
PESTICIDE RESIDUES IN FOOD - 1983
Sponsored jointly by FAO and WHO
EVALUATIONS 1983
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Geneva, 5 - 14 December 1983
Food and Agriculture Organization of the United Nations
Rome 1985
METHIOCARB
TOXICOLOGY
Explanation
Methiocarb was evaluated by the 1981 Meeting and a full acceptable
daily intake (ADI) was allocated.1/ Observations in humans,
considered by the previous Meeting to be desirable information, are
still not available. Other additional toxicological data have been
submitted and are summarized and discussed in the following monograph
addendum.
In 1981, recommendations were made for maximum residue levels (MRLs)
(and temporary MRLs pending further information on GAP) in a range of
commodities on which methiocarb was used for snail and slug control,
spray application as a bird repellent or as a seed treatment.
Information on good agricultural practice for commodities on which
bait applications are recommended or used, was required, and it was
noted that additional metabolism and residue data would be necessary
if future uses of the compound on animal feeds could result in
potential residues in meat, milk, poultry and eggs. Information on
levels of methiocarb residues in foods in commerce or at consumption
was also listed as desirable.
A considerable amount of additional information, not all relevant to
the above requests was evaluated by the meeting and is presented in
this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Elimination and Biotransformation
In male Wistar rats given a single oral dose of approximately 5 mg/rat
(body weight of the animals not given) of methiocarb, only a small
amount of the administered dose was eliminated in the urine, mainly
within 48 hours of treatment, as the unchanged parent compound
(<2.3 percent) and its metabolites (3.3 percent) (van Hoof &
Heyndrickx
1975).
1/ See Annex 2 for FAO and WHO documentation.
Under aerobic conditions and in the presence of NADPH, methiocarb was
oxidized by the flavin-adenine dinucleotide-dependent monoxygenase of
pig liver microsomes. The rate of sulphoxidation of methiocarb was low
as compared to other thioether-containing pesticides such as
disulfoton, an organophosphate, and thiofanox, a carbamate (Hajjar &
Hodgson 1982).
Effects on Enzymes and Other Biochemical Parameters
Rat
Groups of 15 female Sprague-Dawley-derived rats were intubated with
technical methiocarb (97 percent pure) or methiocarb sulphoxide (95.2
percent pure) in Carbowax at 0, 0.5 or 2 mg/kg b.w./day five times
weekly for four weeks. Determination of cholinesterase activity 30
minutes after dosing on days 7, 14, 21 and 28 (i.e. following the two-
day withdrawal period) showed a significant dose-related inhibition of
plasma cholinesterase (21-61 percent) and erythrocyte cholinesterase
(22-46 percent) at practically all intervals following methiocarb
sulphoxide dosing. Treatment with technical methiocarb resulted in
plasma cholinesterase inhibition on days 0, 7 and 14 (23-41 percent)
following the 2 mg/kg b.w. dose and on days 0 (21 percent) following
the 0.5 mg/kg b.w. dose. Erythrocyte cholinesterase was depressed by
29 percent on day 0 with 2 mg/kg b.w. of technical methiocarb. Four
hours after dosing on days 4, 11 and 18, only rats treated with 2
mg/kg b.w. of methiocarb sulphoxide showed a decrease (>20 percent)
in activity of plasma cholinesterase (days 11 and 18) and erythrocyte
cholinesterase (day 11) (Hixson 1981).
Dog
Groups of two male and two female beagles were given daily 0.05 or 0.5
mg/kg b.w. of technical methiocarb (97 percent pure) or methiocarb
sulphoxide (95.2 percent pure) in gelatin capsules for 29 days.
Control groups comprised two males and two females. Slight to heavy
salivation or vomiting was observed in animals (both sexes) given
either test material at 0.5 mg/kg b.w. One female treated with 0.05
mg/kg b.w. of the sulphoxide also exhibited slight salivation on one
occasion. Assay of plasma and erythrocyte cholinesterase from unfasted
animals, using an unspecified method, at various intervals after the
first and second or third dosing each week showed inhibition (> 20
percent) of the enzyme in plasma or erythrocytes by both test
compounds at 0.5 mg/kg b.w. Depression of cholinesterase peaked at
1-2 h and was essentially reversible at 6 h, post-treatment. In
general, the extent of inhibition, which was greater with plasma
cholinesterase than with erythrocyte cholinesterase, did not increase
with time with either compound. Data indicated 0.05 mg/kg b.w. to be a
marginal no-effect level (NOEL) on cholinesterase for technical
methiocarb. A no-effect level for methiocarb sulphoxide was, however,
not demonstrated (Hayes 1981).
TOXICOLOGICAL STUDIES
Special Study on Teratogenicity
Rabbit
Groups of 17-19 female rabbits (New Zealand White), artificially
inseminated, were intubated with methiocarb (a mixture of five
batches, 97.3 percent pure) as a suspension (in distilled water
containing 0.5 percent carboxymethyl cellulose and 0.5 percent
Tween 80) at 0, 1, 3 or 10 mg/kg b.w./day from day 6 to 18 inclusive
of gestation (day 0 = day of insemination). The does were sacrificed
on day 29 of gestation and uterine contents were examined. Foetuses
were examined for external, skeletal and internal abnormalities. Three
to six females per group, including the control, died or were
sacrificed in extremis 6 to 25 days after insemination, mainly owing
to respiratory tract infection and/or gastrointestinal disorder or
accidental tracheal intubation. The number of does found to be
pregnant with viable young on day 29 was only 11 in control group, 13
at 1 mg/kg b.w., 12 at 3 mg/kg b.w. and 10 at 10 mg/kg b.w. Toxic
signs such as increased respiratory rate, muscular tremors, pupillary
constriction, incoordination and prostration were noted at 10 mg/kg
b.w. in maternal animals. Increased respiratory rate was also seen,
although infrequently, at 3 mg/kg b.w. Does of the top-dosage group
exhibited actual weight loss during the first two days of treatment
and growth depression thereafter throughout the dosing period.
Premature parturition occurred in one doe at 3 mg/kg b.w. on day 28
and two does at 10 mg/kg b.w. aborted on day 25. The incidence of
pregnant does with early resorptions was elevated at 10 mg/kg b.w. but
the mean number of early resorptions per litter in this dosage group
was within the ranges of background data submitted.
There were no treatment-related effects on the number of corpora
lutea, implantations, viable young or late resorptions or on pre-
and post- implantation loss, foetal weight and placental weight. An
increase in incidence of foetuses with pale areas in the liver was
noted at 10 mg/kg b.w. but frequency of foetal malformations was not
affected by treatment. The study appeared to give no evidence
suggestive of teratogenic activity of methiocarb under the conditions
of the experiment. Maternal and/or foetal toxicity, however, occurred
at 3 mg/kg b.w. and above (Tesh et al 1981).
Acute Toxicity
The four-hour inhalation LC50 of an aerosol of technical methiocarb
(97.9 percent pure) in male and female Wistar rats (Bor: WISW (Spf
(Cpb)) was greater than 322 mg/cu m air, in terms of analytical
concentration of the test material in the inhalation chamber. No
information was given on the particle size of the aerosol (Thyssen
1982).
Short-Term Studies
In Wistar rats (Bor: WISW (Spf (Cpb)) exposed to an aerosol (particle
size not specified) of technical methiocarb (97.9 percent pure) at
chamber (analytical) concentrations of 0, 27, 92 or 298 mg cu m air, 6
h/day for-five days, the LC50 was greater than 298 mg/cu m air in
males and approximately 300 mg/cu m air in females (Thyssen 1982).
COMMENTS
The cholinesterase studies in both rats and dogs indicated methiocarb
sulphoxide to be a more potent inhibitor than methiocarb. No evidence
of teratogenicity of methiocarb was observed in the rabbit teratology
study.
The Meeting confirmed the ADI estimated at the 1981 Meeting and
further desirable work was recommended.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 25 ppm in the diet, equivalent to 1.3 mg/kg b.w./day.
Dog: 5 ppm in the diet, equivalent to 0.125 mg/kg b.w./day.
Estimation of Acceptable Daily Intake for Man
0 - 0.001 mg/kg b.w.
FURTHER WORK OR INFORMATION
Desirable
1. Data to clarify the significance of the inhibition of plasma
cholinesterase noted in the dog study which led to the estimation of
the no-effect level by the 1981 Meeting, bearing in mind the views of
the 1982 Meeting on the function of plasma cholinesterase.
2. Information on the method of analysis used to estimate plasma and
erythrocyte cholinesterase activities in vivo in the above dog
study.
3. Observations in humans.
REFERENCES-TOXICOLOGY
Hajjar, N.P. & Hodgson, E. Sulfoxidation of thioether-containing
1982 pesticides by the flavin-adeninine
dinucleotide-dependent monooxygenase of
pig liver microsomes. Biochem. Pharmacol
31: 745-752.
Hayes, R.H. Cholinesterase evaluation study of
1981 methiocarb technical and methiocarb
sulphoxide in dogs. Report from Mobay
Chemical Corp. submitted to WHO by Bayer
AG. (Unpublished)
Hixson, E.J. Cholinesterase no-effect level of
1981 RMesurol and RMesurol sulphoxide in
female rats. Report from Mobay Chemical
Corp. submitted to WHO by Bayer AG.
(Unpublished)
Tesh, J.M., Rose, F.W., H321: effects of oral administration
Secker, R.C. & Wilby, O.K. upon pregnancy in the rabbit. 2. Main
1981 study. Report from Life Science
Research, England, submitted to WHO by
Bayer AG. (Unpublished)
Thyssen, J. H321 (Mesurol active ingredient). Acute
1982 inhalation toxicity. Report from Bayer
AG, submitted to WHO by Bayer AG.
(Unpublished)
van Hoof, F. & Heyndrickx. The excretion in urine of four
1975 insecticidal carbamates and their
phenolic metabolites after oral
administration to rats. Arch. Toxicol.
34: 81-88.
RESIDUES
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Further detailed information on use patterns in various countries only
served to confirm those identified in 1981. The only additional uses
brought to light were on sorghum in Africa and South America, where a
single application of 1 - 1.5 kg/ha is made at the dough stage and the
uses in Eastern Africa mentioned below.
Bruggers et al. (1981) summarized the results of field evaluations,
during several years, of methiocarb when used to protect ripening
crops of rice, wheat, sorghum and sunflowers in Eastern Africa from
losses due to birds. The positive results and favourable
cost/effectiveness obtained in nearly all situations justifies the use
of methiocarb under typical farming conditions in Africa. Treatment
rates are in the range of 1-2 kg/ha and application is made at the
dough stage when the crop first becomes vulnerable to damage by birds.
There is world-wide acceptance of methiocarb for use as a bait against
slugs and snails. The baits contain either 2 percent or 4 percent
methiocarb, together with wheat bran or a similar attractant,
formulated into small pellets designed to be scattered by hand or
machine. The treatment is designed to deposit the pellets on the soil
between the crop plants so as to be readily accessible to foraging
molluscs. It is recognized that some pellets will impact upon the
leaves and stems of crop plants and that some may lodge between leaves
of crops such as leafy vegetables. The structure of the pellets is
sufficiently strong that they do not disintegrate and thus contaminate
plant parts to a significant extent. The pellets are generally
coloured distinctively and can be dislodged in preparing crops for
market and cooking.
When methiocarb is used as bait, the rate of application is in the
range of 120-240 g a.i./ha. One application is generally sufficient
but a second may be required. Treatment is usually made early in the
crop cycle to prevent damage to young seedlings or emerging plants. In
the event of snails and slugs invading from adjacent fields, perimeter
baiting is usually effective.
In 1981, the United Stated Environmental Protection Agency granted
authority to use methiocarb to repel birds depredating grapes. The
rate of application is 4 kg/ha with a maximum of four applications per
season. Maximum residue limits (MRLs) were established for grapes,
raisins, raisin waste and grape pomace together with secondary MRLs in
foods of animal origin. This use was extended in 1982. In 1983, almost
900 tonnes of methiocarb active ingredient were used on grapes in 15
states, in addition to quantities used to repel birds from
blueberries, cherries, peaches and cereal grains (Anonymous 1983).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data from supervised trials were evaluated in 1981. The
following additional reports were considered by the Meeting.
Cauliflower
A trial carried out in Germany in 1981 involved the application of 4
percent methiocarb granular snail bait, on two occasions 16 days
apart, at the rate of 0.12 kg a.i./ha. Samples of leaves, flowers and
stalks taken 0, 4, 7, 14 and 28 days after the second treatment were
analysed by a method sensitive to 0.05 mg/kg of methiocarb, its
sulphoxide and sulphone. Though the deposit on the day of treatment
ranged up to 2 mg/kg on stalks, by the fourth day the residue was at
or slightly above the limit of determination. By the seventh day there
was no detectable residue in flowers, leaves or stalks (Bayer 1983).
Maize
Methiocarb is used as a seed treatment against fruit flies and as a
bird repellent. The treatment involves the application of 300 ml of
formulation (500 g/l) per 100 kg of seed. The treated seed is
distributed at a rate equivalent to 150 g of methiocarb/ha. Green
maize plants cut for silage approximately 90 days after planting were
analysed, as was the ripe grain harvested approximately 180 days after
planting. In none of six trials conducted in various regions of
Germany or of two similar trials in the United Kingdom was methiocarb
or its metabolites detected when samples were analysed by methods
sensitive to 0.05 mg/kg or 0.01 mg/kg (Bayer 1983).
Sorghum
Pelissier (1978) working in Senegal showed that when methiocarb was
applied to ripening sorghum at the rate of 2 kg/ha, together with an
adhesive, the pesticide had a half-life of six to seven days. Residue
levels in grain were less than 3.6 mg/kg 20 days after application.
Gras et al. (1981) conducted trials with methiocarb to determine the
level and fate of residues when the pesticide was applied to ripening
sorghum as a bird repellent. Such treatments are finding favour in
West Africa to reduce the depredations wrought by the red-billed
Quelea. Methiocarb was applied at the rate of 2 kg/ha in aqueous
suspension when the sorghym grain was at the "milk" stage. The crop
was subjected to overhead irrigation. Samples (heads on stalk) were
collected 12 and 24 hours, and 5, 10, 15, 20, 25 and 30 days after
treatment. Residues were determined in the entire head (grain and
glume) and in the grain only. The results are given in Table 1.
The normal harvest date is approximately 21 days after treatment (milk
stage). The glumes are usually removed during threshing or prior to
and during milling.
A mathematical analysis of these data indicates that the residual
half-life is 6 days on grain and 7.5 days on grain plus glumes. The
authors drew attention to the fact that sorghum is always cooked prior
to eating. In view of the sensitivity of methiocarb to heat it is
entirely likely that any residues remaining on the sorghum grain would
be partially or wholly degraded during preparing and cooking. However,
the data on residues in grain plus glumes leads to the conclusion that
the residue level on the stubble after harvest would be high. The
consequences of feeding such stubble to livestock have not been
evaluated.
Cherries
Workers in Belgium studied the use of methiocarb sprays for the
protection of cherries against damage by starlings. The concentration
of the residues was determined on cherries treated with methiocarb
spray once, 23 days before harvest and twice, 23 and 6 days before
harvest. The maximum concentrations of methiocarb (13.4 mg/kg) and
methiocarb sulphoxide (1.25 mg/kg) were recorded on cherries sprayed
twice. No methiocarb sulphone was detected in any samples (Hoyoux &
Zenon-Roland, 1979).
Information concerning the use of methiocarb and the residues
resulting from such use was received from the Government of Thailand.
This information is summarized in Table 2. With the exception of
Chinese radish the results were within the MRLs recommended in 1981,
notwithstanding the fact that the treatments had been made by
spraying. In the case of Chinese radish the residue reported (4.25
mg/kg) appears to be high for a root crop but there may be special
reasons for this result. The Meeting proposed an MRL for Chinese
radish.
FATE OF RESIDUES
General
The 1981 evaluation of methiocarb (FAO/WHO 1982b) included a review of
the fate of its residues, which is supplemented below. Revised
versions of the list of identified metabolites and of the diagram of
metabolic pathways given in 1981 are reproduced here for convenience
(Table 3 and Fig. 1, respectively).
In Animals
Some studies on the fate of methiocarb in rats, dogs, cattle, poultry
and fish were evaluated in 1981. It should be noted that the last
sentence describing the study on dogs in FAO/WHO 1982 (p.318) should
end "... tissue residues were below 0.4 mg/kg." Additional studies
are reviewed below.
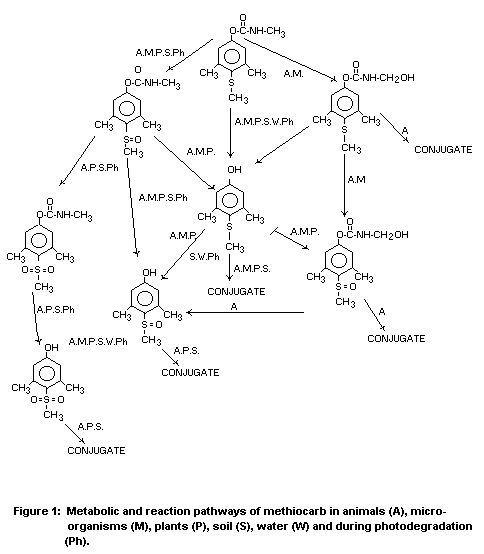 Table 1. Persistence of methiocarb on sorghum, Bambey, Senegal, 1978.
Sample Interval Methiocarb residue (mg/kg)
No. after Thin-layer Gas
application1 chromatography chromatography
(days)
Seed only
1 5 20 36
2 10 17 13
3 15 3.8 8.8
4 20 3.5 4.3
5 25 3.0 4.0
6 30 0.9 2.2
Seed and glumes
7 0 180 200
8 0.5 140 150
9 1 150 170
10 2 100 140
11 5 92 140
12 10 100 140
13 15 11 76
14 20 24 56
15 25 17 24
16 30 8 11
1 Application rate was 2 kg/ha spray.
Table 2. Residues Resulting from Supervised Trials of Methiocarb, Thailand
Application
Crop Pest Rate No. Pre-harvest Residue
(kg/ha) Interval (mg/kg)
(days)
Cabbage Diamond back moth 1.0 1 7 0.16
Aphis
Chinese Kale Aphis 1.0 2 5 N.D.2
Phyllotreta sp.
Edible Rape Thrips 1.0 1 5 0.14
Phyllotreta sp.
Chinese Thrips 1.0 2 7 4.25
Radish Phyllotreta sp.
Broccoli Aphis 1.0 1 7 0.13
Yard long bean Aphis 1.45 1 7 N.D.
Thrips
Multiplier Thrips 2.5 1 7 0.02
onion
Mung bean Aphis 1.0 2 12 N.D.
1 50% wettable powder used in all treatments.
2 N.D. = not detectable
Table 3. Chemical Names, Structures and Designations of Methiocarb and its Metabolites Identified in Animals
Designation Chemical name Structure
Table 1. Persistence of methiocarb on sorghum, Bambey, Senegal, 1978.
Sample Interval Methiocarb residue (mg/kg)
No. after Thin-layer Gas
application1 chromatography chromatography
(days)
Seed only
1 5 20 36
2 10 17 13
3 15 3.8 8.8
4 20 3.5 4.3
5 25 3.0 4.0
6 30 0.9 2.2
Seed and glumes
7 0 180 200
8 0.5 140 150
9 1 150 170
10 2 100 140
11 5 92 140
12 10 100 140
13 15 11 76
14 20 24 56
15 25 17 24
16 30 8 11
1 Application rate was 2 kg/ha spray.
Table 2. Residues Resulting from Supervised Trials of Methiocarb, Thailand
Application
Crop Pest Rate No. Pre-harvest Residue
(kg/ha) Interval (mg/kg)
(days)
Cabbage Diamond back moth 1.0 1 7 0.16
Aphis
Chinese Kale Aphis 1.0 2 5 N.D.2
Phyllotreta sp.
Edible Rape Thrips 1.0 1 5 0.14
Phyllotreta sp.
Chinese Thrips 1.0 2 7 4.25
Radish Phyllotreta sp.
Broccoli Aphis 1.0 1 7 0.13
Yard long bean Aphis 1.45 1 7 N.D.
Thrips
Multiplier Thrips 2.5 1 7 0.02
onion
Mung bean Aphis 1.0 2 12 N.D.
1 50% wettable powder used in all treatments.
2 N.D. = not detectable
Table 3. Chemical Names, Structures and Designations of Methiocarb and its Metabolites Identified in Animals
Designation Chemical name Structure
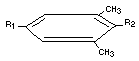 O
"
Methiocarb 3,5-dimethyl-4-(methylthio)=phenyl R1 = -O-C-NH-CH3
methylcarbamate
R2 = -SCH3
O
"
Methiocarb sulphoxide 3,5-dimethyl-4-(methylsul=phenyl)phenyl R1 = -O-C-NH-CH3
methylcarbamate
O
'
R2 = -SCH3
O
"
Methiocarb sulphone 3,5-dimethyl-4-(methylsul=phonyl)phenyl R1 = -O-C-NH-CH3
methylcarbamate
O
'
R2 = -SCH3
'
O
O
"
N-hydroxymethyl-methiocarb 3,5-dimethyl-4-(methylthio)=phenyl R1 = -O-C-NH-CH2OH
N-hydroxymethyl)carbamate
R2 = -SCH3
Table 3 (continued)
Designation Chemical name Structure
O
"
N-hydroxymethyl-methiocarb 3,5-dimethyl-4-(mathylsul=phinyl)phenyl R1 = -O-C-NH-CH2OH
sulphoxide N-hydroxymethyl)carbamate
O
'
R2 = -SCH3
Methiocarb phenol 3,5-dimethyl-4-(methythio)=phenol R1 = -OH
R2 = -SCH3
Methiocarb sulphoxide phenol 3,5-dimethyl-4-(methylsul=phinyl)phenol R1 = OH
O
'
R2 = -SCH3
Methiocarb sulphone phenol 3,5-dimethyl-4-(methylsul=phonyl)phenol R1 = -OH
O
'
R2 = -SCH3
'
O
Rats
Krishna & Casida (1966) studied the elimination of carbonyl-14C
methiocarb after intraperitoneal (i.p.) administration to rats. Within
48 hours, 66.1 percent of the applied radioactivity was expired as
14CO2, 22.3 percent excreted in the urine and 2.5 percent eliminated
in the faeces. Only 8.9 percent remained in the body.
The excretion and distribution of radioactivity were examined in
foetal and maternal tissues of rats after i.p. injection of
carbonyl-14C methiocarb (Wheeler & Strother 1974). The behaviour was
characterized by rapid maternal distribution and placental transfer.
Foetal kidney and heart contained the most radioactivity. Liver was
the maternal tissue with the highest 14C content. From the total
radioactivity recovered in the urine, faeces and exhaled air after 8
h, it appeared that the pregnant rat retained about 10 percent more
radioactivity than the nonpregnant animal.
Following a single oral application of unlabelled methiocarb to rats,
only minor quantities of the unchanged product and its phenol were
excreted in the urine during the 48 hours following administration
(van Hoof & Hendrickx 1975).
In Vitro Studies
The in vitro metabolism of methiocarb by liver, kidney and blood
fractions was studied in man, dog and rat (Strother 1970, 1972;
Wheeler & Strother 1971). Methiocarb was degraded in all preparations.
All species produced essentially the same major metabolites
(methiocarb sulphoxide and N-hydroxymethyl-methiocarb) although
quantitative differences were observed.
In studies with microsomal-NADPH2 systems of houseflies (Tsukamoto &
Casida 1967) and rats (Oonnithan & Casida 1968), sulphoxidation of
methiocarb was also found to be the main degradation pathway. In
vitro studies on houseflies revealed the formation of
N-hydroxymethyl-methiocarb as a further metabolite (Metcalf et al
19670
The request of the 1981 Meeting for analysis of ruminant liver and
kidney for N-hydroxy-methyl-methiocarb was reconsidered since, in one
of the studies cited by that Meeting, essentially all of the
radioactive residue in those tissues was accounted for and none of it
was that metabolite. Even if N-hydroxymethyl-methiocarb were present,
it would be measured by the recommended residue method for meat and
milk.
In view of the need to apply methiocarb to grain crops to prevent
destruction by plagues of birds, consideration needs to be given to
the consequences of feeding the straw to livestock or grazing
livestock on the stubbles. Such limited information as is available
suggests that the residues on the waste plant material would be quite
substantial. The animal transfer studies evaluated by the Meeting in
1981 were not adequate to demonstrate the fate of such residues. The
Meeting therefore was unable to make recommendations for MRLs in meat,
milk and eggs to deal with such practices. Further studies are needed.
In Plants
Beans
Following both foliar application and stem injection, carbonyl -14C
methiocarb was rapidly converted to the sulphoxide and sulphone
(Abdel-Wahab et al 1966). Six days after stem injection, a 64
percent loss of radioactivity was measured, which the authors
considered possibly resulted from expiration as 14CO2 (Table 4).
In Soil
Methiocarb is readily absorbed by soil but not desorbed. The parent
compound is classified as having only low mobility in leaching
studies. The degradation products of methiocarb also appear in only
very small amounts in the leachate.
In laboratory studies, which were conducted in accordance with
BBA-Merkblatt No. 37 (Biologische Bundesanstalt für Land- und
Forstwirtschaft 1980), the leaching behaviour of methiocarb,
formulated as 50 percent WP and as slug bait, methiocarb sulphoxide
and methiocarb sulphone was investigated. After the application of
methiocarb or its sulphone and addition of water equivalent to about
200 mm rainfall in 60 h, residues found in the leachate amounted to
less than 2.5 percent of the applied dose (Bayer 1974a, 1978a). In the
studies with methiocarb sulphoxide, the residues in the leachate
amounted to 3.8 percent (Bayer 1978G).
Laboratory degradation studies with the two standard soils specified
in BBA-Merkblatt No. 36 (Biologische Bundesanstalt für Land- und
Forstwirtschaft, 1976) gave half-lives of about four and six weeks for
methiocarb (Bayer 1974b).
A study of the adsorption of methiocarb sulphoxide from water by sandy
loam (Strankowski & Murphy 1982) was complicated by rapid degradation.
It was not feasible to measure the adsorption because the methiocarb
sulphoxide was rapidly hydrolysed to its phenol. Within half an hour,
24 percent decomposition had occurred in the soil/water system. A
maximum of 4 percent of the methiocarb sulphoxide was adsorbed, and
this decreased to 1 percent during the four-hour study.
In Rotational Crops
Wheat, sugarbeet and spinach were planted as rotational crops one year
after bare soil (sandy loam) had been treated with ring-1-14C
methiocarb at a field application rate of 5.6 kg a.i./ha (Strankowski
& Kottman 1979). Maize was used as the original crop in this soil. At
the time of planting the rotational crops, the soil contained total
radioactivity of 2.62 mg/kg methiocarb equivalents. The rotational
crops were grown to maturity and, at the time of harvest, each crop
matrix contained an average total radioactivity of slightly less than
0.1 mg/kg methiocarb equivalents. Samples of the immature crops
usually contained radioactivity equivalent to more than 0.1 mg/kg. The
carbamate residues found in each of the rotational crops by thin-layer
chromatography were <0.08 mg/kg (wheat heads, 0.022 mg/kg; wheat
stalks, 0.025 mg/kg; wheat forage, 0.080 mg/kg; spinach, 0.016 mg/kg;
sugarbeet roots, <0.014 mg/kg). Methiocarb metabolites identified in
these crops included N-hydroxymethyl-methiocarb, methiocarb
sulphoxide, N-hydroxymethyl-methiocarb sulphoxide, methiocarb
sulphoxide phenol, methiocarb sulphone and methiocarb sulphone phenol.
No methiocarb was detected. The radioactivity in the soil at the final
harvest was 1.57 mg/kg methiocarb equivalents.
In Water
Studies of methiocarb hydrolysis and its degradation in a pond
water/sediment combination were evaluated in 1981 (FAO/WHO 1982).
A further hydrolysis study was carried out according to the OECD Test
Method for determining hydrolysis as a function of pH in compliance
with the requirements of the German Biologische Bundesanstalt für
Land- und Forstwirtschaft (Wilmes 1983). The half-lives at different
temperatures and pH values were as follows:
pH 7 7 9 9
Temperature (°C) 40 50 20 30
Half-life (hours) 26.5 4.6 8.5 1.5
Photodecomposition
Methiocarb in solution and in the adsorbed state is degradable by
light in the laboratory. Owing to the ultraviolet adsorption
properties of the pesticide, direct photodegradation should also be
possible under environmental conditions. The most important primary
degradation reactions are hydrolysis to the phenol and oxidation to
the sulphoxide and sulphone.
Kumar et al (1974) examined the photolysis of methiocarb in aerated
and degassed ethanol and cyclohexane solutions. The exciting
wavelength was above 300 mm. Photolysis yielded only one major
product, methiocarb phenol.
Degradation by light on glass, silica gel and soil surfaces
(Houseworth & Tweedy 1974) was mentioned in the 1981 evaluation.
Crosby et al (1965) irradiated ethanolic solutions of methiocarb in
sunlight and found a number of cholinesterase-inhibiting products,
which were not identified.
Table 4. Distributien of Radioactivity among Methiocarb and its Metabolites in Bean Plants
% of applied label
In organic extract
Treatment Time after Methiocarb Methiocarb methiocarb In Unextracted Loss
last sulphoxide sulphone water
application phase
Foliage Treatment 0 h 99.3 0.4 0.1
14CO 2 h 93.8 4.6 0.2
80 µg - 190 mg/kg 4 h 84.9 8.7 0.5
8 h 70.8 9.3 0.8
1 day 68.8 9.9 1.1
2 days 60.0 8.3 2.2
3 days 55.4 6.7 1.9
Stem Injection 0 h 98.0 1.3 <0.1 0.3 0.4
14CO 12 h 49.0 19.8 2.3 4.2 11.5 13.2
25 µg = 12 mg/kg 1 day 19.8 23.3 8.2 7.2 18.0 23.5
2 days 2.2 5.5 5.5 8.2 25.3 53.3
3 days <0.1 4.7 1.8 8.7 24.0 60.8
4 days <0.1 3.21 8.1 26.1 62.6
6 days <0.1 1.31 7.7 27.5 63.5
1 Total found in chloroform extract after column clean-up. Thin-layer chromatography omitted.
METHODS OF RESIDUE ANALYSIS
In addition to the methods reviewed in 1981 (FAO/WHO 1982) the Meeting
was aware of several other developments that were considered useful.
Blass (1974) described a multi-residue method for the determination of
methiocarb and five other carbamate insecticides in plant material.
This method offers the possibility of determining the unchanged parent
compound following acylation or the total residue after phosporylation
of the phenols. Recoveries from lettuce, potatoes, apples and carrots
in the 0.5-5 mg/kg range were 70-104 percent.
Muth & Erro (1980) described a rapid multi-residue procedure for
determining carbamate residues in vegetable crops, using water as the
extracting solvent and adsorbing the proteinaceous material and
particulate matter on Filter-Cel before filtration. The clarified
extract was passed through a reverse-phase Sep-pak cartridge from
which the residue was eluted with methanol and determined by high
precision liquid chromatography (HPLC). Fifteen crops spiked at a
level of 5 mg/kg were analysed. Interferences were minimal and the
recovery of methiocarb was generally in the range 80-107 percent,
although parsley, artichokes and onions showed lower recoveries.
The method of Strankowski & Stanley, which was reviewed in FAO/WHO
1983, has since been published (Strankowski & Stanley 1981).
A gas-chromatographic method for the determination of carbaryl,
propoxur and methiocarb in vegetables and fruit has been elaborated at
the National Health Institute of The Netherlands (Anon. 1976). It
involves extraction with dichloromethane, followed by hydrolysis with
sodium hydroxide, steam distillation, derivatization with
1-chloro-2,6-dinitro-4-trifluoromethylbenzene and measurement by
gas-liquid chromatography (GLC). The lower limit of determination is
0.1 mg/kg. The sulphoxide and sulphone metabolites are not determined
by this method.
APPRAISAL
A considerable amount of information has been received since the
original evaluation was made in 1981. Some of this information was
directly related to the requests made on that occasion. It is clear
that many countries recognize the use of methiocarb bait (4 percent)
against slugs and snails in vegetables, grains, fruit and sugarbeets.
Some delegations to the 15th Session of the Codex Committee on
Pesticide Residues (CCPR) questioned whether it was good agricultural
practice to use methiocarb as a bird repellent to prevent destruction
of such crops as blueberries, cherries, currants and grapes. There can
be no doubt that numerous countries have accepted such uses as Being
essential to the protection of these crops. Methiocarb is also used to
protect cereal grain crops from attack by birds in Africa, New
Zealand, the United States and elsewhere. Studies made in Belgium
indicated that when methiocarb is used to protect cherries from birds,
the residues can be up to 15 mg/kg.
The Meeting gave special consideration to the toxicological
significance of the residues on apples, blueberries, cherries,
currants, grapes and peaches arising from the use of methiocarb as a
bird repellent. While recognizing that the majority of the fruits
would normally contain methiocarb residues at concentrations
considerably lower than the MRLs, the Meeting considered the
possibility that these commodities, consumed fresh, might contain
residues that could lead to an intake of methiocarb that exceeded the
demonstrated NOEL for plasma cholinesterase in dogs noted by the 1981
Meeting. The Meeting did not have available for review the details of
this dog experiment and therefore was unable to evaluate fully the
significance of this demonstrated inhibition of plasma cholinesterase.
Attention was drawn to para 3.2 of the report of the 1982 Meeting
dealing with the function of plasma cholinesterase. The Meeting agreed
to request that the relevant information should be made available to
it in 1984 to allow full consideration of the safety of the observed
residue levels. The Meeting recommended that the MRLs for the
commodities in question should be withdrawn pending this further
review.
Information concerning the use of methiocarb and the resulting
residues was received from Thailand. This supported several of the
recommendations already made for MRLs but also allowed the Meeting to
estimate a maximum residue level for Chinese radish.
Further information was available on the fate of methiocarb in
animals, plants, soil and water and the effect of light on deposits
and solutions. This only served to confirm the evaluations made in
1981. It is clear that methiocarb is rapidly degraded in plants, water
and soil so that there is little possibility of carry-over in crop
rotation. Similarly, because it is rapidly metabolized in animals,
there is unlikely to be any transfer of residues into foods of animal
origin following the feeding of forage or food offals to livestock.
However, this applies only to current practices and does not allow for
the possibility of livestock being grazed or fed on stubbles or other
plant parts from grain crops sprayed with methiocarb to repel birds.
Further methods of residue analysis have been noted. It was suggested
at the 15th Session of the CCPR that the limit of determination in
regulatory practice should be 0.1 mg/kg, not 0.02 mg/kg as proposed in
1981. The Meeting reconsidered this question and noted that in the
case of several commodities the apparent residue on untreated control
samples was frequently higher than that reported on the treated
samples. It was reasonable to expect that food control officials would
find difficulty in working to a limit of determination as low as 0.02
mg/kg. Bearing in mind the decision published in para. 2.4 of the 1981
Meeting report, the Meeting agreed not to recommend MRLs lower than
0.05 mg/kg.
RECOMMENDATIONS
The further information evaluated enabled the Meeting to recommend
additional MRLs. The previously recommended MRLs for strawberries,
sugarbeets and citrus fruit are increased to reflect the re-evaluated
limit of determination. Some MRLs are withdrawn pending toxicological
re-evaluation. MRLs refer to the sum of methiocarb, its sulphoxide and
its sulphone.
Commodity MRL Pre-harvest intervals
mg/kg on which recommendations
are based, days
*Chinese radishes 5 7
* Sorghum 5 21
Strawberries 0.05** 14
Sugarbeets 0.05** 7
Citrus fruit 0.05** 30
*Meat 0.05**1
*Milk 0.05**1
*Eggs 0.05**1
*Poultry 0.05**1
Apples
Blueberries
Cherries MRLs withdrawn pending
Currants(red) toxicological re-evaluation
Grapes by JMPR.
Peaches
* new recommendations
** at or about the limit of determination
1 These recommendations do not allow for the possible feeding of
straw and similar fodder from crops sprayed with methiocarb to
repel birds.
REFERENCES-RESIDUES
Abdel-Wahah, A.M., Kuhr, R.J. & Fate of C14 -carbonyl-labelled
Casida, J.E. aryl methyl-carbamate insecticide
1966 chemicals in and on bean plants,
J. Agric. Food Chem., 14: 290-298.
Anonymous. The gas-chromatographic
1976 determination of carbaryl, propoxur
and methiocarb in vegetables and
fruit (In Dutch). Report 39/76
TOX-ROB, February 1976.
Rijksinstituut Voor de
Volksgezondheid, Utrecht,
The Netherlands.
Anonymous. Pesticide and Toxic Chemical News,
1983 17 August, p. 20-21.
Bayer. Reports Nos. 2154-2159/74.
1974a
Bayer. Report No. 2153/74.
1974b
Bayer. Reports Nos. 2113-2115/78.
1978a
Bayer. Reports Nos. 2110-2112/78.
1978b
Bayer. Data on methiocarb residues from
1983 supervised trials on cauliflower
and maize. (Unpublished)
Biologische Bundesanstalt für Unterlagen zum Verhalten von
Land- und Forstwirtschaft. Pflanzenbehandlungsmitteln im
1976 Boden im Rahmen des
Zulassungsverfahrens. Merkblatt
Nr. 36, 2. Auflage.
Biologische Bundesanstalt für Prüfung des Versickerungsver
Land- und Forstwirtschaft. haltens von
1980 Pflanzenschutzbehandlungsmitteln.
Merkblatt Nr. 37, 2. Auflage.
Blass, W. The gas-chromatographic deter-
1974 mination of carbamate insecticides
in plant material. Mededelingen van
de Faculteit Landbouwwetenschappen
Rijksuniversiteit Gent,
39: 1337-1358.
Bruggers, R., Matee, J., Reduction of bird damage to field
Miskell, J., Erickson, W., crops in Eastern Africa with
Jaeger, M., Jackson, W.B. & methiocarb. Trop. Pest Manage
Juimale, Y. 27 (2): 230-241.
1981
Crosby, D.G., Leitis, E. & Photodecomposition of carbamate
Winterlin, W.L. insecticides J. Agric. Food Chem.,
1965 13: 204-207.
Gras, G., Hasselman, C., Residue analysis of methiocarb
Pellissier, C. & Bruggers, R. applied to ripening sorghum as a
1981 bird repellent in Senegal. Bull.
Environ. Contam. Toxicol.,
26: 393-400.
Houseworth, L.D. & Tweedy, B.G. Photodecomposition of MESUROL-ring-
1974. 1-14C. Mobay Report 39895
Hoyoux, J.M. & Zenon-Roland, L. Protection of cherries against
1979 damage by starlings. Determination
of methiocarb and its sulphoxide
and sulphone metabolites.
Parasitica, 35 (3): 95-106.
Krishna, J.G., Casida, J.E.: Fate in rats of the radiocarbon
1966 from ten variously labelled methyl-
and dimethylcarbamate-C14
insecticide chemicals and their
hydrolysis products. J. Agric. Food
Chem, 14, 98-105.
Kumar, Y., Semeluk, G.P., The Photochemistry of Carbamates
Silk, P.T., Unger, I.: VI. Chemosphere Vol. 3, 1974,
1974 23-27.
Metcalf, R.L., Osman, M.G., Metabolism of C14 -labelled
Fukuto, T.R.: carbamate insecticides to C14O2
1967 in the housefly. J. econ. Entomol.
60, 445-450.
Muth, G.L., Erro, F.: A rapid carbamate multiresidue
1980 procedure for vegetable crops
Bull. Environm. Contam. Toxicol
24, 759-765.
Oonnithan, E.S., Casida, J.E.: Oxidation of methyl-and
1968 dimethylcarbamate insecticide
chemicals by microsomal enzymes and
anticholinesterase activity of the
metabolites. J. Agric. Food Chem.
16, 28-44.
Pelissier, C., Analyse dans les graminées de
1978 variétés sorghum sp. des résidues
d'un répulsif utilisé dans la lutte
contre le quelia: Le methiocarb
Diplome d'Etudes Approfondes,
Faculté des Sciences, Universite de
Dakar, Dakar, Senegal. 83pp.
Strankowski, K.J., Kottman, R.F.: Radioactive residues of MESUROL in
1979 one-year rotational crops. Mobay
Report No. 66 925, January 1, 1979
(revised: May 18, 1979).
Strankowski, K.J., Murphy, J.J.: Adsorption of MESUROL Sulfoxide by
1982 Sandy Loam. Mobay Report No.
80 858, June 21, 1982.
Strankowski, K.J., and Determination of Residues of
Stanley, C.W. Mesurol and its sulphoxide and
1981 sulphone in plant, animal and soil
samples. J. Agric. Food Chem.
29, 1034-1037.
Strother, A.: Comparative metabolism of selected
1970 N-methylcarbamates by human and rat
liver fractions. Biochem.
Pharmacol. 19, 2525-2529,
Strother, A.: In vitro metabolism of
1972 methylcarbamate insecticides by
human and rat liver fractions.
Toxicol. appl. Pharmacol.
21, 112-129.
Tsukamoto, M., Casida, J.E.: Metabolism of methylcarbamate
1967 insecticides by the NADPH2 -
requiring enzyme system from
houseflies. Nature 7, 49-51.
van Hoof, F. & Henydrickx, A. The excretion in urine of four
1975 insecticidal carbamates and their
phenolic metabolites after oral
administration to rats. Arch.
Toxicol., 34: 81-88.
Wheeler, L., Strother, A.: In vitro metabolism of the N-
1971 methylcarbamates, ZECTRAN and
MESUROL, by liver, kidney and blood
of dogs and rats. J. Pharmacol.
exp. Ther. 178, 371-382.
Wilmes, R.: Hydrolytic stability of methiocarb
1983 as a function of pH. Report to
Biologische Bundesanstalt für Land-
und Forstwirtschaft (15.3.1983).
O
"
Methiocarb 3,5-dimethyl-4-(methylthio)=phenyl R1 = -O-C-NH-CH3
methylcarbamate
R2 = -SCH3
O
"
Methiocarb sulphoxide 3,5-dimethyl-4-(methylsul=phenyl)phenyl R1 = -O-C-NH-CH3
methylcarbamate
O
'
R2 = -SCH3
O
"
Methiocarb sulphone 3,5-dimethyl-4-(methylsul=phonyl)phenyl R1 = -O-C-NH-CH3
methylcarbamate
O
'
R2 = -SCH3
'
O
O
"
N-hydroxymethyl-methiocarb 3,5-dimethyl-4-(methylthio)=phenyl R1 = -O-C-NH-CH2OH
N-hydroxymethyl)carbamate
R2 = -SCH3
Table 3 (continued)
Designation Chemical name Structure
O
"
N-hydroxymethyl-methiocarb 3,5-dimethyl-4-(mathylsul=phinyl)phenyl R1 = -O-C-NH-CH2OH
sulphoxide N-hydroxymethyl)carbamate
O
'
R2 = -SCH3
Methiocarb phenol 3,5-dimethyl-4-(methythio)=phenol R1 = -OH
R2 = -SCH3
Methiocarb sulphoxide phenol 3,5-dimethyl-4-(methylsul=phinyl)phenol R1 = OH
O
'
R2 = -SCH3
Methiocarb sulphone phenol 3,5-dimethyl-4-(methylsul=phonyl)phenol R1 = -OH
O
'
R2 = -SCH3
'
O
Rats
Krishna & Casida (1966) studied the elimination of carbonyl-14C
methiocarb after intraperitoneal (i.p.) administration to rats. Within
48 hours, 66.1 percent of the applied radioactivity was expired as
14CO2, 22.3 percent excreted in the urine and 2.5 percent eliminated
in the faeces. Only 8.9 percent remained in the body.
The excretion and distribution of radioactivity were examined in
foetal and maternal tissues of rats after i.p. injection of
carbonyl-14C methiocarb (Wheeler & Strother 1974). The behaviour was
characterized by rapid maternal distribution and placental transfer.
Foetal kidney and heart contained the most radioactivity. Liver was
the maternal tissue with the highest 14C content. From the total
radioactivity recovered in the urine, faeces and exhaled air after 8
h, it appeared that the pregnant rat retained about 10 percent more
radioactivity than the nonpregnant animal.
Following a single oral application of unlabelled methiocarb to rats,
only minor quantities of the unchanged product and its phenol were
excreted in the urine during the 48 hours following administration
(van Hoof & Hendrickx 1975).
In Vitro Studies
The in vitro metabolism of methiocarb by liver, kidney and blood
fractions was studied in man, dog and rat (Strother 1970, 1972;
Wheeler & Strother 1971). Methiocarb was degraded in all preparations.
All species produced essentially the same major metabolites
(methiocarb sulphoxide and N-hydroxymethyl-methiocarb) although
quantitative differences were observed.
In studies with microsomal-NADPH2 systems of houseflies (Tsukamoto &
Casida 1967) and rats (Oonnithan & Casida 1968), sulphoxidation of
methiocarb was also found to be the main degradation pathway. In
vitro studies on houseflies revealed the formation of
N-hydroxymethyl-methiocarb as a further metabolite (Metcalf et al
19670
The request of the 1981 Meeting for analysis of ruminant liver and
kidney for N-hydroxy-methyl-methiocarb was reconsidered since, in one
of the studies cited by that Meeting, essentially all of the
radioactive residue in those tissues was accounted for and none of it
was that metabolite. Even if N-hydroxymethyl-methiocarb were present,
it would be measured by the recommended residue method for meat and
milk.
In view of the need to apply methiocarb to grain crops to prevent
destruction by plagues of birds, consideration needs to be given to
the consequences of feeding the straw to livestock or grazing
livestock on the stubbles. Such limited information as is available
suggests that the residues on the waste plant material would be quite
substantial. The animal transfer studies evaluated by the Meeting in
1981 were not adequate to demonstrate the fate of such residues. The
Meeting therefore was unable to make recommendations for MRLs in meat,
milk and eggs to deal with such practices. Further studies are needed.
In Plants
Beans
Following both foliar application and stem injection, carbonyl -14C
methiocarb was rapidly converted to the sulphoxide and sulphone
(Abdel-Wahab et al 1966). Six days after stem injection, a 64
percent loss of radioactivity was measured, which the authors
considered possibly resulted from expiration as 14CO2 (Table 4).
In Soil
Methiocarb is readily absorbed by soil but not desorbed. The parent
compound is classified as having only low mobility in leaching
studies. The degradation products of methiocarb also appear in only
very small amounts in the leachate.
In laboratory studies, which were conducted in accordance with
BBA-Merkblatt No. 37 (Biologische Bundesanstalt für Land- und
Forstwirtschaft 1980), the leaching behaviour of methiocarb,
formulated as 50 percent WP and as slug bait, methiocarb sulphoxide
and methiocarb sulphone was investigated. After the application of
methiocarb or its sulphone and addition of water equivalent to about
200 mm rainfall in 60 h, residues found in the leachate amounted to
less than 2.5 percent of the applied dose (Bayer 1974a, 1978a). In the
studies with methiocarb sulphoxide, the residues in the leachate
amounted to 3.8 percent (Bayer 1978G).
Laboratory degradation studies with the two standard soils specified
in BBA-Merkblatt No. 36 (Biologische Bundesanstalt für Land- und
Forstwirtschaft, 1976) gave half-lives of about four and six weeks for
methiocarb (Bayer 1974b).
A study of the adsorption of methiocarb sulphoxide from water by sandy
loam (Strankowski & Murphy 1982) was complicated by rapid degradation.
It was not feasible to measure the adsorption because the methiocarb
sulphoxide was rapidly hydrolysed to its phenol. Within half an hour,
24 percent decomposition had occurred in the soil/water system. A
maximum of 4 percent of the methiocarb sulphoxide was adsorbed, and
this decreased to 1 percent during the four-hour study.
In Rotational Crops
Wheat, sugarbeet and spinach were planted as rotational crops one year
after bare soil (sandy loam) had been treated with ring-1-14C
methiocarb at a field application rate of 5.6 kg a.i./ha (Strankowski
& Kottman 1979). Maize was used as the original crop in this soil. At
the time of planting the rotational crops, the soil contained total
radioactivity of 2.62 mg/kg methiocarb equivalents. The rotational
crops were grown to maturity and, at the time of harvest, each crop
matrix contained an average total radioactivity of slightly less than
0.1 mg/kg methiocarb equivalents. Samples of the immature crops
usually contained radioactivity equivalent to more than 0.1 mg/kg. The
carbamate residues found in each of the rotational crops by thin-layer
chromatography were <0.08 mg/kg (wheat heads, 0.022 mg/kg; wheat
stalks, 0.025 mg/kg; wheat forage, 0.080 mg/kg; spinach, 0.016 mg/kg;
sugarbeet roots, <0.014 mg/kg). Methiocarb metabolites identified in
these crops included N-hydroxymethyl-methiocarb, methiocarb
sulphoxide, N-hydroxymethyl-methiocarb sulphoxide, methiocarb
sulphoxide phenol, methiocarb sulphone and methiocarb sulphone phenol.
No methiocarb was detected. The radioactivity in the soil at the final
harvest was 1.57 mg/kg methiocarb equivalents.
In Water
Studies of methiocarb hydrolysis and its degradation in a pond
water/sediment combination were evaluated in 1981 (FAO/WHO 1982).
A further hydrolysis study was carried out according to the OECD Test
Method for determining hydrolysis as a function of pH in compliance
with the requirements of the German Biologische Bundesanstalt für
Land- und Forstwirtschaft (Wilmes 1983). The half-lives at different
temperatures and pH values were as follows:
pH 7 7 9 9
Temperature (°C) 40 50 20 30
Half-life (hours) 26.5 4.6 8.5 1.5
Photodecomposition
Methiocarb in solution and in the adsorbed state is degradable by
light in the laboratory. Owing to the ultraviolet adsorption
properties of the pesticide, direct photodegradation should also be
possible under environmental conditions. The most important primary
degradation reactions are hydrolysis to the phenol and oxidation to
the sulphoxide and sulphone.
Kumar et al (1974) examined the photolysis of methiocarb in aerated
and degassed ethanol and cyclohexane solutions. The exciting
wavelength was above 300 mm. Photolysis yielded only one major
product, methiocarb phenol.
Degradation by light on glass, silica gel and soil surfaces
(Houseworth & Tweedy 1974) was mentioned in the 1981 evaluation.
Crosby et al (1965) irradiated ethanolic solutions of methiocarb in
sunlight and found a number of cholinesterase-inhibiting products,
which were not identified.
Table 4. Distributien of Radioactivity among Methiocarb and its Metabolites in Bean Plants
% of applied label
In organic extract
Treatment Time after Methiocarb Methiocarb methiocarb In Unextracted Loss
last sulphoxide sulphone water
application phase
Foliage Treatment 0 h 99.3 0.4 0.1
14CO 2 h 93.8 4.6 0.2
80 µg - 190 mg/kg 4 h 84.9 8.7 0.5
8 h 70.8 9.3 0.8
1 day 68.8 9.9 1.1
2 days 60.0 8.3 2.2
3 days 55.4 6.7 1.9
Stem Injection 0 h 98.0 1.3 <0.1 0.3 0.4
14CO 12 h 49.0 19.8 2.3 4.2 11.5 13.2
25 µg = 12 mg/kg 1 day 19.8 23.3 8.2 7.2 18.0 23.5
2 days 2.2 5.5 5.5 8.2 25.3 53.3
3 days <0.1 4.7 1.8 8.7 24.0 60.8
4 days <0.1 3.21 8.1 26.1 62.6
6 days <0.1 1.31 7.7 27.5 63.5
1 Total found in chloroform extract after column clean-up. Thin-layer chromatography omitted.
METHODS OF RESIDUE ANALYSIS
In addition to the methods reviewed in 1981 (FAO/WHO 1982) the Meeting
was aware of several other developments that were considered useful.
Blass (1974) described a multi-residue method for the determination of
methiocarb and five other carbamate insecticides in plant material.
This method offers the possibility of determining the unchanged parent
compound following acylation or the total residue after phosporylation
of the phenols. Recoveries from lettuce, potatoes, apples and carrots
in the 0.5-5 mg/kg range were 70-104 percent.
Muth & Erro (1980) described a rapid multi-residue procedure for
determining carbamate residues in vegetable crops, using water as the
extracting solvent and adsorbing the proteinaceous material and
particulate matter on Filter-Cel before filtration. The clarified
extract was passed through a reverse-phase Sep-pak cartridge from
which the residue was eluted with methanol and determined by high
precision liquid chromatography (HPLC). Fifteen crops spiked at a
level of 5 mg/kg were analysed. Interferences were minimal and the
recovery of methiocarb was generally in the range 80-107 percent,
although parsley, artichokes and onions showed lower recoveries.
The method of Strankowski & Stanley, which was reviewed in FAO/WHO
1983, has since been published (Strankowski & Stanley 1981).
A gas-chromatographic method for the determination of carbaryl,
propoxur and methiocarb in vegetables and fruit has been elaborated at
the National Health Institute of The Netherlands (Anon. 1976). It
involves extraction with dichloromethane, followed by hydrolysis with
sodium hydroxide, steam distillation, derivatization with
1-chloro-2,6-dinitro-4-trifluoromethylbenzene and measurement by
gas-liquid chromatography (GLC). The lower limit of determination is
0.1 mg/kg. The sulphoxide and sulphone metabolites are not determined
by this method.
APPRAISAL
A considerable amount of information has been received since the
original evaluation was made in 1981. Some of this information was
directly related to the requests made on that occasion. It is clear
that many countries recognize the use of methiocarb bait (4 percent)
against slugs and snails in vegetables, grains, fruit and sugarbeets.
Some delegations to the 15th Session of the Codex Committee on
Pesticide Residues (CCPR) questioned whether it was good agricultural
practice to use methiocarb as a bird repellent to prevent destruction
of such crops as blueberries, cherries, currants and grapes. There can
be no doubt that numerous countries have accepted such uses as Being
essential to the protection of these crops. Methiocarb is also used to
protect cereal grain crops from attack by birds in Africa, New
Zealand, the United States and elsewhere. Studies made in Belgium
indicated that when methiocarb is used to protect cherries from birds,
the residues can be up to 15 mg/kg.
The Meeting gave special consideration to the toxicological
significance of the residues on apples, blueberries, cherries,
currants, grapes and peaches arising from the use of methiocarb as a
bird repellent. While recognizing that the majority of the fruits
would normally contain methiocarb residues at concentrations
considerably lower than the MRLs, the Meeting considered the
possibility that these commodities, consumed fresh, might contain
residues that could lead to an intake of methiocarb that exceeded the
demonstrated NOEL for plasma cholinesterase in dogs noted by the 1981
Meeting. The Meeting did not have available for review the details of
this dog experiment and therefore was unable to evaluate fully the
significance of this demonstrated inhibition of plasma cholinesterase.
Attention was drawn to para 3.2 of the report of the 1982 Meeting
dealing with the function of plasma cholinesterase. The Meeting agreed
to request that the relevant information should be made available to
it in 1984 to allow full consideration of the safety of the observed
residue levels. The Meeting recommended that the MRLs for the
commodities in question should be withdrawn pending this further
review.
Information concerning the use of methiocarb and the resulting
residues was received from Thailand. This supported several of the
recommendations already made for MRLs but also allowed the Meeting to
estimate a maximum residue level for Chinese radish.
Further information was available on the fate of methiocarb in
animals, plants, soil and water and the effect of light on deposits
and solutions. This only served to confirm the evaluations made in
1981. It is clear that methiocarb is rapidly degraded in plants, water
and soil so that there is little possibility of carry-over in crop
rotation. Similarly, because it is rapidly metabolized in animals,
there is unlikely to be any transfer of residues into foods of animal
origin following the feeding of forage or food offals to livestock.
However, this applies only to current practices and does not allow for
the possibility of livestock being grazed or fed on stubbles or other
plant parts from grain crops sprayed with methiocarb to repel birds.
Further methods of residue analysis have been noted. It was suggested
at the 15th Session of the CCPR that the limit of determination in
regulatory practice should be 0.1 mg/kg, not 0.02 mg/kg as proposed in
1981. The Meeting reconsidered this question and noted that in the
case of several commodities the apparent residue on untreated control
samples was frequently higher than that reported on the treated
samples. It was reasonable to expect that food control officials would
find difficulty in working to a limit of determination as low as 0.02
mg/kg. Bearing in mind the decision published in para. 2.4 of the 1981
Meeting report, the Meeting agreed not to recommend MRLs lower than
0.05 mg/kg.
RECOMMENDATIONS
The further information evaluated enabled the Meeting to recommend
additional MRLs. The previously recommended MRLs for strawberries,
sugarbeets and citrus fruit are increased to reflect the re-evaluated
limit of determination. Some MRLs are withdrawn pending toxicological
re-evaluation. MRLs refer to the sum of methiocarb, its sulphoxide and
its sulphone.
Commodity MRL Pre-harvest intervals
mg/kg on which recommendations
are based, days
*Chinese radishes 5 7
* Sorghum 5 21
Strawberries 0.05** 14
Sugarbeets 0.05** 7
Citrus fruit 0.05** 30
*Meat 0.05**1
*Milk 0.05**1
*Eggs 0.05**1
*Poultry 0.05**1
Apples
Blueberries
Cherries MRLs withdrawn pending
Currants(red) toxicological re-evaluation
Grapes by JMPR.
Peaches
* new recommendations
** at or about the limit of determination
1 These recommendations do not allow for the possible feeding of
straw and similar fodder from crops sprayed with methiocarb to
repel birds.
REFERENCES-RESIDUES
Abdel-Wahah, A.M., Kuhr, R.J. & Fate of C14 -carbonyl-labelled
Casida, J.E. aryl methyl-carbamate insecticide
1966 chemicals in and on bean plants,
J. Agric. Food Chem., 14: 290-298.
Anonymous. The gas-chromatographic
1976 determination of carbaryl, propoxur
and methiocarb in vegetables and
fruit (In Dutch). Report 39/76
TOX-ROB, February 1976.
Rijksinstituut Voor de
Volksgezondheid, Utrecht,
The Netherlands.
Anonymous. Pesticide and Toxic Chemical News,
1983 17 August, p. 20-21.
Bayer. Reports Nos. 2154-2159/74.
1974a
Bayer. Report No. 2153/74.
1974b
Bayer. Reports Nos. 2113-2115/78.
1978a
Bayer. Reports Nos. 2110-2112/78.
1978b
Bayer. Data on methiocarb residues from
1983 supervised trials on cauliflower
and maize. (Unpublished)
Biologische Bundesanstalt für Unterlagen zum Verhalten von
Land- und Forstwirtschaft. Pflanzenbehandlungsmitteln im
1976 Boden im Rahmen des
Zulassungsverfahrens. Merkblatt
Nr. 36, 2. Auflage.
Biologische Bundesanstalt für Prüfung des Versickerungsver
Land- und Forstwirtschaft. haltens von
1980 Pflanzenschutzbehandlungsmitteln.
Merkblatt Nr. 37, 2. Auflage.
Blass, W. The gas-chromatographic deter-
1974 mination of carbamate insecticides
in plant material. Mededelingen van
de Faculteit Landbouwwetenschappen
Rijksuniversiteit Gent,
39: 1337-1358.
Bruggers, R., Matee, J., Reduction of bird damage to field
Miskell, J., Erickson, W., crops in Eastern Africa with
Jaeger, M., Jackson, W.B. & methiocarb. Trop. Pest Manage
Juimale, Y. 27 (2): 230-241.
1981
Crosby, D.G., Leitis, E. & Photodecomposition of carbamate
Winterlin, W.L. insecticides J. Agric. Food Chem.,
1965 13: 204-207.
Gras, G., Hasselman, C., Residue analysis of methiocarb
Pellissier, C. & Bruggers, R. applied to ripening sorghum as a
1981 bird repellent in Senegal. Bull.
Environ. Contam. Toxicol.,
26: 393-400.
Houseworth, L.D. & Tweedy, B.G. Photodecomposition of MESUROL-ring-
1974. 1-14C. Mobay Report 39895
Hoyoux, J.M. & Zenon-Roland, L. Protection of cherries against
1979 damage by starlings. Determination
of methiocarb and its sulphoxide
and sulphone metabolites.
Parasitica, 35 (3): 95-106.
Krishna, J.G., Casida, J.E.: Fate in rats of the radiocarbon
1966 from ten variously labelled methyl-
and dimethylcarbamate-C14
insecticide chemicals and their
hydrolysis products. J. Agric. Food
Chem, 14, 98-105.
Kumar, Y., Semeluk, G.P., The Photochemistry of Carbamates
Silk, P.T., Unger, I.: VI. Chemosphere Vol. 3, 1974,
1974 23-27.
Metcalf, R.L., Osman, M.G., Metabolism of C14 -labelled
Fukuto, T.R.: carbamate insecticides to C14O2
1967 in the housefly. J. econ. Entomol.
60, 445-450.
Muth, G.L., Erro, F.: A rapid carbamate multiresidue
1980 procedure for vegetable crops
Bull. Environm. Contam. Toxicol
24, 759-765.
Oonnithan, E.S., Casida, J.E.: Oxidation of methyl-and
1968 dimethylcarbamate insecticide
chemicals by microsomal enzymes and
anticholinesterase activity of the
metabolites. J. Agric. Food Chem.
16, 28-44.
Pelissier, C., Analyse dans les graminées de
1978 variétés sorghum sp. des résidues
d'un répulsif utilisé dans la lutte
contre le quelia: Le methiocarb
Diplome d'Etudes Approfondes,
Faculté des Sciences, Universite de
Dakar, Dakar, Senegal. 83pp.
Strankowski, K.J., Kottman, R.F.: Radioactive residues of MESUROL in
1979 one-year rotational crops. Mobay
Report No. 66 925, January 1, 1979
(revised: May 18, 1979).
Strankowski, K.J., Murphy, J.J.: Adsorption of MESUROL Sulfoxide by
1982 Sandy Loam. Mobay Report No.
80 858, June 21, 1982.
Strankowski, K.J., and Determination of Residues of
Stanley, C.W. Mesurol and its sulphoxide and
1981 sulphone in plant, animal and soil
samples. J. Agric. Food Chem.
29, 1034-1037.
Strother, A.: Comparative metabolism of selected
1970 N-methylcarbamates by human and rat
liver fractions. Biochem.
Pharmacol. 19, 2525-2529,
Strother, A.: In vitro metabolism of
1972 methylcarbamate insecticides by
human and rat liver fractions.
Toxicol. appl. Pharmacol.
21, 112-129.
Tsukamoto, M., Casida, J.E.: Metabolism of methylcarbamate
1967 insecticides by the NADPH2 -
requiring enzyme system from
houseflies. Nature 7, 49-51.
van Hoof, F. & Henydrickx, A. The excretion in urine of four
1975 insecticidal carbamates and their
phenolic metabolites after oral
administration to rats. Arch.
Toxicol., 34: 81-88.
Wheeler, L., Strother, A.: In vitro metabolism of the N-
1971 methylcarbamates, ZECTRAN and
MESUROL, by liver, kidney and blood
of dogs and rats. J. Pharmacol.
exp. Ther. 178, 371-382.
Wilmes, R.: Hydrolytic stability of methiocarb
1983 as a function of pH. Report to
Biologische Bundesanstalt für Land-
und Forstwirtschaft (15.3.1983).
 Table 1. Persistence of methiocarb on sorghum, Bambey, Senegal, 1978.
Sample Interval Methiocarb residue (mg/kg)
No. after Thin-layer Gas
application1 chromatography chromatography
(days)
Seed only
1 5 20 36
2 10 17 13
3 15 3.8 8.8
4 20 3.5 4.3
5 25 3.0 4.0
6 30 0.9 2.2
Seed and glumes
7 0 180 200
8 0.5 140 150
9 1 150 170
10 2 100 140
11 5 92 140
12 10 100 140
13 15 11 76
14 20 24 56
15 25 17 24
16 30 8 11
1 Application rate was 2 kg/ha spray.
Table 2. Residues Resulting from Supervised Trials of Methiocarb, Thailand
Application
Crop Pest Rate No. Pre-harvest Residue
(kg/ha) Interval (mg/kg)
(days)
Cabbage Diamond back moth 1.0 1 7 0.16
Aphis
Chinese Kale Aphis 1.0 2 5 N.D.2
Phyllotreta sp.
Edible Rape Thrips 1.0 1 5 0.14
Phyllotreta sp.
Chinese Thrips 1.0 2 7 4.25
Radish Phyllotreta sp.
Broccoli Aphis 1.0 1 7 0.13
Yard long bean Aphis 1.45 1 7 N.D.
Thrips
Multiplier Thrips 2.5 1 7 0.02
onion
Mung bean Aphis 1.0 2 12 N.D.
1 50% wettable powder used in all treatments.
2 N.D. = not detectable
Table 3. Chemical Names, Structures and Designations of Methiocarb and its Metabolites Identified in Animals
Designation Chemical name Structure
Table 1. Persistence of methiocarb on sorghum, Bambey, Senegal, 1978.
Sample Interval Methiocarb residue (mg/kg)
No. after Thin-layer Gas
application1 chromatography chromatography
(days)
Seed only
1 5 20 36
2 10 17 13
3 15 3.8 8.8
4 20 3.5 4.3
5 25 3.0 4.0
6 30 0.9 2.2
Seed and glumes
7 0 180 200
8 0.5 140 150
9 1 150 170
10 2 100 140
11 5 92 140
12 10 100 140
13 15 11 76
14 20 24 56
15 25 17 24
16 30 8 11
1 Application rate was 2 kg/ha spray.
Table 2. Residues Resulting from Supervised Trials of Methiocarb, Thailand
Application
Crop Pest Rate No. Pre-harvest Residue
(kg/ha) Interval (mg/kg)
(days)
Cabbage Diamond back moth 1.0 1 7 0.16
Aphis
Chinese Kale Aphis 1.0 2 5 N.D.2
Phyllotreta sp.
Edible Rape Thrips 1.0 1 5 0.14
Phyllotreta sp.
Chinese Thrips 1.0 2 7 4.25
Radish Phyllotreta sp.
Broccoli Aphis 1.0 1 7 0.13
Yard long bean Aphis 1.45 1 7 N.D.
Thrips
Multiplier Thrips 2.5 1 7 0.02
onion
Mung bean Aphis 1.0 2 12 N.D.
1 50% wettable powder used in all treatments.
2 N.D. = not detectable
Table 3. Chemical Names, Structures and Designations of Methiocarb and its Metabolites Identified in Animals
Designation Chemical name Structure
 O
"
Methiocarb 3,5-dimethyl-4-(methylthio)=phenyl R1 = -O-C-NH-CH3
methylcarbamate
R2 = -SCH3
O
"
Methiocarb sulphoxide 3,5-dimethyl-4-(methylsul=phenyl)phenyl R1 = -O-C-NH-CH3
methylcarbamate
O
'
R2 = -SCH3
O
"
Methiocarb sulphone 3,5-dimethyl-4-(methylsul=phonyl)phenyl R1 = -O-C-NH-CH3
methylcarbamate
O
'
R2 = -SCH3
'
O
O
"
N-hydroxymethyl-methiocarb 3,5-dimethyl-4-(methylthio)=phenyl R1 = -O-C-NH-CH2OH
N-hydroxymethyl)carbamate
R2 = -SCH3
Table 3 (continued)
Designation Chemical name Structure
O
"
N-hydroxymethyl-methiocarb 3,5-dimethyl-4-(mathylsul=phinyl)phenyl R1 = -O-C-NH-CH2OH
sulphoxide N-hydroxymethyl)carbamate
O
'
R2 = -SCH3
Methiocarb phenol 3,5-dimethyl-4-(methythio)=phenol R1 = -OH
R2 = -SCH3
Methiocarb sulphoxide phenol 3,5-dimethyl-4-(methylsul=phinyl)phenol R1 = OH
O
'
R2 = -SCH3
Methiocarb sulphone phenol 3,5-dimethyl-4-(methylsul=phonyl)phenol R1 = -OH
O
'
R2 = -SCH3
'
O
Rats
Krishna & Casida (1966) studied the elimination of carbonyl-14C
methiocarb after intraperitoneal (i.p.) administration to rats. Within
48 hours, 66.1 percent of the applied radioactivity was expired as
14CO2, 22.3 percent excreted in the urine and 2.5 percent eliminated
in the faeces. Only 8.9 percent remained in the body.
The excretion and distribution of radioactivity were examined in
foetal and maternal tissues of rats after i.p. injection of
carbonyl-14C methiocarb (Wheeler & Strother 1974). The behaviour was
characterized by rapid maternal distribution and placental transfer.
Foetal kidney and heart contained the most radioactivity. Liver was
the maternal tissue with the highest 14C content. From the total
radioactivity recovered in the urine, faeces and exhaled air after 8
h, it appeared that the pregnant rat retained about 10 percent more
radioactivity than the nonpregnant animal.
Following a single oral application of unlabelled methiocarb to rats,
only minor quantities of the unchanged product and its phenol were
excreted in the urine during the 48 hours following administration
(van Hoof & Hendrickx 1975).
In Vitro Studies
The in vitro metabolism of methiocarb by liver, kidney and blood
fractions was studied in man, dog and rat (Strother 1970, 1972;
Wheeler & Strother 1971). Methiocarb was degraded in all preparations.
All species produced essentially the same major metabolites
(methiocarb sulphoxide and N-hydroxymethyl-methiocarb) although
quantitative differences were observed.
In studies with microsomal-NADPH2 systems of houseflies (Tsukamoto &
Casida 1967) and rats (Oonnithan & Casida 1968), sulphoxidation of
methiocarb was also found to be the main degradation pathway. In
vitro studies on houseflies revealed the formation of
N-hydroxymethyl-methiocarb as a further metabolite (Metcalf et al
19670
The request of the 1981 Meeting for analysis of ruminant liver and
kidney for N-hydroxy-methyl-methiocarb was reconsidered since, in one
of the studies cited by that Meeting, essentially all of the
radioactive residue in those tissues was accounted for and none of it
was that metabolite. Even if N-hydroxymethyl-methiocarb were present,
it would be measured by the recommended residue method for meat and
milk.
In view of the need to apply methiocarb to grain crops to prevent
destruction by plagues of birds, consideration needs to be given to
the consequences of feeding the straw to livestock or grazing
livestock on the stubbles. Such limited information as is available
suggests that the residues on the waste plant material would be quite
substantial. The animal transfer studies evaluated by the Meeting in
1981 were not adequate to demonstrate the fate of such residues. The
Meeting therefore was unable to make recommendations for MRLs in meat,
milk and eggs to deal with such practices. Further studies are needed.
In Plants
Beans
Following both foliar application and stem injection, carbonyl -14C
methiocarb was rapidly converted to the sulphoxide and sulphone
(Abdel-Wahab et al 1966). Six days after stem injection, a 64
percent loss of radioactivity was measured, which the authors
considered possibly resulted from expiration as 14CO2 (Table 4).
In Soil
Methiocarb is readily absorbed by soil but not desorbed. The parent
compound is classified as having only low mobility in leaching
studies. The degradation products of methiocarb also appear in only
very small amounts in the leachate.
In laboratory studies, which were conducted in accordance with
BBA-Merkblatt No. 37 (Biologische Bundesanstalt für Land- und
Forstwirtschaft 1980), the leaching behaviour of methiocarb,
formulated as 50 percent WP and as slug bait, methiocarb sulphoxide
and methiocarb sulphone was investigated. After the application of
methiocarb or its sulphone and addition of water equivalent to about
200 mm rainfall in 60 h, residues found in the leachate amounted to
less than 2.5 percent of the applied dose (Bayer 1974a, 1978a). In the
studies with methiocarb sulphoxide, the residues in the leachate
amounted to 3.8 percent (Bayer 1978G).
Laboratory degradation studies with the two standard soils specified
in BBA-Merkblatt No. 36 (Biologische Bundesanstalt für Land- und
Forstwirtschaft, 1976) gave half-lives of about four and six weeks for
methiocarb (Bayer 1974b).
A study of the adsorption of methiocarb sulphoxide from water by sandy
loam (Strankowski & Murphy 1982) was complicated by rapid degradation.
It was not feasible to measure the adsorption because the methiocarb
sulphoxide was rapidly hydrolysed to its phenol. Within half an hour,
24 percent decomposition had occurred in the soil/water system. A
maximum of 4 percent of the methiocarb sulphoxide was adsorbed, and
this decreased to 1 percent during the four-hour study.
In Rotational Crops
Wheat, sugarbeet and spinach were planted as rotational crops one year
after bare soil (sandy loam) had been treated with ring-1-14C
methiocarb at a field application rate of 5.6 kg a.i./ha (Strankowski
& Kottman 1979). Maize was used as the original crop in this soil. At
the time of planting the rotational crops, the soil contained total
radioactivity of 2.62 mg/kg methiocarb equivalents. The rotational
crops were grown to maturity and, at the time of harvest, each crop
matrix contained an average total radioactivity of slightly less than
0.1 mg/kg methiocarb equivalents. Samples of the immature crops
usually contained radioactivity equivalent to more than 0.1 mg/kg. The
carbamate residues found in each of the rotational crops by thin-layer
chromatography were <0.08 mg/kg (wheat heads, 0.022 mg/kg; wheat
stalks, 0.025 mg/kg; wheat forage, 0.080 mg/kg; spinach, 0.016 mg/kg;
sugarbeet roots, <0.014 mg/kg). Methiocarb metabolites identified in
these crops included N-hydroxymethyl-methiocarb, methiocarb
sulphoxide, N-hydroxymethyl-methiocarb sulphoxide, methiocarb
sulphoxide phenol, methiocarb sulphone and methiocarb sulphone phenol.
No methiocarb was detected. The radioactivity in the soil at the final
harvest was 1.57 mg/kg methiocarb equivalents.
In Water
Studies of methiocarb hydrolysis and its degradation in a pond
water/sediment combination were evaluated in 1981 (FAO/WHO 1982).
A further hydrolysis study was carried out according to the OECD Test
Method for determining hydrolysis as a function of pH in compliance
with the requirements of the German Biologische Bundesanstalt für
Land- und Forstwirtschaft (Wilmes 1983). The half-lives at different
temperatures and pH values were as follows:
pH 7 7 9 9
Temperature (°C) 40 50 20 30
Half-life (hours) 26.5 4.6 8.5 1.5
Photodecomposition
Methiocarb in solution and in the adsorbed state is degradable by
light in the laboratory. Owing to the ultraviolet adsorption
properties of the pesticide, direct photodegradation should also be
possible under environmental conditions. The most important primary
degradation reactions are hydrolysis to the phenol and oxidation to
the sulphoxide and sulphone.
Kumar et al (1974) examined the photolysis of methiocarb in aerated
and degassed ethanol and cyclohexane solutions. The exciting
wavelength was above 300 mm. Photolysis yielded only one major
product, methiocarb phenol.
Degradation by light on glass, silica gel and soil surfaces
(Houseworth & Tweedy 1974) was mentioned in the 1981 evaluation.
Crosby et al (1965) irradiated ethanolic solutions of methiocarb in
sunlight and found a number of cholinesterase-inhibiting products,
which were not identified.
Table 4. Distributien of Radioactivity among Methiocarb and its Metabolites in Bean Plants
% of applied label
In organic extract
Treatment Time after Methiocarb Methiocarb methiocarb In Unextracted Loss
last sulphoxide sulphone water
application phase
Foliage Treatment 0 h 99.3 0.4 0.1
14CO 2 h 93.8 4.6 0.2
80 µg - 190 mg/kg 4 h 84.9 8.7 0.5
8 h 70.8 9.3 0.8
1 day 68.8 9.9 1.1
2 days 60.0 8.3 2.2
3 days 55.4 6.7 1.9
Stem Injection 0 h 98.0 1.3 <0.1 0.3 0.4
14CO 12 h 49.0 19.8 2.3 4.2 11.5 13.2
25 µg = 12 mg/kg 1 day 19.8 23.3 8.2 7.2 18.0 23.5
2 days 2.2 5.5 5.5 8.2 25.3 53.3
3 days <0.1 4.7 1.8 8.7 24.0 60.8
4 days <0.1 3.21 8.1 26.1 62.6
6 days <0.1 1.31 7.7 27.5 63.5
1 Total found in chloroform extract after column clean-up. Thin-layer chromatography omitted.
METHODS OF RESIDUE ANALYSIS
In addition to the methods reviewed in 1981 (FAO/WHO 1982) the Meeting
was aware of several other developments that were considered useful.
Blass (1974) described a multi-residue method for the determination of
methiocarb and five other carbamate insecticides in plant material.
This method offers the possibility of determining the unchanged parent
compound following acylation or the total residue after phosporylation
of the phenols. Recoveries from lettuce, potatoes, apples and carrots
in the 0.5-5 mg/kg range were 70-104 percent.
Muth & Erro (1980) described a rapid multi-residue procedure for
determining carbamate residues in vegetable crops, using water as the
extracting solvent and adsorbing the proteinaceous material and
particulate matter on Filter-Cel before filtration. The clarified
extract was passed through a reverse-phase Sep-pak cartridge from
which the residue was eluted with methanol and determined by high
precision liquid chromatography (HPLC). Fifteen crops spiked at a
level of 5 mg/kg were analysed. Interferences were minimal and the
recovery of methiocarb was generally in the range 80-107 percent,
although parsley, artichokes and onions showed lower recoveries.
The method of Strankowski & Stanley, which was reviewed in FAO/WHO
1983, has since been published (Strankowski & Stanley 1981).
A gas-chromatographic method for the determination of carbaryl,
propoxur and methiocarb in vegetables and fruit has been elaborated at
the National Health Institute of The Netherlands (Anon. 1976). It
involves extraction with dichloromethane, followed by hydrolysis with
sodium hydroxide, steam distillation, derivatization with
1-chloro-2,6-dinitro-4-trifluoromethylbenzene and measurement by
gas-liquid chromatography (GLC). The lower limit of determination is
0.1 mg/kg. The sulphoxide and sulphone metabolites are not determined
by this method.
APPRAISAL
A considerable amount of information has been received since the
original evaluation was made in 1981. Some of this information was
directly related to the requests made on that occasion. It is clear
that many countries recognize the use of methiocarb bait (4 percent)
against slugs and snails in vegetables, grains, fruit and sugarbeets.
Some delegations to the 15th Session of the Codex Committee on
Pesticide Residues (CCPR) questioned whether it was good agricultural
practice to use methiocarb as a bird repellent to prevent destruction
of such crops as blueberries, cherries, currants and grapes. There can
be no doubt that numerous countries have accepted such uses as Being
essential to the protection of these crops. Methiocarb is also used to
protect cereal grain crops from attack by birds in Africa, New
Zealand, the United States and elsewhere. Studies made in Belgium
indicated that when methiocarb is used to protect cherries from birds,
the residues can be up to 15 mg/kg.
The Meeting gave special consideration to the toxicological
significance of the residues on apples, blueberries, cherries,
currants, grapes and peaches arising from the use of methiocarb as a
bird repellent. While recognizing that the majority of the fruits
would normally contain methiocarb residues at concentrations
considerably lower than the MRLs, the Meeting considered the
possibility that these commodities, consumed fresh, might contain
residues that could lead to an intake of methiocarb that exceeded the
demonstrated NOEL for plasma cholinesterase in dogs noted by the 1981
Meeting. The Meeting did not have available for review the details of
this dog experiment and therefore was unable to evaluate fully the
significance of this demonstrated inhibition of plasma cholinesterase.
Attention was drawn to para 3.2 of the report of the 1982 Meeting
dealing with the function of plasma cholinesterase. The Meeting agreed
to request that the relevant information should be made available to
it in 1984 to allow full consideration of the safety of the observed
residue levels. The Meeting recommended that the MRLs for the
commodities in question should be withdrawn pending this further
review.
Information concerning the use of methiocarb and the resulting
residues was received from Thailand. This supported several of the
recommendations already made for MRLs but also allowed the Meeting to
estimate a maximum residue level for Chinese radish.
Further information was available on the fate of methiocarb in
animals, plants, soil and water and the effect of light on deposits
and solutions. This only served to confirm the evaluations made in
1981. It is clear that methiocarb is rapidly degraded in plants, water
and soil so that there is little possibility of carry-over in crop
rotation. Similarly, because it is rapidly metabolized in animals,
there is unlikely to be any transfer of residues into foods of animal
origin following the feeding of forage or food offals to livestock.
However, this applies only to current practices and does not allow for
the possibility of livestock being grazed or fed on stubbles or other
plant parts from grain crops sprayed with methiocarb to repel birds.
Further methods of residue analysis have been noted. It was suggested
at the 15th Session of the CCPR that the limit of determination in
regulatory practice should be 0.1 mg/kg, not 0.02 mg/kg as proposed in
1981. The Meeting reconsidered this question and noted that in the
case of several commodities the apparent residue on untreated control
samples was frequently higher than that reported on the treated
samples. It was reasonable to expect that food control officials would
find difficulty in working to a limit of determination as low as 0.02
mg/kg. Bearing in mind the decision published in para. 2.4 of the 1981
Meeting report, the Meeting agreed not to recommend MRLs lower than
0.05 mg/kg.
RECOMMENDATIONS
The further information evaluated enabled the Meeting to recommend
additional MRLs. The previously recommended MRLs for strawberries,
sugarbeets and citrus fruit are increased to reflect the re-evaluated
limit of determination. Some MRLs are withdrawn pending toxicological
re-evaluation. MRLs refer to the sum of methiocarb, its sulphoxide and
its sulphone.
Commodity MRL Pre-harvest intervals
mg/kg on which recommendations
are based, days
*Chinese radishes 5 7
* Sorghum 5 21
Strawberries 0.05** 14
Sugarbeets 0.05** 7
Citrus fruit 0.05** 30
*Meat 0.05**1
*Milk 0.05**1
*Eggs 0.05**1
*Poultry 0.05**1
Apples
Blueberries
Cherries MRLs withdrawn pending
Currants(red) toxicological re-evaluation
Grapes by JMPR.
Peaches
* new recommendations
** at or about the limit of determination
1 These recommendations do not allow for the possible feeding of
straw and similar fodder from crops sprayed with methiocarb to
repel birds.
REFERENCES-RESIDUES
Abdel-Wahah, A.M., Kuhr, R.J. & Fate of C14 -carbonyl-labelled
Casida, J.E. aryl methyl-carbamate insecticide
1966 chemicals in and on bean plants,
J. Agric. Food Chem., 14: 290-298.
Anonymous. The gas-chromatographic
1976 determination of carbaryl, propoxur
and methiocarb in vegetables and
fruit (In Dutch). Report 39/76
TOX-ROB, February 1976.
Rijksinstituut Voor de
Volksgezondheid, Utrecht,
The Netherlands.
Anonymous. Pesticide and Toxic Chemical News,
1983 17 August, p. 20-21.
Bayer. Reports Nos. 2154-2159/74.
1974a
Bayer. Report No. 2153/74.
1974b
Bayer. Reports Nos. 2113-2115/78.
1978a
Bayer. Reports Nos. 2110-2112/78.
1978b
Bayer. Data on methiocarb residues from
1983 supervised trials on cauliflower
and maize. (Unpublished)
Biologische Bundesanstalt für Unterlagen zum Verhalten von
Land- und Forstwirtschaft. Pflanzenbehandlungsmitteln im
1976 Boden im Rahmen des
Zulassungsverfahrens. Merkblatt
Nr. 36, 2. Auflage.
Biologische Bundesanstalt für Prüfung des Versickerungsver
Land- und Forstwirtschaft. haltens von
1980 Pflanzenschutzbehandlungsmitteln.
Merkblatt Nr. 37, 2. Auflage.
Blass, W. The gas-chromatographic deter-
1974 mination of carbamate insecticides
in plant material. Mededelingen van
de Faculteit Landbouwwetenschappen
Rijksuniversiteit Gent,
39: 1337-1358.
Bruggers, R., Matee, J., Reduction of bird damage to field
Miskell, J., Erickson, W., crops in Eastern Africa with
Jaeger, M., Jackson, W.B. & methiocarb. Trop. Pest Manage
Juimale, Y. 27 (2): 230-241.
1981
Crosby, D.G., Leitis, E. & Photodecomposition of carbamate
Winterlin, W.L. insecticides J. Agric. Food Chem.,
1965 13: 204-207.
Gras, G., Hasselman, C., Residue analysis of methiocarb
Pellissier, C. & Bruggers, R. applied to ripening sorghum as a
1981 bird repellent in Senegal. Bull.
Environ. Contam. Toxicol.,
26: 393-400.
Houseworth, L.D. & Tweedy, B.G. Photodecomposition of MESUROL-ring-
1974. 1-14C. Mobay Report 39895
Hoyoux, J.M. & Zenon-Roland, L. Protection of cherries against
1979 damage by starlings. Determination
of methiocarb and its sulphoxide
and sulphone metabolites.
Parasitica, 35 (3): 95-106.
Krishna, J.G., Casida, J.E.: Fate in rats of the radiocarbon
1966 from ten variously labelled methyl-
and dimethylcarbamate-C14
insecticide chemicals and their
hydrolysis products. J. Agric. Food
Chem, 14, 98-105.
Kumar, Y., Semeluk, G.P., The Photochemistry of Carbamates
Silk, P.T., Unger, I.: VI. Chemosphere Vol. 3, 1974,
1974 23-27.
Metcalf, R.L., Osman, M.G., Metabolism of C14 -labelled
Fukuto, T.R.: carbamate insecticides to C14O2
1967 in the housefly. J. econ. Entomol.
60, 445-450.
Muth, G.L., Erro, F.: A rapid carbamate multiresidue
1980 procedure for vegetable crops
Bull. Environm. Contam. Toxicol
24, 759-765.
Oonnithan, E.S., Casida, J.E.: Oxidation of methyl-and
1968 dimethylcarbamate insecticide
chemicals by microsomal enzymes and
anticholinesterase activity of the
metabolites. J. Agric. Food Chem.
16, 28-44.
Pelissier, C., Analyse dans les graminées de
1978 variétés sorghum sp. des résidues
d'un répulsif utilisé dans la lutte
contre le quelia: Le methiocarb
Diplome d'Etudes Approfondes,
Faculté des Sciences, Universite de
Dakar, Dakar, Senegal. 83pp.
Strankowski, K.J., Kottman, R.F.: Radioactive residues of MESUROL in
1979 one-year rotational crops. Mobay
Report No. 66 925, January 1, 1979
(revised: May 18, 1979).
Strankowski, K.J., Murphy, J.J.: Adsorption of MESUROL Sulfoxide by
1982 Sandy Loam. Mobay Report No.
80 858, June 21, 1982.
Strankowski, K.J., and Determination of Residues of
Stanley, C.W. Mesurol and its sulphoxide and
1981 sulphone in plant, animal and soil
samples. J. Agric. Food Chem.
29, 1034-1037.
Strother, A.: Comparative metabolism of selected
1970 N-methylcarbamates by human and rat
liver fractions. Biochem.
Pharmacol. 19, 2525-2529,
Strother, A.: In vitro metabolism of
1972 methylcarbamate insecticides by
human and rat liver fractions.
Toxicol. appl. Pharmacol.
21, 112-129.
Tsukamoto, M., Casida, J.E.: Metabolism of methylcarbamate
1967 insecticides by the NADPH2 -
requiring enzyme system from
houseflies. Nature 7, 49-51.
van Hoof, F. & Henydrickx, A. The excretion in urine of four
1975 insecticidal carbamates and their
phenolic metabolites after oral
administration to rats. Arch.
Toxicol., 34: 81-88.
Wheeler, L., Strother, A.: In vitro metabolism of the N-
1971 methylcarbamates, ZECTRAN and
MESUROL, by liver, kidney and blood
of dogs and rats. J. Pharmacol.
exp. Ther. 178, 371-382.
Wilmes, R.: Hydrolytic stability of methiocarb
1983 as a function of pH. Report to
Biologische Bundesanstalt für Land-
und Forstwirtschaft (15.3.1983).
O
"
Methiocarb 3,5-dimethyl-4-(methylthio)=phenyl R1 = -O-C-NH-CH3
methylcarbamate
R2 = -SCH3
O
"
Methiocarb sulphoxide 3,5-dimethyl-4-(methylsul=phenyl)phenyl R1 = -O-C-NH-CH3
methylcarbamate
O
'
R2 = -SCH3
O
"
Methiocarb sulphone 3,5-dimethyl-4-(methylsul=phonyl)phenyl R1 = -O-C-NH-CH3
methylcarbamate
O
'
R2 = -SCH3
'
O
O
"
N-hydroxymethyl-methiocarb 3,5-dimethyl-4-(methylthio)=phenyl R1 = -O-C-NH-CH2OH
N-hydroxymethyl)carbamate
R2 = -SCH3
Table 3 (continued)
Designation Chemical name Structure
O
"
N-hydroxymethyl-methiocarb 3,5-dimethyl-4-(mathylsul=phinyl)phenyl R1 = -O-C-NH-CH2OH
sulphoxide N-hydroxymethyl)carbamate
O
'
R2 = -SCH3
Methiocarb phenol 3,5-dimethyl-4-(methythio)=phenol R1 = -OH
R2 = -SCH3
Methiocarb sulphoxide phenol 3,5-dimethyl-4-(methylsul=phinyl)phenol R1 = OH
O
'
R2 = -SCH3
Methiocarb sulphone phenol 3,5-dimethyl-4-(methylsul=phonyl)phenol R1 = -OH
O
'
R2 = -SCH3
'
O
Rats
Krishna & Casida (1966) studied the elimination of carbonyl-14C
methiocarb after intraperitoneal (i.p.) administration to rats. Within
48 hours, 66.1 percent of the applied radioactivity was expired as
14CO2, 22.3 percent excreted in the urine and 2.5 percent eliminated
in the faeces. Only 8.9 percent remained in the body.
The excretion and distribution of radioactivity were examined in
foetal and maternal tissues of rats after i.p. injection of
carbonyl-14C methiocarb (Wheeler & Strother 1974). The behaviour was
characterized by rapid maternal distribution and placental transfer.
Foetal kidney and heart contained the most radioactivity. Liver was
the maternal tissue with the highest 14C content. From the total
radioactivity recovered in the urine, faeces and exhaled air after 8
h, it appeared that the pregnant rat retained about 10 percent more
radioactivity than the nonpregnant animal.
Following a single oral application of unlabelled methiocarb to rats,
only minor quantities of the unchanged product and its phenol were
excreted in the urine during the 48 hours following administration
(van Hoof & Hendrickx 1975).
In Vitro Studies
The in vitro metabolism of methiocarb by liver, kidney and blood
fractions was studied in man, dog and rat (Strother 1970, 1972;
Wheeler & Strother 1971). Methiocarb was degraded in all preparations.
All species produced essentially the same major metabolites
(methiocarb sulphoxide and N-hydroxymethyl-methiocarb) although
quantitative differences were observed.
In studies with microsomal-NADPH2 systems of houseflies (Tsukamoto &
Casida 1967) and rats (Oonnithan & Casida 1968), sulphoxidation of
methiocarb was also found to be the main degradation pathway. In
vitro studies on houseflies revealed the formation of
N-hydroxymethyl-methiocarb as a further metabolite (Metcalf et al
19670
The request of the 1981 Meeting for analysis of ruminant liver and
kidney for N-hydroxy-methyl-methiocarb was reconsidered since, in one
of the studies cited by that Meeting, essentially all of the
radioactive residue in those tissues was accounted for and none of it
was that metabolite. Even if N-hydroxymethyl-methiocarb were present,
it would be measured by the recommended residue method for meat and
milk.
In view of the need to apply methiocarb to grain crops to prevent
destruction by plagues of birds, consideration needs to be given to
the consequences of feeding the straw to livestock or grazing
livestock on the stubbles. Such limited information as is available
suggests that the residues on the waste plant material would be quite
substantial. The animal transfer studies evaluated by the Meeting in
1981 were not adequate to demonstrate the fate of such residues. The
Meeting therefore was unable to make recommendations for MRLs in meat,
milk and eggs to deal with such practices. Further studies are needed.
In Plants
Beans
Following both foliar application and stem injection, carbonyl -14C
methiocarb was rapidly converted to the sulphoxide and sulphone
(Abdel-Wahab et al 1966). Six days after stem injection, a 64
percent loss of radioactivity was measured, which the authors
considered possibly resulted from expiration as 14CO2 (Table 4).
In Soil
Methiocarb is readily absorbed by soil but not desorbed. The parent
compound is classified as having only low mobility in leaching
studies. The degradation products of methiocarb also appear in only
very small amounts in the leachate.
In laboratory studies, which were conducted in accordance with
BBA-Merkblatt No. 37 (Biologische Bundesanstalt für Land- und
Forstwirtschaft 1980), the leaching behaviour of methiocarb,
formulated as 50 percent WP and as slug bait, methiocarb sulphoxide
and methiocarb sulphone was investigated. After the application of
methiocarb or its sulphone and addition of water equivalent to about
200 mm rainfall in 60 h, residues found in the leachate amounted to
less than 2.5 percent of the applied dose (Bayer 1974a, 1978a). In the
studies with methiocarb sulphoxide, the residues in the leachate
amounted to 3.8 percent (Bayer 1978G).
Laboratory degradation studies with the two standard soils specified
in BBA-Merkblatt No. 36 (Biologische Bundesanstalt für Land- und
Forstwirtschaft, 1976) gave half-lives of about four and six weeks for
methiocarb (Bayer 1974b).
A study of the adsorption of methiocarb sulphoxide from water by sandy
loam (Strankowski & Murphy 1982) was complicated by rapid degradation.
It was not feasible to measure the adsorption because the methiocarb
sulphoxide was rapidly hydrolysed to its phenol. Within half an hour,
24 percent decomposition had occurred in the soil/water system. A
maximum of 4 percent of the methiocarb sulphoxide was adsorbed, and
this decreased to 1 percent during the four-hour study.
In Rotational Crops
Wheat, sugarbeet and spinach were planted as rotational crops one year
after bare soil (sandy loam) had been treated with ring-1-14C
methiocarb at a field application rate of 5.6 kg a.i./ha (Strankowski
& Kottman 1979). Maize was used as the original crop in this soil. At
the time of planting the rotational crops, the soil contained total
radioactivity of 2.62 mg/kg methiocarb equivalents. The rotational
crops were grown to maturity and, at the time of harvest, each crop
matrix contained an average total radioactivity of slightly less than
0.1 mg/kg methiocarb equivalents. Samples of the immature crops
usually contained radioactivity equivalent to more than 0.1 mg/kg. The
carbamate residues found in each of the rotational crops by thin-layer
chromatography were <0.08 mg/kg (wheat heads, 0.022 mg/kg; wheat
stalks, 0.025 mg/kg; wheat forage, 0.080 mg/kg; spinach, 0.016 mg/kg;
sugarbeet roots, <0.014 mg/kg). Methiocarb metabolites identified in
these crops included N-hydroxymethyl-methiocarb, methiocarb
sulphoxide, N-hydroxymethyl-methiocarb sulphoxide, methiocarb
sulphoxide phenol, methiocarb sulphone and methiocarb sulphone phenol.
No methiocarb was detected. The radioactivity in the soil at the final
harvest was 1.57 mg/kg methiocarb equivalents.
In Water
Studies of methiocarb hydrolysis and its degradation in a pond
water/sediment combination were evaluated in 1981 (FAO/WHO 1982).
A further hydrolysis study was carried out according to the OECD Test
Method for determining hydrolysis as a function of pH in compliance
with the requirements of the German Biologische Bundesanstalt für
Land- und Forstwirtschaft (Wilmes 1983). The half-lives at different
temperatures and pH values were as follows:
pH 7 7 9 9
Temperature (°C) 40 50 20 30
Half-life (hours) 26.5 4.6 8.5 1.5
Photodecomposition
Methiocarb in solution and in the adsorbed state is degradable by
light in the laboratory. Owing to the ultraviolet adsorption
properties of the pesticide, direct photodegradation should also be
possible under environmental conditions. The most important primary
degradation reactions are hydrolysis to the phenol and oxidation to
the sulphoxide and sulphone.
Kumar et al (1974) examined the photolysis of methiocarb in aerated
and degassed ethanol and cyclohexane solutions. The exciting
wavelength was above 300 mm. Photolysis yielded only one major
product, methiocarb phenol.
Degradation by light on glass, silica gel and soil surfaces
(Houseworth & Tweedy 1974) was mentioned in the 1981 evaluation.
Crosby et al (1965) irradiated ethanolic solutions of methiocarb in
sunlight and found a number of cholinesterase-inhibiting products,
which were not identified.
Table 4. Distributien of Radioactivity among Methiocarb and its Metabolites in Bean Plants
% of applied label
In organic extract
Treatment Time after Methiocarb Methiocarb methiocarb In Unextracted Loss
last sulphoxide sulphone water
application phase
Foliage Treatment 0 h 99.3 0.4 0.1
14CO 2 h 93.8 4.6 0.2
80 µg - 190 mg/kg 4 h 84.9 8.7 0.5
8 h 70.8 9.3 0.8
1 day 68.8 9.9 1.1
2 days 60.0 8.3 2.2
3 days 55.4 6.7 1.9
Stem Injection 0 h 98.0 1.3 <0.1 0.3 0.4
14CO 12 h 49.0 19.8 2.3 4.2 11.5 13.2
25 µg = 12 mg/kg 1 day 19.8 23.3 8.2 7.2 18.0 23.5
2 days 2.2 5.5 5.5 8.2 25.3 53.3
3 days <0.1 4.7 1.8 8.7 24.0 60.8
4 days <0.1 3.21 8.1 26.1 62.6
6 days <0.1 1.31 7.7 27.5 63.5
1 Total found in chloroform extract after column clean-up. Thin-layer chromatography omitted.
METHODS OF RESIDUE ANALYSIS
In addition to the methods reviewed in 1981 (FAO/WHO 1982) the Meeting
was aware of several other developments that were considered useful.
Blass (1974) described a multi-residue method for the determination of
methiocarb and five other carbamate insecticides in plant material.
This method offers the possibility of determining the unchanged parent
compound following acylation or the total residue after phosporylation
of the phenols. Recoveries from lettuce, potatoes, apples and carrots
in the 0.5-5 mg/kg range were 70-104 percent.
Muth & Erro (1980) described a rapid multi-residue procedure for
determining carbamate residues in vegetable crops, using water as the
extracting solvent and adsorbing the proteinaceous material and
particulate matter on Filter-Cel before filtration. The clarified
extract was passed through a reverse-phase Sep-pak cartridge from
which the residue was eluted with methanol and determined by high
precision liquid chromatography (HPLC). Fifteen crops spiked at a
level of 5 mg/kg were analysed. Interferences were minimal and the
recovery of methiocarb was generally in the range 80-107 percent,
although parsley, artichokes and onions showed lower recoveries.
The method of Strankowski & Stanley, which was reviewed in FAO/WHO
1983, has since been published (Strankowski & Stanley 1981).
A gas-chromatographic method for the determination of carbaryl,
propoxur and methiocarb in vegetables and fruit has been elaborated at
the National Health Institute of The Netherlands (Anon. 1976). It
involves extraction with dichloromethane, followed by hydrolysis with
sodium hydroxide, steam distillation, derivatization with
1-chloro-2,6-dinitro-4-trifluoromethylbenzene and measurement by
gas-liquid chromatography (GLC). The lower limit of determination is
0.1 mg/kg. The sulphoxide and sulphone metabolites are not determined
by this method.
APPRAISAL
A considerable amount of information has been received since the
original evaluation was made in 1981. Some of this information was
directly related to the requests made on that occasion. It is clear
that many countries recognize the use of methiocarb bait (4 percent)
against slugs and snails in vegetables, grains, fruit and sugarbeets.
Some delegations to the 15th Session of the Codex Committee on
Pesticide Residues (CCPR) questioned whether it was good agricultural
practice to use methiocarb as a bird repellent to prevent destruction
of such crops as blueberries, cherries, currants and grapes. There can
be no doubt that numerous countries have accepted such uses as Being
essential to the protection of these crops. Methiocarb is also used to
protect cereal grain crops from attack by birds in Africa, New
Zealand, the United States and elsewhere. Studies made in Belgium
indicated that when methiocarb is used to protect cherries from birds,
the residues can be up to 15 mg/kg.
The Meeting gave special consideration to the toxicological
significance of the residues on apples, blueberries, cherries,
currants, grapes and peaches arising from the use of methiocarb as a
bird repellent. While recognizing that the majority of the fruits
would normally contain methiocarb residues at concentrations
considerably lower than the MRLs, the Meeting considered the
possibility that these commodities, consumed fresh, might contain
residues that could lead to an intake of methiocarb that exceeded the
demonstrated NOEL for plasma cholinesterase in dogs noted by the 1981
Meeting. The Meeting did not have available for review the details of
this dog experiment and therefore was unable to evaluate fully the
significance of this demonstrated inhibition of plasma cholinesterase.
Attention was drawn to para 3.2 of the report of the 1982 Meeting
dealing with the function of plasma cholinesterase. The Meeting agreed
to request that the relevant information should be made available to
it in 1984 to allow full consideration of the safety of the observed
residue levels. The Meeting recommended that the MRLs for the
commodities in question should be withdrawn pending this further
review.
Information concerning the use of methiocarb and the resulting
residues was received from Thailand. This supported several of the
recommendations already made for MRLs but also allowed the Meeting to
estimate a maximum residue level for Chinese radish.
Further information was available on the fate of methiocarb in
animals, plants, soil and water and the effect of light on deposits
and solutions. This only served to confirm the evaluations made in
1981. It is clear that methiocarb is rapidly degraded in plants, water
and soil so that there is little possibility of carry-over in crop
rotation. Similarly, because it is rapidly metabolized in animals,
there is unlikely to be any transfer of residues into foods of animal
origin following the feeding of forage or food offals to livestock.
However, this applies only to current practices and does not allow for
the possibility of livestock being grazed or fed on stubbles or other
plant parts from grain crops sprayed with methiocarb to repel birds.
Further methods of residue analysis have been noted. It was suggested
at the 15th Session of the CCPR that the limit of determination in
regulatory practice should be 0.1 mg/kg, not 0.02 mg/kg as proposed in
1981. The Meeting reconsidered this question and noted that in the
case of several commodities the apparent residue on untreated control
samples was frequently higher than that reported on the treated
samples. It was reasonable to expect that food control officials would
find difficulty in working to a limit of determination as low as 0.02
mg/kg. Bearing in mind the decision published in para. 2.4 of the 1981
Meeting report, the Meeting agreed not to recommend MRLs lower than
0.05 mg/kg.
RECOMMENDATIONS
The further information evaluated enabled the Meeting to recommend
additional MRLs. The previously recommended MRLs for strawberries,
sugarbeets and citrus fruit are increased to reflect the re-evaluated
limit of determination. Some MRLs are withdrawn pending toxicological
re-evaluation. MRLs refer to the sum of methiocarb, its sulphoxide and
its sulphone.
Commodity MRL Pre-harvest intervals
mg/kg on which recommendations
are based, days
*Chinese radishes 5 7
* Sorghum 5 21
Strawberries 0.05** 14
Sugarbeets 0.05** 7
Citrus fruit 0.05** 30
*Meat 0.05**1
*Milk 0.05**1
*Eggs 0.05**1
*Poultry 0.05**1
Apples
Blueberries
Cherries MRLs withdrawn pending
Currants(red) toxicological re-evaluation
Grapes by JMPR.
Peaches
* new recommendations
** at or about the limit of determination
1 These recommendations do not allow for the possible feeding of
straw and similar fodder from crops sprayed with methiocarb to
repel birds.
REFERENCES-RESIDUES
Abdel-Wahah, A.M., Kuhr, R.J. & Fate of C14 -carbonyl-labelled
Casida, J.E. aryl methyl-carbamate insecticide
1966 chemicals in and on bean plants,
J. Agric. Food Chem., 14: 290-298.
Anonymous. The gas-chromatographic
1976 determination of carbaryl, propoxur
and methiocarb in vegetables and
fruit (In Dutch). Report 39/76
TOX-ROB, February 1976.
Rijksinstituut Voor de
Volksgezondheid, Utrecht,
The Netherlands.
Anonymous. Pesticide and Toxic Chemical News,
1983 17 August, p. 20-21.
Bayer. Reports Nos. 2154-2159/74.
1974a
Bayer. Report No. 2153/74.
1974b
Bayer. Reports Nos. 2113-2115/78.
1978a
Bayer. Reports Nos. 2110-2112/78.
1978b
Bayer. Data on methiocarb residues from
1983 supervised trials on cauliflower
and maize. (Unpublished)
Biologische Bundesanstalt für Unterlagen zum Verhalten von
Land- und Forstwirtschaft. Pflanzenbehandlungsmitteln im
1976 Boden im Rahmen des
Zulassungsverfahrens. Merkblatt
Nr. 36, 2. Auflage.
Biologische Bundesanstalt für Prüfung des Versickerungsver
Land- und Forstwirtschaft. haltens von
1980 Pflanzenschutzbehandlungsmitteln.
Merkblatt Nr. 37, 2. Auflage.
Blass, W. The gas-chromatographic deter-
1974 mination of carbamate insecticides
in plant material. Mededelingen van
de Faculteit Landbouwwetenschappen
Rijksuniversiteit Gent,
39: 1337-1358.
Bruggers, R., Matee, J., Reduction of bird damage to field
Miskell, J., Erickson, W., crops in Eastern Africa with
Jaeger, M., Jackson, W.B. & methiocarb. Trop. Pest Manage
Juimale, Y. 27 (2): 230-241.
1981
Crosby, D.G., Leitis, E. & Photodecomposition of carbamate
Winterlin, W.L. insecticides J. Agric. Food Chem.,
1965 13: 204-207.
Gras, G., Hasselman, C., Residue analysis of methiocarb
Pellissier, C. & Bruggers, R. applied to ripening sorghum as a
1981 bird repellent in Senegal. Bull.
Environ. Contam. Toxicol.,
26: 393-400.
Houseworth, L.D. & Tweedy, B.G. Photodecomposition of MESUROL-ring-
1974. 1-14C. Mobay Report 39895
Hoyoux, J.M. & Zenon-Roland, L. Protection of cherries against
1979 damage by starlings. Determination
of methiocarb and its sulphoxide
and sulphone metabolites.
Parasitica, 35 (3): 95-106.
Krishna, J.G., Casida, J.E.: Fate in rats of the radiocarbon
1966 from ten variously labelled methyl-
and dimethylcarbamate-C14
insecticide chemicals and their
hydrolysis products. J. Agric. Food
Chem, 14, 98-105.
Kumar, Y., Semeluk, G.P., The Photochemistry of Carbamates
Silk, P.T., Unger, I.: VI. Chemosphere Vol. 3, 1974,
1974 23-27.
Metcalf, R.L., Osman, M.G., Metabolism of C14 -labelled
Fukuto, T.R.: carbamate insecticides to C14O2
1967 in the housefly. J. econ. Entomol.
60, 445-450.
Muth, G.L., Erro, F.: A rapid carbamate multiresidue
1980 procedure for vegetable crops
Bull. Environm. Contam. Toxicol
24, 759-765.
Oonnithan, E.S., Casida, J.E.: Oxidation of methyl-and
1968 dimethylcarbamate insecticide
chemicals by microsomal enzymes and
anticholinesterase activity of the
metabolites. J. Agric. Food Chem.
16, 28-44.
Pelissier, C., Analyse dans les graminées de
1978 variétés sorghum sp. des résidues
d'un répulsif utilisé dans la lutte
contre le quelia: Le methiocarb
Diplome d'Etudes Approfondes,
Faculté des Sciences, Universite de
Dakar, Dakar, Senegal. 83pp.
Strankowski, K.J., Kottman, R.F.: Radioactive residues of MESUROL in
1979 one-year rotational crops. Mobay
Report No. 66 925, January 1, 1979
(revised: May 18, 1979).
Strankowski, K.J., Murphy, J.J.: Adsorption of MESUROL Sulfoxide by
1982 Sandy Loam. Mobay Report No.
80 858, June 21, 1982.
Strankowski, K.J., and Determination of Residues of
Stanley, C.W. Mesurol and its sulphoxide and
1981 sulphone in plant, animal and soil
samples. J. Agric. Food Chem.
29, 1034-1037.
Strother, A.: Comparative metabolism of selected
1970 N-methylcarbamates by human and rat
liver fractions. Biochem.
Pharmacol. 19, 2525-2529,
Strother, A.: In vitro metabolism of
1972 methylcarbamate insecticides by
human and rat liver fractions.
Toxicol. appl. Pharmacol.
21, 112-129.
Tsukamoto, M., Casida, J.E.: Metabolism of methylcarbamate
1967 insecticides by the NADPH2 -
requiring enzyme system from
houseflies. Nature 7, 49-51.
van Hoof, F. & Henydrickx, A. The excretion in urine of four
1975 insecticidal carbamates and their
phenolic metabolites after oral
administration to rats. Arch.
Toxicol., 34: 81-88.
Wheeler, L., Strother, A.: In vitro metabolism of the N-
1971 methylcarbamates, ZECTRAN and
MESUROL, by liver, kidney and blood
of dogs and rats. J. Pharmacol.
exp. Ther. 178, 371-382.
Wilmes, R.: Hydrolytic stability of methiocarb
1983 as a function of pH. Report to
Biologische Bundesanstalt für Land-
und Forstwirtschaft (15.3.1983).
