ministry of Agriculture, Fisheries, and Food, Her
Majesty's Stationery Office, 58 pp (Food Surveillance Paper
No. 26).
MAHANTY, H.K. (1975) A study of the effects of polychlorinated
biphenyl (Aroclor 1242) on an aquatic plant Spirodela oligorrhiza
(Kurz) Hegelm. Bull. environ. Contam. Toxicol., 14: 558-565.
MAHANTY, H.K. & FINERAN, B.A. (1976) Effects of a polychlorinated
biphenyl (Aroclor 1242) on the ultrastructure of frond cells in the
aquatic plant Spirodela oligorrhiza (Kurz) Hegelm. N. Z. J. Bot.,
14: 13-18.
MAHANTY, H.K. & MCWHA, J.A. (1976) Sensitivity of Spirodela
oligorrhiza (Kurz) Hegelm. to a polychlorinated biphenyl (Aroclor
1242). N. Z. J. Bot., 14: 9-12.
MAKI, A.W. & JOHNSON, H.E. (1975) Effects of PCB (Aroclor 1254) and
p,p'DDT on production and survival of Daphnia magna Strauss.
Bull. environ. Contam. Toxicol., 13: 412-416.
MAKIURA, S., AOE, H., SUGIHARA, S., HIARO, K., ARAI, M., & ITO, N.
(1974) Inhibitory effect of polychlorinated biphenyls on liver
tumorigenesis in rats treated with 3'-methyl-4-dimethyl-
aminobenzene, N-2-fluorenylacetamide and diethylnitrosamine. J.
Natl Cancer Inst., 53: 1253-1257.
MALINS, D.C., MCCAIN, B.B., BROWN, D.W., VARANASI, U., KRAHN, M.M.,
MYERS, M.S., & CHAN, S.-L. (1987) Sediment-associated contaminants
and liver diseases in bottom-dwelling fish. Hydrobiologia,
149(1): 64-74.
MANCHESTER-NEESVIG, J.B. & ANDREN, A.W. (1989) Seasonal variation
in the atmospheric concentration of polychlorinated biphenyl
congeners. Environ. Sci. Technol., 23: 1138-1148.
MANSKE, D.D. & JOHNSON, R.D. (1975) Pesticide residues in total
diet samples (VIII). Pestic. monit. J., 9(2): 94-105.
MANSKE, D.D. & JOHNSON, R.D. (1977) Pesticide and other chemical
residues in total diet samples (X). Pestic. monit. J.,
10(4): 134-148.
MARCUS, J.M. & MATHEWS, T.D. (1987) Polychlorinated biphenyls in
blue crabs from South Carolina. Bull. environ. Contam. Toxicol.,
39(5): 857-862.
MARINUCCI, A.C. & BARTHA, R. (1982) Accumulation of the
polychlorinated biphenyl Aroclor 1242 from contaminated detritus
and water by the saltmarsh detritivore, Uca pugnax. Bull.
environ. Contam. Toxicol., 29: 326-333.
MARKARD, C. (1988) [Organic substances in sewage sludge - a danger
to the food chain?] Korresp. Abwasser, 35(5): 449-455 (in German).
MARKS, T.A., KIMMEL, G.L., & STAPLES, R.E. (1981) Influence of
symmetrical polychlorinated biphenyl isomers on embryo and fetal
development in mice I. Teratogenicity of 3,3',4,4',5,5'-
hexachlorobiphenyl. Toxicol. appl. Pharmacol., 61: 269-276.
MARONI, M., COLUMBI, A., CANTONI, S., FERIOLI, E., & FOA, V.
(1981a) Occupational exposure to polychlorinated biphenyls in
electrical workers. I. Environmental and blood polychlorinated
biphenyl concentrations. Br. J. ind. Med., 38(1): 49-54.
MARONI, M., COLUMBI, A., ARBOSTI, G., CANTONI, S., & FOA, V.
(1981b) Occupational exposure to polychlorinated biphenyls in
electrical workers II. Health effects. Br. J. ind. Med.,
38(1): 55-60.
MARONI, M., COLOMBI, A., FERIOLI, A., & FOA, V. (1984) Evaluation
of porphyrinogenesis and enzyme induction in workers exposed to
PCB. Med. Lav., 75(3): 188-199.
MARTINEAU, D., BELAND, P., DESJARDINS, C., & LAGACE, A. (1987)
Levels of organochlorine chemicals in tissues of beluga whales
(Delphinapterus leucas) from the St. Lawrence Estuary, Quebec,
Canada. Arch. environ. Contam. Toxicol., 16: 137-147.
MASSACHUSETTS DEPARTMENT OF PUBLIC HEALTH (1987) The greater New
Bedford PCB health effects study 1984-1987, Boston, Massachusetts,
Massachusetts Department of Public Health.
MASUDA, Y., KAGAWA, R., & KURATSUNE, M. (1972) Polychlorinated
biphenyls in carbonless copying paper. Nature (Lond.), 237: 41-42.
MASUDA, Y., KAGAWA, R., & KURATSUNE, M. (1974a) [Polychlorinated
biphenyls in Yusho patients and ordinary persons.] Fukuoka Acta
med., 65: 17-24 (in Japanese).
MASUDA, Y., KAGAWA, R., SHIMAMURA, K., TAKADA, M., & KURATSUNE, M.
(1974b) [Polychlorinated biphenyls in the blood of Yusho patients
and ordinary persons.] Fukuoka Acta. med., 65: 25-27 (in Japanese).
MASUDA, Y., KAGAWA, R., KUROKI, H., KURATSUNE, M., YOSHIMURA, T.,
TAKI, I., KUSUDA, M., YAMASHITA, F., & HAYASHI, M. (1978a) Transfer
of polychlorinated biphenyls from mothers to foetuses and infants.
Bull. environ. Contam. Toxicol., 16: 543-546.
MASUDA, Y., KAGAWA, R., TOKUDOME, S., & KURATSUNE, M. (1978b)
Transfer of polychlorinated biphenyls to the foetuses and offspring
of mice. Toxicology, 6: 331-340.
MASUDA, Y., KAGAWA, R., KUROKI, H., TOKUDOME, S. & KURATSUNE, M.
(1979) Transfer of various polychlorinated biphenyls to the fetuses
and offspring of mice. Food Cosmet. Toxicol., 17(6): 623-627.
MASUDA, Y., KUROKI, H., HARAGUCHI, K., & NAGAYAMA, J. (1985) PCB
and PCDF congeners in the blood and tissues of Yusho and Yu-Cheng
patients. Environ. health Perspect., 59: 53-58.
MATTHEWS, H.B. & ANDERSON, M.W. (1975a) Effect of chlorination on
the distribution and excretion of polychlorinated biphenyls. Drug
Metab. Dispos., 3: 371-380.
MATTHEWS, H.B. & ANDERSON, M.W. (1975b) The distribution and
excretion of 2,4,5,2',5'-pentachlorobiphenyl in the rat. Drug
Metab. Dispos., 3: 211-219.
MATTHEWS, H.B. & ANDERSON, M.W. (1976) PCB chlorination versus PCB
distribution and excretion. In: Proceedings of the National
Conference on Polychlorinated Biphenyls, Chicago, 19-21 November
1975, Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances, pp. 50-56 (EPA-560/6-75-004).
MATTHEWS, H.B. & DEDRICK, R.L. (1984) Pharmacokinetics of PCBs.
Annu. Rev. Pharmacol. Toxicol., 24: 85-103.
MATTHEWS, H.B. & TUEY, D.B. (1980) The effect of chlorine position
on the distribution and excretion of four hexachlorobiphenyl
isomers. Toxicol. appl. Pharmacol., 53: 377-388.
MATTSSON, R., MATTSSON, A., KIHLSTRÖM, J.E., & LINDAHL-KIESSLING,
K. (1981) Effects of a hexachlorinated biphenyl on lymphoid organs
and resorption of foetuses in pregnant mice. Arch. environ. Contam.
Toxicol., 10: 281-288.
MAUCK, W.L., MEHRLE, P.M., & MAYER, F.L. (1978) Effects of the
polychlorinated biphenyl Aroclor 1254 on growth, survival, and bone
development in brook trout (Salvelinus fontinalis). J. Fish Res.
Board Can., 35: 1084-1088.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Washington, DC, US Department of Commerce,
National Technical Information Service, 274 pp ((NTIS)
PB87-188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior,
Fish & Wildlife Service, pp. 506-553 (Resource Publication
No. 160).
MAYER, F.L, MEHRLE, P.M., & SANDERS, H.O. (1977) Residue dynamics
and biological effects of polychlorinated biphenyls in aquatic
organisms. Arch. environ. Contam. Toxicol., 5: 501-511.
MEHLMAN, M.A., YIN, L., & NIELSEN, R.C. (1974) Metabolic changes in
"frozen-clamped" livers of rats caused by ingestion of
polychlorinated biphenyls. Toxicol. appl. Pharmacol., 27: 300-307.
MEIER, P.G. & REDISKE, R.R. (1984) Oil and PCB interactions on the
uptake and excretion in midges. Bull. environ. Contam. Toxicol.,
33: 223-232.
MEIGS, J.W., ALBOM, J.J., & KARTIN, B.L. (1954) Chloracne from an
unusual exposure to Aroclor. J. Am. Med. Assoc., 154: 1417-1418.
MELVÅS, B. & BRANDT, I. (1973) The distribution and metabolism of
labelled polychlorinated biphenyls in mice and quails. In:
Proceedings of the Polychlorinated Biphenyls Conference II,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 87-90 (Publication No. 4E).
MENDOZA-FIGUEROA, T., LOPEZ-REVILLA, R., & VILLA-TREVINO, S. (1985)
Aroclor 1254 increases the genotoxicity of several carcinogens to
liver primary cell cultures. J. Toxicol. environ. Health,
15: 245-254.
MERKENS, L.S. & KINTER, W.B. (1971) Acute toxicity of a mixture of
polychlorinated biphenyls (Aroclor 1221) and DDT in a marine
teleost (Fundulus heteroclitus) and effect on serum osmolality,
Na+ and K+. Bull. Mt Desert Isl. biol. Lab., 11: 64-68.
MERSON, M.H. & KIRKPATRICK, R.L. (1976) Reproductive performance of
Captive White-footed mice fed a PCB. Bull. environ. Contam.
Toxicol., 16: 392-398.
MES, J. (1987) Polychlorobiphenyl in children's blood. Environ.
Res., 44: 213-220.
MES, J. & MARCHAND, L. (1987) Comparison of some specific
polychlorinated biphenyl isomers in human and monkey milk. Bull.
environ. Contam. Toxicol., 39: 736-742.
MES, J., COFFIN, D.E., & CAMPBELL, D. (1974) Polychlorinated
biphenyl and organochlorine pesticide residues in Canadian chicken
eggs. Pestic. monit. J., 8: 8-11.
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 28: 97-104.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine pesticides in
milk and blood of Canadian women during lactation. Arch. environ.
Contam. Toxicol., 13: 217-223.
MES, J., DAVIES, D.J., TURTON, D., & SUN, W.-F. (1986) Levels and
trends of chlorinated hydrocarbon contaminants in the breast milk
of Canadian women. Food Addit. Contam., 3(4): 313-322.
MES, J., ARNOLD, D.L., BRYCE, F., DAVIES, D.J., & KARPINSKI, K.
(1989a) The effect of long-term feeding of Aroclor 1254 to female
Rhesus monkeys on their polychlorinated biphenyl tissue levels.
Arch. environ. Contam. Toxicol., 18: 858-865.
MES, J., NEWSOME, W.H., & CONACHER, H.B.S. (1989b) Determination of
some specific isomers of polychlorinated biphenyl congeners in
fatty foods of the Canadian diet. Food Addit. Contam.,
6(3): 365-375.
MICHAELS, R.A., ROWLAND, G., & WURSTER, C.F. (1982) Polychlorinated
biphenyls (PCB) inhibit photosynthesis per cell in the marine
diatom Thalassiosira pseudonana. Environ. Pollut., 27: 9-14.
MILLER, D.L. & OGILVIE, D.M. (1975) Temperature selection in brook
trout (Salvelinus fontinalis) following exposure to DDT, PCB, or
phenol. Bull. environ. Contam. Toxicol., 14: 545-551.
MILLER, M.M., GHODBANE, S., WASIK, S.P., TEWARI, Y.B., & MARTIRE,
D.E. (1984) Aqueous solubilities, octanol/water partition
coefficients, and entropies of melting of chlorinated benzenes and
biphenyls. J. chem. Eng. Data, 29: 184-190.
MILLING, A., MULLER, W.F., COULSTON, F., & KORTE, F. (1979)
Comparative metabolism of 2,2'-dichlorobiphenyl-14C in mice, rats
and Rhesus monkeys after a single oral application. Chemosphere,
1: 15-19.
MINEAU, P., FOX, G.A., NORSTROM, R.J., WESELOH, D.V., HALLETT,
D.J., & ELLENTON, J.A. (1984) Using the herring gull to monitor
levels and effects of organochlorine contamination in the Canadian
Great Lakes. In: Nriagu, J.O., Nriagu, J.O., & Simmons, M.S., ed.
Toxic contaminants in the Great Lakes, New York, John Wiley and
Sons, pp. 425-452.
MIO, T. & SUMINO, K. (1985) Mechanism of biosynthesis of
methylsulfones from PCBs and related compounds. Environ. Health
Perspect., 59: 129-135.
MIO, T., SUMINO K., & MIZUTANI, T. (1976) Sulfur-containing
metabolites of 2,5,2',5'-tetrachlorobiphenyl, a major component of
commercial PCBs. Chem. pharm. Bull., 24: 1958-1960.
MIRANDA, C.L., HENDERSON, M.C., WANG, J.L., NAKAUE, H.S., & BUHLER,
D.R. (1987) Effects of polychlorinated biphenyls on porphyrin
synthesis and cytochrome P-450-dependent mono-oxygenase in small
intestine and liver of Japanese quail. J. Toxicol. environ. Health,
20: 27-35.
MIYATA, H., FUKUSHIMA, S., KASHIMOTO, T., & HUNITA, N. (1985) PCBs,
PCQs, and PCDFs in tissues of Yusho and Yu-Cheng patients. Environ.
Health Perspect., 59: 67-72.
MIYATA, H., TAKAYAMA, K., OGAKI, J., KASHIMOTO, T., & FUKUSHIMA, S.
(1987) Polychlorinated dibenzo- p-dioxins in blue mussel from
marine coastal water in Japan. Bull. environ. Contam. Toxicol.,
39(5): 877-883.
MIYAZAKI, A., HOTTA, T., KATAYAMA, J., & KIMURA, Y. (1975)
Absorption and translocation of PCB into crops. Bull. Osaka agric.
Res. Cent., 12: 135-142.
MIZUNOYA, Y., TANIGUCHI, S., KUSUMOTO, K., MORITA, S., YAMADA, A.,
BABA, T., & OGAKI, S. (1974) [Effects of polychlorinated biphenyls
on fetuses and offspring in rats.] J. Food Hyg. Soc. Jpn,
15: 252-260 (in Japanese).
MIZUTANI, T., HIDAKA, K., OHE, T., & MATSUMOTO, M. (1977) A
comparative study on accumulation and elimination of
tetrachlorobiphenyl isomers in mice. Bull. environ. Contam.
Toxicol., 18: 452-461.
MOILANEN, R., PYYSALO, H., WICKSTROM, K., & LINKO, R. (1982) Time
trends of chlordane, DDT, and PCB concentrations in pike (Esox
lucius) and Baltic herring (Clupea harengus) in the Turku
Archipelago, Northern Baltic Sea for the period 1971-1982. Bull.
environ. Contam. Toxicol., 29: 334-340.
MOKSNES, M.T. & NORHEIM, G. (1986) Levels of chlorinated
hydrocarbons and composition of PCB in herring gull Larus
argentatus eggs collected in Norway in 1969 compared to 1979-81.
Environ. Pollut., B11: 109-116.
MOORE, S.A. & HARRISS, R.C. (1972) Effects of polychlorinated
biphenyl on marine phytoplankton communities. Nature (Lond.),
240: 356-357.
MOORE, S.A. & HARRISS, R.C. (1974) Differential sensitivity to PCB
by phytoplankton. Mar. Pollut. Bull., 5: 174-176.
MORGAN, R.W., WARD, J.M., & HARTMAN, P.E. (1981) Aroclor
1254-induced intestinal metaplasia and adenocarcinoma in the
glandular stomach of F344 rats. Cancer Res., 41: 5052-5059.
MORIARTY, F. (1969) The effects of polychlorobiphenyls on
Chorthippus brunneus (Saltatoria: Acrididae). Entomol. Exp.
Appl., 12: 206-210.
MORITA, M., NAKAGAVA J., & RAPPE, C. (1978) Polychlorinated
dibenzofuran (PCDF) formation from PCB mixture by heat and oxygen.
Bull. environ. Contam. Toxicol., 19: 665-670.
MORSE, R.A., CULLINEY, T.W., GUTENMANN, W.H., LITTMAN, C.B., &
LISK, D.J. (1987) Polychlorinated biphenyls in honey bees. Bull.
environ. Contam. Toxicol., 38: 271-276.
MOSELEY, C.L., GERACI, C.L., & BURG, J. (1982) Polychlorinated
biphenyl exposure in transformer maintenance operations. Am. Ind.
Hyg. Assoc. J., 43: 170-174.
MOSSER, J.L., TENG, T., FISHERR, N.S., & WURSTER, C.F. (1972)
Polychlorinated biphenyls: Toxicity to certain phytoplankters.
Science, 175: 191-192.
MOSSER, J.L., TENG, T., WALTHER, W.G., & WURSTER, C.F. (1974)
Interactions of PCBs, DDT, and DDE in a marine diatom. Bull.
environ. Contam. Toxicol., 12: 665-668.
MOZA, P., WEISBERGER, I., KLEIN, W., & KORTE, F. (1974) Metabolism
of 2,2'-dichlorobiphenyl-14C in two plant-water-soil-systems. Bull.
environ. Contam. Toxicol., 12: 541-546.
MOZA, P., WEISBERGER, I., & KLEIN, W. (1976a) Fate of
2,2'-dichlorobiphenyl-14C in carrots, sugar beet, and soil under
outdoor conditions. J. agric. food Chem., 24: 881-885.
MOZA, P., KILZER, L., WEISGERBER, I., & KLEIN, W. (1976b)
Contribution to ecological chemistry CXV. Metabolism of
2,5,4'-trichlorobiphenyl-14C and 2,4,6,2',4'-pentachloro-
biphenyl-14C in the marsh plant Veronica beccabunga. Bull.
environ. Contam. Toxicol., 16: 454-463.
MOZA, P., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1979a) Studies
with 2,4,5-trichlorobiphenyl-14C and 2,2,4,4,6-penta-
chlorobiphenyl-14C in carrots, sugar, beet, and soil. J. agric.
food Chem., 27: 1120-1124.
MOZA, P.N., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1979b) Long-term
uptake of lower chlorinated biphenyls and their conversion products
by spruce trees (Picea abies) from soil treated with sewage
sludge. Chemosphere, 8: 373-375.
MROZEK, E. & LEIDY, R.B. (1981) Investigation of selective uptake
of polychlorinated biphenyls by Spartina alterniflora Loisel.
Bull. environ. Contam. Toxicol., 27: 481-488.
MROZEK, E., SENECA, E.D., & HOBBS, L.L. (1982) Polychlorinated
biphenyl uptake and translation by Spartina alterniflora Loisel.
Water Air Soil Pollut., 17: 3-15.
MROZEK, E., QUEEN, W.H., & HOBBS, L.L. (1983) Effects of
polychlorinated biphenyls on growth of Spartina alterniflora
Loisel. Environ. exp. Bot., 23: 285-292.
MUEHLEBACK, S. & BICKEL, M.H. (1981) Pharmacokinetics in rats of
2,4,5,2',4',5'-hexachlorobiphenyl an unmetabolisable lipophilic
model compound. Xenobiotica, 11: 249-259.
MUIR, D.C.G., NORSTROM, R.J., & SIMON, M. (1988) Organochlorine
contaminants in Arctic marine food-chains: Accumulation of specific
polychlorinated biphenyls and chlordane-related compounds. Environ.
Sci. Technol., 22: 1071-1077.
MULHERN, B.M., CROMARTIE, E., REICHEL, W.L., & BELISLE, A.A. (1971)
Semiquantitative determination of polychlorinated biphenyls in
tissue samples by thin layer chromatography. J. Assoc. Off. Agric.
Chem., 54: 548-550.
MULLER, W.F., HOBSON, W., FULLER, G.B., KNAUF, W., COULSTON, F., &
KORTE, F. (1978) Endocrine effects of chlorinated hydrocarbons in
Rhesus monkeys. Ecotoxicol. Environ. Saf., 2: 161-172.
MULLIN, M.D., POCHINI, C.M., MCCRINDLE, S., ROMKES, M., SAFE, S.H.,
& SAFE, L.M. (1984) High-resolution PCB analysis: Synthesis and
chromatographic properties of all 209 PCB congeners. Environ. Sci.
Technol., 18: 468-476.
MURADO, M.A., TEJEDOR, M.C., & BALUJA, G. (1976) Interactions
between polychlorinated biphenyls (PCBs) and soil microfungi.
Effects of Aroclor 1254 and other PCBs on Aspergillus flavus
cultures. Bull. environ. Contam. Toxicol., 15: 768-774.
MURAI, Y. & KUROIWA, Y. (1971) Peripheral neuropathy in
chlorobiphenyl poisoning. Neurology, 21: 1173-1176.
MURPHY, T.J. (1984) Atmospheric inputs of chlorinated hydrocarbons
to the Great Lakes. In: Nriagu, J.O., Nriagu, J.O., & Simmons,
M.S., ed. Toxic contaminants in the Great Lakes, New York, John
Wiley and Sons, pp. 53-79.
MURPHY, T.J., FORMANSKI, L.J., BROWNAWELL, B., & MEYER, J.A. (1985)
Polychlorinated biphenyl emissions to the atmosphere in the Great
Lakes region. Municipal landfills and incinerators. Environ. Sci.
Technol., 19(10): 942-946.
MUSSALO-RAUHAMAA, H., PYYSALO, H., & MOILANEN, R. (1984) Influence
of diet and other factors on the levels of organochlorine compounds
in human adipose tissue in Finland. J. Toxicol. environ. Health,
13: 689-704.
MUSSALO-RAUHAMAA, H., PYYSALO, H., & ANTERVO, K. (1988) Relation
between the content of organochlorine compounds in Finnish human
milk and characteristics of the mothers. J. Toxicol. environ.
Health, 25: 1-19.
NAGAI, J., FURUKAWA, M., YAE, Y., & IKEDA, Y. (1969)
[Clinicochemical investigation of chlorobiphenyls poisoning.
Especially on the serum lipid analysis of the patients.] Fukuoka
Acta med., 60: 475-488 (in Japanese).
NAGASAKI, H., TOMII, S., MEGA, T., SUGIHARA, S., MIYATA, Y., & ITO,
N. (1974) [Analysis of various factors on liver carcinogenesis in
mice induced by benzene hexachloride (BHC) and technical
polychlorinated biphenyls (PCBs).] J. Nara Med. Assoc., 25: 635-648
(in Japanese).
NAGASAKI, H., KAWABATA, H., MIYATA, Y., INOUE, K., AOE, H., & ITO,
N. (1975) Effects of various factors on induction of liver tumours
in animals by the alpha-isomer of benzenehexachloride. Gann,
66: 185-191.
NAGAYAMA, J. (1975) Chlorinated dibenzofurans in Kanechlors and
rice oils used by patients with Yusho. Fukuoka Acta med., 66: 593.
NAGAYAMA, L., KURATSUNE, M., & MASUDA, Y. (1976) Determination of
chlorinated dibenzofurans in Kanechlors and "Yusho oil". Bull.
environ. Contam. Toxicol., 15(1): 9-13.
NAGAYAMA, J., KURATSUNE, M., & MASUDA, Y. (1981) Formation of
polychlorinated dibenzofurans by heating polychlorinated biphenyls.
Fukuoka Acta med., 72: 136-141.
NAKANISHI, Y., SHIGEMATSU, N., KURITA, Y., MATSUBA, K., KANEGAE,
H., ISHIMURA, S., & KAWAZOE, Y. (1985) Respiratory involvement and
immune status in Yusho patients. Environ. health Perspect.,
59: 31-36.
NARBONNE, J.F. (1979) Determination of the "no effect level" of
Phenoclor DP6 on five microsomal parameters in rat livers.
Toxicology, 14: 91-93.
NARBONNE, J.F. (1980) Time course of induction of microsomal
enzymes following dietary administration of a polychlorinated
biphenyl (Phenoclor DP6). Toxicol. appl. Pharmacol., 56: 1-7.
NARBONNE, J.F., BOURDICHON, M., GALLIS, J.L., & DAUBEZE, M. (1978)
Polychlorinated biphenyls: effect of diet level on ATPase activity
in rats. Bull. environ. Contam. Toxicol., 20: 184-190.
NAS (1979) Polychlorinated biphenyls, Washington, DC, National
Academy of Sciences, 182 pp.
NAU-RITTER, G.M., WURSTER, C.F., & ROWLAND, R.G. (1982)
Partitioning of [14C]PCB between water and particulates with
various organic contents. Water Res., 16: 1615-1618.
NCI (1975) Third national cancer survey, Bethesda, Maryland,
National Cancer Institute.
NCI (1978) Bioassay of Aroclor (trademark) 1254 for possible
carcinogenicity (CAS
The number of different PCTs theoretically possible is orders of
magnitude higher than that for PCBs, but, in practice, as for PCBs,
PCTs are not sold on a composition specification, but on their
physical properties, which depend on the degree of chlorination.
Common Polychlorinated terphenyls - PCTs
name:
Major trade The trade names are generally similar to those
names: given for PCBs. In the Aroclor series, terphenyls are
indicated by 54 in the first two places of the four
digit code. In Japan, the PCTs are coded Kanechlor
KC-C.
1.2 Physical and chemical properties
The physical and chemical properties of PCTs are very close to those
of PCBs, and depend on the degree of chlorination.
1.3 Analytical methods
Extraction and clean-up procedures are similar to those used for PCBs
(sections 2.3.1.1 and 2.3.1.2).
The gas-liquid chromatographic details are different from those of
PCBs, because of the lower volatility of the PCTs. Zitko et al. (1972)
used 3% OV 210 as the stationary phase with a column temperature of
200°C. Thomas & Reynolds (1973) also used OV 210 with a column
temperature of 250°C and another system with 3% Dexsil as a stationary
phase at 300°C with a 63Ni electron capture detector; this was also
used by Addison et al. (1972). Sosa-Lucero et al. (1973) used OV 210
and SE 30 at 255°C and Freudenthal & Greve (1973) used OV 17 with a
temperature programmed from 200°C to 285°C. Thomas & Reynolds (1973)
confirmed the identity by chlorination to tetradecachloroterphenyl
with antimony pentachloride.
2. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
No specific information available. See PCBs.
3. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
Atmospheric input into the Great Lakes has been studied, because the
lakes, as a whole, represent the largest surface area of any
freshwater body in the world. Wingender & Williams (1984) found that
atmospheric transport was a major pathway for the deposition of
polychlorinated terphenyls into the Great Lakes (see section 4.1.1.1).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 Residues in the environment
Relatively few studies have been carried out to determine
polychlorinated terphenyls in biota. Freudenthal & Greve (1973) found
levels of 0.12 mg/kg (wet weight) in oysters and 0.4 mg/kg (fat basis)
in eels from the Netherlands. Levels of PCTs were generally lower than
PCBs in the same samples. Renberg et al. (1981) analysed biota from
the Baltic Sea and found levels of 2.8-17.2 mg/kg (wet weight) in
white-tailed eagles, 0.5-1 mg/kg in grey seals, and 0.08 mg/kg in
eels. In fish, Jan & Malnersic (1978) found PCT levels of
0.003-0.005 mg/kg in trout from the Soca River, Yugoslavia. Mean PCT
levels of 0.0025 mg/kg (Doguchi, 1977) and 0.01 mg/kg (Takai et al.,
1979) have been found in the freshwater and marine environment of
Japan.
Several bird species have been monitored for PCTs; levels of
0.03-2.2 mg/kg have been found in Japanese birds (Doguchi, 1977).
Zitko et al. (1972) found levels of 1.4 mg/kg fat (wet weight) and
0.1 mg/kg in eggs of herring gulls from the Bay of Fundy, Canada.
Hassell & Holmes (1977) analysed the livers of various birds of prey
in the United Kingdom; residues ranged from <0.05 to 1.2 mg/kg. PCT
levels of between 0.61 and 10.51 mg/kg were found in the fat of gulls
from Italy, (Vannucchi et al., 1978) and black-headed gulls from the
Baltic contained mean residues of 1.8 mg/kg in adipose tissue
(Falandysz, 1980).
PCTs were also measured in the monitoring programme carried out all
over Japan in the period 1974-81. In 1974, 1976, and 1978 no PCTs were
found in 60, 156, and 75 samples of water (limit of determination
0.0001 mg/litre). No PCTs were found in sediment samples in 1974, but,
in 1976 and 1978, 21/151 and 37/75 samples were positive, with PCT
levels of 0.001-0.2 and 0.001-1.0 mg/kg, respectively. In fish in
1974, 1976, and 1978, PCTs were found in 3/11, 0/39, and 3/66 samples,
in concentrations of 0.0002-0.2 mg/kg (Environment Agency Japan,
1983).
4.2 Residues in food
Ushio & Doguchi (1977) analysed cereal products, vegetable products
including vegetable oils, seasonings, and seaweed, marine animal
products, and terrestrial animal products including milk and eggs, for
the presence of PCTs. Only the vegetable products contained average
concentration of 0.05 µg/kg. Other authors referred to by Ushio &
Doguchi failed to detect PCTs in edible oil, vegetables, meat, or
fish.
No PCTs could be detected in a Canadian survey on eggs, domestic and
imported cheese (Villeneuve et al., 1973b).
In Japan, the PCT contents of a number of foods were determined. The
PCT contents of fish were lower than the PCB contents (Fukano et al.,
1974). Villeneuve et al. (1973a) analysed packaged food in Canada and
found that 94.5% of the samples contained less than 0.01 mg PCTs/kg
and 5.5% contained 0.01-0.05 mg PCTs/kg.
4.3 Concentrations in adipose tissue
In Japan, Doguchi et al. (1974) found an average PCT level of
0.6 mg/kg in human fat, with a range of 0.1-2.1 mg/kg. In the same
country, Takizawa & Minagawa (1974) found PCT levels of 0.02 mg/kg in
the human liver (n = 6), 0.01 mg/kg in the kidney (n = 2), 0.02 mg/kg
in the brain (n = 3), and 0.04 mg/kg in the pancreas (n = 1). Thirty
samples of adipose tissue (from 18 males and 12 females), obtained in
Tokyo in 1974, were analysed for PCTs. The average level of PCTs was
1.11 mg/kg (range 0.04-9.20 mg/kg), on a fat basis (Fukano & Doguchi,
1977). In the Netherlands, PCTs were found in human fat at levels of
0-1 mg/kg (Freudenthal & Greve, 1973).
4.4 Concentrations in blood
An average PCT level of 5.0 µg/litre was recorded in the blood of
non-occupationally exposed volunteers in Japan (Doguchi & Fukano,
1975). Human blood samples were collected from 10 subjects in Tokyo in
1975 out of 27 subjects from whom blood had been obtained in 1973. The
average concentration of PCTs in whole blood was 6.45 µg/litre
(0.7-19.6 µg/litre) in 1973, and 5.32 µg/litre (1.1-9.4 µg/litre) in
1975 (Fukano & Doguchi, 1977).
5. KINETICS AND METABOLISM
5.1 Absorption
PCTs have been shown to be absorbed from the intestinal tract
(Sosa-Lucero et al., 1973), but very little information is available
on the rate of absorption.
5.2 Distribution
Diets containing Aroclor 5460 at levels of 10, 100, or 1000 mg/kg were
administered to rats for 7 days. The greatest concentration (611 mg/kg
at 1000 mg/kg diet) was in the liver, while the blood level was
5.85 mg/litre at 1000 mg/kg diet. PCT administration did not affect
body weight, but a significant increase in liver weight occurred in
the rats fed 1000 mg/kg diet (Sosa-Lucero et al., 1973). Table 54
shows the tissue distribution obtained in this study in rats fed with
Aroclor 5460 and in another study using Aroclor 1254 (Curley et al.,
1971).
Addison et al. (1972) dosed cod Gadus morhua by gavage with the
polychlorinated terphenyl (PCT) Aroclor 5460 (in herring oil) at
0.5 g/ml. After one week of starvation, the PCTs were present in all
the tissues analysed. Uptake efficiency appeared to be low with a
total of 1-10 mg of Aroclor 5460 being distributed through all tissues
out of 1 g administered. Liver was found to be the organ richest in
PCTs, and probably contained most of the absorbed material. In a
separate group of fish, analysed 70 days later (fish were fed during
this period), PCT residues were not significantly lower.
Table 54. Tissue distribution (mg/kg wet weight) of PCTs
(Aroclor 5460) in rats fed dietary levels of 100 mg/kg
for 7 days and fed PCBs (Aroclor 1254) at 100 mg/kg
for 9 daysa
Tissue Aroclor 5460 Aroclor 1254
Blood 1.32 0.1
Liver 47 6
Brain 5.1 4
Kidney 15.1 5
Heart 21.5 -
Fat - 180
a From: Curley et al. (1971); Sosa-Lucero et al. (1973).
5.3 Biotransformation
There is little information on the biotransformation of PCTs. Addison
et al. (1972), using gas-liquid chromatography, noted a loss of PCTs
with a shorter retention time in the excreta of a cod dosed orally
with Aroclor 5460; the same loss was observed in rat faeces after the
administration of a diet containing Aroclor 5460 (Sosa-Lucero et al.,
1973).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Marine and estuarine organisms
PCTs, Aroclor 5460, did not show any toxic effects at either 1 or
100 mg/litre on Dunaliella, Olisthodiscus, or Thalassiorsira, the
only 3 species tested with this mixture (Craigie & Hutzinger, 1975).
6.2 Terrestrial invertebrates
Lichtenstein et al. (1969) exposed Drosophila melanogaster to the
dry residues of various PCTs. No mortality was observed after a 48-h
exposure to 2999 µg of Aroclor 4465, 5442, or 5460.
6.3 Birds
A single study on the toxicity of Aroclor 5442, produced a 5-day LC50
of 4477 mg/kg (1301-15402 mg/kg) in Japanese quail (aged 14 days)
(Coturnix coturnix) (Hill & Camardese, 1986).
Cecil et al. (1974) found that Aroclor 5442 at a dose level of
20 mg/kg diet did not change the hatchability of chicken eggs.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single oral exposure
Early data, reported in abstract, indicated that the approximate oral
LD50 values of the PCT-mixtures Aroclor 5442 and 5460, in corn oil,
in rats were 10.6 and 19.2 g/kg body weight, respectively. For 3:1
mixtures of PCBs and PCTs, Aroclor 4465 and 2562, in corn oil, the
LD50 values in rats were 16 and 6.3 g/kg body weight, respectively
(US FDA, 1970).
7.2 Short-term oral exposure
7.2.1 Rat
Modifications in the liver were studied in groups of male
Sprague-Dawley rats fed a diet containing Aroclor 5460 at a level of 0
or 10 000 mg/kg diet (equivalent to 0 or 400 mg/kg body weight). Body
weights were slightly decreased after 3 weeks of exposure. The
enlarged livers showed proliferation of the endoplasmic reticulum and
formation of large concentric membrane arrays. Evidence of fatty
degeneration was observed by Toftgard et al. (1980). Biochemical
changes included an increase in microsomal protein and phospholipid,
and a decrease in RNA and cholesterol. The specific esterase
activities, N-demethylase and nitroreductase, were increased and
those of glucose-6-phosphatase and aryl hydrocarbon hydroxylase
decreased (Norback & Allen, 1972).
Sosa-Lucero et al. (1973) did not observe any signs of toxicity in
groups of male Wistar rats exposed to a diet containing Aroclor 5460
at levels of up to 1000 mg/kg diet (equivalent to 50 mg/kg body
weight) for 7 days. At 1000 mg/kg diet, relative liver weights were
increased as well as microsomal protein, cytochrome P-450, and the
specific activities of aniline hydroxylase and aminopyrine-
N-demethylase. Mixed type induction of hepatic microsomal enzymes in
rats exposed to PCTs has been observed by several investigators
(Ahotupa & Aitio, 1980; Toftgard et al., 1980; Nilsen & Toftgard,
1981).
Kiriyama et al. (1974) fed groups of male Wistar rats a control diet
or diets with ortho-, meta-, or para-PCTs at a level of 2000 mg/kg
diet (equivalent to 100 mg/kg body weight) for 2 weeks. Ortho- and
meta-PCTs reduced growth and increased relative kidney weights,
while only meta-PCTs decreased food intake and increased the
relative liver weights. All mixtures increased plasma, but not liver,
cholesterol levels. There was evidence of adrenal hypertrophy.
7.2.2 Monkey
A dietary level of Aroclor 5460 of 5000 mg/kg (equivalent to 200 mg/kg
body weight) over 3 months caused growth retardation and increased
relative liver weights in 6 Rhesus monkeys compared with 3 controls.
After 6 weeks of exposure, the toxic signs observed were similar to
those found within 1 month in a group of monkeys exposed to 300 mg of
Aroclor 1248/kg diet (equivalent to 12 mg/kg body weight), i.e.,
alopecia, facial oedema, swollen eyelids and lips, and purulent eye
discharge. After exposure of both groups for 3 months, proliferation
of the smooth endoplasmic reticulum was observed as well as
hypertrophy and hyperplasia of the gastric mucosa (Allen & Norback,
1973).
7.3 Teratogenicity
Groups of 15 or 16 pregnant ddY mice were fed diets containing 0, 100,
500, or 2500 mg PCTs/kg (not specified) during gestation. The animals
were sacrificed on day 18 and examined for embryonic effects. The
fetuses of dams receiving the 500 and 2500 mg/kg diet showed a higher
incidence of cleft palate in comparison with the controls. Pregnant
ddY mice were administered 0, 50, or 100 mg PCTs/kg with
corticosterone administered subcutaneously on days 11, 12 and 13. A
significant increase was seen in corticosterone levels in the plasma
in the PCT-treated animals on day 14. Furthermore, when pregnant ddY
mice were adrenalectomized on day 10, it did not suppress the
development of cleft palate, but metapyrone, an inhibitor of
corticosterone synthesis, significantly reduced the incidence of cleft
palate in the fetuses. The results suggest that cleft palate induced
by PCTs is not due to a direct effect, but that an increase in the
corticosterone level in the maternal plasma is involved in the
mechanism of its development (Kaneko, 1988).
Pregnant Wistar rats were fed PCTs at levels of 0, 500, or 2500 mg/kg
diet during gestation and the animals were sacrificed on day 20.
Systemic oedema was observed in the fetuses of the animals fed 500 and
2500 mg PCTs/kg diet, but no cleft palate was found (Kaneko, 1988).
7.4 Carcinogenicity
Groups of 35 male ICR mice received a diet containing Kanechlor C
(a mixture of 95% PCTs and 5% PCBs) at levels of 0, 250, or 500 mg/kg
(equivalent to 0, 36, and 70 mg/kg body weight), for 24 weeks. The
mice were sacrificed following 16 exposure-free weeks. Survivors
numbering 28, 28, and 21 mice at 0, 250, and 500 mg/kg diet,
respectively, were autopsied. A dose-related reduction in body weight
gain and a dose-related increase in absolute liver weights were
observed. Neoplastic nodules (nodular hyperplasia) were found in the
livers of 3/28 mice at 250 mg/kg diet and 6/21 mice at 500 mg/kg diet.
Hepatocellular carcinomas were observed in 3/21 mice at 500 mg/kg
diet. No neoplastic nodules were noted in the controls. The increases
at the higher dose level were statistically significant (Shirai et
al., 1978).
7.5 Miscellaneous effects
Evidence for the estrogenic activity of Aroclor 5442 was found using
the glycogen response of the immature rat uterus. Aroclor 5460 was
inactive in this test (Bitman & Cecil, 1970; Bitman et al., 1972).
The mixed type inducer, Aroclor 5460, increased the metabolism of
4-androstene-3,17-dione in male Sprague-Dawley rats intraperitoneally
injected with 4 doses of 300 mg/kg body weight in 4 days (Nilsen &
Toftgard, 1981). Reproductive effects have not been investigated.
Groups of pregnant ddY mice received a control diet or diets
containing PCTs (not specified) at levels of 50 or 500 mg/kg diet
(equivalent to 7 or 70 mg/kg body weight). Increased incidence of
cleft palate and other malformations was reported in the fetuses. In
neonates, reduced growth and survival as well as hyperactivity were
observed (Kimura & Miyake, 1976). (No details available).
REFERENCES
ABBOTT, D.C., COLLIN, G.B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom, 1969-71. Br.
med. J., 2: 553-556.
ABE, S., INOU, Y., & TAKAMATSU, M. (1975) [Polychlorinated biphenyl
residues in plasma of Yusho children born to mothers who had
consumed oil contaminated by PCBs.] Fukuoka Acta med., 66: 605-609
(in Japanese).
ABRAHAMSON, L.J. & ALLEN, J.R. (1973) The biological response of
infant nonhuman primates to a polychlorinated biphenyl. Environ.
health Perspect., 4: 81-86.
ACHILLES, A. (1983) Fire hazard of polychlorinated biphenyls. In:
Barros, M.C., Könemann H., & Visser, R., ed. Proceedings of the PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 187-191.
ACKER, L. & SCHULTE, E. (1970) [The occurrence of chlorinated
biphenyls and hexachlorobenzene alongside chlorinated insecticides
in breastmilk and human fatty tissue.] Naturwissenschaften, 57: 497
(in German).
ACKER, L. & SCHULTE, E. (1974) [Chlorinated hydrocarbons in human
fatty tissue.] Naturwissenschaften, 61: 1-4 (in German).
ACKER, L., BARKE, E., HAPKE, H.-J., HEESCHEN, W., KORANSKY, W.,
KUBLER, W., & REINHARDT, D. (1984) [Residues and impurities in
breast milk. German Association for the Encouragement of Research:
Communication XII of the Committee on Testing of Residues in
Foods], Weinheim, Verlag Chemie, pp. 1-97.
ACQUAVELLA, J.F., HANIS, N.M., NICOLIC, M.J., & PHILLIPS, S.C.
(1986) Assessment of clinical, metabolic, dietary, and occupational
correlations with serum polychlorinated biphenyl levels among
employees at an electrical capacitor manufacturing plant. J. occup.
Med., 28(11): 1177-1180.
ADAMS, J.A. (1983) Effect of PCB (Aroclor 1254) on early
development and mortality in Arbacia eggs. Water air soil
Pollut., 20: 1-5.
ADDISON, R.F. & BRODIE, P.F. (1977) Organochlorine residues in
maternal blubber, milk, and pup blubber from grey seals
(Halichoerus grypus) from Sable Island, Nova Scotia. J. Fish Res.
Board Can., 34: 937-941.
ADDISON, R.F. & SMITH, T.G. (1974) Organochlorine residue levels in
Arctic ringed seals: variation with age and sex. Oikos,
25: 335-337.
ADDISON, R.F., FLETCHER, G.L., RAY, S., & DOANE, J. (1972) Analysis
of a chlorinated terphenyl (Aroclor 5460) and its deposition in
tissues of cod (Gadus morhua). Bull. environ. Contam. Toxicol.,
8: 52-60.
ADDISON, R.F., KERR, S.R., DALE, J., & SERGEANT, D.E. (1973)
Variation of organochlorine residue levels with age in Gulf of St.
Lawrence harp seals (Pagophilus groenlandicus). J. Fish. Res.
Board Can., 30: 595-600.
ADDISON, R.F., ZINCK, M.E., & SMITH, T.G. (1986) PCBs have declined
more than DDT-group residues in Arctic ringed seals (Phoca
hispida) between 1972 and 1981. Environ. Sci. Technol.,
20: 253-256.
AGRAWAL, A.K., TILSON, H.A., & BONDY, S.C. (1981)
3,4,3',4'-tetrachlorobiphenyl given to mice prenatally produces
long-term decreases in striatal dopamine and receptor binding sites
in the caudate nucleus. Toxicol. Lett., 7: 417-424.
AGUILAR, A. & BORRELL, A. (1988) Age- and sex-related changes in
organochlorine compound levels in fin whales (Balaenoptera
physalus) from the Eastern North Atlantic. Mar. environ. Res.,
25: 195-211.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of polychlorinated biphenyl (PCB) and chlorinated
pesticides in water. Anal. Chem., 42: 1483-1486.
AHMED, M. & FOCHT, D.D. (1973a) Degradation of polychlorinated
biphenyls by two species of Achromobacter. Can. J. Microbiol.,
19: 47-52.
AHMED, M. & FOCHT, D.D. (1973b) Oxidation of polychlorinated
biphenyls by Achromobacter PCB. Bull. environ. Contam. Toxicol.,
10: 70-72.
AHNOFF, M. & JOSEFSSON, B. (1973) Confirmation studies on
polychlorinated biphenyls (PCBs) from river waters using mass
fragmentography. Anal. Lett., 6: 1083-1093.
AHNOFF, M. & JOSEFSSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AHNOFF, M. & JOSEFSSON, B. (1975) Clean-up procedures for PCB
analysis on river water extracts. Bull. environ. Contam. Toxicol.,
13: 159-166.
AHOTUPA, M. & AITIO, A. (1980) Effect of chlorinated naphthalenes
and terphenyl on the activities of drug metabolizing enzymes in rat
liver. Biochem. biophys. Res. Commun., 93: 250-257.
AKAGI, K. & OKUMURA, M. (1985) Association of blood pressure and
PCB level in Yusho patients. Environ. health Perspect., 59: 37-39.
AKIYAMA, K., OHI, G., FUJITANI, K., & YAGYU, H. (1975)
Polychlorinated biphenyl residues in maternal and cord blood in
Tokyo metropolitan area. Bull. environ. Contam. Toxicol.,
14: 588-592.
ALBAIGES, J., FARRAN, A., SOLER, M., GALLIFA, A., & MARTIN, P.
(1987) Accumulation and distribution of biogenic and pollutant
hydrocarbons, PCBs and DDT in tissues of Western Mediterranean
fishes. Mar. environ. Res., 22: 1-18.
ALBRO, P.W. & FISHBEIN, L. (1972) Intestinal absorption of
polychlorinated biphenyls in rats. Bull. environ. Contam. Toxicol.,
8: 26-31.
ALBRO, P.W. & PARKER, C.E. (1979) Comparison of the compositions of
Aroclor 1242 and Aroclor 1016. J. Chromatogr., 169: 161-166.
ALBRO, P.W., CORBETT, J.T., & SCHROEDER, J.L. (1981) Quantitative
characterization of polychlorinated biphenyl mixtures (Aroclors
1248, 1254 and 1260) by gas chromatography using capillary columns.
J. Chromatogr., 205: 103-111.
ALENCASTRO, L.F. DE, PRELAZ, V., & TARRADELLAS, J. (1984)
Contamination of silos in Switzerland by PCB residues in coatings.
Bull. environ. Contam. Toxicol., 33(3): 270-276.
ALFORD-STEVENS, A.L. (1986) Analyzing PCBs. Environ. Sci. Technol.,
20(12): 1194-1199.
ALLEN, J.R. (1975) Response of the non-human primate to
polychlorinated biphenyl exposure. Fed. Proc., 34: 1675-1679.
ALLEN, J.R. & ABRAHAMSON, L.J. (1973) Morphological and biochemical
changes in the liver of rats fed polychlorinated biphenyls. Arch.
environ. Contam. Toxicol., 1: 265-280.
ALLEN, J.R. & BARSOTTI, D.A. (1976) The effects of transplacental
and mammary movement of PCBs on infant rhesus monkeys. Toxicology,
6: 331-340.
ALLEN, J.R. & NORBACK, D.H. (1973) Polychlorinated biphenyl- and
triphenyl-induced gastric mucosal hyperplasia in primates. Science,
179: 498-499.
ALLEN, J.R. & NORBACK, D.H. (1976) Pathobiological responses of
primates to polychlorinated biphenyl exposure. In: Proceedings of
the National Conference on Polychlorinated Biphenyls, Chicago,
19-21 November 1975, Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances, pp. 43-49 (EPA-560/6-75-004).
ALLEN, J.R., CARSTENS, L.A., & BARSOTTI, D.A. (1974) Residual
effects of short-term low level exposures of nonhuman primates to
polychlorinated biphenyls. Toxicol. appl. Pharmacol., 30: 440-451.
ALLEN, J.R., CARSTENS, L.A., ABRAHAMSON, L.J., & MARLAR, R.J.
(1975) Responses of rats and non-human primates to
2,5,2',5',-tetrachlorobiphenyl. Environ. Res., 9: 265-273.
ALLEN, J.R., CARSTENS, L.A., & ABRAHAMSON, L.J. (1976) Responses of
rats to polychlorinated biphenyls for fifty-two weeks. Arch.
environ. Contam., 4: 404-419.
ALLEN, J.R., BARSOTTI, D.A., LAMBRECHT, L.K., & VAN MILLER, J.P.
(1979) Reproduction effects of halogenated aromatic hydrocarbons on
nonhuman primates. Ann. NY Acad. Sci., 320: 419-425.
ALLEN, J.R., BARSOTTI, D.A., & CARSTENS, L.A. (1980) Residual
effects of polychlorinated biphenyls on adult nonhuman primates and
their offspring. J. Toxicol. environ. Health, 6: 55-66.
ALTMAN, N.H., NEW, A.E., MCCONNELL, E.E., & FERRELL, T.L. (1979) A
spontaneous outbreak of polychlorinated biphenyl (PCB) toxicity in
Rhesus monkeys (Macaca mulatta): Clinical observations. Lab.
anim. Sci., 29: 661-665.
ALVARES, A.P. (1977) Stimulatory effects of polychlorinated
biphenyls (PCB) on cytochromes P-450 and P-448 mediated microsomal
oxidations. In: Microsomes and drug oxidations, Oxford, New York,
Pergamon Press, pp. 476-483.
ALVARES, A.P. & KAPPAS, A. (1975) Induction of aryl hydrocarbon
hydroxylase by polychlorinated biphenyls in the foeto-placental
unit and neonatal livers during lactation. FEBS Lett., 50: 172-174.
ALVARES, A.P. & KAPPAS, A. (1977) Heterogenicity of cytochrome
P-450s induced by polychlorinated biphenyls. J. biol. Chem.,
252: 6373-6378.
ALVARES, A.P., FISCHBEIN, A., ANDERSON, K.E., & KAPPAS, A. (1977)
Alterations in drug metabolism in workers exposed to
polychlorinated biphenyls. Clin. Pharmacol. Ther., 22(2): 140-145.
ALVARES, A.P., EISEMAN, J.L., UENG, T.-H., & KAPPAS, A. (1982)
Polychlorinated biphenyls: species and tissue specifications of the
induction of monooxygenases in rats, mice, rabbits, and humans. In:
Proceedings of the Workshop on the Combined Effects of Xenobiotics,
Ottawa, 22-23 June 1981, Ottawa, National Research Council of
Canada, pp. 127-149 (Publication No. NRCC 18978).
ANDERSON, M.R. & PANKOW, J.F. (1986) A case study of a chemical
spill: Polychlorinated biphenyls (PCBs). 3. PCB sorption and
retardation in soil underlying the site. Water Resour. Res.,
22(7): 1051-1057.
ANDERSON, L.M., VAN HAVERE, K., & BUDINGER, J.M. (1983) Effects of
polychlorinated biphenyls on lung and liver tumours initiated in
suckling mice by N-nitrosodimethylamine. J. Natl Cancer Inst.,
71: 157-163.
ANDO, M. (1978) Transfer of 2,4,5,2',4',5'-hexachlorobiphenyl and
2,2-bis(p-chlorophenyl), 1,1,1 -trichloroethane ( p-p'DDT) from
maternal to newborn and suckling rats. Arch. Toxicol., 41: 179-186.
ANDO, M., SAITO, H., & WAKISAKA, I. (1984) [Transfer of PCBs from
mother to newborn baby through placenta and milk.] Res. Rep. Natl
Inst. Environ. Stud. Jpn, 67: 333-345 (in Japanese).
ANDO, M., SAITO, H., & WAKISAKA, I. (1985) Transfer of
polychlorinated biphenyls to newborn infants through the placenta
and mother's milk. Arch. environ. Contam. Toxicol., 14(1): 51-57.
ANDREN, A.W. (1982) Processes determining the flux of PCBs across
air/water interfaces. In: Mackay, D., ed. Physical behavior of PCBs
in the Great Lakes; Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., pp. 127-140.
ANDRES, J., LAMBERT, I., ROBERTSON, L., BANDIERA, S., SAWYER, T.,
LOVERING, S., & SAFE, S. (1983) The comparative biologic and toxic
potencies of polychlorinated biphenyls and polybrominated
biphenyls. Toxicol. appl. Pharmacol., 70: 204-215.
ANDREWS, J.E. (1989) Polychlorinated biphenyl (Aroclor 1254)
induced changes in femur morphometry calcium metabolism and
nephrotoxicity. Toxicology, 57: 83-96.
ANON. (1983a) PCB found in Czechoslovakian canned hams during each
of last four years. Food Chem. News, 18 April: 32.
ANON. (1983b) USDA blocks all Czechoslovakian meat imports because
of PCB residues. Food Chem. News, 2 May: 30-31.
ANON. (1985a) Summary of results of the first test to determine
residual contaminants in the air and on surface in floors 2 through
18 of the Binghamton State Office Building. Test report,
Springfield, Virginia, Versar Inc.
ANON. (1985b) Polychlorinated biphenyl transformer incident New
Mexico. Morb. Mortal. wkly Rep., 34(36): 557-559.
ANON. (1987) Polychlorinated biphenyl in fluorescent lighting.
Environ. Health Saf. News, 33(1): 3-18.
ARMOUR, J.A. & BURKE, J.A. (1970) Method for separating
polychlorinated biphenyls from DDT and its analogs. J. Assoc. Off.
Anal. Chem., 53: 761-768.
ARMBRUSTER, G., GEROW, K.G., GUTENMANN, W.H., LITTMAN, C.B., &
LISK, D.J. (1987) The effects of several methods of fish
preparation on residues of polychlorinated biphenyls and sensory
characteristics in striped bass. J. Food Saf., 8: 235-243.
ARNOLD, D.L., MES, J., ZAWIDZKA, Z.Z., & KARPINSKI, K. (1984)
Toxicity of PCB (Aroclor 1254) as a consequence of continuous
exposure during pregnancy and nursing (PA-24). Presented at the
27th Annual Meeting of the Canadian Federation of Biological
Societies, University of Saskatchewan, Canada, 18-22 June 1984.
ATKINSON, S.A. (1979) Chemical contamination of human milk: A
review of current knowledge. J. Can. Diet. Assoc., 40(3): 223-226.
ATLAS, E. & GIAM, C.S. (1981) Global transport of organic
pollutants: Ambient concentrations in the marine atmosphere.
Science, 211: 163-165.
ATSDR (1989) Toxicological profile for selected PCBs (Aroclor 1260,
1254, 1248, 1242, 1232, 1221 and 1016), Atlanta, Georgia, Agency
for Toxic Substances and Disease Registry (ATSDR/TP-88/21).
AULERICH, R.J. & RINGER, R.K. (1977) Current status of PCB toxicity
to mink and effect on their reproduction. Arch. environ. Contam.
Toxicol., 6: 279-292.
AULERICH, R.J., BURSIAN, S.J., BRESLIN, W.J., OLSON, B.A., &
RINGER, R.K. (1985) Toxicological manifestations of
2,4,5,2',4',5',-; 2,3,6,2',3',6',-; and 3,4,5,3',4',5',-
hexachlorobiphenyl and Aroclor 1254 in mink. J. Toxicol. environ.
Health., 15: 63-79.
AX, R.L. & HANSEN, L.G. (1975) Effects of purified polychlorinated
biphenyl analogs on chicken reproduction. Poult. Sci., 54: 895-900.
BACCI, E. & GAGGI, C. (1985) Polychlorinated biphenyls in plant
foliage: Translocation or volatilization from contaminated soils?
Bull. environ. Contam. Toxicol., 35: 673-681.
BACHE, C.A., SERUM, J.W., YOUNGS, W.D., & LISK, D.J. (1972)
Polychlorinated biphenyl residues: Accumulation in Cayuga lake
trout with age. Science, 177: 1191-1192.
BADSHA, K. & EDULJEE, G. (1986) PCB in the UK environment - A
preliminary survey. Chemosphere, 15(2): 211-215.
BADSHA, K., EDULJEE, G., & SCUDAMORE, N. (1986) Environmental
monitoring for PCB and trace metals in the vicinity of a chemical
waste disposal facility - III. Chemosphere, 15(7): 947-957.
BAGLEY, G.E., REICHEL, W.L., & CROMARTIE, E. (1970) Identification
of polychlorinated biphenyls in two bald eagles by combined
gas-liquid chromatography-mass spectroscopy. J. Assoc. Off. Anal.
Chem., 53: 251-261.
BAHN, A.K., ROSENWAIKE, I., HERRMAN, N., GROVER, P., STELLMAN, J.,
& O'LEARY, K. (1976) Melanomas after exposure to PCBs. New Engl. J.
Med., 295: 450.
BAILEY, S. & BUNYAN, P.J. (1972) Interpretation of persistence and
effects of polychlorinated biphenyls in birds. Nature (Lond.),
236: 34-36.
BAILEY, J., KNAUF, V., MUELLER, W., & HOBSON, W. (1980) Transfer of
hexachlorobenzene and polychlorinated biphenyls to nursing infant
Rhesus monkeys: enhanced toxicity. Environ. Res., 21: 190-196.
BAKER, F.D., BUSH, B., TUMASONIS, C.F., & LO, F.-C. (1977) Toxicity
and persistence of low-level PCB in adult Wistar rats, fetuses, and
young. Arch. environ. Contam. Toxicol., 5: 143-156.
BAKER, E.L., Jr., LANDRIGUN, P.J., GLUECK, C.J., ZACK, M.M., Jr.,
LIDDLE, J.A., BURSE, V.W., HOUSEWORTH, W.J., & NEEDHAM, L.L. (1980)
Metabolic consequences of exposure to polychlorinated biphenyls
(PCBs) in sewage sludge. Am. J. Epidemiol., 112(4): 553-563.
BALLSCHMITER, K. & ZELL, M. (1980) Analysis of polychlorinated
biphenyls (PCB) by glass capillary gas chromatography. Composition
of technical Aroclor- and Clophen-PCB mixtures. Fresenius' Z. anal.
Chem., 302: 20-31.
BALLSCHMITER, K., BUCHERT, H., BIHLER, S., SCHOTT, P., RÖPER, H.P.,
& PACHUR, H.J. (1981) Organochlorine pollutant analysis of
contaminated and uncontaminated lake sediments by high resolution
gas chromatography. Chemosphere, 10: 945-956.
BANDIERA, S., SAFE, S., & OKEY, A.B. (1982) Binding of
polychlorinated biphenyls classified as either phenobarbitone-,
3-methylcholanthrene-, or mixed-type inducers to cytosolic Ah
receptor. Chem. biol. Interact., 39: 259-277.
BANDIERA, S., FARRELL, K., MASON, G., KELLEY, M., ROMKES, M.,
BANNISTER, R., & SAFE, S. (1984) Comparative toxicities of the
polychlorinated dibenzofuran (PCDF) and biphenyl (PCB) mixtures
which persist in Yusho victims. Chemosphere, 13(4): 507-512.
BARSOTTI, D.A. & VAN MILLER, J.P. (1984) Accumulation of a
commercial polychlorinated biphenyl mixture (Aroclor 1016) in adult
Rhesus monkeys and their nursing infants. Toxicology, 30: 31-44.
BARSOTTI, D.A., MARLAR, R.J., & ALLEN, J.R. (1976) Reproductive
dysfunction in Rhesus monkeys exposed to low levels of
polychlorinated biphenyls (Aroclor 1248). Food Cosmet. Toxicol.,
14: 99-103.
BASTOMSKY, C.H. (1974) Effects of polychlorinated biphenyl mixture
(Aroclor 1254) and DDT on biliary excretion in rats. Endocrinology,
95: 1150-1155.
BASTOMSKY, C.H. (1977) Goitres in rats fed polychlorinated
biphenyls. Can. J. Physiol. Pharmacol., 55: 288-292.
BASTOMSKY, C.H. & MURTHY, P.V.N. (1976) Enhanced in vitro hepatic
glucuronidation of thyroxine in rats following cutaneous
application or ingestion of polychlorinated biphenyls. Can. J.
Physiol. Pharmacol., 54: 23-26.
BASTOMSKY, C.H., SOLYMOSS, B., ZSIGMOND, G., & WYSE, J.M. (1975) On
the mechanism of polychlorinated biphenyl-induced hypobili-
rubinaemia. Clin. chim. Acta, 61: 171-174.
BAUMANN, M., DEML, E., SCHAEFFER, E., & GREIM, H. (1983) Effects of
polychlorinated biphenyls at low dose levels in rats. Arch.
environ. Contam. Toxicol., 12: 509-515.
BAXTER, R.A., GILBERT, P.E., LIDGETT, R.A., MAINPRIZE, J.H., &
VODDEN, H.A. (1975) The degradation of polychlorinated biphenyls by
microorganisms. Sci. total Environ., 4: 53-61.
BECK, H. & MATHAR, W. (1985) [Analytical procedures for the
determination of selected components of PCBs in foodstuffs.]
Bundesggesundheitsblatt, 28(1): 1-12 (in German).
BECKER, G.M., MCNULTY, W.P., & BELL, M. (1979) Polychlorinated
biphenyl-induced morphologic changes in the gastric mucosa of the
Rhesus monkey. Lab. Invest., 40: 373-383.
BENGTSON, S.A. & SÖDERGREN, A. (1974) DDT and PCB residues in
airborne fallout and animals in Iceland. Ambio, 3: 84-86.
BENGTSSON, B.E. (1979) Increased growth in minnows exposed to PCBs.
Ambio, 8: 169-170.
BENGTSSON, B.E. (1980) Long-term effects of PCB (Clophen A50) on
growth, reproduction and swimming performance in the minnow,
Phoxinus phoxinus. Water Res., 14: 681-687.
BENTHE, H.F., KNOP, J., & SCHMOLDT, A. (1972) [Absorption and
distribution of polychlorinated biphenyls (PCB) after inhalatory
application.] Arch. Toxikol., 29: 85-95 (in German).
BENTLEY, J. (1983) Incineration of PCBs. In: Barros, M.C.,
Könemann, H., & Visser, R., ed. Proceedings of the PCB Seminar, The
Hague, 28-30 September 1983, The Hague, Ministry of the
Environment, pp. 281-288.
BERAN, M., BRANDT, I., & SLANINA, P. (1983) Distribution and effect
of some polychlorinated biphenyls in the hemopoietic tissues. J.
Toxicol. environ. Health., 12: 521-532.
BERCOVICI, B., WASSERMANN, M., CUCOS, S., RON, M., WASSERMAN, D.,
& PINES, A. (1983) Serum levels of polychlorinated biphenyls and
some organochlorine insecticides in women with recent and former
missed abortions. Environ. Res., 30(1): 169-174.
BERG, O.W., DIOSADY, P.L., & REES, G.A.V. (1972) Column
chromatographic separation of polychlorinated biphenyls from
chlorinated hydrocarbon pesticides and their subsequent gas
chromatographic quantitation in terms of derivatives. Bull.
environ. Contam. Toxicol., 7: 338-347.
BERGH, A.K. & PEOPLE, R.S. (1977) PCB distribution in sewage wastes
and their environmental and community effects. Proceedings of the
1977 National Conference on Treatment and Disposal of Industrial
Wastewaters and Residues, Houston, Texas, pp. 4-6.
BERGLUND, F. (1972) Levels of polychlorinated biphenyls in foods in
Sweden. Environ. health Perspect., 1: 67-69.
BERLIN, M., GAGE, J.C., & HOLM, S. (1973) The metabolism and
distribution of 2,4,5,2',5'-pentachlorobiphenyl in the mouse. In:
Proceedings of the Polychlorinated Biphenyls II Conference,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 101-108 (Publication No. 4E).
BERLIN, M., GAGE, J.C., & HOLM, S. (1975) Distribution and
metabolism of polychlorobiphenyls. In: Proceedings of an
International Symposium on Recent Advances in the Assessment of the
Health Effects of Environmental Pollution, Paris, 24-26 June 1974,
Luxembourg, Commission of the European Communities, Vol. 2,
pp. 895-902.
BERRY, D.L., SLAGA, T.J., DIGIOVANNI, J., & JUCHAU, M.R. (1979)
Studies with chlorinated dibenzo- p-dioxins, polybrominated
biphenyls, and polychlorinated biphenyls in a two-stage system of
mouse skin tumorigenesis: potent anticarcinogenic effects. Ann. NY
Acad. Sci., 320: 405-414.
BERRY, D.L., DIGIOVANNI, J., JUCHAU, M.R., BRACKEN, W.M., GLAESON,
G.L., & SLAGA, T.J. (1978) Lack of tumour promoting ability of
certain environmental chemicals in a two-stage mouse skin
tumorigenesis assay. Res. Commun. chem. Pathol. Pharmacol.,
20: 101-108.
BERTAZZI, P.A., ZOCHETTI, C., GUERCILENA, S., FOGLIA, M.D.,
PESATORI, A., & RIBOLDI, L. (1982) Mortality study of male and
female workers exposed to PCBs. In: Prevention of Occupational
Cancer: International Symposium, Geneva, International Labour
Office, pp. 242-248 (Occupational Safety and Health Series No. 46).
BERTAZZI, P.A., RIBOLDI, L., PESATORI, A., RADICE, L., & ZOCHETTI,
C. (1987) Cancer mortality of capacitor manufacturing workers. Am.
J. ind. Med., 11(2): 165-176.
BICKERS, D.R., HARBER, L.C., KAPPAS, A., & ALVARES, A.P. (1972)
Polychlorinated biphenyls: Comparative effects of high and low
chlorine containing Aroclors on hepatic mixed function oxidase.
Res. Commun. Chem. Pathol. Pharmacol., 3: 505-512.
BIDLEMAN, T.F. & OLNEY, C.E. (1974) Chlorinated hydrocarbons in the
Sargasso Sea atmosphere and surface water. Science, 183: 516-518.
BIDLEMAN, T.F., RICE, C.P., & OLNEY, C.E. (1978) High molecular
weight chlorinated hydrocarbons in the air and sea: Rates and
mechanisms of air/sea transfer. In: Windom, H. & Duce, R.A., ed.
Marine pollutant transfer, Lexington, D.C. Heath and Co.,
pp. 323-351.
BIESSMANN, A. (1982) Effects of PCBs on gonads, sex hormone balance
and reproduction processes of Japanese quail Coturnix coturnix
japonica after ingestion during sexual maturation. Environ.
Pollut., 27: 15-30.
BIGGS, D.C., ROWLAND, R.G., POWERS, C.D., O'CONNERS, H., & WURSTER,
C. (1978) A comparison of the effects of chlordane and PCB on the
growth, photosynthesis, and cell size of estuarine phytoplankton.
Environ. Pollut., 15: 253-263.
BIGGS, D.C., ROWLAND, R.G., & WURSTER, C.F. (1979) Effects of
trichloroethylene, hexachlorobenzene and polychlorinated biphenyls
on the growth and cell size of marine phytoplankton. Bull. environ.
Contam. Toxicol., 21: 196-201.
BIGGS, D.C., POWERS, C.D., ROWLAND, R.G., O'CONNORS, H.B., &
WURSTER, C.F. (1980) Uptake of polychlorinated biphenyls by natural
phytoplankton assemblages: field and laboratory determinations of
14C-PCB particle-water index of sorption. Environ. Pollut.,
22: 101-110.
BILLINGS, W.N., BIDLEMAN, T.F., & VERNBERG, W.B. (1978) Movement of
PCB from a contaminated reservoir into a drinking water supply.
Bull. environ. Contam. Toxicol., 19: 215-222.
BIOCCA, M., GUPTA, B.N., CHAE, K., MCKINNEY, J.D., & MOORE, J.A.
(1981) Toxicity of selected symmetrical hexachlorobiphenyl isomers
in the mouse. Toxicol. appl. Pharmacol., 58: 461-474.
BIRGE, W.J., BLACK, J.A., & WESTERMAN, A.G. (1978) Effects of
polychlorinated biphenyl compounds and proposed PCB-replacement
products on embryo-larval stages of fish and amphibians,
Washington, DC, US Department of the Interior, p. 33 (Research
Report No. 118).
BIRNBAUM, L.S. (1983) Distribution and excretion of 2,3,6,2',3',6'-
and 2,4,5,2',4',5'-hexachlorobiphenyl in senescent rats. Toxicol.
appl. Pharmacol., 70: 262-272.
BIRNBAUM, L. & BAIRD, M.B. (1978) Induction of hepatic mixed
function oxidases in senescent rodents-II. Effect of
polychlorinated biphenyls. Exp. Gerontol., 13: 469-477.
BIRNBAUM, L.S., WEBER, H., HARRIS, M.W., LAMB, J.C., IV, &
MCKINNEY, J.D. (1985) Toxic interaction of specific polychlorinated
biphenyls and 2,3,7,8-tetrachlorodibenzo- p-dioxin: increased
incidence of cleft palate in mice. Toxicol. appl. Pharmacol.,
77: 292-302.
BITMAN, J. & CECIL, H.C. (1970) Estrogenic activity of DDT analogs
and polychlorinated biphenyls. J. agric. food Chem., 18: 1108-1112.
BITMAN, J., CECIL, H.C., & HARRIS, S.J. (1972) Biological effects
of polychlorinated biphenyl in rats and quail. Environ. health
Perspect., 1: 145-149.
BJERK, J.E. (1972) [Residues of DDT and polychlorinated biphenyls
in Norwegian human material.] Tidsskr. Nor. Laegeforen., 92: 15-19
(in Norwegian).
BLEAVINS, M.R., AULERICH, R.J., & RINGER, R.K. (1980)
Polychlorinated biphenyls (Aroclors 1016 and 1242): Effects on
survival and reproduction in mink and ferrets. Arch. environ.
Contam. Toxicol., 9: 627-635.
BLEAVINS, M.R., AULERICH, R.J., RINGER, R.K., & BELL, T.G. (1982)
Excessive nail growth in the European ferret induced by Aroclor
1242. Arch. environ. Contam. Toxicol., 11: 305-312.
BLEAVINS, M.R., AULERICH, R.J., & RINGER, R.K. (1981) Placental and
mammary transfer of polychlorinated and polybrominated biphenyls in
the mink and ferret. In: Proceedings of the 2nd Conference on Avian
and Mammalian Wildlife Toxicology, Philadelphia, Pennsylvania,
American Society for Testing and Material, pp. 121-131
(ASTM STP 757).
BLEAVINS, M.R., BRESLIN, W.J., AULERICH, R.J., & RINGER, R.K.
(1984) Placental and mammary transfer of a polychlorinated biphenyl
mixture (Aroclor 1254) in the European ferret (Mustela putorius
furo). Environ. Toxicol. Chem., 3: 637-644.
BLETCHLY, J.D. (1983) Polychlorinated biphenyls. Production,
current use, and possible rates of future disposal in OECD
countries. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of PCB Seminar, The Hague, 28-30 September 1983, The
Hague, Ministry of the Environment, pp. 343-365.
BLETCHLY, J.D. (1985) Report to the Commission of the European
Communities on a study of measures to avoid dispersion into the
environment of polychlorinated biphenyls (PCBs) and polychlorinated
terphenyls (PCTs) from existing installations, Luxembourg,
Commission of the European Communities, pp. 22-33.
BLOK, S.M.G., GREVE, P.A., SANGSTER, B., SAVELKOUL, T.J.F., &
WEGMAN, R.C.C. (1984) [Study of normally occurring values of a
number of organochlorine pesticides and related compounds and their
metabolites, of polychlorine biphenyls and of chlorophenols in the
blood or plasma of healthy volunteers.] Bilthoven, The Netherlands,
National Institute of Public Health and Environmental Hygiene
(Unpublished report No. 638101001) (in Dutch).
BLUS, L.J., LAMONT, T.G., & NEELY, B.S. (1979) Fish, wildlife, and
estuaries: Effects of organochlorine residues on eggshell
thickness, reproduction, and population status of Brown Pelicans
(Pelecanus occidentalis) in South Carolina and Florida, 1969-76.
Pestic. monit. J., 12: 172-184.
BONNYNS, M. & BASTOMSKY, C.H. (1976) Polychlorinated
biphenyl-induced modification of lymphocyte response to plant
mitogens in rats. Experientia (Basel), 32: 522-523.
BORN, E.W., KRAUL, I., & KRISTENSEN, T. (1981) Mercury, DDT, and
PCB in the Atlantic Walrus (Odobenus rosmarus rosmarus) from the
Thule District, North Greenland. Arctic, 34: 255-260.
BOURQUIN, A.W. & CASSIDY, S. (1975) Effect of polychlorinated
biphenyl formulations on the growth of estuarine bacteria. Appl.
microbiol., 29: 125-127.
BOURQUIN, A.W. & KIEFER, L.A. (1975) Inhibition of estuarine
microorganisms by polychlorinated biphenyls. Dev. ind. Microbiol.,
16: 256-261.
BOWES, G.W. & JONKEL, C.J. (1975) Presence and distribution of
polychlorinated biphenyls (PCBs) in arctic and subarctic marine
food chains. J. Fish Res. Board Can., 32: 2111-2123.
BOWES, G.W., MULVIHILL, M.J., SIMONEIT, B.R.T., BURLINGAME, A.L.,
& RISEBROUGH, R.W. (1975) Identification of chlorinated
dibenzofurans in American polychlorinated biphenyls. Nature
(Lond.), 256: 305-307.
BOWMAN, R.E. & HEIRONIMUS, M.P. (1981) Hypoactivity in adolescent
monkeys perinatally exposed to PCBs and hyperactive as juveniles.
Neurobehav. Toxicol. Teratol., 3: 15-18.
BOWMAN, R.E., HEIRONIMUS, M.P., & ALLEN, J.R. (1978) Correlation of
PCB body burden with behavioural toxicology in monkeys. Pharmacol.
Biochem. Behav., 9: 49-56.
BOWMAN, R.E., HEIRONIMUS, M.P., & BARSOTTI, D.A. (1982) Locomotor
hyperactivity in PCB-exposed monkeys. Neurotoxicology, 2: 251-268.
BRANDT-RAUF, P.W. & NIMAN, H.L. (1988) Serum screening for oncogene
proteins in workers exposed to PCBs. Br. J. ind. Med.,
45(10): 689-693.
BRAUN, F. & MEYHOFER, B. (1977) [Investigations on the
concentration of polychlorinated biphenyls (Clophen C) in fish
organs under laboratory conditions.] Fisch Umwelt., 3: 1-11
(in German).
BRAZELTON, T.B. (1973) Neonatal behavioural assessment scale,
Philadelphia, Pennsylvania, J.B. Lippincott.
BREZNER, E., TERKEL, J., & PERRY, A.S. (1984) The effect of Aroclor
1254 (PCB) on the physiology of reproduction in the female rat-I.
Comp. Biochem. Physiol., 77C: 65-70.
BRIGGS, D.M. & HARRIS, J.R. (1973) Polychlorinated biphenyls
influence on hatchability. Poult. Sci., 52: 1291-1294.
BRINKMAN, U.A. & DE KOK, A. (1980) Production properties and usage.
In: Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins
and related products, Amsterdam, Elsevier Biomedical Press,
pp. 1-40.
BRINKMAN, M., FOGELMAN, K., HOEFLEIN, J., LINDH, T., PATEL, M.,
TRENCH, W.C., & AIKENS, D.A. (1980) Distribution of polychlorinated
biphenyls in the Fort Edward, New York, water system. Environ.
Manage., 4(6): 511-520.
BRINKMAN, M., FOGELMAN, K., HOEFLEIN, J., LINDH, T., PATEL, M.,
TRENCH, W.C., & AIKENS, D.A. (1981) Levels of polychlorinated
biphenyls in the Fort Edward, New York, water system. Adv. Identif.
Anal. Org. Pollut. Water, 2(50): 1001-1015.
BRITTON, W.M. & HUSTON, T.M. (1972) Yolk content and hatchability
of egg from hens fed Aroclor 1242. Poult. Sci., 51: 1869.
BROADHURST, M.G. (1972) Use and replaceability of polychlorinated
biphenyls. Environ. health Perspect., 2: 81-102.
BROUWER, A., VAN DEN BERG, K.J., & KUKLER, A. (1985) Time and dose
responses of the reduction in retinoid concentrations in C57BL/Rij
and DBA/2 mice induced by 3,4,3',4'-tetrachlorobiphenyl. Toxico.
appl. Pharmacol., 78: 180-189.
BROUWER, A., KUKLER, A., & VAN DEN BERG, K.J. (1988) Alterations in
retinoid concentrations in several extrahepatic organs of rats by
3,4,3',4'-tetrachlorobiphenyl. Toxicology, 50: 317-330.
BROUWER, A., REIJNDERS, P.J.H., & KOEMAN, J.H. (1989)
Polychlorinated biphenyl (PCB)-contaminated fish induces vitamin A
and thyroid hormone deficiency in the common seal (Phoca
vitulina). Aquat. Toxicol., 15: 99-106.
BROWN, D.P. (1987) Mortality of workers exposed to polychlorinated
biphenyls - An update. Arch. environ. Health, 42(6): 333-339.
BROWN, D.P. & JONES, M. (1981) Mortality and industrial hygiene
study of workers exposed to polychlorinated biphenyls. Arch.
environ. Health, 36: 120-129.
BROWN, J.F. & LAWTON, R.W. (1984) Polychlorinated biphenyl (PCB)
partitioning between adipose tissue and serum. Bull. environ.
Contam. Toxicol., 33: 277-280.
BROWN, J.F., BEDARD, D.L., BRENNAN, M.J., CARNAHAN, J.C., FENG, H.,
& WAGNER, R.E. (1987a) Polychlorinated biphenyl dechlorination in
aquatic sediments. Science, 236: 709-712.
BROWN, J.F., WAGNER, R.E., FENG, H., BEDARD, D.L., BRENNAN, M.J.,
CARNAHAN, J.C., & MAY, R.J. (1987b) Environmental dechlorination of
PCBs. Environ. Toxicol. Chem., 6: 579-593.
BROWN, J.F., CARNAHAN, J.C., DORN, S.B., GROVES, J.T., LIGON, W.V.,
MAY, R.J., WAGNER, R.E., & HAMILTON, S.B. (1988) Levels of
bioactive PCDF congeners in PCB dieletric fluids from capacitors
and transformers. Chemosphere, 17(9): 1697-1702.
BRUCE, R.W. & HEDDLE, J.A. (1979) The mutagenic activity of 61
agents as determined by the micronucleus, Salmonella, and sperm
abnormality assays. Can. J. Genet. Cytol., 21: 319-334.
BRUCKNER, J.V., KHANNA, K.L., & CORNISH, H.H. (1973) Biological
responses of the rat to polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 24: 434-448.
BRUCKNER, J.V., KHANNA, K.L., & CORNISH, H.H. (1974) Effect of
prolonged ingestion of polychlorinated biphenyls on the rat. Food
Cosmet. Toxicol., 12: 323-330.
BRUCKNER, J.V., JIANG, W.-D., BROWN, J.M., PUTCHA, L., CHU, C.K.,
& STELLA, V.J. (1977) The influence of ingestion of environmentally
encountered levels of a commercial polychlorinated biphenyl mixture
(Aroclor 1254) on drug metabolism in the rat. J. Pharmacol. exp.
Ther., 202: 22-31.
BRUNSTROM, B. & DARNERUD, P.O. (1983) Toxicity and distribution in
chick embryos of 3,3,4,4-tetrachlorobiphenyl injected into the
eggs. Toxicology, 27: 103-110.
BRUNSTROM, B. & REUTERGARDH, L. (1986) Differences in sensitivity
of some avian species to the embryotoxicity of a PCB,
3,3',4,4'-tetrachlorobiphenyl, injected into the eggs. Environ.
Pollut., 42: 37-45.
BRUNSTROM, B., KIHLSTROM, I., & LUNDKVIST, U. (1982) Studies of
foetal death and foetal weight in guinea-pigs fed polychlorinated
biphenyls (PCB). Acta pharmacol. toxicol., 50: 100-103.
BRUNSTRÖM, B., DARNERUD, P.O., BRANDT, I., & ORBERG, J. (1982a)
Distribution, metabolism and toxicity of 2,2,4,5-tetrachloro-
biphenyl after injection into the yolk of embryonated hens' eggs.
Ambio, 11: 212-214.
BRUNSTRÖM, B., KIHLSTRÖM, I., & LUNDKVIST, U. (1982b) Studies of
foetal death and foetal weight in guinea-pigs fed polychlorinated
biphenyls (PCB). Acta pharmacol. toxicol., 50: 100-103.
BUCKLEY, E.H. (1982) Accumulation of airborne polychlorinated
biphenyls in foliage. Science, 216: 520-522.
BUCKLEY, E.H. (1983) Decline of background PCB concentrations in
vegetation in New York state. Northeast. environ. Sci., 2: 181-187.
BUHLER, F., SCHMID, P., & SCHLATTER, Ch. (1988) Kinetics of PCB
elimination in man. Chemosphere, 17(9): 1717-1726.
BUNCE, N.J. (1978) Photolysis of 2-chlorobiphenyl in aqueous
acetonitrile. Chemosphere, 7: 653-656.
BUNCE, N.J., KUMAR, Y., & BROWNLEE, B.G. (1978) An assessment of
the impact of solar degradation of polychlorinated biphenyls in the
aquatic environment. Chemosphere, 7: 155-164.
BURKHARD, L.P., ARMSTRONG, D.E., & ANDREN, A.W. (1985) Henry's Law
Constants for the polychlorinated biphenyls. Environ. Sci.
Technol., 19(7): 590-596.
BURNS, J.E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue-Texas 1969-72.
Pestic. monit. J., 7(3/4): 122-126.
BURSE, V.W., KIMBROUGH, R.D., VILLANUEVA, E.C., JENNINGS, R.W.,
LINDER, R.E., & SOVOCOOL, G.W. (1974) Polychlorinated biphenyls;
storage, distribution, excretion, and recovery: liver morphology
after prolonged dietary ingestion. Arch. environ. Health,
29: 301-307.
BUSER, H.R. (1979) Formation of polychlorinated dibenzofurans
(PCDFs) and dibenzo- p-dioxins (PCDDs) from the pyrolysis of
chlorobenzenes. Chemosphere, 8: 415-424.
BUSER, H.R. (1985) Formation, occurrence, and analysis of
polychlorinated dibenzofurans, dioxins and related compounds.
Environ. health Perspect., 60: 259-267.
BUSER, H.R. & BOSSHARDT, H.P. (1978) [Polychlorinated
dibenzo- p-dioxins, dibenzofurans and benzenes in the ash of
community and industrial incineration plants.] Mitt. Geb. Lebensm.
Hyg., 69: 191-199 (in German).
BUSER, H.R. & RAPPE, C. (1979) Formation of polychlorinated
dibenzofurans (PCDFs) from the pyrolysis of individual PCB isomers.
Chemosphere, 3: 157-174.
BUSER, H.R., BOSSHARDT, H.P., & RAPPE, C. (1978a) Formation of
polychlorinated dibenzofurans (PCDF's) from the pyrolysis of PCBs.
Chemosphere, 7: 109-119.
BUSER, H.R., BOSSHARDT, H.P., RAPPE, C., & LINDAHL, R. (1978b)
Identification of polychlorinated dibenzofuran isomers in fly ash
and PCB pyrolysis. Chemosphere, 7: 419-429.
BUSH, B., TUMASONIS, C.F., & BAKER, F.D. (1974) Toxicity and
persistence of PCB homologs and isomers in the avian system. Arch.
environ. Contam. Toxicol., 2: 195-212.
BUSH, B., SNOW, J., & KOBLINTZ, R. (1984) Polychlorobiphenyl (PCB)
congeners, p,p'-DDE, and hexachlorobenzene in maternal and fetal
cord blood from mothers in upstate New York. Arch. environ. Contam.
Toxicol., 13(5): 517-527.
BUSH, B., SNOW, J., CONNOR, S., & KOBLINTZ, R. (1985)
Polychlorinated biphenyl congeners (PCBs), p,p'-DDE and
hexachlorobenzene in human milk in three areas of upstate New York.
Arch. environ. Contam. Toxicol., 14: 443-450.
BUSH, B., SIMPSON, K.W., SHANE, L., & KOBLINTZ, R.R. (1985) PCB
congener analysis of water and caddisfly larvae (Insecta:
Trichoptera) in the Upper Hudson river by glass capillary
chromatography. Bull. environ. Contam. Toxicol., 34: 96-105.
BYRNE, J.J. & SEPKOVIC, D.W. (1987) Inhibition of monovalent cation
transport across the cell membrane by polychlorinated biphenyl but
not by polybrominated biphenyl. Arch. environ. Contam. Toxicol.,
16: 573-577.
BYRNE, J.J., CARBONE, J.P., & PEPE, M.G. (1988) Suppression of
serum adrenal cortex hormones by chronic low-dose polychloro-
biphenyl of polybromobiphenyl treatments. Arch. environ. Contam.
Toxicol., 17: 47-53.
CAIN, B.W. (1981) Nationwide residues of organochlorine compounds
in wings of adult mallards and black ducks, 1979-80. Pestic. monit.
J., 15: 128-134.
CAIN, B.W. & BUNCK, C.M. (1983) Residues of organochlorine
compounds in starlings (Sturnus vulgaris), 1979. Environ. monit.
Assess., 3: 161-172.
CALANDRA, J.C. (1976) Summary of toxicological studies on
commercial PCBs. In: Proceedings of the National Conference of
polychlorinated biphenyls, Washington, DC, US Environmental
Protection Agency (560/6-75-004).
CALIFANO, R.J., O'CONNOR, J.M., & PETERS, L.S. (1980) Uptake,
retention, and elimination of PCB (Aroclor 1254) by larval striped
bass (Morone saxatilis). Bull. environ. Contam. Toxicol.,
24: 467-472.
CALL, D.J. & HARRELL, B.E. (1974) Effects of dieldrin and PCBs upon
the production and morphology of Japanese quail eggs. Bull.
environ. Contam. Toxicol., 11: 70-77.
CALLAHAN, M.A.A., HAMMERSTROM, K.A., & SCHWEER, G. (1983) Present
PCB uses and their potential for release to the environment. In:
Barros, M.C., Könemann, H., & Visser, R., ed. Proceedings of PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 152-172.
CAMP, B.J., HEJTMANCIK, E., ARMOUR, C., & LEWIS, D.H. (1974) Acute
effects of Aroclor 1254 (PCB) on Ictalurus punctatus (catfish).
Bull. environ. Contam. Toxicol., 12: 204-208.
CAMPS, M., PLANAS, J., GOMEZ-CATALAN, J., SABROSO, M., TO-FIGUERAS,
J., & CORBELLA, J. (1989) Organochlorine residues in human adipose
tissue in Spain: Study of an agrarian area. Bull. environ. Contam.
Toxicol., 42: 195-201.
CANTONI, C., FABBRIS, F., ROGLEDI, R., & CAMPAGNARI, A. (1988)
[Organochlorine pesticides found in foods of animal origin in the
1985-1987 biennium.] Ind. Aliment., XXVII(1): 6-8 (in Italian).
CARLSON, R.W. & DUBY, R.T. (1973) Embryotoxic effects of three
PCB's in the chicken. Bull. environ. Contam. Toxicol., 9: 261-266.
CARNES, R.A., DOERGER, J.U., & SPARKS, H.L. (1973) Polychlorinated
biphenyls in solid waste and solid-waste-related materials. Arch.
environ. Contam. Toxicol., 1: 27-35.
CAREY, A.E. & HARVEY, G.R. (1978) Metabolism of polychlorinated
biphenyls by marine bacteria. Bull. environ. Contam. Toxicol.,
20: 527-534.
CARTER, J.W. (1985) Effects of dietary in PCBs (Aroclor 1254) on
serum levels of lipoprotein cholesterol in Fischer rats. Bull.
environ. Contam. Toxicol., 34: 427-431.
CARTER, J.W. & CLANCY, J. (1980) Acutely administered
polychlorinated biphenyls (PCBs) decrease splenic cellularity but
increase its ability to cause graft-versus host reactions in BALB/c
mice. Immunopharmacology, 2: 341-347.
CATELANI, D., SORLINI, C., & TRECCANI, V. (1971) The metabolism of
biphenyl by Pseudomonas putida. Experientia (Basel),
27: 1173-1174.
CECIL, H.C., BITMAN, J., LILLIE, R.J., FRIES, G.F., & VERRETT, J.
(1974) Embryotoxic and teratogenic effects in unhatched fertile
eggs from hens fed polychlorinated biphenyls (PCBs). Bull. environ.
Contam. Toxicol., 11: 489-495.
CETINKAYA, M., GABEL, B., PODBIELSKI, A., & THIEMANN, W. (1984)
[Investigation on the association between nutrition and living
conditions of breastfeeding mothers and the contamination of
breastmilk with organochlorine compounds of low volatility.] Akt.
Ernähr., 9: 157-162 (in German).
CHAKRABORTY, D., BHATTACHARYYA, A., CHATTERJEE, J., CHATTERJEE, K.,
SEN, A., CHATTERJEE, S., MAJUMDAR, K., & CHATTERJEE, G.C. (1978)
Biochemical studies on polychlorinated biphenyl toxicity in rats:
manipulation by vitamin C. Int. J. Vitam. Nutr. Res., 48: 22-31.
CHANG, K.-T., CHENG, J.-S., HUANG, P.-C., & TUNG, T.-C. (1980a)
Study of patients with PCB poisoning. J. Formosan Med. Assoc.,
79: 304-313.
CHANG, K.-J., LU, F.-J., TUNG, T.-C., & LEE, T.-P. (1980b) Studies
on patients with PCB poisoning. Determination of urinary
coproporphyrin, uroporphyrin, delta-aminolaevulinic acid and
prophobilinogen. Res. Commun. chem. Pathol. Pharmacol.,
30(3): 547-554.
CHANG, K.-J., HSIEH, K.-H., LEE, T.-P., TANG, S.-Y., & TUNG, T.-C.
(1981) Immunologic evaluation of patients with polychlorinated
biphenyls poisoning. Determination of lymphocyte subpopulations.
Toxicol. appl. Pharmacol., 61: 58-63.
CHASE, K.H., WONG, O., THOMAS, D., BERNEY, B.W., & SIMON, R.K.
(1982) Clinical and metabolic abnormalities associated with
occupational exposure to polychlorinated biphenyls (PCBs). J.
occup. Med., 24(2): 109-114.
CHEN, P.H., GRAW, J.M., WONG, C.K., & CHEN, C.J. (1980) Levels and
gas chromatography patterns of PCBs in blood of patients after PCB
poisoning in Taiwan. Bull. environ. Contam. Toxicol., 25: 325-329.
CHEN, P.H., CHANG, K.T., & LU, Y.D. (1981) Polychlorinated
biphenyls and polychlorinated benzofurans in the toxic rice-bran
oil caused PCB poisoning in Taichyung. Bull. environ. Contam.
Toxicol., 26(4): 489-495.
CHEN, P.H., LUO, M.L., WONG, C.K., & CHEN, C.J. (1982) Comparative
rates of elimination of some individual polychlorinated biphenyls
from the blood of PCB-poisoned patients in Taiwan. Food chem.
Toxicol., 20(4): 417-425.
CHEN, P.H., WONG, C.-K., RAPPE, C., & NYGREN, M. (1985)
Polychlorinated biphenyls, dibenzofurans and quaterphenyls in toxic
rice-bran oil and in the blood and tissues of patients with PCB
poisoning (Yu-Cheng) in Taiwan. Environ. Health Perspect.,
59: 59-65.
CHEN, P.R., MCKINNEY, J.D., & MATTHEWS, H.B. (1976) Metabolism of
2,4,5,2',5'-pentachlorobiphenyl in the rat. Drug. Metab. Dispos.,
4: 362-367.
CHEN, R.-C., TANG, S.-Y., MIYATA, H., KASHIMOTO, T., CHANG, Y.-C.,
CHANG, K.-J., & TUNG, T.-C. (1985) Polychlorinated biphenyl
poisoning: Correlation of sensory and motor nerve conduction,
neurologic symptoms and blood levels of polychlorinated biphenyls,
quaterphenyls, and dibenzofurans. Environ. Res., 37: 340-348.
CHEN, T.S. & DUBOIS, K.P. (1973) Studies on the enzyme inducing
effects of polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
26: 504-512.
CHEN HSI-SUNG, P., LUO, M.-L., WONG, C.-K., & CHEN, C.-J. (1984)
Polychlorinated biphenyls, dibenzofurans, and quaterphenyls in the
toxic rice-bran oil and PCBs in the blood of patients with PCB
poisoning in Taiwan. Am. J. ind. Med., 5: 133-145.
CHESNEY, C.F. & ALLEN, J.R. (1974) Oxidative phosphorylation and
respiration by liver mitochondria from polychlorinated
biphenyl-intoxicated rats. Biochem. Pharmacol., 23: 1577-1582.
CHIA, L.-G. & CHU, F.-L. (1984) Neurological studies on
polychlorinated biphenyl (PCB)-poisoned patients. Am. J. ind. Med.,
5: 117-126.
CHOU, S.M., MIKE, T., PAYNE, W.M., & DAVIS, G.J. (1979)
Neuropathology of "spinning syndrome" induced by prenatal
intoxication with a PCB in mice. Ann. NY Acad. Sci., 320: 373-395.
CHOW, C.K., THACKER, R., & GAIROLA, C.C. (1979) Increased level of
L-ascorbic acid in the plasma of polychlorobiphenyls-treated rats
and its inhibition by dietary vitamin E. Res. Commun. chem. Pathol.
Pharmacol., 26: 605-608.
CHRISTIANI, D.C., KRIEBEL, D., FOX, N.J., & BAKER, E.L. (1986)
Persistently elevated polychlorinated biphenyl levels from residual
contamination of workplace surfaces. Am. J. ind. Med., 10: 143-151.
CHU, C.K., STELLA, V.J., BRUCKNER, J.V., & JIANG, W.D. (1977)
Effects of long-term exposure to environmental levels of
polychlorinated biphenyls on pharmacokinetics of pentobarbital in
rats. J. pharm. Sci., 66(2): 238-241.
CLARK, D.R. (1978) Uptake of dietary PCB by pregnant Big Brown Bats
(Eptesicus fuscus) and their fetuses. Bull. environ. Contam.
Toxicol., 19: 707-714.
CLARK, D.R. & LAMONT, T.G. (1976) Organochlorine residues and
reproduction in the big brown bat. J. Wildl. Manage., 40: 249-254.
CLARK, D.R. & PROUTY, R.M. (1977) Experimental feeding of DDT and
PCB to female Big Brown Bats (Eptesicus fuscus). J. Toxicol.
environ. Health, 2: 917-928.
CLARK, R.R., CHIAN, E.S.K., & GRIFFIN, R.A. (1979) Degradation of
polychlorinated biphenyls by mixed microbial cultures. Appl.
environ. Microbiol., 37: 680-685.
CLARK, J.R., PATRICK, J.M., MOORE, J.C., & FORESTER, J. (1986)
Accumulation of sediment-bound PCBs by Fiddler crabs. Bull.
environ. Contam. Toxicol., 36: 571-578.
CLAUS, B. & ACKER, L. (1975) [Contamination of milk and milk
products with chlorinated hydrocarbons in Westphalia. II. Results
and discussion.] Z. Lebensmittelunters. Forsch., 159(3): 129-137
(in German).
CLEVENGER, M.A., ROBERTS, S.M., LATTIN, D.L., HARBINSON, R.D., &
JAMES, R.C. (1989) The pharmacokinetics of 2,2,5,5'-tetrachloro-
biphenyl and 3,3',4,4'-tetrachlorobiphenyl and its relationship to
toxicity. Toxicol. appl. Pharmacol., 100: 315-327.
COLE, D.R. & PLAPP, F.W. (1974) Inhibition of growth and
photosynthesis in Chlorella pyrenoidosa by a polychlorinated
biphenyl and several insecticides. Environ. Entomol., 3: 217-220.
COLLINS, W.T. & CAPEN, C.C. (1980a) Ultrastructural and functional
alterations of the rat's thyroid gland produced by polychlorinated
biphenyls compared with iodide excess and deficiency, and
thyrotropin and thyroxine administration. Virchows Arch. cell.
Pathol., B33: 213-231.
COLLINS, W.T. & CAPEN, C.C. (1980b) Biliary excretion of 125
I-thyroxine and fine structural alterations in the thyroid glands
of Gunn rats fed polychlorinated biphenyls (PCB). Lab. Invest.,
43: 158-164.
COLLINS, G.B., HOLMES, D.C., & JACKSON, F.J. (1972) The estimation
of polychlorobiphenyls. J. Chromatogr., 71: 443-449.
COLLINS, W.T., CAPEN, C.C., KASZA, L., CARTER, C., & DAILEY, R.E.
(1977) Effect of polychlorinated biphenyl (PCB) on the thyroid
gland of rats. Am. J. Pathol., 89: 119-136.
CONNELL, D.W. (1987) Age to PCB concentration relationship with the
striped bass (Morone saxatilis) in the Hudson River and Long
Island Sound. Chemosphere, 16: 1469-1474.
COOKE, A.S. (1973) Shell thinning in avian eggs by environmental
pollutants. Environ. Pollut., 4: 85-152.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides and pollution, Huntingdon, United Kingdom, Natural
Environment Research Council, Institute of Terrestrial Ecology,
74 pp.
COOLEY, N.R., KELTNER, J.M., & FORESTER, J. (1972) Mirex and
Aroclor 1254: Effect on accumulation by Tetrahymena pyriformis
strain W. J. Protozool., 19: 636-638.
COOLEY, N.R., KELTNER, J.M., & FORESTER, J. (1973) The
polychlorinated biphenyls, Aroclors 1248 and 1260: Effect on and
accumulation by Tetrahymena pyriformis. J. Protozool.,
20: 443-445.
COSPER, E.M., WURSTER, C.F., & ROWLAND, R.G. (1984) PCB resistance
within phytoplankton populations in polluted and unpolluted marine
environments. Mar. environ. Res., 12: 209-223.
COTE, M.G., PLAA, G.L., VALLI, V.E., & VILLENEUVE, D.C. (1985)
Subchronic effects of a mixture of "persistent" chemicals found in
the Great Lakes. Bull. environ. Contam. Toxicol., 34: 285-290.
COURTNEY, W.A.M. & DENTON, G.R.W. (1976) Persistence of
polychlorinated biphenyls in the hard-clam (Mercenaria mercenaria)
and the effect upon the distribution of these pollutants in the
estuarine environment. Environ. Pollut., 10: 55-64.
COURTNEY, W.A.M. & LANGSTON, W.J. (1978) Uptake of polychlorinated
biphenyl (Aroclor 1254) from sediment and seawater in two
intertidal polychaetes. Environ. Pollut., 15: 303-309.
CRAIGIE, J.S. & HUTZINGER, O. (1975) Effects of commercial
chlorinated hydrocarbons and specific chlorobiphenyls on the growth
of seven species of marine phytoplankton. Chemosphere, 3: 139-144.
CROSBY, D.G. & MOILANEN, K.W. (1973) Photodecomposition of
chlorinated biphenyls and dibenzofurans. Bull. environ. Contam.
Toxicol., 10: 372-377.
CURLEY, A., BURSE, V.W., GRIM, M.E., JENNINGS, R.W., & LINDER, R.E.
(1971) Polychlorinated biphenyls: Distribution and storage in body
fluids and tissues of Sherman rats. Environ. Res., 4: 481-495.
CURLEY, A., BURSE, V.W., & GRIM, M.E. (1973a) Polychlorinated
biphenyls: Evidence of transplacental passage in the Sherman rats.
Food Cosmet. Toxicol., 11: 471-476.
CURLEY, A., BURSE, V.W., JENNINGS, R.W., VILLANUEVA, E.C., TOMATIS,
L., & AKAZAKI, K. (1973b) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond.), 242: 338-340.
DAHLGREN, R.B. & LINDER, R.L. (1971) Effects of polychlorinated
biphenyls on pheasant reproduction, behavior, and survival. J.
Wildl. Manage., 35: 315-319.
DAHLGREN, R.B., LINDER, R.L., & CARLSON, C.W. (1972)
Polychlorinated biphenyls: Their effects on penned pheasants.
Environ. health Perspect., 1: 89-101.
DAHLGREN, R.B., BURY, R.J., LINDER, R.L., & REIDINGER, R.F. (1972)
Residue levels and histopathology in Pheasants given
polychlorinated biphenyls. J. Wildl. Manage., 36: 524-533.
DAVIDORF, F.H. & KNUPP, J.A. (1979) Epidemiology of ocular
melanoma. Incidence and geographic relationship in Ohio
(1967-1977). Ohio State med. J., 75: 561-564.
DAVIES, K. (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17(2): 263-276.
DAVIES, D. & MES, J. (1987) Comparison of the residue levels of
some organochlorine compounds in breast milk of the general and
indigenous Canadian populations. Bull. environ. Contam. Toxicol.,
39: 743-749.
DAVIS, D. & SAFE, S. (1989) Dose-response immunotoxicities of
commercial polychlorinated biphenyls (PCBs) and their interaction
with 2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol. Lett.,
48: 35-43.
DEFOE, D.L., VEITH, G.D., & CARLSON, R.W. (1978) Effects of Aroclor
1248 and 1260 on the Fathead Minnow (Pimephales promelas). J.
Fish Res. Board Can., 35: 997-1002.
DEFREITAS, A.S.W., NORSTRÖM, R.J., & HUTZINGER, O. (1972) Dynamics
and metabolism of polychlorinated biphenyls (PCBs) in cold-exposed
pigeons. Am. Chem. Soc., 12: 118-123.
DE KOCK, A.C. & LORD, D.A. (1988) Kinetics of the uptake and
elimination of polychlorinated biphenyls by an estuarine fish
species (Rhabdosargus holubi) after aqueous exposure.
Chemosphere, 17: 2381-2390.
DELFINO, J.J. (1979) Toxic substances in the Great Lakes. Environ.
Sci. Technol., 13: 1462-1468.
DELONG, R.L., GILMARTIN, W.G., & SIMPSON, J.G. (1973) Premature
births in California sea lions: Association with high
organochlorine pollutant residue levels. Science, 181: 1168-1169.
DEML, E. & OESTERLE, D. (1982) Sex-dependent promoting effect of
polychlorinated biphenyls on enzyme-altered islands induced by
diethylnitrosamine in rat liver. Carcinogenesis, 3: 1449-1453.
DEML, E. & OESTERLE, D. (1987) Dose-response of promotion by
polychlorinated biphenyls and chloroform in rat liver foci
bioassay. Arch. Toxicol., 60: 209-211.
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels of
organochlorine pesticides on microsomal liver enzymes in short-term
toxicity experiments. Toxicology, 2: 371-380.
DE VOS, R.H. & PEET, E.W. (1971) Thin-layer chromatography of
polychlorinated biphenyls. Bull. environ. Contam. Toxicol.,
6: 164-170.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRIJNS, J.K., MUYS,
T., & VAN DER POLL, J.M. (1984) Pesticides and other chemical
residues in Dutch total diet samples (June 1976-July 1978). Food
Chem. Toxicol., 22(1): 11-21.
DFG (1988) [Polychlorinated biphenyls. Stocktaking on analysis,
occurrence, kinetics and toxicology.] German Association for the
Encouragement of Research: Communication XIII of the Government
Committee on the Testing of Residues in Foods], Weinheim, Verlag
Chemie (in German).
DICKHUT, R.M., ANDREN, A.W., & ARMSTRONG, D.E. (1986) Aqueous
solubilities of six polychlorinated biphenyl congeners at four
temperatures. Environ. Sci. Technol., 20(8): 807-810.
DICKHUT, R.M., ANDREN, A.W., & ARMSTRONG, D.E. (1987) Comment on
"Aqueous solubilities of six polychlorinated biphenyl congeners at
four temperatures". Environ. Sci. Technol., 21(9): 926-928.
DIERINGER, C.S., LAMARTINIERE, C.A., SCHILLER, C.M., & LUCIER, G.W.
(1979) Altered ontogeny of hepatic steroid-metabolizing enzymes by
pure polychlorinated biphenyl congeners. Biochem. Pharmacol.,
28: 2511-2514.
DIETER, M.P. (1974) Plasma enzyme activities in Coturnix quail fed
graded doses of DDE, polychlorinated biphenyls, malathion, and
mercuric chloride. Toxicol. appl. Pharmacol., 27: 86-98.
DIGERNES, V. & ASTRUP, E.G. (1982) Are datascreen terminals a
source of increased PCB-concentrations in the working atmosphere?
Int. Arch. occup. Health, 49: 193-197.
DIGIOVANNI, J., BERRY, D.L., SLAGA, T.J., & JUCHAU, M.R. (1979)
Studies on the relationship between induction of biotransformation
and tumour-initiating activity of 7,12-dimethylbenz(a)anthracene in
mouse skin. In: Jones, P.W. & Leber, P., ed. Polynuclear aromatic
hydrocarbons, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 553-568.
DILLON, J.C., MARTIN, G.B., & O'BRIEN, H.T. (1981) Pesticide
residues in human milk. Food Cosmet. Toxicol., 19: 437-442.
DIVE, D., ERB, F., LECLERC, H., PRIEM, M.N., & COLEIN, M.P. (1976)
Toxicité et bioaccumulation d'isomers de polychlorobiphenyles par
le protozoaire cilie Colpidium campylum. Eur. J. Toxicol.
environ. Hyg., 9: 105-111.
DOBSON, S. (1981) Physiological and behavioural effects of
organochlorines on pigeons. Oekol. Voegel, 3: 39-43.
DOGRA, S., FILSER, J.G., COJOCEL, C., GREIM, H., REGEL, U., OESCH,
F., & ROBERTSON, L.W. (1988) Long-term effects of commercial and
congeneric polychlorinated biphenyls on ethane production and
malondialdehyde levels, indicators of in vivo lipid peroxidation.
Arch. Toxicol., 62: 369-374.
DOGUCHI, M. (1977) Polychlorinated terphenyls as an environmental
pollutant in Japan. Ecotoxicol. environ. Saf., 1: 239-248.
DOGUCHI, M. & FUKANO, S. (1975) Residue levels of polychlorinated
terphenyls, polychlorinated biphenyls and DDT in human blood. Bull.
environ. Contam. Toxicol., 13: 57-63.
DOGUCHI, M., FUKANO, S., & USHIO, F. (1974) Polychlorinated
terphenyls in the human fat. Bull. environ. Contam. Toxicol.,
11(2): 157-158.
DOMMARCO, R., DI MUCCIO, A., COMONI, I., & GIGLI, B. (1987)
Organochlorine pesticide and polychlorinated biphenyl residues in
human milk from Rome (Italy) and surroundings. Bull. environ.
Contam. Toxicol., 39: 919-925.
DONKIN, P., MANN, S., & HAMILTON, E.I. (1981) Polychlorinated
biphenyl, DDT, and dieldrin residues in grey seal (Halichoerus
grypus) males, females and mother-foetus pairs sampled at the
Farne islands, England, during the breeding season. Sci. total
Environ., 19: 121-142.
DOUCETTE, W.J. & ANDREN, A.W. (1988) Aqueous solubility of selected
biphenyl, furan and dioxin congeners. Chemosphere, 17(2): 243-252.
DRIJVER, M., DUIJKERS, T.J., KROMHOUT, D., VISSER, T.J., MULDER,
P., & LOUW, R. (1988) Determinations of polychlorinated biphenyls
(PCBs) in human milk. Acta paediatr. Scand., 77: 30-36.
DRILL, V.A., FREISS, S.L., HAYS, H.W., LOOMIS, T.A., & SCHAFFER,
C.B. (1981) Potential health effects in the human from exposure to
polychlorinated biphenyls (PCBs) and related impurities, Arlington,
Virginia, Drill, Freiss, Hays, Loomis and Schaffer, Inc.
(Unpublished report).
DUINKER, J.C. & BOUCHERTALL, F. (1989) On the distribution of
atmospheric polychlorinated biphenyl congeners between vapor phase,
aerosols and rain. Environ. Sci. Technol., 23: 57-62.
DUINKER, J.C. & HILLEBRAND, M.T.J. (1979) Mobilization of
organochlorines from female lipid tissue and transplacental
transfer to fetus in a harbour porpoise (Phocoena phocoena) in a
contaminated area. Bull. environ. Contam. Toxicol., 23: 728-732.
DUINKER, J.C. & HILLEBRAND, M.T.J. (1983) Characterization of PCB
components in Clophen formulations by capillary GC-MS and GC-ECD
techniques. Environ. Sci. Technol., 17: 449-456.
DUINKER, J.C., SCHULTZ, D.E., & PETRICK, G. (1988) Selection of
chlorinated biphenyl congeners for analysis in environmental
samples. Mar. Pollut. Bull., 19(1): 19-25.
DUKE, T.W., LOWE, J.I., & WILSON, A.J. (1970) A polychlorinated
biphenyl (Aroclor 1254) in the water, sediment, and biota of
Escambia Bay, Florida. Bull. environ. Contam. Toxicol., 5: 171-180.
DUNN, W.I., III, STELLING, D.L., SCHWARTZ, T.R., HOGAN, J.W.,
PETTY, J.D., JOHANSSON, E., & WOLD, S. (1984) Pattern recognition
for classification and determination of polychlorinated biphenyls
in environmental samples. Anal. Chem., 56: 1308-1313.
DUNNIVANT, F.M. & ELZERMAN, A.W. (1988) Aqueous solubility and
Henry's Law Constant data for PCB congeners for evaluation of
quantitative structure-property relationships (QSPRs). Chemosphere,
17(3): 525-541.
DURHAM, S.K. & BROUWER, A. (1989) 3,4,3',4'-tetrachloro-
biphenyl-induced effects in the rat liver. I. Serum and hepatic
retinoid reduction and morphologic changes. Toxicol. Pathol.,
17(3): 536-544.
DUTCH AGRICULTURAL ADVISORY COMMISSION ON ENVIRONMENTAL POLLUTANTS
(1983) Annual report, The Hague, Ministry of Agriculture,
Management of Nature, and Fisheries (Unpublished).
DZOGBEFIA, V.P., KLING, D., & GAMBLE, W. (1978) Polychlorinated
biphenyls: in vivo and in vitro modifications of phospholipid
and glyceride biosynthesis. J. environ. Pathol. Toxicol.,
1: 841-856.
ECOBICHON, D.J. & COMEAU, A.M. (1974) Comparative effects of
commercial Aroclor on rat liver enzyme activities. Chem. biol.
Interact., 9: 341-350.
ECOBICHON, D.J. & COMEAU, A.M. (1975) Isomerically pure
chlorobiphenyl congeners and hepatic function in the rat. Influence
of position and degree of chlorination. Toxicol. appl. Pharmacol.,
33: 94-105.
ECOBICHON, D.J. & MACKENZIE, D.O. (1974) The uterotropic activity
of commercial and isomerically-pure chlorobiphenyls in the rat.
Res. Commun. chem. Pathol. Pharmacol., 9: 85-95.
EDULJEE, G., BADSHA, K., & SCUDAMORE, N. (1986) Environmental
monitoring for PCB and trace metals in the vicinity of a chemical
waste disposal facility - II. Chemosphere, 15(1): 81-93.
EISENREICH, S.J., LOONEY, B.B., & THORNTON, J.D. (1981) Airborne
organic contaminants in the Great Lakes ecosystem. Environ. Sci.
Technol., 15: 30-38.
EISLER, R. (1986) Polychlorinated biphenyl hazards to fish,
wildlife, and invertebrates: A synoptic review, Washington, DC, US
Department of Interior, Fish & Wildlife Service, 72 pp (Biology
Report No. 85).
EKSTEDT, J. & ODEN, S. (1974) Chlorinated hydrocarbons in the lower
atmosphere in Sweden, Uppsala, Sweden, Royal Agricultural College,
Department of Soil Science, pp. 1-16.
ELDER, D.L., FOWLER, S.W., & POLIKARPOV, G.G. (1979) Remobilization
of sediment-associated PCBs by the worm Nereis diversicolor.
Bull. environ. Contam. Toxicol., 21: 448-452.
ELLIOTT, J.E., NORSTRÖM, R.J., & KEITH, J.A. (1988) Organochlorines
and eggshell thinning in Northern Gannets (Sula bassanus) from
Eastern Canada, 1968-1984. Environ. Pollut., 52: 81-102.
ELO, O., VUOJOLAHTI, P., JANHUNEN, H., & RANTANEN, J. (1985) Recent
PCB accidents in Finland. Environ. health Perspect., 60: 315-319.
EMMETT, E.A. (1985) Polychlorinated biphenyl exposure and effects
in transformer repair workers. Environ. health Perspect.,
60: 185-192.
EMMETT, E.A., MARONI, M., SCHMITH, J.M., LEVIN, B.K., & JEFFERYS,
J. (1988a) Studies of transformer repair workers exposed to PCBs:
I. Study design, PCB concentrations, Questionnaire, and clinical
examination results. Am. J. ind. Med., 13: 415-427.
EMMETT, E.A., MARONI, M., JEFFERYS, J., SCHMITH, J., LEVIN, B.K.,
& ALVARES, A. (1988b) Studies of transformer repair workers exposed
to PCBs: II. Results of clinical laboratory investigations. Am. J.
ind. Med., 14: 47-62.
ERICKSON, M.D. (1985) The analytical chemistry of PCBs, Boston,
Massachusetts, Butterworth.
ERICKSON, M.D., STANLEY, J.S., TURMAN, J.K., GOING, J.E., REDFORD,
D.P., & HEGGEM, D.T. (1988) Determination of byproduct
polychlorobiphenyls in commercial products and wastes by
high-resolution gas chromatography/electron impact mass
spectrometry. Environ. Sci. Technol., 22(1): 71-76.
ESCHENROEDER, A.Q., DOYLE, C.P., & FAEDER, E.J. (1986) Health risks
of PCB spills from electrical equipment. Risk Anal., 6(2): 213-221.
EWALD, W.G., FRENCH, J.E., & CHAMP, M.A. (1976) Toxicity of
polychlorinated biphenyls (PCBs) to Euglena gracilis: Cell
population growth, carbon fixation, chlorophyll level, oxygen
consumption, and protein and nucleic acid synthesis. Bull. environ.
Contam. Toxicol., 16: 71-80.
EXON, J.H., TALCOTT, P.A., & KOLLER, L.D. (1985) Effect of lead,
polychlorinated biphenyls, and cyclophosphamide on rat natural
killer cells, interleukine 2, and antibody synthesis. Fundam. appl.
Toxicol., 5: 158-164.
FAIT, A., GROSSMAN, E., SELF, S., FEFFRIES, J., PELLIZZARI, E.D.,
& EMMETT, E.A. (1989) Polychlorinated biphenyl congeners in adipose
tissue lipid and serum of past and present transformer repair
workers and a comparison group. Fundam. appl. Toxicol., 12: 42-55.
FALANDYSZ, J. (1980) Chlorinated hydrocarbons in gulls from the
Baltic south coast. Mar. Pollut. Bull., 11: 75-80.
FARBER, E. (1984a) Perspectives in cancer research. The multistep
nature of cancer development. Cancer Res., 44: 4217-4223.
FARBER, E. (1984b) Cellular biochemistry of the step-wise
development of cancer with chemicals. G.H. Clowes Memorial Lecture.
Cancer Res., 44: 5463-5474.
FARBER, E. (1986) Some emerging general principles in the
pathogenesis of hepatocellular carcinoma. Cancer Surv., 5: 695-718.
FDA (1970) Status report on the chemistry and toxicology of
polychlorinated biphenyls (PCBs) or Aroclors as of 1 June 1970,
Washington, DC, Food and Drug Administration, 16 pp.
FDA (1979) Polychlorinated biphenyls (PCBs): Reduction of
tolerances, Fed. Reg., 44(127): 126-136.
FEIN, G.G. (1984) Intrauterine exposure of humans to PCBs: newborn
effects, Duluth, Minnesota, US Environmental Protection Agency
(EPA 600/53-84-060).
FEIN, G.G., JACOBSON, J.L., JACOBSON, S.W., SCHWARTZ, P.M., &
DOWLER, J.K. (1984) Prenatal exposure to polychlorinated biphenyls:
Effects on birth size and gestational age. J. Pediatr.,
105(2): 315-320.
FELT, G.R., MUELLER, W.F., IATROPOULOS, M.J., COULSTON, F., &
KORTE, F. (1977) Chronic toxicity of 2,5,4'-trichlorobiphenyl in
young rhesus monkeys. I. Body distribution, elimination and
metabolism. Toxicol. appl. Pharmacol., 41(3): 619-627.
FELT, G.R., MUELLER, W.F., COULSTON, F., & KORTE, F. (1979)
Distribution and excretion of 2,2',4,4',6-pentachlorobiphenyl in
the rat. Bull. environ. Contam. Toxicol., 22: 582-585.
FINGERMAN, S.W. (1980) Differences in the effects of fuel oil, an
oil dispersant, and three polychlorinated biphenyls on fin
regeneration in the Gulf Coast kill fish, Fundulus grandis. Bull.
environ. Contam. Toxicol., 25: 234-240.
FINGERMAN, S.W. & FINGERMAN, M. (1977) Effects of a polychlorinated
biphenyl and a polychlorinated dibenzofuran on molting of the
fiddler crab, Uca pugilator. Bull. environ. Contam. Toxicol.,
18: 138-142.
FINGERMAN, S.W. & FINGERMAN, M. (1979) Comparison of the effects of
fourteen-day and chronic exposures to a polychlorinated biphenyl,
Aroclor 1242, on molting of the fiddler crab, Uca pugilator.
Bull. environ. Contam. Toxicol., 21: 352-357.
FINGERMAN, S.W. & RUSSELL, L.C. (1980) Effects of the
polychlorinated biphenyl Aroclor 1242 on locomotor activity and on
the neurotransmitters dopamine and norepinephrine in the brain of
the gulf killifish, Fundulus grandis. Bull. environ. Contam.
Toxicol., 25: 682-687.
FINKLEA, J., PRIESTER, L.E., CREASON, J.P., HAUSER, T., HINNERS,
T., & HAMMER, D.I. (1972) Polychlorinated biphenyl residues in
human plasma expose a major urban pollution problem. Am. J. public
Health, 62(5): 645-651.
FIORE, B.J., ANDERSON, H.A., HANRAHAN, L.P., OLSON, L.J., &
SONZOGNI, W.C. (1989) Sport fish consumption and body burden levels
of chlorinated hydrocarbons: A study of Wisconsin anglers. Arch.
environ. Health, 44(2): 82-88.
FISCHBEIN, A. (1985) Liver function tests in workers with
occupational exposure to polychlorinated biphenyls (PCBs):
Comparison with Yusho and Yu-Cheng. Environ. health Perspect.,
60: 145-150.
FISCHBEIN, A. & WOLFF, M.S. (1987) Conjugal exposure to
polychlorinated biphenyls (PCBs). Br. J. ind. Med., 44: 284-286.
FISCHBEIN, A., WOLFF, M.S., LILIS, R., THORNTON, J., & SELIKOFF,
I.J. (1979) Clinical findings among PCB-exposed workers in a
capacitor manufacturing facility. Ann. NY Acad. Sci., 320: 703-715.
FISCHBEIN, A., WOLFF, M.S., BERNSTEIN, J., SELIKOFF, I.J., &
THORNTON, J. (1982) Dermatological findings in capacitor
manufacturing workers exposed to dielectric fluids containing
polychlorinated biphenyls (PCBs). Arch. environ. Health,
37(2): 69-74.
FISCHBEIN, A., RIZZO, J.N., SOLOMON, S.J., & WOLFF, M.S. (1985)
Oculodermatological findings in workers with occupational exposure
to polychlorinated biphenyls (PCBs). Br. J. ind. Med., 42: 426-430.
FISHBEIN, L. (1974) Toxicity of chlorinated biphenyls. Annu. Rev.
Pharmacol., 14: 139-156.
FISHER, N.S. (1975) Chlorinated hydrocarbon pollutants and
photosynthesis of marine phytoplankton: A reassessment. Science,
189: 463-464.
FISHER, N.S. & WURSTER, C.F. (1973) Individual and combined effects
of temperature and polychlorinated biphenyls on the growth of three
species of phytoplankton. Environ. Pollut., 5: 205-212.
FISHER, N.S., GRAHAM, L.B., & CARPENTER, E.J. (1973) Geographic
differences in phytoplankton sensitivity to PCBs. Nature (Lond.),
241: 548-549.
FISHER, N.S., REMSEN, C.C., WURSTER, C.R., & CARPENTER, E.J. (1974)
Effects of PCB on interspecific competition in natural and
gnotobiotic phytoplankton communities in continuous and batch
cultures. Microbiol. Ecol., 1: 39-50.
FISHER, N.S., GUILLARD, R.R., & WURSTER, C.R. (1976) Effects of a
chlorinated hydrocarbon pollutant on the growth kinetics of a
marine diatom. In: Modeling biochemical processes in aquatic
ecosystems, Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp. 305-317.
FISHER, J.B., PETTY, R.L., & LUCK, W. (1983) Release of
polychlorinated biphenyls from contaminated lake sediments: Flux
and apparent diffusivities of four individual PCBs. Environ.
Pollut., B5: 121-132.
FITZGERALD, E.F., WEINSTEIN, A.L., YOUNGBLOOD, L.G., STANDFAST,
S.J., & MELIUS, J.M. (1989) Health effects three years after
potential exposure to the toxic contaminants of an electrical
transformer fire. Arch. environ. Health, 44(4): 214-221.
FLICK, D.F., O'DELL, R.G., & CHILDS, V.A. (1965) Studies of the
chick oedema disease. 3. Similarity of symptoms produced by feeding
chlorinated biphenyl. Poult. Sci., 44: 1460-1465.
FOCARDI, S. & ROMEI, R. (1987) Fingerprint of polychlorinated
biphenyl congeners in samples of human subcutaneous adipose tissue.
Chemosphere, 16(10/12): 2315-2320.
FOCARDI, S., FOSSI, C., LEONZIO, C., & ROMEI, R. (1986) PCB
congeners, hexachlorobenzene, and organochlorine insecticides in
human fat in Italy. Bull. environ. Contam. Toxicol., 36: 644-650.
FOCARDI, S., LEONZIO, C., & FOSSI, C. (1988) Variations in
polychlorinated biphenyl congener composition in eggs of
Mediterranean water birds in relation to their position in the food
chain. Environ. Pollut., 52: 243-255.
FOLMAR, L.C., DICKHOFF, W.W., ZAUGG, W.S., & HODGKINS, H.O. (1982)
The effects of Aroclor 1254 and No. 2 fuel oil on smoltification
and sea-water adaptation of Coho salmon (Oncorhynchus kisutch).
Aquat. Toxicol., 2: 291-299.
FOOKEN, C. & BUTTE, W. (1987) Organochlorine pesticides and
polychlorinated biphenyls in human milk during lactation.
Chemosphere, 16(6): 1301-1309.
FOREMAN, W.T. & BIDLEMAN, T.F. (1985) Vapor pressure estimates of
individual polychlorinated biphenyls and commercial fluids using
gas chromatographic retention data. J. Chromatogr., 330: 203-216.
FORGUE, S.T., PRESTON, B.D., HARGRAVES, W.A., REICH, I.L. & ALLEN,
J.R. (1980) Direct evidence that an arene oxide is a metabolic
intermediate of 2,2',5,5'-tetrachlorobiphenyl. In: Abstracts of the
Nineteenth Annual Meeting of the Society of Toxicology (Abstract
No. 383).
FOWLER, S.W., POLIKARPOV, G.G., ELDER, D.L., PARSI, P., &
VILLENEUVE, J.P. (1978) Polychlorinated biphenyls: Accumulation
from contaminated sediments and water by the polychaete Nereis
diversicolor. Mar. Biol., 48: 303-309.
FRANK, R., RONALD, K., & BRAUN, H.E. (1973) Organochlorine residues
in harp seals (Pagophilus groenlandicus) caught in Eastern
Canadian waters. J. Fish Res. Board Can., 30: 1053-1063.
FRANK, R., HOLDRINET, M.V.H., & RAPLEY, W.A. (1975) Residue of
organochlorine compounds and mercury in birds' eggs from the
Niagara Peninsula, Ontario. Arch. environ. Contam. Toxicol.,
3: 205-218.
FRANK, R., HOLDRINET, M., BRAUN, H.E., DODGE, D.P., & SPRANGLER,
G.E. (1978) Residues of organochlorine insecticides and
polychlorinated biphenyls in fish from Lakes Huron and Superior,
Canada, 1968-76. Pestic. monit. J., 12: 60-68.
FRANK, R., RASPER, J., SMOUT, M.S., & BRAUN, H.E. (1988)
Organochlorine residues in adipose tissues, blood and milk from
Ontario residents, 1976-1985. Can. J. public Health, 79: 150-158.
FRANKLIN, A. (1987) The concentration of metals, organochlorine
pesticide and PCB residues in marine fish and shellfish: Results
from MAFF fish and shellfish monitoring programmes, 1977-1984,
London, Ministry of Agriculture, Fisheries and Food, Directorate of
Fisheries Research (Aquatic Environment Monitoring Report No. 16).
FREEMAN, H.C., SANGALANG, G., & FLEMMING, B. (1982) The sublethal
effects of a polychlorinated biphenyl (Aroclor 1254) diet on the
Atlantic cod (Gadus morhua). Sci. total Environ., 24: 1-11.
FRENCH, J.E. (1976) The effect of the chlorinated polycyclic
hydrocarbons, p,p'-DDT and polychlorinated biphenyls (PCBs) on the
flagellated protozoan, Crithidia fasciculata. Diss. Abstr. Int.
Sci. Eng., B37: 96-97.
FREUDENTHAL, J. & GREVE, P.A. (1973) Polychlorinated terphenyls in
the environment. Bull. environ. Contam. Toxicol., 10: 108-111.
FRIEND M. & TRAINER, D.O. (1970) Polychlorinated biphenyl:
interaction with duck hepatitis virus. Science, 170: 1314-1316.
FRIES, G.F. (1972) Degradation of chlorinated hydrocarbons under
anaerobic conditions. Adv. Chem. Ser., 111: 256-270.
FRIES, G.F. & MARROW, G.S. (1981) Chlorobiphenyl movement from soil
to soybean plants. J. agric. food Chem., 29: 757-759.
FRIES, G.F., MARROW, G.S., Jr, & GORDON, C.H. (1973) Long-term
studies of residue retention and excretion by cows fed a
polychlorinated biphenyl (Aroclor 1254). J. agric. food Chem.,
21: 117-121.
FU, Y.A. (1984) Ocular manifestation of polychlorinated biphenyls
intoxication. Am. J. ind. Med., 5: 127-132.
FUJIWARA, K. (1975) Environmental and food contamination with PCB's
in Japan. Sci. total Environ., 4: 219-247.
FUJIWARA, M. & KURIYAMA, K. (1977) Effect of PCB (polychloro-
biphenyls) on L-ascorbic acid, pyridoxal phosphate and riboflavin
contents in various organs and on hepatic metabolism of L-ascorbic
acid in the rat. Jpn. J. Pharmacol., 27: 621-627.
FUKADA, K., INUYAMA, Y., TAKESHITA, T., & YAMAMOTO, S. (1973)
[Present state of environmental pollution by PCB in the Shimane
Prefecture.] Shimare Igaku, 5: 1-25 (in Japanese).
FUKANO, S. & DOGUCHI, M. (1977) PCT, PCB and pesticide residues in
human fat and blood. Bull. environ. Contam. Toxicol.,
17(5): 613-617.
FUKANO, S., USHIO, F., & DOGUCHI, M. (1974) [PCB, PCT and pesticide
residues in fish collected from the Tama river.] Annu. Rep. Tokyo
Metrop. Res. Lab. P.H., 25: 297-305 (in Japanese).
FUKUSHIMA, M. & KAWAI, S. (1981) Variation of organochlorine
concentration and burden in striped dolphin (Stenella
coeruleoalba) with growth. In: Fujiyama, T., ed. Studies on the
levels of organochlorine compounds and heavy metals in the marine
organisms, Ryukyus, University of Ryukyus, pp. 97-114.
FULLER, G.B., KNAUF, V., MUELLER, W., & HOBSON, W.C. (1980) PCB
augments LH induced progesteron synthesis. Bull. environ. Contam.
Toxicol., 25: 65-68.
FUNATSU, I., YAMASHITA, F., ITO, Y., TZUGAWA, S., FUNATSU, T.,
YOSHIKANE, T., HAYASHI, M., KATO, T., YAKUSHIJI, M., OKAMOTO, G.,
YAMASAKI, S., ARIMA, T., KUNO, T., IDE, H., & IBE, I. (1972) PCB
induced fetopathy. I. Clinical observation. Kurume med. J.,
919: 43-51.
FURUKAWA, K., TONOMURA, K., & KAMIBAYASHI, A. (1978a) Effect of
chlorine substitution on the biodegradability of polychlorinated
biphenyls. Appl. environ. Microbiol., 35: 223-227.
FURUKAWA, K., TOMIZUKA, N., & KAMIBAYASHI, A. (1978b) Effect of
chlorine substitution on the bacterial metabolism of various
polychlorinated biphenyls. Appl. environ. Microbiol., 38: 301-310.
GAGE, J.C. & HOLM, S. (1976) The influence of molecular structure
on the retention and excretion of polychlorinated biphenyls by the
mouse. Toxicol. appl. Pharmacol., 36: 555-560.
GALLENBERG, L.A. & VODICNIK, M.J. (1987) Potential mechanisms for
redistribution of polychlorinated biphenyls during pregnancy and
lactation. Xenobiotica, 17(3): 299-310.
GALLENBERG, L.A., RING, B.J., & VODICNIK, M.J. (1987) Influence of
lipolysis on the mobilization of 2,4,5,2',4',5'-hexachlorobiphenyl
from adipocytes in vitro. J. Toxicol. environ. Health,
20: 163-171.
GARDNER, A.M., RICHTER, H.F., & ROACH, J.A.G. (1976) Excretion of
hydroxylated polychlorinated biphenyl metabolites in cow's milk. J.
Assoc. Off. Anal. Chem., 59: 273-277.
GARTHOFF, L.H., FRIEDMAN, L., FARBER, T.M., LOCKE, K.K., SOBOTKA,
T.J., GREEN, S., HURLEY, N.E., PETERS, E.L., STORY, G.E., MORELAND,
F.M., GRAHAM, C.H., KEYS, J.E., TAYLOR, M.J., SCALERA, J.V.,
ROTHLEIN, J.E., MARKS, E.M., CERRA, F.E., RODI, S.B., & SPORN, E.M.
(1977) Biochemical and cytogenetic effects in rats caused by
short-term ingestion of Aroclor 1254 or Firemaster BP6. J. Toxicol.
environ. Health, 3: 769-796.
GARTHOFF, L.H., CERRA, F.E., & MARKS, E.M. (1981) Blood chemistry
alterations in rats after single and multiple gavage administration
of polychlorinated biphenyl. Toxicol. appl. Pharmacol., 60: 33-44.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985) Pesticides, selected elements and other chemicals in adult
total diet samples, October 1979-September 1980. J. Assoc. Off.
Anal. Chem., 68(6): 1184-1195.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements and other chemicals in adult
total diet samples, October 1980-March 1982. J. Assoc. Off. Anal.
Chem., 69(1): 146-161.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements and other chemicals in infant
and toddler total diet samples, October 1980-March 1982. J. Assoc.
Off. Anal. Chem., 69(1): 123-145.
GASKIN, D.E., FRANK, R., & HOLDRINET, M. (1983) Polychlorinated
biphenyls in harbor porpoises Phocoena phocoena (L.) from the Bay
of Fundy, Canada and adjacent waters, with some information on
chlordane and hexachlorobenzene levels. Arch. environ. Contam.
Toxicol., 12: 211-219.
GELLERT, R.J. (1978) Uterotropic activity of polychlorinated
biphenyls (PCB) and induction of precocious reproductive aging in
neonatally treated female rats. Environ. Res., 16: 123-130.
GELLERT, R.J. & WILSON, C. (1979) Reproductive function in rats
exposed prenatally to pesticides and polychlorinated biphenyls
(PCBs). Environ. Res., 18: 437-443.
GEZONDHEIDSRAAD (1985) [First recommendation on the quality of
breastmilk. Contamination of breastmilk with polychlorinated
biphenyls (PCBs). Recommendation to the Minister and the Secretary
of State for Welfare, Public Health and Culture], Rijswijk, Public
Health Council of the Netherlands (in Dutch).
GIAM, C.S., CHAN, H.S., NEFF, G.S., & ATLAS, E.L. (1978) Phthalate
ester plasticizers: A new class of marine pollutant. Science,
199: 419-420.
GIAM, C.S., ATLAS, E., CHAN, H.S., & NEFF, G. (1980) Phthalate
esters, PCB and DDT residues in the Gulf of Mexico atmosphere.
Atmos. Environ., 14: 65-69.
GIBSON, D.T., ROBERTS, R.L., WELLS, M.C., & KOBAL, V.M. (1973)
Oxidation of biphenyl by a Beijerinckia species. Biochem.
biophys. Res. Commun., 50: 211-219.
GILBERTSON, M. & FOX, G.A. (1977) Pollutant-associated embryonic
mortality of Great Lakes herring gulls. Environ. Pollut.,
12: 211-216.
GILBERTSON, M. & HALE, R. (1974) Characteristics of the breeding
failure of a colony of herring gulls in Lake Ontario. Can.
Field-Nat., 88: 356-358.
GILBERTSON, M., MORRIS, R.D., & HUNTER, R.A. (1976) Abnormal chicks
and PCB residue levels in eggs of colonial birds on the lower Great
Lakes (1971-73). Auk, 93: 434-442.
GLADEN, B.C., ROGAN, W.J., RAGAN, N.B., & SPIERTO, F.W. (1988a)
Urinary porphyrins in children exposed transplacentally to
polyhalogenated aromatics in Taiwan. Arch. environ. Health,
43(1): 54-58.
GLADEN, B.C., ROGAN, W.J., HARDY, P., THULLEN, J., TINGELSTAD, J.,
& TULLY, M. (1988b) Development after exposure to polychlorinated
biphenyls and dichlorodiphenyl dichloroethane transplacentally and
through human milk. J. Pediatr., 113(6): 991-995.
GLOOSCHENKO, V. & GLOOSCHENKO, W. (1975) Effect of polychlorinated
biphenyl compounds on growth of great lakes phytoplankton. Can. J.
Bot., 53: 653-659.
GLOOSHENKO, W.A., STRACHAN, W.M., & SAMPSON, R.C.J. (1976) Residues
in water distribution of pesticides and polychlorinated biphenyls
in water, sediments and session of the Upper Great Lakes. Pestic.
monit. J., 10: 61-67.
GOLDSTEIN, J.A. (1980) Structure-activity relationships for the
biochemical effects and the relationships to toxicity. In:
Kimbrough, R.D., ed. Halogenated biphenyls, terphenyls,
naphthalenes, dibenzodioxins and related products, Amsterdam,
Elsevier/North-Holland Biomedical Press, pp. 151-190 (Topics in
Environmental Health).
GOLDSTEIN, J.A., HICKMAN, P., & JUE, D.L. (1974) Experimental
hepatic porphyria induced by polychlorinated biphenyls. Toxicol.
appl. Pharmacol., 27: 437-448.
GOLDSTEIN, J.A., HICKMAN, P., BURSE, V.W., & BERGMAN, H. (1975) A
comparative study of two polychlorinated biphenyl mixtures
(Aroclors 1242 and 1016) containing 42% chlorine on induction of
hepatic porphyria and drug metabolizing enzymes. Toxicol. appl.
Pharmacol., 32: 461-473.
GOLDSTEIN, J.A., HICKMAN, P., BERGMAN, H., MCKINNEY, J.D., &
WALKER, M.P. (1977) Separation of pure polychlorinated biphenyl
isomers into two types of inducers on the basis of induction of
cytochrome P-450 or P-448. Chem.-biol. Interact., 17: 69-87.
GORCHEV, H.G. & JELINEK, C.F. (1985) A review of the dietary
intakes of chemical contaminants. Bull. World Health Organ.,
63(5): 945-962.
GOTO, M. & HIGUCHI, K. (1969) The symptomatology of Yusho
(chlorobiphenyls poisoning), in dermatology. Fuoka Act. Med.,
60: 409-431.
GOTO, M., SUGIURA, K., HATTORI, M., MIYAGAWA, T., & OKAMURA, M.
(1973) Hydroxylation of dichlorobiphenyls in rats. In: New
collection of papers presented at the Research Conference on New
Methodology in Ecological Chemistry, Susono, Japan, 23-25 November,
Tokyo, International Academic Printing Co. Ltd, pp. 299-302.
GOTO, M., SUGIURA, K., HATTORI, M., MIYAGAWA, T., & OKAMURA, M.
(1974) Metabolism of 2,3-dichlorobiphenyl-14C and
2,3,6-trichlorobiphenyl-14C in the rat. Chemosphere, 3: 227-232.
GOTO, M., HATTORI, M., & SUGIURA, K. (1975) Metabolism of
pentachloro- and hexachlorobiphenyls in the rat. Chemosphere,
4: 177-180.
GRANT, D.L. & PHILLIPS, W.E.J. (1974) The effect of age and sex on
the toxicity of Aroclor 1254, a polychlorinated biphenyl, in the
rat. Bull. environ. Contam. Toxicol., 12(2): 145-152.
GRANT, D.L., PHILLIPS, W.E.J., & VILLENEUVE, D.C. (1971a)
Metabolism of polychlorinated biphenyl (Aroclor 1254) mixture in
the rat. Bull. environ. Contam. Toxicol., 6: 102-112.
GRANT, D.L., VILLENEUVE, D.C., MCCULLY, K.A., & PHILLIPS, W.E.J.
(1971b) Placental transfer of polychlorinated biphenyls in the
rabbit. Environ. Physiol., 1: 61-66.
GRANT, D.L., MOODIE, C.A., & PHILLIPS, W.E.J. (1974) Toxicodynamics
of Aroclor 1254 in the male rat. Environ. Physiol. Biochem.,
4: 214-225.
GREB, W., KLEIN, W., COULSTON, F., GOLBERG, L., & KORTE, F. (1975)
Metabolism of lower polychlorinated biphenyls-C-14 in the Rhesus
monkey. Bull. environ. Contam. Toxicol., 13: 471-76.
GREEN, S., CARR, J.V., PALMER, K.A., & OSWALD, E.J. (1975a) Lack of
cytogenetic effects in bone marrow and spermatogonial cells in rats
treated with polychlorinated biphenyls (Aroclors 1242 and 1254).
Bull. environ. Contam. Toxicol., 13: 14-22.
GREEN, S., SAURO, F.M., & FRIEDMAN, L. (1975b) Lack of dominant
lethality in rats treated with polychlorinated biphenyls (Aroclors
1242 and 1254). Food Cosmet. Toxicol., 13: 507-510.
GREICHUS, Y.A., CALL, D.J., & AMMANN, B.M. (1975) Physiological
effects of polychlorinated biphenyls or a combination of DDT, DDD,
and DDE in penned white Pelicans. Arch. environ. Contam. Toxicol.,
3: 330-343.
GREIG, R.A. & SENNEFELDER, G. (1987) PCB concentration in winter
flounder from Long Island Sound, 1984-1986. Bull. environ. Contam.
Toxicol., 39(5): 863-868.
GREVE, P.A. & VAN HULST, S.J. (1977) [Organochlorine pesticides and
PCBs in total diets], Bilthoven, The Netherlands, National
Institute of Public Health (Report No. 192/77 Tox-Rob) (in Dutch).
GREVE, P.A. & VAN HARTEN, D.C. (1983a) [Organochlorine pesticides
and polychlorobiphenyls in the fatty tissue of the Dutch population
(period 1980).] Bilthoven, The Netherlands, National Institute of
Public Health (Report No. 638205001) (in Dutch).
GREVE, P.A. & VAN HARTEN, D.C. (1983b) [Association between
organochlorine pesticide and PCB contents in fat and blood in man],
Bilthoven, The Netherlands, National Institute of Public Health
(Report No. 638219001) (in Dutch).
GREVE, P.A. & WEGMAN, R.C.C. (1983) PCB residues in animal fats,
human tissues, duplicate 24-hours' diets, eel and sediments. In:
Barros, M.C., Könemann, H., & Visser, R., ed. Proceedings of the
PCB Seminar, The Hague, 28-30 September 1983, The Hague, Ministry
of the Environment, pp. 54-66.
GREVE, P.A. & WEGMAN, R.C.C. (1984) Organochlorine compounds in
human milk: data from a recent investigation in the Netherlands.
In: Report on the WHO Consultation on Organochlorine Compounds in
Human Milk and Related Hazards, Bilthoven, 9-11 January 1985,
Copenhagen World Health Organization, Regional Office for Europe,
Annex 8.
GREVE, P.A., VAN HARTEN, D.C., HEUSINKVELD, H.A.G., LEUSSINK, A.B.,
& VERSCHRAAGEN, C. (1985) [Chemical contaminants in breastmilk.
Section 2: PCBs (determination of total)], Bilthoven, The
Netherlands, National Institute of Public Health and Environmental
Hygiene (Report No. 638307002) (in Dutch).
GROLIER, P., CASSAND, P., ANTIGNAC, E., NARBONNE, J.F., ALBRECHT,
R., AZAIS, V., ROBERTSON, L.W., & OESCH, F. (1989) Effects of
prototypic PCBs on benzo(a)pyrene mutagenic activity related to
vitamin A intake. Mutat. Res., 211: 139-145.
GROTE, W., SCHMOLDT, A., & BENTHE, H.F. (1975) Hepatic porphyrin
synthesis in rat after pretreatment with polychlorinated biphenyls.
Acta. pharmacol. toxicol., 36: 215-224.
GRUGER, E.H., KARRICK, N.L., DAVIDSON, A.I., & HRUBY, T. (1975)
Accumulation of 3,4,3',4'-tetrachlorobiphenyl and 2,4,5,2',4',5'-,
and 2,4,6,2',4',6'-hexachlorobiphenyl in juvenile coho salmon.
Environ. Sci. Technol., 9: 121-127.
GRUGER, E.H., HRUBY, T., & KARRICK, N.L. (1976) Sublethal effects
of structurally related tetrachloro-, pentachloro-, and
hexachlorobiphenyl on juvenile Coho Salmon. Environ. Sci. Technol.,
10: 1033-1037.
GUINEY, P.D. & PETERSON, R.E. (1980) Distribution and elimination
of a polychlorinated biphenyl after dietary exposure in yellow
perch and rainbow trout. Arch. environ. Contam. Toxicol.,
9: 667-674.
GUINEY, P.D., PETERSON, R.E., MELANCON, M.J., & LECH, J.J. (1977)
The distribution and elimination of 2,5,2,5-[14C]Tetrachloro-
biphenyl in Rainbow Trout (Salmo gairdneri). Toxicol. appl.
Pharmacol., 39: 329-338.
GUINEY, P.D., MELANCON, M.J., LECH, J.J., & PETERSON, R.E. (1979)
Effects of egg and sperm maturation and spawning on the
distribution and elimination of a polychlorinated biphenyl in
rainbow Trout (Salmo gairdneri). Toxicol. appl. Pharmacol.,
47: 261-272.
GUNDERSON, E.L. (1988b) FDA total diet study, April 1982-April
1984, dietary intakes of pesticides, selected elements and other
chemicals. J. Assoc. Off. Anal. Chem., 71(6): 1200-1209.
GUO, Y.L., EMMETT, E.A., PELLIZZARI, E.D., & ROHDE, C.A. (1987)
Influence of serum cholesterol and albumine on partitioning of PCB
congeners between human serum and adipose tissue. Toxicol. appl.
Pharmacol., 87: 48-56.
GUOTH, J., KACHMAR, P., TELEHA, M., & VASIL, M. (1984) [The
influence of polychlorinated biphenyls (PCB) on the activity of
liver aniline hydroxylase and on some metabolic parameters in pig
blood.] Vet. Med. (Prague), 29: 29-38 (in Czech).
GUSTAVSSON, P., HOGSTEDT, C., & RAPPE, C. (1986) Short-term
mortality and cancer incidence in capacitor manufacturing workers
exposed to polychlorinated biphenyls (PCBs). Am. J. ind. Med.,
10: 341-344.
GYORKOS, J., DENOMME, M.A., LEECE, B., HOMONKO, K., VALLI, V.E., &
SAFE, S. (1985) Reconstituted halogenated hydrocarbon pesticide and
pollutant mixtures found in human tissues: effects on the immature
male Wistar rat after short-term exposure. Can. J. Physiol.
Pharmacol., 63(1): 36-43.
HAAHTI, H. & PERTTILA, M. (1988) Levels and trends of
organochlorines in Cod and Herring in the Northern Baltic. Mar.
Pollut. Bull., 19: 29-32.
HAAKE, J.M., SAFE, S., MAYURA, K., & PHILLIPS, T.D. (1987) Aroclor
1254 as an antagonist of the teratogenicity of 2,3,7,8-tetrachloro-
dibenzo- p-dioxin. Toxicol. Lett., 38: 299-306.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
Coturnix. Bull. environ. Contam. Toxicol., 11: 98-101.
HALTER, M.T. & JOHNSON, H.E. (1974) Acute toxicities of a
polychlorinated biphenyl (PCB) and DDT alone and in combination to
early life stages of Coho salmon (Oncorhynchus kisutch). J. Fish
Res. Board Can., 31: 1543-1547.
HALVERSON, M.R., PHILLIPS, T.D., SAFE, S.H., & ROBERTSON, L.W.
(1985) Metabolism of aflatoxin B1 by rat hepatic microsomes induced
by polyhalogenated biphenyl congeners. Appl. environ. Microbiol.,
49: 882-886.
HAMMOND, A.L. (1972) Chemical pollution: Polychlorinated biphenyls.
Science, 175: 155-156.
HANSEN, D.J., PARRISH, P.R., LOWE, J.I., & WILSON, A.J. (1971)
Chronic toxicity, uptake, and retention of Aroclor 1254 in two
estuarine fishes. Bull. environ. Contam. Toxicol., 6: 113-119.
HANSEN, D.J. (1974) Aroclor 1254: Effect on composition of
developing estuarine animal communities in the laboratory. Contrib.
mar. Sci., 18: 19-33.
HANSEN, D.J., SCHIMMEL, S.C., & MATTHEWS, E. (1974a) Avoidance of
Aroclor 1254 by shrimp and fishes. Bull. environ. Contam. Toxicol.,
12: 253-256.
HANSEN, D.J., PARRISH, P.R., & FORESTER, J. (1974b) Aroclor 1016:
Toxicity to and uptake by estuarine animals. Environ. Res.,
7: 363-373.
HANSEN, D.J., SCHIMMEL, S.C., & FORESTER, J. (1975) Effects of
Aroclor 1016 on embryos, fry, juveniles, and adults of sheepshead
minnow (Cyprinodon variegatus). Trans. Am. Fish Soc.,
104: 584-588.
HANSEN, L.G., WIEKHORST, W.B., & SIMON, J. (1976a) Effects of
dietary Aroclor 1242 on channel catfish (Ictalurus punctatus) and
the selective accumulation of PCB components. J. Fish Res. Board
Can., 33: 1343-1352.
HANSEN, L.G., WILSON, D.W., & BYERLY, C.S. (1976b) Effects on
growing swine and sheep of two polychlorinated biphenyls. Am. J.
vet. Res., 37: 1021-1024.
HANSEN, L.G., WASHKO, P.W., TUINSTRA, L.G.M.TH., DORN, S.B., &
HINESLY, T.D. (1981) Polychlorinated biphenyl, pesticide, and heavy
metal residues in swine foraging on sewage sludge amended soils. J.
agric. food Chem., 29: 1012-1017.
HAQUE, R. & SCHMEDDING, D.W. (1975) A method of measuring the water
solubility of hydrophobic chemicals: Solubility of five
polychlorinated biphenyls. Bull. environ. Contam. Toxicol.,
14: 13-18.
HAQUE, R., SCHMEDDING, D.W., & FREED, V.H. (1974) Aqueous
solubility adsorption and vapor behaviour of polychlorinated
biphenyl Aroclor 1254. Environ. Sci. Technol., 8: 139-142.
HARA, I. (1985) Health status and PCBs in blood of workers exposed
to PCBs and their children. Environ. health Perspect., 59: 85-90.
HARA, I., HARADA, H., KIMURA, S., ENDO, T., & KAWANO, K. (1974)
[Follow-up health examination in an electric condenser factory
after cessation of PCBs usage (1st report).] Jpn. J. ind. Health,
16: 365-366 (in Japanese).
HARBISON, R.D., JAMES, R.C., & ROBERTS, S.M. (1987) Biological data
relevant to the evaluation of carcinogenic risk to humans, Little
Rock, Arkansas, University of Arkansas, Division of
Interdisciplinary Toxicology, (Prepared for Scientific Advisory
Panel, Safe Drinking Water and Toxic Enforcement Act, State of
California).
HARDING, L.W. & PHILLIPS, J.H. (1978a) Polychlorinated biphenyl
(PCB) effects on marine phytoplankton photosynthesis and cell
division. Mar. Biol., 49: 93-101.
HARDING, L.W. & PHILLIPS, J.H. (1978b) Polychlorinated biphenyl
(PCB) uptake by marine phytoplankton. Mar. Biol., 49: 103-111.
HARDWICK, J.P., LINKO, P., & GOLDSTEIN, J.A. (1985) Dose response
for induction of two cytochrome P-450 isoenzymes and their mRNAs by
3,4,5,3',4',5'-hexachlorobiphenyl indicating coordinate regulation
in rat liver. Mol. Pharmacol., 27: 676-682.
HARRIS, M.P. & OSBORN, D. (1981) Effect of a polychlorinated
biphenyl on the survival and breeding of puffins. J. appl. Ecol.,
18: 471-479.
HARRIS, J.R. & ROSE, L. (1972) Toxicity of polychlorinated
biphenyls in poultry. J. Am. Vet. Med. Assoc., 161: 1584-1586.
HARVEY, G.R. & STEINHAUER, W.G. (1974) Atmospheric transport of
polychlorobiphenyls to the North Atlantic. Atmos. Environ.,
8: 777-782.
HARVEY, G.R., STEINHAUER, W.G., & TEAL, J.M. (1973)
Polychlorobiphenyls in North Atlantic Ocean water. Science,
180: 643-644.
HASEGAWA, M. (1973) [Studies on the wild animals as
"Contamination-index". Part 1 - The effects of PCB on frog tadpoles
(Rana chensinensis).] Hokkaidoritsu Eisei Kenkyushoho, 23: 6-9
(in Japanese).
HASEGAWA, H., SATO, M., & TSURUTA, H. (1972a) [PCB concentration in
the blood of workers handling PCB.] Occup. Health, 13(10): 50-55
(in Japanese).
HASEGAWA, H., SATO, M., & TSURUTA, H. (1972b) [PCB concentration in
air of PCB-using plants and health examination of workers.] In:
[Report on special research on prevention of environmental
pollution by PCB-like substances], Tokyo, Science and Technology
Agency, Research Co-ordination Bureau, pp. 141-149 (in Japanese).
HASELTINE, S.D. & PROUTY, R.M. (1980) Aroclor 1242 and reproductive
success of adult mallards (Anas platyrhynchos). Environ. Res.,
23: 29-34.
HASELTINE, S.D., HEINZ, G.H., REICHEL, W.L., & MOORE, J.F. (1981)
Organochlorine and metal residues in eggs of wildfowl nesting on
islands in Lake Michigan off Door county, Wisconsin, 1977-78.
Pestic. monit. J., 15: 90-97.
HASHIMOTO, K., AKASAKA, S., TAKAGI, Y., KATAOKA, M., OTAKA, T.,
MURATA, Y., ABURADA, S., KITAURA, T., & UDA, H. (1976) Distribution
and excretion of (14C)polychlorinated biphenyls after their
prolonged administration to male rats. Toxicol. appl. Pharmacol.,
37: 415-423.
HASSELL, K.D. & HOLMES, D.C. (1977) Polychlorinated terphenyls
(PCT) in some British birds. Bull. environ. Contam. Toxicol.,
17: 618-621.
HATCH, W.I. & ALLEN, D.W. (1979) Alterations in calcium
accumulation behavior in response to calcium availability and
polychlorinated biphenyl administration. Bull. environ. Contam.
Toxicol., 22: 172-174.
HATTULA, M.L. (1985) Mutagenicity of PCBs and their pyrosynthetic
derivatives in cell-mediated assay. Environ. health Perspect.,
60: 255-257.
HATTULA, M.L. & KARLOG, O. (1973) Absorption and elimination of
polychlorinated biphenyls (PCB) in goldfish. Acta pharmacol.
toxicol., 32: 237-245.
HAWES, M.L., KRICHER, J.C., & UREY, J.C. (1976a) The effects of
various Aroclor fractions on the population growth of Chlorella
pyrenoidosa. Bull. environ. Contam. Toxicol., 15: 14-18.
HAWES, M.L., KRICHER, J.C., & UREY, J.C. (1976b) The effects of
various Aroclor fractions on the productivity of Chlorella
pyrenoidosa. Bull. environ. Contam. Toxicol., 15: 588-590.
HAWKER, D.W. (1989) Vapour pressures and Henry's Law Constants of
polychlorinated biphenyls. Environ. Sci. Technol., 23: 1250-1253.
HAWKER, D.W. & CONNELL, D.W. (1988) Octanol-water partition
coefficients of polychlorinated biphenyl congeners. Environ. Sci.
Technol., 22: 382-387.
HAYES, M.A., SAFE, S.H., ARMSTRONG, D., & CAMERON, R.G. (1985)
Influence of cell proliferation on initiating activity of pure
polychlorinated biphenyls and complex mixtures in resistant
hepatocyte in vivo assays for carcinogenicity. J. Natl Cancer
Inst., 74: 1037-1041.
HAYES, M.A., ROBERTS, E., SAFE, S.H., FARBER, E., & CAMERON, R.G.
(1986) Influences of different polychlorinated biphenyls on
cytocidal, mitiinhibitory and nodule-selecting activities of
N-2-fluorenylacetamide in rat liver. J. Natl Cancer Inst.,
76: 683-691.
HAYES, M.A. (1987) Carcinogenic and mutagenic effects of PCBs. In:
Safe, S., ed. Polychlorinated biphenyls (PCBs): Mammalian and
environmental toxicology, Berlin, Heidelberg, New York,
Springer-Verlag, pp. 77-95 (Environmental Toxin Series, Vol. I).
HAYS, H. & RISEBROUGH, R.W. (1972) Pollutant concentrations in
abnormal young terns from Long Island Sound. Auk, 89: 19-35.
HEATH, R.G., SPANN, J.W., KREITZER, J.F., & VANCE, C. (1972)
Effects of polychlorinated biphenyls on birds. In: Proceedings of
the XVth International Ornithological Congress, The Hague, 30
August-5 September 1970, pp. 475-485.
HEDDLE, J.A. & BRUCE, W.R. (1977) Comparison of tests for
mutagenicity or carcinogenicity using assays for sperm
abnormalities, formation of micronuclei and mutation in Salmonella.
In: Origins of human cancer, Book C: Human risk assessment, Section
16. Short-term assays - Predictive value, Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp. 1549-1557.
HEESCHEN, W., BLUTHGEN, A., & NIJHUIS, H. (1986) [Milk hygiene:
Trends in the residue situation.] Kieler Milchwirtsch
Forschungsber., 38(2): 131-145 (in German).
HEINZ, G.H., HILL, E.F., & CONTERA, J.F. (1980) Dopamine and
norepinephrine depletion in ring doves fed DDE, dieldrin, and
Aroclor 1254. Toxicol. appl. Pharmacol., 53: 75-82.
HEINZ, G.H., HASELTINE, S.D., REICHEL, W.L., & HENSLER, G.L. (1983)
Relationships of environmental contaminants to reproductive success
in red-breasted mergansers Mergus serrator from Lake Michigan.
Environ. Pollut., 32: 211-232.
HEINZOW, B.G.J., TINNEBERG, H.R., & BOIE, C. (1988) Effects of some
lipophilic xenobiotics on T-lymphocytes using a modified
erythrocyte rosette inhibition test. Res. Commun. chem. Patho.
Pharmacol., 61(2): 277-280.
HEIRONIMUS, M.P., LAUGHLIN, N.K., & BOWMAN, R.E. (1981) Effects of
early exposure to PCBs on learned irrelevancy of cues in Rhesus
monkeys. An incidental learning paradigm. Teratology, 24: 55A.
HELLE, E., OLSSON, M., & JENSEN, S. (1976a) DDT and PCB levels and
reproduction in ringed seals from the Bothnian bay. Ambio,
5: 188-189.
HELLE, E., OLSSON, M., & JENSEN, S. (1976b) PCB levels correlated
with pathological changes in seal uteri. Ambio, 5: 261-263.
HELLE, E., HYVARINEN, H., PYYSALO, H., & WICKSTROM, K. (1983)
Levels of organochlorine compounds in an inland seal population in
Eastern Finland. Mar. Pollut. Bull., 14: 256-260.
HENDRICKS, D., PUTNAM, T.P., BILLS, D.D., & SINNHUBER, R.O. (1977)
Inhibitory effect of a polychlorinated biphenyl (Aroclor 1254) on
aflatoxin B1 carcinogenesis in rainbow trout (Salmo gairdneri).
J. Natl Cancer Inst., 59: 1545-1551.
HERRING, J.L., HANNAN, E.J., & BILLS, D.D. (1972) UV irradiation of
Aroclor 1254. Bull. environ. Contam. Toxicol., 8: 153-157.
HESSELBERG, R.J. & SCHERR, D.D. (1974) PCBs and p,p' DDE in the
blood of cachectic patients. Bull. environ. Contam. Toxicol.,
11: 202-205.
HILL, H.R., Jr, (1985) Effects of polyhalogenated aromatic
compounds on porphyrin metabolism. Environ. health Perspect.,
60: 139-143.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington,
DC, US Department of the Interior, Fish and Wildlife Service, 147
pp (Fish and Wildlife Technical Report No. 2).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1974)
Polychlorinated biphenyl toxicity to Japanese quail as related to
degree of chlorination. Poult. Sci., 53: 597-604.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 61 pp (Special Scientific Report No. 191).
HILL, E.F., HEATH, R.G., & WILLIAMS, J.D. (1976) Effect of dieldrin
and Aroclor 1242 on Japanese quail eggshell thickness. Bull.
environ. Contam. Toxicol., 16: 445-453.
HINTON, D.E., GLAUMANN, H., & TRUMP, B.F. (1978) Studies on the
cellular toxicity of polychlorinated biphenyls (PCBs) I. Effect of
PCBs on microsomal enzymes and on synthesis and turnover of
microsomal and cytoplasmic lipids of rat liver - A morphological
and biochemical study. Virchows Arch. cell. Pathol., B27: 279-306.
HIRAYAMA, C., IRISA, T., & YAMAMOTO, T. (1969) Fine structural
changes of the liver in a patient with chlorobiphenyls
intoxication. Fukuoka Acta med., 60: 445-461.
HIRAYAMA, C., OKUMURA, M., NAGAI, J., & MASUDA, Y. (1974)
Hypobilirubinemia in patients with polychlorinated biphenyls
poisoning. Clin. Chim. Acta., 55: 97-100.
HIROSE, M., SHIRAI, T., TSUCA, H., FUKUSHIMA, S., OGISO, T., & ITO,
N. (1981) Effect of phenobarbital, polychlorinated biphenyl and
sodium saccharin on hepatic and renal carcinogenesis in
unilaterally nephrectomized rats given n-ethyl- N-hydroxy-
ethylnitrosamine orally. Carcinogenesis, 2: 1299-1302.
HLADKA, A., TAKACHOVA, T., & LISHKA, D. (1983) Exposure to
polychlorinated biphenyls and its effect on selected biochemical
functions. Czech. Med., 5: 8-14.
HODSON, K. (1975) Some aspects of the nesting ecology of
Richardson's merlin (Falco columbarius richardsonii) on the
Canadian prairies, Columbia, Vancouver, University of British
Columbia (MSc Thesis).
HOFFMAN, D.J., RATTNER, B.A., BUNCK, C.M., & KRYNITSKY, A. (1986)
Association between PCBs and lower embryonic weight in
black-crowned night herons in San Francisco Bay. J. Toxicol.
environ. Health, 19: 383-391.
HOGAN, J.W. & BRAUHN, J.L. (1975) Abnormal rainbow trout fry from
eggs containing high residues of a PCB (Aroclor 1242). Prog.
Fish-Cult., 37: 229-230.
HOLA, N. & REZNICEK, J. (1985) [The genetic hazard involved in
occupational exposure to high concentrations of polychlorinated
biphenyls.] Prac. Lek., 37: 386-391 (in Czech).
HOLDEN, A.V. (1970) Source of polychlorinated contamination in the
marine environment. Nature (Lond.), 228: 1220-1221.
HOLDEN, A.V. (1973) Monitoring PCBs in water and wildlife. In:
Proceedings of the Polychlorinated Biphenyl II Conference,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 23-33 (Publication No. 4E).
HOLDEN, A.V. & MARSDEN, K. (1969) Single-stage clean-up of animal
tissue extracts for organochlorine residue analysis. J.
Chromatogr., 44: 481-492.
HOLDGATE, M.W. (1971) The sea bird wreck in the Irish Sea. Autumn
1969, London, Natural Environment Research Council (Publication
Series C4).
HOLDRINET, M.V., BRAUN, H.E., FRANK, R., STOPPS, G.J., SMOUT, M.S.,
& MCWADE, J.W. (1977) Organochlorine residues in human adipose
tissue and milk from Ontario residents 1969-1974. Can. J. public
Health, 68: 74-80.
HOLLEMAN, K.A., BARNETT, B.D., & WICKER, G.W. (1976) Response of
chicks and turkey poults to Aroclor 1242. Poult. Sci.,
55: 2354-2356.
HOLT, R.L., CRUSE, S., & DREER, E.S. (1986) Pesticide and
polychlorinated biphenyl residues in human adipose tissue from
Northeast Louisiana. Bull. environ. Contam. Toxicol., 36: 651-655.
HOM, W., RISEBROUGH, R.W., SOUTAR, A., & YOUNG, D.R. (1974)
Deposition of DDE and polychlorinated biphenyls in dated sediments
of the Santa Barbara Basin. Science, 184: 1197-1199.
HONDA, T., NONAKA, S., MURAYAMA, F., OHGAMI, T., SHIMOYAMA, T., &
YOSHIDA, H. (1983) Effects of KC-400 (polychlorinated biphenyls) on
porphyrin metabolism. J. Dermatol., 10: 259-265.
HOOPINGARNER, R., SAMUEL, A., & KRAUSE, D. (1972) Polychlorinated
biphenyl interactions with tissue culture cells. Environ. health
Perspect., 1: 155-158.
HORI, S., OBANA, H., KASHIMOTO, T., OTAKE, T., NISHIMURA, H.,
IKEGAMI, N., KUNITA, N., & UDA, H. (1982) Effect of polychlorinated
biphenyls and polychlorinated quarterphenyls in Cynomolgus monkey
(Macaca fascicularis). Toxicology, 24: 123-139.
HORNSHAW, T.C., AULERICH, R.J., & JOHNSON, H.E. (1983) Feeding
great lakes fish to mink: Effects on mink and accumulation and
elimination of PCBs by mink. J. Toxicol. environ. Health,
11: 933-946.
HORNSHAW, T.C., SAFRONOFF, J., RINGER, R.K., & AULERICH, R.J.
(1986) LC50 test results in polychlorinated biphenyl-fed mink: Age,
season, and diet comparisons. Arch. Environ. Contam. Toxicol.,
15: 717-723.
HORZEMPA, L.M. & DI TORO, D.M. (1983) The extent of reversibility
of polychlorinated biphenyl adsorption. Water Res., 17: 851-859.
HSIA, M.T.S., LIN, F.S.D., & ALLEN, J.R. (1978) Comparative
mutagenicity and toxic effects of 2,2',5,5'-tetrachlorobiphenyl and
its metabolites in bacterial and mammalian test systems. Res.
Commun. chem. Pathol. Pharmacol., 21: 485-496.
HSU, I.C., VAN MILLER, J.P., SEYMOUR, J.L., & ALLEN, J.R. (1975a)
Urinary metabolites of 2,5,2',5'-tetrachlorobiphenyl in the
non-human primate. Proc. Soc. Exp. Biol. Med., 150: 185-188.
HSU, I.C., VAN MILLER, J.P., & ALLEN, J.R. (1975b) Metabolic fate
of 3H 2,5,2',5'-tetrachlorobiphenyls in infant non-human primates.
Bull. environ. Contam. Toxicol., 14: 233-240.
HSU, S.-T., MA, C.-I., HSU, S.K.-H., WU, S.-S., HSU, N.H.-M., YEH,
C.-C., & WU, S.-B. (1985) Discovery and epidemiology of PCB
poisoning in Taiwan: A four-year follow-up. Environ. health
Perspect., 59: 5-10.
HUCKINS, J.N., SCHWARTZ, T.R., PETTY, J.D. & SMITH, L.M. (1988)
Determination, fate and potential significance of PCBs in fish and
sediment samples with emphasis on selected AHH-inducing congeners.
Chemosphere, 17(10): 1995-2016.
HUDECOVA, A., KOSHINOVA, A., & MADARICH, A. (1979) [Investigations
of the effect of polychlorinated biphenyls on the dynamics of the
changes of the vitamin A and E content in rat liver.] Czech. Hyg.,
24: 59-64 (in Czech).
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife, Washington, DC, US Department
of the Interior, Fish and Wildlife Service, 90 pp (Resource
Publication No. 153).
HURST, J.G., NEWCOMER, W.S., & MORRISON, J.A. (1973) The effects of
polychlorinated biphenyl on longevity of bobwhite quail (Collinus
virginianus): A sex differential. Proc. Soc. Exp. Biol. Med.,
144: 431-435.
HURST, J.G., NEWCOMER, W.S., & MORRISON, J.A. (1974) Some effects
of DDT, toxaphene and polychlorinated biphenyl on thyroid function
in bobwhite quail. Poult. Sci., 53: 125-133.
HUSTERT, K. & KORTE, F. (1972) [Contributions to ecological
chemistry. XXXVIII: Synthesis of polychlorinated biphenyls and
their reactions to UV irradiation.] Chemosphere, 1: 7-10
(in German).
HUTZINGER, O., SAFE, S., & ZITKO, V. (1971) Polychlorinated
biphenyls: photolysis of 2,4,6,2',4',6'-hexachlorobiphenyl,
chlorobiphenyl. Nature (Lond.), 232: 15.
HUTZINGER, O., NASH, D.M., SAFE, S., DEFREITAS, A.S.W., NORSTRÖM,
R.J., WILDISH, D.J., & ZITKO, V. (1972) Polychlorinated
biphenyls-metabolic behaviour of pure isomers in pigeons, rats, and
brook trout. Science, 178: 312-314.
HUTZINGER, O., SAFE, S., & ZITKO, V. (1974) The chemistry of PCBs,
Cleveland, Ohio, CRC Press.
HUTZINGER, O., CHOUDHRY, G.G., CHITTIM, B.G., & JOHNSTON, L.E.
(1985) Formation of polychlorinated dibenzofurans and dioxins
during combustion, electrical equipment fires, and PCB
incineration. Environ. health Perspect., 60: 3-9.
IARC (1978) Polychlorinated biphenyls and polybrominated biphenyls,
Lyon, International Agency for Research on Cancer, pp. 43-103 (IARC
Monographs on the Evaluation of the Carcinogenic Risks of Chemicals
to Humans, Volume 18).
IARC (1987) Overall evaluation of carcinogenicity: An updating of
IARC monographs Volumes 1 to 42, Lyon, International Agency for
Research on Cancer, (IARC Monographs on the Evaluation of the
Carcinogenic Risks of Chemicals to Humans, Supplement 7).
IATROPOULOS, M.J., FELT, G.R., ADAMS, H.P., KORTE, F., & COULSTON,
F. (1977) Chronic toxicity of 2,5,4'-trichlorobiphenyl in young
Rhesus monkeys. II. Histopathology. Toxicol. appl. Pharmacol., 41:
629-638.
IATROPOULOS, M.J., BAILEY, J., ADAMS, H.P., COULSTON, F., & HOBSON,
W. (1978) Response of nursing infant Rhesus to Clophen A30 or
hexachlorobenzene given to lactating mothers. Environ. Res.,
16: 38-47.
IKEDA, M., KURATSUNE, M., NAKAMURA, Y., & HIROHATA, T. (1987) A
cohort study on mortality of Yusho patients - a preliminary report.
Fukuoka Acta med., 78: 297-300.
IMANISHI, J., NOMURA, H., MATSUBARA, M., KITA, M., WON, S.-J.,
MIZUTANI, T., & KISHIDA, T. (1980) Effect of polychlorinated
biphenyl on viral infections in mice. Infect. Immun., 29: 275-277.
IMANISHI, J., OKU, T., OISHI, K., NOMURA, H., & MIZUTANI, T. (1984)
Reduced resistance to experimental viral and bacterial infections
of mice treated with polychlorinated biphenyl. Biken J.,
27: 195-198.
INNAMI, S., NAKAMURA, A., MIYAZAKI, M., NAGAYAMA, S., & NISHIDE, E.
(1976) Further studies on the reduction of vitamin A content in the
livers of rats given polychlorinated biphenyls. J. nutr. Sci.
Vitaminol., 22: 409-418.
INOUE, K., TAKANAKA, A., MIZOKAMI, K., FUJIMORI, K., SUNOUCHI, M.,
KASUYA, Y., & OMORI, Y. (1981) Effects of polychlorinated biphenyls
on the monooxygenase system in fetal livers of rats. Toxicol. appl.
Pharmacol., 59: 540-547.
INTERNATIONAL COUNCIL FOR THE EXPLORATION OF THE SEA (1974) Report
of a working group for the international study of the pollution of
the North Sea and its effects on living resources and their
exploitation, Charlottenlund, Denmark, International Council for
the Exploration of the Sea (Co-operative Research Report No. 39).
IRPTC (1986) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme.
ISEKI, K., TAKAHASHI, M., BAUERFEIND, E., & WONG, C.S. (1981)
Effects of polychlorinated biphenyls (PCBs) on a marine plankton
population and sedimentation in controlled ecosystem enclosures.
Mar. Ecol. Prog. Ser., 5: 207-214.
ISHI, H. (1972) PCB pollution in Japan, Tokyo, Japanese Public
Health Association, pp. 13-28 (Environmental Health Report No. 14).
ISHIDATE, K., YOSHIDA, M., & NAKAZAWA, Y. (1978) Effect of typical
inducers of microsomal drug-metabolizing enzymes on phospholipid
metabolism in rat liver. Biochem. Pharmacol., 27: 2595-2603.
ISHIKAWA, T.T., MCNEELY, S., STEINER, P.M., GLUECK, C.J., MELLIES,
M., GARTSIDE, P.S., & MCMILLIN, C. (1978) Effects of chlorinated
hydrocarbons on plasma alpha-lipoprotein cholesterol in rats.
Metabolism, 27: 89-96.
ITO, N., NAGASAKI, H., ARAI, M., MAKIURA, S., SUGIHARA, S., &
HIRAO, K. (1973) Histopathologic studies on liver tumorigenesis
induced in mice by technical polychlorinated biphenyl and its
promoting effect on liver tumours induced by benzenehexachloride.
J. Natl Cancer Inst., 51(5): 1637-1646.
ITO, N., NAGASAKI, H., MAKIURA, S., & ARAI, M. (1974) Histological
studies on liver tumorigenesis in rats treated with polychlorinated
biphenyls. Gann., 65: 545-549.
ITOKAWA, Y., YAGI, N., KAITO, H., KAMOHARA, K., & FUJIWARA, K.
(1976) Influence of diet on the induction of hepatic ceroid pigment
in rats by polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
36: 131-141.
IVERSON, F., VILLENEUVE, D.C., GRANT, D.L., & HATINA, G.V. (1975)
Effect of Aroclor 1016 and 1242 on selected enzyme systems in the
rat. Bull. environ. Contam. Toxicol., 13: 456-463.
IVERSON, F., TRUELOVE, J., & HIERLIHY, S.L. (1982) Hepatic
microsomal enzyme induction by Aroclors 1248 and 1254 in Cynomolgus
monkeys. Food chem. Toxicol., 20: 307-310.
IWATA, Y. & GUNTHER, F.A. (1976) Translocation of the
polychlorinated biphenyl Aroclor 1254 from soil into carrots under
field conditions. Arch. environ. Contam. Toxicol., 4: 44-59.
IWATA, Y., WESTLAKE, W.E., & GUNTHER, F.A. (1973) Varying
persistence of polychlorinated biphenyls in six California soils
under laboratory conditions. Bull. environ. Contam. Toxicol.,
9: 204-211.
IWATA, Y., GUNTHER, F.A., & WESTLAKE, W.E. (1974) Uptake of a PCB
(Aroclor 1254) from soil by carrots under field conditions. Bull.
environ. Contam. Toxicol., 11: 523-528.
JACOBSON, J.L., JACOBSON, S.W., SCHWARTZ, P.M., FEIN, G.G., &
DOWLER, J.K. (1984a) Prenatal exposure to an environmental toxic:
A test of the multiple effects model. Dev. Psychol., 20: 523-532.
JACOBSON, J.L., FEIN, G.G., JACOBSON, S.W., SCHWARTZ, P.M., &
DOWLER, J.K. (1984b) The transfer of polychlorinated biphenyls
(PCBs) and polybrominated biphenyls-(PBBs) across the human
placenta and into maternal milk. Am. J. public Health,
74(4): 378-379.
JACOBSON, S.W., FEIN, G.G., JACOBSON, J.L., SCHWARTZ, P.M., &
DOWLER, J.K. (1985) The effect of intrauterine PCB exposure on
visual recognition memory. Child Dev., 56: 856-860.
JAN, J. & MALNERSIC, S. (1978) Determination of PCB and PCT
residues in fish by tissue acid hydrolysis and destructive clean-up
of the extract. Bull. environ. Contam. Toxicol., 19: 772-780.
JAN, J. & TRATNIK, M. (1988a) Polychlorinated biphenyls in
residents around the River Krupa, Slovenia, Yugoslavia. Bull.
environ. Contam. Toxicol., 41: 809-814.
JAN, J., TRATNIK, M., & KENDA, A. (1988b) Atmospheric contamination
with polychlorinated biphenyls in Bela Krajina (Yugoslavia);
Emissions from industrial plant, landfill and river areas.
Chemosphere, 17(4): 809-813.
JANI, J.P., PATEL, J.S., SHAH, M.P., GUPTA, S.K., & KASHYAP, S.K.
(1988) Levels of organochlorine pesticides in human milk in
Ahmedabad, India. Int. Arch. occup. environ. Health., 60: 111-113.
JANNETTI, R.A. & ANDERSON, L.M. (1981) Dimethylnitrosamine
demethylase activity in fetal, suckling, and maternal mouse liver
and its transplacental and transmammary induction by
polychlorinated biphenyls. J. Natl Cancer Inst., 67: 461-466.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTRÖM, G., &
VAZ, R. (1975) Identification by GC-MS of phenolic metabolites of
PCB and p,p'-DDE isolated from Baltic guillemot and seal. Ambio,
4: 93-97.
JAPAN ENVIRONMENT AGENCY (1983) Environmental monitoring of
chemicals; Environmental survey report of F.Y. 1980 and 1981.
Tokyo, Japan Environment Agency, Department of Environmental
Health, Offices of Health Studies.
JAPAN ENVIRONMENTAL SANITATION BUREAU (1973) [Survey on food
contamination: Report of a comprehensive investigation on the
prevention of pollution by PCBs], Tokyo, Ministry of Health &
Welfare, Environmental Sanitation Bureau, pp. 191-210 (in
Japanese).
JEFFERIES, D.J. & PARSLOW, J.L.F. (1972) Effect of one
polychlorinated biphenyl on size and activity of the gull thyroid.
Bull. environ. Contam. Toxicol., 8: 306-310.
JEFFERIES, D.J. & PARSLOW, J.L.F. (1976) Thyroid changes in
PCB-dosed guillemots and their indication of one of the mechanisms
of action of these materials. Environ. Pollut., 10: 293-311.
JENSEN, A.A. (1983a) Chemical contaminants in human milk. Res.
Rev., 89: 1-128.
JENSEN, A.A. (1983b) PCB in human milk: An Overview. In: Barros,
M.C., Könemann, H., & Visser, R., ed. Proceedings of the PCB
Seminar, The Hague, 28-30 September 1983, The Hague, Ministry of
the Environment, pp. 81-98.
JENSEN, A.A. (1984a) Current data of levels of organohalogen
compounds in human milk in countries outside CEC. In: Report on the
WHO Consultation on Organohalogen Compounds in Human Milk and
Related Hazards, Bilthoven, 9-11 January 1985, Copenhagen, World
Health Organization, Regional Office for Europe, Annex 3.
JENSEN, A.A. (1984b) Data extracted from document on "Assessment of
the presence of potentially toxic substances in breast milk with
special emphasis on the European Community" submitted by the
Commission of the European Communities (CEC Study No. 830465,
CEC/V/E/2/lux/35/84). In: Report on the WHO Consultation on
Organohalogen Compounds in Human Milk and Related Hazards,
Bilthoven, 9-11 January 1985, Copenhagen, World Health
Organization, Regional Office for Europe, Annex 7.
JENSEN, A.A. (1987) Polychlorobiphenyls (PCBs),
polychlorodibenzo- p-dioxins (PCDDs) and polychlorodibenzofurans
(PCDFs) in human milk, blood, and adipose tissue. Sci. total
Environ., 64: 259-293.
JENSEN, S. & JANSSON, B. (1976) Methylsulfone metabolites of PCB
and DDE in seals from the Baltic. Ambio, 5: 257-260.
JENSEN, S. & SUNDSTROM, G. (1974a) Structures and levels of most
chlorobiphenyls in two technical PCB products and in human adipose
tissue. Ambio, 3: 70-76.
JENSEN, S. & SUNDSTROM, G. (1974b) Metabolic hydroxylation of a
chlorobiphenyl containing only isolated unsubstituted
positions-2,2',4,4',5,5'-hexachlorobiphenyl. Nature (Lond.),
251: 219-220.
JENSEN, S., JOHNELS, A.G., OLSSON, M., & OTTERLIND, G. (1969) DDT
and PCB in marine animals from Swedish waters. Nature (Lond.),
224: 247-250.
JENSEN, S., JOHNELS, A.G., OLSSON, M., & OTTERLIND, G. (1972a) DDT
and PCB in herring and cod from the Baltic, the Kattegat and the
Skaggerrak. Ambio, 1(Spec. Rep.): 71-85.
JENSEN, S., JOHNELS, A.G., OLSSEN, M., & WESTERMARK, T. (1972b) The
avifauna of Sweden as indicators of environmental contamination
with mercury and chlorinated hydrocarbons. In: Proceedings of the
15th International Ornithology Congress, Leiden, pp. 455-465.
JENSEN, S., RENBERG, L., & VAZ, R. (1973) Problems in
quantification of PCB in biological material. In: Proceedings of
the Polychlorinated Biphenyls Conference II, Stockholm, 1972,
Solna, Sweden, National Environmental Protection Board, pp. 7-13
(Publication No. 4E).
JENSEN, S., ORBERG, J., KIHLSTROM, J.E., OLSSON, M., & LUNDBERG, C.
(1977) Effects of PCB and DDT on mink (Mustela vision) during the
reproductive season. Ambio, 6: 239.
JFCMP (1985) FAO/WHO Collaborating Centres for Food Contamination
Monitoring. PCB's residues in food, Rome, Food and Agriculture
Organization of the United Nations, FAO/WHO Food Contamination
Monitoring Programme (Document EFP/85,3 prepared for the 17th
Session of the Codex Committee on Pesticide Residues).
JOHANSSON, N., LARSSON, A., & LEWANDER, K. (1972) Metabolic effects
of PCB (Polychlorinated biphenyls) on the brown trout (Salmo
trutta). Comp. gen. Pharmacol., 3: 310-314.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S. (1979)
Pesticides and other chemical residues in infant and toddler total
diet samples (I), August 1974-July 1975. Pestic. monit. J.,
13(3): 87-98.
JOHNSTONE, G.J., ECOBICHON, D.J., & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, D.C.L., DAVIS, W.E., Jr, NEWELL, G.W., SASMORE, D.P., &
ROSEN, V.J. (1974) Modification of hexachlorophene toxicity by
dieldrin and Aroclor 1254. Toxicology, 2: 309-318.
JONES, K.C. (1988) Determination of polychlorinated biphenyls in
human foodstuffs and tissues: suggestions for a selective congener
analytical approach. Sci. total Environ., 68: 141-159.
JONES, K.C. (1989) Polychlorinated biphenyls in Welsh soils: a
survey of typical levels. Chemosphere, 18(7-8): 1665-1672.
JONES, J.W. & ALDEN, H.S. (1936) An acneform dermatergosis. Arch.
Dermato. Syphilol., 33: 1022-1034.
JONSSON, H.T., KEIL, J.E., GADDY, R.G., LOADHOLT, C.B., HENNIGAR,
G.R., & WALKER, E.M. (1976) Prolonged ingestion of commercial DDT
and PCB; effects on progesterone levels and reproduction in the
mature female rat. Arch. environ. Contam. Toxicol., 3: 479-490.
JONSSON, H.T., Jr, WALKER, E.M., Jr, GREENE, W.B., HUGHSON, M.D.,
& HENNIGAR, G.R. (1981) Effects of prolonged exposure to dietary
DDT and PCB on rat liver morphology. Arch. environ. Contam.
Toxicol., 10: 171-183.
JURY, W.A., WINER, A.M., SPENCER, W.F., & FOCHT, D.D. (1987)
Transport and transformations of organic chemicals in the
soil-air-water ecosystem. Rev. environ. Contam. Toxicol.,
99: 119-164.
KAISER, K.L.E. (1974) On the optical activity of polychlorinated
biphenyls. Environ. Pollut., 7: 93-101.
KAISER, K.L.E. & WONG, P.T.S. (1974) Bacterial degradation of
polychlorinated biphenyls. I. Identification of some metabolic
products from Aroclor 1242. Bull. environ. Contam. Toxicol.,
11: 291-296.
KAMAL, M., KLEIN, M., & KORTE, F. (1976) Isolation and
identification of metabolites after long-term feeding of
2,2'-dichlorobiphenyl-C-14. Chemosphere, 5: 349-356.
KAMOHARA, T., YAGI, N., & ITOKAWA, Y. (1984) Mechanism of lipid
peroxide formation in polychlorinated biphenyls (PCB) and
dichlorodiphenyltrichloroethane (DDT)-poisoned rats. Environ. Res.,
34: 18-23.
KANEKO, T. (1988) [Polychlorinated terphenyls (PCTs). A study on
the induction of cleft palate by polychlorinated terphenyls (PCTs)
administered maternally with special reference to the role of
corticosterone.] Pharmacometrics, 36(4): 309-327 (in Japanese).
KANNAN, N., TANABE, S., WAKIMOTO, T., & TATSUKAWA, R. (1987)
Coplanar polychlorinated biphenyls in Aroclor and Kanechlor
mixtures. J. Assoc. Off. Anal. Chem., 70(3): 451-454.
KANNAN, N., TANABE, S., & TATSUKAWA, R. (1988) Potentially
hazardous residues of non- ortho chlorine substituted coplanar
PCBs in human adipose tissue. Arch. environ. Health, 43(1): 11-14.
KANNAN, N., WAKIMOTO, T., & TATSUKAWA, R. (1989) Possible
involvement of frontier (n) electrons in the metabolism of
polychlorinated biphenyls (PCBs). Chemosphere, 18(9/10): 1955-1963.
KARLSSON, B., PERSSON, B., SÖDERGREN, A., & ULFSTRAND, S. (1974)
Locomotory and dehydrogenase activities of redstarts Phoenicurus
phoenicurus L. (Aves) given PCB and DDT. Environ. Pollut.,
7: 53-63.
KARPPANEN, E. & KOLHO, L. (1973) The concentration of PCB in human
blood and adipose tissue in three different research groups.
Presented at the Polychlorinated Biphenyls Conference II,
Stockholm, 1972 (Unpublished document).
KASHIMOTO, T., MIYATA, H., FUKUSHIMA, S., KUNITA, N., OHI, G., &
TUNG, T.-C. (1985) PCBs, PCQs, and PCDFs in blood of Yusho and
Yu-Cheng patients. Environ. health Perspect., 59: 73-78.
KASZA, L., WEINBERGER, M.A., HINTON, D.E., TRUMP, B.F., PATEL, C.,
FRIEDMAN, L., & GARTHOFF, L.H. (1978a) Comparative toxicity of
polychlorinated biphenyl and polybrominated biphenyl in the rat
liver: light and electron microscopic alterations after subacute
dietary exposure. J. environ. Pathol. Toxicol., 1: 241-257.
KASZA, L., COLLINS, W.T., CAPEN, C.C., GARTHOFF, L.H., & FRIEDMAN,
L. (1978b) Comparative toxicity of polychlorinated biphenyl and
polybrominated biphenyl in the rat thyroid gland: light and
electron microscopic alterations after subacute dietary exposure.
J. environ. Pathol. Toxicol., 1: 587-599.
KATO, N. & YOSHIDA, A. (1980) Effect of dietary PCB on hepatic
cholesterogenesis in rats. Nutr. Rep. Int., 21: 107-112.
KATO, N. & YOSHIDA, A. (1981) Effect of various dietary xenobiotics
on serum total cholesterol and high density lipoprotein cholesterol
in rats. Nutr. Rep. Int., 23: 825-831.
KATO, N., KATO, M., KIMURA, T., & YOSHIDA, A. (1978) Effect of
dietary addition of PCB, DDT, or BHT and dietary protein on vitamin
A and cholesterol metabolism. Nutr. Rep. Int., 18: 437-446.
KATO, N., KAWAI, K., & YOSHIDA, A. (1982) Effects of dietary
polychlorinated biphenyls and protein level on liver and serum
lipid metabolism of rats. Agric. biol. Chem., 46: 703-707.
KATO, S., MCKINNEY, J.D., & MATTHEWS, H.B. (1980) Metabolism of
symmetrical hexachlorobiphenyl isomers in the rat. Toxicol. appl.
Pharmacol., 53: 389-398.
KATSUKI, S. (1969) Foreword. Fukuoka Acta med., 60: 403-407.
KAWAI, S., FUKUSHIMA, M., MIYAZAKI, N., & TATSUKAWA, R. (1988)
Relationship between lipid composition and organochlorine levels in
the tissues of striped Dolphin. Mar. Pollut. Bull., 19: 129-133.
KAWANISHI, S., SANO, S., MIZUTANI, T., & MATSUMOTO M. (1973)
[Experimental porphyria induced by polychlorinated biphenyls.] Jpn.
J. Hyg., 28: 84 (in Japanese).
KAWANISHI, S., SANO, S., MIZUTANI, T., & MATSUMOTO M. (1974)
[Experimental studies on toxicity of synthetic tetrachlorobiphenyl
isomers.] Jpn. J. Hyg., 29: 81 (in Japanese).
KAWANISHI, S., SANO, S., & MIZUTANI, T. (1975) [A toxicological
study on synthetic hexachlorobiphenyl isomers.] Jpn. J. Hyg.,
30: 124 (in Japanese).
KAWANISHI, S., SEKI, Y., & SANO, S. (1983) Uroporphyrinogen
decarboxylase. Purification, properties, and inhibition by
polychlorinated biphenyl isomers. J. biol. Chem., 258: 4285-4292.
KECK, G. (1981) Effets de la contamination par les
polychlorobiphényles (PCBs) sur le développement de la tumeur
d'Ehrlich chez la souris Swiss. Toxicol. Eur. Res., 3(5): 229-236.
KERKVLIET, N.I. & BAECHER-STEPPAN, L. (1988a) Suppression of
allograft immunity by 3,4,5,3',4',5-hexachlorobiphenyl. I. Effects
of exposure on tumor rejection and cytotoxic T-cell activity
in vivo. Immunopharmacology, 16: 1-12.
KERKVLIET, N.I. & BAECHER-STEPPAN, L. (1988b) Suppression of
allograft immunity by 3,4,5,3',4',5-hexachlorobiphenyl. II. Effects
of exposure on mixed lymphocyte reactivity and induction of
suppressor cell activity in vitro. Immunopharmacology, 16: 13-23.
KERKVLIET, N.I. & KIMELDORF, D.J. (1977) Anti-tumour activity of a
polychlorinate biphenyl mixture, Aroclor 1254, in rats inoculated
with Walker 256 carcinosarcoma cells. J. Natl Cancer Inst.,
59: 951-955.
KHAN, M.A., RAO, R.M., & NOVAK, A.F. (1976) Polychlorinated
biphenyls (PCBs) in food. January 1976. Crit. Rev. food Sci. Nutr.,
January: 103-145.
KHAN, A.V., LASHNEVA, N.V., KUMOK, S.T., GURVICH, YA.A., &
TUTELYAN, V.A. (1985) Study into the effect of ionol on cytochrome
P-450 induction in rat liver by polychlorinated diphenyls. Vopr.
pitan., 4(1): 66-69.
KIESSLING, A., PART, P., RING, O., & LINDAHL-KIESSLING, K. (1983)
Effects of PCB on the adrenergic response in perfused gills and on
levels of muscle glycogen in rainbow trout (Salmo gairdneri
Rich.). Bull. environ. Contam. Toxicol., 31: 712-718.
KIHLSTROM, J.E., LUNDBERG, C., ORBERG, J., DANIELSSON, P.O., &
SYDHOFF, J. (1975) Sexual functions of mice neonatally exposed to
DDT or PCB. Environ. Physiol. Biochem., 5: 54-57.
KIKUCHI, M. (1984) Autopsy of patients with Yusho. Am. J. ind.
Med., 5: 19-30.
KIMBROUGH, R.D. (1980) Occupational exposure. In: Halogenated
biphenyls, terphenyls, naphthalenes, dibenzodioxins and related
products, Amsterdam, Elsevier, North-Holland Biomedical Press,
pp. 373-395 (Topics in Environmental Health, Vol. 4).
KIMBROUGH, R.D. (1987) Human health effects of polychlorinated
biphenyls (PCBs) and polybrominated biphenyls (PBBs). Annu. Rev.
Pharmacol. Toxicol., 27: 87-111.
KIMBROUGH, R.D., LINDER, R.E., & GAINES, T.B. (1972) Morphological
changes in livers of rats fed polychlorinated biphenyls. Light
microscopy and ultrastructure. Arch. environ. Health, 25: 354-364.
KIMBROUGH, R.D. & LINDER, R.E. (1974) Induction of adenofibrosis
and hepatomas of the liver in BALB/cJ mice by polychlorinated
biphenyls (Aroclor 1254). J. Natl Cancer Inst., 53: 547-552.
KIMBROUGH, R.D., SQUIRE, R.A., LINDER, R.E., STRANBERG, J.D.,
MONTALI, R.J., & BURSE, V.W. (1975) Induction of liver tumours in
Sherman strain female rats by polychlorinated biphenyl Aroclor
1260. J. Natl Cancer Inst., 55: 1453-1459.
KIMURA, N.T. & BABA, T. (1973) Neoplastic changes in the rat liver
induced by polychlorinated biphenyls. Gann, 64: 105-108.
KIMURA, I. & MIYAKE, T. (1976) Teratogenic and postnatal growth-
suppressive effects of polychloro-triphenyl (PCT) in dd/Y mice.
Teratology, 14: 243-244 (Abstract).
KIMURA, S., KUMADA, H., & MATIDA, Y. (1974) [Acute toxicity and
accumulation of PCB (KC 300) in freshwater fish. Bull. freshwater
Fish Res. Lab. (Tokyo), 23: 115-123 (in Japanese).
KIMURA, N.T., KANEMATSU, T., & BABA, T. (1976) Polychlorinated
biphenyl(s) as a promoter in experimental hepatocarcinogenesis in
rats. Z. Krebsforsch., 87: 257-266.
KIRIYAMA, S., BANJO, M., & MATSUSHIMA, H. (1974) Effect of
polychlorinated biphenyls (PCB) and related compounds on the weight
of various organs and plasma and liver cholesterol in the rat.
Nutr. Rep. Int., 10: 79-88.
KITAMURA, M., TSUKAMOTO, T., SUMINO, K., HAYAKAWA, K., SHIBITA, T.,
& HIRANO, I. (1973) [The PCB levels in the blood of workers
employed in a condenser factory.] Jpn. J. ind. Health, 47: 354-355
(in Japanese).
KLAAS, E.E. & SWINEFORD, D.M. (1976) Chemical residue content and
hatchability of screech owl eggs. Wilson Bull., 88: 421-426.
KLASSON-WEHLER, E., BERGMAN, A., KOWALSKI, B., & BRANDT, I. (1987)
Metabolism of 2,3,4'6,-tetrachlorobiphenyl: Formation and tissue
localization of mercapturic acid pathway metabolites in mice.
Xenobiotica, 17(4): 477-486.
KLEIN, H. (1983) The PCB situation in the Federal Republic of
Germany. In: Barros, M.C., Könemann, H., & Visser, R., ed.
Proceedings of PCB Seminar, The Hague, 28-30 September 1983, The
Hague, Ministry of the Environment, pp. 66-79.
KLEKOWSKI, E.J. (1982) Mutation in ferns growing in an environment
contaminated with polychlorinated biphenyls, Amherst,
Massachusetts, University of Massachusetts, Water Resources
Research Center, 23 pp ((NTIS) PB83-150011).
KLEPPEL, G.S. & MCLAUGHLIN, J.J.A. (1980) PCB toxicity to
phytoplankton: Effects of dose and density-dependent recovery
responses. Bull. environ. Contam. Toxicol., 24: 696-703.
KLING, D. & GAMBLE, W. (1982) Cholesterol biosynthesis in
polychlorinated biphenyl-treated rats. Environ. Res., 27: 10-15.
KOBAYASHI, Y. (1972) [Answer report to the questionary paper on
"Regulation on residual levels in foods".] Biol. Pollut., 4: 93-116
(in Japanese).
KODAMA, H. & OTA, H. (1977) Studies on the transfer of PCB to
infants from their mothers (1). Jpn. J. Hyg., 32: 567-573.
KODAMA, H. & OTA, H. (1980) Transfer of polychlorinated biphenyls
to infants from mothers. Arch. environ. Health, 35: 95-100.
KOEMAN, J.H., TEN HOEVER DE BRAUW, M.C., & DE VOS, R.H. (1969)
Chlorinated biphenyls in fish, mussels and birds from the River
Rhine and the Netherlands coastal area. Nature (Lond.),
221: 1126-1128.
KOEMAN, J.H., PEETERS, W.H.M., SMIT, C.J., TJIOE, P.S., & DE GOEIJ,
J.J.M. (1972) Persistent chemicals in marine mammals. TNO-nieuws,
27: 570-578.
KOEMAN, J.H., VAN VELZEN-BLAD, H.C.W., DE VRIES, R., & VOS, J.G.
(1973) Effects of PCB and DDE in cormorants and evaluation of PCB
residues from an experimental study. J. Reprod. Fertil.,
19(Suppl.): 353-364.
KOHLI, K.K., GUPTA, B.N., ALBRO, P.W., MUKHTAR, H., & MCKINNEY,
J.D. (1979) Biochemical effects of pure isomers of hexachloro-
biphenyl: fatty livers and cell structure. Chem. biol. Interact.,
25: 139-156.
KOHLI, K.K., PHILPOT, R.M., ALBRO, P.W., & MCKINNEY, J.D. (1980)
Induction of different species of cytochrome P-450 by coplanar and
noncoplanar isomers of hexachlorobiphenyl. Life Sci., 26: 945-952.
KOLBYE, A.C., Jr (1972) Food exposure to polychlorinated biphenyls.
Environ. health Perspect., 1: 85-88.
KOLLER, L.D. (1977) Enhanced polychlorinated biphenyls lesions in
Moloney leukemia virus-infected mice. Clin. Toxicol.,
11(1): 107-116.
KOLLER, L.D. & THIGPEN, J.E. (1973) Biphenyl-exposed rabbits. Am.
J. vet. Res., 34: 1605-1606.
KOLLER, L.D. & ZINKL, J.G. (1973) Pathology of polychlorinated
biphenyls in rabbits. Am. J. Pathol., 70: 363-378.
KOMATSU, F. & KIKUCHI, M. (1972) [Skin lesion by 3,4,3',4'-
tetrachlorobiphenyl in rabbits.] Fukuoka Acta med., 63: 384-386
(in Japanese).
KORACH, K.S., SARVER, P., CHAE, K., MCLACHLAN, J.A., & MCKINNEY,
J.D. (1987) Estrogen receptor-binding activity of polychlorinated
hydroxybiphenyls: Conformationally restricted structural probes.
Mol. Pharmacol., 33: 120-126.
KOSHIOKA, M., KANAZAWA, J., IIZUKA, H., & MURAI, T. (1987)
Photodegradation of decachlorobiphenyl. Bull. environ. Contam.
Toxicol., 38: 409-415.
KOSS, G., SEUBERT, S., SEUBERT, A., HERBERT, M., KORANSKY, W., &
IPPEN, H. (1980) Hexachlorobenzene and 2,4,5,2',4',5'-hexachloro-
biphenyl - A comparison of their distribution, biotransformation
and prophyrinogenic action in female rats. In: Holmstedt, B.,
Lauwerys, R., Mercier, M., & Roberfroid, M., ed. Mechanisms of
toxicity and hazard evaluation, Amsterdam, Elsevier/North-Holland
Biomedical Press, pp. 517-520.
KOSUTZKY, J., ADAMEC, O., & BOBAKOVA, E. (1979) Effects of
polychlorinated biphenyls on poultry reproduction. Bull. environ.
Contam. Toxicol., 21: 737-742.
KREISS, K. (1985) Studies on populations exposed to polychlorinated
biphenyls. Environ. health Perspect., 60: 193-199.
KREISS, K., ZACK, M.M., KIMBROUGH, R.D., NEEDHAM, L.L., SMRED,
A.L., & JONES, B.T. (1981) Association of blood pressure and
polychlorinated biphenyl levels. J. Am. Med. Assoc.,
245(24): 2505-2509.
KREITZER, J.F. & HEINZ, G.H. (1974) The effect of sublethal dosages
of five pesticides and a polychlorinated biphenyl on the avoidance
response of Coturnix quail chicks. Environ. Pollut., 6: 21-29.
KREITZER, J.F. & SPANN, J.W. (1973) Tests of pesticidal synergism
with young pheasants and Japanese quail. Bull. environ. Contam.
Toxicol., 9: 250-256.
KRICHER, J.C., BAYER, C.L., & MARTIN, D.A. (1979) Effects of two
Aroclor fractions on the productivity and diversity of algae from
two lentic ecosystems. Int. J. environ. Stud., 13: 159-167.
KROGH DERR, S. (1978) In vivo metabolism of exogenous
progesterone by PCB treated female rats. Bull. environ. Contam.
Toxicol., 19: 729-732.
KUNITA, N., HORI, S., OBANA, H., OTAKE, T., NISHIMURA, H.,
KASHIMOTO, T., & IKEGAMI, N. (1985) Biological effects of PCBs PCQs
and PCDFs present in the oil causing Yusho and Yu-Cheng. Environ.
Health Perspect., 59: 79-84.
KURACHI, M. (1983) A new sulfur-containing derivative and
possibility of conjugate formation of PCBs in mice or rats. Agric.
biol. Med., 47(6): 1183-1191.
KURACHI, M. & MIO, T. (1983) On fluctuation of PCBs under various
unnatural conditions in mice. Agric. biol. Med., 47(6): 1173-1181.
KURATSUNE, M. & MASUDA, Y. (1972) Polychlorinated biphenyls in
non-carbon copypaper. Environ. health Perspect., 1: 61-62.
KURATSUNE, M., YOSHIMURA, T., MATSUZAKA, J., & YAMAGUCHI, A. (1972)
Epidemiologic study on Yusho, a poisoning caused by ingestion of
rice-oil contaminated with a commercial brand of polychlorinated
biphenyls. Environ. health Perspect., 1: 119-128.
KURATSUNE, M., MASUDA, Y., & NAGAYAMA, J. (1976) Some of the recent
findings concerning Yusho. In: Proceedings of the National
Conference on Polychlorinated Biphenyls, Chicago, 19-21 November
1975, Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances, pp. 14-29 (EPA-560/6-75-004).
KUROKI, H. & MASUDA, Y. (1977) Structures and concentrations of the
main components of polychlorinated biphenyls retained in patients
with Yusho. Chemosphere, 6: 469-474.
KUSUDA, M. (1971) [Study on the female sexual function suffering
from the chlorobiphenyls poisoning.] Sanka Fujinka, 4: 1063-1072
(in Japanese).
KUWABARA, K., YAKUSHIJI, T., WATANABE, I., YOSHIDA, S., KOYAMA, K.,
KUNITA, N., & HARA, I. (1978) Relationship between breast feeding
and PCB residues in blood of the children whose mothers were
occupationally exposed to PCBs. Int. Arch. occup. environ. Health,
41: 189-197.
KUWABARA, K., YAKUSHIJI, T., WATANABE, I., YOSHIDA, S., KOYAMA, K.,
& KUNITA, N. (1979) Levels of polychlorinated biphenyls in blood of
breast-fed children whose mothers are non-occupationally exposed to
PCBs. Bull. environ. Contam. Toxicol., 21: 458-462.
LAKE, B.G., COLLINS, M.A., HARRIS, R.A., & GANGOLLI, S.D. (1979)
The induction of hepatic and extrahepatic xenobiotic metabolism in
the rat and ferret by a polychlorinated biphenyl mixture (Aroclor
1254). Xenobiotica, 9: 723-731.
LAN, S.-J., YEN, Y.-Y., KO, Y.-C., & CHEN, E.-R. (1989) Growth and
development of permanent teeth germ of transplacental Yu-Cheng
babies in Taiwan. Bull. environ. Contam. Toxicol., 42: 931-934.
LA ROCCA, P. & CARLSON, G.P. (1979) The effect of polychlorinated
biphenyls on adenosine triphosphatase activity. Toxicol. appl.
Pharmacol., 48: 185-192.
LARSEN, P.F., GADBOIS, D.F., & JOHNSON, A.C. (1985) Observations on
the distribution of PCBs in the deepwater sediments of the Gulf of
Maine. Mar. Pollut. Bull., 16: 439-442.
LARSSON, R. (1984) Transport of PCBs from aquatic to terrestrial
environments by emerging chironomids. Environ. Pollut.,
34: 283-289.
LARSSON, P. (1985a) Contaminated sediments of lakes and oceans act
as sources of chlorinated hydrocarbons for release to water and
atmosphere. Nature (Lond.), 317: 347-349.
LARSSON, P. (1985b) Change in PCB (Clophen A 50) composition when
transported from sediment to air in aquatic model systems. Environ.
Pollut., B9: 81-94.
LARSSON, P. (1987) Uptake of polychlorinated biphenyls (PCBs) by
the macroalga, Cladophora glomerata. Bull. environ. Contam.
Toxicol., 38: 58-62.
LARSSON, P. & OKLA, L. (1987) An attempt to measure the flow of
chlorinated hydrocarbons, such as PCBs, from water to air in the
field. Environ. Pollut., 44: 219-225.
LARSSON, C.M. & TILLBERG, J.E. (1975) Effects of the commercial
polychlorinated biphenyl mixture Aroclor 1242 on growth, viability,
phosphate uptake, respiration and oxygen evolution in Scenedesmus.
Physiol. Plant., 33: 256-260.
LASHNEVA, N.V. & TUTELYAN, V.A. (1984) Induction of cytochrome
P-450 in rat liver by polychlorinated diphenyls. Farmakol.
Toksikol., 6: 77-80.
LASHNEVA, N.V., KHAN, A.V., & TUTELYAN, V.A. (1985) The functional
state of monooxygenase system in rat liver tissue after effect of
ionol and polychlorinated diphenyls. Vopr. med. khim. 5: 7-22.
LASHNEVA, N.V., CHICHILANOVA, G.V., SOROKOVAYA, G.K., & TUTELYAN,
V.A. (1987) Effects of polychlorinated biphenyls on rat liver
cytochrome P-450 system formation at early postnatal period. In:
Abstracts of the USSR-All-Union Conference on Cytochrome P-450 and
Environment, Novosibirsk, 27-31 July 1987, Novosibirsk, USSR
Academy of Medical Sciences, Siberian Division.
LAWRENCE, J. & TOSINE, H.M. (1977) Polychlorinated biphenyl
concentrations in sewage and sludges of some waste treatment plants
in Southern Ontario. Bull. environ. Contam. Toxicol., 17: 49-56.
LAWTON, R.W., ROSS, M.R., FEINGOLD, J., & BROWN, J.F., Jr (1985)
Effects of PCB exposure on biochemical and hematological findings
in capacitor workers. Environ. health Perspect., 60: 165-184.
LAY, J.P., KLEIN, W., & KORTE, F. (1975) Excretion, storage, and
metabolism of 2,3,6,2',4'-pentachlorobiphenyl-14-C after a
long-term feeding experiment on rats. Chemosphere, 4: 161-68.
LAY, J.P., KAMAL, M., KLEIN, W., & KORTE, F. (1979) Fate of
2.5.4'-trichlorobiphenyl in rats. Xenobiotica, 12: 713-721.
LEATHERLAND, J.F. & SONSTEGARD, R.A. (1978) Lowering of serum
thyroxine and triiodothyronine levels in yearling coho salmon,
Oncorhynchus kisutch, by dietary mirex and PCBs. J. Fish Res.
Board Can., 35: 1285-1289.
LEATHERLAND, J.F. & SONSTEGARD, R.A. (1979) Effect of dietary mirex
and PCB (Aroclor 1254) on thyroid activity and lipid reserves in
rainbow trout Salmo gairdneri Richardson. J. Fish Dis., 2: 43-48.
LEDERMAN, T.C. & RHEE, G.-Y. (1982) Bioconcentration of a
hexachlorobiphenyl in Great Lakes planktonic algae. Can. J. Fish.
aquat. Sci., 39: 380-387.
LEECE, B., DENOMME, M.A., TOWNER, R., & LI, S.M.A. (1985)
Polychlorinated biphenyls: correlation between in vivo and
in vitro quantitative structure-activity relationships (QSARs).
J. Toxicol. environ. Health, 16: 379-388.
LEES, P.S.J., CORN, M., & BREYSSE, P.N. (1987) Evidence for dermal
absorption as the major route of body entry during exposure of
transformer maintenance and repairmen to PCBs. Am. Ind. Hyg. Assoc.
J., 48(3): 257-264.
LEMMETYINEN, R., RANTAMAKI, P., & KARLIN, A. (1982) Levels of DDT
and PCB's in different stages of life cycle of the Arctic tern
Sterna paradisaea and the herring gull Larus argentatus.
Chemosphere, 11: 1059-1068.
LEONI, V., FABIANI, L., MARINELLI, G., PUCCETTI, G., TARSITANI,
G.F., DE CAROLIS, A., VESCIA, N., MORINI, A., ALEANDRI, V., POZZI,
V., CAPPA, F., & BARBATI, D. (1989) PCB and other organochlorine
compounds in blood of women with or without miscarriage: A
hypothesis of correlation. Ecotoxicol. environ. Saf., 17: 1-11.
LEVIN, B.D. & BOWMAN, R.E. (1983) Polychlorinated biphenyl effects
on delayed spatial alternation in monkeys. Toxicologist, 3: 68.
LEVIN, E.D., SCHANTZ, S.L., & BOWMAN, R.E. (1988) Delayed spatial
alteration deficits resulting from perinatal PCB exposure in
monkeys. Arch. Toxicol., 62: 267-273.
LEWIN, V., MCBLAIN, W.A., & WOLFE, F.H. (1972) Acute
intraperitoneal toxicity of DDT and PCBs in mice using two
solvents. Bull. environ. Contam. Toxicol., 8(4): 245-250.
LICHTENSTEIN, E.P., SCHULZ, K.R., FUHREMANN, T.W., & LIANG, T.T.
(1969) Biological interaction between plasticizers and
insecticides. J. econ. Entomol., 62: 761-765.
LICHTENSTEIN, E.P. (1972) PCBs and interactions with insecticides.
Environ. health Perspect., 1: 151-153.
LIEB, A.J., BILLS, D.D., & SINNHUBER, R.O. (1974) Accumulation of
dietary polychlorinated biphenyls (Aroclor 1254) by rainbow trout
(Salmo gairdneri). J. agric. food Chem., 22: 638-642.
LILLIE, R.J., HARRIS, S.J., CECIL, H.C., & BITMAN, J. (1974) Normal
reproductive performance of mature cockerels fed Aroclor 1248.
Poult. Sci., 53: 1604-1607.
LIN, F.S., HSIA, M.T., & ALLEN, J.R. (1979) Acute hepatotoxicity of
a tetrachlorobiphenyl-changes in the hepatocyte ultrastructure and
plasma membrane-bound enzymes. Arch. environ. Contam. Toxicol.,
8: 321-333.
LIN, P.S., NYQUIST, S.E., & HACLERODE, J. (1982) Interactions of
polychlorinated biphenyls and delta-9-tetrahydrocannabinol with
testosterone hydroxylation and the hepatic microsomal system in the
rat. Proc. Pa. Acad. Sci., 56: 31-35.
LINDER, R.E., GAINES, T.B., & KIMBROUGH, R.D. (1974) The effect of
polychlorinated biphenyls on rat reproduction. Food Cosmet.
Toxicol., 12: 63-76.
LINDSEY, A.S. & WAGSTAFFE, P.J. (1989) Production and certification
of ten high-purity polychlorinated biphenyls as reference
materials. Analyst, 114(5): 553-557.
LINZEY, A.V. (1987a) Effects of chronic polychlorinated biphenyls
exposure on the reproductive success of white-footed mice
(Peromyscus leucopus). Arch. environ. Contam. Toxicol.,
16: 455-460.
LINZEY, A.V. (1987b) Effects of chronic polychlorinated biphenyls
exposure on growth and reproduction of second generation
white-footed mice (Peromyscus leucopus). Arch. environ. Contam.
Toxicol., 17: 39-45.
LITTERST, C.L. & VAN LOON, E.J. (1974) Time-course of induction of
microsomal enzymes following treatment with polychlorinated
biphenyl. Bull. environ. Contam. Toxicol., 11: 206-212.
LITTERST, C.L., FARBER, T.M., BAKER, A.M., & VAN LOON, E.J. (1972)
Effect of polychlorinated biphenyls on hepatic microsomal enzymes
in the rat. Toxicol. appl. Pharmacol., 23: 112-122.
LIU, D. (1980) Enhancement of PCBs biodegradation by sodium
ligninsulfonate. Water Res., 14: 1467-1475.
LIU, D. (1981) Biodegradation of Aroclor 1221 type PCBs in sewage
wastewater. Bull. environ. Contam. Toxicol., 27: 695-703.
LIU, D. (1982) Assessment of continuous biodegradation of
commercial PCB formulations. Bull. environ. Contam. Toxicol.,
29: 200-207.
LLORENTE, G.A., FARRAN, A., RUIZ, X., & ALBAIGES, J. (1987)
Accumulation and distribution of hydrocarbons, polychlorobiphenyls,
and DDT in tissues of three species of Anatidae from the Ebro Delta
(Spain). Arch. environ. Contam. Toxicol., 16: 563-572.
LOOSE, L.D., PITTMAN, K.A., BENITZ, K.-F., & SILKWORTH, J.B. (1977)
Polychlorinated biphenyl and hexachlorobenzene induced humoral
immunosuppression. J. Reticulendothel. Soc., 22: 253-271.
LOOSE, L.D., SILKWORTH, J.B., PITTMAN, K.A., BENITZ, K.-F., &
MUELLER, W. (1978) Impaired host resistance to endotoxin and
malaria in polychlorinated biphenyl- and hexachlorobenzene-treated
mice. Infect. Immun., 20: 30-35.
LORENZ, H. & NEUMEIER, G. (1983) [Polychlorinated biphenyls (PCBs).
A joint report of the Federal Health Office and the Federal
Environment Office.] Munich, MMV Medizin Press (BGA Publication
No. 4/83) (in German).
LOWE, J.I., PARRISH, P.R., PATRICK, J.M., & FORESTER, J. (1972)
Effects of the polychlorinated biphenyl Aroclor 1254 on the
American oyster Crassostrea virginica. Mar. Biol., 17: 209-214.
LU, Y.-C & WONG P.-N (1984) Dermatological, medical and laboratory
findings of patients in Taiwan and their treatments. Am. J. ind.
Med., 5: 81-115.
LU, Y.-C. & WU, Y.-C. (1985) Clinical findings and immunological
abnormalities in Yu-Cheng patients. Environ. health Perspect.,
59: 17-29.
LUARD, E.J. (1973) Sensitivity of Dunaliella and Scenedesmus
(Chlorophyceae) to chlorinated hydrocarbons. Phycologia,
12: 29-33.
LUBET, R.A., LEMAIRE, B.N., AVERY, D., & KOURI, R.E. (1986)
Induction of immunotoxicity in mice by polychlorinated biphenyls.
Arch. Toxicol., 59: 71-77.
LUND, J., ANDERSSON, O., POELLINGER, L., & GUSTAFSSON, J.A. (1986)
In vitro characterization of possible mechanisms underlying the
selective in vivo accumulation of the PCB metabolite
4,4'-bis(methyl-sulphonyl)-2,5,2',5'-tetrachlorobiphenyl in the
lung. Food chem. Toxicol., 24(6/7): 563-566.
LUNDY, P., WURSTER, C.F., & ROWLAND, R.G. (1984) A two-species
marine algal bioassay for detecting aquatic toxicity of chemical
pollutants. Water Res., 18: 187-194.
LUNT, D. & EVANS, W.C. (1970) Microbial metabolism of biphenyl.
Biochem. J., 118: 54-55.
LUOTAMO, M., JÄRVISALO, J., & AITIO, A. (1985) Analysis of
polychlorinated biphenyls (PCBs) in human serum. Environ. health
Perspect., 60: 327-332.
LUTZ, R.J., DEDRICK, R.L., MATTHEWS, H.B., ELING, T., & ANDERSON,
M.W. (1977) A preliminary pharmacokinetic model for several
chlorinated biphenyls in rat. Drug Metab. Dispos., 5: 386-396.
LYNCH, T.R. & JOHNSON, H.E. (1982) Availability of a
hexachlorobiphenyl isomer to benthic amphipods from experimentally
contaminated natural sediments, Philadelphia, Pennsylvania,
American Society for Testing Materials, Aquatic Toxicology and
Hazard Assessment, pp. 273-287 (STP No. 76).
MAACK, L. & SONZOGNI, W.C. (1988) Analysis of polychlorobiphenyl
congeners in Wisconsin fish. Arch. environ. Contam. Toxicol.,
17: 711-719.
MAC, M.J. & SEELYE, J.G. (1981) Patterns of PCB accumulation by fry
of Lake Trout. Bull. environ. Contam. Toxicol., 27: 368-375.
MCCLURE, V.E. (1976) Transport of heavy chlorinated hydrocarbons in
the atmosphere. Environ. Sci. Technol., 10: 1223-1228.
MCCLURG, T.P. (1984) Trace metals and chlorinated hydrocarbons in
Ross seals from Antarctica. Mar. Pollut. Bull., 15: 384-389.
MCCONNELL, E.E., HASS, J.R., ALTMAN, N., & MOORE, J.A. (1979) A
spontaneous outbreak of polychlorinated biphenyl (PCB) toxicity in
Rhesus monkeys (Macaca mulatta): toxicopathology. Lab. anim.
Sci., 29: 666-673.
MCFARLAND, V.A. & CLARKE, J.U. (1989) Environmental occurrence,
abundance and potential toxicity of polychlorinated biphenyl
congeners: Considerations for a congener-specific analysis.
Environ. health Perspect., 81: 225-239.
MACKAY, D. (1989) Modeling the long-term behavior of an organic
contaminant in a large lake: Application to PCBs in Lake Ontario.
J. Great Lakes Res., 15(2): 283-297.
MCKINNEY, J.D. & SINGH, P. (1981) Structure-activity relationships
in halogenated biphenyls: unifying hypothesis for structural
specificity. Chem. biol. Interact., 33: 271-283.
MCKINNEY, J.D., CHAE, K., MCCONNELL, E.E., & BIRNBAUM, L.S. (1985)
Structure-induction versus structure-toxicity relationships for
polychlorinated biphenyls and related aromatic hydrocarbons.
Environ. Health Perspect., 60: 57-68.
MCKINNEY, J.D., KORACH, K.S., & MCLACHLAN, J.A. (1990)
Detoxification of polychlorinated biphenyls. January 27. Lancet,
335: 222-223.
MCLANE, M.A.R. & HUGHES, D.L. (1980) Reproductive success of
screech owls fed Aroclor 1248. Arch. environ. Contam. Toxicol.,
9: 661-665.
MCLEESE, D.W., METCALFE, C.D., & PEZZACK, D.S. (1980) Uptake of
PCBs from sediment by Nereis virens and Crangon septemspinosa.
Arch. environ. Contam. Toxicol., 9: 507-518.
MACLEOD, H.A., SMITH, D.C., & BLUMAN, N. (1980) Pesticide residues
in the total diet in Canada, V: 1976 to 1978. J. Food Saf.,
2: 141-164.
MCMANUS, G.B., WYMAN, K.D., PETERSON, W.T., & WURSTER, C.F. (1983)
Factors affecting the elimination of PCBs in the marine copepod
Acartia tonsa. Estuarine coastal Shelf Sci., 17: 421-430.
MCNULTY, W.P. (1985) Toxicity and fetotoxicity of TCDD, TCDF and
PCB isomers in Rhesus macaques (Macaca mulatta). Environ. Health
Perspect., 60: 77-88.
MCNULTY, W.P., BECKER, G.M., & CORY, H.T. (1980) Chronic toxicity
of 3,4,3',4'- and 2,5,2',5'-tetrachlorobiphenyls in Rhesus
macaques. Toxicol. appl. Pharmacol., 56: 182-190.
MACLEOD, K.E. (1981) Polychlorinated biphenyls in indoor air.
Environ. Sci. Technol., 15(8): 926-928.
MAFF (1986) Report of file working party on pesticide residues
(1982-1985): 16th report of the Steering Group on Food
Surveillance, London, Ministry of Agriculture, Fisheries, and Food,
Her Majesty's Stationery Office, (Food Surveillance Paper No. 16).
MAFF (1989) Migration of substances from food contact materials
into food: 26th report of the Steering Group on Food Surveillance.
Progress report of the Working Party on Chemical Contaminants from
Food, London, Ministry of Agriculture, Fisheries, and Food, Her
Majesty's Stationery Office, 58 pp (Food Surveillance Paper
No. 26).
MAHANTY, H.K. (1975) A study of the effects of polychlorinated
biphenyl (Aroclor 1242) on an aquatic plant Spirodela oligorrhiza
(Kurz) Hegelm. Bull. environ. Contam. Toxicol., 14: 558-565.
MAHANTY, H.K. & FINERAN, B.A. (1976) Effects of a polychlorinated
biphenyl (Aroclor 1242) on the ultrastructure of frond cells in the
aquatic plant Spirodela oligorrhiza (Kurz) Hegelm. N. Z. J. Bot.,
14: 13-18.
MAHANTY, H.K. & MCWHA, J.A. (1976) Sensitivity of Spirodela
oligorrhiza (Kurz) Hegelm. to a polychlorinated biphenyl (Aroclor
1242). N. Z. J. Bot., 14: 9-12.
MAKI, A.W. & JOHNSON, H.E. (1975) Effects of PCB (Aroclor 1254) and
p,p'DDT on production and survival of Daphnia magna Strauss.
Bull. environ. Contam. Toxicol., 13: 412-416.
MAKIURA, S., AOE, H., SUGIHARA, S., HIARO, K., ARAI, M., & ITO, N.
(1974) Inhibitory effect of polychlorinated biphenyls on liver
tumorigenesis in rats treated with 3'-methyl-4-dimethyl-
aminobenzene, N-2-fluorenylacetamide and diethylnitrosamine. J.
Natl Cancer Inst., 53: 1253-1257.
MALINS, D.C., MCCAIN, B.B., BROWN, D.W., VARANASI, U., KRAHN, M.M.,
MYERS, M.S., & CHAN, S.-L. (1987) Sediment-associated contaminants
and liver diseases in bottom-dwelling fish. Hydrobiologia,
149(1): 64-74.
MANCHESTER-NEESVIG, J.B. & ANDREN, A.W. (1989) Seasonal variation
in the atmospheric concentration of polychlorinated biphenyl
congeners. Environ. Sci. Technol., 23: 1138-1148.
MANSKE, D.D. & JOHNSON, R.D. (1975) Pesticide residues in total
diet samples (VIII). Pestic. monit. J., 9(2): 94-105.
MANSKE, D.D. & JOHNSON, R.D. (1977) Pesticide and other chemical
residues in total diet samples (X). Pestic. monit. J.,
10(4): 134-148.
MARCUS, J.M. & MATHEWS, T.D. (1987) Polychlorinated biphenyls in
blue crabs from South Carolina. Bull. environ. Contam. Toxicol.,
39(5): 857-862.
MARINUCCI, A.C. & BARTHA, R. (1982) Accumulation of the
polychlorinated biphenyl Aroclor 1242 from contaminated detritus
and water by the saltmarsh detritivore, Uca pugnax. Bull.
environ. Contam. Toxicol., 29: 326-333.
MARKARD, C. (1988) [Organic substances in sewage sludge - a danger
to the food chain?] Korresp. Abwasser, 35(5): 449-455 (in German).
MARKS, T.A., KIMMEL, G.L., & STAPLES, R.E. (1981) Influence of
symmetrical polychlorinated biphenyl isomers on embryo and fetal
development in mice I. Teratogenicity of 3,3',4,4',5,5'-
hexachlorobiphenyl. Toxicol. appl. Pharmacol., 61: 269-276.
MARONI, M., COLUMBI, A., CANTONI, S., FERIOLI, E., & FOA, V.
(1981a) Occupational exposure to polychlorinated biphenyls in
electrical workers. I. Environmental and blood polychlorinated
biphenyl concentrations. Br. J. ind. Med., 38(1): 49-54.
MARONI, M., COLUMBI, A., ARBOSTI, G., CANTONI, S., & FOA, V.
(1981b) Occupational exposure to polychlorinated biphenyls in
electrical workers II. Health effects. Br. J. ind. Med.,
38(1): 55-60.
MARONI, M., COLOMBI, A., FERIOLI, A., & FOA, V. (1984) Evaluation
of porphyrinogenesis and enzyme induction in workers exposed to
PCB. Med. Lav., 75(3): 188-199.
MARTINEAU, D., BELAND, P., DESJARDINS, C., & LAGACE, A. (1987)
Levels of organochlorine chemicals in tissues of beluga whales
(Delphinapterus leucas) from the St. Lawrence Estuary, Quebec,
Canada. Arch. environ. Contam. Toxicol., 16: 137-147.
MASSACHUSETTS DEPARTMENT OF PUBLIC HEALTH (1987) The greater New
Bedford PCB health effects study 1984-1987, Boston, Massachusetts,
Massachusetts Department of Public Health.
MASUDA, Y., KAGAWA, R., & KURATSUNE, M. (1972) Polychlorinated
biphenyls in carbonless copying paper. Nature (Lond.), 237: 41-42.
MASUDA, Y., KAGAWA, R., & KURATSUNE, M. (1974a) [Polychlorinated
biphenyls in Yusho patients and ordinary persons.] Fukuoka Acta
med., 65: 17-24 (in Japanese).
MASUDA, Y., KAGAWA, R., SHIMAMURA, K., TAKADA, M., & KURATSUNE, M.
(1974b) [Polychlorinated biphenyls in the blood of Yusho patients
and ordinary persons.] Fukuoka Acta. med., 65: 25-27 (in Japanese).
MASUDA, Y., KAGAWA, R., KUROKI, H., KURATSUNE, M., YOSHIMURA, T.,
TAKI, I., KUSUDA, M., YAMASHITA, F., & HAYASHI, M. (1978a) Transfer
of polychlorinated biphenyls from mothers to foetuses and infants.
Bull. environ. Contam. Toxicol., 16: 543-546.
MASUDA, Y., KAGAWA, R., TOKUDOME, S., & KURATSUNE, M. (1978b)
Transfer of polychlorinated biphenyls to the foetuses and offspring
of mice. Toxicology, 6: 331-340.
MASUDA, Y., KAGAWA, R., KUROKI, H., TOKUDOME, S. & KURATSUNE, M.
(1979) Transfer of various polychlorinated biphenyls to the fetuses
and offspring of mice. Food Cosmet. Toxicol., 17(6): 623-627.
MASUDA, Y., KUROKI, H., HARAGUCHI, K., & NAGAYAMA, J. (1985) PCB
and PCDF congeners in the blood and tissues of Yusho and Yu-Cheng
patients. Environ. health Perspect., 59: 53-58.
MATTHEWS, H.B. & ANDERSON, M.W. (1975a) Effect of chlorination on
the distribution and excretion of polychlorinated biphenyls. Drug
Metab. Dispos., 3: 371-380.
MATTHEWS, H.B. & ANDERSON, M.W. (1975b) The distribution and
excretion of 2,4,5,2',5'-pentachlorobiphenyl in the rat. Drug
Metab. Dispos., 3: 211-219.
MATTHEWS, H.B. & ANDERSON, M.W. (1976) PCB chlorination versus PCB
distribution and excretion. In: Proceedings of the National
Conference on Polychlorinated Biphenyls, Chicago, 19-21 November
1975, Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances, pp. 50-56 (EPA-560/6-75-004).
MATTHEWS, H.B. & DEDRICK, R.L. (1984) Pharmacokinetics of PCBs.
Annu. Rev. Pharmacol. Toxicol., 24: 85-103.
MATTHEWS, H.B. & TUEY, D.B. (1980) The effect of chlorine position
on the distribution and excretion of four hexachlorobiphenyl
isomers. Toxicol. appl. Pharmacol., 53: 377-388.
MATTSSON, R., MATTSSON, A., KIHLSTRÖM, J.E., & LINDAHL-KIESSLING,
K. (1981) Effects of a hexachlorinated biphenyl on lymphoid organs
and resorption of foetuses in pregnant mice. Arch. environ. Contam.
Toxicol., 10: 281-288.
MAUCK, W.L., MEHRLE, P.M., & MAYER, F.L. (1978) Effects of the
polychlorinated biphenyl Aroclor 1254 on growth, survival, and bone
development in brook trout (Salvelinus fontinalis). J. Fish Res.
Board Can., 35: 1084-1088.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Washington, DC, US Department of Commerce,
National Technical Information Service, 274 pp ((NTIS)
PB87-188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior,
Fish & Wildlife Service, pp. 506-553 (Resource Publication
No. 160).
MAYER, F.L, MEHRLE, P.M., & SANDERS, H.O. (1977) Residue dynamics
and biological effects of polychlorinated biphenyls in aquatic
organisms. Arch. environ. Contam. Toxicol., 5: 501-511.
MEHLMAN, M.A., YIN, L., & NIELSEN, R.C. (1974) Metabolic changes in
"frozen-clamped" livers of rats caused by ingestion of
polychlorinated biphenyls. Toxicol. appl. Pharmacol., 27: 300-307.
MEIER, P.G. & REDISKE, R.R. (1984) Oil and PCB interactions on the
uptake and excretion in midges. Bull. environ. Contam. Toxicol.,
33: 223-232.
MEIGS, J.W., ALBOM, J.J., & KARTIN, B.L. (1954) Chloracne from an
unusual exposure to Aroclor. J. Am. Med. Assoc., 154: 1417-1418.
MELVÅS, B. & BRANDT, I. (1973) The distribution and metabolism of
labelled polychlorinated biphenyls in mice and quails. In:
Proceedings of the Polychlorinated Biphenyls Conference II,
Stockholm, 1972, Solna, Sweden, National Environmental Protection
Board, pp. 87-90 (Publication No. 4E).
MENDOZA-FIGUEROA, T., LOPEZ-REVILLA, R., & VILLA-TREVINO, S. (1985)
Aroclor 1254 increases the genotoxicity of several carcinogens to
liver primary cell cultures. J. Toxicol. environ. Health,
15: 245-254.
MERKENS, L.S. & KINTER, W.B. (1971) Acute toxicity of a mixture of
polychlorinated biphenyls (Aroclor 1221) and DDT in a marine
teleost (Fundulus heteroclitus) and effect on serum osmolality,
Na+ and K+. Bull. Mt Desert Isl. biol. Lab., 11: 64-68.
MERSON, M.H. & KIRKPATRICK, R.L. (1976) Reproductive performance of
Captive White-footed mice fed a PCB. Bull. environ. Contam.
Toxicol., 16: 392-398.
MES, J. (1987) Polychlorobiphenyl in children's blood. Environ.
Res., 44: 213-220.
MES, J. & MARCHAND, L. (1987) Comparison of some specific
polychlorinated biphenyl isomers in human and monkey milk. Bull.
environ. Contam. Toxicol., 39: 736-742.
MES, J., COFFIN, D.E., & CAMPBELL, D. (1974) Polychlorinated
biphenyl and organochlorine pesticide residues in Canadian chicken
eggs. Pestic. monit. J., 8: 8-11.
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 28: 97-104.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine pesticides in
milk and blood of Canadian women during lactation. Arch. environ.
Contam. Toxicol., 13: 217-223.
MES, J., DAVIES, D.J., TURTON, D., & SUN, W.-F. (1986) Levels and
trends of chlorinated hydrocarbon contaminants in the breast milk
of Canadian women. Food Addit. Contam., 3(4): 313-322.
MES, J., ARNOLD, D.L., BRYCE, F., DAVIES, D.J., & KARPINSKI, K.
(1989a) The effect of long-term feeding of Aroclor 1254 to female
Rhesus monkeys on their polychlorinated biphenyl tissue levels.
Arch. environ. Contam. Toxicol., 18: 858-865.
MES, J., NEWSOME, W.H., & CONACHER, H.B.S. (1989b) Determination of
some specific isomers of polychlorinated biphenyl congeners in
fatty foods of the Canadian diet. Food Addit. Contam.,
6(3): 365-375.
MICHAELS, R.A., ROWLAND, G., & WURSTER, C.F. (1982) Polychlorinated
biphenyls (PCB) inhibit photosynthesis per cell in the marine
diatom Thalassiosira pseudonana. Environ. Pollut., 27: 9-14.
MILLER, D.L. & OGILVIE, D.M. (1975) Temperature selection in brook
trout (Salvelinus fontinalis) following exposure to DDT, PCB, or
phenol. Bull. environ. Contam. Toxicol., 14: 545-551.
MILLER, M.M., GHODBANE, S., WASIK, S.P., TEWARI, Y.B., & MARTIRE,
D.E. (1984) Aqueous solubilities, octanol/water partition
coefficients, and entropies of melting of chlorinated benzenes and
biphenyls. J. chem. Eng. Data, 29: 184-190.
MILLING, A., MULLER, W.F., COULSTON, F., & KORTE, F. (1979)
Comparative metabolism of 2,2'-dichlorobiphenyl-14C in mice, rats
and Rhesus monkeys after a single oral application. Chemosphere,
1: 15-19.
MINEAU, P., FOX, G.A., NORSTROM, R.J., WESELOH, D.V., HALLETT,
D.J., & ELLENTON, J.A. (1984) Using the herring gull to monitor
levels and effects of organochlorine contamination in the Canadian
Great Lakes. In: Nriagu, J.O., Nriagu, J.O., & Simmons, M.S., ed.
Toxic contaminants in the Great Lakes, New York, John Wiley and
Sons, pp. 425-452.
MIO, T. & SUMINO, K. (1985) Mechanism of biosynthesis of
methylsulfones from PCBs and related compounds. Environ. Health
Perspect., 59: 129-135.
MIO, T., SUMINO K., & MIZUTANI, T. (1976) Sulfur-containing
metabolites of 2,5,2',5'-tetrachlorobiphenyl, a major component of
commercial PCBs. Chem. pharm. Bull., 24: 1958-1960.
MIRANDA, C.L., HENDERSON, M.C., WANG, J.L., NAKAUE, H.S., & BUHLER,
D.R. (1987) Effects of polychlorinated biphenyls on porphyrin
synthesis and cytochrome P-450-dependent mono-oxygenase in small
intestine and liver of Japanese quail. J. Toxicol. environ. Health,
20: 27-35.
MIYATA, H., FUKUSHIMA, S., KASHIMOTO, T., & HUNITA, N. (1985) PCBs,
PCQs, and PCDFs in tissues of Yusho and Yu-Cheng patients. Environ.
Health Perspect., 59: 67-72.
MIYATA, H., TAKAYAMA, K., OGAKI, J., KASHIMOTO, T., & FUKUSHIMA, S.
(1987) Polychlorinated dibenzo- p-dioxins in blue mussel from
marine coastal water in Japan. Bull. environ. Contam. Toxicol.,
39(5): 877-883.
MIYAZAKI, A., HOTTA, T., KATAYAMA, J., & KIMURA, Y. (1975)
Absorption and translocation of PCB into crops. Bull. Osaka agric.
Res. Cent., 12: 135-142.
MIZUNOYA, Y., TANIGUCHI, S., KUSUMOTO, K., MORITA, S., YAMADA, A.,
BABA, T., & OGAKI, S. (1974) [Effects of polychlorinated biphenyls
on fetuses and offspring in rats.] J. Food Hyg. Soc. Jpn,
15: 252-260 (in Japanese).
MIZUTANI, T., HIDAKA, K., OHE, T., & MATSUMOTO, M. (1977) A
comparative study on accumulation and elimination of
tetrachlorobiphenyl isomers in mice. Bull. environ. Contam.
Toxicol., 18: 452-461.
MOILANEN, R., PYYSALO, H., WICKSTROM, K., & LINKO, R. (1982) Time
trends of chlordane, DDT, and PCB concentrations in pike (Esox
lucius) and Baltic herring (Clupea harengus) in the Turku
Archipelago, Northern Baltic Sea for the period 1971-1982. Bull.
environ. Contam. Toxicol., 29: 334-340.
MOKSNES, M.T. & NORHEIM, G. (1986) Levels of chlorinated
hydrocarbons and composition of PCB in herring gull Larus
argentatus eggs collected in Norway in 1969 compared to 1979-81.
Environ. Pollut., B11: 109-116.
MOORE, S.A. & HARRISS, R.C. (1972) Effects of polychlorinated
biphenyl on marine phytoplankton communities. Nature (Lond.),
240: 356-357.
MOORE, S.A. & HARRISS, R.C. (1974) Differential sensitivity to PCB
by phytoplankton. Mar. Pollut. Bull., 5: 174-176.
MORGAN, R.W., WARD, J.M., & HARTMAN, P.E. (1981) Aroclor
1254-induced intestinal metaplasia and adenocarcinoma in the
glandular stomach of F344 rats. Cancer Res., 41: 5052-5059.
MORIARTY, F. (1969) The effects of polychlorobiphenyls on
Chorthippus brunneus (Saltatoria: Acrididae). Entomol. Exp.
Appl., 12: 206-210.
MORITA, M., NAKAGAVA J., & RAPPE, C. (1978) Polychlorinated
dibenzofuran (PCDF) formation from PCB mixture by heat and oxygen.
Bull. environ. Contam. Toxicol., 19: 665-670.
MORSE, R.A., CULLINEY, T.W., GUTENMANN, W.H., LITTMAN, C.B., &
LISK, D.J. (1987) Polychlorinated biphenyls in honey bees. Bull.
environ. Contam. Toxicol., 38: 271-276.
MOSELEY, C.L., GERACI, C.L., & BURG, J. (1982) Polychlorinated
biphenyl exposure in transformer maintenance operations. Am. Ind.
Hyg. Assoc. J., 43: 170-174.
MOSSER, J.L., TENG, T., FISHERR, N.S., & WURSTER, C.F. (1972)
Polychlorinated biphenyls: Toxicity to certain phytoplankters.
Science, 175: 191-192.
MOSSER, J.L., TENG, T., WALTHER, W.G., & WURSTER, C.F. (1974)
Interactions of PCBs, DDT, and DDE in a marine diatom. Bull.
environ. Contam. Toxicol., 12: 665-668.
MOZA, P., WEISBERGER, I., KLEIN, W., & KORTE, F. (1974) Metabolism
of 2,2'-dichlorobiphenyl-14C in two plant-water-soil-systems. Bull.
environ. Contam. Toxicol., 12: 541-546.
MOZA, P., WEISBERGER, I., & KLEIN, W. (1976a) Fate of
2,2'-dichlorobiphenyl-14C in carrots, sugar beet, and soil under
outdoor conditions. J. agric. food Chem., 24: 881-885.
MOZA, P., KILZER, L., WEISGERBER, I., & KLEIN, W. (1976b)
Contribution to ecological chemistry CXV. Metabolism of
2,5,4'-trichlorobiphenyl-14C and 2,4,6,2',4'-pentachloro-
biphenyl-14C in the marsh plant Veronica beccabunga. Bull.
environ. Contam. Toxicol., 16: 454-463.
MOZA, P., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1979a) Studies
with 2,4,5-trichlorobiphenyl-14C and 2,2,4,4,6-penta-
chlorobiphenyl-14C in carrots, sugar, beet, and soil. J. agric.
food Chem., 27: 1120-1124.
MOZA, P.N., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1979b) Long-term
uptake of lower chlorinated biphenyls and their conversion products
by spruce trees (Picea abies) from soil treated with sewage
sludge. Chemosphere, 8: 373-375.
MROZEK, E. & LEIDY, R.B. (1981) Investigation of selective uptake
of polychlorinated biphenyls by Spartina alterniflora Loisel.
Bull. environ. Contam. Toxicol., 27: 481-488.
MROZEK, E., SENECA, E.D., & HOBBS, L.L. (1982) Polychlorinated
biphenyl uptake and translation by Spartina alterniflora Loisel.
Water Air Soil Pollut., 17: 3-15.
MROZEK, E., QUEEN, W.H., & HOBBS, L.L. (1983) Effects of
polychlorinated biphenyls on growth of Spartina alterniflora
Loisel. Environ. exp. Bot., 23: 285-292.
MUEHLEBACK, S. & BICKEL, M.H. (1981) Pharmacokinetics in rats of
2,4,5,2',4',5'-hexachlorobiphenyl an unmetabolisable lipophilic
model compound. Xenobiotica, 11: 249-259.
MUIR, D.C.G., NORSTROM, R.J., & SIMON, M. (1988) Organochlorine
contaminants in Arctic marine food-chains: Accumulation of specific
polychlorinated biphenyls and chlordane-related compounds. Environ.
Sci. Technol., 22: 1071-1077.
MULHERN, B.M., CROMARTIE, E., REICHEL, W.L., & BELISLE, A.A. (1971)
Semiquantitative determination of polychlorinated biphenyls in
tissue samples by thin layer chromatography. J. Assoc. Off. Agric.
Chem., 54: 548-550.
MULLER, W.F., HOBSON, W., FULLER, G.B., KNAUF, W., COULSTON, F., &
KORTE, F. (1978) Endocrine effects of chlorinated hydrocarbons in
Rhesus monkeys. Ecotoxicol. Environ. Saf., 2: 161-172.
MULLIN, M.D., POCHINI, C.M., MCCRINDLE, S., ROMKES, M., SAFE, S.H.,
& SAFE, L.M. (1984) High-resolution PCB analysis: Synthesis and
chromatographic properties of all 209 PCB congeners. Environ. Sci.
Technol., 18: 468-476.
MURADO, M.A., TEJEDOR, M.C., & BALUJA, G. (1976) Interactions
between polychlorinated biphenyls (PCBs) and soil microfungi.
Effects of Aroclor 1254 and other PCBs on Aspergillus flavus
cultures. Bull. environ. Contam. Toxicol., 15: 768-774.
MURAI, Y. & KUROIWA, Y. (1971) Peripheral neuropathy in
chlorobiphenyl poisoning. Neurology, 21: 1173-1176.
MURPHY, T.J. (1984) Atmospheric inputs of chlorinated hydrocarbons
to the Great Lakes. In: Nriagu, J.O., Nriagu, J.O., & Simmons,
M.S., ed. Toxic contaminants in the Great Lakes, New York, John
Wiley and Sons, pp. 53-79.
MURPHY, T.J., FORMANSKI, L.J., BROWNAWELL, B., & MEYER, J.A. (1985)
Polychlorinated biphenyl emissions to the atmosphere in the Great
Lakes region. Municipal landfills and incinerators. Environ. Sci.
Technol., 19(10): 942-946.
MUSSALO-RAUHAMAA, H., PYYSALO, H., & MOILANEN, R. (1984) Influence
of diet and other factors on the levels of organochlorine compounds
in human adipose tissue in Finland. J. Toxicol. environ. Health,
13: 689-704.
MUSSALO-RAUHAMAA, H., PYYSALO, H., & ANTERVO, K. (1988) Relation
between the content of organochlorine compounds in Finnish human
milk and characteristics of the mothers. J. Toxicol. environ.
Health, 25: 1-19.
NAGAI, J., FURUKAWA, M., YAE, Y., & IKEDA, Y. (1969)
[Clinicochemical investigation of chlorobiphenyls poisoning.
Especially on the serum lipid analysis of the patients.] Fukuoka
Acta med., 60: 475-488 (in Japanese).
NAGASAKI, H., TOMII, S., MEGA, T., SUGIHARA, S., MIYATA, Y., & ITO,
N. (1974) [Analysis of various factors on liver carcinogenesis in
mice induced by benzene hexachloride (BHC) and technical
polychlorinated biphenyls (PCBs).] J. Nara Med. Assoc., 25: 635-648
(in Japanese).
NAGASAKI, H., KAWABATA, H., MIYATA, Y., INOUE, K., AOE, H., & ITO,
N. (1975) Effects of various factors on induction of liver tumours
in animals by the alpha-isomer of benzenehexachloride. Gann,
66: 185-191.
NAGAYAMA, J. (1975) Chlorinated dibenzofurans in Kanechlors and
rice oils used by patients with Yusho. Fukuoka Acta med., 66: 593.
NAGAYAMA, L., KURATSUNE, M., & MASUDA, Y. (1976) Determination of
chlorinated dibenzofurans in Kanechlors and "Yusho oil". Bull.
environ. Contam. Toxicol., 15(1): 9-13.
NAGAYAMA, J., KURATSUNE, M., & MASUDA, Y. (1981) Formation of
polychlorinated dibenzofurans by heating polychlorinated biphenyls.
Fukuoka Acta med., 72: 136-141.
NAKANISHI, Y., SHIGEMATSU, N., KURITA, Y., MATSUBA, K., KANEGAE,
H., ISHIMURA, S., & KAWAZOE, Y. (1985) Respiratory involvement and
immune status in Yusho patients. Environ. health Perspect.,
59: 31-36.
NARBONNE, J.F. (1979) Determination of the "no effect level" of
Phenoclor DP6 on five microsomal parameters in rat livers.
Toxicology, 14: 91-93.
NARBONNE, J.F. (1980) Time course of induction of microsomal
enzymes following dietary administration of a polychlorinated
biphenyl (Phenoclor DP6). Toxicol. appl. Pharmacol., 56: 1-7.
NARBONNE, J.F., BOURDICHON, M., GALLIS, J.L., & DAUBEZE, M. (1978)
Polychlorinated biphenyls: effect of diet level on ATPase activity
in rats. Bull. environ. Contam. Toxicol., 20: 184-190.
NAS (1979) Polychlorinated biphenyls, Washington, DC, National
Academy of Sciences, 182 pp.
NAU-RITTER, G.M., WURSTER, C.F., & ROWLAND, R.G. (1982)
Partitioning of [14C]PCB between water and particulates with
various organic contents. Water Res., 16: 1615-1618.
NCI (1975) Third national cancer survey, Bethesda, Maryland,
National Cancer Institute.
NCI (1978) Bioassay of Aroclor (trademark) 1254 for possible
carcinogenicity (CAS 