
Pesticide residues in food - 2003 - Joint FAO/WHO Meeting on Pesticide Residues
First draft prepared by
G. Wolterink and P.H. van Hoeven Arentzen
Centre For Substances and Integrated Risk Assessment
National Institute of Public Health and the Environment, Bilthoven, The Netherlands
|
Effects of carbosulfan on semen characteristics and serum testosterone |
Carbosulfan is a carbamate insecticide that acts by inhibiting the activity of acetylcholinesterase. It was evaluated by the Joint Meeting in 1984 and 1986. A toxicological monograph was prepared by the Joint Meeting in 1984 and a monograph addendum was prepared in 1986 (Annex 1, references 43, 47). In 1986, an acceptable daily intake (ADI) of 0-0.01 mg/kg bw was established on the basis of a NOAEL of 1.3 mg/kg bw per day in a 2-year study in mice, a NOAEL of 1.0 mg/kg bw per day in a 2-year study in rats, and a NOAEL of 1.3 mg/kg bw per day in a 6-month study in dogs. One of the metabolites of carbosulfan is carbofuran, which is itself used as a pesticide and which was evaluated by JMPR in 1976, 1979, 1980, 1982, 1996 and 2002. The 1996 JMPR established an ADI of 0-0.002 mg/kg bw and the 2002 JMPR established an acute reference dose (RfD) of 0.009 mg/kg bw for carbofuran.
Carbosulfan was re-evaluated by the present Meeting within the periodic review programme of the Codex Committee on Pesticide Residues. The Meeting reviewed new data on carbosulfan that had not been considered previously and relevant data from the previous evaluation. Conclusions of studies evaluated by the JMPR in 1984 and that were not available for the present evaluation are included.
Rats
The kinetics of [14C]carbosulfan (purity, >97%) were studied in male and female Sprague-Dawley rats, in a study that complied with the United States Environmental Protection Agency (EPA) guideline 85-1. Carbosulfan was labelled either in the phenyl or in the dibutylamino moiety. In a preliminary test it was shown that excretion of radiolabel via expiration only occurred when carbosulfan was labelled in the dibutylamino group. The definitive experimental design was based on the preliminary test. Groups of five male and five female rats received unlabelled carbosulfan as a single oral dose at 4 or 30 mg/kg bw, or as repeated oral doses (4 mg/kg bw per day for 14 days), followed by [14C]carbosulfan as a single oral dose at 4 mg/kg bw on day 15. Corn oil was used as vehicle. Urine, cage rinses and faeces were collected at 0-4, 4-8, 8-12, 12-24, 24-36 h intervals and every 24 h thereafter for 168 h. At 168 h after dosing, the rats were killed, blood was sampled and bone (femur), brain, fat, kidneys, liver, lungs, muscle (thighs), ovaries, testes, heart, spleen, skin (shaven, dorsal), uterus and remaining carcass were collected. For rats treated with carbosulfan labelled in the dibutylamino group, expired carbon dioxide was collected. Statements of compliance with good laboratory practice (GLP) and quality assurance (QA) were provided.
Total recovery of radioactivity was 90-98%. No marked sex differences in patterns of excretion were observed. The major route of excretion was in the urine, excretion also occurring in the faeces in expired air. Patterns of excretion were similar in rats treated with carbosulfan at a single low or high dose. In the rats treated with single low or high doses of phenyl-labelled carbosulfan, 72-83% of the radiolabel was excreted in the urine. In the groups given dibutylamine-labelled carbosulfan at a low or high dose, 65-66% and 10-17% of the radiolabel was excreted in the urine and expired air, respectively. Total excretion of radiolabel in the faeces of rats treated with single low or high doses was about 8-22%. Excretion was relatively rapid. Most (80-90%) radiolabel was excreted within 48 h in animals at the lower dose and 72 h in animals at the higher dose. Excretion of radiolabel in the urine tended to be higher (phenyl group: 79-88%, dibutylamino group 71%) in rats receiving repeated doses of carbosulfan at 4 mg/kg bw than in rats treated with a single (low) dose at 4 mg/kg bw, while excretion in faeces tended to be lower (5-15%) in rats receiving repeated doses of carbosulfan at 4 mg/kg bw, indicating that absorption was slightly increased. Furthermore, in the rats treated with repeated doses, the rate of excretion of radiolabel was increased, i.e. 80-87% of the radiolabel was excreted within 24 h in the rats treated with repeated doses versus 72-78% in the those treated with a single low dose, which indicates that induction of metabolism may have occurred. At 168 h, <0.3% of the administered dose remained in blood and tissue, and up to about 2% remained in the carcass. During the first 24 h after dosing, rats at the highest dose showed behavioural signs indicative of cholinesterase inhibition. Remarkably, a high proportion (7-11%) of administered radiolabel was found in the cage wash of animals treated with carbosulfan labelled on the phenyl group, while in animals treated with carbosulfan labelled on the dibutylamino group only 1-3% of the radiolabel was found in the cage wash (Fang & El Naggar, 1995).
Goats
The kinetics of [14C]carbosulfan was studied in lactating Nubian goats (aged 2 years; body weight, 36-43 kg), in a study that complied with EPA guideline 171-4(a)(3). Carbosulfan was labelled with 14C either in the phenyl ring (purity, 99.2%) or in the dibutylamino side-chain (purity, 98.8%). Gelatin capsules containing carbosulfan at a dose of 44.7mg/day (phenyl label) or 40.9 mg/day (dibutylamino label) (approximate dietary concentration, 25 mg/kg) were administered orally by balling gun to groups of two goats, once daily for 7 days. A single goat received only vehicle (cellulose). Urine and faeces were collected daily and milk was collected twice daily. Blood was collected on days -1, 1, 3, and 7, immediately before dosing and immediately before sacrifice. The animals were killed 22 h after the last dose and edible tissues (liver, kidney, leg and lumbar muscle, peripheral and omental fat) were collected and total radioactive residue was determined. Excretion of radiolabel in expired air was not measured. Statements of compliance with GLP and QA were provided.
The major route of elimination was in the urine. For the goats receiving carbosulfan labelled on the phenyl group, excretion of radiolabel was 81-84% in urine, 7% in faeces, 1% in cage rinse and 0.2% in milk. For the goats receiving carbosulfan labelled on the dibutylamino side-chain, the pattern of excretion was 66-70% in urine, 3-4% in faeces, 0.3-0.4% in cage rinse and 2-3% in milk. For carbosulfan labelled on the phenyl group, total radioactive residue in tissue was highest in kidney (0.15-0.21 mg/kg) and liver (0.06 mg/kg). For carbosulfan labelled on the dibutylamino side-chain, total radioactive residue was highest in omental fat (1.1-1.3 mg/kg), liver (1.0-1.3 mg/kg), kidney (0.7-0.8 mg/kg) and peripheral fat (0.7-0.8 mg/kg). In animals of both groups, concentrations of radiolabel in the blood increased over 7 days, and highest concentrations of radiolabel in blood (phenyl group, 0.02-0.04 mg/kg; dibutylamino side-chain, 0.24-0.27 mg/kg) were observed just before termination (Curry & Weintraub, 1996).
Rats
The metabolism of [14C]carbosulfan (purity, >97%) was studied in male and female Sprague-Dawley rats in vivo, in a study that complied with EPA guideline 85-1. Carbosulfan was labelled either in the phenyl moiety or in the dibutylamino moiety. Groups of five male and five female rats received unlabelled carbosulfan as a single oral dose at 4 (low) or 30 (high) mg/kg bw, or as repeated oral doses at 4 mg/kg bw per day for 14 days, followed by [14C]carbosulfan as a single oral dose at 4 mg/kg bw by gavage on day 15. Control rats received vehicle only (corn oil). Urine, cage rinses and faeces were collected at 0-4, 4-8, 8-12, 12-24, 24-36 h intervals and every 24 h thereafter for 168 h. At 168 h after dosing, the rats were killed, blood was sampled and bone (femur), brain, fat, kidneys, liver, lungs, muscle (thighs), ovaries, testes, heart, spleen, skin (shaven, dorsal), uterus and remaining carcass were collected. For rats treated with carbosulfan labelled in the dibutylamino side-chain, expired CO2 was collected. Urine, faeces and exhaled air were analysed for radio-label by liquid scintillation counting (LSC). Metabolites were identified by thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC). Identity of the major metabolites was confirmed by gas chromatography-mass spectrometry (GC-MS). Statements of compliance with GLP and QA were provided.
Table 1. Quantitative excretion of carbosulfan and metabolites (% of administered radioactivity)
|
Dose (mg/kg bw) |
||||||||||||
|
4 (single dose) |
4 (repeated doses) |
30 (single dose) |
||||||||||
|
Urine |
Faeces |
Urine |
Faeces |
Urine |
Faeces |
|||||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
|
|
14C-phenyl label |
||||||||||||
|
Carbosulfan |
ND |
ND |
2.5 |
3.4 |
ND |
ND |
0.9 |
0.4 |
ND |
0.3 |
1.7 |
4.4 |
|
3-OH-7-phenol |
14.2 |
12.7 |
1.2 |
0.7 |
25.3 |
25.6 |
0.9 |
0.3 |
17.9 |
17.9 |
0.6 |
0.5 |
|
3-OH-carbofuran |
11.0 |
10.0 |
6.4 |
3.8 |
17.6 |
20.1 |
4.5 |
1.0 |
16.0 |
11.5 |
1.3 |
1.9 |
|
3-keto-7-phenol |
20.3 |
26.3 |
0.4 |
0.3 |
14.4 |
20.7 |
0.2 |
0.1 |
25.7 |
23.9 |
0.2 |
0.4 |
|
7-phenol |
23.5 |
22.8 |
0.4 |
0.3 |
8.8 |
11.6 |
0.1 |
0.0 |
7.3 |
4.8 |
0.1 |
0.3 |
|
14C-dibutylamino label |
||||||||||||
|
Carbosulfan |
ND |
ND |
6.3 |
8.3 |
ND |
ND |
3.5 |
4.2 |
ND |
ND |
4.1 |
6.9 |
|
Dibutylamine |
33.9 |
36.2 |
4.5 |
6.3 |
37.5 |
42.2 |
3.4 |
2.5 |
36.3 |
46.5 |
2.7 |
4.3 |
|
OH-dibutylamine |
23.8 |
22.5 |
ND |
ND |
25.8 |
24.7 |
ND |
ND |
23.5 |
17.1 |
ND |
ND |
From Fang & El Naggar (1995) ND, not detected
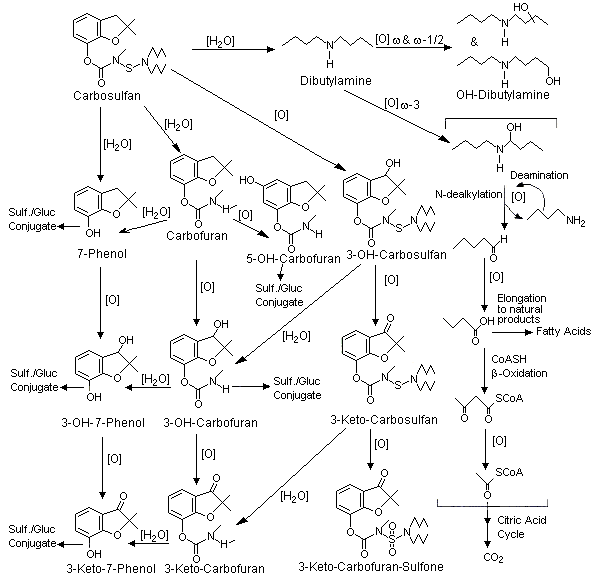
In total, 10 metabolites were identified. The distribution of radiolabel among the major metabolites is presented in Table 1. Other metabolites identified were carbofuran, 5-OH-carbofuran, 3-keto-carbosulfan, 3-OH-carbosulfan, 3-keto-carbosulfan-sulfone and 3-keto-carbofuran. Each of these metabolites or the seven unidentified metabolites was present at <5% of the total administered dose. The authors postulated that metabolites of the dibutylamino moiety may be incorporated in fatty acids. The metabolites resulting from hydrolysis and oxidation were excreted mainly as sulfate/glucuronide conjugates in urine. There was no obvious sex difference in metabolite formation. In the rats treated with single low or high doses of carbosulfan, the relative quantities of metabolites were similar. Compared with rats treated with single doses, the rats treated repeatedly with a low dose of carbosulfan had more 3-OH-7 phenol and 3-OH-carbofuran, and less 3-keto-7-phenol and 7-phenol. This may indicate that repeated dosing leads to induction of metabolizing enzyme systems. A proposed metabolic pathway is depicted in Figure 1 (Fang & El Naggar, 1995).

Figure 1. Proposed metabolic pathways for carbosulfan in rats
From Fang & El Naggar (1995)
In a study reported in the public literature, the biotransformation of carbosulfan in the female rat stomach was assessed at short intervals after oral administration. Three rats received 14C-labelled carbosulfan, dissolved in 0.2 ml of propylene glycol, at a dose of 30 mg/kg bw. The rats were killed 15, 35 or 80 min after treatment, the stomach was removed and the stomach contents were analysed by TLC. Total recovery of radioactivity was 75.7%, 55.1% and 44.6% at 15, 35 and 80 min, respectively. Of the recovered radioactivity, 60%, 47% and 50% represented carbosulfan at 15, 35 and 80 min respectively. Major biotransformation products at 15, 35 and 80 min, respectively, were carbofuran (15%, 20% and 16%), biscarbofuran N,N'-disulfide (11%, 17% and 18%) and an unidentified compound (7%, 8% and 9%); the data indicate that carbosulfan is relatively stable in the stomach (Umetsu & Fukuto, 1982).
Goats
The kinetics of [14C]carbosulfan were studied in lactating Nubian goats (aged 2 years; body weight, 36-43kg), in a study that complied with EPA guideline 171-4(a)(3). Carbosulfan was labelled with 14C, either in the phenyl ring (purity, 99.2%) or in the dibutylamino side-chain (purity, 98.8%). Gelatin capsules containing carbosulfan at a dose of 44.7 mg/day (phenyl label) or 40.9 mg/day (dibutylamino label) (approximate dietary concentration, 25 mg/kg) were administered orally by balling gun to groups of two goats, once daily for 7 days. A single goat received vehicle only (cellulose). Urine and faeces were collected daily and milk was collected twice daily. Blood was collected on days -1, 1, 3, and 7, immediately before dosing and immediately before sacrifice. The animals were killed 22 h after the last dose and edible tissues (liver, kidney, leg and lumbar muscle, peripheral and omental fat) were collected. Samples of milk and tissues were extracted and metabolites were identified by HPLC and TLC; additional information was sometimes obtained by size-exclusion chromatography and GC-MS. Statements of compliance with GLP and QA were provided.
The major metabolites of phenyl- or dibutylamino-labelled carbosulfan are presented in Table 2. The metabolites containing the phenyl label were found in conjugated, protein-bound or non-conjugated form. Greater quantities were found of metabolites containing the dibutylamino label than of metabolites containing the dibutylamino label. This is attributed to the fact that a large part of the dibutylamino residue is broken into smaller fragments and incorporated into natural components, such as fatty acids, triglycerides, carbohydrates and protein (Curry & Weintraub, 1996).
Table 2. Quantitative distribution of metabolites of carbosulfan in goats (% of total radioactive residue in the sample)
|
Metabolite |
Sample |
||||
|
Milk |
Liver |
Kidney |
Fat |
Muscle |
|
|
14C-phenyl label |
|||||
|
3-OH-carbofuran |
34.2 |
9.5 |
21.5 |
ND |
ND |
|
3-OH-7-phenol |
21.1 |
15.6 |
13.3 |
ND |
ND |
|
3-keto-7-phenol |
29.9 |
3.0 |
9.3 |
ND |
ND |
|
7-phenol |
9.2 |
4.6 |
8.9 |
ND |
ND |
|
Minor metabolites |
1.2 |
4.4 |
7.6 |
ND |
ND |
|
Characterized organosolubles |
2.3 |
17.3 |
17.4 |
ND |
ND |
|
Protein-associated metabolites |
NA |
22.6 |
2.5 |
ND |
ND |
|
Polar aqueous metabolites |
0.7 |
10.4 |
18.4 |
ND |
ND |
|
Non-extractable residues |
1.4 |
12.7 |
2.1 |
ND |
ND |
|
14C-dibutylamino label |
|||||
|
Aminobutanols |
29.7 |
8.1 |
11.9 |
0.8 |
ND |
|
Dibutylamine and related metabolites |
6.7 |
13.4 |
10.5 |
0.6 |
9.6 |
|
Natural constituents |
30.2 |
29.1 |
13.8 |
87.3 |
32.0 |
|
Non-conjugated amines |
11.8 |
6.3 |
24.3 |
ND |
5.9 |
|
Conjugated or bound amines |
10.5 |
18.0 |
12.3 |
ND |
14.7 |
|
Lipophilic metabolites |
0.6 |
1.3 |
4.5 |
0.5 |
1.2 |
|
Polar aqueous metabolites |
7.6 |
16.6 |
18.5 |
0.2 |
26.5 |
|
Non-extractable residues |
2.9 |
7.2 |
4.2 |
10.5 |
10.0 |
From Curry & Weintraub (1996)
NA, not applicable; ND, not detected
(a) Oral, dermal and inhalation administration
For the present evaluation, no studies of acute toxicity with technical-grade carbosulfan were available. All available studies of oral, inhalatory and dermal toxicity were performed with formulations of carbosulfan that contained 25-50% active ingredient (a.i.). Data on the composition of the formulations were not provided in the studies. The acute toxicity of formulations containing carbosulfan is summarized in Table 3. The LD50s for the formulations and the recalculated values for the active ingredient are presented, assuming that the active ingredient is responsible for the toxic effect. In most of the studies with carbosulfan administered orally, the LD50 tended to be lower for females than for males. In general, the clinical signs observed resemble those of cholinesterase inhibition. Studies of acute toxicity with technical-grade carbosulfan were evaluated by the JMPR in 1984. The oral LD50s in rats ranged from 90 to 250 mg/kg bw. The LD50 for carbosulfan was >2000 mg/kg bw in rabbits treated dermally, and the LC50 was 0.61 mg/l for carbosulfan in rats treated by inhalation.
The Meeting noted that in the studies of dermal toxicity the formulation was moistened with water or saline. In view of the high lipophilicity of carbosulfan (log pow = 5.4), it is not certain that good contact with the skin was maintained with the vehicles used.
Table 3. Acute toxicity of formulations containing carbosulfan
|
Species |
Strain |
Sex |
Formulation codea |
Purity (%) |
Route and vehicle |
LD50 (mg/kg bw) or LC50 (mg/l) |
Reference |
|
|
Formulation |
Active ingredient |
|||||||
|
Mouse |
SW |
Male |
25 ST |
28.9 |
Oral, in water |
1077 |
311 |
Freeman (1985b)b |
|
Female |
869 |
251 |
||||||
|
Rat |
SD |
Male |
4 EC |
49.7 |
Oral, undiluted |
69 |
34 |
Seaman (1981)d |
|
Female |
<55 |
<27 |
||||||
|
Rat |
COBS(SD) |
Male & Female |
40 DB |
40 |
Oral, in corn oil |
>50 |
>20 |
Sabol (1981a)d |
|
Rat |
SD |
Male |
40 DB |
40 |
Oral, in corn oil |
348 |
134 |
Freeman (1984a)b |
|
Female |
159 |
64 |
||||||
|
Rat |
SD |
Male |
25 ST |
28.9 |
Oral, in water |
278 |
80 |
Freeman (1985a)b |
|
Female |
107 |
31 |
||||||
|
Rat |
Wistar |
Male |
35 STD |
35 |
Oral, in water |
113 |
40 |
Daamen (1991a)b |
|
Female |
147 |
51 |
||||||
|
Rat |
SD |
Male |
25 STW |
25 |
Oral, in water |
258 |
65 |
Freeman (1989a)b |
|
Female |
120 |
30 |
||||||
|
Rat |
Him:OFA |
Male& Female |
25 CS |
25 |
Oral, in water |
238 |
60 |
Klein (1993a)b |
|
Rat |
Wistar |
Male& Female |
400 SC |
40.3 |
Oral, in water |
42 |
17 |
Mello dos Santos (1998a)c |
|
Rat |
Wistar |
Male& Female |
35STD |
35 |
Dermal, in water |
>2000 |
>700 |
Daamen (1991b)b |
|
Rat |
Him:OFA |
Male& Female |
25 CS |
25 |
Dermal, undiluted |
>2000 |
>700 |
Klein (1993b)b |
|
Rat |
Wistar |
Male |
400 SC |
40.3 |
Dermal, in water |
563 |
227 |
Mello dos Santos (1998b)c |
|
Female |
688 |
269 |
||||||
|
Rabbit |
NZW |
Male& Female |
40 BD |
40 |
Dermal, in saline |
>200 |
>80 |
Sabol (1981b)d |
|
Rabbit |
NZW |
Male& Female |
25 ST |
28.9 |
Dermal, in saline |
>2000 |
>578 |
Freeman (1985c)b |
|
Rabbit |
NZW |
Male& Female |
25 STW |
25 |
Dermal, in saline |
>2000 |
>500 |
Freeman (1989b)b |
|
Rat |
Crl:CD |
Male& Female |
40 DB |
40 |
Inhalation (1 h)h |
<5.0 |
<2 |
Morgan (1981)d |
|
Rat |
Crl:CD |
Male& Female |
4 EC |
47.2 |
Inhalation (1 h)d |
<2.2 |
<1 |
Horath (1982)b |
|
Rat |
SD |
Male& Female |
25 STW |
25 |
Inhalation (4 h)a |
>0.11 |
>0.028 |
Blagden (1997)b |
|
Rat |
SD |
Male |
25 ST |
28.9 |
Inhalation (4 h)f |
0.26 |
0.075 |
Dudek (1985) |
|
Female |
0.10 |
0.029 |
||||||
|
Rat |
SD |
Male& Female |
25 STD |
35 |
Inhalation (1 h)i |
>0.43 |
>0.15 |
Signorin (1993)b |
|
Rat |
Wistar |
Male |
400 SC |
40.3 |
Inhalation (4 h)g |
1.37 |
0.55 |
Mello dos Santos (1998c)c |
|
Female |
1.72 |
0.69 |
||||||
NSW, New Zealand white; SD, Sprague-Dawley
a All formulations are named "Marshal", followed by the codes indicated in the table
b Statements of compliance with GLP and QA were provided
c A statement of compliance with GLP was provided
d A statement of compliance with QA was provided
e Nose only; mass median aerodynamic diameter (MMAD), 7.2 µm; inhalable fraction, 16.6%
f Whole body; MMAD not presented
g Nose only; actual concentration and MMAD aerosol not measured
h Whole body; actual concentration and MMAD not assessed
i Whole body; gravimetric concentration, 0.43 mg/l; MMAD, 8.74 µm
For the present evaluation, no studies of ocular irritation with pure or technical-grade carbosulfan were available. Five studies of ocular irritation with formulations of carbosulfan were performed. The proportion of active ingredient in these formulations ranged from 25 to 40.3%.
In the studies of ocular irritation in rabbits, performed in compliance with guideline 81-4 and OECD 405, the irritating properties of Marshal 25 STW (25% a.i), Marshal 25 ST (28.9% a.i.), Marshal 25 CS (25% a.i), Marshal 400 SC (40.3% a.i.) and Marshal 35 STD (35% a.i.) were assessed. The conclusions of these studies were that Marshal 25 STW is minimally irritating to the eye, Marshal 35 STD is mildly irritating to the eye and that the other formulations do not induce ocular irritation (Freeman, 1985d, 1989d; Pels Rijcken, 1991a; Klein, 1993d; Mello dos Santos, 1998e). Statements of compliance with GLP and QA were provided.
For the present evaluation by the Meeting, no studies of dermal irritation with pure or technical-grade carbosulfan were available. Five studies on dermal irritation with formulations of carbosulfan were performed. The level of active ingredient in these formulations ranged from 25 to 40.3%.
In the studies of dermal irritation in rabbits, performed in complience with guideline 81-5 or OECD 404, the irritating properties of Marshal 25 STW (25% a.i), Marshal 25 ST (28.9% a.i.), Marshal 25 CS (25% a.i), Marshal 400 SC (40.3% a.i.) and Marshal 35 STD (35% a.i.) were assessed. The conclusions of these studies were that Marshal 25 ST is minimally irritating to the skin, Marshal 400 SC is slightly irritating to the skin, and that the other formulations do not induce dermal irritation (Freeman, 1985e, 1989e; Pels Rijcken 1991b; Klein, 1993c; Mello dos Santos, 1998f). Statements of compliance with GLP and QA were provided.
For the present evaluation by the Meeting, no studies of sensitization with pure or technical-grade carbosulfan were available. Four studies on the skin-sensitizing properties of formulations of carbosulfan were performed. The level of active ingredient in these formulations ranged from 25 to 40.3%.
In three standard Buehler assays for skin sensitization, performed according to guideline 81-6, the sensitizing properties of 0.3 g of Marshal 25 STW (25% a.i), 0.4 g of Marshal 25 ST (28.9% a.i.), and 0.4 g of Marshal 40 DB (40% a.i) were assessed. The formulations were moistened with saline and the above-mentioned quantities were used in induction as well as in the challenge phase. None of the formulations induced dermal sensitization. Statements of compliance with GLP and QA were provided (Freeman, 1984b, 1985f, 1989c).
A study of dermal sensitization in guinea-pigs (three males, two females; strain unknown) that received intradermal applications of Marshal 400 SC (40.3% a.i.) was considered to be inadequate by the Meeting, since this test was not performed according to the currently required guidelines, the number of animals was insufficient to perform a sound statistical evaluation, and the methods and results sections were poorly described (Mello dos Santos, 1998d).
Rabbits
In a study that complied with EPA guideline 82-2, groups of six male and six female New Zealand white rabbits received carbosulfan (purity, 91.6%) at a dose of 0, 50, 200 or 800 mg/kg bw per day for 21 consecutive days in daily applications, under an occlusive wrapping, to the clipped skin of the back (doses were selected on the basis of a range-finding study). The test material, being a liquid, was applied undiluted to the skin, covering about 5%, 10-15% or 75% of the test site in the groups receiving the lowest, intermediate and highest dose, respectively. Animals in the control group were treated in the same manner, but received no test material. Each day, the test material was removed 6h after application by wiping the treated skin with a gauze pad moistened with methanol and subsequently rinsing it with tepid tap water. The animals were observed daily for mortality, clinical signs or signs of dermal toxicity. Body weight and food consumption were measured weekly. After 21 days, the rabbits were killed and necropsies were performed. One female in the group receiving the highest dose died on day 3. Data from this animal were not included in the analysis and the animal was replaced. Clinical chemistry was performed before the start of treatment and at termination. Plasma and erythrocyte cholinesterase activities were determined before initiation of the study and on days 10 and 21, during the 6 h period of exposure. Cholinesterase activity in the brain was determined at termination, within 2 h after the end of the exposure. Statements of compliance with GLP and QA were provided.
One male and two female rabbits in the groups receiving the highest dose died during the study. The death of one animal was attributed to bronchopneumonia. The cause of death of the other two animals could not be established, but was not considered to be treatment-related. Some rabbits in the group receiving the lowest dose had soft stools. In the animals in the groups receiving the intermediate and highest doses, diarrhoea was frequently observed. In the latter group, one male and several females displayed decreased limb tone, and nasal discharge was observed in two females. All treatment groups showed slight erythema (incidence was dose-dependent) and oedema. No signs of dermal irritation were observed in the control animals. Body weight and food consumption were not affected. Apart from inhibition of cholinesterase activity, no treatment-related haematological and biochemical changes were observed. Organ weights were not affected. Except for dermal effects at the site of application, no microscopic changes were observed. The effect of carbosulfan on cholinesterase activity is summarized in Table 4.
Table 4. Effects of carbosulfan on cholinesterase activitya in rabbits after dermal application
|
Substrate |
Dose (mg/kg bw) |
||||||
|
50 |
200 |
800 |
|||||
|
Males |
Females |
Males |
Females |
Males |
Females |
||
|
Erythrocytes |
Day 10 |
86 |
91 |
94 |
94 |
94 |
75 |
|
Terminal |
84 |
88 |
78* |
84 |
84 |
72* |
|
|
Plasma |
Day 10 |
63* |
75 |
38* |
25* |
25* |
38* |
|
Terminal |
60* |
80 |
40* |
20* |
20* |
30* |
|
|
Brain |
Terminal |
90 |
78 |
78 |
40* |
40* |
44* |
From Goldenthal (1990a)
aAs cholinesterase activities varied considerably over time, cholinesterase activity of animals in the treatment groups is expressed as per cent of the value for concurrent controls rather than as per cent of the pretest value
* Significantly different from control
At the highest dose (800 mg/kg bw), erythrocyte cholinesterase activity appeared to be far less sensitive to treatment with carbosulfan than plasma or brain cholinesterase activity. No consistent sex differences in cholinesterase inhibition were found. On the basis of the statistically significant reduction in erythrocyte cholinesterase activity (22%) in females at the intermediate dose, and the statistically not significant decrease in brain cholinesterase activity (30% and 22% in males and females respectively) at the intermediate dose, the study author considered the NOAEL to be 50 mg/kg bw per day (Goldenthal, 1990a).
The Meeting noted that it was not specified at which time-point during the 6 h period of exposure the blood samples for determination of erythrocyte and plasma cholinesterase activity were drawn, and no data on the peak plasma concentrations of carbosulfan are available. Furthermore, it was reported that brain cholinesterase activity was assessed "within 2 h after the end of exposure". The effects of carbamates being known to be transient, blood and brain concentrations of carbosulfan at this time may be below peak concentrations. Therefore, measurements of plasma, erythrocyte and brain cholinesterase activity in this study may underestimate the level of inhibition induced by carbosulfan. The variability in the assay and the relatively small group size may explain why decreases of 20-30% in cholinesterase activity were often not statistically significant. In a second study by the same author (Goldenthal, 1990b), the lowest-observed-adverse-effect level (LOAEL) was 50 mg/kg bw per day in male rabbits given carbosulfan by dermal application (see below). In view of the potential underestimation of the inhibition of cholinesterase activity and the small number of animals per group in the present study, and taking into account data from the second study by Goldenthal, the Meeting considered that the statistically non-significant reduction (22%) in cholinesterase activity in the brain of females at 50 mg/kg bw (the lowest dose) to be an adverse effect. Therefore, the LOAEL for carbosulfan in this study was 50 mg/kg bw per day.
In a study performed in compliance with EPA guideline 82-2, groups of six male New Zealand white rabbits were given carbosulfan (purity, 91.6%) at a dose of 0, 5, 50 or 100 mg/kg bw per day for 21 consecutive days by daily dermal application, under an occlusive wrapping, to the clipped skin of the back (doses were selected on the basis of a range-finding study). The test material, being a liquid, was applied undiluted to the skin, covering <5%, 5% and 10% of the test site in animals in the groups receiving the lowest, intermediate and highest doses, respectively. Animals in the control group were treated in the same manner, but received no test material. Each day, the test material was removed 6 h after application, by wiping the treated skin with a gauze pad moistened with methanol and subsequently rinsing it with tepid tap water. The animals were observed daily for mortality, clinical signs or signs of dermal toxicity. Body weight and food consumption were measured weekly. After 21 days, the rabbits were killed and necropsies were performed. Clinical chemistry was performed before the start of treatment and at termination. Plasma and erythrocyte cholinesterase activity was determined before the initiation of the study and on days 10 and 21, during the 6 h period of exposure. Cholinesterase activity in one-half brain sections was determined at termination, within 2 h after the end of the exposure. Statements of compliance with GLP and QA were provided. No clinical signs of toxicity were observed. Dose-related slight (all treatment groups) to moderate (highest dose) erythema and oedema were observed. Body weight and food consumption were not affected. No treatment-related haematological and biochemical changes were observed. Organ weight was not affected. Except for dermal effects at the site of application, no microscopic changes in the organs were observed. The effects of carbosulfan on cholinesterase activity are summarized in Table 5.
Table 5. Effects of carbosulfan on cholinesterase activitya in male rabbits after dermal application
|
Substrate |
Time-point |
Dose (mg/kg bw) |
||
|
5 |
50 |
200 |
||
|
Erythrocytes |
Day 10 |
81 |
86 |
74 |
|
Terminal |
93 |
100 |
90 |
|
|
Plasma |
Day 10 |
89* |
67* |
67* |
|
Terminal |
75* |
63* |
63* |
|
|
Brain |
Terminal |
92 |
77* |
69* |
From Goldenthal (1990b)
aAs cholinesterase activities varied considerably over time, cholinesterase activity of animals in the treatment groups is expressed as per cent of the value for concurrent controls rather than as per cent of the pretest value
* Significantly different from control
Erythrocyte cholinesterase activity appeared to be far less sensitive to treatment with carbosulfan than plasma or brain cholinesterase activity. On the basis of the statistically significant reduction in brain cholinesterase activity in animals in the group receiving 50 mg/kg bw per day, the NOAEL was 5 mg/kg bw per day (Goldenthal, 1990b).
The Meeting noted that it was not specified at which time-point during the 6 h period of exposure the blood samples for determination of erythrocyte and plasma cholinesterase activity were drawn, and no data on the peak plasma concentrations of carbosulfan are available. Furthermore, it as reported that brain cholinesterase activity was assessed "within 2 h after the end of exposure". The effects of carbamates being known to be transient, blood and brain concentrations of carbosulfan may be below peak concentrations at this time. Therefore, measurements of plasma, erythrocyte and brain cholinesterase activity in this study may underestimate the level of inhibition induced by carbosulfan.
Rats
Groups of five male and five female Sprague-Dawley rats received whole-body exposure to air containing liquid droplets of carbosulfan technical (purity, 89.6%) stabilized in epoxidized soybean oil, 6 h per day, for 5 days. Mean actual concentrations to which the animals were exposed were 5.4, 15.6, and 47 µg/l. A control group was exposed to room air. An additional group of five rats of each sex were killed before treatment to establish cholinesterase activity before exposure to the test material. During exposure, the groups of animals were observed regularly for clinical signs. Detailed observations of individual animals were carried out before and after exposure. Body weights were measured before exposure on day 1 and after exposure on day 5. After the exposure on day 5, the animals were killed and necropsies were performed, and cholinesterase activity was determined in erythrocytes, plasma and brain. Histopathology was not performed. Statements of compliance with GLP and QA were provided.
The mass median aerodynamic diameter (MMAD) ranged from 1.9 to 2.6 µm. Effects were observed in all treatment groups, these effects increasing in incidence and severity with increasing dose. Females tended to be more sensitive to exposure to carbosulfan than males. During exposure, fasciculation was observed in all treatment groups. Animals in the groups receiving the intermediate and highest doses also displayed irregular breathing and decreased activity. Additionally, salivation, lacrimation, tremors and anogenital staining were observed in the group receiving the highest dose. After exposure, anogenital staining, decreased activity, lacrimation and irregular breathing were observed to occur in a dose-dependent manner in all groups. On day 5, body weights in all treatment groups tended to be less than those of controls; this effect reached statistical significance in males at the highest dose. Except for discoloration of the anogenital and nasal area, no treatment-related macroscopic changes in the organs were observed. The effects of carbosulfan on cholinesterase activity are summarized in Table 6.
Table 6. Effects of carbosulfan on cholinesterase activitya in rats exposed by inhalation for 5 days
|
Substrate |
Dose (µg/l) |
|||||
|
5.4 |
15.6 |
47 |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Erythrocyte |
95 |
95 |
92* |
90* |
94 |
87* |
|
Plasma |
61* |
53 |
60* |
42* |
49* |
37* |
|
Brain |
59* |
52* |
54* |
52* |
38* |
40* |
From Whitman (1990a)
a % of value for controls
* Significantly different from control
Erythrocyte cholinesterase activity appeared to be far less sensitive to treatment with carbosulfan than plasma or brain cholinesterase activity. No consistent sex differences in cholinesterase inhibition were found. Owing to the reduction in brain cholinesterase activity and the clinical signs observed during and after exposure in the groups receiving the lowest dose, a no-observed-adverse-effect concentration (NOAEC) could not be identified. The lowest-observed-adverse-effect concentration (LOAEC) was 5.4 mg/l (Whitman, 1990a).
The Meeting noted that the animals were killed within 3 h 20 min after the end of the final exposure. The effects of carbamates being known to be transient, blood and brain plasma concentrations of carbosulfan at this time may be below peak concentrations. Therefore, measurements of plasma, erythrocyte and brain cholinesterase activities in this study may underestimate the level of inhibition induced by carbosulfan.
Groups of 10 male and 10 female Sprague-Dawley rats received whole-body exposure to air containing liquid droplets of carbosulfan technical (purity, 89.6%) stabilized in epoxidized soybean oil, 6 h per day, 5 days per week for 3 weeks. Mean actual concentrations to which the animals were exposed were 0.15, 0.65 and 5.34 µg/l. An additional group of 10 rats of each sex was killed before treatment, to establish cholinesterase activity before exposure. During exposure, the groups of animals were observed hourly for clinical signs. Detailed observations of individual animals were carried out before and after exposure. Body weights were measured before study initiation, weekly at the start of the exposure, and before and after exposure on the last day. Ophthalmological examinations were performed before study initiation and during the last week of exposure. Within 3 h 20 min after the last exposure, the animals were killed and necropsies were performed. Blood and brain tissue were collected for haematology and clinical chemistry, including determination of cholinesterase activity in erythrocytes, plasma and brain. Organs were dissected and weighed, and microscopical examination was performed on a full range of organs and tissues. Statements of compliance with GLP and QA were provided. The MMAD ranged from 1.9 to 2.0 µm. No clinical signs were observed in animals at the lowest and intermediate doses. At the highest dose, fasciculations, decreased activity, irregular and laboured breathing, salivation, lacrimation, and dried nasal discharge, anogenital staining, soft stool were observed during or after exposure. No treatment-related effects on ophthalmology and body weight were observed. Occasional findings on macroscopical and histopathological examinations, on organ weights and on blood urea nitrogen values were considered to be incidental and not related to treatment. The effects of carbosulfan on cholinesterase activity are summarized in Table 7.
Table 7. Effects of carbosulfan on cholinesterase activitya in rats exposed by inhalation for 5 days
|
Substrate |
Dose (µg/l) |
|||||
|
0.15 |
0.65 |
5.34 |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Erythrocyte |
101 |
95 |
100 |
96 |
92 |
91 |
|
Plasma |
100 |
81 |
97 |
102 |
64* |
63* |
|
Brain |
98 |
98 |
93* |
89* |
57* |
64* |
From Whitman (1990b)
a % of value for controls
* Significantly different from control
Erythrocyte cholinesterase activity appeared to be far less sensitive to treatment with carbosulfan than plasma or brain cholinesterase activity. No consistent sex differences in cholinesterase inhibition were found. Although statistically significant, only small decreases in brain cholinesterase activity were observed at the intermediate dose (7% in males, 11% in females). Moreover, no clinical signs of toxicity were observed in this group. On the basis of the occurrence of clinical signs and inhibition of brain cholinesterase activity in animals at the highest dose, the NOAEC was 0.65 µg/l (Whitman, 1990b).
The Meeting noted that the animals were killed within 5 h 52 min after the end of the final exposure. The effects of carbamates being known to be transient, plasma concentrations of carbosulfan at this time may be below peak concentrations. Therefore, measurements of plasma, erythrocyte and brain cholinesterase activity in this study may underestimate the level of inhibition induced by carbosulfan.
Mice
Groups of 100 male and 100 female Charles River CD-1 mice were given diets containing carbosulfan (purity, 94.5-95.6%, with 0.6-2.4% carbofuran) at a concentration of 0, 10, 20, 500 or 2500 mg/kg (equal to 0, 1.3, 2.5, 62 and 320 mg/kg bw per day for males, and 0, 1.5, 3.1, 72 and 337 mg/kg bw per day for females) for 24 months. All animals were observed daily for signs of toxicity, moribundity and mortality. Body weight and food consumption values were recorded weekly for the first 14 weeks and every two weeks thereafter. Water consumption was determined monthly. Haematological and biochemical measurements and urine analyses, including cholinesterase activity, were performed for 10 unfasted mice of each sex per group at the 6, 12, 18 and 24 month sacrifices. Ophthalmoscopic evaluations were performed in all survivors at 24 months. Selected organs were weighed, gross necropsies conducted and a complete list of tissues and organs examined microscopically. A statement of compliance with QA was provided.
There were no measurable treatment-related effects on mortality or survival. Mean body weights were reduced throughout the study for males at 500 and 2500 mg/kg and for females at 2500 mg/kg. For females, body-weight changes at other dietary concentrations were sporadic and not related to treatment. Males at 20 mg/kg, however, had decreased body-weight gains from week 42 to terminal sacrifice at 104 weeks. Food consumption was depressed for males and females at 2500 mg/kg, but only sporadic decreases were noted at other dietary concentrations.
There were no measurable differences with regard to general appearance and behaviour, except for increased eye irregularities at 2500 mg/kg. These included corneal opacity, eccentric pupil, and white, cloudy eyes. There were no treatment-related effects or haematological changes, except for a tendency towards slightly increased segmented neutrophils and decreased lymphocyte counts in males at 2500 mg/kg. There were no demonstrated effects on glucose, blood urea nitrogen, alanine aminotransferase or alkaline phosphatase in either sex at any dose. The effects of carbosulfan on cholinesterase activity are summarized in Table 8. Since the observed inhibition of cholinesterase activity in the treated groups was not dependent on the time of sampling (i.e. 6, 12, 18 and 24 months), the mean cholinesterase activity (expressed as a percentage of the control value) and range of the means for the four time-points are shown in Table 8.
Table 8. Effects of carbosulfan on cholinesterase activitya in mice after administration in the diet
|
Substrate |
Dietary concentration (mg/kg) |
|||
|
10 |
20 |
500 |
2500 |
|
|
Males |
||||
|
Erythrocytes |
98(91-104) |
92 (89-96) |
64 (56-73)* |
52 (40-63)* |
|
Plasma |
93(86-100) |
95 (86-100) |
52 (36-73)* |
38 (36-41)* |
|
Brain |
100 (89-107) |
105 (93-114) |
51 (39-57)* |
35 (32-43)* |
|
Females |
||||
|
Erythrocyte |
96(90-100) |
93 (88-98) |
66 (64-70)* |
56 (48-60)* |
|
Plasma |
99(85-109) |
96 (79-105) |
77 (72-81)* |
49 (41-53)* |
|
Brain |
101 (98-102) |
97 (88-106) |
50 (35-79)* |
40 (29-61)* |
From DeProspo et al. (1982a)
aOverall mean % activity, relative to controls (range of means)
* Significantly different from control
Cholinesterase activity for plasma, erythrocytes and brain tissue was significantly depressed in both males and females at 500 and 2500 mg/kg, at all time-points.
Ophthalmoscopic examinations indicated an increase in punctuate opacities of the iris at 500 and 2500 mg/kg. Females in the group receiving the highest dose were observed to have focal retinal degeneration. An apparent difference of opinion was expressed by two ophthalmologists, regarding the incidence of cataracts in male mice. An independent ophthalmological examination was subsequently performed, the results of which were considered by the JMPR in 1986. It was concluded that there was no evidence for cataractogenesis in the study of carcinogenicity in mice. Therefore, the concern expressed by the Meeting in 1984 with regard to the cataractogenic potential of carbosulfan was alleviated. Special evaluation and concern for the iris, because of compound-related effects in the rat (DeProspo et al., 1982b) did not indicate similar effects in mice.
Absolute organ weight changes were variably affected in both males and females, except for decreased spleen weight in females at 500 and 2500 mg/kg. Relative spleen weights were also significantly decreased in females at 500 and 2500 mg/kg. Relative brain weights were significantly increased throughout the study for both sexes at 2500 mg/kg. This is considered a reflection of the significant effects on body weight at the higher doses.
The results of gross and histopathological examinations were essentially unremarkable. The most common findings reported were malignant lymphoma and bronchio-alveolar adenoma, which were equally distributed among all groups, except for females at the lowest dose; the latter had a significant increase in the number of metastatic malignant lymphomas of mediastinal and mesenteric lymph nodes, as well as thymus and spleen. Generally, the incidence of malignant lymphomas was highest in females in the control group and in the group receiving the lowest dose. The results of the histopathological examination indicated that the type and incidence of non-neoplastic and neoplastic lesions were normal findings in the mouse and were unrelated to treatment. Carbosulfan was not carcinogenic in mice at dietary concentrations up to and including 2500 mg/kg, equal to 320 and 337 mg/kg bw per day for males and females respectively.
On the basis of the decrease in body weight, the NOAEL was 10 mg/kg, equal to 1.3 mg/kg bw per day (DeProspo et al., 1982a).
Rats
Groups of 90 male and 90 female Charles River CD rats were given diets containing carbosulfan (purity, 94.5-95.6%) at a concentration of 0, 10, 20, 500 or 2500 mg/kg (equal to 0, 0.5, 1.0, 27 and 153 mg/kg bw for males, and 0, 0.6, 1.2, 35 and 213 mg/kg bw for females) for 104 weeks. Carbofuran was present in the technical material at a concentration of 0.6-2.4%. Growth was observed by body-weight changes and data on food consumption that were recorded weekly for the first 14 weeks and every two weeks thereafter. Daily observations were made with respect to behavioural changes and mortality. At periodic intervals throughout the study, haematological, biochemical and cholinesterase analyses were performed on unfasted animals. Urine analysis was conducted on fasted animals.
Eyes were examined at 12, 18 and 24 months. At 6, 12 and 18 months of the study, 10 males and 10 females per group were sacrificed and necropsies were performed. At the termination of the study, all surviving animals were sacrificed and gross pathological and microscopic examinations of tissues and organs were made, and selected organs were weighed. A statement of compliance with QA was provided.
Tremors, laboured breathing and eye-related changes were more frequently observed in animals receiving the diets containing carbosulfan at a concentration of 500 and 2500 mg/kg. Mean body weight and food consumption values of animals at 500 and 2500 mg/kg were significantly lower than control values throughout the study, except for female food consumption, which was comparable to controls. Survival was not apparently affected by treatment. Measurement of haematology parameters at 6, 12, 18 and 24 months demonstrated a compound-related effect at 18 months in males and females at 2500 mg/kg, including a significantly increased leukocyte count (primarily segmented neutrophils), and platelet count, slightly increased reticulocyte count and significantly decreased lymphocyte count. After 24 months, haemoglobin, erythrocyte cell volume, mean corpuscular volume (MCV) and mean corpuscular haemoglobin (MCH) were all lower, although not significantly, in males at the highest dose than in controls. After 18 months, urine analysis showed that males and females at the highest dose had higher concentrations of ketones than did controls. At terminal sacrifice, there was an increase in mononuclear leukocyte infiltrates in the kidney in animals receiving the intermediate and highest doses, and an increase in pigmentation (haemosiderin) in the mediastinal lymph nodes of treated females. All these data are indicative of leukocytosis and toxic neutrophilia. During early phases of neutrophilia, there is a tendency toward acidosis, which is demonstrated by the presence of ketone bodies in the urine. The elevated platelet count is also a reflection of hyperactivity of the bone marrow. However, histopathology of bone marrow, spleen and liver was otherwise unremarkable. Biochemical analyses were generally comparable with those for controls, except for males and females at the highest dose; decreased albumin, total protein and globulin were reported in these animals. Plasma, erythrocyte and brain cholinesterase activity was significantly decreased in males and females at a concentration of 500 or 2500 mg/kg. Significantly increased relative brain, heart, liver and kidney weights in males and females at the intermediate and highest doses were attributed to lower body weights. Absolute spleen, adrenal and thyroid weights were uniformly lower for groups receiving carbosulfan at 500 and 2500 mg/kg, but were not different from those of controls on an organ-to-body weight basis. Carbosulfan produced compound-related effects on the eye, which were described pathologically as focal iris atrophy, iris coloboma and absence of iris tissues in males and females at 500 and 2500 mg/kg, as well as degenerative retinopathy in females at 2500 mg/kg. The atrophy of the iris was attributed, in part, to an extensive anticholinesterase effect. There were no treatment-related effects on the eye at 10 or 20 mg/kg. Gross and histopathologic examinations of all tissues, except the eye, revealed no compound-related effects on the incidence or type of neoplastic or non-neoplastic changes. Carbosulfan was not carcinogenic to rats at dietary concentrations of up to and including 2500 mg/kg (equal to 153 and 213 mg/kg bw per day for males and females, respectively, the highest dose tested). The NOAEL was 20 mg/kg, equal to 1 mg/kg bw per day, on the basis of pathology of the eye, clinical signs and cholinesterase inhibition (DeProspo et al., 1982b).
A range of tests for genotoxicity in vitro and in vivo was performed with carbosulfan or formulations of carbosulfan. A number of these studies were reported in the public literature. Some were poorly described and did not comply with current guidelines, GLP or QA. Positive effects were reported in a number of studies from public literature. The results of tests for genotoxicity are summarized in Table 9.
Table 9. Results of studies of genotoxicity with carbosulfan
|
End-point |
Test object |
Concentration |
Purity (%) |
Results |
Reference |
|
In vitro |
|||||
|
Reverse mutation |
Bacillus subtilis |
0.0005-2 mg/plate |
NR |
Negative |
Jagannath (1979)c |
|
Reverse mutation |
S. typhimurium. |
0.1-10 µl/plate |
93 |
Negative |
Haworth et al. (1980)a |
|
Reverse mutation |
E. coli. WP2, |
0.025, 0.05, 0.1 & |
93 |
Negative |
Haworth et al. (1981)a,d |
|
Reverse mutation |
S. typhimurium |
0.001-5 mg/plate |
40.3 |
Negative |
Gava (1988)a,e |
|
Gene mutation |
Mouse lymphoma |
0.0024-0.032 µl/ml, |
93 |
Negative |
Kirby et al. (1981)a |
|
Chromosomal aberration |
Human lymphocytes |
1 × 10-6 to 5 × 10-5 |
NR |
Positive |
Topaktas & Rencüzoğullari (1993) |
|
Sister chromaid exchange |
Human lymphocytes |
25, 50, 100 µmol/l |
NR |
Positive |
Rencüzoğullari & Topaktas (1998)g |
|
Gene conversion, reverse mutation, aneuploidy |
S. cerevisiae D7, D61.M |
4.9-980 µmol/l |
93 |
Negative |
Stehrer-Schmid & Wolf (1995)h |
|
In vivo |
|||||
|
Micronucleus formation |
Mouse bone-marrow cells |
43.5 & 87 mg/kg bw |
93 |
Negative |
Kirkhart et al. (1979)b |
|
Micronucleus formation |
Mouse bone-marrow cells |
20.3, 40.6 & |
40.3 |
Negative |
Franco Perina (1998)a,j |
|
Micronucleus formation |
Mouse bone-marrow cells |
29.6, 59.2 mg/kg bw |
93 |
Positive |
Stehrer-Schmid & Wolf (1995)k |
|
Chromosomal aberration |
Rat bone-marrow cells |
0, 5, 12, 30 mg/kg bw for 5 consecutive days |
93 |
Negative |
Putnam & Schechtman (1981)b |
|
Chromosomal aberrations |
Rat bone-marrow cells |
12.5, 25, 50 mg/kg bw |
NR |
Positive |
Topaktas & Rencüzoğullari (1996)m |
|
Dominant lethal mutation |
Mice |
0, 7, 20, 60 mg/kg bw for 5 consecutive days |
93 |
Negative |
Preache et al. (1981)a,n |
Positive and negative (solvent) controls were included in all studies
NR, not reported; S9, 9000 × g supernatant of induced rat liver
* Statements of compliance with GLP and QA were provided
b A statement of compliance with QA was provided
cTest substance, FMC 35001. S9 fraction of Aroclor 1254-induced rat liver
dPreferential kill of repair-deficient E. coli CM611 without liver microsomes, at 0.025-0.2 ml/plate
eTest substance was Marshal 400 SC. S9 fraction of Aroclor 1254-induced rat liver
f Study from the public literature. Both carbosulfan (purity not reported) and a formulation of carbosulfan, identified as Marshal (not further specified) were tested. Chromosomal aberrations were reported at all concentrations of carbosulfan and at the two highest concentrations of Marshal
g Study from the public literature. Carbosulfan (purity not reported) induced sister chromatid exchange after 48 h treatment but not after 24 h
h Study from the public literature
iTest compound was administered orally. A dose of 174 mg/kg bw caused excess mortality and was excluded
j Marshal 400 SC was administered intraperitoneally, in two doses of 75, 50 and 25% of the LD50, separated by a 24h interval. No deaths were reported. The ratio of polychromatic erythrocytes to normochromatic erythrocytes (PCE: NCE) was not affected (at the highest dose, carbosulfan induced a 14% decrease in the PCE:NCE ratio in males)
k Study from the public literature. Carbosulfan was administered intraperitoneally. Treatment with carbosulfan resulted in a modest, time-dependent increase in micronucleus formation
lTest compound was administered orally in single doses
m Study from the public literature. A formulation of carbosulfan, identified as Marshal (not further specified) was administered intraperitoneally. Chromosomal aberrations were reported at all concentrations and time-points
nThe test compound was administered orally. Parameters evaluated were fertility index, total number of implantations, number of live implantations, number of early and late implantation deaths, body weights and clinical signs
A test for sister chromatid exchange in human lymphocytes (Rencüzoğullari & Topaktas, 1996) was deemed to be inadequate, since positive controls were lacking and the test was only performed in the absence of metabolic activation. A study in which the genotoxicity of mixtures of carbosulfan and other substances were tested was not evaluated (Rencüzoğullari & Topaktas, 2000). One study that reported negative effects with carbosulfan in an Ames test and positive effects in a test with Saccharomyces cerevisiae D61.M was not evaluated, because only the abstract was available (Wiedenmann et al., 1990).
Rats
Groups of 15 male and 30 female Charles River CD rats were given diets containing carbosulfan at a concentration of 0, 10, 20 and 250 mg/kg (equivalent to 0, 0.67, 1.3 and 16.7 mg/kg bw per day) for three generations. Two successive litters were reared from each female. General condition and behaviour were observed routinely and individual body weights were recorded throughout the study. The number of pups in each litter was examined and pups were culled to 10 per litter at age 4 days. Individual pup weights were measured on days 0, 4, 7, 14 and 21. Ten male and 10 female F1b, F2b, and F3b weanlings were randomly selected for gross necropsy and tissue collection. The F1a and F2a litters were discarded at weaning, and the F1b and F2b litters were used to produce succeeding generations. Weanlings not selected for continuation in the study (F1a, F2a, F1b, F2b, F3a and F3b) were subjected to gross external examination and sacrificed and discarded. A statement of compliance with QA was provided.
Body weights of F0 males and females at 20 and 250 mg/kg showed initial decreases. These decreases were associated with reduced food consumption, and both recovered to normal levels between week 4 and sacrifice. Body weights of parental males (F1 and F2) receiving the diet containing carbosulfan at 250 mg/kg were consistently lower than those of animals in the corresponding control group. A similar effect was observed in females at 250 mg/kg at sometimes during gestation and lactation, but not during the growth phases. Mating index, gestation index and number of viable fetuses were essentially normal throughout the study, except for F2b dams at 250 mg/kg, for which values for these parameters were decreased. Litter size, pup weights and/or pup weight gains for all litter sets in the group receiving carbosulfan at 250 mg/kg were significantly lower at most age intervals between birth and weaning than for the concurrent control group. Neonatal survival at 250 mg/kg was also significantly lower for the first four litters (F1a, F1b, F2a and F2b). There were no treatment-related gross or histological changes observed among the F0, F1b and F2b adults or the F1b, F2b and F3b weanlings. Carbosulfan did not have an adverse effect on reproductive performance. Pup weight, litter size, and pup survival was decreased at 250 mg/kg. In parental animals, the NOAEL was 20 mg/kg (equivalent to 1.3 mg/kg bw per day), on the basis of the decreases in body weight. The NOAEL for fetotoxicity was 20 mg/kg (equivalent to 1.3 mg/kg bw per day) on the basis of reductions in litter size, pup weight and pup weight gain. The NOAEL for reproductive toxicity was 250 mg/kg (equivalent to 16.7 mg/kg bw per day, the highest dose tested) (Kehoe & MacKenzie, 1982).
Rats
Groups of 25 female Charles River CD rats were given carbosulfan at a dose of 0, 2, 10 and 20 mg/kg bw per day by oral gavage on days 6-19 of gestation. Necropsies were performed on surviving females on day 20 and on fetuses delivered by hysterotomy. The number and position of viable/non-viable fetuses, early/late resorptions, mean number of corpora lutea and total number of implantations were recorded. External, internal and skeletal examinations of fetuses were performed. One-third of the fetuses were evaluated for soft-tissue anomalies and the remaining two-thirds for skeletal effects. A statement of compliance with QA was provided.
There was no dose-related increase in mortality in any treated group. Body tremors were reported at 20 mg/kg bw per day after administration of each dose. Clear oral discharge was observed in dams at 10 and 20 mg/kg bw per day. Mean maternal body-weight gains were slightly reduced at 10 mg/kg bw per day and significantly reduced at 20 mg/kg during gestation. There were no differences in number of pregnancies, early/late resorptions, viable fetuses, postimplantation loss or sex distribution. The number of corpora lutea per dam was increased in all treated groups, while the mean fetal body weight was significantly reduced at 10 and 20 mg/kg bw per day. There was also an increase in the number of litters with developmental variations at 20 mg/kg bw per day; these variations included reduced ossification of the skull, unossified hyoid body, unossified sternebrae 5 and 6, and undeveloped renal papilla and/or distended ureters. There were no reported effects on the number or per cent of fetuses or litters with external, internal or skeletal malformations or anomalies at any dose. On the basis of the clinical signs and reduction in body weight, the NOAEL for maternal toxicity was 2 mg/kg bw per day. On the basis of the reduction in fetal body weight, the NOAEL for fetotoxicity was 2 mg/kg bw per day. The NOAEL for developmental toxicity was 20 mg/kg bw per day (the highest dose tested) (Janes et al., 1980a).
Rabbits
Groups of 16 New Zealand albino rabbits were given carbosulfan at a dose of 0, 2, 5, and 10 mg/kg bw per day by gavage on days 6-28 of gestation. Pups were delivered by caesarean section on day 29, and the number, location, and distribution of viable/non-viable fetuses, corpora lutea, early/late resorptions and total implantations were recorded. All fetuses were examined grossly, sectioned for visceral anomalies and stained for skeletal anomalies. A statement of compliance with QA was provided.
No treatment-related effects on appearance and behaviour were observed in the dams. There were three deaths of animals treated at 10 mg/kg, one death in the control group and one death in the group receiving the lowest dose. A cause of death could not be established for two dams in the group receiving the highest dose and in the dam in the group receiving the lowest dose. The deaths of one of the dams at the highest dose and the dam at the lowest dose were attributed to enteritis. The numbers of dams with viable fetuses were 12, 10, 15 and 11 at 0 (control), 2, 5 and 10 mg/kg, respectively. There were no compound-related effects on the number of viable fetuses, corpora lutea, fetal sex distribution or total implantations per dam. There were slight, non-significant increases in postimplantation losses (6, 9, 11 and 13 at 0, 2, 5 and 10 mg/kg bw per day, respectively) and early resorption rate (3, 8, 9 and 11 at 0, 2, 5 and 10 mg/kg bw per day, respectively). Also, the mean fetal body weight was slightly decreased (7%) at the highest dose. There was a single occurrence of scoliosis in each of the three treated groups, but none was reported in the controls. There were no compound-related effects on skeletal variations, such as delayed ossification. Major vessel variations, identified primarily as left carotid arising from the innominate, were observed in 16.7, 100, 46.7 and 72.7% of the litters at 0, 2, 5, and 10 mg/kg bw per day, respectively (mean of data for historical controls in the test laboratory, 51%). The proportions of fetuses presenting this defect were 4.9, 44, 12.8 and 20.8%, respectively (mean of data for historical controls in the test laboratory, 14%). The increase in major vessel variations was not dose-dependent. Furthermore, the Meeting noted that data for historical controls (MARTA, 2003) show that these major vessel variations are very common and occur at highly variable incidences in rabbits of this strain. Therefore the Meeting considered these effects to be incidental. The NOAEL for maternal and fetotoxicity was 10 mg/kg bw per day (the highest dose tested). Carbosulfan was not teratogenic in rabbits. The NOAEL for developmental toxicity was 10 mg/kg bw per day (the highest dose tested) (Janes et al., 1980b).
The neurotoxic effects of acute oral exposure to carbosulfan (purity, 88%) were assessed in groups of 27 male and 27 female Crl: CD BR rats treated by gavage in a study that complied with EPA guideline 81-8-SS. The animals received vehicle (corn oil), or carbosulfan at a dose of 0.5, 5 or 30 mg/kg bw. All animals were checked daily for viability and clinical signs. Body weights were measured on days -6, 0, 7, 14 and 15. The doses and time-points were selected on the basis of a preliminary study. Seven animals of each sex per dose were allocated to undergo neuropathological examination on day 15; this consisted of assessment of brain weight and dimensions, evaluation for gross changes, and histopathological examination of a range of central and peripheral nervous system tissues (including eyes, ganglions and nerve fibres). In groups of five animals of each sex per dose, brain, erythrocyte and plasma cholinesterase activities were measured before treatment, and 4 h after dosing on days 0, 7 and 15. Additionally, at termination the brains of animals in these four groups were weighed, and six brain regions (see Table 10) were dissected. The animals that were allocated to undergo neuropathological examination and the animals for which cholinesterase activity was measured on day 15 were also tested in a functional observational battery (FOB) and for locomotor activity before treatment, 4 h after dosing, and on days 7 and 14. Statements of compliance with GLP and QA were provided.
No mortality was observed. During the first week after treatment, body-weight gain was lower in males at the highest dose. Also at the highest dose, some animals had staining of the ventral abdomen and urogenital area on days 1 and 2. Home cage observations revealed one male at the highest dose with tremors on day 0. At 4 h after dosing, body temperatures were decreased in males and females at the highest dose, a few animals with slight tremors and impaired gait were observed during open-field testing, and sensory tests revealed a slow tail-pinch response in males. Furthermore, motor activity was decreased in animals at the highest dose. Effects on brain weight, gross changes and histopathology were considered not to be treatment-related. Data on cholinesterase activity at 4 h after dosing in the groups treated with carbosulfan are summarized in Table 10.
Table 10. Effects of carbosulfan on cholinesterase activitya in rats, 4 h after dosing by oral gavage
|
Substrate |
Dose (mg/kg bw) |
|||||
|
0.5 |
5 |
30 |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Blood |
||||||
|
Plasma |
96 |
135 |
76* |
116 |
61* |
88 |
|
Erythrocytes |
101 |
89 |
62* |
64* |
54* |
55* |
|
Brain |
||||||
|
Hippocampus |
86 |
108 |
73 |
62* |
54* |
62* |
|
Olfactory region |
93 |
88 |
66 |
66* |
52* |
58* |
|
Midbrain |
97 |
92 |
64* |
61* |
53* |
53* |
|
Brain stem |
84 |
94 |
56* |
59* |
48* |
52* |
|
Cerebellum |
95 |
94 |
74* |
67* |
61* |
68* |
|
Cortex |
88* |
94 |
62* |
66* |
52* |
51* |
From Knapp (1996)
a % of value for controls
* Significantly different from control
In females, plasma cholinesterase activity appeared to be less sensitive than erythrocyte and brain cholinesterase activity to inhibition by carbosulfan. Brain and erythrocyte cholinesterase activity was reduced in males and females at the intermediate and highest doses, 4 h after administration of carbosulfan. No consistent differences in cholinesterase activity were observed before treatment or on days 7 and 14. Occasional significant differences at these time-points were considered not to be treatment-related.
On the basis of the effects on cholinesterase activity in brain and erythrocytes at the intermediate dose, the NOAEL was 0.5 mg/kg bw (Knapp, 1996).
(ii) Neurotoxicity after repeated doses
Groups of 10 male and 10 female Sprague-Dawley CD rats were given diets containing carbosulfan (purity, 88.0%) at a concentration of 0, 20, 1000 or 2000 mg/kg (equal to 0, 1.2, 65 and 131 mg/kg bw per day in males, and 0, 1.4, 79 and 152 mg/kg bw per day in females) for 13 weeks, in a study that complied with EPA guidelines 81, 82 and 83. Clinical signs were recorded daily. Body weights and food consumption were measured weekly. The animals were subjected to FOB and motor activity tests before treatment and 4, 8 and 13 weeks after the start of treatment. At termination, the animals were killed and necropsies were performed. The nervous systems of five animals of each sex in the control group and in the group receiving the highest dose were examined for neuropathological lesions. Statements of compliance with GLP and QA were provided.
At the intermediate and highest doses (dietary concentrations, 1000 or 2000 mg/kg respectively), body weight and body-weight gains were decreased in males and females. At these doses, food consumption in males was decreased throughout the study and food consumption in females was decreased during weeks 2 and 3. Chromodacryorrhoea and decreased faeces were also observed in males at these doses. Additionally, tremors, exophthalmus, chromorhinorrhoea and dehydration were observed in males at the highest dose. In females at the intermediate and highest doses, exophthalmus, tremors, abdominogenital staining, reddish brown staining of the cage-pan liner, chromorhinorrhoea, chromodacryorrhoea, decreased faeces, dehydration, diarrhoea and unthriftiness were observed. In the FOB, males at the highest dose (2000 mg/kg) showed decrease in foot splay, hindlimb grip strength and urine pools. In females at the highest dose, localized spasms/twitching or tremors, exophthalmos, walking on toes, and slightly impaired gait were observed. A reduction in motor activity was observed in females at the two higher doses at weeks 4 and 13 respectively. No treatment-related effects on necropsy and neuropathological examination were found. Measurements of cholinesterase activity in brain, erythrocyte or plasma were not included in this study. On the basis of the clinical signs, observations in the FOB, and effects on body weight, body-weight gain and food consumption in animals at the two higher doses, the NOAEL was 20 mg/kg, equal to 1.2 mg/kg bw per day (Freeman, 1995).
(b) Effects of carbosulfan on semen characteristics and serum testosterone
A study reported in the public literature described the effects of carbosulfan on semen characteristics and serum testosterone concentrations in male rabbits. The study report was confounded by lack of information on the purity of the compound, the doses used, and data on body weight and nutritional state of the animals. Therefore, the Meeting concluded that the significance of the reported effects could not be established (El-Zarkouny et al., 1999).
The absorption of radiolabelled carbosulfan administered orally is rapid and almost complete in male and female rats. Elimination is also relatively rapid; most (80-90%) of the absorbed radioactivity is excreted in the urine within 48-72 h, depending on the dose administered. After repeated dosing of rats with carbosulfan, the rate of excretion appeared to be increased (80-87% within 24 h), which may indicate that induction of metabolism has occurred.
Carbosulfan is metabolized by hydrolysis to the 7-phenol or to carbofuran and dibutylamine, and is subsequently further metabolized via hydrolysis, oxidation and conjugation to a variety of metabolites. Metabolites of the dibutylamino moiety may enter the carbon pool and be incorporated into natural constituents of the body. No marked sex-specific differences were observed in rats with regard to the excretion pattern, tissue distribution and metabolite profile of carbosulfan.
Carbosulfan (technical material) is highly toxic when administered orally, with LD50s ranging from 90 to 250 mg/kg bw in rats. The LD50 for carbosulfan was >2000 mg/kg bw in rabbits treated dermally and the LC50 was 0.61 mg/l in rats treated by inhalation.
Carbosulfan is minimally irritating to the eye, slightly irritating to the skin and is a dermal sensitizer.
In general, in short-term and long-term studies of toxicity, the most sensitive effect of the oral administration of carbosulfan was the inhibition of cholinesterase activity, accompanied at the same or higher doses by clinical signs indicative of cholinesterase inhibition (e.g. salivation, lacrimation, ataxia, tremors, anogenital staining, diarrhoea). In a study of acute oral neurotoxicity in rats, the NOAEL was 0.5 mg/kg bw on the basis of effects on brain cholinesterase activity as measured 4 h after dosing. In a 90-day study in rats, the NOAEL was 20 mg/kg, equivalent to 1 mg/kg bw per day on the basis of inhibition of brain and erythrocyte cholinesterase activity. In a second 90-day study of rats fed with carbosulfan, the NOAEL was 20 mg/kg, equal to 1.2 mg/kg bw per day, on the basis of clinical signs, observations in FOB and effects on body weight, body-weight gain and food consumption at a dose of 62 mg/kg bw per day. In this study, cholinesterase activity was not determined.
In a 6-month study in dogs, the NOAEL was 50 mg/kg, equivalent to 1.3 mg/kg bw per day, on the basis of effects on blood chemistry parameters and occasional reductions in food consumption and body-weight gain.
In long-term studies in mice and rats, carbosulfan was not carcinogenic at dietary concentrations of up to and including the highest dose tested, 2500 mg/kg, equal to 320 and 153 mg/kg bw per day for mouse and rat, respectively. In the study in mice, the NOAEL was 20 mg/kg, equal to 2.5 mg/kg bw per day, on the basis of reductions in body weight, inhibition of brain and erythrocyte cholinesterase activity and reductions in absolute and relative spleen weight. In the study in rats, the NOAEL was 20 mg/kg, equal to 1 mg/kg bw per day, on the basis of inhibition of brain and erythrocyte cholinesterase activity and pathological changes in the eye, i.e. focal iris atrophy, iris coloboma and absence of iris tissue. The mechanism by which these pathological changes in the eye were induced is not clear.
The genotoxic potential of carbosulfan was investigated in a wide range of tests. Primarily negative results were obtained in a number of tests in vitro and in vivo. Positive effects were observed in a few tests, however these tests were confounded by the use of very high doses in vivo, the occurrence of marked cytotoxicity in vitro and the lack of information on the purity of the test compound. The Meeting concluded that carbosulfan is unlikely to be genotoxic.
In view of the lack of genotoxicity and the absence of carcinogenicity in rats and mice the Meeting concluded that carbosulfan is unlikely to pose a carcinogenic risk to humans.
In a three-generation study of reproductive toxicity, carbosulfan was administered at a dose of 10, 20 or 250 mg/kg of diet. No effects on mating index, gestation index and number of viable fetuses were observed. At 250 mg/kg, pup weight, litter size and pup survival were decreased, as were the body weights of parental males and females at this dose. In parental animals, the NOAEL was 20 mg/kg, equivalent to 1.3 mg/kg bw per day, on the basis of the decreases in body weight. The NOAEL for pup toxicity was 20 mg/kg on the basis of the reductions in litter size, pup body weight and pup body-weight gain. The NOAEL for reproductive toxicity was 250 mg/kg, equivalent to 17 mg/kg bw per day, the highest dose tested.
In studies of developmental toxicity in rats and rabbits, carbosulfan was not teratogenic. The NOAEL for maternal toxicity was 2 mg/kg bw per day in the study in rats, on the basis of clinical signs and reduction in body weight. The NOAEL for toxicity in offspring was 2 mg/kg bw per day, on the basis of the reduction in fetal body weight. In the study in rabbits, the NOAEL for maternal and offspring toxicity was 10 mg/kg bw per day, the highest dose tested.
When tested in hens, carbosulfan did not induce delayed polyneuropathy after a single exposure, according to a study evaluated by the Meeting in 1984.
No new data were available for humans.
The Meeting concluded that the present database is sufficient to characterize the potential hazard of carbosulfan to fetuses, infants and children.
Toxicological evaluation
The Meeting established an ADI of 0-0.01 mg/kg bw per day based on a NOAEL of 1 mg/kg bw per day, on the basis of pathological changes in the eye, inhibition of brain and erythrocyte cholinesterase activity and body-weight reduction in the 2-year study in rats, with a safety factor of 100. This safety factor was used because the pathological changes in the eye could not definitely be attributed to inhibition of cholinesterase.
After considering the data available to the present Meeting, as well as the previous evaluations, the Meeting established an acute RfD of 0.02 mg/kg bw. This was based on the NOAEL of 0.5 mg/kg bw per day for inhibition of brain cholinesterase activity in a study of acute neurotoxicity in rats, and a safety factor of 25, as the relevant toxic effects of carbosulfan are dependent on the Cmax (Annex 1, reference 95).
Levels relevant to risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
2-year study of toxicity and carcinogenicitya |
Toxicity |
20 mg/kg, equal to |
500 mg/kg, equal to |
|
Carcinogenicity |
2500 mg/kg, equal to |
|||
|
Rat |
Three-generation study of reproductive toxicitya |
Parental and offspring toxicity |
20 mg/kg, equivalent to |
250 mg/kg, equivalent to |
|
Reproductive toxicity |
250 mg/kg, equivalent to |
|
||
|
Study of developmental toxicityb, |
Maternal toxicity |
2 mg/kg bw per day |
10 mg/kg bw per day |
|
|
Embryo- and fetotoxicity |
2 mg/kg bw per day |
10 mg/kg bw per day |
||
|
Study of acute neurotoxicityb |
Neurotoxicity |
0.5 mg/kg bw |
5 mg/kg bw |
|
|
90-day study of neurotoxicitya |
Neurotoxicity |
20 mg/kg, equivalent to |
500 mg/kg, equivalent to |
|
|
2-year study of toxicity and carcinogenicitya |
Toxicity |
20 mg/kg, equal to |
500 mg/kg, equal to |
|
|
Carcinogenicity |
2500 mg/kg, equal to |
|
||
|
Rabbit |
Study of developmental toxicityb |
Maternal toxicity |
10 mg/kg bw per dayc |
_ |
|
Embryo- and fetotoxicity |
10 mg/kg bw per dayc |
|
||
|
Dog |
6-month study of toxicitya |
Toxicity |
50 mg/kg, equivalent to |
500 mg/kg, equivalent to 13 mg/kg bw per day |
a Diet
b Gavage
c Highest dose tested
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
0.02 mg/kg bw
Studies that would provide information useful for continued evaluation of the compound
Further observations in humans
Summary of critical end-points for carbosulfan
|
Absorption, distribution, excretion and metabolism in animals |
|
|
Rate and extent of absorption |
Rapid and extensive |
|
Dermal absorption |
No data (rabbit: reduction in brain cholinesterase activity at 50 mg/kg bw per day) |
|
Distribution |
Extensive; highest concentrations in liver, kidney, omental fat, peripheral fat |
|
Potential for accumulation |
Low |
|
Rate and extent of excretion |
Relatively rapid (80-90% within 48-72 h in rats, mainly in urine) |
|
Metabolism in animals |
Major metabolites: 3-OH-7-phenol, carbofuran, 3-OH-carbofuran, 3-keto-7-phenol, 7-phenol, dibutylamine (rat) |
|
Toxicologically significant compounds |
Carbosulfan, carbofuran |
|
Acute toxicity |
|
|
Rat, LD50, oral |
90-250 mg/kg bw |
|
Rabbit, LD50, dermal |
>2000 mg/kg bw |
|
Rat, LC50, inhalation |
0.61 mg/l |
|
Rabbit, dermal irritation |
A mild irritant |
|
Rabbit, ocular irritation |
A mild irritant |
|
Dermal sensitization |
Sensitizing (Buehler) |
|
Short-term studies of toxicity |
|
|
Target/critical effect |
Inhibition of cholinesterase activity in brain and erythrocytes |
|
Lowest relevant oral NOAEL |
1 mg/kg bw per day (rats) |
|
Lowest relevant dermal NOAEL |
5 mg/kg bw per day (rabbits) |
|
Lowest relevant inhalatory NOAEC |
0.00065 mg/l (rats) |
|
Genotoxicity |
Negative in most tests; unlikely to be genotoxic |
|
Long-term studies of toxicity and carcinogenicity |
|
|
Target/critical effect |
Inhibition of cholinesterase activity in brain and erythrocytes, pathological changes in the eye |
|
Lowest relevant NOAEL |
1 mg/kg bw per day (rats) |
|
Carcinogenicity |
Unlikely to pose a carcinogenic risk to humans |
|
Reproductive toxicity |
|
|
Reproduction target/critical effect |
Reduction of pup weight, litter size and pup survival (in the presence of parental toxicity) |
|
Lowest relevant reproductive NOAEL |
1.3 mg/kg bw per day (rats) |
|
Developmental target |
Reduction in pup weight (in the presence of maternal toxicity) |
|
Lowest relevant developmental NOAEL |
2 mg/kg bw per day (rats) |
|
Neurotoxicity/delayed neurotoxicity |
|
|
Neurotoxicity |
Inhibition of cholinesterase activity in brain and erythrocytes, and clinical and behavioural effects associated with cholinesterase inhibition |
|
Lowest relevant oral NOAEL |
0.5 mg/kg bw (rats) |
|
Delayed neurotoxicity |
Negative |
|
Medical data |
None |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0-0.01 mg/kg bw |
Rat, long-term toxicity |
100 |
|
Acute RfD |
0.02 mg/kg bw |
Rat, acute neurotoxicity |
25 |
Blagden, S.M. (1997) Acute inhalation toxicity (nose only) study in the rat with Marshal 25 STW/DS. Unpublished report No. A97-4684 May 23 1997 prepared by Safepharm Laboratories Ltd for FMC Corporation. Submitted to WHO by FMC Corporation.
Curry, S. & Weintraub, R.A. (1996) Nature of the residue in livestock: metabolism of carbosulfan in lactating goats (FMC study No. 151GOA94M1, March 1996). Unpublished report prepared by Southwest Bio-Labs for FMC Corporation. Submitted to WHO by FMC Corporation.
Daamen, P.A.M. (1991a) Assessment of acute oral toxicity with Marshal 35 STD in the rat. Unpublished report No. A91-3493 August 13 1991 prepared by RCC NOTOX for FMC Corporation. Submitted to WHO by FMC Corporation.
Daamen, P.A.M. (1991b) Assessment of acute dermal toxicity with Marshal 35 STD in the rat. Unpublished report No. A91-3478 August 19 1991 prepared by RCC NOTOX for FMC Corporation. Submitted to WHO by FMC Corporation.
DeProspo, J.R., Norvell, M.J. & Fletcher, M.J. (1982a) Twenty-four month dietary toxicity/oncogenicity study in mice with FMC 35001. Unpublished report No. A79-334 from FMC Corporate Toxicology Department. Submitted to WHO by FMC Corporation.
DeProspo, J.R., Norvell, M.J. & Fletcher, M.J. (1982b) Twenty-four month dietary toxicity/oncogenicity study in rats with FMC 35001. Unpublished report NoA79-333 from FMC Corporate Toxicology Department. Submitted to WHO by FMC Corporation.
Dudek, R. (1985) Four hour acute dust inhalation toxicity study in rats of Marshal 25 ST (Advantage 25 ST). Unpublished report No. A84-1406 June 3 1985 prepared by Toxigenics Inc. for FMC Corporation. Submitted to WHO by FMC Corporation.
El-Zarkouny, S.A., Ayoub, M.A., Ishak, M.H.G., El-Nouty, F.D., Hassan, G.A., Zahraa R. Abo El-Ezz & Salem, M.H. (1999) Effect of carbosulfan pesticide and selenium on some semen characteristics and serum testosterone in male rabbits. Int. J. Environ. Health Res., 9, 117-124.
Fang, X. & El Naggar, S. (1995) Carbosulfan rat metabolism study. Unpublished report No. 151RAT93M1 September 1995 prepared by FMC Corporation. Submitted to WHO by FMC Corporation.
Franco Perina, V.C. (1998) A micronucleus study in mice for MARSHAL 400 SC. Unpublished report No. A99-4984 September 21 1998 prepared by BioAgri Laboratorios Ltda. for FMC do Brasil Ind. e Com. Ltda. Submitted to WHO by FMC Corporation.
Freeman, C. (1984a) Acute oral toxicity of Marshal 40 DB in rats. Unpublished report No. A84-1216 May 29 1984 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1984b) Skin sensitization of Marshal 40 DB in guinea pigs. Unpublished report No. A84-1217 May 25 1984 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1985a) Acute oral toxicity study in rats with Marshal 25 ST (Advantage 25 ST). Unpublished report No. A84-1439 January 30 1985 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1985b) Acute oral toxicity study in mice with Marshal 25 ST (Advantage 25 ST). Unpublished report No. A84-1438 May 14 1985 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1985c) Acute dermal toxicity study in rabbits with Marshal 25 ST (Advantage 25 ST). Unpublished report No. A84-1440 March 12 1985 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1985d) Primary eye irritation study in rabbits with Marshal 25 ST (Advantage 25 ST). Unpublished report No. A84-1442 February 26 1985 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1985e) Primary skin irritation study in rabbits with Marshal 25 ST (Advantage 25 ST). Unpublished report No. A84-1441 January 30 1985 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1985f) Skin sensitization of Marshal 25 ST (Advantage 25 ST) in guinea pigs. Unpublished report No. A84-1443 March 12 1985 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1989a) Acute oral toxicity study in rats with Marshal 25STW. Unpublished report No. A88-2800 February 24 1989 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1989b) Acute dermal toxicity study in rats with Marshal 25 STW. Unpublished report No. A88-2801 February 16 1989 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1989c) Skin sensitization in guinea pigs with Marshal 25 STW. Unpublished report No. A88-2804 February 23 1989 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1989d) Primary eye irritation study in rabbits with Marshal 25 STW. Unpublished report No. A88-2802 February 17 1989 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1989e) Primary skin irritation study in rabbits with Marshal 25 STW. Unpublished report No. A88-2803 February 16 1989 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Freeman, C. (1995) Carbosulfan technical subchronic neurotoxicity screen in rats. FMC Report No.: A95-4099. November 14, 1995. Unpublished report prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Gava, M.A. (1988) The Salmonella typhimurium reverse mutation by Marshal 400 SC. Unpublished report No. A99-4983 May 27 1998 prepared by BioAgri Laboratorios Ltda. for FMC do Brasil Ind. e Com. Ltda. Submitted to WHO by FMC Corporation.
Goldenthal, E.I. (1990a) 21-day repeated dose dermal toxicity study in rabbits of FMC 35001 technical. Unpublished report No. A90-2960 February 5 1990 prepared by International Research and Development Corporation for FMC Corporation. Submitted to WHO by FMC Corporation.
Goldenthal, E.I. (1990b) 21-day repeated dose dermal toxicity study in rabbits of FMC 35001 technical. Unpublished report No. A90-3187 October 11 1990 prepared by International Research and Development Corporation for FMC Corporation. Submitted to WHO by FMC Corporation.
Haworth, S., Lawlor, T.E., Smith, J.K., Williams, N.A., Burke, P.J., Simmons, R.T. & Reichard, G.L. (1980) Salmonella/mammalian-microsome plate incorporation mutagenesis assay: FMC 35001. Unpublished report No. A79-396 from E.G. & G. Mason Research Institute. Submitted to WHO by FMC Corporation.
Haworth, S., Lawlor, T.E., Smith, J.K., Williams, N.A., Burke, P.J., Simmons, R.T. Hans, L.J. & Reichard, G.L. (1981) DNA damage/repair suspension assay: FMC 35001. Unpublished report No. A80-397 from E.G. & G. Mason Research Institute. Submitted to WHO by FMC Corporation.
Horath, L. (1982) One-hour acute inhalation study in rats of FMC 35001 4 EC. Unpublished report No. A82-677 April 12 1982, prepared by Toxigenics Inc. for FMC Corporation. Submitted to WHO by FMC Corporation.
Jagannath, D.R. (1979) Mutagenicity evaluation of FMC 35001 in the Rec-assay with Bacillus subtilis. Unpublished report No. A95-4247 July 1979 prepared by Litton Bionetics Inc. for FMC Corporation. Submitted to WHO by FMC Corporation.
Janes, J.M., D.E. & Blair, M. (1980a) Teratology study in rats: FMC 35001 technical. Unpublished report No. ACT 265.33 from International Research and Development Corporation. Submitted to WHO by FMC Corporation.
Janes, J.M., Rodwell, D.E. & Blair, M. (1980b) Teratology study in rabbits: FMC 35001 technical. Unpublished report No. ACT 265.33 from International Research and Development Corporation. Submitted to WHO by FMC Corporation.
Kehoe, D.F. & MacKenzie, K.M. (1982) Three-generation rat reproduction study with two litters in each generation: FMC 35001. Unpublished report No. A80-401 from Hazleton Raltech, Inc. Submitted to WHO by FMC Corporation.
Kirby, P.E., Pizzarello, R.F., Brauninger, R.M., Vega, R.A., Cohen, A., Reichard, G.L., Williams, P.E., Wattam, R.E., Johnson, J.L. & Clarke, J.J. (1981) Evaluation of test article FMC 35001 (MRI# 556) for mutagenic potential employing the L51 78Y TK+/- mutagenesis assay. Unpublished report No. A80-426 from E.G. & G. Mason Research Institute. Submitted to WHO by FMC Corporation.
Kirkhart, B., Jones, D.C.L. & Skinner, W.A. (1979) Micronucleus test on FMC 35001. Unpublished report No. A79-366 from S.R.I. International. Submitted to WHO by FMC Corporation.
Klein, W. (1993a) Acute oral toxicity of Marshal 25 CS in rats. Unpublished report No. A94-4044 May 10 1993 prepared by Osterreichisches Forschungszentrum Seibersdorf Ges. mbH (Austria) for FMC Corporation. Submitted to WHO by FMC Corporation.
Klein, W. (1993b) Acute dermal toxicity of Marshal 25 CS in rats (limit test). Unpublished report No. A94-4041 June 24 1993 prepared by Osterreichisches Forschungszentrum Seibersdorf Ges. mbH (Austria) for FMC Corporation. Submitted to WHO by FMC Corporation.
Klein, W. (1993c) Acute dermal irritation/corrosion study with Marshal 25 CS. Unpublished report No. A94-4042 May 25 1993 prepared by Osterreichisches Forschungszentrum Seibersdorf Ges. mbH (Austria) for FMC Corporation. Submitted to WHO by FMC Corporation.
Klein, W. (1993d) Acute eye irritation/corrosion study with Marshal 25 CS. Unpublished report No. A94-4043 May 25 1993 prepared by Osterreichisches Forschungszentrum Seibersdorf Ges. mbH (Austria) for FMC Corporation. Submitted to WHO by FMC Corporation.
Knapp, J.F. (1996) An acute neurotoxicity study of carbosulfan technical in rats. Unpublished report No. A94-4100 April 29 1996 prepared by WIL Research Laboratories Inc. for FMC Corporation. Submitted to WHO by FMC Corporation.
MARTA (2003) Historical control database of pre-clinical developmental teratology and reproductive toxicity parameters. The Middle Atlantic Reproduction and Teratology Association and the Midwest Teratology Association (http//:www.hcd.org/, accessed 2 June 2004).
Mello dos Santos, A.E. (1998a) Acute oral toxicity test in rats by the chemical product Marshal 400 SC. Unpublished report No. A99-4973 May 25 1998 prepared by BioplanBiotechnologia Planalto Ltda for FMC do Brasil Ind. e Com. Ltda. Submitted to WHO by FMC Corporation.
Mello dos Santos, A.E. (1998b) Acute cutaneous toxicity test in rats by the chemical product Marshal 400 SC. Unpublished report No. A99-4976 May 25 1998 prepared by BioplanBiotechnologia Planalto Ltda for FMC do Brasil Ind. e Com. Ltda. Submitted to WHO by FMC Corporation.
Mello dos Santos, A.E. (1998c) Acute inhalation toxicity test in rats by the chemical product Marshal 400 SC. Unpublished report No. A99-4971 May 27 1998 prepared by BioplanBiotechnologia Planalto Ltda for FMC do Brasil Ind. e Com. Ltda. Submitted to WHO by FMC Corporation.
Mello dos Santos, A.E. (1998d) Dermal sensitization test in guinea pigs by the chemical product Marshal 400 SC. Unpublished report No. A2001-5492 October 22 1998 prepared by BioplanBiotechnologia Planalto Ltda for FMC do Brasil Ind. e Com. Ltda. Submitted to WHO by FMC Corporation.
Mello dos Santos, A.E. (1998e) Primary eye irritation test in rabbits by the chemical product Marshal 400 SC. Unpublished report No. A99-4974 May 19 1998 prepared by BioplanBiotechnologia Planalto Ltda for FMC do Brasil Ind. e Com. Ltda. Submitted to WHO by FMC Corporation.
Mello dos Santos, A.E. (1998f) Primary dermal irritation test in rabbits by the chemical product Marshal 400 SC. Unpublished report No. A99-4975 June 23 1998 prepared by BioplanBiotechnologia Planalto Ltda for FMC do Brasil Ind. e Com. Ltda. Submitted to WHO by FMC Corporation.
Morgan, J.M. (1981) One-hour acute dust inhalation toxicity study in rats of FMC 35001 (Marshal) 40DB. Unpublished report No. A81-592 October 6 1981 prepared by Stillmeadow Inc. for FMC Corporation. Submitted to WHO by FMC Corporation.
Pels Rijcken Jr., W.R. (1991a) Acute eye irritation/corrosion study with Marshal 35 STD in the rabbit. Unpublished report No. A91-3477 August 12 1991 prepared by RCC NOTOX for FMC Corporation. Submitted to WHO by FMC Corporation.
Pels Rijcken Jr., W.R. (1991b) Acute skin irritation/corrosion study with Marshal 35 STD in the rabbit (4-hour semi-occlusive application). Unpublished report No. A91-3476 August 12 1991 prepared by RCC NOTOX for FMC Corporation. Submitted to WHO by FMC Corporation.
Preache, M.M., Shefner, A.M. & Reed, J.M. (1981) Dominant lethal assay of FMC 35001 in mice. Unpublished report No. A80-424 from IIT Research Institute. Submitted to WHO by FMC Corporation.
Putnam, D.L. & Schechtman, L.M. (1981) Activity of T1638 (FMC 35001) in the in vivo cytogenetics assay in rodents. Unpublished report No. A80-425 from Microbiological Associates. Submitted to WHO by FMC Corporation.
Rencüzoğullari, E. & Topaktas, M. (1996) The effects of Marshal and its effective ingredient carbosulfan on SCE, MI and RI in cultured human lymphocytes. Turk. J. Biol., 20, 1-12.
Rencüzoğullari, E. & Topaktas, M. (1998) Sister chromatid exchange in cultured human lymphocytes treated with carbosulfan, ethyl carbamate and ethyl methanesulfonate separately and in mixtures. Turk. J. Biol. 22, 369-387.
Rencüzoğullari, E. & Topaktas, M. (2000) Chromosomal aberrations in cultured human lymphocytes treated with the mixtures of carbosulfan, ethyl carbamate and ethyl methanesulfonate. Cytologia, 65, 83-92.
Seaman, L. (1981) Acute oral toxicity study with FMC 35001Advantage 4 EC. Unpublished report No. A81-627 December 2 1981 prepared by the FMC Toxicology Laboratory for FMC Corporation. Submitted to WHO by FMC Corporation.
Sabol, E. (1981a) Rat acute oral toxicity study with Marshal 40 DB. Unpublished report No. A81-593 July 31 1981 prepared by Stillmeadow Inc. for FMC Corporation. Submitted to WHO by FMC Corporation.
Sabol, E. (1981b) Rabbit acute dermal toxicity study with Marshal 40 DB. Unpublished report No. A81-594 July 31 1981 prepared by Stillmeadow Inc. for FMC Corporation. Submitted to WHO by FMC Corporation.
Signorin, J. (1993) Acute inhalation toxicity study in rats with Marshal 25 STD. Unpublished report No. A93-3825 November 2 1993 prepared by the FMC Toxicology Laboratory for FMC Corporation.
Stehrer-Schmid, P. & Wolf, H.U. (1995) Genotoxic evaluation of three heterocyclic N-methylcarbamate pesticides using the mouse bone marrow micronucleus assay and the Saccharomyces cerevisiae strains D7 and D61.M. Mutat. Res., 345, 111-125.
Topaktas, M. & Rencüzoğ
ullari, E. (1993) Chromosome aberrations in cultured human lymphocytes treated with Marshal and its effective ingredient carbosulfan. Mutat. Res., 319, 103-111.Topaktas, M., Rencüzoğullari, E. & İla, H.B. (1996) In vivo chromosomal aberrations in bone marrow cells of rats treated with Marshal. Mutat. Res., 371, 259-264.
Umetsu, N. & Fukuto, T.R. (1982) Alteration of carbosulfan [2,3-dihydro-2,3-dimethyl-7-benzofuranyl (di-n-butylaminosulfenyl) methylcarbamate] in the rat stomach. J. Agric. Food Chem., 30, 555-557.
Whitman, F.T. (1990a) Five-day inhalation toxicity in rats of carbosulfan technical stabilized in epoxidized soybean oil. (laboratory project ID 248017). Unpublished report No. A89-2961-01 February 6 1990 prepared by Exxon Biomedical Sciences Inc. for FMC Corporation. Submitted to WHO by FMC Corporation.
Whitman, F.T. (1990b) Subacute (3-week) inhalation toxicity of carbosulfan technical stabilized in epoxidized soybean oil (laboratory project ID 248018). Unpublished report No. A89-2961-01 February 6 1990 prepared by Exxon Biomedical Sciences Inc. for FMC Corporation. Submitted to WHO by FMC Corporation.
Wiedenmann, D., Stehrer-Schmid, P. & Wolf, H.U. (1990) Mutagenic effects of carbosulfan and furathiocarbin the Ames test and yeast assay. Naunyn-Schmiedebergs Archives of Pharmacology 341, Abstract 113, R 29.
See Also:
Toxicological Abbreviations
Carbosulfan (Pesticide residues in food: 1984 evaluations)
Carbosulfan (Pesticide residues in food: 1984 evaluations)
Carbosulfan (Pesticide residues in food: 1986 evaluations Part II Toxicology)