
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 1
1,2-Dichloroethane
INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS
A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and
OECD
This report contains the collective views of an international
group of experts and does not necessarily represent the decisions
or the stated policy of the United Nations Environment Programme,
the International Labour Organisation, or the World Health
Organization.
First draft prepared by Ms K. Hughes and Ms M.E. Meek,
Environmental Health Directorate,
Health Canada
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and
the World Health Organization, and produced within the framework
of the Inter-Organization Programme for the Sound Management of
Chemicals.
World Health Organization
Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour
Organisation (ILO), and the World Health Organization (WHO). The
overall objectives of the IPCS are to establish the scientific
basis for assessment of the risk to human health and the
environment from exposure to chemicals, through international
peer review processes, as a prerequisite for the promotion of
chemical safety, and to provide technical assistance in
strengthening national capacities for the sound management of
chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food
and Agriculture Organization of the United Nations, WHO, the
United Nations Industrial Development Organization, and the
Organisation for Economic Co-operation and Development
(Participating Organizations), following recommendations made by
the 1992 UN Conference on Environment and Development to
strengthen cooperation and increase coordination in the field of
chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve
the sound management of chemicals in relation to human health and
the environment.
WHO Library Cataloguing in Publication Data
1,2-Dichloroethane.
(Concise international chemical assessment document ; 1)
1.Ethylene dichlorides - toxicity 2.Ethylene dichlorides -
administration and dosage 3.Dose-response relationship, Drug
4.Environmental exposure I.International Programme for
Chemical Safety II.Series
ISBN 92 4 153001 4 (NLM Classification: QV 633)
ISSN 1020-6167
The World Health Organization welcomes requests for
permission to reproduce or translate its publications, in part or
in full. Applications and enquiries should be addressed to the
Office of Publications, World Health Organization, Geneva,
Switzerland, which will be glad to provide the latest information
on any changes made to the text, plans for new editions, and
reprints and translations already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy
copyright protection in accordance with the provisions of
Protocol 2 of the Universal Copyright Convention. All rights
reserved.
The designations employed and the presentation of the
material in this publication do not imply the expression of any
opinion whatsoever on the part of the Secretariat of the World
Health Organization concerning the legal status of any country,
territory, city, or area or of its authorities, or concerning the
delimitation of its frontiers or boundaries.
The mention of specific companies or of certain
manufacturers' products does not imply that they are endorsed or
recommended by the World Health Organization in preference to
others of a similar nature that are not mentioned. Errors and
omissions excepted, the names of proprietary products are
distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Germany, provided financial
support for the printing of this publication.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
9.1. Case reports
9.2. Epidemiological studies
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for 1,2-dichloroethane
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Health surveillance advice
13.4. Explosion and fire hazards
13.4.1. Explosion hazards
13.4.2. Fire hazards
13.4.3. Prevention
13.5. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 - SOURCE DOCUMENTS
APPENDIX 2 - CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs)
are the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) - a cooperative programme of
the World Health Organization (WHO), the International Labour
Organisation (ILO), and the United Nations Environment Programme
(UNEP). CICADs join the Environmental Health Criteria documents
(EHCs) as authoritative documents on the risk assessment of
chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects
of chemicals upon human health and/or the environment. They are
based on selected national or regional evaluation documents or on
existing EHCs. Before acceptance for publication as CICADs by
IPCS, these documents have undergone extensive peer review by
internationally selected experts to ensure their completeness,
accuracy in the way in which the original data are represented,
and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of
hazard and dose-response from exposure to a chemical. CICADs are
not a summary of all available data on a particular chemical;
rather, they include only that information considered critical
for characterization of the risk posed by the chemical. The
critical studies are, however, presented in sufficient detail to
support the conclusions drawn. For additional information, the
reader should consult the identified source documents upon which
the CICAD has been based.
Risks to human health and the environment will vary
considerably depending upon the type and extent of exposure.
Responsible authorities are strongly encouraged to characterize
risk on the basis of locally measured or predicted exposure
scenarios. To assist the reader, examples of exposure estimation
and risk characterization are provided in CICADs, whenever
possible. These examples cannot be considered as representing
all possible exposure situations, but are provided as guidance
only. The reader is referred to EHC 1701 for advice on the
derivation of health-based guidance values.
1 International Programme on Chemical Safety (1994) Assessing
human health risks of chemicals: derivation of guidance values
for health-based exposure limits. Geneva, World Health
Organization (Environmental Health Criteria 170).
While every effort is made to ensure that CICADs represent
the current status of knowledge, new information is being
developed constantly. Unless otherwise stated, CICADs are based
on a search of the scientific literature to the date shown in the
executive summary. In the event that a reader becomes aware of
new information that would change the conclusions drawn in a
CICAD, the reader is requested to contact the IPCS to inform it
of the new information.
Procedures
The flow chart shows the procedures followed to produce a
CICAD. These procedures are designed to take advantage of the
expertise that exists around the world - expertise that is
required to produce the high-quality evaluations of
toxicological, exposure, and other data that are necessary for
assessing risks to human health and/or the environment.
The first draft is based on an existing national, regional,
or international review. Authors of the first draft are usually,
but not necessarily, from the institution that developed the
original review. A standard outline has been developed to
encourage consistency in form. The first draft undergoes primary
review by IPCS and one or more experienced authors of criteria
documents to ensure that it meets the specified criteria for
CICADs.
The second stage involves international peer review by
scientists known for their particular expertise and by scientists
selected from an international roster compiled by IPCS through
recommendations from IPCS national Contact Points and from IPCS
Participating Institutions. Adequate time is allowed for the
selected experts to undertake a thorough review. Authors are
required to take reviewers' comments into account and revise
their draft, if necessary. The resulting second draft is
submitted to a Final Review Board together with the reviewers'
comments.
The CICAD Final Review Board has several important
functions:
- to ensure that each CICAD has been subjected to an
appropriate and thorough peer review;
- to verify that the peer reviewers' comments have been
addressed appropriately;
- to provide guidance to those responsible for the preparation
of CICADs on how to resolve any remaining issues if, in the
opinion of the Board, the author has not adequately
addressed all comments of the reviewers; and
- to approve CICADs as international assessments.
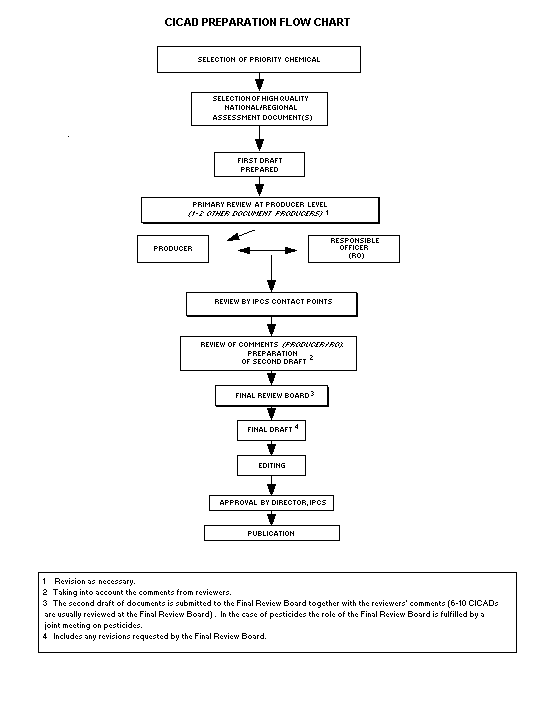 Board members serve in their personal capacity, not as
representatives of any organization, government, or industry.
They are selected because of their expertise in human and
environmental toxicology or because of their experience in the
regulation of chemicals. Boards are chosen according to the
range of expertise required for a meeting and the need for
balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers
who participate in the preparation of a CICAD are required to
declare any real or potential conflict of interest in relation to
the subjects under discussion at any stage of the process.
Representatives of nongovernmental organizations may be invited
to observe the proceedings of the Final Review Board. Observers
may participate in Board discussions only at the invitation of
the Chairperson, and they may not participate in the final
decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on 1,2-dichloroethane was prepared by the
Environmental Health Directorate of Health Canada based on an
International Programme on Chemical Safety (IPCS) Environmental
Health Criteria (EHC) document (IPCS, 1995), which assesses the
potential effects on human health of indirect exposure to
1,2-dichloroethane in the general environment as well as the
chemical's environmental effects. Data identified as of May 1993
(human health effects) and October 1994 (environmental effects)
were considered in these reviews. Information on the nature of
the peer review process and the availability of the EHC document
is presented in Appendix 1. For this CICAD, the peer review
process prior to consideration by the Final Review Board was
covered by the peer review carried out for the EHC. This CICAD
on 1,2-dichloroethane was finalized and approved for publication,
through correspondence, by members of the Final Review Board, who
also considered the peer review comments provided during the
development of the EHC. The composition of the Final Review
Board is outlined in Appendix 2. The International Chemical
Safety Card (ICSC 0250) produced by the IPCS (1993) has also been
reproduced in this document.
1,2-Dichloroethane (CAS no. 107-06-2) is a volatile,
synthetic hydrocarbon that is used principally in the synthesis
of vinyl chloride monomer and other chlorinated solvents. It has
also been used as a leaded gasoline additive and a fumigant,
although its use as a gasoline additive is declining. The
majority of environmental releases are to ambient air, where it
is moderately persistent. However, it is not expected to
contribute to ozone depletion. 1,2-Dichloroethane has a low
potential for bioaccumulation; inhalation in air is likely the
primary source of human exposure.
Little information is available on the effects of
1,2-dichloroethane in humans. The few identified epidemiological
investigations on its potential carcinogenicity are inconclusive.
1,2-Dichloroethane is moderately acutely toxic in
experimental animals. Limited information on non-neoplastic
effects presented in short-term, subchronic, and chronic studies
indicates that the liver and kidneys are the principal target
organs; lowest reported effect levels for ingestion and
inhalation were 49-82 mg/kg body weight per day (increases in
liver weight in rats exposed for 13 weeks) and 202 mg/m3
(effects on liver and kidney function in rats exposed for 12
months), respectively. Based on the results of a limited number
of studies, there is no evidence that 1,2-dichloroethane is
teratogenic in experimental animals or that it induces
reproductive or developmental effects at levels of exposure lower
than those that cause other systemic effects.
Exposure to 1,2-dichloroethane by gavage for 78 weeks
induced a significant increase in the incidence of tumours at
several sites (including haemangiosarcomas and tumours of the
stomach, mammary gland, liver, lung, and endometrium) in both
rats and mice. Although there were no significant increases in
tumour incidence in rats or mice exposed via inhalation, repeated
dermal or intraperitoneal application of 1,2-dichloroethane
resulted in an increase in lung tumours in mice.
1,2-Dichloro-ethane has been consistently genotoxic in numerous
in vitro assays in prokaryotes, fungi, and mammalian
(including human) cells. Similarly, results were consistently
positive for genotoxic activity (as well as binding to DNA) in
in vivo studies in rats, mice, and insects.
The lowest reported IC50s and EC50s for various end-points
in aquatic organisms were 25 and 105 mg/litre, respectively. The
lowest reported LC50 value for Daphnia was 220 mg/litre,
whereas effects on reproduction occurred at 20.7 mg/litre. The
most sensitive freshwater vertebrate tested was the northwestern
salamander (Ambystoma gracile), in which reduced larval
survival was observed at 2.5 mg/litre. Only limited data are
available on the effects of 1,2-dichloroethane on terrestrial
species.
Based on available data, 1,2-dichloroethane is considered to
be a probable human carcinogen, and therefore exposure should be
reduced to the extent possible. The carcinogenic potency
(expressed as the dose associated with a 5% increase in tumour
incidence), derived on the basis of studies in which animals were
exposed by gavage, was calculated to be 6.2-34 mg/kg body weight
per day. Guidance values for air (the principal source of human
exposure) of 3.6-20 µg/m3 or 0.36-2.0 µg/m3, calculated on the
basis of a margin 5000- or 50 000-fold less than the estimated
carcinogenic potency, have been derived; however, it should be
noted that risks are overestimated on this basis, as available
data indicate that 1,2-dichloroethane is less potent when
inhaled. (Corresponding values for ingestion are 1.2-6.8 µg/kg
body weight per day or 0.12-0.68 µg/kg body weight per day.)
These values correspond to those considered by some agencies to
represent "essentially negligible" risk (i.e. 10-5 to 10-6 for a
genotoxic carcinogen). Based on a sample estimate, indirect
exposure in the general environment is up to approximately 300
times less than these values.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
1,2-Dichloroethane (CAS no. 107-06-2; ethylene dichloride,
dichloro-1,2-ethane; see structural diagram below) is a synthetic
chemical that is a colourless liquid at room temperature. It is
also highly volatile, with a vapour pressure of 8.5 kPa (at
20°C), and soluble in water, with a solubility of 8690 mg/litre
(at 20°C). The log octanol/water partition coefficient of
1,2-dichloroethane is 1.76. Additional physical/chemical
properties are presented in the International Chemical Safety
Card, reproduced in this document.
H H
' '
Cl - C - C - Cl
' '
H H
3. ANALYTICAL METHODS
Analysis for 1,2-dichloroethane in environmental media is
usually by gas chromatography, in combination with electron
capture detection, flame ionization detection, or mass
spectrometry. Detection limits range from 0.016 to >4 µg/m3
for air, from 0.001 to 4.7 µg/litre for water, and from 6 to 10
µg/kg for various foodstuffs (ATSDR, 1992).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of 1,2-dichloroethane.
The principal use for 1,2-dichloroethane is in the synthesis of
vinyl chloride monomer and, to a lesser extent, in the
manufacture of various chlorinated solvents. It is also
incorporated into antiknock gasoline additives (although this use
is declining with the phase-out of leaded gasoline in some
countries) and has been used as a fumigant. Total annual
production of 1,2-dichloroethane in Canada (1990) and the USA
(1991) is about 922 and 6318 kt, respectively (CPI, 1991;
Chemical Marketing Reporter, 1992).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The majority of 1,2-dichloroethane released to the
environment is in emissions to air. 1,2-Dichloroethane is
moderately persistent in air; its estimated atmospheric lifetime
is between 43 and 111 days. Small amounts of 1,2-dichloroethane
are transported to the stratosphere, where photolysis may produce
chlorine radicals, which may in turn react with ozone (Spence &
Hanst, 1978; Callaghan et al., 1979). Some 1,2-dichloroethane
may be released in industrial effluents to the aquatic
environment, from where it is removed rapidly by volatilization
(Dilling et al., 1975). 1,2-Dichloroethane may also leach to
groundwater near industrial waste sites. It is not expected to
bioconcentrate in aquatic or terrestrial species.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Data considered to be most representative of current levels
of 1,2-dichloroethane in environmental media are summarized in
Table 1. Mean concentrations of 1,2-dichloroethane in surveys of
ambient air in non-source-dominated areas in cities are 0.07-0.28
µg/m3 in Canada, <0.004-3.8 µg/m3 in Japan, and 1.2 µg/m3 in
the United Kingdom and the Netherlands. Earlier surveys in the
USA reported mean levels of 0.33-6.05 µg/m3; however, peak
levels near chemical manufacturing plants have ranged as high as
736 µg/m3 (US EPA, 1985). Mean levels in residential indoor air
are reported to be <0.1 µg/m3 in Canada, 0.1-0.5 µg/m3 in the
USA, and 3.4 µg/m3 in the Netherlands.
In drinking-water, mean 1,2-dichloroethane concentrations
are generally less than 0.5 µg/litre, based on the results of
surveys in Canada, the USA, Japan, and Spain. Although there are
few recent data, 1,2-dichloroethane has only very rarely been
detected in surface water at concentrations greater than 10
µg/litre.
1,2-Dichloroethane has only rarely been detected in
foodstuffs in extensive surveys in Canada and the USA. Also, as
1,2-dichloroethane has low potential for bioaccumulation, food is
unlikely to be a major source of exposure.
6.2 Human exposure
An example of estimated indirect exposure in the general
environment is presented here. Exposure of the general
population to 1,2-dichloroethane in environmental media may be
estimated based on concentrations determined in various media and
reference values for body weight and consumption patterns. Owing
to the availability of relevant data, exposure has been estimated
based primarily on data from North America. However, countries
are encouraged to estimate total exposure on the basis of
national data, possibly in a manner similar to that outlined
here.
Table 1: Levels of 1,2-dichloroethane in environmental media.
Medium Location Year Concentrations Reference
Ambient air Canada 1988-1990 0.07-0.28 µg/m3 (means) T. Dann, unpublished data, 1992
Ambient air Japan 1992 <0.004-3.8 µg/m3 (means) Environment Agency Japan, 1993
Ambient air UK 1982, 1983 1.2 µg/m3 (mean) Clark et al., 1984a,b
Ambient air Netherlands 1980 1.2 µg/m3 (mean) Guicherit & Schulting, 1985
Ambient air USA 1980-1982 0.33-6.05 µg/m3 (means) Singh et al., 1980, 1981, 1982
Indoor air (residential) Canada 1991 <0.1 µg/m3 (mean) Fellin et al., 1992
Indoor air (residential) USA 0.1-0.5 µg/m3 (means) US EPA, 1992
Indoor air (residential) Netherlands 1984-1985 3.4 µg/m3 (mean) Kliest et al., 1989
Drinking-water Canada 1988-1991 <0.05-0.139 µg/litre P. Lachmaniuk, personal
(mean) communication, 1991
1990 <0.2 µg/litre (mean) Ecobichon & Allen, 1990
1982-1983 <0.1 µg/litre (mean) Otson, 1987
Drinking-water USA Early 1980s NDa - 19 µg/litre Letkiewicz et al., 1982
ND - 0.05 µg/litre Barkley et al., 1980
Drinking-water Japan 1976 <0.5-0.9 µg/litre Fujii, 1977
Drinking-water Spain 1987 2-22 µg/litre Freiria-Gandara et al., 1992
Table 1 (continued)
Medium Location Year Concentrations Reference
Surface water Canada 1981-1985 <0.08 µg/litre Kaiser et al., 1983; Comba &
Kaiser, 1985; Kaiser & Comba,
1986; Lum & Kaiser, 1986
Surface water Japan 1992 0.01-3.4 µg/litre Environment Agency Japan, 1993
Food (34 groups) Canada 1991 <50 µg/kg (solids); Enviro-Test Laboratories, 1991
<1 µg/litre (liquids)
1992 <5 µg/kg (solids); Enviro-Test Laboratories, 1992
<1 µg/litre (liquids)
Food (19 items) USA Not specified ND - 0.31 µg/kg Heikes, 1987, 1990
Not specified ND - 8.2 µg/kg Heikes, 1987
Food (231 items) USA Not specified <9-30 µg/kg Daft, 1988
a Detection limit not reported.
Based on a daily inhalation volume for adults of 22 m3, a
mean body weight for males and females of 64 kg, the assumption
that 4 of 24 hours are spent outdoors (IPCS, 1994), and the range
of mean levels of 1,2-dichloroethane in ambient air of 0.07-0.28
µg/m3 in a survey of cities across Canada, the mean intake of
1,2-dichloroethane from ambient air for the general population is
estimated to range from 0.004 to 0.02 µg/kg body weight per day.
The mean intake of 1,2-dichloroethane in indoor air, based on the
assumption that 20 of 24 hours are spent indoors (IPCS, 1994) and
the range of concentrations in indoor or "personal" air in Canada
and the USA of <0.1-0.5 µg/m3, is estimated to range from
<0.03 to 0.1 µg/kg body weight per day. Based on a daily volume
of water consumption for adults of 1.4 litres, a mean body weight
of 64 kg (IPCS, 1994), and the mean levels of 1,2-dichloroethane
in provincial surveys in Canada of <0.05-0.139 µg/litre, the
mean intake from drinking-water is estimated to range from
<0.001 to 0.003 µg/kg body weight per day. Intake of
1,2-dichloroethane in food is likely to be negligible, as it has
not been detected in extensive surveys and as it has low
potential for bioaccumulation. Therefore, the principal source
of exposure of the general population to 1,2-dichloroethane is
indoor and outdoor air, with only minor amounts being contributed
by drinking-water.
Few data on occupational exposure to 1,2-dichloroethane were
identified. In North America, workers are exposed to
1,2-dichloroethane principally in the manufacture of other
chemical substances; in such situations, the principal route of
exposure is most likely inhalation and, possibly, dermal contact.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
1,2-Dichloroethane is readily absorbed following inhalation,
ingestion, and dermal exposure and is rapidly and widely
distributed throughout the body. Relative distribution of
radioactivity (presumably as metabolites) was similar in rats
administered a single oral dose of 150 mg/kg body weight and
those exposed by inhalation to 150 ppm (600 mg/m3) for 6 hours
(Reitz et al., 1982). 1,2-Dichloroethane is rapidly and
extensively metabolized in rats and mice, with principally
sulfur-containing metabolites being eliminated in the urine in a
dose-dependent manner. Metabolism appears to be saturated or
limited in rats at levels of exposure resulting in concentrations
in blood of 5-10 µg/ml (Reitz et al., 1982). Levels of DNA
alkylation were higher following exposure to a bolus dose of 150
mg/kg body weight by gavage compared with inhalation of 150 ppm
(600 mg/m3) over a 6-hour period (Reitz et al., 1982).
Available data suggest that 1,2-dichloroethane is
metabolized via two principal pathways. The first involves a
saturable microsomal oxidation mediated by cytochrome P-450 to
2-chloroacetaldehyde and 2-chloroethanol, followed by conjugation
with glutathione. The second pathway entails direct conjugation
with glutathione to form S-(2-chloroethyl)-glutathione, which
may be non-enzymatically converted to a glutathione episulfonium
ion; this ion can form adducts with proteins, DNA, or RNA.
Although DNA damage has been induced by the P-450 pathway in
vitro (Banerjee et al., 1980; Guengerich et al., 1980; Lin et
al., 1985), several lines of evidence indicate that the
glutathione conjugation pathway is probably of greater
significance than the P-450 pathway as the major route for DNA
damage (Guengerich et al., 1980; Rannug, 1980; Sundheimer et al.,
1982; Inskeep et al., 1986; Koga et al., 1986; Simula et al.,
1993).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
1,2-Dichloroethane is moderately acutely toxic in
experimental animals. For example, LC50s for rats exposed by
inhalation for 6 or 7.25 hours ranged from 4000 mg/m3 (Spencer
et al., 1951) to 6600 mg/m3 (Bonnet et al., 1980), whereas oral
LD50s for rats, mice, dogs, and rabbits ranged from 413 to 2500
mg/kg body weight (Barsoum & Saad, 1934; McCollister et al.,
1956; Smyth, 1969; Larionov and Kokarovtseva, 1976; Munson et
al., 1982; NIOSH, 1994a).
8.2 Irritation and sensitization
Application of 1,2-dichloroethane to the skin of
experimental animals has resulted in microscopic changes and
moderate oedema (Duprat et al., 1976; Kronevi et al., 1981;
Jakobson et al., 1982). Similarly, histological changes and mild
irritation in the eye have been observed in animals following
direct application (Kuwabara et al., 1968; Duprat et al., 1976).
No information on the sensitization potential of this substance
was identified.
8.3 Short-term exposure
Few data were identified on the toxicity of
1,2-dichloroethane following short-term exposure. Degeneration
and necrosis of the liver and kidneys, accompanied by congestion
and haemorrhage of the lungs and adrenal glands, were observed in
small groups of rats, rabbits, guinea-pigs, dogs, and pigs
exposed to 1,2-dichloroethane by inhalation at 6000 mg/m3, 7
hours/day, for 6 days (Heppel et al., 1945). No effects on body
or organ weights, histology, or clinical chemistry were noted in
rats administered oral doses of up to 150 mg/kg body weight per
day for 2 weeks (van Esch et al., 1977; Reitz et al., 1982).
8.4 Long-term exposure
8.4.1 Subchronic exposure
The results of subchronic studies in several species of
experimental animals indicate that the liver and kidneys are the
target organs of 1,2-dichloroethane exposure; however, most of
these studies were inadequate to serve as a basis for
establishing reliable no-observed-effect levels or lowest-
observed-effect levels, generally because of the inadequate
documentation and the limited range of end-points examined in
small groups of animals. In a series of early limited studies,
morphological changes in the liver were observed in several
species following subchronic exposure (7 hours/day) to airborne
concentrations as low as 800 mg/m3 (Heppel et al., 1946; Spencer
et al., 1951; Hofmann et al., 1971). Increases in relative liver
weight have been observed in rats following subchronic oral
administration of doses of 49-82 mg/kg body weight per day and
above for 13 weeks (van Esch et al., 1977; NTP, 1991).
8.4.2 Chronic exposure and carcinogenicity
Little information was presented on non-neoplastic effects
in available chronic studies. Changes in serum parameters
indicative of liver and kidney toxicity were observed in groups
of 8-10 male or female Sprague-Dawley rats exposed to airborne
concentrations as low as 202 mg/m3 for 12 months, although
histopathological examinations were not conducted in this study
(Spreafico et al., 1980).
The carcinogenicity of 1,2-dichloroethane has been
investigated in a few limited bioassays in experimental animals
(limitations include short duration of exposure and high
mortality). In an inhalation study, no significant increase in
the incidence of any type of tumour was reported in groups of 90
male or female Sprague-Dawley rats exposed to concentrations of
1,2-dichloroethane up to 150 ppm (607 mg/m3), 7 hours/ day, 5
days/week, for 78 weeks and observed until spontaneous death
(Maltoni et al., 1980). However, mortality was high in this
study, although it was not related to concentration, and
incidence rates were not adjusted for differential mortality
among groups. There was a non-significant increase in the
incidence of mammary gland adenomas and fibroadenomas in female
Sprague-Dawley rats ( n = 50) exposed to 1,2-dichloroethane at
50 ppm (200 mg/m3), 7 hours/day, 5 days/ week, for 2 years in an
assay in which no other compound-related toxicity was observed
(Cheever et al., 1990). No increase in the incidence of any type
of tumour was observed in groups of 90 male or female Swiss mice
exposed to concentrations of 1,2-dichloroethane up to 150 ppm
(607 mg/m3), 7 hours/day, 5 days/ week, for 78 weeks and
observed until spontaneous death (Maltoni et al., 1980).
In contrast, there has been convincing evidence of increases
in tumour incidence in two species following ingestion. There
were significant increases in the incidence of tumours at several
sites in Osborne-Mendel rats ( n = 50 of each sex in exposed
groups; n = 20 matched controls; n = 60 pooled controls)
administered time-weighted-average doses of 47 or 95 mg/kg body
weight per day in corn oil by gavage, 5 days/week, for 78 weeks,
followed by 32 weeks of observation. The incidence of squamous
cell carcinomas of the stomach was significantly increased in
both groups of exposed males (0/60, 0/20, 3/50, and 9/50 in
pooled [from concurrent studies] vehicle controls, matched
vehicle controls, low-dose group, and high-dose group,
respectively). There were also significant increases in the
incidence of haemangiosarcomas in exposed males (1/60, 0/20,
9/50, and 7/50) and females (0/59, 0/20, 4/50, and 4/50). The
incidence of fibromas of the subcutaneous tissue was
significantly increased in exposed males (0/60, 0/20, 5/50, and
6/50). In females, there were significant increases in the
incidences of adenocarcinomas and fibroadenomas (combined) of the
mammary gland (6/59, 0/20, 15/50, and 24/50). Mortality was
significantly higher in both males and females in the high-dose
group, and there was a greater frequency of clinical signs of
toxicity in exposed rats compared with controls. Chronic murine
pneumonia was present in 60-94% of rats in each group, although
the incidence was not related to dose (NCI, 1978).
In a similar bioassay, B6C3F1 mice ( n = 50 of each sex in
exposed groups; n = 20 matched controls; n = 60 pooled
controls) were administered time-weighted-average doses of 97 or
195 mg/kg body weight per day (males) and 149 or 299 mg/kg body
weight per day (females) in corn oil by gavage, 5 days/week, for
78 weeks, followed by 13 weeks of observation. The incidence of
hepatocellular carcinomas was significantly increased in exposed
males (4/59, 1/19, 6/47, and 12/48 in pooled vehicle controls,
matched vehicle controls, low-dose group, and high-dose group,
respectively), although the authors noted that the increase in
the incidence of this tumour could not be convincingly attributed
to the test chemical, owing to the high variability of
hepatocellular neoplasms among historical controls. The
incidence of alveolar/bronchiolar adenomas was significantly
increased in males in the high-dose group (0/59, 0/19, 1/47, and
15/48) and in both groups of exposed females (2/60, 1/20, 7/50,
and 15/48); one female in the high-dose group had an
alveolar/bronchiolar carcinoma. The incidence of mammary gland
adenocarcinomas was significantly increased in females at both
doses (0/60, 0/20, 9/50, and 7/48). The incidence of endometrial
stromal polyp or endometrial stromal sarcoma (combined) in
females was significantly elevated at both doses (0/60, 0/20,
5/49, and 5/47). There was a dose-related increase in mortality
in females, but not in males; in addition, body weight was
decreased in females receiving the higher dose (NCI, 1978).
The incidence of lung tumours (benign lung papillomas) was
significantly increased in female non-inbred Ha:ICR mice ( n =
30) following repeated dermal application of 1,2-dichloroethane,
3 times/week, for 440-594 days (van Duuren et al., 1979).
Repeated intraperitoneal injections of 1,2-dichloroethane
resulted in a dose-related increase in the number of pulmonary
adenomas per mouse in a screening bioassay in a susceptible
strain (A/St), although none of these increases was significant
(Theiss et al., 1977). Concomitant exposure to inhaled
1,2-dichloroethane and disulfiram in the diet resulted in an
increased incidence of intrahepatic bile duct cholangiomas and
cysts, subcutaneous fibromas, hepatic neoplastic nodules,
interstitial cell tumours in the testes, and mammary
adenocarcinomas in rats, compared with rats administered either
compound alone or untreated controls (Cheever et al., 1990). No
potential to initiate or promote tumour development was evident
in three bioassays (van Duuren et al., 1979; Klaunig et al.,
1986; Story et al., 1986; Milman et al., 1988), although the
extent of histopathological examination was limited in these
studies.
8.5 Genotoxicity and related end-points
1,2-Dichloroethane has been consistently demonstrated to be
genotoxic in numerous in vitro (Table 2) and in vivo (Table
3) assays for a wide range of end-points. It has been mutagenic
in Salmonella typhimurium, especially in the presence of an
exogenous activation system, and induces unscheduled DNA
synthesis, induces gene mutation, and forms adducts with DNA in
mammalian cells in vitro. It binds to DNA in all reported in
vivo studies in rats and mice. 1,2-Dichloroethane has also
induced somatic cell and sex-linked recessive lethal mutations in
Drosophila melanogaster.
Available data on genotoxicity are consistent with the
hypothesis that the glutathione pathway of conjugation (i.e.
production of the glutathione episulfonium ion) is probably of
greater significance than the P-450 pathway as the major route
for DNA damage (Guengerich et al., 1980; Rannug, 1980; Sundheimer
et al., 1982; Inskeep et al., 1986; Koga et al., 1986; Simula et
al., 1993); mutation frequency in human cell lines has been
correlated with variations in levels of
glutathione- S-transferase activities (Crespi et al., 1985).
8.6 Reproductive and developmental toxicity
Based on the results of a limited number of studies, there
is no evidence that 1,2-dichloroethane is teratogenic in
experimental animals and little convincing evidence that it
induces reproductive or developmental effects at doses below
those that cause other systemic effects (Alumot et al., 1976;
Vozovaya, 1977; Kavlock et al., 1979; Rao et al., 1980; Lane et
al., 1982).
8.7 Immunological and neurological effects
Immunological effects, including reduced resistance to
streptococcal challenge, decreased pulmonary bactericidal
activity in mice, and altered levels of antibody production in
rabbits, have been observed following acute or subchronic
exposure to 1,2-dichloroethane at 20 and 10 mg/m3 and above,
respectively (Shmuter, 1977; Sherwood et al., 1987), whereas
there were no effects in rats exposed to up to 800 mg/m3 for
several days (Sherwood et al., 1987). Effects on antibody levels
and reversible effects on cell-mediated responses were also noted
in mice exposed to 1,2-dichloroethane in drinking-water at
concentrations equivalent to doses of 3 mg/kg body weight per day
and above for 14 or 90 days (Munson et al., 1982).
Data on the neurological effects of 1,2-dichloroethane have
not been identified.
9. EFFECTS ON HUMANS
9.1 Case reports
Acute incidental exposure to 1,2-dichloroethane by
inhalation or ingestion has resulted in a variety of effects in
humans, including effects on the central nervous system, liver,
kidney, lung, and cardiovascular system (e.g. Hinkel, 1965;
Suveev & Babichenko, 1969; Dorndorf et al., 1975; Andriukin,
1979; Nouchi et al., 1984). Based on limited available data in
humans, the lethal oral dose of 1,2-dichloroethane has been
estimated to be between 20 and 50 ml.
9.2 Epidemiological studies
The potential carcinogenicity of 1,2-dichloroethane in
exposed human populations has not been extensively investigated.
Mortality due to pancreatic cancer was significantly increased
(standardized mortality ratio [SMR] = 492, based on eight cases)
in a group of 278 workers at a chemical production plant who had
been principally exposed to 1,2-dichloroethane in combination
with other chemicals. Mortality due to this cause increased with
duration of exposure. In addition, although the number of cases
was small (i.e. four) and the association with duration of
exposure was less consistent, mortality due to leukaemia was also
increased in these workers (Benson & Teta, 1993).
No association between occupational exposure to
1,2-dichloroethane and brain cancer was noted in a small
case-control study (Austin & Schnatter, 1983). Although the
incidence of colon and rectal cancer increased with concentration
of 1,2-dichloroethane in drinking-water in an inherently limited
ecological study, concomitant exposure to other substances may
have contributed to the observed effects (Isacson et al., 1985).
Table 2: Genotoxicity of 1,2-dichloroethane in vitro
(modified from ATSDR, 1992).
Result
Species (test system) End-point With Without
activation activation Reference
PROKARYOTIC SYSTEMS
Salmonella typhimurium Gene mutation + + Milman et al., 1988
+ + Barber et al., 1981
+ + Kanada & Uyeta, 1978
+ + Nestmann et al., 1980
+ + Rannug et al., 1978
+ + van Bladeren et al., 1981
+ NT Rannug & Beije, 1979
+ - Cheh et al., 1980
+ - Moriya et al., 1983
- - King et al., 1979
+ + Strobel & Grummt, 1987
NT +a Simula et al., 1993
S. typhimurium/spot test Gene mutation NT (+) Brem et al., 1974
(+) - Principe et al., 1981
NT - Buijs et al., 1984
S. typhimurium/Ara test (standard) Gene mutation + - Roldan-Arjona et al., 1991
S. typhimurium/Ara test (liquid) Gene mutation (+) (+) Roldan-Arjona et al., 1991
Streptomyces coelicolor Gene mutation NT - Principe et al., 1981
Escherichia coli K12/343/113 Gene mutation - - King et al., 1979
E. coli wp2 Gene mutation NT (+) Hemminki et al., 1980
- - Moriya et al., 1983
E. coli Pol A DNA damage NT (+) Brem et al., 1974
Bacillus subtilis/rec- assay DNA damage NT - Kanada & Uyeta, 1978
Table 2 (continued)
Result
Species (test system) End-point With Without
activation activation Reference
EUKARYOTIC ORGANISMS
- FUNGI
Aspergillus nidulans Gene mutation NT - Crebelli & Carere, 1988
NT - Principe et al., 1981
A. nidulans Mitotic segregation
aberrations NT + Crebelli et al., 1984
A. nidulans Aneuploidy induction NT + Crebelli et al., 1988
Saccharomyces cerevisiae Mitotic recombination NT (+) Simmon, 1980
- ANIMAL SYSTEMS
Hamster CHO/HGPRT Gene mutation + (+) Tan & Hsie, 1981
+ (+) Zamora et al., 1983
Rat hepatocytes Unscheduled DNA synthesis NT + Williams et al., 1989
Mouse hepatocytes Unscheduled DNA synthesis NT + Milman et al., 1988
Mouse liver DNA DNA binding + NT Banerjee, 1988
Calf thymus DNA DNA binding + NT Prodi et al., 1986
Salmon sperm DNA DND binding + - Banerjee & van Duuren, 1979;
Banerjee et al., 1980
Mouse BALB/c-3T3 Cell transformation NT - Milman et al., 1988
NT - Tu et al., 1985
Mouse C3H1OT´ Cell transformation NT +b Schultz et al., 1992
Syrian hamster embryo cells Cell transformation NT + Hatch et al., 1983
Table 2 (continued)
Result
Species (test system) End-point With Without
activation activation Reference
- HUMAN CELLS
Human lymphoblasts AHH-1 Gene mutation NT + Crespi et al., 1985
Human lymphoblasts TK6 Gene mutation NT + Crespi et al., 1985
Human embryo epithelial-like
EUE cells Gene mutation NT + Ferreri et al., 1983
Human peripheral lymphocytes Unscheduled DNA synthesis + - Perocco & Prodi, 1981
NT = not tested - = negative result + = positive result (+) = weakly positive or marginal result
a Increase in cells expressing GSTA1-1.
b Transformed cells induced tumours in nude mice.
Table 3: Genotoxicity of 1,2-dichloroethane in vivo
(modified from ATSDR, 1992).
Species (test system) End-point Results Reference
MAMMALIAN ASSAYS
Mouse Dominant lethal mutations - Lane et al., 1982
Mouse/spot test Gene mutation (+) Gocke et al., 1983
Mouse bone marrow Sister chromatid exchange + Giri & Que Hee, 1988
Mouse bone marrow Micronuclei - King et al., 1979;
Jenssen & Ramal, 1980
Mouse peripheral erythrocytes Micronuclei - Armstrong & Galloway, 1993
Mouse liver, kidney, lung, and stomach DNA binding + Prodi et al., 1986
Mouse liver, kidney, lung, and stomach DNA binding + Arfellini et al., 1984
Mouse forestomach and kidney DNA binding + Hellman & Brandt, 1986
Mouse liver DNA binding + Banerjee, 1988
Rat liver, kidney, spleen, lung,
forestomach, and stomach DNA binding + Reitz et al., 1982
Rat liver, kidney, lung, and stomach DNA binding + Arfellini et al., 1984
Rat liver, kidney, lung, and stomach DNA binding + Prodi et al., 1986
Rat liver and kidney DNA binding + Inskeep et al., 1986
Rat liver and lung DNA binding + Baertsch et al., 1991
Rat liver DNA binding + Banerjee, 1988
Rat liver DNA binding + Cheever et al., 1990
Mouse liver DNA damage + Storer & Conolly 1983,
1985;
Storer et al., 1984
Mouse liver DNA damage + Taningher et al., 1991
Table 3 (continued)
Species (test system) End-point Results Reference
INSECT ASSAYS
Drosophila melanogaster/somatic
mutation Gene mutation + Nylander et al., 1978
D. melanogaster/somatic mutation Gene mutation + Romert et al., 1990
D. melanogaster/somatic mutation Gene mutation + Kramers et al., 1991
D. melanogaster/somatic mutation Gene mutation (+) Ballering et al., 1993
D. melanogaster/recessive lethal Gene mutation + Ballering et al., 1993
D. melanogaster/vermilion locus Gene mutation + Ballering et al., 1993
D. melanogaster/sex-linked recessive Gene mutation + King et al., 1979
D. melanogaster/sex-linked recessive Gene mutation + Kramers et al., 1991
D. melanogaster Chromosomal loss/gain +/+ Valencia et al., 1984
HOST-MEDIATED ASSAYS
Escherichia coli K12/343/113 mouse
host-mediated assay Gene mutation - King et al., 1979
- = negative result + = positive result (+) = weakly positive or marginal result
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
The effects of exposure to 1,2-dichloroethane on a number of
aquatic organisms in the laboratory and field have also been
investigated. In bacteria, the lowest reported IC50s for
inhibition of gas production and ammonia consumption were 25 and
29 mg/litre in methanogens and Nitrosomonas, respectively (Blum
& Speece, 1991). The most sensitive freshwater alga studied was
Microcystis aeruginosa, in which an EC50 for inhibition of
cell multiplication of 105 mg/litre was observed (Bringmann &
Kühn, 1978); in the only identified study in marine algae, an
EC50 for carbon uptake of 340 mg/litre was reported in
Phaeodactylum tricornutum (Pearson & McConnell, 1975).
Toxicity thresholds (cell multiplication inhibition) were above
800 mg/litre for three species of aquatic protozoa (Bringmann &
Kühn, 1980).
The lowest reported LC50 value for Daphnia was 220
mg/litre (Leblanc, 1980), whereas the lowest EC50 (for 10%
immobilization) was 150 mg/litre (Freitag et al., 1994). Effects
on reproductive success and growth were observed in Daphnia at
20.7 and 71.7 mg/litre, respectively. There were no effects on
these end-points at 10.6 and 41.6 mg/litre, respectively (Richter
et al., 1983).
Based on available data, the most sensitive freshwater
vertebrate species appears to be the northwestern salamander, in
which 9-day larval survival (4 days post-hatch) was reduced at
2.5 mg/litre (Black et al., 1982).
10.2 Terrestrial environment
Identified data on the toxicity of 1,2-dichloroethane to
terrestrial organisms are inadequate to permit assessment.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Based on limited available data in humans, the lethal oral
dose of 1,2-dichloroethane has been estimated to be between 20
and 50 ml. 1,2-Dichloroethane is moderately acutely toxic by
inhalation, based on the results of studies in experimental
animals. Skin and eye irritation may also be induced by
1,2-dichloroethane.
Owing to the limitations of the available studies in humans,
it is necessary to rely on available experimental data in animal
species as a basis for derivation of no-effect levels or
quantitative estimates of carcinogenic potency. However, in most
of the identified short-term and subchronic studies, only a
limited range of end-points was examined and documentation was
incomplete. Similarly, little information on non-neoplastic
effects was presented in the long-term carcinogenicity bioassays.
Lowest reported effect levels were 49-82 mg/kg body weight per
day for 13 weeks (increases in liver weight in rats) for
ingestion (NTP, 1991) and 202 mg/m3 (effects on liver and kidney
function in rats exposed for 12 months) for inhalation (Spreafico
et al., 1980). Based on limited data, there is no evidence that
1,2-dichloroethane is teratogenic in experimental animals or that
it induces reproductive or developmental effects at doses below
those that cause other systemic effects.
Based on the limited evidence of carcinogenicity in workers
exposed principally to 1,2-dichloroethane in the most reliable
epidemiological study conducted to date (Benson & Teta, 1993),
the induction of both rare and common tumours in rats and mice
exposed by ingestion (NCI, 1978) and supporting evidence in other
limited bioassays, the production of a reactive intermediate that
alkylates DNA in vivo, and positive results in a range of in
vitro assays for genotoxicity, 1,2-dichloroethane is considered
to be a probable human carcinogen.
The carcinogenic potency of 1,2-dichloroethane has been
calculated based on the increased incidence of squamous cell
carcinomas of the stomach, haemangiosarcomas, fibromas of the
subcutaneous tissue, and adenocarcinomas or fibroadenomas
(combined) of the mammary gland in Osborne-Mendel rats exposed
orally by gavage, as well as the increased incidence of
alveolar/bronchiolar adenomas, hepatocellular carcinomas, mammary
gland adenocarcinomas, and endometrial stromal polyps or sarcomas
(combined) in similarly exposed B6C3F1 mice; data from both the
matched (same study) and pooled (concurrent studies) vehicle
controls were incorporated. It should be noted, however, that
mortality was higher at the high dose in female mice and rats of
both sexes than in other dose groups in this study. Therefore,
these high-dose groups were not included in the derivation of
quantitative estimates of carcinogenic potency.
Based on multistage modelling of these data, amortized for
continuous exposure for a standard duration of 104 weeks and
corrected for the expected rate of increase in tumour formation
in rodents in a standard bioassay of 104 weeks, the doses
associated with a 5% increase in tumour incidence (TD0.05s) range
from 6.2 to 34 mg/kg body weight per day. Incorporation of a
scaling factor for the differences in body surface area between
rodents and humans was not considered appropriate, as it is
likely that the carcinogenicity of 1,2-dichloroethane is due to a
metabolite, rather than to the parent compound.
11.1.2 Criteria for setting guidance values for
1,2-dichloroethane
As available data indicate that 1,2-dichloroethane is a
genotoxic carcinogen, exposure should be reduced to the extent
possible. The following guidance is provided as a possible basis
for derivation of limits of exposure and judgement of the quality
of environmental media by relevant authorities, based on the
potential carcinogenicity of 1,2-dichloroethane in humans. Based
on available data, air is believed to be the principal source of
exposure in the general environment (see section 6.2) and,
therefore, is the principal medium addressed here. Available
data are considered inadequate to serve as a basis for
development of tolerable intakes for non-neoplastic effects.
Although it is desirable to reduce exposure to genotoxic
carcinogens to the extent possible, a value, for example, 5000 or
50 000 times less than the TD0.05s might be considered
appropriate as a guidance value. This margin (5000-50 000)
affords protection similar to that associated with the range for
low-dose risk estimates generally considered by various agencies
to be "essentially negligible" (i.e. 10-5 to 10-6). This
corresponds to a range of airborne concentrations of 3.6-20
µg/m3 or 0.36-2.0 µg/m3. (Corresponding values for ingestion
are 1.2-6.8 µg/kg body weight per day or 0.12-0.68 µg/kg body
weight per day.)
It should be noted, however, that the risk of exposure in
air is most likely overestimated, as the TD0.05s were based on a
study in which the experimental animals were administered bolus
doses of 1,2-dichloroethane by gavage, whereas exposure in the
general population is likely to be mostly via inhalation. Based
on available data, the carcinogenic potency of 1,2-dichloroethane
appears to be less following inhalation than following ingestion
of bolus doses, most likely because of inter-route variations in
toxicokinetics.
11.1.3 Sample risk characterization
Non-neoplastic effects in animals have been observed only at
concentrations more than 700 000 times greater than those in the
principal medium of exposure (air) in the general environment,
based on the sample estimation of exposure presented in section
6.2 for indirect exposure in the general environment. Identified
data are inadequate to allow an estimation of exposure to
1,2-dichloroethane in the occupational environment.
Although, wherever possible, exposure to genotoxic
carcinogens should be reduced to the extent possible, indirect
population exposure in the general environment, based on the
sample estimate presented in section 6.2, is up to approximately
300 times less than a guidance value that might be considered
appropriate on the basis of available dose-response data for
carcinogenicity (i.e. 3.6-20 µg/m3 or 0.36-2.0 µg/m3, the
TD0.05s divided by 5000 or 50 000). Identified data are
inadequate to estimate exposure to 1,2-dichloroethane in the
occupational environment.
11.2 Evaluation of environmental effects
Because 1,2-dichloroethane is released principally in
emissions from industrial sources and because of its high
volatility, the atmosphere is the predominant environmental sink
for 1,2-dichloroethane. It is moderately persistent in air.
Stratospheric photolysis may produce chlorine radicals, which may
in turn react with ozone. However, the ozone depleting potential
is low (0.001 relative to CFC-11), and the compound is not listed
in the Montreal Protocol on Substances that Deplete the Ozone
Layer.
Terrestrial organisms will have the greatest potential for
exposure to 1,2-dichloroethane in ambient air. However,
available data on the effects of 1,2-dichloroethane are
inadequate to allow the characterization of risks in terrestrial
species.
Although 1,2-dichloroethane may be released to surface
waters or soil through industrial processes and disposal, and
although hydrolysis and microbial degradation are slow, the
substance is not likely to persist in these media because of its
volatility. A range of toxicity tests in aquatic species have
indicated that effect levels are generally above 10 mg/litre. As
concentrations in surface waters are generally several orders of
magnitude less than those demonstrated to cause effects, it is
likely that 1,2-dichloroethane poses negligible risk to aquatic
organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1979)
has classified 1,2-dichloroethane in group 2B (possibly
carcinogenic to humans) based on sufficient evidence of
carcinogenicity in experimental animals.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA)
has evaluated 1,2-dichloroethane on three occasions (WHO, 1971,
1980, 1992). In its last evaluation, the Committee concluded
that this compound is genotoxic in both in vitro and in vivo
test systems and carcinogenic in mice and rats when administered
by the oral route. No acceptable daily intake (ADI) was
therefore allocated. The Committee expressed the opinion that
1,2-dichloroethane should not be used in food.
In the current WHO Guidelines for drinking-water quality
(WHO, 1993), the concentrations of 1,2-dichloroethane in
drinking-water estimated to be associated with excess risks of
10-4, 10-5, and 10-6 are 300, 30, and 3 µg/litre, respectively,
based on linear multistage modelling of the incidence of
haemangiosarcomas in male rats in the NCI (1978) study.
Information on international hazard classification and
labelling is included in the International Chemical Safety Card
reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and
protective measures and first aid recommendations, are presented
on the International Chemical Safety Card (ICSC 0250) reproduced
in this document.
13.1 Human health hazards
1,2-Dichloroethane is highly flammable. On long-term or
repeated exposure, it is considered to be a probable human
carcinogen.
13.2 Advice to physicians
In case of emergency, it is important to wash skin with soap
and water after removing contaminated clothing. In the event of
poisoning, the treatment is symptomatic and supportive. Survival
for 48 hours usually implies complete recovery, although deaths
have occurred up to 5 days after exposure.
13.3 Health surveillance advice
Monitoring of both liver and kidney functions should be
included in the health surveillance programme of humans exposed
to 1,2-dichloroethane.
13.4 Explosion and fire hazards
13.4.1 Explosion hazards
1,2-Dichloroethane vapours of between 6 and 12% in air form
an explosive mixture.
13.4.2 Fire hazards
1,2-Dichloroethane is highly flammable.
13.4.3 Prevention
Because of its low electroconductivity, 1,2-dichloroethane
can generate electrostatic charges as a result of flow or
agitation. Use only closed systems, ventilation, and
explosion-proof electrical equipment. All equipment must be
grounded.
13.5 Spillage
1,2-Dichloroethane is highly flammable. In the event of
spillage, eliminate all sources of ignition in the vicinity.
Because the substance is absorbed through the skin, do not touch
or walk through the spilled material without proper equipment.
To avoid the flammability hazard, remove wet or contaminated
clothing immediately, and use non-sparking tools for clean-up.
Do not let the substance enter the drains or watercourses.
The IDLH (Immediately Dangerous to Life or Health) value for
this substance is very low, at 50 ppm (200 mg/m3) (NIOSH,
1994b).
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and
standards is available from the International Register of
Potentially Toxic Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only
in the framework of the legislation of that country. The
regulations and guidelines of all countries are subject to change
and should always be verified with appropriate regulatory
authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
1,2-DICHLOROETHANE ICSC:0250
1,2-DICHLOROETHANE
Ethylene dichloride
1,2-Ethylene dichloride
Ethanedichloride
ClCH2CH2Cl/C2H4Cl2
Molecular mass: 98.96
CAS # 107-06-2
RTECS # KI0525000
ICSC # 0250
UN # 1184
EC # 602-012-00-7
Board members serve in their personal capacity, not as
representatives of any organization, government, or industry.
They are selected because of their expertise in human and
environmental toxicology or because of their experience in the
regulation of chemicals. Boards are chosen according to the
range of expertise required for a meeting and the need for
balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers
who participate in the preparation of a CICAD are required to
declare any real or potential conflict of interest in relation to
the subjects under discussion at any stage of the process.
Representatives of nongovernmental organizations may be invited
to observe the proceedings of the Final Review Board. Observers
may participate in Board discussions only at the invitation of
the Chairperson, and they may not participate in the final
decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on 1,2-dichloroethane was prepared by the
Environmental Health Directorate of Health Canada based on an
International Programme on Chemical Safety (IPCS) Environmental
Health Criteria (EHC) document (IPCS, 1995), which assesses the
potential effects on human health of indirect exposure to
1,2-dichloroethane in the general environment as well as the
chemical's environmental effects. Data identified as of May 1993
(human health effects) and October 1994 (environmental effects)
were considered in these reviews. Information on the nature of
the peer review process and the availability of the EHC document
is presented in Appendix 1. For this CICAD, the peer review
process prior to consideration by the Final Review Board was
covered by the peer review carried out for the EHC. This CICAD
on 1,2-dichloroethane was finalized and approved for publication,
through correspondence, by members of the Final Review Board, who
also considered the peer review comments provided during the
development of the EHC. The composition of the Final Review
Board is outlined in Appendix 2. The International Chemical
Safety Card (ICSC 0250) produced by the IPCS (1993) has also been
reproduced in this document.
1,2-Dichloroethane (CAS no. 107-06-2) is a volatile,
synthetic hydrocarbon that is used principally in the synthesis
of vinyl chloride monomer and other chlorinated solvents. It has
also been used as a leaded gasoline additive and a fumigant,
although its use as a gasoline additive is declining. The
majority of environmental releases are to ambient air, where it
is moderately persistent. However, it is not expected to
contribute to ozone depletion. 1,2-Dichloroethane has a low
potential for bioaccumulation; inhalation in air is likely the
primary source of human exposure.
Little information is available on the effects of
1,2-dichloroethane in humans. The few identified epidemiological
investigations on its potential carcinogenicity are inconclusive.
1,2-Dichloroethane is moderately acutely toxic in
experimental animals. Limited information on non-neoplastic
effects presented in short-term, subchronic, and chronic studies
indicates that the liver and kidneys are the principal target
organs; lowest reported effect levels for ingestion and
inhalation were 49-82 mg/kg body weight per day (increases in
liver weight in rats exposed for 13 weeks) and 202 mg/m3
(effects on liver and kidney function in rats exposed for 12
months), respectively. Based on the results of a limited number
of studies, there is no evidence that 1,2-dichloroethane is
teratogenic in experimental animals or that it induces
reproductive or developmental effects at levels of exposure lower
than those that cause other systemic effects.
Exposure to 1,2-dichloroethane by gavage for 78 weeks
induced a significant increase in the incidence of tumours at
several sites (including haemangiosarcomas and tumours of the
stomach, mammary gland, liver, lung, and endometrium) in both
rats and mice. Although there were no significant increases in
tumour incidence in rats or mice exposed via inhalation, repeated
dermal or intraperitoneal application of 1,2-dichloroethane
resulted in an increase in lung tumours in mice.
1,2-Dichloro-ethane has been consistently genotoxic in numerous
in vitro assays in prokaryotes, fungi, and mammalian
(including human) cells. Similarly, results were consistently
positive for genotoxic activity (as well as binding to DNA) in
in vivo studies in rats, mice, and insects.
The lowest reported IC50s and EC50s for various end-points
in aquatic organisms were 25 and 105 mg/litre, respectively. The
lowest reported LC50 value for Daphnia was 220 mg/litre,
whereas effects on reproduction occurred at 20.7 mg/litre. The
most sensitive freshwater vertebrate tested was the northwestern
salamander (Ambystoma gracile), in which reduced larval
survival was observed at 2.5 mg/litre. Only limited data are
available on the effects of 1,2-dichloroethane on terrestrial
species.
Based on available data, 1,2-dichloroethane is considered to
be a probable human carcinogen, and therefore exposure should be
reduced to the extent possible. The carcinogenic potency
(expressed as the dose associated with a 5% increase in tumour
incidence), derived on the basis of studies in which animals were
exposed by gavage, was calculated to be 6.2-34 mg/kg body weight
per day. Guidance values for air (the principal source of human
exposure) of 3.6-20 µg/m3 or 0.36-2.0 µg/m3, calculated on the
basis of a margin 5000- or 50 000-fold less than the estimated
carcinogenic potency, have been derived; however, it should be
noted that risks are overestimated on this basis, as available
data indicate that 1,2-dichloroethane is less potent when
inhaled. (Corresponding values for ingestion are 1.2-6.8 µg/kg
body weight per day or 0.12-0.68 µg/kg body weight per day.)
These values correspond to those considered by some agencies to
represent "essentially negligible" risk (i.e. 10-5 to 10-6 for a
genotoxic carcinogen). Based on a sample estimate, indirect
exposure in the general environment is up to approximately 300
times less than these values.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
1,2-Dichloroethane (CAS no. 107-06-2; ethylene dichloride,
dichloro-1,2-ethane; see structural diagram below) is a synthetic
chemical that is a colourless liquid at room temperature. It is
also highly volatile, with a vapour pressure of 8.5 kPa (at
20°C), and soluble in water, with a solubility of 8690 mg/litre
(at 20°C). The log octanol/water partition coefficient of
1,2-dichloroethane is 1.76. Additional physical/chemical
properties are presented in the International Chemical Safety
Card, reproduced in this document.
H H
' '
Cl - C - C - Cl
' '
H H
3. ANALYTICAL METHODS
Analysis for 1,2-dichloroethane in environmental media is
usually by gas chromatography, in combination with electron
capture detection, flame ionization detection, or mass
spectrometry. Detection limits range from 0.016 to >4 µg/m3
for air, from 0.001 to 4.7 µg/litre for water, and from 6 to 10
µg/kg for various foodstuffs (ATSDR, 1992).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of 1,2-dichloroethane.
The principal use for 1,2-dichloroethane is in the synthesis of
vinyl chloride monomer and, to a lesser extent, in the
manufacture of various chlorinated solvents. It is also
incorporated into antiknock gasoline additives (although this use
is declining with the phase-out of leaded gasoline in some
countries) and has been used as a fumigant. Total annual
production of 1,2-dichloroethane in Canada (1990) and the USA
(1991) is about 922 and 6318 kt, respectively (CPI, 1991;
Chemical Marketing Reporter, 1992).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The majority of 1,2-dichloroethane released to the
environment is in emissions to air. 1,2-Dichloroethane is
moderately persistent in air; its estimated atmospheric lifetime
is between 43 and 111 days. Small amounts of 1,2-dichloroethane
are transported to the stratosphere, where photolysis may produce
chlorine radicals, which may in turn react with ozone (Spence &
Hanst, 1978; Callaghan et al., 1979). Some 1,2-dichloroethane
may be released in industrial effluents to the aquatic
environment, from where it is removed rapidly by volatilization
(Dilling et al., 1975). 1,2-Dichloroethane may also leach to
groundwater near industrial waste sites. It is not expected to
bioconcentrate in aquatic or terrestrial species.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Data considered to be most representative of current levels
of 1,2-dichloroethane in environmental media are summarized in
Table 1. Mean concentrations of 1,2-dichloroethane in surveys of
ambient air in non-source-dominated areas in cities are 0.07-0.28
µg/m3 in Canada, <0.004-3.8 µg/m3 in Japan, and 1.2 µg/m3 in
the United Kingdom and the Netherlands. Earlier surveys in the
USA reported mean levels of 0.33-6.05 µg/m3; however, peak
levels near chemical manufacturing plants have ranged as high as
736 µg/m3 (US EPA, 1985). Mean levels in residential indoor air
are reported to be <0.1 µg/m3 in Canada, 0.1-0.5 µg/m3 in the
USA, and 3.4 µg/m3 in the Netherlands.
In drinking-water, mean 1,2-dichloroethane concentrations
are generally less than 0.5 µg/litre, based on the results of
surveys in Canada, the USA, Japan, and Spain. Although there are
few recent data, 1,2-dichloroethane has only very rarely been
detected in surface water at concentrations greater than 10
µg/litre.
1,2-Dichloroethane has only rarely been detected in
foodstuffs in extensive surveys in Canada and the USA. Also, as
1,2-dichloroethane has low potential for bioaccumulation, food is
unlikely to be a major source of exposure.
6.2 Human exposure
An example of estimated indirect exposure in the general
environment is presented here. Exposure of the general
population to 1,2-dichloroethane in environmental media may be
estimated based on concentrations determined in various media and
reference values for body weight and consumption patterns. Owing
to the availability of relevant data, exposure has been estimated
based primarily on data from North America. However, countries
are encouraged to estimate total exposure on the basis of
national data, possibly in a manner similar to that outlined
here.
Table 1: Levels of 1,2-dichloroethane in environmental media.
Medium Location Year Concentrations Reference
Ambient air Canada 1988-1990 0.07-0.28 µg/m3 (means) T. Dann, unpublished data, 1992
Ambient air Japan 1992 <0.004-3.8 µg/m3 (means) Environment Agency Japan, 1993
Ambient air UK 1982, 1983 1.2 µg/m3 (mean) Clark et al., 1984a,b
Ambient air Netherlands 1980 1.2 µg/m3 (mean) Guicherit & Schulting, 1985
Ambient air USA 1980-1982 0.33-6.05 µg/m3 (means) Singh et al., 1980, 1981, 1982
Indoor air (residential) Canada 1991 <0.1 µg/m3 (mean) Fellin et al., 1992
Indoor air (residential) USA 0.1-0.5 µg/m3 (means) US EPA, 1992
Indoor air (residential) Netherlands 1984-1985 3.4 µg/m3 (mean) Kliest et al., 1989
Drinking-water Canada 1988-1991 <0.05-0.139 µg/litre P. Lachmaniuk, personal
(mean) communication, 1991
1990 <0.2 µg/litre (mean) Ecobichon & Allen, 1990
1982-1983 <0.1 µg/litre (mean) Otson, 1987
Drinking-water USA Early 1980s NDa - 19 µg/litre Letkiewicz et al., 1982
ND - 0.05 µg/litre Barkley et al., 1980
Drinking-water Japan 1976 <0.5-0.9 µg/litre Fujii, 1977
Drinking-water Spain 1987 2-22 µg/litre Freiria-Gandara et al., 1992
Table 1 (continued)
Medium Location Year Concentrations Reference
Surface water Canada 1981-1985 <0.08 µg/litre Kaiser et al., 1983; Comba &
Kaiser, 1985; Kaiser & Comba,
1986; Lum & Kaiser, 1986
Surface water Japan 1992 0.01-3.4 µg/litre Environment Agency Japan, 1993
Food (34 groups) Canada 1991 <50 µg/kg (solids); Enviro-Test Laboratories, 1991
<1 µg/litre (liquids)
1992 <5 µg/kg (solids); Enviro-Test Laboratories, 1992
<1 µg/litre (liquids)
Food (19 items) USA Not specified ND - 0.31 µg/kg Heikes, 1987, 1990
Not specified ND - 8.2 µg/kg Heikes, 1987
Food (231 items) USA Not specified <9-30 µg/kg Daft, 1988
a Detection limit not reported.
Based on a daily inhalation volume for adults of 22 m3, a
mean body weight for males and females of 64 kg, the assumption
that 4 of 24 hours are spent outdoors (IPCS, 1994), and the range
of mean levels of 1,2-dichloroethane in ambient air of 0.07-0.28
µg/m3 in a survey of cities across Canada, the mean intake of
1,2-dichloroethane from ambient air for the general population is
estimated to range from 0.004 to 0.02 µg/kg body weight per day.
The mean intake of 1,2-dichloroethane in indoor air, based on the
assumption that 20 of 24 hours are spent indoors (IPCS, 1994) and
the range of concentrations in indoor or "personal" air in Canada
and the USA of <0.1-0.5 µg/m3, is estimated to range from
<0.03 to 0.1 µg/kg body weight per day. Based on a daily volume
of water consumption for adults of 1.4 litres, a mean body weight
of 64 kg (IPCS, 1994), and the mean levels of 1,2-dichloroethane
in provincial surveys in Canada of <0.05-0.139 µg/litre, the
mean intake from drinking-water is estimated to range from
<0.001 to 0.003 µg/kg body weight per day. Intake of
1,2-dichloroethane in food is likely to be negligible, as it has
not been detected in extensive surveys and as it has low
potential for bioaccumulation. Therefore, the principal source
of exposure of the general population to 1,2-dichloroethane is
indoor and outdoor air, with only minor amounts being contributed
by drinking-water.
Few data on occupational exposure to 1,2-dichloroethane were
identified. In North America, workers are exposed to
1,2-dichloroethane principally in the manufacture of other
chemical substances; in such situations, the principal route of
exposure is most likely inhalation and, possibly, dermal contact.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
1,2-Dichloroethane is readily absorbed following inhalation,
ingestion, and dermal exposure and is rapidly and widely
distributed throughout the body. Relative distribution of
radioactivity (presumably as metabolites) was similar in rats
administered a single oral dose of 150 mg/kg body weight and
those exposed by inhalation to 150 ppm (600 mg/m3) for 6 hours
(Reitz et al., 1982). 1,2-Dichloroethane is rapidly and
extensively metabolized in rats and mice, with principally
sulfur-containing metabolites being eliminated in the urine in a
dose-dependent manner. Metabolism appears to be saturated or
limited in rats at levels of exposure resulting in concentrations
in blood of 5-10 µg/ml (Reitz et al., 1982). Levels of DNA
alkylation were higher following exposure to a bolus dose of 150
mg/kg body weight by gavage compared with inhalation of 150 ppm
(600 mg/m3) over a 6-hour period (Reitz et al., 1982).
Available data suggest that 1,2-dichloroethane is
metabolized via two principal pathways. The first involves a
saturable microsomal oxidation mediated by cytochrome P-450 to
2-chloroacetaldehyde and 2-chloroethanol, followed by conjugation
with glutathione. The second pathway entails direct conjugation
with glutathione to form S-(2-chloroethyl)-glutathione, which
may be non-enzymatically converted to a glutathione episulfonium
ion; this ion can form adducts with proteins, DNA, or RNA.
Although DNA damage has been induced by the P-450 pathway in
vitro (Banerjee et al., 1980; Guengerich et al., 1980; Lin et
al., 1985), several lines of evidence indicate that the
glutathione conjugation pathway is probably of greater
significance than the P-450 pathway as the major route for DNA
damage (Guengerich et al., 1980; Rannug, 1980; Sundheimer et al.,
1982; Inskeep et al., 1986; Koga et al., 1986; Simula et al.,
1993).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
1,2-Dichloroethane is moderately acutely toxic in
experimental animals. For example, LC50s for rats exposed by
inhalation for 6 or 7.25 hours ranged from 4000 mg/m3 (Spencer
et al., 1951) to 6600 mg/m3 (Bonnet et al., 1980), whereas oral
LD50s for rats, mice, dogs, and rabbits ranged from 413 to 2500
mg/kg body weight (Barsoum & Saad, 1934; McCollister et al.,
1956; Smyth, 1969; Larionov and Kokarovtseva, 1976; Munson et
al., 1982; NIOSH, 1994a).
8.2 Irritation and sensitization
Application of 1,2-dichloroethane to the skin of
experimental animals has resulted in microscopic changes and
moderate oedema (Duprat et al., 1976; Kronevi et al., 1981;
Jakobson et al., 1982). Similarly, histological changes and mild
irritation in the eye have been observed in animals following
direct application (Kuwabara et al., 1968; Duprat et al., 1976).
No information on the sensitization potential of this substance
was identified.
8.3 Short-term exposure
Few data were identified on the toxicity of
1,2-dichloroethane following short-term exposure. Degeneration
and necrosis of the liver and kidneys, accompanied by congestion
and haemorrhage of the lungs and adrenal glands, were observed in
small groups of rats, rabbits, guinea-pigs, dogs, and pigs
exposed to 1,2-dichloroethane by inhalation at 6000 mg/m3, 7
hours/day, for 6 days (Heppel et al., 1945). No effects on body
or organ weights, histology, or clinical chemistry were noted in
rats administered oral doses of up to 150 mg/kg body weight per
day for 2 weeks (van Esch et al., 1977; Reitz et al., 1982).
8.4 Long-term exposure
8.4.1 Subchronic exposure
The results of subchronic studies in several species of
experimental animals indicate that the liver and kidneys are the
target organs of 1,2-dichloroethane exposure; however, most of
these studies were inadequate to serve as a basis for
establishing reliable no-observed-effect levels or lowest-
observed-effect levels, generally because of the inadequate
documentation and the limited range of end-points examined in
small groups of animals. In a series of early limited studies,
morphological changes in the liver were observed in several
species following subchronic exposure (7 hours/day) to airborne
concentrations as low as 800 mg/m3 (Heppel et al., 1946; Spencer
et al., 1951; Hofmann et al., 1971). Increases in relative liver
weight have been observed in rats following subchronic oral
administration of doses of 49-82 mg/kg body weight per day and
above for 13 weeks (van Esch et al., 1977; NTP, 1991).
8.4.2 Chronic exposure and carcinogenicity
Little information was presented on non-neoplastic effects
in available chronic studies. Changes in serum parameters
indicative of liver and kidney toxicity were observed in groups
of 8-10 male or female Sprague-Dawley rats exposed to airborne
concentrations as low as 202 mg/m3 for 12 months, although
histopathological examinations were not conducted in this study
(Spreafico et al., 1980).
The carcinogenicity of 1,2-dichloroethane has been
investigated in a few limited bioassays in experimental animals
(limitations include short duration of exposure and high
mortality). In an inhalation study, no significant increase in
the incidence of any type of tumour was reported in groups of 90
male or female Sprague-Dawley rats exposed to concentrations of
1,2-dichloroethane up to 150 ppm (607 mg/m3), 7 hours/ day, 5
days/week, for 78 weeks and observed until spontaneous death
(Maltoni et al., 1980). However, mortality was high in this
study, although it was not related to concentration, and
incidence rates were not adjusted for differential mortality
among groups. There was a non-significant increase in the
incidence of mammary gland adenomas and fibroadenomas in female
Sprague-Dawley rats ( n = 50) exposed to 1,2-dichloroethane at
50 ppm (200 mg/m3), 7 hours/day, 5 days/ week, for 2 years in an
assay in which no other compound-related toxicity was observed
(Cheever et al., 1990). No increase in the incidence of any type
of tumour was observed in groups of 90 male or female Swiss mice
exposed to concentrations of 1,2-dichloroethane up to 150 ppm
(607 mg/m3), 7 hours/day, 5 days/ week, for 78 weeks and
observed until spontaneous death (Maltoni et al., 1980).
In contrast, there has been convincing evidence of increases
in tumour incidence in two species following ingestion. There
were significant increases in the incidence of tumours at several
sites in Osborne-Mendel rats ( n = 50 of each sex in exposed
groups; n = 20 matched controls; n = 60 pooled controls)
administered time-weighted-average doses of 47 or 95 mg/kg body
weight per day in corn oil by gavage, 5 days/week, for 78 weeks,
followed by 32 weeks of observation. The incidence of squamous
cell carcinomas of the stomach was significantly increased in
both groups of exposed males (0/60, 0/20, 3/50, and 9/50 in
pooled [from concurrent studies] vehicle controls, matched
vehicle controls, low-dose group, and high-dose group,
respectively). There were also significant increases in the
incidence of haemangiosarcomas in exposed males (1/60, 0/20,
9/50, and 7/50) and females (0/59, 0/20, 4/50, and 4/50). The
incidence of fibromas of the subcutaneous tissue was
significantly increased in exposed males (0/60, 0/20, 5/50, and
6/50). In females, there were significant increases in the
incidences of adenocarcinomas and fibroadenomas (combined) of the
mammary gland (6/59, 0/20, 15/50, and 24/50). Mortality was
significantly higher in both males and females in the high-dose
group, and there was a greater frequency of clinical signs of
toxicity in exposed rats compared with controls. Chronic murine
pneumonia was present in 60-94% of rats in each group, although
the incidence was not related to dose (NCI, 1978).
In a similar bioassay, B6C3F1 mice ( n = 50 of each sex in
exposed groups; n = 20 matched controls; n = 60 pooled
controls) were administered time-weighted-average doses of 97 or
195 mg/kg body weight per day (males) and 149 or 299 mg/kg body
weight per day (females) in corn oil by gavage, 5 days/week, for
78 weeks, followed by 13 weeks of observation. The incidence of
hepatocellular carcinomas was significantly increased in exposed
males (4/59, 1/19, 6/47, and 12/48 in pooled vehicle controls,
matched vehicle controls, low-dose group, and high-dose group,
respectively), although the authors noted that the increase in
the incidence of this tumour could not be convincingly attributed
to the test chemical, owing to the high variability of
hepatocellular neoplasms among historical controls. The
incidence of alveolar/bronchiolar adenomas was significantly
increased in males in the high-dose group (0/59, 0/19, 1/47, and
15/48) and in both groups of exposed females (2/60, 1/20, 7/50,
and 15/48); one female in the high-dose group had an
alveolar/bronchiolar carcinoma. The incidence of mammary gland
adenocarcinomas was significantly increased in females at both
doses (0/60, 0/20, 9/50, and 7/48). The incidence of endometrial
stromal polyp or endometrial stromal sarcoma (combined) in
females was significantly elevated at both doses (0/60, 0/20,
5/49, and 5/47). There was a dose-related increase in mortality
in females, but not in males; in addition, body weight was
decreased in females receiving the higher dose (NCI, 1978).
The incidence of lung tumours (benign lung papillomas) was
significantly increased in female non-inbred Ha:ICR mice ( n =
30) following repeated dermal application of 1,2-dichloroethane,
3 times/week, for 440-594 days (van Duuren et al., 1979).
Repeated intraperitoneal injections of 1,2-dichloroethane
resulted in a dose-related increase in the number of pulmonary
adenomas per mouse in a screening bioassay in a susceptible
strain (A/St), although none of these increases was significant
(Theiss et al., 1977). Concomitant exposure to inhaled
1,2-dichloroethane and disulfiram in the diet resulted in an
increased incidence of intrahepatic bile duct cholangiomas and
cysts, subcutaneous fibromas, hepatic neoplastic nodules,
interstitial cell tumours in the testes, and mammary
adenocarcinomas in rats, compared with rats administered either
compound alone or untreated controls (Cheever et al., 1990). No
potential to initiate or promote tumour development was evident
in three bioassays (van Duuren et al., 1979; Klaunig et al.,
1986; Story et al., 1986; Milman et al., 1988), although the
extent of histopathological examination was limited in these
studies.
8.5 Genotoxicity and related end-points
1,2-Dichloroethane has been consistently demonstrated to be
genotoxic in numerous in vitro (Table 2) and in vivo (Table
3) assays for a wide range of end-points. It has been mutagenic
in Salmonella typhimurium, especially in the presence of an
exogenous activation system, and induces unscheduled DNA
synthesis, induces gene mutation, and forms adducts with DNA in
mammalian cells in vitro. It binds to DNA in all reported in
vivo studies in rats and mice. 1,2-Dichloroethane has also
induced somatic cell and sex-linked recessive lethal mutations in
Drosophila melanogaster.
Available data on genotoxicity are consistent with the
hypothesis that the glutathione pathway of conjugation (i.e.
production of the glutathione episulfonium ion) is probably of
greater significance than the P-450 pathway as the major route
for DNA damage (Guengerich et al., 1980; Rannug, 1980; Sundheimer
et al., 1982; Inskeep et al., 1986; Koga et al., 1986; Simula et
al., 1993); mutation frequency in human cell lines has been
correlated with variations in levels of
glutathione- S-transferase activities (Crespi et al., 1985).
8.6 Reproductive and developmental toxicity
Based on the results of a limited number of studies, there
is no evidence that 1,2-dichloroethane is teratogenic in
experimental animals and little convincing evidence that it
induces reproductive or developmental effects at doses below
those that cause other systemic effects (Alumot et al., 1976;
Vozovaya, 1977; Kavlock et al., 1979; Rao et al., 1980; Lane et
al., 1982).
8.7 Immunological and neurological effects
Immunological effects, including reduced resistance to
streptococcal challenge, decreased pulmonary bactericidal
activity in mice, and altered levels of antibody production in
rabbits, have been observed following acute or subchronic
exposure to 1,2-dichloroethane at 20 and 10 mg/m3 and above,
respectively (Shmuter, 1977; Sherwood et al., 1987), whereas
there were no effects in rats exposed to up to 800 mg/m3 for
several days (Sherwood et al., 1987). Effects on antibody levels
and reversible effects on cell-mediated responses were also noted
in mice exposed to 1,2-dichloroethane in drinking-water at
concentrations equivalent to doses of 3 mg/kg body weight per day
and above for 14 or 90 days (Munson et al., 1982).
Data on the neurological effects of 1,2-dichloroethane have
not been identified.
9. EFFECTS ON HUMANS
9.1 Case reports
Acute incidental exposure to 1,2-dichloroethane by
inhalation or ingestion has resulted in a variety of effects in
humans, including effects on the central nervous system, liver,
kidney, lung, and cardiovascular system (e.g. Hinkel, 1965;
Suveev & Babichenko, 1969; Dorndorf et al., 1975; Andriukin,
1979; Nouchi et al., 1984). Based on limited available data in
humans, the lethal oral dose of 1,2-dichloroethane has been
estimated to be between 20 and 50 ml.
9.2 Epidemiological studies
The potential carcinogenicity of 1,2-dichloroethane in
exposed human populations has not been extensively investigated.
Mortality due to pancreatic cancer was significantly increased
(standardized mortality ratio [SMR] = 492, based on eight cases)
in a group of 278 workers at a chemical production plant who had
been principally exposed to 1,2-dichloroethane in combination
with other chemicals. Mortality due to this cause increased with
duration of exposure. In addition, although the number of cases
was small (i.e. four) and the association with duration of
exposure was less consistent, mortality due to leukaemia was also
increased in these workers (Benson & Teta, 1993).
No association between occupational exposure to
1,2-dichloroethane and brain cancer was noted in a small
case-control study (Austin & Schnatter, 1983). Although the
incidence of colon and rectal cancer increased with concentration
of 1,2-dichloroethane in drinking-water in an inherently limited
ecological study, concomitant exposure to other substances may
have contributed to the observed effects (Isacson et al., 1985).
Table 2: Genotoxicity of 1,2-dichloroethane in vitro
(modified from ATSDR, 1992).
Result
Species (test system) End-point With Without
activation activation Reference
PROKARYOTIC SYSTEMS
Salmonella typhimurium Gene mutation + + Milman et al., 1988
+ + Barber et al., 1981
+ + Kanada & Uyeta, 1978
+ + Nestmann et al., 1980
+ + Rannug et al., 1978
+ + van Bladeren et al., 1981
+ NT Rannug & Beije, 1979
+ - Cheh et al., 1980
+ - Moriya et al., 1983
- - King et al., 1979
+ + Strobel & Grummt, 1987
NT +a Simula et al., 1993
S. typhimurium/spot test Gene mutation NT (+) Brem et al., 1974
(+) - Principe et al., 1981
NT - Buijs et al., 1984
S. typhimurium/Ara test (standard) Gene mutation + - Roldan-Arjona et al., 1991
S. typhimurium/Ara test (liquid) Gene mutation (+) (+) Roldan-Arjona et al., 1991
Streptomyces coelicolor Gene mutation NT - Principe et al., 1981
Escherichia coli K12/343/113 Gene mutation - - King et al., 1979
E. coli wp2 Gene mutation NT (+) Hemminki et al., 1980
- - Moriya et al., 1983
E. coli Pol A DNA damage NT (+) Brem et al., 1974
Bacillus subtilis/rec- assay DNA damage NT - Kanada & Uyeta, 1978
Table 2 (continued)
Result
Species (test system) End-point With Without
activation activation Reference
EUKARYOTIC ORGANISMS
- FUNGI
Aspergillus nidulans Gene mutation NT - Crebelli & Carere, 1988
NT - Principe et al., 1981
A. nidulans Mitotic segregation
aberrations NT + Crebelli et al., 1984
A. nidulans Aneuploidy induction NT + Crebelli et al., 1988
Saccharomyces cerevisiae Mitotic recombination NT (+) Simmon, 1980
- ANIMAL SYSTEMS
Hamster CHO/HGPRT Gene mutation + (+) Tan & Hsie, 1981
+ (+) Zamora et al., 1983
Rat hepatocytes Unscheduled DNA synthesis NT + Williams et al., 1989
Mouse hepatocytes Unscheduled DNA synthesis NT + Milman et al., 1988
Mouse liver DNA DNA binding + NT Banerjee, 1988
Calf thymus DNA DNA binding + NT Prodi et al., 1986
Salmon sperm DNA DND binding + - Banerjee & van Duuren, 1979;
Banerjee et al., 1980
Mouse BALB/c-3T3 Cell transformation NT - Milman et al., 1988
NT - Tu et al., 1985
Mouse C3H1OT´ Cell transformation NT +b Schultz et al., 1992
Syrian hamster embryo cells Cell transformation NT + Hatch et al., 1983
Table 2 (continued)
Result
Species (test system) End-point With Without
activation activation Reference
- HUMAN CELLS
Human lymphoblasts AHH-1 Gene mutation NT + Crespi et al., 1985
Human lymphoblasts TK6 Gene mutation NT + Crespi et al., 1985
Human embryo epithelial-like
EUE cells Gene mutation NT + Ferreri et al., 1983
Human peripheral lymphocytes Unscheduled DNA synthesis + - Perocco & Prodi, 1981
NT = not tested - = negative result + = positive result (+) = weakly positive or marginal result
a Increase in cells expressing GSTA1-1.
b Transformed cells induced tumours in nude mice.
Table 3: Genotoxicity of 1,2-dichloroethane in vivo
(modified from ATSDR, 1992).
Species (test system) End-point Results Reference
MAMMALIAN ASSAYS
Mouse Dominant lethal mutations - Lane et al., 1982
Mouse/spot test Gene mutation (+) Gocke et al., 1983
Mouse bone marrow Sister chromatid exchange + Giri & Que Hee, 1988
Mouse bone marrow Micronuclei - King et al., 1979;
Jenssen & Ramal, 1980
Mouse peripheral erythrocytes Micronuclei - Armstrong & Galloway, 1993
Mouse liver, kidney, lung, and stomach DNA binding + Prodi et al., 1986
Mouse liver, kidney, lung, and stomach DNA binding + Arfellini et al., 1984
Mouse forestomach and kidney DNA binding + Hellman & Brandt, 1986
Mouse liver DNA binding + Banerjee, 1988
Rat liver, kidney, spleen, lung,
forestomach, and stomach DNA binding + Reitz et al., 1982
Rat liver, kidney, lung, and stomach DNA binding + Arfellini et al., 1984
Rat liver, kidney, lung, and stomach DNA binding + Prodi et al., 1986
Rat liver and kidney DNA binding + Inskeep et al., 1986
Rat liver and lung DNA binding + Baertsch et al., 1991
Rat liver DNA binding + Banerjee, 1988
Rat liver DNA binding + Cheever et al., 1990
Mouse liver DNA damage + Storer & Conolly 1983,
1985;
Storer et al., 1984
Mouse liver DNA damage + Taningher et al., 1991
Table 3 (continued)
Species (test system) End-point Results Reference
INSECT ASSAYS
Drosophila melanogaster/somatic
mutation Gene mutation + Nylander et al., 1978
D. melanogaster/somatic mutation Gene mutation + Romert et al., 1990
D. melanogaster/somatic mutation Gene mutation + Kramers et al., 1991
D. melanogaster/somatic mutation Gene mutation (+) Ballering et al., 1993
D. melanogaster/recessive lethal Gene mutation + Ballering et al., 1993
D. melanogaster/vermilion locus Gene mutation + Ballering et al., 1993
D. melanogaster/sex-linked recessive Gene mutation + King et al., 1979
D. melanogaster/sex-linked recessive Gene mutation + Kramers et al., 1991
D. melanogaster Chromosomal loss/gain +/+ Valencia et al., 1984
HOST-MEDIATED ASSAYS
Escherichia coli K12/343/113 mouse
host-mediated assay Gene mutation - King et al., 1979
- = negative result + = positive result (+) = weakly positive or marginal result
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
The effects of exposure to 1,2-dichloroethane on a number of
aquatic organisms in the laboratory and field have also been
investigated. In bacteria, the lowest reported IC50s for
inhibition of gas production and ammonia consumption were 25 and
29 mg/litre in methanogens and Nitrosomonas, respectively (Blum
& Speece, 1991). The most sensitive freshwater alga studied was
Microcystis aeruginosa, in which an EC50 for inhibition of
cell multiplication of 105 mg/litre was observed (Bringmann &
Kühn, 1978); in the only identified study in marine algae, an
EC50 for carbon uptake of 340 mg/litre was reported in
Phaeodactylum tricornutum (Pearson & McConnell, 1975).
Toxicity thresholds (cell multiplication inhibition) were above
800 mg/litre for three species of aquatic protozoa (Bringmann &
Kühn, 1980).
The lowest reported LC50 value for Daphnia was 220
mg/litre (Leblanc, 1980), whereas the lowest EC50 (for 10%
immobilization) was 150 mg/litre (Freitag et al., 1994). Effects
on reproductive success and growth were observed in Daphnia at
20.7 and 71.7 mg/litre, respectively. There were no effects on
these end-points at 10.6 and 41.6 mg/litre, respectively (Richter
et al., 1983).
Based on available data, the most sensitive freshwater
vertebrate species appears to be the northwestern salamander, in
which 9-day larval survival (4 days post-hatch) was reduced at
2.5 mg/litre (Black et al., 1982).
10.2 Terrestrial environment
Identified data on the toxicity of 1,2-dichloroethane to
terrestrial organisms are inadequate to permit assessment.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Based on limited available data in humans, the lethal oral
dose of 1,2-dichloroethane has been estimated to be between 20
and 50 ml. 1,2-Dichloroethane is moderately acutely toxic by
inhalation, based on the results of studies in experimental
animals. Skin and eye irritation may also be induced by
1,2-dichloroethane.
Owing to the limitations of the available studies in humans,
it is necessary to rely on available experimental data in animal
species as a basis for derivation of no-effect levels or
quantitative estimates of carcinogenic potency. However, in most
of the identified short-term and subchronic studies, only a
limited range of end-points was examined and documentation was
incomplete. Similarly, little information on non-neoplastic
effects was presented in the long-term carcinogenicity bioassays.
Lowest reported effect levels were 49-82 mg/kg body weight per
day for 13 weeks (increases in liver weight in rats) for
ingestion (NTP, 1991) and 202 mg/m3 (effects on liver and kidney
function in rats exposed for 12 months) for inhalation (Spreafico
et al., 1980). Based on limited data, there is no evidence that
1,2-dichloroethane is teratogenic in experimental animals or that
it induces reproductive or developmental effects at doses below
those that cause other systemic effects.
Based on the limited evidence of carcinogenicity in workers
exposed principally to 1,2-dichloroethane in the most reliable
epidemiological study conducted to date (Benson & Teta, 1993),
the induction of both rare and common tumours in rats and mice
exposed by ingestion (NCI, 1978) and supporting evidence in other
limited bioassays, the production of a reactive intermediate that
alkylates DNA in vivo, and positive results in a range of in
vitro assays for genotoxicity, 1,2-dichloroethane is considered
to be a probable human carcinogen.
The carcinogenic potency of 1,2-dichloroethane has been
calculated based on the increased incidence of squamous cell
carcinomas of the stomach, haemangiosarcomas, fibromas of the
subcutaneous tissue, and adenocarcinomas or fibroadenomas
(combined) of the mammary gland in Osborne-Mendel rats exposed
orally by gavage, as well as the increased incidence of
alveolar/bronchiolar adenomas, hepatocellular carcinomas, mammary
gland adenocarcinomas, and endometrial stromal polyps or sarcomas
(combined) in similarly exposed B6C3F1 mice; data from both the
matched (same study) and pooled (concurrent studies) vehicle
controls were incorporated. It should be noted, however, that
mortality was higher at the high dose in female mice and rats of
both sexes than in other dose groups in this study. Therefore,
these high-dose groups were not included in the derivation of
quantitative estimates of carcinogenic potency.
Based on multistage modelling of these data, amortized for
continuous exposure for a standard duration of 104 weeks and
corrected for the expected rate of increase in tumour formation
in rodents in a standard bioassay of 104 weeks, the doses
associated with a 5% increase in tumour incidence (TD0.05s) range
from 6.2 to 34 mg/kg body weight per day. Incorporation of a
scaling factor for the differences in body surface area between
rodents and humans was not considered appropriate, as it is
likely that the carcinogenicity of 1,2-dichloroethane is due to a
metabolite, rather than to the parent compound.
11.1.2 Criteria for setting guidance values for
1,2-dichloroethane
As available data indicate that 1,2-dichloroethane is a
genotoxic carcinogen, exposure should be reduced to the extent
possible. The following guidance is provided as a possible basis
for derivation of limits of exposure and judgement of the quality
of environmental media by relevant authorities, based on the
potential carcinogenicity of 1,2-dichloroethane in humans. Based
on available data, air is believed to be the principal source of
exposure in the general environment (see section 6.2) and,
therefore, is the principal medium addressed here. Available
data are considered inadequate to serve as a basis for
development of tolerable intakes for non-neoplastic effects.
Although it is desirable to reduce exposure to genotoxic
carcinogens to the extent possible, a value, for example, 5000 or
50 000 times less than the TD0.05s might be considered
appropriate as a guidance value. This margin (5000-50 000)
affords protection similar to that associated with the range for
low-dose risk estimates generally considered by various agencies
to be "essentially negligible" (i.e. 10-5 to 10-6). This
corresponds to a range of airborne concentrations of 3.6-20
µg/m3 or 0.36-2.0 µg/m3. (Corresponding values for ingestion
are 1.2-6.8 µg/kg body weight per day or 0.12-0.68 µg/kg body
weight per day.)
It should be noted, however, that the risk of exposure in
air is most likely overestimated, as the TD0.05s were based on a
study in which the experimental animals were administered bolus
doses of 1,2-dichloroethane by gavage, whereas exposure in the
general population is likely to be mostly via inhalation. Based
on available data, the carcinogenic potency of 1,2-dichloroethane
appears to be less following inhalation than following ingestion
of bolus doses, most likely because of inter-route variations in
toxicokinetics.
11.1.3 Sample risk characterization
Non-neoplastic effects in animals have been observed only at
concentrations more than 700 000 times greater than those in the
principal medium of exposure (air) in the general environment,
based on the sample estimation of exposure presented in section
6.2 for indirect exposure in the general environment. Identified
data are inadequate to allow an estimation of exposure to
1,2-dichloroethane in the occupational environment.
Although, wherever possible, exposure to genotoxic
carcinogens should be reduced to the extent possible, indirect
population exposure in the general environment, based on the
sample estimate presented in section 6.2, is up to approximately
300 times less than a guidance value that might be considered
appropriate on the basis of available dose-response data for
carcinogenicity (i.e. 3.6-20 µg/m3 or 0.36-2.0 µg/m3, the
TD0.05s divided by 5000 or 50 000). Identified data are
inadequate to estimate exposure to 1,2-dichloroethane in the
occupational environment.
11.2 Evaluation of environmental effects
Because 1,2-dichloroethane is released principally in
emissions from industrial sources and because of its high
volatility, the atmosphere is the predominant environmental sink
for 1,2-dichloroethane. It is moderately persistent in air.
Stratospheric photolysis may produce chlorine radicals, which may
in turn react with ozone. However, the ozone depleting potential
is low (0.001 relative to CFC-11), and the compound is not listed
in the Montreal Protocol on Substances that Deplete the Ozone
Layer.
Terrestrial organisms will have the greatest potential for
exposure to 1,2-dichloroethane in ambient air. However,
available data on the effects of 1,2-dichloroethane are
inadequate to allow the characterization of risks in terrestrial
species.
Although 1,2-dichloroethane may be released to surface
waters or soil through industrial processes and disposal, and
although hydrolysis and microbial degradation are slow, the
substance is not likely to persist in these media because of its
volatility. A range of toxicity tests in aquatic species have
indicated that effect levels are generally above 10 mg/litre. As
concentrations in surface waters are generally several orders of
magnitude less than those demonstrated to cause effects, it is
likely that 1,2-dichloroethane poses negligible risk to aquatic
organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1979)
has classified 1,2-dichloroethane in group 2B (possibly
carcinogenic to humans) based on sufficient evidence of
carcinogenicity in experimental animals.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA)
has evaluated 1,2-dichloroethane on three occasions (WHO, 1971,
1980, 1992). In its last evaluation, the Committee concluded
that this compound is genotoxic in both in vitro and in vivo
test systems and carcinogenic in mice and rats when administered
by the oral route. No acceptable daily intake (ADI) was
therefore allocated. The Committee expressed the opinion that
1,2-dichloroethane should not be used in food.
In the current WHO Guidelines for drinking-water quality
(WHO, 1993), the concentrations of 1,2-dichloroethane in
drinking-water estimated to be associated with excess risks of
10-4, 10-5, and 10-6 are 300, 30, and 3 µg/litre, respectively,
based on linear multistage modelling of the incidence of
haemangiosarcomas in male rats in the NCI (1978) study.
Information on international hazard classification and
labelling is included in the International Chemical Safety Card
reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and
protective measures and first aid recommendations, are presented
on the International Chemical Safety Card (ICSC 0250) reproduced
in this document.
13.1 Human health hazards
1,2-Dichloroethane is highly flammable. On long-term or
repeated exposure, it is considered to be a probable human
carcinogen.
13.2 Advice to physicians
In case of emergency, it is important to wash skin with soap
and water after removing contaminated clothing. In the event of
poisoning, the treatment is symptomatic and supportive. Survival
for 48 hours usually implies complete recovery, although deaths
have occurred up to 5 days after exposure.
13.3 Health surveillance advice
Monitoring of both liver and kidney functions should be
included in the health surveillance programme of humans exposed
to 1,2-dichloroethane.
13.4 Explosion and fire hazards
13.4.1 Explosion hazards
1,2-Dichloroethane vapours of between 6 and 12% in air form
an explosive mixture.
13.4.2 Fire hazards
1,2-Dichloroethane is highly flammable.
13.4.3 Prevention
Because of its low electroconductivity, 1,2-dichloroethane
can generate electrostatic charges as a result of flow or
agitation. Use only closed systems, ventilation, and
explosion-proof electrical equipment. All equipment must be
grounded.
13.5 Spillage
1,2-Dichloroethane is highly flammable. In the event of
spillage, eliminate all sources of ignition in the vicinity.
Because the substance is absorbed through the skin, do not touch
or walk through the spilled material without proper equipment.
To avoid the flammability hazard, remove wet or contaminated
clothing immediately, and use non-sparking tools for clean-up.
Do not let the substance enter the drains or watercourses.
The IDLH (Immediately Dangerous to Life or Health) value for
this substance is very low, at 50 ppm (200 mg/m3) (NIOSH,
1994b).
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and
standards is available from the International Register of
Potentially Toxic Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only
in the framework of the legislation of that country. The
regulations and guidelines of all countries are subject to change
and should always be verified with appropriate regulatory
authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
1,2-DICHLOROETHANE ICSC:0250
1,2-DICHLOROETHANE
Ethylene dichloride
1,2-Ethylene dichloride
Ethanedichloride
ClCH2CH2Cl/C2H4Cl2
Molecular mass: 98.96
CAS # 107-06-2
RTECS # KI0525000
ICSC # 0250
UN # 1184
EC # 602-012-00-7
 TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Highly flammable. Gives off No open flames, NO sparks, Powder, water spray, foam,
irritating or toxic fumes (or and NO smoking. carbon dioxide.
gases) in a fire).
EXPLOSION Vapour/air mixtures are Closed system, ventilation, In case of fire: keep drums,
explosive. explosion-proof electrical etc., cool by spraying with
equipment and lighting. water.
Prevent build-up of
electrostatic charges (e.g.,
by grounding) (see Notes).
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTONS FIRE FIGHTING
EXPOSURE
EXPOSURE AVOID ALL CONTACT! IN ALL CASES CONSULT A
DOCTOR!
* INHALATION Abdominal pain. Cough. Ventilation, local exhaust, Fresh air, rest. Half-upright
Dizziness. Drowsiness. or breathing protection. position. Artificial
Headache. Nausea. Sore respiration if indicated.
throat. Unconsciousness. Refer for medical attention.
Vomiting. Symptoms may be
delayed (see Notes).
* SKIN Redness. Protective gloves. Remove contaminated clothes.
Rinse and then wash skin with
water and soap. Refer for
medical attention.
* EYES Redness. Pain. Blurred Safety goggles, face shield, First rinse with plenty of
vision. or eye protection in water for several minutes
combination with breathing (remove contact lenses if
protection. easily possible), then take
to a doctor.
* INGESTION Abdominal cramps. Diarrhoea Do no eat, drink, or smoke Give nothing to drink. Refer
(further see Inhalation). during work. Wash hands for medical attention.
before eating.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Evacuate danger area! Collect leaking Fireproof. Separated from strong Unbreakable packaging; put breakable
and spilled liquid in sealable oxidants, food and feedstuffs and packaging into closed unbreakable
containers as far as possible. Absorb other incompatible substances (see container. Do not transport with food
remaining liquid in sand or inert Chemical Dangers). Cool. Dry. and feedstuffs.
absorbent and remove to safe place. Do F symbol
NOT wash away into sewer (extra Y symbol
personal protection: self-contained R: 45-11-22-36/37/38
breathing apparatus). S: 53-45
Note: E
UN Hazard Class: 3
UN Subsidiary Risks: 6.1
UN Packing Group: 11
Marine Pollutant.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: INHALATION RISK:
COLOURLESS, VISCOUS LIQUID, WITH A harmful contamination of the air can be
CHARACTERISTIC ODOUR, TURNS DARK ON EXPOSURE reached very quickly on evaporation of this
TO AIR, MOISTURE AND LIGHT substance at 20°C.
PHYSICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The vapour is heavier than air and may travel The vapour irritates the eyes, the skin and
along the ground; distant ignition possible. the respiratory tract. Inhalation of the
As a result of flow, agitation, etc., vapour may cause lung oedema (see Notes). The
electrostatic charges can be generated. substance may cause effects on the central
nervous system, kidneys, liver, resulting in
CHEMICAL DANGERS: impaired functions.
The substance decomposes on heating and on EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
burning producing toxic and corrosive fumes
including hydrogen chloride (ICSC #0163) and Repeated or prolonged contact with skin may
phosgene (ICSC #0007). Reacts violently with cause dermatitis. This substance is probably
aluminium, alkali metals, alkali amides, carcinogenic to humans.
ammonia bases, strong oxidants. Attacks many
metals in presence of water. Attacks plastic.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV: 10 ppm; 40 mg/m3 (as TWA)
(ACGIH 1994-1995).
ROUTES OF EXPOSURE:
The substance can be absorbed into the body by
inhalation of its vapour, through the skin and
by ingestion.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 83.5°C Flash point: 13°C c.c.
PROPERTIES Melting point: -35.7°C Auto-ignition temperature: 413°C
Relative density (water = 1): 1.235 Explosive limits, vol% in air: 6.2-16
Solubility in water, g/100 ml: 0.87 Octanol/water partition coefficient
Vapour pressure, kPa at 20°C: 8.7 as logPow: 1.48
Relative vapour density (air = 1): 3.42
Relative density of the vapour/
air-mixture at 20°C (air = 1): 1.2
ENVIRONMENTAL
DATA
NOTES
Depending on the degree of exposure, periodic medical examination is indicated.
The symptoms of lung oedema often do not become manifest until a few hours have passed and they are aggravated by physical effort.
Rest and medical observation are tehrefore essential.
Immediate administration of an appropriate spray, by a doctor or a person authorized by him/her, should be considered.
EXPLOSION/PREVENTION: Do NOT use compressed air for filling, discharging, or handling.
ICSC: 0250 1.1 Transport Emergency Card: TEC (R)-605
NFPA Code: H 2; F 3; R 0
REFERENCES
Alumot E, Nachtomi E, Mandel E, Holstein P (1976) Tolerance and
acceptable daily intake of chlorinated fumigants in the rat diet.
Food and cosmetics toxicology, 14:105-110.
Andriukin AA (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klinicheskaya Meditsina (Moscow),
57:43-47 (in Russian).
Arfellini G, Bartoli S, Colacci A, Mazzullo M, Galli MC, Prodi G,
Grilli S (1984) In vivo and in vitro binding of
1,2-dibromoethane and 1,2-dichloroethane to macromolecules in rat
and mouse organs. Journal of cancer research and clinical
oncology, 108:204-213.
Armstrong MJ, Galloway SM (1993) Micronuclei induced in
peripheral blood of Eµ-PIM-1 transgenic mice by chronic oral
treatment with 2-acetylaminofluorene or benzene but not with
diethylnitrosamine or 1,2-dichloroethane. Mutation research,
302:61-70.
ATSDR (1992) Toxicological profile for 1,2-dichloroethane
(draft). Atlanta, GA, US Department of Health and Human Services,
Public Health Service, Agency for Toxic Substances and Disease
Registry.
Austin SG, Schnatter AR (1983) A case-control study of chemistry
exposures and brain tumors in petrochemical workers. Journal
of occupational medicine, 25(4):313-320.
Baertsch A, Lutz WK, Schlatter C (1991) Effect of inhalation
exposure regimen on DNA binding potency of 1,2-dichloroethane in
the rat. Archives of toxicology, 65:169-176.
Ballering LAP, Nivard MJM, Vogel EW (1993) Characterization of
the genotoxic action of three structurally related
1,2-dihaloalkanes in Drosophila melanogaster. Mutation
research, 285:209-217.
Banerjee S (1988) DNA damage in rodent liver by
1,2-dichloroethane, a hepatocarcinogen. Cancer biochemistry
and biophysics, 10:165-173.
Banerjee S, van Duuren BL (1979) Binding of carcinogenic
halogenated hydrocarbons to cell macromolecules. Journal of
the National Cancer Institute, 63:707-711.
Banerjee S, van Duuren BL, Oruambo FI (1980) Microsome-mediated
covalent binding of 1,2-dichloroethane to lung microsomal protein
and salmon sperm DNA. Cancer research, 40:2170-2173.
Barber RD, Donish WH, Mueller KR (1981) A procedure for the
quantitative measurement of the mutagenicity of volatile liquids
in the Ames Salmonella microsome assay. Mutation research,
90:31-48.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizzari ED, Ray M, Smith D, Tomer KB, Wagner R, Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - A
multimedia environmental study. Biomedical and environmental
mass spectrometry, 7:139-147.
Barsoum GS, Saad K (1934) Relative toxicity of certain chlorine
derivatives of the aliphatic series. Quarterly journal of
pharmacy and pharmacology, 7: 205-214.
Benson LO, Teta MJ (1993) Mortality due to pancreatic and
lymphopoietic cancers in chlorohydrin production workers.
British journal of industrial medicine, 50:710-716.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, Bruser
DM (1982) The aquatic toxicity of organic compounds to
embryo-larval stages of fish and amphibians. Lexington, KY,
University of Kentucky (Research Report No. 133).
Blum DJW, Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons
and correlations. Research journal of the Water Pollution
Control Federation, 63(3):198-207.
Bonnet P, Francin J-M, Grakiski D, Raoult G, Zissu D (1980)
Détermination de la concentration léthale50 des principaux
hydrocarbures aliphatiques chlorés chez le rat. Archives des
maladies professionnelles de medecine du travail et de
securité sociale (Paris), 41(6-7):317-321.
Brem H, Stein AB, Rosenkranz HS (1974) The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer research,
34:2576-2579.
Bringmann G, Kühn R (1978) Testing of substances for their
toxicity threshold: model organisms Microcystis (Diplocystis)
aeruginosa and Scenedesmus quadricauda. Mitteilungen
Internationale Vereinigung für Theoretische und Angewandte
Limnologie, 21:275-284.
Bringmann G, Kühn R (1980) Comparison of the toxicity thresholds
of water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water research, 14:231-241.
Buijs W, van der Gen A, Mohn GR, Breimer DD (1984) The direct
mutagenic activity of alpha, omega-dihalogenoalkanes in
Salmonella typhimurium. Mutation research, 141:11-14.
Callaghan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR,
Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR,
Gould C (1979) Water-related fate of 129 priority pollutants.
Vol. II. Washington, DC, US Environmental Protection Agency
(EPA 40/4-79-029b).
Cheever KL, Cholakis JM, El-Hawari AM, Kovatch RM, Weisburger EK
(1990) Ethylene dichloride: the influence of disulfiram or
ethanol on oncogenicity, metabolism and DNA covalent binding in
rats. Fundamental and applied toxicology, 14:243-261.
Cheh AM, Hooper AB, Skochdopole J, Henke CA, McKinnell RG (1980)
A comparison of the ability of frog and rat S-9 to activate
promutagens in the Ames test. Environmental and molecular
mutagenesis, 2:487-508.
Chemical Marketing Reporter (1992) Chemical profile: ethylene
dichloride. Chemical Marketing Reporter magazine, 241(19):42.
Clark AI, McIntyre AE, Lester JN, Perry R (1984a) Ambient air
measurement of aromatic and halogenated hydrocarbons at urban,
rural, and motorway locations. The science of the total
environment, 39:265-279.
Clark AI, McIntyre AE, Perry R, Lester JN (1984b) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural, and motorway locations.
Environmental pollution series B, 7:141-158.
Comba ME, Kaiser KLE (1985) Volatile halocarbons in the Detroit
River and their relationship with contaminant sources. Journal
of Great Lakes research, 11(3):404-418.
CPI (1991) CPI product profiles: ethylene dichloride. Don
Mills, Ontario, Canadian Process Industries, Corpus Information
Services.
Crebelli R, Carere A (1988) Genotoxic activity of halogenated
aliphatic hydrocarbons in Aspergillus nidulans. Journal of
occupational toxicology, 8:437-442.
Crebelli R, Conti G, Conti L, Carere A (1984) Induction of
somatic segregation by halogenated aliphatic hydrocarbons in
Aspergillus nidulans. Mutation research, 138:33-38.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, Carere A
(1988) Induction of chromosome malsegregation by halogenated
organic solvents in Aspergillus nidulans: unspecific or
specific mechanism? Mutation research, 201:401-411.
Crespi CL, Seixas GM, Turner TR, Ryan CG, Penman BW (1985)
Mutagenicity of 1,2-dichloroethane and 1,2-dibromoethane in two
human lymphoblastoid cell lines. Mutation research,
142:133-140.
Daft JL (1988) Rapid determination of fumigant and industrial
chemical residues in food. Journal of the Association of
Official Analytical Chemists, 71(4):748-760.
Dilling WL, Tefertiller NB, Kalos GJ (1975) Evaporation rates and
reactivities of methylene chloride, chloroform,
1,1,1-trichloroethane, trichloroethylene, tetrachloroethylene,
and other chlorinated compounds in dilute aqueous solutions.
Environmental science and technology, 9(9):833-838.
Dorndorf W, Kresse M, Christian W, Katritzki KG (1975)
[Dichloroethane poisoning with myoclonic syndrome, epileptic
attacks, and irreversible cerebral effects.] Archiv für
Psychiatrie und Nervenkrankheiten, 220:373-379 (in German).
Duprat P, Delsaut L, Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. European journal of toxicology,
9:171-177.
Ecobichon DJ, Allen MC (1990) New Brunswick - Water Quality
Surveillance Program, New Brunswick public water supplies,
data summary report 1990. New Brunswick Department of Health
and Community Services.
Environment Agency Japan (1993) Chemicals in the environment -
1993. Tokyo.
Enviro-Test Laboratories (1991) Cayley background study:
analysis of food products for target organic and inorganic
parameters. Edmonton (Report No. 91-E1208).
Enviro-Test Laboratories (1992) Windsor area background study:
analysis of food products for target organic and inorganic
parameters. Edmonton (Report No. E1052).
Fellin P, Barnett SE, Tran QA (1992) Results of a national
pilot survey of airborne volatile organic compounds in
Canadian residences. Vol. 1. Prepared by Concord Environmental
Corporation for Health and Welfare Canada, Ottawa, Ontario.
Ferreri AM, Rocchi P, Capucci A, Prodi G (1983) Induction of
diphtheria toxin-resistant mutants in human cells by halogenated
compounds. Journal of cancer research and clinical oncology,
105:111-112.
Freiria-Gandara MJ, Lorenzo-Ferreira RA, Alvarez-Devesa A,
Bermejo F (1992) Occurrence of halogenated hydrocarbons in the
water supply of different cities of Galicia (Spain).
Environmental technology, 13:437-447.
Freitag D, Ballhorn L, Behechti A, Fischer K, Thumm W (1994)
Structural configuration and toxicity of chlorinated alkanes.
Chemosphere, 28(2):253-259.
Fujii T (1977) Direct aqueous injection gas chromatography-mass
spectrometry for analysis of organohalides in water at
concentrations below the parts per billion level. Journal of
chromatography, 139:297-302.
Giri AK, Que Hee SS (1988) In vitro sister chromatid exchange
induced by 1,2-dichloroethane on bone marrow cells of mice.
Environmental and molecular mutagenesis, 12:331-334.
Gocke E, Wild D, Eckhardt K, King MT (1983) Mutagenicity studies
with the mouse spot test. Mutation research, 117:201-212.
Guengerich FP, Crawford WM, Domoradzki JY, MacDonald TL, Watanabe
PG (1980) In vitro activation of 1,2-dichloroethane by
microsomal and cytosolic enzyme. Toxicology and applied
pharmacology, 55:303-317.
Guicherit R, Schulting FL (1985) The occurrence of organic
chemicals in the atmosphere of the Netherlands. The science of
the total environment, 43:193-219.
Hatch GG, Mamay PD, Ayer ML, Casto BC, Nesnow W (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo
cells by gaseous and volatile chlorinated methanes and ethanes.
Cancer research, 43:1945-1950.
Heikes DL (1987) Pesticide and industrial chemical residues -
purge and trap method for determination of volatile halocarbons
and carbon disulfide in table-ready foods. Journal of the
Association of Official Analytical Chemists, 70(2):215-226.
Heikes DL (1990) Environmental contaminants in table-ready foods
from the Total Diet Program of the Food and Drug Administration.
In: Food contamination from environmental sources. New York,
NY, John Wiley and Sons, pp. 31-57 (Advances in Environmental
Science and Technology Series No. 23).
Hellman B, Brandt I (1986) Effects of carcinogenic halogenated
aliphatic hydrocarbons on [3H]thymidine incorporation into
various organs of the mouse. A comparison between
1,2-dibromoethane and 1,2-dichloroethane. Mutation research,
163:193-199.
Hemminki K, Falck K, Vaino H (1980) Comparison of alkylation
rates and mutagenicity of directly acting industrial and
laboratory chemicals: epoxides, glycidyl ethers, methylating and
ethylating agents, halogenated hydrocarbons, hydrazine
derivatives, aldehydes, thiouram and dithiocarbamate derivatives.
Archives of toxicology, 46:277-285.
Heppel LA, Neal PA, Perrin TL, Endicott KM, Porterfield VT (1945)
The toxicology of 1,2-dichloroethane (ethylene dichloride). III.
Its acute toxicity and the effect of protective agents.
Journal of pharmacology and experimental therapeutics,
84:53-63.
Heppel LA, Neal PA, Perrin TL, Endicott KM, Porterfield VT (1946)
The toxicology of 1,2-dichloroethane (ethylene dichloride). V.
The effects of daily inhalations. Journal of industrial
hygiene and toxicology, 28(4):113-120.
Hinkel GK (1965) [Oral dichloroethane intoxication of children.]
Deutsche Gesundheitswesen, 20:1327-1331 (in German).
Hofmann HT, Birnsteil H, Jobst P (1971) Zur inhalations toxicitat
von 1,1- und 1,2-dichloroathan. [The inhalation toxicity of 1,1-
and 1,2-dichloroethane.] Archiv für Toxikologie, 27:248-265.
IARC (1979) Some halogenated hydrocarbons. Lyon, International
Agency for Research on Cancer (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Vol. 20).
Inskeep PB, Koga N, Cmarik JL, Guengerich FP (1986) Covalent
binding of 1,2-dihaloalkanes to DNA and stability of the major
DNA adduct, S-[2[(N7-guanyl)ethyl]glutathione. Cancer
research, 46:2839-2844.
IPCS (1993) International Chemical Safety Card -
1,2-Dichloroethane. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 0250).
IPCS (1994) Environmental health criteria for assessing health
risks of chemicals: derivation of guidance values for
health-based exposure limits. Geneva, World Health
Organization, International Programme on Chemical Safety.
IPCS (1995) 1,2-Dichloroethane. Geneva, World Health
Organization, International Programme on Chemical Safety
(Environmental Health Criteria No. 176).
Isacson P, Bean JA, Splinter R, Olson DB, Kohler J (1985)
Drinking water and cancer incidence in Iowa: III. Association of
cancer with indices of contamination. American journal of
epidemiology, 121(6):856-869.
Jakobson I, Wahlberg JE, Holmberg B, Johansson G (1982) Uptake
via the blood and elimination of 10 organic solvents following
epicutaneous exposure of anesthetized guinea pigs. Toxicology
and applied pharmacology, 63:181-187.
Jenssen D, Ramel C (1980) The micronucleus test as part of a
short-term mutagenicity test programme for the prediction of
carcinogenicity evaluated by 43 agents tested. Mutation
research, 75:191-202.
Kaiser KLE, Comba ME (1986) Volatile hydrocarbon contaminant
survey of the St. Clair River. Water pollution research
journal of Canada, 21(3):323-331.
Kaiser KLE, Comba ME, Huneault H (1983) Volatile hydrocarbon
contaminants in the Niagara River and in Lake Ontario.
Journal of Great Lakes research, 9(2):212-223.
Kanada T, Uyeta M (1978) Mutagenicity screening of organic
solvents in microbial systems. Mutation research, 54:215.
Kavlock R, Chernoff N, Carver B, Kopfler F (1979) Teratology
studies in mice exposed to municipal drinking water concentrates
during organogenesis. Food and cosmetics toxicology,
17:343-347.
King M-T, Beikirch H, Eckhardt K, Gocke E, Wild D (1979)
Mutagenicity studies with X-ray-contrast media, analgesics,
antipyretics, antirheumatics and some other pharmaceutical drugs
in bacterial, Drosophila and mammalian test systems.
Mutation research, 66:33-43.
Klaunig JE, Ruch RJ, Pereira MA (1986) Carcinogenicity of
chlorinated methane and ethane compounds administered in drinking
water to mice. Environmental health perspectives, 69:89-95.
Kliest J, Fast T, Boley JSM, van de Wiel H, Bloemen H (1989) The
relationship between soil contaminated with volatile organic
compounds and indoor air pollution. Environment international,
15:419-525.
Koga N, Inskeep PB, Harris TM, Guengerich FP (1986)
S-[2-(N7-guanyl)ethyl] glutathione, the major DNA
adduct formed from 1,2-dibromoethane. Biochemistry, 25:2192-2198.
Kramers PG, Mout HC, Bissumbhar B, Mulder CR (1991) Inhalation
exposure in Drosophila mutagenesis assays: experiments with
aliphatic halogenated hydrocarbons, with emphasis on the genetic
activity profile of 1,2-dichloroethane. Mutation research,
252:17-33.
Kronevi T, Wahlberg JE, Holmberg B (1981) Skin pathology
following epicutaneous exposure to seven organic solvents.
International journal of tissue reactions, 3:21-30.
Kuwabara T, Quevedo AR, Cogan DG (1968) An experimental study of
dichloroethane poisoning. Archives of ophthalmology,
79:321-330.
Lane RW, Riddle BL, Borzelleca JF (1982) Effects of
1,2-dichloroethane and 1,1,1-trichloroethane in drinking water on
reproduction and development in mice. Toxicology and applied
pharmacology, 63:409-421.
Larionov VG, Kokarovtseva MG (1976) Morphological constitution of
peripheral blood in intoxication with dichloroethane and its
metabolites. In: Actual problems of pesticide application in
different climatographic zones. Yerevan, Aiastan Publishers,
pp. 131-133.
Leblanc GA (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bulletin of environmental contamination
and toxicology, 24:684-691.
Letkiewicz F, Johnston P, Coleman J (1982) Occurrence of
1,2-dichloroethane in drinking water, food and air. Prepared by
JRB Associates, Washington DC, under Contract No. 68-01-6185 for
the US Environmental Protection Agency.
Lin ELC, Mattox JK, Pereira MA (1985) Glutathione plus
cytosol- and microsome-mediated binding of 1,2-dichloroethane to
polynucleotides. Toxicology and applied pharmacology,
78:428-435.
Lum KR, Kaiser KLE (1986) Organic and inorganic contaminants in
the St. Lawrence River: some preliminary results on their
distribution. Water pollution research journal of Canada,
21(4):592-603.
Maltoni C, Valgimigli L, Scarnato C (1980) Long-term carcinogenic
bioassays on ethylene dichloride administered by inhalation to
rats and mice. In: Ames BN, Infante P, Reitz R, eds. Ethylene
dichloride: a potential health risk? Cold Spring Harbor, NY,
Cold Spring Harbor Laboratory, pp. 3-33 (Banbury Report No. 5).
McCollister DD, Hollingsworth RL, Oyen F, Rowe VK (1956)
Comparative inhalation toxicity of fumigant mixtures. Individual
and joint effect of ethylene dichloride, carbon tetrachloride,
and ethylene dibromide. Archives of industrial health, 13:1-7.
Milman HA, Storey DL, Riccio ES, Sivak A, Tu AS, Williams GM,
Tong C, Tyson CA (1988) Rat liver foci and in vitro assays to
detect initiating and promoting effects of chlorinated ethanes
and ethylenes. Annals of the New York Academy of Sciences,
534:521-530.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, Shirasu Y
(1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation research, 116:185-216.
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, White
KL Jr (1982) In vivo assessment of immunotoxicity.
Environmental health perspectives, 43:41-52.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity. Bethesda, MD, US Department of Health,
Education and Welfare, Public Health Services, National
Institutes of Health, National Cancer Institute (HEW (NIH)
Publication No. 78-1305).
Nestmann ER, Lee EGH, Matula TI, Douglas GR, Mueller JC (1980)
Mutagenicity of constituents identified in pulp mill effluents
using Salmonella/mammalian-microsome assay. Mutation
research, 79:203-212.
NIOSH (1994a) Registry of toxic effects of chemical
substances. Cincinnati, OH, National Institute for Occupational
Safety and Health (last revision May 1994).
NIOSH (1994b) NIOSH pocket guide to chemical hazards. US
Department of Health and Human Services, Public Health Service,
Center for Disease Control and Prevention, National Institute for
Occupational Safety and Health, June.
Nouchi T, Miura H, Kanayama M, Mizuguchi O, Takano T (1984) Fatal
intoxication by 1,2-dichloroethane - A case report.
International archives of occupational and environmental
health, 54:111-113.
NTP (1991) Toxicity studies of 1,2-dichloroethane (ethylene
dichloride) in F344/N rats, Sprague Dawley rats,
Osborne-Mendel rats, and B6C3F1 mice (drinking water and
gavage studies). Research Triangle Park, NC, National
Institutes of Health, National Toxicology Program (NIH
Publication No. 91-3123).
Nylander P-O, Olofsson H, Rasmuson B, Svahlin H (1978) Mutagenic
effects of petrol in Drosophila melanogaster. I. Effects of
benzene and 1,2-dichloroethane. Mutation research, 57:163-167.
Otson R (1987) Purgeable organics in Great Lakes raw and treated
water. International journal of environmental analytical
chemistry, 31(1):41-53.
Pearson CR, McConnell G (1975) Chlorinated C1 and C2
hydrocarbons in the marine environment. Proceedings of the
Royal Society of London B, 189:305-332.
Perocco P, Prodi G (1981) DNA damage by haloalkanes in human
lymphocytes cultured in vitro. Cancer letters, 13:213-218.
Principe P, Dogliotti E, Bignami M, Crebelli R, Falcone E,
Fabrizi M, Conti G, Comba P (1981) Mutagenicity of chemicals of
industrial and agricultural relevance in Salmonella,
Streptomyces, and Aspergillus. Journal of the science of
food and agriculture, 32:826-832.
Prodi G, Arfelini G, Colacci A, Grilli S, Mazzulo M (1986)
Interaction of halocompounds with nucleic acids. Toxicologic
pathology, 14:438-444.
Rannug U (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichloroethane. Mutation research, 76:269-295.
Rannug U, Beije B (1979) The mutagenic effect of
1,2-dichloro-ethane on Salmonella typhimurium. II. Activation
by the isolated perfused rat liver. Chemico-biological
interactions, 24:265-285.
Rannug U, Sundvall A, Ramel C (1978) The mutagenic effect
of 1,2-dichloroethane on Salmonella typhimurium. I. Activation
through conjugation with glutathione in vitro.
Chemico-biological interactions, 20:1-16.
Rao KS, Murray JS, Deacon MM, John JA, Calhoun LL, Young JT
(1980) Teratogenicity and reproduction studies in animals
inhaling ethylene dichloride. In: Ames BN, Infante P, Reitz R,
eds. Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 149-166 (Banbury
Report No. 5).
Reitz RH, Fox TR, Ramsey JC, Quast JF, Langvardt PW, Watanabe PG
(1982) Pharmacokinetics and macro-molecular interactions of
ethylene dichloride in rats after inhalation or gavage.
Toxicology and applied pharmacology, 62:190-204.
Richter JE, Peterson SF, Kleiner CF (1983) Acute and chronic
toxicity of some chlorinated benzenes, chlorinated ethanes, and
tetrachloroethylene to Daphnia magna. Archives of
environmental contamination and toxicology, 12:679-684.
Roldan-Arjona T, Garcia-Pedrajas MD, Luque-Romero RL, Hera C,
Pueyo C (1991) An association between mutagenicity of the Ara
test of Salmonella typhimurium and carcinogenicity in rodents
for 16 halogenated aliphatic hydrocarbons. Mutagenesis,
6:199-205.
Romert L, Magnusson J, Ramel C (1990) The importance of
glutathione and glutathione transferase for somatic mutations in
Drosophila melanogaster induced in vivo by
1,2-dichloroethane. Carcinogenesis, 11:1399-1402.
Schultz K, Ghosh L, Banerjee S (1992) Neoplastic expression in
murine cells induced by halogenated hydrocarbons. In vitro
cellular and developmental biology, 28A:267-272.
Sherwood RL, O'Shea PT, Thomas W, Ratajczak HV, Aranyi C, Graham
JA (1987) Effects of inhalation of ethylene dichloride on
pulmonary defences of mice and rats. Toxicology and applied
pharmacology, 91:491-496.
Shmuter LM (1977) [Effects of chronic exposure to low
concentrations of chlorinated hydrocarbons of the ethane series on
specific and non-specific reactivity of animals in vivo.]
Gigiena Truda i Professional'nye Zabolevaniya, 8:38-42 (in
Russian).
Simmon VF (1980) Review of non-bacterial tests of the genotoxic
activity of ethylene dichloride. In: Ames BN, Infante P, Reitz R,
eds. Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 97-103 (Banbury
Report No. 5).
Simula TP, Glancey MG, Wolf CR (1993) Human glutathione
S-transferase-expressing Salmonella typhimurium tester
strains to study the activation/detoxification of mutagenic
compounds: studies with halogenated compounds, aromatic amines
and aflatoxin B1. Carcinogenesis, 14:1371-1376.
Singh HB, Salas LJ, Smith AJ, Shigeishi H (1980) Atmospheric
measurements of selected toxic organic chemicals. Research
Triangle Park, NC, US Environmental Protection Agency,
Environmental Sciences Research Laboratory (EPA-600/3-80-072;
PB80-198989).
Singh HB, Salas LJ, Smith AJ, Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban
environments. Atmospheric environment, 15:601-612.
Singh HB, Salas LF, Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environmental science and technology, 16:872-880.
Smyth HF Jr (1969) Acute toxicity of ethyl compounds. In: Spector
WC, ed. Handbook of toxicology. Vol. 1. Philadelphia, PA, W.B.
Saunders Company.
Spence JW, Hanst PL (1978) Oxidation of chlorinated ethanes.
Journal of the Air Pollution Control Association,
28(3):250-253.
Spencer HC, Rowe VK, Adams EM, McCollister DD, Irish DD (1951)
Vapor toxicity of ethylene dichloride determined by experiments
on laboratory animals. American Medical Association archives
of industrial hygiene and occupational medicine, 4:482-493.
Spreafico F, Zuccato E, Marcucci F, Sironi M, Paglialunga S,
Madonna M, Mussini E (1980) Pharmacokinetics of ethylene
dichloride in rats treated by different routes and its long-term
inhalatory toxicity. In: Ames BN, Infante P, Reitz R, eds.
Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 107-133 (Banbury
Report No. 5).
Storer RD, Conolly RB (1983) Comparative in vivo genotoxicity
and acute hepatotoxicity of three 1,2-dihaloethanes.
Carcinogenesis, 4:1491-1494.
Storer RD, Conolly RB (1985) An investigation of the role of
microsomal oxidative metabolism in the in vivo genotoxicity of
1,2-dichloroethane. Toxicology and applied pharmacology,
77:36-46.
Storer RD, Jackson NM, Conolly RB (1984) In vivo genotoxicity
and acute hepatotoxicity of 1,2-dichloroethane in mice:
comparison of oral, intraperitoneal, and inhalation routes of
exposure. Cancer research, 44:4267-4271.
Story DL, Meierhenry EF, Tyson CA, Milman HA (1986) Differences
in rat liver enzyme-positive foci produced by chlorinated
aliphatics and phenobarbital. Toxicology and industrial
health, 2:351-362.
Strobel K, Grummt T (1987) Aliphatic and aromatic halocarbons as
potential mutagens in drinking water. III. Halogenated ethanes
and ethenes. Toxicology and environmental chemistry,
15:101-128.
Sundheimer DW, White RD, Brendel K, Sipes IC (1982) The
bioactivation of 1,2-dibromoethane in rat hepatocytes: covalent
binding to nucleic acids. Carcinogenesis, 3:1129-1133.
Suveev IM, Babichenko ME (1969) [On the clinic and cure of acute
intoxication with dichloroethane vapours.] Gigiena Truda i
Professional'nye Zabolevaniya, 13:50-51 (in Russian).
Tan E-L, Hsie AW (1981) Mutagenicity and cytotoxicity of
haloethanes as studied in the CHO/HGPRT system. Mutation
research, 90:183-191.
Taningher M, Parodi S, Grilli S, Colacci A, Mazzullo M, Bordone
R, Santi L (1991) Lack of correlation between alkaline DNA
fragmentation and DNA covalent binding induced by
polychloroethanes after in vivo administration. Problems
related to the assessment of a carcinogenic hazard. Cancer
detection and prevention, 15:35-39.
Theiss JC, Stoner GD, Shimkin MB, Weisberger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer
research, 37:2717-2720.
Tu AS, Murray TA, Hatch KM, Sivak A, Milman HA (1985) In vitro
transformation of BALB/c-3T3 cells by chlorinated ethanes and
ethylenes. Cancer letters, 28:85-92.
US EPA (1985) Health assessment document for
1,2-dichloroethane (ethylene dichloride). Final report.
Washington, DC, US Environmental Protection Agency
(EPA/600/8-84/006F; NTIS PB86-122702).
US EPA (1992) Indoor air quality data base for organic
compounds. Research Triangle Park, NC, US Environmental
Protection Agency (EPA-600-R-92-025; NTIS PB92-158468).
Valencia R, Abrahamson S, Lee WR, von Halle ES, Woodruff RC,
Wurgler FE, Zimmering S (1984) Chromosome mutations for
mutagenesis in Drosophila melanogaster: a report of the US
Environmental Protection Agency Gene-Tox Program. Mutation
research, 134:61-88.
van Bladeren PJ, Breimer DD, Rotteveel-Smijs RMT, de Knijff P,
Mohn GV, van Meeteren-Walchli B, Buijs W, van der Gen A (1981)
The relation between the structure of vicinal dihalogen compounds
and their mutagenic activation via conjugation to glutathione.
Carcinogenesis, 2:499-505.
van Duuren BL, Goldschmidt BM, Loewengart G, Smith A, Mechionne
S, Seldman I, Roth D (1979) Carcinogenicity of halogenated
olefinic and aliphatic hydrocarbons in mice. Journal of the
National Cancer Institute, 63:1433-1439.
van Esch GJ, Kroes R, van Logten MJ, den Tonkelaar EM (1977)
Ninety-day toxicity study with 1,2-dichloroethane (DCE) in
rats. Bilthoven, National Institute of Public Health and
Environmental Hygiene (Report 195/77 Al. Tox.).
Vozovaya M (1977) [The effect of dichloroethane on the sexual
cycle and embryogenesis of experimental animals.] Akusherstvo
i Ginekologiya (Moscow), 2:57-59 (in Russian).
WHO (1971) Evaluation of food additives: specifications for
the identity and purity of food additives and their
toxicological evaluation: some extraction solvents and
certain other substances; a review of the technological
efficacy of some antimicrobial agents. Fourteenth report of
the Expert Committee. Geneva, World Health Organization (WHO
Technical Report Series No. 462).
WHO (1980) Evaluation of certain food additives.
Twenty-third report of the Joint FAO/WHO Expert Committee
on Food Additives. Geneva, World Health Organization (WHO
Technical Report Series No. 648 and corrigenda).
WHO (1992) Evaluation of certain food additives and
naturally occurring toxicants. Thirty-ninth report of the
Joint FAO/WHO Expert Committee on Food Additives. Geneva, World
Health Organization (WHO Technical Report Series No. 828).
WHO (1993) Guidelines for drinking-water quality, 2nd ed.
Vol. 1. Recommendations. Geneva, World Health Organization.
Williams GM, Mori H, McQueen CA (1989) Structure-activity
relationships in the rat hepatocyte DNA-repair test for 300
chemicals. Mutation research, 221:263-286.
Zamora PO, Benson JM, Li AP, Brooks AL (1983) Evaluation of an
exposure system using cells grown on collagen gels for detecting
highly volatile mutagens in the CHO/HGPRT mutation assay.
Environmental mutagenesis, 5:795-801.
APPENDIX 1 - SOURCE DOCUMENTS
International Programme on Chemical Safety - Environmental Health
Criteria Monograph No. 176 (1995)
Copies of the Environmental Health Criteria document on
1,2-dichloroethane, prepared by the International Programme on
Chemical Safety, as well as the Health and Safety Guide (1991)
and the International Chemical Safety Card (1993), may be
obtained from:
International Programme on Chemical Safety
World Health Organization
Geneva, Switzerland
The first draft of the monograph, prepared by Ms K. Hughes
of the Environmental Health Directorate, Health Canada, was
circulated to IPCS Contact Points (approximately 150 government,
industrial, academic, and independent organizations and
individuals) for comment in June 1994. The second draft, revised
on the basis of the comments received, was also prepared by Ms K.
Hughes. Dr E. Smith and Dr P.G. Jenkins, both members of the
IPCS Central Unit, were responsible for the scientific content
and technical editing, respectively.
The monograph on 1,2-dichloroethane was finalized by the
Core Assessment Group (CAG) of the Joint Meeting on Pesticides
(JMP), which met in Geneva from 25 October to 3 November 1994.
The Core Assessment Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health
and the environment from exposure to 1,2-dichloroethane.
Participants at the Core Assessment Group meeting were:
Dr T. Bailey, Ecological Effects Branch, Environmental Fate
and Effects Division, US Environmental Protection Agency,
Washington, DC, USA
Dr A.L. Black, Department of Human Services and Health,
Canberra, ACT, Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairperson)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution,
London, United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, Office of Pesticide Programs, US
Environmental Protection Agency, Washington, DC, USA
Dr R. Hailey, National Institute of Environmental Health
Sciences, National Institutes of Health, Public Health
Service, Department of Health and Human Services, Research
Triangle Park, NC, USA
Ms K. Hughes, Priority Substances Section, Environmental
Health Directorate, Health Canada, Ottawa, Ontario, Canada
(EHC Joint Rapporteur)
Dr D. Kanungo, Division of Medical Toxicology, Central
Insecticides Laboratory, Government of India, Ministry of
Agriculture & Cooperation, Directorate of Plant Protection,
Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group
Ltd, Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Fontwell, Arundel,
West Sussex, United Kingdom
Professor M. Lotti, Institute of Occupational Medicine,
University of Padua, Padua, Italy (Chairperson)
Dr D.R. Mattison, University of Pittsburgh, Graduate School
of Public Health, Pittsburgh, PA, USA
Dr J. Sekizawa, Division of Information on Chemical Safety,
National Institute of Health Sciences, Setagaya-ku, Tokyo,
Japan
Dr P. Sinhaseni, Department of Pharmacology, Chulalongkorn
University, Bangkok, Thailand
Dr S.A. Soliman, Pesticide Chemicals & Toxicology, King Saud
University, Bureidah, Saudi Arabia
Dr M. Tasheva, Department of Toxicology, National Center of
Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Department of Toxicology, Wageningen
Agricultural Unviersity, Wageningen, The Netherlands
Dr M.I. Willems, Department of Occupational Toxicology, TNO
Nutrition and Food Research Institute, AJ Zeist, The
Netherlands
Procedures for the preparation of an Environmental Health
Criteria document
The order of procedures that result in the publication of an
EHC monograph is shown in the flow chart. A designated staff
member of IPCS, responsible for the scientific quality of the
document, serves as Responsible Officer (RO). The IPCS Editor is
responsible for layout and language. The first draft, prepared
by consultants or, more usually, staff from an IPCS Participating
Institution, is based initially on data provided from the
International Register of Potentially Toxic Chemicals and
reference databases such as Medline and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its
scientific quality and objectivity. Once the RO finds the
document acceptable as a first draft, it is distributed, in its
unedited form, to well over 150 EHC Contact Points throughout the
world who are asked to comment on its completeness and accuracy
and, where necessary, provide additional material. The Contact
Points, usually designated by governments, may be Participating
Institutions, IPCS Focal Points, or individual scientists known
for their particular expertise. Generally, some four months are
allowed before the comments are considered by the RO and
author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not
as representatives of any organization, government, or industry.
Their function is to evaluate the accuracy, significance, and
relevance of the information in the document and to assess the
health and environmental risks from exposure to the chemical. A
summary and recommendations for further research and improved
safety aspects are also required. The composition of the Task
Group is dictated by the range of expertise required for the
subject of the meeting and by the need for a balanced
geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international
associations may be invited to join the Task Group as observers.
While observers may provide a valuable contribution to the
process, they can speak only at the invitation of the
Chairperson. Observers do not participate in the final
evaluation of the chemical; this is the sole responsibility of
the Task Group members. When the Task Group considers it to be
appropriate, it may meet in camera.
All individuals who as authors, consultants, or advisers
participate in the preparation of the EHC monograph must, in
addition to serving in their personal capacity as scientists,
inform the RO if at any time a conflict of interest, whether
actual or potential, could be perceived in their work. They are
required to sign a conflict of interest statement. Such a
procedure ensures the transparency and probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of
the document, it then goes for language editing, reference
checking, and preparation of camera-ready copy. After approval
by the Director, IPCS, the monograph is submitted to the WHO
Office of Publications for printing. At this time, a copy of the
final draft is sent to the Chairperson and Rapporteur of the Task
Group to check for any errors.
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Highly flammable. Gives off No open flames, NO sparks, Powder, water spray, foam,
irritating or toxic fumes (or and NO smoking. carbon dioxide.
gases) in a fire).
EXPLOSION Vapour/air mixtures are Closed system, ventilation, In case of fire: keep drums,
explosive. explosion-proof electrical etc., cool by spraying with
equipment and lighting. water.
Prevent build-up of
electrostatic charges (e.g.,
by grounding) (see Notes).
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTONS FIRE FIGHTING
EXPOSURE
EXPOSURE AVOID ALL CONTACT! IN ALL CASES CONSULT A
DOCTOR!
* INHALATION Abdominal pain. Cough. Ventilation, local exhaust, Fresh air, rest. Half-upright
Dizziness. Drowsiness. or breathing protection. position. Artificial
Headache. Nausea. Sore respiration if indicated.
throat. Unconsciousness. Refer for medical attention.
Vomiting. Symptoms may be
delayed (see Notes).
* SKIN Redness. Protective gloves. Remove contaminated clothes.
Rinse and then wash skin with
water and soap. Refer for
medical attention.
* EYES Redness. Pain. Blurred Safety goggles, face shield, First rinse with plenty of
vision. or eye protection in water for several minutes
combination with breathing (remove contact lenses if
protection. easily possible), then take
to a doctor.
* INGESTION Abdominal cramps. Diarrhoea Do no eat, drink, or smoke Give nothing to drink. Refer
(further see Inhalation). during work. Wash hands for medical attention.
before eating.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Evacuate danger area! Collect leaking Fireproof. Separated from strong Unbreakable packaging; put breakable
and spilled liquid in sealable oxidants, food and feedstuffs and packaging into closed unbreakable
containers as far as possible. Absorb other incompatible substances (see container. Do not transport with food
remaining liquid in sand or inert Chemical Dangers). Cool. Dry. and feedstuffs.
absorbent and remove to safe place. Do F symbol
NOT wash away into sewer (extra Y symbol
personal protection: self-contained R: 45-11-22-36/37/38
breathing apparatus). S: 53-45
Note: E
UN Hazard Class: 3
UN Subsidiary Risks: 6.1
UN Packing Group: 11
Marine Pollutant.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: INHALATION RISK:
COLOURLESS, VISCOUS LIQUID, WITH A harmful contamination of the air can be
CHARACTERISTIC ODOUR, TURNS DARK ON EXPOSURE reached very quickly on evaporation of this
TO AIR, MOISTURE AND LIGHT substance at 20°C.
PHYSICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The vapour is heavier than air and may travel The vapour irritates the eyes, the skin and
along the ground; distant ignition possible. the respiratory tract. Inhalation of the
As a result of flow, agitation, etc., vapour may cause lung oedema (see Notes). The
electrostatic charges can be generated. substance may cause effects on the central
nervous system, kidneys, liver, resulting in
CHEMICAL DANGERS: impaired functions.
The substance decomposes on heating and on EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
burning producing toxic and corrosive fumes
including hydrogen chloride (ICSC #0163) and Repeated or prolonged contact with skin may
phosgene (ICSC #0007). Reacts violently with cause dermatitis. This substance is probably
aluminium, alkali metals, alkali amides, carcinogenic to humans.
ammonia bases, strong oxidants. Attacks many
metals in presence of water. Attacks plastic.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV: 10 ppm; 40 mg/m3 (as TWA)
(ACGIH 1994-1995).
ROUTES OF EXPOSURE:
The substance can be absorbed into the body by
inhalation of its vapour, through the skin and
by ingestion.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 83.5°C Flash point: 13°C c.c.
PROPERTIES Melting point: -35.7°C Auto-ignition temperature: 413°C
Relative density (water = 1): 1.235 Explosive limits, vol% in air: 6.2-16
Solubility in water, g/100 ml: 0.87 Octanol/water partition coefficient
Vapour pressure, kPa at 20°C: 8.7 as logPow: 1.48
Relative vapour density (air = 1): 3.42
Relative density of the vapour/
air-mixture at 20°C (air = 1): 1.2
ENVIRONMENTAL
DATA
NOTES
Depending on the degree of exposure, periodic medical examination is indicated.
The symptoms of lung oedema often do not become manifest until a few hours have passed and they are aggravated by physical effort.
Rest and medical observation are tehrefore essential.
Immediate administration of an appropriate spray, by a doctor or a person authorized by him/her, should be considered.
EXPLOSION/PREVENTION: Do NOT use compressed air for filling, discharging, or handling.
ICSC: 0250 1.1 Transport Emergency Card: TEC (R)-605
NFPA Code: H 2; F 3; R 0
REFERENCES
Alumot E, Nachtomi E, Mandel E, Holstein P (1976) Tolerance and
acceptable daily intake of chlorinated fumigants in the rat diet.
Food and cosmetics toxicology, 14:105-110.
Andriukin AA (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klinicheskaya Meditsina (Moscow),
57:43-47 (in Russian).
Arfellini G, Bartoli S, Colacci A, Mazzullo M, Galli MC, Prodi G,
Grilli S (1984) In vivo and in vitro binding of
1,2-dibromoethane and 1,2-dichloroethane to macromolecules in rat
and mouse organs. Journal of cancer research and clinical
oncology, 108:204-213.
Armstrong MJ, Galloway SM (1993) Micronuclei induced in
peripheral blood of Eµ-PIM-1 transgenic mice by chronic oral
treatment with 2-acetylaminofluorene or benzene but not with
diethylnitrosamine or 1,2-dichloroethane. Mutation research,
302:61-70.
ATSDR (1992) Toxicological profile for 1,2-dichloroethane
(draft). Atlanta, GA, US Department of Health and Human Services,
Public Health Service, Agency for Toxic Substances and Disease
Registry.
Austin SG, Schnatter AR (1983) A case-control study of chemistry
exposures and brain tumors in petrochemical workers. Journal
of occupational medicine, 25(4):313-320.
Baertsch A, Lutz WK, Schlatter C (1991) Effect of inhalation
exposure regimen on DNA binding potency of 1,2-dichloroethane in
the rat. Archives of toxicology, 65:169-176.
Ballering LAP, Nivard MJM, Vogel EW (1993) Characterization of
the genotoxic action of three structurally related
1,2-dihaloalkanes in Drosophila melanogaster. Mutation
research, 285:209-217.
Banerjee S (1988) DNA damage in rodent liver by
1,2-dichloroethane, a hepatocarcinogen. Cancer biochemistry
and biophysics, 10:165-173.
Banerjee S, van Duuren BL (1979) Binding of carcinogenic
halogenated hydrocarbons to cell macromolecules. Journal of
the National Cancer Institute, 63:707-711.
Banerjee S, van Duuren BL, Oruambo FI (1980) Microsome-mediated
covalent binding of 1,2-dichloroethane to lung microsomal protein
and salmon sperm DNA. Cancer research, 40:2170-2173.
Barber RD, Donish WH, Mueller KR (1981) A procedure for the
quantitative measurement of the mutagenicity of volatile liquids
in the Ames Salmonella microsome assay. Mutation research,
90:31-48.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizzari ED, Ray M, Smith D, Tomer KB, Wagner R, Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - A
multimedia environmental study. Biomedical and environmental
mass spectrometry, 7:139-147.
Barsoum GS, Saad K (1934) Relative toxicity of certain chlorine
derivatives of the aliphatic series. Quarterly journal of
pharmacy and pharmacology, 7: 205-214.
Benson LO, Teta MJ (1993) Mortality due to pancreatic and
lymphopoietic cancers in chlorohydrin production workers.
British journal of industrial medicine, 50:710-716.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, Bruser
DM (1982) The aquatic toxicity of organic compounds to
embryo-larval stages of fish and amphibians. Lexington, KY,
University of Kentucky (Research Report No. 133).
Blum DJW, Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons
and correlations. Research journal of the Water Pollution
Control Federation, 63(3):198-207.
Bonnet P, Francin J-M, Grakiski D, Raoult G, Zissu D (1980)
Détermination de la concentration léthale50 des principaux
hydrocarbures aliphatiques chlorés chez le rat. Archives des
maladies professionnelles de medecine du travail et de
securité sociale (Paris), 41(6-7):317-321.
Brem H, Stein AB, Rosenkranz HS (1974) The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer research,
34:2576-2579.
Bringmann G, Kühn R (1978) Testing of substances for their
toxicity threshold: model organisms Microcystis (Diplocystis)
aeruginosa and Scenedesmus quadricauda. Mitteilungen
Internationale Vereinigung für Theoretische und Angewandte
Limnologie, 21:275-284.
Bringmann G, Kühn R (1980) Comparison of the toxicity thresholds
of water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water research, 14:231-241.
Buijs W, van der Gen A, Mohn GR, Breimer DD (1984) The direct
mutagenic activity of alpha, omega-dihalogenoalkanes in
Salmonella typhimurium. Mutation research, 141:11-14.
Callaghan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR,
Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR,
Gould C (1979) Water-related fate of 129 priority pollutants.
Vol. II. Washington, DC, US Environmental Protection Agency
(EPA 40/4-79-029b).
Cheever KL, Cholakis JM, El-Hawari AM, Kovatch RM, Weisburger EK
(1990) Ethylene dichloride: the influence of disulfiram or
ethanol on oncogenicity, metabolism and DNA covalent binding in
rats. Fundamental and applied toxicology, 14:243-261.
Cheh AM, Hooper AB, Skochdopole J, Henke CA, McKinnell RG (1980)
A comparison of the ability of frog and rat S-9 to activate
promutagens in the Ames test. Environmental and molecular
mutagenesis, 2:487-508.
Chemical Marketing Reporter (1992) Chemical profile: ethylene
dichloride. Chemical Marketing Reporter magazine, 241(19):42.
Clark AI, McIntyre AE, Lester JN, Perry R (1984a) Ambient air
measurement of aromatic and halogenated hydrocarbons at urban,
rural, and motorway locations. The science of the total
environment, 39:265-279.
Clark AI, McIntyre AE, Perry R, Lester JN (1984b) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural, and motorway locations.
Environmental pollution series B, 7:141-158.
Comba ME, Kaiser KLE (1985) Volatile halocarbons in the Detroit
River and their relationship with contaminant sources. Journal
of Great Lakes research, 11(3):404-418.
CPI (1991) CPI product profiles: ethylene dichloride. Don
Mills, Ontario, Canadian Process Industries, Corpus Information
Services.
Crebelli R, Carere A (1988) Genotoxic activity of halogenated
aliphatic hydrocarbons in Aspergillus nidulans. Journal of
occupational toxicology, 8:437-442.
Crebelli R, Conti G, Conti L, Carere A (1984) Induction of
somatic segregation by halogenated aliphatic hydrocarbons in
Aspergillus nidulans. Mutation research, 138:33-38.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, Carere A
(1988) Induction of chromosome malsegregation by halogenated
organic solvents in Aspergillus nidulans: unspecific or
specific mechanism? Mutation research, 201:401-411.
Crespi CL, Seixas GM, Turner TR, Ryan CG, Penman BW (1985)
Mutagenicity of 1,2-dichloroethane and 1,2-dibromoethane in two
human lymphoblastoid cell lines. Mutation research,
142:133-140.
Daft JL (1988) Rapid determination of fumigant and industrial
chemical residues in food. Journal of the Association of
Official Analytical Chemists, 71(4):748-760.
Dilling WL, Tefertiller NB, Kalos GJ (1975) Evaporation rates and
reactivities of methylene chloride, chloroform,
1,1,1-trichloroethane, trichloroethylene, tetrachloroethylene,
and other chlorinated compounds in dilute aqueous solutions.
Environmental science and technology, 9(9):833-838.
Dorndorf W, Kresse M, Christian W, Katritzki KG (1975)
[Dichloroethane poisoning with myoclonic syndrome, epileptic
attacks, and irreversible cerebral effects.] Archiv für
Psychiatrie und Nervenkrankheiten, 220:373-379 (in German).
Duprat P, Delsaut L, Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. European journal of toxicology,
9:171-177.
Ecobichon DJ, Allen MC (1990) New Brunswick - Water Quality
Surveillance Program, New Brunswick public water supplies,
data summary report 1990. New Brunswick Department of Health
and Community Services.
Environment Agency Japan (1993) Chemicals in the environment -
1993. Tokyo.
Enviro-Test Laboratories (1991) Cayley background study:
analysis of food products for target organic and inorganic
parameters. Edmonton (Report No. 91-E1208).
Enviro-Test Laboratories (1992) Windsor area background study:
analysis of food products for target organic and inorganic
parameters. Edmonton (Report No. E1052).
Fellin P, Barnett SE, Tran QA (1992) Results of a national
pilot survey of airborne volatile organic compounds in
Canadian residences. Vol. 1. Prepared by Concord Environmental
Corporation for Health and Welfare Canada, Ottawa, Ontario.
Ferreri AM, Rocchi P, Capucci A, Prodi G (1983) Induction of
diphtheria toxin-resistant mutants in human cells by halogenated
compounds. Journal of cancer research and clinical oncology,
105:111-112.
Freiria-Gandara MJ, Lorenzo-Ferreira RA, Alvarez-Devesa A,
Bermejo F (1992) Occurrence of halogenated hydrocarbons in the
water supply of different cities of Galicia (Spain).
Environmental technology, 13:437-447.
Freitag D, Ballhorn L, Behechti A, Fischer K, Thumm W (1994)
Structural configuration and toxicity of chlorinated alkanes.
Chemosphere, 28(2):253-259.
Fujii T (1977) Direct aqueous injection gas chromatography-mass
spectrometry for analysis of organohalides in water at
concentrations below the parts per billion level. Journal of
chromatography, 139:297-302.
Giri AK, Que Hee SS (1988) In vitro sister chromatid exchange
induced by 1,2-dichloroethane on bone marrow cells of mice.
Environmental and molecular mutagenesis, 12:331-334.
Gocke E, Wild D, Eckhardt K, King MT (1983) Mutagenicity studies
with the mouse spot test. Mutation research, 117:201-212.
Guengerich FP, Crawford WM, Domoradzki JY, MacDonald TL, Watanabe
PG (1980) In vitro activation of 1,2-dichloroethane by
microsomal and cytosolic enzyme. Toxicology and applied
pharmacology, 55:303-317.
Guicherit R, Schulting FL (1985) The occurrence of organic
chemicals in the atmosphere of the Netherlands. The science of
the total environment, 43:193-219.
Hatch GG, Mamay PD, Ayer ML, Casto BC, Nesnow W (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo
cells by gaseous and volatile chlorinated methanes and ethanes.
Cancer research, 43:1945-1950.
Heikes DL (1987) Pesticide and industrial chemical residues -
purge and trap method for determination of volatile halocarbons
and carbon disulfide in table-ready foods. Journal of the
Association of Official Analytical Chemists, 70(2):215-226.
Heikes DL (1990) Environmental contaminants in table-ready foods
from the Total Diet Program of the Food and Drug Administration.
In: Food contamination from environmental sources. New York,
NY, John Wiley and Sons, pp. 31-57 (Advances in Environmental
Science and Technology Series No. 23).
Hellman B, Brandt I (1986) Effects of carcinogenic halogenated
aliphatic hydrocarbons on [3H]thymidine incorporation into
various organs of the mouse. A comparison between
1,2-dibromoethane and 1,2-dichloroethane. Mutation research,
163:193-199.
Hemminki K, Falck K, Vaino H (1980) Comparison of alkylation
rates and mutagenicity of directly acting industrial and
laboratory chemicals: epoxides, glycidyl ethers, methylating and
ethylating agents, halogenated hydrocarbons, hydrazine
derivatives, aldehydes, thiouram and dithiocarbamate derivatives.
Archives of toxicology, 46:277-285.
Heppel LA, Neal PA, Perrin TL, Endicott KM, Porterfield VT (1945)
The toxicology of 1,2-dichloroethane (ethylene dichloride). III.
Its acute toxicity and the effect of protective agents.
Journal of pharmacology and experimental therapeutics,
84:53-63.
Heppel LA, Neal PA, Perrin TL, Endicott KM, Porterfield VT (1946)
The toxicology of 1,2-dichloroethane (ethylene dichloride). V.
The effects of daily inhalations. Journal of industrial
hygiene and toxicology, 28(4):113-120.
Hinkel GK (1965) [Oral dichloroethane intoxication of children.]
Deutsche Gesundheitswesen, 20:1327-1331 (in German).
Hofmann HT, Birnsteil H, Jobst P (1971) Zur inhalations toxicitat
von 1,1- und 1,2-dichloroathan. [The inhalation toxicity of 1,1-
and 1,2-dichloroethane.] Archiv für Toxikologie, 27:248-265.
IARC (1979) Some halogenated hydrocarbons. Lyon, International
Agency for Research on Cancer (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Vol. 20).
Inskeep PB, Koga N, Cmarik JL, Guengerich FP (1986) Covalent
binding of 1,2-dihaloalkanes to DNA and stability of the major
DNA adduct, S-[2[(N7-guanyl)ethyl]glutathione. Cancer
research, 46:2839-2844.
IPCS (1993) International Chemical Safety Card -
1,2-Dichloroethane. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 0250).
IPCS (1994) Environmental health criteria for assessing health
risks of chemicals: derivation of guidance values for
health-based exposure limits. Geneva, World Health
Organization, International Programme on Chemical Safety.
IPCS (1995) 1,2-Dichloroethane. Geneva, World Health
Organization, International Programme on Chemical Safety
(Environmental Health Criteria No. 176).
Isacson P, Bean JA, Splinter R, Olson DB, Kohler J (1985)
Drinking water and cancer incidence in Iowa: III. Association of
cancer with indices of contamination. American journal of
epidemiology, 121(6):856-869.
Jakobson I, Wahlberg JE, Holmberg B, Johansson G (1982) Uptake
via the blood and elimination of 10 organic solvents following
epicutaneous exposure of anesthetized guinea pigs. Toxicology
and applied pharmacology, 63:181-187.
Jenssen D, Ramel C (1980) The micronucleus test as part of a
short-term mutagenicity test programme for the prediction of
carcinogenicity evaluated by 43 agents tested. Mutation
research, 75:191-202.
Kaiser KLE, Comba ME (1986) Volatile hydrocarbon contaminant
survey of the St. Clair River. Water pollution research
journal of Canada, 21(3):323-331.
Kaiser KLE, Comba ME, Huneault H (1983) Volatile hydrocarbon
contaminants in the Niagara River and in Lake Ontario.
Journal of Great Lakes research, 9(2):212-223.
Kanada T, Uyeta M (1978) Mutagenicity screening of organic
solvents in microbial systems. Mutation research, 54:215.
Kavlock R, Chernoff N, Carver B, Kopfler F (1979) Teratology
studies in mice exposed to municipal drinking water concentrates
during organogenesis. Food and cosmetics toxicology,
17:343-347.
King M-T, Beikirch H, Eckhardt K, Gocke E, Wild D (1979)
Mutagenicity studies with X-ray-contrast media, analgesics,
antipyretics, antirheumatics and some other pharmaceutical drugs
in bacterial, Drosophila and mammalian test systems.
Mutation research, 66:33-43.
Klaunig JE, Ruch RJ, Pereira MA (1986) Carcinogenicity of
chlorinated methane and ethane compounds administered in drinking
water to mice. Environmental health perspectives, 69:89-95.
Kliest J, Fast T, Boley JSM, van de Wiel H, Bloemen H (1989) The
relationship between soil contaminated with volatile organic
compounds and indoor air pollution. Environment international,
15:419-525.
Koga N, Inskeep PB, Harris TM, Guengerich FP (1986)
S-[2-(N7-guanyl)ethyl] glutathione, the major DNA
adduct formed from 1,2-dibromoethane. Biochemistry, 25:2192-2198.
Kramers PG, Mout HC, Bissumbhar B, Mulder CR (1991) Inhalation
exposure in Drosophila mutagenesis assays: experiments with
aliphatic halogenated hydrocarbons, with emphasis on the genetic
activity profile of 1,2-dichloroethane. Mutation research,
252:17-33.
Kronevi T, Wahlberg JE, Holmberg B (1981) Skin pathology
following epicutaneous exposure to seven organic solvents.
International journal of tissue reactions, 3:21-30.
Kuwabara T, Quevedo AR, Cogan DG (1968) An experimental study of
dichloroethane poisoning. Archives of ophthalmology,
79:321-330.
Lane RW, Riddle BL, Borzelleca JF (1982) Effects of
1,2-dichloroethane and 1,1,1-trichloroethane in drinking water on
reproduction and development in mice. Toxicology and applied
pharmacology, 63:409-421.
Larionov VG, Kokarovtseva MG (1976) Morphological constitution of
peripheral blood in intoxication with dichloroethane and its
metabolites. In: Actual problems of pesticide application in
different climatographic zones. Yerevan, Aiastan Publishers,
pp. 131-133.
Leblanc GA (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bulletin of environmental contamination
and toxicology, 24:684-691.
Letkiewicz F, Johnston P, Coleman J (1982) Occurrence of
1,2-dichloroethane in drinking water, food and air. Prepared by
JRB Associates, Washington DC, under Contract No. 68-01-6185 for
the US Environmental Protection Agency.
Lin ELC, Mattox JK, Pereira MA (1985) Glutathione plus
cytosol- and microsome-mediated binding of 1,2-dichloroethane to
polynucleotides. Toxicology and applied pharmacology,
78:428-435.
Lum KR, Kaiser KLE (1986) Organic and inorganic contaminants in
the St. Lawrence River: some preliminary results on their
distribution. Water pollution research journal of Canada,
21(4):592-603.
Maltoni C, Valgimigli L, Scarnato C (1980) Long-term carcinogenic
bioassays on ethylene dichloride administered by inhalation to
rats and mice. In: Ames BN, Infante P, Reitz R, eds. Ethylene
dichloride: a potential health risk? Cold Spring Harbor, NY,
Cold Spring Harbor Laboratory, pp. 3-33 (Banbury Report No. 5).
McCollister DD, Hollingsworth RL, Oyen F, Rowe VK (1956)
Comparative inhalation toxicity of fumigant mixtures. Individual
and joint effect of ethylene dichloride, carbon tetrachloride,
and ethylene dibromide. Archives of industrial health, 13:1-7.
Milman HA, Storey DL, Riccio ES, Sivak A, Tu AS, Williams GM,
Tong C, Tyson CA (1988) Rat liver foci and in vitro assays to
detect initiating and promoting effects of chlorinated ethanes
and ethylenes. Annals of the New York Academy of Sciences,
534:521-530.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, Shirasu Y
(1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation research, 116:185-216.
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, White
KL Jr (1982) In vivo assessment of immunotoxicity.
Environmental health perspectives, 43:41-52.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity. Bethesda, MD, US Department of Health,
Education and Welfare, Public Health Services, National
Institutes of Health, National Cancer Institute (HEW (NIH)
Publication No. 78-1305).
Nestmann ER, Lee EGH, Matula TI, Douglas GR, Mueller JC (1980)
Mutagenicity of constituents identified in pulp mill effluents
using Salmonella/mammalian-microsome assay. Mutation
research, 79:203-212.
NIOSH (1994a) Registry of toxic effects of chemical
substances. Cincinnati, OH, National Institute for Occupational
Safety and Health (last revision May 1994).
NIOSH (1994b) NIOSH pocket guide to chemical hazards. US
Department of Health and Human Services, Public Health Service,
Center for Disease Control and Prevention, National Institute for
Occupational Safety and Health, June.
Nouchi T, Miura H, Kanayama M, Mizuguchi O, Takano T (1984) Fatal
intoxication by 1,2-dichloroethane - A case report.
International archives of occupational and environmental
health, 54:111-113.
NTP (1991) Toxicity studies of 1,2-dichloroethane (ethylene
dichloride) in F344/N rats, Sprague Dawley rats,
Osborne-Mendel rats, and B6C3F1 mice (drinking water and
gavage studies). Research Triangle Park, NC, National
Institutes of Health, National Toxicology Program (NIH
Publication No. 91-3123).
Nylander P-O, Olofsson H, Rasmuson B, Svahlin H (1978) Mutagenic
effects of petrol in Drosophila melanogaster. I. Effects of
benzene and 1,2-dichloroethane. Mutation research, 57:163-167.
Otson R (1987) Purgeable organics in Great Lakes raw and treated
water. International journal of environmental analytical
chemistry, 31(1):41-53.
Pearson CR, McConnell G (1975) Chlorinated C1 and C2
hydrocarbons in the marine environment. Proceedings of the
Royal Society of London B, 189:305-332.
Perocco P, Prodi G (1981) DNA damage by haloalkanes in human
lymphocytes cultured in vitro. Cancer letters, 13:213-218.
Principe P, Dogliotti E, Bignami M, Crebelli R, Falcone E,
Fabrizi M, Conti G, Comba P (1981) Mutagenicity of chemicals of
industrial and agricultural relevance in Salmonella,
Streptomyces, and Aspergillus. Journal of the science of
food and agriculture, 32:826-832.
Prodi G, Arfelini G, Colacci A, Grilli S, Mazzulo M (1986)
Interaction of halocompounds with nucleic acids. Toxicologic
pathology, 14:438-444.
Rannug U (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichloroethane. Mutation research, 76:269-295.
Rannug U, Beije B (1979) The mutagenic effect of
1,2-dichloro-ethane on Salmonella typhimurium. II. Activation
by the isolated perfused rat liver. Chemico-biological
interactions, 24:265-285.
Rannug U, Sundvall A, Ramel C (1978) The mutagenic effect
of 1,2-dichloroethane on Salmonella typhimurium. I. Activation
through conjugation with glutathione in vitro.
Chemico-biological interactions, 20:1-16.
Rao KS, Murray JS, Deacon MM, John JA, Calhoun LL, Young JT
(1980) Teratogenicity and reproduction studies in animals
inhaling ethylene dichloride. In: Ames BN, Infante P, Reitz R,
eds. Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 149-166 (Banbury
Report No. 5).
Reitz RH, Fox TR, Ramsey JC, Quast JF, Langvardt PW, Watanabe PG
(1982) Pharmacokinetics and macro-molecular interactions of
ethylene dichloride in rats after inhalation or gavage.
Toxicology and applied pharmacology, 62:190-204.
Richter JE, Peterson SF, Kleiner CF (1983) Acute and chronic
toxicity of some chlorinated benzenes, chlorinated ethanes, and
tetrachloroethylene to Daphnia magna. Archives of
environmental contamination and toxicology, 12:679-684.
Roldan-Arjona T, Garcia-Pedrajas MD, Luque-Romero RL, Hera C,
Pueyo C (1991) An association between mutagenicity of the Ara
test of Salmonella typhimurium and carcinogenicity in rodents
for 16 halogenated aliphatic hydrocarbons. Mutagenesis,
6:199-205.
Romert L, Magnusson J, Ramel C (1990) The importance of
glutathione and glutathione transferase for somatic mutations in
Drosophila melanogaster induced in vivo by
1,2-dichloroethane. Carcinogenesis, 11:1399-1402.
Schultz K, Ghosh L, Banerjee S (1992) Neoplastic expression in
murine cells induced by halogenated hydrocarbons. In vitro
cellular and developmental biology, 28A:267-272.
Sherwood RL, O'Shea PT, Thomas W, Ratajczak HV, Aranyi C, Graham
JA (1987) Effects of inhalation of ethylene dichloride on
pulmonary defences of mice and rats. Toxicology and applied
pharmacology, 91:491-496.
Shmuter LM (1977) [Effects of chronic exposure to low
concentrations of chlorinated hydrocarbons of the ethane series on
specific and non-specific reactivity of animals in vivo.]
Gigiena Truda i Professional'nye Zabolevaniya, 8:38-42 (in
Russian).
Simmon VF (1980) Review of non-bacterial tests of the genotoxic
activity of ethylene dichloride. In: Ames BN, Infante P, Reitz R,
eds. Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 97-103 (Banbury
Report No. 5).
Simula TP, Glancey MG, Wolf CR (1993) Human glutathione
S-transferase-expressing Salmonella typhimurium tester
strains to study the activation/detoxification of mutagenic
compounds: studies with halogenated compounds, aromatic amines
and aflatoxin B1. Carcinogenesis, 14:1371-1376.
Singh HB, Salas LJ, Smith AJ, Shigeishi H (1980) Atmospheric
measurements of selected toxic organic chemicals. Research
Triangle Park, NC, US Environmental Protection Agency,
Environmental Sciences Research Laboratory (EPA-600/3-80-072;
PB80-198989).
Singh HB, Salas LJ, Smith AJ, Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban
environments. Atmospheric environment, 15:601-612.
Singh HB, Salas LF, Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environmental science and technology, 16:872-880.
Smyth HF Jr (1969) Acute toxicity of ethyl compounds. In: Spector
WC, ed. Handbook of toxicology. Vol. 1. Philadelphia, PA, W.B.
Saunders Company.
Spence JW, Hanst PL (1978) Oxidation of chlorinated ethanes.
Journal of the Air Pollution Control Association,
28(3):250-253.
Spencer HC, Rowe VK, Adams EM, McCollister DD, Irish DD (1951)
Vapor toxicity of ethylene dichloride determined by experiments
on laboratory animals. American Medical Association archives
of industrial hygiene and occupational medicine, 4:482-493.
Spreafico F, Zuccato E, Marcucci F, Sironi M, Paglialunga S,
Madonna M, Mussini E (1980) Pharmacokinetics of ethylene
dichloride in rats treated by different routes and its long-term
inhalatory toxicity. In: Ames BN, Infante P, Reitz R, eds.
Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 107-133 (Banbury
Report No. 5).
Storer RD, Conolly RB (1983) Comparative in vivo genotoxicity
and acute hepatotoxicity of three 1,2-dihaloethanes.
Carcinogenesis, 4:1491-1494.
Storer RD, Conolly RB (1985) An investigation of the role of
microsomal oxidative metabolism in the in vivo genotoxicity of
1,2-dichloroethane. Toxicology and applied pharmacology,
77:36-46.
Storer RD, Jackson NM, Conolly RB (1984) In vivo genotoxicity
and acute hepatotoxicity of 1,2-dichloroethane in mice:
comparison of oral, intraperitoneal, and inhalation routes of
exposure. Cancer research, 44:4267-4271.
Story DL, Meierhenry EF, Tyson CA, Milman HA (1986) Differences
in rat liver enzyme-positive foci produced by chlorinated
aliphatics and phenobarbital. Toxicology and industrial
health, 2:351-362.
Strobel K, Grummt T (1987) Aliphatic and aromatic halocarbons as
potential mutagens in drinking water. III. Halogenated ethanes
and ethenes. Toxicology and environmental chemistry,
15:101-128.
Sundheimer DW, White RD, Brendel K, Sipes IC (1982) The
bioactivation of 1,2-dibromoethane in rat hepatocytes: covalent
binding to nucleic acids. Carcinogenesis, 3:1129-1133.
Suveev IM, Babichenko ME (1969) [On the clinic and cure of acute
intoxication with dichloroethane vapours.] Gigiena Truda i
Professional'nye Zabolevaniya, 13:50-51 (in Russian).
Tan E-L, Hsie AW (1981) Mutagenicity and cytotoxicity of
haloethanes as studied in the CHO/HGPRT system. Mutation
research, 90:183-191.
Taningher M, Parodi S, Grilli S, Colacci A, Mazzullo M, Bordone
R, Santi L (1991) Lack of correlation between alkaline DNA
fragmentation and DNA covalent binding induced by
polychloroethanes after in vivo administration. Problems
related to the assessment of a carcinogenic hazard. Cancer
detection and prevention, 15:35-39.
Theiss JC, Stoner GD, Shimkin MB, Weisberger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer
research, 37:2717-2720.
Tu AS, Murray TA, Hatch KM, Sivak A, Milman HA (1985) In vitro
transformation of BALB/c-3T3 cells by chlorinated ethanes and
ethylenes. Cancer letters, 28:85-92.
US EPA (1985) Health assessment document for
1,2-dichloroethane (ethylene dichloride). Final report.
Washington, DC, US Environmental Protection Agency
(EPA/600/8-84/006F; NTIS PB86-122702).
US EPA (1992) Indoor air quality data base for organic
compounds. Research Triangle Park, NC, US Environmental
Protection Agency (EPA-600-R-92-025; NTIS PB92-158468).
Valencia R, Abrahamson S, Lee WR, von Halle ES, Woodruff RC,
Wurgler FE, Zimmering S (1984) Chromosome mutations for
mutagenesis in Drosophila melanogaster: a report of the US
Environmental Protection Agency Gene-Tox Program. Mutation
research, 134:61-88.
van Bladeren PJ, Breimer DD, Rotteveel-Smijs RMT, de Knijff P,
Mohn GV, van Meeteren-Walchli B, Buijs W, van der Gen A (1981)
The relation between the structure of vicinal dihalogen compounds
and their mutagenic activation via conjugation to glutathione.
Carcinogenesis, 2:499-505.
van Duuren BL, Goldschmidt BM, Loewengart G, Smith A, Mechionne
S, Seldman I, Roth D (1979) Carcinogenicity of halogenated
olefinic and aliphatic hydrocarbons in mice. Journal of the
National Cancer Institute, 63:1433-1439.
van Esch GJ, Kroes R, van Logten MJ, den Tonkelaar EM (1977)
Ninety-day toxicity study with 1,2-dichloroethane (DCE) in
rats. Bilthoven, National Institute of Public Health and
Environmental Hygiene (Report 195/77 Al. Tox.).
Vozovaya M (1977) [The effect of dichloroethane on the sexual
cycle and embryogenesis of experimental animals.] Akusherstvo
i Ginekologiya (Moscow), 2:57-59 (in Russian).
WHO (1971) Evaluation of food additives: specifications for
the identity and purity of food additives and their
toxicological evaluation: some extraction solvents and
certain other substances; a review of the technological
efficacy of some antimicrobial agents. Fourteenth report of
the Expert Committee. Geneva, World Health Organization (WHO
Technical Report Series No. 462).
WHO (1980) Evaluation of certain food additives.
Twenty-third report of the Joint FAO/WHO Expert Committee
on Food Additives. Geneva, World Health Organization (WHO
Technical Report Series No. 648 and corrigenda).
WHO (1992) Evaluation of certain food additives and
naturally occurring toxicants. Thirty-ninth report of the
Joint FAO/WHO Expert Committee on Food Additives. Geneva, World
Health Organization (WHO Technical Report Series No. 828).
WHO (1993) Guidelines for drinking-water quality, 2nd ed.
Vol. 1. Recommendations. Geneva, World Health Organization.
Williams GM, Mori H, McQueen CA (1989) Structure-activity
relationships in the rat hepatocyte DNA-repair test for 300
chemicals. Mutation research, 221:263-286.
Zamora PO, Benson JM, Li AP, Brooks AL (1983) Evaluation of an
exposure system using cells grown on collagen gels for detecting
highly volatile mutagens in the CHO/HGPRT mutation assay.
Environmental mutagenesis, 5:795-801.
APPENDIX 1 - SOURCE DOCUMENTS
International Programme on Chemical Safety - Environmental Health
Criteria Monograph No. 176 (1995)
Copies of the Environmental Health Criteria document on
1,2-dichloroethane, prepared by the International Programme on
Chemical Safety, as well as the Health and Safety Guide (1991)
and the International Chemical Safety Card (1993), may be
obtained from:
International Programme on Chemical Safety
World Health Organization
Geneva, Switzerland
The first draft of the monograph, prepared by Ms K. Hughes
of the Environmental Health Directorate, Health Canada, was
circulated to IPCS Contact Points (approximately 150 government,
industrial, academic, and independent organizations and
individuals) for comment in June 1994. The second draft, revised
on the basis of the comments received, was also prepared by Ms K.
Hughes. Dr E. Smith and Dr P.G. Jenkins, both members of the
IPCS Central Unit, were responsible for the scientific content
and technical editing, respectively.
The monograph on 1,2-dichloroethane was finalized by the
Core Assessment Group (CAG) of the Joint Meeting on Pesticides
(JMP), which met in Geneva from 25 October to 3 November 1994.
The Core Assessment Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health
and the environment from exposure to 1,2-dichloroethane.
Participants at the Core Assessment Group meeting were:
Dr T. Bailey, Ecological Effects Branch, Environmental Fate
and Effects Division, US Environmental Protection Agency,
Washington, DC, USA
Dr A.L. Black, Department of Human Services and Health,
Canberra, ACT, Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairperson)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution,
London, United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, Office of Pesticide Programs, US
Environmental Protection Agency, Washington, DC, USA
Dr R. Hailey, National Institute of Environmental Health
Sciences, National Institutes of Health, Public Health
Service, Department of Health and Human Services, Research
Triangle Park, NC, USA
Ms K. Hughes, Priority Substances Section, Environmental
Health Directorate, Health Canada, Ottawa, Ontario, Canada
(EHC Joint Rapporteur)
Dr D. Kanungo, Division of Medical Toxicology, Central
Insecticides Laboratory, Government of India, Ministry of
Agriculture & Cooperation, Directorate of Plant Protection,
Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group
Ltd, Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Fontwell, Arundel,
West Sussex, United Kingdom
Professor M. Lotti, Institute of Occupational Medicine,
University of Padua, Padua, Italy (Chairperson)
Dr D.R. Mattison, University of Pittsburgh, Graduate School
of Public Health, Pittsburgh, PA, USA
Dr J. Sekizawa, Division of Information on Chemical Safety,
National Institute of Health Sciences, Setagaya-ku, Tokyo,
Japan
Dr P. Sinhaseni, Department of Pharmacology, Chulalongkorn
University, Bangkok, Thailand
Dr S.A. Soliman, Pesticide Chemicals & Toxicology, King Saud
University, Bureidah, Saudi Arabia
Dr M. Tasheva, Department of Toxicology, National Center of
Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Department of Toxicology, Wageningen
Agricultural Unviersity, Wageningen, The Netherlands
Dr M.I. Willems, Department of Occupational Toxicology, TNO
Nutrition and Food Research Institute, AJ Zeist, The
Netherlands
Procedures for the preparation of an Environmental Health
Criteria document
The order of procedures that result in the publication of an
EHC monograph is shown in the flow chart. A designated staff
member of IPCS, responsible for the scientific quality of the
document, serves as Responsible Officer (RO). The IPCS Editor is
responsible for layout and language. The first draft, prepared
by consultants or, more usually, staff from an IPCS Participating
Institution, is based initially on data provided from the
International Register of Potentially Toxic Chemicals and
reference databases such as Medline and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its
scientific quality and objectivity. Once the RO finds the
document acceptable as a first draft, it is distributed, in its
unedited form, to well over 150 EHC Contact Points throughout the
world who are asked to comment on its completeness and accuracy
and, where necessary, provide additional material. The Contact
Points, usually designated by governments, may be Participating
Institutions, IPCS Focal Points, or individual scientists known
for their particular expertise. Generally, some four months are
allowed before the comments are considered by the RO and
author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not
as representatives of any organization, government, or industry.
Their function is to evaluate the accuracy, significance, and
relevance of the information in the document and to assess the
health and environmental risks from exposure to the chemical. A
summary and recommendations for further research and improved
safety aspects are also required. The composition of the Task
Group is dictated by the range of expertise required for the
subject of the meeting and by the need for a balanced
geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international
associations may be invited to join the Task Group as observers.
While observers may provide a valuable contribution to the
process, they can speak only at the invitation of the
Chairperson. Observers do not participate in the final
evaluation of the chemical; this is the sole responsibility of
the Task Group members. When the Task Group considers it to be
appropriate, it may meet in camera.
All individuals who as authors, consultants, or advisers
participate in the preparation of the EHC monograph must, in
addition to serving in their personal capacity as scientists,
inform the RO if at any time a conflict of interest, whether
actual or potential, could be perceived in their work. They are
required to sign a conflict of interest statement. Such a
procedure ensures the transparency and probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of
the document, it then goes for language editing, reference
checking, and preparation of camera-ready copy. After approval
by the Director, IPCS, the monograph is submitted to the WHO
Office of Publications for printing. At this time, a copy of the
final draft is sent to the Chairperson and Rapporteur of the Task
Group to check for any errors.
 APPENDIX 2 - CICAD FINAL REVIEW BOARD
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit,
Health and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and
Environmental Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental
Assessment, Office of Research and Development, US Environmental
Protection Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of
Consumers & Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of
Hygiene and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences,
Hanover, Germany
Ms E. Meek, Head, Priority Substances Section, Environmental
Health Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National
Institute of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim,
Germany
Professor S. Tarkowski, Department of Environmental Health
Hazards, The Nofer Institute of Occupational Medicine, Lodz,
Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna,
Sweden
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on
Chemical Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé
ce CICAD (Document international succinct sur l'évaluation des
risques chimiques) sur le 1,2-dichloréthane en s'inspirant d'un
document de la série Critères d'hygiène de l'environnement (CHE)
du Programme international sur la Sécurité chimique (PISC) (IPCS,
1995), qui évalue les conséquences potentielles pour la santé
humaine d'une exposition indirecte au 1,2-dichloréthane dans
l'environnement général ainsi que les effets de cette substance
sur l'environnement. Les données prises en compte dans cette
analyse sont celles qui étaient disponibles en mai 1993 (effets
sur la santé humaine) ou en octobre 1994 (effets sur
l'environnement). L'appendice 1 donne des informations sur la
nature du processus d'évaluation par les pairs et sur la
disponibilité du document CHE. Pour ce CICAD, c'est l'évaluation
pratiquée lors de l'élaboration du document CHE qui a fait office
d'évaluation par les pairs préalable à l'examen du Comité
d'évaluation finale. Les membres du Comité d'évaluation finale
ont apporté les dernières corrections à ce CICAD et en ont
approuvé la publication par correspondance, après avoir examiné
les observations présentées lors de l'élaboration du CHE dans le
cadre de l'évaluation par les pairs. La composition du Comité
d'évaluation finale est indiquée à l'appendice 2. La fiche
internationale sur la sécurité chimique (ICSC 0250) produite par
le PISC (IPCS, 1993) est également reproduite dans ce document.
Le 1,2-dichloréthane (CAS N° 107-06-2) est un hydrocarbure
synthétique volatil utilisé principalement dans la synthèse du
chlorure de vinyle monomère et d'autres solvants chlorés. Il a
également été utilisé comme additif de l'essence au plomb et
comme fumigant, mais son utilisation comme additif de l'essence
est en déclin. La plus grande partie du 1,2-dichloréthane libéré
dans l'environnement se retrouve dans l'air ambiant où sa
persistance est modérée. Toutefois, il ne devrait pas contribuer
à la destruction de l'ozone. Le potentiel de bioaccumulation du
1,2-dichloréthane est faible; son inhalation avec l'air est
probablement la principale source d'exposition humaine.
On dispose de peu d'information sur les effets du
1,2-dichloréthane chez l'homme. Les quelques études
épidémiologiques qui ont été faites sur sa cancérogénicité
potentielle ne sont guère concluantes.
Le 1,2-dichloréthane présente une toxicité aiguë modérée
chez les animaux d'expérience. Les quelques données que l'on
trouve sur ses effets non néoplasiques dans des études de
chronicité à court terme, subchronique ou chronique indiquent que
les principaux organes cibles sont le foie et le rein; les doses
les plus faibles pour lesquelles on ait signalé un effet après
ingestion et inhalation sont respectivement de 49-82 mg/kg de
poids corporel par jour (augmentation du poids du foie chez des
rats exposés pendant 13 semaines) et 202 mg/m3 (effets sur les
fonctions hépatique et rénale chez des rats exposés pendant
12 mois). D'après les résultats d'un petit nombre d'études, il
n'y a pas de preuve que le 1,2-dichloréthane soit tératogène chez
les animaux de laboratoire, ni qu'il induise des effets sur la
reproduction ou le développement lorsque les niveaux d'exposition
sont inférieurs aux niveaux qui entraînent d'autres effets
systémiques.
L'exposition au 1,2-dichloréthane par gavage pendant
78 semaines a été suivie d'une augmentation significative de
l'incidence des tumeurs de différents organes (notamment des
hémangiosarcomes et des tumeurs de l'estomac, des glandes
mammaires, du foie, des poumons et de l'endomètre), tant chez le
rat que chez la souris. Il n'y a eu aucune augmentation
significative de l'incidence des tumeurs chez des rats ou des
souris exposés par inhalation, mais l'administration répétée par
voie dermique ou intrapéritonéale a provoqué une augmentation du
nombre des tumeurs des poumons chez la souris. Le
1,2-dichloréthane s'est constamment révélé génotoxique dans de
nombreuses épreuves in vitro sur des cellules de procaryotes,
de champignons et de mammifères (y compris des cellules
humaines). De même, les résultats ont été constamment positifs
en ce qui concerne l'activité génotoxique (ainsi que la fixation
sur l'ADN) dans des études in vivo chez le rat, la souris et
les insectes.
Les valeurs les plus faibles de la CI50s et de la CE50s qui
aient été signalées pour différents effets sur des organismes
aquatiques sont respectivement de 25 et 105 mg/litre. La CL50
la plus faible pour les daphnies était de 220 mg/litre, des
effets ayant toutefois été observés sur la reproduction à
20,7 mg/litre. Le vertébré d'eau douce le plus sensible a été
une salamandre (Ambystoma gracile) chez laquelle on a constaté
une réduction de la survie des larves à 2,5 mg/litre. On ne
dispose que de données limitées sur la toxicité du
1,2-dichloréthane pour les espèces terrestres.
D'après les données disponibles, le 1,2-dichloréthane peut
être considéré comme un cancérogène probable pour l'homme, de
sorte que l'exposition doit être réduite dans toute la mesure
possible. Le potentiel cancérogène (exprimé par la dose associée
à une augmentation de 5 % de l'incidence des tumeurs), calculé à
partir d'études de gavage, a été évalué à 6,2-34 mg/kg de poids
corporel par jour. En appliquant un facteur de sécurité de 5000
ou de 50 000 au potentiel cancérogène estimé, on arrive à une
valeur guide pour l'air (principale source d'exposition humaine)
de 3,6-20 µg/m3 ou 0,36-2,0 µg/m3; il faut cependant noter que
cette méthode surestime les risques, car les données disponibles
montrent que le 1,2-dichloréthane est moins actif lorsqu'il est
inhalé. (Les valeurs correspondantes pour l'ingestion sont
respectivement de 1,2-6,8 ou 0,12-0,68 µg/kg de poids corporel
par jour.) Ces valeurs correspondent à ce que certains
organismes considèrent comme un risque "pratiquement négligeable"
(c'est-à-dire 10-5-10-6 pour un cancérogène génotoxique).
D'après une des estimations qui ont été faites, l'exposition
indirecte dans un environnement normal est approximativement
300 fois inférieure à ces valeurs.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional del
1,2-dicloroetano ha sido preparada por la Dirección de Higiene
del Medio de Health Canada sobre la base de un documento de la
serie "Criterios de Salud Ambiental" (EHC) del Programa
Internacional de Seguridad de las Sustancias Químicas (IPCS,
1995) en el que se evalúan los efectos potenciales sobre la salud
humana de la exposición indirecta al 1,2-dicloroetano en el medio
ambiente general, así como los efectos ambientales de dicha
sustancia química. En este análisis se examinan datos obtenidos
en mayo de 1993 (efectos sobre la salud humana) y octubre de 1994
(efectos ambientales). En el apéndice 1 se proporciona
información sobre el proceso de revisión científica y los
documentos disponibles de la serie EHC. Por lo que respecta a
esta reseña de la evaluación química internacional, el proceso de
revisión científica previo al examen realizado por el Comité de
Revisión Final se ha cumplido mediante la revisión científica
efectuada para la elaboración del documento de la serie EHC.
Comunicándose por correspondencia, los miembros del Comité de
Revisión Final ultimaron esta reseña de la evaluación química
internacional del 1,2-dicloroetano, aprobaron su publicación y
examinaron las observaciones dimanantes de la revisión científica
efectuada durante la preparación del documento de la serie EHC.
La composición del Comité de Revisión Final figura en el apéndice
2. En el presente documento también se reproduce la Ficha
Internacional de Seguridad Química (ICSC 0250) emitida por el
IPCS (1993).
El 1,2-dicloroetano (CAS n° 107-06-2) es un hidrocarburo
sintético volátil que se utiliza principalmente en la síntesis
del monómero cloruro de vinilo y de otros disolventes clorados.
También se ha utilizado como aditivo de la gasolina con plomo y
como fumigante, aunque su uso como aditivo de la gasolina se está
reduciendo. La mayor parte de la liberación en el entorno se
produce en el aire ambiente, donde es moderadamente persistente.
Sin embargo, no parece que contribuya al agotamiento de la capa
de ozono. El 1,2-dicloroetano tiene un potencial de
bioacumulación bajo; la inhalación con el aire probablemente sea
la principal fuente de exposición humana.
Se dispone de poca información sobre los efectos del
1,2-dicloroetano en el ser humano. Las pocas investigaciones
epidemiológicas conocidas sobre su carcinogenicidad potencial no
son concluyentes.
El 1,2-dicloroetano tiene una toxicidad aguda moderada en
animales de experimentación. La escasa información sobre efectos
no neoplásicos presentada en estudios de corta duración,
subcrónicos y crónicos indica que los principales órganos
afectados son el hígado y los riñones; los niveles mínimos de
ingestión e inhalación con efectos comunicados fueron de 49-82
mg/kg de peso corporal por día (aumento de peso del hígado en las
ratas expuestas durante 13 semanas) y 202 mg/m3 (efectos sobre
la función hepática y renal en las ratas expuestas durante 12
meses), respectivamente. Los resultados de un número limitado de
estudios, no aportaron indicios de que el 1,2-dicloroetano sea
teratogénico en animales de experimentación o que tenga efectos
sobre la reproducción o el desarrollo a niveles de exposición
inferiores a los que causan otros efectos sistémicos.
La exposición al 1,2-dicloroetano administrado por sonda
durante 78 semanas produjo un aumento significativo de la
incidencia de tumores en distintos lugares (hemangiosarcomas y
tumores en el estómago, las glándulas mamarias, el hígado, los
pulmones y el endometrio) tanto en ratas como en ratones. Aunque
no hubo un aumento significativo de la incidencia de tumores en
las ratas y los ratones expuestos por inhalación, la aplicación
cutánea o intraperitoneal repetida de 1,2-dicloroetano se ha
revelado sistemáticamente genotóxica en numerosas pruebas
realizadas in vitro en células de procariotas, hongos y
mamíferos (incluidas células humanas). Análogamente, se han
obtenido resultados invariablemente positivos indicadores de
actividad genotóxica (así como enlaces con el ADN) en estudios
realizados in vivo con ratas, ratones e insectos.
Las CI50 y CE50 más bajas notificadas en relación con
diversos puntos finales en organismos acuáticos fueron de 25 y
105 mg/litro respectivamente. La CL50 más baja notificada para
Daphnia fue de 220 mg/litro, mientras que los efectos sobre la
reproducción se produjeron con concentraciones de 20,7 mg/litro.
El vertebrado de agua dulce más sensible estudiado fue la
salamandra noroccidental (Ambystoma gracile), en la que se
observó una reducción de la supervivencia de las larvas con
niveles de 2,5 mg/litro. Se dispone sólo de datos limitados
sobre los efectos del 1,2-dicloroetano en especies terrestres.
Teniendo en cuenta los datos disponibles, es probable que el
1,2-dicloroetano sea carcinógeno para el ser humano y, por lo
tanto, la exposición a éste debería reducirse en la medida de lo
posible. Sobre la base de estudios realizados en animales
expuestos a una administración por sonda, se ha calculado que la
potencia carcinogénica (expresada como la dosis asociada a un
aumento del 5% en la incidencia de tumores) oscila entre 6,2 y 34
mg/kg de peso corporal por día. Con respecto al aire (fuente
principal de exposición humana), se han obtenido valores
orientativos 3,6-20 g/m3 o 0,36-2,0 g/m3, calculados para un
margen 5.000 a 50.000 veces inferior a la potencia carcinogénica
estimada; sin embargo, hay que tener en cuenta que se han
sobreestimado los riesgos, ya que los datos disponibles indican
que el 1,2-dicloroetano inhalado resulta menos potente. (En
cuanto a la ingestión, los valores correspondientes son de
1,2-6,8 g/m3 de peso corporal por día o 0,12-0,68 g/m3 de peso
corporal por día.) Estos valores comportan lo que algunas
entidades consideran como un riesgo "prácticamente
insignificante" (es decir, 10-5 - 10-6 para un carcinógeno
genotóxico). Según las estimaciones estadísticas, la exposición
indirecta a través del medio ambiente general es aproximadamente
300 veces inferior a esos valores.
APPENDIX 2 - CICAD FINAL REVIEW BOARD
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit,
Health and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and
Environmental Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental
Assessment, Office of Research and Development, US Environmental
Protection Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of
Consumers & Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of
Hygiene and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences,
Hanover, Germany
Ms E. Meek, Head, Priority Substances Section, Environmental
Health Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National
Institute of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim,
Germany
Professor S. Tarkowski, Department of Environmental Health
Hazards, The Nofer Institute of Occupational Medicine, Lodz,
Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna,
Sweden
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on
Chemical Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé
ce CICAD (Document international succinct sur l'évaluation des
risques chimiques) sur le 1,2-dichloréthane en s'inspirant d'un
document de la série Critères d'hygiène de l'environnement (CHE)
du Programme international sur la Sécurité chimique (PISC) (IPCS,
1995), qui évalue les conséquences potentielles pour la santé
humaine d'une exposition indirecte au 1,2-dichloréthane dans
l'environnement général ainsi que les effets de cette substance
sur l'environnement. Les données prises en compte dans cette
analyse sont celles qui étaient disponibles en mai 1993 (effets
sur la santé humaine) ou en octobre 1994 (effets sur
l'environnement). L'appendice 1 donne des informations sur la
nature du processus d'évaluation par les pairs et sur la
disponibilité du document CHE. Pour ce CICAD, c'est l'évaluation
pratiquée lors de l'élaboration du document CHE qui a fait office
d'évaluation par les pairs préalable à l'examen du Comité
d'évaluation finale. Les membres du Comité d'évaluation finale
ont apporté les dernières corrections à ce CICAD et en ont
approuvé la publication par correspondance, après avoir examiné
les observations présentées lors de l'élaboration du CHE dans le
cadre de l'évaluation par les pairs. La composition du Comité
d'évaluation finale est indiquée à l'appendice 2. La fiche
internationale sur la sécurité chimique (ICSC 0250) produite par
le PISC (IPCS, 1993) est également reproduite dans ce document.
Le 1,2-dichloréthane (CAS N° 107-06-2) est un hydrocarbure
synthétique volatil utilisé principalement dans la synthèse du
chlorure de vinyle monomère et d'autres solvants chlorés. Il a
également été utilisé comme additif de l'essence au plomb et
comme fumigant, mais son utilisation comme additif de l'essence
est en déclin. La plus grande partie du 1,2-dichloréthane libéré
dans l'environnement se retrouve dans l'air ambiant où sa
persistance est modérée. Toutefois, il ne devrait pas contribuer
à la destruction de l'ozone. Le potentiel de bioaccumulation du
1,2-dichloréthane est faible; son inhalation avec l'air est
probablement la principale source d'exposition humaine.
On dispose de peu d'information sur les effets du
1,2-dichloréthane chez l'homme. Les quelques études
épidémiologiques qui ont été faites sur sa cancérogénicité
potentielle ne sont guère concluantes.
Le 1,2-dichloréthane présente une toxicité aiguë modérée
chez les animaux d'expérience. Les quelques données que l'on
trouve sur ses effets non néoplasiques dans des études de
chronicité à court terme, subchronique ou chronique indiquent que
les principaux organes cibles sont le foie et le rein; les doses
les plus faibles pour lesquelles on ait signalé un effet après
ingestion et inhalation sont respectivement de 49-82 mg/kg de
poids corporel par jour (augmentation du poids du foie chez des
rats exposés pendant 13 semaines) et 202 mg/m3 (effets sur les
fonctions hépatique et rénale chez des rats exposés pendant
12 mois). D'après les résultats d'un petit nombre d'études, il
n'y a pas de preuve que le 1,2-dichloréthane soit tératogène chez
les animaux de laboratoire, ni qu'il induise des effets sur la
reproduction ou le développement lorsque les niveaux d'exposition
sont inférieurs aux niveaux qui entraînent d'autres effets
systémiques.
L'exposition au 1,2-dichloréthane par gavage pendant
78 semaines a été suivie d'une augmentation significative de
l'incidence des tumeurs de différents organes (notamment des
hémangiosarcomes et des tumeurs de l'estomac, des glandes
mammaires, du foie, des poumons et de l'endomètre), tant chez le
rat que chez la souris. Il n'y a eu aucune augmentation
significative de l'incidence des tumeurs chez des rats ou des
souris exposés par inhalation, mais l'administration répétée par
voie dermique ou intrapéritonéale a provoqué une augmentation du
nombre des tumeurs des poumons chez la souris. Le
1,2-dichloréthane s'est constamment révélé génotoxique dans de
nombreuses épreuves in vitro sur des cellules de procaryotes,
de champignons et de mammifères (y compris des cellules
humaines). De même, les résultats ont été constamment positifs
en ce qui concerne l'activité génotoxique (ainsi que la fixation
sur l'ADN) dans des études in vivo chez le rat, la souris et
les insectes.
Les valeurs les plus faibles de la CI50s et de la CE50s qui
aient été signalées pour différents effets sur des organismes
aquatiques sont respectivement de 25 et 105 mg/litre. La CL50
la plus faible pour les daphnies était de 220 mg/litre, des
effets ayant toutefois été observés sur la reproduction à
20,7 mg/litre. Le vertébré d'eau douce le plus sensible a été
une salamandre (Ambystoma gracile) chez laquelle on a constaté
une réduction de la survie des larves à 2,5 mg/litre. On ne
dispose que de données limitées sur la toxicité du
1,2-dichloréthane pour les espèces terrestres.
D'après les données disponibles, le 1,2-dichloréthane peut
être considéré comme un cancérogène probable pour l'homme, de
sorte que l'exposition doit être réduite dans toute la mesure
possible. Le potentiel cancérogène (exprimé par la dose associée
à une augmentation de 5 % de l'incidence des tumeurs), calculé à
partir d'études de gavage, a été évalué à 6,2-34 mg/kg de poids
corporel par jour. En appliquant un facteur de sécurité de 5000
ou de 50 000 au potentiel cancérogène estimé, on arrive à une
valeur guide pour l'air (principale source d'exposition humaine)
de 3,6-20 µg/m3 ou 0,36-2,0 µg/m3; il faut cependant noter que
cette méthode surestime les risques, car les données disponibles
montrent que le 1,2-dichloréthane est moins actif lorsqu'il est
inhalé. (Les valeurs correspondantes pour l'ingestion sont
respectivement de 1,2-6,8 ou 0,12-0,68 µg/kg de poids corporel
par jour.) Ces valeurs correspondent à ce que certains
organismes considèrent comme un risque "pratiquement négligeable"
(c'est-à-dire 10-5-10-6 pour un cancérogène génotoxique).
D'après une des estimations qui ont été faites, l'exposition
indirecte dans un environnement normal est approximativement
300 fois inférieure à ces valeurs.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional del
1,2-dicloroetano ha sido preparada por la Dirección de Higiene
del Medio de Health Canada sobre la base de un documento de la
serie "Criterios de Salud Ambiental" (EHC) del Programa
Internacional de Seguridad de las Sustancias Químicas (IPCS,
1995) en el que se evalúan los efectos potenciales sobre la salud
humana de la exposición indirecta al 1,2-dicloroetano en el medio
ambiente general, así como los efectos ambientales de dicha
sustancia química. En este análisis se examinan datos obtenidos
en mayo de 1993 (efectos sobre la salud humana) y octubre de 1994
(efectos ambientales). En el apéndice 1 se proporciona
información sobre el proceso de revisión científica y los
documentos disponibles de la serie EHC. Por lo que respecta a
esta reseña de la evaluación química internacional, el proceso de
revisión científica previo al examen realizado por el Comité de
Revisión Final se ha cumplido mediante la revisión científica
efectuada para la elaboración del documento de la serie EHC.
Comunicándose por correspondencia, los miembros del Comité de
Revisión Final ultimaron esta reseña de la evaluación química
internacional del 1,2-dicloroetano, aprobaron su publicación y
examinaron las observaciones dimanantes de la revisión científica
efectuada durante la preparación del documento de la serie EHC.
La composición del Comité de Revisión Final figura en el apéndice
2. En el presente documento también se reproduce la Ficha
Internacional de Seguridad Química (ICSC 0250) emitida por el
IPCS (1993).
El 1,2-dicloroetano (CAS n° 107-06-2) es un hidrocarburo
sintético volátil que se utiliza principalmente en la síntesis
del monómero cloruro de vinilo y de otros disolventes clorados.
También se ha utilizado como aditivo de la gasolina con plomo y
como fumigante, aunque su uso como aditivo de la gasolina se está
reduciendo. La mayor parte de la liberación en el entorno se
produce en el aire ambiente, donde es moderadamente persistente.
Sin embargo, no parece que contribuya al agotamiento de la capa
de ozono. El 1,2-dicloroetano tiene un potencial de
bioacumulación bajo; la inhalación con el aire probablemente sea
la principal fuente de exposición humana.
Se dispone de poca información sobre los efectos del
1,2-dicloroetano en el ser humano. Las pocas investigaciones
epidemiológicas conocidas sobre su carcinogenicidad potencial no
son concluyentes.
El 1,2-dicloroetano tiene una toxicidad aguda moderada en
animales de experimentación. La escasa información sobre efectos
no neoplásicos presentada en estudios de corta duración,
subcrónicos y crónicos indica que los principales órganos
afectados son el hígado y los riñones; los niveles mínimos de
ingestión e inhalación con efectos comunicados fueron de 49-82
mg/kg de peso corporal por día (aumento de peso del hígado en las
ratas expuestas durante 13 semanas) y 202 mg/m3 (efectos sobre
la función hepática y renal en las ratas expuestas durante 12
meses), respectivamente. Los resultados de un número limitado de
estudios, no aportaron indicios de que el 1,2-dicloroetano sea
teratogénico en animales de experimentación o que tenga efectos
sobre la reproducción o el desarrollo a niveles de exposición
inferiores a los que causan otros efectos sistémicos.
La exposición al 1,2-dicloroetano administrado por sonda
durante 78 semanas produjo un aumento significativo de la
incidencia de tumores en distintos lugares (hemangiosarcomas y
tumores en el estómago, las glándulas mamarias, el hígado, los
pulmones y el endometrio) tanto en ratas como en ratones. Aunque
no hubo un aumento significativo de la incidencia de tumores en
las ratas y los ratones expuestos por inhalación, la aplicación
cutánea o intraperitoneal repetida de 1,2-dicloroetano se ha
revelado sistemáticamente genotóxica en numerosas pruebas
realizadas in vitro en células de procariotas, hongos y
mamíferos (incluidas células humanas). Análogamente, se han
obtenido resultados invariablemente positivos indicadores de
actividad genotóxica (así como enlaces con el ADN) en estudios
realizados in vivo con ratas, ratones e insectos.
Las CI50 y CE50 más bajas notificadas en relación con
diversos puntos finales en organismos acuáticos fueron de 25 y
105 mg/litro respectivamente. La CL50 más baja notificada para
Daphnia fue de 220 mg/litro, mientras que los efectos sobre la
reproducción se produjeron con concentraciones de 20,7 mg/litro.
El vertebrado de agua dulce más sensible estudiado fue la
salamandra noroccidental (Ambystoma gracile), en la que se
observó una reducción de la supervivencia de las larvas con
niveles de 2,5 mg/litro. Se dispone sólo de datos limitados
sobre los efectos del 1,2-dicloroetano en especies terrestres.
Teniendo en cuenta los datos disponibles, es probable que el
1,2-dicloroetano sea carcinógeno para el ser humano y, por lo
tanto, la exposición a éste debería reducirse en la medida de lo
posible. Sobre la base de estudios realizados en animales
expuestos a una administración por sonda, se ha calculado que la
potencia carcinogénica (expresada como la dosis asociada a un
aumento del 5% en la incidencia de tumores) oscila entre 6,2 y 34
mg/kg de peso corporal por día. Con respecto al aire (fuente
principal de exposición humana), se han obtenido valores
orientativos 3,6-20 g/m3 o 0,36-2,0 g/m3, calculados para un
margen 5.000 a 50.000 veces inferior a la potencia carcinogénica
estimada; sin embargo, hay que tener en cuenta que se han
sobreestimado los riesgos, ya que los datos disponibles indican
que el 1,2-dicloroetano inhalado resulta menos potente. (En
cuanto a la ingestión, los valores correspondientes son de
1,2-6,8 g/m3 de peso corporal por día o 0,12-0,68 g/m3 de peso
corporal por día.) Estos valores comportan lo que algunas
entidades consideran como un riesgo "prácticamente
insignificante" (es decir, 10-5 - 10-6 para un carcinógeno
genotóxico). Según las estimaciones estadísticas, la exposición
indirecta a través del medio ambiente general es aproximadamente
300 veces inferior a esos valores.
 Board members serve in their personal capacity, not as
representatives of any organization, government, or industry.
They are selected because of their expertise in human and
environmental toxicology or because of their experience in the
regulation of chemicals. Boards are chosen according to the
range of expertise required for a meeting and the need for
balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers
who participate in the preparation of a CICAD are required to
declare any real or potential conflict of interest in relation to
the subjects under discussion at any stage of the process.
Representatives of nongovernmental organizations may be invited
to observe the proceedings of the Final Review Board. Observers
may participate in Board discussions only at the invitation of
the Chairperson, and they may not participate in the final
decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on 1,2-dichloroethane was prepared by the
Environmental Health Directorate of Health Canada based on an
International Programme on Chemical Safety (IPCS) Environmental
Health Criteria (EHC) document (IPCS, 1995), which assesses the
potential effects on human health of indirect exposure to
1,2-dichloroethane in the general environment as well as the
chemical's environmental effects. Data identified as of May 1993
(human health effects) and October 1994 (environmental effects)
were considered in these reviews. Information on the nature of
the peer review process and the availability of the EHC document
is presented in Appendix 1. For this CICAD, the peer review
process prior to consideration by the Final Review Board was
covered by the peer review carried out for the EHC. This CICAD
on 1,2-dichloroethane was finalized and approved for publication,
through correspondence, by members of the Final Review Board, who
also considered the peer review comments provided during the
development of the EHC. The composition of the Final Review
Board is outlined in Appendix 2. The International Chemical
Safety Card (ICSC 0250) produced by the IPCS (1993) has also been
reproduced in this document.
1,2-Dichloroethane (CAS no.
Board members serve in their personal capacity, not as
representatives of any organization, government, or industry.
They are selected because of their expertise in human and
environmental toxicology or because of their experience in the
regulation of chemicals. Boards are chosen according to the
range of expertise required for a meeting and the need for
balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers
who participate in the preparation of a CICAD are required to
declare any real or potential conflict of interest in relation to
the subjects under discussion at any stage of the process.
Representatives of nongovernmental organizations may be invited
to observe the proceedings of the Final Review Board. Observers
may participate in Board discussions only at the invitation of
the Chairperson, and they may not participate in the final
decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on 1,2-dichloroethane was prepared by the
Environmental Health Directorate of Health Canada based on an
International Programme on Chemical Safety (IPCS) Environmental
Health Criteria (EHC) document (IPCS, 1995), which assesses the
potential effects on human health of indirect exposure to
1,2-dichloroethane in the general environment as well as the
chemical's environmental effects. Data identified as of May 1993
(human health effects) and October 1994 (environmental effects)
were considered in these reviews. Information on the nature of
the peer review process and the availability of the EHC document
is presented in Appendix 1. For this CICAD, the peer review
process prior to consideration by the Final Review Board was
covered by the peer review carried out for the EHC. This CICAD
on 1,2-dichloroethane was finalized and approved for publication,
through correspondence, by members of the Final Review Board, who
also considered the peer review comments provided during the
development of the EHC. The composition of the Final Review
Board is outlined in Appendix 2. The International Chemical
Safety Card (ICSC 0250) produced by the IPCS (1993) has also been
reproduced in this document.
1,2-Dichloroethane (CAS no.  TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Highly flammable. Gives off No open flames, NO sparks, Powder, water spray, foam,
irritating or toxic fumes (or and NO smoking. carbon dioxide.
gases) in a fire).
EXPLOSION Vapour/air mixtures are Closed system, ventilation, In case of fire: keep drums,
explosive. explosion-proof electrical etc., cool by spraying with
equipment and lighting. water.
Prevent build-up of
electrostatic charges (e.g.,
by grounding) (see Notes).
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTONS FIRE FIGHTING
EXPOSURE
EXPOSURE AVOID ALL CONTACT! IN ALL CASES CONSULT A
DOCTOR!
* INHALATION Abdominal pain. Cough. Ventilation, local exhaust, Fresh air, rest. Half-upright
Dizziness. Drowsiness. or breathing protection. position. Artificial
Headache. Nausea. Sore respiration if indicated.
throat. Unconsciousness. Refer for medical attention.
Vomiting. Symptoms may be
delayed (see Notes).
* SKIN Redness. Protective gloves. Remove contaminated clothes.
Rinse and then wash skin with
water and soap. Refer for
medical attention.
* EYES Redness. Pain. Blurred Safety goggles, face shield, First rinse with plenty of
vision. or eye protection in water for several minutes
combination with breathing (remove contact lenses if
protection. easily possible), then take
to a doctor.
* INGESTION Abdominal cramps. Diarrhoea Do no eat, drink, or smoke Give nothing to drink. Refer
(further see Inhalation). during work. Wash hands for medical attention.
before eating.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Evacuate danger area! Collect leaking Fireproof. Separated from strong Unbreakable packaging; put breakable
and spilled liquid in sealable oxidants, food and feedstuffs and packaging into closed unbreakable
containers as far as possible. Absorb other incompatible substances (see container. Do not transport with food
remaining liquid in sand or inert Chemical Dangers). Cool. Dry. and feedstuffs.
absorbent and remove to safe place. Do F symbol
NOT wash away into sewer (extra Y symbol
personal protection: self-contained R: 45-11-22-36/37/38
breathing apparatus). S: 53-45
Note: E
UN Hazard Class: 3
UN Subsidiary Risks: 6.1
UN Packing Group: 11
Marine Pollutant.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: INHALATION RISK:
COLOURLESS, VISCOUS LIQUID, WITH A harmful contamination of the air can be
CHARACTERISTIC ODOUR, TURNS DARK ON EXPOSURE reached very quickly on evaporation of this
TO AIR, MOISTURE AND LIGHT substance at 20°C.
PHYSICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The vapour is heavier than air and may travel The vapour irritates the eyes, the skin and
along the ground; distant ignition possible. the respiratory tract. Inhalation of the
As a result of flow, agitation, etc., vapour may cause lung oedema (see Notes). The
electrostatic charges can be generated. substance may cause effects on the central
nervous system, kidneys, liver, resulting in
CHEMICAL DANGERS: impaired functions.
The substance decomposes on heating and on EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
burning producing toxic and corrosive fumes
including hydrogen chloride (ICSC #0163) and Repeated or prolonged contact with skin may
phosgene (ICSC #0007). Reacts violently with cause dermatitis. This substance is probably
aluminium, alkali metals, alkali amides, carcinogenic to humans.
ammonia bases, strong oxidants. Attacks many
metals in presence of water. Attacks plastic.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV: 10 ppm; 40 mg/m3 (as TWA)
(ACGIH 1994-1995).
ROUTES OF EXPOSURE:
The substance can be absorbed into the body by
inhalation of its vapour, through the skin and
by ingestion.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 83.5°C Flash point: 13°C c.c.
PROPERTIES Melting point: -35.7°C Auto-ignition temperature: 413°C
Relative density (water = 1): 1.235 Explosive limits, vol% in air: 6.2-16
Solubility in water, g/100 ml: 0.87 Octanol/water partition coefficient
Vapour pressure, kPa at 20°C: 8.7 as logPow: 1.48
Relative vapour density (air = 1): 3.42
Relative density of the vapour/
air-mixture at 20°C (air = 1): 1.2
ENVIRONMENTAL
DATA
NOTES
Depending on the degree of exposure, periodic medical examination is indicated.
The symptoms of lung oedema often do not become manifest until a few hours have passed and they are aggravated by physical effort.
Rest and medical observation are tehrefore essential.
Immediate administration of an appropriate spray, by a doctor or a person authorized by him/her, should be considered.
EXPLOSION/PREVENTION: Do NOT use compressed air for filling, discharging, or handling.
ICSC: 0250 1.1 Transport Emergency Card: TEC (R)-605
NFPA Code: H 2; F 3; R 0
REFERENCES
Alumot E, Nachtomi E, Mandel E, Holstein P (1976) Tolerance and
acceptable daily intake of chlorinated fumigants in the rat diet.
Food and cosmetics toxicology, 14:105-110.
Andriukin AA (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klinicheskaya Meditsina (Moscow),
57:43-47 (in Russian).
Arfellini G, Bartoli S, Colacci A, Mazzullo M, Galli MC, Prodi G,
Grilli S (1984) In vivo and in vitro binding of
1,2-dibromoethane and 1,2-dichloroethane to macromolecules in rat
and mouse organs. Journal of cancer research and clinical
oncology, 108:204-213.
Armstrong MJ, Galloway SM (1993) Micronuclei induced in
peripheral blood of Eµ-PIM-1 transgenic mice by chronic oral
treatment with 2-acetylaminofluorene or benzene but not with
diethylnitrosamine or 1,2-dichloroethane. Mutation research,
302:61-70.
ATSDR (1992) Toxicological profile for 1,2-dichloroethane
(draft). Atlanta, GA, US Department of Health and Human Services,
Public Health Service, Agency for Toxic Substances and Disease
Registry.
Austin SG, Schnatter AR (1983) A case-control study of chemistry
exposures and brain tumors in petrochemical workers. Journal
of occupational medicine, 25(4):313-320.
Baertsch A, Lutz WK, Schlatter C (1991) Effect of inhalation
exposure regimen on DNA binding potency of 1,2-dichloroethane in
the rat. Archives of toxicology, 65:169-176.
Ballering LAP, Nivard MJM, Vogel EW (1993) Characterization of
the genotoxic action of three structurally related
1,2-dihaloalkanes in Drosophila melanogaster. Mutation
research, 285:209-217.
Banerjee S (1988) DNA damage in rodent liver by
1,2-dichloroethane, a hepatocarcinogen. Cancer biochemistry
and biophysics, 10:165-173.
Banerjee S, van Duuren BL (1979) Binding of carcinogenic
halogenated hydrocarbons to cell macromolecules. Journal of
the National Cancer Institute, 63:707-711.
Banerjee S, van Duuren BL, Oruambo FI (1980) Microsome-mediated
covalent binding of 1,2-dichloroethane to lung microsomal protein
and salmon sperm DNA. Cancer research, 40:2170-2173.
Barber RD, Donish WH, Mueller KR (1981) A procedure for the
quantitative measurement of the mutagenicity of volatile liquids
in the Ames Salmonella microsome assay. Mutation research,
90:31-48.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizzari ED, Ray M, Smith D, Tomer KB, Wagner R, Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - A
multimedia environmental study. Biomedical and environmental
mass spectrometry, 7:139-147.
Barsoum GS, Saad K (1934) Relative toxicity of certain chlorine
derivatives of the aliphatic series. Quarterly journal of
pharmacy and pharmacology, 7: 205-214.
Benson LO, Teta MJ (1993) Mortality due to pancreatic and
lymphopoietic cancers in chlorohydrin production workers.
British journal of industrial medicine, 50:710-716.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, Bruser
DM (1982) The aquatic toxicity of organic compounds to
embryo-larval stages of fish and amphibians. Lexington, KY,
University of Kentucky (Research Report No. 133).
Blum DJW, Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons
and correlations. Research journal of the Water Pollution
Control Federation, 63(3):198-207.
Bonnet P, Francin J-M, Grakiski D, Raoult G, Zissu D (1980)
Détermination de la concentration léthale50 des principaux
hydrocarbures aliphatiques chlorés chez le rat. Archives des
maladies professionnelles de medecine du travail et de
securité sociale (Paris), 41(6-7):317-321.
Brem H, Stein AB, Rosenkranz HS (1974) The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer research,
34:2576-2579.
Bringmann G, Kühn R (1978) Testing of substances for their
toxicity threshold: model organisms Microcystis (Diplocystis)
aeruginosa and Scenedesmus quadricauda. Mitteilungen
Internationale Vereinigung für Theoretische und Angewandte
Limnologie, 21:275-284.
Bringmann G, Kühn R (1980) Comparison of the toxicity thresholds
of water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water research, 14:231-241.
Buijs W, van der Gen A, Mohn GR, Breimer DD (1984) The direct
mutagenic activity of alpha, omega-dihalogenoalkanes in
Salmonella typhimurium. Mutation research, 141:11-14.
Callaghan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR,
Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR,
Gould C (1979) Water-related fate of 129 priority pollutants.
Vol. II. Washington, DC, US Environmental Protection Agency
(EPA 40/4-79-029b).
Cheever KL, Cholakis JM, El-Hawari AM, Kovatch RM, Weisburger EK
(1990) Ethylene dichloride: the influence of disulfiram or
ethanol on oncogenicity, metabolism and DNA covalent binding in
rats. Fundamental and applied toxicology, 14:243-261.
Cheh AM, Hooper AB, Skochdopole J, Henke CA, McKinnell RG (1980)
A comparison of the ability of frog and rat S-9 to activate
promutagens in the Ames test. Environmental and molecular
mutagenesis, 2:487-508.
Chemical Marketing Reporter (1992) Chemical profile: ethylene
dichloride. Chemical Marketing Reporter magazine, 241(19):42.
Clark AI, McIntyre AE, Lester JN, Perry R (1984a) Ambient air
measurement of aromatic and halogenated hydrocarbons at urban,
rural, and motorway locations. The science of the total
environment, 39:265-279.
Clark AI, McIntyre AE, Perry R, Lester JN (1984b) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural, and motorway locations.
Environmental pollution series B, 7:141-158.
Comba ME, Kaiser KLE (1985) Volatile halocarbons in the Detroit
River and their relationship with contaminant sources. Journal
of Great Lakes research, 11(3):404-418.
CPI (1991) CPI product profiles: ethylene dichloride. Don
Mills, Ontario, Canadian Process Industries, Corpus Information
Services.
Crebelli R, Carere A (1988) Genotoxic activity of halogenated
aliphatic hydrocarbons in Aspergillus nidulans. Journal of
occupational toxicology, 8:437-442.
Crebelli R, Conti G, Conti L, Carere A (1984) Induction of
somatic segregation by halogenated aliphatic hydrocarbons in
Aspergillus nidulans. Mutation research, 138:33-38.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, Carere A
(1988) Induction of chromosome malsegregation by halogenated
organic solvents in Aspergillus nidulans: unspecific or
specific mechanism? Mutation research, 201:401-411.
Crespi CL, Seixas GM, Turner TR, Ryan CG, Penman BW (1985)
Mutagenicity of 1,2-dichloroethane and 1,2-dibromoethane in two
human lymphoblastoid cell lines. Mutation research,
142:133-140.
Daft JL (1988) Rapid determination of fumigant and industrial
chemical residues in food. Journal of the Association of
Official Analytical Chemists, 71(4):748-760.
Dilling WL, Tefertiller NB, Kalos GJ (1975) Evaporation rates and
reactivities of methylene chloride, chloroform,
1,1,1-trichloroethane, trichloroethylene, tetrachloroethylene,
and other chlorinated compounds in dilute aqueous solutions.
Environmental science and technology, 9(9):833-838.
Dorndorf W, Kresse M, Christian W, Katritzki KG (1975)
[Dichloroethane poisoning with myoclonic syndrome, epileptic
attacks, and irreversible cerebral effects.] Archiv für
Psychiatrie und Nervenkrankheiten, 220:373-379 (in German).
Duprat P, Delsaut L, Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. European journal of toxicology,
9:171-177.
Ecobichon DJ, Allen MC (1990) New Brunswick - Water Quality
Surveillance Program, New Brunswick public water supplies,
data summary report 1990. New Brunswick Department of Health
and Community Services.
Environment Agency Japan (1993) Chemicals in the environment -
1993. Tokyo.
Enviro-Test Laboratories (1991) Cayley background study:
analysis of food products for target organic and inorganic
parameters. Edmonton (Report No. 91-E1208).
Enviro-Test Laboratories (1992) Windsor area background study:
analysis of food products for target organic and inorganic
parameters. Edmonton (Report No. E1052).
Fellin P, Barnett SE, Tran QA (1992) Results of a national
pilot survey of airborne volatile organic compounds in
Canadian residences. Vol. 1. Prepared by Concord Environmental
Corporation for Health and Welfare Canada, Ottawa, Ontario.
Ferreri AM, Rocchi P, Capucci A, Prodi G (1983) Induction of
diphtheria toxin-resistant mutants in human cells by halogenated
compounds. Journal of cancer research and clinical oncology,
105:111-112.
Freiria-Gandara MJ, Lorenzo-Ferreira RA, Alvarez-Devesa A,
Bermejo F (1992) Occurrence of halogenated hydrocarbons in the
water supply of different cities of Galicia (Spain).
Environmental technology, 13:437-447.
Freitag D, Ballhorn L, Behechti A, Fischer K, Thumm W (1994)
Structural configuration and toxicity of chlorinated alkanes.
Chemosphere, 28(2):253-259.
Fujii T (1977) Direct aqueous injection gas chromatography-mass
spectrometry for analysis of organohalides in water at
concentrations below the parts per billion level. Journal of
chromatography, 139:297-302.
Giri AK, Que Hee SS (1988) In vitro sister chromatid exchange
induced by 1,2-dichloroethane on bone marrow cells of mice.
Environmental and molecular mutagenesis, 12:331-334.
Gocke E, Wild D, Eckhardt K, King MT (1983) Mutagenicity studies
with the mouse spot test. Mutation research, 117:201-212.
Guengerich FP, Crawford WM, Domoradzki JY, MacDonald TL, Watanabe
PG (1980) In vitro activation of 1,2-dichloroethane by
microsomal and cytosolic enzyme. Toxicology and applied
pharmacology, 55:303-317.
Guicherit R, Schulting FL (1985) The occurrence of organic
chemicals in the atmosphere of the Netherlands. The science of
the total environment, 43:193-219.
Hatch GG, Mamay PD, Ayer ML, Casto BC, Nesnow W (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo
cells by gaseous and volatile chlorinated methanes and ethanes.
Cancer research, 43:1945-1950.
Heikes DL (1987) Pesticide and industrial chemical residues -
purge and trap method for determination of volatile halocarbons
and carbon disulfide in table-ready foods. Journal of the
Association of Official Analytical Chemists, 70(2):215-226.
Heikes DL (1990) Environmental contaminants in table-ready foods
from the Total Diet Program of the Food and Drug Administration.
In: Food contamination from environmental sources. New York,
NY, John Wiley and Sons, pp. 31-57 (Advances in Environmental
Science and Technology Series No. 23).
Hellman B, Brandt I (1986) Effects of carcinogenic halogenated
aliphatic hydrocarbons on [3H]thymidine incorporation into
various organs of the mouse. A comparison between
1,2-dibromoethane and 1,2-dichloroethane. Mutation research,
163:193-199.
Hemminki K, Falck K, Vaino H (1980) Comparison of alkylation
rates and mutagenicity of directly acting industrial and
laboratory chemicals: epoxides, glycidyl ethers, methylating and
ethylating agents, halogenated hydrocarbons, hydrazine
derivatives, aldehydes, thiouram and dithiocarbamate derivatives.
Archives of toxicology, 46:277-285.
Heppel LA, Neal PA, Perrin TL, Endicott KM, Porterfield VT (1945)
The toxicology of 1,2-dichloroethane (ethylene dichloride). III.
Its acute toxicity and the effect of protective agents.
Journal of pharmacology and experimental therapeutics,
84:53-63.
Heppel LA, Neal PA, Perrin TL, Endicott KM, Porterfield VT (1946)
The toxicology of 1,2-dichloroethane (ethylene dichloride). V.
The effects of daily inhalations. Journal of industrial
hygiene and toxicology, 28(4):113-120.
Hinkel GK (1965) [Oral dichloroethane intoxication of children.]
Deutsche Gesundheitswesen, 20:1327-1331 (in German).
Hofmann HT, Birnsteil H, Jobst P (1971) Zur inhalations toxicitat
von 1,1- und 1,2-dichloroathan. [The inhalation toxicity of 1,1-
and 1,2-dichloroethane.] Archiv für Toxikologie, 27:248-265.
IARC (1979) Some halogenated hydrocarbons. Lyon, International
Agency for Research on Cancer (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Vol. 20).
Inskeep PB, Koga N, Cmarik JL, Guengerich FP (1986) Covalent
binding of 1,2-dihaloalkanes to DNA and stability of the major
DNA adduct, S-[2[(N7-guanyl)ethyl]glutathione. Cancer
research, 46:2839-2844.
IPCS (1993) International Chemical Safety Card -
1,2-Dichloroethane. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 0250).
IPCS (1994) Environmental health criteria for assessing health
risks of chemicals: derivation of guidance values for
health-based exposure limits. Geneva, World Health
Organization, International Programme on Chemical Safety.
IPCS (1995) 1,2-Dichloroethane. Geneva, World Health
Organization, International Programme on Chemical Safety
(Environmental Health Criteria No. 176).
Isacson P, Bean JA, Splinter R, Olson DB, Kohler J (1985)
Drinking water and cancer incidence in Iowa: III. Association of
cancer with indices of contamination. American journal of
epidemiology, 121(6):856-869.
Jakobson I, Wahlberg JE, Holmberg B, Johansson G (1982) Uptake
via the blood and elimination of 10 organic solvents following
epicutaneous exposure of anesthetized guinea pigs. Toxicology
and applied pharmacology, 63:181-187.
Jenssen D, Ramel C (1980) The micronucleus test as part of a
short-term mutagenicity test programme for the prediction of
carcinogenicity evaluated by 43 agents tested. Mutation
research, 75:191-202.
Kaiser KLE, Comba ME (1986) Volatile hydrocarbon contaminant
survey of the St. Clair River. Water pollution research
journal of Canada, 21(3):323-331.
Kaiser KLE, Comba ME, Huneault H (1983) Volatile hydrocarbon
contaminants in the Niagara River and in Lake Ontario.
Journal of Great Lakes research, 9(2):212-223.
Kanada T, Uyeta M (1978) Mutagenicity screening of organic
solvents in microbial systems. Mutation research, 54:215.
Kavlock R, Chernoff N, Carver B, Kopfler F (1979) Teratology
studies in mice exposed to municipal drinking water concentrates
during organogenesis. Food and cosmetics toxicology,
17:343-347.
King M-T, Beikirch H, Eckhardt K, Gocke E, Wild D (1979)
Mutagenicity studies with X-ray-contrast media, analgesics,
antipyretics, antirheumatics and some other pharmaceutical drugs
in bacterial, Drosophila and mammalian test systems.
Mutation research, 66:33-43.
Klaunig JE, Ruch RJ, Pereira MA (1986) Carcinogenicity of
chlorinated methane and ethane compounds administered in drinking
water to mice. Environmental health perspectives, 69:89-95.
Kliest J, Fast T, Boley JSM, van de Wiel H, Bloemen H (1989) The
relationship between soil contaminated with volatile organic
compounds and indoor air pollution. Environment international,
15:419-525.
Koga N, Inskeep PB, Harris TM, Guengerich FP (1986)
S-[2-(N7-guanyl)ethyl] glutathione, the major DNA
adduct formed from 1,2-dibromoethane. Biochemistry, 25:2192-2198.
Kramers PG, Mout HC, Bissumbhar B, Mulder CR (1991) Inhalation
exposure in Drosophila mutagenesis assays: experiments with
aliphatic halogenated hydrocarbons, with emphasis on the genetic
activity profile of 1,2-dichloroethane. Mutation research,
252:17-33.
Kronevi T, Wahlberg JE, Holmberg B (1981) Skin pathology
following epicutaneous exposure to seven organic solvents.
International journal of tissue reactions, 3:21-30.
Kuwabara T, Quevedo AR, Cogan DG (1968) An experimental study of
dichloroethane poisoning. Archives of ophthalmology,
79:321-330.
Lane RW, Riddle BL, Borzelleca JF (1982) Effects of
1,2-dichloroethane and 1,1,1-trichloroethane in drinking water on
reproduction and development in mice. Toxicology and applied
pharmacology, 63:409-421.
Larionov VG, Kokarovtseva MG (1976) Morphological constitution of
peripheral blood in intoxication with dichloroethane and its
metabolites. In: Actual problems of pesticide application in
different climatographic zones. Yerevan, Aiastan Publishers,
pp. 131-133.
Leblanc GA (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bulletin of environmental contamination
and toxicology, 24:684-691.
Letkiewicz F, Johnston P, Coleman J (1982) Occurrence of
1,2-dichloroethane in drinking water, food and air. Prepared by
JRB Associates, Washington DC, under Contract No. 68-01-6185 for
the US Environmental Protection Agency.
Lin ELC, Mattox JK, Pereira MA (1985) Glutathione plus
cytosol- and microsome-mediated binding of 1,2-dichloroethane to
polynucleotides. Toxicology and applied pharmacology,
78:428-435.
Lum KR, Kaiser KLE (1986) Organic and inorganic contaminants in
the St. Lawrence River: some preliminary results on their
distribution. Water pollution research journal of Canada,
21(4):592-603.
Maltoni C, Valgimigli L, Scarnato C (1980) Long-term carcinogenic
bioassays on ethylene dichloride administered by inhalation to
rats and mice. In: Ames BN, Infante P, Reitz R, eds. Ethylene
dichloride: a potential health risk? Cold Spring Harbor, NY,
Cold Spring Harbor Laboratory, pp. 3-33 (Banbury Report No. 5).
McCollister DD, Hollingsworth RL, Oyen F, Rowe VK (1956)
Comparative inhalation toxicity of fumigant mixtures. Individual
and joint effect of ethylene dichloride, carbon tetrachloride,
and ethylene dibromide. Archives of industrial health, 13:1-7.
Milman HA, Storey DL, Riccio ES, Sivak A, Tu AS, Williams GM,
Tong C, Tyson CA (1988) Rat liver foci and in vitro assays to
detect initiating and promoting effects of chlorinated ethanes
and ethylenes. Annals of the New York Academy of Sciences,
534:521-530.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, Shirasu Y
(1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation research, 116:185-216.
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, White
KL Jr (1982) In vivo assessment of immunotoxicity.
Environmental health perspectives, 43:41-52.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity. Bethesda, MD, US Department of Health,
Education and Welfare, Public Health Services, National
Institutes of Health, National Cancer Institute (HEW (NIH)
Publication No. 78-1305).
Nestmann ER, Lee EGH, Matula TI, Douglas GR, Mueller JC (1980)
Mutagenicity of constituents identified in pulp mill effluents
using Salmonella/mammalian-microsome assay. Mutation
research, 79:203-212.
NIOSH (1994a) Registry of toxic effects of chemical
substances. Cincinnati, OH, National Institute for Occupational
Safety and Health (last revision May 1994).
NIOSH (1994b) NIOSH pocket guide to chemical hazards. US
Department of Health and Human Services, Public Health Service,
Center for Disease Control and Prevention, National Institute for
Occupational Safety and Health, June.
Nouchi T, Miura H, Kanayama M, Mizuguchi O, Takano T (1984) Fatal
intoxication by 1,2-dichloroethane - A case report.
International archives of occupational and environmental
health, 54:111-113.
NTP (1991) Toxicity studies of 1,2-dichloroethane (ethylene
dichloride) in F344/N rats, Sprague Dawley rats,
Osborne-Mendel rats, and B6C3F1 mice (drinking water and
gavage studies). Research Triangle Park, NC, National
Institutes of Health, National Toxicology Program (NIH
Publication No. 91-3123).
Nylander P-O, Olofsson H, Rasmuson B, Svahlin H (1978) Mutagenic
effects of petrol in Drosophila melanogaster. I. Effects of
benzene and 1,2-dichloroethane. Mutation research, 57:163-167.
Otson R (1987) Purgeable organics in Great Lakes raw and treated
water. International journal of environmental analytical
chemistry, 31(1):41-53.
Pearson CR, McConnell G (1975) Chlorinated C1 and C2
hydrocarbons in the marine environment. Proceedings of the
Royal Society of London B, 189:305-332.
Perocco P, Prodi G (1981) DNA damage by haloalkanes in human
lymphocytes cultured in vitro. Cancer letters, 13:213-218.
Principe P, Dogliotti E, Bignami M, Crebelli R, Falcone E,
Fabrizi M, Conti G, Comba P (1981) Mutagenicity of chemicals of
industrial and agricultural relevance in Salmonella,
Streptomyces, and Aspergillus. Journal of the science of
food and agriculture, 32:826-832.
Prodi G, Arfelini G, Colacci A, Grilli S, Mazzulo M (1986)
Interaction of halocompounds with nucleic acids. Toxicologic
pathology, 14:438-444.
Rannug U (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichloroethane. Mutation research, 76:269-295.
Rannug U, Beije B (1979) The mutagenic effect of
1,2-dichloro-ethane on Salmonella typhimurium. II. Activation
by the isolated perfused rat liver. Chemico-biological
interactions, 24:265-285.
Rannug U, Sundvall A, Ramel C (1978) The mutagenic effect
of 1,2-dichloroethane on Salmonella typhimurium. I. Activation
through conjugation with glutathione in vitro.
Chemico-biological interactions, 20:1-16.
Rao KS, Murray JS, Deacon MM, John JA, Calhoun LL, Young JT
(1980) Teratogenicity and reproduction studies in animals
inhaling ethylene dichloride. In: Ames BN, Infante P, Reitz R,
eds. Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 149-166 (Banbury
Report No. 5).
Reitz RH, Fox TR, Ramsey JC, Quast JF, Langvardt PW, Watanabe PG
(1982) Pharmacokinetics and macro-molecular interactions of
ethylene dichloride in rats after inhalation or gavage.
Toxicology and applied pharmacology, 62:190-204.
Richter JE, Peterson SF, Kleiner CF (1983) Acute and chronic
toxicity of some chlorinated benzenes, chlorinated ethanes, and
tetrachloroethylene to Daphnia magna. Archives of
environmental contamination and toxicology, 12:679-684.
Roldan-Arjona T, Garcia-Pedrajas MD, Luque-Romero RL, Hera C,
Pueyo C (1991) An association between mutagenicity of the Ara
test of Salmonella typhimurium and carcinogenicity in rodents
for 16 halogenated aliphatic hydrocarbons. Mutagenesis,
6:199-205.
Romert L, Magnusson J, Ramel C (1990) The importance of
glutathione and glutathione transferase for somatic mutations in
Drosophila melanogaster induced in vivo by
1,2-dichloroethane. Carcinogenesis, 11:1399-1402.
Schultz K, Ghosh L, Banerjee S (1992) Neoplastic expression in
murine cells induced by halogenated hydrocarbons. In vitro
cellular and developmental biology, 28A:267-272.
Sherwood RL, O'Shea PT, Thomas W, Ratajczak HV, Aranyi C, Graham
JA (1987) Effects of inhalation of ethylene dichloride on
pulmonary defences of mice and rats. Toxicology and applied
pharmacology, 91:491-496.
Shmuter LM (1977) [Effects of chronic exposure to low
concentrations of chlorinated hydrocarbons of the ethane series on
specific and non-specific reactivity of animals in vivo.]
Gigiena Truda i Professional'nye Zabolevaniya, 8:38-42 (in
Russian).
Simmon VF (1980) Review of non-bacterial tests of the genotoxic
activity of ethylene dichloride. In: Ames BN, Infante P, Reitz R,
eds. Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 97-103 (Banbury
Report No. 5).
Simula TP, Glancey MG, Wolf CR (1993) Human glutathione
S-transferase-expressing Salmonella typhimurium tester
strains to study the activation/detoxification of mutagenic
compounds: studies with halogenated compounds, aromatic amines
and aflatoxin B1. Carcinogenesis, 14:1371-1376.
Singh HB, Salas LJ, Smith AJ, Shigeishi H (1980) Atmospheric
measurements of selected toxic organic chemicals. Research
Triangle Park, NC, US Environmental Protection Agency,
Environmental Sciences Research Laboratory (EPA-600/3-80-072;
PB80-198989).
Singh HB, Salas LJ, Smith AJ, Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban
environments. Atmospheric environment, 15:601-612.
Singh HB, Salas LF, Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environmental science and technology, 16:872-880.
Smyth HF Jr (1969) Acute toxicity of ethyl compounds. In: Spector
WC, ed. Handbook of toxicology. Vol. 1. Philadelphia, PA, W.B.
Saunders Company.
Spence JW, Hanst PL (1978) Oxidation of chlorinated ethanes.
Journal of the Air Pollution Control Association,
28(3):250-253.
Spencer HC, Rowe VK, Adams EM, McCollister DD, Irish DD (1951)
Vapor toxicity of ethylene dichloride determined by experiments
on laboratory animals. American Medical Association archives
of industrial hygiene and occupational medicine, 4:482-493.
Spreafico F, Zuccato E, Marcucci F, Sironi M, Paglialunga S,
Madonna M, Mussini E (1980) Pharmacokinetics of ethylene
dichloride in rats treated by different routes and its long-term
inhalatory toxicity. In: Ames BN, Infante P, Reitz R, eds.
Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 107-133 (Banbury
Report No. 5).
Storer RD, Conolly RB (1983) Comparative in vivo genotoxicity
and acute hepatotoxicity of three 1,2-dihaloethanes.
Carcinogenesis, 4:1491-1494.
Storer RD, Conolly RB (1985) An investigation of the role of
microsomal oxidative metabolism in the in vivo genotoxicity of
1,2-dichloroethane. Toxicology and applied pharmacology,
77:36-46.
Storer RD, Jackson NM, Conolly RB (1984) In vivo genotoxicity
and acute hepatotoxicity of 1,2-dichloroethane in mice:
comparison of oral, intraperitoneal, and inhalation routes of
exposure. Cancer research, 44:4267-4271.
Story DL, Meierhenry EF, Tyson CA, Milman HA (1986) Differences
in rat liver enzyme-positive foci produced by chlorinated
aliphatics and phenobarbital. Toxicology and industrial
health, 2:351-362.
Strobel K, Grummt T (1987) Aliphatic and aromatic halocarbons as
potential mutagens in drinking water. III. Halogenated ethanes
and ethenes. Toxicology and environmental chemistry,
15:101-128.
Sundheimer DW, White RD, Brendel K, Sipes IC (1982) The
bioactivation of 1,2-dibromoethane in rat hepatocytes: covalent
binding to nucleic acids. Carcinogenesis, 3:1129-1133.
Suveev IM, Babichenko ME (1969) [On the clinic and cure of acute
intoxication with dichloroethane vapours.] Gigiena Truda i
Professional'nye Zabolevaniya, 13:50-51 (in Russian).
Tan E-L, Hsie AW (1981) Mutagenicity and cytotoxicity of
haloethanes as studied in the CHO/HGPRT system. Mutation
research, 90:183-191.
Taningher M, Parodi S, Grilli S, Colacci A, Mazzullo M, Bordone
R, Santi L (1991) Lack of correlation between alkaline DNA
fragmentation and DNA covalent binding induced by
polychloroethanes after in vivo administration. Problems
related to the assessment of a carcinogenic hazard. Cancer
detection and prevention, 15:35-39.
Theiss JC, Stoner GD, Shimkin MB, Weisberger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer
research, 37:2717-2720.
Tu AS, Murray TA, Hatch KM, Sivak A, Milman HA (1985) In vitro
transformation of BALB/c-3T3 cells by chlorinated ethanes and
ethylenes. Cancer letters, 28:85-92.
US EPA (1985) Health assessment document for
1,2-dichloroethane (ethylene dichloride). Final report.
Washington, DC, US Environmental Protection Agency
(EPA/600/8-84/006F; NTIS PB86-122702).
US EPA (1992) Indoor air quality data base for organic
compounds. Research Triangle Park, NC, US Environmental
Protection Agency (EPA-600-R-92-025; NTIS PB92-158468).
Valencia R, Abrahamson S, Lee WR, von Halle ES, Woodruff RC,
Wurgler FE, Zimmering S (1984) Chromosome mutations for
mutagenesis in Drosophila melanogaster: a report of the US
Environmental Protection Agency Gene-Tox Program. Mutation
research, 134:61-88.
van Bladeren PJ, Breimer DD, Rotteveel-Smijs RMT, de Knijff P,
Mohn GV, van Meeteren-Walchli B, Buijs W, van der Gen A (1981)
The relation between the structure of vicinal dihalogen compounds
and their mutagenic activation via conjugation to glutathione.
Carcinogenesis, 2:499-505.
van Duuren BL, Goldschmidt BM, Loewengart G, Smith A, Mechionne
S, Seldman I, Roth D (1979) Carcinogenicity of halogenated
olefinic and aliphatic hydrocarbons in mice. Journal of the
National Cancer Institute, 63:1433-1439.
van Esch GJ, Kroes R, van Logten MJ, den Tonkelaar EM (1977)
Ninety-day toxicity study with 1,2-dichloroethane (DCE) in
rats. Bilthoven, National Institute of Public Health and
Environmental Hygiene (Report 195/77 Al. Tox.).
Vozovaya M (1977) [The effect of dichloroethane on the sexual
cycle and embryogenesis of experimental animals.] Akusherstvo
i Ginekologiya (Moscow), 2:57-59 (in Russian).
WHO (1971) Evaluation of food additives: specifications for
the identity and purity of food additives and their
toxicological evaluation: some extraction solvents and
certain other substances; a review of the technological
efficacy of some antimicrobial agents. Fourteenth report of
the Expert Committee. Geneva, World Health Organization (WHO
Technical Report Series No. 462).
WHO (1980) Evaluation of certain food additives.
Twenty-third report of the Joint FAO/WHO Expert Committee
on Food Additives. Geneva, World Health Organization (WHO
Technical Report Series No. 648 and corrigenda).
WHO (1992) Evaluation of certain food additives and
naturally occurring toxicants. Thirty-ninth report of the
Joint FAO/WHO Expert Committee on Food Additives. Geneva, World
Health Organization (WHO Technical Report Series No. 828).
WHO (1993) Guidelines for drinking-water quality, 2nd ed.
Vol. 1. Recommendations. Geneva, World Health Organization.
Williams GM, Mori H, McQueen CA (1989) Structure-activity
relationships in the rat hepatocyte DNA-repair test for 300
chemicals. Mutation research, 221:263-286.
Zamora PO, Benson JM, Li AP, Brooks AL (1983) Evaluation of an
exposure system using cells grown on collagen gels for detecting
highly volatile mutagens in the CHO/HGPRT mutation assay.
Environmental mutagenesis, 5:795-801.
APPENDIX 1 - SOURCE DOCUMENTS
International Programme on Chemical Safety - Environmental Health
Criteria Monograph No. 176 (1995)
Copies of the Environmental Health Criteria document on
1,2-dichloroethane, prepared by the International Programme on
Chemical Safety, as well as the Health and Safety Guide (1991)
and the International Chemical Safety Card (1993), may be
obtained from:
International Programme on Chemical Safety
World Health Organization
Geneva, Switzerland
The first draft of the monograph, prepared by Ms K. Hughes
of the Environmental Health Directorate, Health Canada, was
circulated to IPCS Contact Points (approximately 150 government,
industrial, academic, and independent organizations and
individuals) for comment in June 1994. The second draft, revised
on the basis of the comments received, was also prepared by Ms K.
Hughes. Dr E. Smith and Dr P.G. Jenkins, both members of the
IPCS Central Unit, were responsible for the scientific content
and technical editing, respectively.
The monograph on 1,2-dichloroethane was finalized by the
Core Assessment Group (CAG) of the Joint Meeting on Pesticides
(JMP), which met in Geneva from 25 October to 3 November 1994.
The Core Assessment Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health
and the environment from exposure to 1,2-dichloroethane.
Participants at the Core Assessment Group meeting were:
Dr T. Bailey, Ecological Effects Branch, Environmental Fate
and Effects Division, US Environmental Protection Agency,
Washington, DC, USA
Dr A.L. Black, Department of Human Services and Health,
Canberra, ACT, Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairperson)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution,
London, United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, Office of Pesticide Programs, US
Environmental Protection Agency, Washington, DC, USA
Dr R. Hailey, National Institute of Environmental Health
Sciences, National Institutes of Health, Public Health
Service, Department of Health and Human Services, Research
Triangle Park, NC, USA
Ms K. Hughes, Priority Substances Section, Environmental
Health Directorate, Health Canada, Ottawa, Ontario, Canada
(EHC Joint Rapporteur)
Dr D. Kanungo, Division of Medical Toxicology, Central
Insecticides Laboratory, Government of India, Ministry of
Agriculture & Cooperation, Directorate of Plant Protection,
Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group
Ltd, Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Fontwell, Arundel,
West Sussex, United Kingdom
Professor M. Lotti, Institute of Occupational Medicine,
University of Padua, Padua, Italy (Chairperson)
Dr D.R. Mattison, University of Pittsburgh, Graduate School
of Public Health, Pittsburgh, PA, USA
Dr J. Sekizawa, Division of Information on Chemical Safety,
National Institute of Health Sciences, Setagaya-ku, Tokyo,
Japan
Dr P. Sinhaseni, Department of Pharmacology, Chulalongkorn
University, Bangkok, Thailand
Dr S.A. Soliman, Pesticide Chemicals & Toxicology, King Saud
University, Bureidah, Saudi Arabia
Dr M. Tasheva, Department of Toxicology, National Center of
Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Department of Toxicology, Wageningen
Agricultural Unviersity, Wageningen, The Netherlands
Dr M.I. Willems, Department of Occupational Toxicology, TNO
Nutrition and Food Research Institute, AJ Zeist, The
Netherlands
Procedures for the preparation of an Environmental Health
Criteria document
The order of procedures that result in the publication of an
EHC monograph is shown in the flow chart. A designated staff
member of IPCS, responsible for the scientific quality of the
document, serves as Responsible Officer (RO). The IPCS Editor is
responsible for layout and language. The first draft, prepared
by consultants or, more usually, staff from an IPCS Participating
Institution, is based initially on data provided from the
International Register of Potentially Toxic Chemicals and
reference databases such as Medline and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its
scientific quality and objectivity. Once the RO finds the
document acceptable as a first draft, it is distributed, in its
unedited form, to well over 150 EHC Contact Points throughout the
world who are asked to comment on its completeness and accuracy
and, where necessary, provide additional material. The Contact
Points, usually designated by governments, may be Participating
Institutions, IPCS Focal Points, or individual scientists known
for their particular expertise. Generally, some four months are
allowed before the comments are considered by the RO and
author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not
as representatives of any organization, government, or industry.
Their function is to evaluate the accuracy, significance, and
relevance of the information in the document and to assess the
health and environmental risks from exposure to the chemical. A
summary and recommendations for further research and improved
safety aspects are also required. The composition of the Task
Group is dictated by the range of expertise required for the
subject of the meeting and by the need for a balanced
geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international
associations may be invited to join the Task Group as observers.
While observers may provide a valuable contribution to the
process, they can speak only at the invitation of the
Chairperson. Observers do not participate in the final
evaluation of the chemical; this is the sole responsibility of
the Task Group members. When the Task Group considers it to be
appropriate, it may meet in camera.
All individuals who as authors, consultants, or advisers
participate in the preparation of the EHC monograph must, in
addition to serving in their personal capacity as scientists,
inform the RO if at any time a conflict of interest, whether
actual or potential, could be perceived in their work. They are
required to sign a conflict of interest statement. Such a
procedure ensures the transparency and probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of
the document, it then goes for language editing, reference
checking, and preparation of camera-ready copy. After approval
by the Director, IPCS, the monograph is submitted to the WHO
Office of Publications for printing. At this time, a copy of the
final draft is sent to the Chairperson and Rapporteur of the Task
Group to check for any errors.
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Highly flammable. Gives off No open flames, NO sparks, Powder, water spray, foam,
irritating or toxic fumes (or and NO smoking. carbon dioxide.
gases) in a fire).
EXPLOSION Vapour/air mixtures are Closed system, ventilation, In case of fire: keep drums,
explosive. explosion-proof electrical etc., cool by spraying with
equipment and lighting. water.
Prevent build-up of
electrostatic charges (e.g.,
by grounding) (see Notes).
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTONS FIRE FIGHTING
EXPOSURE
EXPOSURE AVOID ALL CONTACT! IN ALL CASES CONSULT A
DOCTOR!
* INHALATION Abdominal pain. Cough. Ventilation, local exhaust, Fresh air, rest. Half-upright
Dizziness. Drowsiness. or breathing protection. position. Artificial
Headache. Nausea. Sore respiration if indicated.
throat. Unconsciousness. Refer for medical attention.
Vomiting. Symptoms may be
delayed (see Notes).
* SKIN Redness. Protective gloves. Remove contaminated clothes.
Rinse and then wash skin with
water and soap. Refer for
medical attention.
* EYES Redness. Pain. Blurred Safety goggles, face shield, First rinse with plenty of
vision. or eye protection in water for several minutes
combination with breathing (remove contact lenses if
protection. easily possible), then take
to a doctor.
* INGESTION Abdominal cramps. Diarrhoea Do no eat, drink, or smoke Give nothing to drink. Refer
(further see Inhalation). during work. Wash hands for medical attention.
before eating.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Evacuate danger area! Collect leaking Fireproof. Separated from strong Unbreakable packaging; put breakable
and spilled liquid in sealable oxidants, food and feedstuffs and packaging into closed unbreakable
containers as far as possible. Absorb other incompatible substances (see container. Do not transport with food
remaining liquid in sand or inert Chemical Dangers). Cool. Dry. and feedstuffs.
absorbent and remove to safe place. Do F symbol
NOT wash away into sewer (extra Y symbol
personal protection: self-contained R: 45-11-22-36/37/38
breathing apparatus). S: 53-45
Note: E
UN Hazard Class: 3
UN Subsidiary Risks: 6.1
UN Packing Group: 11
Marine Pollutant.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: INHALATION RISK:
COLOURLESS, VISCOUS LIQUID, WITH A harmful contamination of the air can be
CHARACTERISTIC ODOUR, TURNS DARK ON EXPOSURE reached very quickly on evaporation of this
TO AIR, MOISTURE AND LIGHT substance at 20°C.
PHYSICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The vapour is heavier than air and may travel The vapour irritates the eyes, the skin and
along the ground; distant ignition possible. the respiratory tract. Inhalation of the
As a result of flow, agitation, etc., vapour may cause lung oedema (see Notes). The
electrostatic charges can be generated. substance may cause effects on the central
nervous system, kidneys, liver, resulting in
CHEMICAL DANGERS: impaired functions.
The substance decomposes on heating and on EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
burning producing toxic and corrosive fumes
including hydrogen chloride (ICSC #0163) and Repeated or prolonged contact with skin may
phosgene (ICSC #0007). Reacts violently with cause dermatitis. This substance is probably
aluminium, alkali metals, alkali amides, carcinogenic to humans.
ammonia bases, strong oxidants. Attacks many
metals in presence of water. Attacks plastic.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV: 10 ppm; 40 mg/m3 (as TWA)
(ACGIH 1994-1995).
ROUTES OF EXPOSURE:
The substance can be absorbed into the body by
inhalation of its vapour, through the skin and
by ingestion.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 83.5°C Flash point: 13°C c.c.
PROPERTIES Melting point: -35.7°C Auto-ignition temperature: 413°C
Relative density (water = 1): 1.235 Explosive limits, vol% in air: 6.2-16
Solubility in water, g/100 ml: 0.87 Octanol/water partition coefficient
Vapour pressure, kPa at 20°C: 8.7 as logPow: 1.48
Relative vapour density (air = 1): 3.42
Relative density of the vapour/
air-mixture at 20°C (air = 1): 1.2
ENVIRONMENTAL
DATA
NOTES
Depending on the degree of exposure, periodic medical examination is indicated.
The symptoms of lung oedema often do not become manifest until a few hours have passed and they are aggravated by physical effort.
Rest and medical observation are tehrefore essential.
Immediate administration of an appropriate spray, by a doctor or a person authorized by him/her, should be considered.
EXPLOSION/PREVENTION: Do NOT use compressed air for filling, discharging, or handling.
ICSC: 0250 1.1 Transport Emergency Card: TEC (R)-605
NFPA Code: H 2; F 3; R 0
REFERENCES
Alumot E, Nachtomi E, Mandel E, Holstein P (1976) Tolerance and
acceptable daily intake of chlorinated fumigants in the rat diet.
Food and cosmetics toxicology, 14:105-110.
Andriukin AA (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klinicheskaya Meditsina (Moscow),
57:43-47 (in Russian).
Arfellini G, Bartoli S, Colacci A, Mazzullo M, Galli MC, Prodi G,
Grilli S (1984) In vivo and in vitro binding of
1,2-dibromoethane and 1,2-dichloroethane to macromolecules in rat
and mouse organs. Journal of cancer research and clinical
oncology, 108:204-213.
Armstrong MJ, Galloway SM (1993) Micronuclei induced in
peripheral blood of Eµ-PIM-1 transgenic mice by chronic oral
treatment with 2-acetylaminofluorene or benzene but not with
diethylnitrosamine or 1,2-dichloroethane. Mutation research,
302:61-70.
ATSDR (1992) Toxicological profile for 1,2-dichloroethane
(draft). Atlanta, GA, US Department of Health and Human Services,
Public Health Service, Agency for Toxic Substances and Disease
Registry.
Austin SG, Schnatter AR (1983) A case-control study of chemistry
exposures and brain tumors in petrochemical workers. Journal
of occupational medicine, 25(4):313-320.
Baertsch A, Lutz WK, Schlatter C (1991) Effect of inhalation
exposure regimen on DNA binding potency of 1,2-dichloroethane in
the rat. Archives of toxicology, 65:169-176.
Ballering LAP, Nivard MJM, Vogel EW (1993) Characterization of
the genotoxic action of three structurally related
1,2-dihaloalkanes in Drosophila melanogaster. Mutation
research, 285:209-217.
Banerjee S (1988) DNA damage in rodent liver by
1,2-dichloroethane, a hepatocarcinogen. Cancer biochemistry
and biophysics, 10:165-173.
Banerjee S, van Duuren BL (1979) Binding of carcinogenic
halogenated hydrocarbons to cell macromolecules. Journal of
the National Cancer Institute, 63:707-711.
Banerjee S, van Duuren BL, Oruambo FI (1980) Microsome-mediated
covalent binding of 1,2-dichloroethane to lung microsomal protein
and salmon sperm DNA. Cancer research, 40:2170-2173.
Barber RD, Donish WH, Mueller KR (1981) A procedure for the
quantitative measurement of the mutagenicity of volatile liquids
in the Ames Salmonella microsome assay. Mutation research,
90:31-48.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizzari ED, Ray M, Smith D, Tomer KB, Wagner R, Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - A
multimedia environmental study. Biomedical and environmental
mass spectrometry, 7:139-147.
Barsoum GS, Saad K (1934) Relative toxicity of certain chlorine
derivatives of the aliphatic series. Quarterly journal of
pharmacy and pharmacology, 7: 205-214.
Benson LO, Teta MJ (1993) Mortality due to pancreatic and
lymphopoietic cancers in chlorohydrin production workers.
British journal of industrial medicine, 50:710-716.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, Bruser
DM (1982) The aquatic toxicity of organic compounds to
embryo-larval stages of fish and amphibians. Lexington, KY,
University of Kentucky (Research Report No. 133).
Blum DJW, Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons
and correlations. Research journal of the Water Pollution
Control Federation, 63(3):198-207.
Bonnet P, Francin J-M, Grakiski D, Raoult G, Zissu D (1980)
Détermination de la concentration léthale50 des principaux
hydrocarbures aliphatiques chlorés chez le rat. Archives des
maladies professionnelles de medecine du travail et de
securité sociale (Paris), 41(6-7):317-321.
Brem H, Stein AB, Rosenkranz HS (1974) The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer research,
34:2576-2579.
Bringmann G, Kühn R (1978) Testing of substances for their
toxicity threshold: model organisms Microcystis (Diplocystis)
aeruginosa and Scenedesmus quadricauda. Mitteilungen
Internationale Vereinigung für Theoretische und Angewandte
Limnologie, 21:275-284.
Bringmann G, Kühn R (1980) Comparison of the toxicity thresholds
of water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water research, 14:231-241.
Buijs W, van der Gen A, Mohn GR, Breimer DD (1984) The direct
mutagenic activity of alpha, omega-dihalogenoalkanes in
Salmonella typhimurium. Mutation research, 141:11-14.
Callaghan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR,
Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR,
Gould C (1979) Water-related fate of 129 priority pollutants.
Vol. II. Washington, DC, US Environmental Protection Agency
(EPA 40/4-79-029b).
Cheever KL, Cholakis JM, El-Hawari AM, Kovatch RM, Weisburger EK
(1990) Ethylene dichloride: the influence of disulfiram or
ethanol on oncogenicity, metabolism and DNA covalent binding in
rats. Fundamental and applied toxicology, 14:243-261.
Cheh AM, Hooper AB, Skochdopole J, Henke CA, McKinnell RG (1980)
A comparison of the ability of frog and rat S-9 to activate
promutagens in the Ames test. Environmental and molecular
mutagenesis, 2:487-508.
Chemical Marketing Reporter (1992) Chemical profile: ethylene
dichloride. Chemical Marketing Reporter magazine, 241(19):42.
Clark AI, McIntyre AE, Lester JN, Perry R (1984a) Ambient air
measurement of aromatic and halogenated hydrocarbons at urban,
rural, and motorway locations. The science of the total
environment, 39:265-279.
Clark AI, McIntyre AE, Perry R, Lester JN (1984b) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural, and motorway locations.
Environmental pollution series B, 7:141-158.
Comba ME, Kaiser KLE (1985) Volatile halocarbons in the Detroit
River and their relationship with contaminant sources. Journal
of Great Lakes research, 11(3):404-418.
CPI (1991) CPI product profiles: ethylene dichloride. Don
Mills, Ontario, Canadian Process Industries, Corpus Information
Services.
Crebelli R, Carere A (1988) Genotoxic activity of halogenated
aliphatic hydrocarbons in Aspergillus nidulans. Journal of
occupational toxicology, 8:437-442.
Crebelli R, Conti G, Conti L, Carere A (1984) Induction of
somatic segregation by halogenated aliphatic hydrocarbons in
Aspergillus nidulans. Mutation research, 138:33-38.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, Carere A
(1988) Induction of chromosome malsegregation by halogenated
organic solvents in Aspergillus nidulans: unspecific or
specific mechanism? Mutation research, 201:401-411.
Crespi CL, Seixas GM, Turner TR, Ryan CG, Penman BW (1985)
Mutagenicity of 1,2-dichloroethane and 1,2-dibromoethane in two
human lymphoblastoid cell lines. Mutation research,
142:133-140.
Daft JL (1988) Rapid determination of fumigant and industrial
chemical residues in food. Journal of the Association of
Official Analytical Chemists, 71(4):748-760.
Dilling WL, Tefertiller NB, Kalos GJ (1975) Evaporation rates and
reactivities of methylene chloride, chloroform,
1,1,1-trichloroethane, trichloroethylene, tetrachloroethylene,
and other chlorinated compounds in dilute aqueous solutions.
Environmental science and technology, 9(9):833-838.
Dorndorf W, Kresse M, Christian W, Katritzki KG (1975)
[Dichloroethane poisoning with myoclonic syndrome, epileptic
attacks, and irreversible cerebral effects.] Archiv für
Psychiatrie und Nervenkrankheiten, 220:373-379 (in German).
Duprat P, Delsaut L, Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. European journal of toxicology,
9:171-177.
Ecobichon DJ, Allen MC (1990) New Brunswick - Water Quality
Surveillance Program, New Brunswick public water supplies,
data summary report 1990. New Brunswick Department of Health
and Community Services.
Environment Agency Japan (1993) Chemicals in the environment -
1993. Tokyo.
Enviro-Test Laboratories (1991) Cayley background study:
analysis of food products for target organic and inorganic
parameters. Edmonton (Report No. 91-E1208).
Enviro-Test Laboratories (1992) Windsor area background study:
analysis of food products for target organic and inorganic
parameters. Edmonton (Report No. E1052).
Fellin P, Barnett SE, Tran QA (1992) Results of a national
pilot survey of airborne volatile organic compounds in
Canadian residences. Vol. 1. Prepared by Concord Environmental
Corporation for Health and Welfare Canada, Ottawa, Ontario.
Ferreri AM, Rocchi P, Capucci A, Prodi G (1983) Induction of
diphtheria toxin-resistant mutants in human cells by halogenated
compounds. Journal of cancer research and clinical oncology,
105:111-112.
Freiria-Gandara MJ, Lorenzo-Ferreira RA, Alvarez-Devesa A,
Bermejo F (1992) Occurrence of halogenated hydrocarbons in the
water supply of different cities of Galicia (Spain).
Environmental technology, 13:437-447.
Freitag D, Ballhorn L, Behechti A, Fischer K, Thumm W (1994)
Structural configuration and toxicity of chlorinated alkanes.
Chemosphere, 28(2):253-259.
Fujii T (1977) Direct aqueous injection gas chromatography-mass
spectrometry for analysis of organohalides in water at
concentrations below the parts per billion level. Journal of
chromatography, 139:297-302.
Giri AK, Que Hee SS (1988) In vitro sister chromatid exchange
induced by 1,2-dichloroethane on bone marrow cells of mice.
Environmental and molecular mutagenesis, 12:331-334.
Gocke E, Wild D, Eckhardt K, King MT (1983) Mutagenicity studies
with the mouse spot test. Mutation research, 117:201-212.
Guengerich FP, Crawford WM, Domoradzki JY, MacDonald TL, Watanabe
PG (1980) In vitro activation of 1,2-dichloroethane by
microsomal and cytosolic enzyme. Toxicology and applied
pharmacology, 55:303-317.
Guicherit R, Schulting FL (1985) The occurrence of organic
chemicals in the atmosphere of the Netherlands. The science of
the total environment, 43:193-219.
Hatch GG, Mamay PD, Ayer ML, Casto BC, Nesnow W (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo
cells by gaseous and volatile chlorinated methanes and ethanes.
Cancer research, 43:1945-1950.
Heikes DL (1987) Pesticide and industrial chemical residues -
purge and trap method for determination of volatile halocarbons
and carbon disulfide in table-ready foods. Journal of the
Association of Official Analytical Chemists, 70(2):215-226.
Heikes DL (1990) Environmental contaminants in table-ready foods
from the Total Diet Program of the Food and Drug Administration.
In: Food contamination from environmental sources. New York,
NY, John Wiley and Sons, pp. 31-57 (Advances in Environmental
Science and Technology Series No. 23).
Hellman B, Brandt I (1986) Effects of carcinogenic halogenated
aliphatic hydrocarbons on [3H]thymidine incorporation into
various organs of the mouse. A comparison between
1,2-dibromoethane and 1,2-dichloroethane. Mutation research,
163:193-199.
Hemminki K, Falck K, Vaino H (1980) Comparison of alkylation
rates and mutagenicity of directly acting industrial and
laboratory chemicals: epoxides, glycidyl ethers, methylating and
ethylating agents, halogenated hydrocarbons, hydrazine
derivatives, aldehydes, thiouram and dithiocarbamate derivatives.
Archives of toxicology, 46:277-285.
Heppel LA, Neal PA, Perrin TL, Endicott KM, Porterfield VT (1945)
The toxicology of 1,2-dichloroethane (ethylene dichloride). III.
Its acute toxicity and the effect of protective agents.
Journal of pharmacology and experimental therapeutics,
84:53-63.
Heppel LA, Neal PA, Perrin TL, Endicott KM, Porterfield VT (1946)
The toxicology of 1,2-dichloroethane (ethylene dichloride). V.
The effects of daily inhalations. Journal of industrial
hygiene and toxicology, 28(4):113-120.
Hinkel GK (1965) [Oral dichloroethane intoxication of children.]
Deutsche Gesundheitswesen, 20:1327-1331 (in German).
Hofmann HT, Birnsteil H, Jobst P (1971) Zur inhalations toxicitat
von 1,1- und 1,2-dichloroathan. [The inhalation toxicity of 1,1-
and 1,2-dichloroethane.] Archiv für Toxikologie, 27:248-265.
IARC (1979) Some halogenated hydrocarbons. Lyon, International
Agency for Research on Cancer (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Vol. 20).
Inskeep PB, Koga N, Cmarik JL, Guengerich FP (1986) Covalent
binding of 1,2-dihaloalkanes to DNA and stability of the major
DNA adduct, S-[2[(N7-guanyl)ethyl]glutathione. Cancer
research, 46:2839-2844.
IPCS (1993) International Chemical Safety Card -
1,2-Dichloroethane. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 0250).
IPCS (1994) Environmental health criteria for assessing health
risks of chemicals: derivation of guidance values for
health-based exposure limits. Geneva, World Health
Organization, International Programme on Chemical Safety.
IPCS (1995) 1,2-Dichloroethane. Geneva, World Health
Organization, International Programme on Chemical Safety
(Environmental Health Criteria No. 176).
Isacson P, Bean JA, Splinter R, Olson DB, Kohler J (1985)
Drinking water and cancer incidence in Iowa: III. Association of
cancer with indices of contamination. American journal of
epidemiology, 121(6):856-869.
Jakobson I, Wahlberg JE, Holmberg B, Johansson G (1982) Uptake
via the blood and elimination of 10 organic solvents following
epicutaneous exposure of anesthetized guinea pigs. Toxicology
and applied pharmacology, 63:181-187.
Jenssen D, Ramel C (1980) The micronucleus test as part of a
short-term mutagenicity test programme for the prediction of
carcinogenicity evaluated by 43 agents tested. Mutation
research, 75:191-202.
Kaiser KLE, Comba ME (1986) Volatile hydrocarbon contaminant
survey of the St. Clair River. Water pollution research
journal of Canada, 21(3):323-331.
Kaiser KLE, Comba ME, Huneault H (1983) Volatile hydrocarbon
contaminants in the Niagara River and in Lake Ontario.
Journal of Great Lakes research, 9(2):212-223.
Kanada T, Uyeta M (1978) Mutagenicity screening of organic
solvents in microbial systems. Mutation research, 54:215.
Kavlock R, Chernoff N, Carver B, Kopfler F (1979) Teratology
studies in mice exposed to municipal drinking water concentrates
during organogenesis. Food and cosmetics toxicology,
17:343-347.
King M-T, Beikirch H, Eckhardt K, Gocke E, Wild D (1979)
Mutagenicity studies with X-ray-contrast media, analgesics,
antipyretics, antirheumatics and some other pharmaceutical drugs
in bacterial, Drosophila and mammalian test systems.
Mutation research, 66:33-43.
Klaunig JE, Ruch RJ, Pereira MA (1986) Carcinogenicity of
chlorinated methane and ethane compounds administered in drinking
water to mice. Environmental health perspectives, 69:89-95.
Kliest J, Fast T, Boley JSM, van de Wiel H, Bloemen H (1989) The
relationship between soil contaminated with volatile organic
compounds and indoor air pollution. Environment international,
15:419-525.
Koga N, Inskeep PB, Harris TM, Guengerich FP (1986)
S-[2-(N7-guanyl)ethyl] glutathione, the major DNA
adduct formed from 1,2-dibromoethane. Biochemistry, 25:2192-2198.
Kramers PG, Mout HC, Bissumbhar B, Mulder CR (1991) Inhalation
exposure in Drosophila mutagenesis assays: experiments with
aliphatic halogenated hydrocarbons, with emphasis on the genetic
activity profile of 1,2-dichloroethane. Mutation research,
252:17-33.
Kronevi T, Wahlberg JE, Holmberg B (1981) Skin pathology
following epicutaneous exposure to seven organic solvents.
International journal of tissue reactions, 3:21-30.
Kuwabara T, Quevedo AR, Cogan DG (1968) An experimental study of
dichloroethane poisoning. Archives of ophthalmology,
79:321-330.
Lane RW, Riddle BL, Borzelleca JF (1982) Effects of
1,2-dichloroethane and 1,1,1-trichloroethane in drinking water on
reproduction and development in mice. Toxicology and applied
pharmacology, 63:409-421.
Larionov VG, Kokarovtseva MG (1976) Morphological constitution of
peripheral blood in intoxication with dichloroethane and its
metabolites. In: Actual problems of pesticide application in
different climatographic zones. Yerevan, Aiastan Publishers,
pp. 131-133.
Leblanc GA (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bulletin of environmental contamination
and toxicology, 24:684-691.
Letkiewicz F, Johnston P, Coleman J (1982) Occurrence of
1,2-dichloroethane in drinking water, food and air. Prepared by
JRB Associates, Washington DC, under Contract No. 68-01-6185 for
the US Environmental Protection Agency.
Lin ELC, Mattox JK, Pereira MA (1985) Glutathione plus
cytosol- and microsome-mediated binding of 1,2-dichloroethane to
polynucleotides. Toxicology and applied pharmacology,
78:428-435.
Lum KR, Kaiser KLE (1986) Organic and inorganic contaminants in
the St. Lawrence River: some preliminary results on their
distribution. Water pollution research journal of Canada,
21(4):592-603.
Maltoni C, Valgimigli L, Scarnato C (1980) Long-term carcinogenic
bioassays on ethylene dichloride administered by inhalation to
rats and mice. In: Ames BN, Infante P, Reitz R, eds. Ethylene
dichloride: a potential health risk? Cold Spring Harbor, NY,
Cold Spring Harbor Laboratory, pp. 3-33 (Banbury Report No. 5).
McCollister DD, Hollingsworth RL, Oyen F, Rowe VK (1956)
Comparative inhalation toxicity of fumigant mixtures. Individual
and joint effect of ethylene dichloride, carbon tetrachloride,
and ethylene dibromide. Archives of industrial health, 13:1-7.
Milman HA, Storey DL, Riccio ES, Sivak A, Tu AS, Williams GM,
Tong C, Tyson CA (1988) Rat liver foci and in vitro assays to
detect initiating and promoting effects of chlorinated ethanes
and ethylenes. Annals of the New York Academy of Sciences,
534:521-530.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, Shirasu Y
(1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation research, 116:185-216.
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, White
KL Jr (1982) In vivo assessment of immunotoxicity.
Environmental health perspectives, 43:41-52.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity. Bethesda, MD, US Department of Health,
Education and Welfare, Public Health Services, National
Institutes of Health, National Cancer Institute (HEW (NIH)
Publication No. 78-1305).
Nestmann ER, Lee EGH, Matula TI, Douglas GR, Mueller JC (1980)
Mutagenicity of constituents identified in pulp mill effluents
using Salmonella/mammalian-microsome assay. Mutation
research, 79:203-212.
NIOSH (1994a) Registry of toxic effects of chemical
substances. Cincinnati, OH, National Institute for Occupational
Safety and Health (last revision May 1994).
NIOSH (1994b) NIOSH pocket guide to chemical hazards. US
Department of Health and Human Services, Public Health Service,
Center for Disease Control and Prevention, National Institute for
Occupational Safety and Health, June.
Nouchi T, Miura H, Kanayama M, Mizuguchi O, Takano T (1984) Fatal
intoxication by 1,2-dichloroethane - A case report.
International archives of occupational and environmental
health, 54:111-113.
NTP (1991) Toxicity studies of 1,2-dichloroethane (ethylene
dichloride) in F344/N rats, Sprague Dawley rats,
Osborne-Mendel rats, and B6C3F1 mice (drinking water and
gavage studies). Research Triangle Park, NC, National
Institutes of Health, National Toxicology Program (NIH
Publication No. 91-3123).
Nylander P-O, Olofsson H, Rasmuson B, Svahlin H (1978) Mutagenic
effects of petrol in Drosophila melanogaster. I. Effects of
benzene and 1,2-dichloroethane. Mutation research, 57:163-167.
Otson R (1987) Purgeable organics in Great Lakes raw and treated
water. International journal of environmental analytical
chemistry, 31(1):41-53.
Pearson CR, McConnell G (1975) Chlorinated C1 and C2
hydrocarbons in the marine environment. Proceedings of the
Royal Society of London B, 189:305-332.
Perocco P, Prodi G (1981) DNA damage by haloalkanes in human
lymphocytes cultured in vitro. Cancer letters, 13:213-218.
Principe P, Dogliotti E, Bignami M, Crebelli R, Falcone E,
Fabrizi M, Conti G, Comba P (1981) Mutagenicity of chemicals of
industrial and agricultural relevance in Salmonella,
Streptomyces, and Aspergillus. Journal of the science of
food and agriculture, 32:826-832.
Prodi G, Arfelini G, Colacci A, Grilli S, Mazzulo M (1986)
Interaction of halocompounds with nucleic acids. Toxicologic
pathology, 14:438-444.
Rannug U (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichloroethane. Mutation research, 76:269-295.
Rannug U, Beije B (1979) The mutagenic effect of
1,2-dichloro-ethane on Salmonella typhimurium. II. Activation
by the isolated perfused rat liver. Chemico-biological
interactions, 24:265-285.
Rannug U, Sundvall A, Ramel C (1978) The mutagenic effect
of 1,2-dichloroethane on Salmonella typhimurium. I. Activation
through conjugation with glutathione in vitro.
Chemico-biological interactions, 20:1-16.
Rao KS, Murray JS, Deacon MM, John JA, Calhoun LL, Young JT
(1980) Teratogenicity and reproduction studies in animals
inhaling ethylene dichloride. In: Ames BN, Infante P, Reitz R,
eds. Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 149-166 (Banbury
Report No. 5).
Reitz RH, Fox TR, Ramsey JC, Quast JF, Langvardt PW, Watanabe PG
(1982) Pharmacokinetics and macro-molecular interactions of
ethylene dichloride in rats after inhalation or gavage.
Toxicology and applied pharmacology, 62:190-204.
Richter JE, Peterson SF, Kleiner CF (1983) Acute and chronic
toxicity of some chlorinated benzenes, chlorinated ethanes, and
tetrachloroethylene to Daphnia magna. Archives of
environmental contamination and toxicology, 12:679-684.
Roldan-Arjona T, Garcia-Pedrajas MD, Luque-Romero RL, Hera C,
Pueyo C (1991) An association between mutagenicity of the Ara
test of Salmonella typhimurium and carcinogenicity in rodents
for 16 halogenated aliphatic hydrocarbons. Mutagenesis,
6:199-205.
Romert L, Magnusson J, Ramel C (1990) The importance of
glutathione and glutathione transferase for somatic mutations in
Drosophila melanogaster induced in vivo by
1,2-dichloroethane. Carcinogenesis, 11:1399-1402.
Schultz K, Ghosh L, Banerjee S (1992) Neoplastic expression in
murine cells induced by halogenated hydrocarbons. In vitro
cellular and developmental biology, 28A:267-272.
Sherwood RL, O'Shea PT, Thomas W, Ratajczak HV, Aranyi C, Graham
JA (1987) Effects of inhalation of ethylene dichloride on
pulmonary defences of mice and rats. Toxicology and applied
pharmacology, 91:491-496.
Shmuter LM (1977) [Effects of chronic exposure to low
concentrations of chlorinated hydrocarbons of the ethane series on
specific and non-specific reactivity of animals in vivo.]
Gigiena Truda i Professional'nye Zabolevaniya, 8:38-42 (in
Russian).
Simmon VF (1980) Review of non-bacterial tests of the genotoxic
activity of ethylene dichloride. In: Ames BN, Infante P, Reitz R,
eds. Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 97-103 (Banbury
Report No. 5).
Simula TP, Glancey MG, Wolf CR (1993) Human glutathione
S-transferase-expressing Salmonella typhimurium tester
strains to study the activation/detoxification of mutagenic
compounds: studies with halogenated compounds, aromatic amines
and aflatoxin B1. Carcinogenesis, 14:1371-1376.
Singh HB, Salas LJ, Smith AJ, Shigeishi H (1980) Atmospheric
measurements of selected toxic organic chemicals. Research
Triangle Park, NC, US Environmental Protection Agency,
Environmental Sciences Research Laboratory (EPA-600/3-80-072;
PB80-198989).
Singh HB, Salas LJ, Smith AJ, Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban
environments. Atmospheric environment, 15:601-612.
Singh HB, Salas LF, Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environmental science and technology, 16:872-880.
Smyth HF Jr (1969) Acute toxicity of ethyl compounds. In: Spector
WC, ed. Handbook of toxicology. Vol. 1. Philadelphia, PA, W.B.
Saunders Company.
Spence JW, Hanst PL (1978) Oxidation of chlorinated ethanes.
Journal of the Air Pollution Control Association,
28(3):250-253.
Spencer HC, Rowe VK, Adams EM, McCollister DD, Irish DD (1951)
Vapor toxicity of ethylene dichloride determined by experiments
on laboratory animals. American Medical Association archives
of industrial hygiene and occupational medicine, 4:482-493.
Spreafico F, Zuccato E, Marcucci F, Sironi M, Paglialunga S,
Madonna M, Mussini E (1980) Pharmacokinetics of ethylene
dichloride in rats treated by different routes and its long-term
inhalatory toxicity. In: Ames BN, Infante P, Reitz R, eds.
Ethylene dichloride: a potential health risk? Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, pp. 107-133 (Banbury
Report No. 5).
Storer RD, Conolly RB (1983) Comparative in vivo genotoxicity
and acute hepatotoxicity of three 1,2-dihaloethanes.
Carcinogenesis, 4:1491-1494.
Storer RD, Conolly RB (1985) An investigation of the role of
microsomal oxidative metabolism in the in vivo genotoxicity of
1,2-dichloroethane. Toxicology and applied pharmacology,
77:36-46.
Storer RD, Jackson NM, Conolly RB (1984) In vivo genotoxicity
and acute hepatotoxicity of 1,2-dichloroethane in mice:
comparison of oral, intraperitoneal, and inhalation routes of
exposure. Cancer research, 44:4267-4271.
Story DL, Meierhenry EF, Tyson CA, Milman HA (1986) Differences
in rat liver enzyme-positive foci produced by chlorinated
aliphatics and phenobarbital. Toxicology and industrial
health, 2:351-362.
Strobel K, Grummt T (1987) Aliphatic and aromatic halocarbons as
potential mutagens in drinking water. III. Halogenated ethanes
and ethenes. Toxicology and environmental chemistry,
15:101-128.
Sundheimer DW, White RD, Brendel K, Sipes IC (1982) The
bioactivation of 1,2-dibromoethane in rat hepatocytes: covalent
binding to nucleic acids. Carcinogenesis, 3:1129-1133.
Suveev IM, Babichenko ME (1969) [On the clinic and cure of acute
intoxication with dichloroethane vapours.] Gigiena Truda i
Professional'nye Zabolevaniya, 13:50-51 (in Russian).
Tan E-L, Hsie AW (1981) Mutagenicity and cytotoxicity of
haloethanes as studied in the CHO/HGPRT system. Mutation
research, 90:183-191.
Taningher M, Parodi S, Grilli S, Colacci A, Mazzullo M, Bordone
R, Santi L (1991) Lack of correlation between alkaline DNA
fragmentation and DNA covalent binding induced by
polychloroethanes after in vivo administration. Problems
related to the assessment of a carcinogenic hazard. Cancer
detection and prevention, 15:35-39.
Theiss JC, Stoner GD, Shimkin MB, Weisberger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer
research, 37:2717-2720.
Tu AS, Murray TA, Hatch KM, Sivak A, Milman HA (1985) In vitro
transformation of BALB/c-3T3 cells by chlorinated ethanes and
ethylenes. Cancer letters, 28:85-92.
US EPA (1985) Health assessment document for
1,2-dichloroethane (ethylene dichloride). Final report.
Washington, DC, US Environmental Protection Agency
(EPA/600/8-84/006F; NTIS PB86-122702).
US EPA (1992) Indoor air quality data base for organic
compounds. Research Triangle Park, NC, US Environmental
Protection Agency (EPA-600-R-92-025; NTIS PB92-158468).
Valencia R, Abrahamson S, Lee WR, von Halle ES, Woodruff RC,
Wurgler FE, Zimmering S (1984) Chromosome mutations for
mutagenesis in Drosophila melanogaster: a report of the US
Environmental Protection Agency Gene-Tox Program. Mutation
research, 134:61-88.
van Bladeren PJ, Breimer DD, Rotteveel-Smijs RMT, de Knijff P,
Mohn GV, van Meeteren-Walchli B, Buijs W, van der Gen A (1981)
The relation between the structure of vicinal dihalogen compounds
and their mutagenic activation via conjugation to glutathione.
Carcinogenesis, 2:499-505.
van Duuren BL, Goldschmidt BM, Loewengart G, Smith A, Mechionne
S, Seldman I, Roth D (1979) Carcinogenicity of halogenated
olefinic and aliphatic hydrocarbons in mice. Journal of the
National Cancer Institute, 63:1433-1439.
van Esch GJ, Kroes R, van Logten MJ, den Tonkelaar EM (1977)
Ninety-day toxicity study with 1,2-dichloroethane (DCE) in
rats. Bilthoven, National Institute of Public Health and
Environmental Hygiene (Report 195/77 Al. Tox.).
Vozovaya M (1977) [The effect of dichloroethane on the sexual
cycle and embryogenesis of experimental animals.] Akusherstvo
i Ginekologiya (Moscow), 2:57-59 (in Russian).
WHO (1971) Evaluation of food additives: specifications for
the identity and purity of food additives and their
toxicological evaluation: some extraction solvents and
certain other substances; a review of the technological
efficacy of some antimicrobial agents. Fourteenth report of
the Expert Committee. Geneva, World Health Organization (WHO
Technical Report Series No. 462).
WHO (1980) Evaluation of certain food additives.
Twenty-third report of the Joint FAO/WHO Expert Committee
on Food Additives. Geneva, World Health Organization (WHO
Technical Report Series No. 648 and corrigenda).
WHO (1992) Evaluation of certain food additives and
naturally occurring toxicants. Thirty-ninth report of the
Joint FAO/WHO Expert Committee on Food Additives. Geneva, World
Health Organization (WHO Technical Report Series No. 828).
WHO (1993) Guidelines for drinking-water quality, 2nd ed.
Vol. 1. Recommendations. Geneva, World Health Organization.
Williams GM, Mori H, McQueen CA (1989) Structure-activity
relationships in the rat hepatocyte DNA-repair test for 300
chemicals. Mutation research, 221:263-286.
Zamora PO, Benson JM, Li AP, Brooks AL (1983) Evaluation of an
exposure system using cells grown on collagen gels for detecting
highly volatile mutagens in the CHO/HGPRT mutation assay.
Environmental mutagenesis, 5:795-801.
APPENDIX 1 - SOURCE DOCUMENTS
International Programme on Chemical Safety - Environmental Health
Criteria Monograph No. 176 (1995)
Copies of the Environmental Health Criteria document on
1,2-dichloroethane, prepared by the International Programme on
Chemical Safety, as well as the Health and Safety Guide (1991)
and the International Chemical Safety Card (1993), may be
obtained from:
International Programme on Chemical Safety
World Health Organization
Geneva, Switzerland
The first draft of the monograph, prepared by Ms K. Hughes
of the Environmental Health Directorate, Health Canada, was
circulated to IPCS Contact Points (approximately 150 government,
industrial, academic, and independent organizations and
individuals) for comment in June 1994. The second draft, revised
on the basis of the comments received, was also prepared by Ms K.
Hughes. Dr E. Smith and Dr P.G. Jenkins, both members of the
IPCS Central Unit, were responsible for the scientific content
and technical editing, respectively.
The monograph on 1,2-dichloroethane was finalized by the
Core Assessment Group (CAG) of the Joint Meeting on Pesticides
(JMP), which met in Geneva from 25 October to 3 November 1994.
The Core Assessment Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health
and the environment from exposure to 1,2-dichloroethane.
Participants at the Core Assessment Group meeting were:
Dr T. Bailey, Ecological Effects Branch, Environmental Fate
and Effects Division, US Environmental Protection Agency,
Washington, DC, USA
Dr A.L. Black, Department of Human Services and Health,
Canberra, ACT, Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairperson)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution,
London, United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, Office of Pesticide Programs, US
Environmental Protection Agency, Washington, DC, USA
Dr R. Hailey, National Institute of Environmental Health
Sciences, National Institutes of Health, Public Health
Service, Department of Health and Human Services, Research
Triangle Park, NC, USA
Ms K. Hughes, Priority Substances Section, Environmental
Health Directorate, Health Canada, Ottawa, Ontario, Canada
(EHC Joint Rapporteur)
Dr D. Kanungo, Division of Medical Toxicology, Central
Insecticides Laboratory, Government of India, Ministry of
Agriculture & Cooperation, Directorate of Plant Protection,
Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group
Ltd, Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Fontwell, Arundel,
West Sussex, United Kingdom
Professor M. Lotti, Institute of Occupational Medicine,
University of Padua, Padua, Italy (Chairperson)
Dr D.R. Mattison, University of Pittsburgh, Graduate School
of Public Health, Pittsburgh, PA, USA
Dr J. Sekizawa, Division of Information on Chemical Safety,
National Institute of Health Sciences, Setagaya-ku, Tokyo,
Japan
Dr P. Sinhaseni, Department of Pharmacology, Chulalongkorn
University, Bangkok, Thailand
Dr S.A. Soliman, Pesticide Chemicals & Toxicology, King Saud
University, Bureidah, Saudi Arabia
Dr M. Tasheva, Department of Toxicology, National Center of
Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Department of Toxicology, Wageningen
Agricultural Unviersity, Wageningen, The Netherlands
Dr M.I. Willems, Department of Occupational Toxicology, TNO
Nutrition and Food Research Institute, AJ Zeist, The
Netherlands
Procedures for the preparation of an Environmental Health
Criteria document
The order of procedures that result in the publication of an
EHC monograph is shown in the flow chart. A designated staff
member of IPCS, responsible for the scientific quality of the
document, serves as Responsible Officer (RO). The IPCS Editor is
responsible for layout and language. The first draft, prepared
by consultants or, more usually, staff from an IPCS Participating
Institution, is based initially on data provided from the
International Register of Potentially Toxic Chemicals and
reference databases such as Medline and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its
scientific quality and objectivity. Once the RO finds the
document acceptable as a first draft, it is distributed, in its
unedited form, to well over 150 EHC Contact Points throughout the
world who are asked to comment on its completeness and accuracy
and, where necessary, provide additional material. The Contact
Points, usually designated by governments, may be Participating
Institutions, IPCS Focal Points, or individual scientists known
for their particular expertise. Generally, some four months are
allowed before the comments are considered by the RO and
author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not
as representatives of any organization, government, or industry.
Their function is to evaluate the accuracy, significance, and
relevance of the information in the document and to assess the
health and environmental risks from exposure to the chemical. A
summary and recommendations for further research and improved
safety aspects are also required. The composition of the Task
Group is dictated by the range of expertise required for the
subject of the meeting and by the need for a balanced
geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international
associations may be invited to join the Task Group as observers.
While observers may provide a valuable contribution to the
process, they can speak only at the invitation of the
Chairperson. Observers do not participate in the final
evaluation of the chemical; this is the sole responsibility of
the Task Group members. When the Task Group considers it to be
appropriate, it may meet in camera.
All individuals who as authors, consultants, or advisers
participate in the preparation of the EHC monograph must, in
addition to serving in their personal capacity as scientists,
inform the RO if at any time a conflict of interest, whether
actual or potential, could be perceived in their work. They are
required to sign a conflict of interest statement. Such a
procedure ensures the transparency and probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of
the document, it then goes for language editing, reference
checking, and preparation of camera-ready copy. After approval
by the Director, IPCS, the monograph is submitted to the WHO
Office of Publications for printing. At this time, a copy of the
final draft is sent to the Chairperson and Rapporteur of the Task
Group to check for any errors.
 APPENDIX 2 - CICAD FINAL REVIEW BOARD
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit,
Health and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and
Environmental Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental
Assessment, Office of Research and Development, US Environmental
Protection Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of
Consumers & Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of
Hygiene and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences,
Hanover, Germany
Ms E. Meek, Head, Priority Substances Section, Environmental
Health Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National
Institute of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim,
Germany
Professor S. Tarkowski, Department of Environmental Health
Hazards, The Nofer Institute of Occupational Medicine, Lodz,
Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna,
Sweden
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on
Chemical Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé
ce CICAD (Document international succinct sur l'évaluation des
risques chimiques) sur le 1,2-dichloréthane en s'inspirant d'un
document de la série Critères d'hygiène de l'environnement (CHE)
du Programme international sur la Sécurité chimique (PISC) (IPCS,
1995), qui évalue les conséquences potentielles pour la santé
humaine d'une exposition indirecte au 1,2-dichloréthane dans
l'environnement général ainsi que les effets de cette substance
sur l'environnement. Les données prises en compte dans cette
analyse sont celles qui étaient disponibles en mai 1993 (effets
sur la santé humaine) ou en octobre 1994 (effets sur
l'environnement). L'appendice 1 donne des informations sur la
nature du processus d'évaluation par les pairs et sur la
disponibilité du document CHE. Pour ce CICAD, c'est l'évaluation
pratiquée lors de l'élaboration du document CHE qui a fait office
d'évaluation par les pairs préalable à l'examen du Comité
d'évaluation finale. Les membres du Comité d'évaluation finale
ont apporté les dernières corrections à ce CICAD et en ont
approuvé la publication par correspondance, après avoir examiné
les observations présentées lors de l'élaboration du CHE dans le
cadre de l'évaluation par les pairs. La composition du Comité
d'évaluation finale est indiquée à l'appendice 2. La fiche
internationale sur la sécurité chimique (ICSC 0250) produite par
le PISC (IPCS, 1993) est également reproduite dans ce document.
Le 1,2-dichloréthane (CAS N°
APPENDIX 2 - CICAD FINAL REVIEW BOARD
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit,
Health and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and
Environmental Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental
Assessment, Office of Research and Development, US Environmental
Protection Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of
Consumers & Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of
Hygiene and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences,
Hanover, Germany
Ms E. Meek, Head, Priority Substances Section, Environmental
Health Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National
Institute of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim,
Germany
Professor S. Tarkowski, Department of Environmental Health
Hazards, The Nofer Institute of Occupational Medicine, Lodz,
Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna,
Sweden
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on
Chemical Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé
ce CICAD (Document international succinct sur l'évaluation des
risques chimiques) sur le 1,2-dichloréthane en s'inspirant d'un
document de la série Critères d'hygiène de l'environnement (CHE)
du Programme international sur la Sécurité chimique (PISC) (IPCS,
1995), qui évalue les conséquences potentielles pour la santé
humaine d'une exposition indirecte au 1,2-dichloréthane dans
l'environnement général ainsi que les effets de cette substance
sur l'environnement. Les données prises en compte dans cette
analyse sont celles qui étaient disponibles en mai 1993 (effets
sur la santé humaine) ou en octobre 1994 (effets sur
l'environnement). L'appendice 1 donne des informations sur la
nature du processus d'évaluation par les pairs et sur la
disponibilité du document CHE. Pour ce CICAD, c'est l'évaluation
pratiquée lors de l'élaboration du document CHE qui a fait office
d'évaluation par les pairs préalable à l'examen du Comité
d'évaluation finale. Les membres du Comité d'évaluation finale
ont apporté les dernières corrections à ce CICAD et en ont
approuvé la publication par correspondance, après avoir examiné
les observations présentées lors de l'élaboration du CHE dans le
cadre de l'évaluation par les pairs. La composition du Comité
d'évaluation finale est indiquée à l'appendice 2. La fiche
internationale sur la sécurité chimique (ICSC 0250) produite par
le PISC (IPCS, 1993) est également reproduite dans ce document.
Le 1,2-dichloréthane (CAS N° 