
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
DDT
IDENTITY
Synonyms
Chlorophenothane, Dicophane; Zeidane, Gesarol(R), Neocid(R).
Explanation
Except where indicated otherwise in the text, the data here reviewed
relate to technical DDT (i.e. dichlorodiphenyltrichloroethane), which
is a mixture containing 75-80 per cent of the para para isomer to
which the following particulars apply:
Chemical name
1,1,1-trichloro-2,2-di-(p-chlorophenyl) ethane;
trichloro-di-(4'-chlorophenyl) ethane,
Formula
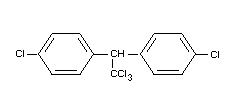 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
DDT is only slightly absorbed through the skin, but the degree of
absorption depends on the vehicle used (Cameron & Burgess, 1945).
After oral administration, most of the dose is found unchanged in the
faeces, but some absorption may occur, particularly in the presence of
lipids. The absorbed DDT is transformed into
2,2-di-(p-chlorophenyl)-1, 1-dichloro-ethylene (DDE) (Pearce et al.,
1952; Mattson et al., 1953) and into 2,2-di-(p-chlorophenyl)-acetic
acid (DDA) (Spicer et al., 1947; Judah, 1949, Hayes et al., 1956).
Some unknown compounds are also found (Spicer et al., 1947; Hayes et
al., 1956; Rothe et al., 1957; Cueto et al., 1956). Both DDT and DDE
accumulate in the fat (see Observations on Man). DDA is excreted in
the urine and in a combined form in the bile (Durham et al., 1963,
1965; Pinto et al., 1965).
A related insecticide, 1,1-dichloro-2,2-di-(p-chlorophenyl)-ethane
(DDD) was found in the liver of rats fed DDT (Klein et al., 1964).
Evidence that the conversion from DDT to DDD is accomplished by the
intestinal flora of the rat has also been presented (Mendel & Walton,
1966).
A level of 500 ppm of DDT in the diet of rats for 2 weeks or injection
of 25 mg/kg daily for 3 days increased the hepatic microsomal enzymic
metabolism of a number of drugs (Harts & Fouts, 1963). A similar
effect was observed in monkeys injected with 5 mg/kg/day for 7 days
(Juchau et al., 1966). As little as 1 mg/kg to rats was sufficient to
reduce pentobarbital sleeping time (Gerboth & Schwabe, 1964). Also
Morello (1966) showed that 3-4 days after a single dose of DDT in the
rat the ability of the liver microsomes to metabolize DDT was markedly
increased. It has been postulated by Street & Blau (1966) that DDT
also enhances the metabolism of dieldrin by microsomal enzymes; as
little as 5 ppm of DDT in the diet of rats decreased the fat storage
of simultaneously ingested dieldrin. However, a single dose of 75
mg/kg a week before treatment with carbon tetrachloride reduced the
LD50 of the latter in rats given either a complete or a protein-free
diet (McLean & McLean, 1966). Furthermore, dietary levels of 5-200 ppm
of DDT decreased the liver glucose-6-phosphate dehydrogenase activity
in rats (Tinsley, 1965).
In adult rats, concentrations of 10 or 100 ppm of DDT in the diet have
been shown to interfere with the storage and metabolism of vitamin A
in the liver; this was not observed in new-born or young rats which
have poor reserves of vitamin A (Phillips, 1963; Read et al., 1965).
In addition, whereas liver carboxylesterase activity was markedly
increased by DDT in weanling and adult rats, the same effect could not
be demonstrated in new-born rats (Read et al., 1965).
The toxic effects, revealing involvement of the central nervous system
(such as hypersensitivity, excitability, generalized trembling,
convulsions, paralysis) appear within 5-10 minutes of intravenous
administration and after a latent period of some hours following oral
administration. Death occurs as a result of respiratory arrest in the
rat, rabbit, cat and monkey, and of ventricular fibrillation in the
dog. It is the consensus of the literature that the significant action
of DDT is on the nervous system; when ventricular fibrillation occurs,
it is precipitated by adrenaline released from the adrenal medulla by
stimulation of the sympathetic nervous system (Phillips & Gilman,
1946).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 150-400* Draize et al., 1944
Bishopp, 1946
Rat new-born Intragastric >4 000 Lu et al., 1965
Rat pre-weanling Oral 437 Lu et al., 1965
Rat adult Oral 194 Lu et al., 1965
Rat Oral 150-420* Smith & Stohlman, 1944
Woodard et al., 1944
Rat Oral 800 Cameron & Burgess, 1945
Guinea-pig Oral 400 Draize et al., 1944
Rabbit Oral 250-500* Cameron & Burgess, 1945
Bishopp, 1946
Dog Intravenous Approximately Philips & Gilman, 1946
50
Cat Oral 400-600* Philips & Gilman, 1946
Monkey Oral >200 Bishopp, 1946
Horse Oral >300 Bishopp, 1946
Chicken Oral >1 300 Bishopp, 1946
* The LD50 dose of DDT varies within wide limits, depending on sex and the
type of vehicle used.
In rats given a single oral dose of DDT sufficient to kill about half
of them, the severity of symptoms corresponded with the concentration
of the unchanged compound in the brain (Dale et al., 1963).
Furthermore, approximately the same concentration of DDT is found in
the brain of rats killed by DDT no matter whether the dosage is acute,
subacute, or chronic (Hayes & Dale, 1964).
The fatal dose for man is difficult to establish but is generally
taken to be of the order of 500 mg/kg body-weight. A dose of the order
of 10 mg/kg body-weight can give rise in some subjects, but not in
all, to toxic symptoms (nausea, headache, sweating), and with doses of
16 mg/kg body-weight upwards, convulsions often occur (Anon., 1951).
Special studies
Daily oral doses of 0.2 mg/kg for 10 days produced functional changes
in the conditioned reflex pattern which appeared after 5-7 doses and
persisted 5-7 days after the end of exposure (Andronova, 1956).
Behavioural studies have also been made on groups of rats fed diets
containing 100, 200, 400 and 600 ppm DDT. Problem-solving and speed of
locomotion were unaffected by these doses of DDT. There was a
significant alteration in the patterns of locomotion in these rats and
their reactions to stress involving visual stimuli were reduced
(Khairy, 1959).
Short-term studies
Rat. Rats, in groups of 8 males and 8 females, were given diets with
1, 5, 10, and 50 ppm of DDT for 23 weeks. At concentrations of 5 ppm
and higher, histopathological changes in the liver were found (Laug et
al., 1950).
A series of experiments was carried out with small groups of rats, 178
males and 104 females in all, which were given diets containing DDT in
concentrations from traces up to 5000 ppm for periods ranging from one
to 14 months. In some cases observation of the animals continued after
cessation of treatment. At levels above 400 ppm changes in growth rate
were noted. Above 200 ppm liver enlargement was seen. In the male
animals with intakes of 5 ppm and above, specific histological lesions
in the liver were seen. These lesions consisted of hypertrophy of the
parenchymal cells, increased lipid deposits, marginal localization of
cytoplasmic granules and, above all, the appearance of complex
cytoplasmic inclusions of a lipid nature, called "lipospheres". In the
females these liver lesions were only seen in diets containing 200 ppm
or more. Necrotic lesions were seen only at concentrations above 1000
ppm. The authors noted that lipospheres and other changes considered
characteristic of DDT were more prominent in male rats although
females store more of the compound and were more susceptible to
poisoning. For this and other reasons, they speculated that the
changes might be adaptive. They noted also that earlier work had
failed to demonstrate similar changes in other species (Ortega et al.,
1956).
In an electron-microscopical examination of the liver of rats fed
diets containing 5-2500 ppm of DDT for 2-18 months, the following
cytoplasmic abnormalities were found: slight proliferation of the
endoplasmic reticulum, peripheral distribution of the ribosomes and
intracytoplasmic inclusions consisting of aggregates of membranes very
rich in lipids. At 100 ppm changes were seen after 2 months (Ortega,
1962, 1966).
Similar changes were found in another study in which the dietary
intake of DDT was 10 ppm. The accumulation of endoplasmic reticulum
has been regarded as evidence of cellular hypertrophy (Stemmer &
Hamdi, 1964).
In one study in which obesity was induced in rats with a high fat
diet, DDT was added into the diet at a dose level of 3.6 mg/kg/day for
up to 10 months. A comparable incidence of chloroleukemia was observed
in rats receiving DDT and in those given the high fat diet only
(Kimbrough et al., 1964).
Dog. Dogs were given DDT orally. Those receiving 100 mg/kg
body-weight daily died within 7 weeks. Animals subjected to lower
doses survived and seemed normal even after 50 weeks (Draize et al.,
1944). When the experiment was continued for 3 years, the animals
receiving doses of 50 and 80 mg/kg bodyweight developed jaundice and
haemorrhagic symptoms. Those receiving 10 mg/kg body-weight daily
showed no ill effects (Hayes et al., 1956; Lehman, 1952).
Dogs were given DDT by stomach-tube, in the form of a 10 per cent
solution in peanut oil in doses ranging from 150 to 350 mg/kg
body-weight for about 90 days. Some of the animals died. The authors
noted neurological symptoms, showing involvement of the cerebellum as
revealed by histological lesions (Haymaker et al., 1946).
Three dogs were given intramuscular injections of DDT in the form of a
10 per cent solution in olive oil at the rate of 100 mg/kg body-weight
daily for 25 to 30 days. A control animal received 1 ml of olive oil
in the same way. The experimental animals showed a temporary loss of
weight. The kidneys were discoloured and on histological examination
showed tubular damage. At the same time, proliferation was observed in
the lymphoid tissues (lymph nodes, spleen, bone marrow) and the wall of
the small intestine, but the blood showed no leukaemic characteristics
(Gerebtzoff et al., 1950; Gerebtzoff & Philippot, 1952).
Monkey. Monkeys given DDT orally in a dose of 0.2 mg/kg body-weight
for 7-9 months developed symptoms of hepatitis. After a year, the
animals showed liver enlargement and hyperglycaemia (Shillinger et
al., 1955). These liver changes were not confirmed in another
experiment on monkeys lasting 7.5 years (Durham et al., 1963).
Long-term studies
Mouse. In one experiment, 683 mice, spread over 5 generations, were
given DDT at 0.3-0.6 mg/kg daily. The background DDT content of the
diet given to 406 controls corresponded to an intake of 0.03-0.05
mg/kg daily. The over-all incidence of leukaemia and other malignant
tumours was 3.5 and 5.4 per cent respectively in the experimental
animals compared to 0.2 and 0.9 per cent in the controls. The
difference was obvious within each generation. The greatest tumour
incidence was seen in the fourth and fifth generations (Kemény &
Tarján, 1966).
Rat. Groups of 12 male rats were subjected for 2 years to diets
containing 0, 100, 200, 400 and 800 ppm of DDT, in the form of a 10
per cent solution in corn oil. In another experiment, groups each of
24 rats (12 males and 12 females) were given, during the same period,
diets containing 0, 100, 200, 400 and 800 ppm. Also additional groups
of 24 animals received 600 and 800 ppm incorporated in their feed in a
dry state. In the groups receiving 400 ppm and above, an increase in
the mortality rate was seen in relation to the dose. Apart from
nervous symptoms at doses of 400 ppm and above, typical liver lesions
were found at all concentrations. Hepatic cell tumours were seen in 4
out of 75 animals and 11 other rats showed nodular adenomatoid
hyperplasia (Fitzhugh & Nelson, 1947).
In an experiment with rats fed for 2 years on a diet containing 10 ppm
of DDT, histological liver lesions were also observed (Fitzhugh,
1948). Groups of 80 young rats each (40 male and 40 female) were fed
0, 0.25, 12.5 and 25 ppm of DDT. The histological lesions of the liver
observed were always slight and, according to the authors,
non-specific, but nevertheless they were more frequent with DDT than
with aldrin or dieldrin, which were administered in the same
concentrations for comparative purposes (Treon & Cleveland, 1955).
Experiments with 25 young rats did not show any harmful effects after
daily administration by stomach-tube of a dose of 10 µg/kg for 17
months (Klimmer, 1955).
Monkey. Twenty-four Rhesus monkeys, 12 males and 12 females, were
divided into groups and fed over periods as long as 7.5 years or more
on diets containing respectively 0, 5, 50, 200 and 5000 ppm of DDT.
All the animals subjected to the concentration of 5000 ppm showed
convulsions, accompanied by loss of appetite and a fall in weight. At
a concentration of 200 ppm (corresponding to daily doses of 2.2-5.54
mg/kg body-weight), no harmful effects were observed and, in
particular, no histological lesions in the liver or disturbances in
the functioning of that organ, as shown by the bromsulfthalein test
(Durham et al., 1963).
Observations on man. An experimental study was carried out of the
effects on man of prolonged ingestion of small doses of DDT (in the
form of oily solutions in capsules or emulsions in milk). The authors
used 51 volunteers for this study; 17 received a normal diet, 17
received 3.5 mg/kg body-weight and 17 received 35 mg DDT daily. The
last dose is approximately 0.5 mg/kg body-weight. Administration was
continued for as long as 18 months. The authors noted that the
accumulation of the insecticide in the fatty tissues and the urinary
excretion of its metabolite, DDA, were proportional to the dose of DDT
ingested. A state of equilibrium was reached after about a year and
the concentration of DDT accumulated in the fatty tissues reached an
average of 234 ppm (101-367 ppm) in subjects who had ingested 35 mg of
DDT per day. Throughout the whole experiment, no subject complained of
malaise, nor did any ill-effects appear that could be attributed to
the ingestion of DDT (Hayes et al., 1956). The essential results were
confirmed in a separate investigation in which dosage with DDT lasted
for 21 months and the volunteers were observed for an additional 27
months. Fourteen men received 35 mg/man/ day; 6 received 3.5
mg/man/day, and 4 men served as controls. The study also revealed the
relationship between storage of DDT and the urinary excretion of DDA
and demonstrated that the loss of DDT from storage in man following
cessation of dosage is slow - only about two-thirds in 27 months
(Hayes et al., 1964).
Observations were made on 40 workers engaged, over a period of years,
in the manufacturing of specialities based on DDT, under conditions
where suitable precautions had not been taken to protect their skin.
According to the DDA concentration found in the urine, these subjects
had absorbed daily as much DDT as volunteers who had ingested 35 mg of
the insecticide per day in the experiment mentioned above. Thorough
medical and biological tests failed to reveal any toxic symptoms, even
in workers exposed to the toxic product for 6.5 years (Ortelee, 1958).
Several studies on DDT and DDE storage in human fat in several parts
of the world have been presented (Laug, 1951; Hayes et al., 1956;
Hayes et al., 1958; Denes 1962; Maier-Bode, 1960; Dale et al., 1963;
Hunter et al., 1963; Hoffmann et al., 1964; Dale et al., 1965; Egan et
al., 1965; Halacka, et al., 1965; Quinby et al., 1965b; Robinson et
al., 1965; Zavon et al., 1965). DDT was also found in a stillborn
infant and in very young babies (Halacka et al., 1965).
In these studies the average total content in human fat ranged between
2 and 30 ppm; the percentage of DDE of total DDT stored ranged between
34 and 77 per cent. It has been shown that the storage of DDT in man
is directly proportional to intake over a wide range of doses (0.04 to
35.0 mg/man/day), so that from the level in the fat the daily dose can
be estimated. Thus, it can be calculated that the highest average
intake of DDT of any human population yet observed is about 0.7-0.8
mg/man/day. From the data which have been reported, it is also
apparent that in the United States of America, where the level of DDT
in human fat has been repeatedly investigated in the general
population over a significant number of years, it has not increased
since 1950 (Durham et al., 1965).
It has also been repeatedly indicated that women and cows secrete DDT
in the milk. Two recent studies indicated an average of total DDT
(i.e. DDT + DDE) of 0.128 ppm (57 per cent as DDE) and 0.170 ppm (58
per cent as DDE). The content of DDT in cows' milk was found to be
lower (Egan et al., 1965; Quinby et al., 1965). The percentage of the
total DDT intake which is eliminated in the milk has been demonstrated
to be smaller in cows than in women (Quinby et al., 1965).
Comments
The evaluation of DDT is difficult because wide species differences
occur. The rat appears to be the most susceptible species; liver
cellular changes were produced by 5 ppm in the diet. In dogs and
monkeys these changes were not seen. Dogs given 10 mg/kg
body-weight/day for 3 years and monkeys given 2-5 mg/kg
body-weight/day for up to 7 years showed no ill-effects. However, in
another study, monkeys given 0.2 mg/kg body-weight/day for one year
showed signs of hepatitis and liver enlargement. In man, as much as
0.5 mg/kg body-weight/day for up to 21 months were without effect
apart from storage in the fat. However, the possibility that the
latter might have deleterious consequences later in life cannot be
ruled out. In addition, it has not been demonstrated that metabolic
changes in the liver cells comparable to those observed in rats do not
take place in man. The Committee realizes that the figure reached as
an estimate for an ADI can be less than the actual intake for humans
in some parts of the world. It might correspond to the intake of DDT
of a baby exclusively breast-fed. (In this respect the significance of
the lower acute toxicity of DDT for new-born rats is uncertain.)
Furthermore, in addition to unchanged DDT, the residues also contain
metabolites of DDT. There is a need for uniformity in reporting the
results of residue analyses. The reports should at least indicate the
percentages of unchanged DDT, DDE and TDE. Some of the metabolites are
probably less toxic than DDT itself. However, more information is
necessary on this point. At the present time the Committee remains
concerned about the storage of DDT which occurs in all species and
about the cellular changes produced in the liver of rats by DDT and by
other compounds chemically related to it.
TOXICOLOGICAL EVALUATION
Estimate of acceptable daily intake in man
0-0.01 mg/kg body-weight
Further work required
Elucidation of the significance of the finding that DDT is one of the
compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
DDT is used to a minor extent as a soil treatment, primarily for the
control of cutworms which attack vegetable crops. It is suggested for
use in many countries on a wide variety of food crops. It is suggested
for the control of about 20 different insects which attack cane
fruits, about 50 different insects which attack vegetables, 50
different insects which attack tree fruits, as well as for control of
insects of nut trees and other food crops. The usual rate of
application is about 1 to 2 lb of the active chemical per acre;
however, some treatments may go as high as 10 lb per acre.
(b) Post-harvest treatments
Direct applications to stored food products are no longer advised.
However, there is some use in and around storage premises and
transport facilities, which may permit incurrence of small amounts in
products during storage and shipping.
(c) Other uses
DDT is used in many home gardens, in many households (forbidden in The
Netherlands), as a mothproofing agent in rugs and clothing in
restaurants and in public buildings. Many mosquito abatement
programmes make use of DDT.
National tolerances
Austria 7 ppm - a general informal tolerance.
Canada 7 ppm on many fruits and vegetables, fat of meat
animals.
United States 7 ppm on many fruits and vegetables, fat of meat
of America animals.
3.5 ppm on sweet corn.
1 ppm on potatoes.
Residues resulting from supervised trials
Although there have been many analyses made for DDT in agricultural
products, many of them were not made on controlled experiments
designed explicitly to ascertain the fate of the residue following
application. A summary of numerous data, which is too long to
reproduce here and which contains the related bibliography, is held at
the FAO headquarters in Rome. Table 1 has been prepared from this
summary. It contains estimates of the average high residues likely to
result from the practical use of DDT to control insects which attack
the different food commodities or that in animal products from animals
exposed to "unavoidable" or very limited feed residues.
TABLE 1. RESIDUES OF DDT EXPECTED FROM PRACTICAL USE
Food Residue
ppm
Vegetables
leafy 7
others 1-7
Meat, fish, poultry 7 (in fat)
Tree fruits 7
Berries (cane) 1
Citrus 4
Shelled nuts 1
Residues in food moving in commerce
Even though there is a continuing and widespread use of DDT, a very
large proportion of the food in commerce has very little DDT present;
seldom is a sample found with residue as high as the tolerance figures
quoted.
Of the large number of "total diet" samples analysed in the United
States of America since 1961, a high proportion have had detectable
amounts of DDT but no sample of any commodity grouping has exceeded
0.05 ppm DDT. (Mills 1963; Williams 1964; Cummings 1965.)
The Government Chemist Laboratory of Great Britain has analysed a
large number of samples of food products since 1962. They chose those
samples most likely to contain residues of DDT such as milk, butter
and fat of different meat animals. The residues of DDT, DDE, and DDD
for the years 1964 and 1965 for over 900 samples average less than
0.15 ppm, with the highest value found being 4.6 ppm. (Lewis 1964 and
1965; Egan et al 1966.)
The Netherlands Government has analysed a number of imported cereal
products and has found a large proportion to contain some residue of
DDT. Of 227 samples examined during 1964 and 1965, 36 per cent
contained DDT. The highest sample residue was 2.85 ppm, but most
samples ran from 0 to 0.5 ppm (Report CCPR 66-17 January 1966 Ministry
of Social Affairs and Public Health, Ministry of Agriculture,
Committee on Phytopharmacy.)
Fate of residues
Although DDT and some of its metabolic products can be altered
readily by chemical means, they are quite comparatively stable
compounds in many biological media. This low biodegradability coupled
with high fat solubility, in part, accounts for the persistence of
residues and their propensity to concentrate in fat tissue. The
stability and solubility also account for its concentration in certain
forms of life when it is part of the food chain (Metcalf 1966).
(a) In animals
It has been known for many years that DDE and DDA form from DDT
(Menzie 1966). DDA is an acid, is relatively water soluble, and thus
is eliminated much more readily than the fat soluble forms and does
not appear nearly so readily in the edible portion of food products of
animal origin. On the other hand, DDE is fat soluble and almost always
appears as a residue in animal products with DDT.
Only recently has it been well established that DDD (TDE) is a
metabolic product of DDT. (Kallman and Andrews 1963; Peterson and
Robison 1964; Klein et al 1964; Miskus and Blair 1965.) It has been
found as a residue associated with DDT in animal products for many
years, but since it has also had some use as a commercial pesticide,
it was not considered to be a metabolic product by most workers.
Whether it forms in animals as a result of enzymes endogenous to the
animal or only from intestinal bacteria or other organisms associated
with the animal has not been fully established.
Work by Klein et al 1964 has shown that o,p-DDT converts to p,p'-DDT
in vivo.
Both DDE and DDD are more stable in many biological systems than DDT;
therefore, in many instances they accumulate in time to a
concentration equal to or greater than DDT in the fat of animal
tissues. The hydroxy analogues of DDT (kelthane) and DDD can form from
DDT and DDD respectively and 4,4'-dichlorobenzophenone can form from
each (Menzie 1966).
(b) In plants
The meeting was not aware of any data on the effect of plant enzyme
systems on DDT; however, it is known that DDE does appear as a very
low level residue on many food crops which contain DDT (U.S.F.D.A.,
unpublished data).
(c) In storage and processing
DDT is stable under most of the conditions which prevail when it is a
residue on food products. Therefore, residues in food products will
not normally diminish greatly from most food products during shipping
and storage. It is especially stable in a fatty medium.
Recent studies by the National Canners Association (United States of
America) has shown that the amount of DDT on green beans does not
change over a 2-week storage period at 45° F (personal communication).
Since DDT does not penetrate significantly into non-fatty food
products, a large loss is shown when the surface is removed such as in
peeling, shelling, harsh brushing, milling, etc.
Recent studies by Farrow et al., 1966, showed that during the normal
heat processing of canned spinach approximately 50 per cent of the
original DDT was destroyed. However, DDD was formed equivalent to
about 20 per cent of the original DDT resulting in a net loss of about
30 per cent.
Methods of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of DDT (together with residues of a number
of other compounds including DDE and DDD). An example is the AOAC
system (1966) in which acetonitrile partition and Florisil column
clean-up are identified and measured by gas chromatography coupled
with thin layer or paper chromatography. Alternative clean-up systems
e.g. that of de Faubert Maunder et al. (1964) using dimethylformamide,
and other methods of confirmation of identity e.g. using infra-red
spectrophotometry, are also available. The methods are in general
sensitive to 0.003 ppm in milk and 0.05 ppm in most other foods,
though under favourable conditions greater sensitivity can, if
appropriate, be obtained.
In those cases where analyses have provided information on the
contents of degradation products of DDT, such as DDE and DDD, the
figures for these compounds should be included in the analytical
report.
RECOMMENDATION FOR TOLERANCES
The recommendations for tolerances are shown in Table 2.
These tolerance values are considered to be temporary, will be kept
under study, and will be reconsidered no later than three years hence.
These values are in excess of values that would be calculated from the
factors utilized in considering tolerance values for other compounds
in this booklet.
New studies in toxicology, metabolism or residues may permit, within
the next three years a more permanent recognition of these levels, or
on the other hand, it may be necessary to markedly lower the tolerance
recommendations. It is unlikely that future tolerance recommendations
will be higher than those prescribed here.
TABLE 2. RECOMMENDED TOLERANCES FOR DDT
Food type Temporary
Restricted to 3 years
ppm
Vegetables 1.0-7.0
Meat, fish, poultry 7.0 (in fat)
Tree fruits 7.0
Berries (cane) 1.0
Citrus 4.0
Shelled nuts 1.0
Milk (whole) Administrative decision .0045 ppm
Milk products Practical residue 0.2 ppm
The "administrative decision" and the "practical residue"
recommendations are made because the widespread use and stability of
DDT have resulted in small residues being ubiquitous. Small residues
have been found to be present in most dairy products. This is
considered to be undesirable but is also unavoidable at the present
time. Since this residue is generally not present from direct
application to the animals or their feed, no tolerance recommendation
is made. However, to assist regulatory officials in identifying those
samples which have residues much in excess of the unavoidable level, a
"practical residue level" of 0.20 ppm DDT is suggested.
Residues of DDT in animal products are invariably associated with
varying amounts of the DDT metabolites DDE and DDD. In many instances
the residues of either of these or the sum of the two exceeds the
residue of DDT. The WHO Expert Committee on Pesticide Residues
estimated an ADI for DDT but not for the other two. They noted that
residues of all three should be determined, but they postponed
consideration of DDE and DDD. Therefore, only DDT residues were taken
into consideration in preparing the temporary tolerance for DDT.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Andronova, G. P. (1956) Dissertation, F. F. Erisman's State Research
Institute for Hygiene, Moscow
Anon. (1951) Report of the Committee on Pesticides of the Council on
Pharmacy and Chemistry, J. Amer. med. Ass., 145, 728
Bishopp, F. C. (1946) Amer. J. Publ. Hlth, 36, 593
Cameron, G. R. & Burgess, F. (1945) Brit. med. J., 1, 865
Cueto, C., Barnes, A. G. & Mattson, A. M. (1956) J. Agr. Food Chem.,
4 (2), 94.3
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Dale, W. E., Gaines, T. B. & Hayes, W. J., jr (1965) Science, 142,
1474
Denes, A. (1962) Nahrung, 6, 48
Draize, J. H., Woodard, G., Fitzhugh, O. G., Nelson, A. A., Smith,
R. B. & Calvery, H. O. (1944) Chem. Eng. News, 22, 1503
Durham, W. F., Armstrong, J. F. & Quinby, G. E. (1965) Arch.
Environm. Hlth., 11, 16
Durham, W. F., Ortega, P. & Hayes, W. J., Jr (1963) Arch. int.
Pharmacodyn., 141, 111
Egan, H., Goulding, R., Roburn, J. & Tatton, J. (1965) Brit. med.
J., ii, 66
Fitzhugh, O. G. (1948) Ind. Eng. Chem., 704
Fitzhugh, O. G. & Nelson, A. A. (1947) J. Pharmacol. exp. Ther.,
89, 18
Gerboth, G. & Schwabe, U. (1964) Arch. exp. Path., 246, 469
Gerebtzoff, M. A. & Philippot, E. (1952) Experientia (Basel), 8
(10), 395
Gerebtzoff, M. A., Dallemagne, M. J. & Philippot, E. (1950) C.R.
Soc. Biol. (Paris), 144, 1135
Halacka, K., Hakl, J. & Vymetal, F. (1965) Cs. Hyg., 10, 188
Hart, L. G. & Fouts, J. R. (1963) Proc. Soc. exp. Biol. (N.Y.),
114, 388
Hayes, W. J., jr & Dale, W. E. (1964) Toxicol. Appl. Pharmacol., 6,
349
Hayes, W. J., jr, Dale, E. & Pirkle, C. I. (1964) Unpublished report
Hayes, W. J., jr, Durham, W. F. & Cueto, C., jr (1956) J. Amer. med.
Ass., 162 (9), 890
Hayes, W. J. jr., Walker, K. C., Elliott, J. W. & Durham, W. F. (1958)
Arch. industr. Hlth, 18, 398
Haymaker, W., Ginzler, A. M. & Ferguson, R. L. (1946) Amer. J. Med.
Sci., 212, 423
Hoffmann, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
Environm. Hlth, 9, 387
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Juchau, M. R., Gram, T. E. & Fouts, J. R. (1966) Gastroenterology,
51, 213
Judah, J D. (1949) Brit. J. Pharmacol., 4, 120
Kemény, T. & Tarján, R. (1966) Orvosi Hetilap, 107 (30), 1407
Khairy, M. (1959) Quart. J. exp. Psychol., 11, 84
Kimbrough, R., Gaines, T. B. & Sherman, J. D. (1964) J. Nat. Cancer
Inst., 33, 215
Klein, A. K., Laug, E. P., Datta, P. R., Watts, J. O. & Chen, J. T.
(1964) J. Assoc. Offic. Agr. Chem., 47, 1129
Laug, E. P., Kunze, F. M. & Prickett, C. S. (1951) Arch. industr.
Hyg., 3, 245
Laug, E. P., Nelson, A. A., Fitzhugh, O. G. & Kunze, F. M. (1950) J.
Pharmacol. exp. Ther., 98, 268
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials,
U.S., 16, 3, 47, 126
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Fd. Cosmet. Toxicol.,
3, 591
Maier-Bode, H. (1960) Med. exp. (Basel), 3, 284
Mattson, A. M., Spillane, J. T., Baker, C. & Pearce, G. W. (1953)
Anal. Chem., 25, 1065
McLean, A. E. & McLean, E. K. (1966) Biochem, J., 100, 564
Mendel, J. L. & Walton, M. S. (1966) Science, 151, 1527
Morello, A. (1965) Canad. J. Biochem., 43 (8), 1289
Ortega, P. (1962) Fed. Proc., 21, 306
Ortega, P. (1966) Lab. Invest., 15, 657
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. (1956)
Publ. Hlth Monogr., 43, 27
Ortelee, M. F. (1958) Arch. industr. Hlth, 18, 433
Pearce, G. W., Mattson, A. M. & Hayes W. J., jr (1952) Science,
116, 254
Philips, F. & Gilman, A. (1946) J. Pharmacol. exp. Ther., 86, 213
Phillips, W. E. G. (1963) Canad. J. Biochem., 41, 1793
Pinto, J. D., Camien, M. N. & Dunn, M. S. (1965) J. biol. Chem.,
240, 2148
Quinby, G. E., Armstrong, J. F. & Durham, W. F. (1965) Nature,
207, 726
Quinby, G. E., Hayes, W. J., jr, Armstrong, J. F. & Durham, W. F.
(1965) J. Amer. med. Ass., 191, 175
Read, S. I., Murray, T. K. & McKinley, W. P. (1965) Canad. J.
Biochem., 43, 317
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. Ind. Med., 22, 220
Rothe, C. F., Mattson, A. M., Nuelsein, R. M. & Hayes, W. J., jr
(1957) Arch. industr. Hlth, 16 (1), 82
Shillinger, Y. I., Naumova, L. P. & Pekerman, S. W. (1955) Vop.
Pitan., 14, 41
Smith, M. I. & Stohlman, E. F. (1944) Publ. Hlth Rep. (Wash.), 59,
984
Spicer, S. S., Sweeney, T. R., von Oettingen, W. F., Lillie, H. D. &
Neal, P. A. (1947) Vet. med., 42 (8), 289
Stemmer, K. L. & Hamdi, E. (1964) Unpublished report submitted by
Kettering laboratory
Street, J. C. & Blau, A. D. (1966) Toxicol. Appl. Pharmacol., 8,
497
Tinsley, I. J. (1965) Biochem. Pharmacol., 14, 847
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Woodard, G., Nelson, A. A. & Calvery, H. O. (1944) J. Pharm. exp.
Ther., 82, 152
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
AOAC (1966) Changes in Methods of Analysis, J. Assoc. Offic. Anal.
Chem. 49, 222-230
Cummings, J. G. (1965) Pesticide Residues in Total Diet Samples, J.
Assoc. Offic. Agr. Chem. 48: 1177-1180
de Faubert Maunder, M. J., Egan, H., Godly, E. W., Hammond, E. W.,
Roburn, J., Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues, Analyst,
89, 168-174
Egan, H., Holmes, D. C., Roburn, J. and Tatton, J. O'G. (1966)
Pesticide Residues in Foodstuffs in Great Britain. II-Persistent
Organochlorine Pesticide Residues in Selected Foods, J. Sci. Fd.
Agric., 17: 563-569
Farrow, R. P., Elkins, E. R. Jr. and Cook, R. W. (1966) Conversion of
DDT to TDE in Canned Spinach. J. Agr. Food Chem. 14/4: 430-434
Kallman, B. J. and Andrews, A. K. (1963) Reductive Dechlorination of
DDT to DDD by Yeast, Science 141: 1050-1051
Klein, A. K., Laug, E. P., Datta, P. R., Watts, J. O., and Chem, J. T.
(1964) Metabolites: Reductive Dechlorination of DDT to DDD and
Isomeric Transformation of o,p'-DDT to p,p'-DDT in vivo, J. Assoc.
Offic. Agr. Chem. 47: 515-531
Lewis, D. T. (1964, 1965) Report of the Government Chemist, H.M.S.O.
London
Menzie, C. M. (1966) Metabolism of Pesticides Special Scientific
Report - Wildlife No. 96, Fish and Wildlife Service, Department of the
Interior Washington, D.C.
Metcalf, R. L. (1966) Metabolism and Fate of Pesticides in Plants and
Animals. Pages 230-250, Scientific Aspects of Pesticide Control.
Publication 1402, National Academy of Sciences - National Research
Council, Washington, D.C.
Mills, P. A. (1963) Total Diet Study: C. Pesticide Content. J. Assoc.
Offic. Agr. Chem. 46: 762-767
Miskus, R. P., and Blair, D. B. (1965) Conversion of DDT to DDD by
Bovine Rumen Fluid, Lake Water, and Reduced Porphyrins, J. Agr. Food
Chem. 13/5: 481-483
Peterson, J. E. and Robison W. H. (1964) Metabolic Products of
p,p'-DDT in the Rat, Toxicology and Applied Pharmacology 6: 321-327
Williams, S. (1964) Pesticide Residues in Total Diet Samples, J.
Assoc. Offic. Agr. Chem. 47: 815-821
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
DDT is only slightly absorbed through the skin, but the degree of
absorption depends on the vehicle used (Cameron & Burgess, 1945).
After oral administration, most of the dose is found unchanged in the
faeces, but some absorption may occur, particularly in the presence of
lipids. The absorbed DDT is transformed into
2,2-di-(p-chlorophenyl)-1, 1-dichloro-ethylene (DDE) (Pearce et al.,
1952; Mattson et al., 1953) and into 2,2-di-(p-chlorophenyl)-acetic
acid (DDA) (Spicer et al., 1947; Judah, 1949, Hayes et al., 1956).
Some unknown compounds are also found (Spicer et al., 1947; Hayes et
al., 1956; Rothe et al., 1957; Cueto et al., 1956). Both DDT and DDE
accumulate in the fat (see Observations on Man). DDA is excreted in
the urine and in a combined form in the bile (Durham et al., 1963,
1965; Pinto et al., 1965).
A related insecticide, 1,1-dichloro-2,2-di-(p-chlorophenyl)-ethane
(DDD) was found in the liver of rats fed DDT (Klein et al., 1964).
Evidence that the conversion from DDT to DDD is accomplished by the
intestinal flora of the rat has also been presented (Mendel & Walton,
1966).
A level of 500 ppm of DDT in the diet of rats for 2 weeks or injection
of 25 mg/kg daily for 3 days increased the hepatic microsomal enzymic
metabolism of a number of drugs (Harts & Fouts, 1963). A similar
effect was observed in monkeys injected with 5 mg/kg/day for 7 days
(Juchau et al., 1966). As little as 1 mg/kg to rats was sufficient to
reduce pentobarbital sleeping time (Gerboth & Schwabe, 1964). Also
Morello (1966) showed that 3-4 days after a single dose of DDT in the
rat the ability of the liver microsomes to metabolize DDT was markedly
increased. It has been postulated by Street & Blau (1966) that DDT
also enhances the metabolism of dieldrin by microsomal enzymes; as
little as 5 ppm of DDT in the diet of rats decreased the fat storage
of simultaneously ingested dieldrin. However, a single dose of 75
mg/kg a week before treatment with carbon tetrachloride reduced the
LD50 of the latter in rats given either a complete or a protein-free
diet (McLean & McLean, 1966). Furthermore, dietary levels of 5-200 ppm
of DDT decreased the liver glucose-6-phosphate dehydrogenase activity
in rats (Tinsley, 1965).
In adult rats, concentrations of 10 or 100 ppm of DDT in the diet have
been shown to interfere with the storage and metabolism of vitamin A
in the liver; this was not observed in new-born or young rats which
have poor reserves of vitamin A (Phillips, 1963; Read et al., 1965).
In addition, whereas liver carboxylesterase activity was markedly
increased by DDT in weanling and adult rats, the same effect could not
be demonstrated in new-born rats (Read et al., 1965).
The toxic effects, revealing involvement of the central nervous system
(such as hypersensitivity, excitability, generalized trembling,
convulsions, paralysis) appear within 5-10 minutes of intravenous
administration and after a latent period of some hours following oral
administration. Death occurs as a result of respiratory arrest in the
rat, rabbit, cat and monkey, and of ventricular fibrillation in the
dog. It is the consensus of the literature that the significant action
of DDT is on the nervous system; when ventricular fibrillation occurs,
it is precipitated by adrenaline released from the adrenal medulla by
stimulation of the sympathetic nervous system (Phillips & Gilman,
1946).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 150-400* Draize et al., 1944
Bishopp, 1946
Rat new-born Intragastric >4 000 Lu et al., 1965
Rat pre-weanling Oral 437 Lu et al., 1965
Rat adult Oral 194 Lu et al., 1965
Rat Oral 150-420* Smith & Stohlman, 1944
Woodard et al., 1944
Rat Oral 800 Cameron & Burgess, 1945
Guinea-pig Oral 400 Draize et al., 1944
Rabbit Oral 250-500* Cameron & Burgess, 1945
Bishopp, 1946
Dog Intravenous Approximately Philips & Gilman, 1946
50
Cat Oral 400-600* Philips & Gilman, 1946
Monkey Oral >200 Bishopp, 1946
Horse Oral >300 Bishopp, 1946
Chicken Oral >1 300 Bishopp, 1946
* The LD50 dose of DDT varies within wide limits, depending on sex and the
type of vehicle used.
In rats given a single oral dose of DDT sufficient to kill about half
of them, the severity of symptoms corresponded with the concentration
of the unchanged compound in the brain (Dale et al., 1963).
Furthermore, approximately the same concentration of DDT is found in
the brain of rats killed by DDT no matter whether the dosage is acute,
subacute, or chronic (Hayes & Dale, 1964).
The fatal dose for man is difficult to establish but is generally
taken to be of the order of 500 mg/kg body-weight. A dose of the order
of 10 mg/kg body-weight can give rise in some subjects, but not in
all, to toxic symptoms (nausea, headache, sweating), and with doses of
16 mg/kg body-weight upwards, convulsions often occur (Anon., 1951).
Special studies
Daily oral doses of 0.2 mg/kg for 10 days produced functional changes
in the conditioned reflex pattern which appeared after 5-7 doses and
persisted 5-7 days after the end of exposure (Andronova, 1956).
Behavioural studies have also been made on groups of rats fed diets
containing 100, 200, 400 and 600 ppm DDT. Problem-solving and speed of
locomotion were unaffected by these doses of DDT. There was a
significant alteration in the patterns of locomotion in these rats and
their reactions to stress involving visual stimuli were reduced
(Khairy, 1959).
Short-term studies
Rat. Rats, in groups of 8 males and 8 females, were given diets with
1, 5, 10, and 50 ppm of DDT for 23 weeks. At concentrations of 5 ppm
and higher, histopathological changes in the liver were found (Laug et
al., 1950).
A series of experiments was carried out with small groups of rats, 178
males and 104 females in all, which were given diets containing DDT in
concentrations from traces up to 5000 ppm for periods ranging from one
to 14 months. In some cases observation of the animals continued after
cessation of treatment. At levels above 400 ppm changes in growth rate
were noted. Above 200 ppm liver enlargement was seen. In the male
animals with intakes of 5 ppm and above, specific histological lesions
in the liver were seen. These lesions consisted of hypertrophy of the
parenchymal cells, increased lipid deposits, marginal localization of
cytoplasmic granules and, above all, the appearance of complex
cytoplasmic inclusions of a lipid nature, called "lipospheres". In the
females these liver lesions were only seen in diets containing 200 ppm
or more. Necrotic lesions were seen only at concentrations above 1000
ppm. The authors noted that lipospheres and other changes considered
characteristic of DDT were more prominent in male rats although
females store more of the compound and were more susceptible to
poisoning. For this and other reasons, they speculated that the
changes might be adaptive. They noted also that earlier work had
failed to demonstrate similar changes in other species (Ortega et al.,
1956).
In an electron-microscopical examination of the liver of rats fed
diets containing 5-2500 ppm of DDT for 2-18 months, the following
cytoplasmic abnormalities were found: slight proliferation of the
endoplasmic reticulum, peripheral distribution of the ribosomes and
intracytoplasmic inclusions consisting of aggregates of membranes very
rich in lipids. At 100 ppm changes were seen after 2 months (Ortega,
1962, 1966).
Similar changes were found in another study in which the dietary
intake of DDT was 10 ppm. The accumulation of endoplasmic reticulum
has been regarded as evidence of cellular hypertrophy (Stemmer &
Hamdi, 1964).
In one study in which obesity was induced in rats with a high fat
diet, DDT was added into the diet at a dose level of 3.6 mg/kg/day for
up to 10 months. A comparable incidence of chloroleukemia was observed
in rats receiving DDT and in those given the high fat diet only
(Kimbrough et al., 1964).
Dog. Dogs were given DDT orally. Those receiving 100 mg/kg
body-weight daily died within 7 weeks. Animals subjected to lower
doses survived and seemed normal even after 50 weeks (Draize et al.,
1944). When the experiment was continued for 3 years, the animals
receiving doses of 50 and 80 mg/kg bodyweight developed jaundice and
haemorrhagic symptoms. Those receiving 10 mg/kg body-weight daily
showed no ill effects (Hayes et al., 1956; Lehman, 1952).
Dogs were given DDT by stomach-tube, in the form of a 10 per cent
solution in peanut oil in doses ranging from 150 to 350 mg/kg
body-weight for about 90 days. Some of the animals died. The authors
noted neurological symptoms, showing involvement of the cerebellum as
revealed by histological lesions (Haymaker et al., 1946).
Three dogs were given intramuscular injections of DDT in the form of a
10 per cent solution in olive oil at the rate of 100 mg/kg body-weight
daily for 25 to 30 days. A control animal received 1 ml of olive oil
in the same way. The experimental animals showed a temporary loss of
weight. The kidneys were discoloured and on histological examination
showed tubular damage. At the same time, proliferation was observed in
the lymphoid tissues (lymph nodes, spleen, bone marrow) and the wall of
the small intestine, but the blood showed no leukaemic characteristics
(Gerebtzoff et al., 1950; Gerebtzoff & Philippot, 1952).
Monkey. Monkeys given DDT orally in a dose of 0.2 mg/kg body-weight
for 7-9 months developed symptoms of hepatitis. After a year, the
animals showed liver enlargement and hyperglycaemia (Shillinger et
al., 1955). These liver changes were not confirmed in another
experiment on monkeys lasting 7.5 years (Durham et al., 1963).
Long-term studies
Mouse. In one experiment, 683 mice, spread over 5 generations, were
given DDT at 0.3-0.6 mg/kg daily. The background DDT content of the
diet given to 406 controls corresponded to an intake of 0.03-0.05
mg/kg daily. The over-all incidence of leukaemia and other malignant
tumours was 3.5 and 5.4 per cent respectively in the experimental
animals compared to 0.2 and 0.9 per cent in the controls. The
difference was obvious within each generation. The greatest tumour
incidence was seen in the fourth and fifth generations (Kemény &
Tarján, 1966).
Rat. Groups of 12 male rats were subjected for 2 years to diets
containing 0, 100, 200, 400 and 800 ppm of DDT, in the form of a 10
per cent solution in corn oil. In another experiment, groups each of
24 rats (12 males and 12 females) were given, during the same period,
diets containing 0, 100, 200, 400 and 800 ppm. Also additional groups
of 24 animals received 600 and 800 ppm incorporated in their feed in a
dry state. In the groups receiving 400 ppm and above, an increase in
the mortality rate was seen in relation to the dose. Apart from
nervous symptoms at doses of 400 ppm and above, typical liver lesions
were found at all concentrations. Hepatic cell tumours were seen in 4
out of 75 animals and 11 other rats showed nodular adenomatoid
hyperplasia (Fitzhugh & Nelson, 1947).
In an experiment with rats fed for 2 years on a diet containing 10 ppm
of DDT, histological liver lesions were also observed (Fitzhugh,
1948). Groups of 80 young rats each (40 male and 40 female) were fed
0, 0.25, 12.5 and 25 ppm of DDT. The histological lesions of the liver
observed were always slight and, according to the authors,
non-specific, but nevertheless they were more frequent with DDT than
with aldrin or dieldrin, which were administered in the same
concentrations for comparative purposes (Treon & Cleveland, 1955).
Experiments with 25 young rats did not show any harmful effects after
daily administration by stomach-tube of a dose of 10 µg/kg for 17
months (Klimmer, 1955).
Monkey. Twenty-four Rhesus monkeys, 12 males and 12 females, were
divided into groups and fed over periods as long as 7.5 years or more
on diets containing respectively 0, 5, 50, 200 and 5000 ppm of DDT.
All the animals subjected to the concentration of 5000 ppm showed
convulsions, accompanied by loss of appetite and a fall in weight. At
a concentration of 200 ppm (corresponding to daily doses of 2.2-5.54
mg/kg body-weight), no harmful effects were observed and, in
particular, no histological lesions in the liver or disturbances in
the functioning of that organ, as shown by the bromsulfthalein test
(Durham et al., 1963).
Observations on man. An experimental study was carried out of the
effects on man of prolonged ingestion of small doses of DDT (in the
form of oily solutions in capsules or emulsions in milk). The authors
used 51 volunteers for this study; 17 received a normal diet, 17
received 3.5 mg/kg body-weight and 17 received 35 mg DDT daily. The
last dose is approximately 0.5 mg/kg body-weight. Administration was
continued for as long as 18 months. The authors noted that the
accumulation of the insecticide in the fatty tissues and the urinary
excretion of its metabolite, DDA, were proportional to the dose of DDT
ingested. A state of equilibrium was reached after about a year and
the concentration of DDT accumulated in the fatty tissues reached an
average of 234 ppm (101-367 ppm) in subjects who had ingested 35 mg of
DDT per day. Throughout the whole experiment, no subject complained of
malaise, nor did any ill-effects appear that could be attributed to
the ingestion of DDT (Hayes et al., 1956). The essential results were
confirmed in a separate investigation in which dosage with DDT lasted
for 21 months and the volunteers were observed for an additional 27
months. Fourteen men received 35 mg/man/ day; 6 received 3.5
mg/man/day, and 4 men served as controls. The study also revealed the
relationship between storage of DDT and the urinary excretion of DDA
and demonstrated that the loss of DDT from storage in man following
cessation of dosage is slow - only about two-thirds in 27 months
(Hayes et al., 1964).
Observations were made on 40 workers engaged, over a period of years,
in the manufacturing of specialities based on DDT, under conditions
where suitable precautions had not been taken to protect their skin.
According to the DDA concentration found in the urine, these subjects
had absorbed daily as much DDT as volunteers who had ingested 35 mg of
the insecticide per day in the experiment mentioned above. Thorough
medical and biological tests failed to reveal any toxic symptoms, even
in workers exposed to the toxic product for 6.5 years (Ortelee, 1958).
Several studies on DDT and DDE storage in human fat in several parts
of the world have been presented (Laug, 1951; Hayes et al., 1956;
Hayes et al., 1958; Denes 1962; Maier-Bode, 1960; Dale et al., 1963;
Hunter et al., 1963; Hoffmann et al., 1964; Dale et al., 1965; Egan et
al., 1965; Halacka, et al., 1965; Quinby et al., 1965b; Robinson et
al., 1965; Zavon et al., 1965). DDT was also found in a stillborn
infant and in very young babies (Halacka et al., 1965).
In these studies the average total content in human fat ranged between
2 and 30 ppm; the percentage of DDE of total DDT stored ranged between
34 and 77 per cent. It has been shown that the storage of DDT in man
is directly proportional to intake over a wide range of doses (0.04 to
35.0 mg/man/day), so that from the level in the fat the daily dose can
be estimated. Thus, it can be calculated that the highest average
intake of DDT of any human population yet observed is about 0.7-0.8
mg/man/day. From the data which have been reported, it is also
apparent that in the United States of America, where the level of DDT
in human fat has been repeatedly investigated in the general
population over a significant number of years, it has not increased
since 1950 (Durham et al., 1965).
It has also been repeatedly indicated that women and cows secrete DDT
in the milk. Two recent studies indicated an average of total DDT
(i.e. DDT + DDE) of 0.128 ppm (57 per cent as DDE) and 0.170 ppm (58
per cent as DDE). The content of DDT in cows' milk was found to be
lower (Egan et al., 1965; Quinby et al., 1965). The percentage of the
total DDT intake which is eliminated in the milk has been demonstrated
to be smaller in cows than in women (Quinby et al., 1965).
Comments
The evaluation of DDT is difficult because wide species differences
occur. The rat appears to be the most susceptible species; liver
cellular changes were produced by 5 ppm in the diet. In dogs and
monkeys these changes were not seen. Dogs given 10 mg/kg
body-weight/day for 3 years and monkeys given 2-5 mg/kg
body-weight/day for up to 7 years showed no ill-effects. However, in
another study, monkeys given 0.2 mg/kg body-weight/day for one year
showed signs of hepatitis and liver enlargement. In man, as much as
0.5 mg/kg body-weight/day for up to 21 months were without effect
apart from storage in the fat. However, the possibility that the
latter might have deleterious consequences later in life cannot be
ruled out. In addition, it has not been demonstrated that metabolic
changes in the liver cells comparable to those observed in rats do not
take place in man. The Committee realizes that the figure reached as
an estimate for an ADI can be less than the actual intake for humans
in some parts of the world. It might correspond to the intake of DDT
of a baby exclusively breast-fed. (In this respect the significance of
the lower acute toxicity of DDT for new-born rats is uncertain.)
Furthermore, in addition to unchanged DDT, the residues also contain
metabolites of DDT. There is a need for uniformity in reporting the
results of residue analyses. The reports should at least indicate the
percentages of unchanged DDT, DDE and TDE. Some of the metabolites are
probably less toxic than DDT itself. However, more information is
necessary on this point. At the present time the Committee remains
concerned about the storage of DDT which occurs in all species and
about the cellular changes produced in the liver of rats by DDT and by
other compounds chemically related to it.
TOXICOLOGICAL EVALUATION
Estimate of acceptable daily intake in man
0-0.01 mg/kg body-weight
Further work required
Elucidation of the significance of the finding that DDT is one of the
compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
DDT is used to a minor extent as a soil treatment, primarily for the
control of cutworms which attack vegetable crops. It is suggested for
use in many countries on a wide variety of food crops. It is suggested
for the control of about 20 different insects which attack cane
fruits, about 50 different insects which attack vegetables, 50
different insects which attack tree fruits, as well as for control of
insects of nut trees and other food crops. The usual rate of
application is about 1 to 2 lb of the active chemical per acre;
however, some treatments may go as high as 10 lb per acre.
(b) Post-harvest treatments
Direct applications to stored food products are no longer advised.
However, there is some use in and around storage premises and
transport facilities, which may permit incurrence of small amounts in
products during storage and shipping.
(c) Other uses
DDT is used in many home gardens, in many households (forbidden in The
Netherlands), as a mothproofing agent in rugs and clothing in
restaurants and in public buildings. Many mosquito abatement
programmes make use of DDT.
National tolerances
Austria 7 ppm - a general informal tolerance.
Canada 7 ppm on many fruits and vegetables, fat of meat
animals.
United States 7 ppm on many fruits and vegetables, fat of meat
of America animals.
3.5 ppm on sweet corn.
1 ppm on potatoes.
Residues resulting from supervised trials
Although there have been many analyses made for DDT in agricultural
products, many of them were not made on controlled experiments
designed explicitly to ascertain the fate of the residue following
application. A summary of numerous data, which is too long to
reproduce here and which contains the related bibliography, is held at
the FAO headquarters in Rome. Table 1 has been prepared from this
summary. It contains estimates of the average high residues likely to
result from the practical use of DDT to control insects which attack
the different food commodities or that in animal products from animals
exposed to "unavoidable" or very limited feed residues.
TABLE 1. RESIDUES OF DDT EXPECTED FROM PRACTICAL USE
Food Residue
ppm
Vegetables
leafy 7
others 1-7
Meat, fish, poultry 7 (in fat)
Tree fruits 7
Berries (cane) 1
Citrus 4
Shelled nuts 1
Residues in food moving in commerce
Even though there is a continuing and widespread use of DDT, a very
large proportion of the food in commerce has very little DDT present;
seldom is a sample found with residue as high as the tolerance figures
quoted.
Of the large number of "total diet" samples analysed in the United
States of America since 1961, a high proportion have had detectable
amounts of DDT but no sample of any commodity grouping has exceeded
0.05 ppm DDT. (Mills 1963; Williams 1964; Cummings 1965.)
The Government Chemist Laboratory of Great Britain has analysed a
large number of samples of food products since 1962. They chose those
samples most likely to contain residues of DDT such as milk, butter
and fat of different meat animals. The residues of DDT, DDE, and DDD
for the years 1964 and 1965 for over 900 samples average less than
0.15 ppm, with the highest value found being 4.6 ppm. (Lewis 1964 and
1965; Egan et al 1966.)
The Netherlands Government has analysed a number of imported cereal
products and has found a large proportion to contain some residue of
DDT. Of 227 samples examined during 1964 and 1965, 36 per cent
contained DDT. The highest sample residue was 2.85 ppm, but most
samples ran from 0 to 0.5 ppm (Report CCPR 66-17 January 1966 Ministry
of Social Affairs and Public Health, Ministry of Agriculture,
Committee on Phytopharmacy.)
Fate of residues
Although DDT and some of its metabolic products can be altered
readily by chemical means, they are quite comparatively stable
compounds in many biological media. This low biodegradability coupled
with high fat solubility, in part, accounts for the persistence of
residues and their propensity to concentrate in fat tissue. The
stability and solubility also account for its concentration in certain
forms of life when it is part of the food chain (Metcalf 1966).
(a) In animals
It has been known for many years that DDE and DDA form from DDT
(Menzie 1966). DDA is an acid, is relatively water soluble, and thus
is eliminated much more readily than the fat soluble forms and does
not appear nearly so readily in the edible portion of food products of
animal origin. On the other hand, DDE is fat soluble and almost always
appears as a residue in animal products with DDT.
Only recently has it been well established that DDD (TDE) is a
metabolic product of DDT. (Kallman and Andrews 1963; Peterson and
Robison 1964; Klein et al 1964; Miskus and Blair 1965.) It has been
found as a residue associated with DDT in animal products for many
years, but since it has also had some use as a commercial pesticide,
it was not considered to be a metabolic product by most workers.
Whether it forms in animals as a result of enzymes endogenous to the
animal or only from intestinal bacteria or other organisms associated
with the animal has not been fully established.
Work by Klein et al 1964 has shown that o,p-DDT converts to p,p'-DDT
in vivo.
Both DDE and DDD are more stable in many biological systems than DDT;
therefore, in many instances they accumulate in time to a
concentration equal to or greater than DDT in the fat of animal
tissues. The hydroxy analogues of DDT (kelthane) and DDD can form from
DDT and DDD respectively and 4,4'-dichlorobenzophenone can form from
each (Menzie 1966).
(b) In plants
The meeting was not aware of any data on the effect of plant enzyme
systems on DDT; however, it is known that DDE does appear as a very
low level residue on many food crops which contain DDT (U.S.F.D.A.,
unpublished data).
(c) In storage and processing
DDT is stable under most of the conditions which prevail when it is a
residue on food products. Therefore, residues in food products will
not normally diminish greatly from most food products during shipping
and storage. It is especially stable in a fatty medium.
Recent studies by the National Canners Association (United States of
America) has shown that the amount of DDT on green beans does not
change over a 2-week storage period at 45° F (personal communication).
Since DDT does not penetrate significantly into non-fatty food
products, a large loss is shown when the surface is removed such as in
peeling, shelling, harsh brushing, milling, etc.
Recent studies by Farrow et al., 1966, showed that during the normal
heat processing of canned spinach approximately 50 per cent of the
original DDT was destroyed. However, DDD was formed equivalent to
about 20 per cent of the original DDT resulting in a net loss of about
30 per cent.
Methods of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of DDT (together with residues of a number
of other compounds including DDE and DDD). An example is the AOAC
system (1966) in which acetonitrile partition and Florisil column
clean-up are identified and measured by gas chromatography coupled
with thin layer or paper chromatography. Alternative clean-up systems
e.g. that of de Faubert Maunder et al. (1964) using dimethylformamide,
and other methods of confirmation of identity e.g. using infra-red
spectrophotometry, are also available. The methods are in general
sensitive to 0.003 ppm in milk and 0.05 ppm in most other foods,
though under favourable conditions greater sensitivity can, if
appropriate, be obtained.
In those cases where analyses have provided information on the
contents of degradation products of DDT, such as DDE and DDD, the
figures for these compounds should be included in the analytical
report.
RECOMMENDATION FOR TOLERANCES
The recommendations for tolerances are shown in Table 2.
These tolerance values are considered to be temporary, will be kept
under study, and will be reconsidered no later than three years hence.
These values are in excess of values that would be calculated from the
factors utilized in considering tolerance values for other compounds
in this booklet.
New studies in toxicology, metabolism or residues may permit, within
the next three years a more permanent recognition of these levels, or
on the other hand, it may be necessary to markedly lower the tolerance
recommendations. It is unlikely that future tolerance recommendations
will be higher than those prescribed here.
TABLE 2. RECOMMENDED TOLERANCES FOR DDT
Food type Temporary
Restricted to 3 years
ppm
Vegetables 1.0-7.0
Meat, fish, poultry 7.0 (in fat)
Tree fruits 7.0
Berries (cane) 1.0
Citrus 4.0
Shelled nuts 1.0
Milk (whole) Administrative decision .0045 ppm
Milk products Practical residue 0.2 ppm
The "administrative decision" and the "practical residue"
recommendations are made because the widespread use and stability of
DDT have resulted in small residues being ubiquitous. Small residues
have been found to be present in most dairy products. This is
considered to be undesirable but is also unavoidable at the present
time. Since this residue is generally not present from direct
application to the animals or their feed, no tolerance recommendation
is made. However, to assist regulatory officials in identifying those
samples which have residues much in excess of the unavoidable level, a
"practical residue level" of 0.20 ppm DDT is suggested.
Residues of DDT in animal products are invariably associated with
varying amounts of the DDT metabolites DDE and DDD. In many instances
the residues of either of these or the sum of the two exceeds the
residue of DDT. The WHO Expert Committee on Pesticide Residues
estimated an ADI for DDT but not for the other two. They noted that
residues of all three should be determined, but they postponed
consideration of DDE and DDD. Therefore, only DDT residues were taken
into consideration in preparing the temporary tolerance for DDT.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Andronova, G. P. (1956) Dissertation, F. F. Erisman's State Research
Institute for Hygiene, Moscow
Anon. (1951) Report of the Committee on Pesticides of the Council on
Pharmacy and Chemistry, J. Amer. med. Ass., 145, 728
Bishopp, F. C. (1946) Amer. J. Publ. Hlth, 36, 593
Cameron, G. R. & Burgess, F. (1945) Brit. med. J., 1, 865
Cueto, C., Barnes, A. G. & Mattson, A. M. (1956) J. Agr. Food Chem.,
4 (2), 94.3
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Dale, W. E., Gaines, T. B. & Hayes, W. J., jr (1965) Science, 142,
1474
Denes, A. (1962) Nahrung, 6, 48
Draize, J. H., Woodard, G., Fitzhugh, O. G., Nelson, A. A., Smith,
R. B. & Calvery, H. O. (1944) Chem. Eng. News, 22, 1503
Durham, W. F., Armstrong, J. F. & Quinby, G. E. (1965) Arch.
Environm. Hlth., 11, 16
Durham, W. F., Ortega, P. & Hayes, W. J., Jr (1963) Arch. int.
Pharmacodyn., 141, 111
Egan, H., Goulding, R., Roburn, J. & Tatton, J. (1965) Brit. med.
J., ii, 66
Fitzhugh, O. G. (1948) Ind. Eng. Chem., 704
Fitzhugh, O. G. & Nelson, A. A. (1947) J. Pharmacol. exp. Ther.,
89, 18
Gerboth, G. & Schwabe, U. (1964) Arch. exp. Path., 246, 469
Gerebtzoff, M. A. & Philippot, E. (1952) Experientia (Basel), 8
(10), 395
Gerebtzoff, M. A., Dallemagne, M. J. & Philippot, E. (1950) C.R.
Soc. Biol. (Paris), 144, 1135
Halacka, K., Hakl, J. & Vymetal, F. (1965) Cs. Hyg., 10, 188
Hart, L. G. & Fouts, J. R. (1963) Proc. Soc. exp. Biol. (N.Y.),
114, 388
Hayes, W. J., jr & Dale, W. E. (1964) Toxicol. Appl. Pharmacol., 6,
349
Hayes, W. J., jr, Dale, E. & Pirkle, C. I. (1964) Unpublished report
Hayes, W. J., jr, Durham, W. F. & Cueto, C., jr (1956) J. Amer. med.
Ass., 162 (9), 890
Hayes, W. J. jr., Walker, K. C., Elliott, J. W. & Durham, W. F. (1958)
Arch. industr. Hlth, 18, 398
Haymaker, W., Ginzler, A. M. & Ferguson, R. L. (1946) Amer. J. Med.
Sci., 212, 423
Hoffmann, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
Environm. Hlth, 9, 387
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Juchau, M. R., Gram, T. E. & Fouts, J. R. (1966) Gastroenterology,
51, 213
Judah, J D. (1949) Brit. J. Pharmacol., 4, 120
Kemény, T. & Tarján, R. (1966) Orvosi Hetilap, 107 (30), 1407
Khairy, M. (1959) Quart. J. exp. Psychol., 11, 84
Kimbrough, R., Gaines, T. B. & Sherman, J. D. (1964) J. Nat. Cancer
Inst., 33, 215
Klein, A. K., Laug, E. P., Datta, P. R., Watts, J. O. & Chen, J. T.
(1964) J. Assoc. Offic. Agr. Chem., 47, 1129
Laug, E. P., Kunze, F. M. & Prickett, C. S. (1951) Arch. industr.
Hyg., 3, 245
Laug, E. P., Nelson, A. A., Fitzhugh, O. G. & Kunze, F. M. (1950) J.
Pharmacol. exp. Ther., 98, 268
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials,
U.S., 16, 3, 47, 126
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Fd. Cosmet. Toxicol.,
3, 591
Maier-Bode, H. (1960) Med. exp. (Basel), 3, 284
Mattson, A. M., Spillane, J. T., Baker, C. & Pearce, G. W. (1953)
Anal. Chem., 25, 1065
McLean, A. E. & McLean, E. K. (1966) Biochem, J., 100, 564
Mendel, J. L. & Walton, M. S. (1966) Science, 151, 1527
Morello, A. (1965) Canad. J. Biochem., 43 (8), 1289
Ortega, P. (1962) Fed. Proc., 21, 306
Ortega, P. (1966) Lab. Invest., 15, 657
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. (1956)
Publ. Hlth Monogr., 43, 27
Ortelee, M. F. (1958) Arch. industr. Hlth, 18, 433
Pearce, G. W., Mattson, A. M. & Hayes W. J., jr (1952) Science,
116, 254
Philips, F. & Gilman, A. (1946) J. Pharmacol. exp. Ther., 86, 213
Phillips, W. E. G. (1963) Canad. J. Biochem., 41, 1793
Pinto, J. D., Camien, M. N. & Dunn, M. S. (1965) J. biol. Chem.,
240, 2148
Quinby, G. E., Armstrong, J. F. & Durham, W. F. (1965) Nature,
207, 726
Quinby, G. E., Hayes, W. J., jr, Armstrong, J. F. & Durham, W. F.
(1965) J. Amer. med. Ass., 191, 175
Read, S. I., Murray, T. K. & McKinley, W. P. (1965) Canad. J.
Biochem., 43, 317
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. Ind. Med., 22, 220
Rothe, C. F., Mattson, A. M., Nuelsein, R. M. & Hayes, W. J., jr
(1957) Arch. industr. Hlth, 16 (1), 82
Shillinger, Y. I., Naumova, L. P. & Pekerman, S. W. (1955) Vop.
Pitan., 14, 41
Smith, M. I. & Stohlman, E. F. (1944) Publ. Hlth Rep. (Wash.), 59,
984
Spicer, S. S., Sweeney, T. R., von Oettingen, W. F., Lillie, H. D. &
Neal, P. A. (1947) Vet. med., 42 (8), 289
Stemmer, K. L. & Hamdi, E. (1964) Unpublished report submitted by
Kettering laboratory
Street, J. C. & Blau, A. D. (1966) Toxicol. Appl. Pharmacol., 8,
497
Tinsley, I. J. (1965) Biochem. Pharmacol., 14, 847
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Woodard, G., Nelson, A. A. & Calvery, H. O. (1944) J. Pharm. exp.
Ther., 82, 152
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
AOAC (1966) Changes in Methods of Analysis, J. Assoc. Offic. Anal.
Chem. 49, 222-230
Cummings, J. G. (1965) Pesticide Residues in Total Diet Samples, J.
Assoc. Offic. Agr. Chem. 48: 1177-1180
de Faubert Maunder, M. J., Egan, H., Godly, E. W., Hammond, E. W.,
Roburn, J., Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues, Analyst,
89, 168-174
Egan, H., Holmes, D. C., Roburn, J. and Tatton, J. O'G. (1966)
Pesticide Residues in Foodstuffs in Great Britain. II-Persistent
Organochlorine Pesticide Residues in Selected Foods, J. Sci. Fd.
Agric., 17: 563-569
Farrow, R. P., Elkins, E. R. Jr. and Cook, R. W. (1966) Conversion of
DDT to TDE in Canned Spinach. J. Agr. Food Chem. 14/4: 430-434
Kallman, B. J. and Andrews, A. K. (1963) Reductive Dechlorination of
DDT to DDD by Yeast, Science 141: 1050-1051
Klein, A. K., Laug, E. P., Datta, P. R., Watts, J. O., and Chem, J. T.
(1964) Metabolites: Reductive Dechlorination of DDT to DDD and
Isomeric Transformation of o,p'-DDT to p,p'-DDT in vivo, J. Assoc.
Offic. Agr. Chem. 47: 515-531
Lewis, D. T. (1964, 1965) Report of the Government Chemist, H.M.S.O.
London
Menzie, C. M. (1966) Metabolism of Pesticides Special Scientific
Report - Wildlife No. 96, Fish and Wildlife Service, Department of the
Interior Washington, D.C.
Metcalf, R. L. (1966) Metabolism and Fate of Pesticides in Plants and
Animals. Pages 230-250, Scientific Aspects of Pesticide Control.
Publication 1402, National Academy of Sciences - National Research
Council, Washington, D.C.
Mills, P. A. (1963) Total Diet Study: C. Pesticide Content. J. Assoc.
Offic. Agr. Chem. 46: 762-767
Miskus, R. P., and Blair, D. B. (1965) Conversion of DDT to DDD by
Bovine Rumen Fluid, Lake Water, and Reduced Porphyrins, J. Agr. Food
Chem. 13/5: 481-483
Peterson, J. E. and Robison W. H. (1964) Metabolic Products of
p,p'-DDT in the Rat, Toxicology and Applied Pharmacology 6: 321-327
Williams, S. (1964) Pesticide Residues in Total Diet Samples, J.
Assoc. Offic. Agr. Chem. 47: 815-821
 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
DDT is only slightly absorbed through the skin, but the degree of
absorption depends on the vehicle used (Cameron & Burgess, 1945).
After oral administration, most of the dose is found unchanged in the
faeces, but some absorption may occur, particularly in the presence of
lipids. The absorbed DDT is transformed into
2,2-di-(p-chlorophenyl)-1, 1-dichloro-ethylene (DDE) (Pearce et al.,
1952; Mattson et al., 1953) and into 2,2-di-(p-chlorophenyl)-acetic
acid (DDA) (Spicer et al., 1947; Judah, 1949, Hayes et al., 1956).
Some unknown compounds are also found (Spicer et al., 1947; Hayes et
al., 1956; Rothe et al., 1957; Cueto et al., 1956). Both DDT and DDE
accumulate in the fat (see Observations on Man). DDA is excreted in
the urine and in a combined form in the bile (Durham et al., 1963,
1965; Pinto et al., 1965).
A related insecticide, 1,1-dichloro-2,2-di-(p-chlorophenyl)-ethane
(DDD) was found in the liver of rats fed DDT (Klein et al., 1964).
Evidence that the conversion from DDT to DDD is accomplished by the
intestinal flora of the rat has also been presented (Mendel & Walton,
1966).
A level of 500 ppm of DDT in the diet of rats for 2 weeks or injection
of 25 mg/kg daily for 3 days increased the hepatic microsomal enzymic
metabolism of a number of drugs (Harts & Fouts, 1963). A similar
effect was observed in monkeys injected with 5 mg/kg/day for 7 days
(Juchau et al., 1966). As little as 1 mg/kg to rats was sufficient to
reduce pentobarbital sleeping time (Gerboth & Schwabe, 1964). Also
Morello (1966) showed that 3-4 days after a single dose of DDT in the
rat the ability of the liver microsomes to metabolize DDT was markedly
increased. It has been postulated by Street & Blau (1966) that DDT
also enhances the metabolism of dieldrin by microsomal enzymes; as
little as 5 ppm of DDT in the diet of rats decreased the fat storage
of simultaneously ingested dieldrin. However, a single dose of 75
mg/kg a week before treatment with carbon tetrachloride reduced the
LD50 of the latter in rats given either a complete or a protein-free
diet (McLean & McLean, 1966). Furthermore, dietary levels of 5-200 ppm
of DDT decreased the liver glucose-6-phosphate dehydrogenase activity
in rats (Tinsley, 1965).
In adult rats, concentrations of 10 or 100 ppm of DDT in the diet have
been shown to interfere with the storage and metabolism of vitamin A
in the liver; this was not observed in new-born or young rats which
have poor reserves of vitamin A (Phillips, 1963; Read et al., 1965).
In addition, whereas liver carboxylesterase activity was markedly
increased by DDT in weanling and adult rats, the same effect could not
be demonstrated in new-born rats (Read et al., 1965).
The toxic effects, revealing involvement of the central nervous system
(such as hypersensitivity, excitability, generalized trembling,
convulsions, paralysis) appear within 5-10 minutes of intravenous
administration and after a latent period of some hours following oral
administration. Death occurs as a result of respiratory arrest in the
rat, rabbit, cat and monkey, and of ventricular fibrillation in the
dog. It is the consensus of the literature that the significant action
of DDT is on the nervous system; when ventricular fibrillation occurs,
it is precipitated by adrenaline released from the adrenal medulla by
stimulation of the sympathetic nervous system (Phillips & Gilman,
1946).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 150-400* Draize et al., 1944
Bishopp, 1946
Rat new-born Intragastric >4 000 Lu et al., 1965
Rat pre-weanling Oral 437 Lu et al., 1965
Rat adult Oral 194 Lu et al., 1965
Rat Oral 150-420* Smith & Stohlman, 1944
Woodard et al., 1944
Rat Oral 800 Cameron & Burgess, 1945
Guinea-pig Oral 400 Draize et al., 1944
Rabbit Oral 250-500* Cameron & Burgess, 1945
Bishopp, 1946
Dog Intravenous Approximately Philips & Gilman, 1946
50
Cat Oral 400-600* Philips & Gilman, 1946
Monkey Oral >200 Bishopp, 1946
Horse Oral >300 Bishopp, 1946
Chicken Oral >1 300 Bishopp, 1946
* The LD50 dose of DDT varies within wide limits, depending on sex and the
type of vehicle used.
In rats given a single oral dose of DDT sufficient to kill about half
of them, the severity of symptoms corresponded with the concentration
of the unchanged compound in the brain (Dale et al., 1963).
Furthermore, approximately the same concentration of DDT is found in
the brain of rats killed by DDT no matter whether the dosage is acute,
subacute, or chronic (Hayes & Dale, 1964).
The fatal dose for man is difficult to establish but is generally
taken to be of the order of 500 mg/kg body-weight. A dose of the order
of 10 mg/kg body-weight can give rise in some subjects, but not in
all, to toxic symptoms (nausea, headache, sweating), and with doses of
16 mg/kg body-weight upwards, convulsions often occur (Anon., 1951).
Special studies
Daily oral doses of 0.2 mg/kg for 10 days produced functional changes
in the conditioned reflex pattern which appeared after 5-7 doses and
persisted 5-7 days after the end of exposure (Andronova, 1956).
Behavioural studies have also been made on groups of rats fed diets
containing 100, 200, 400 and 600 ppm DDT. Problem-solving and speed of
locomotion were unaffected by these doses of DDT. There was a
significant alteration in the patterns of locomotion in these rats and
their reactions to stress involving visual stimuli were reduced
(Khairy, 1959).
Short-term studies
Rat. Rats, in groups of 8 males and 8 females, were given diets with
1, 5, 10, and 50 ppm of DDT for 23 weeks. At concentrations of 5 ppm
and higher, histopathological changes in the liver were found (Laug et
al., 1950).
A series of experiments was carried out with small groups of rats, 178
males and 104 females in all, which were given diets containing DDT in
concentrations from traces up to 5000 ppm for periods ranging from one
to 14 months. In some cases observation of the animals continued after
cessation of treatment. At levels above 400 ppm changes in growth rate
were noted. Above 200 ppm liver enlargement was seen. In the male
animals with intakes of 5 ppm and above, specific histological lesions
in the liver were seen. These lesions consisted of hypertrophy of the
parenchymal cells, increased lipid deposits, marginal localization of
cytoplasmic granules and, above all, the appearance of complex
cytoplasmic inclusions of a lipid nature, called "lipospheres". In the
females these liver lesions were only seen in diets containing 200 ppm
or more. Necrotic lesions were seen only at concentrations above 1000
ppm. The authors noted that lipospheres and other changes considered
characteristic of DDT were more prominent in male rats although
females store more of the compound and were more susceptible to
poisoning. For this and other reasons, they speculated that the
changes might be adaptive. They noted also that earlier work had
failed to demonstrate similar changes in other species (Ortega et al.,
1956).
In an electron-microscopical examination of the liver of rats fed
diets containing 5-2500 ppm of DDT for 2-18 months, the following
cytoplasmic abnormalities were found: slight proliferation of the
endoplasmic reticulum, peripheral distribution of the ribosomes and
intracytoplasmic inclusions consisting of aggregates of membranes very
rich in lipids. At 100 ppm changes were seen after 2 months (Ortega,
1962, 1966).
Similar changes were found in another study in which the dietary
intake of DDT was 10 ppm. The accumulation of endoplasmic reticulum
has been regarded as evidence of cellular hypertrophy (Stemmer &
Hamdi, 1964).
In one study in which obesity was induced in rats with a high fat
diet, DDT was added into the diet at a dose level of 3.6 mg/kg/day for
up to 10 months. A comparable incidence of chloroleukemia was observed
in rats receiving DDT and in those given the high fat diet only
(Kimbrough et al., 1964).
Dog. Dogs were given DDT orally. Those receiving 100 mg/kg
body-weight daily died within 7 weeks. Animals subjected to lower
doses survived and seemed normal even after 50 weeks (Draize et al.,
1944). When the experiment was continued for 3 years, the animals
receiving doses of 50 and 80 mg/kg bodyweight developed jaundice and
haemorrhagic symptoms. Those receiving 10 mg/kg body-weight daily
showed no ill effects (Hayes et al., 1956; Lehman, 1952).
Dogs were given DDT by stomach-tube, in the form of a 10 per cent
solution in peanut oil in doses ranging from 150 to 350 mg/kg
body-weight for about 90 days. Some of the animals died. The authors
noted neurological symptoms, showing involvement of the cerebellum as
revealed by histological lesions (Haymaker et al., 1946).
Three dogs were given intramuscular injections of DDT in the form of a
10 per cent solution in olive oil at the rate of 100 mg/kg body-weight
daily for 25 to 30 days. A control animal received 1 ml of olive oil
in the same way. The experimental animals showed a temporary loss of
weight. The kidneys were discoloured and on histological examination
showed tubular damage. At the same time, proliferation was observed in
the lymphoid tissues (lymph nodes, spleen, bone marrow) and the wall of
the small intestine, but the blood showed no leukaemic characteristics
(Gerebtzoff et al., 1950; Gerebtzoff & Philippot, 1952).
Monkey. Monkeys given DDT orally in a dose of 0.2 mg/kg body-weight
for 7-9 months developed symptoms of hepatitis. After a year, the
animals showed liver enlargement and hyperglycaemia (Shillinger et
al., 1955). These liver changes were not confirmed in another
experiment on monkeys lasting 7.5 years (Durham et al., 1963).
Long-term studies
Mouse. In one experiment, 683 mice, spread over 5 generations, were
given DDT at 0.3-0.6 mg/kg daily. The background DDT content of the
diet given to 406 controls corresponded to an intake of 0.03-0.05
mg/kg daily. The over-all incidence of leukaemia and other malignant
tumours was 3.5 and 5.4 per cent respectively in the experimental
animals compared to 0.2 and 0.9 per cent in the controls. The
difference was obvious within each generation. The greatest tumour
incidence was seen in the fourth and fifth generations (Kemény &
Tarján, 1966).
Rat. Groups of 12 male rats were subjected for 2 years to diets
containing 0, 100, 200, 400 and 800 ppm of DDT, in the form of a 10
per cent solution in corn oil. In another experiment, groups each of
24 rats (12 males and 12 females) were given, during the same period,
diets containing 0, 100, 200, 400 and 800 ppm. Also additional groups
of 24 animals received 600 and 800 ppm incorporated in their feed in a
dry state. In the groups receiving 400 ppm and above, an increase in
the mortality rate was seen in relation to the dose. Apart from
nervous symptoms at doses of 400 ppm and above, typical liver lesions
were found at all concentrations. Hepatic cell tumours were seen in 4
out of 75 animals and 11 other rats showed nodular adenomatoid
hyperplasia (Fitzhugh & Nelson, 1947).
In an experiment with rats fed for 2 years on a diet containing 10 ppm
of DDT, histological liver lesions were also observed (Fitzhugh,
1948). Groups of 80 young rats each (40 male and 40 female) were fed
0, 0.25, 12.5 and 25 ppm of DDT. The histological lesions of the liver
observed were always slight and, according to the authors,
non-specific, but nevertheless they were more frequent with DDT than
with aldrin or dieldrin, which were administered in the same
concentrations for comparative purposes (Treon & Cleveland, 1955).
Experiments with 25 young rats did not show any harmful effects after
daily administration by stomach-tube of a dose of 10 µg/kg for 17
months (Klimmer, 1955).
Monkey. Twenty-four Rhesus monkeys, 12 males and 12 females, were
divided into groups and fed over periods as long as 7.5 years or more
on diets containing respectively 0, 5, 50, 200 and 5000 ppm of DDT.
All the animals subjected to the concentration of 5000 ppm showed
convulsions, accompanied by loss of appetite and a fall in weight. At
a concentration of 200 ppm (corresponding to daily doses of 2.2-5.54
mg/kg body-weight), no harmful effects were observed and, in
particular, no histological lesions in the liver or disturbances in
the functioning of that organ, as shown by the bromsulfthalein test
(Durham et al., 1963).
Observations on man. An experimental study was carried out of the
effects on man of prolonged ingestion of small doses of DDT (in the
form of oily solutions in capsules or emulsions in milk). The authors
used 51 volunteers for this study; 17 received a normal diet, 17
received 3.5 mg/kg body-weight and 17 received 35 mg DDT daily. The
last dose is approximately 0.5 mg/kg body-weight. Administration was
continued for as long as 18 months. The authors noted that the
accumulation of the insecticide in the fatty tissues and the urinary
excretion of its metabolite, DDA, were proportional to the dose of DDT
ingested. A state of equilibrium was reached after about a year and
the concentration of DDT accumulated in the fatty tissues reached an
average of 234 ppm (101-367 ppm) in subjects who had ingested 35 mg of
DDT per day. Throughout the whole experiment, no subject complained of
malaise, nor did any ill-effects appear that could be attributed to
the ingestion of DDT (Hayes et al., 1956). The essential results were
confirmed in a separate investigation in which dosage with DDT lasted
for 21 months and the volunteers were observed for an additional 27
months. Fourteen men received 35 mg/man/ day; 6 received 3.5
mg/man/day, and 4 men served as controls. The study also revealed the
relationship between storage of DDT and the urinary excretion of DDA
and demonstrated that the loss of DDT from storage in man following
cessation of dosage is slow - only about two-thirds in 27 months
(Hayes et al., 1964).
Observations were made on 40 workers engaged, over a period of years,
in the manufacturing of specialities based on DDT, under conditions
where suitable precautions had not been taken to protect their skin.
According to the DDA concentration found in the urine, these subjects
had absorbed daily as much DDT as volunteers who had ingested 35 mg of
the insecticide per day in the experiment mentioned above. Thorough
medical and biological tests failed to reveal any toxic symptoms, even
in workers exposed to the toxic product for 6.5 years (Ortelee, 1958).
Several studies on DDT and DDE storage in human fat in several parts
of the world have been presented (Laug, 1951; Hayes et al., 1956;
Hayes et al., 1958; Denes 1962; Maier-Bode, 1960; Dale et al., 1963;
Hunter et al., 1963; Hoffmann et al., 1964; Dale et al., 1965; Egan et
al., 1965; Halacka, et al., 1965; Quinby et al., 1965b; Robinson et
al., 1965; Zavon et al., 1965). DDT was also found in a stillborn
infant and in very young babies (Halacka et al., 1965).
In these studies the average total content in human fat ranged between
2 and 30 ppm; the percentage of DDE of total DDT stored ranged between
34 and 77 per cent. It has been shown that the storage of DDT in man
is directly proportional to intake over a wide range of doses (0.04 to
35.0 mg/man/day), so that from the level in the fat the daily dose can
be estimated. Thus, it can be calculated that the highest average
intake of DDT of any human population yet observed is about 0.7-0.8
mg/man/day. From the data which have been reported, it is also
apparent that in the United States of America, where the level of DDT
in human fat has been repeatedly investigated in the general
population over a significant number of years, it has not increased
since 1950 (Durham et al., 1965).
It has also been repeatedly indicated that women and cows secrete DDT
in the milk. Two recent studies indicated an average of total DDT
(i.e. DDT + DDE) of 0.128 ppm (57 per cent as DDE) and 0.170 ppm (58
per cent as DDE). The content of DDT in cows' milk was found to be
lower (Egan et al., 1965; Quinby et al., 1965). The percentage of the
total DDT intake which is eliminated in the milk has been demonstrated
to be smaller in cows than in women (Quinby et al., 1965).
Comments
The evaluation of DDT is difficult because wide species differences
occur. The rat appears to be the most susceptible species; liver
cellular changes were produced by 5 ppm in the diet. In dogs and
monkeys these changes were not seen. Dogs given 10 mg/kg
body-weight/day for 3 years and monkeys given 2-5 mg/kg
body-weight/day for up to 7 years showed no ill-effects. However, in
another study, monkeys given 0.2 mg/kg body-weight/day for one year
showed signs of hepatitis and liver enlargement. In man, as much as
0.5 mg/kg body-weight/day for up to 21 months were without effect
apart from storage in the fat. However, the possibility that the
latter might have deleterious consequences later in life cannot be
ruled out. In addition, it has not been demonstrated that metabolic
changes in the liver cells comparable to those observed in rats do not
take place in man. The Committee realizes that the figure reached as
an estimate for an ADI can be less than the actual intake for humans
in some parts of the world. It might correspond to the intake of DDT
of a baby exclusively breast-fed. (In this respect the significance of
the lower acute toxicity of DDT for new-born rats is uncertain.)
Furthermore, in addition to unchanged DDT, the residues also contain
metabolites of DDT. There is a need for uniformity in reporting the
results of residue analyses. The reports should at least indicate the
percentages of unchanged DDT, DDE and TDE. Some of the metabolites are
probably less toxic than DDT itself. However, more information is
necessary on this point. At the present time the Committee remains
concerned about the storage of DDT which occurs in all species and
about the cellular changes produced in the liver of rats by DDT and by
other compounds chemically related to it.
TOXICOLOGICAL EVALUATION
Estimate of acceptable daily intake in man
0-0.01 mg/kg body-weight
Further work required
Elucidation of the significance of the finding that DDT is one of the
compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
DDT is used to a minor extent as a soil treatment, primarily for the
control of cutworms which attack vegetable crops. It is suggested for
use in many countries on a wide variety of food crops. It is suggested
for the control of about 20 different insects which attack cane
fruits, about 50 different insects which attack vegetables, 50
different insects which attack tree fruits, as well as for control of
insects of nut trees and other food crops. The usual rate of
application is about 1 to 2 lb of the active chemical per acre;
however, some treatments may go as high as 10 lb per acre.
(b) Post-harvest treatments
Direct applications to stored food products are no longer advised.
However, there is some use in and around storage premises and
transport facilities, which may permit incurrence of small amounts in
products during storage and shipping.
(c) Other uses
DDT is used in many home gardens, in many households (forbidden in The
Netherlands), as a mothproofing agent in rugs and clothing in
restaurants and in public buildings. Many mosquito abatement
programmes make use of DDT.
National tolerances
Austria 7 ppm - a general informal tolerance.
Canada 7 ppm on many fruits and vegetables, fat of meat
animals.
United States 7 ppm on many fruits and vegetables, fat of meat
of America animals.
3.5 ppm on sweet corn.
1 ppm on potatoes.
Residues resulting from supervised trials
Although there have been many analyses made for DDT in agricultural
products, many of them were not made on controlled experiments
designed explicitly to ascertain the fate of the residue following
application. A summary of numerous data, which is too long to
reproduce here and which contains the related bibliography, is held at
the FAO headquarters in Rome. Table 1 has been prepared from this
summary. It contains estimates of the average high residues likely to
result from the practical use of DDT to control insects which attack
the different food commodities or that in animal products from animals
exposed to "unavoidable" or very limited feed residues.
TABLE 1. RESIDUES OF DDT EXPECTED FROM PRACTICAL USE
Food Residue
ppm
Vegetables
leafy 7
others 1-7
Meat, fish, poultry 7 (in fat)
Tree fruits 7
Berries (cane) 1
Citrus 4
Shelled nuts 1
Residues in food moving in commerce
Even though there is a continuing and widespread use of DDT, a very
large proportion of the food in commerce has very little DDT present;
seldom is a sample found with residue as high as the tolerance figures
quoted.
Of the large number of "total diet" samples analysed in the United
States of America since 1961, a high proportion have had detectable
amounts of DDT but no sample of any commodity grouping has exceeded
0.05 ppm DDT. (Mills 1963; Williams 1964; Cummings 1965.)
The Government Chemist Laboratory of Great Britain has analysed a
large number of samples of food products since 1962. They chose those
samples most likely to contain residues of DDT such as milk, butter
and fat of different meat animals. The residues of DDT, DDE, and DDD
for the years 1964 and 1965 for over 900 samples average less than
0.15 ppm, with the highest value found being 4.6 ppm. (Lewis 1964 and
1965; Egan et al 1966.)
The Netherlands Government has analysed a number of imported cereal
products and has found a large proportion to contain some residue of
DDT. Of 227 samples examined during 1964 and 1965, 36 per cent
contained DDT. The highest sample residue was 2.85 ppm, but most
samples ran from 0 to 0.5 ppm (Report CCPR 66-17 January 1966 Ministry
of Social Affairs and Public Health, Ministry of Agriculture,
Committee on Phytopharmacy.)
Fate of residues
Although DDT and some of its metabolic products can be altered
readily by chemical means, they are quite comparatively stable
compounds in many biological media. This low biodegradability coupled
with high fat solubility, in part, accounts for the persistence of
residues and their propensity to concentrate in fat tissue. The
stability and solubility also account for its concentration in certain
forms of life when it is part of the food chain (Metcalf 1966).
(a) In animals
It has been known for many years that DDE and DDA form from DDT
(Menzie 1966). DDA is an acid, is relatively water soluble, and thus
is eliminated much more readily than the fat soluble forms and does
not appear nearly so readily in the edible portion of food products of
animal origin. On the other hand, DDE is fat soluble and almost always
appears as a residue in animal products with DDT.
Only recently has it been well established that DDD (TDE) is a
metabolic product of DDT. (Kallman and Andrews 1963; Peterson and
Robison 1964; Klein et al 1964; Miskus and Blair 1965.) It has been
found as a residue associated with DDT in animal products for many
years, but since it has also had some use as a commercial pesticide,
it was not considered to be a metabolic product by most workers.
Whether it forms in animals as a result of enzymes endogenous to the
animal or only from intestinal bacteria or other organisms associated
with the animal has not been fully established.
Work by Klein et al 1964 has shown that o,p-DDT converts to p,p'-DDT
in vivo.
Both DDE and DDD are more stable in many biological systems than DDT;
therefore, in many instances they accumulate in time to a
concentration equal to or greater than DDT in the fat of animal
tissues. The hydroxy analogues of DDT (kelthane) and DDD can form from
DDT and DDD respectively and 4,4'-dichlorobenzophenone can form from
each (Menzie 1966).
(b) In plants
The meeting was not aware of any data on the effect of plant enzyme
systems on DDT; however, it is known that DDE does appear as a very
low level residue on many food crops which contain DDT (U.S.F.D.A.,
unpublished data).
(c) In storage and processing
DDT is stable under most of the conditions which prevail when it is a
residue on food products. Therefore, residues in food products will
not normally diminish greatly from most food products during shipping
and storage. It is especially stable in a fatty medium.
Recent studies by the National Canners Association (United States of
America) has shown that the amount of DDT on green beans does not
change over a 2-week storage period at 45° F (personal communication).
Since DDT does not penetrate significantly into non-fatty food
products, a large loss is shown when the surface is removed such as in
peeling, shelling, harsh brushing, milling, etc.
Recent studies by Farrow et al., 1966, showed that during the normal
heat processing of canned spinach approximately 50 per cent of the
original DDT was destroyed. However, DDD was formed equivalent to
about 20 per cent of the original DDT resulting in a net loss of about
30 per cent.
Methods of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of DDT (together with residues of a number
of other compounds including DDE and DDD). An example is the AOAC
system (1966) in which acetonitrile partition and Florisil column
clean-up are identified and measured by gas chromatography coupled
with thin layer or paper chromatography. Alternative clean-up systems
e.g. that of de Faubert Maunder et al. (1964) using dimethylformamide,
and other methods of confirmation of identity e.g. using infra-red
spectrophotometry, are also available. The methods are in general
sensitive to 0.003 ppm in milk and 0.05 ppm in most other foods,
though under favourable conditions greater sensitivity can, if
appropriate, be obtained.
In those cases where analyses have provided information on the
contents of degradation products of DDT, such as DDE and DDD, the
figures for these compounds should be included in the analytical
report.
RECOMMENDATION FOR TOLERANCES
The recommendations for tolerances are shown in Table 2.
These tolerance values are considered to be temporary, will be kept
under study, and will be reconsidered no later than three years hence.
These values are in excess of values that would be calculated from the
factors utilized in considering tolerance values for other compounds
in this booklet.
New studies in toxicology, metabolism or residues may permit, within
the next three years a more permanent recognition of these levels, or
on the other hand, it may be necessary to markedly lower the tolerance
recommendations. It is unlikely that future tolerance recommendations
will be higher than those prescribed here.
TABLE 2. RECOMMENDED TOLERANCES FOR DDT
Food type Temporary
Restricted to 3 years
ppm
Vegetables 1.0-7.0
Meat, fish, poultry 7.0 (in fat)
Tree fruits 7.0
Berries (cane) 1.0
Citrus 4.0
Shelled nuts 1.0
Milk (whole) Administrative decision .0045 ppm
Milk products Practical residue 0.2 ppm
The "administrative decision" and the "practical residue"
recommendations are made because the widespread use and stability of
DDT have resulted in small residues being ubiquitous. Small residues
have been found to be present in most dairy products. This is
considered to be undesirable but is also unavoidable at the present
time. Since this residue is generally not present from direct
application to the animals or their feed, no tolerance recommendation
is made. However, to assist regulatory officials in identifying those
samples which have residues much in excess of the unavoidable level, a
"practical residue level" of 0.20 ppm DDT is suggested.
Residues of DDT in animal products are invariably associated with
varying amounts of the DDT metabolites DDE and DDD. In many instances
the residues of either of these or the sum of the two exceeds the
residue of DDT. The WHO Expert Committee on Pesticide Residues
estimated an ADI for DDT but not for the other two. They noted that
residues of all three should be determined, but they postponed
consideration of DDE and DDD. Therefore, only DDT residues were taken
into consideration in preparing the temporary tolerance for DDT.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Andronova, G. P. (1956) Dissertation, F. F. Erisman's State Research
Institute for Hygiene, Moscow
Anon. (1951) Report of the Committee on Pesticides of the Council on
Pharmacy and Chemistry, J. Amer. med. Ass., 145, 728
Bishopp, F. C. (1946) Amer. J. Publ. Hlth, 36, 593
Cameron, G. R. & Burgess, F. (1945) Brit. med. J., 1, 865
Cueto, C., Barnes, A. G. & Mattson, A. M. (1956) J. Agr. Food Chem.,
4 (2), 94.3
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Dale, W. E., Gaines, T. B. & Hayes, W. J., jr (1965) Science, 142,
1474
Denes, A. (1962) Nahrung, 6, 48
Draize, J. H., Woodard, G., Fitzhugh, O. G., Nelson, A. A., Smith,
R. B. & Calvery, H. O. (1944) Chem. Eng. News, 22, 1503
Durham, W. F., Armstrong, J. F. & Quinby, G. E. (1965) Arch.
Environm. Hlth., 11, 16
Durham, W. F., Ortega, P. & Hayes, W. J., Jr (1963) Arch. int.
Pharmacodyn., 141, 111
Egan, H., Goulding, R., Roburn, J. & Tatton, J. (1965) Brit. med.
J., ii, 66
Fitzhugh, O. G. (1948) Ind. Eng. Chem., 704
Fitzhugh, O. G. & Nelson, A. A. (1947) J. Pharmacol. exp. Ther.,
89, 18
Gerboth, G. & Schwabe, U. (1964) Arch. exp. Path., 246, 469
Gerebtzoff, M. A. & Philippot, E. (1952) Experientia (Basel), 8
(10), 395
Gerebtzoff, M. A., Dallemagne, M. J. & Philippot, E. (1950) C.R.
Soc. Biol. (Paris), 144, 1135
Halacka, K., Hakl, J. & Vymetal, F. (1965) Cs. Hyg., 10, 188
Hart, L. G. & Fouts, J. R. (1963) Proc. Soc. exp. Biol. (N.Y.),
114, 388
Hayes, W. J., jr & Dale, W. E. (1964) Toxicol. Appl. Pharmacol., 6,
349
Hayes, W. J., jr, Dale, E. & Pirkle, C. I. (1964) Unpublished report
Hayes, W. J., jr, Durham, W. F. & Cueto, C., jr (1956) J. Amer. med.
Ass., 162 (9), 890
Hayes, W. J. jr., Walker, K. C., Elliott, J. W. & Durham, W. F. (1958)
Arch. industr. Hlth, 18, 398
Haymaker, W., Ginzler, A. M. & Ferguson, R. L. (1946) Amer. J. Med.
Sci., 212, 423
Hoffmann, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
Environm. Hlth, 9, 387
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Juchau, M. R., Gram, T. E. & Fouts, J. R. (1966) Gastroenterology,
51, 213
Judah, J D. (1949) Brit. J. Pharmacol., 4, 120
Kemény, T. & Tarján, R. (1966) Orvosi Hetilap, 107 (30), 1407
Khairy, M. (1959) Quart. J. exp. Psychol., 11, 84
Kimbrough, R., Gaines, T. B. & Sherman, J. D. (1964) J. Nat. Cancer
Inst., 33, 215
Klein, A. K., Laug, E. P., Datta, P. R., Watts, J. O. & Chen, J. T.
(1964) J. Assoc. Offic. Agr. Chem., 47, 1129
Laug, E. P., Kunze, F. M. & Prickett, C. S. (1951) Arch. industr.
Hyg., 3, 245
Laug, E. P., Nelson, A. A., Fitzhugh, O. G. & Kunze, F. M. (1950) J.
Pharmacol. exp. Ther., 98, 268
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials,
U.S., 16, 3, 47, 126
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Fd. Cosmet. Toxicol.,
3, 591
Maier-Bode, H. (1960) Med. exp. (Basel), 3, 284
Mattson, A. M., Spillane, J. T., Baker, C. & Pearce, G. W. (1953)
Anal. Chem., 25, 1065
McLean, A. E. & McLean, E. K. (1966) Biochem, J., 100, 564
Mendel, J. L. & Walton, M. S. (1966) Science, 151, 1527
Morello, A. (1965) Canad. J. Biochem., 43 (8), 1289
Ortega, P. (1962) Fed. Proc., 21, 306
Ortega, P. (1966) Lab. Invest., 15, 657
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. (1956)
Publ. Hlth Monogr., 43, 27
Ortelee, M. F. (1958) Arch. industr. Hlth, 18, 433
Pearce, G. W., Mattson, A. M. & Hayes W. J., jr (1952) Science,
116, 254
Philips, F. & Gilman, A. (1946) J. Pharmacol. exp. Ther., 86, 213
Phillips, W. E. G. (1963) Canad. J. Biochem., 41, 1793
Pinto, J. D., Camien, M. N. & Dunn, M. S. (1965) J. biol. Chem.,
240, 2148
Quinby, G. E., Armstrong, J. F. & Durham, W. F. (1965) Nature,
207, 726
Quinby, G. E., Hayes, W. J., jr, Armstrong, J. F. & Durham, W. F.
(1965) J. Amer. med. Ass., 191, 175
Read, S. I., Murray, T. K. & McKinley, W. P. (1965) Canad. J.
Biochem., 43, 317
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. Ind. Med., 22, 220
Rothe, C. F., Mattson, A. M., Nuelsein, R. M. & Hayes, W. J., jr
(1957) Arch. industr. Hlth, 16 (1), 82
Shillinger, Y. I., Naumova, L. P. & Pekerman, S. W. (1955) Vop.
Pitan., 14, 41
Smith, M. I. & Stohlman, E. F. (1944) Publ. Hlth Rep. (Wash.), 59,
984
Spicer, S. S., Sweeney, T. R., von Oettingen, W. F., Lillie, H. D. &
Neal, P. A. (1947) Vet. med., 42 (8), 289
Stemmer, K. L. & Hamdi, E. (1964) Unpublished report submitted by
Kettering laboratory
Street, J. C. & Blau, A. D. (1966) Toxicol. Appl. Pharmacol., 8,
497
Tinsley, I. J. (1965) Biochem. Pharmacol., 14, 847
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Woodard, G., Nelson, A. A. & Calvery, H. O. (1944) J. Pharm. exp.
Ther., 82, 152
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
AOAC (1966) Changes in Methods of Analysis, J. Assoc. Offic. Anal.
Chem. 49, 222-230
Cummings, J. G. (1965) Pesticide Residues in Total Diet Samples, J.
Assoc. Offic. Agr. Chem. 48: 1177-1180
de Faubert Maunder, M. J., Egan, H., Godly, E. W., Hammond, E. W.,
Roburn, J., Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues, Analyst,
89, 168-174
Egan, H., Holmes, D. C., Roburn, J. and Tatton, J. O'G. (1966)
Pesticide Residues in Foodstuffs in Great Britain. II-Persistent
Organochlorine Pesticide Residues in Selected Foods, J. Sci. Fd.
Agric., 17: 563-569
Farrow, R. P., Elkins, E. R. Jr. and Cook, R. W. (1966) Conversion of
DDT to TDE in Canned Spinach. J. Agr. Food Chem. 14/4: 430-434
Kallman, B. J. and Andrews, A. K. (1963) Reductive Dechlorination of
DDT to DDD by Yeast, Science 141: 1050-1051
Klein, A. K., Laug, E. P., Datta, P. R., Watts, J. O., and Chem, J. T.
(1964) Metabolites: Reductive Dechlorination of DDT to DDD and
Isomeric Transformation of o,p'-DDT to p,p'-DDT in vivo, J. Assoc.
Offic. Agr. Chem. 47: 515-531
Lewis, D. T. (1964, 1965) Report of the Government Chemist, H.M.S.O.
London
Menzie, C. M. (1966) Metabolism of Pesticides Special Scientific
Report - Wildlife No. 96, Fish and Wildlife Service, Department of the
Interior Washington, D.C.
Metcalf, R. L. (1966) Metabolism and Fate of Pesticides in Plants and
Animals. Pages 230-250, Scientific Aspects of Pesticide Control.
Publication 1402, National Academy of Sciences - National Research
Council, Washington, D.C.
Mills, P. A. (1963) Total Diet Study: C. Pesticide Content. J. Assoc.
Offic. Agr. Chem. 46: 762-767
Miskus, R. P., and Blair, D. B. (1965) Conversion of DDT to DDD by
Bovine Rumen Fluid, Lake Water, and Reduced Porphyrins, J. Agr. Food
Chem. 13/5: 481-483
Peterson, J. E. and Robison W. H. (1964) Metabolic Products of
p,p'-DDT in the Rat, Toxicology and Applied Pharmacology 6: 321-327
Williams, S. (1964) Pesticide Residues in Total Diet Samples, J.
Assoc. Offic. Agr. Chem. 47: 815-821
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
DDT is only slightly absorbed through the skin, but the degree of
absorption depends on the vehicle used (Cameron & Burgess, 1945).
After oral administration, most of the dose is found unchanged in the
faeces, but some absorption may occur, particularly in the presence of
lipids. The absorbed DDT is transformed into
2,2-di-(p-chlorophenyl)-1, 1-dichloro-ethylene (DDE) (Pearce et al.,
1952; Mattson et al., 1953) and into 2,2-di-(p-chlorophenyl)-acetic
acid (DDA) (Spicer et al., 1947; Judah, 1949, Hayes et al., 1956).
Some unknown compounds are also found (Spicer et al., 1947; Hayes et
al., 1956; Rothe et al., 1957; Cueto et al., 1956). Both DDT and DDE
accumulate in the fat (see Observations on Man). DDA is excreted in
the urine and in a combined form in the bile (Durham et al., 1963,
1965; Pinto et al., 1965).
A related insecticide, 1,1-dichloro-2,2-di-(p-chlorophenyl)-ethane
(DDD) was found in the liver of rats fed DDT (Klein et al., 1964).
Evidence that the conversion from DDT to DDD is accomplished by the
intestinal flora of the rat has also been presented (Mendel & Walton,
1966).
A level of 500 ppm of DDT in the diet of rats for 2 weeks or injection
of 25 mg/kg daily for 3 days increased the hepatic microsomal enzymic
metabolism of a number of drugs (Harts & Fouts, 1963). A similar
effect was observed in monkeys injected with 5 mg/kg/day for 7 days
(Juchau et al., 1966). As little as 1 mg/kg to rats was sufficient to
reduce pentobarbital sleeping time (Gerboth & Schwabe, 1964). Also
Morello (1966) showed that 3-4 days after a single dose of DDT in the
rat the ability of the liver microsomes to metabolize DDT was markedly
increased. It has been postulated by Street & Blau (1966) that DDT
also enhances the metabolism of dieldrin by microsomal enzymes; as
little as 5 ppm of DDT in the diet of rats decreased the fat storage
of simultaneously ingested dieldrin. However, a single dose of 75
mg/kg a week before treatment with carbon tetrachloride reduced the
LD50 of the latter in rats given either a complete or a protein-free
diet (McLean & McLean, 1966). Furthermore, dietary levels of 5-200 ppm
of DDT decreased the liver glucose-6-phosphate dehydrogenase activity
in rats (Tinsley, 1965).
In adult rats, concentrations of 10 or 100 ppm of DDT in the diet have
been shown to interfere with the storage and metabolism of vitamin A
in the liver; this was not observed in new-born or young rats which
have poor reserves of vitamin A (Phillips, 1963; Read et al., 1965).
In addition, whereas liver carboxylesterase activity was markedly
increased by DDT in weanling and adult rats, the same effect could not
be demonstrated in new-born rats (Read et al., 1965).
The toxic effects, revealing involvement of the central nervous system
(such as hypersensitivity, excitability, generalized trembling,
convulsions, paralysis) appear within 5-10 minutes of intravenous
administration and after a latent period of some hours following oral
administration. Death occurs as a result of respiratory arrest in the
rat, rabbit, cat and monkey, and of ventricular fibrillation in the
dog. It is the consensus of the literature that the significant action
of DDT is on the nervous system; when ventricular fibrillation occurs,
it is precipitated by adrenaline released from the adrenal medulla by
stimulation of the sympathetic nervous system (Phillips & Gilman,
1946).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 150-400* Draize et al., 1944
Bishopp, 1946
Rat new-born Intragastric >4 000 Lu et al., 1965
Rat pre-weanling Oral 437 Lu et al., 1965
Rat adult Oral 194 Lu et al., 1965
Rat Oral 150-420* Smith & Stohlman, 1944
Woodard et al., 1944
Rat Oral 800 Cameron & Burgess, 1945
Guinea-pig Oral 400 Draize et al., 1944
Rabbit Oral 250-500* Cameron & Burgess, 1945
Bishopp, 1946
Dog Intravenous Approximately Philips & Gilman, 1946
50
Cat Oral 400-600* Philips & Gilman, 1946
Monkey Oral >200 Bishopp, 1946
Horse Oral >300 Bishopp, 1946
Chicken Oral >1 300 Bishopp, 1946
* The LD50 dose of DDT varies within wide limits, depending on sex and the
type of vehicle used.
In rats given a single oral dose of DDT sufficient to kill about half
of them, the severity of symptoms corresponded with the concentration
of the unchanged compound in the brain (Dale et al., 1963).
Furthermore, approximately the same concentration of DDT is found in
the brain of rats killed by DDT no matter whether the dosage is acute,
subacute, or chronic (Hayes & Dale, 1964).
The fatal dose for man is difficult to establish but is generally
taken to be of the order of 500 mg/kg body-weight. A dose of the order
of 10 mg/kg body-weight can give rise in some subjects, but not in
all, to toxic symptoms (nausea, headache, sweating), and with doses of
16 mg/kg body-weight upwards, convulsions often occur (Anon., 1951).
Special studies
Daily oral doses of 0.2 mg/kg for 10 days produced functional changes
in the conditioned reflex pattern which appeared after 5-7 doses and
persisted 5-7 days after the end of exposure (Andronova, 1956).
Behavioural studies have also been made on groups of rats fed diets
containing 100, 200, 400 and 600 ppm DDT. Problem-solving and speed of
locomotion were unaffected by these doses of DDT. There was a
significant alteration in the patterns of locomotion in these rats and
their reactions to stress involving visual stimuli were reduced
(Khairy, 1959).
Short-term studies
Rat. Rats, in groups of 8 males and 8 females, were given diets with
1, 5, 10, and 50 ppm of DDT for 23 weeks. At concentrations of 5 ppm
and higher, histopathological changes in the liver were found (Laug et
al., 1950).
A series of experiments was carried out with small groups of rats, 178
males and 104 females in all, which were given diets containing DDT in
concentrations from traces up to 5000 ppm for periods ranging from one
to 14 months. In some cases observation of the animals continued after
cessation of treatment. At levels above 400 ppm changes in growth rate
were noted. Above 200 ppm liver enlargement was seen. In the male
animals with intakes of 5 ppm and above, specific histological lesions
in the liver were seen. These lesions consisted of hypertrophy of the
parenchymal cells, increased lipid deposits, marginal localization of
cytoplasmic granules and, above all, the appearance of complex
cytoplasmic inclusions of a lipid nature, called "lipospheres". In the
females these liver lesions were only seen in diets containing 200 ppm
or more. Necrotic lesions were seen only at concentrations above 1000
ppm. The authors noted that lipospheres and other changes considered
characteristic of DDT were more prominent in male rats although
females store more of the compound and were more susceptible to
poisoning. For this and other reasons, they speculated that the
changes might be adaptive. They noted also that earlier work had
failed to demonstrate similar changes in other species (Ortega et al.,
1956).
In an electron-microscopical examination of the liver of rats fed
diets containing 5-2500 ppm of DDT for 2-18 months, the following
cytoplasmic abnormalities were found: slight proliferation of the
endoplasmic reticulum, peripheral distribution of the ribosomes and
intracytoplasmic inclusions consisting of aggregates of membranes very
rich in lipids. At 100 ppm changes were seen after 2 months (Ortega,
1962, 1966).
Similar changes were found in another study in which the dietary
intake of DDT was 10 ppm. The accumulation of endoplasmic reticulum
has been regarded as evidence of cellular hypertrophy (Stemmer &
Hamdi, 1964).
In one study in which obesity was induced in rats with a high fat
diet, DDT was added into the diet at a dose level of 3.6 mg/kg/day for
up to 10 months. A comparable incidence of chloroleukemia was observed
in rats receiving DDT and in those given the high fat diet only
(Kimbrough et al., 1964).
Dog. Dogs were given DDT orally. Those receiving 100 mg/kg
body-weight daily died within 7 weeks. Animals subjected to lower
doses survived and seemed normal even after 50 weeks (Draize et al.,
1944). When the experiment was continued for 3 years, the animals
receiving doses of 50 and 80 mg/kg bodyweight developed jaundice and
haemorrhagic symptoms. Those receiving 10 mg/kg body-weight daily
showed no ill effects (Hayes et al., 1956; Lehman, 1952).
Dogs were given DDT by stomach-tube, in the form of a 10 per cent
solution in peanut oil in doses ranging from 150 to 350 mg/kg
body-weight for about 90 days. Some of the animals died. The authors
noted neurological symptoms, showing involvement of the cerebellum as
revealed by histological lesions (Haymaker et al., 1946).
Three dogs were given intramuscular injections of DDT in the form of a
10 per cent solution in olive oil at the rate of 100 mg/kg body-weight
daily for 25 to 30 days. A control animal received 1 ml of olive oil
in the same way. The experimental animals showed a temporary loss of
weight. The kidneys were discoloured and on histological examination
showed tubular damage. At the same time, proliferation was observed in
the lymphoid tissues (lymph nodes, spleen, bone marrow) and the wall of
the small intestine, but the blood showed no leukaemic characteristics
(Gerebtzoff et al., 1950; Gerebtzoff & Philippot, 1952).
Monkey. Monkeys given DDT orally in a dose of 0.2 mg/kg body-weight
for 7-9 months developed symptoms of hepatitis. After a year, the
animals showed liver enlargement and hyperglycaemia (Shillinger et
al., 1955). These liver changes were not confirmed in another
experiment on monkeys lasting 7.5 years (Durham et al., 1963).
Long-term studies
Mouse. In one experiment, 683 mice, spread over 5 generations, were
given DDT at 0.3-0.6 mg/kg daily. The background DDT content of the
diet given to 406 controls corresponded to an intake of 0.03-0.05
mg/kg daily. The over-all incidence of leukaemia and other malignant
tumours was 3.5 and 5.4 per cent respectively in the experimental
animals compared to 0.2 and 0.9 per cent in the controls. The
difference was obvious within each generation. The greatest tumour
incidence was seen in the fourth and fifth generations (Kemény &
Tarján, 1966).
Rat. Groups of 12 male rats were subjected for 2 years to diets
containing 0, 100, 200, 400 and 800 ppm of DDT, in the form of a 10
per cent solution in corn oil. In another experiment, groups each of
24 rats (12 males and 12 females) were given, during the same period,
diets containing 0, 100, 200, 400 and 800 ppm. Also additional groups
of 24 animals received 600 and 800 ppm incorporated in their feed in a
dry state. In the groups receiving 400 ppm and above, an increase in
the mortality rate was seen in relation to the dose. Apart from
nervous symptoms at doses of 400 ppm and above, typical liver lesions
were found at all concentrations. Hepatic cell tumours were seen in 4
out of 75 animals and 11 other rats showed nodular adenomatoid
hyperplasia (Fitzhugh & Nelson, 1947).
In an experiment with rats fed for 2 years on a diet containing 10 ppm
of DDT, histological liver lesions were also observed (Fitzhugh,
1948). Groups of 80 young rats each (40 male and 40 female) were fed
0, 0.25, 12.5 and 25 ppm of DDT. The histological lesions of the liver
observed were always slight and, according to the authors,
non-specific, but nevertheless they were more frequent with DDT than
with aldrin or dieldrin, which were administered in the same
concentrations for comparative purposes (Treon & Cleveland, 1955).
Experiments with 25 young rats did not show any harmful effects after
daily administration by stomach-tube of a dose of 10 µg/kg for 17
months (Klimmer, 1955).
Monkey. Twenty-four Rhesus monkeys, 12 males and 12 females, were
divided into groups and fed over periods as long as 7.5 years or more
on diets containing respectively 0, 5, 50, 200 and 5000 ppm of DDT.
All the animals subjected to the concentration of 5000 ppm showed
convulsions, accompanied by loss of appetite and a fall in weight. At
a concentration of 200 ppm (corresponding to daily doses of 2.2-5.54
mg/kg body-weight), no harmful effects were observed and, in
particular, no histological lesions in the liver or disturbances in
the functioning of that organ, as shown by the bromsulfthalein test
(Durham et al., 1963).
Observations on man. An experimental study was carried out of the
effects on man of prolonged ingestion of small doses of DDT (in the
form of oily solutions in capsules or emulsions in milk). The authors
used 51 volunteers for this study; 17 received a normal diet, 17
received 3.5 mg/kg body-weight and 17 received 35 mg DDT daily. The
last dose is approximately 0.5 mg/kg body-weight. Administration was
continued for as long as 18 months. The authors noted that the
accumulation of the insecticide in the fatty tissues and the urinary
excretion of its metabolite, DDA, were proportional to the dose of DDT
ingested. A state of equilibrium was reached after about a year and
the concentration of DDT accumulated in the fatty tissues reached an
average of 234 ppm (101-367 ppm) in subjects who had ingested 35 mg of
DDT per day. Throughout the whole experiment, no subject complained of
malaise, nor did any ill-effects appear that could be attributed to
the ingestion of DDT (Hayes et al., 1956). The essential results were
confirmed in a separate investigation in which dosage with DDT lasted
for 21 months and the volunteers were observed for an additional 27
months. Fourteen men received 35 mg/man/ day; 6 received 3.5
mg/man/day, and 4 men served as controls. The study also revealed the
relationship between storage of DDT and the urinary excretion of DDA
and demonstrated that the loss of DDT from storage in man following
cessation of dosage is slow - only about two-thirds in 27 months
(Hayes et al., 1964).
Observations were made on 40 workers engaged, over a period of years,
in the manufacturing of specialities based on DDT, under conditions
where suitable precautions had not been taken to protect their skin.
According to the DDA concentration found in the urine, these subjects
had absorbed daily as much DDT as volunteers who had ingested 35 mg of
the insecticide per day in the experiment mentioned above. Thorough
medical and biological tests failed to reveal any toxic symptoms, even
in workers exposed to the toxic product for 6.5 years (Ortelee, 1958).
Several studies on DDT and DDE storage in human fat in several parts
of the world have been presented (Laug, 1951; Hayes et al., 1956;
Hayes et al., 1958; Denes 1962; Maier-Bode, 1960; Dale et al., 1963;
Hunter et al., 1963; Hoffmann et al., 1964; Dale et al., 1965; Egan et
al., 1965; Halacka, et al., 1965; Quinby et al., 1965b; Robinson et
al., 1965; Zavon et al., 1965). DDT was also found in a stillborn
infant and in very young babies (Halacka et al., 1965).
In these studies the average total content in human fat ranged between
2 and 30 ppm; the percentage of DDE of total DDT stored ranged between
34 and 77 per cent. It has been shown that the storage of DDT in man
is directly proportional to intake over a wide range of doses (0.04 to
35.0 mg/man/day), so that from the level in the fat the daily dose can
be estimated. Thus, it can be calculated that the highest average
intake of DDT of any human population yet observed is about 0.7-0.8
mg/man/day. From the data which have been reported, it is also
apparent that in the United States of America, where the level of DDT
in human fat has been repeatedly investigated in the general
population over a significant number of years, it has not increased
since 1950 (Durham et al., 1965).
It has also been repeatedly indicated that women and cows secrete DDT
in the milk. Two recent studies indicated an average of total DDT
(i.e. DDT + DDE) of 0.128 ppm (57 per cent as DDE) and 0.170 ppm (58
per cent as DDE). The content of DDT in cows' milk was found to be
lower (Egan et al., 1965; Quinby et al., 1965). The percentage of the
total DDT intake which is eliminated in the milk has been demonstrated
to be smaller in cows than in women (Quinby et al., 1965).
Comments
The evaluation of DDT is difficult because wide species differences
occur. The rat appears to be the most susceptible species; liver
cellular changes were produced by 5 ppm in the diet. In dogs and
monkeys these changes were not seen. Dogs given 10 mg/kg
body-weight/day for 3 years and monkeys given 2-5 mg/kg
body-weight/day for up to 7 years showed no ill-effects. However, in
another study, monkeys given 0.2 mg/kg body-weight/day for one year
showed signs of hepatitis and liver enlargement. In man, as much as
0.5 mg/kg body-weight/day for up to 21 months were without effect
apart from storage in the fat. However, the possibility that the
latter might have deleterious consequences later in life cannot be
ruled out. In addition, it has not been demonstrated that metabolic
changes in the liver cells comparable to those observed in rats do not
take place in man. The Committee realizes that the figure reached as
an estimate for an ADI can be less than the actual intake for humans
in some parts of the world. It might correspond to the intake of DDT
of a baby exclusively breast-fed. (In this respect the significance of
the lower acute toxicity of DDT for new-born rats is uncertain.)
Furthermore, in addition to unchanged DDT, the residues also contain
metabolites of DDT. There is a need for uniformity in reporting the
results of residue analyses. The reports should at least indicate the
percentages of unchanged DDT, DDE and TDE. Some of the metabolites are
probably less toxic than DDT itself. However, more information is
necessary on this point. At the present time the Committee remains
concerned about the storage of DDT which occurs in all species and
about the cellular changes produced in the liver of rats by DDT and by
other compounds chemically related to it.
TOXICOLOGICAL EVALUATION
Estimate of acceptable daily intake in man
0-0.01 mg/kg body-weight
Further work required
Elucidation of the significance of the finding that DDT is one of the
compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
DDT is used to a minor extent as a soil treatment, primarily for the
control of cutworms which attack vegetable crops. It is suggested for
use in many countries on a wide variety of food crops. It is suggested
for the control of about 20 different insects which attack cane
fruits, about 50 different insects which attack vegetables, 50
different insects which attack tree fruits, as well as for control of
insects of nut trees and other food crops. The usual rate of
application is about 1 to 2 lb of the active chemical per acre;
however, some treatments may go as high as 10 lb per acre.
(b) Post-harvest treatments
Direct applications to stored food products are no longer advised.
However, there is some use in and around storage premises and
transport facilities, which may permit incurrence of small amounts in
products during storage and shipping.
(c) Other uses
DDT is used in many home gardens, in many households (forbidden in The
Netherlands), as a mothproofing agent in rugs and clothing in
restaurants and in public buildings. Many mosquito abatement
programmes make use of DDT.
National tolerances
Austria 7 ppm - a general informal tolerance.
Canada 7 ppm on many fruits and vegetables, fat of meat
animals.
United States 7 ppm on many fruits and vegetables, fat of meat
of America animals.
3.5 ppm on sweet corn.
1 ppm on potatoes.
Residues resulting from supervised trials
Although there have been many analyses made for DDT in agricultural
products, many of them were not made on controlled experiments
designed explicitly to ascertain the fate of the residue following
application. A summary of numerous data, which is too long to
reproduce here and which contains the related bibliography, is held at
the FAO headquarters in Rome. Table 1 has been prepared from this
summary. It contains estimates of the average high residues likely to
result from the practical use of DDT to control insects which attack
the different food commodities or that in animal products from animals
exposed to "unavoidable" or very limited feed residues.
TABLE 1. RESIDUES OF DDT EXPECTED FROM PRACTICAL USE
Food Residue
ppm
Vegetables
leafy 7
others 1-7
Meat, fish, poultry 7 (in fat)
Tree fruits 7
Berries (cane) 1
Citrus 4
Shelled nuts 1
Residues in food moving in commerce
Even though there is a continuing and widespread use of DDT, a very
large proportion of the food in commerce has very little DDT present;
seldom is a sample found with residue as high as the tolerance figures
quoted.
Of the large number of "total diet" samples analysed in the United
States of America since 1961, a high proportion have had detectable
amounts of DDT but no sample of any commodity grouping has exceeded
0.05 ppm DDT. (Mills 1963; Williams 1964; Cummings 1965.)
The Government Chemist Laboratory of Great Britain has analysed a
large number of samples of food products since 1962. They chose those
samples most likely to contain residues of DDT such as milk, butter
and fat of different meat animals. The residues of DDT, DDE, and DDD
for the years 1964 and 1965 for over 900 samples average less than
0.15 ppm, with the highest value found being 4.6 ppm. (Lewis 1964 and
1965; Egan et al 1966.)
The Netherlands Government has analysed a number of imported cereal
products and has found a large proportion to contain some residue of
DDT. Of 227 samples examined during 1964 and 1965, 36 per cent
contained DDT. The highest sample residue was 2.85 ppm, but most
samples ran from 0 to 0.5 ppm (Report CCPR 66-17 January 1966 Ministry
of Social Affairs and Public Health, Ministry of Agriculture,
Committee on Phytopharmacy.)
Fate of residues
Although DDT and some of its metabolic products can be altered
readily by chemical means, they are quite comparatively stable
compounds in many biological media. This low biodegradability coupled
with high fat solubility, in part, accounts for the persistence of
residues and their propensity to concentrate in fat tissue. The
stability and solubility also account for its concentration in certain
forms of life when it is part of the food chain (Metcalf 1966).
(a) In animals
It has been known for many years that DDE and DDA form from DDT
(Menzie 1966). DDA is an acid, is relatively water soluble, and thus
is eliminated much more readily than the fat soluble forms and does
not appear nearly so readily in the edible portion of food products of
animal origin. On the other hand, DDE is fat soluble and almost always
appears as a residue in animal products with DDT.
Only recently has it been well established that DDD (TDE) is a
metabolic product of DDT. (Kallman and Andrews 1963; Peterson and
Robison 1964; Klein et al 1964; Miskus and Blair 1965.) It has been
found as a residue associated with DDT in animal products for many
years, but since it has also had some use as a commercial pesticide,
it was not considered to be a metabolic product by most workers.
Whether it forms in animals as a result of enzymes endogenous to the
animal or only from intestinal bacteria or other organisms associated
with the animal has not been fully established.
Work by Klein et al 1964 has shown that o,p-DDT converts to p,p'-DDT
in vivo.
Both DDE and DDD are more stable in many biological systems than DDT;
therefore, in many instances they accumulate in time to a
concentration equal to or greater than DDT in the fat of animal
tissues. The hydroxy analogues of DDT (kelthane) and DDD can form from
DDT and DDD respectively and 4,4'-dichlorobenzophenone can form from
each (Menzie 1966).
(b) In plants
The meeting was not aware of any data on the effect of plant enzyme
systems on DDT; however, it is known that DDE does appear as a very
low level residue on many food crops which contain DDT (U.S.F.D.A.,
unpublished data).
(c) In storage and processing
DDT is stable under most of the conditions which prevail when it is a
residue on food products. Therefore, residues in food products will
not normally diminish greatly from most food products during shipping
and storage. It is especially stable in a fatty medium.
Recent studies by the National Canners Association (United States of
America) has shown that the amount of DDT on green beans does not
change over a 2-week storage period at 45° F (personal communication).
Since DDT does not penetrate significantly into non-fatty food
products, a large loss is shown when the surface is removed such as in
peeling, shelling, harsh brushing, milling, etc.
Recent studies by Farrow et al., 1966, showed that during the normal
heat processing of canned spinach approximately 50 per cent of the
original DDT was destroyed. However, DDD was formed equivalent to
about 20 per cent of the original DDT resulting in a net loss of about
30 per cent.
Methods of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of DDT (together with residues of a number
of other compounds including DDE and DDD). An example is the AOAC
system (1966) in which acetonitrile partition and Florisil column
clean-up are identified and measured by gas chromatography coupled
with thin layer or paper chromatography. Alternative clean-up systems
e.g. that of de Faubert Maunder et al. (1964) using dimethylformamide,
and other methods of confirmation of identity e.g. using infra-red
spectrophotometry, are also available. The methods are in general
sensitive to 0.003 ppm in milk and 0.05 ppm in most other foods,
though under favourable conditions greater sensitivity can, if
appropriate, be obtained.
In those cases where analyses have provided information on the
contents of degradation products of DDT, such as DDE and DDD, the
figures for these compounds should be included in the analytical
report.
RECOMMENDATION FOR TOLERANCES
The recommendations for tolerances are shown in Table 2.
These tolerance values are considered to be temporary, will be kept
under study, and will be reconsidered no later than three years hence.
These values are in excess of values that would be calculated from the
factors utilized in considering tolerance values for other compounds
in this booklet.
New studies in toxicology, metabolism or residues may permit, within
the next three years a more permanent recognition of these levels, or
on the other hand, it may be necessary to markedly lower the tolerance
recommendations. It is unlikely that future tolerance recommendations
will be higher than those prescribed here.
TABLE 2. RECOMMENDED TOLERANCES FOR DDT
Food type Temporary
Restricted to 3 years
ppm
Vegetables 1.0-7.0
Meat, fish, poultry 7.0 (in fat)
Tree fruits 7.0
Berries (cane) 1.0
Citrus 4.0
Shelled nuts 1.0
Milk (whole) Administrative decision .0045 ppm
Milk products Practical residue 0.2 ppm
The "administrative decision" and the "practical residue"
recommendations are made because the widespread use and stability of
DDT have resulted in small residues being ubiquitous. Small residues
have been found to be present in most dairy products. This is
considered to be undesirable but is also unavoidable at the present
time. Since this residue is generally not present from direct
application to the animals or their feed, no tolerance recommendation
is made. However, to assist regulatory officials in identifying those
samples which have residues much in excess of the unavoidable level, a
"practical residue level" of 0.20 ppm DDT is suggested.
Residues of DDT in animal products are invariably associated with
varying amounts of the DDT metabolites DDE and DDD. In many instances
the residues of either of these or the sum of the two exceeds the
residue of DDT. The WHO Expert Committee on Pesticide Residues
estimated an ADI for DDT but not for the other two. They noted that
residues of all three should be determined, but they postponed
consideration of DDE and DDD. Therefore, only DDT residues were taken
into consideration in preparing the temporary tolerance for DDT.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Andronova, G. P. (1956) Dissertation, F. F. Erisman's State Research
Institute for Hygiene, Moscow
Anon. (1951) Report of the Committee on Pesticides of the Council on
Pharmacy and Chemistry, J. Amer. med. Ass., 145, 728
Bishopp, F. C. (1946) Amer. J. Publ. Hlth, 36, 593
Cameron, G. R. & Burgess, F. (1945) Brit. med. J., 1, 865
Cueto, C., Barnes, A. G. & Mattson, A. M. (1956) J. Agr. Food Chem.,
4 (2), 94.3
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Dale, W. E., Gaines, T. B. & Hayes, W. J., jr (1965) Science, 142,
1474
Denes, A. (1962) Nahrung, 6, 48
Draize, J. H., Woodard, G., Fitzhugh, O. G., Nelson, A. A., Smith,
R. B. & Calvery, H. O. (1944) Chem. Eng. News, 22, 1503
Durham, W. F., Armstrong, J. F. & Quinby, G. E. (1965) Arch.
Environm. Hlth., 11, 16
Durham, W. F., Ortega, P. & Hayes, W. J., Jr (1963) Arch. int.
Pharmacodyn., 141, 111
Egan, H., Goulding, R., Roburn, J. & Tatton, J. (1965) Brit. med.
J., ii, 66
Fitzhugh, O. G. (1948) Ind. Eng. Chem., 704
Fitzhugh, O. G. & Nelson, A. A. (1947) J. Pharmacol. exp. Ther.,
89, 18
Gerboth, G. & Schwabe, U. (1964) Arch. exp. Path., 246, 469
Gerebtzoff, M. A. & Philippot, E. (1952) Experientia (Basel), 8
(10), 395
Gerebtzoff, M. A., Dallemagne, M. J. & Philippot, E. (1950) C.R.
Soc. Biol. (Paris), 144, 1135
Halacka, K., Hakl, J. & Vymetal, F. (1965) Cs. Hyg., 10, 188
Hart, L. G. & Fouts, J. R. (1963) Proc. Soc. exp. Biol. (N.Y.),
114, 388
Hayes, W. J., jr & Dale, W. E. (1964) Toxicol. Appl. Pharmacol., 6,
349
Hayes, W. J., jr, Dale, E. & Pirkle, C. I. (1964) Unpublished report
Hayes, W. J., jr, Durham, W. F. & Cueto, C., jr (1956) J. Amer. med.
Ass., 162 (9), 890
Hayes, W. J. jr., Walker, K. C., Elliott, J. W. & Durham, W. F. (1958)
Arch. industr. Hlth, 18, 398
Haymaker, W., Ginzler, A. M. & Ferguson, R. L. (1946) Amer. J. Med.
Sci., 212, 423
Hoffmann, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
Environm. Hlth, 9, 387
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Juchau, M. R., Gram, T. E. & Fouts, J. R. (1966) Gastroenterology,
51, 213
Judah, J D. (1949) Brit. J. Pharmacol., 4, 120
Kemény, T. & Tarján, R. (1966) Orvosi Hetilap, 107 (30), 1407
Khairy, M. (1959) Quart. J. exp. Psychol., 11, 84
Kimbrough, R., Gaines, T. B. & Sherman, J. D. (1964) J. Nat. Cancer
Inst., 33, 215
Klein, A. K., Laug, E. P., Datta, P. R., Watts, J. O. & Chen, J. T.
(1964) J. Assoc. Offic. Agr. Chem., 47, 1129
Laug, E. P., Kunze, F. M. & Prickett, C. S. (1951) Arch. industr.
Hyg., 3, 245
Laug, E. P., Nelson, A. A., Fitzhugh, O. G. & Kunze, F. M. (1950) J.
Pharmacol. exp. Ther., 98, 268
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials,
U.S., 16, 3, 47, 126
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Fd. Cosmet. Toxicol.,
3, 591
Maier-Bode, H. (1960) Med. exp. (Basel), 3, 284
Mattson, A. M., Spillane, J. T., Baker, C. & Pearce, G. W. (1953)
Anal. Chem., 25, 1065
McLean, A. E. & McLean, E. K. (1966) Biochem, J., 100, 564
Mendel, J. L. & Walton, M. S. (1966) Science, 151, 1527
Morello, A. (1965) Canad. J. Biochem., 43 (8), 1289
Ortega, P. (1962) Fed. Proc., 21, 306
Ortega, P. (1966) Lab. Invest., 15, 657
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. (1956)
Publ. Hlth Monogr., 43, 27
Ortelee, M. F. (1958) Arch. industr. Hlth, 18, 433
Pearce, G. W., Mattson, A. M. & Hayes W. J., jr (1952) Science,
116, 254
Philips, F. & Gilman, A. (1946) J. Pharmacol. exp. Ther., 86, 213
Phillips, W. E. G. (1963) Canad. J. Biochem., 41, 1793
Pinto, J. D., Camien, M. N. & Dunn, M. S. (1965) J. biol. Chem.,
240, 2148
Quinby, G. E., Armstrong, J. F. & Durham, W. F. (1965) Nature,
207, 726
Quinby, G. E., Hayes, W. J., jr, Armstrong, J. F. & Durham, W. F.
(1965) J. Amer. med. Ass., 191, 175
Read, S. I., Murray, T. K. & McKinley, W. P. (1965) Canad. J.
Biochem., 43, 317
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. Ind. Med., 22, 220
Rothe, C. F., Mattson, A. M., Nuelsein, R. M. & Hayes, W. J., jr
(1957) Arch. industr. Hlth, 16 (1), 82
Shillinger, Y. I., Naumova, L. P. & Pekerman, S. W. (1955) Vop.
Pitan., 14, 41
Smith, M. I. & Stohlman, E. F. (1944) Publ. Hlth Rep. (Wash.), 59,
984
Spicer, S. S., Sweeney, T. R., von Oettingen, W. F., Lillie, H. D. &
Neal, P. A. (1947) Vet. med., 42 (8), 289
Stemmer, K. L. & Hamdi, E. (1964) Unpublished report submitted by
Kettering laboratory
Street, J. C. & Blau, A. D. (1966) Toxicol. Appl. Pharmacol., 8,
497
Tinsley, I. J. (1965) Biochem. Pharmacol., 14, 847
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Woodard, G., Nelson, A. A. & Calvery, H. O. (1944) J. Pharm. exp.
Ther., 82, 152
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
AOAC (1966) Changes in Methods of Analysis, J. Assoc. Offic. Anal.
Chem. 49, 222-230
Cummings, J. G. (1965) Pesticide Residues in Total Diet Samples, J.
Assoc. Offic. Agr. Chem. 48: 1177-1180
de Faubert Maunder, M. J., Egan, H., Godly, E. W., Hammond, E. W.,
Roburn, J., Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues, Analyst,
89, 168-174
Egan, H., Holmes, D. C., Roburn, J. and Tatton, J. O'G. (1966)
Pesticide Residues in Foodstuffs in Great Britain. II-Persistent
Organochlorine Pesticide Residues in Selected Foods, J. Sci. Fd.
Agric., 17: 563-569
Farrow, R. P., Elkins, E. R. Jr. and Cook, R. W. (1966) Conversion of
DDT to TDE in Canned Spinach. J. Agr. Food Chem. 14/4: 430-434
Kallman, B. J. and Andrews, A. K. (1963) Reductive Dechlorination of
DDT to DDD by Yeast, Science 141: 1050-1051
Klein, A. K., Laug, E. P., Datta, P. R., Watts, J. O., and Chem, J. T.
(1964) Metabolites: Reductive Dechlorination of DDT to DDD and
Isomeric Transformation of o,p'-DDT to p,p'-DDT in vivo, J. Assoc.
Offic. Agr. Chem. 47: 515-531
Lewis, D. T. (1964, 1965) Report of the Government Chemist, H.M.S.O.
London
Menzie, C. M. (1966) Metabolism of Pesticides Special Scientific
Report - Wildlife No. 96, Fish and Wildlife Service, Department of the
Interior Washington, D.C.
Metcalf, R. L. (1966) Metabolism and Fate of Pesticides in Plants and
Animals. Pages 230-250, Scientific Aspects of Pesticide Control.
Publication 1402, National Academy of Sciences - National Research
Council, Washington, D.C.
Mills, P. A. (1963) Total Diet Study: C. Pesticide Content. J. Assoc.
Offic. Agr. Chem. 46: 762-767
Miskus, R. P., and Blair, D. B. (1965) Conversion of DDT to DDD by
Bovine Rumen Fluid, Lake Water, and Reduced Porphyrins, J. Agr. Food
Chem. 13/5: 481-483
Peterson, J. E. and Robison W. H. (1964) Metabolic Products of
p,p'-DDT in the Rat, Toxicology and Applied Pharmacology 6: 321-327
Williams, S. (1964) Pesticide Residues in Total Diet Samples, J.
Assoc. Offic. Agr. Chem. 47: 815-821
