
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 63
First draft prepared by Mr P.D. Howe, Mr H.M. Malcolm, and Dr S. Dobson,
Centre for Ecology & Hydrology, Monks Wood, United Kingdom
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2004
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Manganese and its compounds : environmental aspects.
(Concise international chemical assessment document ; 63)
1.Manganese - toxicity 2.Risk assessment 3.Environmental
exposure I.International Programme on Chemical Safety
II.Series
ISBN 92 4 153063 4 (LC/NLM Classification: QV 290)
ISSN 1020-6167
©World Health Organization 2004
All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or translate WHO publications whether for sale or for noncommercial distribution should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.
Risk assessment activities of the International Programme on Chemical Safety, including the production of Concise International Chemical Assessment Documents, are supported financially by the Department of Health and Department for Environment, Food & Rural Affairs, UK, Environmental Protection Agency, Food and Drug Administration, and National Institute of Environmental Health Sciences, USA, European Commission, German Federal Ministry of Environment, Nature Conservation and Nuclear Safety, Health Canada, Japanese Ministry of Health, Labour and Welfare, and the Swiss Agency for Environment, Forests and Landscape.
Technically and linguistically edited by Marla Sheffer, Ottawa, Canada, and printed by Wissenchaftliche Verlagsgesellschaft mbH, Stuttgart, Germany
|
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, TRANSFORMATION, AND ACCUMULATION |
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are usually based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and doseresponse from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., a standard CICAD or a de novo CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is usually based on an existing national, regional, or international review. When no appropriate source document is available, a CICAD may be produced de novo. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science. When a CICAD is prepared de novo, a consultative group is normally convened.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on manganese and its compounds (environmental aspects) was based primarily on the report Toxicological profile for manganese (update), prepared by the Agency for Toxic Substances and Disease Registry of the US Department of Health and Human Services (ATSDR, 2000). Secondary sources of information included CICAD No. 12 on manganese and its compounds (IPCS, 1999a) and data identified following a comprehensive literature search of relevant databases conducted up to December 2002 to identify any relevant references published subsequent to those incorporated in these two reports. For information regarding the assessment of human health effects of manganese, the reader should refer to CICAD No. 12 (IPCS, 1999a). Manganese fungicides have been referred to in the document for source and fate information only, and no attempt has been made to evaluate this group of chemicals for environmental effect. Information on the preparation and peer review of the source document is presented in Appendix 1. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was considered and approved as an international assessment at a meeting of the Final Review Board, held in Varna, Bulgaria, on 811 September 2003. Participants at the Final Review Board meeting are presented in Appendix 3. International Chemical Safety Cards on selected manganese compounds (ICSCs 174, 175, 290, 754, 977, 1169, and 1398), produced by the International Programme on Chemical Safety in a separate, peer-reviewed process (IPCS, 1999b,c, 2001, 2003a,b,c,d), have also been reproduced in this document.
Manganese (Mn) is a naturally occurring element that is found in rock, soil, and water. It is ubiquitous in the environment and comprises about 0.1% of the Earths crust. Crustal rock is a major source of manganese found in the atmosphere. Ocean spray, forest fires, vegetation, and volcanic activity are other major natural atmospheric sources of manganese. Important sources of dissolved manganese are anaerobic environments where particulate manganese oxides are reduced, the direct reduction of particulate manganese oxides in aerobic environments, the natural weathering of Mn(II)-containing minerals, and acidic environments. The major pool of manganese in soils originates from crustal sources, with other sources including direct atmospheric deposition, wash-off from plant and other surfaces, leaching from plant tissues, and the shedding or excretion of material such as leaves, dead plant and animal material, and animal excrement. The major anthropogenic sources of environmental manganese include municipal wastewater discharges, sewage sludge, mining and mineral processing, emissions from alloy, steel, and iron production, combustion of fossil fuels, and, to a much lesser extent, emissions from the combustion of fuel additives.
Manganese is released to air mainly as particulate matter, and the fate and transport of the particles depend on their size and density and on wind speed and direction. Some manganese compounds are readily soluble in water. Manganese exists in the aquatic environment in two main forms: Mn(II) and Mn(IV). Movement between these two forms occurs via oxidation and reduction reactions that may be abiotic or microbially mediated. The environmental chemistry of manganese is largely governed by pH and redox conditions; Mn(II) dominates at lower pH and redox potential, with an increasing proportion of colloidal manganese oxyhydroxides above pH 5.5 in non-dystrophic waters. Primary chemical factors controlling sedimentary manganese cycling are the oxygen content of the overlying water, the penetration of oxygen into the sediments, and benthic organic carbon supply. Manganese in soil can migrate as particulate matter to air or water, or soluble manganese compounds can be leached from the soil. In soils, manganese solubility is determined by two major variables: pH and redox potential.
Manganese in water can be significantly bioconcentrated by aquatic biota at lower trophic levels. Bioconcentration factors (BCFs) of 200020 000 for marine and freshwater plants, 25006300 for phytoplankton, 3005500 for marine macroalgae, 800830 for intertidal mussels, and 35930 for fish have been estimated. Uptake of manganese by aquatic invertebrates and fish significantly increases with temperature and decreases with pH, whereas dissolved oxygen has no significant effect. Uptake of manganese has been found to increase with decreasing salinity.
Manganese concentrations in air tend to be lowest in remote locations (about 0.514 ng/m3 on average), higher in rural areas (40 ng/m3 on average), and still higher in urban areas (about 65166 ng/m3 on average). Manganese concentrations in air tend to be highest in source-dominated areas, where values can reach 8000 ng/m3. Annual averages of manganese concentrations may rise to 200300 ng/m3 in air near foundries and to over 500 ng/m3 in air near ferro- and silicomanganese industries.
Concentrations of dissolved manganese in natural waters that are essentially free of anthropogenic inputs can range from 10 to >10 000 ΅g/litre. However, dissolved manganese concentrations in natural surface waters rarely exceed 1000 ΅g/litre and are usually less than 200 ΅g/litre.
Manganese concentrations in river sediments ranged from 410 to 6700 mg/kg dry weight; sediment from an urban lake receiving inputs from industrial and residential areas, as well as windborne dust from old mine dumps, contained manganese at concentrations ranging up to 13 400 mg/kg dry weight. Sediment manganese concentrations of 1001000 mg/kg dry weight have been reported for intertidal mudflats; similar total manganese values were found in the northern Adriatic Sea. Surface sediments in the Baltic Sea contained manganese at mean concentrations of 35508960 mg/kg dry weight; the high manganese concentrations were thought to be due to ferromanganese concretions and riverine loads.
Natural ("background") levels of total manganese in soil range from <1 to 4000 mg/kg dry weight, with mean values around 300600 mg/kg dry weight.
Mean manganese concentrations in seaweed range from 130 to 735 mg/kg dry weight, whereas concentrations in shellfish range from 3 to 660 mg/kg dry weight; higher concentrations in shellfish are associated with manganese-rich sediment. Concentrations of manganese found in tissues of marine and freshwater fish tend to range from <0.2 to 19 mg/kg dry weight. Higher manganese concentrations above 100 mg/kg dry weight have been reported for fish in polluted surface waters.
Concentrations of manganese in terrestrial plants tend to range from 20 to 500 mg/kg. Members of the Ericaceae family, which includes blueberries, are regarded as manganese accumulators. There are numerous reports of foliar manganese levels in excess of 20004000 mg/kg. Mean manganese concentrations in birds eggs from a variety of geographical areas range from 1 to 5 mg/kg dry weight, mean liver concentrations range from 3 to 11 mg/kg dry weight, and mean feather concentrations range from 0.3 to 40 mg/kg dry weight. Mean manganese concentrations of up to 17 mg/kg dry weight have been found in tissues (liver, kidney, and whole body) from a variety of reptiles and wild mammals.
Manganese is an essential nutrient for microorganisms, plants, and animals. Nutritional manganese requirements for terrestrial plants are around 1050 mg/kg tissue. Critical nutritional levels vary widely between species and among cultivars of a species. Calcareous soils, especially those with poor drainage and high organic matter, are the types of soil that produce manganese-deficient plants.
Most toxicity tests have been carried out using ionic manganese. Little is known about the aquatic toxicity of colloidal, particulate, and complexed manganese; in general, however, toxicities of metals bound into these forms are assumed to be less than those of the aquo-ionic forms. For algae and protozoa, there is a wide range of toxicity values; the most sensitive species appear to be the marine diatom Ditylum brightwellii, with a 5-day EC50, based on growth inhibition, of 1.5 mg/litre, and a freshwater alga Scenedesmus quadricauda, with a 12-day EC50, based on total chlorophyll reduction, of 1.9 mg/litre. Tests on aquatic invertebrates reveal 48-h LC50/EC50 values ranging from 0.8 mg/litre (Daphnia magna) to 1389 mg/litre (Crangonyx pseudogracilis), the lowest LC50 being observed under soft water conditions (25 mg calcium carbonate/litre). A significant reduction in survival and hatching of yellow crab (Cancer anthonyi) embryos at >0.01 mg manganese/litre was found in 7-day tests in seawater. For fish, 96-h LC50s range from 2.4 mg manganese/litre for coho salmon (Oncorhynchus kisutch) to 3350 mg/litre for Indian catfish (Heteropneustes fossilis), with the lowest LC50 values obtained under soft water conditions (25 mg calcium carbonate/litre). Significant embryonic mortality was observed in rainbow trout (Oncorhynchus mykiss) eggs exposed to 1 mg manganese sulfate/litre for 29 days. A single embryo-larval test with a 7-day LC50 of 1.4 mg manganese/litre was identified for amphibians. Acute toxicity in aquatic invertebrates and fish decreased with increasing water hardness; the addition of chelating agents can reduce the toxicity of manganese. There is evidence that manganese can protect organisms against the effects of more toxic metals.
In the field, the high frequency of blue crabs (Callinectes sapidus) with shell disease (lesions) in a metal-contaminated estuary was ascribed to manganese toxicity, and the deposition of manganese dioxide on the gills of Norway lobster (Nephrops norvegicus) gave rise to a brown or black discoloration of the gills and black corroded areas on the carapace following hypoxic conditions in the south-east Kattegat, Sweden. Increased mortality of rainbow trout (Oncorhynchus mykiss) at a hatchery was found to be positively correlated with manganese concentration (<0.51 mg/litre). Acid precipitation has caused acid episodes and elevated concentrations of metals. Cage experiments with yearling brown trout (Salmo trutta) showed that pH (4.55.4) and the concentration of labile inorganic manganese (0.10.4 mg/litre) explained all of the observed mortality.
Symptoms of manganese toxicity to terrestrial plants vary widely with species and include marginal chloroses, necrotic lesions, and distorted development of the leaves. Toxic manganese concentrations in crop plant tissues vary widely, with critical values ranging from 100 to 5000 mg/kg. Manganese toxicity is a major factor limiting crop growth on acidic, poorly drained, or steam-sterilized mineral soils. There is a wide range of variation in tolerance to manganese between and within plant species. Factors affecting manganese tolerance include genotype (inter- and intraspecific variation), silicon concentration, temperature, light intensity, physiological leaf age, microbial activity, and the characteristics of the rhizosphere.
Surface freshwater data suggest that higher manganese concentrations occur during periods of higher stream flow, such as spring runoff, and lower concentrations tend to occur downstream of lakes that act as settling areas for sediment. Soft water streams, rivers, and lakes appear to be the most sensitive freshwater environments, with laboratory tests and field observations showing that dissolved manganese concentrations of around 1 mg/litre can cause toxic effects in aquatic organisms. An overall guidance value for the protection of 95% of species with 50% confidence was derived at 0.2 mg manganese/litre for soft waters for the freshwater environment. Other factors, such as acid precipitation, acid mine drainage, land use, and municipal wastewater discharges, can increase dissolved manganese levels and thus increase the risk to sensitive species, especially in soft water areas. Evaluation of the likely toxicity of manganese to organisms in the field has to take account of speciation conditions in both the test and the specific field area. In the marine environment, manganese can be taken up and accumulated by organisms during hypoxic releases of dissolved manganese from manganese-rich sediments. Even taking into account the possible mitigating effects of suspended sediment, salinity, and oxygen levels in natural environments, adverse effects in the field have been observed. An overall guidance value for the protection of 95% of species with 50% confidence was derived at 0.3 mg manganese/litre for the marine environment.
When evaluating the risk to the terrestrial environment from anthropogenic releases of manganese, account must be taken of local natural ("background") levels, which are in turn controlled by a variety of physical and chemical parameters. Different communities and ecosystems would also respond differently, depending on their "normal" exposure to manganese. For these reasons, deriving a single guidance value for the terrestrial environment is inappropriate.
Table 1 lists common synonyms and other relevant information on the chemical identity and properties of manganese and several of its most important compounds. Manganese is a naturally occurring element that is found in rock, soil, water, and food. It can exist in 11 oxidation states ranging from 3 to +7, but the most common ones are +2 (e.g., manganese chloride [MnCl2]), +4 (e.g., manganese dioxide [MnO2]), and +7 (e.g., potassium permanganate [KMnO4]). Manganese and its compounds can exist as solids in the soil and as solutes or small particles in water. Most manganese salts are readily soluble in water, with only the phosphate and the carbonate having low solubilities. The manganese oxides (manganese dioxide and manganese tetroxide) are poorly soluble in water. Manganese can also be present in small dust-like particles in the air.
Table 1: Chemical identity of manganese and its compounds.a
|
Manganese |
Manganese chlorideb |
Manganese sulfate |
Manganese tetroxide |
Manganese dioxide |
Potassium permanganate |
MMTc |
Manebd |
Mancozeb |
|
|
Synonyms |
Elemental manganese |
Manganous chloride |
Manganous sulfate |
Trimanganese tetroxide |
Manganese peroxide |
Permanganic acid, potassium salte |
Methylcyclopentadienyl manganese tricarbonylf |
Manganese ethylene-bis-dithiocarbamate |
Manganese ethylene-bis-(dithiocarbamate) (polymeric) complex with zinc salt |
|
Chemical formula |
Mn |
MnCl2 |
MnSO4 |
Mn3O4 |
MnO2 |
KMnO4 |
C9H7MnO3 |
C4H6MnN2S4 |
C4H6MnN2S4·C4H6N2S4Zn |
|
CAS No. |
|
|
|
|
|
|
|
|
|
|
Relative molecular mass |
54.94e |
125.85e |
151.00e |
228.81g |
86.94e |
158.04e |
218.10 |
265.31 |
541.03 |
|
Colour |
Grey-whiteg |
Pinkg |
Pale rose-red |
Blackg |
Black |
Purple |
Dark orange-redh |
Yellow-brown |
Greyish-yellow |
|
Physical state |
Solid |
Solid |
Solid |
Solid |
Solid |
Solid |
Liquidh |
Powder |
Powder |
|
Melting point |
1244 °Cg |
650 °C |
700 °C |
1564 °C |
535 °C |
<240 °C (decomposes) |
1.5 °C |
Decomposes on heating |
Decomposes without heating |
|
Boiling point |
1962 °Cg |
1190 °Cg |
Decomposes at 850 °C |
No data |
No data |
No data |
232.8 °C |
No data |
No data |
|
Solubility |
Dissolves in dilute mineral acidsg |
Very soluble in water (723 g/litre at 25 °C)b; soluble in alcohol |
Soluble in water (520 g/litre at 5 °C)b and alcohol |
Insoluble in water; soluble in hydrochloric acid |
Soluble in hydrochloric acid; insoluble in water |
Soluble in water (64 g/litre at 20 °C),b acetone, and sulfuric acid |
Practically insoluble in water (0.029 g/litre at 25 °C)b; completely soluble in hydrocarbons |
Slightly soluble in water; soluble in chloroform |
Practically insoluble in water (0.006 g/litre at 25 °C)b as well as most organic solvents |
|
Log Kowi |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
3.7j |
1.33b |
|
|
HLCi |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
0.019j |
a Adapted from ATSDR (2000). All information obtained from Sax & Lewis (1987), except where noted.
b HSDB (1998).
c Zayed et al. (1994).
d Ferraz et al. (1988).
e Windholz (1983).
f NTP (1999).
g Lide (1993).
h Verschueren (1983).
i Kow = octanolwater partition coefficient; HLC = Henrys law constant.
j Garrison et al. (1995).
Additional physical/chemical properties for manganese and select manganese compounds are presented in the International Chemical Safety Cards reproduced in this document.
Atomic absorption spectrophotometric analysis is the most widely used method for determining manganese in biological materials and environmental samples. Fluorimetric, colorimetric, neutron activation analysis, and plasma atomic emission techniques are also recommended for measuring manganese in such samples. Inductively coupled plasma (ICP) atomic emission analysis is frequently employed for multianalyte analyses that include manganese. In most cases, distinguishing between different oxidation states of manganese is impossible, so total manganese is measured. The detection limits of these methods range from <0.01 to 0.2 mg/kg for biological tissues and fluids, from 5 to 10 ΅g/m3 for air, and from 0.01 to 50 ΅g/litre for water (Kucera et al., 1986; Abbasi, 1988; Lavi et al., 1989; Mori et al., 1989; Chin et al., 1992; ATSDR, 2000).
Determination of manganese requires an acid extraction/digestion step before analysis. The details vary with the specific characteristics of the sample, but treatment usually involves heating in nitric acid, oxidation with hydrogen peroxide, and filtration and/or centrifugation to remove insoluble matter (ATSDR, 2000).
Meneses et al. (1999) and Llobet et al. (2002) used ICP mass spectrometry with a detection limit of 0.02 mg/kg for soil and herbage. Pandey et al. (1998) used a sequential ICP optical emission spectrometer with an ultrasonic nebulizer for atmospheric particulates at a detection limit of 0.001 ΅g/litre, whereas ICP with atomic emission spectrophotometry was used for atmospheric particulates (Brewer & Belzer, 2001; Espinosa et al., 2001), sediment (Leivuori, 1998; Leivuori & Vallius, 1998), shellfish (Blackmore et al., 1998; Blackmore, 1999; Rainbow & Blackmore, 2001), feathers (Connell et al., 2002), and liver tissue (Mason & Stephenson, 2001).
Beklemishev et al. (1997) used the catalytic kinetic method for analysis of manganese in water. The method relies on an indicator reaction that is catalysed by Mn(II) (the oxidation of 3,3',5,5'-tetramethylbenzidine by potassium periodate [KIO4]) and is carried out on the surface of a paper-based sorbent. The method has a detection limit (0.005 ΅g/litre) that is much lower than those of other, more established methods.
A nuclear magnetic resonance method (Kellar & Foster, 1991) and a method using on-line concentration analysis (Resing & Mottl, 1992) were used to determine both free and complexed manganese ions in aqueous media. The latter method was highly sensitive, with a detection limit of 36 pmol/litre (1.98 ng/litre when concentrating 15 ml of seawater). A similar detection limit was achieved by Sarzanini et al. (2001) for seawater using flow-injection preconcentration coupled with electrothermal atomic absorption spectrometry.
The technique of diffusive gradients in thin films (DGT) has been used for in situ trace manganese speciation measurements. A concentrationdepth profile of labile manganese was obtained in a stratified estuary by deployment of a string of DGT devices across the redoxcline (Denney et al., 1999). Gauthreaux et al. (2001) used a modified sequential extraction procedure to speciate the chemical forms of manganese in sediment using flame atomic absorption spectrophotometry. Concentrations were determined in five different fractions for each sample: manganese in the exchangeable form, manganese bound to carbonates, manganese bound to manganese/iron oxides, manganese bound to organic matter, and manganese in the residual form. Techniques such as X-ray absorption near-edge structure spectroscopy enable in situ quantification of the oxidation state of manganese (Bargar et al., 2000).
Manganese is ubiquitous in the environment. It comprises about 0.1% of the Earth's crust (NAS, 1973; Graedel, 1978). Manganese does not occur naturally as a base metal but is a component of more than 100 minerals, including various sulfides, oxides, carbonates, silicates, phosphates, and borates (NAS, 1973). The most commonly occurring manganese-bearing minerals include pyrolusite (manganese dioxide), rhodocrosite (manganese carbonate [MnCO3]), rhodonite (manganese silicate), and hausmannite (manganese tetroxide [Mn3O4]) (NAS, 1973; Windholz, 1983; US EPA, 1984; HSDB, 1998).
Ferromanganese minerals such as biotite mica and amphiboles contain large amounts of manganese, and manganese-rich nodules (especially found in the North-east Pacific; Schiele, 1991) have been identified on the seafloor in conjunction with cobalt, nickel, and copper (Reimer, 1999; Ahnert & Borowski, 2000). Similarly, manganese crusts occur as pavement-like encrustations of ferromanganese oxides on exposed abyssal hard substrates related to all types of submarine elevations (Ahnert & Borowski, 2000). Black smokers (hydrothermal vents on the seafloor releasing predominantly iron and sulfide) are an additional source releasing manganese into the (oceanic) hydrosphere (Gamo et al., 2001). Important sources of manganese include soils, sediments, and metamorphic and sedimentary rocks (Reimer, 1999).
Crustal rock is a major source of manganese found in the atmosphere. Ocean spray, forest fires, vegetation, and volcanic activity are other major natural sources of manganese in the atmosphere (Schroeder et al., 1987; Stokes et al., 1988). Stokes et al. (1988) estimated that two-thirds of manganese air emissions were from natural sources. Atmospheric particulate matter collected in the Antarctic indicated that manganese was derived from either crustal weathering or the ocean (Zoller et al., 1974). Air erosion of dusts and soils is also an important atmospheric source of manganese, but no quantitative estimates of manganese release to air from this source were identified (US EPA, 1984). An important source of dissolved manganese is anaerobic environments where particulate manganese oxides are reduced, such as some soils and sediments, wetlands, and the anaerobic hypolimnia of lakes and fjords. Other possible sources include the direct reduction of particulate manganese oxides in aerobic environments by organics, with or without ultraviolet light, the natural weathering of Mn(II)-containing minerals, and acid drainage and other acidic environments. The major pool of manganese in soils originates from crustal sources. Addition of manganese to soils can also result from direct atmospheric deposition, wash-off from plant and other surfaces, leaching from plant tissues, and the shedding or excretion of material such as leaves, dead plant and animal material, and animal excrement (Stokes et al., 1988).
The major anthropogenic sources of environmental manganese include municipal wastewater discharges, sewage sludge, mining and mineral processing (particularly nickel), emissions from alloy, steel, and iron production, combustion of fossil fuels, and, to a much lesser extent, emissions from the combustion of fuel additives.
The manganese content in ore produced worldwide was estimated to be 8.8 million tonnes in 1986. Production levels of manganese ore and its total manganese metal content remained nearly the same through 1990 (US Department of the Interior, 1993). Levels of ore produced worldwide in 1995, 1996, and 1997 declined slightly, with total manganese metal content declining proportionately to 8.0, 8.1, and 7.7 million tonnes, respectively (US Department of the Interior, 1996, 1998). Sites of substantial workable manganeseiron deposits include the former USSR, South and North Africa, South America, India, and China (Schiele, 1991). Most manganese is mined in open-pit or shallow mines (NAS, 1973). Although modern steelmaking technologies call for lower unit consumption of manganese, worldwide demand for steel is projected to increase moderately in the future, particularly in developing countries (US Department of the Interior, 1995, 1998). The demand for manganese in other industries (e.g., dry-cell battery manufacturing) might also increase, but the overall effect of these other uses on global trends in manganese production and use is minor (EM, 1993; US Department of the Interior, 1995, 1998).
Manganese compounds are produced from manganese ores or from manganese metal. Metallic manganese (ferromanganese) is used principally in steel production along with cast iron and superalloys to improve hardness, stiffness, and strength (NAS, 1973; US EPA, 1984; HSDB, 1998). The predominant portion (approximately 90%) of manganese is processed into ferromanganese in blast furnaces (Schiele, 1991). Manganese compounds have a variety of uses. Manganese dioxide is commonly used in the production of dry-cell batteries, matches, fireworks, porcelain and glass-bonding materials, and amethyst glass; it is also used as the starting material for the production of other manganese compounds (NAS, 1973; Venugopal & Luckey, 1978; US EPA, 1984). Manganese chloride is used as a precursor for other manganese compounds, as a catalyst in the chlorination of organic compounds, in animal feed to supply essential trace minerals, and in dry-cell batteries (US EPA, 1984; HSDB, 1998). Manganese sulfate (MnSO4) is used primarily as a fertilizer and as a livestock supplement; it is also used in some glazes, varnishes, ceramics, and fungicides (Windholz, 1983; US EPA, 1984; HSDB, 1998). Maneb (manganese ethylene-bis-dithiocarbamate) is used as a broad-spectrum contact fungicide and is also used for seed treatment of small grains such as wheat. Maneb is therefore a potential source of manganese in soil and plants (Ferraz et al., 1988; Ruijten et al., 1994). Potassium permanganate is used as an oxidizing agent, disinfectant, and antialgal agent; for metal cleaning, tanning, and bleaching; as a purifier in water and waste treatment plants; and as a preservative for fresh flowers and fruits (HSDB, 1998). The organomanganese compound MMT (methylcyclopentadienyl manganese tricarbonyl), an antiknock additive in unleaded gasoline, is produced by the addition of molten sodium metal to methylcyclopentadiene to give methylcyclopentadienylsodium. Anhydrous manganese dichloride is then added to afford methylcyclopentadienylmanganese, which is subsequently reacted with carbon monoxide to give MMT (NAS, 1973; US EPA, 1984; Sax & Lewis, 1987; HSDB, 1998; Kirk & Othmer, 2001). MMT has been approved for use in Argentina, Australia, Bulgaria, the USA, France, and the Russian Federation and has been conditionally approved for use in New Zealand (Zayed et al., 1999; Zayed, 2001); more recently, Ethyl Corp. (a major producer of MMT) noted that MMT is now sold in 25 countries (Kaiser, 2003).
The main anthropogenic sources of manganese release to air are industrial emissions (such as ferroalloy production and iron and steel foundries, power plants, and coke ovens), combustion of fossil fuels, and re-entrainment of manganese-containing soils (Lioy, 1983; US EPA, 1983, 1984, 1985a,b; Ruijten et al., 1994; ATSDR, 2000). Problems with air pollution especially dust and smoke containing manganese dioxide and manganese tetroxide arise during the mining, crushing, and smelting of ores as well as during steel production (Schiele, 1991). Approximately 2 tonnes of manganese ore are required to make 1 tonne of ferromanganese alloy (NAS, 1973). Steel emissions were found to be the predominant source of manganese in urban particulate matter (Sweet et al., 1993). Manganese can also be released to the air during other anthropogenic processes, such as welding and fungicide application (Ferraz et al., 1988; MAK, 1994; Ruijten et al., 1994). Nriagu & Pacyna (1988) estimated that total worldwide emissions of manganese in 1983 ranged from 10 560 to 65 970 tonnes, with the predominant sources being coal combustion, secondary non-ferrous metal production, and sewage sludge incineration. Total emissions to air from anthropogenic sources in the USA were estimated to be 16 400 tonnes in 1978, with about 80% (13 200 tonnes) from industrial facilities and 20% (3200 tonnes) from fossil fuel combustion (US EPA, 1983). Air emissions by US industrial sources reportable to the Toxics Release Inventory (TRI) for 1987 totalled 1200 tonnes (TRI87, 1989). In 1991, air emissions from TRI facilities in the USA ranged from 0 to 74 tonnes, with several US states reporting no emissions (TRI91, 1993). Estimated releases of manganese to air in 1996 were 4000 tonnes, representing 15% of total environmental releases (TRI96, 1998). Figures in Table 2 (see section 6) show decreasing emissions of manganese to air in the USA as a result of air pollution control.
Table 2: Average levels of manganese in air.
|
Type of location |
Year |
Average concentration (ng/m3) |
Range (ng/m3) |
|
Atmospheric air (worldwide)a |
reported in 1982 |
||
|
Remote |
|||
|
- Continental |
3.4 |
<0.189.30 |
|
|
- Oceanic |
14.2 |
0.0279 |
|
|
- Polar |
0.5 |
0.011.5 |
|
|
Rural |
40 |
6.5199 |
|
|
Urban |
|||
|
- Canada |
65 |
20.0270 |
|
|
- USA |
93 |
5.0390 |
|
|
- Europe |
166 |
23.0850 |
|
|
- Other |
149 |
10.0590 |
|
|
US ambient airb |
|||
|
Non-urban |
19531957 |
60 |
|
|
19651967 |
12 |
||
|
1982 |
5 |
||
|
Urban |
19531957 |
110 |
|
|
19651967 |
73 |
||
|
1982 |
33 |
||
|
Source dominated |
19531957 |
No data |
|
|
19651967 |
2508300 |
||
|
1982 |
130140 |
a Adapted from Stokes et al. (1988).
b Adapted from US EPA (1984).
Combustion of MMT leads to the emission of manganese phosphates and manganese sulfate, with manganese oxides such as manganese tetroxide a minor component (NICNAS, 2003). The size of particles emitted to the atmosphere varies from 0.1 to 0.45 ΅m (Waldron, 1980). Combustion products of MMT also include manganese phosphate and manganese sulfide (Zayed et al., 1999; Zayed, 2001). One of the principal sources of inorganic manganese as a pollutant in the urban atmosphere is the combustion of MMT, particularly in areas of high traffic density (Sierra et al., 1998). MMT was used as a gasoline additive in the USA for a number of years, resulting in manganese emissions. Davis et al. (1988) found that motor vehicles made a significant contribution to levels of airborne manganese in areas such as southern California (around 40% of total airborne manganese) compared with, for example, central and northern California, where the addition of manganese to gasoline was much lower. According to a statistical model of source apportionment, the calculated average vehicular contribution of manganese in southern California was about 13 ng/m3, around 4 times the value calculated for both central and northern California.
In Canada, MMT use as a fuel additive has gradually increased since 1977. Manganese emissions from gasoline combustion rose sharply from 1977 through the early 1980s, reaching an estimated 220 tonnes by 1985 (Jaques, 1984). In 1990, lead was completely replaced by MMT in gasoline in Canada (Loranger & Zayed, 1994). MMT use peaked in 1989 at over 400 tonnes, which was more than twice the usage in 1983 and 1.5 times the usage in 1986. MMT use declined to about 300 tonnes by 1992, owing to reductions in its concentration in gasoline. However, ambient monitoring data for manganese in Canadian cities without industrial sources for the 19891992 period did not reflect this peak in MMT use. Air manganese levels (PM2.5, or particulate matter with an aerodynamic diameter less than or equal to 2.5 ΅m) remained constant at 1113 ng/m3 for small cities and 2025 ng/m3 for large cities (Health Canada, 1994; Egyed & Wood, 1996). Manganese emission levels can vary depending on the concentration of MMT in gasoline and gasoline usage patterns. One study reported a correlation between atmospheric manganese concentrations in 1990 air samples and traffic density in Montreal, Canada (Loranger & Zayed, 1994). However, a later study by these investigators reported that atmospheric manganese concentrations in Montreal decreased in 1991 and 1992, despite an estimated 100% increase in manganese emission rates from MMT in gasoline (Loranger & Zayed, 1994). Another study suggested that the high manganese levels in Montreal were, in part, due to the presence of a silico- and ferromanganese facility that ceased operation in 1991 (Egyed & Wood, 1996).
It is clear that the contribution of MMT to overall manganese levels in the environment is complex. The contribution of MMT to atmospheric manganese concentrations is difficult to establish, since it may be masked by more substantial variation associated with other industrial activities as well as road dust and windblown dust (Bankovitch et al., 2003). However, even though manganese may be a small percentage of total suspended particulate matter measured in cities, such as Montreal, the contribution of MMT to air manganese levels could be significant, in that it may account for stable manganese levels in the face of declining total suspended particulate concentrations. Factors such as unfavourable meteorological conditions and high traffic density could lead to an increase in manganese levels (PM2.5) attributable to MMT (Wallace & Slonecker, 1997; Davis et al., 1998).
Manganese can be released to water by discharge from industrial facilities or as leachate from landfills and soil (US EPA, 1979, 1984; Francis & White, 1987; TRI91, 1993). Sea disposal of mine tailings and liquor is another source of manganese to the marine environment, particularly in tropical areas (Florence et al., 1994). Nriagu & Pacyna (1988) estimated that total worldwide anthropogenic inputs of manganese to aquatic ecosystems during 1983 ranged from 109 000 to 414 000 tonnes, with the predominant sources being domestic wastewater and sewage sludge disposal. In the USA, reported industrial discharges of manganese in 1991 ranged from 0 to 17.2 tonnes for surface water, from 0 to 57.3 tonnes for transfers to public sewage, and from 0 to 0.114 tonnes for underground injection (TRI91, 1993). An estimated total of 58.6 tonnes, or 1% of the total environmental release of manganese in the USA, was discharged to water in 1991 (TRI91, 1993). In 1996, the estimated release of manganese to water was 870 tonnes (TRI96, 1998).
Land disposal of manganese-containing wastes is the principal source of manganese releases to soil. Nriagu & Pacyna (1988) estimated that total worldwide anthropogenic releases of manganese to soils during 1983 ranged from 706 000 to 2 633 000 tonnes, with the predominant source being coal fly ash. In 1991, reported industrial releases to land in the USA ranged from 0 to 1000 tonnes. More than 50% of the total environmental release of manganese (3753 tonnes) was to land (TRI91, 1993). Estimated releases of manganese to soil in 1996 were 21 600 tonnes, representing 80% of total environmental releases (TRI96, 1998).
Elemental manganese and inorganic manganese compounds have negligible vapour pressures but can exist in air as suspended particulate matter derived from industrial emissions or the erosion of soils (US EPA, 1984). In the troposphere, manganese is likely to be found in oxide, sulfate, or nitrate forms or as mineral complexes related to its natural origin in soil or rock (Stokes et al., 1988). Manganese-containing particles are removed from the atmosphere mainly by gravitational settling or by rain (US EPA, 1984).
Soil particulate matter containing manganese can be transported in air. The fate and transport of manganese in air are largely determined by the size and density of the particles and by wind speed and direction. An estimated 80% of the manganese in suspended particulate matter is associated with particles with a mass median equivalent diameter (MMED) of <5 ΅m, and 50% of this manganese is estimated to be associated with particles that are <2 ΅m in MMED. Whether these data are for particles in urban or rural areas is unclear. However, it is known that the size of manganese particles in the air tends to vary by source; small particles dominate around ferromanganese and dry-cell battery plants, whereas large particles tend to predominate near mining operations (WHO, 1999). Airborne particles (>2 ΅m) collected over the oceans contained a mean manganese concentration of 1338 mg/kg (Lee & Duffield, 1979). Based on these data, widespread airborne distribution would be expected (IPCS, 1981). Fergusson & Stewart (1992) stated that unlike deposition of other metals, such as copper, lead, cadmium, and zinc, manganese deposition showed little spatial variation between urban and rural areas; however, Mielke et al. (2002) reported a 4-fold increase in manganese concentrations between rural and urban areas of New Orleans, Louisiana, USA. Very little information is available on atmospheric reactions of manganese (US EPA, 1984). Although manganese can react with sulfur dioxide and nitrogen dioxide, the occurrence of such reactions in the atmosphere has not been demonstrated.
The arithmetically averaged annual wet flux of manganese was 1190 ΅g/m2 for Chesapeake Bay, USA (Scudlark et al., 1994), and 1900 ΅g/m2 for a Scottish sea loch (Hall et al., 1996). Rates of atmospheric deposition of manganese into the western Mediterranean Sea (northwestern Corsica) between 1985 and 1987 ranged between 0.0023 and 0.0072 ΅g/cm2 per day. Sporadic but intense Saharan sandstorms were responsible for the highest atmospheric deposition of manganese (Remoudaki et al., 1991). A manganese deposition rate of 350 ΅g/m2 per day was calculated for Burnaby Lake, British Columbia, Canada. The estimated annual deposition rate was estimated to be 7.7 tonnes for the entire watershed (Brewer & Belzer, 2001).
Manganese exists in the aquatic environment in two main forms: Mn(II) and Mn(IV). Transition between these two forms occurs via oxidation and reduction reactions that may be abiotic or microbially mediated (Nealson, 1983; Thamdrup et al., 2000; Heal, 2001). The environmental chemistry of manganese is largely governed by pH and redox conditions; Mn(II) dominates at lower pH and redox potential, with an increasing proportion of colloidal manganese oxyhydroxides above pH 5.5 in non-dystrophic waters (LaZerte & Burling, 1990). In waters receiving acid mine drainage, dissolved manganese concentrations were <40 ΅g/litre above pH 5.5; however, below pH 3, dissolved manganese concentrations ranged from 250 to 4400 ΅g/litre (Filipek et al., 1987). Similarly, in another study, sediment concentrations of manganese decreased from 400 mg/kg at pH 5.65.9 to 8 mg/kg below pH 3 due to manganese dissolution influenced by acid mine drainage (Cherry et al., 2001).
A complex series of oxidation/precipitation and adsorption reactions occurs when Mn(II) is present in aerobic environments, which eventually renders the manganese biologically unavailable as insoluble manganese dioxide. However, the kinetics of Mn(II) oxidation are slow in waters with pH below 8.5 (Zaw & Chiswell, 1999). The time required for the oxidation and precipitation of manganese ranges from days in natural waters to years in synthetic waters (Stokes et al., 1988). However, oxidation rates of manganese increase with increasing pH or the presence of catalytic surfaces such as manganese dioxide (Huntsman & Sunda, 1980). In a stream receiving manganese-rich inflows caused by acid mine drainage, there was rapid oxidation and precipitation of manganese oxides (Scott et al., 2002). The sequence of reactions involving the oxidation of Mn(II) and subsequent precipitation as manganese dioxide includes simultaneous occurrence of several manganese forms (i.e., dissolved Mn(II), hydrous oxides of Mn(III), Mn(II) adsorbed to particulates, and Mn(II)ligand complexes), with individual concentrations dependent on factors that include pH, inorganic carbon, organic carbon, sulfate, chloride, temperature, and time (Stokes et al., 1988). In groundwater with low oxygen levels, Mn(IV) can be reduced both chemically and bacterially to the Mn(II) oxidation state (Jaudon et al., 1989).
There is little evidence for manganeseorganic associations in natural waters, with manganese only weakly bound to dissolved organic carbon (L'Her Roux et al., 1998). Hence, organic complexation does not play a major role in controlling manganese speciation in natural waters. Field studies have confirmed that organically bound manganese is minor, even with high natural dissolved organic carbon levels (Laxen et al., 1984). The Mn(II) ion is more soluble than Mn(IV); therefore, manganese will tend to become more bioavailable with decreasing pH and redox potential (Heal, 2001). The presence of chlorides, nitrates, and sulfates can increase manganese solubility and thus increase aqueous mobility and uptake by plants (Reimer, 1999). Hart et al. (1992) studied the speciation of manganese in Magela Creek in tropical north Australia. They hypothesized that higher temperatures (30 °C) and increased rates of bacterially mediated oxidation could result in equilibrium between Mn(II) and oxidized species within the normal residence time of water in the creek. This was one mechanism by which colloidal manganese could dominate speciation.
There is evidence that afforestation of upland areas has increased manganese concentrations in surface waters. Analysis of sites in the United Kingdom between 1988 and 1996 shows a significant positive correlation between mean manganese concentrations and the percentage of conifer cover in the catchment (Heal, 2001). Enhanced manganese concentrations arise from foliar leaching and wash-off of manganese in fine mist and dry particles that are captured from the atmosphere by the trees (Shanley, 1986; Heal, 2001). Litter from conifer plantations may also enhance manganese leaching from soil into runoff. Soil and water acidification in catchments planted with conifers has been widely documented and is associated with enhanced manganese concentrations in surface waters (Heal, 2001). The extent to which land use influences manganese concentrations in upland catchments is modified by catchment hydrology and soil type (Heal, 2001; Heal et al., 2002). Heal et al. (2002) identified summer baseflow and the summerautumn hydrological transition as critical periods for increased manganese concentrations in runoff. It is only when manganese enters lakes, estuaries, and the ocean, where residence times are considerably longer, that chemical processes will become dominant and the system will approach an equilibrium speciation (Laxen et al., 1984).
Manganese is often transported in rivers adsorbed to suspended sediments. Most of the manganese from industrial sources (metallurgical and chemical plants) found in the Paraiba do Sul-Guandu River, Rio de Janeiro, Brazil, was bound to suspended particles (Malm et al., 1988). A positive correlation between manganese concentrations and suspended sediment levels has been reported for a wide variety of rivers in the United Kingdom (Laxen et al., 1984; Neal et al., 1998, 2000). The tendency of soluble manganese compounds to adsorb to soils and sediments can be highly variable, depending mainly on the cation exchange capacity and the organic composition of the soil (Hemstock & Low, 1953; Schnitzer, 1969; McBride, 1979; Curtin et al., 1980; Baes & Sharp, 1983; Kabata-Pendias & Pendias, 1984). Laxen et al. (1984) proposed that the "particulate" and "dissolved" phases for rivers and streams can be decoupled with weathering processes, leading to suspended sediment and influxes of Mn(II) species leaching from anoxic soil and groundwaters. The speciation in any particular river or stream will depend principally on the hydrogeological conditions of the catchment at time of sampling. Suspended sediment, with a manganese content dependent upon the catchment geology, will be mixed with Mn(II) species in varying proportions.
Primary chemical factors controlling sedimentary manganese cycling are the oxygen content of the overlying water, the penetration of oxygen into the sediments, and benthic organic carbon supply (Lynn & Bonatti, 1965; Grill, 1978; Balzer, 1982; Sundby et al., 1986; Hunt & Kelly, 1988). Manganese exchange between water and sediment is an interdependent process. A cycle between sediment and water is maintained, since dissolved Mn(II) is particle-reactive (Hunt, 1983). Once incorporated into sediments, solid-phase manganese oxides (manganese dioxide) undergo reduction to soluble Mn(II) during anaerobic decomposition of organic matter (Pohl et al., 1998). Release from sediment to water occurs by diffusion processes as a result of a steep Mn(II) concentration gradient across the sediment pore water and bottom water interface (Balzer, 1982; Kremling, 1983; Jung et al., 1996). Recycling at a redox boundary is involved in the formation of enriched manganese horizons. Manganese precipitating on the oxic side of a redox boundary consists of a Mn(IV) oxide. If the boundary is displaced towards the sediment surface or into the water column, the oxide undergoes rapid reduction and dissolution. Removal of Mn(II) by diffusion in the pore water is a slow process, and so supersaturation and precipitation of carbonate are likely to occur, transforming labilized oxide to stable carbonate. Under intermittently anoxic conditions, fixation of an enriched horizon may occur by precipitation of manganese dioxide from the water column during oxic periods, burial in sediment, and transformation to carbonate (Schaanning et al., 1988). A clear enrichment of dissolved manganese was observed at low salinities (<7.5) during estuarine mixing (L'Her Roux et al., 1998).
In soils, manganese solubility is determined by two major variables: pH and redox potential. Water-soluble manganese in soils is directly proportional to pH, with oxidation state being another major determinant of manganese solubility. The lower oxidation state, Mn(II), predominates in reducing conditions, resulting in higher concentrations of dissolved manganese in flooded soils or other reducing situations (Stokes et al., 1988). This is normally reflected in higher manganese bioavailability in flooded soils; in some situations, however, there is competition by iron, and plant absorption of manganese is decreased or unaffected by flooding (Adriano, 1986). The oxidation state of manganese in soils and sediments can be altered by microbial activity (Geering et al., 1969; Francis, 1985). Geering et al. (1969) observed that Mn(II) in suspensions of silt or clay loams was oxidized by microorganisms, leading to precipitation of manganese minerals. Fungi are known to enhance the bioavailability of micronutrients. Accordingly, the solubilization of the sparingly soluble manganese dioxide by the fungus Trichoderma harzianum was reported by Altomare et al. (1999). Herzl & Roevros (1998) found that microbial uptake represented around 60% of the transfer of dissolved manganese to the particulate phase in the Scheldt estuary, Belgium. While microorganisms are believed to play an important role in the cycling of manganese in aquatic environments, specific microbial groups indigenous to these systems have not been well characterized (Thamdrup et al., 2000; Stein et al., 2001). There are two main mechanisms involved in the retention of manganese by soil. Firstly, through cation exchange reactions, manganese ions and the charged surface of soil particles form manganese oxides, hydroxides, and oxyhydroxides, which in turn form adsorption sites for other metals. Secondly, manganese can be adsorbed to other oxides, hydroxides, and oxyhydroxides through ligand exchange reactions (Evans, 1989).
MMT degradation in natural aquifers and sediment systems was determined to be very slow under anaerobic conditions. MMT has been found to be persistent in natural aquatic and soil environments in the absence of sunlight, with a tendency to sorb to soil and sediment particles. Calculated half-lives of MMT in aquatic and soil environments range from approximately 0.2 to 1.5 years at 25 °C (Garrison et al., 1995). In the presence of light, photodegradation of MMT is rapid, with identified products including a manganese carbonyl that readily oxidizes to manganese tetroxide (Garrison et al., 1995). MMT is photolysed rapidly by sunlight in the atmosphere, with a very short half-life of less than 2 min (Ter Haar et al., 1975; Garrison et al., 1995). MMT is photolysed rapidly in purified, distilled water exposed to sunlight, with degradation following first-order kinetics and a calculated half-life of less than 1 min (Garrison et al., 1995). Maneb released to water may be subject to abiotic degradation, with the rate of degradation dependent on the aeration of the water and the pH. In addition, maneb may undergo some photodegradation in sunlit water. Maneb is not expected to undergo significant volatilization from water. Mancozeb hydrolyses rapidly in water, with a half-life of less than 12 days at pH 59 (ATSDR, 2000).
The hydrophobicity of MMT (octanolwater partition coefficient [log Kow] = 3.7) suggests that it can sorb to soil or sediment particles (Garrison et al., 1995). MMT was found to be stable in stream bottom sediments under anaerobic conditions. Photodegradation of MMT is not likely to occur in sediments, and MMT may equilibrate between the sediment, sediment pore water, and water column manganese (Garrison et al., 1995). Calumpang et al. (1993) reported a half-life of 2.9 days for mancozeb determined in a silty clay loam soil. In other studies, the half-life of maneb in soil was estimated to be between 20 and 60 days (Rhodes, 1977; Nash & Beall, 1980). Using chemical and physical properties, Beach et al. (1995) estimated the half-life of maneb and mancozeb in soils to be 70 days.
In the laboratory, microorganisms have been shown to transform both soluble and solid manganese; thus, they potentially have substantial effects on local manganese cycles. Physiological, biochemical, and structural studies of manganese oxidizers and reducers in the laboratory form the basis on which models of the participation of microorganisms in the cycling of manganese have been proposed. Field analyses of the distribution of manganese oxidizers and reducers, structural properties of manganese precipitates, and in situ activity measurements support the hypothesis that microorganisms play an integral role in the cycling of manganese in some environments (Nealson, 1983). Microbial oxidation of Mn(II) occurs at rates up to 5 orders of magnitude greater than those of abiotic Mn(II) oxidation (Tebo, 1991). Johnson et al. (1995) found that microbial catalysis was overwhelmingly responsible for manganese oxidation in the lower epilimnion of a freshwater dam during the summer months. Microbial oxidation of Mn(II) to Mn(IV) by spores of the marine Bacillus sp. was observed by Bargar et al. (2000), whereas Stein et al. (2001) found three freshwater bacterial isolates capable of manganese oxidation. Elevated manganese levels on the carapace of crayfish (Cherax destructor) are thought to be the result of manganese-oxidizing bacteria forming biofilms (King et al., 1999). Nealson et al. (1991) isolated and identified manganese-reducing bacteria in the Black Sea. The major group of organisms isolated from the 80- to 90-m (manganese reduction) zone were in the genus Shewanella. Microbially mediated reduction of complexed Mn(III) has also been observed in the laboratory (Kostka et al., 1995). Further studies have isolated manganese-reducing bacteria in marine sediments, oxic regions of lake water columns, and the rhizosphere of non-mycorrhizal plants (Posta et al., 1994; Bratina et al., 1998; Thamdrup et al., 2000).
Manganese is an essential element (see section 7.1) and is, therefore, actively assimilated and utilized by both plants and animals; however, it can be significantly bioconcentrated by aquatic biota at lower trophic levels. Bioconcentration factors (BCFs) of 200020 000 for marine and freshwater plants, 25006300 for phytoplankton, 3005500 for marine macroalgae, 800830 for intertidal mussels, and 35930 for fish have been estimated (Folsom et al., 1963; Thompson et al., 1972; Bryan & Hummerstone, 1973; Pentreath, 1973; Rai & Chandra, 1992). Ichikawa (1961) reported that marine fish did not accumulate manganese to the same extent as organisms at lower trophic levels, with typical BCFs of about 100. Uptake of manganese by aquatic invertebrates and fish significantly increases with temperature (Miller et al., 1980) and decreases with pH (Rouleau et al., 1996), whereas dissolved oxygen has no significant effect (Miller et al., 1980; Baden et al., 1995). BCFs of 140 000300 000 were reported for annelids (Lamellibrachia satsuma) living near hydrothermal vents (Kagoshima Bay, Japan) (Ando et al., 2002). Uptake of manganese has been found to increase with decreasing salinity (Struck et al., 1997). There are conflicting reports on whether biomagnification of manganese (i.e., increasing concentrations up the food-chain) occurs. Kwasnik et al. (1978) found that there was no biomagnification in a simple freshwater food-chain, with maximum BCFs of 911, 65, and 23 for algae, Daphnia magna, and fathead minnows (Pimephales promelas), respectively. In contrast, other authors have found weak biomagnification (Stokes et al., 1988).
Manganese in its reduced form, Mn(II), is bioavailable and can be readily taken up by benthic fauna. Lobsters living on fine cohesive mud sediments rich in manganese can accumulate manganese following autumnal hypoxia in the south-east Kattegat, Sweden (Eriksson, 2000a). Entry of dissolved manganese occurs mainly via gill transport (Baden et al., 1995), and when individuals are exposed to Mn(II) concentrations above 1.8 mg/litre, they accumulate manganese (Baden et al., 1995; Baden & Neil, 1998). Laboratory experiments with dissolved manganese have shown that the majority of the manganese taken up by lobsters is lost from the inner tissues after 5 days of excretion in "clean" seawater (Baden et al., 1995, 1999). Manganese concentrations in lobster eggs remained stable at around 5 mg/kg dry weight during oocyte maturation and throughout most of embryogenesis; however, concentrations started to increase at the end of embryonic development and had reached 120 mg/kg dry weight at the time of hatching (Eriksson, 2000b). Sea stars (Asterias rubens) accumulated dissolved 54Mn linearly with time to a BCF of 19 after 23 days. Manganese accumulated from seawater was eliminated according to first-order kinetics, with a half-life of 36 days. Sea stars fed a diet containing 54Mn assimilated 6983%. Elimination of manganese accumulated from food was described as a two-compartment system, with half-lives of 1.8 and 25 days (Hansen & Bjerregaard, 1995). Bioaccumulation studies of wastewater from a thermo-mechanical paper mill using the freshwater crayfish Cherax destructor consistently demonstrated elevated levels of manganese in the crayfish. However, the authors suggest that the elevated levels observed were due to manganese-oxidizing bacteria forming biofilms on the carapace followed by manganese dioxide precipitation rather than active uptake by the crayfish (King et al., 1999).
Manganese was readily accumulated in all tissues of brown trout (Salmo trutta) at concentrations reflecting the lowest natural concentrations found in circumneutral lakes. Trout exposed to 0.1 ΅g 54Mn/litre accumulated 1.78 mg manganese/kg (whole body) within 6 weeks, representing 95% of the steady-state concentration. The depuration rate was initially rapid, with 22% loss of radiolabelled manganese after 1 week (Rouleau et al., 1995). The addition of humic and fulvic acids had little effect on the uptake of manganese; however, other chelating agents, such as potassium ethylxanthate, sodium diethyldithiophosphate, and sodium dimethyl- and diethyldithiocarbamate, decreased bioaccumulation by 40% (Rouleau et al., 1992).
Terrestrial plant species vary a great deal in their ability to accumulate manganese. The absolute concentration of manganese in soils is generally less important to plants than the availability of manganese, which is determined by pH, cation exchange capacity, concentration of other cations, organic content, temperature, and microbial activity. Plants take up manganese from soil primarily in the divalent state. Differences in plant uptake can be explained in part by differences in the ability of plants to bring about the dissolution of oxidized manganese (Stokes et al., 1988). The application of chelating agents significantly reduced the uptake of manganese in roots, stems, and leaves of okra (Abelmoschus esculentus) at manganese concentrations of 500 and 1000 mg/kg (Denduluri, 1994).
A BCF of 2 was calculated for earthworms (Lumbricus terrestris) exposed to 54Mn in litter for 20 days, with a depuration half-time of 40 days (Sheppard et al., 1997).
According to a National Research Council of Canada report (Stokes et al., 1988), manganese concentrations in air tend to be lowest in remote locations (about 0.514 ng/m3 on average), higher in rural areas (40 ng/m3 on average), and still higher in urban areas (about 65166 ng/m3 on average) (see Table 2). Similar concentrations have been reported elsewhere, leading to the conclusion that annual manganese concentrations average 1030 ng/m3 in areas far from known sources and 1070 ng/m3 in urban and rural areas without major point sources of manganese (WHO, 1999). Manganese concentrations in air tend to be highest in source-dominated areas (e.g., those with foundries), where values can reach 8000 ng/m3 (US EPA, 1984; Stokes et al., 1988). Annual averages of manganese concentrations may rise to 200300 ng/m3 in air near foundries and to over 500 ng/m3 in air near ferro- and silicomanganese industries (WHO, 1999). Manganese concentrations in air have been measured in many specific locations. In the Vancouver, Canada, area, for example, annual geometric mean concentrations of manganese ranged from <10 to 30 ng/m3 in 1984 (Stokes et al., 1988). Over the period 19811992, Loranger & Zayed (1994) found average manganese concentrations in Montreal, Canada, of 20 and 60 ng/m3 in areas of low and high traffic density, respectively. More recently, Loranger & Zayed (1997) found the average concentration of total manganese in an urban site in Montreal to be 27 ng/m3. In selected periods in the 1970s, annual mean concentrations of manganese were reported to range from 3 to 16 ng/m3 in two German cities (Frankfurt and Munich), from 42 to 455 ng/m3 in Belgium, and from 20 to 800 ng/m3 in Japanese cities (WHO, 1999). More recent analysis of urban aerosols revealed mean manganese concentrations ranging from 80 to 350 ng/m3 for the city of Kayseri, Turkey (Kartal et al., 1993), 154 ng/m3 for Bhilai, India (19951996) (Pandey et al., 1998), and 16.5 ng/m3 for Seville, Spain (Espinosa et al., 2001).
As Table 2 shows, manganese concentrations in air in the USA have decreased over the past three decades (Kleinman et al., 1980; US EPA, 1984), a trend believed to be due primarily to the installation of industrial emission controls (US EPA, 1984, 1985a). In Ontario, Canada, as well, annual average manganese concentrations in air have decreased, along with total suspended particulate levels (Stokes et al., 1988).
In a review of worldwide data on trace metals in precipitation, median concentrations of manganese in wet deposition were 23, 5.7, and 0.19 ΅g/litre for urban, rural, and remote locations, respectively (Galloway et al., 1982).
Concentrations of manganese in open seawater range from 0.4 to 10 ΅g/litre (US EPA, 1984; Zeri et al., 2000). In the North Sea, the north-east Atlantic Ocean, the English Channel, and the Indian Ocean, manganese content was reported to range from 0.03 to 4.0 ΅g/litre. Levels found in coastal waters of the Irish Sea and in the North Sea off the coast of the United Kingdom ranged from 0.2 to 25.5 ΅g/litre (Alessio & Lucchini, 1996). Higher concentrations (up to 500 ΅g/litre) have been reported for anaerobic layers of open seawater (Lewis & Landing, 1991, 1992). Hypoxic concentrations below 16% saturation can increase the concentration of dissolved manganese above that normally found in seawater to concentrations approaching 1500 ΅g/litre (Balzer, 1982).
Concentrations of dissolved manganese in natural waters that are essentially free of anthropogenic sources can range from 10 to >10 000 ΅g/litre (Reimer, 1999). However, manganese concentrations in natural surface waters rarely exceed 1000 ΅g/litre and are usually less than 200 ΅g/litre (Reimer, 1999). In a 19741981 survey of 286 US river water samples, concentrations of dissolved manganese ranged from less than 11 ΅g/litre (25th percentile) to more than 51 ΅g/litre (75th percentile) (Smith et al., 1987), with a median of 24 ΅g/litre. Mean groundwater concentrations were 20 and 90 ΅g/litre from two geological zones in California, USA (Deverel & Millard, 1988). In a number of cases, higher levels in water (in excess of 1000 ΅g/litre) have been detected at US hazardous waste sites, suggesting that, in some instances, wastes from industrial sources can lead to significant contamination of water (ATSDR, 2000). Concentrations of dissolved manganese of up to 4400 ΅g/litre have been recorded in waters receiving acid mine drainage (pH <2.5) (Filipek et al., 1987). The surface waters of Welsh rivers were reported to contain from 0.8 to 28 ΅g manganese/litre (Alessio & Lucchini, 1996). Neal et al. (1986) reported mean manganese concentrations of 41 and 30 ΅g/litre for two Welsh streams with mean rainfall concentrations measured at 2 ΅g manganese/litre. They found that stormflow waters were acidic (pH ~4.5) and enriched in soluble manganese. Concentrations of manganese ranged from 1 to 530 ΅g/litre in 37 rivers in the United Kingdom and in the Rhine and the Maas and their tributaries (Alessio & Lucchini, 1996). Mean dissolved manganese concentrations ranging from 6 to 117 ΅g/litre were found for six United Kingdom rivers with particulate manganese concentrations of 793 ΅g/litre (Neal et al., 2000). Higher manganese concentrations were associated with increasing suspended sediment levels caused by higher river flows. In Chesapeake Bay, USA, manganese concentrations of up to 237 ΅g/litre have been recorded during anoxic conditions (Eaton, 1979), and Kremling (1983) found manganese concentrations of around 700800 ΅g/litre in anoxic bottom waters in the Baltic Sea. During the late summer, Horsetooth Reservoir, Colorado, USA, is fully stratified and exhibits seasonally high fluxes of iron, manganese, and metal-rich particles into the water column. Stein et al. (2002) monitored manganese concentrations during August 1999. The total manganese concentration in water prior to filtration and measured by atomic absorption spectrometry was 93 ΅g/litre; after sedimentation of particles, the total manganese concentration measured by ICP with atomic emission spectrophotometry was 213 ΅g/litre.
Manganese has been measured in snow core samples dated from 1967 to 1989 collected in central Greenland at concentrations ranging from 0.016 to 0.236 ΅g/kg. A large fraction of the manganese was found to originate from rock and soil dust; "excess" manganese sources suggested include volcanoes, natural vegetation fires, continental biogenic emissions, and anthropogenic sources such as industrial outputs and MMT (Veysseyre et al., 1998).
Manganese concentrations in river sediments from the South Platte River Basin, USA (19921993), ranged from 410 to 6700 mg/kg dry weight, with a geometric mean of 1260 mg/kg dry weight (Heiny & Tate, 1997). Total manganese levels in sediment of a Chesapeake Bay, USA, tributary ranged from 940 to 2400 mg/kg dry weight (Hartwell et al., 2000). Sediment from an urban lake (Germiston Lake, South Africa) receiving inputs from industrial and residential areas, as well as windborne dust from old mine dumps, contained manganese concentrations ranging from 970 to 13 400 mg/kg dry weight (Sanders et al., 1998). Mangrove sediments in the Arabian Gulf contained manganese concentrations ranging from 29 to 170 mg/kg dry weight, with higher concentrations associated with the local geology (Shriadah, 1999). Sediment manganese concentrations of 1001000 mg/kg dry weight have been reported for an intertidal flat off Korea (Jung et al., 1996). Similar total manganese values were found in the northern Adriatic Sea (Italy), ranging from 200 to 800 mg/kg dry weight, with a mean of 370 mg/kg dry weight (Fabbri et al., 2001). Surface sediments in the Baltic Sea contained mean manganese concentrations of 3550 (Bothnian Sea), 5070 (Gulf of Finland), and 8960 (Bothnian Bay) mg/kg dry weight; the high manganese concentrations were thought to be due to ferromanganese concretions and riverine loads (Leivuori, 1998). Sediment pore water may contain dissolved manganese at concentrations of 0.224 mg/litre (Bryan & Hummerstone, 1973; Aller, 1994; Eriksson & Baden, 1998), whereas bottom water concentrations are normally around 0.217 ΅g/litre (Hall et al., 1996).
Natural ("background") levels of total manganese in soil range from <1 to 4000 mg/kg dry weight, with mean values around 300600 mg/kg dry weight (Shacklette et al., 1971; Cooper, 1984; US EPA, 1985b; Adriano, 1986; Schroeder et al., 1987; Eckel & Langley, 1988; Rope et al., 1988). Reimer (1999) reported mean total manganese concentrations from uncontaminated soils for British Columbia, Canada, ranging from 284 to 1359 mg/kg dry weight. Similar total manganese concentrations were found in volcanic soils in Romania at varying distances from a lead smelter (Donisa et al., 2000). Median manganese concentrations of up to 1980 mg/kg dry weight were reported for urban areas of Honolulu, Hawaii, USA (Sutherland & Tolosa, 2001). Accumulation of manganese in soil usually occurs in the subsoil and not on the soil surface (IPCS, 1981). Manganese was found at higher concentrations in the upper layers of soil adjacent to highways in Canada following 25 years of the use of MMT as a fuel additive. However, this use has not led to a significant increase in either total or exchangeable manganese in these soils. There was a trend of decreasing manganese concentrations with distance from the highway, but this was not statistically significant (Bhuie et al., 2000; Bhuie & Roy, 2001). In general, soil manganese concentrations in the Kola Peninsula, Russian Federation, ranged from 100 to 500 mg/kg dry weight, except for an extremely contaminated (copper/ nickel smelter complex) and eroded podzol containing 3500 mg/kg (Barcan & Kovnatsky, 1998). Higher concentrations have been reported for some acid soils; for example, a major portion of agricultural land on Oahu, Hawaii, USA, consists of oxisols of basaltic origin, with total manganese concentrations ranging from 10 000 to 40 000 mg/kg (Fujimoto & Sherman, 1948).
Geometric mean manganese concentrations in seaweed from south-west England ranged from 128 to 392 mg/kg dry weight (Bryan & Hummerstone, 1973). Manganese concentrations in seaweed (Fucus vesiculosus) and mussels (Mytilus edulis) were 350 and 29 mg/kg dry weight for the North Sea for the two species, respectively, and 735 and 46 mg/kg dry weight for the Baltic Sea (Struck et al., 1997). High manganese concentrations have been found in all tissues of blue crabs (Callinectes sapidus) from a metal-contaminated estuary in North Carolina, USA (Weinstein et al., 1992), and in Norway lobsters (Nephrops norvegicus) following autumnal hypoxia in the south-east Kattegat, Sweden. Lobsters living on sediments rich in manganese contained whole-body mean manganese concentrations of 92 mg/kg dry weight (Eriksson, 2000a). Similarly, river crabs (Potamonautes warreni) in an urban lake with manganese-rich sediment contained a mean manganese concentration of 662 mg/kg dry weight (Sanders et al., 1998).
Mean manganese levels in mussels (Mytilus trossulus and Crenomytilus grayanus) from the north-west Pacific Ocean ranged from 2.8 to 9.3 mg/kg dry weight (Kavun et al., 2002). Manganese concentrations in barnacles (Balanus amphitrite and Tetraclita squamosa) from Xiamen Harbour and Hong Kong coastal waters ranged from 5.9 to 277 mg/kg dry weight (Blackmore et al., 1998; Blackmore, 1999; Rainbow & Blackmore, 2001). During the 1990s, manganese concentrations in barnacles had increased at some sampling sites, probably due to the resuspension of metal-rich sediments from dredging and reclamation associated with major construction projects (Blackmore, 1999). A manganese concentration of 6 mg/kg for the muscle tissue of annelids (Lamellibrachia satsuma) living near hydrothermal vents was reported by Ando et al. (2002).
Concentrations of manganese found in tissues of marine and freshwater fish tend to range from <0.2 to 19 mg/kg dry weight (Greichus et al., 1977, 1978; Capelli et al., 1987; Sindayigaya et al., 1994; Heiny & Tate, 1997). Higher manganese concentrations of up to 40 mg/kg wet weight (equivalent to dry weight concentrations of >100 mg/kg) were reported for fish from the Lower Savannah River, USA, probably related to acid mine drainage (Winger et al., 1990), and mean liver concentrations of 54 mg/kg dry weight were reported for a polluted lake (Saad et al., 1981). Manganese levels in the opercula and scales of brook trout (Salvelinus fontinalis) were 1.6 times higher in fish from acidified lakes (pH 5.25.5) than in fish from non-acidified lakes (pH 6.87.0) (Moreau et al., 1983). Bendell-Young & Harvey (1986) found significantly more manganese in all tissues of white suckers (Catostomus commersoni) from an acidified lake (pH 4.8) compared with other lakes (pH 5.06.0) in south-central Ontario, Canada.
Concentrations of manganese in terrestrial plants tend to range from 20 to 500 mg/kg (Stokes et al., 1988). Rautio et al. (1998) analysed scots pine (Pinus sylvestris) needles at various distances from a smelter complex (Finnish Lapland and the Kola Peninsula, Russian Federation). Manganese concentrations ranged from <50 to >1200 mg/kg dry weight, with the lowest concentrations in the vicinity of the smelter, possibly because of increased foliar leakage and/or leaching of cations from the upper soil layers due to sulfur and heavy metal deposition. Mean levels of manganese in soil and vegetation near a municipal incinerator in Montcada, Spain, were 390420 mg/kg dry weight and 4954 mg/kg dry weight, respectively (Meneses et al., 1999), whereas seven species of mushroom in the vicinity of a nickel/copper smelter contained 1167 mg/kg dry weight (Barcan et al., 1998). Members of the Ericaceae family, which includes blueberries, are regarded as manganese accumulators. There are numerous reports of foliar manganese levels in excess of 20004000 mg/kg, particularly for Vaccinium angustifolium and V. vitis-idaea (Korcak, 1988). Creeping snowberry (Gaultheria hispidula) and velvet-leafed blueberry (V. myrtilloidies) from a bog soil (pH 4.0) contained 3000 and 2200 mg manganese/kg, respectively (NAS, 1973).
Menta & Parisi (2001) found mean manganese concentrations of 200300 mg/kg dry weight in the digestive gland of terrestrial snails (Helix pomatia and H. aspersa) collected from a semirural area in northern Italy and 1020 mg/kg in the foot; however, manganese concentrations for the slug Arion rufus were 92 mg/kg for the digestive gland and 394 mg/kg for the foot. Similar concentrations were found in the slug Arion ater at a site with vegetation levels of 150 mg manganese/kg; however, at a contaminated site near a disused lead/zinc mine (with vegetation levels of 207 mg/kg), manganese concentrations in the digestive gland and foot were 210 and 26 000 mg/kg dry weight, respectively (Ireland, 1979). The authors hypothesized that the lower levels of manganese found in the foot of the snail compared with the slug were related to the quantity of calcium required for shell formation. Earthworms (Lumbricus rubellus) from the Rhine Delta floodplain contained mean manganese concentrations of 100120 mg/kg dry weight (Hendricks et al., 1995).
Mean manganese concentrations in birds' eggs from a variety of geographical areas range from 1 to 5 mg/kg dry weight (Hothem et al., 1995; Hui et al., 1998; Burger et al., 1999); mean liver concentrations range from 3 to 11 mg/kg dry weight (Hui et al., 1998; Burger & Gochfeld, 1999, 2000b). Burger & Gochfeld (2000a) analysed the feathers of 12 species of seabird from the northern Pacific Ocean and found mean manganese concentrations ranging from 0.3 to 2 mg/kg dry weight. Higher manganese concentrations (4.5 mg/kg) were found in Laysan albatross (Diomedea immutabilis) feathers from the same location (Burger & Gochfeld, 2000b). Little egret (Egretta garzetta) and black-crowned night-heron (Nycticorax nycticorax) feathers contained 1.722.6 mg manganese/kg dry weight (Connell et al., 2002). Mean levels of manganese were lower than previously reported for egrets and herons in the early 1990s (4.263.1 mg/kg) (Burger & Gochfeld, 1993). Great tit (Parus major) feathers had mean concentrations ranging from 17.4 to 43.8 mg/kg dry weight (Janssens et al., 2001).
American alligators (Alligator mississippiensis) from lakes in central Florida, USA, contained a mean manganese concentration of 1.2 mg/kg wet weight in liver tissue; highest concentrations (3.1 mg/kg) were found in the regenerated tail (Burger et al., 2000). Slider turtle (Trachemys scripta) eggs contained 4.5 mg manganese/kg dry weight (Burger & Gibbons, 1998), whereas pine snakes (Pituophis melanoleucus) contained whole-body mean concentrations ranging from 11.9 to 17 mg manganese/kg dry weight (Burger, 1992).
Mean manganese concentrations in Baikal seal (Phoca sibirica) muscle, liver, and kidney tissue were 0.12, 2.1, and 0.84 mg/kg wet weight, respectively; manganese concentrations decreased with age (Watanabe et al., 1998). Mean manganese concentrations in European otter (Lutra lutra) livers ranged from 3.5 to 7.4 mg/kg dry weight (Mason & Stephenson, 2001). Similar mean concentrations were found in shrew (Crocidura russula and Sorex araneus) kidneys, ranging from 7.3 to 8.8 mg manganese/kg dry weight (Hendricks et al., 1995).
Manganese is an essential nutrient for microorganisms, plants, and animals (Underwood, 1977; Woolhouse, 1983). Manganese must be provided as a micronutrient in the culture media for growing algae (McLachlan, 1973) and in the diet of captive fish (Satoh et al., 1987), birds, and mammals (Underwood, 1977). Many calcareous soils require the application of manganese fertilizers for optimum crop growth (Woolhouse, 1983).
Manganese deficiency has been shown to limit the growth rate of marine phytoplankton, particularly where manganese-depleted deep seawater is upwelled to the surface (Huntsman & Sunda, 1980). Manganese levels can affect the microflora species composition of streams. In experiments, it has been shown that under low manganese concentrations (<0.04 mg/litre), blue-green and green algal flora can predominate, whereas at higher manganese levels, diatoms dominate (Patrick et al., 1969). Neutral streams with elevated levels of iron and manganese can develop blooms of ferromanganese-depositing bacteria with oxide deposition zones. Algal abundance declines within these blooms (Wellnitz & Sheldon, 1995).
Overall assemblage composition of macroinvertebrates in upland streams (Wales and Cornwall, United Kingdom) correlated with manganese concentration, pH, and nitrate concentration, with assemblage scores (relative abundance, species richness, and diversity) increasing with increasing manganese concentrations (0.0030.6 mg/litre) (Hirst et al., 2002).
Rainbow trout (Oncorhynchus mykiss) fed on a manganese-deficient diet (4.4 mg manganese/kg diet) for 60 weeks developed lens cataracts and short body dwarfism, although growth was not affected (Yamamoto et al., 1983). Knox et al. (1981) found no effect of low dietary manganese (1.3 mg/kg diet) on trout growth during a 24-week feeding period; there were effects on plasma ion levels, hepatic mineral levels, and hepatic enzyme activity. Experiments have shown that the addition of 10 mg manganese/kg (as manganese sulfate or manganese chloride) to white fishmeal diets (containing 23 mg manganese/kg) is necessary to obtain normal growth of captive carp (Cyprinus carpio) (Satoh et al., 1987). The dietary uptake of manganese dioxide and manganese carbonate by carp was found to be low.
Manganese is an essential nutrient for plant growth as a constituent of a number of metalloenzymes that occupy key roles in metabolism (Clarkson & Hanson, 1980; Woolhouse, 1983; Burnell, 1988). Nutritional requirements for terrestrial plants are around 1050 mg manganese/kg tissue (Hannam & Ohki, 1988; Reisenauer, 1988). Critical nutritional levels vary widely between species and among cultivars of a species (Reisenauer, 1988). Calcareous soils, especially those with poor drainage and high organic matter, are the types of soil that produce manganese-deficient plants. There are numerous examples of manganese deficiency, especially among crop plants. Manganese deficiency of peanut (Arachis hypogaea) is a common problem on some soils of the coastal plain region of the southern USA. Manganese deficiency occurred at pH levels of 6.8; maintaining soil pH at 6 provided a desirable medium for plant growth without the need for manganese fertilizer. Critical manganese levels ranged between 12 and 15 mg/kg in leaves (Parker & Walker, 1986). Application of manganese sulfate (15 mg manganese/kg) to highly calcareous soils enhanced the growth of soybean (Glycine max) plants (Ahangar et al., 1995). Critical limits for a variety of plant species have been calculated: for example, for corn (Zea mays), 10.6 mg manganese/kg in the ear leaf and 4.9 mg manganese/kg in the grain (Uribe et al., 1988); for oats (Avena sativa), 4.5 mg diethylenetriaminepentaacetic acid (DTPA)-extractable manganese/kg soil and 19 mg manganese/kg dry matter for mature leaf blades; and for cowpea (Vigna unguiculata), 2.4 mg DTPA-extractable manganese/kg soil and 41 mg manganese/kg dry matter for leaf blades (Bansal & Nayyar, 1996, 1998).
The importance of manganese for birds has been recognized for almost 50 years, ever since Wilgus et al. (1936) demonstrated that manganese could prevent perosis (a disease causing bone deformities) in the chicken. Subsequent research has shown that manganese is vital for growth, egg production, and proper development of the chick embryo and is essential in the activation of numerous enzymes (Underwood, 1977). Experiments have shown that the minimal manganese requirement for chicks on a casein-dextrose diet was 14 mg/kg diet (Halpin & Baker, 1986).
Plants and animals require manganese as an essential nutrient up to a certain level often referred to as the deficiency limit; however, at concentrations higher than this level, toxic effects are often observed.
Most toxicity tests have been carried out using ionic manganese. Little is known about the aquatic toxicity of colloidal, particulate, and complexed manganese; in general, however, toxicities of metals bound into these forms are assumed to be less than those of the aquo-ionic forms. Manganese fungicides have been referred to in this CICAD for source and fate information only, and no attempt has been made to evaluate this group of chemicals for environmental effect. Toxicity tests for the effects of manganese on aquatic biota are summarized in Table 3. For algae, there is a wide range of toxicity values; the most sensitive species appear to be the marine diatom Ditylum brightwellii, with a 5-day EC50, based on growth, of 1.5 mg manganese/litre, and a freshwater alga Scenedesmus quadricauda, with a 12-day EC50, based on chlorophyll inhibition, of 1.9 mg manganese/litre.
Table 3: Toxicity of manganese to aquatic species.a
|
Organism |
End-point |
Salt |
Manganese concentration (mg/litre) |
Reference |
|
Microalgae |
||||
|
Marine |
||||
|
Diatom (Ditylum brightwellii) |
5-day EC50 (growth inhibition) |
Cl2 |
1.5 |
Canterford & Canterford (1980) |
|
Diatom (Nitzschia closterium) |
96-h EC50 (growth inhibition) |
SO4 |
25.7 |
Rosko & Rachlin (1975) |
|
Diatom (Asterionella japonica) |
72-h EC50 (growth inhibition) |
Cl2 |
4.9 |
Fisher & Jones (1981) |
|
Green alga (Chlorella stigmatophora) |
21-day EC50 (total cell volume reduction) |
Cl2 |
50 |
Christensen et al. (1979) |
|
Freshwater |
||||
|
Alga (Scenedesmus quadricauda) |
12-day EC50 (growth inhibition) |
SO4 |
5 |
Fargaová et al. (1999) |
|
12-day EC50 (total chlorophyll reduction) |
SO4 |
1.9 |
Fargaová et al. (1999) |
|
|
Alga (Pseudokirchneriella subcapitata)b |
72-h EC50 (growth inhibition) |
8.3 |
Reimer (1999) |
|
|
14-day EC50 (total cell volume reduction) |
Cl2 |
3.1 |
Christensen et al. (1979) |
|
|
Protozoa |
||||
|
Ciliated protozoan (Tetrahymena pyriformis) |
1-h EC50 (non-specific esterase inhibition) |
SO4 |
27 |
Bogaerts et al. (1998) |
|
9-h EC50 (proliferation rate inhibition) |
SO4 |
210 |
Bogaerts et al. (1998) |
|
|
Ciliated protozoan (Spirostomum ambiguum) |
24-h LC50 |
Cl2 |
148 |
Nalecz-Jawecki & Sawicki (1998) |
|
24-h EC50 (deformations) |
Cl2 |
92.8 |
Nalecz-Jawecki & Sawicki (1998) |
|
|
Invertebrates |
||||
|
Marine |
||||
|
American oyster (Crassostrea virginica) |
48-h LC50 |
Cl2 |
16 |
Calabrese et al. (1973) |
|
Softshell clam (Mya arenaria) |
168-h LC50 |
Cl2 |
300 |
Eisler (1977) |
|
Mussel (Mytilus edulis) |
48-h EC50 (abnormal larvae) |
SO4 |
30 |
Morgan et al. (1986) |
|
Sea urchin (Heliocidaris tuberculata) |
72-h EC50 (abnormal larvae) |
5.2c |
Doyle et al. (2003) |
|
|
Brine shrimp (Artemia salina) |
48-h LC50 |
Cl2 |
51.8 |
Gajbhiye & Hirota (1990) |
|
Copepod (Nitocra spinipes) |
96-h LC50 |
70 |
Bengtsson (1978) |
|
|
Freshwater |
||||
|
Sludge worm (Tubifex tubifex) |
48-h LC50 |
SO4 |
208.1 |
Khangarot (1991) |
|
96-h LC50 |
SO4 |
170.6 |
Khangarot (1991) |
|
|
48-h LC50 |
SO4 |
171.4350.2d |
Rathore & Khangarot (2002) |
|
|
96-h LC50 |
SO4 |
164.6275.7d |
Rathore & Khangarot (2002) |
|
|
Daphnid (Daphnia magna) |
48-h LC50 |
Cl2 |
9.8 |
Biesinger & Christensen (1972) |
|
21-day LC50 |
Cl2 |
5.7 |
Biesinger & Christensen (1972) |
|
|
48-h EC50 (immobilization) |
SO4 |
8.3 |
Khangarot & Ray (1989) |
|
|
48-h LC50 |
0.876.3e |
Reimer (1999) |
||
|
48-h EC50 (immobilization) |
Cl2 |
4.756.1f |
Baird et al. (1991) |
|
|
48-h EC50 (immobilization) |
Cl2 |
2.0 |
Sheedy et al. (1991) |
|
|
48-h EC50 (immobilization) |
Cl2 |
40 |
Bowmer et al. (1998) |
|
|
48-h EC50 (immobilization) |
lactate |
44 |
Bowmer et al. (1998) |
|
|
24-h EC50 (immobilization) |
Cl2 |
56 |
Sorvari & Sillanpää (1996) |
|
|
Daphnid (Daphnia obtusa) |
48-h EC50 (immobilization) |
SO4 |
37.4 |
Rossini & Ronco (1996) |
|
Daphnid (Ceriodaphnia dubia) |
48-h LC50 |
9.1 |
Boucher & Watzin (1999) |
|
|
48-h LC50 |
SO4 |
5.714.5g |
Lasier et al. (2000) |
|
|
Amphipod (Hyalella azteca) |
96-h LC50 |
3.631e |
Reimer (1999) |
|
|
96-h LC50 |
SO4 |
3.013.7g |
Lasier et al. (2000) |
|
|
Rotifer (Brachionus calyciflorus) |
24-h LC50 |
Cl2 |
38.7 |
Couillard et al. (1989) |
|
Copepod (Canthocamptus spp.) |
48-h LC50 |
54 |
Rao & Nath (1983) |
|
|
Isopod (Asellus aquaticus) |
48-h LC50 |
Cl2 |
771 |
Martin & Holdich (1986) |
|
96-h LC50 |
Cl2 |
333 |
Martin & Holdich (1986) |
|
|
Amphipod (Crangonyx pseudogracilis) |
48-h LC50 |
Cl2 |
1389 |
Martin & Holdich (1986) |
|
96-h LC50 |
Cl2 |
694 |
Martin & Holdich (1986) |
|
|
Midge (Chironomus tentans) |
96-h LC50 |
5.894.3e |
Reimer (1999) |
|
|
Fish |
||||
|
Freshwater |
||||
|
Rainbow trout (Oncorhynchus mykiss) |
96-h LC50 |
4.8h |
Davies & Brinkman (1994) |
|
|
28-day LC50 (embryo-larval test) |
Cl2 |
2.9 |
Birge (1978) |
|
|
Brown trout (Salmo trutta) |
96-h LC50 |
3.849.9e |
Davies & Brinkman (1994, 1995) |
|
|
Coho salmon (Oncorhynchus kisutch) |
96-h LC50 |
2.417.4e |
Reimer (1999) |
|
|
Goldfish (Carassius auratus) |
7-day LC50 (embryo-larval test) |
Cl2 |
8.2 |
Birge (1978) |
|
Indian catfish (Heteropneustes fossilis) |
96-h LC50 |
Cl2 |
3350 |
Garg et al. (1989b) |
|
Indian freshwater murrel (Channa punctatus) |
96-h LC50 |
Cl2 |
3010 |
Garg et al. (1989a) |
|
Giant gourami (Colisa fasciatus) |
96-h LC50 |
SO4 |
2850 |
Agrawal & Srivastava (1980) |
|
96-h LC50 |
SO4 |
3230 |
Nath & Kumar (1988) |
|
|
Amphibians |
||||
|
Narrow-mouthed toad (Gastrophryne carolinensis) |
7-day LC50 (embryo-larval test) |
Cl2 |
1.4 |
Birge (1978) |
|
a |
All freshwater tests were performed at pH 78. |
|
b |
Formerly known as Selenastrum capricornutum. |
|
c |
NOEC = 1.3 mg/litre. |
|
d |
Range of mean LC50 values for temperatures ranging from 15 to 30 °C. |
|
e |
Range of LC50 values for hardness ranging from 25 to 250 mg calcium carbonate/litre. |
|
f |
Range of mean EC50 values for six different clones. |
|
g |
Range of mean LC50 values for hardness ranging from 26 to 184 mg calcium carbonate/litre. |
|
h |
Soft water. |
Manganese can induce iron deficiency in some algae, notably blue-green algae, and this can lead to inhibition of chlorophyll synthesis (Csatorday et al., 1984). The mechanism is thought to be competition for an active site where iron is necessary for functional integrity. Csatorday et al. (1984) found that in the alga Anacystis nidulans, manganese blocks access of iron ions to some functional site involved in the magnesium branch of the tetrapyrrole synthesis pathway in the synthesis of the pigment phycobiliprotein. The site of action was the step after the insertion of magnesium into the protoporphyrin ring. Rousch & Sommerfeld (1999) showed that the chlorophyll a content in two filamentous green algae (Ulothrix minuta and U. fimbriata) decreased at 20 mg manganese/litre over 15 days. The decreased chlorophyll a content may have been due to an effect on chlorophyll a synthesis or increased activity of the enzyme chlorophyllase, which breaks down chlorophyll. Abdel-Basset et al. (1995) also found that the activity of the enzyme chlorophyllase (isolated from two green algae Chlorella fusca and Kirchneriella lunaris) was increased in vitro in the presence of 0.1 mg manganese/litre. Filamentous green algal species (Ulothrix minuta and U. fimbriata) common in streams receiving acid mine drainage showed significant growth reductions at 20 mg manganese/litre (as manganese sulfate) in 15-day tests; algae were unaffected by pH levels typical of contaminated streams (Rousch & Sommerfeld, 1999). Wang (1986) reported a 4-day EC50, based on growth, of 31 mg manganese/litre for the common duckweed (Lemna minor). No significant effect on the growth of the aquatic plant Hydrilla verticillata was observed at 10 mg manganese/litre (as manganese chloride) in 5-day tests; however, a significant increase in enzymatic activity was found at 1 mg manganese/litre (Byl et al., 1994).
There are several reports that manganese can ameliorate the toxicity of other metals to microalgae. For example, Sunda & Huntsman (1998a,b,c) showed that manganese had a protective effect against cadmium uptake in the diatom Thalassiosira pseudonana and the green alga Chlorella pyrenoidosa, as cadmium uptake was inversely proportional to the free manganese ion concentration. Skowroński et al. (1988) also showed that manganese (50 mg/litre) ameliorated the toxicity of cadmium in the green microalga Stichococcus bacillaris. Similarly, in Chlamydomonas sp., cellular zinc increased as external manganese concentrations decreased (Sunda & Huntsman, 1998a). Manganese (4 ΅g/litre) has also been shown to ameliorate the toxicity of copper to the marine alga Nitzschia closterium (Stauber & Florence, 1985). Manganese oxides are efficient scavengers of metals due to their low solubility and large surface area. However, manganese is only slowly oxidized in seawater to manganese dioxide. Manganese added to seawater was found to stay as Mn(II), with only 10% oxidized over 3 months. However, in the presence of algae, Mn(II) may be oxidized at the cell surface to Mn(III), probably by superoxide. Manganese associated with the cells (as Mn(II) or Mn(III) hydroxides) adsorbed copper and prevented copper penetration into the cells. For Nitzschia, although there was competitive binding at the cell surface between copper and manganese, copper did not affect intracellular manganese. Manganese was also shown to be an effective scavenger of superoxide radical produced in the chloroplast by the reduction of molecular oxygen. Manganese catalysed the dismutation of superoxide to hydrogen peroxide and oxygen, providing further protection for the algal cell.
Tests on aquatic invertebrates reveal 48-h LC50/EC50 values ranging from 0.8 mg manganese/litre (Daphnia magna) to 1389 mg manganese/litre (Crangonyx pseudogracilis), with the lowest LC50 being observed under soft water conditions (25 mg calcium carbonate/litre) (see Table 3). It appears that freshwater molluscs and crustaceans are the most manganese-sensitive freshwater invertebrates, followed by arthropods and oligochaetes. For marine species, however, with the exception of crab embryos, crustaceans are relatively insensitive to manganese. In studies with platyhelminths, Palladini et al. (1980) showed that Mn(II) was neurotoxic, blocking presynaptic release of dopamine, leading ultimately to irreversible damage to the dopaminergic cells. Concentrations of manganese (added as manganese sulfate or manganese chloride) of 1001000 mg/litre immediately induced screw-like movements of the worms due to dopaminergic overstimulation. This was followed by death within 2448 h. The 24-h EC50 for Daphnia magna was increased from 56 to 940 mg/litre by the addition of 10 g ethylenediaminetetraacetic acid (EDTA)/litre and to 2300 mg/litre by the addition of 100 g DTPA/litre (Sorvari & Sillanpää, 1996; van Dam et al., 1999). The toxicity of manganese in acute tests on the sludge worm (Tubifex tubifex) was not influenced by temperature between 20 and 30 °C; however, there was an increase in toxicity at 15 °C (Rathore & Khangarot, 2002). Acute toxicity decreased with increasing water hardness; for example, 96-h LC50s determined for Hyalella azteca progressively increased from 3.0 to 13.7 mg/litre with increasing water hardness (from 26 to 164 mg calcium carbonate/litre) (Lasier et al., 2000). Similarly, acute toxicity tests on Daphnia magna showed 48-h LC50s increasing from 0.8 to 76.3 mg manganese/litre with water hardness increasing from 25 to 250 mg calcium carbonate/litre (Reimer, 1999).
Sea stars (Asterias rubens) showed no mortality at 10 and 25 mg manganese/litre (as manganese chloride); sea stars exposed to 50, 100, or 200 mg/litre had median survival times of 72, 18, and 14.4 h, respectively (Hansen & Bjerregaard, 1995). Manganese (0.5 mg/litre) had no significant effect on the feeding rate of Gammarus pulex in 6-day tests (Maltby & Crane, 1994). Macdonald et al. (1988) reported a significant reduction in survival and hatching of yellow crab (Cancer anthonyi) embryos at >0.01 mg manganese/litre (as manganese chloride) in 7-day seawater tests. However, it should be noted that because this species broods embryos externally on the abdomen, the embryos are exposed to contaminants in waters and sediments continuously. Concentrations of >100 mg/litre gave 100% mortality of crab embryos over 7 days. Concentrations of 0.0110 mg/litre gave 2745% mortality; however, the response was not concentration-dependent. Hatching of embryos was also decreased at manganese concentrations of 0.0110 mg/litre, compared with controls. Eggs of the crab Carcinus maenas accumulate manganese (and other metals) during ovogenesis, and, when the eggs are extruded, manganese also adsorbs to the chitinous vitelline membrane (Martin, 1976). This bioconcentration of manganese may explain why crab embryos are killed and larval hatching is impaired at lower manganese concentrations compared with other invertebrates.
Sediments of Outer Malletts Bay (Vermont, USA) in Lake Champlain were found to contain arsenic, manganese, and nickel levels above proposed sediment quality guidelines. Ceriodaphnia dubia exposed to sediment pore water showed acute mortality, and this was correlated with manganese concentrations (2339 mg total manganese/litre). Adding EDTA or temporarily increasing the pH from 7 to 11 reduced the toxicity of the pore water (Boucher & Watzin, 1999). In a metal-contaminated estuary in North Carolina, USA, the high frequency of blue crabs (Callinectes sapidus) with shell disease (lesions) was ascribed to manganese toxicity, since the manganese concentration was always highest in the diseased crabs; however, no statistical causeeffect relationship was shown (Weinstein et al., 1992). Deposition of manganese dioxide on the gills of Norway lobster (Nephrops norvegicus) following hypoxic conditions in the south-east Kattegat, Sweden, has on occasion given rise to a brown or black discoloration of the gills and black corroded areas on the carapace (Baden et al., 1990).
For fish, 96-h LC50s range from 2.4 mg manganese/litre for coho salmon (Oncorhynchus kisutch) to 3350 mg manganese/litre for Indian catfish (Heteropneustes fossilis), with the lowest LC50 values obtained under soft water conditions (25 mg calcium carbonate/litre) (see Table 3). A single embryo-larval test with a 7-day LC50 of 1.4 mg manganese/litre was identified for amphibians. Water hardness significantly affected manganese toxicity in early life stage tests (62 days) on brown trout (Salmo trutta), with toxicity decreasing with increasing hardness (30450 mg calcium carbonate/litre) (Stubblefield et al., 1997). IC25 values, based on survival and growth, ranged from 4.7 mg manganese/litre at 30 mg calcium carbonate/litre to 8.7 mg manganese/litre at 450 mg calcium carbonate/litre. However, it should be noted that a similar trend was not observed for no-observed-effect concentrations (NOECs), with the lowest being reported at 2.8 mg manganese/litre at a hardness of 150 mg calcium carbonate/litre. Most reports on the mechanisms of toxicity of manganese to fish have used unrealistically high manganese concentrations to obtain observable effects. However, Wepener et al. (1992) examined the mechanism of toxicity of manganese (added as manganese chloride) to the banded tilapia (Tilapia sparrmanii) in South Africa. In contrast to the previous studies, manganese was tested at an environmentally relevant concentration of 4.43 mg/litre, the mean level of local waters of the Witwatersrand, at both pH 7.4 and pH 5. There were no mortalities after 96-h exposures. However, there were significant decreases in red blood cells, haemoglobin, mean cell volume, haematocrit, and white blood cells. The decrease in red blood cells and haematocrit was due to internal haemorrhaging, possibly as a result of necrosis of the intestinal mucosa and kidneys. Manganese-induced anaemia was also evident, possibly from damage to haematopoietic tissue in spleen and kidney. Decreased mean cell volume was due to the release of immature red blood cells as a result of bleeding. The enzyme delta-aminolevulinic dehydratase (ALA-D), a key enzyme in haem biosynthesis, showed increased activity to compensate for the hypoxic conditions experienced by the fish. The authors suggested that the decreases in white blood cells may be due to increased secretion of corticosteroids, a non-specific response to environmental stress.
In soft water tests, significant embryonic mortality was observed in rainbow trout (Oncorhynchus mykiss) eggs exposed to 1 mg manganese sulfate/litre for 29 days (Lewis, 1976), and brown trout (Salmo trutta) yolk sac fry showed significant reductions in calcium and sodium levels after 30 days' exposure to 6400 nmol manganese/litre (as manganese chloride) at pH 6.5 (Reader et al., 1989). Rainbow trout fry did not significantly avoid manganese sulfate concentrations of up to 10 mg/litre (Lewis, 1976).
Increased mortality of rainbow trout (Oncorhynchus mykiss) was observed at a fish hatchery in Arkansas, USA, during 1966. A positive correlation was found between mortality and manganese concentrations (<0.51 mg/litre); the presence of oxidized forms of manganese at the interface between anoxic and oxygenated zones was proposed as a possible explanation for the mortalities (Nix & Ingols, 1981). Acid precipitation has caused acid episodes and elevated concentrations of metals such as iron, aluminium, and manganese in streams in mountain regions of Sweden. Cage experiments were carried out with yearling brown trout (Salmo trutta) in the 1980s. Canonical distribution analysis showed that pH (4.55.4) and the concentration of labile inorganic manganese (0.10.4 mg/litre) explained all the observed mortality. The rate of accumulation on/in trout gills was correlated with the concentration of labile inorganic manganese (Nyberg et al., 1995).
Symptoms of manganese toxicity to terrestrial plants vary widely with species and include marginal chloroses, necrotic lesions, and distorted development of the leaves (Woolhouse, 1983). Manganese is the cause of recognizable disorders in some crops, such as crinkle leaf in cotton (Adams & Wear, 1957) and stem streak necrosis in potato (Robinson & Hodgson, 1961). In such instances, induced deficiencies of other mineral nutrients, such as iron, magnesium, and calcium, are involved to various degrees. Toxic manganese concentrations in crop plant tissues vary widely, with critical values ranging from 100 to 5000 mg/kg (Hannam & Ohki, 1988).
Manganese toxicity is a major factor limiting crop growth on acidic, poorly drained, or steam-sterilized mineral soils. Acidic (pH 4.4) oxisol soils in Hawaii, USA, containing 15 g total manganese/kg need to be limed to raise the pH to 5.7 or above, resulting in soluble manganese concentrations of less than 2 mg/litre, for normal watermelon (Citrullus lanatus) growth (Hue & Mai, 2002). Soil acidification enhances the solubility of metals such as aluminium and manganese. Soil acidification can be caused by acidic deposition, acid soils brought to the surface by construction projects, and industrial acid deposition. The pH of soils containing toxic levels of manganese is generally higher (<5.5) than the pH of soils containing toxic levels of aluminium (<4) (Foy et al., 1978, 1988). Manganese tends to accumulate in shoots rather than roots, leading to visible symptoms in leaves (Loneragan, 1988), whereas aluminium toxicity predominantly causes damage to roots without definitive foliar symptoms. Foliar symptoms of manganese toxicity have been noted in hydroponic studies at 50 mg manganese/litre for chlorosis of entire young leaves (white birch Betula platyphylla japonica) and at >1 mg manganese/litre for brown speckle at the leaf marginal and interveinal area in older leaves (Kitao et al., 2001). Kaus & Wild (1998) found that Douglas-fir (Pseudotsuga menziesii viridis) showing symptoms similar to iron deficiency chlorosis had accumulated significantly more manganese than trees without chlorosis. Excess manganese in soil has been found to accumulate in leaves of broad-leaved deciduous plants of northern Japan. This resulted in declines in light-stimulated net photosynthetic rate (Kitao et al., 1997).
Negative correlations have been found between the cover of the foliose epiphytic lichen Hypogymnia physodes and the manganese content in Norway spruce (Picea abies) bark in the Harz Mountains, northern Germany (Hauck et al., 2001). Culture experiments have shown that the growth of soredia of H. physodes is inhibited by ambient manganese concentrations (Hauck et al., 2002b). In further toxicity assays on H. physodes, it was found that the alleviating effects of calcium and magnesium on manganese toxicity were, at least in part, due to reduced manganese uptake (Hauck et al., 2002a).
There is a wide range of variation in tolerance to manganese between and within plant species (Woolhouse, 1983). Factors affecting manganese tolerance include genotype (inter- and intraspecific variation), silicon concentration, temperature, light intensity, physiological leaf age, microbial activity, and the characteristics of the rhizosphere (Horst, 1988). Substantial genetic variation for manganese tolerance exists in crop plants such as common beans (Phaseolus vulgaris) (Foy et al., 1988; González & Lynch, 1999), mung beans (Vigna radiata) (Rout et al., 2001), and rice (Oryza sativa) (Rout et al., 2001; Lidon, 2002). Members of the Ericaceae family are regarded as manganese accumulators (see section 5.3). High foliar manganese levels (in excess of 20004000 mg/kg) have not been associated with either visual toxicity symptoms or depressed growth (Korcak, 1988). Manganese tolerance in the cowpea (Vigna unguiculata) depends on the control of the free Mn(II) concentration and of Mn(II)-mediated oxidation/reduction reactions in the leaf apoplast (Horst et al., 1999). In the early stages of growth, rice tolerance to excess manganese was closely related with exclusion from root cells and with the physiological control of its translocation to the shoot in a process that implicates an alteration of stomata dimensions (Lidon, 2002). Silicon has been found to increase the tolerance of some plant species to manganese (Bowen, 1972; Horst & Marschner, 1978). Rogalla & Römheld (2002) concluded that silicon-mediated tolerance to manganese in cucumber (Cucumis sativus) was a consequence of stronger binding of manganese to cell walls and a lowering of the manganese concentration within the symplast. Hauck et al. (2002b) found that the epiphytic lichen Hypogymnia physodes is susceptible to manganese toxicity in both the laboratory and field. They also found that the lower extracellular uptake of manganese combined with lower losses of calcium and magnesium and a higher intracellular magnesium/manganese ratio may contribute to the higher manganese tolerance of the crustose lichen Lecanora conizaeoides in the field, which was observed in Norway spruce (Picea abies) forests of the Harz Mountains of northern Germany.
In 48-h contact tests, LC50s for earthworms (Eisenia fetida) were 230 ΅g/cm2 for manganese nitrate and 600 ΅g/cm2 for potassium permanganate (Diaz-Lopez & Mancha, 1994). In 8-week toxicity tests using activated sludge, no significant effect on the growth of earthworms (E. fetida) was observed at the highest concentration tested (22 000 mg manganese/kg, as manganese sulfate) (Hartenstein et al., 1981). Worms (E. fetida) exposed for 8 weeks to manure dosed with manganese (4.3 ΅mol/g) contained 9.3 mg/kg body weight, compared with 1.9 mg/kg body weight in controls, and showed marked cellular damage to spermatozoa (Reinecke & Reinecke, 1997). Twenty-four-hour LC50s were reported for free-living soil nematodes (Caenorhabditis elegans) at 98132 mg/kg for total manganese and 76108 mg/kg for the free ion (as manganese nitrate) (Tatara et al., 1997, 1998).
Significant adverse effects on growth and behaviour of herring gull (Larus argentatus) chicks were observed following a single intraperitoneal injection of manganese acetate (25 mg/kg body weight) (Burger & Gochfeld, 1995). Sierra et al. (1998) reported no significant toxic effects on feral pigeons (Columba livia) exposed to manganese tetroxide dust at concentrations of 239 ΅g manganese/m3 (7 h/day, 5 days/week) for 13 weeks.
Manganese is ubiquitous in the environment and comprises about 0.1% of the Earth's crust. Crustal rock is a major source of manganese found in the atmosphere. Ocean spray, forest fires, vegetation, and volcanic activity are other major natural atmospheric sources of manganese, whereas anthropogenic releases include emissions from alloy, steel, and iron production, combustion of fossil fuels, and, to a much lesser extent, emissions from the combustion of fuel additives. Manganese concentrations in air tend to be lowest in remote locations (about 0.514 ng/m3 on average), higher in rural areas (40 ng/m3 on average), and still higher in urban areas (about 65166 ng/m3 on average). Manganese concentrations in air tend to be highest in source-dominated areas. There is relatively little information on the uptake and effects of manganese in organisms via the atmosphere. However, the atmosphere can act as a major source of manganese to the aquatic and terrestrial compartments.
The environmental chemistry of manganese in the aquatic environment is largely governed by pH and redox conditions; Mn(II) dominates at lower pH and redox potential, with an increasing proportion of colloidal manganese oxyhydroxides above pH 5.5 in non-dystrophic waters. Concentrations of dissolved manganese in natural waters that are essentially free of anthropogenic sources can range from 10 ΅g/litre to >10 mg/litre. However, manganese concentrations in natural surface waters rarely exceed 1 mg/litre and are usually less than 0.2 mg/litre. Concentrations of dissolved manganese of up to 4 mg/litre have been recorded in waters receiving acid mine drainage. Primary chemical factors controlling sedimentary manganese cycling are the oxygen content of the overlying water, the penetration of oxygen into the sediments, and benthic organic carbon supply. Under hypoxic conditions, dissolved manganese concentrations can increase in water overlying sediment rich in manganese and be taken up by organisms. Manganese in water can be significantly bioconcentrated by biota at lower trophic levels. Uptake of manganese by aquatic invertebrates and fish significantly increases with temperature and decreases with pH. Uptake of manganese has been found to increase with decreasing salinity.
Most toxicity tests have been carried out using soluble Mn(II) salts. Little is known about the aquatic toxicity of colloidal, particulate, and complexed manganese, but, in general, toxicities of metals bound into these forms are assumed to be less than those of the aquo-ionic forms. Acute and chronic toxicity tests are summarized in Figure 1. Toxicity tests have been almost exclusively conducted at near-neutral pH. For algae and protozoa, there is a wide range of toxicity values; the most sensitive species were marine diatoms, with a 5-day EC50, based on growth, of 1.5 mg manganese/litre, and freshwater algae, with a 12-day EC50, based on chlorophyll inhibition, of 1.9 mg manganese/litre. Tests on aquatic invertebrates reveal 48-h LC50/EC50 values ranging from 0.8 to 1389 mg manganese/litre. A significant reduction in survival and hatching of crab embryos at >0.01 mg manganese/litre in seawater was found; however, these data were not used in the risk assessment due to the lack of a concentrationresponse relationship. For fish, 96-h LC50s range from 2.4 to 3350 mg manganese/litre. In chronic toxicity tests, significant embryonic mortality was observed in trout eggs at 1 mg manganese sulfate/litre. A single embryo-larval test with a 7-day LC50 of 1.4 mg manganese/litre was identified for amphibians. Manganese toxicity in aquatic invertebrates and fish is heavily influenced by water hardness. Figure 2 is a plot of toxicity test results against water hardness. It is clear from the figure that the most sensitive aquatic organisms are most susceptible to the toxic effects of manganese at hardness concentrations below 50 mg calcium carbonate/litre.
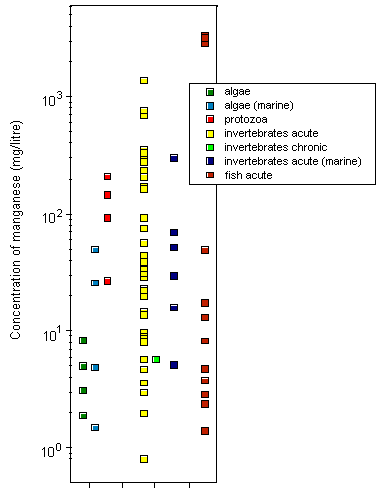
Fig. 1: Toxicity of manganese to aquatic organisms
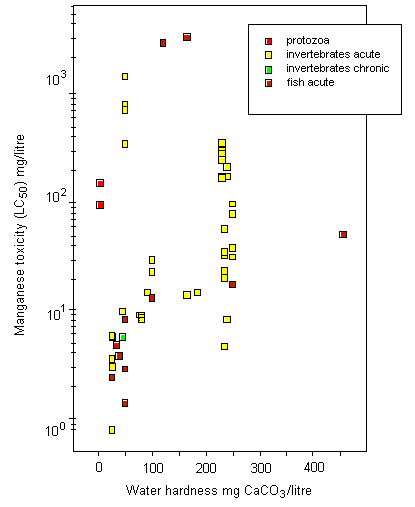
Fig. 2: A plot of manganese toxicity against water hardness
In the field, the high frequency of shell disease in crabs in a metal-contaminated estuary was ascribed to manganese toxicity, and the deposition of manganese dioxide on the gills of lobsters gave rise to a brown or black discoloration of the gills and black corroded areas on the carapace following hypoxic conditions in the south-east Kattegat, Sweden. Increased mortality of rainbow trout (Oncorhynchus mykiss) at a hatchery was positively correlated with manganese concentrations (<0.51 mg/litre). Acid precipitation has caused acid episodes and elevated concentrations of metals. Cage experiments with yearling brown trout (Salmo trutta) showed that pH (4.55.4) and the concentration of labile inorganic manganese (0.10.4 mg/litre) explained all of the observed mortality.
Surface freshwater data suggest that higher manganese concentrations occur during periods of higher stream flow, such as spring runoff, and lower concentrations tend to occur downstream of lakes that act as settling areas for sediment. Soft water streams, rivers, and lakes appear to be the most sensitive freshwater environments, with laboratory tests and field observations showing that dissolved manganese concentrations of around 1 mg/litre can cause toxic effects in aquatic organisms. Other factors, such as acid precipitation, acid mine drainage, land use, and municipal wastewater discharges, can increase dissolved manganese levels and thus increase the risk to sensitive species, especially in soft water areas. Evaluation of the likely toxicity of manganese to organisms in the field has to take account of speciation conditions in both the test and the specific field area. Setting single guidance values globally for manganese would be of limited value. In the marine environment, manganese can be taken up and accumulated by organisms during hypoxic releases of dissolved manganese from manganese-rich sediments. Laboratory tests with crabs suggest that manganese concentrations as low as 0.01 mg/litre can cause adverse effects, although accumulation of manganese may be involved here. Even taking into account the possible mitigating effects of suspended sediment, salinity, and oxygen levels, adverse effects in the field have been observed.
Within the limitations outlined above, suggestive guidance values for manganese toxicity in the marine and freshwater environments can be derived using a probabilistic approach, since the data set is sufficiently large to warrant it. Appendix 4 details the methodology used.
For the marine environment, nine toxicity values were chosen to derive a guidance value. The criteria for choosing the toxicity values and the values are presented in Appendix 4. These acute values have been first converted to chronic estimates and then converted to estimates of NOEC using the factors described in Appendix 4 (see Table A-1). An overall guidance value for the protection of 95% of marine species with 50% confidence was derived at 0.3 mg manganese/litre (see Figure A-1, Appendix 4).2
For the freshwater environment, 21 data points were used in the derivation in the same way. For further details, refer to Appendix 4 and Table A-2. The guidance value for freshwater species in soft waters is 0.2 mg manganese/litre (see Figure A-2 in Appendix 4).
Natural ("background") levels of total manganese in soil range from <1 to 4000 mg/kg, with mean values around 300600 mg/kg. In soils, manganese solubility is determined by two major variables: pH and redox potential. Concentrations of manganese in terrestrial plants tend to range from 20 to 500 mg/kg. Members of the Ericaceae family, which includes blueberries, are regarded as manganese accumulators. There are numerous reports of foliar manganese values in excess of 20004000 mg/kg. These high foliar levels have not been associated with either visual toxicity symptoms or depressed growth. Manganese is an essential nutrient for terrestrial plants, with nutritional requirements around 1050 mg manganese/kg tissue. Critical nutritional levels vary widely between species and among cultivars of a species. Calcareous soils, especially those with poor drainage and high organic matter, are the types of soil that produce manganese-deficient plants; in such areas, manganese fertilizers are often used to enhance crop growth.
Symptoms of manganese toxicity to terrestrial plants vary widely with species and include marginal chloroses, necrotic lesions, and distorted development of the leaves. Toxic manganese concentrations in crop plant tissues vary widely, with critical values ranging from 100 to 5000 mg/kg. Manganese toxicity is a major factor limiting crop growth on acidic, poorly drained, or steam-sterilized mineral soils. The disposal of sewage sludge can be a further source of manganese to plants, depending on the soil characteristics of the local area. There is a wide range of variation in tolerance to manganese between and within plant species. Factors affecting manganese tolerance include genotype (inter- and intraspecific variation), silicon concentration, temperature, light intensity, physiological leaf age, microbial activity, and the characteristics of the rhizosphere.
When evaluating the environmental risk from anthropogenic releases of manganese, account must be taken of local natural ("background") levels, which are in turn controlled by a variety of physical and chemical parameters. Furthermore, anthropogenic activities, such as the release of acidifying chemicals, land use, and dredging, may also have an impact on the local conditions that determine manganese speciation and, therefore, bioavailability. Different communities and ecosystems would also respond differently, depending on their "normal" exposure to manganese. For these reasons, deriving a single guidance value for the terrestrial environment is inappropriate.
There do not appear to be any previous international evaluations of the environmental effects of manganese and manganese compounds.
Abbasi SA (1988) Synergistic extraction and atomic absorption spectrometric determination of manganese in environmental samples using N-p-aminophenyl-2-furyl-acrylohydroxamic acid and trioctylmethyl ammonium cation. Analytical Letters, 21(10):19351944.
Abdel-Basset R, Issa AA, Adam MS (1995) Chlorophyllase activity: effects of heavy metals and calcium. Photosynthetica, 31:421425.
Adams F, Wear JI (1957) Manganese toxicity and soil acidity in relation to crinkle leaf of cotton. Soil Science Society of America Proceedings, 21:305308.
Adriano DC (1986) Trace elements in the terrestrial environment. New York, NY, Springer-Verlag.
Agrawal SJ, Srivastava AK (1980) Haematological responses in a fresh water fish to experimental manganese poisoning. Toxicology, 17(1):97100.
Ahangar AG, Karimian N, Abtahi A, Assad MT, Emam Y (1995) Growth and manganese uptake by soybean in highly calcareous soils as affected by native and applied manganese and predicted by nine different extractants. Communications in Soil Science and Plant Analysis, 26(910):14411454.
Ahnert A, Borowski C (2000) Environmental risk assessment of anthropogenic activity in the deep sea. Journal of Aquatic Ecosystem Stress and Recovery, 7(4):299315.
Aldenberg T, Slob W (1993) Confidence limits for hazardous concentrations based on logistically distributed NOEC toxicity data. Ecotoxicology and Environmental Safety, 25:4863.
Alessio L, Lucchini R (1996) Manganese and manganese compounds. In: Argentesi F, Roi R, Sevilla Marcos JM, eds. Data profiles for selected chemicals and pharmaceuticals 3, 4, 5. Ispra, European Commission, Joint Research Centre.
Aller RC (1994) The sedimentary Mn cycle in Long Island Sound: Its role as intermediate oxidant and the influence of bioturbation, O2, and Corg flux on diagenetic reaction balances. Journal of Marine Research, 52:259295.
Altomare C, Norvell WA, Björkman T, Harman GE (1999) Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Applied Environmental Microbiology, 65:29262933.
Ando T, Yamamoto M, Tomiyasu T, Hashimoto J, Miura T, Nakano A, Akiba S (2002) Bioaccumulation of mercury in a vestimentiferan worm living in Kagoshima Bay, Japan. Chemosphere, 49:477484.
ANZECC/ARMCANZ (2000) Australian and New Zealand guidelines for fresh and marine water quality. National Water Quality Management Strategy, Australian and New Zealand Environment Conservation Council, and Agriculture and Resource Management Council of Australia and New Zealand (further information can be found at http://www.environment.gov.au/water/quality/).
ATSDR (2000) Toxicological profile for manganese (update). Draft for public comment. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
Baden SP, Neil DM (1998) Accumulation of manganese in the haemolymph, nerve and muscle tissue of Nephrops norvegicus (L.) and its effect on neuromuscular performance. Comparative Biochemistry and Physiology, 119A(1):351359.
Baden SP, Pihl L, Rosenberg R (1990) Effects of oxygen depletion on the ecology, blood physiology and fishery of the Norway lobster Nephrops norvegicus (L.). Marine Ecology Progress Series, 67:141155.
Baden SP, Eriksson SP, Weeks JM (1995) Uptake, accumulation and regulation of manganese during experimental hypoxia and normoxia by the decapod Nephrops norvegicus (L.). Marine Pollution Bulletin, 31(13):93102.
Baden SP, Eriksson SP, Gerhardt L (1999) Accumulation and elimination kinetics of manganese from different tissues of the Norway lobster Nephrops norvegicus (L.). Aquatic Toxicology, 46(2):127137.
Baes CFI, Sharp RD (1983) A proposal for estimation of soil leaching and leaching constants for use in assessment models. Journal of Environmental Quality, 12:1728.
Baird DJ, Barber I, Bradley M, Soares AMVM, Calow P (1991) A comparative study of genotype sensitivity to acute toxic stress using clones of Daphnia magna Straus. Ecotoxicology and Environmental Safety, 21(3):257265.
Balzer W (1982) On the distribution of iron and manganese at the sediment/water interface: thermodynamic versus kinetic control. Geochimica et Cosmochimica Acta, 46:11531161.
Bankovitch V, Carrier G, Gagnon C, Normandin L, Kennedy G, Zayed J (2003) Total suspended particulate manganese in ambient air in Montreal 19812000. Science of the Total Environment, 308(13):185193.
Bansal RL, Nayyar VK (1996) Critical deficiency level of manganese for cowpea (Vigna unguiculata) grown on loamy sand. Acta Agronomica Hungarica, 44(2):191195.
Bansal RL, Nayyar VK (1998) Determination of critical limit with built-up levels of manganese for oats (Avena sativa) grown on loamy sand. Acta Agronomica Hungarica, 46(1):7176.
Barcan V, Kovnatsky E (1998) Soil surface geochemical anomaly around the coppernickel metallurgical smelter. Water, Air and Soil Pollution, 103(14):197218.
Barcan VS, Kovnatsky EF, Smetannikova MS (1998) Absorption of heavy metals in wild berries and edible mushrooms in an area affected by smelter emissions. Water, Air and Soil Pollution, 103(14):173195.
Bargar JR, Tebo BM, Villinski JE (2000) In situ characterization of Mn(II) oxidation by spores of the marine Bacillus sp. strain SG-1. Geochimica et Cosmochimica Acta, 64(16):27752778.
Beach ED, Fernandez-Cornejo J, Huang WY (1995) The potential risks of groundwater and surface water contamination by agricultural chemicals used in vegetable production. Journal of Environmental Science and Health, A30(6):12951325.
Beklemishev MK, Stoyan TA, Dolmanova IF (1997) Sorption-catalytic determination of manganese directly on a paper-based chelating sorbent. Analyst, 122:11611165.
Bendell-Young LI, Harvey HH (1986) Uptake and tissue distribution of manganese in the white sucker (Catostomus commersoni) under conditions of low pH. Hydrobiologia, 133:117125.
Bengtsson BE (1978) Use of a harpacticoid copepod in toxicity tests. Marine Pollution Bulletin, 9:238241.
Bhuie AK, Roy DN (2001) Deposition of Mn from automotive combustion of methylcyclopentadienyl manganese tricarbonyl beside the major highways in the Greater Toronto Area, Canada. Journal of the Air & Waste Management Association, 51:12881301.
Bhuie AK, McLaughlin D, Roy DN (2000) Exposure of urban ecosystems to Mn and Pb contaminants from gasoline additives beside a major highway in the Greater Toronto Area, Canada. Forestry Chronicle, 76:251258.
Biesinger KE, Christensen GM (1972) Effects of various metals on survival, growth, reproduction, and metabolism of Daphnia magna. Journal of the Fisheries Research Board of Canada, 29:16911700.
Birge WJ (1978) Aquatic toxicology of trace elements of coal and fly ash. In: Thorp JH, Gibbons JW, eds. Energy and environmental stress in aquatic systems. Augusta, GA, US Department of Energy, pp. 219240 (US Department of Energy Symposium Series 48; CONF-771114).
Blackmore G (1999) Temporal and spatial biomonitoring of heavy metals in Hong Kong coastal waters using Tetraclita squamosa. Environmental Pollution, 106(3):273283.
Blackmore G, Morton B, Huang ZG (1998) Heavy metals in Balanus amphitrite and Tetraclita squamosa (Crustacea: Cirripedia) collected from the coastal waters of Xiamen, China. Marine Pollution Bulletin, 36(1):3240.
Bogaerts P, Senaud J, Bohatier J (1998) Bioassay technique using nonspecific esterase activities of Tetrahymena pyriformis for screening and assessing cytotoxicity of xenobiotics. Environmental Toxicology and Chemistry, 17(8):16001605.
Boucher AM, Watzin MC (1999) Toxicity identification evaluation of metal-contaminated sediments using an artificial pore water containing dissolved organic carbons. Environmental Toxicology and Chemistry, 18(3):509518.
Bowen JE (1972) Manganesesilicon interaction and its effect on growth of Sudangrass. Plant and Soil, 37:577588.
Bowmer CT, Hooftman RN, Hanstveit AO, Venderbosch PWM, van der Hoeven N (1998) The ecotoxicity and the biodegradability of lactic acid, alkyl lactate esters and lactate salts. Chemosphere, 37(7):13171333.
Bratina BJ, Stevenson BS, Green WJ, Schmidt TM (1998) Manganese reduction by microbes from oxic regions of the Lake Vanda (Antarctica) water column. Applied and Environmental Microbiology, 64(10):37913797.
Brewer R, Belzer W (2001) Assessment of metal concentrations in atmospheric particles from Burnaby Lake, British Columbia, Canada. Atmospheric Environment, 35(30):52235233.
Bryan GW, Hummerstone LG (1973) Brown seaweed as an indicator of heavy metals in estuaries in south-west England. Journal of the Marine Biological Association of the U.K., 53:705720.
Burger J (1992) Trace element levels in pine snake hatchlings: tissue and temporal differences. Archives of Environmental Contamination and Toxicology, 22:209213.
Burger J, Gibbons JW (1998) Trace elements in egg contents and egg shells of slider turtles (Trachemys scripta) from the Savannah River site. Archives of Environmental Contamination and Toxicology, 34(4):382386.
Burger J, Gochfeld DJ (1993) Heavy metal and selenium levels in feathers of young egrets and herons from Hong Kong and Szechuan China. Archives of Environmental Contamination and Toxicology, 25:322327.
Burger J, Gochfeld M (1995) Growth and behavioral effects of early postnatal chromium and manganese exposure in herring gull (Larus argentatus) chicks. Pharmacology, Biochemistry and Behavior, 50(4):607612.
Burger J, Gochfeld M (1999) Heavy metals in Franklin's gull tissues: Age and tissue differences. Environmental Toxicology and Chemistry, 18(4):673678.
Burger J, Gochfeld M (2000a) Metal levels in feathers of 12 species of seabirds from Midway Atoll in the northern Pacific Ocean. Science of the Total Environment, 257(1):3752.
Burger J, Gochfeld M (2000b) Metals in Laysan albatrosses from Midway Atoll. Archives of Environmental Contamination and Toxicology, 38(2):254259.
Burger J, Woolfenden GE, Gochfeld M (1999) Metal concentrations in the eggs of endangered Florida scrub-jays from central Florida. Archives of Environmental Contamination and Toxicology, 37(3):385388.
Burger J, Gochfeld M, Rooney AA, Orlando EF, Woodward AR, Guillette LJ (2000) Metals and metalloids in tissues of American alligators in three Florida lakes. Archives of Environmental Contamination and Toxicology, 38(4):501508.
Burnell JN (1988) The biochemistry of manganese in plants. In: Graham RD, Hannam RJ, Uren NC, eds. Manganese in soils and plants. Dordrecht, Kluwer Publishers, pp. 125137.
Byl TD, Sutton HD, Klaine SJ (1994) Evaluation of peroxidase as a biochemical indicator of toxic chemical exposure in the aquatic plant Hydrilla verticillata, Royle. Environmental Toxicology and Chemistry, 13(3):509515.
Calabrese A, Collier RS, Nelson DA, MacInnes JR (1973) The toxicity of heavy metals to embryos of the American oyster Crassostrea virginica. Marine Biology, 18:162166.
Calumpang SMF, Medina MJB, Roxas NP (1993) Movement and degradation of mancozeb fungicide and its metabolites, ethylenethiourea and ethyleneurea, in silty clay loam soils. International Journal of Pest Management, 39:161166.
Canterford GS, Canterford DR (1980) Toxicity of heavy metals to the marine diatom Ditylum brightwellii (West) Grunow: correlation between toxicity and metal speciation. Journal of the Marine Biological Association of the U.K., 60:227242.
Capelli R, Minganti V, Bernhard M (1987) Total mercury, organic mercury, copper, manganese, selenium and zinc in Sarda from the Gulf of Genoa. Science of the Total Environment, 63:8399.
Chapman PM, Fairbrother A, Brown D (1998) A critical evaluation of safety (uncertainty) factors for ecological risk assessment. Environmental Toxicology and Chemistry, 17:99108.
Cherry DS, Currie RJ, Soucek DJ, Latimer HA, Trent GC (2001) An integrative assessment of a watershed impacted by abandoned mined land discharges. Environmental Pollution, 111(3):377388.
Chin CS, Johnson KS, Coale KH (1992) Spectrophotometric determination of dissolved manganese in natural waters with 1-(2-pyridylazo)-2-naphthol: application to analysis in situ in hydrothermal plumes. Marine Chemistry, 37(12):6582.
Christensen ER, Scherfig J, Dixon PS (1979) Effects of manganese, copper and lead on Selenastrum capricornutum and Chlorella stigmatophora. Water Research, 13(1):7992.
Clarkson DT, Hanson JB (1980) The mineral nutrition of higher plants. Annual Review of Plant Physiology, 31:239298.
Connell DW, Wong BSF, Lam PKS, Poon KF, Lam MHW, Wu RSS, Richardson BJ, Yen YF (2002) Risk to breeding success of Ardeids by contaminants in Hong Kong: Evidence from trace metals in feathers. Ecotoxicology, 11(1):4959.
Cooper WC (1984) The health implications of increased manganese in the environment resulting from the combustion of fuel additives: a review of the literature. Journal of Toxicology and Environmental Health, 14:2346.
Couillard Y, Ross P, Pinel-Alloul B (1989) Acute toxicity of six metals to the rotifer Brachionus calyciflorus, with comparisons to other freshwater organisms. Toxicity Assessment, 4(4):451462.
Csatorday K, Gombos Z, Szalontai B (1984) Mn2+ and Co2+ toxicity in chlorophyll biosynthesis. Cell Biology, 81:476478.
Curtin D, Ryan J, Chaudhary RA (1980) Manganese adsorption and desorption in calcareous Lebanese soils. Journal of the Soil Science Society of America, 44:947950.
Davies PH, Brinkman SF (1994) Acute and chronic toxicity of manganese to exposed and unexposed rainbow and brown trout. Fort Collins, CO, Colorado Division of Wildlife (Federal Aid Project #F-243R-1) [cited in Reimer, 1999].
Davies PH, Brinkman SF (1995) Acute and chronic toxicity of manganese to brown trout (Salmo trutta) in hard water. Fort Collins, CO, Colorado Division of Wildlife (Federal Aid Project #F-243R-2) [cited in Reimer, 1999].
Davis DW, Hsiao K, Ingels R, Shiklya J (1988) Origins of manganese in air particulates in California. Journal of the Air Pollution Control Association, 38(9):11521157.
Davis JM, Jarabek AM, Mage DT, Graham JA (1998) The EPA health risk assessment of methylcyclopentadienyl manganese tricarbonyl (MMT). Risk Analysis, 18(1):5770.
Denduluri S (1994) Reduction of manganese accumulation by ethylenediamine tetraacetic acid and nitrilo triacetic acid in okra (Abelmoschus esculentus L.) grown in sewage-irrigated soil. Bulletin of Environmental Contamination and Toxicology, 52(3):438443.
Denney S, Sherwood J, Leyden J (1999) In situ measurements of labile Cu, Cd and Mn in river waters using DGT. Science of the Total Environment, 239(13):7180.
Deverel SJ, Millard SP (1988) Distribution and mobility of selenium and other trace elements in shallow groundwater of the western San Joaquin Valley, CA. Environmental Science & Technology, 22:697702.
Diaz-Lopez G, Mancha R (1994) Usefulness of testing with Eisenia fetida for the evaluation of agrochemicals in soils. In: Donker MH, Eijsackers H, Heimbach F, eds. Ecotoxicology of soil organisms. Boca Raton, FL, Lewis Publishers, pp. 251256.
Donisa C, Mocanu R, Steinnes E, Vasu A (2000) Heavy metal pollution by atmospheric transport in natural soils from the northern part of Eastern Carpathians. Water, Air and Soil Pollution, 120(34):347358.
Doyle CJ, Pablo F, Lim RP, Hyne RV (2003) Assessment of metal toxicity in sediment pore water from Lake Macquarie, Australia. Archives of Environmental Contamination and Toxicology, 44:343350.
Eaton A (1979) The impact of anoxia on Mn fluxes in the Chesapeake Bay. Geochimica et Cosmochimica Acta, 43:429432.
Eckel WP, Langley WD (1988) A background-based ranking technique for assessment of elemental enrichment in soils at hazardous waste sites. In: Superfund '88: Proceedings of the 9th National Conference, Washington, DC. Silver Spring, MD, Hazardous Materials Control Research Institute, pp. 282286.
Egyed M, Wood GC (1996) Risk assessment for combustion products of the gasoline additive MMT in Canada. Science of the Total Environment, 189190:1120.
Eisler R (1977) Acute toxicities of selected heavy metals to the softshell clam, Mya arenaria. Bulletin of Environmental Contamination and Toxicology, 17(2):137144.
EM (1993) The economics of manganese, 7th ed. London, Roskill Information Services, Ltd.
Eriksson SP (2000a) Temporal variations of manganese in the haemolymph and tissues of the Norway lobster, Nephrops norvegicus (L.). Aquatic Toxicology, 48(23):297307.
Eriksson SP (2000b) Variations of manganese in the eggs of the Norway lobster, Nephrops norvegicus (L.). Aquatic Toxicology, 48(23):291295.
Eriksson SP, Baden SP (1998) Manganese in the haemolymph and tissues of the Norway lobster, Nephrops norvegicus, along the Swedish west-coast, 199395. Hydrobiologia, 375376:255295.
Espinosa AJF, Ternero-Rodriguez M, Barragan de la Rosa FJ, Jimenez-Sanchez JC (2001) Size distribution of metals in urban aerosols in Seville (Spain). Atmospheric Environment, 35(14):25952601.
Evans LJ (1989) Chemistry of metal retention by soils: Several processes are explained. Environmental Science & Technology, 23:10481056.
Fabbri D, Gabbianelli G, Locatelli C, Lubrano D, Trombini C, Vassura I (2001) Distribution of mercury and other heavy metals in core sediments of the northern Adriatic Sea. Water, Air and Soil Pollution, 129(14):143153.
Fargaová A, Bumbalova A, Havranek E (1999) Ecotoxicological effects and uptake of metals (Cu+, Cu2+, Mn2+, Mo6+, Ni2+, V5+) in freshwater alga Scenedesmus quadricauda. Chemosphere, 38(5):11651173.
Fergusson JE, Stewart C (1992) The transport of airborne trace elements copper, lead, cadmium, zinc and manganese from a city into rural areas. Science of the Total Environment, 121:247269.
Ferraz HB, Bertolucci PHF, Pereira JS, Lima JGC, Andrade LAF (1988) Chronic exposure to the fungicide maneb may produce symptoms and signs of CNS manganese intoxication. Neurology, 38(4):550553.
Filipek LH, Nordstrom DK, Ficklin WH (1987) Interaction of acid mine drainage with waters and sediments of West Squaw Creek in the West Shasta Mining District, California. Environmental Science & Technology, 21:388396.
Fisher NS, Jones GJ (1981) Heavy metals and marine phytoplankton: Correlation of toxicity and sulfhydryl-binding. Journal of Phycology, 17:108111.
Florence TM, Stauber JL, Ahsanullah M (1994) Toxicity of nickel ores to marine organisms. Science of the Total Environment, 148:139155.
Folsom TR, Young DR, Johnson JN, Pillai KC (1963) Manganese-54 and zinc-65 in coastal organisms of California. Nature, 200:327329.
Foy CD, Chaney RL, White MC (1978) The physiology of metal toxicity in plants. Annual Review of Plant Physiology, 29:511566.
Foy CD, Scott BJ, Fisher JA (1988) Genetic differences in plant tolerance to manganese toxicity. In: Graham RD, Hannam RJ, Uren NC, eds. Manganese in soils and plants. Dordrecht, Kluwer Publishers, pp. 293307.
Francis AJ (1985) Anaerobic microbial dissolution of toxic metals in subsurface environments. Upton, NY, Brookhaven National Laboratory (Report No. BNL-36571).
Francis CW, White GH (1987) Leaching of toxic metals from incinerator ashes. Journal of the Water Pollution Control Federation, 59:979986.
Fujimoto C, Sherman GD (1948) Behavior of manganese in the soil and the manganese cycle. Soil Science, 66:131145.
Gajbhiye SN, Hirota R (1990) Toxicity of heavy metals to brine shrimp Artemia. Journal of the Indian Fisheries Association, 20:4350.
Galloway JN, Thornton JD, Norton SA, Volchock HL, McLean RAN (1982) Trace metals in atmospheric deposition: A review and assessment. Atmospheric Environment, 16(7):16771700.
Gamo T, Chiba H, Yamanaka T, Okudaira T, Hashimoto J, Tsuchida S, Ishibashi J, Kataoika S, Tsunogai U, Okamura K, Sano Y, Shinjyo R (2001) Chemical characteristics of newly discovered black smoker fluids and associated hydrothermal plumes at the Rodrigues Triple Junction, Central Indian Ridge. Earth & Planetary Science Letters, 193:371379.
Garg VK, Garg SK, Tyagi SK (1989a) Hematological parameters in fish Channa punctatus under the stress of manganese. Environment & Ecology, 7(3):752755.
Garg VK, Garg SK, Tyagi SK (1989b) Manganese induced haematological and biochemical anomalies in Heteropneustes fossilis. Journal of Environmental Biology, 10(4):349353.
Garrison AW, Cipollone MG, Wolfe NL, Swank RR (1995) Environmental fate of methylcyclopentadienyl manganese tricarbonyl. Environmental Toxicology and Chemistry, 14(11):18591864.
Gauthreaux K, Hardaway C, Falgoust T, Noble CO, Sneddon J, Beck MJ, Beck JN (2001) Manganese species migration in soil at the Sabine National Wildlife Refuge, Louisiana. Journal of Environmental Monitoring, 3(5):487492.
Geering HR, Hodgson JF, Sdano C (1969) Micronutrient cation complexes in soil solution. IV. The chemical state of manganese in soil solution. Soil Science Society of America Proceedings, 33:8185.
González A, Lynch J (1999) Tolerance of tropical common bean genotypes to manganese toxicity: performance under different growing conditions. Journal of Plant Nutrition, 22(3):511525.
Graedel TE (1978) Inorganic elements, hydrides, oxides, and carbonates. In: Chemical compounds in the atmosphere. New York, NY, Academic Press, pp. 3541, 4449.
Greichus YA, Greichus A, Amman BA, Call DJ, Hamman DCD, Potts RM (1977) Insecticides, polychlorinated biphenyls and metals in African lake ecosystems. I: Hartbeenspoort Dam, Transvaal and Voëlvlei Dam, Cape Province, Republic of South Africa. Archives of Environmental Contamination and Toxicology, 6:371383.
Greichus YA, Greichus A, Amman BD, Hopcraft J (1978) Insecticides, polychlorinated biphenyls and metals in African lake ecosystems. III. Lake McIlwaine, Rhodesia. Bulletin of Environmental Contamination and Toxicology, 19:454461.
Grill EV (1978) The effect of sedimentwater exchange on manganese deposition and nodule growth in Jervis Inlet, British Columbia. Geochimica et Cosmochimica Acta, 42:485494.
Hall IR, Hydes DJ, Statham PJ, Overnell J (1996) Dissolved and particulate trace metals in a Scottish sea loch: an example of a pristine environment? Marine Pollution Bulletin, 32:846854.
Halpin KM, Baker DH (1986) Manganese utilization in the chick: effects of corn, soybean meal, fish meal, wheat bran, and rice bran on tissue uptake of manganese. Poultry Science, 65(5):9951003.
Hamilton K (1995) The Australian directory of registered pesticides. Brisbane, University of Queensland, Centre for Pesticide Application and Safety.
Hannam RJ, Ohki K (1988) Detection of manganese deficiency and toxicity in plants. In: Graham RD, Hannam RJ, Uren NC, eds. Manganese in soils and plants. Dordrecht, Kluwer Publishers, pp. 243259.
Hansen SN, Bjerregaard P (1995) Manganese kinetics in the sea star Asterias rubens (L.) exposed via food or water. Marine Pollution Bulletin, 31(13):127132.
Hart BA, Currey NA, Jones MJ (1992) Biogeochemistry and effects of copper, manganese and zinc added to enclosures in Island Billabong, Magela Creek, northern Australia. Hydrobiologia, 230:98134.
Hartenstein R, Neuhauser EF, Narahara A (1981) Effects of heavy metal and other elemental additives to activated sludge on growth of Eisenia foetida. Journal of Environmental Quality, 10(3):372376.
Hartwell SI, Alden RW, Wright DA, Ailstock S, Kerhin R (2000) Correlation of measures of ambient toxicity and fish community diversity in a Chesapeake Bay tributary, Maryland, USA: A biological, chemical, and geological assessment. Environmental Toxicology and Chemistry, 19(7):17531763.
Hauck M, Jung R, Runge M (2001) Relevance of element content of bark for the distribution of epiphytic lichens in a montane spruce forest affected by forest dieback. Environmental Pollution, 112:221227.
Hauck M, Mulack C, Paul A (2002a) Manganese uptake in the epiphytic lichens Hypogymnia physodes and Lecanora conizaeoides. Environmental and Experimental Botany, 48(2):107117.
Hauck M, Paul A, Mulack C, Fritz E, Runge M (2002b) Effects of manganese on the viability of vegetative diaspores of the epiphytic lichen Hypogymnia physodes. Environmental and Experimental Botany, 47:127142.
Heal KV (2001) Manganese and land-use in upland catchments in Scotland. Science of the Total Environment, 265(13):169179.
Heal KV, Kneale PE, McDonald AT (2002) Manganese in runoff from upland catchments: temporal patterns and controls on mobilization. Hydrological Sciences Journal, 47(5):769780.
Health Canada (1994) Canadian Environmental Protection Act. Human health risk assessment for priority substances. Ottawa, Ontario, Minister of Supply and Services Canada.
Heiny JS, Tate CM (1997) Concentration, distribution, and comparison of selected trace elements in bed sediment and fish tissue in the South Platte River Basin, USA, 19921993. Archives of Environmental Contamination and Toxicology, 32:246259.
Hemstock GA, Low PF (1953) Mechanisms responsible for retention of manganese in the colloidal fraction of soil. Soil Science, 76:331343.
Hendricks AJ, Ma WC, Brouns JJ, de Ruiter-Dijkman EM, Gast R (1995) Modelling and monitoring organochlorine and heavy metal accumulation in soils, earthworms, and shrews in Rhine Delta floodplains. Archives of Environmental Contamination and Toxicology, 29:115127.
Herzl V, Roevros N (1998) Kinetic study of manganese behavior in the Scheldt estuary. Journal of Radioanalytical and Nuclear Chemistry, 235(12):261265.
Hirst H, Juttner I, Ormerod SJ (2002) Comparing the responses of diatoms and macroinvertebrates to metals in upland streams of Wales and Cornwall. Freshwater Biology, 47(9):17521765.
Horst WJ (1988) The physiology of manganese toxicity. In: Graham RD, Hannam RJ, Uren NC, eds. Manganese in soils and plants. Dordrecht, Kluwer Publishers, pp. 175188.
Horst WJ, Marschner H (1978) Effect of silicon on manganese tolerance of bean plants (Phaseolus vulgaris L.). Plant and Soil, 50:287303.
Horst WJ, Maier P, Fecht M, Naumann A, Wissemeier AH (1999) The physiology of manganese toxicity and tolerance in Vigna unguiculata (L.) Walp. Journal of Plant Nutrition and Soil Science, 162:263274.
Hothem RL, Roster DL, King KA, Keldsen TJ, Marois KC, Wainwright SE (1995) Spatial and temporal trends of contaminants in eggs of wading birds from San Francisco Bay, California. Environmental Toxicology and Chemistry, 14:13191331.
HSDB (1998) Hazardous substances data bank. Bethesda, MD, National Institutes of Health, National Library of Medicine.
Hue NV, Mai Y (2002) Manganese toxicity in watermelon as affected by lime and compost amended to a Hawaiian acid oxisol. Hortscience, 37(4):656661.
Hui CA, Takekawa JY, Baranyuk VV, Litvin KV (1998) Trace element concentrations in two subpopulations of lesser snow geese from Wrangel Island, Russia. Archives of Environmental Contamination and Toxicology, 34(2):197203.
Hunt CD (1983) Incorporation and deposition of Mn and other trace metals by flocculent organic matter in a controlled marine ecosystem. Limnology and Oceanography, 28:302308.
Hunt CD, Kelly JR (1988) Manganese cycling in coastal regions: Response to eutrophication. Estuarine, Coastal and Shelf Science, 26:527558.
Huntsman SA, Sunda WG (1980) The role of trace metals in regulating phytoplankton. In: Morris I, ed. The physiological ecology of phytoplankton. Berkeley, CA, University of California, pp. 285328.
Ichikawa R (1961) On the concentration factors of some important radionuclides in marine food organisms. Bulletin of the Japanese Society of Scientific Fisheries, 27:6674.
IPCS (1981) Manganese. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 17).
IPCS (1999a) Manganese and its compounds. Geneva, World Health Organization, International Programme on Chemical Safety (Concise International Chemical Assessment Document 12).
IPCS (1999b) Manganese sulphate monohydrate. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 0290).
IPCS (1999c) Manganese, cyclopentadienyltricarbonyl. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 0977).
IPCS (2001) Manganese oxide. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 1398).
IPCS (2003a) Manganese. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 0174).
IPCS (2003b) Manganese dioxide. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 0175).
IPCS (2003c) Methylcyclopentadienyl manganese tricarbonyl. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 1169).
IPCS (2003d) Mancozeb. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 0754).
Ireland MP (1979) Distribution of essential and toxic metals in the terrestrial gastropod Arion ater. Environmental Pollution, 20:271278.
Janssens E, Dauwe T, Bervoets L, Eens M (2001) Heavy metals and selenium in feathers of great tits (Parus major) along a pollution gradient. Environmental Toxicology and Chemistry, 20(12):28152820.
Jaques AP (1984) National inventory of sources and emissions of manganese 1984. Ottawa, Ontario, Environment Canada, Conservation and Protection, Environmental Protection Service.
Jaudon P, Massiani C, Galea J, Rey J, Vacelet E (1989) Groundwater pollution by manganese manganese speciation application to the selection and discussion of an in situ groundwater treatment. Science of the Total Environment, 84:169183.
Johnson D, Chiswell B, Ohalloran K (1995) Microorganisms and manganese cycling in a seasonally stratified fresh-water dam. Water Research, 29(12):27392745.
Jung HS, Lee CB, Cho YG, Kang JK (1996) A mechanism for the enrichment of Cu and depletion of Mn in anoxic marine sediments, Banweol intertidal flat, Korea. Marine Pollution Bulletin, 32:782787.
Kabata-Pendias A, Pendias H (1984) Trace elements in soils and plants. Boca Raton, FL, CRC Press.
Kaiser J (2003) Manganese: A high-octane dispute. Science, 300:926928.
Kartal S, Dogan M, Rojas CM, Van Grieken RE (1993) Composition and sources of atmospheric particulate matter at Kayseri, central Turkey. Science of the Total Environment, 133:8397.
Kaus A, Wild A (1998) Nutrient disturbance through manganese accumulation in Douglas fir. Chemosphere, 36(45):961964.
Kavun VY, Shulkin VM, Khristoforova NK (2002) Metal accumulation in mussels of the Kuril Islands, north-west Pacific Ocean. Marine Environmental Research, 53(3):219226.
Kellar KE, Foster N (1991) Determination of the relative amounts of free and complexed manganese ions in aqueous solution by nuclear magnetic resonance. Analytical Chemistry, 63(24):29192924.
Khangarot BS (1991) Toxicity of metals to a freshwater tubificid worm, Tubifex tubifex (Muller). Bulletin of Environmental Contamination and Toxicology, 46:906912.
Khangarot BS, Ray PK (1989) Investigation of correlation between physicochemical properties of metals and their toxicity to the water flea Daphnia magna Straus. Ecotoxicology and Environmental Safety, 18(2):109120.
King HM, Baldwin DS, Rees GN, McDonald S (1999) Apparent bioaccumulation of Mn derived from paper-mill effluent by the freshwater crayfish Cherax destructor The role of Mn oxidising bacteria. Science of the Total Environment, 226(23):261267.
Kirk RE, Othmer F, eds. (2001) Kirk-Othmer encyclopedia of chemical technology, 4th ed. Vol. 15. New York, NY, John Wiley & Sons, p. 996.
Kitao M, Lei TT, Koike T (1997) Comparison of photosynthetic responses to manganese toxicity of deciduous broad leaved trees in northern Japan. Environmental Pollution, 97(12):113118.
Kitao M, Lei TT, Nakamura T, Koike T (2001) Manganese toxicity as indicated by visible foliar symptoms of Japanese white birch (Betula platyphylla var. japonica). Environmental Pollution, 111(1):8994.
Kleinman MT, Pasternack BS, Eisenbud M, Kneip T (1980) Identifying and estimating the relative importance of airborne particulates. Environmental Science & Technology, 14:6265.
Knox D, Cowey CB, Adron JW (1981) The effect of low dietary manganese intake on rainbow-trout (Salmo gairdneri). British Journal of Nutrition, 46(3):495501.
Korcak RF (1988) Response of blueberry species to excessive manganese. Journal of the American Society for Horticultural Science, 113(2):189193.
Kostka JE, Luther GW, Nealson KH (1995) Chemical and biological reduction of Mn(III)pyrophosphate complexes potential importance of dissolved Mn(III) as an environmental oxidant. Geochimica et Cosmochimica Acta, 59(5):885894.
Kremling K (1983) The behaviour of Zn, Cd, Cu, Ni, Co, Fe, and Mn in anoxic Baltic waters. Marine Chemistry, 13:87108.
Kucera J, Soukal L, Faltejsek J (1986) Low level determination of manganese in biological reference materials by neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry, 107(6):361369.
Kwasnik GM, Vetter RJ, Atchison GJ (1978) The uptake of manganese-54 by green algae (Protococcoidal chlorella), Daphnia magna, and fathead minnows (Pimephales promelas). Hydrobiologia, 59:181185.
Lasier PJ, Winger PV, Bogenrieder KJ (2000) Toxicity of manganese to Ceriodaphnia dubia and Hyalella azteca. Archives of Environmental Contamination and Toxicology, 38(3):298304.
Lavi N, Lux F, Al-Fassi ZB (1989) Determination of magnesium, aluminum, phosphorus, copper and manganese in biological fluids by neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry, 129(1):93102.
Laxen DPH, Davison W, Woof C (1984) Manganese chemistry in rivers and streams. Geochimica et Cosmochimica Acta, 48:21072111.
LaZerte BD, Burling K (1990) Manganese speciation in dilute waters of the Precambrian Shield, Canada. Water Research, 24:10971101.
Lee RE, Duffield FV (1979) Sources of environmentally important metals in the atmosphere. Advances in Chemistry Series, 172:146171.
Leivuori M (1998) Heavy metal contamination in surface sediments in the Gulf of Finland and comparison with the Gulf of Bothnia. Chemosphere, 36(1):4359.
Leivuori M, Vallius H (1998) A case study of seasonal variation in the chemical composition of accumulating suspended sediments in the central Gulf of Finland. Chemosphere, 36(10):24172435.
Lewis BL, Landing WM (1991) The biogeochemistry of Mn and Fe in the Black Sea. Deep-Sea Research, 38(Suppl. 2):773803.
Lewis BL, Landing WM (1992) The investigation of dissolved and suspended particulate trace metal fractionation in the Black Sea. Marine Chemistry, 40(12):105141.
Lewis M (1976) Effects of low concentrations of manganous sulfate on eggs and fry of rainbow trout. Progressive Fish-Culturist, 38(2):6365.
L'Her Roux L, Le Roux S, Appriou P (1998) Behaviour and speciation of metallic species Cu, Cd, Mn and Fe during estuarine mixing. Marine Pollution Bulletin, 36(1):5664.
Lide DR, ed. (1993) CRC handbook of chemistry and physics. Boca Raton, FL, CRC Press.
Lidon FC (2002) Rice plant structural changes by addition of excess manganese. Journal of Plant Nutrition, 25(2):287296.
Lioy PJ (1983) Air pollution emission profiles of toxic and trace elements from energy related sources: status and needs. Neurotoxicology, 4(3):103112.
Llobet JM, Schuhmacher M, Domingo JL (2002) Spatial distribution and temporal variation of metals in the vicinity of a municipal solid waste incinerator after a modernization of the flue gas cleaning systems of the facility. Science of the Total Environment, 284(13):205214.
Loneragan JF (1988) Distribution and movement of manganese in plants. In: Graham RD, Hannam RJ, Uren NC, eds. Manganese in soils and plants. Dordrecht, Kluwer Publishers, pp. 113124.
Loranger S, Zayed J (1994) Manganese and lead concentrations in ambient air and emission rates from unleaded and leaded gasoline between 1981 and 1992 in Canada: a comparative study. Atmospheric Environment, 28(9):16451651.
Loranger S, Zayed J (1997) Environmental contamination and human exposure to airborne total and respirable manganese in Montreal. Air and Waste Management, 47:983989.
Lynn DC, Bonatti E (1965) Mobility of manganese in diagenesis of deep-sea sediments. Marine Geology, 3:457474.
Macdonald JM, Shields JD, Zimmer-Faust RK (1988) Acute toxicities of eleven metals to early life-history stages of the yellow crab Cancer anthonyi. Marine Biology, 98(2):201207.
MAK (1994) Manganese and its inorganic compounds. Deutsche Forschungsgemeinschaft (DFG), Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK). Weinheim, VCH.
Malm O, Pfeiffer WC, Fiszman M, Azcue JM (1988) Transport and availability of heavy metals in the Paraiba do Sul-Guandu River system, Rio de Janeiro State, Brazil. Science of the Total Environment, 75:201209.
Maltby L, Crane M (1994) Responses of Gammarus pulex (Amphipoda, Crustacea) to metalliferous effluents: Identification of toxic components and the importance of interpopulation variation. Environmental Pollution, 84(1):4552.
Martin JL (1976) Accumulation de Fe, Cu, Zn, Mg, Mn, et Co dans l'ovaire de Carcinus maenus L. au cours de l'ovogenèse. Comptes Rendus des Séances de la Société de Biologie et de ses Filiales, 170:157162.
Martin TR, Holdich DM (1986) The acute lethal toxicity of heavy metals to peracarid crustaceans (with particular reference to fresh-water Asellids and Gammarids). Water Research, 20(9):11371147.
Mason CF, Stephenson A (2001) Metals in tissues of European otters (Lutra lutra) from Denmark, Great Britain and Ireland. Chemosphere, 44(3):351353.
McBride MB (1979) Chemisorption and precipitation of Mn2+ at CaCO3 surfaces. Journal of the Soil Science Society of America, 43:693698.
McLachlan J (1973) Growth media marine. In: Stein JR, ed. Handbook of phycological methods, culture methods and growth measurements. Cambridge, Cambridge University Press, pp. 2551.
Meneses M, Llobet JM, Granero S, Schuhmacher M, Domingo JL (1999) Monitoring metals in the vicinity of a municipal waste incinerator: Temporal variation in soils and vegetation. Science of the Total Environment, 226(23):157164.
Menta C, Parisi V (2001) Metal concentrations in Helix pomatia, Helix aspersa and Arion rufus: A comparative study. Environmental Pollution, 115(2):205208.
Mielke HW, Gonzales CR, Powell E, Shah A, Mielke PW (2002) Natural and anthropogenic processes that concentrate Mn in rural and urban environments of the Lower Mississippi River Delta. Environmental Research, 90(2):157168.
Miller DW, Vetter RJ, Atchinson GJ (1980) Effect of temperature and dissolved oxygen on uptake and retention of 54Mn in fish. Health Physics, 38(2):221225.
Moreau G, Barbeau C, Frenette JJ, Saintonge J, Simoneau M (1983) Zinc, manganese, and strontium in opercula and scales of brook trout (Salvelinus fontinalis) as indicators of lake acidification. Canadian Journal of Fisheries and Aquatic Sciences, 40(10):16851691.
Morgan JD, Mitchell DG, Chapman PM (1986) Individual and combined toxicity of manganese and molybdenum to mussel, Mytilus edulis, larvae. Bulletin of Environmental Contamination and Toxicology, 37(2):303307.
Mori I, Fujita Y, Ikuta K, Nakahashi Y, Kato K (1989) Highly sensitive and selective fluorimetric methods for the determination of iron (III) and manganese (II) using fluoresceinhydrogen peroxidetriethylenetetramine and fluoresceinhydrogen peroxidetriethylenetetramine/iron, respectively. Fresenius Journal of Analytical Chemistry, 334(3):252255.
Nalecz-Jawecki G, Sawicki J (1998) Toxicity of inorganic compounds in the spirotox test: A miniaturized version of the Spirostomum ambiguum test. Archives of Environmental Contamination and Toxicology, 34(1):15.
NAS (1973) Medical and biological effects of environmental pollutants: manganese. Washington, DC, National Academy of Sciences, National Academy Press.
Nash RG, Beall ML (1980) Fate of maneb and zineb fungicides in microagroecosystem chambers. Journal of Agricultural and Food Chemistry, 28:322330.
Nath K, Kumar N (1988) Impact of manganese intoxication on certain parameters of carbohydrate metabolism of a freshwater tropical perch, Colisa fasciatus. Chemosphere, 17(3):617624.
Neal C, Smith CJ, Walls J, Dunn S (1986) Major, minor and trace element mobility in the acidic upland forested catchment of the upper River Severn, mid-Wales. Journal of the Geological Society, London, 143:635648.
Neal C, Robson AJ, Wass P, Wade AJ, Ryland GP, Leach DV, Leeks GJL (1998) Major, minor, trace element and suspended sediment variations in the River Derwent. Science of the Total Environment, 210211:163172.
Neal C, Jarvie HP, Whitton BA, Gemmell J (2000) The water quality of the River Wear, north-east England. Science of the Total Environment, 251252:153172.
Nealson KH (1983) The microbial manganese cycle. In: Krumbein WE, ed. Microbial geochemistry. Oxford, Blackwell, pp. 191221.
Nealson KH, Myers CR, Wimpee BB (1991) Isolation and identification of manganese-reducing bacteria and estimates of microbial Mn(IV)-reducing potential in the Black Sea. Deep-Sea Research Part A Oceanographic Research Papers, 38:S907S920.
NICNAS (2003) Methylcyclopentadienyl manganese tricarbonyl (MMT). Sydney, National Industrial Chemicals Notification and Assessment Scheme, 150 pp. (Priority Existing Chemical Assessment Report No. 24).
Nix J, Ingols R (1981) Oxidized manganese from hypolimnetic water as a possible cause of trout mortality in hatcheries. Progressive Fish-Culturist, 43(1):3236.
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature, 333:134139.
NTP (1999) NTP chemical health and safety data. Research Triangle Park, NC, US Department of Health and Human Services, National Toxicology Program.
Nyberg P, Andersson P, Degerman E, Borg H, Olofsson E (1995) Labile inorganic manganese An overlooked reason for fish mortality in acidified streams? Water, Air and Soil Pollution, 85(2):333340.
OECD (1992) Report of the OECD workshop on extrapolation of laboratory aquatic toxicity data to the real environment. Paris, Organisation for Economic Co-operation and Development (OECD Environment Monograph No. 59).
OECD (1995) Guidance document for aquatic effects assessment. Paris, Organisation for Economic Co-operation and Development (OECD Environment Monograph No. 92).
Palladini G, Margotta V, Carolei A, Hernandez MC (1980) Dopamine agonist performance in Planaria after manganese treatment. Experientia, 36:449450.
Pandey PK, Patel KS, Subrt P (1998) Trace elemental composition of atmospheric particulate at Bhilai in central-east India. Science of the Total Environment, 215(12):123134.
Parker MB, Walker ME (1986) Soil pH and manganese effects on manganese nutrition of peanut. Agronomy Journal, 78:614620.
Patrick R, Crum B, Coles J (1969) Temperature and manganese as determining factors in the presence of diatom or blue-green algal floras in streams. Proceedings of the National Academy of Sciences of the United States of America, 64(2):472478.
Pentreath RJ (1973) The accumulation from water of 65Zn, 54Mn, 58Co, and 59Fe by the mussel, Mytilus edulis. Journal of the Marine Biological Association of the U.K., 53:127143.
Pohl C, Hennings U, Petersohn I, Siegel H (1998) Trace metal budget, transport, modification and sink in the transition area between the Oder and Peene rivers and the southern Pomeranian Bight. Marine Pollution Bulletin, 36(8):598616.
Posta K, Marschner H, Romheld V (1994) Manganese reduction in the rhizosphere of mycorrhizal and nonmycorrhizal maize. Mycorrhiza, 5(2):119124.
Rai UN, Chandra P (1992) Accumulation of copper, lead, manganese and iron by field populations of Hydrodictyon reticulatum (Linn.) Lagerheim. Science of the Total Environment, 116(3):203211.
Rainbow PS, Blackmore G (2001) Barnacles as biomonitors of trace metal availabilities in Hong Kong coastal waters: changes in space and time. Marine Environmental Research, 51(5):441463.
Rao SVR, Nath KJ (1983) Biological effect of some poisons on Canthocamptus (Crustacea spp.). International Journal of Environmental Studies, 21(34):271275.
Rathore RS, Khangarot BS (2002) Effects of temperature on the sensitivity of sludge worm Tubifex tubifex Müller to selected heavy metals. Ecotoxicology and Environmental Safety, 53(1):2736.
Rautio P, Huttunen S, Lamppu J (1998) Effects of sulphur and heavy metal deposition on foliar chemistry of Scots pines in Finnish Lapland and on the Kola peninsula. Chemosphere, 36(45):979984.
Reader JP, Everall NC, Sayer MDJ, Morris R (1989) The effects of eight trace metals in acid soft water on survival, mineral uptake and skeletal calcium deposition in yolk-sac fry of brown trout, Salmo trutta L. Journal of Fish Biology, 35:187198.
Reimer PS (1999) Environmental effects of manganese and proposed freshwater guidelines to protect aquatic life in British Columbia [MSc thesis]. Vancouver, B.C., University of British Columbia.
Reinecke SA, Reinecke AJ (1997) The influence of lead and manganese on spermatozoa of Eisenia fetida (Oligochaeta). Soil Biology and Biochemistry, 29(34):737742.
Reisenauer HM (1988) Determination of plant-available soil manganese. In: Graham RD, Hannam RJ, Uren NC, eds. Manganese in soils and plants. Dordrecht, Kluwer Publishers, pp. 8798.
Remoudaki E, Bergametti G, Losno R (1991) On the dynamic of the atmospheric input of copper and manganese into the western Mediterranean Sea. Atmospheric Environment, 25A(34):733744.
Resing JA, Mottl MJ (1992) Determination of manganese in seawater using flow injection analysis with on-line preconcentration and spectrophotometric detection. Analytical Chemistry, 64(22):26822687.
Rhodes RC (1977) Studies with manganese [14C]ethylenebis(dithiocarbamate) ([14C]maneb) fungicide and [14C]ethylenethiourea ([14C]ETU) in plants, soil, and water. Journal of Agricultural and Food Chemistry, 25:528533.
Robinson DB, Hodgson WA (1961) The effect of some amino acids on manganese toxicity in potato. Canadian Journal of Plant Science, 41:436437.
Rogalla H, Römheld V (2002) Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell and Environment, 25(4):549555.
Rope SK, Arthur WJ, Craig TH, Craig EH (1988) Nutrient and trace elements in soil and desert vegetation of southern Idaho. Environmental Monitoring and Assessment, 10:124.
Rosko JJ, Rachlin JW (1975) The effect of copper, zinc, cobalt and manganese on the growth of the marine diatom Nitzschia closterium. Bulletin of the Torrey Botanical Club, 102(3):100106.
Rossini GDB, Ronco AE (1996) Acute toxicity bioassay using Daphnia obtusa as a test organism. Environmental Toxicology and Water Quality, 11(3):255258.
Rouleau C, Pelletier E, Tjälve H (1992) Bioconcentration and distribution of 54Mn2+ and effects of chelating agents in the brown trout, Salmo trutta. Canadian Technical Report of Fisheries and Aquatic Sciences, 1863:330339.
Rouleau C, Tjälve H, Gottofrey J, Pelletier E (1995) Uptake, distribution and elimination of 54Mn(II) in the brown trout (Salmo trutta). Environmental Toxicology and Chemistry, 14(3):483490.
Rouleau C, Tjälve H, Gottofrey J (1996) Effects of low pH on the uptake and distribution of 54Mn(II) in brown trout (Salmo trutta). Environmental Toxicology and Chemistry, 15(5):708710.
Rousch JM, Sommerfeld MR (1999) Effect of manganese and nickel on growth of selected algae in pH buffered medium. Water Research, 33(10):24482454.
Rout GR, Samantaray S, Das P (2001) Studies on differential manganese tolerance of mung bean and rice genotypes in hydroponic culture. Agronomie, 21(8):725733.
Ruijten MWMM, Sall HJA, Verberk MM, Smink M (1994) Effect of chronic mixed pesticide exposure on peripheral and autonomic nerve function. Archives of Environmental Health, 49(3):188195.
Saad MAH, Erzat AA, el-Rayio OA, Hafez H (1981) Occurrence and distribution of chemical pollutants in Lake Maryut, Egypt. II: Heavy metals. Water, Air and Soil Pollution, 16:401407.
Sanders MJ, Du Preez HH, Van Vuren JHJ (1998) The freshwater river crab, Potamonautes warreni, as a bioaccumulative indicator of iron and manganese pollution in two aquatic systems. Ecotoxicology and Environmental Safety, 41(2):203214.
Sarzanini C, Abollino O, Mentasti E (2001) Flow-injection preconcentration and electrothermal atomic absorption spectrometry determination of manganese in seawater. Analytica Chimica Acta, 435(2):343350.
Satoh S, Takeuchi T, Watanabe T (1987) Availability to carp of manganese in white fish-meal and of various manganese compounds. Nippon Suisan Gakkaishi, 53(5):825832.
Sax NI, Lewis RJ (1987) Hawley's condensed chemical dictionary. New York, NY, Van Nostrand Reinhold Company.
Schaanning M, Næs K, Egeberg PK, Bomé F (1988) Cycling of manganese in the permanently anoxic Drammensfjord. Marine Chemistry, 23:365382.
Schiele R (1991) Manganese. In: Merian E, ed. Metals and their compounds in the environment Occurrence, analysis and biological relevance. Weinheim, VCH, pp. 10351044.
Schnitzer M (1969) Reactions between fulvic acid, a soil humic compound and inorganic soil constituents. Soil Science Society of America Proceedings, 33:7581.
Schroeder WH, Dobson M, Kane DM (1987) Toxic trace elements associated with airborne particulate matter: a review. Journal of the Air Pollution Control Association, 37:12671285.
Scott DT, McKnight DM, Voelker BM, Hrncir DC (2002) Redox processes controlling manganese fate and transport in a mountain stream. Environmental Science & Technology, 36(3):453459.
Scudlark JR, Conko KM, Church TM (1994) Atmospheric wet deposition of trace elements to Chesapeake Bay: CBAD study year 1 results. Atmospheric Environment, 28(8):14871498.
Shacklette HT, Hamilton JC, Boerngen JG, Bowles JM (1971) Elemental composition of surficial materials in the coterminous United States. Washington, DC, US Geological Survey (Professional Paper 574-D).
Shanley JB (1986) Manganese biogeochemistry in a small Adirondack forested lake watershed. Water Resources Research, 22(12):16471656.
Sheedy BR, Lazorchak JM, Grunwald DJ, Pickering QH, Pilli A, Hall D, Webb R (1991) Effect of pollution on freshwater organisms. Research Journal of the Water Pollution Control Federation, 63:619696.
Sheppard SC, Evenden WG, Cornwell TC (1997) Depuration and uptake kinetics of I, Cs, Mn, Zn and Cd by the earthworm (Lumbricus terrestris) in radiotracer-spiked litter. Environmental Toxicology and Chemistry, 16(10):21062112.
Shriadah MMA (1999) Heavy metals in mangrove sediments of the United Arab Emirates shoreline (Arabian Gulf). Water, Air and Soil Pollution, 116(34):523534.
Sierra P, Chakrabarti S, Tounkara R, Loranger S, Kennedy G, Zayed J (1998) Bioaccumulation of manganese and its toxicity in feral pigeons (Columba livia) exposed to manganese oxide dust (Mn3O4). Environmental Research, 79(2):94101.
Sindayigaya E, Van Cauwenbergh R, Robberecht H, Deelstra H (1994) Copper, zinc, manganese, iron, lead, cadmium, mercury and arsenic in fish from Lake Tanganyika, Burundi. Science of the Total Environment, 144:103115.
Skowroński T, Pawlik B, Jakubowski M (1988) Reduction of cadmium toxicity to green microalga Stichococcus bacillaris by manganese. Bulletin of Environmental Contamination and Toxicology, 41:915920.
Smith RA, Alexander RB, Wolman MG (1987) Water-quality trends in the nation's rivers. Science, 235:16071615.
Sorvari J, Sillanpää M (1996) Influence of metal complex formation on heavy metal and free EDTA and DTPA acute toxicity determined by Daphnia magna. Chemosphere, 33(6):11191127.
Stauber JL, Florence TM (1985) Interactions of copper and manganese: A mechanism by which manganese alleviates copper toxicity to the marine diatom, Nitzschia closterium (Ehrenberg) W. Smith. Aquatic Toxicology, 7:241254.
Stein LY, La Duc MT, Grundl TJ, Nealson KH (2001) Bacterial and archeal populations associated with freshwater ferromanganous micronodules and sediments. Environmental Microbiology, 3(1):1018.
Stein LY, Jones G, Alexander B, Elmund K, Wright-Jones C, Nealson KH (2002) Intriguing microbial diversity associated with metal-rich particles from a freshwater reservoir. FEMS Microbiology Ecology, 42(3):431440.
Stokes PM, Campbell PGC, Schroeder WH, Trick C, France RL, Puckett KJ, LaZerte B, Speyer M, Hanna JE, Donaldson J (1988) Manganese in the Canadian environment. Ottawa, Ontario, National Research Council of Canada, Associate Committee on Scientific Criteria for Environmental Quality (NRCC No. 26193).
Struck BD, Pelzer R, Ostapczuk P, Emons H, Mohl C (1997) Statistical evaluation of ecosystem properties influencing the uptake of As, Cd, Co, Cu, Hg, Mn, Ni, Pb and Zn in seaweed (Fucus vesiculosus) and common mussel (Mytilus edulis). Science of the Total Environment, 207:2942.
Stubblefield WA, Brinkman SE, Davies PH, Garrison TD (1997) Effects of water hardness on the toxicity of manganese to developing brown trout (Salmo trutta). Environmental Toxicology and Chemistry, 16(10):20822089.
Sunda WG, Huntsman SA (1998a) Interactions among Cu2+, Zn2+ and Mn2+ in controlling cellular Mn, Zn and growth rate in the coastal alga Chlamydomonas. Limnology and Oceanography, 43:10551064.
Sunda WG, Huntsman SA (1998b) Control of Cd concentrations in a coastal diatom by interactions among free ionic Cd, Zn, and Mn in seawater. Environmental Science and Technology, 32:29612968.
Sunda WG, Huntsman SA (1998c) Interactive effects of external manganese, the toxic metals copper and zinc, and light in controlling cellular manganese and growth in a coastal diatom. Limnology and Oceanography, 43:14671475.
Sundby B, Anderson LG, Hall POJ, Iverfeldt Å, van der Loeff MMR, Westerlund SFG (1986) The effect of oxygen on release and uptake of cobalt, manganese, iron and phosphate at the sedimentwater interface. Geochimica et Cosmochimica Acta, 50:12811288.
Sutherland RS, Tolosa CA (2001) Variation in total and extractable elements with distance from roads in an urban watershed, Honolulu, Hawaii. Water, Air and Soil Pollution, 127(14):315338.
Sweet CW, Vermette SJ, Lansberger S (1993) Sources of toxic trace elements in urban air in Illinois. Environmental Science & Technology, 27:25022510.
Tatara CP, Newman MC, McCloskey JT, Williams PL (1997) Predicting relative metal toxicity with ion characteristics: Caenorhabditis elegans LC50. Aquatic Toxicology, 39(34):279290.
Tatara CP, Newman MC, McCloskey JT, Williams PL (1998) Use of ion characteristics to predict relative toxicity of mono-, di- and trivalent metal ions: Caenorhabditis elegans. Aquatic Toxicology, 42(4):255269.
Tebo BM (1991) Manganese(II) oxidation in the suboxic zone of the Black Sea. Deep-Sea Research Part A Oceanographic Research Papers, 38:S883S905.
Ter Haar GL, Griffing ME, Brandt M (1975) Methylcyclopentadienyl manganese tricarbonyl as an antiknock: Composition and fate of manganese exhaust products. Journal of the Air Pollution Control Association, 25:858860.
Thamdrup B, Rossello-Mora R, Amann R (2000) Microbial manganese and sulfate reduction in Black Sea shelf sediments. Applied and Environmental Microbiology, 66(7):28882897.
Thompson SE, Burton CA, Quinn DJ, Ng YC (1972) Concentration factors of chemical elements in edible aquatic organisms. Livermore, CA, University of California, Lawrence Livermore Laboratory, Bio-Medical Division.
TRI87 (1989) Toxics release inventory. Washington, DC, US Environmental Protection Agency, Office of Toxic Substances.
TRI91 (1993) Toxics release inventory. Washington, DC, US Environmental Protection Agency, Office of Toxic Substances.
TRI96 (1998) Toxics release inventory. Washington, DC, US Environmental Protection Agency, Office of Toxic Substances.
Underwood EJ (1977) Trace elements in human and animal nutrition. New York, NY, Academic Press.
Uribe E, Martens DC, Brann DE (1988) Response of corn (Zea mays L.) to manganese application on Atlantic Coastal Plain soils. Plant and Soil, 112(1):8388.
US Department of the Interior (1993) Manganese statistical compendium. Washington, DC, US Bureau of Mines.
US Department of the Interior (1995) Mineral industry surveys. Manganese. Washington, DC, US Bureau of Mines/US Geological Survey.
US Department of the Interior (1996) Manganese commodity summary (annual). Washington, DC, US Bureau of Mines/US Geological Survey.
US Department of the Interior (1998) Mineral industry surveys. Manganese. Washington, DC, US Bureau of Mines/US Geological Survey.
US EPA (1979) Sources of toxic pollutants found in influents to sewage treatment plants. VI. Integrated interpretation. Washington, DC, US Environmental Protection Agency, Office of Water Planning and Standards (Report No. EPA 440/4-008; NTIS No. PB81-219685).
US EPA (1983) Human exposure to atmospheric concentrations of selected chemicals. Vol. II. Report prepared for Office of Air Quality Planning and Standards, US Environmental Protection Agency, Research Triangle Park, NC, by Systems Applications, Inc., San Rafael, CA (NTIS No. PB83-265249).
US EPA (1984) Health assessment document for manganese. Final draft. Cincinnati, OH, US Environmental Protection Agency, Office of Research and Development (Report No. EPA/600/8-83/013F).
US EPA (1985a) Decision not to regulate manganese under the Clean Air Act. US Environmental Protection Agency. Federal Register, 50:3262732628.
US EPA (1985b) Locating and emitting air emissions from sources of manganese. Research Triangle Park, NC, US Environmental Protection Agency, Office of Air Quality Planning and Standards (Report No. EPA/450/4-84/007h).
van Dam RA, Barry MJ, Ahokas JT, Holdway DA (1999) Investigating mechanisms of diethylenetriamine pentaacetic acid toxicity to the cladoceran, Daphnia carinata. Aquatic Toxicology, 46(34):191210.
Venugopal B, Luckey TD (1978) Toxicity of group VII metals. In: Metal toxicity in mammals. 2. Chemical toxicity of metals and metalloids. New York, NY, Plenum Press, pp. 261271.
Verschueren K (1983) Handbook of environmental data on organic chemicals. New York, NY, Van Nostrand Reinhold.
Veysseyre A, van de Velde K, Ferrari C, Boutron C (1998) Searching for manganese pollution from MMT anti-knock gasoline additives in snow from central Greenland. Science of the Total Environment, 221(23):149158.
Waldron HA (1980) Metals in the environment. London, Academic Press.
Wallace L, Slonecker T (1997) Ambient air concentrations of fine (PM2.5) manganese in US national parks and in California and Canadian cities: The possible impact of adding MMT to unleaded gasoline. Journal of the Air & Waste Management Association, 47(6):642652.
Wang W (1986) Toxicity tests of aquatic pollutants by using common duckweed. Environmental Pollution (Series B), 11(1):114.
Warne MS (1998) Critical review of methods to derive water quality guidelines for toxicants and a proposal for a new framework. Canberra, Environment Australia (Supervising Scientist Report 135).
Watanabe I, Tanabe S, Amano M, Miyazaki N, Petrov EA, Tatsukawa R (1998) Age-dependent accumulation of heavy metals in Baikal seal (Phoca sibirica) from the Lake Baikal. Archives of Environmental Contamination and Toxicology, 35:518526.
Weinstein JE, West TL, Bray JT (1992) Shell disease and metal content of blue crabs, Callinectes sapidus, from the AlbemarlePamlico estuarine system, North Carolina. Archives of Environmental Contamination and Toxicology, 23:355362.
Wellnitz TA, Sheldon SP (1995) The effects of iron and manganese on diatom colonization in a Vermont stream. Freshwater Biology, 34(3):465470.
Wepener V, Van Vuren JHJ, Du Preez HH (1992) Effect of manganese and iron at a neutral and acidic pH on the hematology of the banded tilapia (Tilapia sparrmanii). Bulletin of Environmental Contamination and Toxicology, 49:613619.
WHO (1999) WHO air quality guidelines for Europe, 2nd ed. Copenhagen, World Health Organization, Regional Office for Europe.
Wilgus HS, Norris LC, Heuser GF (1936) The role of certain inorganic elements in the cause and prevention of perosis. Science, 84:252253.
Windholz M, ed. (1983) The Merck index: An encyclopedia of chemicals, drugs and biologicals. Rahway, NJ, Merck and Company.
Winger PV, Schultz DP, Johnson WW (1990) Environmental contaminant concentrations in biota from the Lower Savannah River, Georgia and South Carolina. Archives of Environmental Contamination and Toxicology, 19:101117.
Woolhouse HW (1983) Toxicity and tolerance in the responses of plants to metals. In: Lange OC, Nobel PS, Osmond CB, Ziegler H, eds. Encyclopedia of plant physiology II. Vol. 12B. New York, NY, Springer-Verlag, pp. 245300.
Yamamoto H, Satoh S, Takeuchi T, Watanabe T (1983) [Effects on rainbow trout of deletion of manganese or trace elements from fish meal diet.] Bulletin of the Japanese Society of Scientific Fisheries, 49(2):287293 (in Japanese).
Zaw M, Chiswell B (1999) Iron and manganese dynamics in lake water. Water Research, 33(8):19001910.
Zayed J (2001) Use of MMT in Canadian gasoline: health and environmental issues. American Journal of Industrial Medicine, 39(4):426433.
Zayed J, Gérin G, Loranger S, Sierra P, Bégin D, Kennedy G (1994) Occupational and environmnetal exposure of garage workers and taxi drivers to airborne manganese arising from the use of methylcyclopentadienyl manganese tricarbonyl in unleaded gasoline. American Industrial Hygiene Association Journal, 55:5358.
Zayed J, Vyskocil A, Kennedy G (1999) Environmental contamination and human exposure to manganese Contribution of methylcyclopentadienyl manganese tricarbonyl in unleaded gasoline. International Archives of Occupational and Environmental Health, 72(1):713.
Zeri C, Voutsinou-Taliadouri F, Romanov AS, Ovsjany EI, Moriki A (2000) A comparative approach of dissolved trace element exchange in two interconnected basins: Black Sea and Aegean Sea. Marine Pollution Bulletin, 40(8):666673.
Zoller WH, Gladney ES, Duce RA (1974) Atmospheric concentrations and sources of trace metals at the South Pole. Science, 183:198200.
ATSDR (2000) Toxicological profile for manganese (update). Draft for public comment. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry
The Toxicological profile for manganese (update) (ATSDR, 2000) was prepared by the Agency for Toxic Substances and Disease Registry (ATSDR) through a contract with the Research Triangle Institute. Copies of the profile can be obtained from the ATSDR website (http://www.atsdr.cdc.gov/toxprofiles/) or from:
Division of Toxicology
Agency for Toxic Substances and Disease Registry
Public Health Service
US Department of Health and Human Services
1600 Clifton Road NE, Mailstop E-29
Atlanta, Georgia 30333
USA
M. Williams-Johnson, Division of Toxicology, ATSDR, and K.B. Altshuler, S.W. Rhodes, and L. Kolb, Sciences International, Inc., Alexandria, Virginia, contributed to the development of the toxicological profile as chemical manager and authors. The profile has undergone three ATSDR internal reviews, including a Health Effects Review, a Minimal Risk Level Review, and a Data Needs Review. An external peer review panel was assembled for the updated profile for manganese. The panel consisted of the following members: Professor M. Aschner, Wake Forest University, Winston-Salem, North Carolina; Professor C. Newland, Auburn University, Auburn, Alabama; Professor D. Mergler, CINBOISE, Université du Québec à Montréal, Montreal, Quebec, Canada; and Professor J. Zayed, University of Montreal, Montreal, Quebec, Canada. These experts collectively have knowledge of manganese's physical and chemical properties, toxicokinetics, key health end-points, mechanisms of action, human and animal exposure, and quantification of risk to humans. All reviewers were selected in conformity with the conditions for peer review specified in Section 104(I)(13) of the US Comprehensive Environmental Response, Compensation, and Liability Act, as amended.
Scientists from ATSDR reviewed the peer reviewers' comments and determined which comments were to be included in the profile. A listing of the peer reviewers' comments not incorporated in the profile, with a brief explanation of the rationale for their exclusion, exists as part of the administrative record for this compound. A list of databases reviewed and a list of unpublished documents cited are also included in the administrative record. The citation of the peer review panel should not be understood to imply its approval of the profile's final content.
The draft CICAD on environmental aspects of manganese and its compounds was sent for review to IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
J. Ahlers, Umweltsbundesamt, Berlin, Germany
M. Baril, Institut de Recherche en Santé et en Sécurité du Travail du Québec, Montreal, Canada
R. Benson, Drinking Water Program, US Environmental Protection Agency, Denver, CO, USA
C. Cooke, Health & Safety Executive, Bootle, Merseyside, United Kingdom
P. Copestake, TAC Ltd., Surrey, United Kingdom
J.M. Davis, US Environmental Protection Agency, Research Triangle Park, NC, USA
I. Desi, University of Szeged, Szeged, Hungary
L. Fishbein, Fairfax, VA, USA
A. Juhasz, University of South Australia, Mawson Lakes, SA, Australia
U. Kierdorf, Justus-Liebig-University of Giessen, Giessen, Germany
M.-T. Lo, Health Canada, Ottawa, Ontario, Canada
D.R. Lynam, Ethyl Corporation, Richmond, VA, USA
M. Nordberg, Karolinska Institute, Stockholm, Sweden
S. Schmidt, Fraunhofer Institute for Toxicology and Experimental Medicine, Hanover, Germany
P. Schulte, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
J. Stauber, CSIRO Energy Technology, Bangor, NSW, Australia
K. Victorin, Karolinska Institute, Stockholm, Sweden
M. Vojtisek, National Institute of Public Health, Prague, Czech Republic
A. Weyers, BUA Office of Ecotoxicology, Dresden, Germany
M. Williams-Johnson, Agency for Toxic Substances and Disease Registry, Atlanta, GA, USA
Following the advice of the peer reviewers, the Final Review Board recommended the generation of a probabilistic risk characterization for aqueous environments. This was prepared by the authors and then peer reviewed by Dr J. Stauber (CSIRO Energy Technology, NSW, Australia) and Drs J. Chapman and M. Warne (Department of Environment and Conservation, NSW, Australia), and Dr R. Smith, Hydrobiology Pty Ltd, Brisbane, Australia.
Varna, Bulgaria
811 September 2003
Members
Dr I. Benchev, Sofia, Bulgaria
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G. Dura, National Institute of Environment, József Fodor Public Health Centre, Budapest, Hungary
Dr L. Fishbein, Fairfax, VA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Risk Assessment, Berlin, Germany
Mr P. Howe, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr S. Ishimitsu, Division of Safety Information on Drug, Food and Chemicals, National Institute of Hygienic Sciences, Tokyo, Japan
Dr D. Kanungo, Central Insecticides Board, Directorate of Plant Protection, Quarantine & Storage, Ministry of Agriculture, Haryana, India
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Experimental Medicine, Hanover, Germany
Ms B. Meek, Environmental Health Directorate, Health Canada, Ottawa, Ontario, Canada
Dr T. Morita, Division of Safety Information on Drug, Food and Chemicals, National Institute of Hygienic Sciences, Tokyo, Japan
Mr F.K. Muchiri, Directorate of Occupational Health and Safety Services, Nairobi, Kenya
Dr L. Olsen, Biological Monitoring & Health Assessment Branch, Division of Applied Research & Technology, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr N. Rizov, National Center of Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria
Dr P. Schulte, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr J. Sekizawa, Faculty of Integrated Arts and Sciences, Tokushima University, Tokushima, Japan
Dr F.P. Simeonova, Sofia, Bulgaria
Dr S. Soliman, Faculty of Agriculture, Alexandria University, El Shatby, Alexandria, Egypt
Dr J. Stauber, CSIRO Energy Technology, Centre for Advanced Analytical Chemistry, Bangor, NSW, Australia
Mr P. Watts, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, NSW, Australia
Dr K. Ziegler-Skylakakis, European Commission, Luxembourg
Observers
Dr S. Jacobi, Degussa AG, Fine Chemicals, Hanau-Wolfgang, Germany
Mr M. Southern, Shell International Petroleum Company Ltd, London, United Kingdom
Dr W. ten Berge, DSM, Heerlen, The Netherlands
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Introduction
The traditional approach to using single-species toxicity data to protect field ecosystems has been to apply standardized assessment factors, safety factors, or application factors to the lowest toxicity figure for a particular chemical. The magnitude of these safety factors depends on whether acute or chronic toxicity figures are available and the degree of confidence that one has in whether the figures reflect the field situation. Most of the factors are multiples of 10, and larger factors are applied where there is less certainty in the data. For example, a factor of 1000 is generally used for acute data, except for essential elements, including manganese, where a factor of 200 is applied. This factor of 200 includes a factor of 10 for extrapolating from laboratory to field, a further factor of 10 for a limited data set, and a factor of 2 for conversion of an acute end-point to a chronic end-point for an essential metal.
Concerns have often been raised as to the arbitrary nature of assessment factors (Chapman et al., 1998) and the fact that they do not conform to risk assessment principles. OECD (1992) recommended that assessment factors be used only when there are inadequate data to allow statistical extrapolation methods to be used.
The following sections briefly outline the statistical extrapolation method used to derive the manganese guidance values for the protection of marine and freshwater aquatic organisms for this CICAD. Much of the text is taken directly from the Australian and New Zealand Guidelines for Fresh and Marine Water Quality (ANZECC/ARMCANZ, 2000).
Use of statistical extrapolation methods
New methods using statistical risk-based approaches have been developed over the last decade for deriving guideline (trigger) values. These are based on calculations of a statistical distribution of laboratory ecotoxicity data and attempt to offer a predetermined level of protection, usually 95%. The approach of Aldenberg & Slob (1993) has been adopted in the Netherlands, Australia, and New Zealand for guideline derivation and is recommended for use by the Organisation for Economic Co-operation and Development (OECD). It was chosen because of its theoretical basis, its ease of use, and the fact that it has been extensively evaluated. Warne (1998) compared in detail the risk-based and assessment factor approaches used in various countries.
The Aldenberg & Slob (1993) method uses a statistical approach to protect 95% of species with a predetermined level of confidence, provided there is an adequate data set. This approach uses available data from all tested species (not just the most sensitive species) and considers these data to be a subsample of the range of concentrations at which effects would occur in all species in the environment. The method may be applied if toxicity data, usually chronic NOEC values, are available for at least five different species from at least four taxonomic groups. Data are entered into a computer program and generally fitted to a log-logistic distribution. A hazardous concentration for p per cent of the species (HCp) is derived. HCp is a value such that the probability of selecting a species from the community with a NOEC lower than HCp is equal to p (e.g., 5%, HC5). HC5 is the estimated concentration that should protect 95% of species. A level of uncertainty is associated with this derived value, and so values with a given confidence level (e.g., 50% or 95%) are computed in the program by attaching a distribution to the error in the tail (Figure A-1). The ANZECC/ ARMCANZ (2000) guidelines use the median of 50% confidence.

Fig. A-1: The Dutch statistical approach for the derivation of trigger values (from Aldenberg & Slob, 1993)
HC5 is estimated by dividing the geometric mean of the NOEC values for m species by an extrapolation factor k (OECD, 1995), where:
K = exp(sm x k)
and where:
sm is the sample standard deviation of natural logarithm of the NOEC values for m species,
k is the one-sided tolerance limit factor for a logistic or normal distribution (from computer simulations).
Where acute LC50 data are used to derive a guidance value, the acute data are converted to chronic values using a conversion factor of 2 for essential metals.
The Aldenberg & Slob (1993) extrapolation method is based on several critical assumptions, outlined below. Many of these are common to other statistical distribution methods:
The ecosystem is sufficiently protected if theoretically 95% of the species in the system are fully protected.
The distribution of the NOECs is symmetrical (not required in the ANZECC/ARMCANZ [2000] modification).
The available data are derived from independent random trials of the total distribution of sensitivities in the ecosystem.
Toxicity data are distributed log-logistically, i.e., a logistic distribution is the most appropriate to use.
There are no interactions between species in the ecosystem.
NOEC data are the most appropriate data to use to set ambient environmental guidelines.
NOEC data for five species are a sufficient data set.
Modification of the Aldenberg & Slob (1993) approach
The Aldenberg & Slob (1993) approach assumes the data are best fitted to a log-logistic distribution. For some data sets, however, a better fit is obtained with other models. By using a program developed by CSIRO Biometrics, the data are compared with a range of statistical distributions called the Burr family of distributions, of which the log-logistic distribution is one case. The program determines the distribution that best fits the available toxicity data and calculates the HC5 with 50% confidence (ANZECC/ARMCANZ, 2000); this method has been used to calculate the HC5 for manganese.
Application to the data set for manganese
For both the marine and freshwater risk assessments, acute LC50 values were converted to chronic values using an acute to chronic ratio (ACR) of 2 (normally an ACR of 10 is used; however, because manganese is an essential element, a factor of 2 was used following ANZECC/ARMCANZ [2000] guidelines). Both these extrapolated acute values and chronic EC50s were then converted to chronic NOECs by applying a factor of 5, according to ANZECC/ARMCANZ (2000) guidelines. In other words, each acute LC50 was divided by 10 (i.e., 2 Χ 5) and each algal EC50 value (regarded as chronic) was divided by 5 prior to the species sensitivity distribution being undertaken. It would be better to use experimentally derived acute to chronic conversion factors, but these were not available for manganese.
Marine guidance value
Nine marine data were used from Table 3 (see section 7.2), and from these data were calculated chronic NOECs (see Table A-1). Non-standard test end-points or end-points of uncertain significance, such as total cell reduction volume, were not included.
Table A-1: Toxicity end-points and calculated chronic NOECs used in the derivation of a marine guidance value.
|
Organism |
End-point |
Manganese concentration (mg/litre) |
Calculated chronic NOEC (mg/litre) |
|
Microalgae |
|||
|
Diatom (Ditylum brightwellii) |
5-day EC50 (growth inhibition) |
1.5 |
0.3 |
|
Diatom (Nitzschia closterium) |
96-h EC50 (growth inhibition) |
25.7 |
5.1 |
|
Diatom (Asterionella japonica) |
72-h EC50 (growth inhibition) |
4.9 |
1 |
|
Invertebrates |
|||
|
American oyster (Crassostrea virginica) |
48-h LC50 |
16 |
1.6 |
|
Softshell clam (Mya arenaria) |
168-h LC50 |
300 |
30 |
|
Mussel (Mytilus edulis) |
48-h EC50 (abnormal larvae) |
30 |
6 |
|
Sea urchin (Heliocidaris tuberculata) |
72-h EC50 (abnormal larvae) |
5.2 |
1 |
|
Brine shrimp (Artemia salina) |
48-h LC50 |
51.8 |
5.2 |
|
Copepod (Nitocra spinipes) |
96-h LC50 |
70 |
7 |
Using the calculated chronic NOECs, the HC5(50), i.e., the hazardous concentration to protect 95% of species with 50% confidence a "safe" value to ensure protection against chronic toxicity for most marine species was 0.3 mg manganese/litre (see Figure A-2).
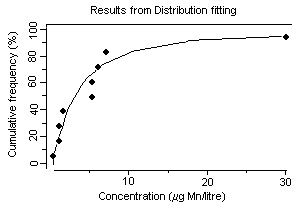
Fig. A-2: Probability curve for manganese in the
marine environment using actual and derived data from Table A-1
It should be noted that further Australian studies were provided after the cut-off date. Adding these data a NOEC for Nitzschia closterium (algal) growth rate inhibition over 72 h of 18 mg manganese/litre, a 96-h LC50 for tiger prawn (Penaeus monodon) survival of 26.1 mg manganese/litre, a 96-h LC50 for Australian bass (Macquaria novemaculeata) of 100 mg manganese/litre, and a 60-h NOEC for rock oyster (Saccostrea glomerata) larval abnormalities of 1 mg manganese/litre and converting them in the same way to chronic NOECs (18, 2.6, 10, and 1 mg manganese/litre, respectively) gives an HC5(50) of 0.4 mg manganese/litre. These extra data points improve the distribution of the data (the method requires unimodality of distribution) and decrease uncertainty.
Freshwater guidance value
Twenty-one freshwater data were used from Table 3 (see section 7.2), and from these data, chronic NOECs were calculated (see Table A-2). Non-standard test end-points or end-points of uncertain significance, such as total cell volume reduction and deformations, were not included. Geometric means of multiple test results from the same species over the same time period were calculated.
Using the calculated chronic NOECs, the HC5(50), i.e., the hazardous concentration to protect 95% of species with 50% confidence a "safe" value to ensure protection against chronic toxicity for most freshwater species in soft water was 0.2 mg manganese/litre (see Figure A-3).
Table A-2: Toxicity end-points and calculated chronic NOECs used in the derivation of a freshwater guidance value.
|
Organism |
End-point |
Manganese concentration (mg/litre) |
Calculated chronic NOEC (mg/litre) |
|
Microalgae |
|||
|
Alga (Scenedesmus quadricauda) |
12-day EC50 (growth inhibition) |
5 |
1 |
|
Alga (Pseudokirchneriella subcapitata)a |
72-h EC50 (growth inhibition) |
8.3 |
1.7 |
|
Protozoa |
|||
|
Ciliated protozoan (Spirostomum ambiguum) |
24-h LC50 |
148 |
14.8 |
|
Invertebrates |
|||
|
Sludge worm (Tubifex tubifex) |
96-h LC50 |
168b |
16.8 |
|
Daphnid (Daphnia magna) |
48-h EC50 (immobilization) |
9.4c |
0.9 |
|
Daphnid (Daphnia obtusa) |
48-h EC50 (immobilization) |
37.4 |
3.7 |
|
Daphnid (Ceriodaphnia dubia) |
48-h LC50 |
7.2b |
0.7 |
|
Amphipod (Hyalella azteca) |
96-h LC50 |
3.3b |
0.3 |
|
Rotifer (Brachionus calyciflorus) |
24-h LC50 |
38.7 |
3.9 |
|
Copepod (Canthocamptus spp.) |
48-h LC50 |
54 |
5.4 |
|
Isopod (Asellus aquaticus) |
96-h LC50 |
333 |
33.3 |
|
Amphipod (Crangonyx pseudogracilis) |
96-h LC50 |
694 |
6.9 |
|
Midge (Chironomus tentans) |
96-h LC50 |
5.8 |
0.6 |
|
Fish |
|||
|
Rainbow trout (Oncorhynchus mykiss) |
28-day LC50 (embryo-larval test) |
2.9 |
0.3 |
|
Brown trout (Salmo trutta) |
96-h LC50 |
3.8 |
0.4 |
|
Coho salmon (Oncorhynchus kisutch) |
96-h LC50 |
2.4 |
0.2 |
|
Goldfish (Carassius auratus) |
7-day LC50 (embryo-larval test) |
8.2 |
0.8 |
|
Indian catfish (Heteropneustes fossilis) |
96-h LC50 |
3350 |
335 |
|
Indian freshwater murrel (Channa punctatus) |
96-h LC50 |
3010 |
301 |
|
Giant gourami (Colisa fasciatus) |
96-h LC50 |
3034b |
303.4 |
|
Amphibians |
|||
|
Narrow-mouthed toad (Gastrophryne carolinensis) |
7-day LC50 (embryo-larval test) |
1.4 |
0.1 |
a Formerly known as Selenastrum capricornutum.
b Geometric mean of LC50 values for this species for the same time period.
c Geometric mean of EC50 values for this species for the same time period.
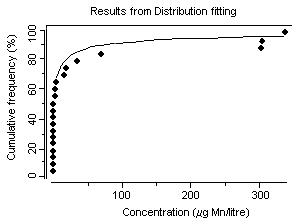
Fig. A-3: Probability curve for manganese in the freshwater environment
using actual and derived data from Table A-2
|
ACR |
acute to chronic ratio |
|
ALA-D |
delta-aminolevulinic dehydratase |
|
ATSDR |
Agency for Toxic Substances and Disease Registry (USA) |
|
BCF |
bioconcentration factor |
|
CICAD |
Concise International Chemical Assessment Document |
|
DGT |
diffusive gradients in thin films |
|
DTPA |
diethylenetriaminepentaacetic acid |
|
EC50 |
median effective concentration |
|
EDTA |
ethylenediaminetetraacetic acid |
|
EHC |
Environmental Health Criteria monograph |
|
HCp |
hazardous concentration for p per cent of species |
|
HC5(50) |
hazardous concentration to protect 95% of species with 50% confidence |
|
HLC |
Henry's law constant |
|
IC25 |
inhibitory concentration at which a 25% effect occurs |
|
ICP |
inductively coupled plasma |
|
ICSC |
International Chemical Safety Card |
|
ILO |
International Labour Organization |
|
IPCS |
International Programme on Chemical Safety |
|
Kow |
octanolwater partition coefficient |
|
LC50 |
median lethal concentration |
|
MMED |
mass median equivalent diameter |
|
MMT |
methylcyclopentadienyl manganese tricarbonyl |
|
NOEC |
no-observed-effect concentration |
|
OECD |
Organisation for Economic Co-operation and Development |
|
PIM |
Poison Information Monograph |
|
PM2.5 |
particulate matter with an aerodynamic diameter less than or equal to 2.5 ΅m |
|
TRI |
Toxics Release Inventory (USA) |
|
UNEP |
United Nations Environment Programme |
|
USA |
United States of America |
|
WHO |
World Health Organization |
MANGANESE SULPHATE MONOHYDRATE ICSC:0290
MANGANESE, CYCLOPENTADIENYLTRICARBONYL ICSC:0977
METHYLCYCLOPENTADIENYL MANGANESE TRICARBONYL ICSC:1169
Le présent CICAD sur le manganèse et ses dérivés (aspects environnementaux) repose essentiellement sur le Profil toxicologique du manganèse (mise à jour) préparé par l'Agence pour les produits toxiques et le Registre des maladies du Département de la santé et des services humains des Etats-Unis (Agency for Toxic Substances and Disease Registry of the US Department of Health and Human Services) (ATSDR, 2000). Parmi les autres sources d'information utilisées figurent le CICAD No 12 consacré au manganèse et à ses composés (IPCS, 1999a) ainsi que les données obtenues à la suite d'une recherche bibliographique exhaustive effectuée jusqu'à décembre 2002 sur les bases de données pertinentes afin de retrouver toute référence interessante publiée postérieurement à celles qui sont utilisées dans ces deux documents. Pour l'évaluation des effets du manganèse sur la santé humaine, le lecteur est prié de se reporter au CICAD No 12 (IPCS, 1999a). En ce qui concerne les fongicides à base de manganèse, on s'est borné à indiquer leurs sources et leur devenir dans l'environnement, sans chercher à évaluer les effets environnementaux de ce groupe de composés. Des renseignements sur la préparation du document de base et son examen par des pairs sont donnés à l'appendice 1. L'appendice 2 donne des indications sur l'examen par des pairs du présent CICAD. Ce CICAD a été examiné et approuvé en tant qu'évaluation internationale lors de la réunion du Comité d'évaluation finale qui s'est tenue à Varna (Bulgarie) du 8 au 11 septembre 2003. La liste des participants à la réunion du Comité d'évaluation finale figure à l'appendice 3. Les fiches internationales sur la sécurité chimique d'un certain nombre de dérivés du manganèse (ICSC 174, 175, 290, 754, 977, 1169 et 1398) établies par le Programme international sur la sécurité chimique lors d'un examen distinct par des pairs (IPCS, 1999b,c, 2001, 2003a,b,c,d) sont également reproduites dans ce document.
Le manganèse (Mn) est naturellement présent dans les roches, le sol et l'eau. Il est omniprésent dans l'environnement et représente environ 0,1 % de l'écorce terrestre. Le manganèse présent dans l'atmosphère provient principalement des roches constitutives de l'écorce terrestre. Les embruns océaniques, les feux de forêt et l'activité volcanique contribuent également de manière importante à la présence de manganèse dans l'atmosphère. D'importantes sources de manganèse en solution sont constituées par les milieux anaérobies où des particules d'oxydes de manganèse subissent une réduction, les milieux aérobies où cette réduction est directe, l'érosion naturelle des minéraux contenant des dérivés du Mn (II) ou encore les milieux acides. Le manganèse présent dans le sol provient essentiellement des roches constituant l'écorce terrestre, mais peut également trouver son origine dans le dépôt direct de particules aéroportées, l'eau de ruissellement provenant de surfaces végétales ou autres, le lessivage de tissus végétaux ou encore l'élimination ou l'excrétion de matières organiques telles que feuilles, plantes mortes, produits d'origine animale ou excréments. Les principales sources anthropogéniques de manganèse sont les décharges municipales d'eaux usées, les boues d'égout, les industries d'extraction et de traitement de substances minérales, les vapeurs émises lors de la production d'alliages, de fer ou d'acier ou de l'utilisation de combustibles fossiles et dans une bien moindre mesure, la combustion des additifs pour combustibles.
Lorsque du manganèse est libéré dans l'air, c'est principalement sous forme de particules dont la granulométrie et la densité, de même que la vitesse et la direction du vent, déterminent le devenir et le transport . Certains dérivés du manganèse sont facilement solubles dans l'eau. Dans le milieu aquatique, le manganèse est essentiellement présent sous la forme de Mn (II) et de Mn (IV). Le passage d'une forme à l'autre s'effectue par des réactions d'oxydation ou de réduction qui peuvent être abiotiques ou d'origine microbienne. Dans l'environnement, la chimie du manganèse est largement tributaire de la valeur du pH et du potentiel d'oxydoréduction ou potentiel rédox; c'est le Mn (II) qui prédomine aux faibles valeurs du pH et du potentiel rédox, la proportion d'oxyhydroxydes colloïdaux étant en augmentation dans les eaux non dystrophiques lorsque le pH dépasse 5,5. Les principaux facteurs chimiques qui déterminent le cycle du manganèse sédimentaire sont la teneur en oxygène de la colonne d'eau, la pénétration de l'oxygène dans les sédiments et l'apport de carbone organique d'origine benthique. Le manganèse présent dans le sol peut migrer dans l'air ou dans l'eau soit sous la forme de particules, soit par lessivage de composés hydrosolubles. Dans le sol, la solubilité du manganèse dépend essentiellement du pH et du potentiel rédox.
Dans l'eau, le manganèse peut subir une concentration importante par les organismes aquatiques aux premiers stades de la chaîne alimentaire. L'estimation des facteurs de bioconcentration donne des valeurs de 2000 à 20 000 pour les plantes marines et dulçaquicoles, de 2500 à 6300 pour le phytoplancton, de 300 à 5500 pour les algues macroscopiques, de 800 à 830 pour les colonies intertidales de moules, et de 35 à 930 pour les poissons. L'absorption de manganèse par les invertébrés aquatiques et les poissons augmente sensiblement avec la température et diminue avec le pH, mais l'oxygène dissous est sans effet notable. On a constaté que l'absorption du manganèse augmente lorsque la salinité augmente.
Dans l'air, la concentration du manganèse, qui tend vers un minimum dans les zones écartées (environ 0,5 à 14 ng/m3), s'élève dans les zones rurales pour atteindre
40 ng/m3 en moyenne, et encore davantage en milieu urbain (environ 65-166 ng/m3 en moyenne). C'est dans l'air des zones où prédominent diverses sources de manganèse que la concentration en manganèse tend vers ses valeurs maximales et peut atteindre 8000 ng/m3. La teneur de l'air en manganèse peut atteindre une valeur annuelle moyenne de 200 à 300 ng/m3 à proximité des fonderies et dépasser 500 ng/m3 à proximité des unités de production de ferro- et de silicomanganèse.
Dans les eaux naturelles peu exposées à la pollution anthropogénique, la concentration du manganèse en solution peut varier de 10 à > 10 000 μg/litre. Toutefois, dans les eaux de surface naturelles, cette concentration excθde rarement 1000 μg/litre et elle est gιnéralement inférieure à 200 μg/litre.
Dans les sédiments des cours d'eau, la concentration du manganèse va de 410 à 6700 mg/kg de matière sèche; dans les sédiments d'un lac urbain pollué par des décharges industrielles ou résidentielles et les poussières de vieux déchets miniers apportées par le vent, on a mesuré des concentrations de manganèse pouvant atteindre 13 400 mg/kg de poids sec. Des concentrations sédimentaires de manganèse comprises entre 100 et 1000 mg/kg de poids sec ont été relevées dans des vasières intertidales; des concentration en manganèse total du même ordre ont été observées dans le nord de l'Adriatique. Dans les sédiments de surface de la Baltique, on a relevé des concentrations moyennes de manganèse allant de 3550 à 8960 mg/kg de poids sec; on pense que ces fortes teneurs en manganèse s'expliquent par la présence de concrétions de ferromanganèse et par la charge manganique des cours d'eau.
La concentration de fond du manganèse total dans le sol va de < 1 à 4000 mg/kg de poids sec, avec une valeur moyenne qui se situe aux alentours de 300 à 600 mg/kg de poids sec.
Dans la végétation marine, la concentration moyenne du manganèse va de 130 à 735 mg/kg de tissu sec, la concentration dans les invertébrés marins se situant entre 3 et 660 mg/kg de matière sèche; il y a un lien entre une forte teneur des invertébrés marins en manganèse et la présence de sédiments riches en manganèse. Dans les tissus de poissons de mer et d'eau douce, on a trouvé des concentrations allant de moins de 0,2 à 19 mg/kg de poids sec. Chez des poissons vivant dans des eaux de surface fortement polluées, on a relevé des concentrations plus élevées, à savoir supérieures à 100 mg/kg de poids sec.
Dans les plantes terrestres, la concentration du manganèse varie de 20 à 500 mg/kg. On considère que les plantes de la famille des éricacées, dont fait partie la myrtille, accumulent le manganèse. De nombreuses observations font état de concentrations foliaires supérieures à 2000-4000 mg/kg. Dans des oeufs d'oiseaux provenant de diverses zones géographiques, on a trouvé des concentrations allant de 1 à 5 mg/kg de poids sec. Dans le foie des volatiles, la concentration moyenne variait de de 3 à 11 mg/kg de poids sec et dans les plumes, elle allait de 0,3 à 40 mg/kg de poids sec. Dans les tissus de divers reptiles et mammifères sauvages (foie, rein et corps entier), la concentration moyenne du manganèse peut atteindre 17 mg/kg de poids sec.
Le manganèse est un nutriment essentiel pour les microorganismes, les végétaux et les animaux. Les besoins nutritionnels en manganèse des plantes terrestres sont d'environ 10 à 50 mg/kg de tissu. Les besoins nutritionnels minimaux varient largement d'une espèce à une autre et à l'intérieur d'une même espèce, d'une variété culturale à l'autre. Les sols calcaires, et notamment ceux qui sont mal drainés et ont une forte teneur en matières organiques, sont le genre de sols qui produisent des plantes carencées en manganèse.
La plupart des épreuves de toxicité ont été effectuées avec du manganèse sous forme ionique. On connaît mal la toxicité du manganèse colloïdal, particulaire ou complexé dans le milieu aquatique; en règle générale, on estime que les métaux qui se trouvent sous ces formes sont moins toxiques que les formes aquo-ioniques. Dans le cas des algues et des protozoaires, la toxicité varie dans de larges proportions; les espèces les plus sensibles semblent être une diatomée marine, Ditylum brightwellii, dont la CE50 à cinq jours, basée sur l'inhibition de la croissance, est égale à 1,5 mg/litre, et une algue d'eau douce, Scenesdesmus quadricauda, dont la CE50 à 12 jours, basée sur la réduction totale de la chlorophylle, est de 1,9 mg/litre. Les tests toxicologiques pratiqués sur des invertébrés aquatiques mettent en évidence des valeurs de la CL50 ou de la CE50 à 48 h allant de 0,8 mg/litre (Daphnia magna) à 1389 mg/litre (Crangonyx pseudogracilis), les valeurs les plus faibles de la CL50 étant observées dans des eaux de faible dureté (25 mg de carbonate de calcium par litre). Au cours de tests d'une durée de 7 jours dans une eau de mer contenant > 0,01 mg de manganèse par litre, on a observé une réduction sensible de la survie et des éclosions chez des embryons de crabes jaunes (Cancer anthonyi). Chez les poissons, les valeurs de la CL50 à 96 h varient de 2,4 mg/litre pour le saumon coho (Oncorhynchus kisutch), à 3350 mg/litre pour le silure indien (Heteropneustes fossilis), les valeurs les plus faibles de la CL50 étant observées dans une eau de faible dureté (25 mg de carbonate de calcium par litre). On a constaté une importante mortalité embryonnaire chez des oeufs de truite arc-en-ciel (Oncorhyncus mykiss) exposés pendant 29 jours à une concentration de sulfate de manganèse égale à 1 mg/litre. En ce qui concerne les amphibiens, on n'a trouvé d'autre test que celui pratiqué sur les stades embryono-larvaires et qui donne une valeur de 1,4 mg/litre pour la CL50 à 7 jours. La toxicité aiguë du manganèse pour les invertébrés et les poissons diminue à mesure qu'augmente la dureté de l'eau; l'adjonction d'agents chélatants peut réduire la toxicité du manganèse. On est porté à croire que le manganèse protège contre les effets de métaux plus toxiques.
Dans un estuaire pollué par des métaux, on attribue à l'action toxique du manganèse la forte proportion de crabes bleus (Callinectes sapidus) dont la carapace présente des anomalies pathologiques (lésions exosquelettiques). Par ailleurs, le dépôt d'oxyde de manganèse sur les branchies du homard de Norvège (Nephrops norvegicus), a provoqué un brunissement ou un noircissement du tissu branchial et entraîné la présence de zones de corrosion noirâtres sur la carapace, à la suite d'une situation d'hypoxie dans le sud-est du Kattegat (Suède). On a également constaté une corrélation positive entre la mortalité de truites arc-en-ciel (Oncorhyncus mykiss) observée dans un éclosoir et la présence de manganèse à une concentration comprise entre < 0,5 et 1 mg/litre. Des pluies acides avaient provoqué une augmentation de l'acidité du milieu et une élévation de la concentration des métaux. Des expériences effectuées sur les truites brunes (Salmo trutta) de l'année en captivité ont montré que le pH (4,5-5,4) et la concentration en manganèse minéral labile (0,1-0,4 mg/litre) expliquent entièrement la mortalité observée.
Chez les végétaux terrestres, la toxicité du manganèse se manifeste par des symptômes très variables d'une espèce à l'autre et qui consistent en une chlorose marginale, une nécrose et des anomalies du développement foliaire. Chez les plantes vivrières, la présence de manganèse à des concentrations toxiques est également très variable, avec des valeurs critiques qui vont de 100 à 5000 mg/kg. La toxicité du manganèse est un facteur important qui limite la croissance des plantes cultivées sur des sols acides, mal drainés ou des sols minéraux stérilisés à la vapeur. D'une espèce à l'autre et au sein d'une même espèce, la tolérance au manganèse varie dans d'importantes proportions. Parmi les facteurs qui influent sur cette tolérance, on peut citer le génotype (variations inter- et intraspécifiques), la concentration en silicium, l'intensité de la lumière, l'âge physiologique des feuilles, l'activité microbienne et les caractéristiques de la rhizosphère.
Les données relatives aux eaux douces de surface incitent à penser que la concentration du manganèse est plus élevée lorsque le débit du cours d'eau augmente, comme c'est le cas au printemps et les concentrations les plus faibles ont tendance à se produire en aval des lacs qui se comportent comme des bassins de sédimentation. Les eaux douces les plus sensibles sont celles des rivières, fleuves et lacs dont l'eau est de faible dureté, les épreuves de laboratoire et les observations en milieu naturel montrant qu'une concentration de manganèse en solution d'environ 1 mg/litre peut provoquer des effets toxiques chez les organismes aquatiques. Dans le cas des eaux douces de faible dureté, on est parvenu à une valeur-guide générale de 0,2 mg de manganèse par litre pour la protection de 95 % des espèces avec une confiance de 50 %. D'autres facteurs, comme les pluies acides, les eaux de drainage acides des sites miniers, l'occupation des sols ou encore la décharge d'eaux usées municipales, sont susceptibles d'accroître le risque pour les espèces sensibles, notamment dans les zones où l'eau présente une faible dureté. Pour évaluer la toxicité probable du manganèse pour les êtres vivants dans leur milieu naturel, il faut prendre en compte les conditions de spéciation de cet élément tant dans la zone expérimentale que dans le secteur en cause. Dans le milieu marin, le manganèse peut être absorbé et accumulé par les êtres vivants à l'occasion de sa libération hypoxique dans l'eau à partir de sédiments riches où il est présent en quantité importante. Malgré l'effet atténuateur éventuel des sédiments en suspension, de la salinité et de la concentration en oxygène du milieu naturel, des effets toxiques sont observés. Dans le cas du milieu marin, on est parvenu à une valeur-guide générale de 0,3 mg de manganèse par litre pour la protection de 95 % des espèces avec une confiance de 50 %.
Pour évaluer le risque que les émissions anthropogéniques de manganèse représentent pour l'environnement terrestre, il faut prendre en considération la concentration locale naturelle (concentration de fond), laquelle dépend à son tour de divers paramètres physico-chimiques. La réaction des diverses communautés ou écosystèmes variera également en fonction de leur exposition « normale » au manganèse. C'est pourquoi il n'est pas justifié de chercher à obtenir une valeur-guide unique pour l'ensemble de l'environnement terrestre.
Este CICAD sobre el manganeso y sus compuestos (aspectos ambientales) se basó fundamentalmente en el informe titulado Perfil toxicológico del manganeso (actualización), preparado por la Agencia para el Registro de Sustancias Tóxicas y Enfermedades del Departamento de Salud y Servicios Sociales de los Estados Unidos (ATSDR, 2000). Se utilizaron como fuentes secundarias de información el CICAD NΊ 12 sobre el manganeso y sus compuestos (IPCS, 1999a) y los datos obtenidos tras una búsqueda bibliográfica amplia de las bases de datos pertinentes realizada hasta diciembre de 2002 con objeto de determinar cualquier referencia de interés publicada después de las incorporadas a estos dos informes. Para la información relativa a la evaluación de los efectos del manganeso en la salud humana, el lector debe consultar el CICAD NΊ 12 (IPCS, 1999a). En el documento se han mencionado los fungicidas de manganeso sólo con fines de información en relación con la fuente y el destino final, pero no se han intentado evaluar los efectos de este grupo de productos químicos en el medio ambiente. La información sobre la preparación y el examen colegiado del documento original figura en el apéndice 1. La información acerca del examen colegiado de este CICAD se presenta en el apéndice 2. Este CICAD se examinó y aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final, celebrada en Varna (Bulgaria) del 8 al 11 de septiembre de 2003. La lista de participantes en esta reunión figura en el apéndice 3. También se reproduce en el presente documento la Ficha internacional de seguridad química para determinados compuestos de manganeso (ICSCs 174, 175, 290, 754, 977, 1169 y 1398), preparada por el Programa Internacional de Seguridad de las Sustancias Químicas en un proceso de examen colegiado realizado por separado (IPCS, 1999b, c, 2001, 2003a, b, c, d).
El manganeso (Mn) es un elemento que está presente en la naturaleza en las rocas, el suelo y el agua. Es ubicuo en el medio ambiente y representa alrededor del 0,1% de la corteza terrestre. Las rocas de la corteza son una fuente importante del manganeso que se encuentra en la atmósfera. Otras fuentes naturales de manganeso atmosférico dignas de consideración son la pulverización de los océanos, los incendios forestales, la vegetación y la actividad volcánica. Son fuentes importantes de manganeso disuelto los entornos anaerobios, en los que se reducen las partículas de óxidos de manganeso, la reducción directa de las partículas de óxidos de manganeso en entornos aerobios, la meteorización natural de minerales que contienen Mn (II) y los entornos ácidos. La reserva principal de manganeso en el suelo procede de fuentes de la corteza, aunque hay otras, como la deposición atmosférica directa, el lavado de superficies vegetales y de otro tipo, la lixiviación a partir de los tejidos vegetales y la diseminación o excreción de material como hojas, materiales muertos de origen vegetal y animal y los excrementos de los animales. Las principales fuentes antropogénicas de manganeso para el medio ambiente son los vertidos de aguas residuales municipales, los fangos cloacales, la extracción de minerales y su elaboración, las emisiones procedentes de la fabricación de aleaciones, de acero y de hierro, el consumo de combustibles fósiles y, en una medida mucho menor, las emisiones procedentes de la combustión de aditivos de los combustibles.
El manganeso se libera en el aire fundamentalmente en forma de partículas, cuyo destino y transporte dependen de su tamaño y densidad, así como de la velocidad y la dirección del viento. Algunos compuestos de manganeso se disuelven fácilmente en agua. El manganeso se encuentra en el medio acuático en dos formas principales: Mn (II) y Mn (IV). La conversión de una forma en otra se produce mediante reacciones de oxidación y reducción, que pueden ser abióticas o mediadas por microorganismos. La química del manganeso en el medio ambiente se rige fundamentalmente por el pH y las condiciones redox; el Mn (II) predomina en condiciones de pH y potencial redox más bajos, aumentando la proporción de oxihidróxidos de manganeso coloidal en aguas no distróficas con valores del pH superiores a 5,5. Los factores químicos primarios que controlan el ciclo sedimentario del manganeso son el contenido de oxígeno del agua situada encima, la penetración de oxígeno en los sedimentos y el suministro de carbono orgánico bentónico. El manganeso del suelo se puede desplazar hacia el aire o el agua en forma de partículas o filtrarse como compuestos solubles. En los suelos, la solubilidad del manganeso depende de dos variables principales: el pH y el potencial redox.
El manganeso puede experimentar una bioconcentración significativa en el agua mediante la biota acuática en los niveles tróficos más bajos. Se han estimado factores de bioconcentración de 2000-20 000 para las plantas marinas y de agua dulce, de 2500-6300 para el fitoplancton, de 300-5500 para las macroalgas marinas, de 800-830 para los mejillones de zonas de oscilación de las mareas y de 35-930 para los peces. La absorción de manganeso por los invertebrados acuáticos y los peces aumenta considerablemente con la temperatura y disminuye con el pH, mientras que el oxígeno disuelto no tiene un efecto importante. Se ha observado que la absorción de manganeso aumenta al disminuir la salinidad.
Las concentraciones de manganeso en el aire tienden a ser más bajas en las zonas más remotas (promedio de unos 0,5-14 ng/m3), más altas en las zonas rurales (40 ng/m3 como promedio) y todavía más altas en las zonas urbanas (promedio de unos 65-166 ng/m3). Las concentraciones de manganeso en el aire tienden a ser más elevadas en zonas donde existe alguna fuente, con valores de hasta 8000 ng/m3. Los promedios anuales de las concentraciones de manganeso pueden aumentar a 200-300 ng/m3 en el aire cercano a fundiciones y a más de 500 ng/m3 en el aire próximo a industrias de ferromanganeso y silicomanganeso.
Las concentraciones de manganeso disuelto en aguas naturales que están básicamente libres de aportaciones antropogénicas pueden oscilar entre 10 y > de 10 000 ΅g/l. Sin embargo, las concentraciones de manganeso disuelto en las aguas superficiales naturales raramente alcanzan un valor superior a 1000 ΅g/l y suelen ser inferiores a 200 ΅g/l.
Las concentraciones de manganeso en los sedimentos de los ríos iban de 410 a 6700 mg/kg de peso seco; los sedimentos de un lago urbano con aportaciones de zonas industriales y residenciales, así como polvo de los vertederos de unas antiguas minas transportado por el viento, contenían concentraciones de manganeso de hasta 13 400 mg/kg de peso seco. Se han notificado concentraciones de manganeso en los sedimentos de 100-1000 mg/kg de peso seco para el lecho fangoso de la zona de oscilación de las mareas; se encontraron valores semejantes de manganeso total en la región septentrional del mar Adriático. Los sedimentos superficiales en el mar Báltico contenían concentraciones medias de manganeso de 3550-8960 mg/kg de peso seco; se estimó que las concentraciones elevadas de manganeso se debían a concreciones de ferromanganeso y a la carga fluvial.
Los niveles naturales ("de fondo") de manganeso total en el suelo oscilan entre < 1 y 4000 mg/kg de peso seco, con valores medios de unos 300-600 mg/kg de peso seco.
Las concentraciones medias de manganeso en las algas marinas oscilan entre 130 y 735 mg/kg de peso seco, mientras que en moluscos y crustáceos son de 3 a 660 mg/kg de peso seco; las concentraciones más altas en moluscos y crustáceos están asociadas a sedimentos ricos en manganeso. Las concentraciones de manganeso observadas en tejidos de peces marinos y de agua dulce tienden a variar entre < 0,2 y 19 mg/kg de peso seco. Se ha informado de concentraciones más altas de manganeso, superiores a 100 mg/kg de peso seco, en peces de aguas superficiales contaminadas.
Las concentraciones de manganeso en las plantas terrestres suelen alcanzar valores de entre 20 y 500 mg/kg. Se considera que las plantas de la familia Ericaceae, que incluye el arándano, son acumuladoras de manganeso. Hay numerosos informes sobre niveles de manganeso foliar superiores a 2000-4000 mg/kg. Las concentraciones medias de manganeso en los huevos de aves procedentes de diversas zonas geográficas varían de 1 a 5 mg/kg de peso seco; las concentraciones medias en el hígado son de 3 a 11 mg/kg de peso seco y las concentraciones medias en las plumas de 0,3 a 40 mg/kg de peso seco. Se han encontrado concentraciones medias de manganeso de hasta 17 mg/kg de peso seco en los tejidos (hígado, riñón y todo el organismo) de diversos reptiles y mamíferos silvestres.
El manganeso es un nutriente esencial de los microorganismos, las plantas y los animales. Las necesidades nutricionales de manganeso para las plantas terrestres son de unos 10-50 mg/kg de tejido. Los niveles nutricionales críticos varían ampliamente entre las especies y entre cultivares de una misma especie. Los suelos calcáreos, especialmente los de escaso drenaje y alto contenido de materia orgánica, son los tipos de suelos que producen plantas con déficit de manganeso.
La mayor parte de las pruebas de toxicidad se han realizado con manganeso iónico. Los conocimientos sobre la toxicidad acuática del manganeso en forma coloidal, en partículas y formando complejos son escasos; sin embargo, en general se supone que la toxicidad de los metales unidos a esos estados es inferior a la de las formas iónicas en medio acuoso. Para las algas y los protozoos hay una gran variedad de valores de toxicidad; las especies más sensibles parecen ser la diatomea marina Ditylum brightwellii, con una CE50 basada en la inhibición del crecimiento de 1,5 mg/l, y el alga de agua dulce Scenedesmus quadricauda, con una CE50 a los 12 días basada en la reducción total de la clorofila de 1,9 mg/l. Las pruebas sobre invertebrados acuáticos ponen de manifiesto valores de la CL50/CE50 a las 48 horas entre 0,8 mg/l (Daphnia magna) y 1389 mg/l (Crangonyx pseudogracilis), habiéndose observado los valores más bajos de la CL50 en condiciones de agua blanda (25 mg de carbonato de calcio/l). En una prueba de siete días en agua marina se observó una reducción significativa de la supervivencia y la eclosión de embriones de cangrejo amarillo (Cancer anthonyi) con 0,01 mg de manganeso/l. Para los peces, las CL50 a las 96 horas van de 2,4 mg de manganeso/l para el salmón plateado (Oncorhynchus kisutch) a 3350 mg/l para el bagre de la India (Heteropneustes fossilis), habiéndose obtenido los valores más bajos de la CL50 en condiciones de agua blanda (25 mg de carbonato de calcio/l). Se observó una mortalidad embrionaria significativa en los huevos de trucha arco iris (Oncorhynchus mykiss) expuestos a 1 mg de sulfato de manganeso/l durante 29 días. En anfibios se realizó una prueba embriolarvaria única, con una CL50 a los siete días de 1,4 mg de manganeso/l. La toxicidad aguda en los invertebrados acuáticos y los peces disminuyó al aumentar la dureza del agua; la adición de agentes quelantes puede reducir la toxicidad del manganeso. Hay pruebas de que el manganeso puede proteger a los organismos contra los efectos de metales más tóxicos.
Sobre el terreno, la elevada frecuencia de cangrejos azules (Callinectes sapidus) con enfermedades del caparazón (lesiones) en un estuario contaminado por metales se atribuyó a la toxicidad del manganeso, y la deposición de dióxido de manganeso en las branquias de la cigala (Nephrops norvegicus) dio origen a la aparición en ellas de una decoloración parda o negra y a zonas negras de corrosión en el caparazón tras la exposición a condiciones hipóxicas en la región sudoriental de Kattegat (Suecia). Se observó que el aumento de la mortalidad de la trucha arco iris (Oncorhynchus mykiss) en un vivero tenía una correlación positiva con las concentraciones de manganeso (< 0,5-1 mg/l). La precipitación ácida ha causado fenómenos de acidez y concentraciones elevadas de metales. En experimentos en jaulas con la trucha marina (Salmo trutta) se comprobó que el pH (4,5-5,4) y la concentración de manganeso inorgánico lábil (0,1-0,4 mg/l) explicaban toda la mortalidad observada.
Los síntomas de la toxicidad del manganeso en las plantas terrestres varían ampliamente en función de la especie e incluyen clorosis marginales, lesiones necróticas y desarrollo deforme de las hojas. Las concentraciones tóxicas del manganeso en los tejidos de las plantas cultivadas son muy variables, con valores críticos que van de 100 a 5000 mg/kg. La toxicidad del manganeso es un factor importante que limita el crecimiento de los cultivos en suelos minerales ácidos, con poco drenaje o esterilizados por vapor. Hay una amplia variación en la tolerancia al manganeso entre distintas especies y dentro de la misma. Los factores que afectan a la tolerancia al manganeso son el genotipo (variación interespecífica e intraespecífica), la concentración de silicio, la temperatura, la intensidad luminosa, la edad fisiológica de la hoja, la actividad microbiana y las características de rizosfera.
Los datos sobre el agua dulce superficial parecen indicar que se producen concentraciones más altas de manganeso durante los períodos de flujo más elevado de las corrientes, como la escorrentía de primavera, y que las concentraciones más bajas suelen darse aguas abajo de los lagos, que actúan como zonas de decantación de los sedimentos. Los arroyos, ríos y lagos de agua blanda parecen ser los entornos de agua dulce más sensibles, mostrando las pruebas de laboratorio y las observaciones sobre el terreno que las concentraciones de manganeso disuelto de alrededor de 1 mg/l pueden provocar efectos tóxicos en los organismos acuáticos. En las aguas blandas de entornos de agua dulce se obtuvo un valor guía global para la protección del 95% de las especies con un 50% de confianza de 0,2 mg de manganeso/l. Otros factores, como la precipitación ácida, el drenaje de minas ácidas, la utilización de la tierra y los vertidos de aguas residuales municipales, pueden elevar los niveles de manganeso disuelto y de esta manera aumentar el riesgo de las especies sensibles, especialmente en zonas de aguas blandas. En la evaluación sobre el terreno de la probabilidad de toxicidad del manganeso para los organismos se han de tener en cuenta las condiciones de especiación tanto en la prueba como en la zona específica de terreno. En el medio marino, los organismos pueden absorber y acumular manganeso durante las emisiones hipóxicas de manganeso disuelto procedente de sedimentos ricos en él. Incluso teniendo en cuenta los posibles efectos amortiguadores del sedimento suspendido, la salinidad y los niveles de oxígeno en el entorno natural, se han observado efectos adversos sobre el terreno. En el entorno marino se obtuvo un valor guía global para la protección del 95% de las especies con un 50% de confianza de 0,3 mg de manganeso/l.
Al evaluar el riesgo de las emisiones antropogénicas de manganeso para el medio terrestre, se han de tener en cuenta los niveles naturales locales ("de fondo") que, a su vez, dependen de una serie de parámetros físicos y químicos. Las distintas comunidades y ecosistemas también responderían de manera diferente en función de su exposición "normal" al manganeso. Por estos motivos, no es apropiado obtener un valor guía único para el medio terrestre.
ENDNOTES:
See Also:
Toxicological Abbreviations