
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 45
First draft prepared by R. Gomes, R. Liteplo, and M.E. Meek, Health Canada, Ottawa, Canada
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2002
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Ethylene glycol : human health aspects.
(Concise international chemical assessment document ; 45)
1. Ethylene glycol - toxicity 2. Ethylene glycol - adverse effects
3. Risk assessment 4. Environmental exposure
5. No-observed-adverse-effect level I.International Programme on Chemical Safety II.Series
ISBN 92 4 153045 6 (NLM Classification: QD 305.A4)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available.
©World Health Organization 2002
Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city, or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany, provided financial support for the printing of this publication.
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
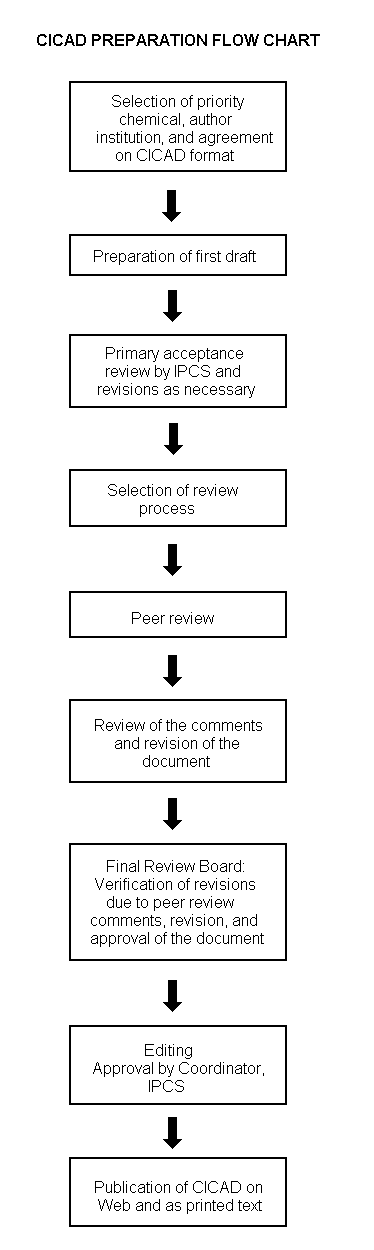
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, a priority chemical typically
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., EHC or CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is based on an existing national, regional, or international review. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science.

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on ethylene glycol (human health aspects) was prepared by the Environmental Health Directorate of Health Canada based on documentation prepared as part of the Priority Substances Program under the Canadian Environmental Protection Act (CEPA). The objective of assessments on priority substances under CEPA is to assess potential effects of indirect exposure in the general environment on human health as well as environmental effects, although only aspects related to human health are considered herein. Data identified as of the end of January 20002 were considered in this review. Information on the nature of the peer review and availability of the source documents is presented in Appendix 1. Other reviews that were also consulted include those prepared by the Environmental Criteria Assessment Office of the US Environmental Protection Agency (US EPA, 1987), the Agency for Toxic Substances and Disease Registry of the US Department of Health and Human Services (ATSDR, 1997), and the German Chemical Society (BUA, 1994), as well as reviews prepared under contract by BIBRA International (1996, 1998). Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Ottawa, Canada, on 29 October – 1 November 2001. Participants at the Final Review Board meeting are presented in Appendix 3. The International Chemical Safety Card (ICSC 0270) on ethylene glycol, produced by the International Programme on Chemical Safety (IPCS, 2000a), has also been reproduced in this document. The effects of ethylene glycol on the environment are not considered herein, since they were addressed in CICAD No. 22 (IPCS, 2000b).
Ethylene glycol (CAS No.
Ethylene glycol is used in the manufacture of polyethylene terephthalate, in natural gas processing, and as an antifreeze agent. Monitoring data upon which to base estimates of exposure of the general population to ethylene glycol are extremely limited. In a sample estimate of exposure, intakes in air and soil in the vicinity of a point source were estimated based on modelled data, and that in food was based on reported concentrations in a very limited range of foodstuffs from various countries. Dermal absorption was also estimated for a limited range of products for which data on the proportion of ethylene glycol in the products were identified.
There is convincing evidence that the toxicity of ethylene glycol is mediated principally through metabolites (notably, glycolate and oxalate). Available data also indicate that the likely pathways involved in the metabolism of ethylene glycol are qualitatively similar in humans and other mammalian species; potential quantitative differences have not been well studied.
Ethylene glycol has low acute toxicity in experimental animals following oral, inhalation, or dermal exposure. In both humans and animals, ethylene glycol has induced only minimal dermal irritation. Nasal and/or throat irritation were reported in a small number of subjects inhaling ethylene glycol, while higher concentrations produced severe irritation. In experimental animals, ethylene glycol induces only minimal conjunctival irritation, without permanent corneal damage. Data on the potential of ethylene glycol to induce sensitization have not been identified.
Ethylene glycol has not been carcinogenic in a 2-year bioassay in rats and mice, in primarily limited, early bioassays. In the limited number of identified in vitro and in vivo studies, ethylene glycol has not been genotoxic.
Available data from acute poisoning cases (humans) and repeated-dose toxicity studies (experimental animals) indicate that the kidney is a critical organ for the toxicity of ethylene glycol in both humans and experimental animals. Consistently, metabolic acidosis and degenerative non-neoplastic changes in the kidney (including dilation, degeneration, and deposition of calcium oxalate in the tubules) have been observed at lowest doses in a range of species.
Based on a rather extensive database, ethylene glycol induces developmental effects in rats and mice by all routes of exposure, although at doses greater than those associated with renal effects in male rats. Indeed, ethylene glycol is teratogenic, inducing primarily skeletal variations and external malformations, sometimes at doses less than those that are maternally toxic, with mice being more sensitive than rats. The reproductive toxicity of ethylene glycol has been extensively investigated in adequate studies in mice and rats. In repeated-dose toxicity studies, there has been no evidence of adverse impact on reproductive organs; in specialized studies, including a three-generation study in rats and continuous-breeding protocols in mice, evidence of reproductive effects has been restricted to mice (but not rats or rabbits) exposed to doses considerably greater than those associated with developmental effects in this species or renal effects in rats.
Available data are inadequate to assess potential adverse neurological or immunological effects associated with long-term exposure to ethylene glycol, although neurobehavioural and neurological disorders have been reported in cases of acute ethylene glycol poisoning in humans. In the limited number of investigations identified to date, neurological effects have not been observed at doses below those that have induced renal toxicity. Consistent treatment-related effects on immune system-related parameters have not been observed in available repeated-dose toxicity studies, in which several species have been exposed to ethylene glycol either orally or by inhalation.
A tolerable intake of 0.05 mg/kg body weight per day has been derived for this substance, based on a benchmark dose of 49 mg/kg body weight per day calculated for non-neoplastic renal effects in animals and an uncertainty factor of 1000. However, this tolerable intake is uncertain, owing primarily to lack of information on progression of renal lesions in the most sensitive animal model. Highly uncertain sample estimates of exposure of some age groups in the vicinity of a point source or of adults through absorption from some consumer products approach or exceed the tolerable intake. Additional study to better characterize progression of the renal lesions and to refine exposure estimates is recommended.
Ethylene glycol (CAS No.
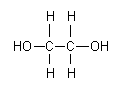
Figure 1: Chemical structure of ethylene glycol
Ethylene glycol is a clear, colourless, odourless, relatively non-volatile, viscous liquid. It has a sweet taste and imparts a warming sensation to the tongue when swallowed. Ethylene glycol has a relatively low vapour pressure (7–12 Pa at 20 °C) and a low Henry’s law constant of 5.8 × 10–6 – 6.0 × 10–3 Pa·m3/mol. It is completely miscible in water. It is very hygroscopic and will absorb up to 200% of its weight in water at 100% relative humidity. The octanol/water partition coefficient of ethylene glycol is very low (i.e., log Kow = –1.36). The conversion factors for airborne ethylene glycol at 101.3 kPa and 20 °C are 1 ppm = 2.6 mg/m3 and 1 mg/m3 = 0.39 ppm (Health Canada, 2000). Information on other physical/chemical properties of ethylene glycol is included in the International Chemical Safety Card (ICSC 0270) reproduced in this document.
The common analytical methods used for determining ethylene glycol in biological and environmental samples are presented in Table 1. The primary method is derivatization followed by gas chromatography using either a flame ionization detector or mass spectrometry for quantification. Detection limits for ethylene glycol by these methods range from sub- to low milligrams per kilogram or milligrams per litre (ATSDR, 1997). Ethylene glycol and its metabolites, such as glycolate, hippurate, and oxalate, are determined in blood and urine samples by high-performance liquid chromatography. Water samples may be analysed with preparation, but the determination of ethylene glycol in air requires adsorption onto a surface and subsequent extraction. Ethylene glycol in foods and drugs is usually analysed by chromatography of the sample after hexane is used to extract fat from the sample.
Table 1: Analytical methods for determining ethylene glycol in biological and environmental samples.a,b
|
Sample matrix |
Preparation method |
Analytical method |
Sample detection limit |
Percent recovery |
Reference |
|
Human plasma |
Deproteinization with acetic acid; vortex; centrifugation; supernatant spiked with internal standard; reaction with butylboronic acid; neutralize with NH4OH, extraction with dichloromethane; concentration |
HRGC/MS |
5 ppm (mg/litre) |
94–106 |
Giachetti et al., 1989 |
|
Human serum |
Internal standard (in acetonitrile) added to sample; centrifugation to remove protein precipitate; esterification with butylboronic acid and 2,2-dimethoxypropane; neutralization with NH4OH in acetonitrile |
HRGC/FID |
NR |
95 |
Smith, 1984 |
|
Human serum (glycolic acid) |
Extraction from salted, acidified serum using methyl ethyl ketone followed by removal of organic phase and evaporation to dryness and derivatization with PNBDI |
HPLC/UV |
0.05 mmol/litre (3 ppm, w/v); 1% RSD |
NR |
Hewlett et al., 1983 |
|
Urine |
Acidification; extraction with CHCl3; concentration; TLC |
TLC |
NR |
NR |
Riley et al., 1982 |
|
Human plasma, urine (oxalate) |
Heparinized blood deproteinated by addition of acetonitrile and phosphate buffer (pH 7), centrifugation, removal of solvent and evaporation to dryness; derivatization as for urine |
HPLC/UV |
Plasma: |
85 |
Brega et al., 1992 |
|
Urine acidified and derivatized using 1,2-diaminobenzene, adjustment of pH to 5–6, centrifugation |
Urine: |
||||
|
Kidney tissue, dog (hippurate) |
Tissue ground with acidic methanol; filtration; concentration; spot on 254-nm TLC plate |
TLC |
NR |
NR |
Riley et al., 1982 |
|
Air |
Sample collection on XAD-7 OVS tube (OVS tube contains a 13-mm glass fibre filter, followed by XAD-7 sorbent material) (NIOSH Method 5523) |
GC/FID |
0.12 mg/m3 for a 60-litre sample |
93–101 |
NIOSH, 1996 |
|
Water |
Direct injection (Method 8015b) |
GC/FID |
NR |
NR |
US EPA, 1995a |
|
Direct injection (Method 8430) |
GC/FTIR |
120 mg/litre |
NR |
US EPA, 1995b |
|
|
Food |
Addition of hot water to sample to obtain slurry; extraction with hexane; precipitation of sugars with calcium hydroxide; concentration; derivatization with BSTFA |
HRGC/FID |
10 ppm (mg/kg) |
78–107 |
Castle et al., 1988b |
|
Plastics |
Sample extraction from plastic with carbon disulfide |
GC/FID |
16.5 ng |
58–61 |
Muzeni, 1985 |
|
a |
From ATSDR (1997). |
|
b |
Abbreviations used: BSTFA = bis(trimethylsilyl)trifluoroacetamide; CHCl3 = chloroform; FID = flame ionization detector; FTIR = Fourier transform infrared spectrometry; GC = gas chromatography; HPLC = high-performance liquid chromatography; HRGC = high-resolution gas chromatography; MS = mass spectrometry; NH4OH = ammonium hydroxide; NR = not reported; PNBDI = o,p-nitrobenzyl-N,N’-diisopropylisourea; RSD = relative standard deviation; TLC = thin-layer chromatography; UV = ultraviolet detector; w/v = weight:volume. |
Limited information on production and use from the source country of the national assessment on which this CICAD is based (i.e., Canada) is presented here, principally to set context for the sample risk characterization. Additional information on sources, production, and use is presented in CICAD No. 22: Ethylene glycol: Environmental aspects (IPCS, 2000b).
Ethylene glycol was identified as one of the substances in the edible fungus Tricholoma matsutake (Ahn & Lee, 1986) and has been identified as a metabolite of ethylene, a natural plant growth regulator (Blomstrom & Beyer, 1980).
Based on a review by CIS (1997), total worldwide capacity for ethylene glycol is over 10 000 kilotonnes per year, with major increases expected in the future. World-wide, ethylene glycol is used primarily in the production of polyesters for fibre and polyethylene terephthalate (PETE). It is also used in smaller quantities in a broad range of products, including paints, lacquers and resins, coolants and heat transfer fluids, and medicinals and adhesives (ATSDR, 1993; Lewis, 1993).
Available data indicate that projected annual production capacity for the ethylene glycols (mono-, di-, and triethylene glycols) in Canada increased to 907 kilotonnes in 1999 from 524 kilotonnes in 1992 (CIS, 1997). In 1996, approximately 810 kilotonnes of ethylene glycols (mono-, di-, and tri-) were exported from Canada. Import volumes in 1996 were estimated at 31.3 kilotonnes (CIS, 1997).
In Canada, the majority of ethylene glycol is used in antifreeze formulations (primarily for automotive vehicle engines, but also for aircraft deicing), at 66% (105 kilotonnes) of domestic consumption (CIS, 1997). An estimated 7.7 kilotonnes of ethylene glycol were used in 1996 for aircraft deicing/anti-icing (Environment Canada, 1997). The amount used for the production of the polyester PETE in 1996 was relatively small, at 25 kilotonnes (15.7% of domestic consumption). Six per cent or 9.5 kilotonnes were used in natural gas processing to assist in the removal of water and to prevent ice formation. The remaining 19.5 kilotonnes were used in the production of solvents, including use as a component in latex paint formulations to guard against paint freezing and as an antifreeze liquid injected into hoses used to pump liquid explosives (CIS, 1997). In 1995 and 1996, 1.4 kilotonnes and 2.0 kilotonnes were used in the Canadian paints and coatings sector, respectively (Environment Canada, 1997).
Data on environmental levels other than those directly linked to human exposure have been addressed in the source documents and CICAD No. 22 (IPCS, 2000b). Data on concentrations in the environment primarily from the source country of the national assessment on which this CICAD is based (i.e., Canada) are presented here, as a basis for the sample risk characterization for human health.
Only those media for which relevant data have been identified are addressed below. Information on concentrations in indoor air and drinking-water were not identified.
Based on ChemCAN 4.0 modelling and attribution of the reported largest emission to the atmosphere in Canada in 1996 (374 tonnes from ethylene glycol manufacturing plants in Alberta) to one plant, the predicted average airborne concentration in the prairie region of Alberta from this emission would be 1.2 ng/m3. Predicted maximum daily average ground-level concentrations downwind from the plant, responsible for approximately 99% of all releases from manufacturing, were 100, 50, and 25 µg/m3 at distances of 1.8, 4.0, and 6.8 km, respectively, from the property boundary, although annual frequency of occurrence was not presented (Environment Canada, 1997).
Percy (1992) reported concentrations of ethylene glycol in air of 3.2 and 4.1 mg/m3 at the Thunder Bay, Ontario, airport. In Louisiana, USA, during bridge deicing operations, total airborne concentrations were between <0.05 and 10.57 mg/m3; aerosol concentrations were lower (<0.05–0.33 mg/m3) (Abdelghani et al., 1990). Proximity of measurements to source of release and the time period represented by individual measurements were not reported for either of these studies.
The presence of ethylene glycol has been demonstrated in only a small number of foodstuffs. In Italy, ethylene glycol was detected in all 44 samples of wine analysed by gas chromatography–mass spectrometry. The average and maximum concentrations were 2.8 and 6.25 mg/litre, respectively (Gaetano & Matta, 1987). However, the source of ethylene glycol in wines is not known (Gaetano & Matta, 1987; Kaiser & Rieder, 1987). In Japan, ethylene glycol was present in the headspace volatiles of roasted sesame seeds, but quantitative data were not provided (Takei, 1988).
Foods that have been disinfected or preserved with ethylene oxide may contain residual ethylene glycol. In France, Buquet & Manchon (1970) sampled 150 bread loaves preserved with carbonic anhydride and ethylene oxide and packaged in airtight plastic bags. The initial concentrations of ethylene glycol in bread ranged from not detected (detection limit not reported) to 92.2 mg/kg but quickly subsided. In France, Chaigneau & Muraz (1993) sampled 16 spices that had been disinfected using ethylene oxide. Concentrations of ethylene glycol were not reported, and the authors indicated that residual ethylene glycol was rapidly lost.
The potential for ethylene glycol to migrate into beverages contained in PETE bottles and into foods packaged in regenerated cellulose film (RCF) has been demonstrated, resulting from small amounts of unreacted ethylene glycol in such products (Kashtock & Breder, 1980; Castle et al., 1988a; Kim et al., 1990). Kashtock & Breder (1980) measured the migration of ethylene glycol at 32 °C from PETE bottles into 3% acetic acid (intended to simulate carbonated beverages). Time-dependent increases in average concentrations were measured, resulting in a maximum concentration of 104 µg/litre after 6 months’ storage at this elevated temperature.
RCF is widely used as a food packaging material, since its permeability, sealability, and ease of application for twist wrapping are desirable for packing certain foods. In the United Kingdom, Castle et al. (1988a) measured the ethylene glycol content of several foodstuffs wrapped in RCF at random intervals up to the end of their usual maximum shelf-lives. Four samples of boiled sweets contained ethylene glycol at concentrations ranging from 14 to 34 mg/kg. Three of four samples of toffee contained ethylene glycol, with a maximum concentration of 22 mg/kg. Two of four samples of Madeira cake contained ethylene glycol, with a maximum concentration of 22 mg/kg. All four samples of fruit cake contained ethylene glycol, with a maximum concentration of 34 mg/kg. Ethylene glycol was not detected in any of the six samples of meat pie, with a limit of detection of 10 mg/kg.
Several products used in the operation or maintenance of automobiles typically contain ethylene glycol. Concentrations ranging up to 85% may have been present in older automotive brake fluids (US EPA, 1986); however, the ethylene glycol content of current brake fluids is less than 0.1% (ATSDR, 1997). Antifreeze solutions in automobile coolant systems typically have an ethylene glycol content of 50% (Franklin Associates Ltd., 1995). Windshield washer fluids intended for use during winter may contain ethylene glycol at up to 14% by weight (Flick, 1986, 1989). The ethylene glycol content of automobile wax and polish can range up to 3% by weight (US EPA, 1986).
Flick (1986) reported concentrations of ethylene glycol ranging from 1.1% to 1.4% in four types of floor polish intended for use in the home. Concentrations ranging up to 3.5% may be present in floor wax and polishes, according to US EPA (1986).
Ethylene glycol may be present as a slow-evaporating solvent or freeze-thaw stabilizer in latex paints (US EPA, 1986). Chang et al. (1997) estimated that latex paints comprised over 85% of the interior coatings used in the USA in 1992 and reported concentrations of ethylene glycol ranging from 23.3 to 25.8 mg/g (from 2.3% to 2.6% by weight) in four samples of medium-priced paints. Eleven Canadian paint and coatings companies reported that their products may contain up to 5% of ethylene glycol by weight (Environment Canada, 1997).
Flick (1986) also reported that other consumer products that may contain ethylene glycol include tub and tile cleaners (3% by weight) and cement sealer (2.2% by weight). Confirmation of ethylene glycol’s use as a tub and tile cleaner in Canada is currently being sought under CEPA.
Ophthalmic solutions (eyedrops) that have been treated with ethylene oxide as a sterilant may contain ethylene glycol and ethylene chlorohydrin as residues. In the USA, Manius (1979) detected ethylene glycol in 4 of 15 samples of ophthalmic solution, with a range of 10–28 mg/litre (detection limit 6 mg/litre).
The only cosmetic registered for use in Canada that lists ethylene glycol as an ingredient is a solid stick foundation, distributed from Quebec. The concentration of ethylene glycol in this product was not available (C. Denman, personal communication, 1999).
Data on levels of ethylene glycol in environmental media in Canada to serve as a basis for development of estimates of population exposure were identified only for areas near industrial point sources in Alberta. These data are limited to a few predicted concentrations in ambient air at ground level and to measured concentrations in soil. No data were identified concerning the presence or concentrations of ethylene glycol in drinking-water in Canada or elsewhere.
Very meaningful deterministic estimates of average exposure for the general population are precluded due to the limitations of the available data. Worst-case intakes have been estimated for populations near industrial point sources of ethylene glycol, although the limitations of the available data, which serve as the basis for these upper-bound estimates, must be borne in mind in their interpretation. On this basis, intake is estimated to range from 22 to 88 µg/kg body weight per day, as summarized in Table 2.
Table 2: Deterministic estimates of worst-case daily intakes of ethylene glycol for a highly exposed
population in the immediate vicinity of an industrial point source.
|
Route of exposure |
Intakes of ethylene glycol for various age groups in the exposed population (µg/kg body weight per day) |
|||||
|
0–6 monthsa |
7 months – 4 yearsb |
5–11 yearsc |
12–19 yearsd |
20–59 yearse |
60+ yearsf |
|
|
Inhalationg |
28 |
60 |
47 |
27 |
23 |
20 |
|
Ingestion of soilh |
17 |
28 |
9 |
2 |
2 |
2 |
|
Total daily intake |
45 |
88 |
56 |
29 |
25 |
22 |
|
a |
Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, and to ingest 30 mg of soil per day (EHD, 1998). |
|
b |
Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, and to ingest 100 mg of soil per day (EHD, 1998). |
|
c |
Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, and to ingest 65 mg of soil per day (EHD, 1998). |
|
d |
Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, and to ingest 30 mg of soil per day (EHD, 1998). |
|
e |
Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, and to ingest 30 mg of soil per day (EHD, 1998). |
|
f |
Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, and to ingest 30 mg of soil per day (EHD, 1998). |
|
g |
Based on the maximum daily average concentration (100 µg/m3) predicted in ambient air at ground level at a distance of 1.8 km from the facility perimeter of an industrial point source of discharge to the atmosphere (Environment Canada, 1997). The same concentration is assumed for indoor air. |
|
h |
Based on the maximum reported concentration (4290 mg/kg) in soil near an industrial point source of discharge (G. Dinwoodie, personal communication, 1996). |
Data on levels of ethylene glycol in food are limited to results of two earlier studies of migration from RCF and PETE bottles in other countries and to reported concentrations in Italian wines. Worst-case intakes from ingestion of foods assumed to be contaminated with ethylene glycol through contact with food packaging materials have been estimated for the general population. On this basis, intake is estimated to range from less than 2.5 to 41.0 µg/kg body weight per day, as summarized in Table 3. Migration to food from RCF accounts for most of the estimated intakes.
Table 3: Deterministic estimates of reasonable worst-case daily intakes of ethylene glycol from ingestion of foods.
|
Food item |
Intakes of ethylene glycol for various age groups in the general population (µg/kg body weight per day) |
|||||
|
0–6 monthsa |
7 months – 4 yearsb |
5–11 yearsc |
12–19 yearsd |
20–59 yearse |
60+ yearsf |
|
|
Cakeg |
0.3 |
19.4 |
27.8 |
23.6 |
10.7 |
7.9 |
|
Pie, otherh |
1.0 |
2.4 |
3.3 |
1.8 |
1.7 |
1.6 |
|
Candy, otheri |
1.1 |
11.8 |
9.2 |
5.9 |
2.5 |
1.7 |
|
Soft drinksj |
<0.1 |
0.7 |
0.6 |
0.4 |
0.2 |
0.1 |
|
Winek |
– |
<0.1 |
0.1 |
0.2 |
1.6 |
1.0 |
|
Total daily intakel |
<2.5 |
<34.4 |
41.0 |
31.9 |
16.7 |
12.3 |
|
a |
Assumed to weigh 7.5 kg and to consume food items at average daily rates indicated in EHD (1998). |
|
b |
Assumed to weigh 15.5 kg and to consume food items at average daily rates indicated in EHD (1998). |
|
c |
Assumed to weigh 31.0 kg and to consume food items at average daily rates indicated in EHD (1998). |
|
d |
Assumed to weigh 59.4 kg and to consume food items at average daily rates indicated in EHD (1998). |
|
e |
Assumed to weigh 70.9 kg and to consume food items at average daily rates indicated in EHD (1998). |
|
f |
Assumed to weigh 72.0 kg and to consume food items at average daily rates indicated in EHD (1998). |
|
g |
Assumed to contain ethylene glycol due to contact with RCF. Based on a maximum reported concentration of 34 mg/kg in fruit cake in the United Kingdom (Castle et al., 1988a). |
|
h |
Assumed to contain ethylene glycol due to contact with RCF. Based on the limit of detection (10 mg/kg) for analysis of meat pies in the United Kingdom (Castle et al., 1988a). |
|
i |
Assumed to contain ethylene glycol due to contact with RCF. Based on a maximum reported concentration of 34 mg/kg in boiled sweets in the United Kingdom (Castle et al., 1988a). |
|
j |
Assumed to contain ethylene glycol due to migration from PETE bottles. Based on a maximum reported concentration of 0.104 mg/litre in 3% acetic acid (used to simulate carbonated beverages) following storage for 6 months at 32 °C (Kashtock & Breder, 1980). |
|
k |
Based on the maximum reported concentration (6.25 mg/litre) of ethylene glycol in wine in Italy (Gaetano & Matta, 1987). |
|
l |
It is assumed that there are no daily intakes of ethylene glycol from the remaining 176 food items for which daily rates of consumption are available in EHD (1998), since no data are available concerning concentrations of ethylene glycol in these food items. |
The general population is also exposed periodically to ethylene glycol through the use of several consumer products, including, for example, automobile antifreeze, wax, polish, and windshield washer solution, floor wax and polish, possibly tub and tile cleaner, and latex paint. Estimation of exposure from such products is incomplete, due to the lack of adequate data on the proportions that include ethylene glycol as an ingredient and on the concentrations in the various products available. Although automobile coolant (i.e., antifreeze) and winter windshield washer fluid are expected to contain the highest concentrations of ethylene glycol, human exposure to these products is expected to be infrequent and of short duration for a small number of individuals and negligible for the majority of the general population. Also, while some inhalation exposure is expected during use of the products mentioned above, intakes by inhalation were not estimated, since the physicochemical properties of ethylene glycol limit its rate of evaporation from liquid products and the application of these products does not generally involve formation of aerosols.
Intakes by dermal absorption have been estimated for adults using these consumer products (Health Canada, 2000). Generic estimates of the maximum concentrations of ethylene glycol in these products are assumed, as data from analyses of specific products in Canada are not available for this purpose. Based on the worst-case assumptions of 100% dermal absorption of ethylene glycol from thin films of liquid products containing the maximum expected concentrations of ethylene glycol in standardized scenarios using estimates of frequencies of use and areas of exposed skin, the upper-bounding estimates of daily intakes by adults range from 0.09 to 236 µg/kg body weight per day for the four product types for which generic estimates of ethylene glycol content are available. This information is summarized in Table 4.
Table 4: Deterministic estimates of upper-bounding daily intakes for adults by dermal absorption
from consumer products.a
|
Consumer product |
Maximum concentration of ethylene glycol in product |
Event description |
Event frequency |
Exposed skin area (cm2)b |
Estimated maximum average daily intakec (µg/kg body weight per day) |
|
Latex paint |
5% |
Roller application to walls and ceiling of an average-sized roomd |
1.4g |
220 |
7.2 |
|
Floor polish/ wax |
3.5% |
Manual application of undiluted product to an average-sized floor with a rag or spongee |
4h |
400 |
4.6 |
|
Auto polish/ wax |
0.03% |
Manual application to an automobile with a sponge-like foam padf |
6i |
400 |
0.09 |
|
Tub and tile cleaner |
3% |
Manual application to bathroom sink and bathtube |
156j |
400 |
180.8 |
|
Manual application to tiled wall in bathroom or elsewheree |
48j |
400 |
55.6 |
||
|
Total estimated intake from tub and tile cleaner |
236.4 |
||||
|
a |
These estimates are based on Standard scenarios for estimating exposure to chemical substances during use of consumer products (US EPA, 1986). It is assumed that thin films of liquid products form on the exposed skin surface and that complete dermal absorption of ethylene glycol present in the thin films occurs. |
|
b |
Estimates of areas of exposed skin are from US EPA (1986). An area of 220 cm2 is approximately 10% of the surface area of the face, hands, and forearms. An area of 400 cm2 is approximately the combined area of the palms and outstretched fingers of two adult hands. |
|
c |
Reasonable worst-case daily intakes are based on the assumption of complete dermal absorption of ethylene glycol present in thin films contacting the skin. Minimum average daily intakes based on penetration rates that are proportional to the ethylene glycol content of the products are several orders of magnitude less for each of these products (Health Canada, 2000). |
|
d |
Assuming a resulting film thickness on the hands of 0.0098 cm (US EPA, 1986). |
|
e |
Assuming a resulting film thickness on the hands of 0.0021 cm (US EPA, 1986). |
|
f |
Assuming a resulting film thickness on the hands of 0.0032 cm (US EPA, 1986). |
|
g |
US EPA (1986) indicates that seven events per year is the 95th percentile number of rooms painted of the 20% of respondents painting during the survey year. This value pertains to the year in which the activity is performed. If it is assumed that each room is painted every 5 years (US EPA, 1986), the event frequency is 7 per year. |
|
h |
It is not indicated whether this is a mid-point or upper-percentile estimate of event frequency (US EPA, 1986). |
|
i |
This is a conservative estimate, based on the assumption that 5.4% of the US population used automotive wax 6 or more times per year (US EPA, 1986). |
|
j |
Based on average event frequencies from US EPA (1997). |
The highest estimates of daily intake by adults due to dermal absorption of ethylene glycol from consumer products result from the standardized scenarios involving the use of tub and tile cleaner. Although mid-point estimates of event frequencies are assumed (i.e., 156 and 48 events per year), these frequencies are considerably higher than the conservative estimates of event frequencies assumed in the scenarios involving the three other consumer product types in Table 4. Therefore, daily intakes are higher for the tub and tile cleaner, even though higher concentrations of ethylene glycol may be present in the other product types.
It should be noted that these values represent worst-case estimates. Estimated daily intakes by dermal absorption of ethylene glycol from use of these consumer products are several orders of magnitude less when based on less conservative assumptions. These include the assumptions that dermal absorption is proportional to the concentration of ethylene glycol in the products and that steady-state penetration occurs for periods equivalent to the average durations of product uses in the standardized scenarios (Health Canada, 2000). However, available data on permeability through human skin are inadequate as a basis for confident estimation of exposure, and, as a result, these estimates are not presented here. This is due to lack of evidence of adequate viability of the skin in the most comprehensive investigation conducted to date, i.e., that by Sun et al. (1995), which may have been compromised due to use of samples of full thickness and lack of identified data on uptake from product formulations (R. Moody, personal communication, 1999; Health Canada, 2000).
A proportion of the population is also exposed to ethylene glycol as passengers during deicing operations for aircraft. While the pattern of exposure of individuals in the population is expected to be highly variable, depending upon frequency of aeroplane travel during the winter season, identified data are inadequate to quantitatively estimate intake from this source.
Based on the US National Occupational Exposure Survey conducted by the National Institute of Occupational Safety and Health (NIOSH, 1990) during 1981–1983, an estimated 1.5 million workers are potentially exposed to ethylene glycol each year. Contact with the skin and eyes is the most likely route of worker exposure to ethylene glycol.
Workers with greatest potential for exposure to ethylene glycol are those in industries involved in the manufacture or use of products containing high concentrations (e.g., antifreeze, coolants, deicing fluids, brake fluids, solvents), particularly in operations involving heating or spraying of these materials (e.g., aircraft deicing). In these cases, inhalation may be an important route of human exposure (Rowe & Wolf, 1982). Air samples taken from the breathing zones of workers applying deicing fluids (50% ethylene glycol) to bridge surfaces contained the compound at concentrations of <0.05–2.33 mg/m3 as aerosols and <0.05–3.37 mg/m3 as vapours (LDOTD, 1990). Trace quantities of ethylene glycol have been detected in artificial theatrical smoke used in plays, concerts, and amusement parks (NIOSH, 1994).
As a small molecular weight alcohol, ethylene glycol readily passes through biological membranes and will be effectively absorbed from the gastrointestinal tract and via inhalation exposure. It is rapidly distributed in body water (Jacobsen et al., 1988).
Based on studies involving oral administration of single doses of ethylene glycol by gavage, absorption is rapid and nearly complete, with peak plasma concentrations increasing linearly with dose among various species (i.e., rats, mice, monkeys) (Carney, 1994). Following the oral administration of 10–1000 mg ethylene glycol/kg body weight per day to Sprague-Dawley rats (both sexes) and CD-1 mice (females), peak plasma concentrations in each species were observed within 1–4 h, with absorption of 90–100% of the administered dose within 24 h (Frantz et al., 1996a,b). Ethylene glycol is rapidly cleared from the blood following absorption into the systemic circulation, with reported plasma half-lives in rodents, monkeys, and dogs (receiving 1–1000 mg/kg body weight) ranging from 1 to 4 h (McChesney et al., 1971; Hewlett et al, 1989; Frantz et al., 1996b).
Following the inhalation exposure (nose only) of F344 rats (n = 15 per sex) to [14C]ethylene glycol vapour (32 mg/m3) for 30 min or [14C]ethylene glycol aerosol (184 mg/m3) for 17 min, approximately 60% of the administered radioactivity was absorbed into the systemic circulation (Marshall & Cheng, 1983). Peak plasma levels were observed within 1 h, with a half-life for plasma clearance of 34–39 h (Marshall & Cheng, 1983).
The results of available studies also indicate that, compared with oral exposure, ethylene glycol was absorbed more slowly and to a lesser extent into the systemic circulation following dermal contact. Unlike the extensive bioavailability of ethylene glycol administered orally, the bioavailability of (unchanged) ethylene glycol within the first 6 h following dermal exposure was only 20–30% and 5% in rats and mice, respectively, administered a dose of 1000 mg/kg body weight (Frantz et al., 1996a,b).
Ethylene glycol is oxidized in experimental animals and in humans in successive steps, first to glycoaldehyde (in a reaction catalysed by alcohol dehydrogenase), then to glycolic acid, glyoxylic acid, and oxalic acid (Figure 2). Glyoxylic acid is metabolized in intermediary metabolism to malate, formate, and glycine. Ethylene glycol, glycolic acid, calcium oxalate, and glycine (and its conjugate, hippurate) are excreted in urine. The metabolites of ethylene glycol that have been typically detected are carbon dioxide, glycolic acid, and oxalic acid.
In an early (limited) investigation conducted in one male subject orally administered 8, 10, or 12 ml of ethylene glycol, the serum half-life for this compound was 4.5 h, with 25% of the administered dose (not specified) eliminated in the urine as the parent compound and 2.3% eliminated as oxalic acid (Reif, 1950). Other reported values for the half-life of ethylene glycol in serum following acute ingestion have ranged from 2.5 h (in children) to 8.4 h (in adults) (ATSDR, 1997). In one study of four individuals presenting with acute ethylene glycol poisoning, the half-life for the elimination of glycolic acid from the blood (without haemodialysis) was approximately 10 h (Moreau et al., 1998).
Quantitative information on the absorption of inhaled ethylene glycol by humans was not identified. In a clinical study reported by Wills et al. (1974), in which male volunteers were exposed continuously (via inhalation) to mean daily (20–22 h/day) concentrations of 17–49 mg/m3 (range 0.8–66.8 mg/m3) aerosol for 30 days, ethylene glycol was considered to be poorly absorbed from the respiratory tract, based on comparison of haematological and clinical chemistry parameters and the similar levels of ethylene glycol measured in the blood and urine of exposed (n = 20) and unexposed subjects; the level of ethylene glycol metabolites was not determined.
Reliable quantitative information on the absorption of ethylene glycol following dermal exposure in vivo in humans or in vitro in human skin has not been identified. However, this compound is likely to be absorbed through the skin, based upon its physicochemical properties (Fiserova-Bergerova et al., 1990) and the results of in vitro permeability studies conducted with samples of human skin (Loden, 1986; Driver et al., 1993; Sun et al., 1995).3
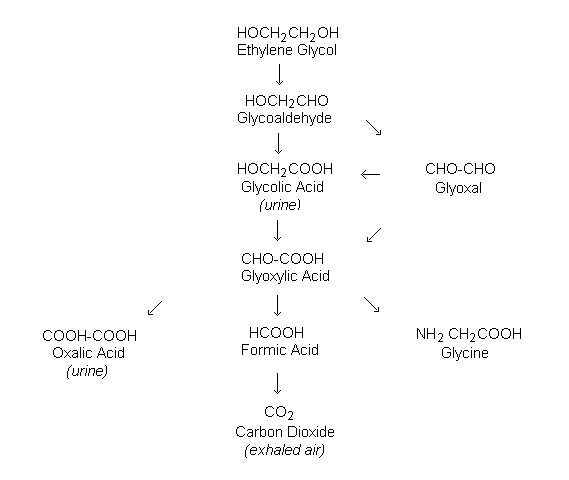
Figure 2: Metabolism of ethylene glycol
Ethylene glycol has low acute toxicity via oral, inhalation, or dermal exposure. LD50s for the oral administration of ethylene glycol in rats range from 4000 to 10 020 mg/kg body weight, while reported values in guinea-pigs and mice are 6610 mg/kg body weight and 5500–8350 mg/kg body weight, respectively. The minimum lethal oral dose in rats is 3.8 g/kg body weight (Clark et al., 1979). Oral LD50s of 5500 and 1650 mg ethylene glycol/kg body weight have also been reported in dogs and cats, respectively. A dermal LD50 of 10 600 mg/kg body weight has been reported for rabbits. In rats and mice, the lethal concentration following 2-h inhalation exposure has been reported to be >200 mg/m3.
Signs of acute ingestion of ethylene glycol are dose dependent and include central nervous system depression, paralysis, ataxia, respiratory arrest, tachycardia, tachypnoea, coma, and death (BUA, 1994). Based on numerous case-studies of accidental acute ingestion of ethylene glycol or ethylene glycol-containing antifreeze in domestic animals (i.e., dogs and cats), metabolic acidosis has been consistently observed; morphologically, congestion and haemorrhage in the lungs, haemorrhage in the stomach, degeneration in the renal tubules, focal necrosis in the liver, and calcium oxalate in the kidneys and brain have been reported (DFG, 1991; BUA, 1994; Health Canada, 2000). Histopathological lesions observed in the kidney included mild tubular nephrosis, necrosis, sloughing of cells, vacuolation, and deposition of oxalate crystals in the cortex and medulla. Electron microscopic myocardial changes have also been noted in rats following acute oral administration (by gavage) of ethylene glycol (7.2 g/kg body weight), including mitochondrial swelling, myofibrillar oedema and necrosis, and enlargement of the smooth endoplasmic reticulum (Bielnik & Szram, 1992; Bielnik et al., 1992).
Studies were not identified concerning the potential of ethylene glycol to induce sensitization in experimental animals. Ethylene glycol induces mild dermal irritation in rabbits and guinea-pigs (Clark et al., 1979; Guillot et al., 1982; Anderson et al., 1986). Single or short-term ocular exposure to ethylene glycol (liquid or vapour) produces minimal conjunctival irritation without permanent corneal damage in rabbits (McDonald et al., 1972; Clark et al., 1979; Guillot et al., 1982; Grant & Schuman, 1993).
The results of short-term studies (in agreement with longer-term studies) confirm that the kidney is the principal target organ following oral exposure to ethylene glycol. In a 10-day study in which a wide range of end-points was examined in Sprague-Dawley rats administered drinking-water containing 0.5–4.0% ethylene glycol (650–5300 mg/kg body weight per day in males; 800–7300 mg/kg body weight per day in females), significant alterations in serum chemistry parameters were observed at all doses in males and at ł 1500 mg/kg body weight per day in females (Robinson et al., 1990). The incidence and severity of histopathological lesions in the kidney were significantly increased in males at >2600 mg/kg body weight per day and in females at 7300 mg/kg body weight per day.
In a 4-week study in Wistar rats administered (by gavage) 2000 mg ethylene glycol/kg body weight per day, effects in the kidney (including discoloration, tubulopathy, hyperplasia, and crystalline deposits), changes in urinary parameters, and increased relative kidney weights (10–14%) were observed in both sexes (Schladt et al., 1998).
In studies in which groups (n = 5–10 per sex) of B6C3F1 mice were administered (by gavage) 50, 100, or 250 mg ethylene glycol/kg body weight per day (in water) for 4 days, no clear treatment-related effects on survival, relative organ weights, haematology, or histopathology in major organs (including the liver, kidney, lung, and bone marrow) were observed. However, exposure produced depression of progenitor cells at all doses, hypocellularity at >100 mg ethylene glycol/kg body weight per day, and decreased erythropoiesis at 250 mg/kg body weight per day in the bone marrow (Hong et al., 1988). As effects on haematological parameters in blood were not observed, the biological significance of these effects is unclear.
In a study for which reporting was limited, male macaque monkeys exposed to 0.25–10% (1–152 g/kg body weight) ethylene glycol in drinking-water for 6–157 days had dose-related renal effects at >17 g ethylene glycol/kg body weight (Roberts & Seibold, 1969). No significant renal histopathology was observed among females receiving up to 152 g ethylene glycol/kg body weight.
In a 90-day study in which Sprague-Dawley rats ingested drinking-water containing 0.25–2.0% ethylene glycol (205–3130 mg/kg body weight per day in males; 600–5750 mg/kg body weight per day in females), alterations in haematological parameters in females (P < 0.05) were observed at lowest doses (Robinson et al., 1990). There were increased relative kidney weights in males at >950 mg/kg body weight per day, decreased body weights in males at 3130 mg/kg body weight per day, and dose-related histopathological changes (tubular dilation and degeneration, intratubular crystals) in the kidney in males (>950 mg/kg body weight per day) and females (>3100 mg/kg body weight per day).
In a well conducted study in which Fischer 344 rats (both sexes) were administered 165, 325, 640, 1300, or 2600 mg ethylene glycol/kg body weight per day in the diet for 13 weeks, significant effects were observed at 1300 mg/kg body weight per day and above, including reduced growth in males, increased kidney weight in both sexes, renal histopathology (dilation, necrosis, fibrosis, and crystal deposition in renal tubules) in males, and alterations in serum clinical chemistry parameters in males (Melnick, 1984). At 2600 mg/kg body weight per day, in males, mortality was increased and relative thymus weights were decreased, and in females, microscopic changes in the kidney (infiltration of inflammatory cells, increased vacuolation, and enlarged nuclei in renal tubules) were increased.
In an unpublished study by Gaunt et al. (1974), in which a wide range of end-points was examined in Wistar rats (n = 25 per sex per dose) administered ethylene glycol in the diet (males: 35, 71, 180, or 715 mg/kg body weight per day; females: 38, 85, 185, or 1128 mg/kg body weight per day) for up to 16 weeks, statistically significant specific microscopic changes in the kidney (i.e., dilation, degeneration, protein casts, deposition of calcium oxalate crystals in nephrons) were observed at the highest dose. Among male rats, histopathological changes were observed in the two highest dose groups (see Table 5).4 With one exception, severe tubular damage was observed in all male rats having oxalate crystals in the kidney. An increased incidence of kidney damage, although not statistically significant, was observed among females receiving the highest dose, 1128 mg ethylene glycol/kg body weight per day. The occurrence of inflammation of the Harderian gland in the exposed males, "pneumonial changes" in the lungs of males and females, and salivary adenitis was not considered related to exposure to ethylene glycol. [No-observed-adverse-effect level (NOAEL) = 71 mg/kg body weight per day (males); lowest-observed-adverse-effect level (LOAEL) = 180 mg/kg body weight per day (males)]
Table 5: Effect of 16-week dietary exposure to ethylene glycol in Wistar rats.a
|
Species |
Protocol |
Results |
Effect level |
|
Rat |
Animals (weanling rats housed five per cage) received 0, 0.05%, 0.1%, 0.25%, or 1.0% ethylene glycol in the diet for up to 16 weeks (reported intake was 0, 35, 71, 180, or 715 mg/kg body weight per day in males; 0, 38, 85, 185, or 1128 mg/kg body weight per day in females). The concentration of ethylene glycol in the diet was constant and was not adjusted to food consumption. The basic food was Spratts Laboratory Diet #1, and water was given ad libitum. Animals were weighed prior to treatment, on days 1, 2, 3, and 7, then twice weekly to 21 days, followed by once weekly to week 14. Food and water intakes were measured during the 24-h periods before rats were weighed. Individual data were reported for body weights, while pooled data (n = 5 per cage) were reported for food consumption. Groups of five rats of each sex were killed after 2 or 6 weeks. Haematology (haemoglobin, haematocrit, erythrocytes, leukocytes, reticulocytes), serum clinical chemistry (urea, glucose, protein, albumin, glutamic–oxaloacetic and glutamic–pyruvic transaminases, lactic dehydrogenase), and urine were assessed at 16 weeks. Samples of urine collected during the treatment period were examined for oxalic acid. Renal function was assessed during weeks 2–16 (although results were not reported). Major organs were weighed (brain, heart, liver, spleen, kidneys, stomach, intestines, adrenal, gonads, pituitary, thyroid), and microscopic examination was conducted in selected tissues (lung, salivary gland, liver, heart, lymph nodes, Harderian glands, thyroid, testes, bladder, kidney) in all groups. |
Animals exposed to ethylene glycol had no treatment-related effects on survival or body weight gain and no overt signs of toxicity. Compared with controls, no consistent dose-related changes in absolute or relative organ weights were observed among animals exposed to ethylene glycol. Haematological and serum chemistry analysis revealed no treatment-related effects. The 24-h urinary elimination of oxalic acid was increased (P < 0.01 or P < 0.05) in both sexes at the highest dose, with the magnitude of the effect appearing markedly greater in males than in females. The only increase in oxalic acid elimination at lower doses occurred in males at 180 mg ethylene glycol/kg body weight per day for 6 weeks. Microscopic examination revealed treatment-related effects in the kidney. Among male rats receiving 0, 35, 71, 180, or 715 mg ethylene glycol/kg body weight per day for 16 weeks, the incidence of specific histological changes within the kidney was 0/15, 0/15, 0/15, 1/15, and 0/15 (individual nephrons with dilated tubules and protein casts); 0/15, 1/15, 1/15, 2/15, and 5/15 (P < 0.05) (individual nephrons with degenerative changes); 0/15, 0/15, 0/15, 1/15, and 4/15 (P < 0.05) (individual nephrons with degenerative changes and occasional oxalate crystal); 0/15, 0/15, 0/15, 0/15, and 2/15 (several nephrons with degenerative changes and frequent crystals); and 0/15, 0/15, 0/15, 0/15, and 4/15 (P < 0.05) (generalized tubular damage and heavy crystals), respectively. The overall incidence of male rats with tubular damage in groups receiving 0, 35, 71, 180, or 715 mg ethylene glycol/kg body weight per day in the diet for 16 weeks was 0/15, 1/15, 1/15, 4/15 (P < 0.05), and 15/15 (P < 0.001), respectively. Females had an increased incidence of kidney damage at the highest dose, although not statistically significant. With one exception, severe tubular damage was observed in all male rats having oxalate crystals in the kidney. Histopathological analyses of the kidneys in small (n = 5) groups of animals exposed for 2 or 6 weeks revealed no statistically significant increase in the incidence of specific histological changes, although the overall incidence of animals with tubular damage was significantly elevated (P < 0.01) in the high-dose group after 6 weeks’ exposure. The occurrence of inflammation of the Harderian gland in the exposed males, "pneumonial changes" in the lungs of males and females, and salivary adenitis was not considered related to exposure to ethylene glycol. |
NOAEL (males): 71 mg/kg body weight per day |
a From Gaunt et al. (1974); P.G. Brantom, personal communication (2000).
Histopathological analyses of the kidneys in small (n = 5) groups of animals terminated at interim sacrifice at 2 or 6 weeks revealed no statistically significant increase in the incidence of specific histological changes, although the overall incidence of animals with tubular damage was significantly elevated in the high-dose group after 6 weeks’ exposure (Gaunt et al., 1974).
In B6C3F1 mice receiving 400–6700 mg ethylene glycol/kg body weight per day in the diet for 13 weeks, treatment-related effects were limited to hyaline degeneration in the liver and minimal to mild tubule dilation, cytoplasmic vacuolation, and regenerative hyperplasia in the kidney in males at >3300 mg/kg body weight per day. There was no evidence of deposition of oxalate crystals in renal tubules. No effects on body or organ weights, clinical chemistry, haematology and urinary parameters, gross pathology, or histopathology in a wide range of organs were observed in females (Melnick, 1984; NTP, 1993).
Identified data on effects following inhalation are restricted to a few early, limited, short- and medium-term studies; in none of these was intake of ethylene glycol resulting from ingestion or via dermal absorption (i.e., see Tyl et al., 1995a,b) assessed. No treatment-related effects on survival, behaviour, physical appearance, locomotor activity, haematology, clinical chemistry parameters, or histopathology in selected organs (including lung, kidney, and liver) were observed in rats, guinea-pigs, rabbits, dogs, or monkeys exposed (whole body) to 10 or 57 mg ethylene glycol vapour/m3 for 8 h/day, 5 days/week, for 6 weeks (Coon et al., 1970). In a review by Browning (1965), it was reported that exposure of rats to 500 mg/m3 for 28 h over 5 days resulted in slight narcosis.
No clear treatment-related effects on histopathology in lung, liver, or kidney, on haematology, or on clinical chemistry parameters were observed in rats (n = 15), guinea-pigs (n = 15), rabbits (n = 3), dogs (n = 2), or monkeys (n = 3) exposed continuously to 12 mg ethylene glycol vapour/m3 for 90 days (Coon et al., 1970). Although mortality was observed among rabbits (n = 1), guinea-pigs (n = 3), and rats (n = 1) exposed in this study, none of the deceased animals was reported to exhibit "any specific signs of toxicity" (Coon et al., 1970). Moderate to severe irritation of the eyes was reported for the continuously exposed rabbits (i.e., erythema, oedema, discharge) and rats (i.e., corneal opacity and apparent blindness in 2 of 15 animals); however, these effects were not observed in a separate study involving exposure of these species to 57 mg ethylene glycol/m3, 8 h/day, 5 days/week, for 6 weeks (Coon et al., 1970).
In a carcinogenicity bioassay reported by DePass et al. (1986a), no tumours were observed in Fischer 344 rats (n = 130 per sex per dose) receiving 40, 200, or 1000 mg ethylene glycol/kg body weight per day in the diet for up to 2 years, based on microscopic examination of an extensive range of organs. At >200 mg/kg body weight per day, calcium oxalate crystals were observed in the urine of both sexes. At 1000 mg/kg body weight per day, females had a transient increase in kidney weight and mild fatty changes in the liver,5 while males had 100% mortality by 15 months (attributed to calcium oxalate nephrosis), reduced growth, organ weight changes (liver and kidney), microscopic lesions in the kidney (including dilation, proteinosis, glomerular shrinkage, hyperplasia, nephritis), and alterations in haematological, clinical chemistry, and urinary parameters. Among male rats, the incidence of tubular dilation, hydronephrosis, oxalate nephrosis, and calcium oxalate crystalluria was significantly elevated in the highest dose group (Table 6).
Table 6: Incidence of renal lesions in male rats administered ethylene glycol for 2 years.
|
|
Dose of ethylene glycol |
|||||||||||||||||||||
|
0 (A)a |
0 (B)a |
40 |
200 |
1000 |
||||||||||||||||||
|
Incidence of calcium oxalate crystalluria in male rats |
||||||||||||||||||||||
|
Incidence at 6-month interim sacrifice (W. Snellings, personal communication, 2000) |
0/10 |
0/10 |
0/10 |
0/10 |
6/10 |
|||||||||||||||||
|
Incidence at 12-month interim sacrifice (W. Snellings, personal communication, 2000) |
0/10 |
0/10 |
0/10 |
0/10 |
10/10 |
|||||||||||||||||
|
Overall incidence reported in DePass et al. (1986a) |
0/128 |
0/128 |
0/129 |
0/129 |
16/116 |
|||||||||||||||||
|
Incidence of tubular hyperplasia in male rats |
||||||||||||||||||||||
|
Incidence at 6-month interim sacrifice (W. Snellings, personal communication, 2000) |
1/10 |
3/10 |
2/10 |
2/10 |
10/10 |
|||||||||||||||||
|
Incidence at 12-month interim sacrifice (W. Snellings, personal communication, 2000) |
9/10 |
8/10 |
8/10 |
8/10 |
0/10 |
|||||||||||||||||
|
Overall incidence reported in DePass et al. (1986a) |
10/128 |
11/128 |
10/129 |
10/129 |
10/116 |
|||||||||||||||||
|
Incidence of tubular dilation in male rats |
||||||||||||||||||||||
|
Incidence at 6-month interim sacrifice (W. Snellings, personal communication, 2000) |
0/10 |
0/10 |
0/10 |
1/10 |
10/10 |
|||||||||||||||||
|
Overall incidence reported in DePass et al. (1986a) |
0/128 |
0/128 |
0/129 |
1/129 |
10/116 |
|||||||||||||||||
|
Incidence of peritubular nephritis in male rats |
||||||||||||||||||||||
|
Incidence at 6-month interim sacrifice (W. Snellings, personal communication, 2000) |
0/10 |
0/10 |
0/10 |
0/10 |
6/10 |
|||||||||||||||||
|
Incidence at 12-month interim sacrifice (W. Snellings, personal communication, 2000) |
2/10 |
4/10 |
4/10 |
7/10 |
0/10 |
|||||||||||||||||
|
Overall incidence reported in DePass et al. (1986a) |
2/128 |
4/128 |
4/129 |
7/129 |
6/116 |
|||||||||||||||||
|
Incidence of oxalate nephrosis in male rats |
||||||||||||||||||||||
|
Incidence in animals dead or sacrificed when moribund (W. Snellings, personal communication, 2000) |
0/19 |
0/18 |
0/19 |
0/16 |
95/96 |
|||||||||||||||||
|
Overall incidence reported in DePass et al. (1986a) |
0/128 |
0/128 |
0/129 |
0/129 |
95/116 |
|||||||||||||||||
|
Incidence of hydronephrosis in male rats |
||||||||||||||||||||||
|
Incidence (unilateral) at 6-month interim sacrifice (W. Snellings, personal communication, 2000) |
0/10 |
0/10 |
0/10 |
0/10 |
1/10 |
|||||||||||||||||
|
Incidence at 24-month sacrifice (W. Snellings, personal communication, 2000) |
1/69 |
1/70 |
0/70 |
0/17 |
no data |
|||||||||||||||||
|
Incidence in animals dead or sacrificed when moribund (W. Snellings, personal communication, 2000) |
0/19 |
3/18 |
0/19 |
3/16 |
71/96 |
|||||||||||||||||
|
Overall incidence reported in DePass et al. (1986a) |
1/128 |
4/128 |
0/129 |
3/129 |
72/116 |
|||||||||||||||||
|
Incidence of glomerulonephrosis in male rats |
||||||||||||||||||||||
|
Incidence at 18-month sacrifice (W. Snellings, personal communication, 2000) |
20/20 |
19/20 |
20/20 |
20/20 |
no data |
|||||||||||||||||
|
Incidence at 24-month sacrifice (W. Snellings, personal communication, 2000) |
69/69 |
70/70 |
70/70 |
73/73 |
no data |
|||||||||||||||||
|
Incidence in animals dead or sacrificed when moribund (W. Snellings, personal communication, 2000) |
17/19 |
17/18 |
13/19 |
14/16 |
5/96b |
|||||||||||||||||
|
Overall incidence reported in DePass et al. (1986a) |
106/128 |
106/128 |
103/129 |
107/129 |
5/116b |
|||||||||||||||||
a 0 (A) and 0 (B) are control groups.
b Low incidence due to high mortality in the top dose group.
Data on the incidence of lesions at interim and final sacrifice additional to those presented in the publication of DePass et al. (1986a) indicate that the terminology for intermediate- and end-stage histological renal lesions in this bioassay was inconsistent (Table 6). Indeed, based on this additional information, the reported incidence of early-stage lesions (tubular hyperplasia, tubular dilation, and calcium oxalate crystalluria) in DePass et al. (1986a) is based on the numbers of animals with these lesions in the small groups killed at interim sacrifice divided by the total number of animals on test, although these lesions were not scored at late stages in the study. Moreover, after 18 months on study, all male rats in the high-dose group either had died or were sacrificed when moribund.
Renal histopathology (calcification or oxalate-containing calculi), reduced growth, and mortality were observed in male and female rats exposed to >250 mg ethylene glycol/kg body weight per day in the diet for 2 years, with effects consistently observed at lower doses in males than in females (Blood, 1965). No tumours were observed in the limited number of tissues examined (including liver, kidney, and lung).
Similarly, calcium oxalate-related renal pathology and slight liver damage but no increase in tumours were presented in an early (limited) chronic bioassay in which only survival, growth, and histopathology in selected organs were examined in small groups of male (n = 6 per group) and female (n = 4 per group) albino rats (strain not specified) fed diets containing 1% or 2% ethylene glycol (500 or 1000 mg/kg body weight per day) for 2 years (Morris et al., 1942).
In an NTP (1993) bioassay, no tumours were observed in B6C3F1 mice (n = 60 per sex per dose) administered ethylene glycol in the diet (males: 1500, 3000, or 6000 mg/kg body weight per day; females: 3000, 6000, or 12 000 mg/kg body weight per day) for 103 weeks. Females had a treatment-related increase in arterial medial hyperplasia in the lungs at all levels of exposure and hyaline degeneration in the liver at 12 000 mg/kg body weight per day. At 3000 and 6000 mg/kg body weight per day, male mice had dose-related hepatocellular hyaline degeneration and transient kidney damage (nephropathy). No clear evidence of treatment-related effects on survival, body weight, or tissue histopathology was observed in CD-1 mice (n = 80 per sex per dose) given 40, 200, or 1000 mg ethylene glycol/kg body weight per day in the diet for 2 years, based on microscopic examination of an extensive range of organs at 80 weeks and 2 years (DePass et al., 1986a). However, as indicated above in relation to the bioassay in rats reported by the same authors, histological reporting in this study was inadequate.
In a limited early investigation reported by Blood et al. (1962), male (n = 2) and female (n = 1) rhesus monkeys receiving 80 or 200 mg ethylene glycol/kg body weight per day in the diet, respectively, for 3 years had no overt signs of toxicity and no abnormal calcium deposits or histopathological changes in the major tissues examined (including the urogenital system, liver, and bone marrow).
In two studies conducted by subcutaneous injection, no tumours were observed in Fischer 344 rats (Mason et al., 1971) or NMRI mice (Dunkelberg, 1987) following repeated administration of up to 1000 mg ethylene glycol/kg body weight for 52 or 106 weeks.
In in vitro mutagenicity studies in bacterial cells, results have been consistently negative (Clark et al., 1979; Pfeiffer & Dunkelberg, 1980; Zeiger et al., 1987; JETOC, 1996), with and without S9 activation. Results have also been negative for mutagenicity in mouse lymphoma L51784Y cells (with and without activation) (McGregor et al., 1991). Results have been negative for chromosomal aberrations and sister chromatid exchange in cultured Chinese hamster ovary cells (with and without activation) (NTP, 1993) and for DNA damage in rat hepatocytes (Storer et al., 1996) and Escherichia coli (McCarroll et al., 1981; von der Hude et al., 1988).
In in vivo genotoxicity studies, results have been negative for dominant lethal mutations in F344 rats following administration in F2 males (from a multigeneration study) of up to 1000 mg ethylene glycol/kg body weight per day for 155 days (DePass et al., 1986b). Results have also been negative for chromosomal aberrations in bone marrow cells of male Swiss mice exposed (by intraperitoneal injection) to 638 mg ethylene glycol/kg body weight per day for 2 days (Conan et al., 1979). There was only a slight increase in the incidence of micronuclei in the erythrocytes of Swiss mice administered >1250 mg ethylene glycol/kg body weight by gavage (or by intraperitoneal injection) (Conan et al., 1979). However, it should be noted that the magnitude of the effect was small, was not dose related, and was based on pooled data for treated groups.
In a three-generation reproduction study in which F344 rats (both sexes) received 40, 200, or 1000 mg ethylene glycol/kg body weight per day in the diet, there were no treatment-related parental effects (based on survival, body weight, food consumption, appearance, behaviour, and histopathology in major organs) or effects on fertility index, gestation index, gestation survival index, pup weight, appearance, behaviour, or histopathology in major organs (DePass et al., 1986b).
In F344 rats administered 40, 200, or 1000 mg ethylene glycol/kg body weight per day in the diet during days 6–15 of gestation, statistically significant increases of poorly ossified and unossified vertebral centra were observed in the offspring of dams exposed to 1000 mg ethylene glycol/kg body weight per day (Maronpot et al., 1983). Exposure to ethylene glycol had no effect upon pregnancy rate or number of corpora lutea, litters, live and dead fetuses, total implantations, pre-implantation loss, or resorptions; there was also no evidence of maternal toxicity.
In studies in which pregnant CD rats were admin-istered (by gavage) 150, 500, 1000, or 2500 mg ethylene glycol/kg body weight per day on days 6–15 of gestation, significant dose-related developmental effects were observed at 1000 mg/kg body weight per day and above, including reduced fetal body weight per litter, reduced skeletal ossification, and malformations in the skeleton (missing arches, missing and extra ribs) (Neeper-Bradley et al., 1995). Exposure had no effect upon the number of corpora lutea, live and dead fetuses, or resorption sites. Maternal toxicity was observed at 2500 mg/kg body weight per day, based on an increase (10%, P < 0.001) in relative kidney weight. [NOAEL (offspring) = 500 mg/kg body weight per day; LOAEL (offspring) = 1000 mg/kg body weight per day; NOAEL (maternal) = 1000 mg/kg body weight per day]
Other oral studies conducted in rats (Price et al., 1985; NTP, 1988; Marr et al., 1992) do not contribute additionally to the weight of evidence or dose–response for the reproductive toxicity of ethylene glycol, due to conduct of the studies at very high doses at which there was clear evidence of maternal toxicity (>1250 mg/kg body weight per day by stomach tube).
In a continuous-breeding study in which male and female CD-1 mice received 410, 840, 1640, or 2800 mg ethylene glycol/kg body weight per day in drinking-water, F1 female pup weight was decreased at >840 mg/kg body weight per day (Lamb et al., 1985; NTP, 1986; Morrissey et al., 1989). Slight reductions in the number of F1 litters per fertile pair (8%, P < 0.01) and number of live F1 pups per litter (6%, P < 0.05), as well as unusual facial features and skeletal changes in the skull, sternebrae, ribs, and vertebrae, were observed at 1640 mg/kg body weight per day; however, incidence rates were not reported. There were no clear treatment-related effects on parental survival, body weight gain, or water intake, and no overt signs of toxicity were observed (Lamb et al., 1985; NTP, 1986; Morrissey et al., 1989).
Following the oral administration (by gavage) in pregnant CD rats of 2500 mg ethylene glycol/kg body weight per day on days 6–15 of gestation, extensive skeletal malformations and variations in the skull, vertebrae, ribs, sternebrae, and centra and significant external (meningoencephalocele, exencephaly, omphalocele, cleft palate, and cleft lip) and visceral (dilated cerebral ventricles) malformations were observed in fetuses from treated dams (Carney et al., 1999). Maternal effects included reduced food consumption and body weight gain and increased liver and kidney weights. Most of the skeletal effects induced by ethylene glycol were similar to those observed among fetuses from dams receiving an equivalent "teratogenic dose" of either glycolic acid (650 mg/kg body weight per day; presence of metabolic acidosis) or sodium glycolate (833 mg/kg body weight per day; absence of metabolic acidosis), while significant external or visceral malformations were not observed among animals exposed to glycolate or sodium glycolate. A reduction in metabolic acidosis (demonstrated with the use of sodium glycolate) ameliorated, but did not completely eliminate, skeletal effects.
Following administration (by gavage) in pregnant CD-1 mice of 750, 1500, or 3000 mg ethylene glycol/kg body weight per day during days 6–15 of gestation, there was a dose-related reduction in average fetal body weights per litter (9–27%) and marked dose-related increases in the incidence of malformed live fetuses per litter (0.25% in the controls and 10–57% in treated groups) and of skeletal malformations in the ribs, arches, centra, and sternebrae (4% in controls and 63–96% in treated groups) at all dose levels (Price et al., 1985). Exposure had no effect on the number of implantations, resorption sites, or live and dead fetuses. At 1500 mg/kg body weight per day and above, there was a significant decrease in maternal body weight gain (32%, P < 0.01) and absolute liver weight (9%, P < 0.01).
In studies in which pregnant CD-1 mice were administered (by gavage) 0, 50, 150, 500, or 1500 mg ethylene glycol/kg body weight per day on days 6–15 of gestation (Neeper-Bradley et al., 1995), exposure to the highest dose produced a statistically significant increase in the incidence of 25 of the 27 skeletal malformations/variations examined. At 1500 mg/kg body weight per day, an increased incidence of skeletal malformations and variations and a reduction in fetal body weight per litter were observed. The administration of 500 mg ethylene glycol/kg body weight produced a statistically significant increase in the incidence of the occurrence of an extra 14th rib. Exposure produced no effects on the number of corpora lutea or viable implantation sites, pre-implantation loss or sex ratio, or maternal toxicity. [No-observed-effect level (NOEL) (offspring) = 150 mg/kg body weight per day; NOAEL (offspring) = 500 mg/kg body weight per day; LOAEL (offspring) = 1500 mg/kg body weight per day; NOAEL (maternal) = 1500 mg/kg body weight per day]
In a study in which sperm counts and motility, histopathology and organ weights in the testes and epididymis, percentage of pregnant females, and number of live and dead implantation sites were examined in CD-1 mice exposed (both sexes) by gavage to 250–2500 mg ethylene glycol/kg body weight per day for up to 19 days, a significant reduction in live implantations per female was observed (Harris et al., 1992). No signs of parental toxicity were observed, based on examination of survival, body weight, clinical signs, and histopathology in selected organs.
Other oral studies conducted in mice (Nagano et al., 1973, 1984; Morrissey et al., 1989; Harris et al., 1992) do not contribute additionally to the weight of evidence of developmental/reproductive toxicity of ethylene glycol, due to the limited range of end-points examined, limited reporting of results, the absence of data on maternal toxicity, or conduct of the studies at higher doses than used in similar studies addressed herein.
A single study has been identified in which the developmental and reproductive toxicities of ethylene glycol have been examined in rabbits following oral exposure. Despite the presence of severe maternal toxicity (mortality and degenerative changes in the kidney) at the highest dose level, there was no evidence of developmental or reproductive effects in fetuses derived from New Zealand white rabbits administered (by gavage) 100–2000 mg ethylene glycol/kg body weight per day during days 6–19 of gestation (Tyl et al., 1993). Parameters examined included the number of corpora lutea, pre- and post-implantation loss, number of fetuses, fetal body weight per litter, litter sex ratio, and external, visceral, or skeletal variations or malformations.
Identified inhalation studies conducted in CD rats and CD-1 mice, in which pregnant animals were exposed (whole body) to up to 2090 mg ethylene glycol/m3 for 6 h/day on days 6–15 of gestation, provide some evidence of treatment-related skeletal variations (reduced ossification, extra ribs, dilated lateral ventricles, malaligned centra) and malformations of the head (exencephaly), face (cleft palate, abnormal face and facial bones), and skeleton (vertebral fusion, and fused, forked, missing, and extra ribs) (Tyl et al., 1995a,b). However, in each of these studies, there was likely considerable intake due to ingestion after grooming and/or percutaneous absorption; it was estimated by the authors that rats and mice received at least 620 mg/kg body weight per day and 910–1400 mg/kg body weight per day, respectively, in this manner.
In a "nose-only" inhalation study, exposure of pregnant CD-1 mice to 360, 779, or 2505 mg ethylene glycol/m3 for 6 h/day on days 6–15 of gestation yielded increases in some skeletal variations (e.g., decreased ossification in centra and sternebrae, unossified phalanges of the forelimb, extra ribs, extra ossification site in the skull)6 and a statistically significant 8-fold increase in the number of litters with animals exhibiting 2–12 fused ribs at the highest concentration (i.e., 2505 mg/m3) (Tyl et al., 1995b). Ethylene glycol had no effect upon the incidence of external or visceral malformations or reproductive parameters (including number of corpora lutea, total and viable implantations per litter, pre- and post-implantation loss, and sex ratio). Only minimal maternal toxicity was observed at 2505 mg/m3, based on a slight increase (7%, P < 0.05) in relative kidney weight, without evidence of cellular injury.
In the single dermal study identified, in which pregnant CD-1 mice were exposed (by occluded cutaneous application) to aqueous solutions of 0, 12.5, 50, or 100% ethylene glycol (estimated doses of 0, 400, 1700, or 3500 mg/kg body weight per day) during days 6–15 of gestation, reported effects were limited to maternal toxicity (based on minimal-grade renal lesions and increased corrected gestational body weight change) and a significant increase in the incidence of poorly ossified skull bone and unossified intermediate phalanges of the hindlimb at 3500 mg/kg body weight per day (Tyl et al., 1995c). Dermal exposure to ethylene glycol had no effect upon the number of corpora lutea, implantation and resorption sites, or live and dead fetuses.
Although data are limited, results of identified toxicity studies conducted (via oral, inhalation, or dermal routes) in rodents, rabbits, and monkeys do not indicate that neurological or immunological effects are critical end-points for ethylene glycol. Neurological effects have not been observed at doses below those that have induced renal toxicity (Penumarthy & Oehme, 1975; Clark et al., 1979; Grauer et al., 1987), and consistent treatment-related effects on immune system-related parameters have not been observed in available repeated-dose toxicity studies in which several species have been exposed to ethylene glycol either orally or by inhalation.
The effects observed in laboratory animals and humans are due primarily to the actions of one or more of its metabolites, rather than to the parent compound per se (see Figure 2). In studies in which inhibitors of alcohol dehydrogenase (the enzyme catalysing the first rate-limiting step in the metabolism of ethylene glycol) have been administered in both animals and humans, toxicity has been minimized. In laboratory animals, the concurrent ingestion or infusion of ethanol, pyrazole, or fomepizole prevents the renal toxicity and mortality observed following exposure to ethylene glycol (Grauer et al., 1987; US EPA, 1987). In humans, therapy for acute ethylene glycol poisoning includes administration of ethanol or 4-methylpyrazole to inhibit ethylene glycol metabolism by competition for alcohol dehydrogenase activity, sodium bicarbonate to reduce metabolic acidosis, and dialysis to remove the toxin (Jacobsen & McMartin, 1986; Grant & Schuman, 1993; Brent et al., 1999).
Based upon the available information, toxicological effects resulting from exposure to ethylene glycol may involve one or a combination of the following: development of an increased osmolal gap,7 metabolic acidosis,8 the formation of calcium oxalate crystals9 and their deposition in various tissues, or possible direct toxic effects produced by one or more of its metabolites.
A mode of action involving the formation and deposition of calcium oxalate crystals as requisite steps in the induction of renal effects in animals and humans is consistent with available metabolic and histopathological data. For example, species differences in sensitivity to renal effects are consistent with the limited available data on the comparative proportions of ethylene glycol excreted as oxalic acid, these being greater in rats than in mice (7–8% versus not detected at 24 h; Frantz et al., 1996a,b). Based on limited information, values for monkeys are intermediate, i.e., between those for rats and mice (0.3% at 48 h; McChesney et al., 1971), and those for humans are within the range of values reported for rats (Reif, 1950).
Indeed, calcium oxalate crystals are considered to be important etiological agents in the development of the renal failure in humans acutely poisoned by the ingestion of ethylene glycol (Jacobsen & McMartin, 1986; Wiley, 1999). In addition, in almost all cases where examined, extensive renal damage in experimental animals has been observed only where such crystals are present (Gaunt et al., 1974; Melnick, 1984). Also, in all cases where examined, ethylene glycol-associated renal damage has been observed only at doses greater than those at which there were increases in urinary excretion of oxalate or calcium oxalate crystals (Gaunt et al., 1974; DePass et al., 1986a). However, the possible role of less frequently observed hippuric acid crystals and direct cytotoxicity of other metabolites such as glycoaldehyde, glycolic acid, and glyoxylic acid cannot be precluded (Parry & Wallach, 1974; Marshall, 1982).
Sex-related variations in sensitivity to the renal effects of ethylene glycol may be a function of both toxicokinetic and toxicodynamic differences. Although there have been variations in the proportion of metabolites excreted as oxalic acid in male versus female rats in repeated-dose studies, proportions have been similar following single administration. In Sprague-Dawley rats administered (orally via gavage) a single dose of 1000 mg [14C]ethylene glycol/kg body weight, similar amounts of the administered radioactivity (i.e., 7–8%) were eliminated in the urine as [14C]oxalic acid in the males and females (Frantz et al., 1996a,b). Similar amounts of radioactivity have been measured in the kidneys of male and female rats administered single doses of [14C]ethylene glycol (Frantz et al., 1996a,b). In two studies in which Sprague-Dawley rats were provided drinking-water containing 0.5% ethylene glycol for 28 days, the elimination of oxalic acid (expressed as either mg/litre or µmol/litre per 24 h) in the urine was approximately 2.6- and 4.3-fold higher in males than in females (Lee et al., 1992, 1996).10 In a study in which male and female Wistar rats received similar doses (i.e., from 35 to 180 mg/kg body weight per day) of ethylene glycol from the diet over a 14- to 16-week period, slightly lower (i.e., 1.3- to 2.8-fold) levels of oxalic acid were excreted in the urine of females than in that of males (Gaunt et al., 1974).
In male Sprague-Dawley rats administered drinking-water containing 0.5% ethylene glycol for 28 days, the occurrence of kidney stones (as well as the excretion of oxalic acid in the urine) was reduced in castrated males, compared with controls (Lee et al., 1992, 1996). The effects of castration upon kidney stone formation (and excretion of oxalic acid) were reversed by the administration of exogenous testosterone to the castrated animals (Lee et al., 1996). Based upon the results of a study using an ethylene glycol/vitamin D induced urolithiasis model in oophorectomized Wistar female rats, Iguchi et al. (1999) suggested that the sex-related differences in the occurrence of kidney stones in rats administered ethylene glycol may be attributed to the female sex hormone-induced suppression of urinary oxalate excretion and suppression of osteopontin11 expression in the kidney.
Based on the limited identified data, including those on acute doses that induce renal toxicity in humans, the comparative proportions of total metabolites excreted as the putatively toxic entity (i.e., oxalic acid) in humans and rats (Reif, 1950; Frantz et al., 1996a,b), and the specific activity of relevant enzymes in hepatic extracts of rats versus humans, it is expected that the sensitivity of humans to renal effects is similar to or greater than that of rats. Available data indicate that humans may be more sensitive than rodents to the acute toxicity of ethylene glycol, with available information on reported minimum lethal doses being consistent with an approximately 10-fold greater sensitivity in humans compared with rodents. The specific activity of alcohol dehydrogenase (the first rate-limiting step in the metabolism of ethylene glycol, considered essential in producing the toxicological effects associated with exposure to this substance) has been slightly higher in hepatic extracts obtained from humans, compared with rats (Zorzano & Herrera, 1990).
Less is known about the potential mode of induction of developmental effects, including the role of putatively toxic metabolites, although research conducted to date has focused on glycolic acid (Carney, 1994; Carney et al., 1999).
Evidence implicating a role for glycolic acid as the principal teratogenic agent has been derived from in vivo studies in which developmental effects have been observed in rats administered glycolic acid at doses lower than those for ethylene glycol inducing similar effects (Munley & Hurrt, 1996; Carney et al., 1999).
In studies in rats, a reduction in the metabolic acidosis typically associated with oral exposure to ethylene glycol ameliorated, but did not completely eliminate, teratogenic effects (Khera, 1991; Carney et al., 1999). Most variations and malformations induced by 2500 mg ethylene glycol/kg body weight administered on days 6–15 of gestation were similar to those observed among fetuses from dams receiving an equivalent "teratogenic dose" of either glycolic acid (presence of metabolic acidosis) or sodium glycolate (absence of metabolic acidosis). However, increased incidence of several external malformations (e.g., meningoencephalocele, exencephaly, omphalocele, cleft lip, cleft palate) among the offspring of ethylene glycol-exposed pregnant rats could not be explained on this basis (Carney et al., 1999).
There are numerous case reports concerning the accidental or deliberate ingestion of this compound in humans; mortality in these cases was associated with renal failure due to marked renal pathology (characterized by calcium oxalate deposition and degeneration in the tubules) (HSDB, 2001). However, available data from these studies are generally inadequate as a basis for quantification of intake associated with observed effects and can provide only crude information as a basis for comparison of the sensitivity of experimental animals with that of humans. Published values for the minimum lethal oral dose in humans have ranged from approximately 0.4 g/kg body weight (RTECS, 1999) to 1.3 g/kg body weight (ATSDR, 1997).12 Systemic signs of toxicity following ingestion generally progress in three stages, commencing with effects on the central nervous system (intoxication, lethargy, seizures, and coma) and metabolic disturbances (acidosis, hyperkalaemia, hypocalcaemia), progressing to effects on the heart and lungs (tachycardia, hypertension, degenerative changes), and ending with renal toxicity (deposition of calcium oxalate, haematuria, necrosis, renal failure). In addition to the immediate central nervous system effects, neurological effects (including facial paralysis, slurred speech, loss of motor skills, and impaired vision due to "bilateral optic atrophy") have been observed up to several weeks following ingestion, suggestive of cranial nerve damage. Ethylene glycol may also produce a local irritant effect on the gut and cause pain and bleeding secondary to gastric erosion. The type and severity of toxicological effects observed following ingestion vary with the amount of ethylene glycol consumed, the interval between ingestion and treatment, and whether there has been concurrent ingestion of ethanol (Health Canada, 2000).
Information concerning adverse effects following inhalation of ethylene glycol is limited to observational data in a single case report (Hodgman et al., 1997) and the results of one laboratory study in which a range of end-points (physical examinations, psychological testing, and analysis of haematological, serum clinical chemistry, and urinary parameters) was examined in 20 male volunteers exposed (whole body) to ethylene glycol aerosol (Wills et al., 1974). In the latter study, no significant adverse effects were observed among individuals exposed (to up to 67 mg/m3) continuously for periods up to 30 days, although some individuals experienced throat irritation, headache, and back pain. Following a gradual increase in the exposure concentration, nasal and/or throat irritation were noted in all test subjects at 140 mg/m3 and above, while concentrations above 200 mg ethylene glycol/m3 produced severe irritation and could not be tolerated (Wills et al., 1974).
Ethylene glycol is a mild ocular irritant in humans. In dermal patch tests, results have been consistently negative in healthy volunteers (Meneghini et al., 1971; Hindson & Ratcliffe, 1975; Seidenari et al., 1990), while dermal irritation has been noted in individuals with dermal sensitivity, including eczema patients (Hannuksela et al., 1975) and occupationally exposed workers with a history of dermatitis (Hindson & Ratcliffe, 1975; Dawson, 1976).
In a cross-sectional survey, there was no evidence of effects on kidney function (based on urinary concentrations of albumin, beta-N-acetyl-glucosaminidase, beta-2-microglobulin, and retinol-binding protein) in a small group of aircraft workers (some of whom wore protective breathing equipment) exposed to ethylene glycol vapour or mist during deicing operations (Gérin et al., 1997). In a case–control study of 26 cases of kidney cancer in a chemical plant, where an excess of this cancer had been observed (Bond et al., 1985), there was no association between presumptive inhalation exposure to ethylene glycol and renal cancer. Quantitative data on exposure to ethylene glycol were not presented.
There is convincing evidence that the toxicity of ethylene glycol is mediated principally through metabolites. Available data also indicate that the likely pathways involved in the metabolism of ethylene glycol are qualitatively similar in humans and other mammalian species; potential quantitative differences have not been well studied.
Identified data in humans are inadequate as a basis for assessment of the weight of evidence for causality of the potential carcinogenicity of ethylene glycol, being limited to the results of a single case–control study of chemical production workers, in which there was no association between presumptive exposure to ethylene glycol and renal cancer (Bond et al., 1985). Ethylene glycol has not been carcinogenic in a 2-year bioassay in rats for which sensitivity was somewhat compromised at the top dose in males due to high mortality (DePass et al., 1986a) or in a comprehensive bioassay in mice (NTP, 1993) exposed in the diet, nor in early (more limited) dietary studies in rats (Morris et al., 1942; Blood, 1965) or monkeys (Blood et al., 1962). In the limited number of identified in vitro and in vivo studies, ethylene glycol has not been genotoxic.
Ethylene glycol has low acute toxicity in experimental animals following oral, inhalation, or dermal exposure. Based upon comparison of published values for the minimum lethal dose in humans (ranging from approximately 0.4 g/kg body weight [RTECS, 1999] to 1.3 g/kg body weight [ATSDR, 1997]) and rats (3.8 g/kg body weight; Clark et al., 1979), humans may be more sensitive to the lethal effects of acute ethylene glycol poisoning than experimental animals, although the limitations of characterization of exposure in human poisoning cases need to be recognized.
In both humans and animals, ethylene glycol has induced only minimal dermal irritation. Nasal and/or throat irritation were reported in a small number of subjects inhaling ethylene glycol, while higher concentrations produced severe irritation (Wills et al., 1974). In experimental animals, ethylene glycol induces only minimal conjunctival irritation, without permanent corneal damage. Data on the potential of ethylene glycol to induce sensitization have not been identified.
Although there was no evidence of effects on renal function in a small group of aircraft workers (some of whom wore protective breathing equipment) exposed in a cross-sectional survey to ethylene glycol vapour or mist during deicing operations (Gérin et al., 1997), available data from acute poisoning cases (humans) and repeated-dose toxicity studies (experimental animals) indicate that the kidney is a critical organ for the toxicity of ethylene glycol in both humans and experimental animals. Data in humans are inadequate for quantification of doses that result in renal effects (deposition of calcium oxalate, haematuria, necrosis, renal failure). However, in experimental animals, although also observed in female rats and males and females of other species in short-, medium-, and long-term oral studies, the male rat has been most sensitive in the development of degenerative changes in the kidney (including dilation, necrosis, fibrosis, inflammation, and deposition of calcium oxalate crystals). Available data are consistent with the metabolism of ethylene glycol to oxalic acid and the subsequent formation and deposition of calcium oxalate crystals being important requisite steps in the induction of these renal lesions, although a potential role of other metabolites cannot be excluded.
In rodents, mild liver damage (including fatty degeneration, hyaline degeneration, bile duct proliferation, diffuse or centrilobular atrophy) has also been observed at doses higher than those associated with the induction of effects in the kidney of male rats in longer-term studies.
Effects of ethylene glycol on other systems (including the blood, lung, and heart) have not been consistently observed at lowest doses.
Based on a rather extensive database, ethylene glycol induces developmental effects in rats and mice by all routes of exposure, although at doses greater than those associated with renal effects in male rats. Indeed, ethylene glycol is teratogenic, inducing primarily skeletal variations and malformations, sometimes at doses less than those that are maternally toxic, with mice being more sensitive than rats. Although most research has focused on the possible role of glycolic acid and/or associated metabolic acidosis in the induction of developmental effects by ethylene glycol, a possible role for other metabolites and/or ethylene glycol itself cannot be excluded on the basis of available data.
The effects of ethylene glycol on fertility have been extensively investigated in adequate studies in mice and rats. In repeated-dose toxicity studies, there has been no evidence of adverse impact on reproductive organs; in specialized studies, including a three-generation study in rats and continuous-breeding protocols in mice, evidence of reproductive effects has been restricted to mice (but not rats or rabbits) exposed to doses considerably greater than those associated with developmental effects in this species or renal effects in rats.
Available data are inadequate to assess potential adverse neurological or immunological effects associated with long-term exposure to ethylene glycol, although neurobehavioural and neurological disorders have been reported in cases of acute ethylene glycol poisoning in humans. In the limited number of investigations identified to date, neurological effects have not been observed at doses below those that have induced renal toxicity. Consistent treatment-related effects on immune system-related parameters have not been observed in available repeated-dose toxicity studies, in which several species have been exposed to ethylene glycol either orally or by inhalation.
Data on dose–response in humans are limited. They include acute poisoning cases in which exposure has not been well quantified. A short-term study in which a range of end-points (physical examinations, psychological testing, and analyses of haematological, serum clinical chemistry, and urinary parameters) has been examined in a small number of human volunteers exposed continuously by whole-body inhalation for periods up to 30 days has also been conducted (Wills et al., 1974). Although inadequate as a basis for characterization of exposure–response, available data in humans are at least somewhat helpful as a basis for crudely characterizing potential relative sensitivity of experi-mental animals and humans. Dose–response has therefore been characterized primarily on the basis of results in studies in experimental animals, but including comparison of relative sensitivity with humans where permitted by available data.
It is histopathological effects on the kidney that have typically been observed at lowest doses in laboratory animals exposed to ethylene glycol in repeated-dose toxicity studies in rats, mice, and monkeys. Based on medium- and long-term studies by the oral route in rats and mice (Gaunt et al., 1974; Melnick, 1984; DePass et al., 1986a; Robinson et al., 1990; NTP, 1993), male rats have been the most sensitive sex and species, with data being consistent with the deposition of calcium oxalate crystals within the renal tissue being a requisite step.
In the absence of maternal toxicity, developmental effects (i.e., skeletal changes and, at higher doses, malformations) have also been observed at lowest doses in mice (NOEL = 150 mg/kg body weight per day;13 NOAEL = 500 mg/kg body weight per day14) and rats (NOAEL = 500 mg/kg body weight per day) administered ethylene glycol orally during gestation (Neeper-Bradley et al., 1995). Effect levels for slight reproductive effects and developmental toxicity in mice in continuous-breeding studies in which ethylene glycol was administered in drinking-water were higher (skeletal malformations and slight reproductive effects at 1640 mg/kg body weight per day) (Lamb et al., 1985; Morrissey et al., 1989).
Emphasis in the following sections is on characterization of exposure–response for the end-point observed at lowest doses in laboratory animals — i.e., renal effects. Developmental effects, which generally occur at somewhat higher concentrations, are also addressed.
The most informative data set for characterization of exposure–response for renal lesions in the most sensitive sex and species (i.e., male rats) is that from the study of Gaunt et al. (1974). In this investigation, there were four dose levels of 35, 71, 180, and 715 mg/kg body weight per day, including two at which there was a significant increase of ethylene glycol-related tubular damage (NOAEL = 71 mg/kg body weight per day [males]; LOAEL = 180 mg/kg body weight per day [males]). Compared with other relevant studies (Melnick, 1984; DePass et al., 1986a; Robinson et al., 1990), the protocol of this investigation included larger numbers of doses in the lower dose range in the vicinity of reported no-effect levels (i.e., 200 mg/kg body weight per day) and optimum dose spacing (2- to 3-fold compared with 5-fold in longer-term studies). Incidences of both individual lesions and total animals with tubular damage were also reported.
While group sizes were relatively small (n = 15 at termination of exposure) and exposures less than long term (16 weeks) in the investigation by Gaunt et al. (1974), data from the most recent chronic bioassay in larger groups of animals (DePass et al., 1986a) are not considered adequate as a basis for characterization of dose–response, for several reasons. Histological reporting of non-cancer lesions in this study was inadequate due to lack of application of consistent diagnostic criteria to assess the onset and progression of treatment-related changes. Terminology for intermediate- and end-stage histological renal lesions was inconsistent, and, as a result, the incidence of early-stage lesions was inadequately reported and likely considerably underestimated. More appropriately, consistent terminology would have been applied across the study with an indication of severity over time to provide an adequate basis for determination of an appropriate effect level or benchmark dose. Moreover, in this chronic bioassay in which there were three dose levels, after 18 months on study, all males in the high-dose group either had died or were sacrificed when moribund. Also, the incidence of end-stage lesions (which likely include several changes, including tubular hyperplasia, dilated tubules, protein casts, basement membrane thickening, etc.) was almost 100% in the top dose group (1000 mg/kg body weight per day) and minimal at the intermediate dose (200 mg/kg body weight per day).
A tolerable intake for ethylene glycol, based on the development of histopathological changes in the kidney of male rats, has been derived based on a benchmark dose05 (i.e., the dose estimated to cause a 5% increase in incidence over the background response rate; BMD05), divided by an uncertainty factor. The BMD05 was calculated by first fitting the following polynomial model to the dose–response data (Howe, 1995):
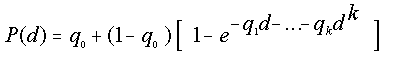
where d is dose, k is the number of dose groups in the study, P(d) is the probability of the animal developing the effect at dose d, and qi > 0, i = 1,...,k are parameters to be estimated.
The models were fit to the incidence data using THRESH (Howe, 1995), and the BMD05s were calculated as the dose D for extra risk that satisfies
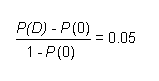
A chi-square lack of fit test was performed for each of the model fits. The degrees of freedom for this test are equal to k minus the number of qi’s whose estimates are non-zero. A P-value less than 0.05 indicates a significant lack of fit. For none of the models was there significant lack of fit.
BMD05s and associated lower 95% confidence limits (95% LCLs) have been derived for histopathological changes in the kidneys of male rats ingesting diets containing ethylene glycol for 16 weeks (Gaunt et al., 1974). The BMD05s range from 84 mg/kg body weight per day (95% LCL = 45 mg/kg body weight per day) to 550 mg/kg body weight per day (95% LCL = 180 mg/kg body weight per day) for individual lesions. Based on total animals with tubular damage, the respective BMD05 and 95% LCL are 49 mg/kg body weight per day and 22 mg/kg body weight per day (Figure 3); the corresponding P-value, chi-square, degrees of freedom, and degrees of polynomial on these estimates were 0.62, 0.94, 2, and 4, respectively. Based on the BMD05, a tolerable intake of 0.05 mg/kg body weight can be derived (49 mg/kg body weight per day divided by 1000, which is the uncertainty factor [×10 for interspecies variation, ×10 for intraspecies variation, ×10 to account for less than long-term exposure]). Available data are inadequate to further address toxicokinetic and toxicodynamic aspects of components of uncertainty with data-derived values. Although the putatively toxic metabolite in induction of renal lesions in rats and humans is likely oxalic acid, the role of other metabolites cannot be excluded; moreover, there are no comparative kinetic or dynamic data in rats and humans to serve as a basis for reliable quantitative scaling. Limited identified relevant data include those on doses that induce acute toxicity in humans (often not well documented) and rats, the exceedingly limited data (particularly for humans) on comparative proportions of total metabolites excreted as the putatively toxic entity (i.e., oxalic acid) in humans and rats (Reif, 1950; Frantz et al., 1996a,b), and the specific activity of relevant enzymes in hepatic extracts of rats versus humans. Based on this limited information, it is expected that the sensitivity of humans to renal effects is similar to or greater than that of rats; indeed, data for acute poisonings are consistent with the magnitude of this greater sensitivity of humans being in the range of 10-fold. The specific activity of alcohol dehydrogenase (the first rate-limiting step in the metabolism of ethylene glycol, considered essential in producing the toxicological effects associated with exposure to this substance) has been slightly higher in hepatic extracts obtained from humans than in those from rats (Zorzano & Herrera, 1990). The additional factor of 10 to account for less than long-term exposure is necessitated due to lack of reliable available data to serve as a basis for quantification of dose–response following long-term exposure, likely progression of the effects with continued exposure, and decrease in renal function with age.
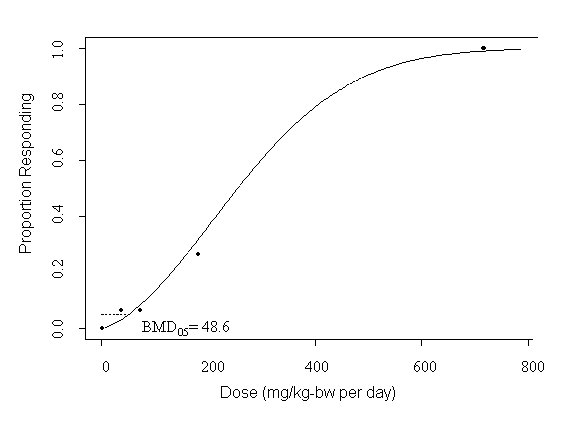
Figure 3: Dose–response analysis (BMD05) of kidney damage in
male Wistar rats based on total animals with tubular damage (Gaunt et al., 1974)
Tolerable intakes developed on the basis of histopathological changes within the kidneys of male rats receiving ethylene glycol are expected to be protective for potential developmental effects. Owing to the lack of reported information on litter-specific developmental effects observed at the lowest doses in mice (Neeper-Bradley et al., 1995), a tolerable intake for this end-point has been developed on the basis of an effect level rather than a benchmark dose.15 Calculation of a tolerable intake based upon the NOAEL for developmental effects in mice (i.e., 500 mg/kg body weight per day) divided by an uncertainty factor of 100 (×10 for interspecies variation, ×10 for intraspecies variation) results in a value of 5 mg/kg body weight per day. Parenthetically, derivation of a tolerable intake based upon division of a putative NOEL (150 mg/kg body weight per day) in this study (i.e., a significant increase in the incidence of only 1 of 27 skeletal malformations/variations — the occurrence of an extra 14th rib on the first lumbar arch — at the next highest dose of 500 mg/kg body weight per day) by an uncertainty factor of 100 (×10 for interspecies variation, ×10 for intraspecies variation) would yield a value of 1.5 mg/kg body weight per day. This is more than an order of magnitude higher than that based on the development of histopathological changes within the kidneys of male rats exposed for 16 weeks (Gaunt et al., 1974).
Data available on tissue- or organ-specific toxicities associated with the inhalation of ethylene glycol following repeated exposure are limited to one short-term (intermittent) and one medium-term (continuous) study in which a limited range of end-points was examined in rats, guinea-pigs, rabbits, dogs, and monkeys exposed (whole body) to ethylene glycol vapour (Coon et al., 1970). In these investigations, reported adverse effects were not observed consistently, and results are considered inadequate as a basis for characterization of exposure–response for critical effects. Information from laboratory studies in humans, limited to examination of a range of end-points following relatively short-term exposures of a small number of volunteers, is also considered inadequate as a basis for characterization of exposure–response (Wills et al., 1974).
Developmental effects have been observed in rats and mice exposed via inhalation to ethylene glycol; however, interpretation is complicated somewhat by the possibility of significant additional intake via ingestion from grooming and/or percutaneous absorption in studies conducted using whole-body exposure (Tyl et al., 1995a,b). The incidence of developmental effects was increased over that of a water aerosol-exposed control group at the highest concentration (2505 mg/m3) in an investigation in which CD-1 mice were exposed nose only for 6 h/day on days 6 through 15 of gestation (Tyl et al., 1995b). Based on examination of pelage washes in satellite groups, it was confirmed that deposition on the fur in surviving animals exposed at this concentration was considerably less than that in animals exposed via whole-body inhalation (Tyl et al., 1995b). The estimated intake (assuming 60% absorption; Marshall & Cheng, 1983) of ethylene glycol, derived (Health Canada, 1994) on the basis of a putative NOAEL of 779 mg/m3 from this study — approximately 156 mg/kg body weight per day16 — is within the range reported for no-effect levels associated with the occurrence of developmental changes in mice administered the substance orally by gavage (Neeper-Bradley et al., 1995) or the induction of histopathological changes in the kidneys of male rats receiving the substance in the diet (Gaunt et al., 1974).
The single repeated-dose toxicity study in which the effects of ethylene glycol have been examined following direct dermal application in experimental species (Berenblum & Haran, 1955) is insufficient to provide a meaningful basis for characterization of exposure–response. In one developmental toxicity study, no effects upon fetal development were observed following exposure of pregnant CD-1 mice to up to 1700 mg ethylene glycol/kg body weight per day (Tyl et al., 1995c). In this study, no effects on reproductive indices were observed following dermal exposure to up to 3500 mg ethylene glycol/kg body weight per day (Tyl et al., 1995c). Based on available data, therefore, the tolerable intake developed for the induction of histopathological changes in the kidneys of male rats receiving the substance in the diet (Gaunt et al., 1974) is likely protective for effects of ethylene glycol administered via the dermal route.
Available data on the concentrations of ethylene glycol in both the occupational and general environments are exceedingly limited. Based on these exceedingly limited data, estimated total daily intakes of ethylene glycol from various media (i.e., ambient air, soil, food, and consumer products) for different age groups in the vicinity of a point source or from consumer products for adults approach or exceed the tolerable intake. Limitations of available data do not permit development of additional exposure scenarios. Moreover, the overall degree of confidence in the population exposure estimates presented here as an example is low, owing principally to the lack of current representative monitoring data for air, drinking-water, food, and consumer products.
For several age groups, estimated intake in air and soil in the vicinity of point sources slightly exceeds the tolerable intake. Estimated total daily intake is greatest for adults (the longest proportion of the life span) due to inclusion of upper-bounding estimates from consumer products for this subgroup of the population. Indeed, the single most important source of exposure to ethylene glycol for adults is via dermal exposure from consumer products. Based on estimated intake from polish/wax, latex paint, and tub and tile cleaner, the maximum estimated total daily intake of ethylene glycol in consumer products is 248.3 µg/kg body weight per day (i.e., 0.25 mg/kg body weight per day). However, it should be noted that this maximum reasonable worst-case estimate of total daily intake is based on assumptions of maximum concentrations of ethylene glycol in these products, 100% dermal absorption, and standardized use scenarios, since the limitations of available data preclude further refinement with sufficient confidence. When based on the assumption that dermal absorption is proportional to the concentration of ethylene glycol in the product and that steady-state penetration occurs for periods equivalent to the average duration of product use in the standardized scenario, estimated daily intakes of ethylene glycol from the use of these products are several orders of magnitude lower. However, available data on permeability through human skin from product formulations are inadequate as a basis for confident estimation of exposure.
Confidence in the estimates of intake of ethylene glycol in ambient air by a subpopulation exposed due to its proximity to a source of discharge to the atmosphere is low, since these estimates are based on a predicted maximum daily average concentration at a fixed distance from the source and not on measured concentrations. The frequency with which the predicted concentration in ambient air can occur is not reported, and there is no information concerning proximity to residences. Nevertheless, there is a moderate degree of certainty that the average daily intake by inhalation for the general population is much lower, since very few industrial point sources of discharge to the atmosphere are present in Canada and since the physicochemical properties of ethylene glycol indicate a tendency to remain in water or soil if discharged to these media.
There is a high degree of certainty that the estimates of intake from ingestion of soil by a population exposed due to its proximity to a source of discharge to the atmosphere are upper bounding. Estimated intakes were based on the maximum reported concentration in soil, which was more than 30 times greater than the next highest value (i.e., 119 mg/kg versus 4290 mg/kg). Levels of ethylene glycol were less than the detection limit (5 mg/kg) in the remaining 97% of soil samples collected near the manufacturing plant.
Confidence in the estimates of intake by ingestion of foods is low, since the few available data are from earlier studies in other countries. There is a high degree of uncertainty regarding the extent of migration of ethylene glycol from RCF and PETE bottles currently used in Canada. Although ethylene glycol was present in all Italian wines sampled by Gaetano & Matta (1987), at a maximum concentration of 6.25 mg/litre, the source of the ethylene glycol was not identified, and no other data concerning its presence in wine or other beverages were identified. Considerable additional uncertainty in the estimates of intake by ingestion of food arises from the assumption that the vast majority of foods consumed in Canada contain no ethylene glycol.
There is a high degree of uncertainty concerning exposure to ethylene glycol during use of various consumer products, related primarily to lack of information on the ranges and distributions of concentrations in any currently available consumer products in Canada. Indeed, the estimates presented herein may not be representative of the range of exposures of the general population to ethylene glycol in consumer products in Canada. For the few product classes considered, there is a reasonable degree of certainty that the estimates of intake by dermal absorption are upper bounding, since they are based on several conservative assumptions, including the maximum expected concentrations of ethylene glycol in these products and the assumption of complete dermal absorption. However, potential additional exposure through inhalation during use of these products has not been estimated.
The overall degree of confidence in the population exposure estimates is, therefore, low, owing principally to the lack of current representative monitoring data for air, drinking-water, food, and consumer products.
Available data are also inadequate to serve as a basis for quantitative estimation of exposure of aircraft passengers to ethylene glycol during deicing operations.
Additional investigation of absorption through human skin from product formulations in assays where there has been attention to maintenance and characterization of viability would likely reduce uncertainty in the estimates of exposure for products.
The degree of confidence in the database on toxicity that serves as the basis for development of the tolerable intake for ethylene glycol is moderate. Although clinical and epidemiological data are inadequate to serve as a basis for characterization of exposure–response for critical effects, available data in humans are at least sufficient for crudely characterizing potential sensitivity relative to experimental species. However, additional characterization of the metabolic profile of ethylene glycol in humans through determination of metabolites in blood and urine would provide additional relevant information.
There is reasonable confidence that ethylene glycol is unlikely to be carcinogenic to humans, based on negative results in two species (mice and rats) and lack of genotoxicity in a limited number of identified in vitro and in vivo assays. This conclusion must be qualified to some extent, though, due to limitations of dose selection in the bioassay in rats, which reduced its sensitivity.
There is a moderately high degree of confidence that the critical effect of ethylene glycol relevant to long-term exposure in the general environment is renal effects. This is based on their being observed at lowest concentrations in short- and medium-term studies for which histopathological reporting was adequate in a relatively robust data set in experimental animals and the observation of renal effects in acute poisoning cases in humans. Confidence that the tolerable intake developed on the basis of renal effects is protective for other adverse effects of ethylene glycol such as teratogenicity is moderate. Indeed, should more extensive information become available to serve as the basis for estimation of exposure through consumer products, averaging time in relation to relevant end-points needs to be considered additionally. Currently, estimates of intake in products, for example, have been averaged over a year, whereas for developmental/reproductive effects, peak exposures during product use may be a more appropriate basis for comparison.
The lack of data on progression of the renal lesions following long-term exposure in the most sensitive animal model examined to date is also a source of considerable uncertainty. This has been addressed in the development of the tolerable intake through application of an additional factor to account for less than long-term exposure. Protocols to address this important area of further research are being developed.
CICAD No. 22 (IPCS, 2000b) contains information on the environmental aspects of ethylene glycol. No previous evaluations of the human health aspects of ethylene glycol by international bodies were identified in the literature.
Abdelghani AA, Anderson AC, Khoury GA, Chang SN (1990) Fate of ethylene glycol in the environment. Prepared for the Louisiana Department of Transportation and Development. New Orleans, LA, Tulane University, 125 pp. (NU-FHWA/LA-90/228).
Ahn J-S, Lee K-H (1986) Studies on the volatile aroma components of edible mushroom (Tricholoma matsutake) of Korea. Journal of the Korean Society of Food and Nutrition, 15:253–257 [cited in BUA, 1994].
Anderson C, Sundberg K, Groth O (1986) Animal model for assessment of skin irritancy. Contact Dermatitis, 15:143–151.
ATSDR (1993) Draft technical report for ethylene glycol/propylene glycol. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, 101 pp. + appendices.
ATSDR (1997) Toxicological profile for ethylene glycol and propylene glycol. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, 249 pp.
Berenblum I, Haran N (1955) The initiating action of ethyl carbamate (urethane) on mouse skin. British Journal of Cancer, 9:453–456.
BIBRA International (1996) Ethylene glycol. Contract report prepared for Health Canada by BIBRA International, Surrey, October.
BIBRA International (1998) Ethylene glycol — Update to 1996 summary. Contract report prepared for Health Canada by BIBRA International, Surrey, November.
Bielnik K, Szram S (1992) Ultrastructural signs of myocardial damage due to acute experimental intoxication with ethylene glycol. Patologia Polska, 43:157–159.
Bielnik K, Szram S, Koktysz R (1992) Morphometric evaluation of myofibrillar mitochondria in experimental administration of ethylene glycol. Patologia Polska, 43:153–155.
Blomstrom DC, Beyer EM Jr (1980) Plants metabolise ethylene to ethylene glycol. Nature, 283:66–68.
Blood FR (1965) Chronic toxicity of ethylene glycol in the rat. Food and Cosmetics Toxicology, 3:229–234.
Blood FR, Elliott GA, Wright MA (1962) Chronic toxicity of ethylene glycol in the monkey. Toxicology and Applied Pharmacology, 4:489–491.
Bond GG, Shellenberger RJ, Flores GH, Cook RR, Fishbeck WA (1985) A case–control study of renal cancer mortality at a Texas chemical plant. American Journal of Industrial Medicine, 7:123–139.
Brega AA, Quadri P, Villa P, et al. (1992) Improved HPLC determination of plasma and urine oxalate in the clinical diagnostic laboratory. Journal of Liquid Chromatography, 15(3):501–511 [cited in ATSDR, 1997].
Brent J, McMartin K, Phillips S, Burkhart KK, Donovan JW, Wells M, Kulig K (1999) Fomepizole for the treatment of ethylene glycol poisoning. New England Journal of Medicine, 340:832–838.
Browning E (1965) Toxicity and metabolism of industrial solvents. Amsterdam, Elsevier, pp. 594–690.
BUA (1994) Ethylene glycol. German Chemical Society (GDCh) Advisory Committee on Existing Chemicals of Environmental Relevance (Beratergremium für Umweltrelevante Altstoffe). S. Hirzel, Wissenschaftliche Verlagsgesellschaft (BUA Report 92).
Buquet A, Manchon P (1970) Recherche et dosage des résidus et dérivés, dans un pain conservé à l’aide d’oxyde d’éthylène. Chimie Analytique, 52:978–983.
Carney EW (1994) An integrated perspective on the developmental toxicity of ethylene glycol. Reproductive Toxicology, 8:99–113.
Carney EW, Freshour NL, Dittenber DA, Dryzga MD (1999) Ethylene glycol developmental toxicity: unraveling the roles of glycolic acid and metabolic acidosis. Toxicological Sciences, 50:117–126.
Castle L, Cloke HR, Crews C, Gilbert J (1988a) The migration of propylene glycol, mono-, di-, and triethylene glycols from regenerated cellulose film into food. Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 187:463–467.
Castle L, Cloke HR, Startin JR, et al. (1988b) Gas chromatographic determination of monoethylene glycol and diethylene glycol in chocolate packaged in regenerated cellulose film. Journal of the Association of Official Analytical Chemists, 71(3):499–502 [cited in ATSDR, 1997].
Chaigneau M, Muraz B (1993) [Disinfection by ethylene glycol of some species.] Annals of Pharmacology, 51:47–53 (in French).
Chang JCS, Tichenor BA, Guo Z, Krebs KA (1997) Substrate effects on VOC emissions from a latex paint. Indoor Air, 7:241–247.
CIS (1997) CPI Product Profile: Ethylene glycols (mono, di, triethylene glycols). Don Mills, Ontario, Camford Information Services, 4 pp.
Clark CR, Marshall TC, Merickel BS, Sanchez A, Brownstein D, Hobbs CH (1979) Toxicological assessment of heat transfer fluids proposed for use in solar energy applications. Toxicology and Applied Pharmacology, 51:529–535.
Conan L, Foucault B, Siou G, Chaigneau M, Le Moan G (1979) Contribution à la recherche d’une action mutagène des résidus d’oxyde d’éthylène, d’éthylène glycol et de chloro-2-éthanol dans le matériel plastique stérilisé par l’oxyde d’éthylène. Annales des Falsifications et de l’Expertise Chimique, 72:141–151.
Coon RA, Jones RA, Jenkins LJ Jr, Siegel J (1970) Animal inhalation studies on ammonia, ethylene glycol, formaldehyde, dimethylamine, and ethanol. Toxicology and Applied Pharmacology, 16:646–655.
Dawson TAJ (1976) Ethylene glycol sensitivity. Contact Dermatitis, 2:233.
DePass LR, Garman RH, Woodside MD, Giddens WE, Maronpot RR, Weil CS (1986a) Chronic toxicity and oncogenicity studies of ethylene glycol in rats and mice. Fundamental and Applied Toxicology, 7:547–565.
DePass LR, Woodside MD, Maronpot RR, Weil CS (1986b) Three-generation reproduction and dominant lethal mutagenesis studies of ethylene glycol in the rat. Fundamental and Applied Toxicology, 7:566–572.
DFG (1991) Occupational toxicants. Critical data evaluation for MAK values and classification of carcinogens. Volume 4. Bonn, Deutsche Forschungsgemeinschaft, Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area, pp. 224–245.
Driver J, Tardiff RG, Sedik L (1993) In vitro percutaneous absorption of [14C] ethylene glycol. Journal of Exposure Analysis and Environmental Epidemiology, 3:277–284.
Dunkelberg H (1987) Carcinogenic activity of ethylene oxide and its reaction products 2-chloroethanol, 2-bromoethanol, ethylene glycol and diethylene glycol. III. Testing of ethylene glycol and diethylene glycol for carcinogenicity. Zentralblatt für Bakteriologie und Hygiene B, 183:358–365.
EHD (1998) Draft internal report on exposure factors for assessing total daily intake of priority substances by the general population of Canada. Ottawa, Ontario, Health Canada, Environmental Health Directorate, Bureau of Chemical Hazards.
Environment Canada (1997) Results of the CEPA Section 16 Notice respecting the second Priority Substances List and di(2-ethylhexyl) phthalate. Hull, Quebec, Environment Canada, Commercial Chemicals Evaluation Branch, Use Patterns Section.
Environment Canada, Health Canada (2000) Canadian Environmental Protection Act, 1999 — Priority Substances List — State of the science report for ethylene glycol. Hull, Quebec, Environment Canada; and Ottawa, Ontario, Health Canada.
Fiserove-Bergerova V, Pierce JT, Droz PO (1990) Dermal absorption potential of industrial chemicals: criteria for skin notation. American Journal of Industrial Medicine, 17:617–635.
Flick EW (1986) Household and automotive cleaners and polishes, 3rd ed. Park Ridge, NJ, Noyes Publications.
Flick EW (1989) Advanced cleaning product formulations: household, industrial, automotive. Volume I. Park Ridge, NJ, Noyes Publications, 339 pp.
Franklin Associates Ltd. (1995) Life cycle assessment of ethylene glycol and propylene glycol based heat transfer fluids. Final report and peer review. Prepared for Union Carbide Corporation by Franklin Associates Ltd.
Frantz SW, Beskitt JL, Tallant MJ, Zourelias LA, Ballantyne B (1996a) Pharmacokinetics of ethylene glycol. III. Plasma disposition and metabolic fate after single increasing intravenous, peroral, or percutaneous doses in the male Sprague-Dawley rat. Xenobiotica, 26:515–539.
Frantz SW, Beskitt JL, Grosse CM, Tallant MJ, Dietz FK, Ballantyne B (1996b) Pharmacokinetics of ethylene glycol. II. Tissue distribution, dose-dependent elimination, and identification of urinary metabolites following single intravenous, peroral or percutaneous doses in female Sprague-Dawley rats and CD-1 mice. Xenobiotica, 26:1195–1220.
Gaetano G, Matta M (1987) Identification and quantitative evaluation of 1,2-ethanediol in wines. Vini d’Italia, 29:7–10.
Gaunt IF, Hardy J, Gangolli SD, Butterworth KR, Lloyd AG (1974) Short-term toxicity of monoethylene glycol in the rat. Carshalton, Surrey, BIBRA International, pp. 1–31 (Research Report 4/1974).
Gérin M, Patrice S, Bégin D, Goldberg MS, Vysjicuk A, Adib G, Drilet D, Viau C (1997) A study of ethylene glycol exposure and kidney function of aircraft de-icing workers. International Archives of Occupational and Environmental Health, 69:255–265.
Giachetti C, Zanolo G, Assandri A, et al. (1989) Determination of cyclic butylboronate esters of some 1,2- and 2,3-diols in plasma by high-resolution gas chromatography/mass spectrometry. Biomedical and Environmental Mass Spectrometry, 18(8):592–597 [cited in ATSDR, 1997].
Grant WM, Schuman JS (1993) Toxicology of the eye, 4th ed. Springfield, IL, Charles C. Thomas Publisher, pp. 663–669.
Grauer GF, Thrall MAH, Henre BA, Hjelle JJ (1987) Comparison of the effects of ethanol and 4-methylpyrazole on the pharmacokinetics and toxicity of ethylene glycol in the dog. Toxicology Letters, 35:307–314.
Guillot JP, Martini MC, Giauffret JY, Gonnet JF, Guyot JY (1982) Safety evaluation of some humectants and moisturizers used in cosmetic formulations. International Journal of Cosmetological Science, 4:67–80.
Hannuksela M, Pirilä V, Salo OP (1975) Skin reactions to propylene glycol. Contact Dermatitis, 1:112–116.
Harris MW, Chapin RE, Lockhart AC, Jokinen MP (1992) Assessment of a short-term reproductive and developmental toxicity screen. Fundamental and Applied Toxicology, 19:186–196.
Health Canada (1994) Canadian Environmental Protection Act — Human health risk assessment for priority substances. Ottawa, Ontario, Canada Communication Group.
Health Canada (2000) Canadian Environmental Protection Act — Priority Substances List — Supporting document for the health assessment of ethylene glycol. Ottawa, Ontario, Health Canada, Environmental Health Directorate, Bureau of Chemical Hazards.
Hewlett TP, Ray AC, Reagor JC (1983) Diagnosis of ethylene glycol (antifreeze) intoxication in dogs by determination of glycolic acid in serum and urine with high pressure liquid chromatography and gas chromatography–mass spectrometry. Journal of the Association of Official Analytical Chemists, 66(2):276–283 [cited in ATSDR, 1997].
Hewlett TP, Jacobsen P, Collins TD, McMartin KE (1989) Ethylene glycol and glycolate kinetics in rats and dogs. Veterinary and Human Toxicology, 31:116–120.
Hindson C, Ratcliffe G (1975) Ethylene glycol in glass lens cutting. Contact Dermatitis, 1:386–387.
Hodgman MJ, Wezorek C, Krenzelok E (1997) Toxic inhalation of ethylene glycol: a pharmacological improbability. Clinical Toxicology, 35:109–111.
Hong HL, Canipe J, Jameson CW, Boorman GA (1988) Comparative effects of ethylene glycol and ethylene glycol monomethyl ether exposure on hematopoiesis and histopathology in B6C3F1 mice. Journal of Environmental Pathology, Toxicology, and Oncology, 8:27–38.
Howe R (1995) THRESH: A computer program to compute a reference dose from quantal animal toxicity data using the benchmark dose method. Ruston, LA, ICF Kaiser Engineers, Inc.
HSDB (2001) Hazardous substances data bank. Bethesda, MD, US National Library of Medicine.
Iguchi M, Takamura C, Umekawa T, Kurita T, Kohri K (1999) Inhibitory effects of female sex hormones on urinary stone formation in rats. Kidney International, 56:479–485.
IPCS (2000a) International Chemical Safety Card — Ethylene glycol. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0270).
IPCS (2000b) Ethylene glycol: Environmental aspects. Geneva, World Health Organization, International Programme on Chemical Safety (Concise International Chemical Assessment Document No. 22).
Jacobsen D, McMartin KE (1986) Methanol and ethylene glycol poisonings. Mechanisms of toxicity, clinical course, diagnosis, and treatment. Medical Toxicology, 1:309–334.
Jacobsen D, Hewlett TP, Webb R, Brown ST, Ordinario AT, McMartin KE (1988) Ethylene glycol intoxication: evaluation of kinetics and crystalluria. American Journal of Medicine, 84:145–152.
JETOC (1996) Mutagenicity test data of existing chemical substances. Based on the toxicity investigation system of the Industrial Safety and Health Law. Tokyo, Japanese Chemical Industry Ecology-Toxicology and Information Center, pp. 190–191.
Kaiser RE, Rieder RI (1987) Native ethylene glycol in wine. Application of a dead volume free, very fast "Deans heart-cut" system on-line with multi-chromatography. Journal of High Resolution Chromatography and Chromatography Communications, 10:240–243.
Kashtock M, Breder CV (1980) Migration of ethylene glycol from polyethylene terephthalate bottles into 3% acetic acid. Journal of the Association of Offical Analytical Chemists, 63:168–172.
Khera KS (1991) Chemically induced alterations in maternal homeostasis and histology of conceptus: their etiologic significance in rat fetal anomalies. Teratology, 44:259–297.
Kim H, Gilbert SG, Johnson JB (1990) Determination of potential migrants from commercial amber polyethylene terephthalate bottle wall. Pharmacological Research, 7:176–179.
Lamb JA, Maronpot RR, Gulati DK, Russell VS, Hommel-Barnes L, Sabharwal PS (1985) Reproductive and developmental toxicity of ethylene glycol in the mouse. Toxicology and Applied Pharmacology, 81:100–112.
LDOTD (1990) Fate of ethylene glycol in the environment. Baton Rouge, LA, Louisiana Department of Transportation and Development, Louisiana Transportation Research Center [cited in ATSDR, 1997].
Lee YH, Huang WC, Chiang H, Chen MT, Huang JK, Chang L (1992) Determinant role of testosterone in the pathogenesis of urolithiasis in rats. Journal of Urology, 147:1134–1138.
Lee YH, Huang WC, Huang JK, Chang LS (1996) Testosterone enhances whereas estrogen inhibits calcium oxalate stone formation in ethylene glycol treated rats. Journal of Urology, 156:502–505.
Lewis RJ, ed. (1993) Hawley’s condensed chemical dictionary, 12th ed. New York, NY, Van Nostrand Reinhold Co.
Loden M (1986) The in vitro permeability of human skin to benzene, ethylene glycol, formaldehyde, and n-hexane. Acta Pharmacologica et Toxicologica, 58:382–389.
Manius GJ (1979) Determination of ethylene oxide, ethylene chlorohydrin, and ethylene glycol residues in ophthalmic solutions at proposed concentration limits. Journal of Pharmacological Science, 68:1547–1549.
Maronpot RR, Zelenak JP, Weaver EV, Smith JN (1983) Teratogenicity study of ethylene glycol in rats. Drug and Chemical Toxicology, 6:579–594.
Marr MC, Price CJ, Myers CB, Morrissey RE (1992) Developmental stages of the CD7 (Sprague-Dawley) rat skeleton after maternal exposure to ethylene glycol. Teratology, 46:169–181.
Marshall TC (1982) Dose-dependent disposition of ethylene glycol in the rat after intravenous administration. Journal of Toxicological and Environmental Health, 10:397–409.
Marshall TC, Cheng YS (1983) Deposition and fate of inhaled ethylene glycol vapor and condensation aerosol in the rat. Fundamental and Applied Toxicology, 3:175–181.
Mason MM, Cate CC, Baker J (1971) Toxicology and carcinogenesis of various chemicals used in the preparation of vaccine. Clinical Toxicology, 4:185–204.
McCarroll NE, Piper CE, Keech BH (1981) An E. coli microsuspension assay for the detection of DNA damage induced by direct-acting agents and promutagens. Environmental Mutagenesis, 3:429–444.
McChesney EW, Goldberg L, Pakekh CK, Russell JC, Min BH (1971) Reappraisal of the toxicology of ethylene glycol. II. Metabolism studies in laboratory animals. Food and Cosmetics Toxicology, 9:21–38.
McDonald TO, Roberts MD, Borgmann AR (1972) Ocular toxicity of ethylene chlorohydrin and ethylene glycol in rabbit eyes. Toxicology and Applied Pharmacology, 21:143–150.
McGregor DB, Brown AG, Howgate S, McBride D, Riach C, Caspary WJ (1991) Responses of the L5178Y mouse lymphoma cell forward mutation assay. V.27 coded chemicals. Environmental and Molecular Mutagenesis, 17:196–219.
Melnick RL (1984) Toxicities of ethylene glycol and ethylene glycol monoethyl ether in Fischer 344/N rats and B6C3F1 mice. Environmental Health Perspectives, 57:147–155.
Meneghini CL, Rantuccio F, Lomuto M (1971) Additives, vehicles and active drugs of topical medicaments as causes of delayed-type allergic dermatitis. Dermatologica, 143:137–147.
Moreau CL, Kerns W, Tomaszewski CA, McMartin KE, Rose SR, Ford MD, Brent J (1998) Glycolate kinetics and haemodialysis clearance in ethylene glycol poisoning. Clinical Toxicology, 36:659–666.
Morris HJ, Nelson AA, Calvery HO (1942) Observations on the chronic toxicities of propylene glycol, ethylene glycol, diethylene glycol, ethylene glycol monoethyl ether and diethylene glycol monoethyl ether. Journal of Pharmacology and Experimental Therapeutics, 74:266–273.
Morrissey RE, Lamb JC IV, Morris RW, Chapin RE, Gulati DK, Heindel JJ (1989) Results and evaluations of 48 continuous breeding reproduction studies conducted in mice. Fundamental and Applied Toxicology, 13:747–777.
Munley SM, Hurrt ME (1996) Developmental toxicity study of glycolic acid in rats. Teratology, 53:117.
Muzeni RJ (1985) Rapid gas chromatographic determination of ethylene oxide, ethylene chlorohydrin, and ethylene glycol residues in rubber catheters. Journal of the Association of Official Analytical Chemists, 68(3):506–508 [cited in ATSDR, 1997].
Nagano K, Nakayama E, Koyano M, Oobayashi H, Adachi H, Yamada T (1973) Testicular atrophy of mice induced by ethylene glycol mono alkyl ethers. Japanese Journal of Industrial Health, 21:29–35.
Nagano K, Nakayama E, Oobayashi H, Nishizawa T, Okuda H, Yamazaki K (1984) Experimental studies on toxicity of ethylene glycol alkyl ethers in Japan. Environmental Health Perspectives, 57:75–84.
Neeper-Bradley TL, Tyl RW, Fisher LC, Kubena MF, Vrbanic MA, Losco PE (1995) Determination of a no-observed-effect level for developmental toxicity of ethylene glycol administered by gavage to CD rats and CD-1 mice. Fundamental and Applied Toxicology, 27:121–130.
NIOSH (1990) National Occupational Exposure Survey (NOES) 1981–1983. Rockville, MD, US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health [cited in ATSDR, 1997].
NIOSH (1994) NIOSH health hazard evaluation report. New York, NY, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health (HETA 90-0355-2449).
NIOSH (1996) Glycols. Method 5523. In: NIOSH manual of analytical methods, 4th ed. Washington, DC, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health.
NTP (1986) Ethylene glycol: Reproduction and fertility assessment in CD-1 mice when administered in drinking water. Final report. Prepared by Environmental Health Research and Testing Inc., Lexington, KY, for National Toxicology Program, National Institutes of Health, Research Triangle Park, NC, 464 pp. (PB86-177383).
NTP (1988) Developmental toxicity evaluation of ethylene glycol (CAS
NTP (1993) NTP technical report on the toxicology and carcinogenesis studies of ethylene glycol (CAS Nos.
Parry MF, Wallach R (1974) Ethylene glycol poisoning. American Journal of Medicine, 57:143–149.
Penumarthy L, Oehme FW (1975) Treatment of ethylene glycol toxicosis in cats. American Journal of Veterinary Research, 36:209–212.
Percy R (1992) A report for Transport Canada on ethylene glycol exposure levels at Thunder Bay airport. Ottawa, Ontario, Health and Welfare Canada, Medical Services Branch, 3 pp.
Pfeiffer EH, Dunkelberg H (1980) Mutagenicity of ethylene oxide and propylene oxide and of the glycols and halohydrins formed from them during the fumigation of foodstuffs. Food and Cosmetics Toxicology, 18:115–118.
Price CJ, Kimmel CA, Tyl RW, Marr MC (1985) The developmental toxicity of ethylene glycol in rats and mice. Toxicology and Applied Pharmacology, 81:113–127.
Reif G (1950) Selbstversuche Äthylenglykol. Pharmazie, 5:276–278 [cited in McChesney et al., 1971; DFG, 1991].
Riley JH, O’Brien S, Riley MG (1982) Urine and tissue oxalate and hippurate levels in ethylene glycol intoxication in the dog. Veterinary and Human Toxicology, 24:331–334.
Roberts JA, Seibold HR (1969) Ethylene glycol toxicity in the monkey. Toxicology and Applied Pharmacology, 15:624–631.
Robinson M, Pond CL, Laurie RD, Bercz JP, Henningsen G, Condie LW (1990) Subacute and subchronic toxicity of ethylene glycol administered in drinking water to Sprague-Dawley rats. Drug and Chemical Toxicology, 13:43–70.
Rowe VK, Wolf MA (1982) Glycols. In: Clayton GD, Clayton FE, eds. Patty’s industrial hygiene and toxicology, 3rd ed. Volume 2C. Toxicology. New York, NY, John Wiley & Sons, pp. 3817–3853 [cited in ATSDR, 1997].
RTECS (1999) Data profile for ethylene glycol. Cincinnati, OH, National Institute of Occupational Safety and Health, Registry of Toxic Effects of Chemical Substances.
Schladt L, Ivens I, Karbe E, Rühl-Fehlert C, Bomhard E (1998) Subacute oral toxicity of tetraethylene glycol and ethylene glycol administered to Wistar rats. Experimental Toxicology and Pathology, 50:257–265.
Seidenari S, Manzini BM, Danse P, Motolese A (1990) Patch and prick test study of 593 healthy subjects. Contact Dermatitis, 23:162–167.
Smith NB (1984) Determination of serum ethylene glycol by capillary gas chromatography. Clinica Chimica Acta, 162(1):269–272 [cited in ATSDR, 1997].
Storer RD, McKelvey TW, Kraynak AR, Elia MC, Barnum JE, Harmon LS, Nichols WW, DeLuca JG (1996) Revalidation of the in vitro alkaline elution/rat hepatocyte assay for DNA damage: improved criteria for assessment of cytotoxicity and genotoxicity and results for 81 compounds. Mutation Research, 368:59–101.
Sun JD, Frantz SW, Beskitt J (1995) In vitro skin penetration of ethylene glycol using excised skin from mice and humans. Journal of Toxicology, 14:273–286.
Takei Y (1988) [Aroma components of roasted sesame seed and roasted huskless sesame seed.] Nippon Kasei Gakkaishi, 39:803–815 (in Japanese).
Tyl RW, Price CJ, Marr MC, Myers CB, Seely JC, Heindel JJ, Schwetz BA (1993) Developmental toxicity evaluation of ethylene glycol by gavage in New Zealand white rabbits. Fundamental and Applied Toxicology, 20:402–412.
Tyl RW, Ballantyne B, Fisher LC, Fait DL, Savine TA, Dodd DE, Klonne DR, Pritts IM (1995a) Evaluation of the developmental toxicity of ethylene glycol aerosol in the CD rat and CD-1 mouse by whole-body exposure. Fundamental and Applied Toxicology, 24:57–75.
Tyl RW, Ballantyne B, Fisher LC, Fait DL, Dodd DE, Klonne DR, Pritts IM, Losco PE (1995b) Evaluation of the developmental toxicity of ethylene glycol aerosol in CD-1 mice by nose-only exposure. Fundamental and Applied Toxicology, 27:49–62.
Tyl RW, Fisher LC, Kubena MF, Vrbanic MA, Losco PE (1995c) Assessment of the developmental toxicity of ethylene glycol applied cutaneously to CD-1 mice. Fundamental and Applied Toxicology, 27:155–166.
US EPA (1986) Standard scenarios for estimating exposure to chemical substances during use of consumer products. Volumes I and II. Prepared by Versar Inc. for Exposure Evaluation Division, Office of Toxic Substances, US Environmental Protection Agency, Washington, DC (EPA Contract No. 68-02-3968).
US EPA (1987) Health effects assessment for ethylene glycol. Cincinnati, OH, US Environmental Protection Agency, Environmental Criteria and Assessment Office, 44 pp. (EPA/600/8-88/038).
US EPA (1995a) Test methods for evaluating solid waste; Method 8015b, revision 2, January 1995; Nonhalogenated organics using GC/FID SW 846. Washington, DC, US Environmental Protection Agency [cited in ATSDR, 1997].
US EPA (1995b) Test methods for evaluating solid waste; Method 8430, revision 0, January 1995; Nonhalogenated organics using GC/FID SW 846. Washington, DC, US Environmental Protection Agency [cited in ATSDR, 1997].
US EPA (1997) Exposure factor handbook volume III — activity factors. Washington, DC, US Environmental Protection Agency, Office of Reseach and Development, National Center for Environmental Assessment, August 1997 (EPA/600/P-95/002Fc).
von der Hude W, Behm C, Gurtler R, Basler A (1988) Evaluation of the SOS chromotest. Mutation Research, 203:81–94.
Wiley JF (1999) Novel therapies for ethylene glycol intoxication. Current Opinion in Pediatrics, 11:269–273.
Wills JH, Coulston F, Harris ES, McChesney EW, Russell JC, Serrone DM (1974) Inhalation of aerosolized ethylene glycol by man. Clinical Toxicology, 7:463–476.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W (1987) Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environmental Mutagenesis, 9(Suppl. 9):1–110.
Zorzano A, Herrera E (1990) Differences in kinetic characteristics and in sensitivity to inhibitors between human and rat liver alcohol dehydrogenase and aldehyde dehydrogenase. General Pharmacology, 21:697.
Environment Canada & Health Canada (2000); Health Canada (2000)
The Canadian Environmental Protection Act, 1999 — Priority Substances List — State of the science report for ethylene glycol (Environment Canada & Health Canada, 2000) is available at the following URL:
www.ec.gc.ca/cceb1/eng/public/index_e.html
More detailed supporting documentation (Health Canada, 2000) may be obtained from:
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney’s Pasture
Ottawa, Ontario
Canada
K1A 0L2
Initial drafts of the health-related sections of the supporting documentation and State of the science report for ethylene glycol were prepared by staff of Health Canada. H. Hirtle contributed additional information in the preparation of the draft CICAD. Studies related to dermal absorption relevant to this assessment were reviewed by R. Moody of the Product Safety Bureau of Health Canada. Advice on interpretation of histopathological lesions reported in critical studies was provided by D. Wolf, National Health and Environmental Effects Research Laboratory, US Environmental Protection Agency, and R. Maronpot, US National Institute of Environmental Health Sciences and National Toxicology Program. M. Wade, Environmental and Occupational Toxicology Division of Health Canada, contributed to the interpretation of data on reproductive/developmental toxicity.
Sections of the supporting documentation pertaining to human health were reviewed externally by the Ethylene Glycol Panel of the Chemical Manufacturers Association, primarily to address adequacy of coverage. Members of the panel included W. Snellings, Union Carbide Corporation; W. Faber, Eastman Kodak; R. Gingell, Shell Chemical Company; and S. Jasti, BASF Corporation.
Accuracy of reporting, adequacy of coverage, and defensibility of conclusions with respect to hazard characterization and dose–response analyses were considered at a panel meeting of the following members, convened by Toxicology Excellence in Risk Assessment (TERA), on 14 February 2000, in Ottawa, Ontario, and during an additional teleconference, held 29 March 2000:
M.S. Abdel-Rahman, University of Medicine and Dentistry of New Jersey
C. Abernathy, Office of Water, US Environmental Protection Agency
J.P. Christopher, California Environmental Protection Agency
J.C. Collins, Solutia, Inc.
J.T. Colman, Syracuse Research Corporation
M. Mumtaz, Agency for Toxic Substances and Disease Registry
K.A. Poirier, TERA
J.E. Whalan, US Environmental Protection Agency
R. Maronpot, US National Institute of Environmental Health Sciences and National Toxicology Program, and E. Ohanian, Office of Water, US Environmental Protection Agency, provided advice on adequacy of histopathological reporting in one of the critical studies during the teleconference.
The draft CICAD on ethylene glycol was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
American Chemistry Council, USA
M. Baril, Institut de Recherche en Santé et en Sécurité du Travail du Québec, Canada
R. Beliles, Office of Research and Development, US Environmental Protection Agency, USA
B. Benson, Drinking Water Program, US Environmental Protection Agency, USA
J. Brent, Health Sciences Center, University of Colorado, USA
R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, USA
C. Elliott-Minty, Industrial Chemicals Unit, Health and Safety Executive, United Kingdom
E. Frantik, Centre of Industrial Hygiene and Occupational Diseases, National Institute of Public Health, Czech Republic
R. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Germany
C. Hiremath, Office of Research and Development, US Environmental Protection Agency, USA
Japanese Chemical Industry Association, Japan
M. Matisons, Environmental Health Service, Department of Health, Australia
H. Nagy, National Institute of Occupational Safety and Health, USA
K. Ziegler-Skylakakis, Commission of the European Communities, Luxembourg
Ottawa, Canada,
29 October – 1 November 2001
Members
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr T. Chakrabarti, National Environmental Engineering Research Institute, Nehru Marg, India
Dr B.-H. Chen, School of Public Health, Fudan University (formerly Shanghai Medical University), Shanghai, China
Dr R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA (teleconference participant)
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA (Chairman)
Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Vice-Chairman)
Dr O. Faroon, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Ms R. Gomes, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr M. Gulumian, National Centre for Occupational Health, Johannesburg, South Africa
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Dr A. Hirose, National Institute of Health Sciences, Tokyo, Japan
Mr P. Howe, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Co-Rapporteur)
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany (Co-Rapporteur)
Dr S.-H. Lee, College of Medicine, The Catholic University of Korea, Seoul, Korea
Ms B. Meek, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr J.A. Menezes Filho, Faculty of Pharmacy, Federal University of Bahia, Salvador, Bahia, Brazil
Dr R. Rolecki, Nofer Institute of Occupational Medicine, Lodz, Poland
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan
Dr S.A. Soliman, Faculty of Agriculture, Alexandria University, Alexandria, Egypt
Dr M.H. Sweeney, Document Development Branch, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr J. Temmink, Department of Agrotechnology & Food Sciences, Wageningen University, Wageningen, The Netherlands
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme (NICNAS), Sydney, Australia
Representative of the European Union
Dr K. Ziegler-Skylakakis, European Commission, DG Employment and Social Affairs, Luxembourg
Observers
Dr R.M. David, Eastman Kodak Company, Rochester, NY, USA
Dr R.J. Golden, ToxLogic LC, Potomac, MD, USA
Mr J.W. Gorsuch, Eastman Kodak Company, Rochester, NY, USA
Mr W. Gulledge, American Chemistry Council, Arlington, VA, USA
Mr S.B. Hamilton, General Electric Company, Fairfield, CN, USA
Dr J.B. Silkworth, GE Corporate Research and Development, Schenectady, NY, USA
Dr W.M. Snellings, Union Carbide Corporation, Danbury, CN, USA
Dr E. Watson, American Chemistry Council, Arlington, VA, USA
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Ce CICAD sur l’éthylène-glycol (aspects touchant la santé humaine) a été préparé par la Direction de l’Hygiène du milieu de Santé Canada sur la base d’une documentation rédigée dans le cadre du Programme d’évaluation des produits chimiques prioritaires prévu par la Loi canadienne sur la protection de l’environnement (LCPE). Les évaluations des substances prioritaires effectuées en application de cette loi portent sur les effets que pourraient avoir ces produits sur la santé humaine en cas d’exposition indirecte dans l’environnement général ainsi que sur leurs effets sur l’environnement lui-même. Dans le présent document toutefois, seuls les effets sur la santé humaine sont pris en considération. La présente mise au point prend en compte les données jusqu’à fin janvier 2000.17 L’appendice 1 donne des informations sur la nature de l’examen par des pairs et sur les sources documentaires. D’autres études ont également été utilisées, à savoir celles de l’Environmental Health Criteria Assessment Office de l’US Environmental Protection Agency (US EPA, 1987), de l’Agency for Toxic Substances and Disease Registry de l’US Department of Health and Human Services (ATSDR, 1997) et de la Société allemande de Chimie (BUA, 1994) ainsi que celles que BIBRA International (1996, 1998) a effectuées sous contrat. Des renseignements sur l’examen par des pairs du présent CICAD sont donnés à l’appendice 2. Ce CICAD a été adopté en tant qu’évaluation internationale lors de la réunion du Comité d’évaluation finale qui s’est tenue à Ottawa (Canada) du 29 octobre au 1er novembre 2001. La liste des participants à cette réunion figure à l’appendice 3. La fiche internationale sur la sécurité chimique (ICSC 0270) de l’éthylène-glycol, établie par le Programme international sur la sécurité chimique (IPCS, 2000a), est également reproduite dans le présent document. Les effets de ce composé sur l’environnement ne sont pas examinés car ils font l’objet du CICAD No 22 (IPCS, 2000b).
L’éthylène-glycol (No CAS
On l’utilise pour la production de téréphtalate de polyéthylène, le traitement du gaz naturel ou encore comme antigel. Les données de surveillance sur lesquelles s’appuyer pour évaluer l’exposition de la population générale sont extrêmement limitées. Il existe par exemple une estimation de l’exposition dans laquelle l’absorption à partir de l’air et du sol à proximité d’une source ponctuelle du produit a été évaluée sur la base de données modellisées, l’absorption à partir des aliments étant établie sur la base de la concentration observée dans divers pays dans un nombre très limité de denrées alimentaires. L’absorption par voie percutanée a été également évaluée pour les quelques produits dont on connaissait la teneur en éthylène-glycol.
On a tout lieu de penser que l’effet toxique de l’éthylène-glycol est principalement dû à ses métabolites (glycolate et oxalate, notamment). Les données disponibles montrent également que la métabolisation de ce composé s’effectue sans doute selon des voies qui sont qualitativement analogues chez l’Homme et les autres mammifères; les différences quantitatives qui pourraient exister n’ont pas été étudiées de façon approfondie.
Administré à des animaux de laboratoire par voie orale, respiratoire ou cutanée, l’éthylène-glycol ne présente qu’une faible toxicité aiguë. Chez l’Homme comme chez l’animal, il est très peu irritant pour la peau. Une irritation nasale et laryngée a été observée chez un petit nombre de sujet ayant inhalé ce composé, l’irritation étant très marquée à forte concentration. Chez les animaux de laboratoire, une exposition à l’éthylène-glycol ne produit qu’une irritation conjonctivale minime, sans atteinte cornéenne permanente. On n’a pas trouvé de données relatives au pouvoir sensibisateur de ce composé.
Une étude biologique de 2 ans sur le rat et la souris, de même que d’autres travaux plus anciens et plus limités, n’ont pas permis de mettre en évidence une activité cancérogène. L’éthylène-glycol n’est pas non plus génotoxique, du moins d’après les études in vitro et in vivo en nombre limité dont on dispose.
Les données existantes relatives à des cas humains d’intoxication aiguë, de même que les résultats d’études toxicologique comportant l’administration de doses répétées d’éthylène-glycol à des animaux de laboratoire, montrent que l’organecible de ce composé est le rein chez l’Homme comme chez l’animal. Après administration de faibles doses du composé, on observe d’ailleurs régulièrement une acidose métabolique ainsi que des lésions dégénératives non néoplasiques au niveau de cet organe (dilatation, dégénérescence et dépôt d’oxalates dans les tubules) chez diverses espèces.
Il existe au sujet de l’éthylène-glycol une base de données plutôt abondante selon laquelle cette molécule exerce des effets sur le développement chez le rat et la souris, encore qu’à des doses plus élevées que celles qui produisent des anomalies rénales chez le rat mâle. En fait, l’éthylène-glycol est tératogène et provoque surtout des anomalies du squelette et des malformations externes, parfois à des doses plus faibles que celles qui sont toxiques pour la mère, la souris étant à cet égard plus sensible que le rat. La toxicité génésique de cette molécule a été largement et valablement étudiée chez le rat et la souris. Après administration répétée d’éthylène-glycol, on n’a pas constaté d’effets indésirables sur l’appareil reproducteur. Des études spécialisées et en particulier une étude portant sur trois générations de rats et d’autres effectuées sur des élevages continus de souris, ont montré que les effets sur la reproduction ne s’observaient que chez la souris (à l’exclusion du rat et du lapin) et seulement à des doses très supérieures à celles qui provoquaient des effets sur le développement de cet animal ou des anomalies rénales chez le rat.
Les données disponibles ne sont pas suffisantes pour permettre de se prononcer sur la possibilité d’effets neurologiques ou immunologiques consécutifs à une exposition de longue durée, mais on a fait état de troubles neurocomportementaux et neurologiques lors d’intoxications aiguës par l’éthylène-glycol chez l’Homme. Selon le petit nombre d’études dont on a eu connaissance jusqu’ici, ces effets neurologiques ne s’observent pas aux doses inférieures aux doses néphrotoxiques. Lors des études toxicologiques au cours desquelles plusieurs espèces ont été exposées de façon répétée à de l’éthylène-glycol par voie orale ou respiratoire, on n’a pas observé d’effets systématiques sur les paramètres du système immunitaire qui soient imputables au traitement par ce composé.
On a attribué à cette substance une dose journalière tolérable de 0,05 mg/kg de poids corporel en prenant comme référence la dose journalière de 49 mg/kg p.c. calculée dans le cas des effets rénaux non néoplasiques et en lui appliquant un coefficient d’incertitude de 1000. Cette dose tolérable reste néanmoins entachée d’incertitude, principalement en raison du manque de données sur l’évolution des lésions rénales chez le modèle animal le plus sensible. Quelques estimations très peu sûres portant sur l’exposition de sujets appartenant à certaines tranches d’âge et vivant à proximité de sources ponctuelles d’éthylène-glycol ou concernant des adultes ayant utilisé certains produits de consommation, font état de valeurs qui approchent ou dépassent celle de la dose tolérable. Il est recommandé de procéder à d’autres études pour mieux caractériser l’évolution des lésions rénales et affiner les estimations relatives à l’exposition.
Este CICAD sobre el etilenglicol (aspectos relativos a la salud humana), preparado por la Dirección de Higiene del Medio del Ministerio de Sanidad del Canadá, se basó en la documentación preparada como parte del Programa de Sustancias Prioritarias en el marco de la Ley Canadiense de Protección del Medio Ambiente (CEPA). Las evaluaciones de sustancias prioritarias previstas en la CEPA tienen por objeto valorar los efectos potenciales para la salud humana de la exposición indirecta en el medio ambiente general, así como los efectos ecológicos, aunque sólo se examinan los aspectos relativos a la salud humana. En este examen se incluyen los datos identificados hasta el final de enero de 2000.18 La información relativa al carácter del examen colegiado del documento original y su disponibilidad figura en el apéndice 1. También se consultaron otros exámenes, entre ellos los de la Oficina de Prevención de la Contaminación y de Sustancias Tóxicas de la Agencia para la Protección del Medio Ambiente de los Estados Unidos (US EPA, 1987), la Agencia para el Registro de Sustancias Tóxicas y Enfermedades, Departamento de Salud y Servicios Sociales de los Estados Unidos (ATSDR, 1997) y la Sociedad Alemana de Química (BUA, 1994), así como los exámenes preparados por contrato por BIBRA International (1996, 1998). La información sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final celebrada en Ottawa, Canadá, del 29 de octubre al 1ş de noviembre de 2001. La lista de participantes en esta reunión figura en el apéndice 3. La Ficha internacional de seguridad química (ICSC 0270) para el etilenglicol, preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2000a), también se reproduce en este documento. No se examinan los efectos del etilenglicol en el medio ambiente, porque ya se abordaron en el CICAD Nş 22 (IPCS, 2000b).
El etilenglicol (CAS Nş
El etilenglicol se utiliza en la fabricación del tereftalato de polietileno, en el tratamiento del gas natural y como agente anticongelante. Los datos de vigilancia en los cuales se basan las estimaciones de la exposición de la población general al etilenglicol son muy limitados. En una estimación de muestra de la exposición se calculó la absorción a partir del aire y el suelo en las cercanías de una fuente puntual basándose en los datos obtenidos de modelos, y la relativa a los alimentos se basó en las concentraciones notificadas en una gama muy limitada de productos alimenticios de diversos países. También se estimó la absorción cutánea para una serie limitada de productos cuyo contenido de etilenglicol se había determinado.
Hay pruebas convincentes de que la toxicidad del etilenglicol está mediada principalmente por metabolitos (en particular, el glicolato y el oxalato). Los datos disponibles indican asimismo que las vías que intervienen en su metabolismo son cualitativamente semejantes en el ser humano y en otras especies de mamíferos; no se han estudiado bien las posibles diferencias cuantitativas.
La toxicidad aguda del etilenglicol en los animales de experimentación es baja tras la exposición oral, por inhalación o cutánea. Tanto en las personas como en los animales sólo indujo una irritación cutánea mínima. Se notificó irritación nasal y/o de la garganta en un pequeño número de personas sometidas a su inhalación, mientras que las concentraciones más elevadas produjeron una irritación grave. En animales de experimentación, el etilenglicol sólo induce una irritación conjuntival mínima, sin daños permanentes de la córnea. No se han identificado datos sobre su potencial para inducir sensibilización.
El etilenglicol no ha sido carcinogénico en una biovaloración de dos años con ratas y ratones, siendo las biovaloraciones iniciales fundamentalmente limitadas. No se ha observado genotoxicidad en el limitado número de estudios in vitro e in vivo identificados.
Los datos obtenidos a partir de casos de intoxicación aguda (en personas) y estudios de toxicidad con dosis repetidas (en animales de experimentación) ponen de manifiesto que el riñón es un órgano fundamental en la toxicidad del etilenglicol tanto en personas como en animales de experimentación. En diversas especies se han observado de manera sistemática acidosis metabólica y cambios degenerativos no neoplásicos en el riñón (en particular dilatación, degeneración y deposición de oxalato de calcio en los túbulos) con las dosis más bajas.
Conforme a una base de datos bastante amplia, el etilenglicol induce efectos en el desarrollo en ratas y ratones por todas las vías de exposición, aunque con dosis más elevadas que las asociadas con los efectos renales en las ratas macho. En efecto, el etilenglicol es teratogénico, induciendo sobre todo variaciones esqueléticas y malformaciones externas, a veces con dosis inferiores a las que producen toxicidad materna, y los ratones son más sensibles que las ratas. Se ha investigado ampliamente su toxicidad reproductiva en estudios adecuados con ratones y ratas. En estudios de toxicidad con dosis repetidas, no se han obtenido pruebas de efectos adversos en los órganos reproductivos; en estudios especializados, entre ellos un estudio de tres generaciones en ratas y protocolos de la reproducción continua en ratones, sólo se han puesto de manifiesto efectos reproductivos en los ratones (pero no en las ratas o los conejos) expuestos a dosis considerablemente superiores a las asociadas con los efectos en el desarrollo en esta especie o con los efectos renales en las ratas.
Los datos disponibles no son suficientes para evaluar los posibles efectos neurológicos o inmunológicos adversos asociados con la exposición prolongada al etilenglicol, aunque se han notificado trastornos del neurocomportamiento y neurológicos en casos de intoxicación aguda en personas. En el limitado número de investigaciones identificadas hasta el momento, no se han observado efectos neurológicos con dosis inferiores a las que inducen toxicidad renal. En los estudios disponibles de toxicidad con dosis repetidas, en los que se expusieron varias especies al etilenglicol por vía oral o por inhalación, no se observaron efectos sistemáticos relacionados con el tratamiento en los parámetros del sistema inmunitario.
Se ha establecido para esta sustancia una ingesta tolerable de 0,05 mg/kg de peso corporal al día, basada en una dosis de referencia de 49 mg/kg de peso corporal al día calculada para los efectos renales no neoplásicos en animales y un factor de incertidumbre de 1000. Sin embargo, esta ingesta tolerable es incierta, debido fundamentalmente a la falta de información sobre la progresión de las lesiones renales en el modelo animal más sensible. Las estimaciones de muestra muy inciertas de la exposición de algunos grupos de edad en las cercanías de una fuente puntual o de adultos mediante la absorción a partir de algunos productos de consumo se acercan a la ingesta tolerable o son superiores a ella. Se recomienda un estudio adicional para caracterizar mejor la progresión de las lesiones renales y definir mejor las estimaciones de la exposición.
ENDNOTES:
|
International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170) (also available at http://www.who.int/pcs/). |
|
|
New information flagged by the reviewers or found in a literature search conducted prior to the Final Review Board meeting has been scoped to indicate its likely impact on the essential conclusions of this assessment, primarily to establish priority for its consideration in an update. More recent information not critical to the hazard characterization of exposure–response analysis, considered by reviewers to add to informational content, has been added. |
|
|
Inadequate as a basis for quantification of absorption (R. Moody, personal communication, 1999). |
|
|
That the total incidence of animals with tubular damage was independently assessed has been verified (Brantom, 2000). |
|
|
Information presented in the text, although not in Table 6 of the published account of this study (DePass et al., 1986a), indicated that the incidence of fatty metamorphosis in the liver of female rats receiving 200 mg/kg body weight per day was significantly increased, compared with controls. |
|
|
Statistical analysis was not provided. |
|
|
In the initial stages following systemic exposure, the concentration of ethylene glycol in extracellular fluids increases, leading to hyperosmolality and an increased osmolal gap. |
|
|
The accumulation of acidic products (e.g., glycolic acid, oxalic acid, and lactic acid) due to the metabolism of ethylene glycol results in metabolic acidosis, a state that is characterized by an actual or relative decrease of alkali in body fluids in relation to acid content. The major determinant of acidosis is the degree of glycolic acid accumulation in the blood. |
|
|
Although only a minor metabolite, oxalic acid is of toxicological significance since it chelates with calcium ions, resulting in the precipitation of (insoluble) calcium oxalate monohydrate in tissues (notably, in the kidney and brain). |
|
|
However, in one of these studies (Lee et al., 1992), the reported intake of drinking-water was slightly lower in the females (18.3 ± 7.2 ml/day) than in the males (25.1 ± 9.3 ml/day). |
|
|
A glycoprotein that is part of the calcium oxalate crystal matrix, considered to promote the formation of kidney stones (Iguchi et al., 1999). |
|
|
The minimum lethal oral dose in rats is 3.8 mg/kg body weight (Clark et al., 1979). |
|
|
Based upon a statistically significant increase in the incidence of one of 27 skeletal malformations/variations (i.e., extra 14th rib on first lumbar arch) at the next highest dose of 500 mg/kg body weight per day. |
|
|
Based upon a statistically significant increase in the incidence of 25 of 27 skeletal malformations/variations at the next highest dose of 1500 mg/kg body weight per day. |
|
|
For comparison with the effect level, the BMD05 for the developmental effect that occurred at lowest dose in this study was approximately 140–245 mg/kg body weight per day; confidence in this value is low, though, due to lack of litter-specific data. |
|
|
Based on theoretical maximum estimated intake through ingestion at the top dose, estimated intake at the NOAEL would be no greater than that via inhalation. |
|
|
Les nouvelles données notées par les auteurs ou obtenues par un dépouillement de la littérature effectué avant la réunion du Comité d’évaluation finale, ont été examinées compte tenu de leur influence probable sur les conclusions essentielles de la présente évaluation, le but étant d’établir si leur prise en compte serait prioritaire lors d’une prochaine mise à jour. Les auteurs ayant estimé qu’elles apportaient des éléments d’information supplémentaires, on a ajouté des données plus récentes encore que non essentielles pour la caractérisation des dangers ou l’analyse des relations dose-réponse. |
|
|
Se ha incluido nueva información destacada por los examinadores u obtenida en una búsqueda bibliográfica realizada antes de la reunión de la Junta de Evaluación Final para señalar sus probables repercusiones en las conclusiones esenciales de esta evaluación, principalmente con objeto de establecer la prioridad para su examen en una actualización. Se ha añadido información más reciente no decisiva para la caracterización del peligro o el análisis de la exposición-respuesta que los examinadores consideraban que aumentaba el contenido informativo. |
See Also:
Toxicological Abbreviations