
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 176
1,2-DICHLOROETHANE
(SECOND EDITION)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Ms K. Hughes, Environmental Health
Directorate, Health Canada
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1995
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
develop-ment of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
1,2-Dichloroethane - 2nd ed.
(Environmental health criteria ; 176)
1.Ethylene dichlorides - toxicity I.Series
ISBN 92 4 157176 4 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROETHANE
Preamble
1. SUMMARY
1.1. Identity, physical and chemical properties,
and analytical methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution and
transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism in laboratory animals
1.6. Effects on laboratory mammals and in vitro
test systems
1.7. Effects on humans
1.8. Effects on non-target organisms in the
laboratory and field
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND
TRANSFORMATION
4.1. Transport and fate in the environment
5. ENVIRONMENTAL LEVELS AND POPULATION EXPOSURE
5.1. Environmental levels
5.1.1. Ambient air
5.1.2. Indoor air
5.1.3. Drinking-water
5.1.4. Surface water
5.1.5. Food
5.1.6. Soils and sediments
5.1.7. Consumer products
5.2. General population exposure
5.2.1. Ambient air
5.2.2. Indoor air
5.2.3. Drinking-water
5.2.4. Food
5.2.5. Other media
5.3. Occupational exposure during manufacture,
formulation or use
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
6.1. Absorption
6.2. Distribution
6.3. Metabolic transformation
6.4. Elimination and excretion
6.5. Retention and bioaccumulation
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO
TEST SYSTEMS
7.1. Single exposure
7.2. Skin and eye irritation
7.3. Short-term exposure
7.4. Subchronic exposure
7.4.1. Inhalation
7.4.2. Ingestion
7.5. Chronic exposure and carcinogenicity
7.5.1. Inhalation
7.5.2. Ingestion
7.5.3. Other routes of administration
7.5.4. Initiation/promotion bioassays
7.6. Mutagenicity and related end-points
7.7. Reproductive toxicity, embryotoxicity and
teratogenicity
7.8. Immunological effects
7.9. Toxicological interactions with other agents
8. EFFECTS ON HUMANS
8.1. Case reports
8.2. Epidemiological studies
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY
AND FIELD
9.1. Aquatic organisms
9.1.1. Microorganisms
9.1.2. Invertebrates
9.1.3. Vertebrates
9.2. Terrestrial organisms
9.2.1. Invertebrates
9.2.2. Vertebrates
9.2.3. Plants
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON
THE ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Environmental assessment
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF
HUMAN HEALTH AND THE ENVIRONMENT
12. FURTHER RESEARCH
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme
was initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an everincreasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World
Health Assembly resolutions and the recommendations of the 1972 UN
Conference on the Human Environment. Subsequently the work became an
integral part of the International Programme on Chemical Safety
(IPCS), a cooperative programme of UNEP, ILO and WHO. In this manner,
with the strong support of the new partners, the importance of
occupational health and environmental effects was fully recognized.
The EHC monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined
below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
Sources of exposure
* Environmental transport, distribution and transformation
Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC,
JECFA, JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
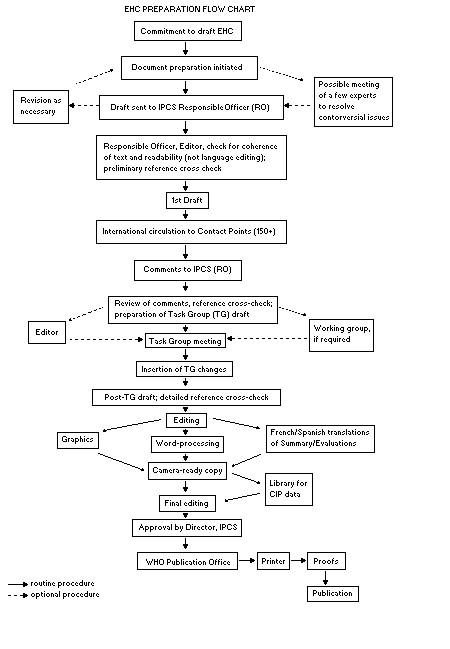 It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROETHANE
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency,
Washington DC, USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist, The
Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROETHANE
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met in Geneva from 25 October to 3 November 1994.
Dr W. Kreisel, Executive Director, welcomed the participants on behalf
of WHO, and Dr M. Mercier, Director, IPCS, on behalf of the IPCS and
its cooperating organizations (UNEP/ILO/WHO). The Core Assessment
Group reviewed and revised the draft monograph and made an evaluation
of the risks for human health and the environment from exposure to
1,2-dichloroethane (ethylene dichloride).
The first draft of the monograph was prepared by Ms K. Hughes,
Environmental Health Directorate, Health Canada. The second draft,
revised in the light of international comment, was also prepared by
Ms K. Hughes. Dr E. Smith and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the scientific content and
technical editing respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
1,2-Dichloroethane was previously evaluated by a WHO Task Group
in 1986 and published by WHO in 1987 as Environmental Health Criteria
62.
ABBREVIATIONS
BCF bioconcentration factor
BUN blood urea nitrogen
ECD electron capture detector
FID flame ionization detector
GC gas chromatography
GSH glutathione
gamma-GT gamma-glutamyltranspeptidase
HECD Hall electron capture detector
LOEL lowest-observed-effect level
MS mass spectrometry
NOEL no-observed-effect level
PIB piperonyl butoxide
SGOT serum glutamic-oxalic transaminase
SGPT serum glutamic-pyruvic transaminase
TEAM total exposure assessment methodology
TWA time-weighted average
1. Summary
1.1 Identity, physical and chemical properties, and analytical
methods
1,2-Dichloroethane (ethylene dichloride) is a synthetic chemical
which is a colourless liquid at room temperature. It is also highly
volatile, with a vapour pressure of 8.5 kPa (at 20°C), and is soluble
in water, with a solubility of 8690 mg/litre (at 20°C). The log
octanol/water partition coefficient is 1.76.
Analysis for 1,2-dichloroethane in environmental media is usually
by gas chromatography, in combination with electron capture or flame
ionization detection or mass spectrometry. Detection limits range
from 0.016 to > 4 µg/m3 in air, 0.001 to 4.7 µg/litre in water, and
from 6 to 10 µg/kg in various foodstuffs.
1.2 Sources of human and environmental exposure
The principal use of 1,2-dichloroethane is in the synthesis of
vinyl chloride monomer, and to a lesser extent in the manufacture of
various chlorinated solvents. It is also incorporated into anti-knock
gasoline additives (although this use is declining with the phase-out
of leaded gasoline in some countries), and has been used as a
fumigant. Total annual production of 1,2-dichloroethane in Canada in
1990 and the USA in 1991 was 922 and 6318 kilotonnes, respectively.
1.3 Environmental transport, distribution and transformation
The majority of 1,2-dichloroethane released to the environment is
in emissions to air. It is moderately persistent in air; the
estimated atmospheric lifetime is between 43 and 111 days.
1,2-Dichloroethane is transported to the stratosphere, where
photolysis may produce chlorine radicals which may in turn react with
ozone. Some 1,2-dichloroethane may be released in industrial
effluents to the aquatic environment, from which it is removed rapidly
by volatilization. 1,2-Dichloroethane may also leach to groundwater
near industrial waste sites. It is not expected to bioconcentrate in
aquatic or terrestrial species.
1.4 Environmental levels and human exposure
Mean concentrations of 1,2-dichloroethane in recent surveys of
ambient air in non-source-dominated areas of cities range from 0.07 to
0.28 µg/m3, while mean levels in residential indoor air are reported
to range from < 0.1 to 3.4 µg/m3. In drinking-water, mean
concentrations are generally less than 0.5 µg/litre.
1,2-Dichloroethane has only rarely been detected in foodstuffs in
recent surveys and, since it has low potential for bioaccumulation,
food is unlikely to be a major source of exposure.
Based on estimates of mean exposure from various media, the
predominant source of exposure to 1,2-dichloroethane by the general
population is indoor and outdoor air, only minor amounts being
contributed by drinking-water. Intake of 1,2-dichloroethane from food
is probably negligible. The amount inhaled in ambient air may be
greater in the vicinity of industrial sources.
1.5 Kinetics and metabolism in laboratory animals
1,2-Dichloroethane is readily absorbed following inhalation,
ingestion or dermal exposure and is rapidly and widely distributed
throughout the body. It is rapidly and extensively metabolized in
rats and mice, with principally sulfur-containing metabolites being
eliminated in the urine in a dose-dependent manner. Metabolism
appears to be saturated or limited in rats at levels of exposure
resulting in blood concentrations of 5 to 10 µg/ml. Levels of DNA
alkylation were higher following exposure to a bolus dose by gavage
than in the case of inhalation over a 6-h period.
1,2-Dichloroethane appears to be metabolized via two principal
pathways; the first involves a saturable microsomal oxidation mediated
by cytochrome P-450 to 2-chloroacetaldehyde and 2-chloroethanol
followed by conjugation with glutathione. The second pathway entails
direct conjugation with glutathione to form S-(2-chloroethyl)-
glutathione, which may be non-enzymatically converted to a glutathione
episulfonium ion; this ion can form adducts with DNA. Although DNA
damage has been induced by the P-450 pathway in vitro, several lines
of evidence indicate that the glutathione conjugation pathway is
probably of greater significance than the P-450 pathway as the major
route for DNA damage.
1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of 1,2-dichloroethane is low in experimental
animals. For example, inhalation LC50s for rats exposed for 6 or
7.25 h ranged from 4000 mg/m3 to 6600 mg/m3, while oral LD50s
for rats, mice, dogs and rabbits ranged from 413 to 2500 mg/kg body
weight.
The results of short-term and subchronic studies in several
species of experimental animals indicate that the liver and kidneys
are the target organs; reliable NOELs or LOELs were not attained in
general due to inadequate documentation and the limited range of
end-points examined in small groups of animals. In a series of early
limited studies, morphological changes in the liver were observed in
several species following subchronic exposure to airborne
concentrations as low as 800 mg/m3. Increases in the relative liver
weight have been observed in rats following subchronic oral
administration of doses of 49 to 82 mg/kg body weight per day or more
for 13 weeks. Little information was presented on non-neoplastic
effects in available chronic studies. Changes in serum parameters
indicative of liver and kidney toxicity were observed in rats exposed
to airborne concentrations as low as 202 mg/m3 for 12 months,
although histopathological examinations were not conducted in this
study.
The carcinogenicity of 1,2-dichloroethane has been investigated
in a few limited bioassays on experimental animals (limitations
include short duration of exposure and high mortality). Significant
increases were not reported in the incidence of any type of tumour in
Sprague-Dawley rats or Swiss mice exposed to up to 607 mg/m3 for 78
weeks and observed until spontaneous death. Mortality was high in
rats in this study, although it was not related to concentration, and
the incidence rates were not adjusted for differential mortality among
groups. There was a nonsignificant increase in the incidence of
mammary gland adenomas and fibroadenomas in female Sprague-Dawley rats
exposed to 200 mg/m3 for 2 years in an assay in which no other
compound-related toxicity was observed.
In contrast, there was convincing evidence of increases in tumour
incidence in two species following ingestion. Significant increases
in the incidence of tumours at several sites (including squamous cell
carcinomas of the stomach (males), haemangiosarcomas (males and
females), fibromas of the subcutaneous tissue (males), adenocarcinomas
and fibroadenomas of the mammary gland (females)) were observed in
Osborne-Mendel rats administered TWA daily doses of 47 or 95 mg/kg
body weight per day by gavage for 78 weeks. Similar increases in the
incidences of tumours at multiple sites (including
alveolar/bronchiolar adenomas (males and females), mammary gland
adenocarcinomas (females) and endometrial stromal polyp or endometrial
stromal sarcoma combined (females) and hepatocellular carcinomas
(males)) occurred in B6C3F1 mice administered TWA daily doses of 97 or
195 mg/kg body weight per day (males) or 149 or 299 mg/kg body weight
per day (females) by gavage for 78 weeks.
The incidence of lung tumours (benign papillomas) was
significantly increased in female mice following repeated dermal
application of 1,2-dichloroethane for 440 to 594 days. Repeated
intraperitoneal injections of 1,2-dichloroethane resulted in
dose-related increases in the number of pulmonary adenomas per mouse
in a susceptible strain, although none of these increases was
significant. Concomitant exposure to inhaled 1,2-dichloroethane and
disulfiram in the diet resulted in an increased incidence of
intrahepatic bile duct cholangiomas and cysts, subcutaneous fibromas,
hepatic neoplastic nodules, interstitial cell tumours in the testes
and mammary adenocarcinomas in rats, compared to rats administered
either compound alone or untreated controls. No potential to initiate
or promote tumour development was evident in three bioassays, although
the extent of histopathological examination was limited in these
studies.
In in vitro assays, 1,2-dichloroethane has been consistently
positive in mutagenicity bioassays in Salmonella typhimurium.
Responses have been greater in the presence of an exogenous activation
system (possibly due to activation by the cytochrome system) than in
its absence, and mutagenicity was more than doubled in S. typhimurium
expressing the human GSTA1-1 gene. In cultured mammalian cells,
1,2-dichloroethane forms adducts with DNA. It also induces
unscheduled DNA synthesis in primary cultures of rodent and human
cells and gene mutation in several cell lines. Mutation frequency in
human cell lines has been correlated with differences in
glutathione- S-transferase activity. In in vivo studies,
1,2-dichloroethane induced somatic cell and sex-linked recessive
lethal mutations in Drosophila melanogaster and the compound bound
to DNA in all reported studies in rats and mice. Although primary DNA
damage in liver and sister chromatid exchange has been observed in
studies in mice, there has been no evidence for micronucleus
induction.
Based on the results of a limited number of studies, there is no
evidence that 1,2-dichloroethane is teratogenic in experimental
animals. There is also little convincing evidence that it induces
reproductive or developmental effects at doses below those which cause
other systemic effects. Available data on the immunotoxicity of
1,2-dichloroethane are limited.
1.7 Effects on humans
Acute incidental exposure to 1,2-dichloroethane by inhalation or
ingestion has resulted in a variety of effects in humans, including
effects on the central nervous system, liver, kidney, lung and
cardiovascular system.
The potential carcinogenicity of 1,2-dichloroethane in exposed
human populations has not been extensively investigated. Mortality
due to pancreatic cancer was significantly increased in a group of
workers at a chemical production plant who had been exposed
principally to 1,2-dichloroethane (in combination with other
chemicals). Mortality due to pancreatic cancer increased with
duration of exposure. In addition, although the number of cases was
small, and the association with duration of exposure was less
consistent, mortality due to leukaemia was also increased in these
workers. No association between occupational exposure to
1,2-dichloroethane and brain cancer was noted in a small case-control
study. Although the incidence of colon and rectal cancer increased
with the concentration of 1,2-dichloroethane in drinking-water in an
inherently limited ecological study, concomitant exposure to other
substances may have contributed to the observed effects.
1.8 Effects on non-target organisms in the laboratory and field
The effects of exposure to 1,2-dichloroethane on a number of
other organisms in the laboratory and field have been investigated.
For aquatic microorganisms, IC50s or EC50s for various end-points
have been reported to range from 25 to 770 mg/litre. The lowest
reported LC50 value for Daphnia was 220 mg/litre, while effects on
reproductive success and growth were observed at 20.7 and
71.7 mg/litre, respectively. Based on available data, the most
sensitive freshwater vertebrate species appears to be the northwestern
salamander (Ambystoma gracile), in which 9-day larval survival (4
days post-hatch) was reduced at 2.54 mg/litre. Only limited data are
available on the toxicity of 1,2-dichloroethane to terrestrial
organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
The empirical formula for 1,2-dichloroethane (ethylene
dichloride) is C2H4Cl2 and the molecular structure is as
follows:
H H
' '
Cl - C - C - Cl
' '
H H
Synonyms include EDC, 1,2-DCE, 1,2-bichloroethane, 1,2-ethylene
dichloride, acethylenchlorid, alpha, beta-dichloroethane, bichlorure
d'ethelene, ethyleen dichloride, ethylene chloride, glycol dichloride,
and sym-dichlorothane. Trade names include: Borer sol, Brocide,
Destruxo,l Di-chlor-mulson, Dichlor-mulsion, Dutch liquid, Dutch oil,
ENT 1656, Freon 150, Gaze Olefiant and Granosan (which also contains
carbon tetrachloride).
The Chemical Abstract Service (CAS) registry number for
1,2-dichloroethane is 107-06-2.
2.2 Physical and chemical properties
1,2-Dichloroethane is a clear, colourless liquid at room
temperature. It is a highly volatile and flammable synthetic chemical
which absorbs infrared light at several wavelengths (7, 12 and 13 µm).
Other properties of 1,2-dichloroethane are presented in Table 1.
2.3 Conversion factors
1 ppm = 4 mg/m3
1 mg/m3 = 0.25 ppm (at 25°C and 760 mmHg)
Table 1. Physical properties of 1,2-dichloroethanea
Physical state liquid
Colour colourless
Odour sweet, chloroform-like
Relative molecular mass 98.96
Density d20 1.253
Refractive index r20 1.4449
D
Boiling point 83°C
Melting point -35°C
Water solubility 8690 mg/litre (20°C)
Vapour pressure 8.5 kPa (20°C)
Saturation concentration in air 350 g/m3 (20°C)
537 g/m3 (30°C)
log Kow 1.76
log Koc 1.28
Henry's law constant 111.5 Pa.m3/mol (25°C)
Flash point 12-15°C
Limits of flammability in air 275-700 mg/litre
a From: Archer (1979); Chiou et al. (1979); Konemann (1981);
Warner et al. (1987); Worthing & Hance (1991)
2.4 Analytical methods
Methods of analysis of 1,2-dichloroethane in various
environmental media are described in Table 2. Gas chromatography,
coupled with electron capture or flame ionization detection or mass
spectrometry, is commonly used for analysis of 1,2-dichloroethane in
most media.
Table 2. Analytical methods for 1,2-dichloroethane in environmental mediaa
Sample matrix Preparation method Analytical method Sample detection Percentage Reference
limit recovery
Air collect sample on Tenax(R)-GC absorbent GC/MS 100 ng/m3 not available Wallace et al.
(1984)
not available GC/MS < 20 ng/m3 ± 5% precision Grimsrud &
(< 5 ppt) Rasmussen (1975)
collect in 6-litres canisters; direct GC/ECD-MS > 4 µg/m3 not available Pleil et al.
injection (> 1 ppb) (1988)
collect air sample in tubes filled with GC/MS 30 pg/sample 98-108% Jonsson & Berg
solid absorbent; heat sample tubes; (1980)
monitor for 1,2-dichloroethane using
selected ion monitoring
collect sample on Tenax(R) TA; thermal GC/ECD 16 ng/m3 not available Class &
desorption (4 ppt) Ballschmiter (1986)
charcoal-tube sampler; desorption with GC/FID 10 µg/sample not available NIOSH (1984)
CS2 solvent
continuous monitoring and breath infra-red not available not available Baretta et al.
analysis spectroscopy (1969)
sampling on charcoal or Chromosorb GC/FID 1.2 µg/m3 not available Parkes et al.
(1976)
Table 2. cont'd.
Sample matrix Preparation method Analytical method Sample detection Percentage Reference
limit recovery
collect sample on Tenax(R) polymeric GC/MS 32 ng/m3 not available Krost et al. (1982)
beads
Water purge-and-trap GC/MS 5 ng/litre not available Wallace et al.
(1984)
purge-and-trap GC/FID 0.1 µg/litre 99% Warner & Beasley
(0.1 ppb) (1984)
headspace/cryogenic trapping HR capillary 80 ng/litre 75% Comba & Kaiser
GC/ECD (1983)
Water and purge-and-trap GC 30 ng/litre 1.04-1.06C US EPA (1982b)
wastewater 97.8% (method 601)
grab sample GC/MS 4.7 µg/litre 1.02 + 0.45C US EPA (1982b)
99% (method 624)
modified purge-and-trap GD/HECD and FID FID 0.1 µg/litre; FID 78%; Otson & Williams
simultaneous HECD < HECD 79% (1982)
0.1 µg/litre
stripping by helium adsorption on GC/FID or MS 1 ng/litre not available Sauer (1981)
Tenax(R)
stripping by helium or nitrogen, GC with 0.1-0.4 µg/litre not available Symons et al.
sorption on Tenax(R) or chromosorb microcoulometric (1975)
detection
not available GC/MS 0.5 µg/litre not available Fujii (1977)
Table 2. cont'd.
Sample matrix Preparation method Analytical method Sample detection Percentage Reference
limit recovery
Grains, legumes, acidified acetone-water extraction; GC/ECD not available 14-75% Daft (1987, 1988,
spices, citrus isooctane back extraction; for liquids, 1989, 1991, 1993)
fruits, isooctane extraction
beverages,
dairy
products, meat
Table-ready stirred with water; purge-and-trap GC/ECD 6 µg/kg 85-104% Heikes (1987b);
foods on Tenax(R) GC; hexane desorption (6 ppb) Heikes & Hopper
(1986)
Fish add fish tissue to reagent grade water; GC/MS 10 µg/kg 85 ± 11% Easley et al.
disrupt cells ultrasonically; analyse (1981)
sample by purge-and-trap method
spiked samples of ground fish tissue; GC/MS not available 92 ± 5%c Hiatt (1981)
vaporize VOCs from fish sample under
vacuum and condense in purge-and-trap
homogenize fish sample; remove residual GC/MS-fused not available not available Hiatt (1983)
moisture by vacuum distillation silica capillary column
Sediment spiked samples; vaporize VOCs under GC/MS not available 96 ± 17%c Hiatt (1981)
vacuum and condense in purge-and-trap
a Modified from: ATSDR (1994); CS2 = carbon disulfide; ECD = electron capture detector; FID = flame ionization detector; GC = gas
chromatography; HECD = Hall electron capture detector; MS = mass spectrometry;
b VOCs = volatile organic carbon compounds
c Reported as percentage spike recoveries for 25 µg/kg (ppb) spikes
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
1,2-Dichloroethane is a synthetic chemical which has no known
natural sources.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
1,2-Dichloroethane, first produced in 1795, was the first
chlorinated hydrocarbon to be synthesized (IARC, 1979). It is
manufactured by either the catalytic vapour-phase or liquid-phase
chlorination of ethylene or by oxychlorination of ethylene
(Archer, 1979). Most commercial grade 1,2-dichloroethane is 97-99%
pure (Drury & Hammons, 1979).
The total annual production of 1,2-dichloroethane in Canada in
1990 was estimated to be 922 000 tonnes (CPI, 1991), while the total
production in the USA in 1991 was 6 318 000 tonnes (Chemical Marketing
Reporter, 1992), increasing from a production value of 5 038 000
tonnes in 1980 (Kirschmer & Ballschmiter, 1983). More than 1 million
tonnes of 1,2-dichloroethane was produced in the United Kingdom in
1991 (UK HSE, 1992). 1,2-Dichloroethane is released to the
environment principally through emissions to ambient air during its
production and that of vinyl chloride monomer. 1,2-Dichloroethane is
recovered from waste streams of manufacturing facilities in a
two-stage distillation operation. This waste stream is then
incinerated (McPherson et al., 1979), the estimated destruction
efficiency being 99.99% (US EPA, 1986).
Release of 1,2-dichloroethane to the atmosphere from production
facilities can occur from a number of sources. Incidental emissions
usually comprise around 50% of the total, while releases from
secondary sources, such as losses from process wastewater, valves and
vents, such as thermal oxidizer vents, handling and storage, and other
sources result in release of the balance. The US EPA estimated that
18 000 tonnes of 1,2-dichloroethane was released to the atmosphere in
the USA in 1982 from fugitive sources (e.g., valves, etc.), storage
tanks, secondary sources (e.g., emissions from wastewater treatment
processes), process vents and shipping operations (US DHHS, 1994).
1,2-Dichloroethane is also released to the atmosphere from
automobile emissions due to its incorporation into anti-knock
formulations for leaded petrol (gasoline).
1,2-Dichloroethane may enter surface waters via effluents from
industries that manufacture or use the substance. In addition, it may
enter the atmosphere or groundwater following disposal in waste sites.
3.2.2 Uses
The predominant uses of 1,2-dichloroethane is as an intermediate
in the synthesis of vinyl chloride; 99% of total demand in Canada, 90%
in Japan and 88% of total production in the USA is used for this
purpose (CPI, 1991; Chemical Marketing Reporter, 1992). It has also
been used in the production of chlorinated solvents such as
trichloroethylene, tetrachloroethylene, 1,1,1-trichloroethane,
ethyleneamines and vinylidene chloride, and in the manufacture of
anti-knock fluids containing tetraethyllead, although this latter use
has declined with the phase-out of leaded petrol. 1,2-Dichloroethane
has been used as a fumigant. However, it is no longer registered for
use on agricultural products in Canada, the USA, the United Kingdom
and Belize.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and fate in the environment
Due to the high vapour pressure of 1,2-dichloroethane, the
atmosphere is expected to be the predominant environmental sink for
the compound. The rate of reaction of 1,2-dichloroethane with hydroxyl
radicals has been predicted to be 3.63 × 10-13 cm3/mol-sec at
25°C (Atkinson, 1987) and 5.42 × 10-13 cm3/mol-sec at 4°C
(Nimitz & Skaggs, 1992). It was experimentally determined to be 2.09
× 10-13 cm3/mol-sec at 19°C (Qiu et al., 1992). Based on these
values, and assuming an atmospheric hydroxyl radical concentration
representative of a moderately polluted area (Finlayson-Pitts &
Pitts, 1986), the estimated atmospheric lifetime of 1,2-dichloroethane
is between 43 and 111 days. Due to the moderate persistence of
1,2-dichloroethane in the troposphere, long-range transport is
possible. Indeed, 1,2-dichloroethane has been detected in the lower
troposphere over the northern Atlantic Ocean and over the Pacific
Ocean (Singh et al., 1983; Class & Ballschmiter, 1986).
Once 1,2-dichloroethane reaches the troposphere, it undergoes
photo-oxidation to produce formyl chloride, chloroacetyl chloride,
hydrochloric acid, carbon monoxide and carbon dioxide (Spence &
Hanst, 1978). Any 1,2-dichloroethane that reaches the stratosphere
may be photolysed to produce chlorine radicals that may, in turn,
react with ozone (Spence & Hanst, 1978; Callahan et al., 1979).
However, 1,2-dichloroethane is not expected to contribute
significantly to the depletion of the stratospheric ozone layer,
since, based on either the experimental or predicted rates of reaction
between hydroxyl radicals and 1,2-dichloroethane, its ozone depletion
potential is very much less than 0.001 relative to the
chlorofluorocarbon, CFC-11. 1,2-Dichloroethane was not included as a
controlled substance in the "Montreal Protocol on Substances that
Deplete the Ozone Layer".
Volatilization is the major removal process of 1,2-dichloroethane
from the aquatic environment (Dilling et al., 1975). The half-life in
a stirred aqueous solution, at varying depths and surface areas,
ranged between 5 and 29 min (Dilling et al., 1975; Chiou et al.,
1980). Based on fate modelling (EXAMS), the predicted half-life of
1,2-dichloroethane was 9 days in a eutrophic lake and one day in a
300-km stretch of a river system (assuming a loading rate of 0.1 kg
1,2-dichloroethane in both cases) (US EPA, 1982a).
Although hydrolysis of 1,2-dichloroethane may also occur in the
aquatic environment, this is not a significant removal process, since
the half-life for hydrolysis has been estimated to be 72 years at
neutral pH and 25°C (Barbash & Reinhard, 1989). In conditions similar
to those of groundwater (i.e. in the presence of sodium sulfide, a pH
of 7, and a temperature of 15°C), the estimated half-life of
1,2-dichloroethane was 23 years (Barbash & Reinhard, 1989). The
primary products of hydrolysis are vinyl chloride and 2-chloroethanol
(Jeffers et al., 1989); vinyl chloride can be further degraded to
acetylene and acetaldehyde (Hill et al., 1976), while 2-chloroethanol
may be degraded to ethylene glycol (Ellington et al., 1988).
Microbial degradation of 1,2-dichloroethane in water has been
observed, but it is a slow process, probably due to the insufficient
time before volatilization of the substance to allow for microbial
adaptation (US EPA, 1982a). In a static flask study with initial
1,2-dichloroethane concentrations of 5 and 10 mg/litre, there was a
loss due to aerobic degradation of 20 to 63% within 7 days following
an acclimation period. However, 5 to 27% of the total loss was
attributed to volatilization (Tabak et al., 1981). The methanotrophic
bacterium Methylosinus trichosporium (Oldenhuis et al., 1989),
methylotrophic bacterium Ancylobacter aquaticus (van den Wijngaard et
al., 1992) and a nitrogen-fixing bacterium Xanthobacter autotrophicus
(Janssen et al., 1985) have been identified as microorganisms capable
of biodegrading 1,2-dichloroethane under aerobic conditions. In a
batch experiment under anaerobic conditions, Bouwer & McCarty (1983)
reported a 63% reduction in 25 days, but were unable to induce
transformation in a flow-through system when initial concentrations of
1,2-dichloroethane were 174 and 22 µg/litre, respectively.
No biodegradation was observed after 35 days of incubation in an
anoxic sediment-water system in which the initial concentration of
1,2-dichloroethane was 1.0 mg/litre (pH not reported) (Jafvert &
Wolfe, 1987).
Based on its low sorption coefficient, 1,2-dichloroethane is not
expected to adsorb appreciably to soil, suspended solids or sediments.
In one study, 1,2-dichloroethane rapidly percolated through sandy soil
with a low organic matter content; no degradation was observed, and
72-74% of the initial amount was reported to have volatilized (Wilson
et al., 1981). 1,2-Dichloroethane may leach to groundwater, based on
its solubility in water, low Koc value and high mobility in soil.
Reductive dechlorination of 1,2-dichloroethane in leachates under
anaerobic conditions has been demonstrated (Lesage et al., 1993).
1,2-Dichloroethane has low potential for bioaccumulation, based
on experimental data and modelling predictions. The bioconcentration
factor (BCF) was determined to be 2, with a clearance half-life in
tissues of less than 2 days, in freshwater bluegill (Lepomis
macrochirus) exposed to 95.6 µg 1,2-dichloroethane/litre for 14 days
(Barrows et al., 1980). This is identical to the value predicted by
Isnard & Lambert (1988). Accumulation and loss of radiolabelled
1,2-dichloroethane was studied in the dab (Limanda limanda) liver
and in the oyster (Ostrea edulis). Following exposure to 3 mg/litre
for 20 days, the level in the dab liver rose rapidly to approximately
80 mg/kg and then remained stable. Following cessation of exposure,
1,2-dichloroethane levels decreased to about 12 mg/kg at 40 days. In
the oyster, the level rose to approximately 9 mg/kg in 4 days, reached
a plateau, and decreased to 3 mg/kg by 40 days after cessation of
exposure (Pearson & McConnell, 1975).
5. ENVIRONMENTAL LEVELS AND POPULATION EXPOSURE
5.1 Environmental levels
5.1.1 Ambient air
The mean concentrations of 1,2-dichloroethane in 1412 samples of
ambient air from 23 sites in 12 cities across Canada taken between
1988 to 1990 ranged from 0.07 to 0.28 µg/m3, with an overall mean of
0.13 µg/m3 and a maximum single value of 2.78 µg/m3 (Dann, 1992).
1,2-Dichloroethane was detected in 55 out of 62 samples of ambient air
from 19 out of 21 areas of Japan surveyed in 1992 at concentrations
ranging from non-detectable (i.e. < 0.004 µg/m3) to 3.8 µg/m3
(Environment Agency Japan, 1993). In the United Kingdom and the
Netherlands, average levels of 1,2-dichloroethane in rural areas were
0.08 and 0.2 µg/m3, respectively (Clark et al., 1984a,b; Guicherit &
Schulting, 1985). In both of these countries, the average
concentration in urban air was 1.2 µg/m3 (Clark et al., 1984a,b;
Guicherit & Schulting, 1985).
The US Environmental Protection Agency (US EPA, 1987) reported
levels of 1,2-dichloroethane in urban/suburban air to be generally
< 0.8 µg/m3 (< 0.2 ppb). Concentrations of 1,2-dichloroethane in
ambient air reported in several early studies conducted in 10 cities
in the USA between 1980 and 1982 were somewhat higher, mean
concentrations ranging from 0.33 µg/m3 (83 ppt) to 6.05 µg/m3
(1512 ppt) (Singh et al., 1980, 1981, 1982). Median concentrations of
1,2-dichloroethane in air of rural/remote areas, urban/suburban areas
and source-dominated areas in the USA were 0 µg/m3, 0.49 µg/m3 and
4.9 µg/m3, respectively; the maximum level was 240 µg/m3
(Brodzinsky & Singh, 1982).
Concentrations of 1,2-dichloroethane in air near areas where
chemicals are manufactured or used in the USA were found to be as high
as 736 µg/m3 (184 ppb), with an average of 110 µg/m3 (27.5 ppb)
(US EPA, 1985a). Concentrations were also high (300 µg/m3) near a
vinyl chloride manufacturing plant in the Netherlands (Kretzschmar et
al., 1976). The annual mean concentrations of 1,2-dichloroethane in
250 samples of ambient air from 12 sites in Hamburg, Germany, surveyed
in 1986-1987 ranged from 0.2 to 119 µg/m3, the highest levels being
detected in an industrial region where lubrication oil was produced
(Bruckmann et al., 1988). Levels of 1,2-dichloroethane ranged from
0.09 to 3.5 µg/m3 in heavily industrialized areas in Japan in
1980/1981 (Environment Agency, Japan, 1983). In New Jersey, USA,
where several petrochemical industries were located and there had been
substantial chemical dumping activity in the past, the mean and
maximum values for five hazardous waste sites (14 to 24 samples each)
ranged up to 1.12 µg/m3 (0.28 ppb) and 20.6 µg/m3 (5.15 ppb),
respectively (LaRegina & Bozzelli, 1986). 1,2-Dichloroethane was also
detected in air at a waste disposal site in New Jersey at levels
ranging from trace to 27 µg/m3 (6.8 ppb) (detection limit not
specified) (Pellizzari, 1982).
5.1.2 Indoor air
In a pilot study of samples taken for 1 year beginning in
mid-January 1991, indoor air of approximately 750 residences from 10
provinces across Canada was analysed. The mean concentration of
1,2-dichloroethane was < 0.1 µg/m3, and the maximum value
1.7 µg/m3 (detection limit not specified) (Fellin et al., 1992).
In the US EPA Total Exposure Assessment Methodology
(TEAM) study, samples of "personal" and outdoor air were taken in 600
residences of New Jersey, North Carolina, North Dakota and California.
1,2-Dichloroethane was detected only occasionally at low
concentrations, and the levels in personal air (range of mean values,
0.1 to 0.5 µg/m3) were higher than those in outdoor air (range of
mean values, 0.05 to 0.2 µg/m3) (quantifiable limit approximately
1 µg/m3) (Wallace, 1986). Based on a recent review of available
literature, mean concentrations of 1,2-dichloroethane in indoor air in
the USA ranged from 1.49 to 2.21 µg/m3 in hospitals and 4.51 µg/m3
in office buildings (US EPA, 1992).
The mean concentration of 1,2-dichloroethane in the air of 20
homes in areas in the Netherlands with "non-contaminated" soil was
3.4 µg/m3, compared to a mean outdoor level of 4.9 µg/m3. In the
crawl space or cellar of these homes, the mean concentration was
2.5 µg/m3 (Kliest et al., 1989).
1,2-Dichloroethane was also detected in the indoor air of two out
of nine residences from the Love Canal area of Niagara, New York
(0.100 µg/m3 and 0.130 µg/m3), while only trace levels were
detected in samples of outdoor air (detection limit not specified)
(Barkley et al., 1980).
5.1.3 Drinking-water
In Ontario, Canada, 1,2-dichloroethane was detected in 15 out of
> 2000 samples of drinking-water from 85 sites surveyed between 1988
and 1991; mean concentrations ranged from nondetectable (detection
limit 0.050 µg/litre) to 0.139 µg/litre, with a maximum single value
of 0.850 µg/litre, in treated water (it was not detected in untreated
water) (Ministry of Environment, 1991). 1,2-Dichloroethane was not
detected in 237 samples of drinking-water taken from 171 sites across
New Brunswick during the summer months of 1990 (detection limit
0.2 µg/litre) (Ecobichon & Allen, 1990).
In a survey of untreated and treated water from 10 potable water
treatment plants along the Great Lakes system in Ontario in 1982-1983,
1,2-dichloroethane was detected (< 0.1 µg/litre) in one sample each
for untreated and treated water in the summer, not at all in the
winter, and in two samples of each (<0.1 µg/litre) in the spring
(Otson, 1987). In an earlier survey of 30 potable water treatment
facilities serving major population centres across Canada sampled in
1979, 1,2-dichloroethane was detected frequently in both untreated and
treated water at mean concentrations of up to 2 µg/litre and
5 µg/litre, respectively (Otson et al., 1982).
Based on a summary of data on levels of 1,2-dichloroethane in
groundwater and surface water supplies from six US Federal surveys,
1,2-dichloroethane was detected in 24 out of 1973 samples of
groundwater at concentrations up to 18 µg/litre and in 12 of 589
samples of surface water at concentrations up to 19 µg/litre
(detection limits not specified) (Letkiewicz et al., 1982).
The US EPA (1987) estimated that 0.3% of groundwater and 3% of
surface water supplies contain concentrations of 1,2-dichloroethane in
the range of 0.5 to 5 µg/litre and 0.5 to 20 µg/litre, respectively
(the basis for these estimates was not specified). 1,2-Dichloroethane
was detected (detection limit not clearly specified) in 7 out of 1792
wells in Wisconsin, USA in the early 1980s; in two of the wells,
concentrations exceeded 7 µg/litre) (Krill & Sonzongni, 1986). In the
Love Canal district of Niagara, New York, 1,2-dichloroethane was
detected in the drinking-water in three out of nine residences
surveyed, at a concentration of 50 ng/litre (Barkley et al., 1980).
Concentrations of 1,2-dichloroethane in drinking-water from five
locations in Japan ranged from non-detectable (i.e. < 0.5 µg/litre)
to 0.9 µg/litre (Fujii, 1977). It was not detected in the
drinking-water samples from 100 cities in Germany (detection limit,
1.0 µg/litre) (Bauer, 1981). 1,2-Dichloroethane was not detected
(detection limit, 0.5 µg/litre) in 229 out of 232 groundwater stations
in the Netherlands surveyed from 1976 to 1978; in the other three
stations concentrations ranged from 0.8 to 1.7 µg/litre (Zoetman et
al., 1979). Concentrations of 1,2-dichloroethane ranged from 2 to
22 µg/litre in 400 samples of drinking-water from six cities in Spain
in 1987 (Freiria-Gandara et al., 1992).
5.1.4 Surface water
1,2-Dichloroethane was detected in 2% of samples in surveys in
the early 1980s of Canadian surface waters (Government of Canada,
1994), but it was not detected (detection limit of 0.08 µg/litre) in
351 samples from several lakes and rivers in Ontario (Kaiser et al.,
1983; Comba & Kaiser, 1985; Kaiser & Comba, 1986; Lum & Kaiser, 1986).
It was detected 300 m downstream of a plant manufacturing
1,2-dichloroethane in Ontario, with a maximum concentration of
16 µg/litre (Environment Canada, 1986).
1,2-Dichloroethane was detected (detection limit not specified)
in 53 of 204 sites from six river basins in the USA surveyed before
1977 at concentrations ranging from 1 to 15 µg/litre and one site
containing 90 µg/litre (HSDB, 1993). It was detected (detection limit
not specified) in 7% of 4972 samples of surface water from the Ohio
River basin in the USA in 1980-1981; concentrations ranged from 1 to
10 µg/litre in 44 samples (HSDB, 1993).
1,2-Dichloroethane was detected in 39 of 102 samples of surface
water from 14 of 34 sites in Japan in 1992 at concentrations ranging
from non-detectable (i.e., < 0.01 µg/litre) to 3.4 µg/litre
(Environment Agency Japan, 1993).
Concentrations of 1,2-dichloroethane in the influent of six
wastewater treatment plants in the Netherlands ranged from < 2 to
400 µg/litre, while levels in the effluents ranged from < 2 to
74 µg/litre. The variation was determined to be due to industrial
discharges (van Luin & van Starkenburg, 1984).
5.1.5 Food
1,2-Dichloroethane was not detected in any samples of 34 food
groups (consisting of dairy products, meats, eggs, fish, soup, bread,
cereal, pasta, fruit, vegetables, cooking oil, peanut butter,
sugar/jam, coffee/tea, soft drinks, wine/beer and tap water) collected
in Calgary, Canada, in 1991 (detection limit 50 µg/kg for solids and
1.0 µg/litre for liquids) (Enviro-Test Laboratories, 1991). In
January 1992, the study was repeated for the same 34 food groups
collected in Windsor, Canada, using more sensitive analytical
methodology (detection limit 5 µg/kg for solids and 1 µg/litre for
liquids). Based on preliminary results, 1,2-dichloroethane was not
detected in any of the samples analysed (Enviro-Test Laboratories,
1992).
In a Total Diet Study conducted by the US Food and Drug
Administration (FDA), 1,2-dichloroethane was not detected in 11
decaffeinated instant coffees or in 14 decaffeinated ground coffees
(detection limit not specified) (Heikes, 1987a).
1,2-Dichloroethane was detected only in one ready-to-eat cereal
(mean 0.31 µg/kg) out of 19 table-ready food items, including butter,
margarine, ready-to-eat cereals, cheese, peanut butter, processed
foods and drinking-water, which were selected to be representative of
the 234 table-ready food items examined in the Total Diet Study
(Heikes, 1987b, 1990). In further analysis of these foodstuffs,
1,2-dichloroethane was detected only in plain granola and shredded
wheat cereal at concentrations of 12 and 8.2 µg/kg, respectively
(Heikes, 1987b).
1,2-Dichloroethane was detected only in one item (whisky, at a
concentration of 30 µg/kg) in an additional Total Diet Study in the
USA of 231 different table-ready foods (quantification limit 9 µg/kg).
The food types examined included off-the-shelf cooked and uncooked
grain-based items, dairy products, fresh and canned fruits and
vegetables, meats and meat dishes, infant and junior blends, baked
goods, nuts and nut products, clear beverages, sugars, jams, and
candies (Daft, 1988). 1,2-Dichloroethane was not detected in four
earlier composite market basket surveys of dairy products, meats, oils
and fats, and beverage products (detection limit not specified) in the
USA (Entz et al., 1982).
In Germany, the mean concentrations of 1,2-dichloroethane in 12
samples of milk-products containing fruits (i.e. ice-cream, yoghurt,
curds and buttermilk) was 0.8 µg/kg fresh weight, with a maximum
concentration of 3.5 µg/kg fresh weight (detection limit not
specified) (Bauer, 1981).
Prior to 1984, 1,2-dichloroethane was used in Canada as a grain
fumigant (Lange, personal communication to the IPCS). In an early
survey, 1,2-dichloroethane concentrations ranged from 23 to 38 mg/kg
in wheat which had been treated with a fumigant containing
1,2-dichloroethane (Wit et al., 1969). 1,2-Dichloroethane could not
be "determined satisfactorily" in wheat which had been fumigated with
a mixture containing 30% of the compound (limit of detection specified
as 1.5 ng); similarly, it was not detected or determined only at trace
levels (not further specified) in samples of cereals (Berck, 1974).
1,2-Dichloroethane is currently not registered for use in
agricultural products in the USA. It was detected in wheat and
bleached flour samples at concentrations of 110 and 180 µg/kg and 6.1
and 6.5 µg/kg, respectively (limit of quantification 6 µg/kg), in a
survey of compounds used as fumigants in whole grains, milled grain
products and intermediate grain-based foods (Heikes & Hopper, 1986).
In 1979, it was detected at a concentration of 290 mg/kg in 1 out of
71 samples of wheat grown in the USA, but was not detected in 61
samples of wheat exported from England to the USA (Bailey et al.,
1982). Cooking (steaming, baking, etc.) tends to reduce levels of
1,2-dichloroethane in most foods contaminated during fumigation (Bond,
1984).
The use of 1,2-dichloroethane in agricultural products in the
United Kingdom has been discontinued. In earlier surveys, it was
detected in one out of 155 samples of wheat grown in the United
Kingdom in 1978-1979 at a concentration of 290 mg/kg and in none of
126 samples of imported wheat (MAFF, 1982); in 1981 and 1982,
1,2-dichloroethane was not detected in 47 and 59 samples of wheat,
respectively (MAFF, 1984). It was also not detected in 84 samples of
brown rice, 107 samples of rye products and 71 samples of processed
oats collected in 1985-1986 (MAFF, 1989). More recently,
1,2-dichloroethane was not detected (detection limit 0.4 mg/kg) in 24
samples of rice analysed in 1992 (UK HSE, 1992; MAFF/HSE, 1993).
No information on concentrations of 1,2-dichloroethane in breast
milk of women in the general population is available.
5.1.6 Soils and sediments
1,2-Dichloroethane was not detected (detection limit 0.01 mg/kg)
in 30 samples of soil taken from "typical" urban residential and
parkland locations in southern Ontario, Canada in 1987 (Golder
Associates, 1987). The mean concentration of 1,2-dichloroethane in
soil near 20 homes in areas of the Netherlands with "uncontaminated"
soil was 11 mg/kg, while samples of soil in the vicinity of a garage
and a waste site contained < 5 and 30 mg/kg, respectively (Kliest et
al., 1989). The US EPA (1988) reported that 1,2-dichloroethane has
been detected in soil samples from 1.5% of 2783 hazardous waste sites
sampled in the USA (concentrations and detection limits were not
reported).
1,2-Dichloroethane was not detected (detection limit, 0.01 µg/kg)
in sediments downstream of two facilities in Canada which manufactured
the compound (Oliver & Pugsley, 1986; AEC, 1989). It was detected in
11 out of 99 samples of sediment from 5 out of 33 areas surveyed in
Japan in 1992 at concentrations ranging from non-detectable (i.e.,
< 0.4 µg/kg) to 0.7 µg/kg (dry weight) (Environment Agency Japan,
1993).
5.1.7 Consumer products
In studies conducted in the USA, 1,2-dichloroethane was released
from cleaning agents and pesticides, glued wallpaper and glued carpets
in environmental chambers, while it was not emitted by painted
sheetrock (detection limit not specified) (Wallace et al., 1987).
More recently, 1,2-dichloroethane was detected in 5 out of 1043
household products tested in the USA; concentrations ranged up to 0.1%
(by weight) in automotive products, oils, greases and lubricants, and
miscellaneous products (Sack et al., 1992). It should be noted that
the use of 1,2-dichloroethane in products such as upholstery and
carpet fumigants, soap and scouring compound ingredients, wetting and
penetrating agents and degreasing fluid has been largely discontinued
in the USA. In addition it is not used in any registered drug
products in the USA (Drury & Hammons, 1979).
In a survey in Germany, 1,2-dichloroethane was not detected in
facial soap, mouthwash or toothpaste (detection limit not specified).
However, it was detected in shampoo and shaving cream at levels
ranging up to 7.6 µg/litre and 122 µg/litre, respectively, and in 1
out of 7 cough-syrups at a concentration of 12.9 µg/kg (Bauer, 1981).
No data on concentrations of 1,2-dichloroethane in cigarettes are
available. No difference was reported between the median air
concentrations of 1,2-dichloroethane in air in the offices of smokers
and those in the offices of non-smokers in southern England (Proctor
et al., 1989).
5.2 General population exposure
Based on estimates of mean exposure from various media, the
principal source of exposure to 1,2-dichloroethane by the general
population is indoor and outdoor air (< 0.03 to 0.1 µg/kg body weight
per day and 0.004 to 0.02 µg/kg body weight per day, respectively),
with only minor amounts being contributed by drinking-water (< 0.001
to 0.003 µg/kg body weight per day). Intake of 1,2-dichloroethane
from food is probably negligible. For some individuals residing in
the vicinity of industrial sources of airborne 1,2-dichloroethane,
intake from ambient air may be substantially greater than that for the
general population.
5.2.1 Ambient air
Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg, the assumption that 4 out
of 24 h are spent outdoors (IPCS, 1994), and the range of mean
1,2-dichloroethane levels found in a recent survey of cities across
Canada (0.07-0.28 µg/m3 as presented in section 5.1.1), mean intake
of 1,2-dichloroethane from ambient air for the general population is
estimated to range from 0.004 to 0.02 µg/kg body weight per day.
5.2.2 Indoor air
Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg, the assumption that 20 out
of 24 h are spent indoors (IPCS, 1993), and the range of
1,2-dichloroethane concentrations in indoor air or "personal" air in
surveys in Canada and the USA (< 0.1 to 0.5 µg/m3 as presented in
section 5.1.2), mean intake of 1,2-dichloroethane from indoor air for
the general population is estimated to range from < 0.03 to 0.1 µg/kg
body weight per day.
5.2.3 Drinking-water
Based on a daily volume of water consumption for adults of 1.4
litres, a mean body weight for males and females of 64 kg (IPCS,
1993), and the mean levels of 1,2-dichloroethane in provincial surveys
in Canada (< 0.05 to 0.139 µg/litre as presented in section 5.1.3),
mean intake of 1,2-dichloroethane from drinking-water for the general
population is estimated to range from < 0.001 to 0.003 µg/kg body
weight per day.
5.2.4 Food
Based on its low octanol/water partition coefficient,
1,2-dichloroethane is unlikely to bioaccumulate, and therefore it is
considered that food does not represent a significant source of
exposure for the general population. It has only rarely been detected
in individual samples of foodstuffs in North America (see section
5.1.5). Even if the compound was assumed to be present in foods at
concentrations up to the limit of detection in the surveys with the
more sensitive analytical methodology, the daily intake of
1,2-dichloroethane from food would still be negligible compared to
that from air.
5.2.5 Other media
Available data were considered insufficient to estimate intake of
1,2-dichloroethane from soil or consumer products.
5.3 Occupational exposure during manufacture, formulation or use
Based on a review of available information, current occupational
exposure to 1,2-dichloroethane in North America occurs predominantly
during the manufacture of other chemicals, such as vinyl chloride,
where 1,2-dichloroethane is used as an intermediate. In a 1982
National Occupational Exposure Survey by the US National Institute for
Occupational Safety and Health (NIOSH), 28% of employees working with
adhesives and solvents were exposed to 1,2-dichloroethane, while
between 5 and 9% of workers were exposed to the substance in the
medicinals and botanicals, biological products, petroleum refining and
organic chemicals industries, and in museums and art galleries (US
Department of Labour, 1989).
Mean concentrations of 1,2-dichloroethane at three production
plants in the United Kingdom in 1990 were 2.8, 3.2 and 6.8 mg/m3
(0.7, 0.8 and 1.7 ppm); 95% of samples contained less than 20 mg/m3
(5 ppm), while maximum values at the plants were 18, 80 and
160 mg/m3 (4.5, 20 and 40 ppm) (UK HSE, 1992).
The time-weighted average concentration of 1,2-dichloroethane in
an electron microscopy preparation laboratory in Hong Kong, in which
the chemical was used as a solvent, was 19.8 mg/m3 (4.9 ppm). The
concentration in the breathing zone of the operator was 52.87 mg/m3
(13.06 ppm) while the average concentration in the preparation room
was 35.1 mg/m3 (8.67 ppm) (Li & Cheng, 1991).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Case reports of acute effects following inhalation exposure to
1,2-dichloroethane in the workplace indicate that it is readily
absorbed (Nouchi et al., 1984).
In experimental animals, absorption following ingestion of
1,2-dichloroethane is rapid and complete. Spreafico et al. (1980) and
Reitz et al. (1982) reported that peak levels in blood (13 to
67 mg/litre) occurred within 10 or 15 min in rats administered single
oral doses of 25, 50 or 150 mg/kg body weight in corn oil. A plot of
administered dose against peak blood level appeared linear up to
50 mg/kg, with a perceptible decrease in steepness thereafter,
possibly indicating a relative saturation in gastrointestinal
absorption at doses of 100 to 150 mg/kg body weight. (The authors
noted that there were no significant differences in kinetic parameters
following single and 10 daily administrations of 50 mg/kg body
weight). Gastrointestinal absorption in rats was more rapid and
efficient following administration in water, compared to corn oil
(Withey et al., 1983).
Absorption following inhalation in experimental animals is also
rapid. In rats, levels of 1,2-dichloroethane in the blood peaked (8
to 10 mg/litre) within 1-2 h of continuous inhalation of 600 mg/m3
(150 ppm) for 6 h (Reitz et al., 1982).
The rate of dermal absorption of 1,2-dichloroethane by mice was
479.3 ± 38.3 nmol/min per cm2 following covered application of 0.5 ml
of the undiluted solvent (Tsuruta, 1975), while the rate of absorption
of 1,2-dichloroethane in 0.9% NaCl in vitro in excised skin of rats
was 169 ± 0.44 nmoles/min per cm2 (Tsuruta, 1977). Dermal
absorption of 1,2-dichloroethane in aqueous solution (1000 mg/litre)
was found to be similar in human and rat epidermis in vitro within
one hour of occluded application (20.3 µg/cm2 per h versus 33.1 µg/cm2
per h), whereas when the substance was applied neat (uncovered),
absorption within the first 15 min was approximately four to ten-fold
greater in the rat epidermis than in the human epidermis. In
addition, absorption increased with applied dose in the rat epidermis,
whereas absorption was not dependent upon dose in the human epidermis
(Ward, 1992).
The concentration of 1,2-dichloroethane in the blood of
guinea-pigs increased rapidly (up to approximately 7 mg/litre) during
the first 30 min following covered application of 1.0 ml of the
undiluted compound to shaved skin; the level in blood then began to
decrease abruptly to a minimum (approximately 5 mg/litre) after one
hour, at which point it began to gradually increase again (up to about
17 mg/litre after 12 h) (Jakobson et al., 1982). 1,2-Dichloroethane
was also rapidly absorbed when applied in aqueous solution to the skin
of rats in vivo, with the levels in blood being directly related to
the concentration of the solution (Morgan et al., 1991).
6.2 Distribution
Absorbed 1,2-dichloroethane is widely distributed throughout the
human body, based on analysis of several tissues of humans who died
following acute oral poisonings with the substance. Concentrations
ranged from 1 to 50 mg/kg in the spleen and 100 to 1000 mg/kg in the
stomach; levels in the liver and kidney were approximately 10 times
less than those in the stomach (Luznikov et al., 1985). The
metabolite 2-chloroacetaldehyde was not detected; detectable
quantities of 2-chloroethanol and monochloroacetic acid were reported,
though levels were too low to compare among tissues.
1,2-Dichloroethane has been detected in the breast milk of women
occupationally exposed via inhalation and dermal contact (Urusova,
1953).
Similarly, 1,2-dichloroethane is widely distributed throughout
the body in experimental animals exposed via inhalation or ingestion.
The highest concentrations were usually found in adipose tissue,
although it was also detected in blood, liver, kidney, brain and
spleen. 1,2-Dichloroethane accumulated most rapidly in the liver of
rats administered single oral doses of 25, 50 or 150 mg/kg body weight
in corn oil, although concentrations were greatest in adipose tissue.
Peak levels in adipose tissue, at 45 to 60 min, exceeded those in
blood by 3.9 to 8.3 times, whereas peak levels in the liver, 10 min
after exposure, exceeded those in the blood by 1.3 to 2.2 times
(Spreafico et al., 1980). Accumulation was less than expected at the
two higher exposure levels, indicating saturation of the tissues.
Similar accumulation in adipose tissue in rats was noted following
inhalation of 200 or 1000 mg/m3 (50 or 250 ppm) for up to 6 h.
During inhalation, steady state levels were reached within 2 to 3 h
and increased 20-to 30-fold when the exposure increased from 202 to
1012 mg/m3, suggesting saturable metabolic capacity. Levels of
1,2-dichloroethane in the spleen, brain and kidney were similar to
those in the blood, irrespective of the route of exposure (Spreafico
et al., 1980).
Reitz et al. (1982) reported that the relative distribution of
radioactivity at 48 h (assumed to be primarily in the form of
metabolites) was similar in rats administered [14C]-labelled
1,2-dichloroethane orally (single dose of 150 mg/kg body weight) or by
inhalation (600 mg/m3 or 150 ppm for 6 h). Residual reactivity in
selected tissues was 1.5 to 2 times higher after oral exposure than
following inhalation. There was also a higher residual activity in
the forestomach after the oral exposure. The distribution pattern for
macromolecular binding was similar, as determined 4 h after oral
ingestion or directly after inhalation. Oral exposure produced lower
(i.e. 1.5 to 2 times less) levels of total macromolecular binding but
higher (i.e. 3 to 5 times more) levels of DNA alkylation than
inhalation, though the absolute levels were considered low.
Arfellini et al. (1984) reported a greater degree of binding to
DNA in organs (liver, kidneys, lung and stomach) of mice than in those
of rats (1.45 to 2.26 fold) 22 h after intraperitoneal administration
of equivalent single doses of 8.7 µmoles/kg body weight.
In periods from 1 min to 4 days following intravenous
administration of a single dose (0.73 mg/kg body weight) of
radiolabelled 1,2-dichloroethane to mice, the highest levels of
radioactivity (non-volatile and bound metabolites) determined by whole
body autoradiography were present in the nasal olfactory mucosa and
the tracheo-bronchial epithelium. Low levels of metabolites were also
present in the epithelium of the upper alimentary tract, vagina and
eyelid and in the liver and kidney. Mucosal and epithelial binding
was decreased by pretreatment with metapyrone, indicating that binding
might be due to oxidative metabolism. In in vitro studies in
tissues from the same strain of mice, reactive products of
1,2-dichloroethane were irreversibly bound to the nasal mucosa, lung
and liver but not to the oesophagus, forestomach or vagina. The level
of binding in the nasal mucosa was twice that in the lung and 1.4
times that in the liver. On the basis of their results, the authors
suggested that the epithelium of the respiratory tract may be a
potential target for the toxic effects of 1,2-dichloroethane due to in
situ metabolism to reactive intermediates (Brittebo et al., 1989).
1,2-Dichloroethane was detected in fetal tissue of rats following
maternal exposure to airborne concentrations ranging from 612-
7996 mg/m3 (153-1999 ppm) on day 17 of gestation, the detected
concentrations in fetal tissues being related to the level of exposure
as well as the position on the uterine horn (Withey & Karpinski,
1985).
6.3 Metabolic transformation
1,2-Dichloroethane is metabolized extensively in rats and mice.
It is principally sulfur-containing metabolites that are eliminated in
the urine. Mitoma et al. (1985) reported slightly more complete
metabolism in mice than in rats, based on 100% recovery of metabolites
as expired CO2 and in the excreta and carcasses of mice administered
an oral dose of 150 mg/kg body weight [14C]-labelled
1,2-dichloroethane, compared to about 85% in rats administered
100 mg/kg body weight. This difference may have been due to
experimental variation or error. Reitz et al. (1982) reported 70 and
91% transformation of 1,2-dichloroethane in the rat following oral
(150 mg/kg body weight) and inhalation (607 mg/m3, 6 h) exposures,
respectively, with 85% of the metabolites appearing in the urine.
Proposed metabolic pathways for 1,2-dichloroethane are
illustrated in Fig. 1. Metabolism appears to occur via two principal
pathways for which the reactions and subsequent metabolism of the
products can account for all of the identified sulfur-containing
metabolites in the urine of 1,2-dichloroethane-exposed animals. One
pathway begins with cytochrome P-450-mediated oxidation, and the other
with glutathione conjugation. In the first pathway, cytochrome P-450
enzymes catalyse an oxidative transformation of 1,2-dichloroethane to
form reactive intermediates, which result in the formation of
2-chloroacetal-dehyde and 2-chloroethanol (Guengerich et al., 1980),
which are conjugated both enzymatically and non-enzymatically with
glutathione (GSH) and excreted in the urine. Guengerich et al. (1991)
concluded that cytochrome P-450 IIE1 is a major catalyst in the
oxidation of 1,2-dichloroethane in human microsomes.
The other pathway involves direct conjugation with glutathione to
form S-(2-chloroethyl)-glutathione, which is a half mustard with a
half-life of 69 min at 20°C (Schasteen & Reed, 1983) and less than 15
min at 37°C (Foureman & Reed, 1985). Non-enzymic conversion of the
half mustard to the corresponding episulfonium ion gives a putative
alkylating agent (episulfonium ion) that has several fates. Reaction
can occur with water to form S-(2hydroxyethyl) glutathione, with
thiols such as GSH to form ethene bis-glutathione, or with DNA to form
adducts. With the exception of the precursors which form DNA adducts,
the reaction products are considered non-toxic and undergo further
metabolism.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROETHANE
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency,
Washington DC, USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist, The
Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROETHANE
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met in Geneva from 25 October to 3 November 1994.
Dr W. Kreisel, Executive Director, welcomed the participants on behalf
of WHO, and Dr M. Mercier, Director, IPCS, on behalf of the IPCS and
its cooperating organizations (UNEP/ILO/WHO). The Core Assessment
Group reviewed and revised the draft monograph and made an evaluation
of the risks for human health and the environment from exposure to
1,2-dichloroethane (ethylene dichloride).
The first draft of the monograph was prepared by Ms K. Hughes,
Environmental Health Directorate, Health Canada. The second draft,
revised in the light of international comment, was also prepared by
Ms K. Hughes. Dr E. Smith and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the scientific content and
technical editing respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
1,2-Dichloroethane was previously evaluated by a WHO Task Group
in 1986 and published by WHO in 1987 as Environmental Health Criteria
62.
ABBREVIATIONS
BCF bioconcentration factor
BUN blood urea nitrogen
ECD electron capture detector
FID flame ionization detector
GC gas chromatography
GSH glutathione
gamma-GT gamma-glutamyltranspeptidase
HECD Hall electron capture detector
LOEL lowest-observed-effect level
MS mass spectrometry
NOEL no-observed-effect level
PIB piperonyl butoxide
SGOT serum glutamic-oxalic transaminase
SGPT serum glutamic-pyruvic transaminase
TEAM total exposure assessment methodology
TWA time-weighted average
1. Summary
1.1 Identity, physical and chemical properties, and analytical
methods
1,2-Dichloroethane (ethylene dichloride) is a synthetic chemical
which is a colourless liquid at room temperature. It is also highly
volatile, with a vapour pressure of 8.5 kPa (at 20°C), and is soluble
in water, with a solubility of 8690 mg/litre (at 20°C). The log
octanol/water partition coefficient is 1.76.
Analysis for 1,2-dichloroethane in environmental media is usually
by gas chromatography, in combination with electron capture or flame
ionization detection or mass spectrometry. Detection limits range
from 0.016 to > 4 µg/m3 in air, 0.001 to 4.7 µg/litre in water, and
from 6 to 10 µg/kg in various foodstuffs.
1.2 Sources of human and environmental exposure
The principal use of 1,2-dichloroethane is in the synthesis of
vinyl chloride monomer, and to a lesser extent in the manufacture of
various chlorinated solvents. It is also incorporated into anti-knock
gasoline additives (although this use is declining with the phase-out
of leaded gasoline in some countries), and has been used as a
fumigant. Total annual production of 1,2-dichloroethane in Canada in
1990 and the USA in 1991 was 922 and 6318 kilotonnes, respectively.
1.3 Environmental transport, distribution and transformation
The majority of 1,2-dichloroethane released to the environment is
in emissions to air. It is moderately persistent in air; the
estimated atmospheric lifetime is between 43 and 111 days.
1,2-Dichloroethane is transported to the stratosphere, where
photolysis may produce chlorine radicals which may in turn react with
ozone. Some 1,2-dichloroethane may be released in industrial
effluents to the aquatic environment, from which it is removed rapidly
by volatilization. 1,2-Dichloroethane may also leach to groundwater
near industrial waste sites. It is not expected to bioconcentrate in
aquatic or terrestrial species.
1.4 Environmental levels and human exposure
Mean concentrations of 1,2-dichloroethane in recent surveys of
ambient air in non-source-dominated areas of cities range from 0.07 to
0.28 µg/m3, while mean levels in residential indoor air are reported
to range from < 0.1 to 3.4 µg/m3. In drinking-water, mean
concentrations are generally less than 0.5 µg/litre.
1,2-Dichloroethane has only rarely been detected in foodstuffs in
recent surveys and, since it has low potential for bioaccumulation,
food is unlikely to be a major source of exposure.
Based on estimates of mean exposure from various media, the
predominant source of exposure to 1,2-dichloroethane by the general
population is indoor and outdoor air, only minor amounts being
contributed by drinking-water. Intake of 1,2-dichloroethane from food
is probably negligible. The amount inhaled in ambient air may be
greater in the vicinity of industrial sources.
1.5 Kinetics and metabolism in laboratory animals
1,2-Dichloroethane is readily absorbed following inhalation,
ingestion or dermal exposure and is rapidly and widely distributed
throughout the body. It is rapidly and extensively metabolized in
rats and mice, with principally sulfur-containing metabolites being
eliminated in the urine in a dose-dependent manner. Metabolism
appears to be saturated or limited in rats at levels of exposure
resulting in blood concentrations of 5 to 10 µg/ml. Levels of DNA
alkylation were higher following exposure to a bolus dose by gavage
than in the case of inhalation over a 6-h period.
1,2-Dichloroethane appears to be metabolized via two principal
pathways; the first involves a saturable microsomal oxidation mediated
by cytochrome P-450 to 2-chloroacetaldehyde and 2-chloroethanol
followed by conjugation with glutathione. The second pathway entails
direct conjugation with glutathione to form S-(2-chloroethyl)-
glutathione, which may be non-enzymatically converted to a glutathione
episulfonium ion; this ion can form adducts with DNA. Although DNA
damage has been induced by the P-450 pathway in vitro, several lines
of evidence indicate that the glutathione conjugation pathway is
probably of greater significance than the P-450 pathway as the major
route for DNA damage.
1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of 1,2-dichloroethane is low in experimental
animals. For example, inhalation LC50s for rats exposed for 6 or
7.25 h ranged from 4000 mg/m3 to 6600 mg/m3, while oral LD50s
for rats, mice, dogs and rabbits ranged from 413 to 2500 mg/kg body
weight.
The results of short-term and subchronic studies in several
species of experimental animals indicate that the liver and kidneys
are the target organs; reliable NOELs or LOELs were not attained in
general due to inadequate documentation and the limited range of
end-points examined in small groups of animals. In a series of early
limited studies, morphological changes in the liver were observed in
several species following subchronic exposure to airborne
concentrations as low as 800 mg/m3. Increases in the relative liver
weight have been observed in rats following subchronic oral
administration of doses of 49 to 82 mg/kg body weight per day or more
for 13 weeks. Little information was presented on non-neoplastic
effects in available chronic studies. Changes in serum parameters
indicative of liver and kidney toxicity were observed in rats exposed
to airborne concentrations as low as 202 mg/m3 for 12 months,
although histopathological examinations were not conducted in this
study.
The carcinogenicity of 1,2-dichloroethane has been investigated
in a few limited bioassays on experimental animals (limitations
include short duration of exposure and high mortality). Significant
increases were not reported in the incidence of any type of tumour in
Sprague-Dawley rats or Swiss mice exposed to up to 607 mg/m3 for 78
weeks and observed until spontaneous death. Mortality was high in
rats in this study, although it was not related to concentration, and
the incidence rates were not adjusted for differential mortality among
groups. There was a nonsignificant increase in the incidence of
mammary gland adenomas and fibroadenomas in female Sprague-Dawley rats
exposed to 200 mg/m3 for 2 years in an assay in which no other
compound-related toxicity was observed.
In contrast, there was convincing evidence of increases in tumour
incidence in two species following ingestion. Significant increases
in the incidence of tumours at several sites (including squamous cell
carcinomas of the stomach (males), haemangiosarcomas (males and
females), fibromas of the subcutaneous tissue (males), adenocarcinomas
and fibroadenomas of the mammary gland (females)) were observed in
Osborne-Mendel rats administered TWA daily doses of 47 or 95 mg/kg
body weight per day by gavage for 78 weeks. Similar increases in the
incidences of tumours at multiple sites (including
alveolar/bronchiolar adenomas (males and females), mammary gland
adenocarcinomas (females) and endometrial stromal polyp or endometrial
stromal sarcoma combined (females) and hepatocellular carcinomas
(males)) occurred in B6C3F1 mice administered TWA daily doses of 97 or
195 mg/kg body weight per day (males) or 149 or 299 mg/kg body weight
per day (females) by gavage for 78 weeks.
The incidence of lung tumours (benign papillomas) was
significantly increased in female mice following repeated dermal
application of 1,2-dichloroethane for 440 to 594 days. Repeated
intraperitoneal injections of 1,2-dichloroethane resulted in
dose-related increases in the number of pulmonary adenomas per mouse
in a susceptible strain, although none of these increases was
significant. Concomitant exposure to inhaled 1,2-dichloroethane and
disulfiram in the diet resulted in an increased incidence of
intrahepatic bile duct cholangiomas and cysts, subcutaneous fibromas,
hepatic neoplastic nodules, interstitial cell tumours in the testes
and mammary adenocarcinomas in rats, compared to rats administered
either compound alone or untreated controls. No potential to initiate
or promote tumour development was evident in three bioassays, although
the extent of histopathological examination was limited in these
studies.
In in vitro assays, 1,2-dichloroethane has been consistently
positive in mutagenicity bioassays in Salmonella typhimurium.
Responses have been greater in the presence of an exogenous activation
system (possibly due to activation by the cytochrome system) than in
its absence, and mutagenicity was more than doubled in S. typhimurium
expressing the human GSTA1-1 gene. In cultured mammalian cells,
1,2-dichloroethane forms adducts with DNA. It also induces
unscheduled DNA synthesis in primary cultures of rodent and human
cells and gene mutation in several cell lines. Mutation frequency in
human cell lines has been correlated with differences in
glutathione- S-transferase activity. In in vivo studies,
1,2-dichloroethane induced somatic cell and sex-linked recessive
lethal mutations in Drosophila melanogaster and the compound bound
to DNA in all reported studies in rats and mice. Although primary DNA
damage in liver and sister chromatid exchange has been observed in
studies in mice, there has been no evidence for micronucleus
induction.
Based on the results of a limited number of studies, there is no
evidence that 1,2-dichloroethane is teratogenic in experimental
animals. There is also little convincing evidence that it induces
reproductive or developmental effects at doses below those which cause
other systemic effects. Available data on the immunotoxicity of
1,2-dichloroethane are limited.
1.7 Effects on humans
Acute incidental exposure to 1,2-dichloroethane by inhalation or
ingestion has resulted in a variety of effects in humans, including
effects on the central nervous system, liver, kidney, lung and
cardiovascular system.
The potential carcinogenicity of 1,2-dichloroethane in exposed
human populations has not been extensively investigated. Mortality
due to pancreatic cancer was significantly increased in a group of
workers at a chemical production plant who had been exposed
principally to 1,2-dichloroethane (in combination with other
chemicals). Mortality due to pancreatic cancer increased with
duration of exposure. In addition, although the number of cases was
small, and the association with duration of exposure was less
consistent, mortality due to leukaemia was also increased in these
workers. No association between occupational exposure to
1,2-dichloroethane and brain cancer was noted in a small case-control
study. Although the incidence of colon and rectal cancer increased
with the concentration of 1,2-dichloroethane in drinking-water in an
inherently limited ecological study, concomitant exposure to other
substances may have contributed to the observed effects.
1.8 Effects on non-target organisms in the laboratory and field
The effects of exposure to 1,2-dichloroethane on a number of
other organisms in the laboratory and field have been investigated.
For aquatic microorganisms, IC50s or EC50s for various end-points
have been reported to range from 25 to 770 mg/litre. The lowest
reported LC50 value for Daphnia was 220 mg/litre, while effects on
reproductive success and growth were observed at 20.7 and
71.7 mg/litre, respectively. Based on available data, the most
sensitive freshwater vertebrate species appears to be the northwestern
salamander (Ambystoma gracile), in which 9-day larval survival (4
days post-hatch) was reduced at 2.54 mg/litre. Only limited data are
available on the toxicity of 1,2-dichloroethane to terrestrial
organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
The empirical formula for 1,2-dichloroethane (ethylene
dichloride) is C2H4Cl2 and the molecular structure is as
follows:
H H
' '
Cl - C - C - Cl
' '
H H
Synonyms include EDC, 1,2-DCE, 1,2-bichloroethane, 1,2-ethylene
dichloride, acethylenchlorid, alpha, beta-dichloroethane, bichlorure
d'ethelene, ethyleen dichloride, ethylene chloride, glycol dichloride,
and sym-dichlorothane. Trade names include: Borer sol, Brocide,
Destruxo,l Di-chlor-mulson, Dichlor-mulsion, Dutch liquid, Dutch oil,
ENT 1656, Freon 150, Gaze Olefiant and Granosan (which also contains
carbon tetrachloride).
The Chemical Abstract Service (CAS) registry number for
1,2-dichloroethane is 107-06-2.
2.2 Physical and chemical properties
1,2-Dichloroethane is a clear, colourless liquid at room
temperature. It is a highly volatile and flammable synthetic chemical
which absorbs infrared light at several wavelengths (7, 12 and 13 µm).
Other properties of 1,2-dichloroethane are presented in Table 1.
2.3 Conversion factors
1 ppm = 4 mg/m3
1 mg/m3 = 0.25 ppm (at 25°C and 760 mmHg)
Table 1. Physical properties of 1,2-dichloroethanea
Physical state liquid
Colour colourless
Odour sweet, chloroform-like
Relative molecular mass 98.96
Density d20 1.253
Refractive index r20 1.4449
D
Boiling point 83°C
Melting point -35°C
Water solubility 8690 mg/litre (20°C)
Vapour pressure 8.5 kPa (20°C)
Saturation concentration in air 350 g/m3 (20°C)
537 g/m3 (30°C)
log Kow 1.76
log Koc 1.28
Henry's law constant 111.5 Pa.m3/mol (25°C)
Flash point 12-15°C
Limits of flammability in air 275-700 mg/litre
a From: Archer (1979); Chiou et al. (1979); Konemann (1981);
Warner et al. (1987); Worthing & Hance (1991)
2.4 Analytical methods
Methods of analysis of 1,2-dichloroethane in various
environmental media are described in Table 2. Gas chromatography,
coupled with electron capture or flame ionization detection or mass
spectrometry, is commonly used for analysis of 1,2-dichloroethane in
most media.
Table 2. Analytical methods for 1,2-dichloroethane in environmental mediaa
Sample matrix Preparation method Analytical method Sample detection Percentage Reference
limit recovery
Air collect sample on Tenax(R)-GC absorbent GC/MS 100 ng/m3 not available Wallace et al.
(1984)
not available GC/MS < 20 ng/m3 ± 5% precision Grimsrud &
(< 5 ppt) Rasmussen (1975)
collect in 6-litres canisters; direct GC/ECD-MS > 4 µg/m3 not available Pleil et al.
injection (> 1 ppb) (1988)
collect air sample in tubes filled with GC/MS 30 pg/sample 98-108% Jonsson & Berg
solid absorbent; heat sample tubes; (1980)
monitor for 1,2-dichloroethane using
selected ion monitoring
collect sample on Tenax(R) TA; thermal GC/ECD 16 ng/m3 not available Class &
desorption (4 ppt) Ballschmiter (1986)
charcoal-tube sampler; desorption with GC/FID 10 µg/sample not available NIOSH (1984)
CS2 solvent
continuous monitoring and breath infra-red not available not available Baretta et al.
analysis spectroscopy (1969)
sampling on charcoal or Chromosorb GC/FID 1.2 µg/m3 not available Parkes et al.
(1976)
Table 2. cont'd.
Sample matrix Preparation method Analytical method Sample detection Percentage Reference
limit recovery
collect sample on Tenax(R) polymeric GC/MS 32 ng/m3 not available Krost et al. (1982)
beads
Water purge-and-trap GC/MS 5 ng/litre not available Wallace et al.
(1984)
purge-and-trap GC/FID 0.1 µg/litre 99% Warner & Beasley
(0.1 ppb) (1984)
headspace/cryogenic trapping HR capillary 80 ng/litre 75% Comba & Kaiser
GC/ECD (1983)
Water and purge-and-trap GC 30 ng/litre 1.04-1.06C US EPA (1982b)
wastewater 97.8% (method 601)
grab sample GC/MS 4.7 µg/litre 1.02 + 0.45C US EPA (1982b)
99% (method 624)
modified purge-and-trap GD/HECD and FID FID 0.1 µg/litre; FID 78%; Otson & Williams
simultaneous HECD < HECD 79% (1982)
0.1 µg/litre
stripping by helium adsorption on GC/FID or MS 1 ng/litre not available Sauer (1981)
Tenax(R)
stripping by helium or nitrogen, GC with 0.1-0.4 µg/litre not available Symons et al.
sorption on Tenax(R) or chromosorb microcoulometric (1975)
detection
not available GC/MS 0.5 µg/litre not available Fujii (1977)
Table 2. cont'd.
Sample matrix Preparation method Analytical method Sample detection Percentage Reference
limit recovery
Grains, legumes, acidified acetone-water extraction; GC/ECD not available 14-75% Daft (1987, 1988,
spices, citrus isooctane back extraction; for liquids, 1989, 1991, 1993)
fruits, isooctane extraction
beverages,
dairy
products, meat
Table-ready stirred with water; purge-and-trap GC/ECD 6 µg/kg 85-104% Heikes (1987b);
foods on Tenax(R) GC; hexane desorption (6 ppb) Heikes & Hopper
(1986)
Fish add fish tissue to reagent grade water; GC/MS 10 µg/kg 85 ± 11% Easley et al.
disrupt cells ultrasonically; analyse (1981)
sample by purge-and-trap method
spiked samples of ground fish tissue; GC/MS not available 92 ± 5%c Hiatt (1981)
vaporize VOCs from fish sample under
vacuum and condense in purge-and-trap
homogenize fish sample; remove residual GC/MS-fused not available not available Hiatt (1983)
moisture by vacuum distillation silica capillary column
Sediment spiked samples; vaporize VOCs under GC/MS not available 96 ± 17%c Hiatt (1981)
vacuum and condense in purge-and-trap
a Modified from: ATSDR (1994); CS2 = carbon disulfide; ECD = electron capture detector; FID = flame ionization detector; GC = gas
chromatography; HECD = Hall electron capture detector; MS = mass spectrometry;
b VOCs = volatile organic carbon compounds
c Reported as percentage spike recoveries for 25 µg/kg (ppb) spikes
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
1,2-Dichloroethane is a synthetic chemical which has no known
natural sources.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
1,2-Dichloroethane, first produced in 1795, was the first
chlorinated hydrocarbon to be synthesized (IARC, 1979). It is
manufactured by either the catalytic vapour-phase or liquid-phase
chlorination of ethylene or by oxychlorination of ethylene
(Archer, 1979). Most commercial grade 1,2-dichloroethane is 97-99%
pure (Drury & Hammons, 1979).
The total annual production of 1,2-dichloroethane in Canada in
1990 was estimated to be 922 000 tonnes (CPI, 1991), while the total
production in the USA in 1991 was 6 318 000 tonnes (Chemical Marketing
Reporter, 1992), increasing from a production value of 5 038 000
tonnes in 1980 (Kirschmer & Ballschmiter, 1983). More than 1 million
tonnes of 1,2-dichloroethane was produced in the United Kingdom in
1991 (UK HSE, 1992). 1,2-Dichloroethane is released to the
environment principally through emissions to ambient air during its
production and that of vinyl chloride monomer. 1,2-Dichloroethane is
recovered from waste streams of manufacturing facilities in a
two-stage distillation operation. This waste stream is then
incinerated (McPherson et al., 1979), the estimated destruction
efficiency being 99.99% (US EPA, 1986).
Release of 1,2-dichloroethane to the atmosphere from production
facilities can occur from a number of sources. Incidental emissions
usually comprise around 50% of the total, while releases from
secondary sources, such as losses from process wastewater, valves and
vents, such as thermal oxidizer vents, handling and storage, and other
sources result in release of the balance. The US EPA estimated that
18 000 tonnes of 1,2-dichloroethane was released to the atmosphere in
the USA in 1982 from fugitive sources (e.g., valves, etc.), storage
tanks, secondary sources (e.g., emissions from wastewater treatment
processes), process vents and shipping operations (US DHHS, 1994).
1,2-Dichloroethane is also released to the atmosphere from
automobile emissions due to its incorporation into anti-knock
formulations for leaded petrol (gasoline).
1,2-Dichloroethane may enter surface waters via effluents from
industries that manufacture or use the substance. In addition, it may
enter the atmosphere or groundwater following disposal in waste sites.
3.2.2 Uses
The predominant uses of 1,2-dichloroethane is as an intermediate
in the synthesis of vinyl chloride; 99% of total demand in Canada, 90%
in Japan and 88% of total production in the USA is used for this
purpose (CPI, 1991; Chemical Marketing Reporter, 1992). It has also
been used in the production of chlorinated solvents such as
trichloroethylene, tetrachloroethylene, 1,1,1-trichloroethane,
ethyleneamines and vinylidene chloride, and in the manufacture of
anti-knock fluids containing tetraethyllead, although this latter use
has declined with the phase-out of leaded petrol. 1,2-Dichloroethane
has been used as a fumigant. However, it is no longer registered for
use on agricultural products in Canada, the USA, the United Kingdom
and Belize.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and fate in the environment
Due to the high vapour pressure of 1,2-dichloroethane, the
atmosphere is expected to be the predominant environmental sink for
the compound. The rate of reaction of 1,2-dichloroethane with hydroxyl
radicals has been predicted to be 3.63 × 10-13 cm3/mol-sec at
25°C (Atkinson, 1987) and 5.42 × 10-13 cm3/mol-sec at 4°C
(Nimitz & Skaggs, 1992). It was experimentally determined to be 2.09
× 10-13 cm3/mol-sec at 19°C (Qiu et al., 1992). Based on these
values, and assuming an atmospheric hydroxyl radical concentration
representative of a moderately polluted area (Finlayson-Pitts &
Pitts, 1986), the estimated atmospheric lifetime of 1,2-dichloroethane
is between 43 and 111 days. Due to the moderate persistence of
1,2-dichloroethane in the troposphere, long-range transport is
possible. Indeed, 1,2-dichloroethane has been detected in the lower
troposphere over the northern Atlantic Ocean and over the Pacific
Ocean (Singh et al., 1983; Class & Ballschmiter, 1986).
Once 1,2-dichloroethane reaches the troposphere, it undergoes
photo-oxidation to produce formyl chloride, chloroacetyl chloride,
hydrochloric acid, carbon monoxide and carbon dioxide (Spence &
Hanst, 1978). Any 1,2-dichloroethane that reaches the stratosphere
may be photolysed to produce chlorine radicals that may, in turn,
react with ozone (Spence & Hanst, 1978; Callahan et al., 1979).
However, 1,2-dichloroethane is not expected to contribute
significantly to the depletion of the stratospheric ozone layer,
since, based on either the experimental or predicted rates of reaction
between hydroxyl radicals and 1,2-dichloroethane, its ozone depletion
potential is very much less than 0.001 relative to the
chlorofluorocarbon, CFC-11. 1,2-Dichloroethane was not included as a
controlled substance in the "Montreal Protocol on Substances that
Deplete the Ozone Layer".
Volatilization is the major removal process of 1,2-dichloroethane
from the aquatic environment (Dilling et al., 1975). The half-life in
a stirred aqueous solution, at varying depths and surface areas,
ranged between 5 and 29 min (Dilling et al., 1975; Chiou et al.,
1980). Based on fate modelling (EXAMS), the predicted half-life of
1,2-dichloroethane was 9 days in a eutrophic lake and one day in a
300-km stretch of a river system (assuming a loading rate of 0.1 kg
1,2-dichloroethane in both cases) (US EPA, 1982a).
Although hydrolysis of 1,2-dichloroethane may also occur in the
aquatic environment, this is not a significant removal process, since
the half-life for hydrolysis has been estimated to be 72 years at
neutral pH and 25°C (Barbash & Reinhard, 1989). In conditions similar
to those of groundwater (i.e. in the presence of sodium sulfide, a pH
of 7, and a temperature of 15°C), the estimated half-life of
1,2-dichloroethane was 23 years (Barbash & Reinhard, 1989). The
primary products of hydrolysis are vinyl chloride and 2-chloroethanol
(Jeffers et al., 1989); vinyl chloride can be further degraded to
acetylene and acetaldehyde (Hill et al., 1976), while 2-chloroethanol
may be degraded to ethylene glycol (Ellington et al., 1988).
Microbial degradation of 1,2-dichloroethane in water has been
observed, but it is a slow process, probably due to the insufficient
time before volatilization of the substance to allow for microbial
adaptation (US EPA, 1982a). In a static flask study with initial
1,2-dichloroethane concentrations of 5 and 10 mg/litre, there was a
loss due to aerobic degradation of 20 to 63% within 7 days following
an acclimation period. However, 5 to 27% of the total loss was
attributed to volatilization (Tabak et al., 1981). The methanotrophic
bacterium Methylosinus trichosporium (Oldenhuis et al., 1989),
methylotrophic bacterium Ancylobacter aquaticus (van den Wijngaard et
al., 1992) and a nitrogen-fixing bacterium Xanthobacter autotrophicus
(Janssen et al., 1985) have been identified as microorganisms capable
of biodegrading 1,2-dichloroethane under aerobic conditions. In a
batch experiment under anaerobic conditions, Bouwer & McCarty (1983)
reported a 63% reduction in 25 days, but were unable to induce
transformation in a flow-through system when initial concentrations of
1,2-dichloroethane were 174 and 22 µg/litre, respectively.
No biodegradation was observed after 35 days of incubation in an
anoxic sediment-water system in which the initial concentration of
1,2-dichloroethane was 1.0 mg/litre (pH not reported) (Jafvert &
Wolfe, 1987).
Based on its low sorption coefficient, 1,2-dichloroethane is not
expected to adsorb appreciably to soil, suspended solids or sediments.
In one study, 1,2-dichloroethane rapidly percolated through sandy soil
with a low organic matter content; no degradation was observed, and
72-74% of the initial amount was reported to have volatilized (Wilson
et al., 1981). 1,2-Dichloroethane may leach to groundwater, based on
its solubility in water, low Koc value and high mobility in soil.
Reductive dechlorination of 1,2-dichloroethane in leachates under
anaerobic conditions has been demonstrated (Lesage et al., 1993).
1,2-Dichloroethane has low potential for bioaccumulation, based
on experimental data and modelling predictions. The bioconcentration
factor (BCF) was determined to be 2, with a clearance half-life in
tissues of less than 2 days, in freshwater bluegill (Lepomis
macrochirus) exposed to 95.6 µg 1,2-dichloroethane/litre for 14 days
(Barrows et al., 1980). This is identical to the value predicted by
Isnard & Lambert (1988). Accumulation and loss of radiolabelled
1,2-dichloroethane was studied in the dab (Limanda limanda) liver
and in the oyster (Ostrea edulis). Following exposure to 3 mg/litre
for 20 days, the level in the dab liver rose rapidly to approximately
80 mg/kg and then remained stable. Following cessation of exposure,
1,2-dichloroethane levels decreased to about 12 mg/kg at 40 days. In
the oyster, the level rose to approximately 9 mg/kg in 4 days, reached
a plateau, and decreased to 3 mg/kg by 40 days after cessation of
exposure (Pearson & McConnell, 1975).
5. ENVIRONMENTAL LEVELS AND POPULATION EXPOSURE
5.1 Environmental levels
5.1.1 Ambient air
The mean concentrations of 1,2-dichloroethane in 1412 samples of
ambient air from 23 sites in 12 cities across Canada taken between
1988 to 1990 ranged from 0.07 to 0.28 µg/m3, with an overall mean of
0.13 µg/m3 and a maximum single value of 2.78 µg/m3 (Dann, 1992).
1,2-Dichloroethane was detected in 55 out of 62 samples of ambient air
from 19 out of 21 areas of Japan surveyed in 1992 at concentrations
ranging from non-detectable (i.e. < 0.004 µg/m3) to 3.8 µg/m3
(Environment Agency Japan, 1993). In the United Kingdom and the
Netherlands, average levels of 1,2-dichloroethane in rural areas were
0.08 and 0.2 µg/m3, respectively (Clark et al., 1984a,b; Guicherit &
Schulting, 1985). In both of these countries, the average
concentration in urban air was 1.2 µg/m3 (Clark et al., 1984a,b;
Guicherit & Schulting, 1985).
The US Environmental Protection Agency (US EPA, 1987) reported
levels of 1,2-dichloroethane in urban/suburban air to be generally
< 0.8 µg/m3 (< 0.2 ppb). Concentrations of 1,2-dichloroethane in
ambient air reported in several early studies conducted in 10 cities
in the USA between 1980 and 1982 were somewhat higher, mean
concentrations ranging from 0.33 µg/m3 (83 ppt) to 6.05 µg/m3
(1512 ppt) (Singh et al., 1980, 1981, 1982). Median concentrations of
1,2-dichloroethane in air of rural/remote areas, urban/suburban areas
and source-dominated areas in the USA were 0 µg/m3, 0.49 µg/m3 and
4.9 µg/m3, respectively; the maximum level was 240 µg/m3
(Brodzinsky & Singh, 1982).
Concentrations of 1,2-dichloroethane in air near areas where
chemicals are manufactured or used in the USA were found to be as high
as 736 µg/m3 (184 ppb), with an average of 110 µg/m3 (27.5 ppb)
(US EPA, 1985a). Concentrations were also high (300 µg/m3) near a
vinyl chloride manufacturing plant in the Netherlands (Kretzschmar et
al., 1976). The annual mean concentrations of 1,2-dichloroethane in
250 samples of ambient air from 12 sites in Hamburg, Germany, surveyed
in 1986-1987 ranged from 0.2 to 119 µg/m3, the highest levels being
detected in an industrial region where lubrication oil was produced
(Bruckmann et al., 1988). Levels of 1,2-dichloroethane ranged from
0.09 to 3.5 µg/m3 in heavily industrialized areas in Japan in
1980/1981 (Environment Agency, Japan, 1983). In New Jersey, USA,
where several petrochemical industries were located and there had been
substantial chemical dumping activity in the past, the mean and
maximum values for five hazardous waste sites (14 to 24 samples each)
ranged up to 1.12 µg/m3 (0.28 ppb) and 20.6 µg/m3 (5.15 ppb),
respectively (LaRegina & Bozzelli, 1986). 1,2-Dichloroethane was also
detected in air at a waste disposal site in New Jersey at levels
ranging from trace to 27 µg/m3 (6.8 ppb) (detection limit not
specified) (Pellizzari, 1982).
5.1.2 Indoor air
In a pilot study of samples taken for 1 year beginning in
mid-January 1991, indoor air of approximately 750 residences from 10
provinces across Canada was analysed. The mean concentration of
1,2-dichloroethane was < 0.1 µg/m3, and the maximum value
1.7 µg/m3 (detection limit not specified) (Fellin et al., 1992).
In the US EPA Total Exposure Assessment Methodology
(TEAM) study, samples of "personal" and outdoor air were taken in 600
residences of New Jersey, North Carolina, North Dakota and California.
1,2-Dichloroethane was detected only occasionally at low
concentrations, and the levels in personal air (range of mean values,
0.1 to 0.5 µg/m3) were higher than those in outdoor air (range of
mean values, 0.05 to 0.2 µg/m3) (quantifiable limit approximately
1 µg/m3) (Wallace, 1986). Based on a recent review of available
literature, mean concentrations of 1,2-dichloroethane in indoor air in
the USA ranged from 1.49 to 2.21 µg/m3 in hospitals and 4.51 µg/m3
in office buildings (US EPA, 1992).
The mean concentration of 1,2-dichloroethane in the air of 20
homes in areas in the Netherlands with "non-contaminated" soil was
3.4 µg/m3, compared to a mean outdoor level of 4.9 µg/m3. In the
crawl space or cellar of these homes, the mean concentration was
2.5 µg/m3 (Kliest et al., 1989).
1,2-Dichloroethane was also detected in the indoor air of two out
of nine residences from the Love Canal area of Niagara, New York
(0.100 µg/m3 and 0.130 µg/m3), while only trace levels were
detected in samples of outdoor air (detection limit not specified)
(Barkley et al., 1980).
5.1.3 Drinking-water
In Ontario, Canada, 1,2-dichloroethane was detected in 15 out of
> 2000 samples of drinking-water from 85 sites surveyed between 1988
and 1991; mean concentrations ranged from nondetectable (detection
limit 0.050 µg/litre) to 0.139 µg/litre, with a maximum single value
of 0.850 µg/litre, in treated water (it was not detected in untreated
water) (Ministry of Environment, 1991). 1,2-Dichloroethane was not
detected in 237 samples of drinking-water taken from 171 sites across
New Brunswick during the summer months of 1990 (detection limit
0.2 µg/litre) (Ecobichon & Allen, 1990).
In a survey of untreated and treated water from 10 potable water
treatment plants along the Great Lakes system in Ontario in 1982-1983,
1,2-dichloroethane was detected (< 0.1 µg/litre) in one sample each
for untreated and treated water in the summer, not at all in the
winter, and in two samples of each (<0.1 µg/litre) in the spring
(Otson, 1987). In an earlier survey of 30 potable water treatment
facilities serving major population centres across Canada sampled in
1979, 1,2-dichloroethane was detected frequently in both untreated and
treated water at mean concentrations of up to 2 µg/litre and
5 µg/litre, respectively (Otson et al., 1982).
Based on a summary of data on levels of 1,2-dichloroethane in
groundwater and surface water supplies from six US Federal surveys,
1,2-dichloroethane was detected in 24 out of 1973 samples of
groundwater at concentrations up to 18 µg/litre and in 12 of 589
samples of surface water at concentrations up to 19 µg/litre
(detection limits not specified) (Letkiewicz et al., 1982).
The US EPA (1987) estimated that 0.3% of groundwater and 3% of
surface water supplies contain concentrations of 1,2-dichloroethane in
the range of 0.5 to 5 µg/litre and 0.5 to 20 µg/litre, respectively
(the basis for these estimates was not specified). 1,2-Dichloroethane
was detected (detection limit not clearly specified) in 7 out of 1792
wells in Wisconsin, USA in the early 1980s; in two of the wells,
concentrations exceeded 7 µg/litre) (Krill & Sonzongni, 1986). In the
Love Canal district of Niagara, New York, 1,2-dichloroethane was
detected in the drinking-water in three out of nine residences
surveyed, at a concentration of 50 ng/litre (Barkley et al., 1980).
Concentrations of 1,2-dichloroethane in drinking-water from five
locations in Japan ranged from non-detectable (i.e. < 0.5 µg/litre)
to 0.9 µg/litre (Fujii, 1977). It was not detected in the
drinking-water samples from 100 cities in Germany (detection limit,
1.0 µg/litre) (Bauer, 1981). 1,2-Dichloroethane was not detected
(detection limit, 0.5 µg/litre) in 229 out of 232 groundwater stations
in the Netherlands surveyed from 1976 to 1978; in the other three
stations concentrations ranged from 0.8 to 1.7 µg/litre (Zoetman et
al., 1979). Concentrations of 1,2-dichloroethane ranged from 2 to
22 µg/litre in 400 samples of drinking-water from six cities in Spain
in 1987 (Freiria-Gandara et al., 1992).
5.1.4 Surface water
1,2-Dichloroethane was detected in 2% of samples in surveys in
the early 1980s of Canadian surface waters (Government of Canada,
1994), but it was not detected (detection limit of 0.08 µg/litre) in
351 samples from several lakes and rivers in Ontario (Kaiser et al.,
1983; Comba & Kaiser, 1985; Kaiser & Comba, 1986; Lum & Kaiser, 1986).
It was detected 300 m downstream of a plant manufacturing
1,2-dichloroethane in Ontario, with a maximum concentration of
16 µg/litre (Environment Canada, 1986).
1,2-Dichloroethane was detected (detection limit not specified)
in 53 of 204 sites from six river basins in the USA surveyed before
1977 at concentrations ranging from 1 to 15 µg/litre and one site
containing 90 µg/litre (HSDB, 1993). It was detected (detection limit
not specified) in 7% of 4972 samples of surface water from the Ohio
River basin in the USA in 1980-1981; concentrations ranged from 1 to
10 µg/litre in 44 samples (HSDB, 1993).
1,2-Dichloroethane was detected in 39 of 102 samples of surface
water from 14 of 34 sites in Japan in 1992 at concentrations ranging
from non-detectable (i.e., < 0.01 µg/litre) to 3.4 µg/litre
(Environment Agency Japan, 1993).
Concentrations of 1,2-dichloroethane in the influent of six
wastewater treatment plants in the Netherlands ranged from < 2 to
400 µg/litre, while levels in the effluents ranged from < 2 to
74 µg/litre. The variation was determined to be due to industrial
discharges (van Luin & van Starkenburg, 1984).
5.1.5 Food
1,2-Dichloroethane was not detected in any samples of 34 food
groups (consisting of dairy products, meats, eggs, fish, soup, bread,
cereal, pasta, fruit, vegetables, cooking oil, peanut butter,
sugar/jam, coffee/tea, soft drinks, wine/beer and tap water) collected
in Calgary, Canada, in 1991 (detection limit 50 µg/kg for solids and
1.0 µg/litre for liquids) (Enviro-Test Laboratories, 1991). In
January 1992, the study was repeated for the same 34 food groups
collected in Windsor, Canada, using more sensitive analytical
methodology (detection limit 5 µg/kg for solids and 1 µg/litre for
liquids). Based on preliminary results, 1,2-dichloroethane was not
detected in any of the samples analysed (Enviro-Test Laboratories,
1992).
In a Total Diet Study conducted by the US Food and Drug
Administration (FDA), 1,2-dichloroethane was not detected in 11
decaffeinated instant coffees or in 14 decaffeinated ground coffees
(detection limit not specified) (Heikes, 1987a).
1,2-Dichloroethane was detected only in one ready-to-eat cereal
(mean 0.31 µg/kg) out of 19 table-ready food items, including butter,
margarine, ready-to-eat cereals, cheese, peanut butter, processed
foods and drinking-water, which were selected to be representative of
the 234 table-ready food items examined in the Total Diet Study
(Heikes, 1987b, 1990). In further analysis of these foodstuffs,
1,2-dichloroethane was detected only in plain granola and shredded
wheat cereal at concentrations of 12 and 8.2 µg/kg, respectively
(Heikes, 1987b).
1,2-Dichloroethane was detected only in one item (whisky, at a
concentration of 30 µg/kg) in an additional Total Diet Study in the
USA of 231 different table-ready foods (quantification limit 9 µg/kg).
The food types examined included off-the-shelf cooked and uncooked
grain-based items, dairy products, fresh and canned fruits and
vegetables, meats and meat dishes, infant and junior blends, baked
goods, nuts and nut products, clear beverages, sugars, jams, and
candies (Daft, 1988). 1,2-Dichloroethane was not detected in four
earlier composite market basket surveys of dairy products, meats, oils
and fats, and beverage products (detection limit not specified) in the
USA (Entz et al., 1982).
In Germany, the mean concentrations of 1,2-dichloroethane in 12
samples of milk-products containing fruits (i.e. ice-cream, yoghurt,
curds and buttermilk) was 0.8 µg/kg fresh weight, with a maximum
concentration of 3.5 µg/kg fresh weight (detection limit not
specified) (Bauer, 1981).
Prior to 1984, 1,2-dichloroethane was used in Canada as a grain
fumigant (Lange, personal communication to the IPCS). In an early
survey, 1,2-dichloroethane concentrations ranged from 23 to 38 mg/kg
in wheat which had been treated with a fumigant containing
1,2-dichloroethane (Wit et al., 1969). 1,2-Dichloroethane could not
be "determined satisfactorily" in wheat which had been fumigated with
a mixture containing 30% of the compound (limit of detection specified
as 1.5 ng); similarly, it was not detected or determined only at trace
levels (not further specified) in samples of cereals (Berck, 1974).
1,2-Dichloroethane is currently not registered for use in
agricultural products in the USA. It was detected in wheat and
bleached flour samples at concentrations of 110 and 180 µg/kg and 6.1
and 6.5 µg/kg, respectively (limit of quantification 6 µg/kg), in a
survey of compounds used as fumigants in whole grains, milled grain
products and intermediate grain-based foods (Heikes & Hopper, 1986).
In 1979, it was detected at a concentration of 290 mg/kg in 1 out of
71 samples of wheat grown in the USA, but was not detected in 61
samples of wheat exported from England to the USA (Bailey et al.,
1982). Cooking (steaming, baking, etc.) tends to reduce levels of
1,2-dichloroethane in most foods contaminated during fumigation (Bond,
1984).
The use of 1,2-dichloroethane in agricultural products in the
United Kingdom has been discontinued. In earlier surveys, it was
detected in one out of 155 samples of wheat grown in the United
Kingdom in 1978-1979 at a concentration of 290 mg/kg and in none of
126 samples of imported wheat (MAFF, 1982); in 1981 and 1982,
1,2-dichloroethane was not detected in 47 and 59 samples of wheat,
respectively (MAFF, 1984). It was also not detected in 84 samples of
brown rice, 107 samples of rye products and 71 samples of processed
oats collected in 1985-1986 (MAFF, 1989). More recently,
1,2-dichloroethane was not detected (detection limit 0.4 mg/kg) in 24
samples of rice analysed in 1992 (UK HSE, 1992; MAFF/HSE, 1993).
No information on concentrations of 1,2-dichloroethane in breast
milk of women in the general population is available.
5.1.6 Soils and sediments
1,2-Dichloroethane was not detected (detection limit 0.01 mg/kg)
in 30 samples of soil taken from "typical" urban residential and
parkland locations in southern Ontario, Canada in 1987 (Golder
Associates, 1987). The mean concentration of 1,2-dichloroethane in
soil near 20 homes in areas of the Netherlands with "uncontaminated"
soil was 11 mg/kg, while samples of soil in the vicinity of a garage
and a waste site contained < 5 and 30 mg/kg, respectively (Kliest et
al., 1989). The US EPA (1988) reported that 1,2-dichloroethane has
been detected in soil samples from 1.5% of 2783 hazardous waste sites
sampled in the USA (concentrations and detection limits were not
reported).
1,2-Dichloroethane was not detected (detection limit, 0.01 µg/kg)
in sediments downstream of two facilities in Canada which manufactured
the compound (Oliver & Pugsley, 1986; AEC, 1989). It was detected in
11 out of 99 samples of sediment from 5 out of 33 areas surveyed in
Japan in 1992 at concentrations ranging from non-detectable (i.e.,
< 0.4 µg/kg) to 0.7 µg/kg (dry weight) (Environment Agency Japan,
1993).
5.1.7 Consumer products
In studies conducted in the USA, 1,2-dichloroethane was released
from cleaning agents and pesticides, glued wallpaper and glued carpets
in environmental chambers, while it was not emitted by painted
sheetrock (detection limit not specified) (Wallace et al., 1987).
More recently, 1,2-dichloroethane was detected in 5 out of 1043
household products tested in the USA; concentrations ranged up to 0.1%
(by weight) in automotive products, oils, greases and lubricants, and
miscellaneous products (Sack et al., 1992). It should be noted that
the use of 1,2-dichloroethane in products such as upholstery and
carpet fumigants, soap and scouring compound ingredients, wetting and
penetrating agents and degreasing fluid has been largely discontinued
in the USA. In addition it is not used in any registered drug
products in the USA (Drury & Hammons, 1979).
In a survey in Germany, 1,2-dichloroethane was not detected in
facial soap, mouthwash or toothpaste (detection limit not specified).
However, it was detected in shampoo and shaving cream at levels
ranging up to 7.6 µg/litre and 122 µg/litre, respectively, and in 1
out of 7 cough-syrups at a concentration of 12.9 µg/kg (Bauer, 1981).
No data on concentrations of 1,2-dichloroethane in cigarettes are
available. No difference was reported between the median air
concentrations of 1,2-dichloroethane in air in the offices of smokers
and those in the offices of non-smokers in southern England (Proctor
et al., 1989).
5.2 General population exposure
Based on estimates of mean exposure from various media, the
principal source of exposure to 1,2-dichloroethane by the general
population is indoor and outdoor air (< 0.03 to 0.1 µg/kg body weight
per day and 0.004 to 0.02 µg/kg body weight per day, respectively),
with only minor amounts being contributed by drinking-water (< 0.001
to 0.003 µg/kg body weight per day). Intake of 1,2-dichloroethane
from food is probably negligible. For some individuals residing in
the vicinity of industrial sources of airborne 1,2-dichloroethane,
intake from ambient air may be substantially greater than that for the
general population.
5.2.1 Ambient air
Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg, the assumption that 4 out
of 24 h are spent outdoors (IPCS, 1994), and the range of mean
1,2-dichloroethane levels found in a recent survey of cities across
Canada (0.07-0.28 µg/m3 as presented in section 5.1.1), mean intake
of 1,2-dichloroethane from ambient air for the general population is
estimated to range from 0.004 to 0.02 µg/kg body weight per day.
5.2.2 Indoor air
Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg, the assumption that 20 out
of 24 h are spent indoors (IPCS, 1993), and the range of
1,2-dichloroethane concentrations in indoor air or "personal" air in
surveys in Canada and the USA (< 0.1 to 0.5 µg/m3 as presented in
section 5.1.2), mean intake of 1,2-dichloroethane from indoor air for
the general population is estimated to range from < 0.03 to 0.1 µg/kg
body weight per day.
5.2.3 Drinking-water
Based on a daily volume of water consumption for adults of 1.4
litres, a mean body weight for males and females of 64 kg (IPCS,
1993), and the mean levels of 1,2-dichloroethane in provincial surveys
in Canada (< 0.05 to 0.139 µg/litre as presented in section 5.1.3),
mean intake of 1,2-dichloroethane from drinking-water for the general
population is estimated to range from < 0.001 to 0.003 µg/kg body
weight per day.
5.2.4 Food
Based on its low octanol/water partition coefficient,
1,2-dichloroethane is unlikely to bioaccumulate, and therefore it is
considered that food does not represent a significant source of
exposure for the general population. It has only rarely been detected
in individual samples of foodstuffs in North America (see section
5.1.5). Even if the compound was assumed to be present in foods at
concentrations up to the limit of detection in the surveys with the
more sensitive analytical methodology, the daily intake of
1,2-dichloroethane from food would still be negligible compared to
that from air.
5.2.5 Other media
Available data were considered insufficient to estimate intake of
1,2-dichloroethane from soil or consumer products.
5.3 Occupational exposure during manufacture, formulation or use
Based on a review of available information, current occupational
exposure to 1,2-dichloroethane in North America occurs predominantly
during the manufacture of other chemicals, such as vinyl chloride,
where 1,2-dichloroethane is used as an intermediate. In a 1982
National Occupational Exposure Survey by the US National Institute for
Occupational Safety and Health (NIOSH), 28% of employees working with
adhesives and solvents were exposed to 1,2-dichloroethane, while
between 5 and 9% of workers were exposed to the substance in the
medicinals and botanicals, biological products, petroleum refining and
organic chemicals industries, and in museums and art galleries (US
Department of Labour, 1989).
Mean concentrations of 1,2-dichloroethane at three production
plants in the United Kingdom in 1990 were 2.8, 3.2 and 6.8 mg/m3
(0.7, 0.8 and 1.7 ppm); 95% of samples contained less than 20 mg/m3
(5 ppm), while maximum values at the plants were 18, 80 and
160 mg/m3 (4.5, 20 and 40 ppm) (UK HSE, 1992).
The time-weighted average concentration of 1,2-dichloroethane in
an electron microscopy preparation laboratory in Hong Kong, in which
the chemical was used as a solvent, was 19.8 mg/m3 (4.9 ppm). The
concentration in the breathing zone of the operator was 52.87 mg/m3
(13.06 ppm) while the average concentration in the preparation room
was 35.1 mg/m3 (8.67 ppm) (Li & Cheng, 1991).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Case reports of acute effects following inhalation exposure to
1,2-dichloroethane in the workplace indicate that it is readily
absorbed (Nouchi et al., 1984).
In experimental animals, absorption following ingestion of
1,2-dichloroethane is rapid and complete. Spreafico et al. (1980) and
Reitz et al. (1982) reported that peak levels in blood (13 to
67 mg/litre) occurred within 10 or 15 min in rats administered single
oral doses of 25, 50 or 150 mg/kg body weight in corn oil. A plot of
administered dose against peak blood level appeared linear up to
50 mg/kg, with a perceptible decrease in steepness thereafter,
possibly indicating a relative saturation in gastrointestinal
absorption at doses of 100 to 150 mg/kg body weight. (The authors
noted that there were no significant differences in kinetic parameters
following single and 10 daily administrations of 50 mg/kg body
weight). Gastrointestinal absorption in rats was more rapid and
efficient following administration in water, compared to corn oil
(Withey et al., 1983).
Absorption following inhalation in experimental animals is also
rapid. In rats, levels of 1,2-dichloroethane in the blood peaked (8
to 10 mg/litre) within 1-2 h of continuous inhalation of 600 mg/m3
(150 ppm) for 6 h (Reitz et al., 1982).
The rate of dermal absorption of 1,2-dichloroethane by mice was
479.3 ± 38.3 nmol/min per cm2 following covered application of 0.5 ml
of the undiluted solvent (Tsuruta, 1975), while the rate of absorption
of 1,2-dichloroethane in 0.9% NaCl in vitro in excised skin of rats
was 169 ± 0.44 nmoles/min per cm2 (Tsuruta, 1977). Dermal
absorption of 1,2-dichloroethane in aqueous solution (1000 mg/litre)
was found to be similar in human and rat epidermis in vitro within
one hour of occluded application (20.3 µg/cm2 per h versus 33.1 µg/cm2
per h), whereas when the substance was applied neat (uncovered),
absorption within the first 15 min was approximately four to ten-fold
greater in the rat epidermis than in the human epidermis. In
addition, absorption increased with applied dose in the rat epidermis,
whereas absorption was not dependent upon dose in the human epidermis
(Ward, 1992).
The concentration of 1,2-dichloroethane in the blood of
guinea-pigs increased rapidly (up to approximately 7 mg/litre) during
the first 30 min following covered application of 1.0 ml of the
undiluted compound to shaved skin; the level in blood then began to
decrease abruptly to a minimum (approximately 5 mg/litre) after one
hour, at which point it began to gradually increase again (up to about
17 mg/litre after 12 h) (Jakobson et al., 1982). 1,2-Dichloroethane
was also rapidly absorbed when applied in aqueous solution to the skin
of rats in vivo, with the levels in blood being directly related to
the concentration of the solution (Morgan et al., 1991).
6.2 Distribution
Absorbed 1,2-dichloroethane is widely distributed throughout the
human body, based on analysis of several tissues of humans who died
following acute oral poisonings with the substance. Concentrations
ranged from 1 to 50 mg/kg in the spleen and 100 to 1000 mg/kg in the
stomach; levels in the liver and kidney were approximately 10 times
less than those in the stomach (Luznikov et al., 1985). The
metabolite 2-chloroacetaldehyde was not detected; detectable
quantities of 2-chloroethanol and monochloroacetic acid were reported,
though levels were too low to compare among tissues.
1,2-Dichloroethane has been detected in the breast milk of women
occupationally exposed via inhalation and dermal contact (Urusova,
1953).
Similarly, 1,2-dichloroethane is widely distributed throughout
the body in experimental animals exposed via inhalation or ingestion.
The highest concentrations were usually found in adipose tissue,
although it was also detected in blood, liver, kidney, brain and
spleen. 1,2-Dichloroethane accumulated most rapidly in the liver of
rats administered single oral doses of 25, 50 or 150 mg/kg body weight
in corn oil, although concentrations were greatest in adipose tissue.
Peak levels in adipose tissue, at 45 to 60 min, exceeded those in
blood by 3.9 to 8.3 times, whereas peak levels in the liver, 10 min
after exposure, exceeded those in the blood by 1.3 to 2.2 times
(Spreafico et al., 1980). Accumulation was less than expected at the
two higher exposure levels, indicating saturation of the tissues.
Similar accumulation in adipose tissue in rats was noted following
inhalation of 200 or 1000 mg/m3 (50 or 250 ppm) for up to 6 h.
During inhalation, steady state levels were reached within 2 to 3 h
and increased 20-to 30-fold when the exposure increased from 202 to
1012 mg/m3, suggesting saturable metabolic capacity. Levels of
1,2-dichloroethane in the spleen, brain and kidney were similar to
those in the blood, irrespective of the route of exposure (Spreafico
et al., 1980).
Reitz et al. (1982) reported that the relative distribution of
radioactivity at 48 h (assumed to be primarily in the form of
metabolites) was similar in rats administered [14C]-labelled
1,2-dichloroethane orally (single dose of 150 mg/kg body weight) or by
inhalation (600 mg/m3 or 150 ppm for 6 h). Residual reactivity in
selected tissues was 1.5 to 2 times higher after oral exposure than
following inhalation. There was also a higher residual activity in
the forestomach after the oral exposure. The distribution pattern for
macromolecular binding was similar, as determined 4 h after oral
ingestion or directly after inhalation. Oral exposure produced lower
(i.e. 1.5 to 2 times less) levels of total macromolecular binding but
higher (i.e. 3 to 5 times more) levels of DNA alkylation than
inhalation, though the absolute levels were considered low.
Arfellini et al. (1984) reported a greater degree of binding to
DNA in organs (liver, kidneys, lung and stomach) of mice than in those
of rats (1.45 to 2.26 fold) 22 h after intraperitoneal administration
of equivalent single doses of 8.7 µmoles/kg body weight.
In periods from 1 min to 4 days following intravenous
administration of a single dose (0.73 mg/kg body weight) of
radiolabelled 1,2-dichloroethane to mice, the highest levels of
radioactivity (non-volatile and bound metabolites) determined by whole
body autoradiography were present in the nasal olfactory mucosa and
the tracheo-bronchial epithelium. Low levels of metabolites were also
present in the epithelium of the upper alimentary tract, vagina and
eyelid and in the liver and kidney. Mucosal and epithelial binding
was decreased by pretreatment with metapyrone, indicating that binding
might be due to oxidative metabolism. In in vitro studies in
tissues from the same strain of mice, reactive products of
1,2-dichloroethane were irreversibly bound to the nasal mucosa, lung
and liver but not to the oesophagus, forestomach or vagina. The level
of binding in the nasal mucosa was twice that in the lung and 1.4
times that in the liver. On the basis of their results, the authors
suggested that the epithelium of the respiratory tract may be a
potential target for the toxic effects of 1,2-dichloroethane due to in
situ metabolism to reactive intermediates (Brittebo et al., 1989).
1,2-Dichloroethane was detected in fetal tissue of rats following
maternal exposure to airborne concentrations ranging from 612-
7996 mg/m3 (153-1999 ppm) on day 17 of gestation, the detected
concentrations in fetal tissues being related to the level of exposure
as well as the position on the uterine horn (Withey & Karpinski,
1985).
6.3 Metabolic transformation
1,2-Dichloroethane is metabolized extensively in rats and mice.
It is principally sulfur-containing metabolites that are eliminated in
the urine. Mitoma et al. (1985) reported slightly more complete
metabolism in mice than in rats, based on 100% recovery of metabolites
as expired CO2 and in the excreta and carcasses of mice administered
an oral dose of 150 mg/kg body weight [14C]-labelled
1,2-dichloroethane, compared to about 85% in rats administered
100 mg/kg body weight. This difference may have been due to
experimental variation or error. Reitz et al. (1982) reported 70 and
91% transformation of 1,2-dichloroethane in the rat following oral
(150 mg/kg body weight) and inhalation (607 mg/m3, 6 h) exposures,
respectively, with 85% of the metabolites appearing in the urine.
Proposed metabolic pathways for 1,2-dichloroethane are
illustrated in Fig. 1. Metabolism appears to occur via two principal
pathways for which the reactions and subsequent metabolism of the
products can account for all of the identified sulfur-containing
metabolites in the urine of 1,2-dichloroethane-exposed animals. One
pathway begins with cytochrome P-450-mediated oxidation, and the other
with glutathione conjugation. In the first pathway, cytochrome P-450
enzymes catalyse an oxidative transformation of 1,2-dichloroethane to
form reactive intermediates, which result in the formation of
2-chloroacetal-dehyde and 2-chloroethanol (Guengerich et al., 1980),
which are conjugated both enzymatically and non-enzymatically with
glutathione (GSH) and excreted in the urine. Guengerich et al. (1991)
concluded that cytochrome P-450 IIE1 is a major catalyst in the
oxidation of 1,2-dichloroethane in human microsomes.
The other pathway involves direct conjugation with glutathione to
form S-(2-chloroethyl)-glutathione, which is a half mustard with a
half-life of 69 min at 20°C (Schasteen & Reed, 1983) and less than 15
min at 37°C (Foureman & Reed, 1985). Non-enzymic conversion of the
half mustard to the corresponding episulfonium ion gives a putative
alkylating agent (episulfonium ion) that has several fates. Reaction
can occur with water to form S-(2hydroxyethyl) glutathione, with
thiols such as GSH to form ethene bis-glutathione, or with DNA to form
adducts. With the exception of the precursors which form DNA adducts,
the reaction products are considered non-toxic and undergo further
metabolism.
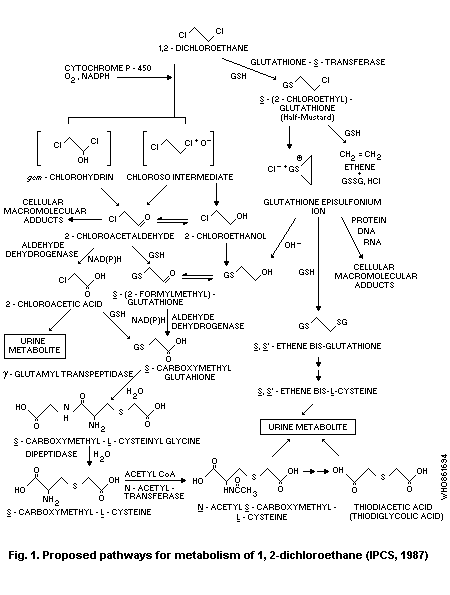 Although some DNA damage has been induced via the P-450 pathway
in vitro (Banerjee et al., 1980; Guengerich et al., 1980; Lin et
al., 1985), several lines of evidence suggest that the GSH conjugation
pathway is probably of greater significance than the P-450 pathway as
the major route for DNA damage (Guengerich et al., 1980; Rannug, 1980;
Sundheimer et al., 1982; Inskeep et al., 1986; Koga et al., 1986).
The P-450-dependent pathway can, however, presumably form
considerable quantities of 2-haloacetaldehydes, which readily bind to
protein and non-protein thiols, as shown for vinyl bromide and vinyl
chloride (Guengerich et al., 1981) and dibromoethane (DBE) (van
Bladeren et al., 1981). However, these authors concluded that 2H
and 18O studies on the formation of 2-haloethanols and
2-haloacetaldehydes from 1,2-dihaloethanes are inconsistent with a
major role of such a mechanism for DNA damage (Guengerich et al.,
1986; Koga et al., 1986).
The 1,2-dichloroethane-induced mutation frequency of two human
cell lines has been correlated with the difference in levels of
glutathione- S-transferase activities. AHH-1 cell line mutation
frequency was 25 times that in the TK6 cell line in the presence of
1,2-dichloroethane. The difference was attributed to the fact that
the AHH-1 cell line possesses 5 times more glutathione- S-transferase
activity than the TK6 cell line (Crespi et al., 1985).
Moreover, although the significance of the reported results is
uncertain, the results of an additional study by Storer & Conolly
(1985) are not inconsistent with the hypothesis that reduction of GSH
levels is associated with a reduction in DNA damage. Male B6C3F1
mice pretreated with piperonyl butoxide (PIB), a P-450 inhibitor, were
examined for the extent of hepatic DNA damage produced 4 h after
1,2-dichloroethane administration. Hepatic DNA damage, as measured by
alkali-labile lesions, was potentiated by PIB. Treatment of mice with
high doses of 2-chloroethanol failed to produce DNA damage, as
measured by this assay. Diethylmaleate, a GSH depletor, potentiated
the hepatotoxicity of 2-chloroethanol but not DNA damage.
In addition, Cheever et al. (1990) reported that although the
levels of hepatic DNA covalent binding of metabolites of
14C-1,2-dichloroethane injected (single dose) to rats which had been
exposed by inhalation to 1,2-dichloroethane in a long-term bioassay
were significant (p < 0.05), these levels were not different in rats
with concomitant exposure to disulfiram in the diet over two years.
Evidence suggests that the putative episulfonium ion, resulting
from non-enzymatic conversion of S-(2-chloroethyl) glutathione, is a
major intermediate in the formation of DNA adducts in vivo from
1,2-dichloroethane exposure (Inskeep et al., 1986). When rats were
administered single does of 14C-1,2-dichloroethane in vivo and the
liver was analysed 8 h later, 78% of the DNA adducts (0.25 nmol/mg
DNA) could be released by neutral thermal hydrolysis. A major adduct
and several minor adducts were present; the major adduct
co-chromatographed with S-[2-(N7-guanyl)ethyl] glutathione. The
postulated adduct of liver DNA after 14C-1,2-dichloroethane
exposure, S-[2-(N7-guanyl)ethyl] glutathione, appears to be
chromatographically identical to the major adduct in rats after
exposure to 1,2-dibromoethane (Koga et al., 1986). This
1,2-dibromoethane adduct, which has been isolated and characterized by
NMR and mass spectrometry, gives strong support to an identical adduct
being the principal DNA adduct from exposure to 1,2-dihaloethanes.
Reitz et al. (1982) found (based on consideration of results of
their own work as well as that of Spreafico et al., 1980) that
metabolism of 1,2-dichloroethane appears to be saturated or limited in
rats at levels of exposure resulting in blood concentrations of 5 to
10 mg/litre, based on an observed non-linear relationship between
levels in blood and administered doses or concentrations.
Administration by gavage resulted in the formation of about twice the
amount of "total" metabolites as did exposure by inhalation, based on
recovery in excreta, expired air and the carcass. Oral exposure
produced 1.5- to 2-fold lower levels of total macromolecular binding
but 3- to 5-fold higher levels of DNA alkylation than inhalation,
though the absolute levels of DNA alkylation were considered low.
Based on examination of DNA binding in the liver and lung of rats
exposed by inhalation to a low constant concentration (0.3 mg/litre)
of 1,2-dichloroethane for 12 h or to a peak concentration (up to
18 mg/litre) for a few minutes, Baertsch et al. (1991) concluded that
DNA damage by 1,2-dichloroethane depends upon the concentration time
profile, with bolus doses causing disproportionately greater damage.
6.4 Elimination and excretion
Unmetabolized 1,2-dichloroethane is eliminated in expired air,
while its metabolites are largely excreted in the urine. Unchanged
1,2-dichloroethane was detected in the exhaled breath of women exposed
dermally and to airborne concentrations of 0.252 mg/m3 (0.063 ppm)
in the workplace; the amount of 1,2-dichloroethane expired was greater
immediately following exposure and decreased over time (Urusova,
1953).
A single dose of 150 mg/kg body weight radiolabelled
1,2-dichloroethane was injected into rats that had been exposed via
inhalation at a concentration of 200 mg/m3 (50 ppm) for 2 years. The
proportion of radioactivity present in the urine within 24 h was 42.5
and 33.9% (in males and females, respectively), while 27.3 and 40.3%
were eliminated as the unchanged parent compound in the breath. Only a
very small amount of radioactivity was detected as 14CO2 or in the
faeces. In rats that had been concomitantly exposed to disulfiram
during the 2-year period, the proportion of unchanged
1,2-dichloroethane eliminated in the breath increased significantly
(i.e. 57.6 and 57.7%; p < 0.05), while the proportion eliminated in
the urine decreased correspondingly (27.6 and 24.9%). Levels of
unchanged 1,2-dichloroethane in blood were significantly (p < 0.05)
increased in rats exposed to 1,2-dichloroethane and disulfiram
compared to those exposed to 1,2-dichloroethane alone (see section
7.10) (Cheever et al., 1990).
The pattern of elimination of metabolites was similar in rats and
mice 48 h after administration of oral doses of radiolabelled
1,2-dichloroethane (100 and 150 mg/kg body weight, respectively). In
rats, 8.2 and 69.51% of the radiolabelled dose was recovered as CO2
and in the excreta (principally urine), respectively, compared to
18.21 and 81.11% in mice. The overall recovery was less in rats than
in mice (96.26 versus 110.12%) (Mitoma et al., 1985).
In rats exposed to 600 mg/m3 (150 ppm) 1,2-dichloroethane for
6 h or administered 150 mg/kg body weight by gavage, there was no
significant difference in the route of excretion of non-volatile
metabolites. After 48 h, in each case, more than 84% of total
metabolites was eliminated in the urine, 7-8% was excreted as carbon
dioxide in expired air, 2% was excreted unchanged in the faeces, and
4% remained in the carcass (Reitz et al., 1982). The major urinary
metabolites identified following exposure of rats by either route were
thiodiacetic acid (70%) and thiodiacetic acid sulfoxide (26 to 28%).
The rate of elimination following oral (gavage) administration or
inhalation was such that 1,2-dichloroethane was not detected in the
blood a few hours after exposure and only small amounts were detected
in tissues (liver, kidney, lung, spleen, forestomach, stomach and
carcass) 48 h after exposure (Reitz et al., 1982). The rate of
elimination from blood and tissues appeared to depend on the exposure
level; the higher the exposure level, the lower the elimination rate
of 1,2-dichloroethane, after both oral and inhalation exposure.
Elimination from the liver was reported to be biphasic, a higher
elimination rate occurring just after the peak levels of
1,2-dichloroethane were reached. Elimination from other organs was
monophasic. Following inhalation up to an exposure level of
1012 mg/m3, elimination was slowest in adipose tissue and most rapid
in the lung (Spreafico et al., 1980).
Withey & Collins (1980) also reported that the elimination of
1,2-dichloroethane was dose-dependent. After intravenous
administration of from 3 to 15 mg/kg body weight to male Wistar rats,
the authors found that the elimination fitted a twocompartment model
at a low dose level and a three-compartment model at high dose levels.
The percentage of administered radioactivity excreted in the
urine over a 24-h period in rats decreased with increasing single
doses (0.25 to 8.08 mmol 1,2-dichloroethane/kg body weight)
administered by gavage in mineral oil (Payan et al., 1993). The
authors attributed these results to saturation of metabolism rather
than kidney damage, as there were no variations in biochemical
parameters of nephrotoxicity between the controls and groups exposed
to doses up to 4.04 mmol/kg body weight. Urinary thiodiglycolic acid
increased as a linear function of the dose of 1,2-dichloroethane until
at least 1.01 mmol/kg body weight; it accounted for 63% of the total
metabolites in urine at this dose.
6.5 Retention and bioaccumulation
Although 1,2-dichloroethane is eliminated more slowly from
adipose tissue than from blood or other tissues (lung and liver)
following exposure, it is unlikely to bioaccumulate significantly, as
no difference was observed between levels in blood or tissues (data
not presented) following single or repeated (10 days) oral doses of
50 mg/kg body weight in rats (Spreafico et al., 1980). Only 71 and
75%, respectively, of an administered oral dose of radiolabelled
1,2-dichloroethane was recovered in the excreta and exhaled breath of
rats administered 150 mg/kg body weight by gavage following 2 years of
exposure via inhalation (200 mg/m3 or 50 ppm); the authors
speculated that the remainder may have been sequestered in the body
fat. Recovery in the excreta and exhaled breath was complete in
younger rats (4 months old) receiving the same oral dose (Cheever et
al., 1990).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Data on the acute toxicity of 1,2-dichloroethane in experimental
animals are summarized in Table 3. These data indicate that
1,2-dichloroethane is of relatively low acute toxicity.
LC50 values in rats exposed to 1,2-dichloroethane for 6 or
7.25 h ranged from 4000 mg/m3 (1000 ppm) (Spencer et al., 1951) to
6600 mg/m3 (1650 ppm) (Bonnet et al., 1980). The 6-h LC50 in mice
was 1050 mg/m3 (Gradiski et al., 1978). LC50 values decreased
with increasing duration of exposure in rats exposed to concentrations
ranging from 1200 to 80 000 mg/m3 (300 to 20 000 ppm)
1,2-dichloroethane for 1 to 8 h (Spencer et al., 1951). Various
non-lethal effects have been reported in animals following acute
exposure to 1,2-dichloroethane, including central nervous system
depression, cardiovascular collapse, altered behaviour, pulmonary
congestion and oedema, histological damage in the liver, kidneys and
adrenal glands and myocardial failure, at concentrations ranging from
4000 mg/m3 for 1.5 or 4 h to 80 000 mg/m3 (20 000 ppm) for 30 min
(Heppel et al., 1945; Spencer et al., 1951; Alumot et al., 1976a;
Wolff et al., 1979; ATSDR, 1989). Central nervous system depression
occurred at much higher concentrations than those which induce effects
in other organs.
Oral LD50 values for rats, mice, dogs and rabbits ranged from
413 mg/kg body weight in female mice to 2500 mg/kg body weight in dogs
(Barsoum & Saad, 1934; McCollister et al., 1956; Smyth, 1969; Larionov
& Kokarovtseva, 1976; Munson et al., 1982; NIOSH, 1994). Non-lethal
effects observed in rats and rabbits following single oral doses of
1,2-dichloroethane ranging from 615 to 1476 mg/kg body weight include
hepatic effects (fatty degeneration, cloudy swelling, congestion,
haemorrhagic lesions, dystrophy in the cytoplasm and hyperchromatosis
in the nuclei of hepatocytes), degeneration of the renal tubular
epithelium, altered levels of enzymes in the serum and liver, oedema
and haemorrhaging in the walls of the coronary vessels, stasis and
thrombi in the myocardium, altered fibrinolytic activity in the blood,
and altered haematological parameters. A single dose of 0.5 ml
altered the ratio of the oxidized and reduced forms of nicotinamide
coenzymes in the liver and myocardium of rats (Natsyuk & Chekman,
1975). Electrocardiographic changes were reported at doses of 1, 1.5
and 2 mg/kg body weight, although these effects have not been
confirmed in other studies (Saitanov & Arsenieva, 1969).
The LD50 for dermal exposure in rabbits was estimated to be
between 2.8 and 4.9 g/kg (Torkelson & Rowe, 1981; NIOSH,
1994).
Table 3. Acute toxicity of 1,2-dichloroethane in experimental animals
Species Numbers/sex Duration/vehicle LC50 or LD50 Reference
Inhalation
Rats (Wistar equal no. of m & f) 10-54 0.53 h 48 000 mg/m3 (12 000 ppm) Spencer et al. (1951)
20-51 2.75 h 12 000 mg/m3 (3000 ppm)
31-32 7.20 h 4000 mg/m3 (1000 ppm)
Rats (albino, strain, number and sex not not specified 30 000 mg/m3 Nevrotsky et al. (1971)
specified)
Rats (Sprague-Dawley, 12 per group, sex not 6 h 6600 mg/m3 (1646 ppm) Bonnet et al. (1980)
specified)
Mice (OF1, 20 f per group) 6 h 1050 mg/m3 (262 ppm) Gradiski et al. (1978)
Ingestion
Rats (strain, number and sex not specified) not specified 850 mg/kg body weight Larionov &
Kokarovtseva (1976)
Rats (6 per group, strain and sex not not specified 770 mg/kg body weight Smyth (1969)
specified)
Rats (young adult albino, 80 m & f) corn oil 680 mg/kg body weight McCollister et al. (1956)
Mice, 6-week old (CD-1, number not male water 489 mg/kg body weight Munson et al. (1982)
specified) female 413 mg/kg body weight
Table 3. (cont'd).
Species Numbers/sex Duration/vehicle LC50 or LD50 Reference
Dogs (strain, number and sex not specified) acacia gum 2500 mg/kg body weight Barsoum & Saad (1934)
Rabbits (strain, number and sex not not specified 860 mg/kg body weight NIOSH (1994)
specified)
Dermal
Rabbits (strain, number and sex not not specified 2800 mg/kg body weight NIOSH (1994)
specified)
Rabbits (strain, number and sex not olive oil; duration 2800-4900 mg/kg body weight Torkelson & Rowe
specified) and area of skin (1981)
exposed not
specified
7.2 Skin and eye irritation
When 1.0 ml undiluted 1,2-dichloroethane was applied directly to
the clipped skin of guinea-pigs for up to 12 h in occluded patch
tests, no gross skin reactions were visible (Jakobson et al., 1982).
Microscopic changes appeared 4 h after application, comprising
karyopyknosis, perinuclear oedema, spongiosis and junctional
separation (Kronevi et al., 1981). In Draize tests on rabbits,
moderate erythema and oedema were observed 24 h after
application (dose not specified). Microscopy on the third day
revealed necrosis and other lesions such as ulcerations and
acanthosis. The severity of the changes was not indicated (Duprat et
al., 1976).
Instillation of 0.1 ml undiluted 1,2-dichloroethane into the
conjunctival sac of the eye of rabbits generated reversible, mild
irritation characterized by conjunctivitis and epithelial abrasion.
Epithelial keratitis, described as being "in a state of repair", was
observed microscopically 7 days after application (Duprat et al.,
1976). Reversible clouding of the cornea was observed in dogs within
10 h of subcutaneous administration of undiluted 1,2-dichloroethane at
0.9 mg/kg body weight. The clouding continued up to 48 h, but the
corneas appeared clear after 5 days. Histological changes, including
necrosis of the corneal endothelium, partially denuded Descemet's
membrane, formation of excess basement membrane, and swelling of the
corneal stroma, were also observed in dogs, cats and rabbits after
ocular injection of 1.8 mg 1,2-dichloroethane (0.15 ml of a 1%
solution) into the anterior chamber (Kuwabara et al., 1968).
7.3 Short-term exposure
Small groups of Wistar rats, rabbits, guinea-pigs, dogs and pigs
(n = 1 to 21) were exposed to 6000 mg/m3 (1500 ppm)
1,2-dichloroethane, 7 h/day for 6 days. Sections of the liver, heart,
lungs, kidney adrenal glands and spleen were examined microscopically.
In most animals, degeneration or necrosis of the kidney and liver,
along with congestion and haemorrhage of the lungs and adrenal glands,
were observed (Heppel et al., 1945).
No significant changes in organ or body weights, histology or
clinical chemistry and haematological parameters were observed in rats
administered 1,2-dichloroethane doses of up to 150 mg/kg body weight
per day in corn oil by gavage, 5 times/week for 2 weeks (van Esch et
al., 1977; Reitz et al., 1982).
7.4 Subchronic exposure
7.4.1 Inhalation
The subchronic toxicity of inhaled 1,2-dichloroethane was
investigated in three early limited studies in multiple species, as
presented in Table 4. Heppel et al. (1946) exposed groups of rats,
mice, rabbits, guinea-pigs, dogs, cats and monkeys to 4000 mg/m3
(1000 ppm) for up to 66 days. Mice, rats, rabbits and guinea-pigs
were the most sensitive species, based on mortality after only one or
a few exposures. Various effects were noted in these animals,
including pulmonary congestion (guinea-pigs, cats and rats), fatty
changes in the kidney (rats and monkeys), fatty changes in the
livera (cats, dogs and monkeys) and clouded corneas (dogs). At
1600 mg/m3 (400 ppm), observed effects included fatty degeneration
of the liver, kidney or heart (guinea-pigs and one rat), fatty changes
in the liver (dogs) and pulmonary congestion (rats), while at
800 mg/m3 (200 ppm), rats and guinea-pigs had mild pulmonary
congestion and one rat had fatty degeneration in the kidneys. No
effects on growth were noted in mice and rats exposed to 400 mg/m3
(100 ppm).
In a similar study, rats, guinea-pigs, rabbits and monkeys were
exposed to 400, 800 or 1600 mg/m3 (100, 200 or 400 ppm)
1,2-dichloroethane for 6 to 9 months (Spencer et al., 1951). Severe
effects, including hepatotoxicity, and deaths were observed in rats
and guinea-pigs exposed at the highest level, while monkeys also
showed degeneration of the liver and kidneys at this concentration.
No effects were noted in rabbits. At 800 mg/m3 (200 ppm) no adverse
effects were observed in rats, but slight degeneration of the liver
was noted in guinea-pigs. At 400 mg/m3 (100 ppm), no adverse
effects were observed in any of the four species. The authors
considered the "maximum concentrations without adverse effects" to be
1600 mg/m3 (400 ppm) in the rabbit, 800 mg/m3 (200 ppm) in the
rat, and 400 mg/m3 (100 ppm) in the monkey and guinea-pig, based on
a limited range of end-points.
a It has been suggested, on the basis of in vitro investigations,
that fatty accumulation in the liver may be due to the ability of
1,2-dichloroethane to block the secretion of hepatocellular
(Cotalasso et al., 1994).
Table 4. Subchronic toxicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats (26, strain and animals were exposed to 0 or There was high mortality in exposed rats (20/26), rabbits (5/6) and Heppel
sex not specified) 4000 mg/m3 (0 or 1000 ppm), guinea-pigs (36/41) after a few exposures. All mice died after one et al.
Mice (22, strain and 7 h/day, 5 days/week for up exposure. Survival was higher among cats and dogs (4/6 of either (1946)
sex not specified) to 66 exposures; sections of species survived more than 23 exposures). One monkey died after 2
Rabbits (5 m & 1 f, liver, heart, lungs, kidney, days and the other after 32 exposures. Pulmonary congestion was
strain not specified) adrenal glands and spleen noted in guinea-pigs, cats and rats. Rats and monkeys had fatty
Guinea-pigs (10-16, strain were examined changes in the kidney. Cats and monkeys had fatty changes in the
and sex not specified) microscopically; haematological liver. Dogs had cloudy corneas; one dog had fatty degeneration of
Dogs (3 f, strain not and urinary parameters were the liver. No effects on haematological or urinary parameters were
specified) assessed in dogs observed in dogs. Rabbits had no obvious effects on postmortem.
Cats (6 f, strain not
specified)
Monkeys (Rhesus, 2,
sex not specified)
Rats (15 m & 1 f, animals were exposed to 0 or All dogs and puppies survived 177 exposures. All rabbits died, after Heppel
strain not specified) 1600 mg/m3 (0 or 400 ppm), 1 to 97 exposures; 14/20 and 9/16 guinea-pigs and rats died by the et al.
Rabbits (2 m & 3 f, 7 h/day, 5 days/week for up to 60th exposure. Rats had pulmonary congestion and 1 rat and 6 (1946)
strain not specified) 177 exposures; sections of guinea-pigs had fatty degeneration of the liver, kidney and heart.
Guinea-pigs (8-10 m & liver, heart, lungs, kidney, Dogs had slight fatty changes in the liver. No effects were noted in
2 f, strain not specified) adrenal glands and spleen were rabbits on postmortem. There were no significant differences in
Dogs (6 f, strain not examined microscopically; haematological parameters in exposed dogs and rabbits compared
specified) and puppies haematological parameters to controls.
(3 m, strain not were also assessed in dogs
specified) and rabbits
Table 4. (cont'd).
Species Protocol Results Reference
Rats (Wistar 1 m & 11 f) animals were exposed to 0 or 5/14 guinea-pigs, 7/12 rats and 8/12 rats died after 1 to 115 Heppel
Osborne-Mendel rats (12 m) 800 mg/m3 (0 or 200 ppm), 7 h/ exposures. Rats and guinea-pigs had mild pulmonary congestion, et al.
Rabbits (5 m, strain day, 5 days/week, for up to and 1 rat had fatty degeneration in the kidneys. There were no (1946)
not specified) 125 exposures; sections of significant differences in haematological parameters in exposed
Guinea-pigs (12 m & 2 f, liver, heart, lungs, kidney, rats and rabbits, compared to controls.
strain not specified) adrenal glands and spleen
Monkeys (2 m, strain were examined microscopically;
not specified) haematological parameters were
assessed in rats and rabbits
Rats (23 m & 16 f, animals were exposed to 0 or All animals survived. There were no differences in the rate of Heppel
strain not specified) 400 mg/m3 (0 or 100 ppm), 7 h/ growth in rats or mice. et al.
Mice (19, strain and day, 5 days/week for 74 (rats) (1946)
sex not specified) and 19 (mice) exposures;
sections of liver, heart,
lungs, kidney, adrenal glands
and spleen were examined
microscopically
Rats (Wistar, 15-20 m & animals were exposed to 0, 400, 1600 mg/m3: All female rats and male guinea-pigs died by 10 Spencer
15-20 f) 800 or 1600 mg/m3 (0, 100, 200, exposures; all male rats died by 40 exposures. All female et al.
Guinea-pigs (2-8 m & or 400 ppm), 7 h/day, 5 days/ guinea-pigs died by 24 exposures. Rats and guinea-pigs had rapid body (1951)
8 m, strain not specified) week for 6 to 9 months; animals weight loss, slight increases in liver and kidney weights, and
Rabbits (albino, number were killed at various times some fatty changes in the liver. Guinea-pigs had swelling of the
and sex not specified) throughout the study; tubular epithelium of the kidneys and alterations in levels of
Monkeys (Rhesus, number haematological parameters were non-protein nitrogen urea nitrogen in the blood. Monkeys had
and sex not specified) assessed and histopathological degeneration of the liver and kidneys and increased fat content
examinations of several tissues of the liver. No effects were noted in rabbits.
were conducted
Table 4. (cont'd).
Species Protocol Results Reference
800 mg/m3: Guinea-pigs tolerated up to 180 exposures (246 days).
Male guinea-pigs had significantly (p=0.001) decreased body
weight gain; both sexes of guinea-pigs had slight degeneration
and fatty accumulation in the liver. Rats tolerated up to 151
exposures (212 days) with no observed adverse effects.
400 mg/m3: No effects were noted in rats, guinea-pigs, rabbits
or monkeys.
Rats (Sprague-Dawley, were exposed to 400 At the higher concentration, rats were dyspnoeic and guinea-pigs Hofman
10, sex not specified) or 2000 mg/m3 (100 or 500 were "apathetic". 3/4 rabbits died after 10-17 exposures; 9/10 et al.
Guinea-pigs (10, strain ppm), 6 h/day, 5 days/week guinea-pigs died after 4-14 exposures. Rats died after 1-5 exposures, (1971)
and sex not specified) for up to 17 weeks; while all cats survived 30 exposures. Rats had pulmonary hyperaemia
Rabbits (4, strain and histological examinations were and oedema, fatty liver and adrenal and myocardial necrosis. Cats and
sex not specified) conducted, and several rabbits had heart lesions, and guinea-pigs had fatty changes in the
Cats (2 per group, strain bio-chemical parameters were myocardium, liver and adrenals, and necrosis in the myocardium and
and sex not specified) assessed liver. At 400 mg/m3, there were no clinical or histological
changes after exposure for 17 weeks.
Table 4. (cont'd).
Species Protocol Results Reference
Ingestion
Rats F344/N, animals were administered 0, Drinking-water: F344/N rats: Body weight was significantly decreased NTP
Sprague-Dawley and 500, 1000, 2000, 4000 and in males at 4000 and 8000 mg/litre (>8%, p<0.001). Relative kidney (1991)
Osborne Mendel, mice, 8000 mg/litre in the weight was significantly increased at >1000 mg/litre in both sexes
B6C3F1 (10 or 20 m & f drinking-water (equivalent to (>11%, p<0.001). Relative liver weight was significantly increased at
per group) doses of of 49-82, 86-126, >2000 mg/litre in males (>14%, p<0.05) and 4000 mg/litre in females
146-213, 259-428, and (>9%, p<0.001). Alterations in haematological and serum parameters
492-727 mg/kg body weight per at the higher concentrations reflected dehydration caused by
day in rats and 244-249, decreased water consumption. Renal tubular "regeneration" observed
448-647, 781-1182, 2478-2710 in all groups of males at similar frequencies and severity, but
and 4207-4926 mg/kg body frequency was related to dose in females (0/10, 0/10, 1/10, 2/10
weight per day in mice) for 3/10 and 9/10 with increasing concentration).
13 weeks
Rats F344/N (10 or rats were administered 0, 30, Sprague-Dawley rats: Body weight was significantly decreased in
20 m & f per group) 60, 120, 240 or 480 mg/kg body males at 8000 ppm (9%, p<0.05). Relative kidney weight was
per day (males) or 0, 18, 37, significantly increased at >1000 mg/litre in males (>7%, p<0.05)
75, 150 or 300 mg/kg body and at >500 mg/litre in females (>8%, p<0.05). Relative liver
weight per day (females) in weight was increased in males at >500 mg/litre (>6%, p<0.05)
corn oil by gavage, and at 8000 mg/litre in females (13%, p<0.001). Alterations in
5 days/week for 13 weeks haematological and serum parameters at the higher
concentrations reflected dehydration caused by decreased water
consumption. Renal tubular "regeneration" was observed in all
groups at similar frequencies and severity.
Table 4. (cont'd).
Species Protocol Results Reference
haematological and serum Osborne-Mendel rats: Body weight was significantly decreased
chemistry parameters were at 8000 mg/litre in males (15%, p<0.05). Relative kidney weight
examined at several times was increased at 4000 and 8000 mg/litre in males (>8%, p<0.05)
during the course of the study. and >500 mg/litre in females (>12%, p<0.001). Relative liver weight
Extensive histopathological was increased in males at 1000 and 2000 mg/litre (>14%, p<0.05).
examinations were conducted Alterations in haematological and serum parameters at the higher
for controls and all animals concentrations reflected dehydration caused by decreased water
at the highest concentration consumption. Although the incidence of renal tubular "regeneration"
in drinking-water and in female was increased at the higher concentrations, the increases were
mice at 4000 ppm, as well as in not related to dose, and severity was similar in all groups.
male rats receiving doses of
120 or 240 mg/kg body weight B6C3F1 mice: 9/10 females exposed to 8000 mg/litre died. Body
per day by gavage and in weight was significantly decreased in males at 8000 mg/litre
female rats at 150 mg/kg body (17.5%, p<0.001). Relative kidney weight was significantly increased
weight per day in males at >1000 mg/litre (>12%, p<0.001) and in females at >500
mg/litre (>16%, p<0.001). Relative liver weight was significantly
increased in males at 500 mg/litre and above and in females at 1000
mg/litre or more. The incidence of renal tubular cell regeneration
was significantly increased in males at 4000 and 8000 mg/litre;
karyomegaly was observed in all males at 8000 mg/litre.
Gavage: F344/N rats: All males receiving 240 or 480 mg/kg body
weight per day and 9/10 female receiving 300 mg/kg body weight per
day died before the end of the study. The incidence of hyperplasia
and inflammation of the forestomach was significantly increased in
males at 240 mg/kg body weight per day; necrosis of the thymus was
observed in 10/10 males at 480 mg/kg body weight per day and 5/10
females at 300 mg/kg body weight per day, compared to none
in controls.
Table 4. (cont'd).
Species Protocol Results Reference
Rats (Osborne-Mendel, animals were administered At the highest dose, three males and one female died. In males, NCI
5 m & 5 f per group) doses of 0, 40, 63, 100, 159 or mean body weight was only significantly decreased (by 50%) at (1978)
251 mg/kg body weight per day 251 mg/kg body weight per day. In females mean body weight was
in corn oil by gavage, 5 days depressed (10% at 40 mg/kg body weight per day to 17% at 100
week for 6 weeks followed by a mg/kg body weight per day and 32% at 159 mg/kg body weight
2-week observation period per day). No other parameters were investigated.
Mice (B6C3F1 5 m & animals were administered All males receiving 398 mg/kg body weight per day and all females NCI
5 f per group) doses of 0, 159, 251, 398, receiving 631 mg/kg body weight per day died. Mean body weight (1978)
631 or 1000 mg/kg body weight depression was only observed in females receiving 398 mg/kg body
per day in corn oil by weight per day, in which "drastic weight loss" was reported. No
gavage, 5 days/week for other parameters were investigated.
6 weeks followed by a 2-week
observation period
Rats (6 per group, rats were fed mash fumigated The only effect noted, based on examination of lipid content Alumot
strain and sex not with 1,2-dichloroethane, of the liver and liver weight, was an increase in liver fat at et al.
specified) resulting in initial 1600 mg/kg. Chronic respiratory disease was evident in all exposure (1976a)
concentrations of 200 or groups (incidence data not presented, but the disease was not
600 mg/kg (approximately believed to be associated with exposure); mortality was higher in
equivalent to doses of 10 and males than females, with the number of rats surviving after 21
30 mg/kg body weight per day, months ranging from 3 to 14.
respectively) for 5 weeks, or
1600 mg/kg (approximately
equivalent to a dose of
80 mg/kg body weight per day)
for 7 weeks; the authors
noted that the dose ingested
was approximately 60 to 70% of
the initial amount after loss
Table 4. (cont'd).
Species Protocol Results Reference
to volatilization was
considered; lipid content of
liver and liver weight were
measured, and hepatic
triglycerides were measured at
the highest dose; no
histopathological examinations
were conducted
Rats (m & f, strain and rats were administered doses Decreased weight gain was observed at the two highest doses. Van Esch
number not specified of 0, 10, 30 or 90 mg/kg body Males and females had increased relative kidney weight at 90 et al.
in secondary account) weight per day (method of oral mg/kg body weight per day. Females also had increased relative (1977)
administration not specified weights of liver and brain at this dose. No effects on histology
in secondary account), 5 times or clinical chemistry were noted. Alterations in some
per week for 90 days; haematological parameters observed, although these did not occur
histopathological examinations in a dose-related manner.
were conducted, along with
assessment of haematological
and clinical chemistry
parameters, although the
extent of the examinations
was not specified in the
secondary account
Rats (m, strain and rats were orally There was a statistically significant (p < 0.02) increase in the Apostolov
number not specified) administered doses activity of the serum lysosome enzyme, &
equivalent to 1/5000, 1/1000 beta-N-acetylglucosa-minidase at the two highest doses. Mihaylova
or 1/200 the LD50 (not further (1975)
specified) for 3 months (abstract
only)
Hofmann et al. (1971) exposed rats, guinea-pigs, rabbits and cats
to 400 or 2000 mg/m3 (100 or 500 ppm) for up to 17 weeks. Mortality
was high in rats, guinea-pigs and rabbits exposed to the higher
concentration. At 2000 mg/m3 (500 ppm) pulmonary hyperaemia and
oedema, fatty liver and adrenal and myocardial necrosis were noted in
rats, while heart lesions were observed in cats and rabbits.
Guinea-pigs had fatty changes in the myocardium, liver and adrenals
and necrosis in the myocardium and liver at the higher concentration.
No clinical or histological effects were noted at 400 mg/m3
(100 ppm).
7.4.2 Ingestion
Available data on the subchronic toxicity of ingested
1,2-dichloroethane are presented in Table 4. In a recent study
conducted by the National Toxicology Program (NTP, 1991) and partially
reported by Morgan et al. (1990), the relative susceptibility of three
strains of rats (F344/N, Sprague-Dawley and Osborne-Mendel) and one
strain of mice (B6C3F1) exposed to 1,2-dichloroethane in
drinking-water at concentrations of up to 8000 mg/litre for 13 weeks,
and one of the same strains of rats (F344/N) exposed to doses of up to
480 mg/kg body weight per day by gavage in corn oil for 13 weeks, was
investigated. Based on increased relative organ weights, the liver
and kidneys were the target organs in both rats and mice, although
treatment-related microscopic lesions were noted only in female F344/N
rats and male B6C3F1 mice. Administration of 1,2-dichloroethane to
F344/N rats by gavage resulted in more severe toxic effects (including
death) than administration of similar doses in drinking-water,
probably due to greater peak levels of the compound in the blood and
saturation of elimination mechanisms. The authors considered the
no-observed-effect levels (NOEL) for 1,2-dichloroethane administered
to F344/N rats by gavage to be 120 and 150 mg/kg body weight per day
in males and females, respectively, based on mortality and chemically
related lesions in the forestomach. The NOEL of B6C3F1 mice exposed
via drinking-water was considered to be 780 mg/kg body weight per day)
(2000 ppm) in males, based on kidney lesions, and (2500 mg/kg body
weight per day) (4000 ppm) in females, based on mortality. The
authors did not consider the doses to which the three strains of rats
were exposed in the drinking-water to be high enough to result in
biologically significant toxic effect, although increases in organ
weights without accompanying histopathological alterations were
observed at doses as low as 49 to 82 mg/kg body weight per day in some
strains (i.e. Sprague-Dawley and Osborne-Mendel).
In limited subchronic studies preliminary to long-term
carcinogenesis bioassays, groups of Osborne-Mendel rats and B6C3F1
mice were administered doses of up to 251 and 1000 mg/kg body weight
per day, respectively, by gavage for 6 weeks. Significant mean body
weight loss was noted in male rats at 251 mg/kg body weight per day
and in female rats at > 40 mg/kg body weight per day. In mice,
mean body weight was decreased only in females receiving 398 mg/kg
body weight per day. No other parameters were investigated in this
study (NCI, 1978).
Decreased body weight gain was observed in rats orally
administered doses of 30 or 90 mg/kg body weight per day for 90 days,
but not in those receiving 10 mg/kg body weight per day. Increased
relative weights of kidneys (both sexes), liver and brain (females
only) were noted at the highest dose, although no histopathological
effects or alterations in clinical chemistry parameters were noted.
Changes in some haematological parameters were noted, but these were
not related to dose (Van Esch et al., 1977).
A slight increase in the fat content of the livers was reported
in rats consuming feed which had been fumigated with
1,2-dichloroethane, resulting in an initial concentration of
1600 mg/kg (approximately equivalent to a dose of 80 mg/kg body weight
per day) for 7 weeks, while no effects were observed at 600 mg/kg
(approximately equivalent to a dose of 30 mg/kg body weight per day)
after 5 weeks (Alumot et al., 1976a). The parameters examined were
limited to hepatic lipid content and liver weight. The activity of
the serum lysosome enzyme, ß- N-acetylglucosaminidase, was
significantly increased in rats administered oral doses equivalent to
1/1000 and 1/200 the LD50 (not further specified in abstract)
(Apostolov & Mihaylova, 1975).
7.5 Chronic exposure and carcinogenicity
7.5.1 Inhalation
Groups of 90 Sprague-Dawley rats of each sex were exposed by
inhalation to 1,2-dichloroethane (99.92% pure) at concentrations of 0,
20, 40, 202 and 1012 mg/m3 (0, 5, 10, 50 and 250 ppm), 7 h/day, 5
days/week for 78 weeks, and observed until spontaneous death (due to
severe toxicity, the highest concentration was reduced to 607 mg/m3
or 150 ppm after several days). "Incidence" was reported as the
number of animals developing specific tumours over the number of
animals alive at the time the first tumour of that type was detected
(i.e. incidences have not been adjusted for differential survival (see
Table 5)). The only tumour types for which the authors reported an
increase in incidence (when compared with controls kept in exposure
chambers, but not when compared with those not kept in chambers) were
fibromas and fibroadenomas (combined) of the mammary gland, which the
authors attributed to the differential survival among the groups
(Maltoni et al., 1980).
Groups of 50 male and 50 female Sprague-Dawley rats were exposed
to 0 or 200 mg/m3 (50 ppm) 1,2-dichloroethane 7 h/day, 5 days/week
for 2 years. No effects on body weight gain or mortality were noted.
There was no significant difference in the incidence of tumours at any
site, although there was a nonsignificant increase in the incidence of
mammary gland adenomas (4 in exposed group versus 2 in control group)
and fibroadenomas (21/50 in exposed group versus 15/50 in control
group) in females. There was an increased incidence of testicular
lesions (not further specified) in males (24% versus 10% in controls,
significance not reported) (Cheever et al., 1990). However, the
sensitivity of this investigation to detect any carcinogenic potential
may have been compromised (the study was designed to investigate the
interaction between 1,2-dichloroethane and other substances), based on
the lack of convincing evidence of compound-related toxicity at the
only concentration to which animals were exposed.
Clinical chemistry and haematological parameters were
investigated in groups of 8 to 10 male or female Sprague-Dawley rats
exposed to 0, 20, 40, 202 or 1012 mg/m3 (decreased to 607 mg/m3
after several days) (0, 5, 10, 50 or 250/150 ppm), 7 h/day, 5
days/week for 3, 6, and 18 months, beginning at 3 months of age. In
addition, groups of 8 to 10 rats were also exposed to these same
concentrations beginning at 14 months of age for 12 months. Although
values were occasionally significantly (p < 0.05) different from
those of controls, no consistent dose-related effects on various
haematological parameters, circulating protein levels or clinical
chemistry parameters were reported in rats exposed from 3 months of
age. In animals exposed for 12 months beginning at 14 months of age,
there were no consistent dose-related effects on haematological
parameters. There were significant (p < 0.05) changes in serum
parameters indicative of effects on liver and kidney function,
including levels of glutamic-pyruvic transaminase (SGPT),
gamma-glutamyltranspeptidase (gamma-GT), glutamic-oxalic transaminase
(SGOT) and cholesterol, and levels of uric acid in the blood and blood
urea nitrogen (BUN) at 202 and 607 mg/m3. Histopathological
examinations were not conducted (Spreafico et al., 1980).
No increase in the incidence of any type of tumour was reported
in groups of 90 male or female Swiss mice exposed to 20, 40, 202 or
1012 (decreased to 607 mg/m3 after a few days) mg/m3 (5, 10, 50 or
250/150 ppm), 7 h/day, 5 days/week for 78 weeks, and observed until
spontaneous death (Maltoni et al., 1980). However, it should be noted
that survival was poor (especially among males).
Table 5. Chronic toxicity and carcinogenicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats Rats were exposed to 0, 20, 40, 202 After several days of exposure to 1012 mg/m3, severe toxic effects, Maltoni
(Sprague-Dawley, and 1012/607 mg/m3 (0, 5, 10, 50 including death were observed and the level of exposure was reduced et al.
90 m & 90 f per and 250/150 ppm) 1,2-dichloroethane to 607 mg/m3. Survival varied among the groups, but was not related (1980)
group, 180 m & (99.92% pure), 7 h/day, 5 days/week to concentration; most rats died by week 140. Survival at 104 weeks
180 f controls) for 78 weeks and observed until of age in controls, chamber controls, and groups exposed to 20, 40,
spontaneous death. One group of 202 and 1012/607 mg/m3 was 17.8, 13.3, 50.0, 14.4, 18.9 and 11.1%
controls was kept in a nearby room, (males) and 40.0, 24.4, 53.3, 28.9, 32.2, and 23.3% (females).
while the other was kept in an "Incidence" was reported as the number of animals developing specific
exposure chamber under the same tumours over the number of animals alive at the time the first tumour
conditions as the exposed groups. of that type was detected (i.e. incidences have not been adjusted for
A complete autopsy was performed differential survival). With the exception of benign mammary tumours,
on each animal, regardless of time there were no significant increases in the incidence of any types of
of death. Several organs were (combined) tumours in exposed rats. The incidence of all mammary
routinely histopathologically tumours (number of animals alive at the time of appearance of the
examined, along with any organs first mammary tumour (12 weeks) was 90 in each exposure group) was
with pathological lesions. 52/90 (57.8%), 38/90 (42.2%), 65/90 (72.2%), 43/90 (47.8%), 58/90
(64.4%) and 52/90 (57.5%) in non-chamber controls, chamber controls,
and groups exposed to 20, 40, 202 and 1012/607 mg/m3, respectively.
The numbers (denominators not specified) of fibromas and
fibroadenomas (combined) of the mammary gland were 47, 27, 56, 33, 49
and 47 in non-chamber controls, chamber controls, and groups exposed
to 20, 40, 202 and 1012/607 mg/m3, respectively. The "incidence"
of these tumours at 20, 40, 202 and 1012/607 mg/m3, was
significantly (p<0.01 or 0.001) different from the incidence in
chamber controls. The "incidences" of benign mammary tumours in the
two control groups were also significantly (p<0.01) different.
Table 5. (cont'd).
Species Protocol Results Reference
Rats Rats were exposed to 0, 20, 40, 202 There were no consistent, exposure-related changes in various Spreafico
(Sprague-Dawley, or 1012-607 mg/m3 (0, 5, 10, 50 or haematological parameters, circulating protein levels or clinical et al.
8-10 or f per 250/150 ppm) 7 h/day, 5 days/week chemistry parameters in animals exposed from 3 months of age, (1980)
group) for 3, 6 or 18 months, beginning at although values occasionally differed significantly from controls.
3 months of age. In addition, groups In animals exposed for 12 months from 14 months of age, there were no
of rats were exposed to 0, 20, 40, consistent exposure-related alterations in haematological parameters.
202 or 1012-607 mg/m3 (0, 5, 10, 50 Levels of serum glutamic-pyruvic transaminase (SGPT) were
or 250/150 ppm) 7 h/day, 5 days/ significantly elevated in both males and females at 202 and 607 mg/m3
week for 12 months, beginning at 14 (p<0.05), and gamma-glutamil transpeptidase (gamma-GT) levels were
months of age. Histopathological also significantly greater in females at the two highest
examinations were not conducted. concentrations (p<0.05). Levels of serum glutamic-oxalic transaminase
(SGOT) were significantly increased in both sexes at 20 and 40 mg/m3
(p<0.05), but significantly decreased in males and females at 202 and
607 mg/m3 (p<0.05). Levels of cholesterol were significantly lower
in males and females at 202 and 607 mg/m3 (p<0.05). Levels of uric
acid in the blood were significantly higher in both sexes at 202 and
607 mg/m3 (p<0.05), while blood urea nitrogen (BUN) values were
significantly elevated at 607 mg/m3 (p<0.05), although there were
no effects on urinary parameters.
Rats Rats were exposed to 200 mg/m3 (0 There were no compound-related effects on body weight gain or Cheever
(Sprague-Dawley, or 50 ppm) 7 h/day, 5 days/week for mortality. There were no significant increases in the incidence of et al.
50 m & 50 f 2 years. Extensive histopathological any type of tumours, although there was a non-significant increase (1990)
per group) examinations were conducted. in the incidence of mammary gland adenomas (4 versus 2 in controls)
and fibroadenomas (21/50 versus 15/50) in females. The incidence of
testicular lesions (not further specified) was increased in exposed
animals (24% versus 10% in controls, significance not reported).
Table 5. (cont'd).
Species Protocol Results Reference
Mice (Swiss, Mice were exposed to 20, 40, 202 or Survival of mice was poor, especially in males, as only 43.4 to 65.6% Maltoni
90 m & 90 f 1012-607 mg/m3 (5, 10, 50 or 250/ of exposed males survived for 52 weeks after exposure commenced. et al.
per group, 150 ppm), 7 h/day, 5 days/week for There were no significant increases in the incidence of any type of (1980)
115 m & 134 f 78 weeks and observed until tumours.
controls) spontaneous death. Controls were
kept in a nearby room. A complete
autopsy was performed on each
animal, regardless of time of death.
Several organs were routinely
histopathologically examined, along
with any organs with pathological
lesions.
Ingestion
Rats Animals were administered time There were no effects on body weight gain in either sex. Mortality NCI
(Osborne-Mendel, weighted average doses of 47 or 95 was significantly higher in both males and females in the high dose (1978)
50 50 m & f per mg/kg body weight per day (initial group, as 50% of exposed rats had died by week 55 (males) and 57
group, 20 m & doses of 50 and 100 mg/kg body (females), compared to week 72 (males) and 88 (females) in controls.
20 f controls; weight per day were increased to 75 Signs of toxicity, including wheezing, nasal discharge, ulcerations,
60 m & 60 f and 150 mg/kg body weight per day localized alopecia, discoloured or stained fur, bloated appearance
pooled controls after 7 weeks then decreased to and swollen areas, occurred at a greater frequency in exposed
from concurrent original doses after 17 weeks) in animals than in controls. Chronic murine pneumonia was present in 60
experiments) corn oil by gavage, 5 days/week, for to 94% of rats in each group (incidence not related to dose).
78 weeks followed by 32 weeks of Acanthosis and hyperkeratosis of the forestomach was present in a
observation. Complete greater proportion of exposed females than controls (1/20, 6/50 and
histopathological examinations were 7/50 in vehicle controls, low and high dose, respectively,
conducted. significance not reported). Other non-neoplastic lesions occurred
at similar frequencies in control and exposed rats. The incidence
of squamous cell carcinomas of the forestomach in males was 0/60,
Table 5. (cont'd).
Species Protocol Results Reference
0/20, 3/50 and 9/50 in pooled controls, matched controls, low and
high dose groups, respectively, significant at the high dose
(p=0.01); only 2/50 females in the low dose group had this tumour.
There were also one leiomyosarcoma of the stomach and one
adenocarcinoma of the small intestine in high dose males (not
significant). The incidence of hemangiosarcomas (mostly in the
spleen) in males was 1/60, 0/20, 9/50 and 7/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively (significant in both exposed groups, p=0.03 (low) and
p=0.016 (high)), and in females was 0/59, 0/20, 4/50 and 4/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively (significant in both exposed groups (p=0.041 in
both)). Both groups of exposed males had an increased incidence of
fibromas of the subcutaneous tissue (0/60, 0/20, 5/50 and 6/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively); no such increase was noted in females. In
females, there was an increased incidence of adenocarcinomas and
fibroadenomas of the mammary gland (1/59, 0/20, 1/50 and 18/50
(adenocarcinoma), 5/59, 0/20, 14/50 and 8/50 (fibroadenoma) and 6/59,
0/20, 15/50 and 24/50 (adenocarcinoma or fibroadenoma)) in pooled
vehicle controls, matched vehicle controls, low and high dose groups,
respectively). Renal tubular cell adenocarcinomas were noted in one
male and one female at the highest dose, while tubular cell adenomas
were present in one male and two females at this dose, and none was
observed in controls (significance not reported).
Rats (18 m & Animals were fed mash fumigated Chronic respiratory disease was reported in all groups in the second Alumot
18 f per group, with 1,2-dichloroethane for 2 years. year of exposure. The number of rats surviving after 21 months ranged et al.
strain not Resulting concentrations were 250 from 2 to 14 per group. No effects on growth or the biochemical (1976a)
specified) and 600 mg/kg. Due to loss of the parameters investigated were observed.
Table 5. (cont'd).
Species Protocol Results Reference
compound through volatilization,
the mash actually consumed was
estimated to contain 60 to 70% of
the initial concentration (estimated
to result in doses of approximately
7.5 to 8.75 and 15 to 17.5 mg/kg
body weight per day). The liver was
analysed for total fat triglyceride
content. Levels of total protein,
albumin, glucose, urea, uric acid
and cholesterol in the serum were
determined. Histopathological
examinations do not appear to have
been conducted on surviving rats
at the end of the exposure period.
Mice (B6C3F1, Animals were administered Mortality in females was related to dose (36 animals in the high dose NCI
50 m & 50 f time-weighted average doses of 97 group died between weeks 60 and 80, which may have been related to (1978)
per group; 20 or 195 mg/kg body weight per day the appearance of tumours as 25 of these animals had tumours); no
m & 20 f (males) or 149 or 299 (females) in similar dose-related trend in mortality was observed in males. Body
controls; 60 m corn oil by gavage, 5 days/week, for weight in females in the high dose group was depressed as early as
& 60 f pooled 78 weeks followed by 13 weeks of week 15 (>10%). The incidence of non-neoplastic lesions was
controls from observation (initial doses in males comparable in exposed and control mice. There was an increased
concurrent of 75 and 150 mg/kg body weight incidence of hepatocellular carcinomas in male mice at the highest
experiments) per day were increased to 100 and dose (4/59, 1/19, 6/47 and 12/48 in pooled vehicle controls, matched
200 mg/kg body weight per day after vehicle controls, low and high dose groups, respectively), but not in
8 weeks; initial doses in females females. The incidence of alveolar/bronchiolar adenomas in males was
Table 5. (cont'd).
Species Protocol Results Reference
of 125 and 250 mg/kg body weight per 0/59, 0/19, 1/47 and 15/48 in pooled vehicle controls, matched
day were increased to 200 and vehicle controls, low and high dose groups, respectively
400 mg/kg body weight per day after (significant at the highest dose (p<0.001)); the incidence of this
8 weeks, then decreased to 150 and tumour in females was 2/60, 1/20, 7/50 and 15/48 in pooled vehicle
300 mg/kg body weight per day after controls, matched vehicle controls, low and high dose groups,
11 weeks). Complete respectively (significant in both exposed groups(p=0.046 (low) and
histopathological examinations were p<0.001 (high)). There was also one alveolar/bronchiolar carcinoma in
conducted. females at 299 mg/kg body weight per day. There was a non-significant
increase in the incidence of squamous cell carcinoma of the
forestomach in females at 299 mg/kg body weight per day (5/48 versus
1/60 or 1/20 in controls). There was a significantly increased
incidence of mammary gland adenocarcinomas in both groups of exposed
females (0/60, 0/20, 9/50 and 7/48 in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively
(p=0.001 (low) and p=0.003 (high)).
Uterine adenocarcinomas occurred in 3/49 low dose and 4/47 high
dose females, compared to none in controls; however, this increase
was not statistically significant. The incidence of endometrial
stromal polyp or endometrial stromal sarcoma (combined) was 0/60,
0/20, 5/49 and 5/47 in pooled vehicle controls, matched vehicle
controls, low and high dose groups, respectively (significant at
both doses, p=0.016 (low) and p=0.014 (high)).
Table 5. (cont'd).
Species Protocol Results Reference
Dermal
application
Swiss mice Doses of 0, 42 or 126 mg/application The incidence of benign lung papillomas was significantly (p<0.0005) van Duuren
(Ha:ICR, 30 f; per mouse in 0.2 ml acetone were increased at the higher dose (26/30 compared to 17/30, 11/30 and et al.
30 vehicle applied 3 times per week to the 30/100 in low dose group, vehicle controls and untreated controls, (1979)
controls and shaved dorsal skin (area of skin respectively). The incidence of stomach tumours was 3/30, 1/30, 2/30
100 naive exposed not specified) of mice for and 5/100 in high dose group, low dose group, vehicle controls and
controls) 440 to 594 days. The skin, liver, untreated controls, respectively (not significant).
kidney and any tissues or
organs appearing abnormal were
examined histopathologically.
7.5.2 Ingestion
In a study conducted by the National Cancer Institute (NCI,
1978), time-weighted average daily doses of 47 or 95 mg/kg body weight
per day of 1,2-dichloroethanea in corn oil were administered by
gavage 5 days/week for 78 weeks to 50 Osborne-Mendel rats of each sex,
followed by 32 weeks of observation. Mortality was significantly
(p < 0.001) higher in both males and females in the high dose group.
Clinical signs of general toxicity occurred at a greater frequency in
exposed animals than in controls. In each group 60-94% of rats had
chronic murine pneumonia, but the incidence was not related to dose.
The incidence of acanthosis and hyperkeratosis of the forestomach was
greater in exposed females than controls.
The incidence of a variety of tumours was increased in exposed
animals compared with controls. The incidence of squamous cell
carcinomas of the stomach was significantly increased in males (3/50
and 9/50 in low and high dose groups, respectively), compared to none
in either group of controls; in females, there were only 2/50 in the
low dose group. The incidence of haemangiosarcoma was significantly
increased in males (1/60, 0/20, 9/50 and 7/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively) and females (0/59, 0/20, 4/50 and 4/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). The incidence of fibromas of the subcutaneous tissue
was significantly increased in males (0/60, 0/20, 5/50 and 6/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively), but not in females. There was a significant
increase in the incidence of adenocarcinomas and fibroadenomas of the
mammary gland in females (1/59, 0/20, 1/50 and 18/50 (adenocarcinoma),
5/59, 0/20, 14/50 and 8/50 (fibroadenoma) and 6/59, 0/20, 15/50 and
24/50 (adenocarcinoma or fibroadenoma)) in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively). It
was concluded that 1,2-dichloroethane was carcinogenic in this strain
of rats, under the conditions of this study.
No effects on growth or biochemical parameters were observed in a
limited study on rats fed mash that had been fumigated with
1,2-dichloroethane, which resulted in doses of approximately 7.5-8.75
and 15-17.5 mg/kg body weight per day. Chronic respiratory disease
was evident in all groups in the second year, the number of rats
surviving after 21 months ranging from 2 to 14 in each of the groups.
The occurrence of respiratory disease and mortality did not appear to
be exposure-related (Alumot et al., 1976a).
a Technical grade with reported purity of > 90% containing 11 minor
contaminants; subsequent analysis indicated a purity of about
98-99% (Hooper et al., 1980 and Ward, 1980).
The National Cancer Institute (NCI, 1978) also conducted a
bioassay in which groups of 50 B6C3F1 mice were administered
time-weighted average daily doses of 97 or 195 mg/kg body weight per
day (males) and 149 or 299 mg/kg body weight per day (females)
1,2-dichloroethane in corn oil by gavage, 5 days/week for 78 weeks,
followed by 13 weeks of observation. A doserelated increase in
mortality was noted in female mice, but not in males. Body weight was
also decreased in females at 299 mg/kg body weight per day.
As in rats, there was a significant increase in the incidence of
several types of tumours in exposed mice. The incidence of
hepatocellular carcinomas was significantly increased in males in the
high dose group (4/59, 1/19, 6/47 and 12/48 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively); no such increase was noted in females. However, the
authors noted that, due to the high variability of incidence of
hepatocellular neoplasms in historical controls (data not presented),
this increase, although statistically significant, was not considered
to be convincing evidence that these tumours were attributable to the
test chemical. The incidence of alveolar/bronchiolar adenomas was
significantly increased in males in the high dose group (0/59, 0/19,
1/47 and 15/48 in pooled vehicle controls, matched vehicle controls,
low and high dose groups, respectively), and in both groups of exposed
females (2/60, 1/20, 7/50 and 15/48 in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively); one
alveolar/bronchiolar carcinoma was noted in a high-dose female mouse.
There was a non-significant increase in the incidence of squamous cell
carcinoma of the forestomach in females in the high dose group. The
incidence of mammary gland adenocarcinomas was significantly increased
in females at both doses (0/60, 0/20, 9/50 and 7/48 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). The incidence of endometrial stromal polyp or
endometrial stromal sarcoma (combined) was significantly elevated at
both doses (0/60, 0/20, 5/49 and 5/47 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). It was concluded that 1,2-dichloroethane was
carcinogenic in this strain of mice, under the conditions of this
study.
It should be noted that the data on tumour incidence presented in
the bioassays by the NCI (1978) do not take into account the increased
early mortality in the high exposure groups; the incidences of several
tumours (and thus the carcinogenic potency of 1,2-dichloroethane) may
have been higher had all animals survived for a long enough period of
time to develop tumours (Ward, 1980; Hooper et al., 1980).
7.5.3 Other routes of administration
1,2-Dichloroethane in acetone was applied to the shaved dorsal
skin of groups of 30 female non-inbred Ha:ICR Swiss mice, 3 times/week
for 440 to 594 days at doses of 0, 42, and 126 mg/application per
mouse. The incidence of lung tumours (benign lung papillomas) was
significantly increased at the higher dose (26/30 compared to 11/30 in
vehicle controls and 30/100 in naive controls, p < 0.0005).
Histopathological examination was limited to the skin, liver, kidney
and any "abnormal-appearing tissues" (van Duuren et al., 1979).
In a bioassay designed to screen the potential of numerous
chemicals to induce pulmonary tumours in a susceptible strain of mice
(A/St), groups of 20 males were administered 24 intraperitoneal
injections of 1,2-dichloroethane (20, 40 or 100 mg/kg body weight) in
tricaprylin, 3 times/week for 8 weeks (total doses of 480, 920 or
2400 mg/kg body weight). All surviving mice were killed 24 weeks
after the first injection. Although there was a dose-related increase
in the number of pulmonary adenomas per mouse (0.39, 0.21, 0.44 and
0.75 in the control, low, mid and high dose groups, respectively),
none of these increases was statistically significant (Theiss et al.,
1977). It should be noted, however, that the duration of the period
of observation may have been insufficient to allow for the development
of most types of tumours.
7.5.4 Initiation/promotion bioassays
In a dermal initiation/promotion protocol, 126 mg
1,2-dichloroethane was applied once to the skin (area exposed not
specified) of 30 female non-inbred Ha:ICR Swiss mice, followed 14 days
later by 5 µg (0.005 mg) of phorbol myristate acetate (PMA) (a
promoter) in 0.2 ml acetone, 3 times/week for 428 to 576 days. Two
PMA control groups of 120 and 90 mice were administered 0.0025 mg and
0.0050 mg PMA/application permouse (number of applications not
specified), respectively. Treatment with 1,2,-dichloroethane did not
significantly increase the incidence of skin papillomas (3/30 versus
9/120 and 6/90 in PMA controls). There were three squamous cell
carcinomas in the control groups, while none was observed in the
exposed group (van Duuren et al., 1979).
In a hepatic initiation/promotion assay, groups of 10
Osborne-Mendel rats were partially hepatectomized and then
administered 1,2-dichloroethane (100 mg/kg body weight) in corn oil by
gavage, followed 5 days later by diets containing phenobarbital for 7
weeks and a control diet for 1 week (initiation protocol). Livers
were examined histopathologically for GGT-positive foci (a putative
preneoplastic indicator). Additional groups of 10 rats were initiated
with an intraperitoneal injection of diethyl-nitrosamine or water
(control) following partial hepatectomy, then administered
1,2-dichloroethane (100 mg/kg body weight) in corn oil by gavage, 5
days/week for 7 weeks (promotion protocol). In rats administered
1,2-dichloroethane in the initiation protocol, there was no increase
in the number of GGT-positive foci.
Similarly, in rats administered 1,2-dichloroethane in the promotion
protocol, there was no significant increase in the number of
GGT-positive foci, either with or without the initiator, when compared
to controls (Story et al., 1986; Milman et al., 1988).
Groups of 35 male B6C3F1 mice were administered
diethylnitrosamine in the drinking-water for 4 weeks, followed by
exposure to 1,2-dichloroethane (835 or 2500 mg/litre) in
drinking-water for 24 or 52 weeks. Only the liver, kidneys and lungs
were examined histopathologically. Drinking-water intake was reduced
at the highest concentration. No significant differences in body
weight gain were noted. However, three mice consuming the highest
concentration of 1,2-dichloroethane died within 52 weeks. There was
no increase in the incidence of liver or lung tumours in exposed mice
either with or without diethylnitrosamine initiation (Klaunig et al.,
1986).
7.6 Mutagenicity and related end-points
The genotoxicity of 1,2-dichloroethane has been extensively
investigated in non-mammalian and mammalian test systems. Data from
in vitro and in vivo studies are summarized in Tables 6 and 7; a
summary of the weight of available evidence is presented here.
1,2-Dichloroethane induced differential toxicity in Escherichia
coli, but it had no effect in a Bacillus subtilis rec assay. It
consistently induced positive responses in mutagenicity assays with
Salmonella typhimurium, whereas it has not produced consistent
responses in mutation assays with E. coli and was negative in a mouse
peritoneal host-mediated assay with E. coli.
In the fungus Aspergillus nidulans, 1,2-dichloroethane induced
errors of mitotic segregation and aneuploidy, but did not induce gene
mutation.
Table 6. Genotoxicity of 1,2-dichloroethane in vitro (modified from ATSDR, 1994)
Species (test system) End-point Resulta Reference
With activation Without activation
Bacterial systems
Salmonella typhimurium Gene mutation + + Milman et al. (1988)
+ + Barber et al. (1981)
+ + Kanada & Uyeta (1978)
+ + Nestmann et al. (1980)
+ + Rannug et al. (1978)
+ + Van Bladeren et al. (1981)
+ NT Rannug & Beije (1979)
+ - Cheh et al. (1980)
+ - Moriya et al. (1983)
- - King et al. (1979)
+ + Strobel & Grummt (1987)
NT +b Simula et al. (1993)
S. typhimurium/spot test NT (+) Brem et al. (1974)
(+) - Principe et al. (1981)
NT - Buijs et al. (1984)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
S. typhimurium/Ara test + - Roldan-Arjona et al. (1991)
(standard)
S. typhimurium/Ara test (+) (+) Roldan-Arjona et al. (1991)
(liquid)
Streptomyces coelicolor Gene mutation NT - Principe et al. (1981)
Escherichia coli/K12/343/113 Gene mutation - - King et al. (1979)
E. coli/wp2 NT (+) Hemminki et al. (1980)
- - Moriya et al. (1983)
E. coli Pol A DNA damage NT (+) Brem et al. (1974)
Bacillus subtilis/rec-assay DNA damage NT - Kanada & Uyeta (1978)
Fungal systems
Aspergillus nidulans Gene mutation NT - Crebelli & Carere (1988)
NT - Principe et al. (1981)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
A. nidulans Mitotic segregation NT + Crebelli et al. (1984)
aberrations
A. nidulans Aneuploidy induction NT + Crebelli et al. (1988)
Saccharomyces cerevisiae Mitotic recombination NT (+) Simmon (1980)
Animal systems
Hamster CHO/HGPRT Gene mutation + (+) Tan & Hsie (1981)
+ (+) Zamora et al. (1983)
Rat hepatocytes Unscheduled DNA synthesis NT + Williams et al. (1989)
Mouse hepatocytes NT + Milman et al. (1988)
Mouse liver DNA DNA binding + NT Banerjee (1988)
Calf thymus DNA + NT Prodi et al. (1986)
Salmon sperm DNA + - Banerjee & Van Duuren (1979);
Banerjee et al. (1980)
Mouse BALBc/3T3 Cell transformation NT - Milman et al. (1988)
NT - Tu et al. (1985)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
Mouse C3H1OT´ Cell transformation NT +c Schultz et al. (1992)
Syrian hamster embryo cells Cell transformation NT + Hatch et al. (1983)
Human cells
Human lymphoblasts AHH-1 Gene mutation NT + Crespi et al. (1985)
Human lymphoblasts TK6 NT + Crespi et al. (1985)
Human embryo epithelial-like NT + Ferreri et al. (1983)
EUE cells
Human peripheral lymphocytes Unscheduled DNA synthesis + - Perocco & Prodi (1981)
a NT = not tested; - = negative result; + = positive result; (+) = weakly positive or marginal result
b increase in cells expressing GSTA1-1
c transformed cells induced tumours in nude mice
Table 7. Genotoxicity of 1,2-dichloroethane in vivo (modified from ATSDR, 1994)
Species (test system) End-point Resultsa Reference
Mammalian assays
Mouse Dominant lethal mutations - Lane et al. (1982)
Mouse/spot test Gene mutation (+) Gocke et al. (1983)
Mouse bone marrow Sister-chromatid exchange + Giri & Que Hee (1988)
Mouse bone marrow Micronuclei - Jenssen & Ramel (1980);
King et al. (1979)
Mouse peripheral erythrocytes - Armstrong & Galloway (1993)
Mouse liver, kidney, lung and stomach DNA binding + Prodi et al. (1986)
Mouse liver, kidney, lung and stomach + Arfellini et al. (1984)
Mouse forestomach and kidney + Hellman & Brandt (1986)
Mouse liver + Banerjee (1988)
Table 7 (contd).
Species (test system) End-point Resultsa Reference
Rat liver, kidney, spleen, lung, + Reitz et al. (1982)
forestomach and stomach
Rat liver, kidney, lung and stomach + Arfellini et al. (1984)
Rat liver, kidney, lung and stomach + Prodi et al. (1986)
Rat liver and kidney + Inskeep et al. (1986)
Rat liver and lung + Baertsch et al. (1991)
Rat liver + Banerjee (1988)
Rat liver + Cheever et al. (1990)
Mouse liver DNA damage + Storer & Conolly 1983, 1985;
Storer et al. (1984)
Mouse liver + Taningher et al. (1991)
Insect assays
Drosophila melanogaster/somatic mutation Gene mutation + Nylander et al. (1978)
Table 7 (contd).
Species (test system) End-point Resultsa Reference
D. melanogaster/somatic mutation + Romert et al. (1990)
D. melanogaster/somatic mutation + Kramers et al. (1991)
D. melanogaster/somatic mutation (+) Ballering et al. (1993)
D. melanogaster/recessive lethal + Ballering et al. (1993)
D. melanogaster/vermilion locus + Ballering et al. (1993)
D. melanogaster/sex-linked recessive + King et al. (1979)
D. melanogaster/sex-linked recessive + Kramers et al. (1991)
D. melanogaster Chromosomal loss/gain +/+ Valencia et al. (1984)
Host-mediated assays
Escherichia coli K12/343/113 mouse Gene mutation - King et al. (1979)
host-mediated assay
a - = negative result; + = positive result; (+) = weakly positive or marginal result
In cultured mammalian cells, 1,2-dichloroethane formed
adducts with DNA. It also induced unscheduled DNA synthesis in primary
cultures of mouse and rat hepatocytes, and human peripheral
lymphocytes (the last in the presence of an exogenous metabolic
activation system), and gene mutation in several cell lines. Mutation
frequency of two human cell lines has been correlated with the
difference in levels of glutathione- S-transferase activities (Crespi
et al., 1985; section 6.3). Cell transformation was induced in
studies with C3H10T´ cells, but not with BALBc/3T3 cells.
1,2-Dichloroethane enhanced SV40 virus transformation of Syrian
hamster embryo cells.
In vivo, both somatic cell and sex-linked recessive lethal
mutations have been consistently induced in Drosophila melanogaster by
1,2-dichloroethane. 1,2-Dichloroethane has been found to bind to DNA
in all reported studies in mice and rats. In other studies with mice,
clearly positive responses have been restricted to primary DNA damage
in liver and sister-chromatid exchange induction in bone marrow. No
evidence for micronucleus induction has emerged from bone marrow
micronucleus studies or for a dominant lethal effect in one study
(although this study may have been insufficiently sensitive), and only
a weak but significant (p < 0.03) response was observed in a single
spot test.
It has been noted that stronger responses were obtained in the
bacterial mutation assay in the presence of an exogenous metabolic
system than in its absence. This could imply the formation of
additional mutagenic metabolites, through either the cytochrome P450
or glutathione-S-transferase pathway. In these in vitro assays with
liver homogenates, activation by the cytochrome system is more likely,
and 2-chloroacetaldehyde, which is a possible metabolite, is known to
be mutagenic (McCann et al., 1975). The mutagenicity of
1,2-dichloroethane in S. typhimurium TA100, in the absence of S9 mix,
was more than doubled if the bacterium expressed the human GSTA1-1
gene, but there was no change in the mutagenic response if the SSTP1-1
gene was expressed (Simula et al., 1993).
7.7 Reproductive toxicity, embryotoxicity and teratogenicity
The reproductive and developmental effects of 1,2-dichloro-ethane
have not been extensively investigated in experimental animals (see
Table 8), although the compound has been detected in fetal tissues in
rats and mice following maternal exposure to 600 mg/m3 for 5 h
(Withey & Karpinski, 1985) and 1000 mg/m3 for 3 days (Vozovaya,
1977).
Table 8. Reproductive and developmental toxicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats Animals were exposed to 0, 101, 304, or No exposure-related histopathological effects in the parents nor Rao
(Sprague-Dawley, 607 mg/m3 (0, 25, 75, or 150 ppm) any alterations in the fertility index and gestation periods were et al.
20 m & 20 f per 6 h/day, 5 days/week for 60 days prior observed compared to controls. There was a significant decrease (1980)
exposed group, to mating, and for an additional 116 in the number of pups per litter in the F1A pups at 304 mg/m3
30 m & 30 f days (7 days/week) after mating. Dams (13%, p<0.05), but not at 607 mg/m3; there was significantly
controls) were not exposed from gestation day 21 increased kidney weight in the F1B male pups at 101 mg/m3
through to day 4 postpartum. The pups (29%, p<0.05), although the authors did not consider this effect
(F1A) were removed after 21 days, and to be related to exposure. There were no significant differences
the females were remated to exposed in growth, sex ratios, survival indices, organ or neonatal body
males following removal of the last weights, or histology in pups.
litter to produce F1B litters. Liver,
kidneys, ovaries , uterus and testes
of parental animals in control and
high exposure group (and other groups
if any changes were noted in high
exposure group) were examined
histopathologically
Rats (Albino, f, Rats were exposed to 57 mg/m3, 4 h/day, Exposed rats had reduced fertility (6.5 fetuses per dam compared Vozovaya
strain and 6 days/week, for 6 or 9 months. The to 9.7 per dam in controls). Newborn pups had reduced body (1974)
number animals were apparently then mated, but weight (5.06 g versus 6.44 g in controls). Perinatal mortality
unspecified it is not clearly stated whether the was increased in the exposed group (data not presented). No
in secondary exposure period extended beyond information was available on maternal effects.
account) mating.
Table 8 (contd).
Species Protocol Results Reference
Rats (f, strain Rats were exposed to 15 mg/m3, 4 h/day, The estrous cycle was longer in exposed rats than in controls. No Vozovaya
and number 6 days/week for 4 months prior to and information on the effects on fertility was presented. Embryonal (1977)
unspecified after mating. mortality increased from approximately 11% in controls to 27% in
in secondary exposed dams. Pre-implantation losses were 5-fold greater in
account) exposed animals than in controls. No fetal abnormalities were
observed, except for haematomas in the region of the head, neck
and anterior extremities (presumably only in pups of exposed
animals, although not clearly stated). No information was
available on maternal effects.
Rats Rats were exposed to 0, 405 or 1215 10/16 dams at 1215 mg/m3 died. At 405 mg/m3, there were no Rao
(Sprague-Dawley, mg/m3 (0, 100 or 300 ppm) for 7 h/day effects on mean litter size, incidence of resorptions, or fetal et al.
16 to 30 pregnant on days 6 to 15 of gestation. body measurements; no significant increase in the incidence of (1980)
f per group) major malformations was observed at this concentration.
Rabbits (New Rabbits were exposed to 0, 405, or 4/21 and 3/19 dams died at 405 and 1215 mg/m3, respectively, Rao
Zealand White, 1215 mg/m3 (0, 100 or 300 ppm) for compared to none in 20 controls. There were no effects on mean et al.
19 to 21 7 h/day on days 6 to 18 of gestation. litter size, incidence of resorptions or fetal body measurements (1980)
pregnant f at either concentration, and there were no significant differences
per group) in the incidence of major malformations.
Table 8 (contd).
Species Protocol Results Reference
Ingestion
Rats (45 m & Animals were fed mash fumigated with There were no significant differences in various reproductive Alumot
90 f, strain 1,2-dichloroethane for 2 years. parameters, including number of dams pregnant, number of dams et al.
unspecified) Resulting concentrations were 250 and with litters, mean litter size, mortality or body weight of young (1976a)
600 mg/kg. Due to loss of the compound at birth and at weaning.
through volatilization, the mash
actually consumed was estimated to
contain 60 to 70% of the initial
concentration (estimated to result in
doses of approximately 7.5 to 8.75 and
15 to 17.5 mg/kg body weight per day).
Exposed females were mated with
exposed males at 2 month intervals.
Mice (ICR Swiss, F0 mice were administered There were no differences in body weight in adults, and there Lane
(10 m & 30 f concentrations of 0, 0.03, 0.09 or 0.29 were no effects on fertility or gestation indices. There were no et al.
per exposed mg/litre drinking-water (equivalent effects on survival, litter size, postnatal body weight or gross (1982)
group, 20 m & to nominal doses of approximately pathology of pups. The incidence of fetal visceral or skeletal
60 f controls) 0, 5, 15 or 50 mg/kg body weight malformations was not increased in exposed animals. F1C litters
per day) for 35 days prior to were not examined for skeletal malformations.
mating. Three sets of offspring
(F1A, F1B and F1C) were produced.
Table 8 (contd).
Species Protocol Results Reference
After weaning and 11 weeks of
exposure to the same concentrations,
the F1B mice were mated (30 female,
10 male) to produce a second generation
of offspring (F2A and F2B). Teratology
screening tests were performed using
F1C and F2B matings where the females
were co-housed with unexposed males.
Mice (f, number Pregnant mice were administered There were no developmental effects and "few discernible effects" Kavlock
and strain not 1,2-dichloroethane in the drinking-water on maternal health. There were no skeletal or visceral anomalies et al.
specified in at a concentration equivalent to a which could be attributed to exposure. (1979)
secondary dose of 510 mg/kg body weight per
account) day on days 7 to 14 of gestation.
No effects on reproductive parameters, including fertility index,
gestation period or histological changes, were reported in a
two-generation study on Sprague-Dawley rats exposed to 0, 101, 304 or
607 mg/m3 (0, 25, 75 or 150 ppm) 1,2-dichloroethane 6 h/day, for 60
days prior to mating and 116 days after mating (except during the
delivery period). There were also no effects on growth, sex ratios,
survival indices, organ or neonatal body weights, or histology in pups
(Rao et al., 1980).
Exposure to 1,2-dichloroethane (15 mg/m3), 4 h/day, for 4
months prior to mating and after mating resulted in a
longer-than-normal estrous cycle in female rats (strain not
specified). Although embryonal mortality and preimplantation losses
were greater in exposed animals than in controls, no fetal
abnormalities were reported, except for haematomas in the area of the
head, neck and anterior extremities (Vozovaya, 1977). Similarly,
increased perinatal mortality and reduced body weight of newborn pups
were observed in the offspring of female albino rats exposed to 57
(± 10) mg/m3 for 6 and 9 months. Exposed females also produced a
lower number of fetuses per dam (Vozovaya, 1974). Information from
these studies was insufficient for evaluation.
Rao et al. (1980) also conducted a developmental study in which
pregnant Sprague-Dawley rats were exposed to 0, 405 or 1215 mg/m3
(0, 100 or 300 ppm) 1,2-dichloroethane, 7 h/day, during gestation.
Mortality was high in dams at 1215 mg/m3 (10/16 rats died); thus the
developmental effects could not be ascertained at this concentration.
No fetotoxic or teratogenic effects were observed at 405 mg/m3. In
pregnant rabbits exposed to the same concentrations, 7 h/day, during
gestation, mortality was increased in exposed animals (4/21 and 3/19
dams died at 405 and 1215 mg/m3, respectively, compared to none in
20 controls). However, no fetotoxic or teratogenic effects were
reported at either concentration. The authors concluded that
1,2-dichloroethane was not teratogenic or fetotoxic in rats at
405 mg/m3 or in rabbits at 405 or 1215 mg/m3.
No effects on male fertility or various reproductive parameters
were noted in rats consuming mash which had been fumigated with
1,2-dichloroethane, the resultant concentrations being 250 and
500 mg/kg (approximately equivalent to doses of 7.5 to 8.75 and 15 to
17.5 mg/kg body weight per day when loss due to volatilization was
taken into account), for up to 2 years (Alumot et al., 1976a). No
effects on reproduction (in terms of fertility and gestation indices)
were reported in a two-generation study on Swiss ICR mice exposed to
1,2-dichloroethane in the drinking-water at concentrations of 0, 0.03,
0.09 or 0.29 g/litre (approximately equivalent to doses of 0, 5, 15 or
50 mg/kg body weight per day). In addition, no fetotoxic or
teratogenic effects were noted in either generation of offspring of
F1C and F2B litters sacrificed on day 18 (Lane et al., 1982). No
developmental effects were reported in a study in which groups of 30
pregnant CD-1 mice were administered drinking-water containing a
mixture of organic compounds, including 0.01% 1,2-dichloroethane
(equivalent to a dose of 5.1 mg/kg body weight per day), during days 7
to 14 of gestation (Kavlock et al., 1979).
7.8 Immunological effects
Groups of 140 female CD1 mice were exposed to airborne
concentrations of 0, 10, 20 and 40 mg/m3 (0, 2.5, 5 and 10 ppm)
1,2-dichloroethane for a 3-h period or to 0 or 10 mg/m3, 3 h/day for
5 consecutive days. After acute exposure to 20 and 40 mg/m3, there
was a significant increase in mortality in mice from streptococcal
challenge (p < 0.05 and p < 0.001, respectively), while no effects
were noted following acute or repeated exposure to 10 mg/m3. In
similarly exposed groups of 18 to 36 mice, significantly (p < 0.01)
decreased pulmonary bactericidal activity to inhaled Klebsiella
pneumoniae was noted only at 40 mg/m3. Single exposure to 40 or
400 mg/m3 (10 or 100 ppm) did not affect the in vitro phagocytic
or cytostatic ability of alveolar macrophages to red blood cells and
tumour target cells, respectively (Sherwood et al., 1987).
Male Sprague-Dawley rats (16 per group) were exposed to 0, 400 or
800 mg/m3 (0, 100 or 200 ppm) 1,2-dichloroethane for 3 h, or to 0,
40, 80, 200 or 400 mg/m3 (0, 10, 20, 50 or 100 ppm), 5 h/day, 5
days/week for 12 days. Pulmonary bactericidal activity was not
affected at any concentration. No effects were noted on in vitro
phagocytic activity to red blood cells, in vitro cytostatis and
cytolysis of tumour target cells, or levels of ectoenzymes in alveolar
macrophages. Blastogenesis of mitogen-stimulated T- and B-lymphocytes
from popliteal and mesenteric lymph nodes was not affected (Sherwood
et al., 1987).
Chinchilla rabbits (number per group not specified but
probably less than 10) were exposed to 2, 10 or 100 mg/m3
1,2-dichloroethane for 3 h/day, 6 days/week, for 7.5 to 8 months. At
the two highest concentrations production of antibodies against
typhoid vaccine was increased, while total antibody production was
reduced at 100 mg/m3 (Shmuter, 1977).
Groups of 10 male CD1 mice (12 per group) (Munson et al., 1982)
were administered 1,2-dichloroethane (4.9 and 49 mg/kg body weight per
day) in water by gavage for 14 days. A significant (p < 0.05)
reduction of 25 and 40% in IgM antibody-forming cells to sheep RBCs
was observed at 4.9 and 49 mg/kg body weight per day, respectively. A
significant (p < 0.05) reduction of cell-mediated responses to sheep
erythrocytes was also observed at both levels, which was not related
to dose, while a significant (p < 0.05) reduction in leucocyte count
was observed at the highest dose. In the same study, groups of 16 to
32 male CD1 mice were administered time-weighted average
1,2-dichloroethane doses of 0, 3, 24 and 189 mg/kg body weight per day
(0, 0.02, 0.2 and 2.0 g/litre) for 90 days in drinking-water; controls
consisted of 24 to 48 mice. The fluid consumption of exposed mice was
decreased in a dose-related manner, which corresponded to a
dosedependent reduction in body weight. No effects on organ weights,
haematology, B-cell mitogen lipopolysaccharide response, spleen cell
response to the T-cell mitogen concanavalin A or cellmediated immunity
(assessed by measuring the delayed-type hypersensitivity responses to
sheep erythrocytes) were noted. There was a tendency towards a
reduction in the serum antibody level after immunization with sheep
erythrocytes, and in the immunoglobulin spleen antibody-forming cells
at all exposure levels (not significant) (Munson et al., 1982).
7.9 Toxicological interactions with other agents
In a recent bioassay designed to determine the influence of
disulfiram or ethanol on the carcinogenicity, metabolism and covalent
binding to DNA of 1,2-dichloroethane, male and female Sprague-Dawley
rats were exposed to 200 mg/m3 (50 ppm) 1,2-dichloroethane for 7 h
per day, 5 days a week, for 2 years. Additional rats were similarly
exposed and administered either 0.05% disulfiram in the diet or 5%
ethanol in the drinking-water, either alone or in combination with
1,2-dichloroethane. The incidence of any type of tumour was not
elevated compared to controls in any groups exposed to these compounds
individually, nor was the incidence of any type of tumour increased in
rats exposed to 1,2-dichloroethane and ethanol in combination compared
to unexposed controls or rats exposed to these compounds alone.
Exposure to disulfiram in combination with 1,2-dichloroethane resulted
in a significant (p<0.05) increase in the incidence of intrahepatic
bile duct cholangiomas (males: 9/49 versus 0/50, females: 17/50 versus
0/50) and cysts (males: 12/49 versus 0/50, females: 24/50 versus 0/50)
in both male and female rats compared to that in rats exposed to
1,2-dichloroethane only. Male rats exposed to this combination also
had a significantly (p<0.05) increased incidence of subcutaneous
fibromas (10/50 versus 0/50), hepatic neoplastic nodules (6/49 versus
2/50) and interstitial cell tumours in the testes (11/50 versus 3/50)
compared to rats exposed to 1,2-dichloroethane alone. Female rats
similarly exposed had a significantly (p<0.05) higher incidence of
mammary adenocarcinomas compared with rats exposed to
1,2-dichloroethane only (12/48 versus 5/50). Combined exposure to
1,2-dichloroethane and disulfiram did not increase the level of
covalent binding to hepatic DNA compared to that found in rats exposed
to 1,2-dichloroethane alone. The profile of urinary metabolites of a
single radiolabelled oral dose of 1,2-dichloroethane in rats
simultaneously exposed to disulfiram and 1,2-dichloroethane for 2
years indicated that the metabolism of 1,2-dichloroethane was
qualitatively similar to that of rats exposed to 1,2-dichloroethane
alone for 2 years. However, a reduced rate of elimination, and
sustained blood levels of unchanged 1,2-dichloroethane were observed
(see section 6.4), which, the authors stated, could be related to the
carcinogenic effects noted following simultaneous exposure (Cheever et
al., 1990).
In addition, concomitant exposure to disulfiram (as may occur in
the rubber industry or in persons undergoing therapy for alcoholism)
in the diet resulted in a synergistic increase in the hepatotoxicity
(as determined by levels of enzymes in serum and increased relative
liver weight (> 30%)) of 1,2-dichloroethane inhaled at
concentrations of 600, 1200 or 1800 mg/m3 (150, 300 and 450 ppm) for
30 days by male Sprague-Dawley rats. However, rats administered
1,2-dichloroethane alone had evidence of liver damage only at the
highest concentration. The enhanced effects were hypothesized to be
due to the inhibition of mixed-function oxidase-mediated metabolism of
1,2-dichloroethane and a compensatory increase in metabolism of
1,2-dichloroethane to reactive metabolites via cytosolic pathways
mediated by glutathione S-transferase, since hepatic cytochrome
P-450 content decreased with increasing concentration of
1,2-dichloroethane only in the presence of disulfiram (Igwe et al.,
1986a). Concomitant exposure to 1,2-dichloroethane by inhalation
(> 1216 mg/m3 or 304 ppm) or intraperitoneal injection
(150 mg/kg body weight per day) and disulfiram administered in the
diet (0.15%) resulted in testicular atrophy in Sprague-Dawley rats
compared to rats exposed to either compound alone (Igwe et al.,
1986b).
The acute toxicity of carbon tetrachloride was potentiated by
1,2-dichloroethane in rats administered single doses of each of 60 and
125 µl/kg body weight perorally, based on determination of serum
hepatic enzymes and indicators of lipid peroxidation at 24 h after
exposure. Pre-treatment with vitamin E prevented hepatotoxicity.
Based on the observation that the hepatic GSH level in the group
concomitantly exposed to both compounds was not significantly
different from that in the group exposed to carbon tetrachloride
alone, the authors concluded that GSH depletion did not play an
important role in the potentiation (Aragno et al., 1992). Concomitant
exposure to oral doses of 1,2-dichloroethane and 1,2-dibromoethane (60
and 20 µl/kg body weight, respectively) did not result in liver
toxicity in rats, based on levels of serum hepatic enzymes and
indicators of lipid peroxidation, although the compounds alone and in
combination resulted in a decrease in hepatic GSH level 2 h after
exposure, which subsequently returned to control values (Danni et al.,
1992).
The in vitro metabolism of 1,2-dichloroethane by liver
homogenates of rats administered ethanol increased with the dose of
ethanol up to 4 g/kg body weight, but declined sharply at 5 g/kg body
weight (Sato et al., 1981).
High doses (1000 to 2000 mg/kg body weight) of several chemicals,
including methionine, p-aminobenzoic acid, sulfanilamide and
aniline, administered orally to mice were protective against the
lethal effects caused by inhalation of 1600 mg/m3 (400 ppm)
1,2-dichloroethane (Heppel et al., 1945).
The acute and subacute toxicity of dichloroethane increased when
it was administered under conditions of high temperature (species and
exposure protocol not specified in abstract) (Mihaylova, 1976).
8. EFFECTS ON HUMANS
8.1 Case reports
The lethal oral dose of 1,2-dichloroethane in humans has been
estimated to be between 20 and 50 ml. Death due to cardiac arrhythmia
has been reported following ingestion of large, single doses
(50-75.2 g) of 1,2-dichloroethane (Hueper & Smith, 1935; Garrison &
Leadingham, 1954; Martin et al., 1969). Effects identified following
ingestion of 1,2-dichloroethane include central nervous system
depression, gastroenteritis, liver, kidney and lung damage,
cardiovascular disorders and haematological effects (Weiss, 1957;
Morozov, 1958; Hinkel, 1965; Bogoyavlenski et al., 1968; Martin et
al., 1969; Schönborn et al., 1970; Yodaiken & Babcock, 1973; Natsyuk &
Mudritsky, 1974; Dorndorf et al., 1975; Andriukin, 1979).
Effects reported following exposure to 1,2-dichloroethane via
inhalation are very similar to those observed after ingestion but are
usually less pronounced. Inhalation of 1,2-dichloroethane vapour
first affects the central nervous system and causes irritation and
inflammation of the respiratory tract. Damage to the liver, kidneys
and lungs (Wirtschafter & Schwartz, 1939; Hadengue & Martin, 1953;
Menschick, 1957; Troisi & Cavallazzi, 1961; Suveev & Babichenko, 1969;
Nouchi et al., 1984) and changes in the heart rhythm (Suveev &
Babichenko, 1969) have been reported in several cases. Death due to
cardiac toxicity has been reported following a 30-min exposure to an
unknown concentration of 1,2-dichloroethane (Nouchi et al., 1984).
Effects on the eyes were observed in several early case reports
(Weiss, 1957; Menschick, 1957; Troisi & Cavallazzi, 1961), while
severe dermatitis has been reported following dermal contact with
1,2-dichloroethane (Wirtschafter & Schwartz, 1939).
8.2 Epidemiological studies
In a study of 278 men working in the chlorohydrin unit of a
chemical production plant between 1940 and 1967 and followed up to
1988, there was a significant (p < 0.01) excess of deaths due to
pancreatic cancer compared to the USA national rates [Standardized
Mortality Ratio (SMR) = 492 (95% CI = 158 - 1140); Observed:Expected
(O:E) = 8:1.6]. The excess was greater when confined to men who
worked in the unit for more than 2 years (SMR = 800). Based on
comparison with two groups of workers in nearby plants, there were
pronounced increases in mortality due to pancreatic cancer as exposure
duration increased. Though an excess of deaths due to "lymphatic and
haemopoietic cancers" was also observed, it appeared to be
attributable principally to leukaemia, for which numbers of observed
cases were small (O=4) and associations with duration of exposure were
less consistent. Although quantitative data were not available, the
authors concluded on the basis of considerable qualitative information
that workers in this unit had been exposed primarily to
1,2-dichloroethane in combination with bis-chloroethyl ether, ethylene
oxide and ethylene chlorohydrin (Benson & Teta, 1993).
In a case-control study, the exposure of 21 male employees at a
petrochemical plant in Texas, USA, whose deaths were
attributed to cancer of the brain, was compared to that of two groups
of 80 controls from the same plant. One control group consisted of
male employees who had died from non-neoplastic causes, while the
second group of controls consisted of those men whose deaths were due
all other causes. Employees were classified as having been exposed to
1,2-dichloroethane if they had ever worked in a department in which
the compound had been used; unexposed workers had never worked in
these departments and the exposure of others was considered to be
unknown (47.6% of cases, 58.8 and 61.3% of the first and second group
of controls, respectively). When those with unknown exposure were
excluded from the analyses, the proportion of cases (all cases or
glioma cases specifically) who were exposed (n = 11 and 10, or 45.5
and 50.0%, respectively) did not differ significantly from the
proportion of controls who had been exposed to 1,2-dichloroethane
(42.4 and 45.2% for the two control groups). When a 15-year latency
period was considered in the analysis, the proportion of cases and
controls exposed still did not differ significantly (40.0 and 44.4%
for cases versus 32.3 and 34.6% for the two control groups) (Austin &
Schnatter, 1983a).
In an accompanying historical cohort study of 6588 workers at
this plant, there was no significant excess in malignant brain tumours
in the overall population of the plant compared to national rates,
although there was a borderline significant (p < 0.05) increase in
hourly employees with more than 6 months of employment (O/E = 10/5).
However, exposure to 1,2-dichloroethane was not specifically
considered in this study, and employees were also exposed to a number
of potentially confounding substances, including benzene, diethyl
sulfate, ethylene oxide and vinyl chloride (Austin & Schnatter,
1983b).
Deschamps & Band (1993) conducted a small case-control study to
investigate a possible association between a spill of
1,2-dichloroethane in 1982 into a river supplying drinking-water to
parts of the city of Vancouver, Canada, and an identified cluster of
childhood leukaemia cases in the city. It was determined that none of
the 15 cases diagnosed between 1975 and 1988 had lived in areas of the
city serviced by the contaminated supply.
In an ecological study in which potential associations between
contamination of drinking-water from groundwater supplies by
particular substances (including 10 volatile organic compounds and 43
inorganic elements) and cancer were investigated, the average annual
age-adjusted incidence (1969 to 1981) of colon and rectal cancer was
statistically significantly greater in men aged > 55 years whose
drinking-water contained > 0.1 µg 1,2-dichloroethane/litre than in
those whose drinking-water contained < 0.1 µg/litre (222.8 per
100 000 versus 170.3 per 100 000 (193 and 633 cases, p = 0.02) and
126.5 per 100 000 versus 92.9 per 100 000 (106 and 337 cases,
p = 0.009), respectively). Rectal cancer in males was also associated
with chlorination of drinking-water. Of the study population over 55
years of age, 50% had lived at the same address for 20 years or more.
There were no significant differences between groups of towns with
respect to eight socioeconomic factors examined, except that the
percentage change in population between 1970 and 1980 was
significantly less in those towns with > 0.1 µg
1,2-dichloroethane/litre. The authors did not suggest that the result
indicated a causal relationship between 1,2-dichloroethane and cancer,
but that cancer incidence may be elevated in populations consuming
water from wells subject to anthropogenic contamination (Isacson et
al., 1985).
The prevalence of subjective symptoms was higher in a group of 10
male oil refinery workers exposed to between 250 and 800 mg/m3 than
in those exposed to lower concentrations (40 to 150 mg/m3); a
"general reduction in body weight" was also noted in both groups.
"Abnormalities" of the liver, central nervous system, gastrointestinal
tract and haematological parameters were reported in some workers
(n = 1 to 8) in the group exposed to the higher concentration,
presumably based upon clinical examination, although this was not
specified in the previous account of this early study. There were no
unexposed controls, and workers were also exposed to benzene (10 to
25 mg/m3) (Cetnarowicz, 1959).
The morbidity during a 5-year period (1951 to 1955) was increased
for all disease categories (not further specified) in a group of
workers (number not specified) at an aircraft factory exposed to
5 mg/m3 or less for 70 to 75% of the working time and 80 to
150 mg/m3 for the remainder, when compared to workers in the entire
factory. Of 83 workers examined further, 19 had disease of the liver
and bile duct, 13 had neurotic conditions, 11 had autonomous dystonia
and 10 had hyperthyroidism and goitre (there were no controls for
comparison) (Kozik, 1957).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
9.1.1 Microorganisms
Blum & Speece (1991) investigated the toxicity of
1,2-dichloroethane to three groups of aquatic bacteria: methanogens,
aerobic heterotrophs and Nitrosomonas. The end-points assessed
included inhibition of gas production (methanogens), oxygen uptake
(aerobic heterotrophs), and ammonia consumption (Nitrosomonas). The
IC50 values for Nitrosomonas and methanogens (29 and 25 mg/litre,
respectively) were considerably lower than that for aerobic
heterotrophs (470 mg/litre). For the bacteria Pseudomonas putida,
the nominal 16-h toxicity threshold for the onset of cell
multiplication inhibition was 135 mg/litre (Bringmann & Kühn, 1981).
Tang et al. (1990) determined the IC50 values based on inhibition of
respiration rate for activated sewage sludge using open and closed
serum bottle methods to be 35 500 mg/litre and 2780 mg/litre,
respectively. The difference was attributed to the volatility of
1,2-dichloroethane which was stripped from the substrate in the open
method.
The freshwater cyanobacterium (blue-green alga) Microcystis
aeruginosa was seven times more sensitive to 1,2-dichloroethane than
the green alga Scenedesmus quadricauda, the nominal 7-day EC50
values for inhibition of cell multiplication at 27°C being 105 and
710 mg/litre, respectively (Bringmann & Kühn, 1978). The 72-h EC50
for inhibition of growth was 189 mg/litre (Freitag et al., 1994).
Bringmann & Kühn (1980) determined the toxicity thresholds for
Scendesmus quadricauda and the protozoan Entosiphon sulcatum to be
710 mg/litre and 1127 mg/litre, respectively. Knie et al. (1983)
reported the acute EC50 for the alga Haematococcus pluvialis to be
130 mg/litre. Based on bioluminescence, the 5-min IC50 was
700 mg/litre in a Microtox test with Photobacterium phosphoreum
(Blum & Speece, 1991). Freitag et al. (1994) reported the 15-min EC50
for inhibition of bioluminescence in this species to be 770 mg/litre.
Bringmann & Kühn (1981) determined the toxicity thresholds in the
holozoic bacteriovorous flagellate protozoan Entosiphon sulcatum, the
holozoic bacteriovorous ciliate protozoan Uronema parduczi, and the
saprozoic flagellate protozoan Chilomonas paramecium to be > 8000
mg/litre, > 16 000 mg/litre and > 800 mg/litre, respectively, using
the cell multiplication inhibition test.
Pearson & McConnell (1975) determined the EC50 (based on carbon
uptake from CO2 during photosynthesis) in the marine unicellular
alga Phaeodactylum tricorhutum to be 340 mg/litre.
9.1.2 Invertebrates
Based on a review of identified acute and chronic toxicity
studies in freshwater invertebrates, Daphnia magna appears to be the
species most sensitive to 1,2-dichloroethane. Under static test
conditions, the measured 48-h LC50 values for fed and unfed first
instar D. magna were 320 and 270 mg/litre, respectively; the 48-h
EC50 values, based on complete immobilization, were 180 and
160 mg/litre for fed and unfed organisms, respectively (Richter et
al., 1983). Leblanc (1980) reported the 24-h and 48-h LC50 values
in D. magna to be 250 and 220 mg/litre, respectively, while the "no
discernible effect concentration" (apparently based on mortality only)
was < 68 mg/litre. Using the Probit method, Ahmad et al. (1984)
determined the 48-h LC50 in unfed D. magna to be 268 mg/litre (95%
CL: 246-293), while the EC50, based on reproductive effects was
155 mg/litre (95% CL: 137-188). Freitag et al. (1994) determined the
24-h EC50 for 10% immobilization of D. magna to be 150 mg/litre.
Knie et al. (1983) reported the EC0, EC50 and EC100 in D. magna
to be 67, 600 and 1075 mg/litre, respectively. Richter et al. (1983)
also examined the effect of 1,2-dichloroethane on reproductive success
and length of first instar D. magna in a 28-day flow-through test.
For reproductive success, the measured lowest-observed-effect level
(LOEL) and no-observed-effect level (NOEL) were 20.7 and
10.6 mg/litre, respectively, while the measured LOEL and NOEL for
growth were 71.7 and 41.6 mg/litre, respectively.
Ahmad et al. (1984) also conducted chronic toxicity studies in
which D. magna were exposed to 0, 10.6, 20.6, 41.6, 71.7, 94.4 and
137 mg 1,2-dichloroethane/litre for 28 days. There was a
concentration-related decrease (as low as 12% of control values) in
the number of young produced (significant (p < 0.05 or 0.01) at
41.6 mg/litre or more) as well as a decrease (as low as 59% of control
values) in the length of adults (significant (p < 0.01) at
71.7 mg/litre or more). Few acute toxicity studies in marine
invertebrates were identified. Under static test conditions, the
nominal 24-h EC50 for immobilization of 30-h posthatch larvae of the
brine shrimp Artemia salina was 93.6 mg/litre (Foster & Tullis,
1984). For marine adult shrimp (Crangon crangon), the measured 24-h
LC50 was 170 mg/litre under static test conditions (Rosenberg et
al., 1975). The 48-h LC50 for barnacle nauplii (Elminuis modestus)
was 186 mg/litre (Pearson & McConnell, 1975). Teratogenic effects
(expressed as surviving larvae with gross debilitating abnormalities)
were observed in the nauplii of the marine brine shrimp (Artemia
salina) at concentrations between 0.25 and 25 mg/litre (Kerster &
Schaeffer, 1983).
9.1.3 Vertebrates
The embryos and larvae of the northwestern salamander (Ambystoma
gracile) and the leopard frog (Rana pipiens) were continuously
exposed to 1,2-dichloroethane from within 30 min of fertilization
(embryos) and maintained for 4 days after hatching (larvae). The
LC50 values for the salamander at the day of hatching (day 5) and 4
days after hatching (day 9) were 6.53 and 2.54 mg/litre, respectively;
the measured LOEL for 23% reduction in egg hatchability was
0.99 mg/litre. The measured 5-day and 9-day LC50 values for the
frog were 4.52 and 4.40 mg/litre, respectively, while the 5-day
posthatch LOEL was 1.07 mg/litre (Black et al., 1982).
Acute toxicity studies have been conducted on several species of
freshwater fish. The most sensitive species was the guppy (Poecilia
reticulata, 2-3 months old), with a nominal 7-day LC50 of
106 mg/litre under static renewal test conditions (Konemann, 1981).
In three studies on 30-day-old fathead minnows (Pimephales promelas),
measured 96-h LC50 values ranged from 116 to 136 mg/litre under
flow-through conditions (Veith et al., 1983; Walbridge et al., 1983;
Geiger et al., 1985). LC50 values after 24, 48, 72 and 96 h in
rainbow trout (Salmo gairdneri) were 362, 340, 337 and 336 mg/litre,
respectively, using the static test method (Bartlett, 1979). Knie et
al. (1983) reported the EC0, EC50 and EC100 in golden orfe
(Leuciscus idus) to be 67, 600 and 1075 mg/litre, respectively.
In marine fish, a nominal 96-h LC50 of 480 mg/litre was
reported in tidewater silversides (Minidia beryllina) under static
test conditions (Dawson et al., 1975/77). Heitmuller et al. (1981)
reported the static test LC50 at 24, 48, 72 and 96 h in sheepshead
minnows (Cyprinodon variegatus) to be between 130 and 230 mg/litre.
The 96-h LC50 for dab (Limanda limanda) was 115 mg/litre (Pearson
& McConnell, 1975).
In a long-term, flow-through study of the early life stages of
fathead minnows (Pimephales promelas), there were no effects on egg
hatchability or larval survival and deformity at 29 mg/litre (NOEL);
however, larval growth was significantly (p < 0.05) reduced by 62% at
59 mg/litre (LOEL) (Benoit et al., 1982). Black et al. (1982) exposed
the embryos and larvae of the rainbow trout (Oncorhynchus mykiss)
continuously to 1,2-dichloroethane under flow-through conditions from
within 30 min of fertilization (embryos) and maintained them until 4
days after hatching. The resulting EC50 for hatchability and 27-day
LC50 for post-hatch survival were both 34 mg/litre, and the LOEL for
a 24% reduction in egg hatchability was 3.49 mg/litre. After 21 days
of continuous exposure to 150 mg 1,2-dichloroethane/litre, the
mortality of coho salmon (Oncorhynchus kisutch) eggs was 46%, while
in alevins, 100% mortality occurred 9 days after hatching at
320 mg/litre (Reid et al., 1982). In addition, premature hatching was
observed at 56 mg/litre, and, within one week of hatching, sublethal
effects, including lethargy and loss of equilibrium, were observed in
alevins exposed to 56 mg/litre; 100% mortality occurred 9 days after
hatching.
Ahmad et al. (1984) conducted chronic toxicity studies in which
fathead minnows were exposed to 300, 4000, 7000, 14 000, 29 000 or
39 000 µg/litre for 32 days. There were no significant effects on
survival, although there was a significant (p < 0.05) decrease in
mean individual net weight at the highest concentration (38% of
control values). The MATC was determined to be 49 000 to
59 000 µg/litre.
Teratogenic effects (expressed as surviving larvae with gross,
debilitating abnormalities) were observed in the larvae of
northwestern salamanders (Ambystoma gracile), leopard frogs
(Rana pipiens) and rainbow trout (Oncorhynchus mykiss) at 21.4,
21.9 and 34.4 mg/litre, respectively (Black et al., 1982).
9.2 Terrestrial organisms
9.2.1 Invertebrates
In an acute contact test, the 48-h LC50 for earthworms (Eisenia
fetida) exposed to 1,2-dichloroethane-treated filter-paper was
60 µg/m2 (Neuhauser et al., 1985).
9.2.2 Vertebrates
Male and female white leghorn chickens were fed mash which had
been fumigated with 1,2-dichloroethane, resulting in concen-trations
in the feed of 250 and 500 mg/kg, for 2 years. The end-points
examined included serum composition, growth, semen characteristics and
fertility. The weight of eggs was significantly reduced at 250 mg/kg
(5 to 10% (p < 0.01)), while both the number and weight of eggs were
reduced at 500 mg/kg (5 to 48% (p < 0.05) and 5 to 13% (p < 0.01),
respectively) (Alumot et al., 1976b).
9.2.3 Plants
1,2-Dichloroethane vapour was both lethal and mutagenic to barley
kernels (two-rowed variety, Bonus) following exposure to 3 mg/m3 for
24 h (Ehrenberg et al., 1974).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Available data on the carcinogenicity of 1,2-dichloroethane in
humans are limited. There is convincing evidence of increases in the
incidence of both common and rare tumours in experimental animals at
several sites (including squamous cell carcinomas of the stomach,
haemangiosarcomas, fibromas of the subcutaneous tissue and
adenocarcinomas and fibroadenomas of the mammary gland in rats; and
alveolar/bronchiolar adenomas, mammary gland adenocarcinomas,
endometrial stromal polyp or endometrial stromal sarcoma combined and
hepatocellular carcinomas in mice) following administration by gavage
for 78 weeks.
The incidence of benign lung papillomas was significantly
increased in mice following long-term dermal application of
1,2-dichloroethane, while a non-significant increase in the number of
pulmonary adenomas per animal was reported in a screening bioassay on
mice and in the incidence of benign mammary gland tumours in rats
exposed by inhalation for 2 years.
1,2-Dichloroethane is genotoxic in in vitro and in vivo
assays, and binds to DNA in rodents in vivo.
Based on the induction of both rare and common tumours in rats
and mice exposed by ingestion and supporting evidence in other limited
bioassays, the production of a reactive intermediate that alkylates
DNA and positive results in a range of in vitro assays for
genotoxicity, 1,2-dichloroethane is considered to be a probable human
carcinogen.
10.2 Environmental assessment
The high volatility of 1,2-dichloroethane makes the atmosphere
the predominant environmental sink. Consequently, measured
concentrations in surface waters are low (around 1 to 10 µg/litre).
Air concentrations are highest around manufacturing plants where they
may reach 300 µg/m3; concentrations in urban air average
< 1 µg/m3. Low adsorption to soil leads to potential leaching to
groundwater; some measurements of low concentrations in drinking-water
(< 0.2 µg/litre) confirm this.
Both hydrolysis and microbial degradation are slow; the
volatility of the compound means that it has low residence time in
media where these processes occur and they are not considered to be of
environmental significance.
The estimated atmospheric lifetime of 1,2-dichloroethane is
between 40 and 110 days. Stratospheric photolysis may produce chlorine
radicals which may in turn react with ozone. However, the
ozone-depleting potential is low (0.001 relative to CFC-11) and the
compound is not listed in the Montreal Convention.
Various toxicity tests have shown LC50s for organisms in the
environment to be generally greater than 10 mg/litre. The difference
(at least 7 orders of magnitude) between measured water concentrations
and these toxic concentrations indicate that 1,2-dichloroethane poses
no risk to organisms since exposure will not occur.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN
HEALTH AND THE ENVIRONMENT
Considering the toxicological characteristics of
1,2-dichloroethane, both qualitatively and quantitatively, an exposure
that would not cause adverse effects in humans by any route of
exposure cannot be estimated. Consequently, all appropriate measures
should be taken to eliminate or minimize human exposure to
1,2-dichloroethane.
12. FURTHER RESEARCH
Although specific studies were not recommended for
1,2-dichloroethane, additional analytical epidemiological studies are
desirable.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1979) has
classified 1,2-dichloroethane in group 2B (possibly carcinogenic to
humans), based on sufficient evidence of carcinogenicity in
experimental animals.
The International Programme on Chemical Safety has previously
evaluated 1,2-dichloroethane (IPCS, 1987). It concluded that
1,2-dichloroethane produces central nervous system depression, and
gastrointestinal and liver abnormalities in humans. The same effects
occur in experimental animals, in addition to possible kidney
abnormalities, lung oedema and cardiovascular disorders.
1,2-Dichloroethane, administered by gavage, is carcinogenic in rats
and mice, and should be regarded, for practical purposes, as if it
presented a carcinogenic risk for humans. 1,2-Dichloroethane was not
considered to accumulate in the environment. In the atmosphere, it is
removed by photo-chemical degradation via hydroxyl radicals and is
eliminated from water by evaporation. It has a low octanol/water
partition coefficient and bioconcentration is unlikely. It was not
considered to pose a hazard to the aquatic environment except in the
case of accidents and inappropriate disposal. Data were insufficient
to evaluate the effects of 1,2-dichloroethane on the terrestrial
environment.
The Joint FAO/WHO Expert Committee on Food Additives
(JECFA) has evaluated 1,2-dichloroethane on three occasions (WHO,
1971, 1980, 1992). When last evaluated, the Committee concluded that
this compound is genotoxic in both in vitro and in vivo test
systems and carcinogenic in mice and rats when administered by the
oral route. No ADI was therefore allocated. The Committee expressed
the opinion that 1,2-dichloroethane should not be used in food.
In the WHO Guidelines for drinking-water quality (WHO, 1993), the
concentrations of 1,2-dichloroethane in drinking-water estimated to be
associated with excess risks of 10-4, 10-5 and 10-6 are 300, 30 and
3 µg/litre, respectively, based on linearized multistage modelling of
the incidence of haemangiosarcomas in male rats in the NCI (1978)
study.
The European Commission published a directive in 1990 in which
limit values for emission of 1,2-dichloroethane were specified for
various types of industrial plants. These limits ranged from
0.1 mg/litre (monthly) for plants using 1,2-dichloroethane for
degreasing metals away from an industrial site to 12 mg/litre (daily)
for plants producing 1,2-dichloroethane and processing or using the
substance at the site (CEC, 1990).
REFERENCES
AEC (Alberta Environmental Centre) (1989) Final effluent analysis of
Dow Chemical, Fort Saskatchewan, Alberta plant. Alberta, Canada,
Alberta Environmental Centre (Unpublished data).
Ahmad N, Benoit D, Brooke L, Call D, Carlson A, DeFoe D, Huot J,
Moriarity A, Richter J, Shubat P, Veith G, & Wallbridge C (1984)
Aquatic toxicity tests to characterize the hazard of volatile organic
chemicals in water: a toxicity data summary - Parts I and II. Duluth,
Minnesota, US Environmental Protection Agency, Office of Research and
Development, Environmental Research Laboratory.
Alumot E, Nachtomi E, Mandel E, & Holstein P (1976a) Tolerance and
acceptable daily intake of chlorinated fumigants in the rat diet. Food
Cosmet Toxicol, 14: 105-110.
Alumot E, Meidler M, Holstein P, & Herzberg M (1976b) Tolerance and
acceptable daily intake of ethylene dichloride in the chicken diet.
Food Cosmet Toxicol, 14: 111-114.
Andriukin AA (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klin Med (Mosk), 57: 43-47 (in Russian).
Apostolov I & Mihaylova A (1975) [Investigation into
beta- N-acetylglucosaminidase activity in the serum of white rats
treated with 1,2-dichloroethane.] Probl Hyg, 1: 161-163
(in Bulgarian).
Aragno M, Tamagno E, Danni O, & Ugazio G (1992) In vivo studies on
halogen compound interactions. III. Effect of carbon tetrachloride
plus 1,2-dichloroethane on liver necrosis and fatty accumulation. Res
Commun Chem Pathol Pharmacol, 76: 341-354.
Archer WJ (1979) Chlorocarbons and chlorohydrocarbons. In: Kirk J &
Othmer DF ed. Encyclopedia of chemical technology, 3rd ed. New York,
John Wiley & Sons, vol 5, pp 723-743.
Arfellini G, Bartoli S, Colacci A, Mazzullo M, Galli MC, Prodi G, &
Grilli S (1984) In vivo and in vitro binding of 1,2-dibromoethane
and 1,2-dichloroethane to macromolecules in rat and mouse organs. J
Cancer Res Clin Oncol, 108: 204-213.
Armstrong MJ & Galloway SM (1993) Micronuclei induced in peripheral
blood of Eµ-PIM-1 transgenic mice by chronic oral treatment with
2-acetylaminofluorene or benzene but not with diethylnitrosamine or
1,2-dichloroethane. Mutat Res, 302: 61-70.
Atkinson R (1987) A structure-activity relationship for the estimation
of rate constants for gas-phase reaction of OH radicals with organic
compounds. Int J Chem Kinet, 19: 799-828.
ATSDR (1989) Toxicological profile for 1,2-dichloroethane. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry, 129 pp.
ATSDR (1994) Toxicological profile for 1,2-dichloroethane (Update).
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry,
192 pp (TP-93/06).
Austin SG & Schnatter AR (1983a) A case-control study of chemistry
exposures and brain tumors in petrochemical workers. J Occup Med,
25(4): 313-320.
Austin SG & Schnatter AR (1983b) A cohort mortality study of
petrochemical workers. J Occup Med, 25(4): 304-312.
Baertsch A, Lutz WK, & Schlatter C (1991) Effect of inhalation
exposure regimen on DNA binding potency of 1,2-dichloroethane in the
rat. Arch Toxicol, 65: 169-176.
Bailey S, Collins GB, Fishwick FB, Hart HV, Horler DF, & Scudamore KA
(1982) Pesticide residues in foodstuffs in Great Britain:
Organochlorine pesticides, organophosphorus pesticides and fumigant
residues in home-produced and imported wheat. Pestic Sci,
13(4): 373-378.
Ballering LAP, Nivard MJM, & Vogel EW (1993) Characterization of the
genotoxic action of three structurally related 1,2-dihaloalkanes in
Drosophila melanogaster. Mutat Res, 285: 209-217.
Ballering LAP, Nivard MJM, & Vogel EW (1994) Mutation spectra of
1,2-dibromoethane, 1,2-dichloroethane and 1-bromo-2-chloroethane in
excision repair proficient and repair deficient strains of Drosophila
melanogaster. Carcinogenesis, 15: 869-875.
Banerjee S (1988) DNA damage in rodent liver by 1,2-dichloroethane, a
hepatocarcinogen. Cancer Biochem Biophys, 10: 165-173.
Banerjee S & Van Duuren BL (1979) Binding of carcinogenic halogenated
hydrocarbons to cell macromolecules. J Natl Cancer Inst, 63: 707-711.
Banerjee S, Van Duuren BL, & Oruambo FI (1980) Microsome-mediated
covalent binding of 1,2-dichloroethane to lung microsomal protein and
salmon sperm DNA. Cancer Res, 40: 2170-2173.
Barbash JE & Reinhard M (1989) Abiotic dehalogenation of
1,2-dichloroethane and 1,2-dibromoethane in aqueous solution
containing hydrogen disulfide. Environ Sci Technol, 23(11): 1349-1357.
Barber RD, Donish WH, & Mueller KR (1981) A procedure for the
quantitative measurement of the mutagenicity of volatile liquids in
the Ames Salmonella microsome assay. Mutat Res, 90: 31-48.
Baretta ED, Steward RD, & Mutchler JE (1969) Monitoring exposure to
vinyl chloride vapour: breath analysis and continuous air sampling. Am
Ind Hyg Assoc J, 30: 537-544.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizzari ED, Ray M, Smith D, Tomer KB, Wagner R, & Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - A
multimedia environmental study. Biomed Environ Mass Spectrom,
7: 139-147.
Barrows ME, Petrocelli SR, Macek KJ, & Carroll JJ (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Haque R ed. Dynamics,
exposure and hazard assessment of toxic chemicals. Ann Arbor,
Michigan, Ann Arbor Science Publishers, chapter 24, pp 379-392.
Barsoum GS & Saad K (1934) Relative toxicity of certain chlorine
derivatives of the aliphatic series. Q J Pharm Pharmacol, 7: 205-214.
Bartlett EA (1979) Toxicity of ethylene dichloride to rainbow trout.
Midland, Michigan, Dow Chemical (Unpublished report).
Bauer U (1981) [Human exposure to environmental chemicals -
Investigations on volatile organic halogenated compounds in water,
air, food, and human tissues. III. Communication: results of
investigations.] Zbl Bakteriol Hyg I Abt Orig B, 174(3): 200-237 (in
German).
Benoit DA, Puglisi FA, & Olson DL (1982) A fathead minnow Pimephales
promelas early stage toxicity test method evaluation and exposure to
four organic chemicals. Environ Pollut, A28: 189-197.
Benson LO & Teta MJ (1993) Mortality due to pancreatic and
lymphopoietic cancers in chlorohydrin production workers. Br J Ind
Med, 50: 710-716.
Berck B (1974) Fumigant residues of carbon tetrachloride, ethylene
dichloride, and ethylene dibromide in wheat, flour, bran, middlings,
and bread. J Agric Food Chem, 22(6): 977-984.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, & Bruser DM
(1982) The aquatic toxicity of organic compounds to embryo-larval
stages of fish and amphibians. Lexington, Kentucky, University of
Kentucky (Research Report No. 133).
Blum DJW & Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons and
correlations. J Water Pollut Control Fed, 63(3): 198-207.
Bogoyavlenski VF, Salikhova SKH, & Karpova EV (1968) [Clinical aspects
and therapy for ethylene dichloride poisoning.] Sov Med, 31: 107-109
(in Russian).
Bond EJ (1984) Manual of fumigation for insect control. Rome, Food and
Agriculture Organization of the United Nations, 432 pp (FAO Plant
Production and Protection Paper 54).
Bonnet P, Francin J-M, Grakiski D, Raoult G, & Zissu D (1980)
Détermination de la concentration léthale50 des principaux
hydrocarbures aliphatiques chlorés chez le rat. Arch Mal Prof,
41(6-7): 317-321.
Bouwer EJ & McCarty PL (1983) Transformation of 1- and 2-carbon
halogenated aliphatic organic compounds under methanogenic conditions.
Appl Environ Microbiol, 45(4): 1286-1294.
Brem H, Stein AB, & Rosenkranz HS (1974) The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer Res, 34: 2576-2579.
Bringmann G & Kühn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1981) [Toxicity thresholds of pollutants in
ciliate flagellate protozoa, holozoic bacteriovorous ciliate protozoa
and saprozoic flagellate protozoa.] GWF-Wasser/Abwasser, 122: 308-313
(in German).
Brittebo EB, Kowalsky B, Ghantous H, & Brandt I (1989) Epithelial
binding of 1,2-dichloroethane in mice. Toxicology, 56: 35-45.
Brodzinsky R & Singh HB (1982) Volatile organic chemicals in the
atmosphere: An assessment of available data (Contract No. 68-02-3452).
Menlo Park, California, SRI International (Prepared for the US
Environmental Protection Agency, Office of Research and Development,
Environmental Sciences Research Laboratory, Research Triangle Park,
North Carolina, USA).
Bruckmann P, Kersten W, Funcke W, Balfanz E, Konig J, Theisen J, Ball
M, & Papke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17(12): 2363-2380.
Buijs W, van der Gen A, Mohn GR, & Breimer DD (1984) The direct
mutagenic activity of alpha, omega-dihalogenoalkanes in Salmonella
typhimurium. Mutat Res, 141: 11-14.
Callahan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR,
Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR, &
Gould C (1979) Water-related fate of 129 priority pollutants: Volume
II. Washington, DC, US Environmental Protection Agency
(EPA 40/4-79-029b).
Cetnarowicz J (1959) Experimental and clinical investigation on the
action of dichloroethane. Folia Med Crac, 1: 169-192.
Cheever KL, Cholakis JM, El-Hawari AM, Kovatch RM, & Weisburger EK
(1990) Ethylene dichloride: The influence of disulfiram or ethanol on
oncogenicity, metabolism and DNA covalent binding in rats. Fundam Appl
Toxicol, 14: 243-261.
Cheh AM, Hooper AB, Skochdopole J, Henke CA, & McKinnell RG (1980) A
comparison of the ability of frog and rat S-9 to activate promutagens
in the Ames test. Environ Mol Mutagen, 2: 487-508.
Chemical Marketing Reporter (1992) Chemical profile: Ethylene
dichloride. Chem Mark Report Mag, 241(19): 42.
Chiou CT, Peters LJ, & Freed VH (1979) A physical concept of
soil-water equilibria for nonionic organic compounds. Science,
206: 831-832.
Chiou CT, Freed VH, Peters LJ, & Kohnert RL (1980) Evaporation of
solutes from water. Environ Int, 3: 231-236.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984a) Ambient air
measurement of aromatic and halogenated hydrocarbons at urban, rural,
and motorway locations. Sci Total Environ, 39: 265-279.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984b) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural, and motorway locations.
Environ Pollut, B7: 141-158.
Class T & Ballschmiter K (1986) Chemistry of organic traces in air.
VI: Distribution of chlorinated C1 - C4 hydrocarbons in air over the
northern and southern Atlantic Ocean. Chemosphere, 15(4): 413-427.
Comba ME & Kaiser KLE (1983) Determination of volatile contaminants at
the ng-L-1 level in water by capillary gas chromatography with
electron capture detection. Int J Environ Anal Chem, 16: 17-31.
Comba ME & Kaiser KLE (1985) Volatile halocarbons in the Detroit River
and their relationship with contaminant sources. J Great Lakes Res,
11(3): 404-418.
Cottalasso D, Barisione G, Fontana L, Domenicotti C, Pronzato MA, &
Nanni G (1994) Impairment of lipoglycoprotein metabolism in rat liver
cells induced by 1,2-dichloro-ethane. J Occup Environ Med,
51: 281-285.
CEC (1990) Council directive of 27 July 1990 amending annex II to
directive 86/280/EEC on limit values and quality objectives for
discharges of certain dangerous substances included in List I of the
annex to directive 76/464/EEC. Brussels, Commission of the European
Communities.
CPI (Canadian Process Industries) (1991) CPI product profiles:
Ethylene dichloride. Don Mills, Ontario, Corpus Information Services.
Crebelli R & Carere A (1988) Genotoxic activity of halogenated
aliphatic hydrocarbons in Aspergillus nidulans. J Occup Toxicol,
8: 437-442.
Crebelli R, Conti G, Conti L, & Carere A (1984) Induction of somatic
segregation by halogenated aliphatic hydrocarbons in Aspergillus
nidulans. Mutat Res, 138: 33-38.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, & Carere A (1988)
Induction of chromosome malsegregation by halogenated organic solvents
in Aspergillus nidulans: Unspecific or specific mechanism? Mutat
Res, 201: 401-411.
Crespi CL, Seixas GM, Turner TR, Ryan CG & Penman BW (1985)
Mutagenicity of 1,2-dichloroethane and 1,2-dibromoethane in two human
lymphoblastoid cell lines. Mutat Res, 142: 133-140.
Daft JL (1987) Determining multifumigants in whole grains and legumes,
milled and low-fat grain products, spices, citrus fruit, and
beverages. J Assoc Off Anal Chem, 70: 734-739.
Daft JL (1988) Rapid determination of fumigant and industrial chemical
residues in food. J Assoc Off Anal Chem, 71(4): 748-760.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and nonfatty foods. J Agric Food Chem, 37: 560-564.
Daft JL (1991) Fumigants and related chemicals in foods: Review of
residue findings, contamination sources, and analytical methods. Sci
Total Environ, 100: 501-518.
Daft JL (1993) Fumigant analysis of foods. In: Sherman J & Cairns T
ed. Comprehensive analytical profiles of important pesticides. Boca
Raton, Florida, CRC Press, pp 235-281.
Dann T (1992) Unpublished data on concentrations of 1,2-dichloroethane
in ambient air across Canada. Ottawa, Ontario, Environment Canada.
Danni O, Aragno M, Tamagno E, & Ugazio G (1992) In vivo studies on
halogen compound interactions. IV. Interaction among different halogen
derivatives with and without synergistic action on liver toxicity. Res
Commun Chem Pathol Pharmacol, 76(3): 355-366.
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1975/77) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J
Hazard Mater, 1(4): 303-318.
Deschamps M & Band P (1993) Study of a cluster of childhood leukemia.
Health Rep, 5(1): 81-85.
Dilling WL, Tefertiller NB, & Kalos GJ (1975) Evaporation rates and
reactivities of methylene chloride, chloroform, 1,1,1-trichloroethane,
trichloroethylene, tetrachloroethylene, and other chlorinated
compounds in dilute aqueous solutions. Environ Sci Technol,
9(9): 833-838.
Dorndorf W, Kresse M, Christian W, & Katritzki KG (1975)
[Dichloroethane poisoning with myoclonic syndrome, epileptic attacks,
and irreversible cerebral effects.] Arch Psychiatr Nervenkr,
220: 373-379 (in German).
Drury JS & Hammons AS (1979) Investigations of selected environmental
pollutants: 1,2-Dichloroethane. Oak Ridge National Laboratory, Oak
Ridge, Tennessee; Environmental Protection Agency, Washington, D.C.
Office of Toxic Substances; Department of Energy, Washington, DC.
Washington, DC, US Environmental Protection Agency (EPA 560/2-78-006).
D'Souza R, Francis WR, Bruce RD, & Anderson ME (1987) Physiologically
based pharmacokinetic model for ethylene dichloride and its
application in risk assessment. In: Pharmacokinetics in risk
assessment - Drinking water and health. Washington, DC, National
Academy Press, vol 8, pp 286-301.
D'Souza RW, Francis WR, & Andersen ME (1988) Physiological model for
tissue glutathione depletion and increased resynthesis after ethylene
dichloride exposure. J Pharmacol Exp Ther, 245: 563-568.
Duprat P, Delsaut L, & Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les muqueuses
oculaires du lapin. Eur J Toxicol, 9: 171-177.
Easley DM, Kleopfer RD, & Carasea AM (1981) Gas chromatographic-mass
spectrometric determination of volatile organic compounds in fish. J
Assoc Off Anal Chem, 64: 653-656.
Ecobichon DJ & Allen MC (1990) New Brunswick - Water Quality
Surveillance Program. New Brunswick Public Water Supplies: Data
summary report 1990. Fredericton, New Brunswick, Canada, Department of
Health and Community Services, 66 pp.
Ehrenberg L, Osterman-Golkar S, Singh D, & Lundqvist U (1974) On the
reaction kinetics and mutagenic activity of methylating and
ß-halogenoethylating gasoline additives. Radiat Bot, 15: 185-194.
Ellington JJ, Stancil FE, Payne WD, & Trusty CD (1988) Measurement of
hydrolysis rate constants for evaluation of hazardous waste land
disposal: Volume III - Data on 70 chemicals. Athens, Georgia, US
Environmental Protection Agency (EPA/600/3-88/028. Entz RC, Thomas KW,
& Diachenko GW (1982) Residues of volatile halocarbons in foods using
headspace gas chromatography. J Agric Food Chem, 30(5): 846-849.
Environment Agency Japan (1983) Environmental monitoring of chemicals:
Environmental survey report of fiscal years 1980 and 1981. Tokyo,
Japan, Environment Agency.
Environment Agency Japan (1993) Chemicals in the environment - 1993.
Tokyo, Japan, Environment Agency.
Environment Canada (1986) Canada-Ontario agreement respecting Great
Lakes water quality. St. Clair river pollution investigation (Sarnia
area). Ottawa, Ontario, Environment Canada, 80 pp.
Enviro-Test Laboratories (1991) Cayley background study: Analysis of
food products for target organic and inorganic parameters. Edmonton,
Alberta, Canada, Enviro-Test Laboratories, 37 pp
(Report No. 91-E1208).
Enviro-Test Laboratories (1992) Windsor area background study:
Analysis of food products for target organic and inorganic parameters.
Edmonton, Alberta, Enviro-Test Laboratories, 36 pp
(Report No. 92-E1052).
FAO/WHO (1971) Evaluation of food additives. Specifications for the
identity and purity of food additives and their toxicological
evaluation: some extraction solvents and certain other substances; and
a review of the technological efficacy of some antimicrobial agents.
Fourteenth report of the Joint FAO/WHO Expert Committee on Food
Additives. Geneva, World Health Organization, 36 pp (WHO Technical
Report Series No. 462).
FAO/WHO (1980) Evaluation of certain food additives. Twenty-third
report of the Joint FAO/WHO Expert Committee on Food Additives.
Geneva, World Health Organization (WHO Technical Report Series No. 648
and corrigenda).
FAO/WHO (1992) Evaluation of certain food additives and naturally
occurring toxicants. Thirty-ninth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization (WHO
Technical Report Series No. 828).
Fellin P, Barnett SE, & Tran QA (1992) Results of a national pilot
survey of airborne volatile organic compounds in Canadian residences:
Volume 1. Downsview, Ontario, Canada, Concord Environmental
Corporation, 156 pp (prepared for Health and Welfare Canada, Ottawa).
Ferreri AM, Rocchi P, Capucci A, & Prodi G (1983) Induction of
diphtheria toxin-resistant mutants in human cells by halogenated
compounds. J Cancer Res Clin Oncol, 105: 111-112.
Finlayson-Pitts BJ & Pitts JN (1986) Atmospheric chemistry:
Fundamentals and experimental techniques. New York, Wiley-Interscience
Publications.
Foster GD & Tullis RE (1984) A quantitative structure-activity
relationship between partition coefficients and the acute toxicity of
naphthalene derivatives in Artemia salina nauplii. Aquat Toxicol,
5: 245-254.
Foureman GL & Reed DJ (1985) Evidence for a non-episulfonium ion
intermediate during alkylation by S-[2-(N7-guanyl)ethyl]
glutathione, the major DNA adduct formed from 1,2-dibromoethane.
Biochemistry, 25: 2192-2198.
Freiria-Gandara MJ, Lorenzo-Ferreira RA, Alvarez-Devesa, A & Bermejo F
(1992) Occurrence of halogenated hydrocarbons in the water supply of
different cities of Galicia (Spain). Environ Technol, 13: 437-447.
Freitag D, Ballhorn L, Behechti A, Fischer K, & Thumm W (1994)
Structural configuration and toxicity of chlorinated alkanes.
Chemosphere, 28(2): 253-259.
Fujii T (1977) Direct aqueous injection gas chromatography-mass
spectrometry for analysis of organohalides in water at concentrations
below the parts per billion level. J Chromatogr, 139: 297-302.
Garrison SC & Leadingham RS (1954) A fatal case of ethylene dichloride
poisoning in an occupational therapy department of a neuropsychiatric
hospital. Am J Phys Med, 33: 230-237.
Geiger DL, Northcott CE, Call DJ, & Brooke LT (1985)
1,2-Dichloroethane. In: Geiger DL, Northcott CE, Call DJ, & Brooke LT
ed. Acute toxicities of organic chemicals to fathead minnow
(Pimephales promelas). Superior, Wisconsin, University of
Wisconsin-Superior, Center for Lake Superior Environmental Studies,
vol 2, pp 41-42.
Giri AK & Que Hee SS (1988) In vivo sister chromatid exchange
induced by 1,2-dichloroethane on bone marrow cells of mice. Environ
Mol Mutagen, 12: 331-334.
Gocke E, Wild D, Eckhardt K, & King MT (1983) Mutagenicity studies
with the mouse spot test. Mutat Res, 117: 201-212.
Golder Associates (1987) Testing of specific organic compounds in
soils in background in urban areas: Port Credit and
Oakville/Burlington, Ontario. Mississauga, Ontario, Canada, Golder
Associates (Working paper to Shell Canada Ltd and Texaco Canada Ltd).
Gradiski D, Bonnet P, Raoult G, & Magadur JL (1978) Toxicité aiguë
comparée par inhalation des principaux solvants aliphatiques chlorés.
Arch Mal Prof, 39(4-5): 249-257.
Grimsrud EP & Rasmussen RA (1975) Survey and analysis of halocarbons
in the atmosphere by gas chromatography-mass spectrometry. Atmos
Environ, 9: 1014-1017.
Guengerich FP, Crawford WM, Domoradzki JY, MacDonald TL, & Watanabe PG
(1980) In vitro activation of 1,2-dichloroethane by microsomal and
cytosolic enzyme. Toxicol Appl Pharmacol, 55: 303-317.
Guengerich FP, Mason PS, Stott WT, Fox TR, & Watanabe PG (1981) Role
of 2-haloethylene oxides and vinylchloride in irreversible binding to
protein and DNA. Cancer Res, 41: 4391-4398.
Guengerich FP, Koga N, Inskeep PB, & Ozawa N (1986) Proceedings of the
Second International Symposium on the Synthesis and Application of
Isotopically Labelled Compounds. New York, Elsevier Science
Publishers.
Guengerich FP, Kim DH, & Iwasaki M (1991) Role of human cytochrome
P-450 IIE1 in the oxidation of many low molecular weight cancer
suspects. Chem Res Toxicol, 4: 168-179.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Hadengue MA & Martin R (1953) Un cas d'intoxication mortelle par le
dichloréthane. Soc Méd Lég, 33: 247-249.
Hatch GG, Mamay PD, Ayer ML, Casto BC, & Nesnow S (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo cells by
gaseous and volatile chlorinated methanes and ethanes. Cancer Res,
43: 1945-1950.
Health Canada (1994) Unpublished update to Canadian Environmental
Protection Act Priority Substances report on 1,2-dichloroethane.
Ottawa, Ontario, Environmental Health Directorate, Priority Substances
Section.
Heikes DL (1987a) Determination of residual chlorinated solvents in
decaffeinated coffee by using purge and trap procedure. J Assoc Off
Anal Chem, 70(1): 176-180.
Heikes DL (1987b) Pesticide and industrial chemical residues - purge
and trap method for determination of volatile halocarbons and carbon
disulfide in table-ready foods. J Assoc Off Anal Chem, 70(2): 215-226.
Heikes DL (1990) Environmental contaminants in table-ready foods from
the total diet program of the Food and Drug Administration. In: Food
contamination from environmental sources. New York, John Wiley and
Sons, pp 31-57 (Advances in Environmental Science and Technology
Series No. 23).
Heikes DL & Hopper ML (1986) Purge and trap method for determination
of fumigants in whole grains, milled grain products, and intermediate
grain-based foods. J Assoc Off Anal Chem, 69(6): 990-998.
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hellman B & Brandt I (1986) Effects of carcinogenic halogenated
aliphatic hydrocarbons on [3H]thymidine incorporation into various
organs of the mouse. A comparison between 1,2-dibromoethane and
1,2-dichloroethane. Mutat Res, 163: 193-199.
Hemminki K, Falck K, & Vainio H (1980) Comparison of alkylation rates
and mutagenicity of directly acting industrial and laboratory
chemicals: epoxides, glycidyl ethers, methylating and ethylating
agents, halogenated hydrocarbons, hydrazine derivatives, aldehydes,
thiouram and dithiocarbamate derivatives. Arch Toxicol, 46: 277-285.
Heppel LA, Neal PA, Perrin TL, Endicott KM, & Porterfield VT (1945)
The toxicology of 1,2-dichloroethane (ethylene). III. Its acute
toxicity and the effect of protective agents. J Pharmacol Exp Ther,
84: 53-63.
Heppel LA, Neal PA, Perrin TL, Endicott KM, & Porterfield VT (1946)
The toxicology of 1,2-dichloroethane (ethylene dichloride). V. The
effects of daily inhalations. J Ind Hyg, Toxicol, 28(4): 113-120.
Hiatt MH (1981) Analysis of fish and sediment for volatile priority
pollutants. Anal Chem, 53: 1541-1543.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography/mass spectrometry. Anal Chem, 55: 506-516.
Hill J, Kollig HP, Paris DF, Wolfe NL, & Zepp RG (1976) Dynamic
behaviour of vinyl chloride in aquatic ecosystems. Athens, Georgia, US
Environmental Protection Agency (EPA/600/3-76/001).
Hinkel GK (1965) [Oral dichloroethane intoxication of children.] Dtsch
Gesudheitswes, 20: 1327-1331 (in German).
Hofmann HT, Birnsteil H, & Jobst P (1971) [The inhalation toxicity of
1,1- and 1,2-dichloroethane.] Arch Toxikol, 27: 248-265 (in German).
Hooper K, Gold LS, & Ames BN (1980) The carcinogenic potency of
ethylene dichloride in two animal bioassays: A comparison of
inhalation and gavage studies. In: Ames B, Infante P, & Reitz R ed.
Ethylene dichloride: a potential health risk? Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp 65-80 (Banbury Report No. 5).
HSDB (1993) Hazardous substances data bank (revision 9/2/93).
Bethesda, Maryland, National Library of Medicine, National Toxicology
Information Program.
Hueper WC & Smith C (1935) Fatal ethylene dichloride poisoning. Am J
Med Sci, 189: 778-784.
IARC (1979) Some halogenated hydrocarbons. Lyon, International Agency
for Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 20).
Igwe OJ, Que Hee SS, & Wagner WD (1986a) Interaction between
1,2-dichloroethane and tetraethylthiuram disulfide (disulfiram). II.
Hepatotoxic manifestations with possible mechanism of action. Toxicol
Appl Pharmacol, 86: 286-297.
Igwe OJ, Que Hee SS, & Wagner WD (1986b) Interaction between
1,2-dichloroethane and disulfiram. I. Toxicologic effects. Fundam Appl
Toxicol, 6: 733-746.
Inskeep PB, Koga N, Cmarik JL, & Guengerich FP (1986) Covalent binding
of 1,2-dihaloalkanes to DNA and stability of the major DNA adduct,
S-[2(N7-guanyl)ethyl] glutathione. Cancer Res, 46: 2839-2844.
IPCS (International Programme on Chemical Safety) (1987) Environmental
health criteria 62: 1,2-Dichloroethane. Geneva, World Health
Organization.
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: The derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization.
Isacson P, Bean JA, Splinter R, Olson DB, & Kohler J (1985) Drinking
water and cancer incidence in Iowa. III. Association of cancer with
indices of contamination. Am J Epidemiol, 121(6): 856-869.
Isnard P & Lambert S (1988) Estimating bioconcentration factors from
octanol-water partition coefficient and aqueous solubility.
Chemosphere, 17(1): 21-34.
Jafvert CT & Wolfe NL (1987) Degradation of selected halogenated
ethanes in anoxic sediment-water systems. Environ Toxicol Chem,
6: 827-837.
Jakobson I, Wahlberg JE, Holmberg B, & Johansson G (1982) Uptake via
the blood and elimination of 10 organic solvents following
epicutaneous exposure of anesthetized guinea pigs. Toxicol Appl
Pharmacol, 63: 181-187.
Janssen DB, Scheper A, Dijkhuizen L, & Witholt B (1985) Degradation of
halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10.
Appl Environ Microbiol, 49(3): 673-677.
Jeffers PM, Ward L, Woytowich L, & Wolfe NL (1989) Homogeneous
hydrolysis rate constants for selected chlorinated methanes, ethanes,
ethenes and propanes. Environ Sci Technol, 23(8): 965-969.
Jenssen D & Ramel C (1980) The micronucleus test as part of a
short-term mutagenicity test programme for the prediction of
carcinogenicity evaluated by 43 agents tested. Mutat Res, 75: 191-202.
Jonsson A & Berg S (1980) Determination of 1,2-dibromoethane,
1,2-dichloroethane, and benzene in ambient air using porous polymer
traps and gas chromatographic-mass spectrometric analysis with
selected ion monitoring. J Chromatogr, 190: 97-106.
Kaiser KLE & Comba ME (1986) Volatile hydrocarbon contaminant survey
of the St. Clair River. Water Pollut Res J Can, 21(3): 323-331.
Kaiser KLE, Comba ME, & Huneault H (1983) Volatile hydrocarbon
contaminants in the Niagara River and in Lake Ontario. J Great Lakes
Res, 9(2): 212-223.
Kanada T & Uyeta M (1978) Mutagenicity screening of organic solvents
in microbial systems. Mutat Res, 54: 215.
Kavlock R, Chernoff N, Carver B, & Kopfler F (1979) Teratology studies
in mice exposed to municipal drinking water concentrates during
organogenesis. Food Cosmet Toxicol, 17: 343-347.
Kerster HW & Schaeffer DJ (1983) Brine shrimp (Artemia salina)
nauplii as a teratogen test system. Ecotoxicol Environ Saf,
7: 342-349.
King MT, Beikirch H, Eckhardt K, Gocke E, & Wild D (1979) Mutagenicity
studies with X-ray-contrast media, analgesics, antipyretics,
antirheumatics and some other pharmaceutical drugs in bacterial,
Drosophila and mammalian test systems. Mutat Res, 66: 33-43.
Kirschmer P & Ballschmiter K (1983) Baseline studies of global
pollution. VIII. The complex pattern of C1 - C4 organohalogens in
continental and marine background air. Int J Environ Anal Chem,
14: 275-284.
Klaunig JE, Ruch RJ, & Pereira MA (1986) Carcinogenicity of
chlorinated methane and ethane compounds administered in drinking
water to mice. Environ Health Perspect, 69: 89-95.
Kliest J, Fast T, Boley JSM, van de Wiel H, & Bloemen H (1989) The
relationship between soil contaminated with volatile organic compounds
and indoor air pollution. Environ Int, 15: 419-425.
Knie VJ, Halke A, Juhnke I, & Schiller W (1983) Results of studies on
chemical substances with four biotests. Dtsch Gewasserkd Mitt,
27(3): 77-79.
Koga N, Inskeep PB, Harris TM, & Guengerich FP (1986)
S-[2-(N7-guanyl)ethyl] glutathione, the major DNA adduct formed
from 1,2-dibromoethane. Biochemistry, 25: 2192-2198.
Konemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. Part 1: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-221.
Kozik IV (1957) [Some problems of occupational hygiene in the use of
dichloroethane in the aircraft industry.] Gig Tr Prof Zabol, 1: 31-38
(in Russian).
Kramers PG, Mout HC, Bissumbhar B, & Mulder CR (1991) Inhalation
exposure in Drosophila mutagenesis assays: experiments with aliphatic
halogenated hydrocarbons, with emphasis on the genetic activity
profile of 1,2-dichloroethane. Mutat Res, 252: 17-33.
Kretzschmar JG, Peperstraete H, & Tymen T (1976) Air pollution in and
around Tessenderlo (The Netherlands). Neth Extern, 5: 147-178.
Krill RM & Sonzongni WC (1986) Chemical monitoring of Wisconsin's
groundwater. J Am Water Works Assoc, 78(9): 70-75.
Kronevi T, Wahlberg JE, & Holmberg B (1981) Skin pathology following
epicutaneous exposure to seven organic solvents. Int J Tissue React,
3: 21-30.
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Kuwabara T, Quevedo AR, & Cogan DG (1968) An experimental study of
dichloroethane poisoning. Arch Ophthalmol, 79: 321-330.
Lane RW, Riddle BL, & Borzelleca JF (1982) Effects of
1,2-dichloroethane and 1,1,1-trichloroethane in drinking water on
reproduction and development in mice. Toxicol Appl Pharmacol,
63: 409-421.
LaRegina J & Bozzelli JW (1986) Volatile organic compounds at
hazardous waste sites and a sanitary landfill in New Jersey - An
up-to-date review of the present situation. Environ Prog,
5(1): 18-27.
Larionov VG & Kokarovtseva MG (1976) Morphological constitution of
peripheral blood in intoxication with dichloroethane and its
metabolites. In: Actual problems of pesticide application in different
climatographic zones. Yerevan, Aiastan Publishers, pp 131-133.
Leblanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lesage S, McBride RA, Cureton PM, & Brown S (1993) Fate of organic
solvents in landfill leachates under simulated field conditions and in
anaerobic microcosms. Waste Manage Res, 11: 215-226.
Letkiewicz F, Johnston P, & Coleman J (1982) Occurrence of
1,2-dichloroethane in drinking water, food and air (Contract
68-01-6185). Washington, DC, JRB Associates (Prepared for the US
Environmental Protection Agency).
Li KM & Cheng WT (1991) The occupational exposure level of
1,2-dichloroethane in an EM preparation laboratory. J R Soc Health,
111(5): 169.
Lin ELC, Mattox JK, & Pereira MA (1985) Glutathione plus cytosol- and
microme-mediated binding of 1,2-dichloroethane to polynucleotides.
Toxicol Appl Pharmacol, 78: 428-435.
Lum KR & Kaiser KLE (1986) Organic and inorganic contaminants in the
St. Lawrence River: Some preliminary results on their distribution.
Water Pollut Res J Can, 21(4): 592-603.
Luznikov EA, Lisovik GA, & Novikovskaya TV (1985) [Metabolism of
1,2-dichloroethane in human organisms after acute poisonings.]
Forensic Med Expert, 2: 47-49 (in Russian).
McCann J, Simmon V, Streitwieser D, & Ames BN (1975) Mutagenicity of
chloroacetaldehyde, a possible metabolic product of 1,2-dichloroethane
(ethylene dichloride), chloroethane (ethylene chlorohydrin), vinyl
chloride and cyclophosphamide. Proc Natl Acad Sci (USA),
72: 3190-3191.
McCollister DD, Hollingsworth RL, Oyen F, & Rowe VK (1956) Comparative
inhalation toxicity of fumigant mixtures. Individual and joint effect
of ethylene dichloride, carbon tetrachloride, and ethylene dibromide.
Arch Ind Health, 13: 1-7.
McPherson RW, Starks CM, & Fryar GJ (1979) Vinyl chloride
monomer...what you should know. Hydrocarb Process, 3: 75-88.
MAFF (Ministry of Agriculture, Fisheries and Food) (1982) Food
surveillance paper No. 9. London, Her Majesty's Stationery Office.
MAFF (Ministry of Agriculture, Fisheries and Food) (1984) Food
surveillance paper No. 16. London, Her Majesty's Stationery Office.
MAFF (Ministry of Agriculture, Fisheries and Food) (1989) Food
surveillance paper No. 25. London, Her Majesty's Stationery Office.
MAFF/HSE (Ministry of Agriculture, Fisheries and Food/Health and
Safety Executive) (1993) Annual Report of the Working Party on
Pesticide Residues: 1992. Supplement to the Pesticides Register, 1993.
London, Her Majesty's Stationery Office.
Maltoni C, Valgimigli L, & Scarnato C (1980) Long-term carcinogenic
bioassays on ethylene dichloride administered by inhalation to rats
and mice. In: Ames BN, Infante P, & Reitz R ed. Ethylene dichloride: A
potential health risk? Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 3-33 (Banbury Report No. 5).
Martin G, Knorpp K, Huth K, Heinrich F, & Mittermayer C (1969)
Clinical features, pathogenesis and management of dichloroethane
poisoning. Germ Med Mon, 14: 62-67.
Menschick H (1957) [Acute inhalation intoxications by symmetric
dichloroethane.] Arch Gewerbepathol Gewerbehyg, 15: 241-252
(in German).
Mihaylova A (1976) [On the action of high temperature of the
environment on the acute and subacute toxicity of dichloroethane.] Gig
Zdraveopaz, 1: 32-36 (in Bulgarian).
Milman HA, Storystorey DL, Riccio ES, Sivak A, Tu AS, Williams GM,
Tong C, & Tyson CA (1988) Rat liver foci and in vitro assays to
detect initiating and promoting effects of chlorinated ethanes and
ethylenes. Ann NY Acad Sci, 534: 521-530.
Ministry of the Environment (1991) Drinking water surveillance program
data base. Ottawa, Canada, Ministry of the Environment, Water
Resources Branch, 27 pp (105 entries).
Mitoma C, Steeger T, Jackson SE, Wheeler KP, Rogers JH, & Milman HA
(1985) Metabolic disposition study of chlorinated hydrocarbons in rats
and mice. Drug Chem Toxicol, 8: 183-194.
Morgan DL, Bucher JR, Elwell MR, Lilja HS, & Murthy AS (1990)
Comparative toxicity of ethylene dichloride in F344/N, Sprague-Dawley
and Osborne-Mendel rats. Food Chem Toxicol, 28: 839-845.
Morgan DL, Cooper SW, Carlock DL, Sykora JJ, Sutton B, Mattie DR, &
McDougal JN (1991) Dermal absorption of neat and aqueous volatile
organic chemicals in Fischer 344 rat. Environ Res, 55: 51-63.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116:185-216.
Morozov GN (1958) [On acute dichloroethane poisoning.] Farmakol
Toksicol, 21(1): 76-78 (in Russian).
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, & White KL
(1982) In vivo assessment of immunotoxicity. Environ Health
Perspect, 43: 41-52.
Natsyuk MV & Chekman IS (1975) The content of nicotinamide coenzymes
in the liver and myocardium of rats poisoned with dichloroethane. Bull
Exp Biol Med, 4: 58-60.
Natsyuk MV & Mudritsky AD (1974) Clinical and biochemical
characteristics of hepatic and renal disorders in acute dichloroethane
intoxications. Mil Med J, 10: 48-50.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity. Bethesda, Maryland, National Cancer Institute (HEW
(NIH) Publication No. 78-1305).
Nestmann ER, Lee EGH, Matula TI, Douglas GR, & Mueller JC (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using Salmonella/mammalian-microsome assay. Mutat Res,
79: 203-212.
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR (1985)
The toxicity of selected organic chemicals to the earthworm Eisenia
foetida. J Environ Qual, 14(3): 383-388.
Nevrotsky VK, Kashin LM, Kulinskaya IL, Mikhailovskaya LF, Shmuter LM,
Burlaka-Vouk ZI, & Zadorozhny BV (1971) Comparative toxicity
evaluation of several industrial poisons in protracted inhalation
exposure to low concentrations. In: Proceedings of the 8th Ukranian
Hygiene Congress, Kiev, 1971, pp 224-226.
Nimitz JS & Skaggs SR (1992) Estimating tropospheric lifetimes and
ozone-depletion potentials of one- and two-carbon hydrofluorocarbons
and hydrochlorofluorocarbons. Environ Sci Technol, 26(4): 739-744.
NIOSH (1994) Registry of toxic effects of chemical substances.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
NIOSH (1984) Manual of analytical methods: Method 1003. Cincinnati,
Ohio, National Institute for Occupational Safety and Health.
Nouchi T, Miura H, Kanayama M, Mizuguchi O, & Takano T (1984) Fatal
intoxication by 1,2-dichloroethane - A case report. Int Arch Occup
Environ Health, 54: 111-113.
NTP (1991) Toxicity studies of 1,2-dichloroethane (ethylene
dichloride) in F344/N rats, Sprague Dawley rats, Osborne-Mendel rats,
and B6C3F1 mice (drinking water and gavage studies). Research
Triangle Park, North Carolina, National Toxicology Program (NIH
Publication No. 91-3123).
Nylander PO, Olofsson H, Rasmuson B, & Svahlin H (1978) Mutagenic
effects of petrol in Drosophila melanogaster. I. Effects of benzene
and 1,2-dichloroethane. Mutat Res, 57: 163-167.
Oldenhuis R, Vink RLJM, Janssen DB, & Witholt B (1989) Degradation of
chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b
expressing soluble methane monooxygenase. Appl Environ Microbiol,
55(11): 2819-2826.
Oliver BG & Pugsley CW (1986) Chlorinated contaminants in St. Clair
River sediments. Water Pollut Res J Can, 21(3): 368-379.
Otson R (1987) Purgeable organics in Great Lakes raw and treated
water. Int J Environ Anal Chem, 31(1): 41-53.
Otson R & Williams DT (1982) Headspace chromatographic determination
of water pollutants. Anal Chem, 54: 942-946.
Otson R, Williams DT, & Bothwell PD (1982) Industrial chemicals -
Volatile organic compounds in water at thirty Canadian potable water
treatment facilities. J Assoc Off Anal Chem, 65(6): 1370-1374.
Parkes DG, Ganz CR, Polinsky A, & Schulze J (1976) A simple gas
chromatographic method for the analysis of trace organics in ambient
air. Am Ind Hyg Assoc J, 37: 165-173.
Payan JP, Beydon D, Fabry JP, Brondeau MT, Ban M, & de Ceaurriz J
(1993) Urinary thiodiglycolic acid and thioether excretion in male
rats dosed with 1,2-dichloroethane. J Appl Toxicol, 13: 417-422.
Pearson CR & McConnell G (1975) Chlorinated C1 and C2 hydrocarbons
in the marine environment. Proc R Soc (Lond), B189: 305-332.
Pellizzari ED (1982) Analysis for organic vapor emissions near
industrial and chemical waste disposal sites. Environ Sci Technol,
16(11): 781-785.
Perocco P & Prodi G (1981) DNA damage by haloalkanes in human
lymphocytes cultured in vitro. Cancer Lett, 13: 213-218.
Pleil JD, Oliver KD, & McClenny WA (1988) Ambient air analyses using
nonspecific flame ionization and electron capture detection compared
by mass spectroscopy. J Air Pollut Control Assoc, 38: 1006-1010.
Principe P, Dogliotti E, Bignami M, Crebelli R, Falcone E, Fabrizi M,
Conti G, & Comba P (1981) Mutagenicity of chemicals of industrial and
agricultural relevance in Salmonella, Streptomyces, and Aspergillus.
J Sci Food Agric, 32: 826-832.
Proctor CJ, Warren ND, & Bevan MAJ (1989) Measurements of
environmental tobacco smoke in an air-conditioned office building.
Environ Technol Lett, 10: 1003-1018.
Prodi G, Arfelini G, Colacci A, Grilli S, & Mazzulo M (1986)
Interaction of halocompounds with nucleic acids. Toxicol Pathol, 14:
438-444.
Qiu LX, Shi SH, Xing SB, & Chen SG (1992) Rate constants for the
reactions of OH with five halogenated substituted ethanes from 292 K
to 366 K. J Phys Chem, 96: 685-689.
Rannug U (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichloroethane. Mutat Res, 76: 269-295.
Rannug U & Beije B (1979) The mutagenic effect of 1,2-dichloroethane
on Salmonella typhimurium. II. Activation by the isolated perfused
rat liver. Chem-Biol Interact, 24: 265-285.
Rannug U, Sundvall A, & Ramel C (1978) The mutagenic effect of
1,2-dichloroethane on Salmonella typhimurium. I. Activation through
conjugation with glutathione in vitro. Chem-Biol Interact, 20: 1-16.
Rao KS, Murray JS, Deacon MM, John JA, Calhoun LL, & Young JT (1980)
Teratogenicity and reproduction studies in animals inhaling ethylene
dichloride. In: Ames B, Infante P, & Reitz R ed. Ethylene dichloride:
A potential health risk? Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 149-166 (Banbury Report No. 5).
Reid BJ, Morgan JD, & Whelen MA (1982) A preliminary examination of
the effects of ethylene dichloride on the hatchability of Coho salmon
eggs (Oncorhynchus kisutch). Can Tech J Fish Aquat Sci,
1163: 145-153.
Reitz RH, Fox TR, Ramsey JC, Quast JF, Langvardt PW, & Watanabe PG
(1982) Pharmacokinetics and macro-molecular interactions of ethylene
dichloride in rats after inhalation or gavage. Toxicol Appl Pharmacol,
62: 190-204.
Reitz RH, Fox TR, Domordzki JY, Quast JF, Langvardt PW, & Watanabe PG
(1980) Pharmacokinetics and macromolecular interactions of ethylene
dichloride: Comparison of oral and inhalation exposures. In: Ames BN,
Infante P, & Reitz R. ed. Ethylene dichloride: A potential health
risk? Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp 135-148 (Banbury Report No. 5).
Richter JE, Peterson SF, & Kleiner CF (1983) Acute and chronic
toxicity of some chlorinated benzenes, chlorinated ethanes, and
tetrachloroethylene to Daphnia magna. Arch Environ Contam Toxicol,
12: 679-684.
Roldan-Arjona T, Garcia-Pedrajas MD, Luque-Romero RL, Hera C, & Pueyo
C (1991) An association between mutagenicity of the Ara test of
Salmonella typhimurium and carcinogenicity in rodents for 16
halogenated aliphatic hydrocarbons. Mutagenesis, 6: 199-205.
Romert L, Magnusson J, & Ramel C (1990) The importance of glutathione
and glutathione transferase for somatic mutations in Drosophila
melanogaster induced in vivo by 1,2-dichloroethane.
Carcinogenesis, 11: 1399-1402.
Rosenberg R, Grahn O, & Johansson L (1975) Toxic effects of aliphatic
chlorinated by-products from vinyl chloride production on marine
animals. Water Res, 9: 607-612.
Sack TM, Steele DH, Hammerstrom K, & Remmers J (1992) A survey of
household products for volatile organic compounds. Atmos Environ,
26A(6): 1063-1070.
Saitanov AO & Arsenieva SS (1969) [Electrocardiographic changes due to
acute dichloroethane poisoning: experimental investigation.] Gig Tr
Prof Zabol, 7: 49-50 (in Russian).
Sato A, Nakajima T, & Koyama Y (1981) Dose-related effects of a single
dose of ethanol on the metabolism in rat liver of some aromatic and
chlorinated hydrocarbons. Toxicol Appl Pharmacol, 60: 8-15.
Sauer TC (1981) Volatile organic compounds in open ocean and coastal
surface waters. Org Geochem, 3: 91-101.
Schasteen CS & Reed DJ (1983) The hydrolysis and alkylation activities
of S-(2-haloethyl)-L-cysteine analogues - evidence for extended
half-life. Toxicol Appl Pharmacol, 70: 423-432.
Schönborn H, Prellwitz W, & Baum P (1970) [Consumption coagulation
pathology of 1,2-dichloroethane poisoning.] Klin Wochenschr,
48: 822-824 (in German).
Schultz K, Ghosh L, & Banerjee S (1992) Neoplastic expression in
murine cells induced by halogenated hydrocarbons. In vitro Cell Dev
Biol, 28A: 267-272.
Sherwood RL, O'Shea PT, Thomas W, Ratajczak HV, Aranyi C, & Graham JA
(1987) Effects of inhalation of ethylene dichloride on pulmonary
defenses of mice and rats. Toxicol Appl Pharmacol, 91: 491-496.
Shmuter LM (1977) [Effect of chronic exposure to low concentrations of
chlorinated hydrocarbons of the ethane series on specific and
non-specific reactivity of animals in vivo.] Gig Tr Prof Zabol,
8: 38-42 (in Russian).
Simmon VF (1980) Review of non-bacterial tests of the genotoxic
activity of ethylene dichloride. In: Ames B, Infante P, & Reitz R ed.
Ethylene dichloride: A potential health risk? Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp 97-103 (Banbury Report No. 5).
Simula TP, Glancey MG, & Wolf CR (1993) Human glutathione
S-transferase-expressing Salmonella typhimurium tester strains to
study the activation/detoxification of mutagenic compounds: studies
with halogenated compounds, aromatic amines and aflatoxin B1.
Carcinogenesis, 14: 1371-1376.
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1980) Atmospheric
measurements of selected toxic organic chemicals. Washington, DC, US
Environmental Protection Agency, 35 pp (EPA-600/3-80-072;
NTIS PB80-89).
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban environments.
Atmos Environ, 15: 601-612.
Singh HB, Salas LJ, & Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environ Sci Technol, 16: 872-880. Singh HB, Salas IJ, & Stiles RE
(1983) Selected man-made halogenated chemicals in the air and oceanic
environment. J Geophys Res, 88(C6): 3675-3683.
Smyth HF (1969) Acute toxicity of ethyl compounds. In: Spector WC ed.
Handbook of toxicology. Philadelphia, Pennsylvania, W.B. Saunders,
vol 1, pp 134-135.
Spence JW & Hanst PL (1978) Oxidation of chlorinated ethanes. J Air
Pollut Control Assoc, 28(3): 250-253.
Spencer HC, Rowe VK, Adams EM, McCollister DD, & Irish DD (1951) Vapor
toxicity of ethylene dichloride determined by experiments on
laboratory animals. Am Med Assoc Arch Ind Hyg Occup Med, 4: 482-493.
Spreafico F, Zuccato E, Marcucci F, Sironi M, Paglialunga S, Madonna
M, & Mussini E (1980) Pharmacokinetics of ethylene dichloride in rats
treated by different routes and its long-term inhalatory toxicity. In:
Ames B, Infante P, & Reitz R ed. Ethylene dichloride: A potential
health risk? Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp 107-133 (Banbury Report No. 5).
Storer RD & Conolly RB (1983) Comparative in vivo genotoxicity and
acute hepatotoxicity of three 1,2-dihaloethanes. Carcinogenesis,
4: 1491-1494.
Storer RD & Conolly RB (1985) An investigation of the role of
microsomal oxidative metabolism in the in vivo genotoxicity of
1,2-dichloroethane. Toxicol Appl Pharmacol, 77: 36-46.
Storer RD, Jackson NM, & Conolly RB (1984) In vivo genotoxicity and
acute hepatotoxicity of 1,2-dichloroethane in mice: Comparison of
oral, intraperitoneal, and inhalation routes of exposure. Cancer Res,
44: 4267-4271.
Story DL, Meierhenry EF, Tyson CA, & Milman HA (1986) Differences in
rat liver enzyme-positive foci produced by chlorinated aliphatics and
phenobarbital. Toxicol Ind Health, 2: 351-362.
Strobel K & Grummt T (1987) Aliphatic and aromatic halocarbons as
potential mutagens in drinking water. III. Halogenated ethanes and
ethenes. Toxicol Environ Chem, 15: 101-128.
Sundheimer DW, White RD, Brendel K, & Sipes IC (1982) The
bioactivation of 1,2-dibromoethane in rat hepatocytes: covalent
binding to nucleic acids. Carcinogenesis, 3: 1129-1133.
Suveev IM & Babichenko ME (1969) [On the clinic and cure of acute
intoxication with dichloroethane vapours.] Gig Tr Prof Zabol,
13: 50-51 (in Russian).
Symons JM, Bellar TA, Carswell JK, Demarco J, Kropp KL, Robeck GG,
Seeger DR, Slocum CJ, Smith BL, & Stevens AA (1975) National organics
reconnaissance survey for halogenated organics. J Am Water Works
Assoc, 67: 634-648.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53(10): 1503-1518.
Tan EL & Hsie AW (1981) Mutagenicity and cytotoxicity of haloethanes
as studied in the CHO/HGPRT system. Mutat Res, 90: 183-191.
Tang NH, Blum DJW, & Speece RE (1990) Comparison of serum bottle
toxicity test with OECD method. J Environ Eng, 116(6): 1076-1084.
Taningher M, Parodi S, Grilli S, Colacci A, Mazzullo M, Bordone R, &
Santi L (1991) Lack of correlation between alkaline DNA fragmentation
and DNA covalent binding induced by polychloroethanes after in vivo
administration. Problems related to the assessment of a carcinogenic
hazard. Cancer Detect Prev, 15: 35-39.
Theiss JC, Stoner GD, Shimkin MB, & Weisberger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer Res,
37: 2717-2720.
Torkelson TR & Rowe VK (1981) Halogenated aliphatic hydrocarbons. In:
Clayton GD & Clayton FE ed. Toxicology: Patty's industrial hygiene
and toxicology, 3rd ed. New York, John Wiley and Sons, vol 2B,
pp 3491-3497.
Troisi FM & Cavallazzi D (1961) [A fatal case of poisoning from
inhalation of dichloroethane vapours.] Med Lav, 52: 612-618
(in Italian).
Tsuruta H (1975) Percutaneous absorption of organic solvents: 1)
Comparative study of the in vivo percutaneous absorption of
chlorinated solvents in mice. Ind Health, 13: 227-236.
Tsuruta H (1977) Percutaneous absorption of organic solvents: 2) A
method for measuring the penetration rate of chlorinated solvents
through excised rat skin. Ind Health, 15: 131-139.
Tu AS, Murray TA, Hatch KM, Sivak A, & Milman HA (1985) In vitro
transformation of BALB/c-3T3 cells by chlorinated ethanes and
ethylenes. Cancer Lett, 28: 85-92.
UK HSE (Health and Safety Executive) (1992) Criteria document for an
occupational exposure limit: 1,2-Dichloroethane. London, Her Majesty's
Stationery Office, 34 pp.
Urusova TP (1953) [The possible presence of dichloroethane in human
milk with exposure in industrial conditions.] Gig i Sanit, 18: 36-37
(in Russian).
US Department of Labor (1989) Industrial exposure and control
technologies for OSHA regulated hazardous substances: Volume I.
Washington, DC, US Department of Labor.
US DHHS (Department of Health and Human Services) (1994) Seventh
annual report on carcinogens -1994: Summary. Research Triangle Park,
North Carolina, National Institute of Environmental Health Sciences,
165 pp.
US EPA (1982a) An exposure and risk assessment for dichloroethanes.
Washington, DC, US Environmental Protection Agency, Office of Water
Regulations and Standards, 175 pp (EPA-440/4-85-009).
US EPA (1982b) Test methods for organic chemical analysis of municipal
and environmental wastewater. Cincinnati, Ohio, US Environmental
Protection Agency, Monitoring and Support Laboratories, 76 pp
(EPA 600/4-82-057).
US EPA (1985a) Health assessment document for 1,2-dichloroethane
(ethylene dichloride). Final Report. Washington, DC, US Environmental
Protection Agency, 95 pp (EPA/600/8-84/006F; NTIS PB86-122702).
US EPA (1985b) Health and environmental effects profile for
1,2-dichloroethane. Washington, DC, US Environmental Protection
Agency, 137 pp (EPA/600/X-85/359; NTIS PB88-178777).
US EPA (1986) Handbook: Permit writer's guide to test burn data,
hazardous waste incineration. Cincinnati, Ohio, US Environmental
Protection Agency, 73 pp (EPA/625/6-86/012).
US EPA (1987) Health advisories for 25 organics. Washington, DC, US
Environmental Protection Agency (NTIS PB87-235578).
US EPA (1988) Contract laboratory program database. Washington, DC, US
Environmental Protection Agency, Contract Laboratory Program.
US EPA (1992) Indoor air quality data base for organic compounds.
Research Triangle Park, North Carolina, US Environmental Protection
Agency (EPA/600/R/92-025; PB92-158468).
Valencia R, Abrahamson S, Lee WR, Von Halle ES, Woodruff RC, Wurgler
FE, & Zimmering S (1984) Chromosome mutations for mutagenesis in
Drosophila melanogaster: A report of the US Environmental Protection
Agency Gene-Tox Program. Mutat Res, 134: 61-88.
Van Bladeren PJ, Breimer DD, Rotteveel-Smijs RMT, De Knijff P, Mohn
GR, van Meeteren-Walchli B, Buijs W, & van der Gen A (1981) The
relation between the structure of vicinal dihalogen compounds and
their mutagenic activation via conjugation to glutathione.
Carcinogenesis, 2: 499-505.
Van den Wijngaard AJ, van der Kamp KWJ, van der Ploeg J, Pries F,
Kazemier B, & Janssen DB (1992) Degradation of 1,2-dichloroethane by
Ancylobacter aquaticus and other facultative methylotrophs. Appl
Environ Microbiol, 58: 976-983.
Van Duuren BL, Goldschmidt BM, Loewengart G, Smith A, Mechionne S,
Seldman I, & Roth D (1979) Carcinogenicity of halogenated olefinic and
aliphatic hydrocarbons in mice. J Natl Cancer Inst, 63: 1433-1439.
Van Esch GJ, Kroes R, Van Logten MJ, & Den Tonkelaar EM (1977)
Ninety-day toxicity study with 1,2-dichloroethane (DCE) in rats.
Bilthoven, The Netherlands, National Institute of Public Health and
Environmental Protection (RIVM) (Report 195/77).
Van Luin AB & van Starkenburg W (1984) Hazardous substances in waste
water. Water Sci Technol, 17: 843-853.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: Narcotic industrial
chemicals. Can J Fish Aquat Sci, 40: 743-748.
Vozovaya MA (1974) [Development of posterity of two generations
obtained from females subjected to the action of dichloroethane.] Gig
i Sanit, 39: 25-28 (in Russian).
Vozovaya MA (1977) [The effect of dichloroethane on the sexual cycle
and embryogenesis of experimental animals.] Aksuk Ginekol (Moscow),
2: 57-59 (in Russian).
Walbridge CR, Fiandt JT, Phipps GL, & Holcombe GW (1983) Acute
toxicity of ten chlorinated aliphatic hydrocarbons to the fathead
minnow (Pimephales promelas). Arch Environ Contam Toxicol,
12: 661-666.
Wallace LA (1986) Personal exposures, indoor and outdoor air
concentrations, and exhaled breath concentrations of selected volatile
organic compounds measured for 600 residents of New Jersey, North
Dakota, North Carolina and California. Toxicol Environ Chem,
12: 215-236.
Wallace LA, Pellizzari E, Hartwell T, Rozenweig M, Erickson M,
Sparacino C, & Zelon H (1984) Personal exposure to volatile organic
compounds. I. Direct measurements in breathing-zone air, drinking
water, food and exhaled breath. Environ Res, 35: 293-319.
Wallace L, Pellizzari E, Leaderer B, Zelon H, & Sheldon L (1987)
Emissions of volatile organic compounds from building materials and
consumer products. Atmos Environ, 21(2): 385-393.
Ward JM (1980) The carcinogenicity of ethylene dichloride in
Osborne-Mendel rats and B6C3F1 mice. In: Ames BN, Infante P, & Reitz
R ed. Ethylene dichloride: A potential health risk? Cold Spring
Harbor, New York, Cold Spring Harbor Laboratory, pp 35-53 (Banbury
Report No. 5).
Ward RJ (1992) Ethylene dichloride: In vitro absorption through
human and rat epidermis. Alderley Park, United Kingdom, ICI Central
Toxicology Laboratory (Unpublished report).
Warner JM & Beasley RK (1984) Purge and trap chromatographic method
for the determination of acrylonitrile, chlorobenzene,
1,2-dichloroethane and ethylbenzene in aqueous samples. Anal Chem,
56: 1953-1956.
Warner HP, Cohen JM, & Ireland JC (1987) Determination of Henry's Law
Constants of selected priority pollutants. Washington, DC, US
Environmental Protection Agency, Office of Science and Development
(EPA-600/D-87/229).
Weiss F (1957) [Lethal oral intoxications by dichloroethane.] Arch
Gewerbepathol Gewerbehyg, 15: 253-264 (in German).
WHO (1993) Guidelines for drinking-water quality. Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization.
Williams GM, Mori H, & McQueen CA (1989) Structure-activity
relationships in the rat hepatocyte DNA-repair test for 300 chemical.
Mutat Res, 221: 263-286.
Wilson JT, Enfield CG, Dunlap WJ, Cosby RL, Foster DA, & Baskin LB
(1981) Transport and fate of selected organic pollutants in a sandy
soil. J Environ Qual, 10(4): 501-506.
Wirtschafter ZT & Schwartz WD (1939) Acute ethylene dichloride
poisoning. J Ind Hyg Toxicol, 21: 126-131.
Wit SL, Besemer AFH, Das HA, Goedkoop W, Loosjes FE, & Meppelink EK
(1969) Toxicology. Bilthoven, The Netherlands, National Institute of
Public Health (Report No. 36/69).
Withey JR & Collins BT (1980) Chlorinated aliphatic hydrocarbons used
in the foods industry: the comparative pharmacokinetics of methylene
chloride, 1,2-dichloroethane, chloroform and trichloroethylene after
I.V. administration in the rat. J Environ Pathol Toxicol, 3: 313-332.
Withey JR & Karpinski K (1985) The fetal distribution of some
aliphatic chlorinated hydrocarbons in the rat after vapor phase
exposure. Biol Res Pregnancy Perinatol, 6: 79-88.
Withey JR, Collins BT, & Collins PG (1983) Effect of vehicle on the
pharmacokinetics and uptake of four halogenated hydrocarbons from the
gastrointestinal tract of the rat. J Appl Toxicol, 3: 249-253.
Wolff DL, Ivanov NG, Kljackina AM, & Mel'Nikova LV (1979) [Evidence of
the effect of significant industrial toxicological substances on the
nervous system by animal behavioural tests.] Zool Jahrb Physiol,
83: 82-91 (in German).
Worthing CR ed. (1991) The pesticide manual. Farnham, Surrey, The
British Crop Protection Council.
Yodaiken RE & Babcock JR (1973) 1,2-Dichloroethane poisoning. Arch
Environ Health, 26: 281-284.
Zamora PO, Benson JM, Li AP, & Brooks AL (1983) Evaluation of an
exposure system using cells grown on collagen gels for detecting
highly volatile mutagens in the CHD/HGPRT mutation assay. Environ
Mutagen, 5: 795-801.
Zoetman BCJ, Piet GJ, Slingerland P, Fonds AW, & Wegman RCC (1979)
[Investigation into the presence of volatile organic halogenated
compounds in groundwater used as a drinking water supply. Final
report: results on the period November (1976) to August (1978).]
Bilthoven, The Netherlands, National Institute of Drinking Water
Supply, National Institute of Public Health and Environmental Hygiene
(Reports Nos RID-CBH-79/01 and RIV 10/79) (in Dutch).
RESUME
1. Identité, propriétés physiques et chimiques, et méthodes d'analyse
Le 1,2-Dichloréthane (ou dichlorure d'éthylène) est un produit
chimique de synthèse qui se présente sous la forme d'un liquide
incolore à la température ambiante. Il est extrêmement volatil, avec
une tension de vapeur de 8,5 kPa à 20°C et il est soluble dans l'eau,
sa solubilité étant de 8690 mg/litre à 20°C. Son coefficient de
partage octanol/eau (log Kow) est égal à 1,76.
Le dosage du dichloréthane dans les différents compartiments de
l'environnement s'effectue généralement par chromatographie en phase
gazeuse, avec détection par capture d'électrons, ionisation de flamme
ou spectrométrie de masse. Les limites de détection vont de 0,016 à
> 4 µg/m3 dans l'air, de 0,001 à 4,7 µg/litre dans l'eau et de 6 à
10 µg/kg dans différentes denrées alimentaires.
2. Sources d'exposition humaine et environnementale
On utilise principalement le 1,2-dichloréthane pour la synthèse
du chlorure de vinyle monomère et dans une moindre mesure pour la
production de divers solvants chlorés. Il entre également dans la
composition des additifs antidétonants de l'essence (encore que cet
usage soit en déclin avec l'élimination progressive de l'essence au
plomb dans certains pays) et on l'utilise aussi pour des fumigations.
La production annuelle totale de 1,2-dichloréthane a été de 922
kilotonnes au Canada en 1990 et de 6318 kilotonnes aux Etats-Unis
d'Amérique en 1991.
3. Transport, distribution et transformation dans l'environnement
La majeure partie du 1,2-dichloréthane rejeté dans
l'environnement provient d'émissions dans l'atmosphère. Il
estmoyennement persistant dans l'air; sa durée de vie estimative dans
l'atmosphère est comprise entre 43 et 111 jours. Le dichloréthane est
transporté vers la stratosphère où, par photolyse, il peut donner
naissance à du chlore radicalaire qui peut à son tour réagir sur
l'ozone. Une partie du 1,2-dichloréthane rejeté dans les effluents
industriels peut passer dans le milieu aquatique dont il s'échappe
rapidement par volatilisation. Il peut également s'infiltrer
jusqu'aux nappes d'eau souterraines à proximité des zones de décharges
industrielles. On ne pense pas qu'il puisse subir une
bioconcentration chez les espèces aquatiques ou terrestres.
4. Concentrations dans l'environnement et exposition humaine
Des enquêtes récentes portant sur l'air ambiant de zones urbaines
non dominées par des sources polluantes ont permis de relever des
concentrations moyennes de 1,2-dichloréthane allant de 0,07 à
0,28 µg/m3, alors que dans l'air intérieur aux habitations des zones
résidentielles, ces valeurs moyennes vont de < 0,1 à 3,4 µg/m3.
Dans l'eau destinée à la consommation, les concentrations moyennes
sont généralement inférieures à 0,5 µg/litre. Lors de récentes
enquêtes, on a rarement décelé du 1,2-dichloréthane dans les denrées
alimentaires et comme il ne présente qu'un faible potentiel de
bioaccumulation, il est peu probable que la nourriture constitue une
source importante d'exposition à ce composé.
La valeur estimative de l'exposition moyenne au 1,2-dichloréthane
à partir de divers milieux montre que la source principale
d'exposition est constituée par l'air intérieur et extérieur, l'eau de
consommation n'y contribuant que pour une très faible part. L'apport
de 1,2-dichloréthane par la voie alimentaire est probablement
négligeable. Les quantités inhalées dans l'air ambiant pourraient
être plus importantes à proximité des sources industrielles.
5. Cinétique et métabolisme chez les animaux de laboratoire
Après inhalation, ingestion ou exposition par voie cutanée, le
1,2-dichloréthane est rapidement absorbé et il se répartit rapidement
et largement dans l'ensemble de l'organisme. Il est rapidement et
largement métabolisé chez le rat et la souris, principalement sous
forme de métabolites soufrés dont l'excrétion s'effectue par la voie
urinaire et dépend de la dose. A des niveaux d'exposition qui
entraînent des concentrations sanguines de 5 à 10 µg/ml, il semble
qu'il y ait saturation ou limitation du métabolisme chez le rat.
Après administration d'une dose unique de dichloréthane par gavage, on
a constaté que le taux d'alkylation de l'ADN était plus important que
lorsque le produit était inhalé sur une période de six heures.
Il existe semble-t-il deux voies principales de métabolisation.
La première est une oxydation saturable qui s'effectue au niveau des
microsomes par l'intermédiaire du cytochrome P-450 et aboutit au
2-chloracétaldéhyde et au 2-chloréthanol, pour s'achever sur une
conjugaison avec le glutathion. La deuxième voie métabolique comporte
une conjugaison directe avec le glutathion pour former du
S-(2-chloréthyl)-glutathion, qui est peut-être ensuite transformé
par voie non enzymatique en un ion glutathion-épitsulfonium
susceptible de former des adduits avec l'ADN. Bien qu'on ait pu
observer in vitro que la voie du P-450 conduisait à des lésions de
l'ADN, il semble bien qu'à cet égard, la voie impliquant la
conjugaison du glutathion soit la plus importante.
6. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Le 1,2-dichloréthane présente une faible toxicité aiguë pour les
animaux de laboratoire. Ainsi, la CL50 par inhalation pour des rats
exposés soit 6 soit 7,25 heures à ce composé allait de 4000 mg/m3 à
6600 mg/m3, la DL50 par voie orale pour le rat, la souris, le
chien et le lapin allant de 413 à 2500 mg/kg de poids corporel.
D'après les résultats d'études à court terme et d'études
subchroniques menées sur différentes espèces d'animaux de laboratoire,
c'est le foie et les reins qui sont les organes cibles; il n'a pas été
possible d'obtenir de valeurs pour la dose sans effets observables
(NOEL) ou La dose la plus faible provoquant un effet (LOEL),
généralement en raison d'une documentation insuffisante et du nombre
trop limité de paramètres biologiques examinés sur un trop petit
nombre d'animaux. Une série d'études limitées anciennes a révélé la
présence de modifications morphologiques au niveau du foie chez
plusieurs espèces après exposition subchronique à des concentrations
atmosphériques ne dépassant pas 800 mg/m3. Après administration
subchronique par voie orale de 1,2-dichloréthane à des doses
quotidiennes allant de 49 à 82 mg/kg de poids corporel ou davantage
pendant 13 semaines, on a observé chez des rats un accroissement du
poids relatif du foie. Les études de toxicité chronique dont on
possède les résultats ne donnent guère d'information sur les effets
non néoplasiques. Chez des rats exposés pendant 12 mois à des
concentrations atmosphériques de 1,2-dichloréthane ne dépassant pas
202 mg/m3, on a observé, au niveau des paramètres sériques, des
modifications indiquant une toxicité hépatique et rénale; toutefois,
aucun examen histopathologique n'a été pratiqué lors de cette étude.
Quelques épreuves limitées ont été effectuées sur des animaux de
laboratoire à la recherche d'une cancérogénicité éventuelle du
1,2-dichloréthane (ces études souffraient d'une trop faible durée
d'exposition et d'une forte mortalité parmi les animaux). Chez des
rats Sprague-Dawley et des souris Swiss exposés pendant 78 semaines à
des concentrations allant jusqu'à 607 mg/m3 et observés jusqu'à ce
qu'ils meurent spontanément, on n'a pas observé d'augmentation
significative dans l'incidence des tumeurs quel qu'en soit le type.
La mortalité était forte parmi les rats, mais sans rapport avec la
concentration du produit et on n'a pas tenu compte des différences de
mortalité entre les groupes pour corriger les taux d'incidence. Des
rattes Sprague-Dawley ont été exposées pendant deux ans à 200 mg/m3
de 1,2-dichloréthane et on a observé à cette occasion une augmentation
de l'incidence des adénomes et des fibroadénomes de la mère, qui
n'était toutefois pas significative; aucun autre effet toxique
attribuable au composé n'a été observé.
En revanche, on a observé, après ingestion, chez deux espèces,
des signes convaincants d'un accroissement de l'incidence tumorale.
Chez des rats Osborne-Mendel à qui l'on avait administré
quotidiennement par gavage pendant 78 semaines des doses de 47 ou
95 mg/kg (en moyenne pondérée par rapport au temps), on a observé une
augmentation significative de l'incidence des tumeurs de différentes
localisations (notamment des carcinomes spinocellulaires de l'estomac
(chez les mâles), des hémangiosarcomes (chez les mâles et les
femelles), des fibromes du tissu sous-cutané (chez les mâles) ainsi
que des adénocarcinomes et des fibroadénomes mammaires chez les
femelles. Chez des souris B6C3F1 à qui l'on avait administré
quotidiennement des doses de 97 ou 195 mg/kg de produit (en moyenne
pondérée par rapport au temps) (mâles) ou de 149 et 299 mg/kg
(femelles) par gavage sur 78 semaines, on a observé une augmentation
similaire de l'incidence des tumeurs de diverses localisations
(notamment des adénomes alvéolaires/bronchiolaires chez les mâles et
les femelles, des adénocarcinomes mammaires chez les femelles, des
polypes ou des sarcomes du stroma de l'endomètre (femelles) et des
carcinomes hépatocellulaires (mâles)).
Chez des souris femelles qui avaient été soumises respectivement
pendant 440 et 594 jours à des applications cutanées répétées de
1,2-dichloréthane, on a observé une incidence sensiblement accrue des
tumeurs pulmonaires (papillomes bénins). Chez une souche sensible de
souris, des injections intrapéritonéales répétées de 1,2-dichloréthane
ont déterminé un accroissement, lié à la dose, du nombre des adénomes
pulmonaires, mais cet accroissement n'était en aucun cas significatif.
Chez des rats à qui l'on faisait simultanément respirer du
1,2-dichloréthane et ingérer du disulfirame avec leur nourriture, on a
observé une incidence accrue des cholangiomes et des kystes dans la
partie intrahépatique des canaux biliaires, et davantage de fibromes
sous-cutanés, de nodules hépatiques malins, de tumeurs du tissu
testiculaire interstitiel et d'adénocarcinomes mammaires, que chez des
rats qui avaient reçu soit l'un, soit l'autre des composés ou aucun
des deux. Trois épreuves biologiques n'ont pas permis de mettre en
évidence une aptitude quelconque de ce composé à se comporter comme un
initiateur ou un promoteur tumoral, encore que l'examen
histopathologique effectué à la suite de ces études ait été de portée
limitée.
Lors d'épreuves de mutagénicité in vitro sur Salmonella
typhimurium, le 1,2-dichloréthane a toujours donné des résultats
positifs. L'effet était plus important en présence d'un système
d'activation exogène (sans doute du fait d'une activation par le
cytochrome) et on constatait que le pouvoir mutagène était plus que
doublé chez S. typhimurium exprimant le gène humain GSTA1-1. Le
1,2-dichloréthane forme des adduits avec l'ADN en cultures de cellules
mammaliennes. Il provoque également une synthèse non programmée de
l'ADN dans des cultures primaires de cellules murines et humaines
ainsi que des mutations géniques dans certaines lignées cellulaires.
On a trouvé une corrélation entre la fréquence des mutations observées
dans des lignées cellulaires humaines et la modification de l'activité
de la glutathion-S-transférase. Des études in vivo ont montré que
le 1,2-dichloréthane produisait des mutations létales récessives dans
les cellules somatiques et germinales de Drosophila melanogaster et
selon toutes les études publiées portant sur des rats et des souris,
il y a liaison du 1,2-dichloréthane à l'ADN. Des lésions directes de
l'ADN des cellules hépatiques ainsi que des échanges entre chromatides
soeurs ont été observés lors d'études sur la souris mais rien
n'indique que le 1,2-dichloréthane provoque la formation de
micronoyaux.
Rien n'indique, à en juger par les résultats d'un nombre limité
d'études, que le 1,2-dichloréthane soit tératogène pour les animaux de
laboratoire. Il n'y a également guère d'éléments en faveur d'effets
sur la reproduction ou le développement à des doses inférieures à
celles qui provoquent d'autres effets généraux. On ne dispose que de
données limitées sur l'immunotoxicité du 1,2-dichloréthane.
7. Effets sur l'homme
Des effets divers ont été observés à la suite d'expositions
accidentelles aiguës à du 1,2-dichloréthane par inhalation ou
ingestion: au niveau du système nerveux central, du foie, des reins,
des poumons et de l'appareil cardio-vasculaire.
On n'a pas beaucoup étudié le pouvoir cancérogène du
1,2-dichloréthane dans les populations humaines exposées. Chez un
groupe d'ouvriers d'un atelier de production de produits chimiques qui
avaient été exposés principalement à du 1,2-dichloréthane, à côté
d'autres substances, on a observé une augmentation significative de la
mortalité par cancer du pancréas. Cette mortalité augmentait avec la
durée de l'exposition. En outre, malgré un nombre limité de cas et
une association moins systématique avec la durée de l'exposition, il y
avait également accroissement de la mortalité par leucémie chez ces
travailleurs. Une petite étude cas-témoins, portant sur l'exposition
à du 1,2-dichloréthane n'a pas permis de mettre en évidence une
corrélation avec l'apparition de tumeurs cérébrales. Une étude
écologique intrinsèquement limitée, portant sur la présence de
1,2-dichloréthane dans de l'eau de consommation, a mis en évidence une
augmentation de l'incidence des cancers colo-rectaux mais il est
possible qu'une exposition simultanée à d'autres substances explique
pour une part les effets observés.
8. Effets sur les organismes non visés au laboratoire et dans leur
milieu naturel
On a étudié les effets d'une exposition au 1,2-dichloréthane sur
un certain nombre d'autres organismes tant au laboratoire que dans
leur milieu naturel. En ce qui concerne les microorganismes
aquatiques, les valeurs de la CI50 et de la CE50 correspondant à
divers paramètres biotoxicologiques vont de 25 à 770 mg/litre. La
valeur de la CL50 la plus faible qui ait été observée pour les
daphnies était de 220 mg/litre, des effets ayant été toutefois
observés sur la fécondité et la croissance aux concentrations
respectives de 20,7 et 71,7 mg/litre. En s'appuyant sur les données
disponibles, on constate que le vertébré d'eau douce le plus sensible
au 1,2-dichloréthane est une espèce de salamandre (Ambystoma gracile),
chez laquelle la survie des larves à neuf jours (quatre jours après
l'éclosion) a accusé une chute à 2,54 mg/litre. On ne possède que des
données limitées sur la toxicité du 1,2-dichloréthane pour les
organismes terrestres.
Resumen
1. Identidad, propiedades físicas y químicas y métodos analíticos
El 1,2-dicloroetano (dicloruro de etileno), producto químico
sintético, es un líquido incoloro a temperatura ambiente. Es también
muy volátil, con una presión de vapor de 8,5 kPa (a 20°C), y soluble
en agua, con una solubilidad de 8690 mg/litro (a 20°C). El log del
coeficiente de reparto octanol/agua es de 1,76.
El análisis del 1,2-dicloroetano en el medio ambiente se realiza
habitualmente por cromatografía de gases, en combinación con la
captura de electrones, la detección de ionización por conductor o bien
la espectrometría de masas. Los límites de la detección oscilan entre
0,016 y > 4 µg/m3 en el aire, entre 0,001 y 4,7 µg/litro en el agua
y entre 6 y 10 µg/kg en diversos productos alimenticios.
2. Fuentes de exposición humana y ambiental
El 1,2-dicloroetano se utiliza principalmente en la síntesis del
monómero cloruro de vinilo y, en menor grado, en la fabricación de
diversos disolventes clorados. Se incorpora también a los aditivos
antidetonantes de la gasolina (aunque este empleo está disminuyendo
con la reducción progresiva en muchos países de la gasolina con plomo)
y se ha usado como fumigante. La producción anual total de
1,2-dicloroetano en el Canadá en 1990 y en los Estados Unidos en 1991
fue de 922 000 y 6 318 000 toneladas, respectivamente.
3. Transporte, distribución y transformación en el medio ambiente
La mayor parte del 1,2-dicloroetano liberado en el medio ambiente
ha sido emitido en el aire. En este medio es moderadamente
persistente; su permanencia en la atmósfera se estima entre 43 y 111
días. Es transportado a la estratosfera, donde, por fotólisis, pueden
producirse radicales de cloro que a su vez pueden reaccionar con el
ozono. Cierta cantidad de 1,2-dicloroetano puede escapar a los
efluentes industriales y de allí pasar al medio ambiente acuático, de
donde desaparece rápidamente por volatilización. También puede
alcanzar por lixiviación las aguas subterráneas próximas a los
vertederos de desechos industriales. No se prevé su bioconcentración
en especies acuáticas o terrestres.
4. Niveles medioambientales y exposición humana
Según estudios recientes del aire ambiental, las concentraciones
medias de 1,2-dicloroetano detectadas en zonas urbanas donde no
abundan sus fuentes de emisión oscilan entre 0,07 y 0,28 µg/m3,
mientras que los niveles medios notificados como presentes en el aire
del interior de las viviendas oscilan entre < 0,1 y 3,4 µg/m3. En
el agua potable, la concentración media suele ser menor de
0,5 µg/litro. En estudios recientes rara vez se ha detectado la
presencia de 1,2-dicloroetano en alimentos y, puesto que el potencial
de bioacumulación de esta sustancia es bajo, los alimentos
probablemente no representen una fuente de exposición importante.
Teniendo en cuenta las estimaciones de la exposición media en
diversos entornos, la principal fuente de exposición de la población
general al 1,2-dicloroetano es el aire de locales cerrados y el aire
exterior, mientras que el agua de bebida contribuye sólo en cantidades
muy pequeñas. La ingestión de 1,2-dicloroetano con los alimentos
probablemente sea insignificante. La cantidad inhalada con el aire
ambiental puede ser más grande en las proximidades de fuentes de
emisión industriales.
5. Cinética y metabolismo en animales de laboratorio
El 1,2-dicloroetano se absorbe fácilmente tras la inhalación, la
ingestión o la exposición cutánea y se distribuye rápida y ampliamente
por todo el organismo. En la rata y el ratón se metaboliza de forma
rápida y completa y por orina se eliminan principalmente metabolitos
azufrados en concentraciones que dependen de la dosis. En la rata, el
metabolismo parece saturado o limitado cuando la exposición alcanza
niveles que dan lugar a concentraciones sanguíneas de 5 a 10 µg/ml.
Después de la administración por sonda de una dosis única, los niveles
de alquilación del ADN eran más elevados que después de la inhalación
durante un periodo de seis horas.
El 1,2-dicloroetano parece metabolizarse siguiendo dos vías
principales: la primera comporta una oxidación microsómica saturable
mediada por el citocromo P-450, que produce 2-cloroacetaldehído y
2-cloroetanol, seguida de conjugación con el glutatión. La segunda
vía entraña la conjugación directa con el glutatión para formar
S-(2-cloroetil)-glutatión, que puede convertirse mediante un proceso
no enzimático en un ion glutatión episulfonio; este ion puede formar
aductos con el ADN. Aunque se han inducido daños en el ADN por la
ruta del P-450 in vitro, hay varias pruebas de que la vía de la
conjugación del glutatión probablemente sea más importante que la otra
en cuanto a los daños que causa en el ADN.
6. Efectos en mamíferos de laboratorio y en sistemas de ensayo
in vitro
La toxicidad aguda del 1,2-cicloroetano en animales de
experimentación es baja. Por ejemplo, la CL50 por inhalación en
ratas expuestas durante 6 ó 7,25 horas oscilaba entre 4000 mg/m3 y
6600 mg/m3, mientras que la DL50 por vía oral en ratas, ratones,
perros y conejos variaba entre 413 y 2500 mg/kg de peso corporal.
Los resultados de estudios de corta duración y subcrónicos
realizados en varias especies de animales de experimentación indican
que los órganos afectados son el hígado y los riñones; en general no
se obtuvieron NOEL ni LOEL fidedignos debido a la documentación
insuficiente y a la gama limitada de puntos finales examinados en
pequeños grupos de animales. En una serie de estudios iniciales
limitados, se observaron cambios morfológicos en el hígado de varias
especies tras la exposición subcrónica a concentraciones de
1,2-dicloroetano en el aire de sólo 800 mg/m3. Tras la
administración subcrónica a ratas por vía oral de dosis comprendidas
entre 49 y 82 mg/kg de peso corporal por día durante más de 13 semanas
se observó un incremento del peso relativo del hígado. En los
estudios crónicos disponibles se ha presentado poca información sobre
efectos no neoplásicos. Las ratas expuestas a concentraciones de sólo
202 mg/m3 en el aire durante 12 meses acusaron en los parámetros del
suero cambios indicativos de toxicidad hepática y renal, pero en ese
estudio no se realizaron exámenes histopatológicos.
La carcinogenicidad del 1,2-dicloroetano se ha investigado en un
pequeño número de biovaloraciones limitadas sobre animales de
experimentación (las limitaciones comprenden una exposición de corta
duración y una mortalidad alta). No se notificaron aumentos
significativos en la incidencia de ningún tipo de tumor en ratas
Sprague-Dawley o en ratones suizos expuestos a concentraciones de
hasta 607 mg/m3 durante 78 semanas; los animales se observaron hasta
que se produjo su muerte espontánea. En este estudio, la mortalidad
de las ratas fue elevada, pero no guardaba relación con la
concentración, y no se realizó un ajuste de la incidencia en función
de la mortalidad diferencial entre los grupos. En un ensayo con ratas
Sprague-Dawley hembra expuestas durante dos años a 200 mg/m3, se
produjo un aumento no significativo de la incidencia de adenomas y
fibroadenomas en las glándulas mamarias, y no se observó otra forma de
toxicidad relacionada con el compuesto.
En cambio, hay pruebas convincentes de un aumento de la
incidencia de tumores en dos especies tras la ingestión del producto.
Las ratas Osborne-Mendel que habían recibido mediante sonda durante 78
semanas dosis diarias de 47 ó 95 mg/kg de peso corporal (promedio
ponderado en función del tiempo) mostraron un aumento significativo de
la incidencia de tumores en diversos lugares (por ejemplo, carcinomas
escamosos del estómago en machos, hemangiosarcomas en machos y
hembras, fibromas del tejido subcutáneo en machos, adenocarcinomas y
fibroadenomas de las glándulas mamarias en hembras. En ratones
B6C3F1 que habían recibido, como promedio ponderado en función del
tiempo, dosis diarias de 97 ó 195 mg/kg de peso corporal (machos) y
149 ó 299 mg/kg de peso corporal (hembras) mediante sonda durante 78
semanas se observaron aumentos semejantes de la incidencia de tumores
en múltiples lugares (adenomas alveolares/bronquiolares en machos y
hembras, adenocarcinomas de las glándulas mamarias en hembras y pólipo
estromal endometrial o sarcoma estromal endometrial combinados en
hembras y carcinomas hepatocelulares en machos).
La incidencia de tumores pulmonares (papilomas benignos)
aumentó significativamente en ratones hembra tras la aplicación
cutánea repetida de 1,2-dicloroetano durante 440 a 594 días. La
administración intraperitoneal repetida produjo en una raza
susceptible aumentos en el número de adenomas pulmonares por ratón;
dichos aumentos estaban relacionados con la dosis, pero ninguno de
ellos fue significativo. En comparación con las ratas que recibieron
el compuesto solo y con el grupo testigo, que no recibió ningún
tratamiento, las ratas expuestas simultáneamente a 1,2-dicloroetano
por inhalación y a disulfiram en los alimentos acusaron una mayor
incidencia de colangiomas y de quistes en el conducto biliar
intrahepático, de fibromas subcutáneos, de nódulos neoplásicos en el
hígado, de tumores de las células intersticiales de los testículos y
de adenocarcinomas mamarios. En tres biovaloraciones realizadas no se
observaron indicios de potencial iniciador o facilitador del
desarrollo de tumores, pero el alcance del examen histopatológico de
esos estudios fue limitado.
In vitro, el 1,2-dicloroetano ha dado constantemente resultados
positivos en biovaloraciones de mutagenicidad en Salmonella
typhimurium. Las respuestas han sido mayores en presencia de un
sistema de activación exógeno (posiblemente debido a la activación por
el sistema del citocromo) que en su ausencia; la mutagenicidad se
duplicó con creces en la cepa de S. typhimurium que contenía el gen
humano GSTA1-1. En cultivos de células mamarias, el 1,2-dicloroetano
forma aductos con el ADN. También induce síntesis no programada del
ADN en cultivos primarios de células de roedores y humanas y mutación
génica en varias líneas celulares. La frecuencia de las mutaciones en
las líneas celulares humanas se ha correlacionado con diferencias en
la actividad de la glutatión-S-transferasa. En estudios in vivo,
el 1,2-dicloroetano indujo mutaciones en células somáticas y
mutaciones letales recesivas ligadas al sexo en Drosophila
melanogaster y, según todos los estudios realizados en ratas y
ratones, el compuesto se había unido al ADN. Aunque en estudios
efectuados en ratones se han observado lesiones primarias del ADN en
el hígado e intercambio de cromátides hermanas, no hay indicaciones de
inducción de micronúcleos.
Teniendo en cuenta los resultados de un número limitado de
estudios, no hay pruebas de que el 1,2-dicloroetano sea teratogénico
en animales de experimentación. Además, hay pocas pruebas
convincentes de que tenga efectos sobre la reproducción o el
desarrollo con dosis inferiores a las que causan otros efectos
sistémicos. Los datos disponibles sobre la inmunotoxicidad del
1,2-dicloroetano son limitados.
7. Efectos en el ser humano
La exposición accidental aguda al 1,2-dicloroetano por inhalación
o por ingestión ha producido diversos efectos en el ser humano, por
ejemplo en el sistema nervioso central, el hígado, el riñón, el pulmón
y el sistema cardiovascular.
No se ha estudiado con detenimiento la carcinogenicidad potencial
del 1,2-dicloroetano en poblaciones humanas expuestas. La mortalidad
por cáncer pancreático aumentó de forma significativa en un grupo de
trabajadores de una planta de producción química que había estado
expuesto sobre todo a 1,2-dicloroetano (en combinación con otros
productos químicos). La mortalidad por cáncer pancreático aumentó con
la duración de la exposición. Además, aunque el número de casos fue
pequeño y la relación con la duración de la exposición menos
constante, en estos trabajadores también aumentó la mortalidad por
leucemia. No se observó relación entre la exposición ocupacional al
1,2-dicloroetano y el cáncer cerebral en un pequeño estudio de casos y
controles. Si bien en un estudio ecológico con limitaciones
inherentes se observó que la incidencia de cáncer de colon y de recto
aumentaba con la concentración del producto en el agua de bebida, la
exposición simultánea a otras sustancias podría haber contribuido a
producir los efectos observados.
8. Efectos en organismos no destinatarios en el laboratorio y
sobre el terreno
Se han investigado los efectos de la exposición al
1,2-dicloroetano en varios otros organismos en el laboratorio y en el
medio ambiente. Con respecto a los microorganismos acuáticos, se ha
informado de que las CI50 y CE50 correspondientes a diversos
puntos finales oscilan entre 25 y 770 mg/litro. La CL50 más baja
notificada para Daphnia fue de 220 mg/litro, mientras que se
detectaron efectos en el éxito reproductivo y el crecimiento a 20,7 y
71,7 mg/litro, respectivamente. Teniendo en cuenta los datos
disponibles, la especie de vertebrados de agua dulce más sensible
parece ser la salamandra noroccidental (Ambystoma gracile), cuyas
larvas de nueve días (cuatro días después de la eclosión) vieron
reducida su supervivencia a concentraciones de 2,54 mg/litro. Se
dispone sólo de datos limitados sobre la toxicidad del
1,2-dicloroetano en organismos terrestres.
Although some DNA damage has been induced via the P-450 pathway
in vitro (Banerjee et al., 1980; Guengerich et al., 1980; Lin et
al., 1985), several lines of evidence suggest that the GSH conjugation
pathway is probably of greater significance than the P-450 pathway as
the major route for DNA damage (Guengerich et al., 1980; Rannug, 1980;
Sundheimer et al., 1982; Inskeep et al., 1986; Koga et al., 1986).
The P-450-dependent pathway can, however, presumably form
considerable quantities of 2-haloacetaldehydes, which readily bind to
protein and non-protein thiols, as shown for vinyl bromide and vinyl
chloride (Guengerich et al., 1981) and dibromoethane (DBE) (van
Bladeren et al., 1981). However, these authors concluded that 2H
and 18O studies on the formation of 2-haloethanols and
2-haloacetaldehydes from 1,2-dihaloethanes are inconsistent with a
major role of such a mechanism for DNA damage (Guengerich et al.,
1986; Koga et al., 1986).
The 1,2-dichloroethane-induced mutation frequency of two human
cell lines has been correlated with the difference in levels of
glutathione- S-transferase activities. AHH-1 cell line mutation
frequency was 25 times that in the TK6 cell line in the presence of
1,2-dichloroethane. The difference was attributed to the fact that
the AHH-1 cell line possesses 5 times more glutathione- S-transferase
activity than the TK6 cell line (Crespi et al., 1985).
Moreover, although the significance of the reported results is
uncertain, the results of an additional study by Storer & Conolly
(1985) are not inconsistent with the hypothesis that reduction of GSH
levels is associated with a reduction in DNA damage. Male B6C3F1
mice pretreated with piperonyl butoxide (PIB), a P-450 inhibitor, were
examined for the extent of hepatic DNA damage produced 4 h after
1,2-dichloroethane administration. Hepatic DNA damage, as measured by
alkali-labile lesions, was potentiated by PIB. Treatment of mice with
high doses of 2-chloroethanol failed to produce DNA damage, as
measured by this assay. Diethylmaleate, a GSH depletor, potentiated
the hepatotoxicity of 2-chloroethanol but not DNA damage.
In addition, Cheever et al. (1990) reported that although the
levels of hepatic DNA covalent binding of metabolites of
14C-1,2-dichloroethane injected (single dose) to rats which had been
exposed by inhalation to 1,2-dichloroethane in a long-term bioassay
were significant (p < 0.05), these levels were not different in rats
with concomitant exposure to disulfiram in the diet over two years.
Evidence suggests that the putative episulfonium ion, resulting
from non-enzymatic conversion of S-(2-chloroethyl) glutathione, is a
major intermediate in the formation of DNA adducts in vivo from
1,2-dichloroethane exposure (Inskeep et al., 1986). When rats were
administered single does of 14C-1,2-dichloroethane in vivo and the
liver was analysed 8 h later, 78% of the DNA adducts (0.25 nmol/mg
DNA) could be released by neutral thermal hydrolysis. A major adduct
and several minor adducts were present; the major adduct
co-chromatographed with S-[2-(N7-guanyl)ethyl] glutathione. The
postulated adduct of liver DNA after 14C-1,2-dichloroethane
exposure, S-[2-(N7-guanyl)ethyl] glutathione, appears to be
chromatographically identical to the major adduct in rats after
exposure to 1,2-dibromoethane (Koga et al., 1986). This
1,2-dibromoethane adduct, which has been isolated and characterized by
NMR and mass spectrometry, gives strong support to an identical adduct
being the principal DNA adduct from exposure to 1,2-dihaloethanes.
Reitz et al. (1982) found (based on consideration of results of
their own work as well as that of Spreafico et al., 1980) that
metabolism of 1,2-dichloroethane appears to be saturated or limited in
rats at levels of exposure resulting in blood concentrations of 5 to
10 mg/litre, based on an observed non-linear relationship between
levels in blood and administered doses or concentrations.
Administration by gavage resulted in the formation of about twice the
amount of "total" metabolites as did exposure by inhalation, based on
recovery in excreta, expired air and the carcass. Oral exposure
produced 1.5- to 2-fold lower levels of total macromolecular binding
but 3- to 5-fold higher levels of DNA alkylation than inhalation,
though the absolute levels of DNA alkylation were considered low.
Based on examination of DNA binding in the liver and lung of rats
exposed by inhalation to a low constant concentration (0.3 mg/litre)
of 1,2-dichloroethane for 12 h or to a peak concentration (up to
18 mg/litre) for a few minutes, Baertsch et al. (1991) concluded that
DNA damage by 1,2-dichloroethane depends upon the concentration time
profile, with bolus doses causing disproportionately greater damage.
6.4 Elimination and excretion
Unmetabolized 1,2-dichloroethane is eliminated in expired air,
while its metabolites are largely excreted in the urine. Unchanged
1,2-dichloroethane was detected in the exhaled breath of women exposed
dermally and to airborne concentrations of 0.252 mg/m3 (0.063 ppm)
in the workplace; the amount of 1,2-dichloroethane expired was greater
immediately following exposure and decreased over time (Urusova,
1953).
A single dose of 150 mg/kg body weight radiolabelled
1,2-dichloroethane was injected into rats that had been exposed via
inhalation at a concentration of 200 mg/m3 (50 ppm) for 2 years. The
proportion of radioactivity present in the urine within 24 h was 42.5
and 33.9% (in males and females, respectively), while 27.3 and 40.3%
were eliminated as the unchanged parent compound in the breath. Only a
very small amount of radioactivity was detected as 14CO2 or in the
faeces. In rats that had been concomitantly exposed to disulfiram
during the 2-year period, the proportion of unchanged
1,2-dichloroethane eliminated in the breath increased significantly
(i.e. 57.6 and 57.7%; p < 0.05), while the proportion eliminated in
the urine decreased correspondingly (27.6 and 24.9%). Levels of
unchanged 1,2-dichloroethane in blood were significantly (p < 0.05)
increased in rats exposed to 1,2-dichloroethane and disulfiram
compared to those exposed to 1,2-dichloroethane alone (see section
7.10) (Cheever et al., 1990).
The pattern of elimination of metabolites was similar in rats and
mice 48 h after administration of oral doses of radiolabelled
1,2-dichloroethane (100 and 150 mg/kg body weight, respectively). In
rats, 8.2 and 69.51% of the radiolabelled dose was recovered as CO2
and in the excreta (principally urine), respectively, compared to
18.21 and 81.11% in mice. The overall recovery was less in rats than
in mice (96.26 versus 110.12%) (Mitoma et al., 1985).
In rats exposed to 600 mg/m3 (150 ppm) 1,2-dichloroethane for
6 h or administered 150 mg/kg body weight by gavage, there was no
significant difference in the route of excretion of non-volatile
metabolites. After 48 h, in each case, more than 84% of total
metabolites was eliminated in the urine, 7-8% was excreted as carbon
dioxide in expired air, 2% was excreted unchanged in the faeces, and
4% remained in the carcass (Reitz et al., 1982). The major urinary
metabolites identified following exposure of rats by either route were
thiodiacetic acid (70%) and thiodiacetic acid sulfoxide (26 to 28%).
The rate of elimination following oral (gavage) administration or
inhalation was such that 1,2-dichloroethane was not detected in the
blood a few hours after exposure and only small amounts were detected
in tissues (liver, kidney, lung, spleen, forestomach, stomach and
carcass) 48 h after exposure (Reitz et al., 1982). The rate of
elimination from blood and tissues appeared to depend on the exposure
level; the higher the exposure level, the lower the elimination rate
of 1,2-dichloroethane, after both oral and inhalation exposure.
Elimination from the liver was reported to be biphasic, a higher
elimination rate occurring just after the peak levels of
1,2-dichloroethane were reached. Elimination from other organs was
monophasic. Following inhalation up to an exposure level of
1012 mg/m3, elimination was slowest in adipose tissue and most rapid
in the lung (Spreafico et al., 1980).
Withey & Collins (1980) also reported that the elimination of
1,2-dichloroethane was dose-dependent. After intravenous
administration of from 3 to 15 mg/kg body weight to male Wistar rats,
the authors found that the elimination fitted a twocompartment model
at a low dose level and a three-compartment model at high dose levels.
The percentage of administered radioactivity excreted in the
urine over a 24-h period in rats decreased with increasing single
doses (0.25 to 8.08 mmol 1,2-dichloroethane/kg body weight)
administered by gavage in mineral oil (Payan et al., 1993). The
authors attributed these results to saturation of metabolism rather
than kidney damage, as there were no variations in biochemical
parameters of nephrotoxicity between the controls and groups exposed
to doses up to 4.04 mmol/kg body weight. Urinary thiodiglycolic acid
increased as a linear function of the dose of 1,2-dichloroethane until
at least 1.01 mmol/kg body weight; it accounted for 63% of the total
metabolites in urine at this dose.
6.5 Retention and bioaccumulation
Although 1,2-dichloroethane is eliminated more slowly from
adipose tissue than from blood or other tissues (lung and liver)
following exposure, it is unlikely to bioaccumulate significantly, as
no difference was observed between levels in blood or tissues (data
not presented) following single or repeated (10 days) oral doses of
50 mg/kg body weight in rats (Spreafico et al., 1980). Only 71 and
75%, respectively, of an administered oral dose of radiolabelled
1,2-dichloroethane was recovered in the excreta and exhaled breath of
rats administered 150 mg/kg body weight by gavage following 2 years of
exposure via inhalation (200 mg/m3 or 50 ppm); the authors
speculated that the remainder may have been sequestered in the body
fat. Recovery in the excreta and exhaled breath was complete in
younger rats (4 months old) receiving the same oral dose (Cheever et
al., 1990).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Data on the acute toxicity of 1,2-dichloroethane in experimental
animals are summarized in Table 3. These data indicate that
1,2-dichloroethane is of relatively low acute toxicity.
LC50 values in rats exposed to 1,2-dichloroethane for 6 or
7.25 h ranged from 4000 mg/m3 (1000 ppm) (Spencer et al., 1951) to
6600 mg/m3 (1650 ppm) (Bonnet et al., 1980). The 6-h LC50 in mice
was 1050 mg/m3 (Gradiski et al., 1978). LC50 values decreased
with increasing duration of exposure in rats exposed to concentrations
ranging from 1200 to 80 000 mg/m3 (300 to 20 000 ppm)
1,2-dichloroethane for 1 to 8 h (Spencer et al., 1951). Various
non-lethal effects have been reported in animals following acute
exposure to 1,2-dichloroethane, including central nervous system
depression, cardiovascular collapse, altered behaviour, pulmonary
congestion and oedema, histological damage in the liver, kidneys and
adrenal glands and myocardial failure, at concentrations ranging from
4000 mg/m3 for 1.5 or 4 h to 80 000 mg/m3 (20 000 ppm) for 30 min
(Heppel et al., 1945; Spencer et al., 1951; Alumot et al., 1976a;
Wolff et al., 1979; ATSDR, 1989). Central nervous system depression
occurred at much higher concentrations than those which induce effects
in other organs.
Oral LD50 values for rats, mice, dogs and rabbits ranged from
413 mg/kg body weight in female mice to 2500 mg/kg body weight in dogs
(Barsoum & Saad, 1934; McCollister et al., 1956; Smyth, 1969; Larionov
& Kokarovtseva, 1976; Munson et al., 1982; NIOSH, 1994). Non-lethal
effects observed in rats and rabbits following single oral doses of
1,2-dichloroethane ranging from 615 to 1476 mg/kg body weight include
hepatic effects (fatty degeneration, cloudy swelling, congestion,
haemorrhagic lesions, dystrophy in the cytoplasm and hyperchromatosis
in the nuclei of hepatocytes), degeneration of the renal tubular
epithelium, altered levels of enzymes in the serum and liver, oedema
and haemorrhaging in the walls of the coronary vessels, stasis and
thrombi in the myocardium, altered fibrinolytic activity in the blood,
and altered haematological parameters. A single dose of 0.5 ml
altered the ratio of the oxidized and reduced forms of nicotinamide
coenzymes in the liver and myocardium of rats (Natsyuk & Chekman,
1975). Electrocardiographic changes were reported at doses of 1, 1.5
and 2 mg/kg body weight, although these effects have not been
confirmed in other studies (Saitanov & Arsenieva, 1969).
The LD50 for dermal exposure in rabbits was estimated to be
between 2.8 and 4.9 g/kg (Torkelson & Rowe, 1981; NIOSH,
1994).
Table 3. Acute toxicity of 1,2-dichloroethane in experimental animals
Species Numbers/sex Duration/vehicle LC50 or LD50 Reference
Inhalation
Rats (Wistar equal no. of m & f) 10-54 0.53 h 48 000 mg/m3 (12 000 ppm) Spencer et al. (1951)
20-51 2.75 h 12 000 mg/m3 (3000 ppm)
31-32 7.20 h 4000 mg/m3 (1000 ppm)
Rats (albino, strain, number and sex not not specified 30 000 mg/m3 Nevrotsky et al. (1971)
specified)
Rats (Sprague-Dawley, 12 per group, sex not 6 h 6600 mg/m3 (1646 ppm) Bonnet et al. (1980)
specified)
Mice (OF1, 20 f per group) 6 h 1050 mg/m3 (262 ppm) Gradiski et al. (1978)
Ingestion
Rats (strain, number and sex not specified) not specified 850 mg/kg body weight Larionov &
Kokarovtseva (1976)
Rats (6 per group, strain and sex not not specified 770 mg/kg body weight Smyth (1969)
specified)
Rats (young adult albino, 80 m & f) corn oil 680 mg/kg body weight McCollister et al. (1956)
Mice, 6-week old (CD-1, number not male water 489 mg/kg body weight Munson et al. (1982)
specified) female 413 mg/kg body weight
Table 3. (cont'd).
Species Numbers/sex Duration/vehicle LC50 or LD50 Reference
Dogs (strain, number and sex not specified) acacia gum 2500 mg/kg body weight Barsoum & Saad (1934)
Rabbits (strain, number and sex not not specified 860 mg/kg body weight NIOSH (1994)
specified)
Dermal
Rabbits (strain, number and sex not not specified 2800 mg/kg body weight NIOSH (1994)
specified)
Rabbits (strain, number and sex not olive oil; duration 2800-4900 mg/kg body weight Torkelson & Rowe
specified) and area of skin (1981)
exposed not
specified
7.2 Skin and eye irritation
When 1.0 ml undiluted 1,2-dichloroethane was applied directly to
the clipped skin of guinea-pigs for up to 12 h in occluded patch
tests, no gross skin reactions were visible (Jakobson et al., 1982).
Microscopic changes appeared 4 h after application, comprising
karyopyknosis, perinuclear oedema, spongiosis and junctional
separation (Kronevi et al., 1981). In Draize tests on rabbits,
moderate erythema and oedema were observed 24 h after
application (dose not specified). Microscopy on the third day
revealed necrosis and other lesions such as ulcerations and
acanthosis. The severity of the changes was not indicated (Duprat et
al., 1976).
Instillation of 0.1 ml undiluted 1,2-dichloroethane into the
conjunctival sac of the eye of rabbits generated reversible, mild
irritation characterized by conjunctivitis and epithelial abrasion.
Epithelial keratitis, described as being "in a state of repair", was
observed microscopically 7 days after application (Duprat et al.,
1976). Reversible clouding of the cornea was observed in dogs within
10 h of subcutaneous administration of undiluted 1,2-dichloroethane at
0.9 mg/kg body weight. The clouding continued up to 48 h, but the
corneas appeared clear after 5 days. Histological changes, including
necrosis of the corneal endothelium, partially denuded Descemet's
membrane, formation of excess basement membrane, and swelling of the
corneal stroma, were also observed in dogs, cats and rabbits after
ocular injection of 1.8 mg 1,2-dichloroethane (0.15 ml of a 1%
solution) into the anterior chamber (Kuwabara et al., 1968).
7.3 Short-term exposure
Small groups of Wistar rats, rabbits, guinea-pigs, dogs and pigs
(n = 1 to 21) were exposed to 6000 mg/m3 (1500 ppm)
1,2-dichloroethane, 7 h/day for 6 days. Sections of the liver, heart,
lungs, kidney adrenal glands and spleen were examined microscopically.
In most animals, degeneration or necrosis of the kidney and liver,
along with congestion and haemorrhage of the lungs and adrenal glands,
were observed (Heppel et al., 1945).
No significant changes in organ or body weights, histology or
clinical chemistry and haematological parameters were observed in rats
administered 1,2-dichloroethane doses of up to 150 mg/kg body weight
per day in corn oil by gavage, 5 times/week for 2 weeks (van Esch et
al., 1977; Reitz et al., 1982).
7.4 Subchronic exposure
7.4.1 Inhalation
The subchronic toxicity of inhaled 1,2-dichloroethane was
investigated in three early limited studies in multiple species, as
presented in Table 4. Heppel et al. (1946) exposed groups of rats,
mice, rabbits, guinea-pigs, dogs, cats and monkeys to 4000 mg/m3
(1000 ppm) for up to 66 days. Mice, rats, rabbits and guinea-pigs
were the most sensitive species, based on mortality after only one or
a few exposures. Various effects were noted in these animals,
including pulmonary congestion (guinea-pigs, cats and rats), fatty
changes in the kidney (rats and monkeys), fatty changes in the
livera (cats, dogs and monkeys) and clouded corneas (dogs). At
1600 mg/m3 (400 ppm), observed effects included fatty degeneration
of the liver, kidney or heart (guinea-pigs and one rat), fatty changes
in the liver (dogs) and pulmonary congestion (rats), while at
800 mg/m3 (200 ppm), rats and guinea-pigs had mild pulmonary
congestion and one rat had fatty degeneration in the kidneys. No
effects on growth were noted in mice and rats exposed to 400 mg/m3
(100 ppm).
In a similar study, rats, guinea-pigs, rabbits and monkeys were
exposed to 400, 800 or 1600 mg/m3 (100, 200 or 400 ppm)
1,2-dichloroethane for 6 to 9 months (Spencer et al., 1951). Severe
effects, including hepatotoxicity, and deaths were observed in rats
and guinea-pigs exposed at the highest level, while monkeys also
showed degeneration of the liver and kidneys at this concentration.
No effects were noted in rabbits. At 800 mg/m3 (200 ppm) no adverse
effects were observed in rats, but slight degeneration of the liver
was noted in guinea-pigs. At 400 mg/m3 (100 ppm), no adverse
effects were observed in any of the four species. The authors
considered the "maximum concentrations without adverse effects" to be
1600 mg/m3 (400 ppm) in the rabbit, 800 mg/m3 (200 ppm) in the
rat, and 400 mg/m3 (100 ppm) in the monkey and guinea-pig, based on
a limited range of end-points.
a It has been suggested, on the basis of in vitro investigations,
that fatty accumulation in the liver may be due to the ability of
1,2-dichloroethane to block the secretion of hepatocellular
(Cotalasso et al., 1994).
Table 4. Subchronic toxicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats (26, strain and animals were exposed to 0 or There was high mortality in exposed rats (20/26), rabbits (5/6) and Heppel
sex not specified) 4000 mg/m3 (0 or 1000 ppm), guinea-pigs (36/41) after a few exposures. All mice died after one et al.
Mice (22, strain and 7 h/day, 5 days/week for up exposure. Survival was higher among cats and dogs (4/6 of either (1946)
sex not specified) to 66 exposures; sections of species survived more than 23 exposures). One monkey died after 2
Rabbits (5 m & 1 f, liver, heart, lungs, kidney, days and the other after 32 exposures. Pulmonary congestion was
strain not specified) adrenal glands and spleen noted in guinea-pigs, cats and rats. Rats and monkeys had fatty
Guinea-pigs (10-16, strain were examined changes in the kidney. Cats and monkeys had fatty changes in the
and sex not specified) microscopically; haematological liver. Dogs had cloudy corneas; one dog had fatty degeneration of
Dogs (3 f, strain not and urinary parameters were the liver. No effects on haematological or urinary parameters were
specified) assessed in dogs observed in dogs. Rabbits had no obvious effects on postmortem.
Cats (6 f, strain not
specified)
Monkeys (Rhesus, 2,
sex not specified)
Rats (15 m & 1 f, animals were exposed to 0 or All dogs and puppies survived 177 exposures. All rabbits died, after Heppel
strain not specified) 1600 mg/m3 (0 or 400 ppm), 1 to 97 exposures; 14/20 and 9/16 guinea-pigs and rats died by the et al.
Rabbits (2 m & 3 f, 7 h/day, 5 days/week for up to 60th exposure. Rats had pulmonary congestion and 1 rat and 6 (1946)
strain not specified) 177 exposures; sections of guinea-pigs had fatty degeneration of the liver, kidney and heart.
Guinea-pigs (8-10 m & liver, heart, lungs, kidney, Dogs had slight fatty changes in the liver. No effects were noted in
2 f, strain not specified) adrenal glands and spleen were rabbits on postmortem. There were no significant differences in
Dogs (6 f, strain not examined microscopically; haematological parameters in exposed dogs and rabbits compared
specified) and puppies haematological parameters to controls.
(3 m, strain not were also assessed in dogs
specified) and rabbits
Table 4. (cont'd).
Species Protocol Results Reference
Rats (Wistar 1 m & 11 f) animals were exposed to 0 or 5/14 guinea-pigs, 7/12 rats and 8/12 rats died after 1 to 115 Heppel
Osborne-Mendel rats (12 m) 800 mg/m3 (0 or 200 ppm), 7 h/ exposures. Rats and guinea-pigs had mild pulmonary congestion, et al.
Rabbits (5 m, strain day, 5 days/week, for up to and 1 rat had fatty degeneration in the kidneys. There were no (1946)
not specified) 125 exposures; sections of significant differences in haematological parameters in exposed
Guinea-pigs (12 m & 2 f, liver, heart, lungs, kidney, rats and rabbits, compared to controls.
strain not specified) adrenal glands and spleen
Monkeys (2 m, strain were examined microscopically;
not specified) haematological parameters were
assessed in rats and rabbits
Rats (23 m & 16 f, animals were exposed to 0 or All animals survived. There were no differences in the rate of Heppel
strain not specified) 400 mg/m3 (0 or 100 ppm), 7 h/ growth in rats or mice. et al.
Mice (19, strain and day, 5 days/week for 74 (rats) (1946)
sex not specified) and 19 (mice) exposures;
sections of liver, heart,
lungs, kidney, adrenal glands
and spleen were examined
microscopically
Rats (Wistar, 15-20 m & animals were exposed to 0, 400, 1600 mg/m3: All female rats and male guinea-pigs died by 10 Spencer
15-20 f) 800 or 1600 mg/m3 (0, 100, 200, exposures; all male rats died by 40 exposures. All female et al.
Guinea-pigs (2-8 m & or 400 ppm), 7 h/day, 5 days/ guinea-pigs died by 24 exposures. Rats and guinea-pigs had rapid body (1951)
8 m, strain not specified) week for 6 to 9 months; animals weight loss, slight increases in liver and kidney weights, and
Rabbits (albino, number were killed at various times some fatty changes in the liver. Guinea-pigs had swelling of the
and sex not specified) throughout the study; tubular epithelium of the kidneys and alterations in levels of
Monkeys (Rhesus, number haematological parameters were non-protein nitrogen urea nitrogen in the blood. Monkeys had
and sex not specified) assessed and histopathological degeneration of the liver and kidneys and increased fat content
examinations of several tissues of the liver. No effects were noted in rabbits.
were conducted
Table 4. (cont'd).
Species Protocol Results Reference
800 mg/m3: Guinea-pigs tolerated up to 180 exposures (246 days).
Male guinea-pigs had significantly (p=0.001) decreased body
weight gain; both sexes of guinea-pigs had slight degeneration
and fatty accumulation in the liver. Rats tolerated up to 151
exposures (212 days) with no observed adverse effects.
400 mg/m3: No effects were noted in rats, guinea-pigs, rabbits
or monkeys.
Rats (Sprague-Dawley, were exposed to 400 At the higher concentration, rats were dyspnoeic and guinea-pigs Hofman
10, sex not specified) or 2000 mg/m3 (100 or 500 were "apathetic". 3/4 rabbits died after 10-17 exposures; 9/10 et al.
Guinea-pigs (10, strain ppm), 6 h/day, 5 days/week guinea-pigs died after 4-14 exposures. Rats died after 1-5 exposures, (1971)
and sex not specified) for up to 17 weeks; while all cats survived 30 exposures. Rats had pulmonary hyperaemia
Rabbits (4, strain and histological examinations were and oedema, fatty liver and adrenal and myocardial necrosis. Cats and
sex not specified) conducted, and several rabbits had heart lesions, and guinea-pigs had fatty changes in the
Cats (2 per group, strain bio-chemical parameters were myocardium, liver and adrenals, and necrosis in the myocardium and
and sex not specified) assessed liver. At 400 mg/m3, there were no clinical or histological
changes after exposure for 17 weeks.
Table 4. (cont'd).
Species Protocol Results Reference
Ingestion
Rats F344/N, animals were administered 0, Drinking-water: F344/N rats: Body weight was significantly decreased NTP
Sprague-Dawley and 500, 1000, 2000, 4000 and in males at 4000 and 8000 mg/litre (>8%, p<0.001). Relative kidney (1991)
Osborne Mendel, mice, 8000 mg/litre in the weight was significantly increased at >1000 mg/litre in both sexes
B6C3F1 (10 or 20 m & f drinking-water (equivalent to (>11%, p<0.001). Relative liver weight was significantly increased at
per group) doses of of 49-82, 86-126, >2000 mg/litre in males (>14%, p<0.05) and 4000 mg/litre in females
146-213, 259-428, and (>9%, p<0.001). Alterations in haematological and serum parameters
492-727 mg/kg body weight per at the higher concentrations reflected dehydration caused by
day in rats and 244-249, decreased water consumption. Renal tubular "regeneration" observed
448-647, 781-1182, 2478-2710 in all groups of males at similar frequencies and severity, but
and 4207-4926 mg/kg body frequency was related to dose in females (0/10, 0/10, 1/10, 2/10
weight per day in mice) for 3/10 and 9/10 with increasing concentration).
13 weeks
Rats F344/N (10 or rats were administered 0, 30, Sprague-Dawley rats: Body weight was significantly decreased in
20 m & f per group) 60, 120, 240 or 480 mg/kg body males at 8000 ppm (9%, p<0.05). Relative kidney weight was
per day (males) or 0, 18, 37, significantly increased at >1000 mg/litre in males (>7%, p<0.05)
75, 150 or 300 mg/kg body and at >500 mg/litre in females (>8%, p<0.05). Relative liver
weight per day (females) in weight was increased in males at >500 mg/litre (>6%, p<0.05)
corn oil by gavage, and at 8000 mg/litre in females (13%, p<0.001). Alterations in
5 days/week for 13 weeks haematological and serum parameters at the higher
concentrations reflected dehydration caused by decreased water
consumption. Renal tubular "regeneration" was observed in all
groups at similar frequencies and severity.
Table 4. (cont'd).
Species Protocol Results Reference
haematological and serum Osborne-Mendel rats: Body weight was significantly decreased
chemistry parameters were at 8000 mg/litre in males (15%, p<0.05). Relative kidney weight
examined at several times was increased at 4000 and 8000 mg/litre in males (>8%, p<0.05)
during the course of the study. and >500 mg/litre in females (>12%, p<0.001). Relative liver weight
Extensive histopathological was increased in males at 1000 and 2000 mg/litre (>14%, p<0.05).
examinations were conducted Alterations in haematological and serum parameters at the higher
for controls and all animals concentrations reflected dehydration caused by decreased water
at the highest concentration consumption. Although the incidence of renal tubular "regeneration"
in drinking-water and in female was increased at the higher concentrations, the increases were
mice at 4000 ppm, as well as in not related to dose, and severity was similar in all groups.
male rats receiving doses of
120 or 240 mg/kg body weight B6C3F1 mice: 9/10 females exposed to 8000 mg/litre died. Body
per day by gavage and in weight was significantly decreased in males at 8000 mg/litre
female rats at 150 mg/kg body (17.5%, p<0.001). Relative kidney weight was significantly increased
weight per day in males at >1000 mg/litre (>12%, p<0.001) and in females at >500
mg/litre (>16%, p<0.001). Relative liver weight was significantly
increased in males at 500 mg/litre and above and in females at 1000
mg/litre or more. The incidence of renal tubular cell regeneration
was significantly increased in males at 4000 and 8000 mg/litre;
karyomegaly was observed in all males at 8000 mg/litre.
Gavage: F344/N rats: All males receiving 240 or 480 mg/kg body
weight per day and 9/10 female receiving 300 mg/kg body weight per
day died before the end of the study. The incidence of hyperplasia
and inflammation of the forestomach was significantly increased in
males at 240 mg/kg body weight per day; necrosis of the thymus was
observed in 10/10 males at 480 mg/kg body weight per day and 5/10
females at 300 mg/kg body weight per day, compared to none
in controls.
Table 4. (cont'd).
Species Protocol Results Reference
Rats (Osborne-Mendel, animals were administered At the highest dose, three males and one female died. In males, NCI
5 m & 5 f per group) doses of 0, 40, 63, 100, 159 or mean body weight was only significantly decreased (by 50%) at (1978)
251 mg/kg body weight per day 251 mg/kg body weight per day. In females mean body weight was
in corn oil by gavage, 5 days depressed (10% at 40 mg/kg body weight per day to 17% at 100
week for 6 weeks followed by a mg/kg body weight per day and 32% at 159 mg/kg body weight
2-week observation period per day). No other parameters were investigated.
Mice (B6C3F1 5 m & animals were administered All males receiving 398 mg/kg body weight per day and all females NCI
5 f per group) doses of 0, 159, 251, 398, receiving 631 mg/kg body weight per day died. Mean body weight (1978)
631 or 1000 mg/kg body weight depression was only observed in females receiving 398 mg/kg body
per day in corn oil by weight per day, in which "drastic weight loss" was reported. No
gavage, 5 days/week for other parameters were investigated.
6 weeks followed by a 2-week
observation period
Rats (6 per group, rats were fed mash fumigated The only effect noted, based on examination of lipid content Alumot
strain and sex not with 1,2-dichloroethane, of the liver and liver weight, was an increase in liver fat at et al.
specified) resulting in initial 1600 mg/kg. Chronic respiratory disease was evident in all exposure (1976a)
concentrations of 200 or groups (incidence data not presented, but the disease was not
600 mg/kg (approximately believed to be associated with exposure); mortality was higher in
equivalent to doses of 10 and males than females, with the number of rats surviving after 21
30 mg/kg body weight per day, months ranging from 3 to 14.
respectively) for 5 weeks, or
1600 mg/kg (approximately
equivalent to a dose of
80 mg/kg body weight per day)
for 7 weeks; the authors
noted that the dose ingested
was approximately 60 to 70% of
the initial amount after loss
Table 4. (cont'd).
Species Protocol Results Reference
to volatilization was
considered; lipid content of
liver and liver weight were
measured, and hepatic
triglycerides were measured at
the highest dose; no
histopathological examinations
were conducted
Rats (m & f, strain and rats were administered doses Decreased weight gain was observed at the two highest doses. Van Esch
number not specified of 0, 10, 30 or 90 mg/kg body Males and females had increased relative kidney weight at 90 et al.
in secondary account) weight per day (method of oral mg/kg body weight per day. Females also had increased relative (1977)
administration not specified weights of liver and brain at this dose. No effects on histology
in secondary account), 5 times or clinical chemistry were noted. Alterations in some
per week for 90 days; haematological parameters observed, although these did not occur
histopathological examinations in a dose-related manner.
were conducted, along with
assessment of haematological
and clinical chemistry
parameters, although the
extent of the examinations
was not specified in the
secondary account
Rats (m, strain and rats were orally There was a statistically significant (p < 0.02) increase in the Apostolov
number not specified) administered doses activity of the serum lysosome enzyme, &
equivalent to 1/5000, 1/1000 beta-N-acetylglucosa-minidase at the two highest doses. Mihaylova
or 1/200 the LD50 (not further (1975)
specified) for 3 months (abstract
only)
Hofmann et al. (1971) exposed rats, guinea-pigs, rabbits and cats
to 400 or 2000 mg/m3 (100 or 500 ppm) for up to 17 weeks. Mortality
was high in rats, guinea-pigs and rabbits exposed to the higher
concentration. At 2000 mg/m3 (500 ppm) pulmonary hyperaemia and
oedema, fatty liver and adrenal and myocardial necrosis were noted in
rats, while heart lesions were observed in cats and rabbits.
Guinea-pigs had fatty changes in the myocardium, liver and adrenals
and necrosis in the myocardium and liver at the higher concentration.
No clinical or histological effects were noted at 400 mg/m3
(100 ppm).
7.4.2 Ingestion
Available data on the subchronic toxicity of ingested
1,2-dichloroethane are presented in Table 4. In a recent study
conducted by the National Toxicology Program (NTP, 1991) and partially
reported by Morgan et al. (1990), the relative susceptibility of three
strains of rats (F344/N, Sprague-Dawley and Osborne-Mendel) and one
strain of mice (B6C3F1) exposed to 1,2-dichloroethane in
drinking-water at concentrations of up to 8000 mg/litre for 13 weeks,
and one of the same strains of rats (F344/N) exposed to doses of up to
480 mg/kg body weight per day by gavage in corn oil for 13 weeks, was
investigated. Based on increased relative organ weights, the liver
and kidneys were the target organs in both rats and mice, although
treatment-related microscopic lesions were noted only in female F344/N
rats and male B6C3F1 mice. Administration of 1,2-dichloroethane to
F344/N rats by gavage resulted in more severe toxic effects (including
death) than administration of similar doses in drinking-water,
probably due to greater peak levels of the compound in the blood and
saturation of elimination mechanisms. The authors considered the
no-observed-effect levels (NOEL) for 1,2-dichloroethane administered
to F344/N rats by gavage to be 120 and 150 mg/kg body weight per day
in males and females, respectively, based on mortality and chemically
related lesions in the forestomach. The NOEL of B6C3F1 mice exposed
via drinking-water was considered to be 780 mg/kg body weight per day)
(2000 ppm) in males, based on kidney lesions, and (2500 mg/kg body
weight per day) (4000 ppm) in females, based on mortality. The
authors did not consider the doses to which the three strains of rats
were exposed in the drinking-water to be high enough to result in
biologically significant toxic effect, although increases in organ
weights without accompanying histopathological alterations were
observed at doses as low as 49 to 82 mg/kg body weight per day in some
strains (i.e. Sprague-Dawley and Osborne-Mendel).
In limited subchronic studies preliminary to long-term
carcinogenesis bioassays, groups of Osborne-Mendel rats and B6C3F1
mice were administered doses of up to 251 and 1000 mg/kg body weight
per day, respectively, by gavage for 6 weeks. Significant mean body
weight loss was noted in male rats at 251 mg/kg body weight per day
and in female rats at > 40 mg/kg body weight per day. In mice,
mean body weight was decreased only in females receiving 398 mg/kg
body weight per day. No other parameters were investigated in this
study (NCI, 1978).
Decreased body weight gain was observed in rats orally
administered doses of 30 or 90 mg/kg body weight per day for 90 days,
but not in those receiving 10 mg/kg body weight per day. Increased
relative weights of kidneys (both sexes), liver and brain (females
only) were noted at the highest dose, although no histopathological
effects or alterations in clinical chemistry parameters were noted.
Changes in some haematological parameters were noted, but these were
not related to dose (Van Esch et al., 1977).
A slight increase in the fat content of the livers was reported
in rats consuming feed which had been fumigated with
1,2-dichloroethane, resulting in an initial concentration of
1600 mg/kg (approximately equivalent to a dose of 80 mg/kg body weight
per day) for 7 weeks, while no effects were observed at 600 mg/kg
(approximately equivalent to a dose of 30 mg/kg body weight per day)
after 5 weeks (Alumot et al., 1976a). The parameters examined were
limited to hepatic lipid content and liver weight. The activity of
the serum lysosome enzyme, ß- N-acetylglucosaminidase, was
significantly increased in rats administered oral doses equivalent to
1/1000 and 1/200 the LD50 (not further specified in abstract)
(Apostolov & Mihaylova, 1975).
7.5 Chronic exposure and carcinogenicity
7.5.1 Inhalation
Groups of 90 Sprague-Dawley rats of each sex were exposed by
inhalation to 1,2-dichloroethane (99.92% pure) at concentrations of 0,
20, 40, 202 and 1012 mg/m3 (0, 5, 10, 50 and 250 ppm), 7 h/day, 5
days/week for 78 weeks, and observed until spontaneous death (due to
severe toxicity, the highest concentration was reduced to 607 mg/m3
or 150 ppm after several days). "Incidence" was reported as the
number of animals developing specific tumours over the number of
animals alive at the time the first tumour of that type was detected
(i.e. incidences have not been adjusted for differential survival (see
Table 5)). The only tumour types for which the authors reported an
increase in incidence (when compared with controls kept in exposure
chambers, but not when compared with those not kept in chambers) were
fibromas and fibroadenomas (combined) of the mammary gland, which the
authors attributed to the differential survival among the groups
(Maltoni et al., 1980).
Groups of 50 male and 50 female Sprague-Dawley rats were exposed
to 0 or 200 mg/m3 (50 ppm) 1,2-dichloroethane 7 h/day, 5 days/week
for 2 years. No effects on body weight gain or mortality were noted.
There was no significant difference in the incidence of tumours at any
site, although there was a nonsignificant increase in the incidence of
mammary gland adenomas (4 in exposed group versus 2 in control group)
and fibroadenomas (21/50 in exposed group versus 15/50 in control
group) in females. There was an increased incidence of testicular
lesions (not further specified) in males (24% versus 10% in controls,
significance not reported) (Cheever et al., 1990). However, the
sensitivity of this investigation to detect any carcinogenic potential
may have been compromised (the study was designed to investigate the
interaction between 1,2-dichloroethane and other substances), based on
the lack of convincing evidence of compound-related toxicity at the
only concentration to which animals were exposed.
Clinical chemistry and haematological parameters were
investigated in groups of 8 to 10 male or female Sprague-Dawley rats
exposed to 0, 20, 40, 202 or 1012 mg/m3 (decreased to 607 mg/m3
after several days) (0, 5, 10, 50 or 250/150 ppm), 7 h/day, 5
days/week for 3, 6, and 18 months, beginning at 3 months of age. In
addition, groups of 8 to 10 rats were also exposed to these same
concentrations beginning at 14 months of age for 12 months. Although
values were occasionally significantly (p < 0.05) different from
those of controls, no consistent dose-related effects on various
haematological parameters, circulating protein levels or clinical
chemistry parameters were reported in rats exposed from 3 months of
age. In animals exposed for 12 months beginning at 14 months of age,
there were no consistent dose-related effects on haematological
parameters. There were significant (p < 0.05) changes in serum
parameters indicative of effects on liver and kidney function,
including levels of glutamic-pyruvic transaminase (SGPT),
gamma-glutamyltranspeptidase (gamma-GT), glutamic-oxalic transaminase
(SGOT) and cholesterol, and levels of uric acid in the blood and blood
urea nitrogen (BUN) at 202 and 607 mg/m3. Histopathological
examinations were not conducted (Spreafico et al., 1980).
No increase in the incidence of any type of tumour was reported
in groups of 90 male or female Swiss mice exposed to 20, 40, 202 or
1012 (decreased to 607 mg/m3 after a few days) mg/m3 (5, 10, 50 or
250/150 ppm), 7 h/day, 5 days/week for 78 weeks, and observed until
spontaneous death (Maltoni et al., 1980). However, it should be noted
that survival was poor (especially among males).
Table 5. Chronic toxicity and carcinogenicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats Rats were exposed to 0, 20, 40, 202 After several days of exposure to 1012 mg/m3, severe toxic effects, Maltoni
(Sprague-Dawley, and 1012/607 mg/m3 (0, 5, 10, 50 including death were observed and the level of exposure was reduced et al.
90 m & 90 f per and 250/150 ppm) 1,2-dichloroethane to 607 mg/m3. Survival varied among the groups, but was not related (1980)
group, 180 m & (99.92% pure), 7 h/day, 5 days/week to concentration; most rats died by week 140. Survival at 104 weeks
180 f controls) for 78 weeks and observed until of age in controls, chamber controls, and groups exposed to 20, 40,
spontaneous death. One group of 202 and 1012/607 mg/m3 was 17.8, 13.3, 50.0, 14.4, 18.9 and 11.1%
controls was kept in a nearby room, (males) and 40.0, 24.4, 53.3, 28.9, 32.2, and 23.3% (females).
while the other was kept in an "Incidence" was reported as the number of animals developing specific
exposure chamber under the same tumours over the number of animals alive at the time the first tumour
conditions as the exposed groups. of that type was detected (i.e. incidences have not been adjusted for
A complete autopsy was performed differential survival). With the exception of benign mammary tumours,
on each animal, regardless of time there were no significant increases in the incidence of any types of
of death. Several organs were (combined) tumours in exposed rats. The incidence of all mammary
routinely histopathologically tumours (number of animals alive at the time of appearance of the
examined, along with any organs first mammary tumour (12 weeks) was 90 in each exposure group) was
with pathological lesions. 52/90 (57.8%), 38/90 (42.2%), 65/90 (72.2%), 43/90 (47.8%), 58/90
(64.4%) and 52/90 (57.5%) in non-chamber controls, chamber controls,
and groups exposed to 20, 40, 202 and 1012/607 mg/m3, respectively.
The numbers (denominators not specified) of fibromas and
fibroadenomas (combined) of the mammary gland were 47, 27, 56, 33, 49
and 47 in non-chamber controls, chamber controls, and groups exposed
to 20, 40, 202 and 1012/607 mg/m3, respectively. The "incidence"
of these tumours at 20, 40, 202 and 1012/607 mg/m3, was
significantly (p<0.01 or 0.001) different from the incidence in
chamber controls. The "incidences" of benign mammary tumours in the
two control groups were also significantly (p<0.01) different.
Table 5. (cont'd).
Species Protocol Results Reference
Rats Rats were exposed to 0, 20, 40, 202 There were no consistent, exposure-related changes in various Spreafico
(Sprague-Dawley, or 1012-607 mg/m3 (0, 5, 10, 50 or haematological parameters, circulating protein levels or clinical et al.
8-10 or f per 250/150 ppm) 7 h/day, 5 days/week chemistry parameters in animals exposed from 3 months of age, (1980)
group) for 3, 6 or 18 months, beginning at although values occasionally differed significantly from controls.
3 months of age. In addition, groups In animals exposed for 12 months from 14 months of age, there were no
of rats were exposed to 0, 20, 40, consistent exposure-related alterations in haematological parameters.
202 or 1012-607 mg/m3 (0, 5, 10, 50 Levels of serum glutamic-pyruvic transaminase (SGPT) were
or 250/150 ppm) 7 h/day, 5 days/ significantly elevated in both males and females at 202 and 607 mg/m3
week for 12 months, beginning at 14 (p<0.05), and gamma-glutamil transpeptidase (gamma-GT) levels were
months of age. Histopathological also significantly greater in females at the two highest
examinations were not conducted. concentrations (p<0.05). Levels of serum glutamic-oxalic transaminase
(SGOT) were significantly increased in both sexes at 20 and 40 mg/m3
(p<0.05), but significantly decreased in males and females at 202 and
607 mg/m3 (p<0.05). Levels of cholesterol were significantly lower
in males and females at 202 and 607 mg/m3 (p<0.05). Levels of uric
acid in the blood were significantly higher in both sexes at 202 and
607 mg/m3 (p<0.05), while blood urea nitrogen (BUN) values were
significantly elevated at 607 mg/m3 (p<0.05), although there were
no effects on urinary parameters.
Rats Rats were exposed to 200 mg/m3 (0 There were no compound-related effects on body weight gain or Cheever
(Sprague-Dawley, or 50 ppm) 7 h/day, 5 days/week for mortality. There were no significant increases in the incidence of et al.
50 m & 50 f 2 years. Extensive histopathological any type of tumours, although there was a non-significant increase (1990)
per group) examinations were conducted. in the incidence of mammary gland adenomas (4 versus 2 in controls)
and fibroadenomas (21/50 versus 15/50) in females. The incidence of
testicular lesions (not further specified) was increased in exposed
animals (24% versus 10% in controls, significance not reported).
Table 5. (cont'd).
Species Protocol Results Reference
Mice (Swiss, Mice were exposed to 20, 40, 202 or Survival of mice was poor, especially in males, as only 43.4 to 65.6% Maltoni
90 m & 90 f 1012-607 mg/m3 (5, 10, 50 or 250/ of exposed males survived for 52 weeks after exposure commenced. et al.
per group, 150 ppm), 7 h/day, 5 days/week for There were no significant increases in the incidence of any type of (1980)
115 m & 134 f 78 weeks and observed until tumours.
controls) spontaneous death. Controls were
kept in a nearby room. A complete
autopsy was performed on each
animal, regardless of time of death.
Several organs were routinely
histopathologically examined, along
with any organs with pathological
lesions.
Ingestion
Rats Animals were administered time There were no effects on body weight gain in either sex. Mortality NCI
(Osborne-Mendel, weighted average doses of 47 or 95 was significantly higher in both males and females in the high dose (1978)
50 50 m & f per mg/kg body weight per day (initial group, as 50% of exposed rats had died by week 55 (males) and 57
group, 20 m & doses of 50 and 100 mg/kg body (females), compared to week 72 (males) and 88 (females) in controls.
20 f controls; weight per day were increased to 75 Signs of toxicity, including wheezing, nasal discharge, ulcerations,
60 m & 60 f and 150 mg/kg body weight per day localized alopecia, discoloured or stained fur, bloated appearance
pooled controls after 7 weeks then decreased to and swollen areas, occurred at a greater frequency in exposed
from concurrent original doses after 17 weeks) in animals than in controls. Chronic murine pneumonia was present in 60
experiments) corn oil by gavage, 5 days/week, for to 94% of rats in each group (incidence not related to dose).
78 weeks followed by 32 weeks of Acanthosis and hyperkeratosis of the forestomach was present in a
observation. Complete greater proportion of exposed females than controls (1/20, 6/50 and
histopathological examinations were 7/50 in vehicle controls, low and high dose, respectively,
conducted. significance not reported). Other non-neoplastic lesions occurred
at similar frequencies in control and exposed rats. The incidence
of squamous cell carcinomas of the forestomach in males was 0/60,
Table 5. (cont'd).
Species Protocol Results Reference
0/20, 3/50 and 9/50 in pooled controls, matched controls, low and
high dose groups, respectively, significant at the high dose
(p=0.01); only 2/50 females in the low dose group had this tumour.
There were also one leiomyosarcoma of the stomach and one
adenocarcinoma of the small intestine in high dose males (not
significant). The incidence of hemangiosarcomas (mostly in the
spleen) in males was 1/60, 0/20, 9/50 and 7/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively (significant in both exposed groups, p=0.03 (low) and
p=0.016 (high)), and in females was 0/59, 0/20, 4/50 and 4/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively (significant in both exposed groups (p=0.041 in
both)). Both groups of exposed males had an increased incidence of
fibromas of the subcutaneous tissue (0/60, 0/20, 5/50 and 6/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively); no such increase was noted in females. In
females, there was an increased incidence of adenocarcinomas and
fibroadenomas of the mammary gland (1/59, 0/20, 1/50 and 18/50
(adenocarcinoma), 5/59, 0/20, 14/50 and 8/50 (fibroadenoma) and 6/59,
0/20, 15/50 and 24/50 (adenocarcinoma or fibroadenoma)) in pooled
vehicle controls, matched vehicle controls, low and high dose groups,
respectively). Renal tubular cell adenocarcinomas were noted in one
male and one female at the highest dose, while tubular cell adenomas
were present in one male and two females at this dose, and none was
observed in controls (significance not reported).
Rats (18 m & Animals were fed mash fumigated Chronic respiratory disease was reported in all groups in the second Alumot
18 f per group, with 1,2-dichloroethane for 2 years. year of exposure. The number of rats surviving after 21 months ranged et al.
strain not Resulting concentrations were 250 from 2 to 14 per group. No effects on growth or the biochemical (1976a)
specified) and 600 mg/kg. Due to loss of the parameters investigated were observed.
Table 5. (cont'd).
Species Protocol Results Reference
compound through volatilization,
the mash actually consumed was
estimated to contain 60 to 70% of
the initial concentration (estimated
to result in doses of approximately
7.5 to 8.75 and 15 to 17.5 mg/kg
body weight per day). The liver was
analysed for total fat triglyceride
content. Levels of total protein,
albumin, glucose, urea, uric acid
and cholesterol in the serum were
determined. Histopathological
examinations do not appear to have
been conducted on surviving rats
at the end of the exposure period.
Mice (B6C3F1, Animals were administered Mortality in females was related to dose (36 animals in the high dose NCI
50 m & 50 f time-weighted average doses of 97 group died between weeks 60 and 80, which may have been related to (1978)
per group; 20 or 195 mg/kg body weight per day the appearance of tumours as 25 of these animals had tumours); no
m & 20 f (males) or 149 or 299 (females) in similar dose-related trend in mortality was observed in males. Body
controls; 60 m corn oil by gavage, 5 days/week, for weight in females in the high dose group was depressed as early as
& 60 f pooled 78 weeks followed by 13 weeks of week 15 (>10%). The incidence of non-neoplastic lesions was
controls from observation (initial doses in males comparable in exposed and control mice. There was an increased
concurrent of 75 and 150 mg/kg body weight incidence of hepatocellular carcinomas in male mice at the highest
experiments) per day were increased to 100 and dose (4/59, 1/19, 6/47 and 12/48 in pooled vehicle controls, matched
200 mg/kg body weight per day after vehicle controls, low and high dose groups, respectively), but not in
8 weeks; initial doses in females females. The incidence of alveolar/bronchiolar adenomas in males was
Table 5. (cont'd).
Species Protocol Results Reference
of 125 and 250 mg/kg body weight per 0/59, 0/19, 1/47 and 15/48 in pooled vehicle controls, matched
day were increased to 200 and vehicle controls, low and high dose groups, respectively
400 mg/kg body weight per day after (significant at the highest dose (p<0.001)); the incidence of this
8 weeks, then decreased to 150 and tumour in females was 2/60, 1/20, 7/50 and 15/48 in pooled vehicle
300 mg/kg body weight per day after controls, matched vehicle controls, low and high dose groups,
11 weeks). Complete respectively (significant in both exposed groups(p=0.046 (low) and
histopathological examinations were p<0.001 (high)). There was also one alveolar/bronchiolar carcinoma in
conducted. females at 299 mg/kg body weight per day. There was a non-significant
increase in the incidence of squamous cell carcinoma of the
forestomach in females at 299 mg/kg body weight per day (5/48 versus
1/60 or 1/20 in controls). There was a significantly increased
incidence of mammary gland adenocarcinomas in both groups of exposed
females (0/60, 0/20, 9/50 and 7/48 in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively
(p=0.001 (low) and p=0.003 (high)).
Uterine adenocarcinomas occurred in 3/49 low dose and 4/47 high
dose females, compared to none in controls; however, this increase
was not statistically significant. The incidence of endometrial
stromal polyp or endometrial stromal sarcoma (combined) was 0/60,
0/20, 5/49 and 5/47 in pooled vehicle controls, matched vehicle
controls, low and high dose groups, respectively (significant at
both doses, p=0.016 (low) and p=0.014 (high)).
Table 5. (cont'd).
Species Protocol Results Reference
Dermal
application
Swiss mice Doses of 0, 42 or 126 mg/application The incidence of benign lung papillomas was significantly (p<0.0005) van Duuren
(Ha:ICR, 30 f; per mouse in 0.2 ml acetone were increased at the higher dose (26/30 compared to 17/30, 11/30 and et al.
30 vehicle applied 3 times per week to the 30/100 in low dose group, vehicle controls and untreated controls, (1979)
controls and shaved dorsal skin (area of skin respectively). The incidence of stomach tumours was 3/30, 1/30, 2/30
100 naive exposed not specified) of mice for and 5/100 in high dose group, low dose group, vehicle controls and
controls) 440 to 594 days. The skin, liver, untreated controls, respectively (not significant).
kidney and any tissues or
organs appearing abnormal were
examined histopathologically.
7.5.2 Ingestion
In a study conducted by the National Cancer Institute (NCI,
1978), time-weighted average daily doses of 47 or 95 mg/kg body weight
per day of 1,2-dichloroethanea in corn oil were administered by
gavage 5 days/week for 78 weeks to 50 Osborne-Mendel rats of each sex,
followed by 32 weeks of observation. Mortality was significantly
(p < 0.001) higher in both males and females in the high dose group.
Clinical signs of general toxicity occurred at a greater frequency in
exposed animals than in controls. In each group 60-94% of rats had
chronic murine pneumonia, but the incidence was not related to dose.
The incidence of acanthosis and hyperkeratosis of the forestomach was
greater in exposed females than controls.
The incidence of a variety of tumours was increased in exposed
animals compared with controls. The incidence of squamous cell
carcinomas of the stomach was significantly increased in males (3/50
and 9/50 in low and high dose groups, respectively), compared to none
in either group of controls; in females, there were only 2/50 in the
low dose group. The incidence of haemangiosarcoma was significantly
increased in males (1/60, 0/20, 9/50 and 7/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively) and females (0/59, 0/20, 4/50 and 4/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). The incidence of fibromas of the subcutaneous tissue
was significantly increased in males (0/60, 0/20, 5/50 and 6/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively), but not in females. There was a significant
increase in the incidence of adenocarcinomas and fibroadenomas of the
mammary gland in females (1/59, 0/20, 1/50 and 18/50 (adenocarcinoma),
5/59, 0/20, 14/50 and 8/50 (fibroadenoma) and 6/59, 0/20, 15/50 and
24/50 (adenocarcinoma or fibroadenoma)) in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively). It
was concluded that 1,2-dichloroethane was carcinogenic in this strain
of rats, under the conditions of this study.
No effects on growth or biochemical parameters were observed in a
limited study on rats fed mash that had been fumigated with
1,2-dichloroethane, which resulted in doses of approximately 7.5-8.75
and 15-17.5 mg/kg body weight per day. Chronic respiratory disease
was evident in all groups in the second year, the number of rats
surviving after 21 months ranging from 2 to 14 in each of the groups.
The occurrence of respiratory disease and mortality did not appear to
be exposure-related (Alumot et al., 1976a).
a Technical grade with reported purity of > 90% containing 11 minor
contaminants; subsequent analysis indicated a purity of about
98-99% (Hooper et al., 1980 and Ward, 1980).
The National Cancer Institute (NCI, 1978) also conducted a
bioassay in which groups of 50 B6C3F1 mice were administered
time-weighted average daily doses of 97 or 195 mg/kg body weight per
day (males) and 149 or 299 mg/kg body weight per day (females)
1,2-dichloroethane in corn oil by gavage, 5 days/week for 78 weeks,
followed by 13 weeks of observation. A doserelated increase in
mortality was noted in female mice, but not in males. Body weight was
also decreased in females at 299 mg/kg body weight per day.
As in rats, there was a significant increase in the incidence of
several types of tumours in exposed mice. The incidence of
hepatocellular carcinomas was significantly increased in males in the
high dose group (4/59, 1/19, 6/47 and 12/48 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively); no such increase was noted in females. However, the
authors noted that, due to the high variability of incidence of
hepatocellular neoplasms in historical controls (data not presented),
this increase, although statistically significant, was not considered
to be convincing evidence that these tumours were attributable to the
test chemical. The incidence of alveolar/bronchiolar adenomas was
significantly increased in males in the high dose group (0/59, 0/19,
1/47 and 15/48 in pooled vehicle controls, matched vehicle controls,
low and high dose groups, respectively), and in both groups of exposed
females (2/60, 1/20, 7/50 and 15/48 in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively); one
alveolar/bronchiolar carcinoma was noted in a high-dose female mouse.
There was a non-significant increase in the incidence of squamous cell
carcinoma of the forestomach in females in the high dose group. The
incidence of mammary gland adenocarcinomas was significantly increased
in females at both doses (0/60, 0/20, 9/50 and 7/48 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). The incidence of endometrial stromal polyp or
endometrial stromal sarcoma (combined) was significantly elevated at
both doses (0/60, 0/20, 5/49 and 5/47 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). It was concluded that 1,2-dichloroethane was
carcinogenic in this strain of mice, under the conditions of this
study.
It should be noted that the data on tumour incidence presented in
the bioassays by the NCI (1978) do not take into account the increased
early mortality in the high exposure groups; the incidences of several
tumours (and thus the carcinogenic potency of 1,2-dichloroethane) may
have been higher had all animals survived for a long enough period of
time to develop tumours (Ward, 1980; Hooper et al., 1980).
7.5.3 Other routes of administration
1,2-Dichloroethane in acetone was applied to the shaved dorsal
skin of groups of 30 female non-inbred Ha:ICR Swiss mice, 3 times/week
for 440 to 594 days at doses of 0, 42, and 126 mg/application per
mouse. The incidence of lung tumours (benign lung papillomas) was
significantly increased at the higher dose (26/30 compared to 11/30 in
vehicle controls and 30/100 in naive controls, p < 0.0005).
Histopathological examination was limited to the skin, liver, kidney
and any "abnormal-appearing tissues" (van Duuren et al., 1979).
In a bioassay designed to screen the potential of numerous
chemicals to induce pulmonary tumours in a susceptible strain of mice
(A/St), groups of 20 males were administered 24 intraperitoneal
injections of 1,2-dichloroethane (20, 40 or 100 mg/kg body weight) in
tricaprylin, 3 times/week for 8 weeks (total doses of 480, 920 or
2400 mg/kg body weight). All surviving mice were killed 24 weeks
after the first injection. Although there was a dose-related increase
in the number of pulmonary adenomas per mouse (0.39, 0.21, 0.44 and
0.75 in the control, low, mid and high dose groups, respectively),
none of these increases was statistically significant (Theiss et al.,
1977). It should be noted, however, that the duration of the period
of observation may have been insufficient to allow for the development
of most types of tumours.
7.5.4 Initiation/promotion bioassays
In a dermal initiation/promotion protocol, 126 mg
1,2-dichloroethane was applied once to the skin (area exposed not
specified) of 30 female non-inbred Ha:ICR Swiss mice, followed 14 days
later by 5 µg (0.005 mg) of phorbol myristate acetate (PMA) (a
promoter) in 0.2 ml acetone, 3 times/week for 428 to 576 days. Two
PMA control groups of 120 and 90 mice were administered 0.0025 mg and
0.0050 mg PMA/application permouse (number of applications not
specified), respectively. Treatment with 1,2,-dichloroethane did not
significantly increase the incidence of skin papillomas (3/30 versus
9/120 and 6/90 in PMA controls). There were three squamous cell
carcinomas in the control groups, while none was observed in the
exposed group (van Duuren et al., 1979).
In a hepatic initiation/promotion assay, groups of 10
Osborne-Mendel rats were partially hepatectomized and then
administered 1,2-dichloroethane (100 mg/kg body weight) in corn oil by
gavage, followed 5 days later by diets containing phenobarbital for 7
weeks and a control diet for 1 week (initiation protocol). Livers
were examined histopathologically for GGT-positive foci (a putative
preneoplastic indicator). Additional groups of 10 rats were initiated
with an intraperitoneal injection of diethyl-nitrosamine or water
(control) following partial hepatectomy, then administered
1,2-dichloroethane (100 mg/kg body weight) in corn oil by gavage, 5
days/week for 7 weeks (promotion protocol). In rats administered
1,2-dichloroethane in the initiation protocol, there was no increase
in the number of GGT-positive foci.
Similarly, in rats administered 1,2-dichloroethane in the promotion
protocol, there was no significant increase in the number of
GGT-positive foci, either with or without the initiator, when compared
to controls (Story et al., 1986; Milman et al., 1988).
Groups of 35 male B6C3F1 mice were administered
diethylnitrosamine in the drinking-water for 4 weeks, followed by
exposure to 1,2-dichloroethane (835 or 2500 mg/litre) in
drinking-water for 24 or 52 weeks. Only the liver, kidneys and lungs
were examined histopathologically. Drinking-water intake was reduced
at the highest concentration. No significant differences in body
weight gain were noted. However, three mice consuming the highest
concentration of 1,2-dichloroethane died within 52 weeks. There was
no increase in the incidence of liver or lung tumours in exposed mice
either with or without diethylnitrosamine initiation (Klaunig et al.,
1986).
7.6 Mutagenicity and related end-points
The genotoxicity of 1,2-dichloroethane has been extensively
investigated in non-mammalian and mammalian test systems. Data from
in vitro and in vivo studies are summarized in Tables 6 and 7; a
summary of the weight of available evidence is presented here.
1,2-Dichloroethane induced differential toxicity in Escherichia
coli, but it had no effect in a Bacillus subtilis rec assay. It
consistently induced positive responses in mutagenicity assays with
Salmonella typhimurium, whereas it has not produced consistent
responses in mutation assays with E. coli and was negative in a mouse
peritoneal host-mediated assay with E. coli.
In the fungus Aspergillus nidulans, 1,2-dichloroethane induced
errors of mitotic segregation and aneuploidy, but did not induce gene
mutation.
Table 6. Genotoxicity of 1,2-dichloroethane in vitro (modified from ATSDR, 1994)
Species (test system) End-point Resulta Reference
With activation Without activation
Bacterial systems
Salmonella typhimurium Gene mutation + + Milman et al. (1988)
+ + Barber et al. (1981)
+ + Kanada & Uyeta (1978)
+ + Nestmann et al. (1980)
+ + Rannug et al. (1978)
+ + Van Bladeren et al. (1981)
+ NT Rannug & Beije (1979)
+ - Cheh et al. (1980)
+ - Moriya et al. (1983)
- - King et al. (1979)
+ + Strobel & Grummt (1987)
NT +b Simula et al. (1993)
S. typhimurium/spot test NT (+) Brem et al. (1974)
(+) - Principe et al. (1981)
NT - Buijs et al. (1984)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
S. typhimurium/Ara test + - Roldan-Arjona et al. (1991)
(standard)
S. typhimurium/Ara test (+) (+) Roldan-Arjona et al. (1991)
(liquid)
Streptomyces coelicolor Gene mutation NT - Principe et al. (1981)
Escherichia coli/K12/343/113 Gene mutation - - King et al. (1979)
E. coli/wp2 NT (+) Hemminki et al. (1980)
- - Moriya et al. (1983)
E. coli Pol A DNA damage NT (+) Brem et al. (1974)
Bacillus subtilis/rec-assay DNA damage NT - Kanada & Uyeta (1978)
Fungal systems
Aspergillus nidulans Gene mutation NT - Crebelli & Carere (1988)
NT - Principe et al. (1981)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
A. nidulans Mitotic segregation NT + Crebelli et al. (1984)
aberrations
A. nidulans Aneuploidy induction NT + Crebelli et al. (1988)
Saccharomyces cerevisiae Mitotic recombination NT (+) Simmon (1980)
Animal systems
Hamster CHO/HGPRT Gene mutation + (+) Tan & Hsie (1981)
+ (+) Zamora et al. (1983)
Rat hepatocytes Unscheduled DNA synthesis NT + Williams et al. (1989)
Mouse hepatocytes NT + Milman et al. (1988)
Mouse liver DNA DNA binding + NT Banerjee (1988)
Calf thymus DNA + NT Prodi et al. (1986)
Salmon sperm DNA + - Banerjee & Van Duuren (1979);
Banerjee et al. (1980)
Mouse BALBc/3T3 Cell transformation NT - Milman et al. (1988)
NT - Tu et al. (1985)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
Mouse C3H1OT´ Cell transformation NT +c Schultz et al. (1992)
Syrian hamster embryo cells Cell transformation NT + Hatch et al. (1983)
Human cells
Human lymphoblasts AHH-1 Gene mutation NT + Crespi et al. (1985)
Human lymphoblasts TK6 NT + Crespi et al. (1985)
Human embryo epithelial-like NT + Ferreri et al. (1983)
EUE cells
Human peripheral lymphocytes Unscheduled DNA synthesis + - Perocco & Prodi (1981)
a NT = not tested; - = negative result; + = positive result; (+) = weakly positive or marginal result
b increase in cells expressing GSTA1-1
c transformed cells induced tumours in nude mice
Table 7. Genotoxicity of 1,2-dichloroethane in vivo (modified from ATSDR, 1994)
Species (test system) End-point Resultsa Reference
Mammalian assays
Mouse Dominant lethal mutations - Lane et al. (1982)
Mouse/spot test Gene mutation (+) Gocke et al. (1983)
Mouse bone marrow Sister-chromatid exchange + Giri & Que Hee (1988)
Mouse bone marrow Micronuclei - Jenssen & Ramel (1980);
King et al. (1979)
Mouse peripheral erythrocytes - Armstrong & Galloway (1993)
Mouse liver, kidney, lung and stomach DNA binding + Prodi et al. (1986)
Mouse liver, kidney, lung and stomach + Arfellini et al. (1984)
Mouse forestomach and kidney + Hellman & Brandt (1986)
Mouse liver + Banerjee (1988)
Table 7 (contd).
Species (test system) End-point Resultsa Reference
Rat liver, kidney, spleen, lung, + Reitz et al. (1982)
forestomach and stomach
Rat liver, kidney, lung and stomach + Arfellini et al. (1984)
Rat liver, kidney, lung and stomach + Prodi et al. (1986)
Rat liver and kidney + Inskeep et al. (1986)
Rat liver and lung + Baertsch et al. (1991)
Rat liver + Banerjee (1988)
Rat liver + Cheever et al. (1990)
Mouse liver DNA damage + Storer & Conolly 1983, 1985;
Storer et al. (1984)
Mouse liver + Taningher et al. (1991)
Insect assays
Drosophila melanogaster/somatic mutation Gene mutation + Nylander et al. (1978)
Table 7 (contd).
Species (test system) End-point Resultsa Reference
D. melanogaster/somatic mutation + Romert et al. (1990)
D. melanogaster/somatic mutation + Kramers et al. (1991)
D. melanogaster/somatic mutation (+) Ballering et al. (1993)
D. melanogaster/recessive lethal + Ballering et al. (1993)
D. melanogaster/vermilion locus + Ballering et al. (1993)
D. melanogaster/sex-linked recessive + King et al. (1979)
D. melanogaster/sex-linked recessive + Kramers et al. (1991)
D. melanogaster Chromosomal loss/gain +/+ Valencia et al. (1984)
Host-mediated assays
Escherichia coli K12/343/113 mouse Gene mutation - King et al. (1979)
host-mediated assay
a - = negative result; + = positive result; (+) = weakly positive or marginal result
In cultured mammalian cells, 1,2-dichloroethane formed
adducts with DNA. It also induced unscheduled DNA synthesis in primary
cultures of mouse and rat hepatocytes, and human peripheral
lymphocytes (the last in the presence of an exogenous metabolic
activation system), and gene mutation in several cell lines. Mutation
frequency of two human cell lines has been correlated with the
difference in levels of glutathione- S-transferase activities (Crespi
et al., 1985; section 6.3). Cell transformation was induced in
studies with C3H10T´ cells, but not with BALBc/3T3 cells.
1,2-Dichloroethane enhanced SV40 virus transformation of Syrian
hamster embryo cells.
In vivo, both somatic cell and sex-linked recessive lethal
mutations have been consistently induced in Drosophila melanogaster by
1,2-dichloroethane. 1,2-Dichloroethane has been found to bind to DNA
in all reported studies in mice and rats. In other studies with mice,
clearly positive responses have been restricted to primary DNA damage
in liver and sister-chromatid exchange induction in bone marrow. No
evidence for micronucleus induction has emerged from bone marrow
micronucleus studies or for a dominant lethal effect in one study
(although this study may have been insufficiently sensitive), and only
a weak but significant (p < 0.03) response was observed in a single
spot test.
It has been noted that stronger responses were obtained in the
bacterial mutation assay in the presence of an exogenous metabolic
system than in its absence. This could imply the formation of
additional mutagenic metabolites, through either the cytochrome P450
or glutathione-S-transferase pathway. In these in vitro assays with
liver homogenates, activation by the cytochrome system is more likely,
and 2-chloroacetaldehyde, which is a possible metabolite, is known to
be mutagenic (McCann et al., 1975). The mutagenicity of
1,2-dichloroethane in S. typhimurium TA100, in the absence of S9 mix,
was more than doubled if the bacterium expressed the human GSTA1-1
gene, but there was no change in the mutagenic response if the SSTP1-1
gene was expressed (Simula et al., 1993).
7.7 Reproductive toxicity, embryotoxicity and teratogenicity
The reproductive and developmental effects of 1,2-dichloro-ethane
have not been extensively investigated in experimental animals (see
Table 8), although the compound has been detected in fetal tissues in
rats and mice following maternal exposure to 600 mg/m3 for 5 h
(Withey & Karpinski, 1985) and 1000 mg/m3 for 3 days (Vozovaya,
1977).
Table 8. Reproductive and developmental toxicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats Animals were exposed to 0, 101, 304, or No exposure-related histopathological effects in the parents nor Rao
(Sprague-Dawley, 607 mg/m3 (0, 25, 75, or 150 ppm) any alterations in the fertility index and gestation periods were et al.
20 m & 20 f per 6 h/day, 5 days/week for 60 days prior observed compared to controls. There was a significant decrease (1980)
exposed group, to mating, and for an additional 116 in the number of pups per litter in the F1A pups at 304 mg/m3
30 m & 30 f days (7 days/week) after mating. Dams (13%, p<0.05), but not at 607 mg/m3; there was significantly
controls) were not exposed from gestation day 21 increased kidney weight in the F1B male pups at 101 mg/m3
through to day 4 postpartum. The pups (29%, p<0.05), although the authors did not consider this effect
(F1A) were removed after 21 days, and to be related to exposure. There were no significant differences
the females were remated to exposed in growth, sex ratios, survival indices, organ or neonatal body
males following removal of the last weights, or histology in pups.
litter to produce F1B litters. Liver,
kidneys, ovaries , uterus and testes
of parental animals in control and
high exposure group (and other groups
if any changes were noted in high
exposure group) were examined
histopathologically
Rats (Albino, f, Rats were exposed to 57 mg/m3, 4 h/day, Exposed rats had reduced fertility (6.5 fetuses per dam compared Vozovaya
strain and 6 days/week, for 6 or 9 months. The to 9.7 per dam in controls). Newborn pups had reduced body (1974)
number animals were apparently then mated, but weight (5.06 g versus 6.44 g in controls). Perinatal mortality
unspecified it is not clearly stated whether the was increased in the exposed group (data not presented). No
in secondary exposure period extended beyond information was available on maternal effects.
account) mating.
Table 8 (contd).
Species Protocol Results Reference
Rats (f, strain Rats were exposed to 15 mg/m3, 4 h/day, The estrous cycle was longer in exposed rats than in controls. No Vozovaya
and number 6 days/week for 4 months prior to and information on the effects on fertility was presented. Embryonal (1977)
unspecified after mating. mortality increased from approximately 11% in controls to 27% in
in secondary exposed dams. Pre-implantation losses were 5-fold greater in
account) exposed animals than in controls. No fetal abnormalities were
observed, except for haematomas in the region of the head, neck
and anterior extremities (presumably only in pups of exposed
animals, although not clearly stated). No information was
available on maternal effects.
Rats Rats were exposed to 0, 405 or 1215 10/16 dams at 1215 mg/m3 died. At 405 mg/m3, there were no Rao
(Sprague-Dawley, mg/m3 (0, 100 or 300 ppm) for 7 h/day effects on mean litter size, incidence of resorptions, or fetal et al.
16 to 30 pregnant on days 6 to 15 of gestation. body measurements; no significant increase in the incidence of (1980)
f per group) major malformations was observed at this concentration.
Rabbits (New Rabbits were exposed to 0, 405, or 4/21 and 3/19 dams died at 405 and 1215 mg/m3, respectively, Rao
Zealand White, 1215 mg/m3 (0, 100 or 300 ppm) for compared to none in 20 controls. There were no effects on mean et al.
19 to 21 7 h/day on days 6 to 18 of gestation. litter size, incidence of resorptions or fetal body measurements (1980)
pregnant f at either concentration, and there were no significant differences
per group) in the incidence of major malformations.
Table 8 (contd).
Species Protocol Results Reference
Ingestion
Rats (45 m & Animals were fed mash fumigated with There were no significant differences in various reproductive Alumot
90 f, strain 1,2-dichloroethane for 2 years. parameters, including number of dams pregnant, number of dams et al.
unspecified) Resulting concentrations were 250 and with litters, mean litter size, mortality or body weight of young (1976a)
600 mg/kg. Due to loss of the compound at birth and at weaning.
through volatilization, the mash
actually consumed was estimated to
contain 60 to 70% of the initial
concentration (estimated to result in
doses of approximately 7.5 to 8.75 and
15 to 17.5 mg/kg body weight per day).
Exposed females were mated with
exposed males at 2 month intervals.
Mice (ICR Swiss, F0 mice were administered There were no differences in body weight in adults, and there Lane
(10 m & 30 f concentrations of 0, 0.03, 0.09 or 0.29 were no effects on fertility or gestation indices. There were no et al.
per exposed mg/litre drinking-water (equivalent effects on survival, litter size, postnatal body weight or gross (1982)
group, 20 m & to nominal doses of approximately pathology of pups. The incidence of fetal visceral or skeletal
60 f controls) 0, 5, 15 or 50 mg/kg body weight malformations was not increased in exposed animals. F1C litters
per day) for 35 days prior to were not examined for skeletal malformations.
mating. Three sets of offspring
(F1A, F1B and F1C) were produced.
Table 8 (contd).
Species Protocol Results Reference
After weaning and 11 weeks of
exposure to the same concentrations,
the F1B mice were mated (30 female,
10 male) to produce a second generation
of offspring (F2A and F2B). Teratology
screening tests were performed using
F1C and F2B matings where the females
were co-housed with unexposed males.
Mice (f, number Pregnant mice were administered There were no developmental effects and "few discernible effects" Kavlock
and strain not 1,2-dichloroethane in the drinking-water on maternal health. There were no skeletal or visceral anomalies et al.
specified in at a concentration equivalent to a which could be attributed to exposure. (1979)
secondary dose of 510 mg/kg body weight per
account) day on days 7 to 14 of gestation.
No effects on reproductive parameters, including fertility index,
gestation period or histological changes, were reported in a
two-generation study on Sprague-Dawley rats exposed to 0, 101, 304 or
607 mg/m3 (0, 25, 75 or 150 ppm) 1,2-dichloroethane 6 h/day, for 60
days prior to mating and 116 days after mating (except during the
delivery period). There were also no effects on growth, sex ratios,
survival indices, organ or neonatal body weights, or histology in pups
(Rao et al., 1980).
Exposure to 1,2-dichloroethane (15 mg/m3), 4 h/day, for 4
months prior to mating and after mating resulted in a
longer-than-normal estrous cycle in female rats (strain not
specified). Although embryonal mortality and preimplantation losses
were greater in exposed animals than in controls, no fetal
abnormalities were reported, except for haematomas in the area of the
head, neck and anterior extremities (Vozovaya, 1977). Similarly,
increased perinatal mortality and reduced body weight of newborn pups
were observed in the offspring of female albino rats exposed to 57
(± 10) mg/m3 for 6 and 9 months. Exposed females also produced a
lower number of fetuses per dam (Vozovaya, 1974). Information from
these studies was insufficient for evaluation.
Rao et al. (1980) also conducted a developmental study in which
pregnant Sprague-Dawley rats were exposed to 0, 405 or 1215 mg/m3
(0, 100 or 300 ppm) 1,2-dichloroethane, 7 h/day, during gestation.
Mortality was high in dams at 1215 mg/m3 (10/16 rats died); thus the
developmental effects could not be ascertained at this concentration.
No fetotoxic or teratogenic effects were observed at 405 mg/m3. In
pregnant rabbits exposed to the same concentrations, 7 h/day, during
gestation, mortality was increased in exposed animals (4/21 and 3/19
dams died at 405 and 1215 mg/m3, respectively, compared to none in
20 controls). However, no fetotoxic or teratogenic effects were
reported at either concentration. The authors concluded that
1,2-dichloroethane was not teratogenic or fetotoxic in rats at
405 mg/m3 or in rabbits at 405 or 1215 mg/m3.
No effects on male fertility or various reproductive parameters
were noted in rats consuming mash which had been fumigated with
1,2-dichloroethane, the resultant concentrations being 250 and
500 mg/kg (approximately equivalent to doses of 7.5 to 8.75 and 15 to
17.5 mg/kg body weight per day when loss due to volatilization was
taken into account), for up to 2 years (Alumot et al., 1976a). No
effects on reproduction (in terms of fertility and gestation indices)
were reported in a two-generation study on Swiss ICR mice exposed to
1,2-dichloroethane in the drinking-water at concentrations of 0, 0.03,
0.09 or 0.29 g/litre (approximately equivalent to doses of 0, 5, 15 or
50 mg/kg body weight per day). In addition, no fetotoxic or
teratogenic effects were noted in either generation of offspring of
F1C and F2B litters sacrificed on day 18 (Lane et al., 1982). No
developmental effects were reported in a study in which groups of 30
pregnant CD-1 mice were administered drinking-water containing a
mixture of organic compounds, including 0.01% 1,2-dichloroethane
(equivalent to a dose of 5.1 mg/kg body weight per day), during days 7
to 14 of gestation (Kavlock et al., 1979).
7.8 Immunological effects
Groups of 140 female CD1 mice were exposed to airborne
concentrations of 0, 10, 20 and 40 mg/m3 (0, 2.5, 5 and 10 ppm)
1,2-dichloroethane for a 3-h period or to 0 or 10 mg/m3, 3 h/day for
5 consecutive days. After acute exposure to 20 and 40 mg/m3, there
was a significant increase in mortality in mice from streptococcal
challenge (p < 0.05 and p < 0.001, respectively), while no effects
were noted following acute or repeated exposure to 10 mg/m3. In
similarly exposed groups of 18 to 36 mice, significantly (p < 0.01)
decreased pulmonary bactericidal activity to inhaled Klebsiella
pneumoniae was noted only at 40 mg/m3. Single exposure to 40 or
400 mg/m3 (10 or 100 ppm) did not affect the in vitro phagocytic
or cytostatic ability of alveolar macrophages to red blood cells and
tumour target cells, respectively (Sherwood et al., 1987).
Male Sprague-Dawley rats (16 per group) were exposed to 0, 400 or
800 mg/m3 (0, 100 or 200 ppm) 1,2-dichloroethane for 3 h, or to 0,
40, 80, 200 or 400 mg/m3 (0, 10, 20, 50 or 100 ppm), 5 h/day, 5
days/week for 12 days. Pulmonary bactericidal activity was not
affected at any concentration. No effects were noted on in vitro
phagocytic activity to red blood cells, in vitro cytostatis and
cytolysis of tumour target cells, or levels of ectoenzymes in alveolar
macrophages. Blastogenesis of mitogen-stimulated T- and B-lymphocytes
from popliteal and mesenteric lymph nodes was not affected (Sherwood
et al., 1987).
Chinchilla rabbits (number per group not specified but
probably less than 10) were exposed to 2, 10 or 100 mg/m3
1,2-dichloroethane for 3 h/day, 6 days/week, for 7.5 to 8 months. At
the two highest concentrations production of antibodies against
typhoid vaccine was increased, while total antibody production was
reduced at 100 mg/m3 (Shmuter, 1977).
Groups of 10 male CD1 mice (12 per group) (Munson et al., 1982)
were administered 1,2-dichloroethane (4.9 and 49 mg/kg body weight per
day) in water by gavage for 14 days. A significant (p < 0.05)
reduction of 25 and 40% in IgM antibody-forming cells to sheep RBCs
was observed at 4.9 and 49 mg/kg body weight per day, respectively. A
significant (p < 0.05) reduction of cell-mediated responses to sheep
erythrocytes was also observed at both levels, which was not related
to dose, while a significant (p < 0.05) reduction in leucocyte count
was observed at the highest dose. In the same study, groups of 16 to
32 male CD1 mice were administered time-weighted average
1,2-dichloroethane doses of 0, 3, 24 and 189 mg/kg body weight per day
(0, 0.02, 0.2 and 2.0 g/litre) for 90 days in drinking-water; controls
consisted of 24 to 48 mice. The fluid consumption of exposed mice was
decreased in a dose-related manner, which corresponded to a
dosedependent reduction in body weight. No effects on organ weights,
haematology, B-cell mitogen lipopolysaccharide response, spleen cell
response to the T-cell mitogen concanavalin A or cellmediated immunity
(assessed by measuring the delayed-type hypersensitivity responses to
sheep erythrocytes) were noted. There was a tendency towards a
reduction in the serum antibody level after immunization with sheep
erythrocytes, and in the immunoglobulin spleen antibody-forming cells
at all exposure levels (not significant) (Munson et al., 1982).
7.9 Toxicological interactions with other agents
In a recent bioassay designed to determine the influence of
disulfiram or ethanol on the carcinogenicity, metabolism and covalent
binding to DNA of 1,2-dichloroethane, male and female Sprague-Dawley
rats were exposed to 200 mg/m3 (50 ppm) 1,2-dichloroethane for 7 h
per day, 5 days a week, for 2 years. Additional rats were similarly
exposed and administered either 0.05% disulfiram in the diet or 5%
ethanol in the drinking-water, either alone or in combination with
1,2-dichloroethane. The incidence of any type of tumour was not
elevated compared to controls in any groups exposed to these compounds
individually, nor was the incidence of any type of tumour increased in
rats exposed to 1,2-dichloroethane and ethanol in combination compared
to unexposed controls or rats exposed to these compounds alone.
Exposure to disulfiram in combination with 1,2-dichloroethane resulted
in a significant (p<0.05) increase in the incidence of intrahepatic
bile duct cholangiomas (males: 9/49 versus 0/50, females: 17/50 versus
0/50) and cysts (males: 12/49 versus 0/50, females: 24/50 versus 0/50)
in both male and female rats compared to that in rats exposed to
1,2-dichloroethane only. Male rats exposed to this combination also
had a significantly (p<0.05) increased incidence of subcutaneous
fibromas (10/50 versus 0/50), hepatic neoplastic nodules (6/49 versus
2/50) and interstitial cell tumours in the testes (11/50 versus 3/50)
compared to rats exposed to 1,2-dichloroethane alone. Female rats
similarly exposed had a significantly (p<0.05) higher incidence of
mammary adenocarcinomas compared with rats exposed to
1,2-dichloroethane only (12/48 versus 5/50). Combined exposure to
1,2-dichloroethane and disulfiram did not increase the level of
covalent binding to hepatic DNA compared to that found in rats exposed
to 1,2-dichloroethane alone. The profile of urinary metabolites of a
single radiolabelled oral dose of 1,2-dichloroethane in rats
simultaneously exposed to disulfiram and 1,2-dichloroethane for 2
years indicated that the metabolism of 1,2-dichloroethane was
qualitatively similar to that of rats exposed to 1,2-dichloroethane
alone for 2 years. However, a reduced rate of elimination, and
sustained blood levels of unchanged 1,2-dichloroethane were observed
(see section 6.4), which, the authors stated, could be related to the
carcinogenic effects noted following simultaneous exposure (Cheever et
al., 1990).
In addition, concomitant exposure to disulfiram (as may occur in
the rubber industry or in persons undergoing therapy for alcoholism)
in the diet resulted in a synergistic increase in the hepatotoxicity
(as determined by levels of enzymes in serum and increased relative
liver weight (> 30%)) of 1,2-dichloroethane inhaled at
concentrations of 600, 1200 or 1800 mg/m3 (150, 300 and 450 ppm) for
30 days by male Sprague-Dawley rats. However, rats administered
1,2-dichloroethane alone had evidence of liver damage only at the
highest concentration. The enhanced effects were hypothesized to be
due to the inhibition of mixed-function oxidase-mediated metabolism of
1,2-dichloroethane and a compensatory increase in metabolism of
1,2-dichloroethane to reactive metabolites via cytosolic pathways
mediated by glutathione S-transferase, since hepatic cytochrome
P-450 content decreased with increasing concentration of
1,2-dichloroethane only in the presence of disulfiram (Igwe et al.,
1986a). Concomitant exposure to 1,2-dichloroethane by inhalation
(> 1216 mg/m3 or 304 ppm) or intraperitoneal injection
(150 mg/kg body weight per day) and disulfiram administered in the
diet (0.15%) resulted in testicular atrophy in Sprague-Dawley rats
compared to rats exposed to either compound alone (Igwe et al.,
1986b).
The acute toxicity of carbon tetrachloride was potentiated by
1,2-dichloroethane in rats administered single doses of each of 60 and
125 µl/kg body weight perorally, based on determination of serum
hepatic enzymes and indicators of lipid peroxidation at 24 h after
exposure. Pre-treatment with vitamin E prevented hepatotoxicity.
Based on the observation that the hepatic GSH level in the group
concomitantly exposed to both compounds was not significantly
different from that in the group exposed to carbon tetrachloride
alone, the authors concluded that GSH depletion did not play an
important role in the potentiation (Aragno et al., 1992). Concomitant
exposure to oral doses of 1,2-dichloroethane and 1,2-dibromoethane (60
and 20 µl/kg body weight, respectively) did not result in liver
toxicity in rats, based on levels of serum hepatic enzymes and
indicators of lipid peroxidation, although the compounds alone and in
combination resulted in a decrease in hepatic GSH level 2 h after
exposure, which subsequently returned to control values (Danni et al.,
1992).
The in vitro metabolism of 1,2-dichloroethane by liver
homogenates of rats administered ethanol increased with the dose of
ethanol up to 4 g/kg body weight, but declined sharply at 5 g/kg body
weight (Sato et al., 1981).
High doses (1000 to 2000 mg/kg body weight) of several chemicals,
including methionine, p-aminobenzoic acid, sulfanilamide and
aniline, administered orally to mice were protective against the
lethal effects caused by inhalation of 1600 mg/m3 (400 ppm)
1,2-dichloroethane (Heppel et al., 1945).
The acute and subacute toxicity of dichloroethane increased when
it was administered under conditions of high temperature (species and
exposure protocol not specified in abstract) (Mihaylova, 1976).
8. EFFECTS ON HUMANS
8.1 Case reports
The lethal oral dose of 1,2-dichloroethane in humans has been
estimated to be between 20 and 50 ml. Death due to cardiac arrhythmia
has been reported following ingestion of large, single doses
(50-75.2 g) of 1,2-dichloroethane (Hueper & Smith, 1935; Garrison &
Leadingham, 1954; Martin et al., 1969). Effects identified following
ingestion of 1,2-dichloroethane include central nervous system
depression, gastroenteritis, liver, kidney and lung damage,
cardiovascular disorders and haematological effects (Weiss, 1957;
Morozov, 1958; Hinkel, 1965; Bogoyavlenski et al., 1968; Martin et
al., 1969; Schönborn et al., 1970; Yodaiken & Babcock, 1973; Natsyuk &
Mudritsky, 1974; Dorndorf et al., 1975; Andriukin, 1979).
Effects reported following exposure to 1,2-dichloroethane via
inhalation are very similar to those observed after ingestion but are
usually less pronounced. Inhalation of 1,2-dichloroethane vapour
first affects the central nervous system and causes irritation and
inflammation of the respiratory tract. Damage to the liver, kidneys
and lungs (Wirtschafter & Schwartz, 1939; Hadengue & Martin, 1953;
Menschick, 1957; Troisi & Cavallazzi, 1961; Suveev & Babichenko, 1969;
Nouchi et al., 1984) and changes in the heart rhythm (Suveev &
Babichenko, 1969) have been reported in several cases. Death due to
cardiac toxicity has been reported following a 30-min exposure to an
unknown concentration of 1,2-dichloroethane (Nouchi et al., 1984).
Effects on the eyes were observed in several early case reports
(Weiss, 1957; Menschick, 1957; Troisi & Cavallazzi, 1961), while
severe dermatitis has been reported following dermal contact with
1,2-dichloroethane (Wirtschafter & Schwartz, 1939).
8.2 Epidemiological studies
In a study of 278 men working in the chlorohydrin unit of a
chemical production plant between 1940 and 1967 and followed up to
1988, there was a significant (p < 0.01) excess of deaths due to
pancreatic cancer compared to the USA national rates [Standardized
Mortality Ratio (SMR) = 492 (95% CI = 158 - 1140); Observed:Expected
(O:E) = 8:1.6]. The excess was greater when confined to men who
worked in the unit for more than 2 years (SMR = 800). Based on
comparison with two groups of workers in nearby plants, there were
pronounced increases in mortality due to pancreatic cancer as exposure
duration increased. Though an excess of deaths due to "lymphatic and
haemopoietic cancers" was also observed, it appeared to be
attributable principally to leukaemia, for which numbers of observed
cases were small (O=4) and associations with duration of exposure were
less consistent. Although quantitative data were not available, the
authors concluded on the basis of considerable qualitative information
that workers in this unit had been exposed primarily to
1,2-dichloroethane in combination with bis-chloroethyl ether, ethylene
oxide and ethylene chlorohydrin (Benson & Teta, 1993).
In a case-control study, the exposure of 21 male employees at a
petrochemical plant in Texas, USA, whose deaths were
attributed to cancer of the brain, was compared to that of two groups
of 80 controls from the same plant. One control group consisted of
male employees who had died from non-neoplastic causes, while the
second group of controls consisted of those men whose deaths were due
all other causes. Employees were classified as having been exposed to
1,2-dichloroethane if they had ever worked in a department in which
the compound had been used; unexposed workers had never worked in
these departments and the exposure of others was considered to be
unknown (47.6% of cases, 58.8 and 61.3% of the first and second group
of controls, respectively). When those with unknown exposure were
excluded from the analyses, the proportion of cases (all cases or
glioma cases specifically) who were exposed (n = 11 and 10, or 45.5
and 50.0%, respectively) did not differ significantly from the
proportion of controls who had been exposed to 1,2-dichloroethane
(42.4 and 45.2% for the two control groups). When a 15-year latency
period was considered in the analysis, the proportion of cases and
controls exposed still did not differ significantly (40.0 and 44.4%
for cases versus 32.3 and 34.6% for the two control groups) (Austin &
Schnatter, 1983a).
In an accompanying historical cohort study of 6588 workers at
this plant, there was no significant excess in malignant brain tumours
in the overall population of the plant compared to national rates,
although there was a borderline significant (p < 0.05) increase in
hourly employees with more than 6 months of employment (O/E = 10/5).
However, exposure to 1,2-dichloroethane was not specifically
considered in this study, and employees were also exposed to a number
of potentially confounding substances, including benzene, diethyl
sulfate, ethylene oxide and vinyl chloride (Austin & Schnatter,
1983b).
Deschamps & Band (1993) conducted a small case-control study to
investigate a possible association between a spill of
1,2-dichloroethane in 1982 into a river supplying drinking-water to
parts of the city of Vancouver, Canada, and an identified cluster of
childhood leukaemia cases in the city. It was determined that none of
the 15 cases diagnosed between 1975 and 1988 had lived in areas of the
city serviced by the contaminated supply.
In an ecological study in which potential associations between
contamination of drinking-water from groundwater supplies by
particular substances (including 10 volatile organic compounds and 43
inorganic elements) and cancer were investigated, the average annual
age-adjusted incidence (1969 to 1981) of colon and rectal cancer was
statistically significantly greater in men aged > 55 years whose
drinking-water contained > 0.1 µg 1,2-dichloroethane/litre than in
those whose drinking-water contained < 0.1 µg/litre (222.8 per
100 000 versus 170.3 per 100 000 (193 and 633 cases, p = 0.02) and
126.5 per 100 000 versus 92.9 per 100 000 (106 and 337 cases,
p = 0.009), respectively). Rectal cancer in males was also associated
with chlorination of drinking-water. Of the study population over 55
years of age, 50% had lived at the same address for 20 years or more.
There were no significant differences between groups of towns with
respect to eight socioeconomic factors examined, except that the
percentage change in population between 1970 and 1980 was
significantly less in those towns with > 0.1 µg
1,2-dichloroethane/litre. The authors did not suggest that the result
indicated a causal relationship between 1,2-dichloroethane and cancer,
but that cancer incidence may be elevated in populations consuming
water from wells subject to anthropogenic contamination (Isacson et
al., 1985).
The prevalence of subjective symptoms was higher in a group of 10
male oil refinery workers exposed to between 250 and 800 mg/m3 than
in those exposed to lower concentrations (40 to 150 mg/m3); a
"general reduction in body weight" was also noted in both groups.
"Abnormalities" of the liver, central nervous system, gastrointestinal
tract and haematological parameters were reported in some workers
(n = 1 to 8) in the group exposed to the higher concentration,
presumably based upon clinical examination, although this was not
specified in the previous account of this early study. There were no
unexposed controls, and workers were also exposed to benzene (10 to
25 mg/m3) (Cetnarowicz, 1959).
The morbidity during a 5-year period (1951 to 1955) was increased
for all disease categories (not further specified) in a group of
workers (number not specified) at an aircraft factory exposed to
5 mg/m3 or less for 70 to 75% of the working time and 80 to
150 mg/m3 for the remainder, when compared to workers in the entire
factory. Of 83 workers examined further, 19 had disease of the liver
and bile duct, 13 had neurotic conditions, 11 had autonomous dystonia
and 10 had hyperthyroidism and goitre (there were no controls for
comparison) (Kozik, 1957).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
9.1.1 Microorganisms
Blum & Speece (1991) investigated the toxicity of
1,2-dichloroethane to three groups of aquatic bacteria: methanogens,
aerobic heterotrophs and Nitrosomonas. The end-points assessed
included inhibition of gas production (methanogens), oxygen uptake
(aerobic heterotrophs), and ammonia consumption (Nitrosomonas). The
IC50 values for Nitrosomonas and methanogens (29 and 25 mg/litre,
respectively) were considerably lower than that for aerobic
heterotrophs (470 mg/litre). For the bacteria Pseudomonas putida,
the nominal 16-h toxicity threshold for the onset of cell
multiplication inhibition was 135 mg/litre (Bringmann & Kühn, 1981).
Tang et al. (1990) determined the IC50 values based on inhibition of
respiration rate for activated sewage sludge using open and closed
serum bottle methods to be 35 500 mg/litre and 2780 mg/litre,
respectively. The difference was attributed to the volatility of
1,2-dichloroethane which was stripped from the substrate in the open
method.
The freshwater cyanobacterium (blue-green alga) Microcystis
aeruginosa was seven times more sensitive to 1,2-dichloroethane than
the green alga Scenedesmus quadricauda, the nominal 7-day EC50
values for inhibition of cell multiplication at 27°C being 105 and
710 mg/litre, respectively (Bringmann & Kühn, 1978). The 72-h EC50
for inhibition of growth was 189 mg/litre (Freitag et al., 1994).
Bringmann & Kühn (1980) determined the toxicity thresholds for
Scendesmus quadricauda and the protozoan Entosiphon sulcatum to be
710 mg/litre and 1127 mg/litre, respectively. Knie et al. (1983)
reported the acute EC50 for the alga Haematococcus pluvialis to be
130 mg/litre. Based on bioluminescence, the 5-min IC50 was
700 mg/litre in a Microtox test with Photobacterium phosphoreum
(Blum & Speece, 1991). Freitag et al. (1994) reported the 15-min EC50
for inhibition of bioluminescence in this species to be 770 mg/litre.
Bringmann & Kühn (1981) determined the toxicity thresholds in the
holozoic bacteriovorous flagellate protozoan Entosiphon sulcatum, the
holozoic bacteriovorous ciliate protozoan Uronema parduczi, and the
saprozoic flagellate protozoan Chilomonas paramecium to be > 8000
mg/litre, > 16 000 mg/litre and > 800 mg/litre, respectively, using
the cell multiplication inhibition test.
Pearson & McConnell (1975) determined the EC50 (based on carbon
uptake from CO2 during photosynthesis) in the marine unicellular
alga Phaeodactylum tricorhutum to be 340 mg/litre.
9.1.2 Invertebrates
Based on a review of identified acute and chronic toxicity
studies in freshwater invertebrates, Daphnia magna appears to be the
species most sensitive to 1,2-dichloroethane. Under static test
conditions, the measured 48-h LC50 values for fed and unfed first
instar D. magna were 320 and 270 mg/litre, respectively; the 48-h
EC50 values, based on complete immobilization, were 180 and
160 mg/litre for fed and unfed organisms, respectively (Richter et
al., 1983). Leblanc (1980) reported the 24-h and 48-h LC50 values
in D. magna to be 250 and 220 mg/litre, respectively, while the "no
discernible effect concentration" (apparently based on mortality only)
was < 68 mg/litre. Using the Probit method, Ahmad et al. (1984)
determined the 48-h LC50 in unfed D. magna to be 268 mg/litre (95%
CL: 246-293), while the EC50, based on reproductive effects was
155 mg/litre (95% CL: 137-188). Freitag et al. (1994) determined the
24-h EC50 for 10% immobilization of D. magna to be 150 mg/litre.
Knie et al. (1983) reported the EC0, EC50 and EC100 in D. magna
to be 67, 600 and 1075 mg/litre, respectively. Richter et al. (1983)
also examined the effect of 1,2-dichloroethane on reproductive success
and length of first instar D. magna in a 28-day flow-through test.
For reproductive success, the measured lowest-observed-effect level
(LOEL) and no-observed-effect level (NOEL) were 20.7 and
10.6 mg/litre, respectively, while the measured LOEL and NOEL for
growth were 71.7 and 41.6 mg/litre, respectively.
Ahmad et al. (1984) also conducted chronic toxicity studies in
which D. magna were exposed to 0, 10.6, 20.6, 41.6, 71.7, 94.4 and
137 mg 1,2-dichloroethane/litre for 28 days. There was a
concentration-related decrease (as low as 12% of control values) in
the number of young produced (significant (p < 0.05 or 0.01) at
41.6 mg/litre or more) as well as a decrease (as low as 59% of control
values) in the length of adults (significant (p < 0.01) at
71.7 mg/litre or more). Few acute toxicity studies in marine
invertebrates were identified. Under static test conditions, the
nominal 24-h EC50 for immobilization of 30-h posthatch larvae of the
brine shrimp Artemia salina was 93.6 mg/litre (Foster & Tullis,
1984). For marine adult shrimp (Crangon crangon), the measured 24-h
LC50 was 170 mg/litre under static test conditions (Rosenberg et
al., 1975). The 48-h LC50 for barnacle nauplii (Elminuis modestus)
was 186 mg/litre (Pearson & McConnell, 1975). Teratogenic effects
(expressed as surviving larvae with gross debilitating abnormalities)
were observed in the nauplii of the marine brine shrimp (Artemia
salina) at concentrations between 0.25 and 25 mg/litre (Kerster &
Schaeffer, 1983).
9.1.3 Vertebrates
The embryos and larvae of the northwestern salamander (Ambystoma
gracile) and the leopard frog (Rana pipiens) were continuously
exposed to 1,2-dichloroethane from within 30 min of fertilization
(embryos) and maintained for 4 days after hatching (larvae). The
LC50 values for the salamander at the day of hatching (day 5) and 4
days after hatching (day 9) were 6.53 and 2.54 mg/litre, respectively;
the measured LOEL for 23% reduction in egg hatchability was
0.99 mg/litre. The measured 5-day and 9-day LC50 values for the
frog were 4.52 and 4.40 mg/litre, respectively, while the 5-day
posthatch LOEL was 1.07 mg/litre (Black et al., 1982).
Acute toxicity studies have been conducted on several species of
freshwater fish. The most sensitive species was the guppy (Poecilia
reticulata, 2-3 months old), with a nominal 7-day LC50 of
106 mg/litre under static renewal test conditions (Konemann, 1981).
In three studies on 30-day-old fathead minnows (Pimephales promelas),
measured 96-h LC50 values ranged from 116 to 136 mg/litre under
flow-through conditions (Veith et al., 1983; Walbridge et al., 1983;
Geiger et al., 1985). LC50 values after 24, 48, 72 and 96 h in
rainbow trout (Salmo gairdneri) were 362, 340, 337 and 336 mg/litre,
respectively, using the static test method (Bartlett, 1979). Knie et
al. (1983) reported the EC0, EC50 and EC100 in golden orfe
(Leuciscus idus) to be 67, 600 and 1075 mg/litre, respectively.
In marine fish, a nominal 96-h LC50 of 480 mg/litre was
reported in tidewater silversides (Minidia beryllina) under static
test conditions (Dawson et al., 1975/77). Heitmuller et al. (1981)
reported the static test LC50 at 24, 48, 72 and 96 h in sheepshead
minnows (Cyprinodon variegatus) to be between 130 and 230 mg/litre.
The 96-h LC50 for dab (Limanda limanda) was 115 mg/litre (Pearson
& McConnell, 1975).
In a long-term, flow-through study of the early life stages of
fathead minnows (Pimephales promelas), there were no effects on egg
hatchability or larval survival and deformity at 29 mg/litre (NOEL);
however, larval growth was significantly (p < 0.05) reduced by 62% at
59 mg/litre (LOEL) (Benoit et al., 1982). Black et al. (1982) exposed
the embryos and larvae of the rainbow trout (Oncorhynchus mykiss)
continuously to 1,2-dichloroethane under flow-through conditions from
within 30 min of fertilization (embryos) and maintained them until 4
days after hatching. The resulting EC50 for hatchability and 27-day
LC50 for post-hatch survival were both 34 mg/litre, and the LOEL for
a 24% reduction in egg hatchability was 3.49 mg/litre. After 21 days
of continuous exposure to 150 mg 1,2-dichloroethane/litre, the
mortality of coho salmon (Oncorhynchus kisutch) eggs was 46%, while
in alevins, 100% mortality occurred 9 days after hatching at
320 mg/litre (Reid et al., 1982). In addition, premature hatching was
observed at 56 mg/litre, and, within one week of hatching, sublethal
effects, including lethargy and loss of equilibrium, were observed in
alevins exposed to 56 mg/litre; 100% mortality occurred 9 days after
hatching.
Ahmad et al. (1984) conducted chronic toxicity studies in which
fathead minnows were exposed to 300, 4000, 7000, 14 000, 29 000 or
39 000 µg/litre for 32 days. There were no significant effects on
survival, although there was a significant (p < 0.05) decrease in
mean individual net weight at the highest concentration (38% of
control values). The MATC was determined to be 49 000 to
59 000 µg/litre.
Teratogenic effects (expressed as surviving larvae with gross,
debilitating abnormalities) were observed in the larvae of
northwestern salamanders (Ambystoma gracile), leopard frogs
(Rana pipiens) and rainbow trout (Oncorhynchus mykiss) at 21.4,
21.9 and 34.4 mg/litre, respectively (Black et al., 1982).
9.2 Terrestrial organisms
9.2.1 Invertebrates
In an acute contact test, the 48-h LC50 for earthworms (Eisenia
fetida) exposed to 1,2-dichloroethane-treated filter-paper was
60 µg/m2 (Neuhauser et al., 1985).
9.2.2 Vertebrates
Male and female white leghorn chickens were fed mash which had
been fumigated with 1,2-dichloroethane, resulting in concen-trations
in the feed of 250 and 500 mg/kg, for 2 years. The end-points
examined included serum composition, growth, semen characteristics and
fertility. The weight of eggs was significantly reduced at 250 mg/kg
(5 to 10% (p < 0.01)), while both the number and weight of eggs were
reduced at 500 mg/kg (5 to 48% (p < 0.05) and 5 to 13% (p < 0.01),
respectively) (Alumot et al., 1976b).
9.2.3 Plants
1,2-Dichloroethane vapour was both lethal and mutagenic to barley
kernels (two-rowed variety, Bonus) following exposure to 3 mg/m3 for
24 h (Ehrenberg et al., 1974).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Available data on the carcinogenicity of 1,2-dichloroethane in
humans are limited. There is convincing evidence of increases in the
incidence of both common and rare tumours in experimental animals at
several sites (including squamous cell carcinomas of the stomach,
haemangiosarcomas, fibromas of the subcutaneous tissue and
adenocarcinomas and fibroadenomas of the mammary gland in rats; and
alveolar/bronchiolar adenomas, mammary gland adenocarcinomas,
endometrial stromal polyp or endometrial stromal sarcoma combined and
hepatocellular carcinomas in mice) following administration by gavage
for 78 weeks.
The incidence of benign lung papillomas was significantly
increased in mice following long-term dermal application of
1,2-dichloroethane, while a non-significant increase in the number of
pulmonary adenomas per animal was reported in a screening bioassay on
mice and in the incidence of benign mammary gland tumours in rats
exposed by inhalation for 2 years.
1,2-Dichloroethane is genotoxic in in vitro and in vivo
assays, and binds to DNA in rodents in vivo.
Based on the induction of both rare and common tumours in rats
and mice exposed by ingestion and supporting evidence in other limited
bioassays, the production of a reactive intermediate that alkylates
DNA and positive results in a range of in vitro assays for
genotoxicity, 1,2-dichloroethane is considered to be a probable human
carcinogen.
10.2 Environmental assessment
The high volatility of 1,2-dichloroethane makes the atmosphere
the predominant environmental sink. Consequently, measured
concentrations in surface waters are low (around 1 to 10 µg/litre).
Air concentrations are highest around manufacturing plants where they
may reach 300 µg/m3; concentrations in urban air average
< 1 µg/m3. Low adsorption to soil leads to potential leaching to
groundwater; some measurements of low concentrations in drinking-water
(< 0.2 µg/litre) confirm this.
Both hydrolysis and microbial degradation are slow; the
volatility of the compound means that it has low residence time in
media where these processes occur and they are not considered to be of
environmental significance.
The estimated atmospheric lifetime of 1,2-dichloroethane is
between 40 and 110 days. Stratospheric photolysis may produce chlorine
radicals which may in turn react with ozone. However, the
ozone-depleting potential is low (0.001 relative to CFC-11) and the
compound is not listed in the Montreal Convention.
Various toxicity tests have shown LC50s for organisms in the
environment to be generally greater than 10 mg/litre. The difference
(at least 7 orders of magnitude) between measured water concentrations
and these toxic concentrations indicate that 1,2-dichloroethane poses
no risk to organisms since exposure will not occur.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN
HEALTH AND THE ENVIRONMENT
Considering the toxicological characteristics of
1,2-dichloroethane, both qualitatively and quantitatively, an exposure
that would not cause adverse effects in humans by any route of
exposure cannot be estimated. Consequently, all appropriate measures
should be taken to eliminate or minimize human exposure to
1,2-dichloroethane.
12. FURTHER RESEARCH
Although specific studies were not recommended for
1,2-dichloroethane, additional analytical epidemiological studies are
desirable.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1979) has
classified 1,2-dichloroethane in group 2B (possibly carcinogenic to
humans), based on sufficient evidence of carcinogenicity in
experimental animals.
The International Programme on Chemical Safety has previously
evaluated 1,2-dichloroethane (IPCS, 1987). It concluded that
1,2-dichloroethane produces central nervous system depression, and
gastrointestinal and liver abnormalities in humans. The same effects
occur in experimental animals, in addition to possible kidney
abnormalities, lung oedema and cardiovascular disorders.
1,2-Dichloroethane, administered by gavage, is carcinogenic in rats
and mice, and should be regarded, for practical purposes, as if it
presented a carcinogenic risk for humans. 1,2-Dichloroethane was not
considered to accumulate in the environment. In the atmosphere, it is
removed by photo-chemical degradation via hydroxyl radicals and is
eliminated from water by evaporation. It has a low octanol/water
partition coefficient and bioconcentration is unlikely. It was not
considered to pose a hazard to the aquatic environment except in the
case of accidents and inappropriate disposal. Data were insufficient
to evaluate the effects of 1,2-dichloroethane on the terrestrial
environment.
The Joint FAO/WHO Expert Committee on Food Additives
(JECFA) has evaluated 1,2-dichloroethane on three occasions (WHO,
1971, 1980, 1992). When last evaluated, the Committee concluded that
this compound is genotoxic in both in vitro and in vivo test
systems and carcinogenic in mice and rats when administered by the
oral route. No ADI was therefore allocated. The Committee expressed
the opinion that 1,2-dichloroethane should not be used in food.
In the WHO Guidelines for drinking-water quality (WHO, 1993), the
concentrations of 1,2-dichloroethane in drinking-water estimated to be
associated with excess risks of 10-4, 10-5 and 10-6 are 300, 30 and
3 µg/litre, respectively, based on linearized multistage modelling of
the incidence of haemangiosarcomas in male rats in the NCI (1978)
study.
The European Commission published a directive in 1990 in which
limit values for emission of 1,2-dichloroethane were specified for
various types of industrial plants. These limits ranged from
0.1 mg/litre (monthly) for plants using 1,2-dichloroethane for
degreasing metals away from an industrial site to 12 mg/litre (daily)
for plants producing 1,2-dichloroethane and processing or using the
substance at the site (CEC, 1990).
REFERENCES
AEC (Alberta Environmental Centre) (1989) Final effluent analysis of
Dow Chemical, Fort Saskatchewan, Alberta plant. Alberta, Canada,
Alberta Environmental Centre (Unpublished data).
Ahmad N, Benoit D, Brooke L, Call D, Carlson A, DeFoe D, Huot J,
Moriarity A, Richter J, Shubat P, Veith G, & Wallbridge C (1984)
Aquatic toxicity tests to characterize the hazard of volatile organic
chemicals in water: a toxicity data summary - Parts I and II. Duluth,
Minnesota, US Environmental Protection Agency, Office of Research and
Development, Environmental Research Laboratory.
Alumot E, Nachtomi E, Mandel E, & Holstein P (1976a) Tolerance and
acceptable daily intake of chlorinated fumigants in the rat diet. Food
Cosmet Toxicol, 14: 105-110.
Alumot E, Meidler M, Holstein P, & Herzberg M (1976b) Tolerance and
acceptable daily intake of ethylene dichloride in the chicken diet.
Food Cosmet Toxicol, 14: 111-114.
Andriukin AA (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klin Med (Mosk), 57: 43-47 (in Russian).
Apostolov I & Mihaylova A (1975) [Investigation into
beta- N-acetylglucosaminidase activity in the serum of white rats
treated with 1,2-dichloroethane.] Probl Hyg, 1: 161-163
(in Bulgarian).
Aragno M, Tamagno E, Danni O, & Ugazio G (1992) In vivo studies on
halogen compound interactions. III. Effect of carbon tetrachloride
plus 1,2-dichloroethane on liver necrosis and fatty accumulation. Res
Commun Chem Pathol Pharmacol, 76: 341-354.
Archer WJ (1979) Chlorocarbons and chlorohydrocarbons. In: Kirk J &
Othmer DF ed. Encyclopedia of chemical technology, 3rd ed. New York,
John Wiley & Sons, vol 5, pp 723-743.
Arfellini G, Bartoli S, Colacci A, Mazzullo M, Galli MC, Prodi G, &
Grilli S (1984) In vivo and in vitro binding of 1,2-dibromoethane
and 1,2-dichloroethane to macromolecules in rat and mouse organs. J
Cancer Res Clin Oncol, 108: 204-213.
Armstrong MJ & Galloway SM (1993) Micronuclei induced in peripheral
blood of Eµ-PIM-1 transgenic mice by chronic oral treatment with
2-acetylaminofluorene or benzene but not with diethylnitrosamine or
1,2-dichloroethane. Mutat Res, 302: 61-70.
Atkinson R (1987) A structure-activity relationship for the estimation
of rate constants for gas-phase reaction of OH radicals with organic
compounds. Int J Chem Kinet, 19: 799-828.
ATSDR (1989) Toxicological profile for 1,2-dichloroethane. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry, 129 pp.
ATSDR (1994) Toxicological profile for 1,2-dichloroethane (Update).
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry,
192 pp (TP-93/06).
Austin SG & Schnatter AR (1983a) A case-control study of chemistry
exposures and brain tumors in petrochemical workers. J Occup Med,
25(4): 313-320.
Austin SG & Schnatter AR (1983b) A cohort mortality study of
petrochemical workers. J Occup Med, 25(4): 304-312.
Baertsch A, Lutz WK, & Schlatter C (1991) Effect of inhalation
exposure regimen on DNA binding potency of 1,2-dichloroethane in the
rat. Arch Toxicol, 65: 169-176.
Bailey S, Collins GB, Fishwick FB, Hart HV, Horler DF, & Scudamore KA
(1982) Pesticide residues in foodstuffs in Great Britain:
Organochlorine pesticides, organophosphorus pesticides and fumigant
residues in home-produced and imported wheat. Pestic Sci,
13(4): 373-378.
Ballering LAP, Nivard MJM, & Vogel EW (1993) Characterization of the
genotoxic action of three structurally related 1,2-dihaloalkanes in
Drosophila melanogaster. Mutat Res, 285: 209-217.
Ballering LAP, Nivard MJM, & Vogel EW (1994) Mutation spectra of
1,2-dibromoethane, 1,2-dichloroethane and 1-bromo-2-chloroethane in
excision repair proficient and repair deficient strains of Drosophila
melanogaster. Carcinogenesis, 15: 869-875.
Banerjee S (1988) DNA damage in rodent liver by 1,2-dichloroethane, a
hepatocarcinogen. Cancer Biochem Biophys, 10: 165-173.
Banerjee S & Van Duuren BL (1979) Binding of carcinogenic halogenated
hydrocarbons to cell macromolecules. J Natl Cancer Inst, 63: 707-711.
Banerjee S, Van Duuren BL, & Oruambo FI (1980) Microsome-mediated
covalent binding of 1,2-dichloroethane to lung microsomal protein and
salmon sperm DNA. Cancer Res, 40: 2170-2173.
Barbash JE & Reinhard M (1989) Abiotic dehalogenation of
1,2-dichloroethane and 1,2-dibromoethane in aqueous solution
containing hydrogen disulfide. Environ Sci Technol, 23(11): 1349-1357.
Barber RD, Donish WH, & Mueller KR (1981) A procedure for the
quantitative measurement of the mutagenicity of volatile liquids in
the Ames Salmonella microsome assay. Mutat Res, 90: 31-48.
Baretta ED, Steward RD, & Mutchler JE (1969) Monitoring exposure to
vinyl chloride vapour: breath analysis and continuous air sampling. Am
Ind Hyg Assoc J, 30: 537-544.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizzari ED, Ray M, Smith D, Tomer KB, Wagner R, & Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - A
multimedia environmental study. Biomed Environ Mass Spectrom,
7: 139-147.
Barrows ME, Petrocelli SR, Macek KJ, & Carroll JJ (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Haque R ed. Dynamics,
exposure and hazard assessment of toxic chemicals. Ann Arbor,
Michigan, Ann Arbor Science Publishers, chapter 24, pp 379-392.
Barsoum GS & Saad K (1934) Relative toxicity of certain chlorine
derivatives of the aliphatic series. Q J Pharm Pharmacol, 7: 205-214.
Bartlett EA (1979) Toxicity of ethylene dichloride to rainbow trout.
Midland, Michigan, Dow Chemical (Unpublished report).
Bauer U (1981) [Human exposure to environmental chemicals -
Investigations on volatile organic halogenated compounds in water,
air, food, and human tissues. III. Communication: results of
investigations.] Zbl Bakteriol Hyg I Abt Orig B, 174(3): 200-237 (in
German).
Benoit DA, Puglisi FA, & Olson DL (1982) A fathead minnow Pimephales
promelas early stage toxicity test method evaluation and exposure to
four organic chemicals. Environ Pollut, A28: 189-197.
Benson LO & Teta MJ (1993) Mortality due to pancreatic and
lymphopoietic cancers in chlorohydrin production workers. Br J Ind
Med, 50: 710-716.
Berck B (1974) Fumigant residues of carbon tetrachloride, ethylene
dichloride, and ethylene dibromide in wheat, flour, bran, middlings,
and bread. J Agric Food Chem, 22(6): 977-984.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, & Bruser DM
(1982) The aquatic toxicity of organic compounds to embryo-larval
stages of fish and amphibians. Lexington, Kentucky, University of
Kentucky (Research Report No. 133).
Blum DJW & Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons and
correlations. J Water Pollut Control Fed, 63(3): 198-207.
Bogoyavlenski VF, Salikhova SKH, & Karpova EV (1968) [Clinical aspects
and therapy for ethylene dichloride poisoning.] Sov Med, 31: 107-109
(in Russian).
Bond EJ (1984) Manual of fumigation for insect control. Rome, Food and
Agriculture Organization of the United Nations, 432 pp (FAO Plant
Production and Protection Paper 54).
Bonnet P, Francin J-M, Grakiski D, Raoult G, & Zissu D (1980)
Détermination de la concentration léthale50 des principaux
hydrocarbures aliphatiques chlorés chez le rat. Arch Mal Prof,
41(6-7): 317-321.
Bouwer EJ & McCarty PL (1983) Transformation of 1- and 2-carbon
halogenated aliphatic organic compounds under methanogenic conditions.
Appl Environ Microbiol, 45(4): 1286-1294.
Brem H, Stein AB, & Rosenkranz HS (1974) The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer Res, 34: 2576-2579.
Bringmann G & Kühn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1981) [Toxicity thresholds of pollutants in
ciliate flagellate protozoa, holozoic bacteriovorous ciliate protozoa
and saprozoic flagellate protozoa.] GWF-Wasser/Abwasser, 122: 308-313
(in German).
Brittebo EB, Kowalsky B, Ghantous H, & Brandt I (1989) Epithelial
binding of 1,2-dichloroethane in mice. Toxicology, 56: 35-45.
Brodzinsky R & Singh HB (1982) Volatile organic chemicals in the
atmosphere: An assessment of available data (Contract No. 68-02-3452).
Menlo Park, California, SRI International (Prepared for the US
Environmental Protection Agency, Office of Research and Development,
Environmental Sciences Research Laboratory, Research Triangle Park,
North Carolina, USA).
Bruckmann P, Kersten W, Funcke W, Balfanz E, Konig J, Theisen J, Ball
M, & Papke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17(12): 2363-2380.
Buijs W, van der Gen A, Mohn GR, & Breimer DD (1984) The direct
mutagenic activity of alpha, omega-dihalogenoalkanes in Salmonella
typhimurium. Mutat Res, 141: 11-14.
Callahan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR,
Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR, &
Gould C (1979) Water-related fate of 129 priority pollutants: Volume
II. Washington, DC, US Environmental Protection Agency
(EPA 40/4-79-029b).
Cetnarowicz J (1959) Experimental and clinical investigation on the
action of dichloroethane. Folia Med Crac, 1: 169-192.
Cheever KL, Cholakis JM, El-Hawari AM, Kovatch RM, & Weisburger EK
(1990) Ethylene dichloride: The influence of disulfiram or ethanol on
oncogenicity, metabolism and DNA covalent binding in rats. Fundam Appl
Toxicol, 14: 243-261.
Cheh AM, Hooper AB, Skochdopole J, Henke CA, & McKinnell RG (1980) A
comparison of the ability of frog and rat S-9 to activate promutagens
in the Ames test. Environ Mol Mutagen, 2: 487-508.
Chemical Marketing Reporter (1992) Chemical profile: Ethylene
dichloride. Chem Mark Report Mag, 241(19): 42.
Chiou CT, Peters LJ, & Freed VH (1979) A physical concept of
soil-water equilibria for nonionic organic compounds. Science,
206: 831-832.
Chiou CT, Freed VH, Peters LJ, & Kohnert RL (1980) Evaporation of
solutes from water. Environ Int, 3: 231-236.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984a) Ambient air
measurement of aromatic and halogenated hydrocarbons at urban, rural,
and motorway locations. Sci Total Environ, 39: 265-279.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984b) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural, and motorway locations.
Environ Pollut, B7: 141-158.
Class T & Ballschmiter K (1986) Chemistry of organic traces in air.
VI: Distribution of chlorinated C1 - C4 hydrocarbons in air over the
northern and southern Atlantic Ocean. Chemosphere, 15(4): 413-427.
Comba ME & Kaiser KLE (1983) Determination of volatile contaminants at
the ng-L-1 level in water by capillary gas chromatography with
electron capture detection. Int J Environ Anal Chem, 16: 17-31.
Comba ME & Kaiser KLE (1985) Volatile halocarbons in the Detroit River
and their relationship with contaminant sources. J Great Lakes Res,
11(3): 404-418.
Cottalasso D, Barisione G, Fontana L, Domenicotti C, Pronzato MA, &
Nanni G (1994) Impairment of lipoglycoprotein metabolism in rat liver
cells induced by 1,2-dichloro-ethane. J Occup Environ Med,
51: 281-285.
CEC (1990) Council directive of 27 July 1990 amending annex II to
directive 86/280/EEC on limit values and quality objectives for
discharges of certain dangerous substances included in List I of the
annex to directive 76/464/EEC. Brussels, Commission of the European
Communities.
CPI (Canadian Process Industries) (1991) CPI product profiles:
Ethylene dichloride. Don Mills, Ontario, Corpus Information Services.
Crebelli R & Carere A (1988) Genotoxic activity of halogenated
aliphatic hydrocarbons in Aspergillus nidulans. J Occup Toxicol,
8: 437-442.
Crebelli R, Conti G, Conti L, & Carere A (1984) Induction of somatic
segregation by halogenated aliphatic hydrocarbons in Aspergillus
nidulans. Mutat Res, 138: 33-38.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, & Carere A (1988)
Induction of chromosome malsegregation by halogenated organic solvents
in Aspergillus nidulans: Unspecific or specific mechanism? Mutat
Res, 201: 401-411.
Crespi CL, Seixas GM, Turner TR, Ryan CG & Penman BW (1985)
Mutagenicity of 1,2-dichloroethane and 1,2-dibromoethane in two human
lymphoblastoid cell lines. Mutat Res, 142: 133-140.
Daft JL (1987) Determining multifumigants in whole grains and legumes,
milled and low-fat grain products, spices, citrus fruit, and
beverages. J Assoc Off Anal Chem, 70: 734-739.
Daft JL (1988) Rapid determination of fumigant and industrial chemical
residues in food. J Assoc Off Anal Chem, 71(4): 748-760.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and nonfatty foods. J Agric Food Chem, 37: 560-564.
Daft JL (1991) Fumigants and related chemicals in foods: Review of
residue findings, contamination sources, and analytical methods. Sci
Total Environ, 100: 501-518.
Daft JL (1993) Fumigant analysis of foods. In: Sherman J & Cairns T
ed. Comprehensive analytical profiles of important pesticides. Boca
Raton, Florida, CRC Press, pp 235-281.
Dann T (1992) Unpublished data on concentrations of 1,2-dichloroethane
in ambient air across Canada. Ottawa, Ontario, Environment Canada.
Danni O, Aragno M, Tamagno E, & Ugazio G (1992) In vivo studies on
halogen compound interactions. IV. Interaction among different halogen
derivatives with and without synergistic action on liver toxicity. Res
Commun Chem Pathol Pharmacol, 76(3): 355-366.
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1975/77) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J
Hazard Mater, 1(4): 303-318.
Deschamps M & Band P (1993) Study of a cluster of childhood leukemia.
Health Rep, 5(1): 81-85.
Dilling WL, Tefertiller NB, & Kalos GJ (1975) Evaporation rates and
reactivities of methylene chloride, chloroform, 1,1,1-trichloroethane,
trichloroethylene, tetrachloroethylene, and other chlorinated
compounds in dilute aqueous solutions. Environ Sci Technol,
9(9): 833-838.
Dorndorf W, Kresse M, Christian W, & Katritzki KG (1975)
[Dichloroethane poisoning with myoclonic syndrome, epileptic attacks,
and irreversible cerebral effects.] Arch Psychiatr Nervenkr,
220: 373-379 (in German).
Drury JS & Hammons AS (1979) Investigations of selected environmental
pollutants: 1,2-Dichloroethane. Oak Ridge National Laboratory, Oak
Ridge, Tennessee; Environmental Protection Agency, Washington, D.C.
Office of Toxic Substances; Department of Energy, Washington, DC.
Washington, DC, US Environmental Protection Agency (EPA 560/2-78-006).
D'Souza R, Francis WR, Bruce RD, & Anderson ME (1987) Physiologically
based pharmacokinetic model for ethylene dichloride and its
application in risk assessment. In: Pharmacokinetics in risk
assessment - Drinking water and health. Washington, DC, National
Academy Press, vol 8, pp 286-301.
D'Souza RW, Francis WR, & Andersen ME (1988) Physiological model for
tissue glutathione depletion and increased resynthesis after ethylene
dichloride exposure. J Pharmacol Exp Ther, 245: 563-568.
Duprat P, Delsaut L, & Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les muqueuses
oculaires du lapin. Eur J Toxicol, 9: 171-177.
Easley DM, Kleopfer RD, & Carasea AM (1981) Gas chromatographic-mass
spectrometric determination of volatile organic compounds in fish. J
Assoc Off Anal Chem, 64: 653-656.
Ecobichon DJ & Allen MC (1990) New Brunswick - Water Quality
Surveillance Program. New Brunswick Public Water Supplies: Data
summary report 1990. Fredericton, New Brunswick, Canada, Department of
Health and Community Services, 66 pp.
Ehrenberg L, Osterman-Golkar S, Singh D, & Lundqvist U (1974) On the
reaction kinetics and mutagenic activity of methylating and
ß-halogenoethylating gasoline additives. Radiat Bot, 15: 185-194.
Ellington JJ, Stancil FE, Payne WD, & Trusty CD (1988) Measurement of
hydrolysis rate constants for evaluation of hazardous waste land
disposal: Volume III - Data on 70 chemicals. Athens, Georgia, US
Environmental Protection Agency (EPA/600/3-88/028. Entz RC, Thomas KW,
& Diachenko GW (1982) Residues of volatile halocarbons in foods using
headspace gas chromatography. J Agric Food Chem, 30(5): 846-849.
Environment Agency Japan (1983) Environmental monitoring of chemicals:
Environmental survey report of fiscal years 1980 and 1981. Tokyo,
Japan, Environment Agency.
Environment Agency Japan (1993) Chemicals in the environment - 1993.
Tokyo, Japan, Environment Agency.
Environment Canada (1986) Canada-Ontario agreement respecting Great
Lakes water quality. St. Clair river pollution investigation (Sarnia
area). Ottawa, Ontario, Environment Canada, 80 pp.
Enviro-Test Laboratories (1991) Cayley background study: Analysis of
food products for target organic and inorganic parameters. Edmonton,
Alberta, Canada, Enviro-Test Laboratories, 37 pp
(Report No. 91-E1208).
Enviro-Test Laboratories (1992) Windsor area background study:
Analysis of food products for target organic and inorganic parameters.
Edmonton, Alberta, Enviro-Test Laboratories, 36 pp
(Report No. 92-E1052).
FAO/WHO (1971) Evaluation of food additives. Specifications for the
identity and purity of food additives and their toxicological
evaluation: some extraction solvents and certain other substances; and
a review of the technological efficacy of some antimicrobial agents.
Fourteenth report of the Joint FAO/WHO Expert Committee on Food
Additives. Geneva, World Health Organization, 36 pp (WHO Technical
Report Series No. 462).
FAO/WHO (1980) Evaluation of certain food additives. Twenty-third
report of the Joint FAO/WHO Expert Committee on Food Additives.
Geneva, World Health Organization (WHO Technical Report Series No. 648
and corrigenda).
FAO/WHO (1992) Evaluation of certain food additives and naturally
occurring toxicants. Thirty-ninth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization (WHO
Technical Report Series No. 828).
Fellin P, Barnett SE, & Tran QA (1992) Results of a national pilot
survey of airborne volatile organic compounds in Canadian residences:
Volume 1. Downsview, Ontario, Canada, Concord Environmental
Corporation, 156 pp (prepared for Health and Welfare Canada, Ottawa).
Ferreri AM, Rocchi P, Capucci A, & Prodi G (1983) Induction of
diphtheria toxin-resistant mutants in human cells by halogenated
compounds. J Cancer Res Clin Oncol, 105: 111-112.
Finlayson-Pitts BJ & Pitts JN (1986) Atmospheric chemistry:
Fundamentals and experimental techniques. New York, Wiley-Interscience
Publications.
Foster GD & Tullis RE (1984) A quantitative structure-activity
relationship between partition coefficients and the acute toxicity of
naphthalene derivatives in Artemia salina nauplii. Aquat Toxicol,
5: 245-254.
Foureman GL & Reed DJ (1985) Evidence for a non-episulfonium ion
intermediate during alkylation by S-[2-(N7-guanyl)ethyl]
glutathione, the major DNA adduct formed from 1,2-dibromoethane.
Biochemistry, 25: 2192-2198.
Freiria-Gandara MJ, Lorenzo-Ferreira RA, Alvarez-Devesa, A & Bermejo F
(1992) Occurrence of halogenated hydrocarbons in the water supply of
different cities of Galicia (Spain). Environ Technol, 13: 437-447.
Freitag D, Ballhorn L, Behechti A, Fischer K, & Thumm W (1994)
Structural configuration and toxicity of chlorinated alkanes.
Chemosphere, 28(2): 253-259.
Fujii T (1977) Direct aqueous injection gas chromatography-mass
spectrometry for analysis of organohalides in water at concentrations
below the parts per billion level. J Chromatogr, 139: 297-302.
Garrison SC & Leadingham RS (1954) A fatal case of ethylene dichloride
poisoning in an occupational therapy department of a neuropsychiatric
hospital. Am J Phys Med, 33: 230-237.
Geiger DL, Northcott CE, Call DJ, & Brooke LT (1985)
1,2-Dichloroethane. In: Geiger DL, Northcott CE, Call DJ, & Brooke LT
ed. Acute toxicities of organic chemicals to fathead minnow
(Pimephales promelas). Superior, Wisconsin, University of
Wisconsin-Superior, Center for Lake Superior Environmental Studies,
vol 2, pp 41-42.
Giri AK & Que Hee SS (1988) In vivo sister chromatid exchange
induced by 1,2-dichloroethane on bone marrow cells of mice. Environ
Mol Mutagen, 12: 331-334.
Gocke E, Wild D, Eckhardt K, & King MT (1983) Mutagenicity studies
with the mouse spot test. Mutat Res, 117: 201-212.
Golder Associates (1987) Testing of specific organic compounds in
soils in background in urban areas: Port Credit and
Oakville/Burlington, Ontario. Mississauga, Ontario, Canada, Golder
Associates (Working paper to Shell Canada Ltd and Texaco Canada Ltd).
Gradiski D, Bonnet P, Raoult G, & Magadur JL (1978) Toxicité aiguë
comparée par inhalation des principaux solvants aliphatiques chlorés.
Arch Mal Prof, 39(4-5): 249-257.
Grimsrud EP & Rasmussen RA (1975) Survey and analysis of halocarbons
in the atmosphere by gas chromatography-mass spectrometry. Atmos
Environ, 9: 1014-1017.
Guengerich FP, Crawford WM, Domoradzki JY, MacDonald TL, & Watanabe PG
(1980) In vitro activation of 1,2-dichloroethane by microsomal and
cytosolic enzyme. Toxicol Appl Pharmacol, 55: 303-317.
Guengerich FP, Mason PS, Stott WT, Fox TR, & Watanabe PG (1981) Role
of 2-haloethylene oxides and vinylchloride in irreversible binding to
protein and DNA. Cancer Res, 41: 4391-4398.
Guengerich FP, Koga N, Inskeep PB, & Ozawa N (1986) Proceedings of the
Second International Symposium on the Synthesis and Application of
Isotopically Labelled Compounds. New York, Elsevier Science
Publishers.
Guengerich FP, Kim DH, & Iwasaki M (1991) Role of human cytochrome
P-450 IIE1 in the oxidation of many low molecular weight cancer
suspects. Chem Res Toxicol, 4: 168-179.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Hadengue MA & Martin R (1953) Un cas d'intoxication mortelle par le
dichloréthane. Soc Méd Lég, 33: 247-249.
Hatch GG, Mamay PD, Ayer ML, Casto BC, & Nesnow S (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo cells by
gaseous and volatile chlorinated methanes and ethanes. Cancer Res,
43: 1945-1950.
Health Canada (1994) Unpublished update to Canadian Environmental
Protection Act Priority Substances report on 1,2-dichloroethane.
Ottawa, Ontario, Environmental Health Directorate, Priority Substances
Section.
Heikes DL (1987a) Determination of residual chlorinated solvents in
decaffeinated coffee by using purge and trap procedure. J Assoc Off
Anal Chem, 70(1): 176-180.
Heikes DL (1987b) Pesticide and industrial chemical residues - purge
and trap method for determination of volatile halocarbons and carbon
disulfide in table-ready foods. J Assoc Off Anal Chem, 70(2): 215-226.
Heikes DL (1990) Environmental contaminants in table-ready foods from
the total diet program of the Food and Drug Administration. In: Food
contamination from environmental sources. New York, John Wiley and
Sons, pp 31-57 (Advances in Environmental Science and Technology
Series No. 23).
Heikes DL & Hopper ML (1986) Purge and trap method for determination
of fumigants in whole grains, milled grain products, and intermediate
grain-based foods. J Assoc Off Anal Chem, 69(6): 990-998.
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hellman B & Brandt I (1986) Effects of carcinogenic halogenated
aliphatic hydrocarbons on [3H]thymidine incorporation into various
organs of the mouse. A comparison between 1,2-dibromoethane and
1,2-dichloroethane. Mutat Res, 163: 193-199.
Hemminki K, Falck K, & Vainio H (1980) Comparison of alkylation rates
and mutagenicity of directly acting industrial and laboratory
chemicals: epoxides, glycidyl ethers, methylating and ethylating
agents, halogenated hydrocarbons, hydrazine derivatives, aldehydes,
thiouram and dithiocarbamate derivatives. Arch Toxicol, 46: 277-285.
Heppel LA, Neal PA, Perrin TL, Endicott KM, & Porterfield VT (1945)
The toxicology of 1,2-dichloroethane (ethylene). III. Its acute
toxicity and the effect of protective agents. J Pharmacol Exp Ther,
84: 53-63.
Heppel LA, Neal PA, Perrin TL, Endicott KM, & Porterfield VT (1946)
The toxicology of 1,2-dichloroethane (ethylene dichloride). V. The
effects of daily inhalations. J Ind Hyg, Toxicol, 28(4): 113-120.
Hiatt MH (1981) Analysis of fish and sediment for volatile priority
pollutants. Anal Chem, 53: 1541-1543.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography/mass spectrometry. Anal Chem, 55: 506-516.
Hill J, Kollig HP, Paris DF, Wolfe NL, & Zepp RG (1976) Dynamic
behaviour of vinyl chloride in aquatic ecosystems. Athens, Georgia, US
Environmental Protection Agency (EPA/600/3-76/001).
Hinkel GK (1965) [Oral dichloroethane intoxication of children.] Dtsch
Gesudheitswes, 20: 1327-1331 (in German).
Hofmann HT, Birnsteil H, & Jobst P (1971) [The inhalation toxicity of
1,1- and 1,2-dichloroethane.] Arch Toxikol, 27: 248-265 (in German).
Hooper K, Gold LS, & Ames BN (1980) The carcinogenic potency of
ethylene dichloride in two animal bioassays: A comparison of
inhalation and gavage studies. In: Ames B, Infante P, & Reitz R ed.
Ethylene dichloride: a potential health risk? Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp 65-80 (Banbury Report No. 5).
HSDB (1993) Hazardous substances data bank (revision 9/2/93).
Bethesda, Maryland, National Library of Medicine, National Toxicology
Information Program.
Hueper WC & Smith C (1935) Fatal ethylene dichloride poisoning. Am J
Med Sci, 189: 778-784.
IARC (1979) Some halogenated hydrocarbons. Lyon, International Agency
for Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 20).
Igwe OJ, Que Hee SS, & Wagner WD (1986a) Interaction between
1,2-dichloroethane and tetraethylthiuram disulfide (disulfiram). II.
Hepatotoxic manifestations with possible mechanism of action. Toxicol
Appl Pharmacol, 86: 286-297.
Igwe OJ, Que Hee SS, & Wagner WD (1986b) Interaction between
1,2-dichloroethane and disulfiram. I. Toxicologic effects. Fundam Appl
Toxicol, 6: 733-746.
Inskeep PB, Koga N, Cmarik JL, & Guengerich FP (1986) Covalent binding
of 1,2-dihaloalkanes to DNA and stability of the major DNA adduct,
S-[2(N7-guanyl)ethyl] glutathione. Cancer Res, 46: 2839-2844.
IPCS (International Programme on Chemical Safety) (1987) Environmental
health criteria 62: 1,2-Dichloroethane. Geneva, World Health
Organization.
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: The derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization.
Isacson P, Bean JA, Splinter R, Olson DB, & Kohler J (1985) Drinking
water and cancer incidence in Iowa. III. Association of cancer with
indices of contamination. Am J Epidemiol, 121(6): 856-869.
Isnard P & Lambert S (1988) Estimating bioconcentration factors from
octanol-water partition coefficient and aqueous solubility.
Chemosphere, 17(1): 21-34.
Jafvert CT & Wolfe NL (1987) Degradation of selected halogenated
ethanes in anoxic sediment-water systems. Environ Toxicol Chem,
6: 827-837.
Jakobson I, Wahlberg JE, Holmberg B, & Johansson G (1982) Uptake via
the blood and elimination of 10 organic solvents following
epicutaneous exposure of anesthetized guinea pigs. Toxicol Appl
Pharmacol, 63: 181-187.
Janssen DB, Scheper A, Dijkhuizen L, & Witholt B (1985) Degradation of
halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10.
Appl Environ Microbiol, 49(3): 673-677.
Jeffers PM, Ward L, Woytowich L, & Wolfe NL (1989) Homogeneous
hydrolysis rate constants for selected chlorinated methanes, ethanes,
ethenes and propanes. Environ Sci Technol, 23(8): 965-969.
Jenssen D & Ramel C (1980) The micronucleus test as part of a
short-term mutagenicity test programme for the prediction of
carcinogenicity evaluated by 43 agents tested. Mutat Res, 75: 191-202.
Jonsson A & Berg S (1980) Determination of 1,2-dibromoethane,
1,2-dichloroethane, and benzene in ambient air using porous polymer
traps and gas chromatographic-mass spectrometric analysis with
selected ion monitoring. J Chromatogr, 190: 97-106.
Kaiser KLE & Comba ME (1986) Volatile hydrocarbon contaminant survey
of the St. Clair River. Water Pollut Res J Can, 21(3): 323-331.
Kaiser KLE, Comba ME, & Huneault H (1983) Volatile hydrocarbon
contaminants in the Niagara River and in Lake Ontario. J Great Lakes
Res, 9(2): 212-223.
Kanada T & Uyeta M (1978) Mutagenicity screening of organic solvents
in microbial systems. Mutat Res, 54: 215.
Kavlock R, Chernoff N, Carver B, & Kopfler F (1979) Teratology studies
in mice exposed to municipal drinking water concentrates during
organogenesis. Food Cosmet Toxicol, 17: 343-347.
Kerster HW & Schaeffer DJ (1983) Brine shrimp (Artemia salina)
nauplii as a teratogen test system. Ecotoxicol Environ Saf,
7: 342-349.
King MT, Beikirch H, Eckhardt K, Gocke E, & Wild D (1979) Mutagenicity
studies with X-ray-contrast media, analgesics, antipyretics,
antirheumatics and some other pharmaceutical drugs in bacterial,
Drosophila and mammalian test systems. Mutat Res, 66: 33-43.
Kirschmer P & Ballschmiter K (1983) Baseline studies of global
pollution. VIII. The complex pattern of C1 - C4 organohalogens in
continental and marine background air. Int J Environ Anal Chem,
14: 275-284.
Klaunig JE, Ruch RJ, & Pereira MA (1986) Carcinogenicity of
chlorinated methane and ethane compounds administered in drinking
water to mice. Environ Health Perspect, 69: 89-95.
Kliest J, Fast T, Boley JSM, van de Wiel H, & Bloemen H (1989) The
relationship between soil contaminated with volatile organic compounds
and indoor air pollution. Environ Int, 15: 419-425.
Knie VJ, Halke A, Juhnke I, & Schiller W (1983) Results of studies on
chemical substances with four biotests. Dtsch Gewasserkd Mitt,
27(3): 77-79.
Koga N, Inskeep PB, Harris TM, & Guengerich FP (1986)
S-[2-(N7-guanyl)ethyl] glutathione, the major DNA adduct formed
from 1,2-dibromoethane. Biochemistry, 25: 2192-2198.
Konemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. Part 1: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-221.
Kozik IV (1957) [Some problems of occupational hygiene in the use of
dichloroethane in the aircraft industry.] Gig Tr Prof Zabol, 1: 31-38
(in Russian).
Kramers PG, Mout HC, Bissumbhar B, & Mulder CR (1991) Inhalation
exposure in Drosophila mutagenesis assays: experiments with aliphatic
halogenated hydrocarbons, with emphasis on the genetic activity
profile of 1,2-dichloroethane. Mutat Res, 252: 17-33.
Kretzschmar JG, Peperstraete H, & Tymen T (1976) Air pollution in and
around Tessenderlo (The Netherlands). Neth Extern, 5: 147-178.
Krill RM & Sonzongni WC (1986) Chemical monitoring of Wisconsin's
groundwater. J Am Water Works Assoc, 78(9): 70-75.
Kronevi T, Wahlberg JE, & Holmberg B (1981) Skin pathology following
epicutaneous exposure to seven organic solvents. Int J Tissue React,
3: 21-30.
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Kuwabara T, Quevedo AR, & Cogan DG (1968) An experimental study of
dichloroethane poisoning. Arch Ophthalmol, 79: 321-330.
Lane RW, Riddle BL, & Borzelleca JF (1982) Effects of
1,2-dichloroethane and 1,1,1-trichloroethane in drinking water on
reproduction and development in mice. Toxicol Appl Pharmacol,
63: 409-421.
LaRegina J & Bozzelli JW (1986) Volatile organic compounds at
hazardous waste sites and a sanitary landfill in New Jersey - An
up-to-date review of the present situation. Environ Prog,
5(1): 18-27.
Larionov VG & Kokarovtseva MG (1976) Morphological constitution of
peripheral blood in intoxication with dichloroethane and its
metabolites. In: Actual problems of pesticide application in different
climatographic zones. Yerevan, Aiastan Publishers, pp 131-133.
Leblanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lesage S, McBride RA, Cureton PM, & Brown S (1993) Fate of organic
solvents in landfill leachates under simulated field conditions and in
anaerobic microcosms. Waste Manage Res, 11: 215-226.
Letkiewicz F, Johnston P, & Coleman J (1982) Occurrence of
1,2-dichloroethane in drinking water, food and air (Contract
68-01-6185). Washington, DC, JRB Associates (Prepared for the US
Environmental Protection Agency).
Li KM & Cheng WT (1991) The occupational exposure level of
1,2-dichloroethane in an EM preparation laboratory. J R Soc Health,
111(5): 169.
Lin ELC, Mattox JK, & Pereira MA (1985) Glutathione plus cytosol- and
microme-mediated binding of 1,2-dichloroethane to polynucleotides.
Toxicol Appl Pharmacol, 78: 428-435.
Lum KR & Kaiser KLE (1986) Organic and inorganic contaminants in the
St. Lawrence River: Some preliminary results on their distribution.
Water Pollut Res J Can, 21(4): 592-603.
Luznikov EA, Lisovik GA, & Novikovskaya TV (1985) [Metabolism of
1,2-dichloroethane in human organisms after acute poisonings.]
Forensic Med Expert, 2: 47-49 (in Russian).
McCann J, Simmon V, Streitwieser D, & Ames BN (1975) Mutagenicity of
chloroacetaldehyde, a possible metabolic product of 1,2-dichloroethane
(ethylene dichloride), chloroethane (ethylene chlorohydrin), vinyl
chloride and cyclophosphamide. Proc Natl Acad Sci (USA),
72: 3190-3191.
McCollister DD, Hollingsworth RL, Oyen F, & Rowe VK (1956) Comparative
inhalation toxicity of fumigant mixtures. Individual and joint effect
of ethylene dichloride, carbon tetrachloride, and ethylene dibromide.
Arch Ind Health, 13: 1-7.
McPherson RW, Starks CM, & Fryar GJ (1979) Vinyl chloride
monomer...what you should know. Hydrocarb Process, 3: 75-88.
MAFF (Ministry of Agriculture, Fisheries and Food) (1982) Food
surveillance paper No. 9. London, Her Majesty's Stationery Office.
MAFF (Ministry of Agriculture, Fisheries and Food) (1984) Food
surveillance paper No. 16. London, Her Majesty's Stationery Office.
MAFF (Ministry of Agriculture, Fisheries and Food) (1989) Food
surveillance paper No. 25. London, Her Majesty's Stationery Office.
MAFF/HSE (Ministry of Agriculture, Fisheries and Food/Health and
Safety Executive) (1993) Annual Report of the Working Party on
Pesticide Residues: 1992. Supplement to the Pesticides Register, 1993.
London, Her Majesty's Stationery Office.
Maltoni C, Valgimigli L, & Scarnato C (1980) Long-term carcinogenic
bioassays on ethylene dichloride administered by inhalation to rats
and mice. In: Ames BN, Infante P, & Reitz R ed. Ethylene dichloride: A
potential health risk? Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 3-33 (Banbury Report No. 5).
Martin G, Knorpp K, Huth K, Heinrich F, & Mittermayer C (1969)
Clinical features, pathogenesis and management of dichloroethane
poisoning. Germ Med Mon, 14: 62-67.
Menschick H (1957) [Acute inhalation intoxications by symmetric
dichloroethane.] Arch Gewerbepathol Gewerbehyg, 15: 241-252
(in German).
Mihaylova A (1976) [On the action of high temperature of the
environment on the acute and subacute toxicity of dichloroethane.] Gig
Zdraveopaz, 1: 32-36 (in Bulgarian).
Milman HA, Storystorey DL, Riccio ES, Sivak A, Tu AS, Williams GM,
Tong C, & Tyson CA (1988) Rat liver foci and in vitro assays to
detect initiating and promoting effects of chlorinated ethanes and
ethylenes. Ann NY Acad Sci, 534: 521-530.
Ministry of the Environment (1991) Drinking water surveillance program
data base. Ottawa, Canada, Ministry of the Environment, Water
Resources Branch, 27 pp (105 entries).
Mitoma C, Steeger T, Jackson SE, Wheeler KP, Rogers JH, & Milman HA
(1985) Metabolic disposition study of chlorinated hydrocarbons in rats
and mice. Drug Chem Toxicol, 8: 183-194.
Morgan DL, Bucher JR, Elwell MR, Lilja HS, & Murthy AS (1990)
Comparative toxicity of ethylene dichloride in F344/N, Sprague-Dawley
and Osborne-Mendel rats. Food Chem Toxicol, 28: 839-845.
Morgan DL, Cooper SW, Carlock DL, Sykora JJ, Sutton B, Mattie DR, &
McDougal JN (1991) Dermal absorption of neat and aqueous volatile
organic chemicals in Fischer 344 rat. Environ Res, 55: 51-63.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116:185-216.
Morozov GN (1958) [On acute dichloroethane poisoning.] Farmakol
Toksicol, 21(1): 76-78 (in Russian).
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, & White KL
(1982) In vivo assessment of immunotoxicity. Environ Health
Perspect, 43: 41-52.
Natsyuk MV & Chekman IS (1975) The content of nicotinamide coenzymes
in the liver and myocardium of rats poisoned with dichloroethane. Bull
Exp Biol Med, 4: 58-60.
Natsyuk MV & Mudritsky AD (1974) Clinical and biochemical
characteristics of hepatic and renal disorders in acute dichloroethane
intoxications. Mil Med J, 10: 48-50.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity. Bethesda, Maryland, National Cancer Institute (HEW
(NIH) Publication No. 78-1305).
Nestmann ER, Lee EGH, Matula TI, Douglas GR, & Mueller JC (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using Salmonella/mammalian-microsome assay. Mutat Res,
79: 203-212.
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR (1985)
The toxicity of selected organic chemicals to the earthworm Eisenia
foetida. J Environ Qual, 14(3): 383-388.
Nevrotsky VK, Kashin LM, Kulinskaya IL, Mikhailovskaya LF, Shmuter LM,
Burlaka-Vouk ZI, & Zadorozhny BV (1971) Comparative toxicity
evaluation of several industrial poisons in protracted inhalation
exposure to low concentrations. In: Proceedings of the 8th Ukranian
Hygiene Congress, Kiev, 1971, pp 224-226.
Nimitz JS & Skaggs SR (1992) Estimating tropospheric lifetimes and
ozone-depletion potentials of one- and two-carbon hydrofluorocarbons
and hydrochlorofluorocarbons. Environ Sci Technol, 26(4): 739-744.
NIOSH (1994) Registry of toxic effects of chemical substances.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
NIOSH (1984) Manual of analytical methods: Method 1003. Cincinnati,
Ohio, National Institute for Occupational Safety and Health.
Nouchi T, Miura H, Kanayama M, Mizuguchi O, & Takano T (1984) Fatal
intoxication by 1,2-dichloroethane - A case report. Int Arch Occup
Environ Health, 54: 111-113.
NTP (1991) Toxicity studies of 1,2-dichloroethane (ethylene
dichloride) in F344/N rats, Sprague Dawley rats, Osborne-Mendel rats,
and B6C3F1 mice (drinking water and gavage studies). Research
Triangle Park, North Carolina, National Toxicology Program (NIH
Publication No. 91-3123).
Nylander PO, Olofsson H, Rasmuson B, & Svahlin H (1978) Mutagenic
effects of petrol in Drosophila melanogaster. I. Effects of benzene
and 1,2-dichloroethane. Mutat Res, 57: 163-167.
Oldenhuis R, Vink RLJM, Janssen DB, & Witholt B (1989) Degradation of
chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b
expressing soluble methane monooxygenase. Appl Environ Microbiol,
55(11): 2819-2826.
Oliver BG & Pugsley CW (1986) Chlorinated contaminants in St. Clair
River sediments. Water Pollut Res J Can, 21(3): 368-379.
Otson R (1987) Purgeable organics in Great Lakes raw and treated
water. Int J Environ Anal Chem, 31(1): 41-53.
Otson R & Williams DT (1982) Headspace chromatographic determination
of water pollutants. Anal Chem, 54: 942-946.
Otson R, Williams DT, & Bothwell PD (1982) Industrial chemicals -
Volatile organic compounds in water at thirty Canadian potable water
treatment facilities. J Assoc Off Anal Chem, 65(6): 1370-1374.
Parkes DG, Ganz CR, Polinsky A, & Schulze J (1976) A simple gas
chromatographic method for the analysis of trace organics in ambient
air. Am Ind Hyg Assoc J, 37: 165-173.
Payan JP, Beydon D, Fabry JP, Brondeau MT, Ban M, & de Ceaurriz J
(1993) Urinary thiodiglycolic acid and thioether excretion in male
rats dosed with 1,2-dichloroethane. J Appl Toxicol, 13: 417-422.
Pearson CR & McConnell G (1975) Chlorinated C1 and C2 hydrocarbons
in the marine environment. Proc R Soc (Lond), B189: 305-332.
Pellizzari ED (1982) Analysis for organic vapor emissions near
industrial and chemical waste disposal sites. Environ Sci Technol,
16(11): 781-785.
Perocco P & Prodi G (1981) DNA damage by haloalkanes in human
lymphocytes cultured in vitro. Cancer Lett, 13: 213-218.
Pleil JD, Oliver KD, & McClenny WA (1988) Ambient air analyses using
nonspecific flame ionization and electron capture detection compared
by mass spectroscopy. J Air Pollut Control Assoc, 38: 1006-1010.
Principe P, Dogliotti E, Bignami M, Crebelli R, Falcone E, Fabrizi M,
Conti G, & Comba P (1981) Mutagenicity of chemicals of industrial and
agricultural relevance in Salmonella, Streptomyces, and Aspergillus.
J Sci Food Agric, 32: 826-832.
Proctor CJ, Warren ND, & Bevan MAJ (1989) Measurements of
environmental tobacco smoke in an air-conditioned office building.
Environ Technol Lett, 10: 1003-1018.
Prodi G, Arfelini G, Colacci A, Grilli S, & Mazzulo M (1986)
Interaction of halocompounds with nucleic acids. Toxicol Pathol, 14:
438-444.
Qiu LX, Shi SH, Xing SB, & Chen SG (1992) Rate constants for the
reactions of OH with five halogenated substituted ethanes from 292 K
to 366 K. J Phys Chem, 96: 685-689.
Rannug U (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichloroethane. Mutat Res, 76: 269-295.
Rannug U & Beije B (1979) The mutagenic effect of 1,2-dichloroethane
on Salmonella typhimurium. II. Activation by the isolated perfused
rat liver. Chem-Biol Interact, 24: 265-285.
Rannug U, Sundvall A, & Ramel C (1978) The mutagenic effect of
1,2-dichloroethane on Salmonella typhimurium. I. Activation through
conjugation with glutathione in vitro. Chem-Biol Interact, 20: 1-16.
Rao KS, Murray JS, Deacon MM, John JA, Calhoun LL, & Young JT (1980)
Teratogenicity and reproduction studies in animals inhaling ethylene
dichloride. In: Ames B, Infante P, & Reitz R ed. Ethylene dichloride:
A potential health risk? Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 149-166 (Banbury Report No. 5).
Reid BJ, Morgan JD, & Whelen MA (1982) A preliminary examination of
the effects of ethylene dichloride on the hatchability of Coho salmon
eggs (Oncorhynchus kisutch). Can Tech J Fish Aquat Sci,
1163: 145-153.
Reitz RH, Fox TR, Ramsey JC, Quast JF, Langvardt PW, & Watanabe PG
(1982) Pharmacokinetics and macro-molecular interactions of ethylene
dichloride in rats after inhalation or gavage. Toxicol Appl Pharmacol,
62: 190-204.
Reitz RH, Fox TR, Domordzki JY, Quast JF, Langvardt PW, & Watanabe PG
(1980) Pharmacokinetics and macromolecular interactions of ethylene
dichloride: Comparison of oral and inhalation exposures. In: Ames BN,
Infante P, & Reitz R. ed. Ethylene dichloride: A potential health
risk? Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp 135-148 (Banbury Report No. 5).
Richter JE, Peterson SF, & Kleiner CF (1983) Acute and chronic
toxicity of some chlorinated benzenes, chlorinated ethanes, and
tetrachloroethylene to Daphnia magna. Arch Environ Contam Toxicol,
12: 679-684.
Roldan-Arjona T, Garcia-Pedrajas MD, Luque-Romero RL, Hera C, & Pueyo
C (1991) An association between mutagenicity of the Ara test of
Salmonella typhimurium and carcinogenicity in rodents for 16
halogenated aliphatic hydrocarbons. Mutagenesis, 6: 199-205.
Romert L, Magnusson J, & Ramel C (1990) The importance of glutathione
and glutathione transferase for somatic mutations in Drosophila
melanogaster induced in vivo by 1,2-dichloroethane.
Carcinogenesis, 11: 1399-1402.
Rosenberg R, Grahn O, & Johansson L (1975) Toxic effects of aliphatic
chlorinated by-products from vinyl chloride production on marine
animals. Water Res, 9: 607-612.
Sack TM, Steele DH, Hammerstrom K, & Remmers J (1992) A survey of
household products for volatile organic compounds. Atmos Environ,
26A(6): 1063-1070.
Saitanov AO & Arsenieva SS (1969) [Electrocardiographic changes due to
acute dichloroethane poisoning: experimental investigation.] Gig Tr
Prof Zabol, 7: 49-50 (in Russian).
Sato A, Nakajima T, & Koyama Y (1981) Dose-related effects of a single
dose of ethanol on the metabolism in rat liver of some aromatic and
chlorinated hydrocarbons. Toxicol Appl Pharmacol, 60: 8-15.
Sauer TC (1981) Volatile organic compounds in open ocean and coastal
surface waters. Org Geochem, 3: 91-101.
Schasteen CS & Reed DJ (1983) The hydrolysis and alkylation activities
of S-(2-haloethyl)-L-cysteine analogues - evidence for extended
half-life. Toxicol Appl Pharmacol, 70: 423-432.
Schönborn H, Prellwitz W, & Baum P (1970) [Consumption coagulation
pathology of 1,2-dichloroethane poisoning.] Klin Wochenschr,
48: 822-824 (in German).
Schultz K, Ghosh L, & Banerjee S (1992) Neoplastic expression in
murine cells induced by halogenated hydrocarbons. In vitro Cell Dev
Biol, 28A: 267-272.
Sherwood RL, O'Shea PT, Thomas W, Ratajczak HV, Aranyi C, & Graham JA
(1987) Effects of inhalation of ethylene dichloride on pulmonary
defenses of mice and rats. Toxicol Appl Pharmacol, 91: 491-496.
Shmuter LM (1977) [Effect of chronic exposure to low concentrations of
chlorinated hydrocarbons of the ethane series on specific and
non-specific reactivity of animals in vivo.] Gig Tr Prof Zabol,
8: 38-42 (in Russian).
Simmon VF (1980) Review of non-bacterial tests of the genotoxic
activity of ethylene dichloride. In: Ames B, Infante P, & Reitz R ed.
Ethylene dichloride: A potential health risk? Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp 97-103 (Banbury Report No. 5).
Simula TP, Glancey MG, & Wolf CR (1993) Human glutathione
S-transferase-expressing Salmonella typhimurium tester strains to
study the activation/detoxification of mutagenic compounds: studies
with halogenated compounds, aromatic amines and aflatoxin B1.
Carcinogenesis, 14: 1371-1376.
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1980) Atmospheric
measurements of selected toxic organic chemicals. Washington, DC, US
Environmental Protection Agency, 35 pp (EPA-600/3-80-072;
NTIS PB80-89).
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban environments.
Atmos Environ, 15: 601-612.
Singh HB, Salas LJ, & Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environ Sci Technol, 16: 872-880. Singh HB, Salas IJ, & Stiles RE
(1983) Selected man-made halogenated chemicals in the air and oceanic
environment. J Geophys Res, 88(C6): 3675-3683.
Smyth HF (1969) Acute toxicity of ethyl compounds. In: Spector WC ed.
Handbook of toxicology. Philadelphia, Pennsylvania, W.B. Saunders,
vol 1, pp 134-135.
Spence JW & Hanst PL (1978) Oxidation of chlorinated ethanes. J Air
Pollut Control Assoc, 28(3): 250-253.
Spencer HC, Rowe VK, Adams EM, McCollister DD, & Irish DD (1951) Vapor
toxicity of ethylene dichloride determined by experiments on
laboratory animals. Am Med Assoc Arch Ind Hyg Occup Med, 4: 482-493.
Spreafico F, Zuccato E, Marcucci F, Sironi M, Paglialunga S, Madonna
M, & Mussini E (1980) Pharmacokinetics of ethylene dichloride in rats
treated by different routes and its long-term inhalatory toxicity. In:
Ames B, Infante P, & Reitz R ed. Ethylene dichloride: A potential
health risk? Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp 107-133 (Banbury Report No. 5).
Storer RD & Conolly RB (1983) Comparative in vivo genotoxicity and
acute hepatotoxicity of three 1,2-dihaloethanes. Carcinogenesis,
4: 1491-1494.
Storer RD & Conolly RB (1985) An investigation of the role of
microsomal oxidative metabolism in the in vivo genotoxicity of
1,2-dichloroethane. Toxicol Appl Pharmacol, 77: 36-46.
Storer RD, Jackson NM, & Conolly RB (1984) In vivo genotoxicity and
acute hepatotoxicity of 1,2-dichloroethane in mice: Comparison of
oral, intraperitoneal, and inhalation routes of exposure. Cancer Res,
44: 4267-4271.
Story DL, Meierhenry EF, Tyson CA, & Milman HA (1986) Differences in
rat liver enzyme-positive foci produced by chlorinated aliphatics and
phenobarbital. Toxicol Ind Health, 2: 351-362.
Strobel K & Grummt T (1987) Aliphatic and aromatic halocarbons as
potential mutagens in drinking water. III. Halogenated ethanes and
ethenes. Toxicol Environ Chem, 15: 101-128.
Sundheimer DW, White RD, Brendel K, & Sipes IC (1982) The
bioactivation of 1,2-dibromoethane in rat hepatocytes: covalent
binding to nucleic acids. Carcinogenesis, 3: 1129-1133.
Suveev IM & Babichenko ME (1969) [On the clinic and cure of acute
intoxication with dichloroethane vapours.] Gig Tr Prof Zabol,
13: 50-51 (in Russian).
Symons JM, Bellar TA, Carswell JK, Demarco J, Kropp KL, Robeck GG,
Seeger DR, Slocum CJ, Smith BL, & Stevens AA (1975) National organics
reconnaissance survey for halogenated organics. J Am Water Works
Assoc, 67: 634-648.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53(10): 1503-1518.
Tan EL & Hsie AW (1981) Mutagenicity and cytotoxicity of haloethanes
as studied in the CHO/HGPRT system. Mutat Res, 90: 183-191.
Tang NH, Blum DJW, & Speece RE (1990) Comparison of serum bottle
toxicity test with OECD method. J Environ Eng, 116(6): 1076-1084.
Taningher M, Parodi S, Grilli S, Colacci A, Mazzullo M, Bordone R, &
Santi L (1991) Lack of correlation between alkaline DNA fragmentation
and DNA covalent binding induced by polychloroethanes after in vivo
administration. Problems related to the assessment of a carcinogenic
hazard. Cancer Detect Prev, 15: 35-39.
Theiss JC, Stoner GD, Shimkin MB, & Weisberger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer Res,
37: 2717-2720.
Torkelson TR & Rowe VK (1981) Halogenated aliphatic hydrocarbons. In:
Clayton GD & Clayton FE ed. Toxicology: Patty's industrial hygiene
and toxicology, 3rd ed. New York, John Wiley and Sons, vol 2B,
pp 3491-3497.
Troisi FM & Cavallazzi D (1961) [A fatal case of poisoning from
inhalation of dichloroethane vapours.] Med Lav, 52: 612-618
(in Italian).
Tsuruta H (1975) Percutaneous absorption of organic solvents: 1)
Comparative study of the in vivo percutaneous absorption of
chlorinated solvents in mice. Ind Health, 13: 227-236.
Tsuruta H (1977) Percutaneous absorption of organic solvents: 2) A
method for measuring the penetration rate of chlorinated solvents
through excised rat skin. Ind Health, 15: 131-139.
Tu AS, Murray TA, Hatch KM, Sivak A, & Milman HA (1985) In vitro
transformation of BALB/c-3T3 cells by chlorinated ethanes and
ethylenes. Cancer Lett, 28: 85-92.
UK HSE (Health and Safety Executive) (1992) Criteria document for an
occupational exposure limit: 1,2-Dichloroethane. London, Her Majesty's
Stationery Office, 34 pp.
Urusova TP (1953) [The possible presence of dichloroethane in human
milk with exposure in industrial conditions.] Gig i Sanit, 18: 36-37
(in Russian).
US Department of Labor (1989) Industrial exposure and control
technologies for OSHA regulated hazardous substances: Volume I.
Washington, DC, US Department of Labor.
US DHHS (Department of Health and Human Services) (1994) Seventh
annual report on carcinogens -1994: Summary. Research Triangle Park,
North Carolina, National Institute of Environmental Health Sciences,
165 pp.
US EPA (1982a) An exposure and risk assessment for dichloroethanes.
Washington, DC, US Environmental Protection Agency, Office of Water
Regulations and Standards, 175 pp (EPA-440/4-85-009).
US EPA (1982b) Test methods for organic chemical analysis of municipal
and environmental wastewater. Cincinnati, Ohio, US Environmental
Protection Agency, Monitoring and Support Laboratories, 76 pp
(EPA 600/4-82-057).
US EPA (1985a) Health assessment document for 1,2-dichloroethane
(ethylene dichloride). Final Report. Washington, DC, US Environmental
Protection Agency, 95 pp (EPA/600/8-84/006F; NTIS PB86-122702).
US EPA (1985b) Health and environmental effects profile for
1,2-dichloroethane. Washington, DC, US Environmental Protection
Agency, 137 pp (EPA/600/X-85/359; NTIS PB88-178777).
US EPA (1986) Handbook: Permit writer's guide to test burn data,
hazardous waste incineration. Cincinnati, Ohio, US Environmental
Protection Agency, 73 pp (EPA/625/6-86/012).
US EPA (1987) Health advisories for 25 organics. Washington, DC, US
Environmental Protection Agency (NTIS PB87-235578).
US EPA (1988) Contract laboratory program database. Washington, DC, US
Environmental Protection Agency, Contract Laboratory Program.
US EPA (1992) Indoor air quality data base for organic compounds.
Research Triangle Park, North Carolina, US Environmental Protection
Agency (EPA/600/R/92-025; PB92-158468).
Valencia R, Abrahamson S, Lee WR, Von Halle ES, Woodruff RC, Wurgler
FE, & Zimmering S (1984) Chromosome mutations for mutagenesis in
Drosophila melanogaster: A report of the US Environmental Protection
Agency Gene-Tox Program. Mutat Res, 134: 61-88.
Van Bladeren PJ, Breimer DD, Rotteveel-Smijs RMT, De Knijff P, Mohn
GR, van Meeteren-Walchli B, Buijs W, & van der Gen A (1981) The
relation between the structure of vicinal dihalogen compounds and
their mutagenic activation via conjugation to glutathione.
Carcinogenesis, 2: 499-505.
Van den Wijngaard AJ, van der Kamp KWJ, van der Ploeg J, Pries F,
Kazemier B, & Janssen DB (1992) Degradation of 1,2-dichloroethane by
Ancylobacter aquaticus and other facultative methylotrophs. Appl
Environ Microbiol, 58: 976-983.
Van Duuren BL, Goldschmidt BM, Loewengart G, Smith A, Mechionne S,
Seldman I, & Roth D (1979) Carcinogenicity of halogenated olefinic and
aliphatic hydrocarbons in mice. J Natl Cancer Inst, 63: 1433-1439.
Van Esch GJ, Kroes R, Van Logten MJ, & Den Tonkelaar EM (1977)
Ninety-day toxicity study with 1,2-dichloroethane (DCE) in rats.
Bilthoven, The Netherlands, National Institute of Public Health and
Environmental Protection (RIVM) (Report 195/77).
Van Luin AB & van Starkenburg W (1984) Hazardous substances in waste
water. Water Sci Technol, 17: 843-853.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: Narcotic industrial
chemicals. Can J Fish Aquat Sci, 40: 743-748.
Vozovaya MA (1974) [Development of posterity of two generations
obtained from females subjected to the action of dichloroethane.] Gig
i Sanit, 39: 25-28 (in Russian).
Vozovaya MA (1977) [The effect of dichloroethane on the sexual cycle
and embryogenesis of experimental animals.] Aksuk Ginekol (Moscow),
2: 57-59 (in Russian).
Walbridge CR, Fiandt JT, Phipps GL, & Holcombe GW (1983) Acute
toxicity of ten chlorinated aliphatic hydrocarbons to the fathead
minnow (Pimephales promelas). Arch Environ Contam Toxicol,
12: 661-666.
Wallace LA (1986) Personal exposures, indoor and outdoor air
concentrations, and exhaled breath concentrations of selected volatile
organic compounds measured for 600 residents of New Jersey, North
Dakota, North Carolina and California. Toxicol Environ Chem,
12: 215-236.
Wallace LA, Pellizzari E, Hartwell T, Rozenweig M, Erickson M,
Sparacino C, & Zelon H (1984) Personal exposure to volatile organic
compounds. I. Direct measurements in breathing-zone air, drinking
water, food and exhaled breath. Environ Res, 35: 293-319.
Wallace L, Pellizzari E, Leaderer B, Zelon H, & Sheldon L (1987)
Emissions of volatile organic compounds from building materials and
consumer products. Atmos Environ, 21(2): 385-393.
Ward JM (1980) The carcinogenicity of ethylene dichloride in
Osborne-Mendel rats and B6C3F1 mice. In: Ames BN, Infante P, & Reitz
R ed. Ethylene dichloride: A potential health risk? Cold Spring
Harbor, New York, Cold Spring Harbor Laboratory, pp 35-53 (Banbury
Report No. 5).
Ward RJ (1992) Ethylene dichloride: In vitro absorption through
human and rat epidermis. Alderley Park, United Kingdom, ICI Central
Toxicology Laboratory (Unpublished report).
Warner JM & Beasley RK (1984) Purge and trap chromatographic method
for the determination of acrylonitrile, chlorobenzene,
1,2-dichloroethane and ethylbenzene in aqueous samples. Anal Chem,
56: 1953-1956.
Warner HP, Cohen JM, & Ireland JC (1987) Determination of Henry's Law
Constants of selected priority pollutants. Washington, DC, US
Environmental Protection Agency, Office of Science and Development
(EPA-600/D-87/229).
Weiss F (1957) [Lethal oral intoxications by dichloroethane.] Arch
Gewerbepathol Gewerbehyg, 15: 253-264 (in German).
WHO (1993) Guidelines for drinking-water quality. Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization.
Williams GM, Mori H, & McQueen CA (1989) Structure-activity
relationships in the rat hepatocyte DNA-repair test for 300 chemical.
Mutat Res, 221: 263-286.
Wilson JT, Enfield CG, Dunlap WJ, Cosby RL, Foster DA, & Baskin LB
(1981) Transport and fate of selected organic pollutants in a sandy
soil. J Environ Qual, 10(4): 501-506.
Wirtschafter ZT & Schwartz WD (1939) Acute ethylene dichloride
poisoning. J Ind Hyg Toxicol, 21: 126-131.
Wit SL, Besemer AFH, Das HA, Goedkoop W, Loosjes FE, & Meppelink EK
(1969) Toxicology. Bilthoven, The Netherlands, National Institute of
Public Health (Report No. 36/69).
Withey JR & Collins BT (1980) Chlorinated aliphatic hydrocarbons used
in the foods industry: the comparative pharmacokinetics of methylene
chloride, 1,2-dichloroethane, chloroform and trichloroethylene after
I.V. administration in the rat. J Environ Pathol Toxicol, 3: 313-332.
Withey JR & Karpinski K (1985) The fetal distribution of some
aliphatic chlorinated hydrocarbons in the rat after vapor phase
exposure. Biol Res Pregnancy Perinatol, 6: 79-88.
Withey JR, Collins BT, & Collins PG (1983) Effect of vehicle on the
pharmacokinetics and uptake of four halogenated hydrocarbons from the
gastrointestinal tract of the rat. J Appl Toxicol, 3: 249-253.
Wolff DL, Ivanov NG, Kljackina AM, & Mel'Nikova LV (1979) [Evidence of
the effect of significant industrial toxicological substances on the
nervous system by animal behavioural tests.] Zool Jahrb Physiol,
83: 82-91 (in German).
Worthing CR ed. (1991) The pesticide manual. Farnham, Surrey, The
British Crop Protection Council.
Yodaiken RE & Babcock JR (1973) 1,2-Dichloroethane poisoning. Arch
Environ Health, 26: 281-284.
Zamora PO, Benson JM, Li AP, & Brooks AL (1983) Evaluation of an
exposure system using cells grown on collagen gels for detecting
highly volatile mutagens in the CHD/HGPRT mutation assay. Environ
Mutagen, 5: 795-801.
Zoetman BCJ, Piet GJ, Slingerland P, Fonds AW, & Wegman RCC (1979)
[Investigation into the presence of volatile organic halogenated
compounds in groundwater used as a drinking water supply. Final
report: results on the period November (1976) to August (1978).]
Bilthoven, The Netherlands, National Institute of Drinking Water
Supply, National Institute of Public Health and Environmental Hygiene
(Reports Nos RID-CBH-79/01 and RIV 10/79) (in Dutch).
RESUME
1. Identité, propriétés physiques et chimiques, et méthodes d'analyse
Le 1,2-Dichloréthane (ou dichlorure d'éthylène) est un produit
chimique de synthèse qui se présente sous la forme d'un liquide
incolore à la température ambiante. Il est extrêmement volatil, avec
une tension de vapeur de 8,5 kPa à 20°C et il est soluble dans l'eau,
sa solubilité étant de 8690 mg/litre à 20°C. Son coefficient de
partage octanol/eau (log Kow) est égal à 1,76.
Le dosage du dichloréthane dans les différents compartiments de
l'environnement s'effectue généralement par chromatographie en phase
gazeuse, avec détection par capture d'électrons, ionisation de flamme
ou spectrométrie de masse. Les limites de détection vont de 0,016 à
> 4 µg/m3 dans l'air, de 0,001 à 4,7 µg/litre dans l'eau et de 6 à
10 µg/kg dans différentes denrées alimentaires.
2. Sources d'exposition humaine et environnementale
On utilise principalement le 1,2-dichloréthane pour la synthèse
du chlorure de vinyle monomère et dans une moindre mesure pour la
production de divers solvants chlorés. Il entre également dans la
composition des additifs antidétonants de l'essence (encore que cet
usage soit en déclin avec l'élimination progressive de l'essence au
plomb dans certains pays) et on l'utilise aussi pour des fumigations.
La production annuelle totale de 1,2-dichloréthane a été de 922
kilotonnes au Canada en 1990 et de 6318 kilotonnes aux Etats-Unis
d'Amérique en 1991.
3. Transport, distribution et transformation dans l'environnement
La majeure partie du 1,2-dichloréthane rejeté dans
l'environnement provient d'émissions dans l'atmosphère. Il
estmoyennement persistant dans l'air; sa durée de vie estimative dans
l'atmosphère est comprise entre 43 et 111 jours. Le dichloréthane est
transporté vers la stratosphère où, par photolyse, il peut donner
naissance à du chlore radicalaire qui peut à son tour réagir sur
l'ozone. Une partie du 1,2-dichloréthane rejeté dans les effluents
industriels peut passer dans le milieu aquatique dont il s'échappe
rapidement par volatilisation. Il peut également s'infiltrer
jusqu'aux nappes d'eau souterraines à proximité des zones de décharges
industrielles. On ne pense pas qu'il puisse subir une
bioconcentration chez les espèces aquatiques ou terrestres.
4. Concentrations dans l'environnement et exposition humaine
Des enquêtes récentes portant sur l'air ambiant de zones urbaines
non dominées par des sources polluantes ont permis de relever des
concentrations moyennes de 1,2-dichloréthane allant de 0,07 à
0,28 µg/m3, alors que dans l'air intérieur aux habitations des zones
résidentielles, ces valeurs moyennes vont de < 0,1 à 3,4 µg/m3.
Dans l'eau destinée à la consommation, les concentrations moyennes
sont généralement inférieures à 0,5 µg/litre. Lors de récentes
enquêtes, on a rarement décelé du 1,2-dichloréthane dans les denrées
alimentaires et comme il ne présente qu'un faible potentiel de
bioaccumulation, il est peu probable que la nourriture constitue une
source importante d'exposition à ce composé.
La valeur estimative de l'exposition moyenne au 1,2-dichloréthane
à partir de divers milieux montre que la source principale
d'exposition est constituée par l'air intérieur et extérieur, l'eau de
consommation n'y contribuant que pour une très faible part. L'apport
de 1,2-dichloréthane par la voie alimentaire est probablement
négligeable. Les quantités inhalées dans l'air ambiant pourraient
être plus importantes à proximité des sources industrielles.
5. Cinétique et métabolisme chez les animaux de laboratoire
Après inhalation, ingestion ou exposition par voie cutanée, le
1,2-dichloréthane est rapidement absorbé et il se répartit rapidement
et largement dans l'ensemble de l'organisme. Il est rapidement et
largement métabolisé chez le rat et la souris, principalement sous
forme de métabolites soufrés dont l'excrétion s'effectue par la voie
urinaire et dépend de la dose. A des niveaux d'exposition qui
entraînent des concentrations sanguines de 5 à 10 µg/ml, il semble
qu'il y ait saturation ou limitation du métabolisme chez le rat.
Après administration d'une dose unique de dichloréthane par gavage, on
a constaté que le taux d'alkylation de l'ADN était plus important que
lorsque le produit était inhalé sur une période de six heures.
Il existe semble-t-il deux voies principales de métabolisation.
La première est une oxydation saturable qui s'effectue au niveau des
microsomes par l'intermédiaire du cytochrome P-450 et aboutit au
2-chloracétaldéhyde et au 2-chloréthanol, pour s'achever sur une
conjugaison avec le glutathion. La deuxième voie métabolique comporte
une conjugaison directe avec le glutathion pour former du
S-(2-chloréthyl)-glutathion, qui est peut-être ensuite transformé
par voie non enzymatique en un ion glutathion-épitsulfonium
susceptible de former des adduits avec l'ADN. Bien qu'on ait pu
observer in vitro que la voie du P-450 conduisait à des lésions de
l'ADN, il semble bien qu'à cet égard, la voie impliquant la
conjugaison du glutathion soit la plus importante.
6. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Le 1,2-dichloréthane présente une faible toxicité aiguë pour les
animaux de laboratoire. Ainsi, la CL50 par inhalation pour des rats
exposés soit 6 soit 7,25 heures à ce composé allait de 4000 mg/m3 à
6600 mg/m3, la DL50 par voie orale pour le rat, la souris, le
chien et le lapin allant de 413 à 2500 mg/kg de poids corporel.
D'après les résultats d'études à court terme et d'études
subchroniques menées sur différentes espèces d'animaux de laboratoire,
c'est le foie et les reins qui sont les organes cibles; il n'a pas été
possible d'obtenir de valeurs pour la dose sans effets observables
(NOEL) ou La dose la plus faible provoquant un effet (LOEL),
généralement en raison d'une documentation insuffisante et du nombre
trop limité de paramètres biologiques examinés sur un trop petit
nombre d'animaux. Une série d'études limitées anciennes a révélé la
présence de modifications morphologiques au niveau du foie chez
plusieurs espèces après exposition subchronique à des concentrations
atmosphériques ne dépassant pas 800 mg/m3. Après administration
subchronique par voie orale de 1,2-dichloréthane à des doses
quotidiennes allant de 49 à 82 mg/kg de poids corporel ou davantage
pendant 13 semaines, on a observé chez des rats un accroissement du
poids relatif du foie. Les études de toxicité chronique dont on
possède les résultats ne donnent guère d'information sur les effets
non néoplasiques. Chez des rats exposés pendant 12 mois à des
concentrations atmosphériques de 1,2-dichloréthane ne dépassant pas
202 mg/m3, on a observé, au niveau des paramètres sériques, des
modifications indiquant une toxicité hépatique et rénale; toutefois,
aucun examen histopathologique n'a été pratiqué lors de cette étude.
Quelques épreuves limitées ont été effectuées sur des animaux de
laboratoire à la recherche d'une cancérogénicité éventuelle du
1,2-dichloréthane (ces études souffraient d'une trop faible durée
d'exposition et d'une forte mortalité parmi les animaux). Chez des
rats Sprague-Dawley et des souris Swiss exposés pendant 78 semaines à
des concentrations allant jusqu'à 607 mg/m3 et observés jusqu'à ce
qu'ils meurent spontanément, on n'a pas observé d'augmentation
significative dans l'incidence des tumeurs quel qu'en soit le type.
La mortalité était forte parmi les rats, mais sans rapport avec la
concentration du produit et on n'a pas tenu compte des différences de
mortalité entre les groupes pour corriger les taux d'incidence. Des
rattes Sprague-Dawley ont été exposées pendant deux ans à 200 mg/m3
de 1,2-dichloréthane et on a observé à cette occasion une augmentation
de l'incidence des adénomes et des fibroadénomes de la mère, qui
n'était toutefois pas significative; aucun autre effet toxique
attribuable au composé n'a été observé.
En revanche, on a observé, après ingestion, chez deux espèces,
des signes convaincants d'un accroissement de l'incidence tumorale.
Chez des rats Osborne-Mendel à qui l'on avait administré
quotidiennement par gavage pendant 78 semaines des doses de 47 ou
95 mg/kg (en moyenne pondérée par rapport au temps), on a observé une
augmentation significative de l'incidence des tumeurs de différentes
localisations (notamment des carcinomes spinocellulaires de l'estomac
(chez les mâles), des hémangiosarcomes (chez les mâles et les
femelles), des fibromes du tissu sous-cutané (chez les mâles) ainsi
que des adénocarcinomes et des fibroadénomes mammaires chez les
femelles. Chez des souris B6C3F1 à qui l'on avait administré
quotidiennement des doses de 97 ou 195 mg/kg de produit (en moyenne
pondérée par rapport au temps) (mâles) ou de 149 et 299 mg/kg
(femelles) par gavage sur 78 semaines, on a observé une augmentation
similaire de l'incidence des tumeurs de diverses localisations
(notamment des adénomes alvéolaires/bronchiolaires chez les mâles et
les femelles, des adénocarcinomes mammaires chez les femelles, des
polypes ou des sarcomes du stroma de l'endomètre (femelles) et des
carcinomes hépatocellulaires (mâles)).
Chez des souris femelles qui avaient été soumises respectivement
pendant 440 et 594 jours à des applications cutanées répétées de
1,2-dichloréthane, on a observé une incidence sensiblement accrue des
tumeurs pulmonaires (papillomes bénins). Chez une souche sensible de
souris, des injections intrapéritonéales répétées de 1,2-dichloréthane
ont déterminé un accroissement, lié à la dose, du nombre des adénomes
pulmonaires, mais cet accroissement n'était en aucun cas significatif.
Chez des rats à qui l'on faisait simultanément respirer du
1,2-dichloréthane et ingérer du disulfirame avec leur nourriture, on a
observé une incidence accrue des cholangiomes et des kystes dans la
partie intrahépatique des canaux biliaires, et davantage de fibromes
sous-cutanés, de nodules hépatiques malins, de tumeurs du tissu
testiculaire interstitiel et d'adénocarcinomes mammaires, que chez des
rats qui avaient reçu soit l'un, soit l'autre des composés ou aucun
des deux. Trois épreuves biologiques n'ont pas permis de mettre en
évidence une aptitude quelconque de ce composé à se comporter comme un
initiateur ou un promoteur tumoral, encore que l'examen
histopathologique effectué à la suite de ces études ait été de portée
limitée.
Lors d'épreuves de mutagénicité in vitro sur Salmonella
typhimurium, le 1,2-dichloréthane a toujours donné des résultats
positifs. L'effet était plus important en présence d'un système
d'activation exogène (sans doute du fait d'une activation par le
cytochrome) et on constatait que le pouvoir mutagène était plus que
doublé chez S. typhimurium exprimant le gène humain GSTA1-1. Le
1,2-dichloréthane forme des adduits avec l'ADN en cultures de cellules
mammaliennes. Il provoque également une synthèse non programmée de
l'ADN dans des cultures primaires de cellules murines et humaines
ainsi que des mutations géniques dans certaines lignées cellulaires.
On a trouvé une corrélation entre la fréquence des mutations observées
dans des lignées cellulaires humaines et la modification de l'activité
de la glutathion-S-transférase. Des études in vivo ont montré que
le 1,2-dichloréthane produisait des mutations létales récessives dans
les cellules somatiques et germinales de Drosophila melanogaster et
selon toutes les études publiées portant sur des rats et des souris,
il y a liaison du 1,2-dichloréthane à l'ADN. Des lésions directes de
l'ADN des cellules hépatiques ainsi que des échanges entre chromatides
soeurs ont été observés lors d'études sur la souris mais rien
n'indique que le 1,2-dichloréthane provoque la formation de
micronoyaux.
Rien n'indique, à en juger par les résultats d'un nombre limité
d'études, que le 1,2-dichloréthane soit tératogène pour les animaux de
laboratoire. Il n'y a également guère d'éléments en faveur d'effets
sur la reproduction ou le développement à des doses inférieures à
celles qui provoquent d'autres effets généraux. On ne dispose que de
données limitées sur l'immunotoxicité du 1,2-dichloréthane.
7. Effets sur l'homme
Des effets divers ont été observés à la suite d'expositions
accidentelles aiguës à du 1,2-dichloréthane par inhalation ou
ingestion: au niveau du système nerveux central, du foie, des reins,
des poumons et de l'appareil cardio-vasculaire.
On n'a pas beaucoup étudié le pouvoir cancérogène du
1,2-dichloréthane dans les populations humaines exposées. Chez un
groupe d'ouvriers d'un atelier de production de produits chimiques qui
avaient été exposés principalement à du 1,2-dichloréthane, à côté
d'autres substances, on a observé une augmentation significative de la
mortalité par cancer du pancréas. Cette mortalité augmentait avec la
durée de l'exposition. En outre, malgré un nombre limité de cas et
une association moins systématique avec la durée de l'exposition, il y
avait également accroissement de la mortalité par leucémie chez ces
travailleurs. Une petite étude cas-témoins, portant sur l'exposition
à du 1,2-dichloréthane n'a pas permis de mettre en évidence une
corrélation avec l'apparition de tumeurs cérébrales. Une étude
écologique intrinsèquement limitée, portant sur la présence de
1,2-dichloréthane dans de l'eau de consommation, a mis en évidence une
augmentation de l'incidence des cancers colo-rectaux mais il est
possible qu'une exposition simultanée à d'autres substances explique
pour une part les effets observés.
8. Effets sur les organismes non visés au laboratoire et dans leur
milieu naturel
On a étudié les effets d'une exposition au 1,2-dichloréthane sur
un certain nombre d'autres organismes tant au laboratoire que dans
leur milieu naturel. En ce qui concerne les microorganismes
aquatiques, les valeurs de la CI50 et de la CE50 correspondant à
divers paramètres biotoxicologiques vont de 25 à 770 mg/litre. La
valeur de la CL50 la plus faible qui ait été observée pour les
daphnies était de 220 mg/litre, des effets ayant été toutefois
observés sur la fécondité et la croissance aux concentrations
respectives de 20,7 et 71,7 mg/litre. En s'appuyant sur les données
disponibles, on constate que le vertébré d'eau douce le plus sensible
au 1,2-dichloréthane est une espèce de salamandre (Ambystoma gracile),
chez laquelle la survie des larves à neuf jours (quatre jours après
l'éclosion) a accusé une chute à 2,54 mg/litre. On ne possède que des
données limitées sur la toxicité du 1,2-dichloréthane pour les
organismes terrestres.
Resumen
1. Identidad, propiedades físicas y químicas y métodos analíticos
El 1,2-dicloroetano (dicloruro de etileno), producto químico
sintético, es un líquido incoloro a temperatura ambiente. Es también
muy volátil, con una presión de vapor de 8,5 kPa (a 20°C), y soluble
en agua, con una solubilidad de 8690 mg/litro (a 20°C). El log del
coeficiente de reparto octanol/agua es de 1,76.
El análisis del 1,2-dicloroetano en el medio ambiente se realiza
habitualmente por cromatografía de gases, en combinación con la
captura de electrones, la detección de ionización por conductor o bien
la espectrometría de masas. Los límites de la detección oscilan entre
0,016 y > 4 µg/m3 en el aire, entre 0,001 y 4,7 µg/litro en el agua
y entre 6 y 10 µg/kg en diversos productos alimenticios.
2. Fuentes de exposición humana y ambiental
El 1,2-dicloroetano se utiliza principalmente en la síntesis del
monómero cloruro de vinilo y, en menor grado, en la fabricación de
diversos disolventes clorados. Se incorpora también a los aditivos
antidetonantes de la gasolina (aunque este empleo está disminuyendo
con la reducción progresiva en muchos países de la gasolina con plomo)
y se ha usado como fumigante. La producción anual total de
1,2-dicloroetano en el Canadá en 1990 y en los Estados Unidos en 1991
fue de 922 000 y 6 318 000 toneladas, respectivamente.
3. Transporte, distribución y transformación en el medio ambiente
La mayor parte del 1,2-dicloroetano liberado en el medio ambiente
ha sido emitido en el aire. En este medio es moderadamente
persistente; su permanencia en la atmósfera se estima entre 43 y 111
días. Es transportado a la estratosfera, donde, por fotólisis, pueden
producirse radicales de cloro que a su vez pueden reaccionar con el
ozono. Cierta cantidad de 1,2-dicloroetano puede escapar a los
efluentes industriales y de allí pasar al medio ambiente acuático, de
donde desaparece rápidamente por volatilización. También puede
alcanzar por lixiviación las aguas subterráneas próximas a los
vertederos de desechos industriales. No se prevé su bioconcentración
en especies acuáticas o terrestres.
4. Niveles medioambientales y exposición humana
Según estudios recientes del aire ambiental, las concentraciones
medias de 1,2-dicloroetano detectadas en zonas urbanas donde no
abundan sus fuentes de emisión oscilan entre 0,07 y 0,28 µg/m3,
mientras que los niveles medios notificados como presentes en el aire
del interior de las viviendas oscilan entre < 0,1 y 3,4 µg/m3. En
el agua potable, la concentración media suele ser menor de
0,5 µg/litro. En estudios recientes rara vez se ha detectado la
presencia de 1,2-dicloroetano en alimentos y, puesto que el potencial
de bioacumulación de esta sustancia es bajo, los alimentos
probablemente no representen una fuente de exposición importante.
Teniendo en cuenta las estimaciones de la exposición media en
diversos entornos, la principal fuente de exposición de la población
general al 1,2-dicloroetano es el aire de locales cerrados y el aire
exterior, mientras que el agua de bebida contribuye sólo en cantidades
muy pequeñas. La ingestión de 1,2-dicloroetano con los alimentos
probablemente sea insignificante. La cantidad inhalada con el aire
ambiental puede ser más grande en las proximidades de fuentes de
emisión industriales.
5. Cinética y metabolismo en animales de laboratorio
El 1,2-dicloroetano se absorbe fácilmente tras la inhalación, la
ingestión o la exposición cutánea y se distribuye rápida y ampliamente
por todo el organismo. En la rata y el ratón se metaboliza de forma
rápida y completa y por orina se eliminan principalmente metabolitos
azufrados en concentraciones que dependen de la dosis. En la rata, el
metabolismo parece saturado o limitado cuando la exposición alcanza
niveles que dan lugar a concentraciones sanguíneas de 5 a 10 µg/ml.
Después de la administración por sonda de una dosis única, los niveles
de alquilación del ADN eran más elevados que después de la inhalación
durante un periodo de seis horas.
El 1,2-dicloroetano parece metabolizarse siguiendo dos vías
principales: la primera comporta una oxidación microsómica saturable
mediada por el citocromo P-450, que produce 2-cloroacetaldehído y
2-cloroetanol, seguida de conjugación con el glutatión. La segunda
vía entraña la conjugación directa con el glutatión para formar
S-(2-cloroetil)-glutatión, que puede convertirse mediante un proceso
no enzimático en un ion glutatión episulfonio; este ion puede formar
aductos con el ADN. Aunque se han inducido daños en el ADN por la
ruta del P-450 in vitro, hay varias pruebas de que la vía de la
conjugación del glutatión probablemente sea más importante que la otra
en cuanto a los daños que causa en el ADN.
6. Efectos en mamíferos de laboratorio y en sistemas de ensayo
in vitro
La toxicidad aguda del 1,2-cicloroetano en animales de
experimentación es baja. Por ejemplo, la CL50 por inhalación en
ratas expuestas durante 6 ó 7,25 horas oscilaba entre 4000 mg/m3 y
6600 mg/m3, mientras que la DL50 por vía oral en ratas, ratones,
perros y conejos variaba entre 413 y 2500 mg/kg de peso corporal.
Los resultados de estudios de corta duración y subcrónicos
realizados en varias especies de animales de experimentación indican
que los órganos afectados son el hígado y los riñones; en general no
se obtuvieron NOEL ni LOEL fidedignos debido a la documentación
insuficiente y a la gama limitada de puntos finales examinados en
pequeños grupos de animales. En una serie de estudios iniciales
limitados, se observaron cambios morfológicos en el hígado de varias
especies tras la exposición subcrónica a concentraciones de
1,2-dicloroetano en el aire de sólo 800 mg/m3. Tras la
administración subcrónica a ratas por vía oral de dosis comprendidas
entre 49 y 82 mg/kg de peso corporal por día durante más de 13 semanas
se observó un incremento del peso relativo del hígado. En los
estudios crónicos disponibles se ha presentado poca información sobre
efectos no neoplásicos. Las ratas expuestas a concentraciones de sólo
202 mg/m3 en el aire durante 12 meses acusaron en los parámetros del
suero cambios indicativos de toxicidad hepática y renal, pero en ese
estudio no se realizaron exámenes histopatológicos.
La carcinogenicidad del 1,2-dicloroetano se ha investigado en un
pequeño número de biovaloraciones limitadas sobre animales de
experimentación (las limitaciones comprenden una exposición de corta
duración y una mortalidad alta). No se notificaron aumentos
significativos en la incidencia de ningún tipo de tumor en ratas
Sprague-Dawley o en ratones suizos expuestos a concentraciones de
hasta 607 mg/m3 durante 78 semanas; los animales se observaron hasta
que se produjo su muerte espontánea. En este estudio, la mortalidad
de las ratas fue elevada, pero no guardaba relación con la
concentración, y no se realizó un ajuste de la incidencia en función
de la mortalidad diferencial entre los grupos. En un ensayo con ratas
Sprague-Dawley hembra expuestas durante dos años a 200 mg/m3, se
produjo un aumento no significativo de la incidencia de adenomas y
fibroadenomas en las glándulas mamarias, y no se observó otra forma de
toxicidad relacionada con el compuesto.
En cambio, hay pruebas convincentes de un aumento de la
incidencia de tumores en dos especies tras la ingestión del producto.
Las ratas Osborne-Mendel que habían recibido mediante sonda durante 78
semanas dosis diarias de 47 ó 95 mg/kg de peso corporal (promedio
ponderado en función del tiempo) mostraron un aumento significativo de
la incidencia de tumores en diversos lugares (por ejemplo, carcinomas
escamosos del estómago en machos, hemangiosarcomas en machos y
hembras, fibromas del tejido subcutáneo en machos, adenocarcinomas y
fibroadenomas de las glándulas mamarias en hembras. En ratones
B6C3F1 que habían recibido, como promedio ponderado en función del
tiempo, dosis diarias de 97 ó 195 mg/kg de peso corporal (machos) y
149 ó 299 mg/kg de peso corporal (hembras) mediante sonda durante 78
semanas se observaron aumentos semejantes de la incidencia de tumores
en múltiples lugares (adenomas alveolares/bronquiolares en machos y
hembras, adenocarcinomas de las glándulas mamarias en hembras y pólipo
estromal endometrial o sarcoma estromal endometrial combinados en
hembras y carcinomas hepatocelulares en machos).
La incidencia de tumores pulmonares (papilomas benignos)
aumentó significativamente en ratones hembra tras la aplicación
cutánea repetida de 1,2-dicloroetano durante 440 a 594 días. La
administración intraperitoneal repetida produjo en una raza
susceptible aumentos en el número de adenomas pulmonares por ratón;
dichos aumentos estaban relacionados con la dosis, pero ninguno de
ellos fue significativo. En comparación con las ratas que recibieron
el compuesto solo y con el grupo testigo, que no recibió ningún
tratamiento, las ratas expuestas simultáneamente a 1,2-dicloroetano
por inhalación y a disulfiram en los alimentos acusaron una mayor
incidencia de colangiomas y de quistes en el conducto biliar
intrahepático, de fibromas subcutáneos, de nódulos neoplásicos en el
hígado, de tumores de las células intersticiales de los testículos y
de adenocarcinomas mamarios. En tres biovaloraciones realizadas no se
observaron indicios de potencial iniciador o facilitador del
desarrollo de tumores, pero el alcance del examen histopatológico de
esos estudios fue limitado.
In vitro, el 1,2-dicloroetano ha dado constantemente resultados
positivos en biovaloraciones de mutagenicidad en Salmonella
typhimurium. Las respuestas han sido mayores en presencia de un
sistema de activación exógeno (posiblemente debido a la activación por
el sistema del citocromo) que en su ausencia; la mutagenicidad se
duplicó con creces en la cepa de S. typhimurium que contenía el gen
humano GSTA1-1. En cultivos de células mamarias, el 1,2-dicloroetano
forma aductos con el ADN. También induce síntesis no programada del
ADN en cultivos primarios de células de roedores y humanas y mutación
génica en varias líneas celulares. La frecuencia de las mutaciones en
las líneas celulares humanas se ha correlacionado con diferencias en
la actividad de la glutatión-S-transferasa. En estudios in vivo,
el 1,2-dicloroetano indujo mutaciones en células somáticas y
mutaciones letales recesivas ligadas al sexo en Drosophila
melanogaster y, según todos los estudios realizados en ratas y
ratones, el compuesto se había unido al ADN. Aunque en estudios
efectuados en ratones se han observado lesiones primarias del ADN en
el hígado e intercambio de cromátides hermanas, no hay indicaciones de
inducción de micronúcleos.
Teniendo en cuenta los resultados de un número limitado de
estudios, no hay pruebas de que el 1,2-dicloroetano sea teratogénico
en animales de experimentación. Además, hay pocas pruebas
convincentes de que tenga efectos sobre la reproducción o el
desarrollo con dosis inferiores a las que causan otros efectos
sistémicos. Los datos disponibles sobre la inmunotoxicidad del
1,2-dicloroetano son limitados.
7. Efectos en el ser humano
La exposición accidental aguda al 1,2-dicloroetano por inhalación
o por ingestión ha producido diversos efectos en el ser humano, por
ejemplo en el sistema nervioso central, el hígado, el riñón, el pulmón
y el sistema cardiovascular.
No se ha estudiado con detenimiento la carcinogenicidad potencial
del 1,2-dicloroetano en poblaciones humanas expuestas. La mortalidad
por cáncer pancreático aumentó de forma significativa en un grupo de
trabajadores de una planta de producción química que había estado
expuesto sobre todo a 1,2-dicloroetano (en combinación con otros
productos químicos). La mortalidad por cáncer pancreático aumentó con
la duración de la exposición. Además, aunque el número de casos fue
pequeño y la relación con la duración de la exposición menos
constante, en estos trabajadores también aumentó la mortalidad por
leucemia. No se observó relación entre la exposición ocupacional al
1,2-dicloroetano y el cáncer cerebral en un pequeño estudio de casos y
controles. Si bien en un estudio ecológico con limitaciones
inherentes se observó que la incidencia de cáncer de colon y de recto
aumentaba con la concentración del producto en el agua de bebida, la
exposición simultánea a otras sustancias podría haber contribuido a
producir los efectos observados.
8. Efectos en organismos no destinatarios en el laboratorio y
sobre el terreno
Se han investigado los efectos de la exposición al
1,2-dicloroetano en varios otros organismos en el laboratorio y en el
medio ambiente. Con respecto a los microorganismos acuáticos, se ha
informado de que las CI50 y CE50 correspondientes a diversos
puntos finales oscilan entre 25 y 770 mg/litro. La CL50 más baja
notificada para Daphnia fue de 220 mg/litro, mientras que se
detectaron efectos en el éxito reproductivo y el crecimiento a 20,7 y
71,7 mg/litro, respectivamente. Teniendo en cuenta los datos
disponibles, la especie de vertebrados de agua dulce más sensible
parece ser la salamandra noroccidental (Ambystoma gracile), cuyas
larvas de nueve días (cuatro días después de la eclosión) vieron
reducida su supervivencia a concentraciones de 2,54 mg/litro. Se
dispone sólo de datos limitados sobre la toxicidad del
1,2-dicloroetano en organismos terrestres.
 It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROETHANE
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency,
Washington DC, USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist, The
Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROETHANE
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met in Geneva from 25 October to 3 November 1994.
Dr W. Kreisel, Executive Director, welcomed the participants on behalf
of WHO, and Dr M. Mercier, Director, IPCS, on behalf of the IPCS and
its cooperating organizations (UNEP/ILO/WHO). The Core Assessment
Group reviewed and revised the draft monograph and made an evaluation
of the risks for human health and the environment from exposure to
1,2-dichloroethane (ethylene dichloride).
The first draft of the monograph was prepared by Ms K. Hughes,
Environmental Health Directorate, Health Canada. The second draft,
revised in the light of international comment, was also prepared by
Ms K. Hughes. Dr E. Smith and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the scientific content and
technical editing respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
1,2-Dichloroethane was previously evaluated by a WHO Task Group
in 1986 and published by WHO in 1987 as Environmental Health Criteria
62.
ABBREVIATIONS
BCF bioconcentration factor
BUN blood urea nitrogen
ECD electron capture detector
FID flame ionization detector
GC gas chromatography
GSH glutathione
gamma-GT gamma-glutamyltranspeptidase
HECD Hall electron capture detector
LOEL lowest-observed-effect level
MS mass spectrometry
NOEL no-observed-effect level
PIB piperonyl butoxide
SGOT serum glutamic-oxalic transaminase
SGPT serum glutamic-pyruvic transaminase
TEAM total exposure assessment methodology
TWA time-weighted average
1. Summary
1.1 Identity, physical and chemical properties, and analytical
methods
1,2-Dichloroethane (ethylene dichloride) is a synthetic chemical
which is a colourless liquid at room temperature. It is also highly
volatile, with a vapour pressure of 8.5 kPa (at 20°C), and is soluble
in water, with a solubility of 8690 mg/litre (at 20°C). The log
octanol/water partition coefficient is 1.76.
Analysis for 1,2-dichloroethane in environmental media is usually
by gas chromatography, in combination with electron capture or flame
ionization detection or mass spectrometry. Detection limits range
from 0.016 to > 4 µg/m3 in air, 0.001 to 4.7 µg/litre in water, and
from 6 to 10 µg/kg in various foodstuffs.
1.2 Sources of human and environmental exposure
The principal use of 1,2-dichloroethane is in the synthesis of
vinyl chloride monomer, and to a lesser extent in the manufacture of
various chlorinated solvents. It is also incorporated into anti-knock
gasoline additives (although this use is declining with the phase-out
of leaded gasoline in some countries), and has been used as a
fumigant. Total annual production of 1,2-dichloroethane in Canada in
1990 and the USA in 1991 was 922 and 6318 kilotonnes, respectively.
1.3 Environmental transport, distribution and transformation
The majority of 1,2-dichloroethane released to the environment is
in emissions to air. It is moderately persistent in air; the
estimated atmospheric lifetime is between 43 and 111 days.
1,2-Dichloroethane is transported to the stratosphere, where
photolysis may produce chlorine radicals which may in turn react with
ozone. Some 1,2-dichloroethane may be released in industrial
effluents to the aquatic environment, from which it is removed rapidly
by volatilization. 1,2-Dichloroethane may also leach to groundwater
near industrial waste sites. It is not expected to bioconcentrate in
aquatic or terrestrial species.
1.4 Environmental levels and human exposure
Mean concentrations of 1,2-dichloroethane in recent surveys of
ambient air in non-source-dominated areas of cities range from 0.07 to
0.28 µg/m3, while mean levels in residential indoor air are reported
to range from < 0.1 to 3.4 µg/m3. In drinking-water, mean
concentrations are generally less than 0.5 µg/litre.
1,2-Dichloroethane has only rarely been detected in foodstuffs in
recent surveys and, since it has low potential for bioaccumulation,
food is unlikely to be a major source of exposure.
Based on estimates of mean exposure from various media, the
predominant source of exposure to 1,2-dichloroethane by the general
population is indoor and outdoor air, only minor amounts being
contributed by drinking-water. Intake of 1,2-dichloroethane from food
is probably negligible. The amount inhaled in ambient air may be
greater in the vicinity of industrial sources.
1.5 Kinetics and metabolism in laboratory animals
1,2-Dichloroethane is readily absorbed following inhalation,
ingestion or dermal exposure and is rapidly and widely distributed
throughout the body. It is rapidly and extensively metabolized in
rats and mice, with principally sulfur-containing metabolites being
eliminated in the urine in a dose-dependent manner. Metabolism
appears to be saturated or limited in rats at levels of exposure
resulting in blood concentrations of 5 to 10 µg/ml. Levels of DNA
alkylation were higher following exposure to a bolus dose by gavage
than in the case of inhalation over a 6-h period.
1,2-Dichloroethane appears to be metabolized via two principal
pathways; the first involves a saturable microsomal oxidation mediated
by cytochrome P-450 to 2-chloroacetaldehyde and 2-chloroethanol
followed by conjugation with glutathione. The second pathway entails
direct conjugation with glutathione to form S-(2-chloroethyl)-
glutathione, which may be non-enzymatically converted to a glutathione
episulfonium ion; this ion can form adducts with DNA. Although DNA
damage has been induced by the P-450 pathway in vitro, several lines
of evidence indicate that the glutathione conjugation pathway is
probably of greater significance than the P-450 pathway as the major
route for DNA damage.
1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of 1,2-dichloroethane is low in experimental
animals. For example, inhalation LC50s for rats exposed for 6 or
7.25 h ranged from 4000 mg/m3 to 6600 mg/m3, while oral LD50s
for rats, mice, dogs and rabbits ranged from 413 to 2500 mg/kg body
weight.
The results of short-term and subchronic studies in several
species of experimental animals indicate that the liver and kidneys
are the target organs; reliable NOELs or LOELs were not attained in
general due to inadequate documentation and the limited range of
end-points examined in small groups of animals. In a series of early
limited studies, morphological changes in the liver were observed in
several species following subchronic exposure to airborne
concentrations as low as 800 mg/m3. Increases in the relative liver
weight have been observed in rats following subchronic oral
administration of doses of 49 to 82 mg/kg body weight per day or more
for 13 weeks. Little information was presented on non-neoplastic
effects in available chronic studies. Changes in serum parameters
indicative of liver and kidney toxicity were observed in rats exposed
to airborne concentrations as low as 202 mg/m3 for 12 months,
although histopathological examinations were not conducted in this
study.
The carcinogenicity of 1,2-dichloroethane has been investigated
in a few limited bioassays on experimental animals (limitations
include short duration of exposure and high mortality). Significant
increases were not reported in the incidence of any type of tumour in
Sprague-Dawley rats or Swiss mice exposed to up to 607 mg/m3 for 78
weeks and observed until spontaneous death. Mortality was high in
rats in this study, although it was not related to concentration, and
the incidence rates were not adjusted for differential mortality among
groups. There was a nonsignificant increase in the incidence of
mammary gland adenomas and fibroadenomas in female Sprague-Dawley rats
exposed to 200 mg/m3 for 2 years in an assay in which no other
compound-related toxicity was observed.
In contrast, there was convincing evidence of increases in tumour
incidence in two species following ingestion. Significant increases
in the incidence of tumours at several sites (including squamous cell
carcinomas of the stomach (males), haemangiosarcomas (males and
females), fibromas of the subcutaneous tissue (males), adenocarcinomas
and fibroadenomas of the mammary gland (females)) were observed in
Osborne-Mendel rats administered TWA daily doses of 47 or 95 mg/kg
body weight per day by gavage for 78 weeks. Similar increases in the
incidences of tumours at multiple sites (including
alveolar/bronchiolar adenomas (males and females), mammary gland
adenocarcinomas (females) and endometrial stromal polyp or endometrial
stromal sarcoma combined (females) and hepatocellular carcinomas
(males)) occurred in B6C3F1 mice administered TWA daily doses of 97 or
195 mg/kg body weight per day (males) or 149 or 299 mg/kg body weight
per day (females) by gavage for 78 weeks.
The incidence of lung tumours (benign papillomas) was
significantly increased in female mice following repeated dermal
application of 1,2-dichloroethane for 440 to 594 days. Repeated
intraperitoneal injections of 1,2-dichloroethane resulted in
dose-related increases in the number of pulmonary adenomas per mouse
in a susceptible strain, although none of these increases was
significant. Concomitant exposure to inhaled 1,2-dichloroethane and
disulfiram in the diet resulted in an increased incidence of
intrahepatic bile duct cholangiomas and cysts, subcutaneous fibromas,
hepatic neoplastic nodules, interstitial cell tumours in the testes
and mammary adenocarcinomas in rats, compared to rats administered
either compound alone or untreated controls. No potential to initiate
or promote tumour development was evident in three bioassays, although
the extent of histopathological examination was limited in these
studies.
In in vitro assays, 1,2-dichloroethane has been consistently
positive in mutagenicity bioassays in Salmonella typhimurium.
Responses have been greater in the presence of an exogenous activation
system (possibly due to activation by the cytochrome system) than in
its absence, and mutagenicity was more than doubled in S. typhimurium
expressing the human GSTA1-1 gene. In cultured mammalian cells,
1,2-dichloroethane forms adducts with DNA. It also induces
unscheduled DNA synthesis in primary cultures of rodent and human
cells and gene mutation in several cell lines. Mutation frequency in
human cell lines has been correlated with differences in
glutathione- S-transferase activity. In in vivo studies,
1,2-dichloroethane induced somatic cell and sex-linked recessive
lethal mutations in Drosophila melanogaster and the compound bound
to DNA in all reported studies in rats and mice. Although primary DNA
damage in liver and sister chromatid exchange has been observed in
studies in mice, there has been no evidence for micronucleus
induction.
Based on the results of a limited number of studies, there is no
evidence that 1,2-dichloroethane is teratogenic in experimental
animals. There is also little convincing evidence that it induces
reproductive or developmental effects at doses below those which cause
other systemic effects. Available data on the immunotoxicity of
1,2-dichloroethane are limited.
1.7 Effects on humans
Acute incidental exposure to 1,2-dichloroethane by inhalation or
ingestion has resulted in a variety of effects in humans, including
effects on the central nervous system, liver, kidney, lung and
cardiovascular system.
The potential carcinogenicity of 1,2-dichloroethane in exposed
human populations has not been extensively investigated. Mortality
due to pancreatic cancer was significantly increased in a group of
workers at a chemical production plant who had been exposed
principally to 1,2-dichloroethane (in combination with other
chemicals). Mortality due to pancreatic cancer increased with
duration of exposure. In addition, although the number of cases was
small, and the association with duration of exposure was less
consistent, mortality due to leukaemia was also increased in these
workers. No association between occupational exposure to
1,2-dichloroethane and brain cancer was noted in a small case-control
study. Although the incidence of colon and rectal cancer increased
with the concentration of 1,2-dichloroethane in drinking-water in an
inherently limited ecological study, concomitant exposure to other
substances may have contributed to the observed effects.
1.8 Effects on non-target organisms in the laboratory and field
The effects of exposure to 1,2-dichloroethane on a number of
other organisms in the laboratory and field have been investigated.
For aquatic microorganisms, IC50s or EC50s for various end-points
have been reported to range from 25 to 770 mg/litre. The lowest
reported LC50 value for Daphnia was 220 mg/litre, while effects on
reproductive success and growth were observed at 20.7 and
71.7 mg/litre, respectively. Based on available data, the most
sensitive freshwater vertebrate species appears to be the northwestern
salamander (Ambystoma gracile), in which 9-day larval survival (4
days post-hatch) was reduced at 2.54 mg/litre. Only limited data are
available on the toxicity of 1,2-dichloroethane to terrestrial
organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
The empirical formula for 1,2-dichloroethane (ethylene
dichloride) is C2H4Cl2 and the molecular structure is as
follows:
H H
' '
Cl - C - C - Cl
' '
H H
Synonyms include EDC, 1,2-DCE, 1,2-bichloroethane, 1,2-ethylene
dichloride, acethylenchlorid, alpha, beta-dichloroethane, bichlorure
d'ethelene, ethyleen dichloride, ethylene chloride, glycol dichloride,
and sym-dichlorothane. Trade names include: Borer sol, Brocide,
Destruxo,l Di-chlor-mulson, Dichlor-mulsion, Dutch liquid, Dutch oil,
ENT 1656, Freon 150, Gaze Olefiant and Granosan (which also contains
carbon tetrachloride).
The Chemical Abstract Service (CAS) registry number for
1,2-dichloroethane is
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROETHANE
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency,
Washington DC, USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist, The
Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROETHANE
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met in Geneva from 25 October to 3 November 1994.
Dr W. Kreisel, Executive Director, welcomed the participants on behalf
of WHO, and Dr M. Mercier, Director, IPCS, on behalf of the IPCS and
its cooperating organizations (UNEP/ILO/WHO). The Core Assessment
Group reviewed and revised the draft monograph and made an evaluation
of the risks for human health and the environment from exposure to
1,2-dichloroethane (ethylene dichloride).
The first draft of the monograph was prepared by Ms K. Hughes,
Environmental Health Directorate, Health Canada. The second draft,
revised in the light of international comment, was also prepared by
Ms K. Hughes. Dr E. Smith and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the scientific content and
technical editing respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
1,2-Dichloroethane was previously evaluated by a WHO Task Group
in 1986 and published by WHO in 1987 as Environmental Health Criteria
62.
ABBREVIATIONS
BCF bioconcentration factor
BUN blood urea nitrogen
ECD electron capture detector
FID flame ionization detector
GC gas chromatography
GSH glutathione
gamma-GT gamma-glutamyltranspeptidase
HECD Hall electron capture detector
LOEL lowest-observed-effect level
MS mass spectrometry
NOEL no-observed-effect level
PIB piperonyl butoxide
SGOT serum glutamic-oxalic transaminase
SGPT serum glutamic-pyruvic transaminase
TEAM total exposure assessment methodology
TWA time-weighted average
1. Summary
1.1 Identity, physical and chemical properties, and analytical
methods
1,2-Dichloroethane (ethylene dichloride) is a synthetic chemical
which is a colourless liquid at room temperature. It is also highly
volatile, with a vapour pressure of 8.5 kPa (at 20°C), and is soluble
in water, with a solubility of 8690 mg/litre (at 20°C). The log
octanol/water partition coefficient is 1.76.
Analysis for 1,2-dichloroethane in environmental media is usually
by gas chromatography, in combination with electron capture or flame
ionization detection or mass spectrometry. Detection limits range
from 0.016 to > 4 µg/m3 in air, 0.001 to 4.7 µg/litre in water, and
from 6 to 10 µg/kg in various foodstuffs.
1.2 Sources of human and environmental exposure
The principal use of 1,2-dichloroethane is in the synthesis of
vinyl chloride monomer, and to a lesser extent in the manufacture of
various chlorinated solvents. It is also incorporated into anti-knock
gasoline additives (although this use is declining with the phase-out
of leaded gasoline in some countries), and has been used as a
fumigant. Total annual production of 1,2-dichloroethane in Canada in
1990 and the USA in 1991 was 922 and 6318 kilotonnes, respectively.
1.3 Environmental transport, distribution and transformation
The majority of 1,2-dichloroethane released to the environment is
in emissions to air. It is moderately persistent in air; the
estimated atmospheric lifetime is between 43 and 111 days.
1,2-Dichloroethane is transported to the stratosphere, where
photolysis may produce chlorine radicals which may in turn react with
ozone. Some 1,2-dichloroethane may be released in industrial
effluents to the aquatic environment, from which it is removed rapidly
by volatilization. 1,2-Dichloroethane may also leach to groundwater
near industrial waste sites. It is not expected to bioconcentrate in
aquatic or terrestrial species.
1.4 Environmental levels and human exposure
Mean concentrations of 1,2-dichloroethane in recent surveys of
ambient air in non-source-dominated areas of cities range from 0.07 to
0.28 µg/m3, while mean levels in residential indoor air are reported
to range from < 0.1 to 3.4 µg/m3. In drinking-water, mean
concentrations are generally less than 0.5 µg/litre.
1,2-Dichloroethane has only rarely been detected in foodstuffs in
recent surveys and, since it has low potential for bioaccumulation,
food is unlikely to be a major source of exposure.
Based on estimates of mean exposure from various media, the
predominant source of exposure to 1,2-dichloroethane by the general
population is indoor and outdoor air, only minor amounts being
contributed by drinking-water. Intake of 1,2-dichloroethane from food
is probably negligible. The amount inhaled in ambient air may be
greater in the vicinity of industrial sources.
1.5 Kinetics and metabolism in laboratory animals
1,2-Dichloroethane is readily absorbed following inhalation,
ingestion or dermal exposure and is rapidly and widely distributed
throughout the body. It is rapidly and extensively metabolized in
rats and mice, with principally sulfur-containing metabolites being
eliminated in the urine in a dose-dependent manner. Metabolism
appears to be saturated or limited in rats at levels of exposure
resulting in blood concentrations of 5 to 10 µg/ml. Levels of DNA
alkylation were higher following exposure to a bolus dose by gavage
than in the case of inhalation over a 6-h period.
1,2-Dichloroethane appears to be metabolized via two principal
pathways; the first involves a saturable microsomal oxidation mediated
by cytochrome P-450 to 2-chloroacetaldehyde and 2-chloroethanol
followed by conjugation with glutathione. The second pathway entails
direct conjugation with glutathione to form S-(2-chloroethyl)-
glutathione, which may be non-enzymatically converted to a glutathione
episulfonium ion; this ion can form adducts with DNA. Although DNA
damage has been induced by the P-450 pathway in vitro, several lines
of evidence indicate that the glutathione conjugation pathway is
probably of greater significance than the P-450 pathway as the major
route for DNA damage.
1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of 1,2-dichloroethane is low in experimental
animals. For example, inhalation LC50s for rats exposed for 6 or
7.25 h ranged from 4000 mg/m3 to 6600 mg/m3, while oral LD50s
for rats, mice, dogs and rabbits ranged from 413 to 2500 mg/kg body
weight.
The results of short-term and subchronic studies in several
species of experimental animals indicate that the liver and kidneys
are the target organs; reliable NOELs or LOELs were not attained in
general due to inadequate documentation and the limited range of
end-points examined in small groups of animals. In a series of early
limited studies, morphological changes in the liver were observed in
several species following subchronic exposure to airborne
concentrations as low as 800 mg/m3. Increases in the relative liver
weight have been observed in rats following subchronic oral
administration of doses of 49 to 82 mg/kg body weight per day or more
for 13 weeks. Little information was presented on non-neoplastic
effects in available chronic studies. Changes in serum parameters
indicative of liver and kidney toxicity were observed in rats exposed
to airborne concentrations as low as 202 mg/m3 for 12 months,
although histopathological examinations were not conducted in this
study.
The carcinogenicity of 1,2-dichloroethane has been investigated
in a few limited bioassays on experimental animals (limitations
include short duration of exposure and high mortality). Significant
increases were not reported in the incidence of any type of tumour in
Sprague-Dawley rats or Swiss mice exposed to up to 607 mg/m3 for 78
weeks and observed until spontaneous death. Mortality was high in
rats in this study, although it was not related to concentration, and
the incidence rates were not adjusted for differential mortality among
groups. There was a nonsignificant increase in the incidence of
mammary gland adenomas and fibroadenomas in female Sprague-Dawley rats
exposed to 200 mg/m3 for 2 years in an assay in which no other
compound-related toxicity was observed.
In contrast, there was convincing evidence of increases in tumour
incidence in two species following ingestion. Significant increases
in the incidence of tumours at several sites (including squamous cell
carcinomas of the stomach (males), haemangiosarcomas (males and
females), fibromas of the subcutaneous tissue (males), adenocarcinomas
and fibroadenomas of the mammary gland (females)) were observed in
Osborne-Mendel rats administered TWA daily doses of 47 or 95 mg/kg
body weight per day by gavage for 78 weeks. Similar increases in the
incidences of tumours at multiple sites (including
alveolar/bronchiolar adenomas (males and females), mammary gland
adenocarcinomas (females) and endometrial stromal polyp or endometrial
stromal sarcoma combined (females) and hepatocellular carcinomas
(males)) occurred in B6C3F1 mice administered TWA daily doses of 97 or
195 mg/kg body weight per day (males) or 149 or 299 mg/kg body weight
per day (females) by gavage for 78 weeks.
The incidence of lung tumours (benign papillomas) was
significantly increased in female mice following repeated dermal
application of 1,2-dichloroethane for 440 to 594 days. Repeated
intraperitoneal injections of 1,2-dichloroethane resulted in
dose-related increases in the number of pulmonary adenomas per mouse
in a susceptible strain, although none of these increases was
significant. Concomitant exposure to inhaled 1,2-dichloroethane and
disulfiram in the diet resulted in an increased incidence of
intrahepatic bile duct cholangiomas and cysts, subcutaneous fibromas,
hepatic neoplastic nodules, interstitial cell tumours in the testes
and mammary adenocarcinomas in rats, compared to rats administered
either compound alone or untreated controls. No potential to initiate
or promote tumour development was evident in three bioassays, although
the extent of histopathological examination was limited in these
studies.
In in vitro assays, 1,2-dichloroethane has been consistently
positive in mutagenicity bioassays in Salmonella typhimurium.
Responses have been greater in the presence of an exogenous activation
system (possibly due to activation by the cytochrome system) than in
its absence, and mutagenicity was more than doubled in S. typhimurium
expressing the human GSTA1-1 gene. In cultured mammalian cells,
1,2-dichloroethane forms adducts with DNA. It also induces
unscheduled DNA synthesis in primary cultures of rodent and human
cells and gene mutation in several cell lines. Mutation frequency in
human cell lines has been correlated with differences in
glutathione- S-transferase activity. In in vivo studies,
1,2-dichloroethane induced somatic cell and sex-linked recessive
lethal mutations in Drosophila melanogaster and the compound bound
to DNA in all reported studies in rats and mice. Although primary DNA
damage in liver and sister chromatid exchange has been observed in
studies in mice, there has been no evidence for micronucleus
induction.
Based on the results of a limited number of studies, there is no
evidence that 1,2-dichloroethane is teratogenic in experimental
animals. There is also little convincing evidence that it induces
reproductive or developmental effects at doses below those which cause
other systemic effects. Available data on the immunotoxicity of
1,2-dichloroethane are limited.
1.7 Effects on humans
Acute incidental exposure to 1,2-dichloroethane by inhalation or
ingestion has resulted in a variety of effects in humans, including
effects on the central nervous system, liver, kidney, lung and
cardiovascular system.
The potential carcinogenicity of 1,2-dichloroethane in exposed
human populations has not been extensively investigated. Mortality
due to pancreatic cancer was significantly increased in a group of
workers at a chemical production plant who had been exposed
principally to 1,2-dichloroethane (in combination with other
chemicals). Mortality due to pancreatic cancer increased with
duration of exposure. In addition, although the number of cases was
small, and the association with duration of exposure was less
consistent, mortality due to leukaemia was also increased in these
workers. No association between occupational exposure to
1,2-dichloroethane and brain cancer was noted in a small case-control
study. Although the incidence of colon and rectal cancer increased
with the concentration of 1,2-dichloroethane in drinking-water in an
inherently limited ecological study, concomitant exposure to other
substances may have contributed to the observed effects.
1.8 Effects on non-target organisms in the laboratory and field
The effects of exposure to 1,2-dichloroethane on a number of
other organisms in the laboratory and field have been investigated.
For aquatic microorganisms, IC50s or EC50s for various end-points
have been reported to range from 25 to 770 mg/litre. The lowest
reported LC50 value for Daphnia was 220 mg/litre, while effects on
reproductive success and growth were observed at 20.7 and
71.7 mg/litre, respectively. Based on available data, the most
sensitive freshwater vertebrate species appears to be the northwestern
salamander (Ambystoma gracile), in which 9-day larval survival (4
days post-hatch) was reduced at 2.54 mg/litre. Only limited data are
available on the toxicity of 1,2-dichloroethane to terrestrial
organisms.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
The empirical formula for 1,2-dichloroethane (ethylene
dichloride) is C2H4Cl2 and the molecular structure is as
follows:
H H
' '
Cl - C - C - Cl
' '
H H
Synonyms include EDC, 1,2-DCE, 1,2-bichloroethane, 1,2-ethylene
dichloride, acethylenchlorid, alpha, beta-dichloroethane, bichlorure
d'ethelene, ethyleen dichloride, ethylene chloride, glycol dichloride,
and sym-dichlorothane. Trade names include: Borer sol, Brocide,
Destruxo,l Di-chlor-mulson, Dichlor-mulsion, Dutch liquid, Dutch oil,
ENT 1656, Freon 150, Gaze Olefiant and Granosan (which also contains
carbon tetrachloride).
The Chemical Abstract Service (CAS) registry number for
1,2-dichloroethane is  Although some DNA damage has been induced via the P-450 pathway
in vitro (Banerjee et al., 1980; Guengerich et al., 1980; Lin et
al., 1985), several lines of evidence suggest that the GSH conjugation
pathway is probably of greater significance than the P-450 pathway as
the major route for DNA damage (Guengerich et al., 1980; Rannug, 1980;
Sundheimer et al., 1982; Inskeep et al., 1986; Koga et al., 1986).
The P-450-dependent pathway can, however, presumably form
considerable quantities of 2-haloacetaldehydes, which readily bind to
protein and non-protein thiols, as shown for vinyl bromide and vinyl
chloride (Guengerich et al., 1981) and dibromoethane (DBE) (van
Bladeren et al., 1981). However, these authors concluded that 2H
and 18O studies on the formation of 2-haloethanols and
2-haloacetaldehydes from 1,2-dihaloethanes are inconsistent with a
major role of such a mechanism for DNA damage (Guengerich et al.,
1986; Koga et al., 1986).
The 1,2-dichloroethane-induced mutation frequency of two human
cell lines has been correlated with the difference in levels of
glutathione- S-transferase activities. AHH-1 cell line mutation
frequency was 25 times that in the TK6 cell line in the presence of
1,2-dichloroethane. The difference was attributed to the fact that
the AHH-1 cell line possesses 5 times more glutathione- S-transferase
activity than the TK6 cell line (Crespi et al., 1985).
Moreover, although the significance of the reported results is
uncertain, the results of an additional study by Storer & Conolly
(1985) are not inconsistent with the hypothesis that reduction of GSH
levels is associated with a reduction in DNA damage. Male B6C3F1
mice pretreated with piperonyl butoxide (PIB), a P-450 inhibitor, were
examined for the extent of hepatic DNA damage produced 4 h after
1,2-dichloroethane administration. Hepatic DNA damage, as measured by
alkali-labile lesions, was potentiated by PIB. Treatment of mice with
high doses of 2-chloroethanol failed to produce DNA damage, as
measured by this assay. Diethylmaleate, a GSH depletor, potentiated
the hepatotoxicity of 2-chloroethanol but not DNA damage.
In addition, Cheever et al. (1990) reported that although the
levels of hepatic DNA covalent binding of metabolites of
14C-1,2-dichloroethane injected (single dose) to rats which had been
exposed by inhalation to 1,2-dichloroethane in a long-term bioassay
were significant (p < 0.05), these levels were not different in rats
with concomitant exposure to disulfiram in the diet over two years.
Evidence suggests that the putative episulfonium ion, resulting
from non-enzymatic conversion of S-(2-chloroethyl) glutathione, is a
major intermediate in the formation of DNA adducts in vivo from
1,2-dichloroethane exposure (Inskeep et al., 1986). When rats were
administered single does of 14C-1,2-dichloroethane in vivo and the
liver was analysed 8 h later, 78% of the DNA adducts (0.25 nmol/mg
DNA) could be released by neutral thermal hydrolysis. A major adduct
and several minor adducts were present; the major adduct
co-chromatographed with S-[2-(N7-guanyl)ethyl] glutathione. The
postulated adduct of liver DNA after 14C-1,2-dichloroethane
exposure, S-[2-(N7-guanyl)ethyl] glutathione, appears to be
chromatographically identical to the major adduct in rats after
exposure to 1,2-dibromoethane (Koga et al., 1986). This
1,2-dibromoethane adduct, which has been isolated and characterized by
NMR and mass spectrometry, gives strong support to an identical adduct
being the principal DNA adduct from exposure to 1,2-dihaloethanes.
Reitz et al. (1982) found (based on consideration of results of
their own work as well as that of Spreafico et al., 1980) that
metabolism of 1,2-dichloroethane appears to be saturated or limited in
rats at levels of exposure resulting in blood concentrations of 5 to
10 mg/litre, based on an observed non-linear relationship between
levels in blood and administered doses or concentrations.
Administration by gavage resulted in the formation of about twice the
amount of "total" metabolites as did exposure by inhalation, based on
recovery in excreta, expired air and the carcass. Oral exposure
produced 1.5- to 2-fold lower levels of total macromolecular binding
but 3- to 5-fold higher levels of DNA alkylation than inhalation,
though the absolute levels of DNA alkylation were considered low.
Based on examination of DNA binding in the liver and lung of rats
exposed by inhalation to a low constant concentration (0.3 mg/litre)
of 1,2-dichloroethane for 12 h or to a peak concentration (up to
18 mg/litre) for a few minutes, Baertsch et al. (1991) concluded that
DNA damage by 1,2-dichloroethane depends upon the concentration time
profile, with bolus doses causing disproportionately greater damage.
6.4 Elimination and excretion
Unmetabolized 1,2-dichloroethane is eliminated in expired air,
while its metabolites are largely excreted in the urine. Unchanged
1,2-dichloroethane was detected in the exhaled breath of women exposed
dermally and to airborne concentrations of 0.252 mg/m3 (0.063 ppm)
in the workplace; the amount of 1,2-dichloroethane expired was greater
immediately following exposure and decreased over time (Urusova,
1953).
A single dose of 150 mg/kg body weight radiolabelled
1,2-dichloroethane was injected into rats that had been exposed via
inhalation at a concentration of 200 mg/m3 (50 ppm) for 2 years. The
proportion of radioactivity present in the urine within 24 h was 42.5
and 33.9% (in males and females, respectively), while 27.3 and 40.3%
were eliminated as the unchanged parent compound in the breath. Only a
very small amount of radioactivity was detected as 14CO2 or in the
faeces. In rats that had been concomitantly exposed to disulfiram
during the 2-year period, the proportion of unchanged
1,2-dichloroethane eliminated in the breath increased significantly
(i.e. 57.6 and 57.7%; p < 0.05), while the proportion eliminated in
the urine decreased correspondingly (27.6 and 24.9%). Levels of
unchanged 1,2-dichloroethane in blood were significantly (p < 0.05)
increased in rats exposed to 1,2-dichloroethane and disulfiram
compared to those exposed to 1,2-dichloroethane alone (see section
7.10) (Cheever et al., 1990).
The pattern of elimination of metabolites was similar in rats and
mice 48 h after administration of oral doses of radiolabelled
1,2-dichloroethane (100 and 150 mg/kg body weight, respectively). In
rats, 8.2 and 69.51% of the radiolabelled dose was recovered as CO2
and in the excreta (principally urine), respectively, compared to
18.21 and 81.11% in mice. The overall recovery was less in rats than
in mice (96.26 versus 110.12%) (Mitoma et al., 1985).
In rats exposed to 600 mg/m3 (150 ppm) 1,2-dichloroethane for
6 h or administered 150 mg/kg body weight by gavage, there was no
significant difference in the route of excretion of non-volatile
metabolites. After 48 h, in each case, more than 84% of total
metabolites was eliminated in the urine, 7-8% was excreted as carbon
dioxide in expired air, 2% was excreted unchanged in the faeces, and
4% remained in the carcass (Reitz et al., 1982). The major urinary
metabolites identified following exposure of rats by either route were
thiodiacetic acid (70%) and thiodiacetic acid sulfoxide (26 to 28%).
The rate of elimination following oral (gavage) administration or
inhalation was such that 1,2-dichloroethane was not detected in the
blood a few hours after exposure and only small amounts were detected
in tissues (liver, kidney, lung, spleen, forestomach, stomach and
carcass) 48 h after exposure (Reitz et al., 1982). The rate of
elimination from blood and tissues appeared to depend on the exposure
level; the higher the exposure level, the lower the elimination rate
of 1,2-dichloroethane, after both oral and inhalation exposure.
Elimination from the liver was reported to be biphasic, a higher
elimination rate occurring just after the peak levels of
1,2-dichloroethane were reached. Elimination from other organs was
monophasic. Following inhalation up to an exposure level of
1012 mg/m3, elimination was slowest in adipose tissue and most rapid
in the lung (Spreafico et al., 1980).
Withey & Collins (1980) also reported that the elimination of
1,2-dichloroethane was dose-dependent. After intravenous
administration of from 3 to 15 mg/kg body weight to male Wistar rats,
the authors found that the elimination fitted a twocompartment model
at a low dose level and a three-compartment model at high dose levels.
The percentage of administered radioactivity excreted in the
urine over a 24-h period in rats decreased with increasing single
doses (0.25 to 8.08 mmol 1,2-dichloroethane/kg body weight)
administered by gavage in mineral oil (Payan et al., 1993). The
authors attributed these results to saturation of metabolism rather
than kidney damage, as there were no variations in biochemical
parameters of nephrotoxicity between the controls and groups exposed
to doses up to 4.04 mmol/kg body weight. Urinary thiodiglycolic acid
increased as a linear function of the dose of 1,2-dichloroethane until
at least 1.01 mmol/kg body weight; it accounted for 63% of the total
metabolites in urine at this dose.
6.5 Retention and bioaccumulation
Although 1,2-dichloroethane is eliminated more slowly from
adipose tissue than from blood or other tissues (lung and liver)
following exposure, it is unlikely to bioaccumulate significantly, as
no difference was observed between levels in blood or tissues (data
not presented) following single or repeated (10 days) oral doses of
50 mg/kg body weight in rats (Spreafico et al., 1980). Only 71 and
75%, respectively, of an administered oral dose of radiolabelled
1,2-dichloroethane was recovered in the excreta and exhaled breath of
rats administered 150 mg/kg body weight by gavage following 2 years of
exposure via inhalation (200 mg/m3 or 50 ppm); the authors
speculated that the remainder may have been sequestered in the body
fat. Recovery in the excreta and exhaled breath was complete in
younger rats (4 months old) receiving the same oral dose (Cheever et
al., 1990).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Data on the acute toxicity of 1,2-dichloroethane in experimental
animals are summarized in Table 3. These data indicate that
1,2-dichloroethane is of relatively low acute toxicity.
LC50 values in rats exposed to 1,2-dichloroethane for 6 or
7.25 h ranged from 4000 mg/m3 (1000 ppm) (Spencer et al., 1951) to
6600 mg/m3 (1650 ppm) (Bonnet et al., 1980). The 6-h LC50 in mice
was 1050 mg/m3 (Gradiski et al., 1978). LC50 values decreased
with increasing duration of exposure in rats exposed to concentrations
ranging from 1200 to 80 000 mg/m3 (300 to 20 000 ppm)
1,2-dichloroethane for 1 to 8 h (Spencer et al., 1951). Various
non-lethal effects have been reported in animals following acute
exposure to 1,2-dichloroethane, including central nervous system
depression, cardiovascular collapse, altered behaviour, pulmonary
congestion and oedema, histological damage in the liver, kidneys and
adrenal glands and myocardial failure, at concentrations ranging from
4000 mg/m3 for 1.5 or 4 h to 80 000 mg/m3 (20 000 ppm) for 30 min
(Heppel et al., 1945; Spencer et al., 1951; Alumot et al., 1976a;
Wolff et al., 1979; ATSDR, 1989). Central nervous system depression
occurred at much higher concentrations than those which induce effects
in other organs.
Oral LD50 values for rats, mice, dogs and rabbits ranged from
413 mg/kg body weight in female mice to 2500 mg/kg body weight in dogs
(Barsoum & Saad, 1934; McCollister et al., 1956; Smyth, 1969; Larionov
& Kokarovtseva, 1976; Munson et al., 1982; NIOSH, 1994). Non-lethal
effects observed in rats and rabbits following single oral doses of
1,2-dichloroethane ranging from 615 to 1476 mg/kg body weight include
hepatic effects (fatty degeneration, cloudy swelling, congestion,
haemorrhagic lesions, dystrophy in the cytoplasm and hyperchromatosis
in the nuclei of hepatocytes), degeneration of the renal tubular
epithelium, altered levels of enzymes in the serum and liver, oedema
and haemorrhaging in the walls of the coronary vessels, stasis and
thrombi in the myocardium, altered fibrinolytic activity in the blood,
and altered haematological parameters. A single dose of 0.5 ml
altered the ratio of the oxidized and reduced forms of nicotinamide
coenzymes in the liver and myocardium of rats (Natsyuk & Chekman,
1975). Electrocardiographic changes were reported at doses of 1, 1.5
and 2 mg/kg body weight, although these effects have not been
confirmed in other studies (Saitanov & Arsenieva, 1969).
The LD50 for dermal exposure in rabbits was estimated to be
between 2.8 and 4.9 g/kg (Torkelson & Rowe, 1981; NIOSH,
1994).
Table 3. Acute toxicity of 1,2-dichloroethane in experimental animals
Species Numbers/sex Duration/vehicle LC50 or LD50 Reference
Inhalation
Rats (Wistar equal no. of m & f) 10-54 0.53 h 48 000 mg/m3 (12 000 ppm) Spencer et al. (1951)
20-51 2.75 h 12 000 mg/m3 (3000 ppm)
31-32 7.20 h 4000 mg/m3 (1000 ppm)
Rats (albino, strain, number and sex not not specified 30 000 mg/m3 Nevrotsky et al. (1971)
specified)
Rats (Sprague-Dawley, 12 per group, sex not 6 h 6600 mg/m3 (1646 ppm) Bonnet et al. (1980)
specified)
Mice (OF1, 20 f per group) 6 h 1050 mg/m3 (262 ppm) Gradiski et al. (1978)
Ingestion
Rats (strain, number and sex not specified) not specified 850 mg/kg body weight Larionov &
Kokarovtseva (1976)
Rats (6 per group, strain and sex not not specified 770 mg/kg body weight Smyth (1969)
specified)
Rats (young adult albino, 80 m & f) corn oil 680 mg/kg body weight McCollister et al. (1956)
Mice, 6-week old (CD-1, number not male water 489 mg/kg body weight Munson et al. (1982)
specified) female 413 mg/kg body weight
Table 3. (cont'd).
Species Numbers/sex Duration/vehicle LC50 or LD50 Reference
Dogs (strain, number and sex not specified) acacia gum 2500 mg/kg body weight Barsoum & Saad (1934)
Rabbits (strain, number and sex not not specified 860 mg/kg body weight NIOSH (1994)
specified)
Dermal
Rabbits (strain, number and sex not not specified 2800 mg/kg body weight NIOSH (1994)
specified)
Rabbits (strain, number and sex not olive oil; duration 2800-4900 mg/kg body weight Torkelson & Rowe
specified) and area of skin (1981)
exposed not
specified
7.2 Skin and eye irritation
When 1.0 ml undiluted 1,2-dichloroethane was applied directly to
the clipped skin of guinea-pigs for up to 12 h in occluded patch
tests, no gross skin reactions were visible (Jakobson et al., 1982).
Microscopic changes appeared 4 h after application, comprising
karyopyknosis, perinuclear oedema, spongiosis and junctional
separation (Kronevi et al., 1981). In Draize tests on rabbits,
moderate erythema and oedema were observed 24 h after
application (dose not specified). Microscopy on the third day
revealed necrosis and other lesions such as ulcerations and
acanthosis. The severity of the changes was not indicated (Duprat et
al., 1976).
Instillation of 0.1 ml undiluted 1,2-dichloroethane into the
conjunctival sac of the eye of rabbits generated reversible, mild
irritation characterized by conjunctivitis and epithelial abrasion.
Epithelial keratitis, described as being "in a state of repair", was
observed microscopically 7 days after application (Duprat et al.,
1976). Reversible clouding of the cornea was observed in dogs within
10 h of subcutaneous administration of undiluted 1,2-dichloroethane at
0.9 mg/kg body weight. The clouding continued up to 48 h, but the
corneas appeared clear after 5 days. Histological changes, including
necrosis of the corneal endothelium, partially denuded Descemet's
membrane, formation of excess basement membrane, and swelling of the
corneal stroma, were also observed in dogs, cats and rabbits after
ocular injection of 1.8 mg 1,2-dichloroethane (0.15 ml of a 1%
solution) into the anterior chamber (Kuwabara et al., 1968).
7.3 Short-term exposure
Small groups of Wistar rats, rabbits, guinea-pigs, dogs and pigs
(n = 1 to 21) were exposed to 6000 mg/m3 (1500 ppm)
1,2-dichloroethane, 7 h/day for 6 days. Sections of the liver, heart,
lungs, kidney adrenal glands and spleen were examined microscopically.
In most animals, degeneration or necrosis of the kidney and liver,
along with congestion and haemorrhage of the lungs and adrenal glands,
were observed (Heppel et al., 1945).
No significant changes in organ or body weights, histology or
clinical chemistry and haematological parameters were observed in rats
administered 1,2-dichloroethane doses of up to 150 mg/kg body weight
per day in corn oil by gavage, 5 times/week for 2 weeks (van Esch et
al., 1977; Reitz et al., 1982).
7.4 Subchronic exposure
7.4.1 Inhalation
The subchronic toxicity of inhaled 1,2-dichloroethane was
investigated in three early limited studies in multiple species, as
presented in Table 4. Heppel et al. (1946) exposed groups of rats,
mice, rabbits, guinea-pigs, dogs, cats and monkeys to 4000 mg/m3
(1000 ppm) for up to 66 days. Mice, rats, rabbits and guinea-pigs
were the most sensitive species, based on mortality after only one or
a few exposures. Various effects were noted in these animals,
including pulmonary congestion (guinea-pigs, cats and rats), fatty
changes in the kidney (rats and monkeys), fatty changes in the
livera (cats, dogs and monkeys) and clouded corneas (dogs). At
1600 mg/m3 (400 ppm), observed effects included fatty degeneration
of the liver, kidney or heart (guinea-pigs and one rat), fatty changes
in the liver (dogs) and pulmonary congestion (rats), while at
800 mg/m3 (200 ppm), rats and guinea-pigs had mild pulmonary
congestion and one rat had fatty degeneration in the kidneys. No
effects on growth were noted in mice and rats exposed to 400 mg/m3
(100 ppm).
In a similar study, rats, guinea-pigs, rabbits and monkeys were
exposed to 400, 800 or 1600 mg/m3 (100, 200 or 400 ppm)
1,2-dichloroethane for 6 to 9 months (Spencer et al., 1951). Severe
effects, including hepatotoxicity, and deaths were observed in rats
and guinea-pigs exposed at the highest level, while monkeys also
showed degeneration of the liver and kidneys at this concentration.
No effects were noted in rabbits. At 800 mg/m3 (200 ppm) no adverse
effects were observed in rats, but slight degeneration of the liver
was noted in guinea-pigs. At 400 mg/m3 (100 ppm), no adverse
effects were observed in any of the four species. The authors
considered the "maximum concentrations without adverse effects" to be
1600 mg/m3 (400 ppm) in the rabbit, 800 mg/m3 (200 ppm) in the
rat, and 400 mg/m3 (100 ppm) in the monkey and guinea-pig, based on
a limited range of end-points.
a It has been suggested, on the basis of in vitro investigations,
that fatty accumulation in the liver may be due to the ability of
1,2-dichloroethane to block the secretion of hepatocellular
(Cotalasso et al., 1994).
Table 4. Subchronic toxicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats (26, strain and animals were exposed to 0 or There was high mortality in exposed rats (20/26), rabbits (5/6) and Heppel
sex not specified) 4000 mg/m3 (0 or 1000 ppm), guinea-pigs (36/41) after a few exposures. All mice died after one et al.
Mice (22, strain and 7 h/day, 5 days/week for up exposure. Survival was higher among cats and dogs (4/6 of either (1946)
sex not specified) to 66 exposures; sections of species survived more than 23 exposures). One monkey died after 2
Rabbits (5 m & 1 f, liver, heart, lungs, kidney, days and the other after 32 exposures. Pulmonary congestion was
strain not specified) adrenal glands and spleen noted in guinea-pigs, cats and rats. Rats and monkeys had fatty
Guinea-pigs (10-16, strain were examined changes in the kidney. Cats and monkeys had fatty changes in the
and sex not specified) microscopically; haematological liver. Dogs had cloudy corneas; one dog had fatty degeneration of
Dogs (3 f, strain not and urinary parameters were the liver. No effects on haematological or urinary parameters were
specified) assessed in dogs observed in dogs. Rabbits had no obvious effects on postmortem.
Cats (6 f, strain not
specified)
Monkeys (Rhesus, 2,
sex not specified)
Rats (15 m & 1 f, animals were exposed to 0 or All dogs and puppies survived 177 exposures. All rabbits died, after Heppel
strain not specified) 1600 mg/m3 (0 or 400 ppm), 1 to 97 exposures; 14/20 and 9/16 guinea-pigs and rats died by the et al.
Rabbits (2 m & 3 f, 7 h/day, 5 days/week for up to 60th exposure. Rats had pulmonary congestion and 1 rat and 6 (1946)
strain not specified) 177 exposures; sections of guinea-pigs had fatty degeneration of the liver, kidney and heart.
Guinea-pigs (8-10 m & liver, heart, lungs, kidney, Dogs had slight fatty changes in the liver. No effects were noted in
2 f, strain not specified) adrenal glands and spleen were rabbits on postmortem. There were no significant differences in
Dogs (6 f, strain not examined microscopically; haematological parameters in exposed dogs and rabbits compared
specified) and puppies haematological parameters to controls.
(3 m, strain not were also assessed in dogs
specified) and rabbits
Table 4. (cont'd).
Species Protocol Results Reference
Rats (Wistar 1 m & 11 f) animals were exposed to 0 or 5/14 guinea-pigs, 7/12 rats and 8/12 rats died after 1 to 115 Heppel
Osborne-Mendel rats (12 m) 800 mg/m3 (0 or 200 ppm), 7 h/ exposures. Rats and guinea-pigs had mild pulmonary congestion, et al.
Rabbits (5 m, strain day, 5 days/week, for up to and 1 rat had fatty degeneration in the kidneys. There were no (1946)
not specified) 125 exposures; sections of significant differences in haematological parameters in exposed
Guinea-pigs (12 m & 2 f, liver, heart, lungs, kidney, rats and rabbits, compared to controls.
strain not specified) adrenal glands and spleen
Monkeys (2 m, strain were examined microscopically;
not specified) haematological parameters were
assessed in rats and rabbits
Rats (23 m & 16 f, animals were exposed to 0 or All animals survived. There were no differences in the rate of Heppel
strain not specified) 400 mg/m3 (0 or 100 ppm), 7 h/ growth in rats or mice. et al.
Mice (19, strain and day, 5 days/week for 74 (rats) (1946)
sex not specified) and 19 (mice) exposures;
sections of liver, heart,
lungs, kidney, adrenal glands
and spleen were examined
microscopically
Rats (Wistar, 15-20 m & animals were exposed to 0, 400, 1600 mg/m3: All female rats and male guinea-pigs died by 10 Spencer
15-20 f) 800 or 1600 mg/m3 (0, 100, 200, exposures; all male rats died by 40 exposures. All female et al.
Guinea-pigs (2-8 m & or 400 ppm), 7 h/day, 5 days/ guinea-pigs died by 24 exposures. Rats and guinea-pigs had rapid body (1951)
8 m, strain not specified) week for 6 to 9 months; animals weight loss, slight increases in liver and kidney weights, and
Rabbits (albino, number were killed at various times some fatty changes in the liver. Guinea-pigs had swelling of the
and sex not specified) throughout the study; tubular epithelium of the kidneys and alterations in levels of
Monkeys (Rhesus, number haematological parameters were non-protein nitrogen urea nitrogen in the blood. Monkeys had
and sex not specified) assessed and histopathological degeneration of the liver and kidneys and increased fat content
examinations of several tissues of the liver. No effects were noted in rabbits.
were conducted
Table 4. (cont'd).
Species Protocol Results Reference
800 mg/m3: Guinea-pigs tolerated up to 180 exposures (246 days).
Male guinea-pigs had significantly (p=0.001) decreased body
weight gain; both sexes of guinea-pigs had slight degeneration
and fatty accumulation in the liver. Rats tolerated up to 151
exposures (212 days) with no observed adverse effects.
400 mg/m3: No effects were noted in rats, guinea-pigs, rabbits
or monkeys.
Rats (Sprague-Dawley, were exposed to 400 At the higher concentration, rats were dyspnoeic and guinea-pigs Hofman
10, sex not specified) or 2000 mg/m3 (100 or 500 were "apathetic". 3/4 rabbits died after 10-17 exposures; 9/10 et al.
Guinea-pigs (10, strain ppm), 6 h/day, 5 days/week guinea-pigs died after 4-14 exposures. Rats died after 1-5 exposures, (1971)
and sex not specified) for up to 17 weeks; while all cats survived 30 exposures. Rats had pulmonary hyperaemia
Rabbits (4, strain and histological examinations were and oedema, fatty liver and adrenal and myocardial necrosis. Cats and
sex not specified) conducted, and several rabbits had heart lesions, and guinea-pigs had fatty changes in the
Cats (2 per group, strain bio-chemical parameters were myocardium, liver and adrenals, and necrosis in the myocardium and
and sex not specified) assessed liver. At 400 mg/m3, there were no clinical or histological
changes after exposure for 17 weeks.
Table 4. (cont'd).
Species Protocol Results Reference
Ingestion
Rats F344/N, animals were administered 0, Drinking-water: F344/N rats: Body weight was significantly decreased NTP
Sprague-Dawley and 500, 1000, 2000, 4000 and in males at 4000 and 8000 mg/litre (>8%, p<0.001). Relative kidney (1991)
Osborne Mendel, mice, 8000 mg/litre in the weight was significantly increased at >1000 mg/litre in both sexes
B6C3F1 (10 or 20 m & f drinking-water (equivalent to (>11%, p<0.001). Relative liver weight was significantly increased at
per group) doses of of 49-82, 86-126, >2000 mg/litre in males (>14%, p<0.05) and 4000 mg/litre in females
146-213, 259-428, and (>9%, p<0.001). Alterations in haematological and serum parameters
492-727 mg/kg body weight per at the higher concentrations reflected dehydration caused by
day in rats and 244-249, decreased water consumption. Renal tubular "regeneration" observed
448-647, 781-1182, 2478-2710 in all groups of males at similar frequencies and severity, but
and 4207-4926 mg/kg body frequency was related to dose in females (0/10, 0/10, 1/10, 2/10
weight per day in mice) for 3/10 and 9/10 with increasing concentration).
13 weeks
Rats F344/N (10 or rats were administered 0, 30, Sprague-Dawley rats: Body weight was significantly decreased in
20 m & f per group) 60, 120, 240 or 480 mg/kg body males at 8000 ppm (9%, p<0.05). Relative kidney weight was
per day (males) or 0, 18, 37, significantly increased at >1000 mg/litre in males (>7%, p<0.05)
75, 150 or 300 mg/kg body and at >500 mg/litre in females (>8%, p<0.05). Relative liver
weight per day (females) in weight was increased in males at >500 mg/litre (>6%, p<0.05)
corn oil by gavage, and at 8000 mg/litre in females (13%, p<0.001). Alterations in
5 days/week for 13 weeks haematological and serum parameters at the higher
concentrations reflected dehydration caused by decreased water
consumption. Renal tubular "regeneration" was observed in all
groups at similar frequencies and severity.
Table 4. (cont'd).
Species Protocol Results Reference
haematological and serum Osborne-Mendel rats: Body weight was significantly decreased
chemistry parameters were at 8000 mg/litre in males (15%, p<0.05). Relative kidney weight
examined at several times was increased at 4000 and 8000 mg/litre in males (>8%, p<0.05)
during the course of the study. and >500 mg/litre in females (>12%, p<0.001). Relative liver weight
Extensive histopathological was increased in males at 1000 and 2000 mg/litre (>14%, p<0.05).
examinations were conducted Alterations in haematological and serum parameters at the higher
for controls and all animals concentrations reflected dehydration caused by decreased water
at the highest concentration consumption. Although the incidence of renal tubular "regeneration"
in drinking-water and in female was increased at the higher concentrations, the increases were
mice at 4000 ppm, as well as in not related to dose, and severity was similar in all groups.
male rats receiving doses of
120 or 240 mg/kg body weight B6C3F1 mice: 9/10 females exposed to 8000 mg/litre died. Body
per day by gavage and in weight was significantly decreased in males at 8000 mg/litre
female rats at 150 mg/kg body (17.5%, p<0.001). Relative kidney weight was significantly increased
weight per day in males at >1000 mg/litre (>12%, p<0.001) and in females at >500
mg/litre (>16%, p<0.001). Relative liver weight was significantly
increased in males at 500 mg/litre and above and in females at 1000
mg/litre or more. The incidence of renal tubular cell regeneration
was significantly increased in males at 4000 and 8000 mg/litre;
karyomegaly was observed in all males at 8000 mg/litre.
Gavage: F344/N rats: All males receiving 240 or 480 mg/kg body
weight per day and 9/10 female receiving 300 mg/kg body weight per
day died before the end of the study. The incidence of hyperplasia
and inflammation of the forestomach was significantly increased in
males at 240 mg/kg body weight per day; necrosis of the thymus was
observed in 10/10 males at 480 mg/kg body weight per day and 5/10
females at 300 mg/kg body weight per day, compared to none
in controls.
Table 4. (cont'd).
Species Protocol Results Reference
Rats (Osborne-Mendel, animals were administered At the highest dose, three males and one female died. In males, NCI
5 m & 5 f per group) doses of 0, 40, 63, 100, 159 or mean body weight was only significantly decreased (by 50%) at (1978)
251 mg/kg body weight per day 251 mg/kg body weight per day. In females mean body weight was
in corn oil by gavage, 5 days depressed (10% at 40 mg/kg body weight per day to 17% at 100
week for 6 weeks followed by a mg/kg body weight per day and 32% at 159 mg/kg body weight
2-week observation period per day). No other parameters were investigated.
Mice (B6C3F1 5 m & animals were administered All males receiving 398 mg/kg body weight per day and all females NCI
5 f per group) doses of 0, 159, 251, 398, receiving 631 mg/kg body weight per day died. Mean body weight (1978)
631 or 1000 mg/kg body weight depression was only observed in females receiving 398 mg/kg body
per day in corn oil by weight per day, in which "drastic weight loss" was reported. No
gavage, 5 days/week for other parameters were investigated.
6 weeks followed by a 2-week
observation period
Rats (6 per group, rats were fed mash fumigated The only effect noted, based on examination of lipid content Alumot
strain and sex not with 1,2-dichloroethane, of the liver and liver weight, was an increase in liver fat at et al.
specified) resulting in initial 1600 mg/kg. Chronic respiratory disease was evident in all exposure (1976a)
concentrations of 200 or groups (incidence data not presented, but the disease was not
600 mg/kg (approximately believed to be associated with exposure); mortality was higher in
equivalent to doses of 10 and males than females, with the number of rats surviving after 21
30 mg/kg body weight per day, months ranging from 3 to 14.
respectively) for 5 weeks, or
1600 mg/kg (approximately
equivalent to a dose of
80 mg/kg body weight per day)
for 7 weeks; the authors
noted that the dose ingested
was approximately 60 to 70% of
the initial amount after loss
Table 4. (cont'd).
Species Protocol Results Reference
to volatilization was
considered; lipid content of
liver and liver weight were
measured, and hepatic
triglycerides were measured at
the highest dose; no
histopathological examinations
were conducted
Rats (m & f, strain and rats were administered doses Decreased weight gain was observed at the two highest doses. Van Esch
number not specified of 0, 10, 30 or 90 mg/kg body Males and females had increased relative kidney weight at 90 et al.
in secondary account) weight per day (method of oral mg/kg body weight per day. Females also had increased relative (1977)
administration not specified weights of liver and brain at this dose. No effects on histology
in secondary account), 5 times or clinical chemistry were noted. Alterations in some
per week for 90 days; haematological parameters observed, although these did not occur
histopathological examinations in a dose-related manner.
were conducted, along with
assessment of haematological
and clinical chemistry
parameters, although the
extent of the examinations
was not specified in the
secondary account
Rats (m, strain and rats were orally There was a statistically significant (p < 0.02) increase in the Apostolov
number not specified) administered doses activity of the serum lysosome enzyme, &
equivalent to 1/5000, 1/1000 beta-N-acetylglucosa-minidase at the two highest doses. Mihaylova
or 1/200 the LD50 (not further (1975)
specified) for 3 months (abstract
only)
Hofmann et al. (1971) exposed rats, guinea-pigs, rabbits and cats
to 400 or 2000 mg/m3 (100 or 500 ppm) for up to 17 weeks. Mortality
was high in rats, guinea-pigs and rabbits exposed to the higher
concentration. At 2000 mg/m3 (500 ppm) pulmonary hyperaemia and
oedema, fatty liver and adrenal and myocardial necrosis were noted in
rats, while heart lesions were observed in cats and rabbits.
Guinea-pigs had fatty changes in the myocardium, liver and adrenals
and necrosis in the myocardium and liver at the higher concentration.
No clinical or histological effects were noted at 400 mg/m3
(100 ppm).
7.4.2 Ingestion
Available data on the subchronic toxicity of ingested
1,2-dichloroethane are presented in Table 4. In a recent study
conducted by the National Toxicology Program (NTP, 1991) and partially
reported by Morgan et al. (1990), the relative susceptibility of three
strains of rats (F344/N, Sprague-Dawley and Osborne-Mendel) and one
strain of mice (B6C3F1) exposed to 1,2-dichloroethane in
drinking-water at concentrations of up to 8000 mg/litre for 13 weeks,
and one of the same strains of rats (F344/N) exposed to doses of up to
480 mg/kg body weight per day by gavage in corn oil for 13 weeks, was
investigated. Based on increased relative organ weights, the liver
and kidneys were the target organs in both rats and mice, although
treatment-related microscopic lesions were noted only in female F344/N
rats and male B6C3F1 mice. Administration of 1,2-dichloroethane to
F344/N rats by gavage resulted in more severe toxic effects (including
death) than administration of similar doses in drinking-water,
probably due to greater peak levels of the compound in the blood and
saturation of elimination mechanisms. The authors considered the
no-observed-effect levels (NOEL) for 1,2-dichloroethane administered
to F344/N rats by gavage to be 120 and 150 mg/kg body weight per day
in males and females, respectively, based on mortality and chemically
related lesions in the forestomach. The NOEL of B6C3F1 mice exposed
via drinking-water was considered to be 780 mg/kg body weight per day)
(2000 ppm) in males, based on kidney lesions, and (2500 mg/kg body
weight per day) (4000 ppm) in females, based on mortality. The
authors did not consider the doses to which the three strains of rats
were exposed in the drinking-water to be high enough to result in
biologically significant toxic effect, although increases in organ
weights without accompanying histopathological alterations were
observed at doses as low as 49 to 82 mg/kg body weight per day in some
strains (i.e. Sprague-Dawley and Osborne-Mendel).
In limited subchronic studies preliminary to long-term
carcinogenesis bioassays, groups of Osborne-Mendel rats and B6C3F1
mice were administered doses of up to 251 and 1000 mg/kg body weight
per day, respectively, by gavage for 6 weeks. Significant mean body
weight loss was noted in male rats at 251 mg/kg body weight per day
and in female rats at > 40 mg/kg body weight per day. In mice,
mean body weight was decreased only in females receiving 398 mg/kg
body weight per day. No other parameters were investigated in this
study (NCI, 1978).
Decreased body weight gain was observed in rats orally
administered doses of 30 or 90 mg/kg body weight per day for 90 days,
but not in those receiving 10 mg/kg body weight per day. Increased
relative weights of kidneys (both sexes), liver and brain (females
only) were noted at the highest dose, although no histopathological
effects or alterations in clinical chemistry parameters were noted.
Changes in some haematological parameters were noted, but these were
not related to dose (Van Esch et al., 1977).
A slight increase in the fat content of the livers was reported
in rats consuming feed which had been fumigated with
1,2-dichloroethane, resulting in an initial concentration of
1600 mg/kg (approximately equivalent to a dose of 80 mg/kg body weight
per day) for 7 weeks, while no effects were observed at 600 mg/kg
(approximately equivalent to a dose of 30 mg/kg body weight per day)
after 5 weeks (Alumot et al., 1976a). The parameters examined were
limited to hepatic lipid content and liver weight. The activity of
the serum lysosome enzyme, ß- N-acetylglucosaminidase, was
significantly increased in rats administered oral doses equivalent to
1/1000 and 1/200 the LD50 (not further specified in abstract)
(Apostolov & Mihaylova, 1975).
7.5 Chronic exposure and carcinogenicity
7.5.1 Inhalation
Groups of 90 Sprague-Dawley rats of each sex were exposed by
inhalation to 1,2-dichloroethane (99.92% pure) at concentrations of 0,
20, 40, 202 and 1012 mg/m3 (0, 5, 10, 50 and 250 ppm), 7 h/day, 5
days/week for 78 weeks, and observed until spontaneous death (due to
severe toxicity, the highest concentration was reduced to 607 mg/m3
or 150 ppm after several days). "Incidence" was reported as the
number of animals developing specific tumours over the number of
animals alive at the time the first tumour of that type was detected
(i.e. incidences have not been adjusted for differential survival (see
Table 5)). The only tumour types for which the authors reported an
increase in incidence (when compared with controls kept in exposure
chambers, but not when compared with those not kept in chambers) were
fibromas and fibroadenomas (combined) of the mammary gland, which the
authors attributed to the differential survival among the groups
(Maltoni et al., 1980).
Groups of 50 male and 50 female Sprague-Dawley rats were exposed
to 0 or 200 mg/m3 (50 ppm) 1,2-dichloroethane 7 h/day, 5 days/week
for 2 years. No effects on body weight gain or mortality were noted.
There was no significant difference in the incidence of tumours at any
site, although there was a nonsignificant increase in the incidence of
mammary gland adenomas (4 in exposed group versus 2 in control group)
and fibroadenomas (21/50 in exposed group versus 15/50 in control
group) in females. There was an increased incidence of testicular
lesions (not further specified) in males (24% versus 10% in controls,
significance not reported) (Cheever et al., 1990). However, the
sensitivity of this investigation to detect any carcinogenic potential
may have been compromised (the study was designed to investigate the
interaction between 1,2-dichloroethane and other substances), based on
the lack of convincing evidence of compound-related toxicity at the
only concentration to which animals were exposed.
Clinical chemistry and haematological parameters were
investigated in groups of 8 to 10 male or female Sprague-Dawley rats
exposed to 0, 20, 40, 202 or 1012 mg/m3 (decreased to 607 mg/m3
after several days) (0, 5, 10, 50 or 250/150 ppm), 7 h/day, 5
days/week for 3, 6, and 18 months, beginning at 3 months of age. In
addition, groups of 8 to 10 rats were also exposed to these same
concentrations beginning at 14 months of age for 12 months. Although
values were occasionally significantly (p < 0.05) different from
those of controls, no consistent dose-related effects on various
haematological parameters, circulating protein levels or clinical
chemistry parameters were reported in rats exposed from 3 months of
age. In animals exposed for 12 months beginning at 14 months of age,
there were no consistent dose-related effects on haematological
parameters. There were significant (p < 0.05) changes in serum
parameters indicative of effects on liver and kidney function,
including levels of glutamic-pyruvic transaminase (SGPT),
gamma-glutamyltranspeptidase (gamma-GT), glutamic-oxalic transaminase
(SGOT) and cholesterol, and levels of uric acid in the blood and blood
urea nitrogen (BUN) at 202 and 607 mg/m3. Histopathological
examinations were not conducted (Spreafico et al., 1980).
No increase in the incidence of any type of tumour was reported
in groups of 90 male or female Swiss mice exposed to 20, 40, 202 or
1012 (decreased to 607 mg/m3 after a few days) mg/m3 (5, 10, 50 or
250/150 ppm), 7 h/day, 5 days/week for 78 weeks, and observed until
spontaneous death (Maltoni et al., 1980). However, it should be noted
that survival was poor (especially among males).
Table 5. Chronic toxicity and carcinogenicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats Rats were exposed to 0, 20, 40, 202 After several days of exposure to 1012 mg/m3, severe toxic effects, Maltoni
(Sprague-Dawley, and 1012/607 mg/m3 (0, 5, 10, 50 including death were observed and the level of exposure was reduced et al.
90 m & 90 f per and 250/150 ppm) 1,2-dichloroethane to 607 mg/m3. Survival varied among the groups, but was not related (1980)
group, 180 m & (99.92% pure), 7 h/day, 5 days/week to concentration; most rats died by week 140. Survival at 104 weeks
180 f controls) for 78 weeks and observed until of age in controls, chamber controls, and groups exposed to 20, 40,
spontaneous death. One group of 202 and 1012/607 mg/m3 was 17.8, 13.3, 50.0, 14.4, 18.9 and 11.1%
controls was kept in a nearby room, (males) and 40.0, 24.4, 53.3, 28.9, 32.2, and 23.3% (females).
while the other was kept in an "Incidence" was reported as the number of animals developing specific
exposure chamber under the same tumours over the number of animals alive at the time the first tumour
conditions as the exposed groups. of that type was detected (i.e. incidences have not been adjusted for
A complete autopsy was performed differential survival). With the exception of benign mammary tumours,
on each animal, regardless of time there were no significant increases in the incidence of any types of
of death. Several organs were (combined) tumours in exposed rats. The incidence of all mammary
routinely histopathologically tumours (number of animals alive at the time of appearance of the
examined, along with any organs first mammary tumour (12 weeks) was 90 in each exposure group) was
with pathological lesions. 52/90 (57.8%), 38/90 (42.2%), 65/90 (72.2%), 43/90 (47.8%), 58/90
(64.4%) and 52/90 (57.5%) in non-chamber controls, chamber controls,
and groups exposed to 20, 40, 202 and 1012/607 mg/m3, respectively.
The numbers (denominators not specified) of fibromas and
fibroadenomas (combined) of the mammary gland were 47, 27, 56, 33, 49
and 47 in non-chamber controls, chamber controls, and groups exposed
to 20, 40, 202 and 1012/607 mg/m3, respectively. The "incidence"
of these tumours at 20, 40, 202 and 1012/607 mg/m3, was
significantly (p<0.01 or 0.001) different from the incidence in
chamber controls. The "incidences" of benign mammary tumours in the
two control groups were also significantly (p<0.01) different.
Table 5. (cont'd).
Species Protocol Results Reference
Rats Rats were exposed to 0, 20, 40, 202 There were no consistent, exposure-related changes in various Spreafico
(Sprague-Dawley, or 1012-607 mg/m3 (0, 5, 10, 50 or haematological parameters, circulating protein levels or clinical et al.
8-10 or f per 250/150 ppm) 7 h/day, 5 days/week chemistry parameters in animals exposed from 3 months of age, (1980)
group) for 3, 6 or 18 months, beginning at although values occasionally differed significantly from controls.
3 months of age. In addition, groups In animals exposed for 12 months from 14 months of age, there were no
of rats were exposed to 0, 20, 40, consistent exposure-related alterations in haematological parameters.
202 or 1012-607 mg/m3 (0, 5, 10, 50 Levels of serum glutamic-pyruvic transaminase (SGPT) were
or 250/150 ppm) 7 h/day, 5 days/ significantly elevated in both males and females at 202 and 607 mg/m3
week for 12 months, beginning at 14 (p<0.05), and gamma-glutamil transpeptidase (gamma-GT) levels were
months of age. Histopathological also significantly greater in females at the two highest
examinations were not conducted. concentrations (p<0.05). Levels of serum glutamic-oxalic transaminase
(SGOT) were significantly increased in both sexes at 20 and 40 mg/m3
(p<0.05), but significantly decreased in males and females at 202 and
607 mg/m3 (p<0.05). Levels of cholesterol were significantly lower
in males and females at 202 and 607 mg/m3 (p<0.05). Levels of uric
acid in the blood were significantly higher in both sexes at 202 and
607 mg/m3 (p<0.05), while blood urea nitrogen (BUN) values were
significantly elevated at 607 mg/m3 (p<0.05), although there were
no effects on urinary parameters.
Rats Rats were exposed to 200 mg/m3 (0 There were no compound-related effects on body weight gain or Cheever
(Sprague-Dawley, or 50 ppm) 7 h/day, 5 days/week for mortality. There were no significant increases in the incidence of et al.
50 m & 50 f 2 years. Extensive histopathological any type of tumours, although there was a non-significant increase (1990)
per group) examinations were conducted. in the incidence of mammary gland adenomas (4 versus 2 in controls)
and fibroadenomas (21/50 versus 15/50) in females. The incidence of
testicular lesions (not further specified) was increased in exposed
animals (24% versus 10% in controls, significance not reported).
Table 5. (cont'd).
Species Protocol Results Reference
Mice (Swiss, Mice were exposed to 20, 40, 202 or Survival of mice was poor, especially in males, as only 43.4 to 65.6% Maltoni
90 m & 90 f 1012-607 mg/m3 (5, 10, 50 or 250/ of exposed males survived for 52 weeks after exposure commenced. et al.
per group, 150 ppm), 7 h/day, 5 days/week for There were no significant increases in the incidence of any type of (1980)
115 m & 134 f 78 weeks and observed until tumours.
controls) spontaneous death. Controls were
kept in a nearby room. A complete
autopsy was performed on each
animal, regardless of time of death.
Several organs were routinely
histopathologically examined, along
with any organs with pathological
lesions.
Ingestion
Rats Animals were administered time There were no effects on body weight gain in either sex. Mortality NCI
(Osborne-Mendel, weighted average doses of 47 or 95 was significantly higher in both males and females in the high dose (1978)
50 50 m & f per mg/kg body weight per day (initial group, as 50% of exposed rats had died by week 55 (males) and 57
group, 20 m & doses of 50 and 100 mg/kg body (females), compared to week 72 (males) and 88 (females) in controls.
20 f controls; weight per day were increased to 75 Signs of toxicity, including wheezing, nasal discharge, ulcerations,
60 m & 60 f and 150 mg/kg body weight per day localized alopecia, discoloured or stained fur, bloated appearance
pooled controls after 7 weeks then decreased to and swollen areas, occurred at a greater frequency in exposed
from concurrent original doses after 17 weeks) in animals than in controls. Chronic murine pneumonia was present in 60
experiments) corn oil by gavage, 5 days/week, for to 94% of rats in each group (incidence not related to dose).
78 weeks followed by 32 weeks of Acanthosis and hyperkeratosis of the forestomach was present in a
observation. Complete greater proportion of exposed females than controls (1/20, 6/50 and
histopathological examinations were 7/50 in vehicle controls, low and high dose, respectively,
conducted. significance not reported). Other non-neoplastic lesions occurred
at similar frequencies in control and exposed rats. The incidence
of squamous cell carcinomas of the forestomach in males was 0/60,
Table 5. (cont'd).
Species Protocol Results Reference
0/20, 3/50 and 9/50 in pooled controls, matched controls, low and
high dose groups, respectively, significant at the high dose
(p=0.01); only 2/50 females in the low dose group had this tumour.
There were also one leiomyosarcoma of the stomach and one
adenocarcinoma of the small intestine in high dose males (not
significant). The incidence of hemangiosarcomas (mostly in the
spleen) in males was 1/60, 0/20, 9/50 and 7/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively (significant in both exposed groups, p=0.03 (low) and
p=0.016 (high)), and in females was 0/59, 0/20, 4/50 and 4/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively (significant in both exposed groups (p=0.041 in
both)). Both groups of exposed males had an increased incidence of
fibromas of the subcutaneous tissue (0/60, 0/20, 5/50 and 6/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively); no such increase was noted in females. In
females, there was an increased incidence of adenocarcinomas and
fibroadenomas of the mammary gland (1/59, 0/20, 1/50 and 18/50
(adenocarcinoma), 5/59, 0/20, 14/50 and 8/50 (fibroadenoma) and 6/59,
0/20, 15/50 and 24/50 (adenocarcinoma or fibroadenoma)) in pooled
vehicle controls, matched vehicle controls, low and high dose groups,
respectively). Renal tubular cell adenocarcinomas were noted in one
male and one female at the highest dose, while tubular cell adenomas
were present in one male and two females at this dose, and none was
observed in controls (significance not reported).
Rats (18 m & Animals were fed mash fumigated Chronic respiratory disease was reported in all groups in the second Alumot
18 f per group, with 1,2-dichloroethane for 2 years. year of exposure. The number of rats surviving after 21 months ranged et al.
strain not Resulting concentrations were 250 from 2 to 14 per group. No effects on growth or the biochemical (1976a)
specified) and 600 mg/kg. Due to loss of the parameters investigated were observed.
Table 5. (cont'd).
Species Protocol Results Reference
compound through volatilization,
the mash actually consumed was
estimated to contain 60 to 70% of
the initial concentration (estimated
to result in doses of approximately
7.5 to 8.75 and 15 to 17.5 mg/kg
body weight per day). The liver was
analysed for total fat triglyceride
content. Levels of total protein,
albumin, glucose, urea, uric acid
and cholesterol in the serum were
determined. Histopathological
examinations do not appear to have
been conducted on surviving rats
at the end of the exposure period.
Mice (B6C3F1, Animals were administered Mortality in females was related to dose (36 animals in the high dose NCI
50 m & 50 f time-weighted average doses of 97 group died between weeks 60 and 80, which may have been related to (1978)
per group; 20 or 195 mg/kg body weight per day the appearance of tumours as 25 of these animals had tumours); no
m & 20 f (males) or 149 or 299 (females) in similar dose-related trend in mortality was observed in males. Body
controls; 60 m corn oil by gavage, 5 days/week, for weight in females in the high dose group was depressed as early as
& 60 f pooled 78 weeks followed by 13 weeks of week 15 (>10%). The incidence of non-neoplastic lesions was
controls from observation (initial doses in males comparable in exposed and control mice. There was an increased
concurrent of 75 and 150 mg/kg body weight incidence of hepatocellular carcinomas in male mice at the highest
experiments) per day were increased to 100 and dose (4/59, 1/19, 6/47 and 12/48 in pooled vehicle controls, matched
200 mg/kg body weight per day after vehicle controls, low and high dose groups, respectively), but not in
8 weeks; initial doses in females females. The incidence of alveolar/bronchiolar adenomas in males was
Table 5. (cont'd).
Species Protocol Results Reference
of 125 and 250 mg/kg body weight per 0/59, 0/19, 1/47 and 15/48 in pooled vehicle controls, matched
day were increased to 200 and vehicle controls, low and high dose groups, respectively
400 mg/kg body weight per day after (significant at the highest dose (p<0.001)); the incidence of this
8 weeks, then decreased to 150 and tumour in females was 2/60, 1/20, 7/50 and 15/48 in pooled vehicle
300 mg/kg body weight per day after controls, matched vehicle controls, low and high dose groups,
11 weeks). Complete respectively (significant in both exposed groups(p=0.046 (low) and
histopathological examinations were p<0.001 (high)). There was also one alveolar/bronchiolar carcinoma in
conducted. females at 299 mg/kg body weight per day. There was a non-significant
increase in the incidence of squamous cell carcinoma of the
forestomach in females at 299 mg/kg body weight per day (5/48 versus
1/60 or 1/20 in controls). There was a significantly increased
incidence of mammary gland adenocarcinomas in both groups of exposed
females (0/60, 0/20, 9/50 and 7/48 in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively
(p=0.001 (low) and p=0.003 (high)).
Uterine adenocarcinomas occurred in 3/49 low dose and 4/47 high
dose females, compared to none in controls; however, this increase
was not statistically significant. The incidence of endometrial
stromal polyp or endometrial stromal sarcoma (combined) was 0/60,
0/20, 5/49 and 5/47 in pooled vehicle controls, matched vehicle
controls, low and high dose groups, respectively (significant at
both doses, p=0.016 (low) and p=0.014 (high)).
Table 5. (cont'd).
Species Protocol Results Reference
Dermal
application
Swiss mice Doses of 0, 42 or 126 mg/application The incidence of benign lung papillomas was significantly (p<0.0005) van Duuren
(Ha:ICR, 30 f; per mouse in 0.2 ml acetone were increased at the higher dose (26/30 compared to 17/30, 11/30 and et al.
30 vehicle applied 3 times per week to the 30/100 in low dose group, vehicle controls and untreated controls, (1979)
controls and shaved dorsal skin (area of skin respectively). The incidence of stomach tumours was 3/30, 1/30, 2/30
100 naive exposed not specified) of mice for and 5/100 in high dose group, low dose group, vehicle controls and
controls) 440 to 594 days. The skin, liver, untreated controls, respectively (not significant).
kidney and any tissues or
organs appearing abnormal were
examined histopathologically.
7.5.2 Ingestion
In a study conducted by the National Cancer Institute (NCI,
1978), time-weighted average daily doses of 47 or 95 mg/kg body weight
per day of 1,2-dichloroethanea in corn oil were administered by
gavage 5 days/week for 78 weeks to 50 Osborne-Mendel rats of each sex,
followed by 32 weeks of observation. Mortality was significantly
(p < 0.001) higher in both males and females in the high dose group.
Clinical signs of general toxicity occurred at a greater frequency in
exposed animals than in controls. In each group 60-94% of rats had
chronic murine pneumonia, but the incidence was not related to dose.
The incidence of acanthosis and hyperkeratosis of the forestomach was
greater in exposed females than controls.
The incidence of a variety of tumours was increased in exposed
animals compared with controls. The incidence of squamous cell
carcinomas of the stomach was significantly increased in males (3/50
and 9/50 in low and high dose groups, respectively), compared to none
in either group of controls; in females, there were only 2/50 in the
low dose group. The incidence of haemangiosarcoma was significantly
increased in males (1/60, 0/20, 9/50 and 7/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively) and females (0/59, 0/20, 4/50 and 4/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). The incidence of fibromas of the subcutaneous tissue
was significantly increased in males (0/60, 0/20, 5/50 and 6/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively), but not in females. There was a significant
increase in the incidence of adenocarcinomas and fibroadenomas of the
mammary gland in females (1/59, 0/20, 1/50 and 18/50 (adenocarcinoma),
5/59, 0/20, 14/50 and 8/50 (fibroadenoma) and 6/59, 0/20, 15/50 and
24/50 (adenocarcinoma or fibroadenoma)) in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively). It
was concluded that 1,2-dichloroethane was carcinogenic in this strain
of rats, under the conditions of this study.
No effects on growth or biochemical parameters were observed in a
limited study on rats fed mash that had been fumigated with
1,2-dichloroethane, which resulted in doses of approximately 7.5-8.75
and 15-17.5 mg/kg body weight per day. Chronic respiratory disease
was evident in all groups in the second year, the number of rats
surviving after 21 months ranging from 2 to 14 in each of the groups.
The occurrence of respiratory disease and mortality did not appear to
be exposure-related (Alumot et al., 1976a).
a Technical grade with reported purity of > 90% containing 11 minor
contaminants; subsequent analysis indicated a purity of about
98-99% (Hooper et al., 1980 and Ward, 1980).
The National Cancer Institute (NCI, 1978) also conducted a
bioassay in which groups of 50 B6C3F1 mice were administered
time-weighted average daily doses of 97 or 195 mg/kg body weight per
day (males) and 149 or 299 mg/kg body weight per day (females)
1,2-dichloroethane in corn oil by gavage, 5 days/week for 78 weeks,
followed by 13 weeks of observation. A doserelated increase in
mortality was noted in female mice, but not in males. Body weight was
also decreased in females at 299 mg/kg body weight per day.
As in rats, there was a significant increase in the incidence of
several types of tumours in exposed mice. The incidence of
hepatocellular carcinomas was significantly increased in males in the
high dose group (4/59, 1/19, 6/47 and 12/48 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively); no such increase was noted in females. However, the
authors noted that, due to the high variability of incidence of
hepatocellular neoplasms in historical controls (data not presented),
this increase, although statistically significant, was not considered
to be convincing evidence that these tumours were attributable to the
test chemical. The incidence of alveolar/bronchiolar adenomas was
significantly increased in males in the high dose group (0/59, 0/19,
1/47 and 15/48 in pooled vehicle controls, matched vehicle controls,
low and high dose groups, respectively), and in both groups of exposed
females (2/60, 1/20, 7/50 and 15/48 in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively); one
alveolar/bronchiolar carcinoma was noted in a high-dose female mouse.
There was a non-significant increase in the incidence of squamous cell
carcinoma of the forestomach in females in the high dose group. The
incidence of mammary gland adenocarcinomas was significantly increased
in females at both doses (0/60, 0/20, 9/50 and 7/48 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). The incidence of endometrial stromal polyp or
endometrial stromal sarcoma (combined) was significantly elevated at
both doses (0/60, 0/20, 5/49 and 5/47 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). It was concluded that 1,2-dichloroethane was
carcinogenic in this strain of mice, under the conditions of this
study.
It should be noted that the data on tumour incidence presented in
the bioassays by the NCI (1978) do not take into account the increased
early mortality in the high exposure groups; the incidences of several
tumours (and thus the carcinogenic potency of 1,2-dichloroethane) may
have been higher had all animals survived for a long enough period of
time to develop tumours (Ward, 1980; Hooper et al., 1980).
7.5.3 Other routes of administration
1,2-Dichloroethane in acetone was applied to the shaved dorsal
skin of groups of 30 female non-inbred Ha:ICR Swiss mice, 3 times/week
for 440 to 594 days at doses of 0, 42, and 126 mg/application per
mouse. The incidence of lung tumours (benign lung papillomas) was
significantly increased at the higher dose (26/30 compared to 11/30 in
vehicle controls and 30/100 in naive controls, p < 0.0005).
Histopathological examination was limited to the skin, liver, kidney
and any "abnormal-appearing tissues" (van Duuren et al., 1979).
In a bioassay designed to screen the potential of numerous
chemicals to induce pulmonary tumours in a susceptible strain of mice
(A/St), groups of 20 males were administered 24 intraperitoneal
injections of 1,2-dichloroethane (20, 40 or 100 mg/kg body weight) in
tricaprylin, 3 times/week for 8 weeks (total doses of 480, 920 or
2400 mg/kg body weight). All surviving mice were killed 24 weeks
after the first injection. Although there was a dose-related increase
in the number of pulmonary adenomas per mouse (0.39, 0.21, 0.44 and
0.75 in the control, low, mid and high dose groups, respectively),
none of these increases was statistically significant (Theiss et al.,
1977). It should be noted, however, that the duration of the period
of observation may have been insufficient to allow for the development
of most types of tumours.
7.5.4 Initiation/promotion bioassays
In a dermal initiation/promotion protocol, 126 mg
1,2-dichloroethane was applied once to the skin (area exposed not
specified) of 30 female non-inbred Ha:ICR Swiss mice, followed 14 days
later by 5 µg (0.005 mg) of phorbol myristate acetate (PMA) (a
promoter) in 0.2 ml acetone, 3 times/week for 428 to 576 days. Two
PMA control groups of 120 and 90 mice were administered 0.0025 mg and
0.0050 mg PMA/application permouse (number of applications not
specified), respectively. Treatment with 1,2,-dichloroethane did not
significantly increase the incidence of skin papillomas (3/30 versus
9/120 and 6/90 in PMA controls). There were three squamous cell
carcinomas in the control groups, while none was observed in the
exposed group (van Duuren et al., 1979).
In a hepatic initiation/promotion assay, groups of 10
Osborne-Mendel rats were partially hepatectomized and then
administered 1,2-dichloroethane (100 mg/kg body weight) in corn oil by
gavage, followed 5 days later by diets containing phenobarbital for 7
weeks and a control diet for 1 week (initiation protocol). Livers
were examined histopathologically for GGT-positive foci (a putative
preneoplastic indicator). Additional groups of 10 rats were initiated
with an intraperitoneal injection of diethyl-nitrosamine or water
(control) following partial hepatectomy, then administered
1,2-dichloroethane (100 mg/kg body weight) in corn oil by gavage, 5
days/week for 7 weeks (promotion protocol). In rats administered
1,2-dichloroethane in the initiation protocol, there was no increase
in the number of GGT-positive foci.
Similarly, in rats administered 1,2-dichloroethane in the promotion
protocol, there was no significant increase in the number of
GGT-positive foci, either with or without the initiator, when compared
to controls (Story et al., 1986; Milman et al., 1988).
Groups of 35 male B6C3F1 mice were administered
diethylnitrosamine in the drinking-water for 4 weeks, followed by
exposure to 1,2-dichloroethane (835 or 2500 mg/litre) in
drinking-water for 24 or 52 weeks. Only the liver, kidneys and lungs
were examined histopathologically. Drinking-water intake was reduced
at the highest concentration. No significant differences in body
weight gain were noted. However, three mice consuming the highest
concentration of 1,2-dichloroethane died within 52 weeks. There was
no increase in the incidence of liver or lung tumours in exposed mice
either with or without diethylnitrosamine initiation (Klaunig et al.,
1986).
7.6 Mutagenicity and related end-points
The genotoxicity of 1,2-dichloroethane has been extensively
investigated in non-mammalian and mammalian test systems. Data from
in vitro and in vivo studies are summarized in Tables 6 and 7; a
summary of the weight of available evidence is presented here.
1,2-Dichloroethane induced differential toxicity in Escherichia
coli, but it had no effect in a Bacillus subtilis rec assay. It
consistently induced positive responses in mutagenicity assays with
Salmonella typhimurium, whereas it has not produced consistent
responses in mutation assays with E. coli and was negative in a mouse
peritoneal host-mediated assay with E. coli.
In the fungus Aspergillus nidulans, 1,2-dichloroethane induced
errors of mitotic segregation and aneuploidy, but did not induce gene
mutation.
Table 6. Genotoxicity of 1,2-dichloroethane in vitro (modified from ATSDR, 1994)
Species (test system) End-point Resulta Reference
With activation Without activation
Bacterial systems
Salmonella typhimurium Gene mutation + + Milman et al. (1988)
+ + Barber et al. (1981)
+ + Kanada & Uyeta (1978)
+ + Nestmann et al. (1980)
+ + Rannug et al. (1978)
+ + Van Bladeren et al. (1981)
+ NT Rannug & Beije (1979)
+ - Cheh et al. (1980)
+ - Moriya et al. (1983)
- - King et al. (1979)
+ + Strobel & Grummt (1987)
NT +b Simula et al. (1993)
S. typhimurium/spot test NT (+) Brem et al. (1974)
(+) - Principe et al. (1981)
NT - Buijs et al. (1984)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
S. typhimurium/Ara test + - Roldan-Arjona et al. (1991)
(standard)
S. typhimurium/Ara test (+) (+) Roldan-Arjona et al. (1991)
(liquid)
Streptomyces coelicolor Gene mutation NT - Principe et al. (1981)
Escherichia coli/K12/343/113 Gene mutation - - King et al. (1979)
E. coli/wp2 NT (+) Hemminki et al. (1980)
- - Moriya et al. (1983)
E. coli Pol A DNA damage NT (+) Brem et al. (1974)
Bacillus subtilis/rec-assay DNA damage NT - Kanada & Uyeta (1978)
Fungal systems
Aspergillus nidulans Gene mutation NT - Crebelli & Carere (1988)
NT - Principe et al. (1981)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
A. nidulans Mitotic segregation NT + Crebelli et al. (1984)
aberrations
A. nidulans Aneuploidy induction NT + Crebelli et al. (1988)
Saccharomyces cerevisiae Mitotic recombination NT (+) Simmon (1980)
Animal systems
Hamster CHO/HGPRT Gene mutation + (+) Tan & Hsie (1981)
+ (+) Zamora et al. (1983)
Rat hepatocytes Unscheduled DNA synthesis NT + Williams et al. (1989)
Mouse hepatocytes NT + Milman et al. (1988)
Mouse liver DNA DNA binding + NT Banerjee (1988)
Calf thymus DNA + NT Prodi et al. (1986)
Salmon sperm DNA + - Banerjee & Van Duuren (1979);
Banerjee et al. (1980)
Mouse BALBc/3T3 Cell transformation NT - Milman et al. (1988)
NT - Tu et al. (1985)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
Mouse C3H1OT´ Cell transformation NT +c Schultz et al. (1992)
Syrian hamster embryo cells Cell transformation NT + Hatch et al. (1983)
Human cells
Human lymphoblasts AHH-1 Gene mutation NT + Crespi et al. (1985)
Human lymphoblasts TK6 NT + Crespi et al. (1985)
Human embryo epithelial-like NT + Ferreri et al. (1983)
EUE cells
Human peripheral lymphocytes Unscheduled DNA synthesis + - Perocco & Prodi (1981)
a NT = not tested; - = negative result; + = positive result; (+) = weakly positive or marginal result
b increase in cells expressing GSTA1-1
c transformed cells induced tumours in nude mice
Table 7. Genotoxicity of 1,2-dichloroethane in vivo (modified from ATSDR, 1994)
Species (test system) End-point Resultsa Reference
Mammalian assays
Mouse Dominant lethal mutations - Lane et al. (1982)
Mouse/spot test Gene mutation (+) Gocke et al. (1983)
Mouse bone marrow Sister-chromatid exchange + Giri & Que Hee (1988)
Mouse bone marrow Micronuclei - Jenssen & Ramel (1980);
King et al. (1979)
Mouse peripheral erythrocytes - Armstrong & Galloway (1993)
Mouse liver, kidney, lung and stomach DNA binding + Prodi et al. (1986)
Mouse liver, kidney, lung and stomach + Arfellini et al. (1984)
Mouse forestomach and kidney + Hellman & Brandt (1986)
Mouse liver + Banerjee (1988)
Table 7 (contd).
Species (test system) End-point Resultsa Reference
Rat liver, kidney, spleen, lung, + Reitz et al. (1982)
forestomach and stomach
Rat liver, kidney, lung and stomach + Arfellini et al. (1984)
Rat liver, kidney, lung and stomach + Prodi et al. (1986)
Rat liver and kidney + Inskeep et al. (1986)
Rat liver and lung + Baertsch et al. (1991)
Rat liver + Banerjee (1988)
Rat liver + Cheever et al. (1990)
Mouse liver DNA damage + Storer & Conolly 1983, 1985;
Storer et al. (1984)
Mouse liver + Taningher et al. (1991)
Insect assays
Drosophila melanogaster/somatic mutation Gene mutation + Nylander et al. (1978)
Table 7 (contd).
Species (test system) End-point Resultsa Reference
D. melanogaster/somatic mutation + Romert et al. (1990)
D. melanogaster/somatic mutation + Kramers et al. (1991)
D. melanogaster/somatic mutation (+) Ballering et al. (1993)
D. melanogaster/recessive lethal + Ballering et al. (1993)
D. melanogaster/vermilion locus + Ballering et al. (1993)
D. melanogaster/sex-linked recessive + King et al. (1979)
D. melanogaster/sex-linked recessive + Kramers et al. (1991)
D. melanogaster Chromosomal loss/gain +/+ Valencia et al. (1984)
Host-mediated assays
Escherichia coli K12/343/113 mouse Gene mutation - King et al. (1979)
host-mediated assay
a - = negative result; + = positive result; (+) = weakly positive or marginal result
In cultured mammalian cells, 1,2-dichloroethane formed
adducts with DNA. It also induced unscheduled DNA synthesis in primary
cultures of mouse and rat hepatocytes, and human peripheral
lymphocytes (the last in the presence of an exogenous metabolic
activation system), and gene mutation in several cell lines. Mutation
frequency of two human cell lines has been correlated with the
difference in levels of glutathione- S-transferase activities (Crespi
et al., 1985; section 6.3). Cell transformation was induced in
studies with C3H10T´ cells, but not with BALBc/3T3 cells.
1,2-Dichloroethane enhanced SV40 virus transformation of Syrian
hamster embryo cells.
In vivo, both somatic cell and sex-linked recessive lethal
mutations have been consistently induced in Drosophila melanogaster by
1,2-dichloroethane. 1,2-Dichloroethane has been found to bind to DNA
in all reported studies in mice and rats. In other studies with mice,
clearly positive responses have been restricted to primary DNA damage
in liver and sister-chromatid exchange induction in bone marrow. No
evidence for micronucleus induction has emerged from bone marrow
micronucleus studies or for a dominant lethal effect in one study
(although this study may have been insufficiently sensitive), and only
a weak but significant (p < 0.03) response was observed in a single
spot test.
It has been noted that stronger responses were obtained in the
bacterial mutation assay in the presence of an exogenous metabolic
system than in its absence. This could imply the formation of
additional mutagenic metabolites, through either the cytochrome P450
or glutathione-S-transferase pathway. In these in vitro assays with
liver homogenates, activation by the cytochrome system is more likely,
and 2-chloroacetaldehyde, which is a possible metabolite, is known to
be mutagenic (McCann et al., 1975). The mutagenicity of
1,2-dichloroethane in S. typhimurium TA100, in the absence of S9 mix,
was more than doubled if the bacterium expressed the human GSTA1-1
gene, but there was no change in the mutagenic response if the SSTP1-1
gene was expressed (Simula et al., 1993).
7.7 Reproductive toxicity, embryotoxicity and teratogenicity
The reproductive and developmental effects of 1,2-dichloro-ethane
have not been extensively investigated in experimental animals (see
Table 8), although the compound has been detected in fetal tissues in
rats and mice following maternal exposure to 600 mg/m3 for 5 h
(Withey & Karpinski, 1985) and 1000 mg/m3 for 3 days (Vozovaya,
1977).
Table 8. Reproductive and developmental toxicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats Animals were exposed to 0, 101, 304, or No exposure-related histopathological effects in the parents nor Rao
(Sprague-Dawley, 607 mg/m3 (0, 25, 75, or 150 ppm) any alterations in the fertility index and gestation periods were et al.
20 m & 20 f per 6 h/day, 5 days/week for 60 days prior observed compared to controls. There was a significant decrease (1980)
exposed group, to mating, and for an additional 116 in the number of pups per litter in the F1A pups at 304 mg/m3
30 m & 30 f days (7 days/week) after mating. Dams (13%, p<0.05), but not at 607 mg/m3; there was significantly
controls) were not exposed from gestation day 21 increased kidney weight in the F1B male pups at 101 mg/m3
through to day 4 postpartum. The pups (29%, p<0.05), although the authors did not consider this effect
(F1A) were removed after 21 days, and to be related to exposure. There were no significant differences
the females were remated to exposed in growth, sex ratios, survival indices, organ or neonatal body
males following removal of the last weights, or histology in pups.
litter to produce F1B litters. Liver,
kidneys, ovaries , uterus and testes
of parental animals in control and
high exposure group (and other groups
if any changes were noted in high
exposure group) were examined
histopathologically
Rats (Albino, f, Rats were exposed to 57 mg/m3, 4 h/day, Exposed rats had reduced fertility (6.5 fetuses per dam compared Vozovaya
strain and 6 days/week, for 6 or 9 months. The to 9.7 per dam in controls). Newborn pups had reduced body (1974)
number animals were apparently then mated, but weight (5.06 g versus 6.44 g in controls). Perinatal mortality
unspecified it is not clearly stated whether the was increased in the exposed group (data not presented). No
in secondary exposure period extended beyond information was available on maternal effects.
account) mating.
Table 8 (contd).
Species Protocol Results Reference
Rats (f, strain Rats were exposed to 15 mg/m3, 4 h/day, The estrous cycle was longer in exposed rats than in controls. No Vozovaya
and number 6 days/week for 4 months prior to and information on the effects on fertility was presented. Embryonal (1977)
unspecified after mating. mortality increased from approximately 11% in controls to 27% in
in secondary exposed dams. Pre-implantation losses were 5-fold greater in
account) exposed animals than in controls. No fetal abnormalities were
observed, except for haematomas in the region of the head, neck
and anterior extremities (presumably only in pups of exposed
animals, although not clearly stated). No information was
available on maternal effects.
Rats Rats were exposed to 0, 405 or 1215 10/16 dams at 1215 mg/m3 died. At 405 mg/m3, there were no Rao
(Sprague-Dawley, mg/m3 (0, 100 or 300 ppm) for 7 h/day effects on mean litter size, incidence of resorptions, or fetal et al.
16 to 30 pregnant on days 6 to 15 of gestation. body measurements; no significant increase in the incidence of (1980)
f per group) major malformations was observed at this concentration.
Rabbits (New Rabbits were exposed to 0, 405, or 4/21 and 3/19 dams died at 405 and 1215 mg/m3, respectively, Rao
Zealand White, 1215 mg/m3 (0, 100 or 300 ppm) for compared to none in 20 controls. There were no effects on mean et al.
19 to 21 7 h/day on days 6 to 18 of gestation. litter size, incidence of resorptions or fetal body measurements (1980)
pregnant f at either concentration, and there were no significant differences
per group) in the incidence of major malformations.
Table 8 (contd).
Species Protocol Results Reference
Ingestion
Rats (45 m & Animals were fed mash fumigated with There were no significant differences in various reproductive Alumot
90 f, strain 1,2-dichloroethane for 2 years. parameters, including number of dams pregnant, number of dams et al.
unspecified) Resulting concentrations were 250 and with litters, mean litter size, mortality or body weight of young (1976a)
600 mg/kg. Due to loss of the compound at birth and at weaning.
through volatilization, the mash
actually consumed was estimated to
contain 60 to 70% of the initial
concentration (estimated to result in
doses of approximately 7.5 to 8.75 and
15 to 17.5 mg/kg body weight per day).
Exposed females were mated with
exposed males at 2 month intervals.
Mice (ICR Swiss, F0 mice were administered There were no differences in body weight in adults, and there Lane
(10 m & 30 f concentrations of 0, 0.03, 0.09 or 0.29 were no effects on fertility or gestation indices. There were no et al.
per exposed mg/litre drinking-water (equivalent effects on survival, litter size, postnatal body weight or gross (1982)
group, 20 m & to nominal doses of approximately pathology of pups. The incidence of fetal visceral or skeletal
60 f controls) 0, 5, 15 or 50 mg/kg body weight malformations was not increased in exposed animals. F1C litters
per day) for 35 days prior to were not examined for skeletal malformations.
mating. Three sets of offspring
(F1A, F1B and F1C) were produced.
Table 8 (contd).
Species Protocol Results Reference
After weaning and 11 weeks of
exposure to the same concentrations,
the F1B mice were mated (30 female,
10 male) to produce a second generation
of offspring (F2A and F2B). Teratology
screening tests were performed using
F1C and F2B matings where the females
were co-housed with unexposed males.
Mice (f, number Pregnant mice were administered There were no developmental effects and "few discernible effects" Kavlock
and strain not 1,2-dichloroethane in the drinking-water on maternal health. There were no skeletal or visceral anomalies et al.
specified in at a concentration equivalent to a which could be attributed to exposure. (1979)
secondary dose of 510 mg/kg body weight per
account) day on days 7 to 14 of gestation.
No effects on reproductive parameters, including fertility index,
gestation period or histological changes, were reported in a
two-generation study on Sprague-Dawley rats exposed to 0, 101, 304 or
607 mg/m3 (0, 25, 75 or 150 ppm) 1,2-dichloroethane 6 h/day, for 60
days prior to mating and 116 days after mating (except during the
delivery period). There were also no effects on growth, sex ratios,
survival indices, organ or neonatal body weights, or histology in pups
(Rao et al., 1980).
Exposure to 1,2-dichloroethane (15 mg/m3), 4 h/day, for 4
months prior to mating and after mating resulted in a
longer-than-normal estrous cycle in female rats (strain not
specified). Although embryonal mortality and preimplantation losses
were greater in exposed animals than in controls, no fetal
abnormalities were reported, except for haematomas in the area of the
head, neck and anterior extremities (Vozovaya, 1977). Similarly,
increased perinatal mortality and reduced body weight of newborn pups
were observed in the offspring of female albino rats exposed to 57
(± 10) mg/m3 for 6 and 9 months. Exposed females also produced a
lower number of fetuses per dam (Vozovaya, 1974). Information from
these studies was insufficient for evaluation.
Rao et al. (1980) also conducted a developmental study in which
pregnant Sprague-Dawley rats were exposed to 0, 405 or 1215 mg/m3
(0, 100 or 300 ppm) 1,2-dichloroethane, 7 h/day, during gestation.
Mortality was high in dams at 1215 mg/m3 (10/16 rats died); thus the
developmental effects could not be ascertained at this concentration.
No fetotoxic or teratogenic effects were observed at 405 mg/m3. In
pregnant rabbits exposed to the same concentrations, 7 h/day, during
gestation, mortality was increased in exposed animals (4/21 and 3/19
dams died at 405 and 1215 mg/m3, respectively, compared to none in
20 controls). However, no fetotoxic or teratogenic effects were
reported at either concentration. The authors concluded that
1,2-dichloroethane was not teratogenic or fetotoxic in rats at
405 mg/m3 or in rabbits at 405 or 1215 mg/m3.
No effects on male fertility or various reproductive parameters
were noted in rats consuming mash which had been fumigated with
1,2-dichloroethane, the resultant concentrations being 250 and
500 mg/kg (approximately equivalent to doses of 7.5 to 8.75 and 15 to
17.5 mg/kg body weight per day when loss due to volatilization was
taken into account), for up to 2 years (Alumot et al., 1976a). No
effects on reproduction (in terms of fertility and gestation indices)
were reported in a two-generation study on Swiss ICR mice exposed to
1,2-dichloroethane in the drinking-water at concentrations of 0, 0.03,
0.09 or 0.29 g/litre (approximately equivalent to doses of 0, 5, 15 or
50 mg/kg body weight per day). In addition, no fetotoxic or
teratogenic effects were noted in either generation of offspring of
F1C and F2B litters sacrificed on day 18 (Lane et al., 1982). No
developmental effects were reported in a study in which groups of 30
pregnant CD-1 mice were administered drinking-water containing a
mixture of organic compounds, including 0.01% 1,2-dichloroethane
(equivalent to a dose of 5.1 mg/kg body weight per day), during days 7
to 14 of gestation (Kavlock et al., 1979).
7.8 Immunological effects
Groups of 140 female CD1 mice were exposed to airborne
concentrations of 0, 10, 20 and 40 mg/m3 (0, 2.5, 5 and 10 ppm)
1,2-dichloroethane for a 3-h period or to 0 or 10 mg/m3, 3 h/day for
5 consecutive days. After acute exposure to 20 and 40 mg/m3, there
was a significant increase in mortality in mice from streptococcal
challenge (p < 0.05 and p < 0.001, respectively), while no effects
were noted following acute or repeated exposure to 10 mg/m3. In
similarly exposed groups of 18 to 36 mice, significantly (p < 0.01)
decreased pulmonary bactericidal activity to inhaled Klebsiella
pneumoniae was noted only at 40 mg/m3. Single exposure to 40 or
400 mg/m3 (10 or 100 ppm) did not affect the in vitro phagocytic
or cytostatic ability of alveolar macrophages to red blood cells and
tumour target cells, respectively (Sherwood et al., 1987).
Male Sprague-Dawley rats (16 per group) were exposed to 0, 400 or
800 mg/m3 (0, 100 or 200 ppm) 1,2-dichloroethane for 3 h, or to 0,
40, 80, 200 or 400 mg/m3 (0, 10, 20, 50 or 100 ppm), 5 h/day, 5
days/week for 12 days. Pulmonary bactericidal activity was not
affected at any concentration. No effects were noted on in vitro
phagocytic activity to red blood cells, in vitro cytostatis and
cytolysis of tumour target cells, or levels of ectoenzymes in alveolar
macrophages. Blastogenesis of mitogen-stimulated T- and B-lymphocytes
from popliteal and mesenteric lymph nodes was not affected (Sherwood
et al., 1987).
Chinchilla rabbits (number per group not specified but
probably less than 10) were exposed to 2, 10 or 100 mg/m3
1,2-dichloroethane for 3 h/day, 6 days/week, for 7.5 to 8 months. At
the two highest concentrations production of antibodies against
typhoid vaccine was increased, while total antibody production was
reduced at 100 mg/m3 (Shmuter, 1977).
Groups of 10 male CD1 mice (12 per group) (Munson et al., 1982)
were administered 1,2-dichloroethane (4.9 and 49 mg/kg body weight per
day) in water by gavage for 14 days. A significant (p < 0.05)
reduction of 25 and 40% in IgM antibody-forming cells to sheep RBCs
was observed at 4.9 and 49 mg/kg body weight per day, respectively. A
significant (p < 0.05) reduction of cell-mediated responses to sheep
erythrocytes was also observed at both levels, which was not related
to dose, while a significant (p < 0.05) reduction in leucocyte count
was observed at the highest dose. In the same study, groups of 16 to
32 male CD1 mice were administered time-weighted average
1,2-dichloroethane doses of 0, 3, 24 and 189 mg/kg body weight per day
(0, 0.02, 0.2 and 2.0 g/litre) for 90 days in drinking-water; controls
consisted of 24 to 48 mice. The fluid consumption of exposed mice was
decreased in a dose-related manner, which corresponded to a
dosedependent reduction in body weight. No effects on organ weights,
haematology, B-cell mitogen lipopolysaccharide response, spleen cell
response to the T-cell mitogen concanavalin A or cellmediated immunity
(assessed by measuring the delayed-type hypersensitivity responses to
sheep erythrocytes) were noted. There was a tendency towards a
reduction in the serum antibody level after immunization with sheep
erythrocytes, and in the immunoglobulin spleen antibody-forming cells
at all exposure levels (not significant) (Munson et al., 1982).
7.9 Toxicological interactions with other agents
In a recent bioassay designed to determine the influence of
disulfiram or ethanol on the carcinogenicity, metabolism and covalent
binding to DNA of 1,2-dichloroethane, male and female Sprague-Dawley
rats were exposed to 200 mg/m3 (50 ppm) 1,2-dichloroethane for 7 h
per day, 5 days a week, for 2 years. Additional rats were similarly
exposed and administered either 0.05% disulfiram in the diet or 5%
ethanol in the drinking-water, either alone or in combination with
1,2-dichloroethane. The incidence of any type of tumour was not
elevated compared to controls in any groups exposed to these compounds
individually, nor was the incidence of any type of tumour increased in
rats exposed to 1,2-dichloroethane and ethanol in combination compared
to unexposed controls or rats exposed to these compounds alone.
Exposure to disulfiram in combination with 1,2-dichloroethane resulted
in a significant (p<0.05) increase in the incidence of intrahepatic
bile duct cholangiomas (males: 9/49 versus 0/50, females: 17/50 versus
0/50) and cysts (males: 12/49 versus 0/50, females: 24/50 versus 0/50)
in both male and female rats compared to that in rats exposed to
1,2-dichloroethane only. Male rats exposed to this combination also
had a significantly (p<0.05) increased incidence of subcutaneous
fibromas (10/50 versus 0/50), hepatic neoplastic nodules (6/49 versus
2/50) and interstitial cell tumours in the testes (11/50 versus 3/50)
compared to rats exposed to 1,2-dichloroethane alone. Female rats
similarly exposed had a significantly (p<0.05) higher incidence of
mammary adenocarcinomas compared with rats exposed to
1,2-dichloroethane only (12/48 versus 5/50). Combined exposure to
1,2-dichloroethane and disulfiram did not increase the level of
covalent binding to hepatic DNA compared to that found in rats exposed
to 1,2-dichloroethane alone. The profile of urinary metabolites of a
single radiolabelled oral dose of 1,2-dichloroethane in rats
simultaneously exposed to disulfiram and 1,2-dichloroethane for 2
years indicated that the metabolism of 1,2-dichloroethane was
qualitatively similar to that of rats exposed to 1,2-dichloroethane
alone for 2 years. However, a reduced rate of elimination, and
sustained blood levels of unchanged 1,2-dichloroethane were observed
(see section 6.4), which, the authors stated, could be related to the
carcinogenic effects noted following simultaneous exposure (Cheever et
al., 1990).
In addition, concomitant exposure to disulfiram (as may occur in
the rubber industry or in persons undergoing therapy for alcoholism)
in the diet resulted in a synergistic increase in the hepatotoxicity
(as determined by levels of enzymes in serum and increased relative
liver weight (> 30%)) of 1,2-dichloroethane inhaled at
concentrations of 600, 1200 or 1800 mg/m3 (150, 300 and 450 ppm) for
30 days by male Sprague-Dawley rats. However, rats administered
1,2-dichloroethane alone had evidence of liver damage only at the
highest concentration. The enhanced effects were hypothesized to be
due to the inhibition of mixed-function oxidase-mediated metabolism of
1,2-dichloroethane and a compensatory increase in metabolism of
1,2-dichloroethane to reactive metabolites via cytosolic pathways
mediated by glutathione S-transferase, since hepatic cytochrome
P-450 content decreased with increasing concentration of
1,2-dichloroethane only in the presence of disulfiram (Igwe et al.,
1986a). Concomitant exposure to 1,2-dichloroethane by inhalation
(> 1216 mg/m3 or 304 ppm) or intraperitoneal injection
(150 mg/kg body weight per day) and disulfiram administered in the
diet (0.15%) resulted in testicular atrophy in Sprague-Dawley rats
compared to rats exposed to either compound alone (Igwe et al.,
1986b).
The acute toxicity of carbon tetrachloride was potentiated by
1,2-dichloroethane in rats administered single doses of each of 60 and
125 µl/kg body weight perorally, based on determination of serum
hepatic enzymes and indicators of lipid peroxidation at 24 h after
exposure. Pre-treatment with vitamin E prevented hepatotoxicity.
Based on the observation that the hepatic GSH level in the group
concomitantly exposed to both compounds was not significantly
different from that in the group exposed to carbon tetrachloride
alone, the authors concluded that GSH depletion did not play an
important role in the potentiation (Aragno et al., 1992). Concomitant
exposure to oral doses of 1,2-dichloroethane and 1,2-dibromoethane (60
and 20 µl/kg body weight, respectively) did not result in liver
toxicity in rats, based on levels of serum hepatic enzymes and
indicators of lipid peroxidation, although the compounds alone and in
combination resulted in a decrease in hepatic GSH level 2 h after
exposure, which subsequently returned to control values (Danni et al.,
1992).
The in vitro metabolism of 1,2-dichloroethane by liver
homogenates of rats administered ethanol increased with the dose of
ethanol up to 4 g/kg body weight, but declined sharply at 5 g/kg body
weight (Sato et al., 1981).
High doses (1000 to 2000 mg/kg body weight) of several chemicals,
including methionine, p-aminobenzoic acid, sulfanilamide and
aniline, administered orally to mice were protective against the
lethal effects caused by inhalation of 1600 mg/m3 (400 ppm)
1,2-dichloroethane (Heppel et al., 1945).
The acute and subacute toxicity of dichloroethane increased when
it was administered under conditions of high temperature (species and
exposure protocol not specified in abstract) (Mihaylova, 1976).
8. EFFECTS ON HUMANS
8.1 Case reports
The lethal oral dose of 1,2-dichloroethane in humans has been
estimated to be between 20 and 50 ml. Death due to cardiac arrhythmia
has been reported following ingestion of large, single doses
(50-75.2 g) of 1,2-dichloroethane (Hueper & Smith, 1935; Garrison &
Leadingham, 1954; Martin et al., 1969). Effects identified following
ingestion of 1,2-dichloroethane include central nervous system
depression, gastroenteritis, liver, kidney and lung damage,
cardiovascular disorders and haematological effects (Weiss, 1957;
Morozov, 1958; Hinkel, 1965; Bogoyavlenski et al., 1968; Martin et
al., 1969; Schönborn et al., 1970; Yodaiken & Babcock, 1973; Natsyuk &
Mudritsky, 1974; Dorndorf et al., 1975; Andriukin, 1979).
Effects reported following exposure to 1,2-dichloroethane via
inhalation are very similar to those observed after ingestion but are
usually less pronounced. Inhalation of 1,2-dichloroethane vapour
first affects the central nervous system and causes irritation and
inflammation of the respiratory tract. Damage to the liver, kidneys
and lungs (Wirtschafter & Schwartz, 1939; Hadengue & Martin, 1953;
Menschick, 1957; Troisi & Cavallazzi, 1961; Suveev & Babichenko, 1969;
Nouchi et al., 1984) and changes in the heart rhythm (Suveev &
Babichenko, 1969) have been reported in several cases. Death due to
cardiac toxicity has been reported following a 30-min exposure to an
unknown concentration of 1,2-dichloroethane (Nouchi et al., 1984).
Effects on the eyes were observed in several early case reports
(Weiss, 1957; Menschick, 1957; Troisi & Cavallazzi, 1961), while
severe dermatitis has been reported following dermal contact with
1,2-dichloroethane (Wirtschafter & Schwartz, 1939).
8.2 Epidemiological studies
In a study of 278 men working in the chlorohydrin unit of a
chemical production plant between 1940 and 1967 and followed up to
1988, there was a significant (p < 0.01) excess of deaths due to
pancreatic cancer compared to the USA national rates [Standardized
Mortality Ratio (SMR) = 492 (95% CI = 158 - 1140); Observed:Expected
(O:E) = 8:1.6]. The excess was greater when confined to men who
worked in the unit for more than 2 years (SMR = 800). Based on
comparison with two groups of workers in nearby plants, there were
pronounced increases in mortality due to pancreatic cancer as exposure
duration increased. Though an excess of deaths due to "lymphatic and
haemopoietic cancers" was also observed, it appeared to be
attributable principally to leukaemia, for which numbers of observed
cases were small (O=4) and associations with duration of exposure were
less consistent. Although quantitative data were not available, the
authors concluded on the basis of considerable qualitative information
that workers in this unit had been exposed primarily to
1,2-dichloroethane in combination with bis-chloroethyl ether, ethylene
oxide and ethylene chlorohydrin (Benson & Teta, 1993).
In a case-control study, the exposure of 21 male employees at a
petrochemical plant in Texas, USA, whose deaths were
attributed to cancer of the brain, was compared to that of two groups
of 80 controls from the same plant. One control group consisted of
male employees who had died from non-neoplastic causes, while the
second group of controls consisted of those men whose deaths were due
all other causes. Employees were classified as having been exposed to
1,2-dichloroethane if they had ever worked in a department in which
the compound had been used; unexposed workers had never worked in
these departments and the exposure of others was considered to be
unknown (47.6% of cases, 58.8 and 61.3% of the first and second group
of controls, respectively). When those with unknown exposure were
excluded from the analyses, the proportion of cases (all cases or
glioma cases specifically) who were exposed (n = 11 and 10, or 45.5
and 50.0%, respectively) did not differ significantly from the
proportion of controls who had been exposed to 1,2-dichloroethane
(42.4 and 45.2% for the two control groups). When a 15-year latency
period was considered in the analysis, the proportion of cases and
controls exposed still did not differ significantly (40.0 and 44.4%
for cases versus 32.3 and 34.6% for the two control groups) (Austin &
Schnatter, 1983a).
In an accompanying historical cohort study of 6588 workers at
this plant, there was no significant excess in malignant brain tumours
in the overall population of the plant compared to national rates,
although there was a borderline significant (p < 0.05) increase in
hourly employees with more than 6 months of employment (O/E = 10/5).
However, exposure to 1,2-dichloroethane was not specifically
considered in this study, and employees were also exposed to a number
of potentially confounding substances, including benzene, diethyl
sulfate, ethylene oxide and vinyl chloride (Austin & Schnatter,
1983b).
Deschamps & Band (1993) conducted a small case-control study to
investigate a possible association between a spill of
1,2-dichloroethane in 1982 into a river supplying drinking-water to
parts of the city of Vancouver, Canada, and an identified cluster of
childhood leukaemia cases in the city. It was determined that none of
the 15 cases diagnosed between 1975 and 1988 had lived in areas of the
city serviced by the contaminated supply.
In an ecological study in which potential associations between
contamination of drinking-water from groundwater supplies by
particular substances (including 10 volatile organic compounds and 43
inorganic elements) and cancer were investigated, the average annual
age-adjusted incidence (1969 to 1981) of colon and rectal cancer was
statistically significantly greater in men aged > 55 years whose
drinking-water contained > 0.1 µg 1,2-dichloroethane/litre than in
those whose drinking-water contained < 0.1 µg/litre (222.8 per
100 000 versus 170.3 per 100 000 (193 and 633 cases, p = 0.02) and
126.5 per 100 000 versus 92.9 per 100 000 (106 and 337 cases,
p = 0.009), respectively). Rectal cancer in males was also associated
with chlorination of drinking-water. Of the study population over 55
years of age, 50% had lived at the same address for 20 years or more.
There were no significant differences between groups of towns with
respect to eight socioeconomic factors examined, except that the
percentage change in population between 1970 and 1980 was
significantly less in those towns with > 0.1 µg
1,2-dichloroethane/litre. The authors did not suggest that the result
indicated a causal relationship between 1,2-dichloroethane and cancer,
but that cancer incidence may be elevated in populations consuming
water from wells subject to anthropogenic contamination (Isacson et
al., 1985).
The prevalence of subjective symptoms was higher in a group of 10
male oil refinery workers exposed to between 250 and 800 mg/m3 than
in those exposed to lower concentrations (40 to 150 mg/m3); a
"general reduction in body weight" was also noted in both groups.
"Abnormalities" of the liver, central nervous system, gastrointestinal
tract and haematological parameters were reported in some workers
(n = 1 to 8) in the group exposed to the higher concentration,
presumably based upon clinical examination, although this was not
specified in the previous account of this early study. There were no
unexposed controls, and workers were also exposed to benzene (10 to
25 mg/m3) (Cetnarowicz, 1959).
The morbidity during a 5-year period (1951 to 1955) was increased
for all disease categories (not further specified) in a group of
workers (number not specified) at an aircraft factory exposed to
5 mg/m3 or less for 70 to 75% of the working time and 80 to
150 mg/m3 for the remainder, when compared to workers in the entire
factory. Of 83 workers examined further, 19 had disease of the liver
and bile duct, 13 had neurotic conditions, 11 had autonomous dystonia
and 10 had hyperthyroidism and goitre (there were no controls for
comparison) (Kozik, 1957).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
9.1.1 Microorganisms
Blum & Speece (1991) investigated the toxicity of
1,2-dichloroethane to three groups of aquatic bacteria: methanogens,
aerobic heterotrophs and Nitrosomonas. The end-points assessed
included inhibition of gas production (methanogens), oxygen uptake
(aerobic heterotrophs), and ammonia consumption (Nitrosomonas). The
IC50 values for Nitrosomonas and methanogens (29 and 25 mg/litre,
respectively) were considerably lower than that for aerobic
heterotrophs (470 mg/litre). For the bacteria Pseudomonas putida,
the nominal 16-h toxicity threshold for the onset of cell
multiplication inhibition was 135 mg/litre (Bringmann & Kühn, 1981).
Tang et al. (1990) determined the IC50 values based on inhibition of
respiration rate for activated sewage sludge using open and closed
serum bottle methods to be 35 500 mg/litre and 2780 mg/litre,
respectively. The difference was attributed to the volatility of
1,2-dichloroethane which was stripped from the substrate in the open
method.
The freshwater cyanobacterium (blue-green alga) Microcystis
aeruginosa was seven times more sensitive to 1,2-dichloroethane than
the green alga Scenedesmus quadricauda, the nominal 7-day EC50
values for inhibition of cell multiplication at 27°C being 105 and
710 mg/litre, respectively (Bringmann & Kühn, 1978). The 72-h EC50
for inhibition of growth was 189 mg/litre (Freitag et al., 1994).
Bringmann & Kühn (1980) determined the toxicity thresholds for
Scendesmus quadricauda and the protozoan Entosiphon sulcatum to be
710 mg/litre and 1127 mg/litre, respectively. Knie et al. (1983)
reported the acute EC50 for the alga Haematococcus pluvialis to be
130 mg/litre. Based on bioluminescence, the 5-min IC50 was
700 mg/litre in a Microtox test with Photobacterium phosphoreum
(Blum & Speece, 1991). Freitag et al. (1994) reported the 15-min EC50
for inhibition of bioluminescence in this species to be 770 mg/litre.
Bringmann & Kühn (1981) determined the toxicity thresholds in the
holozoic bacteriovorous flagellate protozoan Entosiphon sulcatum, the
holozoic bacteriovorous ciliate protozoan Uronema parduczi, and the
saprozoic flagellate protozoan Chilomonas paramecium to be > 8000
mg/litre, > 16 000 mg/litre and > 800 mg/litre, respectively, using
the cell multiplication inhibition test.
Pearson & McConnell (1975) determined the EC50 (based on carbon
uptake from CO2 during photosynthesis) in the marine unicellular
alga Phaeodactylum tricorhutum to be 340 mg/litre.
9.1.2 Invertebrates
Based on a review of identified acute and chronic toxicity
studies in freshwater invertebrates, Daphnia magna appears to be the
species most sensitive to 1,2-dichloroethane. Under static test
conditions, the measured 48-h LC50 values for fed and unfed first
instar D. magna were 320 and 270 mg/litre, respectively; the 48-h
EC50 values, based on complete immobilization, were 180 and
160 mg/litre for fed and unfed organisms, respectively (Richter et
al., 1983). Leblanc (1980) reported the 24-h and 48-h LC50 values
in D. magna to be 250 and 220 mg/litre, respectively, while the "no
discernible effect concentration" (apparently based on mortality only)
was < 68 mg/litre. Using the Probit method, Ahmad et al. (1984)
determined the 48-h LC50 in unfed D. magna to be 268 mg/litre (95%
CL: 246-293), while the EC50, based on reproductive effects was
155 mg/litre (95% CL: 137-188). Freitag et al. (1994) determined the
24-h EC50 for 10% immobilization of D. magna to be 150 mg/litre.
Knie et al. (1983) reported the EC0, EC50 and EC100 in D. magna
to be 67, 600 and 1075 mg/litre, respectively. Richter et al. (1983)
also examined the effect of 1,2-dichloroethane on reproductive success
and length of first instar D. magna in a 28-day flow-through test.
For reproductive success, the measured lowest-observed-effect level
(LOEL) and no-observed-effect level (NOEL) were 20.7 and
10.6 mg/litre, respectively, while the measured LOEL and NOEL for
growth were 71.7 and 41.6 mg/litre, respectively.
Ahmad et al. (1984) also conducted chronic toxicity studies in
which D. magna were exposed to 0, 10.6, 20.6, 41.6, 71.7, 94.4 and
137 mg 1,2-dichloroethane/litre for 28 days. There was a
concentration-related decrease (as low as 12% of control values) in
the number of young produced (significant (p < 0.05 or 0.01) at
41.6 mg/litre or more) as well as a decrease (as low as 59% of control
values) in the length of adults (significant (p < 0.01) at
71.7 mg/litre or more). Few acute toxicity studies in marine
invertebrates were identified. Under static test conditions, the
nominal 24-h EC50 for immobilization of 30-h posthatch larvae of the
brine shrimp Artemia salina was 93.6 mg/litre (Foster & Tullis,
1984). For marine adult shrimp (Crangon crangon), the measured 24-h
LC50 was 170 mg/litre under static test conditions (Rosenberg et
al., 1975). The 48-h LC50 for barnacle nauplii (Elminuis modestus)
was 186 mg/litre (Pearson & McConnell, 1975). Teratogenic effects
(expressed as surviving larvae with gross debilitating abnormalities)
were observed in the nauplii of the marine brine shrimp (Artemia
salina) at concentrations between 0.25 and 25 mg/litre (Kerster &
Schaeffer, 1983).
9.1.3 Vertebrates
The embryos and larvae of the northwestern salamander (Ambystoma
gracile) and the leopard frog (Rana pipiens) were continuously
exposed to 1,2-dichloroethane from within 30 min of fertilization
(embryos) and maintained for 4 days after hatching (larvae). The
LC50 values for the salamander at the day of hatching (day 5) and 4
days after hatching (day 9) were 6.53 and 2.54 mg/litre, respectively;
the measured LOEL for 23% reduction in egg hatchability was
0.99 mg/litre. The measured 5-day and 9-day LC50 values for the
frog were 4.52 and 4.40 mg/litre, respectively, while the 5-day
posthatch LOEL was 1.07 mg/litre (Black et al., 1982).
Acute toxicity studies have been conducted on several species of
freshwater fish. The most sensitive species was the guppy (Poecilia
reticulata, 2-3 months old), with a nominal 7-day LC50 of
106 mg/litre under static renewal test conditions (Konemann, 1981).
In three studies on 30-day-old fathead minnows (Pimephales promelas),
measured 96-h LC50 values ranged from 116 to 136 mg/litre under
flow-through conditions (Veith et al., 1983; Walbridge et al., 1983;
Geiger et al., 1985). LC50 values after 24, 48, 72 and 96 h in
rainbow trout (Salmo gairdneri) were 362, 340, 337 and 336 mg/litre,
respectively, using the static test method (Bartlett, 1979). Knie et
al. (1983) reported the EC0, EC50 and EC100 in golden orfe
(Leuciscus idus) to be 67, 600 and 1075 mg/litre, respectively.
In marine fish, a nominal 96-h LC50 of 480 mg/litre was
reported in tidewater silversides (Minidia beryllina) under static
test conditions (Dawson et al., 1975/77). Heitmuller et al. (1981)
reported the static test LC50 at 24, 48, 72 and 96 h in sheepshead
minnows (Cyprinodon variegatus) to be between 130 and 230 mg/litre.
The 96-h LC50 for dab (Limanda limanda) was 115 mg/litre (Pearson
& McConnell, 1975).
In a long-term, flow-through study of the early life stages of
fathead minnows (Pimephales promelas), there were no effects on egg
hatchability or larval survival and deformity at 29 mg/litre (NOEL);
however, larval growth was significantly (p < 0.05) reduced by 62% at
59 mg/litre (LOEL) (Benoit et al., 1982). Black et al. (1982) exposed
the embryos and larvae of the rainbow trout (Oncorhynchus mykiss)
continuously to 1,2-dichloroethane under flow-through conditions from
within 30 min of fertilization (embryos) and maintained them until 4
days after hatching. The resulting EC50 for hatchability and 27-day
LC50 for post-hatch survival were both 34 mg/litre, and the LOEL for
a 24% reduction in egg hatchability was 3.49 mg/litre. After 21 days
of continuous exposure to 150 mg 1,2-dichloroethane/litre, the
mortality of coho salmon (Oncorhynchus kisutch) eggs was 46%, while
in alevins, 100% mortality occurred 9 days after hatching at
320 mg/litre (Reid et al., 1982). In addition, premature hatching was
observed at 56 mg/litre, and, within one week of hatching, sublethal
effects, including lethargy and loss of equilibrium, were observed in
alevins exposed to 56 mg/litre; 100% mortality occurred 9 days after
hatching.
Ahmad et al. (1984) conducted chronic toxicity studies in which
fathead minnows were exposed to 300, 4000, 7000, 14 000, 29 000 or
39 000 µg/litre for 32 days. There were no significant effects on
survival, although there was a significant (p < 0.05) decrease in
mean individual net weight at the highest concentration (38% of
control values). The MATC was determined to be 49 000 to
59 000 µg/litre.
Teratogenic effects (expressed as surviving larvae with gross,
debilitating abnormalities) were observed in the larvae of
northwestern salamanders (Ambystoma gracile), leopard frogs
(Rana pipiens) and rainbow trout (Oncorhynchus mykiss) at 21.4,
21.9 and 34.4 mg/litre, respectively (Black et al., 1982).
9.2 Terrestrial organisms
9.2.1 Invertebrates
In an acute contact test, the 48-h LC50 for earthworms (Eisenia
fetida) exposed to 1,2-dichloroethane-treated filter-paper was
60 µg/m2 (Neuhauser et al., 1985).
9.2.2 Vertebrates
Male and female white leghorn chickens were fed mash which had
been fumigated with 1,2-dichloroethane, resulting in concen-trations
in the feed of 250 and 500 mg/kg, for 2 years. The end-points
examined included serum composition, growth, semen characteristics and
fertility. The weight of eggs was significantly reduced at 250 mg/kg
(5 to 10% (p < 0.01)), while both the number and weight of eggs were
reduced at 500 mg/kg (5 to 48% (p < 0.05) and 5 to 13% (p < 0.01),
respectively) (Alumot et al., 1976b).
9.2.3 Plants
1,2-Dichloroethane vapour was both lethal and mutagenic to barley
kernels (two-rowed variety, Bonus) following exposure to 3 mg/m3 for
24 h (Ehrenberg et al., 1974).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Available data on the carcinogenicity of 1,2-dichloroethane in
humans are limited. There is convincing evidence of increases in the
incidence of both common and rare tumours in experimental animals at
several sites (including squamous cell carcinomas of the stomach,
haemangiosarcomas, fibromas of the subcutaneous tissue and
adenocarcinomas and fibroadenomas of the mammary gland in rats; and
alveolar/bronchiolar adenomas, mammary gland adenocarcinomas,
endometrial stromal polyp or endometrial stromal sarcoma combined and
hepatocellular carcinomas in mice) following administration by gavage
for 78 weeks.
The incidence of benign lung papillomas was significantly
increased in mice following long-term dermal application of
1,2-dichloroethane, while a non-significant increase in the number of
pulmonary adenomas per animal was reported in a screening bioassay on
mice and in the incidence of benign mammary gland tumours in rats
exposed by inhalation for 2 years.
1,2-Dichloroethane is genotoxic in in vitro and in vivo
assays, and binds to DNA in rodents in vivo.
Based on the induction of both rare and common tumours in rats
and mice exposed by ingestion and supporting evidence in other limited
bioassays, the production of a reactive intermediate that alkylates
DNA and positive results in a range of in vitro assays for
genotoxicity, 1,2-dichloroethane is considered to be a probable human
carcinogen.
10.2 Environmental assessment
The high volatility of 1,2-dichloroethane makes the atmosphere
the predominant environmental sink. Consequently, measured
concentrations in surface waters are low (around 1 to 10 µg/litre).
Air concentrations are highest around manufacturing plants where they
may reach 300 µg/m3; concentrations in urban air average
< 1 µg/m3. Low adsorption to soil leads to potential leaching to
groundwater; some measurements of low concentrations in drinking-water
(< 0.2 µg/litre) confirm this.
Both hydrolysis and microbial degradation are slow; the
volatility of the compound means that it has low residence time in
media where these processes occur and they are not considered to be of
environmental significance.
The estimated atmospheric lifetime of 1,2-dichloroethane is
between 40 and 110 days. Stratospheric photolysis may produce chlorine
radicals which may in turn react with ozone. However, the
ozone-depleting potential is low (0.001 relative to CFC-11) and the
compound is not listed in the Montreal Convention.
Various toxicity tests have shown LC50s for organisms in the
environment to be generally greater than 10 mg/litre. The difference
(at least 7 orders of magnitude) between measured water concentrations
and these toxic concentrations indicate that 1,2-dichloroethane poses
no risk to organisms since exposure will not occur.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN
HEALTH AND THE ENVIRONMENT
Considering the toxicological characteristics of
1,2-dichloroethane, both qualitatively and quantitatively, an exposure
that would not cause adverse effects in humans by any route of
exposure cannot be estimated. Consequently, all appropriate measures
should be taken to eliminate or minimize human exposure to
1,2-dichloroethane.
12. FURTHER RESEARCH
Although specific studies were not recommended for
1,2-dichloroethane, additional analytical epidemiological studies are
desirable.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1979) has
classified 1,2-dichloroethane in group 2B (possibly carcinogenic to
humans), based on sufficient evidence of carcinogenicity in
experimental animals.
The International Programme on Chemical Safety has previously
evaluated 1,2-dichloroethane (IPCS, 1987). It concluded that
1,2-dichloroethane produces central nervous system depression, and
gastrointestinal and liver abnormalities in humans. The same effects
occur in experimental animals, in addition to possible kidney
abnormalities, lung oedema and cardiovascular disorders.
1,2-Dichloroethane, administered by gavage, is carcinogenic in rats
and mice, and should be regarded, for practical purposes, as if it
presented a carcinogenic risk for humans. 1,2-Dichloroethane was not
considered to accumulate in the environment. In the atmosphere, it is
removed by photo-chemical degradation via hydroxyl radicals and is
eliminated from water by evaporation. It has a low octanol/water
partition coefficient and bioconcentration is unlikely. It was not
considered to pose a hazard to the aquatic environment except in the
case of accidents and inappropriate disposal. Data were insufficient
to evaluate the effects of 1,2-dichloroethane on the terrestrial
environment.
The Joint FAO/WHO Expert Committee on Food Additives
(JECFA) has evaluated 1,2-dichloroethane on three occasions (WHO,
1971, 1980, 1992). When last evaluated, the Committee concluded that
this compound is genotoxic in both in vitro and in vivo test
systems and carcinogenic in mice and rats when administered by the
oral route. No ADI was therefore allocated. The Committee expressed
the opinion that 1,2-dichloroethane should not be used in food.
In the WHO Guidelines for drinking-water quality (WHO, 1993), the
concentrations of 1,2-dichloroethane in drinking-water estimated to be
associated with excess risks of 10-4, 10-5 and 10-6 are 300, 30 and
3 µg/litre, respectively, based on linearized multistage modelling of
the incidence of haemangiosarcomas in male rats in the NCI (1978)
study.
The European Commission published a directive in 1990 in which
limit values for emission of 1,2-dichloroethane were specified for
various types of industrial plants. These limits ranged from
0.1 mg/litre (monthly) for plants using 1,2-dichloroethane for
degreasing metals away from an industrial site to 12 mg/litre (daily)
for plants producing 1,2-dichloroethane and processing or using the
substance at the site (CEC, 1990).
REFERENCES
AEC (Alberta Environmental Centre) (1989) Final effluent analysis of
Dow Chemical, Fort Saskatchewan, Alberta plant. Alberta, Canada,
Alberta Environmental Centre (Unpublished data).
Ahmad N, Benoit D, Brooke L, Call D, Carlson A, DeFoe D, Huot J,
Moriarity A, Richter J, Shubat P, Veith G, & Wallbridge C (1984)
Aquatic toxicity tests to characterize the hazard of volatile organic
chemicals in water: a toxicity data summary - Parts I and II. Duluth,
Minnesota, US Environmental Protection Agency, Office of Research and
Development, Environmental Research Laboratory.
Alumot E, Nachtomi E, Mandel E, & Holstein P (1976a) Tolerance and
acceptable daily intake of chlorinated fumigants in the rat diet. Food
Cosmet Toxicol, 14: 105-110.
Alumot E, Meidler M, Holstein P, & Herzberg M (1976b) Tolerance and
acceptable daily intake of ethylene dichloride in the chicken diet.
Food Cosmet Toxicol, 14: 111-114.
Andriukin AA (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klin Med (Mosk), 57: 43-47 (in Russian).
Apostolov I & Mihaylova A (1975) [Investigation into
beta- N-acetylglucosaminidase activity in the serum of white rats
treated with 1,2-dichloroethane.] Probl Hyg, 1: 161-163
(in Bulgarian).
Aragno M, Tamagno E, Danni O, & Ugazio G (1992) In vivo studies on
halogen compound interactions. III. Effect of carbon tetrachloride
plus 1,2-dichloroethane on liver necrosis and fatty accumulation. Res
Commun Chem Pathol Pharmacol, 76: 341-354.
Archer WJ (1979) Chlorocarbons and chlorohydrocarbons. In: Kirk J &
Othmer DF ed. Encyclopedia of chemical technology, 3rd ed. New York,
John Wiley & Sons, vol 5, pp 723-743.
Arfellini G, Bartoli S, Colacci A, Mazzullo M, Galli MC, Prodi G, &
Grilli S (1984) In vivo and in vitro binding of 1,2-dibromoethane
and 1,2-dichloroethane to macromolecules in rat and mouse organs. J
Cancer Res Clin Oncol, 108: 204-213.
Armstrong MJ & Galloway SM (1993) Micronuclei induced in peripheral
blood of Eµ-PIM-1 transgenic mice by chronic oral treatment with
2-acetylaminofluorene or benzene but not with diethylnitrosamine or
1,2-dichloroethane. Mutat Res, 302: 61-70.
Atkinson R (1987) A structure-activity relationship for the estimation
of rate constants for gas-phase reaction of OH radicals with organic
compounds. Int J Chem Kinet, 19: 799-828.
ATSDR (1989) Toxicological profile for 1,2-dichloroethane. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry, 129 pp.
ATSDR (1994) Toxicological profile for 1,2-dichloroethane (Update).
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry,
192 pp (TP-93/06).
Austin SG & Schnatter AR (1983a) A case-control study of chemistry
exposures and brain tumors in petrochemical workers. J Occup Med,
25(4): 313-320.
Austin SG & Schnatter AR (1983b) A cohort mortality study of
petrochemical workers. J Occup Med, 25(4): 304-312.
Baertsch A, Lutz WK, & Schlatter C (1991) Effect of inhalation
exposure regimen on DNA binding potency of 1,2-dichloroethane in the
rat. Arch Toxicol, 65: 169-176.
Bailey S, Collins GB, Fishwick FB, Hart HV, Horler DF, & Scudamore KA
(1982) Pesticide residues in foodstuffs in Great Britain:
Organochlorine pesticides, organophosphorus pesticides and fumigant
residues in home-produced and imported wheat. Pestic Sci,
13(4): 373-378.
Ballering LAP, Nivard MJM, & Vogel EW (1993) Characterization of the
genotoxic action of three structurally related 1,2-dihaloalkanes in
Drosophila melanogaster. Mutat Res, 285: 209-217.
Ballering LAP, Nivard MJM, & Vogel EW (1994) Mutation spectra of
1,2-dibromoethane, 1,2-dichloroethane and 1-bromo-2-chloroethane in
excision repair proficient and repair deficient strains of Drosophila
melanogaster. Carcinogenesis, 15: 869-875.
Banerjee S (1988) DNA damage in rodent liver by 1,2-dichloroethane, a
hepatocarcinogen. Cancer Biochem Biophys, 10: 165-173.
Banerjee S & Van Duuren BL (1979) Binding of carcinogenic halogenated
hydrocarbons to cell macromolecules. J Natl Cancer Inst, 63: 707-711.
Banerjee S, Van Duuren BL, & Oruambo FI (1980) Microsome-mediated
covalent binding of 1,2-dichloroethane to lung microsomal protein and
salmon sperm DNA. Cancer Res, 40: 2170-2173.
Barbash JE & Reinhard M (1989) Abiotic dehalogenation of
1,2-dichloroethane and 1,2-dibromoethane in aqueous solution
containing hydrogen disulfide. Environ Sci Technol, 23(11): 1349-1357.
Barber RD, Donish WH, & Mueller KR (1981) A procedure for the
quantitative measurement of the mutagenicity of volatile liquids in
the Ames Salmonella microsome assay. Mutat Res, 90: 31-48.
Baretta ED, Steward RD, & Mutchler JE (1969) Monitoring exposure to
vinyl chloride vapour: breath analysis and continuous air sampling. Am
Ind Hyg Assoc J, 30: 537-544.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizzari ED, Ray M, Smith D, Tomer KB, Wagner R, & Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - A
multimedia environmental study. Biomed Environ Mass Spectrom,
7: 139-147.
Barrows ME, Petrocelli SR, Macek KJ, & Carroll JJ (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Haque R ed. Dynamics,
exposure and hazard assessment of toxic chemicals. Ann Arbor,
Michigan, Ann Arbor Science Publishers, chapter 24, pp 379-392.
Barsoum GS & Saad K (1934) Relative toxicity of certain chlorine
derivatives of the aliphatic series. Q J Pharm Pharmacol, 7: 205-214.
Bartlett EA (1979) Toxicity of ethylene dichloride to rainbow trout.
Midland, Michigan, Dow Chemical (Unpublished report).
Bauer U (1981) [Human exposure to environmental chemicals -
Investigations on volatile organic halogenated compounds in water,
air, food, and human tissues. III. Communication: results of
investigations.] Zbl Bakteriol Hyg I Abt Orig B, 174(3): 200-237 (in
German).
Benoit DA, Puglisi FA, & Olson DL (1982) A fathead minnow Pimephales
promelas early stage toxicity test method evaluation and exposure to
four organic chemicals. Environ Pollut, A28: 189-197.
Benson LO & Teta MJ (1993) Mortality due to pancreatic and
lymphopoietic cancers in chlorohydrin production workers. Br J Ind
Med, 50: 710-716.
Berck B (1974) Fumigant residues of carbon tetrachloride, ethylene
dichloride, and ethylene dibromide in wheat, flour, bran, middlings,
and bread. J Agric Food Chem, 22(6): 977-984.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, & Bruser DM
(1982) The aquatic toxicity of organic compounds to embryo-larval
stages of fish and amphibians. Lexington, Kentucky, University of
Kentucky (Research Report No. 133).
Blum DJW & Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons and
correlations. J Water Pollut Control Fed, 63(3): 198-207.
Bogoyavlenski VF, Salikhova SKH, & Karpova EV (1968) [Clinical aspects
and therapy for ethylene dichloride poisoning.] Sov Med, 31: 107-109
(in Russian).
Bond EJ (1984) Manual of fumigation for insect control. Rome, Food and
Agriculture Organization of the United Nations, 432 pp (FAO Plant
Production and Protection Paper 54).
Bonnet P, Francin J-M, Grakiski D, Raoult G, & Zissu D (1980)
Détermination de la concentration léthale50 des principaux
hydrocarbures aliphatiques chlorés chez le rat. Arch Mal Prof,
41(6-7): 317-321.
Bouwer EJ & McCarty PL (1983) Transformation of 1- and 2-carbon
halogenated aliphatic organic compounds under methanogenic conditions.
Appl Environ Microbiol, 45(4): 1286-1294.
Brem H, Stein AB, & Rosenkranz HS (1974) The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer Res, 34: 2576-2579.
Bringmann G & Kühn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1981) [Toxicity thresholds of pollutants in
ciliate flagellate protozoa, holozoic bacteriovorous ciliate protozoa
and saprozoic flagellate protozoa.] GWF-Wasser/Abwasser, 122: 308-313
(in German).
Brittebo EB, Kowalsky B, Ghantous H, & Brandt I (1989) Epithelial
binding of 1,2-dichloroethane in mice. Toxicology, 56: 35-45.
Brodzinsky R & Singh HB (1982) Volatile organic chemicals in the
atmosphere: An assessment of available data (Contract No. 68-02-3452).
Menlo Park, California, SRI International (Prepared for the US
Environmental Protection Agency, Office of Research and Development,
Environmental Sciences Research Laboratory, Research Triangle Park,
North Carolina, USA).
Bruckmann P, Kersten W, Funcke W, Balfanz E, Konig J, Theisen J, Ball
M, & Papke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17(12): 2363-2380.
Buijs W, van der Gen A, Mohn GR, & Breimer DD (1984) The direct
mutagenic activity of alpha, omega-dihalogenoalkanes in Salmonella
typhimurium. Mutat Res, 141: 11-14.
Callahan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR,
Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR, &
Gould C (1979) Water-related fate of 129 priority pollutants: Volume
II. Washington, DC, US Environmental Protection Agency
(EPA 40/4-79-029b).
Cetnarowicz J (1959) Experimental and clinical investigation on the
action of dichloroethane. Folia Med Crac, 1: 169-192.
Cheever KL, Cholakis JM, El-Hawari AM, Kovatch RM, & Weisburger EK
(1990) Ethylene dichloride: The influence of disulfiram or ethanol on
oncogenicity, metabolism and DNA covalent binding in rats. Fundam Appl
Toxicol, 14: 243-261.
Cheh AM, Hooper AB, Skochdopole J, Henke CA, & McKinnell RG (1980) A
comparison of the ability of frog and rat S-9 to activate promutagens
in the Ames test. Environ Mol Mutagen, 2: 487-508.
Chemical Marketing Reporter (1992) Chemical profile: Ethylene
dichloride. Chem Mark Report Mag, 241(19): 42.
Chiou CT, Peters LJ, & Freed VH (1979) A physical concept of
soil-water equilibria for nonionic organic compounds. Science,
206: 831-832.
Chiou CT, Freed VH, Peters LJ, & Kohnert RL (1980) Evaporation of
solutes from water. Environ Int, 3: 231-236.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984a) Ambient air
measurement of aromatic and halogenated hydrocarbons at urban, rural,
and motorway locations. Sci Total Environ, 39: 265-279.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984b) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural, and motorway locations.
Environ Pollut, B7: 141-158.
Class T & Ballschmiter K (1986) Chemistry of organic traces in air.
VI: Distribution of chlorinated C1 - C4 hydrocarbons in air over the
northern and southern Atlantic Ocean. Chemosphere, 15(4): 413-427.
Comba ME & Kaiser KLE (1983) Determination of volatile contaminants at
the ng-L-1 level in water by capillary gas chromatography with
electron capture detection. Int J Environ Anal Chem, 16: 17-31.
Comba ME & Kaiser KLE (1985) Volatile halocarbons in the Detroit River
and their relationship with contaminant sources. J Great Lakes Res,
11(3): 404-418.
Cottalasso D, Barisione G, Fontana L, Domenicotti C, Pronzato MA, &
Nanni G (1994) Impairment of lipoglycoprotein metabolism in rat liver
cells induced by 1,2-dichloro-ethane. J Occup Environ Med,
51: 281-285.
CEC (1990) Council directive of 27 July 1990 amending annex II to
directive 86/280/EEC on limit values and quality objectives for
discharges of certain dangerous substances included in List I of the
annex to directive 76/464/EEC. Brussels, Commission of the European
Communities.
CPI (Canadian Process Industries) (1991) CPI product profiles:
Ethylene dichloride. Don Mills, Ontario, Corpus Information Services.
Crebelli R & Carere A (1988) Genotoxic activity of halogenated
aliphatic hydrocarbons in Aspergillus nidulans. J Occup Toxicol,
8: 437-442.
Crebelli R, Conti G, Conti L, & Carere A (1984) Induction of somatic
segregation by halogenated aliphatic hydrocarbons in Aspergillus
nidulans. Mutat Res, 138: 33-38.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, & Carere A (1988)
Induction of chromosome malsegregation by halogenated organic solvents
in Aspergillus nidulans: Unspecific or specific mechanism? Mutat
Res, 201: 401-411.
Crespi CL, Seixas GM, Turner TR, Ryan CG & Penman BW (1985)
Mutagenicity of 1,2-dichloroethane and 1,2-dibromoethane in two human
lymphoblastoid cell lines. Mutat Res, 142: 133-140.
Daft JL (1987) Determining multifumigants in whole grains and legumes,
milled and low-fat grain products, spices, citrus fruit, and
beverages. J Assoc Off Anal Chem, 70: 734-739.
Daft JL (1988) Rapid determination of fumigant and industrial chemical
residues in food. J Assoc Off Anal Chem, 71(4): 748-760.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and nonfatty foods. J Agric Food Chem, 37: 560-564.
Daft JL (1991) Fumigants and related chemicals in foods: Review of
residue findings, contamination sources, and analytical methods. Sci
Total Environ, 100: 501-518.
Daft JL (1993) Fumigant analysis of foods. In: Sherman J & Cairns T
ed. Comprehensive analytical profiles of important pesticides. Boca
Raton, Florida, CRC Press, pp 235-281.
Dann T (1992) Unpublished data on concentrations of 1,2-dichloroethane
in ambient air across Canada. Ottawa, Ontario, Environment Canada.
Danni O, Aragno M, Tamagno E, & Ugazio G (1992) In vivo studies on
halogen compound interactions. IV. Interaction among different halogen
derivatives with and without synergistic action on liver toxicity. Res
Commun Chem Pathol Pharmacol, 76(3): 355-366.
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1975/77) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J
Hazard Mater, 1(4): 303-318.
Deschamps M & Band P (1993) Study of a cluster of childhood leukemia.
Health Rep, 5(1): 81-85.
Dilling WL, Tefertiller NB, & Kalos GJ (1975) Evaporation rates and
reactivities of methylene chloride, chloroform, 1,1,1-trichloroethane,
trichloroethylene, tetrachloroethylene, and other chlorinated
compounds in dilute aqueous solutions. Environ Sci Technol,
9(9): 833-838.
Dorndorf W, Kresse M, Christian W, & Katritzki KG (1975)
[Dichloroethane poisoning with myoclonic syndrome, epileptic attacks,
and irreversible cerebral effects.] Arch Psychiatr Nervenkr,
220: 373-379 (in German).
Drury JS & Hammons AS (1979) Investigations of selected environmental
pollutants: 1,2-Dichloroethane. Oak Ridge National Laboratory, Oak
Ridge, Tennessee; Environmental Protection Agency, Washington, D.C.
Office of Toxic Substances; Department of Energy, Washington, DC.
Washington, DC, US Environmental Protection Agency (EPA 560/2-78-006).
D'Souza R, Francis WR, Bruce RD, & Anderson ME (1987) Physiologically
based pharmacokinetic model for ethylene dichloride and its
application in risk assessment. In: Pharmacokinetics in risk
assessment - Drinking water and health. Washington, DC, National
Academy Press, vol 8, pp 286-301.
D'Souza RW, Francis WR, & Andersen ME (1988) Physiological model for
tissue glutathione depletion and increased resynthesis after ethylene
dichloride exposure. J Pharmacol Exp Ther, 245: 563-568.
Duprat P, Delsaut L, & Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les muqueuses
oculaires du lapin. Eur J Toxicol, 9: 171-177.
Easley DM, Kleopfer RD, & Carasea AM (1981) Gas chromatographic-mass
spectrometric determination of volatile organic compounds in fish. J
Assoc Off Anal Chem, 64: 653-656.
Ecobichon DJ & Allen MC (1990) New Brunswick - Water Quality
Surveillance Program. New Brunswick Public Water Supplies: Data
summary report 1990. Fredericton, New Brunswick, Canada, Department of
Health and Community Services, 66 pp.
Ehrenberg L, Osterman-Golkar S, Singh D, & Lundqvist U (1974) On the
reaction kinetics and mutagenic activity of methylating and
ß-halogenoethylating gasoline additives. Radiat Bot, 15: 185-194.
Ellington JJ, Stancil FE, Payne WD, & Trusty CD (1988) Measurement of
hydrolysis rate constants for evaluation of hazardous waste land
disposal: Volume III - Data on 70 chemicals. Athens, Georgia, US
Environmental Protection Agency (EPA/600/3-88/028. Entz RC, Thomas KW,
& Diachenko GW (1982) Residues of volatile halocarbons in foods using
headspace gas chromatography. J Agric Food Chem, 30(5): 846-849.
Environment Agency Japan (1983) Environmental monitoring of chemicals:
Environmental survey report of fiscal years 1980 and 1981. Tokyo,
Japan, Environment Agency.
Environment Agency Japan (1993) Chemicals in the environment - 1993.
Tokyo, Japan, Environment Agency.
Environment Canada (1986) Canada-Ontario agreement respecting Great
Lakes water quality. St. Clair river pollution investigation (Sarnia
area). Ottawa, Ontario, Environment Canada, 80 pp.
Enviro-Test Laboratories (1991) Cayley background study: Analysis of
food products for target organic and inorganic parameters. Edmonton,
Alberta, Canada, Enviro-Test Laboratories, 37 pp
(Report No. 91-E1208).
Enviro-Test Laboratories (1992) Windsor area background study:
Analysis of food products for target organic and inorganic parameters.
Edmonton, Alberta, Enviro-Test Laboratories, 36 pp
(Report No. 92-E1052).
FAO/WHO (1971) Evaluation of food additives. Specifications for the
identity and purity of food additives and their toxicological
evaluation: some extraction solvents and certain other substances; and
a review of the technological efficacy of some antimicrobial agents.
Fourteenth report of the Joint FAO/WHO Expert Committee on Food
Additives. Geneva, World Health Organization, 36 pp (WHO Technical
Report Series No. 462).
FAO/WHO (1980) Evaluation of certain food additives. Twenty-third
report of the Joint FAO/WHO Expert Committee on Food Additives.
Geneva, World Health Organization (WHO Technical Report Series No. 648
and corrigenda).
FAO/WHO (1992) Evaluation of certain food additives and naturally
occurring toxicants. Thirty-ninth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization (WHO
Technical Report Series No. 828).
Fellin P, Barnett SE, & Tran QA (1992) Results of a national pilot
survey of airborne volatile organic compounds in Canadian residences:
Volume 1. Downsview, Ontario, Canada, Concord Environmental
Corporation, 156 pp (prepared for Health and Welfare Canada, Ottawa).
Ferreri AM, Rocchi P, Capucci A, & Prodi G (1983) Induction of
diphtheria toxin-resistant mutants in human cells by halogenated
compounds. J Cancer Res Clin Oncol, 105: 111-112.
Finlayson-Pitts BJ & Pitts JN (1986) Atmospheric chemistry:
Fundamentals and experimental techniques. New York, Wiley-Interscience
Publications.
Foster GD & Tullis RE (1984) A quantitative structure-activity
relationship between partition coefficients and the acute toxicity of
naphthalene derivatives in Artemia salina nauplii. Aquat Toxicol,
5: 245-254.
Foureman GL & Reed DJ (1985) Evidence for a non-episulfonium ion
intermediate during alkylation by S-[2-(N7-guanyl)ethyl]
glutathione, the major DNA adduct formed from 1,2-dibromoethane.
Biochemistry, 25: 2192-2198.
Freiria-Gandara MJ, Lorenzo-Ferreira RA, Alvarez-Devesa, A & Bermejo F
(1992) Occurrence of halogenated hydrocarbons in the water supply of
different cities of Galicia (Spain). Environ Technol, 13: 437-447.
Freitag D, Ballhorn L, Behechti A, Fischer K, & Thumm W (1994)
Structural configuration and toxicity of chlorinated alkanes.
Chemosphere, 28(2): 253-259.
Fujii T (1977) Direct aqueous injection gas chromatography-mass
spectrometry for analysis of organohalides in water at concentrations
below the parts per billion level. J Chromatogr, 139: 297-302.
Garrison SC & Leadingham RS (1954) A fatal case of ethylene dichloride
poisoning in an occupational therapy department of a neuropsychiatric
hospital. Am J Phys Med, 33: 230-237.
Geiger DL, Northcott CE, Call DJ, & Brooke LT (1985)
1,2-Dichloroethane. In: Geiger DL, Northcott CE, Call DJ, & Brooke LT
ed. Acute toxicities of organic chemicals to fathead minnow
(Pimephales promelas). Superior, Wisconsin, University of
Wisconsin-Superior, Center for Lake Superior Environmental Studies,
vol 2, pp 41-42.
Giri AK & Que Hee SS (1988) In vivo sister chromatid exchange
induced by 1,2-dichloroethane on bone marrow cells of mice. Environ
Mol Mutagen, 12: 331-334.
Gocke E, Wild D, Eckhardt K, & King MT (1983) Mutagenicity studies
with the mouse spot test. Mutat Res, 117: 201-212.
Golder Associates (1987) Testing of specific organic compounds in
soils in background in urban areas: Port Credit and
Oakville/Burlington, Ontario. Mississauga, Ontario, Canada, Golder
Associates (Working paper to Shell Canada Ltd and Texaco Canada Ltd).
Gradiski D, Bonnet P, Raoult G, & Magadur JL (1978) Toxicité aiguë
comparée par inhalation des principaux solvants aliphatiques chlorés.
Arch Mal Prof, 39(4-5): 249-257.
Grimsrud EP & Rasmussen RA (1975) Survey and analysis of halocarbons
in the atmosphere by gas chromatography-mass spectrometry. Atmos
Environ, 9: 1014-1017.
Guengerich FP, Crawford WM, Domoradzki JY, MacDonald TL, & Watanabe PG
(1980) In vitro activation of 1,2-dichloroethane by microsomal and
cytosolic enzyme. Toxicol Appl Pharmacol, 55: 303-317.
Guengerich FP, Mason PS, Stott WT, Fox TR, & Watanabe PG (1981) Role
of 2-haloethylene oxides and vinylchloride in irreversible binding to
protein and DNA. Cancer Res, 41: 4391-4398.
Guengerich FP, Koga N, Inskeep PB, & Ozawa N (1986) Proceedings of the
Second International Symposium on the Synthesis and Application of
Isotopically Labelled Compounds. New York, Elsevier Science
Publishers.
Guengerich FP, Kim DH, & Iwasaki M (1991) Role of human cytochrome
P-450 IIE1 in the oxidation of many low molecular weight cancer
suspects. Chem Res Toxicol, 4: 168-179.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Hadengue MA & Martin R (1953) Un cas d'intoxication mortelle par le
dichloréthane. Soc Méd Lég, 33: 247-249.
Hatch GG, Mamay PD, Ayer ML, Casto BC, & Nesnow S (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo cells by
gaseous and volatile chlorinated methanes and ethanes. Cancer Res,
43: 1945-1950.
Health Canada (1994) Unpublished update to Canadian Environmental
Protection Act Priority Substances report on 1,2-dichloroethane.
Ottawa, Ontario, Environmental Health Directorate, Priority Substances
Section.
Heikes DL (1987a) Determination of residual chlorinated solvents in
decaffeinated coffee by using purge and trap procedure. J Assoc Off
Anal Chem, 70(1): 176-180.
Heikes DL (1987b) Pesticide and industrial chemical residues - purge
and trap method for determination of volatile halocarbons and carbon
disulfide in table-ready foods. J Assoc Off Anal Chem, 70(2): 215-226.
Heikes DL (1990) Environmental contaminants in table-ready foods from
the total diet program of the Food and Drug Administration. In: Food
contamination from environmental sources. New York, John Wiley and
Sons, pp 31-57 (Advances in Environmental Science and Technology
Series No. 23).
Heikes DL & Hopper ML (1986) Purge and trap method for determination
of fumigants in whole grains, milled grain products, and intermediate
grain-based foods. J Assoc Off Anal Chem, 69(6): 990-998.
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hellman B & Brandt I (1986) Effects of carcinogenic halogenated
aliphatic hydrocarbons on [3H]thymidine incorporation into various
organs of the mouse. A comparison between 1,2-dibromoethane and
1,2-dichloroethane. Mutat Res, 163: 193-199.
Hemminki K, Falck K, & Vainio H (1980) Comparison of alkylation rates
and mutagenicity of directly acting industrial and laboratory
chemicals: epoxides, glycidyl ethers, methylating and ethylating
agents, halogenated hydrocarbons, hydrazine derivatives, aldehydes,
thiouram and dithiocarbamate derivatives. Arch Toxicol, 46: 277-285.
Heppel LA, Neal PA, Perrin TL, Endicott KM, & Porterfield VT (1945)
The toxicology of 1,2-dichloroethane (ethylene). III. Its acute
toxicity and the effect of protective agents. J Pharmacol Exp Ther,
84: 53-63.
Heppel LA, Neal PA, Perrin TL, Endicott KM, & Porterfield VT (1946)
The toxicology of 1,2-dichloroethane (ethylene dichloride). V. The
effects of daily inhalations. J Ind Hyg, Toxicol, 28(4): 113-120.
Hiatt MH (1981) Analysis of fish and sediment for volatile priority
pollutants. Anal Chem, 53: 1541-1543.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography/mass spectrometry. Anal Chem, 55: 506-516.
Hill J, Kollig HP, Paris DF, Wolfe NL, & Zepp RG (1976) Dynamic
behaviour of vinyl chloride in aquatic ecosystems. Athens, Georgia, US
Environmental Protection Agency (EPA/600/3-76/001).
Hinkel GK (1965) [Oral dichloroethane intoxication of children.] Dtsch
Gesudheitswes, 20: 1327-1331 (in German).
Hofmann HT, Birnsteil H, & Jobst P (1971) [The inhalation toxicity of
1,1- and 1,2-dichloroethane.] Arch Toxikol, 27: 248-265 (in German).
Hooper K, Gold LS, & Ames BN (1980) The carcinogenic potency of
ethylene dichloride in two animal bioassays: A comparison of
inhalation and gavage studies. In: Ames B, Infante P, & Reitz R ed.
Ethylene dichloride: a potential health risk? Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp 65-80 (Banbury Report No. 5).
HSDB (1993) Hazardous substances data bank (revision 9/2/93).
Bethesda, Maryland, National Library of Medicine, National Toxicology
Information Program.
Hueper WC & Smith C (1935) Fatal ethylene dichloride poisoning. Am J
Med Sci, 189: 778-784.
IARC (1979) Some halogenated hydrocarbons. Lyon, International Agency
for Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 20).
Igwe OJ, Que Hee SS, & Wagner WD (1986a) Interaction between
1,2-dichloroethane and tetraethylthiuram disulfide (disulfiram). II.
Hepatotoxic manifestations with possible mechanism of action. Toxicol
Appl Pharmacol, 86: 286-297.
Igwe OJ, Que Hee SS, & Wagner WD (1986b) Interaction between
1,2-dichloroethane and disulfiram. I. Toxicologic effects. Fundam Appl
Toxicol, 6: 733-746.
Inskeep PB, Koga N, Cmarik JL, & Guengerich FP (1986) Covalent binding
of 1,2-dihaloalkanes to DNA and stability of the major DNA adduct,
S-[2(N7-guanyl)ethyl] glutathione. Cancer Res, 46: 2839-2844.
IPCS (International Programme on Chemical Safety) (1987) Environmental
health criteria 62: 1,2-Dichloroethane. Geneva, World Health
Organization.
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: The derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization.
Isacson P, Bean JA, Splinter R, Olson DB, & Kohler J (1985) Drinking
water and cancer incidence in Iowa. III. Association of cancer with
indices of contamination. Am J Epidemiol, 121(6): 856-869.
Isnard P & Lambert S (1988) Estimating bioconcentration factors from
octanol-water partition coefficient and aqueous solubility.
Chemosphere, 17(1): 21-34.
Jafvert CT & Wolfe NL (1987) Degradation of selected halogenated
ethanes in anoxic sediment-water systems. Environ Toxicol Chem,
6: 827-837.
Jakobson I, Wahlberg JE, Holmberg B, & Johansson G (1982) Uptake via
the blood and elimination of 10 organic solvents following
epicutaneous exposure of anesthetized guinea pigs. Toxicol Appl
Pharmacol, 63: 181-187.
Janssen DB, Scheper A, Dijkhuizen L, & Witholt B (1985) Degradation of
halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10.
Appl Environ Microbiol, 49(3): 673-677.
Jeffers PM, Ward L, Woytowich L, & Wolfe NL (1989) Homogeneous
hydrolysis rate constants for selected chlorinated methanes, ethanes,
ethenes and propanes. Environ Sci Technol, 23(8): 965-969.
Jenssen D & Ramel C (1980) The micronucleus test as part of a
short-term mutagenicity test programme for the prediction of
carcinogenicity evaluated by 43 agents tested. Mutat Res, 75: 191-202.
Jonsson A & Berg S (1980) Determination of 1,2-dibromoethane,
1,2-dichloroethane, and benzene in ambient air using porous polymer
traps and gas chromatographic-mass spectrometric analysis with
selected ion monitoring. J Chromatogr, 190: 97-106.
Kaiser KLE & Comba ME (1986) Volatile hydrocarbon contaminant survey
of the St. Clair River. Water Pollut Res J Can, 21(3): 323-331.
Kaiser KLE, Comba ME, & Huneault H (1983) Volatile hydrocarbon
contaminants in the Niagara River and in Lake Ontario. J Great Lakes
Res, 9(2): 212-223.
Kanada T & Uyeta M (1978) Mutagenicity screening of organic solvents
in microbial systems. Mutat Res, 54: 215.
Kavlock R, Chernoff N, Carver B, & Kopfler F (1979) Teratology studies
in mice exposed to municipal drinking water concentrates during
organogenesis. Food Cosmet Toxicol, 17: 343-347.
Kerster HW & Schaeffer DJ (1983) Brine shrimp (Artemia salina)
nauplii as a teratogen test system. Ecotoxicol Environ Saf,
7: 342-349.
King MT, Beikirch H, Eckhardt K, Gocke E, & Wild D (1979) Mutagenicity
studies with X-ray-contrast media, analgesics, antipyretics,
antirheumatics and some other pharmaceutical drugs in bacterial,
Drosophila and mammalian test systems. Mutat Res, 66: 33-43.
Kirschmer P & Ballschmiter K (1983) Baseline studies of global
pollution. VIII. The complex pattern of C1 - C4 organohalogens in
continental and marine background air. Int J Environ Anal Chem,
14: 275-284.
Klaunig JE, Ruch RJ, & Pereira MA (1986) Carcinogenicity of
chlorinated methane and ethane compounds administered in drinking
water to mice. Environ Health Perspect, 69: 89-95.
Kliest J, Fast T, Boley JSM, van de Wiel H, & Bloemen H (1989) The
relationship between soil contaminated with volatile organic compounds
and indoor air pollution. Environ Int, 15: 419-425.
Knie VJ, Halke A, Juhnke I, & Schiller W (1983) Results of studies on
chemical substances with four biotests. Dtsch Gewasserkd Mitt,
27(3): 77-79.
Koga N, Inskeep PB, Harris TM, & Guengerich FP (1986)
S-[2-(N7-guanyl)ethyl] glutathione, the major DNA adduct formed
from 1,2-dibromoethane. Biochemistry, 25: 2192-2198.
Konemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. Part 1: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-221.
Kozik IV (1957) [Some problems of occupational hygiene in the use of
dichloroethane in the aircraft industry.] Gig Tr Prof Zabol, 1: 31-38
(in Russian).
Kramers PG, Mout HC, Bissumbhar B, & Mulder CR (1991) Inhalation
exposure in Drosophila mutagenesis assays: experiments with aliphatic
halogenated hydrocarbons, with emphasis on the genetic activity
profile of 1,2-dichloroethane. Mutat Res, 252: 17-33.
Kretzschmar JG, Peperstraete H, & Tymen T (1976) Air pollution in and
around Tessenderlo (The Netherlands). Neth Extern, 5: 147-178.
Krill RM & Sonzongni WC (1986) Chemical monitoring of Wisconsin's
groundwater. J Am Water Works Assoc, 78(9): 70-75.
Kronevi T, Wahlberg JE, & Holmberg B (1981) Skin pathology following
epicutaneous exposure to seven organic solvents. Int J Tissue React,
3: 21-30.
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Kuwabara T, Quevedo AR, & Cogan DG (1968) An experimental study of
dichloroethane poisoning. Arch Ophthalmol, 79: 321-330.
Lane RW, Riddle BL, & Borzelleca JF (1982) Effects of
1,2-dichloroethane and 1,1,1-trichloroethane in drinking water on
reproduction and development in mice. Toxicol Appl Pharmacol,
63: 409-421.
LaRegina J & Bozzelli JW (1986) Volatile organic compounds at
hazardous waste sites and a sanitary landfill in New Jersey - An
up-to-date review of the present situation. Environ Prog,
5(1): 18-27.
Larionov VG & Kokarovtseva MG (1976) Morphological constitution of
peripheral blood in intoxication with dichloroethane and its
metabolites. In: Actual problems of pesticide application in different
climatographic zones. Yerevan, Aiastan Publishers, pp 131-133.
Leblanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lesage S, McBride RA, Cureton PM, & Brown S (1993) Fate of organic
solvents in landfill leachates under simulated field conditions and in
anaerobic microcosms. Waste Manage Res, 11: 215-226.
Letkiewicz F, Johnston P, & Coleman J (1982) Occurrence of
1,2-dichloroethane in drinking water, food and air (Contract
68-01-6185). Washington, DC, JRB Associates (Prepared for the US
Environmental Protection Agency).
Li KM & Cheng WT (1991) The occupational exposure level of
1,2-dichloroethane in an EM preparation laboratory. J R Soc Health,
111(5): 169.
Lin ELC, Mattox JK, & Pereira MA (1985) Glutathione plus cytosol- and
microme-mediated binding of 1,2-dichloroethane to polynucleotides.
Toxicol Appl Pharmacol, 78: 428-435.
Lum KR & Kaiser KLE (1986) Organic and inorganic contaminants in the
St. Lawrence River: Some preliminary results on their distribution.
Water Pollut Res J Can, 21(4): 592-603.
Luznikov EA, Lisovik GA, & Novikovskaya TV (1985) [Metabolism of
1,2-dichloroethane in human organisms after acute poisonings.]
Forensic Med Expert, 2: 47-49 (in Russian).
McCann J, Simmon V, Streitwieser D, & Ames BN (1975) Mutagenicity of
chloroacetaldehyde, a possible metabolic product of 1,2-dichloroethane
(ethylene dichloride), chloroethane (ethylene chlorohydrin), vinyl
chloride and cyclophosphamide. Proc Natl Acad Sci (USA),
72: 3190-3191.
McCollister DD, Hollingsworth RL, Oyen F, & Rowe VK (1956) Comparative
inhalation toxicity of fumigant mixtures. Individual and joint effect
of ethylene dichloride, carbon tetrachloride, and ethylene dibromide.
Arch Ind Health, 13: 1-7.
McPherson RW, Starks CM, & Fryar GJ (1979) Vinyl chloride
monomer...what you should know. Hydrocarb Process, 3: 75-88.
MAFF (Ministry of Agriculture, Fisheries and Food) (1982) Food
surveillance paper No. 9. London, Her Majesty's Stationery Office.
MAFF (Ministry of Agriculture, Fisheries and Food) (1984) Food
surveillance paper No. 16. London, Her Majesty's Stationery Office.
MAFF (Ministry of Agriculture, Fisheries and Food) (1989) Food
surveillance paper No. 25. London, Her Majesty's Stationery Office.
MAFF/HSE (Ministry of Agriculture, Fisheries and Food/Health and
Safety Executive) (1993) Annual Report of the Working Party on
Pesticide Residues: 1992. Supplement to the Pesticides Register, 1993.
London, Her Majesty's Stationery Office.
Maltoni C, Valgimigli L, & Scarnato C (1980) Long-term carcinogenic
bioassays on ethylene dichloride administered by inhalation to rats
and mice. In: Ames BN, Infante P, & Reitz R ed. Ethylene dichloride: A
potential health risk? Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 3-33 (Banbury Report No. 5).
Martin G, Knorpp K, Huth K, Heinrich F, & Mittermayer C (1969)
Clinical features, pathogenesis and management of dichloroethane
poisoning. Germ Med Mon, 14: 62-67.
Menschick H (1957) [Acute inhalation intoxications by symmetric
dichloroethane.] Arch Gewerbepathol Gewerbehyg, 15: 241-252
(in German).
Mihaylova A (1976) [On the action of high temperature of the
environment on the acute and subacute toxicity of dichloroethane.] Gig
Zdraveopaz, 1: 32-36 (in Bulgarian).
Milman HA, Storystorey DL, Riccio ES, Sivak A, Tu AS, Williams GM,
Tong C, & Tyson CA (1988) Rat liver foci and in vitro assays to
detect initiating and promoting effects of chlorinated ethanes and
ethylenes. Ann NY Acad Sci, 534: 521-530.
Ministry of the Environment (1991) Drinking water surveillance program
data base. Ottawa, Canada, Ministry of the Environment, Water
Resources Branch, 27 pp (105 entries).
Mitoma C, Steeger T, Jackson SE, Wheeler KP, Rogers JH, & Milman HA
(1985) Metabolic disposition study of chlorinated hydrocarbons in rats
and mice. Drug Chem Toxicol, 8: 183-194.
Morgan DL, Bucher JR, Elwell MR, Lilja HS, & Murthy AS (1990)
Comparative toxicity of ethylene dichloride in F344/N, Sprague-Dawley
and Osborne-Mendel rats. Food Chem Toxicol, 28: 839-845.
Morgan DL, Cooper SW, Carlock DL, Sykora JJ, Sutton B, Mattie DR, &
McDougal JN (1991) Dermal absorption of neat and aqueous volatile
organic chemicals in Fischer 344 rat. Environ Res, 55: 51-63.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116:185-216.
Morozov GN (1958) [On acute dichloroethane poisoning.] Farmakol
Toksicol, 21(1): 76-78 (in Russian).
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, & White KL
(1982) In vivo assessment of immunotoxicity. Environ Health
Perspect, 43: 41-52.
Natsyuk MV & Chekman IS (1975) The content of nicotinamide coenzymes
in the liver and myocardium of rats poisoned with dichloroethane. Bull
Exp Biol Med, 4: 58-60.
Natsyuk MV & Mudritsky AD (1974) Clinical and biochemical
characteristics of hepatic and renal disorders in acute dichloroethane
intoxications. Mil Med J, 10: 48-50.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity. Bethesda, Maryland, National Cancer Institute (HEW
(NIH) Publication No. 78-1305).
Nestmann ER, Lee EGH, Matula TI, Douglas GR, & Mueller JC (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using Salmonella/mammalian-microsome assay. Mutat Res,
79: 203-212.
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR (1985)
The toxicity of selected organic chemicals to the earthworm Eisenia
foetida. J Environ Qual, 14(3): 383-388.
Nevrotsky VK, Kashin LM, Kulinskaya IL, Mikhailovskaya LF, Shmuter LM,
Burlaka-Vouk ZI, & Zadorozhny BV (1971) Comparative toxicity
evaluation of several industrial poisons in protracted inhalation
exposure to low concentrations. In: Proceedings of the 8th Ukranian
Hygiene Congress, Kiev, 1971, pp 224-226.
Nimitz JS & Skaggs SR (1992) Estimating tropospheric lifetimes and
ozone-depletion potentials of one- and two-carbon hydrofluorocarbons
and hydrochlorofluorocarbons. Environ Sci Technol, 26(4): 739-744.
NIOSH (1994) Registry of toxic effects of chemical substances.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
NIOSH (1984) Manual of analytical methods: Method 1003. Cincinnati,
Ohio, National Institute for Occupational Safety and Health.
Nouchi T, Miura H, Kanayama M, Mizuguchi O, & Takano T (1984) Fatal
intoxication by 1,2-dichloroethane - A case report. Int Arch Occup
Environ Health, 54: 111-113.
NTP (1991) Toxicity studies of 1,2-dichloroethane (ethylene
dichloride) in F344/N rats, Sprague Dawley rats, Osborne-Mendel rats,
and B6C3F1 mice (drinking water and gavage studies). Research
Triangle Park, North Carolina, National Toxicology Program (NIH
Publication No. 91-3123).
Nylander PO, Olofsson H, Rasmuson B, & Svahlin H (1978) Mutagenic
effects of petrol in Drosophila melanogaster. I. Effects of benzene
and 1,2-dichloroethane. Mutat Res, 57: 163-167.
Oldenhuis R, Vink RLJM, Janssen DB, & Witholt B (1989) Degradation of
chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b
expressing soluble methane monooxygenase. Appl Environ Microbiol,
55(11): 2819-2826.
Oliver BG & Pugsley CW (1986) Chlorinated contaminants in St. Clair
River sediments. Water Pollut Res J Can, 21(3): 368-379.
Otson R (1987) Purgeable organics in Great Lakes raw and treated
water. Int J Environ Anal Chem, 31(1): 41-53.
Otson R & Williams DT (1982) Headspace chromatographic determination
of water pollutants. Anal Chem, 54: 942-946.
Otson R, Williams DT, & Bothwell PD (1982) Industrial chemicals -
Volatile organic compounds in water at thirty Canadian potable water
treatment facilities. J Assoc Off Anal Chem, 65(6): 1370-1374.
Parkes DG, Ganz CR, Polinsky A, & Schulze J (1976) A simple gas
chromatographic method for the analysis of trace organics in ambient
air. Am Ind Hyg Assoc J, 37: 165-173.
Payan JP, Beydon D, Fabry JP, Brondeau MT, Ban M, & de Ceaurriz J
(1993) Urinary thiodiglycolic acid and thioether excretion in male
rats dosed with 1,2-dichloroethane. J Appl Toxicol, 13: 417-422.
Pearson CR & McConnell G (1975) Chlorinated C1 and C2 hydrocarbons
in the marine environment. Proc R Soc (Lond), B189: 305-332.
Pellizzari ED (1982) Analysis for organic vapor emissions near
industrial and chemical waste disposal sites. Environ Sci Technol,
16(11): 781-785.
Perocco P & Prodi G (1981) DNA damage by haloalkanes in human
lymphocytes cultured in vitro. Cancer Lett, 13: 213-218.
Pleil JD, Oliver KD, & McClenny WA (1988) Ambient air analyses using
nonspecific flame ionization and electron capture detection compared
by mass spectroscopy. J Air Pollut Control Assoc, 38: 1006-1010.
Principe P, Dogliotti E, Bignami M, Crebelli R, Falcone E, Fabrizi M,
Conti G, & Comba P (1981) Mutagenicity of chemicals of industrial and
agricultural relevance in Salmonella, Streptomyces, and Aspergillus.
J Sci Food Agric, 32: 826-832.
Proctor CJ, Warren ND, & Bevan MAJ (1989) Measurements of
environmental tobacco smoke in an air-conditioned office building.
Environ Technol Lett, 10: 1003-1018.
Prodi G, Arfelini G, Colacci A, Grilli S, & Mazzulo M (1986)
Interaction of halocompounds with nucleic acids. Toxicol Pathol, 14:
438-444.
Qiu LX, Shi SH, Xing SB, & Chen SG (1992) Rate constants for the
reactions of OH with five halogenated substituted ethanes from 292 K
to 366 K. J Phys Chem, 96: 685-689.
Rannug U (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichloroethane. Mutat Res, 76: 269-295.
Rannug U & Beije B (1979) The mutagenic effect of 1,2-dichloroethane
on Salmonella typhimurium. II. Activation by the isolated perfused
rat liver. Chem-Biol Interact, 24: 265-285.
Rannug U, Sundvall A, & Ramel C (1978) The mutagenic effect of
1,2-dichloroethane on Salmonella typhimurium. I. Activation through
conjugation with glutathione in vitro. Chem-Biol Interact, 20: 1-16.
Rao KS, Murray JS, Deacon MM, John JA, Calhoun LL, & Young JT (1980)
Teratogenicity and reproduction studies in animals inhaling ethylene
dichloride. In: Ames B, Infante P, & Reitz R ed. Ethylene dichloride:
A potential health risk? Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 149-166 (Banbury Report No. 5).
Reid BJ, Morgan JD, & Whelen MA (1982) A preliminary examination of
the effects of ethylene dichloride on the hatchability of Coho salmon
eggs (Oncorhynchus kisutch). Can Tech J Fish Aquat Sci,
1163: 145-153.
Reitz RH, Fox TR, Ramsey JC, Quast JF, Langvardt PW, & Watanabe PG
(1982) Pharmacokinetics and macro-molecular interactions of ethylene
dichloride in rats after inhalation or gavage. Toxicol Appl Pharmacol,
62: 190-204.
Reitz RH, Fox TR, Domordzki JY, Quast JF, Langvardt PW, & Watanabe PG
(1980) Pharmacokinetics and macromolecular interactions of ethylene
dichloride: Comparison of oral and inhalation exposures. In: Ames BN,
Infante P, & Reitz R. ed. Ethylene dichloride: A potential health
risk? Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp 135-148 (Banbury Report No. 5).
Richter JE, Peterson SF, & Kleiner CF (1983) Acute and chronic
toxicity of some chlorinated benzenes, chlorinated ethanes, and
tetrachloroethylene to Daphnia magna. Arch Environ Contam Toxicol,
12: 679-684.
Roldan-Arjona T, Garcia-Pedrajas MD, Luque-Romero RL, Hera C, & Pueyo
C (1991) An association between mutagenicity of the Ara test of
Salmonella typhimurium and carcinogenicity in rodents for 16
halogenated aliphatic hydrocarbons. Mutagenesis, 6: 199-205.
Romert L, Magnusson J, & Ramel C (1990) The importance of glutathione
and glutathione transferase for somatic mutations in Drosophila
melanogaster induced in vivo by 1,2-dichloroethane.
Carcinogenesis, 11: 1399-1402.
Rosenberg R, Grahn O, & Johansson L (1975) Toxic effects of aliphatic
chlorinated by-products from vinyl chloride production on marine
animals. Water Res, 9: 607-612.
Sack TM, Steele DH, Hammerstrom K, & Remmers J (1992) A survey of
household products for volatile organic compounds. Atmos Environ,
26A(6): 1063-1070.
Saitanov AO & Arsenieva SS (1969) [Electrocardiographic changes due to
acute dichloroethane poisoning: experimental investigation.] Gig Tr
Prof Zabol, 7: 49-50 (in Russian).
Sato A, Nakajima T, & Koyama Y (1981) Dose-related effects of a single
dose of ethanol on the metabolism in rat liver of some aromatic and
chlorinated hydrocarbons. Toxicol Appl Pharmacol, 60: 8-15.
Sauer TC (1981) Volatile organic compounds in open ocean and coastal
surface waters. Org Geochem, 3: 91-101.
Schasteen CS & Reed DJ (1983) The hydrolysis and alkylation activities
of S-(2-haloethyl)-L-cysteine analogues - evidence for extended
half-life. Toxicol Appl Pharmacol, 70: 423-432.
Schönborn H, Prellwitz W, & Baum P (1970) [Consumption coagulation
pathology of 1,2-dichloroethane poisoning.] Klin Wochenschr,
48: 822-824 (in German).
Schultz K, Ghosh L, & Banerjee S (1992) Neoplastic expression in
murine cells induced by halogenated hydrocarbons. In vitro Cell Dev
Biol, 28A: 267-272.
Sherwood RL, O'Shea PT, Thomas W, Ratajczak HV, Aranyi C, & Graham JA
(1987) Effects of inhalation of ethylene dichloride on pulmonary
defenses of mice and rats. Toxicol Appl Pharmacol, 91: 491-496.
Shmuter LM (1977) [Effect of chronic exposure to low concentrations of
chlorinated hydrocarbons of the ethane series on specific and
non-specific reactivity of animals in vivo.] Gig Tr Prof Zabol,
8: 38-42 (in Russian).
Simmon VF (1980) Review of non-bacterial tests of the genotoxic
activity of ethylene dichloride. In: Ames B, Infante P, & Reitz R ed.
Ethylene dichloride: A potential health risk? Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp 97-103 (Banbury Report No. 5).
Simula TP, Glancey MG, & Wolf CR (1993) Human glutathione
S-transferase-expressing Salmonella typhimurium tester strains to
study the activation/detoxification of mutagenic compounds: studies
with halogenated compounds, aromatic amines and aflatoxin B1.
Carcinogenesis, 14: 1371-1376.
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1980) Atmospheric
measurements of selected toxic organic chemicals. Washington, DC, US
Environmental Protection Agency, 35 pp (EPA-600/3-80-072;
NTIS PB80-89).
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban environments.
Atmos Environ, 15: 601-612.
Singh HB, Salas LJ, & Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environ Sci Technol, 16: 872-880. Singh HB, Salas IJ, & Stiles RE
(1983) Selected man-made halogenated chemicals in the air and oceanic
environment. J Geophys Res, 88(C6): 3675-3683.
Smyth HF (1969) Acute toxicity of ethyl compounds. In: Spector WC ed.
Handbook of toxicology. Philadelphia, Pennsylvania, W.B. Saunders,
vol 1, pp 134-135.
Spence JW & Hanst PL (1978) Oxidation of chlorinated ethanes. J Air
Pollut Control Assoc, 28(3): 250-253.
Spencer HC, Rowe VK, Adams EM, McCollister DD, & Irish DD (1951) Vapor
toxicity of ethylene dichloride determined by experiments on
laboratory animals. Am Med Assoc Arch Ind Hyg Occup Med, 4: 482-493.
Spreafico F, Zuccato E, Marcucci F, Sironi M, Paglialunga S, Madonna
M, & Mussini E (1980) Pharmacokinetics of ethylene dichloride in rats
treated by different routes and its long-term inhalatory toxicity. In:
Ames B, Infante P, & Reitz R ed. Ethylene dichloride: A potential
health risk? Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp 107-133 (Banbury Report No. 5).
Storer RD & Conolly RB (1983) Comparative in vivo genotoxicity and
acute hepatotoxicity of three 1,2-dihaloethanes. Carcinogenesis,
4: 1491-1494.
Storer RD & Conolly RB (1985) An investigation of the role of
microsomal oxidative metabolism in the in vivo genotoxicity of
1,2-dichloroethane. Toxicol Appl Pharmacol, 77: 36-46.
Storer RD, Jackson NM, & Conolly RB (1984) In vivo genotoxicity and
acute hepatotoxicity of 1,2-dichloroethane in mice: Comparison of
oral, intraperitoneal, and inhalation routes of exposure. Cancer Res,
44: 4267-4271.
Story DL, Meierhenry EF, Tyson CA, & Milman HA (1986) Differences in
rat liver enzyme-positive foci produced by chlorinated aliphatics and
phenobarbital. Toxicol Ind Health, 2: 351-362.
Strobel K & Grummt T (1987) Aliphatic and aromatic halocarbons as
potential mutagens in drinking water. III. Halogenated ethanes and
ethenes. Toxicol Environ Chem, 15: 101-128.
Sundheimer DW, White RD, Brendel K, & Sipes IC (1982) The
bioactivation of 1,2-dibromoethane in rat hepatocytes: covalent
binding to nucleic acids. Carcinogenesis, 3: 1129-1133.
Suveev IM & Babichenko ME (1969) [On the clinic and cure of acute
intoxication with dichloroethane vapours.] Gig Tr Prof Zabol,
13: 50-51 (in Russian).
Symons JM, Bellar TA, Carswell JK, Demarco J, Kropp KL, Robeck GG,
Seeger DR, Slocum CJ, Smith BL, & Stevens AA (1975) National organics
reconnaissance survey for halogenated organics. J Am Water Works
Assoc, 67: 634-648.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53(10): 1503-1518.
Tan EL & Hsie AW (1981) Mutagenicity and cytotoxicity of haloethanes
as studied in the CHO/HGPRT system. Mutat Res, 90: 183-191.
Tang NH, Blum DJW, & Speece RE (1990) Comparison of serum bottle
toxicity test with OECD method. J Environ Eng, 116(6): 1076-1084.
Taningher M, Parodi S, Grilli S, Colacci A, Mazzullo M, Bordone R, &
Santi L (1991) Lack of correlation between alkaline DNA fragmentation
and DNA covalent binding induced by polychloroethanes after in vivo
administration. Problems related to the assessment of a carcinogenic
hazard. Cancer Detect Prev, 15: 35-39.
Theiss JC, Stoner GD, Shimkin MB, & Weisberger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer Res,
37: 2717-2720.
Torkelson TR & Rowe VK (1981) Halogenated aliphatic hydrocarbons. In:
Clayton GD & Clayton FE ed. Toxicology: Patty's industrial hygiene
and toxicology, 3rd ed. New York, John Wiley and Sons, vol 2B,
pp 3491-3497.
Troisi FM & Cavallazzi D (1961) [A fatal case of poisoning from
inhalation of dichloroethane vapours.] Med Lav, 52: 612-618
(in Italian).
Tsuruta H (1975) Percutaneous absorption of organic solvents: 1)
Comparative study of the in vivo percutaneous absorption of
chlorinated solvents in mice. Ind Health, 13: 227-236.
Tsuruta H (1977) Percutaneous absorption of organic solvents: 2) A
method for measuring the penetration rate of chlorinated solvents
through excised rat skin. Ind Health, 15: 131-139.
Tu AS, Murray TA, Hatch KM, Sivak A, & Milman HA (1985) In vitro
transformation of BALB/c-3T3 cells by chlorinated ethanes and
ethylenes. Cancer Lett, 28: 85-92.
UK HSE (Health and Safety Executive) (1992) Criteria document for an
occupational exposure limit: 1,2-Dichloroethane. London, Her Majesty's
Stationery Office, 34 pp.
Urusova TP (1953) [The possible presence of dichloroethane in human
milk with exposure in industrial conditions.] Gig i Sanit, 18: 36-37
(in Russian).
US Department of Labor (1989) Industrial exposure and control
technologies for OSHA regulated hazardous substances: Volume I.
Washington, DC, US Department of Labor.
US DHHS (Department of Health and Human Services) (1994) Seventh
annual report on carcinogens -1994: Summary. Research Triangle Park,
North Carolina, National Institute of Environmental Health Sciences,
165 pp.
US EPA (1982a) An exposure and risk assessment for dichloroethanes.
Washington, DC, US Environmental Protection Agency, Office of Water
Regulations and Standards, 175 pp (EPA-440/4-85-009).
US EPA (1982b) Test methods for organic chemical analysis of municipal
and environmental wastewater. Cincinnati, Ohio, US Environmental
Protection Agency, Monitoring and Support Laboratories, 76 pp
(EPA 600/4-82-057).
US EPA (1985a) Health assessment document for 1,2-dichloroethane
(ethylene dichloride). Final Report. Washington, DC, US Environmental
Protection Agency, 95 pp (EPA/600/8-84/006F; NTIS PB86-122702).
US EPA (1985b) Health and environmental effects profile for
1,2-dichloroethane. Washington, DC, US Environmental Protection
Agency, 137 pp (EPA/600/X-85/359; NTIS PB88-178777).
US EPA (1986) Handbook: Permit writer's guide to test burn data,
hazardous waste incineration. Cincinnati, Ohio, US Environmental
Protection Agency, 73 pp (EPA/625/6-86/012).
US EPA (1987) Health advisories for 25 organics. Washington, DC, US
Environmental Protection Agency (NTIS PB87-235578).
US EPA (1988) Contract laboratory program database. Washington, DC, US
Environmental Protection Agency, Contract Laboratory Program.
US EPA (1992) Indoor air quality data base for organic compounds.
Research Triangle Park, North Carolina, US Environmental Protection
Agency (EPA/600/R/92-025; PB92-158468).
Valencia R, Abrahamson S, Lee WR, Von Halle ES, Woodruff RC, Wurgler
FE, & Zimmering S (1984) Chromosome mutations for mutagenesis in
Drosophila melanogaster: A report of the US Environmental Protection
Agency Gene-Tox Program. Mutat Res, 134: 61-88.
Van Bladeren PJ, Breimer DD, Rotteveel-Smijs RMT, De Knijff P, Mohn
GR, van Meeteren-Walchli B, Buijs W, & van der Gen A (1981) The
relation between the structure of vicinal dihalogen compounds and
their mutagenic activation via conjugation to glutathione.
Carcinogenesis, 2: 499-505.
Van den Wijngaard AJ, van der Kamp KWJ, van der Ploeg J, Pries F,
Kazemier B, & Janssen DB (1992) Degradation of 1,2-dichloroethane by
Ancylobacter aquaticus and other facultative methylotrophs. Appl
Environ Microbiol, 58: 976-983.
Van Duuren BL, Goldschmidt BM, Loewengart G, Smith A, Mechionne S,
Seldman I, & Roth D (1979) Carcinogenicity of halogenated olefinic and
aliphatic hydrocarbons in mice. J Natl Cancer Inst, 63: 1433-1439.
Van Esch GJ, Kroes R, Van Logten MJ, & Den Tonkelaar EM (1977)
Ninety-day toxicity study with 1,2-dichloroethane (DCE) in rats.
Bilthoven, The Netherlands, National Institute of Public Health and
Environmental Protection (RIVM) (Report 195/77).
Van Luin AB & van Starkenburg W (1984) Hazardous substances in waste
water. Water Sci Technol, 17: 843-853.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: Narcotic industrial
chemicals. Can J Fish Aquat Sci, 40: 743-748.
Vozovaya MA (1974) [Development of posterity of two generations
obtained from females subjected to the action of dichloroethane.] Gig
i Sanit, 39: 25-28 (in Russian).
Vozovaya MA (1977) [The effect of dichloroethane on the sexual cycle
and embryogenesis of experimental animals.] Aksuk Ginekol (Moscow),
2: 57-59 (in Russian).
Walbridge CR, Fiandt JT, Phipps GL, & Holcombe GW (1983) Acute
toxicity of ten chlorinated aliphatic hydrocarbons to the fathead
minnow (Pimephales promelas). Arch Environ Contam Toxicol,
12: 661-666.
Wallace LA (1986) Personal exposures, indoor and outdoor air
concentrations, and exhaled breath concentrations of selected volatile
organic compounds measured for 600 residents of New Jersey, North
Dakota, North Carolina and California. Toxicol Environ Chem,
12: 215-236.
Wallace LA, Pellizzari E, Hartwell T, Rozenweig M, Erickson M,
Sparacino C, & Zelon H (1984) Personal exposure to volatile organic
compounds. I. Direct measurements in breathing-zone air, drinking
water, food and exhaled breath. Environ Res, 35: 293-319.
Wallace L, Pellizzari E, Leaderer B, Zelon H, & Sheldon L (1987)
Emissions of volatile organic compounds from building materials and
consumer products. Atmos Environ, 21(2): 385-393.
Ward JM (1980) The carcinogenicity of ethylene dichloride in
Osborne-Mendel rats and B6C3F1 mice. In: Ames BN, Infante P, & Reitz
R ed. Ethylene dichloride: A potential health risk? Cold Spring
Harbor, New York, Cold Spring Harbor Laboratory, pp 35-53 (Banbury
Report No. 5).
Ward RJ (1992) Ethylene dichloride: In vitro absorption through
human and rat epidermis. Alderley Park, United Kingdom, ICI Central
Toxicology Laboratory (Unpublished report).
Warner JM & Beasley RK (1984) Purge and trap chromatographic method
for the determination of acrylonitrile, chlorobenzene,
1,2-dichloroethane and ethylbenzene in aqueous samples. Anal Chem,
56: 1953-1956.
Warner HP, Cohen JM, & Ireland JC (1987) Determination of Henry's Law
Constants of selected priority pollutants. Washington, DC, US
Environmental Protection Agency, Office of Science and Development
(EPA-600/D-87/229).
Weiss F (1957) [Lethal oral intoxications by dichloroethane.] Arch
Gewerbepathol Gewerbehyg, 15: 253-264 (in German).
WHO (1993) Guidelines for drinking-water quality. Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization.
Williams GM, Mori H, & McQueen CA (1989) Structure-activity
relationships in the rat hepatocyte DNA-repair test for 300 chemical.
Mutat Res, 221: 263-286.
Wilson JT, Enfield CG, Dunlap WJ, Cosby RL, Foster DA, & Baskin LB
(1981) Transport and fate of selected organic pollutants in a sandy
soil. J Environ Qual, 10(4): 501-506.
Wirtschafter ZT & Schwartz WD (1939) Acute ethylene dichloride
poisoning. J Ind Hyg Toxicol, 21: 126-131.
Wit SL, Besemer AFH, Das HA, Goedkoop W, Loosjes FE, & Meppelink EK
(1969) Toxicology. Bilthoven, The Netherlands, National Institute of
Public Health (Report No. 36/69).
Withey JR & Collins BT (1980) Chlorinated aliphatic hydrocarbons used
in the foods industry: the comparative pharmacokinetics of methylene
chloride, 1,2-dichloroethane, chloroform and trichloroethylene after
I.V. administration in the rat. J Environ Pathol Toxicol, 3: 313-332.
Withey JR & Karpinski K (1985) The fetal distribution of some
aliphatic chlorinated hydrocarbons in the rat after vapor phase
exposure. Biol Res Pregnancy Perinatol, 6: 79-88.
Withey JR, Collins BT, & Collins PG (1983) Effect of vehicle on the
pharmacokinetics and uptake of four halogenated hydrocarbons from the
gastrointestinal tract of the rat. J Appl Toxicol, 3: 249-253.
Wolff DL, Ivanov NG, Kljackina AM, & Mel'Nikova LV (1979) [Evidence of
the effect of significant industrial toxicological substances on the
nervous system by animal behavioural tests.] Zool Jahrb Physiol,
83: 82-91 (in German).
Worthing CR ed. (1991) The pesticide manual. Farnham, Surrey, The
British Crop Protection Council.
Yodaiken RE & Babcock JR (1973) 1,2-Dichloroethane poisoning. Arch
Environ Health, 26: 281-284.
Zamora PO, Benson JM, Li AP, & Brooks AL (1983) Evaluation of an
exposure system using cells grown on collagen gels for detecting
highly volatile mutagens in the CHD/HGPRT mutation assay. Environ
Mutagen, 5: 795-801.
Zoetman BCJ, Piet GJ, Slingerland P, Fonds AW, & Wegman RCC (1979)
[Investigation into the presence of volatile organic halogenated
compounds in groundwater used as a drinking water supply. Final
report: results on the period November (1976) to August (1978).]
Bilthoven, The Netherlands, National Institute of Drinking Water
Supply, National Institute of Public Health and Environmental Hygiene
(Reports Nos RID-CBH-79/01 and RIV 10/79) (in Dutch).
RESUME
1. Identité, propriétés physiques et chimiques, et méthodes d'analyse
Le 1,2-Dichloréthane (ou dichlorure d'éthylène) est un produit
chimique de synthèse qui se présente sous la forme d'un liquide
incolore à la température ambiante. Il est extrêmement volatil, avec
une tension de vapeur de 8,5 kPa à 20°C et il est soluble dans l'eau,
sa solubilité étant de 8690 mg/litre à 20°C. Son coefficient de
partage octanol/eau (log Kow) est égal à 1,76.
Le dosage du dichloréthane dans les différents compartiments de
l'environnement s'effectue généralement par chromatographie en phase
gazeuse, avec détection par capture d'électrons, ionisation de flamme
ou spectrométrie de masse. Les limites de détection vont de 0,016 à
> 4 µg/m3 dans l'air, de 0,001 à 4,7 µg/litre dans l'eau et de 6 à
10 µg/kg dans différentes denrées alimentaires.
2. Sources d'exposition humaine et environnementale
On utilise principalement le 1,2-dichloréthane pour la synthèse
du chlorure de vinyle monomère et dans une moindre mesure pour la
production de divers solvants chlorés. Il entre également dans la
composition des additifs antidétonants de l'essence (encore que cet
usage soit en déclin avec l'élimination progressive de l'essence au
plomb dans certains pays) et on l'utilise aussi pour des fumigations.
La production annuelle totale de 1,2-dichloréthane a été de 922
kilotonnes au Canada en 1990 et de 6318 kilotonnes aux Etats-Unis
d'Amérique en 1991.
3. Transport, distribution et transformation dans l'environnement
La majeure partie du 1,2-dichloréthane rejeté dans
l'environnement provient d'émissions dans l'atmosphère. Il
estmoyennement persistant dans l'air; sa durée de vie estimative dans
l'atmosphère est comprise entre 43 et 111 jours. Le dichloréthane est
transporté vers la stratosphère où, par photolyse, il peut donner
naissance à du chlore radicalaire qui peut à son tour réagir sur
l'ozone. Une partie du 1,2-dichloréthane rejeté dans les effluents
industriels peut passer dans le milieu aquatique dont il s'échappe
rapidement par volatilisation. Il peut également s'infiltrer
jusqu'aux nappes d'eau souterraines à proximité des zones de décharges
industrielles. On ne pense pas qu'il puisse subir une
bioconcentration chez les espèces aquatiques ou terrestres.
4. Concentrations dans l'environnement et exposition humaine
Des enquêtes récentes portant sur l'air ambiant de zones urbaines
non dominées par des sources polluantes ont permis de relever des
concentrations moyennes de 1,2-dichloréthane allant de 0,07 à
0,28 µg/m3, alors que dans l'air intérieur aux habitations des zones
résidentielles, ces valeurs moyennes vont de < 0,1 à 3,4 µg/m3.
Dans l'eau destinée à la consommation, les concentrations moyennes
sont généralement inférieures à 0,5 µg/litre. Lors de récentes
enquêtes, on a rarement décelé du 1,2-dichloréthane dans les denrées
alimentaires et comme il ne présente qu'un faible potentiel de
bioaccumulation, il est peu probable que la nourriture constitue une
source importante d'exposition à ce composé.
La valeur estimative de l'exposition moyenne au 1,2-dichloréthane
à partir de divers milieux montre que la source principale
d'exposition est constituée par l'air intérieur et extérieur, l'eau de
consommation n'y contribuant que pour une très faible part. L'apport
de 1,2-dichloréthane par la voie alimentaire est probablement
négligeable. Les quantités inhalées dans l'air ambiant pourraient
être plus importantes à proximité des sources industrielles.
5. Cinétique et métabolisme chez les animaux de laboratoire
Après inhalation, ingestion ou exposition par voie cutanée, le
1,2-dichloréthane est rapidement absorbé et il se répartit rapidement
et largement dans l'ensemble de l'organisme. Il est rapidement et
largement métabolisé chez le rat et la souris, principalement sous
forme de métabolites soufrés dont l'excrétion s'effectue par la voie
urinaire et dépend de la dose. A des niveaux d'exposition qui
entraînent des concentrations sanguines de 5 à 10 µg/ml, il semble
qu'il y ait saturation ou limitation du métabolisme chez le rat.
Après administration d'une dose unique de dichloréthane par gavage, on
a constaté que le taux d'alkylation de l'ADN était plus important que
lorsque le produit était inhalé sur une période de six heures.
Il existe semble-t-il deux voies principales de métabolisation.
La première est une oxydation saturable qui s'effectue au niveau des
microsomes par l'intermédiaire du cytochrome P-450 et aboutit au
2-chloracétaldéhyde et au 2-chloréthanol, pour s'achever sur une
conjugaison avec le glutathion. La deuxième voie métabolique comporte
une conjugaison directe avec le glutathion pour former du
S-(2-chloréthyl)-glutathion, qui est peut-être ensuite transformé
par voie non enzymatique en un ion glutathion-épitsulfonium
susceptible de former des adduits avec l'ADN. Bien qu'on ait pu
observer in vitro que la voie du P-450 conduisait à des lésions de
l'ADN, il semble bien qu'à cet égard, la voie impliquant la
conjugaison du glutathion soit la plus importante.
6. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Le 1,2-dichloréthane présente une faible toxicité aiguë pour les
animaux de laboratoire. Ainsi, la CL50 par inhalation pour des rats
exposés soit 6 soit 7,25 heures à ce composé allait de 4000 mg/m3 à
6600 mg/m3, la DL50 par voie orale pour le rat, la souris, le
chien et le lapin allant de 413 à 2500 mg/kg de poids corporel.
D'après les résultats d'études à court terme et d'études
subchroniques menées sur différentes espèces d'animaux de laboratoire,
c'est le foie et les reins qui sont les organes cibles; il n'a pas été
possible d'obtenir de valeurs pour la dose sans effets observables
(NOEL) ou La dose la plus faible provoquant un effet (LOEL),
généralement en raison d'une documentation insuffisante et du nombre
trop limité de paramètres biologiques examinés sur un trop petit
nombre d'animaux. Une série d'études limitées anciennes a révélé la
présence de modifications morphologiques au niveau du foie chez
plusieurs espèces après exposition subchronique à des concentrations
atmosphériques ne dépassant pas 800 mg/m3. Après administration
subchronique par voie orale de 1,2-dichloréthane à des doses
quotidiennes allant de 49 à 82 mg/kg de poids corporel ou davantage
pendant 13 semaines, on a observé chez des rats un accroissement du
poids relatif du foie. Les études de toxicité chronique dont on
possède les résultats ne donnent guère d'information sur les effets
non néoplasiques. Chez des rats exposés pendant 12 mois à des
concentrations atmosphériques de 1,2-dichloréthane ne dépassant pas
202 mg/m3, on a observé, au niveau des paramètres sériques, des
modifications indiquant une toxicité hépatique et rénale; toutefois,
aucun examen histopathologique n'a été pratiqué lors de cette étude.
Quelques épreuves limitées ont été effectuées sur des animaux de
laboratoire à la recherche d'une cancérogénicité éventuelle du
1,2-dichloréthane (ces études souffraient d'une trop faible durée
d'exposition et d'une forte mortalité parmi les animaux). Chez des
rats Sprague-Dawley et des souris Swiss exposés pendant 78 semaines à
des concentrations allant jusqu'à 607 mg/m3 et observés jusqu'à ce
qu'ils meurent spontanément, on n'a pas observé d'augmentation
significative dans l'incidence des tumeurs quel qu'en soit le type.
La mortalité était forte parmi les rats, mais sans rapport avec la
concentration du produit et on n'a pas tenu compte des différences de
mortalité entre les groupes pour corriger les taux d'incidence. Des
rattes Sprague-Dawley ont été exposées pendant deux ans à 200 mg/m3
de 1,2-dichloréthane et on a observé à cette occasion une augmentation
de l'incidence des adénomes et des fibroadénomes de la mère, qui
n'était toutefois pas significative; aucun autre effet toxique
attribuable au composé n'a été observé.
En revanche, on a observé, après ingestion, chez deux espèces,
des signes convaincants d'un accroissement de l'incidence tumorale.
Chez des rats Osborne-Mendel à qui l'on avait administré
quotidiennement par gavage pendant 78 semaines des doses de 47 ou
95 mg/kg (en moyenne pondérée par rapport au temps), on a observé une
augmentation significative de l'incidence des tumeurs de différentes
localisations (notamment des carcinomes spinocellulaires de l'estomac
(chez les mâles), des hémangiosarcomes (chez les mâles et les
femelles), des fibromes du tissu sous-cutané (chez les mâles) ainsi
que des adénocarcinomes et des fibroadénomes mammaires chez les
femelles. Chez des souris B6C3F1 à qui l'on avait administré
quotidiennement des doses de 97 ou 195 mg/kg de produit (en moyenne
pondérée par rapport au temps) (mâles) ou de 149 et 299 mg/kg
(femelles) par gavage sur 78 semaines, on a observé une augmentation
similaire de l'incidence des tumeurs de diverses localisations
(notamment des adénomes alvéolaires/bronchiolaires chez les mâles et
les femelles, des adénocarcinomes mammaires chez les femelles, des
polypes ou des sarcomes du stroma de l'endomètre (femelles) et des
carcinomes hépatocellulaires (mâles)).
Chez des souris femelles qui avaient été soumises respectivement
pendant 440 et 594 jours à des applications cutanées répétées de
1,2-dichloréthane, on a observé une incidence sensiblement accrue des
tumeurs pulmonaires (papillomes bénins). Chez une souche sensible de
souris, des injections intrapéritonéales répétées de 1,2-dichloréthane
ont déterminé un accroissement, lié à la dose, du nombre des adénomes
pulmonaires, mais cet accroissement n'était en aucun cas significatif.
Chez des rats à qui l'on faisait simultanément respirer du
1,2-dichloréthane et ingérer du disulfirame avec leur nourriture, on a
observé une incidence accrue des cholangiomes et des kystes dans la
partie intrahépatique des canaux biliaires, et davantage de fibromes
sous-cutanés, de nodules hépatiques malins, de tumeurs du tissu
testiculaire interstitiel et d'adénocarcinomes mammaires, que chez des
rats qui avaient reçu soit l'un, soit l'autre des composés ou aucun
des deux. Trois épreuves biologiques n'ont pas permis de mettre en
évidence une aptitude quelconque de ce composé à se comporter comme un
initiateur ou un promoteur tumoral, encore que l'examen
histopathologique effectué à la suite de ces études ait été de portée
limitée.
Lors d'épreuves de mutagénicité in vitro sur Salmonella
typhimurium, le 1,2-dichloréthane a toujours donné des résultats
positifs. L'effet était plus important en présence d'un système
d'activation exogène (sans doute du fait d'une activation par le
cytochrome) et on constatait que le pouvoir mutagène était plus que
doublé chez S. typhimurium exprimant le gène humain GSTA1-1. Le
1,2-dichloréthane forme des adduits avec l'ADN en cultures de cellules
mammaliennes. Il provoque également une synthèse non programmée de
l'ADN dans des cultures primaires de cellules murines et humaines
ainsi que des mutations géniques dans certaines lignées cellulaires.
On a trouvé une corrélation entre la fréquence des mutations observées
dans des lignées cellulaires humaines et la modification de l'activité
de la glutathion-S-transférase. Des études in vivo ont montré que
le 1,2-dichloréthane produisait des mutations létales récessives dans
les cellules somatiques et germinales de Drosophila melanogaster et
selon toutes les études publiées portant sur des rats et des souris,
il y a liaison du 1,2-dichloréthane à l'ADN. Des lésions directes de
l'ADN des cellules hépatiques ainsi que des échanges entre chromatides
soeurs ont été observés lors d'études sur la souris mais rien
n'indique que le 1,2-dichloréthane provoque la formation de
micronoyaux.
Rien n'indique, à en juger par les résultats d'un nombre limité
d'études, que le 1,2-dichloréthane soit tératogène pour les animaux de
laboratoire. Il n'y a également guère d'éléments en faveur d'effets
sur la reproduction ou le développement à des doses inférieures à
celles qui provoquent d'autres effets généraux. On ne dispose que de
données limitées sur l'immunotoxicité du 1,2-dichloréthane.
7. Effets sur l'homme
Des effets divers ont été observés à la suite d'expositions
accidentelles aiguës à du 1,2-dichloréthane par inhalation ou
ingestion: au niveau du système nerveux central, du foie, des reins,
des poumons et de l'appareil cardio-vasculaire.
On n'a pas beaucoup étudié le pouvoir cancérogène du
1,2-dichloréthane dans les populations humaines exposées. Chez un
groupe d'ouvriers d'un atelier de production de produits chimiques qui
avaient été exposés principalement à du 1,2-dichloréthane, à côté
d'autres substances, on a observé une augmentation significative de la
mortalité par cancer du pancréas. Cette mortalité augmentait avec la
durée de l'exposition. En outre, malgré un nombre limité de cas et
une association moins systématique avec la durée de l'exposition, il y
avait également accroissement de la mortalité par leucémie chez ces
travailleurs. Une petite étude cas-témoins, portant sur l'exposition
à du 1,2-dichloréthane n'a pas permis de mettre en évidence une
corrélation avec l'apparition de tumeurs cérébrales. Une étude
écologique intrinsèquement limitée, portant sur la présence de
1,2-dichloréthane dans de l'eau de consommation, a mis en évidence une
augmentation de l'incidence des cancers colo-rectaux mais il est
possible qu'une exposition simultanée à d'autres substances explique
pour une part les effets observés.
8. Effets sur les organismes non visés au laboratoire et dans leur
milieu naturel
On a étudié les effets d'une exposition au 1,2-dichloréthane sur
un certain nombre d'autres organismes tant au laboratoire que dans
leur milieu naturel. En ce qui concerne les microorganismes
aquatiques, les valeurs de la CI50 et de la CE50 correspondant à
divers paramètres biotoxicologiques vont de 25 à 770 mg/litre. La
valeur de la CL50 la plus faible qui ait été observée pour les
daphnies était de 220 mg/litre, des effets ayant été toutefois
observés sur la fécondité et la croissance aux concentrations
respectives de 20,7 et 71,7 mg/litre. En s'appuyant sur les données
disponibles, on constate que le vertébré d'eau douce le plus sensible
au 1,2-dichloréthane est une espèce de salamandre (Ambystoma gracile),
chez laquelle la survie des larves à neuf jours (quatre jours après
l'éclosion) a accusé une chute à 2,54 mg/litre. On ne possède que des
données limitées sur la toxicité du 1,2-dichloréthane pour les
organismes terrestres.
Resumen
1. Identidad, propiedades físicas y químicas y métodos analíticos
El 1,2-dicloroetano (dicloruro de etileno), producto químico
sintético, es un líquido incoloro a temperatura ambiente. Es también
muy volátil, con una presión de vapor de 8,5 kPa (a 20°C), y soluble
en agua, con una solubilidad de 8690 mg/litro (a 20°C). El log del
coeficiente de reparto octanol/agua es de 1,76.
El análisis del 1,2-dicloroetano en el medio ambiente se realiza
habitualmente por cromatografía de gases, en combinación con la
captura de electrones, la detección de ionización por conductor o bien
la espectrometría de masas. Los límites de la detección oscilan entre
0,016 y > 4 µg/m3 en el aire, entre 0,001 y 4,7 µg/litro en el agua
y entre 6 y 10 µg/kg en diversos productos alimenticios.
2. Fuentes de exposición humana y ambiental
El 1,2-dicloroetano se utiliza principalmente en la síntesis del
monómero cloruro de vinilo y, en menor grado, en la fabricación de
diversos disolventes clorados. Se incorpora también a los aditivos
antidetonantes de la gasolina (aunque este empleo está disminuyendo
con la reducción progresiva en muchos países de la gasolina con plomo)
y se ha usado como fumigante. La producción anual total de
1,2-dicloroetano en el Canadá en 1990 y en los Estados Unidos en 1991
fue de 922 000 y 6 318 000 toneladas, respectivamente.
3. Transporte, distribución y transformación en el medio ambiente
La mayor parte del 1,2-dicloroetano liberado en el medio ambiente
ha sido emitido en el aire. En este medio es moderadamente
persistente; su permanencia en la atmósfera se estima entre 43 y 111
días. Es transportado a la estratosfera, donde, por fotólisis, pueden
producirse radicales de cloro que a su vez pueden reaccionar con el
ozono. Cierta cantidad de 1,2-dicloroetano puede escapar a los
efluentes industriales y de allí pasar al medio ambiente acuático, de
donde desaparece rápidamente por volatilización. También puede
alcanzar por lixiviación las aguas subterráneas próximas a los
vertederos de desechos industriales. No se prevé su bioconcentración
en especies acuáticas o terrestres.
4. Niveles medioambientales y exposición humana
Según estudios recientes del aire ambiental, las concentraciones
medias de 1,2-dicloroetano detectadas en zonas urbanas donde no
abundan sus fuentes de emisión oscilan entre 0,07 y 0,28 µg/m3,
mientras que los niveles medios notificados como presentes en el aire
del interior de las viviendas oscilan entre < 0,1 y 3,4 µg/m3. En
el agua potable, la concentración media suele ser menor de
0,5 µg/litro. En estudios recientes rara vez se ha detectado la
presencia de 1,2-dicloroetano en alimentos y, puesto que el potencial
de bioacumulación de esta sustancia es bajo, los alimentos
probablemente no representen una fuente de exposición importante.
Teniendo en cuenta las estimaciones de la exposición media en
diversos entornos, la principal fuente de exposición de la población
general al 1,2-dicloroetano es el aire de locales cerrados y el aire
exterior, mientras que el agua de bebida contribuye sólo en cantidades
muy pequeñas. La ingestión de 1,2-dicloroetano con los alimentos
probablemente sea insignificante. La cantidad inhalada con el aire
ambiental puede ser más grande en las proximidades de fuentes de
emisión industriales.
5. Cinética y metabolismo en animales de laboratorio
El 1,2-dicloroetano se absorbe fácilmente tras la inhalación, la
ingestión o la exposición cutánea y se distribuye rápida y ampliamente
por todo el organismo. En la rata y el ratón se metaboliza de forma
rápida y completa y por orina se eliminan principalmente metabolitos
azufrados en concentraciones que dependen de la dosis. En la rata, el
metabolismo parece saturado o limitado cuando la exposición alcanza
niveles que dan lugar a concentraciones sanguíneas de 5 a 10 µg/ml.
Después de la administración por sonda de una dosis única, los niveles
de alquilación del ADN eran más elevados que después de la inhalación
durante un periodo de seis horas.
El 1,2-dicloroetano parece metabolizarse siguiendo dos vías
principales: la primera comporta una oxidación microsómica saturable
mediada por el citocromo P-450, que produce 2-cloroacetaldehído y
2-cloroetanol, seguida de conjugación con el glutatión. La segunda
vía entraña la conjugación directa con el glutatión para formar
S-(2-cloroetil)-glutatión, que puede convertirse mediante un proceso
no enzimático en un ion glutatión episulfonio; este ion puede formar
aductos con el ADN. Aunque se han inducido daños en el ADN por la
ruta del P-450 in vitro, hay varias pruebas de que la vía de la
conjugación del glutatión probablemente sea más importante que la otra
en cuanto a los daños que causa en el ADN.
6. Efectos en mamíferos de laboratorio y en sistemas de ensayo
in vitro
La toxicidad aguda del 1,2-cicloroetano en animales de
experimentación es baja. Por ejemplo, la CL50 por inhalación en
ratas expuestas durante 6 ó 7,25 horas oscilaba entre 4000 mg/m3 y
6600 mg/m3, mientras que la DL50 por vía oral en ratas, ratones,
perros y conejos variaba entre 413 y 2500 mg/kg de peso corporal.
Los resultados de estudios de corta duración y subcrónicos
realizados en varias especies de animales de experimentación indican
que los órganos afectados son el hígado y los riñones; en general no
se obtuvieron NOEL ni LOEL fidedignos debido a la documentación
insuficiente y a la gama limitada de puntos finales examinados en
pequeños grupos de animales. En una serie de estudios iniciales
limitados, se observaron cambios morfológicos en el hígado de varias
especies tras la exposición subcrónica a concentraciones de
1,2-dicloroetano en el aire de sólo 800 mg/m3. Tras la
administración subcrónica a ratas por vía oral de dosis comprendidas
entre 49 y 82 mg/kg de peso corporal por día durante más de 13 semanas
se observó un incremento del peso relativo del hígado. En los
estudios crónicos disponibles se ha presentado poca información sobre
efectos no neoplásicos. Las ratas expuestas a concentraciones de sólo
202 mg/m3 en el aire durante 12 meses acusaron en los parámetros del
suero cambios indicativos de toxicidad hepática y renal, pero en ese
estudio no se realizaron exámenes histopatológicos.
La carcinogenicidad del 1,2-dicloroetano se ha investigado en un
pequeño número de biovaloraciones limitadas sobre animales de
experimentación (las limitaciones comprenden una exposición de corta
duración y una mortalidad alta). No se notificaron aumentos
significativos en la incidencia de ningún tipo de tumor en ratas
Sprague-Dawley o en ratones suizos expuestos a concentraciones de
hasta 607 mg/m3 durante 78 semanas; los animales se observaron hasta
que se produjo su muerte espontánea. En este estudio, la mortalidad
de las ratas fue elevada, pero no guardaba relación con la
concentración, y no se realizó un ajuste de la incidencia en función
de la mortalidad diferencial entre los grupos. En un ensayo con ratas
Sprague-Dawley hembra expuestas durante dos años a 200 mg/m3, se
produjo un aumento no significativo de la incidencia de adenomas y
fibroadenomas en las glándulas mamarias, y no se observó otra forma de
toxicidad relacionada con el compuesto.
En cambio, hay pruebas convincentes de un aumento de la
incidencia de tumores en dos especies tras la ingestión del producto.
Las ratas Osborne-Mendel que habían recibido mediante sonda durante 78
semanas dosis diarias de 47 ó 95 mg/kg de peso corporal (promedio
ponderado en función del tiempo) mostraron un aumento significativo de
la incidencia de tumores en diversos lugares (por ejemplo, carcinomas
escamosos del estómago en machos, hemangiosarcomas en machos y
hembras, fibromas del tejido subcutáneo en machos, adenocarcinomas y
fibroadenomas de las glándulas mamarias en hembras. En ratones
B6C3F1 que habían recibido, como promedio ponderado en función del
tiempo, dosis diarias de 97 ó 195 mg/kg de peso corporal (machos) y
149 ó 299 mg/kg de peso corporal (hembras) mediante sonda durante 78
semanas se observaron aumentos semejantes de la incidencia de tumores
en múltiples lugares (adenomas alveolares/bronquiolares en machos y
hembras, adenocarcinomas de las glándulas mamarias en hembras y pólipo
estromal endometrial o sarcoma estromal endometrial combinados en
hembras y carcinomas hepatocelulares en machos).
La incidencia de tumores pulmonares (papilomas benignos)
aumentó significativamente en ratones hembra tras la aplicación
cutánea repetida de 1,2-dicloroetano durante 440 a 594 días. La
administración intraperitoneal repetida produjo en una raza
susceptible aumentos en el número de adenomas pulmonares por ratón;
dichos aumentos estaban relacionados con la dosis, pero ninguno de
ellos fue significativo. En comparación con las ratas que recibieron
el compuesto solo y con el grupo testigo, que no recibió ningún
tratamiento, las ratas expuestas simultáneamente a 1,2-dicloroetano
por inhalación y a disulfiram en los alimentos acusaron una mayor
incidencia de colangiomas y de quistes en el conducto biliar
intrahepático, de fibromas subcutáneos, de nódulos neoplásicos en el
hígado, de tumores de las células intersticiales de los testículos y
de adenocarcinomas mamarios. En tres biovaloraciones realizadas no se
observaron indicios de potencial iniciador o facilitador del
desarrollo de tumores, pero el alcance del examen histopatológico de
esos estudios fue limitado.
In vitro, el 1,2-dicloroetano ha dado constantemente resultados
positivos en biovaloraciones de mutagenicidad en Salmonella
typhimurium. Las respuestas han sido mayores en presencia de un
sistema de activación exógeno (posiblemente debido a la activación por
el sistema del citocromo) que en su ausencia; la mutagenicidad se
duplicó con creces en la cepa de S. typhimurium que contenía el gen
humano GSTA1-1. En cultivos de células mamarias, el 1,2-dicloroetano
forma aductos con el ADN. También induce síntesis no programada del
ADN en cultivos primarios de células de roedores y humanas y mutación
génica en varias líneas celulares. La frecuencia de las mutaciones en
las líneas celulares humanas se ha correlacionado con diferencias en
la actividad de la glutatión-S-transferasa. En estudios in vivo,
el 1,2-dicloroetano indujo mutaciones en células somáticas y
mutaciones letales recesivas ligadas al sexo en Drosophila
melanogaster y, según todos los estudios realizados en ratas y
ratones, el compuesto se había unido al ADN. Aunque en estudios
efectuados en ratones se han observado lesiones primarias del ADN en
el hígado e intercambio de cromátides hermanas, no hay indicaciones de
inducción de micronúcleos.
Teniendo en cuenta los resultados de un número limitado de
estudios, no hay pruebas de que el 1,2-dicloroetano sea teratogénico
en animales de experimentación. Además, hay pocas pruebas
convincentes de que tenga efectos sobre la reproducción o el
desarrollo con dosis inferiores a las que causan otros efectos
sistémicos. Los datos disponibles sobre la inmunotoxicidad del
1,2-dicloroetano son limitados.
7. Efectos en el ser humano
La exposición accidental aguda al 1,2-dicloroetano por inhalación
o por ingestión ha producido diversos efectos en el ser humano, por
ejemplo en el sistema nervioso central, el hígado, el riñón, el pulmón
y el sistema cardiovascular.
No se ha estudiado con detenimiento la carcinogenicidad potencial
del 1,2-dicloroetano en poblaciones humanas expuestas. La mortalidad
por cáncer pancreático aumentó de forma significativa en un grupo de
trabajadores de una planta de producción química que había estado
expuesto sobre todo a 1,2-dicloroetano (en combinación con otros
productos químicos). La mortalidad por cáncer pancreático aumentó con
la duración de la exposición. Además, aunque el número de casos fue
pequeño y la relación con la duración de la exposición menos
constante, en estos trabajadores también aumentó la mortalidad por
leucemia. No se observó relación entre la exposición ocupacional al
1,2-dicloroetano y el cáncer cerebral en un pequeño estudio de casos y
controles. Si bien en un estudio ecológico con limitaciones
inherentes se observó que la incidencia de cáncer de colon y de recto
aumentaba con la concentración del producto en el agua de bebida, la
exposición simultánea a otras sustancias podría haber contribuido a
producir los efectos observados.
8. Efectos en organismos no destinatarios en el laboratorio y
sobre el terreno
Se han investigado los efectos de la exposición al
1,2-dicloroetano en varios otros organismos en el laboratorio y en el
medio ambiente. Con respecto a los microorganismos acuáticos, se ha
informado de que las CI50 y CE50 correspondientes a diversos
puntos finales oscilan entre 25 y 770 mg/litro. La CL50 más baja
notificada para Daphnia fue de 220 mg/litro, mientras que se
detectaron efectos en el éxito reproductivo y el crecimiento a 20,7 y
71,7 mg/litro, respectivamente. Teniendo en cuenta los datos
disponibles, la especie de vertebrados de agua dulce más sensible
parece ser la salamandra noroccidental (Ambystoma gracile), cuyas
larvas de nueve días (cuatro días después de la eclosión) vieron
reducida su supervivencia a concentraciones de 2,54 mg/litro. Se
dispone sólo de datos limitados sobre la toxicidad del
1,2-dicloroetano en organismos terrestres.
Although some DNA damage has been induced via the P-450 pathway
in vitro (Banerjee et al., 1980; Guengerich et al., 1980; Lin et
al., 1985), several lines of evidence suggest that the GSH conjugation
pathway is probably of greater significance than the P-450 pathway as
the major route for DNA damage (Guengerich et al., 1980; Rannug, 1980;
Sundheimer et al., 1982; Inskeep et al., 1986; Koga et al., 1986).
The P-450-dependent pathway can, however, presumably form
considerable quantities of 2-haloacetaldehydes, which readily bind to
protein and non-protein thiols, as shown for vinyl bromide and vinyl
chloride (Guengerich et al., 1981) and dibromoethane (DBE) (van
Bladeren et al., 1981). However, these authors concluded that 2H
and 18O studies on the formation of 2-haloethanols and
2-haloacetaldehydes from 1,2-dihaloethanes are inconsistent with a
major role of such a mechanism for DNA damage (Guengerich et al.,
1986; Koga et al., 1986).
The 1,2-dichloroethane-induced mutation frequency of two human
cell lines has been correlated with the difference in levels of
glutathione- S-transferase activities. AHH-1 cell line mutation
frequency was 25 times that in the TK6 cell line in the presence of
1,2-dichloroethane. The difference was attributed to the fact that
the AHH-1 cell line possesses 5 times more glutathione- S-transferase
activity than the TK6 cell line (Crespi et al., 1985).
Moreover, although the significance of the reported results is
uncertain, the results of an additional study by Storer & Conolly
(1985) are not inconsistent with the hypothesis that reduction of GSH
levels is associated with a reduction in DNA damage. Male B6C3F1
mice pretreated with piperonyl butoxide (PIB), a P-450 inhibitor, were
examined for the extent of hepatic DNA damage produced 4 h after
1,2-dichloroethane administration. Hepatic DNA damage, as measured by
alkali-labile lesions, was potentiated by PIB. Treatment of mice with
high doses of 2-chloroethanol failed to produce DNA damage, as
measured by this assay. Diethylmaleate, a GSH depletor, potentiated
the hepatotoxicity of 2-chloroethanol but not DNA damage.
In addition, Cheever et al. (1990) reported that although the
levels of hepatic DNA covalent binding of metabolites of
14C-1,2-dichloroethane injected (single dose) to rats which had been
exposed by inhalation to 1,2-dichloroethane in a long-term bioassay
were significant (p < 0.05), these levels were not different in rats
with concomitant exposure to disulfiram in the diet over two years.
Evidence suggests that the putative episulfonium ion, resulting
from non-enzymatic conversion of S-(2-chloroethyl) glutathione, is a
major intermediate in the formation of DNA adducts in vivo from
1,2-dichloroethane exposure (Inskeep et al., 1986). When rats were
administered single does of 14C-1,2-dichloroethane in vivo and the
liver was analysed 8 h later, 78% of the DNA adducts (0.25 nmol/mg
DNA) could be released by neutral thermal hydrolysis. A major adduct
and several minor adducts were present; the major adduct
co-chromatographed with S-[2-(N7-guanyl)ethyl] glutathione. The
postulated adduct of liver DNA after 14C-1,2-dichloroethane
exposure, S-[2-(N7-guanyl)ethyl] glutathione, appears to be
chromatographically identical to the major adduct in rats after
exposure to 1,2-dibromoethane (Koga et al., 1986). This
1,2-dibromoethane adduct, which has been isolated and characterized by
NMR and mass spectrometry, gives strong support to an identical adduct
being the principal DNA adduct from exposure to 1,2-dihaloethanes.
Reitz et al. (1982) found (based on consideration of results of
their own work as well as that of Spreafico et al., 1980) that
metabolism of 1,2-dichloroethane appears to be saturated or limited in
rats at levels of exposure resulting in blood concentrations of 5 to
10 mg/litre, based on an observed non-linear relationship between
levels in blood and administered doses or concentrations.
Administration by gavage resulted in the formation of about twice the
amount of "total" metabolites as did exposure by inhalation, based on
recovery in excreta, expired air and the carcass. Oral exposure
produced 1.5- to 2-fold lower levels of total macromolecular binding
but 3- to 5-fold higher levels of DNA alkylation than inhalation,
though the absolute levels of DNA alkylation were considered low.
Based on examination of DNA binding in the liver and lung of rats
exposed by inhalation to a low constant concentration (0.3 mg/litre)
of 1,2-dichloroethane for 12 h or to a peak concentration (up to
18 mg/litre) for a few minutes, Baertsch et al. (1991) concluded that
DNA damage by 1,2-dichloroethane depends upon the concentration time
profile, with bolus doses causing disproportionately greater damage.
6.4 Elimination and excretion
Unmetabolized 1,2-dichloroethane is eliminated in expired air,
while its metabolites are largely excreted in the urine. Unchanged
1,2-dichloroethane was detected in the exhaled breath of women exposed
dermally and to airborne concentrations of 0.252 mg/m3 (0.063 ppm)
in the workplace; the amount of 1,2-dichloroethane expired was greater
immediately following exposure and decreased over time (Urusova,
1953).
A single dose of 150 mg/kg body weight radiolabelled
1,2-dichloroethane was injected into rats that had been exposed via
inhalation at a concentration of 200 mg/m3 (50 ppm) for 2 years. The
proportion of radioactivity present in the urine within 24 h was 42.5
and 33.9% (in males and females, respectively), while 27.3 and 40.3%
were eliminated as the unchanged parent compound in the breath. Only a
very small amount of radioactivity was detected as 14CO2 or in the
faeces. In rats that had been concomitantly exposed to disulfiram
during the 2-year period, the proportion of unchanged
1,2-dichloroethane eliminated in the breath increased significantly
(i.e. 57.6 and 57.7%; p < 0.05), while the proportion eliminated in
the urine decreased correspondingly (27.6 and 24.9%). Levels of
unchanged 1,2-dichloroethane in blood were significantly (p < 0.05)
increased in rats exposed to 1,2-dichloroethane and disulfiram
compared to those exposed to 1,2-dichloroethane alone (see section
7.10) (Cheever et al., 1990).
The pattern of elimination of metabolites was similar in rats and
mice 48 h after administration of oral doses of radiolabelled
1,2-dichloroethane (100 and 150 mg/kg body weight, respectively). In
rats, 8.2 and 69.51% of the radiolabelled dose was recovered as CO2
and in the excreta (principally urine), respectively, compared to
18.21 and 81.11% in mice. The overall recovery was less in rats than
in mice (96.26 versus 110.12%) (Mitoma et al., 1985).
In rats exposed to 600 mg/m3 (150 ppm) 1,2-dichloroethane for
6 h or administered 150 mg/kg body weight by gavage, there was no
significant difference in the route of excretion of non-volatile
metabolites. After 48 h, in each case, more than 84% of total
metabolites was eliminated in the urine, 7-8% was excreted as carbon
dioxide in expired air, 2% was excreted unchanged in the faeces, and
4% remained in the carcass (Reitz et al., 1982). The major urinary
metabolites identified following exposure of rats by either route were
thiodiacetic acid (70%) and thiodiacetic acid sulfoxide (26 to 28%).
The rate of elimination following oral (gavage) administration or
inhalation was such that 1,2-dichloroethane was not detected in the
blood a few hours after exposure and only small amounts were detected
in tissues (liver, kidney, lung, spleen, forestomach, stomach and
carcass) 48 h after exposure (Reitz et al., 1982). The rate of
elimination from blood and tissues appeared to depend on the exposure
level; the higher the exposure level, the lower the elimination rate
of 1,2-dichloroethane, after both oral and inhalation exposure.
Elimination from the liver was reported to be biphasic, a higher
elimination rate occurring just after the peak levels of
1,2-dichloroethane were reached. Elimination from other organs was
monophasic. Following inhalation up to an exposure level of
1012 mg/m3, elimination was slowest in adipose tissue and most rapid
in the lung (Spreafico et al., 1980).
Withey & Collins (1980) also reported that the elimination of
1,2-dichloroethane was dose-dependent. After intravenous
administration of from 3 to 15 mg/kg body weight to male Wistar rats,
the authors found that the elimination fitted a twocompartment model
at a low dose level and a three-compartment model at high dose levels.
The percentage of administered radioactivity excreted in the
urine over a 24-h period in rats decreased with increasing single
doses (0.25 to 8.08 mmol 1,2-dichloroethane/kg body weight)
administered by gavage in mineral oil (Payan et al., 1993). The
authors attributed these results to saturation of metabolism rather
than kidney damage, as there were no variations in biochemical
parameters of nephrotoxicity between the controls and groups exposed
to doses up to 4.04 mmol/kg body weight. Urinary thiodiglycolic acid
increased as a linear function of the dose of 1,2-dichloroethane until
at least 1.01 mmol/kg body weight; it accounted for 63% of the total
metabolites in urine at this dose.
6.5 Retention and bioaccumulation
Although 1,2-dichloroethane is eliminated more slowly from
adipose tissue than from blood or other tissues (lung and liver)
following exposure, it is unlikely to bioaccumulate significantly, as
no difference was observed between levels in blood or tissues (data
not presented) following single or repeated (10 days) oral doses of
50 mg/kg body weight in rats (Spreafico et al., 1980). Only 71 and
75%, respectively, of an administered oral dose of radiolabelled
1,2-dichloroethane was recovered in the excreta and exhaled breath of
rats administered 150 mg/kg body weight by gavage following 2 years of
exposure via inhalation (200 mg/m3 or 50 ppm); the authors
speculated that the remainder may have been sequestered in the body
fat. Recovery in the excreta and exhaled breath was complete in
younger rats (4 months old) receiving the same oral dose (Cheever et
al., 1990).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Data on the acute toxicity of 1,2-dichloroethane in experimental
animals are summarized in Table 3. These data indicate that
1,2-dichloroethane is of relatively low acute toxicity.
LC50 values in rats exposed to 1,2-dichloroethane for 6 or
7.25 h ranged from 4000 mg/m3 (1000 ppm) (Spencer et al., 1951) to
6600 mg/m3 (1650 ppm) (Bonnet et al., 1980). The 6-h LC50 in mice
was 1050 mg/m3 (Gradiski et al., 1978). LC50 values decreased
with increasing duration of exposure in rats exposed to concentrations
ranging from 1200 to 80 000 mg/m3 (300 to 20 000 ppm)
1,2-dichloroethane for 1 to 8 h (Spencer et al., 1951). Various
non-lethal effects have been reported in animals following acute
exposure to 1,2-dichloroethane, including central nervous system
depression, cardiovascular collapse, altered behaviour, pulmonary
congestion and oedema, histological damage in the liver, kidneys and
adrenal glands and myocardial failure, at concentrations ranging from
4000 mg/m3 for 1.5 or 4 h to 80 000 mg/m3 (20 000 ppm) for 30 min
(Heppel et al., 1945; Spencer et al., 1951; Alumot et al., 1976a;
Wolff et al., 1979; ATSDR, 1989). Central nervous system depression
occurred at much higher concentrations than those which induce effects
in other organs.
Oral LD50 values for rats, mice, dogs and rabbits ranged from
413 mg/kg body weight in female mice to 2500 mg/kg body weight in dogs
(Barsoum & Saad, 1934; McCollister et al., 1956; Smyth, 1969; Larionov
& Kokarovtseva, 1976; Munson et al., 1982; NIOSH, 1994). Non-lethal
effects observed in rats and rabbits following single oral doses of
1,2-dichloroethane ranging from 615 to 1476 mg/kg body weight include
hepatic effects (fatty degeneration, cloudy swelling, congestion,
haemorrhagic lesions, dystrophy in the cytoplasm and hyperchromatosis
in the nuclei of hepatocytes), degeneration of the renal tubular
epithelium, altered levels of enzymes in the serum and liver, oedema
and haemorrhaging in the walls of the coronary vessels, stasis and
thrombi in the myocardium, altered fibrinolytic activity in the blood,
and altered haematological parameters. A single dose of 0.5 ml
altered the ratio of the oxidized and reduced forms of nicotinamide
coenzymes in the liver and myocardium of rats (Natsyuk & Chekman,
1975). Electrocardiographic changes were reported at doses of 1, 1.5
and 2 mg/kg body weight, although these effects have not been
confirmed in other studies (Saitanov & Arsenieva, 1969).
The LD50 for dermal exposure in rabbits was estimated to be
between 2.8 and 4.9 g/kg (Torkelson & Rowe, 1981; NIOSH,
1994).
Table 3. Acute toxicity of 1,2-dichloroethane in experimental animals
Species Numbers/sex Duration/vehicle LC50 or LD50 Reference
Inhalation
Rats (Wistar equal no. of m & f) 10-54 0.53 h 48 000 mg/m3 (12 000 ppm) Spencer et al. (1951)
20-51 2.75 h 12 000 mg/m3 (3000 ppm)
31-32 7.20 h 4000 mg/m3 (1000 ppm)
Rats (albino, strain, number and sex not not specified 30 000 mg/m3 Nevrotsky et al. (1971)
specified)
Rats (Sprague-Dawley, 12 per group, sex not 6 h 6600 mg/m3 (1646 ppm) Bonnet et al. (1980)
specified)
Mice (OF1, 20 f per group) 6 h 1050 mg/m3 (262 ppm) Gradiski et al. (1978)
Ingestion
Rats (strain, number and sex not specified) not specified 850 mg/kg body weight Larionov &
Kokarovtseva (1976)
Rats (6 per group, strain and sex not not specified 770 mg/kg body weight Smyth (1969)
specified)
Rats (young adult albino, 80 m & f) corn oil 680 mg/kg body weight McCollister et al. (1956)
Mice, 6-week old (CD-1, number not male water 489 mg/kg body weight Munson et al. (1982)
specified) female 413 mg/kg body weight
Table 3. (cont'd).
Species Numbers/sex Duration/vehicle LC50 or LD50 Reference
Dogs (strain, number and sex not specified) acacia gum 2500 mg/kg body weight Barsoum & Saad (1934)
Rabbits (strain, number and sex not not specified 860 mg/kg body weight NIOSH (1994)
specified)
Dermal
Rabbits (strain, number and sex not not specified 2800 mg/kg body weight NIOSH (1994)
specified)
Rabbits (strain, number and sex not olive oil; duration 2800-4900 mg/kg body weight Torkelson & Rowe
specified) and area of skin (1981)
exposed not
specified
7.2 Skin and eye irritation
When 1.0 ml undiluted 1,2-dichloroethane was applied directly to
the clipped skin of guinea-pigs for up to 12 h in occluded patch
tests, no gross skin reactions were visible (Jakobson et al., 1982).
Microscopic changes appeared 4 h after application, comprising
karyopyknosis, perinuclear oedema, spongiosis and junctional
separation (Kronevi et al., 1981). In Draize tests on rabbits,
moderate erythema and oedema were observed 24 h after
application (dose not specified). Microscopy on the third day
revealed necrosis and other lesions such as ulcerations and
acanthosis. The severity of the changes was not indicated (Duprat et
al., 1976).
Instillation of 0.1 ml undiluted 1,2-dichloroethane into the
conjunctival sac of the eye of rabbits generated reversible, mild
irritation characterized by conjunctivitis and epithelial abrasion.
Epithelial keratitis, described as being "in a state of repair", was
observed microscopically 7 days after application (Duprat et al.,
1976). Reversible clouding of the cornea was observed in dogs within
10 h of subcutaneous administration of undiluted 1,2-dichloroethane at
0.9 mg/kg body weight. The clouding continued up to 48 h, but the
corneas appeared clear after 5 days. Histological changes, including
necrosis of the corneal endothelium, partially denuded Descemet's
membrane, formation of excess basement membrane, and swelling of the
corneal stroma, were also observed in dogs, cats and rabbits after
ocular injection of 1.8 mg 1,2-dichloroethane (0.15 ml of a 1%
solution) into the anterior chamber (Kuwabara et al., 1968).
7.3 Short-term exposure
Small groups of Wistar rats, rabbits, guinea-pigs, dogs and pigs
(n = 1 to 21) were exposed to 6000 mg/m3 (1500 ppm)
1,2-dichloroethane, 7 h/day for 6 days. Sections of the liver, heart,
lungs, kidney adrenal glands and spleen were examined microscopically.
In most animals, degeneration or necrosis of the kidney and liver,
along with congestion and haemorrhage of the lungs and adrenal glands,
were observed (Heppel et al., 1945).
No significant changes in organ or body weights, histology or
clinical chemistry and haematological parameters were observed in rats
administered 1,2-dichloroethane doses of up to 150 mg/kg body weight
per day in corn oil by gavage, 5 times/week for 2 weeks (van Esch et
al., 1977; Reitz et al., 1982).
7.4 Subchronic exposure
7.4.1 Inhalation
The subchronic toxicity of inhaled 1,2-dichloroethane was
investigated in three early limited studies in multiple species, as
presented in Table 4. Heppel et al. (1946) exposed groups of rats,
mice, rabbits, guinea-pigs, dogs, cats and monkeys to 4000 mg/m3
(1000 ppm) for up to 66 days. Mice, rats, rabbits and guinea-pigs
were the most sensitive species, based on mortality after only one or
a few exposures. Various effects were noted in these animals,
including pulmonary congestion (guinea-pigs, cats and rats), fatty
changes in the kidney (rats and monkeys), fatty changes in the
livera (cats, dogs and monkeys) and clouded corneas (dogs). At
1600 mg/m3 (400 ppm), observed effects included fatty degeneration
of the liver, kidney or heart (guinea-pigs and one rat), fatty changes
in the liver (dogs) and pulmonary congestion (rats), while at
800 mg/m3 (200 ppm), rats and guinea-pigs had mild pulmonary
congestion and one rat had fatty degeneration in the kidneys. No
effects on growth were noted in mice and rats exposed to 400 mg/m3
(100 ppm).
In a similar study, rats, guinea-pigs, rabbits and monkeys were
exposed to 400, 800 or 1600 mg/m3 (100, 200 or 400 ppm)
1,2-dichloroethane for 6 to 9 months (Spencer et al., 1951). Severe
effects, including hepatotoxicity, and deaths were observed in rats
and guinea-pigs exposed at the highest level, while monkeys also
showed degeneration of the liver and kidneys at this concentration.
No effects were noted in rabbits. At 800 mg/m3 (200 ppm) no adverse
effects were observed in rats, but slight degeneration of the liver
was noted in guinea-pigs. At 400 mg/m3 (100 ppm), no adverse
effects were observed in any of the four species. The authors
considered the "maximum concentrations without adverse effects" to be
1600 mg/m3 (400 ppm) in the rabbit, 800 mg/m3 (200 ppm) in the
rat, and 400 mg/m3 (100 ppm) in the monkey and guinea-pig, based on
a limited range of end-points.
a It has been suggested, on the basis of in vitro investigations,
that fatty accumulation in the liver may be due to the ability of
1,2-dichloroethane to block the secretion of hepatocellular
(Cotalasso et al., 1994).
Table 4. Subchronic toxicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats (26, strain and animals were exposed to 0 or There was high mortality in exposed rats (20/26), rabbits (5/6) and Heppel
sex not specified) 4000 mg/m3 (0 or 1000 ppm), guinea-pigs (36/41) after a few exposures. All mice died after one et al.
Mice (22, strain and 7 h/day, 5 days/week for up exposure. Survival was higher among cats and dogs (4/6 of either (1946)
sex not specified) to 66 exposures; sections of species survived more than 23 exposures). One monkey died after 2
Rabbits (5 m & 1 f, liver, heart, lungs, kidney, days and the other after 32 exposures. Pulmonary congestion was
strain not specified) adrenal glands and spleen noted in guinea-pigs, cats and rats. Rats and monkeys had fatty
Guinea-pigs (10-16, strain were examined changes in the kidney. Cats and monkeys had fatty changes in the
and sex not specified) microscopically; haematological liver. Dogs had cloudy corneas; one dog had fatty degeneration of
Dogs (3 f, strain not and urinary parameters were the liver. No effects on haematological or urinary parameters were
specified) assessed in dogs observed in dogs. Rabbits had no obvious effects on postmortem.
Cats (6 f, strain not
specified)
Monkeys (Rhesus, 2,
sex not specified)
Rats (15 m & 1 f, animals were exposed to 0 or All dogs and puppies survived 177 exposures. All rabbits died, after Heppel
strain not specified) 1600 mg/m3 (0 or 400 ppm), 1 to 97 exposures; 14/20 and 9/16 guinea-pigs and rats died by the et al.
Rabbits (2 m & 3 f, 7 h/day, 5 days/week for up to 60th exposure. Rats had pulmonary congestion and 1 rat and 6 (1946)
strain not specified) 177 exposures; sections of guinea-pigs had fatty degeneration of the liver, kidney and heart.
Guinea-pigs (8-10 m & liver, heart, lungs, kidney, Dogs had slight fatty changes in the liver. No effects were noted in
2 f, strain not specified) adrenal glands and spleen were rabbits on postmortem. There were no significant differences in
Dogs (6 f, strain not examined microscopically; haematological parameters in exposed dogs and rabbits compared
specified) and puppies haematological parameters to controls.
(3 m, strain not were also assessed in dogs
specified) and rabbits
Table 4. (cont'd).
Species Protocol Results Reference
Rats (Wistar 1 m & 11 f) animals were exposed to 0 or 5/14 guinea-pigs, 7/12 rats and 8/12 rats died after 1 to 115 Heppel
Osborne-Mendel rats (12 m) 800 mg/m3 (0 or 200 ppm), 7 h/ exposures. Rats and guinea-pigs had mild pulmonary congestion, et al.
Rabbits (5 m, strain day, 5 days/week, for up to and 1 rat had fatty degeneration in the kidneys. There were no (1946)
not specified) 125 exposures; sections of significant differences in haematological parameters in exposed
Guinea-pigs (12 m & 2 f, liver, heart, lungs, kidney, rats and rabbits, compared to controls.
strain not specified) adrenal glands and spleen
Monkeys (2 m, strain were examined microscopically;
not specified) haematological parameters were
assessed in rats and rabbits
Rats (23 m & 16 f, animals were exposed to 0 or All animals survived. There were no differences in the rate of Heppel
strain not specified) 400 mg/m3 (0 or 100 ppm), 7 h/ growth in rats or mice. et al.
Mice (19, strain and day, 5 days/week for 74 (rats) (1946)
sex not specified) and 19 (mice) exposures;
sections of liver, heart,
lungs, kidney, adrenal glands
and spleen were examined
microscopically
Rats (Wistar, 15-20 m & animals were exposed to 0, 400, 1600 mg/m3: All female rats and male guinea-pigs died by 10 Spencer
15-20 f) 800 or 1600 mg/m3 (0, 100, 200, exposures; all male rats died by 40 exposures. All female et al.
Guinea-pigs (2-8 m & or 400 ppm), 7 h/day, 5 days/ guinea-pigs died by 24 exposures. Rats and guinea-pigs had rapid body (1951)
8 m, strain not specified) week for 6 to 9 months; animals weight loss, slight increases in liver and kidney weights, and
Rabbits (albino, number were killed at various times some fatty changes in the liver. Guinea-pigs had swelling of the
and sex not specified) throughout the study; tubular epithelium of the kidneys and alterations in levels of
Monkeys (Rhesus, number haematological parameters were non-protein nitrogen urea nitrogen in the blood. Monkeys had
and sex not specified) assessed and histopathological degeneration of the liver and kidneys and increased fat content
examinations of several tissues of the liver. No effects were noted in rabbits.
were conducted
Table 4. (cont'd).
Species Protocol Results Reference
800 mg/m3: Guinea-pigs tolerated up to 180 exposures (246 days).
Male guinea-pigs had significantly (p=0.001) decreased body
weight gain; both sexes of guinea-pigs had slight degeneration
and fatty accumulation in the liver. Rats tolerated up to 151
exposures (212 days) with no observed adverse effects.
400 mg/m3: No effects were noted in rats, guinea-pigs, rabbits
or monkeys.
Rats (Sprague-Dawley, were exposed to 400 At the higher concentration, rats were dyspnoeic and guinea-pigs Hofman
10, sex not specified) or 2000 mg/m3 (100 or 500 were "apathetic". 3/4 rabbits died after 10-17 exposures; 9/10 et al.
Guinea-pigs (10, strain ppm), 6 h/day, 5 days/week guinea-pigs died after 4-14 exposures. Rats died after 1-5 exposures, (1971)
and sex not specified) for up to 17 weeks; while all cats survived 30 exposures. Rats had pulmonary hyperaemia
Rabbits (4, strain and histological examinations were and oedema, fatty liver and adrenal and myocardial necrosis. Cats and
sex not specified) conducted, and several rabbits had heart lesions, and guinea-pigs had fatty changes in the
Cats (2 per group, strain bio-chemical parameters were myocardium, liver and adrenals, and necrosis in the myocardium and
and sex not specified) assessed liver. At 400 mg/m3, there were no clinical or histological
changes after exposure for 17 weeks.
Table 4. (cont'd).
Species Protocol Results Reference
Ingestion
Rats F344/N, animals were administered 0, Drinking-water: F344/N rats: Body weight was significantly decreased NTP
Sprague-Dawley and 500, 1000, 2000, 4000 and in males at 4000 and 8000 mg/litre (>8%, p<0.001). Relative kidney (1991)
Osborne Mendel, mice, 8000 mg/litre in the weight was significantly increased at >1000 mg/litre in both sexes
B6C3F1 (10 or 20 m & f drinking-water (equivalent to (>11%, p<0.001). Relative liver weight was significantly increased at
per group) doses of of 49-82, 86-126, >2000 mg/litre in males (>14%, p<0.05) and 4000 mg/litre in females
146-213, 259-428, and (>9%, p<0.001). Alterations in haematological and serum parameters
492-727 mg/kg body weight per at the higher concentrations reflected dehydration caused by
day in rats and 244-249, decreased water consumption. Renal tubular "regeneration" observed
448-647, 781-1182, 2478-2710 in all groups of males at similar frequencies and severity, but
and 4207-4926 mg/kg body frequency was related to dose in females (0/10, 0/10, 1/10, 2/10
weight per day in mice) for 3/10 and 9/10 with increasing concentration).
13 weeks
Rats F344/N (10 or rats were administered 0, 30, Sprague-Dawley rats: Body weight was significantly decreased in
20 m & f per group) 60, 120, 240 or 480 mg/kg body males at 8000 ppm (9%, p<0.05). Relative kidney weight was
per day (males) or 0, 18, 37, significantly increased at >1000 mg/litre in males (>7%, p<0.05)
75, 150 or 300 mg/kg body and at >500 mg/litre in females (>8%, p<0.05). Relative liver
weight per day (females) in weight was increased in males at >500 mg/litre (>6%, p<0.05)
corn oil by gavage, and at 8000 mg/litre in females (13%, p<0.001). Alterations in
5 days/week for 13 weeks haematological and serum parameters at the higher
concentrations reflected dehydration caused by decreased water
consumption. Renal tubular "regeneration" was observed in all
groups at similar frequencies and severity.
Table 4. (cont'd).
Species Protocol Results Reference
haematological and serum Osborne-Mendel rats: Body weight was significantly decreased
chemistry parameters were at 8000 mg/litre in males (15%, p<0.05). Relative kidney weight
examined at several times was increased at 4000 and 8000 mg/litre in males (>8%, p<0.05)
during the course of the study. and >500 mg/litre in females (>12%, p<0.001). Relative liver weight
Extensive histopathological was increased in males at 1000 and 2000 mg/litre (>14%, p<0.05).
examinations were conducted Alterations in haematological and serum parameters at the higher
for controls and all animals concentrations reflected dehydration caused by decreased water
at the highest concentration consumption. Although the incidence of renal tubular "regeneration"
in drinking-water and in female was increased at the higher concentrations, the increases were
mice at 4000 ppm, as well as in not related to dose, and severity was similar in all groups.
male rats receiving doses of
120 or 240 mg/kg body weight B6C3F1 mice: 9/10 females exposed to 8000 mg/litre died. Body
per day by gavage and in weight was significantly decreased in males at 8000 mg/litre
female rats at 150 mg/kg body (17.5%, p<0.001). Relative kidney weight was significantly increased
weight per day in males at >1000 mg/litre (>12%, p<0.001) and in females at >500
mg/litre (>16%, p<0.001). Relative liver weight was significantly
increased in males at 500 mg/litre and above and in females at 1000
mg/litre or more. The incidence of renal tubular cell regeneration
was significantly increased in males at 4000 and 8000 mg/litre;
karyomegaly was observed in all males at 8000 mg/litre.
Gavage: F344/N rats: All males receiving 240 or 480 mg/kg body
weight per day and 9/10 female receiving 300 mg/kg body weight per
day died before the end of the study. The incidence of hyperplasia
and inflammation of the forestomach was significantly increased in
males at 240 mg/kg body weight per day; necrosis of the thymus was
observed in 10/10 males at 480 mg/kg body weight per day and 5/10
females at 300 mg/kg body weight per day, compared to none
in controls.
Table 4. (cont'd).
Species Protocol Results Reference
Rats (Osborne-Mendel, animals were administered At the highest dose, three males and one female died. In males, NCI
5 m & 5 f per group) doses of 0, 40, 63, 100, 159 or mean body weight was only significantly decreased (by 50%) at (1978)
251 mg/kg body weight per day 251 mg/kg body weight per day. In females mean body weight was
in corn oil by gavage, 5 days depressed (10% at 40 mg/kg body weight per day to 17% at 100
week for 6 weeks followed by a mg/kg body weight per day and 32% at 159 mg/kg body weight
2-week observation period per day). No other parameters were investigated.
Mice (B6C3F1 5 m & animals were administered All males receiving 398 mg/kg body weight per day and all females NCI
5 f per group) doses of 0, 159, 251, 398, receiving 631 mg/kg body weight per day died. Mean body weight (1978)
631 or 1000 mg/kg body weight depression was only observed in females receiving 398 mg/kg body
per day in corn oil by weight per day, in which "drastic weight loss" was reported. No
gavage, 5 days/week for other parameters were investigated.
6 weeks followed by a 2-week
observation period
Rats (6 per group, rats were fed mash fumigated The only effect noted, based on examination of lipid content Alumot
strain and sex not with 1,2-dichloroethane, of the liver and liver weight, was an increase in liver fat at et al.
specified) resulting in initial 1600 mg/kg. Chronic respiratory disease was evident in all exposure (1976a)
concentrations of 200 or groups (incidence data not presented, but the disease was not
600 mg/kg (approximately believed to be associated with exposure); mortality was higher in
equivalent to doses of 10 and males than females, with the number of rats surviving after 21
30 mg/kg body weight per day, months ranging from 3 to 14.
respectively) for 5 weeks, or
1600 mg/kg (approximately
equivalent to a dose of
80 mg/kg body weight per day)
for 7 weeks; the authors
noted that the dose ingested
was approximately 60 to 70% of
the initial amount after loss
Table 4. (cont'd).
Species Protocol Results Reference
to volatilization was
considered; lipid content of
liver and liver weight were
measured, and hepatic
triglycerides were measured at
the highest dose; no
histopathological examinations
were conducted
Rats (m & f, strain and rats were administered doses Decreased weight gain was observed at the two highest doses. Van Esch
number not specified of 0, 10, 30 or 90 mg/kg body Males and females had increased relative kidney weight at 90 et al.
in secondary account) weight per day (method of oral mg/kg body weight per day. Females also had increased relative (1977)
administration not specified weights of liver and brain at this dose. No effects on histology
in secondary account), 5 times or clinical chemistry were noted. Alterations in some
per week for 90 days; haematological parameters observed, although these did not occur
histopathological examinations in a dose-related manner.
were conducted, along with
assessment of haematological
and clinical chemistry
parameters, although the
extent of the examinations
was not specified in the
secondary account
Rats (m, strain and rats were orally There was a statistically significant (p < 0.02) increase in the Apostolov
number not specified) administered doses activity of the serum lysosome enzyme, &
equivalent to 1/5000, 1/1000 beta-N-acetylglucosa-minidase at the two highest doses. Mihaylova
or 1/200 the LD50 (not further (1975)
specified) for 3 months (abstract
only)
Hofmann et al. (1971) exposed rats, guinea-pigs, rabbits and cats
to 400 or 2000 mg/m3 (100 or 500 ppm) for up to 17 weeks. Mortality
was high in rats, guinea-pigs and rabbits exposed to the higher
concentration. At 2000 mg/m3 (500 ppm) pulmonary hyperaemia and
oedema, fatty liver and adrenal and myocardial necrosis were noted in
rats, while heart lesions were observed in cats and rabbits.
Guinea-pigs had fatty changes in the myocardium, liver and adrenals
and necrosis in the myocardium and liver at the higher concentration.
No clinical or histological effects were noted at 400 mg/m3
(100 ppm).
7.4.2 Ingestion
Available data on the subchronic toxicity of ingested
1,2-dichloroethane are presented in Table 4. In a recent study
conducted by the National Toxicology Program (NTP, 1991) and partially
reported by Morgan et al. (1990), the relative susceptibility of three
strains of rats (F344/N, Sprague-Dawley and Osborne-Mendel) and one
strain of mice (B6C3F1) exposed to 1,2-dichloroethane in
drinking-water at concentrations of up to 8000 mg/litre for 13 weeks,
and one of the same strains of rats (F344/N) exposed to doses of up to
480 mg/kg body weight per day by gavage in corn oil for 13 weeks, was
investigated. Based on increased relative organ weights, the liver
and kidneys were the target organs in both rats and mice, although
treatment-related microscopic lesions were noted only in female F344/N
rats and male B6C3F1 mice. Administration of 1,2-dichloroethane to
F344/N rats by gavage resulted in more severe toxic effects (including
death) than administration of similar doses in drinking-water,
probably due to greater peak levels of the compound in the blood and
saturation of elimination mechanisms. The authors considered the
no-observed-effect levels (NOEL) for 1,2-dichloroethane administered
to F344/N rats by gavage to be 120 and 150 mg/kg body weight per day
in males and females, respectively, based on mortality and chemically
related lesions in the forestomach. The NOEL of B6C3F1 mice exposed
via drinking-water was considered to be 780 mg/kg body weight per day)
(2000 ppm) in males, based on kidney lesions, and (2500 mg/kg body
weight per day) (4000 ppm) in females, based on mortality. The
authors did not consider the doses to which the three strains of rats
were exposed in the drinking-water to be high enough to result in
biologically significant toxic effect, although increases in organ
weights without accompanying histopathological alterations were
observed at doses as low as 49 to 82 mg/kg body weight per day in some
strains (i.e. Sprague-Dawley and Osborne-Mendel).
In limited subchronic studies preliminary to long-term
carcinogenesis bioassays, groups of Osborne-Mendel rats and B6C3F1
mice were administered doses of up to 251 and 1000 mg/kg body weight
per day, respectively, by gavage for 6 weeks. Significant mean body
weight loss was noted in male rats at 251 mg/kg body weight per day
and in female rats at > 40 mg/kg body weight per day. In mice,
mean body weight was decreased only in females receiving 398 mg/kg
body weight per day. No other parameters were investigated in this
study (NCI, 1978).
Decreased body weight gain was observed in rats orally
administered doses of 30 or 90 mg/kg body weight per day for 90 days,
but not in those receiving 10 mg/kg body weight per day. Increased
relative weights of kidneys (both sexes), liver and brain (females
only) were noted at the highest dose, although no histopathological
effects or alterations in clinical chemistry parameters were noted.
Changes in some haematological parameters were noted, but these were
not related to dose (Van Esch et al., 1977).
A slight increase in the fat content of the livers was reported
in rats consuming feed which had been fumigated with
1,2-dichloroethane, resulting in an initial concentration of
1600 mg/kg (approximately equivalent to a dose of 80 mg/kg body weight
per day) for 7 weeks, while no effects were observed at 600 mg/kg
(approximately equivalent to a dose of 30 mg/kg body weight per day)
after 5 weeks (Alumot et al., 1976a). The parameters examined were
limited to hepatic lipid content and liver weight. The activity of
the serum lysosome enzyme, ß- N-acetylglucosaminidase, was
significantly increased in rats administered oral doses equivalent to
1/1000 and 1/200 the LD50 (not further specified in abstract)
(Apostolov & Mihaylova, 1975).
7.5 Chronic exposure and carcinogenicity
7.5.1 Inhalation
Groups of 90 Sprague-Dawley rats of each sex were exposed by
inhalation to 1,2-dichloroethane (99.92% pure) at concentrations of 0,
20, 40, 202 and 1012 mg/m3 (0, 5, 10, 50 and 250 ppm), 7 h/day, 5
days/week for 78 weeks, and observed until spontaneous death (due to
severe toxicity, the highest concentration was reduced to 607 mg/m3
or 150 ppm after several days). "Incidence" was reported as the
number of animals developing specific tumours over the number of
animals alive at the time the first tumour of that type was detected
(i.e. incidences have not been adjusted for differential survival (see
Table 5)). The only tumour types for which the authors reported an
increase in incidence (when compared with controls kept in exposure
chambers, but not when compared with those not kept in chambers) were
fibromas and fibroadenomas (combined) of the mammary gland, which the
authors attributed to the differential survival among the groups
(Maltoni et al., 1980).
Groups of 50 male and 50 female Sprague-Dawley rats were exposed
to 0 or 200 mg/m3 (50 ppm) 1,2-dichloroethane 7 h/day, 5 days/week
for 2 years. No effects on body weight gain or mortality were noted.
There was no significant difference in the incidence of tumours at any
site, although there was a nonsignificant increase in the incidence of
mammary gland adenomas (4 in exposed group versus 2 in control group)
and fibroadenomas (21/50 in exposed group versus 15/50 in control
group) in females. There was an increased incidence of testicular
lesions (not further specified) in males (24% versus 10% in controls,
significance not reported) (Cheever et al., 1990). However, the
sensitivity of this investigation to detect any carcinogenic potential
may have been compromised (the study was designed to investigate the
interaction between 1,2-dichloroethane and other substances), based on
the lack of convincing evidence of compound-related toxicity at the
only concentration to which animals were exposed.
Clinical chemistry and haematological parameters were
investigated in groups of 8 to 10 male or female Sprague-Dawley rats
exposed to 0, 20, 40, 202 or 1012 mg/m3 (decreased to 607 mg/m3
after several days) (0, 5, 10, 50 or 250/150 ppm), 7 h/day, 5
days/week for 3, 6, and 18 months, beginning at 3 months of age. In
addition, groups of 8 to 10 rats were also exposed to these same
concentrations beginning at 14 months of age for 12 months. Although
values were occasionally significantly (p < 0.05) different from
those of controls, no consistent dose-related effects on various
haematological parameters, circulating protein levels or clinical
chemistry parameters were reported in rats exposed from 3 months of
age. In animals exposed for 12 months beginning at 14 months of age,
there were no consistent dose-related effects on haematological
parameters. There were significant (p < 0.05) changes in serum
parameters indicative of effects on liver and kidney function,
including levels of glutamic-pyruvic transaminase (SGPT),
gamma-glutamyltranspeptidase (gamma-GT), glutamic-oxalic transaminase
(SGOT) and cholesterol, and levels of uric acid in the blood and blood
urea nitrogen (BUN) at 202 and 607 mg/m3. Histopathological
examinations were not conducted (Spreafico et al., 1980).
No increase in the incidence of any type of tumour was reported
in groups of 90 male or female Swiss mice exposed to 20, 40, 202 or
1012 (decreased to 607 mg/m3 after a few days) mg/m3 (5, 10, 50 or
250/150 ppm), 7 h/day, 5 days/week for 78 weeks, and observed until
spontaneous death (Maltoni et al., 1980). However, it should be noted
that survival was poor (especially among males).
Table 5. Chronic toxicity and carcinogenicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats Rats were exposed to 0, 20, 40, 202 After several days of exposure to 1012 mg/m3, severe toxic effects, Maltoni
(Sprague-Dawley, and 1012/607 mg/m3 (0, 5, 10, 50 including death were observed and the level of exposure was reduced et al.
90 m & 90 f per and 250/150 ppm) 1,2-dichloroethane to 607 mg/m3. Survival varied among the groups, but was not related (1980)
group, 180 m & (99.92% pure), 7 h/day, 5 days/week to concentration; most rats died by week 140. Survival at 104 weeks
180 f controls) for 78 weeks and observed until of age in controls, chamber controls, and groups exposed to 20, 40,
spontaneous death. One group of 202 and 1012/607 mg/m3 was 17.8, 13.3, 50.0, 14.4, 18.9 and 11.1%
controls was kept in a nearby room, (males) and 40.0, 24.4, 53.3, 28.9, 32.2, and 23.3% (females).
while the other was kept in an "Incidence" was reported as the number of animals developing specific
exposure chamber under the same tumours over the number of animals alive at the time the first tumour
conditions as the exposed groups. of that type was detected (i.e. incidences have not been adjusted for
A complete autopsy was performed differential survival). With the exception of benign mammary tumours,
on each animal, regardless of time there were no significant increases in the incidence of any types of
of death. Several organs were (combined) tumours in exposed rats. The incidence of all mammary
routinely histopathologically tumours (number of animals alive at the time of appearance of the
examined, along with any organs first mammary tumour (12 weeks) was 90 in each exposure group) was
with pathological lesions. 52/90 (57.8%), 38/90 (42.2%), 65/90 (72.2%), 43/90 (47.8%), 58/90
(64.4%) and 52/90 (57.5%) in non-chamber controls, chamber controls,
and groups exposed to 20, 40, 202 and 1012/607 mg/m3, respectively.
The numbers (denominators not specified) of fibromas and
fibroadenomas (combined) of the mammary gland were 47, 27, 56, 33, 49
and 47 in non-chamber controls, chamber controls, and groups exposed
to 20, 40, 202 and 1012/607 mg/m3, respectively. The "incidence"
of these tumours at 20, 40, 202 and 1012/607 mg/m3, was
significantly (p<0.01 or 0.001) different from the incidence in
chamber controls. The "incidences" of benign mammary tumours in the
two control groups were also significantly (p<0.01) different.
Table 5. (cont'd).
Species Protocol Results Reference
Rats Rats were exposed to 0, 20, 40, 202 There were no consistent, exposure-related changes in various Spreafico
(Sprague-Dawley, or 1012-607 mg/m3 (0, 5, 10, 50 or haematological parameters, circulating protein levels or clinical et al.
8-10 or f per 250/150 ppm) 7 h/day, 5 days/week chemistry parameters in animals exposed from 3 months of age, (1980)
group) for 3, 6 or 18 months, beginning at although values occasionally differed significantly from controls.
3 months of age. In addition, groups In animals exposed for 12 months from 14 months of age, there were no
of rats were exposed to 0, 20, 40, consistent exposure-related alterations in haematological parameters.
202 or 1012-607 mg/m3 (0, 5, 10, 50 Levels of serum glutamic-pyruvic transaminase (SGPT) were
or 250/150 ppm) 7 h/day, 5 days/ significantly elevated in both males and females at 202 and 607 mg/m3
week for 12 months, beginning at 14 (p<0.05), and gamma-glutamil transpeptidase (gamma-GT) levels were
months of age. Histopathological also significantly greater in females at the two highest
examinations were not conducted. concentrations (p<0.05). Levels of serum glutamic-oxalic transaminase
(SGOT) were significantly increased in both sexes at 20 and 40 mg/m3
(p<0.05), but significantly decreased in males and females at 202 and
607 mg/m3 (p<0.05). Levels of cholesterol were significantly lower
in males and females at 202 and 607 mg/m3 (p<0.05). Levels of uric
acid in the blood were significantly higher in both sexes at 202 and
607 mg/m3 (p<0.05), while blood urea nitrogen (BUN) values were
significantly elevated at 607 mg/m3 (p<0.05), although there were
no effects on urinary parameters.
Rats Rats were exposed to 200 mg/m3 (0 There were no compound-related effects on body weight gain or Cheever
(Sprague-Dawley, or 50 ppm) 7 h/day, 5 days/week for mortality. There were no significant increases in the incidence of et al.
50 m & 50 f 2 years. Extensive histopathological any type of tumours, although there was a non-significant increase (1990)
per group) examinations were conducted. in the incidence of mammary gland adenomas (4 versus 2 in controls)
and fibroadenomas (21/50 versus 15/50) in females. The incidence of
testicular lesions (not further specified) was increased in exposed
animals (24% versus 10% in controls, significance not reported).
Table 5. (cont'd).
Species Protocol Results Reference
Mice (Swiss, Mice were exposed to 20, 40, 202 or Survival of mice was poor, especially in males, as only 43.4 to 65.6% Maltoni
90 m & 90 f 1012-607 mg/m3 (5, 10, 50 or 250/ of exposed males survived for 52 weeks after exposure commenced. et al.
per group, 150 ppm), 7 h/day, 5 days/week for There were no significant increases in the incidence of any type of (1980)
115 m & 134 f 78 weeks and observed until tumours.
controls) spontaneous death. Controls were
kept in a nearby room. A complete
autopsy was performed on each
animal, regardless of time of death.
Several organs were routinely
histopathologically examined, along
with any organs with pathological
lesions.
Ingestion
Rats Animals were administered time There were no effects on body weight gain in either sex. Mortality NCI
(Osborne-Mendel, weighted average doses of 47 or 95 was significantly higher in both males and females in the high dose (1978)
50 50 m & f per mg/kg body weight per day (initial group, as 50% of exposed rats had died by week 55 (males) and 57
group, 20 m & doses of 50 and 100 mg/kg body (females), compared to week 72 (males) and 88 (females) in controls.
20 f controls; weight per day were increased to 75 Signs of toxicity, including wheezing, nasal discharge, ulcerations,
60 m & 60 f and 150 mg/kg body weight per day localized alopecia, discoloured or stained fur, bloated appearance
pooled controls after 7 weeks then decreased to and swollen areas, occurred at a greater frequency in exposed
from concurrent original doses after 17 weeks) in animals than in controls. Chronic murine pneumonia was present in 60
experiments) corn oil by gavage, 5 days/week, for to 94% of rats in each group (incidence not related to dose).
78 weeks followed by 32 weeks of Acanthosis and hyperkeratosis of the forestomach was present in a
observation. Complete greater proportion of exposed females than controls (1/20, 6/50 and
histopathological examinations were 7/50 in vehicle controls, low and high dose, respectively,
conducted. significance not reported). Other non-neoplastic lesions occurred
at similar frequencies in control and exposed rats. The incidence
of squamous cell carcinomas of the forestomach in males was 0/60,
Table 5. (cont'd).
Species Protocol Results Reference
0/20, 3/50 and 9/50 in pooled controls, matched controls, low and
high dose groups, respectively, significant at the high dose
(p=0.01); only 2/50 females in the low dose group had this tumour.
There were also one leiomyosarcoma of the stomach and one
adenocarcinoma of the small intestine in high dose males (not
significant). The incidence of hemangiosarcomas (mostly in the
spleen) in males was 1/60, 0/20, 9/50 and 7/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively (significant in both exposed groups, p=0.03 (low) and
p=0.016 (high)), and in females was 0/59, 0/20, 4/50 and 4/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively (significant in both exposed groups (p=0.041 in
both)). Both groups of exposed males had an increased incidence of
fibromas of the subcutaneous tissue (0/60, 0/20, 5/50 and 6/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively); no such increase was noted in females. In
females, there was an increased incidence of adenocarcinomas and
fibroadenomas of the mammary gland (1/59, 0/20, 1/50 and 18/50
(adenocarcinoma), 5/59, 0/20, 14/50 and 8/50 (fibroadenoma) and 6/59,
0/20, 15/50 and 24/50 (adenocarcinoma or fibroadenoma)) in pooled
vehicle controls, matched vehicle controls, low and high dose groups,
respectively). Renal tubular cell adenocarcinomas were noted in one
male and one female at the highest dose, while tubular cell adenomas
were present in one male and two females at this dose, and none was
observed in controls (significance not reported).
Rats (18 m & Animals were fed mash fumigated Chronic respiratory disease was reported in all groups in the second Alumot
18 f per group, with 1,2-dichloroethane for 2 years. year of exposure. The number of rats surviving after 21 months ranged et al.
strain not Resulting concentrations were 250 from 2 to 14 per group. No effects on growth or the biochemical (1976a)
specified) and 600 mg/kg. Due to loss of the parameters investigated were observed.
Table 5. (cont'd).
Species Protocol Results Reference
compound through volatilization,
the mash actually consumed was
estimated to contain 60 to 70% of
the initial concentration (estimated
to result in doses of approximately
7.5 to 8.75 and 15 to 17.5 mg/kg
body weight per day). The liver was
analysed for total fat triglyceride
content. Levels of total protein,
albumin, glucose, urea, uric acid
and cholesterol in the serum were
determined. Histopathological
examinations do not appear to have
been conducted on surviving rats
at the end of the exposure period.
Mice (B6C3F1, Animals were administered Mortality in females was related to dose (36 animals in the high dose NCI
50 m & 50 f time-weighted average doses of 97 group died between weeks 60 and 80, which may have been related to (1978)
per group; 20 or 195 mg/kg body weight per day the appearance of tumours as 25 of these animals had tumours); no
m & 20 f (males) or 149 or 299 (females) in similar dose-related trend in mortality was observed in males. Body
controls; 60 m corn oil by gavage, 5 days/week, for weight in females in the high dose group was depressed as early as
& 60 f pooled 78 weeks followed by 13 weeks of week 15 (>10%). The incidence of non-neoplastic lesions was
controls from observation (initial doses in males comparable in exposed and control mice. There was an increased
concurrent of 75 and 150 mg/kg body weight incidence of hepatocellular carcinomas in male mice at the highest
experiments) per day were increased to 100 and dose (4/59, 1/19, 6/47 and 12/48 in pooled vehicle controls, matched
200 mg/kg body weight per day after vehicle controls, low and high dose groups, respectively), but not in
8 weeks; initial doses in females females. The incidence of alveolar/bronchiolar adenomas in males was
Table 5. (cont'd).
Species Protocol Results Reference
of 125 and 250 mg/kg body weight per 0/59, 0/19, 1/47 and 15/48 in pooled vehicle controls, matched
day were increased to 200 and vehicle controls, low and high dose groups, respectively
400 mg/kg body weight per day after (significant at the highest dose (p<0.001)); the incidence of this
8 weeks, then decreased to 150 and tumour in females was 2/60, 1/20, 7/50 and 15/48 in pooled vehicle
300 mg/kg body weight per day after controls, matched vehicle controls, low and high dose groups,
11 weeks). Complete respectively (significant in both exposed groups(p=0.046 (low) and
histopathological examinations were p<0.001 (high)). There was also one alveolar/bronchiolar carcinoma in
conducted. females at 299 mg/kg body weight per day. There was a non-significant
increase in the incidence of squamous cell carcinoma of the
forestomach in females at 299 mg/kg body weight per day (5/48 versus
1/60 or 1/20 in controls). There was a significantly increased
incidence of mammary gland adenocarcinomas in both groups of exposed
females (0/60, 0/20, 9/50 and 7/48 in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively
(p=0.001 (low) and p=0.003 (high)).
Uterine adenocarcinomas occurred in 3/49 low dose and 4/47 high
dose females, compared to none in controls; however, this increase
was not statistically significant. The incidence of endometrial
stromal polyp or endometrial stromal sarcoma (combined) was 0/60,
0/20, 5/49 and 5/47 in pooled vehicle controls, matched vehicle
controls, low and high dose groups, respectively (significant at
both doses, p=0.016 (low) and p=0.014 (high)).
Table 5. (cont'd).
Species Protocol Results Reference
Dermal
application
Swiss mice Doses of 0, 42 or 126 mg/application The incidence of benign lung papillomas was significantly (p<0.0005) van Duuren
(Ha:ICR, 30 f; per mouse in 0.2 ml acetone were increased at the higher dose (26/30 compared to 17/30, 11/30 and et al.
30 vehicle applied 3 times per week to the 30/100 in low dose group, vehicle controls and untreated controls, (1979)
controls and shaved dorsal skin (area of skin respectively). The incidence of stomach tumours was 3/30, 1/30, 2/30
100 naive exposed not specified) of mice for and 5/100 in high dose group, low dose group, vehicle controls and
controls) 440 to 594 days. The skin, liver, untreated controls, respectively (not significant).
kidney and any tissues or
organs appearing abnormal were
examined histopathologically.
7.5.2 Ingestion
In a study conducted by the National Cancer Institute (NCI,
1978), time-weighted average daily doses of 47 or 95 mg/kg body weight
per day of 1,2-dichloroethanea in corn oil were administered by
gavage 5 days/week for 78 weeks to 50 Osborne-Mendel rats of each sex,
followed by 32 weeks of observation. Mortality was significantly
(p < 0.001) higher in both males and females in the high dose group.
Clinical signs of general toxicity occurred at a greater frequency in
exposed animals than in controls. In each group 60-94% of rats had
chronic murine pneumonia, but the incidence was not related to dose.
The incidence of acanthosis and hyperkeratosis of the forestomach was
greater in exposed females than controls.
The incidence of a variety of tumours was increased in exposed
animals compared with controls. The incidence of squamous cell
carcinomas of the stomach was significantly increased in males (3/50
and 9/50 in low and high dose groups, respectively), compared to none
in either group of controls; in females, there were only 2/50 in the
low dose group. The incidence of haemangiosarcoma was significantly
increased in males (1/60, 0/20, 9/50 and 7/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively) and females (0/59, 0/20, 4/50 and 4/50 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). The incidence of fibromas of the subcutaneous tissue
was significantly increased in males (0/60, 0/20, 5/50 and 6/50 in
pooled vehicle controls, matched vehicle controls, low and high dose
groups, respectively), but not in females. There was a significant
increase in the incidence of adenocarcinomas and fibroadenomas of the
mammary gland in females (1/59, 0/20, 1/50 and 18/50 (adenocarcinoma),
5/59, 0/20, 14/50 and 8/50 (fibroadenoma) and 6/59, 0/20, 15/50 and
24/50 (adenocarcinoma or fibroadenoma)) in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively). It
was concluded that 1,2-dichloroethane was carcinogenic in this strain
of rats, under the conditions of this study.
No effects on growth or biochemical parameters were observed in a
limited study on rats fed mash that had been fumigated with
1,2-dichloroethane, which resulted in doses of approximately 7.5-8.75
and 15-17.5 mg/kg body weight per day. Chronic respiratory disease
was evident in all groups in the second year, the number of rats
surviving after 21 months ranging from 2 to 14 in each of the groups.
The occurrence of respiratory disease and mortality did not appear to
be exposure-related (Alumot et al., 1976a).
a Technical grade with reported purity of > 90% containing 11 minor
contaminants; subsequent analysis indicated a purity of about
98-99% (Hooper et al., 1980 and Ward, 1980).
The National Cancer Institute (NCI, 1978) also conducted a
bioassay in which groups of 50 B6C3F1 mice were administered
time-weighted average daily doses of 97 or 195 mg/kg body weight per
day (males) and 149 or 299 mg/kg body weight per day (females)
1,2-dichloroethane in corn oil by gavage, 5 days/week for 78 weeks,
followed by 13 weeks of observation. A doserelated increase in
mortality was noted in female mice, but not in males. Body weight was
also decreased in females at 299 mg/kg body weight per day.
As in rats, there was a significant increase in the incidence of
several types of tumours in exposed mice. The incidence of
hepatocellular carcinomas was significantly increased in males in the
high dose group (4/59, 1/19, 6/47 and 12/48 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively); no such increase was noted in females. However, the
authors noted that, due to the high variability of incidence of
hepatocellular neoplasms in historical controls (data not presented),
this increase, although statistically significant, was not considered
to be convincing evidence that these tumours were attributable to the
test chemical. The incidence of alveolar/bronchiolar adenomas was
significantly increased in males in the high dose group (0/59, 0/19,
1/47 and 15/48 in pooled vehicle controls, matched vehicle controls,
low and high dose groups, respectively), and in both groups of exposed
females (2/60, 1/20, 7/50 and 15/48 in pooled vehicle controls,
matched vehicle controls, low and high dose groups, respectively); one
alveolar/bronchiolar carcinoma was noted in a high-dose female mouse.
There was a non-significant increase in the incidence of squamous cell
carcinoma of the forestomach in females in the high dose group. The
incidence of mammary gland adenocarcinomas was significantly increased
in females at both doses (0/60, 0/20, 9/50 and 7/48 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). The incidence of endometrial stromal polyp or
endometrial stromal sarcoma (combined) was significantly elevated at
both doses (0/60, 0/20, 5/49 and 5/47 in pooled vehicle
controls, matched vehicle controls, low and high dose groups,
respectively). It was concluded that 1,2-dichloroethane was
carcinogenic in this strain of mice, under the conditions of this
study.
It should be noted that the data on tumour incidence presented in
the bioassays by the NCI (1978) do not take into account the increased
early mortality in the high exposure groups; the incidences of several
tumours (and thus the carcinogenic potency of 1,2-dichloroethane) may
have been higher had all animals survived for a long enough period of
time to develop tumours (Ward, 1980; Hooper et al., 1980).
7.5.3 Other routes of administration
1,2-Dichloroethane in acetone was applied to the shaved dorsal
skin of groups of 30 female non-inbred Ha:ICR Swiss mice, 3 times/week
for 440 to 594 days at doses of 0, 42, and 126 mg/application per
mouse. The incidence of lung tumours (benign lung papillomas) was
significantly increased at the higher dose (26/30 compared to 11/30 in
vehicle controls and 30/100 in naive controls, p < 0.0005).
Histopathological examination was limited to the skin, liver, kidney
and any "abnormal-appearing tissues" (van Duuren et al., 1979).
In a bioassay designed to screen the potential of numerous
chemicals to induce pulmonary tumours in a susceptible strain of mice
(A/St), groups of 20 males were administered 24 intraperitoneal
injections of 1,2-dichloroethane (20, 40 or 100 mg/kg body weight) in
tricaprylin, 3 times/week for 8 weeks (total doses of 480, 920 or
2400 mg/kg body weight). All surviving mice were killed 24 weeks
after the first injection. Although there was a dose-related increase
in the number of pulmonary adenomas per mouse (0.39, 0.21, 0.44 and
0.75 in the control, low, mid and high dose groups, respectively),
none of these increases was statistically significant (Theiss et al.,
1977). It should be noted, however, that the duration of the period
of observation may have been insufficient to allow for the development
of most types of tumours.
7.5.4 Initiation/promotion bioassays
In a dermal initiation/promotion protocol, 126 mg
1,2-dichloroethane was applied once to the skin (area exposed not
specified) of 30 female non-inbred Ha:ICR Swiss mice, followed 14 days
later by 5 µg (0.005 mg) of phorbol myristate acetate (PMA) (a
promoter) in 0.2 ml acetone, 3 times/week for 428 to 576 days. Two
PMA control groups of 120 and 90 mice were administered 0.0025 mg and
0.0050 mg PMA/application permouse (number of applications not
specified), respectively. Treatment with 1,2,-dichloroethane did not
significantly increase the incidence of skin papillomas (3/30 versus
9/120 and 6/90 in PMA controls). There were three squamous cell
carcinomas in the control groups, while none was observed in the
exposed group (van Duuren et al., 1979).
In a hepatic initiation/promotion assay, groups of 10
Osborne-Mendel rats were partially hepatectomized and then
administered 1,2-dichloroethane (100 mg/kg body weight) in corn oil by
gavage, followed 5 days later by diets containing phenobarbital for 7
weeks and a control diet for 1 week (initiation protocol). Livers
were examined histopathologically for GGT-positive foci (a putative
preneoplastic indicator). Additional groups of 10 rats were initiated
with an intraperitoneal injection of diethyl-nitrosamine or water
(control) following partial hepatectomy, then administered
1,2-dichloroethane (100 mg/kg body weight) in corn oil by gavage, 5
days/week for 7 weeks (promotion protocol). In rats administered
1,2-dichloroethane in the initiation protocol, there was no increase
in the number of GGT-positive foci.
Similarly, in rats administered 1,2-dichloroethane in the promotion
protocol, there was no significant increase in the number of
GGT-positive foci, either with or without the initiator, when compared
to controls (Story et al., 1986; Milman et al., 1988).
Groups of 35 male B6C3F1 mice were administered
diethylnitrosamine in the drinking-water for 4 weeks, followed by
exposure to 1,2-dichloroethane (835 or 2500 mg/litre) in
drinking-water for 24 or 52 weeks. Only the liver, kidneys and lungs
were examined histopathologically. Drinking-water intake was reduced
at the highest concentration. No significant differences in body
weight gain were noted. However, three mice consuming the highest
concentration of 1,2-dichloroethane died within 52 weeks. There was
no increase in the incidence of liver or lung tumours in exposed mice
either with or without diethylnitrosamine initiation (Klaunig et al.,
1986).
7.6 Mutagenicity and related end-points
The genotoxicity of 1,2-dichloroethane has been extensively
investigated in non-mammalian and mammalian test systems. Data from
in vitro and in vivo studies are summarized in Tables 6 and 7; a
summary of the weight of available evidence is presented here.
1,2-Dichloroethane induced differential toxicity in Escherichia
coli, but it had no effect in a Bacillus subtilis rec assay. It
consistently induced positive responses in mutagenicity assays with
Salmonella typhimurium, whereas it has not produced consistent
responses in mutation assays with E. coli and was negative in a mouse
peritoneal host-mediated assay with E. coli.
In the fungus Aspergillus nidulans, 1,2-dichloroethane induced
errors of mitotic segregation and aneuploidy, but did not induce gene
mutation.
Table 6. Genotoxicity of 1,2-dichloroethane in vitro (modified from ATSDR, 1994)
Species (test system) End-point Resulta Reference
With activation Without activation
Bacterial systems
Salmonella typhimurium Gene mutation + + Milman et al. (1988)
+ + Barber et al. (1981)
+ + Kanada & Uyeta (1978)
+ + Nestmann et al. (1980)
+ + Rannug et al. (1978)
+ + Van Bladeren et al. (1981)
+ NT Rannug & Beije (1979)
+ - Cheh et al. (1980)
+ - Moriya et al. (1983)
- - King et al. (1979)
+ + Strobel & Grummt (1987)
NT +b Simula et al. (1993)
S. typhimurium/spot test NT (+) Brem et al. (1974)
(+) - Principe et al. (1981)
NT - Buijs et al. (1984)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
S. typhimurium/Ara test + - Roldan-Arjona et al. (1991)
(standard)
S. typhimurium/Ara test (+) (+) Roldan-Arjona et al. (1991)
(liquid)
Streptomyces coelicolor Gene mutation NT - Principe et al. (1981)
Escherichia coli/K12/343/113 Gene mutation - - King et al. (1979)
E. coli/wp2 NT (+) Hemminki et al. (1980)
- - Moriya et al. (1983)
E. coli Pol A DNA damage NT (+) Brem et al. (1974)
Bacillus subtilis/rec-assay DNA damage NT - Kanada & Uyeta (1978)
Fungal systems
Aspergillus nidulans Gene mutation NT - Crebelli & Carere (1988)
NT - Principe et al. (1981)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
A. nidulans Mitotic segregation NT + Crebelli et al. (1984)
aberrations
A. nidulans Aneuploidy induction NT + Crebelli et al. (1988)
Saccharomyces cerevisiae Mitotic recombination NT (+) Simmon (1980)
Animal systems
Hamster CHO/HGPRT Gene mutation + (+) Tan & Hsie (1981)
+ (+) Zamora et al. (1983)
Rat hepatocytes Unscheduled DNA synthesis NT + Williams et al. (1989)
Mouse hepatocytes NT + Milman et al. (1988)
Mouse liver DNA DNA binding + NT Banerjee (1988)
Calf thymus DNA + NT Prodi et al. (1986)
Salmon sperm DNA + - Banerjee & Van Duuren (1979);
Banerjee et al. (1980)
Mouse BALBc/3T3 Cell transformation NT - Milman et al. (1988)
NT - Tu et al. (1985)
Table 6 (contd).
Species (test system) End-point Resulta Reference
With activation Without activation
Mouse C3H1OT´ Cell transformation NT +c Schultz et al. (1992)
Syrian hamster embryo cells Cell transformation NT + Hatch et al. (1983)
Human cells
Human lymphoblasts AHH-1 Gene mutation NT + Crespi et al. (1985)
Human lymphoblasts TK6 NT + Crespi et al. (1985)
Human embryo epithelial-like NT + Ferreri et al. (1983)
EUE cells
Human peripheral lymphocytes Unscheduled DNA synthesis + - Perocco & Prodi (1981)
a NT = not tested; - = negative result; + = positive result; (+) = weakly positive or marginal result
b increase in cells expressing GSTA1-1
c transformed cells induced tumours in nude mice
Table 7. Genotoxicity of 1,2-dichloroethane in vivo (modified from ATSDR, 1994)
Species (test system) End-point Resultsa Reference
Mammalian assays
Mouse Dominant lethal mutations - Lane et al. (1982)
Mouse/spot test Gene mutation (+) Gocke et al. (1983)
Mouse bone marrow Sister-chromatid exchange + Giri & Que Hee (1988)
Mouse bone marrow Micronuclei - Jenssen & Ramel (1980);
King et al. (1979)
Mouse peripheral erythrocytes - Armstrong & Galloway (1993)
Mouse liver, kidney, lung and stomach DNA binding + Prodi et al. (1986)
Mouse liver, kidney, lung and stomach + Arfellini et al. (1984)
Mouse forestomach and kidney + Hellman & Brandt (1986)
Mouse liver + Banerjee (1988)
Table 7 (contd).
Species (test system) End-point Resultsa Reference
Rat liver, kidney, spleen, lung, + Reitz et al. (1982)
forestomach and stomach
Rat liver, kidney, lung and stomach + Arfellini et al. (1984)
Rat liver, kidney, lung and stomach + Prodi et al. (1986)
Rat liver and kidney + Inskeep et al. (1986)
Rat liver and lung + Baertsch et al. (1991)
Rat liver + Banerjee (1988)
Rat liver + Cheever et al. (1990)
Mouse liver DNA damage + Storer & Conolly 1983, 1985;
Storer et al. (1984)
Mouse liver + Taningher et al. (1991)
Insect assays
Drosophila melanogaster/somatic mutation Gene mutation + Nylander et al. (1978)
Table 7 (contd).
Species (test system) End-point Resultsa Reference
D. melanogaster/somatic mutation + Romert et al. (1990)
D. melanogaster/somatic mutation + Kramers et al. (1991)
D. melanogaster/somatic mutation (+) Ballering et al. (1993)
D. melanogaster/recessive lethal + Ballering et al. (1993)
D. melanogaster/vermilion locus + Ballering et al. (1993)
D. melanogaster/sex-linked recessive + King et al. (1979)
D. melanogaster/sex-linked recessive + Kramers et al. (1991)
D. melanogaster Chromosomal loss/gain +/+ Valencia et al. (1984)
Host-mediated assays
Escherichia coli K12/343/113 mouse Gene mutation - King et al. (1979)
host-mediated assay
a - = negative result; + = positive result; (+) = weakly positive or marginal result
In cultured mammalian cells, 1,2-dichloroethane formed
adducts with DNA. It also induced unscheduled DNA synthesis in primary
cultures of mouse and rat hepatocytes, and human peripheral
lymphocytes (the last in the presence of an exogenous metabolic
activation system), and gene mutation in several cell lines. Mutation
frequency of two human cell lines has been correlated with the
difference in levels of glutathione- S-transferase activities (Crespi
et al., 1985; section 6.3). Cell transformation was induced in
studies with C3H10T´ cells, but not with BALBc/3T3 cells.
1,2-Dichloroethane enhanced SV40 virus transformation of Syrian
hamster embryo cells.
In vivo, both somatic cell and sex-linked recessive lethal
mutations have been consistently induced in Drosophila melanogaster by
1,2-dichloroethane. 1,2-Dichloroethane has been found to bind to DNA
in all reported studies in mice and rats. In other studies with mice,
clearly positive responses have been restricted to primary DNA damage
in liver and sister-chromatid exchange induction in bone marrow. No
evidence for micronucleus induction has emerged from bone marrow
micronucleus studies or for a dominant lethal effect in one study
(although this study may have been insufficiently sensitive), and only
a weak but significant (p < 0.03) response was observed in a single
spot test.
It has been noted that stronger responses were obtained in the
bacterial mutation assay in the presence of an exogenous metabolic
system than in its absence. This could imply the formation of
additional mutagenic metabolites, through either the cytochrome P450
or glutathione-S-transferase pathway. In these in vitro assays with
liver homogenates, activation by the cytochrome system is more likely,
and 2-chloroacetaldehyde, which is a possible metabolite, is known to
be mutagenic (McCann et al., 1975). The mutagenicity of
1,2-dichloroethane in S. typhimurium TA100, in the absence of S9 mix,
was more than doubled if the bacterium expressed the human GSTA1-1
gene, but there was no change in the mutagenic response if the SSTP1-1
gene was expressed (Simula et al., 1993).
7.7 Reproductive toxicity, embryotoxicity and teratogenicity
The reproductive and developmental effects of 1,2-dichloro-ethane
have not been extensively investigated in experimental animals (see
Table 8), although the compound has been detected in fetal tissues in
rats and mice following maternal exposure to 600 mg/m3 for 5 h
(Withey & Karpinski, 1985) and 1000 mg/m3 for 3 days (Vozovaya,
1977).
Table 8. Reproductive and developmental toxicity of 1,2-dichloroethane in experimental animals
Species Protocol Results Reference
Inhalation
Rats Animals were exposed to 0, 101, 304, or No exposure-related histopathological effects in the parents nor Rao
(Sprague-Dawley, 607 mg/m3 (0, 25, 75, or 150 ppm) any alterations in the fertility index and gestation periods were et al.
20 m & 20 f per 6 h/day, 5 days/week for 60 days prior observed compared to controls. There was a significant decrease (1980)
exposed group, to mating, and for an additional 116 in the number of pups per litter in the F1A pups at 304 mg/m3
30 m & 30 f days (7 days/week) after mating. Dams (13%, p<0.05), but not at 607 mg/m3; there was significantly
controls) were not exposed from gestation day 21 increased kidney weight in the F1B male pups at 101 mg/m3
through to day 4 postpartum. The pups (29%, p<0.05), although the authors did not consider this effect
(F1A) were removed after 21 days, and to be related to exposure. There were no significant differences
the females were remated to exposed in growth, sex ratios, survival indices, organ or neonatal body
males following removal of the last weights, or histology in pups.
litter to produce F1B litters. Liver,
kidneys, ovaries , uterus and testes
of parental animals in control and
high exposure group (and other groups
if any changes were noted in high
exposure group) were examined
histopathologically
Rats (Albino, f, Rats were exposed to 57 mg/m3, 4 h/day, Exposed rats had reduced fertility (6.5 fetuses per dam compared Vozovaya
strain and 6 days/week, for 6 or 9 months. The to 9.7 per dam in controls). Newborn pups had reduced body (1974)
number animals were apparently then mated, but weight (5.06 g versus 6.44 g in controls). Perinatal mortality
unspecified it is not clearly stated whether the was increased in the exposed group (data not presented). No
in secondary exposure period extended beyond information was available on maternal effects.
account) mating.
Table 8 (contd).
Species Protocol Results Reference
Rats (f, strain Rats were exposed to 15 mg/m3, 4 h/day, The estrous cycle was longer in exposed rats than in controls. No Vozovaya
and number 6 days/week for 4 months prior to and information on the effects on fertility was presented. Embryonal (1977)
unspecified after mating. mortality increased from approximately 11% in controls to 27% in
in secondary exposed dams. Pre-implantation losses were 5-fold greater in
account) exposed animals than in controls. No fetal abnormalities were
observed, except for haematomas in the region of the head, neck
and anterior extremities (presumably only in pups of exposed
animals, although not clearly stated). No information was
available on maternal effects.
Rats Rats were exposed to 0, 405 or 1215 10/16 dams at 1215 mg/m3 died. At 405 mg/m3, there were no Rao
(Sprague-Dawley, mg/m3 (0, 100 or 300 ppm) for 7 h/day effects on mean litter size, incidence of resorptions, or fetal et al.
16 to 30 pregnant on days 6 to 15 of gestation. body measurements; no significant increase in the incidence of (1980)
f per group) major malformations was observed at this concentration.
Rabbits (New Rabbits were exposed to 0, 405, or 4/21 and 3/19 dams died at 405 and 1215 mg/m3, respectively, Rao
Zealand White, 1215 mg/m3 (0, 100 or 300 ppm) for compared to none in 20 controls. There were no effects on mean et al.
19 to 21 7 h/day on days 6 to 18 of gestation. litter size, incidence of resorptions or fetal body measurements (1980)
pregnant f at either concentration, and there were no significant differences
per group) in the incidence of major malformations.
Table 8 (contd).
Species Protocol Results Reference
Ingestion
Rats (45 m & Animals were fed mash fumigated with There were no significant differences in various reproductive Alumot
90 f, strain 1,2-dichloroethane for 2 years. parameters, including number of dams pregnant, number of dams et al.
unspecified) Resulting concentrations were 250 and with litters, mean litter size, mortality or body weight of young (1976a)
600 mg/kg. Due to loss of the compound at birth and at weaning.
through volatilization, the mash
actually consumed was estimated to
contain 60 to 70% of the initial
concentration (estimated to result in
doses of approximately 7.5 to 8.75 and
15 to 17.5 mg/kg body weight per day).
Exposed females were mated with
exposed males at 2 month intervals.
Mice (ICR Swiss, F0 mice were administered There were no differences in body weight in adults, and there Lane
(10 m & 30 f concentrations of 0, 0.03, 0.09 or 0.29 were no effects on fertility or gestation indices. There were no et al.
per exposed mg/litre drinking-water (equivalent effects on survival, litter size, postnatal body weight or gross (1982)
group, 20 m & to nominal doses of approximately pathology of pups. The incidence of fetal visceral or skeletal
60 f controls) 0, 5, 15 or 50 mg/kg body weight malformations was not increased in exposed animals. F1C litters
per day) for 35 days prior to were not examined for skeletal malformations.
mating. Three sets of offspring
(F1A, F1B and F1C) were produced.
Table 8 (contd).
Species Protocol Results Reference
After weaning and 11 weeks of
exposure to the same concentrations,
the F1B mice were mated (30 female,
10 male) to produce a second generation
of offspring (F2A and F2B). Teratology
screening tests were performed using
F1C and F2B matings where the females
were co-housed with unexposed males.
Mice (f, number Pregnant mice were administered There were no developmental effects and "few discernible effects" Kavlock
and strain not 1,2-dichloroethane in the drinking-water on maternal health. There were no skeletal or visceral anomalies et al.
specified in at a concentration equivalent to a which could be attributed to exposure. (1979)
secondary dose of 510 mg/kg body weight per
account) day on days 7 to 14 of gestation.
No effects on reproductive parameters, including fertility index,
gestation period or histological changes, were reported in a
two-generation study on Sprague-Dawley rats exposed to 0, 101, 304 or
607 mg/m3 (0, 25, 75 or 150 ppm) 1,2-dichloroethane 6 h/day, for 60
days prior to mating and 116 days after mating (except during the
delivery period). There were also no effects on growth, sex ratios,
survival indices, organ or neonatal body weights, or histology in pups
(Rao et al., 1980).
Exposure to 1,2-dichloroethane (15 mg/m3), 4 h/day, for 4
months prior to mating and after mating resulted in a
longer-than-normal estrous cycle in female rats (strain not
specified). Although embryonal mortality and preimplantation losses
were greater in exposed animals than in controls, no fetal
abnormalities were reported, except for haematomas in the area of the
head, neck and anterior extremities (Vozovaya, 1977). Similarly,
increased perinatal mortality and reduced body weight of newborn pups
were observed in the offspring of female albino rats exposed to 57
(± 10) mg/m3 for 6 and 9 months. Exposed females also produced a
lower number of fetuses per dam (Vozovaya, 1974). Information from
these studies was insufficient for evaluation.
Rao et al. (1980) also conducted a developmental study in which
pregnant Sprague-Dawley rats were exposed to 0, 405 or 1215 mg/m3
(0, 100 or 300 ppm) 1,2-dichloroethane, 7 h/day, during gestation.
Mortality was high in dams at 1215 mg/m3 (10/16 rats died); thus the
developmental effects could not be ascertained at this concentration.
No fetotoxic or teratogenic effects were observed at 405 mg/m3. In
pregnant rabbits exposed to the same concentrations, 7 h/day, during
gestation, mortality was increased in exposed animals (4/21 and 3/19
dams died at 405 and 1215 mg/m3, respectively, compared to none in
20 controls). However, no fetotoxic or teratogenic effects were
reported at either concentration. The authors concluded that
1,2-dichloroethane was not teratogenic or fetotoxic in rats at
405 mg/m3 or in rabbits at 405 or 1215 mg/m3.
No effects on male fertility or various reproductive parameters
were noted in rats consuming mash which had been fumigated with
1,2-dichloroethane, the resultant concentrations being 250 and
500 mg/kg (approximately equivalent to doses of 7.5 to 8.75 and 15 to
17.5 mg/kg body weight per day when loss due to volatilization was
taken into account), for up to 2 years (Alumot et al., 1976a). No
effects on reproduction (in terms of fertility and gestation indices)
were reported in a two-generation study on Swiss ICR mice exposed to
1,2-dichloroethane in the drinking-water at concentrations of 0, 0.03,
0.09 or 0.29 g/litre (approximately equivalent to doses of 0, 5, 15 or
50 mg/kg body weight per day). In addition, no fetotoxic or
teratogenic effects were noted in either generation of offspring of
F1C and F2B litters sacrificed on day 18 (Lane et al., 1982). No
developmental effects were reported in a study in which groups of 30
pregnant CD-1 mice were administered drinking-water containing a
mixture of organic compounds, including 0.01% 1,2-dichloroethane
(equivalent to a dose of 5.1 mg/kg body weight per day), during days 7
to 14 of gestation (Kavlock et al., 1979).
7.8 Immunological effects
Groups of 140 female CD1 mice were exposed to airborne
concentrations of 0, 10, 20 and 40 mg/m3 (0, 2.5, 5 and 10 ppm)
1,2-dichloroethane for a 3-h period or to 0 or 10 mg/m3, 3 h/day for
5 consecutive days. After acute exposure to 20 and 40 mg/m3, there
was a significant increase in mortality in mice from streptococcal
challenge (p < 0.05 and p < 0.001, respectively), while no effects
were noted following acute or repeated exposure to 10 mg/m3. In
similarly exposed groups of 18 to 36 mice, significantly (p < 0.01)
decreased pulmonary bactericidal activity to inhaled Klebsiella
pneumoniae was noted only at 40 mg/m3. Single exposure to 40 or
400 mg/m3 (10 or 100 ppm) did not affect the in vitro phagocytic
or cytostatic ability of alveolar macrophages to red blood cells and
tumour target cells, respectively (Sherwood et al., 1987).
Male Sprague-Dawley rats (16 per group) were exposed to 0, 400 or
800 mg/m3 (0, 100 or 200 ppm) 1,2-dichloroethane for 3 h, or to 0,
40, 80, 200 or 400 mg/m3 (0, 10, 20, 50 or 100 ppm), 5 h/day, 5
days/week for 12 days. Pulmonary bactericidal activity was not
affected at any concentration. No effects were noted on in vitro
phagocytic activity to red blood cells, in vitro cytostatis and
cytolysis of tumour target cells, or levels of ectoenzymes in alveolar
macrophages. Blastogenesis of mitogen-stimulated T- and B-lymphocytes
from popliteal and mesenteric lymph nodes was not affected (Sherwood
et al., 1987).
Chinchilla rabbits (number per group not specified but
probably less than 10) were exposed to 2, 10 or 100 mg/m3
1,2-dichloroethane for 3 h/day, 6 days/week, for 7.5 to 8 months. At
the two highest concentrations production of antibodies against
typhoid vaccine was increased, while total antibody production was
reduced at 100 mg/m3 (Shmuter, 1977).
Groups of 10 male CD1 mice (12 per group) (Munson et al., 1982)
were administered 1,2-dichloroethane (4.9 and 49 mg/kg body weight per
day) in water by gavage for 14 days. A significant (p < 0.05)
reduction of 25 and 40% in IgM antibody-forming cells to sheep RBCs
was observed at 4.9 and 49 mg/kg body weight per day, respectively. A
significant (p < 0.05) reduction of cell-mediated responses to sheep
erythrocytes was also observed at both levels, which was not related
to dose, while a significant (p < 0.05) reduction in leucocyte count
was observed at the highest dose. In the same study, groups of 16 to
32 male CD1 mice were administered time-weighted average
1,2-dichloroethane doses of 0, 3, 24 and 189 mg/kg body weight per day
(0, 0.02, 0.2 and 2.0 g/litre) for 90 days in drinking-water; controls
consisted of 24 to 48 mice. The fluid consumption of exposed mice was
decreased in a dose-related manner, which corresponded to a
dosedependent reduction in body weight. No effects on organ weights,
haematology, B-cell mitogen lipopolysaccharide response, spleen cell
response to the T-cell mitogen concanavalin A or cellmediated immunity
(assessed by measuring the delayed-type hypersensitivity responses to
sheep erythrocytes) were noted. There was a tendency towards a
reduction in the serum antibody level after immunization with sheep
erythrocytes, and in the immunoglobulin spleen antibody-forming cells
at all exposure levels (not significant) (Munson et al., 1982).
7.9 Toxicological interactions with other agents
In a recent bioassay designed to determine the influence of
disulfiram or ethanol on the carcinogenicity, metabolism and covalent
binding to DNA of 1,2-dichloroethane, male and female Sprague-Dawley
rats were exposed to 200 mg/m3 (50 ppm) 1,2-dichloroethane for 7 h
per day, 5 days a week, for 2 years. Additional rats were similarly
exposed and administered either 0.05% disulfiram in the diet or 5%
ethanol in the drinking-water, either alone or in combination with
1,2-dichloroethane. The incidence of any type of tumour was not
elevated compared to controls in any groups exposed to these compounds
individually, nor was the incidence of any type of tumour increased in
rats exposed to 1,2-dichloroethane and ethanol in combination compared
to unexposed controls or rats exposed to these compounds alone.
Exposure to disulfiram in combination with 1,2-dichloroethane resulted
in a significant (p<0.05) increase in the incidence of intrahepatic
bile duct cholangiomas (males: 9/49 versus 0/50, females: 17/50 versus
0/50) and cysts (males: 12/49 versus 0/50, females: 24/50 versus 0/50)
in both male and female rats compared to that in rats exposed to
1,2-dichloroethane only. Male rats exposed to this combination also
had a significantly (p<0.05) increased incidence of subcutaneous
fibromas (10/50 versus 0/50), hepatic neoplastic nodules (6/49 versus
2/50) and interstitial cell tumours in the testes (11/50 versus 3/50)
compared to rats exposed to 1,2-dichloroethane alone. Female rats
similarly exposed had a significantly (p<0.05) higher incidence of
mammary adenocarcinomas compared with rats exposed to
1,2-dichloroethane only (12/48 versus 5/50). Combined exposure to
1,2-dichloroethane and disulfiram did not increase the level of
covalent binding to hepatic DNA compared to that found in rats exposed
to 1,2-dichloroethane alone. The profile of urinary metabolites of a
single radiolabelled oral dose of 1,2-dichloroethane in rats
simultaneously exposed to disulfiram and 1,2-dichloroethane for 2
years indicated that the metabolism of 1,2-dichloroethane was
qualitatively similar to that of rats exposed to 1,2-dichloroethane
alone for 2 years. However, a reduced rate of elimination, and
sustained blood levels of unchanged 1,2-dichloroethane were observed
(see section 6.4), which, the authors stated, could be related to the
carcinogenic effects noted following simultaneous exposure (Cheever et
al., 1990).
In addition, concomitant exposure to disulfiram (as may occur in
the rubber industry or in persons undergoing therapy for alcoholism)
in the diet resulted in a synergistic increase in the hepatotoxicity
(as determined by levels of enzymes in serum and increased relative
liver weight (> 30%)) of 1,2-dichloroethane inhaled at
concentrations of 600, 1200 or 1800 mg/m3 (150, 300 and 450 ppm) for
30 days by male Sprague-Dawley rats. However, rats administered
1,2-dichloroethane alone had evidence of liver damage only at the
highest concentration. The enhanced effects were hypothesized to be
due to the inhibition of mixed-function oxidase-mediated metabolism of
1,2-dichloroethane and a compensatory increase in metabolism of
1,2-dichloroethane to reactive metabolites via cytosolic pathways
mediated by glutathione S-transferase, since hepatic cytochrome
P-450 content decreased with increasing concentration of
1,2-dichloroethane only in the presence of disulfiram (Igwe et al.,
1986a). Concomitant exposure to 1,2-dichloroethane by inhalation
(> 1216 mg/m3 or 304 ppm) or intraperitoneal injection
(150 mg/kg body weight per day) and disulfiram administered in the
diet (0.15%) resulted in testicular atrophy in Sprague-Dawley rats
compared to rats exposed to either compound alone (Igwe et al.,
1986b).
The acute toxicity of carbon tetrachloride was potentiated by
1,2-dichloroethane in rats administered single doses of each of 60 and
125 µl/kg body weight perorally, based on determination of serum
hepatic enzymes and indicators of lipid peroxidation at 24 h after
exposure. Pre-treatment with vitamin E prevented hepatotoxicity.
Based on the observation that the hepatic GSH level in the group
concomitantly exposed to both compounds was not significantly
different from that in the group exposed to carbon tetrachloride
alone, the authors concluded that GSH depletion did not play an
important role in the potentiation (Aragno et al., 1992). Concomitant
exposure to oral doses of 1,2-dichloroethane and 1,2-dibromoethane (60
and 20 µl/kg body weight, respectively) did not result in liver
toxicity in rats, based on levels of serum hepatic enzymes and
indicators of lipid peroxidation, although the compounds alone and in
combination resulted in a decrease in hepatic GSH level 2 h after
exposure, which subsequently returned to control values (Danni et al.,
1992).
The in vitro metabolism of 1,2-dichloroethane by liver
homogenates of rats administered ethanol increased with the dose of
ethanol up to 4 g/kg body weight, but declined sharply at 5 g/kg body
weight (Sato et al., 1981).
High doses (1000 to 2000 mg/kg body weight) of several chemicals,
including methionine, p-aminobenzoic acid, sulfanilamide and
aniline, administered orally to mice were protective against the
lethal effects caused by inhalation of 1600 mg/m3 (400 ppm)
1,2-dichloroethane (Heppel et al., 1945).
The acute and subacute toxicity of dichloroethane increased when
it was administered under conditions of high temperature (species and
exposure protocol not specified in abstract) (Mihaylova, 1976).
8. EFFECTS ON HUMANS
8.1 Case reports
The lethal oral dose of 1,2-dichloroethane in humans has been
estimated to be between 20 and 50 ml. Death due to cardiac arrhythmia
has been reported following ingestion of large, single doses
(50-75.2 g) of 1,2-dichloroethane (Hueper & Smith, 1935; Garrison &
Leadingham, 1954; Martin et al., 1969). Effects identified following
ingestion of 1,2-dichloroethane include central nervous system
depression, gastroenteritis, liver, kidney and lung damage,
cardiovascular disorders and haematological effects (Weiss, 1957;
Morozov, 1958; Hinkel, 1965; Bogoyavlenski et al., 1968; Martin et
al., 1969; Schönborn et al., 1970; Yodaiken & Babcock, 1973; Natsyuk &
Mudritsky, 1974; Dorndorf et al., 1975; Andriukin, 1979).
Effects reported following exposure to 1,2-dichloroethane via
inhalation are very similar to those observed after ingestion but are
usually less pronounced. Inhalation of 1,2-dichloroethane vapour
first affects the central nervous system and causes irritation and
inflammation of the respiratory tract. Damage to the liver, kidneys
and lungs (Wirtschafter & Schwartz, 1939; Hadengue & Martin, 1953;
Menschick, 1957; Troisi & Cavallazzi, 1961; Suveev & Babichenko, 1969;
Nouchi et al., 1984) and changes in the heart rhythm (Suveev &
Babichenko, 1969) have been reported in several cases. Death due to
cardiac toxicity has been reported following a 30-min exposure to an
unknown concentration of 1,2-dichloroethane (Nouchi et al., 1984).
Effects on the eyes were observed in several early case reports
(Weiss, 1957; Menschick, 1957; Troisi & Cavallazzi, 1961), while
severe dermatitis has been reported following dermal contact with
1,2-dichloroethane (Wirtschafter & Schwartz, 1939).
8.2 Epidemiological studies
In a study of 278 men working in the chlorohydrin unit of a
chemical production plant between 1940 and 1967 and followed up to
1988, there was a significant (p < 0.01) excess of deaths due to
pancreatic cancer compared to the USA national rates [Standardized
Mortality Ratio (SMR) = 492 (95% CI = 158 - 1140); Observed:Expected
(O:E) = 8:1.6]. The excess was greater when confined to men who
worked in the unit for more than 2 years (SMR = 800). Based on
comparison with two groups of workers in nearby plants, there were
pronounced increases in mortality due to pancreatic cancer as exposure
duration increased. Though an excess of deaths due to "lymphatic and
haemopoietic cancers" was also observed, it appeared to be
attributable principally to leukaemia, for which numbers of observed
cases were small (O=4) and associations with duration of exposure were
less consistent. Although quantitative data were not available, the
authors concluded on the basis of considerable qualitative information
that workers in this unit had been exposed primarily to
1,2-dichloroethane in combination with bis-chloroethyl ether, ethylene
oxide and ethylene chlorohydrin (Benson & Teta, 1993).
In a case-control study, the exposure of 21 male employees at a
petrochemical plant in Texas, USA, whose deaths were
attributed to cancer of the brain, was compared to that of two groups
of 80 controls from the same plant. One control group consisted of
male employees who had died from non-neoplastic causes, while the
second group of controls consisted of those men whose deaths were due
all other causes. Employees were classified as having been exposed to
1,2-dichloroethane if they had ever worked in a department in which
the compound had been used; unexposed workers had never worked in
these departments and the exposure of others was considered to be
unknown (47.6% of cases, 58.8 and 61.3% of the first and second group
of controls, respectively). When those with unknown exposure were
excluded from the analyses, the proportion of cases (all cases or
glioma cases specifically) who were exposed (n = 11 and 10, or 45.5
and 50.0%, respectively) did not differ significantly from the
proportion of controls who had been exposed to 1,2-dichloroethane
(42.4 and 45.2% for the two control groups). When a 15-year latency
period was considered in the analysis, the proportion of cases and
controls exposed still did not differ significantly (40.0 and 44.4%
for cases versus 32.3 and 34.6% for the two control groups) (Austin &
Schnatter, 1983a).
In an accompanying historical cohort study of 6588 workers at
this plant, there was no significant excess in malignant brain tumours
in the overall population of the plant compared to national rates,
although there was a borderline significant (p < 0.05) increase in
hourly employees with more than 6 months of employment (O/E = 10/5).
However, exposure to 1,2-dichloroethane was not specifically
considered in this study, and employees were also exposed to a number
of potentially confounding substances, including benzene, diethyl
sulfate, ethylene oxide and vinyl chloride (Austin & Schnatter,
1983b).
Deschamps & Band (1993) conducted a small case-control study to
investigate a possible association between a spill of
1,2-dichloroethane in 1982 into a river supplying drinking-water to
parts of the city of Vancouver, Canada, and an identified cluster of
childhood leukaemia cases in the city. It was determined that none of
the 15 cases diagnosed between 1975 and 1988 had lived in areas of the
city serviced by the contaminated supply.
In an ecological study in which potential associations between
contamination of drinking-water from groundwater supplies by
particular substances (including 10 volatile organic compounds and 43
inorganic elements) and cancer were investigated, the average annual
age-adjusted incidence (1969 to 1981) of colon and rectal cancer was
statistically significantly greater in men aged > 55 years whose
drinking-water contained > 0.1 µg 1,2-dichloroethane/litre than in
those whose drinking-water contained < 0.1 µg/litre (222.8 per
100 000 versus 170.3 per 100 000 (193 and 633 cases, p = 0.02) and
126.5 per 100 000 versus 92.9 per 100 000 (106 and 337 cases,
p = 0.009), respectively). Rectal cancer in males was also associated
with chlorination of drinking-water. Of the study population over 55
years of age, 50% had lived at the same address for 20 years or more.
There were no significant differences between groups of towns with
respect to eight socioeconomic factors examined, except that the
percentage change in population between 1970 and 1980 was
significantly less in those towns with > 0.1 µg
1,2-dichloroethane/litre. The authors did not suggest that the result
indicated a causal relationship between 1,2-dichloroethane and cancer,
but that cancer incidence may be elevated in populations consuming
water from wells subject to anthropogenic contamination (Isacson et
al., 1985).
The prevalence of subjective symptoms was higher in a group of 10
male oil refinery workers exposed to between 250 and 800 mg/m3 than
in those exposed to lower concentrations (40 to 150 mg/m3); a
"general reduction in body weight" was also noted in both groups.
"Abnormalities" of the liver, central nervous system, gastrointestinal
tract and haematological parameters were reported in some workers
(n = 1 to 8) in the group exposed to the higher concentration,
presumably based upon clinical examination, although this was not
specified in the previous account of this early study. There were no
unexposed controls, and workers were also exposed to benzene (10 to
25 mg/m3) (Cetnarowicz, 1959).
The morbidity during a 5-year period (1951 to 1955) was increased
for all disease categories (not further specified) in a group of
workers (number not specified) at an aircraft factory exposed to
5 mg/m3 or less for 70 to 75% of the working time and 80 to
150 mg/m3 for the remainder, when compared to workers in the entire
factory. Of 83 workers examined further, 19 had disease of the liver
and bile duct, 13 had neurotic conditions, 11 had autonomous dystonia
and 10 had hyperthyroidism and goitre (there were no controls for
comparison) (Kozik, 1957).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
9.1.1 Microorganisms
Blum & Speece (1991) investigated the toxicity of
1,2-dichloroethane to three groups of aquatic bacteria: methanogens,
aerobic heterotrophs and Nitrosomonas. The end-points assessed
included inhibition of gas production (methanogens), oxygen uptake
(aerobic heterotrophs), and ammonia consumption (Nitrosomonas). The
IC50 values for Nitrosomonas and methanogens (29 and 25 mg/litre,
respectively) were considerably lower than that for aerobic
heterotrophs (470 mg/litre). For the bacteria Pseudomonas putida,
the nominal 16-h toxicity threshold for the onset of cell
multiplication inhibition was 135 mg/litre (Bringmann & Kühn, 1981).
Tang et al. (1990) determined the IC50 values based on inhibition of
respiration rate for activated sewage sludge using open and closed
serum bottle methods to be 35 500 mg/litre and 2780 mg/litre,
respectively. The difference was attributed to the volatility of
1,2-dichloroethane which was stripped from the substrate in the open
method.
The freshwater cyanobacterium (blue-green alga) Microcystis
aeruginosa was seven times more sensitive to 1,2-dichloroethane than
the green alga Scenedesmus quadricauda, the nominal 7-day EC50
values for inhibition of cell multiplication at 27°C being 105 and
710 mg/litre, respectively (Bringmann & Kühn, 1978). The 72-h EC50
for inhibition of growth was 189 mg/litre (Freitag et al., 1994).
Bringmann & Kühn (1980) determined the toxicity thresholds for
Scendesmus quadricauda and the protozoan Entosiphon sulcatum to be
710 mg/litre and 1127 mg/litre, respectively. Knie et al. (1983)
reported the acute EC50 for the alga Haematococcus pluvialis to be
130 mg/litre. Based on bioluminescence, the 5-min IC50 was
700 mg/litre in a Microtox test with Photobacterium phosphoreum
(Blum & Speece, 1991). Freitag et al. (1994) reported the 15-min EC50
for inhibition of bioluminescence in this species to be 770 mg/litre.
Bringmann & Kühn (1981) determined the toxicity thresholds in the
holozoic bacteriovorous flagellate protozoan Entosiphon sulcatum, the
holozoic bacteriovorous ciliate protozoan Uronema parduczi, and the
saprozoic flagellate protozoan Chilomonas paramecium to be > 8000
mg/litre, > 16 000 mg/litre and > 800 mg/litre, respectively, using
the cell multiplication inhibition test.
Pearson & McConnell (1975) determined the EC50 (based on carbon
uptake from CO2 during photosynthesis) in the marine unicellular
alga Phaeodactylum tricorhutum to be 340 mg/litre.
9.1.2 Invertebrates
Based on a review of identified acute and chronic toxicity
studies in freshwater invertebrates, Daphnia magna appears to be the
species most sensitive to 1,2-dichloroethane. Under static test
conditions, the measured 48-h LC50 values for fed and unfed first
instar D. magna were 320 and 270 mg/litre, respectively; the 48-h
EC50 values, based on complete immobilization, were 180 and
160 mg/litre for fed and unfed organisms, respectively (Richter et
al., 1983). Leblanc (1980) reported the 24-h and 48-h LC50 values
in D. magna to be 250 and 220 mg/litre, respectively, while the "no
discernible effect concentration" (apparently based on mortality only)
was < 68 mg/litre. Using the Probit method, Ahmad et al. (1984)
determined the 48-h LC50 in unfed D. magna to be 268 mg/litre (95%
CL: 246-293), while the EC50, based on reproductive effects was
155 mg/litre (95% CL: 137-188). Freitag et al. (1994) determined the
24-h EC50 for 10% immobilization of D. magna to be 150 mg/litre.
Knie et al. (1983) reported the EC0, EC50 and EC100 in D. magna
to be 67, 600 and 1075 mg/litre, respectively. Richter et al. (1983)
also examined the effect of 1,2-dichloroethane on reproductive success
and length of first instar D. magna in a 28-day flow-through test.
For reproductive success, the measured lowest-observed-effect level
(LOEL) and no-observed-effect level (NOEL) were 20.7 and
10.6 mg/litre, respectively, while the measured LOEL and NOEL for
growth were 71.7 and 41.6 mg/litre, respectively.
Ahmad et al. (1984) also conducted chronic toxicity studies in
which D. magna were exposed to 0, 10.6, 20.6, 41.6, 71.7, 94.4 and
137 mg 1,2-dichloroethane/litre for 28 days. There was a
concentration-related decrease (as low as 12% of control values) in
the number of young produced (significant (p < 0.05 or 0.01) at
41.6 mg/litre or more) as well as a decrease (as low as 59% of control
values) in the length of adults (significant (p < 0.01) at
71.7 mg/litre or more). Few acute toxicity studies in marine
invertebrates were identified. Under static test conditions, the
nominal 24-h EC50 for immobilization of 30-h posthatch larvae of the
brine shrimp Artemia salina was 93.6 mg/litre (Foster & Tullis,
1984). For marine adult shrimp (Crangon crangon), the measured 24-h
LC50 was 170 mg/litre under static test conditions (Rosenberg et
al., 1975). The 48-h LC50 for barnacle nauplii (Elminuis modestus)
was 186 mg/litre (Pearson & McConnell, 1975). Teratogenic effects
(expressed as surviving larvae with gross debilitating abnormalities)
were observed in the nauplii of the marine brine shrimp (Artemia
salina) at concentrations between 0.25 and 25 mg/litre (Kerster &
Schaeffer, 1983).
9.1.3 Vertebrates
The embryos and larvae of the northwestern salamander (Ambystoma
gracile) and the leopard frog (Rana pipiens) were continuously
exposed to 1,2-dichloroethane from within 30 min of fertilization
(embryos) and maintained for 4 days after hatching (larvae). The
LC50 values for the salamander at the day of hatching (day 5) and 4
days after hatching (day 9) were 6.53 and 2.54 mg/litre, respectively;
the measured LOEL for 23% reduction in egg hatchability was
0.99 mg/litre. The measured 5-day and 9-day LC50 values for the
frog were 4.52 and 4.40 mg/litre, respectively, while the 5-day
posthatch LOEL was 1.07 mg/litre (Black et al., 1982).
Acute toxicity studies have been conducted on several species of
freshwater fish. The most sensitive species was the guppy (Poecilia
reticulata, 2-3 months old), with a nominal 7-day LC50 of
106 mg/litre under static renewal test conditions (Konemann, 1981).
In three studies on 30-day-old fathead minnows (Pimephales promelas),
measured 96-h LC50 values ranged from 116 to 136 mg/litre under
flow-through conditions (Veith et al., 1983; Walbridge et al., 1983;
Geiger et al., 1985). LC50 values after 24, 48, 72 and 96 h in
rainbow trout (Salmo gairdneri) were 362, 340, 337 and 336 mg/litre,
respectively, using the static test method (Bartlett, 1979). Knie et
al. (1983) reported the EC0, EC50 and EC100 in golden orfe
(Leuciscus idus) to be 67, 600 and 1075 mg/litre, respectively.
In marine fish, a nominal 96-h LC50 of 480 mg/litre was
reported in tidewater silversides (Minidia beryllina) under static
test conditions (Dawson et al., 1975/77). Heitmuller et al. (1981)
reported the static test LC50 at 24, 48, 72 and 96 h in sheepshead
minnows (Cyprinodon variegatus) to be between 130 and 230 mg/litre.
The 96-h LC50 for dab (Limanda limanda) was 115 mg/litre (Pearson
& McConnell, 1975).
In a long-term, flow-through study of the early life stages of
fathead minnows (Pimephales promelas), there were no effects on egg
hatchability or larval survival and deformity at 29 mg/litre (NOEL);
however, larval growth was significantly (p < 0.05) reduced by 62% at
59 mg/litre (LOEL) (Benoit et al., 1982). Black et al. (1982) exposed
the embryos and larvae of the rainbow trout (Oncorhynchus mykiss)
continuously to 1,2-dichloroethane under flow-through conditions from
within 30 min of fertilization (embryos) and maintained them until 4
days after hatching. The resulting EC50 for hatchability and 27-day
LC50 for post-hatch survival were both 34 mg/litre, and the LOEL for
a 24% reduction in egg hatchability was 3.49 mg/litre. After 21 days
of continuous exposure to 150 mg 1,2-dichloroethane/litre, the
mortality of coho salmon (Oncorhynchus kisutch) eggs was 46%, while
in alevins, 100% mortality occurred 9 days after hatching at
320 mg/litre (Reid et al., 1982). In addition, premature hatching was
observed at 56 mg/litre, and, within one week of hatching, sublethal
effects, including lethargy and loss of equilibrium, were observed in
alevins exposed to 56 mg/litre; 100% mortality occurred 9 days after
hatching.
Ahmad et al. (1984) conducted chronic toxicity studies in which
fathead minnows were exposed to 300, 4000, 7000, 14 000, 29 000 or
39 000 µg/litre for 32 days. There were no significant effects on
survival, although there was a significant (p < 0.05) decrease in
mean individual net weight at the highest concentration (38% of
control values). The MATC was determined to be 49 000 to
59 000 µg/litre.
Teratogenic effects (expressed as surviving larvae with gross,
debilitating abnormalities) were observed in the larvae of
northwestern salamanders (Ambystoma gracile), leopard frogs
(Rana pipiens) and rainbow trout (Oncorhynchus mykiss) at 21.4,
21.9 and 34.4 mg/litre, respectively (Black et al., 1982).
9.2 Terrestrial organisms
9.2.1 Invertebrates
In an acute contact test, the 48-h LC50 for earthworms (Eisenia
fetida) exposed to 1,2-dichloroethane-treated filter-paper was
60 µg/m2 (Neuhauser et al., 1985).
9.2.2 Vertebrates
Male and female white leghorn chickens were fed mash which had
been fumigated with 1,2-dichloroethane, resulting in concen-trations
in the feed of 250 and 500 mg/kg, for 2 years. The end-points
examined included serum composition, growth, semen characteristics and
fertility. The weight of eggs was significantly reduced at 250 mg/kg
(5 to 10% (p < 0.01)), while both the number and weight of eggs were
reduced at 500 mg/kg (5 to 48% (p < 0.05) and 5 to 13% (p < 0.01),
respectively) (Alumot et al., 1976b).
9.2.3 Plants
1,2-Dichloroethane vapour was both lethal and mutagenic to barley
kernels (two-rowed variety, Bonus) following exposure to 3 mg/m3 for
24 h (Ehrenberg et al., 1974).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Available data on the carcinogenicity of 1,2-dichloroethane in
humans are limited. There is convincing evidence of increases in the
incidence of both common and rare tumours in experimental animals at
several sites (including squamous cell carcinomas of the stomach,
haemangiosarcomas, fibromas of the subcutaneous tissue and
adenocarcinomas and fibroadenomas of the mammary gland in rats; and
alveolar/bronchiolar adenomas, mammary gland adenocarcinomas,
endometrial stromal polyp or endometrial stromal sarcoma combined and
hepatocellular carcinomas in mice) following administration by gavage
for 78 weeks.
The incidence of benign lung papillomas was significantly
increased in mice following long-term dermal application of
1,2-dichloroethane, while a non-significant increase in the number of
pulmonary adenomas per animal was reported in a screening bioassay on
mice and in the incidence of benign mammary gland tumours in rats
exposed by inhalation for 2 years.
1,2-Dichloroethane is genotoxic in in vitro and in vivo
assays, and binds to DNA in rodents in vivo.
Based on the induction of both rare and common tumours in rats
and mice exposed by ingestion and supporting evidence in other limited
bioassays, the production of a reactive intermediate that alkylates
DNA and positive results in a range of in vitro assays for
genotoxicity, 1,2-dichloroethane is considered to be a probable human
carcinogen.
10.2 Environmental assessment
The high volatility of 1,2-dichloroethane makes the atmosphere
the predominant environmental sink. Consequently, measured
concentrations in surface waters are low (around 1 to 10 µg/litre).
Air concentrations are highest around manufacturing plants where they
may reach 300 µg/m3; concentrations in urban air average
< 1 µg/m3. Low adsorption to soil leads to potential leaching to
groundwater; some measurements of low concentrations in drinking-water
(< 0.2 µg/litre) confirm this.
Both hydrolysis and microbial degradation are slow; the
volatility of the compound means that it has low residence time in
media where these processes occur and they are not considered to be of
environmental significance.
The estimated atmospheric lifetime of 1,2-dichloroethane is
between 40 and 110 days. Stratospheric photolysis may produce chlorine
radicals which may in turn react with ozone. However, the
ozone-depleting potential is low (0.001 relative to CFC-11) and the
compound is not listed in the Montreal Convention.
Various toxicity tests have shown LC50s for organisms in the
environment to be generally greater than 10 mg/litre. The difference
(at least 7 orders of magnitude) between measured water concentrations
and these toxic concentrations indicate that 1,2-dichloroethane poses
no risk to organisms since exposure will not occur.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN
HEALTH AND THE ENVIRONMENT
Considering the toxicological characteristics of
1,2-dichloroethane, both qualitatively and quantitatively, an exposure
that would not cause adverse effects in humans by any route of
exposure cannot be estimated. Consequently, all appropriate measures
should be taken to eliminate or minimize human exposure to
1,2-dichloroethane.
12. FURTHER RESEARCH
Although specific studies were not recommended for
1,2-dichloroethane, additional analytical epidemiological studies are
desirable.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1979) has
classified 1,2-dichloroethane in group 2B (possibly carcinogenic to
humans), based on sufficient evidence of carcinogenicity in
experimental animals.
The International Programme on Chemical Safety has previously
evaluated 1,2-dichloroethane (IPCS, 1987). It concluded that
1,2-dichloroethane produces central nervous system depression, and
gastrointestinal and liver abnormalities in humans. The same effects
occur in experimental animals, in addition to possible kidney
abnormalities, lung oedema and cardiovascular disorders.
1,2-Dichloroethane, administered by gavage, is carcinogenic in rats
and mice, and should be regarded, for practical purposes, as if it
presented a carcinogenic risk for humans. 1,2-Dichloroethane was not
considered to accumulate in the environment. In the atmosphere, it is
removed by photo-chemical degradation via hydroxyl radicals and is
eliminated from water by evaporation. It has a low octanol/water
partition coefficient and bioconcentration is unlikely. It was not
considered to pose a hazard to the aquatic environment except in the
case of accidents and inappropriate disposal. Data were insufficient
to evaluate the effects of 1,2-dichloroethane on the terrestrial
environment.
The Joint FAO/WHO Expert Committee on Food Additives
(JECFA) has evaluated 1,2-dichloroethane on three occasions (WHO,
1971, 1980, 1992). When last evaluated, the Committee concluded that
this compound is genotoxic in both in vitro and in vivo test
systems and carcinogenic in mice and rats when administered by the
oral route. No ADI was therefore allocated. The Committee expressed
the opinion that 1,2-dichloroethane should not be used in food.
In the WHO Guidelines for drinking-water quality (WHO, 1993), the
concentrations of 1,2-dichloroethane in drinking-water estimated to be
associated with excess risks of 10-4, 10-5 and 10-6 are 300, 30 and
3 µg/litre, respectively, based on linearized multistage modelling of
the incidence of haemangiosarcomas in male rats in the NCI (1978)
study.
The European Commission published a directive in 1990 in which
limit values for emission of 1,2-dichloroethane were specified for
various types of industrial plants. These limits ranged from
0.1 mg/litre (monthly) for plants using 1,2-dichloroethane for
degreasing metals away from an industrial site to 12 mg/litre (daily)
for plants producing 1,2-dichloroethane and processing or using the
substance at the site (CEC, 1990).
REFERENCES
AEC (Alberta Environmental Centre) (1989) Final effluent analysis of
Dow Chemical, Fort Saskatchewan, Alberta plant. Alberta, Canada,
Alberta Environmental Centre (Unpublished data).
Ahmad N, Benoit D, Brooke L, Call D, Carlson A, DeFoe D, Huot J,
Moriarity A, Richter J, Shubat P, Veith G, & Wallbridge C (1984)
Aquatic toxicity tests to characterize the hazard of volatile organic
chemicals in water: a toxicity data summary - Parts I and II. Duluth,
Minnesota, US Environmental Protection Agency, Office of Research and
Development, Environmental Research Laboratory.
Alumot E, Nachtomi E, Mandel E, & Holstein P (1976a) Tolerance and
acceptable daily intake of chlorinated fumigants in the rat diet. Food
Cosmet Toxicol, 14: 105-110.
Alumot E, Meidler M, Holstein P, & Herzberg M (1976b) Tolerance and
acceptable daily intake of ethylene dichloride in the chicken diet.
Food Cosmet Toxicol, 14: 111-114.
Andriukin AA (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klin Med (Mosk), 57: 43-47 (in Russian).
Apostolov I & Mihaylova A (1975) [Investigation into
beta- N-acetylglucosaminidase activity in the serum of white rats
treated with 1,2-dichloroethane.] Probl Hyg, 1: 161-163
(in Bulgarian).
Aragno M, Tamagno E, Danni O, & Ugazio G (1992) In vivo studies on
halogen compound interactions. III. Effect of carbon tetrachloride
plus 1,2-dichloroethane on liver necrosis and fatty accumulation. Res
Commun Chem Pathol Pharmacol, 76: 341-354.
Archer WJ (1979) Chlorocarbons and chlorohydrocarbons. In: Kirk J &
Othmer DF ed. Encyclopedia of chemical technology, 3rd ed. New York,
John Wiley & Sons, vol 5, pp 723-743.
Arfellini G, Bartoli S, Colacci A, Mazzullo M, Galli MC, Prodi G, &
Grilli S (1984) In vivo and in vitro binding of 1,2-dibromoethane
and 1,2-dichloroethane to macromolecules in rat and mouse organs. J
Cancer Res Clin Oncol, 108: 204-213.
Armstrong MJ & Galloway SM (1993) Micronuclei induced in peripheral
blood of Eµ-PIM-1 transgenic mice by chronic oral treatment with
2-acetylaminofluorene or benzene but not with diethylnitrosamine or
1,2-dichloroethane. Mutat Res, 302: 61-70.
Atkinson R (1987) A structure-activity relationship for the estimation
of rate constants for gas-phase reaction of OH radicals with organic
compounds. Int J Chem Kinet, 19: 799-828.
ATSDR (1989) Toxicological profile for 1,2-dichloroethane. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry, 129 pp.
ATSDR (1994) Toxicological profile for 1,2-dichloroethane (Update).
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry,
192 pp (TP-93/06).
Austin SG & Schnatter AR (1983a) A case-control study of chemistry
exposures and brain tumors in petrochemical workers. J Occup Med,
25(4): 313-320.
Austin SG & Schnatter AR (1983b) A cohort mortality study of
petrochemical workers. J Occup Med, 25(4): 304-312.
Baertsch A, Lutz WK, & Schlatter C (1991) Effect of inhalation
exposure regimen on DNA binding potency of 1,2-dichloroethane in the
rat. Arch Toxicol, 65: 169-176.
Bailey S, Collins GB, Fishwick FB, Hart HV, Horler DF, & Scudamore KA
(1982) Pesticide residues in foodstuffs in Great Britain:
Organochlorine pesticides, organophosphorus pesticides and fumigant
residues in home-produced and imported wheat. Pestic Sci,
13(4): 373-378.
Ballering LAP, Nivard MJM, & Vogel EW (1993) Characterization of the
genotoxic action of three structurally related 1,2-dihaloalkanes in
Drosophila melanogaster. Mutat Res, 285: 209-217.
Ballering LAP, Nivard MJM, & Vogel EW (1994) Mutation spectra of
1,2-dibromoethane, 1,2-dichloroethane and 1-bromo-2-chloroethane in
excision repair proficient and repair deficient strains of Drosophila
melanogaster. Carcinogenesis, 15: 869-875.
Banerjee S (1988) DNA damage in rodent liver by 1,2-dichloroethane, a
hepatocarcinogen. Cancer Biochem Biophys, 10: 165-173.
Banerjee S & Van Duuren BL (1979) Binding of carcinogenic halogenated
hydrocarbons to cell macromolecules. J Natl Cancer Inst, 63: 707-711.
Banerjee S, Van Duuren BL, & Oruambo FI (1980) Microsome-mediated
covalent binding of 1,2-dichloroethane to lung microsomal protein and
salmon sperm DNA. Cancer Res, 40: 2170-2173.
Barbash JE & Reinhard M (1989) Abiotic dehalogenation of
1,2-dichloroethane and 1,2-dibromoethane in aqueous solution
containing hydrogen disulfide. Environ Sci Technol, 23(11): 1349-1357.
Barber RD, Donish WH, & Mueller KR (1981) A procedure for the
quantitative measurement of the mutagenicity of volatile liquids in
the Ames Salmonella microsome assay. Mutat Res, 90: 31-48.
Baretta ED, Steward RD, & Mutchler JE (1969) Monitoring exposure to
vinyl chloride vapour: breath analysis and continuous air sampling. Am
Ind Hyg Assoc J, 30: 537-544.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizzari ED, Ray M, Smith D, Tomer KB, Wagner R, & Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - A
multimedia environmental study. Biomed Environ Mass Spectrom,
7: 139-147.
Barrows ME, Petrocelli SR, Macek KJ, & Carroll JJ (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Haque R ed. Dynamics,
exposure and hazard assessment of toxic chemicals. Ann Arbor,
Michigan, Ann Arbor Science Publishers, chapter 24, pp 379-392.
Barsoum GS & Saad K (1934) Relative toxicity of certain chlorine
derivatives of the aliphatic series. Q J Pharm Pharmacol, 7: 205-214.
Bartlett EA (1979) Toxicity of ethylene dichloride to rainbow trout.
Midland, Michigan, Dow Chemical (Unpublished report).
Bauer U (1981) [Human exposure to environmental chemicals -
Investigations on volatile organic halogenated compounds in water,
air, food, and human tissues. III. Communication: results of
investigations.] Zbl Bakteriol Hyg I Abt Orig B, 174(3): 200-237 (in
German).
Benoit DA, Puglisi FA, & Olson DL (1982) A fathead minnow Pimephales
promelas early stage toxicity test method evaluation and exposure to
four organic chemicals. Environ Pollut, A28: 189-197.
Benson LO & Teta MJ (1993) Mortality due to pancreatic and
lymphopoietic cancers in chlorohydrin production workers. Br J Ind
Med, 50: 710-716.
Berck B (1974) Fumigant residues of carbon tetrachloride, ethylene
dichloride, and ethylene dibromide in wheat, flour, bran, middlings,
and bread. J Agric Food Chem, 22(6): 977-984.
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, & Bruser DM
(1982) The aquatic toxicity of organic compounds to embryo-larval
stages of fish and amphibians. Lexington, Kentucky, University of
Kentucky (Research Report No. 133).
Blum DJW & Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons and
correlations. J Water Pollut Control Fed, 63(3): 198-207.
Bogoyavlenski VF, Salikhova SKH, & Karpova EV (1968) [Clinical aspects
and therapy for ethylene dichloride poisoning.] Sov Med, 31: 107-109
(in Russian).
Bond EJ (1984) Manual of fumigation for insect control. Rome, Food and
Agriculture Organization of the United Nations, 432 pp (FAO Plant
Production and Protection Paper 54).
Bonnet P, Francin J-M, Grakiski D, Raoult G, & Zissu D (1980)
Détermination de la concentration léthale50 des principaux
hydrocarbures aliphatiques chlorés chez le rat. Arch Mal Prof,
41(6-7): 317-321.
Bouwer EJ & McCarty PL (1983) Transformation of 1- and 2-carbon
halogenated aliphatic organic compounds under methanogenic conditions.
Appl Environ Microbiol, 45(4): 1286-1294.
Brem H, Stein AB, & Rosenkranz HS (1974) The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer Res, 34: 2576-2579.
Bringmann G & Kühn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Bringmann G & Kühn R (1980) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res, 14: 231-241.
Bringmann G & Kühn R (1981) [Toxicity thresholds of pollutants in
ciliate flagellate protozoa, holozoic bacteriovorous ciliate protozoa
and saprozoic flagellate protozoa.] GWF-Wasser/Abwasser, 122: 308-313
(in German).
Brittebo EB, Kowalsky B, Ghantous H, & Brandt I (1989) Epithelial
binding of 1,2-dichloroethane in mice. Toxicology, 56: 35-45.
Brodzinsky R & Singh HB (1982) Volatile organic chemicals in the
atmosphere: An assessment of available data (Contract No. 68-02-3452).
Menlo Park, California, SRI International (Prepared for the US
Environmental Protection Agency, Office of Research and Development,
Environmental Sciences Research Laboratory, Research Triangle Park,
North Carolina, USA).
Bruckmann P, Kersten W, Funcke W, Balfanz E, Konig J, Theisen J, Ball
M, & Papke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17(12): 2363-2380.
Buijs W, van der Gen A, Mohn GR, & Breimer DD (1984) The direct
mutagenic activity of alpha, omega-dihalogenoalkanes in Salmonella
typhimurium. Mutat Res, 141: 11-14.
Callahan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR,
Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR, &
Gould C (1979) Water-related fate of 129 priority pollutants: Volume
II. Washington, DC, US Environmental Protection Agency
(EPA 40/4-79-029b).
Cetnarowicz J (1959) Experimental and clinical investigation on the
action of dichloroethane. Folia Med Crac, 1: 169-192.
Cheever KL, Cholakis JM, El-Hawari AM, Kovatch RM, & Weisburger EK
(1990) Ethylene dichloride: The influence of disulfiram or ethanol on
oncogenicity, metabolism and DNA covalent binding in rats. Fundam Appl
Toxicol, 14: 243-261.
Cheh AM, Hooper AB, Skochdopole J, Henke CA, & McKinnell RG (1980) A
comparison of the ability of frog and rat S-9 to activate promutagens
in the Ames test. Environ Mol Mutagen, 2: 487-508.
Chemical Marketing Reporter (1992) Chemical profile: Ethylene
dichloride. Chem Mark Report Mag, 241(19): 42.
Chiou CT, Peters LJ, & Freed VH (1979) A physical concept of
soil-water equilibria for nonionic organic compounds. Science,
206: 831-832.
Chiou CT, Freed VH, Peters LJ, & Kohnert RL (1980) Evaporation of
solutes from water. Environ Int, 3: 231-236.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984a) Ambient air
measurement of aromatic and halogenated hydrocarbons at urban, rural,
and motorway locations. Sci Total Environ, 39: 265-279.
Clark AI, McIntyre AE, Perry R, & Lester JN (1984b) Monitoring and
assessment of ambient atmospheric concentrations of aromatic and
halogenated hydrocarbons at urban, rural, and motorway locations.
Environ Pollut, B7: 141-158.
Class T & Ballschmiter K (1986) Chemistry of organic traces in air.
VI: Distribution of chlorinated C1 - C4 hydrocarbons in air over the
northern and southern Atlantic Ocean. Chemosphere, 15(4): 413-427.
Comba ME & Kaiser KLE (1983) Determination of volatile contaminants at
the ng-L-1 level in water by capillary gas chromatography with
electron capture detection. Int J Environ Anal Chem, 16: 17-31.
Comba ME & Kaiser KLE (1985) Volatile halocarbons in the Detroit River
and their relationship with contaminant sources. J Great Lakes Res,
11(3): 404-418.
Cottalasso D, Barisione G, Fontana L, Domenicotti C, Pronzato MA, &
Nanni G (1994) Impairment of lipoglycoprotein metabolism in rat liver
cells induced by 1,2-dichloro-ethane. J Occup Environ Med,
51: 281-285.
CEC (1990) Council directive of 27 July 1990 amending annex II to
directive 86/280/EEC on limit values and quality objectives for
discharges of certain dangerous substances included in List I of the
annex to directive 76/464/EEC. Brussels, Commission of the European
Communities.
CPI (Canadian Process Industries) (1991) CPI product profiles:
Ethylene dichloride. Don Mills, Ontario, Corpus Information Services.
Crebelli R & Carere A (1988) Genotoxic activity of halogenated
aliphatic hydrocarbons in Aspergillus nidulans. J Occup Toxicol,
8: 437-442.
Crebelli R, Conti G, Conti L, & Carere A (1984) Induction of somatic
segregation by halogenated aliphatic hydrocarbons in Aspergillus
nidulans. Mutat Res, 138: 33-38.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, & Carere A (1988)
Induction of chromosome malsegregation by halogenated organic solvents
in Aspergillus nidulans: Unspecific or specific mechanism? Mutat
Res, 201: 401-411.
Crespi CL, Seixas GM, Turner TR, Ryan CG & Penman BW (1985)
Mutagenicity of 1,2-dichloroethane and 1,2-dibromoethane in two human
lymphoblastoid cell lines. Mutat Res, 142: 133-140.
Daft JL (1987) Determining multifumigants in whole grains and legumes,
milled and low-fat grain products, spices, citrus fruit, and
beverages. J Assoc Off Anal Chem, 70: 734-739.
Daft JL (1988) Rapid determination of fumigant and industrial chemical
residues in food. J Assoc Off Anal Chem, 71(4): 748-760.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and nonfatty foods. J Agric Food Chem, 37: 560-564.
Daft JL (1991) Fumigants and related chemicals in foods: Review of
residue findings, contamination sources, and analytical methods. Sci
Total Environ, 100: 501-518.
Daft JL (1993) Fumigant analysis of foods. In: Sherman J & Cairns T
ed. Comprehensive analytical profiles of important pesticides. Boca
Raton, Florida, CRC Press, pp 235-281.
Dann T (1992) Unpublished data on concentrations of 1,2-dichloroethane
in ambient air across Canada. Ottawa, Ontario, Environment Canada.
Danni O, Aragno M, Tamagno E, & Ugazio G (1992) In vivo studies on
halogen compound interactions. IV. Interaction among different halogen
derivatives with and without synergistic action on liver toxicity. Res
Commun Chem Pathol Pharmacol, 76(3): 355-366.
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1975/77) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J
Hazard Mater, 1(4): 303-318.
Deschamps M & Band P (1993) Study of a cluster of childhood leukemia.
Health Rep, 5(1): 81-85.
Dilling WL, Tefertiller NB, & Kalos GJ (1975) Evaporation rates and
reactivities of methylene chloride, chloroform, 1,1,1-trichloroethane,
trichloroethylene, tetrachloroethylene, and other chlorinated
compounds in dilute aqueous solutions. Environ Sci Technol,
9(9): 833-838.
Dorndorf W, Kresse M, Christian W, & Katritzki KG (1975)
[Dichloroethane poisoning with myoclonic syndrome, epileptic attacks,
and irreversible cerebral effects.] Arch Psychiatr Nervenkr,
220: 373-379 (in German).
Drury JS & Hammons AS (1979) Investigations of selected environmental
pollutants: 1,2-Dichloroethane. Oak Ridge National Laboratory, Oak
Ridge, Tennessee; Environmental Protection Agency, Washington, D.C.
Office of Toxic Substances; Department of Energy, Washington, DC.
Washington, DC, US Environmental Protection Agency (EPA 560/2-78-006).
D'Souza R, Francis WR, Bruce RD, & Anderson ME (1987) Physiologically
based pharmacokinetic model for ethylene dichloride and its
application in risk assessment. In: Pharmacokinetics in risk
assessment - Drinking water and health. Washington, DC, National
Academy Press, vol 8, pp 286-301.
D'Souza RW, Francis WR, & Andersen ME (1988) Physiological model for
tissue glutathione depletion and increased resynthesis after ethylene
dichloride exposure. J Pharmacol Exp Ther, 245: 563-568.
Duprat P, Delsaut L, & Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les muqueuses
oculaires du lapin. Eur J Toxicol, 9: 171-177.
Easley DM, Kleopfer RD, & Carasea AM (1981) Gas chromatographic-mass
spectrometric determination of volatile organic compounds in fish. J
Assoc Off Anal Chem, 64: 653-656.
Ecobichon DJ & Allen MC (1990) New Brunswick - Water Quality
Surveillance Program. New Brunswick Public Water Supplies: Data
summary report 1990. Fredericton, New Brunswick, Canada, Department of
Health and Community Services, 66 pp.
Ehrenberg L, Osterman-Golkar S, Singh D, & Lundqvist U (1974) On the
reaction kinetics and mutagenic activity of methylating and
ß-halogenoethylating gasoline additives. Radiat Bot, 15: 185-194.
Ellington JJ, Stancil FE, Payne WD, & Trusty CD (1988) Measurement of
hydrolysis rate constants for evaluation of hazardous waste land
disposal: Volume III - Data on 70 chemicals. Athens, Georgia, US
Environmental Protection Agency (EPA/600/3-88/028. Entz RC, Thomas KW,
& Diachenko GW (1982) Residues of volatile halocarbons in foods using
headspace gas chromatography. J Agric Food Chem, 30(5): 846-849.
Environment Agency Japan (1983) Environmental monitoring of chemicals:
Environmental survey report of fiscal years 1980 and 1981. Tokyo,
Japan, Environment Agency.
Environment Agency Japan (1993) Chemicals in the environment - 1993.
Tokyo, Japan, Environment Agency.
Environment Canada (1986) Canada-Ontario agreement respecting Great
Lakes water quality. St. Clair river pollution investigation (Sarnia
area). Ottawa, Ontario, Environment Canada, 80 pp.
Enviro-Test Laboratories (1991) Cayley background study: Analysis of
food products for target organic and inorganic parameters. Edmonton,
Alberta, Canada, Enviro-Test Laboratories, 37 pp
(Report No. 91-E1208).
Enviro-Test Laboratories (1992) Windsor area background study:
Analysis of food products for target organic and inorganic parameters.
Edmonton, Alberta, Enviro-Test Laboratories, 36 pp
(Report No. 92-E1052).
FAO/WHO (1971) Evaluation of food additives. Specifications for the
identity and purity of food additives and their toxicological
evaluation: some extraction solvents and certain other substances; and
a review of the technological efficacy of some antimicrobial agents.
Fourteenth report of the Joint FAO/WHO Expert Committee on Food
Additives. Geneva, World Health Organization, 36 pp (WHO Technical
Report Series No. 462).
FAO/WHO (1980) Evaluation of certain food additives. Twenty-third
report of the Joint FAO/WHO Expert Committee on Food Additives.
Geneva, World Health Organization (WHO Technical Report Series No. 648
and corrigenda).
FAO/WHO (1992) Evaluation of certain food additives and naturally
occurring toxicants. Thirty-ninth report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization (WHO
Technical Report Series No. 828).
Fellin P, Barnett SE, & Tran QA (1992) Results of a national pilot
survey of airborne volatile organic compounds in Canadian residences:
Volume 1. Downsview, Ontario, Canada, Concord Environmental
Corporation, 156 pp (prepared for Health and Welfare Canada, Ottawa).
Ferreri AM, Rocchi P, Capucci A, & Prodi G (1983) Induction of
diphtheria toxin-resistant mutants in human cells by halogenated
compounds. J Cancer Res Clin Oncol, 105: 111-112.
Finlayson-Pitts BJ & Pitts JN (1986) Atmospheric chemistry:
Fundamentals and experimental techniques. New York, Wiley-Interscience
Publications.
Foster GD & Tullis RE (1984) A quantitative structure-activity
relationship between partition coefficients and the acute toxicity of
naphthalene derivatives in Artemia salina nauplii. Aquat Toxicol,
5: 245-254.
Foureman GL & Reed DJ (1985) Evidence for a non-episulfonium ion
intermediate during alkylation by S-[2-(N7-guanyl)ethyl]
glutathione, the major DNA adduct formed from 1,2-dibromoethane.
Biochemistry, 25: 2192-2198.
Freiria-Gandara MJ, Lorenzo-Ferreira RA, Alvarez-Devesa, A & Bermejo F
(1992) Occurrence of halogenated hydrocarbons in the water supply of
different cities of Galicia (Spain). Environ Technol, 13: 437-447.
Freitag D, Ballhorn L, Behechti A, Fischer K, & Thumm W (1994)
Structural configuration and toxicity of chlorinated alkanes.
Chemosphere, 28(2): 253-259.
Fujii T (1977) Direct aqueous injection gas chromatography-mass
spectrometry for analysis of organohalides in water at concentrations
below the parts per billion level. J Chromatogr, 139: 297-302.
Garrison SC & Leadingham RS (1954) A fatal case of ethylene dichloride
poisoning in an occupational therapy department of a neuropsychiatric
hospital. Am J Phys Med, 33: 230-237.
Geiger DL, Northcott CE, Call DJ, & Brooke LT (1985)
1,2-Dichloroethane. In: Geiger DL, Northcott CE, Call DJ, & Brooke LT
ed. Acute toxicities of organic chemicals to fathead minnow
(Pimephales promelas). Superior, Wisconsin, University of
Wisconsin-Superior, Center for Lake Superior Environmental Studies,
vol 2, pp 41-42.
Giri AK & Que Hee SS (1988) In vivo sister chromatid exchange
induced by 1,2-dichloroethane on bone marrow cells of mice. Environ
Mol Mutagen, 12: 331-334.
Gocke E, Wild D, Eckhardt K, & King MT (1983) Mutagenicity studies
with the mouse spot test. Mutat Res, 117: 201-212.
Golder Associates (1987) Testing of specific organic compounds in
soils in background in urban areas: Port Credit and
Oakville/Burlington, Ontario. Mississauga, Ontario, Canada, Golder
Associates (Working paper to Shell Canada Ltd and Texaco Canada Ltd).
Gradiski D, Bonnet P, Raoult G, & Magadur JL (1978) Toxicité aiguë
comparée par inhalation des principaux solvants aliphatiques chlorés.
Arch Mal Prof, 39(4-5): 249-257.
Grimsrud EP & Rasmussen RA (1975) Survey and analysis of halocarbons
in the atmosphere by gas chromatography-mass spectrometry. Atmos
Environ, 9: 1014-1017.
Guengerich FP, Crawford WM, Domoradzki JY, MacDonald TL, & Watanabe PG
(1980) In vitro activation of 1,2-dichloroethane by microsomal and
cytosolic enzyme. Toxicol Appl Pharmacol, 55: 303-317.
Guengerich FP, Mason PS, Stott WT, Fox TR, & Watanabe PG (1981) Role
of 2-haloethylene oxides and vinylchloride in irreversible binding to
protein and DNA. Cancer Res, 41: 4391-4398.
Guengerich FP, Koga N, Inskeep PB, & Ozawa N (1986) Proceedings of the
Second International Symposium on the Synthesis and Application of
Isotopically Labelled Compounds. New York, Elsevier Science
Publishers.
Guengerich FP, Kim DH, & Iwasaki M (1991) Role of human cytochrome
P-450 IIE1 in the oxidation of many low molecular weight cancer
suspects. Chem Res Toxicol, 4: 168-179.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Hadengue MA & Martin R (1953) Un cas d'intoxication mortelle par le
dichloréthane. Soc Méd Lég, 33: 247-249.
Hatch GG, Mamay PD, Ayer ML, Casto BC, & Nesnow S (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo cells by
gaseous and volatile chlorinated methanes and ethanes. Cancer Res,
43: 1945-1950.
Health Canada (1994) Unpublished update to Canadian Environmental
Protection Act Priority Substances report on 1,2-dichloroethane.
Ottawa, Ontario, Environmental Health Directorate, Priority Substances
Section.
Heikes DL (1987a) Determination of residual chlorinated solvents in
decaffeinated coffee by using purge and trap procedure. J Assoc Off
Anal Chem, 70(1): 176-180.
Heikes DL (1987b) Pesticide and industrial chemical residues - purge
and trap method for determination of volatile halocarbons and carbon
disulfide in table-ready foods. J Assoc Off Anal Chem, 70(2): 215-226.
Heikes DL (1990) Environmental contaminants in table-ready foods from
the total diet program of the Food and Drug Administration. In: Food
contamination from environmental sources. New York, John Wiley and
Sons, pp 31-57 (Advances in Environmental Science and Technology
Series No. 23).
Heikes DL & Hopper ML (1986) Purge and trap method for determination
of fumigants in whole grains, milled grain products, and intermediate
grain-based foods. J Assoc Off Anal Chem, 69(6): 990-998.
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hellman B & Brandt I (1986) Effects of carcinogenic halogenated
aliphatic hydrocarbons on [3H]thymidine incorporation into various
organs of the mouse. A comparison between 1,2-dibromoethane and
1,2-dichloroethane. Mutat Res, 163: 193-199.
Hemminki K, Falck K, & Vainio H (1980) Comparison of alkylation rates
and mutagenicity of directly acting industrial and laboratory
chemicals: epoxides, glycidyl ethers, methylating and ethylating
agents, halogenated hydrocarbons, hydrazine derivatives, aldehydes,
thiouram and dithiocarbamate derivatives. Arch Toxicol, 46: 277-285.
Heppel LA, Neal PA, Perrin TL, Endicott KM, & Porterfield VT (1945)
The toxicology of 1,2-dichloroethane (ethylene). III. Its acute
toxicity and the effect of protective agents. J Pharmacol Exp Ther,
84: 53-63.
Heppel LA, Neal PA, Perrin TL, Endicott KM, & Porterfield VT (1946)
The toxicology of 1,2-dichloroethane (ethylene dichloride). V. The
effects of daily inhalations. J Ind Hyg, Toxicol, 28(4): 113-120.
Hiatt MH (1981) Analysis of fish and sediment for volatile priority
pollutants. Anal Chem, 53: 1541-1543.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography/mass spectrometry. Anal Chem, 55: 506-516.
Hill J, Kollig HP, Paris DF, Wolfe NL, & Zepp RG (1976) Dynamic
behaviour of vinyl chloride in aquatic ecosystems. Athens, Georgia, US
Environmental Protection Agency (EPA/600/3-76/001).
Hinkel GK (1965) [Oral dichloroethane intoxication of children.] Dtsch
Gesudheitswes, 20: 1327-1331 (in German).
Hofmann HT, Birnsteil H, & Jobst P (1971) [The inhalation toxicity of
1,1- and 1,2-dichloroethane.] Arch Toxikol, 27: 248-265 (in German).
Hooper K, Gold LS, & Ames BN (1980) The carcinogenic potency of
ethylene dichloride in two animal bioassays: A comparison of
inhalation and gavage studies. In: Ames B, Infante P, & Reitz R ed.
Ethylene dichloride: a potential health risk? Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp 65-80 (Banbury Report No. 5).
HSDB (1993) Hazardous substances data bank (revision 9/2/93).
Bethesda, Maryland, National Library of Medicine, National Toxicology
Information Program.
Hueper WC & Smith C (1935) Fatal ethylene dichloride poisoning. Am J
Med Sci, 189: 778-784.
IARC (1979) Some halogenated hydrocarbons. Lyon, International Agency
for Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 20).
Igwe OJ, Que Hee SS, & Wagner WD (1986a) Interaction between
1,2-dichloroethane and tetraethylthiuram disulfide (disulfiram). II.
Hepatotoxic manifestations with possible mechanism of action. Toxicol
Appl Pharmacol, 86: 286-297.
Igwe OJ, Que Hee SS, & Wagner WD (1986b) Interaction between
1,2-dichloroethane and disulfiram. I. Toxicologic effects. Fundam Appl
Toxicol, 6: 733-746.
Inskeep PB, Koga N, Cmarik JL, & Guengerich FP (1986) Covalent binding
of 1,2-dihaloalkanes to DNA and stability of the major DNA adduct,
S-[2(N7-guanyl)ethyl] glutathione. Cancer Res, 46: 2839-2844.
IPCS (International Programme on Chemical Safety) (1987) Environmental
health criteria 62: 1,2-Dichloroethane. Geneva, World Health
Organization.
IPCS (International Programme on Chemical Safety) (1994) Environmental
health criteria 170: The derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization.
Isacson P, Bean JA, Splinter R, Olson DB, & Kohler J (1985) Drinking
water and cancer incidence in Iowa. III. Association of cancer with
indices of contamination. Am J Epidemiol, 121(6): 856-869.
Isnard P & Lambert S (1988) Estimating bioconcentration factors from
octanol-water partition coefficient and aqueous solubility.
Chemosphere, 17(1): 21-34.
Jafvert CT & Wolfe NL (1987) Degradation of selected halogenated
ethanes in anoxic sediment-water systems. Environ Toxicol Chem,
6: 827-837.
Jakobson I, Wahlberg JE, Holmberg B, & Johansson G (1982) Uptake via
the blood and elimination of 10 organic solvents following
epicutaneous exposure of anesthetized guinea pigs. Toxicol Appl
Pharmacol, 63: 181-187.
Janssen DB, Scheper A, Dijkhuizen L, & Witholt B (1985) Degradation of
halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10.
Appl Environ Microbiol, 49(3): 673-677.
Jeffers PM, Ward L, Woytowich L, & Wolfe NL (1989) Homogeneous
hydrolysis rate constants for selected chlorinated methanes, ethanes,
ethenes and propanes. Environ Sci Technol, 23(8): 965-969.
Jenssen D & Ramel C (1980) The micronucleus test as part of a
short-term mutagenicity test programme for the prediction of
carcinogenicity evaluated by 43 agents tested. Mutat Res, 75: 191-202.
Jonsson A & Berg S (1980) Determination of 1,2-dibromoethane,
1,2-dichloroethane, and benzene in ambient air using porous polymer
traps and gas chromatographic-mass spectrometric analysis with
selected ion monitoring. J Chromatogr, 190: 97-106.
Kaiser KLE & Comba ME (1986) Volatile hydrocarbon contaminant survey
of the St. Clair River. Water Pollut Res J Can, 21(3): 323-331.
Kaiser KLE, Comba ME, & Huneault H (1983) Volatile hydrocarbon
contaminants in the Niagara River and in Lake Ontario. J Great Lakes
Res, 9(2): 212-223.
Kanada T & Uyeta M (1978) Mutagenicity screening of organic solvents
in microbial systems. Mutat Res, 54: 215.
Kavlock R, Chernoff N, Carver B, & Kopfler F (1979) Teratology studies
in mice exposed to municipal drinking water concentrates during
organogenesis. Food Cosmet Toxicol, 17: 343-347.
Kerster HW & Schaeffer DJ (1983) Brine shrimp (Artemia salina)
nauplii as a teratogen test system. Ecotoxicol Environ Saf,
7: 342-349.
King MT, Beikirch H, Eckhardt K, Gocke E, & Wild D (1979) Mutagenicity
studies with X-ray-contrast media, analgesics, antipyretics,
antirheumatics and some other pharmaceutical drugs in bacterial,
Drosophila and mammalian test systems. Mutat Res, 66: 33-43.
Kirschmer P & Ballschmiter K (1983) Baseline studies of global
pollution. VIII. The complex pattern of C1 - C4 organohalogens in
continental and marine background air. Int J Environ Anal Chem,
14: 275-284.
Klaunig JE, Ruch RJ, & Pereira MA (1986) Carcinogenicity of
chlorinated methane and ethane compounds administered in drinking
water to mice. Environ Health Perspect, 69: 89-95.
Kliest J, Fast T, Boley JSM, van de Wiel H, & Bloemen H (1989) The
relationship between soil contaminated with volatile organic compounds
and indoor air pollution. Environ Int, 15: 419-425.
Knie VJ, Halke A, Juhnke I, & Schiller W (1983) Results of studies on
chemical substances with four biotests. Dtsch Gewasserkd Mitt,
27(3): 77-79.
Koga N, Inskeep PB, Harris TM, & Guengerich FP (1986)
S-[2-(N7-guanyl)ethyl] glutathione, the major DNA adduct formed
from 1,2-dibromoethane. Biochemistry, 25: 2192-2198.
Konemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. Part 1: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-221.
Kozik IV (1957) [Some problems of occupational hygiene in the use of
dichloroethane in the aircraft industry.] Gig Tr Prof Zabol, 1: 31-38
(in Russian).
Kramers PG, Mout HC, Bissumbhar B, & Mulder CR (1991) Inhalation
exposure in Drosophila mutagenesis assays: experiments with aliphatic
halogenated hydrocarbons, with emphasis on the genetic activity
profile of 1,2-dichloroethane. Mutat Res, 252: 17-33.
Kretzschmar JG, Peperstraete H, & Tymen T (1976) Air pollution in and
around Tessenderlo (The Netherlands). Neth Extern, 5: 147-178.
Krill RM & Sonzongni WC (1986) Chemical monitoring of Wisconsin's
groundwater. J Am Water Works Assoc, 78(9): 70-75.
Kronevi T, Wahlberg JE, & Holmberg B (1981) Skin pathology following
epicutaneous exposure to seven organic solvents. Int J Tissue React,
3: 21-30.
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Kuwabara T, Quevedo AR, & Cogan DG (1968) An experimental study of
dichloroethane poisoning. Arch Ophthalmol, 79: 321-330.
Lane RW, Riddle BL, & Borzelleca JF (1982) Effects of
1,2-dichloroethane and 1,1,1-trichloroethane in drinking water on
reproduction and development in mice. Toxicol Appl Pharmacol,
63: 409-421.
LaRegina J & Bozzelli JW (1986) Volatile organic compounds at
hazardous waste sites and a sanitary landfill in New Jersey - An
up-to-date review of the present situation. Environ Prog,
5(1): 18-27.
Larionov VG & Kokarovtseva MG (1976) Morphological constitution of
peripheral blood in intoxication with dichloroethane and its
metabolites. In: Actual problems of pesticide application in different
climatographic zones. Yerevan, Aiastan Publishers, pp 131-133.
Leblanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lesage S, McBride RA, Cureton PM, & Brown S (1993) Fate of organic
solvents in landfill leachates under simulated field conditions and in
anaerobic microcosms. Waste Manage Res, 11: 215-226.
Letkiewicz F, Johnston P, & Coleman J (1982) Occurrence of
1,2-dichloroethane in drinking water, food and air (Contract
68-01-6185). Washington, DC, JRB Associates (Prepared for the US
Environmental Protection Agency).
Li KM & Cheng WT (1991) The occupational exposure level of
1,2-dichloroethane in an EM preparation laboratory. J R Soc Health,
111(5): 169.
Lin ELC, Mattox JK, & Pereira MA (1985) Glutathione plus cytosol- and
microme-mediated binding of 1,2-dichloroethane to polynucleotides.
Toxicol Appl Pharmacol, 78: 428-435.
Lum KR & Kaiser KLE (1986) Organic and inorganic contaminants in the
St. Lawrence River: Some preliminary results on their distribution.
Water Pollut Res J Can, 21(4): 592-603.
Luznikov EA, Lisovik GA, & Novikovskaya TV (1985) [Metabolism of
1,2-dichloroethane in human organisms after acute poisonings.]
Forensic Med Expert, 2: 47-49 (in Russian).
McCann J, Simmon V, Streitwieser D, & Ames BN (1975) Mutagenicity of
chloroacetaldehyde, a possible metabolic product of 1,2-dichloroethane
(ethylene dichloride), chloroethane (ethylene chlorohydrin), vinyl
chloride and cyclophosphamide. Proc Natl Acad Sci (USA),
72: 3190-3191.
McCollister DD, Hollingsworth RL, Oyen F, & Rowe VK (1956) Comparative
inhalation toxicity of fumigant mixtures. Individual and joint effect
of ethylene dichloride, carbon tetrachloride, and ethylene dibromide.
Arch Ind Health, 13: 1-7.
McPherson RW, Starks CM, & Fryar GJ (1979) Vinyl chloride
monomer...what you should know. Hydrocarb Process, 3: 75-88.
MAFF (Ministry of Agriculture, Fisheries and Food) (1982) Food
surveillance paper No. 9. London, Her Majesty's Stationery Office.
MAFF (Ministry of Agriculture, Fisheries and Food) (1984) Food
surveillance paper No. 16. London, Her Majesty's Stationery Office.
MAFF (Ministry of Agriculture, Fisheries and Food) (1989) Food
surveillance paper No. 25. London, Her Majesty's Stationery Office.
MAFF/HSE (Ministry of Agriculture, Fisheries and Food/Health and
Safety Executive) (1993) Annual Report of the Working Party on
Pesticide Residues: 1992. Supplement to the Pesticides Register, 1993.
London, Her Majesty's Stationery Office.
Maltoni C, Valgimigli L, & Scarnato C (1980) Long-term carcinogenic
bioassays on ethylene dichloride administered by inhalation to rats
and mice. In: Ames BN, Infante P, & Reitz R ed. Ethylene dichloride: A
potential health risk? Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 3-33 (Banbury Report No. 5).
Martin G, Knorpp K, Huth K, Heinrich F, & Mittermayer C (1969)
Clinical features, pathogenesis and management of dichloroethane
poisoning. Germ Med Mon, 14: 62-67.
Menschick H (1957) [Acute inhalation intoxications by symmetric
dichloroethane.] Arch Gewerbepathol Gewerbehyg, 15: 241-252
(in German).
Mihaylova A (1976) [On the action of high temperature of the
environment on the acute and subacute toxicity of dichloroethane.] Gig
Zdraveopaz, 1: 32-36 (in Bulgarian).
Milman HA, Storystorey DL, Riccio ES, Sivak A, Tu AS, Williams GM,
Tong C, & Tyson CA (1988) Rat liver foci and in vitro assays to
detect initiating and promoting effects of chlorinated ethanes and
ethylenes. Ann NY Acad Sci, 534: 521-530.
Ministry of the Environment (1991) Drinking water surveillance program
data base. Ottawa, Canada, Ministry of the Environment, Water
Resources Branch, 27 pp (105 entries).
Mitoma C, Steeger T, Jackson SE, Wheeler KP, Rogers JH, & Milman HA
(1985) Metabolic disposition study of chlorinated hydrocarbons in rats
and mice. Drug Chem Toxicol, 8: 183-194.
Morgan DL, Bucher JR, Elwell MR, Lilja HS, & Murthy AS (1990)
Comparative toxicity of ethylene dichloride in F344/N, Sprague-Dawley
and Osborne-Mendel rats. Food Chem Toxicol, 28: 839-845.
Morgan DL, Cooper SW, Carlock DL, Sykora JJ, Sutton B, Mattie DR, &
McDougal JN (1991) Dermal absorption of neat and aqueous volatile
organic chemicals in Fischer 344 rat. Environ Res, 55: 51-63.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116:185-216.
Morozov GN (1958) [On acute dichloroethane poisoning.] Farmakol
Toksicol, 21(1): 76-78 (in Russian).
Munson AE, Sanders VM, Douglas KA, Sain LE, Kauffmann BM, & White KL
(1982) In vivo assessment of immunotoxicity. Environ Health
Perspect, 43: 41-52.
Natsyuk MV & Chekman IS (1975) The content of nicotinamide coenzymes
in the liver and myocardium of rats poisoned with dichloroethane. Bull
Exp Biol Med, 4: 58-60.
Natsyuk MV & Mudritsky AD (1974) Clinical and biochemical
characteristics of hepatic and renal disorders in acute dichloroethane
intoxications. Mil Med J, 10: 48-50.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity. Bethesda, Maryland, National Cancer Institute (HEW
(NIH) Publication No. 78-1305).
Nestmann ER, Lee EGH, Matula TI, Douglas GR, & Mueller JC (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using Salmonella/mammalian-microsome assay. Mutat Res,
79: 203-212.
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR (1985)
The toxicity of selected organic chemicals to the earthworm Eisenia
foetida. J Environ Qual, 14(3): 383-388.
Nevrotsky VK, Kashin LM, Kulinskaya IL, Mikhailovskaya LF, Shmuter LM,
Burlaka-Vouk ZI, & Zadorozhny BV (1971) Comparative toxicity
evaluation of several industrial poisons in protracted inhalation
exposure to low concentrations. In: Proceedings of the 8th Ukranian
Hygiene Congress, Kiev, 1971, pp 224-226.
Nimitz JS & Skaggs SR (1992) Estimating tropospheric lifetimes and
ozone-depletion potentials of one- and two-carbon hydrofluorocarbons
and hydrochlorofluorocarbons. Environ Sci Technol, 26(4): 739-744.
NIOSH (1994) Registry of toxic effects of chemical substances.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health.
NIOSH (1984) Manual of analytical methods: Method 1003. Cincinnati,
Ohio, National Institute for Occupational Safety and Health.
Nouchi T, Miura H, Kanayama M, Mizuguchi O, & Takano T (1984) Fatal
intoxication by 1,2-dichloroethane - A case report. Int Arch Occup
Environ Health, 54: 111-113.
NTP (1991) Toxicity studies of 1,2-dichloroethane (ethylene
dichloride) in F344/N rats, Sprague Dawley rats, Osborne-Mendel rats,
and B6C3F1 mice (drinking water and gavage studies). Research
Triangle Park, North Carolina, National Toxicology Program (NIH
Publication No. 91-3123).
Nylander PO, Olofsson H, Rasmuson B, & Svahlin H (1978) Mutagenic
effects of petrol in Drosophila melanogaster. I. Effects of benzene
and 1,2-dichloroethane. Mutat Res, 57: 163-167.
Oldenhuis R, Vink RLJM, Janssen DB, & Witholt B (1989) Degradation of
chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b
expressing soluble methane monooxygenase. Appl Environ Microbiol,
55(11): 2819-2826.
Oliver BG & Pugsley CW (1986) Chlorinated contaminants in St. Clair
River sediments. Water Pollut Res J Can, 21(3): 368-379.
Otson R (1987) Purgeable organics in Great Lakes raw and treated
water. Int J Environ Anal Chem, 31(1): 41-53.
Otson R & Williams DT (1982) Headspace chromatographic determination
of water pollutants. Anal Chem, 54: 942-946.
Otson R, Williams DT, & Bothwell PD (1982) Industrial chemicals -
Volatile organic compounds in water at thirty Canadian potable water
treatment facilities. J Assoc Off Anal Chem, 65(6): 1370-1374.
Parkes DG, Ganz CR, Polinsky A, & Schulze J (1976) A simple gas
chromatographic method for the analysis of trace organics in ambient
air. Am Ind Hyg Assoc J, 37: 165-173.
Payan JP, Beydon D, Fabry JP, Brondeau MT, Ban M, & de Ceaurriz J
(1993) Urinary thiodiglycolic acid and thioether excretion in male
rats dosed with 1,2-dichloroethane. J Appl Toxicol, 13: 417-422.
Pearson CR & McConnell G (1975) Chlorinated C1 and C2 hydrocarbons
in the marine environment. Proc R Soc (Lond), B189: 305-332.
Pellizzari ED (1982) Analysis for organic vapor emissions near
industrial and chemical waste disposal sites. Environ Sci Technol,
16(11): 781-785.
Perocco P & Prodi G (1981) DNA damage by haloalkanes in human
lymphocytes cultured in vitro. Cancer Lett, 13: 213-218.
Pleil JD, Oliver KD, & McClenny WA (1988) Ambient air analyses using
nonspecific flame ionization and electron capture detection compared
by mass spectroscopy. J Air Pollut Control Assoc, 38: 1006-1010.
Principe P, Dogliotti E, Bignami M, Crebelli R, Falcone E, Fabrizi M,
Conti G, & Comba P (1981) Mutagenicity of chemicals of industrial and
agricultural relevance in Salmonella, Streptomyces, and Aspergillus.
J Sci Food Agric, 32: 826-832.
Proctor CJ, Warren ND, & Bevan MAJ (1989) Measurements of
environmental tobacco smoke in an air-conditioned office building.
Environ Technol Lett, 10: 1003-1018.
Prodi G, Arfelini G, Colacci A, Grilli S, & Mazzulo M (1986)
Interaction of halocompounds with nucleic acids. Toxicol Pathol, 14:
438-444.
Qiu LX, Shi SH, Xing SB, & Chen SG (1992) Rate constants for the
reactions of OH with five halogenated substituted ethanes from 292 K
to 366 K. J Phys Chem, 96: 685-689.
Rannug U (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichloroethane. Mutat Res, 76: 269-295.
Rannug U & Beije B (1979) The mutagenic effect of 1,2-dichloroethane
on Salmonella typhimurium. II. Activation by the isolated perfused
rat liver. Chem-Biol Interact, 24: 265-285.
Rannug U, Sundvall A, & Ramel C (1978) The mutagenic effect of
1,2-dichloroethane on Salmonella typhimurium. I. Activation through
conjugation with glutathione in vitro. Chem-Biol Interact, 20: 1-16.
Rao KS, Murray JS, Deacon MM, John JA, Calhoun LL, & Young JT (1980)
Teratogenicity and reproduction studies in animals inhaling ethylene
dichloride. In: Ames B, Infante P, & Reitz R ed. Ethylene dichloride:
A potential health risk? Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 149-166 (Banbury Report No. 5).
Reid BJ, Morgan JD, & Whelen MA (1982) A preliminary examination of
the effects of ethylene dichloride on the hatchability of Coho salmon
eggs (Oncorhynchus kisutch). Can Tech J Fish Aquat Sci,
1163: 145-153.
Reitz RH, Fox TR, Ramsey JC, Quast JF, Langvardt PW, & Watanabe PG
(1982) Pharmacokinetics and macro-molecular interactions of ethylene
dichloride in rats after inhalation or gavage. Toxicol Appl Pharmacol,
62: 190-204.
Reitz RH, Fox TR, Domordzki JY, Quast JF, Langvardt PW, & Watanabe PG
(1980) Pharmacokinetics and macromolecular interactions of ethylene
dichloride: Comparison of oral and inhalation exposures. In: Ames BN,
Infante P, & Reitz R. ed. Ethylene dichloride: A potential health
risk? Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp 135-148 (Banbury Report No. 5).
Richter JE, Peterson SF, & Kleiner CF (1983) Acute and chronic
toxicity of some chlorinated benzenes, chlorinated ethanes, and
tetrachloroethylene to Daphnia magna. Arch Environ Contam Toxicol,
12: 679-684.
Roldan-Arjona T, Garcia-Pedrajas MD, Luque-Romero RL, Hera C, & Pueyo
C (1991) An association between mutagenicity of the Ara test of
Salmonella typhimurium and carcinogenicity in rodents for 16
halogenated aliphatic hydrocarbons. Mutagenesis, 6: 199-205.
Romert L, Magnusson J, & Ramel C (1990) The importance of glutathione
and glutathione transferase for somatic mutations in Drosophila
melanogaster induced in vivo by 1,2-dichloroethane.
Carcinogenesis, 11: 1399-1402.
Rosenberg R, Grahn O, & Johansson L (1975) Toxic effects of aliphatic
chlorinated by-products from vinyl chloride production on marine
animals. Water Res, 9: 607-612.
Sack TM, Steele DH, Hammerstrom K, & Remmers J (1992) A survey of
household products for volatile organic compounds. Atmos Environ,
26A(6): 1063-1070.
Saitanov AO & Arsenieva SS (1969) [Electrocardiographic changes due to
acute dichloroethane poisoning: experimental investigation.] Gig Tr
Prof Zabol, 7: 49-50 (in Russian).
Sato A, Nakajima T, & Koyama Y (1981) Dose-related effects of a single
dose of ethanol on the metabolism in rat liver of some aromatic and
chlorinated hydrocarbons. Toxicol Appl Pharmacol, 60: 8-15.
Sauer TC (1981) Volatile organic compounds in open ocean and coastal
surface waters. Org Geochem, 3: 91-101.
Schasteen CS & Reed DJ (1983) The hydrolysis and alkylation activities
of S-(2-haloethyl)-L-cysteine analogues - evidence for extended
half-life. Toxicol Appl Pharmacol, 70: 423-432.
Schönborn H, Prellwitz W, & Baum P (1970) [Consumption coagulation
pathology of 1,2-dichloroethane poisoning.] Klin Wochenschr,
48: 822-824 (in German).
Schultz K, Ghosh L, & Banerjee S (1992) Neoplastic expression in
murine cells induced by halogenated hydrocarbons. In vitro Cell Dev
Biol, 28A: 267-272.
Sherwood RL, O'Shea PT, Thomas W, Ratajczak HV, Aranyi C, & Graham JA
(1987) Effects of inhalation of ethylene dichloride on pulmonary
defenses of mice and rats. Toxicol Appl Pharmacol, 91: 491-496.
Shmuter LM (1977) [Effect of chronic exposure to low concentrations of
chlorinated hydrocarbons of the ethane series on specific and
non-specific reactivity of animals in vivo.] Gig Tr Prof Zabol,
8: 38-42 (in Russian).
Simmon VF (1980) Review of non-bacterial tests of the genotoxic
activity of ethylene dichloride. In: Ames B, Infante P, & Reitz R ed.
Ethylene dichloride: A potential health risk? Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, pp 97-103 (Banbury Report No. 5).
Simula TP, Glancey MG, & Wolf CR (1993) Human glutathione
S-transferase-expressing Salmonella typhimurium tester strains to
study the activation/detoxification of mutagenic compounds: studies
with halogenated compounds, aromatic amines and aflatoxin B1.
Carcinogenesis, 14: 1371-1376.
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1980) Atmospheric
measurements of selected toxic organic chemicals. Washington, DC, US
Environmental Protection Agency, 35 pp (EPA-600/3-80-072;
NTIS PB80-89).
Singh HB, Salas LJ, Smith AJ, & Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban environments.
Atmos Environ, 15: 601-612.
Singh HB, Salas LJ, & Stiles RE (1982) Distribution of selected
gaseous organic mutagens and suspect carcinogens in ambient air.
Environ Sci Technol, 16: 872-880. Singh HB, Salas IJ, & Stiles RE
(1983) Selected man-made halogenated chemicals in the air and oceanic
environment. J Geophys Res, 88(C6): 3675-3683.
Smyth HF (1969) Acute toxicity of ethyl compounds. In: Spector WC ed.
Handbook of toxicology. Philadelphia, Pennsylvania, W.B. Saunders,
vol 1, pp 134-135.
Spence JW & Hanst PL (1978) Oxidation of chlorinated ethanes. J Air
Pollut Control Assoc, 28(3): 250-253.
Spencer HC, Rowe VK, Adams EM, McCollister DD, & Irish DD (1951) Vapor
toxicity of ethylene dichloride determined by experiments on
laboratory animals. Am Med Assoc Arch Ind Hyg Occup Med, 4: 482-493.
Spreafico F, Zuccato E, Marcucci F, Sironi M, Paglialunga S, Madonna
M, & Mussini E (1980) Pharmacokinetics of ethylene dichloride in rats
treated by different routes and its long-term inhalatory toxicity. In:
Ames B, Infante P, & Reitz R ed. Ethylene dichloride: A potential
health risk? Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp 107-133 (Banbury Report No. 5).
Storer RD & Conolly RB (1983) Comparative in vivo genotoxicity and
acute hepatotoxicity of three 1,2-dihaloethanes. Carcinogenesis,
4: 1491-1494.
Storer RD & Conolly RB (1985) An investigation of the role of
microsomal oxidative metabolism in the in vivo genotoxicity of
1,2-dichloroethane. Toxicol Appl Pharmacol, 77: 36-46.
Storer RD, Jackson NM, & Conolly RB (1984) In vivo genotoxicity and
acute hepatotoxicity of 1,2-dichloroethane in mice: Comparison of
oral, intraperitoneal, and inhalation routes of exposure. Cancer Res,
44: 4267-4271.
Story DL, Meierhenry EF, Tyson CA, & Milman HA (1986) Differences in
rat liver enzyme-positive foci produced by chlorinated aliphatics and
phenobarbital. Toxicol Ind Health, 2: 351-362.
Strobel K & Grummt T (1987) Aliphatic and aromatic halocarbons as
potential mutagens in drinking water. III. Halogenated ethanes and
ethenes. Toxicol Environ Chem, 15: 101-128.
Sundheimer DW, White RD, Brendel K, & Sipes IC (1982) The
bioactivation of 1,2-dibromoethane in rat hepatocytes: covalent
binding to nucleic acids. Carcinogenesis, 3: 1129-1133.
Suveev IM & Babichenko ME (1969) [On the clinic and cure of acute
intoxication with dichloroethane vapours.] Gig Tr Prof Zabol,
13: 50-51 (in Russian).
Symons JM, Bellar TA, Carswell JK, Demarco J, Kropp KL, Robeck GG,
Seeger DR, Slocum CJ, Smith BL, & Stevens AA (1975) National organics
reconnaissance survey for halogenated organics. J Am Water Works
Assoc, 67: 634-648.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53(10): 1503-1518.
Tan EL & Hsie AW (1981) Mutagenicity and cytotoxicity of haloethanes
as studied in the CHO/HGPRT system. Mutat Res, 90: 183-191.
Tang NH, Blum DJW, & Speece RE (1990) Comparison of serum bottle
toxicity test with OECD method. J Environ Eng, 116(6): 1076-1084.
Taningher M, Parodi S, Grilli S, Colacci A, Mazzullo M, Bordone R, &
Santi L (1991) Lack of correlation between alkaline DNA fragmentation
and DNA covalent binding induced by polychloroethanes after in vivo
administration. Problems related to the assessment of a carcinogenic
hazard. Cancer Detect Prev, 15: 35-39.
Theiss JC, Stoner GD, Shimkin MB, & Weisberger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer Res,
37: 2717-2720.
Torkelson TR & Rowe VK (1981) Halogenated aliphatic hydrocarbons. In:
Clayton GD & Clayton FE ed. Toxicology: Patty's industrial hygiene
and toxicology, 3rd ed. New York, John Wiley and Sons, vol 2B,
pp 3491-3497.
Troisi FM & Cavallazzi D (1961) [A fatal case of poisoning from
inhalation of dichloroethane vapours.] Med Lav, 52: 612-618
(in Italian).
Tsuruta H (1975) Percutaneous absorption of organic solvents: 1)
Comparative study of the in vivo percutaneous absorption of
chlorinated solvents in mice. Ind Health, 13: 227-236.
Tsuruta H (1977) Percutaneous absorption of organic solvents: 2) A
method for measuring the penetration rate of chlorinated solvents
through excised rat skin. Ind Health, 15: 131-139.
Tu AS, Murray TA, Hatch KM, Sivak A, & Milman HA (1985) In vitro
transformation of BALB/c-3T3 cells by chlorinated ethanes and
ethylenes. Cancer Lett, 28: 85-92.
UK HSE (Health and Safety Executive) (1992) Criteria document for an
occupational exposure limit: 1,2-Dichloroethane. London, Her Majesty's
Stationery Office, 34 pp.
Urusova TP (1953) [The possible presence of dichloroethane in human
milk with exposure in industrial conditions.] Gig i Sanit, 18: 36-37
(in Russian).
US Department of Labor (1989) Industrial exposure and control
technologies for OSHA regulated hazardous substances: Volume I.
Washington, DC, US Department of Labor.
US DHHS (Department of Health and Human Services) (1994) Seventh
annual report on carcinogens -1994: Summary. Research Triangle Park,
North Carolina, National Institute of Environmental Health Sciences,
165 pp.
US EPA (1982a) An exposure and risk assessment for dichloroethanes.
Washington, DC, US Environmental Protection Agency, Office of Water
Regulations and Standards, 175 pp (EPA-440/4-85-009).
US EPA (1982b) Test methods for organic chemical analysis of municipal
and environmental wastewater. Cincinnati, Ohio, US Environmental
Protection Agency, Monitoring and Support Laboratories, 76 pp
(EPA 600/4-82-057).
US EPA (1985a) Health assessment document for 1,2-dichloroethane
(ethylene dichloride). Final Report. Washington, DC, US Environmental
Protection Agency, 95 pp (EPA/600/8-84/006F; NTIS PB86-122702).
US EPA (1985b) Health and environmental effects profile for
1,2-dichloroethane. Washington, DC, US Environmental Protection
Agency, 137 pp (EPA/600/X-85/359; NTIS PB88-178777).
US EPA (1986) Handbook: Permit writer's guide to test burn data,
hazardous waste incineration. Cincinnati, Ohio, US Environmental
Protection Agency, 73 pp (EPA/625/6-86/012).
US EPA (1987) Health advisories for 25 organics. Washington, DC, US
Environmental Protection Agency (NTIS PB87-235578).
US EPA (1988) Contract laboratory program database. Washington, DC, US
Environmental Protection Agency, Contract Laboratory Program.
US EPA (1992) Indoor air quality data base for organic compounds.
Research Triangle Park, North Carolina, US Environmental Protection
Agency (EPA/600/R/92-025; PB92-158468).
Valencia R, Abrahamson S, Lee WR, Von Halle ES, Woodruff RC, Wurgler
FE, & Zimmering S (1984) Chromosome mutations for mutagenesis in
Drosophila melanogaster: A report of the US Environmental Protection
Agency Gene-Tox Program. Mutat Res, 134: 61-88.
Van Bladeren PJ, Breimer DD, Rotteveel-Smijs RMT, De Knijff P, Mohn
GR, van Meeteren-Walchli B, Buijs W, & van der Gen A (1981) The
relation between the structure of vicinal dihalogen compounds and
their mutagenic activation via conjugation to glutathione.
Carcinogenesis, 2: 499-505.
Van den Wijngaard AJ, van der Kamp KWJ, van der Ploeg J, Pries F,
Kazemier B, & Janssen DB (1992) Degradation of 1,2-dichloroethane by
Ancylobacter aquaticus and other facultative methylotrophs. Appl
Environ Microbiol, 58: 976-983.
Van Duuren BL, Goldschmidt BM, Loewengart G, Smith A, Mechionne S,
Seldman I, & Roth D (1979) Carcinogenicity of halogenated olefinic and
aliphatic hydrocarbons in mice. J Natl Cancer Inst, 63: 1433-1439.
Van Esch GJ, Kroes R, Van Logten MJ, & Den Tonkelaar EM (1977)
Ninety-day toxicity study with 1,2-dichloroethane (DCE) in rats.
Bilthoven, The Netherlands, National Institute of Public Health and
Environmental Protection (RIVM) (Report 195/77).
Van Luin AB & van Starkenburg W (1984) Hazardous substances in waste
water. Water Sci Technol, 17: 843-853.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: Narcotic industrial
chemicals. Can J Fish Aquat Sci, 40: 743-748.
Vozovaya MA (1974) [Development of posterity of two generations
obtained from females subjected to the action of dichloroethane.] Gig
i Sanit, 39: 25-28 (in Russian).
Vozovaya MA (1977) [The effect of dichloroethane on the sexual cycle
and embryogenesis of experimental animals.] Aksuk Ginekol (Moscow),
2: 57-59 (in Russian).
Walbridge CR, Fiandt JT, Phipps GL, & Holcombe GW (1983) Acute
toxicity of ten chlorinated aliphatic hydrocarbons to the fathead
minnow (Pimephales promelas). Arch Environ Contam Toxicol,
12: 661-666.
Wallace LA (1986) Personal exposures, indoor and outdoor air
concentrations, and exhaled breath concentrations of selected volatile
organic compounds measured for 600 residents of New Jersey, North
Dakota, North Carolina and California. Toxicol Environ Chem,
12: 215-236.
Wallace LA, Pellizzari E, Hartwell T, Rozenweig M, Erickson M,
Sparacino C, & Zelon H (1984) Personal exposure to volatile organic
compounds. I. Direct measurements in breathing-zone air, drinking
water, food and exhaled breath. Environ Res, 35: 293-319.
Wallace L, Pellizzari E, Leaderer B, Zelon H, & Sheldon L (1987)
Emissions of volatile organic compounds from building materials and
consumer products. Atmos Environ, 21(2): 385-393.
Ward JM (1980) The carcinogenicity of ethylene dichloride in
Osborne-Mendel rats and B6C3F1 mice. In: Ames BN, Infante P, & Reitz
R ed. Ethylene dichloride: A potential health risk? Cold Spring
Harbor, New York, Cold Spring Harbor Laboratory, pp 35-53 (Banbury
Report No. 5).
Ward RJ (1992) Ethylene dichloride: In vitro absorption through
human and rat epidermis. Alderley Park, United Kingdom, ICI Central
Toxicology Laboratory (Unpublished report).
Warner JM & Beasley RK (1984) Purge and trap chromatographic method
for the determination of acrylonitrile, chlorobenzene,
1,2-dichloroethane and ethylbenzene in aqueous samples. Anal Chem,
56: 1953-1956.
Warner HP, Cohen JM, & Ireland JC (1987) Determination of Henry's Law
Constants of selected priority pollutants. Washington, DC, US
Environmental Protection Agency, Office of Science and Development
(EPA-600/D-87/229).
Weiss F (1957) [Lethal oral intoxications by dichloroethane.] Arch
Gewerbepathol Gewerbehyg, 15: 253-264 (in German).
WHO (1993) Guidelines for drinking-water quality. Volume 1:
Recommendations, 2nd ed. Geneva, World Health Organization.
Williams GM, Mori H, & McQueen CA (1989) Structure-activity
relationships in the rat hepatocyte DNA-repair test for 300 chemical.
Mutat Res, 221: 263-286.
Wilson JT, Enfield CG, Dunlap WJ, Cosby RL, Foster DA, & Baskin LB
(1981) Transport and fate of selected organic pollutants in a sandy
soil. J Environ Qual, 10(4): 501-506.
Wirtschafter ZT & Schwartz WD (1939) Acute ethylene dichloride
poisoning. J Ind Hyg Toxicol, 21: 126-131.
Wit SL, Besemer AFH, Das HA, Goedkoop W, Loosjes FE, & Meppelink EK
(1969) Toxicology. Bilthoven, The Netherlands, National Institute of
Public Health (Report No. 36/69).
Withey JR & Collins BT (1980) Chlorinated aliphatic hydrocarbons used
in the foods industry: the comparative pharmacokinetics of methylene
chloride, 1,2-dichloroethane, chloroform and trichloroethylene after
I.V. administration in the rat. J Environ Pathol Toxicol, 3: 313-332.
Withey JR & Karpinski K (1985) The fetal distribution of some
aliphatic chlorinated hydrocarbons in the rat after vapor phase
exposure. Biol Res Pregnancy Perinatol, 6: 79-88.
Withey JR, Collins BT, & Collins PG (1983) Effect of vehicle on the
pharmacokinetics and uptake of four halogenated hydrocarbons from the
gastrointestinal tract of the rat. J Appl Toxicol, 3: 249-253.
Wolff DL, Ivanov NG, Kljackina AM, & Mel'Nikova LV (1979) [Evidence of
the effect of significant industrial toxicological substances on the
nervous system by animal behavioural tests.] Zool Jahrb Physiol,
83: 82-91 (in German).
Worthing CR ed. (1991) The pesticide manual. Farnham, Surrey, The
British Crop Protection Council.
Yodaiken RE & Babcock JR (1973) 1,2-Dichloroethane poisoning. Arch
Environ Health, 26: 281-284.
Zamora PO, Benson JM, Li AP, & Brooks AL (1983) Evaluation of an
exposure system using cells grown on collagen gels for detecting
highly volatile mutagens in the CHD/HGPRT mutation assay. Environ
Mutagen, 5: 795-801.
Zoetman BCJ, Piet GJ, Slingerland P, Fonds AW, & Wegman RCC (1979)
[Investigation into the presence of volatile organic halogenated
compounds in groundwater used as a drinking water supply. Final
report: results on the period November (1976) to August (1978).]
Bilthoven, The Netherlands, National Institute of Drinking Water
Supply, National Institute of Public Health and Environmental Hygiene
(Reports Nos RID-CBH-79/01 and RIV 10/79) (in Dutch).
RESUME
1. Identité, propriétés physiques et chimiques, et méthodes d'analyse
Le 1,2-Dichloréthane (ou dichlorure d'éthylène) est un produit
chimique de synthèse qui se présente sous la forme d'un liquide
incolore à la température ambiante. Il est extrêmement volatil, avec
une tension de vapeur de 8,5 kPa à 20°C et il est soluble dans l'eau,
sa solubilité étant de 8690 mg/litre à 20°C. Son coefficient de
partage octanol/eau (log Kow) est égal à 1,76.
Le dosage du dichloréthane dans les différents compartiments de
l'environnement s'effectue généralement par chromatographie en phase
gazeuse, avec détection par capture d'électrons, ionisation de flamme
ou spectrométrie de masse. Les limites de détection vont de 0,016 à
> 4 µg/m3 dans l'air, de 0,001 à 4,7 µg/litre dans l'eau et de 6 à
10 µg/kg dans différentes denrées alimentaires.
2. Sources d'exposition humaine et environnementale
On utilise principalement le 1,2-dichloréthane pour la synthèse
du chlorure de vinyle monomère et dans une moindre mesure pour la
production de divers solvants chlorés. Il entre également dans la
composition des additifs antidétonants de l'essence (encore que cet
usage soit en déclin avec l'élimination progressive de l'essence au
plomb dans certains pays) et on l'utilise aussi pour des fumigations.
La production annuelle totale de 1,2-dichloréthane a été de 922
kilotonnes au Canada en 1990 et de 6318 kilotonnes aux Etats-Unis
d'Amérique en 1991.
3. Transport, distribution et transformation dans l'environnement
La majeure partie du 1,2-dichloréthane rejeté dans
l'environnement provient d'émissions dans l'atmosphère. Il
estmoyennement persistant dans l'air; sa durée de vie estimative dans
l'atmosphère est comprise entre 43 et 111 jours. Le dichloréthane est
transporté vers la stratosphère où, par photolyse, il peut donner
naissance à du chlore radicalaire qui peut à son tour réagir sur
l'ozone. Une partie du 1,2-dichloréthane rejeté dans les effluents
industriels peut passer dans le milieu aquatique dont il s'échappe
rapidement par volatilisation. Il peut également s'infiltrer
jusqu'aux nappes d'eau souterraines à proximité des zones de décharges
industrielles. On ne pense pas qu'il puisse subir une
bioconcentration chez les espèces aquatiques ou terrestres.
4. Concentrations dans l'environnement et exposition humaine
Des enquêtes récentes portant sur l'air ambiant de zones urbaines
non dominées par des sources polluantes ont permis de relever des
concentrations moyennes de 1,2-dichloréthane allant de 0,07 à
0,28 µg/m3, alors que dans l'air intérieur aux habitations des zones
résidentielles, ces valeurs moyennes vont de < 0,1 à 3,4 µg/m3.
Dans l'eau destinée à la consommation, les concentrations moyennes
sont généralement inférieures à 0,5 µg/litre. Lors de récentes
enquêtes, on a rarement décelé du 1,2-dichloréthane dans les denrées
alimentaires et comme il ne présente qu'un faible potentiel de
bioaccumulation, il est peu probable que la nourriture constitue une
source importante d'exposition à ce composé.
La valeur estimative de l'exposition moyenne au 1,2-dichloréthane
à partir de divers milieux montre que la source principale
d'exposition est constituée par l'air intérieur et extérieur, l'eau de
consommation n'y contribuant que pour une très faible part. L'apport
de 1,2-dichloréthane par la voie alimentaire est probablement
négligeable. Les quantités inhalées dans l'air ambiant pourraient
être plus importantes à proximité des sources industrielles.
5. Cinétique et métabolisme chez les animaux de laboratoire
Après inhalation, ingestion ou exposition par voie cutanée, le
1,2-dichloréthane est rapidement absorbé et il se répartit rapidement
et largement dans l'ensemble de l'organisme. Il est rapidement et
largement métabolisé chez le rat et la souris, principalement sous
forme de métabolites soufrés dont l'excrétion s'effectue par la voie
urinaire et dépend de la dose. A des niveaux d'exposition qui
entraînent des concentrations sanguines de 5 à 10 µg/ml, il semble
qu'il y ait saturation ou limitation du métabolisme chez le rat.
Après administration d'une dose unique de dichloréthane par gavage, on
a constaté que le taux d'alkylation de l'ADN était plus important que
lorsque le produit était inhalé sur une période de six heures.
Il existe semble-t-il deux voies principales de métabolisation.
La première est une oxydation saturable qui s'effectue au niveau des
microsomes par l'intermédiaire du cytochrome P-450 et aboutit au
2-chloracétaldéhyde et au 2-chloréthanol, pour s'achever sur une
conjugaison avec le glutathion. La deuxième voie métabolique comporte
une conjugaison directe avec le glutathion pour former du
S-(2-chloréthyl)-glutathion, qui est peut-être ensuite transformé
par voie non enzymatique en un ion glutathion-épitsulfonium
susceptible de former des adduits avec l'ADN. Bien qu'on ait pu
observer in vitro que la voie du P-450 conduisait à des lésions de
l'ADN, il semble bien qu'à cet égard, la voie impliquant la
conjugaison du glutathion soit la plus importante.
6. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Le 1,2-dichloréthane présente une faible toxicité aiguë pour les
animaux de laboratoire. Ainsi, la CL50 par inhalation pour des rats
exposés soit 6 soit 7,25 heures à ce composé allait de 4000 mg/m3 à
6600 mg/m3, la DL50 par voie orale pour le rat, la souris, le
chien et le lapin allant de 413 à 2500 mg/kg de poids corporel.
D'après les résultats d'études à court terme et d'études
subchroniques menées sur différentes espèces d'animaux de laboratoire,
c'est le foie et les reins qui sont les organes cibles; il n'a pas été
possible d'obtenir de valeurs pour la dose sans effets observables
(NOEL) ou La dose la plus faible provoquant un effet (LOEL),
généralement en raison d'une documentation insuffisante et du nombre
trop limité de paramètres biologiques examinés sur un trop petit
nombre d'animaux. Une série d'études limitées anciennes a révélé la
présence de modifications morphologiques au niveau du foie chez
plusieurs espèces après exposition subchronique à des concentrations
atmosphériques ne dépassant pas 800 mg/m3. Après administration
subchronique par voie orale de 1,2-dichloréthane à des doses
quotidiennes allant de 49 à 82 mg/kg de poids corporel ou davantage
pendant 13 semaines, on a observé chez des rats un accroissement du
poids relatif du foie. Les études de toxicité chronique dont on
possède les résultats ne donnent guère d'information sur les effets
non néoplasiques. Chez des rats exposés pendant 12 mois à des
concentrations atmosphériques de 1,2-dichloréthane ne dépassant pas
202 mg/m3, on a observé, au niveau des paramètres sériques, des
modifications indiquant une toxicité hépatique et rénale; toutefois,
aucun examen histopathologique n'a été pratiqué lors de cette étude.
Quelques épreuves limitées ont été effectuées sur des animaux de
laboratoire à la recherche d'une cancérogénicité éventuelle du
1,2-dichloréthane (ces études souffraient d'une trop faible durée
d'exposition et d'une forte mortalité parmi les animaux). Chez des
rats Sprague-Dawley et des souris Swiss exposés pendant 78 semaines à
des concentrations allant jusqu'à 607 mg/m3 et observés jusqu'à ce
qu'ils meurent spontanément, on n'a pas observé d'augmentation
significative dans l'incidence des tumeurs quel qu'en soit le type.
La mortalité était forte parmi les rats, mais sans rapport avec la
concentration du produit et on n'a pas tenu compte des différences de
mortalité entre les groupes pour corriger les taux d'incidence. Des
rattes Sprague-Dawley ont été exposées pendant deux ans à 200 mg/m3
de 1,2-dichloréthane et on a observé à cette occasion une augmentation
de l'incidence des adénomes et des fibroadénomes de la mère, qui
n'était toutefois pas significative; aucun autre effet toxique
attribuable au composé n'a été observé.
En revanche, on a observé, après ingestion, chez deux espèces,
des signes convaincants d'un accroissement de l'incidence tumorale.
Chez des rats Osborne-Mendel à qui l'on avait administré
quotidiennement par gavage pendant 78 semaines des doses de 47 ou
95 mg/kg (en moyenne pondérée par rapport au temps), on a observé une
augmentation significative de l'incidence des tumeurs de différentes
localisations (notamment des carcinomes spinocellulaires de l'estomac
(chez les mâles), des hémangiosarcomes (chez les mâles et les
femelles), des fibromes du tissu sous-cutané (chez les mâles) ainsi
que des adénocarcinomes et des fibroadénomes mammaires chez les
femelles. Chez des souris B6C3F1 à qui l'on avait administré
quotidiennement des doses de 97 ou 195 mg/kg de produit (en moyenne
pondérée par rapport au temps) (mâles) ou de 149 et 299 mg/kg
(femelles) par gavage sur 78 semaines, on a observé une augmentation
similaire de l'incidence des tumeurs de diverses localisations
(notamment des adénomes alvéolaires/bronchiolaires chez les mâles et
les femelles, des adénocarcinomes mammaires chez les femelles, des
polypes ou des sarcomes du stroma de l'endomètre (femelles) et des
carcinomes hépatocellulaires (mâles)).
Chez des souris femelles qui avaient été soumises respectivement
pendant 440 et 594 jours à des applications cutanées répétées de
1,2-dichloréthane, on a observé une incidence sensiblement accrue des
tumeurs pulmonaires (papillomes bénins). Chez une souche sensible de
souris, des injections intrapéritonéales répétées de 1,2-dichloréthane
ont déterminé un accroissement, lié à la dose, du nombre des adénomes
pulmonaires, mais cet accroissement n'était en aucun cas significatif.
Chez des rats à qui l'on faisait simultanément respirer du
1,2-dichloréthane et ingérer du disulfirame avec leur nourriture, on a
observé une incidence accrue des cholangiomes et des kystes dans la
partie intrahépatique des canaux biliaires, et davantage de fibromes
sous-cutanés, de nodules hépatiques malins, de tumeurs du tissu
testiculaire interstitiel et d'adénocarcinomes mammaires, que chez des
rats qui avaient reçu soit l'un, soit l'autre des composés ou aucun
des deux. Trois épreuves biologiques n'ont pas permis de mettre en
évidence une aptitude quelconque de ce composé à se comporter comme un
initiateur ou un promoteur tumoral, encore que l'examen
histopathologique effectué à la suite de ces études ait été de portée
limitée.
Lors d'épreuves de mutagénicité in vitro sur Salmonella
typhimurium, le 1,2-dichloréthane a toujours donné des résultats
positifs. L'effet était plus important en présence d'un système
d'activation exogène (sans doute du fait d'une activation par le
cytochrome) et on constatait que le pouvoir mutagène était plus que
doublé chez S. typhimurium exprimant le gène humain GSTA1-1. Le
1,2-dichloréthane forme des adduits avec l'ADN en cultures de cellules
mammaliennes. Il provoque également une synthèse non programmée de
l'ADN dans des cultures primaires de cellules murines et humaines
ainsi que des mutations géniques dans certaines lignées cellulaires.
On a trouvé une corrélation entre la fréquence des mutations observées
dans des lignées cellulaires humaines et la modification de l'activité
de la glutathion-S-transférase. Des études in vivo ont montré que
le 1,2-dichloréthane produisait des mutations létales récessives dans
les cellules somatiques et germinales de Drosophila melanogaster et
selon toutes les études publiées portant sur des rats et des souris,
il y a liaison du 1,2-dichloréthane à l'ADN. Des lésions directes de
l'ADN des cellules hépatiques ainsi que des échanges entre chromatides
soeurs ont été observés lors d'études sur la souris mais rien
n'indique que le 1,2-dichloréthane provoque la formation de
micronoyaux.
Rien n'indique, à en juger par les résultats d'un nombre limité
d'études, que le 1,2-dichloréthane soit tératogène pour les animaux de
laboratoire. Il n'y a également guère d'éléments en faveur d'effets
sur la reproduction ou le développement à des doses inférieures à
celles qui provoquent d'autres effets généraux. On ne dispose que de
données limitées sur l'immunotoxicité du 1,2-dichloréthane.
7. Effets sur l'homme
Des effets divers ont été observés à la suite d'expositions
accidentelles aiguës à du 1,2-dichloréthane par inhalation ou
ingestion: au niveau du système nerveux central, du foie, des reins,
des poumons et de l'appareil cardio-vasculaire.
On n'a pas beaucoup étudié le pouvoir cancérogène du
1,2-dichloréthane dans les populations humaines exposées. Chez un
groupe d'ouvriers d'un atelier de production de produits chimiques qui
avaient été exposés principalement à du 1,2-dichloréthane, à côté
d'autres substances, on a observé une augmentation significative de la
mortalité par cancer du pancréas. Cette mortalité augmentait avec la
durée de l'exposition. En outre, malgré un nombre limité de cas et
une association moins systématique avec la durée de l'exposition, il y
avait également accroissement de la mortalité par leucémie chez ces
travailleurs. Une petite étude cas-témoins, portant sur l'exposition
à du 1,2-dichloréthane n'a pas permis de mettre en évidence une
corrélation avec l'apparition de tumeurs cérébrales. Une étude
écologique intrinsèquement limitée, portant sur la présence de
1,2-dichloréthane dans de l'eau de consommation, a mis en évidence une
augmentation de l'incidence des cancers colo-rectaux mais il est
possible qu'une exposition simultanée à d'autres substances explique
pour une part les effets observés.
8. Effets sur les organismes non visés au laboratoire et dans leur
milieu naturel
On a étudié les effets d'une exposition au 1,2-dichloréthane sur
un certain nombre d'autres organismes tant au laboratoire que dans
leur milieu naturel. En ce qui concerne les microorganismes
aquatiques, les valeurs de la CI50 et de la CE50 correspondant à
divers paramètres biotoxicologiques vont de 25 à 770 mg/litre. La
valeur de la CL50 la plus faible qui ait été observée pour les
daphnies était de 220 mg/litre, des effets ayant été toutefois
observés sur la fécondité et la croissance aux concentrations
respectives de 20,7 et 71,7 mg/litre. En s'appuyant sur les données
disponibles, on constate que le vertébré d'eau douce le plus sensible
au 1,2-dichloréthane est une espèce de salamandre (Ambystoma gracile),
chez laquelle la survie des larves à neuf jours (quatre jours après
l'éclosion) a accusé une chute à 2,54 mg/litre. On ne possède que des
données limitées sur la toxicité du 1,2-dichloréthane pour les
organismes terrestres.
Resumen
1. Identidad, propiedades físicas y químicas y métodos analíticos
El 1,2-dicloroetano (dicloruro de etileno), producto químico
sintético, es un líquido incoloro a temperatura ambiente. Es también
muy volátil, con una presión de vapor de 8,5 kPa (a 20°C), y soluble
en agua, con una solubilidad de 8690 mg/litro (a 20°C). El log del
coeficiente de reparto octanol/agua es de 1,76.
El análisis del 1,2-dicloroetano en el medio ambiente se realiza
habitualmente por cromatografía de gases, en combinación con la
captura de electrones, la detección de ionización por conductor o bien
la espectrometría de masas. Los límites de la detección oscilan entre
0,016 y > 4 µg/m3 en el aire, entre 0,001 y 4,7 µg/litro en el agua
y entre 6 y 10 µg/kg en diversos productos alimenticios.
2. Fuentes de exposición humana y ambiental
El 1,2-dicloroetano se utiliza principalmente en la síntesis del
monómero cloruro de vinilo y, en menor grado, en la fabricación de
diversos disolventes clorados. Se incorpora también a los aditivos
antidetonantes de la gasolina (aunque este empleo está disminuyendo
con la reducción progresiva en muchos países de la gasolina con plomo)
y se ha usado como fumigante. La producción anual total de
1,2-dicloroetano en el Canadá en 1990 y en los Estados Unidos en 1991
fue de 922 000 y 6 318 000 toneladas, respectivamente.
3. Transporte, distribución y transformación en el medio ambiente
La mayor parte del 1,2-dicloroetano liberado en el medio ambiente
ha sido emitido en el aire. En este medio es moderadamente
persistente; su permanencia en la atmósfera se estima entre 43 y 111
días. Es transportado a la estratosfera, donde, por fotólisis, pueden
producirse radicales de cloro que a su vez pueden reaccionar con el
ozono. Cierta cantidad de 1,2-dicloroetano puede escapar a los
efluentes industriales y de allí pasar al medio ambiente acuático, de
donde desaparece rápidamente por volatilización. También puede
alcanzar por lixiviación las aguas subterráneas próximas a los
vertederos de desechos industriales. No se prevé su bioconcentración
en especies acuáticas o terrestres.
4. Niveles medioambientales y exposición humana
Según estudios recientes del aire ambiental, las concentraciones
medias de 1,2-dicloroetano detectadas en zonas urbanas donde no
abundan sus fuentes de emisión oscilan entre 0,07 y 0,28 µg/m3,
mientras que los niveles medios notificados como presentes en el aire
del interior de las viviendas oscilan entre < 0,1 y 3,4 µg/m3. En
el agua potable, la concentración media suele ser menor de
0,5 µg/litro. En estudios recientes rara vez se ha detectado la
presencia de 1,2-dicloroetano en alimentos y, puesto que el potencial
de bioacumulación de esta sustancia es bajo, los alimentos
probablemente no representen una fuente de exposición importante.
Teniendo en cuenta las estimaciones de la exposición media en
diversos entornos, la principal fuente de exposición de la población
general al 1,2-dicloroetano es el aire de locales cerrados y el aire
exterior, mientras que el agua de bebida contribuye sólo en cantidades
muy pequeñas. La ingestión de 1,2-dicloroetano con los alimentos
probablemente sea insignificante. La cantidad inhalada con el aire
ambiental puede ser más grande en las proximidades de fuentes de
emisión industriales.
5. Cinética y metabolismo en animales de laboratorio
El 1,2-dicloroetano se absorbe fácilmente tras la inhalación, la
ingestión o la exposición cutánea y se distribuye rápida y ampliamente
por todo el organismo. En la rata y el ratón se metaboliza de forma
rápida y completa y por orina se eliminan principalmente metabolitos
azufrados en concentraciones que dependen de la dosis. En la rata, el
metabolismo parece saturado o limitado cuando la exposición alcanza
niveles que dan lugar a concentraciones sanguíneas de 5 a 10 µg/ml.
Después de la administración por sonda de una dosis única, los niveles
de alquilación del ADN eran más elevados que después de la inhalación
durante un periodo de seis horas.
El 1,2-dicloroetano parece metabolizarse siguiendo dos vías
principales: la primera comporta una oxidación microsómica saturable
mediada por el citocromo P-450, que produce 2-cloroacetaldehído y
2-cloroetanol, seguida de conjugación con el glutatión. La segunda
vía entraña la conjugación directa con el glutatión para formar
S-(2-cloroetil)-glutatión, que puede convertirse mediante un proceso
no enzimático en un ion glutatión episulfonio; este ion puede formar
aductos con el ADN. Aunque se han inducido daños en el ADN por la
ruta del P-450 in vitro, hay varias pruebas de que la vía de la
conjugación del glutatión probablemente sea más importante que la otra
en cuanto a los daños que causa en el ADN.
6. Efectos en mamíferos de laboratorio y en sistemas de ensayo
in vitro
La toxicidad aguda del 1,2-cicloroetano en animales de
experimentación es baja. Por ejemplo, la CL50 por inhalación en
ratas expuestas durante 6 ó 7,25 horas oscilaba entre 4000 mg/m3 y
6600 mg/m3, mientras que la DL50 por vía oral en ratas, ratones,
perros y conejos variaba entre 413 y 2500 mg/kg de peso corporal.
Los resultados de estudios de corta duración y subcrónicos
realizados en varias especies de animales de experimentación indican
que los órganos afectados son el hígado y los riñones; en general no
se obtuvieron NOEL ni LOEL fidedignos debido a la documentación
insuficiente y a la gama limitada de puntos finales examinados en
pequeños grupos de animales. En una serie de estudios iniciales
limitados, se observaron cambios morfológicos en el hígado de varias
especies tras la exposición subcrónica a concentraciones de
1,2-dicloroetano en el aire de sólo 800 mg/m3. Tras la
administración subcrónica a ratas por vía oral de dosis comprendidas
entre 49 y 82 mg/kg de peso corporal por día durante más de 13 semanas
se observó un incremento del peso relativo del hígado. En los
estudios crónicos disponibles se ha presentado poca información sobre
efectos no neoplásicos. Las ratas expuestas a concentraciones de sólo
202 mg/m3 en el aire durante 12 meses acusaron en los parámetros del
suero cambios indicativos de toxicidad hepática y renal, pero en ese
estudio no se realizaron exámenes histopatológicos.
La carcinogenicidad del 1,2-dicloroetano se ha investigado en un
pequeño número de biovaloraciones limitadas sobre animales de
experimentación (las limitaciones comprenden una exposición de corta
duración y una mortalidad alta). No se notificaron aumentos
significativos en la incidencia de ningún tipo de tumor en ratas
Sprague-Dawley o en ratones suizos expuestos a concentraciones de
hasta 607 mg/m3 durante 78 semanas; los animales se observaron hasta
que se produjo su muerte espontánea. En este estudio, la mortalidad
de las ratas fue elevada, pero no guardaba relación con la
concentración, y no se realizó un ajuste de la incidencia en función
de la mortalidad diferencial entre los grupos. En un ensayo con ratas
Sprague-Dawley hembra expuestas durante dos años a 200 mg/m3, se
produjo un aumento no significativo de la incidencia de adenomas y
fibroadenomas en las glándulas mamarias, y no se observó otra forma de
toxicidad relacionada con el compuesto.
En cambio, hay pruebas convincentes de un aumento de la
incidencia de tumores en dos especies tras la ingestión del producto.
Las ratas Osborne-Mendel que habían recibido mediante sonda durante 78
semanas dosis diarias de 47 ó 95 mg/kg de peso corporal (promedio
ponderado en función del tiempo) mostraron un aumento significativo de
la incidencia de tumores en diversos lugares (por ejemplo, carcinomas
escamosos del estómago en machos, hemangiosarcomas en machos y
hembras, fibromas del tejido subcutáneo en machos, adenocarcinomas y
fibroadenomas de las glándulas mamarias en hembras. En ratones
B6C3F1 que habían recibido, como promedio ponderado en función del
tiempo, dosis diarias de 97 ó 195 mg/kg de peso corporal (machos) y
149 ó 299 mg/kg de peso corporal (hembras) mediante sonda durante 78
semanas se observaron aumentos semejantes de la incidencia de tumores
en múltiples lugares (adenomas alveolares/bronquiolares en machos y
hembras, adenocarcinomas de las glándulas mamarias en hembras y pólipo
estromal endometrial o sarcoma estromal endometrial combinados en
hembras y carcinomas hepatocelulares en machos).
La incidencia de tumores pulmonares (papilomas benignos)
aumentó significativamente en ratones hembra tras la aplicación
cutánea repetida de 1,2-dicloroetano durante 440 a 594 días. La
administración intraperitoneal repetida produjo en una raza
susceptible aumentos en el número de adenomas pulmonares por ratón;
dichos aumentos estaban relacionados con la dosis, pero ninguno de
ellos fue significativo. En comparación con las ratas que recibieron
el compuesto solo y con el grupo testigo, que no recibió ningún
tratamiento, las ratas expuestas simultáneamente a 1,2-dicloroetano
por inhalación y a disulfiram en los alimentos acusaron una mayor
incidencia de colangiomas y de quistes en el conducto biliar
intrahepático, de fibromas subcutáneos, de nódulos neoplásicos en el
hígado, de tumores de las células intersticiales de los testículos y
de adenocarcinomas mamarios. En tres biovaloraciones realizadas no se
observaron indicios de potencial iniciador o facilitador del
desarrollo de tumores, pero el alcance del examen histopatológico de
esos estudios fue limitado.
In vitro, el 1,2-dicloroetano ha dado constantemente resultados
positivos en biovaloraciones de mutagenicidad en Salmonella
typhimurium. Las respuestas han sido mayores en presencia de un
sistema de activación exógeno (posiblemente debido a la activación por
el sistema del citocromo) que en su ausencia; la mutagenicidad se
duplicó con creces en la cepa de S. typhimurium que contenía el gen
humano GSTA1-1. En cultivos de células mamarias, el 1,2-dicloroetano
forma aductos con el ADN. También induce síntesis no programada del
ADN en cultivos primarios de células de roedores y humanas y mutación
génica en varias líneas celulares. La frecuencia de las mutaciones en
las líneas celulares humanas se ha correlacionado con diferencias en
la actividad de la glutatión-S-transferasa. En estudios in vivo,
el 1,2-dicloroetano indujo mutaciones en células somáticas y
mutaciones letales recesivas ligadas al sexo en Drosophila
melanogaster y, según todos los estudios realizados en ratas y
ratones, el compuesto se había unido al ADN. Aunque en estudios
efectuados en ratones se han observado lesiones primarias del ADN en
el hígado e intercambio de cromátides hermanas, no hay indicaciones de
inducción de micronúcleos.
Teniendo en cuenta los resultados de un número limitado de
estudios, no hay pruebas de que el 1,2-dicloroetano sea teratogénico
en animales de experimentación. Además, hay pocas pruebas
convincentes de que tenga efectos sobre la reproducción o el
desarrollo con dosis inferiores a las que causan otros efectos
sistémicos. Los datos disponibles sobre la inmunotoxicidad del
1,2-dicloroetano son limitados.
7. Efectos en el ser humano
La exposición accidental aguda al 1,2-dicloroetano por inhalación
o por ingestión ha producido diversos efectos en el ser humano, por
ejemplo en el sistema nervioso central, el hígado, el riñón, el pulmón
y el sistema cardiovascular.
No se ha estudiado con detenimiento la carcinogenicidad potencial
del 1,2-dicloroetano en poblaciones humanas expuestas. La mortalidad
por cáncer pancreático aumentó de forma significativa en un grupo de
trabajadores de una planta de producción química que había estado
expuesto sobre todo a 1,2-dicloroetano (en combinación con otros
productos químicos). La mortalidad por cáncer pancreático aumentó con
la duración de la exposición. Además, aunque el número de casos fue
pequeño y la relación con la duración de la exposición menos
constante, en estos trabajadores también aumentó la mortalidad por
leucemia. No se observó relación entre la exposición ocupacional al
1,2-dicloroetano y el cáncer cerebral en un pequeño estudio de casos y
controles. Si bien en un estudio ecológico con limitaciones
inherentes se observó que la incidencia de cáncer de colon y de recto
aumentaba con la concentración del producto en el agua de bebida, la
exposición simultánea a otras sustancias podría haber contribuido a
producir los efectos observados.
8. Efectos en organismos no destinatarios en el laboratorio y
sobre el terreno
Se han investigado los efectos de la exposición al
1,2-dicloroetano en varios otros organismos en el laboratorio y en el
medio ambiente. Con respecto a los microorganismos acuáticos, se ha
informado de que las CI50 y CE50 correspondientes a diversos
puntos finales oscilan entre 25 y 770 mg/litro. La CL50 más baja
notificada para Daphnia fue de 220 mg/litro, mientras que se
detectaron efectos en el éxito reproductivo y el crecimiento a 20,7 y
71,7 mg/litro, respectivamente. Teniendo en cuenta los datos
disponibles, la especie de vertebrados de agua dulce más sensible
parece ser la salamandra noroccidental (Ambystoma gracile), cuyas
larvas de nueve días (cuatro días después de la eclosión) vieron
reducida su supervivencia a concentraciones de 2,54 mg/litro. Se
dispone sólo de datos limitados sobre la toxicidad del
1,2-dicloroetano en organismos terrestres.
