
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 2
POLYCHLORINATED BIPHENYLS AND TERPHENYLS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of either the World Health Organization or the United Nations
Environment Programme.
Published under the joint sponsorship of
the United Nations Environment Programme
and the World Health Organization
World Health Organization
Geneva, 1976
ISBN 92 4 154062 1
(c) World Health Organization 1976
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed of any opinion of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR POLYCHLORINATED BIPHENYLS AND
TERPHENYLS
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1. Introductory note
1.2. Summary
1.2.1. Composition and analytical problems
1.2.2. Sources and pathways in the environment
1.2.3. Concentration in the environment
1.2.4. Metabolism
1.2.5. The extent of human exposure
1.2.6. Experimental studies on the effects of PCBs and
PCTs
1.2.7. Clinical studies of the effects of PCBs in man
1.2.8. Dose-effect relationships
1.3. Recommendations for further research
1.3.1. Analytical methods
1.3.2. Environmental pollution
1.3.3. Effects on man
1.3.4. Experimental studies
1.3.5. PCB substitutes
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Chemical composition
2.2. Purity of products
2.3. Determination of PCB residues
2.3.1. Extraction of sample
2.3.2. Clean-up
2.3.3. Chromatographic separation of PCBs
2.3.4. Quantification of PCB content
2.3.5. Accuracy of PCB determination
2.3.6. Confirmation of identity
2.4. Determination of PCTs
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1. Production and uses of PCBs
3.2. Entry of PCBs into the environment
3.2.1. Release of PCBs into the atmosphere
3.2.2. Leakage and disposal of PCBs in industry
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATION
4.1. Environmental transport
4.1.1. Air transport
4.1.2. Transport in soil
4.1.3. Transport in water
4.1.4. Transport through biota
4.2. Transformation in the environment
4.2.1. Abiotic transformation
4.2.2. Biotransformation
4.2.3. Metabolism in limited ecosystems
4.3. Biological accumulation
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1. Air
5.2. Soil and sediments
5.3. Water
5.4. Living organisms
5.4.1. The influence of local pollution
5.4.2. The influence of fat content of tissues
5.4.3. The influence of the trophic stage in food chains
5.4.4. Indicator organisms
5.5. The extent of human exposure to PCBs and PCTs
5.5.1. Air and water
5.5.2. Food
5.5.3. Occupational exposure
5.5.4. Other sources of exposure
5.5.5. Biological indices of human exposure
5.5.5.1 Body fat
5.5.5.2 Blood
5.5.5.3 Human milk
5.5.6. Estimated daily intake
6. METABOLISM
6.1. Absorption
6.2. Tissue distribution of PCBs
6.3. Tissue distribution of PCTs
6.4. Placental transport
6.5. Excretion and elimination
6.5.1. Milk
6.5.2. Eggs
6.5.3. Urine and faeces
6.6. Biotransformation
6.6.1. Metabolic degradation
6.6.2. The effect of structure on retention
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF PCBs AND PCTs
7.1. Toxic effects in different species
7.1.1. Mammals
7.1.1.1 Acute oral and intravenous toxicity
7.1.1.2 Subacute oral toxicity
7.1.1.3 Chronic oral toxicity
7.1.1.4 Dermal toxicity
7.1.1.5 Inhalation toxicity
7.1.2. Birds
7.1.3. Aquatic organisms
7.1.3.1 Fish
7.1.3.2 Aquatic invertebrates
7.1.3.3 Microorganisms
7.2. Toxicity of impurities in commercial PCBs
7.3. Toxicity of the PCTs
7.4. Biochemical effects
7.4.1. Induction of enzymes
7.4.2. Porphyria
7.4.3. Effects on steroid metabolism
7.4.4. Other biochemical effects
7.4.5. Potentiation and antagonism by PCBs
7.5. Cytotoxic effects
7.6. Immunosuppressive effects
7.7. Effects on reproduction
7.8. Neoplasia and adenofibrosis
8. EFFECTS OF PCBs AND PCTs ON MAN -- EPIDEMIOLOGICAL AND CLINICAL
STUDIES
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO PCBs AND PCTs
9.1. Species variation
9.2. Dose-effect relationships
9.2.1. Body weight
9.2.2. Effects on liver
9.2.3. Reproduction
9.2.4. Immunosuppression
9.2.5. Skin effects
9.3. Nondetected effect levels
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in
criteria documents as accurately as possible without unduly delaying
publication, mistakes might have occurred and are likely to occur in
the future. In the interest of all users of the environmental health
criteria documents, readers are kindly requested to communicate any
errors found to the Division of Environmental Health, World Health
Organization, Geneva, Switzerland, in order that they may be included
in corrigenda which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that information may be considered in the event of
updating and re-evaluating the conclusions contained in the criteria
documents.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR POLYCHLORINATED
BIPHENYLS AND TERPHENYLS
Copenhagen, 20-24 October 1975
Participants
Members
Dr. V. Benes, Department of Toxicology, Institute of Hygiene and
Epidemiology, Prague, Czechoslovakia (Vice-Chairman)
Dr. H. L Falk, National Institute for Environment Health Services,
Research Triangle Park, NC, USA
Mr L. Gordts, Institute of Hygiene and Epidemiology, Brussels, Belgium
Dr. D. L. Grant, Pesticides Section, Toxicology Evaluation Division,
Bureau of Chemical Safety, Department of Health and Welfare,
Ottawa, Ontario, Canada (Rapporteur)
Mr A. V. Holden, Department of Agriculture and Fisheries for Scotland,
Freshwater Fisheries Laboratory, Faskally, Pitlochry, Perthshire,
Scotland
Dr. S. Jensen, Naturvardsverkets Specialanalytiska Laboratorium,
Wallenberg-laboratoriet, Lilla Frescati, Stockholm, Sweden
Dr. Renate Kimbrough, Center for Disease Control, Toxicology Branch,
Atlanta, GA, USA
Professor H. Kuratsune, Department of Public Health, Faculty of
Medicine, Kyushu University, Fukuoka, Japan
Dr. E. Schulte, Institut fur Lebensmittelchemie der Westfälischen
Wilhelms-Universität, Munster/Westf., Federal Republic of Germany
Dr. J. G. Vos, Laboratory for Pathology, National Institute for Public
Health, Bilthoven, Netherlands (Chairman)
Observer
Dr. D. Axelrod, Division of Laboratories and Research, New York State
Department of Health, Albany, NY, USA
Unable to attend
a Dr M. V. Kryznovskaja, All-Union Institute for Research on
Hygiene and Toxicology of Pesticides, Polymers, and Plastics, Kiev,
USSR.
Representatives of other organizations:
International Federation of Pharmaceutical Manufacturers Associations
Professor P. Fabiani, Laboratoire du Chimie et de Toxicologie de
l'Hōtel Die, Paris, France
Permanent Commission and International Association on Occupational
Health
Dr Aa. Grut, State Labour Inspection Service, Hellerup, Denmark
Secretariat:
Dr J. C. Gage, 21 Lambolle Road, London, England (Temporary Adviser)
Dr. M. J. Suess, Environmental Pollution Control, WHO Regional Office
for Copenhagen, Denmark
Dr A. H. Wahba, Health Laboratory Services, WHO Regional Office for
Europe, Copenhagen, Denmark
Dr. G. Vettorazzi, Food Additives Unit, Division of Environmental
Health, World Health Organization, Geneva (Secretary)
Dr. D.C. Villeneuve,a Laboratory of Toxicology, National Institute of
Public Health, Bilthoven, Netherlands (Temporary Adviser)
a On sabbatical leave from: Biochemical Toxicology Unit,
Environmental Toxicology Division, Environmental Health Centre,
Department of Health and Welfare, Ottawa, Ontario, Canada.
ENVIRONMENTAL HEALTH CRITERIA FOR POLYCHLORINATED BIPHENYLS AND
TERPHENYLS
A WHO Task Group on Environmental Health Criteria for
Polychlorinated Biphenyls (PCBs) and Terphenyls (PCTs) met in
Copenhagen from 20-24 October 1975. Dr F. A. Bauhofer, Director of
Health Services of the WHO Regional Office for Europe opened the
meeting on behalf of the Director-General and the Director of the
Regional Office for Europe. The Task Group reviewed and amended the
second draft criteria document and made an evaluation of the health
risks from exposure to these compounds.
The preparation of the first draft criteria document was based on
national reviews of health effects research on polychlorinated
biphenyls, received from the national focal points collaborating in
the WHO Environmental Health Criteria Programme in Canada, the Federal
Republic of Germany, Finland, France, Japan, the Netherlands, New
Zealand, Sweden, the United Kingdom, and the USA. Dr J. C. Gage,
London, England, prepared the first draft as well as the second draft
criteria document which took into account the comments received from
the national focal points in Canada, Czechoslovakia, the Federal
Republic of Germany, France, Japan, New Zealand, Sweden, the United
Kingdom, the USA, and the USSR; from the United Nations Industrial
Development Organization (UNIDO), Vienna, the Food and Agriculture
Organization of the United Nations (FAO) Rome, and from the United
Nations Educational, Scientific and Cultural Organization (UNESCO)
Paris; the Organization for Economic Co-operation and Development,
Paris, and the Health Protection Directorate of the Commission of the
European Communities, Luxembourg.
Comments were also received at the request of the Secretariat,
from Dr K. Kojima, Japan, Dr D. S. May, United Kingdom, and Dr V.
Zitko, Canada.
The collaboration of these national institutions, international
organizations and individual experts is gratefully acknowledged.
Without their assistance the document could not have been completed.
This document is based primarily on original publications listed
in the reference section. In addition, some recent publications
reviewing the environmental and health aspects of polychlorinated
biphenyls were also used. These include reviews by the Commission of
the European Communities (1974), the US Department of Health,
Education and Welfare (1972), the International Agency for Research on
Cancer (1974), the International Council for the Exploration of the
Sea (1974), Jensen (1974), Kimbrough (1974), the National Swedish
Environment Protection Board (1973), the Panel on Hazardous Trace
Substances (1972), the USDA/USDC/EPA/FDA/USDA (1972), and a WHO
working group (1973).
Details about the WHO Environmental Health Criteria Programme
including the definition of some terms frequently used in the document
may be found in the general introduction to the Environmental Health
Criteria Programme published together with the Environmental Health
Criteria Document on mercury (Environmental Health Criteria 1, Geneva
World Health Organization, 1976).
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1 Introductory Note
The commercial production of the polychlorinated biphenyls (PCBs)
began in 1930, and during the 1930s cases of poisoning were reported
among men engaged in their manufacture. The nature of this
occupational disease was characterized by a skin affliction with
acneiform eruptions; occasionally the liver was involved, in some
cases with fatal consequences. Subsequent safety precautions appear
largely to have prevented further outbreaks of this disease in
connection with the manufacture of PCBs, but from 1953 onwards, cases
have been reported in Japanese factories manufacturing condensers.
The distribution of PCBs in the environment was not recognized
until Jensen started an investigation in 1964 to ascertain the origins
of unknown peaks observed during the gas-liquid chromatographic
separation of organochlorine pesticides from wild-life samples. In
1966, he and his colleagues succeeded in attributing these to the
presence of PCBs. Since that time, investigations in many parts of the
world have revealed the widespread distribution of PCBs in
environmental samples.
The serious outbreaks of poisoning in man and in domestic animals
from the ingestion of food accidentally contaminated with PCBs have
stimulated investigations into the toxic effects of PCBs on animals
and on nutritional food chains. This has resulted in limitation of the
commercial exploitation of PCBs and polychlorinated terphenyls (PCTs),
and in regulations to limit the residues in human and animal food.
The environmental impact of the PCBs and PCTs has been the subject
of several reviews, and has been discussed at a number of regional and
international meetings. The relevant publications are mentioned in the
previous section.
1.2 Summary
1.2.1 Composition and analytical problems
The commercial production of PCBs and PCTs by the direct
chlorination of biphenyl and terphenyl leads to a mixture of
components with a range of chlorine contents, the mean percentage
chlorine in the product being controlled to give the required
technical properties. Most of these components have been separated by
gas-liquid chromatography, and the PCBs in the mixtures have been
characterized after synthesis of the components by unequivocal routes.
Techniques are available to analyse environmental samples for PCBs and
PCTs, and experience has that interlaboratory collaborative studies
are necessary to competence to determine residues below the 1 mg/kg
level.
The commercial PCB mixtures contain various quantities of
impurities among which chlorinated dibenzofurans and chlorinated
naphthalenes have been identified.
1.2.2 Sources and pathways in the environment
The estimated cumulative world production of PCBs since 1930 is of
the order of 1 million tonnes. Of this, more than one-half has entered
dumps and landfills, where it is likely to be stable and released only
very slowly. Much of the remainder has entered the environment by the
disposal of industrial fluids into rivers and coastal waters, by
leakage from nonenclosed systems, or by volatilization into the
atmosphere from incineration of PCB-containing material at dumps. The
ultimate reservoirs of PCBs and PCTs that enter the environment are
mainly sediments of rivers and coastal waters. PCBs and PCTs are
stable in the environment, but a small proportion is transformed by
biological action and possibly by photolysis.
1.2.3 Concentration in the environment
Measured concentrations of PCBs in air range from 50 ng/m3 to
less than 1 ng/m3. Nonpolluted fresh waters should contain less than
0.5 ng of PCBs per litre compared with 50 ng per litre in moderately
polluted rivers and estuaries, and 500 ng per litre in highly polluted
rivers. The concentration in living organisms depends upon the extent
of local pollution, the amount of fat in the tissues, and the trophic
stage of the organism in food chains. Highest tissue levels were found
in marine ecosystems with very high values in top predators from
polluted areas though most of the fish caught for human consumption
contains PCBs at levels of less than 0.1 mg/kg in muscle tissue. There
is no information available on the environmental distribution of PCTs.
1.2.4 Metabolism
PCBs are well absorbed by mammals through the gastrointestinal
tract, lungs, and skin. They are stored mainly in adipose tissue and
there is some placental transfer. Excretion in mammals is mainly
through faeces, where the PCBs appear as phenolic metabolites; they
appear unchanged in human milk. In birds, there is a considerable
excretion in eggs. The rate of excretion in faeces is dependent on the
rate of metabolism and this is much influenced by the number and
orientation of the chlorine substituents. As environmental PCBs pass
up biological food chains, there is a progressive loss of the lower
chlorinated components owing to selective biotransformation, and only
traces of PCBs containing less than five chlorine atoms per molecule
are found in human fat.
The smaller amount of information available concerning the PCTs
indicates that they also are absorbed from the gastrointestinal tract
and undergo selective biotransformation, but that the concentration in
fat in relation to that in other tissues appears to be less than is
observed with the PCBs.
1.2.5 The extent of human exposure
Surveys on human adipose tissue in several countries have shown
that most samples contain levels of PCBs in the region of 1 mg/kg or
less, although higher values have been reported from some countries.
Much higher values, up to 700 mg/kg, have been found in fat from men
occupationally exposed. Several national surveys give PCB
concentrations in the blood in the region of 0.3 µg/100 ml but levels
approaching 200 µg/100 ml have been measured in men occupationally
exposed, and these may be associated with skin lesions. Most surveys
on human milk have shown PCB concentrations in the region of
0.02 mg/litre, although concentrations up to 0.1 mg/litre have been
recorded. Results from the very few investigations on PCT
concentrations in fat and blood suggest that these may be equal to
those of PCBs.
An estimate of the total daily intake of PCBs in air, water, and
diet by individuals not occupationally exposed indicates that this
falls within the range of 5-100 µg, which may be supplemented by
unknown amounts from nondietary sources. This estimate has some
support from measurements of concentrations in human milk.
1.2.6 Experimental studies on the effects of PCBs and PCTs
Most of the studies on the toxicity of PCBs have been performed
with the commercial mixtures. The PCBs are of low acute toxicity but
the effects are cumulative with prolonged administration; in mammals,
liver enlargement is observed that may progress to liver damage.
Non-metastasizing neoplastic liver nodules have been produced in rats
and mice, some of which were classified as hepatocellular carcinomas
on the basis of histological criteria in one study in rats and one
study in mice. The monkey is much more sensitive to PCBs than the rat,
showing effects similar to those seen in human Yusho patients (See
section 8, p 65) with a similar order of exposure. Low dose effects on
fertility have been seen in both the monkey and the mink, a species
that is also relatively sensitive to PCBs.
Other effects of PCBs include porphyria, immunosuppression, and
interference with steroid metabolism; some of these maybe attributable
to the increase in microsomal enzyme activity associated with liver
enlargement. Some of the toxic effects can be attributed to impurities
in the commercial products.
The toxicity of PCBs to fish is not high by comparison with that
of some pesticides, but some aquatic invertebrates are more sensitive.
There is little information on the toxicity of the PCTs.
1.2.7 Clinical studies of the effects of PCBs in man
Information on the effects of PCBs in man has been obtained from a
large-scale incident in Japan (Yusho), in which over 1000 individuals
showed signs of poisoning from the ingestion of rice oil contaminated
with PCBs from a heat exchanger liquid. The most striking effects were
hypersecretion in the eyes, pigmentation and acneiform eruptions of
the skin, and disturbances of the respiratory system. Babies born to
Yusho mothers were of less than normal size and initially showed skin
pigmentation. Over a six-year period, the effects on the skin
diminished very gradually, but the nonspecific symptoms tended to
become somewhat more prominent. The smallest dose of PCBs calculated
to produce an effect was approximately 0.5 g over about 120 days, but
as the rice oil contained chlorinated dibenzofurans at a concentration
of 5 mg/kg of rice oil in addition to PCBs at 2000-3000 mg/kg it is
not certain that the symptoms were due solely to PCBs.
1.2.8 Dose effect relationships
Experimental studies on the dose-effect relationship have shown
that no effects on growth, and reproduction are seen in rats receiving
PCB levels of 1 mg/kg body weight per day; there may be liver
enlargement and a reversible induction of microsome enzyme activity at
a level of 1 mg/kg/day but not at 0.1 mg/kg/day. Effects on
reproduction are seen in the monkey with PCB levels of about
0.12 mg/kg/day. Symptoms were reported in some Yusho patients
ingesting less than 0.1 mg/kg/day.
1.3 Recommendations for Further Research
1.3.1 Analytical methods
Collaborative intercalibration studies on the determination of
PCBs, PCTs, and chlorinated dibenzofurans should be established
between all laboratories engaged in determining these compounds in
environmental samples, and adequate standards should be made available
for individual chlorinated biphenyls and dibenzofurans.
Improved analytical techniques, including those involving
capillary gas-liquid chromatography and mass spectroscopy, should be
developed for the determination of PCBs, PCTs, polybrominated
biphenyls, polychlorinated dibenzofurans, and naphthalenes, and their
metabolites and degradation products.
1.3.2 Environmental pollution
The content of chlorinated dibenzofurans should be studied in a
range of commercial PCB mixtures, and in used PCBs from existing or
newly designed heat exchangers, capacitors, and hydraulic
transmissions. The possibility of the formation of chlorinated
dibenzofurans from PCBs in cooking oils before and after use and in
other foods during storage or heating requires investigation.
The current production, use patterns, and methods of disposal of
PCBs should be carefully examined to gain information on the impact of
PCBs on the environment at the present time. The rate of leaching of
PCBs from waste dumps and landfills should be studied, and methods of
incineration should be investigated to ascertain which of the
components survive inefficient combustion, and whether chlorinated
dibenzofurans or other compounds are released into the atmosphere.
Information is required on the metabolism and environmental fate
of the chlorinated dibenzofurans.
1.3.3 Effects on man
The intake of PCBs and PCTs from all sources by typical
populations should be studied, and an attempt should be made to trace
the sources of PCBs and PCTs in those items of the diet that make the
greatest contribution to the daily intake. Further measurements are
required on levels in human body fat, blood, and milk, and an attempt
should be made to relate these levels to the daily intake.
Clinical and epidemiological studies are required on individuals
exposed to relatively high concentrations of PCBs and PCTs, either
occupationally or by virtue of the nature of their diet, and their
status should be correlated with exposure and tissue levels.
1.3.4 Experimental studies
Further toxicological and metabolic studies are required on
polychlorinated biphenyls, dibenzofurans, naphthalenes, and impurities
occurring in commercial products, on a variety of species including
primates, in order to assess the nature of the toxic effects, the
dose-response relationship, and the threshold of toxic effects. Such
investigations should be extended to include dermal and inhalation
exposure.
Carcinogenic and cocarcinogenic studies should be undertaken to
identify the components in commercial PCBs responsible for neoplastic
effects.
1.3.5 PCB substitutes
More information should be made available on the production and
use patterns of PCTs, polybrominated biphenyls, and of other possible
substitutes for PCBs, and when appropriate, these products should be
subjected to adequate toxicological investigations.
2. PROPERTIES AND ANALYTICAL METHODS
2.1 Chemical Composition
The PCBs form a class of chlorinated hydrocarbons and are
manufactured commercially by the progressive chlorination of biphenyl
in the presence of a suitable catalyst. They are known by a variety of
trade names: Aroclor (USA), Phenochlor (France), Clophen (Federal
Republic of Germany), Kanechlor (Japan), Fenchlor (Italy), and Sovol
(USSR). Their value for industrial applications depends upon their
chemical inertness, resistance to heat, non-flammability, low vapour
pressure (particularly with the higher chlorinated compounds), and
high dielectric constant. There are many different trade names for
mixtures of PCBs with other compounds.
Individual manufacturers have their own system of identification
for their products. In the Aroclor series, a four digit code is used;
biphenyls are generally indicated by 12 in the first two positions,
while the last two numbers indicate the percentage by weight of
chlorine in the mixture; thus Aroclor 1260 is a polychlorinated-
biphenyl mixture containing 60% of chlorine. An exception to this
generalization is Aroclor 1016 which is a distillation product of
Aroclor 1242 containing only 60% of components with five or more
chlorine atoms (Burse et al, 1974). With other commercial products the
codes may indicate the approximate mean lumber of chlorine atoms in
the components; thus Clophen A60, Pheno-DP6, and Kanechlor 600 are
biphenyls with an average of about six chlorine atoms per molecule
(equivalent to 59.0% chlorine by weight).
In the Aroclor series, terphenyls are indicated by 54 in the first
two of the four digit code. In Japan, the PCTs are coded Kanechlor.
Individual PCBs have been synthesized for use as reference samples
in the identification of gas-liquid chromatographic peaks, for
toxicological investigations, and for studying their metabolic fate in
living organisms, for which purpose they have been prepared labelled
with carbon-14 (Hutzinger et al., 1971; Tas & de Vos, 1971; Webb &
McCall, 1972; Moron, et al., 1972; Sundström & Wachtmeister, 1973;
Jensen & Sundström, 1974).
The chlorination of biphenyl can lead to the replacement of 1-10
hydrogen atoms by chlorine; the conventional numbering of substituent
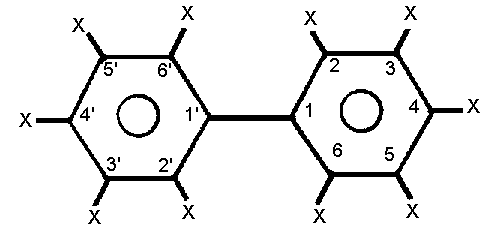
positions is shown in the diagram. It has been calculated that 210
different biphenyls of different chlorine content are theoretically
possible, although Sissons & Welti (1971) have demonstrated that
chlorine substituents in the 3,5- and 2,4,6-positions are not obtained
by the direct chlorination of biphenyl. The proportions of PCBs with
1-9 chlorine substituents in the Aroclors are shown in Table 1.
Table 1. Approximate composition of Aroclors
Aroclor
No. of CI atoms % of chlorine
in molecule weight 1221a 1242b 1248b 1254b 1260b
0 0 12.7
1 18.8 47.1 3
2 31.8 32.3 13 2
3 41.3 28 18
4 48.6 30 40 11
5 54.4 22 36 49 12
6 59.0 4 4 34 38
7 62.8 6 41
8 66.0 8
9 68.8 1
a Willis & Addison (1972).
b Panel on Hazardous Trace Substances (1972).
 There have been several investigations to identify individual PCBs
in commercial products. Sissons & Welti (1971) separated the
components of the Aroclors by column and gas-liquid chromatography,
and characterized many of the peaks by high-resolution mass
spectrometry and nuclear magnetic resonance, and by comparison with 40
synthesized Webb & McCall (1972) identified the gas-liquid
chromatographic with those of synthesized compounds by retention times
and spectrometry (Table 2). The most exhaustive study is that of
Jensen & Sundström (1974). They recognized that conventional
gas-liquid chromatography could not separate all the components, so
they devised preliminary fractionation on a charcoal column, which
separated the component PCBs according to the number of graphs in the
2,2',6 or 6' positions in the molecule ( o-chlorines). They compared
the gas-liquid chromatographic peaks with those of 90 synthesized
PCBs, and were able to characterize and quantify 60 components of
Clophens A50 and A60 (Table 3). Tables 2 and 3 show a considerable
overlap between the components of Aroclor 1254 and Clophen A50.
Table 2. Polychlorinated biphenyls in Aroclors 1221-1254 (Webb & McCall, 1972)
Retention Synthetic Acolor
timea chlorobiphenyl
1221 1232 1242 1248 1254
10 Biphenyl xb Cc x x x
13 2 x C x x x
16.9 3 x Dd
17 4 x C x
17.7
18.5 2,2' x C x x C x
21.3 x x x
21.6 x x x
23.5 2,3' x C x x x
24 2,4' x C x x C x
26 2,6,2' x x D x
29 2,5,2' x x C x
29.2 2,4,2' x x x C
29.5 4,4' x C x x C
34 2,3,2' x x Ce x
38 2,4,3' x x C x
38.5 2,4,4' x x C x
38.7 2,5,4' x x C x
41.5 x x
42.5 3,4,2' x C x
43 x x
44 x x
44.5 x
48 2,5,2',5' x C x x C
49.5 2,4,2',5' x C x x C
50.5 2,4,2',4' x D x
51.5 x x
55 2,3,2',5' x x x C
57 x x
59 2,3,6,2',6' x x x D
60 x x
64 x x
69 x x
69.5 x x
70 2,5,3',4' x Ce x
70.5 2,3,6,2',5' x C
71 2,4,3',4' x Ce x
72 x x
76 2,3,6,2',3' x C
Table 2. (cont'd).
Retention Synthetic Acolor
timea chlorobiphenyl
1221 1232 1242 1248 1254
82 x
83 2,3,6,2',5' x x C
84 x
85 2,4,5,2',5' x x C
87 2,4,5,2',4' x x C
96 x
99 2,4,5,2',3' x x C
101 x x
104 x x
107 x x
115 x
119 x x
125 2,4,5,2',3',6' x C
126 2,4,5,3',4' x C
135 x
147 x
148 2,4,5,2',4',5' x C
149 x
152 x
165 x
178 x
a Relative to p,p'-DDE at 190°C on 1 00' × 0.02' SCOT SE 30 column.
b indicates a GLC peak in this Aroclor at this retention time.
c C means that the synthetic compound in the second column was
confirmed by GLC and IR in this Aroclor. Because of the labour
involved, most compounds were only confirmed in one Aroclor. For
example, biphenyl is probably present in 1232-1248 as well as 1221.
d Only GLC data available.
e Something else also present.
2.2 Purity of Products
Commercial PCBs are not sold on a composition specification, but
on their physical properties. Different batches may vary somewhat from
the compositions shown in Tables 1-3. Impurities known to be present
in commercial PCBs are chlorinated dibenzofurans and chlorinated
naphthalenes (Vos et al., 1970; Bowes et al., 1975). Bowes et al.
(1975) found chlorinated dibenzofurans at 0.8-2.0 mg/kg in samples of
the Aroclor 1248-1260 series, but none in Aroclor 1016, and at levels
of 8.4 mg/kg in Clophen A60 and 13.6 mg/kg in Phenoclor DP-6. Roach &
Pomerantz (1974) found chlorinated dibenzofurans levels of 1 mg/kg and
Nagayama et al. (1976) found 18 mg/kg in different batches of
Kanechlor 400, but no chlorinated dibenzodioxins.
2.3 Determination of PCB Residues
Reviews have been published on methods used for the determination
of organochlorine compounds including PCBs in environmental samples
(Holden, 1973a, Panel on Hazardous Trace Substances, 1972). No two
laboratories have identical methods, although all have features in
common. The techniques appear to be those previously developed for the
determination of organochlorine pesticides with appropriate
modifications for the presence of PCBs, and the studies on PCBs
sometimes form part of a wider programme for monitoring persistent
organochlorine compounds in the environment. The major difficulties in
the determination of PCBs are to separate them from interfering
organochlorine pesticides, and to derive a single quantitative figure
from a variable mixture of components.
2.3.1 Extraction of sample
Air
Particulate fallout from air has been trapped on 200 µm nylon net
coated with silicone oil, and the PCBs then extracted with hexane
(Södergren, 1972a). Separate determinations of particulate and vapour
phase PCBs in air have been made by the passage of a large volume of
air through a filter which was followed by an impinger containing
hexane (Hasegawa et al., 1972b), or a polyurethane plug (Bidleman &
Olney, 1974) or ceramic saddles coated with OV 17 silicone (Harvey &
Steinbauer, 1974) to absorb the vapour.
Table 3. Percentages of polychlorinated biphenyls in Clophen A50 and A60 and in human (Jensen & Sundström, 1974)
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
1 2,5-2'5' 2 0.25 0.30 5.0
2 2,4-2'5' 2 0.26 0.30 1.4
3 2,3-2'5' 2 0.27 0.33 1.9 1.1
4 (2,-2',3',4') 2 0.30 0.43 1.2 0.66
5 (4-2',3'6') 2 0.32 0.43 2.1 0.56
6 2,5-2',3',6' 3 0.35 0.43 4.4b 2.9 1.2
7 2,3-2',3',6' 3 0.38 0.49 2.5b 0.28 0.48
8 3,4-2',5' 1 0.41 0.42 3.9 1.5
9 1 or 2 0.42 2.2c 2.0c
10 2,5-2',3',5' 2 0.45 0.49 2.2 1.1 1.2
11 2,3,6-2',3',6' 4 0.47 0.61 0.50 1.0d
12 2,5-2',4',5' 2 0.48 0.50 7.0b 5.6 4.2
13 2,4-2',4',5' 2 0.51 0.51 1.8b --e 1.9
14 2,3-2',4',5' 2 0.53 0.57 1.4b
15 2,5-2',3',4' 2 0.53 0.58 5.4 1.4 2.3
16 2,3-2',3',4' 2 0.55 0.66 1.0
17 3,4-2',3',6' 2 0.56 0.62 7.6b 2.9 4.7
18 2,3,5-2',3',6' 3 0.60 0.70 1.2 4.2 1.0
19 2,4,5-2',3',6' 3 0.64 0.73 2.0b 6.5d 0.13
20 2,5-2',3',5',6' 3 0.65 0.68 1.3 3.3 0.43
21 2,3-2',3',5',6' 3 0.70 0.74 0.5 0.05
22 2,3,4-2',3',6' 3 0.72 0.83 1.8 3.2 0.15
23 1 or 2 0.76 0.6c
Table 3. (cont'd).
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
24 2,3,6-2',3',5',6' 4 0.77 0.91 0.09 0.96
25 3,4-2',4',5' 1 0.79 0.74 5.0b 1.6 5.4
26 2,3,6-2',3'4',6' 4 0.84 0.95 0.05 0.37
27 2,3,52',4',5' 2 0.85 0.86 0.90 2.9 2.7
28 3,4-2',3',4' 1 0.87 0.86 3.6 1.9
29 2,4,5-2',4',5' 2 0.90 0.86 4.2b 12.9d 21.5
30 2,3,4-2',3',5' 2 0.94 0.97 ) 1.5 --e
31 1 or 2 0.95 0.93 ) 1.1 --e
32 2,3,4-2',4',5' 2 1.00 1.00 5.1 11.3d 14.0
33 2,3,5-2',3',5',6' 3 1.01 1.06 0.04 0.49 0.90
34 4 1.02 0.05c
35 1 or 2 1.04 1.10 1.1c 2.0c 1.5'
36 2,4,5-2',3',5',6' 3 1.05 1.13 0.39 3.3 3.5
37 2,3,4-2',3',4' 2 1.11 1.16 1.3 2.0 0.81
38 2,3,6-2',3',4',5' 3 1.13 1.29 0.33 3.7d --e
39 2,4,5-2',3',4',6' 3 1.14 1.16 0.17 1.8 2.5
40 2,3,4-2',3',5',6' 3 1.19 1.32 0.27 2.1 1.3
41 2,3,5,6-2',3',5',6' 4 1.23 1.34 0.005 0.07
42 2,3,4-2',3',4',6' 3 1.28 1.40 0.13 1.3 0.57
43 2,3,4,6-2',3',5',6' 4 1.34 1.44 0.007 0.09
44 2,4,5-3',4',5' 1 1.41 1.19 0.47 1.0 0.49
45 2,3,6-2',3',4',5',6' 4 1.45 1.59 0.008 0.09
46 2,3,4,5-2',3',4',6' 4 1.46 1.51 --e 0.03
47 3,4-2',3',4',5' 1 1.55 1.37 0.81 1.5 2.0
48 2,3,5-2',3',4',5' 2 1.59 1.49 0.23 0.90 1.2
49 2,4,5-2',3',4',5' 2 1.71 1.56 0.98 7.6d 7.7
Table 3. (cont'd).
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
50 2,3,4-2',3',4',5' 2 1.88 1.82 0.72 4.1d 5.9
51 2,3,4,5-2',3',5',6' 3 1.94 1.99 0.08 0.74 0.77
52 1 or 2 2.02 1.96 0.23c 1.0c --e
53 2,3,4,5-2',3',4',6' 3 2.07 2.05 0.06 0.44 0.94
54 2,4,5-2',3',4',5',6' 3 2.15 2.04 0.01 0.28 0.46
55 (2,3,5,6-2',3',4',5',6') 4 2.23 --e
56 2,3,4-2',3',4',5',6' 3 2.40 2.20 0.01 0.17 0.31
57 2,3,4,6-2',3',4',5',6' 4 2.45 2.56 --e --e
58 3,4,5-2',3',4',5' 1 2.74 2.41 --e --e
59 2,3,4,5-2',3',4',6' 2 3.18 2.81 0.35 0.67 1.7
60 2,3,4,5,6-2',3',4',5',6'4 4 4.12 0.62
Total 86.7% 99.4% 100.0%
p,p'-DDE 0.52 0.58
p,p'-DDT 0.71 0.73
p,p'-DDT 0.90 0.97
a Tentative structures are given in brackets.
b Component found present in Aroclor 1254 by Webb & McCall (1972).
c Figures calculated using responses of chlorobiphenyls with similar retention times in the same fraction
from the charcoal column.
d Component found present in Phenoclor DP6 by Tas & Vos (1971).
e Present in trace amounts only.
Water
PCBs have been extracted from water by passing a sample through a
filter of undecane and Carbowax 400 monostearate supported on
Chromosorb W (Ahling & Jensen, 1970) or a porous plug of polyurethane
coated with a suitable gas-liquid chromatographic stationary phase
(Uthe et al., 1972), or Amberlite XAD-2 resin (Harvey, et al., 1973)
followed by elution of the PCBs with a solvent. Ahnoff & Josefsson
(1975) have described a liquid-liquid extraction into cyclohexane.
Biological samples
Most analysts have used standard methods developed for
organochlorine pesticides, in which the PCBs are extracted together
with the fat; the sample is ground with anhydrous sodium sulphate and
extracted with petroleum ether or hexane. Porter et al. (1970) studied
the optimal conditions for this procedure. A dehydrating solvent may
be included to facilitate the breakdown of cell structures; ethanol
(Norén & Westöö, (1968) and acetone (Jensen et al., 1973) have been
used. Rote & Murp (1971) digested the sample with a mixture of acetic
and perchloric acid prior to hexane extraction.
2.3.2 Clean-up
Methods for the removal of fat from the extract include solve
partitioning between hexane and acetonitrile or dimethylformamide,
treatment with strong sulfuric acid or ethanolic potassium hydroxide
Gel permeation has also been used (Stalling et al., 1972), and Holden
Marsden (1969) removed fat on dry, partially deactivated alumina
column. Certain pesticides such as dieldrin are destroyed by the
sulfuric at treatment, so this method cannot be used if such
pesticides are to determined together with PCBs (Jensen et al., 1973).
PCBs may be separated from organochlorine pesticides by column
chromatography on Florisil (Mulhern et al., 1971), silica gel (Holden
Marsden 1969; Armour & Burke, 1970; Collins et al., 1972) or on
charcoal (Berg et al., 1972; Jensen & Sundström, 1974). Several
laboratories have reported difficulties in repeating results obtained
by other investigators; the ease of separation appears to depend upon
the characteristics of absorbent, of the eluting solvent, and of the
sample extract, though the appears to be no difficulty in separating
all interfering substances except DDE, a metabolite of DDT. Thin-layer
chromatography has been us for separation by Norén & Westöö (1968),
Bagley et al. (1970), and Rein et al. (1973).
In many environmental samples, DDE is present in large excess over
the PCBs, and must be removed before the quantitative determination
PCBs. Oxidation procedures have been used to convert DDE
dichlorobenzophenone; recommended oxidants are potassium dichromate
and sulfuric acid (Westöö & Norén, 1970b) and chromium (II) oxide a
acetic acid (Mulhern et al., 1971). Jensen & Sundström (1974), who we
interested in determining DDT/PCB ratios in environmental sample
preferred sodium dichromate in acetic acid with a trace of sulfuric
acid. They claim that this does not destroy DDT and its metabolite DDD
which may be present in extracts after clean-up with strong sulfuric
acid and that using this mixture makes possible the quantitative
determination of the dichlorobenzophenone from the oxidation of DDE.
Conversion of DDT to DDE may be achieved by treatment with
ethanolic potassium hydroxide, which also removes interference from
elemental sulfur (Ahling & Jensen, 1970). Sulfur may also be removed
activated Raney nickel (Ahnoff & Josefsson, 1975) or by metallic
mercury.
Södergren (1973b) has scaled down the clean-up procedure for small
samples, using microlitre volumes.
2.3.3 Chromatographic separation of PCBs
Gas-liquid chromatography
Most analysts use gas-liquid chromatography with an electron-
capture detector for the separation of PCBs from the extract after
clean-up. Stationary phases commonly used are silicones or their
derivatives, for example, DC 200, SF 96, OV 1, and QF 1, or Apiezon L.
Jensen & Sundström (1974) state that with a mixture of SF 96 and QF 1,
14 peaks can be obtained from Clophen A50, but that Apiezon L gives
much better resolution. They obtained better peak separation by prior
fractionation on a charcoal column, which separated the PCBs according
to the number of o-chlorine substituents; they regard such
refinements as unnecessary in PCB residue analysis, although they may
be of value in the study of the selective, environmental degradation
of PCBs. Column temperatures used ranged between 170°C and 230°C.
Glass capillary columns gave good separation of PCBs from DDT and its
metabolites (Schulte & Acker, 1974).
Thin-layer chromatography
This has been used in the clean-up stage, but it may also provide
semi-quantitative results by visualization of the spots followed by
densitometry, or by comparison with spots produced by known amounts of
PCBs. Mulhern et al. (1971) separated PCBs on a plate coated with
alumina containing silver nitrate, and the spots were developed by
exposure to ultraviolet light; the detection limit was in the region
of 1 µg. Collins et al. (1972) devised a similar method in which the
PCBs remained together in a single spot, and they claimed a limit of
detection of about 50 ng. Reversed-phase chromatography on plates
coated with kieselguhr treated with liquid paraffin has been used to
separate Phenochlor DP6 into several spots with a detection limit of a
few micrograms (de Vos & Peet, 1971).
2.3.4 Quantification of PCB content
The response of the electron capture detector is not equal for all
PCB components, being much affected by the degree of chlorination
(Zitko et al., 1971). This does not lead to difficulties when the
sample under investigation has been directly contaminated by a
commercial PCB mixture, as that mixture can be used as a standard.
Difficulties are encountered when the PCBs in the sample have
undergone selective environmental degradation (see sections 3 and 5).
Several investigators have noted that the pattern of peaks from such
samples resembles fairly closely that of one or other of the higher
chlorinated PCB mixtures such as Aroclor 1254, and they have compared
the total area of the peaks with that of the nearest commercial
product in order to determine the amount of PCBs in the sample (Armour
& Burke, 1970). Collins et al. (1972 observed that, under their
conditions, the area of peaks usually encountered in extracts of
tissue samples was closely similar to that of an equivalent amount of
DDE, thus DDE could be used for calibration. In order to overcome the
uncertainties of these procedures, Rote & Murphy (1971) divided the
peaks into groups according to the number of chlorine atoms in the
molecule, as determined from mass spectrographic data, and calculated
the PCB content of each group from the theoretical response of the
detector to chlorine content. Jensen et al. (1973) selected a
commercial in PCB that included all the peaks from the extract; they
determined the PCB content of each peak by combined mass spectrometry
and coulometry, and determined the total PCBs in the sample by
comparing the height of each peak obtained with the extract with those
obtained with the reference sample. Simpler methods have been used by
Koeman et al. (1969), who compared the height of a single peak
obtained with the extract with that of a peak with the same retention
time obtained with a e commercial PCB mixture; others have averaged
out more than one peak s for this calculation (Reynolds, 1971; Reinke
et al., 1973). Rote & Murphy (1971) have calculated that such
procedures may more than double the values obtained by a more accurate
method.
A different technique has been recommended by Berg et al. (1972);
the PGBs are chlorinated with antimony pentachloride to decachloro-
biphenyl, which can then be measured as a single peak.
2.3.5 Accuracy of PCB determinations
A group of eight analysts engaged in an investigation of pollution
in the North Sea undertook a collaborative study to determine the PCB
content of a sample of fish oil, using the methods currently employed
in their laboratories (International Council for the Exploration of
the Sea, 1974). The PCB values obtained ranged from 1.0 to 3.9 mg/kg
with a mean of 1.97 mg/kg and a standard deviation of 0.93 mg/kg.
Better agreement was obtained with the same fish oil fortified with
PCBs at a concentration of 10 mg/kg; the mean of the results for the
fortified sample was 10.0 mg/kg with a standard deviation of
1.1 mg/kg.
A probable source of error is incomplete initial extraction of
PCBs from the sample (Holden & Marsden, 1969). Another source of
variation between laboratories lies in the method used to quantify
gas-liquid chromatographic peaks (section 2.3.4); van Hove Holdrinet
(1975) considered this to be the major source of error.
It is evident that caution should be exercised in accepting the
analytical results from a laboratory, particularly for samples with a
low PCB content, until the competence of that laboratory has been
established by an inter-laboratory collaborative study.
2.3.6 Confirmation of identity
Since Jensen first identified as PCBs those hitherto unknown
substances that interfered in the gas-liquid chromatographic
determination of organochlorine pesticides using mass spectrographic
data, other investigators have confirmed the presence of PCBs in
environmental samples by combining gas-liquid chromatography with mass
spectrometry (Bagley et al., 1970) and with coulometry to measure the
chlorine content. The conversion of PCBs to bicyclohexyl and
decachlorobiphenyl is further confirmation (Berg et al., 1972). The
widespread distribution of PCBs is now well established, and, as
adequate methods are available to remove interference from
organochlorine pesticides, there is no evidence of the presence of
other interfering substances in the types of sample that have so far
been analysed, down to a limit of detection of around 0.01 mg/kg. This
does not necessarily apply to other types of sample, particularly when
very low levels are being sought; Ahnoff & Josefsson (1973, 1975)
reported a number of unknown interfering substances, when measuring
PCBs in water at levels below 1 ng/litre, one of which was
subsequently identified as elemental sulfur. They recommend
confirmation by mass fragmentography for such samples.
2.4 Determination of PCTs
A few methods have been published for the determination of PCTs;
extraction and clean-up procedures are similar to those used for PCBs,
but the gas-liquid chromatographic details are different because of
the lower volatility of the PCTs. Zitko et al. (1972b) used 3% OV 210
as the stationary phase with a column temperature of 200°C. Thomas &
Reynolds (1973) also used OV 210 with a column temperature of 250°C
and another system with 3% Dexsil as stationary phase at 300°C with a
63Ni electron capture detector; this was also used by Addison et al.
(1972). Sosa-Lucero et al. (1973) used OV 210 and SE 30 at 255°C and
Freudental & Greve (1973) used OV 17 with a temperature programmed
from 200°C to 285°C. Thomas & Reynolds (1973) confirmed the identity
by chlorination to tetradecachloroterphenyl with antimony
pentachloride.
A thin-layer chromatographic technique has also been described
with limit of detection of about 1 µg (Addison et al., 1972).
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1 Production and Uses of PCBs
Details of the production and uses of PCBs in the USA have been
released, and have been summarized by Nisbet & Sarofim (1972). Annual
production has increased steadily since 1930 and reached a maximum in
1970 of 33 000 tonnes. During this peak year, 65% of the production
was of the 42% chlorinated type, 25% was less chlorinated, and the
remainder more chlorinated. After 1970, production sharply decreased
owing to voluntary limitation of sales by the Monsanto Company, the
sole manufacturer in the USA. According to information collected by
the Organization for Economic Co-operation and Development (OECD), the
1970, production in the USA was 18 000 tonnes and the total in OECD
countries in that year was 48 000 tonnes. It has been estimated that
the cumulative total production of PCBs in North America up to 1971
was 0.5 million tonnes, and in the whole world probably double this
figure.
The commercial applications of PCBs have been reviewed in an OECD
report (Organization for Economic Co-operation and Development, 1973)
From an environmental viewpoint these can be divided into three
categories:
Controllable closed systems. PCBs used as dielectrics in
transformers and large capacitors have a life equal to that of the
equipment, and with proper design leakage does not occur. When the
equipment is scrapper the quantity of dielectric is sufficiently large
to justify regeneration.
Uncontrollable closed systems. PCBs are used in heat transfer and
hydraulic systems which, although technically closed, permit leakage.
The need for frequent replacement of small quantities makes recovery
impracticable, PCBs are very widely dispersed in small capacitors, and
there are great difficulties in collecting these items for disposal.
Dissipative uses. PCBs have been used in the formulation of
lubricating and cutting oils, in pesticides, and as plasticizers in
paints, copying paper, adhesives, sealants, and plastics. In these
applications, the PCBs are in direct contact with the environment, and
there is no way of recovering them when the product is scrapped.
The uses of PCBs in the USA in 1970 have been analysed by Nisbet &
Sarofim (1972). Of the total 33 000 tonnes, 56% was used as a
dielectric, with 36% in capacitors and 20% in transformers. Various
plasticizer outlets accounted for 30%, hydraulic fluids and lubricants
12%, and heat transfer liquids 1.5%. Following the restriction of
sales for non-dissipative uses, the percentage of PCBs sold as
dielectrics rose to 77% in 1971 and the proportion of highly
chlorinated products was considerably reduced, Aroclor 1016 replacing
Aroclor 1242. In Japan, 44 800 tonnes of PCBs were used from 1962 to
1971, and of this 65.4% was used in the electrical industry, 11.3% in
heat exchangers, 17.9% in pressure-sensitive duplicating paper, and
5.4% for other dissipative uses (Ishi, 1972). In Sweden, most of the
600 tonnes imported in 1969 was used in the electrical industry and a
large part of the remainder in paints (Jensen, unpublished report
1972).
According to the OECD report, transformers and capacitors provided
the major outlets for PCBs in most OECD countries in 1971. In 1972,
several countries restricted sales; in Sweden the importation and use
of PCBs were restricted by law; in the United Kingdom, as in the USA,
sales were voluntarily restricted to the lower chlorinated PCBs for
use as dielectrics in enclosed systems; in the Federal Republic of
Germany, use in hydraulic and heat transfer fluids was also permitted.
In Japan the production and use of PCBs were banned in 1972.
Limitations on sales were subsequently introduced in other countries.
No information is available on the scale of production and the
uses of PCTs.
3.2 Entry of PCBs into the Environment
Surveys of the sources of environmental pollution with PCBs were
made before production and use were limited, and the information
available may not now apply in North America and elsewhere. Table 4
gives an estimate of the fate of the PCBs produced in 1970 in the USA
(Nisbet & Sarofim, 1972). Only 20% of the annual production can be
regarded as a net increase in current usage, and the remainder is
balanced by a loss to the environment. More than one-half of this
entered dumps and landfills and it has been calculated that 0.3
million tonnes of PCBs have accumulated in such locations in North
America since 1930. Much of this was originally enclosed in containers
such as capacitors or was in plasticized resins and will not be
released until the containing medium decays. The diffusion of PCBs
from landfills is likely to be slow on account of their low volatility
and low water solubility; Carnes et al. (1973) found little leaching
from one site they tested.
Pollution of the environment has occurred mainly from the first
three routes mentioned in Table 4. In addition, there are other
routes, which although involving relatively small amounts,
nevertheless have an influence on the entry of PCBs into food chains.
PCBs have been used in the USA in amounts of about 10 tonnes/year in
pesticide formulations (Panel on Hazardous Trace Substances, 1972),
and the unauthorized use of scrap transformer fluid for this purpose
has led to local contamination of milk supplies.
Table 4. Entry of PCBs into the environmenta
Percentage of
annual PCB type
Route production (% chlorination)
Vaporization from plasticizers 4.5 48-60
Vaporization during incineration 1 42
Leaks and disposal of industrial fluids 13 42-60
Destruction by incineration 9 mainly 42
Disposal in dumps and landfills 52.5 42-60
Net increase in current usage 20 42-54
a From Nisbet & Sarafim, 1972.
Pressure sensitive duplicating paper containing PCBs has found its
way into waste paper supplies and has been recycled into paper and
board used as food packaging materials; paints for coating the bottom
of ships contained 3-5% of PCBs -- about 3% of the annual quantity
imported into Sweden has been used for this purpose -- and this has
been a source of plankton contamination (Jensen et al., 1972a).
3.2.1 Release of PCBs into the atmosphere
There appears to be little widespread atmospheric contamination
during the manufacture and processing of PCBs, but this can occur
during their subsequent use and disposal. Although PCBs have a low
volatility, there may be an appreciable loss to the atmosphere during
the lifetime of a PCB-plasticized resin, particularly of the lower
chlorinated products. Further pollution may occur during the
incineration of industrial and municipal waste. Most municipal
incinerators are not very effective in destroying PCBs; efficient
incinerators can be designed for this purpose (Jensen & Wickberg,
unpublished report 1971; Jensen, unpublished report 1972), although
the higher chlorinated PCBs are more resistant to pyrolysis. Secondary
sources of atmospheric pollution are volatilization from soil, and
from the drying of sewage sludge. Laveskog (1973) found that the PCB
emissions from municipal incineration and from the drying of sewage
sludge each amounted to about 1 kg/year per million inhabitants, a
small amount compared with the 2 tonnes deposited yearly from aerial
fallout in the south of Sweden.
3.2.2 Leakage and disposal of PCBs in industry
The major source of environmental pollution with PCBs, which
eventually affects food chains, is the leakage and the disposal of
industrial fluids. There have been serious cases of poisoning in man
(Kuratsune et al., 1972) and in animals (Panel on Hazardous Trace
Substances, 1972) due to leakage from a heat exchanger. Leakages, or
the unintentional or deliberate discharge of waste, have contaminated
seas, lakes, waterways, and sewers (Duke et al., 1970; Schmidt et al.,
1971; Veith, 1972).
Analysis of solids from factory wastes in Japan have revealed a
wide variation in PCB content. In most, less than 1 mg/kg was
detected, but in: some the contamination was very heavy, the highest
level recorded being 8.26% from a factory manufacturing electrical
equipment (Japanese Environment & Safety Bureau, unpublished report
1972).
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATION
4.1 Environmental Transport
Nisbet & Sarofim (1972) emphasize that the available data are
insufficient to determine anything but a very crude model of the
transfer of PCBs into the environment. Guesses can be made by
referring to the distribution of DDT, which resembles the PCBs to some
extent in its physical and chemical properties and about which more is
known. Much of the following discussion arises from their analysis of
the situation in the North American continent.
4.1.1 Air transport
By analogy with DDT, it might be expected that PCBs entering the
atmosphere in the vapour phase would be adsorbed rapidly on to
particles, which would be deposited or washed out in rain at a rate
depending on their particle size, the average residence time in the
atmosphere being 2-3 days. This has been confirmed by Södergren
(1972a), who measured the deposition in southern Sweden of PCBs that
had originated from municipal incinerators or had been carried over
from Denmark by the prevailing winds, and showed that the amount
deposited in central Sweden was much less. Cames (1973) and Laveskog,
(unpublished report 1973) found mainly particulate PCBs in the
emission from incinerators. Harvey & Steinhauer (1974), however,
considered that the results of their analyses of air in north Atlantic
regions indicated that most of the PCBs carried in the air were in the
vapour phase.
4.1.2 Transport in soil
The PCBs in soil are derived mainly from particulate deposition,
estimated at 1000-2000 tonnes annually in North America, most of which
is in urban areas. Small amounts have originated from the use of
sewage sludge as a fertilizer, from the leaching of landfalls, and
from the se of PCBs in pesticide formulations. Tucker et al. (1975)
found that under experimental conditions, the higher chlorinated PCBs
were not leached from soils by percolating water, and those with a
lower level of chlorination were removed only slowly, particularly
from soils of high lay content. Losses do occur by volatilization and
by biotransformation; by analogy with DDT and its metabolites the
half-time in soil has been estimated at 5 years. Haque et al. (1974)
showed that the rate of evaporation decreased with the clay content of
the soil and he degree of chlorination of the biphenyl, and increased
with temperature. Biotransformation has also been shown to play a part
in the disappearance of the lower chlorinated compounds from soil
(Iwata et al., 1973).
The total amount of PCBs distributed over North America, apart
from that in dumps and landfills, has been estimated at 20 000 tonnes,
of which one quarter has subsequently been transported via the air to
the seas. (Nisbet & Sarofim, 1972).
4.1.3 Transport in water
The entry of PCBs into water occurs mainly at the points of
discharge of industrial and urban wastes into rivers, lakes, and
coastal waters. Sewage treatment appears to remove particulate PCBs
from water, but not PCBs in solution; these are concentrated in the
sludge (Ahling & 1970), which may be dumped into rivers and coastal
waters. Holden (1970b) found a mean PCB content of 3 mg/kg in liquid
sludges from Glasgow, and calculated that PCBs at the rate of
1 tonne/year were released into the Clyde and Thames estuaries; a
similar output was calculated from water treatment plants off the
California coast (Schmidt et al., 1971). Other localized sources of
pollution are leakages or waste disposal from ships. PCBs in water are
attached mainly to particulate matter (Södergren, 1973a) and
eventually fall to the bottom sediment at a rate that depends on the
particle size. PCBs may be leached from the sediment and may reach
coastal waters, but Nimmo et al. (1971) noted little change in the PCB
content of a sediment at a point downstream from a source of
contamination over a period of 9 months. The process may be
accelerated by the dumping of dredging spoil.
4.1.4 Transport through biota
According to the approximate calculations of Nisbet & Sarofim
(1972), less than 1000 tonnes of PCBs are located in living organisms
throughout the world, so that biological transport and degradation
play little part in the fate of PCBs in the environment, though these
factors have great ecotoxicological significance.
4.2 Transformation in the Environment
4.2.1 Abiotic transformation
The fate of the various PCBs in commercial mixtures depends on
their physical and chemical properties. Some fractionation occurs
during the volatilization of PCBs, because of a decrease in vapour
pressure with increasing chlorination. The PCBs are chemically very
stable, and are not likely to be degraded at a significant rate by
hydrolytic or similar reactions under environmental conditions. They
are, however, fairly easily degraded by photolysis under laboratory
conditions; Safe & Hutzinger (1971), and Hutzinger et al. (1972b) have
shown that PCBs are dechlorinated in hexane solution at a rate that
increases with increasing chlorination. PCBs in aqueous-dioxane
suspensions and in thin films give hydroxy and carboxylic acid
derivatives on irradiation. The lower chlorinated biphenyls, at a
vapour concentration in air of 1.5 mg/m3 are readily destroyed by
photolysis under laboratory conditions. There is no direct evidence of
the extent of PCB breakdown in the atmosphere under environmental
conditions, and nothing is known of the persistence and toxicity of
any transformation products.
4.2.2 Biotransformation
The biotransformation of PCBs is discussed in section 6.6. Owing
to small proportion of the total environmental PCBs contained in
living biotransformation does not significantly influence the overall
environmental concentrations of PCBs, though it has a marked influence
on PCBs passing through food chains.
4.2.3 Metabolism in limited ecosystems
The presence of PCBs in sewage sludge suggests that they are not
all readily transformed by microorganisms. Choi et al. (1974) found no
evidence of biotransformation of Aroclor 1254 added to water entering
an aerated biological water treatment system, although much of it was
removed with the sludge; there appeared to be no interference by the
PCBs in the performance of the system. Vodden (1973) quotes
investigations showing that PCBs with four or fewer chlorine atoms are
readily broken down by microorganisms, but that this can be inhibited
by the presence of higher chlorinated PCBs. Mono- and dichlorobiphenyl
can be transformed by Achromobacter isolated from sewage effluent
(Ahmed & Focht, 1973), and a culture of lake bacteria can degrade some
of the lower chlorinated components of Aroclor 1242 to chlorine-free
derivatives (Kaiser & Wong, 1974).
Södergren (1972b) investigated the transport of Clophen A50 added
to a model aquatic ecosystem; this was rapidly taken up by an alga
(Chlorella) and no change was observed in the proportion of the
component PCBs in the first consumer fish; however, there was a
relative loss of the lower chlorinated PCBs in the perch, the second
consumer. No progressive loss of the lower chlorinated PCBs was
observed in sediment, plankton, invertebrates, and fish inhabiting a
Swedish lake (Södergren, 1973a). Evidence on the metabolism of PCBs by
fish is conflicting, but it seems probable that most fish,
particularly those in the lower trophic stages of food chains, cannot
readily degrade the low chlorinated PCBs.
4.3 Biological Accumulation
Although PCB concentrations in living organisms clearly indicate a
progressive accumulation in food chains, the factors discussed in the
previous sections make it impossible to give any reliable figure for
bioaccumulation at each trophic stage. There is also doubt about which
tissue level should be used in the calculation, that of the whole
body, the fat, or the liver.
There is good evidence that all aquatic organisms studied in
aquaria can absorb PCBs directly from water. The accumulation varies
with the duration of exposure and the concentration in the ambient
water. A diatom exposed to Aroclor 1242 showed an accumulation factor
of 1100 (Keil et al., 1971); with Aroclor 1254 the following values
have been obtained: pink shrimp, 6600; blue crab, 4600; oyster, 8100;
pinfish, 980 (Duke et al., 1970); spot, 37 000 (Hansen et al., 1971);
bluegills, up to 71 400 (Stalling & Mayer, 1972). The accumulation
factor in scud exposed to Aroclor 1254 reached a maximum of 24 000
within 4 days and thereafter remained fairly constant (Sanders &
Chandler, 1972). Similar results were obtained with other
invertebrates, although with crayfish the uptake was slower and the
accumulation was still increasing after 21 days. However, it is
probable that most of the PCBs entering aquatic systems are retained
by particulate matter, and the above accumulation factors are not
necessarily applicable to natural ecosystems. Nimmo et al. (1971)
demonstrated that the fiddler crab could ingest PCB contained in the
bottom sediment. When exposed to Aroclor 1254 in water, ciliated
protozoa, stated to be the major benthic input to aquatic food chains,
had an accumulation factor of 60 (Cooley et al., 1972).
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1 Air
Mean concentrations in air, in several locations in Sweden, ranged
from the detection limit of 0.8 ng/m3-3.9 ng/m3. The highest figure
recorded was 12.5 ng/m3 (Ekstedt & Odén, 1974). In the USA, PCB
concentrations in air ranged from 5 ng/m3 near the north-east coast
to 0.05 ng/m3 at a distance of 2000 m out over the Atlantic Ocean
(Harvey & Steinhauer, 1974). Results from the United States
Environmental Protection Agency indicate a range between 1 and
50 ng/m3 (Panel on Hazardous Trace Substances, 1972), and similar
results have been reported from Japan (Tatsukawa & Watanabe, 1972).
5.2 Soil and Sediments
In Sweden, PCBs have been found in natural soil at a concentration
of 15 µg/kg by Odén & Berggren (1973). The same authors also found
0.006-1.4 mg/kg in sediments from areas in the Baltic Sea with
different degrees of pollution. Nimmo et al. (1971) found PCB levels
of 1.461 mg/kg in sediment from an estuary at a point near the site of
an accidental release of PCBs from a factory, and levels of 0.6 mg/kg
at a point 16 km downstream. Soil samples from the bank 6.5 km
downstream from the source contained 1.4-1.7 mg/kg. Less than 1 mg/kg
has been found in Japanese agricultural soil, but as much as 510 mg/kg
in soil near a factory making electrical components (Fukada, et al.,
1973).
5.3 Water
In heavily contaminated waters, PCB concentrations may be several
times greater than their solubility, owing to adsorption on suspended
particles (Duke et al., 1970). Water in a Swedish river contained
0.5 ng/litre as it entered a water treatment plant, and 0.33 ng/litre
in the tap water produced (Ahling & Jensen, 1970). Values of
0.1-0.3 ng/litre have been measured in other Swedish rivers (Ahnoff &
Josefsson, 1974). Södergren (1973a) found a seasonal variation in the
PCB level in a Swedish lake, with a maximum of 2 ng/litre, the
pollution being attributed to aerial fallout. Concentrations of
10-100 ng/litre have been measured in tap water at Kyoto in Japan
(Panel on Hazardous Trace Substances, 1972) In a polluted coastal
area of Lake Michigan in 1970, PCB concentrations of from 450 to less
than 100 ng/litre were measured in 1970, but there was a marked
decrease in 1971, possibly due to the limitation on sales of PCBs
(Panel on Hazardous Trace Substances, 1972). From the scanty
information on PCBs, reinforced by analogy with the more extensive
information on DDT, it has been estimated that nonpolluted fresh
waters should contain not more than 0.5 ng/litre up to 5 ng/litre for
the Great Lakes of North America, 50 ng/litre for moderately polluted
rivers and estuaries, and 500 ng/litre for highly polluted rivers.
5.4 Living Organisms
There is now considerable information from Canada, Japan, Sweden
the United Kingdom, and the USA, on the accumulation of PCB's
biological material. Analytical measurements on different organisms,
and on the same organism from different localities, vary widely and it
is necessary to consider the factors that lead to this variation.
Difference in analytical techniques may contribute to this (see
section 2.3.5) but important influences are exerted by the extent of
local pollution, amount of fat in the organism studied, and its
trophic stage in food chains.
5.4.1 The influence of local pollution
Most of the fish eaten by man is taken from waters with little
pollution. In a collaborative study by seven national (International
Council for the Exploration of the Sea, 1974), the PCB content of
muscle tissue of fish taken from the North Sea was measured. A mean of
0.01 mg/kg was found in cod, herring contained up to 0.48 mg/kg with
most samples in the range of 0.1-0.2 mg/kg, and plaice contained
0.1 mg/kg or less. Similar values were reported by Zitko (1971) for
fish taken from the North Atlantic.
There are many examples of different PCB levels in similar species
collected from areas of high and low pollution. Jensen, et al. (1972b)
found five times as much PCBs in herrings caught in waters off
industrialized areas near Stockholm, as in herrings from the cleaner
waters of the west coast of Sweden. Similarly, levels in plankton
harvested along the Swedish archipelago at various distances from
Stockholm fell progressively, away from the more polluted areas
(Jensen, et al., 1972c); the concentration in pike fell to one-half
(Olsson & Jensen, 1974). Koeman et al., (1972b) found PCB levels of up
to 88 mg/kg in the blubber of toothed whales caught in the North Sea,
but none was detectable in similar species from New Zealand or
Surinam. Holden (1973b) found high PCB concentrations in the blubber
of seals in polluted coastal areas of the United Kingdom (up to
235 mg/kg), and much lower levels in unpolluted areas (down to
2 mg/kg).
Risebrough & de Lappe (1972) studied the PCB content of
extractable lipids from brown pelican eggs collected from areas
throughout North and South America, and showed that the content varied
from 4 mg/kg up to 266 mg/kg in highly industrialized regions. They
also reported levels greater than 3 mg/kg in fish from New York Sound
and Tokyo Bay, both very polluted areas. Even higher levels of PCBs
have been found in fish from polluted lakes and inland waterways, a
level of 20 mg/kg being found in fish from Lake Ontario, and over
200 mg/kg in fish from the Hudson River (Stalling & Mayer, 1972).
Similar correlations between pollution and PCB levels have been
reported from the United Kingdom in fish (Portmann, 1970), and in
mussels (Holdgate, 1971).
The association between high PCB levels and local pollution may be
disturbed by the migratory habits of certain species, particularly in
birds that may be exposed to PCBs in their wintering areas or on the
migration routes. As much as 400 mg/kg has been measured in the fat of
a robin entering Sweden, although the normal value is about 16 mg/kg.
Many migratory birds start egg-laying on arrival at their summer
quarters, so the PCB content of eggs may reflect the bird's previous
exposure rather than the local pollution (Odsjö, 1973). A special case
concerning the effect of pollution is seen in the use of fish-feed in
poultry and fish farming. Kolbye (1972) stated that this may contain
PCB levels of 0.6-4.5 mg/kg.
5.4.2 The influence of the fat content of tissues
PCBs are mainly stored in body fat (see section 5.5.5.1), and the
total PCB content of the body tissues is much influenced by their fat
content (Portmann, 1970; Westöö & Norén, 1970a). Jensen, et al. (1969)
found PCB levels of 0.27 mg/kg and 0.33 mg/kg respectively, in the
muscle tissue of herring and cod from the same area of the Baltic,
although the cod is at a higher trophic stage (see section 5.4.3).
These two species have 4.4 and 0.32% of extractable fat respectively,
and when the PCB level is calculated on the fat content, values of
6.8 mg/kg for the herring and 11 mg/kg for the cod are obtained. Cod
liver has a much higher fat content than cod muscle and Jensen (1973)
has reported the ratio of PCB concentrations in cod liver and muscle
to be over 100, the maximum in liver being 59 mg/kg. Jensen et al.
(1969) have remarked that the considerable seasonal variation in the
fat content of the herring, rising from 1% in spring to 10% in autumn,
influences the tissue level of PCBs. Peakall et al. (1972) noticed a
marked rise in tissue levels in starved birds owing to the
mobilization of fat, and it is possible that the high levels of PCBs
in the livers of birds dying during the "seabird wreck" in the Irish
Sea were secondary to emaciation (Holdgate, 1971). De Freitas &
Norstrom (1974) showed that, in pigeons, pure PCBs left fatty tissues
and accumulated mainly in muscle during the mobilization of fat
associated with starvation.
5.4.3 The influence of the trophic stage in food
Swedish work on the distribution of PCBs in aquatic ecosystems has
been summarized by Jensen et al. (1972b), and Olsson et al. (1973),
and it has been largely confirmed by work in other areas (Risebrough
et al., 1968; Risebrough & de Lappe, 1972: Holdgate, 1971). Zoo- and
phytoplankton readily absorb or adsorb PCBs from their environment;
Södergren (1972) has demonstrated the rapid uptake of Clophen A50 by
the unicellular alga Chlorella. Marine zooplankton may contain PCB
levels of 5 mg/kg in extractable lipids in areas of moderate pollution
(Jensen et al., 1972c; Williams & Holden, 1973), with somewhat lower
values in relatively unpolluted areas. However, results with plankton,
particularly where high levels are found, must be regarded with
caution as the sample could be contaminated with PCB-rich oil or tar
particles. Herring feeding on plankton in areas of moderate pollution
contain PCB levels of about 0.5 mg/kg in muscle tissue (10 mg/kg in
extractable fat); plaice and flounder, both bottom-feeding fish
contain about one-third of this, presumably because the PCB content of
benthic organisms is lower. In predatory fish such as the cod and
pike, the PCB level in extractable fat is about 10 mg/kg and a mean of
10 mg/kg has been measured in the blubber of seals.
Much higher PCB values have been obtained in fish-eating birds;
levels of 18 mg/kg (650 mg/kg in extractable fat), and 17 mg/kg
(420 mg/kg in extractable fat) were found in the herring gull and
comorant respectively. Lower values were found in birds feeding on
invertebrates, such as the long-tailed duck which contained 14 mg/kg
in fat; marine invertebrates contain PCB levels in the region of
0.1-0.2 mg/kg. At the top trophic level, 96 mg/kg (9.7 g/kg in
extractable fat) has been measured in the eagle owl; the highest
recorded PCB values were from eagle owls found dead in the south-east
coastal region of Sweden, 260 mg/kg in the brain (3.4 g/kg in
extractable fat) and 110 mg/kg in muscle (12 g/kg in extractable fat).
(Odsjö, 1973).
Less information is available on terrestrial ecosystems. PCB
concentrations in the region of 0.01 mg/kg have been found in fresh
tissue in slugs, snakes, and ants, and slightly higher concentrations
in earthworms. Tissue levels were generally at the limit of detection
(0.01 mg/kg) in herbivorous mammals (Odsjö, 1973). Bruggemann et al.
(1974) reported a mean PCB concentration of 0.22 mg/kg in 20 out of 72
measurements in the adipose tissues of the hare and a higher value
(2.5 mg/kg) in 1 out of 5 tests on the adipose tissues of the fox.
Higher values have been found in the American mink in Sweden with
0.58 mg/kg in muscle (45 mg/kg in fat) presumably because of a fish
diet (Odsjö, 1973). Tissue levels in wild birds on a mixed diet are
variable and rather low, but those in predatory birds are higher.
Prestt et al. (1970) related the PCB concentration in the liver of
wild birds to their diet; less than 1 mg/kg was found in insectivorous
birds and more than 70 mg/kg in the sparrow hawk. A level of 0.5 mg/kg
has been found in the muscle of the eagle owl in central Sweden, but
this is much less than the tissue concentrations encountered in this
bird in coastal areas (Odsjö, 1973). High tissue concentrations in
predatory and marine birds have also been reported from Canada
(Gilbertson & Reynolds, 1974), the Netherlands (Koeman et al., 1972a),
and from the United Kingdom (Bourne & Bogan, 1972).
5.4.4 Indicator organisms
Several of the organisms, that have been shown to accumulate-PCBs
from the environment, have been suggested as indicators of the extent
of local pollution with PCBs. In aquatic systems, the use of plankton
as an indicator has the advantage that it is at the lowest trophic
stage of food chains but errors may occur in the determination because
of the inclusion in the sample of nonplanktonic particles with a high
PCB content (Jensen et al., 1972a). The herring, which feeds on
plankton, has been suggested as an indicator (Jensen et al., 1972b)
and, at higher trophic stages, the pike, which is a stationary fish
(Olsson & Jensen, unpublished report, 1974), and seabirds (Jensen et
al., 1972c). In fresh waters, the amphipod Gammarus pulex has been
used as an indicator organism for chlorinated hydrocarbons (Södergren
et al., 1972). However, Zitko et. al. (1974) claimed that the
variation between individual fish was so high that Atlantic herring
and yellow perch could be used to detect trends in pollution only if
large numbers were taken for analysis, with an interval between
measurements of at least 4 years.
A series of monitoring studies has been made by OECD, the species
selected covering terrestrial, fresh water, and marine environments.
The analytical results, and the general problems of selecting species
for monitoring, have been discussed by Holden. (1970a, 1973a, 1973b).
5.5 The Extent of Human Exposure to PCBs and PCTs
5.5.1 Air and water
The maximum concentration of PCBs in air is not likely to exceed
50 ng/m3 (section 5.1). The highest concentration of PCBs reported in
domestic tap water is 100 ng/litre in the Kyoto area of Japan (Panel
on Hazardous Trace Substances, 1972), but levels more likely to be
encountered should not exceed 1 ng/litre (section 5.3).
5.5.2 Food
The PCB content of a variety of foods on the Swedish market been
measured by Westöö & Norén (1970a) and Westöö et al. Less than
0.1 mg/kg was found in samples of butter, margarine, oils, eggs, beef,
lamb, chicken, bread, biscuits, and baby of pork out of more than 100
had a PCB content in the range of 0.5 mg/kg. As might be expected from
the discussion in section 5.4 higher values were found in fish
depending on the fat content and pollution of the fishing area (Westöö
& Norén, 1970a; Berglund, 1972] The PCB levels obtained in an
extensive study by the US Food Administration are shown in Table 5.
These values are considerably higher than but they are probably
biased, as they include samples originating areas previously suspected
of having been subject to local pollution. In a Canadian survey, PCB
levels of less than 0.01 mg/kg were found in eggs (Mes et al., 1974)
and a mean of 0.042 mg/kg was found in domestic and imported cheese
with a maximum of 0.27 mg/kg (Villeneuve et al., 1973b). No traces of
PCTs were found.
In Japan, a similar range of PCB contents for most foods has been
reported; however, some high levels have been-reported for rice and
vegetables harvested in fields polluted with PCBs (Environmental
Sanitation Bureau, 1973). The PCB content of most fish on the market
was less than 3 mg/kg, although some contained more than this. The PCT
content of fish was much lower (Fukano et al., 1974). In the
Netherlands, eel has been reported to contain PCT levels of
0.2-0.5 mg/kg and PCB levels of 4.7 mg/kg (Freudenthal & Greve, 1973).
Table 5. PCB levels in food in the USAa
% positive Level in positive samples (mg/kg)
Food (0.1 mg/kg) Mean Maximum
Cheese 6 0.25 1.0
Milk 7 2.3 27.8
Eggs 29 0.55 3.7
Fish 54 1.87 35.3
a From Kolbye (1972).
Samples of butter from the Westphalian area of the Federal
Republic of Germany, obtained in the period 1972-74, contained PCB
levels of 0.38 mg/kg (range 0.25-0.54 mg/kg) (Claus & Acker, 1975).
Relatively high PCB levels in some packaged foods in Sweden,
mainly of imported origin, could be attributed to migration from the
packaging material (Westöö et al., 1971). The highest level
encountered was 11 mg/kg in a children's breakfast cereal; PCB levels
of 70 mg/kg and 700 mg/kg were found in the material of the inner bag
containing this product and in the outer cardboard container
respectively. Up to 2000 mg/kg was found in cartons of other samples.
Villeneuve et al. (1973a) have analysed packaged food in Canada; they
found that 66.7% of the samples contained PCB levels of less than
0.01 mg/kg, 30.7% contained between 0.01 and 1 mg/kg, and 2.6%
contained more than 1 mg/kg. PCT determinations were also made on
these samples; 94.5% of the samples contained less than 0.01 mg/kg and
5.5% contained 0.01-0.05 mg/kg. The highest PCB levels were
encountered in a rice sample with 2.1 mg/kg where the packaging
material contained 31 mg/kg, and in a dried fruit sample with
4.5 mg/kg in a container containing 76 mg/kg. In a survey of packaging
containers, approximately 80% were found to contain PCB and PCT levels
of less than 1 mg/kg, while about 4% contained levels higher than
10 mg/kg. The most likely source of PCBs in packaging materials is the
recycling of paper waste containing pressure-sensitive duplicating
paper (Masuda et al., 1972).
5.5.3 Occupational exposure
Occupational exposure does not only occur during the manufacture
of PCBs and with their use in the electrical industry. It may also be
widespread among mechanics in contact with lubricating oils and
hydraulic fluids, among workers exposed to varnishes and paints, and
among office workers from contact with pressure-sensitive duplicating
paper, some brands of which readily transfer PCBs to skin (Kuratsune &
Masuda, 1972b). Studies in Finland showed that whole blood from
persons with no special exposure to PCBs contained 0.3-1.2 µg/100 ml,
while blood from persons handling PCBs in an analytical laboratory
contained 3.66.3 µg/100 ml and blood from workers in a capacitor
factory had PCB levels of 7.5-190 µg/100 ml in the blood and
30-700 mg/kg in fat. No signs of toxicity were evident in these workers
(Karppanen & Kolho, 1973). Similar plasma values were found in workers
from Japanese capacitor factories, but here skin lesions were noted
(Hasegawa et al., 1972a). This same study reported that air levels of
PCBs of 0.01-0.05 mg/m3 were measured in a factory where KC-300 was
used in the manufacture of electric condensers. PCB levels in serum in
workers ranged from 10 to 65 µg/100 ml.a One month after the use of
PCBs had been suspended, serum levels still ranged from 9 to
74 µg/100 ml. However, in another factory making electric condensers, serum
levels decreased from an average of 80 µg/100 ml to 30 µg/100 ml
within three months of the use of PCBs being suspended (Kitamura et
al., 1973). According to Hara et al. (1974), the half-time of PCBs in
the blood of workers engaged in the manufacture of electric condensers
for less than 5 years was several months, while that of workers
employed for more than 10 years was 2-3 years. Hammer et al. (1972)
found a higher frequency of measurable plasma values in workers
working with refuse burners than in a control group.
a In this document, the concentrations of PCBs in blood and serum
are expressed in µg/100 ml although in some original papers the
values are given in µg/100 g. For practical purposes the
differences, about 5% and 3% respectively, can be neglected.
5.5.4 Other sources of exposure
Broadhurst (1972) has reviewed the many technical applications of
PCBs that appear in the literature and in patent specifications, and
which indicate the possibility of a widespread nonoccupational
low-level exposure to PCBs, other than that deriving from the diet.
PCBs are used in the home in ballast capacitors for fluorescent
lighting, and exposure deriving from pressure-sensitive copying paper
has not been limited to office workers. The valuable properties of
PCBs as plasticizers has led to their use in furnishings, interior
decoration, and building construction; examples are surface treatment
for textiles, adhesive for waterproof wall coatings, paints, and
sealant putties. PCBs have been used as plasticizers for plastic
materials and in the formulation of printing inks.
5.5.5 Biological indices of human exposure
The only surveys of value have been on body fat, blood, and milk.
5.5.5.1 Body fat
In a survey of 637 fat samples taken at autopsy or during surgery
in the USA, 68.9% contained PCB levels of less than 1 mg/kg, 25.9%
contained 1-2 mg/kg, and 5.2% contained more than 2 mg/kg (Yobs,
1972). A similar distribution was found in a smaller survey by Price &
Welch (1972). In the Kochi area of Japan, a mean PCB level of
2.86 mg/kg was recorded with an upper limit of 7.5 mg/kg; about double
these values were found in the Kyoto area (Nishimoto et al., 1972a,
1972b). Bjerk (1972) reported average PCB levels of 0.9 mg/kg
(1.6 mg/kg on a lipid basis) in adipose tissue, taken at 40 autopsies
in the Oslo area. Curley et al. (1973b) found PCB levels ranging from
0.30 to 1.48 mg/kg in a total of 241 human adipose samples in Japan. A
mean value of 5.7 mg/kg has been reported for 20 samples from the
Federal Republic of Germany (Acker & Schulte, 1970); in a more recent
study, 282 adipose tissue samples from different areas were found to
contain PCB levels of 8.3 mg/kg of adipose tissue on a lipid basis
(Acker & Schulte, 1974). In Austria, a range of PCB levels of
0.3-7.3 mg/kg on a lipid weight basis was found in 32 residents in the
Vienna metropolitan area; an increase of the PCB concentration with
age was not observed (Pesendorfer et al., 1973). Detectable levels of
PCBs were found in only a few of 201 human fat samples in the United
Kingdom and in these, the level did not exceed 1 mg/kg (Abbott et al.,
1972). A survey of 51 human fat samples in New Zealand showed that all
samples contained PCB residues with an average of 0.82 mg/kg (Solly &
Shanks, 1974).
Doguchi et al. (1974) found an average PCT level of 0.6 mg/kg in
human fat with a range of 0.1-2.1 mg/kg. Takizawa & Minagawa (1974)
also found PCT levels of 0.02 mg/kg in human liver ( n = 6),
0.01 mg/kg in the kidney ( n = 2), 0.02 mg/kg in the brain ( n = 3)
and 0.04 mg/kg in the pancreas ( n = 1). In the Netherlands, PCTs
were found in human fat at levels of 0-1 mg/kg (Freudenthal & Greve,
1973).
5.5.5.2 Blood
43% of blood plasma samples from 723 volunteers in the USA showed
the presence of PCBs; the mean value in these was about 0.5 µg/100 ml
(Finklea et al., 1972). Studies in Finland on whole blood showed
0.31-1.2 µg/100 ml in persons with no special exposure to PCBs
(Karppanen & Kolho, 1973). In Japan, an average PCB level of
0.32 µg/100 ml and a PCT level of 0.5 µg/100 ml have been recorded in
the blood of non-occupationally exposed volunteers (Doguchi & Fukano,
1975). Inpatients with severe weight loss, high levels of PCBs in the
blood (up to 10 µg/100 ml) were noted (Hesselberg & Scherr, 1974).
This was attributed to the release of PCBs from the mobilization of
fat.
5.5.5.3 Human milk
PCB concentrations measured in whole human milk in Sweden were
0.014 mg/litre in 1967 and 0.025 mg/litre in 1971-72 (Westöö & Norén,
1972), 0.03 mg/litre in Japan Nishimoto et al., 1972a), 0.103 in the
Federal Republic of Germany (Acker & Schulte, 1970) and 0.02 mg/litre
in Canada (Musial et al., 1974) A survey in Colorado, USA, revealed 8
positive samples out of 39, within the range of 0.04-0.1 mg/kg (Savage
et al., 1973).
5.5.6 Estimated daily intake
From the PCB levels encountered in air and drinking water (section
5.5.1), the daily intake from each of these sources is likely to be
less than 1 µg.
It has been stated that the major part of the human dietary intake
of PCBs is from fish (Berglund, 1972; Hammond, 1972). This may well be
true in areas such as Japan or certain localities near the North
American Great Lakes, where fish from polluted waters may form a
relatively large part of the diet. Several investigators from Japan
have measured the daily intake of PCBs in food; the highest mean value
recorded was 48 µg/day, of which 90% was from fish (Kobayashi, 1972);
the lowest was 8 µg/day (Ushio et al., 1974).
In much of Europe and North America, however, the daily intake of
fish is in the region of 30-40 g and most of the fish is taken from
waters of low pollution and contains PCB levels of not more than
0.1 mg/kg. Berglund (1972) has estimated that the daily intake of PCBs
from fish in Sweden is in the region of 1 µg, though if the fish
consumed were solely Baltic herring, the intake would be about 10 µg.
It is difficult to make an assessment of the PCB intake from foods
other than fish. Westöö et al. (1971, 1972), in their extensive study
of the Swedish diet, reported that most foods contained PCB levels of
less than 0.1 mg/kg; it may be concluded that this corresponds to a
daily intake of less than 100 µg. The conclusion that foods other than
fish may make a greater contribution to the PCB content of the diet
can be drawn from the survey in the USA reported by Kolbye (1972), and
also from a Swiss study of three different types of home-prepared
meals, not containing fish, which were found to contain 6, 41, and
84 µg of PCBs, respectively (Zimmerli & Marek, 1973).
The figures for the PCB content of human milk (section 5.5.5.3)
indicate that most nursing mothers excreted about 30 µg/day by this
route, and in some areas up to 100 µg/day. It may be assumed that only
a portion of the PCBs absorbed was excreted by this route, so that the
daily intake could have been more than 100 µg.
It may be concluded that, in the more industrialized countries,
the average daily PCB intake from the diet has rarely been less than
5 µg or greater than 100 µg; it is likely that the non-dietary sources
of exposure detailed in section 5.5.4 have made a significant
contribution, but this cannot be estimated at this time. In any area,
the intake depends not only on the diet, but also on social domestic
and environmental conditions; the influence of these factors on the
daily intake cannot readily be quantified.
6. METABOLISM
6.1 Absorption
The experiments of Vos & Beems (1971) who applied several
commercial PCBs to rabbit skin and found systemic effects (see section
7.1.1.4) indicate that PCBs can penetrate the skin. Early cases of
human poisoning from occupational exposure were probably due to a
combination of skin absorption and inhalation. Experimental studies on
rats by Benthe et al. (1972a) showed that an aerosol containing
Aroclor 1242 (particle size 0.5-3.0 µm) was readily absorbed through
the lungs.
Although one means of entry of PCBs into aquatic food chains is
through the consumption of plankton by fish, aquatic organisms can
also absorb PCBs in solution in the ambient water, presumably mainly
through the gills (see section 4.3). Salmon eggs can also absorb PCBs
from water (Johansson et al., 1970), and Södergren & Svensson 1973
concluded that mayfly nymphs could take in PCBs from water through the
gills and the integument.
Recent work with chlorobiphenyl isomers administered orally to
rodents at levels up 100 mg/kg of body weight for lower chlorinated
compounds and up to 5 mg/kg for the higher chlorinated compounds,
showed that 90% of the compounds were rapidly absorbed (Albro &
Fishbein, 1972; Berlin et al., 1973; Melvås & Brandt, 1973).
Cholestyramine, a basic anion exchange resin, was shown to interfere
with intestinal absorption of KC-400 in mice (Tanaka & Araki, 1974).
PCTs have been shown to be absorbed from the gut (Sosa-Lucero et
al., 1973) but very little information is available on the rate of
absorption.
6.2 Tissue Distribution of PCBs
Grant et al. (1971a) demonstrated that 4 days after an oral dose
of Aroclor 1254 at 500 mg/kg was given to rats, the concentrations of
PCBs in fat, liver, and brain were 996, 116, and 40 mg/kg,
respectively. Similar results showing that the highest concentration
was in fat, were obtained in rats given Aroclor 1254 in the diet
(Curley et al., 1971), in boars (Platonow et al, 1972), cows (Platonow
& Chen, 1973), and in pigeons and quail (Bailey & Bunyan, 1972). In
the experiments of Curley et al. (1971), the tissue concentrations
initially showed a rapid rise and thereafter a slow increase while the
PCB diet was being administered; Grant et al. (1974) fed diets
containing Aroclor 1254 at 0.2, 20, and 100 mg/kg to rats for 8
months, during which period the tissue concentrations reached a steady
state that was dose-dependent (Table 6). Similar tissue distribution
data for Aroclors 1016 and 1242 have been reported by Burse et al.
(1974) and for Kanechlor-400 by Yoshimura et al. (1971). PCB
deposition, in general, depends on the fat content of the tissue
(section 5.4.2). Residues in trout, receiving Aroclor 1254 in doses of
15 mg/kg in the diet, stabilized after 16 weeks while the absolute
quantity continued to increase as the fish grew (Lieb et al., 1974).
More detailed information on the tissue distribution of PCBs and
their metabolites has been obtained by the administration of pure
14C-labelled compounds, using both whole-body autoradiography and
scintillation counting of tissue samples. Berlin et al. (1975)
demonstrated that after a single oral dose of 14C-labelled
2,5,2',4',5'-pentachlorobiphenyl, radioactivity rapidly entered the
circulation of mice and was distributed in the tissues, particularly
in the liver, kidneys, lungs, and adrenals. Subsequently, the
radioactivity in the body fat increased, rising to a maximum within
4-24 h. In most other tissues the radioactivity decreased rapidly
after dosing, but the authors noted a special affinity for the skin,
the bronchiolar epithelium of the lungs, and certain glandular
secreting tissue. Soon after administration of the dose, radioactivity
appeared in bile and was excreted in the faeces. Similar results were
obtained by Melvås & Brandt (1973) with 2,4,2',4'-tetrachlorobiphenyl
in the mouse, and with 2,4,2',3'- and 2,4,3',4'-tetrachlorobiphenyls
in the quail; in the mouse they found a high affinity for the adrenal
cortex, the corpora lutea, and glandular secreting tissue, and in the
quail the radioactivity in egg yolk was high, exceeding that in fat.
Brandt & Ullberg (unpublished report 1973) found a similar pattern
after administration of hexa- and octachlorobiphenyls to mice.
6.3 Tissue Distribution of PCTs
Diets containing Aroclor 5460 at levels of 10, 100, and 1000 mg/kg
were administered to rats for 7 days (Sosa-Lucero et al., 1973). Table
7 shows the tissue distribution obtained in this study in rats fed
with Aroclor 5460 at 100 mg/kg body weight and the values in rats fed
with Aroclor 1254 at 100 mg/kg body weight in a similar study (Curley
et al., 1971). After oral administration of Aroclor 5460 to the cod,
the concentration of PCTs in the liver was more than 100 times that in
muscle on a wet weight basis (Addison et al., 1972), a ratio found by
Jensen et al. (1973) for PCBs in the cod.
Table 7. Tissue distribution (mg/kg wet weight) of PCTs (Aroclor
5460) in rats fed dietary levels of 100 mg/kg for 7 days
(Sosa-Lucero et al., 1 973) and fed PCB (Aroclor 1254) at
100 mg/kg for 9 days (Curley et al., 1971)
Aroclor Aroclor
Tissue 5460 1254
Blood 1.32 0.1
Liver 47 6
Brain 5.1 4
Kidneys 15.1 5
Heart 21.5 --
Fat -- 180
6.4 Placental Transport
Aroclors 1221 and 1254 were found to cross the placenta of
rabbits, when administered orally to does during gestation. The
concentration in fetal tissues was dose-dependent and much less with
Aroclor 1221 than with Aroclor 1254; with the latter, the
concentration in the fetal liver was greater than that in the maternal
liver (Grant et al., 1971b). Curley et al. (1973a) found some
placental transport of Aroclor 1254 in the rat. Platonow & Chen (1973)
demonstrated that the PCBs in the fetal kidney of a cow dosed with
Aroclor 1254 was greater than that in the mother. Placental transfer
of polychlorinated biphenyls has also been reported in the mouse
(Berlin et al., 1975; Melvås & Brandt, 1973; Brandt & Ullberg,
unpublished report 1973).
PCB concentration in human umbilical blood has been shown to be
about 25% of that in maternal blood (Taki et al., 1973). Placental
transfer of PCBs was observed in Yusho patients (Tsukamoto et al.,
1969).
No information is available on the placental transfer of PCTs.
6.5 Excretion and Elimination
6.5.1 Milk
Saschenbrecker et al. (1972) found that after oral administration
of doses of Aroclor 1254 of 10, and 100 mg/kg in diet to cows, 6.27
and 74.5 mg/litre, respectively, appeared in the milk after 24 hours.
These levels were reduced to less than one-half within 3 days but
traces remained at 50 days. Cows receiving 200 mg of Aroclor 1254
daily reached a steady state concentration of 61 mg/kg in milk fat and
42 mg/kg in body fat after 10 days (Fries et al., 1973). The PCBs in
milk survived processing into dairy products, and most was located in
milk fat (Platonow et al., 1971). PCBs have also been found in human
milk (see section 5.5.5.3).
6.5.2 Eggs
Several investigations have demonstrated the presence of high
levels of PCBs in the eggs of seabirds (Riseburgh & de Lappe, 1972).
An incident has been reported in which PCB contamination of poultry
food was toxic to chickens and decreased the hatchability of eggs
which also contained PCB residues (Pichirallo, 1971). In a laboratory
study, Scott et al. (1975) fed Aroclor 1248 to chickens at dietary
concentrations of 0, 0.5, 1.0, 10, and 20 mg/kg. After 8 weeks, the
approximate PCB levels in eggs were 0, 0.22, 0.41, 3.1 and 7.0 mg/kg
respectively. Jensen & Sundström (1974) found 33% of ah oral dose of
2,4,5,T,4',5'-hexa-chlorobiphenyl administered to quails was excreted
in eggs over a period of 10 days; eggs also provided a major route of
excretion in the pheasant (Dahlgren et al., 1971).
6.5.3 Urine and faeces
All investigators agree that only traces of PCBs can be found in
the urine of dosed animals, and that faeces provide a major route of
elimination. When the analysis of faeces is limited to the
determination of unchanged PCBs, the recovery of the dose administered
is incomplete; in boars receiving single or repeated doses of Aroclor
1254, not more than 16% of the dose was recovered from the faeces and
less than 1% in urine (Platonow et al., 1972). Better recoveries have
been obtained with PCB labelled with radioactive isotopes. Yoshimura
et al. (1971) found 70% of the activity from a dose of tritium-
labelled Kanechlor 400 in faeces and 2% in urine over a 4-week period.
Berlin et al. (1974, 1975) found over 75% of the activity from
14C-labelled penta- and hexachlorobiphenyls in faeces and less than
2% in urine; most of the faecal excretion consisted of PCB metabolites
(see section 6.6.1). Similar results were obtained by Melvås & Brandt
(1973) with tetrachlorobiphenyls.
6.6 Biotransformation
6.6.1 Metabolic degradation
Most investigators studying the tissue distribution of PCBs after
administration of commercial mixtures have noted a relative reduction
of the gas-liquid chromatographic peaks with shorter retention times,
corresponding to the lower chlorinated biphenyls. This has been
reported in the rat (Grant et al., 1971a; Curley et al., 1971), the
rabbit (Grant et al, 1971b), the cow (Platonow & Chen, 1973), and in
pigeons and quails (Koeman et al., 1969; Bailey & Bunyan, 1972).
Samples of tissues from animals and man (see table 3, pp. 20-21) that
have absorbed PCBs from the environment have shown, on analysis, a
pattern of peaks approaching that of PCB mixtures with more than 50%
chlorination, although the major manufactured products contain 42% of
chlorine or less. This has led to the belief that the rate of
metabolic attack on PCBs decreases with increasing chlorination.
Studies on single PCBs with 1, 2, 4, or 5 chlorine atoms have shown
that these are more readily excreted as metabolites in faeces by
mammals and birds and remain for a shorter time in fatty tissues than
most PCBs with 6 or more chlorine atoms (Berlin et al., 1975;
Hutzinger et al., 1972a; Melvås & Brandt, 1973; Brandt & Ullberg,
unpublished report 1973). See also section 6.6.2.
The administration to rats of diets containing Aroclors 1016 or
1242 at a concentration of 100 mg/kg resulted in a steady state in
adipose tissue for both compounds in about 4 months. When gas
chromatographic traces were compared with those for standard PCB
mixtures a difference in the gas-liquid chromatographic pattern, that
is disappearance of the peaks with short retention times was noted.
After exposure to PCBs was discontinued, a major portion of the PCBs
was eliminated from the body in 4 months. However, 20% of the total
PCBs in Aroclor 1242 with longer retention times were present after 6
months, and 10% of the total PCBs of Aroclor 1016 with longer
retention times were present in adipose tissue after 5 months. (Burse
et al., 1974).
The excretion of monohydroxy metabolites of 3,4,3',4'-tetrachloro-
biphenyl and 2,4,3',4'-tetrachlorobiphenyl (orally administered) in
rats has been demonstrated by Yoshimura & Yamamoto (1973). Yoshimura
et al. (1973), Yamamoto & Yoshimura (1973), Yoshimura & Yamamoto
(1974), and Yoshimura et al. (1974). They demonstrated that the
metabolites of the first isomer were 2-hydroxy or 5-hydroxy compounds
while the metabolites of the second isomer were 5-hydroxy and
3-hydroxy compounds. All hydroxy metabolites were excreted
non-conjugated via the bile and no parent isomers were found in the
bile. Yoshimura & Yamamoto (1975) found that unchanged
2,4,3',4'-tetrachlorobiphenyl was excreted through the intestine, when
it was intravenously injected in rats with the bile duct ligated,
while no metabolite of this isomer was excreted by this route.
Hutzinger et al. (1972a) demonstrated the presence of hydroxylated
derivatives of mono-, di-, and tetrachlorobiphenyls in the excreta of
rats and pigeons but not of trout and were unable to detect
hydroxylated 2,4,5,2',4',5'-hexachlorobiphenyl. Berlin et al. (1975)
isolated a hydroxy derivative of 2,5,2',4',5'-pentachlorobiphenyl from
mouse faeces, and Jensen & Sundström (1975) have shown that although
2,4,5,2',4',5'-hexachlorobiphenyl is excreted very slowly, a hydroxy
derivative could be detected in rat faeces. Hutzinger et al. (1974),
however, showed that this compound was also dechlorinated by the
rabbit and excreted as the hydroxy derivative of pentachlorobiphenyl.
Gardner et al. (1973) detected hydroxylated metabolites in the urine
of rabbits dosed with 2,5,2',5'-tetrachlorobiphenyl together with a
dihydroxy derivative which they regarded as evidence for the formation
of an arene oxide (epoxide) intermediate (see section 6.5.3). Jansson
et al. (1975) have identified up to 26 mono- and dihydroxylated
metabolites of PCB in the bile and faeces of wild grey seal and
guillemot from the Baltic.
There is little information on the biotransformation of PCTs.
Addison et al. (1972), using gas-liquid chromatography, noted a loss
of PCTs with a shorter retention time in the excreta of a cod dosed
orally with Aroclor 5460; the same loss was observed in rat faeces
after the administration of a diet containing Aroclor 5460
(Sosa-Lucero et al., 1973).
6.6.2 The effect of structure on retention
While each of the components of mixtures of PCBs has a different
pattern of retention and elimination in different species, the
measurement of biological half-times for PCB mixtures in tissues has
provided useful information. Bailey & Bunyan (1972) found the
half-time of PCBs in the fat of quail and pigeon, after cessation of
dosing with Aroclor 1242, or with Aroclor 1254, to be 50 days and 125
days, respectively. A half-time of about 200 days was recorded in the
fat of rats after feeding with Aroclor 1254 (Grant et al., 1974).
Berlin et al. (1975) noted that in mice dosed with a
pentachlorobiphenyl there was an initial fairly rapid elimination
while the PCB level in the liver was high, followed by a slower
elimination when most of the PCB was located in fat. It seems likely
that the mobilization of PCBs from fat, and therefore their half-time
in the body, depends upon their rates of metabolism. Berlin et al.
(1974) investigated the hypothesis that the ability of a PCB to be
readily degraded with a half-time of a few days depended upon the
presence of two adjacent unsubstituted carbon atoms in the molecule
rather than on the number of chlorine atoms, although the presence of
such unsubstituted pairs depends to a large extent on the degree of
chlorination. They came to the conclusion that this hypothesis
probably applied to unsubstituted pairs in the 3,4-position, but that
in the 2,3-position, their susceptibility to metabolic degradation was
much influenced by the presence of chlorines in the o-position of
the ring bridge.
Jensen & Sundström (1974) demonstrated that the retention of PCBs
in human fat is also influenced by o-chlorine substitution (Table 3,
pp. 20-21 ).
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF PCBs AND PCTs
7.1 Toxic Effects in Different Species
Most of the available information on the toxicity of the PCBs has
been obtained from studies on commercial mixtures. The much smaller
amount of information available concerning the impurities in
commercial products and PCTs, is given in sections 7.2 and 7.3.
7.1.1 Mammals
7.1.1.1 Acute oral and intravenous toxicity
Earlier work by the Monsanto Company indicated a low acute oral
toxicity for the Aroclors, the LD50s to rats ranging from 4.0 g/kg
for Aroclor 1221 to 11.3 g/kg for Aroclor 1262 (Panel on Hazardous
Trace Substances, 1972). More recent work has demonstrated a slightly
higher toxicity (Bruckner et al., 1973; Grant & Philips, 1974). With a
single intravenous dose the LD50 for Aroclor 1254 was 358 mg/kg body
weight in adult female Sherman rats (Linder et al., 1974). The acute
oral LD50 for Aroclor 1254 in the same strain and sex was between 4
and 10 g/kg (Kimbrough et al., 1972). According to Bruckner et al.,
(1973), severely poisoned animals showed weight loss, ataxia,
diarrhoea, and chromodacryorrhoea, and they considered progressive
dehydration and central nervous depression were the causes of death.
In rats, vacuolation in the liver and kidneys was observed (Bruckner
et al., 1973) and also ulceration of the gastric and duodenal mucosa
(Kimbrough et al., 1972).
Yamamoto & Yoshimura (1973) found the intraperitoneal LD50 of
2,4,3'4'-tetrachlorobiphenyl in mice to be 2.15 g/kg body weight and
that of the 5-hydroxy derivative, which is the main in vivo
metabolite, to be 0.43 g/kg body weight.
7.1.1.2 Subacute oral toxicity
After repeated administration, the PCBs have a cumulative toxic
action. In a group of rats receiving Aroclor 1254 at a dose of 1 g/kg
of diet, deaths occurred between the 28th and 53rd days of feeding
(Tucker & Crabtree, 1970), and with Phenochlor DP6 at 2 g/kg of diet,
deaths occurred between the 12th and 26th days (Vos & Koeman, 1970).
In the latter experiment enlarged livers, small spleens, and a
progressive chemically-induced hepatic porphyria were seen at autopsy.
Repeated weekly oral administration of 150 mg of Aroclors 1221, 1242
or 1254 to rabbits for 14 weeks produced liver enlargement and damage
with Aroclor 1242 and no effect with Aroclor 1221 (Koller & Zinkl,
1973). Allen et al. (1974) administered diets containing Aroclor 1248
at the concentration of 25 mg/kg of diet to 6 female rhesus monkeys
for two months; facial oedema, loss of hair, and acne developed after
1 month and one animal died with severe gastritis 2 months after
removal from experimental diet. PCB concentrations in the body fat of
the animals, after two months of treatment, averaged 127 mg/kg while 8
months later the value declined to 34-mg/kg.
Mink appear to be unusually sensitive to PCBs. Aulerich et al.
(1973) administered diets containing PCB levels of 30 mg/kg (10 mg/kg
each of Aroclors 1242, 1248, and 1254) to adult mink and demonstrated
100% mortality within 6 months. PCB residues in the brains of these
mink averaged about 11 mg/kg and were approximately twice the level
observed in other tissues. Four months of feeding Aroclor 1254 at
levels of 5 and 10 mg/kg demonstrated a dose-dependent retardation of
weight gain of growing female mink. Female mink fed a diet
supplemented with Aroclor 1254 at 5 mg/kg for 9 months failed to
produce offspring (Ringer et al., 1972).
7.1.1.3 Chronic oral toxicity
Aroclors 1242, 1254, and 1260 have been administered for 18 months
to rats at 1, 10 and 100 mg/kg in the diet (Keplinger et al., 1971).
No adverse effects were recorded with the three Aroclors at 10 mg/kg
but with Aroclors 1242 and 1254 at 100 mg/kg there was an increase in
liver weight and a reduced survival of litters. In similar experiments
on dogs, there was a reduced weight gain with Aroclors 1254 and 1260
in the diet at a level of 100 mg/kg (Keplinger et al., 1971). In the
experiments reported by Kimbrough et al. (1972), male rats survived
Aroclor 1260 in the diet at 1 g/kg (71.4 mg/kg body weight) for 8
months but 8/10 females died at this dose, 2/10 died at 500 mg/kg, and
1/10 at 100 mg/kg (7.2 mg/kg body weight). With Aroclors 1254 and
1260, a dose-dependent increase in liver weight in male rats was
significant down to 20 mg/kg (1.4 mg/kg body weight) in the diet; with
females the liver enlargement occurred only at diet levels of
500 mg/kg and higher. The livers showed an orange fluorescence, the
cells were enlarged and vacuolated with lipid inclusions; there was
also much increased smooth endoplasmic reticulum, and what was termed
"adenofibrosis" was present, being more marked with Aroclor 1254 (see
section 7.8). Grant et al. (1974) fed diets containing Aroclor 1254 at
0, 2, 20, and 100 mg/kg to rats for 246 days followed by 180 days on a
PCB-free diet; after 246 days the body weight of the 100 mg/kg rats
was significantly less than that of the controls and the liver weight
was greater. The most notable change in the livers on histological
examination was the appearance of fat microdroplets in the
centrilobular region; this effect was dose-dependent and not seen in
the rats receiving the 2 mg/kg diet and was reversible when the rats
were returned to a normal diet.
Liver enlargement has been described by a number of authors, in a
number of species, with different PCB mixtures and pure isomers and it
is considered to be due primarily to hypertrophy of the smooth
endoplasmic reticulum of the liver cells (Vos & Beems, 1971; Allen et
al., 1973; Kimbrough, 1974; Nishizumi, 1970) but with large enough
doses (oral intubation of about 150 mg/kg body weight/week for 14
weeks) of Aroclor 1254 and 1242, it progressed to frank liver damage
(Koller & Zinkl, 1973). The smooth endoplasmic reticulum may condense
in the liver cell and form hyalin inclusions and this may be
accompanied by a loss of enzyme activity. Lipid accumulation, pigment
deposition, nuclear changes, and necrosis may also occur (Vos &
Notenboom-Ram, 1972).
The rhesus monkey is the only species reported to show signs of
poisoning similar to those in human Yusho patients (see section 8).
The administration of Aroclor 1248 at 2.5 and 5.0 mg/kg of diet for 1
year produced periorbital oedema, alopecia, erythema, and acneiform
lesions involving the face and neck within 1-2 months. The effects
were less marked in male monkeys. At 25 mg/kg of diet, one out of a
group of six died, and at 100 and 300 mg/kg the mortality approached
100% within 2-3 months. Animals more severely affected showed
hypertrophic hyperplastic gastritis with ulceration, anaemia,
hyperproteinaemia and bone marrow hypoplasia. The survivors still
showed signs of poisoning 8 months after exposure (Allen & Norback,
1973; Allen et al., 1974; Allen 1975).
7.1.1.4 Dermal toxicity
Vos & Beems (1971) have confirmed earlier reports that PCBs damage
the follicular epithelium in experimental animals. They applied three
commercial 60% chlorinated mixtures, Clophen A60, Phenochlor DP6, and
Aroclor 1260 to rabbit skin at a daily dose of 118 mg/50 cm (5 times
per week) for 38 days. After initial reddening, transverse wrinkling
developed with hyperplasia and hyperkeratosis of the epidermal and
follicular epithelium. These effects were more marked with Clophen and
Phenochlor than with the Aroclor.
During these experiments, deaths occurred in the Clophen- and
Phenochlor-treated groups but not in the Aroclor group. Kidney lesions
were seen in all groups; liver damage was least in the Aroclor group.
There was atrophy of the thymus cortex and a reduction of germinal
centres of the lymph nodes as well as lymphopenia, and some animals in
all groups showed oedema of the abdominal and thoracic cavities,
subcutaneous tissue, and pericardium. Faecal excretion of copro- and
protoporphyrins was increased by all three PCBs but was lowest with
Aroclor 1260. Vos & Beems attributed the greater severity of the
effects observed with Clophen and Phenochlor to the presence of toxic
impurities (see section 7.2). In another comparative dermal toxicity
study in rabbits (Vos & Notenboom-Ram, 1972), skin lesions in Aroclor
1260-treated animals were more severe than in animals treated with
2,4,5,2',4',5'-hexachlorobiphenyl. In a Japanese study, no differences
in skin lesions were observed in rabbits after dermal applications of
Kanechlor 400, Kanechlor 500, or 3,4,3',4'-tetrachlorobiphenyl
(Komatsu & Kikuchi, 1972).
7.1.1.5 Inhalation toxicity
Only one inhalation study has been reported using Aroclor 1242 and
1254 (Treon et al., 1956). Rats, mice, rabbits, and guinea pigs were
exposed to Aroclor 1242 or 1254 vapours for five days a week for
several weeks at concentrations ranging from 1.5 to 8.6 mg/m3. At
these concentrations Aroclor 1254 produced liver enlargement in rats.
7.1.2 Birds
The acute oral toxicity of Aroclors 1242 and 1254 is low for the
mallard duck, the LD50s being greater than 2 g/kg body weight (Tucker
& Crabtree, 1970). Published figures for the lethal dose for birds
after repeated administration are very variable; it appears to be
dependent on the species and age of the bird, the method of
administration, the degree of chlorination of the PCBs, and the
presence of impurities (Vos, 1972). Heath et al. (1970) determined the
dietary concentrations of Aroclors 1232 to 1262, administered over a
5-day period, that were required to kill 50% of groups of mallards,
pheasants, and quails. The concentrations were in the range of 500 to
over 5000 mg/kg, with the bobwhite quail the most sensitive and the
Japanese quail the least. There was a positive relationship between
the percentage of chlorine in a technical Aroclor and its toxicity.
The relationship held true for those containing less than 60%
chlorine, Aroclor 1260 and 1262 deviating slightly. Mallards were
relatively less responsive to chlorine content than the three
gallimaceous species. Prestt et al. (1970) found a 50% mortality in
Bengalese finches receiving a daily oral dose of Aroclor 1254 at
254 mg/kg body weight for 56 days; cormorants have been killed with a
cumulative dose of 5.7 g of Clophen A60, but herons were more
resistant (Koeman et al., 1973).
The toxicity to chickens of diets containing Phenochlor DP6,
Clophen A60, or Aroclor 1260 at 400 mg/kg has been studied by Vos &
Koeman (1970). The first two produced a 100% mortality with survival
times of 24.3 and 20.5 days, but there was only 15%o mortality with
the Aroclor over the 60-day test period. The authors demonstrated the
presence of impurities in the Phenochlor and Clophen (Vos et al.,
1970). The results of Flick et al. (1965) and Platonow & Funnel (1971)
indicate that chickens can survive 200 mg/kg in the diet for several
months, but that deaths may occur at 250 mg/kg, though Rehfeld et al.
(1971) found a much higher toxicity, recording 1/30 deaths with
Aroclor 1248 at 30 mg/kg of diet over 25 days and 16/30 at 50 mg/kg.
Keplinger et al. (1971) found decreased growth in chickens receiving
Aroclor 1242 at 10 mg/kg and Aroclor 1254 at 100 mg/kg of diet, but no
effects with Aroclor 1260 at 100 mg/kg. Birds severely poisoned with
PCBs show tremors, ataxia, and ruffling and loss of feathers. Oedema
of the abdominal and peritoneal cavities has been a characteristic
sign at autopsy in some experiments (Flick et al., 1965) and this was
observed in a very large number of chickens killed in Japan by the
contamination of their feed with Kanechlor KC400 (Kohanawa et al.,
1969a, 1969b). Enlargement of the kidneys and sometimes of the liver
has been reported; some authors have noted a reduction in the size of
the spleen, comb, and testes, an enlargement of the adrenals and
thyroid, and a pale pancreas. No pathological signs were observed by
Dahlgren et al. (1972) that could account for the deaths of pheasants,
which occurred with a cumulative intake of about 900 mg of Aroclor
1254 (daily oral administration of 20 or 200 mg to 11-week-old hens);
they found a mean concentration of 520 mg/kg in brain tissue at death
and it is possible that effects in the central nervous system were a
contributory cause.
7.1.3 Aquatic organisms
7.1.3.1 Fish
Stalling & Mayer (1972) report an oral LD50 of more than 1.5 g/kg
for rainbow trout with Aroclors 1242 and 1260. A 15 mg/kg oral dose to
cod affected their ability to maintain an upright position in rotating
water (Lindahl, unpublished report 1974).
The assessment of toxicity to fish by adding PCBs to aquarium
water is subject to considerable error on account of the different
solubilities of the components. Zitko (1970) claims the aqueous
solubility of Aroclor 1221 to be 3.8-5 mg/litre and that of Aroclor
1254 to be 0.3-0.5 mg/litre. Stalling & Mayer (1972) report the
96-hour LC50s on the cut-throat trout to be 1.17 mg/litre for Aroclor
1221 and up to 60 mg/litre for Aroclor 1260, where the solubility is
clearly exceeded. In the more prolonged experiments of these authors
(Table 8), most of the concentrations are within the solubility limits
and therefore more reliable. In more prolonged experiments, Hansen et
al. (1971) found deaths in fish exposed for up to 45 days to Aroclor
1254 at 5 µg/litre, but none at 1 µg/litre. These results show that
the effect of PCBs is cumulative in fish and that the toxicity
decreases with increasing chlorination.
Table 8. Intermittent-flow bioassays of Aroclors against three species of fisha
LC50 (µg/litre)
5 10 15 20 25 30
Aroclor Species days days days days days days
1254 Rainbow trout 156 8 -- -- -- --
1260 -- 240 94 21 -- --
DDT 2.26 0.87 0.26 -- -- --
1242 Bluegills 154 72 54 -- -- --
248 307 160 76 10 -- --
1254 -- 443 204 135 54 --
1260 -- -- -- 245 212 151
1242 Channel catfish -- 174 107 -- -- --
1248 -- 225 127 -- -- --
1254 -- __ 741 300 113 --
1260 -- -- -- 296 166 137
1248b Bluegills 137 76 -- -- -- --
1248c Channel catfish -- 94 57 -- -- --
a From Stalling & Mayer (1972).
b Temperature, 20°C; alkalinity 260 mg/litre pH 7.4.
c Temperature, 27°C.
Young fish appear to be more sensitive to PCBs than adults,
96-hour LC50s for newly hatched fathead minnows were 15 and
8 µg/litre, respectively for Aroclors 1242 and 1254. Growth of young
fathead minnows and flagfish was affected above 2.2 µg/litre (Nebeker
et al., 1974). There is no information on pathological changes in fish
that might be related to the lethal action of PCBs; Hansen et al.
(1971) found that fish survived exposure to Aroclor 1254 at
5 µg/litre, but died later, after being returned to clean water, with
signs of a lowered resistance to infection. Pathological changes were
observed in kidney, spleen, and liver of rainbow trout fed Aroclor
1254 at 10 or 100 mg/kg of diet for up to 330 days (Nestel & Budd,
1975).
7.1.3.2 Aquatic invertebrates
The results of several investigations into the toxicity of PCBs to
aquatic invertebrates have been reported by Stalling & Mayer (1972).
The exposure of a variety of scud (Gammarus pseudolimnaeus) to PCBs
showed a decreased toxicity with increasing chlorination; 4-day LC50s
of 10, 52, and 2400 µg/litre were obtained with Aroclors 1242, 1248
and 1254, respectively. The threshold for survival, growth, and
reproduction of Gammarus pseudolimnaeus exposed to Aroclor 1248 was
about 5 µg/litre and was the same for Daphnia magna. With the
crayfish, 7-day LC50s were 30 µg/litre (Aroclor 1242) and 80 µg/litre
(Aroclor 1254) and with the glass shrimp, 3 µg/litre (Aroclor 1254).
Wildish (1970) found that mortality in Gammarus oceanicus was
dependent on the duration of exposure to Aroclor 1254 at levels above
10 µg/litre. Stalling & Mayer (1972) reported the 15-day LC50 of
Aroclor 1254 for immature pink shrimp to be 0.94 µg/litre.
7.1.3.3 Microorganisms
The growth of certain marine diatoms is inhibited by Aroclor 1254
at 10-25 µg/litre, but marine and freshwater algae are more resistant,
being unaffected by 100 µg/litre (Mosser et al., 1972). Fisher et al.
(1972) found that phytoplankton from the Sargasso Sea did not grow in
Aroclor 1254 at 10 µg/litre, though phytoplankton from estuarine and
coastal waters were not much affected by this concentration. Keil et
al. (1971) report that the growth of a diatom was inhibited by Aroclor
1242 at 100 µg/litre with a reduction of RNA synthesis, but that
10 µg/litre had no effect.
The growth of cultures of lake bacteria was not inhibited by
concentrations of Aroclors 1221, 1242, and 1254 in excess of
solubility, and Aroclors 1221 and 1242 could be utilized as the sole
source of carbon and energy (Wong & Kaiser, 1975).
7.2 Toxicity of Impurities in Commercial PCBs
Vos & Koeman (1970) observed that Phenochlor DP6 and Clophen A60
were more toxic to chickens than was Aroclor 1260 (see section 7.1.2)
and Vos & Beems (1971) showed a similar difference in the dermal
toxicity to the rabbit (see section 7.1.1.4). Vos et al. (1970)
subdivided Clophen A60 and Phenochlor DP6 into non-polar PCBs and
polar fractions and found polar components that were not detectable in
a similar fraction from Aroclor 1260. Phenoclor DP6, Clophen A60, and
the polar fraction from Clophen A60 produced a high mortality in the
chick embryo test but the polar fraction from Aroclor 1260 did not. A
difference between the three fractions was seen in the development of
skin lesions in the rabbit (Vos & Beems, 1971). Mass spectrographic
analysis indicated that the impurities were tetra- and pentachloro-
dibenzofurans. Additional contaminants were chlorinated naphthalenes.
Vos et al. (1970) calculated that the maximum level of chlorinated
dibenzofurans in Clophen A60 was 5 mg/kg and in Phenochlor DP6,
20 mg/kg. They calculated that the chlorinated dibenzofurans were
approximately one order of magnitude less toxic than the chlorinated
benzodioxins, and considered that they were mainly responsible for the
toxicity of the polar fraction and for the difference in toxicity
between the three commercial PCB mixtures.
Recently, chlorinated dibenzofurans were detected in the
PCB-contaminated oil that was responsible for the Yusho disease in
Japan (see section 8, p. 65).
In 1961, Bauer et al. demonstrated the toxicity of a mixture of
tri- and tetrachlorodibenzofurans; a single oral dose of 0.5-1.0 mg/kg
body weight caused severe and often lethal liver necrosis in rabbits.
Application to the rabbit ear resulted in hyperplasia and
hyperkeratosis. Similar toxic effects were found with 2,3,7,8-tetra-
chlorodibenzo- p-dioxin in doses that were 10 times lower than those
found to be toxic in the case of chlorinated dibenzofurans. See also
the review by Kimbrough (1974). 2,3,7,8-Tetrachlorodibenzofuran, which
has recently been shown to be present in PCBs (Bowes et al., 1975)
caused mortality in chickens after 8-15 days when they were dosed
orally with 5 µg/kg/day. A single oral dose of 4000 µg/kg was not
lethal to mice. Porphyria was not observed in chicks or mice
(Goldstein et al., 1975a). In comparison, 8 out of 10 chicks died
after 9-19 days when dosed orally with 2,3,7,8-tetrachloro-
dibenzo- p-dioxin at 1 µg/kg body weight/day (Schwetz et al., 1973).
The oral LD50 of 2,3,7,8-tetrachlorodibenzofuran in guinea pigs is
approximately 7 µg/kg body weight, which is less than one order of
magnitude higher than that of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(Moore, 1975).
Using low resolution mass spectrometry, McKinney (1975) found
metabolites in the excreta of chickens fed 2,4,6,2',4',6'-hexa-
chlorobiphenyl which had correct masses for chlorodibenzofurans. In
addition, by perchlorination, he identified octachlorodibenzofuran
again by low resolution mass spectrometry. The 2,4,6,2',4',6'-hexa-
chlorobiphenyl isomer is not found in commercial mixtures.
Zitko et al. (1972b) looked for the presence of chlorinated
dibenzofurans in fish and fish products taken from a contaminated
coastal area. The samples included shark tissues, cormorant and
herring gull eggs, herring oil, and herring fishmeal. The detection
limit of the method was 0.01-0.02 mg/kg in the sample. No chlorinated
dibenzofurans were detected, although the authors admit that the limit
of detection was not sufficiently low to detect amounts that might
exert a significant toxic action. Curley et al. (1975) detected a
component which had a 4 Cl isotopic cluster and a mass number of 304
corresponding with that of tetrachlorodibenzofuran in the urine of
rats dosed with PCBs, but a positive identification was not made.
7.3 Toxicity of the PCTs
There have not been any systematic studies on the toxicity of the
PCTs. Sosa-Lucero et al. (1973) administered diets containing Aroclor
5460 at 0, 10, 100, and 1000 mg/kg to groups of rats for seven days.
There were no adverse effects on health or body weight; a significant
liver enlargement was recorded at the 1000 mg/kg level. In a test for
estrogenic activity involving the stimulation of glycogen response in
the immature rat uterus, Aroclor 5460 was inactive, as was Aroclor
1260 but Aroclor 5442 was more active than Aroclor 1242 (Bitman et
al., 1972). A dietary level of Aroclor 5460 of 5000 mg/kg for 12 weeks
caused decreased body weight and increased liver weight in rhesus
monkeys (Allen & Norback, 1973); after 6 weeks, facial oedema, hair
loss, and eye discharge were observed as described by the same authors
in experiments with Aroclor 1248 (section 7.1.1.2), and similar
gastric changes were also reported.
7.4 Biochemical Effects
7.4.1 Induction of enzymes
Several investigators have observed an increase in the smooth
endoplasmic reticulum of liver cells after administration of PCBs (see
section 7.1.1.3). This is accompanied by an induction of microsomal
mixed function oxidase. Induction of microsomal enzyme activity is,
like liver enlargement, more marked with the higher chlorinated PCBs
and relatively low with Aroclors 1221 and 1016 (Villeneuve et al.,
1971a, 1972; Bickers et al., 1972; Ecobichon & Corneau, 1974). The
effect has also been demonstrated with single pure PCBs administered
orally (Fujita et al., 1971); more recently this work has been
confirmed by Johnstone et al. (1974) whose results are summarized in
Table 9 and show a greater degree of enzyme induction with the higher
chlorinated compounds.
Grant et al. (1974) found an increase in microsomal enzyme
activity in rats receiving Aroclor 1254 at 20 mg/kg of diet for 246
days, but none at 2 mg/kg. Iverson et al. (1975) and Goldstein et al.
(1975) compared the microsomal enzyme inducing potential of Aroclors
1242 and 1016. Iverson et al. (1975) found increased hepatic
microsomal enzyme activity with both Aroclors in male rats receiving
21 daily oral doses of 1 mg/kg body weight, and with Aroclor 1242 in
females at 10 mg/kg of body weight and with Aroclor 1016 at 100 mg/kg.
PCBs administered to rats during pregnancy can induce microsomal
enzyme activity in the placenta and fetus and this also occurs in the
liver of newborn rats suckled by mothers fed with diets containing
PCBs (Alvares & Kappas, 1975). Benthe et al. (1972b) reported that
when rats were stressed by food deprivation or cold, the PCB residues
in adipose tissue were released during mobilization of the fat and
caused increased hepatic microsomal enzyme activity.
Table 9. Stimulation of microsomal enzyme activity by single chlorinated biphenyls
Hepatic microsomal enzyme activity
Chlorine aniline
substituents O-demethylation N-demethylation hydroxylation nitro-reduction
4 0 0 0 0
2,2' 0 + + 0
2,4' 0 0 + 0
4,4' + + + + + + +
2,5,2',5' 0 + + + +
2,4,2',4' + + + + + + 0
2,4,5,2',4',5' + + + + + + + +
2,3,5,2',3',5' + + + + + + +
2,4,6,2',4',6' + + + + + + + +
2,3,4,5,2',3',4',5' + + + + + + + +
a Johnstone et al. (1974).
0 no activity.
+ slight activity.
+ + marked activity.
7.4.2 Porphyria
Hepatic porphyria has been induced by a number of PCBs (Clophen
A60, Phenoclor DP6, Aroclors 1016, 1242, 1254, and 1260) in the
chicken, rabbit, Japanese quail, and the rat (Vos & Koeman 1970; Vos &
Beems, 1971; Iverson et al., 1975; Vos et al., 1971; Goldstein et al.,
1974, 1975a, 1975b). Porphyrin induction has been studied more
extensively in rats. A dose-dependent increase in liver porphyrins has
been observed in females receiving 21 daily oral doses of Aroclor 1242
at 10 and 100 mg/kg of body weight, but not at 1 mg/kg. Female rats
were more sensitive than males, and Aroclor 1016 had less effect
(Iverson et al., 1975). In rats receiving Aroclor 1254 at 100 mg/kg in
the diet, the increase was usually delayed 2-4 months after the start
of dosing (Goldstein, 1974) and was characterized by high hepatic and
urinary levels of uroporphyrin. Disturbance of porphyrin biosynthesis
had been connected with an increase of the rate limiting enzyme
2.3.1.37-delta-aminolaevulinate synthase (Vos et al., 1971; Goldstein
1974, 1975).
Kawanishi et al. (1973, 1974) have shown that the administration,
in the diet, of Kanechlors KC-300 and KC-500 to rats at 500 mg/kg
produced a marked increase in urinary excretion of copro- and
uroporphyrins, and in faecal excretion of protoporphyrin, but no
increase was observed with KC-400. Experimental administration of pure
tetrachlorobiphenyls did not produce porphyria. However, porphyria did
result from the repeated subcutaneous injection of KC400 (total dose
1.8 g) to rabbits for 55 days (Miura et al., 1973).
7.4.3 Effects on steroid metabolism
PCBs have been shown to stimulate the activity of enzymes
responsible for metabolizing steroids such as estrodiol and
androsterone more effectively than does DDT or DDE (Risebrough et al.,
1968; Lincer & Peakall, 1970). It has been suggested that effects on
reproduction (see section 7.4) may be attributed to the induction of
steroid-metabolizing enzymes (Kihlström et al., 1973). Long-term
administration of daily oral doses of 0.025 mg Clophen A60 to 23
female NMRI strain mice caused a lengthening of the estrous cycle and
a reduction in the frequency of implantation of ova Orberg &
Kihlström, 1973).
7.4.4 Other biochemical effects
Hepatic vitamin A has been reported by Villeneuve et al. (1971a)
to be reduced to half the normal values in pregnant rabbits by Aroclor
1254. Similar observations have been made by Cecil et al. (1973) in
male and female Japanese quails and in rats after feeding Aroclor 1242
at 100 mg/kg of diet. The 50% decrease in hepatic vitamin A found in
male and female rats was also found in male and female quail provided
that the latter were kept in the dark to prevent egg laying.
A lowering of hepatic vitamin A in rats, fed a 0.1% PCB diet (PCB
not specified), was described by Innami et al. (1974). The rats given
vitamin A supplement (3400 IU) plus PCB for 6 weeks showed better
growth than those given PCB alone, while those given PCB and a vitamin
A deficient diet showed significant growth retardation. On the PCB
diet, the hepatic vitamin A level decreased to 20% of the normal level
during the experimental period; supplementing the diet with 1000 IU
did not re-establish the vitamin A level in the liver. Administration
of 3000 IU of vitamin A with the PCB diet, however, allowed better
than normal hepatic vitamin A levels to be re-established. The authors
concluded that vitamin A may play a role in the detoxification of PCB
rather than that PCB plays a role in the destructive metabolism of
vitamin A.
Aroclor 1254 administered intraperitoneally to rats at 25 mg/kg
body weight daily for 4 days caused a 4- to 5-fold increase in the
biliary excretion of thyroxine during a 3-hour period. Biliary
clearance was greatly elevated. Hypobilirubinaemia has been produced
in rats by Bastomsky et al. (1975) who investigated the mechanism by
administering daily intra-peritoneal injections of Aroclor 1254
(25 mg/kg body weight in corn oil) to female rats for 4 days, then
measuring bilirubin glucuronide formation by hepatic microsomes
in vitro. PCB treatment was not effective in increasing
UDPglucuronosyltransferase (2.4.1.7) activity. Serum bilirubin levels
were also significantly decreased by PCB treatment of Gunn rats, which
are genetically deficient in UDPglucuronosyltransferase (2.4.1.7)
activity.
7.4.5 Potentiation and antagonism by PCBs
As PCBs can stimulate microsomal enzyme activity, it is to be
expected that they may potentiate the action of those chemicals that
undergo microsomal activation, and decrease the action of those that
are detoxified. Villeneuve et al. (1973) demonstrated the antagonistic
effect by the reduction of phenobarbital sleeping time in rats
receiving Aroclors 1242, 1254, and 1260 in their diet, but not in
those receiving Aroclor 1221. Johnstone et al. (1974) have confirmed
this with a series of single PCBs. Tanaka & Komatsu (1972) found that
the hexobarbital-induced sleeping time in female rats was reduced to
49% of the control value by daily oral doses of Kanechlor 500 of
2 mg/kg for 3 days (total 6 mg/kg). When a daily dose of 0.4 mg/kg was
given for 15 days (total 6 mg/kg), no reduction in sleeping time was
observed. When this small dose was continued for 45 and 53 days, the
reduction remained at 12-13%. Phillips et al. (1972) did not find any
potentiation of the cholinesterase-inhibitory action of parathion in
rats dosed with Aroclors 1221 and 1254; this does not necessarily
imply that there was no enhanced activation of parathion, as a
stimulation of detoxication may have occurred concurrently. A
stimulation of parathion detoxication but not of activation has been
demonstrated in rabbit microsomes (Villeneuve et al., 1971a).
Lichtenstein (1972) reports a potentiation by PCBs of the toxicity of
parathion to flies.
Cecil et al. (1975) have shown that the ability of PCTs to
decrease phenobarbital sleeping time in quails is rather less than
that of the PCBs.
Aroclor 1254 at 160 mg/kg of diet fed to 5-week old male and
female Fischer-344 rats for 8 weeks reduced mortality due to feeding
hexachlorophene at a concentration of 600 mg/kg of diet from 77% to 7%
and completely prevented the paralysis that was observed in all
animals on the hexachlorophene diet alone. However, in the animals on
the combined treatment, histological changes in the brain
characteristic of hexachlorophene were still apparent and the
possibility of delayed toxicity beyond the 8 weeks of the experiment
could not be eliminated. The protective effect of Aroclor 1254 was
explained by its capacity to enhance detoxification by means of
hepatic microsomal enzyme induction (Jones et al., 1974).
7.5 Cytotoxic Effects
Peakall et al. (1972) found a significant increase in chromosome
abnormalities in the embryos of ring doves when the parents were fed
on a diet containing Aroclor 1254 at 10 mg/kg. Nilsson & Ramel (1974)
found no chromosome breakage in Drosophila melanogaster when either
Clophen A30 or A50 was added to the substrate. Green et al. (1975)
administered Aroclor 1242 orally to rats in single doses of 1250,
2500, or 5000 mg/kg or as a repeated dose of 500 mg/kg/day for 4 days.
Aroclor 1254 was also administered for 5 days at doses of 75, 150, or
300 mg/kg/day; they found no evidence of a mutagenic potential as
assessed by cytogenic analysis of bone marrow and spermatogonia.
7.6 Immunosuppressive Effects
Administration of PCBs leads to an atrophy of lymphoid tissue in
chickens (Flick et al., 1965; Vos & Koeman, 1970), in pheasants
(Dahlgren et al., 1972), and in rabbits (Vos & Beems, 1971). Vos & de
Roij (1972) and Vos & van Driel-Grootenhuis (1972) came to the
conclusion that these effects could be attributed to an
immunosuppressive effect of PCBs. They found that when guinea pigs fed
on diets containing Clophen A60 or Aroclor 1260 at 50 mg/kg were
stimulated with tetanus toxoid, a lower antitoxin titre and a lower
count of antitoxin-producing cells was obtained than in control guinea
pigs, resulting in a significant reduction of immunoglobulins. The
skin reaction after tuberculination in animals immunized with Freund's
complete adjuvant (as a parameter of cell-mediated immunity) was also
depressed at the 50 mg/kg of diet level. Vos & de Roij (1972)
suggested that the ability of PCBs to increase the susceptibility of
ducklings to duck hepatitis virus (Friend & Trainer, 1970) and of fish
to fungal disease (Hansen et al., 1971) could be attributed to this
immunosuppressive effect. Kimuru & Baba (1973) found an increased
incidence of pneumonia, and lung and intracranial abscesses in rats on
a diet containing Kanechlor 400 and suggested that this was due to a
lowered resistance to infection.
7.7 Effects on Reproduction
In a one-generation reproduction study, rats were fed with a diet
containing Aroclor 1242, 1254, or 1260 at levels of 1, 10, and
100 mg/kg. Decreased survival of pups with Aroclors 1242 and 1254 at
100 mg/kg was noted. No effect on reproduction was detected with
Aroclor 1260 (Keplinger et al., 1971). In a two-generation
reproduction study (Linder et al., 1974), rats were fed a diet
containing Aroclor 1254 at levels of 0, 1, 5, 20, and 100 mg/kg and
Aroclor 1260 at levels of 0, 5, 20, and 100 mg/kg. Rats exposed to
Aroclor 1254 at dietary levels of 20 mg/kg or more had fewer pups per
litter. Aroclor 1260 had no effect on reproduction, even at levels of
100 mg/kg. Kihlström et al. (1973) showed that a daily oral dose of
25 µg of Clophen A60 to female mice for 62 days significantly
increased the length of the estrus cycle and decreased the frequency
of implanted ova. In order to study the effect of PCBs on the
development of sexual functions in the early postnatal period they
also mated mice that had been suckled by mothers dosed with Clophen
A60 during the lactation period. A decrease in the frequency of
implanted ova was noted when both parents of the couple had been
suckled with milk containing PCBs.
In the rhesus monkey, Allen (1975) reports a lower fertility and a
diminished weight of the young at birth, after the administration of a
diet containing Aroclor 1248 at 2.5 mg/kg for several months.
Studies by Aulerich et al. (1971), and Ringer et al. (1972) showed
that Aroclor 1254 fed to mink at 5 mg/kg severely affected
reproduction.
There have been several reports that PCBs can adversely affect egg
production and hatchability in birds. Contamination of feed by PCBs
from a heat exchanger was shown to be the cause of reduced
hatchability of hen eggs at a large poultry hatchery in the USA
(Kolbye, 1972), and this has been confirmed in laboratory experiments.
Keplinger et al. (1971) found poor hatchability of eggs from hens
receiving diets containing Aroclor 1242 at 10 mg/kg or Aroclor 1254 at
100 mg/kg, but not with hens receiving Aroclor 1260 at 100 mg/kg. An
adverse effect on eggs has been noted with Aroclor 1248 at 10 mg/kg
(Panel on Hazardous Trace Substances, 1972). Peakall et al. (1972) fed
ring doves on a diet containing Aroclor 1254 at 10 mg/kg for 3 months
and found a marked reduction in egg hatchability 6 months later, due
to embryo mortality.
The viability of salmon eggs bears some relation to their PCB
content; Johansson et al. (1970) found a 12% mortality in eggs
containing PCBs at the rate of 9.2 mg/kg in extractable fat, and a
100% mortality with 34 mg/kg. Adverse effects on the reproduction of
aquatic invertebrates have occurred at water concentrations of PCBs in
the region of 5 µg/litre.
The potential teratogenic effect of the PCBs has been studied by
dosing pregnant females during the gestation period. No fetal
abnormalities were produced in the rat by daily doses of Aroclors
1242, 1254, or 1260 at 10 and 30 mg/kg (Keplinger et al., 1971), or
Aroclor 1254 at 100 mg/kg (Villeneuve et al., 1971b), or in the rabbit
dosed with Aroclor 1254 at 10 and 50 mg/kg (Villeneuve et al., 1971b).
Injection of PCBs into eggs has been reported to produce beak
abnormalities in chicks (McLaughlin et al., 1963). Cecil et al. (1974)
have claimed that the administration of Aroclors 1232 and 1254 to hens
at 20 mg/kg in the diet causes teratogenic effects and a reduction in
hatchability of fertile eggs.
7.8 Neoplasia and Adenofibrosis
The liver is the only organ where tumours have been reported
following the ingestion of PCBs. Ito et al. (1973) fed groups of 12
male dd mice with diets containing 500, 250, 100 and 0 mg/kg of
Kanechlor 500, 400, and 300, respectively. After 1 year, 7/12 mice
developed neoplastic nodules (hyperplastic nodules) and 5/12 developed
hepatocellular carcinomas, all in mice from the group fed with
500 mg/kg Kanechlor 500. Metastases were not observed. In a second
study on mice, combined exposure to Kanechlor 500 and either alpha- or
ß-hexachlorocyclohexane isomers enhanced the development of neoplastic
nodules (hyperplastic nodules) and hepato-cellular carcinomas. A
combination of Kanechlor 500 and gamma-hexachlorocyclohexane did not
produce tumours. Dosing with Kanechlor 500 alone at a dietary level of
250 mg/kg, and ß- or gamma-hexachlorocyclohexane at dietary levels of
250, 100 or 50 mg/kg did not produce tumours. However, alpha-hexa-
chlorocyclohexane produced 8/30 hepata-cellular carcinomas and 23/30
hyperplastic nodules.
Groups of 50 BALB/cj male inbred mice were fed dietary
concentrations of Aroclor 1254 of 0 or 300 mg/kg (49.8 mg/kg body
weight) for 6 or 11 months respectively. No liver tumours were noted
in a total of 58 surviving controls. A total of 10 hepatomas
(neoplastic or hyperplastic nodules) were noted in 9/22 surviving mice
fed Aroclor 1254 for 11 months and in 1/24 surviving mice fed Aroclor
1254 for 6 months. In addition adenofibrosis (cholangiofibrosis) was
observed in all 22 livers of mice fed Aroclor 1254 for 11 months, but
not in the other groups (Kimbrough & Linder 1974).
In another study (Kimbrough et al., 1975), 200 female Sherman
strain COBS random bred rats (descendants of the Osborne Mendel
strain) were given a diet containing Aroclor 1260 at 100 mg/kg
(11.6-4.3 mg/kg body weight) for approximately 21 months; 200 female
rats were kept as controls. The rats were sacrificed when 23 months
old. Hepatocellular carcinomas were found in 26/184 of the
experimental groups and in 1/173 of the control rats. None of the
controls, but 144/184 experimental rats had neoplastic nodules
(hyperplastic nodules). Areas of hepatocellular alteration were noted
in 28/173 controls and 182/184 experimental rats. No effect of the
Aroclor on the incidence of tumours in other organs and no metastases
from the liver tumours were observed. In this and two earlier studies,
adenofibrosis of the liver was observed in male and female rats fed
either Aroclor 1260 or Aroclor 1254 (Kimbrough et al., 1972; 1973);
1975). Adenofibrosis of the liver is a persistent progressive lesion
that consists of a marked proliferation of fibrous tissue and
epithelial glandular cells which are well differentiated in the mouse
but appear atypical in the rat.
In a preliminary study, liver tumours (multiple adenomatous
nodules) were induced by Kanechlor 400 in 6/10 female, but not in male
Donryu rats. The dietary exposure varied throughout the study and the
number of animals used was small (10 experimental and 5 control rats
for each sex) (Kimura & Baba, 1973). Makiura et al. (1974) reported
that PCBs (Kanechlor 500) inhibited the induction of liver tumours in
male Sprague-Dawley rats by the known carcinogens 3' methyl- p-
dimethylaminoazo-benzene, N-2-fluosenyl acetamide, and diethyl-
nitrosamine.
8. EFFECTS OF PCBs AND PCTs ON MAN -- EPIDEMIOLOGICAL AND CLINICAL
STUDIES
In June 1968, patients appeared at the Dermatology Clinic of
Kyushu University Hospital, Fukuoka, Japan suffering from chloracne. A
group at the University undertook intensive clinical, chemical, and
epidemiological investigations and found that the disease originated
from the consumption of a batch of rice oil supplied in February 1968;
the disease was called Yusho (rice oil disease) (Katsuki, 1969). This
batch of rice oil was found to be contaminated with Kanechlor 400, a
48% chlorinated biphenyl, at 2000-3000 mg/kg which entered the oil
through a leak in a heat exchanger (Tsukamoto et al., 1969). The
symptoms and signs of Yusho were described by Goto & Higuchi (1969)
and by Okumura & Katsuki (1969). The earliest signs were enlargement
and hypersecretion of the Meibomian glands of the eyes, swelling of
the eyelids, and pigmentation of the nails and mucous membranes,
occasionally associated with fatigue, nausea, and vomiting. This was
usually followed by hyperkeratosis and darkening of the skin with
follicular enlargement and acneform eruptions, frequently with a
secondary staphylococcal infection. These skin changes were most often
seen on the neck and upper chest, but in severe cases extended to the
whole body. Biopsy skin samples showed hyperkeratosis, dilation of the
follicles, and an accumulation of melanin in the basal cells of the
epidermis; melanin granules have also been observed in biopsy samples
of the conjunctiva. Oedema of the arms and legs was seen in some
patients. There were no definite signs of liver enlargement or liver
disorders (Okumura & Katsuki 1969), but slight rises in serum
transaminases and in alkaline phosphatase were detected, and a liver
sample from a Yusho patient showed an increase in the smooth
endoplasmic reticulum (Hirayama et al., 1969). The majority of the
patients were found to have respiratory symptoms, and suffered from a
chronic bronchitis-like disturbance that persisted for several years
(Shigematsu et al., 1971, 1974).
Yusho patients did not appear to suffer from central nervous
effects, but some complained of numbness of the arms and legs. Mural &
Kuroiwa (1971) found a decrease in the conduction velocity in
peripheral sensory nerves.
Yoshimura (1971) reported diminished growth in boys but not in
girls, who consumed the oil. Babies born to Yusho mothers were smaller
than normal. Newborn babies showed a dark brown skin pigmentation,
which disappeared after a few months (Yagamuchi et al., 1971; Taki et
al., 1969). Funatsu et al. (1972) found spotted and sporadic
ossification of the skull and facial oedema with exophthalmia in four
babies, but there was no evidence of any teratogenic action.
Determinations of PCB concentrations in the tissue of Yusho
patients were made several months after the ingestion of the oil,
apparently by an X-ray fluorescence method for organic chlorine (Goto
& Higuchi, 1969). Abdominal fat contained 13.1 mg/kg, subcutaneous fat
75.5 mg/kg, and nails 59 mg/kg. The mesenteric adipose tissue in six
Yusho patients, analysed by gas-liquid chromatography 1-3 years after
the occurrence of intoxication, contained PCB levels of 2.5 mg/kg on
average, which was considerably higher than the normal value. (Masuda
et al., 1974a). The mean blood level of PCBs in patients was 0.6 or
0.7 µg/100 ml (0.3 µg/100 ml for the general population) five years
after exposure (Masuda et al., 1974b; Takamatsu et al., 1974). These
authors also noted a specific gas-liquid chromatographic pattern,
peculiar to Yusho patients, which is still observed.
Hirayama et al. (1974) also reported that the serum bilirubin
level of patients was significantly lower than the normal level and
was negatively correlated with the blood level of PCBs and the serum
triglyceride level.
A considerable number of patients had elevated serum triglyceride
levels, up to four times the normal values, although this was not
correlated with the severity of the symptoms; these high values were
maintained for three years in many patients (Uzawa, 1972). There were
no marked abnormalities in serum cholesterol and phospholipid levels
(Okumura & Katsuki, 1969; Uzawa et al., 1969). Nagai et al. (1969)
reported an increase in urinary 17-ketosteroid excretion. Kusuda
(1971) also observed changes in the menstrual cycle in approximately
60% of 81 female Yusho patients as compared with their cycles prior to
exposure. Okumura et al. (1974) examined the relationship between the
blood levels of triglycerides and PCBs in 42 patients and observed a
positive correlation. Uzawa et al. (1972) showed that high values of
serum triglycerides were maintained for 3 years in many patients.
Shigematsu et al. (1971) examined serum immunoglobulin levels in 38
patients, 2 years after onset, and observed a decrease in IgA and IgM
and an increase in IgG. Saito et al. (1972) reported lower IgM levels
in patients showing chloracne.
Urabe (1974) reported that the total number of Yusho patients had
reached 1200 by 13 September 1973 and that 22 of them had died.
Mucocutaneous signs had decreased year by year, but neurological and
respiratory signs and symptoms and various complaints such as general
fatigue, anorexia, abdominal pain, and headache had become more
prominent among the patients. The smallest amount of oil that produced
symptoms when ingested over approximately 120 days, contained
approximately 0.5 g of PCBs, or approximately 0.07 mg/kg body weight
per day (Kuratsune, 1972a). Recently chlorinated dibenzofurans at
5 mg/kg were found in three samples of the toxic rice oil that
contained PCB levels of about 1000 mg/kg (Nagayama et al., 1975).
Symptoms similar to those of Yusho have been observed in workers
in a Japanese condenser factory, including pigmentation of the fingers
and nails, and acneiform eruptions on the jaw, back, and thighs. It
was thought that these effects arose from local contact with PCBs;
when the use of PCBs ceased, the symptoms disappeared (Hasegawa et
al., 1972b).
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO PCBs AND PCTs
9.1 Species Variation
The data in sections 7 and 8 indicate that man appears to be the
species most sensitive to PCBs, the consumption of relatively small
amounts having resulted in a severe disease (Yusho) in 1200 persons in
Japan. The monkey is the only experimental species in which effects
qualitatively and quantitatively approaching those in man have been
observed; Allen (1975) attributed this to metabolic differences
leading to a slower elimination than that observed in other species
tested.
Conclusions concerning the specific effects of PCBs on different
species are confused by uncertainty arising from the presence of toxic
impurities. The rice oil that caused the outbreak of Yusho was
contaminated with PCBs containing relatively high amounts of
tetrachlorodibenzofuran (see section 8), but the sample used in the
monkey experiments had a low content of these impurities, so it is not
clear whether PCBs alone were responsible for the Yusho incident.
Further uncertainty arises from reports from Finland of high PCB
concentrations in blood and body fat of occupationally exposed workers
with no indication of adverse effects, while at similar tissue
concentrations Japanese workers showed skin lesions typical of Yusho
(see section 5.5.3).
A species-specific toxic manifestation that can probably be
attributed to toxic impurities, is the abdominal oedema and
hydropericardium seen in birds affected by some commercial PCB
mixtures.
Mink is another species showing a high sensitivity to PCBs. Deaths
have been produced with diets containing PCB levels of 30 mg/kg; no
information is available on any species-specific metabolic pathway in
the mink that would account for this susceptibility.
9.2 Dose-Effect Relationships
The following is a summary of the data in Sections 7 and 8
concerning the relationship between mammalian toxicity and dose.
Approximate calculations of the daily dose in mg/kg body weight
derived from the dietary concentration are given in parentheses.a
a When no food consumption figures were available from the
experimental studies, the following factors were used to transform
mg/kg in the diet to mg/kg body weight: mouse (7), rat (20),
guinea-pig (25), mink (10), rabbit (33), monkey (25).
9.2.1 Body weight
Body weight was reduced in rats after 8 months of dietary intake
of Aroclor 1254 at 100 mg/kg (corresponding to 5 mg/kg body weight);
no effects were observed at 20 mg/kg in the diet (corresponding to
1 mg/kg body weight).
Dose-dependent retardation of weight gain was observed in mink
after 4 months of dietary intake of Aroclor 1254 at 5 and 10 mg/kg
(corresponding to 0.5 and 1.1 mg/kg body weight respectively).
9.2.2 Effects on liver
Liver weight
Dose-dependent increase in liver weight was observed in rats
receiving Aroclors 1242, 1254 and 1260 at concentrations of more than
20 mg/kg in the diet (corresponding to > 1.4 mg/kg body weight). Male
rats were more sensitive than female rats; no effects were observed
with Aroclors 1254 and 1260 at concentrations lower than 20 mg/kg in
the diet (corresponding to < 1.4 mg/kg body weight). Effects were
less marked with the lower chlorinated PCBs.
Liver changes
Smooth endoplasmic reticulum proliferation with fat droplet
inclusions were observed in the liver tissue of rats after 8 months of
dietary intake of Aroclor 1254 at 20 mg/kg (corresponding to 1.4 mg/kg
body weight).
Liver damage was observed with Aroclors 1242 and 1254 in rabbits
receiving 14 weekly oral doses of 150 mg/kg body weight; no effect was
observed with Aroclor 1221.
Liver enzyme activitya
Increase in microsomal enzyme activity was observed in male rats
after 8 months of dietary intake of Aroclor 1254 of 20 mg/kg
(corresponding to 1 mg/kg body weight). No effect was observed at
2 mg/kg in the diet (corresponding to 0.1 mg/kg body weight. Effects
were less marked in female rats.
Increased activity was also observed with Aroclors 1242 and 1016
in male rats receiving 21 daily oral doses of 1 mg/kg body weight.
a According to Litterst, et al. (1972) the dose producing an effect
on nitroreductase activity in the rat corresponds to 0.5 mg/kg in
the diet (corresponding to 0.3 mg/kg body weight).
Liver porphyria
Effects were observed in rats after several months of dietary
intake of Aroclor 1254 at 100 mg/kg (corresponding to 5 mg/kg body
weight); dose-dependent effects were observed in female rats after 21
daily oral doses of Aroclor 1242 at 10 and 100 mg/kg; no effects were
noted at 1 mg/kg body weight.
Liver vitamin A
Reduction of hepatic vitamin A was observed in rats receiving
Aroclor 1242 at the rate of 100 mg/kg in the diet (corresponding to
5 mg/kg body weight).
Liver tumours
Hepatocellular carcinomas were observed in mice after one year of
dietary intake of Kanechlor 500 at 500 mg/kg (corresponding to
75 mg/kg body weight); no carcinomas were observed with Kanechlor 500
at 250 mg/kg in the diet (corresponding to 37.5 mg/kg body weight), or
with Kanechlor 300 and 400 at 500 mg/kg in the diet (corresponding to
75 mg/kg body weight).
Hepatomas were observed in mice after 10 months of daily intake of
Aroclor 1254 at 300 mg/kg in the diet (corresponding to 49.8 mg/kg
body weight).
Hepatocellular carcinomas were observed in rats after 21 months of
daily intake of Aroclor 1260 at 100 mg/kg in the diet (corresponding
to 11.6-4.3 mg/kg body weight).
9.2.3 Reproduction
Effects on reproduction were observed in the mouse at a daily oral
dose of 0.025 mg Clophen A60; in the rat at a dietary level of Aroclor
1254 of 20 mg/kg (corresponding to 1 mg/kg body weight) with the
effects decreasing with higher chlorinated PCBs; in the mink at a
dietary level of Aroclor 1254 of 5 mg/kg (corresponding to 0.5 mg/kg
body weight); and in the monkey at a dietary level of Aroclor 1248 of
2.5 mg/kg (corresponding to 0.1 mg/kg body weight).
9.2.4 Immunosuppression
Immunosuppressive effects were observed in the guinea-pig at a
dietary level of Clophen A60 or Aroclor 1260 of 50 mg/kg
(corresponding to 2 mg/kg body weight).
9.2.5 Skin effects
In man, symptoms of Yusho disease were observed at a dietary level
of 4.2 mg/day of PCBs (corresponding to 0.07 mg/kg body weight/day for
a 60-kg person). A value of 0.50 g was estimated as the quantity of
PCBs consumed over approximately 120 days above which toxic symptoms
were evident. Similar effects were observed in the monkey at a dietary
level of Aroclor 1248 of 2.5 mg/kg (corresponding to 0.1 mg/kg body
weight) after several months.
9.3 Nondetected effect levels
The assessment of non detected effect levels for toxic effects is
complicated by the different activities of the component PCBs and by
the presence of impurities, in addition to the influence of inter- and
intraspecies variation, age, sex, and length of exposure. Moreover,
many of the available experimental studies do not include a
nondetected effect level.
The most sensitive species appears to be man, and effects have
been observed at intake rates of 0.07 mg/kg body weight/day. This may
have been influenced by the intake of impurities more toxic than PCBs,
but similar effects have been produced in the monkey, at the same
order of dosage, with a product containing little of these impurities.
At this dosage level, no effects may be expected on growth, liver
enlargement, and liver enzyme activity in less sensitive species such
as the rat. Although non-detected effect levels are not available for
effects on immunosuppression and reproduction, and for certain
biochemical effects on the liver, it seems unlikely that these effects
would be apparent at intake rates of 0.1 mg/kg body weight/day.
Carcinogenic effects have been observed in rats and mice at doses two
orders of magnitude greater than this, but there is no epidemiological
evidence to suggest that PCBs cause tumours in man. According to Grant
et al. (1974) rats fed on a diet containing Aroclor at the rate of
2 mg/kg (equivalent to about 0.1 mg/kg body weight) showed PCB levels
of 8 µg/100 ml in blood and 26.1 mg/kg in body fat. However, values
much higher than these have been observed in men occupationally
exposed to PCBs without evidence of any toxic effects (see section
5.5.3).
It is not possible at present to resolve this conflict in the
evidence on the toxicity of PCBs to man.
REFERENCES
ABBOT, D.C., COLLINS, G. B. & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom 1969-71.
Br. med. J., 2: 553-556.
ACKER, L. & SCHULTE, E. (1970) Uber das Vorkommen von chlorierten
Biphenylen und Hexachlorbenzol neben chlorierten Insektiziden in
Humanmilch und menschlichem Fettgewebe. Naturwissenschaften,
57: 497 (in German).
ACKER, L. & SCHULTE, E. (1974) Chlorkohlenwasserstoffe in menschlichem
Fettgewebe, Naturwissenschaften, 61: 1-4 (in German).
ADDISON, R. F., FLETCHER, G. L., RAY, S. & DOANE, J. (1972) Analysis
of a chlorinated terphenyl (Aroclor 5460) and its deposition in
tissues of the cod (Gadus morhua). Bull. environ. Contam.
Toxicol., 8: 52-60.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of polychlorinated biphenyl (PCB) and chlorinated
pesticides in water. Anal. Chem., 42: 1483-1486.
AHMED, M. & FOCHT, D. D. (1973) Degradation of polychlorinated
biphenyls by two species of Achromobacter. Can. J. Microbiol.,
19: 47-52.
AHNOFF, M. & JOSEFSSON, B. (1973) Confirmation studies on
polychlorinated biphenyls (PCB) from river waters using mass
fragmentography. Anal. Lett., 6: 1083-1093.
AHNOFF, M. & JOSEFSSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AHNOFF, M. & JOSEFSSON, B. (1975) Clean-up procedures for PCB analysis
on river water extracts. Bull. environ. Contam. Toxicol.,
13: 159-166.
ALBRO, P. W. & FISHBEIN, L. (1972) Intestinal absorption of
polychlorinated biphenyls in rats. Bull. environ. Contam.
Toxicol., 8: 26-31.
ALLEN, J. R. (1975) Response of the non-human primate to
polychlorinated biphenyl exposure. Fed. Proc., 34: 1675-1679.
ALLEN, J. R., ABRAHAMSON, L. J. & NORBACK, D. H. (1973) Biological
effects of polychlorinated biphenyls and triphenyls on the
subhuman primate. Environ. Res., 6: 344-354.
ALLEN, J. R. & NORBACK, D. A. (1973) Polychlorinated biphenyl- and
triphenyl-induced mucosal hyperplasia in primates. Science,
179: 498-499.
ALLEN, J. R., CARSTENS, L. A. & BARSOTTI, D. A. (1974) Residual
effects of short-term, low-level exposure of non-human primates to
polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
30: 440-451.
ALVARES, A. P. & KAPPAS, A. (1975) Induction of aryl hydrocarbon
hydroxylase by chlorinated biphenyls in the foeto-placental unit
and in neonatal livers during lactation. FEBS Lett., 50:172-174.
ARMOUR, J. A. & BURKE, J. A. (1970) Method for separating
polychlorinated biphenyls from DDT and its analogs. J. Assoc.
Off. Anal. Chem., 53: 761-768.
AULERICH, R. J., RINGER, R. K., SEAGRAN, H. L. & YOUATT, W. G. (1971)
Effects of feeding coho salmon and other Great Lakes fish on mink
reproduction. Can. J. Zool., 49: 611-616.
AULERICH, R. J., RINGER, R. K. & IWAMOTO, S. (1973) Reproductive
failure and mortality in mink fed on Great Lake fish. J. Reprod.
Fertil., 19 (Suppl.): 365-376.
BAGLEY, G. E., REICHEL, W. L. & CROMARTIE, E. (1970) Identification of
polychlorinated biphenyls in two bald eagles by combined
gas-liquid chromatography-mass spectroscopy. J. Assoc. Off. Anal.
Chem., 53: 251-261.
BAILEY, S. & BUNYAN, P. J. (1972) Interpretation of persistence and
effects of polychlorinated biphenyls in birds. Nature (Lond.),
236: 34-36.
BASTOMSKY, C. H., SOLYMOSS, a., ZSIGMOND, G. & WYSE, J. M. (1975) On
the mechanism of polychlorinated biphenyl-induced
hypobilirubinaemia. Clin. chim. Acta, 61: 171-174.
BAUER, H., SCHULZ, K. H. & SPIEGELBERG, U. (1961) Berufliche
Vergiftungen bei der Herstellung von Chlorphenol-Verbindungen.
Arch. Gewerbepathol. Gewerbehyg., 18: 538-555.
BENTHE, H. F., KNOP, J. & SCHMOLDT, A. (1972a) Absorption and
distribution of polychlorinated biphenyls (PCB) after inhalatory
application. Arch. Toxikol., 29: 85-95 (in German).
BENTHE, H. F., SCHMOLDT, A. & SCHMIDT, H. (1972b) Induction of
microsomal liver enzymes after polychlorinated biphenyls (PCB) and
following stress. Arch. Toxikol., 29: 97-106 (in German).
BERG, O. W., DIOSADY, P. L. & REES, G. A. V. (1972) Column
chromatographic separation of polychlorinated biphenyls from
chlorinated hydrocarbon pesticides and their subsequent gas
chromatographic quantitation in terms of derivatives. Bull.
environ. Contam. Toxicol., 7: 338-347.
BERGLUND, F. (1972) Levels of polychlorinated biphenyls in foods in
Sweden. Environ. Perspect, 1: 67-69.
BERLIN, M., GAGE, J. C. & HOLM, S. (1973) The metabolism and
distribution of 2,4,5,2',5'-pentachlorobiphenyl in the mouse.
In: PCB Conference II. National Swedish Environment Protection
Board Publications: 4E, pp. 101-107.
BERLIN, M., GAGE, J. C. & HOLM, S. (1974) Distribution and metabolism
of polychlorobiphenyls. In: Proceedings of the International
Symposium on Recent Advances in Environmental Pollution, Paris,
24-28 June. pp. 8.
BERLIN, M., GAGE, J. & HOLM, S. (1975) The distribution and metabolism
of 2,4,5,2',5'-pentachlorobiphenyl. Arch. environ. Health,
30: 141-147.
BICKERS, D. R., HARBER, L. C., KAPPAS, A. & ALVARES, A. P. (1972)
Polychlorinated biphenyls: comparative effects of high and low
chlorine containing Aroclors on hepatic mixed function oxidase.
Res, Comm. Chem. Pathol. Pharmacol., 3: 505-511.
BIDLEMAN, T. F. & OLNEY, C. E. (1974) High-volume collection of
atmospheric polychlorinated biphenyls. Bull. environ. Contam.
Toxicol., 11: 442-450.
BITMAN, J., CECIL, H. C. & HARRIS, S. J. (1972) Biological effects of
polychlorinated biphenyl in rats and quail. Environ. Health
Perspec., 1: 145-149.
BJERK, J. E. (1972) Rester av DDT og polyklorerte bifenyler i Norsk
humant materiale. Tidsskr. Nor-Laegeforen., 92: 15-19 (cited by
Kimbrough, 1974).
BOURNE, W. P. P. & BOGEN, A. A. (1972) Polychlorinated biphenyls in
North Atlantic seabirds, Mar. Pollut. Bull., 3 (11): 172-175.
BOWES, G. W., MULVIHILL, M. J., SIMONEIT, B. R. T., BURLINGAME, A. L.
& RISEBROUGH, R. W. (1975) Identification of chlorinated
dibenzofurans in American polychlorinated biphenyls. Nature
(Lond.), 256, 305-307.
BROADHURST, M. G. (1972) Use and replaceability of polychlorinated
biphenyls. Environ. Health Perspect., 2: 81-102.
BRUCKNER, J. V., KHANNA, K. L. & CORNISH, H. H. (1973) Biological
responses of the rat to polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 24: 434-448.
BRUGGEMAN, VON J., BUSCH, L., DRESCHER-KADAN, U., EISELI, W. & HOPPE,
P. (1974) Pesticid-und PCB-Ruckstunde in Organen von Wildtieren
als Indikatoren fur Umweltkontamination. Z. Jagdwiss.,
20: 70-74.
BURSE, V. W., KIMBROUGH, R. D., VILLANUEVAS, E. C, JENNINGSI R. W.,
LINDER, R. E. & SOVOCOOL, G. W. (1974) Polychlorinated biphenyls.
Storage, distribution, excretion and recovery: liver morphology
after prolonged dietary ingestion. Arch. environ. Health,
29: 301-307.
CARNES, R. A., DOERGER, J. U. & SPARKS, H. L. (1973) Polychlorinated
biphenyls in solid waste and solid-waste-related materials. Arch.
environ. Contam. Toxicol., 1: 27-35.
CECIL, H. C., HARRIS, S. J., BITMAN, J. & FRIES, G. F. (1973)
Polychlorinated biphenyl-induced decrease in liver vitamin A in
Japanese quail and rats. Bull. environ. Contam. Toxicol.,
9: 179-185.
CECIL, H. C., BITMAN, J., LILLIE, R. J., FRIES, G. F. & VERRETT, J.
(1974) Embryotoxic and teratogenic effects in unhatched fertile
eggs from hens fed polychlorinated biphenyls (PCBs). Bull.
environ. Contam. Toxicol., 11: 489-495.
CECIL, H. C., HARRIS, S. J. & BITMAN, J. (1975) Effects of
polychlorinated biphenyls and terphenyls and polybrominated
biphenyls on pentobarbital sleeping times of Japanese quail.
Arch. environ. Contam. Toxicol., 3, 183-192.
CHOI, P.S. K., NACK, H. & FLINN, J. E. (1974) Distribution of
polychlorinated biphenyls in an aerated biological oxidation
wastewater treatment system. Bull. environ. Contam. Toxicol.,
11: 12-17.
CLAUS, B. & ACKER, L. (1975) Zur Kontamination von Milch und
Milcherzeugnissen mit chlorierten Kohlenwasserstoffen im
Westphälischen Raum II Ergebnisse und Diskussion. Zeitschrift fur
Lebensmitteluntersuchung und forschung, 159 (3): 129-137.
COLLINS, G. B., HOLMES, D. C. & JACKSON, F. J. (1972) The estimation
of polychlorobiphenyls. J. Chromatogr., 71: 443-449.
COMMISSION OF THE EUROPEAN COMMUNITIES (1974) European Colloquium.
Problems raised by the contamination of man and his environment
by persistent pesticides and organohalogenated compounds.
Luxembourg, 14-16 May 1964.
COOLEY, N. R., KELTNER, J. M. & FORESTER, J. (1972) Mirex and Aroclor
1254: effect on accumulation by Tetrahymena pyriformis strain
W. J. Protozool., 19: 636-638.
CURLEY, A., BURSE, V. W., GRIM, M. E., JENNINGS, R. W. & LINDER, R. E.
(1971) Polychlorinated biphenyls: distribution and storage in body
fluids and tissues of Sherman rats. Environ. Res., 4: 481-495.
CURLEY, A., BURSE, V. W. & GRIM, M. E. (1973a) Polychlorinated
biphenyls: evidence of transplacental passage in the Sherman rat.
Fd. cosmet. Toxicol., 11: 471-476.
CURLEY, A., BURSE, V. W., JENNINGS, R. W., VILLANUEVA, E. C., TOMATIS,
L. & AKAZAKI, K. (1973b) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond), 242: 338-340.
CURLEY, A., BURSE, W. V., JENNINGS, R. W., VILLANUEVA, E. C. &
KIMBROUGH, R. D. (1975) Evidence of tetrachlorodibenzofuran (TCDF)
in Aroclor 1254 and the urine of rats following dietary exposure
to Aroclor 1254. Bull. environ. Contam. Toxicol., 14: 153-158.
DAHLGREN, R. B., GREICHUS, Y. A. & LINDER, R. L. (1971) Storage and
excretion of polychlorinated biphenyls in the pheasant.
J. Wildlife Manag., 35: 823-828.
DAHLGREN, R. B., LINDER, R. L. & CARLSON, C. W. (1972) Polychlorinated
biphenyls: their effects on penned pheasants. Environ. Health
Perspect., 1: 89-101.
DE FREITAS, A. S. & NORSTROM, R. J. (1974) Turnover and metabolism of
polychlorinated biphenyls in relation to their chemical structure
and the movement of lipids in the pigeon. Can. J. Physiol.
Pharmacol., 52: 1080-1094.
DEPARTMENT OF HEALTH EDUCATION AND WELFARE (1972) Environ. Health
Perspect. Experimental Issue 1, April 1972. DHEW, Bethesda, MD,
USA. Publ. No. (NIH) 72-218.
DE VOS, R. H. & PEET, E. W. (1971) Thin-layer chromatography of
polychlorinated biphenyls. Bull environ. Contam. Toxicol.,
6: 164-170.
DOGUCHI, M. & FUKANO, S. (1975) Residue levels of polychlorinated
terphenyls, polychlorinated biphenyls and DDT in human blood.
Bull. environ. Contam. Toxicol., 13: 57-63.
DOGUCHI, M., FUKANO, S. & USHIO, F. (1974) Polychlorinated terphenyls
in the human fat. Bull. environ. Contam. Toxicol.,
11 (2): 157-158.
DUKE, T. W., LOWE, J. I. & WILSON, A. J. JR (1970) A polychlorinated
biphenyl (Aroclor 1254) in the water, sediment and biota of
Escambia Bay, Florida. Bull. environ. Contam. Toxicol.,
5: 171-180.
ECOBICHON, D. J. & COMEAU, A.M. (1974) Comparative effects of
commercial Aroclors on rat liver enzyme activities. Chem.-Biol.
Interactions, 9: 341-350.
EKSTEDT, J. & ODEN, S. (1974) Chlorinated hydrocarbons in the lower
atmosphere in Sweden. Report from Department of Soil Science,
Royal Agricultural College, S-75007, Uppsala 7. pp. 1-16.
ENVIRONMENTAL SANITATION BUREAU, MINISTRY OF HEALTH & WELFARE (1973)
Survey on foods contamination. Report of comprehensive
investigation on the prevention of pollution by PCBs, 191-210
(in Japanese).
FINKLEA, J., PRIESTER, L. E., CREASON, J.P., HAUSER, T., HINNERS, T. &
HAMMER, D. I. (1972) Polychlorinated biphenyl residues in human
plasma expose a major urban pollution problem. Am. J. pub.
Health, 62: 645-651.
FISHER, N. S., GRAHAM, L. B., CARPENTER, E. J. & WURSTER, C. F. (1972)
Geographic differences in phytoplankton sensitivity to PCBs.
Nature (Lond.), 241: 548-549.
FLICK, D. F., O'DELL, R. G. & CHILDS, V. A. (1965) Studies of the
chick edema disease. Similarity of symptoms produced by feeding
chlorinated biphenyl. Poult. Sci., 44: 1460-1465.
FREUDENTHAL, J. & GREVE, P. A. (1973) Polychlorinated terphenyls in
the environment. Bull. environ. Contam. Toxicol., 10: 108-111.
FRIEND, M. & TRAINER, D. O. (1970) Polychlorinated biphenyl:
interaction with duck hepatitis virus. Science, 170: 1314-1316.
FRIES, G. F., MARROW, G. S. JR & GORDON, C. H. (1973) Long-term
studies of residue retention and excretion by cows fed a
polychlorinated biphenyl (Aroclor 1254). J. agr. Food Chem.,
21: 117-121.
FUJITA, S., TZUJI, H., KATO, K., SAEICI, S. & TSUKAMOTO, H. (1971)
Effect of biphenyl chlorides on rat liver microsomes. Fukuoka
med. Acta., 62: 30-34.
FUKADA, K., INUYAMA, Y., TAKESHITA, T. & YAMAMOTO, S. (1973) Present
state of environmental pollution by PCB in Shimane Prefecture.
Shimare Igaku, 5: 1-25 (in Japanese).
FUKADA, S., USHIO, F. & DOGUCHI, M. (1974) PCB, PCT and pesticides
residues in fish collected from the Tama River. Ann. Rep. Tokyo
Metr. Res. Lab. P.H., 25: 297-305 (in Japanese).
FUNATSU, I., YAMASHITA, F., ITO, Y., TZUGAWA, S., FUNATSU, T.,
YOSHIKANE, T., HAYASHI, M., KATO, T., YAKUSHIJI, M., OKAMOTO, G.,
YAMASAKI, S., ARIMA, T., KUNO, T., IDE, H. & IBE, I. (1972) PCB
induced fetopathy. I. Clinical observation. Kurume med. J.,
19: 43-51 (in English).
GARDNER, A.M., CHEN, J. F., ROACH, J. A. G. & RAEGLIS, E. P. (1973)
Polychlorinated biphenyls. Hydroxylated urinary metabolites of
2,5,2',5'-tetrachlorobiphenyl identified in rabbits. Biochem.
biophys. res. Commun., 55: 1377-1384.
GILBERTSON, M. & REYNOLDS, L. (1974) DDE and PCB in Canadian wild
birds. In: Occasional paper, Canadian Wildlife Service,
Environment, Canada, Ottawa, pp. 1-16.
GOLDSTEIN, J. A., HICKMAN, P. & JUE, D. L. (1974) Experimental hepatic
porphyria induced by polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 27: 437-448.
GOLDSTEIN, J. A., MCKINNEY, J. D., LUCIEN, G. W., MOORE, J. A.,
HICKMAN, P. & BERGMAN, H. (1975a) Effects of hexachlorobiphenyl
isomers and 2,3,7,8-tetrachlorodibenzofuran (TCDF) on hepatic drug
metabolism and porphyria accumulation. Pharmacologist,
16: 239 (Abstract No. 278).
GOLDSTEIN, J. A., HICKMAN, P., BURSE, V. W. & BERGMAN, H. (1975b) A
comparative study of two polychlorinated biphenyl mixture (Aroclor
1242 and 1016) containing 42% chlorine on induction of hepatic
porphyria and drug metabolizing enzymes. Toxicol. appl.
Pharmacol., 32: 461-473.
GOTO, M. & HIGUCHI, K. (1969) The symptomatology of Yusho
(chlorobiphenyls poisoning) in dermatology. Fuoka Act. Med.,
60: 409-431.
GRANT, D. L., MOODIE, C. A. & PHILLIPS, W. E. J. (1974) Toxicodynamics
of Aroclor 1254 in the male rat. Environ. physiol. Biochem.,
4: 214-225.
GRANT, D. L. & PHILLIPS, W. E. J. (1974) The effect of age and sex on
the toxicity of Aroclor 1254, a polychlorinated biphenyl, in the
rat. Bull. environ. Contam. Toxicol., 12: 145-152.
GRANT, D. L., PHILLIPS, W. E. J. & VILLENEUVE, D.C. (1971a) Metabolism
of a polychlorinated biphenyl (Aroclor 1254) mixture in the rat.
Bull. environ. Contam. Toxicol., 6: 102-112.
GRANT, D. L., VILLENEUVE, D.C., MCCULLY, K. A. & PHILLIPS, W. E. J.
(1971b) Placental transfer of polychlorinated biphenyls in the
rabbit. Environ. Physiol., 1: 61-66.
GREEN, S. G., CARR, J. V., PALMER, K. A. & OSWALD, E. J. (1975) Lack
of cytogenetic effects in bone marrow and spermatagonial cells in
rats treated with polychlorinated biphenyls (Aroclor 1242 and
1254). Bull. environ. Contam. Toxicol., 13: 14-22.
HAMMER, D. I., FINKLEA, J. F., PRIESTER, L. E., KEIL, J. E., SANDIFER,
S. H. & BRIDBORN, K. (1972) Polychlorinated biphenyl residues in
the plasma and hair of refuse workers. Environ. Health
Perspect., 1 (15): 83.
HAMMOND, A. L. (1972) Chemical pollution: polychlorinated biphenyls.
Science, 175: 155-156.
HANSEN, D. J., PARRISH, P. R., LOWE, J. I., WILSON, A. J. JR & WILSON,
P. D. (1971) Chronic toxicity, uptake and retention of Aroclor
1254 in two estuarine fishes. Bull. environ. Contam. Toxicol.,
6: 113-119.
HAQUE, R., SCHMEDDING, D. W. & FREED, V. H. (1974) Aqueous solubility,
absorption and vapour behaviour of polychlorinated biphenyl
Aroclor 1254. Environ. Sci. Technol., 8: 139-142.
HARA, I., HARADA, H., KIMURA, S., ENDO, T. & KAWANO, K. (1974) Follow
up health examination in an electric condenser factory after
cessation of PCBs usage (1st report) Jpn. J. Ind. Health,
16: 365-366 (in Japanese).
HARVEY, G. R. & STEINHAUER, W. G. (1974) Atmospheric transport of
polychlorobiphenyls to the North Atlantic. Atmos. Environ.,
8: 777-782.
HARVEY, G. R., STEINHAUER, W. G. & TEAL, J. M. (1973) Polychloro-
biphenyls in North Atlantic Ocean water. Science, 80: 643-644.
HASEGAWA, H., SATO, M. & TSURUTA, H. (1972a) PCBs concentration in the
blood of workers handling PCB. Occup. Health, 10: 50-55
(in Japanese).
HASEGAWA, H., SATO, M. & TSURUTA, H. (1972b) PCB concentration in air
of PCB-using plants and health examination of workers (in
Japanese). In: Report on Special Research on Prevention of
Environmental Pollution by PCB-like Substances. Tokyo, Research
Co-ordination Bureau, Science and Technology Agency, pp. 141-149.
HEATH, R. G., SPANS, J. W., KREITZER, J. F. & VANCE, C. (1970)
Effects of polychlorinated biphenyls on birds In: Proceedings
of the 15th Congress of International. Ornithology, The Hague,
pp. 475-485.
HESSELBERG, R. J. & SCHERR, D. D. (1974) PCBs and p,p' DDE in the
blood of cachectic patients. Bull. environ. Contam. Toxicol.,
11: 202-205.
HIRAYAMA, C., IRISA, T. & YAMAMOTO, T. (1969) Fine structural changes
of the liver in a patient with chlorobiphenyls intoxication.
Fukuoka Acta Med., 60: 455-461 (in Japanese).
HIRAYAMA, C., OKUMURA, M., NAGAI, J. & MASUDA, Y. (1974)
Hypobilirubinemia in patients with polychlorinated biphenyls
poisoning. Clin. chim. Acta., 55: 97-100.
HOLDGATE, M. W. (1971) The sea bird wreck in the Irish Sea, Autumn
1969. Natural Environmental Research Council (Publication
Series C4).
HOLDEN, A. V. (1970a) International co-operative study of
organochlorine pesticide residues in terrestial and aquatic
wildlife 1967/1968. Pest. monit. J., 4: 117-135.
HOLDEN, A. V. (1970b) Source of polychlorinated biphenyl contamination
in the marine environment. Nature (Lond.), 228: 1220-1221.
HOLDEN, A. V. (1973a) International co-operative study of
organochlorine and mercury residues in wildlife, 1969-71. Pest.
monit. J., 7: 37-52.
HOLDEN, A. V. (1973b) Monitoring PCBs in water and wildlife. In:
PCB Conference II, Stockholm, Swedish Environment Protection
Board, Publication: 4E, pp. 23-33.
HOLDEN, A. V. & MARSDEN, K. (1969) Single-stage clean-up of animal
tissue extracts for organochlorine residue analysis.
J. Chromatogr., 44: 481-492.
HUTZINGER, O., JAMIESON, W. D., SAFE, S., PAULMANN, L. & AM , R.
(1974) Identification of metabolic dechlorination of highly
chlorinated bipheny bit. Nature (Lond.), 252: 698-699.
HUTZINGER, O., SASH, D. M., SAFE, S., DE FREITAS, A. S. W., NORSIROM,
R. J., WILDISH, D. J. & ZITKO, V. (1972a) Polychlorinated
biphenyls: metabolic behaviour of pure isomers in pigeons, rats
and brook trout. Science, 178: 312-313.
HUTZINGER, O., SAFE, S. & ZITKO, V. (1971) Polychlorinated biphenyls:
Synthesis of some individual chlorobiphenyls. Bull. environ.
Contam. Toxicol., 6: 209-219.
HUTZINGER, O., SAFE, S. & ZITKO, V. (1972b) Photochemical degradation
of chlorobiphenyls (PCBs). Environ. Health Perspect., 1: 15-20.
INNAMI, S. et al. (1974) PCB toxicity and nutrition II, PCB toxicity
and Vitamin A (2). J. Nutr. Sci-Vitaminol., 20: 363-370.
INTERNATIONAL AGENCY FOR RESEARCH ON CANCER (1974) Polychlorinated
biphenyls in IARC monographs on the evaluation of carcinogenic
risk of chemicals to man, Vol. 7, 261-289.
INTERNATIONAL COUNCIL FOR THE EXPLORATION OF THE SEA (1974) Report of
a Working Group for the international study of the pollution of
the North Sea and its effects on living resources and their
exploitation. Charlottenlund, Denmark (Co-operative Research
Report No. 39).
ISHI, H. (1972) PCB Pollution in Japan. Environ. Health Rep. No. 14,
Jpn. Publ. Health Assoc., pp. 13-28.
ITO, N., NAGASAKI, H, ARAI, M., MAKIURA, S., SUGIHARA, S. & HIRAO, K.
(1973) Histopathologic studies on liver tumorigenesis induced in
mice by technical polychlorinated biphenyls and its promoting
effect on liver tumours induced by benzene hexachloride. J. nat.
Cancer Inst., 51: 1637-1646.
IVERSON, F., VILLENEUVE, D.C., GRANT, D. L. & HATINA, G. V. (1975)
Effect of Aroclor 1016 and 1242 on selected enzyme systems in the
rat. Bull. environ. Contam. Toxicol., 13: 456-463.
IWATA, Y., WESTLAKE, W. E. & GUNTHER, F. A. (1973) Varying persistence
of polychlorinated biphenyls in six California soils under
laboratory conditions. Bull. environ. Contam. Toxicol.,
9: 204-211.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTROM, G. & VAZ,
R. (1975) Identification by GC-MS of phenolic metabolites of PCB
and p,p'-DDE isolated from Baltic guillemot and seal. Ambio.,
4: 93-97.
JENSEN, S. (1973) (no title) Report to Swedish National Environment
Protection Board, Stockholm, 12 October 1973.
JENSEN, S. (1974) Identification of some organic substances
potentially harmful to the environment. National Swedish
Environment Protection Board, University of Stockholm, Kollenberg
Laboratory (Publication SNV-PM 520).
JENSEN, S. & SUNDSTROM, G. (1974) Structures and levels of most
chlorobiphenyls in two technical PCB products and in human adipose
tissue. Ambio, 3: 70-76.
JENSEN, S. & SUNDSTROM, G. (1975) Metabolic hydroxylation of a
chlorobiphenyl containing only isolated unsubstituted positions --
2,2',4,4',5,5'-hexachlorobiphenyl. Nature (Lond.)
(in press).
JENSEN, S., JOHNELS, A. G., OLSSEN, M., OTTERLIND, G. (1969) DDT and
PCB in marine animals from Swedish waters. Nature (Lond.),
224: 247-250.
JENSEN, S., JOHNELS, A. G., OLSSON, M. & OTTERLIND, G. (1972b) DDT and
PCB in herring and cod from the Baltic, the Kattegat and the
Skaggerrak. Ambio spec. Rep., No. 1: 71-85.
JENSEN, S., JOHNELS, A. G., OLSSEN, M. & WESTERMARK, T. (1972c) The
avifaune of Sweden as indicators of environmental contamination
with mercury and chlorinated hydrocarbons. In: Proceedings of
the 15th International Ornithology Congress, Leiden,
pp. 455-465.
JENSEN, S., RENBERG, L. & OLSSON, M. (1972a) PCB contamination from
boat bottom paint and levels of PCB in plankton outside a polluted
area. Nature (London), 240: 358-360.
JENSEN, S., RENBERG, L. & VAZ, R. (1973) Problems in quantification
of PCB in biological material. In: PCB Conference II. National
Swedish Environment Protection Board. Publications: 4E, pp. 7-13.
JOHANSSON, N., JENSEN, S. & OLSSON, M. (1970) PCB indications of
effects on fish. In: PCB Conference, National Swedish
Environment Protection Board, pp. 59-67.
JOHNSTONE, G. J., ECOBICHON, D. J. & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, D.C. L., DAVIS, W. E., JR, NEWELL, G. W., SASMORE, D. P. &
ROSEN, V. J. (1974) Modification of hexachlorophene toxicity by
dieldrin and Aroclor 1254. Toxicology, 2: 309-318.
KAISER, K. L. E. & WONG, P. T. S. (1974) Bacterial degradation of
polychlorinated biphenyls. I. Identification of some metabolic
products from Aroclor 1242. Bull. environ. Contam. Toxicol.,
11: 291-296.
KARPPANEN, E. & KOLHO, L. (1973) The concentration of PCB in human
blood and adipose tissue in three different research groups. In:
PCB Conference II. National Swedish Environment Protection Board
Publications: 4E: pp. 124-127.
KATSUKI, S. (1969) Foreword. Fukuoka Acta Med., 60: 403-407.
KAWANISHI, S., SANO, S., MIZUTANI, T. & MATSUMOTO, M. (1973)
Experimental porphyria induced by polychlorinated biphenyls (in
Japanese). Jpn. J. Hyg., 28: 84.
KAWANISI, S., SANO, S., MIZUTANI, T. & MATSUMOTO, M. (1974)
Experimental studies on toxicity of synthetic tetrachlorobiphenyl
isomers (in Japanese). Jpn. J. Hyg., 29: 81.
KEIL, J. E., PRIESTER, L. E. & SANDIFER, S. H. (1971) Polychlorinated
biphenyl (Aroclor 1242): effects of uptake on growth, nucleic
acids and chlorophyll of a marine diatom. Bull. environ. Contam.
Toxicol., 6: 156-159.
KEPLINGER, M. L., FANeHER, O. E. & CALANDRA, J. C. (1971) Toxicologic
studies with polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 19: 402-403.
KIHLSTROM, J. E., ORBERG, J., LUNDBERG, C., DANIELSSON, P.O. &
SYDHOFF, J. (1973) Effects of PCB on mammalian reproduction. In:
PCB Conference II. National Swedish Environment Protection Board
Publications 4E, pp. 109-111.
KIMBROUGH, R. D. (1974) The toxicity of polychlorinated polycyclic
compounds and related chemicals. Crit. Rev. Toxicol.,
2: 445-498.
KIMBROUGH, R. D. & LINDER, R. E. (1974) Induction of adenofibrosis and
hepatomas of the liver in BALB/cJ mice by polychlorinated
biphenyls (Aroclor 1254). J. nat. Cancer Inst., 53: 547-549.
KIMBROUGH, R. D., LINDER, R. E. & GAINES, T. B. (1972) Morphological
changes in livers of rats fed polychlorinated biphenyls. Arch.
environ. Health, 25: 354-364.
KIMBROUGH, R. D., LINDER, R. E., BURSE, V. W., JENNINGS, R. W. & GA,
C. (1973) Adenofibrosis in the rat liver with persistence of
polychlorinated biphenyls in adipose tissue. Arch. environ.
Health, 27: 389-395.
KIMBROUGH, R. D., SQUIRE, R. A., LINDER, R. E., STRANDBERG, J. D.,
MONTALI, R. J. & BURSE, V. W. (1975) Induction of liver tumours in
Sherman strain female rats by polychlorinated biphenyl Aroclor
1260. J. nat. Cancer Inst., 55: 1453-1459.
KIMURA, N. T. & BABA, T. (1973) Neoplastic changes in the rat liver
induced by polychlorinated biphenyl. GANN, 64: 105-108.
KITAMURA, M., TSUKAMOTO, T., SUMINO, K., HAYAKAWA, K., SHIBITA, T. &
HIRANO, I. (1973) The PCB levels in the blood of workers employed
in a condenser factory. Jpn. J. indust. Health, 47: 354-355
(in Japanese).
KOBAYASHI, Y. (1972) Answer Report to the Questionary paper on
"Regulation of Residual level in Foods". Biol. Pollut.,
4: 93-116 (in Japanese).
KOEMAN, J. H., TEN NOEVER DE BRAUW & DE VOS, R. H. (1969) Chlorinated
biphenyls in fish, mussels and birds from the river Rhine and the
Netherlands coastal area. Nature (Lond), 221: 1126-1128.
KOEMAN, J. H., BOTHOF, TH., DE VRIES, R., VAN VELZEN-BLAD, H. & VOS,
J. G. (1972a) The impact of persistent pollutants on piscivorous
and molluscivorous birds. TNO-nieuws, 27: 561-569.
KOEMAN, J. H., PEETERS, W. H. M., SMIT, C. J., TJIOE, P, S. & DE
GOEIJ, J. J. M. (1972b) Persistent chemicals in marine mammals.
TNO-nieuws, 27: 570-578.
KOEMAN, J. H., VAN VELZEN-BLAD, H. C. W., DE VRIES, R. & Vos, J. G.
(1973) Effects of PCB and DDE in cormorants and evaluation of PCB
residues from an experimental study. J. Reprod. Fertil.,
19 (Suppl.): 353-364.
KOHANAWA, M., SHOYA, S., OGURA, Y., MORIWAKI, M. & KAWASAKI, M.
(1969a) Poisoning due to an oily by-product of rice-bran similar
to chick edema disease. I. Occurrence and toxicity test. Nat.
Inst. anim. Health Quart., 9: 213-219.
KOHANAWA, M., SHOYA, S., YONEMURA, T., NISHIMURA, K. & TSUSHIO, Y.
(1969b) Poisoning due to an oily by-product of rice-bran similar
to chick edema disease. II. Tetrachlorodiphenyl as toxic
substance. Nat. Inst. anim. Health Quart., 9: 220-228.
KOLBYE, A. C. JR (1972) Food exposures to polychlorinated biphenyls.
Environ. Health Perspect., 1: 85-88.
KOLLER, L. D. & ZINKL, J. G. (1973) Pathology of polychlorinated
biphenyls in rabbits. Am. J. Pathol., 70: 363-373.
KOMATSU, F. & KIKUCHI, M. (1972) Skin lesions by 3,4,Y,4'-tetra-
chlorobiphenyl in rabbits. Fukuoka Acta Med., 63: 384-386
(in Japanese).
KURATSUNE, M. (1972a) PCB pollution. Kosei no Shihyo, 19: 11-18
(in Japanese). KURATSUNE, M. & MASUDA, Y. (1972b) Polychlorinated
biphenyls in non-carbon copypaper. Environ. Health Perspect.,
1: 61-62.
KURATSUNE, M., YOSHIMURA, T. & MATSUZAKA, J. (1972) Epidemiologic
study on Yusho, a poisoning caused by ingestion of rice oil
contaminated with a commercial brand of polychlorinated biphenyls.
Environ. Health Perspect., 1: 119-128.
KUSUDA, M. (1971) Study on the female sexual function suffering from
the chlorobiphenyls poisoning. Sanka to Fujinka, 4: 1063-1072
(in Japanese).
LINCER, J. L. & PEAKALL, D. B. (1970) Metabolic effects of
polychlorinated biphenyls in the American kestrel. Nature
(Lond.), 228: 783-784.
LICHTENSTEIN, E. P. (1972) PCBs and interactions with insecticides.
Environ. Health Perspect., 1: 151-153.
LIEB, A. J., BILLS, D. B. & SINNHUBER, R. O. (1974) Accumulation of
dietary polychlorinated biphenyls (Aroclor 1254) by rainbow trout
(Salmo gairdneri). J. agr. Food Chem., 22: 638-642.
LINDER, R. E., GAINES, T. B. & KIMBROUGH, R. D. (1974) The effect of
polychlorinated biphenyls on rat reproduction. Food cosmet.
Toxicol., 12: 63-77.
LITTERST, C. L., FARBER, T. M., BAKER, A.M. & VAN LOON, E. J. (1972)
Effect of polychlorinated biphenyls on hepatic microsomal enzymes
in the rat. Toxicol. appl. Pharmacol., 23: 112-122.
MAKIURA, S., AOE, H., SUGIHARA, S., HIRAO, K., ARAI, M. & ITO, N.
(1974) Inhibitory effect of polychlorinated biphenyls on liver
tumorigenesis in rats treated with 3'-methyl-4-dimethyl-
aminoazobenzene, N-2-fluorenylacetamide, and diethylnitrosamine.
J. nat. Cancer Inst., 53: 1253-1257.
MASUDA, Y., KAGAWA, R. & KURATSUNE, M. (1972) Polychlorinated
biphenyls in carbonless copying paper. Nature (Lond.),
237: 41-42.
MASUDA, Y., KAGAWA, R. & KURATSUNE, M. (1974a) Polychlorinated
biphenyls in Yusho patients and ordinary persons. Fukuoka Acta
Med., 65: 17-24 (in Japanese).
MASUDA, Y., KAGAWA, R., SHIMAMURA, K., TAKADA, M. & KURATSUNE, M.
(1974b) Polychlorinated biphenyls in the blood of Yusho patients
and ordinary persons. Fukuoka Acta Med., 65: 25-27
(in Japanese).
MCKINNEY, J. D. (in press) Toxicology of selected symmetrical
hexachlorobiphenyl isomers : correlating biological effects with
chemical structure. In: Proceedings of the National Conference
on Polychlorinated Biphenyls, Chicago, 19-21 November, 1975.
MCLAUGHLIN, J. JR., MARLIAC, G. P., MERRETT, M. J., MUTCHLER, M. K. &
FITZHUGH, O. G. (1963) The injection of chemicals into the yolk
sac of fertile eggs prior to incubation as toxicity test.
Toxicol. appl. Pharmacol., 5: 760-771.
MELVÅS, B. & BRANDT, I. (1973) The distribution and metabolism of
labelled polychlorinated biphenyls in mice and quails. In: PCB
Conference II. National Swedish Environment Protection Board
Publications: 4E, pp. 87-90.
MES, J., COFFIN, D. E. & CAMPBELL, D. (1974) Polychlorinated biphenyl
and organochlorine pesticide residues in Canadian chicken eggs.
Pestic. Monit. J., 8: 8-11.
MIURA, H., OMORI, S., KATOH, M. (1973) Experimental porphyria in
rabbits induced by PCB. Jpn. J. Hyg, 28: 83 (in Japanese).
MOORE, J. A. (in press) Toxicity of 2,3,7,8-tetrachlorodibenzofuran:
preliminary results. Proceedings of the National Conference on
Polychlorinated Biphenyls, Chicago, 19-21 November.
MOSSER, J. L., FISHER, N. S., TENG, T. & WURSTER, C. F. (1972)
Polychlorinated biphenyls: toxicity to certain phytoplankters.
Science, 175: 191-192.
MULHERN, B. M., CROMARTIE, E., REICHEL, W. L. & BELISLE, A. A. (1971)
Semiquantitative determination of polychlorinated biphenyls in
tissue samples by thin layer chromatography. J. Assoc. Off.
Agric. Chem., 54: 548-550.
MORNS, Y. & KUROIWA, Y. (1971) Peripheral neuropathy in chlorobiphenyl
poisoning. Neurol., 21: 1173-1176.
MUSIAL, C. J., HUTZINGER, O., ZITKO, V. & CROCKER, J. (1974) Presence
of PCB, DDE and DDT in human milk in the provinces of New
Brunswick and Nova Scotia, Canada. Bull. environ. Contam.
Toxicol., 12: 258-267.
NAGAI, J., FURUKAWA, M., YAE, Y. & IKEDA, Y. (1969) Clinicochemical
investigation of chlorobiphenyls poisoning. Especially on the
serum lipid analysis of the patients. Fukuoka Acta Med.,
60: 475-488 (in Japanese).
NAGAYAMA, J., KURATSUNE, M. & MASUDA, Y. (1976) Determination of
chlorinated dibenzofurans in Kanechlors and "Yusho Oil". Bull.
environ. Contam. Toxicol., 15(1): 9-13.
NAGAYAMA, J., MASUDA, Y. & KURATSUNE, M. (1975) Chlorinated
dibenzofurans in Kanechlors and rice oils used by patients with
Yusho, Fukuoka Acta Med., 66: 593-599.
NATIONAL SWEDISH ENVIRONMENT PROTECTION BOARD (1973) PCB Conference
II. Publication 1973: 4E.
NEBEKER, A. V., PUGLISI, F. h. & DEFOE, D. L. (1974) Effect of
polychlorinated biphenyl compounds on survival and reproduction of
the fathead minnow and flagfish. Trans. Am. Fish Soc.,
No. 3, 562-568.
NESTEL, H. & BUDD, J. (1975) Chronic oral exposure of rainbow trout
(Salmo Gairdneri) to a PCB (Aroclor 1254): pathological effects.
Can. J. comp. Med., 39: 208-215.
NILSSON, B. & RAMEL, C. (1974) Genetic tests on Drosophila
melanogaster with polychlorinated biphenyls (PCB). Heriditas,
77: 319-322.
NIMMO, D. R., WILSON, P. D., BLACKMAN, R. R. & WILSON, A. J. JR (1971)
Polychlorinated biphenyl absorbed from sediments by fiddler crabs
and pink shrimp. Nature (Lond.), 231: 50-52.
NISBET, I. C. T. & SAROFIM, A. F. (1972) Rates and routes of transport
of PCBs in the environment. Environ. Health Perspect. 1: 21-38.
NISHIMOTO, T, UEDAM, M, TAUL, S. & CHIKAZAWA, K. (1972a)
Organochlorine pesticide residues and PCB in breast milk. Igaku
no Ayumi (Proc. Med. Sci.), 82: 574-575 (in Japanese).
NISHIMOTO, T., UETA, M., TAUL, S., CHIKAZAWA, K. NISHIUCHI, I. &
KONDO, K. (1972b) Deposition of organochlorine pesticide residues
and PCB in human body fat. Igaku no Ayumi (Proc. med. Sci.),
82: 515-516.
NISHIZUMI, M. (1970) Light and electron microscope study of
chlorobiphenyl poisoning in mouse and monkey liver. Arch.
environ. Health, 21: 620-632.
NORÉN, K. & WESTOO, G. (1968) Determination of some chlorinated
pesticides in vegetable oils, margarine, butter, milk, eggs, meat
and fish by gas chromatography and thin-layer chromatography.
Acta chem. scand., 22: 2289-2293.
ODÉN, S. & BERGGREN, B. (1973) PCB and DDT in Baltic sediments.
Report Dept. Soil Science, Agricultural College, Uppsala,
pp. 1-10.
ODSJO, T. (1973) PCB in some Swedish terrestrial organisms. In: PCB
Conference II. National Swedish Environment Protection Board
Publications: 4E, pp. 45-58. OKUMURA, M. & KATSUKI, S. (1969)
Clinical observation on Yusho (chlorobiphenyls poisoning).
Fukuoka Acta Med., 60: 440-446 (in Japanese).
OKUMURA, M., MASUDA, Y. & NAKAMUTA, S. (1974) Correlation between
blood PCB and serum triglyceride levels in patients with PCB
poisoning. Fukuoka Acta Med., 65: 84-87 (in Japanese).
OLSSON, M, JENSEN, S. & RENBERG, L. (1973) PCB in coastal areas of
the Baltic. PCB Conference H. National Swedish Environment
Protection Board, Publications: 4E, pp. 59-68.
ORBERG, J. & KIHLSTROM, J. E. (1973) Effects of long-term feeding of
polychlorinated biphenyls (PCB, Clopehn A60) on the length of the
oestrous cycle and on the frequency of implanted ova in the mouse.
Environ. Res., 6: 176-179.
ORGANIZATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT (1973)
Polychlorinated biphenyls, their use and control. Environmental
Directorate, Paris, OECD.
PANEL ON HAZARDOUS TRACE SUBSTANCES (1972) PCBs-Environmental Impact.
Environ. Res. 5: 249-362.
PEAKALL, D. B. LIMIAR, J. L. & BLOOM, S. E. (1972) Embryonic mortality
and chromosomal alterations caused by Aroclor 1254 in ring doves.
Environ. Health Perspect., 1: 103-104.
PESENDORFER, VON H., EICHLER, I. & GLOFKE, E. (1973) Informative
analyses or organochlorine pesticide and PCB residues in human
adipose tissue (from the area of Vienna). Wiener klinische
Wochenschr., 85: 218-222 (summary in English).
PHILLIPS, W. E. J., HATINA, G., VILLENEUVE, D.C. & GRANT, D. L. (1972)
Effect of parathion administration in rats following long-term
feeding with PCBs. Environ. physiol. Biochem., 2: 165-169.
PJCHIRALLO, J. (1971) PCBs: leaks of toxic substances raises issue of
effects, regulations. Science, 173: 899-902.
PLATONOW, N. S. & FUNNELL, H. S. (1971) Anti-androgenic-like effect of
polychlorinated biphenyls in cockerels. Vet. Rec, 88: 109-110.
PLATONOW, N. & CHEN, N.Y. (1973) Transplacental transfer of
polychlorinated biphenyls (Aroclor 1254) in a cow. Vet. Rec.,
92: 69-70.
PLATONOW, N. S., FUNNELL, H. S., BULLOCK, D. H., ARNOTT, D. R.,
SASCHENBRECKER, P. W. & GRIEVE, D. O. (1971) Fate of
polychlorinated biphenyls in dairy products processed from the
milk of exposed cows. J. Dairy Sci., 54: 1305-1308.
PLATONOW, N. S., LIPTRAP, R. M. & GEISSINGER, H. D. (1972) The
distribution and excretion of polychlorinated biphenyls (Aroclor
1254) and their effect on urinary gonadal steroid levels in the
boar. Bull. environ. Contam. Toxicol., 7: 358-365.
PORTER, M. L., YOUNG, S. J. V. & BURKE, J. A. (1970) A method for the
analysis of fish, poultry and animal tissue for chlorinated
pesticide residues. J. Am. Off. Anal. Chem., 53: 1300-1303.
PORTMANN, J. E. (1970) Monitoring of organochlorine residues in fish
from around England and Wales, with special reference to
polychlorinated biphenyls. Report CM 1970/E: 9 International
Council for the Exploration of the Sea, Charlottenlund Slot,
DK-2920 Charlottenlund, Denmark.
PRESTT, I., JEFFERIES, D. J. & MOORE, N. W. (1970) Polychlorinated
biphenyls in wild birds in Britain and their avian toxicity.
Environ. Pollut., 1: 3-26.
PRICE, H. A. & WELCH, R. L. (1972) Occurrence of polychlorinated
biphenyls in humans, Environ. Health Perspect., 1: 73-78.
REHFELD, B. M., BRADLEY, R. L. JR & SUNDE, M. L. (1971) Toxicity
studies on polychlorinated biphenyls in the chick. 1. Toxicity and
symptoms. Poult. Sci., 50: 1090-1096.
REINKE, J., UTHE, J. F. & O'BRODOVlCH, H. (1973) Determination of
polychlorinated biphenyls in the presence of organochlorine
pesticides by thin-layer chromatography. Environ. Lett.,
4: 201-210.
REYNOLDS, L. M. (1971) Pesticide residue analysis in the presence of
polychlorobiphenyls (PCBs). Residue Rev., 34: 27-45.
RINGER, R. K., AULERICH, R. J. & ZABIK, M. (1972) Effect of dietary
polychlorinated biphenyls on growth and reproduction of mink.
Proc. Amer. chem. Soc., 164th A.C.S. Meeting, New York,
pp. 149-154.
RISEBROUGH, R. W. & DE LAPPE, B. (1972) Accumulation of
polychlorinated biphenyls in ecosystems. Environ. Health
Perspect., 1: 39-45.
RISEBROUGH, R. W., RIECHE, P., PEAKALL, D. B., HERMAN, S. G. & KIRVEN,
M. N. (1968) Polychlorinated biphenyls in the global ecosystem.
Nature (Lond.), 220: 1098-1102.
ROACH, J. A. G. & POMERANTZ, I. H. (1974) The finding of chlorinated
dibenzofurans in a Japanese polychlorinated biphenyl sample.
Bull. environ. Contam. Toxicol., 12: 338-342.
ROTE, J. W. & MURPHY, P. G. (1971) A method for the quantitation of
polychlorinated biphenyl (PCB) isomers. Bull environ. Contam.
Toxicol., 6: 377-384.
SAFE, S. & HUTZINGER, O. (1971) Polychlorinated biphenyls: photolysis
of 2,4,6,T,4',6'-hexachlorobiphenyl, Nature (Lond.),
232: 641-642.
SAITO, R., SHIGEMATSU, N. & ISHIMARU, S. (1972) Immunoglobulin levels
in serum and sputum of patients with PCB poisoning. Fukuoka Acta
Med., 63: 408-411 (in Japanese).
SANDERS, H. O. & CHANDLER, J. H. (1972) Biological magnification of a
polychlorinated biphenyl (Aroclor 1254) from water by aquatic
invertebrates. Bull. environ. Contam. Toxicol., 7: 257-263.
SASCHENBRECKER, P. W., FUNNELL, H. S. & PLATONOW, N. S. (1972)
Persistence of polychlorinated biphenyls in the milk of exposed
cows. Vet. Rec., 90: 100-102.
SAVAGE, E. P., TESSARI, J. D., MALBERG, J. W., WHEELER, H. W. & BAGBY,
J. R. (1973) A search for polychlorinated biphenyls in human milk
in rural Colorado. Bull. environ. Contam. Toxicol., 9: 222-226.
SCHMIDT, T. T., RISEBROUGH, R. W. & GRESS, F. (1971) Input of
polychlorinated biphenyls into California coastal waters from
urban sewage outfalls. Bull. environ. Contam. Toxicol.,
6: 235-243.
SCHULTE, E. & ACKER, L. (1974) Gas chromatographic unit Glascapillaran
bei Temperaturan bis zu 320°C. Z. Anal. Chem., 268: 261-267.
SCOTT, M. L., ZIMMERMAN, J. R., MARINSKY, S. & MULLENHOFF, P. A.
(1975) Effects of PCBs, DDT and mercury compounds upon egg
production, hatchability and shell quality in chickens and
Japanese quail. Poult. Sci., 54: 350-368.
SCHWETZ, B. A., NORRIS, J. M., SPARSCHU, G. L., ROWE, U. K., GEHRING,
P. J., EMERSON, J. L. & GERBIG, C. G. (1973) Toxicology of
chlorinated dibenzo- p-dioxin. Environ. Health Perspect.,
5: 87-99.
SHIGEMATSU, N., NORIMATSU, Y., ISHIBASHI, T., YOSHIDA, M., SUETSUGU,
S., KAWATSU, T., IKEDA, T., SAITO, R., ISHIMARU, S., SHIRAKISA,
T., KIDO, M., EMORI, K. & TOSHIMITSU, H. (1971) Clinical and
experimental studies on respiratory involvement in chlorobiphenyls
poisoning. Fukuoka Acta Med., 62: 150-156 (in Japanese).
SHIGEMATSU, N., ISHIMARU, S., HIROSE, T., IKEDA, T., EMORI, I. &
MIYAZAKI, N. (1974) Clinical and experimental studies on
respiratory involvement in PCB poisoning. Fukuoka Acta Med.,
65: 88-95 (in Japanese).
SISSONS, D. & WELTI, D. (1971) Structural identification of
polychlorinated biphenyls in commercial mixtures by gas-liquid
chromatography, nuclear magnetic resonance and mass spectrometry.
J. Chromatogr., 60: 15-32.
SODERGREN, A. (1972a) Chlorinated hydrocarbon residues in airborne
fallout. Nature (Lond.), 236: 395-397.
SODERGREN, A. (1972b) Transport, distribution and degradation of
chlorinated hydrocarbon residues in aquatic model ecosystems.
Oikos, 23: 30-41.
SODERGREN, A. (1973a) Transport, distribution and degradation of DDT
and PCB in a south Swedish lake ecosystem. Vatten, 2: 90-108.
SODERGREN, A. (1973b) A simplified clean-up technique for
organochlorine residues at the microliter level. Bull. environ.
Contam. Toxicol., 10: 116-119.
SODERGREN, A. & SVENSSON, Bj. (1973) Uptake and accumulation of DDT
and PCB by Ephemera danica (Ephemeroptera) in continuous-flow
systems. Bull. environ. Contam. Toxicol., 9: 345-350.
SODERGREN, A., SVENSSON, Bj. & ULFSTRAND, S. (1972) DDT and PCB in
South Swedish streams. Environ. Pollut., 3: 25-36.
SOLLY, S. R. B. & SHANKS, V. (1974) Polychlorinated biphenyls and
organochlorine pesticides in human fat in New Zealand. N.Z.J.
Sci., 17: 535-544.
SOSA-LUCERO, J. C. & BE LA IGLESIA, F. A. (1973) Distribution of a
polychlorinated terphenyl (PET) (Aroclor 5460) in rat tissues and
effect on hepatic microsomal mixed function oxidases. Bull.
environ. Contam. Toxicol., 10: 248-256.
STALLING, D. L. & MAYER, F. L. JR (1972) Toxicities of PCBs to fish
and environmental residues. Environ. Health Perspect.,
1: 159-164.
STALLING, D. L., TRINDLE, R. C. & JOHNSON, J. L. (1972) Clean up of
pesticides and polychlorobiphenyl residues in fish extracts by gel
permeation chromatography. J. Assoc. Off. Anal. Chem.,
55: 32-45.
TAKAMATSU, M., INOUE, Y. & ABE, S. (1974) Diagnostic meaning of the
blood PCB. Fukuoka Acta Med., 65:28-31 (in Japanese).
TAKI, I., HISANAGA, S. & AMAGESE, Y. (1969) Report on Yusho
(chlorobiphenyl poisoning). Especially further study of its
dermatological findings. Fukuoka Acta Med., 62: 132-138
(in Japanese).
TAKI, I., KURATSUNE, M., MASUDA, Y. (1973) Studies on the
transmission to the fetus through placenta and the health of
and baby. Special Research Report on the effects of PCBs on human
health such as chronic toxicity for prevention of pollution by
PCBs. Research Co-ordination Bureau, Science and Technology
Agency, pp. 87-95 (in Japanese).
TAKIZAWA, Y. & MINAGAWA, K. (1974) Studies on environmental
accumulation and bio-accumulation of organochloric compounds.
Mainly mentioned PCT. Studies on the body effect of
degradation-registration substances, pp. 29-50 (in Japanese).
TANAKA, K. & ARAKI, Y. (1974) Inhibitory effect of cholestyramine on
the intestinal absorption of PCB. Fukuoka Acta Med., 68: 53-57.
TANAKA, K. & KOMATSU, F. (1972) Shortening of hexobarbital sleeping
time after small doses of PCB in rats. Fukuoka Acta Med.,
63: 360-366 (in Japanese).
TAS, A. C. & DE VOS, R. H. (1971) Characterization of four major
components in a technical polychlorinated biphenyl mixture.
Environ. Sci. Technol., 5: 1216-1218.
TATSUKAWA, R. & WATANABE, I. (1972) Air pollution by PCBs. Shoku No
Kagaku, 8: 55-63.
THOMAS, G. H. & REYNOLDS, L. M. (1973) Polychlorinated terphenyls in
paperboard samples. Bull. environ. Contam. Toxicol.,
10: 37-41.
TREON, J. F., CLEVELAND, F. P., CAPPEL, J. W. & ATCHLEY, R. W. (1956)
The toxicity of the vapours of Aroclor 1242 and Aroclor 1254.
Am. Ind. Hyg. Assoc. Quart., 17: 204-213.
TSUKAMOTO, H. ET AL. (1969) The chemical studies on detection of toxic
compounds in the rice bran oils used by the patients of Yusho.
Fukuoka Acta Med., 60: 496-512 (in Japanese).
TUCKER, R. K. & CRABTREE, D. G. (1970) Handbook of toxicity of
pesticides to wildlife. Bureau of Sport, Fisheries and Wildlife
Resources Pub. No. 84, Washington, DC, US Dept. of Interior,
pp. 130.
TUCKER, E. S., LITSCHGI, W. S. & MEES, W. M. (1975) Migration of
polychlorinated biphenyls in soil induced by percolating water.
Bull. environ. Contam. Toxicol., 13: 86-93.
URABE, H. (1974) Foreword. Fukuoka Acta Med., 65: 1-4 (in Japanese).
USDA/USDC/EPA/FDA/USDI/(1972) Polychlorinated biphenyls and the
environment. Washington DC, Interdepartmental Task Force on
PCBs. (Report ITF-PCB-72-1).
USHIO, F., FUKANO, S., NISHIDA, K., KANI, T. & DOGUCHI, M. (1974) Some
attempt to estimate the total daily intake of pesticides and PCB
residues and trace heavy metals. Ann. Rep. Tokyo Metr. Res. Lab.
P.H., 25: 307-312 (in Japanese).
UTHE, J. F., REINKE, J. & GESSER, H. (1972) Extraction of
organochlorine pesticides from water by porous polyurethane coated
with selective absorbent. Environ. Lett., 3: 117-135.
UZAWA, H., ITO, Y., NOTOMI, A. & KATSUKI, S. (1969) Hyperglyceridemia
resulting from intake of rice oil contaminated with chlorinated
biphenyls. Fukuoka Acta Med., 60: 449-454 (in Japanese).
UZAWA, H., NOTOMI, A., NAKAMUTA, S. & IKEURA, Y. (1972) Consecutive
three year follow up study of serum triglyceride concentrations of
82 subjects with PCB poisoning. Fukuoka Acta Med., 63: 401-404
(in Japanese).
VAN HOVE HOLDRINET, M. (1975) Preliminary results of an inter-
laboratory PCB check sample programme. J. Environ. Qual. (in press).
VEITH, G. D. (1972) Recent fluctuations of chlorobiphenyls (PCBs) in
the Green Bay, Wisconsin, region. Environ. Health Perspect.,
1: 51-54.
VILLENEUVE, D. C., GRANT, D. L., PHILLIPS, W. E. J., CLARK, M. L. &
CLEGG, D. J. (1971 a) Effects of PCB administration on microsomal
enzyme activity in pregnant rabbits. Bull. environ. Contam.
Toxicol., 6: 120-128.
VILLENEUVE, D. C., GRANT, D. L., KHERA, K., CLEGG, D. J., BAER, H. &
PHILLIPS, W. E. J. (1971b) The fetotoxicity of a polychlorinated
biphenyl mixture (Aroclor 1254) in the rabbit and in the rat.
Environ. Physiol., 1: 67-71.
VILLENEUVE, D.C., GRANT, D. L. & PHILLIPS, W. E. J. (1972)
Modification of pentobarbital sleeping times in rats following
chronic PCB ingestion. Bull. environ. Contam. Toxicol.,
7: 264-269.
VILLENEUVE, D.C., REYNOLDS, L. M., THOMAS, G. H. & PHILLIPS, W. E. J.
(1973a) Polychlorinated biphenyls and polychlorinated terphenyls
in Canadian food packaging materials. d. Assoc. Offic. Anal.
Chem., 56: 999-1001.
VILLENEUVE, D.C., REYNOLDS, L. M. & PHILLIPS, W. E. J. (1973b)
Residues of PCBs and PCTs in Canadian and imported European
cheeses, Canada-1972. Pestic. Monit. J., 7: 95-96.
VODDEN, H. A. (1973) Discussion In: PCB Conference II. National
Swedish Environment Protection Board, Publications: 4E, p. 118.
VOS, J. G. (1972) Toxicology of PCBs for mammals and for birds. Era,.
Health Perspect., 1: 105-117.
VOS, J. G. & BEEMS, R. B. (1971) Dermal toxicity studies of technical
polychlorinated biphenyls and fractions thereof in rabbits.
Toxicol. appl. Pharmacol., 19: 617-633.
VOS, J. G. DE ROIJ, Th. (1972) Immunosuppressive activity of a
polychlorinated biphenyl preparation on the humoral immune
response in guinea pigs. Toxicol. appl. Pharmacol., 21: 549-555.
VOS, J. G. & KOEMAN, J. H. (1970) Comparative toxicologic study with
polychlorinated biphenyls in chickens with special reference to
porphyria, edema formation, liver necrosis and tissue residues.
Toxicol. appl. Pharmacol., 17: 656-668.
VOS, J. G. & NOTENBOOM-RAM, E. (1972) Comparative toxicity study of
2,4,5,2',4',5'-hexachlorobiphenyl and a polychlorinated biphenyl
mixture in rabbits. Toxicol. appl. Pharmacol., 23: 563-578.
VOS, J. G. & VAN DRIEL-GROOTENHUIS, L. (1972) PCB-induced suppression
of the humoral and cell-mediated immunity in guinea pigs. Sci.
Total Environ., 1: 289-302.
VOS, J. G., KOEMAN, J. H., VAN DER MAAS, H. L., TEN NOEVER DE BRAUW,
M. G. & DE VOS, R. E. (1970) Identification and toxicological
evaluation of chlorinated dibensofuran and chlorinated naphthalene
in two commercial polychlorinated biphenyls. Fd. cosmet.
Toxicol., 8: 625-633.
VOS, J. G., STRIK, J. J. T. W. A., VAN HOLSTEYN, C. M. W. & PENNINGS,
J. H. (1971) Polychlorinated biphenyls as inducers of hepatic
porphyria in Japanese quail, with special reference to
delta-aminolevulinic acid synthetase activity, fluorescence and
residues in the liver. Toxicol. appl. Pharmacol., 20: 232-240.
WEBB, R. G. & MCCALL, A. C. (1972) Identification of polychlorinated
biphenyl isomers in aroclors. J. Am. Off. Anal. Chem.,
55: 746-752.
WESTOO, G. & NORÉN, K. (1970a) Levels of organochlorine pesticides and
polychlorinated biphenyls in fish caught in Swedish water areas or
kept for sale in Sweden, 1967-1970. Var Föda, 3: 93-146
(summary in English).
WESTOO, G. & NORÉN, K. (1970b) Determination of organochlorine
pesticides and polychlorinated biphenyls in animal foods. Acta
Chem. Scand., 24: 1639-1644.
WESTOO, G. & NORÉN, K. (1972) Levels of organochlorine pesticides and
polychlorinated biphenyls in Swedish human milk. Var Föda,
24: 41-54 (summary in English).
WESTOO, G., NORÉN, K. & ANDERSSON, M. (1971) Levels of organochlorine
pesticides and polychlorinated biphenyls in some cereal products.
Var Föda, 10: 341-360 (summary in English).
WHO WORKING GROUP (1973) The hazards to health and ecological effects
of persistent substances in the environment -- polychlorinated
biphenyls. Report of a Working Group convened by the World
Health Organization, Regional Office for Europe, EURO, 3109(2).
WILDISH, D. J. (1970) The toxicity of polychlorinated biphenyls (PCB)
in sea water to Grammarus oceanicus. Bull. environ. Contam.
Toxicol., 5: 202-204.
WILLIAMS, R. & HOLDEN, A. V. (1973) Organochlorine residues from
plankton. Mar. Bull., 4, 109-111.
WILLIS, D. E. & ADDISON, R. F. (1972) Identification and estimation of
the major components of a commercial polychlorinated biphenyl
mixture, Aroclor 1221. J. Fish. Res. Board Can., 29(5): 592-595.
WONG, P. T. S. & KAISER, K. L. E. (1975) Bacterial degradation of
polychlorinated biphenyls. II. Rate studies. Bull. environ.
Contam. Toxicol, 13: 249-256.
YAGAMUCHI, A., YOSHIMURA, T. & KURATSUNE, M. (1971) A survey on
pregnant women having consumed rice oil contaminated with
chlorobiphenyls and their babies. Fukuoka Acta Med.,
62: 112-117 (in Japanese).
YAMAMOTO, H. & YOSHIMURA, H. (1973) Metabolic studies on
polychlorinated biphenyls. III. Complete structure and acute
toxicity of the metabolites of 2,4,3',4'-tetrachlorobiphenyl.
Chem. pharm. Bull., 21 (10): 2237-2242 (in English).
YOBS, A. R. (1972) Levels of polychlorinated biphenyls in adipose
tissue of the general population of the nation. Environ. Health
Perspect., 1: 79-81.
YOSHIMURA, T. (1971) Epidemiological analysis of "Yusho" patients with
special reference to sex, age, clinical grades and oil
consumption. Fukuoka Acta Med., 62: 109-116 (in Japanese).
YOSHIMURA, H. & YAMAMOTO, H. (1973) Metabolic studies on
polychlorinated biphenyls. I. Metabolic fate of
3,4,3',4'-tetrachlorobiphenyl in rats. Chem. pharm. Bull.,
21: 1168-1169 (in English).
YOSHIMURA, H. & YAMAMOTO, H. (1974) Metabolic studies on
polychlorinated biphenyls. IV. Biotransformation of
3,4,3',4'-tetrachlorobiphenyl, one of the major components of
Kanechlor-400. Fukuoka Acta Med., 65: 5-11 (in Japanese).
YOSHIMURA, H. & YAMAMOTO, H. (1975) A novel route of excretion of
2,4,3',4'-tetrachlorobiphenyl in rats. Bull. environ. Contam.
Toxicol., 13: 681-687.
YOSHIMURA, H., YAMAMOTO, H., NAGAI, J., YAE, Y., UZAWA, H., ITO, Y.,
NOTOMI, A., MIMAKAMI, S., ITO, A., KATO, K. & TSUJI, H. (1971)
Studies on the tissue distribution and the urinary and faecal
excretion of 3H-Kanechlor (chlorobiphenyls) in rats. Fukuoka
Acta Med., 62: 12-19 (in Japanese).
YOSHIMURA, H., YAMAMOTO, H. & SAEKI, S. (1973) Metabolic studies on
polychlorinated biphenyls. II. Metabolic fate of
2,4,3'4'-tetrachlorobiphenyl in rats. Chem. pharm. Bull.,
21: 2231-2236 (in English).
YOSHIMURA, H., YAMAMOTO, H. & KINOSHITA, H. (1974) Metabolic studies
on polychlorinated biphenyls. V. Biliary excretion of
5-hydroxy-2,4,3',4',-tetrachlorobiphenyl, a major metabolite of
2,4,3',4'-tetrachlorobiphenyl. Fukuoka Acta Med., 65: 12-16
(in Japanese).
ZIMMERLI, B. & MARES, B. (1973) Die Belastung der Schweizerischen
Bevolkerung mit Pestiziden. Mitt. Lebensm. Unters. Hyg.,
64: 459-479.
ZITKO, V. (1970) Polychlorinated biphenyls (PCB) solubilized in water
by nonionic surfactants for studies of toxicity to aquatic
animals. Bull. environ. Contam. Toxicol., 5: 279-285.
ZITKO, V. (1971) Polychlorinated biphenyls and organochlorine
pesticides in some fresh-water and marine fishes. Bull. environ.
Contam. Toxicol., 6: 464-470.
ZITKO, V., HUTZINGER, O. & SAFE, S. (1971) Retention times and
electron-capture detector responses of some individual
chlorobiphenyls. Bull. environ. Contam. Toxicol., 6: 160-163.
ZITKO, V., HUTZINGER, O., JAMIESON, W. D. & CHOI, P. M. K. (1972a)
Polychlorinated terphenyls in the environment. Bull. environ.
Contam. Toxicol., 7: 200-201.
ZITKO, V., HUTZINGER, O. & CHOI, P.M. K. (1972b) Contamination of the
Bay of Fundy-Gulf of Maine area with polychlorinated biphenyls,
polychlorinated terphenyls, chlorinated dibenzodioxins and
dibenzofurans. Environ. Health Perspect., 1: 47-50.
ZITKO, V., CHOI, P. M. K., WILDISH, D. J., MONAGHAN, C. F. & LISTER,
N. A. (1974) Distribution of PCBs and p,p'-DDE residues in
Atlantic herring and yellow perch in eastern Canada, 1972.
Post. Mon. J., 8: 105-109.
There have been several investigations to identify individual PCBs
in commercial products. Sissons & Welti (1971) separated the
components of the Aroclors by column and gas-liquid chromatography,
and characterized many of the peaks by high-resolution mass
spectrometry and nuclear magnetic resonance, and by comparison with 40
synthesized Webb & McCall (1972) identified the gas-liquid
chromatographic with those of synthesized compounds by retention times
and spectrometry (Table 2). The most exhaustive study is that of
Jensen & Sundström (1974). They recognized that conventional
gas-liquid chromatography could not separate all the components, so
they devised preliminary fractionation on a charcoal column, which
separated the component PCBs according to the number of graphs in the
2,2',6 or 6' positions in the molecule ( o-chlorines). They compared
the gas-liquid chromatographic peaks with those of 90 synthesized
PCBs, and were able to characterize and quantify 60 components of
Clophens A50 and A60 (Table 3). Tables 2 and 3 show a considerable
overlap between the components of Aroclor 1254 and Clophen A50.
Table 2. Polychlorinated biphenyls in Aroclors 1221-1254 (Webb & McCall, 1972)
Retention Synthetic Acolor
timea chlorobiphenyl
1221 1232 1242 1248 1254
10 Biphenyl xb Cc x x x
13 2 x C x x x
16.9 3 x Dd
17 4 x C x
17.7
18.5 2,2' x C x x C x
21.3 x x x
21.6 x x x
23.5 2,3' x C x x x
24 2,4' x C x x C x
26 2,6,2' x x D x
29 2,5,2' x x C x
29.2 2,4,2' x x x C
29.5 4,4' x C x x C
34 2,3,2' x x Ce x
38 2,4,3' x x C x
38.5 2,4,4' x x C x
38.7 2,5,4' x x C x
41.5 x x
42.5 3,4,2' x C x
43 x x
44 x x
44.5 x
48 2,5,2',5' x C x x C
49.5 2,4,2',5' x C x x C
50.5 2,4,2',4' x D x
51.5 x x
55 2,3,2',5' x x x C
57 x x
59 2,3,6,2',6' x x x D
60 x x
64 x x
69 x x
69.5 x x
70 2,5,3',4' x Ce x
70.5 2,3,6,2',5' x C
71 2,4,3',4' x Ce x
72 x x
76 2,3,6,2',3' x C
Table 2. (cont'd).
Retention Synthetic Acolor
timea chlorobiphenyl
1221 1232 1242 1248 1254
82 x
83 2,3,6,2',5' x x C
84 x
85 2,4,5,2',5' x x C
87 2,4,5,2',4' x x C
96 x
99 2,4,5,2',3' x x C
101 x x
104 x x
107 x x
115 x
119 x x
125 2,4,5,2',3',6' x C
126 2,4,5,3',4' x C
135 x
147 x
148 2,4,5,2',4',5' x C
149 x
152 x
165 x
178 x
a Relative to p,p'-DDE at 190°C on 1 00' × 0.02' SCOT SE 30 column.
b indicates a GLC peak in this Aroclor at this retention time.
c C means that the synthetic compound in the second column was
confirmed by GLC and IR in this Aroclor. Because of the labour
involved, most compounds were only confirmed in one Aroclor. For
example, biphenyl is probably present in 1232-1248 as well as 1221.
d Only GLC data available.
e Something else also present.
2.2 Purity of Products
Commercial PCBs are not sold on a composition specification, but
on their physical properties. Different batches may vary somewhat from
the compositions shown in Tables 1-3. Impurities known to be present
in commercial PCBs are chlorinated dibenzofurans and chlorinated
naphthalenes (Vos et al., 1970; Bowes et al., 1975). Bowes et al.
(1975) found chlorinated dibenzofurans at 0.8-2.0 mg/kg in samples of
the Aroclor 1248-1260 series, but none in Aroclor 1016, and at levels
of 8.4 mg/kg in Clophen A60 and 13.6 mg/kg in Phenoclor DP-6. Roach &
Pomerantz (1974) found chlorinated dibenzofurans levels of 1 mg/kg and
Nagayama et al. (1976) found 18 mg/kg in different batches of
Kanechlor 400, but no chlorinated dibenzodioxins.
2.3 Determination of PCB Residues
Reviews have been published on methods used for the determination
of organochlorine compounds including PCBs in environmental samples
(Holden, 1973a, Panel on Hazardous Trace Substances, 1972). No two
laboratories have identical methods, although all have features in
common. The techniques appear to be those previously developed for the
determination of organochlorine pesticides with appropriate
modifications for the presence of PCBs, and the studies on PCBs
sometimes form part of a wider programme for monitoring persistent
organochlorine compounds in the environment. The major difficulties in
the determination of PCBs are to separate them from interfering
organochlorine pesticides, and to derive a single quantitative figure
from a variable mixture of components.
2.3.1 Extraction of sample
Air
Particulate fallout from air has been trapped on 200 µm nylon net
coated with silicone oil, and the PCBs then extracted with hexane
(Södergren, 1972a). Separate determinations of particulate and vapour
phase PCBs in air have been made by the passage of a large volume of
air through a filter which was followed by an impinger containing
hexane (Hasegawa et al., 1972b), or a polyurethane plug (Bidleman &
Olney, 1974) or ceramic saddles coated with OV 17 silicone (Harvey &
Steinbauer, 1974) to absorb the vapour.
Table 3. Percentages of polychlorinated biphenyls in Clophen A50 and A60 and in human (Jensen & Sundström, 1974)
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
1 2,5-2'5' 2 0.25 0.30 5.0
2 2,4-2'5' 2 0.26 0.30 1.4
3 2,3-2'5' 2 0.27 0.33 1.9 1.1
4 (2,-2',3',4') 2 0.30 0.43 1.2 0.66
5 (4-2',3'6') 2 0.32 0.43 2.1 0.56
6 2,5-2',3',6' 3 0.35 0.43 4.4b 2.9 1.2
7 2,3-2',3',6' 3 0.38 0.49 2.5b 0.28 0.48
8 3,4-2',5' 1 0.41 0.42 3.9 1.5
9 1 or 2 0.42 2.2c 2.0c
10 2,5-2',3',5' 2 0.45 0.49 2.2 1.1 1.2
11 2,3,6-2',3',6' 4 0.47 0.61 0.50 1.0d
12 2,5-2',4',5' 2 0.48 0.50 7.0b 5.6 4.2
13 2,4-2',4',5' 2 0.51 0.51 1.8b --e 1.9
14 2,3-2',4',5' 2 0.53 0.57 1.4b
15 2,5-2',3',4' 2 0.53 0.58 5.4 1.4 2.3
16 2,3-2',3',4' 2 0.55 0.66 1.0
17 3,4-2',3',6' 2 0.56 0.62 7.6b 2.9 4.7
18 2,3,5-2',3',6' 3 0.60 0.70 1.2 4.2 1.0
19 2,4,5-2',3',6' 3 0.64 0.73 2.0b 6.5d 0.13
20 2,5-2',3',5',6' 3 0.65 0.68 1.3 3.3 0.43
21 2,3-2',3',5',6' 3 0.70 0.74 0.5 0.05
22 2,3,4-2',3',6' 3 0.72 0.83 1.8 3.2 0.15
23 1 or 2 0.76 0.6c
Table 3. (cont'd).
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
24 2,3,6-2',3',5',6' 4 0.77 0.91 0.09 0.96
25 3,4-2',4',5' 1 0.79 0.74 5.0b 1.6 5.4
26 2,3,6-2',3'4',6' 4 0.84 0.95 0.05 0.37
27 2,3,52',4',5' 2 0.85 0.86 0.90 2.9 2.7
28 3,4-2',3',4' 1 0.87 0.86 3.6 1.9
29 2,4,5-2',4',5' 2 0.90 0.86 4.2b 12.9d 21.5
30 2,3,4-2',3',5' 2 0.94 0.97 ) 1.5 --e
31 1 or 2 0.95 0.93 ) 1.1 --e
32 2,3,4-2',4',5' 2 1.00 1.00 5.1 11.3d 14.0
33 2,3,5-2',3',5',6' 3 1.01 1.06 0.04 0.49 0.90
34 4 1.02 0.05c
35 1 or 2 1.04 1.10 1.1c 2.0c 1.5'
36 2,4,5-2',3',5',6' 3 1.05 1.13 0.39 3.3 3.5
37 2,3,4-2',3',4' 2 1.11 1.16 1.3 2.0 0.81
38 2,3,6-2',3',4',5' 3 1.13 1.29 0.33 3.7d --e
39 2,4,5-2',3',4',6' 3 1.14 1.16 0.17 1.8 2.5
40 2,3,4-2',3',5',6' 3 1.19 1.32 0.27 2.1 1.3
41 2,3,5,6-2',3',5',6' 4 1.23 1.34 0.005 0.07
42 2,3,4-2',3',4',6' 3 1.28 1.40 0.13 1.3 0.57
43 2,3,4,6-2',3',5',6' 4 1.34 1.44 0.007 0.09
44 2,4,5-3',4',5' 1 1.41 1.19 0.47 1.0 0.49
45 2,3,6-2',3',4',5',6' 4 1.45 1.59 0.008 0.09
46 2,3,4,5-2',3',4',6' 4 1.46 1.51 --e 0.03
47 3,4-2',3',4',5' 1 1.55 1.37 0.81 1.5 2.0
48 2,3,5-2',3',4',5' 2 1.59 1.49 0.23 0.90 1.2
49 2,4,5-2',3',4',5' 2 1.71 1.56 0.98 7.6d 7.7
Table 3. (cont'd).
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
50 2,3,4-2',3',4',5' 2 1.88 1.82 0.72 4.1d 5.9
51 2,3,4,5-2',3',5',6' 3 1.94 1.99 0.08 0.74 0.77
52 1 or 2 2.02 1.96 0.23c 1.0c --e
53 2,3,4,5-2',3',4',6' 3 2.07 2.05 0.06 0.44 0.94
54 2,4,5-2',3',4',5',6' 3 2.15 2.04 0.01 0.28 0.46
55 (2,3,5,6-2',3',4',5',6') 4 2.23 --e
56 2,3,4-2',3',4',5',6' 3 2.40 2.20 0.01 0.17 0.31
57 2,3,4,6-2',3',4',5',6' 4 2.45 2.56 --e --e
58 3,4,5-2',3',4',5' 1 2.74 2.41 --e --e
59 2,3,4,5-2',3',4',6' 2 3.18 2.81 0.35 0.67 1.7
60 2,3,4,5,6-2',3',4',5',6'4 4 4.12 0.62
Total 86.7% 99.4% 100.0%
p,p'-DDE 0.52 0.58
p,p'-DDT 0.71 0.73
p,p'-DDT 0.90 0.97
a Tentative structures are given in brackets.
b Component found present in Aroclor 1254 by Webb & McCall (1972).
c Figures calculated using responses of chlorobiphenyls with similar retention times in the same fraction
from the charcoal column.
d Component found present in Phenoclor DP6 by Tas & Vos (1971).
e Present in trace amounts only.
Water
PCBs have been extracted from water by passing a sample through a
filter of undecane and Carbowax 400 monostearate supported on
Chromosorb W (Ahling & Jensen, 1970) or a porous plug of polyurethane
coated with a suitable gas-liquid chromatographic stationary phase
(Uthe et al., 1972), or Amberlite XAD-2 resin (Harvey, et al., 1973)
followed by elution of the PCBs with a solvent. Ahnoff & Josefsson
(1975) have described a liquid-liquid extraction into cyclohexane.
Biological samples
Most analysts have used standard methods developed for
organochlorine pesticides, in which the PCBs are extracted together
with the fat; the sample is ground with anhydrous sodium sulphate and
extracted with petroleum ether or hexane. Porter et al. (1970) studied
the optimal conditions for this procedure. A dehydrating solvent may
be included to facilitate the breakdown of cell structures; ethanol
(Norén & Westöö, (1968) and acetone (Jensen et al., 1973) have been
used. Rote & Murp (1971) digested the sample with a mixture of acetic
and perchloric acid prior to hexane extraction.
2.3.2 Clean-up
Methods for the removal of fat from the extract include solve
partitioning between hexane and acetonitrile or dimethylformamide,
treatment with strong sulfuric acid or ethanolic potassium hydroxide
Gel permeation has also been used (Stalling et al., 1972), and Holden
Marsden (1969) removed fat on dry, partially deactivated alumina
column. Certain pesticides such as dieldrin are destroyed by the
sulfuric at treatment, so this method cannot be used if such
pesticides are to determined together with PCBs (Jensen et al., 1973).
PCBs may be separated from organochlorine pesticides by column
chromatography on Florisil (Mulhern et al., 1971), silica gel (Holden
Marsden 1969; Armour & Burke, 1970; Collins et al., 1972) or on
charcoal (Berg et al., 1972; Jensen & Sundström, 1974). Several
laboratories have reported difficulties in repeating results obtained
by other investigators; the ease of separation appears to depend upon
the characteristics of absorbent, of the eluting solvent, and of the
sample extract, though the appears to be no difficulty in separating
all interfering substances except DDE, a metabolite of DDT. Thin-layer
chromatography has been us for separation by Norén & Westöö (1968),
Bagley et al. (1970), and Rein et al. (1973).
In many environmental samples, DDE is present in large excess over
the PCBs, and must be removed before the quantitative determination
PCBs. Oxidation procedures have been used to convert DDE
dichlorobenzophenone; recommended oxidants are potassium dichromate
and sulfuric acid (Westöö & Norén, 1970b) and chromium (II) oxide a
acetic acid (Mulhern et al., 1971). Jensen & Sundström (1974), who we
interested in determining DDT/PCB ratios in environmental sample
preferred sodium dichromate in acetic acid with a trace of sulfuric
acid. They claim that this does not destroy DDT and its metabolite DDD
which may be present in extracts after clean-up with strong sulfuric
acid and that using this mixture makes possible the quantitative
determination of the dichlorobenzophenone from the oxidation of DDE.
Conversion of DDT to DDE may be achieved by treatment with
ethanolic potassium hydroxide, which also removes interference from
elemental sulfur (Ahling & Jensen, 1970). Sulfur may also be removed
activated Raney nickel (Ahnoff & Josefsson, 1975) or by metallic
mercury.
Södergren (1973b) has scaled down the clean-up procedure for small
samples, using microlitre volumes.
2.3.3 Chromatographic separation of PCBs
Gas-liquid chromatography
Most analysts use gas-liquid chromatography with an electron-
capture detector for the separation of PCBs from the extract after
clean-up. Stationary phases commonly used are silicones or their
derivatives, for example, DC 200, SF 96, OV 1, and QF 1, or Apiezon L.
Jensen & Sundström (1974) state that with a mixture of SF 96 and QF 1,
14 peaks can be obtained from Clophen A50, but that Apiezon L gives
much better resolution. They obtained better peak separation by prior
fractionation on a charcoal column, which separated the PCBs according
to the number of o-chlorine substituents; they regard such
refinements as unnecessary in PCB residue analysis, although they may
be of value in the study of the selective, environmental degradation
of PCBs. Column temperatures used ranged between 170°C and 230°C.
Glass capillary columns gave good separation of PCBs from DDT and its
metabolites (Schulte & Acker, 1974).
Thin-layer chromatography
This has been used in the clean-up stage, but it may also provide
semi-quantitative results by visualization of the spots followed by
densitometry, or by comparison with spots produced by known amounts of
PCBs. Mulhern et al. (1971) separated PCBs on a plate coated with
alumina containing silver nitrate, and the spots were developed by
exposure to ultraviolet light; the detection limit was in the region
of 1 µg. Collins et al. (1972) devised a similar method in which the
PCBs remained together in a single spot, and they claimed a limit of
detection of about 50 ng. Reversed-phase chromatography on plates
coated with kieselguhr treated with liquid paraffin has been used to
separate Phenochlor DP6 into several spots with a detection limit of a
few micrograms (de Vos & Peet, 1971).
2.3.4 Quantification of PCB content
The response of the electron capture detector is not equal for all
PCB components, being much affected by the degree of chlorination
(Zitko et al., 1971). This does not lead to difficulties when the
sample under investigation has been directly contaminated by a
commercial PCB mixture, as that mixture can be used as a standard.
Difficulties are encountered when the PCBs in the sample have
undergone selective environmental degradation (see sections 3 and 5).
Several investigators have noted that the pattern of peaks from such
samples resembles fairly closely that of one or other of the higher
chlorinated PCB mixtures such as Aroclor 1254, and they have compared
the total area of the peaks with that of the nearest commercial
product in order to determine the amount of PCBs in the sample (Armour
& Burke, 1970). Collins et al. (1972 observed that, under their
conditions, the area of peaks usually encountered in extracts of
tissue samples was closely similar to that of an equivalent amount of
DDE, thus DDE could be used for calibration. In order to overcome the
uncertainties of these procedures, Rote & Murphy (1971) divided the
peaks into groups according to the number of chlorine atoms in the
molecule, as determined from mass spectrographic data, and calculated
the PCB content of each group from the theoretical response of the
detector to chlorine content. Jensen et al. (1973) selected a
commercial in PCB that included all the peaks from the extract; they
determined the PCB content of each peak by combined mass spectrometry
and coulometry, and determined the total PCBs in the sample by
comparing the height of each peak obtained with the extract with those
obtained with the reference sample. Simpler methods have been used by
Koeman et al. (1969), who compared the height of a single peak
obtained with the extract with that of a peak with the same retention
time obtained with a e commercial PCB mixture; others have averaged
out more than one peak s for this calculation (Reynolds, 1971; Reinke
et al., 1973). Rote & Murphy (1971) have calculated that such
procedures may more than double the values obtained by a more accurate
method.
A different technique has been recommended by Berg et al. (1972);
the PGBs are chlorinated with antimony pentachloride to decachloro-
biphenyl, which can then be measured as a single peak.
2.3.5 Accuracy of PCB determinations
A group of eight analysts engaged in an investigation of pollution
in the North Sea undertook a collaborative study to determine the PCB
content of a sample of fish oil, using the methods currently employed
in their laboratories (International Council for the Exploration of
the Sea, 1974). The PCB values obtained ranged from 1.0 to 3.9 mg/kg
with a mean of 1.97 mg/kg and a standard deviation of 0.93 mg/kg.
Better agreement was obtained with the same fish oil fortified with
PCBs at a concentration of 10 mg/kg; the mean of the results for the
fortified sample was 10.0 mg/kg with a standard deviation of
1.1 mg/kg.
A probable source of error is incomplete initial extraction of
PCBs from the sample (Holden & Marsden, 1969). Another source of
variation between laboratories lies in the method used to quantify
gas-liquid chromatographic peaks (section 2.3.4); van Hove Holdrinet
(1975) considered this to be the major source of error.
It is evident that caution should be exercised in accepting the
analytical results from a laboratory, particularly for samples with a
low PCB content, until the competence of that laboratory has been
established by an inter-laboratory collaborative study.
2.3.6 Confirmation of identity
Since Jensen first identified as PCBs those hitherto unknown
substances that interfered in the gas-liquid chromatographic
determination of organochlorine pesticides using mass spectrographic
data, other investigators have confirmed the presence of PCBs in
environmental samples by combining gas-liquid chromatography with mass
spectrometry (Bagley et al., 1970) and with coulometry to measure the
chlorine content. The conversion of PCBs to bicyclohexyl and
decachlorobiphenyl is further confirmation (Berg et al., 1972). The
widespread distribution of PCBs is now well established, and, as
adequate methods are available to remove interference from
organochlorine pesticides, there is no evidence of the presence of
other interfering substances in the types of sample that have so far
been analysed, down to a limit of detection of around 0.01 mg/kg. This
does not necessarily apply to other types of sample, particularly when
very low levels are being sought; Ahnoff & Josefsson (1973, 1975)
reported a number of unknown interfering substances, when measuring
PCBs in water at levels below 1 ng/litre, one of which was
subsequently identified as elemental sulfur. They recommend
confirmation by mass fragmentography for such samples.
2.4 Determination of PCTs
A few methods have been published for the determination of PCTs;
extraction and clean-up procedures are similar to those used for PCBs,
but the gas-liquid chromatographic details are different because of
the lower volatility of the PCTs. Zitko et al. (1972b) used 3% OV 210
as the stationary phase with a column temperature of 200°C. Thomas &
Reynolds (1973) also used OV 210 with a column temperature of 250°C
and another system with 3% Dexsil as stationary phase at 300°C with a
63Ni electron capture detector; this was also used by Addison et al.
(1972). Sosa-Lucero et al. (1973) used OV 210 and SE 30 at 255°C and
Freudental & Greve (1973) used OV 17 with a temperature programmed
from 200°C to 285°C. Thomas & Reynolds (1973) confirmed the identity
by chlorination to tetradecachloroterphenyl with antimony
pentachloride.
A thin-layer chromatographic technique has also been described
with limit of detection of about 1 µg (Addison et al., 1972).
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1 Production and Uses of PCBs
Details of the production and uses of PCBs in the USA have been
released, and have been summarized by Nisbet & Sarofim (1972). Annual
production has increased steadily since 1930 and reached a maximum in
1970 of 33 000 tonnes. During this peak year, 65% of the production
was of the 42% chlorinated type, 25% was less chlorinated, and the
remainder more chlorinated. After 1970, production sharply decreased
owing to voluntary limitation of sales by the Monsanto Company, the
sole manufacturer in the USA. According to information collected by
the Organization for Economic Co-operation and Development (OECD), the
1970, production in the USA was 18 000 tonnes and the total in OECD
countries in that year was 48 000 tonnes. It has been estimated that
the cumulative total production of PCBs in North America up to 1971
was 0.5 million tonnes, and in the whole world probably double this
figure.
The commercial applications of PCBs have been reviewed in an OECD
report (Organization for Economic Co-operation and Development, 1973)
From an environmental viewpoint these can be divided into three
categories:
Controllable closed systems. PCBs used as dielectrics in
transformers and large capacitors have a life equal to that of the
equipment, and with proper design leakage does not occur. When the
equipment is scrapper the quantity of dielectric is sufficiently large
to justify regeneration.
Uncontrollable closed systems. PCBs are used in heat transfer and
hydraulic systems which, although technically closed, permit leakage.
The need for frequent replacement of small quantities makes recovery
impracticable, PCBs are very widely dispersed in small capacitors, and
there are great difficulties in collecting these items for disposal.
Dissipative uses. PCBs have been used in the formulation of
lubricating and cutting oils, in pesticides, and as plasticizers in
paints, copying paper, adhesives, sealants, and plastics. In these
applications, the PCBs are in direct contact with the environment, and
there is no way of recovering them when the product is scrapped.
The uses of PCBs in the USA in 1970 have been analysed by Nisbet &
Sarofim (1972). Of the total 33 000 tonnes, 56% was used as a
dielectric, with 36% in capacitors and 20% in transformers. Various
plasticizer outlets accounted for 30%, hydraulic fluids and lubricants
12%, and heat transfer liquids 1.5%. Following the restriction of
sales for non-dissipative uses, the percentage of PCBs sold as
dielectrics rose to 77% in 1971 and the proportion of highly
chlorinated products was considerably reduced, Aroclor 1016 replacing
Aroclor 1242. In Japan, 44 800 tonnes of PCBs were used from 1962 to
1971, and of this 65.4% was used in the electrical industry, 11.3% in
heat exchangers, 17.9% in pressure-sensitive duplicating paper, and
5.4% for other dissipative uses (Ishi, 1972). In Sweden, most of the
600 tonnes imported in 1969 was used in the electrical industry and a
large part of the remainder in paints (Jensen, unpublished report
1972).
According to the OECD report, transformers and capacitors provided
the major outlets for PCBs in most OECD countries in 1971. In 1972,
several countries restricted sales; in Sweden the importation and use
of PCBs were restricted by law; in the United Kingdom, as in the USA,
sales were voluntarily restricted to the lower chlorinated PCBs for
use as dielectrics in enclosed systems; in the Federal Republic of
Germany, use in hydraulic and heat transfer fluids was also permitted.
In Japan the production and use of PCBs were banned in 1972.
Limitations on sales were subsequently introduced in other countries.
No information is available on the scale of production and the
uses of PCTs.
3.2 Entry of PCBs into the Environment
Surveys of the sources of environmental pollution with PCBs were
made before production and use were limited, and the information
available may not now apply in North America and elsewhere. Table 4
gives an estimate of the fate of the PCBs produced in 1970 in the USA
(Nisbet & Sarofim, 1972). Only 20% of the annual production can be
regarded as a net increase in current usage, and the remainder is
balanced by a loss to the environment. More than one-half of this
entered dumps and landfills and it has been calculated that 0.3
million tonnes of PCBs have accumulated in such locations in North
America since 1930. Much of this was originally enclosed in containers
such as capacitors or was in plasticized resins and will not be
released until the containing medium decays. The diffusion of PCBs
from landfills is likely to be slow on account of their low volatility
and low water solubility; Carnes et al. (1973) found little leaching
from one site they tested.
Pollution of the environment has occurred mainly from the first
three routes mentioned in Table 4. In addition, there are other
routes, which although involving relatively small amounts,
nevertheless have an influence on the entry of PCBs into food chains.
PCBs have been used in the USA in amounts of about 10 tonnes/year in
pesticide formulations (Panel on Hazardous Trace Substances, 1972),
and the unauthorized use of scrap transformer fluid for this purpose
has led to local contamination of milk supplies.
Table 4. Entry of PCBs into the environmenta
Percentage of
annual PCB type
Route production (% chlorination)
Vaporization from plasticizers 4.5 48-60
Vaporization during incineration 1 42
Leaks and disposal of industrial fluids 13 42-60
Destruction by incineration 9 mainly 42
Disposal in dumps and landfills 52.5 42-60
Net increase in current usage 20 42-54
a From Nisbet & Sarafim, 1972.
Pressure sensitive duplicating paper containing PCBs has found its
way into waste paper supplies and has been recycled into paper and
board used as food packaging materials; paints for coating the bottom
of ships contained 3-5% of PCBs -- about 3% of the annual quantity
imported into Sweden has been used for this purpose -- and this has
been a source of plankton contamination (Jensen et al., 1972a).
3.2.1 Release of PCBs into the atmosphere
There appears to be little widespread atmospheric contamination
during the manufacture and processing of PCBs, but this can occur
during their subsequent use and disposal. Although PCBs have a low
volatility, there may be an appreciable loss to the atmosphere during
the lifetime of a PCB-plasticized resin, particularly of the lower
chlorinated products. Further pollution may occur during the
incineration of industrial and municipal waste. Most municipal
incinerators are not very effective in destroying PCBs; efficient
incinerators can be designed for this purpose (Jensen & Wickberg,
unpublished report 1971; Jensen, unpublished report 1972), although
the higher chlorinated PCBs are more resistant to pyrolysis. Secondary
sources of atmospheric pollution are volatilization from soil, and
from the drying of sewage sludge. Laveskog (1973) found that the PCB
emissions from municipal incineration and from the drying of sewage
sludge each amounted to about 1 kg/year per million inhabitants, a
small amount compared with the 2 tonnes deposited yearly from aerial
fallout in the south of Sweden.
3.2.2 Leakage and disposal of PCBs in industry
The major source of environmental pollution with PCBs, which
eventually affects food chains, is the leakage and the disposal of
industrial fluids. There have been serious cases of poisoning in man
(Kuratsune et al., 1972) and in animals (Panel on Hazardous Trace
Substances, 1972) due to leakage from a heat exchanger. Leakages, or
the unintentional or deliberate discharge of waste, have contaminated
seas, lakes, waterways, and sewers (Duke et al., 1970; Schmidt et al.,
1971; Veith, 1972).
Analysis of solids from factory wastes in Japan have revealed a
wide variation in PCB content. In most, less than 1 mg/kg was
detected, but in: some the contamination was very heavy, the highest
level recorded being 8.26% from a factory manufacturing electrical
equipment (Japanese Environment & Safety Bureau, unpublished report
1972).
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATION
4.1 Environmental Transport
Nisbet & Sarofim (1972) emphasize that the available data are
insufficient to determine anything but a very crude model of the
transfer of PCBs into the environment. Guesses can be made by
referring to the distribution of DDT, which resembles the PCBs to some
extent in its physical and chemical properties and about which more is
known. Much of the following discussion arises from their analysis of
the situation in the North American continent.
4.1.1 Air transport
By analogy with DDT, it might be expected that PCBs entering the
atmosphere in the vapour phase would be adsorbed rapidly on to
particles, which would be deposited or washed out in rain at a rate
depending on their particle size, the average residence time in the
atmosphere being 2-3 days. This has been confirmed by Södergren
(1972a), who measured the deposition in southern Sweden of PCBs that
had originated from municipal incinerators or had been carried over
from Denmark by the prevailing winds, and showed that the amount
deposited in central Sweden was much less. Cames (1973) and Laveskog,
(unpublished report 1973) found mainly particulate PCBs in the
emission from incinerators. Harvey & Steinhauer (1974), however,
considered that the results of their analyses of air in north Atlantic
regions indicated that most of the PCBs carried in the air were in the
vapour phase.
4.1.2 Transport in soil
The PCBs in soil are derived mainly from particulate deposition,
estimated at 1000-2000 tonnes annually in North America, most of which
is in urban areas. Small amounts have originated from the use of
sewage sludge as a fertilizer, from the leaching of landfalls, and
from the se of PCBs in pesticide formulations. Tucker et al. (1975)
found that under experimental conditions, the higher chlorinated PCBs
were not leached from soils by percolating water, and those with a
lower level of chlorination were removed only slowly, particularly
from soils of high lay content. Losses do occur by volatilization and
by biotransformation; by analogy with DDT and its metabolites the
half-time in soil has been estimated at 5 years. Haque et al. (1974)
showed that the rate of evaporation decreased with the clay content of
the soil and he degree of chlorination of the biphenyl, and increased
with temperature. Biotransformation has also been shown to play a part
in the disappearance of the lower chlorinated compounds from soil
(Iwata et al., 1973).
The total amount of PCBs distributed over North America, apart
from that in dumps and landfills, has been estimated at 20 000 tonnes,
of which one quarter has subsequently been transported via the air to
the seas. (Nisbet & Sarofim, 1972).
4.1.3 Transport in water
The entry of PCBs into water occurs mainly at the points of
discharge of industrial and urban wastes into rivers, lakes, and
coastal waters. Sewage treatment appears to remove particulate PCBs
from water, but not PCBs in solution; these are concentrated in the
sludge (Ahling & 1970), which may be dumped into rivers and coastal
waters. Holden (1970b) found a mean PCB content of 3 mg/kg in liquid
sludges from Glasgow, and calculated that PCBs at the rate of
1 tonne/year were released into the Clyde and Thames estuaries; a
similar output was calculated from water treatment plants off the
California coast (Schmidt et al., 1971). Other localized sources of
pollution are leakages or waste disposal from ships. PCBs in water are
attached mainly to particulate matter (Södergren, 1973a) and
eventually fall to the bottom sediment at a rate that depends on the
particle size. PCBs may be leached from the sediment and may reach
coastal waters, but Nimmo et al. (1971) noted little change in the PCB
content of a sediment at a point downstream from a source of
contamination over a period of 9 months. The process may be
accelerated by the dumping of dredging spoil.
4.1.4 Transport through biota
According to the approximate calculations of Nisbet & Sarofim
(1972), less than 1000 tonnes of PCBs are located in living organisms
throughout the world, so that biological transport and degradation
play little part in the fate of PCBs in the environment, though these
factors have great ecotoxicological significance.
4.2 Transformation in the Environment
4.2.1 Abiotic transformation
The fate of the various PCBs in commercial mixtures depends on
their physical and chemical properties. Some fractionation occurs
during the volatilization of PCBs, because of a decrease in vapour
pressure with increasing chlorination. The PCBs are chemically very
stable, and are not likely to be degraded at a significant rate by
hydrolytic or similar reactions under environmental conditions. They
are, however, fairly easily degraded by photolysis under laboratory
conditions; Safe & Hutzinger (1971), and Hutzinger et al. (1972b) have
shown that PCBs are dechlorinated in hexane solution at a rate that
increases with increasing chlorination. PCBs in aqueous-dioxane
suspensions and in thin films give hydroxy and carboxylic acid
derivatives on irradiation. The lower chlorinated biphenyls, at a
vapour concentration in air of 1.5 mg/m3 are readily destroyed by
photolysis under laboratory conditions. There is no direct evidence of
the extent of PCB breakdown in the atmosphere under environmental
conditions, and nothing is known of the persistence and toxicity of
any transformation products.
4.2.2 Biotransformation
The biotransformation of PCBs is discussed in section 6.6. Owing
to small proportion of the total environmental PCBs contained in
living biotransformation does not significantly influence the overall
environmental concentrations of PCBs, though it has a marked influence
on PCBs passing through food chains.
4.2.3 Metabolism in limited ecosystems
The presence of PCBs in sewage sludge suggests that they are not
all readily transformed by microorganisms. Choi et al. (1974) found no
evidence of biotransformation of Aroclor 1254 added to water entering
an aerated biological water treatment system, although much of it was
removed with the sludge; there appeared to be no interference by the
PCBs in the performance of the system. Vodden (1973) quotes
investigations showing that PCBs with four or fewer chlorine atoms are
readily broken down by microorganisms, but that this can be inhibited
by the presence of higher chlorinated PCBs. Mono- and dichlorobiphenyl
can be transformed by Achromobacter isolated from sewage effluent
(Ahmed & Focht, 1973), and a culture of lake bacteria can degrade some
of the lower chlorinated components of Aroclor 1242 to chlorine-free
derivatives (Kaiser & Wong, 1974).
Södergren (1972b) investigated the transport of Clophen A50 added
to a model aquatic ecosystem; this was rapidly taken up by an alga
(Chlorella) and no change was observed in the proportion of the
component PCBs in the first consumer fish; however, there was a
relative loss of the lower chlorinated PCBs in the perch, the second
consumer. No progressive loss of the lower chlorinated PCBs was
observed in sediment, plankton, invertebrates, and fish inhabiting a
Swedish lake (Södergren, 1973a). Evidence on the metabolism of PCBs by
fish is conflicting, but it seems probable that most fish,
particularly those in the lower trophic stages of food chains, cannot
readily degrade the low chlorinated PCBs.
4.3 Biological Accumulation
Although PCB concentrations in living organisms clearly indicate a
progressive accumulation in food chains, the factors discussed in the
previous sections make it impossible to give any reliable figure for
bioaccumulation at each trophic stage. There is also doubt about which
tissue level should be used in the calculation, that of the whole
body, the fat, or the liver.
There is good evidence that all aquatic organisms studied in
aquaria can absorb PCBs directly from water. The accumulation varies
with the duration of exposure and the concentration in the ambient
water. A diatom exposed to Aroclor 1242 showed an accumulation factor
of 1100 (Keil et al., 1971); with Aroclor 1254 the following values
have been obtained: pink shrimp, 6600; blue crab, 4600; oyster, 8100;
pinfish, 980 (Duke et al., 1970); spot, 37 000 (Hansen et al., 1971);
bluegills, up to 71 400 (Stalling & Mayer, 1972). The accumulation
factor in scud exposed to Aroclor 1254 reached a maximum of 24 000
within 4 days and thereafter remained fairly constant (Sanders &
Chandler, 1972). Similar results were obtained with other
invertebrates, although with crayfish the uptake was slower and the
accumulation was still increasing after 21 days. However, it is
probable that most of the PCBs entering aquatic systems are retained
by particulate matter, and the above accumulation factors are not
necessarily applicable to natural ecosystems. Nimmo et al. (1971)
demonstrated that the fiddler crab could ingest PCB contained in the
bottom sediment. When exposed to Aroclor 1254 in water, ciliated
protozoa, stated to be the major benthic input to aquatic food chains,
had an accumulation factor of 60 (Cooley et al., 1972).
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1 Air
Mean concentrations in air, in several locations in Sweden, ranged
from the detection limit of 0.8 ng/m3-3.9 ng/m3. The highest figure
recorded was 12.5 ng/m3 (Ekstedt & Odén, 1974). In the USA, PCB
concentrations in air ranged from 5 ng/m3 near the north-east coast
to 0.05 ng/m3 at a distance of 2000 m out over the Atlantic Ocean
(Harvey & Steinhauer, 1974). Results from the United States
Environmental Protection Agency indicate a range between 1 and
50 ng/m3 (Panel on Hazardous Trace Substances, 1972), and similar
results have been reported from Japan (Tatsukawa & Watanabe, 1972).
5.2 Soil and Sediments
In Sweden, PCBs have been found in natural soil at a concentration
of 15 µg/kg by Odén & Berggren (1973). The same authors also found
0.006-1.4 mg/kg in sediments from areas in the Baltic Sea with
different degrees of pollution. Nimmo et al. (1971) found PCB levels
of 1.461 mg/kg in sediment from an estuary at a point near the site of
an accidental release of PCBs from a factory, and levels of 0.6 mg/kg
at a point 16 km downstream. Soil samples from the bank 6.5 km
downstream from the source contained 1.4-1.7 mg/kg. Less than 1 mg/kg
has been found in Japanese agricultural soil, but as much as 510 mg/kg
in soil near a factory making electrical components (Fukada, et al.,
1973).
5.3 Water
In heavily contaminated waters, PCB concentrations may be several
times greater than their solubility, owing to adsorption on suspended
particles (Duke et al., 1970). Water in a Swedish river contained
0.5 ng/litre as it entered a water treatment plant, and 0.33 ng/litre
in the tap water produced (Ahling & Jensen, 1970). Values of
0.1-0.3 ng/litre have been measured in other Swedish rivers (Ahnoff &
Josefsson, 1974). Södergren (1973a) found a seasonal variation in the
PCB level in a Swedish lake, with a maximum of 2 ng/litre, the
pollution being attributed to aerial fallout. Concentrations of
10-100 ng/litre have been measured in tap water at Kyoto in Japan
(Panel on Hazardous Trace Substances, 1972) In a polluted coastal
area of Lake Michigan in 1970, PCB concentrations of from 450 to less
than 100 ng/litre were measured in 1970, but there was a marked
decrease in 1971, possibly due to the limitation on sales of PCBs
(Panel on Hazardous Trace Substances, 1972). From the scanty
information on PCBs, reinforced by analogy with the more extensive
information on DDT, it has been estimated that nonpolluted fresh
waters should contain not more than 0.5 ng/litre up to 5 ng/litre for
the Great Lakes of North America, 50 ng/litre for moderately polluted
rivers and estuaries, and 500 ng/litre for highly polluted rivers.
5.4 Living Organisms
There is now considerable information from Canada, Japan, Sweden
the United Kingdom, and the USA, on the accumulation of PCB's
biological material. Analytical measurements on different organisms,
and on the same organism from different localities, vary widely and it
is necessary to consider the factors that lead to this variation.
Difference in analytical techniques may contribute to this (see
section 2.3.5) but important influences are exerted by the extent of
local pollution, amount of fat in the organism studied, and its
trophic stage in food chains.
5.4.1 The influence of local pollution
Most of the fish eaten by man is taken from waters with little
pollution. In a collaborative study by seven national (International
Council for the Exploration of the Sea, 1974), the PCB content of
muscle tissue of fish taken from the North Sea was measured. A mean of
0.01 mg/kg was found in cod, herring contained up to 0.48 mg/kg with
most samples in the range of 0.1-0.2 mg/kg, and plaice contained
0.1 mg/kg or less. Similar values were reported by Zitko (1971) for
fish taken from the North Atlantic.
There are many examples of different PCB levels in similar species
collected from areas of high and low pollution. Jensen, et al. (1972b)
found five times as much PCBs in herrings caught in waters off
industrialized areas near Stockholm, as in herrings from the cleaner
waters of the west coast of Sweden. Similarly, levels in plankton
harvested along the Swedish archipelago at various distances from
Stockholm fell progressively, away from the more polluted areas
(Jensen, et al., 1972c); the concentration in pike fell to one-half
(Olsson & Jensen, 1974). Koeman et al., (1972b) found PCB levels of up
to 88 mg/kg in the blubber of toothed whales caught in the North Sea,
but none was detectable in similar species from New Zealand or
Surinam. Holden (1973b) found high PCB concentrations in the blubber
of seals in polluted coastal areas of the United Kingdom (up to
235 mg/kg), and much lower levels in unpolluted areas (down to
2 mg/kg).
Risebrough & de Lappe (1972) studied the PCB content of
extractable lipids from brown pelican eggs collected from areas
throughout North and South America, and showed that the content varied
from 4 mg/kg up to 266 mg/kg in highly industrialized regions. They
also reported levels greater than 3 mg/kg in fish from New York Sound
and Tokyo Bay, both very polluted areas. Even higher levels of PCBs
have been found in fish from polluted lakes and inland waterways, a
level of 20 mg/kg being found in fish from Lake Ontario, and over
200 mg/kg in fish from the Hudson River (Stalling & Mayer, 1972).
Similar correlations between pollution and PCB levels have been
reported from the United Kingdom in fish (Portmann, 1970), and in
mussels (Holdgate, 1971).
The association between high PCB levels and local pollution may be
disturbed by the migratory habits of certain species, particularly in
birds that may be exposed to PCBs in their wintering areas or on the
migration routes. As much as 400 mg/kg has been measured in the fat of
a robin entering Sweden, although the normal value is about 16 mg/kg.
Many migratory birds start egg-laying on arrival at their summer
quarters, so the PCB content of eggs may reflect the bird's previous
exposure rather than the local pollution (Odsjö, 1973). A special case
concerning the effect of pollution is seen in the use of fish-feed in
poultry and fish farming. Kolbye (1972) stated that this may contain
PCB levels of 0.6-4.5 mg/kg.
5.4.2 The influence of the fat content of tissues
PCBs are mainly stored in body fat (see section 5.5.5.1), and the
total PCB content of the body tissues is much influenced by their fat
content (Portmann, 1970; Westöö & Norén, 1970a). Jensen, et al. (1969)
found PCB levels of 0.27 mg/kg and 0.33 mg/kg respectively, in the
muscle tissue of herring and cod from the same area of the Baltic,
although the cod is at a higher trophic stage (see section 5.4.3).
These two species have 4.4 and 0.32% of extractable fat respectively,
and when the PCB level is calculated on the fat content, values of
6.8 mg/kg for the herring and 11 mg/kg for the cod are obtained. Cod
liver has a much higher fat content than cod muscle and Jensen (1973)
has reported the ratio of PCB concentrations in cod liver and muscle
to be over 100, the maximum in liver being 59 mg/kg. Jensen et al.
(1969) have remarked that the considerable seasonal variation in the
fat content of the herring, rising from 1% in spring to 10% in autumn,
influences the tissue level of PCBs. Peakall et al. (1972) noticed a
marked rise in tissue levels in starved birds owing to the
mobilization of fat, and it is possible that the high levels of PCBs
in the livers of birds dying during the "seabird wreck" in the Irish
Sea were secondary to emaciation (Holdgate, 1971). De Freitas &
Norstrom (1974) showed that, in pigeons, pure PCBs left fatty tissues
and accumulated mainly in muscle during the mobilization of fat
associated with starvation.
5.4.3 The influence of the trophic stage in food
Swedish work on the distribution of PCBs in aquatic ecosystems has
been summarized by Jensen et al. (1972b), and Olsson et al. (1973),
and it has been largely confirmed by work in other areas (Risebrough
et al., 1968; Risebrough & de Lappe, 1972: Holdgate, 1971). Zoo- and
phytoplankton readily absorb or adsorb PCBs from their environment;
Södergren (1972) has demonstrated the rapid uptake of Clophen A50 by
the unicellular alga Chlorella. Marine zooplankton may contain PCB
levels of 5 mg/kg in extractable lipids in areas of moderate pollution
(Jensen et al., 1972c; Williams & Holden, 1973), with somewhat lower
values in relatively unpolluted areas. However, results with plankton,
particularly where high levels are found, must be regarded with
caution as the sample could be contaminated with PCB-rich oil or tar
particles. Herring feeding on plankton in areas of moderate pollution
contain PCB levels of about 0.5 mg/kg in muscle tissue (10 mg/kg in
extractable fat); plaice and flounder, both bottom-feeding fish
contain about one-third of this, presumably because the PCB content of
benthic organisms is lower. In predatory fish such as the cod and
pike, the PCB level in extractable fat is about 10 mg/kg and a mean of
10 mg/kg has been measured in the blubber of seals.
Much higher PCB values have been obtained in fish-eating birds;
levels of 18 mg/kg (650 mg/kg in extractable fat), and 17 mg/kg
(420 mg/kg in extractable fat) were found in the herring gull and
comorant respectively. Lower values were found in birds feeding on
invertebrates, such as the long-tailed duck which contained 14 mg/kg
in fat; marine invertebrates contain PCB levels in the region of
0.1-0.2 mg/kg. At the top trophic level, 96 mg/kg (9.7 g/kg in
extractable fat) has been measured in the eagle owl; the highest
recorded PCB values were from eagle owls found dead in the south-east
coastal region of Sweden, 260 mg/kg in the brain (3.4 g/kg in
extractable fat) and 110 mg/kg in muscle (12 g/kg in extractable fat).
(Odsjö, 1973).
Less information is available on terrestrial ecosystems. PCB
concentrations in the region of 0.01 mg/kg have been found in fresh
tissue in slugs, snakes, and ants, and slightly higher concentrations
in earthworms. Tissue levels were generally at the limit of detection
(0.01 mg/kg) in herbivorous mammals (Odsjö, 1973). Bruggemann et al.
(1974) reported a mean PCB concentration of 0.22 mg/kg in 20 out of 72
measurements in the adipose tissues of the hare and a higher value
(2.5 mg/kg) in 1 out of 5 tests on the adipose tissues of the fox.
Higher values have been found in the American mink in Sweden with
0.58 mg/kg in muscle (45 mg/kg in fat) presumably because of a fish
diet (Odsjö, 1973). Tissue levels in wild birds on a mixed diet are
variable and rather low, but those in predatory birds are higher.
Prestt et al. (1970) related the PCB concentration in the liver of
wild birds to their diet; less than 1 mg/kg was found in insectivorous
birds and more than 70 mg/kg in the sparrow hawk. A level of 0.5 mg/kg
has been found in the muscle of the eagle owl in central Sweden, but
this is much less than the tissue concentrations encountered in this
bird in coastal areas (Odsjö, 1973). High tissue concentrations in
predatory and marine birds have also been reported from Canada
(Gilbertson & Reynolds, 1974), the Netherlands (Koeman et al., 1972a),
and from the United Kingdom (Bourne & Bogan, 1972).
5.4.4 Indicator organisms
Several of the organisms, that have been shown to accumulate-PCBs
from the environment, have been suggested as indicators of the extent
of local pollution with PCBs. In aquatic systems, the use of plankton
as an indicator has the advantage that it is at the lowest trophic
stage of food chains but errors may occur in the determination because
of the inclusion in the sample of nonplanktonic particles with a high
PCB content (Jensen et al., 1972a). The herring, which feeds on
plankton, has been suggested as an indicator (Jensen et al., 1972b)
and, at higher trophic stages, the pike, which is a stationary fish
(Olsson & Jensen, unpublished report, 1974), and seabirds (Jensen et
al., 1972c). In fresh waters, the amphipod Gammarus pulex has been
used as an indicator organism for chlorinated hydrocarbons (Södergren
et al., 1972). However, Zitko et. al. (1974) claimed that the
variation between individual fish was so high that Atlantic herring
and yellow perch could be used to detect trends in pollution only if
large numbers were taken for analysis, with an interval between
measurements of at least 4 years.
A series of monitoring studies has been made by OECD, the species
selected covering terrestrial, fresh water, and marine environments.
The analytical results, and the general problems of selecting species
for monitoring, have been discussed by Holden. (1970a, 1973a, 1973b).
5.5 The Extent of Human Exposure to PCBs and PCTs
5.5.1 Air and water
The maximum concentration of PCBs in air is not likely to exceed
50 ng/m3 (section 5.1). The highest concentration of PCBs reported in
domestic tap water is 100 ng/litre in the Kyoto area of Japan (Panel
on Hazardous Trace Substances, 1972), but levels more likely to be
encountered should not exceed 1 ng/litre (section 5.3).
5.5.2 Food
The PCB content of a variety of foods on the Swedish market been
measured by Westöö & Norén (1970a) and Westöö et al. Less than
0.1 mg/kg was found in samples of butter, margarine, oils, eggs, beef,
lamb, chicken, bread, biscuits, and baby of pork out of more than 100
had a PCB content in the range of 0.5 mg/kg. As might be expected from
the discussion in section 5.4 higher values were found in fish
depending on the fat content and pollution of the fishing area (Westöö
& Norén, 1970a; Berglund, 1972] The PCB levels obtained in an
extensive study by the US Food Administration are shown in Table 5.
These values are considerably higher than but they are probably
biased, as they include samples originating areas previously suspected
of having been subject to local pollution. In a Canadian survey, PCB
levels of less than 0.01 mg/kg were found in eggs (Mes et al., 1974)
and a mean of 0.042 mg/kg was found in domestic and imported cheese
with a maximum of 0.27 mg/kg (Villeneuve et al., 1973b). No traces of
PCTs were found.
In Japan, a similar range of PCB contents for most foods has been
reported; however, some high levels have been-reported for rice and
vegetables harvested in fields polluted with PCBs (Environmental
Sanitation Bureau, 1973). The PCB content of most fish on the market
was less than 3 mg/kg, although some contained more than this. The PCT
content of fish was much lower (Fukano et al., 1974). In the
Netherlands, eel has been reported to contain PCT levels of
0.2-0.5 mg/kg and PCB levels of 4.7 mg/kg (Freudenthal & Greve, 1973).
Table 5. PCB levels in food in the USAa
% positive Level in positive samples (mg/kg)
Food (0.1 mg/kg) Mean Maximum
Cheese 6 0.25 1.0
Milk 7 2.3 27.8
Eggs 29 0.55 3.7
Fish 54 1.87 35.3
a From Kolbye (1972).
Samples of butter from the Westphalian area of the Federal
Republic of Germany, obtained in the period 1972-74, contained PCB
levels of 0.38 mg/kg (range 0.25-0.54 mg/kg) (Claus & Acker, 1975).
Relatively high PCB levels in some packaged foods in Sweden,
mainly of imported origin, could be attributed to migration from the
packaging material (Westöö et al., 1971). The highest level
encountered was 11 mg/kg in a children's breakfast cereal; PCB levels
of 70 mg/kg and 700 mg/kg were found in the material of the inner bag
containing this product and in the outer cardboard container
respectively. Up to 2000 mg/kg was found in cartons of other samples.
Villeneuve et al. (1973a) have analysed packaged food in Canada; they
found that 66.7% of the samples contained PCB levels of less than
0.01 mg/kg, 30.7% contained between 0.01 and 1 mg/kg, and 2.6%
contained more than 1 mg/kg. PCT determinations were also made on
these samples; 94.5% of the samples contained less than 0.01 mg/kg and
5.5% contained 0.01-0.05 mg/kg. The highest PCB levels were
encountered in a rice sample with 2.1 mg/kg where the packaging
material contained 31 mg/kg, and in a dried fruit sample with
4.5 mg/kg in a container containing 76 mg/kg. In a survey of packaging
containers, approximately 80% were found to contain PCB and PCT levels
of less than 1 mg/kg, while about 4% contained levels higher than
10 mg/kg. The most likely source of PCBs in packaging materials is the
recycling of paper waste containing pressure-sensitive duplicating
paper (Masuda et al., 1972).
5.5.3 Occupational exposure
Occupational exposure does not only occur during the manufacture
of PCBs and with their use in the electrical industry. It may also be
widespread among mechanics in contact with lubricating oils and
hydraulic fluids, among workers exposed to varnishes and paints, and
among office workers from contact with pressure-sensitive duplicating
paper, some brands of which readily transfer PCBs to skin (Kuratsune &
Masuda, 1972b). Studies in Finland showed that whole blood from
persons with no special exposure to PCBs contained 0.3-1.2 µg/100 ml,
while blood from persons handling PCBs in an analytical laboratory
contained 3.66.3 µg/100 ml and blood from workers in a capacitor
factory had PCB levels of 7.5-190 µg/100 ml in the blood and
30-700 mg/kg in fat. No signs of toxicity were evident in these workers
(Karppanen & Kolho, 1973). Similar plasma values were found in workers
from Japanese capacitor factories, but here skin lesions were noted
(Hasegawa et al., 1972a). This same study reported that air levels of
PCBs of 0.01-0.05 mg/m3 were measured in a factory where KC-300 was
used in the manufacture of electric condensers. PCB levels in serum in
workers ranged from 10 to 65 µg/100 ml.a One month after the use of
PCBs had been suspended, serum levels still ranged from 9 to
74 µg/100 ml. However, in another factory making electric condensers, serum
levels decreased from an average of 80 µg/100 ml to 30 µg/100 ml
within three months of the use of PCBs being suspended (Kitamura et
al., 1973). According to Hara et al. (1974), the half-time of PCBs in
the blood of workers engaged in the manufacture of electric condensers
for less than 5 years was several months, while that of workers
employed for more than 10 years was 2-3 years. Hammer et al. (1972)
found a higher frequency of measurable plasma values in workers
working with refuse burners than in a control group.
a In this document, the concentrations of PCBs in blood and serum
are expressed in µg/100 ml although in some original papers the
values are given in µg/100 g. For practical purposes the
differences, about 5% and 3% respectively, can be neglected.
5.5.4 Other sources of exposure
Broadhurst (1972) has reviewed the many technical applications of
PCBs that appear in the literature and in patent specifications, and
which indicate the possibility of a widespread nonoccupational
low-level exposure to PCBs, other than that deriving from the diet.
PCBs are used in the home in ballast capacitors for fluorescent
lighting, and exposure deriving from pressure-sensitive copying paper
has not been limited to office workers. The valuable properties of
PCBs as plasticizers has led to their use in furnishings, interior
decoration, and building construction; examples are surface treatment
for textiles, adhesive for waterproof wall coatings, paints, and
sealant putties. PCBs have been used as plasticizers for plastic
materials and in the formulation of printing inks.
5.5.5 Biological indices of human exposure
The only surveys of value have been on body fat, blood, and milk.
5.5.5.1 Body fat
In a survey of 637 fat samples taken at autopsy or during surgery
in the USA, 68.9% contained PCB levels of less than 1 mg/kg, 25.9%
contained 1-2 mg/kg, and 5.2% contained more than 2 mg/kg (Yobs,
1972). A similar distribution was found in a smaller survey by Price &
Welch (1972). In the Kochi area of Japan, a mean PCB level of
2.86 mg/kg was recorded with an upper limit of 7.5 mg/kg; about double
these values were found in the Kyoto area (Nishimoto et al., 1972a,
1972b). Bjerk (1972) reported average PCB levels of 0.9 mg/kg
(1.6 mg/kg on a lipid basis) in adipose tissue, taken at 40 autopsies
in the Oslo area. Curley et al. (1973b) found PCB levels ranging from
0.30 to 1.48 mg/kg in a total of 241 human adipose samples in Japan. A
mean value of 5.7 mg/kg has been reported for 20 samples from the
Federal Republic of Germany (Acker & Schulte, 1970); in a more recent
study, 282 adipose tissue samples from different areas were found to
contain PCB levels of 8.3 mg/kg of adipose tissue on a lipid basis
(Acker & Schulte, 1974). In Austria, a range of PCB levels of
0.3-7.3 mg/kg on a lipid weight basis was found in 32 residents in the
Vienna metropolitan area; an increase of the PCB concentration with
age was not observed (Pesendorfer et al., 1973). Detectable levels of
PCBs were found in only a few of 201 human fat samples in the United
Kingdom and in these, the level did not exceed 1 mg/kg (Abbott et al.,
1972). A survey of 51 human fat samples in New Zealand showed that all
samples contained PCB residues with an average of 0.82 mg/kg (Solly &
Shanks, 1974).
Doguchi et al. (1974) found an average PCT level of 0.6 mg/kg in
human fat with a range of 0.1-2.1 mg/kg. Takizawa & Minagawa (1974)
also found PCT levels of 0.02 mg/kg in human liver ( n = 6),
0.01 mg/kg in the kidney ( n = 2), 0.02 mg/kg in the brain ( n = 3)
and 0.04 mg/kg in the pancreas ( n = 1). In the Netherlands, PCTs
were found in human fat at levels of 0-1 mg/kg (Freudenthal & Greve,
1973).
5.5.5.2 Blood
43% of blood plasma samples from 723 volunteers in the USA showed
the presence of PCBs; the mean value in these was about 0.5 µg/100 ml
(Finklea et al., 1972). Studies in Finland on whole blood showed
0.31-1.2 µg/100 ml in persons with no special exposure to PCBs
(Karppanen & Kolho, 1973). In Japan, an average PCB level of
0.32 µg/100 ml and a PCT level of 0.5 µg/100 ml have been recorded in
the blood of non-occupationally exposed volunteers (Doguchi & Fukano,
1975). Inpatients with severe weight loss, high levels of PCBs in the
blood (up to 10 µg/100 ml) were noted (Hesselberg & Scherr, 1974).
This was attributed to the release of PCBs from the mobilization of
fat.
5.5.5.3 Human milk
PCB concentrations measured in whole human milk in Sweden were
0.014 mg/litre in 1967 and 0.025 mg/litre in 1971-72 (Westöö & Norén,
1972), 0.03 mg/litre in Japan Nishimoto et al., 1972a), 0.103 in the
Federal Republic of Germany (Acker & Schulte, 1970) and 0.02 mg/litre
in Canada (Musial et al., 1974) A survey in Colorado, USA, revealed 8
positive samples out of 39, within the range of 0.04-0.1 mg/kg (Savage
et al., 1973).
5.5.6 Estimated daily intake
From the PCB levels encountered in air and drinking water (section
5.5.1), the daily intake from each of these sources is likely to be
less than 1 µg.
It has been stated that the major part of the human dietary intake
of PCBs is from fish (Berglund, 1972; Hammond, 1972). This may well be
true in areas such as Japan or certain localities near the North
American Great Lakes, where fish from polluted waters may form a
relatively large part of the diet. Several investigators from Japan
have measured the daily intake of PCBs in food; the highest mean value
recorded was 48 µg/day, of which 90% was from fish (Kobayashi, 1972);
the lowest was 8 µg/day (Ushio et al., 1974).
In much of Europe and North America, however, the daily intake of
fish is in the region of 30-40 g and most of the fish is taken from
waters of low pollution and contains PCB levels of not more than
0.1 mg/kg. Berglund (1972) has estimated that the daily intake of PCBs
from fish in Sweden is in the region of 1 µg, though if the fish
consumed were solely Baltic herring, the intake would be about 10 µg.
It is difficult to make an assessment of the PCB intake from foods
other than fish. Westöö et al. (1971, 1972), in their extensive study
of the Swedish diet, reported that most foods contained PCB levels of
less than 0.1 mg/kg; it may be concluded that this corresponds to a
daily intake of less than 100 µg. The conclusion that foods other than
fish may make a greater contribution to the PCB content of the diet
can be drawn from the survey in the USA reported by Kolbye (1972), and
also from a Swiss study of three different types of home-prepared
meals, not containing fish, which were found to contain 6, 41, and
84 µg of PCBs, respectively (Zimmerli & Marek, 1973).
The figures for the PCB content of human milk (section 5.5.5.3)
indicate that most nursing mothers excreted about 30 µg/day by this
route, and in some areas up to 100 µg/day. It may be assumed that only
a portion of the PCBs absorbed was excreted by this route, so that the
daily intake could have been more than 100 µg.
It may be concluded that, in the more industrialized countries,
the average daily PCB intake from the diet has rarely been less than
5 µg or greater than 100 µg; it is likely that the non-dietary sources
of exposure detailed in section 5.5.4 have made a significant
contribution, but this cannot be estimated at this time. In any area,
the intake depends not only on the diet, but also on social domestic
and environmental conditions; the influence of these factors on the
daily intake cannot readily be quantified.
6. METABOLISM
6.1 Absorption
The experiments of Vos & Beems (1971) who applied several
commercial PCBs to rabbit skin and found systemic effects (see section
7.1.1.4) indicate that PCBs can penetrate the skin. Early cases of
human poisoning from occupational exposure were probably due to a
combination of skin absorption and inhalation. Experimental studies on
rats by Benthe et al. (1972a) showed that an aerosol containing
Aroclor 1242 (particle size 0.5-3.0 µm) was readily absorbed through
the lungs.
Although one means of entry of PCBs into aquatic food chains is
through the consumption of plankton by fish, aquatic organisms can
also absorb PCBs in solution in the ambient water, presumably mainly
through the gills (see section 4.3). Salmon eggs can also absorb PCBs
from water (Johansson et al., 1970), and Södergren & Svensson 1973
concluded that mayfly nymphs could take in PCBs from water through the
gills and the integument.
Recent work with chlorobiphenyl isomers administered orally to
rodents at levels up 100 mg/kg of body weight for lower chlorinated
compounds and up to 5 mg/kg for the higher chlorinated compounds,
showed that 90% of the compounds were rapidly absorbed (Albro &
Fishbein, 1972; Berlin et al., 1973; Melvås & Brandt, 1973).
Cholestyramine, a basic anion exchange resin, was shown to interfere
with intestinal absorption of KC-400 in mice (Tanaka & Araki, 1974).
PCTs have been shown to be absorbed from the gut (Sosa-Lucero et
al., 1973) but very little information is available on the rate of
absorption.
6.2 Tissue Distribution of PCBs
Grant et al. (1971a) demonstrated that 4 days after an oral dose
of Aroclor 1254 at 500 mg/kg was given to rats, the concentrations of
PCBs in fat, liver, and brain were 996, 116, and 40 mg/kg,
respectively. Similar results showing that the highest concentration
was in fat, were obtained in rats given Aroclor 1254 in the diet
(Curley et al., 1971), in boars (Platonow et al, 1972), cows (Platonow
& Chen, 1973), and in pigeons and quail (Bailey & Bunyan, 1972). In
the experiments of Curley et al. (1971), the tissue concentrations
initially showed a rapid rise and thereafter a slow increase while the
PCB diet was being administered; Grant et al. (1974) fed diets
containing Aroclor 1254 at 0.2, 20, and 100 mg/kg to rats for 8
months, during which period the tissue concentrations reached a steady
state that was dose-dependent (Table 6). Similar tissue distribution
data for Aroclors 1016 and 1242 have been reported by Burse et al.
(1974) and for Kanechlor-400 by Yoshimura et al. (1971). PCB
deposition, in general, depends on the fat content of the tissue
(section 5.4.2). Residues in trout, receiving Aroclor 1254 in doses of
15 mg/kg in the diet, stabilized after 16 weeks while the absolute
quantity continued to increase as the fish grew (Lieb et al., 1974).
More detailed information on the tissue distribution of PCBs and
their metabolites has been obtained by the administration of pure
14C-labelled compounds, using both whole-body autoradiography and
scintillation counting of tissue samples. Berlin et al. (1975)
demonstrated that after a single oral dose of 14C-labelled
2,5,2',4',5'-pentachlorobiphenyl, radioactivity rapidly entered the
circulation of mice and was distributed in the tissues, particularly
in the liver, kidneys, lungs, and adrenals. Subsequently, the
radioactivity in the body fat increased, rising to a maximum within
4-24 h. In most other tissues the radioactivity decreased rapidly
after dosing, but the authors noted a special affinity for the skin,
the bronchiolar epithelium of the lungs, and certain glandular
secreting tissue. Soon after administration of the dose, radioactivity
appeared in bile and was excreted in the faeces. Similar results were
obtained by Melvås & Brandt (1973) with 2,4,2',4'-tetrachlorobiphenyl
in the mouse, and with 2,4,2',3'- and 2,4,3',4'-tetrachlorobiphenyls
in the quail; in the mouse they found a high affinity for the adrenal
cortex, the corpora lutea, and glandular secreting tissue, and in the
quail the radioactivity in egg yolk was high, exceeding that in fat.
Brandt & Ullberg (unpublished report 1973) found a similar pattern
after administration of hexa- and octachlorobiphenyls to mice.
6.3 Tissue Distribution of PCTs
Diets containing Aroclor 5460 at levels of 10, 100, and 1000 mg/kg
were administered to rats for 7 days (Sosa-Lucero et al., 1973). Table
7 shows the tissue distribution obtained in this study in rats fed
with Aroclor 5460 at 100 mg/kg body weight and the values in rats fed
with Aroclor 1254 at 100 mg/kg body weight in a similar study (Curley
et al., 1971). After oral administration of Aroclor 5460 to the cod,
the concentration of PCTs in the liver was more than 100 times that in
muscle on a wet weight basis (Addison et al., 1972), a ratio found by
Jensen et al. (1973) for PCBs in the cod.
Table 7. Tissue distribution (mg/kg wet weight) of PCTs (Aroclor
5460) in rats fed dietary levels of 100 mg/kg for 7 days
(Sosa-Lucero et al., 1 973) and fed PCB (Aroclor 1254) at
100 mg/kg for 9 days (Curley et al., 1971)
Aroclor Aroclor
Tissue 5460 1254
Blood 1.32 0.1
Liver 47 6
Brain 5.1 4
Kidneys 15.1 5
Heart 21.5 --
Fat -- 180
6.4 Placental Transport
Aroclors 1221 and 1254 were found to cross the placenta of
rabbits, when administered orally to does during gestation. The
concentration in fetal tissues was dose-dependent and much less with
Aroclor 1221 than with Aroclor 1254; with the latter, the
concentration in the fetal liver was greater than that in the maternal
liver (Grant et al., 1971b). Curley et al. (1973a) found some
placental transport of Aroclor 1254 in the rat. Platonow & Chen (1973)
demonstrated that the PCBs in the fetal kidney of a cow dosed with
Aroclor 1254 was greater than that in the mother. Placental transfer
of polychlorinated biphenyls has also been reported in the mouse
(Berlin et al., 1975; Melvås & Brandt, 1973; Brandt & Ullberg,
unpublished report 1973).
PCB concentration in human umbilical blood has been shown to be
about 25% of that in maternal blood (Taki et al., 1973). Placental
transfer of PCBs was observed in Yusho patients (Tsukamoto et al.,
1969).
No information is available on the placental transfer of PCTs.
6.5 Excretion and Elimination
6.5.1 Milk
Saschenbrecker et al. (1972) found that after oral administration
of doses of Aroclor 1254 of 10, and 100 mg/kg in diet to cows, 6.27
and 74.5 mg/litre, respectively, appeared in the milk after 24 hours.
These levels were reduced to less than one-half within 3 days but
traces remained at 50 days. Cows receiving 200 mg of Aroclor 1254
daily reached a steady state concentration of 61 mg/kg in milk fat and
42 mg/kg in body fat after 10 days (Fries et al., 1973). The PCBs in
milk survived processing into dairy products, and most was located in
milk fat (Platonow et al., 1971). PCBs have also been found in human
milk (see section 5.5.5.3).
6.5.2 Eggs
Several investigations have demonstrated the presence of high
levels of PCBs in the eggs of seabirds (Riseburgh & de Lappe, 1972).
An incident has been reported in which PCB contamination of poultry
food was toxic to chickens and decreased the hatchability of eggs
which also contained PCB residues (Pichirallo, 1971). In a laboratory
study, Scott et al. (1975) fed Aroclor 1248 to chickens at dietary
concentrations of 0, 0.5, 1.0, 10, and 20 mg/kg. After 8 weeks, the
approximate PCB levels in eggs were 0, 0.22, 0.41, 3.1 and 7.0 mg/kg
respectively. Jensen & Sundström (1974) found 33% of ah oral dose of
2,4,5,T,4',5'-hexa-chlorobiphenyl administered to quails was excreted
in eggs over a period of 10 days; eggs also provided a major route of
excretion in the pheasant (Dahlgren et al., 1971).
6.5.3 Urine and faeces
All investigators agree that only traces of PCBs can be found in
the urine of dosed animals, and that faeces provide a major route of
elimination. When the analysis of faeces is limited to the
determination of unchanged PCBs, the recovery of the dose administered
is incomplete; in boars receiving single or repeated doses of Aroclor
1254, not more than 16% of the dose was recovered from the faeces and
less than 1% in urine (Platonow et al., 1972). Better recoveries have
been obtained with PCB labelled with radioactive isotopes. Yoshimura
et al. (1971) found 70% of the activity from a dose of tritium-
labelled Kanechlor 400 in faeces and 2% in urine over a 4-week period.
Berlin et al. (1974, 1975) found over 75% of the activity from
14C-labelled penta- and hexachlorobiphenyls in faeces and less than
2% in urine; most of the faecal excretion consisted of PCB metabolites
(see section 6.6.1). Similar results were obtained by Melvås & Brandt
(1973) with tetrachlorobiphenyls.
6.6 Biotransformation
6.6.1 Metabolic degradation
Most investigators studying the tissue distribution of PCBs after
administration of commercial mixtures have noted a relative reduction
of the gas-liquid chromatographic peaks with shorter retention times,
corresponding to the lower chlorinated biphenyls. This has been
reported in the rat (Grant et al., 1971a; Curley et al., 1971), the
rabbit (Grant et al, 1971b), the cow (Platonow & Chen, 1973), and in
pigeons and quails (Koeman et al., 1969; Bailey & Bunyan, 1972).
Samples of tissues from animals and man (see table 3, pp. 20-21) that
have absorbed PCBs from the environment have shown, on analysis, a
pattern of peaks approaching that of PCB mixtures with more than 50%
chlorination, although the major manufactured products contain 42% of
chlorine or less. This has led to the belief that the rate of
metabolic attack on PCBs decreases with increasing chlorination.
Studies on single PCBs with 1, 2, 4, or 5 chlorine atoms have shown
that these are more readily excreted as metabolites in faeces by
mammals and birds and remain for a shorter time in fatty tissues than
most PCBs with 6 or more chlorine atoms (Berlin et al., 1975;
Hutzinger et al., 1972a; Melvås & Brandt, 1973; Brandt & Ullberg,
unpublished report 1973). See also section 6.6.2.
The administration to rats of diets containing Aroclors 1016 or
1242 at a concentration of 100 mg/kg resulted in a steady state in
adipose tissue for both compounds in about 4 months. When gas
chromatographic traces were compared with those for standard PCB
mixtures a difference in the gas-liquid chromatographic pattern, that
is disappearance of the peaks with short retention times was noted.
After exposure to PCBs was discontinued, a major portion of the PCBs
was eliminated from the body in 4 months. However, 20% of the total
PCBs in Aroclor 1242 with longer retention times were present after 6
months, and 10% of the total PCBs of Aroclor 1016 with longer
retention times were present in adipose tissue after 5 months. (Burse
et al., 1974).
The excretion of monohydroxy metabolites of 3,4,3',4'-tetrachloro-
biphenyl and 2,4,3',4'-tetrachlorobiphenyl (orally administered) in
rats has been demonstrated by Yoshimura & Yamamoto (1973). Yoshimura
et al. (1973), Yamamoto & Yoshimura (1973), Yoshimura & Yamamoto
(1974), and Yoshimura et al. (1974). They demonstrated that the
metabolites of the first isomer were 2-hydroxy or 5-hydroxy compounds
while the metabolites of the second isomer were 5-hydroxy and
3-hydroxy compounds. All hydroxy metabolites were excreted
non-conjugated via the bile and no parent isomers were found in the
bile. Yoshimura & Yamamoto (1975) found that unchanged
2,4,3',4'-tetrachlorobiphenyl was excreted through the intestine, when
it was intravenously injected in rats with the bile duct ligated,
while no metabolite of this isomer was excreted by this route.
Hutzinger et al. (1972a) demonstrated the presence of hydroxylated
derivatives of mono-, di-, and tetrachlorobiphenyls in the excreta of
rats and pigeons but not of trout and were unable to detect
hydroxylated 2,4,5,2',4',5'-hexachlorobiphenyl. Berlin et al. (1975)
isolated a hydroxy derivative of 2,5,2',4',5'-pentachlorobiphenyl from
mouse faeces, and Jensen & Sundström (1975) have shown that although
2,4,5,2',4',5'-hexachlorobiphenyl is excreted very slowly, a hydroxy
derivative could be detected in rat faeces. Hutzinger et al. (1974),
however, showed that this compound was also dechlorinated by the
rabbit and excreted as the hydroxy derivative of pentachlorobiphenyl.
Gardner et al. (1973) detected hydroxylated metabolites in the urine
of rabbits dosed with 2,5,2',5'-tetrachlorobiphenyl together with a
dihydroxy derivative which they regarded as evidence for the formation
of an arene oxide (epoxide) intermediate (see section 6.5.3). Jansson
et al. (1975) have identified up to 26 mono- and dihydroxylated
metabolites of PCB in the bile and faeces of wild grey seal and
guillemot from the Baltic.
There is little information on the biotransformation of PCTs.
Addison et al. (1972), using gas-liquid chromatography, noted a loss
of PCTs with a shorter retention time in the excreta of a cod dosed
orally with Aroclor 5460; the same loss was observed in rat faeces
after the administration of a diet containing Aroclor 5460
(Sosa-Lucero et al., 1973).
6.6.2 The effect of structure on retention
While each of the components of mixtures of PCBs has a different
pattern of retention and elimination in different species, the
measurement of biological half-times for PCB mixtures in tissues has
provided useful information. Bailey & Bunyan (1972) found the
half-time of PCBs in the fat of quail and pigeon, after cessation of
dosing with Aroclor 1242, or with Aroclor 1254, to be 50 days and 125
days, respectively. A half-time of about 200 days was recorded in the
fat of rats after feeding with Aroclor 1254 (Grant et al., 1974).
Berlin et al. (1975) noted that in mice dosed with a
pentachlorobiphenyl there was an initial fairly rapid elimination
while the PCB level in the liver was high, followed by a slower
elimination when most of the PCB was located in fat. It seems likely
that the mobilization of PCBs from fat, and therefore their half-time
in the body, depends upon their rates of metabolism. Berlin et al.
(1974) investigated the hypothesis that the ability of a PCB to be
readily degraded with a half-time of a few days depended upon the
presence of two adjacent unsubstituted carbon atoms in the molecule
rather than on the number of chlorine atoms, although the presence of
such unsubstituted pairs depends to a large extent on the degree of
chlorination. They came to the conclusion that this hypothesis
probably applied to unsubstituted pairs in the 3,4-position, but that
in the 2,3-position, their susceptibility to metabolic degradation was
much influenced by the presence of chlorines in the o-position of
the ring bridge.
Jensen & Sundström (1974) demonstrated that the retention of PCBs
in human fat is also influenced by o-chlorine substitution (Table 3,
pp. 20-21 ).
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF PCBs AND PCTs
7.1 Toxic Effects in Different Species
Most of the available information on the toxicity of the PCBs has
been obtained from studies on commercial mixtures. The much smaller
amount of information available concerning the impurities in
commercial products and PCTs, is given in sections 7.2 and 7.3.
7.1.1 Mammals
7.1.1.1 Acute oral and intravenous toxicity
Earlier work by the Monsanto Company indicated a low acute oral
toxicity for the Aroclors, the LD50s to rats ranging from 4.0 g/kg
for Aroclor 1221 to 11.3 g/kg for Aroclor 1262 (Panel on Hazardous
Trace Substances, 1972). More recent work has demonstrated a slightly
higher toxicity (Bruckner et al., 1973; Grant & Philips, 1974). With a
single intravenous dose the LD50 for Aroclor 1254 was 358 mg/kg body
weight in adult female Sherman rats (Linder et al., 1974). The acute
oral LD50 for Aroclor 1254 in the same strain and sex was between 4
and 10 g/kg (Kimbrough et al., 1972). According to Bruckner et al.,
(1973), severely poisoned animals showed weight loss, ataxia,
diarrhoea, and chromodacryorrhoea, and they considered progressive
dehydration and central nervous depression were the causes of death.
In rats, vacuolation in the liver and kidneys was observed (Bruckner
et al., 1973) and also ulceration of the gastric and duodenal mucosa
(Kimbrough et al., 1972).
Yamamoto & Yoshimura (1973) found the intraperitoneal LD50 of
2,4,3'4'-tetrachlorobiphenyl in mice to be 2.15 g/kg body weight and
that of the 5-hydroxy derivative, which is the main in vivo
metabolite, to be 0.43 g/kg body weight.
7.1.1.2 Subacute oral toxicity
After repeated administration, the PCBs have a cumulative toxic
action. In a group of rats receiving Aroclor 1254 at a dose of 1 g/kg
of diet, deaths occurred between the 28th and 53rd days of feeding
(Tucker & Crabtree, 1970), and with Phenochlor DP6 at 2 g/kg of diet,
deaths occurred between the 12th and 26th days (Vos & Koeman, 1970).
In the latter experiment enlarged livers, small spleens, and a
progressive chemically-induced hepatic porphyria were seen at autopsy.
Repeated weekly oral administration of 150 mg of Aroclors 1221, 1242
or 1254 to rabbits for 14 weeks produced liver enlargement and damage
with Aroclor 1242 and no effect with Aroclor 1221 (Koller & Zinkl,
1973). Allen et al. (1974) administered diets containing Aroclor 1248
at the concentration of 25 mg/kg of diet to 6 female rhesus monkeys
for two months; facial oedema, loss of hair, and acne developed after
1 month and one animal died with severe gastritis 2 months after
removal from experimental diet. PCB concentrations in the body fat of
the animals, after two months of treatment, averaged 127 mg/kg while 8
months later the value declined to 34-mg/kg.
Mink appear to be unusually sensitive to PCBs. Aulerich et al.
(1973) administered diets containing PCB levels of 30 mg/kg (10 mg/kg
each of Aroclors 1242, 1248, and 1254) to adult mink and demonstrated
100% mortality within 6 months. PCB residues in the brains of these
mink averaged about 11 mg/kg and were approximately twice the level
observed in other tissues. Four months of feeding Aroclor 1254 at
levels of 5 and 10 mg/kg demonstrated a dose-dependent retardation of
weight gain of growing female mink. Female mink fed a diet
supplemented with Aroclor 1254 at 5 mg/kg for 9 months failed to
produce offspring (Ringer et al., 1972).
7.1.1.3 Chronic oral toxicity
Aroclors 1242, 1254, and 1260 have been administered for 18 months
to rats at 1, 10 and 100 mg/kg in the diet (Keplinger et al., 1971).
No adverse effects were recorded with the three Aroclors at 10 mg/kg
but with Aroclors 1242 and 1254 at 100 mg/kg there was an increase in
liver weight and a reduced survival of litters. In similar experiments
on dogs, there was a reduced weight gain with Aroclors 1254 and 1260
in the diet at a level of 100 mg/kg (Keplinger et al., 1971). In the
experiments reported by Kimbrough et al. (1972), male rats survived
Aroclor 1260 in the diet at 1 g/kg (71.4 mg/kg body weight) for 8
months but 8/10 females died at this dose, 2/10 died at 500 mg/kg, and
1/10 at 100 mg/kg (7.2 mg/kg body weight). With Aroclors 1254 and
1260, a dose-dependent increase in liver weight in male rats was
significant down to 20 mg/kg (1.4 mg/kg body weight) in the diet; with
females the liver enlargement occurred only at diet levels of
500 mg/kg and higher. The livers showed an orange fluorescence, the
cells were enlarged and vacuolated with lipid inclusions; there was
also much increased smooth endoplasmic reticulum, and what was termed
"adenofibrosis" was present, being more marked with Aroclor 1254 (see
section 7.8). Grant et al. (1974) fed diets containing Aroclor 1254 at
0, 2, 20, and 100 mg/kg to rats for 246 days followed by 180 days on a
PCB-free diet; after 246 days the body weight of the 100 mg/kg rats
was significantly less than that of the controls and the liver weight
was greater. The most notable change in the livers on histological
examination was the appearance of fat microdroplets in the
centrilobular region; this effect was dose-dependent and not seen in
the rats receiving the 2 mg/kg diet and was reversible when the rats
were returned to a normal diet.
Liver enlargement has been described by a number of authors, in a
number of species, with different PCB mixtures and pure isomers and it
is considered to be due primarily to hypertrophy of the smooth
endoplasmic reticulum of the liver cells (Vos & Beems, 1971; Allen et
al., 1973; Kimbrough, 1974; Nishizumi, 1970) but with large enough
doses (oral intubation of about 150 mg/kg body weight/week for 14
weeks) of Aroclor 1254 and 1242, it progressed to frank liver damage
(Koller & Zinkl, 1973). The smooth endoplasmic reticulum may condense
in the liver cell and form hyalin inclusions and this may be
accompanied by a loss of enzyme activity. Lipid accumulation, pigment
deposition, nuclear changes, and necrosis may also occur (Vos &
Notenboom-Ram, 1972).
The rhesus monkey is the only species reported to show signs of
poisoning similar to those in human Yusho patients (see section 8).
The administration of Aroclor 1248 at 2.5 and 5.0 mg/kg of diet for 1
year produced periorbital oedema, alopecia, erythema, and acneiform
lesions involving the face and neck within 1-2 months. The effects
were less marked in male monkeys. At 25 mg/kg of diet, one out of a
group of six died, and at 100 and 300 mg/kg the mortality approached
100% within 2-3 months. Animals more severely affected showed
hypertrophic hyperplastic gastritis with ulceration, anaemia,
hyperproteinaemia and bone marrow hypoplasia. The survivors still
showed signs of poisoning 8 months after exposure (Allen & Norback,
1973; Allen et al., 1974; Allen 1975).
7.1.1.4 Dermal toxicity
Vos & Beems (1971) have confirmed earlier reports that PCBs damage
the follicular epithelium in experimental animals. They applied three
commercial 60% chlorinated mixtures, Clophen A60, Phenochlor DP6, and
Aroclor 1260 to rabbit skin at a daily dose of 118 mg/50 cm (5 times
per week) for 38 days. After initial reddening, transverse wrinkling
developed with hyperplasia and hyperkeratosis of the epidermal and
follicular epithelium. These effects were more marked with Clophen and
Phenochlor than with the Aroclor.
During these experiments, deaths occurred in the Clophen- and
Phenochlor-treated groups but not in the Aroclor group. Kidney lesions
were seen in all groups; liver damage was least in the Aroclor group.
There was atrophy of the thymus cortex and a reduction of germinal
centres of the lymph nodes as well as lymphopenia, and some animals in
all groups showed oedema of the abdominal and thoracic cavities,
subcutaneous tissue, and pericardium. Faecal excretion of copro- and
protoporphyrins was increased by all three PCBs but was lowest with
Aroclor 1260. Vos & Beems attributed the greater severity of the
effects observed with Clophen and Phenochlor to the presence of toxic
impurities (see section 7.2). In another comparative dermal toxicity
study in rabbits (Vos & Notenboom-Ram, 1972), skin lesions in Aroclor
1260-treated animals were more severe than in animals treated with
2,4,5,2',4',5'-hexachlorobiphenyl. In a Japanese study, no differences
in skin lesions were observed in rabbits after dermal applications of
Kanechlor 400, Kanechlor 500, or 3,4,3',4'-tetrachlorobiphenyl
(Komatsu & Kikuchi, 1972).
7.1.1.5 Inhalation toxicity
Only one inhalation study has been reported using Aroclor 1242 and
1254 (Treon et al., 1956). Rats, mice, rabbits, and guinea pigs were
exposed to Aroclor 1242 or 1254 vapours for five days a week for
several weeks at concentrations ranging from 1.5 to 8.6 mg/m3. At
these concentrations Aroclor 1254 produced liver enlargement in rats.
7.1.2 Birds
The acute oral toxicity of Aroclors 1242 and 1254 is low for the
mallard duck, the LD50s being greater than 2 g/kg body weight (Tucker
& Crabtree, 1970). Published figures for the lethal dose for birds
after repeated administration are very variable; it appears to be
dependent on the species and age of the bird, the method of
administration, the degree of chlorination of the PCBs, and the
presence of impurities (Vos, 1972). Heath et al. (1970) determined the
dietary concentrations of Aroclors 1232 to 1262, administered over a
5-day period, that were required to kill 50% of groups of mallards,
pheasants, and quails. The concentrations were in the range of 500 to
over 5000 mg/kg, with the bobwhite quail the most sensitive and the
Japanese quail the least. There was a positive relationship between
the percentage of chlorine in a technical Aroclor and its toxicity.
The relationship held true for those containing less than 60%
chlorine, Aroclor 1260 and 1262 deviating slightly. Mallards were
relatively less responsive to chlorine content than the three
gallimaceous species. Prestt et al. (1970) found a 50% mortality in
Bengalese finches receiving a daily oral dose of Aroclor 1254 at
254 mg/kg body weight for 56 days; cormorants have been killed with a
cumulative dose of 5.7 g of Clophen A60, but herons were more
resistant (Koeman et al., 1973).
The toxicity to chickens of diets containing Phenochlor DP6,
Clophen A60, or Aroclor 1260 at 400 mg/kg has been studied by Vos &
Koeman (1970). The first two produced a 100% mortality with survival
times of 24.3 and 20.5 days, but there was only 15%o mortality with
the Aroclor over the 60-day test period. The authors demonstrated the
presence of impurities in the Phenochlor and Clophen (Vos et al.,
1970). The results of Flick et al. (1965) and Platonow & Funnel (1971)
indicate that chickens can survive 200 mg/kg in the diet for several
months, but that deaths may occur at 250 mg/kg, though Rehfeld et al.
(1971) found a much higher toxicity, recording 1/30 deaths with
Aroclor 1248 at 30 mg/kg of diet over 25 days and 16/30 at 50 mg/kg.
Keplinger et al. (1971) found decreased growth in chickens receiving
Aroclor 1242 at 10 mg/kg and Aroclor 1254 at 100 mg/kg of diet, but no
effects with Aroclor 1260 at 100 mg/kg. Birds severely poisoned with
PCBs show tremors, ataxia, and ruffling and loss of feathers. Oedema
of the abdominal and peritoneal cavities has been a characteristic
sign at autopsy in some experiments (Flick et al., 1965) and this was
observed in a very large number of chickens killed in Japan by the
contamination of their feed with Kanechlor KC400 (Kohanawa et al.,
1969a, 1969b). Enlargement of the kidneys and sometimes of the liver
has been reported; some authors have noted a reduction in the size of
the spleen, comb, and testes, an enlargement of the adrenals and
thyroid, and a pale pancreas. No pathological signs were observed by
Dahlgren et al. (1972) that could account for the deaths of pheasants,
which occurred with a cumulative intake of about 900 mg of Aroclor
1254 (daily oral administration of 20 or 200 mg to 11-week-old hens);
they found a mean concentration of 520 mg/kg in brain tissue at death
and it is possible that effects in the central nervous system were a
contributory cause.
7.1.3 Aquatic organisms
7.1.3.1 Fish
Stalling & Mayer (1972) report an oral LD50 of more than 1.5 g/kg
for rainbow trout with Aroclors 1242 and 1260. A 15 mg/kg oral dose to
cod affected their ability to maintain an upright position in rotating
water (Lindahl, unpublished report 1974).
The assessment of toxicity to fish by adding PCBs to aquarium
water is subject to considerable error on account of the different
solubilities of the components. Zitko (1970) claims the aqueous
solubility of Aroclor 1221 to be 3.8-5 mg/litre and that of Aroclor
1254 to be 0.3-0.5 mg/litre. Stalling & Mayer (1972) report the
96-hour LC50s on the cut-throat trout to be 1.17 mg/litre for Aroclor
1221 and up to 60 mg/litre for Aroclor 1260, where the solubility is
clearly exceeded. In the more prolonged experiments of these authors
(Table 8), most of the concentrations are within the solubility limits
and therefore more reliable. In more prolonged experiments, Hansen et
al. (1971) found deaths in fish exposed for up to 45 days to Aroclor
1254 at 5 µg/litre, but none at 1 µg/litre. These results show that
the effect of PCBs is cumulative in fish and that the toxicity
decreases with increasing chlorination.
Table 8. Intermittent-flow bioassays of Aroclors against three species of fisha
LC50 (µg/litre)
5 10 15 20 25 30
Aroclor Species days days days days days days
1254 Rainbow trout 156 8 -- -- -- --
1260 -- 240 94 21 -- --
DDT 2.26 0.87 0.26 -- -- --
1242 Bluegills 154 72 54 -- -- --
248 307 160 76 10 -- --
1254 -- 443 204 135 54 --
1260 -- -- -- 245 212 151
1242 Channel catfish -- 174 107 -- -- --
1248 -- 225 127 -- -- --
1254 -- __ 741 300 113 --
1260 -- -- -- 296 166 137
1248b Bluegills 137 76 -- -- -- --
1248c Channel catfish -- 94 57 -- -- --
a From Stalling & Mayer (1972).
b Temperature, 20°C; alkalinity 260 mg/litre pH 7.4.
c Temperature, 27°C.
Young fish appear to be more sensitive to PCBs than adults,
96-hour LC50s for newly hatched fathead minnows were 15 and
8 µg/litre, respectively for Aroclors 1242 and 1254. Growth of young
fathead minnows and flagfish was affected above 2.2 µg/litre (Nebeker
et al., 1974). There is no information on pathological changes in fish
that might be related to the lethal action of PCBs; Hansen et al.
(1971) found that fish survived exposure to Aroclor 1254 at
5 µg/litre, but died later, after being returned to clean water, with
signs of a lowered resistance to infection. Pathological changes were
observed in kidney, spleen, and liver of rainbow trout fed Aroclor
1254 at 10 or 100 mg/kg of diet for up to 330 days (Nestel & Budd,
1975).
7.1.3.2 Aquatic invertebrates
The results of several investigations into the toxicity of PCBs to
aquatic invertebrates have been reported by Stalling & Mayer (1972).
The exposure of a variety of scud (Gammarus pseudolimnaeus) to PCBs
showed a decreased toxicity with increasing chlorination; 4-day LC50s
of 10, 52, and 2400 µg/litre were obtained with Aroclors 1242, 1248
and 1254, respectively. The threshold for survival, growth, and
reproduction of Gammarus pseudolimnaeus exposed to Aroclor 1248 was
about 5 µg/litre and was the same for Daphnia magna. With the
crayfish, 7-day LC50s were 30 µg/litre (Aroclor 1242) and 80 µg/litre
(Aroclor 1254) and with the glass shrimp, 3 µg/litre (Aroclor 1254).
Wildish (1970) found that mortality in Gammarus oceanicus was
dependent on the duration of exposure to Aroclor 1254 at levels above
10 µg/litre. Stalling & Mayer (1972) reported the 15-day LC50 of
Aroclor 1254 for immature pink shrimp to be 0.94 µg/litre.
7.1.3.3 Microorganisms
The growth of certain marine diatoms is inhibited by Aroclor 1254
at 10-25 µg/litre, but marine and freshwater algae are more resistant,
being unaffected by 100 µg/litre (Mosser et al., 1972). Fisher et al.
(1972) found that phytoplankton from the Sargasso Sea did not grow in
Aroclor 1254 at 10 µg/litre, though phytoplankton from estuarine and
coastal waters were not much affected by this concentration. Keil et
al. (1971) report that the growth of a diatom was inhibited by Aroclor
1242 at 100 µg/litre with a reduction of RNA synthesis, but that
10 µg/litre had no effect.
The growth of cultures of lake bacteria was not inhibited by
concentrations of Aroclors 1221, 1242, and 1254 in excess of
solubility, and Aroclors 1221 and 1242 could be utilized as the sole
source of carbon and energy (Wong & Kaiser, 1975).
7.2 Toxicity of Impurities in Commercial PCBs
Vos & Koeman (1970) observed that Phenochlor DP6 and Clophen A60
were more toxic to chickens than was Aroclor 1260 (see section 7.1.2)
and Vos & Beems (1971) showed a similar difference in the dermal
toxicity to the rabbit (see section 7.1.1.4). Vos et al. (1970)
subdivided Clophen A60 and Phenochlor DP6 into non-polar PCBs and
polar fractions and found polar components that were not detectable in
a similar fraction from Aroclor 1260. Phenoclor DP6, Clophen A60, and
the polar fraction from Clophen A60 produced a high mortality in the
chick embryo test but the polar fraction from Aroclor 1260 did not. A
difference between the three fractions was seen in the development of
skin lesions in the rabbit (Vos & Beems, 1971). Mass spectrographic
analysis indicated that the impurities were tetra- and pentachloro-
dibenzofurans. Additional contaminants were chlorinated naphthalenes.
Vos et al. (1970) calculated that the maximum level of chlorinated
dibenzofurans in Clophen A60 was 5 mg/kg and in Phenochlor DP6,
20 mg/kg. They calculated that the chlorinated dibenzofurans were
approximately one order of magnitude less toxic than the chlorinated
benzodioxins, and considered that they were mainly responsible for the
toxicity of the polar fraction and for the difference in toxicity
between the three commercial PCB mixtures.
Recently, chlorinated dibenzofurans were detected in the
PCB-contaminated oil that was responsible for the Yusho disease in
Japan (see section 8, p. 65).
In 1961, Bauer et al. demonstrated the toxicity of a mixture of
tri- and tetrachlorodibenzofurans; a single oral dose of 0.5-1.0 mg/kg
body weight caused severe and often lethal liver necrosis in rabbits.
Application to the rabbit ear resulted in hyperplasia and
hyperkeratosis. Similar toxic effects were found with 2,3,7,8-tetra-
chlorodibenzo- p-dioxin in doses that were 10 times lower than those
found to be toxic in the case of chlorinated dibenzofurans. See also
the review by Kimbrough (1974). 2,3,7,8-Tetrachlorodibenzofuran, which
has recently been shown to be present in PCBs (Bowes et al., 1975)
caused mortality in chickens after 8-15 days when they were dosed
orally with 5 µg/kg/day. A single oral dose of 4000 µg/kg was not
lethal to mice. Porphyria was not observed in chicks or mice
(Goldstein et al., 1975a). In comparison, 8 out of 10 chicks died
after 9-19 days when dosed orally with 2,3,7,8-tetrachloro-
dibenzo- p-dioxin at 1 µg/kg body weight/day (Schwetz et al., 1973).
The oral LD50 of 2,3,7,8-tetrachlorodibenzofuran in guinea pigs is
approximately 7 µg/kg body weight, which is less than one order of
magnitude higher than that of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(Moore, 1975).
Using low resolution mass spectrometry, McKinney (1975) found
metabolites in the excreta of chickens fed 2,4,6,2',4',6'-hexa-
chlorobiphenyl which had correct masses for chlorodibenzofurans. In
addition, by perchlorination, he identified octachlorodibenzofuran
again by low resolution mass spectrometry. The 2,4,6,2',4',6'-hexa-
chlorobiphenyl isomer is not found in commercial mixtures.
Zitko et al. (1972b) looked for the presence of chlorinated
dibenzofurans in fish and fish products taken from a contaminated
coastal area. The samples included shark tissues, cormorant and
herring gull eggs, herring oil, and herring fishmeal. The detection
limit of the method was 0.01-0.02 mg/kg in the sample. No chlorinated
dibenzofurans were detected, although the authors admit that the limit
of detection was not sufficiently low to detect amounts that might
exert a significant toxic action. Curley et al. (1975) detected a
component which had a 4 Cl isotopic cluster and a mass number of 304
corresponding with that of tetrachlorodibenzofuran in the urine of
rats dosed with PCBs, but a positive identification was not made.
7.3 Toxicity of the PCTs
There have not been any systematic studies on the toxicity of the
PCTs. Sosa-Lucero et al. (1973) administered diets containing Aroclor
5460 at 0, 10, 100, and 1000 mg/kg to groups of rats for seven days.
There were no adverse effects on health or body weight; a significant
liver enlargement was recorded at the 1000 mg/kg level. In a test for
estrogenic activity involving the stimulation of glycogen response in
the immature rat uterus, Aroclor 5460 was inactive, as was Aroclor
1260 but Aroclor 5442 was more active than Aroclor 1242 (Bitman et
al., 1972). A dietary level of Aroclor 5460 of 5000 mg/kg for 12 weeks
caused decreased body weight and increased liver weight in rhesus
monkeys (Allen & Norback, 1973); after 6 weeks, facial oedema, hair
loss, and eye discharge were observed as described by the same authors
in experiments with Aroclor 1248 (section 7.1.1.2), and similar
gastric changes were also reported.
7.4 Biochemical Effects
7.4.1 Induction of enzymes
Several investigators have observed an increase in the smooth
endoplasmic reticulum of liver cells after administration of PCBs (see
section 7.1.1.3). This is accompanied by an induction of microsomal
mixed function oxidase. Induction of microsomal enzyme activity is,
like liver enlargement, more marked with the higher chlorinated PCBs
and relatively low with Aroclors 1221 and 1016 (Villeneuve et al.,
1971a, 1972; Bickers et al., 1972; Ecobichon & Corneau, 1974). The
effect has also been demonstrated with single pure PCBs administered
orally (Fujita et al., 1971); more recently this work has been
confirmed by Johnstone et al. (1974) whose results are summarized in
Table 9 and show a greater degree of enzyme induction with the higher
chlorinated compounds.
Grant et al. (1974) found an increase in microsomal enzyme
activity in rats receiving Aroclor 1254 at 20 mg/kg of diet for 246
days, but none at 2 mg/kg. Iverson et al. (1975) and Goldstein et al.
(1975) compared the microsomal enzyme inducing potential of Aroclors
1242 and 1016. Iverson et al. (1975) found increased hepatic
microsomal enzyme activity with both Aroclors in male rats receiving
21 daily oral doses of 1 mg/kg body weight, and with Aroclor 1242 in
females at 10 mg/kg of body weight and with Aroclor 1016 at 100 mg/kg.
PCBs administered to rats during pregnancy can induce microsomal
enzyme activity in the placenta and fetus and this also occurs in the
liver of newborn rats suckled by mothers fed with diets containing
PCBs (Alvares & Kappas, 1975). Benthe et al. (1972b) reported that
when rats were stressed by food deprivation or cold, the PCB residues
in adipose tissue were released during mobilization of the fat and
caused increased hepatic microsomal enzyme activity.
Table 9. Stimulation of microsomal enzyme activity by single chlorinated biphenyls
Hepatic microsomal enzyme activity
Chlorine aniline
substituents O-demethylation N-demethylation hydroxylation nitro-reduction
4 0 0 0 0
2,2' 0 + + 0
2,4' 0 0 + 0
4,4' + + + + + + +
2,5,2',5' 0 + + + +
2,4,2',4' + + + + + + 0
2,4,5,2',4',5' + + + + + + + +
2,3,5,2',3',5' + + + + + + +
2,4,6,2',4',6' + + + + + + + +
2,3,4,5,2',3',4',5' + + + + + + + +
a Johnstone et al. (1974).
0 no activity.
+ slight activity.
+ + marked activity.
7.4.2 Porphyria
Hepatic porphyria has been induced by a number of PCBs (Clophen
A60, Phenoclor DP6, Aroclors 1016, 1242, 1254, and 1260) in the
chicken, rabbit, Japanese quail, and the rat (Vos & Koeman 1970; Vos &
Beems, 1971; Iverson et al., 1975; Vos et al., 1971; Goldstein et al.,
1974, 1975a, 1975b). Porphyrin induction has been studied more
extensively in rats. A dose-dependent increase in liver porphyrins has
been observed in females receiving 21 daily oral doses of Aroclor 1242
at 10 and 100 mg/kg of body weight, but not at 1 mg/kg. Female rats
were more sensitive than males, and Aroclor 1016 had less effect
(Iverson et al., 1975). In rats receiving Aroclor 1254 at 100 mg/kg in
the diet, the increase was usually delayed 2-4 months after the start
of dosing (Goldstein, 1974) and was characterized by high hepatic and
urinary levels of uroporphyrin. Disturbance of porphyrin biosynthesis
had been connected with an increase of the rate limiting enzyme
2.3.1.37-delta-aminolaevulinate synthase (Vos et al., 1971; Goldstein
1974, 1975).
Kawanishi et al. (1973, 1974) have shown that the administration,
in the diet, of Kanechlors KC-300 and KC-500 to rats at 500 mg/kg
produced a marked increase in urinary excretion of copro- and
uroporphyrins, and in faecal excretion of protoporphyrin, but no
increase was observed with KC-400. Experimental administration of pure
tetrachlorobiphenyls did not produce porphyria. However, porphyria did
result from the repeated subcutaneous injection of KC400 (total dose
1.8 g) to rabbits for 55 days (Miura et al., 1973).
7.4.3 Effects on steroid metabolism
PCBs have been shown to stimulate the activity of enzymes
responsible for metabolizing steroids such as estrodiol and
androsterone more effectively than does DDT or DDE (Risebrough et al.,
1968; Lincer & Peakall, 1970). It has been suggested that effects on
reproduction (see section 7.4) may be attributed to the induction of
steroid-metabolizing enzymes (Kihlström et al., 1973). Long-term
administration of daily oral doses of 0.025 mg Clophen A60 to 23
female NMRI strain mice caused a lengthening of the estrous cycle and
a reduction in the frequency of implantation of ova Orberg &
Kihlström, 1973).
7.4.4 Other biochemical effects
Hepatic vitamin A has been reported by Villeneuve et al. (1971a)
to be reduced to half the normal values in pregnant rabbits by Aroclor
1254. Similar observations have been made by Cecil et al. (1973) in
male and female Japanese quails and in rats after feeding Aroclor 1242
at 100 mg/kg of diet. The 50% decrease in hepatic vitamin A found in
male and female rats was also found in male and female quail provided
that the latter were kept in the dark to prevent egg laying.
A lowering of hepatic vitamin A in rats, fed a 0.1% PCB diet (PCB
not specified), was described by Innami et al. (1974). The rats given
vitamin A supplement (3400 IU) plus PCB for 6 weeks showed better
growth than those given PCB alone, while those given PCB and a vitamin
A deficient diet showed significant growth retardation. On the PCB
diet, the hepatic vitamin A level decreased to 20% of the normal level
during the experimental period; supplementing the diet with 1000 IU
did not re-establish the vitamin A level in the liver. Administration
of 3000 IU of vitamin A with the PCB diet, however, allowed better
than normal hepatic vitamin A levels to be re-established. The authors
concluded that vitamin A may play a role in the detoxification of PCB
rather than that PCB plays a role in the destructive metabolism of
vitamin A.
Aroclor 1254 administered intraperitoneally to rats at 25 mg/kg
body weight daily for 4 days caused a 4- to 5-fold increase in the
biliary excretion of thyroxine during a 3-hour period. Biliary
clearance was greatly elevated. Hypobilirubinaemia has been produced
in rats by Bastomsky et al. (1975) who investigated the mechanism by
administering daily intra-peritoneal injections of Aroclor 1254
(25 mg/kg body weight in corn oil) to female rats for 4 days, then
measuring bilirubin glucuronide formation by hepatic microsomes
in vitro. PCB treatment was not effective in increasing
UDPglucuronosyltransferase (2.4.1.7) activity. Serum bilirubin levels
were also significantly decreased by PCB treatment of Gunn rats, which
are genetically deficient in UDPglucuronosyltransferase (2.4.1.7)
activity.
7.4.5 Potentiation and antagonism by PCBs
As PCBs can stimulate microsomal enzyme activity, it is to be
expected that they may potentiate the action of those chemicals that
undergo microsomal activation, and decrease the action of those that
are detoxified. Villeneuve et al. (1973) demonstrated the antagonistic
effect by the reduction of phenobarbital sleeping time in rats
receiving Aroclors 1242, 1254, and 1260 in their diet, but not in
those receiving Aroclor 1221. Johnstone et al. (1974) have confirmed
this with a series of single PCBs. Tanaka & Komatsu (1972) found that
the hexobarbital-induced sleeping time in female rats was reduced to
49% of the control value by daily oral doses of Kanechlor 500 of
2 mg/kg for 3 days (total 6 mg/kg). When a daily dose of 0.4 mg/kg was
given for 15 days (total 6 mg/kg), no reduction in sleeping time was
observed. When this small dose was continued for 45 and 53 days, the
reduction remained at 12-13%. Phillips et al. (1972) did not find any
potentiation of the cholinesterase-inhibitory action of parathion in
rats dosed with Aroclors 1221 and 1254; this does not necessarily
imply that there was no enhanced activation of parathion, as a
stimulation of detoxication may have occurred concurrently. A
stimulation of parathion detoxication but not of activation has been
demonstrated in rabbit microsomes (Villeneuve et al., 1971a).
Lichtenstein (1972) reports a potentiation by PCBs of the toxicity of
parathion to flies.
Cecil et al. (1975) have shown that the ability of PCTs to
decrease phenobarbital sleeping time in quails is rather less than
that of the PCBs.
Aroclor 1254 at 160 mg/kg of diet fed to 5-week old male and
female Fischer-344 rats for 8 weeks reduced mortality due to feeding
hexachlorophene at a concentration of 600 mg/kg of diet from 77% to 7%
and completely prevented the paralysis that was observed in all
animals on the hexachlorophene diet alone. However, in the animals on
the combined treatment, histological changes in the brain
characteristic of hexachlorophene were still apparent and the
possibility of delayed toxicity beyond the 8 weeks of the experiment
could not be eliminated. The protective effect of Aroclor 1254 was
explained by its capacity to enhance detoxification by means of
hepatic microsomal enzyme induction (Jones et al., 1974).
7.5 Cytotoxic Effects
Peakall et al. (1972) found a significant increase in chromosome
abnormalities in the embryos of ring doves when the parents were fed
on a diet containing Aroclor 1254 at 10 mg/kg. Nilsson & Ramel (1974)
found no chromosome breakage in Drosophila melanogaster when either
Clophen A30 or A50 was added to the substrate. Green et al. (1975)
administered Aroclor 1242 orally to rats in single doses of 1250,
2500, or 5000 mg/kg or as a repeated dose of 500 mg/kg/day for 4 days.
Aroclor 1254 was also administered for 5 days at doses of 75, 150, or
300 mg/kg/day; they found no evidence of a mutagenic potential as
assessed by cytogenic analysis of bone marrow and spermatogonia.
7.6 Immunosuppressive Effects
Administration of PCBs leads to an atrophy of lymphoid tissue in
chickens (Flick et al., 1965; Vos & Koeman, 1970), in pheasants
(Dahlgren et al., 1972), and in rabbits (Vos & Beems, 1971). Vos & de
Roij (1972) and Vos & van Driel-Grootenhuis (1972) came to the
conclusion that these effects could be attributed to an
immunosuppressive effect of PCBs. They found that when guinea pigs fed
on diets containing Clophen A60 or Aroclor 1260 at 50 mg/kg were
stimulated with tetanus toxoid, a lower antitoxin titre and a lower
count of antitoxin-producing cells was obtained than in control guinea
pigs, resulting in a significant reduction of immunoglobulins. The
skin reaction after tuberculination in animals immunized with Freund's
complete adjuvant (as a parameter of cell-mediated immunity) was also
depressed at the 50 mg/kg of diet level. Vos & de Roij (1972)
suggested that the ability of PCBs to increase the susceptibility of
ducklings to duck hepatitis virus (Friend & Trainer, 1970) and of fish
to fungal disease (Hansen et al., 1971) could be attributed to this
immunosuppressive effect. Kimuru & Baba (1973) found an increased
incidence of pneumonia, and lung and intracranial abscesses in rats on
a diet containing Kanechlor 400 and suggested that this was due to a
lowered resistance to infection.
7.7 Effects on Reproduction
In a one-generation reproduction study, rats were fed with a diet
containing Aroclor 1242, 1254, or 1260 at levels of 1, 10, and
100 mg/kg. Decreased survival of pups with Aroclors 1242 and 1254 at
100 mg/kg was noted. No effect on reproduction was detected with
Aroclor 1260 (Keplinger et al., 1971). In a two-generation
reproduction study (Linder et al., 1974), rats were fed a diet
containing Aroclor 1254 at levels of 0, 1, 5, 20, and 100 mg/kg and
Aroclor 1260 at levels of 0, 5, 20, and 100 mg/kg. Rats exposed to
Aroclor 1254 at dietary levels of 20 mg/kg or more had fewer pups per
litter. Aroclor 1260 had no effect on reproduction, even at levels of
100 mg/kg. Kihlström et al. (1973) showed that a daily oral dose of
25 µg of Clophen A60 to female mice for 62 days significantly
increased the length of the estrus cycle and decreased the frequency
of implanted ova. In order to study the effect of PCBs on the
development of sexual functions in the early postnatal period they
also mated mice that had been suckled by mothers dosed with Clophen
A60 during the lactation period. A decrease in the frequency of
implanted ova was noted when both parents of the couple had been
suckled with milk containing PCBs.
In the rhesus monkey, Allen (1975) reports a lower fertility and a
diminished weight of the young at birth, after the administration of a
diet containing Aroclor 1248 at 2.5 mg/kg for several months.
Studies by Aulerich et al. (1971), and Ringer et al. (1972) showed
that Aroclor 1254 fed to mink at 5 mg/kg severely affected
reproduction.
There have been several reports that PCBs can adversely affect egg
production and hatchability in birds. Contamination of feed by PCBs
from a heat exchanger was shown to be the cause of reduced
hatchability of hen eggs at a large poultry hatchery in the USA
(Kolbye, 1972), and this has been confirmed in laboratory experiments.
Keplinger et al. (1971) found poor hatchability of eggs from hens
receiving diets containing Aroclor 1242 at 10 mg/kg or Aroclor 1254 at
100 mg/kg, but not with hens receiving Aroclor 1260 at 100 mg/kg. An
adverse effect on eggs has been noted with Aroclor 1248 at 10 mg/kg
(Panel on Hazardous Trace Substances, 1972). Peakall et al. (1972) fed
ring doves on a diet containing Aroclor 1254 at 10 mg/kg for 3 months
and found a marked reduction in egg hatchability 6 months later, due
to embryo mortality.
The viability of salmon eggs bears some relation to their PCB
content; Johansson et al. (1970) found a 12% mortality in eggs
containing PCBs at the rate of 9.2 mg/kg in extractable fat, and a
100% mortality with 34 mg/kg. Adverse effects on the reproduction of
aquatic invertebrates have occurred at water concentrations of PCBs in
the region of 5 µg/litre.
The potential teratogenic effect of the PCBs has been studied by
dosing pregnant females during the gestation period. No fetal
abnormalities were produced in the rat by daily doses of Aroclors
1242, 1254, or 1260 at 10 and 30 mg/kg (Keplinger et al., 1971), or
Aroclor 1254 at 100 mg/kg (Villeneuve et al., 1971b), or in the rabbit
dosed with Aroclor 1254 at 10 and 50 mg/kg (Villeneuve et al., 1971b).
Injection of PCBs into eggs has been reported to produce beak
abnormalities in chicks (McLaughlin et al., 1963). Cecil et al. (1974)
have claimed that the administration of Aroclors 1232 and 1254 to hens
at 20 mg/kg in the diet causes teratogenic effects and a reduction in
hatchability of fertile eggs.
7.8 Neoplasia and Adenofibrosis
The liver is the only organ where tumours have been reported
following the ingestion of PCBs. Ito et al. (1973) fed groups of 12
male dd mice with diets containing 500, 250, 100 and 0 mg/kg of
Kanechlor 500, 400, and 300, respectively. After 1 year, 7/12 mice
developed neoplastic nodules (hyperplastic nodules) and 5/12 developed
hepatocellular carcinomas, all in mice from the group fed with
500 mg/kg Kanechlor 500. Metastases were not observed. In a second
study on mice, combined exposure to Kanechlor 500 and either alpha- or
ß-hexachlorocyclohexane isomers enhanced the development of neoplastic
nodules (hyperplastic nodules) and hepato-cellular carcinomas. A
combination of Kanechlor 500 and gamma-hexachlorocyclohexane did not
produce tumours. Dosing with Kanechlor 500 alone at a dietary level of
250 mg/kg, and ß- or gamma-hexachlorocyclohexane at dietary levels of
250, 100 or 50 mg/kg did not produce tumours. However, alpha-hexa-
chlorocyclohexane produced 8/30 hepata-cellular carcinomas and 23/30
hyperplastic nodules.
Groups of 50 BALB/cj male inbred mice were fed dietary
concentrations of Aroclor 1254 of 0 or 300 mg/kg (49.8 mg/kg body
weight) for 6 or 11 months respectively. No liver tumours were noted
in a total of 58 surviving controls. A total of 10 hepatomas
(neoplastic or hyperplastic nodules) were noted in 9/22 surviving mice
fed Aroclor 1254 for 11 months and in 1/24 surviving mice fed Aroclor
1254 for 6 months. In addition adenofibrosis (cholangiofibrosis) was
observed in all 22 livers of mice fed Aroclor 1254 for 11 months, but
not in the other groups (Kimbrough & Linder 1974).
In another study (Kimbrough et al., 1975), 200 female Sherman
strain COBS random bred rats (descendants of the Osborne Mendel
strain) were given a diet containing Aroclor 1260 at 100 mg/kg
(11.6-4.3 mg/kg body weight) for approximately 21 months; 200 female
rats were kept as controls. The rats were sacrificed when 23 months
old. Hepatocellular carcinomas were found in 26/184 of the
experimental groups and in 1/173 of the control rats. None of the
controls, but 144/184 experimental rats had neoplastic nodules
(hyperplastic nodules). Areas of hepatocellular alteration were noted
in 28/173 controls and 182/184 experimental rats. No effect of the
Aroclor on the incidence of tumours in other organs and no metastases
from the liver tumours were observed. In this and two earlier studies,
adenofibrosis of the liver was observed in male and female rats fed
either Aroclor 1260 or Aroclor 1254 (Kimbrough et al., 1972; 1973);
1975). Adenofibrosis of the liver is a persistent progressive lesion
that consists of a marked proliferation of fibrous tissue and
epithelial glandular cells which are well differentiated in the mouse
but appear atypical in the rat.
In a preliminary study, liver tumours (multiple adenomatous
nodules) were induced by Kanechlor 400 in 6/10 female, but not in male
Donryu rats. The dietary exposure varied throughout the study and the
number of animals used was small (10 experimental and 5 control rats
for each sex) (Kimura & Baba, 1973). Makiura et al. (1974) reported
that PCBs (Kanechlor 500) inhibited the induction of liver tumours in
male Sprague-Dawley rats by the known carcinogens 3' methyl- p-
dimethylaminoazo-benzene, N-2-fluosenyl acetamide, and diethyl-
nitrosamine.
8. EFFECTS OF PCBs AND PCTs ON MAN -- EPIDEMIOLOGICAL AND CLINICAL
STUDIES
In June 1968, patients appeared at the Dermatology Clinic of
Kyushu University Hospital, Fukuoka, Japan suffering from chloracne. A
group at the University undertook intensive clinical, chemical, and
epidemiological investigations and found that the disease originated
from the consumption of a batch of rice oil supplied in February 1968;
the disease was called Yusho (rice oil disease) (Katsuki, 1969). This
batch of rice oil was found to be contaminated with Kanechlor 400, a
48% chlorinated biphenyl, at 2000-3000 mg/kg which entered the oil
through a leak in a heat exchanger (Tsukamoto et al., 1969). The
symptoms and signs of Yusho were described by Goto & Higuchi (1969)
and by Okumura & Katsuki (1969). The earliest signs were enlargement
and hypersecretion of the Meibomian glands of the eyes, swelling of
the eyelids, and pigmentation of the nails and mucous membranes,
occasionally associated with fatigue, nausea, and vomiting. This was
usually followed by hyperkeratosis and darkening of the skin with
follicular enlargement and acneform eruptions, frequently with a
secondary staphylococcal infection. These skin changes were most often
seen on the neck and upper chest, but in severe cases extended to the
whole body. Biopsy skin samples showed hyperkeratosis, dilation of the
follicles, and an accumulation of melanin in the basal cells of the
epidermis; melanin granules have also been observed in biopsy samples
of the conjunctiva. Oedema of the arms and legs was seen in some
patients. There were no definite signs of liver enlargement or liver
disorders (Okumura & Katsuki 1969), but slight rises in serum
transaminases and in alkaline phosphatase were detected, and a liver
sample from a Yusho patient showed an increase in the smooth
endoplasmic reticulum (Hirayama et al., 1969). The majority of the
patients were found to have respiratory symptoms, and suffered from a
chronic bronchitis-like disturbance that persisted for several years
(Shigematsu et al., 1971, 1974).
Yusho patients did not appear to suffer from central nervous
effects, but some complained of numbness of the arms and legs. Mural &
Kuroiwa (1971) found a decrease in the conduction velocity in
peripheral sensory nerves.
Yoshimura (1971) reported diminished growth in boys but not in
girls, who consumed the oil. Babies born to Yusho mothers were smaller
than normal. Newborn babies showed a dark brown skin pigmentation,
which disappeared after a few months (Yagamuchi et al., 1971; Taki et
al., 1969). Funatsu et al. (1972) found spotted and sporadic
ossification of the skull and facial oedema with exophthalmia in four
babies, but there was no evidence of any teratogenic action.
Determinations of PCB concentrations in the tissue of Yusho
patients were made several months after the ingestion of the oil,
apparently by an X-ray fluorescence method for organic chlorine (Goto
& Higuchi, 1969). Abdominal fat contained 13.1 mg/kg, subcutaneous fat
75.5 mg/kg, and nails 59 mg/kg. The mesenteric adipose tissue in six
Yusho patients, analysed by gas-liquid chromatography 1-3 years after
the occurrence of intoxication, contained PCB levels of 2.5 mg/kg on
average, which was considerably higher than the normal value. (Masuda
et al., 1974a). The mean blood level of PCBs in patients was 0.6 or
0.7 µg/100 ml (0.3 µg/100 ml for the general population) five years
after exposure (Masuda et al., 1974b; Takamatsu et al., 1974). These
authors also noted a specific gas-liquid chromatographic pattern,
peculiar to Yusho patients, which is still observed.
Hirayama et al. (1974) also reported that the serum bilirubin
level of patients was significantly lower than the normal level and
was negatively correlated with the blood level of PCBs and the serum
triglyceride level.
A considerable number of patients had elevated serum triglyceride
levels, up to four times the normal values, although this was not
correlated with the severity of the symptoms; these high values were
maintained for three years in many patients (Uzawa, 1972). There were
no marked abnormalities in serum cholesterol and phospholipid levels
(Okumura & Katsuki, 1969; Uzawa et al., 1969). Nagai et al. (1969)
reported an increase in urinary 17-ketosteroid excretion. Kusuda
(1971) also observed changes in the menstrual cycle in approximately
60% of 81 female Yusho patients as compared with their cycles prior to
exposure. Okumura et al. (1974) examined the relationship between the
blood levels of triglycerides and PCBs in 42 patients and observed a
positive correlation. Uzawa et al. (1972) showed that high values of
serum triglycerides were maintained for 3 years in many patients.
Shigematsu et al. (1971) examined serum immunoglobulin levels in 38
patients, 2 years after onset, and observed a decrease in IgA and IgM
and an increase in IgG. Saito et al. (1972) reported lower IgM levels
in patients showing chloracne.
Urabe (1974) reported that the total number of Yusho patients had
reached 1200 by 13 September 1973 and that 22 of them had died.
Mucocutaneous signs had decreased year by year, but neurological and
respiratory signs and symptoms and various complaints such as general
fatigue, anorexia, abdominal pain, and headache had become more
prominent among the patients. The smallest amount of oil that produced
symptoms when ingested over approximately 120 days, contained
approximately 0.5 g of PCBs, or approximately 0.07 mg/kg body weight
per day (Kuratsune, 1972a). Recently chlorinated dibenzofurans at
5 mg/kg were found in three samples of the toxic rice oil that
contained PCB levels of about 1000 mg/kg (Nagayama et al., 1975).
Symptoms similar to those of Yusho have been observed in workers
in a Japanese condenser factory, including pigmentation of the fingers
and nails, and acneiform eruptions on the jaw, back, and thighs. It
was thought that these effects arose from local contact with PCBs;
when the use of PCBs ceased, the symptoms disappeared (Hasegawa et
al., 1972b).
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO PCBs AND PCTs
9.1 Species Variation
The data in sections 7 and 8 indicate that man appears to be the
species most sensitive to PCBs, the consumption of relatively small
amounts having resulted in a severe disease (Yusho) in 1200 persons in
Japan. The monkey is the only experimental species in which effects
qualitatively and quantitatively approaching those in man have been
observed; Allen (1975) attributed this to metabolic differences
leading to a slower elimination than that observed in other species
tested.
Conclusions concerning the specific effects of PCBs on different
species are confused by uncertainty arising from the presence of toxic
impurities. The rice oil that caused the outbreak of Yusho was
contaminated with PCBs containing relatively high amounts of
tetrachlorodibenzofuran (see section 8), but the sample used in the
monkey experiments had a low content of these impurities, so it is not
clear whether PCBs alone were responsible for the Yusho incident.
Further uncertainty arises from reports from Finland of high PCB
concentrations in blood and body fat of occupationally exposed workers
with no indication of adverse effects, while at similar tissue
concentrations Japanese workers showed skin lesions typical of Yusho
(see section 5.5.3).
A species-specific toxic manifestation that can probably be
attributed to toxic impurities, is the abdominal oedema and
hydropericardium seen in birds affected by some commercial PCB
mixtures.
Mink is another species showing a high sensitivity to PCBs. Deaths
have been produced with diets containing PCB levels of 30 mg/kg; no
information is available on any species-specific metabolic pathway in
the mink that would account for this susceptibility.
9.2 Dose-Effect Relationships
The following is a summary of the data in Sections 7 and 8
concerning the relationship between mammalian toxicity and dose.
Approximate calculations of the daily dose in mg/kg body weight
derived from the dietary concentration are given in parentheses.a
a When no food consumption figures were available from the
experimental studies, the following factors were used to transform
mg/kg in the diet to mg/kg body weight: mouse (7), rat (20),
guinea-pig (25), mink (10), rabbit (33), monkey (25).
9.2.1 Body weight
Body weight was reduced in rats after 8 months of dietary intake
of Aroclor 1254 at 100 mg/kg (corresponding to 5 mg/kg body weight);
no effects were observed at 20 mg/kg in the diet (corresponding to
1 mg/kg body weight).
Dose-dependent retardation of weight gain was observed in mink
after 4 months of dietary intake of Aroclor 1254 at 5 and 10 mg/kg
(corresponding to 0.5 and 1.1 mg/kg body weight respectively).
9.2.2 Effects on liver
Liver weight
Dose-dependent increase in liver weight was observed in rats
receiving Aroclors 1242, 1254 and 1260 at concentrations of more than
20 mg/kg in the diet (corresponding to > 1.4 mg/kg body weight). Male
rats were more sensitive than female rats; no effects were observed
with Aroclors 1254 and 1260 at concentrations lower than 20 mg/kg in
the diet (corresponding to < 1.4 mg/kg body weight). Effects were
less marked with the lower chlorinated PCBs.
Liver changes
Smooth endoplasmic reticulum proliferation with fat droplet
inclusions were observed in the liver tissue of rats after 8 months of
dietary intake of Aroclor 1254 at 20 mg/kg (corresponding to 1.4 mg/kg
body weight).
Liver damage was observed with Aroclors 1242 and 1254 in rabbits
receiving 14 weekly oral doses of 150 mg/kg body weight; no effect was
observed with Aroclor 1221.
Liver enzyme activitya
Increase in microsomal enzyme activity was observed in male rats
after 8 months of dietary intake of Aroclor 1254 of 20 mg/kg
(corresponding to 1 mg/kg body weight). No effect was observed at
2 mg/kg in the diet (corresponding to 0.1 mg/kg body weight. Effects
were less marked in female rats.
Increased activity was also observed with Aroclors 1242 and 1016
in male rats receiving 21 daily oral doses of 1 mg/kg body weight.
a According to Litterst, et al. (1972) the dose producing an effect
on nitroreductase activity in the rat corresponds to 0.5 mg/kg in
the diet (corresponding to 0.3 mg/kg body weight).
Liver porphyria
Effects were observed in rats after several months of dietary
intake of Aroclor 1254 at 100 mg/kg (corresponding to 5 mg/kg body
weight); dose-dependent effects were observed in female rats after 21
daily oral doses of Aroclor 1242 at 10 and 100 mg/kg; no effects were
noted at 1 mg/kg body weight.
Liver vitamin A
Reduction of hepatic vitamin A was observed in rats receiving
Aroclor 1242 at the rate of 100 mg/kg in the diet (corresponding to
5 mg/kg body weight).
Liver tumours
Hepatocellular carcinomas were observed in mice after one year of
dietary intake of Kanechlor 500 at 500 mg/kg (corresponding to
75 mg/kg body weight); no carcinomas were observed with Kanechlor 500
at 250 mg/kg in the diet (corresponding to 37.5 mg/kg body weight), or
with Kanechlor 300 and 400 at 500 mg/kg in the diet (corresponding to
75 mg/kg body weight).
Hepatomas were observed in mice after 10 months of daily intake of
Aroclor 1254 at 300 mg/kg in the diet (corresponding to 49.8 mg/kg
body weight).
Hepatocellular carcinomas were observed in rats after 21 months of
daily intake of Aroclor 1260 at 100 mg/kg in the diet (corresponding
to 11.6-4.3 mg/kg body weight).
9.2.3 Reproduction
Effects on reproduction were observed in the mouse at a daily oral
dose of 0.025 mg Clophen A60; in the rat at a dietary level of Aroclor
1254 of 20 mg/kg (corresponding to 1 mg/kg body weight) with the
effects decreasing with higher chlorinated PCBs; in the mink at a
dietary level of Aroclor 1254 of 5 mg/kg (corresponding to 0.5 mg/kg
body weight); and in the monkey at a dietary level of Aroclor 1248 of
2.5 mg/kg (corresponding to 0.1 mg/kg body weight).
9.2.4 Immunosuppression
Immunosuppressive effects were observed in the guinea-pig at a
dietary level of Clophen A60 or Aroclor 1260 of 50 mg/kg
(corresponding to 2 mg/kg body weight).
9.2.5 Skin effects
In man, symptoms of Yusho disease were observed at a dietary level
of 4.2 mg/day of PCBs (corresponding to 0.07 mg/kg body weight/day for
a 60-kg person). A value of 0.50 g was estimated as the quantity of
PCBs consumed over approximately 120 days above which toxic symptoms
were evident. Similar effects were observed in the monkey at a dietary
level of Aroclor 1248 of 2.5 mg/kg (corresponding to 0.1 mg/kg body
weight) after several months.
9.3 Nondetected effect levels
The assessment of non detected effect levels for toxic effects is
complicated by the different activities of the component PCBs and by
the presence of impurities, in addition to the influence of inter- and
intraspecies variation, age, sex, and length of exposure. Moreover,
many of the available experimental studies do not include a
nondetected effect level.
The most sensitive species appears to be man, and effects have
been observed at intake rates of 0.07 mg/kg body weight/day. This may
have been influenced by the intake of impurities more toxic than PCBs,
but similar effects have been produced in the monkey, at the same
order of dosage, with a product containing little of these impurities.
At this dosage level, no effects may be expected on growth, liver
enlargement, and liver enzyme activity in less sensitive species such
as the rat. Although non-detected effect levels are not available for
effects on immunosuppression and reproduction, and for certain
biochemical effects on the liver, it seems unlikely that these effects
would be apparent at intake rates of 0.1 mg/kg body weight/day.
Carcinogenic effects have been observed in rats and mice at doses two
orders of magnitude greater than this, but there is no epidemiological
evidence to suggest that PCBs cause tumours in man. According to Grant
et al. (1974) rats fed on a diet containing Aroclor at the rate of
2 mg/kg (equivalent to about 0.1 mg/kg body weight) showed PCB levels
of 8 µg/100 ml in blood and 26.1 mg/kg in body fat. However, values
much higher than these have been observed in men occupationally
exposed to PCBs without evidence of any toxic effects (see section
5.5.3).
It is not possible at present to resolve this conflict in the
evidence on the toxicity of PCBs to man.
REFERENCES
ABBOT, D.C., COLLINS, G. B. & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom 1969-71.
Br. med. J., 2: 553-556.
ACKER, L. & SCHULTE, E. (1970) Uber das Vorkommen von chlorierten
Biphenylen und Hexachlorbenzol neben chlorierten Insektiziden in
Humanmilch und menschlichem Fettgewebe. Naturwissenschaften,
57: 497 (in German).
ACKER, L. & SCHULTE, E. (1974) Chlorkohlenwasserstoffe in menschlichem
Fettgewebe, Naturwissenschaften, 61: 1-4 (in German).
ADDISON, R. F., FLETCHER, G. L., RAY, S. & DOANE, J. (1972) Analysis
of a chlorinated terphenyl (Aroclor 5460) and its deposition in
tissues of the cod (Gadus morhua). Bull. environ. Contam.
Toxicol., 8: 52-60.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of polychlorinated biphenyl (PCB) and chlorinated
pesticides in water. Anal. Chem., 42: 1483-1486.
AHMED, M. & FOCHT, D. D. (1973) Degradation of polychlorinated
biphenyls by two species of Achromobacter. Can. J. Microbiol.,
19: 47-52.
AHNOFF, M. & JOSEFSSON, B. (1973) Confirmation studies on
polychlorinated biphenyls (PCB) from river waters using mass
fragmentography. Anal. Lett., 6: 1083-1093.
AHNOFF, M. & JOSEFSSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AHNOFF, M. & JOSEFSSON, B. (1975) Clean-up procedures for PCB analysis
on river water extracts. Bull. environ. Contam. Toxicol.,
13: 159-166.
ALBRO, P. W. & FISHBEIN, L. (1972) Intestinal absorption of
polychlorinated biphenyls in rats. Bull. environ. Contam.
Toxicol., 8: 26-31.
ALLEN, J. R. (1975) Response of the non-human primate to
polychlorinated biphenyl exposure. Fed. Proc., 34: 1675-1679.
ALLEN, J. R., ABRAHAMSON, L. J. & NORBACK, D. H. (1973) Biological
effects of polychlorinated biphenyls and triphenyls on the
subhuman primate. Environ. Res., 6: 344-354.
ALLEN, J. R. & NORBACK, D. A. (1973) Polychlorinated biphenyl- and
triphenyl-induced mucosal hyperplasia in primates. Science,
179: 498-499.
ALLEN, J. R., CARSTENS, L. A. & BARSOTTI, D. A. (1974) Residual
effects of short-term, low-level exposure of non-human primates to
polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
30: 440-451.
ALVARES, A. P. & KAPPAS, A. (1975) Induction of aryl hydrocarbon
hydroxylase by chlorinated biphenyls in the foeto-placental unit
and in neonatal livers during lactation. FEBS Lett., 50:172-174.
ARMOUR, J. A. & BURKE, J. A. (1970) Method for separating
polychlorinated biphenyls from DDT and its analogs. J. Assoc.
Off. Anal. Chem., 53: 761-768.
AULERICH, R. J., RINGER, R. K., SEAGRAN, H. L. & YOUATT, W. G. (1971)
Effects of feeding coho salmon and other Great Lakes fish on mink
reproduction. Can. J. Zool., 49: 611-616.
AULERICH, R. J., RINGER, R. K. & IWAMOTO, S. (1973) Reproductive
failure and mortality in mink fed on Great Lake fish. J. Reprod.
Fertil., 19 (Suppl.): 365-376.
BAGLEY, G. E., REICHEL, W. L. & CROMARTIE, E. (1970) Identification of
polychlorinated biphenyls in two bald eagles by combined
gas-liquid chromatography-mass spectroscopy. J. Assoc. Off. Anal.
Chem., 53: 251-261.
BAILEY, S. & BUNYAN, P. J. (1972) Interpretation of persistence and
effects of polychlorinated biphenyls in birds. Nature (Lond.),
236: 34-36.
BASTOMSKY, C. H., SOLYMOSS, a., ZSIGMOND, G. & WYSE, J. M. (1975) On
the mechanism of polychlorinated biphenyl-induced
hypobilirubinaemia. Clin. chim. Acta, 61: 171-174.
BAUER, H., SCHULZ, K. H. & SPIEGELBERG, U. (1961) Berufliche
Vergiftungen bei der Herstellung von Chlorphenol-Verbindungen.
Arch. Gewerbepathol. Gewerbehyg., 18: 538-555.
BENTHE, H. F., KNOP, J. & SCHMOLDT, A. (1972a) Absorption and
distribution of polychlorinated biphenyls (PCB) after inhalatory
application. Arch. Toxikol., 29: 85-95 (in German).
BENTHE, H. F., SCHMOLDT, A. & SCHMIDT, H. (1972b) Induction of
microsomal liver enzymes after polychlorinated biphenyls (PCB) and
following stress. Arch. Toxikol., 29: 97-106 (in German).
BERG, O. W., DIOSADY, P. L. & REES, G. A. V. (1972) Column
chromatographic separation of polychlorinated biphenyls from
chlorinated hydrocarbon pesticides and their subsequent gas
chromatographic quantitation in terms of derivatives. Bull.
environ. Contam. Toxicol., 7: 338-347.
BERGLUND, F. (1972) Levels of polychlorinated biphenyls in foods in
Sweden. Environ. Perspect, 1: 67-69.
BERLIN, M., GAGE, J. C. & HOLM, S. (1973) The metabolism and
distribution of 2,4,5,2',5'-pentachlorobiphenyl in the mouse.
In: PCB Conference II. National Swedish Environment Protection
Board Publications: 4E, pp. 101-107.
BERLIN, M., GAGE, J. C. & HOLM, S. (1974) Distribution and metabolism
of polychlorobiphenyls. In: Proceedings of the International
Symposium on Recent Advances in Environmental Pollution, Paris,
24-28 June. pp. 8.
BERLIN, M., GAGE, J. & HOLM, S. (1975) The distribution and metabolism
of 2,4,5,2',5'-pentachlorobiphenyl. Arch. environ. Health,
30: 141-147.
BICKERS, D. R., HARBER, L. C., KAPPAS, A. & ALVARES, A. P. (1972)
Polychlorinated biphenyls: comparative effects of high and low
chlorine containing Aroclors on hepatic mixed function oxidase.
Res, Comm. Chem. Pathol. Pharmacol., 3: 505-511.
BIDLEMAN, T. F. & OLNEY, C. E. (1974) High-volume collection of
atmospheric polychlorinated biphenyls. Bull. environ. Contam.
Toxicol., 11: 442-450.
BITMAN, J., CECIL, H. C. & HARRIS, S. J. (1972) Biological effects of
polychlorinated biphenyl in rats and quail. Environ. Health
Perspec., 1: 145-149.
BJERK, J. E. (1972) Rester av DDT og polyklorerte bifenyler i Norsk
humant materiale. Tidsskr. Nor-Laegeforen., 92: 15-19 (cited by
Kimbrough, 1974).
BOURNE, W. P. P. & BOGEN, A. A. (1972) Polychlorinated biphenyls in
North Atlantic seabirds, Mar. Pollut. Bull., 3 (11): 172-175.
BOWES, G. W., MULVIHILL, M. J., SIMONEIT, B. R. T., BURLINGAME, A. L.
& RISEBROUGH, R. W. (1975) Identification of chlorinated
dibenzofurans in American polychlorinated biphenyls. Nature
(Lond.), 256, 305-307.
BROADHURST, M. G. (1972) Use and replaceability of polychlorinated
biphenyls. Environ. Health Perspect., 2: 81-102.
BRUCKNER, J. V., KHANNA, K. L. & CORNISH, H. H. (1973) Biological
responses of the rat to polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 24: 434-448.
BRUGGEMAN, VON J., BUSCH, L., DRESCHER-KADAN, U., EISELI, W. & HOPPE,
P. (1974) Pesticid-und PCB-Ruckstunde in Organen von Wildtieren
als Indikatoren fur Umweltkontamination. Z. Jagdwiss.,
20: 70-74.
BURSE, V. W., KIMBROUGH, R. D., VILLANUEVAS, E. C, JENNINGSI R. W.,
LINDER, R. E. & SOVOCOOL, G. W. (1974) Polychlorinated biphenyls.
Storage, distribution, excretion and recovery: liver morphology
after prolonged dietary ingestion. Arch. environ. Health,
29: 301-307.
CARNES, R. A., DOERGER, J. U. & SPARKS, H. L. (1973) Polychlorinated
biphenyls in solid waste and solid-waste-related materials. Arch.
environ. Contam. Toxicol., 1: 27-35.
CECIL, H. C., HARRIS, S. J., BITMAN, J. & FRIES, G. F. (1973)
Polychlorinated biphenyl-induced decrease in liver vitamin A in
Japanese quail and rats. Bull. environ. Contam. Toxicol.,
9: 179-185.
CECIL, H. C., BITMAN, J., LILLIE, R. J., FRIES, G. F. & VERRETT, J.
(1974) Embryotoxic and teratogenic effects in unhatched fertile
eggs from hens fed polychlorinated biphenyls (PCBs). Bull.
environ. Contam. Toxicol., 11: 489-495.
CECIL, H. C., HARRIS, S. J. & BITMAN, J. (1975) Effects of
polychlorinated biphenyls and terphenyls and polybrominated
biphenyls on pentobarbital sleeping times of Japanese quail.
Arch. environ. Contam. Toxicol., 3, 183-192.
CHOI, P.S. K., NACK, H. & FLINN, J. E. (1974) Distribution of
polychlorinated biphenyls in an aerated biological oxidation
wastewater treatment system. Bull. environ. Contam. Toxicol.,
11: 12-17.
CLAUS, B. & ACKER, L. (1975) Zur Kontamination von Milch und
Milcherzeugnissen mit chlorierten Kohlenwasserstoffen im
Westphälischen Raum II Ergebnisse und Diskussion. Zeitschrift fur
Lebensmitteluntersuchung und forschung, 159 (3): 129-137.
COLLINS, G. B., HOLMES, D. C. & JACKSON, F. J. (1972) The estimation
of polychlorobiphenyls. J. Chromatogr., 71: 443-449.
COMMISSION OF THE EUROPEAN COMMUNITIES (1974) European Colloquium.
Problems raised by the contamination of man and his environment
by persistent pesticides and organohalogenated compounds.
Luxembourg, 14-16 May 1964.
COOLEY, N. R., KELTNER, J. M. & FORESTER, J. (1972) Mirex and Aroclor
1254: effect on accumulation by Tetrahymena pyriformis strain
W. J. Protozool., 19: 636-638.
CURLEY, A., BURSE, V. W., GRIM, M. E., JENNINGS, R. W. & LINDER, R. E.
(1971) Polychlorinated biphenyls: distribution and storage in body
fluids and tissues of Sherman rats. Environ. Res., 4: 481-495.
CURLEY, A., BURSE, V. W. & GRIM, M. E. (1973a) Polychlorinated
biphenyls: evidence of transplacental passage in the Sherman rat.
Fd. cosmet. Toxicol., 11: 471-476.
CURLEY, A., BURSE, V. W., JENNINGS, R. W., VILLANUEVA, E. C., TOMATIS,
L. & AKAZAKI, K. (1973b) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond), 242: 338-340.
CURLEY, A., BURSE, W. V., JENNINGS, R. W., VILLANUEVA, E. C. &
KIMBROUGH, R. D. (1975) Evidence of tetrachlorodibenzofuran (TCDF)
in Aroclor 1254 and the urine of rats following dietary exposure
to Aroclor 1254. Bull. environ. Contam. Toxicol., 14: 153-158.
DAHLGREN, R. B., GREICHUS, Y. A. & LINDER, R. L. (1971) Storage and
excretion of polychlorinated biphenyls in the pheasant.
J. Wildlife Manag., 35: 823-828.
DAHLGREN, R. B., LINDER, R. L. & CARLSON, C. W. (1972) Polychlorinated
biphenyls: their effects on penned pheasants. Environ. Health
Perspect., 1: 89-101.
DE FREITAS, A. S. & NORSTROM, R. J. (1974) Turnover and metabolism of
polychlorinated biphenyls in relation to their chemical structure
and the movement of lipids in the pigeon. Can. J. Physiol.
Pharmacol., 52: 1080-1094.
DEPARTMENT OF HEALTH EDUCATION AND WELFARE (1972) Environ. Health
Perspect. Experimental Issue 1, April 1972. DHEW, Bethesda, MD,
USA. Publ. No. (NIH) 72-218.
DE VOS, R. H. & PEET, E. W. (1971) Thin-layer chromatography of
polychlorinated biphenyls. Bull environ. Contam. Toxicol.,
6: 164-170.
DOGUCHI, M. & FUKANO, S. (1975) Residue levels of polychlorinated
terphenyls, polychlorinated biphenyls and DDT in human blood.
Bull. environ. Contam. Toxicol., 13: 57-63.
DOGUCHI, M., FUKANO, S. & USHIO, F. (1974) Polychlorinated terphenyls
in the human fat. Bull. environ. Contam. Toxicol.,
11 (2): 157-158.
DUKE, T. W., LOWE, J. I. & WILSON, A. J. JR (1970) A polychlorinated
biphenyl (Aroclor 1254) in the water, sediment and biota of
Escambia Bay, Florida. Bull. environ. Contam. Toxicol.,
5: 171-180.
ECOBICHON, D. J. & COMEAU, A.M. (1974) Comparative effects of
commercial Aroclors on rat liver enzyme activities. Chem.-Biol.
Interactions, 9: 341-350.
EKSTEDT, J. & ODEN, S. (1974) Chlorinated hydrocarbons in the lower
atmosphere in Sweden. Report from Department of Soil Science,
Royal Agricultural College, S-75007, Uppsala 7. pp. 1-16.
ENVIRONMENTAL SANITATION BUREAU, MINISTRY OF HEALTH & WELFARE (1973)
Survey on foods contamination. Report of comprehensive
investigation on the prevention of pollution by PCBs, 191-210
(in Japanese).
FINKLEA, J., PRIESTER, L. E., CREASON, J.P., HAUSER, T., HINNERS, T. &
HAMMER, D. I. (1972) Polychlorinated biphenyl residues in human
plasma expose a major urban pollution problem. Am. J. pub.
Health, 62: 645-651.
FISHER, N. S., GRAHAM, L. B., CARPENTER, E. J. & WURSTER, C. F. (1972)
Geographic differences in phytoplankton sensitivity to PCBs.
Nature (Lond.), 241: 548-549.
FLICK, D. F., O'DELL, R. G. & CHILDS, V. A. (1965) Studies of the
chick edema disease. Similarity of symptoms produced by feeding
chlorinated biphenyl. Poult. Sci., 44: 1460-1465.
FREUDENTHAL, J. & GREVE, P. A. (1973) Polychlorinated terphenyls in
the environment. Bull. environ. Contam. Toxicol., 10: 108-111.
FRIEND, M. & TRAINER, D. O. (1970) Polychlorinated biphenyl:
interaction with duck hepatitis virus. Science, 170: 1314-1316.
FRIES, G. F., MARROW, G. S. JR & GORDON, C. H. (1973) Long-term
studies of residue retention and excretion by cows fed a
polychlorinated biphenyl (Aroclor 1254). J. agr. Food Chem.,
21: 117-121.
FUJITA, S., TZUJI, H., KATO, K., SAEICI, S. & TSUKAMOTO, H. (1971)
Effect of biphenyl chlorides on rat liver microsomes. Fukuoka
med. Acta., 62: 30-34.
FUKADA, K., INUYAMA, Y., TAKESHITA, T. & YAMAMOTO, S. (1973) Present
state of environmental pollution by PCB in Shimane Prefecture.
Shimare Igaku, 5: 1-25 (in Japanese).
FUKADA, S., USHIO, F. & DOGUCHI, M. (1974) PCB, PCT and pesticides
residues in fish collected from the Tama River. Ann. Rep. Tokyo
Metr. Res. Lab. P.H., 25: 297-305 (in Japanese).
FUNATSU, I., YAMASHITA, F., ITO, Y., TZUGAWA, S., FUNATSU, T.,
YOSHIKANE, T., HAYASHI, M., KATO, T., YAKUSHIJI, M., OKAMOTO, G.,
YAMASAKI, S., ARIMA, T., KUNO, T., IDE, H. & IBE, I. (1972) PCB
induced fetopathy. I. Clinical observation. Kurume med. J.,
19: 43-51 (in English).
GARDNER, A.M., CHEN, J. F., ROACH, J. A. G. & RAEGLIS, E. P. (1973)
Polychlorinated biphenyls. Hydroxylated urinary metabolites of
2,5,2',5'-tetrachlorobiphenyl identified in rabbits. Biochem.
biophys. res. Commun., 55: 1377-1384.
GILBERTSON, M. & REYNOLDS, L. (1974) DDE and PCB in Canadian wild
birds. In: Occasional paper, Canadian Wildlife Service,
Environment, Canada, Ottawa, pp. 1-16.
GOLDSTEIN, J. A., HICKMAN, P. & JUE, D. L. (1974) Experimental hepatic
porphyria induced by polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 27: 437-448.
GOLDSTEIN, J. A., MCKINNEY, J. D., LUCIEN, G. W., MOORE, J. A.,
HICKMAN, P. & BERGMAN, H. (1975a) Effects of hexachlorobiphenyl
isomers and 2,3,7,8-tetrachlorodibenzofuran (TCDF) on hepatic drug
metabolism and porphyria accumulation. Pharmacologist,
16: 239 (Abstract No. 278).
GOLDSTEIN, J. A., HICKMAN, P., BURSE, V. W. & BERGMAN, H. (1975b) A
comparative study of two polychlorinated biphenyl mixture (Aroclor
1242 and 1016) containing 42% chlorine on induction of hepatic
porphyria and drug metabolizing enzymes. Toxicol. appl.
Pharmacol., 32: 461-473.
GOTO, M. & HIGUCHI, K. (1969) The symptomatology of Yusho
(chlorobiphenyls poisoning) in dermatology. Fuoka Act. Med.,
60: 409-431.
GRANT, D. L., MOODIE, C. A. & PHILLIPS, W. E. J. (1974) Toxicodynamics
of Aroclor 1254 in the male rat. Environ. physiol. Biochem.,
4: 214-225.
GRANT, D. L. & PHILLIPS, W. E. J. (1974) The effect of age and sex on
the toxicity of Aroclor 1254, a polychlorinated biphenyl, in the
rat. Bull. environ. Contam. Toxicol., 12: 145-152.
GRANT, D. L., PHILLIPS, W. E. J. & VILLENEUVE, D.C. (1971a) Metabolism
of a polychlorinated biphenyl (Aroclor 1254) mixture in the rat.
Bull. environ. Contam. Toxicol., 6: 102-112.
GRANT, D. L., VILLENEUVE, D.C., MCCULLY, K. A. & PHILLIPS, W. E. J.
(1971b) Placental transfer of polychlorinated biphenyls in the
rabbit. Environ. Physiol., 1: 61-66.
GREEN, S. G., CARR, J. V., PALMER, K. A. & OSWALD, E. J. (1975) Lack
of cytogenetic effects in bone marrow and spermatagonial cells in
rats treated with polychlorinated biphenyls (Aroclor 1242 and
1254). Bull. environ. Contam. Toxicol., 13: 14-22.
HAMMER, D. I., FINKLEA, J. F., PRIESTER, L. E., KEIL, J. E., SANDIFER,
S. H. & BRIDBORN, K. (1972) Polychlorinated biphenyl residues in
the plasma and hair of refuse workers. Environ. Health
Perspect., 1 (15): 83.
HAMMOND, A. L. (1972) Chemical pollution: polychlorinated biphenyls.
Science, 175: 155-156.
HANSEN, D. J., PARRISH, P. R., LOWE, J. I., WILSON, A. J. JR & WILSON,
P. D. (1971) Chronic toxicity, uptake and retention of Aroclor
1254 in two estuarine fishes. Bull. environ. Contam. Toxicol.,
6: 113-119.
HAQUE, R., SCHMEDDING, D. W. & FREED, V. H. (1974) Aqueous solubility,
absorption and vapour behaviour of polychlorinated biphenyl
Aroclor 1254. Environ. Sci. Technol., 8: 139-142.
HARA, I., HARADA, H., KIMURA, S., ENDO, T. & KAWANO, K. (1974) Follow
up health examination in an electric condenser factory after
cessation of PCBs usage (1st report) Jpn. J. Ind. Health,
16: 365-366 (in Japanese).
HARVEY, G. R. & STEINHAUER, W. G. (1974) Atmospheric transport of
polychlorobiphenyls to the North Atlantic. Atmos. Environ.,
8: 777-782.
HARVEY, G. R., STEINHAUER, W. G. & TEAL, J. M. (1973) Polychloro-
biphenyls in North Atlantic Ocean water. Science, 80: 643-644.
HASEGAWA, H., SATO, M. & TSURUTA, H. (1972a) PCBs concentration in the
blood of workers handling PCB. Occup. Health, 10: 50-55
(in Japanese).
HASEGAWA, H., SATO, M. & TSURUTA, H. (1972b) PCB concentration in air
of PCB-using plants and health examination of workers (in
Japanese). In: Report on Special Research on Prevention of
Environmental Pollution by PCB-like Substances. Tokyo, Research
Co-ordination Bureau, Science and Technology Agency, pp. 141-149.
HEATH, R. G., SPANS, J. W., KREITZER, J. F. & VANCE, C. (1970)
Effects of polychlorinated biphenyls on birds In: Proceedings
of the 15th Congress of International. Ornithology, The Hague,
pp. 475-485.
HESSELBERG, R. J. & SCHERR, D. D. (1974) PCBs and p,p' DDE in the
blood of cachectic patients. Bull. environ. Contam. Toxicol.,
11: 202-205.
HIRAYAMA, C., IRISA, T. & YAMAMOTO, T. (1969) Fine structural changes
of the liver in a patient with chlorobiphenyls intoxication.
Fukuoka Acta Med., 60: 455-461 (in Japanese).
HIRAYAMA, C., OKUMURA, M., NAGAI, J. & MASUDA, Y. (1974)
Hypobilirubinemia in patients with polychlorinated biphenyls
poisoning. Clin. chim. Acta., 55: 97-100.
HOLDGATE, M. W. (1971) The sea bird wreck in the Irish Sea, Autumn
1969. Natural Environmental Research Council (Publication
Series C4).
HOLDEN, A. V. (1970a) International co-operative study of
organochlorine pesticide residues in terrestial and aquatic
wildlife 1967/1968. Pest. monit. J., 4: 117-135.
HOLDEN, A. V. (1970b) Source of polychlorinated biphenyl contamination
in the marine environment. Nature (Lond.), 228: 1220-1221.
HOLDEN, A. V. (1973a) International co-operative study of
organochlorine and mercury residues in wildlife, 1969-71. Pest.
monit. J., 7: 37-52.
HOLDEN, A. V. (1973b) Monitoring PCBs in water and wildlife. In:
PCB Conference II, Stockholm, Swedish Environment Protection
Board, Publication: 4E, pp. 23-33.
HOLDEN, A. V. & MARSDEN, K. (1969) Single-stage clean-up of animal
tissue extracts for organochlorine residue analysis.
J. Chromatogr., 44: 481-492.
HUTZINGER, O., JAMIESON, W. D., SAFE, S., PAULMANN, L. & AM , R.
(1974) Identification of metabolic dechlorination of highly
chlorinated bipheny bit. Nature (Lond.), 252: 698-699.
HUTZINGER, O., SASH, D. M., SAFE, S., DE FREITAS, A. S. W., NORSIROM,
R. J., WILDISH, D. J. & ZITKO, V. (1972a) Polychlorinated
biphenyls: metabolic behaviour of pure isomers in pigeons, rats
and brook trout. Science, 178: 312-313.
HUTZINGER, O., SAFE, S. & ZITKO, V. (1971) Polychlorinated biphenyls:
Synthesis of some individual chlorobiphenyls. Bull. environ.
Contam. Toxicol., 6: 209-219.
HUTZINGER, O., SAFE, S. & ZITKO, V. (1972b) Photochemical degradation
of chlorobiphenyls (PCBs). Environ. Health Perspect., 1: 15-20.
INNAMI, S. et al. (1974) PCB toxicity and nutrition II, PCB toxicity
and Vitamin A (2). J. Nutr. Sci-Vitaminol., 20: 363-370.
INTERNATIONAL AGENCY FOR RESEARCH ON CANCER (1974) Polychlorinated
biphenyls in IARC monographs on the evaluation of carcinogenic
risk of chemicals to man, Vol. 7, 261-289.
INTERNATIONAL COUNCIL FOR THE EXPLORATION OF THE SEA (1974) Report of
a Working Group for the international study of the pollution of
the North Sea and its effects on living resources and their
exploitation. Charlottenlund, Denmark (Co-operative Research
Report No. 39).
ISHI, H. (1972) PCB Pollution in Japan. Environ. Health Rep. No. 14,
Jpn. Publ. Health Assoc., pp. 13-28.
ITO, N., NAGASAKI, H, ARAI, M., MAKIURA, S., SUGIHARA, S. & HIRAO, K.
(1973) Histopathologic studies on liver tumorigenesis induced in
mice by technical polychlorinated biphenyls and its promoting
effect on liver tumours induced by benzene hexachloride. J. nat.
Cancer Inst., 51: 1637-1646.
IVERSON, F., VILLENEUVE, D.C., GRANT, D. L. & HATINA, G. V. (1975)
Effect of Aroclor 1016 and 1242 on selected enzyme systems in the
rat. Bull. environ. Contam. Toxicol., 13: 456-463.
IWATA, Y., WESTLAKE, W. E. & GUNTHER, F. A. (1973) Varying persistence
of polychlorinated biphenyls in six California soils under
laboratory conditions. Bull. environ. Contam. Toxicol.,
9: 204-211.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTROM, G. & VAZ,
R. (1975) Identification by GC-MS of phenolic metabolites of PCB
and p,p'-DDE isolated from Baltic guillemot and seal. Ambio.,
4: 93-97.
JENSEN, S. (1973) (no title) Report to Swedish National Environment
Protection Board, Stockholm, 12 October 1973.
JENSEN, S. (1974) Identification of some organic substances
potentially harmful to the environment. National Swedish
Environment Protection Board, University of Stockholm, Kollenberg
Laboratory (Publication SNV-PM 520).
JENSEN, S. & SUNDSTROM, G. (1974) Structures and levels of most
chlorobiphenyls in two technical PCB products and in human adipose
tissue. Ambio, 3: 70-76.
JENSEN, S. & SUNDSTROM, G. (1975) Metabolic hydroxylation of a
chlorobiphenyl containing only isolated unsubstituted positions --
2,2',4,4',5,5'-hexachlorobiphenyl. Nature (Lond.)
(in press).
JENSEN, S., JOHNELS, A. G., OLSSEN, M., OTTERLIND, G. (1969) DDT and
PCB in marine animals from Swedish waters. Nature (Lond.),
224: 247-250.
JENSEN, S., JOHNELS, A. G., OLSSON, M. & OTTERLIND, G. (1972b) DDT and
PCB in herring and cod from the Baltic, the Kattegat and the
Skaggerrak. Ambio spec. Rep., No. 1: 71-85.
JENSEN, S., JOHNELS, A. G., OLSSEN, M. & WESTERMARK, T. (1972c) The
avifaune of Sweden as indicators of environmental contamination
with mercury and chlorinated hydrocarbons. In: Proceedings of
the 15th International Ornithology Congress, Leiden,
pp. 455-465.
JENSEN, S., RENBERG, L. & OLSSON, M. (1972a) PCB contamination from
boat bottom paint and levels of PCB in plankton outside a polluted
area. Nature (London), 240: 358-360.
JENSEN, S., RENBERG, L. & VAZ, R. (1973) Problems in quantification
of PCB in biological material. In: PCB Conference II. National
Swedish Environment Protection Board. Publications: 4E, pp. 7-13.
JOHANSSON, N., JENSEN, S. & OLSSON, M. (1970) PCB indications of
effects on fish. In: PCB Conference, National Swedish
Environment Protection Board, pp. 59-67.
JOHNSTONE, G. J., ECOBICHON, D. J. & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, D.C. L., DAVIS, W. E., JR, NEWELL, G. W., SASMORE, D. P. &
ROSEN, V. J. (1974) Modification of hexachlorophene toxicity by
dieldrin and Aroclor 1254. Toxicology, 2: 309-318.
KAISER, K. L. E. & WONG, P. T. S. (1974) Bacterial degradation of
polychlorinated biphenyls. I. Identification of some metabolic
products from Aroclor 1242. Bull. environ. Contam. Toxicol.,
11: 291-296.
KARPPANEN, E. & KOLHO, L. (1973) The concentration of PCB in human
blood and adipose tissue in three different research groups. In:
PCB Conference II. National Swedish Environment Protection Board
Publications: 4E: pp. 124-127.
KATSUKI, S. (1969) Foreword. Fukuoka Acta Med., 60: 403-407.
KAWANISHI, S., SANO, S., MIZUTANI, T. & MATSUMOTO, M. (1973)
Experimental porphyria induced by polychlorinated biphenyls (in
Japanese). Jpn. J. Hyg., 28: 84.
KAWANISI, S., SANO, S., MIZUTANI, T. & MATSUMOTO, M. (1974)
Experimental studies on toxicity of synthetic tetrachlorobiphenyl
isomers (in Japanese). Jpn. J. Hyg., 29: 81.
KEIL, J. E., PRIESTER, L. E. & SANDIFER, S. H. (1971) Polychlorinated
biphenyl (Aroclor 1242): effects of uptake on growth, nucleic
acids and chlorophyll of a marine diatom. Bull. environ. Contam.
Toxicol., 6: 156-159.
KEPLINGER, M. L., FANeHER, O. E. & CALANDRA, J. C. (1971) Toxicologic
studies with polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 19: 402-403.
KIHLSTROM, J. E., ORBERG, J., LUNDBERG, C., DANIELSSON, P.O. &
SYDHOFF, J. (1973) Effects of PCB on mammalian reproduction. In:
PCB Conference II. National Swedish Environment Protection Board
Publications 4E, pp. 109-111.
KIMBROUGH, R. D. (1974) The toxicity of polychlorinated polycyclic
compounds and related chemicals. Crit. Rev. Toxicol.,
2: 445-498.
KIMBROUGH, R. D. & LINDER, R. E. (1974) Induction of adenofibrosis and
hepatomas of the liver in BALB/cJ mice by polychlorinated
biphenyls (Aroclor 1254). J. nat. Cancer Inst., 53: 547-549.
KIMBROUGH, R. D., LINDER, R. E. & GAINES, T. B. (1972) Morphological
changes in livers of rats fed polychlorinated biphenyls. Arch.
environ. Health, 25: 354-364.
KIMBROUGH, R. D., LINDER, R. E., BURSE, V. W., JENNINGS, R. W. & GA,
C. (1973) Adenofibrosis in the rat liver with persistence of
polychlorinated biphenyls in adipose tissue. Arch. environ.
Health, 27: 389-395.
KIMBROUGH, R. D., SQUIRE, R. A., LINDER, R. E., STRANDBERG, J. D.,
MONTALI, R. J. & BURSE, V. W. (1975) Induction of liver tumours in
Sherman strain female rats by polychlorinated biphenyl Aroclor
1260. J. nat. Cancer Inst., 55: 1453-1459.
KIMURA, N. T. & BABA, T. (1973) Neoplastic changes in the rat liver
induced by polychlorinated biphenyl. GANN, 64: 105-108.
KITAMURA, M., TSUKAMOTO, T., SUMINO, K., HAYAKAWA, K., SHIBITA, T. &
HIRANO, I. (1973) The PCB levels in the blood of workers employed
in a condenser factory. Jpn. J. indust. Health, 47: 354-355
(in Japanese).
KOBAYASHI, Y. (1972) Answer Report to the Questionary paper on
"Regulation of Residual level in Foods". Biol. Pollut.,
4: 93-116 (in Japanese).
KOEMAN, J. H., TEN NOEVER DE BRAUW & DE VOS, R. H. (1969) Chlorinated
biphenyls in fish, mussels and birds from the river Rhine and the
Netherlands coastal area. Nature (Lond), 221: 1126-1128.
KOEMAN, J. H., BOTHOF, TH., DE VRIES, R., VAN VELZEN-BLAD, H. & VOS,
J. G. (1972a) The impact of persistent pollutants on piscivorous
and molluscivorous birds. TNO-nieuws, 27: 561-569.
KOEMAN, J. H., PEETERS, W. H. M., SMIT, C. J., TJIOE, P, S. & DE
GOEIJ, J. J. M. (1972b) Persistent chemicals in marine mammals.
TNO-nieuws, 27: 570-578.
KOEMAN, J. H., VAN VELZEN-BLAD, H. C. W., DE VRIES, R. & Vos, J. G.
(1973) Effects of PCB and DDE in cormorants and evaluation of PCB
residues from an experimental study. J. Reprod. Fertil.,
19 (Suppl.): 353-364.
KOHANAWA, M., SHOYA, S., OGURA, Y., MORIWAKI, M. & KAWASAKI, M.
(1969a) Poisoning due to an oily by-product of rice-bran similar
to chick edema disease. I. Occurrence and toxicity test. Nat.
Inst. anim. Health Quart., 9: 213-219.
KOHANAWA, M., SHOYA, S., YONEMURA, T., NISHIMURA, K. & TSUSHIO, Y.
(1969b) Poisoning due to an oily by-product of rice-bran similar
to chick edema disease. II. Tetrachlorodiphenyl as toxic
substance. Nat. Inst. anim. Health Quart., 9: 220-228.
KOLBYE, A. C. JR (1972) Food exposures to polychlorinated biphenyls.
Environ. Health Perspect., 1: 85-88.
KOLLER, L. D. & ZINKL, J. G. (1973) Pathology of polychlorinated
biphenyls in rabbits. Am. J. Pathol., 70: 363-373.
KOMATSU, F. & KIKUCHI, M. (1972) Skin lesions by 3,4,Y,4'-tetra-
chlorobiphenyl in rabbits. Fukuoka Acta Med., 63: 384-386
(in Japanese).
KURATSUNE, M. (1972a) PCB pollution. Kosei no Shihyo, 19: 11-18
(in Japanese). KURATSUNE, M. & MASUDA, Y. (1972b) Polychlorinated
biphenyls in non-carbon copypaper. Environ. Health Perspect.,
1: 61-62.
KURATSUNE, M., YOSHIMURA, T. & MATSUZAKA, J. (1972) Epidemiologic
study on Yusho, a poisoning caused by ingestion of rice oil
contaminated with a commercial brand of polychlorinated biphenyls.
Environ. Health Perspect., 1: 119-128.
KUSUDA, M. (1971) Study on the female sexual function suffering from
the chlorobiphenyls poisoning. Sanka to Fujinka, 4: 1063-1072
(in Japanese).
LINCER, J. L. & PEAKALL, D. B. (1970) Metabolic effects of
polychlorinated biphenyls in the American kestrel. Nature
(Lond.), 228: 783-784.
LICHTENSTEIN, E. P. (1972) PCBs and interactions with insecticides.
Environ. Health Perspect., 1: 151-153.
LIEB, A. J., BILLS, D. B. & SINNHUBER, R. O. (1974) Accumulation of
dietary polychlorinated biphenyls (Aroclor 1254) by rainbow trout
(Salmo gairdneri). J. agr. Food Chem., 22: 638-642.
LINDER, R. E., GAINES, T. B. & KIMBROUGH, R. D. (1974) The effect of
polychlorinated biphenyls on rat reproduction. Food cosmet.
Toxicol., 12: 63-77.
LITTERST, C. L., FARBER, T. M., BAKER, A.M. & VAN LOON, E. J. (1972)
Effect of polychlorinated biphenyls on hepatic microsomal enzymes
in the rat. Toxicol. appl. Pharmacol., 23: 112-122.
MAKIURA, S., AOE, H., SUGIHARA, S., HIRAO, K., ARAI, M. & ITO, N.
(1974) Inhibitory effect of polychlorinated biphenyls on liver
tumorigenesis in rats treated with 3'-methyl-4-dimethyl-
aminoazobenzene, N-2-fluorenylacetamide, and diethylnitrosamine.
J. nat. Cancer Inst., 53: 1253-1257.
MASUDA, Y., KAGAWA, R. & KURATSUNE, M. (1972) Polychlorinated
biphenyls in carbonless copying paper. Nature (Lond.),
237: 41-42.
MASUDA, Y., KAGAWA, R. & KURATSUNE, M. (1974a) Polychlorinated
biphenyls in Yusho patients and ordinary persons. Fukuoka Acta
Med., 65: 17-24 (in Japanese).
MASUDA, Y., KAGAWA, R., SHIMAMURA, K., TAKADA, M. & KURATSUNE, M.
(1974b) Polychlorinated biphenyls in the blood of Yusho patients
and ordinary persons. Fukuoka Acta Med., 65: 25-27
(in Japanese).
MCKINNEY, J. D. (in press) Toxicology of selected symmetrical
hexachlorobiphenyl isomers : correlating biological effects with
chemical structure. In: Proceedings of the National Conference
on Polychlorinated Biphenyls, Chicago, 19-21 November, 1975.
MCLAUGHLIN, J. JR., MARLIAC, G. P., MERRETT, M. J., MUTCHLER, M. K. &
FITZHUGH, O. G. (1963) The injection of chemicals into the yolk
sac of fertile eggs prior to incubation as toxicity test.
Toxicol. appl. Pharmacol., 5: 760-771.
MELVÅS, B. & BRANDT, I. (1973) The distribution and metabolism of
labelled polychlorinated biphenyls in mice and quails. In: PCB
Conference II. National Swedish Environment Protection Board
Publications: 4E, pp. 87-90.
MES, J., COFFIN, D. E. & CAMPBELL, D. (1974) Polychlorinated biphenyl
and organochlorine pesticide residues in Canadian chicken eggs.
Pestic. Monit. J., 8: 8-11.
MIURA, H., OMORI, S., KATOH, M. (1973) Experimental porphyria in
rabbits induced by PCB. Jpn. J. Hyg, 28: 83 (in Japanese).
MOORE, J. A. (in press) Toxicity of 2,3,7,8-tetrachlorodibenzofuran:
preliminary results. Proceedings of the National Conference on
Polychlorinated Biphenyls, Chicago, 19-21 November.
MOSSER, J. L., FISHER, N. S., TENG, T. & WURSTER, C. F. (1972)
Polychlorinated biphenyls: toxicity to certain phytoplankters.
Science, 175: 191-192.
MULHERN, B. M., CROMARTIE, E., REICHEL, W. L. & BELISLE, A. A. (1971)
Semiquantitative determination of polychlorinated biphenyls in
tissue samples by thin layer chromatography. J. Assoc. Off.
Agric. Chem., 54: 548-550.
MORNS, Y. & KUROIWA, Y. (1971) Peripheral neuropathy in chlorobiphenyl
poisoning. Neurol., 21: 1173-1176.
MUSIAL, C. J., HUTZINGER, O., ZITKO, V. & CROCKER, J. (1974) Presence
of PCB, DDE and DDT in human milk in the provinces of New
Brunswick and Nova Scotia, Canada. Bull. environ. Contam.
Toxicol., 12: 258-267.
NAGAI, J., FURUKAWA, M., YAE, Y. & IKEDA, Y. (1969) Clinicochemical
investigation of chlorobiphenyls poisoning. Especially on the
serum lipid analysis of the patients. Fukuoka Acta Med.,
60: 475-488 (in Japanese).
NAGAYAMA, J., KURATSUNE, M. & MASUDA, Y. (1976) Determination of
chlorinated dibenzofurans in Kanechlors and "Yusho Oil". Bull.
environ. Contam. Toxicol., 15(1): 9-13.
NAGAYAMA, J., MASUDA, Y. & KURATSUNE, M. (1975) Chlorinated
dibenzofurans in Kanechlors and rice oils used by patients with
Yusho, Fukuoka Acta Med., 66: 593-599.
NATIONAL SWEDISH ENVIRONMENT PROTECTION BOARD (1973) PCB Conference
II. Publication 1973: 4E.
NEBEKER, A. V., PUGLISI, F. h. & DEFOE, D. L. (1974) Effect of
polychlorinated biphenyl compounds on survival and reproduction of
the fathead minnow and flagfish. Trans. Am. Fish Soc.,
No. 3, 562-568.
NESTEL, H. & BUDD, J. (1975) Chronic oral exposure of rainbow trout
(Salmo Gairdneri) to a PCB (Aroclor 1254): pathological effects.
Can. J. comp. Med., 39: 208-215.
NILSSON, B. & RAMEL, C. (1974) Genetic tests on Drosophila
melanogaster with polychlorinated biphenyls (PCB). Heriditas,
77: 319-322.
NIMMO, D. R., WILSON, P. D., BLACKMAN, R. R. & WILSON, A. J. JR (1971)
Polychlorinated biphenyl absorbed from sediments by fiddler crabs
and pink shrimp. Nature (Lond.), 231: 50-52.
NISBET, I. C. T. & SAROFIM, A. F. (1972) Rates and routes of transport
of PCBs in the environment. Environ. Health Perspect. 1: 21-38.
NISHIMOTO, T, UEDAM, M, TAUL, S. & CHIKAZAWA, K. (1972a)
Organochlorine pesticide residues and PCB in breast milk. Igaku
no Ayumi (Proc. Med. Sci.), 82: 574-575 (in Japanese).
NISHIMOTO, T., UETA, M., TAUL, S., CHIKAZAWA, K. NISHIUCHI, I. &
KONDO, K. (1972b) Deposition of organochlorine pesticide residues
and PCB in human body fat. Igaku no Ayumi (Proc. med. Sci.),
82: 515-516.
NISHIZUMI, M. (1970) Light and electron microscope study of
chlorobiphenyl poisoning in mouse and monkey liver. Arch.
environ. Health, 21: 620-632.
NORÉN, K. & WESTOO, G. (1968) Determination of some chlorinated
pesticides in vegetable oils, margarine, butter, milk, eggs, meat
and fish by gas chromatography and thin-layer chromatography.
Acta chem. scand., 22: 2289-2293.
ODÉN, S. & BERGGREN, B. (1973) PCB and DDT in Baltic sediments.
Report Dept. Soil Science, Agricultural College, Uppsala,
pp. 1-10.
ODSJO, T. (1973) PCB in some Swedish terrestrial organisms. In: PCB
Conference II. National Swedish Environment Protection Board
Publications: 4E, pp. 45-58. OKUMURA, M. & KATSUKI, S. (1969)
Clinical observation on Yusho (chlorobiphenyls poisoning).
Fukuoka Acta Med., 60: 440-446 (in Japanese).
OKUMURA, M., MASUDA, Y. & NAKAMUTA, S. (1974) Correlation between
blood PCB and serum triglyceride levels in patients with PCB
poisoning. Fukuoka Acta Med., 65: 84-87 (in Japanese).
OLSSON, M, JENSEN, S. & RENBERG, L. (1973) PCB in coastal areas of
the Baltic. PCB Conference H. National Swedish Environment
Protection Board, Publications: 4E, pp. 59-68.
ORBERG, J. & KIHLSTROM, J. E. (1973) Effects of long-term feeding of
polychlorinated biphenyls (PCB, Clopehn A60) on the length of the
oestrous cycle and on the frequency of implanted ova in the mouse.
Environ. Res., 6: 176-179.
ORGANIZATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT (1973)
Polychlorinated biphenyls, their use and control. Environmental
Directorate, Paris, OECD.
PANEL ON HAZARDOUS TRACE SUBSTANCES (1972) PCBs-Environmental Impact.
Environ. Res. 5: 249-362.
PEAKALL, D. B. LIMIAR, J. L. & BLOOM, S. E. (1972) Embryonic mortality
and chromosomal alterations caused by Aroclor 1254 in ring doves.
Environ. Health Perspect., 1: 103-104.
PESENDORFER, VON H., EICHLER, I. & GLOFKE, E. (1973) Informative
analyses or organochlorine pesticide and PCB residues in human
adipose tissue (from the area of Vienna). Wiener klinische
Wochenschr., 85: 218-222 (summary in English).
PHILLIPS, W. E. J., HATINA, G., VILLENEUVE, D.C. & GRANT, D. L. (1972)
Effect of parathion administration in rats following long-term
feeding with PCBs. Environ. physiol. Biochem., 2: 165-169.
PJCHIRALLO, J. (1971) PCBs: leaks of toxic substances raises issue of
effects, regulations. Science, 173: 899-902.
PLATONOW, N. S. & FUNNELL, H. S. (1971) Anti-androgenic-like effect of
polychlorinated biphenyls in cockerels. Vet. Rec, 88: 109-110.
PLATONOW, N. & CHEN, N.Y. (1973) Transplacental transfer of
polychlorinated biphenyls (Aroclor 1254) in a cow. Vet. Rec.,
92: 69-70.
PLATONOW, N. S., FUNNELL, H. S., BULLOCK, D. H., ARNOTT, D. R.,
SASCHENBRECKER, P. W. & GRIEVE, D. O. (1971) Fate of
polychlorinated biphenyls in dairy products processed from the
milk of exposed cows. J. Dairy Sci., 54: 1305-1308.
PLATONOW, N. S., LIPTRAP, R. M. & GEISSINGER, H. D. (1972) The
distribution and excretion of polychlorinated biphenyls (Aroclor
1254) and their effect on urinary gonadal steroid levels in the
boar. Bull. environ. Contam. Toxicol., 7: 358-365.
PORTER, M. L., YOUNG, S. J. V. & BURKE, J. A. (1970) A method for the
analysis of fish, poultry and animal tissue for chlorinated
pesticide residues. J. Am. Off. Anal. Chem., 53: 1300-1303.
PORTMANN, J. E. (1970) Monitoring of organochlorine residues in fish
from around England and Wales, with special reference to
polychlorinated biphenyls. Report CM 1970/E: 9 International
Council for the Exploration of the Sea, Charlottenlund Slot,
DK-2920 Charlottenlund, Denmark.
PRESTT, I., JEFFERIES, D. J. & MOORE, N. W. (1970) Polychlorinated
biphenyls in wild birds in Britain and their avian toxicity.
Environ. Pollut., 1: 3-26.
PRICE, H. A. & WELCH, R. L. (1972) Occurrence of polychlorinated
biphenyls in humans, Environ. Health Perspect., 1: 73-78.
REHFELD, B. M., BRADLEY, R. L. JR & SUNDE, M. L. (1971) Toxicity
studies on polychlorinated biphenyls in the chick. 1. Toxicity and
symptoms. Poult. Sci., 50: 1090-1096.
REINKE, J., UTHE, J. F. & O'BRODOVlCH, H. (1973) Determination of
polychlorinated biphenyls in the presence of organochlorine
pesticides by thin-layer chromatography. Environ. Lett.,
4: 201-210.
REYNOLDS, L. M. (1971) Pesticide residue analysis in the presence of
polychlorobiphenyls (PCBs). Residue Rev., 34: 27-45.
RINGER, R. K., AULERICH, R. J. & ZABIK, M. (1972) Effect of dietary
polychlorinated biphenyls on growth and reproduction of mink.
Proc. Amer. chem. Soc., 164th A.C.S. Meeting, New York,
pp. 149-154.
RISEBROUGH, R. W. & DE LAPPE, B. (1972) Accumulation of
polychlorinated biphenyls in ecosystems. Environ. Health
Perspect., 1: 39-45.
RISEBROUGH, R. W., RIECHE, P., PEAKALL, D. B., HERMAN, S. G. & KIRVEN,
M. N. (1968) Polychlorinated biphenyls in the global ecosystem.
Nature (Lond.), 220: 1098-1102.
ROACH, J. A. G. & POMERANTZ, I. H. (1974) The finding of chlorinated
dibenzofurans in a Japanese polychlorinated biphenyl sample.
Bull. environ. Contam. Toxicol., 12: 338-342.
ROTE, J. W. & MURPHY, P. G. (1971) A method for the quantitation of
polychlorinated biphenyl (PCB) isomers. Bull environ. Contam.
Toxicol., 6: 377-384.
SAFE, S. & HUTZINGER, O. (1971) Polychlorinated biphenyls: photolysis
of 2,4,6,T,4',6'-hexachlorobiphenyl, Nature (Lond.),
232: 641-642.
SAITO, R., SHIGEMATSU, N. & ISHIMARU, S. (1972) Immunoglobulin levels
in serum and sputum of patients with PCB poisoning. Fukuoka Acta
Med., 63: 408-411 (in Japanese).
SANDERS, H. O. & CHANDLER, J. H. (1972) Biological magnification of a
polychlorinated biphenyl (Aroclor 1254) from water by aquatic
invertebrates. Bull. environ. Contam. Toxicol., 7: 257-263.
SASCHENBRECKER, P. W., FUNNELL, H. S. & PLATONOW, N. S. (1972)
Persistence of polychlorinated biphenyls in the milk of exposed
cows. Vet. Rec., 90: 100-102.
SAVAGE, E. P., TESSARI, J. D., MALBERG, J. W., WHEELER, H. W. & BAGBY,
J. R. (1973) A search for polychlorinated biphenyls in human milk
in rural Colorado. Bull. environ. Contam. Toxicol., 9: 222-226.
SCHMIDT, T. T., RISEBROUGH, R. W. & GRESS, F. (1971) Input of
polychlorinated biphenyls into California coastal waters from
urban sewage outfalls. Bull. environ. Contam. Toxicol.,
6: 235-243.
SCHULTE, E. & ACKER, L. (1974) Gas chromatographic unit Glascapillaran
bei Temperaturan bis zu 320°C. Z. Anal. Chem., 268: 261-267.
SCOTT, M. L., ZIMMERMAN, J. R., MARINSKY, S. & MULLENHOFF, P. A.
(1975) Effects of PCBs, DDT and mercury compounds upon egg
production, hatchability and shell quality in chickens and
Japanese quail. Poult. Sci., 54: 350-368.
SCHWETZ, B. A., NORRIS, J. M., SPARSCHU, G. L., ROWE, U. K., GEHRING,
P. J., EMERSON, J. L. & GERBIG, C. G. (1973) Toxicology of
chlorinated dibenzo- p-dioxin. Environ. Health Perspect.,
5: 87-99.
SHIGEMATSU, N., NORIMATSU, Y., ISHIBASHI, T., YOSHIDA, M., SUETSUGU,
S., KAWATSU, T., IKEDA, T., SAITO, R., ISHIMARU, S., SHIRAKISA,
T., KIDO, M., EMORI, K. & TOSHIMITSU, H. (1971) Clinical and
experimental studies on respiratory involvement in chlorobiphenyls
poisoning. Fukuoka Acta Med., 62: 150-156 (in Japanese).
SHIGEMATSU, N., ISHIMARU, S., HIROSE, T., IKEDA, T., EMORI, I. &
MIYAZAKI, N. (1974) Clinical and experimental studies on
respiratory involvement in PCB poisoning. Fukuoka Acta Med.,
65: 88-95 (in Japanese).
SISSONS, D. & WELTI, D. (1971) Structural identification of
polychlorinated biphenyls in commercial mixtures by gas-liquid
chromatography, nuclear magnetic resonance and mass spectrometry.
J. Chromatogr., 60: 15-32.
SODERGREN, A. (1972a) Chlorinated hydrocarbon residues in airborne
fallout. Nature (Lond.), 236: 395-397.
SODERGREN, A. (1972b) Transport, distribution and degradation of
chlorinated hydrocarbon residues in aquatic model ecosystems.
Oikos, 23: 30-41.
SODERGREN, A. (1973a) Transport, distribution and degradation of DDT
and PCB in a south Swedish lake ecosystem. Vatten, 2: 90-108.
SODERGREN, A. (1973b) A simplified clean-up technique for
organochlorine residues at the microliter level. Bull. environ.
Contam. Toxicol., 10: 116-119.
SODERGREN, A. & SVENSSON, Bj. (1973) Uptake and accumulation of DDT
and PCB by Ephemera danica (Ephemeroptera) in continuous-flow
systems. Bull. environ. Contam. Toxicol., 9: 345-350.
SODERGREN, A., SVENSSON, Bj. & ULFSTRAND, S. (1972) DDT and PCB in
South Swedish streams. Environ. Pollut., 3: 25-36.
SOLLY, S. R. B. & SHANKS, V. (1974) Polychlorinated biphenyls and
organochlorine pesticides in human fat in New Zealand. N.Z.J.
Sci., 17: 535-544.
SOSA-LUCERO, J. C. & BE LA IGLESIA, F. A. (1973) Distribution of a
polychlorinated terphenyl (PET) (Aroclor 5460) in rat tissues and
effect on hepatic microsomal mixed function oxidases. Bull.
environ. Contam. Toxicol., 10: 248-256.
STALLING, D. L. & MAYER, F. L. JR (1972) Toxicities of PCBs to fish
and environmental residues. Environ. Health Perspect.,
1: 159-164.
STALLING, D. L., TRINDLE, R. C. & JOHNSON, J. L. (1972) Clean up of
pesticides and polychlorobiphenyl residues in fish extracts by gel
permeation chromatography. J. Assoc. Off. Anal. Chem.,
55: 32-45.
TAKAMATSU, M., INOUE, Y. & ABE, S. (1974) Diagnostic meaning of the
blood PCB. Fukuoka Acta Med., 65:28-31 (in Japanese).
TAKI, I., HISANAGA, S. & AMAGESE, Y. (1969) Report on Yusho
(chlorobiphenyl poisoning). Especially further study of its
dermatological findings. Fukuoka Acta Med., 62: 132-138
(in Japanese).
TAKI, I., KURATSUNE, M., MASUDA, Y. (1973) Studies on the
transmission to the fetus through placenta and the health of
and baby. Special Research Report on the effects of PCBs on human
health such as chronic toxicity for prevention of pollution by
PCBs. Research Co-ordination Bureau, Science and Technology
Agency, pp. 87-95 (in Japanese).
TAKIZAWA, Y. & MINAGAWA, K. (1974) Studies on environmental
accumulation and bio-accumulation of organochloric compounds.
Mainly mentioned PCT. Studies on the body effect of
degradation-registration substances, pp. 29-50 (in Japanese).
TANAKA, K. & ARAKI, Y. (1974) Inhibitory effect of cholestyramine on
the intestinal absorption of PCB. Fukuoka Acta Med., 68: 53-57.
TANAKA, K. & KOMATSU, F. (1972) Shortening of hexobarbital sleeping
time after small doses of PCB in rats. Fukuoka Acta Med.,
63: 360-366 (in Japanese).
TAS, A. C. & DE VOS, R. H. (1971) Characterization of four major
components in a technical polychlorinated biphenyl mixture.
Environ. Sci. Technol., 5: 1216-1218.
TATSUKAWA, R. & WATANABE, I. (1972) Air pollution by PCBs. Shoku No
Kagaku, 8: 55-63.
THOMAS, G. H. & REYNOLDS, L. M. (1973) Polychlorinated terphenyls in
paperboard samples. Bull. environ. Contam. Toxicol.,
10: 37-41.
TREON, J. F., CLEVELAND, F. P., CAPPEL, J. W. & ATCHLEY, R. W. (1956)
The toxicity of the vapours of Aroclor 1242 and Aroclor 1254.
Am. Ind. Hyg. Assoc. Quart., 17: 204-213.
TSUKAMOTO, H. ET AL. (1969) The chemical studies on detection of toxic
compounds in the rice bran oils used by the patients of Yusho.
Fukuoka Acta Med., 60: 496-512 (in Japanese).
TUCKER, R. K. & CRABTREE, D. G. (1970) Handbook of toxicity of
pesticides to wildlife. Bureau of Sport, Fisheries and Wildlife
Resources Pub. No. 84, Washington, DC, US Dept. of Interior,
pp. 130.
TUCKER, E. S., LITSCHGI, W. S. & MEES, W. M. (1975) Migration of
polychlorinated biphenyls in soil induced by percolating water.
Bull. environ. Contam. Toxicol., 13: 86-93.
URABE, H. (1974) Foreword. Fukuoka Acta Med., 65: 1-4 (in Japanese).
USDA/USDC/EPA/FDA/USDI/(1972) Polychlorinated biphenyls and the
environment. Washington DC, Interdepartmental Task Force on
PCBs. (Report ITF-PCB-72-1).
USHIO, F., FUKANO, S., NISHIDA, K., KANI, T. & DOGUCHI, M. (1974) Some
attempt to estimate the total daily intake of pesticides and PCB
residues and trace heavy metals. Ann. Rep. Tokyo Metr. Res. Lab.
P.H., 25: 307-312 (in Japanese).
UTHE, J. F., REINKE, J. & GESSER, H. (1972) Extraction of
organochlorine pesticides from water by porous polyurethane coated
with selective absorbent. Environ. Lett., 3: 117-135.
UZAWA, H., ITO, Y., NOTOMI, A. & KATSUKI, S. (1969) Hyperglyceridemia
resulting from intake of rice oil contaminated with chlorinated
biphenyls. Fukuoka Acta Med., 60: 449-454 (in Japanese).
UZAWA, H., NOTOMI, A., NAKAMUTA, S. & IKEURA, Y. (1972) Consecutive
three year follow up study of serum triglyceride concentrations of
82 subjects with PCB poisoning. Fukuoka Acta Med., 63: 401-404
(in Japanese).
VAN HOVE HOLDRINET, M. (1975) Preliminary results of an inter-
laboratory PCB check sample programme. J. Environ. Qual. (in press).
VEITH, G. D. (1972) Recent fluctuations of chlorobiphenyls (PCBs) in
the Green Bay, Wisconsin, region. Environ. Health Perspect.,
1: 51-54.
VILLENEUVE, D. C., GRANT, D. L., PHILLIPS, W. E. J., CLARK, M. L. &
CLEGG, D. J. (1971 a) Effects of PCB administration on microsomal
enzyme activity in pregnant rabbits. Bull. environ. Contam.
Toxicol., 6: 120-128.
VILLENEUVE, D. C., GRANT, D. L., KHERA, K., CLEGG, D. J., BAER, H. &
PHILLIPS, W. E. J. (1971b) The fetotoxicity of a polychlorinated
biphenyl mixture (Aroclor 1254) in the rabbit and in the rat.
Environ. Physiol., 1: 67-71.
VILLENEUVE, D.C., GRANT, D. L. & PHILLIPS, W. E. J. (1972)
Modification of pentobarbital sleeping times in rats following
chronic PCB ingestion. Bull. environ. Contam. Toxicol.,
7: 264-269.
VILLENEUVE, D.C., REYNOLDS, L. M., THOMAS, G. H. & PHILLIPS, W. E. J.
(1973a) Polychlorinated biphenyls and polychlorinated terphenyls
in Canadian food packaging materials. d. Assoc. Offic. Anal.
Chem., 56: 999-1001.
VILLENEUVE, D.C., REYNOLDS, L. M. & PHILLIPS, W. E. J. (1973b)
Residues of PCBs and PCTs in Canadian and imported European
cheeses, Canada-1972. Pestic. Monit. J., 7: 95-96.
VODDEN, H. A. (1973) Discussion In: PCB Conference II. National
Swedish Environment Protection Board, Publications: 4E, p. 118.
VOS, J. G. (1972) Toxicology of PCBs for mammals and for birds. Era,.
Health Perspect., 1: 105-117.
VOS, J. G. & BEEMS, R. B. (1971) Dermal toxicity studies of technical
polychlorinated biphenyls and fractions thereof in rabbits.
Toxicol. appl. Pharmacol., 19: 617-633.
VOS, J. G. DE ROIJ, Th. (1972) Immunosuppressive activity of a
polychlorinated biphenyl preparation on the humoral immune
response in guinea pigs. Toxicol. appl. Pharmacol., 21: 549-555.
VOS, J. G. & KOEMAN, J. H. (1970) Comparative toxicologic study with
polychlorinated biphenyls in chickens with special reference to
porphyria, edema formation, liver necrosis and tissue residues.
Toxicol. appl. Pharmacol., 17: 656-668.
VOS, J. G. & NOTENBOOM-RAM, E. (1972) Comparative toxicity study of
2,4,5,2',4',5'-hexachlorobiphenyl and a polychlorinated biphenyl
mixture in rabbits. Toxicol. appl. Pharmacol., 23: 563-578.
VOS, J. G. & VAN DRIEL-GROOTENHUIS, L. (1972) PCB-induced suppression
of the humoral and cell-mediated immunity in guinea pigs. Sci.
Total Environ., 1: 289-302.
VOS, J. G., KOEMAN, J. H., VAN DER MAAS, H. L., TEN NOEVER DE BRAUW,
M. G. & DE VOS, R. E. (1970) Identification and toxicological
evaluation of chlorinated dibensofuran and chlorinated naphthalene
in two commercial polychlorinated biphenyls. Fd. cosmet.
Toxicol., 8: 625-633.
VOS, J. G., STRIK, J. J. T. W. A., VAN HOLSTEYN, C. M. W. & PENNINGS,
J. H. (1971) Polychlorinated biphenyls as inducers of hepatic
porphyria in Japanese quail, with special reference to
delta-aminolevulinic acid synthetase activity, fluorescence and
residues in the liver. Toxicol. appl. Pharmacol., 20: 232-240.
WEBB, R. G. & MCCALL, A. C. (1972) Identification of polychlorinated
biphenyl isomers in aroclors. J. Am. Off. Anal. Chem.,
55: 746-752.
WESTOO, G. & NORÉN, K. (1970a) Levels of organochlorine pesticides and
polychlorinated biphenyls in fish caught in Swedish water areas or
kept for sale in Sweden, 1967-1970. Var Föda, 3: 93-146
(summary in English).
WESTOO, G. & NORÉN, K. (1970b) Determination of organochlorine
pesticides and polychlorinated biphenyls in animal foods. Acta
Chem. Scand., 24: 1639-1644.
WESTOO, G. & NORÉN, K. (1972) Levels of organochlorine pesticides and
polychlorinated biphenyls in Swedish human milk. Var Föda,
24: 41-54 (summary in English).
WESTOO, G., NORÉN, K. & ANDERSSON, M. (1971) Levels of organochlorine
pesticides and polychlorinated biphenyls in some cereal products.
Var Föda, 10: 341-360 (summary in English).
WHO WORKING GROUP (1973) The hazards to health and ecological effects
of persistent substances in the environment -- polychlorinated
biphenyls. Report of a Working Group convened by the World
Health Organization, Regional Office for Europe, EURO, 3109(2).
WILDISH, D. J. (1970) The toxicity of polychlorinated biphenyls (PCB)
in sea water to Grammarus oceanicus. Bull. environ. Contam.
Toxicol., 5: 202-204.
WILLIAMS, R. & HOLDEN, A. V. (1973) Organochlorine residues from
plankton. Mar. Bull., 4, 109-111.
WILLIS, D. E. & ADDISON, R. F. (1972) Identification and estimation of
the major components of a commercial polychlorinated biphenyl
mixture, Aroclor 1221. J. Fish. Res. Board Can., 29(5): 592-595.
WONG, P. T. S. & KAISER, K. L. E. (1975) Bacterial degradation of
polychlorinated biphenyls. II. Rate studies. Bull. environ.
Contam. Toxicol, 13: 249-256.
YAGAMUCHI, A., YOSHIMURA, T. & KURATSUNE, M. (1971) A survey on
pregnant women having consumed rice oil contaminated with
chlorobiphenyls and their babies. Fukuoka Acta Med.,
62: 112-117 (in Japanese).
YAMAMOTO, H. & YOSHIMURA, H. (1973) Metabolic studies on
polychlorinated biphenyls. III. Complete structure and acute
toxicity of the metabolites of 2,4,3',4'-tetrachlorobiphenyl.
Chem. pharm. Bull., 21 (10): 2237-2242 (in English).
YOBS, A. R. (1972) Levels of polychlorinated biphenyls in adipose
tissue of the general population of the nation. Environ. Health
Perspect., 1: 79-81.
YOSHIMURA, T. (1971) Epidemiological analysis of "Yusho" patients with
special reference to sex, age, clinical grades and oil
consumption. Fukuoka Acta Med., 62: 109-116 (in Japanese).
YOSHIMURA, H. & YAMAMOTO, H. (1973) Metabolic studies on
polychlorinated biphenyls. I. Metabolic fate of
3,4,3',4'-tetrachlorobiphenyl in rats. Chem. pharm. Bull.,
21: 1168-1169 (in English).
YOSHIMURA, H. & YAMAMOTO, H. (1974) Metabolic studies on
polychlorinated biphenyls. IV. Biotransformation of
3,4,3',4'-tetrachlorobiphenyl, one of the major components of
Kanechlor-400. Fukuoka Acta Med., 65: 5-11 (in Japanese).
YOSHIMURA, H. & YAMAMOTO, H. (1975) A novel route of excretion of
2,4,3',4'-tetrachlorobiphenyl in rats. Bull. environ. Contam.
Toxicol., 13: 681-687.
YOSHIMURA, H., YAMAMOTO, H., NAGAI, J., YAE, Y., UZAWA, H., ITO, Y.,
NOTOMI, A., MIMAKAMI, S., ITO, A., KATO, K. & TSUJI, H. (1971)
Studies on the tissue distribution and the urinary and faecal
excretion of 3H-Kanechlor (chlorobiphenyls) in rats. Fukuoka
Acta Med., 62: 12-19 (in Japanese).
YOSHIMURA, H., YAMAMOTO, H. & SAEKI, S. (1973) Metabolic studies on
polychlorinated biphenyls. II. Metabolic fate of
2,4,3'4'-tetrachlorobiphenyl in rats. Chem. pharm. Bull.,
21: 2231-2236 (in English).
YOSHIMURA, H., YAMAMOTO, H. & KINOSHITA, H. (1974) Metabolic studies
on polychlorinated biphenyls. V. Biliary excretion of
5-hydroxy-2,4,3',4',-tetrachlorobiphenyl, a major metabolite of
2,4,3',4'-tetrachlorobiphenyl. Fukuoka Acta Med., 65: 12-16
(in Japanese).
ZIMMERLI, B. & MARES, B. (1973) Die Belastung der Schweizerischen
Bevolkerung mit Pestiziden. Mitt. Lebensm. Unters. Hyg.,
64: 459-479.
ZITKO, V. (1970) Polychlorinated biphenyls (PCB) solubilized in water
by nonionic surfactants for studies of toxicity to aquatic
animals. Bull. environ. Contam. Toxicol., 5: 279-285.
ZITKO, V. (1971) Polychlorinated biphenyls and organochlorine
pesticides in some fresh-water and marine fishes. Bull. environ.
Contam. Toxicol., 6: 464-470.
ZITKO, V., HUTZINGER, O. & SAFE, S. (1971) Retention times and
electron-capture detector responses of some individual
chlorobiphenyls. Bull. environ. Contam. Toxicol., 6: 160-163.
ZITKO, V., HUTZINGER, O., JAMIESON, W. D. & CHOI, P. M. K. (1972a)
Polychlorinated terphenyls in the environment. Bull. environ.
Contam. Toxicol., 7: 200-201.
ZITKO, V., HUTZINGER, O. & CHOI, P.M. K. (1972b) Contamination of the
Bay of Fundy-Gulf of Maine area with polychlorinated biphenyls,
polychlorinated terphenyls, chlorinated dibenzodioxins and
dibenzofurans. Environ. Health Perspect., 1: 47-50.
ZITKO, V., CHOI, P. M. K., WILDISH, D. J., MONAGHAN, C. F. & LISTER,
N. A. (1974) Distribution of PCBs and p,p'-DDE residues in
Atlantic herring and yellow perch in eastern Canada, 1972.
Post. Mon. J., 8: 105-109.
 There have been several investigations to identify individual PCBs
in commercial products. Sissons & Welti (1971) separated the
components of the Aroclors by column and gas-liquid chromatography,
and characterized many of the peaks by high-resolution mass
spectrometry and nuclear magnetic resonance, and by comparison with 40
synthesized Webb & McCall (1972) identified the gas-liquid
chromatographic with those of synthesized compounds by retention times
and spectrometry (Table 2). The most exhaustive study is that of
Jensen & Sundström (1974). They recognized that conventional
gas-liquid chromatography could not separate all the components, so
they devised preliminary fractionation on a charcoal column, which
separated the component PCBs according to the number of graphs in the
2,2',6 or 6' positions in the molecule ( o-chlorines). They compared
the gas-liquid chromatographic peaks with those of 90 synthesized
PCBs, and were able to characterize and quantify 60 components of
Clophens A50 and A60 (Table 3). Tables 2 and 3 show a considerable
overlap between the components of Aroclor 1254 and Clophen A50.
Table 2. Polychlorinated biphenyls in Aroclors 1221-1254 (Webb & McCall, 1972)
Retention Synthetic Acolor
timea chlorobiphenyl
1221 1232 1242 1248 1254
10 Biphenyl xb Cc x x x
13 2 x C x x x
16.9 3 x Dd
17 4 x C x
17.7
18.5 2,2' x C x x C x
21.3 x x x
21.6 x x x
23.5 2,3' x C x x x
24 2,4' x C x x C x
26 2,6,2' x x D x
29 2,5,2' x x C x
29.2 2,4,2' x x x C
29.5 4,4' x C x x C
34 2,3,2' x x Ce x
38 2,4,3' x x C x
38.5 2,4,4' x x C x
38.7 2,5,4' x x C x
41.5 x x
42.5 3,4,2' x C x
43 x x
44 x x
44.5 x
48 2,5,2',5' x C x x C
49.5 2,4,2',5' x C x x C
50.5 2,4,2',4' x D x
51.5 x x
55 2,3,2',5' x x x C
57 x x
59 2,3,6,2',6' x x x D
60 x x
64 x x
69 x x
69.5 x x
70 2,5,3',4' x Ce x
70.5 2,3,6,2',5' x C
71 2,4,3',4' x Ce x
72 x x
76 2,3,6,2',3' x C
Table 2. (cont'd).
Retention Synthetic Acolor
timea chlorobiphenyl
1221 1232 1242 1248 1254
82 x
83 2,3,6,2',5' x x C
84 x
85 2,4,5,2',5' x x C
87 2,4,5,2',4' x x C
96 x
99 2,4,5,2',3' x x C
101 x x
104 x x
107 x x
115 x
119 x x
125 2,4,5,2',3',6' x C
126 2,4,5,3',4' x C
135 x
147 x
148 2,4,5,2',4',5' x C
149 x
152 x
165 x
178 x
a Relative to p,p'-DDE at 190°C on 1 00' × 0.02' SCOT SE 30 column.
b indicates a GLC peak in this Aroclor at this retention time.
c C means that the synthetic compound in the second column was
confirmed by GLC and IR in this Aroclor. Because of the labour
involved, most compounds were only confirmed in one Aroclor. For
example, biphenyl is probably present in 1232-1248 as well as 1221.
d Only GLC data available.
e Something else also present.
2.2 Purity of Products
Commercial PCBs are not sold on a composition specification, but
on their physical properties. Different batches may vary somewhat from
the compositions shown in Tables 1-3. Impurities known to be present
in commercial PCBs are chlorinated dibenzofurans and chlorinated
naphthalenes (Vos et al., 1970; Bowes et al., 1975). Bowes et al.
(1975) found chlorinated dibenzofurans at 0.8-2.0 mg/kg in samples of
the Aroclor 1248-1260 series, but none in Aroclor 1016, and at levels
of 8.4 mg/kg in Clophen A60 and 13.6 mg/kg in Phenoclor DP-6. Roach &
Pomerantz (1974) found chlorinated dibenzofurans levels of 1 mg/kg and
Nagayama et al. (1976) found 18 mg/kg in different batches of
Kanechlor 400, but no chlorinated dibenzodioxins.
2.3 Determination of PCB Residues
Reviews have been published on methods used for the determination
of organochlorine compounds including PCBs in environmental samples
(Holden, 1973a, Panel on Hazardous Trace Substances, 1972). No two
laboratories have identical methods, although all have features in
common. The techniques appear to be those previously developed for the
determination of organochlorine pesticides with appropriate
modifications for the presence of PCBs, and the studies on PCBs
sometimes form part of a wider programme for monitoring persistent
organochlorine compounds in the environment. The major difficulties in
the determination of PCBs are to separate them from interfering
organochlorine pesticides, and to derive a single quantitative figure
from a variable mixture of components.
2.3.1 Extraction of sample
Air
Particulate fallout from air has been trapped on 200 µm nylon net
coated with silicone oil, and the PCBs then extracted with hexane
(Södergren, 1972a). Separate determinations of particulate and vapour
phase PCBs in air have been made by the passage of a large volume of
air through a filter which was followed by an impinger containing
hexane (Hasegawa et al., 1972b), or a polyurethane plug (Bidleman &
Olney, 1974) or ceramic saddles coated with OV 17 silicone (Harvey &
Steinbauer, 1974) to absorb the vapour.
Table 3. Percentages of polychlorinated biphenyls in Clophen A50 and A60 and in human (Jensen & Sundström, 1974)
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
1 2,5-2'5' 2 0.25 0.30 5.0
2 2,4-2'5' 2 0.26 0.30 1.4
3 2,3-2'5' 2 0.27 0.33 1.9 1.1
4 (2,-2',3',4') 2 0.30 0.43 1.2 0.66
5 (4-2',3'6') 2 0.32 0.43 2.1 0.56
6 2,5-2',3',6' 3 0.35 0.43 4.4b 2.9 1.2
7 2,3-2',3',6' 3 0.38 0.49 2.5b 0.28 0.48
8 3,4-2',5' 1 0.41 0.42 3.9 1.5
9 1 or 2 0.42 2.2c 2.0c
10 2,5-2',3',5' 2 0.45 0.49 2.2 1.1 1.2
11 2,3,6-2',3',6' 4 0.47 0.61 0.50 1.0d
12 2,5-2',4',5' 2 0.48 0.50 7.0b 5.6 4.2
13 2,4-2',4',5' 2 0.51 0.51 1.8b --e 1.9
14 2,3-2',4',5' 2 0.53 0.57 1.4b
15 2,5-2',3',4' 2 0.53 0.58 5.4 1.4 2.3
16 2,3-2',3',4' 2 0.55 0.66 1.0
17 3,4-2',3',6' 2 0.56 0.62 7.6b 2.9 4.7
18 2,3,5-2',3',6' 3 0.60 0.70 1.2 4.2 1.0
19 2,4,5-2',3',6' 3 0.64 0.73 2.0b 6.5d 0.13
20 2,5-2',3',5',6' 3 0.65 0.68 1.3 3.3 0.43
21 2,3-2',3',5',6' 3 0.70 0.74 0.5 0.05
22 2,3,4-2',3',6' 3 0.72 0.83 1.8 3.2 0.15
23 1 or 2 0.76 0.6c
Table 3. (cont'd).
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
24 2,3,6-2',3',5',6' 4 0.77 0.91 0.09 0.96
25 3,4-2',4',5' 1 0.79 0.74 5.0b 1.6 5.4
26 2,3,6-2',3'4',6' 4 0.84 0.95 0.05 0.37
27 2,3,52',4',5' 2 0.85 0.86 0.90 2.9 2.7
28 3,4-2',3',4' 1 0.87 0.86 3.6 1.9
29 2,4,5-2',4',5' 2 0.90 0.86 4.2b 12.9d 21.5
30 2,3,4-2',3',5' 2 0.94 0.97 ) 1.5 --e
31 1 or 2 0.95 0.93 ) 1.1 --e
32 2,3,4-2',4',5' 2 1.00 1.00 5.1 11.3d 14.0
33 2,3,5-2',3',5',6' 3 1.01 1.06 0.04 0.49 0.90
34 4 1.02 0.05c
35 1 or 2 1.04 1.10 1.1c 2.0c 1.5'
36 2,4,5-2',3',5',6' 3 1.05 1.13 0.39 3.3 3.5
37 2,3,4-2',3',4' 2 1.11 1.16 1.3 2.0 0.81
38 2,3,6-2',3',4',5' 3 1.13 1.29 0.33 3.7d --e
39 2,4,5-2',3',4',6' 3 1.14 1.16 0.17 1.8 2.5
40 2,3,4-2',3',5',6' 3 1.19 1.32 0.27 2.1 1.3
41 2,3,5,6-2',3',5',6' 4 1.23 1.34 0.005 0.07
42 2,3,4-2',3',4',6' 3 1.28 1.40 0.13 1.3 0.57
43 2,3,4,6-2',3',5',6' 4 1.34 1.44 0.007 0.09
44 2,4,5-3',4',5' 1 1.41 1.19 0.47 1.0 0.49
45 2,3,6-2',3',4',5',6' 4 1.45 1.59 0.008 0.09
46 2,3,4,5-2',3',4',6' 4 1.46 1.51 --e 0.03
47 3,4-2',3',4',5' 1 1.55 1.37 0.81 1.5 2.0
48 2,3,5-2',3',4',5' 2 1.59 1.49 0.23 0.90 1.2
49 2,4,5-2',3',4',5' 2 1.71 1.56 0.98 7.6d 7.7
Table 3. (cont'd).
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
50 2,3,4-2',3',4',5' 2 1.88 1.82 0.72 4.1d 5.9
51 2,3,4,5-2',3',5',6' 3 1.94 1.99 0.08 0.74 0.77
52 1 or 2 2.02 1.96 0.23c 1.0c --e
53 2,3,4,5-2',3',4',6' 3 2.07 2.05 0.06 0.44 0.94
54 2,4,5-2',3',4',5',6' 3 2.15 2.04 0.01 0.28 0.46
55 (2,3,5,6-2',3',4',5',6') 4 2.23 --e
56 2,3,4-2',3',4',5',6' 3 2.40 2.20 0.01 0.17 0.31
57 2,3,4,6-2',3',4',5',6' 4 2.45 2.56 --e --e
58 3,4,5-2',3',4',5' 1 2.74 2.41 --e --e
59 2,3,4,5-2',3',4',6' 2 3.18 2.81 0.35 0.67 1.7
60 2,3,4,5,6-2',3',4',5',6'4 4 4.12 0.62
Total 86.7% 99.4% 100.0%
p,p'-DDE 0.52 0.58
p,p'-DDT 0.71 0.73
p,p'-DDT 0.90 0.97
a Tentative structures are given in brackets.
b Component found present in Aroclor 1254 by Webb & McCall (1972).
c Figures calculated using responses of chlorobiphenyls with similar retention times in the same fraction
from the charcoal column.
d Component found present in Phenoclor DP6 by Tas & Vos (1971).
e Present in trace amounts only.
Water
PCBs have been extracted from water by passing a sample through a
filter of undecane and Carbowax 400 monostearate supported on
Chromosorb W (Ahling & Jensen, 1970) or a porous plug of polyurethane
coated with a suitable gas-liquid chromatographic stationary phase
(Uthe et al., 1972), or Amberlite XAD-2 resin (Harvey, et al., 1973)
followed by elution of the PCBs with a solvent. Ahnoff & Josefsson
(1975) have described a liquid-liquid extraction into cyclohexane.
Biological samples
Most analysts have used standard methods developed for
organochlorine pesticides, in which the PCBs are extracted together
with the fat; the sample is ground with anhydrous sodium sulphate and
extracted with petroleum ether or hexane. Porter et al. (1970) studied
the optimal conditions for this procedure. A dehydrating solvent may
be included to facilitate the breakdown of cell structures; ethanol
(Norén & Westöö, (1968) and acetone (Jensen et al., 1973) have been
used. Rote & Murp (1971) digested the sample with a mixture of acetic
and perchloric acid prior to hexane extraction.
2.3.2 Clean-up
Methods for the removal of fat from the extract include solve
partitioning between hexane and acetonitrile or dimethylformamide,
treatment with strong sulfuric acid or ethanolic potassium hydroxide
Gel permeation has also been used (Stalling et al., 1972), and Holden
Marsden (1969) removed fat on dry, partially deactivated alumina
column. Certain pesticides such as dieldrin are destroyed by the
sulfuric at treatment, so this method cannot be used if such
pesticides are to determined together with PCBs (Jensen et al., 1973).
PCBs may be separated from organochlorine pesticides by column
chromatography on Florisil (Mulhern et al., 1971), silica gel (Holden
Marsden 1969; Armour & Burke, 1970; Collins et al., 1972) or on
charcoal (Berg et al., 1972; Jensen & Sundström, 1974). Several
laboratories have reported difficulties in repeating results obtained
by other investigators; the ease of separation appears to depend upon
the characteristics of absorbent, of the eluting solvent, and of the
sample extract, though the appears to be no difficulty in separating
all interfering substances except DDE, a metabolite of DDT. Thin-layer
chromatography has been us for separation by Norén & Westöö (1968),
Bagley et al. (1970), and Rein et al. (1973).
In many environmental samples, DDE is present in large excess over
the PCBs, and must be removed before the quantitative determination
PCBs. Oxidation procedures have been used to convert DDE
dichlorobenzophenone; recommended oxidants are potassium dichromate
and sulfuric acid (Westöö & Norén, 1970b) and chromium (II) oxide a
acetic acid (Mulhern et al., 1971). Jensen & Sundström (1974), who we
interested in determining DDT/PCB ratios in environmental sample
preferred sodium dichromate in acetic acid with a trace of sulfuric
acid. They claim that this does not destroy DDT and its metabolite DDD
which may be present in extracts after clean-up with strong sulfuric
acid and that using this mixture makes possible the quantitative
determination of the dichlorobenzophenone from the oxidation of DDE.
Conversion of DDT to DDE may be achieved by treatment with
ethanolic potassium hydroxide, which also removes interference from
elemental sulfur (Ahling & Jensen, 1970). Sulfur may also be removed
activated Raney nickel (Ahnoff & Josefsson, 1975) or by metallic
mercury.
Södergren (1973b) has scaled down the clean-up procedure for small
samples, using microlitre volumes.
2.3.3 Chromatographic separation of PCBs
Gas-liquid chromatography
Most analysts use gas-liquid chromatography with an electron-
capture detector for the separation of PCBs from the extract after
clean-up. Stationary phases commonly used are silicones or their
derivatives, for example, DC 200, SF 96, OV 1, and QF 1, or Apiezon L.
Jensen & Sundström (1974) state that with a mixture of SF 96 and QF 1,
14 peaks can be obtained from Clophen A50, but that Apiezon L gives
much better resolution. They obtained better peak separation by prior
fractionation on a charcoal column, which separated the PCBs according
to the number of o-chlorine substituents; they regard such
refinements as unnecessary in PCB residue analysis, although they may
be of value in the study of the selective, environmental degradation
of PCBs. Column temperatures used ranged between 170°C and 230°C.
Glass capillary columns gave good separation of PCBs from DDT and its
metabolites (Schulte & Acker, 1974).
Thin-layer chromatography
This has been used in the clean-up stage, but it may also provide
semi-quantitative results by visualization of the spots followed by
densitometry, or by comparison with spots produced by known amounts of
PCBs. Mulhern et al. (1971) separated PCBs on a plate coated with
alumina containing silver nitrate, and the spots were developed by
exposure to ultraviolet light; the detection limit was in the region
of 1 µg. Collins et al. (1972) devised a similar method in which the
PCBs remained together in a single spot, and they claimed a limit of
detection of about 50 ng. Reversed-phase chromatography on plates
coated with kieselguhr treated with liquid paraffin has been used to
separate Phenochlor DP6 into several spots with a detection limit of a
few micrograms (de Vos & Peet, 1971).
2.3.4 Quantification of PCB content
The response of the electron capture detector is not equal for all
PCB components, being much affected by the degree of chlorination
(Zitko et al., 1971). This does not lead to difficulties when the
sample under investigation has been directly contaminated by a
commercial PCB mixture, as that mixture can be used as a standard.
Difficulties are encountered when the PCBs in the sample have
undergone selective environmental degradation (see sections 3 and 5).
Several investigators have noted that the pattern of peaks from such
samples resembles fairly closely that of one or other of the higher
chlorinated PCB mixtures such as Aroclor 1254, and they have compared
the total area of the peaks with that of the nearest commercial
product in order to determine the amount of PCBs in the sample (Armour
& Burke, 1970). Collins et al. (1972 observed that, under their
conditions, the area of peaks usually encountered in extracts of
tissue samples was closely similar to that of an equivalent amount of
DDE, thus DDE could be used for calibration. In order to overcome the
uncertainties of these procedures, Rote & Murphy (1971) divided the
peaks into groups according to the number of chlorine atoms in the
molecule, as determined from mass spectrographic data, and calculated
the PCB content of each group from the theoretical response of the
detector to chlorine content. Jensen et al. (1973) selected a
commercial in PCB that included all the peaks from the extract; they
determined the PCB content of each peak by combined mass spectrometry
and coulometry, and determined the total PCBs in the sample by
comparing the height of each peak obtained with the extract with those
obtained with the reference sample. Simpler methods have been used by
Koeman et al. (1969), who compared the height of a single peak
obtained with the extract with that of a peak with the same retention
time obtained with a e commercial PCB mixture; others have averaged
out more than one peak s for this calculation (Reynolds, 1971; Reinke
et al., 1973). Rote & Murphy (1971) have calculated that such
procedures may more than double the values obtained by a more accurate
method.
A different technique has been recommended by Berg et al. (1972);
the PGBs are chlorinated with antimony pentachloride to decachloro-
biphenyl, which can then be measured as a single peak.
2.3.5 Accuracy of PCB determinations
A group of eight analysts engaged in an investigation of pollution
in the North Sea undertook a collaborative study to determine the PCB
content of a sample of fish oil, using the methods currently employed
in their laboratories (International Council for the Exploration of
the Sea, 1974). The PCB values obtained ranged from 1.0 to 3.9 mg/kg
with a mean of 1.97 mg/kg and a standard deviation of 0.93 mg/kg.
Better agreement was obtained with the same fish oil fortified with
PCBs at a concentration of 10 mg/kg; the mean of the results for the
fortified sample was 10.0 mg/kg with a standard deviation of
1.1 mg/kg.
A probable source of error is incomplete initial extraction of
PCBs from the sample (Holden & Marsden, 1969). Another source of
variation between laboratories lies in the method used to quantify
gas-liquid chromatographic peaks (section 2.3.4); van Hove Holdrinet
(1975) considered this to be the major source of error.
It is evident that caution should be exercised in accepting the
analytical results from a laboratory, particularly for samples with a
low PCB content, until the competence of that laboratory has been
established by an inter-laboratory collaborative study.
2.3.6 Confirmation of identity
Since Jensen first identified as PCBs those hitherto unknown
substances that interfered in the gas-liquid chromatographic
determination of organochlorine pesticides using mass spectrographic
data, other investigators have confirmed the presence of PCBs in
environmental samples by combining gas-liquid chromatography with mass
spectrometry (Bagley et al., 1970) and with coulometry to measure the
chlorine content. The conversion of PCBs to bicyclohexyl and
decachlorobiphenyl is further confirmation (Berg et al., 1972). The
widespread distribution of PCBs is now well established, and, as
adequate methods are available to remove interference from
organochlorine pesticides, there is no evidence of the presence of
other interfering substances in the types of sample that have so far
been analysed, down to a limit of detection of around 0.01 mg/kg. This
does not necessarily apply to other types of sample, particularly when
very low levels are being sought; Ahnoff & Josefsson (1973, 1975)
reported a number of unknown interfering substances, when measuring
PCBs in water at levels below 1 ng/litre, one of which was
subsequently identified as elemental sulfur. They recommend
confirmation by mass fragmentography for such samples.
2.4 Determination of PCTs
A few methods have been published for the determination of PCTs;
extraction and clean-up procedures are similar to those used for PCBs,
but the gas-liquid chromatographic details are different because of
the lower volatility of the PCTs. Zitko et al. (1972b) used 3% OV 210
as the stationary phase with a column temperature of 200°C. Thomas &
Reynolds (1973) also used OV 210 with a column temperature of 250°C
and another system with 3% Dexsil as stationary phase at 300°C with a
63Ni electron capture detector; this was also used by Addison et al.
(1972). Sosa-Lucero et al. (1973) used OV 210 and SE 30 at 255°C and
Freudental & Greve (1973) used OV 17 with a temperature programmed
from 200°C to 285°C. Thomas & Reynolds (1973) confirmed the identity
by chlorination to tetradecachloroterphenyl with antimony
pentachloride.
A thin-layer chromatographic technique has also been described
with limit of detection of about 1 µg (Addison et al., 1972).
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1 Production and Uses of PCBs
Details of the production and uses of PCBs in the USA have been
released, and have been summarized by Nisbet & Sarofim (1972). Annual
production has increased steadily since 1930 and reached a maximum in
1970 of 33 000 tonnes. During this peak year, 65% of the production
was of the 42% chlorinated type, 25% was less chlorinated, and the
remainder more chlorinated. After 1970, production sharply decreased
owing to voluntary limitation of sales by the Monsanto Company, the
sole manufacturer in the USA. According to information collected by
the Organization for Economic Co-operation and Development (OECD), the
1970, production in the USA was 18 000 tonnes and the total in OECD
countries in that year was 48 000 tonnes. It has been estimated that
the cumulative total production of PCBs in North America up to 1971
was 0.5 million tonnes, and in the whole world probably double this
figure.
The commercial applications of PCBs have been reviewed in an OECD
report (Organization for Economic Co-operation and Development, 1973)
From an environmental viewpoint these can be divided into three
categories:
Controllable closed systems. PCBs used as dielectrics in
transformers and large capacitors have a life equal to that of the
equipment, and with proper design leakage does not occur. When the
equipment is scrapper the quantity of dielectric is sufficiently large
to justify regeneration.
Uncontrollable closed systems. PCBs are used in heat transfer and
hydraulic systems which, although technically closed, permit leakage.
The need for frequent replacement of small quantities makes recovery
impracticable, PCBs are very widely dispersed in small capacitors, and
there are great difficulties in collecting these items for disposal.
Dissipative uses. PCBs have been used in the formulation of
lubricating and cutting oils, in pesticides, and as plasticizers in
paints, copying paper, adhesives, sealants, and plastics. In these
applications, the PCBs are in direct contact with the environment, and
there is no way of recovering them when the product is scrapped.
The uses of PCBs in the USA in 1970 have been analysed by Nisbet &
Sarofim (1972). Of the total 33 000 tonnes, 56% was used as a
dielectric, with 36% in capacitors and 20% in transformers. Various
plasticizer outlets accounted for 30%, hydraulic fluids and lubricants
12%, and heat transfer liquids 1.5%. Following the restriction of
sales for non-dissipative uses, the percentage of PCBs sold as
dielectrics rose to 77% in 1971 and the proportion of highly
chlorinated products was considerably reduced, Aroclor 1016 replacing
Aroclor 1242. In Japan, 44 800 tonnes of PCBs were used from 1962 to
1971, and of this 65.4% was used in the electrical industry, 11.3% in
heat exchangers, 17.9% in pressure-sensitive duplicating paper, and
5.4% for other dissipative uses (Ishi, 1972). In Sweden, most of the
600 tonnes imported in 1969 was used in the electrical industry and a
large part of the remainder in paints (Jensen, unpublished report
1972).
According to the OECD report, transformers and capacitors provided
the major outlets for PCBs in most OECD countries in 1971. In 1972,
several countries restricted sales; in Sweden the importation and use
of PCBs were restricted by law; in the United Kingdom, as in the USA,
sales were voluntarily restricted to the lower chlorinated PCBs for
use as dielectrics in enclosed systems; in the Federal Republic of
Germany, use in hydraulic and heat transfer fluids was also permitted.
In Japan the production and use of PCBs were banned in 1972.
Limitations on sales were subsequently introduced in other countries.
No information is available on the scale of production and the
uses of PCTs.
3.2 Entry of PCBs into the Environment
Surveys of the sources of environmental pollution with PCBs were
made before production and use were limited, and the information
available may not now apply in North America and elsewhere. Table 4
gives an estimate of the fate of the PCBs produced in 1970 in the USA
(Nisbet & Sarofim, 1972). Only 20% of the annual production can be
regarded as a net increase in current usage, and the remainder is
balanced by a loss to the environment. More than one-half of this
entered dumps and landfills and it has been calculated that 0.3
million tonnes of PCBs have accumulated in such locations in North
America since 1930. Much of this was originally enclosed in containers
such as capacitors or was in plasticized resins and will not be
released until the containing medium decays. The diffusion of PCBs
from landfills is likely to be slow on account of their low volatility
and low water solubility; Carnes et al. (1973) found little leaching
from one site they tested.
Pollution of the environment has occurred mainly from the first
three routes mentioned in Table 4. In addition, there are other
routes, which although involving relatively small amounts,
nevertheless have an influence on the entry of PCBs into food chains.
PCBs have been used in the USA in amounts of about 10 tonnes/year in
pesticide formulations (Panel on Hazardous Trace Substances, 1972),
and the unauthorized use of scrap transformer fluid for this purpose
has led to local contamination of milk supplies.
Table 4. Entry of PCBs into the environmenta
Percentage of
annual PCB type
Route production (% chlorination)
Vaporization from plasticizers 4.5 48-60
Vaporization during incineration 1 42
Leaks and disposal of industrial fluids 13 42-60
Destruction by incineration 9 mainly 42
Disposal in dumps and landfills 52.5 42-60
Net increase in current usage 20 42-54
a From Nisbet & Sarafim, 1972.
Pressure sensitive duplicating paper containing PCBs has found its
way into waste paper supplies and has been recycled into paper and
board used as food packaging materials; paints for coating the bottom
of ships contained 3-5% of PCBs -- about 3% of the annual quantity
imported into Sweden has been used for this purpose -- and this has
been a source of plankton contamination (Jensen et al., 1972a).
3.2.1 Release of PCBs into the atmosphere
There appears to be little widespread atmospheric contamination
during the manufacture and processing of PCBs, but this can occur
during their subsequent use and disposal. Although PCBs have a low
volatility, there may be an appreciable loss to the atmosphere during
the lifetime of a PCB-plasticized resin, particularly of the lower
chlorinated products. Further pollution may occur during the
incineration of industrial and municipal waste. Most municipal
incinerators are not very effective in destroying PCBs; efficient
incinerators can be designed for this purpose (Jensen & Wickberg,
unpublished report 1971; Jensen, unpublished report 1972), although
the higher chlorinated PCBs are more resistant to pyrolysis. Secondary
sources of atmospheric pollution are volatilization from soil, and
from the drying of sewage sludge. Laveskog (1973) found that the PCB
emissions from municipal incineration and from the drying of sewage
sludge each amounted to about 1 kg/year per million inhabitants, a
small amount compared with the 2 tonnes deposited yearly from aerial
fallout in the south of Sweden.
3.2.2 Leakage and disposal of PCBs in industry
The major source of environmental pollution with PCBs, which
eventually affects food chains, is the leakage and the disposal of
industrial fluids. There have been serious cases of poisoning in man
(Kuratsune et al., 1972) and in animals (Panel on Hazardous Trace
Substances, 1972) due to leakage from a heat exchanger. Leakages, or
the unintentional or deliberate discharge of waste, have contaminated
seas, lakes, waterways, and sewers (Duke et al., 1970; Schmidt et al.,
1971; Veith, 1972).
Analysis of solids from factory wastes in Japan have revealed a
wide variation in PCB content. In most, less than 1 mg/kg was
detected, but in: some the contamination was very heavy, the highest
level recorded being 8.26% from a factory manufacturing electrical
equipment (Japanese Environment & Safety Bureau, unpublished report
1972).
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATION
4.1 Environmental Transport
Nisbet & Sarofim (1972) emphasize that the available data are
insufficient to determine anything but a very crude model of the
transfer of PCBs into the environment. Guesses can be made by
referring to the distribution of DDT, which resembles the PCBs to some
extent in its physical and chemical properties and about which more is
known. Much of the following discussion arises from their analysis of
the situation in the North American continent.
4.1.1 Air transport
By analogy with DDT, it might be expected that PCBs entering the
atmosphere in the vapour phase would be adsorbed rapidly on to
particles, which would be deposited or washed out in rain at a rate
depending on their particle size, the average residence time in the
atmosphere being 2-3 days. This has been confirmed by Södergren
(1972a), who measured the deposition in southern Sweden of PCBs that
had originated from municipal incinerators or had been carried over
from Denmark by the prevailing winds, and showed that the amount
deposited in central Sweden was much less. Cames (1973) and Laveskog,
(unpublished report 1973) found mainly particulate PCBs in the
emission from incinerators. Harvey & Steinhauer (1974), however,
considered that the results of their analyses of air in north Atlantic
regions indicated that most of the PCBs carried in the air were in the
vapour phase.
4.1.2 Transport in soil
The PCBs in soil are derived mainly from particulate deposition,
estimated at 1000-2000 tonnes annually in North America, most of which
is in urban areas. Small amounts have originated from the use of
sewage sludge as a fertilizer, from the leaching of landfalls, and
from the se of PCBs in pesticide formulations. Tucker et al. (1975)
found that under experimental conditions, the higher chlorinated PCBs
were not leached from soils by percolating water, and those with a
lower level of chlorination were removed only slowly, particularly
from soils of high lay content. Losses do occur by volatilization and
by biotransformation; by analogy with DDT and its metabolites the
half-time in soil has been estimated at 5 years. Haque et al. (1974)
showed that the rate of evaporation decreased with the clay content of
the soil and he degree of chlorination of the biphenyl, and increased
with temperature. Biotransformation has also been shown to play a part
in the disappearance of the lower chlorinated compounds from soil
(Iwata et al., 1973).
The total amount of PCBs distributed over North America, apart
from that in dumps and landfills, has been estimated at 20 000 tonnes,
of which one quarter has subsequently been transported via the air to
the seas. (Nisbet & Sarofim, 1972).
4.1.3 Transport in water
The entry of PCBs into water occurs mainly at the points of
discharge of industrial and urban wastes into rivers, lakes, and
coastal waters. Sewage treatment appears to remove particulate PCBs
from water, but not PCBs in solution; these are concentrated in the
sludge (Ahling & 1970), which may be dumped into rivers and coastal
waters. Holden (1970b) found a mean PCB content of 3 mg/kg in liquid
sludges from Glasgow, and calculated that PCBs at the rate of
1 tonne/year were released into the Clyde and Thames estuaries; a
similar output was calculated from water treatment plants off the
California coast (Schmidt et al., 1971). Other localized sources of
pollution are leakages or waste disposal from ships. PCBs in water are
attached mainly to particulate matter (Södergren, 1973a) and
eventually fall to the bottom sediment at a rate that depends on the
particle size. PCBs may be leached from the sediment and may reach
coastal waters, but Nimmo et al. (1971) noted little change in the PCB
content of a sediment at a point downstream from a source of
contamination over a period of 9 months. The process may be
accelerated by the dumping of dredging spoil.
4.1.4 Transport through biota
According to the approximate calculations of Nisbet & Sarofim
(1972), less than 1000 tonnes of PCBs are located in living organisms
throughout the world, so that biological transport and degradation
play little part in the fate of PCBs in the environment, though these
factors have great ecotoxicological significance.
4.2 Transformation in the Environment
4.2.1 Abiotic transformation
The fate of the various PCBs in commercial mixtures depends on
their physical and chemical properties. Some fractionation occurs
during the volatilization of PCBs, because of a decrease in vapour
pressure with increasing chlorination. The PCBs are chemically very
stable, and are not likely to be degraded at a significant rate by
hydrolytic or similar reactions under environmental conditions. They
are, however, fairly easily degraded by photolysis under laboratory
conditions; Safe & Hutzinger (1971), and Hutzinger et al. (1972b) have
shown that PCBs are dechlorinated in hexane solution at a rate that
increases with increasing chlorination. PCBs in aqueous-dioxane
suspensions and in thin films give hydroxy and carboxylic acid
derivatives on irradiation. The lower chlorinated biphenyls, at a
vapour concentration in air of 1.5 mg/m3 are readily destroyed by
photolysis under laboratory conditions. There is no direct evidence of
the extent of PCB breakdown in the atmosphere under environmental
conditions, and nothing is known of the persistence and toxicity of
any transformation products.
4.2.2 Biotransformation
The biotransformation of PCBs is discussed in section 6.6. Owing
to small proportion of the total environmental PCBs contained in
living biotransformation does not significantly influence the overall
environmental concentrations of PCBs, though it has a marked influence
on PCBs passing through food chains.
4.2.3 Metabolism in limited ecosystems
The presence of PCBs in sewage sludge suggests that they are not
all readily transformed by microorganisms. Choi et al. (1974) found no
evidence of biotransformation of Aroclor 1254 added to water entering
an aerated biological water treatment system, although much of it was
removed with the sludge; there appeared to be no interference by the
PCBs in the performance of the system. Vodden (1973) quotes
investigations showing that PCBs with four or fewer chlorine atoms are
readily broken down by microorganisms, but that this can be inhibited
by the presence of higher chlorinated PCBs. Mono- and dichlorobiphenyl
can be transformed by Achromobacter isolated from sewage effluent
(Ahmed & Focht, 1973), and a culture of lake bacteria can degrade some
of the lower chlorinated components of Aroclor 1242 to chlorine-free
derivatives (Kaiser & Wong, 1974).
Södergren (1972b) investigated the transport of Clophen A50 added
to a model aquatic ecosystem; this was rapidly taken up by an alga
(Chlorella) and no change was observed in the proportion of the
component PCBs in the first consumer fish; however, there was a
relative loss of the lower chlorinated PCBs in the perch, the second
consumer. No progressive loss of the lower chlorinated PCBs was
observed in sediment, plankton, invertebrates, and fish inhabiting a
Swedish lake (Södergren, 1973a). Evidence on the metabolism of PCBs by
fish is conflicting, but it seems probable that most fish,
particularly those in the lower trophic stages of food chains, cannot
readily degrade the low chlorinated PCBs.
4.3 Biological Accumulation
Although PCB concentrations in living organisms clearly indicate a
progressive accumulation in food chains, the factors discussed in the
previous sections make it impossible to give any reliable figure for
bioaccumulation at each trophic stage. There is also doubt about which
tissue level should be used in the calculation, that of the whole
body, the fat, or the liver.
There is good evidence that all aquatic organisms studied in
aquaria can absorb PCBs directly from water. The accumulation varies
with the duration of exposure and the concentration in the ambient
water. A diatom exposed to Aroclor 1242 showed an accumulation factor
of 1100 (Keil et al., 1971); with Aroclor 1254 the following values
have been obtained: pink shrimp, 6600; blue crab, 4600; oyster, 8100;
pinfish, 980 (Duke et al., 1970); spot, 37 000 (Hansen et al., 1971);
bluegills, up to 71 400 (Stalling & Mayer, 1972). The accumulation
factor in scud exposed to Aroclor 1254 reached a maximum of 24 000
within 4 days and thereafter remained fairly constant (Sanders &
Chandler, 1972). Similar results were obtained with other
invertebrates, although with crayfish the uptake was slower and the
accumulation was still increasing after 21 days. However, it is
probable that most of the PCBs entering aquatic systems are retained
by particulate matter, and the above accumulation factors are not
necessarily applicable to natural ecosystems. Nimmo et al. (1971)
demonstrated that the fiddler crab could ingest PCB contained in the
bottom sediment. When exposed to Aroclor 1254 in water, ciliated
protozoa, stated to be the major benthic input to aquatic food chains,
had an accumulation factor of 60 (Cooley et al., 1972).
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1 Air
Mean concentrations in air, in several locations in Sweden, ranged
from the detection limit of 0.8 ng/m3-3.9 ng/m3. The highest figure
recorded was 12.5 ng/m3 (Ekstedt & Odén, 1974). In the USA, PCB
concentrations in air ranged from 5 ng/m3 near the north-east coast
to 0.05 ng/m3 at a distance of 2000 m out over the Atlantic Ocean
(Harvey & Steinhauer, 1974). Results from the United States
Environmental Protection Agency indicate a range between 1 and
50 ng/m3 (Panel on Hazardous Trace Substances, 1972), and similar
results have been reported from Japan (Tatsukawa & Watanabe, 1972).
5.2 Soil and Sediments
In Sweden, PCBs have been found in natural soil at a concentration
of 15 µg/kg by Odén & Berggren (1973). The same authors also found
0.006-1.4 mg/kg in sediments from areas in the Baltic Sea with
different degrees of pollution. Nimmo et al. (1971) found PCB levels
of 1.461 mg/kg in sediment from an estuary at a point near the site of
an accidental release of PCBs from a factory, and levels of 0.6 mg/kg
at a point 16 km downstream. Soil samples from the bank 6.5 km
downstream from the source contained 1.4-1.7 mg/kg. Less than 1 mg/kg
has been found in Japanese agricultural soil, but as much as 510 mg/kg
in soil near a factory making electrical components (Fukada, et al.,
1973).
5.3 Water
In heavily contaminated waters, PCB concentrations may be several
times greater than their solubility, owing to adsorption on suspended
particles (Duke et al., 1970). Water in a Swedish river contained
0.5 ng/litre as it entered a water treatment plant, and 0.33 ng/litre
in the tap water produced (Ahling & Jensen, 1970). Values of
0.1-0.3 ng/litre have been measured in other Swedish rivers (Ahnoff &
Josefsson, 1974). Södergren (1973a) found a seasonal variation in the
PCB level in a Swedish lake, with a maximum of 2 ng/litre, the
pollution being attributed to aerial fallout. Concentrations of
10-100 ng/litre have been measured in tap water at Kyoto in Japan
(Panel on Hazardous Trace Substances, 1972) In a polluted coastal
area of Lake Michigan in 1970, PCB concentrations of from 450 to less
than 100 ng/litre were measured in 1970, but there was a marked
decrease in 1971, possibly due to the limitation on sales of PCBs
(Panel on Hazardous Trace Substances, 1972). From the scanty
information on PCBs, reinforced by analogy with the more extensive
information on DDT, it has been estimated that nonpolluted fresh
waters should contain not more than 0.5 ng/litre up to 5 ng/litre for
the Great Lakes of North America, 50 ng/litre for moderately polluted
rivers and estuaries, and 500 ng/litre for highly polluted rivers.
5.4 Living Organisms
There is now considerable information from Canada, Japan, Sweden
the United Kingdom, and the USA, on the accumulation of PCB's
biological material. Analytical measurements on different organisms,
and on the same organism from different localities, vary widely and it
is necessary to consider the factors that lead to this variation.
Difference in analytical techniques may contribute to this (see
section 2.3.5) but important influences are exerted by the extent of
local pollution, amount of fat in the organism studied, and its
trophic stage in food chains.
5.4.1 The influence of local pollution
Most of the fish eaten by man is taken from waters with little
pollution. In a collaborative study by seven national (International
Council for the Exploration of the Sea, 1974), the PCB content of
muscle tissue of fish taken from the North Sea was measured. A mean of
0.01 mg/kg was found in cod, herring contained up to 0.48 mg/kg with
most samples in the range of 0.1-0.2 mg/kg, and plaice contained
0.1 mg/kg or less. Similar values were reported by Zitko (1971) for
fish taken from the North Atlantic.
There are many examples of different PCB levels in similar species
collected from areas of high and low pollution. Jensen, et al. (1972b)
found five times as much PCBs in herrings caught in waters off
industrialized areas near Stockholm, as in herrings from the cleaner
waters of the west coast of Sweden. Similarly, levels in plankton
harvested along the Swedish archipelago at various distances from
Stockholm fell progressively, away from the more polluted areas
(Jensen, et al., 1972c); the concentration in pike fell to one-half
(Olsson & Jensen, 1974). Koeman et al., (1972b) found PCB levels of up
to 88 mg/kg in the blubber of toothed whales caught in the North Sea,
but none was detectable in similar species from New Zealand or
Surinam. Holden (1973b) found high PCB concentrations in the blubber
of seals in polluted coastal areas of the United Kingdom (up to
235 mg/kg), and much lower levels in unpolluted areas (down to
2 mg/kg).
Risebrough & de Lappe (1972) studied the PCB content of
extractable lipids from brown pelican eggs collected from areas
throughout North and South America, and showed that the content varied
from 4 mg/kg up to 266 mg/kg in highly industrialized regions. They
also reported levels greater than 3 mg/kg in fish from New York Sound
and Tokyo Bay, both very polluted areas. Even higher levels of PCBs
have been found in fish from polluted lakes and inland waterways, a
level of 20 mg/kg being found in fish from Lake Ontario, and over
200 mg/kg in fish from the Hudson River (Stalling & Mayer, 1972).
Similar correlations between pollution and PCB levels have been
reported from the United Kingdom in fish (Portmann, 1970), and in
mussels (Holdgate, 1971).
The association between high PCB levels and local pollution may be
disturbed by the migratory habits of certain species, particularly in
birds that may be exposed to PCBs in their wintering areas or on the
migration routes. As much as 400 mg/kg has been measured in the fat of
a robin entering Sweden, although the normal value is about 16 mg/kg.
Many migratory birds start egg-laying on arrival at their summer
quarters, so the PCB content of eggs may reflect the bird's previous
exposure rather than the local pollution (Odsjö, 1973). A special case
concerning the effect of pollution is seen in the use of fish-feed in
poultry and fish farming. Kolbye (1972) stated that this may contain
PCB levels of 0.6-4.5 mg/kg.
5.4.2 The influence of the fat content of tissues
PCBs are mainly stored in body fat (see section 5.5.5.1), and the
total PCB content of the body tissues is much influenced by their fat
content (Portmann, 1970; Westöö & Norén, 1970a). Jensen, et al. (1969)
found PCB levels of 0.27 mg/kg and 0.33 mg/kg respectively, in the
muscle tissue of herring and cod from the same area of the Baltic,
although the cod is at a higher trophic stage (see section 5.4.3).
These two species have 4.4 and 0.32% of extractable fat respectively,
and when the PCB level is calculated on the fat content, values of
6.8 mg/kg for the herring and 11 mg/kg for the cod are obtained. Cod
liver has a much higher fat content than cod muscle and Jensen (1973)
has reported the ratio of PCB concentrations in cod liver and muscle
to be over 100, the maximum in liver being 59 mg/kg. Jensen et al.
(1969) have remarked that the considerable seasonal variation in the
fat content of the herring, rising from 1% in spring to 10% in autumn,
influences the tissue level of PCBs. Peakall et al. (1972) noticed a
marked rise in tissue levels in starved birds owing to the
mobilization of fat, and it is possible that the high levels of PCBs
in the livers of birds dying during the "seabird wreck" in the Irish
Sea were secondary to emaciation (Holdgate, 1971). De Freitas &
Norstrom (1974) showed that, in pigeons, pure PCBs left fatty tissues
and accumulated mainly in muscle during the mobilization of fat
associated with starvation.
5.4.3 The influence of the trophic stage in food
Swedish work on the distribution of PCBs in aquatic ecosystems has
been summarized by Jensen et al. (1972b), and Olsson et al. (1973),
and it has been largely confirmed by work in other areas (Risebrough
et al., 1968; Risebrough & de Lappe, 1972: Holdgate, 1971). Zoo- and
phytoplankton readily absorb or adsorb PCBs from their environment;
Södergren (1972) has demonstrated the rapid uptake of Clophen A50 by
the unicellular alga Chlorella. Marine zooplankton may contain PCB
levels of 5 mg/kg in extractable lipids in areas of moderate pollution
(Jensen et al., 1972c; Williams & Holden, 1973), with somewhat lower
values in relatively unpolluted areas. However, results with plankton,
particularly where high levels are found, must be regarded with
caution as the sample could be contaminated with PCB-rich oil or tar
particles. Herring feeding on plankton in areas of moderate pollution
contain PCB levels of about 0.5 mg/kg in muscle tissue (10 mg/kg in
extractable fat); plaice and flounder, both bottom-feeding fish
contain about one-third of this, presumably because the PCB content of
benthic organisms is lower. In predatory fish such as the cod and
pike, the PCB level in extractable fat is about 10 mg/kg and a mean of
10 mg/kg has been measured in the blubber of seals.
Much higher PCB values have been obtained in fish-eating birds;
levels of 18 mg/kg (650 mg/kg in extractable fat), and 17 mg/kg
(420 mg/kg in extractable fat) were found in the herring gull and
comorant respectively. Lower values were found in birds feeding on
invertebrates, such as the long-tailed duck which contained 14 mg/kg
in fat; marine invertebrates contain PCB levels in the region of
0.1-0.2 mg/kg. At the top trophic level, 96 mg/kg (9.7 g/kg in
extractable fat) has been measured in the eagle owl; the highest
recorded PCB values were from eagle owls found dead in the south-east
coastal region of Sweden, 260 mg/kg in the brain (3.4 g/kg in
extractable fat) and 110 mg/kg in muscle (12 g/kg in extractable fat).
(Odsjö, 1973).
Less information is available on terrestrial ecosystems. PCB
concentrations in the region of 0.01 mg/kg have been found in fresh
tissue in slugs, snakes, and ants, and slightly higher concentrations
in earthworms. Tissue levels were generally at the limit of detection
(0.01 mg/kg) in herbivorous mammals (Odsjö, 1973). Bruggemann et al.
(1974) reported a mean PCB concentration of 0.22 mg/kg in 20 out of 72
measurements in the adipose tissues of the hare and a higher value
(2.5 mg/kg) in 1 out of 5 tests on the adipose tissues of the fox.
Higher values have been found in the American mink in Sweden with
0.58 mg/kg in muscle (45 mg/kg in fat) presumably because of a fish
diet (Odsjö, 1973). Tissue levels in wild birds on a mixed diet are
variable and rather low, but those in predatory birds are higher.
Prestt et al. (1970) related the PCB concentration in the liver of
wild birds to their diet; less than 1 mg/kg was found in insectivorous
birds and more than 70 mg/kg in the sparrow hawk. A level of 0.5 mg/kg
has been found in the muscle of the eagle owl in central Sweden, but
this is much less than the tissue concentrations encountered in this
bird in coastal areas (Odsjö, 1973). High tissue concentrations in
predatory and marine birds have also been reported from Canada
(Gilbertson & Reynolds, 1974), the Netherlands (Koeman et al., 1972a),
and from the United Kingdom (Bourne & Bogan, 1972).
5.4.4 Indicator organisms
Several of the organisms, that have been shown to accumulate-PCBs
from the environment, have been suggested as indicators of the extent
of local pollution with PCBs. In aquatic systems, the use of plankton
as an indicator has the advantage that it is at the lowest trophic
stage of food chains but errors may occur in the determination because
of the inclusion in the sample of nonplanktonic particles with a high
PCB content (Jensen et al., 1972a). The herring, which feeds on
plankton, has been suggested as an indicator (Jensen et al., 1972b)
and, at higher trophic stages, the pike, which is a stationary fish
(Olsson & Jensen, unpublished report, 1974), and seabirds (Jensen et
al., 1972c). In fresh waters, the amphipod Gammarus pulex has been
used as an indicator organism for chlorinated hydrocarbons (Södergren
et al., 1972). However, Zitko et. al. (1974) claimed that the
variation between individual fish was so high that Atlantic herring
and yellow perch could be used to detect trends in pollution only if
large numbers were taken for analysis, with an interval between
measurements of at least 4 years.
A series of monitoring studies has been made by OECD, the species
selected covering terrestrial, fresh water, and marine environments.
The analytical results, and the general problems of selecting species
for monitoring, have been discussed by Holden. (1970a, 1973a, 1973b).
5.5 The Extent of Human Exposure to PCBs and PCTs
5.5.1 Air and water
The maximum concentration of PCBs in air is not likely to exceed
50 ng/m3 (section 5.1). The highest concentration of PCBs reported in
domestic tap water is 100 ng/litre in the Kyoto area of Japan (Panel
on Hazardous Trace Substances, 1972), but levels more likely to be
encountered should not exceed 1 ng/litre (section 5.3).
5.5.2 Food
The PCB content of a variety of foods on the Swedish market been
measured by Westöö & Norén (1970a) and Westöö et al. Less than
0.1 mg/kg was found in samples of butter, margarine, oils, eggs, beef,
lamb, chicken, bread, biscuits, and baby of pork out of more than 100
had a PCB content in the range of 0.5 mg/kg. As might be expected from
the discussion in section 5.4 higher values were found in fish
depending on the fat content and pollution of the fishing area (Westöö
& Norén, 1970a; Berglund, 1972] The PCB levels obtained in an
extensive study by the US Food Administration are shown in Table 5.
These values are considerably higher than but they are probably
biased, as they include samples originating areas previously suspected
of having been subject to local pollution. In a Canadian survey, PCB
levels of less than 0.01 mg/kg were found in eggs (Mes et al., 1974)
and a mean of 0.042 mg/kg was found in domestic and imported cheese
with a maximum of 0.27 mg/kg (Villeneuve et al., 1973b). No traces of
PCTs were found.
In Japan, a similar range of PCB contents for most foods has been
reported; however, some high levels have been-reported for rice and
vegetables harvested in fields polluted with PCBs (Environmental
Sanitation Bureau, 1973). The PCB content of most fish on the market
was less than 3 mg/kg, although some contained more than this. The PCT
content of fish was much lower (Fukano et al., 1974). In the
Netherlands, eel has been reported to contain PCT levels of
0.2-0.5 mg/kg and PCB levels of 4.7 mg/kg (Freudenthal & Greve, 1973).
Table 5. PCB levels in food in the USAa
% positive Level in positive samples (mg/kg)
Food (0.1 mg/kg) Mean Maximum
Cheese 6 0.25 1.0
Milk 7 2.3 27.8
Eggs 29 0.55 3.7
Fish 54 1.87 35.3
a From Kolbye (1972).
Samples of butter from the Westphalian area of the Federal
Republic of Germany, obtained in the period 1972-74, contained PCB
levels of 0.38 mg/kg (range 0.25-0.54 mg/kg) (Claus & Acker, 1975).
Relatively high PCB levels in some packaged foods in Sweden,
mainly of imported origin, could be attributed to migration from the
packaging material (Westöö et al., 1971). The highest level
encountered was 11 mg/kg in a children's breakfast cereal; PCB levels
of 70 mg/kg and 700 mg/kg were found in the material of the inner bag
containing this product and in the outer cardboard container
respectively. Up to 2000 mg/kg was found in cartons of other samples.
Villeneuve et al. (1973a) have analysed packaged food in Canada; they
found that 66.7% of the samples contained PCB levels of less than
0.01 mg/kg, 30.7% contained between 0.01 and 1 mg/kg, and 2.6%
contained more than 1 mg/kg. PCT determinations were also made on
these samples; 94.5% of the samples contained less than 0.01 mg/kg and
5.5% contained 0.01-0.05 mg/kg. The highest PCB levels were
encountered in a rice sample with 2.1 mg/kg where the packaging
material contained 31 mg/kg, and in a dried fruit sample with
4.5 mg/kg in a container containing 76 mg/kg. In a survey of packaging
containers, approximately 80% were found to contain PCB and PCT levels
of less than 1 mg/kg, while about 4% contained levels higher than
10 mg/kg. The most likely source of PCBs in packaging materials is the
recycling of paper waste containing pressure-sensitive duplicating
paper (Masuda et al., 1972).
5.5.3 Occupational exposure
Occupational exposure does not only occur during the manufacture
of PCBs and with their use in the electrical industry. It may also be
widespread among mechanics in contact with lubricating oils and
hydraulic fluids, among workers exposed to varnishes and paints, and
among office workers from contact with pressure-sensitive duplicating
paper, some brands of which readily transfer PCBs to skin (Kuratsune &
Masuda, 1972b). Studies in Finland showed that whole blood from
persons with no special exposure to PCBs contained 0.3-1.2 µg/100 ml,
while blood from persons handling PCBs in an analytical laboratory
contained 3.66.3 µg/100 ml and blood from workers in a capacitor
factory had PCB levels of 7.5-190 µg/100 ml in the blood and
30-700 mg/kg in fat. No signs of toxicity were evident in these workers
(Karppanen & Kolho, 1973). Similar plasma values were found in workers
from Japanese capacitor factories, but here skin lesions were noted
(Hasegawa et al., 1972a). This same study reported that air levels of
PCBs of 0.01-0.05 mg/m3 were measured in a factory where KC-300 was
used in the manufacture of electric condensers. PCB levels in serum in
workers ranged from 10 to 65 µg/100 ml.a One month after the use of
PCBs had been suspended, serum levels still ranged from 9 to
74 µg/100 ml. However, in another factory making electric condensers, serum
levels decreased from an average of 80 µg/100 ml to 30 µg/100 ml
within three months of the use of PCBs being suspended (Kitamura et
al., 1973). According to Hara et al. (1974), the half-time of PCBs in
the blood of workers engaged in the manufacture of electric condensers
for less than 5 years was several months, while that of workers
employed for more than 10 years was 2-3 years. Hammer et al. (1972)
found a higher frequency of measurable plasma values in workers
working with refuse burners than in a control group.
a In this document, the concentrations of PCBs in blood and serum
are expressed in µg/100 ml although in some original papers the
values are given in µg/100 g. For practical purposes the
differences, about 5% and 3% respectively, can be neglected.
5.5.4 Other sources of exposure
Broadhurst (1972) has reviewed the many technical applications of
PCBs that appear in the literature and in patent specifications, and
which indicate the possibility of a widespread nonoccupational
low-level exposure to PCBs, other than that deriving from the diet.
PCBs are used in the home in ballast capacitors for fluorescent
lighting, and exposure deriving from pressure-sensitive copying paper
has not been limited to office workers. The valuable properties of
PCBs as plasticizers has led to their use in furnishings, interior
decoration, and building construction; examples are surface treatment
for textiles, adhesive for waterproof wall coatings, paints, and
sealant putties. PCBs have been used as plasticizers for plastic
materials and in the formulation of printing inks.
5.5.5 Biological indices of human exposure
The only surveys of value have been on body fat, blood, and milk.
5.5.5.1 Body fat
In a survey of 637 fat samples taken at autopsy or during surgery
in the USA, 68.9% contained PCB levels of less than 1 mg/kg, 25.9%
contained 1-2 mg/kg, and 5.2% contained more than 2 mg/kg (Yobs,
1972). A similar distribution was found in a smaller survey by Price &
Welch (1972). In the Kochi area of Japan, a mean PCB level of
2.86 mg/kg was recorded with an upper limit of 7.5 mg/kg; about double
these values were found in the Kyoto area (Nishimoto et al., 1972a,
1972b). Bjerk (1972) reported average PCB levels of 0.9 mg/kg
(1.6 mg/kg on a lipid basis) in adipose tissue, taken at 40 autopsies
in the Oslo area. Curley et al. (1973b) found PCB levels ranging from
0.30 to 1.48 mg/kg in a total of 241 human adipose samples in Japan. A
mean value of 5.7 mg/kg has been reported for 20 samples from the
Federal Republic of Germany (Acker & Schulte, 1970); in a more recent
study, 282 adipose tissue samples from different areas were found to
contain PCB levels of 8.3 mg/kg of adipose tissue on a lipid basis
(Acker & Schulte, 1974). In Austria, a range of PCB levels of
0.3-7.3 mg/kg on a lipid weight basis was found in 32 residents in the
Vienna metropolitan area; an increase of the PCB concentration with
age was not observed (Pesendorfer et al., 1973). Detectable levels of
PCBs were found in only a few of 201 human fat samples in the United
Kingdom and in these, the level did not exceed 1 mg/kg (Abbott et al.,
1972). A survey of 51 human fat samples in New Zealand showed that all
samples contained PCB residues with an average of 0.82 mg/kg (Solly &
Shanks, 1974).
Doguchi et al. (1974) found an average PCT level of 0.6 mg/kg in
human fat with a range of 0.1-2.1 mg/kg. Takizawa & Minagawa (1974)
also found PCT levels of 0.02 mg/kg in human liver ( n = 6),
0.01 mg/kg in the kidney ( n = 2), 0.02 mg/kg in the brain ( n = 3)
and 0.04 mg/kg in the pancreas ( n = 1). In the Netherlands, PCTs
were found in human fat at levels of 0-1 mg/kg (Freudenthal & Greve,
1973).
5.5.5.2 Blood
43% of blood plasma samples from 723 volunteers in the USA showed
the presence of PCBs; the mean value in these was about 0.5 µg/100 ml
(Finklea et al., 1972). Studies in Finland on whole blood showed
0.31-1.2 µg/100 ml in persons with no special exposure to PCBs
(Karppanen & Kolho, 1973). In Japan, an average PCB level of
0.32 µg/100 ml and a PCT level of 0.5 µg/100 ml have been recorded in
the blood of non-occupationally exposed volunteers (Doguchi & Fukano,
1975). Inpatients with severe weight loss, high levels of PCBs in the
blood (up to 10 µg/100 ml) were noted (Hesselberg & Scherr, 1974).
This was attributed to the release of PCBs from the mobilization of
fat.
5.5.5.3 Human milk
PCB concentrations measured in whole human milk in Sweden were
0.014 mg/litre in 1967 and 0.025 mg/litre in 1971-72 (Westöö & Norén,
1972), 0.03 mg/litre in Japan Nishimoto et al., 1972a), 0.103 in the
Federal Republic of Germany (Acker & Schulte, 1970) and 0.02 mg/litre
in Canada (Musial et al., 1974) A survey in Colorado, USA, revealed 8
positive samples out of 39, within the range of 0.04-0.1 mg/kg (Savage
et al., 1973).
5.5.6 Estimated daily intake
From the PCB levels encountered in air and drinking water (section
5.5.1), the daily intake from each of these sources is likely to be
less than 1 µg.
It has been stated that the major part of the human dietary intake
of PCBs is from fish (Berglund, 1972; Hammond, 1972). This may well be
true in areas such as Japan or certain localities near the North
American Great Lakes, where fish from polluted waters may form a
relatively large part of the diet. Several investigators from Japan
have measured the daily intake of PCBs in food; the highest mean value
recorded was 48 µg/day, of which 90% was from fish (Kobayashi, 1972);
the lowest was 8 µg/day (Ushio et al., 1974).
In much of Europe and North America, however, the daily intake of
fish is in the region of 30-40 g and most of the fish is taken from
waters of low pollution and contains PCB levels of not more than
0.1 mg/kg. Berglund (1972) has estimated that the daily intake of PCBs
from fish in Sweden is in the region of 1 µg, though if the fish
consumed were solely Baltic herring, the intake would be about 10 µg.
It is difficult to make an assessment of the PCB intake from foods
other than fish. Westöö et al. (1971, 1972), in their extensive study
of the Swedish diet, reported that most foods contained PCB levels of
less than 0.1 mg/kg; it may be concluded that this corresponds to a
daily intake of less than 100 µg. The conclusion that foods other than
fish may make a greater contribution to the PCB content of the diet
can be drawn from the survey in the USA reported by Kolbye (1972), and
also from a Swiss study of three different types of home-prepared
meals, not containing fish, which were found to contain 6, 41, and
84 µg of PCBs, respectively (Zimmerli & Marek, 1973).
The figures for the PCB content of human milk (section 5.5.5.3)
indicate that most nursing mothers excreted about 30 µg/day by this
route, and in some areas up to 100 µg/day. It may be assumed that only
a portion of the PCBs absorbed was excreted by this route, so that the
daily intake could have been more than 100 µg.
It may be concluded that, in the more industrialized countries,
the average daily PCB intake from the diet has rarely been less than
5 µg or greater than 100 µg; it is likely that the non-dietary sources
of exposure detailed in section 5.5.4 have made a significant
contribution, but this cannot be estimated at this time. In any area,
the intake depends not only on the diet, but also on social domestic
and environmental conditions; the influence of these factors on the
daily intake cannot readily be quantified.
6. METABOLISM
6.1 Absorption
The experiments of Vos & Beems (1971) who applied several
commercial PCBs to rabbit skin and found systemic effects (see section
7.1.1.4) indicate that PCBs can penetrate the skin. Early cases of
human poisoning from occupational exposure were probably due to a
combination of skin absorption and inhalation. Experimental studies on
rats by Benthe et al. (1972a) showed that an aerosol containing
Aroclor 1242 (particle size 0.5-3.0 µm) was readily absorbed through
the lungs.
Although one means of entry of PCBs into aquatic food chains is
through the consumption of plankton by fish, aquatic organisms can
also absorb PCBs in solution in the ambient water, presumably mainly
through the gills (see section 4.3). Salmon eggs can also absorb PCBs
from water (Johansson et al., 1970), and Södergren & Svensson 1973
concluded that mayfly nymphs could take in PCBs from water through the
gills and the integument.
Recent work with chlorobiphenyl isomers administered orally to
rodents at levels up 100 mg/kg of body weight for lower chlorinated
compounds and up to 5 mg/kg for the higher chlorinated compounds,
showed that 90% of the compounds were rapidly absorbed (Albro &
Fishbein, 1972; Berlin et al., 1973; Melvås & Brandt, 1973).
Cholestyramine, a basic anion exchange resin, was shown to interfere
with intestinal absorption of KC-400 in mice (Tanaka & Araki, 1974).
PCTs have been shown to be absorbed from the gut (Sosa-Lucero et
al., 1973) but very little information is available on the rate of
absorption.
6.2 Tissue Distribution of PCBs
Grant et al. (1971a) demonstrated that 4 days after an oral dose
of Aroclor 1254 at 500 mg/kg was given to rats, the concentrations of
PCBs in fat, liver, and brain were 996, 116, and 40 mg/kg,
respectively. Similar results showing that the highest concentration
was in fat, were obtained in rats given Aroclor 1254 in the diet
(Curley et al., 1971), in boars (Platonow et al, 1972), cows (Platonow
& Chen, 1973), and in pigeons and quail (Bailey & Bunyan, 1972). In
the experiments of Curley et al. (1971), the tissue concentrations
initially showed a rapid rise and thereafter a slow increase while the
PCB diet was being administered; Grant et al. (1974) fed diets
containing Aroclor 1254 at 0.2, 20, and 100 mg/kg to rats for 8
months, during which period the tissue concentrations reached a steady
state that was dose-dependent (Table 6). Similar tissue distribution
data for Aroclors 1016 and 1242 have been reported by Burse et al.
(1974) and for Kanechlor-400 by Yoshimura et al. (1971). PCB
deposition, in general, depends on the fat content of the tissue
(section 5.4.2). Residues in trout, receiving Aroclor 1254 in doses of
15 mg/kg in the diet, stabilized after 16 weeks while the absolute
quantity continued to increase as the fish grew (Lieb et al., 1974).
More detailed information on the tissue distribution of PCBs and
their metabolites has been obtained by the administration of pure
14C-labelled compounds, using both whole-body autoradiography and
scintillation counting of tissue samples. Berlin et al. (1975)
demonstrated that after a single oral dose of 14C-labelled
2,5,2',4',5'-pentachlorobiphenyl, radioactivity rapidly entered the
circulation of mice and was distributed in the tissues, particularly
in the liver, kidneys, lungs, and adrenals. Subsequently, the
radioactivity in the body fat increased, rising to a maximum within
4-24 h. In most other tissues the radioactivity decreased rapidly
after dosing, but the authors noted a special affinity for the skin,
the bronchiolar epithelium of the lungs, and certain glandular
secreting tissue. Soon after administration of the dose, radioactivity
appeared in bile and was excreted in the faeces. Similar results were
obtained by Melvås & Brandt (1973) with 2,4,2',4'-tetrachlorobiphenyl
in the mouse, and with 2,4,2',3'- and 2,4,3',4'-tetrachlorobiphenyls
in the quail; in the mouse they found a high affinity for the adrenal
cortex, the corpora lutea, and glandular secreting tissue, and in the
quail the radioactivity in egg yolk was high, exceeding that in fat.
Brandt & Ullberg (unpublished report 1973) found a similar pattern
after administration of hexa- and octachlorobiphenyls to mice.
6.3 Tissue Distribution of PCTs
Diets containing Aroclor 5460 at levels of 10, 100, and 1000 mg/kg
were administered to rats for 7 days (Sosa-Lucero et al., 1973). Table
7 shows the tissue distribution obtained in this study in rats fed
with Aroclor 5460 at 100 mg/kg body weight and the values in rats fed
with Aroclor 1254 at 100 mg/kg body weight in a similar study (Curley
et al., 1971). After oral administration of Aroclor 5460 to the cod,
the concentration of PCTs in the liver was more than 100 times that in
muscle on a wet weight basis (Addison et al., 1972), a ratio found by
Jensen et al. (1973) for PCBs in the cod.
Table 7. Tissue distribution (mg/kg wet weight) of PCTs (Aroclor
5460) in rats fed dietary levels of 100 mg/kg for 7 days
(Sosa-Lucero et al., 1 973) and fed PCB (Aroclor 1254) at
100 mg/kg for 9 days (Curley et al., 1971)
Aroclor Aroclor
Tissue 5460 1254
Blood 1.32 0.1
Liver 47 6
Brain 5.1 4
Kidneys 15.1 5
Heart 21.5 --
Fat -- 180
6.4 Placental Transport
Aroclors 1221 and 1254 were found to cross the placenta of
rabbits, when administered orally to does during gestation. The
concentration in fetal tissues was dose-dependent and much less with
Aroclor 1221 than with Aroclor 1254; with the latter, the
concentration in the fetal liver was greater than that in the maternal
liver (Grant et al., 1971b). Curley et al. (1973a) found some
placental transport of Aroclor 1254 in the rat. Platonow & Chen (1973)
demonstrated that the PCBs in the fetal kidney of a cow dosed with
Aroclor 1254 was greater than that in the mother. Placental transfer
of polychlorinated biphenyls has also been reported in the mouse
(Berlin et al., 1975; Melvås & Brandt, 1973; Brandt & Ullberg,
unpublished report 1973).
PCB concentration in human umbilical blood has been shown to be
about 25% of that in maternal blood (Taki et al., 1973). Placental
transfer of PCBs was observed in Yusho patients (Tsukamoto et al.,
1969).
No information is available on the placental transfer of PCTs.
6.5 Excretion and Elimination
6.5.1 Milk
Saschenbrecker et al. (1972) found that after oral administration
of doses of Aroclor 1254 of 10, and 100 mg/kg in diet to cows, 6.27
and 74.5 mg/litre, respectively, appeared in the milk after 24 hours.
These levels were reduced to less than one-half within 3 days but
traces remained at 50 days. Cows receiving 200 mg of Aroclor 1254
daily reached a steady state concentration of 61 mg/kg in milk fat and
42 mg/kg in body fat after 10 days (Fries et al., 1973). The PCBs in
milk survived processing into dairy products, and most was located in
milk fat (Platonow et al., 1971). PCBs have also been found in human
milk (see section 5.5.5.3).
6.5.2 Eggs
Several investigations have demonstrated the presence of high
levels of PCBs in the eggs of seabirds (Riseburgh & de Lappe, 1972).
An incident has been reported in which PCB contamination of poultry
food was toxic to chickens and decreased the hatchability of eggs
which also contained PCB residues (Pichirallo, 1971). In a laboratory
study, Scott et al. (1975) fed Aroclor 1248 to chickens at dietary
concentrations of 0, 0.5, 1.0, 10, and 20 mg/kg. After 8 weeks, the
approximate PCB levels in eggs were 0, 0.22, 0.41, 3.1 and 7.0 mg/kg
respectively. Jensen & Sundström (1974) found 33% of ah oral dose of
2,4,5,T,4',5'-hexa-chlorobiphenyl administered to quails was excreted
in eggs over a period of 10 days; eggs also provided a major route of
excretion in the pheasant (Dahlgren et al., 1971).
6.5.3 Urine and faeces
All investigators agree that only traces of PCBs can be found in
the urine of dosed animals, and that faeces provide a major route of
elimination. When the analysis of faeces is limited to the
determination of unchanged PCBs, the recovery of the dose administered
is incomplete; in boars receiving single or repeated doses of Aroclor
1254, not more than 16% of the dose was recovered from the faeces and
less than 1% in urine (Platonow et al., 1972). Better recoveries have
been obtained with PCB labelled with radioactive isotopes. Yoshimura
et al. (1971) found 70% of the activity from a dose of tritium-
labelled Kanechlor 400 in faeces and 2% in urine over a 4-week period.
Berlin et al. (1974, 1975) found over 75% of the activity from
14C-labelled penta- and hexachlorobiphenyls in faeces and less than
2% in urine; most of the faecal excretion consisted of PCB metabolites
(see section 6.6.1). Similar results were obtained by Melvås & Brandt
(1973) with tetrachlorobiphenyls.
6.6 Biotransformation
6.6.1 Metabolic degradation
Most investigators studying the tissue distribution of PCBs after
administration of commercial mixtures have noted a relative reduction
of the gas-liquid chromatographic peaks with shorter retention times,
corresponding to the lower chlorinated biphenyls. This has been
reported in the rat (Grant et al., 1971a; Curley et al., 1971), the
rabbit (Grant et al, 1971b), the cow (Platonow & Chen, 1973), and in
pigeons and quails (Koeman et al., 1969; Bailey & Bunyan, 1972).
Samples of tissues from animals and man (see table 3, pp. 20-21) that
have absorbed PCBs from the environment have shown, on analysis, a
pattern of peaks approaching that of PCB mixtures with more than 50%
chlorination, although the major manufactured products contain 42% of
chlorine or less. This has led to the belief that the rate of
metabolic attack on PCBs decreases with increasing chlorination.
Studies on single PCBs with 1, 2, 4, or 5 chlorine atoms have shown
that these are more readily excreted as metabolites in faeces by
mammals and birds and remain for a shorter time in fatty tissues than
most PCBs with 6 or more chlorine atoms (Berlin et al., 1975;
Hutzinger et al., 1972a; Melvås & Brandt, 1973; Brandt & Ullberg,
unpublished report 1973). See also section 6.6.2.
The administration to rats of diets containing Aroclors 1016 or
1242 at a concentration of 100 mg/kg resulted in a steady state in
adipose tissue for both compounds in about 4 months. When gas
chromatographic traces were compared with those for standard PCB
mixtures a difference in the gas-liquid chromatographic pattern, that
is disappearance of the peaks with short retention times was noted.
After exposure to PCBs was discontinued, a major portion of the PCBs
was eliminated from the body in 4 months. However, 20% of the total
PCBs in Aroclor 1242 with longer retention times were present after 6
months, and 10% of the total PCBs of Aroclor 1016 with longer
retention times were present in adipose tissue after 5 months. (Burse
et al., 1974).
The excretion of monohydroxy metabolites of 3,4,3',4'-tetrachloro-
biphenyl and 2,4,3',4'-tetrachlorobiphenyl (orally administered) in
rats has been demonstrated by Yoshimura & Yamamoto (1973). Yoshimura
et al. (1973), Yamamoto & Yoshimura (1973), Yoshimura & Yamamoto
(1974), and Yoshimura et al. (1974). They demonstrated that the
metabolites of the first isomer were 2-hydroxy or 5-hydroxy compounds
while the metabolites of the second isomer were 5-hydroxy and
3-hydroxy compounds. All hydroxy metabolites were excreted
non-conjugated via the bile and no parent isomers were found in the
bile. Yoshimura & Yamamoto (1975) found that unchanged
2,4,3',4'-tetrachlorobiphenyl was excreted through the intestine, when
it was intravenously injected in rats with the bile duct ligated,
while no metabolite of this isomer was excreted by this route.
Hutzinger et al. (1972a) demonstrated the presence of hydroxylated
derivatives of mono-, di-, and tetrachlorobiphenyls in the excreta of
rats and pigeons but not of trout and were unable to detect
hydroxylated 2,4,5,2',4',5'-hexachlorobiphenyl. Berlin et al. (1975)
isolated a hydroxy derivative of 2,5,2',4',5'-pentachlorobiphenyl from
mouse faeces, and Jensen & Sundström (1975) have shown that although
2,4,5,2',4',5'-hexachlorobiphenyl is excreted very slowly, a hydroxy
derivative could be detected in rat faeces. Hutzinger et al. (1974),
however, showed that this compound was also dechlorinated by the
rabbit and excreted as the hydroxy derivative of pentachlorobiphenyl.
Gardner et al. (1973) detected hydroxylated metabolites in the urine
of rabbits dosed with 2,5,2',5'-tetrachlorobiphenyl together with a
dihydroxy derivative which they regarded as evidence for the formation
of an arene oxide (epoxide) intermediate (see section 6.5.3). Jansson
et al. (1975) have identified up to 26 mono- and dihydroxylated
metabolites of PCB in the bile and faeces of wild grey seal and
guillemot from the Baltic.
There is little information on the biotransformation of PCTs.
Addison et al. (1972), using gas-liquid chromatography, noted a loss
of PCTs with a shorter retention time in the excreta of a cod dosed
orally with Aroclor 5460; the same loss was observed in rat faeces
after the administration of a diet containing Aroclor 5460
(Sosa-Lucero et al., 1973).
6.6.2 The effect of structure on retention
While each of the components of mixtures of PCBs has a different
pattern of retention and elimination in different species, the
measurement of biological half-times for PCB mixtures in tissues has
provided useful information. Bailey & Bunyan (1972) found the
half-time of PCBs in the fat of quail and pigeon, after cessation of
dosing with Aroclor 1242, or with Aroclor 1254, to be 50 days and 125
days, respectively. A half-time of about 200 days was recorded in the
fat of rats after feeding with Aroclor 1254 (Grant et al., 1974).
Berlin et al. (1975) noted that in mice dosed with a
pentachlorobiphenyl there was an initial fairly rapid elimination
while the PCB level in the liver was high, followed by a slower
elimination when most of the PCB was located in fat. It seems likely
that the mobilization of PCBs from fat, and therefore their half-time
in the body, depends upon their rates of metabolism. Berlin et al.
(1974) investigated the hypothesis that the ability of a PCB to be
readily degraded with a half-time of a few days depended upon the
presence of two adjacent unsubstituted carbon atoms in the molecule
rather than on the number of chlorine atoms, although the presence of
such unsubstituted pairs depends to a large extent on the degree of
chlorination. They came to the conclusion that this hypothesis
probably applied to unsubstituted pairs in the 3,4-position, but that
in the 2,3-position, their susceptibility to metabolic degradation was
much influenced by the presence of chlorines in the o-position of
the ring bridge.
Jensen & Sundström (1974) demonstrated that the retention of PCBs
in human fat is also influenced by o-chlorine substitution (Table 3,
pp. 20-21 ).
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF PCBs AND PCTs
7.1 Toxic Effects in Different Species
Most of the available information on the toxicity of the PCBs has
been obtained from studies on commercial mixtures. The much smaller
amount of information available concerning the impurities in
commercial products and PCTs, is given in sections 7.2 and 7.3.
7.1.1 Mammals
7.1.1.1 Acute oral and intravenous toxicity
Earlier work by the Monsanto Company indicated a low acute oral
toxicity for the Aroclors, the LD50s to rats ranging from 4.0 g/kg
for Aroclor 1221 to 11.3 g/kg for Aroclor 1262 (Panel on Hazardous
Trace Substances, 1972). More recent work has demonstrated a slightly
higher toxicity (Bruckner et al., 1973; Grant & Philips, 1974). With a
single intravenous dose the LD50 for Aroclor 1254 was 358 mg/kg body
weight in adult female Sherman rats (Linder et al., 1974). The acute
oral LD50 for Aroclor 1254 in the same strain and sex was between 4
and 10 g/kg (Kimbrough et al., 1972). According to Bruckner et al.,
(1973), severely poisoned animals showed weight loss, ataxia,
diarrhoea, and chromodacryorrhoea, and they considered progressive
dehydration and central nervous depression were the causes of death.
In rats, vacuolation in the liver and kidneys was observed (Bruckner
et al., 1973) and also ulceration of the gastric and duodenal mucosa
(Kimbrough et al., 1972).
Yamamoto & Yoshimura (1973) found the intraperitoneal LD50 of
2,4,3'4'-tetrachlorobiphenyl in mice to be 2.15 g/kg body weight and
that of the 5-hydroxy derivative, which is the main in vivo
metabolite, to be 0.43 g/kg body weight.
7.1.1.2 Subacute oral toxicity
After repeated administration, the PCBs have a cumulative toxic
action. In a group of rats receiving Aroclor 1254 at a dose of 1 g/kg
of diet, deaths occurred between the 28th and 53rd days of feeding
(Tucker & Crabtree, 1970), and with Phenochlor DP6 at 2 g/kg of diet,
deaths occurred between the 12th and 26th days (Vos & Koeman, 1970).
In the latter experiment enlarged livers, small spleens, and a
progressive chemically-induced hepatic porphyria were seen at autopsy.
Repeated weekly oral administration of 150 mg of Aroclors 1221, 1242
or 1254 to rabbits for 14 weeks produced liver enlargement and damage
with Aroclor 1242 and no effect with Aroclor 1221 (Koller & Zinkl,
1973). Allen et al. (1974) administered diets containing Aroclor 1248
at the concentration of 25 mg/kg of diet to 6 female rhesus monkeys
for two months; facial oedema, loss of hair, and acne developed after
1 month and one animal died with severe gastritis 2 months after
removal from experimental diet. PCB concentrations in the body fat of
the animals, after two months of treatment, averaged 127 mg/kg while 8
months later the value declined to 34-mg/kg.
Mink appear to be unusually sensitive to PCBs. Aulerich et al.
(1973) administered diets containing PCB levels of 30 mg/kg (10 mg/kg
each of Aroclors 1242, 1248, and 1254) to adult mink and demonstrated
100% mortality within 6 months. PCB residues in the brains of these
mink averaged about 11 mg/kg and were approximately twice the level
observed in other tissues. Four months of feeding Aroclor 1254 at
levels of 5 and 10 mg/kg demonstrated a dose-dependent retardation of
weight gain of growing female mink. Female mink fed a diet
supplemented with Aroclor 1254 at 5 mg/kg for 9 months failed to
produce offspring (Ringer et al., 1972).
7.1.1.3 Chronic oral toxicity
Aroclors 1242, 1254, and 1260 have been administered for 18 months
to rats at 1, 10 and 100 mg/kg in the diet (Keplinger et al., 1971).
No adverse effects were recorded with the three Aroclors at 10 mg/kg
but with Aroclors 1242 and 1254 at 100 mg/kg there was an increase in
liver weight and a reduced survival of litters. In similar experiments
on dogs, there was a reduced weight gain with Aroclors 1254 and 1260
in the diet at a level of 100 mg/kg (Keplinger et al., 1971). In the
experiments reported by Kimbrough et al. (1972), male rats survived
Aroclor 1260 in the diet at 1 g/kg (71.4 mg/kg body weight) for 8
months but 8/10 females died at this dose, 2/10 died at 500 mg/kg, and
1/10 at 100 mg/kg (7.2 mg/kg body weight). With Aroclors 1254 and
1260, a dose-dependent increase in liver weight in male rats was
significant down to 20 mg/kg (1.4 mg/kg body weight) in the diet; with
females the liver enlargement occurred only at diet levels of
500 mg/kg and higher. The livers showed an orange fluorescence, the
cells were enlarged and vacuolated with lipid inclusions; there was
also much increased smooth endoplasmic reticulum, and what was termed
"adenofibrosis" was present, being more marked with Aroclor 1254 (see
section 7.8). Grant et al. (1974) fed diets containing Aroclor 1254 at
0, 2, 20, and 100 mg/kg to rats for 246 days followed by 180 days on a
PCB-free diet; after 246 days the body weight of the 100 mg/kg rats
was significantly less than that of the controls and the liver weight
was greater. The most notable change in the livers on histological
examination was the appearance of fat microdroplets in the
centrilobular region; this effect was dose-dependent and not seen in
the rats receiving the 2 mg/kg diet and was reversible when the rats
were returned to a normal diet.
Liver enlargement has been described by a number of authors, in a
number of species, with different PCB mixtures and pure isomers and it
is considered to be due primarily to hypertrophy of the smooth
endoplasmic reticulum of the liver cells (Vos & Beems, 1971; Allen et
al., 1973; Kimbrough, 1974; Nishizumi, 1970) but with large enough
doses (oral intubation of about 150 mg/kg body weight/week for 14
weeks) of Aroclor 1254 and 1242, it progressed to frank liver damage
(Koller & Zinkl, 1973). The smooth endoplasmic reticulum may condense
in the liver cell and form hyalin inclusions and this may be
accompanied by a loss of enzyme activity. Lipid accumulation, pigment
deposition, nuclear changes, and necrosis may also occur (Vos &
Notenboom-Ram, 1972).
The rhesus monkey is the only species reported to show signs of
poisoning similar to those in human Yusho patients (see section 8).
The administration of Aroclor 1248 at 2.5 and 5.0 mg/kg of diet for 1
year produced periorbital oedema, alopecia, erythema, and acneiform
lesions involving the face and neck within 1-2 months. The effects
were less marked in male monkeys. At 25 mg/kg of diet, one out of a
group of six died, and at 100 and 300 mg/kg the mortality approached
100% within 2-3 months. Animals more severely affected showed
hypertrophic hyperplastic gastritis with ulceration, anaemia,
hyperproteinaemia and bone marrow hypoplasia. The survivors still
showed signs of poisoning 8 months after exposure (Allen & Norback,
1973; Allen et al., 1974; Allen 1975).
7.1.1.4 Dermal toxicity
Vos & Beems (1971) have confirmed earlier reports that PCBs damage
the follicular epithelium in experimental animals. They applied three
commercial 60% chlorinated mixtures, Clophen A60, Phenochlor DP6, and
Aroclor 1260 to rabbit skin at a daily dose of 118 mg/50 cm (5 times
per week) for 38 days. After initial reddening, transverse wrinkling
developed with hyperplasia and hyperkeratosis of the epidermal and
follicular epithelium. These effects were more marked with Clophen and
Phenochlor than with the Aroclor.
During these experiments, deaths occurred in the Clophen- and
Phenochlor-treated groups but not in the Aroclor group. Kidney lesions
were seen in all groups; liver damage was least in the Aroclor group.
There was atrophy of the thymus cortex and a reduction of germinal
centres of the lymph nodes as well as lymphopenia, and some animals in
all groups showed oedema of the abdominal and thoracic cavities,
subcutaneous tissue, and pericardium. Faecal excretion of copro- and
protoporphyrins was increased by all three PCBs but was lowest with
Aroclor 1260. Vos & Beems attributed the greater severity of the
effects observed with Clophen and Phenochlor to the presence of toxic
impurities (see section 7.2). In another comparative dermal toxicity
study in rabbits (Vos & Notenboom-Ram, 1972), skin lesions in Aroclor
1260-treated animals were more severe than in animals treated with
2,4,5,2',4',5'-hexachlorobiphenyl. In a Japanese study, no differences
in skin lesions were observed in rabbits after dermal applications of
Kanechlor 400, Kanechlor 500, or 3,4,3',4'-tetrachlorobiphenyl
(Komatsu & Kikuchi, 1972).
7.1.1.5 Inhalation toxicity
Only one inhalation study has been reported using Aroclor 1242 and
1254 (Treon et al., 1956). Rats, mice, rabbits, and guinea pigs were
exposed to Aroclor 1242 or 1254 vapours for five days a week for
several weeks at concentrations ranging from 1.5 to 8.6 mg/m3. At
these concentrations Aroclor 1254 produced liver enlargement in rats.
7.1.2 Birds
The acute oral toxicity of Aroclors 1242 and 1254 is low for the
mallard duck, the LD50s being greater than 2 g/kg body weight (Tucker
& Crabtree, 1970). Published figures for the lethal dose for birds
after repeated administration are very variable; it appears to be
dependent on the species and age of the bird, the method of
administration, the degree of chlorination of the PCBs, and the
presence of impurities (Vos, 1972). Heath et al. (1970) determined the
dietary concentrations of Aroclors 1232 to 1262, administered over a
5-day period, that were required to kill 50% of groups of mallards,
pheasants, and quails. The concentrations were in the range of 500 to
over 5000 mg/kg, with the bobwhite quail the most sensitive and the
Japanese quail the least. There was a positive relationship between
the percentage of chlorine in a technical Aroclor and its toxicity.
The relationship held true for those containing less than 60%
chlorine, Aroclor 1260 and 1262 deviating slightly. Mallards were
relatively less responsive to chlorine content than the three
gallimaceous species. Prestt et al. (1970) found a 50% mortality in
Bengalese finches receiving a daily oral dose of Aroclor 1254 at
254 mg/kg body weight for 56 days; cormorants have been killed with a
cumulative dose of 5.7 g of Clophen A60, but herons were more
resistant (Koeman et al., 1973).
The toxicity to chickens of diets containing Phenochlor DP6,
Clophen A60, or Aroclor 1260 at 400 mg/kg has been studied by Vos &
Koeman (1970). The first two produced a 100% mortality with survival
times of 24.3 and 20.5 days, but there was only 15%o mortality with
the Aroclor over the 60-day test period. The authors demonstrated the
presence of impurities in the Phenochlor and Clophen (Vos et al.,
1970). The results of Flick et al. (1965) and Platonow & Funnel (1971)
indicate that chickens can survive 200 mg/kg in the diet for several
months, but that deaths may occur at 250 mg/kg, though Rehfeld et al.
(1971) found a much higher toxicity, recording 1/30 deaths with
Aroclor 1248 at 30 mg/kg of diet over 25 days and 16/30 at 50 mg/kg.
Keplinger et al. (1971) found decreased growth in chickens receiving
Aroclor 1242 at 10 mg/kg and Aroclor 1254 at 100 mg/kg of diet, but no
effects with Aroclor 1260 at 100 mg/kg. Birds severely poisoned with
PCBs show tremors, ataxia, and ruffling and loss of feathers. Oedema
of the abdominal and peritoneal cavities has been a characteristic
sign at autopsy in some experiments (Flick et al., 1965) and this was
observed in a very large number of chickens killed in Japan by the
contamination of their feed with Kanechlor KC400 (Kohanawa et al.,
1969a, 1969b). Enlargement of the kidneys and sometimes of the liver
has been reported; some authors have noted a reduction in the size of
the spleen, comb, and testes, an enlargement of the adrenals and
thyroid, and a pale pancreas. No pathological signs were observed by
Dahlgren et al. (1972) that could account for the deaths of pheasants,
which occurred with a cumulative intake of about 900 mg of Aroclor
1254 (daily oral administration of 20 or 200 mg to 11-week-old hens);
they found a mean concentration of 520 mg/kg in brain tissue at death
and it is possible that effects in the central nervous system were a
contributory cause.
7.1.3 Aquatic organisms
7.1.3.1 Fish
Stalling & Mayer (1972) report an oral LD50 of more than 1.5 g/kg
for rainbow trout with Aroclors 1242 and 1260. A 15 mg/kg oral dose to
cod affected their ability to maintain an upright position in rotating
water (Lindahl, unpublished report 1974).
The assessment of toxicity to fish by adding PCBs to aquarium
water is subject to considerable error on account of the different
solubilities of the components. Zitko (1970) claims the aqueous
solubility of Aroclor 1221 to be 3.8-5 mg/litre and that of Aroclor
1254 to be 0.3-0.5 mg/litre. Stalling & Mayer (1972) report the
96-hour LC50s on the cut-throat trout to be 1.17 mg/litre for Aroclor
1221 and up to 60 mg/litre for Aroclor 1260, where the solubility is
clearly exceeded. In the more prolonged experiments of these authors
(Table 8), most of the concentrations are within the solubility limits
and therefore more reliable. In more prolonged experiments, Hansen et
al. (1971) found deaths in fish exposed for up to 45 days to Aroclor
1254 at 5 µg/litre, but none at 1 µg/litre. These results show that
the effect of PCBs is cumulative in fish and that the toxicity
decreases with increasing chlorination.
Table 8. Intermittent-flow bioassays of Aroclors against three species of fisha
LC50 (µg/litre)
5 10 15 20 25 30
Aroclor Species days days days days days days
1254 Rainbow trout 156 8 -- -- -- --
1260 -- 240 94 21 -- --
DDT 2.26 0.87 0.26 -- -- --
1242 Bluegills 154 72 54 -- -- --
248 307 160 76 10 -- --
1254 -- 443 204 135 54 --
1260 -- -- -- 245 212 151
1242 Channel catfish -- 174 107 -- -- --
1248 -- 225 127 -- -- --
1254 -- __ 741 300 113 --
1260 -- -- -- 296 166 137
1248b Bluegills 137 76 -- -- -- --
1248c Channel catfish -- 94 57 -- -- --
a From Stalling & Mayer (1972).
b Temperature, 20°C; alkalinity 260 mg/litre pH 7.4.
c Temperature, 27°C.
Young fish appear to be more sensitive to PCBs than adults,
96-hour LC50s for newly hatched fathead minnows were 15 and
8 µg/litre, respectively for Aroclors 1242 and 1254. Growth of young
fathead minnows and flagfish was affected above 2.2 µg/litre (Nebeker
et al., 1974). There is no information on pathological changes in fish
that might be related to the lethal action of PCBs; Hansen et al.
(1971) found that fish survived exposure to Aroclor 1254 at
5 µg/litre, but died later, after being returned to clean water, with
signs of a lowered resistance to infection. Pathological changes were
observed in kidney, spleen, and liver of rainbow trout fed Aroclor
1254 at 10 or 100 mg/kg of diet for up to 330 days (Nestel & Budd,
1975).
7.1.3.2 Aquatic invertebrates
The results of several investigations into the toxicity of PCBs to
aquatic invertebrates have been reported by Stalling & Mayer (1972).
The exposure of a variety of scud (Gammarus pseudolimnaeus) to PCBs
showed a decreased toxicity with increasing chlorination; 4-day LC50s
of 10, 52, and 2400 µg/litre were obtained with Aroclors 1242, 1248
and 1254, respectively. The threshold for survival, growth, and
reproduction of Gammarus pseudolimnaeus exposed to Aroclor 1248 was
about 5 µg/litre and was the same for Daphnia magna. With the
crayfish, 7-day LC50s were 30 µg/litre (Aroclor 1242) and 80 µg/litre
(Aroclor 1254) and with the glass shrimp, 3 µg/litre (Aroclor 1254).
Wildish (1970) found that mortality in Gammarus oceanicus was
dependent on the duration of exposure to Aroclor 1254 at levels above
10 µg/litre. Stalling & Mayer (1972) reported the 15-day LC50 of
Aroclor 1254 for immature pink shrimp to be 0.94 µg/litre.
7.1.3.3 Microorganisms
The growth of certain marine diatoms is inhibited by Aroclor 1254
at 10-25 µg/litre, but marine and freshwater algae are more resistant,
being unaffected by 100 µg/litre (Mosser et al., 1972). Fisher et al.
(1972) found that phytoplankton from the Sargasso Sea did not grow in
Aroclor 1254 at 10 µg/litre, though phytoplankton from estuarine and
coastal waters were not much affected by this concentration. Keil et
al. (1971) report that the growth of a diatom was inhibited by Aroclor
1242 at 100 µg/litre with a reduction of RNA synthesis, but that
10 µg/litre had no effect.
The growth of cultures of lake bacteria was not inhibited by
concentrations of Aroclors 1221, 1242, and 1254 in excess of
solubility, and Aroclors 1221 and 1242 could be utilized as the sole
source of carbon and energy (Wong & Kaiser, 1975).
7.2 Toxicity of Impurities in Commercial PCBs
Vos & Koeman (1970) observed that Phenochlor DP6 and Clophen A60
were more toxic to chickens than was Aroclor 1260 (see section 7.1.2)
and Vos & Beems (1971) showed a similar difference in the dermal
toxicity to the rabbit (see section 7.1.1.4). Vos et al. (1970)
subdivided Clophen A60 and Phenochlor DP6 into non-polar PCBs and
polar fractions and found polar components that were not detectable in
a similar fraction from Aroclor 1260. Phenoclor DP6, Clophen A60, and
the polar fraction from Clophen A60 produced a high mortality in the
chick embryo test but the polar fraction from Aroclor 1260 did not. A
difference between the three fractions was seen in the development of
skin lesions in the rabbit (Vos & Beems, 1971). Mass spectrographic
analysis indicated that the impurities were tetra- and pentachloro-
dibenzofurans. Additional contaminants were chlorinated naphthalenes.
Vos et al. (1970) calculated that the maximum level of chlorinated
dibenzofurans in Clophen A60 was 5 mg/kg and in Phenochlor DP6,
20 mg/kg. They calculated that the chlorinated dibenzofurans were
approximately one order of magnitude less toxic than the chlorinated
benzodioxins, and considered that they were mainly responsible for the
toxicity of the polar fraction and for the difference in toxicity
between the three commercial PCB mixtures.
Recently, chlorinated dibenzofurans were detected in the
PCB-contaminated oil that was responsible for the Yusho disease in
Japan (see section 8, p. 65).
In 1961, Bauer et al. demonstrated the toxicity of a mixture of
tri- and tetrachlorodibenzofurans; a single oral dose of 0.5-1.0 mg/kg
body weight caused severe and often lethal liver necrosis in rabbits.
Application to the rabbit ear resulted in hyperplasia and
hyperkeratosis. Similar toxic effects were found with 2,3,7,8-tetra-
chlorodibenzo- p-dioxin in doses that were 10 times lower than those
found to be toxic in the case of chlorinated dibenzofurans. See also
the review by Kimbrough (1974). 2,3,7,8-Tetrachlorodibenzofuran, which
has recently been shown to be present in PCBs (Bowes et al., 1975)
caused mortality in chickens after 8-15 days when they were dosed
orally with 5 µg/kg/day. A single oral dose of 4000 µg/kg was not
lethal to mice. Porphyria was not observed in chicks or mice
(Goldstein et al., 1975a). In comparison, 8 out of 10 chicks died
after 9-19 days when dosed orally with 2,3,7,8-tetrachloro-
dibenzo- p-dioxin at 1 µg/kg body weight/day (Schwetz et al., 1973).
The oral LD50 of 2,3,7,8-tetrachlorodibenzofuran in guinea pigs is
approximately 7 µg/kg body weight, which is less than one order of
magnitude higher than that of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(Moore, 1975).
Using low resolution mass spectrometry, McKinney (1975) found
metabolites in the excreta of chickens fed 2,4,6,2',4',6'-hexa-
chlorobiphenyl which had correct masses for chlorodibenzofurans. In
addition, by perchlorination, he identified octachlorodibenzofuran
again by low resolution mass spectrometry. The 2,4,6,2',4',6'-hexa-
chlorobiphenyl isomer is not found in commercial mixtures.
Zitko et al. (1972b) looked for the presence of chlorinated
dibenzofurans in fish and fish products taken from a contaminated
coastal area. The samples included shark tissues, cormorant and
herring gull eggs, herring oil, and herring fishmeal. The detection
limit of the method was 0.01-0.02 mg/kg in the sample. No chlorinated
dibenzofurans were detected, although the authors admit that the limit
of detection was not sufficiently low to detect amounts that might
exert a significant toxic action. Curley et al. (1975) detected a
component which had a 4 Cl isotopic cluster and a mass number of 304
corresponding with that of tetrachlorodibenzofuran in the urine of
rats dosed with PCBs, but a positive identification was not made.
7.3 Toxicity of the PCTs
There have not been any systematic studies on the toxicity of the
PCTs. Sosa-Lucero et al. (1973) administered diets containing Aroclor
5460 at 0, 10, 100, and 1000 mg/kg to groups of rats for seven days.
There were no adverse effects on health or body weight; a significant
liver enlargement was recorded at the 1000 mg/kg level. In a test for
estrogenic activity involving the stimulation of glycogen response in
the immature rat uterus, Aroclor 5460 was inactive, as was Aroclor
1260 but Aroclor 5442 was more active than Aroclor 1242 (Bitman et
al., 1972). A dietary level of Aroclor 5460 of 5000 mg/kg for 12 weeks
caused decreased body weight and increased liver weight in rhesus
monkeys (Allen & Norback, 1973); after 6 weeks, facial oedema, hair
loss, and eye discharge were observed as described by the same authors
in experiments with Aroclor 1248 (section 7.1.1.2), and similar
gastric changes were also reported.
7.4 Biochemical Effects
7.4.1 Induction of enzymes
Several investigators have observed an increase in the smooth
endoplasmic reticulum of liver cells after administration of PCBs (see
section 7.1.1.3). This is accompanied by an induction of microsomal
mixed function oxidase. Induction of microsomal enzyme activity is,
like liver enlargement, more marked with the higher chlorinated PCBs
and relatively low with Aroclors 1221 and 1016 (Villeneuve et al.,
1971a, 1972; Bickers et al., 1972; Ecobichon & Corneau, 1974). The
effect has also been demonstrated with single pure PCBs administered
orally (Fujita et al., 1971); more recently this work has been
confirmed by Johnstone et al. (1974) whose results are summarized in
Table 9 and show a greater degree of enzyme induction with the higher
chlorinated compounds.
Grant et al. (1974) found an increase in microsomal enzyme
activity in rats receiving Aroclor 1254 at 20 mg/kg of diet for 246
days, but none at 2 mg/kg. Iverson et al. (1975) and Goldstein et al.
(1975) compared the microsomal enzyme inducing potential of Aroclors
1242 and 1016. Iverson et al. (1975) found increased hepatic
microsomal enzyme activity with both Aroclors in male rats receiving
21 daily oral doses of 1 mg/kg body weight, and with Aroclor 1242 in
females at 10 mg/kg of body weight and with Aroclor 1016 at 100 mg/kg.
PCBs administered to rats during pregnancy can induce microsomal
enzyme activity in the placenta and fetus and this also occurs in the
liver of newborn rats suckled by mothers fed with diets containing
PCBs (Alvares & Kappas, 1975). Benthe et al. (1972b) reported that
when rats were stressed by food deprivation or cold, the PCB residues
in adipose tissue were released during mobilization of the fat and
caused increased hepatic microsomal enzyme activity.
Table 9. Stimulation of microsomal enzyme activity by single chlorinated biphenyls
Hepatic microsomal enzyme activity
Chlorine aniline
substituents O-demethylation N-demethylation hydroxylation nitro-reduction
4 0 0 0 0
2,2' 0 + + 0
2,4' 0 0 + 0
4,4' + + + + + + +
2,5,2',5' 0 + + + +
2,4,2',4' + + + + + + 0
2,4,5,2',4',5' + + + + + + + +
2,3,5,2',3',5' + + + + + + +
2,4,6,2',4',6' + + + + + + + +
2,3,4,5,2',3',4',5' + + + + + + + +
a Johnstone et al. (1974).
0 no activity.
+ slight activity.
+ + marked activity.
7.4.2 Porphyria
Hepatic porphyria has been induced by a number of PCBs (Clophen
A60, Phenoclor DP6, Aroclors 1016, 1242, 1254, and 1260) in the
chicken, rabbit, Japanese quail, and the rat (Vos & Koeman 1970; Vos &
Beems, 1971; Iverson et al., 1975; Vos et al., 1971; Goldstein et al.,
1974, 1975a, 1975b). Porphyrin induction has been studied more
extensively in rats. A dose-dependent increase in liver porphyrins has
been observed in females receiving 21 daily oral doses of Aroclor 1242
at 10 and 100 mg/kg of body weight, but not at 1 mg/kg. Female rats
were more sensitive than males, and Aroclor 1016 had less effect
(Iverson et al., 1975). In rats receiving Aroclor 1254 at 100 mg/kg in
the diet, the increase was usually delayed 2-4 months after the start
of dosing (Goldstein, 1974) and was characterized by high hepatic and
urinary levels of uroporphyrin. Disturbance of porphyrin biosynthesis
had been connected with an increase of the rate limiting enzyme
2.3.1.37-delta-aminolaevulinate synthase (Vos et al., 1971; Goldstein
1974, 1975).
Kawanishi et al. (1973, 1974) have shown that the administration,
in the diet, of Kanechlors KC-300 and KC-500 to rats at 500 mg/kg
produced a marked increase in urinary excretion of copro- and
uroporphyrins, and in faecal excretion of protoporphyrin, but no
increase was observed with KC-400. Experimental administration of pure
tetrachlorobiphenyls did not produce porphyria. However, porphyria did
result from the repeated subcutaneous injection of KC400 (total dose
1.8 g) to rabbits for 55 days (Miura et al., 1973).
7.4.3 Effects on steroid metabolism
PCBs have been shown to stimulate the activity of enzymes
responsible for metabolizing steroids such as estrodiol and
androsterone more effectively than does DDT or DDE (Risebrough et al.,
1968; Lincer & Peakall, 1970). It has been suggested that effects on
reproduction (see section 7.4) may be attributed to the induction of
steroid-metabolizing enzymes (Kihlström et al., 1973). Long-term
administration of daily oral doses of 0.025 mg Clophen A60 to 23
female NMRI strain mice caused a lengthening of the estrous cycle and
a reduction in the frequency of implantation of ova Orberg &
Kihlström, 1973).
7.4.4 Other biochemical effects
Hepatic vitamin A has been reported by Villeneuve et al. (1971a)
to be reduced to half the normal values in pregnant rabbits by Aroclor
1254. Similar observations have been made by Cecil et al. (1973) in
male and female Japanese quails and in rats after feeding Aroclor 1242
at 100 mg/kg of diet. The 50% decrease in hepatic vitamin A found in
male and female rats was also found in male and female quail provided
that the latter were kept in the dark to prevent egg laying.
A lowering of hepatic vitamin A in rats, fed a 0.1% PCB diet (PCB
not specified), was described by Innami et al. (1974). The rats given
vitamin A supplement (3400 IU) plus PCB for 6 weeks showed better
growth than those given PCB alone, while those given PCB and a vitamin
A deficient diet showed significant growth retardation. On the PCB
diet, the hepatic vitamin A level decreased to 20% of the normal level
during the experimental period; supplementing the diet with 1000 IU
did not re-establish the vitamin A level in the liver. Administration
of 3000 IU of vitamin A with the PCB diet, however, allowed better
than normal hepatic vitamin A levels to be re-established. The authors
concluded that vitamin A may play a role in the detoxification of PCB
rather than that PCB plays a role in the destructive metabolism of
vitamin A.
Aroclor 1254 administered intraperitoneally to rats at 25 mg/kg
body weight daily for 4 days caused a 4- to 5-fold increase in the
biliary excretion of thyroxine during a 3-hour period. Biliary
clearance was greatly elevated. Hypobilirubinaemia has been produced
in rats by Bastomsky et al. (1975) who investigated the mechanism by
administering daily intra-peritoneal injections of Aroclor 1254
(25 mg/kg body weight in corn oil) to female rats for 4 days, then
measuring bilirubin glucuronide formation by hepatic microsomes
in vitro. PCB treatment was not effective in increasing
UDPglucuronosyltransferase (2.4.1.7) activity. Serum bilirubin levels
were also significantly decreased by PCB treatment of Gunn rats, which
are genetically deficient in UDPglucuronosyltransferase (2.4.1.7)
activity.
7.4.5 Potentiation and antagonism by PCBs
As PCBs can stimulate microsomal enzyme activity, it is to be
expected that they may potentiate the action of those chemicals that
undergo microsomal activation, and decrease the action of those that
are detoxified. Villeneuve et al. (1973) demonstrated the antagonistic
effect by the reduction of phenobarbital sleeping time in rats
receiving Aroclors 1242, 1254, and 1260 in their diet, but not in
those receiving Aroclor 1221. Johnstone et al. (1974) have confirmed
this with a series of single PCBs. Tanaka & Komatsu (1972) found that
the hexobarbital-induced sleeping time in female rats was reduced to
49% of the control value by daily oral doses of Kanechlor 500 of
2 mg/kg for 3 days (total 6 mg/kg). When a daily dose of 0.4 mg/kg was
given for 15 days (total 6 mg/kg), no reduction in sleeping time was
observed. When this small dose was continued for 45 and 53 days, the
reduction remained at 12-13%. Phillips et al. (1972) did not find any
potentiation of the cholinesterase-inhibitory action of parathion in
rats dosed with Aroclors 1221 and 1254; this does not necessarily
imply that there was no enhanced activation of parathion, as a
stimulation of detoxication may have occurred concurrently. A
stimulation of parathion detoxication but not of activation has been
demonstrated in rabbit microsomes (Villeneuve et al., 1971a).
Lichtenstein (1972) reports a potentiation by PCBs of the toxicity of
parathion to flies.
Cecil et al. (1975) have shown that the ability of PCTs to
decrease phenobarbital sleeping time in quails is rather less than
that of the PCBs.
Aroclor 1254 at 160 mg/kg of diet fed to 5-week old male and
female Fischer-344 rats for 8 weeks reduced mortality due to feeding
hexachlorophene at a concentration of 600 mg/kg of diet from 77% to 7%
and completely prevented the paralysis that was observed in all
animals on the hexachlorophene diet alone. However, in the animals on
the combined treatment, histological changes in the brain
characteristic of hexachlorophene were still apparent and the
possibility of delayed toxicity beyond the 8 weeks of the experiment
could not be eliminated. The protective effect of Aroclor 1254 was
explained by its capacity to enhance detoxification by means of
hepatic microsomal enzyme induction (Jones et al., 1974).
7.5 Cytotoxic Effects
Peakall et al. (1972) found a significant increase in chromosome
abnormalities in the embryos of ring doves when the parents were fed
on a diet containing Aroclor 1254 at 10 mg/kg. Nilsson & Ramel (1974)
found no chromosome breakage in Drosophila melanogaster when either
Clophen A30 or A50 was added to the substrate. Green et al. (1975)
administered Aroclor 1242 orally to rats in single doses of 1250,
2500, or 5000 mg/kg or as a repeated dose of 500 mg/kg/day for 4 days.
Aroclor 1254 was also administered for 5 days at doses of 75, 150, or
300 mg/kg/day; they found no evidence of a mutagenic potential as
assessed by cytogenic analysis of bone marrow and spermatogonia.
7.6 Immunosuppressive Effects
Administration of PCBs leads to an atrophy of lymphoid tissue in
chickens (Flick et al., 1965; Vos & Koeman, 1970), in pheasants
(Dahlgren et al., 1972), and in rabbits (Vos & Beems, 1971). Vos & de
Roij (1972) and Vos & van Driel-Grootenhuis (1972) came to the
conclusion that these effects could be attributed to an
immunosuppressive effect of PCBs. They found that when guinea pigs fed
on diets containing Clophen A60 or Aroclor 1260 at 50 mg/kg were
stimulated with tetanus toxoid, a lower antitoxin titre and a lower
count of antitoxin-producing cells was obtained than in control guinea
pigs, resulting in a significant reduction of immunoglobulins. The
skin reaction after tuberculination in animals immunized with Freund's
complete adjuvant (as a parameter of cell-mediated immunity) was also
depressed at the 50 mg/kg of diet level. Vos & de Roij (1972)
suggested that the ability of PCBs to increase the susceptibility of
ducklings to duck hepatitis virus (Friend & Trainer, 1970) and of fish
to fungal disease (Hansen et al., 1971) could be attributed to this
immunosuppressive effect. Kimuru & Baba (1973) found an increased
incidence of pneumonia, and lung and intracranial abscesses in rats on
a diet containing Kanechlor 400 and suggested that this was due to a
lowered resistance to infection.
7.7 Effects on Reproduction
In a one-generation reproduction study, rats were fed with a diet
containing Aroclor 1242, 1254, or 1260 at levels of 1, 10, and
100 mg/kg. Decreased survival of pups with Aroclors 1242 and 1254 at
100 mg/kg was noted. No effect on reproduction was detected with
Aroclor 1260 (Keplinger et al., 1971). In a two-generation
reproduction study (Linder et al., 1974), rats were fed a diet
containing Aroclor 1254 at levels of 0, 1, 5, 20, and 100 mg/kg and
Aroclor 1260 at levels of 0, 5, 20, and 100 mg/kg. Rats exposed to
Aroclor 1254 at dietary levels of 20 mg/kg or more had fewer pups per
litter. Aroclor 1260 had no effect on reproduction, even at levels of
100 mg/kg. Kihlström et al. (1973) showed that a daily oral dose of
25 µg of Clophen A60 to female mice for 62 days significantly
increased the length of the estrus cycle and decreased the frequency
of implanted ova. In order to study the effect of PCBs on the
development of sexual functions in the early postnatal period they
also mated mice that had been suckled by mothers dosed with Clophen
A60 during the lactation period. A decrease in the frequency of
implanted ova was noted when both parents of the couple had been
suckled with milk containing PCBs.
In the rhesus monkey, Allen (1975) reports a lower fertility and a
diminished weight of the young at birth, after the administration of a
diet containing Aroclor 1248 at 2.5 mg/kg for several months.
Studies by Aulerich et al. (1971), and Ringer et al. (1972) showed
that Aroclor 1254 fed to mink at 5 mg/kg severely affected
reproduction.
There have been several reports that PCBs can adversely affect egg
production and hatchability in birds. Contamination of feed by PCBs
from a heat exchanger was shown to be the cause of reduced
hatchability of hen eggs at a large poultry hatchery in the USA
(Kolbye, 1972), and this has been confirmed in laboratory experiments.
Keplinger et al. (1971) found poor hatchability of eggs from hens
receiving diets containing Aroclor 1242 at 10 mg/kg or Aroclor 1254 at
100 mg/kg, but not with hens receiving Aroclor 1260 at 100 mg/kg. An
adverse effect on eggs has been noted with Aroclor 1248 at 10 mg/kg
(Panel on Hazardous Trace Substances, 1972). Peakall et al. (1972) fed
ring doves on a diet containing Aroclor 1254 at 10 mg/kg for 3 months
and found a marked reduction in egg hatchability 6 months later, due
to embryo mortality.
The viability of salmon eggs bears some relation to their PCB
content; Johansson et al. (1970) found a 12% mortality in eggs
containing PCBs at the rate of 9.2 mg/kg in extractable fat, and a
100% mortality with 34 mg/kg. Adverse effects on the reproduction of
aquatic invertebrates have occurred at water concentrations of PCBs in
the region of 5 µg/litre.
The potential teratogenic effect of the PCBs has been studied by
dosing pregnant females during the gestation period. No fetal
abnormalities were produced in the rat by daily doses of Aroclors
1242, 1254, or 1260 at 10 and 30 mg/kg (Keplinger et al., 1971), or
Aroclor 1254 at 100 mg/kg (Villeneuve et al., 1971b), or in the rabbit
dosed with Aroclor 1254 at 10 and 50 mg/kg (Villeneuve et al., 1971b).
Injection of PCBs into eggs has been reported to produce beak
abnormalities in chicks (McLaughlin et al., 1963). Cecil et al. (1974)
have claimed that the administration of Aroclors 1232 and 1254 to hens
at 20 mg/kg in the diet causes teratogenic effects and a reduction in
hatchability of fertile eggs.
7.8 Neoplasia and Adenofibrosis
The liver is the only organ where tumours have been reported
following the ingestion of PCBs. Ito et al. (1973) fed groups of 12
male dd mice with diets containing 500, 250, 100 and 0 mg/kg of
Kanechlor 500, 400, and 300, respectively. After 1 year, 7/12 mice
developed neoplastic nodules (hyperplastic nodules) and 5/12 developed
hepatocellular carcinomas, all in mice from the group fed with
500 mg/kg Kanechlor 500. Metastases were not observed. In a second
study on mice, combined exposure to Kanechlor 500 and either alpha- or
ß-hexachlorocyclohexane isomers enhanced the development of neoplastic
nodules (hyperplastic nodules) and hepato-cellular carcinomas. A
combination of Kanechlor 500 and gamma-hexachlorocyclohexane did not
produce tumours. Dosing with Kanechlor 500 alone at a dietary level of
250 mg/kg, and ß- or gamma-hexachlorocyclohexane at dietary levels of
250, 100 or 50 mg/kg did not produce tumours. However, alpha-hexa-
chlorocyclohexane produced 8/30 hepata-cellular carcinomas and 23/30
hyperplastic nodules.
Groups of 50 BALB/cj male inbred mice were fed dietary
concentrations of Aroclor 1254 of 0 or 300 mg/kg (49.8 mg/kg body
weight) for 6 or 11 months respectively. No liver tumours were noted
in a total of 58 surviving controls. A total of 10 hepatomas
(neoplastic or hyperplastic nodules) were noted in 9/22 surviving mice
fed Aroclor 1254 for 11 months and in 1/24 surviving mice fed Aroclor
1254 for 6 months. In addition adenofibrosis (cholangiofibrosis) was
observed in all 22 livers of mice fed Aroclor 1254 for 11 months, but
not in the other groups (Kimbrough & Linder 1974).
In another study (Kimbrough et al., 1975), 200 female Sherman
strain COBS random bred rats (descendants of the Osborne Mendel
strain) were given a diet containing Aroclor 1260 at 100 mg/kg
(11.6-4.3 mg/kg body weight) for approximately 21 months; 200 female
rats were kept as controls. The rats were sacrificed when 23 months
old. Hepatocellular carcinomas were found in 26/184 of the
experimental groups and in 1/173 of the control rats. None of the
controls, but 144/184 experimental rats had neoplastic nodules
(hyperplastic nodules). Areas of hepatocellular alteration were noted
in 28/173 controls and 182/184 experimental rats. No effect of the
Aroclor on the incidence of tumours in other organs and no metastases
from the liver tumours were observed. In this and two earlier studies,
adenofibrosis of the liver was observed in male and female rats fed
either Aroclor 1260 or Aroclor 1254 (Kimbrough et al., 1972; 1973);
1975). Adenofibrosis of the liver is a persistent progressive lesion
that consists of a marked proliferation of fibrous tissue and
epithelial glandular cells which are well differentiated in the mouse
but appear atypical in the rat.
In a preliminary study, liver tumours (multiple adenomatous
nodules) were induced by Kanechlor 400 in 6/10 female, but not in male
Donryu rats. The dietary exposure varied throughout the study and the
number of animals used was small (10 experimental and 5 control rats
for each sex) (Kimura & Baba, 1973). Makiura et al. (1974) reported
that PCBs (Kanechlor 500) inhibited the induction of liver tumours in
male Sprague-Dawley rats by the known carcinogens 3' methyl- p-
dimethylaminoazo-benzene, N-2-fluosenyl acetamide, and diethyl-
nitrosamine.
8. EFFECTS OF PCBs AND PCTs ON MAN -- EPIDEMIOLOGICAL AND CLINICAL
STUDIES
In June 1968, patients appeared at the Dermatology Clinic of
Kyushu University Hospital, Fukuoka, Japan suffering from chloracne. A
group at the University undertook intensive clinical, chemical, and
epidemiological investigations and found that the disease originated
from the consumption of a batch of rice oil supplied in February 1968;
the disease was called Yusho (rice oil disease) (Katsuki, 1969). This
batch of rice oil was found to be contaminated with Kanechlor 400, a
48% chlorinated biphenyl, at 2000-3000 mg/kg which entered the oil
through a leak in a heat exchanger (Tsukamoto et al., 1969). The
symptoms and signs of Yusho were described by Goto & Higuchi (1969)
and by Okumura & Katsuki (1969). The earliest signs were enlargement
and hypersecretion of the Meibomian glands of the eyes, swelling of
the eyelids, and pigmentation of the nails and mucous membranes,
occasionally associated with fatigue, nausea, and vomiting. This was
usually followed by hyperkeratosis and darkening of the skin with
follicular enlargement and acneform eruptions, frequently with a
secondary staphylococcal infection. These skin changes were most often
seen on the neck and upper chest, but in severe cases extended to the
whole body. Biopsy skin samples showed hyperkeratosis, dilation of the
follicles, and an accumulation of melanin in the basal cells of the
epidermis; melanin granules have also been observed in biopsy samples
of the conjunctiva. Oedema of the arms and legs was seen in some
patients. There were no definite signs of liver enlargement or liver
disorders (Okumura & Katsuki 1969), but slight rises in serum
transaminases and in alkaline phosphatase were detected, and a liver
sample from a Yusho patient showed an increase in the smooth
endoplasmic reticulum (Hirayama et al., 1969). The majority of the
patients were found to have respiratory symptoms, and suffered from a
chronic bronchitis-like disturbance that persisted for several years
(Shigematsu et al., 1971, 1974).
Yusho patients did not appear to suffer from central nervous
effects, but some complained of numbness of the arms and legs. Mural &
Kuroiwa (1971) found a decrease in the conduction velocity in
peripheral sensory nerves.
Yoshimura (1971) reported diminished growth in boys but not in
girls, who consumed the oil. Babies born to Yusho mothers were smaller
than normal. Newborn babies showed a dark brown skin pigmentation,
which disappeared after a few months (Yagamuchi et al., 1971; Taki et
al., 1969). Funatsu et al. (1972) found spotted and sporadic
ossification of the skull and facial oedema with exophthalmia in four
babies, but there was no evidence of any teratogenic action.
Determinations of PCB concentrations in the tissue of Yusho
patients were made several months after the ingestion of the oil,
apparently by an X-ray fluorescence method for organic chlorine (Goto
& Higuchi, 1969). Abdominal fat contained 13.1 mg/kg, subcutaneous fat
75.5 mg/kg, and nails 59 mg/kg. The mesenteric adipose tissue in six
Yusho patients, analysed by gas-liquid chromatography 1-3 years after
the occurrence of intoxication, contained PCB levels of 2.5 mg/kg on
average, which was considerably higher than the normal value. (Masuda
et al., 1974a). The mean blood level of PCBs in patients was 0.6 or
0.7 µg/100 ml (0.3 µg/100 ml for the general population) five years
after exposure (Masuda et al., 1974b; Takamatsu et al., 1974). These
authors also noted a specific gas-liquid chromatographic pattern,
peculiar to Yusho patients, which is still observed.
Hirayama et al. (1974) also reported that the serum bilirubin
level of patients was significantly lower than the normal level and
was negatively correlated with the blood level of PCBs and the serum
triglyceride level.
A considerable number of patients had elevated serum triglyceride
levels, up to four times the normal values, although this was not
correlated with the severity of the symptoms; these high values were
maintained for three years in many patients (Uzawa, 1972). There were
no marked abnormalities in serum cholesterol and phospholipid levels
(Okumura & Katsuki, 1969; Uzawa et al., 1969). Nagai et al. (1969)
reported an increase in urinary 17-ketosteroid excretion. Kusuda
(1971) also observed changes in the menstrual cycle in approximately
60% of 81 female Yusho patients as compared with their cycles prior to
exposure. Okumura et al. (1974) examined the relationship between the
blood levels of triglycerides and PCBs in 42 patients and observed a
positive correlation. Uzawa et al. (1972) showed that high values of
serum triglycerides were maintained for 3 years in many patients.
Shigematsu et al. (1971) examined serum immunoglobulin levels in 38
patients, 2 years after onset, and observed a decrease in IgA and IgM
and an increase in IgG. Saito et al. (1972) reported lower IgM levels
in patients showing chloracne.
Urabe (1974) reported that the total number of Yusho patients had
reached 1200 by 13 September 1973 and that 22 of them had died.
Mucocutaneous signs had decreased year by year, but neurological and
respiratory signs and symptoms and various complaints such as general
fatigue, anorexia, abdominal pain, and headache had become more
prominent among the patients. The smallest amount of oil that produced
symptoms when ingested over approximately 120 days, contained
approximately 0.5 g of PCBs, or approximately 0.07 mg/kg body weight
per day (Kuratsune, 1972a). Recently chlorinated dibenzofurans at
5 mg/kg were found in three samples of the toxic rice oil that
contained PCB levels of about 1000 mg/kg (Nagayama et al., 1975).
Symptoms similar to those of Yusho have been observed in workers
in a Japanese condenser factory, including pigmentation of the fingers
and nails, and acneiform eruptions on the jaw, back, and thighs. It
was thought that these effects arose from local contact with PCBs;
when the use of PCBs ceased, the symptoms disappeared (Hasegawa et
al., 1972b).
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO PCBs AND PCTs
9.1 Species Variation
The data in sections 7 and 8 indicate that man appears to be the
species most sensitive to PCBs, the consumption of relatively small
amounts having resulted in a severe disease (Yusho) in 1200 persons in
Japan. The monkey is the only experimental species in which effects
qualitatively and quantitatively approaching those in man have been
observed; Allen (1975) attributed this to metabolic differences
leading to a slower elimination than that observed in other species
tested.
Conclusions concerning the specific effects of PCBs on different
species are confused by uncertainty arising from the presence of toxic
impurities. The rice oil that caused the outbreak of Yusho was
contaminated with PCBs containing relatively high amounts of
tetrachlorodibenzofuran (see section 8), but the sample used in the
monkey experiments had a low content of these impurities, so it is not
clear whether PCBs alone were responsible for the Yusho incident.
Further uncertainty arises from reports from Finland of high PCB
concentrations in blood and body fat of occupationally exposed workers
with no indication of adverse effects, while at similar tissue
concentrations Japanese workers showed skin lesions typical of Yusho
(see section 5.5.3).
A species-specific toxic manifestation that can probably be
attributed to toxic impurities, is the abdominal oedema and
hydropericardium seen in birds affected by some commercial PCB
mixtures.
Mink is another species showing a high sensitivity to PCBs. Deaths
have been produced with diets containing PCB levels of 30 mg/kg; no
information is available on any species-specific metabolic pathway in
the mink that would account for this susceptibility.
9.2 Dose-Effect Relationships
The following is a summary of the data in Sections 7 and 8
concerning the relationship between mammalian toxicity and dose.
Approximate calculations of the daily dose in mg/kg body weight
derived from the dietary concentration are given in parentheses.a
a When no food consumption figures were available from the
experimental studies, the following factors were used to transform
mg/kg in the diet to mg/kg body weight: mouse (7), rat (20),
guinea-pig (25), mink (10), rabbit (33), monkey (25).
9.2.1 Body weight
Body weight was reduced in rats after 8 months of dietary intake
of Aroclor 1254 at 100 mg/kg (corresponding to 5 mg/kg body weight);
no effects were observed at 20 mg/kg in the diet (corresponding to
1 mg/kg body weight).
Dose-dependent retardation of weight gain was observed in mink
after 4 months of dietary intake of Aroclor 1254 at 5 and 10 mg/kg
(corresponding to 0.5 and 1.1 mg/kg body weight respectively).
9.2.2 Effects on liver
Liver weight
Dose-dependent increase in liver weight was observed in rats
receiving Aroclors 1242, 1254 and 1260 at concentrations of more than
20 mg/kg in the diet (corresponding to > 1.4 mg/kg body weight). Male
rats were more sensitive than female rats; no effects were observed
with Aroclors 1254 and 1260 at concentrations lower than 20 mg/kg in
the diet (corresponding to < 1.4 mg/kg body weight). Effects were
less marked with the lower chlorinated PCBs.
Liver changes
Smooth endoplasmic reticulum proliferation with fat droplet
inclusions were observed in the liver tissue of rats after 8 months of
dietary intake of Aroclor 1254 at 20 mg/kg (corresponding to 1.4 mg/kg
body weight).
Liver damage was observed with Aroclors 1242 and 1254 in rabbits
receiving 14 weekly oral doses of 150 mg/kg body weight; no effect was
observed with Aroclor 1221.
Liver enzyme activitya
Increase in microsomal enzyme activity was observed in male rats
after 8 months of dietary intake of Aroclor 1254 of 20 mg/kg
(corresponding to 1 mg/kg body weight). No effect was observed at
2 mg/kg in the diet (corresponding to 0.1 mg/kg body weight. Effects
were less marked in female rats.
Increased activity was also observed with Aroclors 1242 and 1016
in male rats receiving 21 daily oral doses of 1 mg/kg body weight.
a According to Litterst, et al. (1972) the dose producing an effect
on nitroreductase activity in the rat corresponds to 0.5 mg/kg in
the diet (corresponding to 0.3 mg/kg body weight).
Liver porphyria
Effects were observed in rats after several months of dietary
intake of Aroclor 1254 at 100 mg/kg (corresponding to 5 mg/kg body
weight); dose-dependent effects were observed in female rats after 21
daily oral doses of Aroclor 1242 at 10 and 100 mg/kg; no effects were
noted at 1 mg/kg body weight.
Liver vitamin A
Reduction of hepatic vitamin A was observed in rats receiving
Aroclor 1242 at the rate of 100 mg/kg in the diet (corresponding to
5 mg/kg body weight).
Liver tumours
Hepatocellular carcinomas were observed in mice after one year of
dietary intake of Kanechlor 500 at 500 mg/kg (corresponding to
75 mg/kg body weight); no carcinomas were observed with Kanechlor 500
at 250 mg/kg in the diet (corresponding to 37.5 mg/kg body weight), or
with Kanechlor 300 and 400 at 500 mg/kg in the diet (corresponding to
75 mg/kg body weight).
Hepatomas were observed in mice after 10 months of daily intake of
Aroclor 1254 at 300 mg/kg in the diet (corresponding to 49.8 mg/kg
body weight).
Hepatocellular carcinomas were observed in rats after 21 months of
daily intake of Aroclor 1260 at 100 mg/kg in the diet (corresponding
to 11.6-4.3 mg/kg body weight).
9.2.3 Reproduction
Effects on reproduction were observed in the mouse at a daily oral
dose of 0.025 mg Clophen A60; in the rat at a dietary level of Aroclor
1254 of 20 mg/kg (corresponding to 1 mg/kg body weight) with the
effects decreasing with higher chlorinated PCBs; in the mink at a
dietary level of Aroclor 1254 of 5 mg/kg (corresponding to 0.5 mg/kg
body weight); and in the monkey at a dietary level of Aroclor 1248 of
2.5 mg/kg (corresponding to 0.1 mg/kg body weight).
9.2.4 Immunosuppression
Immunosuppressive effects were observed in the guinea-pig at a
dietary level of Clophen A60 or Aroclor 1260 of 50 mg/kg
(corresponding to 2 mg/kg body weight).
9.2.5 Skin effects
In man, symptoms of Yusho disease were observed at a dietary level
of 4.2 mg/day of PCBs (corresponding to 0.07 mg/kg body weight/day for
a 60-kg person). A value of 0.50 g was estimated as the quantity of
PCBs consumed over approximately 120 days above which toxic symptoms
were evident. Similar effects were observed in the monkey at a dietary
level of Aroclor 1248 of 2.5 mg/kg (corresponding to 0.1 mg/kg body
weight) after several months.
9.3 Nondetected effect levels
The assessment of non detected effect levels for toxic effects is
complicated by the different activities of the component PCBs and by
the presence of impurities, in addition to the influence of inter- and
intraspecies variation, age, sex, and length of exposure. Moreover,
many of the available experimental studies do not include a
nondetected effect level.
The most sensitive species appears to be man, and effects have
been observed at intake rates of 0.07 mg/kg body weight/day. This may
have been influenced by the intake of impurities more toxic than PCBs,
but similar effects have been produced in the monkey, at the same
order of dosage, with a product containing little of these impurities.
At this dosage level, no effects may be expected on growth, liver
enlargement, and liver enzyme activity in less sensitive species such
as the rat. Although non-detected effect levels are not available for
effects on immunosuppression and reproduction, and for certain
biochemical effects on the liver, it seems unlikely that these effects
would be apparent at intake rates of 0.1 mg/kg body weight/day.
Carcinogenic effects have been observed in rats and mice at doses two
orders of magnitude greater than this, but there is no epidemiological
evidence to suggest that PCBs cause tumours in man. According to Grant
et al. (1974) rats fed on a diet containing Aroclor at the rate of
2 mg/kg (equivalent to about 0.1 mg/kg body weight) showed PCB levels
of 8 µg/100 ml in blood and 26.1 mg/kg in body fat. However, values
much higher than these have been observed in men occupationally
exposed to PCBs without evidence of any toxic effects (see section
5.5.3).
It is not possible at present to resolve this conflict in the
evidence on the toxicity of PCBs to man.
REFERENCES
ABBOT, D.C., COLLINS, G. B. & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom 1969-71.
Br. med. J., 2: 553-556.
ACKER, L. & SCHULTE, E. (1970) Uber das Vorkommen von chlorierten
Biphenylen und Hexachlorbenzol neben chlorierten Insektiziden in
Humanmilch und menschlichem Fettgewebe. Naturwissenschaften,
57: 497 (in German).
ACKER, L. & SCHULTE, E. (1974) Chlorkohlenwasserstoffe in menschlichem
Fettgewebe, Naturwissenschaften, 61: 1-4 (in German).
ADDISON, R. F., FLETCHER, G. L., RAY, S. & DOANE, J. (1972) Analysis
of a chlorinated terphenyl (Aroclor 5460) and its deposition in
tissues of the cod (Gadus morhua). Bull. environ. Contam.
Toxicol., 8: 52-60.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of polychlorinated biphenyl (PCB) and chlorinated
pesticides in water. Anal. Chem., 42: 1483-1486.
AHMED, M. & FOCHT, D. D. (1973) Degradation of polychlorinated
biphenyls by two species of Achromobacter. Can. J. Microbiol.,
19: 47-52.
AHNOFF, M. & JOSEFSSON, B. (1973) Confirmation studies on
polychlorinated biphenyls (PCB) from river waters using mass
fragmentography. Anal. Lett., 6: 1083-1093.
AHNOFF, M. & JOSEFSSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AHNOFF, M. & JOSEFSSON, B. (1975) Clean-up procedures for PCB analysis
on river water extracts. Bull. environ. Contam. Toxicol.,
13: 159-166.
ALBRO, P. W. & FISHBEIN, L. (1972) Intestinal absorption of
polychlorinated biphenyls in rats. Bull. environ. Contam.
Toxicol., 8: 26-31.
ALLEN, J. R. (1975) Response of the non-human primate to
polychlorinated biphenyl exposure. Fed. Proc., 34: 1675-1679.
ALLEN, J. R., ABRAHAMSON, L. J. & NORBACK, D. H. (1973) Biological
effects of polychlorinated biphenyls and triphenyls on the
subhuman primate. Environ. Res., 6: 344-354.
ALLEN, J. R. & NORBACK, D. A. (1973) Polychlorinated biphenyl- and
triphenyl-induced mucosal hyperplasia in primates. Science,
179: 498-499.
ALLEN, J. R., CARSTENS, L. A. & BARSOTTI, D. A. (1974) Residual
effects of short-term, low-level exposure of non-human primates to
polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
30: 440-451.
ALVARES, A. P. & KAPPAS, A. (1975) Induction of aryl hydrocarbon
hydroxylase by chlorinated biphenyls in the foeto-placental unit
and in neonatal livers during lactation. FEBS Lett., 50:172-174.
ARMOUR, J. A. & BURKE, J. A. (1970) Method for separating
polychlorinated biphenyls from DDT and its analogs. J. Assoc.
Off. Anal. Chem., 53: 761-768.
AULERICH, R. J., RINGER, R. K., SEAGRAN, H. L. & YOUATT, W. G. (1971)
Effects of feeding coho salmon and other Great Lakes fish on mink
reproduction. Can. J. Zool., 49: 611-616.
AULERICH, R. J., RINGER, R. K. & IWAMOTO, S. (1973) Reproductive
failure and mortality in mink fed on Great Lake fish. J. Reprod.
Fertil., 19 (Suppl.): 365-376.
BAGLEY, G. E., REICHEL, W. L. & CROMARTIE, E. (1970) Identification of
polychlorinated biphenyls in two bald eagles by combined
gas-liquid chromatography-mass spectroscopy. J. Assoc. Off. Anal.
Chem., 53: 251-261.
BAILEY, S. & BUNYAN, P. J. (1972) Interpretation of persistence and
effects of polychlorinated biphenyls in birds. Nature (Lond.),
236: 34-36.
BASTOMSKY, C. H., SOLYMOSS, a., ZSIGMOND, G. & WYSE, J. M. (1975) On
the mechanism of polychlorinated biphenyl-induced
hypobilirubinaemia. Clin. chim. Acta, 61: 171-174.
BAUER, H., SCHULZ, K. H. & SPIEGELBERG, U. (1961) Berufliche
Vergiftungen bei der Herstellung von Chlorphenol-Verbindungen.
Arch. Gewerbepathol. Gewerbehyg., 18: 538-555.
BENTHE, H. F., KNOP, J. & SCHMOLDT, A. (1972a) Absorption and
distribution of polychlorinated biphenyls (PCB) after inhalatory
application. Arch. Toxikol., 29: 85-95 (in German).
BENTHE, H. F., SCHMOLDT, A. & SCHMIDT, H. (1972b) Induction of
microsomal liver enzymes after polychlorinated biphenyls (PCB) and
following stress. Arch. Toxikol., 29: 97-106 (in German).
BERG, O. W., DIOSADY, P. L. & REES, G. A. V. (1972) Column
chromatographic separation of polychlorinated biphenyls from
chlorinated hydrocarbon pesticides and their subsequent gas
chromatographic quantitation in terms of derivatives. Bull.
environ. Contam. Toxicol., 7: 338-347.
BERGLUND, F. (1972) Levels of polychlorinated biphenyls in foods in
Sweden. Environ. Perspect, 1: 67-69.
BERLIN, M., GAGE, J. C. & HOLM, S. (1973) The metabolism and
distribution of 2,4,5,2',5'-pentachlorobiphenyl in the mouse.
In: PCB Conference II. National Swedish Environment Protection
Board Publications: 4E, pp. 101-107.
BERLIN, M., GAGE, J. C. & HOLM, S. (1974) Distribution and metabolism
of polychlorobiphenyls. In: Proceedings of the International
Symposium on Recent Advances in Environmental Pollution, Paris,
24-28 June. pp. 8.
BERLIN, M., GAGE, J. & HOLM, S. (1975) The distribution and metabolism
of 2,4,5,2',5'-pentachlorobiphenyl. Arch. environ. Health,
30: 141-147.
BICKERS, D. R., HARBER, L. C., KAPPAS, A. & ALVARES, A. P. (1972)
Polychlorinated biphenyls: comparative effects of high and low
chlorine containing Aroclors on hepatic mixed function oxidase.
Res, Comm. Chem. Pathol. Pharmacol., 3: 505-511.
BIDLEMAN, T. F. & OLNEY, C. E. (1974) High-volume collection of
atmospheric polychlorinated biphenyls. Bull. environ. Contam.
Toxicol., 11: 442-450.
BITMAN, J., CECIL, H. C. & HARRIS, S. J. (1972) Biological effects of
polychlorinated biphenyl in rats and quail. Environ. Health
Perspec., 1: 145-149.
BJERK, J. E. (1972) Rester av DDT og polyklorerte bifenyler i Norsk
humant materiale. Tidsskr. Nor-Laegeforen., 92: 15-19 (cited by
Kimbrough, 1974).
BOURNE, W. P. P. & BOGEN, A. A. (1972) Polychlorinated biphenyls in
North Atlantic seabirds, Mar. Pollut. Bull., 3 (11): 172-175.
BOWES, G. W., MULVIHILL, M. J., SIMONEIT, B. R. T., BURLINGAME, A. L.
& RISEBROUGH, R. W. (1975) Identification of chlorinated
dibenzofurans in American polychlorinated biphenyls. Nature
(Lond.), 256, 305-307.
BROADHURST, M. G. (1972) Use and replaceability of polychlorinated
biphenyls. Environ. Health Perspect., 2: 81-102.
BRUCKNER, J. V., KHANNA, K. L. & CORNISH, H. H. (1973) Biological
responses of the rat to polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 24: 434-448.
BRUGGEMAN, VON J., BUSCH, L., DRESCHER-KADAN, U., EISELI, W. & HOPPE,
P. (1974) Pesticid-und PCB-Ruckstunde in Organen von Wildtieren
als Indikatoren fur Umweltkontamination. Z. Jagdwiss.,
20: 70-74.
BURSE, V. W., KIMBROUGH, R. D., VILLANUEVAS, E. C, JENNINGSI R. W.,
LINDER, R. E. & SOVOCOOL, G. W. (1974) Polychlorinated biphenyls.
Storage, distribution, excretion and recovery: liver morphology
after prolonged dietary ingestion. Arch. environ. Health,
29: 301-307.
CARNES, R. A., DOERGER, J. U. & SPARKS, H. L. (1973) Polychlorinated
biphenyls in solid waste and solid-waste-related materials. Arch.
environ. Contam. Toxicol., 1: 27-35.
CECIL, H. C., HARRIS, S. J., BITMAN, J. & FRIES, G. F. (1973)
Polychlorinated biphenyl-induced decrease in liver vitamin A in
Japanese quail and rats. Bull. environ. Contam. Toxicol.,
9: 179-185.
CECIL, H. C., BITMAN, J., LILLIE, R. J., FRIES, G. F. & VERRETT, J.
(1974) Embryotoxic and teratogenic effects in unhatched fertile
eggs from hens fed polychlorinated biphenyls (PCBs). Bull.
environ. Contam. Toxicol., 11: 489-495.
CECIL, H. C., HARRIS, S. J. & BITMAN, J. (1975) Effects of
polychlorinated biphenyls and terphenyls and polybrominated
biphenyls on pentobarbital sleeping times of Japanese quail.
Arch. environ. Contam. Toxicol., 3, 183-192.
CHOI, P.S. K., NACK, H. & FLINN, J. E. (1974) Distribution of
polychlorinated biphenyls in an aerated biological oxidation
wastewater treatment system. Bull. environ. Contam. Toxicol.,
11: 12-17.
CLAUS, B. & ACKER, L. (1975) Zur Kontamination von Milch und
Milcherzeugnissen mit chlorierten Kohlenwasserstoffen im
Westphälischen Raum II Ergebnisse und Diskussion. Zeitschrift fur
Lebensmitteluntersuchung und forschung, 159 (3): 129-137.
COLLINS, G. B., HOLMES, D. C. & JACKSON, F. J. (1972) The estimation
of polychlorobiphenyls. J. Chromatogr., 71: 443-449.
COMMISSION OF THE EUROPEAN COMMUNITIES (1974) European Colloquium.
Problems raised by the contamination of man and his environment
by persistent pesticides and organohalogenated compounds.
Luxembourg, 14-16 May 1964.
COOLEY, N. R., KELTNER, J. M. & FORESTER, J. (1972) Mirex and Aroclor
1254: effect on accumulation by Tetrahymena pyriformis strain
W. J. Protozool., 19: 636-638.
CURLEY, A., BURSE, V. W., GRIM, M. E., JENNINGS, R. W. & LINDER, R. E.
(1971) Polychlorinated biphenyls: distribution and storage in body
fluids and tissues of Sherman rats. Environ. Res., 4: 481-495.
CURLEY, A., BURSE, V. W. & GRIM, M. E. (1973a) Polychlorinated
biphenyls: evidence of transplacental passage in the Sherman rat.
Fd. cosmet. Toxicol., 11: 471-476.
CURLEY, A., BURSE, V. W., JENNINGS, R. W., VILLANUEVA, E. C., TOMATIS,
L. & AKAZAKI, K. (1973b) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond), 242: 338-340.
CURLEY, A., BURSE, W. V., JENNINGS, R. W., VILLANUEVA, E. C. &
KIMBROUGH, R. D. (1975) Evidence of tetrachlorodibenzofuran (TCDF)
in Aroclor 1254 and the urine of rats following dietary exposure
to Aroclor 1254. Bull. environ. Contam. Toxicol., 14: 153-158.
DAHLGREN, R. B., GREICHUS, Y. A. & LINDER, R. L. (1971) Storage and
excretion of polychlorinated biphenyls in the pheasant.
J. Wildlife Manag., 35: 823-828.
DAHLGREN, R. B., LINDER, R. L. & CARLSON, C. W. (1972) Polychlorinated
biphenyls: their effects on penned pheasants. Environ. Health
Perspect., 1: 89-101.
DE FREITAS, A. S. & NORSTROM, R. J. (1974) Turnover and metabolism of
polychlorinated biphenyls in relation to their chemical structure
and the movement of lipids in the pigeon. Can. J. Physiol.
Pharmacol., 52: 1080-1094.
DEPARTMENT OF HEALTH EDUCATION AND WELFARE (1972) Environ. Health
Perspect. Experimental Issue 1, April 1972. DHEW, Bethesda, MD,
USA. Publ. No. (NIH) 72-218.
DE VOS, R. H. & PEET, E. W. (1971) Thin-layer chromatography of
polychlorinated biphenyls. Bull environ. Contam. Toxicol.,
6: 164-170.
DOGUCHI, M. & FUKANO, S. (1975) Residue levels of polychlorinated
terphenyls, polychlorinated biphenyls and DDT in human blood.
Bull. environ. Contam. Toxicol., 13: 57-63.
DOGUCHI, M., FUKANO, S. & USHIO, F. (1974) Polychlorinated terphenyls
in the human fat. Bull. environ. Contam. Toxicol.,
11 (2): 157-158.
DUKE, T. W., LOWE, J. I. & WILSON, A. J. JR (1970) A polychlorinated
biphenyl (Aroclor 1254) in the water, sediment and biota of
Escambia Bay, Florida. Bull. environ. Contam. Toxicol.,
5: 171-180.
ECOBICHON, D. J. & COMEAU, A.M. (1974) Comparative effects of
commercial Aroclors on rat liver enzyme activities. Chem.-Biol.
Interactions, 9: 341-350.
EKSTEDT, J. & ODEN, S. (1974) Chlorinated hydrocarbons in the lower
atmosphere in Sweden. Report from Department of Soil Science,
Royal Agricultural College, S-75007, Uppsala 7. pp. 1-16.
ENVIRONMENTAL SANITATION BUREAU, MINISTRY OF HEALTH & WELFARE (1973)
Survey on foods contamination. Report of comprehensive
investigation on the prevention of pollution by PCBs, 191-210
(in Japanese).
FINKLEA, J., PRIESTER, L. E., CREASON, J.P., HAUSER, T., HINNERS, T. &
HAMMER, D. I. (1972) Polychlorinated biphenyl residues in human
plasma expose a major urban pollution problem. Am. J. pub.
Health, 62: 645-651.
FISHER, N. S., GRAHAM, L. B., CARPENTER, E. J. & WURSTER, C. F. (1972)
Geographic differences in phytoplankton sensitivity to PCBs.
Nature (Lond.), 241: 548-549.
FLICK, D. F., O'DELL, R. G. & CHILDS, V. A. (1965) Studies of the
chick edema disease. Similarity of symptoms produced by feeding
chlorinated biphenyl. Poult. Sci., 44: 1460-1465.
FREUDENTHAL, J. & GREVE, P. A. (1973) Polychlorinated terphenyls in
the environment. Bull. environ. Contam. Toxicol., 10: 108-111.
FRIEND, M. & TRAINER, D. O. (1970) Polychlorinated biphenyl:
interaction with duck hepatitis virus. Science, 170: 1314-1316.
FRIES, G. F., MARROW, G. S. JR & GORDON, C. H. (1973) Long-term
studies of residue retention and excretion by cows fed a
polychlorinated biphenyl (Aroclor 1254). J. agr. Food Chem.,
21: 117-121.
FUJITA, S., TZUJI, H., KATO, K., SAEICI, S. & TSUKAMOTO, H. (1971)
Effect of biphenyl chlorides on rat liver microsomes. Fukuoka
med. Acta., 62: 30-34.
FUKADA, K., INUYAMA, Y., TAKESHITA, T. & YAMAMOTO, S. (1973) Present
state of environmental pollution by PCB in Shimane Prefecture.
Shimare Igaku, 5: 1-25 (in Japanese).
FUKADA, S., USHIO, F. & DOGUCHI, M. (1974) PCB, PCT and pesticides
residues in fish collected from the Tama River. Ann. Rep. Tokyo
Metr. Res. Lab. P.H., 25: 297-305 (in Japanese).
FUNATSU, I., YAMASHITA, F., ITO, Y., TZUGAWA, S., FUNATSU, T.,
YOSHIKANE, T., HAYASHI, M., KATO, T., YAKUSHIJI, M., OKAMOTO, G.,
YAMASAKI, S., ARIMA, T., KUNO, T., IDE, H. & IBE, I. (1972) PCB
induced fetopathy. I. Clinical observation. Kurume med. J.,
19: 43-51 (in English).
GARDNER, A.M., CHEN, J. F., ROACH, J. A. G. & RAEGLIS, E. P. (1973)
Polychlorinated biphenyls. Hydroxylated urinary metabolites of
2,5,2',5'-tetrachlorobiphenyl identified in rabbits. Biochem.
biophys. res. Commun., 55: 1377-1384.
GILBERTSON, M. & REYNOLDS, L. (1974) DDE and PCB in Canadian wild
birds. In: Occasional paper, Canadian Wildlife Service,
Environment, Canada, Ottawa, pp. 1-16.
GOLDSTEIN, J. A., HICKMAN, P. & JUE, D. L. (1974) Experimental hepatic
porphyria induced by polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 27: 437-448.
GOLDSTEIN, J. A., MCKINNEY, J. D., LUCIEN, G. W., MOORE, J. A.,
HICKMAN, P. & BERGMAN, H. (1975a) Effects of hexachlorobiphenyl
isomers and 2,3,7,8-tetrachlorodibenzofuran (TCDF) on hepatic drug
metabolism and porphyria accumulation. Pharmacologist,
16: 239 (Abstract No. 278).
GOLDSTEIN, J. A., HICKMAN, P., BURSE, V. W. & BERGMAN, H. (1975b) A
comparative study of two polychlorinated biphenyl mixture (Aroclor
1242 and 1016) containing 42% chlorine on induction of hepatic
porphyria and drug metabolizing enzymes. Toxicol. appl.
Pharmacol., 32: 461-473.
GOTO, M. & HIGUCHI, K. (1969) The symptomatology of Yusho
(chlorobiphenyls poisoning) in dermatology. Fuoka Act. Med.,
60: 409-431.
GRANT, D. L., MOODIE, C. A. & PHILLIPS, W. E. J. (1974) Toxicodynamics
of Aroclor 1254 in the male rat. Environ. physiol. Biochem.,
4: 214-225.
GRANT, D. L. & PHILLIPS, W. E. J. (1974) The effect of age and sex on
the toxicity of Aroclor 1254, a polychlorinated biphenyl, in the
rat. Bull. environ. Contam. Toxicol., 12: 145-152.
GRANT, D. L., PHILLIPS, W. E. J. & VILLENEUVE, D.C. (1971a) Metabolism
of a polychlorinated biphenyl (Aroclor 1254) mixture in the rat.
Bull. environ. Contam. Toxicol., 6: 102-112.
GRANT, D. L., VILLENEUVE, D.C., MCCULLY, K. A. & PHILLIPS, W. E. J.
(1971b) Placental transfer of polychlorinated biphenyls in the
rabbit. Environ. Physiol., 1: 61-66.
GREEN, S. G., CARR, J. V., PALMER, K. A. & OSWALD, E. J. (1975) Lack
of cytogenetic effects in bone marrow and spermatagonial cells in
rats treated with polychlorinated biphenyls (Aroclor 1242 and
1254). Bull. environ. Contam. Toxicol., 13: 14-22.
HAMMER, D. I., FINKLEA, J. F., PRIESTER, L. E., KEIL, J. E., SANDIFER,
S. H. & BRIDBORN, K. (1972) Polychlorinated biphenyl residues in
the plasma and hair of refuse workers. Environ. Health
Perspect., 1 (15): 83.
HAMMOND, A. L. (1972) Chemical pollution: polychlorinated biphenyls.
Science, 175: 155-156.
HANSEN, D. J., PARRISH, P. R., LOWE, J. I., WILSON, A. J. JR & WILSON,
P. D. (1971) Chronic toxicity, uptake and retention of Aroclor
1254 in two estuarine fishes. Bull. environ. Contam. Toxicol.,
6: 113-119.
HAQUE, R., SCHMEDDING, D. W. & FREED, V. H. (1974) Aqueous solubility,
absorption and vapour behaviour of polychlorinated biphenyl
Aroclor 1254. Environ. Sci. Technol., 8: 139-142.
HARA, I., HARADA, H., KIMURA, S., ENDO, T. & KAWANO, K. (1974) Follow
up health examination in an electric condenser factory after
cessation of PCBs usage (1st report) Jpn. J. Ind. Health,
16: 365-366 (in Japanese).
HARVEY, G. R. & STEINHAUER, W. G. (1974) Atmospheric transport of
polychlorobiphenyls to the North Atlantic. Atmos. Environ.,
8: 777-782.
HARVEY, G. R., STEINHAUER, W. G. & TEAL, J. M. (1973) Polychloro-
biphenyls in North Atlantic Ocean water. Science, 80: 643-644.
HASEGAWA, H., SATO, M. & TSURUTA, H. (1972a) PCBs concentration in the
blood of workers handling PCB. Occup. Health, 10: 50-55
(in Japanese).
HASEGAWA, H., SATO, M. & TSURUTA, H. (1972b) PCB concentration in air
of PCB-using plants and health examination of workers (in
Japanese). In: Report on Special Research on Prevention of
Environmental Pollution by PCB-like Substances. Tokyo, Research
Co-ordination Bureau, Science and Technology Agency, pp. 141-149.
HEATH, R. G., SPANS, J. W., KREITZER, J. F. & VANCE, C. (1970)
Effects of polychlorinated biphenyls on birds In: Proceedings
of the 15th Congress of International. Ornithology, The Hague,
pp. 475-485.
HESSELBERG, R. J. & SCHERR, D. D. (1974) PCBs and p,p' DDE in the
blood of cachectic patients. Bull. environ. Contam. Toxicol.,
11: 202-205.
HIRAYAMA, C., IRISA, T. & YAMAMOTO, T. (1969) Fine structural changes
of the liver in a patient with chlorobiphenyls intoxication.
Fukuoka Acta Med., 60: 455-461 (in Japanese).
HIRAYAMA, C., OKUMURA, M., NAGAI, J. & MASUDA, Y. (1974)
Hypobilirubinemia in patients with polychlorinated biphenyls
poisoning. Clin. chim. Acta., 55: 97-100.
HOLDGATE, M. W. (1971) The sea bird wreck in the Irish Sea, Autumn
1969. Natural Environmental Research Council (Publication
Series C4).
HOLDEN, A. V. (1970a) International co-operative study of
organochlorine pesticide residues in terrestial and aquatic
wildlife 1967/1968. Pest. monit. J., 4: 117-135.
HOLDEN, A. V. (1970b) Source of polychlorinated biphenyl contamination
in the marine environment. Nature (Lond.), 228: 1220-1221.
HOLDEN, A. V. (1973a) International co-operative study of
organochlorine and mercury residues in wildlife, 1969-71. Pest.
monit. J., 7: 37-52.
HOLDEN, A. V. (1973b) Monitoring PCBs in water and wildlife. In:
PCB Conference II, Stockholm, Swedish Environment Protection
Board, Publication: 4E, pp. 23-33.
HOLDEN, A. V. & MARSDEN, K. (1969) Single-stage clean-up of animal
tissue extracts for organochlorine residue analysis.
J. Chromatogr., 44: 481-492.
HUTZINGER, O., JAMIESON, W. D., SAFE, S., PAULMANN, L. & AM , R.
(1974) Identification of metabolic dechlorination of highly
chlorinated bipheny bit. Nature (Lond.), 252: 698-699.
HUTZINGER, O., SASH, D. M., SAFE, S., DE FREITAS, A. S. W., NORSIROM,
R. J., WILDISH, D. J. & ZITKO, V. (1972a) Polychlorinated
biphenyls: metabolic behaviour of pure isomers in pigeons, rats
and brook trout. Science, 178: 312-313.
HUTZINGER, O., SAFE, S. & ZITKO, V. (1971) Polychlorinated biphenyls:
Synthesis of some individual chlorobiphenyls. Bull. environ.
Contam. Toxicol., 6: 209-219.
HUTZINGER, O., SAFE, S. & ZITKO, V. (1972b) Photochemical degradation
of chlorobiphenyls (PCBs). Environ. Health Perspect., 1: 15-20.
INNAMI, S. et al. (1974) PCB toxicity and nutrition II, PCB toxicity
and Vitamin A (2). J. Nutr. Sci-Vitaminol., 20: 363-370.
INTERNATIONAL AGENCY FOR RESEARCH ON CANCER (1974) Polychlorinated
biphenyls in IARC monographs on the evaluation of carcinogenic
risk of chemicals to man, Vol. 7, 261-289.
INTERNATIONAL COUNCIL FOR THE EXPLORATION OF THE SEA (1974) Report of
a Working Group for the international study of the pollution of
the North Sea and its effects on living resources and their
exploitation. Charlottenlund, Denmark (Co-operative Research
Report No. 39).
ISHI, H. (1972) PCB Pollution in Japan. Environ. Health Rep. No. 14,
Jpn. Publ. Health Assoc., pp. 13-28.
ITO, N., NAGASAKI, H, ARAI, M., MAKIURA, S., SUGIHARA, S. & HIRAO, K.
(1973) Histopathologic studies on liver tumorigenesis induced in
mice by technical polychlorinated biphenyls and its promoting
effect on liver tumours induced by benzene hexachloride. J. nat.
Cancer Inst., 51: 1637-1646.
IVERSON, F., VILLENEUVE, D.C., GRANT, D. L. & HATINA, G. V. (1975)
Effect of Aroclor 1016 and 1242 on selected enzyme systems in the
rat. Bull. environ. Contam. Toxicol., 13: 456-463.
IWATA, Y., WESTLAKE, W. E. & GUNTHER, F. A. (1973) Varying persistence
of polychlorinated biphenyls in six California soils under
laboratory conditions. Bull. environ. Contam. Toxicol.,
9: 204-211.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTROM, G. & VAZ,
R. (1975) Identification by GC-MS of phenolic metabolites of PCB
and p,p'-DDE isolated from Baltic guillemot and seal. Ambio.,
4: 93-97.
JENSEN, S. (1973) (no title) Report to Swedish National Environment
Protection Board, Stockholm, 12 October 1973.
JENSEN, S. (1974) Identification of some organic substances
potentially harmful to the environment. National Swedish
Environment Protection Board, University of Stockholm, Kollenberg
Laboratory (Publication SNV-PM 520).
JENSEN, S. & SUNDSTROM, G. (1974) Structures and levels of most
chlorobiphenyls in two technical PCB products and in human adipose
tissue. Ambio, 3: 70-76.
JENSEN, S. & SUNDSTROM, G. (1975) Metabolic hydroxylation of a
chlorobiphenyl containing only isolated unsubstituted positions --
2,2',4,4',5,5'-hexachlorobiphenyl. Nature (Lond.)
(in press).
JENSEN, S., JOHNELS, A. G., OLSSEN, M., OTTERLIND, G. (1969) DDT and
PCB in marine animals from Swedish waters. Nature (Lond.),
224: 247-250.
JENSEN, S., JOHNELS, A. G., OLSSON, M. & OTTERLIND, G. (1972b) DDT and
PCB in herring and cod from the Baltic, the Kattegat and the
Skaggerrak. Ambio spec. Rep., No. 1: 71-85.
JENSEN, S., JOHNELS, A. G., OLSSEN, M. & WESTERMARK, T. (1972c) The
avifaune of Sweden as indicators of environmental contamination
with mercury and chlorinated hydrocarbons. In: Proceedings of
the 15th International Ornithology Congress, Leiden,
pp. 455-465.
JENSEN, S., RENBERG, L. & OLSSON, M. (1972a) PCB contamination from
boat bottom paint and levels of PCB in plankton outside a polluted
area. Nature (London), 240: 358-360.
JENSEN, S., RENBERG, L. & VAZ, R. (1973) Problems in quantification
of PCB in biological material. In: PCB Conference II. National
Swedish Environment Protection Board. Publications: 4E, pp. 7-13.
JOHANSSON, N., JENSEN, S. & OLSSON, M. (1970) PCB indications of
effects on fish. In: PCB Conference, National Swedish
Environment Protection Board, pp. 59-67.
JOHNSTONE, G. J., ECOBICHON, D. J. & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, D.C. L., DAVIS, W. E., JR, NEWELL, G. W., SASMORE, D. P. &
ROSEN, V. J. (1974) Modification of hexachlorophene toxicity by
dieldrin and Aroclor 1254. Toxicology, 2: 309-318.
KAISER, K. L. E. & WONG, P. T. S. (1974) Bacterial degradation of
polychlorinated biphenyls. I. Identification of some metabolic
products from Aroclor 1242. Bull. environ. Contam. Toxicol.,
11: 291-296.
KARPPANEN, E. & KOLHO, L. (1973) The concentration of PCB in human
blood and adipose tissue in three different research groups. In:
PCB Conference II. National Swedish Environment Protection Board
Publications: 4E: pp. 124-127.
KATSUKI, S. (1969) Foreword. Fukuoka Acta Med., 60: 403-407.
KAWANISHI, S., SANO, S., MIZUTANI, T. & MATSUMOTO, M. (1973)
Experimental porphyria induced by polychlorinated biphenyls (in
Japanese). Jpn. J. Hyg., 28: 84.
KAWANISI, S., SANO, S., MIZUTANI, T. & MATSUMOTO, M. (1974)
Experimental studies on toxicity of synthetic tetrachlorobiphenyl
isomers (in Japanese). Jpn. J. Hyg., 29: 81.
KEIL, J. E., PRIESTER, L. E. & SANDIFER, S. H. (1971) Polychlorinated
biphenyl (Aroclor 1242): effects of uptake on growth, nucleic
acids and chlorophyll of a marine diatom. Bull. environ. Contam.
Toxicol., 6: 156-159.
KEPLINGER, M. L., FANeHER, O. E. & CALANDRA, J. C. (1971) Toxicologic
studies with polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 19: 402-403.
KIHLSTROM, J. E., ORBERG, J., LUNDBERG, C., DANIELSSON, P.O. &
SYDHOFF, J. (1973) Effects of PCB on mammalian reproduction. In:
PCB Conference II. National Swedish Environment Protection Board
Publications 4E, pp. 109-111.
KIMBROUGH, R. D. (1974) The toxicity of polychlorinated polycyclic
compounds and related chemicals. Crit. Rev. Toxicol.,
2: 445-498.
KIMBROUGH, R. D. & LINDER, R. E. (1974) Induction of adenofibrosis and
hepatomas of the liver in BALB/cJ mice by polychlorinated
biphenyls (Aroclor 1254). J. nat. Cancer Inst., 53: 547-549.
KIMBROUGH, R. D., LINDER, R. E. & GAINES, T. B. (1972) Morphological
changes in livers of rats fed polychlorinated biphenyls. Arch.
environ. Health, 25: 354-364.
KIMBROUGH, R. D., LINDER, R. E., BURSE, V. W., JENNINGS, R. W. & GA,
C. (1973) Adenofibrosis in the rat liver with persistence of
polychlorinated biphenyls in adipose tissue. Arch. environ.
Health, 27: 389-395.
KIMBROUGH, R. D., SQUIRE, R. A., LINDER, R. E., STRANDBERG, J. D.,
MONTALI, R. J. & BURSE, V. W. (1975) Induction of liver tumours in
Sherman strain female rats by polychlorinated biphenyl Aroclor
1260. J. nat. Cancer Inst., 55: 1453-1459.
KIMURA, N. T. & BABA, T. (1973) Neoplastic changes in the rat liver
induced by polychlorinated biphenyl. GANN, 64: 105-108.
KITAMURA, M., TSUKAMOTO, T., SUMINO, K., HAYAKAWA, K., SHIBITA, T. &
HIRANO, I. (1973) The PCB levels in the blood of workers employed
in a condenser factory. Jpn. J. indust. Health, 47: 354-355
(in Japanese).
KOBAYASHI, Y. (1972) Answer Report to the Questionary paper on
"Regulation of Residual level in Foods". Biol. Pollut.,
4: 93-116 (in Japanese).
KOEMAN, J. H., TEN NOEVER DE BRAUW & DE VOS, R. H. (1969) Chlorinated
biphenyls in fish, mussels and birds from the river Rhine and the
Netherlands coastal area. Nature (Lond), 221: 1126-1128.
KOEMAN, J. H., BOTHOF, TH., DE VRIES, R., VAN VELZEN-BLAD, H. & VOS,
J. G. (1972a) The impact of persistent pollutants on piscivorous
and molluscivorous birds. TNO-nieuws, 27: 561-569.
KOEMAN, J. H., PEETERS, W. H. M., SMIT, C. J., TJIOE, P, S. & DE
GOEIJ, J. J. M. (1972b) Persistent chemicals in marine mammals.
TNO-nieuws, 27: 570-578.
KOEMAN, J. H., VAN VELZEN-BLAD, H. C. W., DE VRIES, R. & Vos, J. G.
(1973) Effects of PCB and DDE in cormorants and evaluation of PCB
residues from an experimental study. J. Reprod. Fertil.,
19 (Suppl.): 353-364.
KOHANAWA, M., SHOYA, S., OGURA, Y., MORIWAKI, M. & KAWASAKI, M.
(1969a) Poisoning due to an oily by-product of rice-bran similar
to chick edema disease. I. Occurrence and toxicity test. Nat.
Inst. anim. Health Quart., 9: 213-219.
KOHANAWA, M., SHOYA, S., YONEMURA, T., NISHIMURA, K. & TSUSHIO, Y.
(1969b) Poisoning due to an oily by-product of rice-bran similar
to chick edema disease. II. Tetrachlorodiphenyl as toxic
substance. Nat. Inst. anim. Health Quart., 9: 220-228.
KOLBYE, A. C. JR (1972) Food exposures to polychlorinated biphenyls.
Environ. Health Perspect., 1: 85-88.
KOLLER, L. D. & ZINKL, J. G. (1973) Pathology of polychlorinated
biphenyls in rabbits. Am. J. Pathol., 70: 363-373.
KOMATSU, F. & KIKUCHI, M. (1972) Skin lesions by 3,4,Y,4'-tetra-
chlorobiphenyl in rabbits. Fukuoka Acta Med., 63: 384-386
(in Japanese).
KURATSUNE, M. (1972a) PCB pollution. Kosei no Shihyo, 19: 11-18
(in Japanese). KURATSUNE, M. & MASUDA, Y. (1972b) Polychlorinated
biphenyls in non-carbon copypaper. Environ. Health Perspect.,
1: 61-62.
KURATSUNE, M., YOSHIMURA, T. & MATSUZAKA, J. (1972) Epidemiologic
study on Yusho, a poisoning caused by ingestion of rice oil
contaminated with a commercial brand of polychlorinated biphenyls.
Environ. Health Perspect., 1: 119-128.
KUSUDA, M. (1971) Study on the female sexual function suffering from
the chlorobiphenyls poisoning. Sanka to Fujinka, 4: 1063-1072
(in Japanese).
LINCER, J. L. & PEAKALL, D. B. (1970) Metabolic effects of
polychlorinated biphenyls in the American kestrel. Nature
(Lond.), 228: 783-784.
LICHTENSTEIN, E. P. (1972) PCBs and interactions with insecticides.
Environ. Health Perspect., 1: 151-153.
LIEB, A. J., BILLS, D. B. & SINNHUBER, R. O. (1974) Accumulation of
dietary polychlorinated biphenyls (Aroclor 1254) by rainbow trout
(Salmo gairdneri). J. agr. Food Chem., 22: 638-642.
LINDER, R. E., GAINES, T. B. & KIMBROUGH, R. D. (1974) The effect of
polychlorinated biphenyls on rat reproduction. Food cosmet.
Toxicol., 12: 63-77.
LITTERST, C. L., FARBER, T. M., BAKER, A.M. & VAN LOON, E. J. (1972)
Effect of polychlorinated biphenyls on hepatic microsomal enzymes
in the rat. Toxicol. appl. Pharmacol., 23: 112-122.
MAKIURA, S., AOE, H., SUGIHARA, S., HIRAO, K., ARAI, M. & ITO, N.
(1974) Inhibitory effect of polychlorinated biphenyls on liver
tumorigenesis in rats treated with 3'-methyl-4-dimethyl-
aminoazobenzene, N-2-fluorenylacetamide, and diethylnitrosamine.
J. nat. Cancer Inst., 53: 1253-1257.
MASUDA, Y., KAGAWA, R. & KURATSUNE, M. (1972) Polychlorinated
biphenyls in carbonless copying paper. Nature (Lond.),
237: 41-42.
MASUDA, Y., KAGAWA, R. & KURATSUNE, M. (1974a) Polychlorinated
biphenyls in Yusho patients and ordinary persons. Fukuoka Acta
Med., 65: 17-24 (in Japanese).
MASUDA, Y., KAGAWA, R., SHIMAMURA, K., TAKADA, M. & KURATSUNE, M.
(1974b) Polychlorinated biphenyls in the blood of Yusho patients
and ordinary persons. Fukuoka Acta Med., 65: 25-27
(in Japanese).
MCKINNEY, J. D. (in press) Toxicology of selected symmetrical
hexachlorobiphenyl isomers : correlating biological effects with
chemical structure. In: Proceedings of the National Conference
on Polychlorinated Biphenyls, Chicago, 19-21 November, 1975.
MCLAUGHLIN, J. JR., MARLIAC, G. P., MERRETT, M. J., MUTCHLER, M. K. &
FITZHUGH, O. G. (1963) The injection of chemicals into the yolk
sac of fertile eggs prior to incubation as toxicity test.
Toxicol. appl. Pharmacol., 5: 760-771.
MELVÅS, B. & BRANDT, I. (1973) The distribution and metabolism of
labelled polychlorinated biphenyls in mice and quails. In: PCB
Conference II. National Swedish Environment Protection Board
Publications: 4E, pp. 87-90.
MES, J., COFFIN, D. E. & CAMPBELL, D. (1974) Polychlorinated biphenyl
and organochlorine pesticide residues in Canadian chicken eggs.
Pestic. Monit. J., 8: 8-11.
MIURA, H., OMORI, S., KATOH, M. (1973) Experimental porphyria in
rabbits induced by PCB. Jpn. J. Hyg, 28: 83 (in Japanese).
MOORE, J. A. (in press) Toxicity of 2,3,7,8-tetrachlorodibenzofuran:
preliminary results. Proceedings of the National Conference on
Polychlorinated Biphenyls, Chicago, 19-21 November.
MOSSER, J. L., FISHER, N. S., TENG, T. & WURSTER, C. F. (1972)
Polychlorinated biphenyls: toxicity to certain phytoplankters.
Science, 175: 191-192.
MULHERN, B. M., CROMARTIE, E., REICHEL, W. L. & BELISLE, A. A. (1971)
Semiquantitative determination of polychlorinated biphenyls in
tissue samples by thin layer chromatography. J. Assoc. Off.
Agric. Chem., 54: 548-550.
MORNS, Y. & KUROIWA, Y. (1971) Peripheral neuropathy in chlorobiphenyl
poisoning. Neurol., 21: 1173-1176.
MUSIAL, C. J., HUTZINGER, O., ZITKO, V. & CROCKER, J. (1974) Presence
of PCB, DDE and DDT in human milk in the provinces of New
Brunswick and Nova Scotia, Canada. Bull. environ. Contam.
Toxicol., 12: 258-267.
NAGAI, J., FURUKAWA, M., YAE, Y. & IKEDA, Y. (1969) Clinicochemical
investigation of chlorobiphenyls poisoning. Especially on the
serum lipid analysis of the patients. Fukuoka Acta Med.,
60: 475-488 (in Japanese).
NAGAYAMA, J., KURATSUNE, M. & MASUDA, Y. (1976) Determination of
chlorinated dibenzofurans in Kanechlors and "Yusho Oil". Bull.
environ. Contam. Toxicol., 15(1): 9-13.
NAGAYAMA, J., MASUDA, Y. & KURATSUNE, M. (1975) Chlorinated
dibenzofurans in Kanechlors and rice oils used by patients with
Yusho, Fukuoka Acta Med., 66: 593-599.
NATIONAL SWEDISH ENVIRONMENT PROTECTION BOARD (1973) PCB Conference
II. Publication 1973: 4E.
NEBEKER, A. V., PUGLISI, F. h. & DEFOE, D. L. (1974) Effect of
polychlorinated biphenyl compounds on survival and reproduction of
the fathead minnow and flagfish. Trans. Am. Fish Soc.,
No. 3, 562-568.
NESTEL, H. & BUDD, J. (1975) Chronic oral exposure of rainbow trout
(Salmo Gairdneri) to a PCB (Aroclor 1254): pathological effects.
Can. J. comp. Med., 39: 208-215.
NILSSON, B. & RAMEL, C. (1974) Genetic tests on Drosophila
melanogaster with polychlorinated biphenyls (PCB). Heriditas,
77: 319-322.
NIMMO, D. R., WILSON, P. D., BLACKMAN, R. R. & WILSON, A. J. JR (1971)
Polychlorinated biphenyl absorbed from sediments by fiddler crabs
and pink shrimp. Nature (Lond.), 231: 50-52.
NISBET, I. C. T. & SAROFIM, A. F. (1972) Rates and routes of transport
of PCBs in the environment. Environ. Health Perspect. 1: 21-38.
NISHIMOTO, T, UEDAM, M, TAUL, S. & CHIKAZAWA, K. (1972a)
Organochlorine pesticide residues and PCB in breast milk. Igaku
no Ayumi (Proc. Med. Sci.), 82: 574-575 (in Japanese).
NISHIMOTO, T., UETA, M., TAUL, S., CHIKAZAWA, K. NISHIUCHI, I. &
KONDO, K. (1972b) Deposition of organochlorine pesticide residues
and PCB in human body fat. Igaku no Ayumi (Proc. med. Sci.),
82: 515-516.
NISHIZUMI, M. (1970) Light and electron microscope study of
chlorobiphenyl poisoning in mouse and monkey liver. Arch.
environ. Health, 21: 620-632.
NORÉN, K. & WESTOO, G. (1968) Determination of some chlorinated
pesticides in vegetable oils, margarine, butter, milk, eggs, meat
and fish by gas chromatography and thin-layer chromatography.
Acta chem. scand., 22: 2289-2293.
ODÉN, S. & BERGGREN, B. (1973) PCB and DDT in Baltic sediments.
Report Dept. Soil Science, Agricultural College, Uppsala,
pp. 1-10.
ODSJO, T. (1973) PCB in some Swedish terrestrial organisms. In: PCB
Conference II. National Swedish Environment Protection Board
Publications: 4E, pp. 45-58. OKUMURA, M. & KATSUKI, S. (1969)
Clinical observation on Yusho (chlorobiphenyls poisoning).
Fukuoka Acta Med., 60: 440-446 (in Japanese).
OKUMURA, M., MASUDA, Y. & NAKAMUTA, S. (1974) Correlation between
blood PCB and serum triglyceride levels in patients with PCB
poisoning. Fukuoka Acta Med., 65: 84-87 (in Japanese).
OLSSON, M, JENSEN, S. & RENBERG, L. (1973) PCB in coastal areas of
the Baltic. PCB Conference H. National Swedish Environment
Protection Board, Publications: 4E, pp. 59-68.
ORBERG, J. & KIHLSTROM, J. E. (1973) Effects of long-term feeding of
polychlorinated biphenyls (PCB, Clopehn A60) on the length of the
oestrous cycle and on the frequency of implanted ova in the mouse.
Environ. Res., 6: 176-179.
ORGANIZATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT (1973)
Polychlorinated biphenyls, their use and control. Environmental
Directorate, Paris, OECD.
PANEL ON HAZARDOUS TRACE SUBSTANCES (1972) PCBs-Environmental Impact.
Environ. Res. 5: 249-362.
PEAKALL, D. B. LIMIAR, J. L. & BLOOM, S. E. (1972) Embryonic mortality
and chromosomal alterations caused by Aroclor 1254 in ring doves.
Environ. Health Perspect., 1: 103-104.
PESENDORFER, VON H., EICHLER, I. & GLOFKE, E. (1973) Informative
analyses or organochlorine pesticide and PCB residues in human
adipose tissue (from the area of Vienna). Wiener klinische
Wochenschr., 85: 218-222 (summary in English).
PHILLIPS, W. E. J., HATINA, G., VILLENEUVE, D.C. & GRANT, D. L. (1972)
Effect of parathion administration in rats following long-term
feeding with PCBs. Environ. physiol. Biochem., 2: 165-169.
PJCHIRALLO, J. (1971) PCBs: leaks of toxic substances raises issue of
effects, regulations. Science, 173: 899-902.
PLATONOW, N. S. & FUNNELL, H. S. (1971) Anti-androgenic-like effect of
polychlorinated biphenyls in cockerels. Vet. Rec, 88: 109-110.
PLATONOW, N. & CHEN, N.Y. (1973) Transplacental transfer of
polychlorinated biphenyls (Aroclor 1254) in a cow. Vet. Rec.,
92: 69-70.
PLATONOW, N. S., FUNNELL, H. S., BULLOCK, D. H., ARNOTT, D. R.,
SASCHENBRECKER, P. W. & GRIEVE, D. O. (1971) Fate of
polychlorinated biphenyls in dairy products processed from the
milk of exposed cows. J. Dairy Sci., 54: 1305-1308.
PLATONOW, N. S., LIPTRAP, R. M. & GEISSINGER, H. D. (1972) The
distribution and excretion of polychlorinated biphenyls (Aroclor
1254) and their effect on urinary gonadal steroid levels in the
boar. Bull. environ. Contam. Toxicol., 7: 358-365.
PORTER, M. L., YOUNG, S. J. V. & BURKE, J. A. (1970) A method for the
analysis of fish, poultry and animal tissue for chlorinated
pesticide residues. J. Am. Off. Anal. Chem., 53: 1300-1303.
PORTMANN, J. E. (1970) Monitoring of organochlorine residues in fish
from around England and Wales, with special reference to
polychlorinated biphenyls. Report CM 1970/E: 9 International
Council for the Exploration of the Sea, Charlottenlund Slot,
DK-2920 Charlottenlund, Denmark.
PRESTT, I., JEFFERIES, D. J. & MOORE, N. W. (1970) Polychlorinated
biphenyls in wild birds in Britain and their avian toxicity.
Environ. Pollut., 1: 3-26.
PRICE, H. A. & WELCH, R. L. (1972) Occurrence of polychlorinated
biphenyls in humans, Environ. Health Perspect., 1: 73-78.
REHFELD, B. M., BRADLEY, R. L. JR & SUNDE, M. L. (1971) Toxicity
studies on polychlorinated biphenyls in the chick. 1. Toxicity and
symptoms. Poult. Sci., 50: 1090-1096.
REINKE, J., UTHE, J. F. & O'BRODOVlCH, H. (1973) Determination of
polychlorinated biphenyls in the presence of organochlorine
pesticides by thin-layer chromatography. Environ. Lett.,
4: 201-210.
REYNOLDS, L. M. (1971) Pesticide residue analysis in the presence of
polychlorobiphenyls (PCBs). Residue Rev., 34: 27-45.
RINGER, R. K., AULERICH, R. J. & ZABIK, M. (1972) Effect of dietary
polychlorinated biphenyls on growth and reproduction of mink.
Proc. Amer. chem. Soc., 164th A.C.S. Meeting, New York,
pp. 149-154.
RISEBROUGH, R. W. & DE LAPPE, B. (1972) Accumulation of
polychlorinated biphenyls in ecosystems. Environ. Health
Perspect., 1: 39-45.
RISEBROUGH, R. W., RIECHE, P., PEAKALL, D. B., HERMAN, S. G. & KIRVEN,
M. N. (1968) Polychlorinated biphenyls in the global ecosystem.
Nature (Lond.), 220: 1098-1102.
ROACH, J. A. G. & POMERANTZ, I. H. (1974) The finding of chlorinated
dibenzofurans in a Japanese polychlorinated biphenyl sample.
Bull. environ. Contam. Toxicol., 12: 338-342.
ROTE, J. W. & MURPHY, P. G. (1971) A method for the quantitation of
polychlorinated biphenyl (PCB) isomers. Bull environ. Contam.
Toxicol., 6: 377-384.
SAFE, S. & HUTZINGER, O. (1971) Polychlorinated biphenyls: photolysis
of 2,4,6,T,4',6'-hexachlorobiphenyl, Nature (Lond.),
232: 641-642.
SAITO, R., SHIGEMATSU, N. & ISHIMARU, S. (1972) Immunoglobulin levels
in serum and sputum of patients with PCB poisoning. Fukuoka Acta
Med., 63: 408-411 (in Japanese).
SANDERS, H. O. & CHANDLER, J. H. (1972) Biological magnification of a
polychlorinated biphenyl (Aroclor 1254) from water by aquatic
invertebrates. Bull. environ. Contam. Toxicol., 7: 257-263.
SASCHENBRECKER, P. W., FUNNELL, H. S. & PLATONOW, N. S. (1972)
Persistence of polychlorinated biphenyls in the milk of exposed
cows. Vet. Rec., 90: 100-102.
SAVAGE, E. P., TESSARI, J. D., MALBERG, J. W., WHEELER, H. W. & BAGBY,
J. R. (1973) A search for polychlorinated biphenyls in human milk
in rural Colorado. Bull. environ. Contam. Toxicol., 9: 222-226.
SCHMIDT, T. T., RISEBROUGH, R. W. & GRESS, F. (1971) Input of
polychlorinated biphenyls into California coastal waters from
urban sewage outfalls. Bull. environ. Contam. Toxicol.,
6: 235-243.
SCHULTE, E. & ACKER, L. (1974) Gas chromatographic unit Glascapillaran
bei Temperaturan bis zu 320°C. Z. Anal. Chem., 268: 261-267.
SCOTT, M. L., ZIMMERMAN, J. R., MARINSKY, S. & MULLENHOFF, P. A.
(1975) Effects of PCBs, DDT and mercury compounds upon egg
production, hatchability and shell quality in chickens and
Japanese quail. Poult. Sci., 54: 350-368.
SCHWETZ, B. A., NORRIS, J. M., SPARSCHU, G. L., ROWE, U. K., GEHRING,
P. J., EMERSON, J. L. & GERBIG, C. G. (1973) Toxicology of
chlorinated dibenzo- p-dioxin. Environ. Health Perspect.,
5: 87-99.
SHIGEMATSU, N., NORIMATSU, Y., ISHIBASHI, T., YOSHIDA, M., SUETSUGU,
S., KAWATSU, T., IKEDA, T., SAITO, R., ISHIMARU, S., SHIRAKISA,
T., KIDO, M., EMORI, K. & TOSHIMITSU, H. (1971) Clinical and
experimental studies on respiratory involvement in chlorobiphenyls
poisoning. Fukuoka Acta Med., 62: 150-156 (in Japanese).
SHIGEMATSU, N., ISHIMARU, S., HIROSE, T., IKEDA, T., EMORI, I. &
MIYAZAKI, N. (1974) Clinical and experimental studies on
respiratory involvement in PCB poisoning. Fukuoka Acta Med.,
65: 88-95 (in Japanese).
SISSONS, D. & WELTI, D. (1971) Structural identification of
polychlorinated biphenyls in commercial mixtures by gas-liquid
chromatography, nuclear magnetic resonance and mass spectrometry.
J. Chromatogr., 60: 15-32.
SODERGREN, A. (1972a) Chlorinated hydrocarbon residues in airborne
fallout. Nature (Lond.), 236: 395-397.
SODERGREN, A. (1972b) Transport, distribution and degradation of
chlorinated hydrocarbon residues in aquatic model ecosystems.
Oikos, 23: 30-41.
SODERGREN, A. (1973a) Transport, distribution and degradation of DDT
and PCB in a south Swedish lake ecosystem. Vatten, 2: 90-108.
SODERGREN, A. (1973b) A simplified clean-up technique for
organochlorine residues at the microliter level. Bull. environ.
Contam. Toxicol., 10: 116-119.
SODERGREN, A. & SVENSSON, Bj. (1973) Uptake and accumulation of DDT
and PCB by Ephemera danica (Ephemeroptera) in continuous-flow
systems. Bull. environ. Contam. Toxicol., 9: 345-350.
SODERGREN, A., SVENSSON, Bj. & ULFSTRAND, S. (1972) DDT and PCB in
South Swedish streams. Environ. Pollut., 3: 25-36.
SOLLY, S. R. B. & SHANKS, V. (1974) Polychlorinated biphenyls and
organochlorine pesticides in human fat in New Zealand. N.Z.J.
Sci., 17: 535-544.
SOSA-LUCERO, J. C. & BE LA IGLESIA, F. A. (1973) Distribution of a
polychlorinated terphenyl (PET) (Aroclor 5460) in rat tissues and
effect on hepatic microsomal mixed function oxidases. Bull.
environ. Contam. Toxicol., 10: 248-256.
STALLING, D. L. & MAYER, F. L. JR (1972) Toxicities of PCBs to fish
and environmental residues. Environ. Health Perspect.,
1: 159-164.
STALLING, D. L., TRINDLE, R. C. & JOHNSON, J. L. (1972) Clean up of
pesticides and polychlorobiphenyl residues in fish extracts by gel
permeation chromatography. J. Assoc. Off. Anal. Chem.,
55: 32-45.
TAKAMATSU, M., INOUE, Y. & ABE, S. (1974) Diagnostic meaning of the
blood PCB. Fukuoka Acta Med., 65:28-31 (in Japanese).
TAKI, I., HISANAGA, S. & AMAGESE, Y. (1969) Report on Yusho
(chlorobiphenyl poisoning). Especially further study of its
dermatological findings. Fukuoka Acta Med., 62: 132-138
(in Japanese).
TAKI, I., KURATSUNE, M., MASUDA, Y. (1973) Studies on the
transmission to the fetus through placenta and the health of
and baby. Special Research Report on the effects of PCBs on human
health such as chronic toxicity for prevention of pollution by
PCBs. Research Co-ordination Bureau, Science and Technology
Agency, pp. 87-95 (in Japanese).
TAKIZAWA, Y. & MINAGAWA, K. (1974) Studies on environmental
accumulation and bio-accumulation of organochloric compounds.
Mainly mentioned PCT. Studies on the body effect of
degradation-registration substances, pp. 29-50 (in Japanese).
TANAKA, K. & ARAKI, Y. (1974) Inhibitory effect of cholestyramine on
the intestinal absorption of PCB. Fukuoka Acta Med., 68: 53-57.
TANAKA, K. & KOMATSU, F. (1972) Shortening of hexobarbital sleeping
time after small doses of PCB in rats. Fukuoka Acta Med.,
63: 360-366 (in Japanese).
TAS, A. C. & DE VOS, R. H. (1971) Characterization of four major
components in a technical polychlorinated biphenyl mixture.
Environ. Sci. Technol., 5: 1216-1218.
TATSUKAWA, R. & WATANABE, I. (1972) Air pollution by PCBs. Shoku No
Kagaku, 8: 55-63.
THOMAS, G. H. & REYNOLDS, L. M. (1973) Polychlorinated terphenyls in
paperboard samples. Bull. environ. Contam. Toxicol.,
10: 37-41.
TREON, J. F., CLEVELAND, F. P., CAPPEL, J. W. & ATCHLEY, R. W. (1956)
The toxicity of the vapours of Aroclor 1242 and Aroclor 1254.
Am. Ind. Hyg. Assoc. Quart., 17: 204-213.
TSUKAMOTO, H. ET AL. (1969) The chemical studies on detection of toxic
compounds in the rice bran oils used by the patients of Yusho.
Fukuoka Acta Med., 60: 496-512 (in Japanese).
TUCKER, R. K. & CRABTREE, D. G. (1970) Handbook of toxicity of
pesticides to wildlife. Bureau of Sport, Fisheries and Wildlife
Resources Pub. No. 84, Washington, DC, US Dept. of Interior,
pp. 130.
TUCKER, E. S., LITSCHGI, W. S. & MEES, W. M. (1975) Migration of
polychlorinated biphenyls in soil induced by percolating water.
Bull. environ. Contam. Toxicol., 13: 86-93.
URABE, H. (1974) Foreword. Fukuoka Acta Med., 65: 1-4 (in Japanese).
USDA/USDC/EPA/FDA/USDI/(1972) Polychlorinated biphenyls and the
environment. Washington DC, Interdepartmental Task Force on
PCBs. (Report ITF-PCB-72-1).
USHIO, F., FUKANO, S., NISHIDA, K., KANI, T. & DOGUCHI, M. (1974) Some
attempt to estimate the total daily intake of pesticides and PCB
residues and trace heavy metals. Ann. Rep. Tokyo Metr. Res. Lab.
P.H., 25: 307-312 (in Japanese).
UTHE, J. F., REINKE, J. & GESSER, H. (1972) Extraction of
organochlorine pesticides from water by porous polyurethane coated
with selective absorbent. Environ. Lett., 3: 117-135.
UZAWA, H., ITO, Y., NOTOMI, A. & KATSUKI, S. (1969) Hyperglyceridemia
resulting from intake of rice oil contaminated with chlorinated
biphenyls. Fukuoka Acta Med., 60: 449-454 (in Japanese).
UZAWA, H., NOTOMI, A., NAKAMUTA, S. & IKEURA, Y. (1972) Consecutive
three year follow up study of serum triglyceride concentrations of
82 subjects with PCB poisoning. Fukuoka Acta Med., 63: 401-404
(in Japanese).
VAN HOVE HOLDRINET, M. (1975) Preliminary results of an inter-
laboratory PCB check sample programme. J. Environ. Qual. (in press).
VEITH, G. D. (1972) Recent fluctuations of chlorobiphenyls (PCBs) in
the Green Bay, Wisconsin, region. Environ. Health Perspect.,
1: 51-54.
VILLENEUVE, D. C., GRANT, D. L., PHILLIPS, W. E. J., CLARK, M. L. &
CLEGG, D. J. (1971 a) Effects of PCB administration on microsomal
enzyme activity in pregnant rabbits. Bull. environ. Contam.
Toxicol., 6: 120-128.
VILLENEUVE, D. C., GRANT, D. L., KHERA, K., CLEGG, D. J., BAER, H. &
PHILLIPS, W. E. J. (1971b) The fetotoxicity of a polychlorinated
biphenyl mixture (Aroclor 1254) in the rabbit and in the rat.
Environ. Physiol., 1: 67-71.
VILLENEUVE, D.C., GRANT, D. L. & PHILLIPS, W. E. J. (1972)
Modification of pentobarbital sleeping times in rats following
chronic PCB ingestion. Bull. environ. Contam. Toxicol.,
7: 264-269.
VILLENEUVE, D.C., REYNOLDS, L. M., THOMAS, G. H. & PHILLIPS, W. E. J.
(1973a) Polychlorinated biphenyls and polychlorinated terphenyls
in Canadian food packaging materials. d. Assoc. Offic. Anal.
Chem., 56: 999-1001.
VILLENEUVE, D.C., REYNOLDS, L. M. & PHILLIPS, W. E. J. (1973b)
Residues of PCBs and PCTs in Canadian and imported European
cheeses, Canada-1972. Pestic. Monit. J., 7: 95-96.
VODDEN, H. A. (1973) Discussion In: PCB Conference II. National
Swedish Environment Protection Board, Publications: 4E, p. 118.
VOS, J. G. (1972) Toxicology of PCBs for mammals and for birds. Era,.
Health Perspect., 1: 105-117.
VOS, J. G. & BEEMS, R. B. (1971) Dermal toxicity studies of technical
polychlorinated biphenyls and fractions thereof in rabbits.
Toxicol. appl. Pharmacol., 19: 617-633.
VOS, J. G. DE ROIJ, Th. (1972) Immunosuppressive activity of a
polychlorinated biphenyl preparation on the humoral immune
response in guinea pigs. Toxicol. appl. Pharmacol., 21: 549-555.
VOS, J. G. & KOEMAN, J. H. (1970) Comparative toxicologic study with
polychlorinated biphenyls in chickens with special reference to
porphyria, edema formation, liver necrosis and tissue residues.
Toxicol. appl. Pharmacol., 17: 656-668.
VOS, J. G. & NOTENBOOM-RAM, E. (1972) Comparative toxicity study of
2,4,5,2',4',5'-hexachlorobiphenyl and a polychlorinated biphenyl
mixture in rabbits. Toxicol. appl. Pharmacol., 23: 563-578.
VOS, J. G. & VAN DRIEL-GROOTENHUIS, L. (1972) PCB-induced suppression
of the humoral and cell-mediated immunity in guinea pigs. Sci.
Total Environ., 1: 289-302.
VOS, J. G., KOEMAN, J. H., VAN DER MAAS, H. L., TEN NOEVER DE BRAUW,
M. G. & DE VOS, R. E. (1970) Identification and toxicological
evaluation of chlorinated dibensofuran and chlorinated naphthalene
in two commercial polychlorinated biphenyls. Fd. cosmet.
Toxicol., 8: 625-633.
VOS, J. G., STRIK, J. J. T. W. A., VAN HOLSTEYN, C. M. W. & PENNINGS,
J. H. (1971) Polychlorinated biphenyls as inducers of hepatic
porphyria in Japanese quail, with special reference to
delta-aminolevulinic acid synthetase activity, fluorescence and
residues in the liver. Toxicol. appl. Pharmacol., 20: 232-240.
WEBB, R. G. & MCCALL, A. C. (1972) Identification of polychlorinated
biphenyl isomers in aroclors. J. Am. Off. Anal. Chem.,
55: 746-752.
WESTOO, G. & NORÉN, K. (1970a) Levels of organochlorine pesticides and
polychlorinated biphenyls in fish caught in Swedish water areas or
kept for sale in Sweden, 1967-1970. Var Föda, 3: 93-146
(summary in English).
WESTOO, G. & NORÉN, K. (1970b) Determination of organochlorine
pesticides and polychlorinated biphenyls in animal foods. Acta
Chem. Scand., 24: 1639-1644.
WESTOO, G. & NORÉN, K. (1972) Levels of organochlorine pesticides and
polychlorinated biphenyls in Swedish human milk. Var Föda,
24: 41-54 (summary in English).
WESTOO, G., NORÉN, K. & ANDERSSON, M. (1971) Levels of organochlorine
pesticides and polychlorinated biphenyls in some cereal products.
Var Föda, 10: 341-360 (summary in English).
WHO WORKING GROUP (1973) The hazards to health and ecological effects
of persistent substances in the environment -- polychlorinated
biphenyls. Report of a Working Group convened by the World
Health Organization, Regional Office for Europe, EURO, 3109(2).
WILDISH, D. J. (1970) The toxicity of polychlorinated biphenyls (PCB)
in sea water to Grammarus oceanicus. Bull. environ. Contam.
Toxicol., 5: 202-204.
WILLIAMS, R. & HOLDEN, A. V. (1973) Organochlorine residues from
plankton. Mar. Bull., 4, 109-111.
WILLIS, D. E. & ADDISON, R. F. (1972) Identification and estimation of
the major components of a commercial polychlorinated biphenyl
mixture, Aroclor 1221. J. Fish. Res. Board Can., 29(5): 592-595.
WONG, P. T. S. & KAISER, K. L. E. (1975) Bacterial degradation of
polychlorinated biphenyls. II. Rate studies. Bull. environ.
Contam. Toxicol, 13: 249-256.
YAGAMUCHI, A., YOSHIMURA, T. & KURATSUNE, M. (1971) A survey on
pregnant women having consumed rice oil contaminated with
chlorobiphenyls and their babies. Fukuoka Acta Med.,
62: 112-117 (in Japanese).
YAMAMOTO, H. & YOSHIMURA, H. (1973) Metabolic studies on
polychlorinated biphenyls. III. Complete structure and acute
toxicity of the metabolites of 2,4,3',4'-tetrachlorobiphenyl.
Chem. pharm. Bull., 21 (10): 2237-2242 (in English).
YOBS, A. R. (1972) Levels of polychlorinated biphenyls in adipose
tissue of the general population of the nation. Environ. Health
Perspect., 1: 79-81.
YOSHIMURA, T. (1971) Epidemiological analysis of "Yusho" patients with
special reference to sex, age, clinical grades and oil
consumption. Fukuoka Acta Med., 62: 109-116 (in Japanese).
YOSHIMURA, H. & YAMAMOTO, H. (1973) Metabolic studies on
polychlorinated biphenyls. I. Metabolic fate of
3,4,3',4'-tetrachlorobiphenyl in rats. Chem. pharm. Bull.,
21: 1168-1169 (in English).
YOSHIMURA, H. & YAMAMOTO, H. (1974) Metabolic studies on
polychlorinated biphenyls. IV. Biotransformation of
3,4,3',4'-tetrachlorobiphenyl, one of the major components of
Kanechlor-400. Fukuoka Acta Med., 65: 5-11 (in Japanese).
YOSHIMURA, H. & YAMAMOTO, H. (1975) A novel route of excretion of
2,4,3',4'-tetrachlorobiphenyl in rats. Bull. environ. Contam.
Toxicol., 13: 681-687.
YOSHIMURA, H., YAMAMOTO, H., NAGAI, J., YAE, Y., UZAWA, H., ITO, Y.,
NOTOMI, A., MIMAKAMI, S., ITO, A., KATO, K. & TSUJI, H. (1971)
Studies on the tissue distribution and the urinary and faecal
excretion of 3H-Kanechlor (chlorobiphenyls) in rats. Fukuoka
Acta Med., 62: 12-19 (in Japanese).
YOSHIMURA, H., YAMAMOTO, H. & SAEKI, S. (1973) Metabolic studies on
polychlorinated biphenyls. II. Metabolic fate of
2,4,3'4'-tetrachlorobiphenyl in rats. Chem. pharm. Bull.,
21: 2231-2236 (in English).
YOSHIMURA, H., YAMAMOTO, H. & KINOSHITA, H. (1974) Metabolic studies
on polychlorinated biphenyls. V. Biliary excretion of
5-hydroxy-2,4,3',4',-tetrachlorobiphenyl, a major metabolite of
2,4,3',4'-tetrachlorobiphenyl. Fukuoka Acta Med., 65: 12-16
(in Japanese).
ZIMMERLI, B. & MARES, B. (1973) Die Belastung der Schweizerischen
Bevolkerung mit Pestiziden. Mitt. Lebensm. Unters. Hyg.,
64: 459-479.
ZITKO, V. (1970) Polychlorinated biphenyls (PCB) solubilized in water
by nonionic surfactants for studies of toxicity to aquatic
animals. Bull. environ. Contam. Toxicol., 5: 279-285.
ZITKO, V. (1971) Polychlorinated biphenyls and organochlorine
pesticides in some fresh-water and marine fishes. Bull. environ.
Contam. Toxicol., 6: 464-470.
ZITKO, V., HUTZINGER, O. & SAFE, S. (1971) Retention times and
electron-capture detector responses of some individual
chlorobiphenyls. Bull. environ. Contam. Toxicol., 6: 160-163.
ZITKO, V., HUTZINGER, O., JAMIESON, W. D. & CHOI, P. M. K. (1972a)
Polychlorinated terphenyls in the environment. Bull. environ.
Contam. Toxicol., 7: 200-201.
ZITKO, V., HUTZINGER, O. & CHOI, P.M. K. (1972b) Contamination of the
Bay of Fundy-Gulf of Maine area with polychlorinated biphenyls,
polychlorinated terphenyls, chlorinated dibenzodioxins and
dibenzofurans. Environ. Health Perspect., 1: 47-50.
ZITKO, V., CHOI, P. M. K., WILDISH, D. J., MONAGHAN, C. F. & LISTER,
N. A. (1974) Distribution of PCBs and p,p'-DDE residues in
Atlantic herring and yellow perch in eastern Canada, 1972.
Post. Mon. J., 8: 105-109.
There have been several investigations to identify individual PCBs
in commercial products. Sissons & Welti (1971) separated the
components of the Aroclors by column and gas-liquid chromatography,
and characterized many of the peaks by high-resolution mass
spectrometry and nuclear magnetic resonance, and by comparison with 40
synthesized Webb & McCall (1972) identified the gas-liquid
chromatographic with those of synthesized compounds by retention times
and spectrometry (Table 2). The most exhaustive study is that of
Jensen & Sundström (1974). They recognized that conventional
gas-liquid chromatography could not separate all the components, so
they devised preliminary fractionation on a charcoal column, which
separated the component PCBs according to the number of graphs in the
2,2',6 or 6' positions in the molecule ( o-chlorines). They compared
the gas-liquid chromatographic peaks with those of 90 synthesized
PCBs, and were able to characterize and quantify 60 components of
Clophens A50 and A60 (Table 3). Tables 2 and 3 show a considerable
overlap between the components of Aroclor 1254 and Clophen A50.
Table 2. Polychlorinated biphenyls in Aroclors 1221-1254 (Webb & McCall, 1972)
Retention Synthetic Acolor
timea chlorobiphenyl
1221 1232 1242 1248 1254
10 Biphenyl xb Cc x x x
13 2 x C x x x
16.9 3 x Dd
17 4 x C x
17.7
18.5 2,2' x C x x C x
21.3 x x x
21.6 x x x
23.5 2,3' x C x x x
24 2,4' x C x x C x
26 2,6,2' x x D x
29 2,5,2' x x C x
29.2 2,4,2' x x x C
29.5 4,4' x C x x C
34 2,3,2' x x Ce x
38 2,4,3' x x C x
38.5 2,4,4' x x C x
38.7 2,5,4' x x C x
41.5 x x
42.5 3,4,2' x C x
43 x x
44 x x
44.5 x
48 2,5,2',5' x C x x C
49.5 2,4,2',5' x C x x C
50.5 2,4,2',4' x D x
51.5 x x
55 2,3,2',5' x x x C
57 x x
59 2,3,6,2',6' x x x D
60 x x
64 x x
69 x x
69.5 x x
70 2,5,3',4' x Ce x
70.5 2,3,6,2',5' x C
71 2,4,3',4' x Ce x
72 x x
76 2,3,6,2',3' x C
Table 2. (cont'd).
Retention Synthetic Acolor
timea chlorobiphenyl
1221 1232 1242 1248 1254
82 x
83 2,3,6,2',5' x x C
84 x
85 2,4,5,2',5' x x C
87 2,4,5,2',4' x x C
96 x
99 2,4,5,2',3' x x C
101 x x
104 x x
107 x x
115 x
119 x x
125 2,4,5,2',3',6' x C
126 2,4,5,3',4' x C
135 x
147 x
148 2,4,5,2',4',5' x C
149 x
152 x
165 x
178 x
a Relative to p,p'-DDE at 190°C on 1 00' × 0.02' SCOT SE 30 column.
b indicates a GLC peak in this Aroclor at this retention time.
c C means that the synthetic compound in the second column was
confirmed by GLC and IR in this Aroclor. Because of the labour
involved, most compounds were only confirmed in one Aroclor. For
example, biphenyl is probably present in 1232-1248 as well as 1221.
d Only GLC data available.
e Something else also present.
2.2 Purity of Products
Commercial PCBs are not sold on a composition specification, but
on their physical properties. Different batches may vary somewhat from
the compositions shown in Tables 1-3. Impurities known to be present
in commercial PCBs are chlorinated dibenzofurans and chlorinated
naphthalenes (Vos et al., 1970; Bowes et al., 1975). Bowes et al.
(1975) found chlorinated dibenzofurans at 0.8-2.0 mg/kg in samples of
the Aroclor 1248-1260 series, but none in Aroclor 1016, and at levels
of 8.4 mg/kg in Clophen A60 and 13.6 mg/kg in Phenoclor DP-6. Roach &
Pomerantz (1974) found chlorinated dibenzofurans levels of 1 mg/kg and
Nagayama et al. (1976) found 18 mg/kg in different batches of
Kanechlor 400, but no chlorinated dibenzodioxins.
2.3 Determination of PCB Residues
Reviews have been published on methods used for the determination
of organochlorine compounds including PCBs in environmental samples
(Holden, 1973a, Panel on Hazardous Trace Substances, 1972). No two
laboratories have identical methods, although all have features in
common. The techniques appear to be those previously developed for the
determination of organochlorine pesticides with appropriate
modifications for the presence of PCBs, and the studies on PCBs
sometimes form part of a wider programme for monitoring persistent
organochlorine compounds in the environment. The major difficulties in
the determination of PCBs are to separate them from interfering
organochlorine pesticides, and to derive a single quantitative figure
from a variable mixture of components.
2.3.1 Extraction of sample
Air
Particulate fallout from air has been trapped on 200 µm nylon net
coated with silicone oil, and the PCBs then extracted with hexane
(Södergren, 1972a). Separate determinations of particulate and vapour
phase PCBs in air have been made by the passage of a large volume of
air through a filter which was followed by an impinger containing
hexane (Hasegawa et al., 1972b), or a polyurethane plug (Bidleman &
Olney, 1974) or ceramic saddles coated with OV 17 silicone (Harvey &
Steinbauer, 1974) to absorb the vapour.
Table 3. Percentages of polychlorinated biphenyls in Clophen A50 and A60 and in human (Jensen & Sundström, 1974)
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
1 2,5-2'5' 2 0.25 0.30 5.0
2 2,4-2'5' 2 0.26 0.30 1.4
3 2,3-2'5' 2 0.27 0.33 1.9 1.1
4 (2,-2',3',4') 2 0.30 0.43 1.2 0.66
5 (4-2',3'6') 2 0.32 0.43 2.1 0.56
6 2,5-2',3',6' 3 0.35 0.43 4.4b 2.9 1.2
7 2,3-2',3',6' 3 0.38 0.49 2.5b 0.28 0.48
8 3,4-2',5' 1 0.41 0.42 3.9 1.5
9 1 or 2 0.42 2.2c 2.0c
10 2,5-2',3',5' 2 0.45 0.49 2.2 1.1 1.2
11 2,3,6-2',3',6' 4 0.47 0.61 0.50 1.0d
12 2,5-2',4',5' 2 0.48 0.50 7.0b 5.6 4.2
13 2,4-2',4',5' 2 0.51 0.51 1.8b --e 1.9
14 2,3-2',4',5' 2 0.53 0.57 1.4b
15 2,5-2',3',4' 2 0.53 0.58 5.4 1.4 2.3
16 2,3-2',3',4' 2 0.55 0.66 1.0
17 3,4-2',3',6' 2 0.56 0.62 7.6b 2.9 4.7
18 2,3,5-2',3',6' 3 0.60 0.70 1.2 4.2 1.0
19 2,4,5-2',3',6' 3 0.64 0.73 2.0b 6.5d 0.13
20 2,5-2',3',5',6' 3 0.65 0.68 1.3 3.3 0.43
21 2,3-2',3',5',6' 3 0.70 0.74 0.5 0.05
22 2,3,4-2',3',6' 3 0.72 0.83 1.8 3.2 0.15
23 1 or 2 0.76 0.6c
Table 3. (cont'd).
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
24 2,3,6-2',3',5',6' 4 0.77 0.91 0.09 0.96
25 3,4-2',4',5' 1 0.79 0.74 5.0b 1.6 5.4
26 2,3,6-2',3'4',6' 4 0.84 0.95 0.05 0.37
27 2,3,52',4',5' 2 0.85 0.86 0.90 2.9 2.7
28 3,4-2',3',4' 1 0.87 0.86 3.6 1.9
29 2,4,5-2',4',5' 2 0.90 0.86 4.2b 12.9d 21.5
30 2,3,4-2',3',5' 2 0.94 0.97 ) 1.5 --e
31 1 or 2 0.95 0.93 ) 1.1 --e
32 2,3,4-2',4',5' 2 1.00 1.00 5.1 11.3d 14.0
33 2,3,5-2',3',5',6' 3 1.01 1.06 0.04 0.49 0.90
34 4 1.02 0.05c
35 1 or 2 1.04 1.10 1.1c 2.0c 1.5'
36 2,4,5-2',3',5',6' 3 1.05 1.13 0.39 3.3 3.5
37 2,3,4-2',3',4' 2 1.11 1.16 1.3 2.0 0.81
38 2,3,6-2',3',4',5' 3 1.13 1.29 0.33 3.7d --e
39 2,4,5-2',3',4',6' 3 1.14 1.16 0.17 1.8 2.5
40 2,3,4-2',3',5',6' 3 1.19 1.32 0.27 2.1 1.3
41 2,3,5,6-2',3',5',6' 4 1.23 1.34 0.005 0.07
42 2,3,4-2',3',4',6' 3 1.28 1.40 0.13 1.3 0.57
43 2,3,4,6-2',3',5',6' 4 1.34 1.44 0.007 0.09
44 2,4,5-3',4',5' 1 1.41 1.19 0.47 1.0 0.49
45 2,3,6-2',3',4',5',6' 4 1.45 1.59 0.008 0.09
46 2,3,4,5-2',3',4',6' 4 1.46 1.51 --e 0.03
47 3,4-2',3',4',5' 1 1.55 1.37 0.81 1.5 2.0
48 2,3,5-2',3',4',5' 2 1.59 1.49 0.23 0.90 1.2
49 2,4,5-2',3',4',5' 2 1.71 1.56 0.98 7.6d 7.7
Table 3. (cont'd).
Percentage in
Relative
Compound No. of retention time Clophen
orthochlorines Human
No. Structurea Apiezon L SF 96 A50 A60 tissue
50 2,3,4-2',3',4',5' 2 1.88 1.82 0.72 4.1d 5.9
51 2,3,4,5-2',3',5',6' 3 1.94 1.99 0.08 0.74 0.77
52 1 or 2 2.02 1.96 0.23c 1.0c --e
53 2,3,4,5-2',3',4',6' 3 2.07 2.05 0.06 0.44 0.94
54 2,4,5-2',3',4',5',6' 3 2.15 2.04 0.01 0.28 0.46
55 (2,3,5,6-2',3',4',5',6') 4 2.23 --e
56 2,3,4-2',3',4',5',6' 3 2.40 2.20 0.01 0.17 0.31
57 2,3,4,6-2',3',4',5',6' 4 2.45 2.56 --e --e
58 3,4,5-2',3',4',5' 1 2.74 2.41 --e --e
59 2,3,4,5-2',3',4',6' 2 3.18 2.81 0.35 0.67 1.7
60 2,3,4,5,6-2',3',4',5',6'4 4 4.12 0.62
Total 86.7% 99.4% 100.0%
p,p'-DDE 0.52 0.58
p,p'-DDT 0.71 0.73
p,p'-DDT 0.90 0.97
a Tentative structures are given in brackets.
b Component found present in Aroclor 1254 by Webb & McCall (1972).
c Figures calculated using responses of chlorobiphenyls with similar retention times in the same fraction
from the charcoal column.
d Component found present in Phenoclor DP6 by Tas & Vos (1971).
e Present in trace amounts only.
Water
PCBs have been extracted from water by passing a sample through a
filter of undecane and Carbowax 400 monostearate supported on
Chromosorb W (Ahling & Jensen, 1970) or a porous plug of polyurethane
coated with a suitable gas-liquid chromatographic stationary phase
(Uthe et al., 1972), or Amberlite XAD-2 resin (Harvey, et al., 1973)
followed by elution of the PCBs with a solvent. Ahnoff & Josefsson
(1975) have described a liquid-liquid extraction into cyclohexane.
Biological samples
Most analysts have used standard methods developed for
organochlorine pesticides, in which the PCBs are extracted together
with the fat; the sample is ground with anhydrous sodium sulphate and
extracted with petroleum ether or hexane. Porter et al. (1970) studied
the optimal conditions for this procedure. A dehydrating solvent may
be included to facilitate the breakdown of cell structures; ethanol
(Norén & Westöö, (1968) and acetone (Jensen et al., 1973) have been
used. Rote & Murp (1971) digested the sample with a mixture of acetic
and perchloric acid prior to hexane extraction.
2.3.2 Clean-up
Methods for the removal of fat from the extract include solve
partitioning between hexane and acetonitrile or dimethylformamide,
treatment with strong sulfuric acid or ethanolic potassium hydroxide
Gel permeation has also been used (Stalling et al., 1972), and Holden
Marsden (1969) removed fat on dry, partially deactivated alumina
column. Certain pesticides such as dieldrin are destroyed by the
sulfuric at treatment, so this method cannot be used if such
pesticides are to determined together with PCBs (Jensen et al., 1973).
PCBs may be separated from organochlorine pesticides by column
chromatography on Florisil (Mulhern et al., 1971), silica gel (Holden
Marsden 1969; Armour & Burke, 1970; Collins et al., 1972) or on
charcoal (Berg et al., 1972; Jensen & Sundström, 1974). Several
laboratories have reported difficulties in repeating results obtained
by other investigators; the ease of separation appears to depend upon
the characteristics of absorbent, of the eluting solvent, and of the
sample extract, though the appears to be no difficulty in separating
all interfering substances except DDE, a metabolite of DDT. Thin-layer
chromatography has been us for separation by Norén & Westöö (1968),
Bagley et al. (1970), and Rein et al. (1973).
In many environmental samples, DDE is present in large excess over
the PCBs, and must be removed before the quantitative determination
PCBs. Oxidation procedures have been used to convert DDE
dichlorobenzophenone; recommended oxidants are potassium dichromate
and sulfuric acid (Westöö & Norén, 1970b) and chromium (II) oxide a
acetic acid (Mulhern et al., 1971). Jensen & Sundström (1974), who we
interested in determining DDT/PCB ratios in environmental sample
preferred sodium dichromate in acetic acid with a trace of sulfuric
acid. They claim that this does not destroy DDT and its metabolite DDD
which may be present in extracts after clean-up with strong sulfuric
acid and that using this mixture makes possible the quantitative
determination of the dichlorobenzophenone from the oxidation of DDE.
Conversion of DDT to DDE may be achieved by treatment with
ethanolic potassium hydroxide, which also removes interference from
elemental sulfur (Ahling & Jensen, 1970). Sulfur may also be removed
activated Raney nickel (Ahnoff & Josefsson, 1975) or by metallic
mercury.
Södergren (1973b) has scaled down the clean-up procedure for small
samples, using microlitre volumes.
2.3.3 Chromatographic separation of PCBs
Gas-liquid chromatography
Most analysts use gas-liquid chromatography with an electron-
capture detector for the separation of PCBs from the extract after
clean-up. Stationary phases commonly used are silicones or their
derivatives, for example, DC 200, SF 96, OV 1, and QF 1, or Apiezon L.
Jensen & Sundström (1974) state that with a mixture of SF 96 and QF 1,
14 peaks can be obtained from Clophen A50, but that Apiezon L gives
much better resolution. They obtained better peak separation by prior
fractionation on a charcoal column, which separated the PCBs according
to the number of o-chlorine substituents; they regard such
refinements as unnecessary in PCB residue analysis, although they may
be of value in the study of the selective, environmental degradation
of PCBs. Column temperatures used ranged between 170°C and 230°C.
Glass capillary columns gave good separation of PCBs from DDT and its
metabolites (Schulte & Acker, 1974).
Thin-layer chromatography
This has been used in the clean-up stage, but it may also provide
semi-quantitative results by visualization of the spots followed by
densitometry, or by comparison with spots produced by known amounts of
PCBs. Mulhern et al. (1971) separated PCBs on a plate coated with
alumina containing silver nitrate, and the spots were developed by
exposure to ultraviolet light; the detection limit was in the region
of 1 µg. Collins et al. (1972) devised a similar method in which the
PCBs remained together in a single spot, and they claimed a limit of
detection of about 50 ng. Reversed-phase chromatography on plates
coated with kieselguhr treated with liquid paraffin has been used to
separate Phenochlor DP6 into several spots with a detection limit of a
few micrograms (de Vos & Peet, 1971).
2.3.4 Quantification of PCB content
The response of the electron capture detector is not equal for all
PCB components, being much affected by the degree of chlorination
(Zitko et al., 1971). This does not lead to difficulties when the
sample under investigation has been directly contaminated by a
commercial PCB mixture, as that mixture can be used as a standard.
Difficulties are encountered when the PCBs in the sample have
undergone selective environmental degradation (see sections 3 and 5).
Several investigators have noted that the pattern of peaks from such
samples resembles fairly closely that of one or other of the higher
chlorinated PCB mixtures such as Aroclor 1254, and they have compared
the total area of the peaks with that of the nearest commercial
product in order to determine the amount of PCBs in the sample (Armour
& Burke, 1970). Collins et al. (1972 observed that, under their
conditions, the area of peaks usually encountered in extracts of
tissue samples was closely similar to that of an equivalent amount of
DDE, thus DDE could be used for calibration. In order to overcome the
uncertainties of these procedures, Rote & Murphy (1971) divided the
peaks into groups according to the number of chlorine atoms in the
molecule, as determined from mass spectrographic data, and calculated
the PCB content of each group from the theoretical response of the
detector to chlorine content. Jensen et al. (1973) selected a
commercial in PCB that included all the peaks from the extract; they
determined the PCB content of each peak by combined mass spectrometry
and coulometry, and determined the total PCBs in the sample by
comparing the height of each peak obtained with the extract with those
obtained with the reference sample. Simpler methods have been used by
Koeman et al. (1969), who compared the height of a single peak
obtained with the extract with that of a peak with the same retention
time obtained with a e commercial PCB mixture; others have averaged
out more than one peak s for this calculation (Reynolds, 1971; Reinke
et al., 1973). Rote & Murphy (1971) have calculated that such
procedures may more than double the values obtained by a more accurate
method.
A different technique has been recommended by Berg et al. (1972);
the PGBs are chlorinated with antimony pentachloride to decachloro-
biphenyl, which can then be measured as a single peak.
2.3.5 Accuracy of PCB determinations
A group of eight analysts engaged in an investigation of pollution
in the North Sea undertook a collaborative study to determine the PCB
content of a sample of fish oil, using the methods currently employed
in their laboratories (International Council for the Exploration of
the Sea, 1974). The PCB values obtained ranged from 1.0 to 3.9 mg/kg
with a mean of 1.97 mg/kg and a standard deviation of 0.93 mg/kg.
Better agreement was obtained with the same fish oil fortified with
PCBs at a concentration of 10 mg/kg; the mean of the results for the
fortified sample was 10.0 mg/kg with a standard deviation of
1.1 mg/kg.
A probable source of error is incomplete initial extraction of
PCBs from the sample (Holden & Marsden, 1969). Another source of
variation between laboratories lies in the method used to quantify
gas-liquid chromatographic peaks (section 2.3.4); van Hove Holdrinet
(1975) considered this to be the major source of error.
It is evident that caution should be exercised in accepting the
analytical results from a laboratory, particularly for samples with a
low PCB content, until the competence of that laboratory has been
established by an inter-laboratory collaborative study.
2.3.6 Confirmation of identity
Since Jensen first identified as PCBs those hitherto unknown
substances that interfered in the gas-liquid chromatographic
determination of organochlorine pesticides using mass spectrographic
data, other investigators have confirmed the presence of PCBs in
environmental samples by combining gas-liquid chromatography with mass
spectrometry (Bagley et al., 1970) and with coulometry to measure the
chlorine content. The conversion of PCBs to bicyclohexyl and
decachlorobiphenyl is further confirmation (Berg et al., 1972). The
widespread distribution of PCBs is now well established, and, as
adequate methods are available to remove interference from
organochlorine pesticides, there is no evidence of the presence of
other interfering substances in the types of sample that have so far
been analysed, down to a limit of detection of around 0.01 mg/kg. This
does not necessarily apply to other types of sample, particularly when
very low levels are being sought; Ahnoff & Josefsson (1973, 1975)
reported a number of unknown interfering substances, when measuring
PCBs in water at levels below 1 ng/litre, one of which was
subsequently identified as elemental sulfur. They recommend
confirmation by mass fragmentography for such samples.
2.4 Determination of PCTs
A few methods have been published for the determination of PCTs;
extraction and clean-up procedures are similar to those used for PCBs,
but the gas-liquid chromatographic details are different because of
the lower volatility of the PCTs. Zitko et al. (1972b) used 3% OV 210
as the stationary phase with a column temperature of 200°C. Thomas &
Reynolds (1973) also used OV 210 with a column temperature of 250°C
and another system with 3% Dexsil as stationary phase at 300°C with a
63Ni electron capture detector; this was also used by Addison et al.
(1972). Sosa-Lucero et al. (1973) used OV 210 and SE 30 at 255°C and
Freudental & Greve (1973) used OV 17 with a temperature programmed
from 200°C to 285°C. Thomas & Reynolds (1973) confirmed the identity
by chlorination to tetradecachloroterphenyl with antimony
pentachloride.
A thin-layer chromatographic technique has also been described
with limit of detection of about 1 µg (Addison et al., 1972).
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1 Production and Uses of PCBs
Details of the production and uses of PCBs in the USA have been
released, and have been summarized by Nisbet & Sarofim (1972). Annual
production has increased steadily since 1930 and reached a maximum in
1970 of 33 000 tonnes. During this peak year, 65% of the production
was of the 42% chlorinated type, 25% was less chlorinated, and the
remainder more chlorinated. After 1970, production sharply decreased
owing to voluntary limitation of sales by the Monsanto Company, the
sole manufacturer in the USA. According to information collected by
the Organization for Economic Co-operation and Development (OECD), the
1970, production in the USA was 18 000 tonnes and the total in OECD
countries in that year was 48 000 tonnes. It has been estimated that
the cumulative total production of PCBs in North America up to 1971
was 0.5 million tonnes, and in the whole world probably double this
figure.
The commercial applications of PCBs have been reviewed in an OECD
report (Organization for Economic Co-operation and Development, 1973)
From an environmental viewpoint these can be divided into three
categories:
Controllable closed systems. PCBs used as dielectrics in
transformers and large capacitors have a life equal to that of the
equipment, and with proper design leakage does not occur. When the
equipment is scrapper the quantity of dielectric is sufficiently large
to justify regeneration.
Uncontrollable closed systems. PCBs are used in heat transfer and
hydraulic systems which, although technically closed, permit leakage.
The need for frequent replacement of small quantities makes recovery
impracticable, PCBs are very widely dispersed in small capacitors, and
there are great difficulties in collecting these items for disposal.
Dissipative uses. PCBs have been used in the formulation of
lubricating and cutting oils, in pesticides, and as plasticizers in
paints, copying paper, adhesives, sealants, and plastics. In these
applications, the PCBs are in direct contact with the environment, and
there is no way of recovering them when the product is scrapped.
The uses of PCBs in the USA in 1970 have been analysed by Nisbet &
Sarofim (1972). Of the total 33 000 tonnes, 56% was used as a
dielectric, with 36% in capacitors and 20% in transformers. Various
plasticizer outlets accounted for 30%, hydraulic fluids and lubricants
12%, and heat transfer liquids 1.5%. Following the restriction of
sales for non-dissipative uses, the percentage of PCBs sold as
dielectrics rose to 77% in 1971 and the proportion of highly
chlorinated products was considerably reduced, Aroclor 1016 replacing
Aroclor 1242. In Japan, 44 800 tonnes of PCBs were used from 1962 to
1971, and of this 65.4% was used in the electrical industry, 11.3% in
heat exchangers, 17.9% in pressure-sensitive duplicating paper, and
5.4% for other dissipative uses (Ishi, 1972). In Sweden, most of the
600 tonnes imported in 1969 was used in the electrical industry and a
large part of the remainder in paints (Jensen, unpublished report
1972).
According to the OECD report, transformers and capacitors provided
the major outlets for PCBs in most OECD countries in 1971. In 1972,
several countries restricted sales; in Sweden the importation and use
of PCBs were restricted by law; in the United Kingdom, as in the USA,
sales were voluntarily restricted to the lower chlorinated PCBs for
use as dielectrics in enclosed systems; in the Federal Republic of
Germany, use in hydraulic and heat transfer fluids was also permitted.
In Japan the production and use of PCBs were banned in 1972.
Limitations on sales were subsequently introduced in other countries.
No information is available on the scale of production and the
uses of PCTs.
3.2 Entry of PCBs into the Environment
Surveys of the sources of environmental pollution with PCBs were
made before production and use were limited, and the information
available may not now apply in North America and elsewhere. Table 4
gives an estimate of the fate of the PCBs produced in 1970 in the USA
(Nisbet & Sarofim, 1972). Only 20% of the annual production can be
regarded as a net increase in current usage, and the remainder is
balanced by a loss to the environment. More than one-half of this
entered dumps and landfills and it has been calculated that 0.3
million tonnes of PCBs have accumulated in such locations in North
America since 1930. Much of this was originally enclosed in containers
such as capacitors or was in plasticized resins and will not be
released until the containing medium decays. The diffusion of PCBs
from landfills is likely to be slow on account of their low volatility
and low water solubility; Carnes et al. (1973) found little leaching
from one site they tested.
Pollution of the environment has occurred mainly from the first
three routes mentioned in Table 4. In addition, there are other
routes, which although involving relatively small amounts,
nevertheless have an influence on the entry of PCBs into food chains.
PCBs have been used in the USA in amounts of about 10 tonnes/year in
pesticide formulations (Panel on Hazardous Trace Substances, 1972),
and the unauthorized use of scrap transformer fluid for this purpose
has led to local contamination of milk supplies.
Table 4. Entry of PCBs into the environmenta
Percentage of
annual PCB type
Route production (% chlorination)
Vaporization from plasticizers 4.5 48-60
Vaporization during incineration 1 42
Leaks and disposal of industrial fluids 13 42-60
Destruction by incineration 9 mainly 42
Disposal in dumps and landfills 52.5 42-60
Net increase in current usage 20 42-54
a From Nisbet & Sarafim, 1972.
Pressure sensitive duplicating paper containing PCBs has found its
way into waste paper supplies and has been recycled into paper and
board used as food packaging materials; paints for coating the bottom
of ships contained 3-5% of PCBs -- about 3% of the annual quantity
imported into Sweden has been used for this purpose -- and this has
been a source of plankton contamination (Jensen et al., 1972a).
3.2.1 Release of PCBs into the atmosphere
There appears to be little widespread atmospheric contamination
during the manufacture and processing of PCBs, but this can occur
during their subsequent use and disposal. Although PCBs have a low
volatility, there may be an appreciable loss to the atmosphere during
the lifetime of a PCB-plasticized resin, particularly of the lower
chlorinated products. Further pollution may occur during the
incineration of industrial and municipal waste. Most municipal
incinerators are not very effective in destroying PCBs; efficient
incinerators can be designed for this purpose (Jensen & Wickberg,
unpublished report 1971; Jensen, unpublished report 1972), although
the higher chlorinated PCBs are more resistant to pyrolysis. Secondary
sources of atmospheric pollution are volatilization from soil, and
from the drying of sewage sludge. Laveskog (1973) found that the PCB
emissions from municipal incineration and from the drying of sewage
sludge each amounted to about 1 kg/year per million inhabitants, a
small amount compared with the 2 tonnes deposited yearly from aerial
fallout in the south of Sweden.
3.2.2 Leakage and disposal of PCBs in industry
The major source of environmental pollution with PCBs, which
eventually affects food chains, is the leakage and the disposal of
industrial fluids. There have been serious cases of poisoning in man
(Kuratsune et al., 1972) and in animals (Panel on Hazardous Trace
Substances, 1972) due to leakage from a heat exchanger. Leakages, or
the unintentional or deliberate discharge of waste, have contaminated
seas, lakes, waterways, and sewers (Duke et al., 1970; Schmidt et al.,
1971; Veith, 1972).
Analysis of solids from factory wastes in Japan have revealed a
wide variation in PCB content. In most, less than 1 mg/kg was
detected, but in: some the contamination was very heavy, the highest
level recorded being 8.26% from a factory manufacturing electrical
equipment (Japanese Environment & Safety Bureau, unpublished report
1972).
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATION
4.1 Environmental Transport
Nisbet & Sarofim (1972) emphasize that the available data are
insufficient to determine anything but a very crude model of the
transfer of PCBs into the environment. Guesses can be made by
referring to the distribution of DDT, which resembles the PCBs to some
extent in its physical and chemical properties and about which more is
known. Much of the following discussion arises from their analysis of
the situation in the North American continent.
4.1.1 Air transport
By analogy with DDT, it might be expected that PCBs entering the
atmosphere in the vapour phase would be adsorbed rapidly on to
particles, which would be deposited or washed out in rain at a rate
depending on their particle size, the average residence time in the
atmosphere being 2-3 days. This has been confirmed by Södergren
(1972a), who measured the deposition in southern Sweden of PCBs that
had originated from municipal incinerators or had been carried over
from Denmark by the prevailing winds, and showed that the amount
deposited in central Sweden was much less. Cames (1973) and Laveskog,
(unpublished report 1973) found mainly particulate PCBs in the
emission from incinerators. Harvey & Steinhauer (1974), however,
considered that the results of their analyses of air in north Atlantic
regions indicated that most of the PCBs carried in the air were in the
vapour phase.
4.1.2 Transport in soil
The PCBs in soil are derived mainly from particulate deposition,
estimated at 1000-2000 tonnes annually in North America, most of which
is in urban areas. Small amounts have originated from the use of
sewage sludge as a fertilizer, from the leaching of landfalls, and
from the se of PCBs in pesticide formulations. Tucker et al. (1975)
found that under experimental conditions, the higher chlorinated PCBs
were not leached from soils by percolating water, and those with a
lower level of chlorination were removed only slowly, particularly
from soils of high lay content. Losses do occur by volatilization and
by biotransformation; by analogy with DDT and its metabolites the
half-time in soil has been estimated at 5 years. Haque et al. (1974)
showed that the rate of evaporation decreased with the clay content of
the soil and he degree of chlorination of the biphenyl, and increased
with temperature. Biotransformation has also been shown to play a part
in the disappearance of the lower chlorinated compounds from soil
(Iwata et al., 1973).
The total amount of PCBs distributed over North America, apart
from that in dumps and landfills, has been estimated at 20 000 tonnes,
of which one quarter has subsequently been transported via the air to
the seas. (Nisbet & Sarofim, 1972).
4.1.3 Transport in water
The entry of PCBs into water occurs mainly at the points of
discharge of industrial and urban wastes into rivers, lakes, and
coastal waters. Sewage treatment appears to remove particulate PCBs
from water, but not PCBs in solution; these are concentrated in the
sludge (Ahling & 1970), which may be dumped into rivers and coastal
waters. Holden (1970b) found a mean PCB content of 3 mg/kg in liquid
sludges from Glasgow, and calculated that PCBs at the rate of
1 tonne/year were released into the Clyde and Thames estuaries; a
similar output was calculated from water treatment plants off the
California coast (Schmidt et al., 1971). Other localized sources of
pollution are leakages or waste disposal from ships. PCBs in water are
attached mainly to particulate matter (Södergren, 1973a) and
eventually fall to the bottom sediment at a rate that depends on the
particle size. PCBs may be leached from the sediment and may reach
coastal waters, but Nimmo et al. (1971) noted little change in the PCB
content of a sediment at a point downstream from a source of
contamination over a period of 9 months. The process may be
accelerated by the dumping of dredging spoil.
4.1.4 Transport through biota
According to the approximate calculations of Nisbet & Sarofim
(1972), less than 1000 tonnes of PCBs are located in living organisms
throughout the world, so that biological transport and degradation
play little part in the fate of PCBs in the environment, though these
factors have great ecotoxicological significance.
4.2 Transformation in the Environment
4.2.1 Abiotic transformation
The fate of the various PCBs in commercial mixtures depends on
their physical and chemical properties. Some fractionation occurs
during the volatilization of PCBs, because of a decrease in vapour
pressure with increasing chlorination. The PCBs are chemically very
stable, and are not likely to be degraded at a significant rate by
hydrolytic or similar reactions under environmental conditions. They
are, however, fairly easily degraded by photolysis under laboratory
conditions; Safe & Hutzinger (1971), and Hutzinger et al. (1972b) have
shown that PCBs are dechlorinated in hexane solution at a rate that
increases with increasing chlorination. PCBs in aqueous-dioxane
suspensions and in thin films give hydroxy and carboxylic acid
derivatives on irradiation. The lower chlorinated biphenyls, at a
vapour concentration in air of 1.5 mg/m3 are readily destroyed by
photolysis under laboratory conditions. There is no direct evidence of
the extent of PCB breakdown in the atmosphere under environmental
conditions, and nothing is known of the persistence and toxicity of
any transformation products.
4.2.2 Biotransformation
The biotransformation of PCBs is discussed in section 6.6. Owing
to small proportion of the total environmental PCBs contained in
living biotransformation does not significantly influence the overall
environmental concentrations of PCBs, though it has a marked influence
on PCBs passing through food chains.
4.2.3 Metabolism in limited ecosystems
The presence of PCBs in sewage sludge suggests that they are not
all readily transformed by microorganisms. Choi et al. (1974) found no
evidence of biotransformation of Aroclor 1254 added to water entering
an aerated biological water treatment system, although much of it was
removed with the sludge; there appeared to be no interference by the
PCBs in the performance of the system. Vodden (1973) quotes
investigations showing that PCBs with four or fewer chlorine atoms are
readily broken down by microorganisms, but that this can be inhibited
by the presence of higher chlorinated PCBs. Mono- and dichlorobiphenyl
can be transformed by Achromobacter isolated from sewage effluent
(Ahmed & Focht, 1973), and a culture of lake bacteria can degrade some
of the lower chlorinated components of Aroclor 1242 to chlorine-free
derivatives (Kaiser & Wong, 1974).
Södergren (1972b) investigated the transport of Clophen A50 added
to a model aquatic ecosystem; this was rapidly taken up by an alga
(Chlorella) and no change was observed in the proportion of the
component PCBs in the first consumer fish; however, there was a
relative loss of the lower chlorinated PCBs in the perch, the second
consumer. No progressive loss of the lower chlorinated PCBs was
observed in sediment, plankton, invertebrates, and fish inhabiting a
Swedish lake (Södergren, 1973a). Evidence on the metabolism of PCBs by
fish is conflicting, but it seems probable that most fish,
particularly those in the lower trophic stages of food chains, cannot
readily degrade the low chlorinated PCBs.
4.3 Biological Accumulation
Although PCB concentrations in living organisms clearly indicate a
progressive accumulation in food chains, the factors discussed in the
previous sections make it impossible to give any reliable figure for
bioaccumulation at each trophic stage. There is also doubt about which
tissue level should be used in the calculation, that of the whole
body, the fat, or the liver.
There is good evidence that all aquatic organisms studied in
aquaria can absorb PCBs directly from water. The accumulation varies
with the duration of exposure and the concentration in the ambient
water. A diatom exposed to Aroclor 1242 showed an accumulation factor
of 1100 (Keil et al., 1971); with Aroclor 1254 the following values
have been obtained: pink shrimp, 6600; blue crab, 4600; oyster, 8100;
pinfish, 980 (Duke et al., 1970); spot, 37 000 (Hansen et al., 1971);
bluegills, up to 71 400 (Stalling & Mayer, 1972). The accumulation
factor in scud exposed to Aroclor 1254 reached a maximum of 24 000
within 4 days and thereafter remained fairly constant (Sanders &
Chandler, 1972). Similar results were obtained with other
invertebrates, although with crayfish the uptake was slower and the
accumulation was still increasing after 21 days. However, it is
probable that most of the PCBs entering aquatic systems are retained
by particulate matter, and the above accumulation factors are not
necessarily applicable to natural ecosystems. Nimmo et al. (1971)
demonstrated that the fiddler crab could ingest PCB contained in the
bottom sediment. When exposed to Aroclor 1254 in water, ciliated
protozoa, stated to be the major benthic input to aquatic food chains,
had an accumulation factor of 60 (Cooley et al., 1972).
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1 Air
Mean concentrations in air, in several locations in Sweden, ranged
from the detection limit of 0.8 ng/m3-3.9 ng/m3. The highest figure
recorded was 12.5 ng/m3 (Ekstedt & Odén, 1974). In the USA, PCB
concentrations in air ranged from 5 ng/m3 near the north-east coast
to 0.05 ng/m3 at a distance of 2000 m out over the Atlantic Ocean
(Harvey & Steinhauer, 1974). Results from the United States
Environmental Protection Agency indicate a range between 1 and
50 ng/m3 (Panel on Hazardous Trace Substances, 1972), and similar
results have been reported from Japan (Tatsukawa & Watanabe, 1972).
5.2 Soil and Sediments
In Sweden, PCBs have been found in natural soil at a concentration
of 15 µg/kg by Odén & Berggren (1973). The same authors also found
0.006-1.4 mg/kg in sediments from areas in the Baltic Sea with
different degrees of pollution. Nimmo et al. (1971) found PCB levels
of 1.461 mg/kg in sediment from an estuary at a point near the site of
an accidental release of PCBs from a factory, and levels of 0.6 mg/kg
at a point 16 km downstream. Soil samples from the bank 6.5 km
downstream from the source contained 1.4-1.7 mg/kg. Less than 1 mg/kg
has been found in Japanese agricultural soil, but as much as 510 mg/kg
in soil near a factory making electrical components (Fukada, et al.,
1973).
5.3 Water
In heavily contaminated waters, PCB concentrations may be several
times greater than their solubility, owing to adsorption on suspended
particles (Duke et al., 1970). Water in a Swedish river contained
0.5 ng/litre as it entered a water treatment plant, and 0.33 ng/litre
in the tap water produced (Ahling & Jensen, 1970). Values of
0.1-0.3 ng/litre have been measured in other Swedish rivers (Ahnoff &
Josefsson, 1974). Södergren (1973a) found a seasonal variation in the
PCB level in a Swedish lake, with a maximum of 2 ng/litre, the
pollution being attributed to aerial fallout. Concentrations of
10-100 ng/litre have been measured in tap water at Kyoto in Japan
(Panel on Hazardous Trace Substances, 1972) In a polluted coastal
area of Lake Michigan in 1970, PCB concentrations of from 450 to less
than 100 ng/litre were measured in 1970, but there was a marked
decrease in 1971, possibly due to the limitation on sales of PCBs
(Panel on Hazardous Trace Substances, 1972). From the scanty
information on PCBs, reinforced by analogy with the more extensive
information on DDT, it has been estimated that nonpolluted fresh
waters should contain not more than 0.5 ng/litre up to 5 ng/litre for
the Great Lakes of North America, 50 ng/litre for moderately polluted
rivers and estuaries, and 500 ng/litre for highly polluted rivers.
5.4 Living Organisms
There is now considerable information from Canada, Japan, Sweden
the United Kingdom, and the USA, on the accumulation of PCB's
biological material. Analytical measurements on different organisms,
and on the same organism from different localities, vary widely and it
is necessary to consider the factors that lead to this variation.
Difference in analytical techniques may contribute to this (see
section 2.3.5) but important influences are exerted by the extent of
local pollution, amount of fat in the organism studied, and its
trophic stage in food chains.
5.4.1 The influence of local pollution
Most of the fish eaten by man is taken from waters with little
pollution. In a collaborative study by seven national (International
Council for the Exploration of the Sea, 1974), the PCB content of
muscle tissue of fish taken from the North Sea was measured. A mean of
0.01 mg/kg was found in cod, herring contained up to 0.48 mg/kg with
most samples in the range of 0.1-0.2 mg/kg, and plaice contained
0.1 mg/kg or less. Similar values were reported by Zitko (1971) for
fish taken from the North Atlantic.
There are many examples of different PCB levels in similar species
collected from areas of high and low pollution. Jensen, et al. (1972b)
found five times as much PCBs in herrings caught in waters off
industrialized areas near Stockholm, as in herrings from the cleaner
waters of the west coast of Sweden. Similarly, levels in plankton
harvested along the Swedish archipelago at various distances from
Stockholm fell progressively, away from the more polluted areas
(Jensen, et al., 1972c); the concentration in pike fell to one-half
(Olsson & Jensen, 1974). Koeman et al., (1972b) found PCB levels of up
to 88 mg/kg in the blubber of toothed whales caught in the North Sea,
but none was detectable in similar species from New Zealand or
Surinam. Holden (1973b) found high PCB concentrations in the blubber
of seals in polluted coastal areas of the United Kingdom (up to
235 mg/kg), and much lower levels in unpolluted areas (down to
2 mg/kg).
Risebrough & de Lappe (1972) studied the PCB content of
extractable lipids from brown pelican eggs collected from areas
throughout North and South America, and showed that the content varied
from 4 mg/kg up to 266 mg/kg in highly industrialized regions. They
also reported levels greater than 3 mg/kg in fish from New York Sound
and Tokyo Bay, both very polluted areas. Even higher levels of PCBs
have been found in fish from polluted lakes and inland waterways, a
level of 20 mg/kg being found in fish from Lake Ontario, and over
200 mg/kg in fish from the Hudson River (Stalling & Mayer, 1972).
Similar correlations between pollution and PCB levels have been
reported from the United Kingdom in fish (Portmann, 1970), and in
mussels (Holdgate, 1971).
The association between high PCB levels and local pollution may be
disturbed by the migratory habits of certain species, particularly in
birds that may be exposed to PCBs in their wintering areas or on the
migration routes. As much as 400 mg/kg has been measured in the fat of
a robin entering Sweden, although the normal value is about 16 mg/kg.
Many migratory birds start egg-laying on arrival at their summer
quarters, so the PCB content of eggs may reflect the bird's previous
exposure rather than the local pollution (Odsjö, 1973). A special case
concerning the effect of pollution is seen in the use of fish-feed in
poultry and fish farming. Kolbye (1972) stated that this may contain
PCB levels of 0.6-4.5 mg/kg.
5.4.2 The influence of the fat content of tissues
PCBs are mainly stored in body fat (see section 5.5.5.1), and the
total PCB content of the body tissues is much influenced by their fat
content (Portmann, 1970; Westöö & Norén, 1970a). Jensen, et al. (1969)
found PCB levels of 0.27 mg/kg and 0.33 mg/kg respectively, in the
muscle tissue of herring and cod from the same area of the Baltic,
although the cod is at a higher trophic stage (see section 5.4.3).
These two species have 4.4 and 0.32% of extractable fat respectively,
and when the PCB level is calculated on the fat content, values of
6.8 mg/kg for the herring and 11 mg/kg for the cod are obtained. Cod
liver has a much higher fat content than cod muscle and Jensen (1973)
has reported the ratio of PCB concentrations in cod liver and muscle
to be over 100, the maximum in liver being 59 mg/kg. Jensen et al.
(1969) have remarked that the considerable seasonal variation in the
fat content of the herring, rising from 1% in spring to 10% in autumn,
influences the tissue level of PCBs. Peakall et al. (1972) noticed a
marked rise in tissue levels in starved birds owing to the
mobilization of fat, and it is possible that the high levels of PCBs
in the livers of birds dying during the "seabird wreck" in the Irish
Sea were secondary to emaciation (Holdgate, 1971). De Freitas &
Norstrom (1974) showed that, in pigeons, pure PCBs left fatty tissues
and accumulated mainly in muscle during the mobilization of fat
associated with starvation.
5.4.3 The influence of the trophic stage in food
Swedish work on the distribution of PCBs in aquatic ecosystems has
been summarized by Jensen et al. (1972b), and Olsson et al. (1973),
and it has been largely confirmed by work in other areas (Risebrough
et al., 1968; Risebrough & de Lappe, 1972: Holdgate, 1971). Zoo- and
phytoplankton readily absorb or adsorb PCBs from their environment;
Södergren (1972) has demonstrated the rapid uptake of Clophen A50 by
the unicellular alga Chlorella. Marine zooplankton may contain PCB
levels of 5 mg/kg in extractable lipids in areas of moderate pollution
(Jensen et al., 1972c; Williams & Holden, 1973), with somewhat lower
values in relatively unpolluted areas. However, results with plankton,
particularly where high levels are found, must be regarded with
caution as the sample could be contaminated with PCB-rich oil or tar
particles. Herring feeding on plankton in areas of moderate pollution
contain PCB levels of about 0.5 mg/kg in muscle tissue (10 mg/kg in
extractable fat); plaice and flounder, both bottom-feeding fish
contain about one-third of this, presumably because the PCB content of
benthic organisms is lower. In predatory fish such as the cod and
pike, the PCB level in extractable fat is about 10 mg/kg and a mean of
10 mg/kg has been measured in the blubber of seals.
Much higher PCB values have been obtained in fish-eating birds;
levels of 18 mg/kg (650 mg/kg in extractable fat), and 17 mg/kg
(420 mg/kg in extractable fat) were found in the herring gull and
comorant respectively. Lower values were found in birds feeding on
invertebrates, such as the long-tailed duck which contained 14 mg/kg
in fat; marine invertebrates contain PCB levels in the region of
0.1-0.2 mg/kg. At the top trophic level, 96 mg/kg (9.7 g/kg in
extractable fat) has been measured in the eagle owl; the highest
recorded PCB values were from eagle owls found dead in the south-east
coastal region of Sweden, 260 mg/kg in the brain (3.4 g/kg in
extractable fat) and 110 mg/kg in muscle (12 g/kg in extractable fat).
(Odsjö, 1973).
Less information is available on terrestrial ecosystems. PCB
concentrations in the region of 0.01 mg/kg have been found in fresh
tissue in slugs, snakes, and ants, and slightly higher concentrations
in earthworms. Tissue levels were generally at the limit of detection
(0.01 mg/kg) in herbivorous mammals (Odsjö, 1973). Bruggemann et al.
(1974) reported a mean PCB concentration of 0.22 mg/kg in 20 out of 72
measurements in the adipose tissues of the hare and a higher value
(2.5 mg/kg) in 1 out of 5 tests on the adipose tissues of the fox.
Higher values have been found in the American mink in Sweden with
0.58 mg/kg in muscle (45 mg/kg in fat) presumably because of a fish
diet (Odsjö, 1973). Tissue levels in wild birds on a mixed diet are
variable and rather low, but those in predatory birds are higher.
Prestt et al. (1970) related the PCB concentration in the liver of
wild birds to their diet; less than 1 mg/kg was found in insectivorous
birds and more than 70 mg/kg in the sparrow hawk. A level of 0.5 mg/kg
has been found in the muscle of the eagle owl in central Sweden, but
this is much less than the tissue concentrations encountered in this
bird in coastal areas (Odsjö, 1973). High tissue concentrations in
predatory and marine birds have also been reported from Canada
(Gilbertson & Reynolds, 1974), the Netherlands (Koeman et al., 1972a),
and from the United Kingdom (Bourne & Bogan, 1972).
5.4.4 Indicator organisms
Several of the organisms, that have been shown to accumulate-PCBs
from the environment, have been suggested as indicators of the extent
of local pollution with PCBs. In aquatic systems, the use of plankton
as an indicator has the advantage that it is at the lowest trophic
stage of food chains but errors may occur in the determination because
of the inclusion in the sample of nonplanktonic particles with a high
PCB content (Jensen et al., 1972a). The herring, which feeds on
plankton, has been suggested as an indicator (Jensen et al., 1972b)
and, at higher trophic stages, the pike, which is a stationary fish
(Olsson & Jensen, unpublished report, 1974), and seabirds (Jensen et
al., 1972c). In fresh waters, the amphipod Gammarus pulex has been
used as an indicator organism for chlorinated hydrocarbons (Södergren
et al., 1972). However, Zitko et. al. (1974) claimed that the
variation between individual fish was so high that Atlantic herring
and yellow perch could be used to detect trends in pollution only if
large numbers were taken for analysis, with an interval between
measurements of at least 4 years.
A series of monitoring studies has been made by OECD, the species
selected covering terrestrial, fresh water, and marine environments.
The analytical results, and the general problems of selecting species
for monitoring, have been discussed by Holden. (1970a, 1973a, 1973b).
5.5 The Extent of Human Exposure to PCBs and PCTs
5.5.1 Air and water
The maximum concentration of PCBs in air is not likely to exceed
50 ng/m3 (section 5.1). The highest concentration of PCBs reported in
domestic tap water is 100 ng/litre in the Kyoto area of Japan (Panel
on Hazardous Trace Substances, 1972), but levels more likely to be
encountered should not exceed 1 ng/litre (section 5.3).
5.5.2 Food
The PCB content of a variety of foods on the Swedish market been
measured by Westöö & Norén (1970a) and Westöö et al. Less than
0.1 mg/kg was found in samples of butter, margarine, oils, eggs, beef,
lamb, chicken, bread, biscuits, and baby of pork out of more than 100
had a PCB content in the range of 0.5 mg/kg. As might be expected from
the discussion in section 5.4 higher values were found in fish
depending on the fat content and pollution of the fishing area (Westöö
& Norén, 1970a; Berglund, 1972] The PCB levels obtained in an
extensive study by the US Food Administration are shown in Table 5.
These values are considerably higher than but they are probably
biased, as they include samples originating areas previously suspected
of having been subject to local pollution. In a Canadian survey, PCB
levels of less than 0.01 mg/kg were found in eggs (Mes et al., 1974)
and a mean of 0.042 mg/kg was found in domestic and imported cheese
with a maximum of 0.27 mg/kg (Villeneuve et al., 1973b). No traces of
PCTs were found.
In Japan, a similar range of PCB contents for most foods has been
reported; however, some high levels have been-reported for rice and
vegetables harvested in fields polluted with PCBs (Environmental
Sanitation Bureau, 1973). The PCB content of most fish on the market
was less than 3 mg/kg, although some contained more than this. The PCT
content of fish was much lower (Fukano et al., 1974). In the
Netherlands, eel has been reported to contain PCT levels of
0.2-0.5 mg/kg and PCB levels of 4.7 mg/kg (Freudenthal & Greve, 1973).
Table 5. PCB levels in food in the USAa
% positive Level in positive samples (mg/kg)
Food (0.1 mg/kg) Mean Maximum
Cheese 6 0.25 1.0
Milk 7 2.3 27.8
Eggs 29 0.55 3.7
Fish 54 1.87 35.3
a From Kolbye (1972).
Samples of butter from the Westphalian area of the Federal
Republic of Germany, obtained in the period 1972-74, contained PCB
levels of 0.38 mg/kg (range 0.25-0.54 mg/kg) (Claus & Acker, 1975).
Relatively high PCB levels in some packaged foods in Sweden,
mainly of imported origin, could be attributed to migration from the
packaging material (Westöö et al., 1971). The highest level
encountered was 11 mg/kg in a children's breakfast cereal; PCB levels
of 70 mg/kg and 700 mg/kg were found in the material of the inner bag
containing this product and in the outer cardboard container
respectively. Up to 2000 mg/kg was found in cartons of other samples.
Villeneuve et al. (1973a) have analysed packaged food in Canada; they
found that 66.7% of the samples contained PCB levels of less than
0.01 mg/kg, 30.7% contained between 0.01 and 1 mg/kg, and 2.6%
contained more than 1 mg/kg. PCT determinations were also made on
these samples; 94.5% of the samples contained less than 0.01 mg/kg and
5.5% contained 0.01-0.05 mg/kg. The highest PCB levels were
encountered in a rice sample with 2.1 mg/kg where the packaging
material contained 31 mg/kg, and in a dried fruit sample with
4.5 mg/kg in a container containing 76 mg/kg. In a survey of packaging
containers, approximately 80% were found to contain PCB and PCT levels
of less than 1 mg/kg, while about 4% contained levels higher than
10 mg/kg. The most likely source of PCBs in packaging materials is the
recycling of paper waste containing pressure-sensitive duplicating
paper (Masuda et al., 1972).
5.5.3 Occupational exposure
Occupational exposure does not only occur during the manufacture
of PCBs and with their use in the electrical industry. It may also be
widespread among mechanics in contact with lubricating oils and
hydraulic fluids, among workers exposed to varnishes and paints, and
among office workers from contact with pressure-sensitive duplicating
paper, some brands of which readily transfer PCBs to skin (Kuratsune &
Masuda, 1972b). Studies in Finland showed that whole blood from
persons with no special exposure to PCBs contained 0.3-1.2 µg/100 ml,
while blood from persons handling PCBs in an analytical laboratory
contained 3.66.3 µg/100 ml and blood from workers in a capacitor
factory had PCB levels of 7.5-190 µg/100 ml in the blood and
30-700 mg/kg in fat. No signs of toxicity were evident in these workers
(Karppanen & Kolho, 1973). Similar plasma values were found in workers
from Japanese capacitor factories, but here skin lesions were noted
(Hasegawa et al., 1972a). This same study reported that air levels of
PCBs of 0.01-0.05 mg/m3 were measured in a factory where KC-300 was
used in the manufacture of electric condensers. PCB levels in serum in
workers ranged from 10 to 65 µg/100 ml.a One month after the use of
PCBs had been suspended, serum levels still ranged from 9 to
74 µg/100 ml. However, in another factory making electric condensers, serum
levels decreased from an average of 80 µg/100 ml to 30 µg/100 ml
within three months of the use of PCBs being suspended (Kitamura et
al., 1973). According to Hara et al. (1974), the half-time of PCBs in
the blood of workers engaged in the manufacture of electric condensers
for less than 5 years was several months, while that of workers
employed for more than 10 years was 2-3 years. Hammer et al. (1972)
found a higher frequency of measurable plasma values in workers
working with refuse burners than in a control group.
a In this document, the concentrations of PCBs in blood and serum
are expressed in µg/100 ml although in some original papers the
values are given in µg/100 g. For practical purposes the
differences, about 5% and 3% respectively, can be neglected.
5.5.4 Other sources of exposure
Broadhurst (1972) has reviewed the many technical applications of
PCBs that appear in the literature and in patent specifications, and
which indicate the possibility of a widespread nonoccupational
low-level exposure to PCBs, other than that deriving from the diet.
PCBs are used in the home in ballast capacitors for fluorescent
lighting, and exposure deriving from pressure-sensitive copying paper
has not been limited to office workers. The valuable properties of
PCBs as plasticizers has led to their use in furnishings, interior
decoration, and building construction; examples are surface treatment
for textiles, adhesive for waterproof wall coatings, paints, and
sealant putties. PCBs have been used as plasticizers for plastic
materials and in the formulation of printing inks.
5.5.5 Biological indices of human exposure
The only surveys of value have been on body fat, blood, and milk.
5.5.5.1 Body fat
In a survey of 637 fat samples taken at autopsy or during surgery
in the USA, 68.9% contained PCB levels of less than 1 mg/kg, 25.9%
contained 1-2 mg/kg, and 5.2% contained more than 2 mg/kg (Yobs,
1972). A similar distribution was found in a smaller survey by Price &
Welch (1972). In the Kochi area of Japan, a mean PCB level of
2.86 mg/kg was recorded with an upper limit of 7.5 mg/kg; about double
these values were found in the Kyoto area (Nishimoto et al., 1972a,
1972b). Bjerk (1972) reported average PCB levels of 0.9 mg/kg
(1.6 mg/kg on a lipid basis) in adipose tissue, taken at 40 autopsies
in the Oslo area. Curley et al. (1973b) found PCB levels ranging from
0.30 to 1.48 mg/kg in a total of 241 human adipose samples in Japan. A
mean value of 5.7 mg/kg has been reported for 20 samples from the
Federal Republic of Germany (Acker & Schulte, 1970); in a more recent
study, 282 adipose tissue samples from different areas were found to
contain PCB levels of 8.3 mg/kg of adipose tissue on a lipid basis
(Acker & Schulte, 1974). In Austria, a range of PCB levels of
0.3-7.3 mg/kg on a lipid weight basis was found in 32 residents in the
Vienna metropolitan area; an increase of the PCB concentration with
age was not observed (Pesendorfer et al., 1973). Detectable levels of
PCBs were found in only a few of 201 human fat samples in the United
Kingdom and in these, the level did not exceed 1 mg/kg (Abbott et al.,
1972). A survey of 51 human fat samples in New Zealand showed that all
samples contained PCB residues with an average of 0.82 mg/kg (Solly &
Shanks, 1974).
Doguchi et al. (1974) found an average PCT level of 0.6 mg/kg in
human fat with a range of 0.1-2.1 mg/kg. Takizawa & Minagawa (1974)
also found PCT levels of 0.02 mg/kg in human liver ( n = 6),
0.01 mg/kg in the kidney ( n = 2), 0.02 mg/kg in the brain ( n = 3)
and 0.04 mg/kg in the pancreas ( n = 1). In the Netherlands, PCTs
were found in human fat at levels of 0-1 mg/kg (Freudenthal & Greve,
1973).
5.5.5.2 Blood
43% of blood plasma samples from 723 volunteers in the USA showed
the presence of PCBs; the mean value in these was about 0.5 µg/100 ml
(Finklea et al., 1972). Studies in Finland on whole blood showed
0.31-1.2 µg/100 ml in persons with no special exposure to PCBs
(Karppanen & Kolho, 1973). In Japan, an average PCB level of
0.32 µg/100 ml and a PCT level of 0.5 µg/100 ml have been recorded in
the blood of non-occupationally exposed volunteers (Doguchi & Fukano,
1975). Inpatients with severe weight loss, high levels of PCBs in the
blood (up to 10 µg/100 ml) were noted (Hesselberg & Scherr, 1974).
This was attributed to the release of PCBs from the mobilization of
fat.
5.5.5.3 Human milk
PCB concentrations measured in whole human milk in Sweden were
0.014 mg/litre in 1967 and 0.025 mg/litre in 1971-72 (Westöö & Norén,
1972), 0.03 mg/litre in Japan Nishimoto et al., 1972a), 0.103 in the
Federal Republic of Germany (Acker & Schulte, 1970) and 0.02 mg/litre
in Canada (Musial et al., 1974) A survey in Colorado, USA, revealed 8
positive samples out of 39, within the range of 0.04-0.1 mg/kg (Savage
et al., 1973).
5.5.6 Estimated daily intake
From the PCB levels encountered in air and drinking water (section
5.5.1), the daily intake from each of these sources is likely to be
less than 1 µg.
It has been stated that the major part of the human dietary intake
of PCBs is from fish (Berglund, 1972; Hammond, 1972). This may well be
true in areas such as Japan or certain localities near the North
American Great Lakes, where fish from polluted waters may form a
relatively large part of the diet. Several investigators from Japan
have measured the daily intake of PCBs in food; the highest mean value
recorded was 48 µg/day, of which 90% was from fish (Kobayashi, 1972);
the lowest was 8 µg/day (Ushio et al., 1974).
In much of Europe and North America, however, the daily intake of
fish is in the region of 30-40 g and most of the fish is taken from
waters of low pollution and contains PCB levels of not more than
0.1 mg/kg. Berglund (1972) has estimated that the daily intake of PCBs
from fish in Sweden is in the region of 1 µg, though if the fish
consumed were solely Baltic herring, the intake would be about 10 µg.
It is difficult to make an assessment of the PCB intake from foods
other than fish. Westöö et al. (1971, 1972), in their extensive study
of the Swedish diet, reported that most foods contained PCB levels of
less than 0.1 mg/kg; it may be concluded that this corresponds to a
daily intake of less than 100 µg. The conclusion that foods other than
fish may make a greater contribution to the PCB content of the diet
can be drawn from the survey in the USA reported by Kolbye (1972), and
also from a Swiss study of three different types of home-prepared
meals, not containing fish, which were found to contain 6, 41, and
84 µg of PCBs, respectively (Zimmerli & Marek, 1973).
The figures for the PCB content of human milk (section 5.5.5.3)
indicate that most nursing mothers excreted about 30 µg/day by this
route, and in some areas up to 100 µg/day. It may be assumed that only
a portion of the PCBs absorbed was excreted by this route, so that the
daily intake could have been more than 100 µg.
It may be concluded that, in the more industrialized countries,
the average daily PCB intake from the diet has rarely been less than
5 µg or greater than 100 µg; it is likely that the non-dietary sources
of exposure detailed in section 5.5.4 have made a significant
contribution, but this cannot be estimated at this time. In any area,
the intake depends not only on the diet, but also on social domestic
and environmental conditions; the influence of these factors on the
daily intake cannot readily be quantified.
6. METABOLISM
6.1 Absorption
The experiments of Vos & Beems (1971) who applied several
commercial PCBs to rabbit skin and found systemic effects (see section
7.1.1.4) indicate that PCBs can penetrate the skin. Early cases of
human poisoning from occupational exposure were probably due to a
combination of skin absorption and inhalation. Experimental studies on
rats by Benthe et al. (1972a) showed that an aerosol containing
Aroclor 1242 (particle size 0.5-3.0 µm) was readily absorbed through
the lungs.
Although one means of entry of PCBs into aquatic food chains is
through the consumption of plankton by fish, aquatic organisms can
also absorb PCBs in solution in the ambient water, presumably mainly
through the gills (see section 4.3). Salmon eggs can also absorb PCBs
from water (Johansson et al., 1970), and Södergren & Svensson 1973
concluded that mayfly nymphs could take in PCBs from water through the
gills and the integument.
Recent work with chlorobiphenyl isomers administered orally to
rodents at levels up 100 mg/kg of body weight for lower chlorinated
compounds and up to 5 mg/kg for the higher chlorinated compounds,
showed that 90% of the compounds were rapidly absorbed (Albro &
Fishbein, 1972; Berlin et al., 1973; Melvås & Brandt, 1973).
Cholestyramine, a basic anion exchange resin, was shown to interfere
with intestinal absorption of KC-400 in mice (Tanaka & Araki, 1974).
PCTs have been shown to be absorbed from the gut (Sosa-Lucero et
al., 1973) but very little information is available on the rate of
absorption.
6.2 Tissue Distribution of PCBs
Grant et al. (1971a) demonstrated that 4 days after an oral dose
of Aroclor 1254 at 500 mg/kg was given to rats, the concentrations of
PCBs in fat, liver, and brain were 996, 116, and 40 mg/kg,
respectively. Similar results showing that the highest concentration
was in fat, were obtained in rats given Aroclor 1254 in the diet
(Curley et al., 1971), in boars (Platonow et al, 1972), cows (Platonow
& Chen, 1973), and in pigeons and quail (Bailey & Bunyan, 1972). In
the experiments of Curley et al. (1971), the tissue concentrations
initially showed a rapid rise and thereafter a slow increase while the
PCB diet was being administered; Grant et al. (1974) fed diets
containing Aroclor 1254 at 0.2, 20, and 100 mg/kg to rats for 8
months, during which period the tissue concentrations reached a steady
state that was dose-dependent (Table 6). Similar tissue distribution
data for Aroclors 1016 and 1242 have been reported by Burse et al.
(1974) and for Kanechlor-400 by Yoshimura et al. (1971). PCB
deposition, in general, depends on the fat content of the tissue
(section 5.4.2). Residues in trout, receiving Aroclor 1254 in doses of
15 mg/kg in the diet, stabilized after 16 weeks while the absolute
quantity continued to increase as the fish grew (Lieb et al., 1974).
More detailed information on the tissue distribution of PCBs and
their metabolites has been obtained by the administration of pure
14C-labelled compounds, using both whole-body autoradiography and
scintillation counting of tissue samples. Berlin et al. (1975)
demonstrated that after a single oral dose of 14C-labelled
2,5,2',4',5'-pentachlorobiphenyl, radioactivity rapidly entered the
circulation of mice and was distributed in the tissues, particularly
in the liver, kidneys, lungs, and adrenals. Subsequently, the
radioactivity in the body fat increased, rising to a maximum within
4-24 h. In most other tissues the radioactivity decreased rapidly
after dosing, but the authors noted a special affinity for the skin,
the bronchiolar epithelium of the lungs, and certain glandular
secreting tissue. Soon after administration of the dose, radioactivity
appeared in bile and was excreted in the faeces. Similar results were
obtained by Melvås & Brandt (1973) with 2,4,2',4'-tetrachlorobiphenyl
in the mouse, and with 2,4,2',3'- and 2,4,3',4'-tetrachlorobiphenyls
in the quail; in the mouse they found a high affinity for the adrenal
cortex, the corpora lutea, and glandular secreting tissue, and in the
quail the radioactivity in egg yolk was high, exceeding that in fat.
Brandt & Ullberg (unpublished report 1973) found a similar pattern
after administration of hexa- and octachlorobiphenyls to mice.
6.3 Tissue Distribution of PCTs
Diets containing Aroclor 5460 at levels of 10, 100, and 1000 mg/kg
were administered to rats for 7 days (Sosa-Lucero et al., 1973). Table
7 shows the tissue distribution obtained in this study in rats fed
with Aroclor 5460 at 100 mg/kg body weight and the values in rats fed
with Aroclor 1254 at 100 mg/kg body weight in a similar study (Curley
et al., 1971). After oral administration of Aroclor 5460 to the cod,
the concentration of PCTs in the liver was more than 100 times that in
muscle on a wet weight basis (Addison et al., 1972), a ratio found by
Jensen et al. (1973) for PCBs in the cod.
Table 7. Tissue distribution (mg/kg wet weight) of PCTs (Aroclor
5460) in rats fed dietary levels of 100 mg/kg for 7 days
(Sosa-Lucero et al., 1 973) and fed PCB (Aroclor 1254) at
100 mg/kg for 9 days (Curley et al., 1971)
Aroclor Aroclor
Tissue 5460 1254
Blood 1.32 0.1
Liver 47 6
Brain 5.1 4
Kidneys 15.1 5
Heart 21.5 --
Fat -- 180
6.4 Placental Transport
Aroclors 1221 and 1254 were found to cross the placenta of
rabbits, when administered orally to does during gestation. The
concentration in fetal tissues was dose-dependent and much less with
Aroclor 1221 than with Aroclor 1254; with the latter, the
concentration in the fetal liver was greater than that in the maternal
liver (Grant et al., 1971b). Curley et al. (1973a) found some
placental transport of Aroclor 1254 in the rat. Platonow & Chen (1973)
demonstrated that the PCBs in the fetal kidney of a cow dosed with
Aroclor 1254 was greater than that in the mother. Placental transfer
of polychlorinated biphenyls has also been reported in the mouse
(Berlin et al., 1975; Melvås & Brandt, 1973; Brandt & Ullberg,
unpublished report 1973).
PCB concentration in human umbilical blood has been shown to be
about 25% of that in maternal blood (Taki et al., 1973). Placental
transfer of PCBs was observed in Yusho patients (Tsukamoto et al.,
1969).
No information is available on the placental transfer of PCTs.
6.5 Excretion and Elimination
6.5.1 Milk
Saschenbrecker et al. (1972) found that after oral administration
of doses of Aroclor 1254 of 10, and 100 mg/kg in diet to cows, 6.27
and 74.5 mg/litre, respectively, appeared in the milk after 24 hours.
These levels were reduced to less than one-half within 3 days but
traces remained at 50 days. Cows receiving 200 mg of Aroclor 1254
daily reached a steady state concentration of 61 mg/kg in milk fat and
42 mg/kg in body fat after 10 days (Fries et al., 1973). The PCBs in
milk survived processing into dairy products, and most was located in
milk fat (Platonow et al., 1971). PCBs have also been found in human
milk (see section 5.5.5.3).
6.5.2 Eggs
Several investigations have demonstrated the presence of high
levels of PCBs in the eggs of seabirds (Riseburgh & de Lappe, 1972).
An incident has been reported in which PCB contamination of poultry
food was toxic to chickens and decreased the hatchability of eggs
which also contained PCB residues (Pichirallo, 1971). In a laboratory
study, Scott et al. (1975) fed Aroclor 1248 to chickens at dietary
concentrations of 0, 0.5, 1.0, 10, and 20 mg/kg. After 8 weeks, the
approximate PCB levels in eggs were 0, 0.22, 0.41, 3.1 and 7.0 mg/kg
respectively. Jensen & Sundström (1974) found 33% of ah oral dose of
2,4,5,T,4',5'-hexa-chlorobiphenyl administered to quails was excreted
in eggs over a period of 10 days; eggs also provided a major route of
excretion in the pheasant (Dahlgren et al., 1971).
6.5.3 Urine and faeces
All investigators agree that only traces of PCBs can be found in
the urine of dosed animals, and that faeces provide a major route of
elimination. When the analysis of faeces is limited to the
determination of unchanged PCBs, the recovery of the dose administered
is incomplete; in boars receiving single or repeated doses of Aroclor
1254, not more than 16% of the dose was recovered from the faeces and
less than 1% in urine (Platonow et al., 1972). Better recoveries have
been obtained with PCB labelled with radioactive isotopes. Yoshimura
et al. (1971) found 70% of the activity from a dose of tritium-
labelled Kanechlor 400 in faeces and 2% in urine over a 4-week period.
Berlin et al. (1974, 1975) found over 75% of the activity from
14C-labelled penta- and hexachlorobiphenyls in faeces and less than
2% in urine; most of the faecal excretion consisted of PCB metabolites
(see section 6.6.1). Similar results were obtained by Melvås & Brandt
(1973) with tetrachlorobiphenyls.
6.6 Biotransformation
6.6.1 Metabolic degradation
Most investigators studying the tissue distribution of PCBs after
administration of commercial mixtures have noted a relative reduction
of the gas-liquid chromatographic peaks with shorter retention times,
corresponding to the lower chlorinated biphenyls. This has been
reported in the rat (Grant et al., 1971a; Curley et al., 1971), the
rabbit (Grant et al, 1971b), the cow (Platonow & Chen, 1973), and in
pigeons and quails (Koeman et al., 1969; Bailey & Bunyan, 1972).
Samples of tissues from animals and man (see table 3, pp. 20-21) that
have absorbed PCBs from the environment have shown, on analysis, a
pattern of peaks approaching that of PCB mixtures with more than 50%
chlorination, although the major manufactured products contain 42% of
chlorine or less. This has led to the belief that the rate of
metabolic attack on PCBs decreases with increasing chlorination.
Studies on single PCBs with 1, 2, 4, or 5 chlorine atoms have shown
that these are more readily excreted as metabolites in faeces by
mammals and birds and remain for a shorter time in fatty tissues than
most PCBs with 6 or more chlorine atoms (Berlin et al., 1975;
Hutzinger et al., 1972a; Melvås & Brandt, 1973; Brandt & Ullberg,
unpublished report 1973). See also section 6.6.2.
The administration to rats of diets containing Aroclors 1016 or
1242 at a concentration of 100 mg/kg resulted in a steady state in
adipose tissue for both compounds in about 4 months. When gas
chromatographic traces were compared with those for standard PCB
mixtures a difference in the gas-liquid chromatographic pattern, that
is disappearance of the peaks with short retention times was noted.
After exposure to PCBs was discontinued, a major portion of the PCBs
was eliminated from the body in 4 months. However, 20% of the total
PCBs in Aroclor 1242 with longer retention times were present after 6
months, and 10% of the total PCBs of Aroclor 1016 with longer
retention times were present in adipose tissue after 5 months. (Burse
et al., 1974).
The excretion of monohydroxy metabolites of 3,4,3',4'-tetrachloro-
biphenyl and 2,4,3',4'-tetrachlorobiphenyl (orally administered) in
rats has been demonstrated by Yoshimura & Yamamoto (1973). Yoshimura
et al. (1973), Yamamoto & Yoshimura (1973), Yoshimura & Yamamoto
(1974), and Yoshimura et al. (1974). They demonstrated that the
metabolites of the first isomer were 2-hydroxy or 5-hydroxy compounds
while the metabolites of the second isomer were 5-hydroxy and
3-hydroxy compounds. All hydroxy metabolites were excreted
non-conjugated via the bile and no parent isomers were found in the
bile. Yoshimura & Yamamoto (1975) found that unchanged
2,4,3',4'-tetrachlorobiphenyl was excreted through the intestine, when
it was intravenously injected in rats with the bile duct ligated,
while no metabolite of this isomer was excreted by this route.
Hutzinger et al. (1972a) demonstrated the presence of hydroxylated
derivatives of mono-, di-, and tetrachlorobiphenyls in the excreta of
rats and pigeons but not of trout and were unable to detect
hydroxylated 2,4,5,2',4',5'-hexachlorobiphenyl. Berlin et al. (1975)
isolated a hydroxy derivative of 2,5,2',4',5'-pentachlorobiphenyl from
mouse faeces, and Jensen & Sundström (1975) have shown that although
2,4,5,2',4',5'-hexachlorobiphenyl is excreted very slowly, a hydroxy
derivative could be detected in rat faeces. Hutzinger et al. (1974),
however, showed that this compound was also dechlorinated by the
rabbit and excreted as the hydroxy derivative of pentachlorobiphenyl.
Gardner et al. (1973) detected hydroxylated metabolites in the urine
of rabbits dosed with 2,5,2',5'-tetrachlorobiphenyl together with a
dihydroxy derivative which they regarded as evidence for the formation
of an arene oxide (epoxide) intermediate (see section 6.5.3). Jansson
et al. (1975) have identified up to 26 mono- and dihydroxylated
metabolites of PCB in the bile and faeces of wild grey seal and
guillemot from the Baltic.
There is little information on the biotransformation of PCTs.
Addison et al. (1972), using gas-liquid chromatography, noted a loss
of PCTs with a shorter retention time in the excreta of a cod dosed
orally with Aroclor 5460; the same loss was observed in rat faeces
after the administration of a diet containing Aroclor 5460
(Sosa-Lucero et al., 1973).
6.6.2 The effect of structure on retention
While each of the components of mixtures of PCBs has a different
pattern of retention and elimination in different species, the
measurement of biological half-times for PCB mixtures in tissues has
provided useful information. Bailey & Bunyan (1972) found the
half-time of PCBs in the fat of quail and pigeon, after cessation of
dosing with Aroclor 1242, or with Aroclor 1254, to be 50 days and 125
days, respectively. A half-time of about 200 days was recorded in the
fat of rats after feeding with Aroclor 1254 (Grant et al., 1974).
Berlin et al. (1975) noted that in mice dosed with a
pentachlorobiphenyl there was an initial fairly rapid elimination
while the PCB level in the liver was high, followed by a slower
elimination when most of the PCB was located in fat. It seems likely
that the mobilization of PCBs from fat, and therefore their half-time
in the body, depends upon their rates of metabolism. Berlin et al.
(1974) investigated the hypothesis that the ability of a PCB to be
readily degraded with a half-time of a few days depended upon the
presence of two adjacent unsubstituted carbon atoms in the molecule
rather than on the number of chlorine atoms, although the presence of
such unsubstituted pairs depends to a large extent on the degree of
chlorination. They came to the conclusion that this hypothesis
probably applied to unsubstituted pairs in the 3,4-position, but that
in the 2,3-position, their susceptibility to metabolic degradation was
much influenced by the presence of chlorines in the o-position of
the ring bridge.
Jensen & Sundström (1974) demonstrated that the retention of PCBs
in human fat is also influenced by o-chlorine substitution (Table 3,
pp. 20-21 ).
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF PCBs AND PCTs
7.1 Toxic Effects in Different Species
Most of the available information on the toxicity of the PCBs has
been obtained from studies on commercial mixtures. The much smaller
amount of information available concerning the impurities in
commercial products and PCTs, is given in sections 7.2 and 7.3.
7.1.1 Mammals
7.1.1.1 Acute oral and intravenous toxicity
Earlier work by the Monsanto Company indicated a low acute oral
toxicity for the Aroclors, the LD50s to rats ranging from 4.0 g/kg
for Aroclor 1221 to 11.3 g/kg for Aroclor 1262 (Panel on Hazardous
Trace Substances, 1972). More recent work has demonstrated a slightly
higher toxicity (Bruckner et al., 1973; Grant & Philips, 1974). With a
single intravenous dose the LD50 for Aroclor 1254 was 358 mg/kg body
weight in adult female Sherman rats (Linder et al., 1974). The acute
oral LD50 for Aroclor 1254 in the same strain and sex was between 4
and 10 g/kg (Kimbrough et al., 1972). According to Bruckner et al.,
(1973), severely poisoned animals showed weight loss, ataxia,
diarrhoea, and chromodacryorrhoea, and they considered progressive
dehydration and central nervous depression were the causes of death.
In rats, vacuolation in the liver and kidneys was observed (Bruckner
et al., 1973) and also ulceration of the gastric and duodenal mucosa
(Kimbrough et al., 1972).
Yamamoto & Yoshimura (1973) found the intraperitoneal LD50 of
2,4,3'4'-tetrachlorobiphenyl in mice to be 2.15 g/kg body weight and
that of the 5-hydroxy derivative, which is the main in vivo
metabolite, to be 0.43 g/kg body weight.
7.1.1.2 Subacute oral toxicity
After repeated administration, the PCBs have a cumulative toxic
action. In a group of rats receiving Aroclor 1254 at a dose of 1 g/kg
of diet, deaths occurred between the 28th and 53rd days of feeding
(Tucker & Crabtree, 1970), and with Phenochlor DP6 at 2 g/kg of diet,
deaths occurred between the 12th and 26th days (Vos & Koeman, 1970).
In the latter experiment enlarged livers, small spleens, and a
progressive chemically-induced hepatic porphyria were seen at autopsy.
Repeated weekly oral administration of 150 mg of Aroclors 1221, 1242
or 1254 to rabbits for 14 weeks produced liver enlargement and damage
with Aroclor 1242 and no effect with Aroclor 1221 (Koller & Zinkl,
1973). Allen et al. (1974) administered diets containing Aroclor 1248
at the concentration of 25 mg/kg of diet to 6 female rhesus monkeys
for two months; facial oedema, loss of hair, and acne developed after
1 month and one animal died with severe gastritis 2 months after
removal from experimental diet. PCB concentrations in the body fat of
the animals, after two months of treatment, averaged 127 mg/kg while 8
months later the value declined to 34-mg/kg.
Mink appear to be unusually sensitive to PCBs. Aulerich et al.
(1973) administered diets containing PCB levels of 30 mg/kg (10 mg/kg
each of Aroclors 1242, 1248, and 1254) to adult mink and demonstrated
100% mortality within 6 months. PCB residues in the brains of these
mink averaged about 11 mg/kg and were approximately twice the level
observed in other tissues. Four months of feeding Aroclor 1254 at
levels of 5 and 10 mg/kg demonstrated a dose-dependent retardation of
weight gain of growing female mink. Female mink fed a diet
supplemented with Aroclor 1254 at 5 mg/kg for 9 months failed to
produce offspring (Ringer et al., 1972).
7.1.1.3 Chronic oral toxicity
Aroclors 1242, 1254, and 1260 have been administered for 18 months
to rats at 1, 10 and 100 mg/kg in the diet (Keplinger et al., 1971).
No adverse effects were recorded with the three Aroclors at 10 mg/kg
but with Aroclors 1242 and 1254 at 100 mg/kg there was an increase in
liver weight and a reduced survival of litters. In similar experiments
on dogs, there was a reduced weight gain with Aroclors 1254 and 1260
in the diet at a level of 100 mg/kg (Keplinger et al., 1971). In the
experiments reported by Kimbrough et al. (1972), male rats survived
Aroclor 1260 in the diet at 1 g/kg (71.4 mg/kg body weight) for 8
months but 8/10 females died at this dose, 2/10 died at 500 mg/kg, and
1/10 at 100 mg/kg (7.2 mg/kg body weight). With Aroclors 1254 and
1260, a dose-dependent increase in liver weight in male rats was
significant down to 20 mg/kg (1.4 mg/kg body weight) in the diet; with
females the liver enlargement occurred only at diet levels of
500 mg/kg and higher. The livers showed an orange fluorescence, the
cells were enlarged and vacuolated with lipid inclusions; there was
also much increased smooth endoplasmic reticulum, and what was termed
"adenofibrosis" was present, being more marked with Aroclor 1254 (see
section 7.8). Grant et al. (1974) fed diets containing Aroclor 1254 at
0, 2, 20, and 100 mg/kg to rats for 246 days followed by 180 days on a
PCB-free diet; after 246 days the body weight of the 100 mg/kg rats
was significantly less than that of the controls and the liver weight
was greater. The most notable change in the livers on histological
examination was the appearance of fat microdroplets in the
centrilobular region; this effect was dose-dependent and not seen in
the rats receiving the 2 mg/kg diet and was reversible when the rats
were returned to a normal diet.
Liver enlargement has been described by a number of authors, in a
number of species, with different PCB mixtures and pure isomers and it
is considered to be due primarily to hypertrophy of the smooth
endoplasmic reticulum of the liver cells (Vos & Beems, 1971; Allen et
al., 1973; Kimbrough, 1974; Nishizumi, 1970) but with large enough
doses (oral intubation of about 150 mg/kg body weight/week for 14
weeks) of Aroclor 1254 and 1242, it progressed to frank liver damage
(Koller & Zinkl, 1973). The smooth endoplasmic reticulum may condense
in the liver cell and form hyalin inclusions and this may be
accompanied by a loss of enzyme activity. Lipid accumulation, pigment
deposition, nuclear changes, and necrosis may also occur (Vos &
Notenboom-Ram, 1972).
The rhesus monkey is the only species reported to show signs of
poisoning similar to those in human Yusho patients (see section 8).
The administration of Aroclor 1248 at 2.5 and 5.0 mg/kg of diet for 1
year produced periorbital oedema, alopecia, erythema, and acneiform
lesions involving the face and neck within 1-2 months. The effects
were less marked in male monkeys. At 25 mg/kg of diet, one out of a
group of six died, and at 100 and 300 mg/kg the mortality approached
100% within 2-3 months. Animals more severely affected showed
hypertrophic hyperplastic gastritis with ulceration, anaemia,
hyperproteinaemia and bone marrow hypoplasia. The survivors still
showed signs of poisoning 8 months after exposure (Allen & Norback,
1973; Allen et al., 1974; Allen 1975).
7.1.1.4 Dermal toxicity
Vos & Beems (1971) have confirmed earlier reports that PCBs damage
the follicular epithelium in experimental animals. They applied three
commercial 60% chlorinated mixtures, Clophen A60, Phenochlor DP6, and
Aroclor 1260 to rabbit skin at a daily dose of 118 mg/50 cm (5 times
per week) for 38 days. After initial reddening, transverse wrinkling
developed with hyperplasia and hyperkeratosis of the epidermal and
follicular epithelium. These effects were more marked with Clophen and
Phenochlor than with the Aroclor.
During these experiments, deaths occurred in the Clophen- and
Phenochlor-treated groups but not in the Aroclor group. Kidney lesions
were seen in all groups; liver damage was least in the Aroclor group.
There was atrophy of the thymus cortex and a reduction of germinal
centres of the lymph nodes as well as lymphopenia, and some animals in
all groups showed oedema of the abdominal and thoracic cavities,
subcutaneous tissue, and pericardium. Faecal excretion of copro- and
protoporphyrins was increased by all three PCBs but was lowest with
Aroclor 1260. Vos & Beems attributed the greater severity of the
effects observed with Clophen and Phenochlor to the presence of toxic
impurities (see section 7.2). In another comparative dermal toxicity
study in rabbits (Vos & Notenboom-Ram, 1972), skin lesions in Aroclor
1260-treated animals were more severe than in animals treated with
2,4,5,2',4',5'-hexachlorobiphenyl. In a Japanese study, no differences
in skin lesions were observed in rabbits after dermal applications of
Kanechlor 400, Kanechlor 500, or 3,4,3',4'-tetrachlorobiphenyl
(Komatsu & Kikuchi, 1972).
7.1.1.5 Inhalation toxicity
Only one inhalation study has been reported using Aroclor 1242 and
1254 (Treon et al., 1956). Rats, mice, rabbits, and guinea pigs were
exposed to Aroclor 1242 or 1254 vapours for five days a week for
several weeks at concentrations ranging from 1.5 to 8.6 mg/m3. At
these concentrations Aroclor 1254 produced liver enlargement in rats.
7.1.2 Birds
The acute oral toxicity of Aroclors 1242 and 1254 is low for the
mallard duck, the LD50s being greater than 2 g/kg body weight (Tucker
& Crabtree, 1970). Published figures for the lethal dose for birds
after repeated administration are very variable; it appears to be
dependent on the species and age of the bird, the method of
administration, the degree of chlorination of the PCBs, and the
presence of impurities (Vos, 1972). Heath et al. (1970) determined the
dietary concentrations of Aroclors 1232 to 1262, administered over a
5-day period, that were required to kill 50% of groups of mallards,
pheasants, and quails. The concentrations were in the range of 500 to
over 5000 mg/kg, with the bobwhite quail the most sensitive and the
Japanese quail the least. There was a positive relationship between
the percentage of chlorine in a technical Aroclor and its toxicity.
The relationship held true for those containing less than 60%
chlorine, Aroclor 1260 and 1262 deviating slightly. Mallards were
relatively less responsive to chlorine content than the three
gallimaceous species. Prestt et al. (1970) found a 50% mortality in
Bengalese finches receiving a daily oral dose of Aroclor 1254 at
254 mg/kg body weight for 56 days; cormorants have been killed with a
cumulative dose of 5.7 g of Clophen A60, but herons were more
resistant (Koeman et al., 1973).
The toxicity to chickens of diets containing Phenochlor DP6,
Clophen A60, or Aroclor 1260 at 400 mg/kg has been studied by Vos &
Koeman (1970). The first two produced a 100% mortality with survival
times of 24.3 and 20.5 days, but there was only 15%o mortality with
the Aroclor over the 60-day test period. The authors demonstrated the
presence of impurities in the Phenochlor and Clophen (Vos et al.,
1970). The results of Flick et al. (1965) and Platonow & Funnel (1971)
indicate that chickens can survive 200 mg/kg in the diet for several
months, but that deaths may occur at 250 mg/kg, though Rehfeld et al.
(1971) found a much higher toxicity, recording 1/30 deaths with
Aroclor 1248 at 30 mg/kg of diet over 25 days and 16/30 at 50 mg/kg.
Keplinger et al. (1971) found decreased growth in chickens receiving
Aroclor 1242 at 10 mg/kg and Aroclor 1254 at 100 mg/kg of diet, but no
effects with Aroclor 1260 at 100 mg/kg. Birds severely poisoned with
PCBs show tremors, ataxia, and ruffling and loss of feathers. Oedema
of the abdominal and peritoneal cavities has been a characteristic
sign at autopsy in some experiments (Flick et al., 1965) and this was
observed in a very large number of chickens killed in Japan by the
contamination of their feed with Kanechlor KC400 (Kohanawa et al.,
1969a, 1969b). Enlargement of the kidneys and sometimes of the liver
has been reported; some authors have noted a reduction in the size of
the spleen, comb, and testes, an enlargement of the adrenals and
thyroid, and a pale pancreas. No pathological signs were observed by
Dahlgren et al. (1972) that could account for the deaths of pheasants,
which occurred with a cumulative intake of about 900 mg of Aroclor
1254 (daily oral administration of 20 or 200 mg to 11-week-old hens);
they found a mean concentration of 520 mg/kg in brain tissue at death
and it is possible that effects in the central nervous system were a
contributory cause.
7.1.3 Aquatic organisms
7.1.3.1 Fish
Stalling & Mayer (1972) report an oral LD50 of more than 1.5 g/kg
for rainbow trout with Aroclors 1242 and 1260. A 15 mg/kg oral dose to
cod affected their ability to maintain an upright position in rotating
water (Lindahl, unpublished report 1974).
The assessment of toxicity to fish by adding PCBs to aquarium
water is subject to considerable error on account of the different
solubilities of the components. Zitko (1970) claims the aqueous
solubility of Aroclor 1221 to be 3.8-5 mg/litre and that of Aroclor
1254 to be 0.3-0.5 mg/litre. Stalling & Mayer (1972) report the
96-hour LC50s on the cut-throat trout to be 1.17 mg/litre for Aroclor
1221 and up to 60 mg/litre for Aroclor 1260, where the solubility is
clearly exceeded. In the more prolonged experiments of these authors
(Table 8), most of the concentrations are within the solubility limits
and therefore more reliable. In more prolonged experiments, Hansen et
al. (1971) found deaths in fish exposed for up to 45 days to Aroclor
1254 at 5 µg/litre, but none at 1 µg/litre. These results show that
the effect of PCBs is cumulative in fish and that the toxicity
decreases with increasing chlorination.
Table 8. Intermittent-flow bioassays of Aroclors against three species of fisha
LC50 (µg/litre)
5 10 15 20 25 30
Aroclor Species days days days days days days
1254 Rainbow trout 156 8 -- -- -- --
1260 -- 240 94 21 -- --
DDT 2.26 0.87 0.26 -- -- --
1242 Bluegills 154 72 54 -- -- --
248 307 160 76 10 -- --
1254 -- 443 204 135 54 --
1260 -- -- -- 245 212 151
1242 Channel catfish -- 174 107 -- -- --
1248 -- 225 127 -- -- --
1254 -- __ 741 300 113 --
1260 -- -- -- 296 166 137
1248b Bluegills 137 76 -- -- -- --
1248c Channel catfish -- 94 57 -- -- --
a From Stalling & Mayer (1972).
b Temperature, 20°C; alkalinity 260 mg/litre pH 7.4.
c Temperature, 27°C.
Young fish appear to be more sensitive to PCBs than adults,
96-hour LC50s for newly hatched fathead minnows were 15 and
8 µg/litre, respectively for Aroclors 1242 and 1254. Growth of young
fathead minnows and flagfish was affected above 2.2 µg/litre (Nebeker
et al., 1974). There is no information on pathological changes in fish
that might be related to the lethal action of PCBs; Hansen et al.
(1971) found that fish survived exposure to Aroclor 1254 at
5 µg/litre, but died later, after being returned to clean water, with
signs of a lowered resistance to infection. Pathological changes were
observed in kidney, spleen, and liver of rainbow trout fed Aroclor
1254 at 10 or 100 mg/kg of diet for up to 330 days (Nestel & Budd,
1975).
7.1.3.2 Aquatic invertebrates
The results of several investigations into the toxicity of PCBs to
aquatic invertebrates have been reported by Stalling & Mayer (1972).
The exposure of a variety of scud (Gammarus pseudolimnaeus) to PCBs
showed a decreased toxicity with increasing chlorination; 4-day LC50s
of 10, 52, and 2400 µg/litre were obtained with Aroclors 1242, 1248
and 1254, respectively. The threshold for survival, growth, and
reproduction of Gammarus pseudolimnaeus exposed to Aroclor 1248 was
about 5 µg/litre and was the same for Daphnia magna. With the
crayfish, 7-day LC50s were 30 µg/litre (Aroclor 1242) and 80 µg/litre
(Aroclor 1254) and with the glass shrimp, 3 µg/litre (Aroclor 1254).
Wildish (1970) found that mortality in Gammarus oceanicus was
dependent on the duration of exposure to Aroclor 1254 at levels above
10 µg/litre. Stalling & Mayer (1972) reported the 15-day LC50 of
Aroclor 1254 for immature pink shrimp to be 0.94 µg/litre.
7.1.3.3 Microorganisms
The growth of certain marine diatoms is inhibited by Aroclor 1254
at 10-25 µg/litre, but marine and freshwater algae are more resistant,
being unaffected by 100 µg/litre (Mosser et al., 1972). Fisher et al.
(1972) found that phytoplankton from the Sargasso Sea did not grow in
Aroclor 1254 at 10 µg/litre, though phytoplankton from estuarine and
coastal waters were not much affected by this concentration. Keil et
al. (1971) report that the growth of a diatom was inhibited by Aroclor
1242 at 100 µg/litre with a reduction of RNA synthesis, but that
10 µg/litre had no effect.
The growth of cultures of lake bacteria was not inhibited by
concentrations of Aroclors 1221, 1242, and 1254 in excess of
solubility, and Aroclors 1221 and 1242 could be utilized as the sole
source of carbon and energy (Wong & Kaiser, 1975).
7.2 Toxicity of Impurities in Commercial PCBs
Vos & Koeman (1970) observed that Phenochlor DP6 and Clophen A60
were more toxic to chickens than was Aroclor 1260 (see section 7.1.2)
and Vos & Beems (1971) showed a similar difference in the dermal
toxicity to the rabbit (see section 7.1.1.4). Vos et al. (1970)
subdivided Clophen A60 and Phenochlor DP6 into non-polar PCBs and
polar fractions and found polar components that were not detectable in
a similar fraction from Aroclor 1260. Phenoclor DP6, Clophen A60, and
the polar fraction from Clophen A60 produced a high mortality in the
chick embryo test but the polar fraction from Aroclor 1260 did not. A
difference between the three fractions was seen in the development of
skin lesions in the rabbit (Vos & Beems, 1971). Mass spectrographic
analysis indicated that the impurities were tetra- and pentachloro-
dibenzofurans. Additional contaminants were chlorinated naphthalenes.
Vos et al. (1970) calculated that the maximum level of chlorinated
dibenzofurans in Clophen A60 was 5 mg/kg and in Phenochlor DP6,
20 mg/kg. They calculated that the chlorinated dibenzofurans were
approximately one order of magnitude less toxic than the chlorinated
benzodioxins, and considered that they were mainly responsible for the
toxicity of the polar fraction and for the difference in toxicity
between the three commercial PCB mixtures.
Recently, chlorinated dibenzofurans were detected in the
PCB-contaminated oil that was responsible for the Yusho disease in
Japan (see section 8, p. 65).
In 1961, Bauer et al. demonstrated the toxicity of a mixture of
tri- and tetrachlorodibenzofurans; a single oral dose of 0.5-1.0 mg/kg
body weight caused severe and often lethal liver necrosis in rabbits.
Application to the rabbit ear resulted in hyperplasia and
hyperkeratosis. Similar toxic effects were found with 2,3,7,8-tetra-
chlorodibenzo- p-dioxin in doses that were 10 times lower than those
found to be toxic in the case of chlorinated dibenzofurans. See also
the review by Kimbrough (1974). 2,3,7,8-Tetrachlorodibenzofuran, which
has recently been shown to be present in PCBs (Bowes et al., 1975)
caused mortality in chickens after 8-15 days when they were dosed
orally with 5 µg/kg/day. A single oral dose of 4000 µg/kg was not
lethal to mice. Porphyria was not observed in chicks or mice
(Goldstein et al., 1975a). In comparison, 8 out of 10 chicks died
after 9-19 days when dosed orally with 2,3,7,8-tetrachloro-
dibenzo- p-dioxin at 1 µg/kg body weight/day (Schwetz et al., 1973).
The oral LD50 of 2,3,7,8-tetrachlorodibenzofuran in guinea pigs is
approximately 7 µg/kg body weight, which is less than one order of
magnitude higher than that of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(Moore, 1975).
Using low resolution mass spectrometry, McKinney (1975) found
metabolites in the excreta of chickens fed 2,4,6,2',4',6'-hexa-
chlorobiphenyl which had correct masses for chlorodibenzofurans. In
addition, by perchlorination, he identified octachlorodibenzofuran
again by low resolution mass spectrometry. The 2,4,6,2',4',6'-hexa-
chlorobiphenyl isomer is not found in commercial mixtures.
Zitko et al. (1972b) looked for the presence of chlorinated
dibenzofurans in fish and fish products taken from a contaminated
coastal area. The samples included shark tissues, cormorant and
herring gull eggs, herring oil, and herring fishmeal. The detection
limit of the method was 0.01-0.02 mg/kg in the sample. No chlorinated
dibenzofurans were detected, although the authors admit that the limit
of detection was not sufficiently low to detect amounts that might
exert a significant toxic action. Curley et al. (1975) detected a
component which had a 4 Cl isotopic cluster and a mass number of 304
corresponding with that of tetrachlorodibenzofuran in the urine of
rats dosed with PCBs, but a positive identification was not made.
7.3 Toxicity of the PCTs
There have not been any systematic studies on the toxicity of the
PCTs. Sosa-Lucero et al. (1973) administered diets containing Aroclor
5460 at 0, 10, 100, and 1000 mg/kg to groups of rats for seven days.
There were no adverse effects on health or body weight; a significant
liver enlargement was recorded at the 1000 mg/kg level. In a test for
estrogenic activity involving the stimulation of glycogen response in
the immature rat uterus, Aroclor 5460 was inactive, as was Aroclor
1260 but Aroclor 5442 was more active than Aroclor 1242 (Bitman et
al., 1972). A dietary level of Aroclor 5460 of 5000 mg/kg for 12 weeks
caused decreased body weight and increased liver weight in rhesus
monkeys (Allen & Norback, 1973); after 6 weeks, facial oedema, hair
loss, and eye discharge were observed as described by the same authors
in experiments with Aroclor 1248 (section 7.1.1.2), and similar
gastric changes were also reported.
7.4 Biochemical Effects
7.4.1 Induction of enzymes
Several investigators have observed an increase in the smooth
endoplasmic reticulum of liver cells after administration of PCBs (see
section 7.1.1.3). This is accompanied by an induction of microsomal
mixed function oxidase. Induction of microsomal enzyme activity is,
like liver enlargement, more marked with the higher chlorinated PCBs
and relatively low with Aroclors 1221 and 1016 (Villeneuve et al.,
1971a, 1972; Bickers et al., 1972; Ecobichon & Corneau, 1974). The
effect has also been demonstrated with single pure PCBs administered
orally (Fujita et al., 1971); more recently this work has been
confirmed by Johnstone et al. (1974) whose results are summarized in
Table 9 and show a greater degree of enzyme induction with the higher
chlorinated compounds.
Grant et al. (1974) found an increase in microsomal enzyme
activity in rats receiving Aroclor 1254 at 20 mg/kg of diet for 246
days, but none at 2 mg/kg. Iverson et al. (1975) and Goldstein et al.
(1975) compared the microsomal enzyme inducing potential of Aroclors
1242 and 1016. Iverson et al. (1975) found increased hepatic
microsomal enzyme activity with both Aroclors in male rats receiving
21 daily oral doses of 1 mg/kg body weight, and with Aroclor 1242 in
females at 10 mg/kg of body weight and with Aroclor 1016 at 100 mg/kg.
PCBs administered to rats during pregnancy can induce microsomal
enzyme activity in the placenta and fetus and this also occurs in the
liver of newborn rats suckled by mothers fed with diets containing
PCBs (Alvares & Kappas, 1975). Benthe et al. (1972b) reported that
when rats were stressed by food deprivation or cold, the PCB residues
in adipose tissue were released during mobilization of the fat and
caused increased hepatic microsomal enzyme activity.
Table 9. Stimulation of microsomal enzyme activity by single chlorinated biphenyls
Hepatic microsomal enzyme activity
Chlorine aniline
substituents O-demethylation N-demethylation hydroxylation nitro-reduction
4 0 0 0 0
2,2' 0 + + 0
2,4' 0 0 + 0
4,4' + + + + + + +
2,5,2',5' 0 + + + +
2,4,2',4' + + + + + + 0
2,4,5,2',4',5' + + + + + + + +
2,3,5,2',3',5' + + + + + + +
2,4,6,2',4',6' + + + + + + + +
2,3,4,5,2',3',4',5' + + + + + + + +
a Johnstone et al. (1974).
0 no activity.
+ slight activity.
+ + marked activity.
7.4.2 Porphyria
Hepatic porphyria has been induced by a number of PCBs (Clophen
A60, Phenoclor DP6, Aroclors 1016, 1242, 1254, and 1260) in the
chicken, rabbit, Japanese quail, and the rat (Vos & Koeman 1970; Vos &
Beems, 1971; Iverson et al., 1975; Vos et al., 1971; Goldstein et al.,
1974, 1975a, 1975b). Porphyrin induction has been studied more
extensively in rats. A dose-dependent increase in liver porphyrins has
been observed in females receiving 21 daily oral doses of Aroclor 1242
at 10 and 100 mg/kg of body weight, but not at 1 mg/kg. Female rats
were more sensitive than males, and Aroclor 1016 had less effect
(Iverson et al., 1975). In rats receiving Aroclor 1254 at 100 mg/kg in
the diet, the increase was usually delayed 2-4 months after the start
of dosing (Goldstein, 1974) and was characterized by high hepatic and
urinary levels of uroporphyrin. Disturbance of porphyrin biosynthesis
had been connected with an increase of the rate limiting enzyme
2.3.1.37-delta-aminolaevulinate synthase (Vos et al., 1971; Goldstein
1974, 1975).
Kawanishi et al. (1973, 1974) have shown that the administration,
in the diet, of Kanechlors KC-300 and KC-500 to rats at 500 mg/kg
produced a marked increase in urinary excretion of copro- and
uroporphyrins, and in faecal excretion of protoporphyrin, but no
increase was observed with KC-400. Experimental administration of pure
tetrachlorobiphenyls did not produce porphyria. However, porphyria did
result from the repeated subcutaneous injection of KC400 (total dose
1.8 g) to rabbits for 55 days (Miura et al., 1973).
7.4.3 Effects on steroid metabolism
PCBs have been shown to stimulate the activity of enzymes
responsible for metabolizing steroids such as estrodiol and
androsterone more effectively than does DDT or DDE (Risebrough et al.,
1968; Lincer & Peakall, 1970). It has been suggested that effects on
reproduction (see section 7.4) may be attributed to the induction of
steroid-metabolizing enzymes (Kihlström et al., 1973). Long-term
administration of daily oral doses of 0.025 mg Clophen A60 to 23
female NMRI strain mice caused a lengthening of the estrous cycle and
a reduction in the frequency of implantation of ova Orberg &
Kihlström, 1973).
7.4.4 Other biochemical effects
Hepatic vitamin A has been reported by Villeneuve et al. (1971a)
to be reduced to half the normal values in pregnant rabbits by Aroclor
1254. Similar observations have been made by Cecil et al. (1973) in
male and female Japanese quails and in rats after feeding Aroclor 1242
at 100 mg/kg of diet. The 50% decrease in hepatic vitamin A found in
male and female rats was also found in male and female quail provided
that the latter were kept in the dark to prevent egg laying.
A lowering of hepatic vitamin A in rats, fed a 0.1% PCB diet (PCB
not specified), was described by Innami et al. (1974). The rats given
vitamin A supplement (3400 IU) plus PCB for 6 weeks showed better
growth than those given PCB alone, while those given PCB and a vitamin
A deficient diet showed significant growth retardation. On the PCB
diet, the hepatic vitamin A level decreased to 20% of the normal level
during the experimental period; supplementing the diet with 1000 IU
did not re-establish the vitamin A level in the liver. Administration
of 3000 IU of vitamin A with the PCB diet, however, allowed better
than normal hepatic vitamin A levels to be re-established. The authors
concluded that vitamin A may play a role in the detoxification of PCB
rather than that PCB plays a role in the destructive metabolism of
vitamin A.
Aroclor 1254 administered intraperitoneally to rats at 25 mg/kg
body weight daily for 4 days caused a 4- to 5-fold increase in the
biliary excretion of thyroxine during a 3-hour period. Biliary
clearance was greatly elevated. Hypobilirubinaemia has been produced
in rats by Bastomsky et al. (1975) who investigated the mechanism by
administering daily intra-peritoneal injections of Aroclor 1254
(25 mg/kg body weight in corn oil) to female rats for 4 days, then
measuring bilirubin glucuronide formation by hepatic microsomes
in vitro. PCB treatment was not effective in increasing
UDPglucuronosyltransferase (2.4.1.7) activity. Serum bilirubin levels
were also significantly decreased by PCB treatment of Gunn rats, which
are genetically deficient in UDPglucuronosyltransferase (2.4.1.7)
activity.
7.4.5 Potentiation and antagonism by PCBs
As PCBs can stimulate microsomal enzyme activity, it is to be
expected that they may potentiate the action of those chemicals that
undergo microsomal activation, and decrease the action of those that
are detoxified. Villeneuve et al. (1973) demonstrated the antagonistic
effect by the reduction of phenobarbital sleeping time in rats
receiving Aroclors 1242, 1254, and 1260 in their diet, but not in
those receiving Aroclor 1221. Johnstone et al. (1974) have confirmed
this with a series of single PCBs. Tanaka & Komatsu (1972) found that
the hexobarbital-induced sleeping time in female rats was reduced to
49% of the control value by daily oral doses of Kanechlor 500 of
2 mg/kg for 3 days (total 6 mg/kg). When a daily dose of 0.4 mg/kg was
given for 15 days (total 6 mg/kg), no reduction in sleeping time was
observed. When this small dose was continued for 45 and 53 days, the
reduction remained at 12-13%. Phillips et al. (1972) did not find any
potentiation of the cholinesterase-inhibitory action of parathion in
rats dosed with Aroclors 1221 and 1254; this does not necessarily
imply that there was no enhanced activation of parathion, as a
stimulation of detoxication may have occurred concurrently. A
stimulation of parathion detoxication but not of activation has been
demonstrated in rabbit microsomes (Villeneuve et al., 1971a).
Lichtenstein (1972) reports a potentiation by PCBs of the toxicity of
parathion to flies.
Cecil et al. (1975) have shown that the ability of PCTs to
decrease phenobarbital sleeping time in quails is rather less than
that of the PCBs.
Aroclor 1254 at 160 mg/kg of diet fed to 5-week old male and
female Fischer-344 rats for 8 weeks reduced mortality due to feeding
hexachlorophene at a concentration of 600 mg/kg of diet from 77% to 7%
and completely prevented the paralysis that was observed in all
animals on the hexachlorophene diet alone. However, in the animals on
the combined treatment, histological changes in the brain
characteristic of hexachlorophene were still apparent and the
possibility of delayed toxicity beyond the 8 weeks of the experiment
could not be eliminated. The protective effect of Aroclor 1254 was
explained by its capacity to enhance detoxification by means of
hepatic microsomal enzyme induction (Jones et al., 1974).
7.5 Cytotoxic Effects
Peakall et al. (1972) found a significant increase in chromosome
abnormalities in the embryos of ring doves when the parents were fed
on a diet containing Aroclor 1254 at 10 mg/kg. Nilsson & Ramel (1974)
found no chromosome breakage in Drosophila melanogaster when either
Clophen A30 or A50 was added to the substrate. Green et al. (1975)
administered Aroclor 1242 orally to rats in single doses of 1250,
2500, or 5000 mg/kg or as a repeated dose of 500 mg/kg/day for 4 days.
Aroclor 1254 was also administered for 5 days at doses of 75, 150, or
300 mg/kg/day; they found no evidence of a mutagenic potential as
assessed by cytogenic analysis of bone marrow and spermatogonia.
7.6 Immunosuppressive Effects
Administration of PCBs leads to an atrophy of lymphoid tissue in
chickens (Flick et al., 1965; Vos & Koeman, 1970), in pheasants
(Dahlgren et al., 1972), and in rabbits (Vos & Beems, 1971). Vos & de
Roij (1972) and Vos & van Driel-Grootenhuis (1972) came to the
conclusion that these effects could be attributed to an
immunosuppressive effect of PCBs. They found that when guinea pigs fed
on diets containing Clophen A60 or Aroclor 1260 at 50 mg/kg were
stimulated with tetanus toxoid, a lower antitoxin titre and a lower
count of antitoxin-producing cells was obtained than in control guinea
pigs, resulting in a significant reduction of immunoglobulins. The
skin reaction after tuberculination in animals immunized with Freund's
complete adjuvant (as a parameter of cell-mediated immunity) was also
depressed at the 50 mg/kg of diet level. Vos & de Roij (1972)
suggested that the ability of PCBs to increase the susceptibility of
ducklings to duck hepatitis virus (Friend & Trainer, 1970) and of fish
to fungal disease (Hansen et al., 1971) could be attributed to this
immunosuppressive effect. Kimuru & Baba (1973) found an increased
incidence of pneumonia, and lung and intracranial abscesses in rats on
a diet containing Kanechlor 400 and suggested that this was due to a
lowered resistance to infection.
7.7 Effects on Reproduction
In a one-generation reproduction study, rats were fed with a diet
containing Aroclor 1242, 1254, or 1260 at levels of 1, 10, and
100 mg/kg. Decreased survival of pups with Aroclors 1242 and 1254 at
100 mg/kg was noted. No effect on reproduction was detected with
Aroclor 1260 (Keplinger et al., 1971). In a two-generation
reproduction study (Linder et al., 1974), rats were fed a diet
containing Aroclor 1254 at levels of 0, 1, 5, 20, and 100 mg/kg and
Aroclor 1260 at levels of 0, 5, 20, and 100 mg/kg. Rats exposed to
Aroclor 1254 at dietary levels of 20 mg/kg or more had fewer pups per
litter. Aroclor 1260 had no effect on reproduction, even at levels of
100 mg/kg. Kihlström et al. (1973) showed that a daily oral dose of
25 µg of Clophen A60 to female mice for 62 days significantly
increased the length of the estrus cycle and decreased the frequency
of implanted ova. In order to study the effect of PCBs on the
development of sexual functions in the early postnatal period they
also mated mice that had been suckled by mothers dosed with Clophen
A60 during the lactation period. A decrease in the frequency of
implanted ova was noted when both parents of the couple had been
suckled with milk containing PCBs.
In the rhesus monkey, Allen (1975) reports a lower fertility and a
diminished weight of the young at birth, after the administration of a
diet containing Aroclor 1248 at 2.5 mg/kg for several months.
Studies by Aulerich et al. (1971), and Ringer et al. (1972) showed
that Aroclor 1254 fed to mink at 5 mg/kg severely affected
reproduction.
There have been several reports that PCBs can adversely affect egg
production and hatchability in birds. Contamination of feed by PCBs
from a heat exchanger was shown to be the cause of reduced
hatchability of hen eggs at a large poultry hatchery in the USA
(Kolbye, 1972), and this has been confirmed in laboratory experiments.
Keplinger et al. (1971) found poor hatchability of eggs from hens
receiving diets containing Aroclor 1242 at 10 mg/kg or Aroclor 1254 at
100 mg/kg, but not with hens receiving Aroclor 1260 at 100 mg/kg. An
adverse effect on eggs has been noted with Aroclor 1248 at 10 mg/kg
(Panel on Hazardous Trace Substances, 1972). Peakall et al. (1972) fed
ring doves on a diet containing Aroclor 1254 at 10 mg/kg for 3 months
and found a marked reduction in egg hatchability 6 months later, due
to embryo mortality.
The viability of salmon eggs bears some relation to their PCB
content; Johansson et al. (1970) found a 12% mortality in eggs
containing PCBs at the rate of 9.2 mg/kg in extractable fat, and a
100% mortality with 34 mg/kg. Adverse effects on the reproduction of
aquatic invertebrates have occurred at water concentrations of PCBs in
the region of 5 µg/litre.
The potential teratogenic effect of the PCBs has been studied by
dosing pregnant females during the gestation period. No fetal
abnormalities were produced in the rat by daily doses of Aroclors
1242, 1254, or 1260 at 10 and 30 mg/kg (Keplinger et al., 1971), or
Aroclor 1254 at 100 mg/kg (Villeneuve et al., 1971b), or in the rabbit
dosed with Aroclor 1254 at 10 and 50 mg/kg (Villeneuve et al., 1971b).
Injection of PCBs into eggs has been reported to produce beak
abnormalities in chicks (McLaughlin et al., 1963). Cecil et al. (1974)
have claimed that the administration of Aroclors 1232 and 1254 to hens
at 20 mg/kg in the diet causes teratogenic effects and a reduction in
hatchability of fertile eggs.
7.8 Neoplasia and Adenofibrosis
The liver is the only organ where tumours have been reported
following the ingestion of PCBs. Ito et al. (1973) fed groups of 12
male dd mice with diets containing 500, 250, 100 and 0 mg/kg of
Kanechlor 500, 400, and 300, respectively. After 1 year, 7/12 mice
developed neoplastic nodules (hyperplastic nodules) and 5/12 developed
hepatocellular carcinomas, all in mice from the group fed with
500 mg/kg Kanechlor 500. Metastases were not observed. In a second
study on mice, combined exposure to Kanechlor 500 and either alpha- or
ß-hexachlorocyclohexane isomers enhanced the development of neoplastic
nodules (hyperplastic nodules) and hepato-cellular carcinomas. A
combination of Kanechlor 500 and gamma-hexachlorocyclohexane did not
produce tumours. Dosing with Kanechlor 500 alone at a dietary level of
250 mg/kg, and ß- or gamma-hexachlorocyclohexane at dietary levels of
250, 100 or 50 mg/kg did not produce tumours. However, alpha-hexa-
chlorocyclohexane produced 8/30 hepata-cellular carcinomas and 23/30
hyperplastic nodules.
Groups of 50 BALB/cj male inbred mice were fed dietary
concentrations of Aroclor 1254 of 0 or 300 mg/kg (49.8 mg/kg body
weight) for 6 or 11 months respectively. No liver tumours were noted
in a total of 58 surviving controls. A total of 10 hepatomas
(neoplastic or hyperplastic nodules) were noted in 9/22 surviving mice
fed Aroclor 1254 for 11 months and in 1/24 surviving mice fed Aroclor
1254 for 6 months. In addition adenofibrosis (cholangiofibrosis) was
observed in all 22 livers of mice fed Aroclor 1254 for 11 months, but
not in the other groups (Kimbrough & Linder 1974).
In another study (Kimbrough et al., 1975), 200 female Sherman
strain COBS random bred rats (descendants of the Osborne Mendel
strain) were given a diet containing Aroclor 1260 at 100 mg/kg
(11.6-4.3 mg/kg body weight) for approximately 21 months; 200 female
rats were kept as controls. The rats were sacrificed when 23 months
old. Hepatocellular carcinomas were found in 26/184 of the
experimental groups and in 1/173 of the control rats. None of the
controls, but 144/184 experimental rats had neoplastic nodules
(hyperplastic nodules). Areas of hepatocellular alteration were noted
in 28/173 controls and 182/184 experimental rats. No effect of the
Aroclor on the incidence of tumours in other organs and no metastases
from the liver tumours were observed. In this and two earlier studies,
adenofibrosis of the liver was observed in male and female rats fed
either Aroclor 1260 or Aroclor 1254 (Kimbrough et al., 1972; 1973);
1975). Adenofibrosis of the liver is a persistent progressive lesion
that consists of a marked proliferation of fibrous tissue and
epithelial glandular cells which are well differentiated in the mouse
but appear atypical in the rat.
In a preliminary study, liver tumours (multiple adenomatous
nodules) were induced by Kanechlor 400 in 6/10 female, but not in male
Donryu rats. The dietary exposure varied throughout the study and the
number of animals used was small (10 experimental and 5 control rats
for each sex) (Kimura & Baba, 1973). Makiura et al. (1974) reported
that PCBs (Kanechlor 500) inhibited the induction of liver tumours in
male Sprague-Dawley rats by the known carcinogens 3' methyl- p-
dimethylaminoazo-benzene, N-2-fluosenyl acetamide, and diethyl-
nitrosamine.
8. EFFECTS OF PCBs AND PCTs ON MAN -- EPIDEMIOLOGICAL AND CLINICAL
STUDIES
In June 1968, patients appeared at the Dermatology Clinic of
Kyushu University Hospital, Fukuoka, Japan suffering from chloracne. A
group at the University undertook intensive clinical, chemical, and
epidemiological investigations and found that the disease originated
from the consumption of a batch of rice oil supplied in February 1968;
the disease was called Yusho (rice oil disease) (Katsuki, 1969). This
batch of rice oil was found to be contaminated with Kanechlor 400, a
48% chlorinated biphenyl, at 2000-3000 mg/kg which entered the oil
through a leak in a heat exchanger (Tsukamoto et al., 1969). The
symptoms and signs of Yusho were described by Goto & Higuchi (1969)
and by Okumura & Katsuki (1969). The earliest signs were enlargement
and hypersecretion of the Meibomian glands of the eyes, swelling of
the eyelids, and pigmentation of the nails and mucous membranes,
occasionally associated with fatigue, nausea, and vomiting. This was
usually followed by hyperkeratosis and darkening of the skin with
follicular enlargement and acneform eruptions, frequently with a
secondary staphylococcal infection. These skin changes were most often
seen on the neck and upper chest, but in severe cases extended to the
whole body. Biopsy skin samples showed hyperkeratosis, dilation of the
follicles, and an accumulation of melanin in the basal cells of the
epidermis; melanin granules have also been observed in biopsy samples
of the conjunctiva. Oedema of the arms and legs was seen in some
patients. There were no definite signs of liver enlargement or liver
disorders (Okumura & Katsuki 1969), but slight rises in serum
transaminases and in alkaline phosphatase were detected, and a liver
sample from a Yusho patient showed an increase in the smooth
endoplasmic reticulum (Hirayama et al., 1969). The majority of the
patients were found to have respiratory symptoms, and suffered from a
chronic bronchitis-like disturbance that persisted for several years
(Shigematsu et al., 1971, 1974).
Yusho patients did not appear to suffer from central nervous
effects, but some complained of numbness of the arms and legs. Mural &
Kuroiwa (1971) found a decrease in the conduction velocity in
peripheral sensory nerves.
Yoshimura (1971) reported diminished growth in boys but not in
girls, who consumed the oil. Babies born to Yusho mothers were smaller
than normal. Newborn babies showed a dark brown skin pigmentation,
which disappeared after a few months (Yagamuchi et al., 1971; Taki et
al., 1969). Funatsu et al. (1972) found spotted and sporadic
ossification of the skull and facial oedema with exophthalmia in four
babies, but there was no evidence of any teratogenic action.
Determinations of PCB concentrations in the tissue of Yusho
patients were made several months after the ingestion of the oil,
apparently by an X-ray fluorescence method for organic chlorine (Goto
& Higuchi, 1969). Abdominal fat contained 13.1 mg/kg, subcutaneous fat
75.5 mg/kg, and nails 59 mg/kg. The mesenteric adipose tissue in six
Yusho patients, analysed by gas-liquid chromatography 1-3 years after
the occurrence of intoxication, contained PCB levels of 2.5 mg/kg on
average, which was considerably higher than the normal value. (Masuda
et al., 1974a). The mean blood level of PCBs in patients was 0.6 or
0.7 µg/100 ml (0.3 µg/100 ml for the general population) five years
after exposure (Masuda et al., 1974b; Takamatsu et al., 1974). These
authors also noted a specific gas-liquid chromatographic pattern,
peculiar to Yusho patients, which is still observed.
Hirayama et al. (1974) also reported that the serum bilirubin
level of patients was significantly lower than the normal level and
was negatively correlated with the blood level of PCBs and the serum
triglyceride level.
A considerable number of patients had elevated serum triglyceride
levels, up to four times the normal values, although this was not
correlated with the severity of the symptoms; these high values were
maintained for three years in many patients (Uzawa, 1972). There were
no marked abnormalities in serum cholesterol and phospholipid levels
(Okumura & Katsuki, 1969; Uzawa et al., 1969). Nagai et al. (1969)
reported an increase in urinary 17-ketosteroid excretion. Kusuda
(1971) also observed changes in the menstrual cycle in approximately
60% of 81 female Yusho patients as compared with their cycles prior to
exposure. Okumura et al. (1974) examined the relationship between the
blood levels of triglycerides and PCBs in 42 patients and observed a
positive correlation. Uzawa et al. (1972) showed that high values of
serum triglycerides were maintained for 3 years in many patients.
Shigematsu et al. (1971) examined serum immunoglobulin levels in 38
patients, 2 years after onset, and observed a decrease in IgA and IgM
and an increase in IgG. Saito et al. (1972) reported lower IgM levels
in patients showing chloracne.
Urabe (1974) reported that the total number of Yusho patients had
reached 1200 by 13 September 1973 and that 22 of them had died.
Mucocutaneous signs had decreased year by year, but neurological and
respiratory signs and symptoms and various complaints such as general
fatigue, anorexia, abdominal pain, and headache had become more
prominent among the patients. The smallest amount of oil that produced
symptoms when ingested over approximately 120 days, contained
approximately 0.5 g of PCBs, or approximately 0.07 mg/kg body weight
per day (Kuratsune, 1972a). Recently chlorinated dibenzofurans at
5 mg/kg were found in three samples of the toxic rice oil that
contained PCB levels of about 1000 mg/kg (Nagayama et al., 1975).
Symptoms similar to those of Yusho have been observed in workers
in a Japanese condenser factory, including pigmentation of the fingers
and nails, and acneiform eruptions on the jaw, back, and thighs. It
was thought that these effects arose from local contact with PCBs;
when the use of PCBs ceased, the symptoms disappeared (Hasegawa et
al., 1972b).
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO PCBs AND PCTs
9.1 Species Variation
The data in sections 7 and 8 indicate that man appears to be the
species most sensitive to PCBs, the consumption of relatively small
amounts having resulted in a severe disease (Yusho) in 1200 persons in
Japan. The monkey is the only experimental species in which effects
qualitatively and quantitatively approaching those in man have been
observed; Allen (1975) attributed this to metabolic differences
leading to a slower elimination than that observed in other species
tested.
Conclusions concerning the specific effects of PCBs on different
species are confused by uncertainty arising from the presence of toxic
impurities. The rice oil that caused the outbreak of Yusho was
contaminated with PCBs containing relatively high amounts of
tetrachlorodibenzofuran (see section 8), but the sample used in the
monkey experiments had a low content of these impurities, so it is not
clear whether PCBs alone were responsible for the Yusho incident.
Further uncertainty arises from reports from Finland of high PCB
concentrations in blood and body fat of occupationally exposed workers
with no indication of adverse effects, while at similar tissue
concentrations Japanese workers showed skin lesions typical of Yusho
(see section 5.5.3).
A species-specific toxic manifestation that can probably be
attributed to toxic impurities, is the abdominal oedema and
hydropericardium seen in birds affected by some commercial PCB
mixtures.
Mink is another species showing a high sensitivity to PCBs. Deaths
have been produced with diets containing PCB levels of 30 mg/kg; no
information is available on any species-specific metabolic pathway in
the mink that would account for this susceptibility.
9.2 Dose-Effect Relationships
The following is a summary of the data in Sections 7 and 8
concerning the relationship between mammalian toxicity and dose.
Approximate calculations of the daily dose in mg/kg body weight
derived from the dietary concentration are given in parentheses.a
a When no food consumption figures were available from the
experimental studies, the following factors were used to transform
mg/kg in the diet to mg/kg body weight: mouse (7), rat (20),
guinea-pig (25), mink (10), rabbit (33), monkey (25).
9.2.1 Body weight
Body weight was reduced in rats after 8 months of dietary intake
of Aroclor 1254 at 100 mg/kg (corresponding to 5 mg/kg body weight);
no effects were observed at 20 mg/kg in the diet (corresponding to
1 mg/kg body weight).
Dose-dependent retardation of weight gain was observed in mink
after 4 months of dietary intake of Aroclor 1254 at 5 and 10 mg/kg
(corresponding to 0.5 and 1.1 mg/kg body weight respectively).
9.2.2 Effects on liver
Liver weight
Dose-dependent increase in liver weight was observed in rats
receiving Aroclors 1242, 1254 and 1260 at concentrations of more than
20 mg/kg in the diet (corresponding to > 1.4 mg/kg body weight). Male
rats were more sensitive than female rats; no effects were observed
with Aroclors 1254 and 1260 at concentrations lower than 20 mg/kg in
the diet (corresponding to < 1.4 mg/kg body weight). Effects were
less marked with the lower chlorinated PCBs.
Liver changes
Smooth endoplasmic reticulum proliferation with fat droplet
inclusions were observed in the liver tissue of rats after 8 months of
dietary intake of Aroclor 1254 at 20 mg/kg (corresponding to 1.4 mg/kg
body weight).
Liver damage was observed with Aroclors 1242 and 1254 in rabbits
receiving 14 weekly oral doses of 150 mg/kg body weight; no effect was
observed with Aroclor 1221.
Liver enzyme activitya
Increase in microsomal enzyme activity was observed in male rats
after 8 months of dietary intake of Aroclor 1254 of 20 mg/kg
(corresponding to 1 mg/kg body weight). No effect was observed at
2 mg/kg in the diet (corresponding to 0.1 mg/kg body weight. Effects
were less marked in female rats.
Increased activity was also observed with Aroclors 1242 and 1016
in male rats receiving 21 daily oral doses of 1 mg/kg body weight.
a According to Litterst, et al. (1972) the dose producing an effect
on nitroreductase activity in the rat corresponds to 0.5 mg/kg in
the diet (corresponding to 0.3 mg/kg body weight).
Liver porphyria
Effects were observed in rats after several months of dietary
intake of Aroclor 1254 at 100 mg/kg (corresponding to 5 mg/kg body
weight); dose-dependent effects were observed in female rats after 21
daily oral doses of Aroclor 1242 at 10 and 100 mg/kg; no effects were
noted at 1 mg/kg body weight.
Liver vitamin A
Reduction of hepatic vitamin A was observed in rats receiving
Aroclor 1242 at the rate of 100 mg/kg in the diet (corresponding to
5 mg/kg body weight).
Liver tumours
Hepatocellular carcinomas were observed in mice after one year of
dietary intake of Kanechlor 500 at 500 mg/kg (corresponding to
75 mg/kg body weight); no carcinomas were observed with Kanechlor 500
at 250 mg/kg in the diet (corresponding to 37.5 mg/kg body weight), or
with Kanechlor 300 and 400 at 500 mg/kg in the diet (corresponding to
75 mg/kg body weight).
Hepatomas were observed in mice after 10 months of daily intake of
Aroclor 1254 at 300 mg/kg in the diet (corresponding to 49.8 mg/kg
body weight).
Hepatocellular carcinomas were observed in rats after 21 months of
daily intake of Aroclor 1260 at 100 mg/kg in the diet (corresponding
to 11.6-4.3 mg/kg body weight).
9.2.3 Reproduction
Effects on reproduction were observed in the mouse at a daily oral
dose of 0.025 mg Clophen A60; in the rat at a dietary level of Aroclor
1254 of 20 mg/kg (corresponding to 1 mg/kg body weight) with the
effects decreasing with higher chlorinated PCBs; in the mink at a
dietary level of Aroclor 1254 of 5 mg/kg (corresponding to 0.5 mg/kg
body weight); and in the monkey at a dietary level of Aroclor 1248 of
2.5 mg/kg (corresponding to 0.1 mg/kg body weight).
9.2.4 Immunosuppression
Immunosuppressive effects were observed in the guinea-pig at a
dietary level of Clophen A60 or Aroclor 1260 of 50 mg/kg
(corresponding to 2 mg/kg body weight).
9.2.5 Skin effects
In man, symptoms of Yusho disease were observed at a dietary level
of 4.2 mg/day of PCBs (corresponding to 0.07 mg/kg body weight/day for
a 60-kg person). A value of 0.50 g was estimated as the quantity of
PCBs consumed over approximately 120 days above which toxic symptoms
were evident. Similar effects were observed in the monkey at a dietary
level of Aroclor 1248 of 2.5 mg/kg (corresponding to 0.1 mg/kg body
weight) after several months.
9.3 Nondetected effect levels
The assessment of non detected effect levels for toxic effects is
complicated by the different activities of the component PCBs and by
the presence of impurities, in addition to the influence of inter- and
intraspecies variation, age, sex, and length of exposure. Moreover,
many of the available experimental studies do not include a
nondetected effect level.
The most sensitive species appears to be man, and effects have
been observed at intake rates of 0.07 mg/kg body weight/day. This may
have been influenced by the intake of impurities more toxic than PCBs,
but similar effects have been produced in the monkey, at the same
order of dosage, with a product containing little of these impurities.
At this dosage level, no effects may be expected on growth, liver
enlargement, and liver enzyme activity in less sensitive species such
as the rat. Although non-detected effect levels are not available for
effects on immunosuppression and reproduction, and for certain
biochemical effects on the liver, it seems unlikely that these effects
would be apparent at intake rates of 0.1 mg/kg body weight/day.
Carcinogenic effects have been observed in rats and mice at doses two
orders of magnitude greater than this, but there is no epidemiological
evidence to suggest that PCBs cause tumours in man. According to Grant
et al. (1974) rats fed on a diet containing Aroclor at the rate of
2 mg/kg (equivalent to about 0.1 mg/kg body weight) showed PCB levels
of 8 µg/100 ml in blood and 26.1 mg/kg in body fat. However, values
much higher than these have been observed in men occupationally
exposed to PCBs without evidence of any toxic effects (see section
5.5.3).
It is not possible at present to resolve this conflict in the
evidence on the toxicity of PCBs to man.
REFERENCES
ABBOT, D.C., COLLINS, G. B. & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom 1969-71.
Br. med. J., 2: 553-556.
ACKER, L. & SCHULTE, E. (1970) Uber das Vorkommen von chlorierten
Biphenylen und Hexachlorbenzol neben chlorierten Insektiziden in
Humanmilch und menschlichem Fettgewebe. Naturwissenschaften,
57: 497 (in German).
ACKER, L. & SCHULTE, E. (1974) Chlorkohlenwasserstoffe in menschlichem
Fettgewebe, Naturwissenschaften, 61: 1-4 (in German).
ADDISON, R. F., FLETCHER, G. L., RAY, S. & DOANE, J. (1972) Analysis
of a chlorinated terphenyl (Aroclor 5460) and its deposition in
tissues of the cod (Gadus morhua). Bull. environ. Contam.
Toxicol., 8: 52-60.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of polychlorinated biphenyl (PCB) and chlorinated
pesticides in water. Anal. Chem., 42: 1483-1486.
AHMED, M. & FOCHT, D. D. (1973) Degradation of polychlorinated
biphenyls by two species of Achromobacter. Can. J. Microbiol.,
19: 47-52.
AHNOFF, M. & JOSEFSSON, B. (1973) Confirmation studies on
polychlorinated biphenyls (PCB) from river waters using mass
fragmentography. Anal. Lett., 6: 1083-1093.
AHNOFF, M. & JOSEFSSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AHNOFF, M. & JOSEFSSON, B. (1975) Clean-up procedures for PCB analysis
on river water extracts. Bull. environ. Contam. Toxicol.,
13: 159-166.
ALBRO, P. W. & FISHBEIN, L. (1972) Intestinal absorption of
polychlorinated biphenyls in rats. Bull. environ. Contam.
Toxicol., 8: 26-31.
ALLEN, J. R. (1975) Response of the non-human primate to
polychlorinated biphenyl exposure. Fed. Proc., 34: 1675-1679.
ALLEN, J. R., ABRAHAMSON, L. J. & NORBACK, D. H. (1973) Biological
effects of polychlorinated biphenyls and triphenyls on the
subhuman primate. Environ. Res., 6: 344-354.
ALLEN, J. R. & NORBACK, D. A. (1973) Polychlorinated biphenyl- and
triphenyl-induced mucosal hyperplasia in primates. Science,
179: 498-499.
ALLEN, J. R., CARSTENS, L. A. & BARSOTTI, D. A. (1974) Residual
effects of short-term, low-level exposure of non-human primates to
polychlorinated biphenyls. Toxicol. appl. Pharmacol.,
30: 440-451.
ALVARES, A. P. & KAPPAS, A. (1975) Induction of aryl hydrocarbon
hydroxylase by chlorinated biphenyls in the foeto-placental unit
and in neonatal livers during lactation. FEBS Lett., 50:172-174.
ARMOUR, J. A. & BURKE, J. A. (1970) Method for separating
polychlorinated biphenyls from DDT and its analogs. J. Assoc.
Off. Anal. Chem., 53: 761-768.
AULERICH, R. J., RINGER, R. K., SEAGRAN, H. L. & YOUATT, W. G. (1971)
Effects of feeding coho salmon and other Great Lakes fish on mink
reproduction. Can. J. Zool., 49: 611-616.
AULERICH, R. J., RINGER, R. K. & IWAMOTO, S. (1973) Reproductive
failure and mortality in mink fed on Great Lake fish. J. Reprod.
Fertil., 19 (Suppl.): 365-376.
BAGLEY, G. E., REICHEL, W. L. & CROMARTIE, E. (1970) Identification of
polychlorinated biphenyls in two bald eagles by combined
gas-liquid chromatography-mass spectroscopy. J. Assoc. Off. Anal.
Chem., 53: 251-261.
BAILEY, S. & BUNYAN, P. J. (1972) Interpretation of persistence and
effects of polychlorinated biphenyls in birds. Nature (Lond.),
236: 34-36.
BASTOMSKY, C. H., SOLYMOSS, a., ZSIGMOND, G. & WYSE, J. M. (1975) On
the mechanism of polychlorinated biphenyl-induced
hypobilirubinaemia. Clin. chim. Acta, 61: 171-174.
BAUER, H., SCHULZ, K. H. & SPIEGELBERG, U. (1961) Berufliche
Vergiftungen bei der Herstellung von Chlorphenol-Verbindungen.
Arch. Gewerbepathol. Gewerbehyg., 18: 538-555.
BENTHE, H. F., KNOP, J. & SCHMOLDT, A. (1972a) Absorption and
distribution of polychlorinated biphenyls (PCB) after inhalatory
application. Arch. Toxikol., 29: 85-95 (in German).
BENTHE, H. F., SCHMOLDT, A. & SCHMIDT, H. (1972b) Induction of
microsomal liver enzymes after polychlorinated biphenyls (PCB) and
following stress. Arch. Toxikol., 29: 97-106 (in German).
BERG, O. W., DIOSADY, P. L. & REES, G. A. V. (1972) Column
chromatographic separation of polychlorinated biphenyls from
chlorinated hydrocarbon pesticides and their subsequent gas
chromatographic quantitation in terms of derivatives. Bull.
environ. Contam. Toxicol., 7: 338-347.
BERGLUND, F. (1972) Levels of polychlorinated biphenyls in foods in
Sweden. Environ. Perspect, 1: 67-69.
BERLIN, M., GAGE, J. C. & HOLM, S. (1973) The metabolism and
distribution of 2,4,5,2',5'-pentachlorobiphenyl in the mouse.
In: PCB Conference II. National Swedish Environment Protection
Board Publications: 4E, pp. 101-107.
BERLIN, M., GAGE, J. C. & HOLM, S. (1974) Distribution and metabolism
of polychlorobiphenyls. In: Proceedings of the International
Symposium on Recent Advances in Environmental Pollution, Paris,
24-28 June. pp. 8.
BERLIN, M., GAGE, J. & HOLM, S. (1975) The distribution and metabolism
of 2,4,5,2',5'-pentachlorobiphenyl. Arch. environ. Health,
30: 141-147.
BICKERS, D. R., HARBER, L. C., KAPPAS, A. & ALVARES, A. P. (1972)
Polychlorinated biphenyls: comparative effects of high and low
chlorine containing Aroclors on hepatic mixed function oxidase.
Res, Comm. Chem. Pathol. Pharmacol., 3: 505-511.
BIDLEMAN, T. F. & OLNEY, C. E. (1974) High-volume collection of
atmospheric polychlorinated biphenyls. Bull. environ. Contam.
Toxicol., 11: 442-450.
BITMAN, J., CECIL, H. C. & HARRIS, S. J. (1972) Biological effects of
polychlorinated biphenyl in rats and quail. Environ. Health
Perspec., 1: 145-149.
BJERK, J. E. (1972) Rester av DDT og polyklorerte bifenyler i Norsk
humant materiale. Tidsskr. Nor-Laegeforen., 92: 15-19 (cited by
Kimbrough, 1974).
BOURNE, W. P. P. & BOGEN, A. A. (1972) Polychlorinated biphenyls in
North Atlantic seabirds, Mar. Pollut. Bull., 3 (11): 172-175.
BOWES, G. W., MULVIHILL, M. J., SIMONEIT, B. R. T., BURLINGAME, A. L.
& RISEBROUGH, R. W. (1975) Identification of chlorinated
dibenzofurans in American polychlorinated biphenyls. Nature
(Lond.), 256, 305-307.
BROADHURST, M. G. (1972) Use and replaceability of polychlorinated
biphenyls. Environ. Health Perspect., 2: 81-102.
BRUCKNER, J. V., KHANNA, K. L. & CORNISH, H. H. (1973) Biological
responses of the rat to polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 24: 434-448.
BRUGGEMAN, VON J., BUSCH, L., DRESCHER-KADAN, U., EISELI, W. & HOPPE,
P. (1974) Pesticid-und PCB-Ruckstunde in Organen von Wildtieren
als Indikatoren fur Umweltkontamination. Z. Jagdwiss.,
20: 70-74.
BURSE, V. W., KIMBROUGH, R. D., VILLANUEVAS, E. C, JENNINGSI R. W.,
LINDER, R. E. & SOVOCOOL, G. W. (1974) Polychlorinated biphenyls.
Storage, distribution, excretion and recovery: liver morphology
after prolonged dietary ingestion. Arch. environ. Health,
29: 301-307.
CARNES, R. A., DOERGER, J. U. & SPARKS, H. L. (1973) Polychlorinated
biphenyls in solid waste and solid-waste-related materials. Arch.
environ. Contam. Toxicol., 1: 27-35.
CECIL, H. C., HARRIS, S. J., BITMAN, J. & FRIES, G. F. (1973)
Polychlorinated biphenyl-induced decrease in liver vitamin A in
Japanese quail and rats. Bull. environ. Contam. Toxicol.,
9: 179-185.
CECIL, H. C., BITMAN, J., LILLIE, R. J., FRIES, G. F. & VERRETT, J.
(1974) Embryotoxic and teratogenic effects in unhatched fertile
eggs from hens fed polychlorinated biphenyls (PCBs). Bull.
environ. Contam. Toxicol., 11: 489-495.
CECIL, H. C., HARRIS, S. J. & BITMAN, J. (1975) Effects of
polychlorinated biphenyls and terphenyls and polybrominated
biphenyls on pentobarbital sleeping times of Japanese quail.
Arch. environ. Contam. Toxicol., 3, 183-192.
CHOI, P.S. K., NACK, H. & FLINN, J. E. (1974) Distribution of
polychlorinated biphenyls in an aerated biological oxidation
wastewater treatment system. Bull. environ. Contam. Toxicol.,
11: 12-17.
CLAUS, B. & ACKER, L. (1975) Zur Kontamination von Milch und
Milcherzeugnissen mit chlorierten Kohlenwasserstoffen im
Westphälischen Raum II Ergebnisse und Diskussion. Zeitschrift fur
Lebensmitteluntersuchung und forschung, 159 (3): 129-137.
COLLINS, G. B., HOLMES, D. C. & JACKSON, F. J. (1972) The estimation
of polychlorobiphenyls. J. Chromatogr., 71: 443-449.
COMMISSION OF THE EUROPEAN COMMUNITIES (1974) European Colloquium.
Problems raised by the contamination of man and his environment
by persistent pesticides and organohalogenated compounds.
Luxembourg, 14-16 May 1964.
COOLEY, N. R., KELTNER, J. M. & FORESTER, J. (1972) Mirex and Aroclor
1254: effect on accumulation by Tetrahymena pyriformis strain
W. J. Protozool., 19: 636-638.
CURLEY, A., BURSE, V. W., GRIM, M. E., JENNINGS, R. W. & LINDER, R. E.
(1971) Polychlorinated biphenyls: distribution and storage in body
fluids and tissues of Sherman rats. Environ. Res., 4: 481-495.
CURLEY, A., BURSE, V. W. & GRIM, M. E. (1973a) Polychlorinated
biphenyls: evidence of transplacental passage in the Sherman rat.
Fd. cosmet. Toxicol., 11: 471-476.
CURLEY, A., BURSE, V. W., JENNINGS, R. W., VILLANUEVA, E. C., TOMATIS,
L. & AKAZAKI, K. (1973b) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond), 242: 338-340.
CURLEY, A., BURSE, W. V., JENNINGS, R. W., VILLANUEVA, E. C. &
KIMBROUGH, R. D. (1975) Evidence of tetrachlorodibenzofuran (TCDF)
in Aroclor 1254 and the urine of rats following dietary exposure
to Aroclor 1254. Bull. environ. Contam. Toxicol., 14: 153-158.
DAHLGREN, R. B., GREICHUS, Y. A. & LINDER, R. L. (1971) Storage and
excretion of polychlorinated biphenyls in the pheasant.
J. Wildlife Manag., 35: 823-828.
DAHLGREN, R. B., LINDER, R. L. & CARLSON, C. W. (1972) Polychlorinated
biphenyls: their effects on penned pheasants. Environ. Health
Perspect., 1: 89-101.
DE FREITAS, A. S. & NORSTROM, R. J. (1974) Turnover and metabolism of
polychlorinated biphenyls in relation to their chemical structure
and the movement of lipids in the pigeon. Can. J. Physiol.
Pharmacol., 52: 1080-1094.
DEPARTMENT OF HEALTH EDUCATION AND WELFARE (1972) Environ. Health
Perspect. Experimental Issue 1, April 1972. DHEW, Bethesda, MD,
USA. Publ. No. (NIH) 72-218.
DE VOS, R. H. & PEET, E. W. (1971) Thin-layer chromatography of
polychlorinated biphenyls. Bull environ. Contam. Toxicol.,
6: 164-170.
DOGUCHI, M. & FUKANO, S. (1975) Residue levels of polychlorinated
terphenyls, polychlorinated biphenyls and DDT in human blood.
Bull. environ. Contam. Toxicol., 13: 57-63.
DOGUCHI, M., FUKANO, S. & USHIO, F. (1974) Polychlorinated terphenyls
in the human fat. Bull. environ. Contam. Toxicol.,
11 (2): 157-158.
DUKE, T. W., LOWE, J. I. & WILSON, A. J. JR (1970) A polychlorinated
biphenyl (Aroclor 1254) in the water, sediment and biota of
Escambia Bay, Florida. Bull. environ. Contam. Toxicol.,
5: 171-180.
ECOBICHON, D. J. & COMEAU, A.M. (1974) Comparative effects of
commercial Aroclors on rat liver enzyme activities. Chem.-Biol.
Interactions, 9: 341-350.
EKSTEDT, J. & ODEN, S. (1974) Chlorinated hydrocarbons in the lower
atmosphere in Sweden. Report from Department of Soil Science,
Royal Agricultural College, S-75007, Uppsala 7. pp. 1-16.
ENVIRONMENTAL SANITATION BUREAU, MINISTRY OF HEALTH & WELFARE (1973)
Survey on foods contamination. Report of comprehensive
investigation on the prevention of pollution by PCBs, 191-210
(in Japanese).
FINKLEA, J., PRIESTER, L. E., CREASON, J.P., HAUSER, T., HINNERS, T. &
HAMMER, D. I. (1972) Polychlorinated biphenyl residues in human
plasma expose a major urban pollution problem. Am. J. pub.
Health, 62: 645-651.
FISHER, N. S., GRAHAM, L. B., CARPENTER, E. J. & WURSTER, C. F. (1972)
Geographic differences in phytoplankton sensitivity to PCBs.
Nature (Lond.), 241: 548-549.
FLICK, D. F., O'DELL, R. G. & CHILDS, V. A. (1965) Studies of the
chick edema disease. Similarity of symptoms produced by feeding
chlorinated biphenyl. Poult. Sci., 44: 1460-1465.
FREUDENTHAL, J. & GREVE, P. A. (1973) Polychlorinated terphenyls in
the environment. Bull. environ. Contam. Toxicol., 10: 108-111.
FRIEND, M. & TRAINER, D. O. (1970) Polychlorinated biphenyl:
interaction with duck hepatitis virus. Science, 170: 1314-1316.
FRIES, G. F., MARROW, G. S. JR & GORDON, C. H. (1973) Long-term
studies of residue retention and excretion by cows fed a
polychlorinated biphenyl (Aroclor 1254). J. agr. Food Chem.,
21: 117-121.
FUJITA, S., TZUJI, H., KATO, K., SAEICI, S. & TSUKAMOTO, H. (1971)
Effect of biphenyl chlorides on rat liver microsomes. Fukuoka
med. Acta., 62: 30-34.
FUKADA, K., INUYAMA, Y., TAKESHITA, T. & YAMAMOTO, S. (1973) Present
state of environmental pollution by PCB in Shimane Prefecture.
Shimare Igaku, 5: 1-25 (in Japanese).
FUKADA, S., USHIO, F. & DOGUCHI, M. (1974) PCB, PCT and pesticides
residues in fish collected from the Tama River. Ann. Rep. Tokyo
Metr. Res. Lab. P.H., 25: 297-305 (in Japanese).
FUNATSU, I., YAMASHITA, F., ITO, Y., TZUGAWA, S., FUNATSU, T.,
YOSHIKANE, T., HAYASHI, M., KATO, T., YAKUSHIJI, M., OKAMOTO, G.,
YAMASAKI, S., ARIMA, T., KUNO, T., IDE, H. & IBE, I. (1972) PCB
induced fetopathy. I. Clinical observation. Kurume med. J.,
19: 43-51 (in English).
GARDNER, A.M., CHEN, J. F., ROACH, J. A. G. & RAEGLIS, E. P. (1973)
Polychlorinated biphenyls. Hydroxylated urinary metabolites of
2,5,2',5'-tetrachlorobiphenyl identified in rabbits. Biochem.
biophys. res. Commun., 55: 1377-1384.
GILBERTSON, M. & REYNOLDS, L. (1974) DDE and PCB in Canadian wild
birds. In: Occasional paper, Canadian Wildlife Service,
Environment, Canada, Ottawa, pp. 1-16.
GOLDSTEIN, J. A., HICKMAN, P. & JUE, D. L. (1974) Experimental hepatic
porphyria induced by polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 27: 437-448.
GOLDSTEIN, J. A., MCKINNEY, J. D., LUCIEN, G. W., MOORE, J. A.,
HICKMAN, P. & BERGMAN, H. (1975a) Effects of hexachlorobiphenyl
isomers and 2,3,7,8-tetrachlorodibenzofuran (TCDF) on hepatic drug
metabolism and porphyria accumulation. Pharmacologist,
16: 239 (Abstract No. 278).
GOLDSTEIN, J. A., HICKMAN, P., BURSE, V. W. & BERGMAN, H. (1975b) A
comparative study of two polychlorinated biphenyl mixture (Aroclor
1242 and 1016) containing 42% chlorine on induction of hepatic
porphyria and drug metabolizing enzymes. Toxicol. appl.
Pharmacol., 32: 461-473.
GOTO, M. & HIGUCHI, K. (1969) The symptomatology of Yusho
(chlorobiphenyls poisoning) in dermatology. Fuoka Act. Med.,
60: 409-431.
GRANT, D. L., MOODIE, C. A. & PHILLIPS, W. E. J. (1974) Toxicodynamics
of Aroclor 1254 in the male rat. Environ. physiol. Biochem.,
4: 214-225.
GRANT, D. L. & PHILLIPS, W. E. J. (1974) The effect of age and sex on
the toxicity of Aroclor 1254, a polychlorinated biphenyl, in the
rat. Bull. environ. Contam. Toxicol., 12: 145-152.
GRANT, D. L., PHILLIPS, W. E. J. & VILLENEUVE, D.C. (1971a) Metabolism
of a polychlorinated biphenyl (Aroclor 1254) mixture in the rat.
Bull. environ. Contam. Toxicol., 6: 102-112.
GRANT, D. L., VILLENEUVE, D.C., MCCULLY, K. A. & PHILLIPS, W. E. J.
(1971b) Placental transfer of polychlorinated biphenyls in the
rabbit. Environ. Physiol., 1: 61-66.
GREEN, S. G., CARR, J. V., PALMER, K. A. & OSWALD, E. J. (1975) Lack
of cytogenetic effects in bone marrow and spermatagonial cells in
rats treated with polychlorinated biphenyls (Aroclor 1242 and
1254). Bull. environ. Contam. Toxicol., 13: 14-22.
HAMMER, D. I., FINKLEA, J. F., PRIESTER, L. E., KEIL, J. E., SANDIFER,
S. H. & BRIDBORN, K. (1972) Polychlorinated biphenyl residues in
the plasma and hair of refuse workers. Environ. Health
Perspect., 1 (15): 83.
HAMMOND, A. L. (1972) Chemical pollution: polychlorinated biphenyls.
Science, 175: 155-156.
HANSEN, D. J., PARRISH, P. R., LOWE, J. I., WILSON, A. J. JR & WILSON,
P. D. (1971) Chronic toxicity, uptake and retention of Aroclor
1254 in two estuarine fishes. Bull. environ. Contam. Toxicol.,
6: 113-119.
HAQUE, R., SCHMEDDING, D. W. & FREED, V. H. (1974) Aqueous solubility,
absorption and vapour behaviour of polychlorinated biphenyl
Aroclor 1254. Environ. Sci. Technol., 8: 139-142.
HARA, I., HARADA, H., KIMURA, S., ENDO, T. & KAWANO, K. (1974) Follow
up health examination in an electric condenser factory after
cessation of PCBs usage (1st report) Jpn. J. Ind. Health,
16: 365-366 (in Japanese).
HARVEY, G. R. & STEINHAUER, W. G. (1974) Atmospheric transport of
polychlorobiphenyls to the North Atlantic. Atmos. Environ.,
8: 777-782.
HARVEY, G. R., STEINHAUER, W. G. & TEAL, J. M. (1973) Polychloro-
biphenyls in North Atlantic Ocean water. Science, 80: 643-644.
HASEGAWA, H., SATO, M. & TSURUTA, H. (1972a) PCBs concentration in the
blood of workers handling PCB. Occup. Health, 10: 50-55
(in Japanese).
HASEGAWA, H., SATO, M. & TSURUTA, H. (1972b) PCB concentration in air
of PCB-using plants and health examination of workers (in
Japanese). In: Report on Special Research on Prevention of
Environmental Pollution by PCB-like Substances. Tokyo, Research
Co-ordination Bureau, Science and Technology Agency, pp. 141-149.
HEATH, R. G., SPANS, J. W., KREITZER, J. F. & VANCE, C. (1970)
Effects of polychlorinated biphenyls on birds In: Proceedings
of the 15th Congress of International. Ornithology, The Hague,
pp. 475-485.
HESSELBERG, R. J. & SCHERR, D. D. (1974) PCBs and p,p' DDE in the
blood of cachectic patients. Bull. environ. Contam. Toxicol.,
11: 202-205.
HIRAYAMA, C., IRISA, T. & YAMAMOTO, T. (1969) Fine structural changes
of the liver in a patient with chlorobiphenyls intoxication.
Fukuoka Acta Med., 60: 455-461 (in Japanese).
HIRAYAMA, C., OKUMURA, M., NAGAI, J. & MASUDA, Y. (1974)
Hypobilirubinemia in patients with polychlorinated biphenyls
poisoning. Clin. chim. Acta., 55: 97-100.
HOLDGATE, M. W. (1971) The sea bird wreck in the Irish Sea, Autumn
1969. Natural Environmental Research Council (Publication
Series C4).
HOLDEN, A. V. (1970a) International co-operative study of
organochlorine pesticide residues in terrestial and aquatic
wildlife 1967/1968. Pest. monit. J., 4: 117-135.
HOLDEN, A. V. (1970b) Source of polychlorinated biphenyl contamination
in the marine environment. Nature (Lond.), 228: 1220-1221.
HOLDEN, A. V. (1973a) International co-operative study of
organochlorine and mercury residues in wildlife, 1969-71. Pest.
monit. J., 7: 37-52.
HOLDEN, A. V. (1973b) Monitoring PCBs in water and wildlife. In:
PCB Conference II, Stockholm, Swedish Environment Protection
Board, Publication: 4E, pp. 23-33.
HOLDEN, A. V. & MARSDEN, K. (1969) Single-stage clean-up of animal
tissue extracts for organochlorine residue analysis.
J. Chromatogr., 44: 481-492.
HUTZINGER, O., JAMIESON, W. D., SAFE, S., PAULMANN, L. & AM , R.
(1974) Identification of metabolic dechlorination of highly
chlorinated bipheny bit. Nature (Lond.), 252: 698-699.
HUTZINGER, O., SASH, D. M., SAFE, S., DE FREITAS, A. S. W., NORSIROM,
R. J., WILDISH, D. J. & ZITKO, V. (1972a) Polychlorinated
biphenyls: metabolic behaviour of pure isomers in pigeons, rats
and brook trout. Science, 178: 312-313.
HUTZINGER, O., SAFE, S. & ZITKO, V. (1971) Polychlorinated biphenyls:
Synthesis of some individual chlorobiphenyls. Bull. environ.
Contam. Toxicol., 6: 209-219.
HUTZINGER, O., SAFE, S. & ZITKO, V. (1972b) Photochemical degradation
of chlorobiphenyls (PCBs). Environ. Health Perspect., 1: 15-20.
INNAMI, S. et al. (1974) PCB toxicity and nutrition II, PCB toxicity
and Vitamin A (2). J. Nutr. Sci-Vitaminol., 20: 363-370.
INTERNATIONAL AGENCY FOR RESEARCH ON CANCER (1974) Polychlorinated
biphenyls in IARC monographs on the evaluation of carcinogenic
risk of chemicals to man, Vol. 7, 261-289.
INTERNATIONAL COUNCIL FOR THE EXPLORATION OF THE SEA (1974) Report of
a Working Group for the international study of the pollution of
the North Sea and its effects on living resources and their
exploitation. Charlottenlund, Denmark (Co-operative Research
Report No. 39).
ISHI, H. (1972) PCB Pollution in Japan. Environ. Health Rep. No. 14,
Jpn. Publ. Health Assoc., pp. 13-28.
ITO, N., NAGASAKI, H, ARAI, M., MAKIURA, S., SUGIHARA, S. & HIRAO, K.
(1973) Histopathologic studies on liver tumorigenesis induced in
mice by technical polychlorinated biphenyls and its promoting
effect on liver tumours induced by benzene hexachloride. J. nat.
Cancer Inst., 51: 1637-1646.
IVERSON, F., VILLENEUVE, D.C., GRANT, D. L. & HATINA, G. V. (1975)
Effect of Aroclor 1016 and 1242 on selected enzyme systems in the
rat. Bull. environ. Contam. Toxicol., 13: 456-463.
IWATA, Y., WESTLAKE, W. E. & GUNTHER, F. A. (1973) Varying persistence
of polychlorinated biphenyls in six California soils under
laboratory conditions. Bull. environ. Contam. Toxicol.,
9: 204-211.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTROM, G. & VAZ,
R. (1975) Identification by GC-MS of phenolic metabolites of PCB
and p,p'-DDE isolated from Baltic guillemot and seal. Ambio.,
4: 93-97.
JENSEN, S. (1973) (no title) Report to Swedish National Environment
Protection Board, Stockholm, 12 October 1973.
JENSEN, S. (1974) Identification of some organic substances
potentially harmful to the environment. National Swedish
Environment Protection Board, University of Stockholm, Kollenberg
Laboratory (Publication SNV-PM 520).
JENSEN, S. & SUNDSTROM, G. (1974) Structures and levels of most
chlorobiphenyls in two technical PCB products and in human adipose
tissue. Ambio, 3: 70-76.
JENSEN, S. & SUNDSTROM, G. (1975) Metabolic hydroxylation of a
chlorobiphenyl containing only isolated unsubstituted positions --
2,2',4,4',5,5'-hexachlorobiphenyl. Nature (Lond.)
(in press).
JENSEN, S., JOHNELS, A. G., OLSSEN, M., OTTERLIND, G. (1969) DDT and
PCB in marine animals from Swedish waters. Nature (Lond.),
224: 247-250.
JENSEN, S., JOHNELS, A. G., OLSSON, M. & OTTERLIND, G. (1972b) DDT and
PCB in herring and cod from the Baltic, the Kattegat and the
Skaggerrak. Ambio spec. Rep., No. 1: 71-85.
JENSEN, S., JOHNELS, A. G., OLSSEN, M. & WESTERMARK, T. (1972c) The
avifaune of Sweden as indicators of environmental contamination
with mercury and chlorinated hydrocarbons. In: Proceedings of
the 15th International Ornithology Congress, Leiden,
pp. 455-465.
JENSEN, S., RENBERG, L. & OLSSON, M. (1972a) PCB contamination from
boat bottom paint and levels of PCB in plankton outside a polluted
area. Nature (London), 240: 358-360.
JENSEN, S., RENBERG, L. & VAZ, R. (1973) Problems in quantification
of PCB in biological material. In: PCB Conference II. National
Swedish Environment Protection Board. Publications: 4E, pp. 7-13.
JOHANSSON, N., JENSEN, S. & OLSSON, M. (1970) PCB indications of
effects on fish. In: PCB Conference, National Swedish
Environment Protection Board, pp. 59-67.
JOHNSTONE, G. J., ECOBICHON, D. J. & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, D.C. L., DAVIS, W. E., JR, NEWELL, G. W., SASMORE, D. P. &
ROSEN, V. J. (1974) Modification of hexachlorophene toxicity by
dieldrin and Aroclor 1254. Toxicology, 2: 309-318.
KAISER, K. L. E. & WONG, P. T. S. (1974) Bacterial degradation of
polychlorinated biphenyls. I. Identification of some metabolic
products from Aroclor 1242. Bull. environ. Contam. Toxicol.,
11: 291-296.
KARPPANEN, E. & KOLHO, L. (1973) The concentration of PCB in human
blood and adipose tissue in three different research groups. In:
PCB Conference II. National Swedish Environment Protection Board
Publications: 4E: pp. 124-127.
KATSUKI, S. (1969) Foreword. Fukuoka Acta Med., 60: 403-407.
KAWANISHI, S., SANO, S., MIZUTANI, T. & MATSUMOTO, M. (1973)
Experimental porphyria induced by polychlorinated biphenyls (in
Japanese). Jpn. J. Hyg., 28: 84.
KAWANISI, S., SANO, S., MIZUTANI, T. & MATSUMOTO, M. (1974)
Experimental studies on toxicity of synthetic tetrachlorobiphenyl
isomers (in Japanese). Jpn. J. Hyg., 29: 81.
KEIL, J. E., PRIESTER, L. E. & SANDIFER, S. H. (1971) Polychlorinated
biphenyl (Aroclor 1242): effects of uptake on growth, nucleic
acids and chlorophyll of a marine diatom. Bull. environ. Contam.
Toxicol., 6: 156-159.
KEPLINGER, M. L., FANeHER, O. E. & CALANDRA, J. C. (1971) Toxicologic
studies with polychlorinated biphenyls. Toxicol. appl.
Pharmacol., 19: 402-403.
KIHLSTROM, J. E., ORBERG, J., LUNDBERG, C., DANIELSSON, P.O. &
SYDHOFF, J. (1973) Effects of PCB on mammalian reproduction. In:
PCB Conference II. National Swedish Environment Protection Board
Publications 4E, pp. 109-111.
KIMBROUGH, R. D. (1974) The toxicity of polychlorinated polycyclic
compounds and related chemicals. Crit. Rev. Toxicol.,
2: 445-498.
KIMBROUGH, R. D. & LINDER, R. E. (1974) Induction of adenofibrosis and
hepatomas of the liver in BALB/cJ mice by polychlorinated
biphenyls (Aroclor 1254). J. nat. Cancer Inst., 53: 547-549.
KIMBROUGH, R. D., LINDER, R. E. & GAINES, T. B. (1972) Morphological
changes in livers of rats fed polychlorinated biphenyls. Arch.
environ. Health, 25: 354-364.
KIMBROUGH, R. D., LINDER, R. E., BURSE, V. W., JENNINGS, R. W. & GA,
C. (1973) Adenofibrosis in the rat liver with persistence of
polychlorinated biphenyls in adipose tissue. Arch. environ.
Health, 27: 389-395.
KIMBROUGH, R. D., SQUIRE, R. A., LINDER, R. E., STRANDBERG, J. D.,
MONTALI, R. J. & BURSE, V. W. (1975) Induction of liver tumours in
Sherman strain female rats by polychlorinated biphenyl Aroclor
1260. J. nat. Cancer Inst., 55: 1453-1459.
KIMURA, N. T. & BABA, T. (1973) Neoplastic changes in the rat liver
induced by polychlorinated biphenyl. GANN, 64: 105-108.
KITAMURA, M., TSUKAMOTO, T., SUMINO, K., HAYAKAWA, K., SHIBITA, T. &
HIRANO, I. (1973) The PCB levels in the blood of workers employed
in a condenser factory. Jpn. J. indust. Health, 47: 354-355
(in Japanese).
KOBAYASHI, Y. (1972) Answer Report to the Questionary paper on
"Regulation of Residual level in Foods". Biol. Pollut.,
4: 93-116 (in Japanese).
KOEMAN, J. H., TEN NOEVER DE BRAUW & DE VOS, R. H. (1969) Chlorinated
biphenyls in fish, mussels and birds from the river Rhine and the
Netherlands coastal area. Nature (Lond), 221: 1126-1128.
KOEMAN, J. H., BOTHOF, TH., DE VRIES, R., VAN VELZEN-BLAD, H. & VOS,
J. G. (1972a) The impact of persistent pollutants on piscivorous
and molluscivorous birds. TNO-nieuws, 27: 561-569.
KOEMAN, J. H., PEETERS, W. H. M., SMIT, C. J., TJIOE, P, S. & DE
GOEIJ, J. J. M. (1972b) Persistent chemicals in marine mammals.
TNO-nieuws, 27: 570-578.
KOEMAN, J. H., VAN VELZEN-BLAD, H. C. W., DE VRIES, R. & Vos, J. G.
(1973) Effects of PCB and DDE in cormorants and evaluation of PCB
residues from an experimental study. J. Reprod. Fertil.,
19 (Suppl.): 353-364.
KOHANAWA, M., SHOYA, S., OGURA, Y., MORIWAKI, M. & KAWASAKI, M.
(1969a) Poisoning due to an oily by-product of rice-bran similar
to chick edema disease. I. Occurrence and toxicity test. Nat.
Inst. anim. Health Quart., 9: 213-219.
KOHANAWA, M., SHOYA, S., YONEMURA, T., NISHIMURA, K. & TSUSHIO, Y.
(1969b) Poisoning due to an oily by-product of rice-bran similar
to chick edema disease. II. Tetrachlorodiphenyl as toxic
substance. Nat. Inst. anim. Health Quart., 9: 220-228.
KOLBYE, A. C. JR (1972) Food exposures to polychlorinated biphenyls.
Environ. Health Perspect., 1: 85-88.
KOLLER, L. D. & ZINKL, J. G. (1973) Pathology of polychlorinated
biphenyls in rabbits. Am. J. Pathol., 70: 363-373.
KOMATSU, F. & KIKUCHI, M. (1972) Skin lesions by 3,4,Y,4'-tetra-
chlorobiphenyl in rabbits. Fukuoka Acta Med., 63: 384-386
(in Japanese).
KURATSUNE, M. (1972a) PCB pollution. Kosei no Shihyo, 19: 11-18
(in Japanese). KURATSUNE, M. & MASUDA, Y. (1972b) Polychlorinated
biphenyls in non-carbon copypaper. Environ. Health Perspect.,
1: 61-62.
KURATSUNE, M., YOSHIMURA, T. & MATSUZAKA, J. (1972) Epidemiologic
study on Yusho, a poisoning caused by ingestion of rice oil
contaminated with a commercial brand of polychlorinated biphenyls.
Environ. Health Perspect., 1: 119-128.
KUSUDA, M. (1971) Study on the female sexual function suffering from
the chlorobiphenyls poisoning. Sanka to Fujinka, 4: 1063-1072
(in Japanese).
LINCER, J. L. & PEAKALL, D. B. (1970) Metabolic effects of
polychlorinated biphenyls in the American kestrel. Nature
(Lond.), 228: 783-784.
LICHTENSTEIN, E. P. (1972) PCBs and interactions with insecticides.
Environ. Health Perspect., 1: 151-153.
LIEB, A. J., BILLS, D. B. & SINNHUBER, R. O. (1974) Accumulation of
dietary polychlorinated biphenyls (Aroclor 1254) by rainbow trout
(Salmo gairdneri). J. agr. Food Chem., 22: 638-642.
LINDER, R. E., GAINES, T. B. & KIMBROUGH, R. D. (1974) The effect of
polychlorinated biphenyls on rat reproduction. Food cosmet.
Toxicol., 12: 63-77.
LITTERST, C. L., FARBER, T. M., BAKER, A.M. & VAN LOON, E. J. (1972)
Effect of polychlorinated biphenyls on hepatic microsomal enzymes
in the rat. Toxicol. appl. Pharmacol., 23: 112-122.
MAKIURA, S., AOE, H., SUGIHARA, S., HIRAO, K., ARAI, M. & ITO, N.
(1974) Inhibitory effect of polychlorinated biphenyls on liver
tumorigenesis in rats treated with 3'-methyl-4-dimethyl-
aminoazobenzene, N-2-fluorenylacetamide, and diethylnitrosamine.
J. nat. Cancer Inst., 53: 1253-1257.
MASUDA, Y., KAGAWA, R. & KURATSUNE, M. (1972) Polychlorinated
biphenyls in carbonless copying paper. Nature (Lond.),
237: 41-42.
MASUDA, Y., KAGAWA, R. & KURATSUNE, M. (1974a) Polychlorinated
biphenyls in Yusho patients and ordinary persons. Fukuoka Acta
Med., 65: 17-24 (in Japanese).
MASUDA, Y., KAGAWA, R., SHIMAMURA, K., TAKADA, M. & KURATSUNE, M.
(1974b) Polychlorinated biphenyls in the blood of Yusho patients
and ordinary persons. Fukuoka Acta Med., 65: 25-27
(in Japanese).
MCKINNEY, J. D. (in press) Toxicology of selected symmetrical
hexachlorobiphenyl isomers : correlating biological effects with
chemical structure. In: Proceedings of the National Conference
on Polychlorinated Biphenyls, Chicago, 19-21 November, 1975.
MCLAUGHLIN, J. JR., MARLIAC, G. P., MERRETT, M. J., MUTCHLER, M. K. &
FITZHUGH, O. G. (1963) The injection of chemicals into the yolk
sac of fertile eggs prior to incubation as toxicity test.
Toxicol. appl. Pharmacol., 5: 760-771.
MELVÅS, B. & BRANDT, I. (1973) The distribution and metabolism of
labelled polychlorinated biphenyls in mice and quails. In: PCB
Conference II. National Swedish Environment Protection Board
Publications: 4E, pp. 87-90.
MES, J., COFFIN, D. E. & CAMPBELL, D. (1974) Polychlorinated biphenyl
and organochlorine pesticide residues in Canadian chicken eggs.
Pestic. Monit. J., 8: 8-11.
MIURA, H., OMORI, S., KATOH, M. (1973) Experimental porphyria in
rabbits induced by PCB. Jpn. J. Hyg, 28: 83 (in Japanese).
MOORE, J. A. (in press) Toxicity of 2,3,7,8-tetrachlorodibenzofuran:
preliminary results. Proceedings of the National Conference on
Polychlorinated Biphenyls, Chicago, 19-21 November.
MOSSER, J. L., FISHER, N. S., TENG, T. & WURSTER, C. F. (1972)
Polychlorinated biphenyls: toxicity to certain phytoplankters.
Science, 175: 191-192.
MULHERN, B. M., CROMARTIE, E., REICHEL, W. L. & BELISLE, A. A. (1971)
Semiquantitative determination of polychlorinated biphenyls in
tissue samples by thin layer chromatography. J. Assoc. Off.
Agric. Chem., 54: 548-550.
MORNS, Y. & KUROIWA, Y. (1971) Peripheral neuropathy in chlorobiphenyl
poisoning. Neurol., 21: 1173-1176.
MUSIAL, C. J., HUTZINGER, O., ZITKO, V. & CROCKER, J. (1974) Presence
of PCB, DDE and DDT in human milk in the provinces of New
Brunswick and Nova Scotia, Canada. Bull. environ. Contam.
Toxicol., 12: 258-267.
NAGAI, J., FURUKAWA, M., YAE, Y. & IKEDA, Y. (1969) Clinicochemical
investigation of chlorobiphenyls poisoning. Especially on the
serum lipid analysis of the patients. Fukuoka Acta Med.,
60: 475-488 (in Japanese).
NAGAYAMA, J., KURATSUNE, M. & MASUDA, Y. (1976) Determination of
chlorinated dibenzofurans in Kanechlors and "Yusho Oil". Bull.
environ. Contam. Toxicol., 15(1): 9-13.
NAGAYAMA, J., MASUDA, Y. & KURATSUNE, M. (1975) Chlorinated
dibenzofurans in Kanechlors and rice oils used by patients with
Yusho, Fukuoka Acta Med., 66: 593-599.
NATIONAL SWEDISH ENVIRONMENT PROTECTION BOARD (1973) PCB Conference
II. Publication 1973: 4E.
NEBEKER, A. V., PUGLISI, F. h. & DEFOE, D. L. (1974) Effect of
polychlorinated biphenyl compounds on survival and reproduction of
the fathead minnow and flagfish. Trans. Am. Fish Soc.,
No. 3, 562-568.
NESTEL, H. & BUDD, J. (1975) Chronic oral exposure of rainbow trout
(Salmo Gairdneri) to a PCB (Aroclor 1254): pathological effects.
Can. J. comp. Med., 39: 208-215.
NILSSON, B. & RAMEL, C. (1974) Genetic tests on Drosophila
melanogaster with polychlorinated biphenyls (PCB). Heriditas,
77: 319-322.
NIMMO, D. R., WILSON, P. D., BLACKMAN, R. R. & WILSON, A. J. JR (1971)
Polychlorinated biphenyl absorbed from sediments by fiddler crabs
and pink shrimp. Nature (Lond.), 231: 50-52.
NISBET, I. C. T. & SAROFIM, A. F. (1972) Rates and routes of transport
of PCBs in the environment. Environ. Health Perspect. 1: 21-38.
NISHIMOTO, T, UEDAM, M, TAUL, S. & CHIKAZAWA, K. (1972a)
Organochlorine pesticide residues and PCB in breast milk. Igaku
no Ayumi (Proc. Med. Sci.), 82: 574-575 (in Japanese).
NISHIMOTO, T., UETA, M., TAUL, S., CHIKAZAWA, K. NISHIUCHI, I. &
KONDO, K. (1972b) Deposition of organochlorine pesticide residues
and PCB in human body fat. Igaku no Ayumi (Proc. med. Sci.),
82: 515-516.
NISHIZUMI, M. (1970) Light and electron microscope study of
chlorobiphenyl poisoning in mouse and monkey liver. Arch.
environ. Health, 21: 620-632.
NORÉN, K. & WESTOO, G. (1968) Determination of some chlorinated
pesticides in vegetable oils, margarine, butter, milk, eggs, meat
and fish by gas chromatography and thin-layer chromatography.
Acta chem. scand., 22: 2289-2293.
ODÉN, S. & BERGGREN, B. (1973) PCB and DDT in Baltic sediments.
Report Dept. Soil Science, Agricultural College, Uppsala,
pp. 1-10.
ODSJO, T. (1973) PCB in some Swedish terrestrial organisms. In: PCB
Conference II. National Swedish Environment Protection Board
Publications: 4E, pp. 45-58. OKUMURA, M. & KATSUKI, S. (1969)
Clinical observation on Yusho (chlorobiphenyls poisoning).
Fukuoka Acta Med., 60: 440-446 (in Japanese).
OKUMURA, M., MASUDA, Y. & NAKAMUTA, S. (1974) Correlation between
blood PCB and serum triglyceride levels in patients with PCB
poisoning. Fukuoka Acta Med., 65: 84-87 (in Japanese).
OLSSON, M, JENSEN, S. & RENBERG, L. (1973) PCB in coastal areas of
the Baltic. PCB Conference H. National Swedish Environment
Protection Board, Publications: 4E, pp. 59-68.
ORBERG, J. & KIHLSTROM, J. E. (1973) Effects of long-term feeding of
polychlorinated biphenyls (PCB, Clopehn A60) on the length of the
oestrous cycle and on the frequency of implanted ova in the mouse.
Environ. Res., 6: 176-179.
ORGANIZATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT (1973)
Polychlorinated biphenyls, their use and control. Environmental
Directorate, Paris, OECD.
PANEL ON HAZARDOUS TRACE SUBSTANCES (1972) PCBs-Environmental Impact.
Environ. Res. 5: 249-362.
PEAKALL, D. B. LIMIAR, J. L. & BLOOM, S. E. (1972) Embryonic mortality
and chromosomal alterations caused by Aroclor 1254 in ring doves.
Environ. Health Perspect., 1: 103-104.
PESENDORFER, VON H., EICHLER, I. & GLOFKE, E. (1973) Informative
analyses or organochlorine pesticide and PCB residues in human
adipose tissue (from the area of Vienna). Wiener klinische
Wochenschr., 85: 218-222 (summary in English).
PHILLIPS, W. E. J., HATINA, G., VILLENEUVE, D.C. & GRANT, D. L. (1972)
Effect of parathion administration in rats following long-term
feeding with PCBs. Environ. physiol. Biochem., 2: 165-169.
PJCHIRALLO, J. (1971) PCBs: leaks of toxic substances raises issue of
effects, regulations. Science, 173: 899-902.
PLATONOW, N. S. & FUNNELL, H. S. (1971) Anti-androgenic-like effect of
polychlorinated biphenyls in cockerels. Vet. Rec, 88: 109-110.
PLATONOW, N. & CHEN, N.Y. (1973) Transplacental transfer of
polychlorinated biphenyls (Aroclor 1254) in a cow. Vet. Rec.,
92: 69-70.
PLATONOW, N. S., FUNNELL, H. S., BULLOCK, D. H., ARNOTT, D. R.,
SASCHENBRECKER, P. W. & GRIEVE, D. O. (1971) Fate of
polychlorinated biphenyls in dairy products processed from the
milk of exposed cows. J. Dairy Sci., 54: 1305-1308.
PLATONOW, N. S., LIPTRAP, R. M. & GEISSINGER, H. D. (1972) The
distribution and excretion of polychlorinated biphenyls (Aroclor
1254) and their effect on urinary gonadal steroid levels in the
boar. Bull. environ. Contam. Toxicol., 7: 358-365.
PORTER, M. L., YOUNG, S. J. V. & BURKE, J. A. (1970) A method for the
analysis of fish, poultry and animal tissue for chlorinated
pesticide residues. J. Am. Off. Anal. Chem., 53: 1300-1303.
PORTMANN, J. E. (1970) Monitoring of organochlorine residues in fish
from around England and Wales, with special reference to
polychlorinated biphenyls. Report CM 1970/E: 9 International
Council for the Exploration of the Sea, Charlottenlund Slot,
DK-2920 Charlottenlund, Denmark.
PRESTT, I., JEFFERIES, D. J. & MOORE, N. W. (1970) Polychlorinated
biphenyls in wild birds in Britain and their avian toxicity.
Environ. Pollut., 1: 3-26.
PRICE, H. A. & WELCH, R. L. (1972) Occurrence of polychlorinated
biphenyls in humans, Environ. Health Perspect., 1: 73-78.
REHFELD, B. M., BRADLEY, R. L. JR & SUNDE, M. L. (1971) Toxicity
studies on polychlorinated biphenyls in the chick. 1. Toxicity and
symptoms. Poult. Sci., 50: 1090-1096.
REINKE, J., UTHE, J. F. & O'BRODOVlCH, H. (1973) Determination of
polychlorinated biphenyls in the presence of organochlorine
pesticides by thin-layer chromatography. Environ. Lett.,
4: 201-210.
REYNOLDS, L. M. (1971) Pesticide residue analysis in the presence of
polychlorobiphenyls (PCBs). Residue Rev., 34: 27-45.
RINGER, R. K., AULERICH, R. J. & ZABIK, M. (1972) Effect of dietary
polychlorinated biphenyls on growth and reproduction of mink.
Proc. Amer. chem. Soc., 164th A.C.S. Meeting, New York,
pp. 149-154.
RISEBROUGH, R. W. & DE LAPPE, B. (1972) Accumulation of
polychlorinated biphenyls in ecosystems. Environ. Health
Perspect., 1: 39-45.
RISEBROUGH, R. W., RIECHE, P., PEAKALL, D. B., HERMAN, S. G. & KIRVEN,
M. N. (1968) Polychlorinated biphenyls in the global ecosystem.
Nature (Lond.), 220: 1098-1102.
ROACH, J. A. G. & POMERANTZ, I. H. (1974) The finding of chlorinated
dibenzofurans in a Japanese polychlorinated biphenyl sample.
Bull. environ. Contam. Toxicol., 12: 338-342.
ROTE, J. W. & MURPHY, P. G. (1971) A method for the quantitation of
polychlorinated biphenyl (PCB) isomers. Bull environ. Contam.
Toxicol., 6: 377-384.
SAFE, S. & HUTZINGER, O. (1971) Polychlorinated biphenyls: photolysis
of 2,4,6,T,4',6'-hexachlorobiphenyl, Nature (Lond.),
232: 641-642.
SAITO, R., SHIGEMATSU, N. & ISHIMARU, S. (1972) Immunoglobulin levels
in serum and sputum of patients with PCB poisoning. Fukuoka Acta
Med., 63: 408-411 (in Japanese).
SANDERS, H. O. & CHANDLER, J. H. (1972) Biological magnification of a
polychlorinated biphenyl (Aroclor 1254) from water by aquatic
invertebrates. Bull. environ. Contam. Toxicol., 7: 257-263.
SASCHENBRECKER, P. W., FUNNELL, H. S. & PLATONOW, N. S. (1972)
Persistence of polychlorinated biphenyls in the milk of exposed
cows. Vet. Rec., 90: 100-102.
SAVAGE, E. P., TESSARI, J. D., MALBERG, J. W., WHEELER, H. W. & BAGBY,
J. R. (1973) A search for polychlorinated biphenyls in human milk
in rural Colorado. Bull. environ. Contam. Toxicol., 9: 222-226.
SCHMIDT, T. T., RISEBROUGH, R. W. & GRESS, F. (1971) Input of
polychlorinated biphenyls into California coastal waters from
urban sewage outfalls. Bull. environ. Contam. Toxicol.,
6: 235-243.
SCHULTE, E. & ACKER, L. (1974) Gas chromatographic unit Glascapillaran
bei Temperaturan bis zu 320°C. Z. Anal. Chem., 268: 261-267.
SCOTT, M. L., ZIMMERMAN, J. R., MARINSKY, S. & MULLENHOFF, P. A.
(1975) Effects of PCBs, DDT and mercury compounds upon egg
production, hatchability and shell quality in chickens and
Japanese quail. Poult. Sci., 54: 350-368.
SCHWETZ, B. A., NORRIS, J. M., SPARSCHU, G. L., ROWE, U. K., GEHRING,
P. J., EMERSON, J. L. & GERBIG, C. G. (1973) Toxicology of
chlorinated dibenzo- p-dioxin. Environ. Health Perspect.,
5: 87-99.
SHIGEMATSU, N., NORIMATSU, Y., ISHIBASHI, T., YOSHIDA, M., SUETSUGU,
S., KAWATSU, T., IKEDA, T., SAITO, R., ISHIMARU, S., SHIRAKISA,
T., KIDO, M., EMORI, K. & TOSHIMITSU, H. (1971) Clinical and
experimental studies on respiratory involvement in chlorobiphenyls
poisoning. Fukuoka Acta Med., 62: 150-156 (in Japanese).
SHIGEMATSU, N., ISHIMARU, S., HIROSE, T., IKEDA, T., EMORI, I. &
MIYAZAKI, N. (1974) Clinical and experimental studies on
respiratory involvement in PCB poisoning. Fukuoka Acta Med.,
65: 88-95 (in Japanese).
SISSONS, D. & WELTI, D. (1971) Structural identification of
polychlorinated biphenyls in commercial mixtures by gas-liquid
chromatography, nuclear magnetic resonance and mass spectrometry.
J. Chromatogr., 60: 15-32.
SODERGREN, A. (1972a) Chlorinated hydrocarbon residues in airborne
fallout. Nature (Lond.), 236: 395-397.
SODERGREN, A. (1972b) Transport, distribution and degradation of
chlorinated hydrocarbon residues in aquatic model ecosystems.
Oikos, 23: 30-41.
SODERGREN, A. (1973a) Transport, distribution and degradation of DDT
and PCB in a south Swedish lake ecosystem. Vatten, 2: 90-108.
SODERGREN, A. (1973b) A simplified clean-up technique for
organochlorine residues at the microliter level. Bull. environ.
Contam. Toxicol., 10: 116-119.
SODERGREN, A. & SVENSSON, Bj. (1973) Uptake and accumulation of DDT
and PCB by Ephemera danica (Ephemeroptera) in continuous-flow
systems. Bull. environ. Contam. Toxicol., 9: 345-350.
SODERGREN, A., SVENSSON, Bj. & ULFSTRAND, S. (1972) DDT and PCB in
South Swedish streams. Environ. Pollut., 3: 25-36.
SOLLY, S. R. B. & SHANKS, V. (1974) Polychlorinated biphenyls and
organochlorine pesticides in human fat in New Zealand. N.Z.J.
Sci., 17: 535-544.
SOSA-LUCERO, J. C. & BE LA IGLESIA, F. A. (1973) Distribution of a
polychlorinated terphenyl (PET) (Aroclor 5460) in rat tissues and
effect on hepatic microsomal mixed function oxidases. Bull.
environ. Contam. Toxicol., 10: 248-256.
STALLING, D. L. & MAYER, F. L. JR (1972) Toxicities of PCBs to fish
and environmental residues. Environ. Health Perspect.,
1: 159-164.
STALLING, D. L., TRINDLE, R. C. & JOHNSON, J. L. (1972) Clean up of
pesticides and polychlorobiphenyl residues in fish extracts by gel
permeation chromatography. J. Assoc. Off. Anal. Chem.,
55: 32-45.
TAKAMATSU, M., INOUE, Y. & ABE, S. (1974) Diagnostic meaning of the
blood PCB. Fukuoka Acta Med., 65:28-31 (in Japanese).
TAKI, I., HISANAGA, S. & AMAGESE, Y. (1969) Report on Yusho
(chlorobiphenyl poisoning). Especially further study of its
dermatological findings. Fukuoka Acta Med., 62: 132-138
(in Japanese).
TAKI, I., KURATSUNE, M., MASUDA, Y. (1973) Studies on the
transmission to the fetus through placenta and the health of
and baby. Special Research Report on the effects of PCBs on human
health such as chronic toxicity for prevention of pollution by
PCBs. Research Co-ordination Bureau, Science and Technology
Agency, pp. 87-95 (in Japanese).
TAKIZAWA, Y. & MINAGAWA, K. (1974) Studies on environmental
accumulation and bio-accumulation of organochloric compounds.
Mainly mentioned PCT. Studies on the body effect of
degradation-registration substances, pp. 29-50 (in Japanese).
TANAKA, K. & ARAKI, Y. (1974) Inhibitory effect of cholestyramine on
the intestinal absorption of PCB. Fukuoka Acta Med., 68: 53-57.
TANAKA, K. & KOMATSU, F. (1972) Shortening of hexobarbital sleeping
time after small doses of PCB in rats. Fukuoka Acta Med.,
63: 360-366 (in Japanese).
TAS, A. C. & DE VOS, R. H. (1971) Characterization of four major
components in a technical polychlorinated biphenyl mixture.
Environ. Sci. Technol., 5: 1216-1218.
TATSUKAWA, R. & WATANABE, I. (1972) Air pollution by PCBs. Shoku No
Kagaku, 8: 55-63.
THOMAS, G. H. & REYNOLDS, L. M. (1973) Polychlorinated terphenyls in
paperboard samples. Bull. environ. Contam. Toxicol.,
10: 37-41.
TREON, J. F., CLEVELAND, F. P., CAPPEL, J. W. & ATCHLEY, R. W. (1956)
The toxicity of the vapours of Aroclor 1242 and Aroclor 1254.
Am. Ind. Hyg. Assoc. Quart., 17: 204-213.
TSUKAMOTO, H. ET AL. (1969) The chemical studies on detection of toxic
compounds in the rice bran oils used by the patients of Yusho.
Fukuoka Acta Med., 60: 496-512 (in Japanese).
TUCKER, R. K. & CRABTREE, D. G. (1970) Handbook of toxicity of
pesticides to wildlife. Bureau of Sport, Fisheries and Wildlife
Resources Pub. No. 84, Washington, DC, US Dept. of Interior,
pp. 130.
TUCKER, E. S., LITSCHGI, W. S. & MEES, W. M. (1975) Migration of
polychlorinated biphenyls in soil induced by percolating water.
Bull. environ. Contam. Toxicol., 13: 86-93.
URABE, H. (1974) Foreword. Fukuoka Acta Med., 65: 1-4 (in Japanese).
USDA/USDC/EPA/FDA/USDI/(1972) Polychlorinated biphenyls and the
environment. Washington DC, Interdepartmental Task Force on
PCBs. (Report ITF-PCB-72-1).
USHIO, F., FUKANO, S., NISHIDA, K., KANI, T. & DOGUCHI, M. (1974) Some
attempt to estimate the total daily intake of pesticides and PCB
residues and trace heavy metals. Ann. Rep. Tokyo Metr. Res. Lab.
P.H., 25: 307-312 (in Japanese).
UTHE, J. F., REINKE, J. & GESSER, H. (1972) Extraction of
organochlorine pesticides from water by porous polyurethane coated
with selective absorbent. Environ. Lett., 3: 117-135.
UZAWA, H., ITO, Y., NOTOMI, A. & KATSUKI, S. (1969) Hyperglyceridemia
resulting from intake of rice oil contaminated with chlorinated
biphenyls. Fukuoka Acta Med., 60: 449-454 (in Japanese).
UZAWA, H., NOTOMI, A., NAKAMUTA, S. & IKEURA, Y. (1972) Consecutive
three year follow up study of serum triglyceride concentrations of
82 subjects with PCB poisoning. Fukuoka Acta Med., 63: 401-404
(in Japanese).
VAN HOVE HOLDRINET, M. (1975) Preliminary results of an inter-
laboratory PCB check sample programme. J. Environ. Qual. (in press).
VEITH, G. D. (1972) Recent fluctuations of chlorobiphenyls (PCBs) in
the Green Bay, Wisconsin, region. Environ. Health Perspect.,
1: 51-54.
VILLENEUVE, D. C., GRANT, D. L., PHILLIPS, W. E. J., CLARK, M. L. &
CLEGG, D. J. (1971 a) Effects of PCB administration on microsomal
enzyme activity in pregnant rabbits. Bull. environ. Contam.
Toxicol., 6: 120-128.
VILLENEUVE, D. C., GRANT, D. L., KHERA, K., CLEGG, D. J., BAER, H. &
PHILLIPS, W. E. J. (1971b) The fetotoxicity of a polychlorinated
biphenyl mixture (Aroclor 1254) in the rabbit and in the rat.
Environ. Physiol., 1: 67-71.
VILLENEUVE, D.C., GRANT, D. L. & PHILLIPS, W. E. J. (1972)
Modification of pentobarbital sleeping times in rats following
chronic PCB ingestion. Bull. environ. Contam. Toxicol.,
7: 264-269.
VILLENEUVE, D.C., REYNOLDS, L. M., THOMAS, G. H. & PHILLIPS, W. E. J.
(1973a) Polychlorinated biphenyls and polychlorinated terphenyls
in Canadian food packaging materials. d. Assoc. Offic. Anal.
Chem., 56: 999-1001.
VILLENEUVE, D.C., REYNOLDS, L. M. & PHILLIPS, W. E. J. (1973b)
Residues of PCBs and PCTs in Canadian and imported European
cheeses, Canada-1972. Pestic. Monit. J., 7: 95-96.
VODDEN, H. A. (1973) Discussion In: PCB Conference II. National
Swedish Environment Protection Board, Publications: 4E, p. 118.
VOS, J. G. (1972) Toxicology of PCBs for mammals and for birds. Era,.
Health Perspect., 1: 105-117.
VOS, J. G. & BEEMS, R. B. (1971) Dermal toxicity studies of technical
polychlorinated biphenyls and fractions thereof in rabbits.
Toxicol. appl. Pharmacol., 19: 617-633.
VOS, J. G. DE ROIJ, Th. (1972) Immunosuppressive activity of a
polychlorinated biphenyl preparation on the humoral immune
response in guinea pigs. Toxicol. appl. Pharmacol., 21: 549-555.
VOS, J. G. & KOEMAN, J. H. (1970) Comparative toxicologic study with
polychlorinated biphenyls in chickens with special reference to
porphyria, edema formation, liver necrosis and tissue residues.
Toxicol. appl. Pharmacol., 17: 656-668.
VOS, J. G. & NOTENBOOM-RAM, E. (1972) Comparative toxicity study of
2,4,5,2',4',5'-hexachlorobiphenyl and a polychlorinated biphenyl
mixture in rabbits. Toxicol. appl. Pharmacol., 23: 563-578.
VOS, J. G. & VAN DRIEL-GROOTENHUIS, L. (1972) PCB-induced suppression
of the humoral and cell-mediated immunity in guinea pigs. Sci.
Total Environ., 1: 289-302.
VOS, J. G., KOEMAN, J. H., VAN DER MAAS, H. L., TEN NOEVER DE BRAUW,
M. G. & DE VOS, R. E. (1970) Identification and toxicological
evaluation of chlorinated dibensofuran and chlorinated naphthalene
in two commercial polychlorinated biphenyls. Fd. cosmet.
Toxicol., 8: 625-633.
VOS, J. G., STRIK, J. J. T. W. A., VAN HOLSTEYN, C. M. W. & PENNINGS,
J. H. (1971) Polychlorinated biphenyls as inducers of hepatic
porphyria in Japanese quail, with special reference to
delta-aminolevulinic acid synthetase activity, fluorescence and
residues in the liver. Toxicol. appl. Pharmacol., 20: 232-240.
WEBB, R. G. & MCCALL, A. C. (1972) Identification of polychlorinated
biphenyl isomers in aroclors. J. Am. Off. Anal. Chem.,
55: 746-752.
WESTOO, G. & NORÉN, K. (1970a) Levels of organochlorine pesticides and
polychlorinated biphenyls in fish caught in Swedish water areas or
kept for sale in Sweden, 1967-1970. Var Föda, 3: 93-146
(summary in English).
WESTOO, G. & NORÉN, K. (1970b) Determination of organochlorine
pesticides and polychlorinated biphenyls in animal foods. Acta
Chem. Scand., 24: 1639-1644.
WESTOO, G. & NORÉN, K. (1972) Levels of organochlorine pesticides and
polychlorinated biphenyls in Swedish human milk. Var Föda,
24: 41-54 (summary in English).
WESTOO, G., NORÉN, K. & ANDERSSON, M. (1971) Levels of organochlorine
pesticides and polychlorinated biphenyls in some cereal products.
Var Föda, 10: 341-360 (summary in English).
WHO WORKING GROUP (1973) The hazards to health and ecological effects
of persistent substances in the environment -- polychlorinated
biphenyls. Report of a Working Group convened by the World
Health Organization, Regional Office for Europe, EURO, 3109(2).
WILDISH, D. J. (1970) The toxicity of polychlorinated biphenyls (PCB)
in sea water to Grammarus oceanicus. Bull. environ. Contam.
Toxicol., 5: 202-204.
WILLIAMS, R. & HOLDEN, A. V. (1973) Organochlorine residues from
plankton. Mar. Bull., 4, 109-111.
WILLIS, D. E. & ADDISON, R. F. (1972) Identification and estimation of
the major components of a commercial polychlorinated biphenyl
mixture, Aroclor 1221. J. Fish. Res. Board Can., 29(5): 592-595.
WONG, P. T. S. & KAISER, K. L. E. (1975) Bacterial degradation of
polychlorinated biphenyls. II. Rate studies. Bull. environ.
Contam. Toxicol, 13: 249-256.
YAGAMUCHI, A., YOSHIMURA, T. & KURATSUNE, M. (1971) A survey on
pregnant women having consumed rice oil contaminated with
chlorobiphenyls and their babies. Fukuoka Acta Med.,
62: 112-117 (in Japanese).
YAMAMOTO, H. & YOSHIMURA, H. (1973) Metabolic studies on
polychlorinated biphenyls. III. Complete structure and acute
toxicity of the metabolites of 2,4,3',4'-tetrachlorobiphenyl.
Chem. pharm. Bull., 21 (10): 2237-2242 (in English).
YOBS, A. R. (1972) Levels of polychlorinated biphenyls in adipose
tissue of the general population of the nation. Environ. Health
Perspect., 1: 79-81.
YOSHIMURA, T. (1971) Epidemiological analysis of "Yusho" patients with
special reference to sex, age, clinical grades and oil
consumption. Fukuoka Acta Med., 62: 109-116 (in Japanese).
YOSHIMURA, H. & YAMAMOTO, H. (1973) Metabolic studies on
polychlorinated biphenyls. I. Metabolic fate of
3,4,3',4'-tetrachlorobiphenyl in rats. Chem. pharm. Bull.,
21: 1168-1169 (in English).
YOSHIMURA, H. & YAMAMOTO, H. (1974) Metabolic studies on
polychlorinated biphenyls. IV. Biotransformation of
3,4,3',4'-tetrachlorobiphenyl, one of the major components of
Kanechlor-400. Fukuoka Acta Med., 65: 5-11 (in Japanese).
YOSHIMURA, H. & YAMAMOTO, H. (1975) A novel route of excretion of
2,4,3',4'-tetrachlorobiphenyl in rats. Bull. environ. Contam.
Toxicol., 13: 681-687.
YOSHIMURA, H., YAMAMOTO, H., NAGAI, J., YAE, Y., UZAWA, H., ITO, Y.,
NOTOMI, A., MIMAKAMI, S., ITO, A., KATO, K. & TSUJI, H. (1971)
Studies on the tissue distribution and the urinary and faecal
excretion of 3H-Kanechlor (chlorobiphenyls) in rats. Fukuoka
Acta Med., 62: 12-19 (in Japanese).
YOSHIMURA, H., YAMAMOTO, H. & SAEKI, S. (1973) Metabolic studies on
polychlorinated biphenyls. II. Metabolic fate of
2,4,3'4'-tetrachlorobiphenyl in rats. Chem. pharm. Bull.,
21: 2231-2236 (in English).
YOSHIMURA, H., YAMAMOTO, H. & KINOSHITA, H. (1974) Metabolic studies
on polychlorinated biphenyls. V. Biliary excretion of
5-hydroxy-2,4,3',4',-tetrachlorobiphenyl, a major metabolite of
2,4,3',4'-tetrachlorobiphenyl. Fukuoka Acta Med., 65: 12-16
(in Japanese).
ZIMMERLI, B. & MARES, B. (1973) Die Belastung der Schweizerischen
Bevolkerung mit Pestiziden. Mitt. Lebensm. Unters. Hyg.,
64: 459-479.
ZITKO, V. (1970) Polychlorinated biphenyls (PCB) solubilized in water
by nonionic surfactants for studies of toxicity to aquatic
animals. Bull. environ. Contam. Toxicol., 5: 279-285.
ZITKO, V. (1971) Polychlorinated biphenyls and organochlorine
pesticides in some fresh-water and marine fishes. Bull. environ.
Contam. Toxicol., 6: 464-470.
ZITKO, V., HUTZINGER, O. & SAFE, S. (1971) Retention times and
electron-capture detector responses of some individual
chlorobiphenyls. Bull. environ. Contam. Toxicol., 6: 160-163.
ZITKO, V., HUTZINGER, O., JAMIESON, W. D. & CHOI, P. M. K. (1972a)
Polychlorinated terphenyls in the environment. Bull. environ.
Contam. Toxicol., 7: 200-201.
ZITKO, V., HUTZINGER, O. & CHOI, P.M. K. (1972b) Contamination of the
Bay of Fundy-Gulf of Maine area with polychlorinated biphenyls,
polychlorinated terphenyls, chlorinated dibenzodioxins and
dibenzofurans. Environ. Health Perspect., 1: 47-50.
ZITKO, V., CHOI, P. M. K., WILDISH, D. J., MONAGHAN, C. F. & LISTER,
N. A. (1974) Distribution of PCBs and p,p'-DDE residues in
Atlantic herring and yellow perch in eastern Canada, 1972.
Post. Mon. J., 8: 105-109.
