
CHLORDIMEFORM JMPR 1978
Explanation
Chlordimeform was evaluated in 1971 and 1975 (FAO/WHO 1972b,
1976b) and a temporary ADI was established. Temporary MRLs were
recommended for a number of foods and five requirements for further
information were listed.
In 1976 the manufacturer temporarily suspended, the sale of the
pesticide. This action was noted by the 1976 and 1977 Joint Meetings
(FAO/WHO 1977a, 1978a).
Having completed a number of long-term toxicological, metabolism
and residue studies, in 1978 the manufacturers approached authorities
in a number of countries to allow limited commercial use in cotton
crops. The results of these studies were evaluated by the meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Effects on Enzymes and other biochemical parameters
Chlordimeform and its major metabolites were tested for their
ability to affect DNA, RNA and protein synthesis. In vitro studies
using HeLa cells exposed to a variety of concentrations of
chlordimeform and its major metabolites showed that the synthesis of
macromolecules were virtually unaffected except at extremely high
concentrations at which RNA synthesis was reduced (Murakami and
Fukami, 1974).
Several studies have been reported to define the mode of action
of chlordimeform. Chlordimeform has shown a complex mechanism of
action eliciting a variety of unusual biochemical and pharmacological
responses. Chlordimeform has shown little activity on cholinergic
transmission although in frog muscle preparations a decrease in
acetylcholine reception sensitivity has been observed. Chlordimeform
has been shown to be an effective uncoupler of oxidative
phosphorylation and inhibitor of electron transport. Physiologically,
chlordimeform acting as a direct depressant on cardiac and vascular
muscle, induced a hypotensive state in dogs. Chlordimeform did not
interfere with the autonomic nervous system. The mechanism of
cardiovascular depression may be related to that noted with frog nerve
preparations treated with procaine, a local anesthetic.
4-chloro-o-toluidine has been shown to interfere with rat cardiac
receptors (Lund et., al., 1978; Chinn et al., 1976; Wang et al., 1975;
Watanabe et al., 1975; Matsumura and Beeman, 1976; Knowles, 1976;
Hollingworth, 1976).
It has been suggested that the increase in biogenetic amines
resulting from inhibition of monoamine oxidase (MAO) activity by
chlordimeform and its metabolites may be a factor in expressing the
complex mode of action. Matsumura and Beeman (1976) have shown that
rats treated with chlordimeform accumulated seratonin and
norepinephrin in the brain while Benezet and Knowles (1978) showed
dopamine to also accumulate. While, as had been suggested in the past
that MAO inhibition is not the primary mechanism in chlordimeform
insecticidal activity, inhibition of this process in concert with
several other biological events has provided some data that may
resolve the toxic mode of action in mammals. In further studies
towards this goal, Knowles and his coworkers have continued to examine
the metabolic fate of chlordimeform. They have also investigated the
physiological significance and toxic action of the metabolic product.
These workers have proposed that sequential oxidative and
N-demethylation of chlordimeform results in the formation of two
formamidine derivatives, the N-mono-desmethyl and the N-didesmethyl
formamidines. These two metabolites appear to be more acutely toxic
and more biologically active than chlordimeform. The onset of acute
signs of poisoning was shown to be faster with these metabolites than
with chlordimeform itself. Chlordimeform and the two formamidine
metabolites have been shown to increase sleeping time in mice.
Chlordimeform may indirectly influence chemically induced sleep or it
may be an active sleep inducer especially through the action of some
of its metabolites. Based on preliminary acute toxicity data, the two
formamidine chlordimeform metabolites appear to be rapidly formed and
may serve to contribute directly to the overall toxic action in rats.
In contrast, two additional metabolites, the N-formyl-4-
chloro-o-toluidine and the 4-chloro-o-toluidine were not as
acutely toxic as chlordimeform but still appear to have contributed to
the complex mode of action. The latter toluidine metabolites have been
shown in vivo to induce a sedative and an anesthetic effect in
addition to their biochemical ability to inhibit MAO activity. To
define the multiple stress on animals treated with chlordimeform,
Knowles and coworkers reported the acute poisoning response in rats of
all of these metabolites following a variety of administrative routes.
The signs of poisoning following acute Intoxication with chlordimeform
and its two major formamidine metabolites included a marked
hyperexcitability which increased with time and was accompanied by a
high degree of sensitivity to external stimuli. Stimulation caused
rapid running and elicited an intense escape behavior in rats. At the
latter stages of poisoning, prostration and hyperextension of the hind
legs occurred and death followed shortly. Signs of poisoning were
observed to be the most rapid with the didesmethyl (the NH2
derivative) formamidine, Hyperexcitability was evident almost
immediately after poisoning and within five to six minutes convulsions
were observed. In sharp contrast, rats treated with N-formyl and the
4-chloro-o-toluidine metabolises responded rapidly as though they
had been sedated. This condition was soon followed by one which
resembled an anesthetic effect (Benezet et al., 1978).
As mentioned above, the increase in biogenic amines resulting
from inhibition of MAO activity undoubtedly has some physiological
significance in defining the overall toxic response. Previous studies
have shown that inhibition of rat MAO activity was relatively rapid
and reversible following chlordimeform intoxication. In contrast to
classical MAO inhibitory chlordimeform exhibited a readily reversible
Inhibition (Maitre et al., 1978). Recovery was complete within two
days in liver and four days in brain following an acute
intraperitoneal dose of 100 mg/kg. In evaluating the sensitivity of
the two types of MAO activity to inhibition with chlordimeform, the
type B seemed to be more sensitive. Subacute, intraperitoneal
administration of 10 mg/chlordimeform/kg body weight for four days did
not appear to result in brain M0 inhibition and only marginally
reduced liver MAO activity. This suggested the low cumulative
potential for MAO inhibition at low or threshold doses. In contrast,
Kaloyanova et al., (1978) observed substantial inhibition when rats
were administered 5 mg/kg daily for 40 days. This inhibition may have
resulted from an observed cumulative effect of prolonged
administration and delayed recovery of MAO activity over the 40 day
treatment interval. Thus, evidently the complex mechanism of action
has not been fully elucidated. Direct diverse actions of chlordimeform
and its metabolites and the indirect action of many biologically
active amines all interacting simultaneously appear to preclude a
rapid resolution of this complicated event.
TOXICOLOGICAL STUDIES
Mutagenicity
In a series of bioassays, chlordimeform and its principle
metabolites were examined for mutagenic effects on
histidine-auxotrophic mutants of Salmonella either alone or after
biological activation. Chlordimeform tested in a series of five tester
strains (TA-98, TA-100, TA-1535, TA-1537 and TA-1538), was found to be
negative at concentrations up to and including 20 mg/ml. The desmethyl
metabolite of chlordimeform was slightly mutagenic when assayed
against TA-1535. The Formyl-metabolite was negative when tested
against the five tester strains although in one instance a weak
response was elicited with TA-100. In contrast, the 4-chloro-o-
toluidine metabolite was found to elicit a positive mutagenic response
at high concentrations with the tester strains TA-100 and TA-1535
(Arni and Muller, 1976a, b and c; Konopka and Haymann, 1977a and b;
Rashid, 1974). Chlordimeform did not elicit a mutagenic response when
examined utilizing a streptomycin dependent E. coli-SD 4 tester
strain (Hurni and Ohder 1970), A potential metabolite of chlordimeform
formed in soil and in the presence of sunlight from the
4-chloro-o-toluidine (4,4-dichloro-2,2-dimethylazobenzene) was found
to be inactive when examined against two tester strains of Salmonella,
TA-1535 and TA-1538 (Rashid, 1974).
Mouse - Dominant Lethal
Chlordimeform was administered to groups of 20 male mice at
dosage levels of 0, 22 and 66 mg/kg/body weight. The males were mated
with two untreated females weekly for six consecutive weeks in a
standard dominant lethal assay. No evidence of dominant lethal effects
was observed in the progeny of the male mice treated with
chlordimeform (Fritz, 1978).
Chlordimeform was bioassayed as a potential carcinogen using the
C3H7OT 1/2 CLB mouse embryo fibroblast oncogenic transformation assay
Nesnow and Heidelberger, 1976) and found to be negative over a range
of 1 to 100 µg/ml. This assay has been shown to have a low rate for
false positive responses suggesting that a positive result is highly
likely to be an animal carcinogen (Nesnow, 1978).
Groups of Chinese hamsters (3 males and 3 females per group) were
administered chlordimeform by gavage on two consecutive days at dose
levels of 0, 60, 120 and 240 mg/kg. A positive control using
cyclophosphamide (128 mg/kg) and a negative control of
carboxymethylcellulose was used in an in vivo, mutagenesis assay
evaluating bone marrow cells. In all dosage groups (sacrificed 24
hours after the second dose) the percentage of cells displaying
changes in nuclei did not differ from the negative controls. The
positive controls showed a marked increase in the percentage of cells
with anomalies of the nuclei (Langeuer and Muller, 1977).
Teratogenicity
Groups of rabbits (group size ranged from 17 to 38 dams per
group) were administered chlordimeform orally from day 6 to day 18 of
pregnancy at dose levels of 0, 10, 30 and 100 mg/kg/body weight. The
administration of chlordimeform at 100 mg/kg/body weight produced a
distinct adverse effect on dams in the first four days of treatment
within four hours of administration of chlorphenamide. Examination of
dams and fetuses (fetuses were removed by caesarian section on day 28
of pregnancy) suggested that the low dose had no teratogenic or embryo
toxic effect. In the high dose group, the number of incompletely
ossified sternebrae showed a slight increase over that observed in the
controls and in the other groups. In addition, the number of live
fetuses with malformations was slightly increased. These malformations
included a median cleft palate and exencephaly and an omphalocele.
Further examination of spontaneous malformations observed in a
cumulative control of 2495 rabbit fetuses suggested that these
abnormalities may be spontaneous and not as a consequence of the
administration of chlordimeform (Fritz, 1971).
Groups of rats (25 females/group, 30 females were used as
controls) were administered chlordimeform in carboxymethylcellulose at
dose levels of up to 0, 10, 25 and 50 mg/kg/day from six to day
fifteen of pregnancy. Examination of fetuses removed by caesarian
section on day 21 showed that a slight delay in growth of the fetuses
had occurred at the two upper treatment levels. This effect was
probably a direct result of a slightly toxic response in the dams as
evidenced by reduced body weight gain and reduced food intake and
somnolence noted on days 6, 7 and 8 of pregnancy. No teratogenic
events were observed in the offspring although an increased incidence
of eternal ossification defects occurred at 25 mg/kg (Fritz, 1975).
Short Term Studies
Monoamineoxidase (MAO) activity of liver, brain and serum was
examined from rats treated with chlordimeform at dosages of 0, 5, 10
and 50 mg/kg/day for 40 consecutive days. The MAO activity was
determined with a variety of substrates. A dose-dependent inhibitory
promotion was observed using several of the substrates representing
different forms (isoenzymes) of MAO activity. The liver, brain and
serum MAO activity was significantly inhibited at 5 mg/kg/day, the
lowest dose level tested. A distinct, dose-response relationship was
observed in both liver, brain and serum with two of the substrates
used in the assay (Kaloyanova et al., 1978).
Mice - 4-chloro-o-toluidine
Groups of mice (30 males and 30 females/group, TIF.: NMRI strain)
were bred and maintained under SPF conditions and fed a diet
containing 4-chloro-o-toluidine at concentrations of 0, 750, 1500,
3000 and 6000 ppm for 60 days. Mortality 50% was observed in the 6000
ppm group. Food intake and growth were retarded at the two highest
dose levels. No abnormal clinical signs of poisoning were observed.
Eye examinations did not indicate adverse occular changes. A toxic
Hemolytic anemia in both sexes of all treated groups was characterized
by reticulocytosis and heinz body formation. In the male mice of all
treated groups, hemoglobin concentration, packed cell volume and
erythrocyte count was slightly below that of controls. In addition,
Leukocytosis was observed in all animals of all dosage groups with the
exception of female at the 750 ppm level. In both sexes at 6000 ppm
and in the females at 3000 ppm total protein concentration was
reduced, blood glucose and urea nitrogen values were increased. Plasma
GPT was increased in male mice at 3000 ppm and in female mice at 1500
ppm. Urinalysis revealed a reduced specific gravity in both sexes at
3000 ppm and above and in females at 1500 ppm. Microscopic examination
of tissues and organs at the conclusion of the studies showed slight
to moderate vacuolar changes of Hepatocytes, which was pronounced in
animals at the 3000 ppm levels and above. There was also a marked
congestion of the spleen at these high dose levels. In addition, the
urinary bladder revealed hyperemia and dilation of the capillaries in
the mucosal layer. These changes were accompanied by edema, multiple
intraepithelial hemorrhage and focal proliferation of the transitional
cell epithelium. On occasion, these changes in the urinary bladder
were noted at the lowest concentration (Suter et al., 1976b).
Rat - 4-chloro-o-toluidine
Groups of rats (30 males and 30 females per group Tif/Rai,
strain) were maintained under SPF-barrier conditions and fed
4-chloro-o-toluidine in the diet at concentrations of 0, 750, 1500,
3000 and 6000 ppm for 60 days. There was no mortality over the course
of the study and clinical signs of poisoning were not observed.
Ophthamological examinations did not suggest changes related to the
presence of 4-chloro-o-toluidine in the diet. Growth was reduced in
the upper three dosed groups at dietary levels of 1500 ppm and above.
Toxic hemolytic anemia in both sexes of all treated groups was
characterized by a variety of hematological parameters including:
reduced hemoglobin content, reduced hematocrit content, reduced blood
cell count, increased methemoglobin content, heinz body formation,
reticulocytosis and polychromatophilia. In the highest dose group, an
increased number of immature red blood cells (normoblasts) were
observed. An increased leukocyte count and prothrombin time was
recorded at the 3000 and 6000 ppm level. Total protein was slightly
reduced at 3000 and 6000 ppm and there was a shift in the globulin
content as observed by electrophoresis. Plasma lambda-glutamyl
transpeptidase of males and alkaline phosphatase of females was
increased at 6000 ppm. Urinalysis was not significantly affected. In
all treated animals, the liver showed an increase in size accompanied
by hypertrophy of the hepatocytes. In the two highest dose groups, the
spleen was enlarged and microscopic examination showed pronounced
congestion and hemorrhage. In the highest dose group, slight or
moderate proliferation of the transitional cell epithelium was noted
in the urinary bladder (Suter et al., 1976b).
Long Term Studies
Mice - Chlordimeform Hydrochloride
Groups of mice (50 males and 50 females per group, Tif: MAG
strain, SPF derived) were fed chlordimeform in the diet at
concentrations of 0, 20, 100 and 500 ppm for 24 months. At the
conclusion of the dietary feeding interval animals were maintained on
control diet until 90% of a group had died, at which time the
remaining animals of the group were sacrificed. There were no acute
signs of toxicity related to chlordimeform in the diet over the course
of the feeding trial. Growth and food consumption were similarly
unaffected by the presence of chlordimeform in the diet. Mortality was
significantly increased in females after 60, and 90 weeks at 500 and
100 ppm respectively. In males, significantly increased mortality was
observed after 70 and 110 weeks at 500 and 100 ppm. However, lifespan
was not significantly affected in males at 100 ppm. The animals fed
dietary levels of 100 ppm and above displayed an increased incidence
of hemorrhagic tissue masses in subcutaneous tissues, retroperitoneum
and some internal organs (kidney, liver and spleen) which upon
examination were classified as malignant haemangioenditheliomas. These
malignancies which were reported to occur rarely in control
populations mere found predominantly in the 100 and 500 ppm dietary
groups. In some animals the tumors were of multiple origin and
metastases were observed in the lungs. There were no other types of
neoplasm observed in the study which were attributable to
chlordimeform in the diet. Under the conditions of this study, 20 ppm
in the diet appears to be a no effect level (Suter et al., 1978).
Mice - N-formyl-4-chloro-o-toluidine
Groups of mice (50 male and 50 female/group, Tif: MAG strain)
were bred and maintained under standard SPF conditions and fed
N-formyl-chloro-o-toluidine in the diet at concentrations of 0, 20,
100 and 500 ppm for 24 months. Alter 24 months all animals were fed a
control diet until the study was concluded when 90% of a group of
animals had died.
There was no sign of adverse behavior and acute mortality was not
noted. Growth and food consumption were unaffected. There were
significant differences noted in survival after one year of age. Both
males and females showed an increased mortality after at 100 and 500
ppm approximately one year of feeding. The onset of increased
mortality occurred earlier in females. The females at the 20 ppm
dietary level showed a slightly higher, non-significant mortality
during the same period. Detailed gross and microscopic examination of
a variety of tissues and organs showed the presence of numerous gross
anatomical lesions. There was an increased number of hemorrhagic
masses in the subcutaneous tissues in the retroperitoneum and in some
internal organs of mice at all treatment levels. Detailed microscopic
examination confirmed that the increased incidence of hemorrhagic
masses were malignant tumors of vascular origin. These tumors were
histologically classified as malignant haemangioenditheliomas. In
addition to the occurrence of tumors, the time to tumor relationship
was decreased as the dietary concentration was increased. Apart from a
significantly higher incidence and earlier appearance of malignant
vascular tumors noted at all dose levels, other neoplasms occurring in
the study were not influenced by the dietary concentration; a
non-effect level was not demonstrated under the conditions of this
experiment (Sachsse at al., 1978a).
Mice - 4-chloro-o-toluidine
Groups of mice (30 males and 30 females per group, ICR-strain)
were fed dietary concentrations of 4-chloro-o-toluidine for 80 weeks
at dietary levels of 0, 20, 100 and 500 ppm. At the conclusion of the
study, gross and microscopic examination of tissues and organs was
performed. Data were not presented with respect to general
observation, behavior changes, food consumption and growth. There
appeared to be no significant changes with respect to organ and tissue
weight in animals sacrificed at 80 weeks. The results of the
histological examination of tissues and organs suggest that there was
a dose-related increase in the number of tumors observed when data
from both sexes are combined. These have been characterized as
reticulum cell sarcoma. In addition, there was a dose-related increase
in the overall tumor incidence at all treatment levels. The study
showed a high incidence of spleenic effects and lung problems,
indicative of probable infection in the colony. A no-effect level has
not been demonstrated in the study based upon the dose-related
increase in tumor incidence at all dietary levels tested.
Unfortunately, a complete analysis of the study was precluded in part
due to the overall tumor incidence in control animals. The control
tumor incidence ranged from 56 to 73% of the animals at eighty weeks.
The data upon which statistical analyses were based utilized a
modified life table (acturial) method. On the basin of the actual
incidence of tumors, the dose/effect relationship is still apparent
when use is made of total tumor incidence data (Ezumi and Nakao,
1974b).
Mice - 4-chloro-o-toluidine
Groups of mice (50 males and 50 females/group, Tif: MAG strain)
were derived and maintained under standard SPF-barrier conditions and
fed a diet containing 4-chloro-o-toluidine at concentrations of 0,
2, 20, 100 and 500 ppm for 24 months. After 24 months all animals were
fed control diets until the study was concluded when 90% of a group of
animals had died. There were no overt toxicological signs of
poisoning. Growth and food consumption were unaffected. An adverse
effect on longevity (lifespan) was noted in both males and females at
the two highest dietary levels. At the conclusion of the study upon
gross examination there was a marked increase number of hemorrhagic
masses in subcutaneous tissue, in the retroperitoneum and in some
internal organs. Microscopic examination revealed an increased
incidence of hemorrhagic malignant tumors of vascular origin at dosage
levels of 20 ppm and above. The incidence in control exceeded the
incidence observed at 2 ppm. The tumors were histologically classified
as malignant haemangioenditheliomas and on occasion metastases were
observed. There was not only a significant dose-dependent increase in
the total incidence of malignant tumors but, the tumors occurred in
animals at the higher concentrations at a markedly earlier date than
those at the lower concentrations. (The time-to-tumor calculations
were dose related). A benign variant of the haemangioma was observed
in all groups. The occurrence of other types of neoplasms in the study
was apparently not influenced by the presence of
4-chloro-o-toluidine in the diet. Under the conditions of this
experiment, 2 ppm in the diet appears to be a non-effect level
(Sachsse et al., 1978b).
Rat - 4-chloro-o-toluidine
Groups of rats (30 males and 30 females/group, Sprague-Dawley
strain) were fed a diet containing 4-chloro-o-toluidine at
concentrations of 0, 20, 100 and 500 ppm for periods of 94 weeks for
the males and 104 weeks for the females. There was a slight increase
in mortality over the course of the study that appeared to be dose
related when calculations of combined mortality data with both sexes
were made. There was no indication of a reduced lifespan as a
consequence of 4-chloro-o-toluidine in the diet. At the conclusion
of the study there seemed to be an increased liver weight observed at
500 ppm. A dose-related decrease in ovarian weight was also observed
at all dose levels. There was a dose-related increase in tumors in the
study. In the animals sacrificed at the conclusion of the study, liver
nodules, described as tumor-like, were observed at 100 and 500 ppm but
not in the controls and in the 20 ppm group. Upon microscopic
examination these nodules were found to be non-malignant. In those
animals that died during the course of the study there were a
significant number of tumor-like nodules and swellings in the liver
which were classified as benign or malignant hepatomas upon
histological examination. On an overall evaluation of the study, it
was concluded that liver tumors were found in all dose groups but not
in controls. The total incidence of liver tumor was related to dietary
dosage and found to be higher in females than in males. However,
incidence of malignant tumors while increasing with dose was not sex
related. Additionally, adenoma of the adrenal gland was found in the
treated groups. A "no-effect level" was not seen in this study (Ezumi
and Nakao, 1974a). As in the previous mouse study, the data
calculations are based on the modified life table (actuarial) method.
Two studies, each using both rats and mice in the bioassay, have
been undertaken under the U.S. National Cancer Institute
carcinogenicity program to evaluate the carcinogenic potential of
4-chloro-o-toluidine. Full reports of these studies are not available.
Mice
In an initial study reported only in summary, groups of 25 male
mice were fed 4-chloro-o-toluidine in the diet for 2 years at levels
of 0, 750 and 1500 ppm. Similar groups of female mice were fed dietary
concentrations of 0, 2000 and 4000 ppm. Further details on the
parameters used in the study are unavailable. There appear to be a
significant number of vascular tumors observed in both male and female
mice. There were no differences reported between the treated and
control animals with regard to other tumors.
In a more well-defined program, reported as a draft report,
groups of mice (50 males and 50 females per group, B6C3F1 strain) were
fed dietary concentrations of 4-chloro-o-toluidine in a lifetime (92
to 99 weeks) carcinogenic bioassay. The females were fed dietary
levels of 0, 3750 and 15,000 ppm while the males were fed dietary
levels of 0, 1250 and 5000 ppm. Body weight of treated animals was
reduced. Mortality was not affected. A significant number of tumors
designated as hemangiosarcoma of the epididimus, testes, urinary
bladder, seminal vesicle and prostate were recorded in the high dosed
male mice receiving 5000 ppm in the diet. A similar neoplastic
condition existed in the reproductive organs of both high and
low-dosed groups of female mice.
Rat
Groups of 25 male rats/group were fed dietary levels of 0, 2000
and 4000 per four months at which time the dietary level was decreased
to 500 and 1000 ppm for the remainder of the study. The rat study
showed no significant differences between the treated and control
animals.
Groups of rat (50 males and 50 females per group, 20 males and 20
females were used in controls, F344 strain) were fed dietary
concentration of 4-chloro-o-toluidine at levels of 0, 1250 and 5000
ppm. A significant number of rats survived the two-year feeding trial
although growth was retarded at the high dose. Gross and microscopic
pathological examinations of the animals revealed no tumours
associated with the presence of 4-chloro-o-toluidine in the diet. It
was concluded that 4-chloro-o-toluidine hydrochloride was not
carcinogenic for F344 rats (Anonymous, 1978b).
Rat - 2 Year dietary-lifetime studies - Interim reports
A series of three one-year interim reports entitled "Lifetime
Feeding Study in Rats" indicate that studies are being performed with
chlordimeform, N-formyl-4-chloro-o-toluidine and with
4-chloro-o-toluidine. Details of the studies are not defined. The
reports, designated "interim reports", contain some brief information
on hematology, blood chemistry, urinalysis urine electrophoresis and
growth and food consumption data for a one year period (Anonymous,
1978a, b and c).
For chlordimeform, the data show a slight growth retardation at
the highest dose level accompanied by a toxic hemalytic anemia.
Methaemoglobin was increased at 4 weeks in a dose related response
from 20 ppm. However, no effects were noted at 13, or 26 weeks, and
minor changes were observed only at 500 ppm after 52 weeks. Total
serum protein was reduced at 500 ppm. Heinz bodies, generally
associated with methemoglobinemia were not observed in male rats but
were observed in females. The dose levels used in these studies are 0,
2, 20, 100 and 500 ppm in the diet with groups of 108 rats/sex/dose
being used. There may have been an interim sacrificed but data are not
reported.
The data for the N-Formyl-metabolite appear to be similar to that
described for chlordimeform. At the highest dose level, hemaglobin
concentration was significantly reduced in both males and females. In
addition, packed cell volume and red blood cell count were decreased
and there was a significant reticulocytosis. There was an increased
methemoglobin formation but heinz bodies were not observed. Blood
chemistry, urine analysis and urine elcotrophoresis were not unusual.
As with the previous study, growth was slightly retarded at the 500
ppm dose level although food consumption did not appear to be
affected.
Preliminary data presented for the dietary 4-chloro-o-toluidine
study show a reduction in weight gain in both sexes at the highest
dietary concentration. This was again associated with a toxic
hemolytic anemia. A marginal decrease was observed in the hemoglobin
concentration in males and females at the highest dose level.
Methemoglobin concentration at the end of one year was increased in
females at the top three dose levels while with the males it was
increased only at the top dose level. Again, heinz bodies were not
noted in male rats but were noted in females. Against blood chemistry,
urine analysis and urine electrophoresis was not affected.
In all studies, there was a suggestion that an interim sacrifice
was made. A statement was made that neither toxic symptoms nor gross
pathology changes were found at this interim sacrifice time. The
reports of these three studies are incomplete and the data could not
be evaluated at the present time. The studies should be considered
when full reports are available (Anonymous, 1978a, b and c).
COMMENTS
Chlordimeform was reviewed and evaluated for acceptable daily
intake in 1971 and 1975 and a temporary ADI was allocated. The
metabolic pathway in animals has been well-defined but no information
is available on either the metabolic fate in man or the primary mode
of toxic action.
In studies performed subsequent to the initial reviews, adverse
effects of long-term exposure were observed. Results of a preliminary
report suggested a carcinogenic response in mice with
4-chloro-o-toluidine, a major metabolite of chlordimeform. At that
time voluntary withdrawal of all agricultural uses of chlordimeform
was instituted by the major manufacturers to allow time to complete
ongoing studies which would permit a complete evaluation of the
hazards associated with continued agricultural use.
An extensive series of short-term, high-level and long-term,
low-level studies in both rats and mice, were reviewed and considered
by the Meeting. Results of long-term studies demonstrate that
chlordimeform and its principal metabolites, the N-formyl
chlordimeform and 4-chloro-o-toluidine, are carcinogenic in the
mouse, producing a dose-related malignancy histologically
characterized as a haemangioendothelioma. In carcinogenicity studies
with rats, results have been conflicting. Chlorimeform was not
mutagenic to bacteria but its main metabolite 4-chloro-o-toluidine was
mutagenic in the same bioassay systems. Because of these concerns, the
temporary ADI was reduced.
The Meeting was informed that there were currently uses of
chlordimeform on cotton from which food residues have not been
detected at the limit of the existing method of analysis. In
consideration of this and the new data expected within one year, the
Meeting recommended chlordimeform be reviewed in 1979.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 100 ppm in the diet equivalent to 5 mg/kg bw
Dog: 250 ppm in the diet equivalent to 6.25 mg/kg bw
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.0001 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
The manufacturers were currently limiting their proposals to
aerial applications to cotton crops under supervised conditions which
limit the uptake by operators, bystanders and consumers in Guatemala,
Colombia, Nicaragua, Salvador and USA. Further such applications to
cotton in Australia, Egypt, Mexico and Sudan were under consideration.
Use pattern on cotton
In 1972, chlordimeform was recommended in cotton at rates of
0.5 - 1.0 kg/ha. Further research revealed that useful control could
be achieved at rates of 0.125 to 0.25 kg/ha by killing eggs and early
instar larvae and in 1973 these rates were recommended. Emulsifiable
solutions of chlordimeform base, or solutions of the hydrochloride in
Australia, USA and several other countries, are need on a 3 - 5 day
schedule when moth flights begin or when eggs appear. In some other
countries including Colombia and Guatamala it has been found necessary
to use 1 kg/ha when mites are to be controlled. The compound is active
again a narrower spectrum of insects than the organo-chlorine
compounds and other compounds used on cotton and is being used
successfully in integrated pest management programmes in the USA and
other countries. Observations have shown that it does little harm to
many beneficial insects and its removal in 1976 created a serious
situation because acceptable alternatives were not available.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Cotton
Reports on supervised trials carried out between 1969 and 1977
(Ciba-Giegy/Schering, 1978) and showing residues in cottonseed and
cotton products processed from seed cotton grown under a range of
treatment regimes were considered.
The analytical methods used were based on the determination of
4-chloro-o-toluidine which is a moiety common to chlordimeform and
its intermediate metabolites as-well as being the principal metabolite
in plants and animals (Morton, 1968; Nor-Am, 1969; Ciba-Geigy, 1976;
Schering, 1973). The limits of determination were in the range 0.01 -
0.05 mg/kg of chlordimeform equivalents with recoveries usually above
80% (69-106%). A gas-chromatographic, method which allows separate
determination of chlordimeform and its metabolites and has a limit of
determination of 0.01 mg/kg and recoveries in the range of 74 - 106%
was also used (Schering, 1975).
Most determinations were concerned with residues in intact
cottonseeds. The application rate ranged from 125 g to 3.6 kg/ha. From
1 to 20 applications were made and the interval between the last
application and harvest ranged from 0 to 89 days. Table 1 summarizes
the results from the many trials reported Ciba-Geigy/Schering, 1978).
There was no direct relationship between the residue levels and
the experimental conditions such as application rate, number of
applications, length of waiting period etc. However, the results
suggest that the application rate had the strongest influence on the
residue level, followed by the interval between last application and
harvest. Tables 2 and 3 summarize the results of typical trials
carried out in the USA.
As shown in Table 3, the decrease of residues with time is most
pronounced during the first 10 days after the last treatment of the
cotton plants. The number of applications in a season appears to have
little influence on the residue present at harvest. When comparing the
dates of application with the residue levels in seed, it was thought
that the later the last application was made in the season, the higher
the residues. To test this hypothesis a study was conducted in South
Carolina in 1977 (Ciba-Geigy, 1978b) in which the interval between
applications and the pre-harvest interval were varied. The date of the
last application was compared with the proportion of open bolls. With
only one exception, the 0.25 kg/ha rate resulted in residues
proportionally higher than the 0.125 kg/ha rate. In addition, the data
showed that the timing of the last application (number of bolls open)
influences the level of residues more than the total number of
applications or the interval between applications.
Though most of the data are from trials using the emulsifiable
concentrate base, in some studies the emulsifiable concentrate and the
soluble powder (hydrochloride) formulations were compared. The results
were essentially the same (see Table 3). Trials in which the fate of
residues were traced through all stages of cottonseed processing
showed that this similarity remains at each stage (Table 4).
TABLE 1. Effect of application rate on chlordimeform residues in
cottonseed.
Application rate, No. of Residue, mg/kg
kg/ha trials
Range Mean
0.125 32 <0.05 - 0.49 0.1
0.25 2 <0.05 <0.05
0.45 2 2.0 - 2.8 2.4
0.5 9 <0.05 - 3.0 1.7
0.6 5 <0.05 - 3.0 1.5
0.75 1 1.3 1.3
1.0 23 <0.05 - 5.7 2.3
1.5 2 0.25 - 3.7 2.0
2.0 6 0.05 - 5.6 1.9
2.25 1 12.0 12.0
3.6 1 13.1 13.1
NOTE: The high figures in the range represent residues in cottonseed
harvested immediately after application.
TABLE 2. Chlordimeform residues in cottonseed, USA.
Application
State Year No. Rate, Formulation 20 30 40 50 60 70 > 70
kg/ a.i./ha
Ala. 1970 8 0.5 EC 0.46
Miss. 1970 9 0.5 EC 0.95
Ark. 1969 9 0.5 EC < 0.05
Tex. 1970 9 0.5 EC 0.22
Cal. 1969 10 0.5 SP 2.7
Cal. 1969 10 0.5 EC 2.3
Geo. 1970 14 0.5 EC 3.0
Cal. 1970 7 0.75 SP 2.6
S. Car. 1969 5 1.0 EC <0.05
Okla. 1970 6 1.0 EC < 0.05
Tex. 1969 1.0 EC 0.22
Cal. 1968 10 1.0 SP 3.3
Miss. 1969 11 1.0 EC 0.81
Tex. 1970 7 1.5 EC 0.25
Tex. 1970 9 1.5 EC 3.7
Tex. 1969 7 2.0 EC 0.05
Miss. 1970 9 2.0 EC 5.6
Miss. 1969 11 2.0 EC 0.62
Tex. 1970 11 2.0 EC 1.5
Tex. 1970 9 2.25 EC 12.0
(3 days)
EC = Emulsion concentrate
SP = Soluble powder
TABLE 3. Effect of pre-harvest interval on level of chlordimeform residues in cottonseeds
Application
State Year No. Rate, kg Formulation 3 7 10 14 21
a.i./ha
Ariz. 1970 6 1.0 EC 1.8 1.6 2.3 1.9
Ariz. 1970 6 1.0 SP 2.0 1.8 2.4 1.6
Florida 1970 14 1.0 EC 3.7 3.0 2.0 3.0
Florida 1970 14 1.0 SP 5.7 5.1 2.3 2.9
Georgia 1975 20 0.5 EC 2.0 1.0 1.0 0.81
Miss. 1975 20 0.5 EC 3.0 1.6 1.3 0.92
EC = Emulsion concentrate
SP = Soluble powder
TABLE 4. Effect of Processing on chlordimeform residues in cottonseed and cottonseed products.
333/ 333/ 333/ 333/ 333/ 333/ 333/ 13/ 13/ 13/ 13/ 13/
Trial no. 107 92 92 105 86 109 08 77 77 77 77 77
Location Texas Calif. Calif. Calif. - Miss. Texas Calif. Calif. Calif. Calif. Miss.
Rate of application
kg/ha 0.5 0.5 0.5 1.0 - 2.0 2.0 1.0 1.0 2.0 2.0 2.0
Formulation EC EC EC SP - EC EC EC SP EC SP EC
No. of applications 9 10 10 10 - 9 11 15 15 15 15 15
Pre-harvest interval
(days after last appl.) 57 84 84 28 - 24 45 21 21 21 21 -
Cottonseed 0.22 2.3 2.7 3.3 - 5.6 1.5 2.9 3.4 - -
Lint cotton 0.37 - - - - 5.2 - - - -
Delinted cottonseed 0.19 - - - - 1.4 - - - -
Linters 0.47 - - - - 2.3 - - - -
Cottonseed hulls 0.20 3.1 3.9 8.0 - 8.5 2.2 3.75 4.0 - -
Solvent-extracted
meal 0.15 1.6 2.5 2.0 - 1.4 0.32 0.15 0.2 - -
Screw press
extracted meal 0.09 0.92 1.1 1.1 - 0.83 0.39 - - - -
Crude
solvent-extracted meal 0.11 - - - 1.2 0.63 0.28 0.41 0.52 0.8 1.1 3.8
TABLE 4. Cont'd.
333/ 333/ 333/ 333/ 333/ 333/ 333/ 13/ 13/ 13/ 13/ 13/
Trial no. 107 92 92 105 86 109 08 77 77 77 77 77
Location Texas Calif. Calif. Calif. - Miss. Texas Calif. Calif. Calif. Calif. Miss.
Refined
solvent-extracted meal 0.09 0.73 0.78 - 1.0 0.21 0.33 0.37 0.38 0.8 0.8 3.3
Crude screw
press-extracted oil 0.17 - - - 4.0 1.7 1.3 - - - - -
Refined screw
press-extracted oil 0.14 1.3 1.7 - 3.6 0.85 0.72 - - - - -
Solvent-extracted
soapstock - 1.2 1.8 - - - - - - - - -
Screw press-extracted
soapstock - 2.8 2.8 - - - - - - - - -
Refined, bleached,
deodorised oil - - - - - - - <0.05 <0.05 0.05 0.06 <0.05
Refined, bleached,
deodorised,
hydrogenated oil - - - - - - - <0.05 <0.05 <0.05 <0.05 <0.05
FATE OF RESIDUES
General
Since the 1971 Meeting FAO/WHO, 1972b there have been a number of
detailed studies on metabolism in cotton plants and on the fate of
residues in cottonseed and products subjected to commercial
processing. There have also been studies of the nature and fate of
residues in lactating cows and a balance and characterization study of
radio-labelled metabolites in a lactating goat. Studies have also been
made into the fate of residues fed to poultry.
Fate in plants
The metabolic behaviour was studies in greenhouse-grown cotton
plants (Gross, 1977). Selected leaves of the plants were treated at a
rate equivalent to 0.6 kg a.i./ha with 14C-phenyl-labelled
chlordimeform at the "first flower" stage and the treatment was
repeated twice at one-week intervals. The distribution of
radioactivity and pattern of metabolites were determined 30 minutes
and one, two, eight and eleven weeks after the first treatment. The
last period corresponded to the normal pre-harvest interval.
Metabolism was relatively slow: six weeks after the last treatment,
18.2% of the radioactivity was still unchanged chlordimeform.
The treated leaves were exhaustively extracted with
acetonitrile/water (8:2). The extractable radioactivity was further
fractionated into hexane, methylene chloride- and water-soluble
fractions. The radioactivity of the organic fractions was found to
consist of at least seven different substances. Four of them were
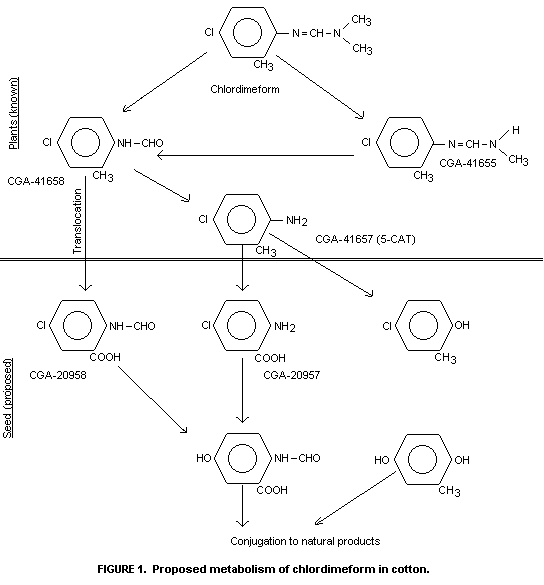
characterized by TLC comparison with reference compounds (Figure 1) as
chlordimeform, N-demethyl-chlordimeform, 4-chloro-o-toluidine and
N-formyl-4-chloro-o-toluidine. Their identIty was confirmed by gas
co-chromatography. A fifth compound, also found by Bull (1973) was not
identical to any of the available reference compounds.
Fifty six per cent of the foliar-applied dose was found in the
plant after one week, the balance being lost by volatilization. Most
of the absorbed radioactivity remained in the treated plant parts
during eight weeks accounted for only 1.8% of the dose. At harvest the
radioactivity calculated as chlordimeform was 0.45 mg/kg in the open
bolls, 0.1 mg/kg in the fibre and 0.3 mg/kg in the seeds. No parent
insecticide was found in the seeds and only 4.5% of the radioactivity
in the seeds still contained the intact aniline moiety. Hydrolysis of
the parent compound to yield N-formyl-4-chloro-o-toluidine and
4-chloro-o-toluidine was the main degradation pathway.
N-demethylation of chlordimeform was of more importance only at later
sampling times. Strong evidence for the formation of the
N-hydroxymethyl derivative of chlordimeform as an intermediate was
found. Some water-soluble and unextractable compounds were produced.
As previously demonstrated by Bull (1973), this metabolic behaviour of
chlordimeform in cotton is similar to that reported in apple seedlings
(Sen Gupta and Knowles 1969) and grapefruit seedlings (Ehrhardt and
Knowles, 1970).
 After hydrolysis and analysis by TLC and GLC, 45% of the
water-soluble radioactivity was found as 4-chloro-o-toluidine.
Another 42% of the radioactivity remaining in the aqueous hydrolysate
was extractable with n-butanol at pH 1 but was not further
characterized.
The unextractable radioactivity in the treated leaves was low at
all time intervals. On the other hand, when the untreated leaves,
stems and green bolls at the 8 week sampling interval were extracted
exhaustively with acetonitrile/hexane (7:3), then 80% aqueous methanol
and finally hot methanol only 15% to 23% of the radioactivity of each
plant part could be extracted. No unchanged chlordimeform was present
in the untreated plant parts. When the plant material was hydrolysed
45%, 17% and 10.5% of the radioactivity of the untreated leaves, stems
and bolls, respectively, was extractable by iso-octane.
Extraction of cottonseeds, taken from mature bolls 11 weeks after
the first application, showed 8% of the radioactivity in the
acetonitrile/hexane phase and an additional 7% of the radioactivity in
the aqueous methanol. Hydrolysis of the seeds showed that only 4.5% of
the radioactivity could be converted to 4-chloro-o-toluidine. When
the nonextractable radioactivity of the weeds was further fractionated
by chemical means into pectin, lignin, hemicellulose and cellulose
fractions, the main part of the radioactivity (78%) was found to be
associated with the cellulose fraction and 14%, 2% and 6% in the
hemicellulose, lignin and pectin fractional respectively.
Gross (1977) concluded that when 14C-chlordimeform was topically
applied to cotton leaves, there was little movement of the
radioactivity and none of chlordimeform itself into the untreated
plant parts. The translocated radioactivity consisted exclusively of
polar, mainly non-extractable substances, suggesting a possible
incorporation of radioactivity into natural products.
Fischer and Cassidy (1976a) reported an experiment in which the
soil of a cotton field plot was treated with 14C-chlordimeform when
the cotton was ten weeks old. The rate of application to the soil
surface was equivalent to 1.0 kg a.i./ha.
Radioactivity in the cotton plants seven weeks after treatment
was equivalent to 0.13 mg/kg chlordimeform. Biphasic extraction showed
42.4% in the organic fraction, 34.9% in the polar fraction and 25.8%
was non-extractable. Thirteen weeks after treatment the mature cotton
contained 0.09 mg/kg in the leaves. The radioactive content of these
mature samples was too low to fractionate for balance determinations.
Treatment of soil surrounding a growing cotton plant showed that
14C-chlordimeform and its soil metabolites are taken up, in small
quantities by cotton, especially the seeds and fibres.
In another experiment Fischer and Cassidy (1976b) treated a
cotton field plot over-the-top with formulated 14C-chlordimeform at a
rate of 1.0 kg/ha when the plants were 10, 12 and 14 weeks old.
Radioactivity in the cotton plants immediately after treatment was
equivalent to 2.44 mg/kg chlordimeform. At harvest the radioactivity
calculated as 14C-chlordimeform was 12.91 mg/kg in the leaves, 0.99
mg/kg in stalks, 0.03 mg/kg in the fibre and 0.26 mg/kg in the seed
with 0.07 mg/kg in the oil and 0.19 mg/kg in the meal. The balance
could be done only on the leaves and stalks at maturity because of the
low radioactivity in the other plant parts. From the leaves and
stalks respectively, the organic fractions contained 57.6% and 72.9%,
the polar fractions 18.9% and 11.4% and the non-extractable fractions
18.8% and 17.8%. Parent 14C-chlordimeform accounted for 31.0% and
45.2% in leaves and stalks respectively. The radioactivity
co-chromatographing with other known standards was minor, accounting
for no more than 8% of the total in either leaves or stalks. This
indicates that there is a large percentage of polar conjugates. The
data show that, although leaf radioactivity is high, there is little
translocation of 14C-chlordimeform metabolites to the seed or fibre.
Application of the method for determining chlordimeform and its
metabolites to cotton leaves showed that 88.9% of the radioactivity in
the leaves but only 29.9% of that in the seeds could be accounted for
as 4-chloro-o-toluidine. This indicates extensive further metabolism
of the translocated metabolites of 14C-chlordimeform.
Fischer and Cassidy (1976c) identified the radioactive
metabolites in leaves after phenyl-14C-chlordimeform was sprayed
over-the-top according to standard agricultural practice. At mature
harvest the total radioactivity in the leaves consisted of parent
chlordimeform 60.3%, demethyl-chlordimeform 4.1%,
4-chloro-o-toluidine 7.6% and its N-formyl derivative 7.0%.
Identification was based on partitioning characteristics and
cochromatography with standards. The metabolism of chlordimeform by
cotton leaves involves, therefore, sequential hydrolysis of terminal
groups with 4-chloro-o-toluidine as the final non-polar product.
These data show that parent chlordimeform will account for the main
part of the material containing the chlorotoluidine moiety in mature
cotton foliage.
Cotton grown in a greenhouse was injected with ring-labelled
chlordimeform in order to characterize the radioactive metabolites,
especially those not extractable with methanol and water, and to
elucidate the metabolic pathway of chlordimeform in cottonseed
(Honeycutt and Cassidy, 1977). The injections were made into the stem
69, 73 and 81 days after planting, the plants were grown to maturity
and the cotton harvested 157 days from planting. The cottonseed
contained radioactivity equivalent to 0.65 mg/kg of chlordimeform at
maturity. Polar, extractable material accounted for 58% of the total
radioactivity in the seed and consisted of 5-chloro-2-formamidobenzoic
acid (N-formyl-5-chloroanthranilic acid) (3%) and eight unknown polar
metabolites, none of which made up more than 8% of the total.
Forty per cent of the radioactivity in the cottonseed was not
extractable and was further fractionated into base-soluble (30%) and
base-insoluble (23%) radioactivity. The base-soluble radioactivity was
characterized as low molecular weight degradation products "A" (1.2%)
and "B" (5.7%), as well as high molecular weight (> 1,000) components
(20%) physicochemically similar to lignin. The base-insoluble
radioactivity was characterized mainly as radioactive conjugates
(17.6%) covalently linked to natural products physicochemically
similar to cellulose. Three percent of the total radioactivity in the
cottonseed could not be solubilized either by strong acid or base
hydrolysis. Application of the total hydrolysis method to both the
polar extractable and the non-extractable radioactivity showed that a
total of only 19.8% of the radioactivity in the cottonseed could be
converted to 4-chloro-o-toluidine (5-CAT).
The above data confirm that the metabolism of chlordimeform in
cottonseed is extensive, processing through 5-CAT, its N-formyl
derivative and N-formyl-5-chloroanthranilic acid to hydroxylated
metabolites which become conjugated to natural products such as
lignin, pectin, cellulose or hemicellulose. This explains why attempts
to define a moiety common to chlordimeform and its metabolites in
cottonseed have not been successful.
The authors have deduced the scheme set out in Figure 2 to
explain the metabolism of chlordimeform in cotton. They contend that
4-chloro-o-toluidine is incorporated into lignin according to the
schemes outlined in Figure 2.
In animals
Burkard (1971a) studied the fate following the application of a
0.5% solution of chlordimeform to the hindquarters of cows. The
treatment was applied three times at 7-day intervals and the results
were determined in milk collected daily until 33 days after the last
treatment. At the end of that period the cows were slaughtered and
samples of meat, liver and fat were analysed.
Generally the total residue in milk was below the limit of
determination (0.03 mg/kg) except on the day following treatment when
it rose to a maximum of 1.0 mg/kg. Thereafter the concentration
declined rapidly reaching the limit of determination on the third day.
The meat and fat contained no detectable residues but liver contained
from 0.3 to 0.6 mg/kg 33 days after the last treatment.
In a further study Burkard (1971b) fed Swiss cows daily with 1 kg
of a concentrate containing from 40 to 240 ppm chlordimeform for
periods up to 42 days. Residues were determined in samples of milk
collected throughout the trial and in samples of meat, fat, liver and
kidney obtained from cows slaughtered after 7, 14, 21 and 42 days
continuous administration.
Total residues of chlordimeform and its metabolites in all milk,
meat and fat samples analysed were below the limit of determination
(0.03 mg/kg). However, the residues in the kidney and liver samples
(mean values of duplicate determinations) were as shown in Table 5.
After hydrolysis and analysis by TLC and GLC, 45% of the
water-soluble radioactivity was found as 4-chloro-o-toluidine.
Another 42% of the radioactivity remaining in the aqueous hydrolysate
was extractable with n-butanol at pH 1 but was not further
characterized.
The unextractable radioactivity in the treated leaves was low at
all time intervals. On the other hand, when the untreated leaves,
stems and green bolls at the 8 week sampling interval were extracted
exhaustively with acetonitrile/hexane (7:3), then 80% aqueous methanol
and finally hot methanol only 15% to 23% of the radioactivity of each
plant part could be extracted. No unchanged chlordimeform was present
in the untreated plant parts. When the plant material was hydrolysed
45%, 17% and 10.5% of the radioactivity of the untreated leaves, stems
and bolls, respectively, was extractable by iso-octane.
Extraction of cottonseeds, taken from mature bolls 11 weeks after
the first application, showed 8% of the radioactivity in the
acetonitrile/hexane phase and an additional 7% of the radioactivity in
the aqueous methanol. Hydrolysis of the seeds showed that only 4.5% of
the radioactivity could be converted to 4-chloro-o-toluidine. When
the nonextractable radioactivity of the weeds was further fractionated
by chemical means into pectin, lignin, hemicellulose and cellulose
fractions, the main part of the radioactivity (78%) was found to be
associated with the cellulose fraction and 14%, 2% and 6% in the
hemicellulose, lignin and pectin fractional respectively.
Gross (1977) concluded that when 14C-chlordimeform was topically
applied to cotton leaves, there was little movement of the
radioactivity and none of chlordimeform itself into the untreated
plant parts. The translocated radioactivity consisted exclusively of
polar, mainly non-extractable substances, suggesting a possible
incorporation of radioactivity into natural products.
Fischer and Cassidy (1976a) reported an experiment in which the
soil of a cotton field plot was treated with 14C-chlordimeform when
the cotton was ten weeks old. The rate of application to the soil
surface was equivalent to 1.0 kg a.i./ha.
Radioactivity in the cotton plants seven weeks after treatment
was equivalent to 0.13 mg/kg chlordimeform. Biphasic extraction showed
42.4% in the organic fraction, 34.9% in the polar fraction and 25.8%
was non-extractable. Thirteen weeks after treatment the mature cotton
contained 0.09 mg/kg in the leaves. The radioactive content of these
mature samples was too low to fractionate for balance determinations.
Treatment of soil surrounding a growing cotton plant showed that
14C-chlordimeform and its soil metabolites are taken up, in small
quantities by cotton, especially the seeds and fibres.
In another experiment Fischer and Cassidy (1976b) treated a
cotton field plot over-the-top with formulated 14C-chlordimeform at a
rate of 1.0 kg/ha when the plants were 10, 12 and 14 weeks old.
Radioactivity in the cotton plants immediately after treatment was
equivalent to 2.44 mg/kg chlordimeform. At harvest the radioactivity
calculated as 14C-chlordimeform was 12.91 mg/kg in the leaves, 0.99
mg/kg in stalks, 0.03 mg/kg in the fibre and 0.26 mg/kg in the seed
with 0.07 mg/kg in the oil and 0.19 mg/kg in the meal. The balance
could be done only on the leaves and stalks at maturity because of the
low radioactivity in the other plant parts. From the leaves and
stalks respectively, the organic fractions contained 57.6% and 72.9%,
the polar fractions 18.9% and 11.4% and the non-extractable fractions
18.8% and 17.8%. Parent 14C-chlordimeform accounted for 31.0% and
45.2% in leaves and stalks respectively. The radioactivity
co-chromatographing with other known standards was minor, accounting
for no more than 8% of the total in either leaves or stalks. This
indicates that there is a large percentage of polar conjugates. The
data show that, although leaf radioactivity is high, there is little
translocation of 14C-chlordimeform metabolites to the seed or fibre.
Application of the method for determining chlordimeform and its
metabolites to cotton leaves showed that 88.9% of the radioactivity in
the leaves but only 29.9% of that in the seeds could be accounted for
as 4-chloro-o-toluidine. This indicates extensive further metabolism
of the translocated metabolites of 14C-chlordimeform.
Fischer and Cassidy (1976c) identified the radioactive
metabolites in leaves after phenyl-14C-chlordimeform was sprayed
over-the-top according to standard agricultural practice. At mature
harvest the total radioactivity in the leaves consisted of parent
chlordimeform 60.3%, demethyl-chlordimeform 4.1%,
4-chloro-o-toluidine 7.6% and its N-formyl derivative 7.0%.
Identification was based on partitioning characteristics and
cochromatography with standards. The metabolism of chlordimeform by
cotton leaves involves, therefore, sequential hydrolysis of terminal
groups with 4-chloro-o-toluidine as the final non-polar product.
These data show that parent chlordimeform will account for the main
part of the material containing the chlorotoluidine moiety in mature
cotton foliage.
Cotton grown in a greenhouse was injected with ring-labelled
chlordimeform in order to characterize the radioactive metabolites,
especially those not extractable with methanol and water, and to
elucidate the metabolic pathway of chlordimeform in cottonseed
(Honeycutt and Cassidy, 1977). The injections were made into the stem
69, 73 and 81 days after planting, the plants were grown to maturity
and the cotton harvested 157 days from planting. The cottonseed
contained radioactivity equivalent to 0.65 mg/kg of chlordimeform at
maturity. Polar, extractable material accounted for 58% of the total
radioactivity in the seed and consisted of 5-chloro-2-formamidobenzoic
acid (N-formyl-5-chloroanthranilic acid) (3%) and eight unknown polar
metabolites, none of which made up more than 8% of the total.
Forty per cent of the radioactivity in the cottonseed was not
extractable and was further fractionated into base-soluble (30%) and
base-insoluble (23%) radioactivity. The base-soluble radioactivity was
characterized as low molecular weight degradation products "A" (1.2%)
and "B" (5.7%), as well as high molecular weight (> 1,000) components
(20%) physicochemically similar to lignin. The base-insoluble
radioactivity was characterized mainly as radioactive conjugates
(17.6%) covalently linked to natural products physicochemically
similar to cellulose. Three percent of the total radioactivity in the
cottonseed could not be solubilized either by strong acid or base
hydrolysis. Application of the total hydrolysis method to both the
polar extractable and the non-extractable radioactivity showed that a
total of only 19.8% of the radioactivity in the cottonseed could be
converted to 4-chloro-o-toluidine (5-CAT).
The above data confirm that the metabolism of chlordimeform in
cottonseed is extensive, processing through 5-CAT, its N-formyl
derivative and N-formyl-5-chloroanthranilic acid to hydroxylated
metabolites which become conjugated to natural products such as
lignin, pectin, cellulose or hemicellulose. This explains why attempts
to define a moiety common to chlordimeform and its metabolites in
cottonseed have not been successful.
The authors have deduced the scheme set out in Figure 2 to
explain the metabolism of chlordimeform in cotton. They contend that
4-chloro-o-toluidine is incorporated into lignin according to the
schemes outlined in Figure 2.
In animals
Burkard (1971a) studied the fate following the application of a
0.5% solution of chlordimeform to the hindquarters of cows. The
treatment was applied three times at 7-day intervals and the results
were determined in milk collected daily until 33 days after the last
treatment. At the end of that period the cows were slaughtered and
samples of meat, liver and fat were analysed.
Generally the total residue in milk was below the limit of
determination (0.03 mg/kg) except on the day following treatment when
it rose to a maximum of 1.0 mg/kg. Thereafter the concentration
declined rapidly reaching the limit of determination on the third day.
The meat and fat contained no detectable residues but liver contained
from 0.3 to 0.6 mg/kg 33 days after the last treatment.
In a further study Burkard (1971b) fed Swiss cows daily with 1 kg
of a concentrate containing from 40 to 240 ppm chlordimeform for
periods up to 42 days. Residues were determined in samples of milk
collected throughout the trial and in samples of meat, fat, liver and
kidney obtained from cows slaughtered after 7, 14, 21 and 42 days
continuous administration.
Total residues of chlordimeform and its metabolites in all milk,
meat and fat samples analysed were below the limit of determination
(0.03 mg/kg). However, the residues in the kidney and liver samples
(mean values of duplicate determinations) were as shown in Table 5.
 TABLE 5. Residues of chlordimeform and its metabolites in cows.
Chlordimeform and metabolites, mg/kg
Duration of
feeding (days)
In concentrate In liver In kidney
21 40 0.09 < 0.03
21 120 0.38 0.07
7 240 0.45 0.05
14 240 0.58 0.13
21 240 0.50 0.13
42 240 0.44 0.09
Honeycutt and Cassidy (1976) fed a lactating goat with cottonseed
containing biosynthesized metabolites of phenyl-14C-chlordimeform at
a level equivalent to 0.032 ppm chlordimeform daily for 4 consecutive
days. The total recovery of radioactivity was 97.1%. It was primarily
distributed between the urine (21.3%), which reached a plateau by the
2nd day, the faeces (35.9%), which reached a plateau by the third day,
and the rumenintestinal contents (38.7%). There was no deposition of
radioactivity in tissues (< 0.002 mg/kg), even though uptake of the
biosynthesized metabolites occurred.
The milk contained 0.9% of the total dose, its radioactivity
plateaued by the second day (0.0004 ± 0.0001 mg/kg chlordimeform
equivalents). The ratio of the concentration in milk to that in the
feed was 0.013. Blood contained < 0.2% of the total dose.
Comparison of the 14C-extractables of the original cottonseed
with those in the faeces, urine and rumen-intestinal contents,
suggests that the goat metabolized the biosynthesized metabolites in
cottonseed. However, metabolism to natural products was not
significant since expired 14CO2 accounted for only 0.3% of the total
dose.
Ciba-Geigy Corp. (1971) conducted experiments in which white
Leghorn hens in active egg production were fed a commercial egg laying
mash to which chlordimeform was added at rates equivalent to 0, 0.25,
0.75 and 1.50 mg/kg of feed. Groups were sacrificed after 7, 14, 21
and 28 days feeding and were subjected to post-mortem examination and
assessment of egg production, body-weight change and food consumption.
There were no significant differences between treated and control
binds in any of the features examined.
Residues in eggs and tissues (breast, fat and liver) were
determined by Jenny (1971). Eggs collected at -3 (pre-treatment), -1
(pre-treatment), 3, 7, 14, 21 and 28 days from each group contained no
detectable amounts of chlordimeform and/or its metabolites containing
the 4-chloro-o-toluidine moiety.
The breasts of birds sacrificed at 7, 14, 21 and 28 days of
feeding treated feed as well as 49 days after returning to untreated
feed contained no significant residues. Average residues of 0.11, 0.09
and 0.22 mg/kg were detected in fat at the feeding levels of 0.25,
0.75 and 1.50 ppm respectively, at the 21-day interval only. No
residues were detected in livers from chickens fed 0.25 ppm
chlordimeform at any of the intervals tested and residue of 0.09 mg/kg
was detected in liver from chickens fed 0.75 ppm only at the 21-day
interval. Residues of 0.20, 0.17, and 0.10 mg/kg were detected in
livers from the 1.5 ppm level at intervals of 7, 14, 21 and 28 days.
Nine days after withdrawal from the feed containing 1.5 ppm, no
residues could be detected in any of the tissues.
In processing
The Meeting reviewed many data on the level and fate of residues
during processing. Cottonseed is subjected to well-defined sequential
processes, details of which were available (Ciba-Geigy, 1978). The
results of some typical trials are set out in Table 4 and 6. The
distribution is independent of the pattern of application as is
apparent from Table 6. Although the major fraction of the residue
remains in the hulls and the meal from which the oil has been removed
there is still a significant concentration in the crude oil. Treatment
of crude cottonseed oil with caustic soda in the refining process does
not reduce the residue concentration significantly but the additional
refining procedures including bleaching, hydrogenating and deodorizing
reduce the residue in the oil to a level below the detection limits of
present analytical methods (< 0.05 mg/kg). Since all the oil used for
human consumption is at least subjected to the bleaching and
deodorizing process, virtually all the chlordimeform will be removed
before the cottonseed oil is consumed.
In soil
In the course of studies on the uptake of chlordimeform and its
soil metabolites from treated soil into cotton Fischer and Cassidy
(1976a) applied radio-labelled chlordimeform to a cotton field plot
thirteen weeks before harvesting the cotton. The rate of application
was equivalent to 1 kg/ha.
TABLE 6. Effect of use pattern on Chlordimeform residues in cottonseed products.
AGA AGA AGA AGA AGA AGA AGA AGA AGA AGA
Trial No. 4397 4408 4409 4449 4450 4502 4981 4981 4981 4981
Location Alab. Ariz. Calif. Georg. Georg. Miss. S.C. S.C. S.C. S.C.
Rate of application 0.125 0.125 0.25 0.125 0.25 0.125 0.125 0.25 0.125 0.25
Formulation EC EC EC EC EC EC EC EC EC EC
No. of applications 7 7 6 11 12 9 12 12 17 17
Pre-harvest interval 28 39 31 22 16 31 21 21 21 21
Cottonseed 0.2 0.82 0.35 0.35 0.35 0.30 0.29 0.20 0.22 0.40
Hulls 0.36 1.8 0.85 0.58 0.55 0.33 0.86 0.50 0.90 0.75
Meal 0.19 1.2 0.45 0.24 0.32 0.45 0.18 0.10 0.17 0.15
Soap stock 0.10 0.84 0.34 0.17 0.23 0.22 0.09 0.07 0.10 0.10
Crude oil 0.10 0.46 0.21 0.15 0.23 0.16 0.10 0.07 0.11 0.08
Refined oil 0.12 0.66 0.32 0.17 0.19 0.19 0.07 <0.05 0.08 0.07
Refined bleached
deodorised oil <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Refined bleached
deodorised
hydrogenated oil <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Radioactivity in the top 75 mm layer of silt loam soil accounted
for 1.23 mg/kg chlordimeform equivalents after treatment. Seven weeks
later this level had decreased to 0.33 mg/kg and at 13 weeks to 0.20
mg/kg. When the 0-75 mm layer was extracted after seven weeks, 32% of
the radioactivity partitioned into the organic fraction, 20%
partitioned into the polar fraction and 44% remained non-extractable,
showing that the chlordimeform had degraded rapidly. The radioactivity
in the samples taken 13 weeks after treatment was too low to
fractionate. Except for one sample, the level of radioactivity as
chlordimeform equivalents in the 75-150 mm and 150-200 mm layers was
less than 0.01 mg/kg; thus leaching did not occur in this silt loam.
In a later study when radio-labelled chlordimeform was applied at
the rate of 1 kg/ha over cotton plants and soil in field plots,
Fischer and Cassidy (1976b) found that the radioactivity in the 0-75
mm layer of silt loam was 1.24 mg/kg immediately after the first
treatment but only 0.21 mg/kg after the final treatment and 0.20 mg/kg
at harvest of the mature cotton. This decrease is due to (1) less
drainage from the leaves onto the soil at the later sprayings because
of the increased cover with plant growth, (2) volatility of the
chlordimeform and (3) soil metabolism. Radioactivity was less than
0.01 mg/kg in the 75-150 and 150-225 mm layers at all-sampling dates.
These data show that metabolism of 14C-chlordimeform in silt-loam is
rapid and that the metabolites are not leached.
Analysis of the 0-75 mm layer of soil taken at harvest showed
that 91.0% of the radioactivity could be converted to
4-chloro-o-toluidine. Therefore, although 57% of the soil
metabolites were polar or non-extractable, they contained this moiety.
Following this study Fischer and Cassidy (1976b) planted soybeans
at a rotation crop 38 weeks after the original treatment with
chlordimeform at 1 kg/ha. At maturity the soybeans were found to
contain radioactivity equivalent to 0.01 mg/kg chlordimeform in the
stalks and less than 0.01 mg/kg in the beans themselves.
Wheat planted in the plots was examined for radioactivity at
maturity. The radioactivity was equivalent to 0.16 mg/kg in the straw
and 0.03 mg/kg in the grain.
METHODS OF RESIDUE ANALYSIS
Although methods have been developed for the determination of
chlordimeform alone, most of the residue data have been based on the
determination of chlordimeform and its metabolites as
4-chloro-o-toluidine.
Geissbuhler et al. (1971) published the method used by Ciba-Geigy
Ltd. and Schering A.G. for the development of chlordimeform
pesticides. This method has been utilized with and without
modification for much of the work reported in this monograph. In this
method chlordimeform and its potential metabolites and/or conjugates
are hydrolyzed to 4-chloro-o-toluidine in the presence of plant and
soil material by successive treatments with acetic acid and sodium
hydroxide. The aromatic amine is steam distilled and extracted into
iso-octane. Diazotization and coupling of 4-chloro-o-toluidine with
N-ethyl-1-naphthylamine yields a stable purple dye, which, after
column chromatography on cellulose, is measured colorimetrically. To
verify the identity of chlordimeform, the azo-dye is separated and
visualized on a cellulose thin-layer plate. Alternatively,
4-chloro-o-toluidine is diazotized and iodinated. The iodine
derivative is determined by electron capture gas chromatography. A
further potential metabolite of chlordimeform
2,2-dimethyl-4-4-dichloroazobenzene, is reduced to
4-chloro-o-toluidine in the presence of zinc and acid 1,4-dioxane
and likewise determined by gas chromatography. Recoveries in various
plant and soil materials have been observed to vary between 75 and
100% for all three quantitative methods described. The limit of
determination is 0.05 mg/kg.
APPRAISAL
Chlordimeform products which had been widely used for many years
as insecticides/acaricides on a large variety of crops and on
livestock, were withdrawn by the manufacturers in 1976. Additional
studies have since been carried out and new proposals have been made
for commercial use on cotton crops under restricted conditions.
Information on those proposed uses in various countries and data on
the nature, level and fate of residues, and including the fate of
residues in cottonseed products used in the rations of dairy cattle
and poultry was also examined.
The influence of rate of application pre-harvest interval and
number of applications were investigated. At the maximum application
rate of 1 kg/ha the residue rarely exceeded 2 mg/kg in cottonseed,
seed meal or crude oil. Solvent-extracted oil contained significantly
lower residues than screwpress-extracted oil. Treatment of crude oil
with caustic sodal did not reduce residues markedly but refining
procedures such as bleaching and deodorization as normally undertaken
in commercial practice reduced the levels to below the analytical
detection limits (< 0.05 mg/kg).
Eggs from hens fed for 28 days on rations containing up to 1.5
ppm contained no detectable residues. Meat, fat and liver from hens
slaughtered at various intervals throughout the study was analysed.
Although nothing was found in any of the meat, small residues were
detected in some samples of fat and liver. Cows fed for up to 42 days
on rations containing excessive levels of chlordimeform (up to 240
ppm) showed no residues (< 0.3 mg/kg) in milk, fat or meat at any
stage during the feeding; but residues in some animals fed at the
highest rates, ranged up to 0.6 mg/kg in liver and 0.1 mg/kg in
kidney. Since there appears to be no likelihood of livestock rations
containing more than 0.5 mg/kg of residue however, detectable residues
are unlikely to occur in foods of animal origin.
The analytical methods used determined chlordimeform, the
intermediate metabolite N-formyl-4-chloro-o-toluidine and the final
metabolite 4-chloro-o-toluidine together as 4-chloro-o-toluidine. No
attempt was made to quantify the amounts of each present and the limit
of determination was 0.05 mg/kg.
Studies of the metabolism of chlordimeform in cotton plants and
goats showed that metabolism in plants and animals proceeds through
sequential hydrolysis of terminal groups to 4-chloro-o-toluidine. In
cotton metabolism proceeds to the formation of polar metabolites which
apparently conjugate to natural products. Sprays applied after the
bolls open would partly avoid translocation of residues as metabolites
from the treated leaves to seed but would result in the retention of
significant residues of the parent compound in fibre and seed. In a
study involving the feeding of cottonseed containing biosynthesised
radioactive metabolites to a goat, no residues were deposited in the
animal tissue (limit of determination 0.002 mg/kg).
In the light of the new data examined, the Meeting decided that
there was no need to amend the temporary MRL formerly proposed for
cottonseed and crude cottonseed oil; but unless direct application to
cattle were to be re-installed, the MRL for foods of animal origin
should be based on the data reflecting the feeding of cottonseed
hulls, cottonseed meal and similar products. As chlordimeform is no
longer used on crops other than cotton, the recommendations for
temporary MRLs previously made for crops other than cotton should be
held in abeyance until such time as the uses on those crops should be
reinstated.
RECOMMENDATIONS
The following new or amended recommendations for temporary MRLs
replace all previous recommendations.
Commodity Temporary MRL mg/kg
Cottonseed 2
Cottonseed (crude) 2
Cottonseed oil (edible) ) No residues are to
) occur at the current
Meat of cattle ) limit of detection
) (0.05 mg/kg)
Meat of pigs )
Meat of poultry ) " " " "
Meat of sheep )
Milk )
Milk products )
FURTHER WORK OR INFORMATION
Required (by 1979)
1. Full submission of all toxicological data.
2. Continued epidemiology data on people exposed to chlordimeform.
3. Reports of continued observation of the possible occurrence of
haemorrhagic cystitis.
REFERENCES
Anonymous Chlordimeform HC1 Life Time Feeding Study in Rats - One
(1978a) Year Interim Report. Unpublished report from
CIBA-GEIGTY, Ltd., Basal, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Anonymous N-Formyl-4-chloro-o-toluidine Life Time Feeding Study in
(1978b) Rats One Year Interim Report. Unpublished
report from CIBA-GEIGY, Ltd., Basel,
Switzerland submitted by CIBA-GEIGY, Ltd. to
the WHO.
Anonymous 4-chloro-o-toluidine HC1 Life Time Feeding Study in Rats
(1978c) - One Year Interim Report. Unpublished report
from CIBA-GEIGY, Ltd., Basel, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Anonymous Bioassay of 4-chloro-o-toluidine Hydrochloride for
(1978d) Possible Carcinogenicity. NCI Carcinogenic
Programme Preliminary Draft Report [ NIH ]
79-1721).
Arni, P. and D. Muller Salmonella/Mammalian-Microsome Mutagenicity
(1976a) Test with C-8513 Chlordimeform HC1.
Unpublished report from CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Arni, P. and D. Muller Salmonella/Mammalian-Microsome Mutagenicity
(1976b) Test with CGA 72651
(N-formyl-4-chloro-o-toluidine).
Unpublished report from CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Arni, P. and D. Muller Salmonella/Mammalian Microsome Mutagenicity
(1976c) Test with CGA 72647 (4-chloro-o-toluidine
HC1). Unpublished report from CIBA-GEIGY,
Ltd., Basil, Switzerland submitted by
CIBA-GEIGY, Ltd. to the WHO.
Benezet, H.J. and C.O. Knowles Inhibition of Rat Brain
(1976) Monoamine Oxidase by Formamidine and Related
Compounds. Neuropharmacol. 15:369.
Benezet, H.J., K.M. Chang and C.O. Knowles Formamidine Pesticides-
(1978) Metabolic Aspects of Neurotoxicity in
Pesticide and Venom Neural Toxicity. Ed. D.L.
Shankland, R.M. Hollingworth and J. Smyth,
Jr. Plenum Press, N.Y. pg. 189-206.
Bull, D.L., Environmental Entomology 2, 869.
(1973)
Burkhard, N. Residues in milk, meat, liver and fat of a Swiss cow
(1971a) washed with a 0.5% solution of
chlordimeform. Report RVA 14/71, Agrochemical
Division, CIBA-GEIGY, Ltd., Basel,
Switzerland. 1 February 1971.
Chinn, C., W.R. Pfister and G.K.W. Yim Local Anesthetic-Like Actions
(1976) of the Pesticide Chlordimeform. Fed. Proc.
35:729.
Ciba-Geigy Chlorphenamidime residues in eggs and poultry tissues.
(1971) Research Report C79910, CIBA-GEIGY, Corp.
Greensboro N.C.
Ciba-Geigy Method for determination of chlordimeform residues -
(1976) Method REM 4/76.
Ciba-Geigy Submission of data for the reevaluation of chlordimeform by
(1978) the 1978 FAO/WHO Joint Meeting on Pesticide
Residues, CIBA-GEIGY, Ltd., Basel and
Schering AG, Berlin. July 24, 1978.
Ciba-Geigy Residues resulting from chlordimeform application to
(1978a) cotton. Report by H.B. Camp and B.G. Tweedy
No ABR-78004 (4 January 1978).
Ehrhardt, D.A. and Knowles, C.O. J. Econ. Entomol. 63, 1306.
(1970)
Ezumi, K. and H. Nakao Effects of 4-chloro-o-toluidine in Oral
(1974a) Prolonged Administration to Rat for 94 (Male)
and 104 (Female) Weeks. Unpublished report
from Nihon Schering Inc., Osaka, Japan
submitted by CIBA-GEIGY, Ltd. to the WHO.
Ezumi, K. and H. Nakao Effects of 4-chloro-o-toluidine in Oral
(1974b) Prolonged Administration to Mice for 80 Weeks
Unpublished report from Nihon Schering, Inc.,
Osaka, Japan submitted by CIBA-GEIGY, Ltd. to
the WHO.
FAO/WHO 1971 Evaluations of some pesticide residues in food.
(1972b) AGP:1971/M/9/1; WHO Pesticide Residues Series
No 1.
FAO/WHO 1975 Evaluations of some pesticide residues in food. AGP:
(1976b) 1975/M/13; WHO Pesticide Residue Series No 5.
FAO/WHO Pesticide residues in food. Report of the 1976 Joint Meeting,
(1977a) of the FAO Panel Experts on Pesticide.
Residues and the Environment and the WHO
Expert Group on Pesticide Residues. FAO Food
and Nutrition Series, No 9; FAO Plant
Production and Protection Series, No 8; WHO
Technical Report Series, No 612.
FAO/WHO Pesticide residues in food - 1977. Report of the FAO Panel of
(1978a) Experts on Pesticide Residues and Environment
and the WHO Expert Committee on Pesticide
Residues. FAO Plant Production and Protection
Paper 10 Rev. par 4.13.
Fischer, W.C. and Cassidy, J.E. Uptake of radio-labelled (1976a)
chlordimeform and its soil metabolites in
cotton in a field prepared for rotation
crops. Report No GAAC-76014 CIBA-GEIGY
Corporation, Greensboro, N.C. March 15, 1976.
Fischer W.C. and Cassidy, J.E. Uptake and characterization of
(1976b) the metabolites of radio-labelled
chlordimeform in spray treated cotton and
field soil. Report of Biochemistry Department
CIBA-GEIGY Corporation Report ABR - 76056
July 27, 1976.
Fischer W.C. and Cassidy, J.E. Identification of the metabolites
(1976c) of chlordimeform in mature leaves of spray
treated field cotton. Report No ABR - 76073
from Biochemistry Department, CIBA-GEIGY,
Corporation, Greensboro, N.C.
Fritz, H. Reproduction study on C8514 Technical Rabbit - Seg II. (Test
(1971) for teratogenic and embryotoxic effects).
Unpublished report from Toxicology/ Pathology
CIBA-GEIGY, Ltd., Basely Switzerland,
submitted by CIBA-GEIGY Ltd to the WHO.
Fritz, H. Reproduction Study on C8514 Technical Rat-Seg II (Test for
(1975) Teratogenic or Embroyotic Effects).
Unpublished report from Toxicology/Pathology
CIBA-GEIGY, Ltd., Basely Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Geisebuhler, H., Kossmann, K., Baunck, I. and Boyd, V.F.
(1971) Determination of total residues of
chlorphenamidine in plant and soil material
by colorimetry and thin layer and electron
capture gas-chromatography. Agric. Food Chem.
19 (2):365.
Gross Metabolism of chlordimeform in cotton. Project Report 19/77 from
(1977) Biochemistry Department, CIBA-GEIGY, Ltd.,
Basel, Switzerland (April 1977).
Hollingworth R.M. Chemistry Biological Activity and Uses of
(1976) Formamidine Pesticides. Environ. Health
Perspect. 14: 57-69.
Honeycutt, R.C. and Cassidy, J.E. Extraction and characterization
(1977) of cottonseed metabolite from cotton
injected with o 14C-chlordimeform. Report No
ABR - 77087 from Biochemistry Department,
CIBA-GEIGY, Corporation, Greensboro, N.C.
Sept. 14, 1977.
Hurni, H. and H. Ohder Report on the Mutagenic Effect of Technical
(1970) C-8514-N. Unpublished report from Tierfarm AG
Biomedical Research, Sisseln, Switzerland
submitted by CIBA-GEIGY, Ltd., to the WHO.
Jenny, N.A. Chlorphenamidine residues in eggs and poultry
(1971) tissues/Analysis of tissue and egg samples.
Knowles, C.O. Chemistry and Toxicology of Quinoxaline Organotin
(1976) Organofluorine and Formamidine Acaracides.
Env. Health Perspect. 14:93-102.
Kalyonova, F.P., Z. Zapryanav and A.I. Baynova Influence of the
(1978) Insecticide Chlordimeform of the Activity of
the Monoamine Oxidase (MAO) of Albino Rats.
Comptes Rendus de l'Academi Bulgare des
Sciences 31 (4): 491-493.
Konopa, E.A. and H. Heymann Salmonella/Microsome Mutagenicity
(1977) Test with Chlordimeform. Unpublished report
from Pharmaceuticals Division, CIBA-GEIGY,
Corporation, Surnmity N.J. submitted by
CIBA-GEIGY, Ltd., to the WHO.
Konopka. E.A. and H. Heymann Salmonella/Microsome Mutagenicity
(1977b) Test with Chlordimeform. Unpublished report
from Pharmaceuticals Division, CIBA-GEIGY
Corporation, Summit, N.J. submitted by
CIBA-GEIGY, Ltd. to the WHO.
Langauer, M. and D. Muller Nucleus Anomaly Test on Somatic
(1977) Interphase Nuclei-C-8513- Chinese Hamster.
Unpublished report from Toxicology/Pathology,
CIBA-GEIGY, Ltd., Basel, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Lund, A.E., G.K.W. Yim and D.L. Shankland The Cardiovascular
(1978) Toxicity of Chlordimeform, a Local
Anesthetic-Like Action in Pesticide and Venom
Neurotoxicity. Ed. D.L. Shankland, R.M.
Hollingworth and T. Smyth, Jr. Plenum Press,
N.Y., pgs. 171-177.
Maitre L., A. Felner, P. Waldmeier and W. Kehr Moccasins Oxidase
(1978) Inhibition in Brain and Liver of Rats Treated
with Chlordimeform. J. Agr. Fd Chem. 26
(2): 442-446.
Matsumura, F. and R.W. Beeman Biochemical and Physiological
(1976) Effects of Chlordimeform. Environ. Health
Perspect. 14: 71-82.
Morton Method for the determination of chlordimeform. Method 333/7
(1968) (7/12/68)
Murakami, J. and J. Fukami Effects of Chlophenamidine and Its
(1974) Metabolites on HeLa Cells. Bull. Env.
Contam. and Tox. 11: 184-188.
Nesnow, S. and C. Heidelberger The Effect of Modifiers of
Microsomal Enzymes on Chemical Oncogenesis in
Cultures of C3H Mouse Cell Lines. Cancer
Res. 36:1801-1808.
Nesnow S. Unpublished bioassay submitted to the WHO.
(1978)
Nor-Am Method of analysis for chlordimeform residues - Research Method
(1969) 333/44. 5/6/69.
Sachsse, K., P. Suter, F. Zak and R. Hess Lifespan Feeding Study in
(1978b) Mice with 4-chloro-o-toludine. Unpublished
report from Toxicology, CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Schering Method of analysis of chlordimeform residues. Method 36 268/8
(1973) (24/4/73).
Schering Method of determination of chlordimeform and its degradation
(1975) products. Method 36268/9 17/11/75.
Sen Gupta, A.K. and Knowles, C.O. J. Agric. Food Chem. 17, 595.
(1969)
Suter, P., H. Luetkemeier, K. Sachsse and F. Zak 60-day Pending
(1976a) Study in the Rat with chloro-o-toluidine
HC1. Unpublished report from
Toxicology/Pathology, CIBA-GEIGY, Ltd.,
Basel, Switzerland, submitted by CIBA-GEIGY,
Ltd. to the WHO.
Suter, P., H. Luetkemeier, K. Sachsse and F. Zak 60-day Pending
(1976b) Study in Mice with 4, chloro-o-toluidine
HC1. Unpublished report from
Toxicology/Pathology, CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Suter, P., F. Zak, K. Sachsse and R. Hess Lifespan Feeding Study
(1979) in Mice with Chlordimeform HC1. Unpublished
report from Toxicology/Pathology,
CIBA-GEIGY, Ltd., Basel, Switzerland,
submitted by CIBA-GEIGY, Ltd. to the WHO.
Wang, C.M., T. Narahashi and J. Fukami Mechanism of Neuromuscular
(1975) Block by Chlordimeform. Pest. Biochem.
Physiol. 5: 119-125.
Watanabe, H., S. Tsuda and J. Fukami. Effects of Chlordimeform
(1975) on Rectusabdominum Muscle in Frog. Pest.
Biochem. Physiol. 5: 150-154.
TABLE 5. Residues of chlordimeform and its metabolites in cows.
Chlordimeform and metabolites, mg/kg
Duration of
feeding (days)
In concentrate In liver In kidney
21 40 0.09 < 0.03
21 120 0.38 0.07
7 240 0.45 0.05
14 240 0.58 0.13
21 240 0.50 0.13
42 240 0.44 0.09
Honeycutt and Cassidy (1976) fed a lactating goat with cottonseed
containing biosynthesized metabolites of phenyl-14C-chlordimeform at
a level equivalent to 0.032 ppm chlordimeform daily for 4 consecutive
days. The total recovery of radioactivity was 97.1%. It was primarily
distributed between the urine (21.3%), which reached a plateau by the
2nd day, the faeces (35.9%), which reached a plateau by the third day,
and the rumenintestinal contents (38.7%). There was no deposition of
radioactivity in tissues (< 0.002 mg/kg), even though uptake of the
biosynthesized metabolites occurred.
The milk contained 0.9% of the total dose, its radioactivity
plateaued by the second day (0.0004 ± 0.0001 mg/kg chlordimeform
equivalents). The ratio of the concentration in milk to that in the
feed was 0.013. Blood contained < 0.2% of the total dose.
Comparison of the 14C-extractables of the original cottonseed
with those in the faeces, urine and rumen-intestinal contents,
suggests that the goat metabolized the biosynthesized metabolites in
cottonseed. However, metabolism to natural products was not
significant since expired 14CO2 accounted for only 0.3% of the total
dose.
Ciba-Geigy Corp. (1971) conducted experiments in which white
Leghorn hens in active egg production were fed a commercial egg laying
mash to which chlordimeform was added at rates equivalent to 0, 0.25,
0.75 and 1.50 mg/kg of feed. Groups were sacrificed after 7, 14, 21
and 28 days feeding and were subjected to post-mortem examination and
assessment of egg production, body-weight change and food consumption.
There were no significant differences between treated and control
binds in any of the features examined.
Residues in eggs and tissues (breast, fat and liver) were
determined by Jenny (1971). Eggs collected at -3 (pre-treatment), -1
(pre-treatment), 3, 7, 14, 21 and 28 days from each group contained no
detectable amounts of chlordimeform and/or its metabolites containing
the 4-chloro-o-toluidine moiety.
The breasts of birds sacrificed at 7, 14, 21 and 28 days of
feeding treated feed as well as 49 days after returning to untreated
feed contained no significant residues. Average residues of 0.11, 0.09
and 0.22 mg/kg were detected in fat at the feeding levels of 0.25,
0.75 and 1.50 ppm respectively, at the 21-day interval only. No
residues were detected in livers from chickens fed 0.25 ppm
chlordimeform at any of the intervals tested and residue of 0.09 mg/kg
was detected in liver from chickens fed 0.75 ppm only at the 21-day
interval. Residues of 0.20, 0.17, and 0.10 mg/kg were detected in
livers from the 1.5 ppm level at intervals of 7, 14, 21 and 28 days.
Nine days after withdrawal from the feed containing 1.5 ppm, no
residues could be detected in any of the tissues.
In processing
The Meeting reviewed many data on the level and fate of residues
during processing. Cottonseed is subjected to well-defined sequential
processes, details of which were available (Ciba-Geigy, 1978). The
results of some typical trials are set out in Table 4 and 6. The
distribution is independent of the pattern of application as is
apparent from Table 6. Although the major fraction of the residue
remains in the hulls and the meal from which the oil has been removed
there is still a significant concentration in the crude oil. Treatment
of crude cottonseed oil with caustic soda in the refining process does
not reduce the residue concentration significantly but the additional
refining procedures including bleaching, hydrogenating and deodorizing
reduce the residue in the oil to a level below the detection limits of
present analytical methods (< 0.05 mg/kg). Since all the oil used for
human consumption is at least subjected to the bleaching and
deodorizing process, virtually all the chlordimeform will be removed
before the cottonseed oil is consumed.
In soil
In the course of studies on the uptake of chlordimeform and its
soil metabolites from treated soil into cotton Fischer and Cassidy
(1976a) applied radio-labelled chlordimeform to a cotton field plot
thirteen weeks before harvesting the cotton. The rate of application
was equivalent to 1 kg/ha.
TABLE 6. Effect of use pattern on Chlordimeform residues in cottonseed products.
AGA AGA AGA AGA AGA AGA AGA AGA AGA AGA
Trial No. 4397 4408 4409 4449 4450 4502 4981 4981 4981 4981
Location Alab. Ariz. Calif. Georg. Georg. Miss. S.C. S.C. S.C. S.C.
Rate of application 0.125 0.125 0.25 0.125 0.25 0.125 0.125 0.25 0.125 0.25
Formulation EC EC EC EC EC EC EC EC EC EC
No. of applications 7 7 6 11 12 9 12 12 17 17
Pre-harvest interval 28 39 31 22 16 31 21 21 21 21
Cottonseed 0.2 0.82 0.35 0.35 0.35 0.30 0.29 0.20 0.22 0.40
Hulls 0.36 1.8 0.85 0.58 0.55 0.33 0.86 0.50 0.90 0.75
Meal 0.19 1.2 0.45 0.24 0.32 0.45 0.18 0.10 0.17 0.15
Soap stock 0.10 0.84 0.34 0.17 0.23 0.22 0.09 0.07 0.10 0.10
Crude oil 0.10 0.46 0.21 0.15 0.23 0.16 0.10 0.07 0.11 0.08
Refined oil 0.12 0.66 0.32 0.17 0.19 0.19 0.07 <0.05 0.08 0.07
Refined bleached
deodorised oil <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Refined bleached
deodorised
hydrogenated oil <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Radioactivity in the top 75 mm layer of silt loam soil accounted
for 1.23 mg/kg chlordimeform equivalents after treatment. Seven weeks
later this level had decreased to 0.33 mg/kg and at 13 weeks to 0.20
mg/kg. When the 0-75 mm layer was extracted after seven weeks, 32% of
the radioactivity partitioned into the organic fraction, 20%
partitioned into the polar fraction and 44% remained non-extractable,
showing that the chlordimeform had degraded rapidly. The radioactivity
in the samples taken 13 weeks after treatment was too low to
fractionate. Except for one sample, the level of radioactivity as
chlordimeform equivalents in the 75-150 mm and 150-200 mm layers was
less than 0.01 mg/kg; thus leaching did not occur in this silt loam.
In a later study when radio-labelled chlordimeform was applied at
the rate of 1 kg/ha over cotton plants and soil in field plots,
Fischer and Cassidy (1976b) found that the radioactivity in the 0-75
mm layer of silt loam was 1.24 mg/kg immediately after the first
treatment but only 0.21 mg/kg after the final treatment and 0.20 mg/kg
at harvest of the mature cotton. This decrease is due to (1) less
drainage from the leaves onto the soil at the later sprayings because
of the increased cover with plant growth, (2) volatility of the
chlordimeform and (3) soil metabolism. Radioactivity was less than
0.01 mg/kg in the 75-150 and 150-225 mm layers at all-sampling dates.
These data show that metabolism of 14C-chlordimeform in silt-loam is
rapid and that the metabolites are not leached.
Analysis of the 0-75 mm layer of soil taken at harvest showed
that 91.0% of the radioactivity could be converted to
4-chloro-o-toluidine. Therefore, although 57% of the soil
metabolites were polar or non-extractable, they contained this moiety.
Following this study Fischer and Cassidy (1976b) planted soybeans
at a rotation crop 38 weeks after the original treatment with
chlordimeform at 1 kg/ha. At maturity the soybeans were found to
contain radioactivity equivalent to 0.01 mg/kg chlordimeform in the
stalks and less than 0.01 mg/kg in the beans themselves.
Wheat planted in the plots was examined for radioactivity at
maturity. The radioactivity was equivalent to 0.16 mg/kg in the straw
and 0.03 mg/kg in the grain.
METHODS OF RESIDUE ANALYSIS
Although methods have been developed for the determination of
chlordimeform alone, most of the residue data have been based on the
determination of chlordimeform and its metabolites as
4-chloro-o-toluidine.
Geissbuhler et al. (1971) published the method used by Ciba-Geigy
Ltd. and Schering A.G. for the development of chlordimeform
pesticides. This method has been utilized with and without
modification for much of the work reported in this monograph. In this
method chlordimeform and its potential metabolites and/or conjugates
are hydrolyzed to 4-chloro-o-toluidine in the presence of plant and
soil material by successive treatments with acetic acid and sodium
hydroxide. The aromatic amine is steam distilled and extracted into
iso-octane. Diazotization and coupling of 4-chloro-o-toluidine with
N-ethyl-1-naphthylamine yields a stable purple dye, which, after
column chromatography on cellulose, is measured colorimetrically. To
verify the identity of chlordimeform, the azo-dye is separated and
visualized on a cellulose thin-layer plate. Alternatively,
4-chloro-o-toluidine is diazotized and iodinated. The iodine
derivative is determined by electron capture gas chromatography. A
further potential metabolite of chlordimeform
2,2-dimethyl-4-4-dichloroazobenzene, is reduced to
4-chloro-o-toluidine in the presence of zinc and acid 1,4-dioxane
and likewise determined by gas chromatography. Recoveries in various
plant and soil materials have been observed to vary between 75 and
100% for all three quantitative methods described. The limit of
determination is 0.05 mg/kg.
APPRAISAL
Chlordimeform products which had been widely used for many years
as insecticides/acaricides on a large variety of crops and on
livestock, were withdrawn by the manufacturers in 1976. Additional
studies have since been carried out and new proposals have been made
for commercial use on cotton crops under restricted conditions.
Information on those proposed uses in various countries and data on
the nature, level and fate of residues, and including the fate of
residues in cottonseed products used in the rations of dairy cattle
and poultry was also examined.
The influence of rate of application pre-harvest interval and
number of applications were investigated. At the maximum application
rate of 1 kg/ha the residue rarely exceeded 2 mg/kg in cottonseed,
seed meal or crude oil. Solvent-extracted oil contained significantly
lower residues than screwpress-extracted oil. Treatment of crude oil
with caustic sodal did not reduce residues markedly but refining
procedures such as bleaching and deodorization as normally undertaken
in commercial practice reduced the levels to below the analytical
detection limits (< 0.05 mg/kg).
Eggs from hens fed for 28 days on rations containing up to 1.5
ppm contained no detectable residues. Meat, fat and liver from hens
slaughtered at various intervals throughout the study was analysed.
Although nothing was found in any of the meat, small residues were
detected in some samples of fat and liver. Cows fed for up to 42 days
on rations containing excessive levels of chlordimeform (up to 240
ppm) showed no residues (< 0.3 mg/kg) in milk, fat or meat at any
stage during the feeding; but residues in some animals fed at the
highest rates, ranged up to 0.6 mg/kg in liver and 0.1 mg/kg in
kidney. Since there appears to be no likelihood of livestock rations
containing more than 0.5 mg/kg of residue however, detectable residues
are unlikely to occur in foods of animal origin.
The analytical methods used determined chlordimeform, the
intermediate metabolite N-formyl-4-chloro-o-toluidine and the final
metabolite 4-chloro-o-toluidine together as 4-chloro-o-toluidine. No
attempt was made to quantify the amounts of each present and the limit
of determination was 0.05 mg/kg.
Studies of the metabolism of chlordimeform in cotton plants and
goats showed that metabolism in plants and animals proceeds through
sequential hydrolysis of terminal groups to 4-chloro-o-toluidine. In
cotton metabolism proceeds to the formation of polar metabolites which
apparently conjugate to natural products. Sprays applied after the
bolls open would partly avoid translocation of residues as metabolites
from the treated leaves to seed but would result in the retention of
significant residues of the parent compound in fibre and seed. In a
study involving the feeding of cottonseed containing biosynthesised
radioactive metabolites to a goat, no residues were deposited in the
animal tissue (limit of determination 0.002 mg/kg).
In the light of the new data examined, the Meeting decided that
there was no need to amend the temporary MRL formerly proposed for
cottonseed and crude cottonseed oil; but unless direct application to
cattle were to be re-installed, the MRL for foods of animal origin
should be based on the data reflecting the feeding of cottonseed
hulls, cottonseed meal and similar products. As chlordimeform is no
longer used on crops other than cotton, the recommendations for
temporary MRLs previously made for crops other than cotton should be
held in abeyance until such time as the uses on those crops should be
reinstated.
RECOMMENDATIONS
The following new or amended recommendations for temporary MRLs
replace all previous recommendations.
Commodity Temporary MRL mg/kg
Cottonseed 2
Cottonseed (crude) 2
Cottonseed oil (edible) ) No residues are to
) occur at the current
Meat of cattle ) limit of detection
) (0.05 mg/kg)
Meat of pigs )
Meat of poultry ) " " " "
Meat of sheep )
Milk )
Milk products )
FURTHER WORK OR INFORMATION
Required (by 1979)
1. Full submission of all toxicological data.
2. Continued epidemiology data on people exposed to chlordimeform.
3. Reports of continued observation of the possible occurrence of
haemorrhagic cystitis.
REFERENCES
Anonymous Chlordimeform HC1 Life Time Feeding Study in Rats - One
(1978a) Year Interim Report. Unpublished report from
CIBA-GEIGTY, Ltd., Basal, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Anonymous N-Formyl-4-chloro-o-toluidine Life Time Feeding Study in
(1978b) Rats One Year Interim Report. Unpublished
report from CIBA-GEIGY, Ltd., Basel,
Switzerland submitted by CIBA-GEIGY, Ltd. to
the WHO.
Anonymous 4-chloro-o-toluidine HC1 Life Time Feeding Study in Rats
(1978c) - One Year Interim Report. Unpublished report
from CIBA-GEIGY, Ltd., Basel, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Anonymous Bioassay of 4-chloro-o-toluidine Hydrochloride for
(1978d) Possible Carcinogenicity. NCI Carcinogenic
Programme Preliminary Draft Report [ NIH ]
79-1721).
Arni, P. and D. Muller Salmonella/Mammalian-Microsome Mutagenicity
(1976a) Test with C-8513 Chlordimeform HC1.
Unpublished report from CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Arni, P. and D. Muller Salmonella/Mammalian-Microsome Mutagenicity
(1976b) Test with CGA 72651
(N-formyl-4-chloro-o-toluidine).
Unpublished report from CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Arni, P. and D. Muller Salmonella/Mammalian Microsome Mutagenicity
(1976c) Test with CGA 72647 (4-chloro-o-toluidine
HC1). Unpublished report from CIBA-GEIGY,
Ltd., Basil, Switzerland submitted by
CIBA-GEIGY, Ltd. to the WHO.
Benezet, H.J. and C.O. Knowles Inhibition of Rat Brain
(1976) Monoamine Oxidase by Formamidine and Related
Compounds. Neuropharmacol. 15:369.
Benezet, H.J., K.M. Chang and C.O. Knowles Formamidine Pesticides-
(1978) Metabolic Aspects of Neurotoxicity in
Pesticide and Venom Neural Toxicity. Ed. D.L.
Shankland, R.M. Hollingworth and J. Smyth,
Jr. Plenum Press, N.Y. pg. 189-206.
Bull, D.L., Environmental Entomology 2, 869.
(1973)
Burkhard, N. Residues in milk, meat, liver and fat of a Swiss cow
(1971a) washed with a 0.5% solution of
chlordimeform. Report RVA 14/71, Agrochemical
Division, CIBA-GEIGY, Ltd., Basel,
Switzerland. 1 February 1971.
Chinn, C., W.R. Pfister and G.K.W. Yim Local Anesthetic-Like Actions
(1976) of the Pesticide Chlordimeform. Fed. Proc.
35:729.
Ciba-Geigy Chlorphenamidime residues in eggs and poultry tissues.
(1971) Research Report C79910, CIBA-GEIGY, Corp.
Greensboro N.C.
Ciba-Geigy Method for determination of chlordimeform residues -
(1976) Method REM 4/76.
Ciba-Geigy Submission of data for the reevaluation of chlordimeform by
(1978) the 1978 FAO/WHO Joint Meeting on Pesticide
Residues, CIBA-GEIGY, Ltd., Basel and
Schering AG, Berlin. July 24, 1978.
Ciba-Geigy Residues resulting from chlordimeform application to
(1978a) cotton. Report by H.B. Camp and B.G. Tweedy
No ABR-78004 (4 January 1978).
Ehrhardt, D.A. and Knowles, C.O. J. Econ. Entomol. 63, 1306.
(1970)
Ezumi, K. and H. Nakao Effects of 4-chloro-o-toluidine in Oral
(1974a) Prolonged Administration to Rat for 94 (Male)
and 104 (Female) Weeks. Unpublished report
from Nihon Schering Inc., Osaka, Japan
submitted by CIBA-GEIGY, Ltd. to the WHO.
Ezumi, K. and H. Nakao Effects of 4-chloro-o-toluidine in Oral
(1974b) Prolonged Administration to Mice for 80 Weeks
Unpublished report from Nihon Schering, Inc.,
Osaka, Japan submitted by CIBA-GEIGY, Ltd. to
the WHO.
FAO/WHO 1971 Evaluations of some pesticide residues in food.
(1972b) AGP:1971/M/9/1; WHO Pesticide Residues Series
No 1.
FAO/WHO 1975 Evaluations of some pesticide residues in food. AGP:
(1976b) 1975/M/13; WHO Pesticide Residue Series No 5.
FAO/WHO Pesticide residues in food. Report of the 1976 Joint Meeting,
(1977a) of the FAO Panel Experts on Pesticide.
Residues and the Environment and the WHO
Expert Group on Pesticide Residues. FAO Food
and Nutrition Series, No 9; FAO Plant
Production and Protection Series, No 8; WHO
Technical Report Series, No 612.
FAO/WHO Pesticide residues in food - 1977. Report of the FAO Panel of
(1978a) Experts on Pesticide Residues and Environment
and the WHO Expert Committee on Pesticide
Residues. FAO Plant Production and Protection
Paper 10 Rev. par 4.13.
Fischer, W.C. and Cassidy, J.E. Uptake of radio-labelled (1976a)
chlordimeform and its soil metabolites in
cotton in a field prepared for rotation
crops. Report No GAAC-76014 CIBA-GEIGY
Corporation, Greensboro, N.C. March 15, 1976.
Fischer W.C. and Cassidy, J.E. Uptake and characterization of
(1976b) the metabolites of radio-labelled
chlordimeform in spray treated cotton and
field soil. Report of Biochemistry Department
CIBA-GEIGY Corporation Report ABR - 76056
July 27, 1976.
Fischer W.C. and Cassidy, J.E. Identification of the metabolites
(1976c) of chlordimeform in mature leaves of spray
treated field cotton. Report No ABR - 76073
from Biochemistry Department, CIBA-GEIGY,
Corporation, Greensboro, N.C.
Fritz, H. Reproduction study on C8514 Technical Rabbit - Seg II. (Test
(1971) for teratogenic and embryotoxic effects).
Unpublished report from Toxicology/ Pathology
CIBA-GEIGY, Ltd., Basely Switzerland,
submitted by CIBA-GEIGY Ltd to the WHO.
Fritz, H. Reproduction Study on C8514 Technical Rat-Seg II (Test for
(1975) Teratogenic or Embroyotic Effects).
Unpublished report from Toxicology/Pathology
CIBA-GEIGY, Ltd., Basely Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Geisebuhler, H., Kossmann, K., Baunck, I. and Boyd, V.F.
(1971) Determination of total residues of
chlorphenamidine in plant and soil material
by colorimetry and thin layer and electron
capture gas-chromatography. Agric. Food Chem.
19 (2):365.
Gross Metabolism of chlordimeform in cotton. Project Report 19/77 from
(1977) Biochemistry Department, CIBA-GEIGY, Ltd.,
Basel, Switzerland (April 1977).
Hollingworth R.M. Chemistry Biological Activity and Uses of
(1976) Formamidine Pesticides. Environ. Health
Perspect. 14: 57-69.
Honeycutt, R.C. and Cassidy, J.E. Extraction and characterization
(1977) of cottonseed metabolite from cotton
injected with o 14C-chlordimeform. Report No
ABR - 77087 from Biochemistry Department,
CIBA-GEIGY, Corporation, Greensboro, N.C.
Sept. 14, 1977.
Hurni, H. and H. Ohder Report on the Mutagenic Effect of Technical
(1970) C-8514-N. Unpublished report from Tierfarm AG
Biomedical Research, Sisseln, Switzerland
submitted by CIBA-GEIGY, Ltd., to the WHO.
Jenny, N.A. Chlorphenamidine residues in eggs and poultry
(1971) tissues/Analysis of tissue and egg samples.
Knowles, C.O. Chemistry and Toxicology of Quinoxaline Organotin
(1976) Organofluorine and Formamidine Acaracides.
Env. Health Perspect. 14:93-102.
Kalyonova, F.P., Z. Zapryanav and A.I. Baynova Influence of the
(1978) Insecticide Chlordimeform of the Activity of
the Monoamine Oxidase (MAO) of Albino Rats.
Comptes Rendus de l'Academi Bulgare des
Sciences 31 (4): 491-493.
Konopa, E.A. and H. Heymann Salmonella/Microsome Mutagenicity
(1977) Test with Chlordimeform. Unpublished report
from Pharmaceuticals Division, CIBA-GEIGY,
Corporation, Surnmity N.J. submitted by
CIBA-GEIGY, Ltd., to the WHO.
Konopka. E.A. and H. Heymann Salmonella/Microsome Mutagenicity
(1977b) Test with Chlordimeform. Unpublished report
from Pharmaceuticals Division, CIBA-GEIGY
Corporation, Summit, N.J. submitted by
CIBA-GEIGY, Ltd. to the WHO.
Langauer, M. and D. Muller Nucleus Anomaly Test on Somatic
(1977) Interphase Nuclei-C-8513- Chinese Hamster.
Unpublished report from Toxicology/Pathology,
CIBA-GEIGY, Ltd., Basel, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Lund, A.E., G.K.W. Yim and D.L. Shankland The Cardiovascular
(1978) Toxicity of Chlordimeform, a Local
Anesthetic-Like Action in Pesticide and Venom
Neurotoxicity. Ed. D.L. Shankland, R.M.
Hollingworth and T. Smyth, Jr. Plenum Press,
N.Y., pgs. 171-177.
Maitre L., A. Felner, P. Waldmeier and W. Kehr Moccasins Oxidase
(1978) Inhibition in Brain and Liver of Rats Treated
with Chlordimeform. J. Agr. Fd Chem. 26
(2): 442-446.
Matsumura, F. and R.W. Beeman Biochemical and Physiological
(1976) Effects of Chlordimeform. Environ. Health
Perspect. 14: 71-82.
Morton Method for the determination of chlordimeform. Method 333/7
(1968) (7/12/68)
Murakami, J. and J. Fukami Effects of Chlophenamidine and Its
(1974) Metabolites on HeLa Cells. Bull. Env.
Contam. and Tox. 11: 184-188.
Nesnow, S. and C. Heidelberger The Effect of Modifiers of
Microsomal Enzymes on Chemical Oncogenesis in
Cultures of C3H Mouse Cell Lines. Cancer
Res. 36:1801-1808.
Nesnow S. Unpublished bioassay submitted to the WHO.
(1978)
Nor-Am Method of analysis for chlordimeform residues - Research Method
(1969) 333/44. 5/6/69.
Sachsse, K., P. Suter, F. Zak and R. Hess Lifespan Feeding Study in
(1978b) Mice with 4-chloro-o-toludine. Unpublished
report from Toxicology, CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Schering Method of analysis of chlordimeform residues. Method 36 268/8
(1973) (24/4/73).
Schering Method of determination of chlordimeform and its degradation
(1975) products. Method 36268/9 17/11/75.
Sen Gupta, A.K. and Knowles, C.O. J. Agric. Food Chem. 17, 595.
(1969)
Suter, P., H. Luetkemeier, K. Sachsse and F. Zak 60-day Pending
(1976a) Study in the Rat with chloro-o-toluidine
HC1. Unpublished report from
Toxicology/Pathology, CIBA-GEIGY, Ltd.,
Basel, Switzerland, submitted by CIBA-GEIGY,
Ltd. to the WHO.
Suter, P., H. Luetkemeier, K. Sachsse and F. Zak 60-day Pending
(1976b) Study in Mice with 4, chloro-o-toluidine
HC1. Unpublished report from
Toxicology/Pathology, CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Suter, P., F. Zak, K. Sachsse and R. Hess Lifespan Feeding Study
(1979) in Mice with Chlordimeform HC1. Unpublished
report from Toxicology/Pathology,
CIBA-GEIGY, Ltd., Basel, Switzerland,
submitted by CIBA-GEIGY, Ltd. to the WHO.
Wang, C.M., T. Narahashi and J. Fukami Mechanism of Neuromuscular
(1975) Block by Chlordimeform. Pest. Biochem.
Physiol. 5: 119-125.
Watanabe, H., S. Tsuda and J. Fukami. Effects of Chlordimeform
(1975) on Rectusabdominum Muscle in Frog. Pest.
Biochem. Physiol. 5: 150-154.
 After hydrolysis and analysis by TLC and GLC, 45% of the
water-soluble radioactivity was found as 4-chloro-o-toluidine.
Another 42% of the radioactivity remaining in the aqueous hydrolysate
was extractable with n-butanol at pH 1 but was not further
characterized.
The unextractable radioactivity in the treated leaves was low at
all time intervals. On the other hand, when the untreated leaves,
stems and green bolls at the 8 week sampling interval were extracted
exhaustively with acetonitrile/hexane (7:3), then 80% aqueous methanol
and finally hot methanol only 15% to 23% of the radioactivity of each
plant part could be extracted. No unchanged chlordimeform was present
in the untreated plant parts. When the plant material was hydrolysed
45%, 17% and 10.5% of the radioactivity of the untreated leaves, stems
and bolls, respectively, was extractable by iso-octane.
Extraction of cottonseeds, taken from mature bolls 11 weeks after
the first application, showed 8% of the radioactivity in the
acetonitrile/hexane phase and an additional 7% of the radioactivity in
the aqueous methanol. Hydrolysis of the seeds showed that only 4.5% of
the radioactivity could be converted to 4-chloro-o-toluidine. When
the nonextractable radioactivity of the weeds was further fractionated
by chemical means into pectin, lignin, hemicellulose and cellulose
fractions, the main part of the radioactivity (78%) was found to be
associated with the cellulose fraction and 14%, 2% and 6% in the
hemicellulose, lignin and pectin fractional respectively.
Gross (1977) concluded that when 14C-chlordimeform was topically
applied to cotton leaves, there was little movement of the
radioactivity and none of chlordimeform itself into the untreated
plant parts. The translocated radioactivity consisted exclusively of
polar, mainly non-extractable substances, suggesting a possible
incorporation of radioactivity into natural products.
Fischer and Cassidy (1976a) reported an experiment in which the
soil of a cotton field plot was treated with 14C-chlordimeform when
the cotton was ten weeks old. The rate of application to the soil
surface was equivalent to 1.0 kg a.i./ha.
Radioactivity in the cotton plants seven weeks after treatment
was equivalent to 0.13 mg/kg chlordimeform. Biphasic extraction showed
42.4% in the organic fraction, 34.9% in the polar fraction and 25.8%
was non-extractable. Thirteen weeks after treatment the mature cotton
contained 0.09 mg/kg in the leaves. The radioactive content of these
mature samples was too low to fractionate for balance determinations.
Treatment of soil surrounding a growing cotton plant showed that
14C-chlordimeform and its soil metabolites are taken up, in small
quantities by cotton, especially the seeds and fibres.
In another experiment Fischer and Cassidy (1976b) treated a
cotton field plot over-the-top with formulated 14C-chlordimeform at a
rate of 1.0 kg/ha when the plants were 10, 12 and 14 weeks old.
Radioactivity in the cotton plants immediately after treatment was
equivalent to 2.44 mg/kg chlordimeform. At harvest the radioactivity
calculated as 14C-chlordimeform was 12.91 mg/kg in the leaves, 0.99
mg/kg in stalks, 0.03 mg/kg in the fibre and 0.26 mg/kg in the seed
with 0.07 mg/kg in the oil and 0.19 mg/kg in the meal. The balance
could be done only on the leaves and stalks at maturity because of the
low radioactivity in the other plant parts. From the leaves and
stalks respectively, the organic fractions contained 57.6% and 72.9%,
the polar fractions 18.9% and 11.4% and the non-extractable fractions
18.8% and 17.8%. Parent 14C-chlordimeform accounted for 31.0% and
45.2% in leaves and stalks respectively. The radioactivity
co-chromatographing with other known standards was minor, accounting
for no more than 8% of the total in either leaves or stalks. This
indicates that there is a large percentage of polar conjugates. The
data show that, although leaf radioactivity is high, there is little
translocation of 14C-chlordimeform metabolites to the seed or fibre.
Application of the method for determining chlordimeform and its
metabolites to cotton leaves showed that 88.9% of the radioactivity in
the leaves but only 29.9% of that in the seeds could be accounted for
as 4-chloro-o-toluidine. This indicates extensive further metabolism
of the translocated metabolites of 14C-chlordimeform.
Fischer and Cassidy (1976c) identified the radioactive
metabolites in leaves after phenyl-14C-chlordimeform was sprayed
over-the-top according to standard agricultural practice. At mature
harvest the total radioactivity in the leaves consisted of parent
chlordimeform 60.3%, demethyl-chlordimeform 4.1%,
4-chloro-o-toluidine 7.6% and its N-formyl derivative 7.0%.
Identification was based on partitioning characteristics and
cochromatography with standards. The metabolism of chlordimeform by
cotton leaves involves, therefore, sequential hydrolysis of terminal
groups with 4-chloro-o-toluidine as the final non-polar product.
These data show that parent chlordimeform will account for the main
part of the material containing the chlorotoluidine moiety in mature
cotton foliage.
Cotton grown in a greenhouse was injected with ring-labelled
chlordimeform in order to characterize the radioactive metabolites,
especially those not extractable with methanol and water, and to
elucidate the metabolic pathway of chlordimeform in cottonseed
(Honeycutt and Cassidy, 1977). The injections were made into the stem
69, 73 and 81 days after planting, the plants were grown to maturity
and the cotton harvested 157 days from planting. The cottonseed
contained radioactivity equivalent to 0.65 mg/kg of chlordimeform at
maturity. Polar, extractable material accounted for 58% of the total
radioactivity in the seed and consisted of 5-chloro-2-formamidobenzoic
acid (N-formyl-5-chloroanthranilic acid) (3%) and eight unknown polar
metabolites, none of which made up more than 8% of the total.
Forty per cent of the radioactivity in the cottonseed was not
extractable and was further fractionated into base-soluble (30%) and
base-insoluble (23%) radioactivity. The base-soluble radioactivity was
characterized as low molecular weight degradation products "A" (1.2%)
and "B" (5.7%), as well as high molecular weight (> 1,000) components
(20%) physicochemically similar to lignin. The base-insoluble
radioactivity was characterized mainly as radioactive conjugates
(17.6%) covalently linked to natural products physicochemically
similar to cellulose. Three percent of the total radioactivity in the
cottonseed could not be solubilized either by strong acid or base
hydrolysis. Application of the total hydrolysis method to both the
polar extractable and the non-extractable radioactivity showed that a
total of only 19.8% of the radioactivity in the cottonseed could be
converted to 4-chloro-o-toluidine (5-CAT).
The above data confirm that the metabolism of chlordimeform in
cottonseed is extensive, processing through 5-CAT, its N-formyl
derivative and N-formyl-5-chloroanthranilic acid to hydroxylated
metabolites which become conjugated to natural products such as
lignin, pectin, cellulose or hemicellulose. This explains why attempts
to define a moiety common to chlordimeform and its metabolites in
cottonseed have not been successful.
The authors have deduced the scheme set out in Figure 2 to
explain the metabolism of chlordimeform in cotton. They contend that
4-chloro-o-toluidine is incorporated into lignin according to the
schemes outlined in Figure 2.
In animals
Burkard (1971a) studied the fate following the application of a
0.5% solution of chlordimeform to the hindquarters of cows. The
treatment was applied three times at 7-day intervals and the results
were determined in milk collected daily until 33 days after the last
treatment. At the end of that period the cows were slaughtered and
samples of meat, liver and fat were analysed.
Generally the total residue in milk was below the limit of
determination (0.03 mg/kg) except on the day following treatment when
it rose to a maximum of 1.0 mg/kg. Thereafter the concentration
declined rapidly reaching the limit of determination on the third day.
The meat and fat contained no detectable residues but liver contained
from 0.3 to 0.6 mg/kg 33 days after the last treatment.
In a further study Burkard (1971b) fed Swiss cows daily with 1 kg
of a concentrate containing from 40 to 240 ppm chlordimeform for
periods up to 42 days. Residues were determined in samples of milk
collected throughout the trial and in samples of meat, fat, liver and
kidney obtained from cows slaughtered after 7, 14, 21 and 42 days
continuous administration.
Total residues of chlordimeform and its metabolites in all milk,
meat and fat samples analysed were below the limit of determination
(0.03 mg/kg). However, the residues in the kidney and liver samples
(mean values of duplicate determinations) were as shown in Table 5.
After hydrolysis and analysis by TLC and GLC, 45% of the
water-soluble radioactivity was found as 4-chloro-o-toluidine.
Another 42% of the radioactivity remaining in the aqueous hydrolysate
was extractable with n-butanol at pH 1 but was not further
characterized.
The unextractable radioactivity in the treated leaves was low at
all time intervals. On the other hand, when the untreated leaves,
stems and green bolls at the 8 week sampling interval were extracted
exhaustively with acetonitrile/hexane (7:3), then 80% aqueous methanol
and finally hot methanol only 15% to 23% of the radioactivity of each
plant part could be extracted. No unchanged chlordimeform was present
in the untreated plant parts. When the plant material was hydrolysed
45%, 17% and 10.5% of the radioactivity of the untreated leaves, stems
and bolls, respectively, was extractable by iso-octane.
Extraction of cottonseeds, taken from mature bolls 11 weeks after
the first application, showed 8% of the radioactivity in the
acetonitrile/hexane phase and an additional 7% of the radioactivity in
the aqueous methanol. Hydrolysis of the seeds showed that only 4.5% of
the radioactivity could be converted to 4-chloro-o-toluidine. When
the nonextractable radioactivity of the weeds was further fractionated
by chemical means into pectin, lignin, hemicellulose and cellulose
fractions, the main part of the radioactivity (78%) was found to be
associated with the cellulose fraction and 14%, 2% and 6% in the
hemicellulose, lignin and pectin fractional respectively.
Gross (1977) concluded that when 14C-chlordimeform was topically
applied to cotton leaves, there was little movement of the
radioactivity and none of chlordimeform itself into the untreated
plant parts. The translocated radioactivity consisted exclusively of
polar, mainly non-extractable substances, suggesting a possible
incorporation of radioactivity into natural products.
Fischer and Cassidy (1976a) reported an experiment in which the
soil of a cotton field plot was treated with 14C-chlordimeform when
the cotton was ten weeks old. The rate of application to the soil
surface was equivalent to 1.0 kg a.i./ha.
Radioactivity in the cotton plants seven weeks after treatment
was equivalent to 0.13 mg/kg chlordimeform. Biphasic extraction showed
42.4% in the organic fraction, 34.9% in the polar fraction and 25.8%
was non-extractable. Thirteen weeks after treatment the mature cotton
contained 0.09 mg/kg in the leaves. The radioactive content of these
mature samples was too low to fractionate for balance determinations.
Treatment of soil surrounding a growing cotton plant showed that
14C-chlordimeform and its soil metabolites are taken up, in small
quantities by cotton, especially the seeds and fibres.
In another experiment Fischer and Cassidy (1976b) treated a
cotton field plot over-the-top with formulated 14C-chlordimeform at a
rate of 1.0 kg/ha when the plants were 10, 12 and 14 weeks old.
Radioactivity in the cotton plants immediately after treatment was
equivalent to 2.44 mg/kg chlordimeform. At harvest the radioactivity
calculated as 14C-chlordimeform was 12.91 mg/kg in the leaves, 0.99
mg/kg in stalks, 0.03 mg/kg in the fibre and 0.26 mg/kg in the seed
with 0.07 mg/kg in the oil and 0.19 mg/kg in the meal. The balance
could be done only on the leaves and stalks at maturity because of the
low radioactivity in the other plant parts. From the leaves and
stalks respectively, the organic fractions contained 57.6% and 72.9%,
the polar fractions 18.9% and 11.4% and the non-extractable fractions
18.8% and 17.8%. Parent 14C-chlordimeform accounted for 31.0% and
45.2% in leaves and stalks respectively. The radioactivity
co-chromatographing with other known standards was minor, accounting
for no more than 8% of the total in either leaves or stalks. This
indicates that there is a large percentage of polar conjugates. The
data show that, although leaf radioactivity is high, there is little
translocation of 14C-chlordimeform metabolites to the seed or fibre.
Application of the method for determining chlordimeform and its
metabolites to cotton leaves showed that 88.9% of the radioactivity in
the leaves but only 29.9% of that in the seeds could be accounted for
as 4-chloro-o-toluidine. This indicates extensive further metabolism
of the translocated metabolites of 14C-chlordimeform.
Fischer and Cassidy (1976c) identified the radioactive
metabolites in leaves after phenyl-14C-chlordimeform was sprayed
over-the-top according to standard agricultural practice. At mature
harvest the total radioactivity in the leaves consisted of parent
chlordimeform 60.3%, demethyl-chlordimeform 4.1%,
4-chloro-o-toluidine 7.6% and its N-formyl derivative 7.0%.
Identification was based on partitioning characteristics and
cochromatography with standards. The metabolism of chlordimeform by
cotton leaves involves, therefore, sequential hydrolysis of terminal
groups with 4-chloro-o-toluidine as the final non-polar product.
These data show that parent chlordimeform will account for the main
part of the material containing the chlorotoluidine moiety in mature
cotton foliage.
Cotton grown in a greenhouse was injected with ring-labelled
chlordimeform in order to characterize the radioactive metabolites,
especially those not extractable with methanol and water, and to
elucidate the metabolic pathway of chlordimeform in cottonseed
(Honeycutt and Cassidy, 1977). The injections were made into the stem
69, 73 and 81 days after planting, the plants were grown to maturity
and the cotton harvested 157 days from planting. The cottonseed
contained radioactivity equivalent to 0.65 mg/kg of chlordimeform at
maturity. Polar, extractable material accounted for 58% of the total
radioactivity in the seed and consisted of 5-chloro-2-formamidobenzoic
acid (N-formyl-5-chloroanthranilic acid) (3%) and eight unknown polar
metabolites, none of which made up more than 8% of the total.
Forty per cent of the radioactivity in the cottonseed was not
extractable and was further fractionated into base-soluble (30%) and
base-insoluble (23%) radioactivity. The base-soluble radioactivity was
characterized as low molecular weight degradation products "A" (1.2%)
and "B" (5.7%), as well as high molecular weight (> 1,000) components
(20%) physicochemically similar to lignin. The base-insoluble
radioactivity was characterized mainly as radioactive conjugates
(17.6%) covalently linked to natural products physicochemically
similar to cellulose. Three percent of the total radioactivity in the
cottonseed could not be solubilized either by strong acid or base
hydrolysis. Application of the total hydrolysis method to both the
polar extractable and the non-extractable radioactivity showed that a
total of only 19.8% of the radioactivity in the cottonseed could be
converted to 4-chloro-o-toluidine (5-CAT).
The above data confirm that the metabolism of chlordimeform in
cottonseed is extensive, processing through 5-CAT, its N-formyl
derivative and N-formyl-5-chloroanthranilic acid to hydroxylated
metabolites which become conjugated to natural products such as
lignin, pectin, cellulose or hemicellulose. This explains why attempts
to define a moiety common to chlordimeform and its metabolites in
cottonseed have not been successful.
The authors have deduced the scheme set out in Figure 2 to
explain the metabolism of chlordimeform in cotton. They contend that
4-chloro-o-toluidine is incorporated into lignin according to the
schemes outlined in Figure 2.
In animals
Burkard (1971a) studied the fate following the application of a
0.5% solution of chlordimeform to the hindquarters of cows. The
treatment was applied three times at 7-day intervals and the results
were determined in milk collected daily until 33 days after the last
treatment. At the end of that period the cows were slaughtered and
samples of meat, liver and fat were analysed.
Generally the total residue in milk was below the limit of
determination (0.03 mg/kg) except on the day following treatment when
it rose to a maximum of 1.0 mg/kg. Thereafter the concentration
declined rapidly reaching the limit of determination on the third day.
The meat and fat contained no detectable residues but liver contained
from 0.3 to 0.6 mg/kg 33 days after the last treatment.
In a further study Burkard (1971b) fed Swiss cows daily with 1 kg
of a concentrate containing from 40 to 240 ppm chlordimeform for
periods up to 42 days. Residues were determined in samples of milk
collected throughout the trial and in samples of meat, fat, liver and
kidney obtained from cows slaughtered after 7, 14, 21 and 42 days
continuous administration.
Total residues of chlordimeform and its metabolites in all milk,
meat and fat samples analysed were below the limit of determination
(0.03 mg/kg). However, the residues in the kidney and liver samples
(mean values of duplicate determinations) were as shown in Table 5.
 TABLE 5. Residues of chlordimeform and its metabolites in cows.
Chlordimeform and metabolites, mg/kg
Duration of
feeding (days)
In concentrate In liver In kidney
21 40 0.09 < 0.03
21 120 0.38 0.07
7 240 0.45 0.05
14 240 0.58 0.13
21 240 0.50 0.13
42 240 0.44 0.09
Honeycutt and Cassidy (1976) fed a lactating goat with cottonseed
containing biosynthesized metabolites of phenyl-14C-chlordimeform at
a level equivalent to 0.032 ppm chlordimeform daily for 4 consecutive
days. The total recovery of radioactivity was 97.1%. It was primarily
distributed between the urine (21.3%), which reached a plateau by the
2nd day, the faeces (35.9%), which reached a plateau by the third day,
and the rumenintestinal contents (38.7%). There was no deposition of
radioactivity in tissues (< 0.002 mg/kg), even though uptake of the
biosynthesized metabolites occurred.
The milk contained 0.9% of the total dose, its radioactivity
plateaued by the second day (0.0004 ± 0.0001 mg/kg chlordimeform
equivalents). The ratio of the concentration in milk to that in the
feed was 0.013. Blood contained < 0.2% of the total dose.
Comparison of the 14C-extractables of the original cottonseed
with those in the faeces, urine and rumen-intestinal contents,
suggests that the goat metabolized the biosynthesized metabolites in
cottonseed. However, metabolism to natural products was not
significant since expired 14CO2 accounted for only 0.3% of the total
dose.
Ciba-Geigy Corp. (1971) conducted experiments in which white
Leghorn hens in active egg production were fed a commercial egg laying
mash to which chlordimeform was added at rates equivalent to 0, 0.25,
0.75 and 1.50 mg/kg of feed. Groups were sacrificed after 7, 14, 21
and 28 days feeding and were subjected to post-mortem examination and
assessment of egg production, body-weight change and food consumption.
There were no significant differences between treated and control
binds in any of the features examined.
Residues in eggs and tissues (breast, fat and liver) were
determined by Jenny (1971). Eggs collected at -3 (pre-treatment), -1
(pre-treatment), 3, 7, 14, 21 and 28 days from each group contained no
detectable amounts of chlordimeform and/or its metabolites containing
the 4-chloro-o-toluidine moiety.
The breasts of birds sacrificed at 7, 14, 21 and 28 days of
feeding treated feed as well as 49 days after returning to untreated
feed contained no significant residues. Average residues of 0.11, 0.09
and 0.22 mg/kg were detected in fat at the feeding levels of 0.25,
0.75 and 1.50 ppm respectively, at the 21-day interval only. No
residues were detected in livers from chickens fed 0.25 ppm
chlordimeform at any of the intervals tested and residue of 0.09 mg/kg
was detected in liver from chickens fed 0.75 ppm only at the 21-day
interval. Residues of 0.20, 0.17, and 0.10 mg/kg were detected in
livers from the 1.5 ppm level at intervals of 7, 14, 21 and 28 days.
Nine days after withdrawal from the feed containing 1.5 ppm, no
residues could be detected in any of the tissues.
In processing
The Meeting reviewed many data on the level and fate of residues
during processing. Cottonseed is subjected to well-defined sequential
processes, details of which were available (Ciba-Geigy, 1978). The
results of some typical trials are set out in Table 4 and 6. The
distribution is independent of the pattern of application as is
apparent from Table 6. Although the major fraction of the residue
remains in the hulls and the meal from which the oil has been removed
there is still a significant concentration in the crude oil. Treatment
of crude cottonseed oil with caustic soda in the refining process does
not reduce the residue concentration significantly but the additional
refining procedures including bleaching, hydrogenating and deodorizing
reduce the residue in the oil to a level below the detection limits of
present analytical methods (< 0.05 mg/kg). Since all the oil used for
human consumption is at least subjected to the bleaching and
deodorizing process, virtually all the chlordimeform will be removed
before the cottonseed oil is consumed.
In soil
In the course of studies on the uptake of chlordimeform and its
soil metabolites from treated soil into cotton Fischer and Cassidy
(1976a) applied radio-labelled chlordimeform to a cotton field plot
thirteen weeks before harvesting the cotton. The rate of application
was equivalent to 1 kg/ha.
TABLE 6. Effect of use pattern on Chlordimeform residues in cottonseed products.
AGA AGA AGA AGA AGA AGA AGA AGA AGA AGA
Trial No. 4397 4408 4409 4449 4450 4502 4981 4981 4981 4981
Location Alab. Ariz. Calif. Georg. Georg. Miss. S.C. S.C. S.C. S.C.
Rate of application 0.125 0.125 0.25 0.125 0.25 0.125 0.125 0.25 0.125 0.25
Formulation EC EC EC EC EC EC EC EC EC EC
No. of applications 7 7 6 11 12 9 12 12 17 17
Pre-harvest interval 28 39 31 22 16 31 21 21 21 21
Cottonseed 0.2 0.82 0.35 0.35 0.35 0.30 0.29 0.20 0.22 0.40
Hulls 0.36 1.8 0.85 0.58 0.55 0.33 0.86 0.50 0.90 0.75
Meal 0.19 1.2 0.45 0.24 0.32 0.45 0.18 0.10 0.17 0.15
Soap stock 0.10 0.84 0.34 0.17 0.23 0.22 0.09 0.07 0.10 0.10
Crude oil 0.10 0.46 0.21 0.15 0.23 0.16 0.10 0.07 0.11 0.08
Refined oil 0.12 0.66 0.32 0.17 0.19 0.19 0.07 <0.05 0.08 0.07
Refined bleached
deodorised oil <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Refined bleached
deodorised
hydrogenated oil <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Radioactivity in the top 75 mm layer of silt loam soil accounted
for 1.23 mg/kg chlordimeform equivalents after treatment. Seven weeks
later this level had decreased to 0.33 mg/kg and at 13 weeks to 0.20
mg/kg. When the 0-75 mm layer was extracted after seven weeks, 32% of
the radioactivity partitioned into the organic fraction, 20%
partitioned into the polar fraction and 44% remained non-extractable,
showing that the chlordimeform had degraded rapidly. The radioactivity
in the samples taken 13 weeks after treatment was too low to
fractionate. Except for one sample, the level of radioactivity as
chlordimeform equivalents in the 75-150 mm and 150-200 mm layers was
less than 0.01 mg/kg; thus leaching did not occur in this silt loam.
In a later study when radio-labelled chlordimeform was applied at
the rate of 1 kg/ha over cotton plants and soil in field plots,
Fischer and Cassidy (1976b) found that the radioactivity in the 0-75
mm layer of silt loam was 1.24 mg/kg immediately after the first
treatment but only 0.21 mg/kg after the final treatment and 0.20 mg/kg
at harvest of the mature cotton. This decrease is due to (1) less
drainage from the leaves onto the soil at the later sprayings because
of the increased cover with plant growth, (2) volatility of the
chlordimeform and (3) soil metabolism. Radioactivity was less than
0.01 mg/kg in the 75-150 and 150-225 mm layers at all-sampling dates.
These data show that metabolism of 14C-chlordimeform in silt-loam is
rapid and that the metabolites are not leached.
Analysis of the 0-75 mm layer of soil taken at harvest showed
that 91.0% of the radioactivity could be converted to
4-chloro-o-toluidine. Therefore, although 57% of the soil
metabolites were polar or non-extractable, they contained this moiety.
Following this study Fischer and Cassidy (1976b) planted soybeans
at a rotation crop 38 weeks after the original treatment with
chlordimeform at 1 kg/ha. At maturity the soybeans were found to
contain radioactivity equivalent to 0.01 mg/kg chlordimeform in the
stalks and less than 0.01 mg/kg in the beans themselves.
Wheat planted in the plots was examined for radioactivity at
maturity. The radioactivity was equivalent to 0.16 mg/kg in the straw
and 0.03 mg/kg in the grain.
METHODS OF RESIDUE ANALYSIS
Although methods have been developed for the determination of
chlordimeform alone, most of the residue data have been based on the
determination of chlordimeform and its metabolites as
4-chloro-o-toluidine.
Geissbuhler et al. (1971) published the method used by Ciba-Geigy
Ltd. and Schering A.G. for the development of chlordimeform
pesticides. This method has been utilized with and without
modification for much of the work reported in this monograph. In this
method chlordimeform and its potential metabolites and/or conjugates
are hydrolyzed to 4-chloro-o-toluidine in the presence of plant and
soil material by successive treatments with acetic acid and sodium
hydroxide. The aromatic amine is steam distilled and extracted into
iso-octane. Diazotization and coupling of 4-chloro-o-toluidine with
N-ethyl-1-naphthylamine yields a stable purple dye, which, after
column chromatography on cellulose, is measured colorimetrically. To
verify the identity of chlordimeform, the azo-dye is separated and
visualized on a cellulose thin-layer plate. Alternatively,
4-chloro-o-toluidine is diazotized and iodinated. The iodine
derivative is determined by electron capture gas chromatography. A
further potential metabolite of chlordimeform
2,2-dimethyl-4-4-dichloroazobenzene, is reduced to
4-chloro-o-toluidine in the presence of zinc and acid 1,4-dioxane
and likewise determined by gas chromatography. Recoveries in various
plant and soil materials have been observed to vary between 75 and
100% for all three quantitative methods described. The limit of
determination is 0.05 mg/kg.
APPRAISAL
Chlordimeform products which had been widely used for many years
as insecticides/acaricides on a large variety of crops and on
livestock, were withdrawn by the manufacturers in 1976. Additional
studies have since been carried out and new proposals have been made
for commercial use on cotton crops under restricted conditions.
Information on those proposed uses in various countries and data on
the nature, level and fate of residues, and including the fate of
residues in cottonseed products used in the rations of dairy cattle
and poultry was also examined.
The influence of rate of application pre-harvest interval and
number of applications were investigated. At the maximum application
rate of 1 kg/ha the residue rarely exceeded 2 mg/kg in cottonseed,
seed meal or crude oil. Solvent-extracted oil contained significantly
lower residues than screwpress-extracted oil. Treatment of crude oil
with caustic sodal did not reduce residues markedly but refining
procedures such as bleaching and deodorization as normally undertaken
in commercial practice reduced the levels to below the analytical
detection limits (< 0.05 mg/kg).
Eggs from hens fed for 28 days on rations containing up to 1.5
ppm contained no detectable residues. Meat, fat and liver from hens
slaughtered at various intervals throughout the study was analysed.
Although nothing was found in any of the meat, small residues were
detected in some samples of fat and liver. Cows fed for up to 42 days
on rations containing excessive levels of chlordimeform (up to 240
ppm) showed no residues (< 0.3 mg/kg) in milk, fat or meat at any
stage during the feeding; but residues in some animals fed at the
highest rates, ranged up to 0.6 mg/kg in liver and 0.1 mg/kg in
kidney. Since there appears to be no likelihood of livestock rations
containing more than 0.5 mg/kg of residue however, detectable residues
are unlikely to occur in foods of animal origin.
The analytical methods used determined chlordimeform, the
intermediate metabolite N-formyl-4-chloro-o-toluidine and the final
metabolite 4-chloro-o-toluidine together as 4-chloro-o-toluidine. No
attempt was made to quantify the amounts of each present and the limit
of determination was 0.05 mg/kg.
Studies of the metabolism of chlordimeform in cotton plants and
goats showed that metabolism in plants and animals proceeds through
sequential hydrolysis of terminal groups to 4-chloro-o-toluidine. In
cotton metabolism proceeds to the formation of polar metabolites which
apparently conjugate to natural products. Sprays applied after the
bolls open would partly avoid translocation of residues as metabolites
from the treated leaves to seed but would result in the retention of
significant residues of the parent compound in fibre and seed. In a
study involving the feeding of cottonseed containing biosynthesised
radioactive metabolites to a goat, no residues were deposited in the
animal tissue (limit of determination 0.002 mg/kg).
In the light of the new data examined, the Meeting decided that
there was no need to amend the temporary MRL formerly proposed for
cottonseed and crude cottonseed oil; but unless direct application to
cattle were to be re-installed, the MRL for foods of animal origin
should be based on the data reflecting the feeding of cottonseed
hulls, cottonseed meal and similar products. As chlordimeform is no
longer used on crops other than cotton, the recommendations for
temporary MRLs previously made for crops other than cotton should be
held in abeyance until such time as the uses on those crops should be
reinstated.
RECOMMENDATIONS
The following new or amended recommendations for temporary MRLs
replace all previous recommendations.
Commodity Temporary MRL mg/kg
Cottonseed 2
Cottonseed (crude) 2
Cottonseed oil (edible) ) No residues are to
) occur at the current
Meat of cattle ) limit of detection
) (0.05 mg/kg)
Meat of pigs )
Meat of poultry ) " " " "
Meat of sheep )
Milk )
Milk products )
FURTHER WORK OR INFORMATION
Required (by 1979)
1. Full submission of all toxicological data.
2. Continued epidemiology data on people exposed to chlordimeform.
3. Reports of continued observation of the possible occurrence of
haemorrhagic cystitis.
REFERENCES
Anonymous Chlordimeform HC1 Life Time Feeding Study in Rats - One
(1978a) Year Interim Report. Unpublished report from
CIBA-GEIGTY, Ltd., Basal, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Anonymous N-Formyl-4-chloro-o-toluidine Life Time Feeding Study in
(1978b) Rats One Year Interim Report. Unpublished
report from CIBA-GEIGY, Ltd., Basel,
Switzerland submitted by CIBA-GEIGY, Ltd. to
the WHO.
Anonymous 4-chloro-o-toluidine HC1 Life Time Feeding Study in Rats
(1978c) - One Year Interim Report. Unpublished report
from CIBA-GEIGY, Ltd., Basel, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Anonymous Bioassay of 4-chloro-o-toluidine Hydrochloride for
(1978d) Possible Carcinogenicity. NCI Carcinogenic
Programme Preliminary Draft Report [ NIH ]
79-1721).
Arni, P. and D. Muller Salmonella/Mammalian-Microsome Mutagenicity
(1976a) Test with C-8513 Chlordimeform HC1.
Unpublished report from CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Arni, P. and D. Muller Salmonella/Mammalian-Microsome Mutagenicity
(1976b) Test with CGA 72651
(N-formyl-4-chloro-o-toluidine).
Unpublished report from CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Arni, P. and D. Muller Salmonella/Mammalian Microsome Mutagenicity
(1976c) Test with CGA 72647 (4-chloro-o-toluidine
HC1). Unpublished report from CIBA-GEIGY,
Ltd., Basil, Switzerland submitted by
CIBA-GEIGY, Ltd. to the WHO.
Benezet, H.J. and C.O. Knowles Inhibition of Rat Brain
(1976) Monoamine Oxidase by Formamidine and Related
Compounds. Neuropharmacol. 15:369.
Benezet, H.J., K.M. Chang and C.O. Knowles Formamidine Pesticides-
(1978) Metabolic Aspects of Neurotoxicity in
Pesticide and Venom Neural Toxicity. Ed. D.L.
Shankland, R.M. Hollingworth and J. Smyth,
Jr. Plenum Press, N.Y. pg. 189-206.
Bull, D.L., Environmental Entomology 2, 869.
(1973)
Burkhard, N. Residues in milk, meat, liver and fat of a Swiss cow
(1971a) washed with a 0.5% solution of
chlordimeform. Report RVA 14/71, Agrochemical
Division, CIBA-GEIGY, Ltd., Basel,
Switzerland. 1 February 1971.
Chinn, C., W.R. Pfister and G.K.W. Yim Local Anesthetic-Like Actions
(1976) of the Pesticide Chlordimeform. Fed. Proc.
35:729.
Ciba-Geigy Chlorphenamidime residues in eggs and poultry tissues.
(1971) Research Report C79910, CIBA-GEIGY, Corp.
Greensboro N.C.
Ciba-Geigy Method for determination of chlordimeform residues -
(1976) Method REM 4/76.
Ciba-Geigy Submission of data for the reevaluation of chlordimeform by
(1978) the 1978 FAO/WHO Joint Meeting on Pesticide
Residues, CIBA-GEIGY, Ltd., Basel and
Schering AG, Berlin. July 24, 1978.
Ciba-Geigy Residues resulting from chlordimeform application to
(1978a) cotton. Report by H.B. Camp and B.G. Tweedy
No ABR-78004 (4 January 1978).
Ehrhardt, D.A. and Knowles, C.O. J. Econ. Entomol. 63, 1306.
(1970)
Ezumi, K. and H. Nakao Effects of 4-chloro-o-toluidine in Oral
(1974a) Prolonged Administration to Rat for 94 (Male)
and 104 (Female) Weeks. Unpublished report
from Nihon Schering Inc., Osaka, Japan
submitted by CIBA-GEIGY, Ltd. to the WHO.
Ezumi, K. and H. Nakao Effects of 4-chloro-o-toluidine in Oral
(1974b) Prolonged Administration to Mice for 80 Weeks
Unpublished report from Nihon Schering, Inc.,
Osaka, Japan submitted by CIBA-GEIGY, Ltd. to
the WHO.
FAO/WHO 1971 Evaluations of some pesticide residues in food.
(1972b) AGP:1971/M/9/1; WHO Pesticide Residues Series
No 1.
FAO/WHO 1975 Evaluations of some pesticide residues in food. AGP:
(1976b) 1975/M/13; WHO Pesticide Residue Series No 5.
FAO/WHO Pesticide residues in food. Report of the 1976 Joint Meeting,
(1977a) of the FAO Panel Experts on Pesticide.
Residues and the Environment and the WHO
Expert Group on Pesticide Residues. FAO Food
and Nutrition Series, No 9; FAO Plant
Production and Protection Series, No 8; WHO
Technical Report Series, No 612.
FAO/WHO Pesticide residues in food - 1977. Report of the FAO Panel of
(1978a) Experts on Pesticide Residues and Environment
and the WHO Expert Committee on Pesticide
Residues. FAO Plant Production and Protection
Paper 10 Rev. par 4.13.
Fischer, W.C. and Cassidy, J.E. Uptake of radio-labelled (1976a)
chlordimeform and its soil metabolites in
cotton in a field prepared for rotation
crops. Report No GAAC-76014 CIBA-GEIGY
Corporation, Greensboro, N.C. March 15, 1976.
Fischer W.C. and Cassidy, J.E. Uptake and characterization of
(1976b) the metabolites of radio-labelled
chlordimeform in spray treated cotton and
field soil. Report of Biochemistry Department
CIBA-GEIGY Corporation Report ABR - 76056
July 27, 1976.
Fischer W.C. and Cassidy, J.E. Identification of the metabolites
(1976c) of chlordimeform in mature leaves of spray
treated field cotton. Report No ABR - 76073
from Biochemistry Department, CIBA-GEIGY,
Corporation, Greensboro, N.C.
Fritz, H. Reproduction study on C8514 Technical Rabbit - Seg II. (Test
(1971) for teratogenic and embryotoxic effects).
Unpublished report from Toxicology/ Pathology
CIBA-GEIGY, Ltd., Basely Switzerland,
submitted by CIBA-GEIGY Ltd to the WHO.
Fritz, H. Reproduction Study on C8514 Technical Rat-Seg II (Test for
(1975) Teratogenic or Embroyotic Effects).
Unpublished report from Toxicology/Pathology
CIBA-GEIGY, Ltd., Basely Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Geisebuhler, H., Kossmann, K., Baunck, I. and Boyd, V.F.
(1971) Determination of total residues of
chlorphenamidine in plant and soil material
by colorimetry and thin layer and electron
capture gas-chromatography. Agric. Food Chem.
19 (2):365.
Gross Metabolism of chlordimeform in cotton. Project Report 19/77 from
(1977) Biochemistry Department, CIBA-GEIGY, Ltd.,
Basel, Switzerland (April 1977).
Hollingworth R.M. Chemistry Biological Activity and Uses of
(1976) Formamidine Pesticides. Environ. Health
Perspect. 14: 57-69.
Honeycutt, R.C. and Cassidy, J.E. Extraction and characterization
(1977) of cottonseed metabolite from cotton
injected with o 14C-chlordimeform. Report No
ABR - 77087 from Biochemistry Department,
CIBA-GEIGY, Corporation, Greensboro, N.C.
Sept. 14, 1977.
Hurni, H. and H. Ohder Report on the Mutagenic Effect of Technical
(1970) C-8514-N. Unpublished report from Tierfarm AG
Biomedical Research, Sisseln, Switzerland
submitted by CIBA-GEIGY, Ltd., to the WHO.
Jenny, N.A. Chlorphenamidine residues in eggs and poultry
(1971) tissues/Analysis of tissue and egg samples.
Knowles, C.O. Chemistry and Toxicology of Quinoxaline Organotin
(1976) Organofluorine and Formamidine Acaracides.
Env. Health Perspect. 14:93-102.
Kalyonova, F.P., Z. Zapryanav and A.I. Baynova Influence of the
(1978) Insecticide Chlordimeform of the Activity of
the Monoamine Oxidase (MAO) of Albino Rats.
Comptes Rendus de l'Academi Bulgare des
Sciences 31 (4): 491-493.
Konopa, E.A. and H. Heymann Salmonella/Microsome Mutagenicity
(1977) Test with Chlordimeform. Unpublished report
from Pharmaceuticals Division, CIBA-GEIGY,
Corporation, Surnmity N.J. submitted by
CIBA-GEIGY, Ltd., to the WHO.
Konopka. E.A. and H. Heymann Salmonella/Microsome Mutagenicity
(1977b) Test with Chlordimeform. Unpublished report
from Pharmaceuticals Division, CIBA-GEIGY
Corporation, Summit, N.J. submitted by
CIBA-GEIGY, Ltd. to the WHO.
Langauer, M. and D. Muller Nucleus Anomaly Test on Somatic
(1977) Interphase Nuclei-C-8513- Chinese Hamster.
Unpublished report from Toxicology/Pathology,
CIBA-GEIGY, Ltd., Basel, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Lund, A.E., G.K.W. Yim and D.L. Shankland The Cardiovascular
(1978) Toxicity of Chlordimeform, a Local
Anesthetic-Like Action in Pesticide and Venom
Neurotoxicity. Ed. D.L. Shankland, R.M.
Hollingworth and T. Smyth, Jr. Plenum Press,
N.Y., pgs. 171-177.
Maitre L., A. Felner, P. Waldmeier and W. Kehr Moccasins Oxidase
(1978) Inhibition in Brain and Liver of Rats Treated
with Chlordimeform. J. Agr. Fd Chem. 26
(2): 442-446.
Matsumura, F. and R.W. Beeman Biochemical and Physiological
(1976) Effects of Chlordimeform. Environ. Health
Perspect. 14: 71-82.
Morton Method for the determination of chlordimeform. Method 333/7
(1968) (7/12/68)
Murakami, J. and J. Fukami Effects of Chlophenamidine and Its
(1974) Metabolites on HeLa Cells. Bull. Env.
Contam. and Tox. 11: 184-188.
Nesnow, S. and C. Heidelberger The Effect of Modifiers of
Microsomal Enzymes on Chemical Oncogenesis in
Cultures of C3H Mouse Cell Lines. Cancer
Res. 36:1801-1808.
Nesnow S. Unpublished bioassay submitted to the WHO.
(1978)
Nor-Am Method of analysis for chlordimeform residues - Research Method
(1969) 333/44. 5/6/69.
Sachsse, K., P. Suter, F. Zak and R. Hess Lifespan Feeding Study in
(1978b) Mice with 4-chloro-o-toludine. Unpublished
report from Toxicology, CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Schering Method of analysis of chlordimeform residues. Method 36 268/8
(1973) (24/4/73).
Schering Method of determination of chlordimeform and its degradation
(1975) products. Method 36268/9 17/11/75.
Sen Gupta, A.K. and Knowles, C.O. J. Agric. Food Chem. 17, 595.
(1969)
Suter, P., H. Luetkemeier, K. Sachsse and F. Zak 60-day Pending
(1976a) Study in the Rat with chloro-o-toluidine
HC1. Unpublished report from
Toxicology/Pathology, CIBA-GEIGY, Ltd.,
Basel, Switzerland, submitted by CIBA-GEIGY,
Ltd. to the WHO.
Suter, P., H. Luetkemeier, K. Sachsse and F. Zak 60-day Pending
(1976b) Study in Mice with 4, chloro-o-toluidine
HC1. Unpublished report from
Toxicology/Pathology, CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Suter, P., F. Zak, K. Sachsse and R. Hess Lifespan Feeding Study
(1979) in Mice with Chlordimeform HC1. Unpublished
report from Toxicology/Pathology,
CIBA-GEIGY, Ltd., Basel, Switzerland,
submitted by CIBA-GEIGY, Ltd. to the WHO.
Wang, C.M., T. Narahashi and J. Fukami Mechanism of Neuromuscular
(1975) Block by Chlordimeform. Pest. Biochem.
Physiol. 5: 119-125.
Watanabe, H., S. Tsuda and J. Fukami. Effects of Chlordimeform
(1975) on Rectusabdominum Muscle in Frog. Pest.
Biochem. Physiol. 5: 150-154.
TABLE 5. Residues of chlordimeform and its metabolites in cows.
Chlordimeform and metabolites, mg/kg
Duration of
feeding (days)
In concentrate In liver In kidney
21 40 0.09 < 0.03
21 120 0.38 0.07
7 240 0.45 0.05
14 240 0.58 0.13
21 240 0.50 0.13
42 240 0.44 0.09
Honeycutt and Cassidy (1976) fed a lactating goat with cottonseed
containing biosynthesized metabolites of phenyl-14C-chlordimeform at
a level equivalent to 0.032 ppm chlordimeform daily for 4 consecutive
days. The total recovery of radioactivity was 97.1%. It was primarily
distributed between the urine (21.3%), which reached a plateau by the
2nd day, the faeces (35.9%), which reached a plateau by the third day,
and the rumenintestinal contents (38.7%). There was no deposition of
radioactivity in tissues (< 0.002 mg/kg), even though uptake of the
biosynthesized metabolites occurred.
The milk contained 0.9% of the total dose, its radioactivity
plateaued by the second day (0.0004 ± 0.0001 mg/kg chlordimeform
equivalents). The ratio of the concentration in milk to that in the
feed was 0.013. Blood contained < 0.2% of the total dose.
Comparison of the 14C-extractables of the original cottonseed
with those in the faeces, urine and rumen-intestinal contents,
suggests that the goat metabolized the biosynthesized metabolites in
cottonseed. However, metabolism to natural products was not
significant since expired 14CO2 accounted for only 0.3% of the total
dose.
Ciba-Geigy Corp. (1971) conducted experiments in which white
Leghorn hens in active egg production were fed a commercial egg laying
mash to which chlordimeform was added at rates equivalent to 0, 0.25,
0.75 and 1.50 mg/kg of feed. Groups were sacrificed after 7, 14, 21
and 28 days feeding and were subjected to post-mortem examination and
assessment of egg production, body-weight change and food consumption.
There were no significant differences between treated and control
binds in any of the features examined.
Residues in eggs and tissues (breast, fat and liver) were
determined by Jenny (1971). Eggs collected at -3 (pre-treatment), -1
(pre-treatment), 3, 7, 14, 21 and 28 days from each group contained no
detectable amounts of chlordimeform and/or its metabolites containing
the 4-chloro-o-toluidine moiety.
The breasts of birds sacrificed at 7, 14, 21 and 28 days of
feeding treated feed as well as 49 days after returning to untreated
feed contained no significant residues. Average residues of 0.11, 0.09
and 0.22 mg/kg were detected in fat at the feeding levels of 0.25,
0.75 and 1.50 ppm respectively, at the 21-day interval only. No
residues were detected in livers from chickens fed 0.25 ppm
chlordimeform at any of the intervals tested and residue of 0.09 mg/kg
was detected in liver from chickens fed 0.75 ppm only at the 21-day
interval. Residues of 0.20, 0.17, and 0.10 mg/kg were detected in
livers from the 1.5 ppm level at intervals of 7, 14, 21 and 28 days.
Nine days after withdrawal from the feed containing 1.5 ppm, no
residues could be detected in any of the tissues.
In processing
The Meeting reviewed many data on the level and fate of residues
during processing. Cottonseed is subjected to well-defined sequential
processes, details of which were available (Ciba-Geigy, 1978). The
results of some typical trials are set out in Table 4 and 6. The
distribution is independent of the pattern of application as is
apparent from Table 6. Although the major fraction of the residue
remains in the hulls and the meal from which the oil has been removed
there is still a significant concentration in the crude oil. Treatment
of crude cottonseed oil with caustic soda in the refining process does
not reduce the residue concentration significantly but the additional
refining procedures including bleaching, hydrogenating and deodorizing
reduce the residue in the oil to a level below the detection limits of
present analytical methods (< 0.05 mg/kg). Since all the oil used for
human consumption is at least subjected to the bleaching and
deodorizing process, virtually all the chlordimeform will be removed
before the cottonseed oil is consumed.
In soil
In the course of studies on the uptake of chlordimeform and its
soil metabolites from treated soil into cotton Fischer and Cassidy
(1976a) applied radio-labelled chlordimeform to a cotton field plot
thirteen weeks before harvesting the cotton. The rate of application
was equivalent to 1 kg/ha.
TABLE 6. Effect of use pattern on Chlordimeform residues in cottonseed products.
AGA AGA AGA AGA AGA AGA AGA AGA AGA AGA
Trial No. 4397 4408 4409 4449 4450 4502 4981 4981 4981 4981
Location Alab. Ariz. Calif. Georg. Georg. Miss. S.C. S.C. S.C. S.C.
Rate of application 0.125 0.125 0.25 0.125 0.25 0.125 0.125 0.25 0.125 0.25
Formulation EC EC EC EC EC EC EC EC EC EC
No. of applications 7 7 6 11 12 9 12 12 17 17
Pre-harvest interval 28 39 31 22 16 31 21 21 21 21
Cottonseed 0.2 0.82 0.35 0.35 0.35 0.30 0.29 0.20 0.22 0.40
Hulls 0.36 1.8 0.85 0.58 0.55 0.33 0.86 0.50 0.90 0.75
Meal 0.19 1.2 0.45 0.24 0.32 0.45 0.18 0.10 0.17 0.15
Soap stock 0.10 0.84 0.34 0.17 0.23 0.22 0.09 0.07 0.10 0.10
Crude oil 0.10 0.46 0.21 0.15 0.23 0.16 0.10 0.07 0.11 0.08
Refined oil 0.12 0.66 0.32 0.17 0.19 0.19 0.07 <0.05 0.08 0.07
Refined bleached
deodorised oil <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Refined bleached
deodorised
hydrogenated oil <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Radioactivity in the top 75 mm layer of silt loam soil accounted
for 1.23 mg/kg chlordimeform equivalents after treatment. Seven weeks
later this level had decreased to 0.33 mg/kg and at 13 weeks to 0.20
mg/kg. When the 0-75 mm layer was extracted after seven weeks, 32% of
the radioactivity partitioned into the organic fraction, 20%
partitioned into the polar fraction and 44% remained non-extractable,
showing that the chlordimeform had degraded rapidly. The radioactivity
in the samples taken 13 weeks after treatment was too low to
fractionate. Except for one sample, the level of radioactivity as
chlordimeform equivalents in the 75-150 mm and 150-200 mm layers was
less than 0.01 mg/kg; thus leaching did not occur in this silt loam.
In a later study when radio-labelled chlordimeform was applied at
the rate of 1 kg/ha over cotton plants and soil in field plots,
Fischer and Cassidy (1976b) found that the radioactivity in the 0-75
mm layer of silt loam was 1.24 mg/kg immediately after the first
treatment but only 0.21 mg/kg after the final treatment and 0.20 mg/kg
at harvest of the mature cotton. This decrease is due to (1) less
drainage from the leaves onto the soil at the later sprayings because
of the increased cover with plant growth, (2) volatility of the
chlordimeform and (3) soil metabolism. Radioactivity was less than
0.01 mg/kg in the 75-150 and 150-225 mm layers at all-sampling dates.
These data show that metabolism of 14C-chlordimeform in silt-loam is
rapid and that the metabolites are not leached.
Analysis of the 0-75 mm layer of soil taken at harvest showed
that 91.0% of the radioactivity could be converted to
4-chloro-o-toluidine. Therefore, although 57% of the soil
metabolites were polar or non-extractable, they contained this moiety.
Following this study Fischer and Cassidy (1976b) planted soybeans
at a rotation crop 38 weeks after the original treatment with
chlordimeform at 1 kg/ha. At maturity the soybeans were found to
contain radioactivity equivalent to 0.01 mg/kg chlordimeform in the
stalks and less than 0.01 mg/kg in the beans themselves.
Wheat planted in the plots was examined for radioactivity at
maturity. The radioactivity was equivalent to 0.16 mg/kg in the straw
and 0.03 mg/kg in the grain.
METHODS OF RESIDUE ANALYSIS
Although methods have been developed for the determination of
chlordimeform alone, most of the residue data have been based on the
determination of chlordimeform and its metabolites as
4-chloro-o-toluidine.
Geissbuhler et al. (1971) published the method used by Ciba-Geigy
Ltd. and Schering A.G. for the development of chlordimeform
pesticides. This method has been utilized with and without
modification for much of the work reported in this monograph. In this
method chlordimeform and its potential metabolites and/or conjugates
are hydrolyzed to 4-chloro-o-toluidine in the presence of plant and
soil material by successive treatments with acetic acid and sodium
hydroxide. The aromatic amine is steam distilled and extracted into
iso-octane. Diazotization and coupling of 4-chloro-o-toluidine with
N-ethyl-1-naphthylamine yields a stable purple dye, which, after
column chromatography on cellulose, is measured colorimetrically. To
verify the identity of chlordimeform, the azo-dye is separated and
visualized on a cellulose thin-layer plate. Alternatively,
4-chloro-o-toluidine is diazotized and iodinated. The iodine
derivative is determined by electron capture gas chromatography. A
further potential metabolite of chlordimeform
2,2-dimethyl-4-4-dichloroazobenzene, is reduced to
4-chloro-o-toluidine in the presence of zinc and acid 1,4-dioxane
and likewise determined by gas chromatography. Recoveries in various
plant and soil materials have been observed to vary between 75 and
100% for all three quantitative methods described. The limit of
determination is 0.05 mg/kg.
APPRAISAL
Chlordimeform products which had been widely used for many years
as insecticides/acaricides on a large variety of crops and on
livestock, were withdrawn by the manufacturers in 1976. Additional
studies have since been carried out and new proposals have been made
for commercial use on cotton crops under restricted conditions.
Information on those proposed uses in various countries and data on
the nature, level and fate of residues, and including the fate of
residues in cottonseed products used in the rations of dairy cattle
and poultry was also examined.
The influence of rate of application pre-harvest interval and
number of applications were investigated. At the maximum application
rate of 1 kg/ha the residue rarely exceeded 2 mg/kg in cottonseed,
seed meal or crude oil. Solvent-extracted oil contained significantly
lower residues than screwpress-extracted oil. Treatment of crude oil
with caustic sodal did not reduce residues markedly but refining
procedures such as bleaching and deodorization as normally undertaken
in commercial practice reduced the levels to below the analytical
detection limits (< 0.05 mg/kg).
Eggs from hens fed for 28 days on rations containing up to 1.5
ppm contained no detectable residues. Meat, fat and liver from hens
slaughtered at various intervals throughout the study was analysed.
Although nothing was found in any of the meat, small residues were
detected in some samples of fat and liver. Cows fed for up to 42 days
on rations containing excessive levels of chlordimeform (up to 240
ppm) showed no residues (< 0.3 mg/kg) in milk, fat or meat at any
stage during the feeding; but residues in some animals fed at the
highest rates, ranged up to 0.6 mg/kg in liver and 0.1 mg/kg in
kidney. Since there appears to be no likelihood of livestock rations
containing more than 0.5 mg/kg of residue however, detectable residues
are unlikely to occur in foods of animal origin.
The analytical methods used determined chlordimeform, the
intermediate metabolite N-formyl-4-chloro-o-toluidine and the final
metabolite 4-chloro-o-toluidine together as 4-chloro-o-toluidine. No
attempt was made to quantify the amounts of each present and the limit
of determination was 0.05 mg/kg.
Studies of the metabolism of chlordimeform in cotton plants and
goats showed that metabolism in plants and animals proceeds through
sequential hydrolysis of terminal groups to 4-chloro-o-toluidine. In
cotton metabolism proceeds to the formation of polar metabolites which
apparently conjugate to natural products. Sprays applied after the
bolls open would partly avoid translocation of residues as metabolites
from the treated leaves to seed but would result in the retention of
significant residues of the parent compound in fibre and seed. In a
study involving the feeding of cottonseed containing biosynthesised
radioactive metabolites to a goat, no residues were deposited in the
animal tissue (limit of determination 0.002 mg/kg).
In the light of the new data examined, the Meeting decided that
there was no need to amend the temporary MRL formerly proposed for
cottonseed and crude cottonseed oil; but unless direct application to
cattle were to be re-installed, the MRL for foods of animal origin
should be based on the data reflecting the feeding of cottonseed
hulls, cottonseed meal and similar products. As chlordimeform is no
longer used on crops other than cotton, the recommendations for
temporary MRLs previously made for crops other than cotton should be
held in abeyance until such time as the uses on those crops should be
reinstated.
RECOMMENDATIONS
The following new or amended recommendations for temporary MRLs
replace all previous recommendations.
Commodity Temporary MRL mg/kg
Cottonseed 2
Cottonseed (crude) 2
Cottonseed oil (edible) ) No residues are to
) occur at the current
Meat of cattle ) limit of detection
) (0.05 mg/kg)
Meat of pigs )
Meat of poultry ) " " " "
Meat of sheep )
Milk )
Milk products )
FURTHER WORK OR INFORMATION
Required (by 1979)
1. Full submission of all toxicological data.
2. Continued epidemiology data on people exposed to chlordimeform.
3. Reports of continued observation of the possible occurrence of
haemorrhagic cystitis.
REFERENCES
Anonymous Chlordimeform HC1 Life Time Feeding Study in Rats - One
(1978a) Year Interim Report. Unpublished report from
CIBA-GEIGTY, Ltd., Basal, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Anonymous N-Formyl-4-chloro-o-toluidine Life Time Feeding Study in
(1978b) Rats One Year Interim Report. Unpublished
report from CIBA-GEIGY, Ltd., Basel,
Switzerland submitted by CIBA-GEIGY, Ltd. to
the WHO.
Anonymous 4-chloro-o-toluidine HC1 Life Time Feeding Study in Rats
(1978c) - One Year Interim Report. Unpublished report
from CIBA-GEIGY, Ltd., Basel, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Anonymous Bioassay of 4-chloro-o-toluidine Hydrochloride for
(1978d) Possible Carcinogenicity. NCI Carcinogenic
Programme Preliminary Draft Report [ NIH ]
79-1721).
Arni, P. and D. Muller Salmonella/Mammalian-Microsome Mutagenicity
(1976a) Test with C-8513 Chlordimeform HC1.
Unpublished report from CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Arni, P. and D. Muller Salmonella/Mammalian-Microsome Mutagenicity
(1976b) Test with CGA 72651
(N-formyl-4-chloro-o-toluidine).
Unpublished report from CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Arni, P. and D. Muller Salmonella/Mammalian Microsome Mutagenicity
(1976c) Test with CGA 72647 (4-chloro-o-toluidine
HC1). Unpublished report from CIBA-GEIGY,
Ltd., Basil, Switzerland submitted by
CIBA-GEIGY, Ltd. to the WHO.
Benezet, H.J. and C.O. Knowles Inhibition of Rat Brain
(1976) Monoamine Oxidase by Formamidine and Related
Compounds. Neuropharmacol. 15:369.
Benezet, H.J., K.M. Chang and C.O. Knowles Formamidine Pesticides-
(1978) Metabolic Aspects of Neurotoxicity in
Pesticide and Venom Neural Toxicity. Ed. D.L.
Shankland, R.M. Hollingworth and J. Smyth,
Jr. Plenum Press, N.Y. pg. 189-206.
Bull, D.L., Environmental Entomology 2, 869.
(1973)
Burkhard, N. Residues in milk, meat, liver and fat of a Swiss cow
(1971a) washed with a 0.5% solution of
chlordimeform. Report RVA 14/71, Agrochemical
Division, CIBA-GEIGY, Ltd., Basel,
Switzerland. 1 February 1971.
Chinn, C., W.R. Pfister and G.K.W. Yim Local Anesthetic-Like Actions
(1976) of the Pesticide Chlordimeform. Fed. Proc.
35:729.
Ciba-Geigy Chlorphenamidime residues in eggs and poultry tissues.
(1971) Research Report C79910, CIBA-GEIGY, Corp.
Greensboro N.C.
Ciba-Geigy Method for determination of chlordimeform residues -
(1976) Method REM 4/76.
Ciba-Geigy Submission of data for the reevaluation of chlordimeform by
(1978) the 1978 FAO/WHO Joint Meeting on Pesticide
Residues, CIBA-GEIGY, Ltd., Basel and
Schering AG, Berlin. July 24, 1978.
Ciba-Geigy Residues resulting from chlordimeform application to
(1978a) cotton. Report by H.B. Camp and B.G. Tweedy
No ABR-78004 (4 January 1978).
Ehrhardt, D.A. and Knowles, C.O. J. Econ. Entomol. 63, 1306.
(1970)
Ezumi, K. and H. Nakao Effects of 4-chloro-o-toluidine in Oral
(1974a) Prolonged Administration to Rat for 94 (Male)
and 104 (Female) Weeks. Unpublished report
from Nihon Schering Inc., Osaka, Japan
submitted by CIBA-GEIGY, Ltd. to the WHO.
Ezumi, K. and H. Nakao Effects of 4-chloro-o-toluidine in Oral
(1974b) Prolonged Administration to Mice for 80 Weeks
Unpublished report from Nihon Schering, Inc.,
Osaka, Japan submitted by CIBA-GEIGY, Ltd. to
the WHO.
FAO/WHO 1971 Evaluations of some pesticide residues in food.
(1972b) AGP:1971/M/9/1; WHO Pesticide Residues Series
No 1.
FAO/WHO 1975 Evaluations of some pesticide residues in food. AGP:
(1976b) 1975/M/13; WHO Pesticide Residue Series No 5.
FAO/WHO Pesticide residues in food. Report of the 1976 Joint Meeting,
(1977a) of the FAO Panel Experts on Pesticide.
Residues and the Environment and the WHO
Expert Group on Pesticide Residues. FAO Food
and Nutrition Series, No 9; FAO Plant
Production and Protection Series, No 8; WHO
Technical Report Series, No 612.
FAO/WHO Pesticide residues in food - 1977. Report of the FAO Panel of
(1978a) Experts on Pesticide Residues and Environment
and the WHO Expert Committee on Pesticide
Residues. FAO Plant Production and Protection
Paper 10 Rev. par 4.13.
Fischer, W.C. and Cassidy, J.E. Uptake of radio-labelled (1976a)
chlordimeform and its soil metabolites in
cotton in a field prepared for rotation
crops. Report No GAAC-76014 CIBA-GEIGY
Corporation, Greensboro, N.C. March 15, 1976.
Fischer W.C. and Cassidy, J.E. Uptake and characterization of
(1976b) the metabolites of radio-labelled
chlordimeform in spray treated cotton and
field soil. Report of Biochemistry Department
CIBA-GEIGY Corporation Report ABR - 76056
July 27, 1976.
Fischer W.C. and Cassidy, J.E. Identification of the metabolites
(1976c) of chlordimeform in mature leaves of spray
treated field cotton. Report No ABR - 76073
from Biochemistry Department, CIBA-GEIGY,
Corporation, Greensboro, N.C.
Fritz, H. Reproduction study on C8514 Technical Rabbit - Seg II. (Test
(1971) for teratogenic and embryotoxic effects).
Unpublished report from Toxicology/ Pathology
CIBA-GEIGY, Ltd., Basely Switzerland,
submitted by CIBA-GEIGY Ltd to the WHO.
Fritz, H. Reproduction Study on C8514 Technical Rat-Seg II (Test for
(1975) Teratogenic or Embroyotic Effects).
Unpublished report from Toxicology/Pathology
CIBA-GEIGY, Ltd., Basely Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Geisebuhler, H., Kossmann, K., Baunck, I. and Boyd, V.F.
(1971) Determination of total residues of
chlorphenamidine in plant and soil material
by colorimetry and thin layer and electron
capture gas-chromatography. Agric. Food Chem.
19 (2):365.
Gross Metabolism of chlordimeform in cotton. Project Report 19/77 from
(1977) Biochemistry Department, CIBA-GEIGY, Ltd.,
Basel, Switzerland (April 1977).
Hollingworth R.M. Chemistry Biological Activity and Uses of
(1976) Formamidine Pesticides. Environ. Health
Perspect. 14: 57-69.
Honeycutt, R.C. and Cassidy, J.E. Extraction and characterization
(1977) of cottonseed metabolite from cotton
injected with o 14C-chlordimeform. Report No
ABR - 77087 from Biochemistry Department,
CIBA-GEIGY, Corporation, Greensboro, N.C.
Sept. 14, 1977.
Hurni, H. and H. Ohder Report on the Mutagenic Effect of Technical
(1970) C-8514-N. Unpublished report from Tierfarm AG
Biomedical Research, Sisseln, Switzerland
submitted by CIBA-GEIGY, Ltd., to the WHO.
Jenny, N.A. Chlorphenamidine residues in eggs and poultry
(1971) tissues/Analysis of tissue and egg samples.
Knowles, C.O. Chemistry and Toxicology of Quinoxaline Organotin
(1976) Organofluorine and Formamidine Acaracides.
Env. Health Perspect. 14:93-102.
Kalyonova, F.P., Z. Zapryanav and A.I. Baynova Influence of the
(1978) Insecticide Chlordimeform of the Activity of
the Monoamine Oxidase (MAO) of Albino Rats.
Comptes Rendus de l'Academi Bulgare des
Sciences 31 (4): 491-493.
Konopa, E.A. and H. Heymann Salmonella/Microsome Mutagenicity
(1977) Test with Chlordimeform. Unpublished report
from Pharmaceuticals Division, CIBA-GEIGY,
Corporation, Surnmity N.J. submitted by
CIBA-GEIGY, Ltd., to the WHO.
Konopka. E.A. and H. Heymann Salmonella/Microsome Mutagenicity
(1977b) Test with Chlordimeform. Unpublished report
from Pharmaceuticals Division, CIBA-GEIGY
Corporation, Summit, N.J. submitted by
CIBA-GEIGY, Ltd. to the WHO.
Langauer, M. and D. Muller Nucleus Anomaly Test on Somatic
(1977) Interphase Nuclei-C-8513- Chinese Hamster.
Unpublished report from Toxicology/Pathology,
CIBA-GEIGY, Ltd., Basel, Switzerland
submitted by CIBA-GEIGY, Ltd. to the WHO.
Lund, A.E., G.K.W. Yim and D.L. Shankland The Cardiovascular
(1978) Toxicity of Chlordimeform, a Local
Anesthetic-Like Action in Pesticide and Venom
Neurotoxicity. Ed. D.L. Shankland, R.M.
Hollingworth and T. Smyth, Jr. Plenum Press,
N.Y., pgs. 171-177.
Maitre L., A. Felner, P. Waldmeier and W. Kehr Moccasins Oxidase
(1978) Inhibition in Brain and Liver of Rats Treated
with Chlordimeform. J. Agr. Fd Chem. 26
(2): 442-446.
Matsumura, F. and R.W. Beeman Biochemical and Physiological
(1976) Effects of Chlordimeform. Environ. Health
Perspect. 14: 71-82.
Morton Method for the determination of chlordimeform. Method 333/7
(1968) (7/12/68)
Murakami, J. and J. Fukami Effects of Chlophenamidine and Its
(1974) Metabolites on HeLa Cells. Bull. Env.
Contam. and Tox. 11: 184-188.
Nesnow, S. and C. Heidelberger The Effect of Modifiers of
Microsomal Enzymes on Chemical Oncogenesis in
Cultures of C3H Mouse Cell Lines. Cancer
Res. 36:1801-1808.
Nesnow S. Unpublished bioassay submitted to the WHO.
(1978)
Nor-Am Method of analysis for chlordimeform residues - Research Method
(1969) 333/44. 5/6/69.
Sachsse, K., P. Suter, F. Zak and R. Hess Lifespan Feeding Study in
(1978b) Mice with 4-chloro-o-toludine. Unpublished
report from Toxicology, CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Schering Method of analysis of chlordimeform residues. Method 36 268/8
(1973) (24/4/73).
Schering Method of determination of chlordimeform and its degradation
(1975) products. Method 36268/9 17/11/75.
Sen Gupta, A.K. and Knowles, C.O. J. Agric. Food Chem. 17, 595.
(1969)
Suter, P., H. Luetkemeier, K. Sachsse and F. Zak 60-day Pending
(1976a) Study in the Rat with chloro-o-toluidine
HC1. Unpublished report from
Toxicology/Pathology, CIBA-GEIGY, Ltd.,
Basel, Switzerland, submitted by CIBA-GEIGY,
Ltd. to the WHO.
Suter, P., H. Luetkemeier, K. Sachsse and F. Zak 60-day Pending
(1976b) Study in Mice with 4, chloro-o-toluidine
HC1. Unpublished report from
Toxicology/Pathology, CIBA-GEIGY, Ltd.,
Basel, Switzerland submitted by CIBA-GEIGY,
Ltd. to the WHO.
Suter, P., F. Zak, K. Sachsse and R. Hess Lifespan Feeding Study
(1979) in Mice with Chlordimeform HC1. Unpublished
report from Toxicology/Pathology,
CIBA-GEIGY, Ltd., Basel, Switzerland,
submitted by CIBA-GEIGY, Ltd. to the WHO.
Wang, C.M., T. Narahashi and J. Fukami Mechanism of Neuromuscular
(1975) Block by Chlordimeform. Pest. Biochem.
Physiol. 5: 119-125.
Watanabe, H., S. Tsuda and J. Fukami. Effects of Chlordimeform
(1975) on Rectusabdominum Muscle in Frog. Pest.
Biochem. Physiol. 5: 150-154.
