
ZIRAM
First draft prepared by
J.-J. Larsen,
Institute of Toxicology, National Food Agency,
Ministry of Health, Soborg, Denmark
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Neurotoxicity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Ziram was evaluated for toxicological effects by the Joint
Meeting in 1965, 1967, 1970, 1974, 1977, and 1980 (Annex 1, references
4, 8, 14, 22, 28, and 34). A temporary ADI of 0-0.025 mg/kg bw for
ziram or ziram in combination with other dimethyldithiocarbamates was
allocated in 1967, on the basis of the NOAEL in a one-year study in
dogs. This temporary ADI was lowered to 0-0.005 mg/kg bw in 1974. A
group ADI of 0-0.02 mg/kg bw for ferbam and ziram was allocated in
1977 and confirmed in 1980. The compound was reviewed by the present
Meeting within the CCPR periodic review programme. This monograph
summarizes the data received since the previous evaluation and
contains relevant summaries from the previous monograph and monograph
addenda on ziram (Annex I, references 4, 9, 15, 23, 29, and 35).
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution and excretion
A group of 71 rats were given a single oral dose of 15 mg/kg bw;
14 daily doses of 15 mg/kg bw nonradiolabeled ziram (purity, 99%)
followed by a single dose of 14C-ziram (radiochemical purity, 96.9%)
on day 15; or a single dose of 352 mg/kg bw 14C-ziram. Urine, faeces,
expired carbon dioxide, and volatile organic compounds were collected
from all animals 0-4, 4-8, 8-12, and 12-24 h after treatment. The rats
were killed seven days after administration of the radiolabelled
dose. The mean recovery of radiolabel was 79-92% of the total dose
administered. Most of the radiolabel was eliminated within 24 h after
administration of the low dose and within 48 h of the high dose. Most
radiolabel (36-53%) was found in expired air, with 17-35% in urine
and 9-18% in faeces. The amounts retained in tissues and carcass
represented 1-2% of the total dose. The highest residue levels were
found in blood, liver, kidney, heart, lungs, spleen, and thyroid
gland. No apparent sex-related differences were observed in the
elimination or distribution of 14C-ziram (Cheng, 1991a).
The extent of absorption of 14C-ziram was studied in 74 rats
given single dermal doses of 0, 1.1, 12, or 91 mg per animal (0.086,
0.95, or 7.25 mg/cm2). Urine, faeces, blood, skin at the application
site, and the carcass were collected from four animals killed 0.5, 1,
2, 4, 10, or 24 h after initiation of exposure to ziram. A mean of
74-101% of the dose was recovered. Most of the radiolabel (68-99%) was
washed off the skin at the application site; only 16% remained on or
in the skin. Less than 0.3% of the administered radiolabel was
retained in the carcass and was eliminated in the excreta within
24 h after exposure. The mean amount absorbed (sum of radiolabel in
urine, carcass, and skin at the test site) by 24 h was 29% of the
administered dose in animals at 1.1 mg, 31% in those at 12 mg, and 5%
for those at 91 mg, indicating non-linear dermal absorption (Cheng,
1991b).
(b) Biotransformation
Three lactating goats weighing about 40 kg were fed 14C-ziram
for six consecutive days at concentrations of 0 or 300 ppm, equal to 0
or 12.5 mg/kg bw per day. All milk, urine, and faeces were collected
for radioanalysis. The animals were killed 6 h after the last dose and
the organs were removed. The concentrations of radiolabel in the milk
appeared to reach a plateau by day 3, at 0.6-1.8 ppm. In the edible
tissues, the concentrations of radiolabel were 22-28 ppm in the liver,
3 ppm in the kidneys, and < 1 ppm in fat and muscle. The total
amounts of radiolabel excreted during the test period were 42-61% of
the administered dose in faeces, 3% in urine, and 0.28-0.51% in milk.
Dithiocarbamates represented maxima of 28 and 10% of the total
radioactive residues in urine and liver, respectively, and
undetectable amounts in other tissues. N-Nitrosodimethylamine
residues (detection limit, 1 ppb) were not found. Radiolabelled
lactose and casein were isolated from milk and radiolabelled urea from
urine, indicating that the metabolism of ziram in goats occurs at
least in part via a single-carbon pathway, which results in extensive
radiolabelling of natural products. The author concluded that the
bound and extractable radioactivity in milk and tissues was derived
largely from radiolabelled natural products rather than from discrete
dithiocarbamate metabolites (Bodden, 1993).
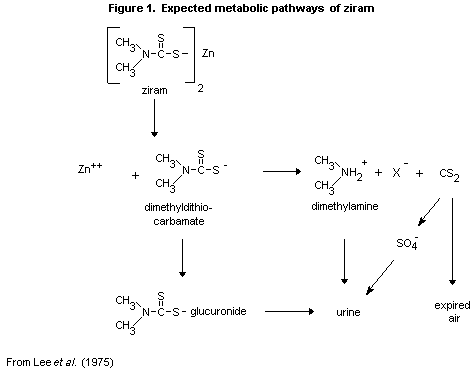
The expected metabolic pathways of ziram, on the basis of data on
other dimethyldithocarbamates, are shown in Figure 1.
 2. Toxicological studies
(a) Acute toxicity
The acute toxicity of ziram is summarized in Table 1.
(b) Short-term toxicity
Mice
Ziram (purity 97.6%) was administered orally to C57B1 and A mice
at doses of 0 or 75 mg/kg bw twice a week (equivalent to 20 mg/kg bw
per day) for 2.5 months. A positive control group was exposed in the
same way to urethane. The presence of adenomas in the lungs and the
liver was studied after the end of the exposure at intervals of 1.5
months for six months; adenomas were found only in the lungs. In the A
mice, adenomas occurred in 100% of animals given urethane, 51% of
those given ziram, and 43% of the controls. The differences were not
statistically significant. The first adenomas were seen after three
months. In C57B1 mice, adenomas occurred in 26% of mice given
urethane, 7.4% of those given ziram, and none of the controls. The
results were statistically significant according to Student's t test
( t = 2.08; p = 0.05) but not according to the chi-squared test
(chi-squared = 2.18; p = 0.15) (Khicenko & Chernev, 1968; Annex 1,
reference 15).
In a range-finding study, groups of five male and five female
Crl.CD(ICR)BR mice were fed ziram (purity, 89.5%) at concentrations of
0, 3000, 4000, or 5000 ppm, equal to 0, 370, 510, or 740 mg/kg bw per
day for males and 0, 420, 660, or 780 mg/kg bw per day for females,
for four weeks. The body weight, food intake, and efficiency of food
use were significantly reduced at all doses. The brain and heart
weights were reduced in all female mice, and the heart weight was also
reduced in males at 5000 ppm. There was no NOAEL (Chambers et al.,
1992a).
In another preliminary study, groups of 10 male and 10 female
Crl:CD-1 mice were fed ziram (purity, 98.7%) at concentrations of 0,
100, 300, 900, or 2700 ppm, equal to 0, 15, 44, 120, or 360 mg/kg bw
per day for males and 0, 19, 49, 130, or 410 mg/kg bw per day for
females, for 13 weeks. Clinical signs, body weight, food and water
consumption, and efficiency of food use were recorded. On completion
of treatment, all mice were sacrificed for macroscopic and microscopic
examination. Over week 1, a reduction was noted in food consumption
and use in animals of each sex and in the body weight of males and the
body-weight gain of females at 2700 ppm. Lower weight gain was also
noted during the study in males and females at 900 ppm. A lower spleen
weight was noted in animals of each sex at 900 and 2700 ppm and in
females at 300 ppm. Uterine weight was reduced at 2700 ppm. An
increased incidence of hyperkeratosis of the non-glandular epithelium
Table 1. Acute toxicity of ziram in experimental animals
Species Sex Route Purity LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Rat Male Oral 98.5 380 Liggett & Allan (1989a)
Female 270
Rat Male Inhalation (4 h) 98.5 0.08 Jackson & Hardy (1989)
Female 0.06
Rat Male Inhalation (4 h) 98.4 0.18 Blagden (1991)
Female 0.08
Rabbit NR Oral NR 400 Annex I, reference 4
Rabbit Male Dermal 98.5 > 2000 Liggett & Allen (1989b)
Female > 2000
NR, not reported
in the stomach was seen among mice given 900 or 2700 ppm. Epithelial
hyperplasia of the limiting ridge in the stomach was also seen at
2700 ppm. Contraction of the spleen and extramedullary haematopoiesis
in the spleen were observed in mice at 900 and 2700 ppm. Corpora lutea
were absent in one mouse at 900 ppm, and recent corpora lutea were
absent in two mice at 2700 ppm and in one at 900 ppm. The uteri of
some of these mice showed changes consistent with the ovarian changes,
i.e. cellular stroma and reduced luminal diameter. These changes were
not seen at 0, 100, or 300 ppm. The NOAEL was 100 ppm, equal to
15 mg/kg bw per day, on the basis of decreased spleen weight at doses
> 300 ppm (Powell et al., 1993).
Rats
Groups of 10 male and 10 female weanling rats were fed ziram
(purity unspecified) at concentrations of 0, 100, 500, 2500, or
5000 ppm (equivalent to 0, 10, 50, 250, or 500 mg/kg bw per day) for
28 days. Growth retardation was marked in animals at 2500 and
5000 ppm; a slight retardation was also seen in those at 500 ppm, but
growth was normal in those at 100 ppm. Except for slight anaemia at
2500 and 5000 ppm, the haematological findings were normal and no
significant histopathological changes were seen in any of the animals.
The NOAEL was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis
of growth retardation at doses > 500 ppm (Annex I, reference 4).
In a separate experiment, 10 male and 10 female rats were
maintained for 28 days on a diet containing 0 or 2500 ppm ziram. No
thyroid abnormalities were seen (Annex I, reference 4).
Groups of 15 male and 15 female Wistar rats were given ziram
(purity, 98%) by gavage at doses of 0, 3, 15, or 100 mg/kg bw per day
for four weeks. Food consumption, efficiency of food use, and body
weights were reduced significantly in males at the highest dose and in
females at 15 and 100 mg/kg bw per day. End-points of haematology and
clinical chemistry did not show clear dose-dependent changes, except
for an increase in aspartate transaminase and alkaline phosphatase
activities and blood urea nitrogen values at the highest dose. The
liver weight was increased in males at this dose, and degenerative
changes in the liver and kidneys were seen in animals of each sex.
Degenerative liver changes were also seen in males and females at
15 mg/kg bw per day. The NOAEL was 3 mg/kg bw per day, on the basis of
decreased body weight, food consumption, and food use and degenerative
liver changes at doses > 15 mg/kg bw per day (Dickhaus & Heisler,
1980).
In a range-finding study, groups of five male and five female
Crl:CD(SD)BR rats were fed ziram (purity, 98.5%) at concentrations of
0, 500, 1000, or 2000 ppm (equal to 0, 46, 90 or 170 mg/kg bw per day
for males and 0, 46, 85, or 170 mg/kg bw per day for females) for four
weeks. The body weight, food intake, and efficiency of food use were
significantly reduced in animals of each sex at all doses. In males,
the absolute weights of the liver, pituitary, and testes were reduced
at all doses. The absolute weights of the brain in animals of each sex
and of the uterus were also reduced at the highest dose. There was no
NOAEL (Chambers et al., 1992b).
In another range-finding study, groups of 10 male and 10 female
Crl:CD(SD)BR rats were fed ziram (purity, 98.7%) at concentrations of
0, 100, 300, or 1000 ppm (equal to 0, 7.4, 21, or 68 mg/kg bw per day
for males and 0, 8.8, 24, or 77 mg/kg bw per day bw for females)
for 13 weeks. The body-weight gain, food intake, and food use were
dose-dependently reduced at 300 and 1000 ppm. Brain weight and spleen
weight were increased and a higher incidence of hair loss was noted at
300 and 1000 ppm. Epithelial hyperplasia of the nonglandular stomach
was seen at 1000 ppm. The NOAEL was 100 ppm, equal to 7.4 mg/kg bw per
day, on the basis of decreased body-weight gain, food intake, and food
use and increased brain and spleen weights and hair loss at doses
> 300 ppm (Powell et al., 1992).
Rabbits
Ziram (purity 98.5%) was applied to the intact skin of groups
of five male and five female New Zealand white rabbits, weighing
2.2-2.6 kg, daily for 21 consecutive days at doses of 0, 100, 300, or
1000 mg/kg bw per day. The test substance was moistened with distilled
water and maintained on the backs of the rabbits for 6 h each day,
after which the dressings were removed and the treated skin washed
with tap-water at 30-40°C and gently blotted dry. No dermal reaction
to the treatment was observed at any dose. Significant losses in body
weight or low body-weight gain and reduced food consumption were
recorded for female rabbits receiving 1000 mg/kg bw. The number of
lymphocytes was reduced in both males and females at 1000 mg/kg bw,
and alanine and aspartate transaminase activities were increased at
300 and 1000 mg/kg bw. At the highest dose, significantly increased
levels of bilirubin were found in females and of cholesterol in
animals of each sex. The NOAEL was 300 mg/kg bw per day, on the basis
of decreased body weight, body-weight gain, food consumption, and
number of lymphocytes and increased bilirubin and cholesterol levels
at 1000 mg/kg bw per day (Edwards et al., 1989).
Dogs
In a range-finding study, single male and female beagle dogs were
fed ziram (purity, 89.5%) at concentrations of 1000, 2000, or 5000 ppm
(equal to 38, 78, or 260 mg/kg bw per day for males and 28, 91 or
270 mg/kg bw per day for females) for four weeks; there was no control
group. Both dogs at 5000 ppm and the female at 2000 ppm were killed
for humane reasons due to loss of body weight and deteriorated
condition. Convulsive episodes of up to 5-min duration were seen in
females at 2000 ppm. Body-weight loss was seen in both animals at
5000 ppm and in the female at 1000 ppm. Food intake and efficiency of
food use were reduced in females at all doses and in males at
5000 ppm. Increased liver weight in comparison with historical
controls in the laboratory was seen in males at all doses. No
histopathological examinations were performed. There was no NOAEL
(McLean et al., 1992a).
In another range-finding study, groups of four male and four
female beagle dogs were fed ziram (purity, 98.5%) in the diet at
concentrations of 0, 100, 300, or 1000 ppm (equal to 0, 4.1, 12, or
42 mg/kg bw per day for males and 0, 4.3, 13, or 41 mg/kg bw per day
for females) for 13 weeks. One of the four dogs at 1000 ppm suffered a
seizure and was killed for humane reasons, and another had tremors.
Animals at this dose showed reduced body weight and food intake;
lowered erythrocyte count, packed cell volume, and haemoglobin; and
elevated circulating reticulocyte counts, with associated slight
polychromasia, hypochromasia, and anisocytosis. Activated partial
thromboplastin time was increased in male dogs at 300 or 100 ppm.
Alkaline phosphatase activity was elevated in females at 1000 ppm, and
the cholesterol level was elevated in females at 300 and 1000 ppm. The
albumin concentration was decreased and that of globulin increased in
some dogs at all doses during week 13. The liver weight was increased
in male and female dogs at 1000 ppm and in one dog at 300 ppm. Focal
myocardial necrosis with acute inflammation and haemorrhage were seen
in the dog at 1000 ppm that was killed for humane reasons. Focal
necrosis in the liver with loss of cells and dilated sinusoids were
seen in one dog at 1000 ppm, and focal necrosis was also seen in a dog
receiving 300 ppm. Minimal amounts of pigment in Kupffer cells were
observed in two dogs at 300 ppm and in three dogs at 1000 ppm. The
NOAEL was 100 ppm, equal to 4.1 mg/kg bw per day, on the basis of
increased liver weight, focal liver necrosis, pigment in Kupffer
cells, increased activated partial thromboplastin time, and an
elevated cholesterol level at doses > 300 ppm (McLean et al., 1992b).
Groups of four male and four female beagle dogs were fed ziram
(purity, 98.5%) in the diet to give concentrations of 0, 50, 180, or
700/500 ppm (equal to 0, 1.6, 6.6, or 17 mg/kg bw per day for males
and 0, 1.9, 6.7, or 21 mg/kg bw per day for females) for one year. The
highest dose was reduced from 700 to 500 ppm in week 12 due to
treatment-related deaths. All animals were killed and necropsied after
one year. Body-weight gain was reduced in females at 180 and 500 ppm.
Alanine transaminase, alkaline phosphatase, and, occasionally,
aspartate transaminase activities were elevated in males receiving 180
or 500 ppm; and the liver weights of males receiving 500 ppm were
increased. Reduced albumin values and raised cholesterol levels were
found for male dogs receiving 500 ppm. In the liver, some foci of
degenerated hepatocytes were seen in a female at 180 ppm and in a
number of dogs at 500 ppm, single-cell necrosis was seen in one male
dog at 500 ppm, and inflammatory-cell infiltration around the central
veins and branches of the hepatic veins was seen in a proportion of
these dogs. An increase in centrilobular fibrocytes was seen in one
male dog at 180 ppm and three males at 500 ppm. Aggregates of
pigmented Kupffer cells were observed in the livers of dogs of each
sex in a dose-related incidence, including one male and one female at
50 ppm. An increased incidence and degree of pigmented macrophages
were seen in the spleens of male dogs at 180 and 500 ppm. The NOAEL
was 50 ppm, equal to 1.6 mg/kg bw per day, on the basis of decreased
body-weight gain, degenerated hepatocytes, and increases in
centrilobular fibrocytes, spleen macrophages, alanine transaminase and
alkaline phosphatase activities, and pigmented Kupffer cells at doses
> 180 ppm (Smith et al., 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 49 or 50 male and female B6C3F1 mice were fed ziram
(purity, 89%; containing 6.5% thiram, 2% other zinc salts, and 2%
unidentified additional impurities) at concentrations of 0, 600, or
1200 ppm (equal to 0, 120, or 200 mg/kg bw per day for males and 0,
130, or 250 mg/kg bw per day for females) for 103 weeks. All animals
were observed daily for clinical signs, and body weight and food
consumption were recorded monthly. At termination, necropsies were
performed on all animals, and selected organs and tissues from most
animals were examined microscopically. The survival of treated mice
was not adversely affected. The body-weight gain of male mice at 600
and 1200 ppm and of female mice at 600 ppm was reduced, and food
consumption was reduced in both males and females at 1200 ppm. The
incidence of alveolar or bronchiolar adenomas was statistical
significantly increased in female mice (2/50 controls, 5/49 at the low
dose, 10/50 at the high dose), and the combined incidence of alveolar
and bronchiolar adenomas and carcinomas in female mice showed a
statistically significant, positive trend. The incidence in males at
1200 ppm was significantly higher than that in the controls (4/50
controls, 6/49 at the low dose, 11/50 at the high dose). Pulmonary
adenomatous hyperplasia consistent with chronic Sendai viral infection
was observed in control and treated animals. The authors concluded
that any interpretation of the increase in lung tumours is complicated
by the presence of intercurrent viral infection (National Toxicology
Program, 1983).
Groups of 50 male and 50 female Crl:CD-1(ICR)BR mice were fed
ziram (purity, 98.7%) in the diet to give concentrations of 0, 25, 75,
225, or 675 ppm (equal to 0, 3, 9, 27, or 82 mg/kg bw per day for
males and 0, 4, 11, 33, or 95 mg/kg bw per day for females) for 80
weeks. The concentrations of ziram given to the groups at 25 and
75 ppm were greater than the nominal concentration in order to
compensate for losses during storage. Statistically significant
reductions in body-weight gain and food intake were seen in males and
females receiving 225 or 675 ppm. Dose-related, statistically
significant decreases in the absolute brain weight of males receiving
225 or 675 ppm and the brain weight adjusted for final body weight
of females receiving 75, 225, or 675 ppm were noted. Macroscopic
examination of all animals that died or were killed at termination
revealed increased incidences of reduced adipose tissue in males
that died, irregular cortical scarring of the kidneys in males at
terminal sacrifice, brown kidneys in males, and roughened and white
forestomachs in females, all receiving the highest dose. An increased
incidence of centrilobular and/or generalized hepatocellular
enlargement was seen in all treated groups, and an increased incidence
of urinary bladder epithelial hyperplasia was seen in males and
females receiving 675 ppm and in males at 225 ppm. No treatment-
related neoplastic effects were seen. The NOAEL was 25 ppm, equal
to 3 mg/kg bw per day, on the basis of reduced brain weight and
hepatocellular enlargement at doses > 75 ppm (Powell et al., 1994a).
Rats
Groups of 25 male and 25 female weanling rats were fed ziram at
concentrations of 0, 25, 250, or 2500 ppm (equivalent to 0, 1.3, 13,
or 125 mg/kg bw per day) for two years. The growth rate and life span
were normal in all groups. Neurological changes were observed at
2500 ppm, but no cystic lesions were seen in the brain post mortem.
No neurological changes were seen at the lower doses. In some males,
the testes were atrophied and there was a slight indication of thyroid
hyperplasia, notably at 2500 ppm. There was no increase in tumour
incidence in the treated animals (Annex I, reference 4).
Groups of 50 Fischer 344/N rats were fed ziram (purity, 89%;
containing 6.5% thiram, 2% other zinc salts, and 2% unidentified
additional impurities) at concentrations of 0, 300, or 600 ppm (equal
to 0, 11, or 22 mg/kg bw per day for males and 0, 13, or 26 mg/kg bw
per day for females) for 103 weeks. All animals were observed daily
for clinical signs, and body weights and food consumption were
recorded monthly. At termination, necropsies were performed on all
animals, and selected organs and tissues from most animals were
examined microscopically. The survival, body weight, and food
consumption of treated rats were not adversely affected. C-Cell
carcinomas of the thyroid occurred in male rats, with a statistically
significant, positive trend ( p < 0.01); the incidence among animals
at 600 ppm was significantly greater than that in controls (0/50
controls, 2/49 at the low dose, 7/49 at the high dose). The combined
incidence of C-cell adenomas and carcinomas in males also showed a
statistically significant, positive trend (4/50 controls, 9/49 at the
low dose, 12/49 at the high dose). No significant histopathological
changes were found in the follicular cells. There was no NOAEL, since
the combined incidence of C-cell adenomas and carcinomas of the
thyroid in males showed a statistically significant, positive trend at
300 ppm, the lowest dose tested (National Toxicology Program, 1983).
In a study of toxicity, involving groups of 20 CD(SD)BR rats of
each sex, and carcinogenicity, involving groups of 50 rats of each
sex, the animals were fed diets containing ziram (purity, 98.7%)
providing concentrations of 0, 60, 180, or 540 ppm (equal to 0,
3.0/2.5, 9.1/7.7, or 27/24 mg/kg bw per day for males and 0, 3.9/3.4,
12/10, or 38/35 mg/kg bw per day for females), for 12 or 24 months.
Clinical signs, body weight, and food consumption were observed
weekly, and ophthalmoscopic, haematological, biochemical, and urinary
investigations were performed at certain intervals. At termination,
the organ weights, haematological parameters, and the results
of clinical chemical, urinary, and macroscopic and microscopic
examinations were recorded. Body-weight gain, food intake, and food
conversion ratios were reduced in a dose-dependent fashion in male and
female rats at 180 and 540 ppm. The erythrocyte count was reduced
dose-dependently in animals of each sex at 60, 180, and 540 ppm, and a
dose-dependent decrease in thromboplastin time was observed in males
at all three doses. At 180 and 540 ppm, packed cell volume and
haemoglobin were reduced in females and thromboplastin time in males.
Tri-iodothyronine, thyroxine, albumin, total protein, and calcium
levels were reduced and that of blood urea nitrogen was increased
at 180 and 540 ppm in animals of each sex. Haemangiomata in the
mesenteric lymph nodes, hypertrophy with vacuolation of the adrenals,
and C-cell hyperplasia of the thyroids were observed in males at
540 ppm. In females, cortical cystic degeneration of the adrenals was
seen at 540 ppm. Dose-dependent adipose replacement, narrowing of the
myofibres in skeletal muscle, haemosiderosis in the spleen, adipose
tissue replacement in the pancreas, prominent ultimobranchial cysts in
the thyroids, and epithelial hyperplasia and subepithelial oedema in
the nonglandular part of the stomach were observed in animals at 60,
180, or 540 ppm. There was no NOAEL, since decreased erythrocyte
counts and thromboplastin time, haemosiderosis in the spleen,
ultimobranchial cysts in the thyroids, and epithelial hyperplasia in
the stomach were seen at 60 ppm, the lowest dose tested (Powell
et al., 1994b).
(d) Reproductive toxicity
Mice
Groups of 10 CH3 or AK male mice were given ziram (purity
unspecified) by gavage at concentrations of 0 or 0.2 mg% (equivalent
to 0 or 0.02 mg/kg bw per day) for 21 days before mating with
untreated females of the same strain. The males were then killed for
histopathological examination. Some of the female mice were killed on
day 17 of gestation in order to examine the numbers of corpora lutea,
nidation places, and dead and live embryos; the other females were
kept alive to breed. Newborn mice were immediately sacrificed and
stained with alizarin for examination of skeletal abnormalities. Ziram
increased the incidence of sterility: only 20% of the C3H and 80% of
the AK females, but all of the controls, were fertilized. The mean
number of embryos was reduced from 11.1 in controls to 7.5 in the CH3
mice and from 11.2 in controls to 9.1 in the AK mice. The calculated
embryonic mortality was 4.5% in the CH3 strain and 6.3% in the AK
strain (Abbota's formula). When the numbers of corpora lutea and of
live and resorbed embryos were included, embryonic mortality rates of
27% for C3H mice and 32% AK mice were found (method of dominant
lethals). Analysis of the testes showed atrophy of the seminiferous
tubules and impaired spermatogenesis. Examination of meiotic
chromosomes in diakinesis showed the presence of tetravalent and
univalent chromosomes and frequent pyknosis, demonstrating the
clastogenic effect of ziram on future gametes. The offspring of both
CH3 and AK strains had anomalies of the spine, cyphosis, scoliosis,
sternum ossification failure, branching ribs (some of which were
incompletely ossified), assymetry of the cranium, microcephalus, and
eviscerations. The bone dimensions of the CH3 mice were greater than
those of the controls. There was no NOAEL for male fertility, because
of decreased fertility, atrophy of seminiferous tubules, and impaired
spermatogenesis, or for developmental toxicity, because anomalies of
the spine, cyphosis, scoliosis, and sternum ossification failure were
seen in the offspring at 0.02 mg/kg bw per day, the lowest dose tested
(Cilievici et al., 1983). In the absence of important information on
the design of the study, this report was not considered in establishing
the ADI.
Groups of six Swiss albino male mice, aged 12-14 weeks, received
ziram (purity unspecified), dissolved in 5% dimethyl sulfoxide,
intraperitoneally as single doses of 0, 50, or 100 mg/kg bw or
repeated doses of 25 mg/kg bw per day for five days. The mice were
killed one month after the treatment. The epididymides were isolated,
minced in 4 ml NaCl, and dispersed by slow aspiration. The tissue
particles were filtered out, and smears were made on clean, dry slides
for microscopy. A minimum of 2000 sperm was scored per animal, and the
abnormalities were classified. The frequency of abnormal sperm was
1.6% in the controls, 5.6% in animals at 50 mg/kg bw, 8.2% in those
at 100 mg/kg bw, and 8.4% following repeated doses of 25 mg/kg bw
per day. The abnormalities included sperm with acrosomes bent
upwards, acrosomes bent downwards, and without acrosomes. Many head
abnormalities, including double heads, banana heads, amorphous heads,
microcephaly, and macrocephaly, were found, and a high frequency of
coiled sperm was seen. The abnormalities observed were considered to
be due either to changes in the genes responsible for spermatogenesis
or to changes in differentiation during gene expression involving
post-transcriptional stages and thereafter in the translation of the
genetic message. There was no NOAEL, as sperm abnormalities were seen
at 25 mg/kg bw per day, the lowest dose tested (Hemavathi & Rahiman,
1993).
Rats
In a two-generation study of reproductive and developmental
neurotoxicity, groups of 30 male and 30 female Crl:CD BR Sprague-
Dawley rats were fed ziram at concentrations of 0, 72, 210, or 540
ppm, equal to 0, 3, 10, or 25 mg/kg bw per day for males and 0, 5, 13,
or 32 mg/kg bw per day for females. The parental animals received the
diets from about six weeks of age for the F0 generation and on day 22
postnatally for the F1 generation, for at least 70 days before mating
and throughout all subsequent phases of the study until termination of
the generation. All animals were observed twice daily for appearance
and behaviour. Body weights and food consumption were recorded at
appropriate intervals. All females were allowed to deliver and rear
their pups to weaning on lactation day 21. Offspring from the pairing
of the F0 animals (30 pups of each sex per group) were selected to
constitute the F1 generation. Thirty F2 pups of each sex per group
were selected for testing of developmental landmarks and behaviour,
neuropathological examination, and brain weight measurement. Surplus
F1 pups were killed and necropsied on postnatal day 28, and surplus
F2 pups were killed and necropsied on postnatal day 22. The F0 and
F1 parental animals and selected F2 pups that were not allocated for
measurements underwent detailed gross necropsy; and the weights of
the brain, kidneys, liver, pituitary gland, ovaries, or testes and
epididymides (F0 and F1 only) were recorded. Designated tissues from
the controls and from F0 and F1 parental animals at 540 ppm and F2
pups selected for neuropathological evaluation were evaluated for
histopathological changes.
Reproductive parameters (fertility, mating, and days between
pairing and coitus, gestation, and parturition) in the F0 and F1
generations were not adversely affected by concentrations of 72, 210,
or 540 ppm ziram. All F0 and F1 parental animals survived to the
scheduled necropsies, and no adverse clinical signs attributable to
treatment were observed. The mean body weights and body-weight gains
of F0 males at 540 ppm were reduced early in the treatment period,
and the mean body weights and body-weight gains of F1 males at
540 ppm were generally reduced. The mean body weights and body-weight
gains of F0 and F1 females at 540 ppm were generally reduced before
breeding, during gestation and lactation, and after weaning. No adverse
effects were seen on the body weights or body-weight gains of animals
in either generation at 72 or 210 ppm. The food consumption of F0 and
F1 males at 540 ppm was generally reduced throughout each generation.
The food consumption of F0 and F1 females at 540 ppm was reduced
before breeding, during gestation and lactation, and after weaning of
the F0 and F2 generations. No adverse effects on food consumption
were observed in males or females given ziram at concentrations of 72
and 210 ppm in either generation. No treatment-related, macroscopic
internal changes were found in treated F0 or F1 animals, and no
adverse effects on organ weights were observed at any dose. No
microscopic lesions attributable to treatment were observed in
tissues from animals at 540 ppm on histopathological examination.
Microscopic examination of gross lesions seen at the scheduled
necropsies of animals at 540 ppm did not reveal any adverse effects.
The mean body weights of F1 and F2 pups in the litters of
animals at 540 ppm were slightly reduced (usually statistically
significantly) during lactation and throughout the remainder of the
study until postnatal day 70 (selected F2 pups). The F1 and F2 pup
sex ratios, live litter sizes, number of dead pups on lactation day 0,
viability indices, general physical condition, and brain weight
(selected F2 pups) were not adversely affected by parental treatment
at any concentration. The findings at necropsy of F1 and F2 pups
that died or were killed at the scheduled postnatal necropsies did not
suggest any correlation with parental treatment. Various indicators
of physical and functional development and behavioural responses
in the selected F2 pups were comparable to those in controls.
Neuropathological examinations on postnatal days 11 and 70 showed no
gross or microscopic treatment-related lesions in the F2 pups. The
NOAEL for maternal toxicity was 210 ppm, equal to 10 mg/kg bw per day,
on the basis of reduced food consumption and body-weight gain; that
for neonatal toxicity was 210 ppm, equal to 10 mg/kg bw per day, on
the basis of reduced body weight; and that for reproductive and
developmental neurotoxicity was 540 ppm, equal to 25 mg/kg bw per day
(Nemec, 1996).
(e) Developmental toxicity
Rats
In a preliminary study, groups of 10 pregnant Crl:CD(SD)BR rats
were given ziram (purity, 98.9%) by gavage at doses of 0, 5, 20, or
80 mg/kg bw per day on days 6-15 of gestation. Dams were observed for
clinical symptoms, body weight, and food and water consumption, and
were killed on day 20 and examined for congenital abnormalities and
macroscopic pathological changes in organs. The ovaries and uteri
were examined to determine the number of corpora lutea, the number
and distribution of live young, and the number of early and late
embryo-fetal deaths and fetal abnormalities. Live young were examined
externally and weighed; no further examinations were done. Dams showed
body-weight loss up to day 8, decreased food intake, and increased
water intake at doses of 5, 20, and 80 mg/kg bw per day. Hair loss
was observed in dams at 20 and 80 mg/kg bw per day. Salivation and
increased postimplantation losses were seen in dams at 80 mg/kg bw per
day. Reduced litter and fetal weights were seen at 20 and 80 mg/kg bw
per day. The dose range was considered to be too high for the main
study (Smith et al., 1990).
Groups of 25 pregnant Crl:CD(SD)BR rats were given ziram (purity,
98.9%) by gavage at doses of 0, 1, 4, 16, or 64 mg/kg bw per day on
days 6-15 of gestation and were then observed for clinical symptoms,
body weight, and food and water consumption. On day 20, the animals
were killed and examined for congenital abnormalities and macroscopic
pathological changes in organs. The ovaries and uteri were examined to
determine the number of corpora lutea, the number and distribution of
live young, and the number of early and late embryo-fetal deaths and
fetal abnormalities. Live young were examined externally and weighed.
One-half of the fetuses were subsequently examined for visceral and
the other half for skeletal abnormalities. Dams showed dose-related
body-weight loss up to day 8, followed by retarded weight gain
throughout treatment; decreased food consumption and increased water
consumption and salivation were seen at 16 and 64 mg/kg bw per day.
Pre- and postimplantation losses were unaffected by treatment. At
4 mg/kg bw per day, only marginally increased water consumption was
observed during treatment. Significantly lower litter and fetal
weights were seen at 64 mg/kg bw per day; litter size and the
incidences of skeletal and visceral anomalies were unaffected by
treatment. The NOAELs were 4 mg/kg bw per day for maternal toxicity,
on the basis of decreased body weight and food intake, increased water
intake, and salivation at doses > 16 mg/kg bw per day, and 16 mg/kg
bw per day for developmental toxicity, on the basis of decreased
litter and fetal weight at 64 mg/kg bw per day. No teratogenicity was
seen (Smith et al., 1990).
Rabbits
In a preliminary study, groups of five pregnant New Zealand white
rabbits were given ziram (purity, 98.0%) by gavage at doses of 0, 5,
10, or 20 mg/kg bw per day on days 7-19 of gestation. All animals
were observed for clinical symptoms, body weight, and food consumption.
On day 28, the animals were killed, dissected, and examined
macroscopically for abnormalities. The ovaries and uteri were removed
and examined to determine the gravid uterine weight, number of corpora
lutea, and the distribution of live young and of early and late
embryo-fetal deaths. Live young were examined externally for
abnormalities and weighed. Two animals at 20 mg/kg bw per day died,
and one aborted. Treatment had no effect on the clinical condition or
findings at necropsy for animals that survived to term. Marked
reductions in weight gain and food intake, particularly at the start
of treatment, were observed in animals at 20 mg/kg bw per day. Slight,
temporary reductions in weight gain and food intake were also observed
at 10 mg/kg bw per day. At 20 mg/kg bw per day, postimplantation
losses were increased and litter size, litter weight, and fetal weight
were reduced. Because maternal toxicity was observed at 20 mg/kg bw
per day, the high dose for the main study was < 20 mg/kg bw per day
(Barker, 1985).
Groups of 16 pregnant New Zealand white rabbits were given ziram
(purity, 98.1%) by gavage at doses of 0, 3, 7.5, or 15 mg/kg bw per
day on days 7-19 of gestation. All animals were observed for clinical
symptoms, body weight, and food consumption. On day 28, the animals
were killed, dissected, and examined macroscopically for abnormalities.
The ovaries and uteri were removed and examined to determine the gravid
uterine weight, the number of corpora lutea, and the distribution of
live young and of early and late embryo-fetal deaths. Live fetuses were
weighed and examined for abnormalities before alizarin staining.
Significant reductions in maternal body-weight gain and food intake
were observed from the start of treatment at 15 mg/kg bw per day;
slight reductions in weight gain and food intake were also observed
during the early part of the treatment period in animals at 7.5 mg/kg
bw per day. At 15 mg/kg bw per day, postimplantation loss was slightly
increased and litter size, litter weight, fetal weight, and the
crown-rump length were reduced. There was no teratogenic effect. The
NOAEL for maternal and developmental toxicity was 7.5 mg/kg bw per day,
on the basis of decreased body-weight gain and food intake, increased
postimplantation loss, and reduced litter size, litter weight, fetal
weight, and crown-rump length at 15 mg/kg bw per day (Barker, 1986).
(f) Genotoxicity
The results of studies on the genotoxicity of ziram are
summarized in Table 2. Ziram is mutagenic in bacteria. It induced
chromosomal aberrations in some, but not all, studies with cultured
mammalian cells and did not induce unscheduled DNA synthesis in
hepatocytes. In vivo, ziram induced DNA single-strand breaks in the
livers of rats but not mice. Chromosomal aberrations were not
induced in mice in vivo in bone-marrow cells or spermatogonia, and
micronuclei were not induced in bone-marrow cells or peripheral
erythrocytes. Studies for clastogenicity have not been conducted in
rats in vivo. Consequently, the Meeting was unable to reach a
conclusion about the genotoxicity of ziram.
Table 2. Results tests for the genotoxicity of ziram
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium < 100 µg/plate NR Positivea Hedenstedt
TA98, TA100, TA1535, at 10, 50, et al. (1979)
TA1537, TA1538 100 µg/
plate
Reverse mutation S. typhimurium < 50 µg/plate 98.5 Positiveb Jones et al.
TA98, TA100, TA1535, at 15, 50 (1990)
TA1537, TA1538 µg/plate
Reverse mutation S. typhimurium < 90 µg/plate 98.5 Positivea Crebelli et al.
TA98, TA100, TA102, TA1537, at 7.5-90 (1992)
TA1950, TA1975, TA1535 µg/plate
Reverse mutation S. typhimurium 15-90 µg/plate NR Positiveb Franekic et al.
TA98, TA100, TA102, (1994)
TA104, TA1535, TA1538
Chromosomal Saccharomyces 5-50 µg/ml NR Negative Franekic et al.
malsegregation cerevisiae (1994)
Cell division Shallot root-tip 0.5-50 µg/ml NR Positive Franekic et al.
cells (1994)
Chromosomal aberration Chinese hamster < 1.5 µg/ml 98.5 Negative Brooker &
ovary cells Akhurst (1989)
Chromosomal aberration Chinese hamster < 1.75 µg/ml 86.2 Positivea Gulati et al.
ovary cells (1989)
Table 2. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Chromosomal aberration Chinese hamster < 10 µg/ml 98.5 Positiveb Mosesso et al.
ovary cells (1994)
Chromosomal aberration Chinese hamster < 2.15 µg/ml 98.5 Positiveb Mosesso et al.
epithelial liver cells (1994)
Unscheduled DNA synthesis Rat hepatocytes < 100 µg/ml 98.5 Negative Proudlock (1989)
In vivo
Chromosomal aberration Male and female NMRI 40, 120, 400 mg/kg bw 98.4 Negative Völkner (1992a)
mice, bone-marrow cells
Chromosomal aberration Male NMRI mice, germ cells 20, 67, 200 mg/kg bw 98.4 Negative Völkner (1992b)
Micronucleus formation Male and female Crl:CD-1 3.8, 11, 34, 101 mg/kg bw 98.8 Negative Proudlock &
mice, erythrocytes Taylor (1992)
Micronucleus formation Male and female B6C3F1 2.5, 5.0, 10, 20 mg/kg bw 98.5 Negative Crebelli et al.
mice, bone-marrow cells (1992)
Single-strand DNA breaks Male Swiss mice, 13, 25, 50, 100 mg/kg bw NR Negative Scarabelli et al.
liver cells (1993)
Single-strand DNA breaks Male Wistar rats, 13, 25, 50, 100 mg/kg bw NR Positive Scarabelli et al.
liver cells (1993)
NR, Not reported
a In the presence or absence of metabolic activation
b In the presence of metabolic activation
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Rabbits
The irritancy of ziram to the skin was tested in New Zealand
white rabbits. About 24 h before application of the test substance,
hair was removed from the dorsal lumbar region. Ziram (purity, 98.5%)
was applied at a dose of 0.5 g under a 2.5-cm2 gauze pad moistened
with 0.5 ml distilled water to one skin site on each of six animals,
and each treatment site was occluded with an elastic adhesive dressing
for 4 h. The animals were not restrained during the exposure and were
returned to their cages. At the end of treatment, the semiocclusive
dressing and gauze pads were removed and the treatment sites were
washed with water to remove any residual test substance. The treated
skin was examined for erythema and oedema on days 1, 2, 3, and 4. None
of the animals showed a response to treatment (Liggett & McRae,
1990a).
The irritancy of ziram to the eye was tested in New Zealand white
rabbits. The eyes of the animals were examined before instillation of
80 mg ziram (purity, 89.5%) in 0.1 ml into the lower everted lid of
one eye. Ocular lesions were graded and scored 1 and 24 h after
instillation. As the first rabbit had a severe ocular reaction
(irridal inflammation and opacity), the study was terminated on humane
grounds (Liggett & McRae, 1990b).
Guinea-pigs
The skin sensitizing potential of ziram (purity, 99.0%) was
examined in 30 adult female Dunkin-Hartley guinea-pigs. After
induction by epicutaneous applications of the test substance (25% w/w
in corn oil) and challenge with 10, 5, or 1% (w/w) in corn oil, six
guinea-pigs showed a positive skin response (grade 2 or more) to the
10% concentration. Five of these animals also reacted positively to
the 5% dose and three to the 1% concentration. No positive reactions
were observed in the control animals. Thus, a sensitization rate of
30% was obtained. The author considered that ziram has moderate
sensitizing properties (Daamen, 1988).
(ii) Neurotoxicity
Rats
The neurotoxicity of repeated doses of ziram was studied in
groups of 10 Crl:CD(SD)BR rats fed diets containing ziram (purity,
97.9%) providing concentrations of 0, 72, 210, or 540 ppm, equal to 0,
5, 14, or 34 mg/kg bw per day for males and 0, 6, 16, or 40 mg/kg bw
per day for females, for 91 days. Five animals of each sex per group
were allocated for evaluation of neurotoxic esterase and five for
evaluation of neuropathological changes. Viability, clinical signs,
body weight, and food consumption were recorded for all animals.
The results of a functional observational battery (home cage,
handling, open field, sensorimotor, neuromuscular, and physiological
observations) and of tests for total and ambulatory motor activity
were recorded for all animals during week 3, 7, and 12 of exposure.
The body weights and food consumption of male and female rats at
540 ppm were reduced, but no treatment-related effects indicative of
neurotoxicity were apparent. Brain neurotoxic esterase activity was
47% lower than that in controls in males and 38% lower in females at
540 ppm. The NOAEL for brain neurotoxic esterase inhibition was
210 ppm, equal to 14 mg/kg bw per day (Nemec, 1993).
The neurotoxicity of a single dose of ziram was studied in groups
of 12-16 Crl:CD(SD)BR rats given single doses of 0, 15, 300, or
600 mg/kg bw ziram (purity, 97.8%). Viability, clinical signs, and
body weight were recorded for all animals. The results of a functional
observational battery (home cage, handling, open field, sensorimotor,
neuromuscular, and physiological observations) and tests for total and
ambulatory motor activity were recorded for all animals before and 4 h
after treatment and on days 7 and 14. Five controls and five animals
at 600 mg/kg bw were selected for evaluation of neuropathological
changes. Four males and seven females at 600 mg/kg bw and one female
at 300 mg/kg bw died. At these doses, body weight was reduced, and
several treatment-related clinical signs (e.g. cyanosis, hypothermia,
enophthalmus, unkempt appearance, gait alterations, hypoactivity,
ptosis, and abdomal respiration) were observed. Remarkable differences
from the controls were seen in the functional observational battery
for animals at 300 and 600 mg/kg bw. Most of the responses were
observed 4 h after treatment (e.g. altered posture, palpebra closure,
and faecal consistency) or daily during the first week after dosing
(e.g. lacrimation, salivation, changes in fur appearance, altered
palpebral closure, changes in respiratory rate, and altered gait).
Ambulatory and total motor activity counts were reduced in males and
females at 300 and 600 mg/kg bw. No treatment-related microscopic
lesions were observed in the central and peripheral nervous tissues
examined. The NOAEL for neurotoxicity was 15 mg/kg bw, on the basis of
mortality, reduced body weight, ambulatory, and total motor activity,
and clinical signs at doses > 300 mg/kg bw (Lamb, 1994).
3. Observations in humans
The ability of ziram to induce chromosomal aberrations in
peripheral leukocytes was studied in four men and five women who had
been exposed for three to five years to ziram at a concentration of
2-4 mg/m3 air. The control group consisted of three women and one
man. The exposed group had 5.9% cells with aberrations and the
controls, 0.75% (Pilinskaya, 1970; Annex I, reference 15).
Comments
In experiments with 14C-labelled ziram in rats, elimination was
essentially complete within 48 h. Excretion occurred mainly in expired
air, urine, and faeces. Less than 2% of the administered dose remained
in the tissues. The biotransformation of ziram has not been studied
in rodents. In goats, it is metabolized at least in part via a
single-carbon pathway, which results in extensive radiolabelling of
natural products.
The primary effects of short- and long-term treatment with ziram
in mice, rats, and dogs were on the liver, thyroid gland, and testis.
The hepatic effects were increased liver weight, degeneration, and
focal-cell necrosis. Effects in the thyroid were C-cell hyperplasia
and carcinomas, and that on the testes was sterility.
Ziram had moderate acute oral toxicity in rats and rabbits (LD50
= 200-400 mg/kg bw). WHO has classified ziram as 'slightly hazardous'
(WHO, 1996).
In a four-week study of toxicity in mice given dietary
concentrations of 0, 3000, 4000, or 5000 ppm, an NOAEL was not
identified. Reductions in body weight, food intake, efficiency of food
use, and brain and heart weight occurred at all doses.
In a 13-week study of toxicity in mice given dietary
concentrations of 0, 100, 300, 900, or 2700 ppm, the NOAEL was
100 ppm, equal to 15 mg/kg bw per day, on the basis of lowered spleen
weight at higher doses. At 900 and 2700 ppm, the number of corpora
lutea was reduced, which was consistent with cellular changes in the
uterus.
In two four-week studies of toxicity in rats either given diets
providing doses of 0, 100, 500, 2500, or 5000 ppm or treated by gavage
with 0, 3, 15 or 100 mg/kg bw per day, the NOAEL was 3 mg/kg bw, on
the basis of degenerative liver changes. At 100 mg/kg bw, degenerative
changes in the kidneys and reductions in body weight, food intake,
efficiency of food use, and absolute weights of the liver, pituitary,
testes, brain, and uterus were seen.
In a 13-week study of toxicity in which rats received dietary
levels of 0, 100, 300, or 1000 ppm, the NOAEL was 100 ppm, equal to
7.4 mg/kg bw per day, on the basis of reduced body-weight gain, food
intake, and food use and increased brain and spleen weights at higher
doses.
In a four-week study of toxicity in dogs given diets providing
doses of 0, 1000, 2000, or 5000 ppm, an NOAEL was not identified.
Increased liver weight occurred at all doses. At 2000 ppm, convulsive
episodes were observed.
In a 13-week study of toxicity in dogs given diets providing 0,
100, 300, or 1000 ppm, the NOAEL was 100 ppm, equal to 4.1 mg/kg bw
per day, on the basis of increased liver weight, focal liver necrosis,
pigment in Kupffer cells, activated partial thromboplastin time, and
elevated cholesterol level at higher doses.
In a one-year study of toxicity in which dogs were fed diets
providing doses of 0, 50, 180, or 500 ppm, the NOAEL was 50 ppm, equal
to 1.6 mg/kg bw per day, on the basis of reductions in body-weight
gain, degeneration of hepatocytes, and increased activity of alanine
and aspartate aminotransferases and alkaline phosphatase at doses
> 185 ppm. At 500 ppm, single liver-cell necrosis was observed, and
the liver weight and cholesterol values were increased; albumin values
were reduced. Inflammatory cell infiltration around the hepatic vein
and its branches and aggregates of pigmented Kupffer cells were
observed in the liver.
Two long-term studies of toxicity and carcinogenicity in mice
have been reported. One was considered inadequate for evaluating
the carcinogenicity of ziram. In the other, mice were given diets
providing doses of 0, 25, 75, 220, or 680 ppm for 80 weeks. The NOAEL
was 25 ppm, equal to 3 mg/kg bw per day, on the basis of reduced brain
weight at doses > 75 ppm. There was no evidence of carcinogenicity.
In a two-year study of toxicity and carcinogenicity in rats at
dietary concentrations of 0, 25, 250, or 2500 ppm, the NOAEL was
250 ppm, equivalent to 12 mg/kg bw per day, on the basis of testicular
atrophy and thyroid hyperplasia at 2500 ppm. There was no evidence of
carcinogenicity.
In a two-year study of toxicity and carcinogenicity in Fischer
344 rats with dietary concentrations of 0, 300, or 600 ppm, an NOAEL
was not identified, since the combined incidence of C-cell adenoma
and carcinoma of the thyroid in males showed a positive trend. This
finding was considered to represent an extension of the known toxicity
of the compound to the thyroid, to which the rat is particularly
sensitive, and not to indicate carcinogenic potential for humans.
In a study of toxicity and carcinogenicity in CD rats treated
with 0, 60, 180, or 540 ppm in the diet for 12-24 months, an NOAEL was
not identified because dose-related changes in organ weights and
histopathological and haematological changes were observed at 60 ppm,
equal to 2.5 mg/kg per day. Other effects included reduced body
weight, erythrocyte count, and triiodothyronine and thyroxine
activity. Cysts in the thyroids, epithelial hyperplasia, hypertrophy
with vacuolation, cortical cystic degeneration of the adrenals, and
C-cell hyperplasia of the thyroid were also demonstrated. The tumour
incidence was not increased.
In a study of sperm quality in mice treated intraperitoneally
with ziram at a single dose of 0, 50, or 100 mg/kg bw or repeated
doses of 25 mg/kg bw per day for five days, severe morphological
abnormalities were observed. The frequency of abnormal sperm was 1.6%
in the controls, 5.6% at 50 mg/kg bw, 8.2% at 100 mg/kg bw, and 8.4%
after repeated doses of 25 mg/kg bw.
In a two-generation study of reproductive toxicity and
developmental neurotoxicity, rats were fed ziram at concentrations of
0, 72, 210, or 540 ppm. The NOAEL for maternal toxicity was 210 ppm,
equal to 10 mg/kg bw per day, on the basis of reduced food consumption
and body-weight gain at 540 ppm. The NOAEL for neonatal toxicity was
210 ppm, equal to 10 mg/kg bw per day, on the basis of reduced body
weight gain at 540 ppm. The NOAEL for reproductive toxicity and
developmental neurotoxicity was 540 ppm, equal to 25 mg/kg bw per day.
In a study of developmental toxicity, rats were given ziram at 0,
1, 4, 16, or 64 mg/kg bw per day on days 6-15 of gestation. The NOAEL
for maternal toxicity was 4 mg/kg bw per day for maternal toxicity, on
the basis of decreased body-weight gain and food intake and increased
water intake and salivation at doses > 16 mg/kg bw per day. The
NOAEL for developmental toxicity was 16 mg/kg bw per day, on the basis
of decreased litter weight and fetal weight at 64 mg/kg bw per day. No
teratogenicity was seen.
In a study of developmental toxicity in rabbits given ziram at
doses of 0, 3, 7.5, or 15 mg/kg bw per day on days 7-19 of gestation,
the NOAEL for maternal toxicity and developmental toxicity was 7.5
mg/kg bw per day, on the basis of decreased body-weight gain and food
intake in the dams and post-implantation loss, reduced litter size,
litter weight, fetal weight, and crown-rump length at 15 mg/kg bw per
day. There was no evidence of teratogenicity.
Ziram is mutagenic in bacteria. It induced chromosomal
aberrations in some, but not all, studies with cultured mammalian
cells but did not induce unscheduled DNA synthesis in hepatocytes.
In vivo, ziram induced single-strand breaks in DNA in the livers of
rats but not mice. Chromosomal aberrations were not induced in mice
in vivo in bone-marrow cells or spermatogonia, and micronuclei were
not induced in bone-marrow cells or peripheral erythrocytes. Studies
for clastogenicity have not been conducted in rats in vivo. In an
old study of nine workers exposed for three to five years to ziram at
a concentration of 2-4 mg/m3 air, the percentage of peripheral
leukocytes with chromosomal aberrations was 5.9%; in a control group,
the percentage was 0.75%. The Meeting was unable to reach a conclusion
about the genotoxicity of ziram.
Ziram caused severe ocular irritation but no dermal irritation in
rabbits and moderate skin sensitization in guinea-pigs.
In two studies of neurotoxicity in rats treated with single doses
of 0, 15, 300, or 600 mg/kg bw or 0, 72, 210, or 540 ppm for 91 days,
behavioural effects indicative of neurotoxicity were apparent after
the single high doses but not after repeated dosing at a lower level.
The NOAEL was 210 ppm, equal to 14 mg/kg bw per day, on the basis of
reduced body weight and food consumption and inhibition of brain
neurotoxic esterase activity at 540 ppm.
An ADI of 0-0.003 mg/kg bw was established on the basis of
long-term toxicity in the rat. In this study, effects were seen at all
doses, the LOAEL being 60 ppm, equal to 2.5 mg/kg bw per day. In view
of the absence of an NOAEL, a safety factor of 1000 was used. The
NOAEL of 1.6 mg/kg bw per day observed in a long-term study of
toxicity in dogs supported this ADI, which served as the basis for the
group ADI that was established for ziram alone or in combination with
ferbam.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3 mg/kg bw per day (80-week study of
toxicity and carcinogenicity)
Rat: NOAEL could not be determined: lowest effective dose,
60 ppm, equal to 2.5 mg/kg bw per day (12-24-month
study of toxicity, various effects)
100 ppm, equal to 7.4 mg/kg bw per day (13-week study
of toxicity)
250 ppm, equivalent to 12 mg/kg bw per day per day
(two-year study of toxicity and carcinogenicity)
210 ppm, equal to 10 mg/kg bw per day (maternal
toxicity in a study of reproductive toxicity)
Rabbit: 7.5 mg/kg bw per day (maternal toxicity and
embryotoxicity in a study of developmental toxicity)
Dog: 50 ppm, equal to 1.6 mg/kg bw per day (one-year study
of toxicity)
100 ppm, equal to 4.1 mg/kg bw per day (13-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw (for ferbam and ziram)
Studies that would provide information useful for continued evaluation
of the compound
1. Further studies on long-term toxicity in the rat
2. Further studies on genotoxicity in rats
3. Further studies on male reproductive toxicity
4. Further observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to ziram
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 270 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.06 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Severely irritating
Dermal, sensitization, guinea-pig Moderately sensitizing
Medium-term (1-26 weeks) Repeated oral, 13 weeks, toxicity, mouse NOAEL = 15 mg/kg bw per day, decreased spleen weight
Repeated oral, 4 weeks, toxicity, rat NOAEL = 3 mg/kg bw per day, reduced body weight and
food consumption, degenerative hepatic changes
Repeated oral, 13 weeks, toxicity, rat NOAEL = 7.4 mg per kg bw per day, reduced body weight
and food consumption, increased brain and spleen weight
Repeated oral, 13 weeks, toxicity, dog NOAEL = 4.1 mg/kg bw per day, hepatic toxicity
Repeated oral, reproductive toxicity and NOAEL = 25 mg/kg bw per day, reproductive toxicity
developmental neurotoxicity, rat and development neurotoxicity
NOAEL = 10 mg/kg bw per day, maternal and neonatal
toxicity (reduced body weight)
Repeated oral, developmental toxicity, rat NOAEL= 16 mg/kg bw per day, developmental toxicity
(reduced fetal weight)
NOAEL = 4 mg/kg bw per day, maternal toxicity
(reduced body weight)
Repeated oral, developmental toxicity, NOAEL = 7.5 mg/kg bw per day, developmental and
rabbit maternal toxicity (reduced fetal and maternal weight)
Repeated oral, neurotoxicity, rat NOAEL = 14 mg/kg bw per day, inhibition of
neuropathy target esterase activity
Long-term (> 1 year) Repeated oral, 18 months, toxicity, mouse NOAEL = 3 mg/kg bw per day, reduced brain weight and
hepatic toxicity
Repeated oral, 2 years, toxicity and No NOAEL; LOAEL = 2.5 mg/kg bw per day, haematological
carcinogenicity, rat toxicity and toxic effects on the thyroid
Repeated oral, 1 year, toxicity, dog NOAEL = 1.6 mg/kg bw per day, reduced body weight and
hepatic toxicity
References
Barker, L. (1985) Ziram: Oral (gavage) range-findings study in the
pregnant rabbit. Unpublished report No. 4588-508/1 from Hazleton
Laboratories Europe, Ltd, North Yorkshire, United Kingdom. Submitted
to WHO by UCB Chemicals, Brussels, Belgium.
Barker, L. (1986) Ziram: Oral (gavage) teratology study in the rabbit.
Unpublished report No. 4913-508/2 from Hazleton Laboratories Europe,
Ltd, North Yorkshire, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Blagden, S.M. (1991) Ziram technical: Acute inhalation toxicity study.
Four hour exposure (nose only) in the rat. Unpublished report
No. 378/2 from Safepharm Laboratories Ltd, Derby, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Bodden, R.M. (1993) Ziram: Nature of the residue in lactating goats.
Unpublished report No. HLA 6225-101 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Brooker, P.C. & Akhurst, L.C. (1989) Analysis of metaphase chromosomes
obtained from CHO cells cultured in vitro and treated with ziram.
Unpublished report No. 7/89675 from Huntingdon Research Centre Ltd,
Cambs, United Kingdom. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Chambers, P.R., Cary, R.J., Gibson, W.A. & Gopinath, C. (1992a) Ziram.
Palatability study in mice for 4 weeks. Unpublished report No. ZIR
3/89720 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Chambers, P.R., Cary, R.J., Gibson, W.A. & Gopinath, C. (1992b) Ziram.
Palatability study in rats for 4 weeks. Unpublished report No. ZIR
2/89719 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Cheng, T. (1991a) Metabolism of ziram in rats. Unpublished report
No. HLA 6225-106 from Hazleton Laboratories America, Inc., WI, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Cheng, T. (1991b) Dermal absorption of 14C-ziram in male rats.
Unpublished report No. HLA 6225-107 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Cilievici, O., Graciun, C. & Ghidus, E. (1983) Decreased fertility,
increased dominant lethals, skeletal malformations induced in the
mouse by ziram fungicide. Rev. Roum. Morphol. Embryol. Physiol.,
29, 159-165.
Crebelli, R., Zijno, A., Conti, L., Crochi, B., Leopardi, P., Marcon,
F., Renzi, L. & Carere, A. (1992) Further in vitro and in vivo
mutagenicity assays with thiram and ziram fungicides: Bacterial
reversion assays and mouse micronucleus test. Teratog. Carcinog. Mutag.,
12, 97-112.
Daamen, P.A.M. (1988) Assessment of the skin sensitization potential
of ziram technical in the guinea-pig (split adjuvant test).
Unpublished report No. RCC NOTOX 0878/1096 from RCC Notox, C.,
's-Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Dickhaus, S. & Heisler, E. (1980) Report of a subacute toxicity study
of the substance 'Ziram' (techn.) for 4 weeks in the rat. Unpublished
report No. 2-4-132-80 from Pharmatox, Forschung und Beratung GmbH,
Hanover, Germany. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Edwards, J.A., Baldrick, P., Gibson, W.A., Crook, D., Offer, J.M. &
Gopinath, C. (1989) Twenty-one day dermal toxicity study in rabbits
with ziram. Unpublished report No. ZIR 4/89689 from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Franekic, J. Bratulic, N., Pavlica, M. & Papes, D. (1994) Genotoxicity
of dithiocarbamates and their metabolites. Mutat. Res., 325, 65-74.
Gulati, D.K., Witt, K., Anderson, B., Zeiger, E. & Shelby, M.D. (1989)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro. III: Results with 27 chemicals.
Environ. Mol. Mutag., 13, 133-193.
Hedenstedt, A., Rannug, U., Ramel, C. & Wachtmeister, C.A. (1979)
Mutagenicity and metabolism studies on 12 thiuram and dithiocarbamate
compounds used as accelerators in the Swedish rubber industry.
Mutat. Res., 68, 313-325.
Hemavathi, E. & Rahiman, M.A. (1993) Toxicological effects of ziram,
thiram, and dithane M-45 assessed by sperm shape abnormalities in
mice. J. Toxicol. Environ. Health, 38, 393-398.
Hodge, H.C., Maynard, E.A, Downs, W.L., Coye, R.D. & Steadman, L.T.
(1956) Chronic oral toxicity of ferric dimethyldithiocarbamate
(ferbam) and zinc dimethyldithiocarbamate (ziram). J. Pharmacol.
Exp. Ther., 118, 174-181.
Jackson, G.C. & Hardy, C.J. (1989) Ziram technical. Acute inhalation
toxicity in rats, 4-hour exposure. Unpublished report No. UCB,
314/89684 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Jones, E., Cook, P.G.S., Gant, R.A. & Kitshing, J. (1990) Ziram
technical: Bacterial mutation assay. Unpublished report No. ZIR
25/891914 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Khicenko, F.F. & Chernov, O.V. (1968) Experimental study of potential
vlastogenic effect of ziram. Gig. Primeniya Toksikol. Pestic. Klin.
Obravleniy (Kiev), 6, 770-776.
Korablev, M.V. (1969) Toxicological characteristics of dithiocarbamate
and derivative employed in the national economy and medicine
(literature survey). Farmakol. Toksikol., 32, 356-362 (in Russian).
Lamb, I.C. (1994) An acute neurotoxicity study of ziram in rats.
Unpublished report No. WIL-223001 from WIL Research Laboratories,
Inc., Ashland, OH, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Lee, C.-C., Russell, J.Q., Minor, J.L, Kowalski, J.J., Sanyer, J.L.,
Kintner, L.D., Hodgson, J.R., Short, R.D., Peters, P.J., Dilley, J.V.,
Murrill, E.A., Holton, D.O. & Ellis, H.V. (1975) Toxicological
evaluation of ferric dimethyldithiocarbamate (ferbam) and dithio-
carbamate (thiram) with acute toxicity of manganese and zinc
ethylenebisdithiocarbamates (maneb and zineb) Unpublished report
No. 3612-B from National Institute of Environmental Health Sciences,
Research Triangle Park, NC, USA. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Liggett, M.P. & Allan, S.A. (1989a) Acute oral toxicity to rats of
ziram. Unpublished report No. 89690D/UCB 315/AC from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Liggett, M.P. & Allen, S.A. (1989b) Acute dermal toxicity to rabbits
with ziram. Unpublished report No. 89338D/UCB 316/AC from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Liggett, M.P. & McRae, L.A. (1990a) Skin irritation to rabbits with
ziram (technical). Unpublished report No. 90500D/UCB 317/SE from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Liggett, M.P. & McRae, L.A. (1990b) Eye irritation to rabbits with
ziram (technical). Unpublished report No. 90499D/UCB 318/SE from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
McLean, T.A., Horner, S.A., Buist, D.P. (1992a) Ziram preliminary
dietary toxicity study in beagle dogs. Unpublished report No. ZIR
1-G/89637 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
McLean, T.A., Horner, S.A., Buist, D.P., Crook, D., Read, R.M.,
Gopinath, C., Anderson, A., Dawe, I.S., Johnson, S.F. & Patel, S.
(1992b) 13-Week dietary toxicity study in beagle dogs. Unpublished
report No. ZIR 8-G/901813 from Huntingdon Research Centre Ltd, Cambs,
United Kingdom. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Mosesso, P., Turchi, G., Cinelli, S., Chiara, D.D., Fiore, M. &
Palitti, F. (1994) Clastogenic effects of the dithiocarbamate
fungicides thiram and ziram in Chinese hamster cell lines cultures
in vitro. Teratog. Carcinog. Mutag., 14, 145-155.
National Toxicology Program (1983) Carcinogenesis Bioassay of Ziram
(CAS No. 137-30-4) in F344/N Rats and B6C3F1 Mice (Feed Study) (NIH report
No. 83-1794), Washington DC, Department of Health and Human Services,
Public Health Service, National Institutes of Health.
Nemec, M.D. (1993) A subchronic (13-week) neurotoxicity study of
ziram in rats. Unpublished report No. WIL-223004 from WIL Research
Laboratories, Inc., Ashland, OH, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Nemec, M.D. (1996) A dietary two-generation reproduction and
developmental neurotoxicity study of ziram in rats. Unpublished report
No. WIL-223003 from WIL Research Laboratories, Inc., Ashland, OH, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Pilinskaya, M.A. (1970) Chromosome aberrations in the persons
contacted with ziram. Genetika, 6, 157-163 (in Russian).
Powell, L.A.J., Crook, D., Gregson, R.L., Gopinath, C., Gibson, W.A.
& Anderson, A. (1992) Preliminary toxicity to rats by dietary
administration for 13 weeks. Unpublished report No. ZIR 5/901840 from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Powell, L.A.J., Gopinath, C., Gibson, W.A. & Anderson, A. (1993) Ziram
preliminary toxicity to mice by dietary administration for 13 weeks.
Unpublished report No. ZIR 11/901841 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Powell, L.A.J., Bottomley, S.M., Crook, D., Majeed, S.K., Gopinath,
C., Gibson, W.A. & Anderson, A. (1994a) Ziram (technical). Potential
oncogenicity to mice by repeated dietary administration for 80 weeks.
Unpublished report No. ZIR 12/932311 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf Autochem
Agri, Brussels, Belgium.
Powell, L.A.J., Bottomley, S.M., Crook, D., Gregson, R.L., Offer,
J.M., Gibson, W.A. & Anderson, A. (1994b) Ziram (technical). Combined
chronic toxicity and oncogenicity of ziram (technical) administered in
the diet to rats. Unpublished report No. ZIR 9/942098 from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB,
Elf Autochem Agri, Brussels, Belgium.
Proudlock, R.J. (1989) Autoradiographic assessment of DNA repair after
in vitro exposure of rat hepatocytes to ziram. Unpublished report
No. ZIR 6/89820 from Huntingdon Research Centre Ltd, Cambs, United
Kingdom. Submitted to WHO by UCB, Elf Autochem Agri, Brussels,
Belgium.
Proudlock, R.J. & Taylor K. (1992) Ziram (technical). Peripheral
blood micronucleus test in the mouse after 89 days dietary exposure.
Unpublished report No. ZIR 27/920513 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf Autochem
Agri, Brussels, Belgium.
Robens, J.F. (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol. Appl.
Pharmacol., 15, 152-163.
Ryazanova, R.A. (1967) Gig. Sanit., 2, 26.
Scarabelli, L., Giannoni, P., Malfatto, C., Bolognesi, C. & Cesarone,
C.F. (1993) Relationship between poly(ADP-ribose) polymerase activity
and DNA damage induced by zinc dithiocarbamates in mouse and rat
liver. Mutat. Res., 302, 1-6.
Smith, J.A., Bryson, A.M., John, D.M. & Anderson, A. (1990) A study of
the effect of ziram on pregnancy of the rat. Unpublished report No.
ZIR 15/24/891371 from Huntingdon Research Centre Ltd, Cambs, United
Kingdom. Submitted to WHO by UCB, Elf Autochem Agri, Brussels,
Belgium.
Smith, T.G., Buist, D.P., Crook, D., Morrow, J. & Gopinath, C. (1993)
Ziram toxicity to dogs by repeated dietary administration for 52
weeks. Unpublished report No. ZIR 10-G/920533 from Huntingdon Research
Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf
Autochem Agri, Brussels, Belgium.
Völkner, W. (1992a) Chromosome aberration assay in bone marrow cells
of the mouse with ziram technical (UCB provenience). Unpublished
report No. CCR 254812 from Cytotest Cell Research GmbH & Co. KG,
Rossdorf, Germany. Submitted to WHO by UCB, Elf Autochem Agri,
Brussels, Belgium.
Völkner, W. (1992b) Mouse germ-cell cytogenetic assay with ziram
technical (UCB provenience). Unpublished report No. CCR 254834 from
Cytotest Cell Research GmbH &Co. KG, Rossdorf, Germany. Submitted to
WHO by UCB, Elf Atocbem Agri, Brussels, Belgium.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of ziram is summarized in Table 1.
(b) Short-term toxicity
Mice
Ziram (purity 97.6%) was administered orally to C57B1 and A mice
at doses of 0 or 75 mg/kg bw twice a week (equivalent to 20 mg/kg bw
per day) for 2.5 months. A positive control group was exposed in the
same way to urethane. The presence of adenomas in the lungs and the
liver was studied after the end of the exposure at intervals of 1.5
months for six months; adenomas were found only in the lungs. In the A
mice, adenomas occurred in 100% of animals given urethane, 51% of
those given ziram, and 43% of the controls. The differences were not
statistically significant. The first adenomas were seen after three
months. In C57B1 mice, adenomas occurred in 26% of mice given
urethane, 7.4% of those given ziram, and none of the controls. The
results were statistically significant according to Student's t test
( t = 2.08; p = 0.05) but not according to the chi-squared test
(chi-squared = 2.18; p = 0.15) (Khicenko & Chernev, 1968; Annex 1,
reference 15).
In a range-finding study, groups of five male and five female
Crl.CD(ICR)BR mice were fed ziram (purity, 89.5%) at concentrations of
0, 3000, 4000, or 5000 ppm, equal to 0, 370, 510, or 740 mg/kg bw per
day for males and 0, 420, 660, or 780 mg/kg bw per day for females,
for four weeks. The body weight, food intake, and efficiency of food
use were significantly reduced at all doses. The brain and heart
weights were reduced in all female mice, and the heart weight was also
reduced in males at 5000 ppm. There was no NOAEL (Chambers et al.,
1992a).
In another preliminary study, groups of 10 male and 10 female
Crl:CD-1 mice were fed ziram (purity, 98.7%) at concentrations of 0,
100, 300, 900, or 2700 ppm, equal to 0, 15, 44, 120, or 360 mg/kg bw
per day for males and 0, 19, 49, 130, or 410 mg/kg bw per day for
females, for 13 weeks. Clinical signs, body weight, food and water
consumption, and efficiency of food use were recorded. On completion
of treatment, all mice were sacrificed for macroscopic and microscopic
examination. Over week 1, a reduction was noted in food consumption
and use in animals of each sex and in the body weight of males and the
body-weight gain of females at 2700 ppm. Lower weight gain was also
noted during the study in males and females at 900 ppm. A lower spleen
weight was noted in animals of each sex at 900 and 2700 ppm and in
females at 300 ppm. Uterine weight was reduced at 2700 ppm. An
increased incidence of hyperkeratosis of the non-glandular epithelium
Table 1. Acute toxicity of ziram in experimental animals
Species Sex Route Purity LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Rat Male Oral 98.5 380 Liggett & Allan (1989a)
Female 270
Rat Male Inhalation (4 h) 98.5 0.08 Jackson & Hardy (1989)
Female 0.06
Rat Male Inhalation (4 h) 98.4 0.18 Blagden (1991)
Female 0.08
Rabbit NR Oral NR 400 Annex I, reference 4
Rabbit Male Dermal 98.5 > 2000 Liggett & Allen (1989b)
Female > 2000
NR, not reported
in the stomach was seen among mice given 900 or 2700 ppm. Epithelial
hyperplasia of the limiting ridge in the stomach was also seen at
2700 ppm. Contraction of the spleen and extramedullary haematopoiesis
in the spleen were observed in mice at 900 and 2700 ppm. Corpora lutea
were absent in one mouse at 900 ppm, and recent corpora lutea were
absent in two mice at 2700 ppm and in one at 900 ppm. The uteri of
some of these mice showed changes consistent with the ovarian changes,
i.e. cellular stroma and reduced luminal diameter. These changes were
not seen at 0, 100, or 300 ppm. The NOAEL was 100 ppm, equal to
15 mg/kg bw per day, on the basis of decreased spleen weight at doses
> 300 ppm (Powell et al., 1993).
Rats
Groups of 10 male and 10 female weanling rats were fed ziram
(purity unspecified) at concentrations of 0, 100, 500, 2500, or
5000 ppm (equivalent to 0, 10, 50, 250, or 500 mg/kg bw per day) for
28 days. Growth retardation was marked in animals at 2500 and
5000 ppm; a slight retardation was also seen in those at 500 ppm, but
growth was normal in those at 100 ppm. Except for slight anaemia at
2500 and 5000 ppm, the haematological findings were normal and no
significant histopathological changes were seen in any of the animals.
The NOAEL was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis
of growth retardation at doses > 500 ppm (Annex I, reference 4).
In a separate experiment, 10 male and 10 female rats were
maintained for 28 days on a diet containing 0 or 2500 ppm ziram. No
thyroid abnormalities were seen (Annex I, reference 4).
Groups of 15 male and 15 female Wistar rats were given ziram
(purity, 98%) by gavage at doses of 0, 3, 15, or 100 mg/kg bw per day
for four weeks. Food consumption, efficiency of food use, and body
weights were reduced significantly in males at the highest dose and in
females at 15 and 100 mg/kg bw per day. End-points of haematology and
clinical chemistry did not show clear dose-dependent changes, except
for an increase in aspartate transaminase and alkaline phosphatase
activities and blood urea nitrogen values at the highest dose. The
liver weight was increased in males at this dose, and degenerative
changes in the liver and kidneys were seen in animals of each sex.
Degenerative liver changes were also seen in males and females at
15 mg/kg bw per day. The NOAEL was 3 mg/kg bw per day, on the basis of
decreased body weight, food consumption, and food use and degenerative
liver changes at doses > 15 mg/kg bw per day (Dickhaus & Heisler,
1980).
In a range-finding study, groups of five male and five female
Crl:CD(SD)BR rats were fed ziram (purity, 98.5%) at concentrations of
0, 500, 1000, or 2000 ppm (equal to 0, 46, 90 or 170 mg/kg bw per day
for males and 0, 46, 85, or 170 mg/kg bw per day for females) for four
weeks. The body weight, food intake, and efficiency of food use were
significantly reduced in animals of each sex at all doses. In males,
the absolute weights of the liver, pituitary, and testes were reduced
at all doses. The absolute weights of the brain in animals of each sex
and of the uterus were also reduced at the highest dose. There was no
NOAEL (Chambers et al., 1992b).
In another range-finding study, groups of 10 male and 10 female
Crl:CD(SD)BR rats were fed ziram (purity, 98.7%) at concentrations of
0, 100, 300, or 1000 ppm (equal to 0, 7.4, 21, or 68 mg/kg bw per day
for males and 0, 8.8, 24, or 77 mg/kg bw per day bw for females)
for 13 weeks. The body-weight gain, food intake, and food use were
dose-dependently reduced at 300 and 1000 ppm. Brain weight and spleen
weight were increased and a higher incidence of hair loss was noted at
300 and 1000 ppm. Epithelial hyperplasia of the nonglandular stomach
was seen at 1000 ppm. The NOAEL was 100 ppm, equal to 7.4 mg/kg bw per
day, on the basis of decreased body-weight gain, food intake, and food
use and increased brain and spleen weights and hair loss at doses
> 300 ppm (Powell et al., 1992).
Rabbits
Ziram (purity 98.5%) was applied to the intact skin of groups
of five male and five female New Zealand white rabbits, weighing
2.2-2.6 kg, daily for 21 consecutive days at doses of 0, 100, 300, or
1000 mg/kg bw per day. The test substance was moistened with distilled
water and maintained on the backs of the rabbits for 6 h each day,
after which the dressings were removed and the treated skin washed
with tap-water at 30-40°C and gently blotted dry. No dermal reaction
to the treatment was observed at any dose. Significant losses in body
weight or low body-weight gain and reduced food consumption were
recorded for female rabbits receiving 1000 mg/kg bw. The number of
lymphocytes was reduced in both males and females at 1000 mg/kg bw,
and alanine and aspartate transaminase activities were increased at
300 and 1000 mg/kg bw. At the highest dose, significantly increased
levels of bilirubin were found in females and of cholesterol in
animals of each sex. The NOAEL was 300 mg/kg bw per day, on the basis
of decreased body weight, body-weight gain, food consumption, and
number of lymphocytes and increased bilirubin and cholesterol levels
at 1000 mg/kg bw per day (Edwards et al., 1989).
Dogs
In a range-finding study, single male and female beagle dogs were
fed ziram (purity, 89.5%) at concentrations of 1000, 2000, or 5000 ppm
(equal to 38, 78, or 260 mg/kg bw per day for males and 28, 91 or
270 mg/kg bw per day for females) for four weeks; there was no control
group. Both dogs at 5000 ppm and the female at 2000 ppm were killed
for humane reasons due to loss of body weight and deteriorated
condition. Convulsive episodes of up to 5-min duration were seen in
females at 2000 ppm. Body-weight loss was seen in both animals at
5000 ppm and in the female at 1000 ppm. Food intake and efficiency of
food use were reduced in females at all doses and in males at
5000 ppm. Increased liver weight in comparison with historical
controls in the laboratory was seen in males at all doses. No
histopathological examinations were performed. There was no NOAEL
(McLean et al., 1992a).
In another range-finding study, groups of four male and four
female beagle dogs were fed ziram (purity, 98.5%) in the diet at
concentrations of 0, 100, 300, or 1000 ppm (equal to 0, 4.1, 12, or
42 mg/kg bw per day for males and 0, 4.3, 13, or 41 mg/kg bw per day
for females) for 13 weeks. One of the four dogs at 1000 ppm suffered a
seizure and was killed for humane reasons, and another had tremors.
Animals at this dose showed reduced body weight and food intake;
lowered erythrocyte count, packed cell volume, and haemoglobin; and
elevated circulating reticulocyte counts, with associated slight
polychromasia, hypochromasia, and anisocytosis. Activated partial
thromboplastin time was increased in male dogs at 300 or 100 ppm.
Alkaline phosphatase activity was elevated in females at 1000 ppm, and
the cholesterol level was elevated in females at 300 and 1000 ppm. The
albumin concentration was decreased and that of globulin increased in
some dogs at all doses during week 13. The liver weight was increased
in male and female dogs at 1000 ppm and in one dog at 300 ppm. Focal
myocardial necrosis with acute inflammation and haemorrhage were seen
in the dog at 1000 ppm that was killed for humane reasons. Focal
necrosis in the liver with loss of cells and dilated sinusoids were
seen in one dog at 1000 ppm, and focal necrosis was also seen in a dog
receiving 300 ppm. Minimal amounts of pigment in Kupffer cells were
observed in two dogs at 300 ppm and in three dogs at 1000 ppm. The
NOAEL was 100 ppm, equal to 4.1 mg/kg bw per day, on the basis of
increased liver weight, focal liver necrosis, pigment in Kupffer
cells, increased activated partial thromboplastin time, and an
elevated cholesterol level at doses > 300 ppm (McLean et al., 1992b).
Groups of four male and four female beagle dogs were fed ziram
(purity, 98.5%) in the diet to give concentrations of 0, 50, 180, or
700/500 ppm (equal to 0, 1.6, 6.6, or 17 mg/kg bw per day for males
and 0, 1.9, 6.7, or 21 mg/kg bw per day for females) for one year. The
highest dose was reduced from 700 to 500 ppm in week 12 due to
treatment-related deaths. All animals were killed and necropsied after
one year. Body-weight gain was reduced in females at 180 and 500 ppm.
Alanine transaminase, alkaline phosphatase, and, occasionally,
aspartate transaminase activities were elevated in males receiving 180
or 500 ppm; and the liver weights of males receiving 500 ppm were
increased. Reduced albumin values and raised cholesterol levels were
found for male dogs receiving 500 ppm. In the liver, some foci of
degenerated hepatocytes were seen in a female at 180 ppm and in a
number of dogs at 500 ppm, single-cell necrosis was seen in one male
dog at 500 ppm, and inflammatory-cell infiltration around the central
veins and branches of the hepatic veins was seen in a proportion of
these dogs. An increase in centrilobular fibrocytes was seen in one
male dog at 180 ppm and three males at 500 ppm. Aggregates of
pigmented Kupffer cells were observed in the livers of dogs of each
sex in a dose-related incidence, including one male and one female at
50 ppm. An increased incidence and degree of pigmented macrophages
were seen in the spleens of male dogs at 180 and 500 ppm. The NOAEL
was 50 ppm, equal to 1.6 mg/kg bw per day, on the basis of decreased
body-weight gain, degenerated hepatocytes, and increases in
centrilobular fibrocytes, spleen macrophages, alanine transaminase and
alkaline phosphatase activities, and pigmented Kupffer cells at doses
> 180 ppm (Smith et al., 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 49 or 50 male and female B6C3F1 mice were fed ziram
(purity, 89%; containing 6.5% thiram, 2% other zinc salts, and 2%
unidentified additional impurities) at concentrations of 0, 600, or
1200 ppm (equal to 0, 120, or 200 mg/kg bw per day for males and 0,
130, or 250 mg/kg bw per day for females) for 103 weeks. All animals
were observed daily for clinical signs, and body weight and food
consumption were recorded monthly. At termination, necropsies were
performed on all animals, and selected organs and tissues from most
animals were examined microscopically. The survival of treated mice
was not adversely affected. The body-weight gain of male mice at 600
and 1200 ppm and of female mice at 600 ppm was reduced, and food
consumption was reduced in both males and females at 1200 ppm. The
incidence of alveolar or bronchiolar adenomas was statistical
significantly increased in female mice (2/50 controls, 5/49 at the low
dose, 10/50 at the high dose), and the combined incidence of alveolar
and bronchiolar adenomas and carcinomas in female mice showed a
statistically significant, positive trend. The incidence in males at
1200 ppm was significantly higher than that in the controls (4/50
controls, 6/49 at the low dose, 11/50 at the high dose). Pulmonary
adenomatous hyperplasia consistent with chronic Sendai viral infection
was observed in control and treated animals. The authors concluded
that any interpretation of the increase in lung tumours is complicated
by the presence of intercurrent viral infection (National Toxicology
Program, 1983).
Groups of 50 male and 50 female Crl:CD-1(ICR)BR mice were fed
ziram (purity, 98.7%) in the diet to give concentrations of 0, 25, 75,
225, or 675 ppm (equal to 0, 3, 9, 27, or 82 mg/kg bw per day for
males and 0, 4, 11, 33, or 95 mg/kg bw per day for females) for 80
weeks. The concentrations of ziram given to the groups at 25 and
75 ppm were greater than the nominal concentration in order to
compensate for losses during storage. Statistically significant
reductions in body-weight gain and food intake were seen in males and
females receiving 225 or 675 ppm. Dose-related, statistically
significant decreases in the absolute brain weight of males receiving
225 or 675 ppm and the brain weight adjusted for final body weight
of females receiving 75, 225, or 675 ppm were noted. Macroscopic
examination of all animals that died or were killed at termination
revealed increased incidences of reduced adipose tissue in males
that died, irregular cortical scarring of the kidneys in males at
terminal sacrifice, brown kidneys in males, and roughened and white
forestomachs in females, all receiving the highest dose. An increased
incidence of centrilobular and/or generalized hepatocellular
enlargement was seen in all treated groups, and an increased incidence
of urinary bladder epithelial hyperplasia was seen in males and
females receiving 675 ppm and in males at 225 ppm. No treatment-
related neoplastic effects were seen. The NOAEL was 25 ppm, equal
to 3 mg/kg bw per day, on the basis of reduced brain weight and
hepatocellular enlargement at doses > 75 ppm (Powell et al., 1994a).
Rats
Groups of 25 male and 25 female weanling rats were fed ziram at
concentrations of 0, 25, 250, or 2500 ppm (equivalent to 0, 1.3, 13,
or 125 mg/kg bw per day) for two years. The growth rate and life span
were normal in all groups. Neurological changes were observed at
2500 ppm, but no cystic lesions were seen in the brain post mortem.
No neurological changes were seen at the lower doses. In some males,
the testes were atrophied and there was a slight indication of thyroid
hyperplasia, notably at 2500 ppm. There was no increase in tumour
incidence in the treated animals (Annex I, reference 4).
Groups of 50 Fischer 344/N rats were fed ziram (purity, 89%;
containing 6.5% thiram, 2% other zinc salts, and 2% unidentified
additional impurities) at concentrations of 0, 300, or 600 ppm (equal
to 0, 11, or 22 mg/kg bw per day for males and 0, 13, or 26 mg/kg bw
per day for females) for 103 weeks. All animals were observed daily
for clinical signs, and body weights and food consumption were
recorded monthly. At termination, necropsies were performed on all
animals, and selected organs and tissues from most animals were
examined microscopically. The survival, body weight, and food
consumption of treated rats were not adversely affected. C-Cell
carcinomas of the thyroid occurred in male rats, with a statistically
significant, positive trend ( p < 0.01); the incidence among animals
at 600 ppm was significantly greater than that in controls (0/50
controls, 2/49 at the low dose, 7/49 at the high dose). The combined
incidence of C-cell adenomas and carcinomas in males also showed a
statistically significant, positive trend (4/50 controls, 9/49 at the
low dose, 12/49 at the high dose). No significant histopathological
changes were found in the follicular cells. There was no NOAEL, since
the combined incidence of C-cell adenomas and carcinomas of the
thyroid in males showed a statistically significant, positive trend at
300 ppm, the lowest dose tested (National Toxicology Program, 1983).
In a study of toxicity, involving groups of 20 CD(SD)BR rats of
each sex, and carcinogenicity, involving groups of 50 rats of each
sex, the animals were fed diets containing ziram (purity, 98.7%)
providing concentrations of 0, 60, 180, or 540 ppm (equal to 0,
3.0/2.5, 9.1/7.7, or 27/24 mg/kg bw per day for males and 0, 3.9/3.4,
12/10, or 38/35 mg/kg bw per day for females), for 12 or 24 months.
Clinical signs, body weight, and food consumption were observed
weekly, and ophthalmoscopic, haematological, biochemical, and urinary
investigations were performed at certain intervals. At termination,
the organ weights, haematological parameters, and the results
of clinical chemical, urinary, and macroscopic and microscopic
examinations were recorded. Body-weight gain, food intake, and food
conversion ratios were reduced in a dose-dependent fashion in male and
female rats at 180 and 540 ppm. The erythrocyte count was reduced
dose-dependently in animals of each sex at 60, 180, and 540 ppm, and a
dose-dependent decrease in thromboplastin time was observed in males
at all three doses. At 180 and 540 ppm, packed cell volume and
haemoglobin were reduced in females and thromboplastin time in males.
Tri-iodothyronine, thyroxine, albumin, total protein, and calcium
levels were reduced and that of blood urea nitrogen was increased
at 180 and 540 ppm in animals of each sex. Haemangiomata in the
mesenteric lymph nodes, hypertrophy with vacuolation of the adrenals,
and C-cell hyperplasia of the thyroids were observed in males at
540 ppm. In females, cortical cystic degeneration of the adrenals was
seen at 540 ppm. Dose-dependent adipose replacement, narrowing of the
myofibres in skeletal muscle, haemosiderosis in the spleen, adipose
tissue replacement in the pancreas, prominent ultimobranchial cysts in
the thyroids, and epithelial hyperplasia and subepithelial oedema in
the nonglandular part of the stomach were observed in animals at 60,
180, or 540 ppm. There was no NOAEL, since decreased erythrocyte
counts and thromboplastin time, haemosiderosis in the spleen,
ultimobranchial cysts in the thyroids, and epithelial hyperplasia in
the stomach were seen at 60 ppm, the lowest dose tested (Powell
et al., 1994b).
(d) Reproductive toxicity
Mice
Groups of 10 CH3 or AK male mice were given ziram (purity
unspecified) by gavage at concentrations of 0 or 0.2 mg% (equivalent
to 0 or 0.02 mg/kg bw per day) for 21 days before mating with
untreated females of the same strain. The males were then killed for
histopathological examination. Some of the female mice were killed on
day 17 of gestation in order to examine the numbers of corpora lutea,
nidation places, and dead and live embryos; the other females were
kept alive to breed. Newborn mice were immediately sacrificed and
stained with alizarin for examination of skeletal abnormalities. Ziram
increased the incidence of sterility: only 20% of the C3H and 80% of
the AK females, but all of the controls, were fertilized. The mean
number of embryos was reduced from 11.1 in controls to 7.5 in the CH3
mice and from 11.2 in controls to 9.1 in the AK mice. The calculated
embryonic mortality was 4.5% in the CH3 strain and 6.3% in the AK
strain (Abbota's formula). When the numbers of corpora lutea and of
live and resorbed embryos were included, embryonic mortality rates of
27% for C3H mice and 32% AK mice were found (method of dominant
lethals). Analysis of the testes showed atrophy of the seminiferous
tubules and impaired spermatogenesis. Examination of meiotic
chromosomes in diakinesis showed the presence of tetravalent and
univalent chromosomes and frequent pyknosis, demonstrating the
clastogenic effect of ziram on future gametes. The offspring of both
CH3 and AK strains had anomalies of the spine, cyphosis, scoliosis,
sternum ossification failure, branching ribs (some of which were
incompletely ossified), assymetry of the cranium, microcephalus, and
eviscerations. The bone dimensions of the CH3 mice were greater than
those of the controls. There was no NOAEL for male fertility, because
of decreased fertility, atrophy of seminiferous tubules, and impaired
spermatogenesis, or for developmental toxicity, because anomalies of
the spine, cyphosis, scoliosis, and sternum ossification failure were
seen in the offspring at 0.02 mg/kg bw per day, the lowest dose tested
(Cilievici et al., 1983). In the absence of important information on
the design of the study, this report was not considered in establishing
the ADI.
Groups of six Swiss albino male mice, aged 12-14 weeks, received
ziram (purity unspecified), dissolved in 5% dimethyl sulfoxide,
intraperitoneally as single doses of 0, 50, or 100 mg/kg bw or
repeated doses of 25 mg/kg bw per day for five days. The mice were
killed one month after the treatment. The epididymides were isolated,
minced in 4 ml NaCl, and dispersed by slow aspiration. The tissue
particles were filtered out, and smears were made on clean, dry slides
for microscopy. A minimum of 2000 sperm was scored per animal, and the
abnormalities were classified. The frequency of abnormal sperm was
1.6% in the controls, 5.6% in animals at 50 mg/kg bw, 8.2% in those
at 100 mg/kg bw, and 8.4% following repeated doses of 25 mg/kg bw
per day. The abnormalities included sperm with acrosomes bent
upwards, acrosomes bent downwards, and without acrosomes. Many head
abnormalities, including double heads, banana heads, amorphous heads,
microcephaly, and macrocephaly, were found, and a high frequency of
coiled sperm was seen. The abnormalities observed were considered to
be due either to changes in the genes responsible for spermatogenesis
or to changes in differentiation during gene expression involving
post-transcriptional stages and thereafter in the translation of the
genetic message. There was no NOAEL, as sperm abnormalities were seen
at 25 mg/kg bw per day, the lowest dose tested (Hemavathi & Rahiman,
1993).
Rats
In a two-generation study of reproductive and developmental
neurotoxicity, groups of 30 male and 30 female Crl:CD BR Sprague-
Dawley rats were fed ziram at concentrations of 0, 72, 210, or 540
ppm, equal to 0, 3, 10, or 25 mg/kg bw per day for males and 0, 5, 13,
or 32 mg/kg bw per day for females. The parental animals received the
diets from about six weeks of age for the F0 generation and on day 22
postnatally for the F1 generation, for at least 70 days before mating
and throughout all subsequent phases of the study until termination of
the generation. All animals were observed twice daily for appearance
and behaviour. Body weights and food consumption were recorded at
appropriate intervals. All females were allowed to deliver and rear
their pups to weaning on lactation day 21. Offspring from the pairing
of the F0 animals (30 pups of each sex per group) were selected to
constitute the F1 generation. Thirty F2 pups of each sex per group
were selected for testing of developmental landmarks and behaviour,
neuropathological examination, and brain weight measurement. Surplus
F1 pups were killed and necropsied on postnatal day 28, and surplus
F2 pups were killed and necropsied on postnatal day 22. The F0 and
F1 parental animals and selected F2 pups that were not allocated for
measurements underwent detailed gross necropsy; and the weights of
the brain, kidneys, liver, pituitary gland, ovaries, or testes and
epididymides (F0 and F1 only) were recorded. Designated tissues from
the controls and from F0 and F1 parental animals at 540 ppm and F2
pups selected for neuropathological evaluation were evaluated for
histopathological changes.
Reproductive parameters (fertility, mating, and days between
pairing and coitus, gestation, and parturition) in the F0 and F1
generations were not adversely affected by concentrations of 72, 210,
or 540 ppm ziram. All F0 and F1 parental animals survived to the
scheduled necropsies, and no adverse clinical signs attributable to
treatment were observed. The mean body weights and body-weight gains
of F0 males at 540 ppm were reduced early in the treatment period,
and the mean body weights and body-weight gains of F1 males at
540 ppm were generally reduced. The mean body weights and body-weight
gains of F0 and F1 females at 540 ppm were generally reduced before
breeding, during gestation and lactation, and after weaning. No adverse
effects were seen on the body weights or body-weight gains of animals
in either generation at 72 or 210 ppm. The food consumption of F0 and
F1 males at 540 ppm was generally reduced throughout each generation.
The food consumption of F0 and F1 females at 540 ppm was reduced
before breeding, during gestation and lactation, and after weaning of
the F0 and F2 generations. No adverse effects on food consumption
were observed in males or females given ziram at concentrations of 72
and 210 ppm in either generation. No treatment-related, macroscopic
internal changes were found in treated F0 or F1 animals, and no
adverse effects on organ weights were observed at any dose. No
microscopic lesions attributable to treatment were observed in
tissues from animals at 540 ppm on histopathological examination.
Microscopic examination of gross lesions seen at the scheduled
necropsies of animals at 540 ppm did not reveal any adverse effects.
The mean body weights of F1 and F2 pups in the litters of
animals at 540 ppm were slightly reduced (usually statistically
significantly) during lactation and throughout the remainder of the
study until postnatal day 70 (selected F2 pups). The F1 and F2 pup
sex ratios, live litter sizes, number of dead pups on lactation day 0,
viability indices, general physical condition, and brain weight
(selected F2 pups) were not adversely affected by parental treatment
at any concentration. The findings at necropsy of F1 and F2 pups
that died or were killed at the scheduled postnatal necropsies did not
suggest any correlation with parental treatment. Various indicators
of physical and functional development and behavioural responses
in the selected F2 pups were comparable to those in controls.
Neuropathological examinations on postnatal days 11 and 70 showed no
gross or microscopic treatment-related lesions in the F2 pups. The
NOAEL for maternal toxicity was 210 ppm, equal to 10 mg/kg bw per day,
on the basis of reduced food consumption and body-weight gain; that
for neonatal toxicity was 210 ppm, equal to 10 mg/kg bw per day, on
the basis of reduced body weight; and that for reproductive and
developmental neurotoxicity was 540 ppm, equal to 25 mg/kg bw per day
(Nemec, 1996).
(e) Developmental toxicity
Rats
In a preliminary study, groups of 10 pregnant Crl:CD(SD)BR rats
were given ziram (purity, 98.9%) by gavage at doses of 0, 5, 20, or
80 mg/kg bw per day on days 6-15 of gestation. Dams were observed for
clinical symptoms, body weight, and food and water consumption, and
were killed on day 20 and examined for congenital abnormalities and
macroscopic pathological changes in organs. The ovaries and uteri
were examined to determine the number of corpora lutea, the number
and distribution of live young, and the number of early and late
embryo-fetal deaths and fetal abnormalities. Live young were examined
externally and weighed; no further examinations were done. Dams showed
body-weight loss up to day 8, decreased food intake, and increased
water intake at doses of 5, 20, and 80 mg/kg bw per day. Hair loss
was observed in dams at 20 and 80 mg/kg bw per day. Salivation and
increased postimplantation losses were seen in dams at 80 mg/kg bw per
day. Reduced litter and fetal weights were seen at 20 and 80 mg/kg bw
per day. The dose range was considered to be too high for the main
study (Smith et al., 1990).
Groups of 25 pregnant Crl:CD(SD)BR rats were given ziram (purity,
98.9%) by gavage at doses of 0, 1, 4, 16, or 64 mg/kg bw per day on
days 6-15 of gestation and were then observed for clinical symptoms,
body weight, and food and water consumption. On day 20, the animals
were killed and examined for congenital abnormalities and macroscopic
pathological changes in organs. The ovaries and uteri were examined to
determine the number of corpora lutea, the number and distribution of
live young, and the number of early and late embryo-fetal deaths and
fetal abnormalities. Live young were examined externally and weighed.
One-half of the fetuses were subsequently examined for visceral and
the other half for skeletal abnormalities. Dams showed dose-related
body-weight loss up to day 8, followed by retarded weight gain
throughout treatment; decreased food consumption and increased water
consumption and salivation were seen at 16 and 64 mg/kg bw per day.
Pre- and postimplantation losses were unaffected by treatment. At
4 mg/kg bw per day, only marginally increased water consumption was
observed during treatment. Significantly lower litter and fetal
weights were seen at 64 mg/kg bw per day; litter size and the
incidences of skeletal and visceral anomalies were unaffected by
treatment. The NOAELs were 4 mg/kg bw per day for maternal toxicity,
on the basis of decreased body weight and food intake, increased water
intake, and salivation at doses > 16 mg/kg bw per day, and 16 mg/kg
bw per day for developmental toxicity, on the basis of decreased
litter and fetal weight at 64 mg/kg bw per day. No teratogenicity was
seen (Smith et al., 1990).
Rabbits
In a preliminary study, groups of five pregnant New Zealand white
rabbits were given ziram (purity, 98.0%) by gavage at doses of 0, 5,
10, or 20 mg/kg bw per day on days 7-19 of gestation. All animals
were observed for clinical symptoms, body weight, and food consumption.
On day 28, the animals were killed, dissected, and examined
macroscopically for abnormalities. The ovaries and uteri were removed
and examined to determine the gravid uterine weight, number of corpora
lutea, and the distribution of live young and of early and late
embryo-fetal deaths. Live young were examined externally for
abnormalities and weighed. Two animals at 20 mg/kg bw per day died,
and one aborted. Treatment had no effect on the clinical condition or
findings at necropsy for animals that survived to term. Marked
reductions in weight gain and food intake, particularly at the start
of treatment, were observed in animals at 20 mg/kg bw per day. Slight,
temporary reductions in weight gain and food intake were also observed
at 10 mg/kg bw per day. At 20 mg/kg bw per day, postimplantation
losses were increased and litter size, litter weight, and fetal weight
were reduced. Because maternal toxicity was observed at 20 mg/kg bw
per day, the high dose for the main study was < 20 mg/kg bw per day
(Barker, 1985).
Groups of 16 pregnant New Zealand white rabbits were given ziram
(purity, 98.1%) by gavage at doses of 0, 3, 7.5, or 15 mg/kg bw per
day on days 7-19 of gestation. All animals were observed for clinical
symptoms, body weight, and food consumption. On day 28, the animals
were killed, dissected, and examined macroscopically for abnormalities.
The ovaries and uteri were removed and examined to determine the gravid
uterine weight, the number of corpora lutea, and the distribution of
live young and of early and late embryo-fetal deaths. Live fetuses were
weighed and examined for abnormalities before alizarin staining.
Significant reductions in maternal body-weight gain and food intake
were observed from the start of treatment at 15 mg/kg bw per day;
slight reductions in weight gain and food intake were also observed
during the early part of the treatment period in animals at 7.5 mg/kg
bw per day. At 15 mg/kg bw per day, postimplantation loss was slightly
increased and litter size, litter weight, fetal weight, and the
crown-rump length were reduced. There was no teratogenic effect. The
NOAEL for maternal and developmental toxicity was 7.5 mg/kg bw per day,
on the basis of decreased body-weight gain and food intake, increased
postimplantation loss, and reduced litter size, litter weight, fetal
weight, and crown-rump length at 15 mg/kg bw per day (Barker, 1986).
(f) Genotoxicity
The results of studies on the genotoxicity of ziram are
summarized in Table 2. Ziram is mutagenic in bacteria. It induced
chromosomal aberrations in some, but not all, studies with cultured
mammalian cells and did not induce unscheduled DNA synthesis in
hepatocytes. In vivo, ziram induced DNA single-strand breaks in the
livers of rats but not mice. Chromosomal aberrations were not
induced in mice in vivo in bone-marrow cells or spermatogonia, and
micronuclei were not induced in bone-marrow cells or peripheral
erythrocytes. Studies for clastogenicity have not been conducted in
rats in vivo. Consequently, the Meeting was unable to reach a
conclusion about the genotoxicity of ziram.
Table 2. Results tests for the genotoxicity of ziram
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium < 100 µg/plate NR Positivea Hedenstedt
TA98, TA100, TA1535, at 10, 50, et al. (1979)
TA1537, TA1538 100 µg/
plate
Reverse mutation S. typhimurium < 50 µg/plate 98.5 Positiveb Jones et al.
TA98, TA100, TA1535, at 15, 50 (1990)
TA1537, TA1538 µg/plate
Reverse mutation S. typhimurium < 90 µg/plate 98.5 Positivea Crebelli et al.
TA98, TA100, TA102, TA1537, at 7.5-90 (1992)
TA1950, TA1975, TA1535 µg/plate
Reverse mutation S. typhimurium 15-90 µg/plate NR Positiveb Franekic et al.
TA98, TA100, TA102, (1994)
TA104, TA1535, TA1538
Chromosomal Saccharomyces 5-50 µg/ml NR Negative Franekic et al.
malsegregation cerevisiae (1994)
Cell division Shallot root-tip 0.5-50 µg/ml NR Positive Franekic et al.
cells (1994)
Chromosomal aberration Chinese hamster < 1.5 µg/ml 98.5 Negative Brooker &
ovary cells Akhurst (1989)
Chromosomal aberration Chinese hamster < 1.75 µg/ml 86.2 Positivea Gulati et al.
ovary cells (1989)
Table 2. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Chromosomal aberration Chinese hamster < 10 µg/ml 98.5 Positiveb Mosesso et al.
ovary cells (1994)
Chromosomal aberration Chinese hamster < 2.15 µg/ml 98.5 Positiveb Mosesso et al.
epithelial liver cells (1994)
Unscheduled DNA synthesis Rat hepatocytes < 100 µg/ml 98.5 Negative Proudlock (1989)
In vivo
Chromosomal aberration Male and female NMRI 40, 120, 400 mg/kg bw 98.4 Negative Völkner (1992a)
mice, bone-marrow cells
Chromosomal aberration Male NMRI mice, germ cells 20, 67, 200 mg/kg bw 98.4 Negative Völkner (1992b)
Micronucleus formation Male and female Crl:CD-1 3.8, 11, 34, 101 mg/kg bw 98.8 Negative Proudlock &
mice, erythrocytes Taylor (1992)
Micronucleus formation Male and female B6C3F1 2.5, 5.0, 10, 20 mg/kg bw 98.5 Negative Crebelli et al.
mice, bone-marrow cells (1992)
Single-strand DNA breaks Male Swiss mice, 13, 25, 50, 100 mg/kg bw NR Negative Scarabelli et al.
liver cells (1993)
Single-strand DNA breaks Male Wistar rats, 13, 25, 50, 100 mg/kg bw NR Positive Scarabelli et al.
liver cells (1993)
NR, Not reported
a In the presence or absence of metabolic activation
b In the presence of metabolic activation
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Rabbits
The irritancy of ziram to the skin was tested in New Zealand
white rabbits. About 24 h before application of the test substance,
hair was removed from the dorsal lumbar region. Ziram (purity, 98.5%)
was applied at a dose of 0.5 g under a 2.5-cm2 gauze pad moistened
with 0.5 ml distilled water to one skin site on each of six animals,
and each treatment site was occluded with an elastic adhesive dressing
for 4 h. The animals were not restrained during the exposure and were
returned to their cages. At the end of treatment, the semiocclusive
dressing and gauze pads were removed and the treatment sites were
washed with water to remove any residual test substance. The treated
skin was examined for erythema and oedema on days 1, 2, 3, and 4. None
of the animals showed a response to treatment (Liggett & McRae,
1990a).
The irritancy of ziram to the eye was tested in New Zealand white
rabbits. The eyes of the animals were examined before instillation of
80 mg ziram (purity, 89.5%) in 0.1 ml into the lower everted lid of
one eye. Ocular lesions were graded and scored 1 and 24 h after
instillation. As the first rabbit had a severe ocular reaction
(irridal inflammation and opacity), the study was terminated on humane
grounds (Liggett & McRae, 1990b).
Guinea-pigs
The skin sensitizing potential of ziram (purity, 99.0%) was
examined in 30 adult female Dunkin-Hartley guinea-pigs. After
induction by epicutaneous applications of the test substance (25% w/w
in corn oil) and challenge with 10, 5, or 1% (w/w) in corn oil, six
guinea-pigs showed a positive skin response (grade 2 or more) to the
10% concentration. Five of these animals also reacted positively to
the 5% dose and three to the 1% concentration. No positive reactions
were observed in the control animals. Thus, a sensitization rate of
30% was obtained. The author considered that ziram has moderate
sensitizing properties (Daamen, 1988).
(ii) Neurotoxicity
Rats
The neurotoxicity of repeated doses of ziram was studied in
groups of 10 Crl:CD(SD)BR rats fed diets containing ziram (purity,
97.9%) providing concentrations of 0, 72, 210, or 540 ppm, equal to 0,
5, 14, or 34 mg/kg bw per day for males and 0, 6, 16, or 40 mg/kg bw
per day for females, for 91 days. Five animals of each sex per group
were allocated for evaluation of neurotoxic esterase and five for
evaluation of neuropathological changes. Viability, clinical signs,
body weight, and food consumption were recorded for all animals.
The results of a functional observational battery (home cage,
handling, open field, sensorimotor, neuromuscular, and physiological
observations) and of tests for total and ambulatory motor activity
were recorded for all animals during week 3, 7, and 12 of exposure.
The body weights and food consumption of male and female rats at
540 ppm were reduced, but no treatment-related effects indicative of
neurotoxicity were apparent. Brain neurotoxic esterase activity was
47% lower than that in controls in males and 38% lower in females at
540 ppm. The NOAEL for brain neurotoxic esterase inhibition was
210 ppm, equal to 14 mg/kg bw per day (Nemec, 1993).
The neurotoxicity of a single dose of ziram was studied in groups
of 12-16 Crl:CD(SD)BR rats given single doses of 0, 15, 300, or
600 mg/kg bw ziram (purity, 97.8%). Viability, clinical signs, and
body weight were recorded for all animals. The results of a functional
observational battery (home cage, handling, open field, sensorimotor,
neuromuscular, and physiological observations) and tests for total and
ambulatory motor activity were recorded for all animals before and 4 h
after treatment and on days 7 and 14. Five controls and five animals
at 600 mg/kg bw were selected for evaluation of neuropathological
changes. Four males and seven females at 600 mg/kg bw and one female
at 300 mg/kg bw died. At these doses, body weight was reduced, and
several treatment-related clinical signs (e.g. cyanosis, hypothermia,
enophthalmus, unkempt appearance, gait alterations, hypoactivity,
ptosis, and abdomal respiration) were observed. Remarkable differences
from the controls were seen in the functional observational battery
for animals at 300 and 600 mg/kg bw. Most of the responses were
observed 4 h after treatment (e.g. altered posture, palpebra closure,
and faecal consistency) or daily during the first week after dosing
(e.g. lacrimation, salivation, changes in fur appearance, altered
palpebral closure, changes in respiratory rate, and altered gait).
Ambulatory and total motor activity counts were reduced in males and
females at 300 and 600 mg/kg bw. No treatment-related microscopic
lesions were observed in the central and peripheral nervous tissues
examined. The NOAEL for neurotoxicity was 15 mg/kg bw, on the basis of
mortality, reduced body weight, ambulatory, and total motor activity,
and clinical signs at doses > 300 mg/kg bw (Lamb, 1994).
3. Observations in humans
The ability of ziram to induce chromosomal aberrations in
peripheral leukocytes was studied in four men and five women who had
been exposed for three to five years to ziram at a concentration of
2-4 mg/m3 air. The control group consisted of three women and one
man. The exposed group had 5.9% cells with aberrations and the
controls, 0.75% (Pilinskaya, 1970; Annex I, reference 15).
Comments
In experiments with 14C-labelled ziram in rats, elimination was
essentially complete within 48 h. Excretion occurred mainly in expired
air, urine, and faeces. Less than 2% of the administered dose remained
in the tissues. The biotransformation of ziram has not been studied
in rodents. In goats, it is metabolized at least in part via a
single-carbon pathway, which results in extensive radiolabelling of
natural products.
The primary effects of short- and long-term treatment with ziram
in mice, rats, and dogs were on the liver, thyroid gland, and testis.
The hepatic effects were increased liver weight, degeneration, and
focal-cell necrosis. Effects in the thyroid were C-cell hyperplasia
and carcinomas, and that on the testes was sterility.
Ziram had moderate acute oral toxicity in rats and rabbits (LD50
= 200-400 mg/kg bw). WHO has classified ziram as 'slightly hazardous'
(WHO, 1996).
In a four-week study of toxicity in mice given dietary
concentrations of 0, 3000, 4000, or 5000 ppm, an NOAEL was not
identified. Reductions in body weight, food intake, efficiency of food
use, and brain and heart weight occurred at all doses.
In a 13-week study of toxicity in mice given dietary
concentrations of 0, 100, 300, 900, or 2700 ppm, the NOAEL was
100 ppm, equal to 15 mg/kg bw per day, on the basis of lowered spleen
weight at higher doses. At 900 and 2700 ppm, the number of corpora
lutea was reduced, which was consistent with cellular changes in the
uterus.
In two four-week studies of toxicity in rats either given diets
providing doses of 0, 100, 500, 2500, or 5000 ppm or treated by gavage
with 0, 3, 15 or 100 mg/kg bw per day, the NOAEL was 3 mg/kg bw, on
the basis of degenerative liver changes. At 100 mg/kg bw, degenerative
changes in the kidneys and reductions in body weight, food intake,
efficiency of food use, and absolute weights of the liver, pituitary,
testes, brain, and uterus were seen.
In a 13-week study of toxicity in which rats received dietary
levels of 0, 100, 300, or 1000 ppm, the NOAEL was 100 ppm, equal to
7.4 mg/kg bw per day, on the basis of reduced body-weight gain, food
intake, and food use and increased brain and spleen weights at higher
doses.
In a four-week study of toxicity in dogs given diets providing
doses of 0, 1000, 2000, or 5000 ppm, an NOAEL was not identified.
Increased liver weight occurred at all doses. At 2000 ppm, convulsive
episodes were observed.
In a 13-week study of toxicity in dogs given diets providing 0,
100, 300, or 1000 ppm, the NOAEL was 100 ppm, equal to 4.1 mg/kg bw
per day, on the basis of increased liver weight, focal liver necrosis,
pigment in Kupffer cells, activated partial thromboplastin time, and
elevated cholesterol level at higher doses.
In a one-year study of toxicity in which dogs were fed diets
providing doses of 0, 50, 180, or 500 ppm, the NOAEL was 50 ppm, equal
to 1.6 mg/kg bw per day, on the basis of reductions in body-weight
gain, degeneration of hepatocytes, and increased activity of alanine
and aspartate aminotransferases and alkaline phosphatase at doses
> 185 ppm. At 500 ppm, single liver-cell necrosis was observed, and
the liver weight and cholesterol values were increased; albumin values
were reduced. Inflammatory cell infiltration around the hepatic vein
and its branches and aggregates of pigmented Kupffer cells were
observed in the liver.
Two long-term studies of toxicity and carcinogenicity in mice
have been reported. One was considered inadequate for evaluating
the carcinogenicity of ziram. In the other, mice were given diets
providing doses of 0, 25, 75, 220, or 680 ppm for 80 weeks. The NOAEL
was 25 ppm, equal to 3 mg/kg bw per day, on the basis of reduced brain
weight at doses > 75 ppm. There was no evidence of carcinogenicity.
In a two-year study of toxicity and carcinogenicity in rats at
dietary concentrations of 0, 25, 250, or 2500 ppm, the NOAEL was
250 ppm, equivalent to 12 mg/kg bw per day, on the basis of testicular
atrophy and thyroid hyperplasia at 2500 ppm. There was no evidence of
carcinogenicity.
In a two-year study of toxicity and carcinogenicity in Fischer
344 rats with dietary concentrations of 0, 300, or 600 ppm, an NOAEL
was not identified, since the combined incidence of C-cell adenoma
and carcinoma of the thyroid in males showed a positive trend. This
finding was considered to represent an extension of the known toxicity
of the compound to the thyroid, to which the rat is particularly
sensitive, and not to indicate carcinogenic potential for humans.
In a study of toxicity and carcinogenicity in CD rats treated
with 0, 60, 180, or 540 ppm in the diet for 12-24 months, an NOAEL was
not identified because dose-related changes in organ weights and
histopathological and haematological changes were observed at 60 ppm,
equal to 2.5 mg/kg per day. Other effects included reduced body
weight, erythrocyte count, and triiodothyronine and thyroxine
activity. Cysts in the thyroids, epithelial hyperplasia, hypertrophy
with vacuolation, cortical cystic degeneration of the adrenals, and
C-cell hyperplasia of the thyroid were also demonstrated. The tumour
incidence was not increased.
In a study of sperm quality in mice treated intraperitoneally
with ziram at a single dose of 0, 50, or 100 mg/kg bw or repeated
doses of 25 mg/kg bw per day for five days, severe morphological
abnormalities were observed. The frequency of abnormal sperm was 1.6%
in the controls, 5.6% at 50 mg/kg bw, 8.2% at 100 mg/kg bw, and 8.4%
after repeated doses of 25 mg/kg bw.
In a two-generation study of reproductive toxicity and
developmental neurotoxicity, rats were fed ziram at concentrations of
0, 72, 210, or 540 ppm. The NOAEL for maternal toxicity was 210 ppm,
equal to 10 mg/kg bw per day, on the basis of reduced food consumption
and body-weight gain at 540 ppm. The NOAEL for neonatal toxicity was
210 ppm, equal to 10 mg/kg bw per day, on the basis of reduced body
weight gain at 540 ppm. The NOAEL for reproductive toxicity and
developmental neurotoxicity was 540 ppm, equal to 25 mg/kg bw per day.
In a study of developmental toxicity, rats were given ziram at 0,
1, 4, 16, or 64 mg/kg bw per day on days 6-15 of gestation. The NOAEL
for maternal toxicity was 4 mg/kg bw per day for maternal toxicity, on
the basis of decreased body-weight gain and food intake and increased
water intake and salivation at doses > 16 mg/kg bw per day. The
NOAEL for developmental toxicity was 16 mg/kg bw per day, on the basis
of decreased litter weight and fetal weight at 64 mg/kg bw per day. No
teratogenicity was seen.
In a study of developmental toxicity in rabbits given ziram at
doses of 0, 3, 7.5, or 15 mg/kg bw per day on days 7-19 of gestation,
the NOAEL for maternal toxicity and developmental toxicity was 7.5
mg/kg bw per day, on the basis of decreased body-weight gain and food
intake in the dams and post-implantation loss, reduced litter size,
litter weight, fetal weight, and crown-rump length at 15 mg/kg bw per
day. There was no evidence of teratogenicity.
Ziram is mutagenic in bacteria. It induced chromosomal
aberrations in some, but not all, studies with cultured mammalian
cells but did not induce unscheduled DNA synthesis in hepatocytes.
In vivo, ziram induced single-strand breaks in DNA in the livers of
rats but not mice. Chromosomal aberrations were not induced in mice
in vivo in bone-marrow cells or spermatogonia, and micronuclei were
not induced in bone-marrow cells or peripheral erythrocytes. Studies
for clastogenicity have not been conducted in rats in vivo. In an
old study of nine workers exposed for three to five years to ziram at
a concentration of 2-4 mg/m3 air, the percentage of peripheral
leukocytes with chromosomal aberrations was 5.9%; in a control group,
the percentage was 0.75%. The Meeting was unable to reach a conclusion
about the genotoxicity of ziram.
Ziram caused severe ocular irritation but no dermal irritation in
rabbits and moderate skin sensitization in guinea-pigs.
In two studies of neurotoxicity in rats treated with single doses
of 0, 15, 300, or 600 mg/kg bw or 0, 72, 210, or 540 ppm for 91 days,
behavioural effects indicative of neurotoxicity were apparent after
the single high doses but not after repeated dosing at a lower level.
The NOAEL was 210 ppm, equal to 14 mg/kg bw per day, on the basis of
reduced body weight and food consumption and inhibition of brain
neurotoxic esterase activity at 540 ppm.
An ADI of 0-0.003 mg/kg bw was established on the basis of
long-term toxicity in the rat. In this study, effects were seen at all
doses, the LOAEL being 60 ppm, equal to 2.5 mg/kg bw per day. In view
of the absence of an NOAEL, a safety factor of 1000 was used. The
NOAEL of 1.6 mg/kg bw per day observed in a long-term study of
toxicity in dogs supported this ADI, which served as the basis for the
group ADI that was established for ziram alone or in combination with
ferbam.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3 mg/kg bw per day (80-week study of
toxicity and carcinogenicity)
Rat: NOAEL could not be determined: lowest effective dose,
60 ppm, equal to 2.5 mg/kg bw per day (12-24-month
study of toxicity, various effects)
100 ppm, equal to 7.4 mg/kg bw per day (13-week study
of toxicity)
250 ppm, equivalent to 12 mg/kg bw per day per day
(two-year study of toxicity and carcinogenicity)
210 ppm, equal to 10 mg/kg bw per day (maternal
toxicity in a study of reproductive toxicity)
Rabbit: 7.5 mg/kg bw per day (maternal toxicity and
embryotoxicity in a study of developmental toxicity)
Dog: 50 ppm, equal to 1.6 mg/kg bw per day (one-year study
of toxicity)
100 ppm, equal to 4.1 mg/kg bw per day (13-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw (for ferbam and ziram)
Studies that would provide information useful for continued evaluation
of the compound
1. Further studies on long-term toxicity in the rat
2. Further studies on genotoxicity in rats
3. Further studies on male reproductive toxicity
4. Further observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to ziram
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 270 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.06 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Severely irritating
Dermal, sensitization, guinea-pig Moderately sensitizing
Medium-term (1-26 weeks) Repeated oral, 13 weeks, toxicity, mouse NOAEL = 15 mg/kg bw per day, decreased spleen weight
Repeated oral, 4 weeks, toxicity, rat NOAEL = 3 mg/kg bw per day, reduced body weight and
food consumption, degenerative hepatic changes
Repeated oral, 13 weeks, toxicity, rat NOAEL = 7.4 mg per kg bw per day, reduced body weight
and food consumption, increased brain and spleen weight
Repeated oral, 13 weeks, toxicity, dog NOAEL = 4.1 mg/kg bw per day, hepatic toxicity
Repeated oral, reproductive toxicity and NOAEL = 25 mg/kg bw per day, reproductive toxicity
developmental neurotoxicity, rat and development neurotoxicity
NOAEL = 10 mg/kg bw per day, maternal and neonatal
toxicity (reduced body weight)
Repeated oral, developmental toxicity, rat NOAEL= 16 mg/kg bw per day, developmental toxicity
(reduced fetal weight)
NOAEL = 4 mg/kg bw per day, maternal toxicity
(reduced body weight)
Repeated oral, developmental toxicity, NOAEL = 7.5 mg/kg bw per day, developmental and
rabbit maternal toxicity (reduced fetal and maternal weight)
Repeated oral, neurotoxicity, rat NOAEL = 14 mg/kg bw per day, inhibition of
neuropathy target esterase activity
Long-term (> 1 year) Repeated oral, 18 months, toxicity, mouse NOAEL = 3 mg/kg bw per day, reduced brain weight and
hepatic toxicity
Repeated oral, 2 years, toxicity and No NOAEL; LOAEL = 2.5 mg/kg bw per day, haematological
carcinogenicity, rat toxicity and toxic effects on the thyroid
Repeated oral, 1 year, toxicity, dog NOAEL = 1.6 mg/kg bw per day, reduced body weight and
hepatic toxicity
References
Barker, L. (1985) Ziram: Oral (gavage) range-findings study in the
pregnant rabbit. Unpublished report No. 4588-508/1 from Hazleton
Laboratories Europe, Ltd, North Yorkshire, United Kingdom. Submitted
to WHO by UCB Chemicals, Brussels, Belgium.
Barker, L. (1986) Ziram: Oral (gavage) teratology study in the rabbit.
Unpublished report No. 4913-508/2 from Hazleton Laboratories Europe,
Ltd, North Yorkshire, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Blagden, S.M. (1991) Ziram technical: Acute inhalation toxicity study.
Four hour exposure (nose only) in the rat. Unpublished report
No. 378/2 from Safepharm Laboratories Ltd, Derby, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Bodden, R.M. (1993) Ziram: Nature of the residue in lactating goats.
Unpublished report No. HLA 6225-101 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Brooker, P.C. & Akhurst, L.C. (1989) Analysis of metaphase chromosomes
obtained from CHO cells cultured in vitro and treated with ziram.
Unpublished report No. 7/89675 from Huntingdon Research Centre Ltd,
Cambs, United Kingdom. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Chambers, P.R., Cary, R.J., Gibson, W.A. & Gopinath, C. (1992a) Ziram.
Palatability study in mice for 4 weeks. Unpublished report No. ZIR
3/89720 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Chambers, P.R., Cary, R.J., Gibson, W.A. & Gopinath, C. (1992b) Ziram.
Palatability study in rats for 4 weeks. Unpublished report No. ZIR
2/89719 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Cheng, T. (1991a) Metabolism of ziram in rats. Unpublished report
No. HLA 6225-106 from Hazleton Laboratories America, Inc., WI, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Cheng, T. (1991b) Dermal absorption of 14C-ziram in male rats.
Unpublished report No. HLA 6225-107 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Cilievici, O., Graciun, C. & Ghidus, E. (1983) Decreased fertility,
increased dominant lethals, skeletal malformations induced in the
mouse by ziram fungicide. Rev. Roum. Morphol. Embryol. Physiol.,
29, 159-165.
Crebelli, R., Zijno, A., Conti, L., Crochi, B., Leopardi, P., Marcon,
F., Renzi, L. & Carere, A. (1992) Further in vitro and in vivo
mutagenicity assays with thiram and ziram fungicides: Bacterial
reversion assays and mouse micronucleus test. Teratog. Carcinog. Mutag.,
12, 97-112.
Daamen, P.A.M. (1988) Assessment of the skin sensitization potential
of ziram technical in the guinea-pig (split adjuvant test).
Unpublished report No. RCC NOTOX 0878/1096 from RCC Notox, C.,
's-Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Dickhaus, S. & Heisler, E. (1980) Report of a subacute toxicity study
of the substance 'Ziram' (techn.) for 4 weeks in the rat. Unpublished
report No. 2-4-132-80 from Pharmatox, Forschung und Beratung GmbH,
Hanover, Germany. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Edwards, J.A., Baldrick, P., Gibson, W.A., Crook, D., Offer, J.M. &
Gopinath, C. (1989) Twenty-one day dermal toxicity study in rabbits
with ziram. Unpublished report No. ZIR 4/89689 from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Franekic, J. Bratulic, N., Pavlica, M. & Papes, D. (1994) Genotoxicity
of dithiocarbamates and their metabolites. Mutat. Res., 325, 65-74.
Gulati, D.K., Witt, K., Anderson, B., Zeiger, E. & Shelby, M.D. (1989)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro. III: Results with 27 chemicals.
Environ. Mol. Mutag., 13, 133-193.
Hedenstedt, A., Rannug, U., Ramel, C. & Wachtmeister, C.A. (1979)
Mutagenicity and metabolism studies on 12 thiuram and dithiocarbamate
compounds used as accelerators in the Swedish rubber industry.
Mutat. Res., 68, 313-325.
Hemavathi, E. & Rahiman, M.A. (1993) Toxicological effects of ziram,
thiram, and dithane M-45 assessed by sperm shape abnormalities in
mice. J. Toxicol. Environ. Health, 38, 393-398.
Hodge, H.C., Maynard, E.A, Downs, W.L., Coye, R.D. & Steadman, L.T.
(1956) Chronic oral toxicity of ferric dimethyldithiocarbamate
(ferbam) and zinc dimethyldithiocarbamate (ziram). J. Pharmacol.
Exp. Ther., 118, 174-181.
Jackson, G.C. & Hardy, C.J. (1989) Ziram technical. Acute inhalation
toxicity in rats, 4-hour exposure. Unpublished report No. UCB,
314/89684 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Jones, E., Cook, P.G.S., Gant, R.A. & Kitshing, J. (1990) Ziram
technical: Bacterial mutation assay. Unpublished report No. ZIR
25/891914 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Khicenko, F.F. & Chernov, O.V. (1968) Experimental study of potential
vlastogenic effect of ziram. Gig. Primeniya Toksikol. Pestic. Klin.
Obravleniy (Kiev), 6, 770-776.
Korablev, M.V. (1969) Toxicological characteristics of dithiocarbamate
and derivative employed in the national economy and medicine
(literature survey). Farmakol. Toksikol., 32, 356-362 (in Russian).
Lamb, I.C. (1994) An acute neurotoxicity study of ziram in rats.
Unpublished report No. WIL-223001 from WIL Research Laboratories,
Inc., Ashland, OH, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Lee, C.-C., Russell, J.Q., Minor, J.L, Kowalski, J.J., Sanyer, J.L.,
Kintner, L.D., Hodgson, J.R., Short, R.D., Peters, P.J., Dilley, J.V.,
Murrill, E.A., Holton, D.O. & Ellis, H.V. (1975) Toxicological
evaluation of ferric dimethyldithiocarbamate (ferbam) and dithio-
carbamate (thiram) with acute toxicity of manganese and zinc
ethylenebisdithiocarbamates (maneb and zineb) Unpublished report
No. 3612-B from National Institute of Environmental Health Sciences,
Research Triangle Park, NC, USA. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Liggett, M.P. & Allan, S.A. (1989a) Acute oral toxicity to rats of
ziram. Unpublished report No. 89690D/UCB 315/AC from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Liggett, M.P. & Allen, S.A. (1989b) Acute dermal toxicity to rabbits
with ziram. Unpublished report No. 89338D/UCB 316/AC from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Liggett, M.P. & McRae, L.A. (1990a) Skin irritation to rabbits with
ziram (technical). Unpublished report No. 90500D/UCB 317/SE from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Liggett, M.P. & McRae, L.A. (1990b) Eye irritation to rabbits with
ziram (technical). Unpublished report No. 90499D/UCB 318/SE from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
McLean, T.A., Horner, S.A., Buist, D.P. (1992a) Ziram preliminary
dietary toxicity study in beagle dogs. Unpublished report No. ZIR
1-G/89637 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
McLean, T.A., Horner, S.A., Buist, D.P., Crook, D., Read, R.M.,
Gopinath, C., Anderson, A., Dawe, I.S., Johnson, S.F. & Patel, S.
(1992b) 13-Week dietary toxicity study in beagle dogs. Unpublished
report No. ZIR 8-G/901813 from Huntingdon Research Centre Ltd, Cambs,
United Kingdom. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Mosesso, P., Turchi, G., Cinelli, S., Chiara, D.D., Fiore, M. &
Palitti, F. (1994) Clastogenic effects of the dithiocarbamate
fungicides thiram and ziram in Chinese hamster cell lines cultures
in vitro. Teratog. Carcinog. Mutag., 14, 145-155.
National Toxicology Program (1983) Carcinogenesis Bioassay of Ziram
(CAS No. 137-30-4) in F344/N Rats and B6C3F1 Mice (Feed Study) (NIH report
No. 83-1794), Washington DC, Department of Health and Human Services,
Public Health Service, National Institutes of Health.
Nemec, M.D. (1993) A subchronic (13-week) neurotoxicity study of
ziram in rats. Unpublished report No. WIL-223004 from WIL Research
Laboratories, Inc., Ashland, OH, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Nemec, M.D. (1996) A dietary two-generation reproduction and
developmental neurotoxicity study of ziram in rats. Unpublished report
No. WIL-223003 from WIL Research Laboratories, Inc., Ashland, OH, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Pilinskaya, M.A. (1970) Chromosome aberrations in the persons
contacted with ziram. Genetika, 6, 157-163 (in Russian).
Powell, L.A.J., Crook, D., Gregson, R.L., Gopinath, C., Gibson, W.A.
& Anderson, A. (1992) Preliminary toxicity to rats by dietary
administration for 13 weeks. Unpublished report No. ZIR 5/901840 from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Powell, L.A.J., Gopinath, C., Gibson, W.A. & Anderson, A. (1993) Ziram
preliminary toxicity to mice by dietary administration for 13 weeks.
Unpublished report No. ZIR 11/901841 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Powell, L.A.J., Bottomley, S.M., Crook, D., Majeed, S.K., Gopinath,
C., Gibson, W.A. & Anderson, A. (1994a) Ziram (technical). Potential
oncogenicity to mice by repeated dietary administration for 80 weeks.
Unpublished report No. ZIR 12/932311 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf Autochem
Agri, Brussels, Belgium.
Powell, L.A.J., Bottomley, S.M., Crook, D., Gregson, R.L., Offer,
J.M., Gibson, W.A. & Anderson, A. (1994b) Ziram (technical). Combined
chronic toxicity and oncogenicity of ziram (technical) administered in
the diet to rats. Unpublished report No. ZIR 9/942098 from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB,
Elf Autochem Agri, Brussels, Belgium.
Proudlock, R.J. (1989) Autoradiographic assessment of DNA repair after
in vitro exposure of rat hepatocytes to ziram. Unpublished report
No. ZIR 6/89820 from Huntingdon Research Centre Ltd, Cambs, United
Kingdom. Submitted to WHO by UCB, Elf Autochem Agri, Brussels,
Belgium.
Proudlock, R.J. & Taylor K. (1992) Ziram (technical). Peripheral
blood micronucleus test in the mouse after 89 days dietary exposure.
Unpublished report No. ZIR 27/920513 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf Autochem
Agri, Brussels, Belgium.
Robens, J.F. (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol. Appl.
Pharmacol., 15, 152-163.
Ryazanova, R.A. (1967) Gig. Sanit., 2, 26.
Scarabelli, L., Giannoni, P., Malfatto, C., Bolognesi, C. & Cesarone,
C.F. (1993) Relationship between poly(ADP-ribose) polymerase activity
and DNA damage induced by zinc dithiocarbamates in mouse and rat
liver. Mutat. Res., 302, 1-6.
Smith, J.A., Bryson, A.M., John, D.M. & Anderson, A. (1990) A study of
the effect of ziram on pregnancy of the rat. Unpublished report No.
ZIR 15/24/891371 from Huntingdon Research Centre Ltd, Cambs, United
Kingdom. Submitted to WHO by UCB, Elf Autochem Agri, Brussels,
Belgium.
Smith, T.G., Buist, D.P., Crook, D., Morrow, J. & Gopinath, C. (1993)
Ziram toxicity to dogs by repeated dietary administration for 52
weeks. Unpublished report No. ZIR 10-G/920533 from Huntingdon Research
Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf
Autochem Agri, Brussels, Belgium.
Völkner, W. (1992a) Chromosome aberration assay in bone marrow cells
of the mouse with ziram technical (UCB provenience). Unpublished
report No. CCR 254812 from Cytotest Cell Research GmbH & Co. KG,
Rossdorf, Germany. Submitted to WHO by UCB, Elf Autochem Agri,
Brussels, Belgium.
Völkner, W. (1992b) Mouse germ-cell cytogenetic assay with ziram
technical (UCB provenience). Unpublished report No. CCR 254834 from
Cytotest Cell Research GmbH &Co. KG, Rossdorf, Germany. Submitted to
WHO by UCB, Elf Atocbem Agri, Brussels, Belgium.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
 2. Toxicological studies
(a) Acute toxicity
The acute toxicity of ziram is summarized in Table 1.
(b) Short-term toxicity
Mice
Ziram (purity 97.6%) was administered orally to C57B1 and A mice
at doses of 0 or 75 mg/kg bw twice a week (equivalent to 20 mg/kg bw
per day) for 2.5 months. A positive control group was exposed in the
same way to urethane. The presence of adenomas in the lungs and the
liver was studied after the end of the exposure at intervals of 1.5
months for six months; adenomas were found only in the lungs. In the A
mice, adenomas occurred in 100% of animals given urethane, 51% of
those given ziram, and 43% of the controls. The differences were not
statistically significant. The first adenomas were seen after three
months. In C57B1 mice, adenomas occurred in 26% of mice given
urethane, 7.4% of those given ziram, and none of the controls. The
results were statistically significant according to Student's t test
( t = 2.08; p = 0.05) but not according to the chi-squared test
(chi-squared = 2.18; p = 0.15) (Khicenko & Chernev, 1968; Annex 1,
reference 15).
In a range-finding study, groups of five male and five female
Crl.CD(ICR)BR mice were fed ziram (purity, 89.5%) at concentrations of
0, 3000, 4000, or 5000 ppm, equal to 0, 370, 510, or 740 mg/kg bw per
day for males and 0, 420, 660, or 780 mg/kg bw per day for females,
for four weeks. The body weight, food intake, and efficiency of food
use were significantly reduced at all doses. The brain and heart
weights were reduced in all female mice, and the heart weight was also
reduced in males at 5000 ppm. There was no NOAEL (Chambers et al.,
1992a).
In another preliminary study, groups of 10 male and 10 female
Crl:CD-1 mice were fed ziram (purity, 98.7%) at concentrations of 0,
100, 300, 900, or 2700 ppm, equal to 0, 15, 44, 120, or 360 mg/kg bw
per day for males and 0, 19, 49, 130, or 410 mg/kg bw per day for
females, for 13 weeks. Clinical signs, body weight, food and water
consumption, and efficiency of food use were recorded. On completion
of treatment, all mice were sacrificed for macroscopic and microscopic
examination. Over week 1, a reduction was noted in food consumption
and use in animals of each sex and in the body weight of males and the
body-weight gain of females at 2700 ppm. Lower weight gain was also
noted during the study in males and females at 900 ppm. A lower spleen
weight was noted in animals of each sex at 900 and 2700 ppm and in
females at 300 ppm. Uterine weight was reduced at 2700 ppm. An
increased incidence of hyperkeratosis of the non-glandular epithelium
Table 1. Acute toxicity of ziram in experimental animals
Species Sex Route Purity LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Rat Male Oral 98.5 380 Liggett & Allan (1989a)
Female 270
Rat Male Inhalation (4 h) 98.5 0.08 Jackson & Hardy (1989)
Female 0.06
Rat Male Inhalation (4 h) 98.4 0.18 Blagden (1991)
Female 0.08
Rabbit NR Oral NR 400 Annex I, reference 4
Rabbit Male Dermal 98.5 > 2000 Liggett & Allen (1989b)
Female > 2000
NR, not reported
in the stomach was seen among mice given 900 or 2700 ppm. Epithelial
hyperplasia of the limiting ridge in the stomach was also seen at
2700 ppm. Contraction of the spleen and extramedullary haematopoiesis
in the spleen were observed in mice at 900 and 2700 ppm. Corpora lutea
were absent in one mouse at 900 ppm, and recent corpora lutea were
absent in two mice at 2700 ppm and in one at 900 ppm. The uteri of
some of these mice showed changes consistent with the ovarian changes,
i.e. cellular stroma and reduced luminal diameter. These changes were
not seen at 0, 100, or 300 ppm. The NOAEL was 100 ppm, equal to
15 mg/kg bw per day, on the basis of decreased spleen weight at doses
> 300 ppm (Powell et al., 1993).
Rats
Groups of 10 male and 10 female weanling rats were fed ziram
(purity unspecified) at concentrations of 0, 100, 500, 2500, or
5000 ppm (equivalent to 0, 10, 50, 250, or 500 mg/kg bw per day) for
28 days. Growth retardation was marked in animals at 2500 and
5000 ppm; a slight retardation was also seen in those at 500 ppm, but
growth was normal in those at 100 ppm. Except for slight anaemia at
2500 and 5000 ppm, the haematological findings were normal and no
significant histopathological changes were seen in any of the animals.
The NOAEL was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis
of growth retardation at doses > 500 ppm (Annex I, reference 4).
In a separate experiment, 10 male and 10 female rats were
maintained for 28 days on a diet containing 0 or 2500 ppm ziram. No
thyroid abnormalities were seen (Annex I, reference 4).
Groups of 15 male and 15 female Wistar rats were given ziram
(purity, 98%) by gavage at doses of 0, 3, 15, or 100 mg/kg bw per day
for four weeks. Food consumption, efficiency of food use, and body
weights were reduced significantly in males at the highest dose and in
females at 15 and 100 mg/kg bw per day. End-points of haematology and
clinical chemistry did not show clear dose-dependent changes, except
for an increase in aspartate transaminase and alkaline phosphatase
activities and blood urea nitrogen values at the highest dose. The
liver weight was increased in males at this dose, and degenerative
changes in the liver and kidneys were seen in animals of each sex.
Degenerative liver changes were also seen in males and females at
15 mg/kg bw per day. The NOAEL was 3 mg/kg bw per day, on the basis of
decreased body weight, food consumption, and food use and degenerative
liver changes at doses > 15 mg/kg bw per day (Dickhaus & Heisler,
1980).
In a range-finding study, groups of five male and five female
Crl:CD(SD)BR rats were fed ziram (purity, 98.5%) at concentrations of
0, 500, 1000, or 2000 ppm (equal to 0, 46, 90 or 170 mg/kg bw per day
for males and 0, 46, 85, or 170 mg/kg bw per day for females) for four
weeks. The body weight, food intake, and efficiency of food use were
significantly reduced in animals of each sex at all doses. In males,
the absolute weights of the liver, pituitary, and testes were reduced
at all doses. The absolute weights of the brain in animals of each sex
and of the uterus were also reduced at the highest dose. There was no
NOAEL (Chambers et al., 1992b).
In another range-finding study, groups of 10 male and 10 female
Crl:CD(SD)BR rats were fed ziram (purity, 98.7%) at concentrations of
0, 100, 300, or 1000 ppm (equal to 0, 7.4, 21, or 68 mg/kg bw per day
for males and 0, 8.8, 24, or 77 mg/kg bw per day bw for females)
for 13 weeks. The body-weight gain, food intake, and food use were
dose-dependently reduced at 300 and 1000 ppm. Brain weight and spleen
weight were increased and a higher incidence of hair loss was noted at
300 and 1000 ppm. Epithelial hyperplasia of the nonglandular stomach
was seen at 1000 ppm. The NOAEL was 100 ppm, equal to 7.4 mg/kg bw per
day, on the basis of decreased body-weight gain, food intake, and food
use and increased brain and spleen weights and hair loss at doses
> 300 ppm (Powell et al., 1992).
Rabbits
Ziram (purity 98.5%) was applied to the intact skin of groups
of five male and five female New Zealand white rabbits, weighing
2.2-2.6 kg, daily for 21 consecutive days at doses of 0, 100, 300, or
1000 mg/kg bw per day. The test substance was moistened with distilled
water and maintained on the backs of the rabbits for 6 h each day,
after which the dressings were removed and the treated skin washed
with tap-water at 30-40°C and gently blotted dry. No dermal reaction
to the treatment was observed at any dose. Significant losses in body
weight or low body-weight gain and reduced food consumption were
recorded for female rabbits receiving 1000 mg/kg bw. The number of
lymphocytes was reduced in both males and females at 1000 mg/kg bw,
and alanine and aspartate transaminase activities were increased at
300 and 1000 mg/kg bw. At the highest dose, significantly increased
levels of bilirubin were found in females and of cholesterol in
animals of each sex. The NOAEL was 300 mg/kg bw per day, on the basis
of decreased body weight, body-weight gain, food consumption, and
number of lymphocytes and increased bilirubin and cholesterol levels
at 1000 mg/kg bw per day (Edwards et al., 1989).
Dogs
In a range-finding study, single male and female beagle dogs were
fed ziram (purity, 89.5%) at concentrations of 1000, 2000, or 5000 ppm
(equal to 38, 78, or 260 mg/kg bw per day for males and 28, 91 or
270 mg/kg bw per day for females) for four weeks; there was no control
group. Both dogs at 5000 ppm and the female at 2000 ppm were killed
for humane reasons due to loss of body weight and deteriorated
condition. Convulsive episodes of up to 5-min duration were seen in
females at 2000 ppm. Body-weight loss was seen in both animals at
5000 ppm and in the female at 1000 ppm. Food intake and efficiency of
food use were reduced in females at all doses and in males at
5000 ppm. Increased liver weight in comparison with historical
controls in the laboratory was seen in males at all doses. No
histopathological examinations were performed. There was no NOAEL
(McLean et al., 1992a).
In another range-finding study, groups of four male and four
female beagle dogs were fed ziram (purity, 98.5%) in the diet at
concentrations of 0, 100, 300, or 1000 ppm (equal to 0, 4.1, 12, or
42 mg/kg bw per day for males and 0, 4.3, 13, or 41 mg/kg bw per day
for females) for 13 weeks. One of the four dogs at 1000 ppm suffered a
seizure and was killed for humane reasons, and another had tremors.
Animals at this dose showed reduced body weight and food intake;
lowered erythrocyte count, packed cell volume, and haemoglobin; and
elevated circulating reticulocyte counts, with associated slight
polychromasia, hypochromasia, and anisocytosis. Activated partial
thromboplastin time was increased in male dogs at 300 or 100 ppm.
Alkaline phosphatase activity was elevated in females at 1000 ppm, and
the cholesterol level was elevated in females at 300 and 1000 ppm. The
albumin concentration was decreased and that of globulin increased in
some dogs at all doses during week 13. The liver weight was increased
in male and female dogs at 1000 ppm and in one dog at 300 ppm. Focal
myocardial necrosis with acute inflammation and haemorrhage were seen
in the dog at 1000 ppm that was killed for humane reasons. Focal
necrosis in the liver with loss of cells and dilated sinusoids were
seen in one dog at 1000 ppm, and focal necrosis was also seen in a dog
receiving 300 ppm. Minimal amounts of pigment in Kupffer cells were
observed in two dogs at 300 ppm and in three dogs at 1000 ppm. The
NOAEL was 100 ppm, equal to 4.1 mg/kg bw per day, on the basis of
increased liver weight, focal liver necrosis, pigment in Kupffer
cells, increased activated partial thromboplastin time, and an
elevated cholesterol level at doses > 300 ppm (McLean et al., 1992b).
Groups of four male and four female beagle dogs were fed ziram
(purity, 98.5%) in the diet to give concentrations of 0, 50, 180, or
700/500 ppm (equal to 0, 1.6, 6.6, or 17 mg/kg bw per day for males
and 0, 1.9, 6.7, or 21 mg/kg bw per day for females) for one year. The
highest dose was reduced from 700 to 500 ppm in week 12 due to
treatment-related deaths. All animals were killed and necropsied after
one year. Body-weight gain was reduced in females at 180 and 500 ppm.
Alanine transaminase, alkaline phosphatase, and, occasionally,
aspartate transaminase activities were elevated in males receiving 180
or 500 ppm; and the liver weights of males receiving 500 ppm were
increased. Reduced albumin values and raised cholesterol levels were
found for male dogs receiving 500 ppm. In the liver, some foci of
degenerated hepatocytes were seen in a female at 180 ppm and in a
number of dogs at 500 ppm, single-cell necrosis was seen in one male
dog at 500 ppm, and inflammatory-cell infiltration around the central
veins and branches of the hepatic veins was seen in a proportion of
these dogs. An increase in centrilobular fibrocytes was seen in one
male dog at 180 ppm and three males at 500 ppm. Aggregates of
pigmented Kupffer cells were observed in the livers of dogs of each
sex in a dose-related incidence, including one male and one female at
50 ppm. An increased incidence and degree of pigmented macrophages
were seen in the spleens of male dogs at 180 and 500 ppm. The NOAEL
was 50 ppm, equal to 1.6 mg/kg bw per day, on the basis of decreased
body-weight gain, degenerated hepatocytes, and increases in
centrilobular fibrocytes, spleen macrophages, alanine transaminase and
alkaline phosphatase activities, and pigmented Kupffer cells at doses
> 180 ppm (Smith et al., 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 49 or 50 male and female B6C3F1 mice were fed ziram
(purity, 89%; containing 6.5% thiram, 2% other zinc salts, and 2%
unidentified additional impurities) at concentrations of 0, 600, or
1200 ppm (equal to 0, 120, or 200 mg/kg bw per day for males and 0,
130, or 250 mg/kg bw per day for females) for 103 weeks. All animals
were observed daily for clinical signs, and body weight and food
consumption were recorded monthly. At termination, necropsies were
performed on all animals, and selected organs and tissues from most
animals were examined microscopically. The survival of treated mice
was not adversely affected. The body-weight gain of male mice at 600
and 1200 ppm and of female mice at 600 ppm was reduced, and food
consumption was reduced in both males and females at 1200 ppm. The
incidence of alveolar or bronchiolar adenomas was statistical
significantly increased in female mice (2/50 controls, 5/49 at the low
dose, 10/50 at the high dose), and the combined incidence of alveolar
and bronchiolar adenomas and carcinomas in female mice showed a
statistically significant, positive trend. The incidence in males at
1200 ppm was significantly higher than that in the controls (4/50
controls, 6/49 at the low dose, 11/50 at the high dose). Pulmonary
adenomatous hyperplasia consistent with chronic Sendai viral infection
was observed in control and treated animals. The authors concluded
that any interpretation of the increase in lung tumours is complicated
by the presence of intercurrent viral infection (National Toxicology
Program, 1983).
Groups of 50 male and 50 female Crl:CD-1(ICR)BR mice were fed
ziram (purity, 98.7%) in the diet to give concentrations of 0, 25, 75,
225, or 675 ppm (equal to 0, 3, 9, 27, or 82 mg/kg bw per day for
males and 0, 4, 11, 33, or 95 mg/kg bw per day for females) for 80
weeks. The concentrations of ziram given to the groups at 25 and
75 ppm were greater than the nominal concentration in order to
compensate for losses during storage. Statistically significant
reductions in body-weight gain and food intake were seen in males and
females receiving 225 or 675 ppm. Dose-related, statistically
significant decreases in the absolute brain weight of males receiving
225 or 675 ppm and the brain weight adjusted for final body weight
of females receiving 75, 225, or 675 ppm were noted. Macroscopic
examination of all animals that died or were killed at termination
revealed increased incidences of reduced adipose tissue in males
that died, irregular cortical scarring of the kidneys in males at
terminal sacrifice, brown kidneys in males, and roughened and white
forestomachs in females, all receiving the highest dose. An increased
incidence of centrilobular and/or generalized hepatocellular
enlargement was seen in all treated groups, and an increased incidence
of urinary bladder epithelial hyperplasia was seen in males and
females receiving 675 ppm and in males at 225 ppm. No treatment-
related neoplastic effects were seen. The NOAEL was 25 ppm, equal
to 3 mg/kg bw per day, on the basis of reduced brain weight and
hepatocellular enlargement at doses > 75 ppm (Powell et al., 1994a).
Rats
Groups of 25 male and 25 female weanling rats were fed ziram at
concentrations of 0, 25, 250, or 2500 ppm (equivalent to 0, 1.3, 13,
or 125 mg/kg bw per day) for two years. The growth rate and life span
were normal in all groups. Neurological changes were observed at
2500 ppm, but no cystic lesions were seen in the brain post mortem.
No neurological changes were seen at the lower doses. In some males,
the testes were atrophied and there was a slight indication of thyroid
hyperplasia, notably at 2500 ppm. There was no increase in tumour
incidence in the treated animals (Annex I, reference 4).
Groups of 50 Fischer 344/N rats were fed ziram (purity, 89%;
containing 6.5% thiram, 2% other zinc salts, and 2% unidentified
additional impurities) at concentrations of 0, 300, or 600 ppm (equal
to 0, 11, or 22 mg/kg bw per day for males and 0, 13, or 26 mg/kg bw
per day for females) for 103 weeks. All animals were observed daily
for clinical signs, and body weights and food consumption were
recorded monthly. At termination, necropsies were performed on all
animals, and selected organs and tissues from most animals were
examined microscopically. The survival, body weight, and food
consumption of treated rats were not adversely affected. C-Cell
carcinomas of the thyroid occurred in male rats, with a statistically
significant, positive trend ( p < 0.01); the incidence among animals
at 600 ppm was significantly greater than that in controls (0/50
controls, 2/49 at the low dose, 7/49 at the high dose). The combined
incidence of C-cell adenomas and carcinomas in males also showed a
statistically significant, positive trend (4/50 controls, 9/49 at the
low dose, 12/49 at the high dose). No significant histopathological
changes were found in the follicular cells. There was no NOAEL, since
the combined incidence of C-cell adenomas and carcinomas of the
thyroid in males showed a statistically significant, positive trend at
300 ppm, the lowest dose tested (National Toxicology Program, 1983).
In a study of toxicity, involving groups of 20 CD(SD)BR rats of
each sex, and carcinogenicity, involving groups of 50 rats of each
sex, the animals were fed diets containing ziram (purity, 98.7%)
providing concentrations of 0, 60, 180, or 540 ppm (equal to 0,
3.0/2.5, 9.1/7.7, or 27/24 mg/kg bw per day for males and 0, 3.9/3.4,
12/10, or 38/35 mg/kg bw per day for females), for 12 or 24 months.
Clinical signs, body weight, and food consumption were observed
weekly, and ophthalmoscopic, haematological, biochemical, and urinary
investigations were performed at certain intervals. At termination,
the organ weights, haematological parameters, and the results
of clinical chemical, urinary, and macroscopic and microscopic
examinations were recorded. Body-weight gain, food intake, and food
conversion ratios were reduced in a dose-dependent fashion in male and
female rats at 180 and 540 ppm. The erythrocyte count was reduced
dose-dependently in animals of each sex at 60, 180, and 540 ppm, and a
dose-dependent decrease in thromboplastin time was observed in males
at all three doses. At 180 and 540 ppm, packed cell volume and
haemoglobin were reduced in females and thromboplastin time in males.
Tri-iodothyronine, thyroxine, albumin, total protein, and calcium
levels were reduced and that of blood urea nitrogen was increased
at 180 and 540 ppm in animals of each sex. Haemangiomata in the
mesenteric lymph nodes, hypertrophy with vacuolation of the adrenals,
and C-cell hyperplasia of the thyroids were observed in males at
540 ppm. In females, cortical cystic degeneration of the adrenals was
seen at 540 ppm. Dose-dependent adipose replacement, narrowing of the
myofibres in skeletal muscle, haemosiderosis in the spleen, adipose
tissue replacement in the pancreas, prominent ultimobranchial cysts in
the thyroids, and epithelial hyperplasia and subepithelial oedema in
the nonglandular part of the stomach were observed in animals at 60,
180, or 540 ppm. There was no NOAEL, since decreased erythrocyte
counts and thromboplastin time, haemosiderosis in the spleen,
ultimobranchial cysts in the thyroids, and epithelial hyperplasia in
the stomach were seen at 60 ppm, the lowest dose tested (Powell
et al., 1994b).
(d) Reproductive toxicity
Mice
Groups of 10 CH3 or AK male mice were given ziram (purity
unspecified) by gavage at concentrations of 0 or 0.2 mg% (equivalent
to 0 or 0.02 mg/kg bw per day) for 21 days before mating with
untreated females of the same strain. The males were then killed for
histopathological examination. Some of the female mice were killed on
day 17 of gestation in order to examine the numbers of corpora lutea,
nidation places, and dead and live embryos; the other females were
kept alive to breed. Newborn mice were immediately sacrificed and
stained with alizarin for examination of skeletal abnormalities. Ziram
increased the incidence of sterility: only 20% of the C3H and 80% of
the AK females, but all of the controls, were fertilized. The mean
number of embryos was reduced from 11.1 in controls to 7.5 in the CH3
mice and from 11.2 in controls to 9.1 in the AK mice. The calculated
embryonic mortality was 4.5% in the CH3 strain and 6.3% in the AK
strain (Abbota's formula). When the numbers of corpora lutea and of
live and resorbed embryos were included, embryonic mortality rates of
27% for C3H mice and 32% AK mice were found (method of dominant
lethals). Analysis of the testes showed atrophy of the seminiferous
tubules and impaired spermatogenesis. Examination of meiotic
chromosomes in diakinesis showed the presence of tetravalent and
univalent chromosomes and frequent pyknosis, demonstrating the
clastogenic effect of ziram on future gametes. The offspring of both
CH3 and AK strains had anomalies of the spine, cyphosis, scoliosis,
sternum ossification failure, branching ribs (some of which were
incompletely ossified), assymetry of the cranium, microcephalus, and
eviscerations. The bone dimensions of the CH3 mice were greater than
those of the controls. There was no NOAEL for male fertility, because
of decreased fertility, atrophy of seminiferous tubules, and impaired
spermatogenesis, or for developmental toxicity, because anomalies of
the spine, cyphosis, scoliosis, and sternum ossification failure were
seen in the offspring at 0.02 mg/kg bw per day, the lowest dose tested
(Cilievici et al., 1983). In the absence of important information on
the design of the study, this report was not considered in establishing
the ADI.
Groups of six Swiss albino male mice, aged 12-14 weeks, received
ziram (purity unspecified), dissolved in 5% dimethyl sulfoxide,
intraperitoneally as single doses of 0, 50, or 100 mg/kg bw or
repeated doses of 25 mg/kg bw per day for five days. The mice were
killed one month after the treatment. The epididymides were isolated,
minced in 4 ml NaCl, and dispersed by slow aspiration. The tissue
particles were filtered out, and smears were made on clean, dry slides
for microscopy. A minimum of 2000 sperm was scored per animal, and the
abnormalities were classified. The frequency of abnormal sperm was
1.6% in the controls, 5.6% in animals at 50 mg/kg bw, 8.2% in those
at 100 mg/kg bw, and 8.4% following repeated doses of 25 mg/kg bw
per day. The abnormalities included sperm with acrosomes bent
upwards, acrosomes bent downwards, and without acrosomes. Many head
abnormalities, including double heads, banana heads, amorphous heads,
microcephaly, and macrocephaly, were found, and a high frequency of
coiled sperm was seen. The abnormalities observed were considered to
be due either to changes in the genes responsible for spermatogenesis
or to changes in differentiation during gene expression involving
post-transcriptional stages and thereafter in the translation of the
genetic message. There was no NOAEL, as sperm abnormalities were seen
at 25 mg/kg bw per day, the lowest dose tested (Hemavathi & Rahiman,
1993).
Rats
In a two-generation study of reproductive and developmental
neurotoxicity, groups of 30 male and 30 female Crl:CD BR Sprague-
Dawley rats were fed ziram at concentrations of 0, 72, 210, or 540
ppm, equal to 0, 3, 10, or 25 mg/kg bw per day for males and 0, 5, 13,
or 32 mg/kg bw per day for females. The parental animals received the
diets from about six weeks of age for the F0 generation and on day 22
postnatally for the F1 generation, for at least 70 days before mating
and throughout all subsequent phases of the study until termination of
the generation. All animals were observed twice daily for appearance
and behaviour. Body weights and food consumption were recorded at
appropriate intervals. All females were allowed to deliver and rear
their pups to weaning on lactation day 21. Offspring from the pairing
of the F0 animals (30 pups of each sex per group) were selected to
constitute the F1 generation. Thirty F2 pups of each sex per group
were selected for testing of developmental landmarks and behaviour,
neuropathological examination, and brain weight measurement. Surplus
F1 pups were killed and necropsied on postnatal day 28, and surplus
F2 pups were killed and necropsied on postnatal day 22. The F0 and
F1 parental animals and selected F2 pups that were not allocated for
measurements underwent detailed gross necropsy; and the weights of
the brain, kidneys, liver, pituitary gland, ovaries, or testes and
epididymides (F0 and F1 only) were recorded. Designated tissues from
the controls and from F0 and F1 parental animals at 540 ppm and F2
pups selected for neuropathological evaluation were evaluated for
histopathological changes.
Reproductive parameters (fertility, mating, and days between
pairing and coitus, gestation, and parturition) in the F0 and F1
generations were not adversely affected by concentrations of 72, 210,
or 540 ppm ziram. All F0 and F1 parental animals survived to the
scheduled necropsies, and no adverse clinical signs attributable to
treatment were observed. The mean body weights and body-weight gains
of F0 males at 540 ppm were reduced early in the treatment period,
and the mean body weights and body-weight gains of F1 males at
540 ppm were generally reduced. The mean body weights and body-weight
gains of F0 and F1 females at 540 ppm were generally reduced before
breeding, during gestation and lactation, and after weaning. No adverse
effects were seen on the body weights or body-weight gains of animals
in either generation at 72 or 210 ppm. The food consumption of F0 and
F1 males at 540 ppm was generally reduced throughout each generation.
The food consumption of F0 and F1 females at 540 ppm was reduced
before breeding, during gestation and lactation, and after weaning of
the F0 and F2 generations. No adverse effects on food consumption
were observed in males or females given ziram at concentrations of 72
and 210 ppm in either generation. No treatment-related, macroscopic
internal changes were found in treated F0 or F1 animals, and no
adverse effects on organ weights were observed at any dose. No
microscopic lesions attributable to treatment were observed in
tissues from animals at 540 ppm on histopathological examination.
Microscopic examination of gross lesions seen at the scheduled
necropsies of animals at 540 ppm did not reveal any adverse effects.
The mean body weights of F1 and F2 pups in the litters of
animals at 540 ppm were slightly reduced (usually statistically
significantly) during lactation and throughout the remainder of the
study until postnatal day 70 (selected F2 pups). The F1 and F2 pup
sex ratios, live litter sizes, number of dead pups on lactation day 0,
viability indices, general physical condition, and brain weight
(selected F2 pups) were not adversely affected by parental treatment
at any concentration. The findings at necropsy of F1 and F2 pups
that died or were killed at the scheduled postnatal necropsies did not
suggest any correlation with parental treatment. Various indicators
of physical and functional development and behavioural responses
in the selected F2 pups were comparable to those in controls.
Neuropathological examinations on postnatal days 11 and 70 showed no
gross or microscopic treatment-related lesions in the F2 pups. The
NOAEL for maternal toxicity was 210 ppm, equal to 10 mg/kg bw per day,
on the basis of reduced food consumption and body-weight gain; that
for neonatal toxicity was 210 ppm, equal to 10 mg/kg bw per day, on
the basis of reduced body weight; and that for reproductive and
developmental neurotoxicity was 540 ppm, equal to 25 mg/kg bw per day
(Nemec, 1996).
(e) Developmental toxicity
Rats
In a preliminary study, groups of 10 pregnant Crl:CD(SD)BR rats
were given ziram (purity, 98.9%) by gavage at doses of 0, 5, 20, or
80 mg/kg bw per day on days 6-15 of gestation. Dams were observed for
clinical symptoms, body weight, and food and water consumption, and
were killed on day 20 and examined for congenital abnormalities and
macroscopic pathological changes in organs. The ovaries and uteri
were examined to determine the number of corpora lutea, the number
and distribution of live young, and the number of early and late
embryo-fetal deaths and fetal abnormalities. Live young were examined
externally and weighed; no further examinations were done. Dams showed
body-weight loss up to day 8, decreased food intake, and increased
water intake at doses of 5, 20, and 80 mg/kg bw per day. Hair loss
was observed in dams at 20 and 80 mg/kg bw per day. Salivation and
increased postimplantation losses were seen in dams at 80 mg/kg bw per
day. Reduced litter and fetal weights were seen at 20 and 80 mg/kg bw
per day. The dose range was considered to be too high for the main
study (Smith et al., 1990).
Groups of 25 pregnant Crl:CD(SD)BR rats were given ziram (purity,
98.9%) by gavage at doses of 0, 1, 4, 16, or 64 mg/kg bw per day on
days 6-15 of gestation and were then observed for clinical symptoms,
body weight, and food and water consumption. On day 20, the animals
were killed and examined for congenital abnormalities and macroscopic
pathological changes in organs. The ovaries and uteri were examined to
determine the number of corpora lutea, the number and distribution of
live young, and the number of early and late embryo-fetal deaths and
fetal abnormalities. Live young were examined externally and weighed.
One-half of the fetuses were subsequently examined for visceral and
the other half for skeletal abnormalities. Dams showed dose-related
body-weight loss up to day 8, followed by retarded weight gain
throughout treatment; decreased food consumption and increased water
consumption and salivation were seen at 16 and 64 mg/kg bw per day.
Pre- and postimplantation losses were unaffected by treatment. At
4 mg/kg bw per day, only marginally increased water consumption was
observed during treatment. Significantly lower litter and fetal
weights were seen at 64 mg/kg bw per day; litter size and the
incidences of skeletal and visceral anomalies were unaffected by
treatment. The NOAELs were 4 mg/kg bw per day for maternal toxicity,
on the basis of decreased body weight and food intake, increased water
intake, and salivation at doses > 16 mg/kg bw per day, and 16 mg/kg
bw per day for developmental toxicity, on the basis of decreased
litter and fetal weight at 64 mg/kg bw per day. No teratogenicity was
seen (Smith et al., 1990).
Rabbits
In a preliminary study, groups of five pregnant New Zealand white
rabbits were given ziram (purity, 98.0%) by gavage at doses of 0, 5,
10, or 20 mg/kg bw per day on days 7-19 of gestation. All animals
were observed for clinical symptoms, body weight, and food consumption.
On day 28, the animals were killed, dissected, and examined
macroscopically for abnormalities. The ovaries and uteri were removed
and examined to determine the gravid uterine weight, number of corpora
lutea, and the distribution of live young and of early and late
embryo-fetal deaths. Live young were examined externally for
abnormalities and weighed. Two animals at 20 mg/kg bw per day died,
and one aborted. Treatment had no effect on the clinical condition or
findings at necropsy for animals that survived to term. Marked
reductions in weight gain and food intake, particularly at the start
of treatment, were observed in animals at 20 mg/kg bw per day. Slight,
temporary reductions in weight gain and food intake were also observed
at 10 mg/kg bw per day. At 20 mg/kg bw per day, postimplantation
losses were increased and litter size, litter weight, and fetal weight
were reduced. Because maternal toxicity was observed at 20 mg/kg bw
per day, the high dose for the main study was < 20 mg/kg bw per day
(Barker, 1985).
Groups of 16 pregnant New Zealand white rabbits were given ziram
(purity, 98.1%) by gavage at doses of 0, 3, 7.5, or 15 mg/kg bw per
day on days 7-19 of gestation. All animals were observed for clinical
symptoms, body weight, and food consumption. On day 28, the animals
were killed, dissected, and examined macroscopically for abnormalities.
The ovaries and uteri were removed and examined to determine the gravid
uterine weight, the number of corpora lutea, and the distribution of
live young and of early and late embryo-fetal deaths. Live fetuses were
weighed and examined for abnormalities before alizarin staining.
Significant reductions in maternal body-weight gain and food intake
were observed from the start of treatment at 15 mg/kg bw per day;
slight reductions in weight gain and food intake were also observed
during the early part of the treatment period in animals at 7.5 mg/kg
bw per day. At 15 mg/kg bw per day, postimplantation loss was slightly
increased and litter size, litter weight, fetal weight, and the
crown-rump length were reduced. There was no teratogenic effect. The
NOAEL for maternal and developmental toxicity was 7.5 mg/kg bw per day,
on the basis of decreased body-weight gain and food intake, increased
postimplantation loss, and reduced litter size, litter weight, fetal
weight, and crown-rump length at 15 mg/kg bw per day (Barker, 1986).
(f) Genotoxicity
The results of studies on the genotoxicity of ziram are
summarized in Table 2. Ziram is mutagenic in bacteria. It induced
chromosomal aberrations in some, but not all, studies with cultured
mammalian cells and did not induce unscheduled DNA synthesis in
hepatocytes. In vivo, ziram induced DNA single-strand breaks in the
livers of rats but not mice. Chromosomal aberrations were not
induced in mice in vivo in bone-marrow cells or spermatogonia, and
micronuclei were not induced in bone-marrow cells or peripheral
erythrocytes. Studies for clastogenicity have not been conducted in
rats in vivo. Consequently, the Meeting was unable to reach a
conclusion about the genotoxicity of ziram.
Table 2. Results tests for the genotoxicity of ziram
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium < 100 µg/plate NR Positivea Hedenstedt
TA98, TA100, TA1535, at 10, 50, et al. (1979)
TA1537, TA1538 100 µg/
plate
Reverse mutation S. typhimurium < 50 µg/plate 98.5 Positiveb Jones et al.
TA98, TA100, TA1535, at 15, 50 (1990)
TA1537, TA1538 µg/plate
Reverse mutation S. typhimurium < 90 µg/plate 98.5 Positivea Crebelli et al.
TA98, TA100, TA102, TA1537, at 7.5-90 (1992)
TA1950, TA1975, TA1535 µg/plate
Reverse mutation S. typhimurium 15-90 µg/plate NR Positiveb Franekic et al.
TA98, TA100, TA102, (1994)
TA104, TA1535, TA1538
Chromosomal Saccharomyces 5-50 µg/ml NR Negative Franekic et al.
malsegregation cerevisiae (1994)
Cell division Shallot root-tip 0.5-50 µg/ml NR Positive Franekic et al.
cells (1994)
Chromosomal aberration Chinese hamster < 1.5 µg/ml 98.5 Negative Brooker &
ovary cells Akhurst (1989)
Chromosomal aberration Chinese hamster < 1.75 µg/ml 86.2 Positivea Gulati et al.
ovary cells (1989)
Table 2. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Chromosomal aberration Chinese hamster < 10 µg/ml 98.5 Positiveb Mosesso et al.
ovary cells (1994)
Chromosomal aberration Chinese hamster < 2.15 µg/ml 98.5 Positiveb Mosesso et al.
epithelial liver cells (1994)
Unscheduled DNA synthesis Rat hepatocytes < 100 µg/ml 98.5 Negative Proudlock (1989)
In vivo
Chromosomal aberration Male and female NMRI 40, 120, 400 mg/kg bw 98.4 Negative Völkner (1992a)
mice, bone-marrow cells
Chromosomal aberration Male NMRI mice, germ cells 20, 67, 200 mg/kg bw 98.4 Negative Völkner (1992b)
Micronucleus formation Male and female Crl:CD-1 3.8, 11, 34, 101 mg/kg bw 98.8 Negative Proudlock &
mice, erythrocytes Taylor (1992)
Micronucleus formation Male and female B6C3F1 2.5, 5.0, 10, 20 mg/kg bw 98.5 Negative Crebelli et al.
mice, bone-marrow cells (1992)
Single-strand DNA breaks Male Swiss mice, 13, 25, 50, 100 mg/kg bw NR Negative Scarabelli et al.
liver cells (1993)
Single-strand DNA breaks Male Wistar rats, 13, 25, 50, 100 mg/kg bw NR Positive Scarabelli et al.
liver cells (1993)
NR, Not reported
a In the presence or absence of metabolic activation
b In the presence of metabolic activation
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Rabbits
The irritancy of ziram to the skin was tested in New Zealand
white rabbits. About 24 h before application of the test substance,
hair was removed from the dorsal lumbar region. Ziram (purity, 98.5%)
was applied at a dose of 0.5 g under a 2.5-cm2 gauze pad moistened
with 0.5 ml distilled water to one skin site on each of six animals,
and each treatment site was occluded with an elastic adhesive dressing
for 4 h. The animals were not restrained during the exposure and were
returned to their cages. At the end of treatment, the semiocclusive
dressing and gauze pads were removed and the treatment sites were
washed with water to remove any residual test substance. The treated
skin was examined for erythema and oedema on days 1, 2, 3, and 4. None
of the animals showed a response to treatment (Liggett & McRae,
1990a).
The irritancy of ziram to the eye was tested in New Zealand white
rabbits. The eyes of the animals were examined before instillation of
80 mg ziram (purity, 89.5%) in 0.1 ml into the lower everted lid of
one eye. Ocular lesions were graded and scored 1 and 24 h after
instillation. As the first rabbit had a severe ocular reaction
(irridal inflammation and opacity), the study was terminated on humane
grounds (Liggett & McRae, 1990b).
Guinea-pigs
The skin sensitizing potential of ziram (purity, 99.0%) was
examined in 30 adult female Dunkin-Hartley guinea-pigs. After
induction by epicutaneous applications of the test substance (25% w/w
in corn oil) and challenge with 10, 5, or 1% (w/w) in corn oil, six
guinea-pigs showed a positive skin response (grade 2 or more) to the
10% concentration. Five of these animals also reacted positively to
the 5% dose and three to the 1% concentration. No positive reactions
were observed in the control animals. Thus, a sensitization rate of
30% was obtained. The author considered that ziram has moderate
sensitizing properties (Daamen, 1988).
(ii) Neurotoxicity
Rats
The neurotoxicity of repeated doses of ziram was studied in
groups of 10 Crl:CD(SD)BR rats fed diets containing ziram (purity,
97.9%) providing concentrations of 0, 72, 210, or 540 ppm, equal to 0,
5, 14, or 34 mg/kg bw per day for males and 0, 6, 16, or 40 mg/kg bw
per day for females, for 91 days. Five animals of each sex per group
were allocated for evaluation of neurotoxic esterase and five for
evaluation of neuropathological changes. Viability, clinical signs,
body weight, and food consumption were recorded for all animals.
The results of a functional observational battery (home cage,
handling, open field, sensorimotor, neuromuscular, and physiological
observations) and of tests for total and ambulatory motor activity
were recorded for all animals during week 3, 7, and 12 of exposure.
The body weights and food consumption of male and female rats at
540 ppm were reduced, but no treatment-related effects indicative of
neurotoxicity were apparent. Brain neurotoxic esterase activity was
47% lower than that in controls in males and 38% lower in females at
540 ppm. The NOAEL for brain neurotoxic esterase inhibition was
210 ppm, equal to 14 mg/kg bw per day (Nemec, 1993).
The neurotoxicity of a single dose of ziram was studied in groups
of 12-16 Crl:CD(SD)BR rats given single doses of 0, 15, 300, or
600 mg/kg bw ziram (purity, 97.8%). Viability, clinical signs, and
body weight were recorded for all animals. The results of a functional
observational battery (home cage, handling, open field, sensorimotor,
neuromuscular, and physiological observations) and tests for total and
ambulatory motor activity were recorded for all animals before and 4 h
after treatment and on days 7 and 14. Five controls and five animals
at 600 mg/kg bw were selected for evaluation of neuropathological
changes. Four males and seven females at 600 mg/kg bw and one female
at 300 mg/kg bw died. At these doses, body weight was reduced, and
several treatment-related clinical signs (e.g. cyanosis, hypothermia,
enophthalmus, unkempt appearance, gait alterations, hypoactivity,
ptosis, and abdomal respiration) were observed. Remarkable differences
from the controls were seen in the functional observational battery
for animals at 300 and 600 mg/kg bw. Most of the responses were
observed 4 h after treatment (e.g. altered posture, palpebra closure,
and faecal consistency) or daily during the first week after dosing
(e.g. lacrimation, salivation, changes in fur appearance, altered
palpebral closure, changes in respiratory rate, and altered gait).
Ambulatory and total motor activity counts were reduced in males and
females at 300 and 600 mg/kg bw. No treatment-related microscopic
lesions were observed in the central and peripheral nervous tissues
examined. The NOAEL for neurotoxicity was 15 mg/kg bw, on the basis of
mortality, reduced body weight, ambulatory, and total motor activity,
and clinical signs at doses > 300 mg/kg bw (Lamb, 1994).
3. Observations in humans
The ability of ziram to induce chromosomal aberrations in
peripheral leukocytes was studied in four men and five women who had
been exposed for three to five years to ziram at a concentration of
2-4 mg/m3 air. The control group consisted of three women and one
man. The exposed group had 5.9% cells with aberrations and the
controls, 0.75% (Pilinskaya, 1970; Annex I, reference 15).
Comments
In experiments with 14C-labelled ziram in rats, elimination was
essentially complete within 48 h. Excretion occurred mainly in expired
air, urine, and faeces. Less than 2% of the administered dose remained
in the tissues. The biotransformation of ziram has not been studied
in rodents. In goats, it is metabolized at least in part via a
single-carbon pathway, which results in extensive radiolabelling of
natural products.
The primary effects of short- and long-term treatment with ziram
in mice, rats, and dogs were on the liver, thyroid gland, and testis.
The hepatic effects were increased liver weight, degeneration, and
focal-cell necrosis. Effects in the thyroid were C-cell hyperplasia
and carcinomas, and that on the testes was sterility.
Ziram had moderate acute oral toxicity in rats and rabbits (LD50
= 200-400 mg/kg bw). WHO has classified ziram as 'slightly hazardous'
(WHO, 1996).
In a four-week study of toxicity in mice given dietary
concentrations of 0, 3000, 4000, or 5000 ppm, an NOAEL was not
identified. Reductions in body weight, food intake, efficiency of food
use, and brain and heart weight occurred at all doses.
In a 13-week study of toxicity in mice given dietary
concentrations of 0, 100, 300, 900, or 2700 ppm, the NOAEL was
100 ppm, equal to 15 mg/kg bw per day, on the basis of lowered spleen
weight at higher doses. At 900 and 2700 ppm, the number of corpora
lutea was reduced, which was consistent with cellular changes in the
uterus.
In two four-week studies of toxicity in rats either given diets
providing doses of 0, 100, 500, 2500, or 5000 ppm or treated by gavage
with 0, 3, 15 or 100 mg/kg bw per day, the NOAEL was 3 mg/kg bw, on
the basis of degenerative liver changes. At 100 mg/kg bw, degenerative
changes in the kidneys and reductions in body weight, food intake,
efficiency of food use, and absolute weights of the liver, pituitary,
testes, brain, and uterus were seen.
In a 13-week study of toxicity in which rats received dietary
levels of 0, 100, 300, or 1000 ppm, the NOAEL was 100 ppm, equal to
7.4 mg/kg bw per day, on the basis of reduced body-weight gain, food
intake, and food use and increased brain and spleen weights at higher
doses.
In a four-week study of toxicity in dogs given diets providing
doses of 0, 1000, 2000, or 5000 ppm, an NOAEL was not identified.
Increased liver weight occurred at all doses. At 2000 ppm, convulsive
episodes were observed.
In a 13-week study of toxicity in dogs given diets providing 0,
100, 300, or 1000 ppm, the NOAEL was 100 ppm, equal to 4.1 mg/kg bw
per day, on the basis of increased liver weight, focal liver necrosis,
pigment in Kupffer cells, activated partial thromboplastin time, and
elevated cholesterol level at higher doses.
In a one-year study of toxicity in which dogs were fed diets
providing doses of 0, 50, 180, or 500 ppm, the NOAEL was 50 ppm, equal
to 1.6 mg/kg bw per day, on the basis of reductions in body-weight
gain, degeneration of hepatocytes, and increased activity of alanine
and aspartate aminotransferases and alkaline phosphatase at doses
> 185 ppm. At 500 ppm, single liver-cell necrosis was observed, and
the liver weight and cholesterol values were increased; albumin values
were reduced. Inflammatory cell infiltration around the hepatic vein
and its branches and aggregates of pigmented Kupffer cells were
observed in the liver.
Two long-term studies of toxicity and carcinogenicity in mice
have been reported. One was considered inadequate for evaluating
the carcinogenicity of ziram. In the other, mice were given diets
providing doses of 0, 25, 75, 220, or 680 ppm for 80 weeks. The NOAEL
was 25 ppm, equal to 3 mg/kg bw per day, on the basis of reduced brain
weight at doses > 75 ppm. There was no evidence of carcinogenicity.
In a two-year study of toxicity and carcinogenicity in rats at
dietary concentrations of 0, 25, 250, or 2500 ppm, the NOAEL was
250 ppm, equivalent to 12 mg/kg bw per day, on the basis of testicular
atrophy and thyroid hyperplasia at 2500 ppm. There was no evidence of
carcinogenicity.
In a two-year study of toxicity and carcinogenicity in Fischer
344 rats with dietary concentrations of 0, 300, or 600 ppm, an NOAEL
was not identified, since the combined incidence of C-cell adenoma
and carcinoma of the thyroid in males showed a positive trend. This
finding was considered to represent an extension of the known toxicity
of the compound to the thyroid, to which the rat is particularly
sensitive, and not to indicate carcinogenic potential for humans.
In a study of toxicity and carcinogenicity in CD rats treated
with 0, 60, 180, or 540 ppm in the diet for 12-24 months, an NOAEL was
not identified because dose-related changes in organ weights and
histopathological and haematological changes were observed at 60 ppm,
equal to 2.5 mg/kg per day. Other effects included reduced body
weight, erythrocyte count, and triiodothyronine and thyroxine
activity. Cysts in the thyroids, epithelial hyperplasia, hypertrophy
with vacuolation, cortical cystic degeneration of the adrenals, and
C-cell hyperplasia of the thyroid were also demonstrated. The tumour
incidence was not increased.
In a study of sperm quality in mice treated intraperitoneally
with ziram at a single dose of 0, 50, or 100 mg/kg bw or repeated
doses of 25 mg/kg bw per day for five days, severe morphological
abnormalities were observed. The frequency of abnormal sperm was 1.6%
in the controls, 5.6% at 50 mg/kg bw, 8.2% at 100 mg/kg bw, and 8.4%
after repeated doses of 25 mg/kg bw.
In a two-generation study of reproductive toxicity and
developmental neurotoxicity, rats were fed ziram at concentrations of
0, 72, 210, or 540 ppm. The NOAEL for maternal toxicity was 210 ppm,
equal to 10 mg/kg bw per day, on the basis of reduced food consumption
and body-weight gain at 540 ppm. The NOAEL for neonatal toxicity was
210 ppm, equal to 10 mg/kg bw per day, on the basis of reduced body
weight gain at 540 ppm. The NOAEL for reproductive toxicity and
developmental neurotoxicity was 540 ppm, equal to 25 mg/kg bw per day.
In a study of developmental toxicity, rats were given ziram at 0,
1, 4, 16, or 64 mg/kg bw per day on days 6-15 of gestation. The NOAEL
for maternal toxicity was 4 mg/kg bw per day for maternal toxicity, on
the basis of decreased body-weight gain and food intake and increased
water intake and salivation at doses > 16 mg/kg bw per day. The
NOAEL for developmental toxicity was 16 mg/kg bw per day, on the basis
of decreased litter weight and fetal weight at 64 mg/kg bw per day. No
teratogenicity was seen.
In a study of developmental toxicity in rabbits given ziram at
doses of 0, 3, 7.5, or 15 mg/kg bw per day on days 7-19 of gestation,
the NOAEL for maternal toxicity and developmental toxicity was 7.5
mg/kg bw per day, on the basis of decreased body-weight gain and food
intake in the dams and post-implantation loss, reduced litter size,
litter weight, fetal weight, and crown-rump length at 15 mg/kg bw per
day. There was no evidence of teratogenicity.
Ziram is mutagenic in bacteria. It induced chromosomal
aberrations in some, but not all, studies with cultured mammalian
cells but did not induce unscheduled DNA synthesis in hepatocytes.
In vivo, ziram induced single-strand breaks in DNA in the livers of
rats but not mice. Chromosomal aberrations were not induced in mice
in vivo in bone-marrow cells or spermatogonia, and micronuclei were
not induced in bone-marrow cells or peripheral erythrocytes. Studies
for clastogenicity have not been conducted in rats in vivo. In an
old study of nine workers exposed for three to five years to ziram at
a concentration of 2-4 mg/m3 air, the percentage of peripheral
leukocytes with chromosomal aberrations was 5.9%; in a control group,
the percentage was 0.75%. The Meeting was unable to reach a conclusion
about the genotoxicity of ziram.
Ziram caused severe ocular irritation but no dermal irritation in
rabbits and moderate skin sensitization in guinea-pigs.
In two studies of neurotoxicity in rats treated with single doses
of 0, 15, 300, or 600 mg/kg bw or 0, 72, 210, or 540 ppm for 91 days,
behavioural effects indicative of neurotoxicity were apparent after
the single high doses but not after repeated dosing at a lower level.
The NOAEL was 210 ppm, equal to 14 mg/kg bw per day, on the basis of
reduced body weight and food consumption and inhibition of brain
neurotoxic esterase activity at 540 ppm.
An ADI of 0-0.003 mg/kg bw was established on the basis of
long-term toxicity in the rat. In this study, effects were seen at all
doses, the LOAEL being 60 ppm, equal to 2.5 mg/kg bw per day. In view
of the absence of an NOAEL, a safety factor of 1000 was used. The
NOAEL of 1.6 mg/kg bw per day observed in a long-term study of
toxicity in dogs supported this ADI, which served as the basis for the
group ADI that was established for ziram alone or in combination with
ferbam.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3 mg/kg bw per day (80-week study of
toxicity and carcinogenicity)
Rat: NOAEL could not be determined: lowest effective dose,
60 ppm, equal to 2.5 mg/kg bw per day (12-24-month
study of toxicity, various effects)
100 ppm, equal to 7.4 mg/kg bw per day (13-week study
of toxicity)
250 ppm, equivalent to 12 mg/kg bw per day per day
(two-year study of toxicity and carcinogenicity)
210 ppm, equal to 10 mg/kg bw per day (maternal
toxicity in a study of reproductive toxicity)
Rabbit: 7.5 mg/kg bw per day (maternal toxicity and
embryotoxicity in a study of developmental toxicity)
Dog: 50 ppm, equal to 1.6 mg/kg bw per day (one-year study
of toxicity)
100 ppm, equal to 4.1 mg/kg bw per day (13-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw (for ferbam and ziram)
Studies that would provide information useful for continued evaluation
of the compound
1. Further studies on long-term toxicity in the rat
2. Further studies on genotoxicity in rats
3. Further studies on male reproductive toxicity
4. Further observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to ziram
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 270 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.06 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Severely irritating
Dermal, sensitization, guinea-pig Moderately sensitizing
Medium-term (1-26 weeks) Repeated oral, 13 weeks, toxicity, mouse NOAEL = 15 mg/kg bw per day, decreased spleen weight
Repeated oral, 4 weeks, toxicity, rat NOAEL = 3 mg/kg bw per day, reduced body weight and
food consumption, degenerative hepatic changes
Repeated oral, 13 weeks, toxicity, rat NOAEL = 7.4 mg per kg bw per day, reduced body weight
and food consumption, increased brain and spleen weight
Repeated oral, 13 weeks, toxicity, dog NOAEL = 4.1 mg/kg bw per day, hepatic toxicity
Repeated oral, reproductive toxicity and NOAEL = 25 mg/kg bw per day, reproductive toxicity
developmental neurotoxicity, rat and development neurotoxicity
NOAEL = 10 mg/kg bw per day, maternal and neonatal
toxicity (reduced body weight)
Repeated oral, developmental toxicity, rat NOAEL= 16 mg/kg bw per day, developmental toxicity
(reduced fetal weight)
NOAEL = 4 mg/kg bw per day, maternal toxicity
(reduced body weight)
Repeated oral, developmental toxicity, NOAEL = 7.5 mg/kg bw per day, developmental and
rabbit maternal toxicity (reduced fetal and maternal weight)
Repeated oral, neurotoxicity, rat NOAEL = 14 mg/kg bw per day, inhibition of
neuropathy target esterase activity
Long-term (> 1 year) Repeated oral, 18 months, toxicity, mouse NOAEL = 3 mg/kg bw per day, reduced brain weight and
hepatic toxicity
Repeated oral, 2 years, toxicity and No NOAEL; LOAEL = 2.5 mg/kg bw per day, haematological
carcinogenicity, rat toxicity and toxic effects on the thyroid
Repeated oral, 1 year, toxicity, dog NOAEL = 1.6 mg/kg bw per day, reduced body weight and
hepatic toxicity
References
Barker, L. (1985) Ziram: Oral (gavage) range-findings study in the
pregnant rabbit. Unpublished report No. 4588-508/1 from Hazleton
Laboratories Europe, Ltd, North Yorkshire, United Kingdom. Submitted
to WHO by UCB Chemicals, Brussels, Belgium.
Barker, L. (1986) Ziram: Oral (gavage) teratology study in the rabbit.
Unpublished report No. 4913-508/2 from Hazleton Laboratories Europe,
Ltd, North Yorkshire, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Blagden, S.M. (1991) Ziram technical: Acute inhalation toxicity study.
Four hour exposure (nose only) in the rat. Unpublished report
No. 378/2 from Safepharm Laboratories Ltd, Derby, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Bodden, R.M. (1993) Ziram: Nature of the residue in lactating goats.
Unpublished report No. HLA 6225-101 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Brooker, P.C. & Akhurst, L.C. (1989) Analysis of metaphase chromosomes
obtained from CHO cells cultured in vitro and treated with ziram.
Unpublished report No. 7/89675 from Huntingdon Research Centre Ltd,
Cambs, United Kingdom. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Chambers, P.R., Cary, R.J., Gibson, W.A. & Gopinath, C. (1992a) Ziram.
Palatability study in mice for 4 weeks. Unpublished report No. ZIR
3/89720 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Chambers, P.R., Cary, R.J., Gibson, W.A. & Gopinath, C. (1992b) Ziram.
Palatability study in rats for 4 weeks. Unpublished report No. ZIR
2/89719 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Cheng, T. (1991a) Metabolism of ziram in rats. Unpublished report
No. HLA 6225-106 from Hazleton Laboratories America, Inc., WI, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Cheng, T. (1991b) Dermal absorption of 14C-ziram in male rats.
Unpublished report No. HLA 6225-107 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Cilievici, O., Graciun, C. & Ghidus, E. (1983) Decreased fertility,
increased dominant lethals, skeletal malformations induced in the
mouse by ziram fungicide. Rev. Roum. Morphol. Embryol. Physiol.,
29, 159-165.
Crebelli, R., Zijno, A., Conti, L., Crochi, B., Leopardi, P., Marcon,
F., Renzi, L. & Carere, A. (1992) Further in vitro and in vivo
mutagenicity assays with thiram and ziram fungicides: Bacterial
reversion assays and mouse micronucleus test. Teratog. Carcinog. Mutag.,
12, 97-112.
Daamen, P.A.M. (1988) Assessment of the skin sensitization potential
of ziram technical in the guinea-pig (split adjuvant test).
Unpublished report No. RCC NOTOX 0878/1096 from RCC Notox, C.,
's-Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Dickhaus, S. & Heisler, E. (1980) Report of a subacute toxicity study
of the substance 'Ziram' (techn.) for 4 weeks in the rat. Unpublished
report No. 2-4-132-80 from Pharmatox, Forschung und Beratung GmbH,
Hanover, Germany. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Edwards, J.A., Baldrick, P., Gibson, W.A., Crook, D., Offer, J.M. &
Gopinath, C. (1989) Twenty-one day dermal toxicity study in rabbits
with ziram. Unpublished report No. ZIR 4/89689 from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Franekic, J. Bratulic, N., Pavlica, M. & Papes, D. (1994) Genotoxicity
of dithiocarbamates and their metabolites. Mutat. Res., 325, 65-74.
Gulati, D.K., Witt, K., Anderson, B., Zeiger, E. & Shelby, M.D. (1989)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro. III: Results with 27 chemicals.
Environ. Mol. Mutag., 13, 133-193.
Hedenstedt, A., Rannug, U., Ramel, C. & Wachtmeister, C.A. (1979)
Mutagenicity and metabolism studies on 12 thiuram and dithiocarbamate
compounds used as accelerators in the Swedish rubber industry.
Mutat. Res., 68, 313-325.
Hemavathi, E. & Rahiman, M.A. (1993) Toxicological effects of ziram,
thiram, and dithane M-45 assessed by sperm shape abnormalities in
mice. J. Toxicol. Environ. Health, 38, 393-398.
Hodge, H.C., Maynard, E.A, Downs, W.L., Coye, R.D. & Steadman, L.T.
(1956) Chronic oral toxicity of ferric dimethyldithiocarbamate
(ferbam) and zinc dimethyldithiocarbamate (ziram). J. Pharmacol.
Exp. Ther., 118, 174-181.
Jackson, G.C. & Hardy, C.J. (1989) Ziram technical. Acute inhalation
toxicity in rats, 4-hour exposure. Unpublished report No. UCB,
314/89684 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Jones, E., Cook, P.G.S., Gant, R.A. & Kitshing, J. (1990) Ziram
technical: Bacterial mutation assay. Unpublished report No. ZIR
25/891914 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Khicenko, F.F. & Chernov, O.V. (1968) Experimental study of potential
vlastogenic effect of ziram. Gig. Primeniya Toksikol. Pestic. Klin.
Obravleniy (Kiev), 6, 770-776.
Korablev, M.V. (1969) Toxicological characteristics of dithiocarbamate
and derivative employed in the national economy and medicine
(literature survey). Farmakol. Toksikol., 32, 356-362 (in Russian).
Lamb, I.C. (1994) An acute neurotoxicity study of ziram in rats.
Unpublished report No. WIL-223001 from WIL Research Laboratories,
Inc., Ashland, OH, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Lee, C.-C., Russell, J.Q., Minor, J.L, Kowalski, J.J., Sanyer, J.L.,
Kintner, L.D., Hodgson, J.R., Short, R.D., Peters, P.J., Dilley, J.V.,
Murrill, E.A., Holton, D.O. & Ellis, H.V. (1975) Toxicological
evaluation of ferric dimethyldithiocarbamate (ferbam) and dithio-
carbamate (thiram) with acute toxicity of manganese and zinc
ethylenebisdithiocarbamates (maneb and zineb) Unpublished report
No. 3612-B from National Institute of Environmental Health Sciences,
Research Triangle Park, NC, USA. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Liggett, M.P. & Allan, S.A. (1989a) Acute oral toxicity to rats of
ziram. Unpublished report No. 89690D/UCB 315/AC from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Liggett, M.P. & Allen, S.A. (1989b) Acute dermal toxicity to rabbits
with ziram. Unpublished report No. 89338D/UCB 316/AC from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Liggett, M.P. & McRae, L.A. (1990a) Skin irritation to rabbits with
ziram (technical). Unpublished report No. 90500D/UCB 317/SE from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Liggett, M.P. & McRae, L.A. (1990b) Eye irritation to rabbits with
ziram (technical). Unpublished report No. 90499D/UCB 318/SE from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
McLean, T.A., Horner, S.A., Buist, D.P. (1992a) Ziram preliminary
dietary toxicity study in beagle dogs. Unpublished report No. ZIR
1-G/89637 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
McLean, T.A., Horner, S.A., Buist, D.P., Crook, D., Read, R.M.,
Gopinath, C., Anderson, A., Dawe, I.S., Johnson, S.F. & Patel, S.
(1992b) 13-Week dietary toxicity study in beagle dogs. Unpublished
report No. ZIR 8-G/901813 from Huntingdon Research Centre Ltd, Cambs,
United Kingdom. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Mosesso, P., Turchi, G., Cinelli, S., Chiara, D.D., Fiore, M. &
Palitti, F. (1994) Clastogenic effects of the dithiocarbamate
fungicides thiram and ziram in Chinese hamster cell lines cultures
in vitro. Teratog. Carcinog. Mutag., 14, 145-155.
National Toxicology Program (1983) Carcinogenesis Bioassay of Ziram
(CAS No. 137-30-4) in F344/N Rats and B6C3F1 Mice (Feed Study) (NIH report
No. 83-1794), Washington DC, Department of Health and Human Services,
Public Health Service, National Institutes of Health.
Nemec, M.D. (1993) A subchronic (13-week) neurotoxicity study of
ziram in rats. Unpublished report No. WIL-223004 from WIL Research
Laboratories, Inc., Ashland, OH, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Nemec, M.D. (1996) A dietary two-generation reproduction and
developmental neurotoxicity study of ziram in rats. Unpublished report
No. WIL-223003 from WIL Research Laboratories, Inc., Ashland, OH, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Pilinskaya, M.A. (1970) Chromosome aberrations in the persons
contacted with ziram. Genetika, 6, 157-163 (in Russian).
Powell, L.A.J., Crook, D., Gregson, R.L., Gopinath, C., Gibson, W.A.
& Anderson, A. (1992) Preliminary toxicity to rats by dietary
administration for 13 weeks. Unpublished report No. ZIR 5/901840 from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Powell, L.A.J., Gopinath, C., Gibson, W.A. & Anderson, A. (1993) Ziram
preliminary toxicity to mice by dietary administration for 13 weeks.
Unpublished report No. ZIR 11/901841 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Powell, L.A.J., Bottomley, S.M., Crook, D., Majeed, S.K., Gopinath,
C., Gibson, W.A. & Anderson, A. (1994a) Ziram (technical). Potential
oncogenicity to mice by repeated dietary administration for 80 weeks.
Unpublished report No. ZIR 12/932311 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf Autochem
Agri, Brussels, Belgium.
Powell, L.A.J., Bottomley, S.M., Crook, D., Gregson, R.L., Offer,
J.M., Gibson, W.A. & Anderson, A. (1994b) Ziram (technical). Combined
chronic toxicity and oncogenicity of ziram (technical) administered in
the diet to rats. Unpublished report No. ZIR 9/942098 from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB,
Elf Autochem Agri, Brussels, Belgium.
Proudlock, R.J. (1989) Autoradiographic assessment of DNA repair after
in vitro exposure of rat hepatocytes to ziram. Unpublished report
No. ZIR 6/89820 from Huntingdon Research Centre Ltd, Cambs, United
Kingdom. Submitted to WHO by UCB, Elf Autochem Agri, Brussels,
Belgium.
Proudlock, R.J. & Taylor K. (1992) Ziram (technical). Peripheral
blood micronucleus test in the mouse after 89 days dietary exposure.
Unpublished report No. ZIR 27/920513 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf Autochem
Agri, Brussels, Belgium.
Robens, J.F. (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol. Appl.
Pharmacol., 15, 152-163.
Ryazanova, R.A. (1967) Gig. Sanit., 2, 26.
Scarabelli, L., Giannoni, P., Malfatto, C., Bolognesi, C. & Cesarone,
C.F. (1993) Relationship between poly(ADP-ribose) polymerase activity
and DNA damage induced by zinc dithiocarbamates in mouse and rat
liver. Mutat. Res., 302, 1-6.
Smith, J.A., Bryson, A.M., John, D.M. & Anderson, A. (1990) A study of
the effect of ziram on pregnancy of the rat. Unpublished report No.
ZIR 15/24/891371 from Huntingdon Research Centre Ltd, Cambs, United
Kingdom. Submitted to WHO by UCB, Elf Autochem Agri, Brussels,
Belgium.
Smith, T.G., Buist, D.P., Crook, D., Morrow, J. & Gopinath, C. (1993)
Ziram toxicity to dogs by repeated dietary administration for 52
weeks. Unpublished report No. ZIR 10-G/920533 from Huntingdon Research
Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf
Autochem Agri, Brussels, Belgium.
Völkner, W. (1992a) Chromosome aberration assay in bone marrow cells
of the mouse with ziram technical (UCB provenience). Unpublished
report No. CCR 254812 from Cytotest Cell Research GmbH & Co. KG,
Rossdorf, Germany. Submitted to WHO by UCB, Elf Autochem Agri,
Brussels, Belgium.
Völkner, W. (1992b) Mouse germ-cell cytogenetic assay with ziram
technical (UCB provenience). Unpublished report No. CCR 254834 from
Cytotest Cell Research GmbH &Co. KG, Rossdorf, Germany. Submitted to
WHO by UCB, Elf Atocbem Agri, Brussels, Belgium.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of ziram is summarized in Table 1.
(b) Short-term toxicity
Mice
Ziram (purity 97.6%) was administered orally to C57B1 and A mice
at doses of 0 or 75 mg/kg bw twice a week (equivalent to 20 mg/kg bw
per day) for 2.5 months. A positive control group was exposed in the
same way to urethane. The presence of adenomas in the lungs and the
liver was studied after the end of the exposure at intervals of 1.5
months for six months; adenomas were found only in the lungs. In the A
mice, adenomas occurred in 100% of animals given urethane, 51% of
those given ziram, and 43% of the controls. The differences were not
statistically significant. The first adenomas were seen after three
months. In C57B1 mice, adenomas occurred in 26% of mice given
urethane, 7.4% of those given ziram, and none of the controls. The
results were statistically significant according to Student's t test
( t = 2.08; p = 0.05) but not according to the chi-squared test
(chi-squared = 2.18; p = 0.15) (Khicenko & Chernev, 1968; Annex 1,
reference 15).
In a range-finding study, groups of five male and five female
Crl.CD(ICR)BR mice were fed ziram (purity, 89.5%) at concentrations of
0, 3000, 4000, or 5000 ppm, equal to 0, 370, 510, or 740 mg/kg bw per
day for males and 0, 420, 660, or 780 mg/kg bw per day for females,
for four weeks. The body weight, food intake, and efficiency of food
use were significantly reduced at all doses. The brain and heart
weights were reduced in all female mice, and the heart weight was also
reduced in males at 5000 ppm. There was no NOAEL (Chambers et al.,
1992a).
In another preliminary study, groups of 10 male and 10 female
Crl:CD-1 mice were fed ziram (purity, 98.7%) at concentrations of 0,
100, 300, 900, or 2700 ppm, equal to 0, 15, 44, 120, or 360 mg/kg bw
per day for males and 0, 19, 49, 130, or 410 mg/kg bw per day for
females, for 13 weeks. Clinical signs, body weight, food and water
consumption, and efficiency of food use were recorded. On completion
of treatment, all mice were sacrificed for macroscopic and microscopic
examination. Over week 1, a reduction was noted in food consumption
and use in animals of each sex and in the body weight of males and the
body-weight gain of females at 2700 ppm. Lower weight gain was also
noted during the study in males and females at 900 ppm. A lower spleen
weight was noted in animals of each sex at 900 and 2700 ppm and in
females at 300 ppm. Uterine weight was reduced at 2700 ppm. An
increased incidence of hyperkeratosis of the non-glandular epithelium
Table 1. Acute toxicity of ziram in experimental animals
Species Sex Route Purity LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Rat Male Oral 98.5 380 Liggett & Allan (1989a)
Female 270
Rat Male Inhalation (4 h) 98.5 0.08 Jackson & Hardy (1989)
Female 0.06
Rat Male Inhalation (4 h) 98.4 0.18 Blagden (1991)
Female 0.08
Rabbit NR Oral NR 400 Annex I, reference 4
Rabbit Male Dermal 98.5 > 2000 Liggett & Allen (1989b)
Female > 2000
NR, not reported
in the stomach was seen among mice given 900 or 2700 ppm. Epithelial
hyperplasia of the limiting ridge in the stomach was also seen at
2700 ppm. Contraction of the spleen and extramedullary haematopoiesis
in the spleen were observed in mice at 900 and 2700 ppm. Corpora lutea
were absent in one mouse at 900 ppm, and recent corpora lutea were
absent in two mice at 2700 ppm and in one at 900 ppm. The uteri of
some of these mice showed changes consistent with the ovarian changes,
i.e. cellular stroma and reduced luminal diameter. These changes were
not seen at 0, 100, or 300 ppm. The NOAEL was 100 ppm, equal to
15 mg/kg bw per day, on the basis of decreased spleen weight at doses
> 300 ppm (Powell et al., 1993).
Rats
Groups of 10 male and 10 female weanling rats were fed ziram
(purity unspecified) at concentrations of 0, 100, 500, 2500, or
5000 ppm (equivalent to 0, 10, 50, 250, or 500 mg/kg bw per day) for
28 days. Growth retardation was marked in animals at 2500 and
5000 ppm; a slight retardation was also seen in those at 500 ppm, but
growth was normal in those at 100 ppm. Except for slight anaemia at
2500 and 5000 ppm, the haematological findings were normal and no
significant histopathological changes were seen in any of the animals.
The NOAEL was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis
of growth retardation at doses > 500 ppm (Annex I, reference 4).
In a separate experiment, 10 male and 10 female rats were
maintained for 28 days on a diet containing 0 or 2500 ppm ziram. No
thyroid abnormalities were seen (Annex I, reference 4).
Groups of 15 male and 15 female Wistar rats were given ziram
(purity, 98%) by gavage at doses of 0, 3, 15, or 100 mg/kg bw per day
for four weeks. Food consumption, efficiency of food use, and body
weights were reduced significantly in males at the highest dose and in
females at 15 and 100 mg/kg bw per day. End-points of haematology and
clinical chemistry did not show clear dose-dependent changes, except
for an increase in aspartate transaminase and alkaline phosphatase
activities and blood urea nitrogen values at the highest dose. The
liver weight was increased in males at this dose, and degenerative
changes in the liver and kidneys were seen in animals of each sex.
Degenerative liver changes were also seen in males and females at
15 mg/kg bw per day. The NOAEL was 3 mg/kg bw per day, on the basis of
decreased body weight, food consumption, and food use and degenerative
liver changes at doses > 15 mg/kg bw per day (Dickhaus & Heisler,
1980).
In a range-finding study, groups of five male and five female
Crl:CD(SD)BR rats were fed ziram (purity, 98.5%) at concentrations of
0, 500, 1000, or 2000 ppm (equal to 0, 46, 90 or 170 mg/kg bw per day
for males and 0, 46, 85, or 170 mg/kg bw per day for females) for four
weeks. The body weight, food intake, and efficiency of food use were
significantly reduced in animals of each sex at all doses. In males,
the absolute weights of the liver, pituitary, and testes were reduced
at all doses. The absolute weights of the brain in animals of each sex
and of the uterus were also reduced at the highest dose. There was no
NOAEL (Chambers et al., 1992b).
In another range-finding study, groups of 10 male and 10 female
Crl:CD(SD)BR rats were fed ziram (purity, 98.7%) at concentrations of
0, 100, 300, or 1000 ppm (equal to 0, 7.4, 21, or 68 mg/kg bw per day
for males and 0, 8.8, 24, or 77 mg/kg bw per day bw for females)
for 13 weeks. The body-weight gain, food intake, and food use were
dose-dependently reduced at 300 and 1000 ppm. Brain weight and spleen
weight were increased and a higher incidence of hair loss was noted at
300 and 1000 ppm. Epithelial hyperplasia of the nonglandular stomach
was seen at 1000 ppm. The NOAEL was 100 ppm, equal to 7.4 mg/kg bw per
day, on the basis of decreased body-weight gain, food intake, and food
use and increased brain and spleen weights and hair loss at doses
> 300 ppm (Powell et al., 1992).
Rabbits
Ziram (purity 98.5%) was applied to the intact skin of groups
of five male and five female New Zealand white rabbits, weighing
2.2-2.6 kg, daily for 21 consecutive days at doses of 0, 100, 300, or
1000 mg/kg bw per day. The test substance was moistened with distilled
water and maintained on the backs of the rabbits for 6 h each day,
after which the dressings were removed and the treated skin washed
with tap-water at 30-40°C and gently blotted dry. No dermal reaction
to the treatment was observed at any dose. Significant losses in body
weight or low body-weight gain and reduced food consumption were
recorded for female rabbits receiving 1000 mg/kg bw. The number of
lymphocytes was reduced in both males and females at 1000 mg/kg bw,
and alanine and aspartate transaminase activities were increased at
300 and 1000 mg/kg bw. At the highest dose, significantly increased
levels of bilirubin were found in females and of cholesterol in
animals of each sex. The NOAEL was 300 mg/kg bw per day, on the basis
of decreased body weight, body-weight gain, food consumption, and
number of lymphocytes and increased bilirubin and cholesterol levels
at 1000 mg/kg bw per day (Edwards et al., 1989).
Dogs
In a range-finding study, single male and female beagle dogs were
fed ziram (purity, 89.5%) at concentrations of 1000, 2000, or 5000 ppm
(equal to 38, 78, or 260 mg/kg bw per day for males and 28, 91 or
270 mg/kg bw per day for females) for four weeks; there was no control
group. Both dogs at 5000 ppm and the female at 2000 ppm were killed
for humane reasons due to loss of body weight and deteriorated
condition. Convulsive episodes of up to 5-min duration were seen in
females at 2000 ppm. Body-weight loss was seen in both animals at
5000 ppm and in the female at 1000 ppm. Food intake and efficiency of
food use were reduced in females at all doses and in males at
5000 ppm. Increased liver weight in comparison with historical
controls in the laboratory was seen in males at all doses. No
histopathological examinations were performed. There was no NOAEL
(McLean et al., 1992a).
In another range-finding study, groups of four male and four
female beagle dogs were fed ziram (purity, 98.5%) in the diet at
concentrations of 0, 100, 300, or 1000 ppm (equal to 0, 4.1, 12, or
42 mg/kg bw per day for males and 0, 4.3, 13, or 41 mg/kg bw per day
for females) for 13 weeks. One of the four dogs at 1000 ppm suffered a
seizure and was killed for humane reasons, and another had tremors.
Animals at this dose showed reduced body weight and food intake;
lowered erythrocyte count, packed cell volume, and haemoglobin; and
elevated circulating reticulocyte counts, with associated slight
polychromasia, hypochromasia, and anisocytosis. Activated partial
thromboplastin time was increased in male dogs at 300 or 100 ppm.
Alkaline phosphatase activity was elevated in females at 1000 ppm, and
the cholesterol level was elevated in females at 300 and 1000 ppm. The
albumin concentration was decreased and that of globulin increased in
some dogs at all doses during week 13. The liver weight was increased
in male and female dogs at 1000 ppm and in one dog at 300 ppm. Focal
myocardial necrosis with acute inflammation and haemorrhage were seen
in the dog at 1000 ppm that was killed for humane reasons. Focal
necrosis in the liver with loss of cells and dilated sinusoids were
seen in one dog at 1000 ppm, and focal necrosis was also seen in a dog
receiving 300 ppm. Minimal amounts of pigment in Kupffer cells were
observed in two dogs at 300 ppm and in three dogs at 1000 ppm. The
NOAEL was 100 ppm, equal to 4.1 mg/kg bw per day, on the basis of
increased liver weight, focal liver necrosis, pigment in Kupffer
cells, increased activated partial thromboplastin time, and an
elevated cholesterol level at doses > 300 ppm (McLean et al., 1992b).
Groups of four male and four female beagle dogs were fed ziram
(purity, 98.5%) in the diet to give concentrations of 0, 50, 180, or
700/500 ppm (equal to 0, 1.6, 6.6, or 17 mg/kg bw per day for males
and 0, 1.9, 6.7, or 21 mg/kg bw per day for females) for one year. The
highest dose was reduced from 700 to 500 ppm in week 12 due to
treatment-related deaths. All animals were killed and necropsied after
one year. Body-weight gain was reduced in females at 180 and 500 ppm.
Alanine transaminase, alkaline phosphatase, and, occasionally,
aspartate transaminase activities were elevated in males receiving 180
or 500 ppm; and the liver weights of males receiving 500 ppm were
increased. Reduced albumin values and raised cholesterol levels were
found for male dogs receiving 500 ppm. In the liver, some foci of
degenerated hepatocytes were seen in a female at 180 ppm and in a
number of dogs at 500 ppm, single-cell necrosis was seen in one male
dog at 500 ppm, and inflammatory-cell infiltration around the central
veins and branches of the hepatic veins was seen in a proportion of
these dogs. An increase in centrilobular fibrocytes was seen in one
male dog at 180 ppm and three males at 500 ppm. Aggregates of
pigmented Kupffer cells were observed in the livers of dogs of each
sex in a dose-related incidence, including one male and one female at
50 ppm. An increased incidence and degree of pigmented macrophages
were seen in the spleens of male dogs at 180 and 500 ppm. The NOAEL
was 50 ppm, equal to 1.6 mg/kg bw per day, on the basis of decreased
body-weight gain, degenerated hepatocytes, and increases in
centrilobular fibrocytes, spleen macrophages, alanine transaminase and
alkaline phosphatase activities, and pigmented Kupffer cells at doses
> 180 ppm (Smith et al., 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 49 or 50 male and female B6C3F1 mice were fed ziram
(purity, 89%; containing 6.5% thiram, 2% other zinc salts, and 2%
unidentified additional impurities) at concentrations of 0, 600, or
1200 ppm (equal to 0, 120, or 200 mg/kg bw per day for males and 0,
130, or 250 mg/kg bw per day for females) for 103 weeks. All animals
were observed daily for clinical signs, and body weight and food
consumption were recorded monthly. At termination, necropsies were
performed on all animals, and selected organs and tissues from most
animals were examined microscopically. The survival of treated mice
was not adversely affected. The body-weight gain of male mice at 600
and 1200 ppm and of female mice at 600 ppm was reduced, and food
consumption was reduced in both males and females at 1200 ppm. The
incidence of alveolar or bronchiolar adenomas was statistical
significantly increased in female mice (2/50 controls, 5/49 at the low
dose, 10/50 at the high dose), and the combined incidence of alveolar
and bronchiolar adenomas and carcinomas in female mice showed a
statistically significant, positive trend. The incidence in males at
1200 ppm was significantly higher than that in the controls (4/50
controls, 6/49 at the low dose, 11/50 at the high dose). Pulmonary
adenomatous hyperplasia consistent with chronic Sendai viral infection
was observed in control and treated animals. The authors concluded
that any interpretation of the increase in lung tumours is complicated
by the presence of intercurrent viral infection (National Toxicology
Program, 1983).
Groups of 50 male and 50 female Crl:CD-1(ICR)BR mice were fed
ziram (purity, 98.7%) in the diet to give concentrations of 0, 25, 75,
225, or 675 ppm (equal to 0, 3, 9, 27, or 82 mg/kg bw per day for
males and 0, 4, 11, 33, or 95 mg/kg bw per day for females) for 80
weeks. The concentrations of ziram given to the groups at 25 and
75 ppm were greater than the nominal concentration in order to
compensate for losses during storage. Statistically significant
reductions in body-weight gain and food intake were seen in males and
females receiving 225 or 675 ppm. Dose-related, statistically
significant decreases in the absolute brain weight of males receiving
225 or 675 ppm and the brain weight adjusted for final body weight
of females receiving 75, 225, or 675 ppm were noted. Macroscopic
examination of all animals that died or were killed at termination
revealed increased incidences of reduced adipose tissue in males
that died, irregular cortical scarring of the kidneys in males at
terminal sacrifice, brown kidneys in males, and roughened and white
forestomachs in females, all receiving the highest dose. An increased
incidence of centrilobular and/or generalized hepatocellular
enlargement was seen in all treated groups, and an increased incidence
of urinary bladder epithelial hyperplasia was seen in males and
females receiving 675 ppm and in males at 225 ppm. No treatment-
related neoplastic effects were seen. The NOAEL was 25 ppm, equal
to 3 mg/kg bw per day, on the basis of reduced brain weight and
hepatocellular enlargement at doses > 75 ppm (Powell et al., 1994a).
Rats
Groups of 25 male and 25 female weanling rats were fed ziram at
concentrations of 0, 25, 250, or 2500 ppm (equivalent to 0, 1.3, 13,
or 125 mg/kg bw per day) for two years. The growth rate and life span
were normal in all groups. Neurological changes were observed at
2500 ppm, but no cystic lesions were seen in the brain post mortem.
No neurological changes were seen at the lower doses. In some males,
the testes were atrophied and there was a slight indication of thyroid
hyperplasia, notably at 2500 ppm. There was no increase in tumour
incidence in the treated animals (Annex I, reference 4).
Groups of 50 Fischer 344/N rats were fed ziram (purity, 89%;
containing 6.5% thiram, 2% other zinc salts, and 2% unidentified
additional impurities) at concentrations of 0, 300, or 600 ppm (equal
to 0, 11, or 22 mg/kg bw per day for males and 0, 13, or 26 mg/kg bw
per day for females) for 103 weeks. All animals were observed daily
for clinical signs, and body weights and food consumption were
recorded monthly. At termination, necropsies were performed on all
animals, and selected organs and tissues from most animals were
examined microscopically. The survival, body weight, and food
consumption of treated rats were not adversely affected. C-Cell
carcinomas of the thyroid occurred in male rats, with a statistically
significant, positive trend ( p < 0.01); the incidence among animals
at 600 ppm was significantly greater than that in controls (0/50
controls, 2/49 at the low dose, 7/49 at the high dose). The combined
incidence of C-cell adenomas and carcinomas in males also showed a
statistically significant, positive trend (4/50 controls, 9/49 at the
low dose, 12/49 at the high dose). No significant histopathological
changes were found in the follicular cells. There was no NOAEL, since
the combined incidence of C-cell adenomas and carcinomas of the
thyroid in males showed a statistically significant, positive trend at
300 ppm, the lowest dose tested (National Toxicology Program, 1983).
In a study of toxicity, involving groups of 20 CD(SD)BR rats of
each sex, and carcinogenicity, involving groups of 50 rats of each
sex, the animals were fed diets containing ziram (purity, 98.7%)
providing concentrations of 0, 60, 180, or 540 ppm (equal to 0,
3.0/2.5, 9.1/7.7, or 27/24 mg/kg bw per day for males and 0, 3.9/3.4,
12/10, or 38/35 mg/kg bw per day for females), for 12 or 24 months.
Clinical signs, body weight, and food consumption were observed
weekly, and ophthalmoscopic, haematological, biochemical, and urinary
investigations were performed at certain intervals. At termination,
the organ weights, haematological parameters, and the results
of clinical chemical, urinary, and macroscopic and microscopic
examinations were recorded. Body-weight gain, food intake, and food
conversion ratios were reduced in a dose-dependent fashion in male and
female rats at 180 and 540 ppm. The erythrocyte count was reduced
dose-dependently in animals of each sex at 60, 180, and 540 ppm, and a
dose-dependent decrease in thromboplastin time was observed in males
at all three doses. At 180 and 540 ppm, packed cell volume and
haemoglobin were reduced in females and thromboplastin time in males.
Tri-iodothyronine, thyroxine, albumin, total protein, and calcium
levels were reduced and that of blood urea nitrogen was increased
at 180 and 540 ppm in animals of each sex. Haemangiomata in the
mesenteric lymph nodes, hypertrophy with vacuolation of the adrenals,
and C-cell hyperplasia of the thyroids were observed in males at
540 ppm. In females, cortical cystic degeneration of the adrenals was
seen at 540 ppm. Dose-dependent adipose replacement, narrowing of the
myofibres in skeletal muscle, haemosiderosis in the spleen, adipose
tissue replacement in the pancreas, prominent ultimobranchial cysts in
the thyroids, and epithelial hyperplasia and subepithelial oedema in
the nonglandular part of the stomach were observed in animals at 60,
180, or 540 ppm. There was no NOAEL, since decreased erythrocyte
counts and thromboplastin time, haemosiderosis in the spleen,
ultimobranchial cysts in the thyroids, and epithelial hyperplasia in
the stomach were seen at 60 ppm, the lowest dose tested (Powell
et al., 1994b).
(d) Reproductive toxicity
Mice
Groups of 10 CH3 or AK male mice were given ziram (purity
unspecified) by gavage at concentrations of 0 or 0.2 mg% (equivalent
to 0 or 0.02 mg/kg bw per day) for 21 days before mating with
untreated females of the same strain. The males were then killed for
histopathological examination. Some of the female mice were killed on
day 17 of gestation in order to examine the numbers of corpora lutea,
nidation places, and dead and live embryos; the other females were
kept alive to breed. Newborn mice were immediately sacrificed and
stained with alizarin for examination of skeletal abnormalities. Ziram
increased the incidence of sterility: only 20% of the C3H and 80% of
the AK females, but all of the controls, were fertilized. The mean
number of embryos was reduced from 11.1 in controls to 7.5 in the CH3
mice and from 11.2 in controls to 9.1 in the AK mice. The calculated
embryonic mortality was 4.5% in the CH3 strain and 6.3% in the AK
strain (Abbota's formula). When the numbers of corpora lutea and of
live and resorbed embryos were included, embryonic mortality rates of
27% for C3H mice and 32% AK mice were found (method of dominant
lethals). Analysis of the testes showed atrophy of the seminiferous
tubules and impaired spermatogenesis. Examination of meiotic
chromosomes in diakinesis showed the presence of tetravalent and
univalent chromosomes and frequent pyknosis, demonstrating the
clastogenic effect of ziram on future gametes. The offspring of both
CH3 and AK strains had anomalies of the spine, cyphosis, scoliosis,
sternum ossification failure, branching ribs (some of which were
incompletely ossified), assymetry of the cranium, microcephalus, and
eviscerations. The bone dimensions of the CH3 mice were greater than
those of the controls. There was no NOAEL for male fertility, because
of decreased fertility, atrophy of seminiferous tubules, and impaired
spermatogenesis, or for developmental toxicity, because anomalies of
the spine, cyphosis, scoliosis, and sternum ossification failure were
seen in the offspring at 0.02 mg/kg bw per day, the lowest dose tested
(Cilievici et al., 1983). In the absence of important information on
the design of the study, this report was not considered in establishing
the ADI.
Groups of six Swiss albino male mice, aged 12-14 weeks, received
ziram (purity unspecified), dissolved in 5% dimethyl sulfoxide,
intraperitoneally as single doses of 0, 50, or 100 mg/kg bw or
repeated doses of 25 mg/kg bw per day for five days. The mice were
killed one month after the treatment. The epididymides were isolated,
minced in 4 ml NaCl, and dispersed by slow aspiration. The tissue
particles were filtered out, and smears were made on clean, dry slides
for microscopy. A minimum of 2000 sperm was scored per animal, and the
abnormalities were classified. The frequency of abnormal sperm was
1.6% in the controls, 5.6% in animals at 50 mg/kg bw, 8.2% in those
at 100 mg/kg bw, and 8.4% following repeated doses of 25 mg/kg bw
per day. The abnormalities included sperm with acrosomes bent
upwards, acrosomes bent downwards, and without acrosomes. Many head
abnormalities, including double heads, banana heads, amorphous heads,
microcephaly, and macrocephaly, were found, and a high frequency of
coiled sperm was seen. The abnormalities observed were considered to
be due either to changes in the genes responsible for spermatogenesis
or to changes in differentiation during gene expression involving
post-transcriptional stages and thereafter in the translation of the
genetic message. There was no NOAEL, as sperm abnormalities were seen
at 25 mg/kg bw per day, the lowest dose tested (Hemavathi & Rahiman,
1993).
Rats
In a two-generation study of reproductive and developmental
neurotoxicity, groups of 30 male and 30 female Crl:CD BR Sprague-
Dawley rats were fed ziram at concentrations of 0, 72, 210, or 540
ppm, equal to 0, 3, 10, or 25 mg/kg bw per day for males and 0, 5, 13,
or 32 mg/kg bw per day for females. The parental animals received the
diets from about six weeks of age for the F0 generation and on day 22
postnatally for the F1 generation, for at least 70 days before mating
and throughout all subsequent phases of the study until termination of
the generation. All animals were observed twice daily for appearance
and behaviour. Body weights and food consumption were recorded at
appropriate intervals. All females were allowed to deliver and rear
their pups to weaning on lactation day 21. Offspring from the pairing
of the F0 animals (30 pups of each sex per group) were selected to
constitute the F1 generation. Thirty F2 pups of each sex per group
were selected for testing of developmental landmarks and behaviour,
neuropathological examination, and brain weight measurement. Surplus
F1 pups were killed and necropsied on postnatal day 28, and surplus
F2 pups were killed and necropsied on postnatal day 22. The F0 and
F1 parental animals and selected F2 pups that were not allocated for
measurements underwent detailed gross necropsy; and the weights of
the brain, kidneys, liver, pituitary gland, ovaries, or testes and
epididymides (F0 and F1 only) were recorded. Designated tissues from
the controls and from F0 and F1 parental animals at 540 ppm and F2
pups selected for neuropathological evaluation were evaluated for
histopathological changes.
Reproductive parameters (fertility, mating, and days between
pairing and coitus, gestation, and parturition) in the F0 and F1
generations were not adversely affected by concentrations of 72, 210,
or 540 ppm ziram. All F0 and F1 parental animals survived to the
scheduled necropsies, and no adverse clinical signs attributable to
treatment were observed. The mean body weights and body-weight gains
of F0 males at 540 ppm were reduced early in the treatment period,
and the mean body weights and body-weight gains of F1 males at
540 ppm were generally reduced. The mean body weights and body-weight
gains of F0 and F1 females at 540 ppm were generally reduced before
breeding, during gestation and lactation, and after weaning. No adverse
effects were seen on the body weights or body-weight gains of animals
in either generation at 72 or 210 ppm. The food consumption of F0 and
F1 males at 540 ppm was generally reduced throughout each generation.
The food consumption of F0 and F1 females at 540 ppm was reduced
before breeding, during gestation and lactation, and after weaning of
the F0 and F2 generations. No adverse effects on food consumption
were observed in males or females given ziram at concentrations of 72
and 210 ppm in either generation. No treatment-related, macroscopic
internal changes were found in treated F0 or F1 animals, and no
adverse effects on organ weights were observed at any dose. No
microscopic lesions attributable to treatment were observed in
tissues from animals at 540 ppm on histopathological examination.
Microscopic examination of gross lesions seen at the scheduled
necropsies of animals at 540 ppm did not reveal any adverse effects.
The mean body weights of F1 and F2 pups in the litters of
animals at 540 ppm were slightly reduced (usually statistically
significantly) during lactation and throughout the remainder of the
study until postnatal day 70 (selected F2 pups). The F1 and F2 pup
sex ratios, live litter sizes, number of dead pups on lactation day 0,
viability indices, general physical condition, and brain weight
(selected F2 pups) were not adversely affected by parental treatment
at any concentration. The findings at necropsy of F1 and F2 pups
that died or were killed at the scheduled postnatal necropsies did not
suggest any correlation with parental treatment. Various indicators
of physical and functional development and behavioural responses
in the selected F2 pups were comparable to those in controls.
Neuropathological examinations on postnatal days 11 and 70 showed no
gross or microscopic treatment-related lesions in the F2 pups. The
NOAEL for maternal toxicity was 210 ppm, equal to 10 mg/kg bw per day,
on the basis of reduced food consumption and body-weight gain; that
for neonatal toxicity was 210 ppm, equal to 10 mg/kg bw per day, on
the basis of reduced body weight; and that for reproductive and
developmental neurotoxicity was 540 ppm, equal to 25 mg/kg bw per day
(Nemec, 1996).
(e) Developmental toxicity
Rats
In a preliminary study, groups of 10 pregnant Crl:CD(SD)BR rats
were given ziram (purity, 98.9%) by gavage at doses of 0, 5, 20, or
80 mg/kg bw per day on days 6-15 of gestation. Dams were observed for
clinical symptoms, body weight, and food and water consumption, and
were killed on day 20 and examined for congenital abnormalities and
macroscopic pathological changes in organs. The ovaries and uteri
were examined to determine the number of corpora lutea, the number
and distribution of live young, and the number of early and late
embryo-fetal deaths and fetal abnormalities. Live young were examined
externally and weighed; no further examinations were done. Dams showed
body-weight loss up to day 8, decreased food intake, and increased
water intake at doses of 5, 20, and 80 mg/kg bw per day. Hair loss
was observed in dams at 20 and 80 mg/kg bw per day. Salivation and
increased postimplantation losses were seen in dams at 80 mg/kg bw per
day. Reduced litter and fetal weights were seen at 20 and 80 mg/kg bw
per day. The dose range was considered to be too high for the main
study (Smith et al., 1990).
Groups of 25 pregnant Crl:CD(SD)BR rats were given ziram (purity,
98.9%) by gavage at doses of 0, 1, 4, 16, or 64 mg/kg bw per day on
days 6-15 of gestation and were then observed for clinical symptoms,
body weight, and food and water consumption. On day 20, the animals
were killed and examined for congenital abnormalities and macroscopic
pathological changes in organs. The ovaries and uteri were examined to
determine the number of corpora lutea, the number and distribution of
live young, and the number of early and late embryo-fetal deaths and
fetal abnormalities. Live young were examined externally and weighed.
One-half of the fetuses were subsequently examined for visceral and
the other half for skeletal abnormalities. Dams showed dose-related
body-weight loss up to day 8, followed by retarded weight gain
throughout treatment; decreased food consumption and increased water
consumption and salivation were seen at 16 and 64 mg/kg bw per day.
Pre- and postimplantation losses were unaffected by treatment. At
4 mg/kg bw per day, only marginally increased water consumption was
observed during treatment. Significantly lower litter and fetal
weights were seen at 64 mg/kg bw per day; litter size and the
incidences of skeletal and visceral anomalies were unaffected by
treatment. The NOAELs were 4 mg/kg bw per day for maternal toxicity,
on the basis of decreased body weight and food intake, increased water
intake, and salivation at doses > 16 mg/kg bw per day, and 16 mg/kg
bw per day for developmental toxicity, on the basis of decreased
litter and fetal weight at 64 mg/kg bw per day. No teratogenicity was
seen (Smith et al., 1990).
Rabbits
In a preliminary study, groups of five pregnant New Zealand white
rabbits were given ziram (purity, 98.0%) by gavage at doses of 0, 5,
10, or 20 mg/kg bw per day on days 7-19 of gestation. All animals
were observed for clinical symptoms, body weight, and food consumption.
On day 28, the animals were killed, dissected, and examined
macroscopically for abnormalities. The ovaries and uteri were removed
and examined to determine the gravid uterine weight, number of corpora
lutea, and the distribution of live young and of early and late
embryo-fetal deaths. Live young were examined externally for
abnormalities and weighed. Two animals at 20 mg/kg bw per day died,
and one aborted. Treatment had no effect on the clinical condition or
findings at necropsy for animals that survived to term. Marked
reductions in weight gain and food intake, particularly at the start
of treatment, were observed in animals at 20 mg/kg bw per day. Slight,
temporary reductions in weight gain and food intake were also observed
at 10 mg/kg bw per day. At 20 mg/kg bw per day, postimplantation
losses were increased and litter size, litter weight, and fetal weight
were reduced. Because maternal toxicity was observed at 20 mg/kg bw
per day, the high dose for the main study was < 20 mg/kg bw per day
(Barker, 1985).
Groups of 16 pregnant New Zealand white rabbits were given ziram
(purity, 98.1%) by gavage at doses of 0, 3, 7.5, or 15 mg/kg bw per
day on days 7-19 of gestation. All animals were observed for clinical
symptoms, body weight, and food consumption. On day 28, the animals
were killed, dissected, and examined macroscopically for abnormalities.
The ovaries and uteri were removed and examined to determine the gravid
uterine weight, the number of corpora lutea, and the distribution of
live young and of early and late embryo-fetal deaths. Live fetuses were
weighed and examined for abnormalities before alizarin staining.
Significant reductions in maternal body-weight gain and food intake
were observed from the start of treatment at 15 mg/kg bw per day;
slight reductions in weight gain and food intake were also observed
during the early part of the treatment period in animals at 7.5 mg/kg
bw per day. At 15 mg/kg bw per day, postimplantation loss was slightly
increased and litter size, litter weight, fetal weight, and the
crown-rump length were reduced. There was no teratogenic effect. The
NOAEL for maternal and developmental toxicity was 7.5 mg/kg bw per day,
on the basis of decreased body-weight gain and food intake, increased
postimplantation loss, and reduced litter size, litter weight, fetal
weight, and crown-rump length at 15 mg/kg bw per day (Barker, 1986).
(f) Genotoxicity
The results of studies on the genotoxicity of ziram are
summarized in Table 2. Ziram is mutagenic in bacteria. It induced
chromosomal aberrations in some, but not all, studies with cultured
mammalian cells and did not induce unscheduled DNA synthesis in
hepatocytes. In vivo, ziram induced DNA single-strand breaks in the
livers of rats but not mice. Chromosomal aberrations were not
induced in mice in vivo in bone-marrow cells or spermatogonia, and
micronuclei were not induced in bone-marrow cells or peripheral
erythrocytes. Studies for clastogenicity have not been conducted in
rats in vivo. Consequently, the Meeting was unable to reach a
conclusion about the genotoxicity of ziram.
Table 2. Results tests for the genotoxicity of ziram
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium < 100 µg/plate NR Positivea Hedenstedt
TA98, TA100, TA1535, at 10, 50, et al. (1979)
TA1537, TA1538 100 µg/
plate
Reverse mutation S. typhimurium < 50 µg/plate 98.5 Positiveb Jones et al.
TA98, TA100, TA1535, at 15, 50 (1990)
TA1537, TA1538 µg/plate
Reverse mutation S. typhimurium < 90 µg/plate 98.5 Positivea Crebelli et al.
TA98, TA100, TA102, TA1537, at 7.5-90 (1992)
TA1950, TA1975, TA1535 µg/plate
Reverse mutation S. typhimurium 15-90 µg/plate NR Positiveb Franekic et al.
TA98, TA100, TA102, (1994)
TA104, TA1535, TA1538
Chromosomal Saccharomyces 5-50 µg/ml NR Negative Franekic et al.
malsegregation cerevisiae (1994)
Cell division Shallot root-tip 0.5-50 µg/ml NR Positive Franekic et al.
cells (1994)
Chromosomal aberration Chinese hamster < 1.5 µg/ml 98.5 Negative Brooker &
ovary cells Akhurst (1989)
Chromosomal aberration Chinese hamster < 1.75 µg/ml 86.2 Positivea Gulati et al.
ovary cells (1989)
Table 2. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Chromosomal aberration Chinese hamster < 10 µg/ml 98.5 Positiveb Mosesso et al.
ovary cells (1994)
Chromosomal aberration Chinese hamster < 2.15 µg/ml 98.5 Positiveb Mosesso et al.
epithelial liver cells (1994)
Unscheduled DNA synthesis Rat hepatocytes < 100 µg/ml 98.5 Negative Proudlock (1989)
In vivo
Chromosomal aberration Male and female NMRI 40, 120, 400 mg/kg bw 98.4 Negative Völkner (1992a)
mice, bone-marrow cells
Chromosomal aberration Male NMRI mice, germ cells 20, 67, 200 mg/kg bw 98.4 Negative Völkner (1992b)
Micronucleus formation Male and female Crl:CD-1 3.8, 11, 34, 101 mg/kg bw 98.8 Negative Proudlock &
mice, erythrocytes Taylor (1992)
Micronucleus formation Male and female B6C3F1 2.5, 5.0, 10, 20 mg/kg bw 98.5 Negative Crebelli et al.
mice, bone-marrow cells (1992)
Single-strand DNA breaks Male Swiss mice, 13, 25, 50, 100 mg/kg bw NR Negative Scarabelli et al.
liver cells (1993)
Single-strand DNA breaks Male Wistar rats, 13, 25, 50, 100 mg/kg bw NR Positive Scarabelli et al.
liver cells (1993)
NR, Not reported
a In the presence or absence of metabolic activation
b In the presence of metabolic activation
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Rabbits
The irritancy of ziram to the skin was tested in New Zealand
white rabbits. About 24 h before application of the test substance,
hair was removed from the dorsal lumbar region. Ziram (purity, 98.5%)
was applied at a dose of 0.5 g under a 2.5-cm2 gauze pad moistened
with 0.5 ml distilled water to one skin site on each of six animals,
and each treatment site was occluded with an elastic adhesive dressing
for 4 h. The animals were not restrained during the exposure and were
returned to their cages. At the end of treatment, the semiocclusive
dressing and gauze pads were removed and the treatment sites were
washed with water to remove any residual test substance. The treated
skin was examined for erythema and oedema on days 1, 2, 3, and 4. None
of the animals showed a response to treatment (Liggett & McRae,
1990a).
The irritancy of ziram to the eye was tested in New Zealand white
rabbits. The eyes of the animals were examined before instillation of
80 mg ziram (purity, 89.5%) in 0.1 ml into the lower everted lid of
one eye. Ocular lesions were graded and scored 1 and 24 h after
instillation. As the first rabbit had a severe ocular reaction
(irridal inflammation and opacity), the study was terminated on humane
grounds (Liggett & McRae, 1990b).
Guinea-pigs
The skin sensitizing potential of ziram (purity, 99.0%) was
examined in 30 adult female Dunkin-Hartley guinea-pigs. After
induction by epicutaneous applications of the test substance (25% w/w
in corn oil) and challenge with 10, 5, or 1% (w/w) in corn oil, six
guinea-pigs showed a positive skin response (grade 2 or more) to the
10% concentration. Five of these animals also reacted positively to
the 5% dose and three to the 1% concentration. No positive reactions
were observed in the control animals. Thus, a sensitization rate of
30% was obtained. The author considered that ziram has moderate
sensitizing properties (Daamen, 1988).
(ii) Neurotoxicity
Rats
The neurotoxicity of repeated doses of ziram was studied in
groups of 10 Crl:CD(SD)BR rats fed diets containing ziram (purity,
97.9%) providing concentrations of 0, 72, 210, or 540 ppm, equal to 0,
5, 14, or 34 mg/kg bw per day for males and 0, 6, 16, or 40 mg/kg bw
per day for females, for 91 days. Five animals of each sex per group
were allocated for evaluation of neurotoxic esterase and five for
evaluation of neuropathological changes. Viability, clinical signs,
body weight, and food consumption were recorded for all animals.
The results of a functional observational battery (home cage,
handling, open field, sensorimotor, neuromuscular, and physiological
observations) and of tests for total and ambulatory motor activity
were recorded for all animals during week 3, 7, and 12 of exposure.
The body weights and food consumption of male and female rats at
540 ppm were reduced, but no treatment-related effects indicative of
neurotoxicity were apparent. Brain neurotoxic esterase activity was
47% lower than that in controls in males and 38% lower in females at
540 ppm. The NOAEL for brain neurotoxic esterase inhibition was
210 ppm, equal to 14 mg/kg bw per day (Nemec, 1993).
The neurotoxicity of a single dose of ziram was studied in groups
of 12-16 Crl:CD(SD)BR rats given single doses of 0, 15, 300, or
600 mg/kg bw ziram (purity, 97.8%). Viability, clinical signs, and
body weight were recorded for all animals. The results of a functional
observational battery (home cage, handling, open field, sensorimotor,
neuromuscular, and physiological observations) and tests for total and
ambulatory motor activity were recorded for all animals before and 4 h
after treatment and on days 7 and 14. Five controls and five animals
at 600 mg/kg bw were selected for evaluation of neuropathological
changes. Four males and seven females at 600 mg/kg bw and one female
at 300 mg/kg bw died. At these doses, body weight was reduced, and
several treatment-related clinical signs (e.g. cyanosis, hypothermia,
enophthalmus, unkempt appearance, gait alterations, hypoactivity,
ptosis, and abdomal respiration) were observed. Remarkable differences
from the controls were seen in the functional observational battery
for animals at 300 and 600 mg/kg bw. Most of the responses were
observed 4 h after treatment (e.g. altered posture, palpebra closure,
and faecal consistency) or daily during the first week after dosing
(e.g. lacrimation, salivation, changes in fur appearance, altered
palpebral closure, changes in respiratory rate, and altered gait).
Ambulatory and total motor activity counts were reduced in males and
females at 300 and 600 mg/kg bw. No treatment-related microscopic
lesions were observed in the central and peripheral nervous tissues
examined. The NOAEL for neurotoxicity was 15 mg/kg bw, on the basis of
mortality, reduced body weight, ambulatory, and total motor activity,
and clinical signs at doses > 300 mg/kg bw (Lamb, 1994).
3. Observations in humans
The ability of ziram to induce chromosomal aberrations in
peripheral leukocytes was studied in four men and five women who had
been exposed for three to five years to ziram at a concentration of
2-4 mg/m3 air. The control group consisted of three women and one
man. The exposed group had 5.9% cells with aberrations and the
controls, 0.75% (Pilinskaya, 1970; Annex I, reference 15).
Comments
In experiments with 14C-labelled ziram in rats, elimination was
essentially complete within 48 h. Excretion occurred mainly in expired
air, urine, and faeces. Less than 2% of the administered dose remained
in the tissues. The biotransformation of ziram has not been studied
in rodents. In goats, it is metabolized at least in part via a
single-carbon pathway, which results in extensive radiolabelling of
natural products.
The primary effects of short- and long-term treatment with ziram
in mice, rats, and dogs were on the liver, thyroid gland, and testis.
The hepatic effects were increased liver weight, degeneration, and
focal-cell necrosis. Effects in the thyroid were C-cell hyperplasia
and carcinomas, and that on the testes was sterility.
Ziram had moderate acute oral toxicity in rats and rabbits (LD50
= 200-400 mg/kg bw). WHO has classified ziram as 'slightly hazardous'
(WHO, 1996).
In a four-week study of toxicity in mice given dietary
concentrations of 0, 3000, 4000, or 5000 ppm, an NOAEL was not
identified. Reductions in body weight, food intake, efficiency of food
use, and brain and heart weight occurred at all doses.
In a 13-week study of toxicity in mice given dietary
concentrations of 0, 100, 300, 900, or 2700 ppm, the NOAEL was
100 ppm, equal to 15 mg/kg bw per day, on the basis of lowered spleen
weight at higher doses. At 900 and 2700 ppm, the number of corpora
lutea was reduced, which was consistent with cellular changes in the
uterus.
In two four-week studies of toxicity in rats either given diets
providing doses of 0, 100, 500, 2500, or 5000 ppm or treated by gavage
with 0, 3, 15 or 100 mg/kg bw per day, the NOAEL was 3 mg/kg bw, on
the basis of degenerative liver changes. At 100 mg/kg bw, degenerative
changes in the kidneys and reductions in body weight, food intake,
efficiency of food use, and absolute weights of the liver, pituitary,
testes, brain, and uterus were seen.
In a 13-week study of toxicity in which rats received dietary
levels of 0, 100, 300, or 1000 ppm, the NOAEL was 100 ppm, equal to
7.4 mg/kg bw per day, on the basis of reduced body-weight gain, food
intake, and food use and increased brain and spleen weights at higher
doses.
In a four-week study of toxicity in dogs given diets providing
doses of 0, 1000, 2000, or 5000 ppm, an NOAEL was not identified.
Increased liver weight occurred at all doses. At 2000 ppm, convulsive
episodes were observed.
In a 13-week study of toxicity in dogs given diets providing 0,
100, 300, or 1000 ppm, the NOAEL was 100 ppm, equal to 4.1 mg/kg bw
per day, on the basis of increased liver weight, focal liver necrosis,
pigment in Kupffer cells, activated partial thromboplastin time, and
elevated cholesterol level at higher doses.
In a one-year study of toxicity in which dogs were fed diets
providing doses of 0, 50, 180, or 500 ppm, the NOAEL was 50 ppm, equal
to 1.6 mg/kg bw per day, on the basis of reductions in body-weight
gain, degeneration of hepatocytes, and increased activity of alanine
and aspartate aminotransferases and alkaline phosphatase at doses
> 185 ppm. At 500 ppm, single liver-cell necrosis was observed, and
the liver weight and cholesterol values were increased; albumin values
were reduced. Inflammatory cell infiltration around the hepatic vein
and its branches and aggregates of pigmented Kupffer cells were
observed in the liver.
Two long-term studies of toxicity and carcinogenicity in mice
have been reported. One was considered inadequate for evaluating
the carcinogenicity of ziram. In the other, mice were given diets
providing doses of 0, 25, 75, 220, or 680 ppm for 80 weeks. The NOAEL
was 25 ppm, equal to 3 mg/kg bw per day, on the basis of reduced brain
weight at doses > 75 ppm. There was no evidence of carcinogenicity.
In a two-year study of toxicity and carcinogenicity in rats at
dietary concentrations of 0, 25, 250, or 2500 ppm, the NOAEL was
250 ppm, equivalent to 12 mg/kg bw per day, on the basis of testicular
atrophy and thyroid hyperplasia at 2500 ppm. There was no evidence of
carcinogenicity.
In a two-year study of toxicity and carcinogenicity in Fischer
344 rats with dietary concentrations of 0, 300, or 600 ppm, an NOAEL
was not identified, since the combined incidence of C-cell adenoma
and carcinoma of the thyroid in males showed a positive trend. This
finding was considered to represent an extension of the known toxicity
of the compound to the thyroid, to which the rat is particularly
sensitive, and not to indicate carcinogenic potential for humans.
In a study of toxicity and carcinogenicity in CD rats treated
with 0, 60, 180, or 540 ppm in the diet for 12-24 months, an NOAEL was
not identified because dose-related changes in organ weights and
histopathological and haematological changes were observed at 60 ppm,
equal to 2.5 mg/kg per day. Other effects included reduced body
weight, erythrocyte count, and triiodothyronine and thyroxine
activity. Cysts in the thyroids, epithelial hyperplasia, hypertrophy
with vacuolation, cortical cystic degeneration of the adrenals, and
C-cell hyperplasia of the thyroid were also demonstrated. The tumour
incidence was not increased.
In a study of sperm quality in mice treated intraperitoneally
with ziram at a single dose of 0, 50, or 100 mg/kg bw or repeated
doses of 25 mg/kg bw per day for five days, severe morphological
abnormalities were observed. The frequency of abnormal sperm was 1.6%
in the controls, 5.6% at 50 mg/kg bw, 8.2% at 100 mg/kg bw, and 8.4%
after repeated doses of 25 mg/kg bw.
In a two-generation study of reproductive toxicity and
developmental neurotoxicity, rats were fed ziram at concentrations of
0, 72, 210, or 540 ppm. The NOAEL for maternal toxicity was 210 ppm,
equal to 10 mg/kg bw per day, on the basis of reduced food consumption
and body-weight gain at 540 ppm. The NOAEL for neonatal toxicity was
210 ppm, equal to 10 mg/kg bw per day, on the basis of reduced body
weight gain at 540 ppm. The NOAEL for reproductive toxicity and
developmental neurotoxicity was 540 ppm, equal to 25 mg/kg bw per day.
In a study of developmental toxicity, rats were given ziram at 0,
1, 4, 16, or 64 mg/kg bw per day on days 6-15 of gestation. The NOAEL
for maternal toxicity was 4 mg/kg bw per day for maternal toxicity, on
the basis of decreased body-weight gain and food intake and increased
water intake and salivation at doses > 16 mg/kg bw per day. The
NOAEL for developmental toxicity was 16 mg/kg bw per day, on the basis
of decreased litter weight and fetal weight at 64 mg/kg bw per day. No
teratogenicity was seen.
In a study of developmental toxicity in rabbits given ziram at
doses of 0, 3, 7.5, or 15 mg/kg bw per day on days 7-19 of gestation,
the NOAEL for maternal toxicity and developmental toxicity was 7.5
mg/kg bw per day, on the basis of decreased body-weight gain and food
intake in the dams and post-implantation loss, reduced litter size,
litter weight, fetal weight, and crown-rump length at 15 mg/kg bw per
day. There was no evidence of teratogenicity.
Ziram is mutagenic in bacteria. It induced chromosomal
aberrations in some, but not all, studies with cultured mammalian
cells but did not induce unscheduled DNA synthesis in hepatocytes.
In vivo, ziram induced single-strand breaks in DNA in the livers of
rats but not mice. Chromosomal aberrations were not induced in mice
in vivo in bone-marrow cells or spermatogonia, and micronuclei were
not induced in bone-marrow cells or peripheral erythrocytes. Studies
for clastogenicity have not been conducted in rats in vivo. In an
old study of nine workers exposed for three to five years to ziram at
a concentration of 2-4 mg/m3 air, the percentage of peripheral
leukocytes with chromosomal aberrations was 5.9%; in a control group,
the percentage was 0.75%. The Meeting was unable to reach a conclusion
about the genotoxicity of ziram.
Ziram caused severe ocular irritation but no dermal irritation in
rabbits and moderate skin sensitization in guinea-pigs.
In two studies of neurotoxicity in rats treated with single doses
of 0, 15, 300, or 600 mg/kg bw or 0, 72, 210, or 540 ppm for 91 days,
behavioural effects indicative of neurotoxicity were apparent after
the single high doses but not after repeated dosing at a lower level.
The NOAEL was 210 ppm, equal to 14 mg/kg bw per day, on the basis of
reduced body weight and food consumption and inhibition of brain
neurotoxic esterase activity at 540 ppm.
An ADI of 0-0.003 mg/kg bw was established on the basis of
long-term toxicity in the rat. In this study, effects were seen at all
doses, the LOAEL being 60 ppm, equal to 2.5 mg/kg bw per day. In view
of the absence of an NOAEL, a safety factor of 1000 was used. The
NOAEL of 1.6 mg/kg bw per day observed in a long-term study of
toxicity in dogs supported this ADI, which served as the basis for the
group ADI that was established for ziram alone or in combination with
ferbam.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3 mg/kg bw per day (80-week study of
toxicity and carcinogenicity)
Rat: NOAEL could not be determined: lowest effective dose,
60 ppm, equal to 2.5 mg/kg bw per day (12-24-month
study of toxicity, various effects)
100 ppm, equal to 7.4 mg/kg bw per day (13-week study
of toxicity)
250 ppm, equivalent to 12 mg/kg bw per day per day
(two-year study of toxicity and carcinogenicity)
210 ppm, equal to 10 mg/kg bw per day (maternal
toxicity in a study of reproductive toxicity)
Rabbit: 7.5 mg/kg bw per day (maternal toxicity and
embryotoxicity in a study of developmental toxicity)
Dog: 50 ppm, equal to 1.6 mg/kg bw per day (one-year study
of toxicity)
100 ppm, equal to 4.1 mg/kg bw per day (13-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw (for ferbam and ziram)
Studies that would provide information useful for continued evaluation
of the compound
1. Further studies on long-term toxicity in the rat
2. Further studies on genotoxicity in rats
3. Further studies on male reproductive toxicity
4. Further observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to ziram
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 270 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.06 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Severely irritating
Dermal, sensitization, guinea-pig Moderately sensitizing
Medium-term (1-26 weeks) Repeated oral, 13 weeks, toxicity, mouse NOAEL = 15 mg/kg bw per day, decreased spleen weight
Repeated oral, 4 weeks, toxicity, rat NOAEL = 3 mg/kg bw per day, reduced body weight and
food consumption, degenerative hepatic changes
Repeated oral, 13 weeks, toxicity, rat NOAEL = 7.4 mg per kg bw per day, reduced body weight
and food consumption, increased brain and spleen weight
Repeated oral, 13 weeks, toxicity, dog NOAEL = 4.1 mg/kg bw per day, hepatic toxicity
Repeated oral, reproductive toxicity and NOAEL = 25 mg/kg bw per day, reproductive toxicity
developmental neurotoxicity, rat and development neurotoxicity
NOAEL = 10 mg/kg bw per day, maternal and neonatal
toxicity (reduced body weight)
Repeated oral, developmental toxicity, rat NOAEL= 16 mg/kg bw per day, developmental toxicity
(reduced fetal weight)
NOAEL = 4 mg/kg bw per day, maternal toxicity
(reduced body weight)
Repeated oral, developmental toxicity, NOAEL = 7.5 mg/kg bw per day, developmental and
rabbit maternal toxicity (reduced fetal and maternal weight)
Repeated oral, neurotoxicity, rat NOAEL = 14 mg/kg bw per day, inhibition of
neuropathy target esterase activity
Long-term (> 1 year) Repeated oral, 18 months, toxicity, mouse NOAEL = 3 mg/kg bw per day, reduced brain weight and
hepatic toxicity
Repeated oral, 2 years, toxicity and No NOAEL; LOAEL = 2.5 mg/kg bw per day, haematological
carcinogenicity, rat toxicity and toxic effects on the thyroid
Repeated oral, 1 year, toxicity, dog NOAEL = 1.6 mg/kg bw per day, reduced body weight and
hepatic toxicity
References
Barker, L. (1985) Ziram: Oral (gavage) range-findings study in the
pregnant rabbit. Unpublished report No. 4588-508/1 from Hazleton
Laboratories Europe, Ltd, North Yorkshire, United Kingdom. Submitted
to WHO by UCB Chemicals, Brussels, Belgium.
Barker, L. (1986) Ziram: Oral (gavage) teratology study in the rabbit.
Unpublished report No. 4913-508/2 from Hazleton Laboratories Europe,
Ltd, North Yorkshire, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Blagden, S.M. (1991) Ziram technical: Acute inhalation toxicity study.
Four hour exposure (nose only) in the rat. Unpublished report
No. 378/2 from Safepharm Laboratories Ltd, Derby, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Bodden, R.M. (1993) Ziram: Nature of the residue in lactating goats.
Unpublished report No. HLA 6225-101 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Brooker, P.C. & Akhurst, L.C. (1989) Analysis of metaphase chromosomes
obtained from CHO cells cultured in vitro and treated with ziram.
Unpublished report No. 7/89675 from Huntingdon Research Centre Ltd,
Cambs, United Kingdom. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Chambers, P.R., Cary, R.J., Gibson, W.A. & Gopinath, C. (1992a) Ziram.
Palatability study in mice for 4 weeks. Unpublished report No. ZIR
3/89720 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Chambers, P.R., Cary, R.J., Gibson, W.A. & Gopinath, C. (1992b) Ziram.
Palatability study in rats for 4 weeks. Unpublished report No. ZIR
2/89719 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Cheng, T. (1991a) Metabolism of ziram in rats. Unpublished report
No. HLA 6225-106 from Hazleton Laboratories America, Inc., WI, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Cheng, T. (1991b) Dermal absorption of 14C-ziram in male rats.
Unpublished report No. HLA 6225-107 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Cilievici, O., Graciun, C. & Ghidus, E. (1983) Decreased fertility,
increased dominant lethals, skeletal malformations induced in the
mouse by ziram fungicide. Rev. Roum. Morphol. Embryol. Physiol.,
29, 159-165.
Crebelli, R., Zijno, A., Conti, L., Crochi, B., Leopardi, P., Marcon,
F., Renzi, L. & Carere, A. (1992) Further in vitro and in vivo
mutagenicity assays with thiram and ziram fungicides: Bacterial
reversion assays and mouse micronucleus test. Teratog. Carcinog. Mutag.,
12, 97-112.
Daamen, P.A.M. (1988) Assessment of the skin sensitization potential
of ziram technical in the guinea-pig (split adjuvant test).
Unpublished report No. RCC NOTOX 0878/1096 from RCC Notox, C.,
's-Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Dickhaus, S. & Heisler, E. (1980) Report of a subacute toxicity study
of the substance 'Ziram' (techn.) for 4 weeks in the rat. Unpublished
report No. 2-4-132-80 from Pharmatox, Forschung und Beratung GmbH,
Hanover, Germany. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Edwards, J.A., Baldrick, P., Gibson, W.A., Crook, D., Offer, J.M. &
Gopinath, C. (1989) Twenty-one day dermal toxicity study in rabbits
with ziram. Unpublished report No. ZIR 4/89689 from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Franekic, J. Bratulic, N., Pavlica, M. & Papes, D. (1994) Genotoxicity
of dithiocarbamates and their metabolites. Mutat. Res., 325, 65-74.
Gulati, D.K., Witt, K., Anderson, B., Zeiger, E. & Shelby, M.D. (1989)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro. III: Results with 27 chemicals.
Environ. Mol. Mutag., 13, 133-193.
Hedenstedt, A., Rannug, U., Ramel, C. & Wachtmeister, C.A. (1979)
Mutagenicity and metabolism studies on 12 thiuram and dithiocarbamate
compounds used as accelerators in the Swedish rubber industry.
Mutat. Res., 68, 313-325.
Hemavathi, E. & Rahiman, M.A. (1993) Toxicological effects of ziram,
thiram, and dithane M-45 assessed by sperm shape abnormalities in
mice. J. Toxicol. Environ. Health, 38, 393-398.
Hodge, H.C., Maynard, E.A, Downs, W.L., Coye, R.D. & Steadman, L.T.
(1956) Chronic oral toxicity of ferric dimethyldithiocarbamate
(ferbam) and zinc dimethyldithiocarbamate (ziram). J. Pharmacol.
Exp. Ther., 118, 174-181.
Jackson, G.C. & Hardy, C.J. (1989) Ziram technical. Acute inhalation
toxicity in rats, 4-hour exposure. Unpublished report No. UCB,
314/89684 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Jones, E., Cook, P.G.S., Gant, R.A. & Kitshing, J. (1990) Ziram
technical: Bacterial mutation assay. Unpublished report No. ZIR
25/891914 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Khicenko, F.F. & Chernov, O.V. (1968) Experimental study of potential
vlastogenic effect of ziram. Gig. Primeniya Toksikol. Pestic. Klin.
Obravleniy (Kiev), 6, 770-776.
Korablev, M.V. (1969) Toxicological characteristics of dithiocarbamate
and derivative employed in the national economy and medicine
(literature survey). Farmakol. Toksikol., 32, 356-362 (in Russian).
Lamb, I.C. (1994) An acute neurotoxicity study of ziram in rats.
Unpublished report No. WIL-223001 from WIL Research Laboratories,
Inc., Ashland, OH, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Lee, C.-C., Russell, J.Q., Minor, J.L, Kowalski, J.J., Sanyer, J.L.,
Kintner, L.D., Hodgson, J.R., Short, R.D., Peters, P.J., Dilley, J.V.,
Murrill, E.A., Holton, D.O. & Ellis, H.V. (1975) Toxicological
evaluation of ferric dimethyldithiocarbamate (ferbam) and dithio-
carbamate (thiram) with acute toxicity of manganese and zinc
ethylenebisdithiocarbamates (maneb and zineb) Unpublished report
No. 3612-B from National Institute of Environmental Health Sciences,
Research Triangle Park, NC, USA. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Liggett, M.P. & Allan, S.A. (1989a) Acute oral toxicity to rats of
ziram. Unpublished report No. 89690D/UCB 315/AC from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Liggett, M.P. & Allen, S.A. (1989b) Acute dermal toxicity to rabbits
with ziram. Unpublished report No. 89338D/UCB 316/AC from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Liggett, M.P. & McRae, L.A. (1990a) Skin irritation to rabbits with
ziram (technical). Unpublished report No. 90500D/UCB 317/SE from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Liggett, M.P. & McRae, L.A. (1990b) Eye irritation to rabbits with
ziram (technical). Unpublished report No. 90499D/UCB 318/SE from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
McLean, T.A., Horner, S.A., Buist, D.P. (1992a) Ziram preliminary
dietary toxicity study in beagle dogs. Unpublished report No. ZIR
1-G/89637 from Huntingdon Research Centre Ltd, Cambs, United Kingdom.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
McLean, T.A., Horner, S.A., Buist, D.P., Crook, D., Read, R.M.,
Gopinath, C., Anderson, A., Dawe, I.S., Johnson, S.F. & Patel, S.
(1992b) 13-Week dietary toxicity study in beagle dogs. Unpublished
report No. ZIR 8-G/901813 from Huntingdon Research Centre Ltd, Cambs,
United Kingdom. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Mosesso, P., Turchi, G., Cinelli, S., Chiara, D.D., Fiore, M. &
Palitti, F. (1994) Clastogenic effects of the dithiocarbamate
fungicides thiram and ziram in Chinese hamster cell lines cultures
in vitro. Teratog. Carcinog. Mutag., 14, 145-155.
National Toxicology Program (1983) Carcinogenesis Bioassay of Ziram
(CAS No. 137-30-4) in F344/N Rats and B6C3F1 Mice (Feed Study) (NIH report
No. 83-1794), Washington DC, Department of Health and Human Services,
Public Health Service, National Institutes of Health.
Nemec, M.D. (1993) A subchronic (13-week) neurotoxicity study of
ziram in rats. Unpublished report No. WIL-223004 from WIL Research
Laboratories, Inc., Ashland, OH, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Nemec, M.D. (1996) A dietary two-generation reproduction and
developmental neurotoxicity study of ziram in rats. Unpublished report
No. WIL-223003 from WIL Research Laboratories, Inc., Ashland, OH, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Pilinskaya, M.A. (1970) Chromosome aberrations in the persons
contacted with ziram. Genetika, 6, 157-163 (in Russian).
Powell, L.A.J., Crook, D., Gregson, R.L., Gopinath, C., Gibson, W.A.
& Anderson, A. (1992) Preliminary toxicity to rats by dietary
administration for 13 weeks. Unpublished report No. ZIR 5/901840 from
Huntingdon Research Centre Ltd, Cambs, United Kingdom. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Powell, L.A.J., Gopinath, C., Gibson, W.A. & Anderson, A. (1993) Ziram
preliminary toxicity to mice by dietary administration for 13 weeks.
Unpublished report No. ZIR 11/901841 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Powell, L.A.J., Bottomley, S.M., Crook, D., Majeed, S.K., Gopinath,
C., Gibson, W.A. & Anderson, A. (1994a) Ziram (technical). Potential
oncogenicity to mice by repeated dietary administration for 80 weeks.
Unpublished report No. ZIR 12/932311 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf Autochem
Agri, Brussels, Belgium.
Powell, L.A.J., Bottomley, S.M., Crook, D., Gregson, R.L., Offer,
J.M., Gibson, W.A. & Anderson, A. (1994b) Ziram (technical). Combined
chronic toxicity and oncogenicity of ziram (technical) administered in
the diet to rats. Unpublished report No. ZIR 9/942098 from Huntingdon
Research Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB,
Elf Autochem Agri, Brussels, Belgium.
Proudlock, R.J. (1989) Autoradiographic assessment of DNA repair after
in vitro exposure of rat hepatocytes to ziram. Unpublished report
No. ZIR 6/89820 from Huntingdon Research Centre Ltd, Cambs, United
Kingdom. Submitted to WHO by UCB, Elf Autochem Agri, Brussels,
Belgium.
Proudlock, R.J. & Taylor K. (1992) Ziram (technical). Peripheral
blood micronucleus test in the mouse after 89 days dietary exposure.
Unpublished report No. ZIR 27/920513 from Huntingdon Research Centre
Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf Autochem
Agri, Brussels, Belgium.
Robens, J.F. (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol. Appl.
Pharmacol., 15, 152-163.
Ryazanova, R.A. (1967) Gig. Sanit., 2, 26.
Scarabelli, L., Giannoni, P., Malfatto, C., Bolognesi, C. & Cesarone,
C.F. (1993) Relationship between poly(ADP-ribose) polymerase activity
and DNA damage induced by zinc dithiocarbamates in mouse and rat
liver. Mutat. Res., 302, 1-6.
Smith, J.A., Bryson, A.M., John, D.M. & Anderson, A. (1990) A study of
the effect of ziram on pregnancy of the rat. Unpublished report No.
ZIR 15/24/891371 from Huntingdon Research Centre Ltd, Cambs, United
Kingdom. Submitted to WHO by UCB, Elf Autochem Agri, Brussels,
Belgium.
Smith, T.G., Buist, D.P., Crook, D., Morrow, J. & Gopinath, C. (1993)
Ziram toxicity to dogs by repeated dietary administration for 52
weeks. Unpublished report No. ZIR 10-G/920533 from Huntingdon Research
Centre Ltd, Cambs, United Kingdom. Submitted to WHO by UCB, Elf
Autochem Agri, Brussels, Belgium.
Völkner, W. (1992a) Chromosome aberration assay in bone marrow cells
of the mouse with ziram technical (UCB provenience). Unpublished
report No. CCR 254812 from Cytotest Cell Research GmbH & Co. KG,
Rossdorf, Germany. Submitted to WHO by UCB, Elf Autochem Agri,
Brussels, Belgium.
Völkner, W. (1992b) Mouse germ-cell cytogenetic assay with ziram
technical (UCB provenience). Unpublished report No. CCR 254834 from
Cytotest Cell Research GmbH &Co. KG, Rossdorf, Germany. Submitted to
WHO by UCB, Elf Atocbem Agri, Brussels, Belgium.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
