
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 99
CYHALOTHRIN
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Cyhalothrin.
(Environmental health criteria ; 99)
1.Pyrethrins - adverse effects 2.Pyrethrins - toxicity
I.Series
ISBN 92 4 154299 3 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CYHALOTHRIN AND LAMBDA-CYHALOTHRIN
INTRODUCTION
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, physical and chemical properties,
analytical methods
1.1.2. Production and use
1.1.3. Human exposure
1.1.4. Environmental exposure and fate
1.1.5. Uptake, metabolism, and excretion
1.1.6. Effects on organisms in the environment
1.1.7. Effects on experimental animals and in vitro
test systems
1.1.8. Effects on humans
1.2. Conclusions
1.3. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
2.3.1. Sampling methods
2.3.2. Sample storage
2.4. Sample preparation
2.5. Gas chromatographic procedures for the determination of
cyhalothrin residues
2.5.1. Extraction
2.5.2. Clean-up
2.5.3. Determination
2.5.4. Limit of determination
2.5.5. Recoveries and interference
2.5.6. Confirmation of residue identity
3. SOURCES AND LEVELS OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production levels and processes
3.2. Uses
3.3. Residues in food
3.4. Levels in the environment
3.4.1. Air
3.4.2. Water
3.4.3. Soil
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Abiotic degradation
4.2.1. Hydrolysis and photodegradation in water
4.2.2. Photodegradation in soil
4.3. Biodegradation in soil
4.3.1. Degradation rate
4.3.2. Degradation pathways
4.4. Metabolism in plants
4.5. Bioaccumulation and biomagnification
4.5.1. n-Octanol-water partition coefficient
4.5.2. Bioaccumulation
5. KINETICS AND METABOLISM
5.1. Absorption, distribution, and excretion
5.1.1. Rat
5.1.2. Dog
5.1.3. Cow
5.2. Metabolism
5.2.1. Rat
5.2.2. Dog
5.2.3. Cow
5.2.4. Goat
5.2.5. Fish
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic organisms
6.1.1. Microorganisms
6.1.2. Invertebrates
6.1.2.1 Acute toxicity
6.1.2.2 Long-term toxicity
6.1.3. Fish
6.1.3.1 Acute toxicity
6.1.3.2 Long-term toxicity
6.1.4. Model ecosystem
6.2. Terrestrial organisms
6.2.1. Birds
6.2.1.1 Acute toxicity
6.2.2. Honey-bees
6.2.3. Earthworms
6.2.4. Higher plants
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposures
7.1.1. Oral
7.1.2. Percutaneous
7.1.3. Intraperitoneal
7.2. Irritation and sensitization
7.2.1. Irritation
7.2.2. Sensitization
7.3. Short-term exposures
7.3.1. Oral
7.3.1.1 Rat
7.3.1.2 Dog
7.3.2. Dermal
7.3.2.1 Rabbit
7.4. Long-term exposures and carcinogenicity
7.4.1. Rat
7.4.2. Mouse
7.5. Reproduction, embryotoxicity, and teratogenicity
7.5.1. Reproduction
7.5.2. Embryotoxicity and teratogenicity
7.5.2.1 Rat
7.5.2.2 Rabbit
7.6. Mutagenicity and related end-points
7.6.1. Microorganisms
7.6.2. In vitro mammalian cells
7.6.3. In vivo mammalian assays
7.7. Mode of action
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
8.2.1. Acute toxicity: poisoning incidents
8.2.2. Effects of short- and long-term exposure
8.2.2.1 Manufacture
8.2.2.2 Formulation and laboratory work
8.2.2.3 Field use
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX
RESUME, EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
RESUMEN, EVALUACION, CONCLUSIONES Y RECOMENDACIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CYHALOTHRIN
Members
Dr V. Benes, Toxicology and Reference Laboratory, Institute of
Hygiene and Epidemiology, Prague, Czechoslovakia
Dr A.J. Browning, Toxicology Evaluation Section, Department of
Community Services and Health, Woden, ACT, Australia
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, United Kingdom
(Chairman)
Dr P. Hurley, Office of Pesticide Programme, US Environmental
Protection Agency, Washington, DC, USA
Dr K. Imaida, Section of Tumour Pathology, Division of Pathology,
National Institute of Hygienic Sciences, Setagaya-Ku, Tokyo,
Japan
Dr S.K. Kashyap, National Institute of Occupational Health,
(I.C.M.R.) Ahmedabad, India (Vice-Chairman)
Dr Yu. I. Kundiev, Research Institute of Labour, Hygiene and
Occupational Diseases, Ul. Saksaganskogo, Kiev, USSR
Dr J.P. Leahey, ICI Agrochemicals, Jealotts Hill Research Station,
Bracknell, United Kingdom (Joint Rapporteur)
Dr M. Matsuo, Sumitomo Chemical Company, Biochemistry and
Toxicology Laboratory, Kasugade-naka, Konohana-Ku, Osaka, Japan
Representatives of Other Organizations
Mr M. L'Hotellier, International Group of National Associations of
Manufacturers of Agricultural Products (GIFAP)
Dr N. Punja, International Group of National Associations of
Manufacturers of Agricultural Products (GIFAP)
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr R. Plestina, Division of Vector Biology and Control, World
Health Organization, Geneva, Switzerland
Dr J. Sekizawa, Division of Information on Chemical Safety,
National Institute of Hygienic Sciences, Setagaya-Ku, Tokyo,
Japan (Joint Rapporteur)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
* * *
The proprietary information contained in this document cannot
replace documentation for registration purposes, because the latter
has to be closely linked to the source, the manufacturing route and
the purity/impurities of the substance to be registered. The data
should be used in accordance with paragraphs 82-84 and
recommendations paragraph 90 of the Second FAO Government
Consultation (1982).
ENVIRONMENTAL HEALTH CRITERIA FOR CYHALOTHRIN
A WHO Task Group meeting on Environmental Health Criteria for
Cyhalothrin was held in Geneva from 24 to 28 October 1988. Dr M.
Mercier, Manager, IPCS, opened the meeting and welcomed the
participants on behalf of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The group reviewed and revised the draft monograph
and made an evaluation of the risks for human health and the
environment from exposure to cyhalothrin.
The first draft was prepared by the IPCS Secretariat, based on
material made available by ICI Agrochemicals, United Kingdom.
The second draft was also prepared by the IPCS Secretariat,
incorporating comments received following circulation of the first
draft to the IPCS contact points for Environmental Health Criteria
monographs. Dr K.W. Jager and Dr P.G. Jenkins, both members of
the IPCS Central Unit, were responsible for the technical
development and editing, respectively, of this monograph.
The assistance of ICI Agrochemicals in making available to the
IPCS and the Task Group its toxicological proprietary information
on cyhalothrin is gratefully acknowledged. This allowed the Task
Group to make its evaluation on the basis of more complete data.
ABBREVIATIONS
ADI acceptable daily intake
ai active ingredient
APDM aminopyrine- N-demethylase
EC emulsifiable concentrate
ECD electron capture detection
GC gas chromatography
HPLC high performance liquid chromatography
LOEL lowest-observed-effect level
MS mass spectrometry
MSD mass selective detection
NOEL no-observed-effect-level
SFS subjective facial sensation
TLC thin-layer chromatography
WP wettable powder
INTRODUCTION
SYNTHETIC PYRETHROIDS - A PROFILE
1. During investigations to modify the chemical structures of
natural pyrethrins, a certain number of synthetic pyrethroids
were produced with improved physical and chemical properties
and greater biological activity. Several of the earlier
synthetic pyrethroids were successfully commercialized, mainly
for the control of household insects. Other more recent
pyrethroids have been introduced as agricultural insecticides
because of their excellent activity against a wide range of
insect pests and their non-persistence in the environment.
2. The pyrethroids constitute another group of insecticides in
addition to organochlorine, organophosphorus, carbamate, and
other compounds. Pyrethroids commercially available to date
include allethrin, resmethrin, d-phenothrin, and tetramethrin
(for insects of public health importance), and cypermethrin,
deltamethrin, fenvalerate, and permethrin (mainly for
agricultural insects). Other pyrethroids are also available
including furamethrin, kadethrin, and tellallethrin (usually
for household insects), fenpropathrin, tralomethrin,
cyhalothrin, lambda-cyhalothrin, tefluthrin, cyfluthrin,
flucythrinate, fluvalinate, and biphenate (for agricultural
insects).
3. Toxicological evaluations of several synthetic pyrethroids have
been performed by the FAO/WHO Joint Meeting on Pesticide
Residues (JMPR). The acceptable daily intake (ADI) has been
estimated by the JMPR for cypermethrin, deltamethrin,
fenvalerate, permethrin, d-phenothrin, cyfluthrin,
cyhalothrin, and flucythrinate.
4. Chemically, synthetic pyrethroids are esters of specific acids
(e.g., chrysanthemic acid, halo-substituted chrysanthemic
acid, 2-(4-chlorophenyl)-3-methylbutyric acid) and alcohols
(e.g., allethrolone, 3-phenoxybenzyl alcohol). For certain
pyrethroids, the asymmetric centre(s) exist in the acid and/or
alcohol moiety, and the commercial products sometimes consist
of a mixture of both optical (1R/1S or d/1) and geometric
(cis/trans) isomers. However, most of the insecticidal
activity of such products may reside in only one or two
isomers. Some of the products (e.g., d-phenothrin,
deltamethrin) consist only of such active isomer(s).
5. Synthetic pyrethroids are neuropoisons acting on the axons in
the peripheral and central nervous systems by interacting with
sodium channels in mammals and/or insects. A single dose
produces toxic signs in mammals, such as tremors,
hyperexcitability, salivation, choreoathetosis, and paralysis.
The signs disappear fairly rapidly, and the animals recover,
generally within a week. At near-lethal dose levels, synthetic
pyrethroids cause transient changes in the nervous system, such
as axonal swelling and/or breaks and myelin degeneration in
sciatic nerves. They are not considered to cause delayed
neurotoxicity of the kind induced by some organophosphorus
compounds. The mechanism of toxicity of synthetic pyrethroids
and their classification into two types are discussed in the
Appendix.
6. Some pyrethroids (e.g., deltamethrin, fenvalerate, cyhalothrin,
lambda-cyhalothrin, flucythrinate, and cypermethrin) may cause
a transient itching and/or burning sensation in exposed human
skin.
7. Synthetic pyrethroids are generally metabolized in mammals
through ester hydrolysis, oxidation, and conjugation, and there
is no tendency to accumulate in tissues. In the environment,
synthetic pyrethroids are fairly rapidly degraded in soil and
in plants. Ester hydrolysis and oxidation at various sites on
the molecule are the major degradation processes. The
pyrethroids are strongly adsorbed on soil and sediments, and
hardly eluted with water. There is little tendency for
bioaccumulation in organisms.
8. Because of low application rates and rapid degradation in the
environment, residues in food are generally low.
9. Synthetic pyrethroids have been shown to be toxic for fish,
aquatic arthropods, and honey-bees in laboratory tests. But, in
practical usage, no serious adverse effects have been noticed
because of the low rates of application and lack of persistence
in the environment. The toxicity of synthetic pyrethroids in
birds and domestic animals is low.
10. In addition to the evaluation documents of FAO/WHO, there are
several good reviews and books on the chemistry, metabolism,
mammalian toxicity, environmental effects, etc., of synthetic
pyrethroids, including those by Elliott (1977), Miyamoto
(1981), Miyamoto & Kearney (1983), and Leahey (1985).
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, physical and chemical properties, analytical methods
Cyhalothrin is formed by esterifying 3-(2-chloro-3,3,3-
trifluoroprop-1-enyl)-2,2- dimethylcyclopropanecarboxylic acid
with alpha-cyano-3-phenoxybenzyl alcohol, and it consists of a
mixture of four stereoisomers. Lambda-cyhalothrin consists of one
enantiomeric pair of isomers and is the more biologically active
form.
Technical grade cyhalothrin is a yellow-brown viscous liquid
(melting point: approximately 10 °C) and contains more than 90%
active material. It is composed of four cis isomers in the ratio
of 1:1:1:1. Although it is insoluble in water, it is soluble in a
range of organic solvents such as aliphatic and aromatic
hydrocarbons. It is stable to light and heat and has a low vapour
pressure.
Technical grade lambda-cyhalothrin is a beige solid (melting
point: 49.2 °C) and contains more than 90% active material. The
enantiomer ratio of the (Z), (1R, 3R), S-ester to the (Z), (1S,
3S), R-ester is 1:1. It is sparingly soluble in water but soluble
in a range of organic solvents and has a low vapour pressure. Both
cyhalothrin and lambda-cyhalothrin are rapidly hydrolysed under
alkaline conditions but not in neutral or acidic media.
Well established methods for residue and environmental analysis
of cyhalothrin and lambda-cyhalothrin are available (the minimum
detectable concentration is 0.005 mg/kg).
1.1.2. Production and use
Cyhalothrin was developed in 1977. It is principally used to
combat a wide range of pests in public health and animal health,
but is also employed in agriculture against pests of pome fruit.
Lambda-cyhalothrin is mainly used as an agricultural pesticide on a
wide range of crops and is being developed for public health.
No data are available on production levels.
1.1.3. Human exposure
Residues in food arising from the use of cyhalothrin and
lambda-cyhalothrin on crops and in animal health are low, usually
less than 0.2 mg/kg. No results are available on the total dietary
intake in humans, but it can be assumed that the dietary exposure
of the general population will not exceed the ADI (0.02 mg/kg body
weight).
1.1.4. Environmental exposure and fate
On soil surfaces and in aqueous solutions at pH 5, lambda-
cyhalothrin degrades in sunlight with a half-life of approximately
30 days. The main degradation products are 3-(2-chloro-3,3,3-
trifluoroprop-1-enyl)-2,2-dimethyl-cyclopropanecarboxylic acid, the
amide derivative of cyhalothrin, and 3-phenoxybenzoic acid.
Degradation in soil occurs primarily by hydroxylation followed
by cleavage of the ester linkage to give two main degradation
products, which are further degraded to carbon dioxide. The
initial half-lives are in the range of 22 to 82 days.
Cyhalothrin and lambda-cyhalothrin are adsorbed on soil
particles and are non-mobile in the environment.
On plants lambda-cyhalothrin degrades at a moderate rate (half-
life of up to 40 days), so that the major constituent of the
residue on plants is usually the parent compound. Lower levels of
metabolites, resulting from a range of hydrolytic and oxidative
reactions, are also found.
No data are available on actual levels in the environment, but
with the low current use pattern and low application rates, these
are expected to be low.
1.1.5. Uptake, metabolism, and excretion
Metabolic studies have been carried out on the rat, dog, cow,
and goat. In rats and dogs, cyhalothrin has been shown to be well
absorbed after oral administration, extensively metabolized, and
eliminated as polar conjugates in urine. Cyhalothrin levels in rat
tissues declined upon cessation of exposure to the compound.
Residues in rat carcasses were low (< 5% of the dose after 7 days)
and were found to be almost entirely due to cyhalothrin contained
in fats. Residues in fats were eliminated with a half-life of 23
days.
After oral administration to lactating cows, cyhalothrin was
rapidly eliminated, an equilibrium between ingestion and
elimination being reached after 3 days. Of the overall dose, 27%
was excreted in the urine, 50% in the faeces, and 0.8% in the milk.
Urinary material consisted entirely of ester cleavage metabolites
and their conjugates, whereas 60-70% of the faecal [14C]-labelled
material was identified as unchanged cyhalothrin. Tissue
residues, 16 h after the last dose, were low, the highest
concentrations being detected in fat. The [14C]-labelled residues
in milk and fatty tissues were almost entirely unchanged
cyhalothrin, no other component being detected.
In all mammalian species investigated, cyhalothrin has been
found to be extensively metabolized as a result of ester cleavage
to the cyclopropanecarboxylic acid and 3-phenoxybenzoic acid, and
eliminated as conjugates.
In fish the main residue in tissues consists of unchanged
cyhalothrin, and there are lower levels of the ester cleavage
products.
1.1.6. Effects on organisms in the environment
Under laboratory conditions of constant toxicant
concentrations, cyhalothrin and lambda-cyhalothrin are highly toxic
to fish and to aquatic invertebrates. The 96-h LC50 values for
fish range between 0.2 and 1.3 µg/litre, whereas for aquatic
invertebrates the 48-h LC50 values range between 0.008 and 0.4
µg/litre.
Accumulation studies conducted under laboratory conditions
with constant concentration show that rapid uptake takes place in
fish (accumulation factor approximately 1000-2000). However, in the
presence of soil and suspended sediment, the bioaccumulation
factors are greatly reduced (to 19 in the case of fish and 194 in
the case of daphnids). When exposed fish and daphnids were placed
in clean water the residues declined rapidly, with half-lives of 7
days and 1 day, respectively. The concentrations of cyhalothrin
and lambda-cyhalothrin that are likely to arise in water from
normal agricultural application will be low. Since the compound is
rapidly adsorbed and degraded under natural conditions, there will
not be any practical problems concerning the accumulation of
residues or the toxicity of cyhalothrin or lambda-cyhalothrin in
aquatic species.
Cyhalothrin and lambda-cyhalothrin are virtually non-toxic to
birds; the single-dose LD50 was greater than 3950 mg/kg in all
species tested and the lowest 5-day dietary LC50 was 3948 mg/kg
(lambda-cyhalothrin fed to 8-day-old mallard ducks).
Under laboratory conditions, cyhalothrin and lambda-cyhalothrin
are toxic to honey-bees; the oral LD50 for lambda-cyhalothrin is
0.97 µg/bee. However, in the field the hazard is lower since
current formulations have a repellant action that causes a
suspension of foraging activity in treated crops. When foraging
restarts there is no significant increase in bee mortality.
1.1.7. Effects on experimental animals and in vitro
test systems
The acute oral toxicity of cyhalothrin is moderate in rats and
mice and low in guinea-pigs and rabbits (LD50 values are as
follows: rat, 144-243 mg/kg; mouse, 37-62 mg/kg; guinea-pig,
> 5000 mg/kg; rabbit, > 1000 mg/kg). The acute oral toxicity of
lambda-cyhalothrin is higher than that of cyhalothrin (LD50 values
are: 56-79 mg/kg for the rat and 20 mg/kg for the mouse). The
dermal toxicities (LD50) are as follows: rat, 200-2000 mg/kg
(cyhalothrin), 632-696 mg/kg (lambda-cyhalothrin); rabbit, > 2000
mg/kg (cyhalothrin). Cyhalothrin and lambda-cyhalothrin are type
II pyrethroids; clinical signs include ataxia, unsteady gait, and
hyperexcitability.
In the rabbit, cyhalothrin is a moderate eye irritant and
lambda-cyhalothrin is a mild eye irritant; both are mild skin
irritants. Cyhalothrin is not a skin irritant in the rat.
However, it is a moderate skin sensitizer in the guinea-pig.
Lambda-cyhalothrin is not a skin sensitizer.
In a 90-day feeding study in which rats were fed cyhalothrin at
dose levels up to 250 mg/kg diet, reduced body weight gains were
observed in males at 250 mg/kg diet. Marginal effects on mean
erythrocyte volumes were noted in some treated groups, as well as
some liver changes, which were considered to be an adaptive
response. In a 90-day feeding study in which rats were fed lambda-
cyhalothrin at dose levels up to 250 mg/kg diet, reduced body
weight gain was observed in both sexes at 250 mg/kg diet. Some
effects on clinical chemistry were observed, as well as liver
effects similar to those noted with cyhalothrin. The no-observed-
effect level was 50 mg/kg diet.
In a 26-week oral study in which cyhalothrin doses of up to 10
mg/kg body weight per day were administered to dogs, signs of
pyrethroid toxicity were observed at 10 mg per kg body weight per
day. The no-observed-effect level was 2.5 mg/kg body weight per
day. A similar study was conducted in which up to 3.5 mg lambda-
cyhalothrin/kg body weight per day was administered to dogs for 52
weeks. Clinical signs of pyrethroid toxicity (neurological signs)
were observed in all animals dosed with 3.5 mg/kg body weight per
day. The no-observed-effect level was 0.5 mg/kg body weight per
day.
In a 21-day dermal study on rabbits using cyhalothrin in
polyethylene glycol at dose levels of up to 1000 mg/kg per day,
clinical signs of toxicity were observed in some animals at the
highest dose level. Slight to severe skin irritation was observed
in all groups, including controls.
Cyhalothrin was tested in two 104-week feeding studies, one on
rats and one on mice. In the rat study, no oncogenic effects were
observed at dose levels up to 250 mg/kg diet (highest level
tested). The no-observed-effect level for systemic toxicity was 50
mg/kg diet (1.8 mg/kg body weight per day). Decreased body weight
gain was observed in both sexes at 250 mg/kg diet. In the mouse
study, no oncogenic effects were observed at dose levels up to 500
mg/kg diet (highest level tested). Clinical signs of pyrethroid
toxicity were observed at 100 and 500 mg/kg diet, and reduced body
weight gain was observed at 500 mg/kg diet. The no-observed-effect
level for systemic toxicity was 20 mg/kg diet (1.9 mg/kg body
weight per day). No histological evidence of damage to the nervous
system was observed in either study.
Cyhalothrin and lambda-cyhalothrin gave negative results in a
range of in vivo and in vitro assays designed to detect gene
mutations, chromosomal damage, and other genotoxic effects. When
orally administered to the rat and rabbit during the period of
major organogenesis, cyhalothrin was neither embryotoxic nor
teratogenic at dose levels that elicited maternal toxicity (15
mg/kg per day for rats and 30 mg/kg per day for rabbits, both
highest dose levels tested).
A three-generation reproduction study was conducted on rats
with cyhalothrin at dose levels of up to 100 mg/kg diet. Minor
decreases in litter size and small reductions in weight gain were
seen at 100 mg/kg diet. The no-observed-effect level for
reproductive effects was 30 mg per kg diet.
1.1.8. Effects on humans
No cases of accidental poisoning have been described.
In manufacturing, formulation, laboratory work, and field
usage, symptoms of subjective facial sensation have been reported.
This effect generally lasts only a few hours, but occasionally
persists for up to 72 h after exposure; medical examination has not
revealed any neurological abnormalities.
Subjective facial skin sensations, which may be experienced by
people who handle cyhalothrin and lambda-cyhalothrin, are believed
to be brought about by repetitive firing of sensory nerve
terminals in the skin. They may be considered as an early warning
signal indicating that overexposure of the skin has occurred.
There are no indications that cyhalothrin and lambda-
cyhalothrin, used under the present recommended conditions and
application rates, will have any adverse effect on humans.
1.2. Conclusions
(a) General population: The exposure of the general population to
cyhalothrin and lambda-cyhalothrin is expected to be very low and
is not likely to present a hazard under recommended conditions of
use.
(b) Occupational exposure: With good work practices, hygiene
measures, and safety precautions, cyhalothrin and lambda-
cyhalothrin are unlikely to present a hazard to those
occupationally exposed.
(c) Environment: It is unlikely that cyhalothrin and lambda-
cyhalothrin or their degradation products will attain levels of
adverse environmental significance with recommended application
rates. Under laboratory conditions cyhalothrin and lambda-
cyhalothrin are highly toxic to fish, aquatic arthropods, and
honey-bees. However, under field conditions, lasting adverse
effects are not likely to occur under recommended conditions of
use.
1.3. Recommendations
Although dietary levels from recommended usage are considered
to be very low, confirmation of this through inclusion of
cyhalothrin and lambda-cyhalothrin in monitoring studies should be
considered.
Although cyhalothrin and lambda-cyhalothrin have been used for
several years and any effects from occupational exposure have been
only transient, observations of human exposure should be
maintained.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Molecular formula: C23H19ClF3NO3
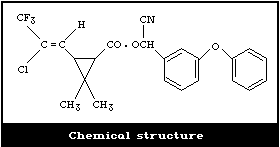 Chemical name: alpha-cyano-3-phenoxybenzyl 3-(2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-dimethyl-
cyclopropanecarboxylate
CAS Chemical ( RS)-alpha-cyano-3-(phenoxyphenyl)methyl
name: ( 1RS)-cis-3-( Z-2-chloro-3,3,3-tri-
fluoroprop-1-enyl)-2,2-dimethylcyclopro-
panecarboxylate
CAS registry: cyhalothrin: 68085-85-8
number lambda-cyhalothrin: 91465-08-6
Common cyhalothrin: R114563, PP563
synonyms: lambda-cyhalothrin: R119321, PP321
Trade names: cyhalothrin: Grenade
lambda-cyhalothrin: Karate, Matador, Icon
Cyhalothrin was developed by ICI in 1977. It is prepared by
esterification of 3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid chloride with alpha-cyano-3-
phenoxybenzyl alcohol.
Cyhalothrin has two asymmetric centres in the acid moiety and
one in the alcohol moiety, as well as Z and E forms. Thus, there
are 16 possible isomeric forms (eight enantiomeric pairs).
However, in practice cyhalothrin is produced only in the Z and cis
forms, reducing the number of isomers to four. These comprise two
cis enantiomeric pairs:
Enantiomer pair A: ( Z), (1R, 3R ), R-alpha-cyano ( Z),
( 1S, 3S) S-alpha-cyano;
Enantiomer pair B: ( Z), (1R, 3R), S-alpha-cyano ( Z),
( 1S, 3S) R-alpha-cyano.
Chemical name: alpha-cyano-3-phenoxybenzyl 3-(2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-dimethyl-
cyclopropanecarboxylate
CAS Chemical ( RS)-alpha-cyano-3-(phenoxyphenyl)methyl
name: ( 1RS)-cis-3-( Z-2-chloro-3,3,3-tri-
fluoroprop-1-enyl)-2,2-dimethylcyclopro-
panecarboxylate
CAS registry: cyhalothrin: 68085-85-8
number lambda-cyhalothrin: 91465-08-6
Common cyhalothrin: R114563, PP563
synonyms: lambda-cyhalothrin: R119321, PP321
Trade names: cyhalothrin: Grenade
lambda-cyhalothrin: Karate, Matador, Icon
Cyhalothrin was developed by ICI in 1977. It is prepared by
esterification of 3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid chloride with alpha-cyano-3-
phenoxybenzyl alcohol.
Cyhalothrin has two asymmetric centres in the acid moiety and
one in the alcohol moiety, as well as Z and E forms. Thus, there
are 16 possible isomeric forms (eight enantiomeric pairs).
However, in practice cyhalothrin is produced only in the Z and cis
forms, reducing the number of isomers to four. These comprise two
cis enantiomeric pairs:
Enantiomer pair A: ( Z), (1R, 3R ), R-alpha-cyano ( Z),
( 1S, 3S) S-alpha-cyano;
Enantiomer pair B: ( Z), (1R, 3R), S-alpha-cyano ( Z),
( 1S, 3S) R-alpha-cyano.
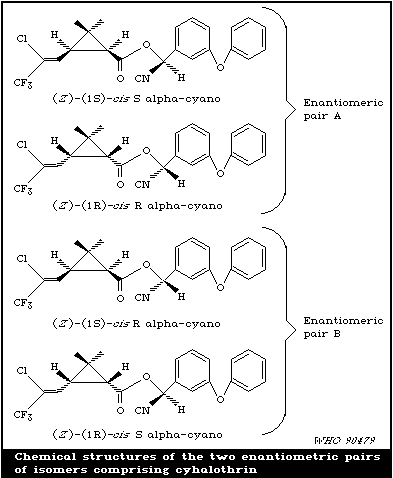 Lambda-cyhalothrin is manufactured by crystallization of the
more active pair of enantiomers from cyhalothrin. The less active
pair of enantiomers is recycled.
Pure lambda-cyhalothrin is a racemic mixture of the enantiomer
pair B isomers. The enantiomer pair A is present in low
concentration in the commercial product.
Technical grade cyhalothrin contains more than 90% of the
pesticide and is formulated in 5%, 10%, and 20% emulsifiable
concentrates. Technical grade lambda-cyhalothrin also contains
more than 90% active ingredient. It is formulated as 2.5%, 5.0%,
8.3%, and 12% emulsifiable concen-trates and as a 0.8% ultra-low
volume concentrate.
2.2. Physical and chemical properties
Some physical and chemical properties of cyhalothrin and
lambda-cyhalothrin are listed in Table 1.
Table 1. Some physical and chemical properties of cyhalothrin and
lambda-cyhalothrin
---------------------------------------------------------------------------
Cyhalothrin Lambda-cyhalothrin
---------------------------------------------------------------------------
Physical state viscous liquid solid
Colour yellow-brown beige
Odour mild mild
Relative molecular mass 449.9 449.9
Melting point glass-like 49.2 °C
below 10 °C
Decomposes > 275 °C > 275 °C
Water solubility 4 x 10-3 mg/litre 5 x 10-3 mg/litre
Solubility in organic solvents soluble soluble
n-octanol water-partition 6.9 7.0
coefficient (log Pow) at 20 °C
Relative density 1.25 1.33
Vapour pressure at 20 °C 1 x 10-9 kPa 2 x 10-10 kPa
Vapour pressure at 80 °C 4 x 10-6 kPa 3 x 10-6 kPa
---------------------------------------------------------------------------
No boiling point data are available as both forms decompose on
heating above 275 °C. Cyhalothrin is highly stable to light and at
temperatures below 220 °C.
Lambda-cyhalothrin is stable in water at pH 5. At pH 7 and pH
9, there is racemization at the alpha-cyano carbon to yield a 1:1
mixture of enantiomer pairs A and B. At pH 9, the ester bond is
fairly readily hydrolysed (half-life, 7 days) (Collis & Leahey,
1984).
Dilute aqueous solutions are subject to photolysis at a
moderate rate (Hall & Leahey, 1983; Curl et al., 1984a).
2.3. Analytical methods
The most widely adopted procedures for analysing cyhalothrin
residues in crops, soil, animal tissues and products, and
environmental samples are based on extraction of the residue with
organic solvent, clean-up of the extract by solvent-solvent
partition and adsorption column chromatography, and determination
of the residue using gas chromatography (GC) with electron capture
detection (GC/ECD). The identity of residues can be confirmed by
GC with mass selective detection (GC-MSD) or by thin-layer
chromatography (TLC) followed by GC/ECD.
2.3.1. Sampling methods
Procedures for obtaining representative samples of crops,
processed commodities, soil, and some animal products have been
described in detail (GIFAP, 1981) and will not be discussed
further.
Particular care is necessary when sampling water because
cyhalothrin is extremely hydrophobic and rapidly adsorbs onto
particulates or container walls from aqueous solution. For this
reason, the whole analytical sample should be taken for analysis
and not subdivided in the field (Sapiets et al., 1984). Collection
of the sample in a clean glass container, with addition of the
extraction solvent before sealing and shaking the bottle, gives
good recoveries. Crossland et al. (1982) described procedures for
sampling surface water (using stainless steel fine mesh discs) and
subsurface water from ponds for separate analysis of cypermethrin.
Precautions were taken to avoid contamination during sampling
(Crossland et al., 1982). These methods, developed for
cypermethrin, are equally applicable to cyhalothrin and other
pyrethroids. During the course of the pond studies, cypermethrin
spray drift deposits were collected by means of horizontally placed
aluminium foil plates and washed from the foils using acetone.
Crossland also described the use of a core sampler to remove pond
sediment for cypermethrin analysis (Crossland, 1982). This method
is equally applicable to cyhalothrin.
Sampling of air in agriculture work space for pyrethroid
aerosol droplets using absorption onto exposed filter papers and
porous glass slides has been described by Girenko & Klisenko
(1984). They quoted limits in the range of 0.05 to 0.5 mg/m3 for
the concentrations of pyrethroid that could be detected without
interference from organophosphorus or organochlorine insecticides.
An alternative and more conventional approach would be to use a
pumped device consisting of a filter (to trap droplets or
particulates) in series with a packed tube (to trap vapour).
The use of a vacuum probe was described by Bengstone et al.
(1983) to subsample pyrethroid-treated grain from several points
within a silo for combination to give a composite sample. However,
care is needed to obtain a representative sample using this
procedure (GIFAP, 1981).
2.3.2. Sample storage
Storage stability experiments using untreated samples fortified
with cyhalothrin (at 1.0 mg/kg) have shown that apples, cabbage,
soil, and products of animal origin can be stored deep frozen at
temperatures of -20 °C for periods of up to one year without
residue loss (Sapiets, 1984a)
Particular problems in the handling of water samples have
already been indicated and samples should be extracted and
analysed as soon after sampling as possible.
2.4. Sample preparation
Forbes & Dutton (1985) reported details of the procedures used
to process crop and soil samples, which have been treated with
pyrethroid insecticides, for sub-sampling before extraction. Crops
of high water content, e.g., fruit and vegetables, were chopped,
dried, or puréed. Grain and oil seed crops (cotton seed and
linseed) were frozen and ground to a powder, while crops of low
water content, e.g., straw and tobacco, were finely divided in a
rotary knife mill. Soil samples were mixed thoroughly and stones
and plant debris removed. In all cases, care was taken to avoid
localized overheating of the sample during processing. Sapiets et
al. (1984) used a similar processing procedure for high water
content crops and also applied this to meat and eggs. However,
they draw attention to the importance of processing materials with
high water content while still frozen to prevent separation of
juice, which leads to sample inhomogeneity. Milk was thoroughly
mixed before subsampling. In the limited number of other studies
where details for preparation of pyrethroid-treated samples have
been given, none of the procedures differ markedly from those
described above. All of the procedures are equally applicable to
cyhalothrin and lambda-cyhalothrin.
2.5. Gas chromatographic procedures for the determination
of cyhalothrin residues
Details of GC procedures are described in the following
subsections (Sapiets, 1984b,c, 1985a,b, 1986a,b).
2.5.1. Extraction
Representative subsamples of prepared crops or meat are blended
for 2-5 min with a mixture of acetone and hexane (1 + 1 by volume).
Dry materials are dampened with water. Soil is similarly extracted
by refluxing with acetonitrile for 1 h. Extracts are then gravity
filtered and partitioned with 5% sodium chloride solution to remove
the acetone. The hexane extract is dried over anhydrous sodium
sulfate.
Eggs are extracted by homogenizing for 5 min with acetonitrile.
An aliquot is evaporated to dryness and redissolved in hexane.
Milk is extracted by homogenizing for 2 min in acetone and
hexane (1:1 by volume). The solvent is dried over anhydrous sodium
sulfate.
2.5.2. Clean-up
Column absorption chromatography on Florisil is used for
cleaning up extracts from crops and soils, and cyhalothrin
residues are eluted with a mixture of diethyl ether in hexane.
Extracts from crops with high lipid content, animal products,
and difficult matrices, e.g., tobacco and hops, require preliminary
clean-up by liquid-liquid partition procedures.
2.5.3. Determination
Residues of cyhalothrin in cleaned-up extracts are determined
by GC/ECD using packed or capillary columns.
A variety of liquid phases have been found suitable for use
with packed columns; these include OV-25, OV-101, OV-210, and OV-
202. These are generally used at low loadings (3-5%) in the
temperature range 230-250 °C, and retention times for cyhalothrin
are normally less than 10 min. Glass columns should be used. On
these packed columns cyhalothrin is eluted as a single peak.
Capillary columns will separate the two pairs of
diastereoisomers (enantiomer pairs) of cyhalothrin to give two
peaks. Lambda-cyhalothrin under the same conditions gives a single
peak. Fused silica columns, 25 m long, coated with OV-101 have
been found suitable when separate determination of diastereoisomers
in the cyhalothrin residue is required. Retention times normally
fall within the range of 10 to 30 min.
2.5.4. Limit of determination
The limit of determination of the methods for cyhalothrin
residues in crops and animal products is set at 0.01 mg/kg on the
basis of recovery experiments at low fortification levels (0.005-
0.02 mg/kg) and background noise in the chromatograms. The limit
of determination for soils is set at 0.005 to 0.01 mg/kg and for
water is 10 ng/litre (ppt).
2.5.5. Recoveries and interference
The internal standardization procedure used in these methods
determines the concentration of cyhalothrin or lambda-cyhalothrin
relative to that of a known concentration of internal standard
added to the sample prior to extraction. Correction for percentage
recovery is thereby inherent for each individual sample. The
repeatability of the procedure for most substrates is 2 to 3%. The
methods have been found to be applicable to the determination of
cyhalothrin in a wide variety of substrates without interference
from endogenous natural products in the GC determination.
2.5.6. Confirmation of residue identity
Qualitative and quantitative confirmation of residue identity
may be achieved by combined GC-MS operated in the selected ion
monitoring mode.
3. SOURCES AND LEVELS OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production levels and processes
No data on industrial production are available.
3.2. Uses
Cyhalothrin is a pyrethroid insecticide with a high level of
activity (application rate up to 20 g/ha) against a wide range of
Lepidoptera, Hemiptera, Diptera, and Coleoptera species. It also
has some miticidal activity. Lambda-cyhalothrin has the same
spectrum of insecticidal activity as cyhalothrin but it is more
active. The compound is a stomach, contact, and residual
insecticide. It shows adulticidal, ovicidal and, particularly,
larvicidal activity.
Like other photostable synthetic pyrethroids, cyhalothrin and
lambda-cyhalothrin are relatively stable to degradation in
sunlight. This permits their use as practical tools in
agriculture. The compound is not plant-systemic and has very
little fumigant or translaminar activity.
Owing, in part, to its short persistence in soil and lack of
systemic effect, the compound is of only limited value when used as
a soil insecticide. It can, however, give useful control of
cutworms when applied as a crop/ground spray. It has no
molluscicidal or nematocidal activity.
Preventive treatments are generally more effective than
curative treatments against major pests such as boring caterpillars
or leaf miners. A programme of sprays is usually required,
particularly during the more active growth stages of the plant and
when the potential for re-infestation remains high.
Cyhalothrin has also found uses in public and animal health
applications where it effectively controls a broad spectrum of
insects, including cockroaches, flies, mosquitos, and ticks. It
has high activity as a residual spray on inert surfaces.
3.3. Residues in food
Supervised trials have been carried out on a wide variety of
crops, and comprehensive summaries of residue analysis in these
trials can be found in the evaluation reports of the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) (FAO/WHO 1985, 1986a).
Data reviewed by the JMPR showed that in studies on apples and
on pears, when different rates of application were used in the same
trial, initial residues reflected the different rates applied.
When the spray programme was doubled from three to six applications
per season, there was no increase in the lambda-cyhalothrin residue
levels over those obtained with the three applications programme at
the same rates. Lambda-cyhalothrin residue levels on apples often
declined relatively slowly, although this was not always the case.
There were no obvious differences in residue levels arising from
the use of the different strengths of emulsion concentrate
formulations or from the use of either low volume or high volume
rates of application (FAO/WHO, 1986a,b).
3.4. Levels in the environment
3.4.1. Air
No specific data on air concentrations are available. Since
cyhalothrin and lambda-cyhalothrin are of low vapour pressure,
atmospheric levels of their vapour will be negligible.
3.4.2. Water
No specific data on water levels are available. Since
cyhalothrin and lambda-cyhalothrin are insoluble in water and not
mobile in soil, they are very unlikely to reach ground water.
3.4.3. Soil
No information on concentrations in soil is available.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transportation and distribution between media
The very low vapour pressure of cyhalothrin means that this
compound will not enter the atmosphere.
Studies have shown that cyhalothrin and its soil degradation
products do not leach through soils. 14C-cyclopropane-labelled
cyhalothrin was aerobically incubated for 30 days with a sandy loam
(4.2% organic matter) and a loamy sand (2.0% organic matter). The
incubated soils (rates equivalent to 0.04 and 0.05 kg cyhalothrin
equivalents per ha for the sandy loam and loamy sand, respectively)
were then applied to soil columns (30 cm long) and leached during a
period of 9 weeks with 66 cm "rain". The radioactive residues more
than 5 cm beneath the surface were below the limit of determination
(i.e. < 0.47 ng cyhalothrin equivalents per g) in all the soil
columns. Radioactive residues in the leachate samples were also
generally below the limit of determination (i.e. < 0.023 ng
cyhalothrin equivalent per ml) and represented less than 0.3% of
the applied radiocarbon. Thus, cyhalothrin, lambda-cyhalothrin,
and their degradation products have very low mobility in soil. On
the basis of these data it is concluded that the agricultural use
of cyhalothrin or lambda-cyhalothrin will not result in the
leaching of either the parent compounds or their degradation
products into ground water (Stevens & Bewick, 1985). Furthermore,
if soils containing cyhalothrin are flooded, there is no release of
cyhalothrin into the water (Hamer & Hill, 1985). Thus, residues of
cyhalothrin in soil resulting from agricultural use will not be
transported into other compartments of the environment.
4.2. Abiotic degradation
4.2.1. Hydrolysis and photodegradation in water
In studies by Collis & Leahey (1984), aqueous solutions of 14C-
cyclopropyl-labelled lambda-cyhalothrin, buffered at pH 5, 7, and
9, were maintained in the dark at 25° C for periods of up to 30
days. Acetonitrile (1%) was used as a co-solvent to facilitate
dissolution in the water. Hydrolysis of lambda-cyhalothrin
occurred rapidly in the pH 9 aqueous buffer solution (half-life,
approxi-mately 7 days) via ester cleavage of the molecule to yield
(1 RS)-cis-3-( Z-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid. Rapid isomerization of the
optical centre at the alpha-CN position also occurred at pH 9. At
pH 7 no hydrolysis was detected, but slow isomerization did occur.
At pH 5 no hydrolysis or isomerization was observed (Collis &
Leahey, 1984).
When quartz flasks containing 14C-cyclopropyl-labelled lambda-
cyhalothrin in pH 5 buffer were exposed to sunlight for 30 days,
the compound underwent photodegradation with a half-life of
approximately 30 days. (1 RS)-cis- and (1 RS)-trans-3-( ZE-2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylic
acids and ( RS)-alpha-amido-3-phenoxybenzyl (1 RS)-cis,trans-3-( ZE-2-
chloro-3,3,3-tri-fluoroprop-1-enyl)-2,2-dimethylcyclopropanecarb-
oxylate were the major degradation products formed. Optical and
geometrical isomerization of lambda-cyhalothrin occurred in the
irradiated flasks. No photodegradation or photoisomerization was
observed in a dark control flask (Curl et al., 1984a).
In studies by Hall & Leahey (1983), 14C-cyhalothrin was
incubated with two river water/sediment mixtures contained in
quartz flasks. The flasks were either exposed to sunlight or were
maintained under dark conditions by covering them with aluminium
foil. In the dark, degradation of cyhalothrin was slow (over 80%
remained unchanged after 32 days). However, when exposed to
sunlight the cyhalothrin degraded with a half-life of approximately
20 days in both river water/sediment mixtures. The rate at which
the parent compound was lost from the aqueous phase was, however,
much faster than its rate of degradation in the whole
water/sediment system. This was due to the ready absorption of
cyhalothrin onto the sediment. The major degradation process was
simple ester cleavage of the molecule, producing (1 RS)-cis- and
(1 RS)-trans-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acids. After 32 days of
irradiation, these compounds together represented 36-47% of the
radioactivity applied to the water/sediment systems. Some
photoisomerization also occurred.
4.2.2. Photodegradation in soil
When thin-layer soil plates were treated with 14C-cyclopropyl-
labelled lambda-cyhalothrin and irradiated in a xenon arc apparatus
or in sunlight, the half-life of lambda-cyhalothrin was less than 2
days in the xenon arc apparatus and less than 30 days in sunlight.
(1 RS)-cis-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropane-carboxylic acid and ( RS)-alpha-amido-3-
phenoxybenzyl (1 RS)-cis,trans-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-
enyl)-2,2-dimethylcyclopropanecarboxylate were the major
degradation products (Curl et al., 1984b).
4.3. Biodegradation in soil
Cyhalothrin degradation in the outdoor environment may occur by
either biological or photochemical processes. In most cases,
biological processes are by far the most important, although
photochemical reactions can sometimes contribute to the degradation
of residues on exposed surfaces.
4.3.1. Degradation rate
At residue levels that are likely to occur under normal field
conditions, cyhalothrin is degraded rapidly in soil. When a sandy
loam soil was treated with 14C-cyclopropyl-labelled cyhalothrin,
only 28% of the recovered radioactivity was present as cyhalothrin
after five weeks of incubation under aerobic conditions; 30% was
evolved as 14C-labelled carbon dioxide and 3.5% of the recovered
radioactivity was due to 1 RS-cis-3-( Z-2-chloro-3,3,3-trifluoroprop-
1-enyl)-2,2-dimethylcyclopropanecarboxylic acid. Approximately 19%
of the radioactivity was not extracted using acetonitrile at room
temperature followed by soxhlet extraction with aqueous
acetonitrile (Bewick & Zinner, 1981).
In a more recent and detailed study, 14C-cyclopropyl-labelled
cyhalothrin was applied to two soils (a sandy loam and a loamy
sand) at 100 g/ha and incubated under both aerobic and flooded
conditions at 20 °C. The sandy loam soil was also treated with
cyhalothrin at 500 g/ha and, in a later experiment, treated
separately with the two enantiomer pairs of cyhalothrin (one of
which constitutes lambda-cyhalothrin). This soil was also
incubated at 10 °C. All the isomers of cyhalothrin, including
those that constitute lambda-cyhalothrin, were readily degraded in
soil under a range of conditions. Half-lives for cyhalothrin at
20 °C in the sandy loam and loamy sand soils were 22 and 82 days,
respectively. Degradation was somewhat slower in the sandy loam at
higher rates (half-life: 42 days), lower temperature (half-life: 56
days) and under flooded conditions (half-life: 74 days). Lambda-
cyhalothrin was degraded at about 70% the rate of the other
enantiomer pair of cyhalothrin in the aerobic soils but at
approximately the same rate under flooded conditions. In the
aerobic soils, the principle degradative reactions were
hydroxylation, yielding up to 11% of the applied radiocarbon as
( RS)-alpha-cyano-3-(4-hydroxyphenoxy) benzyl cis-3-( Z-2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-di-methylcyclopropanecarboxylate
(compound XV), and hydrolysis, yielding up to 7% as ( RS)-cis-3-( Z-
2-chloro-3,3,3-tri-fluoroprop-1-enyl)-2,2-dimethylcyclopropane-
carboxylic acid (compound Ia). In the flooded soil, hydrolysis was
the main degradative reaction (up to 18% of compound Ia in the soil
phase), and hydroxylation was less important (only up to 1.4% of
compound XV). Compound Ia was the only compound detected (up to
17%) in the aqueous phase of the flooded soils. No isomerization
of the parent esters or their hydrolysis product was detected. The
initial degradation products of cyhalothrin and lambda-cyhalothrin
in the aerobic soils were rapidly further degraded with extensive
mineralization (up to 70% in 26 weeks) to 14CO2 (Bharti et al.,
1985). Thus the acid moiety of cyhalothrin is readily mineralized
in soil. The alcohol moiety of this compound is identical to that
of cypermethrin. Studies with cypermethrin show that, after ester
cleavage, the alcohol moiety released is readily mineralized to
14CO2 (FAO/WHO, 1986a).
In a further study, when 14C-cyclopropyl-labelled cyhalothrin
was applied (at 100 g/ha) to a Japanese upland soil (a volcanic
ash) and incubated under aerobic conditions at 20 °C, the half-
life of cyhalothrin was approximately 100 days. The principle
degradative reactions were hydroxylation, yielding up to 7% of the
applied radiocarbon as compound XV, and hydrolysis, yielding up to
ca. 2% as compound Ia. The initial degradation products of
cyhalothrin were further degraded with mineralization (up to 17% in
26 weeks) to 14CO2 (Bharti & Bewick, 1986).
Four sites in the USA were treated with lambda-cyhalothrin
(1.1 kg/ha), and soil was sampled and analysed at intervals up to 9
months after treatment. Residues remained in the top 15 cm of
soil, except at one site where low residues immediately after
treatment and at 2 days were attributed to contamination during
sampling. Initial soil residues of 0.18 to 0.31 mg/kg declined to
extremely low levels (< 0.01 to 0.02 mg/kg) during the course of
the study, and at one site the residues were less than the limit of
determination after 91 days (Fitzpatrick, 1985).
4.3.2. Degradation pathways
Degradation pathways for cyhalothrin and lambda-cyhalothrin in
soil are shown in Figure 2.
Lambda-cyhalothrin is manufactured by crystallization of the
more active pair of enantiomers from cyhalothrin. The less active
pair of enantiomers is recycled.
Pure lambda-cyhalothrin is a racemic mixture of the enantiomer
pair B isomers. The enantiomer pair A is present in low
concentration in the commercial product.
Technical grade cyhalothrin contains more than 90% of the
pesticide and is formulated in 5%, 10%, and 20% emulsifiable
concentrates. Technical grade lambda-cyhalothrin also contains
more than 90% active ingredient. It is formulated as 2.5%, 5.0%,
8.3%, and 12% emulsifiable concen-trates and as a 0.8% ultra-low
volume concentrate.
2.2. Physical and chemical properties
Some physical and chemical properties of cyhalothrin and
lambda-cyhalothrin are listed in Table 1.
Table 1. Some physical and chemical properties of cyhalothrin and
lambda-cyhalothrin
---------------------------------------------------------------------------
Cyhalothrin Lambda-cyhalothrin
---------------------------------------------------------------------------
Physical state viscous liquid solid
Colour yellow-brown beige
Odour mild mild
Relative molecular mass 449.9 449.9
Melting point glass-like 49.2 °C
below 10 °C
Decomposes > 275 °C > 275 °C
Water solubility 4 x 10-3 mg/litre 5 x 10-3 mg/litre
Solubility in organic solvents soluble soluble
n-octanol water-partition 6.9 7.0
coefficient (log Pow) at 20 °C
Relative density 1.25 1.33
Vapour pressure at 20 °C 1 x 10-9 kPa 2 x 10-10 kPa
Vapour pressure at 80 °C 4 x 10-6 kPa 3 x 10-6 kPa
---------------------------------------------------------------------------
No boiling point data are available as both forms decompose on
heating above 275 °C. Cyhalothrin is highly stable to light and at
temperatures below 220 °C.
Lambda-cyhalothrin is stable in water at pH 5. At pH 7 and pH
9, there is racemization at the alpha-cyano carbon to yield a 1:1
mixture of enantiomer pairs A and B. At pH 9, the ester bond is
fairly readily hydrolysed (half-life, 7 days) (Collis & Leahey,
1984).
Dilute aqueous solutions are subject to photolysis at a
moderate rate (Hall & Leahey, 1983; Curl et al., 1984a).
2.3. Analytical methods
The most widely adopted procedures for analysing cyhalothrin
residues in crops, soil, animal tissues and products, and
environmental samples are based on extraction of the residue with
organic solvent, clean-up of the extract by solvent-solvent
partition and adsorption column chromatography, and determination
of the residue using gas chromatography (GC) with electron capture
detection (GC/ECD). The identity of residues can be confirmed by
GC with mass selective detection (GC-MSD) or by thin-layer
chromatography (TLC) followed by GC/ECD.
2.3.1. Sampling methods
Procedures for obtaining representative samples of crops,
processed commodities, soil, and some animal products have been
described in detail (GIFAP, 1981) and will not be discussed
further.
Particular care is necessary when sampling water because
cyhalothrin is extremely hydrophobic and rapidly adsorbs onto
particulates or container walls from aqueous solution. For this
reason, the whole analytical sample should be taken for analysis
and not subdivided in the field (Sapiets et al., 1984). Collection
of the sample in a clean glass container, with addition of the
extraction solvent before sealing and shaking the bottle, gives
good recoveries. Crossland et al. (1982) described procedures for
sampling surface water (using stainless steel fine mesh discs) and
subsurface water from ponds for separate analysis of cypermethrin.
Precautions were taken to avoid contamination during sampling
(Crossland et al., 1982). These methods, developed for
cypermethrin, are equally applicable to cyhalothrin and other
pyrethroids. During the course of the pond studies, cypermethrin
spray drift deposits were collected by means of horizontally placed
aluminium foil plates and washed from the foils using acetone.
Crossland also described the use of a core sampler to remove pond
sediment for cypermethrin analysis (Crossland, 1982). This method
is equally applicable to cyhalothrin.
Sampling of air in agriculture work space for pyrethroid
aerosol droplets using absorption onto exposed filter papers and
porous glass slides has been described by Girenko & Klisenko
(1984). They quoted limits in the range of 0.05 to 0.5 mg/m3 for
the concentrations of pyrethroid that could be detected without
interference from organophosphorus or organochlorine insecticides.
An alternative and more conventional approach would be to use a
pumped device consisting of a filter (to trap droplets or
particulates) in series with a packed tube (to trap vapour).
The use of a vacuum probe was described by Bengstone et al.
(1983) to subsample pyrethroid-treated grain from several points
within a silo for combination to give a composite sample. However,
care is needed to obtain a representative sample using this
procedure (GIFAP, 1981).
2.3.2. Sample storage
Storage stability experiments using untreated samples fortified
with cyhalothrin (at 1.0 mg/kg) have shown that apples, cabbage,
soil, and products of animal origin can be stored deep frozen at
temperatures of -20 °C for periods of up to one year without
residue loss (Sapiets, 1984a)
Particular problems in the handling of water samples have
already been indicated and samples should be extracted and
analysed as soon after sampling as possible.
2.4. Sample preparation
Forbes & Dutton (1985) reported details of the procedures used
to process crop and soil samples, which have been treated with
pyrethroid insecticides, for sub-sampling before extraction. Crops
of high water content, e.g., fruit and vegetables, were chopped,
dried, or puréed. Grain and oil seed crops (cotton seed and
linseed) were frozen and ground to a powder, while crops of low
water content, e.g., straw and tobacco, were finely divided in a
rotary knife mill. Soil samples were mixed thoroughly and stones
and plant debris removed. In all cases, care was taken to avoid
localized overheating of the sample during processing. Sapiets et
al. (1984) used a similar processing procedure for high water
content crops and also applied this to meat and eggs. However,
they draw attention to the importance of processing materials with
high water content while still frozen to prevent separation of
juice, which leads to sample inhomogeneity. Milk was thoroughly
mixed before subsampling. In the limited number of other studies
where details for preparation of pyrethroid-treated samples have
been given, none of the procedures differ markedly from those
described above. All of the procedures are equally applicable to
cyhalothrin and lambda-cyhalothrin.
2.5. Gas chromatographic procedures for the determination
of cyhalothrin residues
Details of GC procedures are described in the following
subsections (Sapiets, 1984b,c, 1985a,b, 1986a,b).
2.5.1. Extraction
Representative subsamples of prepared crops or meat are blended
for 2-5 min with a mixture of acetone and hexane (1 + 1 by volume).
Dry materials are dampened with water. Soil is similarly extracted
by refluxing with acetonitrile for 1 h. Extracts are then gravity
filtered and partitioned with 5% sodium chloride solution to remove
the acetone. The hexane extract is dried over anhydrous sodium
sulfate.
Eggs are extracted by homogenizing for 5 min with acetonitrile.
An aliquot is evaporated to dryness and redissolved in hexane.
Milk is extracted by homogenizing for 2 min in acetone and
hexane (1:1 by volume). The solvent is dried over anhydrous sodium
sulfate.
2.5.2. Clean-up
Column absorption chromatography on Florisil is used for
cleaning up extracts from crops and soils, and cyhalothrin
residues are eluted with a mixture of diethyl ether in hexane.
Extracts from crops with high lipid content, animal products,
and difficult matrices, e.g., tobacco and hops, require preliminary
clean-up by liquid-liquid partition procedures.
2.5.3. Determination
Residues of cyhalothrin in cleaned-up extracts are determined
by GC/ECD using packed or capillary columns.
A variety of liquid phases have been found suitable for use
with packed columns; these include OV-25, OV-101, OV-210, and OV-
202. These are generally used at low loadings (3-5%) in the
temperature range 230-250 °C, and retention times for cyhalothrin
are normally less than 10 min. Glass columns should be used. On
these packed columns cyhalothrin is eluted as a single peak.
Capillary columns will separate the two pairs of
diastereoisomers (enantiomer pairs) of cyhalothrin to give two
peaks. Lambda-cyhalothrin under the same conditions gives a single
peak. Fused silica columns, 25 m long, coated with OV-101 have
been found suitable when separate determination of diastereoisomers
in the cyhalothrin residue is required. Retention times normally
fall within the range of 10 to 30 min.
2.5.4. Limit of determination
The limit of determination of the methods for cyhalothrin
residues in crops and animal products is set at 0.01 mg/kg on the
basis of recovery experiments at low fortification levels (0.005-
0.02 mg/kg) and background noise in the chromatograms. The limit
of determination for soils is set at 0.005 to 0.01 mg/kg and for
water is 10 ng/litre (ppt).
2.5.5. Recoveries and interference
The internal standardization procedure used in these methods
determines the concentration of cyhalothrin or lambda-cyhalothrin
relative to that of a known concentration of internal standard
added to the sample prior to extraction. Correction for percentage
recovery is thereby inherent for each individual sample. The
repeatability of the procedure for most substrates is 2 to 3%. The
methods have been found to be applicable to the determination of
cyhalothrin in a wide variety of substrates without interference
from endogenous natural products in the GC determination.
2.5.6. Confirmation of residue identity
Qualitative and quantitative confirmation of residue identity
may be achieved by combined GC-MS operated in the selected ion
monitoring mode.
3. SOURCES AND LEVELS OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production levels and processes
No data on industrial production are available.
3.2. Uses
Cyhalothrin is a pyrethroid insecticide with a high level of
activity (application rate up to 20 g/ha) against a wide range of
Lepidoptera, Hemiptera, Diptera, and Coleoptera species. It also
has some miticidal activity. Lambda-cyhalothrin has the same
spectrum of insecticidal activity as cyhalothrin but it is more
active. The compound is a stomach, contact, and residual
insecticide. It shows adulticidal, ovicidal and, particularly,
larvicidal activity.
Like other photostable synthetic pyrethroids, cyhalothrin and
lambda-cyhalothrin are relatively stable to degradation in
sunlight. This permits their use as practical tools in
agriculture. The compound is not plant-systemic and has very
little fumigant or translaminar activity.
Owing, in part, to its short persistence in soil and lack of
systemic effect, the compound is of only limited value when used as
a soil insecticide. It can, however, give useful control of
cutworms when applied as a crop/ground spray. It has no
molluscicidal or nematocidal activity.
Preventive treatments are generally more effective than
curative treatments against major pests such as boring caterpillars
or leaf miners. A programme of sprays is usually required,
particularly during the more active growth stages of the plant and
when the potential for re-infestation remains high.
Cyhalothrin has also found uses in public and animal health
applications where it effectively controls a broad spectrum of
insects, including cockroaches, flies, mosquitos, and ticks. It
has high activity as a residual spray on inert surfaces.
3.3. Residues in food
Supervised trials have been carried out on a wide variety of
crops, and comprehensive summaries of residue analysis in these
trials can be found in the evaluation reports of the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) (FAO/WHO 1985, 1986a).
Data reviewed by the JMPR showed that in studies on apples and
on pears, when different rates of application were used in the same
trial, initial residues reflected the different rates applied.
When the spray programme was doubled from three to six applications
per season, there was no increase in the lambda-cyhalothrin residue
levels over those obtained with the three applications programme at
the same rates. Lambda-cyhalothrin residue levels on apples often
declined relatively slowly, although this was not always the case.
There were no obvious differences in residue levels arising from
the use of the different strengths of emulsion concentrate
formulations or from the use of either low volume or high volume
rates of application (FAO/WHO, 1986a,b).
3.4. Levels in the environment
3.4.1. Air
No specific data on air concentrations are available. Since
cyhalothrin and lambda-cyhalothrin are of low vapour pressure,
atmospheric levels of their vapour will be negligible.
3.4.2. Water
No specific data on water levels are available. Since
cyhalothrin and lambda-cyhalothrin are insoluble in water and not
mobile in soil, they are very unlikely to reach ground water.
3.4.3. Soil
No information on concentrations in soil is available.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transportation and distribution between media
The very low vapour pressure of cyhalothrin means that this
compound will not enter the atmosphere.
Studies have shown that cyhalothrin and its soil degradation
products do not leach through soils. 14C-cyclopropane-labelled
cyhalothrin was aerobically incubated for 30 days with a sandy loam
(4.2% organic matter) and a loamy sand (2.0% organic matter). The
incubated soils (rates equivalent to 0.04 and 0.05 kg cyhalothrin
equivalents per ha for the sandy loam and loamy sand, respectively)
were then applied to soil columns (30 cm long) and leached during a
period of 9 weeks with 66 cm "rain". The radioactive residues more
than 5 cm beneath the surface were below the limit of determination
(i.e. < 0.47 ng cyhalothrin equivalents per g) in all the soil
columns. Radioactive residues in the leachate samples were also
generally below the limit of determination (i.e. < 0.023 ng
cyhalothrin equivalent per ml) and represented less than 0.3% of
the applied radiocarbon. Thus, cyhalothrin, lambda-cyhalothrin,
and their degradation products have very low mobility in soil. On
the basis of these data it is concluded that the agricultural use
of cyhalothrin or lambda-cyhalothrin will not result in the
leaching of either the parent compounds or their degradation
products into ground water (Stevens & Bewick, 1985). Furthermore,
if soils containing cyhalothrin are flooded, there is no release of
cyhalothrin into the water (Hamer & Hill, 1985). Thus, residues of
cyhalothrin in soil resulting from agricultural use will not be
transported into other compartments of the environment.
4.2. Abiotic degradation
4.2.1. Hydrolysis and photodegradation in water
In studies by Collis & Leahey (1984), aqueous solutions of 14C-
cyclopropyl-labelled lambda-cyhalothrin, buffered at pH 5, 7, and
9, were maintained in the dark at 25° C for periods of up to 30
days. Acetonitrile (1%) was used as a co-solvent to facilitate
dissolution in the water. Hydrolysis of lambda-cyhalothrin
occurred rapidly in the pH 9 aqueous buffer solution (half-life,
approxi-mately 7 days) via ester cleavage of the molecule to yield
(1 RS)-cis-3-( Z-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid. Rapid isomerization of the
optical centre at the alpha-CN position also occurred at pH 9. At
pH 7 no hydrolysis was detected, but slow isomerization did occur.
At pH 5 no hydrolysis or isomerization was observed (Collis &
Leahey, 1984).
When quartz flasks containing 14C-cyclopropyl-labelled lambda-
cyhalothrin in pH 5 buffer were exposed to sunlight for 30 days,
the compound underwent photodegradation with a half-life of
approximately 30 days. (1 RS)-cis- and (1 RS)-trans-3-( ZE-2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylic
acids and ( RS)-alpha-amido-3-phenoxybenzyl (1 RS)-cis,trans-3-( ZE-2-
chloro-3,3,3-tri-fluoroprop-1-enyl)-2,2-dimethylcyclopropanecarb-
oxylate were the major degradation products formed. Optical and
geometrical isomerization of lambda-cyhalothrin occurred in the
irradiated flasks. No photodegradation or photoisomerization was
observed in a dark control flask (Curl et al., 1984a).
In studies by Hall & Leahey (1983), 14C-cyhalothrin was
incubated with two river water/sediment mixtures contained in
quartz flasks. The flasks were either exposed to sunlight or were
maintained under dark conditions by covering them with aluminium
foil. In the dark, degradation of cyhalothrin was slow (over 80%
remained unchanged after 32 days). However, when exposed to
sunlight the cyhalothrin degraded with a half-life of approximately
20 days in both river water/sediment mixtures. The rate at which
the parent compound was lost from the aqueous phase was, however,
much faster than its rate of degradation in the whole
water/sediment system. This was due to the ready absorption of
cyhalothrin onto the sediment. The major degradation process was
simple ester cleavage of the molecule, producing (1 RS)-cis- and
(1 RS)-trans-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acids. After 32 days of
irradiation, these compounds together represented 36-47% of the
radioactivity applied to the water/sediment systems. Some
photoisomerization also occurred.
4.2.2. Photodegradation in soil
When thin-layer soil plates were treated with 14C-cyclopropyl-
labelled lambda-cyhalothrin and irradiated in a xenon arc apparatus
or in sunlight, the half-life of lambda-cyhalothrin was less than 2
days in the xenon arc apparatus and less than 30 days in sunlight.
(1 RS)-cis-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropane-carboxylic acid and ( RS)-alpha-amido-3-
phenoxybenzyl (1 RS)-cis,trans-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-
enyl)-2,2-dimethylcyclopropanecarboxylate were the major
degradation products (Curl et al., 1984b).
4.3. Biodegradation in soil
Cyhalothrin degradation in the outdoor environment may occur by
either biological or photochemical processes. In most cases,
biological processes are by far the most important, although
photochemical reactions can sometimes contribute to the degradation
of residues on exposed surfaces.
4.3.1. Degradation rate
At residue levels that are likely to occur under normal field
conditions, cyhalothrin is degraded rapidly in soil. When a sandy
loam soil was treated with 14C-cyclopropyl-labelled cyhalothrin,
only 28% of the recovered radioactivity was present as cyhalothrin
after five weeks of incubation under aerobic conditions; 30% was
evolved as 14C-labelled carbon dioxide and 3.5% of the recovered
radioactivity was due to 1 RS-cis-3-( Z-2-chloro-3,3,3-trifluoroprop-
1-enyl)-2,2-dimethylcyclopropanecarboxylic acid. Approximately 19%
of the radioactivity was not extracted using acetonitrile at room
temperature followed by soxhlet extraction with aqueous
acetonitrile (Bewick & Zinner, 1981).
In a more recent and detailed study, 14C-cyclopropyl-labelled
cyhalothrin was applied to two soils (a sandy loam and a loamy
sand) at 100 g/ha and incubated under both aerobic and flooded
conditions at 20 °C. The sandy loam soil was also treated with
cyhalothrin at 500 g/ha and, in a later experiment, treated
separately with the two enantiomer pairs of cyhalothrin (one of
which constitutes lambda-cyhalothrin). This soil was also
incubated at 10 °C. All the isomers of cyhalothrin, including
those that constitute lambda-cyhalothrin, were readily degraded in
soil under a range of conditions. Half-lives for cyhalothrin at
20 °C in the sandy loam and loamy sand soils were 22 and 82 days,
respectively. Degradation was somewhat slower in the sandy loam at
higher rates (half-life: 42 days), lower temperature (half-life: 56
days) and under flooded conditions (half-life: 74 days). Lambda-
cyhalothrin was degraded at about 70% the rate of the other
enantiomer pair of cyhalothrin in the aerobic soils but at
approximately the same rate under flooded conditions. In the
aerobic soils, the principle degradative reactions were
hydroxylation, yielding up to 11% of the applied radiocarbon as
( RS)-alpha-cyano-3-(4-hydroxyphenoxy) benzyl cis-3-( Z-2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-di-methylcyclopropanecarboxylate
(compound XV), and hydrolysis, yielding up to 7% as ( RS)-cis-3-( Z-
2-chloro-3,3,3-tri-fluoroprop-1-enyl)-2,2-dimethylcyclopropane-
carboxylic acid (compound Ia). In the flooded soil, hydrolysis was
the main degradative reaction (up to 18% of compound Ia in the soil
phase), and hydroxylation was less important (only up to 1.4% of
compound XV). Compound Ia was the only compound detected (up to
17%) in the aqueous phase of the flooded soils. No isomerization
of the parent esters or their hydrolysis product was detected. The
initial degradation products of cyhalothrin and lambda-cyhalothrin
in the aerobic soils were rapidly further degraded with extensive
mineralization (up to 70% in 26 weeks) to 14CO2 (Bharti et al.,
1985). Thus the acid moiety of cyhalothrin is readily mineralized
in soil. The alcohol moiety of this compound is identical to that
of cypermethrin. Studies with cypermethrin show that, after ester
cleavage, the alcohol moiety released is readily mineralized to
14CO2 (FAO/WHO, 1986a).
In a further study, when 14C-cyclopropyl-labelled cyhalothrin
was applied (at 100 g/ha) to a Japanese upland soil (a volcanic
ash) and incubated under aerobic conditions at 20 °C, the half-
life of cyhalothrin was approximately 100 days. The principle
degradative reactions were hydroxylation, yielding up to 7% of the
applied radiocarbon as compound XV, and hydrolysis, yielding up to
ca. 2% as compound Ia. The initial degradation products of
cyhalothrin were further degraded with mineralization (up to 17% in
26 weeks) to 14CO2 (Bharti & Bewick, 1986).
Four sites in the USA were treated with lambda-cyhalothrin
(1.1 kg/ha), and soil was sampled and analysed at intervals up to 9
months after treatment. Residues remained in the top 15 cm of
soil, except at one site where low residues immediately after
treatment and at 2 days were attributed to contamination during
sampling. Initial soil residues of 0.18 to 0.31 mg/kg declined to
extremely low levels (< 0.01 to 0.02 mg/kg) during the course of
the study, and at one site the residues were less than the limit of
determination after 91 days (Fitzpatrick, 1985).
4.3.2. Degradation pathways
Degradation pathways for cyhalothrin and lambda-cyhalothrin in
soil are shown in Figure 2.
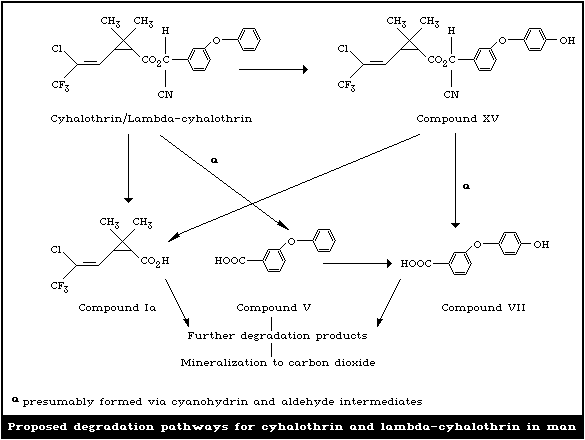 4.4. Metabolism in plants
In studies by Leahey & French (1986a), soya bean plants were
treated with 14C-cyclopropane-labelled and 14C-benzyl-labelled
lambda-cyhalothrin. Two applications (18 days apart) were made by
spraying an EC formulation at a rate of 20 g/ha. The plants were
analysed at maturity, 39 days after the second application, when
radioactive residues on the leaves ranged from 1.2 mg/kg (benzyl-
labelled treatment) to 1.5 mg/kg (cyclopropane-labelled treatment).
Very little radioactivity translocated into seeds (< 0.01 mg/kg).
In a similar experiment, in which cotton plants were treated
with 14C-cyclopropane-labelled and 14C-benzyl-labelled lambda-
cyhalothrin, three applications at a rate of 66 g/ha were made (at
flowering and 3 and 7 weeks after flowering). The plants were
analysed, at maturity, 30 days after the final application. The
radioactive residues on the leaves at harvest were 3.7 mg/kg for
the benzyl-labelled treatment and 4.1 mg/kg for the cyclopropane-
labelled treatment. Very little radioactivity (< 0.03 mg/kg) was
detected in the cotton seeds (Leahey & French, 1986b,c).
At harvest, the major constituents of the radioactive residue
on the leaves of both cotton and soya bean were lambda-cyhalothrin
and other isomeric forms of lambda-cyhalothrin resulting from
photochemically initiated interconversions (soya: 52% benzyl-
label, 45% cyclopropane-label; cotton: 52% benzyl-label, 37%
cyclopropane-label). The metabolites detected on the leaves of both
plants resulted from a range of hydrolytic and oxidative reactions.
A metabolic pathway illustrating these reactions is shown in Fig. 3.
4.4. Metabolism in plants
In studies by Leahey & French (1986a), soya bean plants were
treated with 14C-cyclopropane-labelled and 14C-benzyl-labelled
lambda-cyhalothrin. Two applications (18 days apart) were made by
spraying an EC formulation at a rate of 20 g/ha. The plants were
analysed at maturity, 39 days after the second application, when
radioactive residues on the leaves ranged from 1.2 mg/kg (benzyl-
labelled treatment) to 1.5 mg/kg (cyclopropane-labelled treatment).
Very little radioactivity translocated into seeds (< 0.01 mg/kg).
In a similar experiment, in which cotton plants were treated
with 14C-cyclopropane-labelled and 14C-benzyl-labelled lambda-
cyhalothrin, three applications at a rate of 66 g/ha were made (at
flowering and 3 and 7 weeks after flowering). The plants were
analysed, at maturity, 30 days after the final application. The
radioactive residues on the leaves at harvest were 3.7 mg/kg for
the benzyl-labelled treatment and 4.1 mg/kg for the cyclopropane-
labelled treatment. Very little radioactivity (< 0.03 mg/kg) was
detected in the cotton seeds (Leahey & French, 1986b,c).
At harvest, the major constituents of the radioactive residue
on the leaves of both cotton and soya bean were lambda-cyhalothrin
and other isomeric forms of lambda-cyhalothrin resulting from
photochemically initiated interconversions (soya: 52% benzyl-
label, 45% cyclopropane-label; cotton: 52% benzyl-label, 37%
cyclopropane-label). The metabolites detected on the leaves of both
plants resulted from a range of hydrolytic and oxidative reactions.
A metabolic pathway illustrating these reactions is shown in Fig. 3.
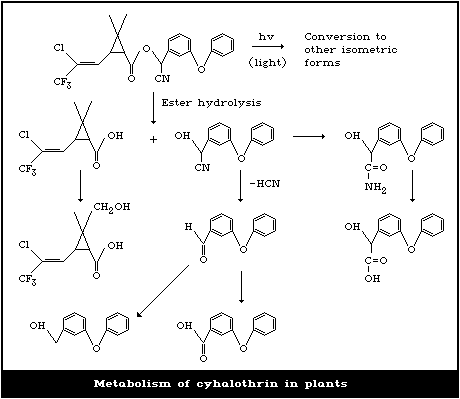 4.5. Bioaccumulation and biomagnification
4.5.1. n-Octanol-water partition coefficient
In common with other synthetic pyrethroids, the n-octanol-water
partition coefficient of cyhalothrin is high; values for log Pow of
6.9 and 7.0 at 20 °C have been obtained for cyhalothrin and lambda-
cyhalothrin, respectively, using a generator column method
(personal communication by ICI Agrochemicals to the IPCS). However,
since the compound is insoluble in water (thus limiting exposures
of aquatic species) and is rapidly metabolized in animal systems to
the cyclopropanecarboxylic acid and 3-phenoxybenzoic acid, both of
which are polar compounds, no problem of bioaccumulation will
occur.
4.5.2. Bioaccumulation
In a study consisting of a 28-day exposure and a 28-day
depuration period, carp (Cyprinus carpio) were exposed to
cyhalothrin in a flow-through water system using 14C-labelled
cyhalothrin at a nominal concentration of 0.02 µg cyhalothrin
equivalent/litre. During the exposure period, the concentration of
the total 14C-labelled cyhalothrin in the carp reached an
equilibrium within 1-2 weeks. The bioconcentration factors
measured were: 4250-7340 in the viscera, 490-850 in the muscle,
1020-2290 in the remainder of the body, and 1660-2240 in the whole
fish. Rapid depuration of residues was observed; the biological
half-life of the total 14C-labelled cyhalothrin was 9 days in the
viscera, muscle, and whole fish (Yamauchi et al., 1984a).
The accumulation of cyhalothrin and its degradation products in
channel catfish and Daphnia magna has been investigated in a soil-
water system. 14C-cyclopropane-labelled cyhalothrin was applied at
50 g ai per ha and aerobically incubated in soil for 3 weeks prior
to flooding. Channel catfish and Daphnia magna were introduced for
exposure periods of 31 and 28 days, respectively, after which the
fish and daphnids were transferred to an uncontaminated system for
depuration periods of 42 and 7 days, respectively. Soil, water,
fish, and daphnids were ana-lysed for 14C-residues. Prior to
flooding, 14C-labelled residues in soil decreased to 60-70% of that
applied, 40% of which remained as extractable cyhalothrin.
Following flooding of the soil, 14C-labelled residues in the water
increased throughout the exposure period, reaching a level of 8% of
the applied radioactivity. No parent cyhalothrin was detected in
the water; the only product comprising more than 1% of the applied
radioactivity was cis-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl-
2,2-dimethylcyclopropane-carboxylic acid, which represented up to
5.3% of the applied radioactivity. During exposure, the maximum
bio-concentration factors in whole fish and daphnids were 19 and
194, respectively (Table 2). The concentration of 14C-residues in
fish and daphnids decreased during the depuration period, the
half-lives being approximately 7 days and 1 day, respectively
(Table 3) (Hamer & Hill, 1985).
Table 2. Bioconcentration factors for
cyhalothrin in Daphnia and fish
---------------------------------------------
Exposure Daphnia Fish Fish Whole
phase muscle viscera fish
(days)
---------------------------------------------
1 93 2 20 17
3 194 3 28 7
7 158 6 45 10
14 62 7 66 19
21 47 5 28 10
28 29 7 49 8
31 NR 7 48 9
---------------------------------------------
From: Hamer & Hill (1985)
NR = not recorded.
Table 3. Concentration of 14C-residues in
Daphnia and fish tissues
--------------------------------------------
Depuration % of tissue level on final day
phase of exposure
(days) Daphnia Fish Fish Whole
muscle viscera fish
--------------------------------------------
1 56 105 60 80
3 29 66 53 64
4 NR NR NR 61
7 11 51 4 38
14 NR 32 4 37
21 NR 31 6 27
42 NR 20 2 14
--------------------------------------------
From: Hamer & Hill (1985)
NR = not recorded.
5. KINETICS AND METABOLISM
5.1. Absorption, distribution, and excretion
5.1.1. Rat
In studies by Harrison (1981, 1984a,b), groups of six male and
six female Alderley Park rats received a single oral dose (1 or 25
mg/kg) of radiolabelled cyhalothrin in corn oil. As it was known
that the metabolism of related pyrethroids involves extensive
cleavage of the ester bond, duplicate experiments were performed
using two forms of cyhalothrin labelled with 14C in the acid (14C-
cyclopropyl) or alcohol (14C-benzyl) portions of the ester.
Excreta (urine, faeces and, in selected animals, expired air) were
collected for up to 7 days after dosing and analysed for total
radioactivity and metabolites by liquid scintillation counting and
thin-layer chromatography. Blood samples were also collected at
various times up to 48 h and analysed for total radioactivity and
unchanged cyhalothrin.
Following oral administration of cyhalothrin, absorption was
variable but accounted for about 55% of the dose. The proportions
absorbed were similar at both dose levels.
Excretion was rapid for both 14C-cyclopropyl- and 14C-benzyl-
labelled cyhalothrin at both dose levels, although excretion rates
were faster with the 14C-benzyl label than with the 14C-cyclopropyl
label. Urinary excretion accounted for approximately 20-40% of the
dose and faecal excretion for 40-65% of the dose during the first 7
days. Peak blood concentrations of radioactivity were reached
within 4-7 h, and by 48 h these concentrations had declined to 10%
or less of peak values. A small proportion of an oral dose (2-3%)
was retained in the animals after seven days; analysis of twelve
different tissues indicated that this radioactivity was present
mainly in white fat.
Results from a study in which rats were dosed subcutaneously
indicated that some of the dose was excreted via the bile.
In a further experiment to study the excretion and tissue
accumulation of cyhalothrin (1 mg/kg per day by gavage), groups of
six male and six female rats received daily doses of 14C-benzyl-
labelled or 14C-cyclopropyl-labelled cyhalothrin for 14 days. Urine
and faecal samples were collected every 24 h up to 7 days after the
final dose. Groups of animals were killed 2, 5, and 7 days after
the final dose and a range of tissues were removed for measurement
of residual radioactivity. Fat samples were analysed by HPLC for
unchanged cyhalothrin. The results demonstrated that the excretion
of 14C-material after multiple oral dosing was similar to that
which followed a single dose. Slightly higher overall excretion in
urine (up to 50% of the administered dose) was probably due to more
consistent oral absorption in this study. A large proportion of
the oral dose of cyhalothrin was rapidly eliminated from the body.
Analysis of tissue residues revealed that the small proportion
(< 5%) of the dose retained in white fat was unchanged
cyhalothrin, which was eliminated from this tissue with a half-life
of about 23 days (Harrison, 1981, 1984a,b).
A further study was undertaken in the rat to explore the
retention, in fat, of cyhalothrin and lambda-cyhalothrin. Groups
of male rats received daily oral doses of 14C-cyclopropyl-labelled
cyhalothrin (1 mg/kg per day) for up to 119 days. At intervals
during and after the dosing period, groups of three rats were
killed and the concentrations of radioactivity in the liver,
kidney, fat, and blood were determined. Additionally the
concentration in fat of lambda-cyhalothrin and its opposite
enantiomer pair (enantiomer pair A) was measured by high-pressure
liquid chromatography. Levels of radioactivity in the blood
remained fairly constant and low (approximately 0.2 µg cyhalothrin
equivalents per g) throughout the dosing period. In the liver and
kidney, the radioactivity reached a plateau, after approximately 70
days, at a level corresponding to approximately 2.5 µg cyhalothrin
equivalents per g liver and 1.2 µg per g kidney. The concentration
of cyhalothrin in fat at the end of the dosing period was
approximately 10 µg/g. After the cessation of dosing, levels of
radioactivity in the liver, kidney, and blood declined rapidly. In
fat, the levels declined more slowly with an elimination half-life
of 30 days. The radioactive material in fat was unchanged
cyhalothrin; the ratio of enantiomeric pairs, one of which was
lambda-cyhalothrin, was not significantly different from that in
the dosing solution, indicating that the rate of metabolism of
lambda-cyhalothrin was the same as cyhalothrin and that there was
no preferential accumulation of lambda-cyhalothrin (Prout, 1984).
A comparison of the absorption, distribution, excretion, and
metabolism of lambda-cyhalothrin and cyhalothrin was made to
establish whether the single enantiomer pair lambda-cyhalothrin
differed from cyhalothrin (a 50:50 mixture of lambda-cyhalothrin
and the opposite enantiomer pair A) (Prout & Howard, 1985). One
group of four male rats was given a single oral dose of 14C-
cyclopropyl-labelled lambda-cyhalothrin (1 mg/kg); a second group
of four male rats was given 14C-cyclopropyl-labelled lambda-
cyhalothrin (1 mg/kg) plus the unlabelled enantiomeric pair A (1
mg/kg); and a third group of four male rats was given a single oral
dose of a 50:50 mixture of 14C-cyclopropyl-labelled lambda-
cyhalothrin and 14C-labelled enantiomeric pair A (i.e. 14C-
cyclopropyl-labelled cyhalothrin at 1 mg/kg). The urinary and
faecal excretion of radioactivity was monitored in all three groups
for three days and the residual radioactivity was then determined
in selected tissues. The metabolite profile of the excreta was
determined by thin-layer chromatography. The results of this study
indicate that co-administration of enantiomer pair A with lambda-
cyhalothrin had little or no effect upon the absorption,
distribution, or tissue retention of radioactivity, and there was
no effect upon the metabolite profile of lambda-cyhalothrin.
Similarly, the absorption, distribution, excretion, and metabolism
of cyhalothrin was indistinguishable from that of lambda-
cyhalothrin, thus confirming the results of the bioaccumulation
study of Prout (1984).
5.1.2. Dog
The absorption, distribution, excretion, and metabolism of
cyhalothrin have been studied in the dog. As in the rat studies,
the experiments were duplicated using cyhalothrin labelled either
in the acid (14C-cyclopro-pyl) or alcohol (14C-benzyl) moieties of
the molecule. Groups of three male and three female beagle dogs
were given a single oral dose of cyhalothrin (1 mg/kg or 10 mg/kg)
and, after a 3-week interval, a further single intravenous
administration of 0.1 mg/kg. Samples of blood and excreta were
collected for 7 days after dosing and were analysed for total
radioactivity. The proportions of unchanged cyhalothrin and of
metabolites in urine and faeces were determined by thin-layer
chromatography. The identity of major metabolites was confirmed by
mass spectrometry.
The absorption of cyhalothrin after oral administration was
variable. The degree of absorption was difficult to assess but was
within the range 48%-80%. Excretion of radioactivity after both
oral and intravenous dosing was initially rapid, with most of the
administered radioactivity being excreted in the first 48 h after
dosing. After 7 days, a mean of 82-93% had been excreted (Harrison,
1984c).
5.1.3. Cow
After twice daily oral ingestion of 14C-benzyl- or 14C-
cyclopropyl-labelled cyhalothrin (1 mg/kg per day for 7 days),
absorption of the insecticide by cows was apparently slow and
incomplete. Approximately 50% of the dosed radioactivity was
excreted in the faeces, mainly as unchanged cyhalothrin, but only
small amounts were detected in the bile. With both labelled forms,
most of the radioactive material was rapidly eliminated in the
urine (27%) and faeces (49%) within 24 h of each daily dose. Only
a very small proportion of the dose was secreted in the milk (0.8%)
and this was found to be unchanged cyhalothrin. Tissue residues of
radioactive material were low and were in the following order: fats
> liver > kidney > blood > muscle. Residues in fat consisted
of unchanged cyhalothrin. The liver and kidney contained small
amounts of cyhalothrin, but the residues were largely due to a
number of ester-cleavage metabolites that were probably present
because the animals were still actively metabolizing and
eliminating a significant fraction of the most recent day's intake
of cyhalothrin. The almost two-fold difference in the plasma
levels of total radiolabelled components obtained with the
different labelled forms suggests that little cyhalothrin was
present in blood. The ester link must therefore be hydrolysed very
rapidly, apart from a small fraction that is distributed into fatty
tissues (Harrison, 1984d).
In studies by Sapiets (1985c), Friesian cows were fed for up to
thirty consecutive days on diets containing lambda-cyhalothrin at
1, 5, and 25 mg/kg. Lambda-cyhalothrin residues in milk correlated
well with dietary inclusion rates, the mean plateau residue levels
being 0.02 mg/kg, 0.09 mg/kg, and 0.52 mg/kg, respectively, for the
three dietary inclusion rates. Lambda-cyhalothrin residue levels
in milk did not accumulate, and they declined when feeding of the
treated diet ceased. At the end of the 30 days, three cows from
each group were sacrificed. The remaining two cows from the high-
dose group were fed an untreated diet for a further 14 days before
they too were slaughtered. Lambda-cyhalothrin residue levels in
the tissues of the sacrificed animals were as shown in Table 4.
Table 4. Lambda-cyhalothrin residues (mg/kg) in cow tissuesa
-----------------------------------------------------------------------------------------------
Dietary feeding Abductor Pectoral Subcutaneous Peritoneal Liver Kidney
rate (mg/kg) muscle muscle fat fat
-----------------------------------------------------------------------------------------------
1.0 < 0.01 < 0.01 0.01-0.21 0.07-0.50 < 0.01-0.03 0.01-0.02
5.0 0.01-0.03 0.03-0.07 0.44-0.81 0.95-1.8 < 0.01 0.01-0.07
25.0 0.08-0.14 0.02-0.41 1.3-4.6 3.9-7.9 0.06-0.10 0.09-0.43
25.0 + 14-day < 0.01-0.05 < 0.01-0.03 0.03-1.1 0.47-2.6 < 0.01 0.10-0.20
recovery period
Control < 0.01 < 0.01 < 0.01-0.02 < 0.01-0.07 < 0.01 < 0.01
-----------------------------------------------------------------------------------------------
a From: Sapiets (1985c).
5.2. Metabolism
The metabolic pathways that have been established for
cyhalothrin in mammals are summarized in Fig. 4.
4.5. Bioaccumulation and biomagnification
4.5.1. n-Octanol-water partition coefficient
In common with other synthetic pyrethroids, the n-octanol-water
partition coefficient of cyhalothrin is high; values for log Pow of
6.9 and 7.0 at 20 °C have been obtained for cyhalothrin and lambda-
cyhalothrin, respectively, using a generator column method
(personal communication by ICI Agrochemicals to the IPCS). However,
since the compound is insoluble in water (thus limiting exposures
of aquatic species) and is rapidly metabolized in animal systems to
the cyclopropanecarboxylic acid and 3-phenoxybenzoic acid, both of
which are polar compounds, no problem of bioaccumulation will
occur.
4.5.2. Bioaccumulation
In a study consisting of a 28-day exposure and a 28-day
depuration period, carp (Cyprinus carpio) were exposed to
cyhalothrin in a flow-through water system using 14C-labelled
cyhalothrin at a nominal concentration of 0.02 µg cyhalothrin
equivalent/litre. During the exposure period, the concentration of
the total 14C-labelled cyhalothrin in the carp reached an
equilibrium within 1-2 weeks. The bioconcentration factors
measured were: 4250-7340 in the viscera, 490-850 in the muscle,
1020-2290 in the remainder of the body, and 1660-2240 in the whole
fish. Rapid depuration of residues was observed; the biological
half-life of the total 14C-labelled cyhalothrin was 9 days in the
viscera, muscle, and whole fish (Yamauchi et al., 1984a).
The accumulation of cyhalothrin and its degradation products in
channel catfish and Daphnia magna has been investigated in a soil-
water system. 14C-cyclopropane-labelled cyhalothrin was applied at
50 g ai per ha and aerobically incubated in soil for 3 weeks prior
to flooding. Channel catfish and Daphnia magna were introduced for
exposure periods of 31 and 28 days, respectively, after which the
fish and daphnids were transferred to an uncontaminated system for
depuration periods of 42 and 7 days, respectively. Soil, water,
fish, and daphnids were ana-lysed for 14C-residues. Prior to
flooding, 14C-labelled residues in soil decreased to 60-70% of that
applied, 40% of which remained as extractable cyhalothrin.
Following flooding of the soil, 14C-labelled residues in the water
increased throughout the exposure period, reaching a level of 8% of
the applied radioactivity. No parent cyhalothrin was detected in
the water; the only product comprising more than 1% of the applied
radioactivity was cis-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl-
2,2-dimethylcyclopropane-carboxylic acid, which represented up to
5.3% of the applied radioactivity. During exposure, the maximum
bio-concentration factors in whole fish and daphnids were 19 and
194, respectively (Table 2). The concentration of 14C-residues in
fish and daphnids decreased during the depuration period, the
half-lives being approximately 7 days and 1 day, respectively
(Table 3) (Hamer & Hill, 1985).
Table 2. Bioconcentration factors for
cyhalothrin in Daphnia and fish
---------------------------------------------
Exposure Daphnia Fish Fish Whole
phase muscle viscera fish
(days)
---------------------------------------------
1 93 2 20 17
3 194 3 28 7
7 158 6 45 10
14 62 7 66 19
21 47 5 28 10
28 29 7 49 8
31 NR 7 48 9
---------------------------------------------
From: Hamer & Hill (1985)
NR = not recorded.
Table 3. Concentration of 14C-residues in
Daphnia and fish tissues
--------------------------------------------
Depuration % of tissue level on final day
phase of exposure
(days) Daphnia Fish Fish Whole
muscle viscera fish
--------------------------------------------
1 56 105 60 80
3 29 66 53 64
4 NR NR NR 61
7 11 51 4 38
14 NR 32 4 37
21 NR 31 6 27
42 NR 20 2 14
--------------------------------------------
From: Hamer & Hill (1985)
NR = not recorded.
5. KINETICS AND METABOLISM
5.1. Absorption, distribution, and excretion
5.1.1. Rat
In studies by Harrison (1981, 1984a,b), groups of six male and
six female Alderley Park rats received a single oral dose (1 or 25
mg/kg) of radiolabelled cyhalothrin in corn oil. As it was known
that the metabolism of related pyrethroids involves extensive
cleavage of the ester bond, duplicate experiments were performed
using two forms of cyhalothrin labelled with 14C in the acid (14C-
cyclopropyl) or alcohol (14C-benzyl) portions of the ester.
Excreta (urine, faeces and, in selected animals, expired air) were
collected for up to 7 days after dosing and analysed for total
radioactivity and metabolites by liquid scintillation counting and
thin-layer chromatography. Blood samples were also collected at
various times up to 48 h and analysed for total radioactivity and
unchanged cyhalothrin.
Following oral administration of cyhalothrin, absorption was
variable but accounted for about 55% of the dose. The proportions
absorbed were similar at both dose levels.
Excretion was rapid for both 14C-cyclopropyl- and 14C-benzyl-
labelled cyhalothrin at both dose levels, although excretion rates
were faster with the 14C-benzyl label than with the 14C-cyclopropyl
label. Urinary excretion accounted for approximately 20-40% of the
dose and faecal excretion for 40-65% of the dose during the first 7
days. Peak blood concentrations of radioactivity were reached
within 4-7 h, and by 48 h these concentrations had declined to 10%
or less of peak values. A small proportion of an oral dose (2-3%)
was retained in the animals after seven days; analysis of twelve
different tissues indicated that this radioactivity was present
mainly in white fat.
Results from a study in which rats were dosed subcutaneously
indicated that some of the dose was excreted via the bile.
In a further experiment to study the excretion and tissue
accumulation of cyhalothrin (1 mg/kg per day by gavage), groups of
six male and six female rats received daily doses of 14C-benzyl-
labelled or 14C-cyclopropyl-labelled cyhalothrin for 14 days. Urine
and faecal samples were collected every 24 h up to 7 days after the
final dose. Groups of animals were killed 2, 5, and 7 days after
the final dose and a range of tissues were removed for measurement
of residual radioactivity. Fat samples were analysed by HPLC for
unchanged cyhalothrin. The results demonstrated that the excretion
of 14C-material after multiple oral dosing was similar to that
which followed a single dose. Slightly higher overall excretion in
urine (up to 50% of the administered dose) was probably due to more
consistent oral absorption in this study. A large proportion of
the oral dose of cyhalothrin was rapidly eliminated from the body.
Analysis of tissue residues revealed that the small proportion
(< 5%) of the dose retained in white fat was unchanged
cyhalothrin, which was eliminated from this tissue with a half-life
of about 23 days (Harrison, 1981, 1984a,b).
A further study was undertaken in the rat to explore the
retention, in fat, of cyhalothrin and lambda-cyhalothrin. Groups
of male rats received daily oral doses of 14C-cyclopropyl-labelled
cyhalothrin (1 mg/kg per day) for up to 119 days. At intervals
during and after the dosing period, groups of three rats were
killed and the concentrations of radioactivity in the liver,
kidney, fat, and blood were determined. Additionally the
concentration in fat of lambda-cyhalothrin and its opposite
enantiomer pair (enantiomer pair A) was measured by high-pressure
liquid chromatography. Levels of radioactivity in the blood
remained fairly constant and low (approximately 0.2 µg cyhalothrin
equivalents per g) throughout the dosing period. In the liver and
kidney, the radioactivity reached a plateau, after approximately 70
days, at a level corresponding to approximately 2.5 µg cyhalothrin
equivalents per g liver and 1.2 µg per g kidney. The concentration
of cyhalothrin in fat at the end of the dosing period was
approximately 10 µg/g. After the cessation of dosing, levels of
radioactivity in the liver, kidney, and blood declined rapidly. In
fat, the levels declined more slowly with an elimination half-life
of 30 days. The radioactive material in fat was unchanged
cyhalothrin; the ratio of enantiomeric pairs, one of which was
lambda-cyhalothrin, was not significantly different from that in
the dosing solution, indicating that the rate of metabolism of
lambda-cyhalothrin was the same as cyhalothrin and that there was
no preferential accumulation of lambda-cyhalothrin (Prout, 1984).
A comparison of the absorption, distribution, excretion, and
metabolism of lambda-cyhalothrin and cyhalothrin was made to
establish whether the single enantiomer pair lambda-cyhalothrin
differed from cyhalothrin (a 50:50 mixture of lambda-cyhalothrin
and the opposite enantiomer pair A) (Prout & Howard, 1985). One
group of four male rats was given a single oral dose of 14C-
cyclopropyl-labelled lambda-cyhalothrin (1 mg/kg); a second group
of four male rats was given 14C-cyclopropyl-labelled lambda-
cyhalothrin (1 mg/kg) plus the unlabelled enantiomeric pair A (1
mg/kg); and a third group of four male rats was given a single oral
dose of a 50:50 mixture of 14C-cyclopropyl-labelled lambda-
cyhalothrin and 14C-labelled enantiomeric pair A (i.e. 14C-
cyclopropyl-labelled cyhalothrin at 1 mg/kg). The urinary and
faecal excretion of radioactivity was monitored in all three groups
for three days and the residual radioactivity was then determined
in selected tissues. The metabolite profile of the excreta was
determined by thin-layer chromatography. The results of this study
indicate that co-administration of enantiomer pair A with lambda-
cyhalothrin had little or no effect upon the absorption,
distribution, or tissue retention of radioactivity, and there was
no effect upon the metabolite profile of lambda-cyhalothrin.
Similarly, the absorption, distribution, excretion, and metabolism
of cyhalothrin was indistinguishable from that of lambda-
cyhalothrin, thus confirming the results of the bioaccumulation
study of Prout (1984).
5.1.2. Dog
The absorption, distribution, excretion, and metabolism of
cyhalothrin have been studied in the dog. As in the rat studies,
the experiments were duplicated using cyhalothrin labelled either
in the acid (14C-cyclopro-pyl) or alcohol (14C-benzyl) moieties of
the molecule. Groups of three male and three female beagle dogs
were given a single oral dose of cyhalothrin (1 mg/kg or 10 mg/kg)
and, after a 3-week interval, a further single intravenous
administration of 0.1 mg/kg. Samples of blood and excreta were
collected for 7 days after dosing and were analysed for total
radioactivity. The proportions of unchanged cyhalothrin and of
metabolites in urine and faeces were determined by thin-layer
chromatography. The identity of major metabolites was confirmed by
mass spectrometry.
The absorption of cyhalothrin after oral administration was
variable. The degree of absorption was difficult to assess but was
within the range 48%-80%. Excretion of radioactivity after both
oral and intravenous dosing was initially rapid, with most of the
administered radioactivity being excreted in the first 48 h after
dosing. After 7 days, a mean of 82-93% had been excreted (Harrison,
1984c).
5.1.3. Cow
After twice daily oral ingestion of 14C-benzyl- or 14C-
cyclopropyl-labelled cyhalothrin (1 mg/kg per day for 7 days),
absorption of the insecticide by cows was apparently slow and
incomplete. Approximately 50% of the dosed radioactivity was
excreted in the faeces, mainly as unchanged cyhalothrin, but only
small amounts were detected in the bile. With both labelled forms,
most of the radioactive material was rapidly eliminated in the
urine (27%) and faeces (49%) within 24 h of each daily dose. Only
a very small proportion of the dose was secreted in the milk (0.8%)
and this was found to be unchanged cyhalothrin. Tissue residues of
radioactive material were low and were in the following order: fats
> liver > kidney > blood > muscle. Residues in fat consisted
of unchanged cyhalothrin. The liver and kidney contained small
amounts of cyhalothrin, but the residues were largely due to a
number of ester-cleavage metabolites that were probably present
because the animals were still actively metabolizing and
eliminating a significant fraction of the most recent day's intake
of cyhalothrin. The almost two-fold difference in the plasma
levels of total radiolabelled components obtained with the
different labelled forms suggests that little cyhalothrin was
present in blood. The ester link must therefore be hydrolysed very
rapidly, apart from a small fraction that is distributed into fatty
tissues (Harrison, 1984d).
In studies by Sapiets (1985c), Friesian cows were fed for up to
thirty consecutive days on diets containing lambda-cyhalothrin at
1, 5, and 25 mg/kg. Lambda-cyhalothrin residues in milk correlated
well with dietary inclusion rates, the mean plateau residue levels
being 0.02 mg/kg, 0.09 mg/kg, and 0.52 mg/kg, respectively, for the
three dietary inclusion rates. Lambda-cyhalothrin residue levels
in milk did not accumulate, and they declined when feeding of the
treated diet ceased. At the end of the 30 days, three cows from
each group were sacrificed. The remaining two cows from the high-
dose group were fed an untreated diet for a further 14 days before
they too were slaughtered. Lambda-cyhalothrin residue levels in
the tissues of the sacrificed animals were as shown in Table 4.
Table 4. Lambda-cyhalothrin residues (mg/kg) in cow tissuesa
-----------------------------------------------------------------------------------------------
Dietary feeding Abductor Pectoral Subcutaneous Peritoneal Liver Kidney
rate (mg/kg) muscle muscle fat fat
-----------------------------------------------------------------------------------------------
1.0 < 0.01 < 0.01 0.01-0.21 0.07-0.50 < 0.01-0.03 0.01-0.02
5.0 0.01-0.03 0.03-0.07 0.44-0.81 0.95-1.8 < 0.01 0.01-0.07
25.0 0.08-0.14 0.02-0.41 1.3-4.6 3.9-7.9 0.06-0.10 0.09-0.43
25.0 + 14-day < 0.01-0.05 < 0.01-0.03 0.03-1.1 0.47-2.6 < 0.01 0.10-0.20
recovery period
Control < 0.01 < 0.01 < 0.01-0.02 < 0.01-0.07 < 0.01 < 0.01
-----------------------------------------------------------------------------------------------
a From: Sapiets (1985c).
5.2. Metabolism
The metabolic pathways that have been established for
cyhalothrin in mammals are summarized in Fig. 4.
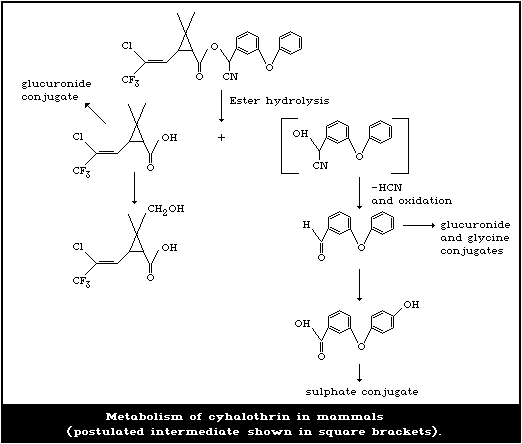 5.2.1. Rat
Identification of the metabolites produced in the rat studies
of Harrison (described in section 5.1.1) revealed that, following
oral administration, unabsorbed cyhalothrin was eliminated
unchanged via the faeces. The absorbed material was rapidly and
extensively metabolized and no unchanged cyhalothrin was present in
urine or bile. The main route of metabolism was, as anticipated,
via hydrolysis of the ester linkage (Fig. 4). The cyclopropane-
carboxylic acid moiety was subsequently excreted via the urine as
the glucuronide conjugate. This material accounted for about 50%
of the radioactivity in urine following dosing with 14C-
cyclopropyl-labelled cyhalothrin. The 3-phenoxybenzyl moiety was
further metabolized by loss of the nitrile group, oxidation of the
aldehyde formed to a carboxylic acid, aromatic hydroxylation at the
4' position, and formation of the 4- O-sulfate conjugate of 3-(4-
hydroxyphenoxy)benzoic acid. This conjugate accounted for
approximately 75% of the urinary radioactivity following dosing
with 14C-benzyl-labelled cyhalothrin. No metabolite containing the
ester function was detected (Harrison, 1983).
5.2.2. Dog
The main route of metabolism after oral administration is, as
in the rat, via cleavage of the ester bond. After intravenous
administration (0.1 mg/kg body weight), the patterns of metabolites
in urine were very similar to those seen in the oral studies. Very
little unchanged compound was present in the faeces or urine. The
phenoxy-benzyl moiety was further metabolized as in the rat; the
main metabolites were N-(3-phenoxybenzyl) glycine, 3-(4-
hydroxyphenoxy)benzoic acid and its sulfate conjugate, 3-
phenoxybenzoyl glucuronide, and a little free 3-phenoxybenzoic
acid. Other conjugated metabolites were also present. The
cyclopropane acid moiety was extensively metabolized to produce 11
metabolites. These included the cyclopropane acid glucuronide and
other conjugated metabolites. Thus, the metabolism of cyhalothrin
is dominated by cleavage of the ester bond (Fig. 4). Subsequent
metabolism of the products is similar both to that of other
pyrethroids and to the fate of cyhalothrin in other species
(Harrison, 1984c).
5.2.3. Cow
In common with other structurally related pyrethroids, the main
routes of metabolism of cyhalothrin in the cow have been found to
be similar to those observed in rats and dogs, i.e cleavage of the
ester bond with subsequent excretion of the cyclopropyl carboxylic
moiety, either free, hydroxylated, or as a glucuronide conjugate.
The phenoxybenzyl moiety was further metabolized by loss of the
nitrile group and excreted as free 3-phenoxybenzoic acid and its
amino acid conjugates, or after aromatic hydroxylation probably at
the 4'position. Cyhalothrin itself gives rise to residues in fats;
this is consistent with the lipophilic properties of cyhalothrin
compared to those of its more polar metabolites (Harrison, 1984d).
5.2.4. Goat
In a study by Leahey et al. (1985), a goat was dosed orally for
seven days with 14C-cyclopropyl-labelled lambda-cyhalothrin at a
rate equivalent to approximately 11 mg/kg diet. During dosing, the
maximum residue level in the milk was 0.27 mg cyhalothrin
equivalents/kg (mean value during days 3-7: 0.21 mg/kg), virtually
all of which was characterized as lambda-cyhalothrin. When the goat
was slaughtered 16 h after receiving the final dose, residues in
the tissues, expressed in cyhalothrin equivalents, were: meat,
0.024-0.028 mg/kg; fat, 0.13-0.44 mg/kg; liver, 0.34-0.35 mg/kg;
kidney, 0.20 mg/kg. The residues in meat and fat were due mainly
to lambda-cyhalothrin. However, in the liver and kidney, intact
pyrethroid accounted for only a small part of the residue. (1 RS)-
cis-(Z-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclo-
propanecarboxylic acid and 3-(2-chloro-3,3,3-trifluoro-prop-1-
enyl)-2-hydroxymethyl-2-methylcyclopropanecarboxylic acid were the
major components of the residue identified in liver and kidney.
5.2.5. Fish
In an accumulation study by Leahey & Parker (1985), carp were
maintained in a flow-through water system con-taining 14C-
cyclopropyl-labelled cyhalothrin (at a level of 20 ng/g) for 28
days. Results showed that radioactive residues in muscle, head,
and viscera were 0.035, 0.050, and 0.115 mg/kg, respectively. The
major part of the 14C-residue (50-65%) was characterized as
cyhalothrin, a further 10-19% consisting of the compound 1a (Fig. 2).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic organisms
6.1.1. Microorganisms
It has been shown that lambda-cyhalothrin, at a concentration
of 1.0 mg/litre, does not affect the growth of the single-celled
green alga Selenastrum capricornutum over a period of 96 h
(Thompson & Williams, 1985).
6.1.2. Invertebrates
6.1.2.1 Acute toxicity
Aquatic invertebrates show a wide range of susceptibility to
cyhalothrin and lambda-cyhalothrin. The data are summarized in
Table 5.
Table 5. Acute toxicity of cyhalothrin and lambda-cyhalothrin for aquatic invertebrates
-----------------------------------------------------------------------------------------
Species Stage Temper- Test substance Test 48-h Reference
ature system LC50
(°C) (ng/
litre)
-----------------------------------------------------------------------------------------
Freshwater
Water flea < 24 h 20 cyhalothrin static 160 Yamauchi et
(Daphnia pulex) 5% WP al. (1984d)
Water flea 12 h 20 technical static 380 Williams &
(Daphnia magna) (± 12 h) cyhalothrin Thompson
(1981)
Water flea < 24 h 20 technical lambda- static 360 Farrelly et
(Daphnia magna) cyhalothrin al. (1984)
< 24 h 20 lambda-cyhalothrin static 90a Farrelly et
5% EC al. (1985)
< 24 h 20 lambda-cyhalothrin static 90a Farrelly et
13% EC al. (1985)
Freshwater shrimp 5 mm 15 14C-lambda- flow- 8 Hamer et al.
(Gammarus pulex) cyhalothrin through (1985a)
Marine
Mysid shrimp < 48 h 25 14C-lambda- flow- 7.5 Thompson
(Mysidopsis bahia) cyhalothrin through (1985)
-----------------------------------------------------------------------------------------
a Concentration of active ingredient.
6.1.2.2 Long-term toxicity
The effects of lambda-cyhalothrin on the survival, growth, and
reproduction of Daphnia magna were investigated for a period of 21
days in a static water test with daily renewal of test solutions.
The nominal concentrations tested were 0, 2.5, 5.0, 10.0, 20.0,
and 40.0 ng/litre. Lambda-cyhalothrin affected all three parameters
at a nominal concentration of 40 ng/litre, but no effects were
noted at 2.5 ng/litre. Chemical analysis suggested that the
daphnids were exposed to about 60% of the nominal concentration.
These results show that the life-cycle no-observed-effect level of
lambda-cyhalothrin for daphnids is of the order of 2.5 ng/litre
(Hamer et al., 1985b).
6.1.3. Fish
6.1.3.1 Acute toxicity
Cyhalothrin and lambda-cyhalothrin are very toxic to fish in
clean water under laboratory conditions. The available data,
summarized in Table 6, demonstrate a similar high acute toxicity
for both cold and warm water species of fish.
6.1.3.2 Long-term toxicity
Sheepshead minnow Cyprinodon variegatus embryos and larvae were
continuously exposed (through 28 days post hatch) to mean measured
lambda-cyhalothrin concentrations of 0.04, 0.07, 0.14, 0.25, and
0.38 µg/litre, in a flow-through system. The test was performed in
duplicate. Assessments were made of percentage hatch and survival
of embryos and of total length and weight of the larvae at the
completion of the study. Hatchability was not affected (P < 0.05)
at any concentration in the carrier dimethylformamide or dilution
water controls, percentage hatch ranging from 81.3% to 100%.
Larval survival was not significantly affected. A significant
effect (P < 0.05) was found on the weight of the larvae at the
highest concen-tration tested, but not at any other concentration.
On the basis of these data the NOEL was 0.25 µg/litre and the
lowest-observed-effect level (LOEL) was 0.38 µg/litre (Hill et al.,
1985).
6.1.4. Model ecosystem
Cyhalothrin is readily adsorbed onto soil and suspended
particles, which in consequence significantly reduces its toxicity
to aquatic organisms (Yamauchi et al., 1984c). Daphnia pulex and
Cyprinus carpio were used in four different systems to test this
experimentally (Table 7). The median lethal concentrations (LC50)
were calculated, immobilization being used as the end-point for
Daphnia pulex.
Table 6. Acute toxicity of cyhalothrin and lambda-cyhalothrin to fish
---------------------------------------------------------------------------------------------------
Species Weight Test substance and vehicle Temper- 96-h Reference
(g) ature LC50
(°C) (µg/
litre)
---------------------------------------------------------------------------------------------------
Rainbow trout 0.32-1.37 technical cyhalothrin 12 0.54 Hill (1981a)
(Salmo gairdneri) dispersed via acetone
0.30-1.48 technical lambda-cyhalothrin 12 0.24 Hill (1984a)
dispersed via acetone
0.99-2.32 lambda-cyhalothrin 2.5% 16 0.39 Hill (1985a)
EC dispersed in water
1.28-4.78 lambda-cyhalothrin 13% EC 12 0.44 Hill (1985b)
dispersed in water
0.87-4.09 lambda-cyhalothrin 5% EC 16 0.93 Hill (1985c)
dispersed in water
Carp 5.2 technical cyhalothrin 23-35 1.34a Takeda Chemical
(Cyprinus carpio) dispersed via Co. Ltd.
dimethylformamide (1979)
5.4 cyhalothrin 5% WP 25 1.1 Yamauchi et al.
(1984b)
1.48-5.8 lambda-cyhalothrin 2.5% 22 0.54 Hill (1985d)
EC dispersed in water
3.92-7.28 lambda-cyhalothrin 5% EC 22 0.50 Hill (1985e)
dispersed in water
Bluegill sunfish 0.23-0.84 technical cyhalothrin 22 0.46 Reynolds (1984)
(Lepomis macrochirus) dispersed via acetone
0.7-2.6 technical lambda- 22 0.21 Hill (1984b)
cyhalothrin dispersed
via acetone
0.47-2.06 lambda-cyhalothrin 13% 22 0.28 Hill (1985f)
EC dispersed in water
Sheephead minnow 0.32-0.91 technical lambda- 22 0.81 Hill (1985g)
(Cyprinodon variegatus) cyhalothrin dispersed
via acetone
---------------------------------------------------------------------------------------------------
a 72-h LC50.
Table 7. Effect of soil on the toxicity (72-h LC50 in µg/
litre) of cyhalothrin to Daphnia pulex and Cyprinus carpioa
--------------------------------------------------------------
Conditions Daphnia pulex Cyprinus carpio
--------------------------------------------------------------
Application to water surface 0.4 9
(without soil)
Application to water surface 1.0 32
(soil undisturbed)
Application to water surface 16 57
(soil suspended)
Application to soil 70 642
(soil undisturbed)
--------------------------------------------------------------
a From: Yamauchi et al (1984c).
6.2. Terrestrial organisms
6.2.1. Birds
6.2.1.1 Acute toxicity
The toxicity of single oral doses of cyhalothrin and lambda-
cyhalothrin for birds is summarized in Table 8.
The 5-day dietary LC50 for cyhalothrin and lambda-cyhalothrin
has been measured in Anas platyrhynchos and Colinus virginianus
(Table 9).
Table 8. Oral toxicity of cyhalothrin and lambda-cyhalothrin for birds
---------------------------------------------------------------------------------------
Species Age Test substance Observation LD50 (mg/kg References
and vehicle period body weight)
---------------------------------------------------------------------------------------
Domestic hen adult cyhalothrin in 14 days > 10 000 Roberts
(Gallus domesticus) corn oil et al.
(1982)
Mallard duck adult cyhalothrin in 14 days > 5000 Roberts &
(Anas platyrhynchos) corn oil Fairley
(1981)
Mallard duck adult lambda-cyhalothrin 14 days > 3950 Roberts &
(Anas platyrhynchos) in corn oil Fairley
(1984)
---------------------------------------------------------------------------------------
Table 9. Dietary LC50 of cyhalothrin and lambda-cyhalothrin for birds
---------------------------------------------------------------------------------------
Species Age Test substance Observation LC50 Reference
period (post (mg/kg
treatment) diet)
---------------------------------------------------------------------------------------
Mallard duck 10 days cyhalothrin 3 days 14 000 Roberts et
(Anas platyrhynchos) al. (1981a)
Mallard duck 8 days lambda-cyhalothrin 4 days 3948 Roberts et
(Anas platyrhynchos) al. (1985a)
Bobwhite quail 10 days cyhalothrin 3 days > 7530 Roberts et
(Colinus virginianus) al. (1981b)
Bobwhite quail 11 days lambda-cyhalothrin 3 days > 5300 Roberts et
(Colinus virginianus) al. (1985b)
---------------------------------------------------------------------------------------
6.2.2. Honey-bees
Cyhalothrin and lambda-cyhalothrin have been shown to be toxic
to honey-bees (Apis mellifera) in laboratory tests (Table 10).
Table 10. Toxicity of cyhalothrin and lambda-cyhalothrin for
honey-bees (expressed as 24-h LD50 in µg ai per bee)
----------------------------------------------------------------------
Formulation Topical Oral Reference
application administration
----------------------------------------------------------------------
Technical cyhalothrin 0.027 - Smart &
Stevenson
(1982)
Technical lambda-cyhalothrin 0.051 0.97 Gough et
al. (1984)
Lambda-cyhalothrin EC (5%) 0.095 0.57 Gough et
al. (1984)
----------------------------------------------------------------------
In common with other pyrethroids, the high laboratory toxicity
of lambda-cyhalothrin is not translated into a significant field
hazard to bees. In two trials on flowering rape, lambda-
cyhalothrin (JF 9509) EC was applied, at midday, by helicopter at a
concentration of 10 g ai/ha to fields where hives of honey-bees
were located. A toxic standard and untreated control were used for
comparison. Bees were actively foraging during spraying, and the
hives were oversprayed. Mortality, foraging activity, activity at
the hive, and brood development were monitored before and after
treatment, and pollen, honey, and wax were analyzed for residues.
Apart from a suppression of foraging lasting up to 1.5 h, the
lambda-cyhalothrin formulation had no effect on the bees, whereas
the toxic standard killed large numbers. Only low levels of
residues were detected (pollen, 0.44 µg/g; honey, 0.01 µg/g; wax,
0.01 µg/g). It was concluded that, at 10 g ai/ha, lambda-
cyhalothrin formulation (JF 9509) EC is non-hazardous to honey-bees
on flowering rape (Gough et al., 1985).
6.2.3. Earthworms
Three annual applications of lambda-cyhalothrin at rates of up
to 250 g ai/ha to field plots had no adverse effect on populations
of individual species of earthworms or total earthworm numbers or
weight (Coulson et al., 1986).
6.2.4. Higher plants
No phytotoxic effects have been reported.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposures
7.1.1. Oral
The acute oral toxicity of cyhalothrin and lambda-cyhalothrin
in corn oil has been determined for several species (Table 11). The
toxicity of cyhalothrin is moderate (LD50 values: rat, 144-243
mg/kg; mouse, 37-62 mg/kg), whereas that of lambda-cyhalothrin is
higher (LD50 values: rat, 56-79 mg/kg; mouse, 20 mg/kg). Signs of
intoxication are characteristic of type II pyrethroid toxicity and
included piloerection, subdued behaviour, ataxia, unsteady gait,
salivation, incontinence, scouring, and chromodacryorrhoea.
Table 11. Acute oral toxicity of technical cyhalothrin and
lambda-cyhalothrin in corn oil
------------------------------------------------------------------
Species Test substance LD50 (mg/kg Reference
body weight)
------------------------------------------------------------------
Rat cyhalothrin 243 (male) Nixon &
144 (female) Jackson (1981a)
Rat lambda-cyhalothrin 79 (male) Southwood (1985)
56 (female)
Mouse cyhalothrin 36.7 (male) Nixon &
62.3 (female) Jackson (1981a)
Mouse lambda-cyhalothrin 19.9 (male) Southwood (1984)
19.9 (female)
Guinea-pig cyhalothrin > 5000 (male) Nixon &
Jackson (1981a)
Rabbit cyhalothrin > 1000 (female) Nixon &
Jackson (1981a)
------------------------------------------------------------------
7.1.2. Percutaneous
The percutaneous toxicity of cyhalothrin and lambda-cyhalothrin
is summarized in Table 12. Signs of intoxication were similar to
those seen after oral ingestion, and included incontinence,
scouring, dehydration, subdued behaviour, curvature of the spine,
unsteady gait, nervous appearance, piloerection, and increased
vocalization when handled.
Table 12. Percutaneous toxicity of technical cyhalothrin and
lambda-cyhalothrin in polyethylene glycol paste
-----------------------------------------------------------------
Species Test substance LD50 (mg/kg Reference
body weight)
-----------------------------------------------------------------
Rat cyhalothrin 1000-2000 (male) Nixon &
200-2000 (female) Jackson (1981a)
Rat lambda-cyhalothrin 632 (male) Barber (1985)
696 (female)
Rabbit cyhalothrin > 2000 (male) Nixon &
> 2000 (female) Jackson (1981a)
-----------------------------------------------------------------
7.1.3. Intraperitoneal
The intraperitoneal LD50 of cyhalothrin to rats is in the range
of 250 to 750 mg/kg. Responses of 0% or 100% mortality were
observed at all but one dose, and so no precise value for the LD50
could be given. The highest dose with no mortality was 250 mg/kg
and the lowest dose with 100% mortality was 750 mg/kg (Nixon &
Jackson, 1981a).
7.2. Irritation and sensitization
7.2.1. Irritation
Undiluted technical cyhalothrin is a mild irritant to occluded
rabbit skin (both intact and abraded) but is non-irritant to
occluded rat skin (intact) (Jackson & Nixon, 1981). Technical
cyhalothrin is a moderate irritant to the rabbit eye without
irrigation and is a mild irritant when irrigated for one minute, 20
to 30 seconds after instillation of the material (Jackson, 1981).
Technical lambda-cyhalothrin is non-irritant to occluded
rabbit skin without abrasion (Pritchard, 1985a). It is a mild
irritant to the rabbit eye (Pritchard, 1985b).
7.2.2. Sensitization
A skin sensitization test with cyhalothrin on guinea-pigs,
using the procedure of Buehler, indicated that cyhalothrin has
skin-sensitizing potential (Nixon & Jackson, 1981b). In guinea-
pigs that had been previously induced with undiluted cyhalothrin
technical material, using the Magnusson and Kligman maximization
test, a moderate sensitization response was elicited. When
lambda-cyhalothrin was tested for skin sensitization on guinea-
pigs, using the maximization procedure of Magnusson and Kligmann,
it was shown to have no sensitization potential (Pritchard, 1984).
7.3. Short-term exposures
7.3.1. Oral
7.3.1.1 Rat
When groups of male and female rats were fed diets containing
cyhalothrin at levels up to 750 mg/kg for 28 days, symptoms of
toxicity shown by animals receiving doses of 250 mg/kg diet or more
included high stepping gait, ataxia, and hypersensitivity to
external stimuli. These effects were dose related and some
mortality occurred at the highest dose level. The clinical signs
were not accompanied by histopathological changes in the nervous
system. Thymic atrophy, adrenal enlargement with vacuolization,
and incomplete spermatogenesis occurred with the highest dose. An
adaptive change was present in the liver as judged by an increase
in liver weight, proliferation of smooth endoplasmic reticulum,
and an increase in the activity of the xenobiotic-metabolizing
enzyme, aminopyrine- N-demethylase (APDM). These effects occurred at
doses of 100 mg/kg or more, and there was some evidence for this
type of change at 20 mg/kg (Tinson et al., 1984).
When groups of 20 male and 20 female Wistar rats were fed
cyhalothrin (89% pure) at dietary concentrations of 0, 10, 50, and
250 mg/kg for 90 days, only male rats fed 250 mg/kg showed
significantly reduced body weight gain. No abnormal clinical signs
were seen. Haemoglobin and haematocrit values were not affected by
the treatment, but there were marginal effects on mean erythrocyte
volume in some treated groups. Male rats fed 50 or 250 mg/kg
showed decreased plasma triglyceride levels and a dose-related
increase in hepatic APDM activity. The latter effect was also seen
in females fed 250 mg/kg. Small increases in urinary glucose
excretion occurred in males fed 50 or 250 mg/kg after 13 weeks. No
treatment-related effects on organ weights and no significant
histopathological changes were reported (Lindsay et al., 1981).
In studies by Lindsay et al. (1982), two groups of 32 male rats
were fed either a control diet or a diet containing 250 mg
cyhalothrin/kg for 28 days. After this period eight rats per group
were killed and examined. The remaining rats were fed the control
diet for periods of 7, 14, or 28 days after cessation of treatment,
and a further eight rats per group were killed and examined at each
of these time intervals. A decrease in body weight gain was seen
in the treated rats, which, although not statistically
significant, was still much reduced at the end of the 28-day
recovery period. Proliferation of the hepatic smooth endoplasmic
reticulum and elevated hepatic APDM activity were also seen in the
treated animals. These effects had reversed 7 days after the
cessation of treatment with cyhalothrin and were considered to be
physiological adaptive changes rather than a toxicological effect.
When groups of 20 male and 20 female Alderley Park rats
received diets containing 0, 10, 50, or 250 mg lambda-
cyhalothrin/kg for 90 days, decreased body weight gain, accompanied
by a reduction in food consumption, was seen in both male and
female rats receiving the highest dose. No abnormal clinical signs
were seen; in particular, there was no evidence of neurological
effects. There were no treatment-related effects on haematological
parameters but reductions in the activities of plasma alanine
trans-aminase (males only) and alkaline phosphatase (females only
at 13 weeks) were apparent in animals fed the highest dose. Plasma
triglycerides were also reduced in males at this feeding level.
Relative liver weights were increased in both sexes at 250 mg/kg
and in males at 50 mg/kg, accompanied by increased activity of
hepatic APDM. No other changes in organ weight or histopathology
were attributed to treatment with lambda-cyhalothrin. These two
effects, particularly in view of the findings with cyhalothrin,
were considered to be adaptive in nature and the toxicological NOEL
was established at 50 mg/kg (equivalent to 2.5 mg/kg body weight
per day (Hart et al., 1985).
7.3.1.2 Dog
In studies by Chesterman et al. (1980), groups of one male and
one female dog (Alderley Park beagles) received 0, 2.5, or 10 mg
cyhalothrin/kg body weight orally in corn oil by gelatine capsule
daily for four weeks. A further group initially received 30 mg/kg
per day, but due to severe clinical signs after 10 days dosing,
which were typical of pyrethroid toxicity (muscular trembling,
unsteadiness, vomiting, and body weight loss), the animals were
rested and then received 20 mg/kg per day for four weeks. Similar
clinical signs were seen, with the exception of body weight loss,
in all animals receiving 10 mg/kg per day or more, the severity
being dose related. Investigation of the sciatic and tibial nerves
and the lumbricalis muscle with special histopathological stains
indicated no changes that could be attributed to treatment with
cyhalothrin. Liquid faeces were produced by all animals receiving
cyhalothrin, the incidence being dose related. These changes were
considered to be of no toxicological significance. No other
changes were observed that could be attributed to the treatment.
When groups of 6 male and 6 female dogs (Alderley Park beagles)
were fed cyhalothrin in corn oil by gelatine capsule at 0, 1, 2.5,
or 10 mg/kg body weight per day for 26 weeks, signs of pyrethroid
toxicity were seen in some dogs at 10 mg/kg. Although liquid
faeces were produced by all animals in the study, including the
controls, the incidence and frequency were higher in treated
animals and were dose related. These changes were considered to be
of no toxicological significance. Macroscopic postmortem
examination, organ weights, and histological investigations
revealed no treatment-related changes. The oral NOEL in dogs was
found to be 2.5 mg/kg per day (Chesterman et al., 1981).
In studies by Hext et al. (1986), groups of 6 male and 6 female
dogs were dosed by gavage in corn oil with 0, 0.1, 0.5, or 3.5 mg
lambda-cyhalothrin/kg body weight daily for 52 weeks. Clinical
signs of neurological effects were evident in all animals fed the
highest dose, which were unaccompanied by histological changes in
the nervous system. There was an increased incidence of fluid
faeces at the highest dose and a slight increase in the group
receiving 0.5 mg/kg per day. This effect was considered to be
related to the method of administration and not to be
toxicologically significant. No histopathological changes
attributable to lambda-cyhalothrin administration were observed at
any of the dose levels employed, and the toxicological NOEL in
this study was 0.5 mg lambda-cyhalothrin per kg per day.
7.3.2. Dermal
7.3.2.1 Rabbit
Cyhalothrin in polyethylene glycol (PEG 300) (10, 100, or 1000
mg/kg body weight per day) was applied to the skin of groups of 10
male and 10 female New Zealand White rabbits and kept in contact
with the skin 6 h/day, 5 days per week for 3 weeks (i.e. a total
of 15 applications) by means of an occlusive dressing. A group of
14 male and 14 female control rabbits was treated with polyethylene
glycol (PEG 300) using the same procedure. The skin of half the
animals in each group was abraded prior to the application of
cyhalothrin. Repeated application of the vehicle alone
(polyethylene glycol) and the vehicle plus cyhalothrin caused
slight to severe skin irritation. At the highest dose level there
was an increased incidence of oedema and erythema. A small number
of animals given the highest dose showed pyrethroid-like symptoms,
but only when the skin was unabraded. The NOEL was considered to be
100 mg/kg per day (Henderson & Jackson, 1982).
7.4. Long-term exposures and carcinogenicity
7.4.1. Rat
In studies by Pigott et al. (1984), groups of 72 male and 72
female Alpk/AP strain rats were fed diets containing cyhalothrin
at levels of 0, 10, 50, or 250 mg/kg diet for up to 104 weeks. All
the surviving animals were sacrificed, and histopathological and
gross postmortem examinations were carried out. Decreased body
weight gain, accompanied by a small decrease in food consumption,
was evident in rats of both sexes fed the highest dose. This was
accompanied by minor changes in blood biochemistry. Increased liver
weight was seen in rats of both sexes fed cyhalothrin at 250 mg/kg
at the interim sacrifice but this was not evident at termination.
There was no histopathological evidence of a chronic toxic effect
due to cyhalothrin. In particular clinical and histopathological
evaluation gave no indication of an effect on the nervous system.
There was no evidence for a carcinogenic effect of cyhalothrin.
The toxicological NOEL for this study was 50 mg cyhalothrin/kg
diet, corresponding to a minimum dose rate of approximately 1.7
mg/kg body weight per day for male rats and 1.9 mg/kg per day for
female rats.
7.4.2. Mouse
Groups of 52 male and 52 female Charles River CD-1 mice were
maintained for 104 weeks on diets containing 0, 20, 100, or 500 mg
cyhalothrin/kg and further groups of 12 males and 12 females were
designated for interim sacrifice after 52 weeks. During the study
there were no deaths attributable to treatment with cyhalothrin.
Signs of toxicity ascribable to cyhalothrin included piloerection
and hunched posture in both sexes at 500 mg/kg and in males at 100
mg/kg and reduced body weight gain, higher food intake, and
reduced efficiency of food utilization in males receiving 500
mg/kg. There was a statistically significant increase, compared to
the controls, in the incidence of mammary adenocarcinoma in females
at the two highest dose levels. However, the frequency of these
tumours was not unduly at variance with that normally seen in the
strain of mouse used, and no dose relationship was apparent. Thus,
there were no neoplastic findings that could be attributed to the
long-term administration of cyhalothrin. There was a clear NOEL of
20 mg/kg, corresponding to a mean calculated daily intake of 1.8
mg/kg body weight per day in males and 2.0 mg/kg body weight per
day in females (Colley et al., 1984).
7.5. Reproduction, embryotoxicity, and teratogenicity
7.5.1. Reproduction
In studies by Milburn et al. (1984), groups of 15 male and 30
female (F0 parents) weaning Alderley Park rats were fed diets
containing 0, 10, 30, or 100 mg cyhalothrin per kg. After 12
weeks, the animals were mated to produce the first (F1a) litter and
subsequently re-mated to produce a second (F1b) litter. The
breeding programme was repeated with F1 parents selected from the
F1b off-spring and F2 parents selected from the F2b offspring.
Test diets were fed continuously throughout the study. There were
minor effects on body weight gain of parents from all generations
receiving 100 mg/kg, but no clinical signs of neurological effects
were seen in either parents or offspring. No effects of treatment
were seen on indices of male and female fertility, gestation
period, live born index, or pup survival. There was a small
reduction in mean total litter weight of the F2 and F3 generations
from rats receiving the highest dose, which was attributable to
minor decreases in litter size and a small reduction in weight gain
of the pups. No effect was seen in litters from rats receiving 30
mg/kg. There was no evidence of gross or histopathological change
attributable to the treatment. The reproductive effects seen in
rats receiving the highest dose were of a minor nature. A clear
NOEL of 30 mg/kg (corresponding to a dosage in the range of 1.5 to
1.9 mg/kg body weight per day) was established (Milburn et al.,
1984).
7.5.2. Embryotoxicity and teratogenicity
7.5.2.1 Rat
When groups of 24 mated female rats (Charles River CD-1) were
given cyhalothrin orally (in corn oil), at 0, 5, 10, or 15 mg/kg
body weight per day, from day 6 to 15 inclusive of gestation and
were killed on day 20, there was reduced body weight gain at the
highest dose level and evidence of mild pyrethroid toxicity in two
of these animals. There were no other effects on the clinical or
litter parameters attributable to treatment with cyhalothrin, and
examination of the viscera and skeletons showed no effects of
treatment. At the highest dose level, there was maternal toxicity,
but there was no effect on any aspect of fetal development at any
dose level (Killick, 1981a).
7.5.2.2 Rabbit
In studies by Killick (1981b), groups of at least 18 pregnant
New Zealand White rabbits received cyhalothrin orally in corn oil
daily at 0, 3, 10, or 30 mg/kg body weight, from days 6 to 18
(inclusive) of gestation and were killed on day 28. There was
reduced body weight gain at the highest dose, accompanied by
reduced food intake during dosing. There were no clinical signs and
no changes in pregnancy incidence or in litter parameters
attributable to treatment with cyhalothrin. Examination of the
viscera and skeletons revealed no effects of treatment. At the
highest dose level, there was maternal toxicity, but there was no
effect on any aspect of fetal development at any dose level.
7.6. Mutagenicity and related end-points
7.6.1. Microorganisms
Five test strains, TA1535, TA1537, TA1538, TA98, and TA100,
were employed to evaluate the mutagenic potential of cyhalothrin
using the salmonella reverse mutation assay of Ames. The assay was
conducted in the presence and absence of metabolic activation (S9
mix) with cyhalothrin at levels up to 2500 µg/plate. The mean
numbers of revertant colonies of Salmonella typhimurium observed in
the five test strains indicated an unequivocal negative response
(Trueman, 1981). Lambda-cyhalothrin at dose levels of up to 5000
µg/plate, both in the presence and absence of metabolic activation,
gave a non-mutagenic response in the same test using the same
strains (Callander, 1984).
7.6.2. In vitro mammalian cells
When cyhalothrin was tested in a modification of the cell
culture transformation test of Styles, using Syrian Hamster kidney
cell line BHK21C13, the response in the presence of metabolic
activation was unequivocally negative. In the absence of metabolic
transformation there was an erratic increase in numbers of
transformed colonies together with a poor dose response. These
data were not thought to indicate a significant positive response,
and it was concluded that cyhalothrin does not appear to possess
significant cell-transforming properties (Richold et al., 1981).
In addition, the significance of the results from the BHK cell
system is doubtful in view of the questionable interlaboratory
reproducibility of this assay.
The mutagenic potential of lambda-cyhalothrin has been assessed
in vitro with L51787 mouse lymphoma cells, both in the presence and
absence of auxiliary metabolic activation (S9) mix, using dose
levels of 125-1000, 2000, and 4000 µg/ml. There was no increase in
mutation frequency either in the presence or absence of S9 mix
(Cross, 1985).
Lambda-cyhalothrin, at dose levels of up to 1000 µg/ml, either
in the presence or absence of metabolic activation, did not induce
statistically significant increases in the incorporation of
tritiated thymidine in cultured human (Hela) cells (Milone, 1986)
or induce chromosomal damage in human lymphocytes stimulated by
phytohaemagglutinin (Sheldon et al., 1985).
7.6.3. In vivo mammalian assays
Male rats were given a single dose or five consecutive daily
doses by gavage of cyhalothrin at levels of 1.5, 7.5, or 15 mg/kg,
and bone marrow samples were taken and examined for chromosomal
abnormalities. The results indicated that cyhalothrin has no
clastogenic potential (Anderson et al., 1981).
In studies by Irvine (1981), three groups of male mice were
dosed with cyhalothrin by gavage, at dose levels of 1, 5, or 10
mg/kg daily, for 5 consecutive days. A further group received the
known mutagen cyclophosphamide intraperitoneally at 200 mg/kg
daily for five days. The animals were then mated with groups of
females at weekly intervals for eight weeks. Pregnancy incidence,
pre- and post-implantation loss, clinical condition, body weight,
and gross necropsy were assessed. There was no evidence of an
increase in the dominant lethal mutation frequency following
treatment. The NOEL was 10 mg/kg per day. Although these studies
showed no clastogenic or mutagenic effect, it is not clear whether
sufficiently high dose levels were used.
When lambda-cyhalothrin was administered to mice at levels of
up to 35 mg/kg and bone marrow preparations were examined for the
formation of micronuclei in polychromatic erythrocytes, there was
no statistically significant increase in the frequency of
micronuclei, compared to control animals. The positive control
substance, cyclophosphamide, showed the expected response (Sheldon
et al., 1984).
7.7. Mode of action
The mode of action of cyhalothrin and lambda-cyhalothrin, both
type II pyrethroids, is basically the same as that of the other
pyrethroids (see Appendix).
8. EFFECTS ON HUMANS
8.1. General population exposure
No poisoning incidents with cyhalothrin or lambda-cyhalothrin
have been reported. There is no information on effects from short-
or long-term exposure and no epidemiological information is
available.
8.2. Occupational exposure
Cyhalothrin is known to produce an effect described as
subjective facial sensation (SFS) in some people working with this
compound. SFS is a transient phenomenon; symptoms are not
associated with objective physical signs and recovery appears to be
complete. It is likely that SFS arises from direct facial contact
with the chemical particularly from touching the face with
contaminated gloves or hands. This would also help to explain the
effect of formulation or concentration as these could affect the
rate of dermal penetration and therefore access of the chemical to
the nerve endings.
It appears unlikely that individual sensitivity is important
in the development of symptoms following exposure. It is more
likely to be related to the amount of the chemical that comes into
contact with the facial skin. The information currently available
(Hart, 1984) is summarized in section 8.2.2.
8.2.1. Acute toxicity: poisoning incidents
No poisoning incidents involving cyhalothrin or lambda-
cyhalothrin have been reported.
8.2.2. Effects of short- and long-term exposure
Laboratory workers and manufacturing plant and field operators
handling natural and synthetic pyrethroids, including cyhalothrin
and lambda-cyhalothrin, have noticed a transient skin sensation in
the periorbital area of the face and other sites after direct skin
exposure. It has been suggested that these sensations are caused
by spontaneous repetitive firing of sensory nerve fibres or nerve
endings, whose threshold has been transiently lowered by the
compound, localized around the sites of exposure.
Symptoms from exposure to a synthetic pyrethroid generally
start 30 min to 1 h after exposure and last for several hours,
sometimes up to 2 days. They almost always affect the facial area,
producing a tingling, burning, or numb sensation that has been
variously described as paraesthesia, dysaesthesia, or SFS. The
latter term appears most appropriate as no objective signs of
abnormality of nerve function have been found in workers suffering
from these effects and the facial area is by far the most commonly
affected.
8.2.2.1 Manufacture
At least 12 workers involved in the manufacture of cyhalothrin
have experienced symptoms of SFS. The symptoms were described as
burning, tingling, or sunburn, but clinical examination revealed no
abnormal neurological signs. The areas of the face affected were
the eyelids, cheeks, nostrils, forehead, and lips. In one case the
penis was affected, probably from contact with contaminated hands.
The symptoms started between 5 min and 3 h from the onset of
exposure and lasted from 5 to 30 h. Wind, washing, and temperature
increase led to a worsening of the symptoms, and relief could be
obtained by using a local anaesthetic. Barrier creams were
relatively ineffective in preventing the symptoms.
8.2.2.2 Formulation and laboratory work
Two workers have been reported to have suffered from SFS. Both
were exposed to technical cyhalothrin and symptoms began 1-6 h
after exposure. The symptoms described were typical of SFS,
consisting of tingling and burning around the eyes and facial area,
and lasted 6-24 h.
Four reports involving three individuals have been received
from laboratories involved with lambda-cyhalothrin. The symptoms
consisted of a facial tingling and burning sensation and in one
case tingling on the tongue. Symptoms began within 30 min of
exposure and lasted from 6 h to 2 days. All three workers were
involved in handling either technical material or concentrated
lambda-cyhalothrin solutions (10% w/v ai).
8.2.2.3 Field use
Virtually no reports have been received on the agricultural or
public health use of cyhalothrin. The only information that has
become available is from the use of cyhalothrin in animal health
products. Skin itching has been reported following exposure to a
5% emulsifiable concentrate of cyhalothrin, but dilutions of 0.1%
and 0.005% (w/v of ai) were without significant effect. More
recently, several incidents of facial irritation have been reported
following the use of cyhalothrin in sheep sprays (Hart, 1984). The
concentration used in these sprays was very low (0.002% w/v), but
the characteristics of the spray used were unclear.
Out of 38 field trials staff who returned questionnaires, only
four reported experiencing any adverse effects as a result of
exposure to lambda-cyhalothrin. Three of these individuals
experienced a burning sensation or irritation around the face,
cheeks, or eyes, which started between 45 and 60 min after initial
exposure and lasted, respectively, for 5, 18, and 72 h. In one case
the symptoms were severe enough to prevent the individual from
working. The other field trials worker experienced a rash on the
hands that started 24 h after exposure and lasted several days. All
four field trials staff were involved in handling concentrated
solutions of lambda-cyhalothrin (2.5% w/v ai) and three of the four
sprayed diluted solutions. None of the three workers experiencing
the facial sensation had experienced similar symptoms before, but
the worker who developed the rash had suffered a similar rash after
exposure to permethrin, cypermethrin, and deltamethrin following
their application to top fruit. Eight of the other 34 field trials
staff returning questionnaires stated that they had previously
suffered symptoms of facial tingling or burning.
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Cyhalothrin was discussed at the 1984 and 1986 FAO/WHO Joint
Meetings on Pesticide Residues (JMPR), where an acceptable daily
intake (ADI) for cyhalothrin of 0-0.02 mg/kg body weight was
established (FAO/WHO, 1985, 1986a).
The JMPR (FAO/WHO, 1985, 1986a,b) has estimated maximum
residue limits (MRLs) for cyhalothrin (sum of the isomers) as
follows:
---------------------------------------------------------
Commodity Maximum Pre-harvest
residue limit interval (days)
---------------------------------------------------------
Pome fruits 0.2 14
Cabbages, head 0.2 3-4
Potatoes 0.02a non specified
Cotton seed 0.02a 21
Cotton seed oil, crude 0.02a non specified
Cotton seed oil, edible 0.02a non specified
---------------------------------------------------------
a At or about the limit of detection.
REFERENCES
ANDERSON, D., RICHARDSON, C.R., HULME, A., MORRIS, J., BANHAM, P.B., &
GODLEY, M.J. (1981) Cyhalothrin: A cytogenic study in the rat (Unpublished
proprietary report No. CTL/P/664, submitted to WHO by ICI).
BARBER, J.E. (1985) PP321: Acute dermal toxicity study (Unpublished
proprietary report No. CTL/P/1119, submitted to WHO by ICI).
BENGSTONE, M., DAVIES, R.A.H., DESMARCHELIER, J.M., HENNING, R., MURRAY,
W., SIMPSON, B.W., SNELSON, J.T., STICKA, R., & WALLBANK, B.E. (1983)
Organophosphorothioates and synergised synthetic pyrethroids as grain
protectants on bulk wheat. Pestic. Sci., 14: 373-384.
BEWICK, D.W. & ZINNER, C.K.J. (1981) Cyhalothrin: Degradation in soil
(Unpublished proprietary report No. RJ0203B, submitted to WHO by ICI).
BHARTI, H. & BEWICK, D.W. (1986) Cyhalothrin: Degradation in a Japanese
soil (Unpublished proprietary report No. RJ0491B, submitted to WHO by ICI).
BHARTI, H., BEWICK, D.W., & WHITE, R.D. (1985) PP563 and PP321: Degradation
in soil (Unpublished proprietary report No. RJ0382B, submitted to WHO by
ICI).
CALLANDER, R.D. (1984) PP321: An evaluation in the Salmonella mutagenicity
assay (Unpublished proprietary report No. CTL/P/1000, submitted to WHO by
ICI).
CHESTERMAN, H., HEYWOOD, R., ALLEN, T.R., STREET, A.E., PRENTICE, D.,
BUCKLEY, P., & OFFER, J. (1980) PP563: Preliminary oral toxicity study in
beagle dogs (Unpublished proprietary report of the Huntingdon Research
Centre No. 325/8074, submitted to WHO by ICI).
CHESTERMAN, H., HAYWOOD, R., ALLEN, T.R., STREET, A.E., KELLY, D.F.,
GOPINATH, C., & PRENTICE, D.E. (1981) Cyhalothrin oral toxicity study in
beagle dogs (Final report: Repeated daily dosing for 26 weeks) (Unpublished
proprietary report of the Huntingdon Research Centre No. ICI/326/8162,
submitted to WHO by ICI).
COLLEY, J., DAWE, S., HEYWOOD, R., ALMOND, R., GIBSON, W.A., GREGSON, R., &
GOPINATH, C. (1984) Cyhalothrin potential tumorigenic and toxic effects in
prolonged dietary administration to mice (Unpublished proprietary report of
the Huntingdon Research Centre No. ICI 395/83668, submitted to WHO by ICI).
COLLIS, W.M.D. & LEAHEY, J.P. (1984) PP321: Hydrolysis in water at pH 5, 7
and 9 (Unpublished proprietary report No. RJ0338B, submitted to WHO by
ICI).
COULSON, J.M., COLLINS, I.G., & EDWARDS, P.J. (1986) PP321: Effects on
earthworms Lumbricidae of repeated annual field applications (Unpublished
proprietary report No. RJ0511B, submitted to WHO by ICI).
CROSS, M. (1985) PP321: Assessment of mutagenic potential using L5178Y
mouse lymphoma cells (Unpublished proprietary report No. CTL/P/1340,
submitted to WHO by ICI).
CROSSLAND, N.O. (1982) Aquatic toxicology of cypermethrin. II. Fate and
biological effects in pond experiments. Aquat. Toxicol., 2: 205-222.
CROSSLAND, N.O., SHIRES, S.W., & BENNETT, D. (1982) Aquatic toxicology of
cypermethrin. III. Fate and biological effects of spray drift deposits in
freshwater adjacent to agricultural land. Aquat. Toxicol., 2: 253-270.
CURL, E.A., LEAHEY, J.P., & LLOYD, S.J. (1984a) PP321: Aqueous photolysis
at pH 5 (Unpublished proprietary report No. RJ0362B, submitted to WHO by
ICI).
CURL, E.A., LEAHEY, J.P., & LLOYD, D. (1984b) PP321: Photodegradation on a
soil surface (Unpublished proprietary report No. RJ0358B, submitted to WHO
by ICI).
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American Chemical
Society, pp. 229 (ACS Symposium Series 42).
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid. Pestic. Biochem. Physiol., 6: 547-550.
FAO (1982) Report of the Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15 October,
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1985) Cyhalothrin. In: 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agricultural Organization of the United Nations,
pp. 173-185, 571-584 (FAO Plant production and protection Paper 67).
FAO/WHO (1986a) Cyhalothrin: In: 1986 Evaluations of some pesticide
residues in food, Part 1: Residues, Rome, Food and Agriculture Organization
of the United Nations, pp. 95-138 (FAO Plant Production and Protection
Paper 78).
FAO/WHO (1986b) Codex maximum limits for pesticide residues, 2nd ed., Rome,
Codex Alimentarius Commission, Food and Agriculture Organization of the
United Nations (CAC XIII).
FARRELLY, E., HAMER, M.J., & HILL, I.R. (1984) PP321: Toxicity to first
instar Daphnia magna (Unpublished proprietary report No. RJ0359B, submitted
to WHO by ICI).
FARRELLY, E., HAMER, M.J., & HILL, I.R. (1985) PP321: Toxicity of
formulations JF9509 and GFU383C to first instar Daphnia magna (Unpublished
proprietary report No. RJ0426B, submitted to WHO by ICI).
FITZPATRICK, R.D. (1985) PP321 dissipation in US soils - 1983 (Unpublished
report No. TMU1809, submitted to WHO by ICI).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous sensation
between synthetic pyrethroid insecticides. Contact dermatitis, 13: 140-147.
FORBES, S. & DUTTON, A.J. (1985) A small scale method for the determination
of pyrethroid insecticide residues in crops and soils. Pestic. Sci., 16:
404-408.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent class
antagonize GABA action at the crayfish neuromuscular junction. Neurosci.
Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of pyrethroid
action in the cockroach. Pestic. Biochem. Physiol., 15: 181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid toxicology:
Protective effects of Diazepam and phenobarbital in the mouse and the
cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
GIFAP (1981) Technical Monograph No. 4: Guidelines on pesticide residue
trials to provide residue data for the registration of pesticides and the
establishment of maximum residue limits, Brussels, International Group of
National Associations of Manufacturers of Agrochemical Products (GIFAP
D/1981/2537/3).
GIRENKO, D.B. & KLISENKO, M.A. (1984) Concentration and determination of
trace quantities of synthetic pyrethroids in the air. Gig. i Sanit., 3: 57-
59.
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4(6):
793-799.
GOUGH, H.J., COLLINS, I.G., EVERETT, C.J., & WILKINSON, W. (1984) PP321:
Acute contact and oral toxicity to honey bees (Apis mellifera) (Unpublished
proprietary report No. RJ0390B, submitted to WHO by ICI).
GOUGH, H.J., COLLINS, I.G., & WILKINSON, W. (1985) PP321: Field test of
toxicity to honey bees (Apis mellifera) on flowering oil-seed rape
(Brassica napus) (Unpublished proprietary report No. RJ0413B, submitted to
WHO by ICI).
HALL, J.S. & LEAHEY, J.P. (1983) Cyhalothrin: Fate in river water
(Unpublished proprietary report No. RJ0320B, submitted to WHO by ICI).
HAMER, M.J. & HILL, I.R. (1985) Cyhalothrin: The accumulation of
cyhalothrin and its degradation products by channel catfish and
Daphnia magna in a soil/water system (Unpublished proprietary
report No. RJ0427B, submitted to WHO by ICI).
HAMER, M.J., FARRELLY, E., & HILL, I.R. (1985a) PP321: Toxicity to Gammarus
pulex (Unpublished proprietary report No. RJ0414B, submitted to WHO by
ICI).
HAMER, M.J., FARRELLY, E., & HILL, I.R. (1985b) PP321: 21 day Daphnia magna
life-cycle study (Unpublished proprietary report No. RJ0451B, submitted to
WHO by ICI).
HARRISON, M.P. (1981) Cyhalothrin: The disposition and metabolism of [ 14 C]-
146814 in rats (Unpublished proprietary report No. 10/HD/007325, submitted
to WHO by ICI).
HARRISON, M.P. (1983) Cyhalothrin: The metabolism and disposition of 146814
in the rat: Part IV. Isolation and identification of the major urinary
metabolites derived from [14 C-benzyl]- or [14 C-cyclopropyl]-146814
following oral administration (Unpublished proprietary report No.
6/HC/005683, submitted to WHO by ICI).
HARRISON, M.P. (1984a) Cyhalothrin (146814): The metabolism and disposition
of 146814 in rats: Part II. Tissue residues derived from [14 C-benzyl] or
[14 C-cyclopropyl]-146814, after a single oral dose of 1 or 25 mg/kg
(Unpublished proprietary report No. KMR 002/2, submitted to WHO by ICI).
HARRISON, M.P. (1984b) Cyhalothrin: The metabolism and disposition of
[14 C]-146814 in rats: Part III. Studies to determine radioactive residues
in the rat following 14 days repeated oral administration (Unpublished
proprietary report No. 146814 KMR 002/03, submitted to WHO by ICI).
HARRISON, M.P. (1984c) Cyhalothrin (146814): The disposition and metabolism
of [14 C]-146814 in the dog (Unpublished proprietary report No. 146814 KMD
005, submitted to WHO by ICI).
HARRISON, M.P. (1984d) Cyhalothrin (146814): The metabolism, excretion and
residues in the cow after 7 days oral administration with two [14 C]-
labelled forms of 146814 at 1 mg/kg/day (Unpublished proprietary report No.
146814 KMC 006/007, submitted to WHO by ICI).
HART, D., BANHAM, P.B., CHART, I.S., EVANS, D.P., GORE, C.W., STONARD,
M.D., MORELAND, S., GODLEY, M.J., & ROBINSON, M. (1985) PP321: 90-day
feeding study in rats (Unpublished proprietary report No. CTL/P/1045,
submitted to WHO by ICI).
HART, T.B. (1984) Review of adverse skin reactions associated with the use
of synthetic pyrethroids (Unpublished proprietary report No. TMF2530,
submitted to WHO by ICI).
HENDERSON, C. & JACKSON, S.J. (1982) Cyhalothrin: Subacute dermal toxicity
study in rabbits (Unpublished proprietary report No. CTL/P/680, submitted
to WHO by ICI).
HEXT, P.M., BRAMMER, A., CHALMERS, D.T., CHART, I.S., GORE, C.W., PATE, I.,
& BANHAM, P.B. (1986) PP321: 1 year oral dosing study in dogs (Unpublished
proprietary report No. CTL/P/1316, submitted to WHO by ICI).
HILL, R.W. (1981) Determination of the acute toxicity of cyhalothrin
(PP563) to Rainbow trout (Salmo gairdneri) (Unpublished proprietary report
No. BL/B/2097, submitted to WHO by ICI).
HILL, R.W. (1984a) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) (Unpublished proprietary report No. BL/B/2405, submitted
to WHO by ICI).
HILL, R.W. (1984b) PP321: Determination of acute toxicity to Bluegill
sunfish (Lepomis macrochirus) (Unpublished proprietary report No.
BL/B/2406, submitted to WHO by ICI).
HILL, R.W. (1985a) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) of 2.5% w/w formulation (Unpublished proprietary report
No. BL/B/2603, submitted to WHO by ICI).
HILL, R.W. (1985b) PP321: Determination of acute toxicity of a 1 lb/US
gallon EC formulation to Rainbow trout (Salmo gairdneri) (Unpublished
proprietary report No. BL/B/2609, submitted to WHO by ICI).
HILL, R.W. (1985c) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) of a 5% EC formulation (Unpublished proprietary report
No. BL/B/2783, submitted to WHO by ICI).
HILL, R.W. (1985d) PP321: Determination of acute toxicity to Mirror carp
(Cyprinus carpio) of a 2.5% w/w formulation (Unpublished proprietary report
No. BL/B/2604, submitted to WHO by ICI).
HILL, R.W. (1985e) PP321: Determination of acute toxicity to Mirror carp
(Cyprinus carpio) of a 5% EC formulation (Unpublished proprietary report
No. BL/B/2784, submitted to WHO by ICI).
HILL, R.W. (1985f) PP321: Determination of acute toxicity of a 1 lb/US
gallon EC formulation to Bluegill sunfish (Lepomis macrochirus)
(Unpublished proprietary report No. BL/B/2610, submitted to WHO by ICI).
HILL, R.W. (1985g) PP321: Determination of acute toxicity to Sheepshead
minnow (Cyprinodon variegatus) (Unpublished proprietary report No.
BL/B/2615, submitted to WHO by ICI).
HILL, R.W., CAUNTER, J.E., & CUMMING, R.I. (1985) PP321: Determination of
the chronic toxicity to Sheepshead minnow (Cyprinodon variegatus) embryos
and larvae (Unpublished proprietary report No. BL/B/2677, submitted to WHO
by ICI).
IRVINE, L.F.H. (1981) Cyhalothrin: Oral (gavage) dominant lethal study in
the male mouse (Unpublished proprietary report of the Hazleton Laboratories
No. 2647-72/213, submitted to WHO by ICI).
JACKSON, S.J. (1981) Cyhalothrin: Eye irritation study in the rabbit
(Unpublished proprietary report No. CTL/T/1502, submitted to WHO by ICI).
JACKSON, S.J. & NIXON, J. (1981) C yhalothrin: Skin irritation studies in
the rabbit and rat (Unpublished proprietary report No. CTL/T/1504,
submitted to WHO by ICI).
KILLICK, M.E. (1981a) Cyhalothrin: Oral (gavage) teratology study in the
rat (Unpublished proprietary report of the Hazleton Laboratories No. 2661-
72/208, submitted to WHO by ICI).
KILLICK, M.E. (1981b) Cyhalothrin: Oral (gavage) teratology study in the
New Zealand white rabbit (Unpublished proprietary report of the Hazleton
Laboratories No. 2700-72/211, submitted to WHO by ICI).
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of pyrethroid
insecticides on the gamma-aminobutyric acid receptor-ionophore complex.
Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding sites.
Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor & Francis
Ltd, 440 pp.
LEAHEY, J.P. & FRENCH, D.A. (1986a) Quantification and characterisation of
radioactive residues found in soya leaves from plants treated with 14 C-
PP321 (Unpublished proprietary report No. RJ0507B, submitted to WHO by
ICI).
LEAHEY, J.P. & FRENCH, D.A. (1986b) Quantification and characterisation of
radioactive residues found in cotton leaves from plants treated with 14 C-
cyclopropane-labelled PP321 (Unpublished proprietary report No. JR0526B,
submitted to WHO by ICI).
LEAHEY, J.P. & FRENCH, D.A. (1986c) Quantification and characterisation of
radioactive residues in cotton leaves from plants treated 14 C-benzyl-
labelled PP321 (Unpublished proprietary report No. RJ0497B, submitted to
WHO by ICI).
LEAHEY, J.P. & PARKER, S. (1985) Cyhalothrin: Characterisation of residues
accumulated by carp continuously exposed to 14 C-cyhalothrin (Unpublished
proprietary report No. RJ0407B, submitted to WHO by ICI).
LEAHEY, J.P., FRENCH, D.A., & HEATH, J. (1985) PP321: Metabolism in a goat
(Unpublished proprietary Report No. RJ0435B, submitted to WHO by ICI).
LINDSAY, S., CHART, I.S., GODLEY, M.J., GORE, C.W., HALL, M., PRATT, I.,
ROBINSON, M., & STONARD, M. (1981) Cyhalothrin: 90-day feeding study in
rats (Unpublished proprietary report No. CTL/P/629, submitted to WHO by
ICI).
LINDSAY, S., DOE, J.E., GODLEY, M.J., HALL, M., PRATT, I., ROBINSON, M., &
STONARD, M.D. (1982) Cyhalothrin induced liver changes: Reversibility study
in male rats (Unpublished proprietary report No. CTL/P/668, submitted to
WHO by ICI).
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel modification
as the basis for the variation in the nerve membrane effects of pyrethroids
and DDT analogs. Pestic. Biochem. Physiol., 20: 203-216.
MILBURN, G.M., BANHAM, P., GODLEY, M.J., PIGOTT, G., & ROBINSON, M. (1984)
Cyhalothrin: Three generation reproduction study in the rat (Unpublished
proprietary report No. CTL/P/906, submitted to WHO by ICI).
MILONE, M.F. (1986) PP321: Unscheduled DNA synthesis in cultured Hela cells
(autoradiographic method) (Unpublished proprietary report of the Institute
di Ricerche Biomediche Antione Marxer No. M914, submitted to WHO by ICI).
MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2922.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare and
the environment. Proceedings of the Fifth International Congress on
Pesticide Chemistry, Kyoto, Japan, 29 August-4 September, 1982, Oxford, New
York, Pergamon Press (Vol. 1-4).
NIXON, J. & JACKSON, S.J. (1981a) Cyhalothrin: Acute toxicity (Unpublished
proprietary report No. CTL/T/1555, submitted to WHO by ICI).
NIXON, J. & JACKSON, S.J. (1981b) Cyhalothrin: Skin sensitisation study in
the guinea-pig (Unpublished proprietary report No. CTL/T/1552, submitted to
WHO by ICI).
PIGOTT, G.H., CHART, I.S., GODLEY, M.J., GORE, C.W., HOLLIS, K.J.,
ROBINSON, M., TAYLOR, K., & TINSTON, D.J. (1984) Cyhalothrin: Two year
feeding study in rats (Unpublished proprietary report No. CTL/P/980,
submitted to WHO by ICI).
PRITCHARD, V.K. (1984) PP321: Skin sensitisation study (Unpublished
proprietary report No. CTL/P/1054, submitted to WHO by ICI).
PRITCHARD, V.K. (1985a) PP321 and cyhalothrin: skin irritation study
(Unpublished proprietary report No. CTL/P/1139, submitted to WHO by ICI).
PRITCHARD, V.K. (1985b) PP321: Eye irritation study (Unpublished
proprietary report No. CTL/P/1207, submitted to WHO by ICI).
PROUT, M.S. (1984) Cyhalothrin: Bioaccumulation in the rat (Unpublished
proprietary report No. CTL/P/1014, submitted to WHO by ICI).
PROUT, M.S. & HOWARD, E.F. (1985) PP321: Comparative absorption study in
the rat (1 mg/kg) (Unpublished proprietary report No. CTL/P/1214, submitted
to WHO by ICI).
REYNOLDS, L.F. (1984) Cyhalothrin: Determination of acute toxicity to
Bluegill sunfish (Lepomis machrochirus) (Unpublished proprietary report No.
BL/B/2514, submitted to WHO by ICI).
RICHOLD, M., ALLEN, J.A., WILLIAMS, A., & RANSOME, S.J. (1981) Cell
transformation test for potential carcinogenicity of Y00102/010/005
(cyhalothrin (PP563)) (Unpublished proprietary report of the Huntingdon
Research Centre No. 391(B)/80948, submitted to WHO by ICI).
ROBERTS, N.L. & FAIRLEY, C. (1981) The acute oral toxicity (LD50) of
cyhalothrin to the Mallard duck (Unpublished proprietary report of the
Huntingdon Research Centre No. 373WL/801024, submitted to WHO by ICI).
ROBERTS, N.L. & FAIRLEY, C. (1984) The acute oral toxicity (LD50) of PP321
to the Mallard duck (Unpublished proprietary report of the Huntingdon
Research Centre No. 438BT/831011, submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., & WOODHOUSE, R.N. (1981a) The subacute dietary
toxicity (LC50 ) of cyhalothrin to the Mallard duck (Unpublished proprietary
report of the Huntingdon Research Centre No. 377WL/8120, submitted to WHO
by ICI).
ROBERTS, N.L., FAIRLEY, C., & WOODHOUSE, R.N. (1981b) The subacute dietary
toxicity (LC50 ) of cyhalothrin to the Bobwhite quail (Unpublished
proprietary report of the Huntingdon Research Centre No. 378WL/8179,
submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., HAKIN, B., PRENTICE, D.E., & WIGHT, D.G.D.
(1982) The acute oral toxicity (LD50 ) and neurotoxic effects of cyhalothrin
to the domestic hen (Unpublished proprietary report of the Huntingdon
Research Centre No. ICI/374 NT/81742, submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., & DAWE, I.S. (1985a) The subacute
dietary toxicity of PP321 to the Mallard duck (Unpublished proprietary
report of the Huntingdon Research Centre No. ISN 46BT/85171, submitted to
WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., & DAWE, I.S. (1985b) The subacute
toxicity of PP321 to the Bobwhite quail (Unpublished proprietary report of
the Huntingdon Research Centre No. ISN 45BT/841287, submitted to WHO by
ICI).
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a nerve
muscle preparation of the clawed frog, Xenopus laevis. Pestic. Biochem.
Physiol., 25: 176-187.
SAPIETS, A. (1984a) Cyhalothrin: Storage stability of the residue on deep
frozen apple, cabbage and soil samples (Unpublished proprietary report No.
M3843B, submitted to WHO by ICI).
SAPIETS, A. (1984b) The determination of residues of PP321 in crops. A gas-
liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 81, submitted to WHO by
ICI).
SAPIETS, A. (1984c) The determination of residues of cyhalothrin
metabolites in crops. A gas-liquid chromatographic method (Unpublished
proprietary residue analytical method No. PPRAM 74, submitted to WHO by
ICI).
SAPIETS, A. (1985a) The determination of residues of cyhalothrin in crops.
A gas-liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 70, submitted to WHO by
ICI).
SAPIETS, A. (1985b) The determination of residues of cyhalothrin in water.
A gas-liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 69, submitted to WHO by
ICI).
SAPIETS, A. (1985c) PP321: Residue transfer study with dairy cows fed on a
diet containing the insecticide (Unpublished proprietary report No. M3936B,
submitted to WHO by ICI).
SAPIETS, A. (1986a) The determination of residues of PP321 in products of
animal origin. A GLC method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 86/1, submitted to WHO by
ICI).
SAPIETS, A. (1986b) The determination of residues of PP321 in soil. A GLC
method using an internal standard (Unpublished proprietary residue
analytical method No. PPRAM 93, submitted to WHO by ICI).
SAPIETS, A., SWAINE, H., & TANDY, M.J. (1984) In: Zweig, G. & Sherma, J.,
ed. Cypermethrin (Chapter 2). Analytical methods for pesticides and plant
growth regulators: Vol XIII, Synthetic pyrethroids and other pesticides,
New York, London, San Francisco, Academic Press.
SHELDON, T., RICHARDSON, C.R., SHAW, J., & BARBER, G. (1984) An evaluation
of PP321 in the mouse micronucleus test (Unpublished proprietary report No.
CTL/P/1090, submitted to WHO by ICI).
SHELDON, T., HOWARD, C.A., & RICHARDSON, C.R. (1985) PP321: A cytogenetic
study in human lymphocytes in vitro (Unpublished proprietary report No.
CTL/P/1333, submitted to WHO by ICI).
SMART, L.E. & STEVENSON, J.H. (1982) Laboratory estimation of toxicity of
pyrethroid insecticides to honeybees: Relevance to hazard in the field. Bee
World, 63(4): 150-152.
SOUTHWOOD, J. (1984) PP321: Acute oral toxicity to the mouse (Unpublished
proprietary report No. CTL/P/1066, submitted to WHO by ICI).
SOUTHWOOD, J. (1985) PP321: Acute oral toxicity studies (Unpublished report
No. CTL/P/1102, submitted to WHO by ICI).
STEVENS, J.E.B. & BEWICK, D.W. (1985) PP563 and PP321: Leaching of PP563
and PP321 and their degradation products in soil columns (Unpublished
proprietary report No. RJ0408B, submitted to WHO by ICI).
TAKEDA CHEMICAL CO. LTD (1979) Test result of fish toxicity of PP-563
(Unpublished proprietary report, submitted to WHO by ICI).
THOMPSON, R.S. (1985) PP321: Determination of acute toxicity to Mysid
shrimps (Mysidopsis bahia) (Unpublished proprietary report No. BL/B/2635,
submitted to WHO by ICI).
THOMPSON, R.S. & WILLIAMS, T.D. (1985) PP321: Toxicity to the green alga
Selenastrum capricornutum (Unpublished proprietary report No. BL/B2584,
submitted to WHO by ICI).
TINSON, D.J., BANHAM, P.D., CHART, I.S., GORE, C.W., PRATT, I., SCALES,
M.D.C., & WEIGHT, T.M. (1984) PP563: 28-day feeding study in rats. Summary
report (Unpublished proprietary report No. CTL/P/1056, submitted to WHO by
ICI).
TRUEMAN, R.W. (1981) Cyhalothrin: Results from the Salmonella reverse
mutation assay (Unpublished proprietary report No. CTL/P/665, submitted to
WHO by ICI).
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies on
the effects of allethrin and DDT on the sodium channels in frog myelinated
nerve membrane. In: Insect neurobiology and pesticide action, London,
Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M. (1973) DDT-
like action of allethrin in the sensory nervous system of Xenopus laevis.
Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A. (1979) Effects of
insecticides on the sensory nervous system. In: Narashashi, T., ed.
Neurotoxicology of insecticides and pheromones, New York, London, Plenum
Publishing Corporation, pp. 183-210.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity relationships
of some pyrethroids in rats. Arch. Toxicol., 45: 325-329.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-dependent effects
of the pyrethroid insecticide decamethrin in frog myelinated nerve fibres.
Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a) Structure-
related effects of pyrethroid insecticides on the lateral-line sense organ
and on peripheral nerves of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel gating in
myelinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN DEN
BERCKEN, J. (1983) Temperature- and structure-dependent interaction of
pyrethroids with the sodium channels in the frog node of Ranvier. Biochem.
Biophys. Acta, 728: 73-82.
WHO (1979) Safe use of pesticides. Third report of the WHO Expert Committee
on Vector Biology and Control, Geneva, World Health Organization (WHO
Technical Report Series, No. 634).
WILLIAMS, T.D. & THOMPSON, R.S. (1981) Determination of the acute toxicity
of cyhalothrin (PP563) to Daphnia magna (Unpublished proprietary report No.
BL/B/2114, submitted to WHO by ICI).
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SAITO, H. (1984a) PP563
(cyhalothrin): Accumulation in fish (carp) in a flow-through water system
(Unpublished proprietary report of MITES No. 58-367, submitted to WHO by
ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984b) PP563
(cyhalothrin) formulation (5% WP): Acute toxicity to carp (Unpublished
proprietary report of MITES No. 58-367, submitted to WHO by ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984c) PP563
(cyhalothrin): Toxicity to daphnia and fish (carp) in the presence and
absence of soil (Unpublished proprietary report of MITES No. 58-367,
submitted to WHO by ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984d) PP563
(cyhalothrin) formulation (5% WP): Acute toxicity to daphnia (Unpublished
proprietary report of MITES No. 58-367, submitted to WHO by ICI).
APPENDIX
On the basis of electrophysiological studies with peripheral
nerve preparations of frogs (Xenopus laevis; Rana temporaria, and
Rana escuelenta), it is possible to distinguish between 2 classes
of pyrethroid insecticides: (Type I and Type II). A similar
distinction between these 2 classes of pyrethroids has been made on
the basis of the symptoms of toxicity in mammals and insects (Van
den Bercken et al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980;
Glickman & Casida, 1982; Lawrence & Casida, 1982). The same
distinction was found in studies on cockroaches (Gammon et al.,
1981).
Based on the binding assay on the gamma-aminobutyric acid
(GABA) receptor-ionophore complex, synthetic pyrethroids can also
be classified into two types: the alpha-cyano-3-phenoxybenzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982;
Gammon & Casida, 1983; Lawrence & Casida, 1983; Lawrence et al.,
1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give
rise to pronounced repetitive activity in sense organs and in
sensory nerve fibres (Van den Bercken et al., 1973). At room
temperature, this repetitive activity usually consists of trains of
3-10 impulses and occasionally up to 25 impulses. Train duration
is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the
insecticide on neurotransmitter release or on the sensitivity of
the subsynaptic membrane, nor on the muscle fibre membrane.
Presynaptic repetitive firing was also observed in the sympathetic
ganglion treated with these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in sensory
nerve fibres, the pyrethroid-induced repetitive activity increases
dramatically as the temperature is lowered, and a decrease of 5 °C
in temperature may cause a more than 3-fold increase in the number
of repetitive impulses per train. This effect is easily reversed
by raising the temperature. The origin of this "negative
temperature coefficient" is not clear (Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve impulse. The
transitional state of the sodium channel is controlled by 2
separately acting gating mechanisms, referred to as the activation
gate and the inactivation gate. Since pyrethroids only appear to
affect the sodium current during depolarization, the rapid opening
of the activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is open,
the activation gate is restrained in the open position by the
pyrethroid molecule. While all pyrethroids have essentially the
same basic mechanism of action, however, the rate of relaxation
differs substantially for the various pyrethroids (Flannigan &
Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation
of the transient increase in sodium permeability of the nerve
membrane during excitation (Van den Bercken & Vijverberg, 1980).
Evidence so far available indicates that allethrin selectively
slows down the closing of the activation gate of a fraction of the
sodium channels that open during depolarization of the membrane.
The time constant of closing of the activation gate in the
allethrin-affected channels is about 100 milliseconds compared with
less than 100 microseconds in the normal sodium channel, i.e., it
is slowed down by a factor of more than 100. This results in a
marked prolongation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is directly
responsible for the repetitive activity induced by allethrin
(Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive
firing, and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral nervous
system of the frog. DDT also causes pronounced repetitive activity
in sense organs, in sensory nerve fibres, and in motor nerve
terminals, due to a prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation.
Recently, it was demonstrated that allethrin and DDT have
essentially the same effect on sodium channels in frog myelinated
nerve membrane. Both compounds slow down the rate of closing of a
fraction of the sodium channels that open on depolarization of the
membrane (Van den Bercken et al., 1973, 1979; Vijverberg et al.,
1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing after-potentials, and evoke repetitive firing with
minimal effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in
sodium permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl
alcohol (deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral line organ in the form of long-
lasting trains of impulses (Vijverberg et al., 1982a). Such a train
may last for up to 1 min and contains thousands of impulses. The
duration of the trains and the number of impulses per train
increase markedly on lowering the temperature. Cypermethrin does
not cause repetitive activity in myelinated nerve fibres. Instead,
this pyrethroid causes a frequency-dependant depression of the
nervous impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of depolarizing
after-potentials during train stimulation (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in
sodium permeability during excitation, presumably by slowing down
the closing of the activation gate of the sodium channel
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983). The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged sodium
current after cypermethrin is too small to induce repetitive
activity in nerve fibres, but is sufficient to cause the long-
lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a long-
lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
cray-fish, the Type II pyrethroids retain sodium channels in a
modified continuous open state persistently, depolarize the
membrane, and block the action potential without causing repetitive
firing (Lund & Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of
the Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant picrotoxin
(PTX). Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin
to rat brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible
relation between the Type II pyrethroid action and the GABA
receptor complex. The stereospecific correlation between the
toxicity of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand, [35S]- t-
butylbicyclophosphorothionate [35S]-TBPS.
Studies with 37 pyrethroids revealed an absolute correlation,
without any false positive or negative, between mouse intracerebral
toxicity and in vitro inhibition: all toxic cyano compounds
including deltamethrin, 1R, cis-cypermethrin, 1R, trans-cypermethrin,
and [2S,alpha]-fenval-erate were inhibitors, but their non-toxic
stereoisomers were not; non-cyano pyrethroids were much less potent
or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies
of pyrethroids were closely related to their mammalian toxicities.
The most toxic pyrethroids of Type II were the most potent
inhibitors of [3H]-Ro 5-4864 specific binding to rat brain
membranes. The [3H]-dihydropicrotoxin and [35S]-TBPS binding
studies with pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an interaction
with a GABA-regulated chloride ionophore-associated binding site.
Moreover, studies with [3H]-Ro 5-4864 support this hypothesis and,
in addition, indicate that the pyrethroid-binding site may be very
closely related to the convulsant benzodiazepine site of action
(Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin and
[2S, alphaS]-fenvalerate) increased the input resistance of
crayfish claw opener muscle fibres bathed in GABA. In contrast,
two non-insecticidal stereoisomers and Type I pyrethroids
(permethrin, resmethrin, allethrin) were inactive. Therefore,
cyanophenoxybenzyl pyrethroids appear to act on the GABA receptor-
ionophore complex (Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type II pyrethroids
(cypermethrin and deltamethrin) induced trains of repetitive
muscle action potentials without presynaptic repetitive activity.
However, an intermediate group of pyrethroids (1R-permethrin,
cyphenothrin, and fenvalerate) caused both types of effect. Thus,
in muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current. But no
clear distinction was observed between non-cyano and alpha-cyano
pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary
target site of pyrethroid insecticides in the vertebrate nervous
system is the sodium channel in the nerve membrane. Pyrethroids
without an alpha-cyano group (allethrin, d-phenothrin, permethrin,
and cismethrin) cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres,
and, in effect, nerve terminals. On the other hand, the alpha-
cyano pyrethroids cause a long-lasting prolongation of the
transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of
repetitive impulses in sense organs and a frequency-dependent
depression of the nerve impulse in nerve fibres. The difference in
effects between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-cyano
group on the phenoxybenzyl alcohol, indicates that it is this
alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation
and conduction are basically the same throughout the entire
nervous system, pyrethroids may also induce repetitive activity in
various parts of the brain. The difference in symptoms of
poisoning by alpha-cyano pyrethroids, compared with the classical
pyrethroids, is not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive activity
in sense organs and possibly in other parts of the nervous system,
which, in a more advance state of poisoning, may be accompanied by
a frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of
the nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane
is also the most important target site of pyrethroids in the
invertebrate nervous system (Wouters & Van den Bercken, 1978; WHO,
1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be
considered restricted to particular animal species, or to a certain
region of the nervous system. Although it has been established that
sense organs and nerve endings are the most vulnerable to the
action of pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions, and on the
chemical structure and physical characteristics of the pyrethroid
molecule (Vijverberg & Van den Bercken, 1982).
RESUME, EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques, méthodes d'analyse
La cyhalothrine s'obtient en estérifiant l'acide (chloro-2
trifluoro-3,3,3 propényl-1)-3 diméthyl-2,2 cyclopropanecarb-
oxylique, par l'alcool alpha-cyanophénoxy-3 benzylique et elle
consiste en un mélange de quatre stéréoisomères formant deux paires
d'énantiomères. La lambda-cyhalothrine est l'une de ces paires
d'énantiomères et constitue la forme la plus active biologiquement.
La cyhalothrine de qualité technique est un liquide visqueux
d'un jaune brunâtre (point de fusion environ 10 °C) qui contient
plus de 90% de matière active. Elle est constituée de quatre
isomères cis dans la proportion de 1:1:1:1. Elle est insoluble
dans l'eau mais soluble dans divers solvants organiques, en
particulier les hydrocarbures aliphatiques et aromatiques. Elle
est stable à la lumière et à la chaleur et possède une faible
tension de vapeur.
La lambda-cyhalothrine technique est un solide beige (point de
fusion 49,2 °C) contenant plus de 90% de matière active. Le
rapport des énantiomères (Z) (1R, 3R) de l'ester S aux énantiomères
(Z) (1S, 3S) de l'ester R est de 1:1. Peu soluble dans l'eau, ce
produit est soluble dans divers solvants organiques et possède une
faible tension de vapeur. La cyhalothrine et la lambda-cyhalothrine
sont rapidement hydrolysées en milieu alcalin mais ne subissent
aucune hydrolyse en milieu neutre ou acide.
On dispose de méthodes confirmées pour la recherche et le
dosage des résidus de cyhalothrine et de lambda-cyhalothrine ainsi
que pour l'analyse des prélèvements effectués dans l'environnement
(limite inférieure de détection: 0,005 mg/kg).
1.2 Production et usage
La cyhalothrine a été mise au point en 1977. On l'utilise
principalement pour détruire diverses espèces nuisibles en santé
publique et santé publique vétérinaire ainsi qu'en agriculture
contre les ravageurs des fruits à pépins. La lambda-cyhalothrine
est utilisée essentiellement en agriculture pour la protection des
récoltes les plus variées et fait actuellement l'objet d'une mise
au point en vue d'être utilisée en santé publique.
On ne dispose d'aucune donnée sur les quantités produites.
1.3 Exposition humaine
Les résidus dans les produits alimentaires, par suite du
traitement des récoltes par la cyhalothrine et la lambda-
cyhalothrine ainsi que de leur utilisation en médecine vétérinaire
sont faibles, généralement inférieurs à 0,2 mg/kg. On ignore quel
est l'apport total d'origine alimentaire chez l'homme mais on peut
supposer que l'exposition de la population générale par cette voie
ne dépasse pas la DJA (0,02 mg/kg de poids corporel).
1.4 Exposition et destinée dans l'environnement
A la surface du sol et en solution aqueuse à pH 5, la lambda-
cyhalothrine se décompose sous l'effet du rayonnement solaire avec
une demi-vie d'environ 30 jours. Les principaux produits de
décomposition sont l'acide (chloro-2 trifluoro-3,3,3 propényl-1)-3
diméthyl-2,2 cyclopropanecarboxylique, l'amide correspondant et
l'acide phénoxy-3 benzoïque.
Dans le sol, la dégradation s'effectue principalement par
hydroxylation puis rupture de la liaison ester aboutissant à deux
produits principaux dont la dégradation se poursuit jusqu'à
obtention de dioxyde de carbone. La demi-vie initiale varie de 22 à
82 jours.
La cyhalothrine et la lambda-cyhalothrine sont adsorbées sur
les particules du sol et ne se déplacent pas dans l'environnement.
Sur les végétaux, la lambda-cyhalothrine se décompose à vitesse
modérée (demi-vie allant jusqu'à 40 jours) de sorte que les résidus
sont principalement constitués du composé initial. On trouve
également des métabolites en faibles quantités qui résultent d'un
certain nombre de réactions d'hydrolyse et d'oxydation.
On ne dispose d'aucune donnée sur les quantités effectivement
présentes dans l'environnement mais compte tenu du fait que ce
produit est relativement peu utilisé et à doses réduites, ces
quantités sont vraisemblablement faibles.
1.5 Absorption, métabolisme, et excrétion
Des études métaboliques ont été effectuées sur des rats, des
chiens, des vaches et des chèvres. Chez les rats et les chiens, on
a montré que la cyhalothrine administrée par voie orale était bien
absorbée, fortement métabolisée, puis éliminée sous forme de
conjugués polaires dans les urines. Chez le rat, on a constaté que
le taux de cyhalothrine tissulaire diminuait après cessation de
l'exposition. Dans les carcasses, les résidus étaient faibles
(moins de 5% de la dose au bout de sept jours) et consistaient
presque entièrement en cyhalothrine accumulée dans le tissu
adipeux. Les résidus présents dans les tissus adipeux étaient
éliminés avec une demi-vie de 23 jours.
Après administration par voie orale de cyhalothrine à des
vaches en lactation, on a constaté que le produit était rapidement
éliminé et qu'un état d'équilibre s'établissait au bout de trois
jours entre ingestion et élimination. Vingt-sept pour cent de la
dose étaient excrétés dans les urines, 50% dans les matières
fécales et 0,8% dans le lait. Les produits retrouvés dans les
urines étaient entièrement constitués de métabolites résultant du
clivage de l'ester et de leurs conjugués, alors que 60 à 70% des
produits marqués au 14C retrouvés dans les matières fécales
consistaient en cyhalothrine non métabolisée. Les résidus
tissulaires, mesurés 16 heures après l'administration de la
dernière dose, étaient faibles, la concentration la plus élevée
étant enregistrée dans le tissu adipeux. Les résidus marqués au 14C
retrouvés dans le lait et les tissus adipeux étaient presque
entièrement constitués de cyhalothrine non métabolisée, à
l'exclusion de tout autre composé.
Chez toutes les espèces mammaliennes étudiées, on a constaté
que la cyhalothrine subissait une forte métabolisation par clivage
de l'ester en acide cyclopropanecarboxylique et acide phénoxy-3
benzoïque et qu'elle était éliminée sous forme de conjugués.
Chez les poissons, les principaux résidus tissulaires
consistent en cyhalothrine non métabolisée à côté de quantités plus
faibles de produits résultant du clivage de l'ester.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Dans les conditions du laboratoire où la concentration est
constante, la cyhalothrine et la lambda-cyhalothrine se révèlent
très toxiques pour les poissons et les invertébrés aquatiques. Les
valeurs de la CL50 à 96 heures pour les poissons vont de 0,2 à 1,3
µg/litre, la CL50 à 48 heures pour les invertébrés aquatiques se
situant entre 0,008 et 0,04 µg/litre.
En étudiant au laboratoire l'accumulation de la cyhalothrine à
concentration constante, on a observé une absorption rapide par les
poissons (facteur d'accumulation d'environ 1000 à 2000). Toutefois
lorsqu'il y a un sol et des sédiments en suspension, on constate
une forte réduction du facteur de bioaccumulation, qui passe à 19
pour les poissons et à 194 pour les daphnies. Lorsqu'on remet les
poissons et les daphnies dans de l'eau propre, on constate une
diminution rapide des résidus, avec une demi-vie respective de 7 et
1 jours. Il est vraisemblable qu'en utilisation agricole normale,
la cyhalothrine et la lambda-cyhalothrine subsistent à faible
concentration dans l'eau. Etant donné que ces composés sont
rapidement adsorbés et dégradés dans les conditions naturelles, il
ne se pose en pratique aucun problème d'accumulation de résidus ni
de toxicité vis-à-vis des espèces aquatiques.
La cyhalothrine et la lambda-cyhalothrine sont pratiquement
inoffensives pour les oiseaux; la DL50 (dose unique) s'est révélée
supérieure à 3950 mg/kg pour toutes les espèces étudiées et la CL50
alimentaire à cinq jours la plus faible a été de 3948 mg/kg pour
des canards sauvages de huit jours qui recevaient de la lambda-
cyhalothrine dans leur alimentation.
Dans les conditions du laboratoire, la cyhalothrine et la
lambda-cyhalothrine sont toxiques pour les abeilles; la DL50 par
voie orale est de 0,97 µg de lambda-cyhalothrine par abeille.
Toutefois, le risque est probablement moins élevé en pratique étant
donné que les formulations actuellement en usage exercent une
action répulsive qui empêche les abeilles de butiner sur les
récoltes traitées. Lorsque les abeilles se remettent à butiner, on
ne constate pas d'augmentation importante de la mortalité.
1.7 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
La toxicité aiguë par voie orale de la cyhalothrine est modérée
chez les rats et les souris et faible chez les cobayes et les
lapins (les valeurs de la DL50 sont les suivantes: rat, 144-243
mg/kg; souris, 37-62 mg/kg; cobaye, plus de 5000 mg/kg; lapin, plus
de 1000 mg/kg). La toxicité aiguë par voie orale de la lambda-
cyhalothrine est plus forte que celle de la cyhalothrine (les
valeurs de la DL50 sont les suivantes: 56-79 mg/kg pour le rat et
20 mg/kg pour la souris). Par voie dermique, on obtient les
toxicités suivantes exprimées par la DL50: rat, 200-2000 mg/kg
(cyhalothrine), 632-696 mg/kg (lambda-cyhalothrine); lapin, plus
de 2000 mg/kg (cyhalothrine). La cyhalothrine et la lambda-
cyhalothrine sont des pyréthroïdes du type II avec pour signes
cliniques d'intoxication une ataxie, une démarche chancelante et
une hyperexcitabilité.
Chez le lapin, la cyhalothrine est modérément irritante pour
l'oeil et la lambda-cyhalothrine peu irritante; les deux composés
sont légèrement irritants pour la peau. La cyhalothrine n'est pas
irritante pour la peau chez le rat. Toutefois elle provoque une
sensibilisation cutanée modérée chez le cobaye. La lambda-
cyhalothrine n'a pas d'action sensibilisatrice sur la peau.
Lors d'une étude d'alimentation de 90 jours au cours de
laquelle des rats ont reçu de la cyhalothrine à des doses allant
jusqu'à 250 mg/kg de nourriture, on a observé une réduction du gain
de poids chez les mâles à la dose la plus forte. Des effets
marginaux sur la valeur moyenne de l'hématocrite ont été
enregistrés chez certains des groupes traités ainsi que quelques
modifications au niveau du foie que l'on a considérées comme
résultant d'une réaction d'adaptation. Lors d'une étude de 90
jours au cours de laquelle des rats ont reçu dans leur alimentation
de la lambda-cyhalothrine à des doses allant jusqu'à 250 mg/kg de
nourriture, on a observé une réduction du gain de poids chez les
deux sexes à la dose la plus forte. On a observé un certain nombre
d'effets sur le chimisme sanguin ainsi que des effets hépatiques
analogues à ceux qui avaient été observés avec la cyhalothrine. La
dose sans effet observable était de 50 mg/kg de nourriture.
Lors d'une étude de 26 semaines au cours de laquelle de la
cyhalothrine a été administrée par voie orale à des chiens à des
doses quotidiennes allant jusqu'à 10 mg/kg de poids corporel, on a
observé des signes d'intoxication de type pyréthroïde à la dose la
plus forte. La dose sans effet observable se situait à 2,5 mg/kg
de poids corporel. Une étude du même genre a été menée sur d'autres
chiens avec administration pendant 52 semaines de 3,5 mg de lambda-
cyhalothrine par kg de poids corporel et par jour. Des signes
cliniques d'intoxication de type pyréthroïde (signes neurologiques)
ont été observés chez tous les ani-maux à la dose quotidienne de
3,5 mg/kg de poids corporel. La dose sans effet observable se
situait à 0,5 mg/kg de poids corporel par jour.
Lors d'une étude de toxicité cutanée de 21 jours pratiquée sur
des lapins au moyen d'une solution de cyhalothrine dans le
polyéthylène-glycol à des doses quotidiennes allant jusqu'à 1000
mg/kg de poids corporel, on a constaté des signes cliniques
d'intoxication chez certains des animaux à la dose la plus forte.
Dans tous les groupes, y compris les témoins, on a observé une
irritation cutanée légère à sévère.
Deux études toxicologiques de 104 semaines ont été consacrées à
la cyhalothrine, l'une sur des rats et l'autre sur des souris.
L'étude sur le rat n'a révélé aucun effet oncogène à des doses
allant jusqu'à 250 mg/kg de nourriture (dose la plus forte
étudiée). La dose sans effet observable en ce qui concerne la
toxicité générale était de 50 mg/kg de nourriture (1,8 mg/kg de
poids corporel par jour). On a relevé une diminution du gain de
poids corporel chez les deux sexes à la dose de 250 mg/kg de
nourriture. Aucun effet oncogène n'a été observé chez la souris à
des doses allant jusqu'à 500 mg/kg de nourriture (dose la plus
forte étudiée). Les signes cliniques d'une intoxication de type
pyréthroïde ont été observés aux doses de 100 et 500 mg/kg de
nourriture, avec réduction du gain de poids corporel à cette
dernière dose. En ce qui concerne la toxicité générale, la dose
sans effet observable était de 20 mg/kg de nourriture (1,9 mg/kg de
poids corporel par jour). Aucun signe histologique de lésion du
système nerveux n'a été observé dans l'une ou l'autre de ces deux
études.
Lors d'une série d'épreuves in vivo et in vitro visant à
déceler des mutations géniques, des lésions chromosomiques et
autres effets génotoxiques, on n'a observé aucun effet de ce type
imputable à la cyhalothrine ou à la lambda-cyhalothrine.
Administrée par voie orale à des rats et à des lapins au cours de
la période cruciale de l'organogénèse, la cyhalothrine ne s'est
révélée ni embryotoxique ni tératogène à des doses quotidiennes qui
étaient toxiques pour la mère (15 mg/kg pour les rats et 30 mg/kg
pour les lapins, c'est-à-dire les deux doses les plus fortes
étudiées).
Lors d'une étude de reproduction portant sur trois générations,
des rats ont reçu de la cyhalothrine dans leur alimentation à des
doses allant jusqu'à 100 mg/kg de nourriture. A la dose de 100
mg/kg de nourriture on a observé une légère diminution de la taille
des portées et une réduction peu importante du gain de poids. En
ce qui concerne les effets sur la reproduction, la dose sans effet
observable était de 30 mg/kg de nourriture.
1.8 Effets sur l'homme
Aucun cas d'intoxication accidentelle n'a été décrit.
Des sensations subjectives au niveau de la face ont été
ressenties lors de la fabrication, de la formulation, du travail de
laboratoire et de l'utilisation sur le terrain. Cet effet ne dure
en général que quelques heures mais il arrive qu'il se prolonge
jusqu'à 72 heures après l'exposition; l'examen médical n'a pas
révélé d'anomalies neurologiques.
Ces sensations cutanées subjectives au niveau de la face, qui
sont ressenties par les personnes qui manipulent de la cyhalothrine
ou de la lambda-cyhalothrine sont, semble-t-il, provoquées par
l'excitation répétée des terminaisons nerveuses de la peau. On
peut les considérer comme un signal d'alarme qui indique une
surexposition de l'épiderme.
Rien n'indique que la cyhalothrine et la lambda-cyhalothrine
puissent avoir des effets nocifs pour l'homme lorsqu'on les utilise
dans les conditions et aux doses qui sont recommandées
actuellement.
2. Conclusions
a) Population générale: l'exposition de la population générale à la
cyhalothrine et à la lambda-cyhalothrine est vraisemblablement très
faible et ne présente probablement aucun danger lorsque ces
produits sont utilisés conformément aux recommandations.
b) Exposition professionnelle: moyennant de bonnes méthodes de
travail, des mesures d'hygiène et pour peu que l'on respecte les
précautions nécessaires, la cyhalothrine et la lambda-cyhalothrine
ne présentent vraisemblablement aucun danger pour les personnes
exposées de par leur profession.
c) Environnement: il ne semble pas que la cyhalothrine, la lambda-
cyhalothrine ou leurs produits de dégradation puissent atteindre
des concentrations nocives pour l'environnement lorsqu'elles sont
utilisées aux doses recommandées. Dans les conditions du
laboratoire, la cyhalothrine et la lambda-cyhalothrine sont très
toxiques pour les poissons, les arthropodes aquatiques et les
abeilles. Toutefois, en pratique, il ne semble pas qu'il puisse se
produire d'effets nocifs durables, lorsque ces insecticides sont
utilisés conformément aux recommandations.
3. Recommandations
Lorsque ces insecticides sont utilisés conformément aux
recommandations, leurs concentrations dans les denrées alimentaires
doivent être très faibles, toutefois il serait bon de le confirmer
en soumettant la cyhalothrine et la lambda-cyhalothrine à une
surveillance.
La cyhalothrine et la lambda-cyhalothrine sont utilisées
depuis des années et seuls des effets passagers ont été observés
lors d'expositions professionnelles; toute-fois il faut continuer à
surveiller l'exposition humaine.
RESUMEN, EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades físicas y químicas, y métodos
analíticos
La cihalotrina se obtiene esterificando ácido 3-(2-cloro-3,3,3-
trifluoroprop-1-enil)-2,2-dimetilciclopropanocarboxílico con
alcohol alpha-ciano-3-fenoxibencílico y se compone de una mezcla de
cuatro estereoisómeros. La lambda-cihalotrina se compone de un par
enantiomérico de isómeros y es la forma más activa biológicamente.
La cihalotrina de calidad técnica es un líquido viscoso, de
color entre amarillo y marrón (punto de fusión: aproximadamente 10
°C), que contiene más del 90% de material activo. Está compuesta
por cuatro isómeros cis en proporción 1:1:1:1. Aunque es insoluble
en el agua, es soluble en toda una gama de disolventes orgánicos
como los hidrocarburos alifáticos y aromáticos. Es estable respecto
de la luz y la temperatura y tiene una baja tensión de vapor.
La lambda-cihalotrina de calidad técnica es un sólido de color
café con leche (punto de fusión: 49,2 °C) que contiene más del 90%
de material activo. La razón enantiomérica del ( Z), (1R, 3R), S-
éster y el ( Z), (1S, 3S), R-éster es 1:1. Es apenas soluble en el
agua pero es soluble en toda una gama de disolventes orgánicos y
tiene una baja tensión de vapor. Tanto la cihalotrina como la
lambda-cihalotrina se hidrolizan rápidamente en medio alcalino pero
no en medio neutro o ácido.
Existen métodos bien establecidos para analizar la cihalotrina
y la lambda-cihalotrina presentes en residuos y en el medio
ambiente (la concentración detectable mínima es 0,005 mg/kg).
1.2 Producción y utilización
La cihalotrina comenzó a producirse en 1977. Se utiliza sobre
todo para combatir múltiples plagas en salud pública y en
veterinaria, pero también se emplea en agricultura contra las
plagas que atacan a los pomos. La lambda-cihalotrina se usa
principalmente como plaguicida agrícola aplicable a muy diversos
cultivos y se está preparando para emplearla en salud pública.
No se dispone de datos sobre los niveles de producción.
1.3 Exposición humana
Los residuos presentes en los alimentos de resultas de la
aplicación de cihalotrina y lamba-cihalotrina a los cultivos y en
veterinaria son bajos, por lo general inferiores a 0,2 mg/kg. No
se dispone de datos sobre la ingesta total en la dieta humana, pero
se puede suponer que la exposición dietética de la población
general no supera la IDA (0,02 mg/kg de peso corporal).
1.4 Exposición y transformación en el medio ambiente
En la superficie del suelo y en soluciones acuosas con un pH de
5, la lambda-cihalotrina se degrada a la luz del sol con una
semivida de unos 30 días. Los principales productos de esa
degradación son ácido 3-(2-cloro-3,3,3-trifluoroprop-1-enil)-2,2-
dimetilciclopropanocarboxílico, el derivado amídico de la
cihalotrina, y ácido 3-fenoxibenzoico.
La degradación en el suelo se produce primordialmente por
hidroxilación seguida de la ruptura del enlace éster para dar lugar
a dos principales productos de degradación, que siguen degradándose
hasta convertirse en bióxido de carbono. Las semividas iniciales
oscilan entre 22 y 82 días.
La cihalotrina y la lambda-cihalotrina se adsorben a las
partículas del suelo y no son móviles en el medio ambiente.
En las plantas, la lambda-cihalotrina se degrada a un ritmo
moderado (semivida de hasta 40 días), por lo que el principal
elemento constituyente de los residuos que en ellas se encuentran
es por lo común el compuesto inicial. Se hallan también niveles más
bajos de metabolitos, resultantes de diversas reacciones
hidrolíticas y oxidantes.
No se dispone de datos sobre los niveles efectivos en el medio
ambiente, pero dadas la escasa utilización habitual en la
actualidad y las reducidas tasas de aplicación, se supone que son
bajos.
1.5 Incorporación, metabolismo y excreción
Se han realizado estudios metabólicos en la rata, el perro, la
vaca y la cabra. En la rata y el perro se ha demostrado que la
cihalotrina administrada por vía oral se absorbe y se metaboliza
bien y se elimina en la orina, en forma de conjugados polares. En
los tejidos de las ratas, disminuyó la concentración de cihalotrina
al interrumpirse la exposición al compuesto. En los cadáveres de
las ratas se encontraron niveles reducidos de residuos (< 5% de la
dosis a los siete días), que se debían casi totalmente a la
cihalotrina contenida en las grasas. Los restos presentes en las
grasas se eliminaron con una semivida de 23 días.
La cihalotrina administrada por vía oral a vacas lactantes se
eliminó rápidamente, alcanzándose un equilibrio entre la ingestión
y la eliminación a los tres días. De la dosis total, el 27% se
excretó en la orina, el 50% en las heces, y el 0,8% en la leche.
El material presente en la orina estaba compuesto exclusivamente
por metabolitos de la disociación de los ésteres y por sus
conjugados, mientras que del 60% al 70% del material fecal marcado
con [14C] se identificó como cihalotrina no alterada. Dieciséis
horas después de la última dosis, los residuos tisulares eran
bajos, hallándose las concentraciones más elevadas en las grasas.
Los residuos marcados con [14C] presentes en la leche y los tejidos
adiposos constaban casi enteramente de cihalotrina no alterada, y
no se detectó ningún otro componente.
En todas las especies de mamíferos investigadas, se ha
descubierto que la cihalotrina se metaboliza ampliamente de
resultas de la ruptura del enlace éster para dar ácido
ciclopropanocarboxílico y ácido 3-fenoxibenzoico, y se elimina en
forma de conjugados.
En los peces, el principal residuo presente en los tejidos es
cihalotrina no alterada y hay niveles más bajos de los productos de
la ruptura del éster.
1.6 Efectos en los organismos del medio ambiente
En condiciones de laboratorio con concentraciones tóxicas
constantes, la cihalotrina y la lambda-cihalotrina son muy tóxicas
para los peces y los invertebrados acuáticos. El valor de las CL50
a las 96 horas para los peces oscila entre 0,2 y 1,3 µg/litro,
mientras que en el caso de los invertebrados acuáticos los valores
de la CL50 a las 48 horas van de 0,008 a 0,4 µg/litro.
Los estudios sobre la acumulación realizados en condiciones de
laboratorio con concentraciones constantes indican que, en los
peces, tiene lugar una rápida incorporación (factor de acumulación
aproximado de 1000 a 2000). No obstante, en presencia de suelo y
sedimentos en suspensión, los factores de bioacumulación quedan muy
reducidos (a 19 en el caso de los peces y a 194 en el caso de los
dáfnidos). Cuando se colocó en agua limpia a los peces y dáfnidos
expuestos, los residuos disminuyeron rápidamente, con semividas de
siete días y un día, respectivamente. Las concentraciones de
cihalotrina y lambda-cihalotrina que pueden hallarse en el agua de
resultas de su aplicación normal con fines agrícolas serán bajas.
Como el compuesto se adsorbe y se degrada rápidamente en
condiciones naturales, la acumulación de residuos o la toxicidad de
la cihalotrina y la lambda-cihalotrina para las especies acuáticas
no plantearán problemas prácticos.
La cihalotrina y la lambda-cihalotrina son prácticamente
inocuas para las aves; en todas las especies sometidas a prueba la
DL50 con una dosis única fue superior a 3950 mg/kg y la CL50
dietética más baja en cinco días fue de 3948 mg/kg (lambda-
cihalotrina administrada en el alimento a patos silvestres de ocho
días de edad).
En condiciones de laboratorio, la cihalotrina y la lambda-
cihalotrina son tóxicas para la abeja; la DL50 de la lambda-
cihalotrina por vía oral es de 0,97 µg/abeja. No obstante, en la
práctica, el riesgo es menor puesto que las formas actualmente
utilizadas tienen un efecto repelente que hace que las abejas
suspendan las actividades de búsqueda en los cultivos tratados.
Cuando éstas vuelven a emprenderse, no hay un aumento significativo
de la mortalidad de las abejas.
1.7 Efectos en animales de experimentación y en sistemas de
prueba in vitro
La toxicidad aguda de la cihalotrina administrada por vía oral
es moderada para las ratas y los ratones y baja para las cobayas y
los conejos (los valores de la DL50 son los siguientes: rata: 144-
243 mg/kg; ratón: 37-62 mg/kg; cobaya: > 5000 mg/kg; conejo: >
1000 mg/kg). La toxicidad aguda de la lambda-cihalotrina por vía
oral es mayor que la de la cihalotrina (los valores de la DL50 son:
56-79 mg/kg para la rata y 20 mg/kg para el ratón). La toxicidad
por vía cutánea (DL50) alcanza los siguientes valores: rata: 200-
2000 mg/kg (cihalotrina), 632-696 mg/kg (lambda-cihalotrina);
conejo: > 2000 mg/kg (cihalotrina). La cihalotrina y la lambda-
cihalotrina son piretroides de tipo II; entre los signos clínicos
figuran la ataxia, el andar vacilante y la hiperexcitabilidad.
En el conejo, la cihalotrina es un irritante ocular moderado y
la lambda-cihalotrina un irritante ocular leve; ambos irritan
levemente la piel. La cihalotrina no irrita la piel de la rata.
Sin embargo, sensibiliza moderadamente la piel de la cobaya. La
lambda-cihalotrina no sensibiliza la piel.
En un estudio de alimentación de 90 días en el que se
administró cihalotrina a ratas en dosis de hasta 250 mg/kg de
alimento, se observó una disminución del aumento del peso corporal
en los machos con la dosis de 250 mg. En algunos de los grupos
tratados, se señalaron efectos marginales en los volúmenes medios
de eritrocitos, así como ciertas alteraciones hepáticas, que se
consideraron una respuesta adaptativa. En otro estudio de
alimentación de 90 días, en el que se administró lambda-cihalotrina
a ratas en dosis de hasta 250 mg/kg de alimento, se observó en
ambos sexos una reducción del aumento del peso corporal con la
dosis de 250 mg. Se señalaron también algunos efectos en la
química clínica, así como efectos hepáticos análogos a los
observados con la cihalotrina. El nivel sin efecto observado fue
de 50 mg/kg de alimento.
En un estudio de 26 semanas en el que se administraron a perros
por vía oral dosis diarias de cihalotrina de hasta 10 mg/kg de peso
corporal, se observaron signos de toxicidad por piretroides con la
dosis de 10 mg. El nivel sin efecto observado fue de 2,5 mg/kg de
peso corporal al día. Se realizó otro estudio similar en el que se
administraron a perros, durante 52 semanas, dosis diarias de
lambda-cihalotrina de hasta 3,5 mg/kg de peso corporal. En todos
los animales que recibieron la dosis de 3,5 mg se observaron signos
clínicos de toxicidad por piretroides (signos neurológicos). El
nivel sin efecto observado fue de 0,5 mg/kg de peso corporal al
día.
En un estudio cutáneo de 21 días con conejos a los que se
aplicó cihalotrina en polietilénglicol en dosis diarias de hasta
1000 mg/kg, se observaron en algunos animales signos clínicos de
toxicidad con la dosis más alta. En todos los grupos, incluidos
los testigos, se señaló irritación de la piel de leve a grave.
Se realizaron dos estudios de alimentación de 104 semanas con
cihalotrina, uno con ratas y otro con ratones. En el estudio con
ratas, no se observaron efectos oncogénicos con dosis de hasta 250
mg/kg de alimento (la dosis más alta ensayada). En cuanto a la
toxicidad sistémica, el nivel sin efecto observado fue de 50 mg/kg
de alimento (1,8 mg/kg de peso corporal al día). Con dosis de 250
mg se señaló en ambos sexos una disminución del aumento del peso
corporal. En el estudio con ratones, no se observaron efectos
oncogénicos con dosis de hasta 500 mg/kg de alimento (la dosis más
alta ensayada). Con dosis de 100 y 500 mg/kg de alimento se
apreciaron signos clínicos de toxicidad por piretroides y, con
dosis de 500 mg, una disminución del aumento del peso corporal. En
cuanto a la toxicidad sistémica, el nivel sin efecto observado fue
de 20 mg/kg de alimento (1,9 mg/kg de peso corporal al día). En
ninguno de los dos estudios se obtuvieron datos histológicos que
indicaran la presencia de lesiones del sistema nervioso.
En una serie de pruebas in vivo e in vitro destinadas a
detectar mutaciones génicas, lesiones cromosómicas y otros efectos
genotóxicos, se obtuvieron resultados negativos con la cihalotrina
y la lambda-cihalotrina. La cihalotrina administrada por vía oral
a ratas y conejos durante el periodo de organogénesis principal no
resultó embriotóxica ni teratogénica en dosis que provocaron
reacciones tóxicas en las madres (15 mg/kg diarios para las ratas y
30 mg/kg diarios para los conejos, en ambos casos los niveles más
altos ensayados).
Se realizó un estudio sobre la reproducción en tres
generaciones de ratas a las que se administró cihalotrina en dosis
de hasta 100 mg/kg de alimento. Con la dosis de 100 mg se
observaron pequeñas reducciones del tamaño de las camadas y del
aumento de peso. En los aspectos relacionados con la reproducción,
el nivel sin efecto observado fue de 30 mg/kg de alimento.
1.8 Efectos en el ser humano
No se ha descrito ningún caso de intoxicación accidental.
En las actividades de fabricación, formulación y laboratorio y
durante el uso sobre el terreno, se han notificado sensaciones
faciales subjetivas. Este efecto sólo suele durar unas horas, pero
ocasionalmente persiste hasta 72 horas después de la exposición; el
examen médico no ha revelado ninguna anomalía neurológica.
Se cree que las sensaciones cutáneas faciales subjetivas que
pueden experimentar las personas que manipulan cihalotrina y
lambda-cihalotrina se deben a la repetida estimulación de las
terminaciones nerviosas de la piel. Pueden considerarse una señal
de alerta que indica que la piel ha sufrido una exposición
excesiva.
No hay ninguna indicación de que la cihalotrina y la lambda-
cihalotrina, utilizadas en las condiciones y con arreglo a las
tasas de aplicación actualmente recomendadas, tengan efectos
adversos en los seres humanos.
2. Conclusiones
(a) Población general: Se cree que la exposición de la población
general a la cihalotrina y la lambda-cihalotrina es muy baja y que
no es probable que represente un riesgo en las condiciones de uso
recomendadas.
(b) Exposición ocupacional: Con prácticas de trabajo, medidas de
higiene y precauciones de seguridad adecuadas, es poco probable que
la cihalotrina y la lambda-cihalotrina representen un riesgo para
las personas ocupacionalmente expuestas.
(c) Medio ambiente: No es probable que la cihalotrina y la lambda-
cihalotrina o los productos de su degradación alcancen niveles
ambientales que puedan producir efectos adversos, si se respetan
las tasas de aplicación recomendadas. En condiciones de
laboratorio, la cihalotrina y la lambda-cihalotrina son muy tóxicas
para los peces, los artrópodos acuáticos y las abejas. No
obstante, en la práctica, no es probable que se produzcan efectos
adversos duraderos en las condiciones de uso recomendadas.
3. Recomendaciones
Aunque se cree que los niveles dietéticos según el uso
recomendado son muy bajos, debe considerarse la posibilidad de
confirmar este extremo incluyendo la cihalotrina y la lambda-
cihalotrina en estudios de vigilancia.
Pese a que ambas sustancias se llevan utilizando varios años y
a que los efectos observados de la exposición ocupacional sólo han
sido transitorios, debe continuar la observación de la exposición
humana.
5.2.1. Rat
Identification of the metabolites produced in the rat studies
of Harrison (described in section 5.1.1) revealed that, following
oral administration, unabsorbed cyhalothrin was eliminated
unchanged via the faeces. The absorbed material was rapidly and
extensively metabolized and no unchanged cyhalothrin was present in
urine or bile. The main route of metabolism was, as anticipated,
via hydrolysis of the ester linkage (Fig. 4). The cyclopropane-
carboxylic acid moiety was subsequently excreted via the urine as
the glucuronide conjugate. This material accounted for about 50%
of the radioactivity in urine following dosing with 14C-
cyclopropyl-labelled cyhalothrin. The 3-phenoxybenzyl moiety was
further metabolized by loss of the nitrile group, oxidation of the
aldehyde formed to a carboxylic acid, aromatic hydroxylation at the
4' position, and formation of the 4- O-sulfate conjugate of 3-(4-
hydroxyphenoxy)benzoic acid. This conjugate accounted for
approximately 75% of the urinary radioactivity following dosing
with 14C-benzyl-labelled cyhalothrin. No metabolite containing the
ester function was detected (Harrison, 1983).
5.2.2. Dog
The main route of metabolism after oral administration is, as
in the rat, via cleavage of the ester bond. After intravenous
administration (0.1 mg/kg body weight), the patterns of metabolites
in urine were very similar to those seen in the oral studies. Very
little unchanged compound was present in the faeces or urine. The
phenoxy-benzyl moiety was further metabolized as in the rat; the
main metabolites were N-(3-phenoxybenzyl) glycine, 3-(4-
hydroxyphenoxy)benzoic acid and its sulfate conjugate, 3-
phenoxybenzoyl glucuronide, and a little free 3-phenoxybenzoic
acid. Other conjugated metabolites were also present. The
cyclopropane acid moiety was extensively metabolized to produce 11
metabolites. These included the cyclopropane acid glucuronide and
other conjugated metabolites. Thus, the metabolism of cyhalothrin
is dominated by cleavage of the ester bond (Fig. 4). Subsequent
metabolism of the products is similar both to that of other
pyrethroids and to the fate of cyhalothrin in other species
(Harrison, 1984c).
5.2.3. Cow
In common with other structurally related pyrethroids, the main
routes of metabolism of cyhalothrin in the cow have been found to
be similar to those observed in rats and dogs, i.e cleavage of the
ester bond with subsequent excretion of the cyclopropyl carboxylic
moiety, either free, hydroxylated, or as a glucuronide conjugate.
The phenoxybenzyl moiety was further metabolized by loss of the
nitrile group and excreted as free 3-phenoxybenzoic acid and its
amino acid conjugates, or after aromatic hydroxylation probably at
the 4'position. Cyhalothrin itself gives rise to residues in fats;
this is consistent with the lipophilic properties of cyhalothrin
compared to those of its more polar metabolites (Harrison, 1984d).
5.2.4. Goat
In a study by Leahey et al. (1985), a goat was dosed orally for
seven days with 14C-cyclopropyl-labelled lambda-cyhalothrin at a
rate equivalent to approximately 11 mg/kg diet. During dosing, the
maximum residue level in the milk was 0.27 mg cyhalothrin
equivalents/kg (mean value during days 3-7: 0.21 mg/kg), virtually
all of which was characterized as lambda-cyhalothrin. When the goat
was slaughtered 16 h after receiving the final dose, residues in
the tissues, expressed in cyhalothrin equivalents, were: meat,
0.024-0.028 mg/kg; fat, 0.13-0.44 mg/kg; liver, 0.34-0.35 mg/kg;
kidney, 0.20 mg/kg. The residues in meat and fat were due mainly
to lambda-cyhalothrin. However, in the liver and kidney, intact
pyrethroid accounted for only a small part of the residue. (1 RS)-
cis-(Z-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclo-
propanecarboxylic acid and 3-(2-chloro-3,3,3-trifluoro-prop-1-
enyl)-2-hydroxymethyl-2-methylcyclopropanecarboxylic acid were the
major components of the residue identified in liver and kidney.
5.2.5. Fish
In an accumulation study by Leahey & Parker (1985), carp were
maintained in a flow-through water system con-taining 14C-
cyclopropyl-labelled cyhalothrin (at a level of 20 ng/g) for 28
days. Results showed that radioactive residues in muscle, head,
and viscera were 0.035, 0.050, and 0.115 mg/kg, respectively. The
major part of the 14C-residue (50-65%) was characterized as
cyhalothrin, a further 10-19% consisting of the compound 1a (Fig. 2).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic organisms
6.1.1. Microorganisms
It has been shown that lambda-cyhalothrin, at a concentration
of 1.0 mg/litre, does not affect the growth of the single-celled
green alga Selenastrum capricornutum over a period of 96 h
(Thompson & Williams, 1985).
6.1.2. Invertebrates
6.1.2.1 Acute toxicity
Aquatic invertebrates show a wide range of susceptibility to
cyhalothrin and lambda-cyhalothrin. The data are summarized in
Table 5.
Table 5. Acute toxicity of cyhalothrin and lambda-cyhalothrin for aquatic invertebrates
-----------------------------------------------------------------------------------------
Species Stage Temper- Test substance Test 48-h Reference
ature system LC50
(°C) (ng/
litre)
-----------------------------------------------------------------------------------------
Freshwater
Water flea < 24 h 20 cyhalothrin static 160 Yamauchi et
(Daphnia pulex) 5% WP al. (1984d)
Water flea 12 h 20 technical static 380 Williams &
(Daphnia magna) (± 12 h) cyhalothrin Thompson
(1981)
Water flea < 24 h 20 technical lambda- static 360 Farrelly et
(Daphnia magna) cyhalothrin al. (1984)
< 24 h 20 lambda-cyhalothrin static 90a Farrelly et
5% EC al. (1985)
< 24 h 20 lambda-cyhalothrin static 90a Farrelly et
13% EC al. (1985)
Freshwater shrimp 5 mm 15 14C-lambda- flow- 8 Hamer et al.
(Gammarus pulex) cyhalothrin through (1985a)
Marine
Mysid shrimp < 48 h 25 14C-lambda- flow- 7.5 Thompson
(Mysidopsis bahia) cyhalothrin through (1985)
-----------------------------------------------------------------------------------------
a Concentration of active ingredient.
6.1.2.2 Long-term toxicity
The effects of lambda-cyhalothrin on the survival, growth, and
reproduction of Daphnia magna were investigated for a period of 21
days in a static water test with daily renewal of test solutions.
The nominal concentrations tested were 0, 2.5, 5.0, 10.0, 20.0,
and 40.0 ng/litre. Lambda-cyhalothrin affected all three parameters
at a nominal concentration of 40 ng/litre, but no effects were
noted at 2.5 ng/litre. Chemical analysis suggested that the
daphnids were exposed to about 60% of the nominal concentration.
These results show that the life-cycle no-observed-effect level of
lambda-cyhalothrin for daphnids is of the order of 2.5 ng/litre
(Hamer et al., 1985b).
6.1.3. Fish
6.1.3.1 Acute toxicity
Cyhalothrin and lambda-cyhalothrin are very toxic to fish in
clean water under laboratory conditions. The available data,
summarized in Table 6, demonstrate a similar high acute toxicity
for both cold and warm water species of fish.
6.1.3.2 Long-term toxicity
Sheepshead minnow Cyprinodon variegatus embryos and larvae were
continuously exposed (through 28 days post hatch) to mean measured
lambda-cyhalothrin concentrations of 0.04, 0.07, 0.14, 0.25, and
0.38 µg/litre, in a flow-through system. The test was performed in
duplicate. Assessments were made of percentage hatch and survival
of embryos and of total length and weight of the larvae at the
completion of the study. Hatchability was not affected (P < 0.05)
at any concentration in the carrier dimethylformamide or dilution
water controls, percentage hatch ranging from 81.3% to 100%.
Larval survival was not significantly affected. A significant
effect (P < 0.05) was found on the weight of the larvae at the
highest concen-tration tested, but not at any other concentration.
On the basis of these data the NOEL was 0.25 µg/litre and the
lowest-observed-effect level (LOEL) was 0.38 µg/litre (Hill et al.,
1985).
6.1.4. Model ecosystem
Cyhalothrin is readily adsorbed onto soil and suspended
particles, which in consequence significantly reduces its toxicity
to aquatic organisms (Yamauchi et al., 1984c). Daphnia pulex and
Cyprinus carpio were used in four different systems to test this
experimentally (Table 7). The median lethal concentrations (LC50)
were calculated, immobilization being used as the end-point for
Daphnia pulex.
Table 6. Acute toxicity of cyhalothrin and lambda-cyhalothrin to fish
---------------------------------------------------------------------------------------------------
Species Weight Test substance and vehicle Temper- 96-h Reference
(g) ature LC50
(°C) (µg/
litre)
---------------------------------------------------------------------------------------------------
Rainbow trout 0.32-1.37 technical cyhalothrin 12 0.54 Hill (1981a)
(Salmo gairdneri) dispersed via acetone
0.30-1.48 technical lambda-cyhalothrin 12 0.24 Hill (1984a)
dispersed via acetone
0.99-2.32 lambda-cyhalothrin 2.5% 16 0.39 Hill (1985a)
EC dispersed in water
1.28-4.78 lambda-cyhalothrin 13% EC 12 0.44 Hill (1985b)
dispersed in water
0.87-4.09 lambda-cyhalothrin 5% EC 16 0.93 Hill (1985c)
dispersed in water
Carp 5.2 technical cyhalothrin 23-35 1.34a Takeda Chemical
(Cyprinus carpio) dispersed via Co. Ltd.
dimethylformamide (1979)
5.4 cyhalothrin 5% WP 25 1.1 Yamauchi et al.
(1984b)
1.48-5.8 lambda-cyhalothrin 2.5% 22 0.54 Hill (1985d)
EC dispersed in water
3.92-7.28 lambda-cyhalothrin 5% EC 22 0.50 Hill (1985e)
dispersed in water
Bluegill sunfish 0.23-0.84 technical cyhalothrin 22 0.46 Reynolds (1984)
(Lepomis macrochirus) dispersed via acetone
0.7-2.6 technical lambda- 22 0.21 Hill (1984b)
cyhalothrin dispersed
via acetone
0.47-2.06 lambda-cyhalothrin 13% 22 0.28 Hill (1985f)
EC dispersed in water
Sheephead minnow 0.32-0.91 technical lambda- 22 0.81 Hill (1985g)
(Cyprinodon variegatus) cyhalothrin dispersed
via acetone
---------------------------------------------------------------------------------------------------
a 72-h LC50.
Table 7. Effect of soil on the toxicity (72-h LC50 in µg/
litre) of cyhalothrin to Daphnia pulex and Cyprinus carpioa
--------------------------------------------------------------
Conditions Daphnia pulex Cyprinus carpio
--------------------------------------------------------------
Application to water surface 0.4 9
(without soil)
Application to water surface 1.0 32
(soil undisturbed)
Application to water surface 16 57
(soil suspended)
Application to soil 70 642
(soil undisturbed)
--------------------------------------------------------------
a From: Yamauchi et al (1984c).
6.2. Terrestrial organisms
6.2.1. Birds
6.2.1.1 Acute toxicity
The toxicity of single oral doses of cyhalothrin and lambda-
cyhalothrin for birds is summarized in Table 8.
The 5-day dietary LC50 for cyhalothrin and lambda-cyhalothrin
has been measured in Anas platyrhynchos and Colinus virginianus
(Table 9).
Table 8. Oral toxicity of cyhalothrin and lambda-cyhalothrin for birds
---------------------------------------------------------------------------------------
Species Age Test substance Observation LD50 (mg/kg References
and vehicle period body weight)
---------------------------------------------------------------------------------------
Domestic hen adult cyhalothrin in 14 days > 10 000 Roberts
(Gallus domesticus) corn oil et al.
(1982)
Mallard duck adult cyhalothrin in 14 days > 5000 Roberts &
(Anas platyrhynchos) corn oil Fairley
(1981)
Mallard duck adult lambda-cyhalothrin 14 days > 3950 Roberts &
(Anas platyrhynchos) in corn oil Fairley
(1984)
---------------------------------------------------------------------------------------
Table 9. Dietary LC50 of cyhalothrin and lambda-cyhalothrin for birds
---------------------------------------------------------------------------------------
Species Age Test substance Observation LC50 Reference
period (post (mg/kg
treatment) diet)
---------------------------------------------------------------------------------------
Mallard duck 10 days cyhalothrin 3 days 14 000 Roberts et
(Anas platyrhynchos) al. (1981a)
Mallard duck 8 days lambda-cyhalothrin 4 days 3948 Roberts et
(Anas platyrhynchos) al. (1985a)
Bobwhite quail 10 days cyhalothrin 3 days > 7530 Roberts et
(Colinus virginianus) al. (1981b)
Bobwhite quail 11 days lambda-cyhalothrin 3 days > 5300 Roberts et
(Colinus virginianus) al. (1985b)
---------------------------------------------------------------------------------------
6.2.2. Honey-bees
Cyhalothrin and lambda-cyhalothrin have been shown to be toxic
to honey-bees (Apis mellifera) in laboratory tests (Table 10).
Table 10. Toxicity of cyhalothrin and lambda-cyhalothrin for
honey-bees (expressed as 24-h LD50 in µg ai per bee)
----------------------------------------------------------------------
Formulation Topical Oral Reference
application administration
----------------------------------------------------------------------
Technical cyhalothrin 0.027 - Smart &
Stevenson
(1982)
Technical lambda-cyhalothrin 0.051 0.97 Gough et
al. (1984)
Lambda-cyhalothrin EC (5%) 0.095 0.57 Gough et
al. (1984)
----------------------------------------------------------------------
In common with other pyrethroids, the high laboratory toxicity
of lambda-cyhalothrin is not translated into a significant field
hazard to bees. In two trials on flowering rape, lambda-
cyhalothrin (JF 9509) EC was applied, at midday, by helicopter at a
concentration of 10 g ai/ha to fields where hives of honey-bees
were located. A toxic standard and untreated control were used for
comparison. Bees were actively foraging during spraying, and the
hives were oversprayed. Mortality, foraging activity, activity at
the hive, and brood development were monitored before and after
treatment, and pollen, honey, and wax were analyzed for residues.
Apart from a suppression of foraging lasting up to 1.5 h, the
lambda-cyhalothrin formulation had no effect on the bees, whereas
the toxic standard killed large numbers. Only low levels of
residues were detected (pollen, 0.44 µg/g; honey, 0.01 µg/g; wax,
0.01 µg/g). It was concluded that, at 10 g ai/ha, lambda-
cyhalothrin formulation (JF 9509) EC is non-hazardous to honey-bees
on flowering rape (Gough et al., 1985).
6.2.3. Earthworms
Three annual applications of lambda-cyhalothrin at rates of up
to 250 g ai/ha to field plots had no adverse effect on populations
of individual species of earthworms or total earthworm numbers or
weight (Coulson et al., 1986).
6.2.4. Higher plants
No phytotoxic effects have been reported.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposures
7.1.1. Oral
The acute oral toxicity of cyhalothrin and lambda-cyhalothrin
in corn oil has been determined for several species (Table 11). The
toxicity of cyhalothrin is moderate (LD50 values: rat, 144-243
mg/kg; mouse, 37-62 mg/kg), whereas that of lambda-cyhalothrin is
higher (LD50 values: rat, 56-79 mg/kg; mouse, 20 mg/kg). Signs of
intoxication are characteristic of type II pyrethroid toxicity and
included piloerection, subdued behaviour, ataxia, unsteady gait,
salivation, incontinence, scouring, and chromodacryorrhoea.
Table 11. Acute oral toxicity of technical cyhalothrin and
lambda-cyhalothrin in corn oil
------------------------------------------------------------------
Species Test substance LD50 (mg/kg Reference
body weight)
------------------------------------------------------------------
Rat cyhalothrin 243 (male) Nixon &
144 (female) Jackson (1981a)
Rat lambda-cyhalothrin 79 (male) Southwood (1985)
56 (female)
Mouse cyhalothrin 36.7 (male) Nixon &
62.3 (female) Jackson (1981a)
Mouse lambda-cyhalothrin 19.9 (male) Southwood (1984)
19.9 (female)
Guinea-pig cyhalothrin > 5000 (male) Nixon &
Jackson (1981a)
Rabbit cyhalothrin > 1000 (female) Nixon &
Jackson (1981a)
------------------------------------------------------------------
7.1.2. Percutaneous
The percutaneous toxicity of cyhalothrin and lambda-cyhalothrin
is summarized in Table 12. Signs of intoxication were similar to
those seen after oral ingestion, and included incontinence,
scouring, dehydration, subdued behaviour, curvature of the spine,
unsteady gait, nervous appearance, piloerection, and increased
vocalization when handled.
Table 12. Percutaneous toxicity of technical cyhalothrin and
lambda-cyhalothrin in polyethylene glycol paste
-----------------------------------------------------------------
Species Test substance LD50 (mg/kg Reference
body weight)
-----------------------------------------------------------------
Rat cyhalothrin 1000-2000 (male) Nixon &
200-2000 (female) Jackson (1981a)
Rat lambda-cyhalothrin 632 (male) Barber (1985)
696 (female)
Rabbit cyhalothrin > 2000 (male) Nixon &
> 2000 (female) Jackson (1981a)
-----------------------------------------------------------------
7.1.3. Intraperitoneal
The intraperitoneal LD50 of cyhalothrin to rats is in the range
of 250 to 750 mg/kg. Responses of 0% or 100% mortality were
observed at all but one dose, and so no precise value for the LD50
could be given. The highest dose with no mortality was 250 mg/kg
and the lowest dose with 100% mortality was 750 mg/kg (Nixon &
Jackson, 1981a).
7.2. Irritation and sensitization
7.2.1. Irritation
Undiluted technical cyhalothrin is a mild irritant to occluded
rabbit skin (both intact and abraded) but is non-irritant to
occluded rat skin (intact) (Jackson & Nixon, 1981). Technical
cyhalothrin is a moderate irritant to the rabbit eye without
irrigation and is a mild irritant when irrigated for one minute, 20
to 30 seconds after instillation of the material (Jackson, 1981).
Technical lambda-cyhalothrin is non-irritant to occluded
rabbit skin without abrasion (Pritchard, 1985a). It is a mild
irritant to the rabbit eye (Pritchard, 1985b).
7.2.2. Sensitization
A skin sensitization test with cyhalothrin on guinea-pigs,
using the procedure of Buehler, indicated that cyhalothrin has
skin-sensitizing potential (Nixon & Jackson, 1981b). In guinea-
pigs that had been previously induced with undiluted cyhalothrin
technical material, using the Magnusson and Kligman maximization
test, a moderate sensitization response was elicited. When
lambda-cyhalothrin was tested for skin sensitization on guinea-
pigs, using the maximization procedure of Magnusson and Kligmann,
it was shown to have no sensitization potential (Pritchard, 1984).
7.3. Short-term exposures
7.3.1. Oral
7.3.1.1 Rat
When groups of male and female rats were fed diets containing
cyhalothrin at levels up to 750 mg/kg for 28 days, symptoms of
toxicity shown by animals receiving doses of 250 mg/kg diet or more
included high stepping gait, ataxia, and hypersensitivity to
external stimuli. These effects were dose related and some
mortality occurred at the highest dose level. The clinical signs
were not accompanied by histopathological changes in the nervous
system. Thymic atrophy, adrenal enlargement with vacuolization,
and incomplete spermatogenesis occurred with the highest dose. An
adaptive change was present in the liver as judged by an increase
in liver weight, proliferation of smooth endoplasmic reticulum,
and an increase in the activity of the xenobiotic-metabolizing
enzyme, aminopyrine- N-demethylase (APDM). These effects occurred at
doses of 100 mg/kg or more, and there was some evidence for this
type of change at 20 mg/kg (Tinson et al., 1984).
When groups of 20 male and 20 female Wistar rats were fed
cyhalothrin (89% pure) at dietary concentrations of 0, 10, 50, and
250 mg/kg for 90 days, only male rats fed 250 mg/kg showed
significantly reduced body weight gain. No abnormal clinical signs
were seen. Haemoglobin and haematocrit values were not affected by
the treatment, but there were marginal effects on mean erythrocyte
volume in some treated groups. Male rats fed 50 or 250 mg/kg
showed decreased plasma triglyceride levels and a dose-related
increase in hepatic APDM activity. The latter effect was also seen
in females fed 250 mg/kg. Small increases in urinary glucose
excretion occurred in males fed 50 or 250 mg/kg after 13 weeks. No
treatment-related effects on organ weights and no significant
histopathological changes were reported (Lindsay et al., 1981).
In studies by Lindsay et al. (1982), two groups of 32 male rats
were fed either a control diet or a diet containing 250 mg
cyhalothrin/kg for 28 days. After this period eight rats per group
were killed and examined. The remaining rats were fed the control
diet for periods of 7, 14, or 28 days after cessation of treatment,
and a further eight rats per group were killed and examined at each
of these time intervals. A decrease in body weight gain was seen
in the treated rats, which, although not statistically
significant, was still much reduced at the end of the 28-day
recovery period. Proliferation of the hepatic smooth endoplasmic
reticulum and elevated hepatic APDM activity were also seen in the
treated animals. These effects had reversed 7 days after the
cessation of treatment with cyhalothrin and were considered to be
physiological adaptive changes rather than a toxicological effect.
When groups of 20 male and 20 female Alderley Park rats
received diets containing 0, 10, 50, or 250 mg lambda-
cyhalothrin/kg for 90 days, decreased body weight gain, accompanied
by a reduction in food consumption, was seen in both male and
female rats receiving the highest dose. No abnormal clinical signs
were seen; in particular, there was no evidence of neurological
effects. There were no treatment-related effects on haematological
parameters but reductions in the activities of plasma alanine
trans-aminase (males only) and alkaline phosphatase (females only
at 13 weeks) were apparent in animals fed the highest dose. Plasma
triglycerides were also reduced in males at this feeding level.
Relative liver weights were increased in both sexes at 250 mg/kg
and in males at 50 mg/kg, accompanied by increased activity of
hepatic APDM. No other changes in organ weight or histopathology
were attributed to treatment with lambda-cyhalothrin. These two
effects, particularly in view of the findings with cyhalothrin,
were considered to be adaptive in nature and the toxicological NOEL
was established at 50 mg/kg (equivalent to 2.5 mg/kg body weight
per day (Hart et al., 1985).
7.3.1.2 Dog
In studies by Chesterman et al. (1980), groups of one male and
one female dog (Alderley Park beagles) received 0, 2.5, or 10 mg
cyhalothrin/kg body weight orally in corn oil by gelatine capsule
daily for four weeks. A further group initially received 30 mg/kg
per day, but due to severe clinical signs after 10 days dosing,
which were typical of pyrethroid toxicity (muscular trembling,
unsteadiness, vomiting, and body weight loss), the animals were
rested and then received 20 mg/kg per day for four weeks. Similar
clinical signs were seen, with the exception of body weight loss,
in all animals receiving 10 mg/kg per day or more, the severity
being dose related. Investigation of the sciatic and tibial nerves
and the lumbricalis muscle with special histopathological stains
indicated no changes that could be attributed to treatment with
cyhalothrin. Liquid faeces were produced by all animals receiving
cyhalothrin, the incidence being dose related. These changes were
considered to be of no toxicological significance. No other
changes were observed that could be attributed to the treatment.
When groups of 6 male and 6 female dogs (Alderley Park beagles)
were fed cyhalothrin in corn oil by gelatine capsule at 0, 1, 2.5,
or 10 mg/kg body weight per day for 26 weeks, signs of pyrethroid
toxicity were seen in some dogs at 10 mg/kg. Although liquid
faeces were produced by all animals in the study, including the
controls, the incidence and frequency were higher in treated
animals and were dose related. These changes were considered to be
of no toxicological significance. Macroscopic postmortem
examination, organ weights, and histological investigations
revealed no treatment-related changes. The oral NOEL in dogs was
found to be 2.5 mg/kg per day (Chesterman et al., 1981).
In studies by Hext et al. (1986), groups of 6 male and 6 female
dogs were dosed by gavage in corn oil with 0, 0.1, 0.5, or 3.5 mg
lambda-cyhalothrin/kg body weight daily for 52 weeks. Clinical
signs of neurological effects were evident in all animals fed the
highest dose, which were unaccompanied by histological changes in
the nervous system. There was an increased incidence of fluid
faeces at the highest dose and a slight increase in the group
receiving 0.5 mg/kg per day. This effect was considered to be
related to the method of administration and not to be
toxicologically significant. No histopathological changes
attributable to lambda-cyhalothrin administration were observed at
any of the dose levels employed, and the toxicological NOEL in
this study was 0.5 mg lambda-cyhalothrin per kg per day.
7.3.2. Dermal
7.3.2.1 Rabbit
Cyhalothrin in polyethylene glycol (PEG 300) (10, 100, or 1000
mg/kg body weight per day) was applied to the skin of groups of 10
male and 10 female New Zealand White rabbits and kept in contact
with the skin 6 h/day, 5 days per week for 3 weeks (i.e. a total
of 15 applications) by means of an occlusive dressing. A group of
14 male and 14 female control rabbits was treated with polyethylene
glycol (PEG 300) using the same procedure. The skin of half the
animals in each group was abraded prior to the application of
cyhalothrin. Repeated application of the vehicle alone
(polyethylene glycol) and the vehicle plus cyhalothrin caused
slight to severe skin irritation. At the highest dose level there
was an increased incidence of oedema and erythema. A small number
of animals given the highest dose showed pyrethroid-like symptoms,
but only when the skin was unabraded. The NOEL was considered to be
100 mg/kg per day (Henderson & Jackson, 1982).
7.4. Long-term exposures and carcinogenicity
7.4.1. Rat
In studies by Pigott et al. (1984), groups of 72 male and 72
female Alpk/AP strain rats were fed diets containing cyhalothrin
at levels of 0, 10, 50, or 250 mg/kg diet for up to 104 weeks. All
the surviving animals were sacrificed, and histopathological and
gross postmortem examinations were carried out. Decreased body
weight gain, accompanied by a small decrease in food consumption,
was evident in rats of both sexes fed the highest dose. This was
accompanied by minor changes in blood biochemistry. Increased liver
weight was seen in rats of both sexes fed cyhalothrin at 250 mg/kg
at the interim sacrifice but this was not evident at termination.
There was no histopathological evidence of a chronic toxic effect
due to cyhalothrin. In particular clinical and histopathological
evaluation gave no indication of an effect on the nervous system.
There was no evidence for a carcinogenic effect of cyhalothrin.
The toxicological NOEL for this study was 50 mg cyhalothrin/kg
diet, corresponding to a minimum dose rate of approximately 1.7
mg/kg body weight per day for male rats and 1.9 mg/kg per day for
female rats.
7.4.2. Mouse
Groups of 52 male and 52 female Charles River CD-1 mice were
maintained for 104 weeks on diets containing 0, 20, 100, or 500 mg
cyhalothrin/kg and further groups of 12 males and 12 females were
designated for interim sacrifice after 52 weeks. During the study
there were no deaths attributable to treatment with cyhalothrin.
Signs of toxicity ascribable to cyhalothrin included piloerection
and hunched posture in both sexes at 500 mg/kg and in males at 100
mg/kg and reduced body weight gain, higher food intake, and
reduced efficiency of food utilization in males receiving 500
mg/kg. There was a statistically significant increase, compared to
the controls, in the incidence of mammary adenocarcinoma in females
at the two highest dose levels. However, the frequency of these
tumours was not unduly at variance with that normally seen in the
strain of mouse used, and no dose relationship was apparent. Thus,
there were no neoplastic findings that could be attributed to the
long-term administration of cyhalothrin. There was a clear NOEL of
20 mg/kg, corresponding to a mean calculated daily intake of 1.8
mg/kg body weight per day in males and 2.0 mg/kg body weight per
day in females (Colley et al., 1984).
7.5. Reproduction, embryotoxicity, and teratogenicity
7.5.1. Reproduction
In studies by Milburn et al. (1984), groups of 15 male and 30
female (F0 parents) weaning Alderley Park rats were fed diets
containing 0, 10, 30, or 100 mg cyhalothrin per kg. After 12
weeks, the animals were mated to produce the first (F1a) litter and
subsequently re-mated to produce a second (F1b) litter. The
breeding programme was repeated with F1 parents selected from the
F1b off-spring and F2 parents selected from the F2b offspring.
Test diets were fed continuously throughout the study. There were
minor effects on body weight gain of parents from all generations
receiving 100 mg/kg, but no clinical signs of neurological effects
were seen in either parents or offspring. No effects of treatment
were seen on indices of male and female fertility, gestation
period, live born index, or pup survival. There was a small
reduction in mean total litter weight of the F2 and F3 generations
from rats receiving the highest dose, which was attributable to
minor decreases in litter size and a small reduction in weight gain
of the pups. No effect was seen in litters from rats receiving 30
mg/kg. There was no evidence of gross or histopathological change
attributable to the treatment. The reproductive effects seen in
rats receiving the highest dose were of a minor nature. A clear
NOEL of 30 mg/kg (corresponding to a dosage in the range of 1.5 to
1.9 mg/kg body weight per day) was established (Milburn et al.,
1984).
7.5.2. Embryotoxicity and teratogenicity
7.5.2.1 Rat
When groups of 24 mated female rats (Charles River CD-1) were
given cyhalothrin orally (in corn oil), at 0, 5, 10, or 15 mg/kg
body weight per day, from day 6 to 15 inclusive of gestation and
were killed on day 20, there was reduced body weight gain at the
highest dose level and evidence of mild pyrethroid toxicity in two
of these animals. There were no other effects on the clinical or
litter parameters attributable to treatment with cyhalothrin, and
examination of the viscera and skeletons showed no effects of
treatment. At the highest dose level, there was maternal toxicity,
but there was no effect on any aspect of fetal development at any
dose level (Killick, 1981a).
7.5.2.2 Rabbit
In studies by Killick (1981b), groups of at least 18 pregnant
New Zealand White rabbits received cyhalothrin orally in corn oil
daily at 0, 3, 10, or 30 mg/kg body weight, from days 6 to 18
(inclusive) of gestation and were killed on day 28. There was
reduced body weight gain at the highest dose, accompanied by
reduced food intake during dosing. There were no clinical signs and
no changes in pregnancy incidence or in litter parameters
attributable to treatment with cyhalothrin. Examination of the
viscera and skeletons revealed no effects of treatment. At the
highest dose level, there was maternal toxicity, but there was no
effect on any aspect of fetal development at any dose level.
7.6. Mutagenicity and related end-points
7.6.1. Microorganisms
Five test strains, TA1535, TA1537, TA1538, TA98, and TA100,
were employed to evaluate the mutagenic potential of cyhalothrin
using the salmonella reverse mutation assay of Ames. The assay was
conducted in the presence and absence of metabolic activation (S9
mix) with cyhalothrin at levels up to 2500 µg/plate. The mean
numbers of revertant colonies of Salmonella typhimurium observed in
the five test strains indicated an unequivocal negative response
(Trueman, 1981). Lambda-cyhalothrin at dose levels of up to 5000
µg/plate, both in the presence and absence of metabolic activation,
gave a non-mutagenic response in the same test using the same
strains (Callander, 1984).
7.6.2. In vitro mammalian cells
When cyhalothrin was tested in a modification of the cell
culture transformation test of Styles, using Syrian Hamster kidney
cell line BHK21C13, the response in the presence of metabolic
activation was unequivocally negative. In the absence of metabolic
transformation there was an erratic increase in numbers of
transformed colonies together with a poor dose response. These
data were not thought to indicate a significant positive response,
and it was concluded that cyhalothrin does not appear to possess
significant cell-transforming properties (Richold et al., 1981).
In addition, the significance of the results from the BHK cell
system is doubtful in view of the questionable interlaboratory
reproducibility of this assay.
The mutagenic potential of lambda-cyhalothrin has been assessed
in vitro with L51787 mouse lymphoma cells, both in the presence and
absence of auxiliary metabolic activation (S9) mix, using dose
levels of 125-1000, 2000, and 4000 µg/ml. There was no increase in
mutation frequency either in the presence or absence of S9 mix
(Cross, 1985).
Lambda-cyhalothrin, at dose levels of up to 1000 µg/ml, either
in the presence or absence of metabolic activation, did not induce
statistically significant increases in the incorporation of
tritiated thymidine in cultured human (Hela) cells (Milone, 1986)
or induce chromosomal damage in human lymphocytes stimulated by
phytohaemagglutinin (Sheldon et al., 1985).
7.6.3. In vivo mammalian assays
Male rats were given a single dose or five consecutive daily
doses by gavage of cyhalothrin at levels of 1.5, 7.5, or 15 mg/kg,
and bone marrow samples were taken and examined for chromosomal
abnormalities. The results indicated that cyhalothrin has no
clastogenic potential (Anderson et al., 1981).
In studies by Irvine (1981), three groups of male mice were
dosed with cyhalothrin by gavage, at dose levels of 1, 5, or 10
mg/kg daily, for 5 consecutive days. A further group received the
known mutagen cyclophosphamide intraperitoneally at 200 mg/kg
daily for five days. The animals were then mated with groups of
females at weekly intervals for eight weeks. Pregnancy incidence,
pre- and post-implantation loss, clinical condition, body weight,
and gross necropsy were assessed. There was no evidence of an
increase in the dominant lethal mutation frequency following
treatment. The NOEL was 10 mg/kg per day. Although these studies
showed no clastogenic or mutagenic effect, it is not clear whether
sufficiently high dose levels were used.
When lambda-cyhalothrin was administered to mice at levels of
up to 35 mg/kg and bone marrow preparations were examined for the
formation of micronuclei in polychromatic erythrocytes, there was
no statistically significant increase in the frequency of
micronuclei, compared to control animals. The positive control
substance, cyclophosphamide, showed the expected response (Sheldon
et al., 1984).
7.7. Mode of action
The mode of action of cyhalothrin and lambda-cyhalothrin, both
type II pyrethroids, is basically the same as that of the other
pyrethroids (see Appendix).
8. EFFECTS ON HUMANS
8.1. General population exposure
No poisoning incidents with cyhalothrin or lambda-cyhalothrin
have been reported. There is no information on effects from short-
or long-term exposure and no epidemiological information is
available.
8.2. Occupational exposure
Cyhalothrin is known to produce an effect described as
subjective facial sensation (SFS) in some people working with this
compound. SFS is a transient phenomenon; symptoms are not
associated with objective physical signs and recovery appears to be
complete. It is likely that SFS arises from direct facial contact
with the chemical particularly from touching the face with
contaminated gloves or hands. This would also help to explain the
effect of formulation or concentration as these could affect the
rate of dermal penetration and therefore access of the chemical to
the nerve endings.
It appears unlikely that individual sensitivity is important
in the development of symptoms following exposure. It is more
likely to be related to the amount of the chemical that comes into
contact with the facial skin. The information currently available
(Hart, 1984) is summarized in section 8.2.2.
8.2.1. Acute toxicity: poisoning incidents
No poisoning incidents involving cyhalothrin or lambda-
cyhalothrin have been reported.
8.2.2. Effects of short- and long-term exposure
Laboratory workers and manufacturing plant and field operators
handling natural and synthetic pyrethroids, including cyhalothrin
and lambda-cyhalothrin, have noticed a transient skin sensation in
the periorbital area of the face and other sites after direct skin
exposure. It has been suggested that these sensations are caused
by spontaneous repetitive firing of sensory nerve fibres or nerve
endings, whose threshold has been transiently lowered by the
compound, localized around the sites of exposure.
Symptoms from exposure to a synthetic pyrethroid generally
start 30 min to 1 h after exposure and last for several hours,
sometimes up to 2 days. They almost always affect the facial area,
producing a tingling, burning, or numb sensation that has been
variously described as paraesthesia, dysaesthesia, or SFS. The
latter term appears most appropriate as no objective signs of
abnormality of nerve function have been found in workers suffering
from these effects and the facial area is by far the most commonly
affected.
8.2.2.1 Manufacture
At least 12 workers involved in the manufacture of cyhalothrin
have experienced symptoms of SFS. The symptoms were described as
burning, tingling, or sunburn, but clinical examination revealed no
abnormal neurological signs. The areas of the face affected were
the eyelids, cheeks, nostrils, forehead, and lips. In one case the
penis was affected, probably from contact with contaminated hands.
The symptoms started between 5 min and 3 h from the onset of
exposure and lasted from 5 to 30 h. Wind, washing, and temperature
increase led to a worsening of the symptoms, and relief could be
obtained by using a local anaesthetic. Barrier creams were
relatively ineffective in preventing the symptoms.
8.2.2.2 Formulation and laboratory work
Two workers have been reported to have suffered from SFS. Both
were exposed to technical cyhalothrin and symptoms began 1-6 h
after exposure. The symptoms described were typical of SFS,
consisting of tingling and burning around the eyes and facial area,
and lasted 6-24 h.
Four reports involving three individuals have been received
from laboratories involved with lambda-cyhalothrin. The symptoms
consisted of a facial tingling and burning sensation and in one
case tingling on the tongue. Symptoms began within 30 min of
exposure and lasted from 6 h to 2 days. All three workers were
involved in handling either technical material or concentrated
lambda-cyhalothrin solutions (10% w/v ai).
8.2.2.3 Field use
Virtually no reports have been received on the agricultural or
public health use of cyhalothrin. The only information that has
become available is from the use of cyhalothrin in animal health
products. Skin itching has been reported following exposure to a
5% emulsifiable concentrate of cyhalothrin, but dilutions of 0.1%
and 0.005% (w/v of ai) were without significant effect. More
recently, several incidents of facial irritation have been reported
following the use of cyhalothrin in sheep sprays (Hart, 1984). The
concentration used in these sprays was very low (0.002% w/v), but
the characteristics of the spray used were unclear.
Out of 38 field trials staff who returned questionnaires, only
four reported experiencing any adverse effects as a result of
exposure to lambda-cyhalothrin. Three of these individuals
experienced a burning sensation or irritation around the face,
cheeks, or eyes, which started between 45 and 60 min after initial
exposure and lasted, respectively, for 5, 18, and 72 h. In one case
the symptoms were severe enough to prevent the individual from
working. The other field trials worker experienced a rash on the
hands that started 24 h after exposure and lasted several days. All
four field trials staff were involved in handling concentrated
solutions of lambda-cyhalothrin (2.5% w/v ai) and three of the four
sprayed diluted solutions. None of the three workers experiencing
the facial sensation had experienced similar symptoms before, but
the worker who developed the rash had suffered a similar rash after
exposure to permethrin, cypermethrin, and deltamethrin following
their application to top fruit. Eight of the other 34 field trials
staff returning questionnaires stated that they had previously
suffered symptoms of facial tingling or burning.
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Cyhalothrin was discussed at the 1984 and 1986 FAO/WHO Joint
Meetings on Pesticide Residues (JMPR), where an acceptable daily
intake (ADI) for cyhalothrin of 0-0.02 mg/kg body weight was
established (FAO/WHO, 1985, 1986a).
The JMPR (FAO/WHO, 1985, 1986a,b) has estimated maximum
residue limits (MRLs) for cyhalothrin (sum of the isomers) as
follows:
---------------------------------------------------------
Commodity Maximum Pre-harvest
residue limit interval (days)
---------------------------------------------------------
Pome fruits 0.2 14
Cabbages, head 0.2 3-4
Potatoes 0.02a non specified
Cotton seed 0.02a 21
Cotton seed oil, crude 0.02a non specified
Cotton seed oil, edible 0.02a non specified
---------------------------------------------------------
a At or about the limit of detection.
REFERENCES
ANDERSON, D., RICHARDSON, C.R., HULME, A., MORRIS, J., BANHAM, P.B., &
GODLEY, M.J. (1981) Cyhalothrin: A cytogenic study in the rat (Unpublished
proprietary report No. CTL/P/664, submitted to WHO by ICI).
BARBER, J.E. (1985) PP321: Acute dermal toxicity study (Unpublished
proprietary report No. CTL/P/1119, submitted to WHO by ICI).
BENGSTONE, M., DAVIES, R.A.H., DESMARCHELIER, J.M., HENNING, R., MURRAY,
W., SIMPSON, B.W., SNELSON, J.T., STICKA, R., & WALLBANK, B.E. (1983)
Organophosphorothioates and synergised synthetic pyrethroids as grain
protectants on bulk wheat. Pestic. Sci., 14: 373-384.
BEWICK, D.W. & ZINNER, C.K.J. (1981) Cyhalothrin: Degradation in soil
(Unpublished proprietary report No. RJ0203B, submitted to WHO by ICI).
BHARTI, H. & BEWICK, D.W. (1986) Cyhalothrin: Degradation in a Japanese
soil (Unpublished proprietary report No. RJ0491B, submitted to WHO by ICI).
BHARTI, H., BEWICK, D.W., & WHITE, R.D. (1985) PP563 and PP321: Degradation
in soil (Unpublished proprietary report No. RJ0382B, submitted to WHO by
ICI).
CALLANDER, R.D. (1984) PP321: An evaluation in the Salmonella mutagenicity
assay (Unpublished proprietary report No. CTL/P/1000, submitted to WHO by
ICI).
CHESTERMAN, H., HEYWOOD, R., ALLEN, T.R., STREET, A.E., PRENTICE, D.,
BUCKLEY, P., & OFFER, J. (1980) PP563: Preliminary oral toxicity study in
beagle dogs (Unpublished proprietary report of the Huntingdon Research
Centre No. 325/8074, submitted to WHO by ICI).
CHESTERMAN, H., HAYWOOD, R., ALLEN, T.R., STREET, A.E., KELLY, D.F.,
GOPINATH, C., & PRENTICE, D.E. (1981) Cyhalothrin oral toxicity study in
beagle dogs (Final report: Repeated daily dosing for 26 weeks) (Unpublished
proprietary report of the Huntingdon Research Centre No. ICI/326/8162,
submitted to WHO by ICI).
COLLEY, J., DAWE, S., HEYWOOD, R., ALMOND, R., GIBSON, W.A., GREGSON, R., &
GOPINATH, C. (1984) Cyhalothrin potential tumorigenic and toxic effects in
prolonged dietary administration to mice (Unpublished proprietary report of
the Huntingdon Research Centre No. ICI 395/83668, submitted to WHO by ICI).
COLLIS, W.M.D. & LEAHEY, J.P. (1984) PP321: Hydrolysis in water at pH 5, 7
and 9 (Unpublished proprietary report No. RJ0338B, submitted to WHO by
ICI).
COULSON, J.M., COLLINS, I.G., & EDWARDS, P.J. (1986) PP321: Effects on
earthworms Lumbricidae of repeated annual field applications (Unpublished
proprietary report No. RJ0511B, submitted to WHO by ICI).
CROSS, M. (1985) PP321: Assessment of mutagenic potential using L5178Y
mouse lymphoma cells (Unpublished proprietary report No. CTL/P/1340,
submitted to WHO by ICI).
CROSSLAND, N.O. (1982) Aquatic toxicology of cypermethrin. II. Fate and
biological effects in pond experiments. Aquat. Toxicol., 2: 205-222.
CROSSLAND, N.O., SHIRES, S.W., & BENNETT, D. (1982) Aquatic toxicology of
cypermethrin. III. Fate and biological effects of spray drift deposits in
freshwater adjacent to agricultural land. Aquat. Toxicol., 2: 253-270.
CURL, E.A., LEAHEY, J.P., & LLOYD, S.J. (1984a) PP321: Aqueous photolysis
at pH 5 (Unpublished proprietary report No. RJ0362B, submitted to WHO by
ICI).
CURL, E.A., LEAHEY, J.P., & LLOYD, D. (1984b) PP321: Photodegradation on a
soil surface (Unpublished proprietary report No. RJ0358B, submitted to WHO
by ICI).
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American Chemical
Society, pp. 229 (ACS Symposium Series 42).
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid. Pestic. Biochem. Physiol., 6: 547-550.
FAO (1982) Report of the Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15 October,
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1985) Cyhalothrin. In: 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agricultural Organization of the United Nations,
pp. 173-185, 571-584 (FAO Plant production and protection Paper 67).
FAO/WHO (1986a) Cyhalothrin: In: 1986 Evaluations of some pesticide
residues in food, Part 1: Residues, Rome, Food and Agriculture Organization
of the United Nations, pp. 95-138 (FAO Plant Production and Protection
Paper 78).
FAO/WHO (1986b) Codex maximum limits for pesticide residues, 2nd ed., Rome,
Codex Alimentarius Commission, Food and Agriculture Organization of the
United Nations (CAC XIII).
FARRELLY, E., HAMER, M.J., & HILL, I.R. (1984) PP321: Toxicity to first
instar Daphnia magna (Unpublished proprietary report No. RJ0359B, submitted
to WHO by ICI).
FARRELLY, E., HAMER, M.J., & HILL, I.R. (1985) PP321: Toxicity of
formulations JF9509 and GFU383C to first instar Daphnia magna (Unpublished
proprietary report No. RJ0426B, submitted to WHO by ICI).
FITZPATRICK, R.D. (1985) PP321 dissipation in US soils - 1983 (Unpublished
report No. TMU1809, submitted to WHO by ICI).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous sensation
between synthetic pyrethroid insecticides. Contact dermatitis, 13: 140-147.
FORBES, S. & DUTTON, A.J. (1985) A small scale method for the determination
of pyrethroid insecticide residues in crops and soils. Pestic. Sci., 16:
404-408.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent class
antagonize GABA action at the crayfish neuromuscular junction. Neurosci.
Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of pyrethroid
action in the cockroach. Pestic. Biochem. Physiol., 15: 181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid toxicology:
Protective effects of Diazepam and phenobarbital in the mouse and the
cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
GIFAP (1981) Technical Monograph No. 4: Guidelines on pesticide residue
trials to provide residue data for the registration of pesticides and the
establishment of maximum residue limits, Brussels, International Group of
National Associations of Manufacturers of Agrochemical Products (GIFAP
D/1981/2537/3).
GIRENKO, D.B. & KLISENKO, M.A. (1984) Concentration and determination of
trace quantities of synthetic pyrethroids in the air. Gig. i Sanit., 3: 57-
59.
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4(6):
793-799.
GOUGH, H.J., COLLINS, I.G., EVERETT, C.J., & WILKINSON, W. (1984) PP321:
Acute contact and oral toxicity to honey bees (Apis mellifera) (Unpublished
proprietary report No. RJ0390B, submitted to WHO by ICI).
GOUGH, H.J., COLLINS, I.G., & WILKINSON, W. (1985) PP321: Field test of
toxicity to honey bees (Apis mellifera) on flowering oil-seed rape
(Brassica napus) (Unpublished proprietary report No. RJ0413B, submitted to
WHO by ICI).
HALL, J.S. & LEAHEY, J.P. (1983) Cyhalothrin: Fate in river water
(Unpublished proprietary report No. RJ0320B, submitted to WHO by ICI).
HAMER, M.J. & HILL, I.R. (1985) Cyhalothrin: The accumulation of
cyhalothrin and its degradation products by channel catfish and
Daphnia magna in a soil/water system (Unpublished proprietary
report No. RJ0427B, submitted to WHO by ICI).
HAMER, M.J., FARRELLY, E., & HILL, I.R. (1985a) PP321: Toxicity to Gammarus
pulex (Unpublished proprietary report No. RJ0414B, submitted to WHO by
ICI).
HAMER, M.J., FARRELLY, E., & HILL, I.R. (1985b) PP321: 21 day Daphnia magna
life-cycle study (Unpublished proprietary report No. RJ0451B, submitted to
WHO by ICI).
HARRISON, M.P. (1981) Cyhalothrin: The disposition and metabolism of [ 14 C]-
146814 in rats (Unpublished proprietary report No. 10/HD/007325, submitted
to WHO by ICI).
HARRISON, M.P. (1983) Cyhalothrin: The metabolism and disposition of 146814
in the rat: Part IV. Isolation and identification of the major urinary
metabolites derived from [14 C-benzyl]- or [14 C-cyclopropyl]-146814
following oral administration (Unpublished proprietary report No.
6/HC/005683, submitted to WHO by ICI).
HARRISON, M.P. (1984a) Cyhalothrin (146814): The metabolism and disposition
of 146814 in rats: Part II. Tissue residues derived from [14 C-benzyl] or
[14 C-cyclopropyl]-146814, after a single oral dose of 1 or 25 mg/kg
(Unpublished proprietary report No. KMR 002/2, submitted to WHO by ICI).
HARRISON, M.P. (1984b) Cyhalothrin: The metabolism and disposition of
[14 C]-146814 in rats: Part III. Studies to determine radioactive residues
in the rat following 14 days repeated oral administration (Unpublished
proprietary report No. 146814 KMR 002/03, submitted to WHO by ICI).
HARRISON, M.P. (1984c) Cyhalothrin (146814): The disposition and metabolism
of [14 C]-146814 in the dog (Unpublished proprietary report No. 146814 KMD
005, submitted to WHO by ICI).
HARRISON, M.P. (1984d) Cyhalothrin (146814): The metabolism, excretion and
residues in the cow after 7 days oral administration with two [14 C]-
labelled forms of 146814 at 1 mg/kg/day (Unpublished proprietary report No.
146814 KMC 006/007, submitted to WHO by ICI).
HART, D., BANHAM, P.B., CHART, I.S., EVANS, D.P., GORE, C.W., STONARD,
M.D., MORELAND, S., GODLEY, M.J., & ROBINSON, M. (1985) PP321: 90-day
feeding study in rats (Unpublished proprietary report No. CTL/P/1045,
submitted to WHO by ICI).
HART, T.B. (1984) Review of adverse skin reactions associated with the use
of synthetic pyrethroids (Unpublished proprietary report No. TMF2530,
submitted to WHO by ICI).
HENDERSON, C. & JACKSON, S.J. (1982) Cyhalothrin: Subacute dermal toxicity
study in rabbits (Unpublished proprietary report No. CTL/P/680, submitted
to WHO by ICI).
HEXT, P.M., BRAMMER, A., CHALMERS, D.T., CHART, I.S., GORE, C.W., PATE, I.,
& BANHAM, P.B. (1986) PP321: 1 year oral dosing study in dogs (Unpublished
proprietary report No. CTL/P/1316, submitted to WHO by ICI).
HILL, R.W. (1981) Determination of the acute toxicity of cyhalothrin
(PP563) to Rainbow trout (Salmo gairdneri) (Unpublished proprietary report
No. BL/B/2097, submitted to WHO by ICI).
HILL, R.W. (1984a) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) (Unpublished proprietary report No. BL/B/2405, submitted
to WHO by ICI).
HILL, R.W. (1984b) PP321: Determination of acute toxicity to Bluegill
sunfish (Lepomis macrochirus) (Unpublished proprietary report No.
BL/B/2406, submitted to WHO by ICI).
HILL, R.W. (1985a) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) of 2.5% w/w formulation (Unpublished proprietary report
No. BL/B/2603, submitted to WHO by ICI).
HILL, R.W. (1985b) PP321: Determination of acute toxicity of a 1 lb/US
gallon EC formulation to Rainbow trout (Salmo gairdneri) (Unpublished
proprietary report No. BL/B/2609, submitted to WHO by ICI).
HILL, R.W. (1985c) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) of a 5% EC formulation (Unpublished proprietary report
No. BL/B/2783, submitted to WHO by ICI).
HILL, R.W. (1985d) PP321: Determination of acute toxicity to Mirror carp
(Cyprinus carpio) of a 2.5% w/w formulation (Unpublished proprietary report
No. BL/B/2604, submitted to WHO by ICI).
HILL, R.W. (1985e) PP321: Determination of acute toxicity to Mirror carp
(Cyprinus carpio) of a 5% EC formulation (Unpublished proprietary report
No. BL/B/2784, submitted to WHO by ICI).
HILL, R.W. (1985f) PP321: Determination of acute toxicity of a 1 lb/US
gallon EC formulation to Bluegill sunfish (Lepomis macrochirus)
(Unpublished proprietary report No. BL/B/2610, submitted to WHO by ICI).
HILL, R.W. (1985g) PP321: Determination of acute toxicity to Sheepshead
minnow (Cyprinodon variegatus) (Unpublished proprietary report No.
BL/B/2615, submitted to WHO by ICI).
HILL, R.W., CAUNTER, J.E., & CUMMING, R.I. (1985) PP321: Determination of
the chronic toxicity to Sheepshead minnow (Cyprinodon variegatus) embryos
and larvae (Unpublished proprietary report No. BL/B/2677, submitted to WHO
by ICI).
IRVINE, L.F.H. (1981) Cyhalothrin: Oral (gavage) dominant lethal study in
the male mouse (Unpublished proprietary report of the Hazleton Laboratories
No. 2647-72/213, submitted to WHO by ICI).
JACKSON, S.J. (1981) Cyhalothrin: Eye irritation study in the rabbit
(Unpublished proprietary report No. CTL/T/1502, submitted to WHO by ICI).
JACKSON, S.J. & NIXON, J. (1981) C yhalothrin: Skin irritation studies in
the rabbit and rat (Unpublished proprietary report No. CTL/T/1504,
submitted to WHO by ICI).
KILLICK, M.E. (1981a) Cyhalothrin: Oral (gavage) teratology study in the
rat (Unpublished proprietary report of the Hazleton Laboratories No. 2661-
72/208, submitted to WHO by ICI).
KILLICK, M.E. (1981b) Cyhalothrin: Oral (gavage) teratology study in the
New Zealand white rabbit (Unpublished proprietary report of the Hazleton
Laboratories No. 2700-72/211, submitted to WHO by ICI).
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of pyrethroid
insecticides on the gamma-aminobutyric acid receptor-ionophore complex.
Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding sites.
Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor & Francis
Ltd, 440 pp.
LEAHEY, J.P. & FRENCH, D.A. (1986a) Quantification and characterisation of
radioactive residues found in soya leaves from plants treated with 14 C-
PP321 (Unpublished proprietary report No. RJ0507B, submitted to WHO by
ICI).
LEAHEY, J.P. & FRENCH, D.A. (1986b) Quantification and characterisation of
radioactive residues found in cotton leaves from plants treated with 14 C-
cyclopropane-labelled PP321 (Unpublished proprietary report No. JR0526B,
submitted to WHO by ICI).
LEAHEY, J.P. & FRENCH, D.A. (1986c) Quantification and characterisation of
radioactive residues in cotton leaves from plants treated 14 C-benzyl-
labelled PP321 (Unpublished proprietary report No. RJ0497B, submitted to
WHO by ICI).
LEAHEY, J.P. & PARKER, S. (1985) Cyhalothrin: Characterisation of residues
accumulated by carp continuously exposed to 14 C-cyhalothrin (Unpublished
proprietary report No. RJ0407B, submitted to WHO by ICI).
LEAHEY, J.P., FRENCH, D.A., & HEATH, J. (1985) PP321: Metabolism in a goat
(Unpublished proprietary Report No. RJ0435B, submitted to WHO by ICI).
LINDSAY, S., CHART, I.S., GODLEY, M.J., GORE, C.W., HALL, M., PRATT, I.,
ROBINSON, M., & STONARD, M. (1981) Cyhalothrin: 90-day feeding study in
rats (Unpublished proprietary report No. CTL/P/629, submitted to WHO by
ICI).
LINDSAY, S., DOE, J.E., GODLEY, M.J., HALL, M., PRATT, I., ROBINSON, M., &
STONARD, M.D. (1982) Cyhalothrin induced liver changes: Reversibility study
in male rats (Unpublished proprietary report No. CTL/P/668, submitted to
WHO by ICI).
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel modification
as the basis for the variation in the nerve membrane effects of pyrethroids
and DDT analogs. Pestic. Biochem. Physiol., 20: 203-216.
MILBURN, G.M., BANHAM, P., GODLEY, M.J., PIGOTT, G., & ROBINSON, M. (1984)
Cyhalothrin: Three generation reproduction study in the rat (Unpublished
proprietary report No. CTL/P/906, submitted to WHO by ICI).
MILONE, M.F. (1986) PP321: Unscheduled DNA synthesis in cultured Hela cells
(autoradiographic method) (Unpublished proprietary report of the Institute
di Ricerche Biomediche Antione Marxer No. M914, submitted to WHO by ICI).
MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2922.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare and
the environment. Proceedings of the Fifth International Congress on
Pesticide Chemistry, Kyoto, Japan, 29 August-4 September, 1982, Oxford, New
York, Pergamon Press (Vol. 1-4).
NIXON, J. & JACKSON, S.J. (1981a) Cyhalothrin: Acute toxicity (Unpublished
proprietary report No. CTL/T/1555, submitted to WHO by ICI).
NIXON, J. & JACKSON, S.J. (1981b) Cyhalothrin: Skin sensitisation study in
the guinea-pig (Unpublished proprietary report No. CTL/T/1552, submitted to
WHO by ICI).
PIGOTT, G.H., CHART, I.S., GODLEY, M.J., GORE, C.W., HOLLIS, K.J.,
ROBINSON, M., TAYLOR, K., & TINSTON, D.J. (1984) Cyhalothrin: Two year
feeding study in rats (Unpublished proprietary report No. CTL/P/980,
submitted to WHO by ICI).
PRITCHARD, V.K. (1984) PP321: Skin sensitisation study (Unpublished
proprietary report No. CTL/P/1054, submitted to WHO by ICI).
PRITCHARD, V.K. (1985a) PP321 and cyhalothrin: skin irritation study
(Unpublished proprietary report No. CTL/P/1139, submitted to WHO by ICI).
PRITCHARD, V.K. (1985b) PP321: Eye irritation study (Unpublished
proprietary report No. CTL/P/1207, submitted to WHO by ICI).
PROUT, M.S. (1984) Cyhalothrin: Bioaccumulation in the rat (Unpublished
proprietary report No. CTL/P/1014, submitted to WHO by ICI).
PROUT, M.S. & HOWARD, E.F. (1985) PP321: Comparative absorption study in
the rat (1 mg/kg) (Unpublished proprietary report No. CTL/P/1214, submitted
to WHO by ICI).
REYNOLDS, L.F. (1984) Cyhalothrin: Determination of acute toxicity to
Bluegill sunfish (Lepomis machrochirus) (Unpublished proprietary report No.
BL/B/2514, submitted to WHO by ICI).
RICHOLD, M., ALLEN, J.A., WILLIAMS, A., & RANSOME, S.J. (1981) Cell
transformation test for potential carcinogenicity of Y00102/010/005
(cyhalothrin (PP563)) (Unpublished proprietary report of the Huntingdon
Research Centre No. 391(B)/80948, submitted to WHO by ICI).
ROBERTS, N.L. & FAIRLEY, C. (1981) The acute oral toxicity (LD50) of
cyhalothrin to the Mallard duck (Unpublished proprietary report of the
Huntingdon Research Centre No. 373WL/801024, submitted to WHO by ICI).
ROBERTS, N.L. & FAIRLEY, C. (1984) The acute oral toxicity (LD50) of PP321
to the Mallard duck (Unpublished proprietary report of the Huntingdon
Research Centre No. 438BT/831011, submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., & WOODHOUSE, R.N. (1981a) The subacute dietary
toxicity (LC50 ) of cyhalothrin to the Mallard duck (Unpublished proprietary
report of the Huntingdon Research Centre No. 377WL/8120, submitted to WHO
by ICI).
ROBERTS, N.L., FAIRLEY, C., & WOODHOUSE, R.N. (1981b) The subacute dietary
toxicity (LC50 ) of cyhalothrin to the Bobwhite quail (Unpublished
proprietary report of the Huntingdon Research Centre No. 378WL/8179,
submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., HAKIN, B., PRENTICE, D.E., & WIGHT, D.G.D.
(1982) The acute oral toxicity (LD50 ) and neurotoxic effects of cyhalothrin
to the domestic hen (Unpublished proprietary report of the Huntingdon
Research Centre No. ICI/374 NT/81742, submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., & DAWE, I.S. (1985a) The subacute
dietary toxicity of PP321 to the Mallard duck (Unpublished proprietary
report of the Huntingdon Research Centre No. ISN 46BT/85171, submitted to
WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., & DAWE, I.S. (1985b) The subacute
toxicity of PP321 to the Bobwhite quail (Unpublished proprietary report of
the Huntingdon Research Centre No. ISN 45BT/841287, submitted to WHO by
ICI).
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a nerve
muscle preparation of the clawed frog, Xenopus laevis. Pestic. Biochem.
Physiol., 25: 176-187.
SAPIETS, A. (1984a) Cyhalothrin: Storage stability of the residue on deep
frozen apple, cabbage and soil samples (Unpublished proprietary report No.
M3843B, submitted to WHO by ICI).
SAPIETS, A. (1984b) The determination of residues of PP321 in crops. A gas-
liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 81, submitted to WHO by
ICI).
SAPIETS, A. (1984c) The determination of residues of cyhalothrin
metabolites in crops. A gas-liquid chromatographic method (Unpublished
proprietary residue analytical method No. PPRAM 74, submitted to WHO by
ICI).
SAPIETS, A. (1985a) The determination of residues of cyhalothrin in crops.
A gas-liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 70, submitted to WHO by
ICI).
SAPIETS, A. (1985b) The determination of residues of cyhalothrin in water.
A gas-liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 69, submitted to WHO by
ICI).
SAPIETS, A. (1985c) PP321: Residue transfer study with dairy cows fed on a
diet containing the insecticide (Unpublished proprietary report No. M3936B,
submitted to WHO by ICI).
SAPIETS, A. (1986a) The determination of residues of PP321 in products of
animal origin. A GLC method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 86/1, submitted to WHO by
ICI).
SAPIETS, A. (1986b) The determination of residues of PP321 in soil. A GLC
method using an internal standard (Unpublished proprietary residue
analytical method No. PPRAM 93, submitted to WHO by ICI).
SAPIETS, A., SWAINE, H., & TANDY, M.J. (1984) In: Zweig, G. & Sherma, J.,
ed. Cypermethrin (Chapter 2). Analytical methods for pesticides and plant
growth regulators: Vol XIII, Synthetic pyrethroids and other pesticides,
New York, London, San Francisco, Academic Press.
SHELDON, T., RICHARDSON, C.R., SHAW, J., & BARBER, G. (1984) An evaluation
of PP321 in the mouse micronucleus test (Unpublished proprietary report No.
CTL/P/1090, submitted to WHO by ICI).
SHELDON, T., HOWARD, C.A., & RICHARDSON, C.R. (1985) PP321: A cytogenetic
study in human lymphocytes in vitro (Unpublished proprietary report No.
CTL/P/1333, submitted to WHO by ICI).
SMART, L.E. & STEVENSON, J.H. (1982) Laboratory estimation of toxicity of
pyrethroid insecticides to honeybees: Relevance to hazard in the field. Bee
World, 63(4): 150-152.
SOUTHWOOD, J. (1984) PP321: Acute oral toxicity to the mouse (Unpublished
proprietary report No. CTL/P/1066, submitted to WHO by ICI).
SOUTHWOOD, J. (1985) PP321: Acute oral toxicity studies (Unpublished report
No. CTL/P/1102, submitted to WHO by ICI).
STEVENS, J.E.B. & BEWICK, D.W. (1985) PP563 and PP321: Leaching of PP563
and PP321 and their degradation products in soil columns (Unpublished
proprietary report No. RJ0408B, submitted to WHO by ICI).
TAKEDA CHEMICAL CO. LTD (1979) Test result of fish toxicity of PP-563
(Unpublished proprietary report, submitted to WHO by ICI).
THOMPSON, R.S. (1985) PP321: Determination of acute toxicity to Mysid
shrimps (Mysidopsis bahia) (Unpublished proprietary report No. BL/B/2635,
submitted to WHO by ICI).
THOMPSON, R.S. & WILLIAMS, T.D. (1985) PP321: Toxicity to the green alga
Selenastrum capricornutum (Unpublished proprietary report No. BL/B2584,
submitted to WHO by ICI).
TINSON, D.J., BANHAM, P.D., CHART, I.S., GORE, C.W., PRATT, I., SCALES,
M.D.C., & WEIGHT, T.M. (1984) PP563: 28-day feeding study in rats. Summary
report (Unpublished proprietary report No. CTL/P/1056, submitted to WHO by
ICI).
TRUEMAN, R.W. (1981) Cyhalothrin: Results from the Salmonella reverse
mutation assay (Unpublished proprietary report No. CTL/P/665, submitted to
WHO by ICI).
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies on
the effects of allethrin and DDT on the sodium channels in frog myelinated
nerve membrane. In: Insect neurobiology and pesticide action, London,
Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M. (1973) DDT-
like action of allethrin in the sensory nervous system of Xenopus laevis.
Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A. (1979) Effects of
insecticides on the sensory nervous system. In: Narashashi, T., ed.
Neurotoxicology of insecticides and pheromones, New York, London, Plenum
Publishing Corporation, pp. 183-210.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity relationships
of some pyrethroids in rats. Arch. Toxicol., 45: 325-329.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-dependent effects
of the pyrethroid insecticide decamethrin in frog myelinated nerve fibres.
Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a) Structure-
related effects of pyrethroid insecticides on the lateral-line sense organ
and on peripheral nerves of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel gating in
myelinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN DEN
BERCKEN, J. (1983) Temperature- and structure-dependent interaction of
pyrethroids with the sodium channels in the frog node of Ranvier. Biochem.
Biophys. Acta, 728: 73-82.
WHO (1979) Safe use of pesticides. Third report of the WHO Expert Committee
on Vector Biology and Control, Geneva, World Health Organization (WHO
Technical Report Series, No. 634).
WILLIAMS, T.D. & THOMPSON, R.S. (1981) Determination of the acute toxicity
of cyhalothrin (PP563) to Daphnia magna (Unpublished proprietary report No.
BL/B/2114, submitted to WHO by ICI).
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SAITO, H. (1984a) PP563
(cyhalothrin): Accumulation in fish (carp) in a flow-through water system
(Unpublished proprietary report of MITES No. 58-367, submitted to WHO by
ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984b) PP563
(cyhalothrin) formulation (5% WP): Acute toxicity to carp (Unpublished
proprietary report of MITES No. 58-367, submitted to WHO by ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984c) PP563
(cyhalothrin): Toxicity to daphnia and fish (carp) in the presence and
absence of soil (Unpublished proprietary report of MITES No. 58-367,
submitted to WHO by ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984d) PP563
(cyhalothrin) formulation (5% WP): Acute toxicity to daphnia (Unpublished
proprietary report of MITES No. 58-367, submitted to WHO by ICI).
APPENDIX
On the basis of electrophysiological studies with peripheral
nerve preparations of frogs (Xenopus laevis; Rana temporaria, and
Rana escuelenta), it is possible to distinguish between 2 classes
of pyrethroid insecticides: (Type I and Type II). A similar
distinction between these 2 classes of pyrethroids has been made on
the basis of the symptoms of toxicity in mammals and insects (Van
den Bercken et al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980;
Glickman & Casida, 1982; Lawrence & Casida, 1982). The same
distinction was found in studies on cockroaches (Gammon et al.,
1981).
Based on the binding assay on the gamma-aminobutyric acid
(GABA) receptor-ionophore complex, synthetic pyrethroids can also
be classified into two types: the alpha-cyano-3-phenoxybenzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982;
Gammon & Casida, 1983; Lawrence & Casida, 1983; Lawrence et al.,
1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give
rise to pronounced repetitive activity in sense organs and in
sensory nerve fibres (Van den Bercken et al., 1973). At room
temperature, this repetitive activity usually consists of trains of
3-10 impulses and occasionally up to 25 impulses. Train duration
is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the
insecticide on neurotransmitter release or on the sensitivity of
the subsynaptic membrane, nor on the muscle fibre membrane.
Presynaptic repetitive firing was also observed in the sympathetic
ganglion treated with these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in sensory
nerve fibres, the pyrethroid-induced repetitive activity increases
dramatically as the temperature is lowered, and a decrease of 5 °C
in temperature may cause a more than 3-fold increase in the number
of repetitive impulses per train. This effect is easily reversed
by raising the temperature. The origin of this "negative
temperature coefficient" is not clear (Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve impulse. The
transitional state of the sodium channel is controlled by 2
separately acting gating mechanisms, referred to as the activation
gate and the inactivation gate. Since pyrethroids only appear to
affect the sodium current during depolarization, the rapid opening
of the activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is open,
the activation gate is restrained in the open position by the
pyrethroid molecule. While all pyrethroids have essentially the
same basic mechanism of action, however, the rate of relaxation
differs substantially for the various pyrethroids (Flannigan &
Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation
of the transient increase in sodium permeability of the nerve
membrane during excitation (Van den Bercken & Vijverberg, 1980).
Evidence so far available indicates that allethrin selectively
slows down the closing of the activation gate of a fraction of the
sodium channels that open during depolarization of the membrane.
The time constant of closing of the activation gate in the
allethrin-affected channels is about 100 milliseconds compared with
less than 100 microseconds in the normal sodium channel, i.e., it
is slowed down by a factor of more than 100. This results in a
marked prolongation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is directly
responsible for the repetitive activity induced by allethrin
(Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive
firing, and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral nervous
system of the frog. DDT also causes pronounced repetitive activity
in sense organs, in sensory nerve fibres, and in motor nerve
terminals, due to a prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation.
Recently, it was demonstrated that allethrin and DDT have
essentially the same effect on sodium channels in frog myelinated
nerve membrane. Both compounds slow down the rate of closing of a
fraction of the sodium channels that open on depolarization of the
membrane (Van den Bercken et al., 1973, 1979; Vijverberg et al.,
1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing after-potentials, and evoke repetitive firing with
minimal effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in
sodium permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl
alcohol (deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral line organ in the form of long-
lasting trains of impulses (Vijverberg et al., 1982a). Such a train
may last for up to 1 min and contains thousands of impulses. The
duration of the trains and the number of impulses per train
increase markedly on lowering the temperature. Cypermethrin does
not cause repetitive activity in myelinated nerve fibres. Instead,
this pyrethroid causes a frequency-dependant depression of the
nervous impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of depolarizing
after-potentials during train stimulation (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in
sodium permeability during excitation, presumably by slowing down
the closing of the activation gate of the sodium channel
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983). The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged sodium
current after cypermethrin is too small to induce repetitive
activity in nerve fibres, but is sufficient to cause the long-
lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a long-
lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
cray-fish, the Type II pyrethroids retain sodium channels in a
modified continuous open state persistently, depolarize the
membrane, and block the action potential without causing repetitive
firing (Lund & Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of
the Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant picrotoxin
(PTX). Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin
to rat brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible
relation between the Type II pyrethroid action and the GABA
receptor complex. The stereospecific correlation between the
toxicity of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand, [35S]- t-
butylbicyclophosphorothionate [35S]-TBPS.
Studies with 37 pyrethroids revealed an absolute correlation,
without any false positive or negative, between mouse intracerebral
toxicity and in vitro inhibition: all toxic cyano compounds
including deltamethrin, 1R, cis-cypermethrin, 1R, trans-cypermethrin,
and [2S,alpha]-fenval-erate were inhibitors, but their non-toxic
stereoisomers were not; non-cyano pyrethroids were much less potent
or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies
of pyrethroids were closely related to their mammalian toxicities.
The most toxic pyrethroids of Type II were the most potent
inhibitors of [3H]-Ro 5-4864 specific binding to rat brain
membranes. The [3H]-dihydropicrotoxin and [35S]-TBPS binding
studies with pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an interaction
with a GABA-regulated chloride ionophore-associated binding site.
Moreover, studies with [3H]-Ro 5-4864 support this hypothesis and,
in addition, indicate that the pyrethroid-binding site may be very
closely related to the convulsant benzodiazepine site of action
(Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin and
[2S, alphaS]-fenvalerate) increased the input resistance of
crayfish claw opener muscle fibres bathed in GABA. In contrast,
two non-insecticidal stereoisomers and Type I pyrethroids
(permethrin, resmethrin, allethrin) were inactive. Therefore,
cyanophenoxybenzyl pyrethroids appear to act on the GABA receptor-
ionophore complex (Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type II pyrethroids
(cypermethrin and deltamethrin) induced trains of repetitive
muscle action potentials without presynaptic repetitive activity.
However, an intermediate group of pyrethroids (1R-permethrin,
cyphenothrin, and fenvalerate) caused both types of effect. Thus,
in muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current. But no
clear distinction was observed between non-cyano and alpha-cyano
pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary
target site of pyrethroid insecticides in the vertebrate nervous
system is the sodium channel in the nerve membrane. Pyrethroids
without an alpha-cyano group (allethrin, d-phenothrin, permethrin,
and cismethrin) cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres,
and, in effect, nerve terminals. On the other hand, the alpha-
cyano pyrethroids cause a long-lasting prolongation of the
transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of
repetitive impulses in sense organs and a frequency-dependent
depression of the nerve impulse in nerve fibres. The difference in
effects between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-cyano
group on the phenoxybenzyl alcohol, indicates that it is this
alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation
and conduction are basically the same throughout the entire
nervous system, pyrethroids may also induce repetitive activity in
various parts of the brain. The difference in symptoms of
poisoning by alpha-cyano pyrethroids, compared with the classical
pyrethroids, is not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive activity
in sense organs and possibly in other parts of the nervous system,
which, in a more advance state of poisoning, may be accompanied by
a frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of
the nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane
is also the most important target site of pyrethroids in the
invertebrate nervous system (Wouters & Van den Bercken, 1978; WHO,
1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be
considered restricted to particular animal species, or to a certain
region of the nervous system. Although it has been established that
sense organs and nerve endings are the most vulnerable to the
action of pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions, and on the
chemical structure and physical characteristics of the pyrethroid
molecule (Vijverberg & Van den Bercken, 1982).
RESUME, EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques, méthodes d'analyse
La cyhalothrine s'obtient en estérifiant l'acide (chloro-2
trifluoro-3,3,3 propényl-1)-3 diméthyl-2,2 cyclopropanecarb-
oxylique, par l'alcool alpha-cyanophénoxy-3 benzylique et elle
consiste en un mélange de quatre stéréoisomères formant deux paires
d'énantiomères. La lambda-cyhalothrine est l'une de ces paires
d'énantiomères et constitue la forme la plus active biologiquement.
La cyhalothrine de qualité technique est un liquide visqueux
d'un jaune brunâtre (point de fusion environ 10 °C) qui contient
plus de 90% de matière active. Elle est constituée de quatre
isomères cis dans la proportion de 1:1:1:1. Elle est insoluble
dans l'eau mais soluble dans divers solvants organiques, en
particulier les hydrocarbures aliphatiques et aromatiques. Elle
est stable à la lumière et à la chaleur et possède une faible
tension de vapeur.
La lambda-cyhalothrine technique est un solide beige (point de
fusion 49,2 °C) contenant plus de 90% de matière active. Le
rapport des énantiomères (Z) (1R, 3R) de l'ester S aux énantiomères
(Z) (1S, 3S) de l'ester R est de 1:1. Peu soluble dans l'eau, ce
produit est soluble dans divers solvants organiques et possède une
faible tension de vapeur. La cyhalothrine et la lambda-cyhalothrine
sont rapidement hydrolysées en milieu alcalin mais ne subissent
aucune hydrolyse en milieu neutre ou acide.
On dispose de méthodes confirmées pour la recherche et le
dosage des résidus de cyhalothrine et de lambda-cyhalothrine ainsi
que pour l'analyse des prélèvements effectués dans l'environnement
(limite inférieure de détection: 0,005 mg/kg).
1.2 Production et usage
La cyhalothrine a été mise au point en 1977. On l'utilise
principalement pour détruire diverses espèces nuisibles en santé
publique et santé publique vétérinaire ainsi qu'en agriculture
contre les ravageurs des fruits à pépins. La lambda-cyhalothrine
est utilisée essentiellement en agriculture pour la protection des
récoltes les plus variées et fait actuellement l'objet d'une mise
au point en vue d'être utilisée en santé publique.
On ne dispose d'aucune donnée sur les quantités produites.
1.3 Exposition humaine
Les résidus dans les produits alimentaires, par suite du
traitement des récoltes par la cyhalothrine et la lambda-
cyhalothrine ainsi que de leur utilisation en médecine vétérinaire
sont faibles, généralement inférieurs à 0,2 mg/kg. On ignore quel
est l'apport total d'origine alimentaire chez l'homme mais on peut
supposer que l'exposition de la population générale par cette voie
ne dépasse pas la DJA (0,02 mg/kg de poids corporel).
1.4 Exposition et destinée dans l'environnement
A la surface du sol et en solution aqueuse à pH 5, la lambda-
cyhalothrine se décompose sous l'effet du rayonnement solaire avec
une demi-vie d'environ 30 jours. Les principaux produits de
décomposition sont l'acide (chloro-2 trifluoro-3,3,3 propényl-1)-3
diméthyl-2,2 cyclopropanecarboxylique, l'amide correspondant et
l'acide phénoxy-3 benzoïque.
Dans le sol, la dégradation s'effectue principalement par
hydroxylation puis rupture de la liaison ester aboutissant à deux
produits principaux dont la dégradation se poursuit jusqu'à
obtention de dioxyde de carbone. La demi-vie initiale varie de 22 à
82 jours.
La cyhalothrine et la lambda-cyhalothrine sont adsorbées sur
les particules du sol et ne se déplacent pas dans l'environnement.
Sur les végétaux, la lambda-cyhalothrine se décompose à vitesse
modérée (demi-vie allant jusqu'à 40 jours) de sorte que les résidus
sont principalement constitués du composé initial. On trouve
également des métabolites en faibles quantités qui résultent d'un
certain nombre de réactions d'hydrolyse et d'oxydation.
On ne dispose d'aucune donnée sur les quantités effectivement
présentes dans l'environnement mais compte tenu du fait que ce
produit est relativement peu utilisé et à doses réduites, ces
quantités sont vraisemblablement faibles.
1.5 Absorption, métabolisme, et excrétion
Des études métaboliques ont été effectuées sur des rats, des
chiens, des vaches et des chèvres. Chez les rats et les chiens, on
a montré que la cyhalothrine administrée par voie orale était bien
absorbée, fortement métabolisée, puis éliminée sous forme de
conjugués polaires dans les urines. Chez le rat, on a constaté que
le taux de cyhalothrine tissulaire diminuait après cessation de
l'exposition. Dans les carcasses, les résidus étaient faibles
(moins de 5% de la dose au bout de sept jours) et consistaient
presque entièrement en cyhalothrine accumulée dans le tissu
adipeux. Les résidus présents dans les tissus adipeux étaient
éliminés avec une demi-vie de 23 jours.
Après administration par voie orale de cyhalothrine à des
vaches en lactation, on a constaté que le produit était rapidement
éliminé et qu'un état d'équilibre s'établissait au bout de trois
jours entre ingestion et élimination. Vingt-sept pour cent de la
dose étaient excrétés dans les urines, 50% dans les matières
fécales et 0,8% dans le lait. Les produits retrouvés dans les
urines étaient entièrement constitués de métabolites résultant du
clivage de l'ester et de leurs conjugués, alors que 60 à 70% des
produits marqués au 14C retrouvés dans les matières fécales
consistaient en cyhalothrine non métabolisée. Les résidus
tissulaires, mesurés 16 heures après l'administration de la
dernière dose, étaient faibles, la concentration la plus élevée
étant enregistrée dans le tissu adipeux. Les résidus marqués au 14C
retrouvés dans le lait et les tissus adipeux étaient presque
entièrement constitués de cyhalothrine non métabolisée, à
l'exclusion de tout autre composé.
Chez toutes les espèces mammaliennes étudiées, on a constaté
que la cyhalothrine subissait une forte métabolisation par clivage
de l'ester en acide cyclopropanecarboxylique et acide phénoxy-3
benzoïque et qu'elle était éliminée sous forme de conjugués.
Chez les poissons, les principaux résidus tissulaires
consistent en cyhalothrine non métabolisée à côté de quantités plus
faibles de produits résultant du clivage de l'ester.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Dans les conditions du laboratoire où la concentration est
constante, la cyhalothrine et la lambda-cyhalothrine se révèlent
très toxiques pour les poissons et les invertébrés aquatiques. Les
valeurs de la CL50 à 96 heures pour les poissons vont de 0,2 à 1,3
µg/litre, la CL50 à 48 heures pour les invertébrés aquatiques se
situant entre 0,008 et 0,04 µg/litre.
En étudiant au laboratoire l'accumulation de la cyhalothrine à
concentration constante, on a observé une absorption rapide par les
poissons (facteur d'accumulation d'environ 1000 à 2000). Toutefois
lorsqu'il y a un sol et des sédiments en suspension, on constate
une forte réduction du facteur de bioaccumulation, qui passe à 19
pour les poissons et à 194 pour les daphnies. Lorsqu'on remet les
poissons et les daphnies dans de l'eau propre, on constate une
diminution rapide des résidus, avec une demi-vie respective de 7 et
1 jours. Il est vraisemblable qu'en utilisation agricole normale,
la cyhalothrine et la lambda-cyhalothrine subsistent à faible
concentration dans l'eau. Etant donné que ces composés sont
rapidement adsorbés et dégradés dans les conditions naturelles, il
ne se pose en pratique aucun problème d'accumulation de résidus ni
de toxicité vis-à-vis des espèces aquatiques.
La cyhalothrine et la lambda-cyhalothrine sont pratiquement
inoffensives pour les oiseaux; la DL50 (dose unique) s'est révélée
supérieure à 3950 mg/kg pour toutes les espèces étudiées et la CL50
alimentaire à cinq jours la plus faible a été de 3948 mg/kg pour
des canards sauvages de huit jours qui recevaient de la lambda-
cyhalothrine dans leur alimentation.
Dans les conditions du laboratoire, la cyhalothrine et la
lambda-cyhalothrine sont toxiques pour les abeilles; la DL50 par
voie orale est de 0,97 µg de lambda-cyhalothrine par abeille.
Toutefois, le risque est probablement moins élevé en pratique étant
donné que les formulations actuellement en usage exercent une
action répulsive qui empêche les abeilles de butiner sur les
récoltes traitées. Lorsque les abeilles se remettent à butiner, on
ne constate pas d'augmentation importante de la mortalité.
1.7 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
La toxicité aiguë par voie orale de la cyhalothrine est modérée
chez les rats et les souris et faible chez les cobayes et les
lapins (les valeurs de la DL50 sont les suivantes: rat, 144-243
mg/kg; souris, 37-62 mg/kg; cobaye, plus de 5000 mg/kg; lapin, plus
de 1000 mg/kg). La toxicité aiguë par voie orale de la lambda-
cyhalothrine est plus forte que celle de la cyhalothrine (les
valeurs de la DL50 sont les suivantes: 56-79 mg/kg pour le rat et
20 mg/kg pour la souris). Par voie dermique, on obtient les
toxicités suivantes exprimées par la DL50: rat, 200-2000 mg/kg
(cyhalothrine), 632-696 mg/kg (lambda-cyhalothrine); lapin, plus
de 2000 mg/kg (cyhalothrine). La cyhalothrine et la lambda-
cyhalothrine sont des pyréthroïdes du type II avec pour signes
cliniques d'intoxication une ataxie, une démarche chancelante et
une hyperexcitabilité.
Chez le lapin, la cyhalothrine est modérément irritante pour
l'oeil et la lambda-cyhalothrine peu irritante; les deux composés
sont légèrement irritants pour la peau. La cyhalothrine n'est pas
irritante pour la peau chez le rat. Toutefois elle provoque une
sensibilisation cutanée modérée chez le cobaye. La lambda-
cyhalothrine n'a pas d'action sensibilisatrice sur la peau.
Lors d'une étude d'alimentation de 90 jours au cours de
laquelle des rats ont reçu de la cyhalothrine à des doses allant
jusqu'à 250 mg/kg de nourriture, on a observé une réduction du gain
de poids chez les mâles à la dose la plus forte. Des effets
marginaux sur la valeur moyenne de l'hématocrite ont été
enregistrés chez certains des groupes traités ainsi que quelques
modifications au niveau du foie que l'on a considérées comme
résultant d'une réaction d'adaptation. Lors d'une étude de 90
jours au cours de laquelle des rats ont reçu dans leur alimentation
de la lambda-cyhalothrine à des doses allant jusqu'à 250 mg/kg de
nourriture, on a observé une réduction du gain de poids chez les
deux sexes à la dose la plus forte. On a observé un certain nombre
d'effets sur le chimisme sanguin ainsi que des effets hépatiques
analogues à ceux qui avaient été observés avec la cyhalothrine. La
dose sans effet observable était de 50 mg/kg de nourriture.
Lors d'une étude de 26 semaines au cours de laquelle de la
cyhalothrine a été administrée par voie orale à des chiens à des
doses quotidiennes allant jusqu'à 10 mg/kg de poids corporel, on a
observé des signes d'intoxication de type pyréthroïde à la dose la
plus forte. La dose sans effet observable se situait à 2,5 mg/kg
de poids corporel. Une étude du même genre a été menée sur d'autres
chiens avec administration pendant 52 semaines de 3,5 mg de lambda-
cyhalothrine par kg de poids corporel et par jour. Des signes
cliniques d'intoxication de type pyréthroïde (signes neurologiques)
ont été observés chez tous les ani-maux à la dose quotidienne de
3,5 mg/kg de poids corporel. La dose sans effet observable se
situait à 0,5 mg/kg de poids corporel par jour.
Lors d'une étude de toxicité cutanée de 21 jours pratiquée sur
des lapins au moyen d'une solution de cyhalothrine dans le
polyéthylène-glycol à des doses quotidiennes allant jusqu'à 1000
mg/kg de poids corporel, on a constaté des signes cliniques
d'intoxication chez certains des animaux à la dose la plus forte.
Dans tous les groupes, y compris les témoins, on a observé une
irritation cutanée légère à sévère.
Deux études toxicologiques de 104 semaines ont été consacrées à
la cyhalothrine, l'une sur des rats et l'autre sur des souris.
L'étude sur le rat n'a révélé aucun effet oncogène à des doses
allant jusqu'à 250 mg/kg de nourriture (dose la plus forte
étudiée). La dose sans effet observable en ce qui concerne la
toxicité générale était de 50 mg/kg de nourriture (1,8 mg/kg de
poids corporel par jour). On a relevé une diminution du gain de
poids corporel chez les deux sexes à la dose de 250 mg/kg de
nourriture. Aucun effet oncogène n'a été observé chez la souris à
des doses allant jusqu'à 500 mg/kg de nourriture (dose la plus
forte étudiée). Les signes cliniques d'une intoxication de type
pyréthroïde ont été observés aux doses de 100 et 500 mg/kg de
nourriture, avec réduction du gain de poids corporel à cette
dernière dose. En ce qui concerne la toxicité générale, la dose
sans effet observable était de 20 mg/kg de nourriture (1,9 mg/kg de
poids corporel par jour). Aucun signe histologique de lésion du
système nerveux n'a été observé dans l'une ou l'autre de ces deux
études.
Lors d'une série d'épreuves in vivo et in vitro visant à
déceler des mutations géniques, des lésions chromosomiques et
autres effets génotoxiques, on n'a observé aucun effet de ce type
imputable à la cyhalothrine ou à la lambda-cyhalothrine.
Administrée par voie orale à des rats et à des lapins au cours de
la période cruciale de l'organogénèse, la cyhalothrine ne s'est
révélée ni embryotoxique ni tératogène à des doses quotidiennes qui
étaient toxiques pour la mère (15 mg/kg pour les rats et 30 mg/kg
pour les lapins, c'est-à-dire les deux doses les plus fortes
étudiées).
Lors d'une étude de reproduction portant sur trois générations,
des rats ont reçu de la cyhalothrine dans leur alimentation à des
doses allant jusqu'à 100 mg/kg de nourriture. A la dose de 100
mg/kg de nourriture on a observé une légère diminution de la taille
des portées et une réduction peu importante du gain de poids. En
ce qui concerne les effets sur la reproduction, la dose sans effet
observable était de 30 mg/kg de nourriture.
1.8 Effets sur l'homme
Aucun cas d'intoxication accidentelle n'a été décrit.
Des sensations subjectives au niveau de la face ont été
ressenties lors de la fabrication, de la formulation, du travail de
laboratoire et de l'utilisation sur le terrain. Cet effet ne dure
en général que quelques heures mais il arrive qu'il se prolonge
jusqu'à 72 heures après l'exposition; l'examen médical n'a pas
révélé d'anomalies neurologiques.
Ces sensations cutanées subjectives au niveau de la face, qui
sont ressenties par les personnes qui manipulent de la cyhalothrine
ou de la lambda-cyhalothrine sont, semble-t-il, provoquées par
l'excitation répétée des terminaisons nerveuses de la peau. On
peut les considérer comme un signal d'alarme qui indique une
surexposition de l'épiderme.
Rien n'indique que la cyhalothrine et la lambda-cyhalothrine
puissent avoir des effets nocifs pour l'homme lorsqu'on les utilise
dans les conditions et aux doses qui sont recommandées
actuellement.
2. Conclusions
a) Population générale: l'exposition de la population générale à la
cyhalothrine et à la lambda-cyhalothrine est vraisemblablement très
faible et ne présente probablement aucun danger lorsque ces
produits sont utilisés conformément aux recommandations.
b) Exposition professionnelle: moyennant de bonnes méthodes de
travail, des mesures d'hygiène et pour peu que l'on respecte les
précautions nécessaires, la cyhalothrine et la lambda-cyhalothrine
ne présentent vraisemblablement aucun danger pour les personnes
exposées de par leur profession.
c) Environnement: il ne semble pas que la cyhalothrine, la lambda-
cyhalothrine ou leurs produits de dégradation puissent atteindre
des concentrations nocives pour l'environnement lorsqu'elles sont
utilisées aux doses recommandées. Dans les conditions du
laboratoire, la cyhalothrine et la lambda-cyhalothrine sont très
toxiques pour les poissons, les arthropodes aquatiques et les
abeilles. Toutefois, en pratique, il ne semble pas qu'il puisse se
produire d'effets nocifs durables, lorsque ces insecticides sont
utilisés conformément aux recommandations.
3. Recommandations
Lorsque ces insecticides sont utilisés conformément aux
recommandations, leurs concentrations dans les denrées alimentaires
doivent être très faibles, toutefois il serait bon de le confirmer
en soumettant la cyhalothrine et la lambda-cyhalothrine à une
surveillance.
La cyhalothrine et la lambda-cyhalothrine sont utilisées
depuis des années et seuls des effets passagers ont été observés
lors d'expositions professionnelles; toute-fois il faut continuer à
surveiller l'exposition humaine.
RESUMEN, EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades físicas y químicas, y métodos
analíticos
La cihalotrina se obtiene esterificando ácido 3-(2-cloro-3,3,3-
trifluoroprop-1-enil)-2,2-dimetilciclopropanocarboxílico con
alcohol alpha-ciano-3-fenoxibencílico y se compone de una mezcla de
cuatro estereoisómeros. La lambda-cihalotrina se compone de un par
enantiomérico de isómeros y es la forma más activa biológicamente.
La cihalotrina de calidad técnica es un líquido viscoso, de
color entre amarillo y marrón (punto de fusión: aproximadamente 10
°C), que contiene más del 90% de material activo. Está compuesta
por cuatro isómeros cis en proporción 1:1:1:1. Aunque es insoluble
en el agua, es soluble en toda una gama de disolventes orgánicos
como los hidrocarburos alifáticos y aromáticos. Es estable respecto
de la luz y la temperatura y tiene una baja tensión de vapor.
La lambda-cihalotrina de calidad técnica es un sólido de color
café con leche (punto de fusión: 49,2 °C) que contiene más del 90%
de material activo. La razón enantiomérica del ( Z), (1R, 3R), S-
éster y el ( Z), (1S, 3S), R-éster es 1:1. Es apenas soluble en el
agua pero es soluble en toda una gama de disolventes orgánicos y
tiene una baja tensión de vapor. Tanto la cihalotrina como la
lambda-cihalotrina se hidrolizan rápidamente en medio alcalino pero
no en medio neutro o ácido.
Existen métodos bien establecidos para analizar la cihalotrina
y la lambda-cihalotrina presentes en residuos y en el medio
ambiente (la concentración detectable mínima es 0,005 mg/kg).
1.2 Producción y utilización
La cihalotrina comenzó a producirse en 1977. Se utiliza sobre
todo para combatir múltiples plagas en salud pública y en
veterinaria, pero también se emplea en agricultura contra las
plagas que atacan a los pomos. La lambda-cihalotrina se usa
principalmente como plaguicida agrícola aplicable a muy diversos
cultivos y se está preparando para emplearla en salud pública.
No se dispone de datos sobre los niveles de producción.
1.3 Exposición humana
Los residuos presentes en los alimentos de resultas de la
aplicación de cihalotrina y lamba-cihalotrina a los cultivos y en
veterinaria son bajos, por lo general inferiores a 0,2 mg/kg. No
se dispone de datos sobre la ingesta total en la dieta humana, pero
se puede suponer que la exposición dietética de la población
general no supera la IDA (0,02 mg/kg de peso corporal).
1.4 Exposición y transformación en el medio ambiente
En la superficie del suelo y en soluciones acuosas con un pH de
5, la lambda-cihalotrina se degrada a la luz del sol con una
semivida de unos 30 días. Los principales productos de esa
degradación son ácido 3-(2-cloro-3,3,3-trifluoroprop-1-enil)-2,2-
dimetilciclopropanocarboxílico, el derivado amídico de la
cihalotrina, y ácido 3-fenoxibenzoico.
La degradación en el suelo se produce primordialmente por
hidroxilación seguida de la ruptura del enlace éster para dar lugar
a dos principales productos de degradación, que siguen degradándose
hasta convertirse en bióxido de carbono. Las semividas iniciales
oscilan entre 22 y 82 días.
La cihalotrina y la lambda-cihalotrina se adsorben a las
partículas del suelo y no son móviles en el medio ambiente.
En las plantas, la lambda-cihalotrina se degrada a un ritmo
moderado (semivida de hasta 40 días), por lo que el principal
elemento constituyente de los residuos que en ellas se encuentran
es por lo común el compuesto inicial. Se hallan también niveles más
bajos de metabolitos, resultantes de diversas reacciones
hidrolíticas y oxidantes.
No se dispone de datos sobre los niveles efectivos en el medio
ambiente, pero dadas la escasa utilización habitual en la
actualidad y las reducidas tasas de aplicación, se supone que son
bajos.
1.5 Incorporación, metabolismo y excreción
Se han realizado estudios metabólicos en la rata, el perro, la
vaca y la cabra. En la rata y el perro se ha demostrado que la
cihalotrina administrada por vía oral se absorbe y se metaboliza
bien y se elimina en la orina, en forma de conjugados polares. En
los tejidos de las ratas, disminuyó la concentración de cihalotrina
al interrumpirse la exposición al compuesto. En los cadáveres de
las ratas se encontraron niveles reducidos de residuos (< 5% de la
dosis a los siete días), que se debían casi totalmente a la
cihalotrina contenida en las grasas. Los restos presentes en las
grasas se eliminaron con una semivida de 23 días.
La cihalotrina administrada por vía oral a vacas lactantes se
eliminó rápidamente, alcanzándose un equilibrio entre la ingestión
y la eliminación a los tres días. De la dosis total, el 27% se
excretó en la orina, el 50% en las heces, y el 0,8% en la leche.
El material presente en la orina estaba compuesto exclusivamente
por metabolitos de la disociación de los ésteres y por sus
conjugados, mientras que del 60% al 70% del material fecal marcado
con [14C] se identificó como cihalotrina no alterada. Dieciséis
horas después de la última dosis, los residuos tisulares eran
bajos, hallándose las concentraciones más elevadas en las grasas.
Los residuos marcados con [14C] presentes en la leche y los tejidos
adiposos constaban casi enteramente de cihalotrina no alterada, y
no se detectó ningún otro componente.
En todas las especies de mamíferos investigadas, se ha
descubierto que la cihalotrina se metaboliza ampliamente de
resultas de la ruptura del enlace éster para dar ácido
ciclopropanocarboxílico y ácido 3-fenoxibenzoico, y se elimina en
forma de conjugados.
En los peces, el principal residuo presente en los tejidos es
cihalotrina no alterada y hay niveles más bajos de los productos de
la ruptura del éster.
1.6 Efectos en los organismos del medio ambiente
En condiciones de laboratorio con concentraciones tóxicas
constantes, la cihalotrina y la lambda-cihalotrina son muy tóxicas
para los peces y los invertebrados acuáticos. El valor de las CL50
a las 96 horas para los peces oscila entre 0,2 y 1,3 µg/litro,
mientras que en el caso de los invertebrados acuáticos los valores
de la CL50 a las 48 horas van de 0,008 a 0,4 µg/litro.
Los estudios sobre la acumulación realizados en condiciones de
laboratorio con concentraciones constantes indican que, en los
peces, tiene lugar una rápida incorporación (factor de acumulación
aproximado de 1000 a 2000). No obstante, en presencia de suelo y
sedimentos en suspensión, los factores de bioacumulación quedan muy
reducidos (a 19 en el caso de los peces y a 194 en el caso de los
dáfnidos). Cuando se colocó en agua limpia a los peces y dáfnidos
expuestos, los residuos disminuyeron rápidamente, con semividas de
siete días y un día, respectivamente. Las concentraciones de
cihalotrina y lambda-cihalotrina que pueden hallarse en el agua de
resultas de su aplicación normal con fines agrícolas serán bajas.
Como el compuesto se adsorbe y se degrada rápidamente en
condiciones naturales, la acumulación de residuos o la toxicidad de
la cihalotrina y la lambda-cihalotrina para las especies acuáticas
no plantearán problemas prácticos.
La cihalotrina y la lambda-cihalotrina son prácticamente
inocuas para las aves; en todas las especies sometidas a prueba la
DL50 con una dosis única fue superior a 3950 mg/kg y la CL50
dietética más baja en cinco días fue de 3948 mg/kg (lambda-
cihalotrina administrada en el alimento a patos silvestres de ocho
días de edad).
En condiciones de laboratorio, la cihalotrina y la lambda-
cihalotrina son tóxicas para la abeja; la DL50 de la lambda-
cihalotrina por vía oral es de 0,97 µg/abeja. No obstante, en la
práctica, el riesgo es menor puesto que las formas actualmente
utilizadas tienen un efecto repelente que hace que las abejas
suspendan las actividades de búsqueda en los cultivos tratados.
Cuando éstas vuelven a emprenderse, no hay un aumento significativo
de la mortalidad de las abejas.
1.7 Efectos en animales de experimentación y en sistemas de
prueba in vitro
La toxicidad aguda de la cihalotrina administrada por vía oral
es moderada para las ratas y los ratones y baja para las cobayas y
los conejos (los valores de la DL50 son los siguientes: rata: 144-
243 mg/kg; ratón: 37-62 mg/kg; cobaya: > 5000 mg/kg; conejo: >
1000 mg/kg). La toxicidad aguda de la lambda-cihalotrina por vía
oral es mayor que la de la cihalotrina (los valores de la DL50 son:
56-79 mg/kg para la rata y 20 mg/kg para el ratón). La toxicidad
por vía cutánea (DL50) alcanza los siguientes valores: rata: 200-
2000 mg/kg (cihalotrina), 632-696 mg/kg (lambda-cihalotrina);
conejo: > 2000 mg/kg (cihalotrina). La cihalotrina y la lambda-
cihalotrina son piretroides de tipo II; entre los signos clínicos
figuran la ataxia, el andar vacilante y la hiperexcitabilidad.
En el conejo, la cihalotrina es un irritante ocular moderado y
la lambda-cihalotrina un irritante ocular leve; ambos irritan
levemente la piel. La cihalotrina no irrita la piel de la rata.
Sin embargo, sensibiliza moderadamente la piel de la cobaya. La
lambda-cihalotrina no sensibiliza la piel.
En un estudio de alimentación de 90 días en el que se
administró cihalotrina a ratas en dosis de hasta 250 mg/kg de
alimento, se observó una disminución del aumento del peso corporal
en los machos con la dosis de 250 mg. En algunos de los grupos
tratados, se señalaron efectos marginales en los volúmenes medios
de eritrocitos, así como ciertas alteraciones hepáticas, que se
consideraron una respuesta adaptativa. En otro estudio de
alimentación de 90 días, en el que se administró lambda-cihalotrina
a ratas en dosis de hasta 250 mg/kg de alimento, se observó en
ambos sexos una reducción del aumento del peso corporal con la
dosis de 250 mg. Se señalaron también algunos efectos en la
química clínica, así como efectos hepáticos análogos a los
observados con la cihalotrina. El nivel sin efecto observado fue
de 50 mg/kg de alimento.
En un estudio de 26 semanas en el que se administraron a perros
por vía oral dosis diarias de cihalotrina de hasta 10 mg/kg de peso
corporal, se observaron signos de toxicidad por piretroides con la
dosis de 10 mg. El nivel sin efecto observado fue de 2,5 mg/kg de
peso corporal al día. Se realizó otro estudio similar en el que se
administraron a perros, durante 52 semanas, dosis diarias de
lambda-cihalotrina de hasta 3,5 mg/kg de peso corporal. En todos
los animales que recibieron la dosis de 3,5 mg se observaron signos
clínicos de toxicidad por piretroides (signos neurológicos). El
nivel sin efecto observado fue de 0,5 mg/kg de peso corporal al
día.
En un estudio cutáneo de 21 días con conejos a los que se
aplicó cihalotrina en polietilénglicol en dosis diarias de hasta
1000 mg/kg, se observaron en algunos animales signos clínicos de
toxicidad con la dosis más alta. En todos los grupos, incluidos
los testigos, se señaló irritación de la piel de leve a grave.
Se realizaron dos estudios de alimentación de 104 semanas con
cihalotrina, uno con ratas y otro con ratones. En el estudio con
ratas, no se observaron efectos oncogénicos con dosis de hasta 250
mg/kg de alimento (la dosis más alta ensayada). En cuanto a la
toxicidad sistémica, el nivel sin efecto observado fue de 50 mg/kg
de alimento (1,8 mg/kg de peso corporal al día). Con dosis de 250
mg se señaló en ambos sexos una disminución del aumento del peso
corporal. En el estudio con ratones, no se observaron efectos
oncogénicos con dosis de hasta 500 mg/kg de alimento (la dosis más
alta ensayada). Con dosis de 100 y 500 mg/kg de alimento se
apreciaron signos clínicos de toxicidad por piretroides y, con
dosis de 500 mg, una disminución del aumento del peso corporal. En
cuanto a la toxicidad sistémica, el nivel sin efecto observado fue
de 20 mg/kg de alimento (1,9 mg/kg de peso corporal al día). En
ninguno de los dos estudios se obtuvieron datos histológicos que
indicaran la presencia de lesiones del sistema nervioso.
En una serie de pruebas in vivo e in vitro destinadas a
detectar mutaciones génicas, lesiones cromosómicas y otros efectos
genotóxicos, se obtuvieron resultados negativos con la cihalotrina
y la lambda-cihalotrina. La cihalotrina administrada por vía oral
a ratas y conejos durante el periodo de organogénesis principal no
resultó embriotóxica ni teratogénica en dosis que provocaron
reacciones tóxicas en las madres (15 mg/kg diarios para las ratas y
30 mg/kg diarios para los conejos, en ambos casos los niveles más
altos ensayados).
Se realizó un estudio sobre la reproducción en tres
generaciones de ratas a las que se administró cihalotrina en dosis
de hasta 100 mg/kg de alimento. Con la dosis de 100 mg se
observaron pequeñas reducciones del tamaño de las camadas y del
aumento de peso. En los aspectos relacionados con la reproducción,
el nivel sin efecto observado fue de 30 mg/kg de alimento.
1.8 Efectos en el ser humano
No se ha descrito ningún caso de intoxicación accidental.
En las actividades de fabricación, formulación y laboratorio y
durante el uso sobre el terreno, se han notificado sensaciones
faciales subjetivas. Este efecto sólo suele durar unas horas, pero
ocasionalmente persiste hasta 72 horas después de la exposición; el
examen médico no ha revelado ninguna anomalía neurológica.
Se cree que las sensaciones cutáneas faciales subjetivas que
pueden experimentar las personas que manipulan cihalotrina y
lambda-cihalotrina se deben a la repetida estimulación de las
terminaciones nerviosas de la piel. Pueden considerarse una señal
de alerta que indica que la piel ha sufrido una exposición
excesiva.
No hay ninguna indicación de que la cihalotrina y la lambda-
cihalotrina, utilizadas en las condiciones y con arreglo a las
tasas de aplicación actualmente recomendadas, tengan efectos
adversos en los seres humanos.
2. Conclusiones
(a) Población general: Se cree que la exposición de la población
general a la cihalotrina y la lambda-cihalotrina es muy baja y que
no es probable que represente un riesgo en las condiciones de uso
recomendadas.
(b) Exposición ocupacional: Con prácticas de trabajo, medidas de
higiene y precauciones de seguridad adecuadas, es poco probable que
la cihalotrina y la lambda-cihalotrina representen un riesgo para
las personas ocupacionalmente expuestas.
(c) Medio ambiente: No es probable que la cihalotrina y la lambda-
cihalotrina o los productos de su degradación alcancen niveles
ambientales que puedan producir efectos adversos, si se respetan
las tasas de aplicación recomendadas. En condiciones de
laboratorio, la cihalotrina y la lambda-cihalotrina son muy tóxicas
para los peces, los artrópodos acuáticos y las abejas. No
obstante, en la práctica, no es probable que se produzcan efectos
adversos duraderos en las condiciones de uso recomendadas.
3. Recomendaciones
Aunque se cree que los niveles dietéticos según el uso
recomendado son muy bajos, debe considerarse la posibilidad de
confirmar este extremo incluyendo la cihalotrina y la lambda-
cihalotrina en estudios de vigilancia.
Pese a que ambas sustancias se llevan utilizando varios años y
a que los efectos observados de la exposición ocupacional sólo han
sido transitorios, debe continuar la observación de la exposición
humana.
 Chemical name: alpha-cyano-3-phenoxybenzyl 3-(2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-dimethyl-
cyclopropanecarboxylate
CAS Chemical ( RS)-alpha-cyano-3-(phenoxyphenyl)methyl
name: ( 1RS)-cis-3-( Z-2-chloro-3,3,3-tri-
fluoroprop-1-enyl)-2,2-dimethylcyclopro-
panecarboxylate
CAS registry: cyhalothrin:
Chemical name: alpha-cyano-3-phenoxybenzyl 3-(2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-dimethyl-
cyclopropanecarboxylate
CAS Chemical ( RS)-alpha-cyano-3-(phenoxyphenyl)methyl
name: ( 1RS)-cis-3-( Z-2-chloro-3,3,3-tri-
fluoroprop-1-enyl)-2,2-dimethylcyclopro-
panecarboxylate
CAS registry: cyhalothrin:  Lambda-cyhalothrin is manufactured by crystallization of the
more active pair of enantiomers from cyhalothrin. The less active
pair of enantiomers is recycled.
Pure lambda-cyhalothrin is a racemic mixture of the enantiomer
pair B isomers. The enantiomer pair A is present in low
concentration in the commercial product.
Technical grade cyhalothrin contains more than 90% of the
pesticide and is formulated in 5%, 10%, and 20% emulsifiable
concentrates. Technical grade lambda-cyhalothrin also contains
more than 90% active ingredient. It is formulated as 2.5%, 5.0%,
8.3%, and 12% emulsifiable concen-trates and as a 0.8% ultra-low
volume concentrate.
2.2. Physical and chemical properties
Some physical and chemical properties of cyhalothrin and
lambda-cyhalothrin are listed in Table 1.
Table 1. Some physical and chemical properties of cyhalothrin and
lambda-cyhalothrin
---------------------------------------------------------------------------
Cyhalothrin Lambda-cyhalothrin
---------------------------------------------------------------------------
Physical state viscous liquid solid
Colour yellow-brown beige
Odour mild mild
Relative molecular mass 449.9 449.9
Melting point glass-like 49.2 °C
below 10 °C
Decomposes > 275 °C > 275 °C
Water solubility 4 x 10-3 mg/litre 5 x 10-3 mg/litre
Solubility in organic solvents soluble soluble
n-octanol water-partition 6.9 7.0
coefficient (log Pow) at 20 °C
Relative density 1.25 1.33
Vapour pressure at 20 °C 1 x 10-9 kPa 2 x 10-10 kPa
Vapour pressure at 80 °C 4 x 10-6 kPa 3 x 10-6 kPa
---------------------------------------------------------------------------
No boiling point data are available as both forms decompose on
heating above 275 °C. Cyhalothrin is highly stable to light and at
temperatures below 220 °C.
Lambda-cyhalothrin is stable in water at pH 5. At pH 7 and pH
9, there is racemization at the alpha-cyano carbon to yield a 1:1
mixture of enantiomer pairs A and B. At pH 9, the ester bond is
fairly readily hydrolysed (half-life, 7 days) (Collis & Leahey,
1984).
Dilute aqueous solutions are subject to photolysis at a
moderate rate (Hall & Leahey, 1983; Curl et al., 1984a).
2.3. Analytical methods
The most widely adopted procedures for analysing cyhalothrin
residues in crops, soil, animal tissues and products, and
environmental samples are based on extraction of the residue with
organic solvent, clean-up of the extract by solvent-solvent
partition and adsorption column chromatography, and determination
of the residue using gas chromatography (GC) with electron capture
detection (GC/ECD). The identity of residues can be confirmed by
GC with mass selective detection (GC-MSD) or by thin-layer
chromatography (TLC) followed by GC/ECD.
2.3.1. Sampling methods
Procedures for obtaining representative samples of crops,
processed commodities, soil, and some animal products have been
described in detail (GIFAP, 1981) and will not be discussed
further.
Particular care is necessary when sampling water because
cyhalothrin is extremely hydrophobic and rapidly adsorbs onto
particulates or container walls from aqueous solution. For this
reason, the whole analytical sample should be taken for analysis
and not subdivided in the field (Sapiets et al., 1984). Collection
of the sample in a clean glass container, with addition of the
extraction solvent before sealing and shaking the bottle, gives
good recoveries. Crossland et al. (1982) described procedures for
sampling surface water (using stainless steel fine mesh discs) and
subsurface water from ponds for separate analysis of cypermethrin.
Precautions were taken to avoid contamination during sampling
(Crossland et al., 1982). These methods, developed for
cypermethrin, are equally applicable to cyhalothrin and other
pyrethroids. During the course of the pond studies, cypermethrin
spray drift deposits were collected by means of horizontally placed
aluminium foil plates and washed from the foils using acetone.
Crossland also described the use of a core sampler to remove pond
sediment for cypermethrin analysis (Crossland, 1982). This method
is equally applicable to cyhalothrin.
Sampling of air in agriculture work space for pyrethroid
aerosol droplets using absorption onto exposed filter papers and
porous glass slides has been described by Girenko & Klisenko
(1984). They quoted limits in the range of 0.05 to 0.5 mg/m3 for
the concentrations of pyrethroid that could be detected without
interference from organophosphorus or organochlorine insecticides.
An alternative and more conventional approach would be to use a
pumped device consisting of a filter (to trap droplets or
particulates) in series with a packed tube (to trap vapour).
The use of a vacuum probe was described by Bengstone et al.
(1983) to subsample pyrethroid-treated grain from several points
within a silo for combination to give a composite sample. However,
care is needed to obtain a representative sample using this
procedure (GIFAP, 1981).
2.3.2. Sample storage
Storage stability experiments using untreated samples fortified
with cyhalothrin (at 1.0 mg/kg) have shown that apples, cabbage,
soil, and products of animal origin can be stored deep frozen at
temperatures of -20 °C for periods of up to one year without
residue loss (Sapiets, 1984a)
Particular problems in the handling of water samples have
already been indicated and samples should be extracted and
analysed as soon after sampling as possible.
2.4. Sample preparation
Forbes & Dutton (1985) reported details of the procedures used
to process crop and soil samples, which have been treated with
pyrethroid insecticides, for sub-sampling before extraction. Crops
of high water content, e.g., fruit and vegetables, were chopped,
dried, or puréed. Grain and oil seed crops (cotton seed and
linseed) were frozen and ground to a powder, while crops of low
water content, e.g., straw and tobacco, were finely divided in a
rotary knife mill. Soil samples were mixed thoroughly and stones
and plant debris removed. In all cases, care was taken to avoid
localized overheating of the sample during processing. Sapiets et
al. (1984) used a similar processing procedure for high water
content crops and also applied this to meat and eggs. However,
they draw attention to the importance of processing materials with
high water content while still frozen to prevent separation of
juice, which leads to sample inhomogeneity. Milk was thoroughly
mixed before subsampling. In the limited number of other studies
where details for preparation of pyrethroid-treated samples have
been given, none of the procedures differ markedly from those
described above. All of the procedures are equally applicable to
cyhalothrin and lambda-cyhalothrin.
2.5. Gas chromatographic procedures for the determination
of cyhalothrin residues
Details of GC procedures are described in the following
subsections (Sapiets, 1984b,c, 1985a,b, 1986a,b).
2.5.1. Extraction
Representative subsamples of prepared crops or meat are blended
for 2-5 min with a mixture of acetone and hexane (1 + 1 by volume).
Dry materials are dampened with water. Soil is similarly extracted
by refluxing with acetonitrile for 1 h. Extracts are then gravity
filtered and partitioned with 5% sodium chloride solution to remove
the acetone. The hexane extract is dried over anhydrous sodium
sulfate.
Eggs are extracted by homogenizing for 5 min with acetonitrile.
An aliquot is evaporated to dryness and redissolved in hexane.
Milk is extracted by homogenizing for 2 min in acetone and
hexane (1:1 by volume). The solvent is dried over anhydrous sodium
sulfate.
2.5.2. Clean-up
Column absorption chromatography on Florisil is used for
cleaning up extracts from crops and soils, and cyhalothrin
residues are eluted with a mixture of diethyl ether in hexane.
Extracts from crops with high lipid content, animal products,
and difficult matrices, e.g., tobacco and hops, require preliminary
clean-up by liquid-liquid partition procedures.
2.5.3. Determination
Residues of cyhalothrin in cleaned-up extracts are determined
by GC/ECD using packed or capillary columns.
A variety of liquid phases have been found suitable for use
with packed columns; these include OV-25, OV-101, OV-210, and OV-
202. These are generally used at low loadings (3-5%) in the
temperature range 230-250 °C, and retention times for cyhalothrin
are normally less than 10 min. Glass columns should be used. On
these packed columns cyhalothrin is eluted as a single peak.
Capillary columns will separate the two pairs of
diastereoisomers (enantiomer pairs) of cyhalothrin to give two
peaks. Lambda-cyhalothrin under the same conditions gives a single
peak. Fused silica columns, 25 m long, coated with OV-101 have
been found suitable when separate determination of diastereoisomers
in the cyhalothrin residue is required. Retention times normally
fall within the range of 10 to 30 min.
2.5.4. Limit of determination
The limit of determination of the methods for cyhalothrin
residues in crops and animal products is set at 0.01 mg/kg on the
basis of recovery experiments at low fortification levels (0.005-
0.02 mg/kg) and background noise in the chromatograms. The limit
of determination for soils is set at 0.005 to 0.01 mg/kg and for
water is 10 ng/litre (ppt).
2.5.5. Recoveries and interference
The internal standardization procedure used in these methods
determines the concentration of cyhalothrin or lambda-cyhalothrin
relative to that of a known concentration of internal standard
added to the sample prior to extraction. Correction for percentage
recovery is thereby inherent for each individual sample. The
repeatability of the procedure for most substrates is 2 to 3%. The
methods have been found to be applicable to the determination of
cyhalothrin in a wide variety of substrates without interference
from endogenous natural products in the GC determination.
2.5.6. Confirmation of residue identity
Qualitative and quantitative confirmation of residue identity
may be achieved by combined GC-MS operated in the selected ion
monitoring mode.
3. SOURCES AND LEVELS OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production levels and processes
No data on industrial production are available.
3.2. Uses
Cyhalothrin is a pyrethroid insecticide with a high level of
activity (application rate up to 20 g/ha) against a wide range of
Lepidoptera, Hemiptera, Diptera, and Coleoptera species. It also
has some miticidal activity. Lambda-cyhalothrin has the same
spectrum of insecticidal activity as cyhalothrin but it is more
active. The compound is a stomach, contact, and residual
insecticide. It shows adulticidal, ovicidal and, particularly,
larvicidal activity.
Like other photostable synthetic pyrethroids, cyhalothrin and
lambda-cyhalothrin are relatively stable to degradation in
sunlight. This permits their use as practical tools in
agriculture. The compound is not plant-systemic and has very
little fumigant or translaminar activity.
Owing, in part, to its short persistence in soil and lack of
systemic effect, the compound is of only limited value when used as
a soil insecticide. It can, however, give useful control of
cutworms when applied as a crop/ground spray. It has no
molluscicidal or nematocidal activity.
Preventive treatments are generally more effective than
curative treatments against major pests such as boring caterpillars
or leaf miners. A programme of sprays is usually required,
particularly during the more active growth stages of the plant and
when the potential for re-infestation remains high.
Cyhalothrin has also found uses in public and animal health
applications where it effectively controls a broad spectrum of
insects, including cockroaches, flies, mosquitos, and ticks. It
has high activity as a residual spray on inert surfaces.
3.3. Residues in food
Supervised trials have been carried out on a wide variety of
crops, and comprehensive summaries of residue analysis in these
trials can be found in the evaluation reports of the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) (FAO/WHO 1985, 1986a).
Data reviewed by the JMPR showed that in studies on apples and
on pears, when different rates of application were used in the same
trial, initial residues reflected the different rates applied.
When the spray programme was doubled from three to six applications
per season, there was no increase in the lambda-cyhalothrin residue
levels over those obtained with the three applications programme at
the same rates. Lambda-cyhalothrin residue levels on apples often
declined relatively slowly, although this was not always the case.
There were no obvious differences in residue levels arising from
the use of the different strengths of emulsion concentrate
formulations or from the use of either low volume or high volume
rates of application (FAO/WHO, 1986a,b).
3.4. Levels in the environment
3.4.1. Air
No specific data on air concentrations are available. Since
cyhalothrin and lambda-cyhalothrin are of low vapour pressure,
atmospheric levels of their vapour will be negligible.
3.4.2. Water
No specific data on water levels are available. Since
cyhalothrin and lambda-cyhalothrin are insoluble in water and not
mobile in soil, they are very unlikely to reach ground water.
3.4.3. Soil
No information on concentrations in soil is available.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transportation and distribution between media
The very low vapour pressure of cyhalothrin means that this
compound will not enter the atmosphere.
Studies have shown that cyhalothrin and its soil degradation
products do not leach through soils. 14C-cyclopropane-labelled
cyhalothrin was aerobically incubated for 30 days with a sandy loam
(4.2% organic matter) and a loamy sand (2.0% organic matter). The
incubated soils (rates equivalent to 0.04 and 0.05 kg cyhalothrin
equivalents per ha for the sandy loam and loamy sand, respectively)
were then applied to soil columns (30 cm long) and leached during a
period of 9 weeks with 66 cm "rain". The radioactive residues more
than 5 cm beneath the surface were below the limit of determination
(i.e. < 0.47 ng cyhalothrin equivalents per g) in all the soil
columns. Radioactive residues in the leachate samples were also
generally below the limit of determination (i.e. < 0.023 ng
cyhalothrin equivalent per ml) and represented less than 0.3% of
the applied radiocarbon. Thus, cyhalothrin, lambda-cyhalothrin,
and their degradation products have very low mobility in soil. On
the basis of these data it is concluded that the agricultural use
of cyhalothrin or lambda-cyhalothrin will not result in the
leaching of either the parent compounds or their degradation
products into ground water (Stevens & Bewick, 1985). Furthermore,
if soils containing cyhalothrin are flooded, there is no release of
cyhalothrin into the water (Hamer & Hill, 1985). Thus, residues of
cyhalothrin in soil resulting from agricultural use will not be
transported into other compartments of the environment.
4.2. Abiotic degradation
4.2.1. Hydrolysis and photodegradation in water
In studies by Collis & Leahey (1984), aqueous solutions of 14C-
cyclopropyl-labelled lambda-cyhalothrin, buffered at pH 5, 7, and
9, were maintained in the dark at 25° C for periods of up to 30
days. Acetonitrile (1%) was used as a co-solvent to facilitate
dissolution in the water. Hydrolysis of lambda-cyhalothrin
occurred rapidly in the pH 9 aqueous buffer solution (half-life,
approxi-mately 7 days) via ester cleavage of the molecule to yield
(1 RS)-cis-3-( Z-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid. Rapid isomerization of the
optical centre at the alpha-CN position also occurred at pH 9. At
pH 7 no hydrolysis was detected, but slow isomerization did occur.
At pH 5 no hydrolysis or isomerization was observed (Collis &
Leahey, 1984).
When quartz flasks containing 14C-cyclopropyl-labelled lambda-
cyhalothrin in pH 5 buffer were exposed to sunlight for 30 days,
the compound underwent photodegradation with a half-life of
approximately 30 days. (1 RS)-cis- and (1 RS)-trans-3-( ZE-2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylic
acids and ( RS)-alpha-amido-3-phenoxybenzyl (1 RS)-cis,trans-3-( ZE-2-
chloro-3,3,3-tri-fluoroprop-1-enyl)-2,2-dimethylcyclopropanecarb-
oxylate were the major degradation products formed. Optical and
geometrical isomerization of lambda-cyhalothrin occurred in the
irradiated flasks. No photodegradation or photoisomerization was
observed in a dark control flask (Curl et al., 1984a).
In studies by Hall & Leahey (1983), 14C-cyhalothrin was
incubated with two river water/sediment mixtures contained in
quartz flasks. The flasks were either exposed to sunlight or were
maintained under dark conditions by covering them with aluminium
foil. In the dark, degradation of cyhalothrin was slow (over 80%
remained unchanged after 32 days). However, when exposed to
sunlight the cyhalothrin degraded with a half-life of approximately
20 days in both river water/sediment mixtures. The rate at which
the parent compound was lost from the aqueous phase was, however,
much faster than its rate of degradation in the whole
water/sediment system. This was due to the ready absorption of
cyhalothrin onto the sediment. The major degradation process was
simple ester cleavage of the molecule, producing (1 RS)-cis- and
(1 RS)-trans-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acids. After 32 days of
irradiation, these compounds together represented 36-47% of the
radioactivity applied to the water/sediment systems. Some
photoisomerization also occurred.
4.2.2. Photodegradation in soil
When thin-layer soil plates were treated with 14C-cyclopropyl-
labelled lambda-cyhalothrin and irradiated in a xenon arc apparatus
or in sunlight, the half-life of lambda-cyhalothrin was less than 2
days in the xenon arc apparatus and less than 30 days in sunlight.
(1 RS)-cis-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropane-carboxylic acid and ( RS)-alpha-amido-3-
phenoxybenzyl (1 RS)-cis,trans-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-
enyl)-2,2-dimethylcyclopropanecarboxylate were the major
degradation products (Curl et al., 1984b).
4.3. Biodegradation in soil
Cyhalothrin degradation in the outdoor environment may occur by
either biological or photochemical processes. In most cases,
biological processes are by far the most important, although
photochemical reactions can sometimes contribute to the degradation
of residues on exposed surfaces.
4.3.1. Degradation rate
At residue levels that are likely to occur under normal field
conditions, cyhalothrin is degraded rapidly in soil. When a sandy
loam soil was treated with 14C-cyclopropyl-labelled cyhalothrin,
only 28% of the recovered radioactivity was present as cyhalothrin
after five weeks of incubation under aerobic conditions; 30% was
evolved as 14C-labelled carbon dioxide and 3.5% of the recovered
radioactivity was due to 1 RS-cis-3-( Z-2-chloro-3,3,3-trifluoroprop-
1-enyl)-2,2-dimethylcyclopropanecarboxylic acid. Approximately 19%
of the radioactivity was not extracted using acetonitrile at room
temperature followed by soxhlet extraction with aqueous
acetonitrile (Bewick & Zinner, 1981).
In a more recent and detailed study, 14C-cyclopropyl-labelled
cyhalothrin was applied to two soils (a sandy loam and a loamy
sand) at 100 g/ha and incubated under both aerobic and flooded
conditions at 20 °C. The sandy loam soil was also treated with
cyhalothrin at 500 g/ha and, in a later experiment, treated
separately with the two enantiomer pairs of cyhalothrin (one of
which constitutes lambda-cyhalothrin). This soil was also
incubated at 10 °C. All the isomers of cyhalothrin, including
those that constitute lambda-cyhalothrin, were readily degraded in
soil under a range of conditions. Half-lives for cyhalothrin at
20 °C in the sandy loam and loamy sand soils were 22 and 82 days,
respectively. Degradation was somewhat slower in the sandy loam at
higher rates (half-life: 42 days), lower temperature (half-life: 56
days) and under flooded conditions (half-life: 74 days). Lambda-
cyhalothrin was degraded at about 70% the rate of the other
enantiomer pair of cyhalothrin in the aerobic soils but at
approximately the same rate under flooded conditions. In the
aerobic soils, the principle degradative reactions were
hydroxylation, yielding up to 11% of the applied radiocarbon as
( RS)-alpha-cyano-3-(4-hydroxyphenoxy) benzyl cis-3-( Z-2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-di-methylcyclopropanecarboxylate
(compound XV), and hydrolysis, yielding up to 7% as ( RS)-cis-3-( Z-
2-chloro-3,3,3-tri-fluoroprop-1-enyl)-2,2-dimethylcyclopropane-
carboxylic acid (compound Ia). In the flooded soil, hydrolysis was
the main degradative reaction (up to 18% of compound Ia in the soil
phase), and hydroxylation was less important (only up to 1.4% of
compound XV). Compound Ia was the only compound detected (up to
17%) in the aqueous phase of the flooded soils. No isomerization
of the parent esters or their hydrolysis product was detected. The
initial degradation products of cyhalothrin and lambda-cyhalothrin
in the aerobic soils were rapidly further degraded with extensive
mineralization (up to 70% in 26 weeks) to 14CO2 (Bharti et al.,
1985). Thus the acid moiety of cyhalothrin is readily mineralized
in soil. The alcohol moiety of this compound is identical to that
of cypermethrin. Studies with cypermethrin show that, after ester
cleavage, the alcohol moiety released is readily mineralized to
14CO2 (FAO/WHO, 1986a).
In a further study, when 14C-cyclopropyl-labelled cyhalothrin
was applied (at 100 g/ha) to a Japanese upland soil (a volcanic
ash) and incubated under aerobic conditions at 20 °C, the half-
life of cyhalothrin was approximately 100 days. The principle
degradative reactions were hydroxylation, yielding up to 7% of the
applied radiocarbon as compound XV, and hydrolysis, yielding up to
ca. 2% as compound Ia. The initial degradation products of
cyhalothrin were further degraded with mineralization (up to 17% in
26 weeks) to 14CO2 (Bharti & Bewick, 1986).
Four sites in the USA were treated with lambda-cyhalothrin
(1.1 kg/ha), and soil was sampled and analysed at intervals up to 9
months after treatment. Residues remained in the top 15 cm of
soil, except at one site where low residues immediately after
treatment and at 2 days were attributed to contamination during
sampling. Initial soil residues of 0.18 to 0.31 mg/kg declined to
extremely low levels (< 0.01 to 0.02 mg/kg) during the course of
the study, and at one site the residues were less than the limit of
determination after 91 days (Fitzpatrick, 1985).
4.3.2. Degradation pathways
Degradation pathways for cyhalothrin and lambda-cyhalothrin in
soil are shown in Figure 2.
Lambda-cyhalothrin is manufactured by crystallization of the
more active pair of enantiomers from cyhalothrin. The less active
pair of enantiomers is recycled.
Pure lambda-cyhalothrin is a racemic mixture of the enantiomer
pair B isomers. The enantiomer pair A is present in low
concentration in the commercial product.
Technical grade cyhalothrin contains more than 90% of the
pesticide and is formulated in 5%, 10%, and 20% emulsifiable
concentrates. Technical grade lambda-cyhalothrin also contains
more than 90% active ingredient. It is formulated as 2.5%, 5.0%,
8.3%, and 12% emulsifiable concen-trates and as a 0.8% ultra-low
volume concentrate.
2.2. Physical and chemical properties
Some physical and chemical properties of cyhalothrin and
lambda-cyhalothrin are listed in Table 1.
Table 1. Some physical and chemical properties of cyhalothrin and
lambda-cyhalothrin
---------------------------------------------------------------------------
Cyhalothrin Lambda-cyhalothrin
---------------------------------------------------------------------------
Physical state viscous liquid solid
Colour yellow-brown beige
Odour mild mild
Relative molecular mass 449.9 449.9
Melting point glass-like 49.2 °C
below 10 °C
Decomposes > 275 °C > 275 °C
Water solubility 4 x 10-3 mg/litre 5 x 10-3 mg/litre
Solubility in organic solvents soluble soluble
n-octanol water-partition 6.9 7.0
coefficient (log Pow) at 20 °C
Relative density 1.25 1.33
Vapour pressure at 20 °C 1 x 10-9 kPa 2 x 10-10 kPa
Vapour pressure at 80 °C 4 x 10-6 kPa 3 x 10-6 kPa
---------------------------------------------------------------------------
No boiling point data are available as both forms decompose on
heating above 275 °C. Cyhalothrin is highly stable to light and at
temperatures below 220 °C.
Lambda-cyhalothrin is stable in water at pH 5. At pH 7 and pH
9, there is racemization at the alpha-cyano carbon to yield a 1:1
mixture of enantiomer pairs A and B. At pH 9, the ester bond is
fairly readily hydrolysed (half-life, 7 days) (Collis & Leahey,
1984).
Dilute aqueous solutions are subject to photolysis at a
moderate rate (Hall & Leahey, 1983; Curl et al., 1984a).
2.3. Analytical methods
The most widely adopted procedures for analysing cyhalothrin
residues in crops, soil, animal tissues and products, and
environmental samples are based on extraction of the residue with
organic solvent, clean-up of the extract by solvent-solvent
partition and adsorption column chromatography, and determination
of the residue using gas chromatography (GC) with electron capture
detection (GC/ECD). The identity of residues can be confirmed by
GC with mass selective detection (GC-MSD) or by thin-layer
chromatography (TLC) followed by GC/ECD.
2.3.1. Sampling methods
Procedures for obtaining representative samples of crops,
processed commodities, soil, and some animal products have been
described in detail (GIFAP, 1981) and will not be discussed
further.
Particular care is necessary when sampling water because
cyhalothrin is extremely hydrophobic and rapidly adsorbs onto
particulates or container walls from aqueous solution. For this
reason, the whole analytical sample should be taken for analysis
and not subdivided in the field (Sapiets et al., 1984). Collection
of the sample in a clean glass container, with addition of the
extraction solvent before sealing and shaking the bottle, gives
good recoveries. Crossland et al. (1982) described procedures for
sampling surface water (using stainless steel fine mesh discs) and
subsurface water from ponds for separate analysis of cypermethrin.
Precautions were taken to avoid contamination during sampling
(Crossland et al., 1982). These methods, developed for
cypermethrin, are equally applicable to cyhalothrin and other
pyrethroids. During the course of the pond studies, cypermethrin
spray drift deposits were collected by means of horizontally placed
aluminium foil plates and washed from the foils using acetone.
Crossland also described the use of a core sampler to remove pond
sediment for cypermethrin analysis (Crossland, 1982). This method
is equally applicable to cyhalothrin.
Sampling of air in agriculture work space for pyrethroid
aerosol droplets using absorption onto exposed filter papers and
porous glass slides has been described by Girenko & Klisenko
(1984). They quoted limits in the range of 0.05 to 0.5 mg/m3 for
the concentrations of pyrethroid that could be detected without
interference from organophosphorus or organochlorine insecticides.
An alternative and more conventional approach would be to use a
pumped device consisting of a filter (to trap droplets or
particulates) in series with a packed tube (to trap vapour).
The use of a vacuum probe was described by Bengstone et al.
(1983) to subsample pyrethroid-treated grain from several points
within a silo for combination to give a composite sample. However,
care is needed to obtain a representative sample using this
procedure (GIFAP, 1981).
2.3.2. Sample storage
Storage stability experiments using untreated samples fortified
with cyhalothrin (at 1.0 mg/kg) have shown that apples, cabbage,
soil, and products of animal origin can be stored deep frozen at
temperatures of -20 °C for periods of up to one year without
residue loss (Sapiets, 1984a)
Particular problems in the handling of water samples have
already been indicated and samples should be extracted and
analysed as soon after sampling as possible.
2.4. Sample preparation
Forbes & Dutton (1985) reported details of the procedures used
to process crop and soil samples, which have been treated with
pyrethroid insecticides, for sub-sampling before extraction. Crops
of high water content, e.g., fruit and vegetables, were chopped,
dried, or puréed. Grain and oil seed crops (cotton seed and
linseed) were frozen and ground to a powder, while crops of low
water content, e.g., straw and tobacco, were finely divided in a
rotary knife mill. Soil samples were mixed thoroughly and stones
and plant debris removed. In all cases, care was taken to avoid
localized overheating of the sample during processing. Sapiets et
al. (1984) used a similar processing procedure for high water
content crops and also applied this to meat and eggs. However,
they draw attention to the importance of processing materials with
high water content while still frozen to prevent separation of
juice, which leads to sample inhomogeneity. Milk was thoroughly
mixed before subsampling. In the limited number of other studies
where details for preparation of pyrethroid-treated samples have
been given, none of the procedures differ markedly from those
described above. All of the procedures are equally applicable to
cyhalothrin and lambda-cyhalothrin.
2.5. Gas chromatographic procedures for the determination
of cyhalothrin residues
Details of GC procedures are described in the following
subsections (Sapiets, 1984b,c, 1985a,b, 1986a,b).
2.5.1. Extraction
Representative subsamples of prepared crops or meat are blended
for 2-5 min with a mixture of acetone and hexane (1 + 1 by volume).
Dry materials are dampened with water. Soil is similarly extracted
by refluxing with acetonitrile for 1 h. Extracts are then gravity
filtered and partitioned with 5% sodium chloride solution to remove
the acetone. The hexane extract is dried over anhydrous sodium
sulfate.
Eggs are extracted by homogenizing for 5 min with acetonitrile.
An aliquot is evaporated to dryness and redissolved in hexane.
Milk is extracted by homogenizing for 2 min in acetone and
hexane (1:1 by volume). The solvent is dried over anhydrous sodium
sulfate.
2.5.2. Clean-up
Column absorption chromatography on Florisil is used for
cleaning up extracts from crops and soils, and cyhalothrin
residues are eluted with a mixture of diethyl ether in hexane.
Extracts from crops with high lipid content, animal products,
and difficult matrices, e.g., tobacco and hops, require preliminary
clean-up by liquid-liquid partition procedures.
2.5.3. Determination
Residues of cyhalothrin in cleaned-up extracts are determined
by GC/ECD using packed or capillary columns.
A variety of liquid phases have been found suitable for use
with packed columns; these include OV-25, OV-101, OV-210, and OV-
202. These are generally used at low loadings (3-5%) in the
temperature range 230-250 °C, and retention times for cyhalothrin
are normally less than 10 min. Glass columns should be used. On
these packed columns cyhalothrin is eluted as a single peak.
Capillary columns will separate the two pairs of
diastereoisomers (enantiomer pairs) of cyhalothrin to give two
peaks. Lambda-cyhalothrin under the same conditions gives a single
peak. Fused silica columns, 25 m long, coated with OV-101 have
been found suitable when separate determination of diastereoisomers
in the cyhalothrin residue is required. Retention times normally
fall within the range of 10 to 30 min.
2.5.4. Limit of determination
The limit of determination of the methods for cyhalothrin
residues in crops and animal products is set at 0.01 mg/kg on the
basis of recovery experiments at low fortification levels (0.005-
0.02 mg/kg) and background noise in the chromatograms. The limit
of determination for soils is set at 0.005 to 0.01 mg/kg and for
water is 10 ng/litre (ppt).
2.5.5. Recoveries and interference
The internal standardization procedure used in these methods
determines the concentration of cyhalothrin or lambda-cyhalothrin
relative to that of a known concentration of internal standard
added to the sample prior to extraction. Correction for percentage
recovery is thereby inherent for each individual sample. The
repeatability of the procedure for most substrates is 2 to 3%. The
methods have been found to be applicable to the determination of
cyhalothrin in a wide variety of substrates without interference
from endogenous natural products in the GC determination.
2.5.6. Confirmation of residue identity
Qualitative and quantitative confirmation of residue identity
may be achieved by combined GC-MS operated in the selected ion
monitoring mode.
3. SOURCES AND LEVELS OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production levels and processes
No data on industrial production are available.
3.2. Uses
Cyhalothrin is a pyrethroid insecticide with a high level of
activity (application rate up to 20 g/ha) against a wide range of
Lepidoptera, Hemiptera, Diptera, and Coleoptera species. It also
has some miticidal activity. Lambda-cyhalothrin has the same
spectrum of insecticidal activity as cyhalothrin but it is more
active. The compound is a stomach, contact, and residual
insecticide. It shows adulticidal, ovicidal and, particularly,
larvicidal activity.
Like other photostable synthetic pyrethroids, cyhalothrin and
lambda-cyhalothrin are relatively stable to degradation in
sunlight. This permits their use as practical tools in
agriculture. The compound is not plant-systemic and has very
little fumigant or translaminar activity.
Owing, in part, to its short persistence in soil and lack of
systemic effect, the compound is of only limited value when used as
a soil insecticide. It can, however, give useful control of
cutworms when applied as a crop/ground spray. It has no
molluscicidal or nematocidal activity.
Preventive treatments are generally more effective than
curative treatments against major pests such as boring caterpillars
or leaf miners. A programme of sprays is usually required,
particularly during the more active growth stages of the plant and
when the potential for re-infestation remains high.
Cyhalothrin has also found uses in public and animal health
applications where it effectively controls a broad spectrum of
insects, including cockroaches, flies, mosquitos, and ticks. It
has high activity as a residual spray on inert surfaces.
3.3. Residues in food
Supervised trials have been carried out on a wide variety of
crops, and comprehensive summaries of residue analysis in these
trials can be found in the evaluation reports of the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) (FAO/WHO 1985, 1986a).
Data reviewed by the JMPR showed that in studies on apples and
on pears, when different rates of application were used in the same
trial, initial residues reflected the different rates applied.
When the spray programme was doubled from three to six applications
per season, there was no increase in the lambda-cyhalothrin residue
levels over those obtained with the three applications programme at
the same rates. Lambda-cyhalothrin residue levels on apples often
declined relatively slowly, although this was not always the case.
There were no obvious differences in residue levels arising from
the use of the different strengths of emulsion concentrate
formulations or from the use of either low volume or high volume
rates of application (FAO/WHO, 1986a,b).
3.4. Levels in the environment
3.4.1. Air
No specific data on air concentrations are available. Since
cyhalothrin and lambda-cyhalothrin are of low vapour pressure,
atmospheric levels of their vapour will be negligible.
3.4.2. Water
No specific data on water levels are available. Since
cyhalothrin and lambda-cyhalothrin are insoluble in water and not
mobile in soil, they are very unlikely to reach ground water.
3.4.3. Soil
No information on concentrations in soil is available.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transportation and distribution between media
The very low vapour pressure of cyhalothrin means that this
compound will not enter the atmosphere.
Studies have shown that cyhalothrin and its soil degradation
products do not leach through soils. 14C-cyclopropane-labelled
cyhalothrin was aerobically incubated for 30 days with a sandy loam
(4.2% organic matter) and a loamy sand (2.0% organic matter). The
incubated soils (rates equivalent to 0.04 and 0.05 kg cyhalothrin
equivalents per ha for the sandy loam and loamy sand, respectively)
were then applied to soil columns (30 cm long) and leached during a
period of 9 weeks with 66 cm "rain". The radioactive residues more
than 5 cm beneath the surface were below the limit of determination
(i.e. < 0.47 ng cyhalothrin equivalents per g) in all the soil
columns. Radioactive residues in the leachate samples were also
generally below the limit of determination (i.e. < 0.023 ng
cyhalothrin equivalent per ml) and represented less than 0.3% of
the applied radiocarbon. Thus, cyhalothrin, lambda-cyhalothrin,
and their degradation products have very low mobility in soil. On
the basis of these data it is concluded that the agricultural use
of cyhalothrin or lambda-cyhalothrin will not result in the
leaching of either the parent compounds or their degradation
products into ground water (Stevens & Bewick, 1985). Furthermore,
if soils containing cyhalothrin are flooded, there is no release of
cyhalothrin into the water (Hamer & Hill, 1985). Thus, residues of
cyhalothrin in soil resulting from agricultural use will not be
transported into other compartments of the environment.
4.2. Abiotic degradation
4.2.1. Hydrolysis and photodegradation in water
In studies by Collis & Leahey (1984), aqueous solutions of 14C-
cyclopropyl-labelled lambda-cyhalothrin, buffered at pH 5, 7, and
9, were maintained in the dark at 25° C for periods of up to 30
days. Acetonitrile (1%) was used as a co-solvent to facilitate
dissolution in the water. Hydrolysis of lambda-cyhalothrin
occurred rapidly in the pH 9 aqueous buffer solution (half-life,
approxi-mately 7 days) via ester cleavage of the molecule to yield
(1 RS)-cis-3-( Z-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid. Rapid isomerization of the
optical centre at the alpha-CN position also occurred at pH 9. At
pH 7 no hydrolysis was detected, but slow isomerization did occur.
At pH 5 no hydrolysis or isomerization was observed (Collis &
Leahey, 1984).
When quartz flasks containing 14C-cyclopropyl-labelled lambda-
cyhalothrin in pH 5 buffer were exposed to sunlight for 30 days,
the compound underwent photodegradation with a half-life of
approximately 30 days. (1 RS)-cis- and (1 RS)-trans-3-( ZE-2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylic
acids and ( RS)-alpha-amido-3-phenoxybenzyl (1 RS)-cis,trans-3-( ZE-2-
chloro-3,3,3-tri-fluoroprop-1-enyl)-2,2-dimethylcyclopropanecarb-
oxylate were the major degradation products formed. Optical and
geometrical isomerization of lambda-cyhalothrin occurred in the
irradiated flasks. No photodegradation or photoisomerization was
observed in a dark control flask (Curl et al., 1984a).
In studies by Hall & Leahey (1983), 14C-cyhalothrin was
incubated with two river water/sediment mixtures contained in
quartz flasks. The flasks were either exposed to sunlight or were
maintained under dark conditions by covering them with aluminium
foil. In the dark, degradation of cyhalothrin was slow (over 80%
remained unchanged after 32 days). However, when exposed to
sunlight the cyhalothrin degraded with a half-life of approximately
20 days in both river water/sediment mixtures. The rate at which
the parent compound was lost from the aqueous phase was, however,
much faster than its rate of degradation in the whole
water/sediment system. This was due to the ready absorption of
cyhalothrin onto the sediment. The major degradation process was
simple ester cleavage of the molecule, producing (1 RS)-cis- and
(1 RS)-trans-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acids. After 32 days of
irradiation, these compounds together represented 36-47% of the
radioactivity applied to the water/sediment systems. Some
photoisomerization also occurred.
4.2.2. Photodegradation in soil
When thin-layer soil plates were treated with 14C-cyclopropyl-
labelled lambda-cyhalothrin and irradiated in a xenon arc apparatus
or in sunlight, the half-life of lambda-cyhalothrin was less than 2
days in the xenon arc apparatus and less than 30 days in sunlight.
(1 RS)-cis-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropane-carboxylic acid and ( RS)-alpha-amido-3-
phenoxybenzyl (1 RS)-cis,trans-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-
enyl)-2,2-dimethylcyclopropanecarboxylate were the major
degradation products (Curl et al., 1984b).
4.3. Biodegradation in soil
Cyhalothrin degradation in the outdoor environment may occur by
either biological or photochemical processes. In most cases,
biological processes are by far the most important, although
photochemical reactions can sometimes contribute to the degradation
of residues on exposed surfaces.
4.3.1. Degradation rate
At residue levels that are likely to occur under normal field
conditions, cyhalothrin is degraded rapidly in soil. When a sandy
loam soil was treated with 14C-cyclopropyl-labelled cyhalothrin,
only 28% of the recovered radioactivity was present as cyhalothrin
after five weeks of incubation under aerobic conditions; 30% was
evolved as 14C-labelled carbon dioxide and 3.5% of the recovered
radioactivity was due to 1 RS-cis-3-( Z-2-chloro-3,3,3-trifluoroprop-
1-enyl)-2,2-dimethylcyclopropanecarboxylic acid. Approximately 19%
of the radioactivity was not extracted using acetonitrile at room
temperature followed by soxhlet extraction with aqueous
acetonitrile (Bewick & Zinner, 1981).
In a more recent and detailed study, 14C-cyclopropyl-labelled
cyhalothrin was applied to two soils (a sandy loam and a loamy
sand) at 100 g/ha and incubated under both aerobic and flooded
conditions at 20 °C. The sandy loam soil was also treated with
cyhalothrin at 500 g/ha and, in a later experiment, treated
separately with the two enantiomer pairs of cyhalothrin (one of
which constitutes lambda-cyhalothrin). This soil was also
incubated at 10 °C. All the isomers of cyhalothrin, including
those that constitute lambda-cyhalothrin, were readily degraded in
soil under a range of conditions. Half-lives for cyhalothrin at
20 °C in the sandy loam and loamy sand soils were 22 and 82 days,
respectively. Degradation was somewhat slower in the sandy loam at
higher rates (half-life: 42 days), lower temperature (half-life: 56
days) and under flooded conditions (half-life: 74 days). Lambda-
cyhalothrin was degraded at about 70% the rate of the other
enantiomer pair of cyhalothrin in the aerobic soils but at
approximately the same rate under flooded conditions. In the
aerobic soils, the principle degradative reactions were
hydroxylation, yielding up to 11% of the applied radiocarbon as
( RS)-alpha-cyano-3-(4-hydroxyphenoxy) benzyl cis-3-( Z-2-chloro-
3,3,3-trifluoroprop-1-enyl)-2,2-di-methylcyclopropanecarboxylate
(compound XV), and hydrolysis, yielding up to 7% as ( RS)-cis-3-( Z-
2-chloro-3,3,3-tri-fluoroprop-1-enyl)-2,2-dimethylcyclopropane-
carboxylic acid (compound Ia). In the flooded soil, hydrolysis was
the main degradative reaction (up to 18% of compound Ia in the soil
phase), and hydroxylation was less important (only up to 1.4% of
compound XV). Compound Ia was the only compound detected (up to
17%) in the aqueous phase of the flooded soils. No isomerization
of the parent esters or their hydrolysis product was detected. The
initial degradation products of cyhalothrin and lambda-cyhalothrin
in the aerobic soils were rapidly further degraded with extensive
mineralization (up to 70% in 26 weeks) to 14CO2 (Bharti et al.,
1985). Thus the acid moiety of cyhalothrin is readily mineralized
in soil. The alcohol moiety of this compound is identical to that
of cypermethrin. Studies with cypermethrin show that, after ester
cleavage, the alcohol moiety released is readily mineralized to
14CO2 (FAO/WHO, 1986a).
In a further study, when 14C-cyclopropyl-labelled cyhalothrin
was applied (at 100 g/ha) to a Japanese upland soil (a volcanic
ash) and incubated under aerobic conditions at 20 °C, the half-
life of cyhalothrin was approximately 100 days. The principle
degradative reactions were hydroxylation, yielding up to 7% of the
applied radiocarbon as compound XV, and hydrolysis, yielding up to
ca. 2% as compound Ia. The initial degradation products of
cyhalothrin were further degraded with mineralization (up to 17% in
26 weeks) to 14CO2 (Bharti & Bewick, 1986).
Four sites in the USA were treated with lambda-cyhalothrin
(1.1 kg/ha), and soil was sampled and analysed at intervals up to 9
months after treatment. Residues remained in the top 15 cm of
soil, except at one site where low residues immediately after
treatment and at 2 days were attributed to contamination during
sampling. Initial soil residues of 0.18 to 0.31 mg/kg declined to
extremely low levels (< 0.01 to 0.02 mg/kg) during the course of
the study, and at one site the residues were less than the limit of
determination after 91 days (Fitzpatrick, 1985).
4.3.2. Degradation pathways
Degradation pathways for cyhalothrin and lambda-cyhalothrin in
soil are shown in Figure 2.
 4.4. Metabolism in plants
In studies by Leahey & French (1986a), soya bean plants were
treated with 14C-cyclopropane-labelled and 14C-benzyl-labelled
lambda-cyhalothrin. Two applications (18 days apart) were made by
spraying an EC formulation at a rate of 20 g/ha. The plants were
analysed at maturity, 39 days after the second application, when
radioactive residues on the leaves ranged from 1.2 mg/kg (benzyl-
labelled treatment) to 1.5 mg/kg (cyclopropane-labelled treatment).
Very little radioactivity translocated into seeds (< 0.01 mg/kg).
In a similar experiment, in which cotton plants were treated
with 14C-cyclopropane-labelled and 14C-benzyl-labelled lambda-
cyhalothrin, three applications at a rate of 66 g/ha were made (at
flowering and 3 and 7 weeks after flowering). The plants were
analysed, at maturity, 30 days after the final application. The
radioactive residues on the leaves at harvest were 3.7 mg/kg for
the benzyl-labelled treatment and 4.1 mg/kg for the cyclopropane-
labelled treatment. Very little radioactivity (< 0.03 mg/kg) was
detected in the cotton seeds (Leahey & French, 1986b,c).
At harvest, the major constituents of the radioactive residue
on the leaves of both cotton and soya bean were lambda-cyhalothrin
and other isomeric forms of lambda-cyhalothrin resulting from
photochemically initiated interconversions (soya: 52% benzyl-
label, 45% cyclopropane-label; cotton: 52% benzyl-label, 37%
cyclopropane-label). The metabolites detected on the leaves of both
plants resulted from a range of hydrolytic and oxidative reactions.
A metabolic pathway illustrating these reactions is shown in Fig. 3.
4.4. Metabolism in plants
In studies by Leahey & French (1986a), soya bean plants were
treated with 14C-cyclopropane-labelled and 14C-benzyl-labelled
lambda-cyhalothrin. Two applications (18 days apart) were made by
spraying an EC formulation at a rate of 20 g/ha. The plants were
analysed at maturity, 39 days after the second application, when
radioactive residues on the leaves ranged from 1.2 mg/kg (benzyl-
labelled treatment) to 1.5 mg/kg (cyclopropane-labelled treatment).
Very little radioactivity translocated into seeds (< 0.01 mg/kg).
In a similar experiment, in which cotton plants were treated
with 14C-cyclopropane-labelled and 14C-benzyl-labelled lambda-
cyhalothrin, three applications at a rate of 66 g/ha were made (at
flowering and 3 and 7 weeks after flowering). The plants were
analysed, at maturity, 30 days after the final application. The
radioactive residues on the leaves at harvest were 3.7 mg/kg for
the benzyl-labelled treatment and 4.1 mg/kg for the cyclopropane-
labelled treatment. Very little radioactivity (< 0.03 mg/kg) was
detected in the cotton seeds (Leahey & French, 1986b,c).
At harvest, the major constituents of the radioactive residue
on the leaves of both cotton and soya bean were lambda-cyhalothrin
and other isomeric forms of lambda-cyhalothrin resulting from
photochemically initiated interconversions (soya: 52% benzyl-
label, 45% cyclopropane-label; cotton: 52% benzyl-label, 37%
cyclopropane-label). The metabolites detected on the leaves of both
plants resulted from a range of hydrolytic and oxidative reactions.
A metabolic pathway illustrating these reactions is shown in Fig. 3.
 4.5. Bioaccumulation and biomagnification
4.5.1. n-Octanol-water partition coefficient
In common with other synthetic pyrethroids, the n-octanol-water
partition coefficient of cyhalothrin is high; values for log Pow of
6.9 and 7.0 at 20 °C have been obtained for cyhalothrin and lambda-
cyhalothrin, respectively, using a generator column method
(personal communication by ICI Agrochemicals to the IPCS). However,
since the compound is insoluble in water (thus limiting exposures
of aquatic species) and is rapidly metabolized in animal systems to
the cyclopropanecarboxylic acid and 3-phenoxybenzoic acid, both of
which are polar compounds, no problem of bioaccumulation will
occur.
4.5.2. Bioaccumulation
In a study consisting of a 28-day exposure and a 28-day
depuration period, carp (Cyprinus carpio) were exposed to
cyhalothrin in a flow-through water system using 14C-labelled
cyhalothrin at a nominal concentration of 0.02 µg cyhalothrin
equivalent/litre. During the exposure period, the concentration of
the total 14C-labelled cyhalothrin in the carp reached an
equilibrium within 1-2 weeks. The bioconcentration factors
measured were: 4250-7340 in the viscera, 490-850 in the muscle,
1020-2290 in the remainder of the body, and 1660-2240 in the whole
fish. Rapid depuration of residues was observed; the biological
half-life of the total 14C-labelled cyhalothrin was 9 days in the
viscera, muscle, and whole fish (Yamauchi et al., 1984a).
The accumulation of cyhalothrin and its degradation products in
channel catfish and Daphnia magna has been investigated in a soil-
water system. 14C-cyclopropane-labelled cyhalothrin was applied at
50 g ai per ha and aerobically incubated in soil for 3 weeks prior
to flooding. Channel catfish and Daphnia magna were introduced for
exposure periods of 31 and 28 days, respectively, after which the
fish and daphnids were transferred to an uncontaminated system for
depuration periods of 42 and 7 days, respectively. Soil, water,
fish, and daphnids were ana-lysed for 14C-residues. Prior to
flooding, 14C-labelled residues in soil decreased to 60-70% of that
applied, 40% of which remained as extractable cyhalothrin.
Following flooding of the soil, 14C-labelled residues in the water
increased throughout the exposure period, reaching a level of 8% of
the applied radioactivity. No parent cyhalothrin was detected in
the water; the only product comprising more than 1% of the applied
radioactivity was cis-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl-
2,2-dimethylcyclopropane-carboxylic acid, which represented up to
5.3% of the applied radioactivity. During exposure, the maximum
bio-concentration factors in whole fish and daphnids were 19 and
194, respectively (Table 2). The concentration of 14C-residues in
fish and daphnids decreased during the depuration period, the
half-lives being approximately 7 days and 1 day, respectively
(Table 3) (Hamer & Hill, 1985).
Table 2. Bioconcentration factors for
cyhalothrin in Daphnia and fish
---------------------------------------------
Exposure Daphnia Fish Fish Whole
phase muscle viscera fish
(days)
---------------------------------------------
1 93 2 20 17
3 194 3 28 7
7 158 6 45 10
14 62 7 66 19
21 47 5 28 10
28 29 7 49 8
31 NR 7 48 9
---------------------------------------------
From: Hamer & Hill (1985)
NR = not recorded.
Table 3. Concentration of 14C-residues in
Daphnia and fish tissues
--------------------------------------------
Depuration % of tissue level on final day
phase of exposure
(days) Daphnia Fish Fish Whole
muscle viscera fish
--------------------------------------------
1 56 105 60 80
3 29 66 53 64
4 NR NR NR 61
7 11 51 4 38
14 NR 32 4 37
21 NR 31 6 27
42 NR 20 2 14
--------------------------------------------
From: Hamer & Hill (1985)
NR = not recorded.
5. KINETICS AND METABOLISM
5.1. Absorption, distribution, and excretion
5.1.1. Rat
In studies by Harrison (1981, 1984a,b), groups of six male and
six female Alderley Park rats received a single oral dose (1 or 25
mg/kg) of radiolabelled cyhalothrin in corn oil. As it was known
that the metabolism of related pyrethroids involves extensive
cleavage of the ester bond, duplicate experiments were performed
using two forms of cyhalothrin labelled with 14C in the acid (14C-
cyclopropyl) or alcohol (14C-benzyl) portions of the ester.
Excreta (urine, faeces and, in selected animals, expired air) were
collected for up to 7 days after dosing and analysed for total
radioactivity and metabolites by liquid scintillation counting and
thin-layer chromatography. Blood samples were also collected at
various times up to 48 h and analysed for total radioactivity and
unchanged cyhalothrin.
Following oral administration of cyhalothrin, absorption was
variable but accounted for about 55% of the dose. The proportions
absorbed were similar at both dose levels.
Excretion was rapid for both 14C-cyclopropyl- and 14C-benzyl-
labelled cyhalothrin at both dose levels, although excretion rates
were faster with the 14C-benzyl label than with the 14C-cyclopropyl
label. Urinary excretion accounted for approximately 20-40% of the
dose and faecal excretion for 40-65% of the dose during the first 7
days. Peak blood concentrations of radioactivity were reached
within 4-7 h, and by 48 h these concentrations had declined to 10%
or less of peak values. A small proportion of an oral dose (2-3%)
was retained in the animals after seven days; analysis of twelve
different tissues indicated that this radioactivity was present
mainly in white fat.
Results from a study in which rats were dosed subcutaneously
indicated that some of the dose was excreted via the bile.
In a further experiment to study the excretion and tissue
accumulation of cyhalothrin (1 mg/kg per day by gavage), groups of
six male and six female rats received daily doses of 14C-benzyl-
labelled or 14C-cyclopropyl-labelled cyhalothrin for 14 days. Urine
and faecal samples were collected every 24 h up to 7 days after the
final dose. Groups of animals were killed 2, 5, and 7 days after
the final dose and a range of tissues were removed for measurement
of residual radioactivity. Fat samples were analysed by HPLC for
unchanged cyhalothrin. The results demonstrated that the excretion
of 14C-material after multiple oral dosing was similar to that
which followed a single dose. Slightly higher overall excretion in
urine (up to 50% of the administered dose) was probably due to more
consistent oral absorption in this study. A large proportion of
the oral dose of cyhalothrin was rapidly eliminated from the body.
Analysis of tissue residues revealed that the small proportion
(< 5%) of the dose retained in white fat was unchanged
cyhalothrin, which was eliminated from this tissue with a half-life
of about 23 days (Harrison, 1981, 1984a,b).
A further study was undertaken in the rat to explore the
retention, in fat, of cyhalothrin and lambda-cyhalothrin. Groups
of male rats received daily oral doses of 14C-cyclopropyl-labelled
cyhalothrin (1 mg/kg per day) for up to 119 days. At intervals
during and after the dosing period, groups of three rats were
killed and the concentrations of radioactivity in the liver,
kidney, fat, and blood were determined. Additionally the
concentration in fat of lambda-cyhalothrin and its opposite
enantiomer pair (enantiomer pair A) was measured by high-pressure
liquid chromatography. Levels of radioactivity in the blood
remained fairly constant and low (approximately 0.2 µg cyhalothrin
equivalents per g) throughout the dosing period. In the liver and
kidney, the radioactivity reached a plateau, after approximately 70
days, at a level corresponding to approximately 2.5 µg cyhalothrin
equivalents per g liver and 1.2 µg per g kidney. The concentration
of cyhalothrin in fat at the end of the dosing period was
approximately 10 µg/g. After the cessation of dosing, levels of
radioactivity in the liver, kidney, and blood declined rapidly. In
fat, the levels declined more slowly with an elimination half-life
of 30 days. The radioactive material in fat was unchanged
cyhalothrin; the ratio of enantiomeric pairs, one of which was
lambda-cyhalothrin, was not significantly different from that in
the dosing solution, indicating that the rate of metabolism of
lambda-cyhalothrin was the same as cyhalothrin and that there was
no preferential accumulation of lambda-cyhalothrin (Prout, 1984).
A comparison of the absorption, distribution, excretion, and
metabolism of lambda-cyhalothrin and cyhalothrin was made to
establish whether the single enantiomer pair lambda-cyhalothrin
differed from cyhalothrin (a 50:50 mixture of lambda-cyhalothrin
and the opposite enantiomer pair A) (Prout & Howard, 1985). One
group of four male rats was given a single oral dose of 14C-
cyclopropyl-labelled lambda-cyhalothrin (1 mg/kg); a second group
of four male rats was given 14C-cyclopropyl-labelled lambda-
cyhalothrin (1 mg/kg) plus the unlabelled enantiomeric pair A (1
mg/kg); and a third group of four male rats was given a single oral
dose of a 50:50 mixture of 14C-cyclopropyl-labelled lambda-
cyhalothrin and 14C-labelled enantiomeric pair A (i.e. 14C-
cyclopropyl-labelled cyhalothrin at 1 mg/kg). The urinary and
faecal excretion of radioactivity was monitored in all three groups
for three days and the residual radioactivity was then determined
in selected tissues. The metabolite profile of the excreta was
determined by thin-layer chromatography. The results of this study
indicate that co-administration of enantiomer pair A with lambda-
cyhalothrin had little or no effect upon the absorption,
distribution, or tissue retention of radioactivity, and there was
no effect upon the metabolite profile of lambda-cyhalothrin.
Similarly, the absorption, distribution, excretion, and metabolism
of cyhalothrin was indistinguishable from that of lambda-
cyhalothrin, thus confirming the results of the bioaccumulation
study of Prout (1984).
5.1.2. Dog
The absorption, distribution, excretion, and metabolism of
cyhalothrin have been studied in the dog. As in the rat studies,
the experiments were duplicated using cyhalothrin labelled either
in the acid (14C-cyclopro-pyl) or alcohol (14C-benzyl) moieties of
the molecule. Groups of three male and three female beagle dogs
were given a single oral dose of cyhalothrin (1 mg/kg or 10 mg/kg)
and, after a 3-week interval, a further single intravenous
administration of 0.1 mg/kg. Samples of blood and excreta were
collected for 7 days after dosing and were analysed for total
radioactivity. The proportions of unchanged cyhalothrin and of
metabolites in urine and faeces were determined by thin-layer
chromatography. The identity of major metabolites was confirmed by
mass spectrometry.
The absorption of cyhalothrin after oral administration was
variable. The degree of absorption was difficult to assess but was
within the range 48%-80%. Excretion of radioactivity after both
oral and intravenous dosing was initially rapid, with most of the
administered radioactivity being excreted in the first 48 h after
dosing. After 7 days, a mean of 82-93% had been excreted (Harrison,
1984c).
5.1.3. Cow
After twice daily oral ingestion of 14C-benzyl- or 14C-
cyclopropyl-labelled cyhalothrin (1 mg/kg per day for 7 days),
absorption of the insecticide by cows was apparently slow and
incomplete. Approximately 50% of the dosed radioactivity was
excreted in the faeces, mainly as unchanged cyhalothrin, but only
small amounts were detected in the bile. With both labelled forms,
most of the radioactive material was rapidly eliminated in the
urine (27%) and faeces (49%) within 24 h of each daily dose. Only
a very small proportion of the dose was secreted in the milk (0.8%)
and this was found to be unchanged cyhalothrin. Tissue residues of
radioactive material were low and were in the following order: fats
> liver > kidney > blood > muscle. Residues in fat consisted
of unchanged cyhalothrin. The liver and kidney contained small
amounts of cyhalothrin, but the residues were largely due to a
number of ester-cleavage metabolites that were probably present
because the animals were still actively metabolizing and
eliminating a significant fraction of the most recent day's intake
of cyhalothrin. The almost two-fold difference in the plasma
levels of total radiolabelled components obtained with the
different labelled forms suggests that little cyhalothrin was
present in blood. The ester link must therefore be hydrolysed very
rapidly, apart from a small fraction that is distributed into fatty
tissues (Harrison, 1984d).
In studies by Sapiets (1985c), Friesian cows were fed for up to
thirty consecutive days on diets containing lambda-cyhalothrin at
1, 5, and 25 mg/kg. Lambda-cyhalothrin residues in milk correlated
well with dietary inclusion rates, the mean plateau residue levels
being 0.02 mg/kg, 0.09 mg/kg, and 0.52 mg/kg, respectively, for the
three dietary inclusion rates. Lambda-cyhalothrin residue levels
in milk did not accumulate, and they declined when feeding of the
treated diet ceased. At the end of the 30 days, three cows from
each group were sacrificed. The remaining two cows from the high-
dose group were fed an untreated diet for a further 14 days before
they too were slaughtered. Lambda-cyhalothrin residue levels in
the tissues of the sacrificed animals were as shown in Table 4.
Table 4. Lambda-cyhalothrin residues (mg/kg) in cow tissuesa
-----------------------------------------------------------------------------------------------
Dietary feeding Abductor Pectoral Subcutaneous Peritoneal Liver Kidney
rate (mg/kg) muscle muscle fat fat
-----------------------------------------------------------------------------------------------
1.0 < 0.01 < 0.01 0.01-0.21 0.07-0.50 < 0.01-0.03 0.01-0.02
5.0 0.01-0.03 0.03-0.07 0.44-0.81 0.95-1.8 < 0.01 0.01-0.07
25.0 0.08-0.14 0.02-0.41 1.3-4.6 3.9-7.9 0.06-0.10 0.09-0.43
25.0 + 14-day < 0.01-0.05 < 0.01-0.03 0.03-1.1 0.47-2.6 < 0.01 0.10-0.20
recovery period
Control < 0.01 < 0.01 < 0.01-0.02 < 0.01-0.07 < 0.01 < 0.01
-----------------------------------------------------------------------------------------------
a From: Sapiets (1985c).
5.2. Metabolism
The metabolic pathways that have been established for
cyhalothrin in mammals are summarized in Fig. 4.
4.5. Bioaccumulation and biomagnification
4.5.1. n-Octanol-water partition coefficient
In common with other synthetic pyrethroids, the n-octanol-water
partition coefficient of cyhalothrin is high; values for log Pow of
6.9 and 7.0 at 20 °C have been obtained for cyhalothrin and lambda-
cyhalothrin, respectively, using a generator column method
(personal communication by ICI Agrochemicals to the IPCS). However,
since the compound is insoluble in water (thus limiting exposures
of aquatic species) and is rapidly metabolized in animal systems to
the cyclopropanecarboxylic acid and 3-phenoxybenzoic acid, both of
which are polar compounds, no problem of bioaccumulation will
occur.
4.5.2. Bioaccumulation
In a study consisting of a 28-day exposure and a 28-day
depuration period, carp (Cyprinus carpio) were exposed to
cyhalothrin in a flow-through water system using 14C-labelled
cyhalothrin at a nominal concentration of 0.02 µg cyhalothrin
equivalent/litre. During the exposure period, the concentration of
the total 14C-labelled cyhalothrin in the carp reached an
equilibrium within 1-2 weeks. The bioconcentration factors
measured were: 4250-7340 in the viscera, 490-850 in the muscle,
1020-2290 in the remainder of the body, and 1660-2240 in the whole
fish. Rapid depuration of residues was observed; the biological
half-life of the total 14C-labelled cyhalothrin was 9 days in the
viscera, muscle, and whole fish (Yamauchi et al., 1984a).
The accumulation of cyhalothrin and its degradation products in
channel catfish and Daphnia magna has been investigated in a soil-
water system. 14C-cyclopropane-labelled cyhalothrin was applied at
50 g ai per ha and aerobically incubated in soil for 3 weeks prior
to flooding. Channel catfish and Daphnia magna were introduced for
exposure periods of 31 and 28 days, respectively, after which the
fish and daphnids were transferred to an uncontaminated system for
depuration periods of 42 and 7 days, respectively. Soil, water,
fish, and daphnids were ana-lysed for 14C-residues. Prior to
flooding, 14C-labelled residues in soil decreased to 60-70% of that
applied, 40% of which remained as extractable cyhalothrin.
Following flooding of the soil, 14C-labelled residues in the water
increased throughout the exposure period, reaching a level of 8% of
the applied radioactivity. No parent cyhalothrin was detected in
the water; the only product comprising more than 1% of the applied
radioactivity was cis-3-( ZE-2-chloro-3,3,3-trifluoroprop-1-enyl-
2,2-dimethylcyclopropane-carboxylic acid, which represented up to
5.3% of the applied radioactivity. During exposure, the maximum
bio-concentration factors in whole fish and daphnids were 19 and
194, respectively (Table 2). The concentration of 14C-residues in
fish and daphnids decreased during the depuration period, the
half-lives being approximately 7 days and 1 day, respectively
(Table 3) (Hamer & Hill, 1985).
Table 2. Bioconcentration factors for
cyhalothrin in Daphnia and fish
---------------------------------------------
Exposure Daphnia Fish Fish Whole
phase muscle viscera fish
(days)
---------------------------------------------
1 93 2 20 17
3 194 3 28 7
7 158 6 45 10
14 62 7 66 19
21 47 5 28 10
28 29 7 49 8
31 NR 7 48 9
---------------------------------------------
From: Hamer & Hill (1985)
NR = not recorded.
Table 3. Concentration of 14C-residues in
Daphnia and fish tissues
--------------------------------------------
Depuration % of tissue level on final day
phase of exposure
(days) Daphnia Fish Fish Whole
muscle viscera fish
--------------------------------------------
1 56 105 60 80
3 29 66 53 64
4 NR NR NR 61
7 11 51 4 38
14 NR 32 4 37
21 NR 31 6 27
42 NR 20 2 14
--------------------------------------------
From: Hamer & Hill (1985)
NR = not recorded.
5. KINETICS AND METABOLISM
5.1. Absorption, distribution, and excretion
5.1.1. Rat
In studies by Harrison (1981, 1984a,b), groups of six male and
six female Alderley Park rats received a single oral dose (1 or 25
mg/kg) of radiolabelled cyhalothrin in corn oil. As it was known
that the metabolism of related pyrethroids involves extensive
cleavage of the ester bond, duplicate experiments were performed
using two forms of cyhalothrin labelled with 14C in the acid (14C-
cyclopropyl) or alcohol (14C-benzyl) portions of the ester.
Excreta (urine, faeces and, in selected animals, expired air) were
collected for up to 7 days after dosing and analysed for total
radioactivity and metabolites by liquid scintillation counting and
thin-layer chromatography. Blood samples were also collected at
various times up to 48 h and analysed for total radioactivity and
unchanged cyhalothrin.
Following oral administration of cyhalothrin, absorption was
variable but accounted for about 55% of the dose. The proportions
absorbed were similar at both dose levels.
Excretion was rapid for both 14C-cyclopropyl- and 14C-benzyl-
labelled cyhalothrin at both dose levels, although excretion rates
were faster with the 14C-benzyl label than with the 14C-cyclopropyl
label. Urinary excretion accounted for approximately 20-40% of the
dose and faecal excretion for 40-65% of the dose during the first 7
days. Peak blood concentrations of radioactivity were reached
within 4-7 h, and by 48 h these concentrations had declined to 10%
or less of peak values. A small proportion of an oral dose (2-3%)
was retained in the animals after seven days; analysis of twelve
different tissues indicated that this radioactivity was present
mainly in white fat.
Results from a study in which rats were dosed subcutaneously
indicated that some of the dose was excreted via the bile.
In a further experiment to study the excretion and tissue
accumulation of cyhalothrin (1 mg/kg per day by gavage), groups of
six male and six female rats received daily doses of 14C-benzyl-
labelled or 14C-cyclopropyl-labelled cyhalothrin for 14 days. Urine
and faecal samples were collected every 24 h up to 7 days after the
final dose. Groups of animals were killed 2, 5, and 7 days after
the final dose and a range of tissues were removed for measurement
of residual radioactivity. Fat samples were analysed by HPLC for
unchanged cyhalothrin. The results demonstrated that the excretion
of 14C-material after multiple oral dosing was similar to that
which followed a single dose. Slightly higher overall excretion in
urine (up to 50% of the administered dose) was probably due to more
consistent oral absorption in this study. A large proportion of
the oral dose of cyhalothrin was rapidly eliminated from the body.
Analysis of tissue residues revealed that the small proportion
(< 5%) of the dose retained in white fat was unchanged
cyhalothrin, which was eliminated from this tissue with a half-life
of about 23 days (Harrison, 1981, 1984a,b).
A further study was undertaken in the rat to explore the
retention, in fat, of cyhalothrin and lambda-cyhalothrin. Groups
of male rats received daily oral doses of 14C-cyclopropyl-labelled
cyhalothrin (1 mg/kg per day) for up to 119 days. At intervals
during and after the dosing period, groups of three rats were
killed and the concentrations of radioactivity in the liver,
kidney, fat, and blood were determined. Additionally the
concentration in fat of lambda-cyhalothrin and its opposite
enantiomer pair (enantiomer pair A) was measured by high-pressure
liquid chromatography. Levels of radioactivity in the blood
remained fairly constant and low (approximately 0.2 µg cyhalothrin
equivalents per g) throughout the dosing period. In the liver and
kidney, the radioactivity reached a plateau, after approximately 70
days, at a level corresponding to approximately 2.5 µg cyhalothrin
equivalents per g liver and 1.2 µg per g kidney. The concentration
of cyhalothrin in fat at the end of the dosing period was
approximately 10 µg/g. After the cessation of dosing, levels of
radioactivity in the liver, kidney, and blood declined rapidly. In
fat, the levels declined more slowly with an elimination half-life
of 30 days. The radioactive material in fat was unchanged
cyhalothrin; the ratio of enantiomeric pairs, one of which was
lambda-cyhalothrin, was not significantly different from that in
the dosing solution, indicating that the rate of metabolism of
lambda-cyhalothrin was the same as cyhalothrin and that there was
no preferential accumulation of lambda-cyhalothrin (Prout, 1984).
A comparison of the absorption, distribution, excretion, and
metabolism of lambda-cyhalothrin and cyhalothrin was made to
establish whether the single enantiomer pair lambda-cyhalothrin
differed from cyhalothrin (a 50:50 mixture of lambda-cyhalothrin
and the opposite enantiomer pair A) (Prout & Howard, 1985). One
group of four male rats was given a single oral dose of 14C-
cyclopropyl-labelled lambda-cyhalothrin (1 mg/kg); a second group
of four male rats was given 14C-cyclopropyl-labelled lambda-
cyhalothrin (1 mg/kg) plus the unlabelled enantiomeric pair A (1
mg/kg); and a third group of four male rats was given a single oral
dose of a 50:50 mixture of 14C-cyclopropyl-labelled lambda-
cyhalothrin and 14C-labelled enantiomeric pair A (i.e. 14C-
cyclopropyl-labelled cyhalothrin at 1 mg/kg). The urinary and
faecal excretion of radioactivity was monitored in all three groups
for three days and the residual radioactivity was then determined
in selected tissues. The metabolite profile of the excreta was
determined by thin-layer chromatography. The results of this study
indicate that co-administration of enantiomer pair A with lambda-
cyhalothrin had little or no effect upon the absorption,
distribution, or tissue retention of radioactivity, and there was
no effect upon the metabolite profile of lambda-cyhalothrin.
Similarly, the absorption, distribution, excretion, and metabolism
of cyhalothrin was indistinguishable from that of lambda-
cyhalothrin, thus confirming the results of the bioaccumulation
study of Prout (1984).
5.1.2. Dog
The absorption, distribution, excretion, and metabolism of
cyhalothrin have been studied in the dog. As in the rat studies,
the experiments were duplicated using cyhalothrin labelled either
in the acid (14C-cyclopro-pyl) or alcohol (14C-benzyl) moieties of
the molecule. Groups of three male and three female beagle dogs
were given a single oral dose of cyhalothrin (1 mg/kg or 10 mg/kg)
and, after a 3-week interval, a further single intravenous
administration of 0.1 mg/kg. Samples of blood and excreta were
collected for 7 days after dosing and were analysed for total
radioactivity. The proportions of unchanged cyhalothrin and of
metabolites in urine and faeces were determined by thin-layer
chromatography. The identity of major metabolites was confirmed by
mass spectrometry.
The absorption of cyhalothrin after oral administration was
variable. The degree of absorption was difficult to assess but was
within the range 48%-80%. Excretion of radioactivity after both
oral and intravenous dosing was initially rapid, with most of the
administered radioactivity being excreted in the first 48 h after
dosing. After 7 days, a mean of 82-93% had been excreted (Harrison,
1984c).
5.1.3. Cow
After twice daily oral ingestion of 14C-benzyl- or 14C-
cyclopropyl-labelled cyhalothrin (1 mg/kg per day for 7 days),
absorption of the insecticide by cows was apparently slow and
incomplete. Approximately 50% of the dosed radioactivity was
excreted in the faeces, mainly as unchanged cyhalothrin, but only
small amounts were detected in the bile. With both labelled forms,
most of the radioactive material was rapidly eliminated in the
urine (27%) and faeces (49%) within 24 h of each daily dose. Only
a very small proportion of the dose was secreted in the milk (0.8%)
and this was found to be unchanged cyhalothrin. Tissue residues of
radioactive material were low and were in the following order: fats
> liver > kidney > blood > muscle. Residues in fat consisted
of unchanged cyhalothrin. The liver and kidney contained small
amounts of cyhalothrin, but the residues were largely due to a
number of ester-cleavage metabolites that were probably present
because the animals were still actively metabolizing and
eliminating a significant fraction of the most recent day's intake
of cyhalothrin. The almost two-fold difference in the plasma
levels of total radiolabelled components obtained with the
different labelled forms suggests that little cyhalothrin was
present in blood. The ester link must therefore be hydrolysed very
rapidly, apart from a small fraction that is distributed into fatty
tissues (Harrison, 1984d).
In studies by Sapiets (1985c), Friesian cows were fed for up to
thirty consecutive days on diets containing lambda-cyhalothrin at
1, 5, and 25 mg/kg. Lambda-cyhalothrin residues in milk correlated
well with dietary inclusion rates, the mean plateau residue levels
being 0.02 mg/kg, 0.09 mg/kg, and 0.52 mg/kg, respectively, for the
three dietary inclusion rates. Lambda-cyhalothrin residue levels
in milk did not accumulate, and they declined when feeding of the
treated diet ceased. At the end of the 30 days, three cows from
each group were sacrificed. The remaining two cows from the high-
dose group were fed an untreated diet for a further 14 days before
they too were slaughtered. Lambda-cyhalothrin residue levels in
the tissues of the sacrificed animals were as shown in Table 4.
Table 4. Lambda-cyhalothrin residues (mg/kg) in cow tissuesa
-----------------------------------------------------------------------------------------------
Dietary feeding Abductor Pectoral Subcutaneous Peritoneal Liver Kidney
rate (mg/kg) muscle muscle fat fat
-----------------------------------------------------------------------------------------------
1.0 < 0.01 < 0.01 0.01-0.21 0.07-0.50 < 0.01-0.03 0.01-0.02
5.0 0.01-0.03 0.03-0.07 0.44-0.81 0.95-1.8 < 0.01 0.01-0.07
25.0 0.08-0.14 0.02-0.41 1.3-4.6 3.9-7.9 0.06-0.10 0.09-0.43
25.0 + 14-day < 0.01-0.05 < 0.01-0.03 0.03-1.1 0.47-2.6 < 0.01 0.10-0.20
recovery period
Control < 0.01 < 0.01 < 0.01-0.02 < 0.01-0.07 < 0.01 < 0.01
-----------------------------------------------------------------------------------------------
a From: Sapiets (1985c).
5.2. Metabolism
The metabolic pathways that have been established for
cyhalothrin in mammals are summarized in Fig. 4.
 5.2.1. Rat
Identification of the metabolites produced in the rat studies
of Harrison (described in section 5.1.1) revealed that, following
oral administration, unabsorbed cyhalothrin was eliminated
unchanged via the faeces. The absorbed material was rapidly and
extensively metabolized and no unchanged cyhalothrin was present in
urine or bile. The main route of metabolism was, as anticipated,
via hydrolysis of the ester linkage (Fig. 4). The cyclopropane-
carboxylic acid moiety was subsequently excreted via the urine as
the glucuronide conjugate. This material accounted for about 50%
of the radioactivity in urine following dosing with 14C-
cyclopropyl-labelled cyhalothrin. The 3-phenoxybenzyl moiety was
further metabolized by loss of the nitrile group, oxidation of the
aldehyde formed to a carboxylic acid, aromatic hydroxylation at the
4' position, and formation of the 4- O-sulfate conjugate of 3-(4-
hydroxyphenoxy)benzoic acid. This conjugate accounted for
approximately 75% of the urinary radioactivity following dosing
with 14C-benzyl-labelled cyhalothrin. No metabolite containing the
ester function was detected (Harrison, 1983).
5.2.2. Dog
The main route of metabolism after oral administration is, as
in the rat, via cleavage of the ester bond. After intravenous
administration (0.1 mg/kg body weight), the patterns of metabolites
in urine were very similar to those seen in the oral studies. Very
little unchanged compound was present in the faeces or urine. The
phenoxy-benzyl moiety was further metabolized as in the rat; the
main metabolites were N-(3-phenoxybenzyl) glycine, 3-(4-
hydroxyphenoxy)benzoic acid and its sulfate conjugate, 3-
phenoxybenzoyl glucuronide, and a little free 3-phenoxybenzoic
acid. Other conjugated metabolites were also present. The
cyclopropane acid moiety was extensively metabolized to produce 11
metabolites. These included the cyclopropane acid glucuronide and
other conjugated metabolites. Thus, the metabolism of cyhalothrin
is dominated by cleavage of the ester bond (Fig. 4). Subsequent
metabolism of the products is similar both to that of other
pyrethroids and to the fate of cyhalothrin in other species
(Harrison, 1984c).
5.2.3. Cow
In common with other structurally related pyrethroids, the main
routes of metabolism of cyhalothrin in the cow have been found to
be similar to those observed in rats and dogs, i.e cleavage of the
ester bond with subsequent excretion of the cyclopropyl carboxylic
moiety, either free, hydroxylated, or as a glucuronide conjugate.
The phenoxybenzyl moiety was further metabolized by loss of the
nitrile group and excreted as free 3-phenoxybenzoic acid and its
amino acid conjugates, or after aromatic hydroxylation probably at
the 4'position. Cyhalothrin itself gives rise to residues in fats;
this is consistent with the lipophilic properties of cyhalothrin
compared to those of its more polar metabolites (Harrison, 1984d).
5.2.4. Goat
In a study by Leahey et al. (1985), a goat was dosed orally for
seven days with 14C-cyclopropyl-labelled lambda-cyhalothrin at a
rate equivalent to approximately 11 mg/kg diet. During dosing, the
maximum residue level in the milk was 0.27 mg cyhalothrin
equivalents/kg (mean value during days 3-7: 0.21 mg/kg), virtually
all of which was characterized as lambda-cyhalothrin. When the goat
was slaughtered 16 h after receiving the final dose, residues in
the tissues, expressed in cyhalothrin equivalents, were: meat,
0.024-0.028 mg/kg; fat, 0.13-0.44 mg/kg; liver, 0.34-0.35 mg/kg;
kidney, 0.20 mg/kg. The residues in meat and fat were due mainly
to lambda-cyhalothrin. However, in the liver and kidney, intact
pyrethroid accounted for only a small part of the residue. (1 RS)-
cis-(Z-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclo-
propanecarboxylic acid and 3-(2-chloro-3,3,3-trifluoro-prop-1-
enyl)-2-hydroxymethyl-2-methylcyclopropanecarboxylic acid were the
major components of the residue identified in liver and kidney.
5.2.5. Fish
In an accumulation study by Leahey & Parker (1985), carp were
maintained in a flow-through water system con-taining 14C-
cyclopropyl-labelled cyhalothrin (at a level of 20 ng/g) for 28
days. Results showed that radioactive residues in muscle, head,
and viscera were 0.035, 0.050, and 0.115 mg/kg, respectively. The
major part of the 14C-residue (50-65%) was characterized as
cyhalothrin, a further 10-19% consisting of the compound 1a (Fig. 2).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic organisms
6.1.1. Microorganisms
It has been shown that lambda-cyhalothrin, at a concentration
of 1.0 mg/litre, does not affect the growth of the single-celled
green alga Selenastrum capricornutum over a period of 96 h
(Thompson & Williams, 1985).
6.1.2. Invertebrates
6.1.2.1 Acute toxicity
Aquatic invertebrates show a wide range of susceptibility to
cyhalothrin and lambda-cyhalothrin. The data are summarized in
Table 5.
Table 5. Acute toxicity of cyhalothrin and lambda-cyhalothrin for aquatic invertebrates
-----------------------------------------------------------------------------------------
Species Stage Temper- Test substance Test 48-h Reference
ature system LC50
(°C) (ng/
litre)
-----------------------------------------------------------------------------------------
Freshwater
Water flea < 24 h 20 cyhalothrin static 160 Yamauchi et
(Daphnia pulex) 5% WP al. (1984d)
Water flea 12 h 20 technical static 380 Williams &
(Daphnia magna) (± 12 h) cyhalothrin Thompson
(1981)
Water flea < 24 h 20 technical lambda- static 360 Farrelly et
(Daphnia magna) cyhalothrin al. (1984)
< 24 h 20 lambda-cyhalothrin static 90a Farrelly et
5% EC al. (1985)
< 24 h 20 lambda-cyhalothrin static 90a Farrelly et
13% EC al. (1985)
Freshwater shrimp 5 mm 15 14C-lambda- flow- 8 Hamer et al.
(Gammarus pulex) cyhalothrin through (1985a)
Marine
Mysid shrimp < 48 h 25 14C-lambda- flow- 7.5 Thompson
(Mysidopsis bahia) cyhalothrin through (1985)
-----------------------------------------------------------------------------------------
a Concentration of active ingredient.
6.1.2.2 Long-term toxicity
The effects of lambda-cyhalothrin on the survival, growth, and
reproduction of Daphnia magna were investigated for a period of 21
days in a static water test with daily renewal of test solutions.
The nominal concentrations tested were 0, 2.5, 5.0, 10.0, 20.0,
and 40.0 ng/litre. Lambda-cyhalothrin affected all three parameters
at a nominal concentration of 40 ng/litre, but no effects were
noted at 2.5 ng/litre. Chemical analysis suggested that the
daphnids were exposed to about 60% of the nominal concentration.
These results show that the life-cycle no-observed-effect level of
lambda-cyhalothrin for daphnids is of the order of 2.5 ng/litre
(Hamer et al., 1985b).
6.1.3. Fish
6.1.3.1 Acute toxicity
Cyhalothrin and lambda-cyhalothrin are very toxic to fish in
clean water under laboratory conditions. The available data,
summarized in Table 6, demonstrate a similar high acute toxicity
for both cold and warm water species of fish.
6.1.3.2 Long-term toxicity
Sheepshead minnow Cyprinodon variegatus embryos and larvae were
continuously exposed (through 28 days post hatch) to mean measured
lambda-cyhalothrin concentrations of 0.04, 0.07, 0.14, 0.25, and
0.38 µg/litre, in a flow-through system. The test was performed in
duplicate. Assessments were made of percentage hatch and survival
of embryos and of total length and weight of the larvae at the
completion of the study. Hatchability was not affected (P < 0.05)
at any concentration in the carrier dimethylformamide or dilution
water controls, percentage hatch ranging from 81.3% to 100%.
Larval survival was not significantly affected. A significant
effect (P < 0.05) was found on the weight of the larvae at the
highest concen-tration tested, but not at any other concentration.
On the basis of these data the NOEL was 0.25 µg/litre and the
lowest-observed-effect level (LOEL) was 0.38 µg/litre (Hill et al.,
1985).
6.1.4. Model ecosystem
Cyhalothrin is readily adsorbed onto soil and suspended
particles, which in consequence significantly reduces its toxicity
to aquatic organisms (Yamauchi et al., 1984c). Daphnia pulex and
Cyprinus carpio were used in four different systems to test this
experimentally (Table 7). The median lethal concentrations (LC50)
were calculated, immobilization being used as the end-point for
Daphnia pulex.
Table 6. Acute toxicity of cyhalothrin and lambda-cyhalothrin to fish
---------------------------------------------------------------------------------------------------
Species Weight Test substance and vehicle Temper- 96-h Reference
(g) ature LC50
(°C) (µg/
litre)
---------------------------------------------------------------------------------------------------
Rainbow trout 0.32-1.37 technical cyhalothrin 12 0.54 Hill (1981a)
(Salmo gairdneri) dispersed via acetone
0.30-1.48 technical lambda-cyhalothrin 12 0.24 Hill (1984a)
dispersed via acetone
0.99-2.32 lambda-cyhalothrin 2.5% 16 0.39 Hill (1985a)
EC dispersed in water
1.28-4.78 lambda-cyhalothrin 13% EC 12 0.44 Hill (1985b)
dispersed in water
0.87-4.09 lambda-cyhalothrin 5% EC 16 0.93 Hill (1985c)
dispersed in water
Carp 5.2 technical cyhalothrin 23-35 1.34a Takeda Chemical
(Cyprinus carpio) dispersed via Co. Ltd.
dimethylformamide (1979)
5.4 cyhalothrin 5% WP 25 1.1 Yamauchi et al.
(1984b)
1.48-5.8 lambda-cyhalothrin 2.5% 22 0.54 Hill (1985d)
EC dispersed in water
3.92-7.28 lambda-cyhalothrin 5% EC 22 0.50 Hill (1985e)
dispersed in water
Bluegill sunfish 0.23-0.84 technical cyhalothrin 22 0.46 Reynolds (1984)
(Lepomis macrochirus) dispersed via acetone
0.7-2.6 technical lambda- 22 0.21 Hill (1984b)
cyhalothrin dispersed
via acetone
0.47-2.06 lambda-cyhalothrin 13% 22 0.28 Hill (1985f)
EC dispersed in water
Sheephead minnow 0.32-0.91 technical lambda- 22 0.81 Hill (1985g)
(Cyprinodon variegatus) cyhalothrin dispersed
via acetone
---------------------------------------------------------------------------------------------------
a 72-h LC50.
Table 7. Effect of soil on the toxicity (72-h LC50 in µg/
litre) of cyhalothrin to Daphnia pulex and Cyprinus carpioa
--------------------------------------------------------------
Conditions Daphnia pulex Cyprinus carpio
--------------------------------------------------------------
Application to water surface 0.4 9
(without soil)
Application to water surface 1.0 32
(soil undisturbed)
Application to water surface 16 57
(soil suspended)
Application to soil 70 642
(soil undisturbed)
--------------------------------------------------------------
a From: Yamauchi et al (1984c).
6.2. Terrestrial organisms
6.2.1. Birds
6.2.1.1 Acute toxicity
The toxicity of single oral doses of cyhalothrin and lambda-
cyhalothrin for birds is summarized in Table 8.
The 5-day dietary LC50 for cyhalothrin and lambda-cyhalothrin
has been measured in Anas platyrhynchos and Colinus virginianus
(Table 9).
Table 8. Oral toxicity of cyhalothrin and lambda-cyhalothrin for birds
---------------------------------------------------------------------------------------
Species Age Test substance Observation LD50 (mg/kg References
and vehicle period body weight)
---------------------------------------------------------------------------------------
Domestic hen adult cyhalothrin in 14 days > 10 000 Roberts
(Gallus domesticus) corn oil et al.
(1982)
Mallard duck adult cyhalothrin in 14 days > 5000 Roberts &
(Anas platyrhynchos) corn oil Fairley
(1981)
Mallard duck adult lambda-cyhalothrin 14 days > 3950 Roberts &
(Anas platyrhynchos) in corn oil Fairley
(1984)
---------------------------------------------------------------------------------------
Table 9. Dietary LC50 of cyhalothrin and lambda-cyhalothrin for birds
---------------------------------------------------------------------------------------
Species Age Test substance Observation LC50 Reference
period (post (mg/kg
treatment) diet)
---------------------------------------------------------------------------------------
Mallard duck 10 days cyhalothrin 3 days 14 000 Roberts et
(Anas platyrhynchos) al. (1981a)
Mallard duck 8 days lambda-cyhalothrin 4 days 3948 Roberts et
(Anas platyrhynchos) al. (1985a)
Bobwhite quail 10 days cyhalothrin 3 days > 7530 Roberts et
(Colinus virginianus) al. (1981b)
Bobwhite quail 11 days lambda-cyhalothrin 3 days > 5300 Roberts et
(Colinus virginianus) al. (1985b)
---------------------------------------------------------------------------------------
6.2.2. Honey-bees
Cyhalothrin and lambda-cyhalothrin have been shown to be toxic
to honey-bees (Apis mellifera) in laboratory tests (Table 10).
Table 10. Toxicity of cyhalothrin and lambda-cyhalothrin for
honey-bees (expressed as 24-h LD50 in µg ai per bee)
----------------------------------------------------------------------
Formulation Topical Oral Reference
application administration
----------------------------------------------------------------------
Technical cyhalothrin 0.027 - Smart &
Stevenson
(1982)
Technical lambda-cyhalothrin 0.051 0.97 Gough et
al. (1984)
Lambda-cyhalothrin EC (5%) 0.095 0.57 Gough et
al. (1984)
----------------------------------------------------------------------
In common with other pyrethroids, the high laboratory toxicity
of lambda-cyhalothrin is not translated into a significant field
hazard to bees. In two trials on flowering rape, lambda-
cyhalothrin (JF 9509) EC was applied, at midday, by helicopter at a
concentration of 10 g ai/ha to fields where hives of honey-bees
were located. A toxic standard and untreated control were used for
comparison. Bees were actively foraging during spraying, and the
hives were oversprayed. Mortality, foraging activity, activity at
the hive, and brood development were monitored before and after
treatment, and pollen, honey, and wax were analyzed for residues.
Apart from a suppression of foraging lasting up to 1.5 h, the
lambda-cyhalothrin formulation had no effect on the bees, whereas
the toxic standard killed large numbers. Only low levels of
residues were detected (pollen, 0.44 µg/g; honey, 0.01 µg/g; wax,
0.01 µg/g). It was concluded that, at 10 g ai/ha, lambda-
cyhalothrin formulation (JF 9509) EC is non-hazardous to honey-bees
on flowering rape (Gough et al., 1985).
6.2.3. Earthworms
Three annual applications of lambda-cyhalothrin at rates of up
to 250 g ai/ha to field plots had no adverse effect on populations
of individual species of earthworms or total earthworm numbers or
weight (Coulson et al., 1986).
6.2.4. Higher plants
No phytotoxic effects have been reported.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposures
7.1.1. Oral
The acute oral toxicity of cyhalothrin and lambda-cyhalothrin
in corn oil has been determined for several species (Table 11). The
toxicity of cyhalothrin is moderate (LD50 values: rat, 144-243
mg/kg; mouse, 37-62 mg/kg), whereas that of lambda-cyhalothrin is
higher (LD50 values: rat, 56-79 mg/kg; mouse, 20 mg/kg). Signs of
intoxication are characteristic of type II pyrethroid toxicity and
included piloerection, subdued behaviour, ataxia, unsteady gait,
salivation, incontinence, scouring, and chromodacryorrhoea.
Table 11. Acute oral toxicity of technical cyhalothrin and
lambda-cyhalothrin in corn oil
------------------------------------------------------------------
Species Test substance LD50 (mg/kg Reference
body weight)
------------------------------------------------------------------
Rat cyhalothrin 243 (male) Nixon &
144 (female) Jackson (1981a)
Rat lambda-cyhalothrin 79 (male) Southwood (1985)
56 (female)
Mouse cyhalothrin 36.7 (male) Nixon &
62.3 (female) Jackson (1981a)
Mouse lambda-cyhalothrin 19.9 (male) Southwood (1984)
19.9 (female)
Guinea-pig cyhalothrin > 5000 (male) Nixon &
Jackson (1981a)
Rabbit cyhalothrin > 1000 (female) Nixon &
Jackson (1981a)
------------------------------------------------------------------
7.1.2. Percutaneous
The percutaneous toxicity of cyhalothrin and lambda-cyhalothrin
is summarized in Table 12. Signs of intoxication were similar to
those seen after oral ingestion, and included incontinence,
scouring, dehydration, subdued behaviour, curvature of the spine,
unsteady gait, nervous appearance, piloerection, and increased
vocalization when handled.
Table 12. Percutaneous toxicity of technical cyhalothrin and
lambda-cyhalothrin in polyethylene glycol paste
-----------------------------------------------------------------
Species Test substance LD50 (mg/kg Reference
body weight)
-----------------------------------------------------------------
Rat cyhalothrin 1000-2000 (male) Nixon &
200-2000 (female) Jackson (1981a)
Rat lambda-cyhalothrin 632 (male) Barber (1985)
696 (female)
Rabbit cyhalothrin > 2000 (male) Nixon &
> 2000 (female) Jackson (1981a)
-----------------------------------------------------------------
7.1.3. Intraperitoneal
The intraperitoneal LD50 of cyhalothrin to rats is in the range
of 250 to 750 mg/kg. Responses of 0% or 100% mortality were
observed at all but one dose, and so no precise value for the LD50
could be given. The highest dose with no mortality was 250 mg/kg
and the lowest dose with 100% mortality was 750 mg/kg (Nixon &
Jackson, 1981a).
7.2. Irritation and sensitization
7.2.1. Irritation
Undiluted technical cyhalothrin is a mild irritant to occluded
rabbit skin (both intact and abraded) but is non-irritant to
occluded rat skin (intact) (Jackson & Nixon, 1981). Technical
cyhalothrin is a moderate irritant to the rabbit eye without
irrigation and is a mild irritant when irrigated for one minute, 20
to 30 seconds after instillation of the material (Jackson, 1981).
Technical lambda-cyhalothrin is non-irritant to occluded
rabbit skin without abrasion (Pritchard, 1985a). It is a mild
irritant to the rabbit eye (Pritchard, 1985b).
7.2.2. Sensitization
A skin sensitization test with cyhalothrin on guinea-pigs,
using the procedure of Buehler, indicated that cyhalothrin has
skin-sensitizing potential (Nixon & Jackson, 1981b). In guinea-
pigs that had been previously induced with undiluted cyhalothrin
technical material, using the Magnusson and Kligman maximization
test, a moderate sensitization response was elicited. When
lambda-cyhalothrin was tested for skin sensitization on guinea-
pigs, using the maximization procedure of Magnusson and Kligmann,
it was shown to have no sensitization potential (Pritchard, 1984).
7.3. Short-term exposures
7.3.1. Oral
7.3.1.1 Rat
When groups of male and female rats were fed diets containing
cyhalothrin at levels up to 750 mg/kg for 28 days, symptoms of
toxicity shown by animals receiving doses of 250 mg/kg diet or more
included high stepping gait, ataxia, and hypersensitivity to
external stimuli. These effects were dose related and some
mortality occurred at the highest dose level. The clinical signs
were not accompanied by histopathological changes in the nervous
system. Thymic atrophy, adrenal enlargement with vacuolization,
and incomplete spermatogenesis occurred with the highest dose. An
adaptive change was present in the liver as judged by an increase
in liver weight, proliferation of smooth endoplasmic reticulum,
and an increase in the activity of the xenobiotic-metabolizing
enzyme, aminopyrine- N-demethylase (APDM). These effects occurred at
doses of 100 mg/kg or more, and there was some evidence for this
type of change at 20 mg/kg (Tinson et al., 1984).
When groups of 20 male and 20 female Wistar rats were fed
cyhalothrin (89% pure) at dietary concentrations of 0, 10, 50, and
250 mg/kg for 90 days, only male rats fed 250 mg/kg showed
significantly reduced body weight gain. No abnormal clinical signs
were seen. Haemoglobin and haematocrit values were not affected by
the treatment, but there were marginal effects on mean erythrocyte
volume in some treated groups. Male rats fed 50 or 250 mg/kg
showed decreased plasma triglyceride levels and a dose-related
increase in hepatic APDM activity. The latter effect was also seen
in females fed 250 mg/kg. Small increases in urinary glucose
excretion occurred in males fed 50 or 250 mg/kg after 13 weeks. No
treatment-related effects on organ weights and no significant
histopathological changes were reported (Lindsay et al., 1981).
In studies by Lindsay et al. (1982), two groups of 32 male rats
were fed either a control diet or a diet containing 250 mg
cyhalothrin/kg for 28 days. After this period eight rats per group
were killed and examined. The remaining rats were fed the control
diet for periods of 7, 14, or 28 days after cessation of treatment,
and a further eight rats per group were killed and examined at each
of these time intervals. A decrease in body weight gain was seen
in the treated rats, which, although not statistically
significant, was still much reduced at the end of the 28-day
recovery period. Proliferation of the hepatic smooth endoplasmic
reticulum and elevated hepatic APDM activity were also seen in the
treated animals. These effects had reversed 7 days after the
cessation of treatment with cyhalothrin and were considered to be
physiological adaptive changes rather than a toxicological effect.
When groups of 20 male and 20 female Alderley Park rats
received diets containing 0, 10, 50, or 250 mg lambda-
cyhalothrin/kg for 90 days, decreased body weight gain, accompanied
by a reduction in food consumption, was seen in both male and
female rats receiving the highest dose. No abnormal clinical signs
were seen; in particular, there was no evidence of neurological
effects. There were no treatment-related effects on haematological
parameters but reductions in the activities of plasma alanine
trans-aminase (males only) and alkaline phosphatase (females only
at 13 weeks) were apparent in animals fed the highest dose. Plasma
triglycerides were also reduced in males at this feeding level.
Relative liver weights were increased in both sexes at 250 mg/kg
and in males at 50 mg/kg, accompanied by increased activity of
hepatic APDM. No other changes in organ weight or histopathology
were attributed to treatment with lambda-cyhalothrin. These two
effects, particularly in view of the findings with cyhalothrin,
were considered to be adaptive in nature and the toxicological NOEL
was established at 50 mg/kg (equivalent to 2.5 mg/kg body weight
per day (Hart et al., 1985).
7.3.1.2 Dog
In studies by Chesterman et al. (1980), groups of one male and
one female dog (Alderley Park beagles) received 0, 2.5, or 10 mg
cyhalothrin/kg body weight orally in corn oil by gelatine capsule
daily for four weeks. A further group initially received 30 mg/kg
per day, but due to severe clinical signs after 10 days dosing,
which were typical of pyrethroid toxicity (muscular trembling,
unsteadiness, vomiting, and body weight loss), the animals were
rested and then received 20 mg/kg per day for four weeks. Similar
clinical signs were seen, with the exception of body weight loss,
in all animals receiving 10 mg/kg per day or more, the severity
being dose related. Investigation of the sciatic and tibial nerves
and the lumbricalis muscle with special histopathological stains
indicated no changes that could be attributed to treatment with
cyhalothrin. Liquid faeces were produced by all animals receiving
cyhalothrin, the incidence being dose related. These changes were
considered to be of no toxicological significance. No other
changes were observed that could be attributed to the treatment.
When groups of 6 male and 6 female dogs (Alderley Park beagles)
were fed cyhalothrin in corn oil by gelatine capsule at 0, 1, 2.5,
or 10 mg/kg body weight per day for 26 weeks, signs of pyrethroid
toxicity were seen in some dogs at 10 mg/kg. Although liquid
faeces were produced by all animals in the study, including the
controls, the incidence and frequency were higher in treated
animals and were dose related. These changes were considered to be
of no toxicological significance. Macroscopic postmortem
examination, organ weights, and histological investigations
revealed no treatment-related changes. The oral NOEL in dogs was
found to be 2.5 mg/kg per day (Chesterman et al., 1981).
In studies by Hext et al. (1986), groups of 6 male and 6 female
dogs were dosed by gavage in corn oil with 0, 0.1, 0.5, or 3.5 mg
lambda-cyhalothrin/kg body weight daily for 52 weeks. Clinical
signs of neurological effects were evident in all animals fed the
highest dose, which were unaccompanied by histological changes in
the nervous system. There was an increased incidence of fluid
faeces at the highest dose and a slight increase in the group
receiving 0.5 mg/kg per day. This effect was considered to be
related to the method of administration and not to be
toxicologically significant. No histopathological changes
attributable to lambda-cyhalothrin administration were observed at
any of the dose levels employed, and the toxicological NOEL in
this study was 0.5 mg lambda-cyhalothrin per kg per day.
7.3.2. Dermal
7.3.2.1 Rabbit
Cyhalothrin in polyethylene glycol (PEG 300) (10, 100, or 1000
mg/kg body weight per day) was applied to the skin of groups of 10
male and 10 female New Zealand White rabbits and kept in contact
with the skin 6 h/day, 5 days per week for 3 weeks (i.e. a total
of 15 applications) by means of an occlusive dressing. A group of
14 male and 14 female control rabbits was treated with polyethylene
glycol (PEG 300) using the same procedure. The skin of half the
animals in each group was abraded prior to the application of
cyhalothrin. Repeated application of the vehicle alone
(polyethylene glycol) and the vehicle plus cyhalothrin caused
slight to severe skin irritation. At the highest dose level there
was an increased incidence of oedema and erythema. A small number
of animals given the highest dose showed pyrethroid-like symptoms,
but only when the skin was unabraded. The NOEL was considered to be
100 mg/kg per day (Henderson & Jackson, 1982).
7.4. Long-term exposures and carcinogenicity
7.4.1. Rat
In studies by Pigott et al. (1984), groups of 72 male and 72
female Alpk/AP strain rats were fed diets containing cyhalothrin
at levels of 0, 10, 50, or 250 mg/kg diet for up to 104 weeks. All
the surviving animals were sacrificed, and histopathological and
gross postmortem examinations were carried out. Decreased body
weight gain, accompanied by a small decrease in food consumption,
was evident in rats of both sexes fed the highest dose. This was
accompanied by minor changes in blood biochemistry. Increased liver
weight was seen in rats of both sexes fed cyhalothrin at 250 mg/kg
at the interim sacrifice but this was not evident at termination.
There was no histopathological evidence of a chronic toxic effect
due to cyhalothrin. In particular clinical and histopathological
evaluation gave no indication of an effect on the nervous system.
There was no evidence for a carcinogenic effect of cyhalothrin.
The toxicological NOEL for this study was 50 mg cyhalothrin/kg
diet, corresponding to a minimum dose rate of approximately 1.7
mg/kg body weight per day for male rats and 1.9 mg/kg per day for
female rats.
7.4.2. Mouse
Groups of 52 male and 52 female Charles River CD-1 mice were
maintained for 104 weeks on diets containing 0, 20, 100, or 500 mg
cyhalothrin/kg and further groups of 12 males and 12 females were
designated for interim sacrifice after 52 weeks. During the study
there were no deaths attributable to treatment with cyhalothrin.
Signs of toxicity ascribable to cyhalothrin included piloerection
and hunched posture in both sexes at 500 mg/kg and in males at 100
mg/kg and reduced body weight gain, higher food intake, and
reduced efficiency of food utilization in males receiving 500
mg/kg. There was a statistically significant increase, compared to
the controls, in the incidence of mammary adenocarcinoma in females
at the two highest dose levels. However, the frequency of these
tumours was not unduly at variance with that normally seen in the
strain of mouse used, and no dose relationship was apparent. Thus,
there were no neoplastic findings that could be attributed to the
long-term administration of cyhalothrin. There was a clear NOEL of
20 mg/kg, corresponding to a mean calculated daily intake of 1.8
mg/kg body weight per day in males and 2.0 mg/kg body weight per
day in females (Colley et al., 1984).
7.5. Reproduction, embryotoxicity, and teratogenicity
7.5.1. Reproduction
In studies by Milburn et al. (1984), groups of 15 male and 30
female (F0 parents) weaning Alderley Park rats were fed diets
containing 0, 10, 30, or 100 mg cyhalothrin per kg. After 12
weeks, the animals were mated to produce the first (F1a) litter and
subsequently re-mated to produce a second (F1b) litter. The
breeding programme was repeated with F1 parents selected from the
F1b off-spring and F2 parents selected from the F2b offspring.
Test diets were fed continuously throughout the study. There were
minor effects on body weight gain of parents from all generations
receiving 100 mg/kg, but no clinical signs of neurological effects
were seen in either parents or offspring. No effects of treatment
were seen on indices of male and female fertility, gestation
period, live born index, or pup survival. There was a small
reduction in mean total litter weight of the F2 and F3 generations
from rats receiving the highest dose, which was attributable to
minor decreases in litter size and a small reduction in weight gain
of the pups. No effect was seen in litters from rats receiving 30
mg/kg. There was no evidence of gross or histopathological change
attributable to the treatment. The reproductive effects seen in
rats receiving the highest dose were of a minor nature. A clear
NOEL of 30 mg/kg (corresponding to a dosage in the range of 1.5 to
1.9 mg/kg body weight per day) was established (Milburn et al.,
1984).
7.5.2. Embryotoxicity and teratogenicity
7.5.2.1 Rat
When groups of 24 mated female rats (Charles River CD-1) were
given cyhalothrin orally (in corn oil), at 0, 5, 10, or 15 mg/kg
body weight per day, from day 6 to 15 inclusive of gestation and
were killed on day 20, there was reduced body weight gain at the
highest dose level and evidence of mild pyrethroid toxicity in two
of these animals. There were no other effects on the clinical or
litter parameters attributable to treatment with cyhalothrin, and
examination of the viscera and skeletons showed no effects of
treatment. At the highest dose level, there was maternal toxicity,
but there was no effect on any aspect of fetal development at any
dose level (Killick, 1981a).
7.5.2.2 Rabbit
In studies by Killick (1981b), groups of at least 18 pregnant
New Zealand White rabbits received cyhalothrin orally in corn oil
daily at 0, 3, 10, or 30 mg/kg body weight, from days 6 to 18
(inclusive) of gestation and were killed on day 28. There was
reduced body weight gain at the highest dose, accompanied by
reduced food intake during dosing. There were no clinical signs and
no changes in pregnancy incidence or in litter parameters
attributable to treatment with cyhalothrin. Examination of the
viscera and skeletons revealed no effects of treatment. At the
highest dose level, there was maternal toxicity, but there was no
effect on any aspect of fetal development at any dose level.
7.6. Mutagenicity and related end-points
7.6.1. Microorganisms
Five test strains, TA1535, TA1537, TA1538, TA98, and TA100,
were employed to evaluate the mutagenic potential of cyhalothrin
using the salmonella reverse mutation assay of Ames. The assay was
conducted in the presence and absence of metabolic activation (S9
mix) with cyhalothrin at levels up to 2500 µg/plate. The mean
numbers of revertant colonies of Salmonella typhimurium observed in
the five test strains indicated an unequivocal negative response
(Trueman, 1981). Lambda-cyhalothrin at dose levels of up to 5000
µg/plate, both in the presence and absence of metabolic activation,
gave a non-mutagenic response in the same test using the same
strains (Callander, 1984).
7.6.2. In vitro mammalian cells
When cyhalothrin was tested in a modification of the cell
culture transformation test of Styles, using Syrian Hamster kidney
cell line BHK21C13, the response in the presence of metabolic
activation was unequivocally negative. In the absence of metabolic
transformation there was an erratic increase in numbers of
transformed colonies together with a poor dose response. These
data were not thought to indicate a significant positive response,
and it was concluded that cyhalothrin does not appear to possess
significant cell-transforming properties (Richold et al., 1981).
In addition, the significance of the results from the BHK cell
system is doubtful in view of the questionable interlaboratory
reproducibility of this assay.
The mutagenic potential of lambda-cyhalothrin has been assessed
in vitro with L51787 mouse lymphoma cells, both in the presence and
absence of auxiliary metabolic activation (S9) mix, using dose
levels of 125-1000, 2000, and 4000 µg/ml. There was no increase in
mutation frequency either in the presence or absence of S9 mix
(Cross, 1985).
Lambda-cyhalothrin, at dose levels of up to 1000 µg/ml, either
in the presence or absence of metabolic activation, did not induce
statistically significant increases in the incorporation of
tritiated thymidine in cultured human (Hela) cells (Milone, 1986)
or induce chromosomal damage in human lymphocytes stimulated by
phytohaemagglutinin (Sheldon et al., 1985).
7.6.3. In vivo mammalian assays
Male rats were given a single dose or five consecutive daily
doses by gavage of cyhalothrin at levels of 1.5, 7.5, or 15 mg/kg,
and bone marrow samples were taken and examined for chromosomal
abnormalities. The results indicated that cyhalothrin has no
clastogenic potential (Anderson et al., 1981).
In studies by Irvine (1981), three groups of male mice were
dosed with cyhalothrin by gavage, at dose levels of 1, 5, or 10
mg/kg daily, for 5 consecutive days. A further group received the
known mutagen cyclophosphamide intraperitoneally at 200 mg/kg
daily for five days. The animals were then mated with groups of
females at weekly intervals for eight weeks. Pregnancy incidence,
pre- and post-implantation loss, clinical condition, body weight,
and gross necropsy were assessed. There was no evidence of an
increase in the dominant lethal mutation frequency following
treatment. The NOEL was 10 mg/kg per day. Although these studies
showed no clastogenic or mutagenic effect, it is not clear whether
sufficiently high dose levels were used.
When lambda-cyhalothrin was administered to mice at levels of
up to 35 mg/kg and bone marrow preparations were examined for the
formation of micronuclei in polychromatic erythrocytes, there was
no statistically significant increase in the frequency of
micronuclei, compared to control animals. The positive control
substance, cyclophosphamide, showed the expected response (Sheldon
et al., 1984).
7.7. Mode of action
The mode of action of cyhalothrin and lambda-cyhalothrin, both
type II pyrethroids, is basically the same as that of the other
pyrethroids (see Appendix).
8. EFFECTS ON HUMANS
8.1. General population exposure
No poisoning incidents with cyhalothrin or lambda-cyhalothrin
have been reported. There is no information on effects from short-
or long-term exposure and no epidemiological information is
available.
8.2. Occupational exposure
Cyhalothrin is known to produce an effect described as
subjective facial sensation (SFS) in some people working with this
compound. SFS is a transient phenomenon; symptoms are not
associated with objective physical signs and recovery appears to be
complete. It is likely that SFS arises from direct facial contact
with the chemical particularly from touching the face with
contaminated gloves or hands. This would also help to explain the
effect of formulation or concentration as these could affect the
rate of dermal penetration and therefore access of the chemical to
the nerve endings.
It appears unlikely that individual sensitivity is important
in the development of symptoms following exposure. It is more
likely to be related to the amount of the chemical that comes into
contact with the facial skin. The information currently available
(Hart, 1984) is summarized in section 8.2.2.
8.2.1. Acute toxicity: poisoning incidents
No poisoning incidents involving cyhalothrin or lambda-
cyhalothrin have been reported.
8.2.2. Effects of short- and long-term exposure
Laboratory workers and manufacturing plant and field operators
handling natural and synthetic pyrethroids, including cyhalothrin
and lambda-cyhalothrin, have noticed a transient skin sensation in
the periorbital area of the face and other sites after direct skin
exposure. It has been suggested that these sensations are caused
by spontaneous repetitive firing of sensory nerve fibres or nerve
endings, whose threshold has been transiently lowered by the
compound, localized around the sites of exposure.
Symptoms from exposure to a synthetic pyrethroid generally
start 30 min to 1 h after exposure and last for several hours,
sometimes up to 2 days. They almost always affect the facial area,
producing a tingling, burning, or numb sensation that has been
variously described as paraesthesia, dysaesthesia, or SFS. The
latter term appears most appropriate as no objective signs of
abnormality of nerve function have been found in workers suffering
from these effects and the facial area is by far the most commonly
affected.
8.2.2.1 Manufacture
At least 12 workers involved in the manufacture of cyhalothrin
have experienced symptoms of SFS. The symptoms were described as
burning, tingling, or sunburn, but clinical examination revealed no
abnormal neurological signs. The areas of the face affected were
the eyelids, cheeks, nostrils, forehead, and lips. In one case the
penis was affected, probably from contact with contaminated hands.
The symptoms started between 5 min and 3 h from the onset of
exposure and lasted from 5 to 30 h. Wind, washing, and temperature
increase led to a worsening of the symptoms, and relief could be
obtained by using a local anaesthetic. Barrier creams were
relatively ineffective in preventing the symptoms.
8.2.2.2 Formulation and laboratory work
Two workers have been reported to have suffered from SFS. Both
were exposed to technical cyhalothrin and symptoms began 1-6 h
after exposure. The symptoms described were typical of SFS,
consisting of tingling and burning around the eyes and facial area,
and lasted 6-24 h.
Four reports involving three individuals have been received
from laboratories involved with lambda-cyhalothrin. The symptoms
consisted of a facial tingling and burning sensation and in one
case tingling on the tongue. Symptoms began within 30 min of
exposure and lasted from 6 h to 2 days. All three workers were
involved in handling either technical material or concentrated
lambda-cyhalothrin solutions (10% w/v ai).
8.2.2.3 Field use
Virtually no reports have been received on the agricultural or
public health use of cyhalothrin. The only information that has
become available is from the use of cyhalothrin in animal health
products. Skin itching has been reported following exposure to a
5% emulsifiable concentrate of cyhalothrin, but dilutions of 0.1%
and 0.005% (w/v of ai) were without significant effect. More
recently, several incidents of facial irritation have been reported
following the use of cyhalothrin in sheep sprays (Hart, 1984). The
concentration used in these sprays was very low (0.002% w/v), but
the characteristics of the spray used were unclear.
Out of 38 field trials staff who returned questionnaires, only
four reported experiencing any adverse effects as a result of
exposure to lambda-cyhalothrin. Three of these individuals
experienced a burning sensation or irritation around the face,
cheeks, or eyes, which started between 45 and 60 min after initial
exposure and lasted, respectively, for 5, 18, and 72 h. In one case
the symptoms were severe enough to prevent the individual from
working. The other field trials worker experienced a rash on the
hands that started 24 h after exposure and lasted several days. All
four field trials staff were involved in handling concentrated
solutions of lambda-cyhalothrin (2.5% w/v ai) and three of the four
sprayed diluted solutions. None of the three workers experiencing
the facial sensation had experienced similar symptoms before, but
the worker who developed the rash had suffered a similar rash after
exposure to permethrin, cypermethrin, and deltamethrin following
their application to top fruit. Eight of the other 34 field trials
staff returning questionnaires stated that they had previously
suffered symptoms of facial tingling or burning.
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Cyhalothrin was discussed at the 1984 and 1986 FAO/WHO Joint
Meetings on Pesticide Residues (JMPR), where an acceptable daily
intake (ADI) for cyhalothrin of 0-0.02 mg/kg body weight was
established (FAO/WHO, 1985, 1986a).
The JMPR (FAO/WHO, 1985, 1986a,b) has estimated maximum
residue limits (MRLs) for cyhalothrin (sum of the isomers) as
follows:
---------------------------------------------------------
Commodity Maximum Pre-harvest
residue limit interval (days)
---------------------------------------------------------
Pome fruits 0.2 14
Cabbages, head 0.2 3-4
Potatoes 0.02a non specified
Cotton seed 0.02a 21
Cotton seed oil, crude 0.02a non specified
Cotton seed oil, edible 0.02a non specified
---------------------------------------------------------
a At or about the limit of detection.
REFERENCES
ANDERSON, D., RICHARDSON, C.R., HULME, A., MORRIS, J., BANHAM, P.B., &
GODLEY, M.J. (1981) Cyhalothrin: A cytogenic study in the rat (Unpublished
proprietary report No. CTL/P/664, submitted to WHO by ICI).
BARBER, J.E. (1985) PP321: Acute dermal toxicity study (Unpublished
proprietary report No. CTL/P/1119, submitted to WHO by ICI).
BENGSTONE, M., DAVIES, R.A.H., DESMARCHELIER, J.M., HENNING, R., MURRAY,
W., SIMPSON, B.W., SNELSON, J.T., STICKA, R., & WALLBANK, B.E. (1983)
Organophosphorothioates and synergised synthetic pyrethroids as grain
protectants on bulk wheat. Pestic. Sci., 14: 373-384.
BEWICK, D.W. & ZINNER, C.K.J. (1981) Cyhalothrin: Degradation in soil
(Unpublished proprietary report No. RJ0203B, submitted to WHO by ICI).
BHARTI, H. & BEWICK, D.W. (1986) Cyhalothrin: Degradation in a Japanese
soil (Unpublished proprietary report No. RJ0491B, submitted to WHO by ICI).
BHARTI, H., BEWICK, D.W., & WHITE, R.D. (1985) PP563 and PP321: Degradation
in soil (Unpublished proprietary report No. RJ0382B, submitted to WHO by
ICI).
CALLANDER, R.D. (1984) PP321: An evaluation in the Salmonella mutagenicity
assay (Unpublished proprietary report No. CTL/P/1000, submitted to WHO by
ICI).
CHESTERMAN, H., HEYWOOD, R., ALLEN, T.R., STREET, A.E., PRENTICE, D.,
BUCKLEY, P., & OFFER, J. (1980) PP563: Preliminary oral toxicity study in
beagle dogs (Unpublished proprietary report of the Huntingdon Research
Centre No. 325/8074, submitted to WHO by ICI).
CHESTERMAN, H., HAYWOOD, R., ALLEN, T.R., STREET, A.E., KELLY, D.F.,
GOPINATH, C., & PRENTICE, D.E. (1981) Cyhalothrin oral toxicity study in
beagle dogs (Final report: Repeated daily dosing for 26 weeks) (Unpublished
proprietary report of the Huntingdon Research Centre No. ICI/326/8162,
submitted to WHO by ICI).
COLLEY, J., DAWE, S., HEYWOOD, R., ALMOND, R., GIBSON, W.A., GREGSON, R., &
GOPINATH, C. (1984) Cyhalothrin potential tumorigenic and toxic effects in
prolonged dietary administration to mice (Unpublished proprietary report of
the Huntingdon Research Centre No. ICI 395/83668, submitted to WHO by ICI).
COLLIS, W.M.D. & LEAHEY, J.P. (1984) PP321: Hydrolysis in water at pH 5, 7
and 9 (Unpublished proprietary report No. RJ0338B, submitted to WHO by
ICI).
COULSON, J.M., COLLINS, I.G., & EDWARDS, P.J. (1986) PP321: Effects on
earthworms Lumbricidae of repeated annual field applications (Unpublished
proprietary report No. RJ0511B, submitted to WHO by ICI).
CROSS, M. (1985) PP321: Assessment of mutagenic potential using L5178Y
mouse lymphoma cells (Unpublished proprietary report No. CTL/P/1340,
submitted to WHO by ICI).
CROSSLAND, N.O. (1982) Aquatic toxicology of cypermethrin. II. Fate and
biological effects in pond experiments. Aquat. Toxicol., 2: 205-222.
CROSSLAND, N.O., SHIRES, S.W., & BENNETT, D. (1982) Aquatic toxicology of
cypermethrin. III. Fate and biological effects of spray drift deposits in
freshwater adjacent to agricultural land. Aquat. Toxicol., 2: 253-270.
CURL, E.A., LEAHEY, J.P., & LLOYD, S.J. (1984a) PP321: Aqueous photolysis
at pH 5 (Unpublished proprietary report No. RJ0362B, submitted to WHO by
ICI).
CURL, E.A., LEAHEY, J.P., & LLOYD, D. (1984b) PP321: Photodegradation on a
soil surface (Unpublished proprietary report No. RJ0358B, submitted to WHO
by ICI).
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American Chemical
Society, pp. 229 (ACS Symposium Series 42).
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid. Pestic. Biochem. Physiol., 6: 547-550.
FAO (1982) Report of the Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15 October,
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1985) Cyhalothrin. In: 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agricultural Organization of the United Nations,
pp. 173-185, 571-584 (FAO Plant production and protection Paper 67).
FAO/WHO (1986a) Cyhalothrin: In: 1986 Evaluations of some pesticide
residues in food, Part 1: Residues, Rome, Food and Agriculture Organization
of the United Nations, pp. 95-138 (FAO Plant Production and Protection
Paper 78).
FAO/WHO (1986b) Codex maximum limits for pesticide residues, 2nd ed., Rome,
Codex Alimentarius Commission, Food and Agriculture Organization of the
United Nations (CAC XIII).
FARRELLY, E., HAMER, M.J., & HILL, I.R. (1984) PP321: Toxicity to first
instar Daphnia magna (Unpublished proprietary report No. RJ0359B, submitted
to WHO by ICI).
FARRELLY, E., HAMER, M.J., & HILL, I.R. (1985) PP321: Toxicity of
formulations JF9509 and GFU383C to first instar Daphnia magna (Unpublished
proprietary report No. RJ0426B, submitted to WHO by ICI).
FITZPATRICK, R.D. (1985) PP321 dissipation in US soils - 1983 (Unpublished
report No. TMU1809, submitted to WHO by ICI).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous sensation
between synthetic pyrethroid insecticides. Contact dermatitis, 13: 140-147.
FORBES, S. & DUTTON, A.J. (1985) A small scale method for the determination
of pyrethroid insecticide residues in crops and soils. Pestic. Sci., 16:
404-408.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent class
antagonize GABA action at the crayfish neuromuscular junction. Neurosci.
Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of pyrethroid
action in the cockroach. Pestic. Biochem. Physiol., 15: 181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid toxicology:
Protective effects of Diazepam and phenobarbital in the mouse and the
cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
GIFAP (1981) Technical Monograph No. 4: Guidelines on pesticide residue
trials to provide residue data for the registration of pesticides and the
establishment of maximum residue limits, Brussels, International Group of
National Associations of Manufacturers of Agrochemical Products (GIFAP
D/1981/2537/3).
GIRENKO, D.B. & KLISENKO, M.A. (1984) Concentration and determination of
trace quantities of synthetic pyrethroids in the air. Gig. i Sanit., 3: 57-
59.
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4(6):
793-799.
GOUGH, H.J., COLLINS, I.G., EVERETT, C.J., & WILKINSON, W. (1984) PP321:
Acute contact and oral toxicity to honey bees (Apis mellifera) (Unpublished
proprietary report No. RJ0390B, submitted to WHO by ICI).
GOUGH, H.J., COLLINS, I.G., & WILKINSON, W. (1985) PP321: Field test of
toxicity to honey bees (Apis mellifera) on flowering oil-seed rape
(Brassica napus) (Unpublished proprietary report No. RJ0413B, submitted to
WHO by ICI).
HALL, J.S. & LEAHEY, J.P. (1983) Cyhalothrin: Fate in river water
(Unpublished proprietary report No. RJ0320B, submitted to WHO by ICI).
HAMER, M.J. & HILL, I.R. (1985) Cyhalothrin: The accumulation of
cyhalothrin and its degradation products by channel catfish and
Daphnia magna in a soil/water system (Unpublished proprietary
report No. RJ0427B, submitted to WHO by ICI).
HAMER, M.J., FARRELLY, E., & HILL, I.R. (1985a) PP321: Toxicity to Gammarus
pulex (Unpublished proprietary report No. RJ0414B, submitted to WHO by
ICI).
HAMER, M.J., FARRELLY, E., & HILL, I.R. (1985b) PP321: 21 day Daphnia magna
life-cycle study (Unpublished proprietary report No. RJ0451B, submitted to
WHO by ICI).
HARRISON, M.P. (1981) Cyhalothrin: The disposition and metabolism of [ 14 C]-
146814 in rats (Unpublished proprietary report No. 10/HD/007325, submitted
to WHO by ICI).
HARRISON, M.P. (1983) Cyhalothrin: The metabolism and disposition of 146814
in the rat: Part IV. Isolation and identification of the major urinary
metabolites derived from [14 C-benzyl]- or [14 C-cyclopropyl]-146814
following oral administration (Unpublished proprietary report No.
6/HC/005683, submitted to WHO by ICI).
HARRISON, M.P. (1984a) Cyhalothrin (146814): The metabolism and disposition
of 146814 in rats: Part II. Tissue residues derived from [14 C-benzyl] or
[14 C-cyclopropyl]-146814, after a single oral dose of 1 or 25 mg/kg
(Unpublished proprietary report No. KMR 002/2, submitted to WHO by ICI).
HARRISON, M.P. (1984b) Cyhalothrin: The metabolism and disposition of
[14 C]-146814 in rats: Part III. Studies to determine radioactive residues
in the rat following 14 days repeated oral administration (Unpublished
proprietary report No. 146814 KMR 002/03, submitted to WHO by ICI).
HARRISON, M.P. (1984c) Cyhalothrin (146814): The disposition and metabolism
of [14 C]-146814 in the dog (Unpublished proprietary report No. 146814 KMD
005, submitted to WHO by ICI).
HARRISON, M.P. (1984d) Cyhalothrin (146814): The metabolism, excretion and
residues in the cow after 7 days oral administration with two [14 C]-
labelled forms of 146814 at 1 mg/kg/day (Unpublished proprietary report No.
146814 KMC 006/007, submitted to WHO by ICI).
HART, D., BANHAM, P.B., CHART, I.S., EVANS, D.P., GORE, C.W., STONARD,
M.D., MORELAND, S., GODLEY, M.J., & ROBINSON, M. (1985) PP321: 90-day
feeding study in rats (Unpublished proprietary report No. CTL/P/1045,
submitted to WHO by ICI).
HART, T.B. (1984) Review of adverse skin reactions associated with the use
of synthetic pyrethroids (Unpublished proprietary report No. TMF2530,
submitted to WHO by ICI).
HENDERSON, C. & JACKSON, S.J. (1982) Cyhalothrin: Subacute dermal toxicity
study in rabbits (Unpublished proprietary report No. CTL/P/680, submitted
to WHO by ICI).
HEXT, P.M., BRAMMER, A., CHALMERS, D.T., CHART, I.S., GORE, C.W., PATE, I.,
& BANHAM, P.B. (1986) PP321: 1 year oral dosing study in dogs (Unpublished
proprietary report No. CTL/P/1316, submitted to WHO by ICI).
HILL, R.W. (1981) Determination of the acute toxicity of cyhalothrin
(PP563) to Rainbow trout (Salmo gairdneri) (Unpublished proprietary report
No. BL/B/2097, submitted to WHO by ICI).
HILL, R.W. (1984a) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) (Unpublished proprietary report No. BL/B/2405, submitted
to WHO by ICI).
HILL, R.W. (1984b) PP321: Determination of acute toxicity to Bluegill
sunfish (Lepomis macrochirus) (Unpublished proprietary report No.
BL/B/2406, submitted to WHO by ICI).
HILL, R.W. (1985a) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) of 2.5% w/w formulation (Unpublished proprietary report
No. BL/B/2603, submitted to WHO by ICI).
HILL, R.W. (1985b) PP321: Determination of acute toxicity of a 1 lb/US
gallon EC formulation to Rainbow trout (Salmo gairdneri) (Unpublished
proprietary report No. BL/B/2609, submitted to WHO by ICI).
HILL, R.W. (1985c) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) of a 5% EC formulation (Unpublished proprietary report
No. BL/B/2783, submitted to WHO by ICI).
HILL, R.W. (1985d) PP321: Determination of acute toxicity to Mirror carp
(Cyprinus carpio) of a 2.5% w/w formulation (Unpublished proprietary report
No. BL/B/2604, submitted to WHO by ICI).
HILL, R.W. (1985e) PP321: Determination of acute toxicity to Mirror carp
(Cyprinus carpio) of a 5% EC formulation (Unpublished proprietary report
No. BL/B/2784, submitted to WHO by ICI).
HILL, R.W. (1985f) PP321: Determination of acute toxicity of a 1 lb/US
gallon EC formulation to Bluegill sunfish (Lepomis macrochirus)
(Unpublished proprietary report No. BL/B/2610, submitted to WHO by ICI).
HILL, R.W. (1985g) PP321: Determination of acute toxicity to Sheepshead
minnow (Cyprinodon variegatus) (Unpublished proprietary report No.
BL/B/2615, submitted to WHO by ICI).
HILL, R.W., CAUNTER, J.E., & CUMMING, R.I. (1985) PP321: Determination of
the chronic toxicity to Sheepshead minnow (Cyprinodon variegatus) embryos
and larvae (Unpublished proprietary report No. BL/B/2677, submitted to WHO
by ICI).
IRVINE, L.F.H. (1981) Cyhalothrin: Oral (gavage) dominant lethal study in
the male mouse (Unpublished proprietary report of the Hazleton Laboratories
No. 2647-72/213, submitted to WHO by ICI).
JACKSON, S.J. (1981) Cyhalothrin: Eye irritation study in the rabbit
(Unpublished proprietary report No. CTL/T/1502, submitted to WHO by ICI).
JACKSON, S.J. & NIXON, J. (1981) C yhalothrin: Skin irritation studies in
the rabbit and rat (Unpublished proprietary report No. CTL/T/1504,
submitted to WHO by ICI).
KILLICK, M.E. (1981a) Cyhalothrin: Oral (gavage) teratology study in the
rat (Unpublished proprietary report of the Hazleton Laboratories No. 2661-
72/208, submitted to WHO by ICI).
KILLICK, M.E. (1981b) Cyhalothrin: Oral (gavage) teratology study in the
New Zealand white rabbit (Unpublished proprietary report of the Hazleton
Laboratories No. 2700-72/211, submitted to WHO by ICI).
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of pyrethroid
insecticides on the gamma-aminobutyric acid receptor-ionophore complex.
Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding sites.
Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor & Francis
Ltd, 440 pp.
LEAHEY, J.P. & FRENCH, D.A. (1986a) Quantification and characterisation of
radioactive residues found in soya leaves from plants treated with 14 C-
PP321 (Unpublished proprietary report No. RJ0507B, submitted to WHO by
ICI).
LEAHEY, J.P. & FRENCH, D.A. (1986b) Quantification and characterisation of
radioactive residues found in cotton leaves from plants treated with 14 C-
cyclopropane-labelled PP321 (Unpublished proprietary report No. JR0526B,
submitted to WHO by ICI).
LEAHEY, J.P. & FRENCH, D.A. (1986c) Quantification and characterisation of
radioactive residues in cotton leaves from plants treated 14 C-benzyl-
labelled PP321 (Unpublished proprietary report No. RJ0497B, submitted to
WHO by ICI).
LEAHEY, J.P. & PARKER, S. (1985) Cyhalothrin: Characterisation of residues
accumulated by carp continuously exposed to 14 C-cyhalothrin (Unpublished
proprietary report No. RJ0407B, submitted to WHO by ICI).
LEAHEY, J.P., FRENCH, D.A., & HEATH, J. (1985) PP321: Metabolism in a goat
(Unpublished proprietary Report No. RJ0435B, submitted to WHO by ICI).
LINDSAY, S., CHART, I.S., GODLEY, M.J., GORE, C.W., HALL, M., PRATT, I.,
ROBINSON, M., & STONARD, M. (1981) Cyhalothrin: 90-day feeding study in
rats (Unpublished proprietary report No. CTL/P/629, submitted to WHO by
ICI).
LINDSAY, S., DOE, J.E., GODLEY, M.J., HALL, M., PRATT, I., ROBINSON, M., &
STONARD, M.D. (1982) Cyhalothrin induced liver changes: Reversibility study
in male rats (Unpublished proprietary report No. CTL/P/668, submitted to
WHO by ICI).
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel modification
as the basis for the variation in the nerve membrane effects of pyrethroids
and DDT analogs. Pestic. Biochem. Physiol., 20: 203-216.
MILBURN, G.M., BANHAM, P., GODLEY, M.J., PIGOTT, G., & ROBINSON, M. (1984)
Cyhalothrin: Three generation reproduction study in the rat (Unpublished
proprietary report No. CTL/P/906, submitted to WHO by ICI).
MILONE, M.F. (1986) PP321: Unscheduled DNA synthesis in cultured Hela cells
(autoradiographic method) (Unpublished proprietary report of the Institute
di Ricerche Biomediche Antione Marxer No. M914, submitted to WHO by ICI).
MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2922.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare and
the environment. Proceedings of the Fifth International Congress on
Pesticide Chemistry, Kyoto, Japan, 29 August-4 September, 1982, Oxford, New
York, Pergamon Press (Vol. 1-4).
NIXON, J. & JACKSON, S.J. (1981a) Cyhalothrin: Acute toxicity (Unpublished
proprietary report No. CTL/T/1555, submitted to WHO by ICI).
NIXON, J. & JACKSON, S.J. (1981b) Cyhalothrin: Skin sensitisation study in
the guinea-pig (Unpublished proprietary report No. CTL/T/1552, submitted to
WHO by ICI).
PIGOTT, G.H., CHART, I.S., GODLEY, M.J., GORE, C.W., HOLLIS, K.J.,
ROBINSON, M., TAYLOR, K., & TINSTON, D.J. (1984) Cyhalothrin: Two year
feeding study in rats (Unpublished proprietary report No. CTL/P/980,
submitted to WHO by ICI).
PRITCHARD, V.K. (1984) PP321: Skin sensitisation study (Unpublished
proprietary report No. CTL/P/1054, submitted to WHO by ICI).
PRITCHARD, V.K. (1985a) PP321 and cyhalothrin: skin irritation study
(Unpublished proprietary report No. CTL/P/1139, submitted to WHO by ICI).
PRITCHARD, V.K. (1985b) PP321: Eye irritation study (Unpublished
proprietary report No. CTL/P/1207, submitted to WHO by ICI).
PROUT, M.S. (1984) Cyhalothrin: Bioaccumulation in the rat (Unpublished
proprietary report No. CTL/P/1014, submitted to WHO by ICI).
PROUT, M.S. & HOWARD, E.F. (1985) PP321: Comparative absorption study in
the rat (1 mg/kg) (Unpublished proprietary report No. CTL/P/1214, submitted
to WHO by ICI).
REYNOLDS, L.F. (1984) Cyhalothrin: Determination of acute toxicity to
Bluegill sunfish (Lepomis machrochirus) (Unpublished proprietary report No.
BL/B/2514, submitted to WHO by ICI).
RICHOLD, M., ALLEN, J.A., WILLIAMS, A., & RANSOME, S.J. (1981) Cell
transformation test for potential carcinogenicity of Y00102/010/005
(cyhalothrin (PP563)) (Unpublished proprietary report of the Huntingdon
Research Centre No. 391(B)/80948, submitted to WHO by ICI).
ROBERTS, N.L. & FAIRLEY, C. (1981) The acute oral toxicity (LD50) of
cyhalothrin to the Mallard duck (Unpublished proprietary report of the
Huntingdon Research Centre No. 373WL/801024, submitted to WHO by ICI).
ROBERTS, N.L. & FAIRLEY, C. (1984) The acute oral toxicity (LD50) of PP321
to the Mallard duck (Unpublished proprietary report of the Huntingdon
Research Centre No. 438BT/831011, submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., & WOODHOUSE, R.N. (1981a) The subacute dietary
toxicity (LC50 ) of cyhalothrin to the Mallard duck (Unpublished proprietary
report of the Huntingdon Research Centre No. 377WL/8120, submitted to WHO
by ICI).
ROBERTS, N.L., FAIRLEY, C., & WOODHOUSE, R.N. (1981b) The subacute dietary
toxicity (LC50 ) of cyhalothrin to the Bobwhite quail (Unpublished
proprietary report of the Huntingdon Research Centre No. 378WL/8179,
submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., HAKIN, B., PRENTICE, D.E., & WIGHT, D.G.D.
(1982) The acute oral toxicity (LD50 ) and neurotoxic effects of cyhalothrin
to the domestic hen (Unpublished proprietary report of the Huntingdon
Research Centre No. ICI/374 NT/81742, submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., & DAWE, I.S. (1985a) The subacute
dietary toxicity of PP321 to the Mallard duck (Unpublished proprietary
report of the Huntingdon Research Centre No. ISN 46BT/85171, submitted to
WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., & DAWE, I.S. (1985b) The subacute
toxicity of PP321 to the Bobwhite quail (Unpublished proprietary report of
the Huntingdon Research Centre No. ISN 45BT/841287, submitted to WHO by
ICI).
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a nerve
muscle preparation of the clawed frog, Xenopus laevis. Pestic. Biochem.
Physiol., 25: 176-187.
SAPIETS, A. (1984a) Cyhalothrin: Storage stability of the residue on deep
frozen apple, cabbage and soil samples (Unpublished proprietary report No.
M3843B, submitted to WHO by ICI).
SAPIETS, A. (1984b) The determination of residues of PP321 in crops. A gas-
liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 81, submitted to WHO by
ICI).
SAPIETS, A. (1984c) The determination of residues of cyhalothrin
metabolites in crops. A gas-liquid chromatographic method (Unpublished
proprietary residue analytical method No. PPRAM 74, submitted to WHO by
ICI).
SAPIETS, A. (1985a) The determination of residues of cyhalothrin in crops.
A gas-liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 70, submitted to WHO by
ICI).
SAPIETS, A. (1985b) The determination of residues of cyhalothrin in water.
A gas-liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 69, submitted to WHO by
ICI).
SAPIETS, A. (1985c) PP321: Residue transfer study with dairy cows fed on a
diet containing the insecticide (Unpublished proprietary report No. M3936B,
submitted to WHO by ICI).
SAPIETS, A. (1986a) The determination of residues of PP321 in products of
animal origin. A GLC method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 86/1, submitted to WHO by
ICI).
SAPIETS, A. (1986b) The determination of residues of PP321 in soil. A GLC
method using an internal standard (Unpublished proprietary residue
analytical method No. PPRAM 93, submitted to WHO by ICI).
SAPIETS, A., SWAINE, H., & TANDY, M.J. (1984) In: Zweig, G. & Sherma, J.,
ed. Cypermethrin (Chapter 2). Analytical methods for pesticides and plant
growth regulators: Vol XIII, Synthetic pyrethroids and other pesticides,
New York, London, San Francisco, Academic Press.
SHELDON, T., RICHARDSON, C.R., SHAW, J., & BARBER, G. (1984) An evaluation
of PP321 in the mouse micronucleus test (Unpublished proprietary report No.
CTL/P/1090, submitted to WHO by ICI).
SHELDON, T., HOWARD, C.A., & RICHARDSON, C.R. (1985) PP321: A cytogenetic
study in human lymphocytes in vitro (Unpublished proprietary report No.
CTL/P/1333, submitted to WHO by ICI).
SMART, L.E. & STEVENSON, J.H. (1982) Laboratory estimation of toxicity of
pyrethroid insecticides to honeybees: Relevance to hazard in the field. Bee
World, 63(4): 150-152.
SOUTHWOOD, J. (1984) PP321: Acute oral toxicity to the mouse (Unpublished
proprietary report No. CTL/P/1066, submitted to WHO by ICI).
SOUTHWOOD, J. (1985) PP321: Acute oral toxicity studies (Unpublished report
No. CTL/P/1102, submitted to WHO by ICI).
STEVENS, J.E.B. & BEWICK, D.W. (1985) PP563 and PP321: Leaching of PP563
and PP321 and their degradation products in soil columns (Unpublished
proprietary report No. RJ0408B, submitted to WHO by ICI).
TAKEDA CHEMICAL CO. LTD (1979) Test result of fish toxicity of PP-563
(Unpublished proprietary report, submitted to WHO by ICI).
THOMPSON, R.S. (1985) PP321: Determination of acute toxicity to Mysid
shrimps (Mysidopsis bahia) (Unpublished proprietary report No. BL/B/2635,
submitted to WHO by ICI).
THOMPSON, R.S. & WILLIAMS, T.D. (1985) PP321: Toxicity to the green alga
Selenastrum capricornutum (Unpublished proprietary report No. BL/B2584,
submitted to WHO by ICI).
TINSON, D.J., BANHAM, P.D., CHART, I.S., GORE, C.W., PRATT, I., SCALES,
M.D.C., & WEIGHT, T.M. (1984) PP563: 28-day feeding study in rats. Summary
report (Unpublished proprietary report No. CTL/P/1056, submitted to WHO by
ICI).
TRUEMAN, R.W. (1981) Cyhalothrin: Results from the Salmonella reverse
mutation assay (Unpublished proprietary report No. CTL/P/665, submitted to
WHO by ICI).
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies on
the effects of allethrin and DDT on the sodium channels in frog myelinated
nerve membrane. In: Insect neurobiology and pesticide action, London,
Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M. (1973) DDT-
like action of allethrin in the sensory nervous system of Xenopus laevis.
Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A. (1979) Effects of
insecticides on the sensory nervous system. In: Narashashi, T., ed.
Neurotoxicology of insecticides and pheromones, New York, London, Plenum
Publishing Corporation, pp. 183-210.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity relationships
of some pyrethroids in rats. Arch. Toxicol., 45: 325-329.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-dependent effects
of the pyrethroid insecticide decamethrin in frog myelinated nerve fibres.
Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a) Structure-
related effects of pyrethroid insecticides on the lateral-line sense organ
and on peripheral nerves of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel gating in
myelinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN DEN
BERCKEN, J. (1983) Temperature- and structure-dependent interaction of
pyrethroids with the sodium channels in the frog node of Ranvier. Biochem.
Biophys. Acta, 728: 73-82.
WHO (1979) Safe use of pesticides. Third report of the WHO Expert Committee
on Vector Biology and Control, Geneva, World Health Organization (WHO
Technical Report Series, No. 634).
WILLIAMS, T.D. & THOMPSON, R.S. (1981) Determination of the acute toxicity
of cyhalothrin (PP563) to Daphnia magna (Unpublished proprietary report No.
BL/B/2114, submitted to WHO by ICI).
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SAITO, H. (1984a) PP563
(cyhalothrin): Accumulation in fish (carp) in a flow-through water system
(Unpublished proprietary report of MITES No. 58-367, submitted to WHO by
ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984b) PP563
(cyhalothrin) formulation (5% WP): Acute toxicity to carp (Unpublished
proprietary report of MITES No. 58-367, submitted to WHO by ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984c) PP563
(cyhalothrin): Toxicity to daphnia and fish (carp) in the presence and
absence of soil (Unpublished proprietary report of MITES No. 58-367,
submitted to WHO by ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984d) PP563
(cyhalothrin) formulation (5% WP): Acute toxicity to daphnia (Unpublished
proprietary report of MITES No. 58-367, submitted to WHO by ICI).
APPENDIX
On the basis of electrophysiological studies with peripheral
nerve preparations of frogs (Xenopus laevis; Rana temporaria, and
Rana escuelenta), it is possible to distinguish between 2 classes
of pyrethroid insecticides: (Type I and Type II). A similar
distinction between these 2 classes of pyrethroids has been made on
the basis of the symptoms of toxicity in mammals and insects (Van
den Bercken et al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980;
Glickman & Casida, 1982; Lawrence & Casida, 1982). The same
distinction was found in studies on cockroaches (Gammon et al.,
1981).
Based on the binding assay on the gamma-aminobutyric acid
(GABA) receptor-ionophore complex, synthetic pyrethroids can also
be classified into two types: the alpha-cyano-3-phenoxybenzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982;
Gammon & Casida, 1983; Lawrence & Casida, 1983; Lawrence et al.,
1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give
rise to pronounced repetitive activity in sense organs and in
sensory nerve fibres (Van den Bercken et al., 1973). At room
temperature, this repetitive activity usually consists of trains of
3-10 impulses and occasionally up to 25 impulses. Train duration
is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the
insecticide on neurotransmitter release or on the sensitivity of
the subsynaptic membrane, nor on the muscle fibre membrane.
Presynaptic repetitive firing was also observed in the sympathetic
ganglion treated with these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in sensory
nerve fibres, the pyrethroid-induced repetitive activity increases
dramatically as the temperature is lowered, and a decrease of 5 °C
in temperature may cause a more than 3-fold increase in the number
of repetitive impulses per train. This effect is easily reversed
by raising the temperature. The origin of this "negative
temperature coefficient" is not clear (Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve impulse. The
transitional state of the sodium channel is controlled by 2
separately acting gating mechanisms, referred to as the activation
gate and the inactivation gate. Since pyrethroids only appear to
affect the sodium current during depolarization, the rapid opening
of the activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is open,
the activation gate is restrained in the open position by the
pyrethroid molecule. While all pyrethroids have essentially the
same basic mechanism of action, however, the rate of relaxation
differs substantially for the various pyrethroids (Flannigan &
Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation
of the transient increase in sodium permeability of the nerve
membrane during excitation (Van den Bercken & Vijverberg, 1980).
Evidence so far available indicates that allethrin selectively
slows down the closing of the activation gate of a fraction of the
sodium channels that open during depolarization of the membrane.
The time constant of closing of the activation gate in the
allethrin-affected channels is about 100 milliseconds compared with
less than 100 microseconds in the normal sodium channel, i.e., it
is slowed down by a factor of more than 100. This results in a
marked prolongation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is directly
responsible for the repetitive activity induced by allethrin
(Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive
firing, and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral nervous
system of the frog. DDT also causes pronounced repetitive activity
in sense organs, in sensory nerve fibres, and in motor nerve
terminals, due to a prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation.
Recently, it was demonstrated that allethrin and DDT have
essentially the same effect on sodium channels in frog myelinated
nerve membrane. Both compounds slow down the rate of closing of a
fraction of the sodium channels that open on depolarization of the
membrane (Van den Bercken et al., 1973, 1979; Vijverberg et al.,
1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing after-potentials, and evoke repetitive firing with
minimal effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in
sodium permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl
alcohol (deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral line organ in the form of long-
lasting trains of impulses (Vijverberg et al., 1982a). Such a train
may last for up to 1 min and contains thousands of impulses. The
duration of the trains and the number of impulses per train
increase markedly on lowering the temperature. Cypermethrin does
not cause repetitive activity in myelinated nerve fibres. Instead,
this pyrethroid causes a frequency-dependant depression of the
nervous impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of depolarizing
after-potentials during train stimulation (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in
sodium permeability during excitation, presumably by slowing down
the closing of the activation gate of the sodium channel
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983). The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged sodium
current after cypermethrin is too small to induce repetitive
activity in nerve fibres, but is sufficient to cause the long-
lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a long-
lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
cray-fish, the Type II pyrethroids retain sodium channels in a
modified continuous open state persistently, depolarize the
membrane, and block the action potential without causing repetitive
firing (Lund & Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of
the Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant picrotoxin
(PTX). Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin
to rat brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible
relation between the Type II pyrethroid action and the GABA
receptor complex. The stereospecific correlation between the
toxicity of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand, [35S]- t-
butylbicyclophosphorothionate [35S]-TBPS.
Studies with 37 pyrethroids revealed an absolute correlation,
without any false positive or negative, between mouse intracerebral
toxicity and in vitro inhibition: all toxic cyano compounds
including deltamethrin, 1R, cis-cypermethrin, 1R, trans-cypermethrin,
and [2S,alpha]-fenval-erate were inhibitors, but their non-toxic
stereoisomers were not; non-cyano pyrethroids were much less potent
or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies
of pyrethroids were closely related to their mammalian toxicities.
The most toxic pyrethroids of Type II were the most potent
inhibitors of [3H]-Ro 5-4864 specific binding to rat brain
membranes. The [3H]-dihydropicrotoxin and [35S]-TBPS binding
studies with pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an interaction
with a GABA-regulated chloride ionophore-associated binding site.
Moreover, studies with [3H]-Ro 5-4864 support this hypothesis and,
in addition, indicate that the pyrethroid-binding site may be very
closely related to the convulsant benzodiazepine site of action
(Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin and
[2S, alphaS]-fenvalerate) increased the input resistance of
crayfish claw opener muscle fibres bathed in GABA. In contrast,
two non-insecticidal stereoisomers and Type I pyrethroids
(permethrin, resmethrin, allethrin) were inactive. Therefore,
cyanophenoxybenzyl pyrethroids appear to act on the GABA receptor-
ionophore complex (Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type II pyrethroids
(cypermethrin and deltamethrin) induced trains of repetitive
muscle action potentials without presynaptic repetitive activity.
However, an intermediate group of pyrethroids (1R-permethrin,
cyphenothrin, and fenvalerate) caused both types of effect. Thus,
in muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current. But no
clear distinction was observed between non-cyano and alpha-cyano
pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary
target site of pyrethroid insecticides in the vertebrate nervous
system is the sodium channel in the nerve membrane. Pyrethroids
without an alpha-cyano group (allethrin, d-phenothrin, permethrin,
and cismethrin) cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres,
and, in effect, nerve terminals. On the other hand, the alpha-
cyano pyrethroids cause a long-lasting prolongation of the
transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of
repetitive impulses in sense organs and a frequency-dependent
depression of the nerve impulse in nerve fibres. The difference in
effects between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-cyano
group on the phenoxybenzyl alcohol, indicates that it is this
alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation
and conduction are basically the same throughout the entire
nervous system, pyrethroids may also induce repetitive activity in
various parts of the brain. The difference in symptoms of
poisoning by alpha-cyano pyrethroids, compared with the classical
pyrethroids, is not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive activity
in sense organs and possibly in other parts of the nervous system,
which, in a more advance state of poisoning, may be accompanied by
a frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of
the nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane
is also the most important target site of pyrethroids in the
invertebrate nervous system (Wouters & Van den Bercken, 1978; WHO,
1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be
considered restricted to particular animal species, or to a certain
region of the nervous system. Although it has been established that
sense organs and nerve endings are the most vulnerable to the
action of pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions, and on the
chemical structure and physical characteristics of the pyrethroid
molecule (Vijverberg & Van den Bercken, 1982).
RESUME, EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques, méthodes d'analyse
La cyhalothrine s'obtient en estérifiant l'acide (chloro-2
trifluoro-3,3,3 propényl-1)-3 diméthyl-2,2 cyclopropanecarb-
oxylique, par l'alcool alpha-cyanophénoxy-3 benzylique et elle
consiste en un mélange de quatre stéréoisomères formant deux paires
d'énantiomères. La lambda-cyhalothrine est l'une de ces paires
d'énantiomères et constitue la forme la plus active biologiquement.
La cyhalothrine de qualité technique est un liquide visqueux
d'un jaune brunâtre (point de fusion environ 10 °C) qui contient
plus de 90% de matière active. Elle est constituée de quatre
isomères cis dans la proportion de 1:1:1:1. Elle est insoluble
dans l'eau mais soluble dans divers solvants organiques, en
particulier les hydrocarbures aliphatiques et aromatiques. Elle
est stable à la lumière et à la chaleur et possède une faible
tension de vapeur.
La lambda-cyhalothrine technique est un solide beige (point de
fusion 49,2 °C) contenant plus de 90% de matière active. Le
rapport des énantiomères (Z) (1R, 3R) de l'ester S aux énantiomères
(Z) (1S, 3S) de l'ester R est de 1:1. Peu soluble dans l'eau, ce
produit est soluble dans divers solvants organiques et possède une
faible tension de vapeur. La cyhalothrine et la lambda-cyhalothrine
sont rapidement hydrolysées en milieu alcalin mais ne subissent
aucune hydrolyse en milieu neutre ou acide.
On dispose de méthodes confirmées pour la recherche et le
dosage des résidus de cyhalothrine et de lambda-cyhalothrine ainsi
que pour l'analyse des prélèvements effectués dans l'environnement
(limite inférieure de détection: 0,005 mg/kg).
1.2 Production et usage
La cyhalothrine a été mise au point en 1977. On l'utilise
principalement pour détruire diverses espèces nuisibles en santé
publique et santé publique vétérinaire ainsi qu'en agriculture
contre les ravageurs des fruits à pépins. La lambda-cyhalothrine
est utilisée essentiellement en agriculture pour la protection des
récoltes les plus variées et fait actuellement l'objet d'une mise
au point en vue d'être utilisée en santé publique.
On ne dispose d'aucune donnée sur les quantités produites.
1.3 Exposition humaine
Les résidus dans les produits alimentaires, par suite du
traitement des récoltes par la cyhalothrine et la lambda-
cyhalothrine ainsi que de leur utilisation en médecine vétérinaire
sont faibles, généralement inférieurs à 0,2 mg/kg. On ignore quel
est l'apport total d'origine alimentaire chez l'homme mais on peut
supposer que l'exposition de la population générale par cette voie
ne dépasse pas la DJA (0,02 mg/kg de poids corporel).
1.4 Exposition et destinée dans l'environnement
A la surface du sol et en solution aqueuse à pH 5, la lambda-
cyhalothrine se décompose sous l'effet du rayonnement solaire avec
une demi-vie d'environ 30 jours. Les principaux produits de
décomposition sont l'acide (chloro-2 trifluoro-3,3,3 propényl-1)-3
diméthyl-2,2 cyclopropanecarboxylique, l'amide correspondant et
l'acide phénoxy-3 benzoïque.
Dans le sol, la dégradation s'effectue principalement par
hydroxylation puis rupture de la liaison ester aboutissant à deux
produits principaux dont la dégradation se poursuit jusqu'à
obtention de dioxyde de carbone. La demi-vie initiale varie de 22 à
82 jours.
La cyhalothrine et la lambda-cyhalothrine sont adsorbées sur
les particules du sol et ne se déplacent pas dans l'environnement.
Sur les végétaux, la lambda-cyhalothrine se décompose à vitesse
modérée (demi-vie allant jusqu'à 40 jours) de sorte que les résidus
sont principalement constitués du composé initial. On trouve
également des métabolites en faibles quantités qui résultent d'un
certain nombre de réactions d'hydrolyse et d'oxydation.
On ne dispose d'aucune donnée sur les quantités effectivement
présentes dans l'environnement mais compte tenu du fait que ce
produit est relativement peu utilisé et à doses réduites, ces
quantités sont vraisemblablement faibles.
1.5 Absorption, métabolisme, et excrétion
Des études métaboliques ont été effectuées sur des rats, des
chiens, des vaches et des chèvres. Chez les rats et les chiens, on
a montré que la cyhalothrine administrée par voie orale était bien
absorbée, fortement métabolisée, puis éliminée sous forme de
conjugués polaires dans les urines. Chez le rat, on a constaté que
le taux de cyhalothrine tissulaire diminuait après cessation de
l'exposition. Dans les carcasses, les résidus étaient faibles
(moins de 5% de la dose au bout de sept jours) et consistaient
presque entièrement en cyhalothrine accumulée dans le tissu
adipeux. Les résidus présents dans les tissus adipeux étaient
éliminés avec une demi-vie de 23 jours.
Après administration par voie orale de cyhalothrine à des
vaches en lactation, on a constaté que le produit était rapidement
éliminé et qu'un état d'équilibre s'établissait au bout de trois
jours entre ingestion et élimination. Vingt-sept pour cent de la
dose étaient excrétés dans les urines, 50% dans les matières
fécales et 0,8% dans le lait. Les produits retrouvés dans les
urines étaient entièrement constitués de métabolites résultant du
clivage de l'ester et de leurs conjugués, alors que 60 à 70% des
produits marqués au 14C retrouvés dans les matières fécales
consistaient en cyhalothrine non métabolisée. Les résidus
tissulaires, mesurés 16 heures après l'administration de la
dernière dose, étaient faibles, la concentration la plus élevée
étant enregistrée dans le tissu adipeux. Les résidus marqués au 14C
retrouvés dans le lait et les tissus adipeux étaient presque
entièrement constitués de cyhalothrine non métabolisée, à
l'exclusion de tout autre composé.
Chez toutes les espèces mammaliennes étudiées, on a constaté
que la cyhalothrine subissait une forte métabolisation par clivage
de l'ester en acide cyclopropanecarboxylique et acide phénoxy-3
benzoïque et qu'elle était éliminée sous forme de conjugués.
Chez les poissons, les principaux résidus tissulaires
consistent en cyhalothrine non métabolisée à côté de quantités plus
faibles de produits résultant du clivage de l'ester.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Dans les conditions du laboratoire où la concentration est
constante, la cyhalothrine et la lambda-cyhalothrine se révèlent
très toxiques pour les poissons et les invertébrés aquatiques. Les
valeurs de la CL50 à 96 heures pour les poissons vont de 0,2 à 1,3
µg/litre, la CL50 à 48 heures pour les invertébrés aquatiques se
situant entre 0,008 et 0,04 µg/litre.
En étudiant au laboratoire l'accumulation de la cyhalothrine à
concentration constante, on a observé une absorption rapide par les
poissons (facteur d'accumulation d'environ 1000 à 2000). Toutefois
lorsqu'il y a un sol et des sédiments en suspension, on constate
une forte réduction du facteur de bioaccumulation, qui passe à 19
pour les poissons et à 194 pour les daphnies. Lorsqu'on remet les
poissons et les daphnies dans de l'eau propre, on constate une
diminution rapide des résidus, avec une demi-vie respective de 7 et
1 jours. Il est vraisemblable qu'en utilisation agricole normale,
la cyhalothrine et la lambda-cyhalothrine subsistent à faible
concentration dans l'eau. Etant donné que ces composés sont
rapidement adsorbés et dégradés dans les conditions naturelles, il
ne se pose en pratique aucun problème d'accumulation de résidus ni
de toxicité vis-à-vis des espèces aquatiques.
La cyhalothrine et la lambda-cyhalothrine sont pratiquement
inoffensives pour les oiseaux; la DL50 (dose unique) s'est révélée
supérieure à 3950 mg/kg pour toutes les espèces étudiées et la CL50
alimentaire à cinq jours la plus faible a été de 3948 mg/kg pour
des canards sauvages de huit jours qui recevaient de la lambda-
cyhalothrine dans leur alimentation.
Dans les conditions du laboratoire, la cyhalothrine et la
lambda-cyhalothrine sont toxiques pour les abeilles; la DL50 par
voie orale est de 0,97 µg de lambda-cyhalothrine par abeille.
Toutefois, le risque est probablement moins élevé en pratique étant
donné que les formulations actuellement en usage exercent une
action répulsive qui empêche les abeilles de butiner sur les
récoltes traitées. Lorsque les abeilles se remettent à butiner, on
ne constate pas d'augmentation importante de la mortalité.
1.7 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
La toxicité aiguë par voie orale de la cyhalothrine est modérée
chez les rats et les souris et faible chez les cobayes et les
lapins (les valeurs de la DL50 sont les suivantes: rat, 144-243
mg/kg; souris, 37-62 mg/kg; cobaye, plus de 5000 mg/kg; lapin, plus
de 1000 mg/kg). La toxicité aiguë par voie orale de la lambda-
cyhalothrine est plus forte que celle de la cyhalothrine (les
valeurs de la DL50 sont les suivantes: 56-79 mg/kg pour le rat et
20 mg/kg pour la souris). Par voie dermique, on obtient les
toxicités suivantes exprimées par la DL50: rat, 200-2000 mg/kg
(cyhalothrine), 632-696 mg/kg (lambda-cyhalothrine); lapin, plus
de 2000 mg/kg (cyhalothrine). La cyhalothrine et la lambda-
cyhalothrine sont des pyréthroïdes du type II avec pour signes
cliniques d'intoxication une ataxie, une démarche chancelante et
une hyperexcitabilité.
Chez le lapin, la cyhalothrine est modérément irritante pour
l'oeil et la lambda-cyhalothrine peu irritante; les deux composés
sont légèrement irritants pour la peau. La cyhalothrine n'est pas
irritante pour la peau chez le rat. Toutefois elle provoque une
sensibilisation cutanée modérée chez le cobaye. La lambda-
cyhalothrine n'a pas d'action sensibilisatrice sur la peau.
Lors d'une étude d'alimentation de 90 jours au cours de
laquelle des rats ont reçu de la cyhalothrine à des doses allant
jusqu'à 250 mg/kg de nourriture, on a observé une réduction du gain
de poids chez les mâles à la dose la plus forte. Des effets
marginaux sur la valeur moyenne de l'hématocrite ont été
enregistrés chez certains des groupes traités ainsi que quelques
modifications au niveau du foie que l'on a considérées comme
résultant d'une réaction d'adaptation. Lors d'une étude de 90
jours au cours de laquelle des rats ont reçu dans leur alimentation
de la lambda-cyhalothrine à des doses allant jusqu'à 250 mg/kg de
nourriture, on a observé une réduction du gain de poids chez les
deux sexes à la dose la plus forte. On a observé un certain nombre
d'effets sur le chimisme sanguin ainsi que des effets hépatiques
analogues à ceux qui avaient été observés avec la cyhalothrine. La
dose sans effet observable était de 50 mg/kg de nourriture.
Lors d'une étude de 26 semaines au cours de laquelle de la
cyhalothrine a été administrée par voie orale à des chiens à des
doses quotidiennes allant jusqu'à 10 mg/kg de poids corporel, on a
observé des signes d'intoxication de type pyréthroïde à la dose la
plus forte. La dose sans effet observable se situait à 2,5 mg/kg
de poids corporel. Une étude du même genre a été menée sur d'autres
chiens avec administration pendant 52 semaines de 3,5 mg de lambda-
cyhalothrine par kg de poids corporel et par jour. Des signes
cliniques d'intoxication de type pyréthroïde (signes neurologiques)
ont été observés chez tous les ani-maux à la dose quotidienne de
3,5 mg/kg de poids corporel. La dose sans effet observable se
situait à 0,5 mg/kg de poids corporel par jour.
Lors d'une étude de toxicité cutanée de 21 jours pratiquée sur
des lapins au moyen d'une solution de cyhalothrine dans le
polyéthylène-glycol à des doses quotidiennes allant jusqu'à 1000
mg/kg de poids corporel, on a constaté des signes cliniques
d'intoxication chez certains des animaux à la dose la plus forte.
Dans tous les groupes, y compris les témoins, on a observé une
irritation cutanée légère à sévère.
Deux études toxicologiques de 104 semaines ont été consacrées à
la cyhalothrine, l'une sur des rats et l'autre sur des souris.
L'étude sur le rat n'a révélé aucun effet oncogène à des doses
allant jusqu'à 250 mg/kg de nourriture (dose la plus forte
étudiée). La dose sans effet observable en ce qui concerne la
toxicité générale était de 50 mg/kg de nourriture (1,8 mg/kg de
poids corporel par jour). On a relevé une diminution du gain de
poids corporel chez les deux sexes à la dose de 250 mg/kg de
nourriture. Aucun effet oncogène n'a été observé chez la souris à
des doses allant jusqu'à 500 mg/kg de nourriture (dose la plus
forte étudiée). Les signes cliniques d'une intoxication de type
pyréthroïde ont été observés aux doses de 100 et 500 mg/kg de
nourriture, avec réduction du gain de poids corporel à cette
dernière dose. En ce qui concerne la toxicité générale, la dose
sans effet observable était de 20 mg/kg de nourriture (1,9 mg/kg de
poids corporel par jour). Aucun signe histologique de lésion du
système nerveux n'a été observé dans l'une ou l'autre de ces deux
études.
Lors d'une série d'épreuves in vivo et in vitro visant à
déceler des mutations géniques, des lésions chromosomiques et
autres effets génotoxiques, on n'a observé aucun effet de ce type
imputable à la cyhalothrine ou à la lambda-cyhalothrine.
Administrée par voie orale à des rats et à des lapins au cours de
la période cruciale de l'organogénèse, la cyhalothrine ne s'est
révélée ni embryotoxique ni tératogène à des doses quotidiennes qui
étaient toxiques pour la mère (15 mg/kg pour les rats et 30 mg/kg
pour les lapins, c'est-à-dire les deux doses les plus fortes
étudiées).
Lors d'une étude de reproduction portant sur trois générations,
des rats ont reçu de la cyhalothrine dans leur alimentation à des
doses allant jusqu'à 100 mg/kg de nourriture. A la dose de 100
mg/kg de nourriture on a observé une légère diminution de la taille
des portées et une réduction peu importante du gain de poids. En
ce qui concerne les effets sur la reproduction, la dose sans effet
observable était de 30 mg/kg de nourriture.
1.8 Effets sur l'homme
Aucun cas d'intoxication accidentelle n'a été décrit.
Des sensations subjectives au niveau de la face ont été
ressenties lors de la fabrication, de la formulation, du travail de
laboratoire et de l'utilisation sur le terrain. Cet effet ne dure
en général que quelques heures mais il arrive qu'il se prolonge
jusqu'à 72 heures après l'exposition; l'examen médical n'a pas
révélé d'anomalies neurologiques.
Ces sensations cutanées subjectives au niveau de la face, qui
sont ressenties par les personnes qui manipulent de la cyhalothrine
ou de la lambda-cyhalothrine sont, semble-t-il, provoquées par
l'excitation répétée des terminaisons nerveuses de la peau. On
peut les considérer comme un signal d'alarme qui indique une
surexposition de l'épiderme.
Rien n'indique que la cyhalothrine et la lambda-cyhalothrine
puissent avoir des effets nocifs pour l'homme lorsqu'on les utilise
dans les conditions et aux doses qui sont recommandées
actuellement.
2. Conclusions
a) Population générale: l'exposition de la population générale à la
cyhalothrine et à la lambda-cyhalothrine est vraisemblablement très
faible et ne présente probablement aucun danger lorsque ces
produits sont utilisés conformément aux recommandations.
b) Exposition professionnelle: moyennant de bonnes méthodes de
travail, des mesures d'hygiène et pour peu que l'on respecte les
précautions nécessaires, la cyhalothrine et la lambda-cyhalothrine
ne présentent vraisemblablement aucun danger pour les personnes
exposées de par leur profession.
c) Environnement: il ne semble pas que la cyhalothrine, la lambda-
cyhalothrine ou leurs produits de dégradation puissent atteindre
des concentrations nocives pour l'environnement lorsqu'elles sont
utilisées aux doses recommandées. Dans les conditions du
laboratoire, la cyhalothrine et la lambda-cyhalothrine sont très
toxiques pour les poissons, les arthropodes aquatiques et les
abeilles. Toutefois, en pratique, il ne semble pas qu'il puisse se
produire d'effets nocifs durables, lorsque ces insecticides sont
utilisés conformément aux recommandations.
3. Recommandations
Lorsque ces insecticides sont utilisés conformément aux
recommandations, leurs concentrations dans les denrées alimentaires
doivent être très faibles, toutefois il serait bon de le confirmer
en soumettant la cyhalothrine et la lambda-cyhalothrine à une
surveillance.
La cyhalothrine et la lambda-cyhalothrine sont utilisées
depuis des années et seuls des effets passagers ont été observés
lors d'expositions professionnelles; toute-fois il faut continuer à
surveiller l'exposition humaine.
RESUMEN, EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades físicas y químicas, y métodos
analíticos
La cihalotrina se obtiene esterificando ácido 3-(2-cloro-3,3,3-
trifluoroprop-1-enil)-2,2-dimetilciclopropanocarboxílico con
alcohol alpha-ciano-3-fenoxibencílico y se compone de una mezcla de
cuatro estereoisómeros. La lambda-cihalotrina se compone de un par
enantiomérico de isómeros y es la forma más activa biológicamente.
La cihalotrina de calidad técnica es un líquido viscoso, de
color entre amarillo y marrón (punto de fusión: aproximadamente 10
°C), que contiene más del 90% de material activo. Está compuesta
por cuatro isómeros cis en proporción 1:1:1:1. Aunque es insoluble
en el agua, es soluble en toda una gama de disolventes orgánicos
como los hidrocarburos alifáticos y aromáticos. Es estable respecto
de la luz y la temperatura y tiene una baja tensión de vapor.
La lambda-cihalotrina de calidad técnica es un sólido de color
café con leche (punto de fusión: 49,2 °C) que contiene más del 90%
de material activo. La razón enantiomérica del ( Z), (1R, 3R), S-
éster y el ( Z), (1S, 3S), R-éster es 1:1. Es apenas soluble en el
agua pero es soluble en toda una gama de disolventes orgánicos y
tiene una baja tensión de vapor. Tanto la cihalotrina como la
lambda-cihalotrina se hidrolizan rápidamente en medio alcalino pero
no en medio neutro o ácido.
Existen métodos bien establecidos para analizar la cihalotrina
y la lambda-cihalotrina presentes en residuos y en el medio
ambiente (la concentración detectable mínima es 0,005 mg/kg).
1.2 Producción y utilización
La cihalotrina comenzó a producirse en 1977. Se utiliza sobre
todo para combatir múltiples plagas en salud pública y en
veterinaria, pero también se emplea en agricultura contra las
plagas que atacan a los pomos. La lambda-cihalotrina se usa
principalmente como plaguicida agrícola aplicable a muy diversos
cultivos y se está preparando para emplearla en salud pública.
No se dispone de datos sobre los niveles de producción.
1.3 Exposición humana
Los residuos presentes en los alimentos de resultas de la
aplicación de cihalotrina y lamba-cihalotrina a los cultivos y en
veterinaria son bajos, por lo general inferiores a 0,2 mg/kg. No
se dispone de datos sobre la ingesta total en la dieta humana, pero
se puede suponer que la exposición dietética de la población
general no supera la IDA (0,02 mg/kg de peso corporal).
1.4 Exposición y transformación en el medio ambiente
En la superficie del suelo y en soluciones acuosas con un pH de
5, la lambda-cihalotrina se degrada a la luz del sol con una
semivida de unos 30 días. Los principales productos de esa
degradación son ácido 3-(2-cloro-3,3,3-trifluoroprop-1-enil)-2,2-
dimetilciclopropanocarboxílico, el derivado amídico de la
cihalotrina, y ácido 3-fenoxibenzoico.
La degradación en el suelo se produce primordialmente por
hidroxilación seguida de la ruptura del enlace éster para dar lugar
a dos principales productos de degradación, que siguen degradándose
hasta convertirse en bióxido de carbono. Las semividas iniciales
oscilan entre 22 y 82 días.
La cihalotrina y la lambda-cihalotrina se adsorben a las
partículas del suelo y no son móviles en el medio ambiente.
En las plantas, la lambda-cihalotrina se degrada a un ritmo
moderado (semivida de hasta 40 días), por lo que el principal
elemento constituyente de los residuos que en ellas se encuentran
es por lo común el compuesto inicial. Se hallan también niveles más
bajos de metabolitos, resultantes de diversas reacciones
hidrolíticas y oxidantes.
No se dispone de datos sobre los niveles efectivos en el medio
ambiente, pero dadas la escasa utilización habitual en la
actualidad y las reducidas tasas de aplicación, se supone que son
bajos.
1.5 Incorporación, metabolismo y excreción
Se han realizado estudios metabólicos en la rata, el perro, la
vaca y la cabra. En la rata y el perro se ha demostrado que la
cihalotrina administrada por vía oral se absorbe y se metaboliza
bien y se elimina en la orina, en forma de conjugados polares. En
los tejidos de las ratas, disminuyó la concentración de cihalotrina
al interrumpirse la exposición al compuesto. En los cadáveres de
las ratas se encontraron niveles reducidos de residuos (< 5% de la
dosis a los siete días), que se debían casi totalmente a la
cihalotrina contenida en las grasas. Los restos presentes en las
grasas se eliminaron con una semivida de 23 días.
La cihalotrina administrada por vía oral a vacas lactantes se
eliminó rápidamente, alcanzándose un equilibrio entre la ingestión
y la eliminación a los tres días. De la dosis total, el 27% se
excretó en la orina, el 50% en las heces, y el 0,8% en la leche.
El material presente en la orina estaba compuesto exclusivamente
por metabolitos de la disociación de los ésteres y por sus
conjugados, mientras que del 60% al 70% del material fecal marcado
con [14C] se identificó como cihalotrina no alterada. Dieciséis
horas después de la última dosis, los residuos tisulares eran
bajos, hallándose las concentraciones más elevadas en las grasas.
Los residuos marcados con [14C] presentes en la leche y los tejidos
adiposos constaban casi enteramente de cihalotrina no alterada, y
no se detectó ningún otro componente.
En todas las especies de mamíferos investigadas, se ha
descubierto que la cihalotrina se metaboliza ampliamente de
resultas de la ruptura del enlace éster para dar ácido
ciclopropanocarboxílico y ácido 3-fenoxibenzoico, y se elimina en
forma de conjugados.
En los peces, el principal residuo presente en los tejidos es
cihalotrina no alterada y hay niveles más bajos de los productos de
la ruptura del éster.
1.6 Efectos en los organismos del medio ambiente
En condiciones de laboratorio con concentraciones tóxicas
constantes, la cihalotrina y la lambda-cihalotrina son muy tóxicas
para los peces y los invertebrados acuáticos. El valor de las CL50
a las 96 horas para los peces oscila entre 0,2 y 1,3 µg/litro,
mientras que en el caso de los invertebrados acuáticos los valores
de la CL50 a las 48 horas van de 0,008 a 0,4 µg/litro.
Los estudios sobre la acumulación realizados en condiciones de
laboratorio con concentraciones constantes indican que, en los
peces, tiene lugar una rápida incorporación (factor de acumulación
aproximado de 1000 a 2000). No obstante, en presencia de suelo y
sedimentos en suspensión, los factores de bioacumulación quedan muy
reducidos (a 19 en el caso de los peces y a 194 en el caso de los
dáfnidos). Cuando se colocó en agua limpia a los peces y dáfnidos
expuestos, los residuos disminuyeron rápidamente, con semividas de
siete días y un día, respectivamente. Las concentraciones de
cihalotrina y lambda-cihalotrina que pueden hallarse en el agua de
resultas de su aplicación normal con fines agrícolas serán bajas.
Como el compuesto se adsorbe y se degrada rápidamente en
condiciones naturales, la acumulación de residuos o la toxicidad de
la cihalotrina y la lambda-cihalotrina para las especies acuáticas
no plantearán problemas prácticos.
La cihalotrina y la lambda-cihalotrina son prácticamente
inocuas para las aves; en todas las especies sometidas a prueba la
DL50 con una dosis única fue superior a 3950 mg/kg y la CL50
dietética más baja en cinco días fue de 3948 mg/kg (lambda-
cihalotrina administrada en el alimento a patos silvestres de ocho
días de edad).
En condiciones de laboratorio, la cihalotrina y la lambda-
cihalotrina son tóxicas para la abeja; la DL50 de la lambda-
cihalotrina por vía oral es de 0,97 µg/abeja. No obstante, en la
práctica, el riesgo es menor puesto que las formas actualmente
utilizadas tienen un efecto repelente que hace que las abejas
suspendan las actividades de búsqueda en los cultivos tratados.
Cuando éstas vuelven a emprenderse, no hay un aumento significativo
de la mortalidad de las abejas.
1.7 Efectos en animales de experimentación y en sistemas de
prueba in vitro
La toxicidad aguda de la cihalotrina administrada por vía oral
es moderada para las ratas y los ratones y baja para las cobayas y
los conejos (los valores de la DL50 son los siguientes: rata: 144-
243 mg/kg; ratón: 37-62 mg/kg; cobaya: > 5000 mg/kg; conejo: >
1000 mg/kg). La toxicidad aguda de la lambda-cihalotrina por vía
oral es mayor que la de la cihalotrina (los valores de la DL50 son:
56-79 mg/kg para la rata y 20 mg/kg para el ratón). La toxicidad
por vía cutánea (DL50) alcanza los siguientes valores: rata: 200-
2000 mg/kg (cihalotrina), 632-696 mg/kg (lambda-cihalotrina);
conejo: > 2000 mg/kg (cihalotrina). La cihalotrina y la lambda-
cihalotrina son piretroides de tipo II; entre los signos clínicos
figuran la ataxia, el andar vacilante y la hiperexcitabilidad.
En el conejo, la cihalotrina es un irritante ocular moderado y
la lambda-cihalotrina un irritante ocular leve; ambos irritan
levemente la piel. La cihalotrina no irrita la piel de la rata.
Sin embargo, sensibiliza moderadamente la piel de la cobaya. La
lambda-cihalotrina no sensibiliza la piel.
En un estudio de alimentación de 90 días en el que se
administró cihalotrina a ratas en dosis de hasta 250 mg/kg de
alimento, se observó una disminución del aumento del peso corporal
en los machos con la dosis de 250 mg. En algunos de los grupos
tratados, se señalaron efectos marginales en los volúmenes medios
de eritrocitos, así como ciertas alteraciones hepáticas, que se
consideraron una respuesta adaptativa. En otro estudio de
alimentación de 90 días, en el que se administró lambda-cihalotrina
a ratas en dosis de hasta 250 mg/kg de alimento, se observó en
ambos sexos una reducción del aumento del peso corporal con la
dosis de 250 mg. Se señalaron también algunos efectos en la
química clínica, así como efectos hepáticos análogos a los
observados con la cihalotrina. El nivel sin efecto observado fue
de 50 mg/kg de alimento.
En un estudio de 26 semanas en el que se administraron a perros
por vía oral dosis diarias de cihalotrina de hasta 10 mg/kg de peso
corporal, se observaron signos de toxicidad por piretroides con la
dosis de 10 mg. El nivel sin efecto observado fue de 2,5 mg/kg de
peso corporal al día. Se realizó otro estudio similar en el que se
administraron a perros, durante 52 semanas, dosis diarias de
lambda-cihalotrina de hasta 3,5 mg/kg de peso corporal. En todos
los animales que recibieron la dosis de 3,5 mg se observaron signos
clínicos de toxicidad por piretroides (signos neurológicos). El
nivel sin efecto observado fue de 0,5 mg/kg de peso corporal al
día.
En un estudio cutáneo de 21 días con conejos a los que se
aplicó cihalotrina en polietilénglicol en dosis diarias de hasta
1000 mg/kg, se observaron en algunos animales signos clínicos de
toxicidad con la dosis más alta. En todos los grupos, incluidos
los testigos, se señaló irritación de la piel de leve a grave.
Se realizaron dos estudios de alimentación de 104 semanas con
cihalotrina, uno con ratas y otro con ratones. En el estudio con
ratas, no se observaron efectos oncogénicos con dosis de hasta 250
mg/kg de alimento (la dosis más alta ensayada). En cuanto a la
toxicidad sistémica, el nivel sin efecto observado fue de 50 mg/kg
de alimento (1,8 mg/kg de peso corporal al día). Con dosis de 250
mg se señaló en ambos sexos una disminución del aumento del peso
corporal. En el estudio con ratones, no se observaron efectos
oncogénicos con dosis de hasta 500 mg/kg de alimento (la dosis más
alta ensayada). Con dosis de 100 y 500 mg/kg de alimento se
apreciaron signos clínicos de toxicidad por piretroides y, con
dosis de 500 mg, una disminución del aumento del peso corporal. En
cuanto a la toxicidad sistémica, el nivel sin efecto observado fue
de 20 mg/kg de alimento (1,9 mg/kg de peso corporal al día). En
ninguno de los dos estudios se obtuvieron datos histológicos que
indicaran la presencia de lesiones del sistema nervioso.
En una serie de pruebas in vivo e in vitro destinadas a
detectar mutaciones génicas, lesiones cromosómicas y otros efectos
genotóxicos, se obtuvieron resultados negativos con la cihalotrina
y la lambda-cihalotrina. La cihalotrina administrada por vía oral
a ratas y conejos durante el periodo de organogénesis principal no
resultó embriotóxica ni teratogénica en dosis que provocaron
reacciones tóxicas en las madres (15 mg/kg diarios para las ratas y
30 mg/kg diarios para los conejos, en ambos casos los niveles más
altos ensayados).
Se realizó un estudio sobre la reproducción en tres
generaciones de ratas a las que se administró cihalotrina en dosis
de hasta 100 mg/kg de alimento. Con la dosis de 100 mg se
observaron pequeñas reducciones del tamaño de las camadas y del
aumento de peso. En los aspectos relacionados con la reproducción,
el nivel sin efecto observado fue de 30 mg/kg de alimento.
1.8 Efectos en el ser humano
No se ha descrito ningún caso de intoxicación accidental.
En las actividades de fabricación, formulación y laboratorio y
durante el uso sobre el terreno, se han notificado sensaciones
faciales subjetivas. Este efecto sólo suele durar unas horas, pero
ocasionalmente persiste hasta 72 horas después de la exposición; el
examen médico no ha revelado ninguna anomalía neurológica.
Se cree que las sensaciones cutáneas faciales subjetivas que
pueden experimentar las personas que manipulan cihalotrina y
lambda-cihalotrina se deben a la repetida estimulación de las
terminaciones nerviosas de la piel. Pueden considerarse una señal
de alerta que indica que la piel ha sufrido una exposición
excesiva.
No hay ninguna indicación de que la cihalotrina y la lambda-
cihalotrina, utilizadas en las condiciones y con arreglo a las
tasas de aplicación actualmente recomendadas, tengan efectos
adversos en los seres humanos.
2. Conclusiones
(a) Población general: Se cree que la exposición de la población
general a la cihalotrina y la lambda-cihalotrina es muy baja y que
no es probable que represente un riesgo en las condiciones de uso
recomendadas.
(b) Exposición ocupacional: Con prácticas de trabajo, medidas de
higiene y precauciones de seguridad adecuadas, es poco probable que
la cihalotrina y la lambda-cihalotrina representen un riesgo para
las personas ocupacionalmente expuestas.
(c) Medio ambiente: No es probable que la cihalotrina y la lambda-
cihalotrina o los productos de su degradación alcancen niveles
ambientales que puedan producir efectos adversos, si se respetan
las tasas de aplicación recomendadas. En condiciones de
laboratorio, la cihalotrina y la lambda-cihalotrina son muy tóxicas
para los peces, los artrópodos acuáticos y las abejas. No
obstante, en la práctica, no es probable que se produzcan efectos
adversos duraderos en las condiciones de uso recomendadas.
3. Recomendaciones
Aunque se cree que los niveles dietéticos según el uso
recomendado son muy bajos, debe considerarse la posibilidad de
confirmar este extremo incluyendo la cihalotrina y la lambda-
cihalotrina en estudios de vigilancia.
Pese a que ambas sustancias se llevan utilizando varios años y
a que los efectos observados de la exposición ocupacional sólo han
sido transitorios, debe continuar la observación de la exposición
humana.
5.2.1. Rat
Identification of the metabolites produced in the rat studies
of Harrison (described in section 5.1.1) revealed that, following
oral administration, unabsorbed cyhalothrin was eliminated
unchanged via the faeces. The absorbed material was rapidly and
extensively metabolized and no unchanged cyhalothrin was present in
urine or bile. The main route of metabolism was, as anticipated,
via hydrolysis of the ester linkage (Fig. 4). The cyclopropane-
carboxylic acid moiety was subsequently excreted via the urine as
the glucuronide conjugate. This material accounted for about 50%
of the radioactivity in urine following dosing with 14C-
cyclopropyl-labelled cyhalothrin. The 3-phenoxybenzyl moiety was
further metabolized by loss of the nitrile group, oxidation of the
aldehyde formed to a carboxylic acid, aromatic hydroxylation at the
4' position, and formation of the 4- O-sulfate conjugate of 3-(4-
hydroxyphenoxy)benzoic acid. This conjugate accounted for
approximately 75% of the urinary radioactivity following dosing
with 14C-benzyl-labelled cyhalothrin. No metabolite containing the
ester function was detected (Harrison, 1983).
5.2.2. Dog
The main route of metabolism after oral administration is, as
in the rat, via cleavage of the ester bond. After intravenous
administration (0.1 mg/kg body weight), the patterns of metabolites
in urine were very similar to those seen in the oral studies. Very
little unchanged compound was present in the faeces or urine. The
phenoxy-benzyl moiety was further metabolized as in the rat; the
main metabolites were N-(3-phenoxybenzyl) glycine, 3-(4-
hydroxyphenoxy)benzoic acid and its sulfate conjugate, 3-
phenoxybenzoyl glucuronide, and a little free 3-phenoxybenzoic
acid. Other conjugated metabolites were also present. The
cyclopropane acid moiety was extensively metabolized to produce 11
metabolites. These included the cyclopropane acid glucuronide and
other conjugated metabolites. Thus, the metabolism of cyhalothrin
is dominated by cleavage of the ester bond (Fig. 4). Subsequent
metabolism of the products is similar both to that of other
pyrethroids and to the fate of cyhalothrin in other species
(Harrison, 1984c).
5.2.3. Cow
In common with other structurally related pyrethroids, the main
routes of metabolism of cyhalothrin in the cow have been found to
be similar to those observed in rats and dogs, i.e cleavage of the
ester bond with subsequent excretion of the cyclopropyl carboxylic
moiety, either free, hydroxylated, or as a glucuronide conjugate.
The phenoxybenzyl moiety was further metabolized by loss of the
nitrile group and excreted as free 3-phenoxybenzoic acid and its
amino acid conjugates, or after aromatic hydroxylation probably at
the 4'position. Cyhalothrin itself gives rise to residues in fats;
this is consistent with the lipophilic properties of cyhalothrin
compared to those of its more polar metabolites (Harrison, 1984d).
5.2.4. Goat
In a study by Leahey et al. (1985), a goat was dosed orally for
seven days with 14C-cyclopropyl-labelled lambda-cyhalothrin at a
rate equivalent to approximately 11 mg/kg diet. During dosing, the
maximum residue level in the milk was 0.27 mg cyhalothrin
equivalents/kg (mean value during days 3-7: 0.21 mg/kg), virtually
all of which was characterized as lambda-cyhalothrin. When the goat
was slaughtered 16 h after receiving the final dose, residues in
the tissues, expressed in cyhalothrin equivalents, were: meat,
0.024-0.028 mg/kg; fat, 0.13-0.44 mg/kg; liver, 0.34-0.35 mg/kg;
kidney, 0.20 mg/kg. The residues in meat and fat were due mainly
to lambda-cyhalothrin. However, in the liver and kidney, intact
pyrethroid accounted for only a small part of the residue. (1 RS)-
cis-(Z-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclo-
propanecarboxylic acid and 3-(2-chloro-3,3,3-trifluoro-prop-1-
enyl)-2-hydroxymethyl-2-methylcyclopropanecarboxylic acid were the
major components of the residue identified in liver and kidney.
5.2.5. Fish
In an accumulation study by Leahey & Parker (1985), carp were
maintained in a flow-through water system con-taining 14C-
cyclopropyl-labelled cyhalothrin (at a level of 20 ng/g) for 28
days. Results showed that radioactive residues in muscle, head,
and viscera were 0.035, 0.050, and 0.115 mg/kg, respectively. The
major part of the 14C-residue (50-65%) was characterized as
cyhalothrin, a further 10-19% consisting of the compound 1a (Fig. 2).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic organisms
6.1.1. Microorganisms
It has been shown that lambda-cyhalothrin, at a concentration
of 1.0 mg/litre, does not affect the growth of the single-celled
green alga Selenastrum capricornutum over a period of 96 h
(Thompson & Williams, 1985).
6.1.2. Invertebrates
6.1.2.1 Acute toxicity
Aquatic invertebrates show a wide range of susceptibility to
cyhalothrin and lambda-cyhalothrin. The data are summarized in
Table 5.
Table 5. Acute toxicity of cyhalothrin and lambda-cyhalothrin for aquatic invertebrates
-----------------------------------------------------------------------------------------
Species Stage Temper- Test substance Test 48-h Reference
ature system LC50
(°C) (ng/
litre)
-----------------------------------------------------------------------------------------
Freshwater
Water flea < 24 h 20 cyhalothrin static 160 Yamauchi et
(Daphnia pulex) 5% WP al. (1984d)
Water flea 12 h 20 technical static 380 Williams &
(Daphnia magna) (± 12 h) cyhalothrin Thompson
(1981)
Water flea < 24 h 20 technical lambda- static 360 Farrelly et
(Daphnia magna) cyhalothrin al. (1984)
< 24 h 20 lambda-cyhalothrin static 90a Farrelly et
5% EC al. (1985)
< 24 h 20 lambda-cyhalothrin static 90a Farrelly et
13% EC al. (1985)
Freshwater shrimp 5 mm 15 14C-lambda- flow- 8 Hamer et al.
(Gammarus pulex) cyhalothrin through (1985a)
Marine
Mysid shrimp < 48 h 25 14C-lambda- flow- 7.5 Thompson
(Mysidopsis bahia) cyhalothrin through (1985)
-----------------------------------------------------------------------------------------
a Concentration of active ingredient.
6.1.2.2 Long-term toxicity
The effects of lambda-cyhalothrin on the survival, growth, and
reproduction of Daphnia magna were investigated for a period of 21
days in a static water test with daily renewal of test solutions.
The nominal concentrations tested were 0, 2.5, 5.0, 10.0, 20.0,
and 40.0 ng/litre. Lambda-cyhalothrin affected all three parameters
at a nominal concentration of 40 ng/litre, but no effects were
noted at 2.5 ng/litre. Chemical analysis suggested that the
daphnids were exposed to about 60% of the nominal concentration.
These results show that the life-cycle no-observed-effect level of
lambda-cyhalothrin for daphnids is of the order of 2.5 ng/litre
(Hamer et al., 1985b).
6.1.3. Fish
6.1.3.1 Acute toxicity
Cyhalothrin and lambda-cyhalothrin are very toxic to fish in
clean water under laboratory conditions. The available data,
summarized in Table 6, demonstrate a similar high acute toxicity
for both cold and warm water species of fish.
6.1.3.2 Long-term toxicity
Sheepshead minnow Cyprinodon variegatus embryos and larvae were
continuously exposed (through 28 days post hatch) to mean measured
lambda-cyhalothrin concentrations of 0.04, 0.07, 0.14, 0.25, and
0.38 µg/litre, in a flow-through system. The test was performed in
duplicate. Assessments were made of percentage hatch and survival
of embryos and of total length and weight of the larvae at the
completion of the study. Hatchability was not affected (P < 0.05)
at any concentration in the carrier dimethylformamide or dilution
water controls, percentage hatch ranging from 81.3% to 100%.
Larval survival was not significantly affected. A significant
effect (P < 0.05) was found on the weight of the larvae at the
highest concen-tration tested, but not at any other concentration.
On the basis of these data the NOEL was 0.25 µg/litre and the
lowest-observed-effect level (LOEL) was 0.38 µg/litre (Hill et al.,
1985).
6.1.4. Model ecosystem
Cyhalothrin is readily adsorbed onto soil and suspended
particles, which in consequence significantly reduces its toxicity
to aquatic organisms (Yamauchi et al., 1984c). Daphnia pulex and
Cyprinus carpio were used in four different systems to test this
experimentally (Table 7). The median lethal concentrations (LC50)
were calculated, immobilization being used as the end-point for
Daphnia pulex.
Table 6. Acute toxicity of cyhalothrin and lambda-cyhalothrin to fish
---------------------------------------------------------------------------------------------------
Species Weight Test substance and vehicle Temper- 96-h Reference
(g) ature LC50
(°C) (µg/
litre)
---------------------------------------------------------------------------------------------------
Rainbow trout 0.32-1.37 technical cyhalothrin 12 0.54 Hill (1981a)
(Salmo gairdneri) dispersed via acetone
0.30-1.48 technical lambda-cyhalothrin 12 0.24 Hill (1984a)
dispersed via acetone
0.99-2.32 lambda-cyhalothrin 2.5% 16 0.39 Hill (1985a)
EC dispersed in water
1.28-4.78 lambda-cyhalothrin 13% EC 12 0.44 Hill (1985b)
dispersed in water
0.87-4.09 lambda-cyhalothrin 5% EC 16 0.93 Hill (1985c)
dispersed in water
Carp 5.2 technical cyhalothrin 23-35 1.34a Takeda Chemical
(Cyprinus carpio) dispersed via Co. Ltd.
dimethylformamide (1979)
5.4 cyhalothrin 5% WP 25 1.1 Yamauchi et al.
(1984b)
1.48-5.8 lambda-cyhalothrin 2.5% 22 0.54 Hill (1985d)
EC dispersed in water
3.92-7.28 lambda-cyhalothrin 5% EC 22 0.50 Hill (1985e)
dispersed in water
Bluegill sunfish 0.23-0.84 technical cyhalothrin 22 0.46 Reynolds (1984)
(Lepomis macrochirus) dispersed via acetone
0.7-2.6 technical lambda- 22 0.21 Hill (1984b)
cyhalothrin dispersed
via acetone
0.47-2.06 lambda-cyhalothrin 13% 22 0.28 Hill (1985f)
EC dispersed in water
Sheephead minnow 0.32-0.91 technical lambda- 22 0.81 Hill (1985g)
(Cyprinodon variegatus) cyhalothrin dispersed
via acetone
---------------------------------------------------------------------------------------------------
a 72-h LC50.
Table 7. Effect of soil on the toxicity (72-h LC50 in µg/
litre) of cyhalothrin to Daphnia pulex and Cyprinus carpioa
--------------------------------------------------------------
Conditions Daphnia pulex Cyprinus carpio
--------------------------------------------------------------
Application to water surface 0.4 9
(without soil)
Application to water surface 1.0 32
(soil undisturbed)
Application to water surface 16 57
(soil suspended)
Application to soil 70 642
(soil undisturbed)
--------------------------------------------------------------
a From: Yamauchi et al (1984c).
6.2. Terrestrial organisms
6.2.1. Birds
6.2.1.1 Acute toxicity
The toxicity of single oral doses of cyhalothrin and lambda-
cyhalothrin for birds is summarized in Table 8.
The 5-day dietary LC50 for cyhalothrin and lambda-cyhalothrin
has been measured in Anas platyrhynchos and Colinus virginianus
(Table 9).
Table 8. Oral toxicity of cyhalothrin and lambda-cyhalothrin for birds
---------------------------------------------------------------------------------------
Species Age Test substance Observation LD50 (mg/kg References
and vehicle period body weight)
---------------------------------------------------------------------------------------
Domestic hen adult cyhalothrin in 14 days > 10 000 Roberts
(Gallus domesticus) corn oil et al.
(1982)
Mallard duck adult cyhalothrin in 14 days > 5000 Roberts &
(Anas platyrhynchos) corn oil Fairley
(1981)
Mallard duck adult lambda-cyhalothrin 14 days > 3950 Roberts &
(Anas platyrhynchos) in corn oil Fairley
(1984)
---------------------------------------------------------------------------------------
Table 9. Dietary LC50 of cyhalothrin and lambda-cyhalothrin for birds
---------------------------------------------------------------------------------------
Species Age Test substance Observation LC50 Reference
period (post (mg/kg
treatment) diet)
---------------------------------------------------------------------------------------
Mallard duck 10 days cyhalothrin 3 days 14 000 Roberts et
(Anas platyrhynchos) al. (1981a)
Mallard duck 8 days lambda-cyhalothrin 4 days 3948 Roberts et
(Anas platyrhynchos) al. (1985a)
Bobwhite quail 10 days cyhalothrin 3 days > 7530 Roberts et
(Colinus virginianus) al. (1981b)
Bobwhite quail 11 days lambda-cyhalothrin 3 days > 5300 Roberts et
(Colinus virginianus) al. (1985b)
---------------------------------------------------------------------------------------
6.2.2. Honey-bees
Cyhalothrin and lambda-cyhalothrin have been shown to be toxic
to honey-bees (Apis mellifera) in laboratory tests (Table 10).
Table 10. Toxicity of cyhalothrin and lambda-cyhalothrin for
honey-bees (expressed as 24-h LD50 in µg ai per bee)
----------------------------------------------------------------------
Formulation Topical Oral Reference
application administration
----------------------------------------------------------------------
Technical cyhalothrin 0.027 - Smart &
Stevenson
(1982)
Technical lambda-cyhalothrin 0.051 0.97 Gough et
al. (1984)
Lambda-cyhalothrin EC (5%) 0.095 0.57 Gough et
al. (1984)
----------------------------------------------------------------------
In common with other pyrethroids, the high laboratory toxicity
of lambda-cyhalothrin is not translated into a significant field
hazard to bees. In two trials on flowering rape, lambda-
cyhalothrin (JF 9509) EC was applied, at midday, by helicopter at a
concentration of 10 g ai/ha to fields where hives of honey-bees
were located. A toxic standard and untreated control were used for
comparison. Bees were actively foraging during spraying, and the
hives were oversprayed. Mortality, foraging activity, activity at
the hive, and brood development were monitored before and after
treatment, and pollen, honey, and wax were analyzed for residues.
Apart from a suppression of foraging lasting up to 1.5 h, the
lambda-cyhalothrin formulation had no effect on the bees, whereas
the toxic standard killed large numbers. Only low levels of
residues were detected (pollen, 0.44 µg/g; honey, 0.01 µg/g; wax,
0.01 µg/g). It was concluded that, at 10 g ai/ha, lambda-
cyhalothrin formulation (JF 9509) EC is non-hazardous to honey-bees
on flowering rape (Gough et al., 1985).
6.2.3. Earthworms
Three annual applications of lambda-cyhalothrin at rates of up
to 250 g ai/ha to field plots had no adverse effect on populations
of individual species of earthworms or total earthworm numbers or
weight (Coulson et al., 1986).
6.2.4. Higher plants
No phytotoxic effects have been reported.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposures
7.1.1. Oral
The acute oral toxicity of cyhalothrin and lambda-cyhalothrin
in corn oil has been determined for several species (Table 11). The
toxicity of cyhalothrin is moderate (LD50 values: rat, 144-243
mg/kg; mouse, 37-62 mg/kg), whereas that of lambda-cyhalothrin is
higher (LD50 values: rat, 56-79 mg/kg; mouse, 20 mg/kg). Signs of
intoxication are characteristic of type II pyrethroid toxicity and
included piloerection, subdued behaviour, ataxia, unsteady gait,
salivation, incontinence, scouring, and chromodacryorrhoea.
Table 11. Acute oral toxicity of technical cyhalothrin and
lambda-cyhalothrin in corn oil
------------------------------------------------------------------
Species Test substance LD50 (mg/kg Reference
body weight)
------------------------------------------------------------------
Rat cyhalothrin 243 (male) Nixon &
144 (female) Jackson (1981a)
Rat lambda-cyhalothrin 79 (male) Southwood (1985)
56 (female)
Mouse cyhalothrin 36.7 (male) Nixon &
62.3 (female) Jackson (1981a)
Mouse lambda-cyhalothrin 19.9 (male) Southwood (1984)
19.9 (female)
Guinea-pig cyhalothrin > 5000 (male) Nixon &
Jackson (1981a)
Rabbit cyhalothrin > 1000 (female) Nixon &
Jackson (1981a)
------------------------------------------------------------------
7.1.2. Percutaneous
The percutaneous toxicity of cyhalothrin and lambda-cyhalothrin
is summarized in Table 12. Signs of intoxication were similar to
those seen after oral ingestion, and included incontinence,
scouring, dehydration, subdued behaviour, curvature of the spine,
unsteady gait, nervous appearance, piloerection, and increased
vocalization when handled.
Table 12. Percutaneous toxicity of technical cyhalothrin and
lambda-cyhalothrin in polyethylene glycol paste
-----------------------------------------------------------------
Species Test substance LD50 (mg/kg Reference
body weight)
-----------------------------------------------------------------
Rat cyhalothrin 1000-2000 (male) Nixon &
200-2000 (female) Jackson (1981a)
Rat lambda-cyhalothrin 632 (male) Barber (1985)
696 (female)
Rabbit cyhalothrin > 2000 (male) Nixon &
> 2000 (female) Jackson (1981a)
-----------------------------------------------------------------
7.1.3. Intraperitoneal
The intraperitoneal LD50 of cyhalothrin to rats is in the range
of 250 to 750 mg/kg. Responses of 0% or 100% mortality were
observed at all but one dose, and so no precise value for the LD50
could be given. The highest dose with no mortality was 250 mg/kg
and the lowest dose with 100% mortality was 750 mg/kg (Nixon &
Jackson, 1981a).
7.2. Irritation and sensitization
7.2.1. Irritation
Undiluted technical cyhalothrin is a mild irritant to occluded
rabbit skin (both intact and abraded) but is non-irritant to
occluded rat skin (intact) (Jackson & Nixon, 1981). Technical
cyhalothrin is a moderate irritant to the rabbit eye without
irrigation and is a mild irritant when irrigated for one minute, 20
to 30 seconds after instillation of the material (Jackson, 1981).
Technical lambda-cyhalothrin is non-irritant to occluded
rabbit skin without abrasion (Pritchard, 1985a). It is a mild
irritant to the rabbit eye (Pritchard, 1985b).
7.2.2. Sensitization
A skin sensitization test with cyhalothrin on guinea-pigs,
using the procedure of Buehler, indicated that cyhalothrin has
skin-sensitizing potential (Nixon & Jackson, 1981b). In guinea-
pigs that had been previously induced with undiluted cyhalothrin
technical material, using the Magnusson and Kligman maximization
test, a moderate sensitization response was elicited. When
lambda-cyhalothrin was tested for skin sensitization on guinea-
pigs, using the maximization procedure of Magnusson and Kligmann,
it was shown to have no sensitization potential (Pritchard, 1984).
7.3. Short-term exposures
7.3.1. Oral
7.3.1.1 Rat
When groups of male and female rats were fed diets containing
cyhalothrin at levels up to 750 mg/kg for 28 days, symptoms of
toxicity shown by animals receiving doses of 250 mg/kg diet or more
included high stepping gait, ataxia, and hypersensitivity to
external stimuli. These effects were dose related and some
mortality occurred at the highest dose level. The clinical signs
were not accompanied by histopathological changes in the nervous
system. Thymic atrophy, adrenal enlargement with vacuolization,
and incomplete spermatogenesis occurred with the highest dose. An
adaptive change was present in the liver as judged by an increase
in liver weight, proliferation of smooth endoplasmic reticulum,
and an increase in the activity of the xenobiotic-metabolizing
enzyme, aminopyrine- N-demethylase (APDM). These effects occurred at
doses of 100 mg/kg or more, and there was some evidence for this
type of change at 20 mg/kg (Tinson et al., 1984).
When groups of 20 male and 20 female Wistar rats were fed
cyhalothrin (89% pure) at dietary concentrations of 0, 10, 50, and
250 mg/kg for 90 days, only male rats fed 250 mg/kg showed
significantly reduced body weight gain. No abnormal clinical signs
were seen. Haemoglobin and haematocrit values were not affected by
the treatment, but there were marginal effects on mean erythrocyte
volume in some treated groups. Male rats fed 50 or 250 mg/kg
showed decreased plasma triglyceride levels and a dose-related
increase in hepatic APDM activity. The latter effect was also seen
in females fed 250 mg/kg. Small increases in urinary glucose
excretion occurred in males fed 50 or 250 mg/kg after 13 weeks. No
treatment-related effects on organ weights and no significant
histopathological changes were reported (Lindsay et al., 1981).
In studies by Lindsay et al. (1982), two groups of 32 male rats
were fed either a control diet or a diet containing 250 mg
cyhalothrin/kg for 28 days. After this period eight rats per group
were killed and examined. The remaining rats were fed the control
diet for periods of 7, 14, or 28 days after cessation of treatment,
and a further eight rats per group were killed and examined at each
of these time intervals. A decrease in body weight gain was seen
in the treated rats, which, although not statistically
significant, was still much reduced at the end of the 28-day
recovery period. Proliferation of the hepatic smooth endoplasmic
reticulum and elevated hepatic APDM activity were also seen in the
treated animals. These effects had reversed 7 days after the
cessation of treatment with cyhalothrin and were considered to be
physiological adaptive changes rather than a toxicological effect.
When groups of 20 male and 20 female Alderley Park rats
received diets containing 0, 10, 50, or 250 mg lambda-
cyhalothrin/kg for 90 days, decreased body weight gain, accompanied
by a reduction in food consumption, was seen in both male and
female rats receiving the highest dose. No abnormal clinical signs
were seen; in particular, there was no evidence of neurological
effects. There were no treatment-related effects on haematological
parameters but reductions in the activities of plasma alanine
trans-aminase (males only) and alkaline phosphatase (females only
at 13 weeks) were apparent in animals fed the highest dose. Plasma
triglycerides were also reduced in males at this feeding level.
Relative liver weights were increased in both sexes at 250 mg/kg
and in males at 50 mg/kg, accompanied by increased activity of
hepatic APDM. No other changes in organ weight or histopathology
were attributed to treatment with lambda-cyhalothrin. These two
effects, particularly in view of the findings with cyhalothrin,
were considered to be adaptive in nature and the toxicological NOEL
was established at 50 mg/kg (equivalent to 2.5 mg/kg body weight
per day (Hart et al., 1985).
7.3.1.2 Dog
In studies by Chesterman et al. (1980), groups of one male and
one female dog (Alderley Park beagles) received 0, 2.5, or 10 mg
cyhalothrin/kg body weight orally in corn oil by gelatine capsule
daily for four weeks. A further group initially received 30 mg/kg
per day, but due to severe clinical signs after 10 days dosing,
which were typical of pyrethroid toxicity (muscular trembling,
unsteadiness, vomiting, and body weight loss), the animals were
rested and then received 20 mg/kg per day for four weeks. Similar
clinical signs were seen, with the exception of body weight loss,
in all animals receiving 10 mg/kg per day or more, the severity
being dose related. Investigation of the sciatic and tibial nerves
and the lumbricalis muscle with special histopathological stains
indicated no changes that could be attributed to treatment with
cyhalothrin. Liquid faeces were produced by all animals receiving
cyhalothrin, the incidence being dose related. These changes were
considered to be of no toxicological significance. No other
changes were observed that could be attributed to the treatment.
When groups of 6 male and 6 female dogs (Alderley Park beagles)
were fed cyhalothrin in corn oil by gelatine capsule at 0, 1, 2.5,
or 10 mg/kg body weight per day for 26 weeks, signs of pyrethroid
toxicity were seen in some dogs at 10 mg/kg. Although liquid
faeces were produced by all animals in the study, including the
controls, the incidence and frequency were higher in treated
animals and were dose related. These changes were considered to be
of no toxicological significance. Macroscopic postmortem
examination, organ weights, and histological investigations
revealed no treatment-related changes. The oral NOEL in dogs was
found to be 2.5 mg/kg per day (Chesterman et al., 1981).
In studies by Hext et al. (1986), groups of 6 male and 6 female
dogs were dosed by gavage in corn oil with 0, 0.1, 0.5, or 3.5 mg
lambda-cyhalothrin/kg body weight daily for 52 weeks. Clinical
signs of neurological effects were evident in all animals fed the
highest dose, which were unaccompanied by histological changes in
the nervous system. There was an increased incidence of fluid
faeces at the highest dose and a slight increase in the group
receiving 0.5 mg/kg per day. This effect was considered to be
related to the method of administration and not to be
toxicologically significant. No histopathological changes
attributable to lambda-cyhalothrin administration were observed at
any of the dose levels employed, and the toxicological NOEL in
this study was 0.5 mg lambda-cyhalothrin per kg per day.
7.3.2. Dermal
7.3.2.1 Rabbit
Cyhalothrin in polyethylene glycol (PEG 300) (10, 100, or 1000
mg/kg body weight per day) was applied to the skin of groups of 10
male and 10 female New Zealand White rabbits and kept in contact
with the skin 6 h/day, 5 days per week for 3 weeks (i.e. a total
of 15 applications) by means of an occlusive dressing. A group of
14 male and 14 female control rabbits was treated with polyethylene
glycol (PEG 300) using the same procedure. The skin of half the
animals in each group was abraded prior to the application of
cyhalothrin. Repeated application of the vehicle alone
(polyethylene glycol) and the vehicle plus cyhalothrin caused
slight to severe skin irritation. At the highest dose level there
was an increased incidence of oedema and erythema. A small number
of animals given the highest dose showed pyrethroid-like symptoms,
but only when the skin was unabraded. The NOEL was considered to be
100 mg/kg per day (Henderson & Jackson, 1982).
7.4. Long-term exposures and carcinogenicity
7.4.1. Rat
In studies by Pigott et al. (1984), groups of 72 male and 72
female Alpk/AP strain rats were fed diets containing cyhalothrin
at levels of 0, 10, 50, or 250 mg/kg diet for up to 104 weeks. All
the surviving animals were sacrificed, and histopathological and
gross postmortem examinations were carried out. Decreased body
weight gain, accompanied by a small decrease in food consumption,
was evident in rats of both sexes fed the highest dose. This was
accompanied by minor changes in blood biochemistry. Increased liver
weight was seen in rats of both sexes fed cyhalothrin at 250 mg/kg
at the interim sacrifice but this was not evident at termination.
There was no histopathological evidence of a chronic toxic effect
due to cyhalothrin. In particular clinical and histopathological
evaluation gave no indication of an effect on the nervous system.
There was no evidence for a carcinogenic effect of cyhalothrin.
The toxicological NOEL for this study was 50 mg cyhalothrin/kg
diet, corresponding to a minimum dose rate of approximately 1.7
mg/kg body weight per day for male rats and 1.9 mg/kg per day for
female rats.
7.4.2. Mouse
Groups of 52 male and 52 female Charles River CD-1 mice were
maintained for 104 weeks on diets containing 0, 20, 100, or 500 mg
cyhalothrin/kg and further groups of 12 males and 12 females were
designated for interim sacrifice after 52 weeks. During the study
there were no deaths attributable to treatment with cyhalothrin.
Signs of toxicity ascribable to cyhalothrin included piloerection
and hunched posture in both sexes at 500 mg/kg and in males at 100
mg/kg and reduced body weight gain, higher food intake, and
reduced efficiency of food utilization in males receiving 500
mg/kg. There was a statistically significant increase, compared to
the controls, in the incidence of mammary adenocarcinoma in females
at the two highest dose levels. However, the frequency of these
tumours was not unduly at variance with that normally seen in the
strain of mouse used, and no dose relationship was apparent. Thus,
there were no neoplastic findings that could be attributed to the
long-term administration of cyhalothrin. There was a clear NOEL of
20 mg/kg, corresponding to a mean calculated daily intake of 1.8
mg/kg body weight per day in males and 2.0 mg/kg body weight per
day in females (Colley et al., 1984).
7.5. Reproduction, embryotoxicity, and teratogenicity
7.5.1. Reproduction
In studies by Milburn et al. (1984), groups of 15 male and 30
female (F0 parents) weaning Alderley Park rats were fed diets
containing 0, 10, 30, or 100 mg cyhalothrin per kg. After 12
weeks, the animals were mated to produce the first (F1a) litter and
subsequently re-mated to produce a second (F1b) litter. The
breeding programme was repeated with F1 parents selected from the
F1b off-spring and F2 parents selected from the F2b offspring.
Test diets were fed continuously throughout the study. There were
minor effects on body weight gain of parents from all generations
receiving 100 mg/kg, but no clinical signs of neurological effects
were seen in either parents or offspring. No effects of treatment
were seen on indices of male and female fertility, gestation
period, live born index, or pup survival. There was a small
reduction in mean total litter weight of the F2 and F3 generations
from rats receiving the highest dose, which was attributable to
minor decreases in litter size and a small reduction in weight gain
of the pups. No effect was seen in litters from rats receiving 30
mg/kg. There was no evidence of gross or histopathological change
attributable to the treatment. The reproductive effects seen in
rats receiving the highest dose were of a minor nature. A clear
NOEL of 30 mg/kg (corresponding to a dosage in the range of 1.5 to
1.9 mg/kg body weight per day) was established (Milburn et al.,
1984).
7.5.2. Embryotoxicity and teratogenicity
7.5.2.1 Rat
When groups of 24 mated female rats (Charles River CD-1) were
given cyhalothrin orally (in corn oil), at 0, 5, 10, or 15 mg/kg
body weight per day, from day 6 to 15 inclusive of gestation and
were killed on day 20, there was reduced body weight gain at the
highest dose level and evidence of mild pyrethroid toxicity in two
of these animals. There were no other effects on the clinical or
litter parameters attributable to treatment with cyhalothrin, and
examination of the viscera and skeletons showed no effects of
treatment. At the highest dose level, there was maternal toxicity,
but there was no effect on any aspect of fetal development at any
dose level (Killick, 1981a).
7.5.2.2 Rabbit
In studies by Killick (1981b), groups of at least 18 pregnant
New Zealand White rabbits received cyhalothrin orally in corn oil
daily at 0, 3, 10, or 30 mg/kg body weight, from days 6 to 18
(inclusive) of gestation and were killed on day 28. There was
reduced body weight gain at the highest dose, accompanied by
reduced food intake during dosing. There were no clinical signs and
no changes in pregnancy incidence or in litter parameters
attributable to treatment with cyhalothrin. Examination of the
viscera and skeletons revealed no effects of treatment. At the
highest dose level, there was maternal toxicity, but there was no
effect on any aspect of fetal development at any dose level.
7.6. Mutagenicity and related end-points
7.6.1. Microorganisms
Five test strains, TA1535, TA1537, TA1538, TA98, and TA100,
were employed to evaluate the mutagenic potential of cyhalothrin
using the salmonella reverse mutation assay of Ames. The assay was
conducted in the presence and absence of metabolic activation (S9
mix) with cyhalothrin at levels up to 2500 µg/plate. The mean
numbers of revertant colonies of Salmonella typhimurium observed in
the five test strains indicated an unequivocal negative response
(Trueman, 1981). Lambda-cyhalothrin at dose levels of up to 5000
µg/plate, both in the presence and absence of metabolic activation,
gave a non-mutagenic response in the same test using the same
strains (Callander, 1984).
7.6.2. In vitro mammalian cells
When cyhalothrin was tested in a modification of the cell
culture transformation test of Styles, using Syrian Hamster kidney
cell line BHK21C13, the response in the presence of metabolic
activation was unequivocally negative. In the absence of metabolic
transformation there was an erratic increase in numbers of
transformed colonies together with a poor dose response. These
data were not thought to indicate a significant positive response,
and it was concluded that cyhalothrin does not appear to possess
significant cell-transforming properties (Richold et al., 1981).
In addition, the significance of the results from the BHK cell
system is doubtful in view of the questionable interlaboratory
reproducibility of this assay.
The mutagenic potential of lambda-cyhalothrin has been assessed
in vitro with L51787 mouse lymphoma cells, both in the presence and
absence of auxiliary metabolic activation (S9) mix, using dose
levels of 125-1000, 2000, and 4000 µg/ml. There was no increase in
mutation frequency either in the presence or absence of S9 mix
(Cross, 1985).
Lambda-cyhalothrin, at dose levels of up to 1000 µg/ml, either
in the presence or absence of metabolic activation, did not induce
statistically significant increases in the incorporation of
tritiated thymidine in cultured human (Hela) cells (Milone, 1986)
or induce chromosomal damage in human lymphocytes stimulated by
phytohaemagglutinin (Sheldon et al., 1985).
7.6.3. In vivo mammalian assays
Male rats were given a single dose or five consecutive daily
doses by gavage of cyhalothrin at levels of 1.5, 7.5, or 15 mg/kg,
and bone marrow samples were taken and examined for chromosomal
abnormalities. The results indicated that cyhalothrin has no
clastogenic potential (Anderson et al., 1981).
In studies by Irvine (1981), three groups of male mice were
dosed with cyhalothrin by gavage, at dose levels of 1, 5, or 10
mg/kg daily, for 5 consecutive days. A further group received the
known mutagen cyclophosphamide intraperitoneally at 200 mg/kg
daily for five days. The animals were then mated with groups of
females at weekly intervals for eight weeks. Pregnancy incidence,
pre- and post-implantation loss, clinical condition, body weight,
and gross necropsy were assessed. There was no evidence of an
increase in the dominant lethal mutation frequency following
treatment. The NOEL was 10 mg/kg per day. Although these studies
showed no clastogenic or mutagenic effect, it is not clear whether
sufficiently high dose levels were used.
When lambda-cyhalothrin was administered to mice at levels of
up to 35 mg/kg and bone marrow preparations were examined for the
formation of micronuclei in polychromatic erythrocytes, there was
no statistically significant increase in the frequency of
micronuclei, compared to control animals. The positive control
substance, cyclophosphamide, showed the expected response (Sheldon
et al., 1984).
7.7. Mode of action
The mode of action of cyhalothrin and lambda-cyhalothrin, both
type II pyrethroids, is basically the same as that of the other
pyrethroids (see Appendix).
8. EFFECTS ON HUMANS
8.1. General population exposure
No poisoning incidents with cyhalothrin or lambda-cyhalothrin
have been reported. There is no information on effects from short-
or long-term exposure and no epidemiological information is
available.
8.2. Occupational exposure
Cyhalothrin is known to produce an effect described as
subjective facial sensation (SFS) in some people working with this
compound. SFS is a transient phenomenon; symptoms are not
associated with objective physical signs and recovery appears to be
complete. It is likely that SFS arises from direct facial contact
with the chemical particularly from touching the face with
contaminated gloves or hands. This would also help to explain the
effect of formulation or concentration as these could affect the
rate of dermal penetration and therefore access of the chemical to
the nerve endings.
It appears unlikely that individual sensitivity is important
in the development of symptoms following exposure. It is more
likely to be related to the amount of the chemical that comes into
contact with the facial skin. The information currently available
(Hart, 1984) is summarized in section 8.2.2.
8.2.1. Acute toxicity: poisoning incidents
No poisoning incidents involving cyhalothrin or lambda-
cyhalothrin have been reported.
8.2.2. Effects of short- and long-term exposure
Laboratory workers and manufacturing plant and field operators
handling natural and synthetic pyrethroids, including cyhalothrin
and lambda-cyhalothrin, have noticed a transient skin sensation in
the periorbital area of the face and other sites after direct skin
exposure. It has been suggested that these sensations are caused
by spontaneous repetitive firing of sensory nerve fibres or nerve
endings, whose threshold has been transiently lowered by the
compound, localized around the sites of exposure.
Symptoms from exposure to a synthetic pyrethroid generally
start 30 min to 1 h after exposure and last for several hours,
sometimes up to 2 days. They almost always affect the facial area,
producing a tingling, burning, or numb sensation that has been
variously described as paraesthesia, dysaesthesia, or SFS. The
latter term appears most appropriate as no objective signs of
abnormality of nerve function have been found in workers suffering
from these effects and the facial area is by far the most commonly
affected.
8.2.2.1 Manufacture
At least 12 workers involved in the manufacture of cyhalothrin
have experienced symptoms of SFS. The symptoms were described as
burning, tingling, or sunburn, but clinical examination revealed no
abnormal neurological signs. The areas of the face affected were
the eyelids, cheeks, nostrils, forehead, and lips. In one case the
penis was affected, probably from contact with contaminated hands.
The symptoms started between 5 min and 3 h from the onset of
exposure and lasted from 5 to 30 h. Wind, washing, and temperature
increase led to a worsening of the symptoms, and relief could be
obtained by using a local anaesthetic. Barrier creams were
relatively ineffective in preventing the symptoms.
8.2.2.2 Formulation and laboratory work
Two workers have been reported to have suffered from SFS. Both
were exposed to technical cyhalothrin and symptoms began 1-6 h
after exposure. The symptoms described were typical of SFS,
consisting of tingling and burning around the eyes and facial area,
and lasted 6-24 h.
Four reports involving three individuals have been received
from laboratories involved with lambda-cyhalothrin. The symptoms
consisted of a facial tingling and burning sensation and in one
case tingling on the tongue. Symptoms began within 30 min of
exposure and lasted from 6 h to 2 days. All three workers were
involved in handling either technical material or concentrated
lambda-cyhalothrin solutions (10% w/v ai).
8.2.2.3 Field use
Virtually no reports have been received on the agricultural or
public health use of cyhalothrin. The only information that has
become available is from the use of cyhalothrin in animal health
products. Skin itching has been reported following exposure to a
5% emulsifiable concentrate of cyhalothrin, but dilutions of 0.1%
and 0.005% (w/v of ai) were without significant effect. More
recently, several incidents of facial irritation have been reported
following the use of cyhalothrin in sheep sprays (Hart, 1984). The
concentration used in these sprays was very low (0.002% w/v), but
the characteristics of the spray used were unclear.
Out of 38 field trials staff who returned questionnaires, only
four reported experiencing any adverse effects as a result of
exposure to lambda-cyhalothrin. Three of these individuals
experienced a burning sensation or irritation around the face,
cheeks, or eyes, which started between 45 and 60 min after initial
exposure and lasted, respectively, for 5, 18, and 72 h. In one case
the symptoms were severe enough to prevent the individual from
working. The other field trials worker experienced a rash on the
hands that started 24 h after exposure and lasted several days. All
four field trials staff were involved in handling concentrated
solutions of lambda-cyhalothrin (2.5% w/v ai) and three of the four
sprayed diluted solutions. None of the three workers experiencing
the facial sensation had experienced similar symptoms before, but
the worker who developed the rash had suffered a similar rash after
exposure to permethrin, cypermethrin, and deltamethrin following
their application to top fruit. Eight of the other 34 field trials
staff returning questionnaires stated that they had previously
suffered symptoms of facial tingling or burning.
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Cyhalothrin was discussed at the 1984 and 1986 FAO/WHO Joint
Meetings on Pesticide Residues (JMPR), where an acceptable daily
intake (ADI) for cyhalothrin of 0-0.02 mg/kg body weight was
established (FAO/WHO, 1985, 1986a).
The JMPR (FAO/WHO, 1985, 1986a,b) has estimated maximum
residue limits (MRLs) for cyhalothrin (sum of the isomers) as
follows:
---------------------------------------------------------
Commodity Maximum Pre-harvest
residue limit interval (days)
---------------------------------------------------------
Pome fruits 0.2 14
Cabbages, head 0.2 3-4
Potatoes 0.02a non specified
Cotton seed 0.02a 21
Cotton seed oil, crude 0.02a non specified
Cotton seed oil, edible 0.02a non specified
---------------------------------------------------------
a At or about the limit of detection.
REFERENCES
ANDERSON, D., RICHARDSON, C.R., HULME, A., MORRIS, J., BANHAM, P.B., &
GODLEY, M.J. (1981) Cyhalothrin: A cytogenic study in the rat (Unpublished
proprietary report No. CTL/P/664, submitted to WHO by ICI).
BARBER, J.E. (1985) PP321: Acute dermal toxicity study (Unpublished
proprietary report No. CTL/P/1119, submitted to WHO by ICI).
BENGSTONE, M., DAVIES, R.A.H., DESMARCHELIER, J.M., HENNING, R., MURRAY,
W., SIMPSON, B.W., SNELSON, J.T., STICKA, R., & WALLBANK, B.E. (1983)
Organophosphorothioates and synergised synthetic pyrethroids as grain
protectants on bulk wheat. Pestic. Sci., 14: 373-384.
BEWICK, D.W. & ZINNER, C.K.J. (1981) Cyhalothrin: Degradation in soil
(Unpublished proprietary report No. RJ0203B, submitted to WHO by ICI).
BHARTI, H. & BEWICK, D.W. (1986) Cyhalothrin: Degradation in a Japanese
soil (Unpublished proprietary report No. RJ0491B, submitted to WHO by ICI).
BHARTI, H., BEWICK, D.W., & WHITE, R.D. (1985) PP563 and PP321: Degradation
in soil (Unpublished proprietary report No. RJ0382B, submitted to WHO by
ICI).
CALLANDER, R.D. (1984) PP321: An evaluation in the Salmonella mutagenicity
assay (Unpublished proprietary report No. CTL/P/1000, submitted to WHO by
ICI).
CHESTERMAN, H., HEYWOOD, R., ALLEN, T.R., STREET, A.E., PRENTICE, D.,
BUCKLEY, P., & OFFER, J. (1980) PP563: Preliminary oral toxicity study in
beagle dogs (Unpublished proprietary report of the Huntingdon Research
Centre No. 325/8074, submitted to WHO by ICI).
CHESTERMAN, H., HAYWOOD, R., ALLEN, T.R., STREET, A.E., KELLY, D.F.,
GOPINATH, C., & PRENTICE, D.E. (1981) Cyhalothrin oral toxicity study in
beagle dogs (Final report: Repeated daily dosing for 26 weeks) (Unpublished
proprietary report of the Huntingdon Research Centre No. ICI/326/8162,
submitted to WHO by ICI).
COLLEY, J., DAWE, S., HEYWOOD, R., ALMOND, R., GIBSON, W.A., GREGSON, R., &
GOPINATH, C. (1984) Cyhalothrin potential tumorigenic and toxic effects in
prolonged dietary administration to mice (Unpublished proprietary report of
the Huntingdon Research Centre No. ICI 395/83668, submitted to WHO by ICI).
COLLIS, W.M.D. & LEAHEY, J.P. (1984) PP321: Hydrolysis in water at pH 5, 7
and 9 (Unpublished proprietary report No. RJ0338B, submitted to WHO by
ICI).
COULSON, J.M., COLLINS, I.G., & EDWARDS, P.J. (1986) PP321: Effects on
earthworms Lumbricidae of repeated annual field applications (Unpublished
proprietary report No. RJ0511B, submitted to WHO by ICI).
CROSS, M. (1985) PP321: Assessment of mutagenic potential using L5178Y
mouse lymphoma cells (Unpublished proprietary report No. CTL/P/1340,
submitted to WHO by ICI).
CROSSLAND, N.O. (1982) Aquatic toxicology of cypermethrin. II. Fate and
biological effects in pond experiments. Aquat. Toxicol., 2: 205-222.
CROSSLAND, N.O., SHIRES, S.W., & BENNETT, D. (1982) Aquatic toxicology of
cypermethrin. III. Fate and biological effects of spray drift deposits in
freshwater adjacent to agricultural land. Aquat. Toxicol., 2: 253-270.
CURL, E.A., LEAHEY, J.P., & LLOYD, S.J. (1984a) PP321: Aqueous photolysis
at pH 5 (Unpublished proprietary report No. RJ0362B, submitted to WHO by
ICI).
CURL, E.A., LEAHEY, J.P., & LLOYD, D. (1984b) PP321: Photodegradation on a
soil surface (Unpublished proprietary report No. RJ0358B, submitted to WHO
by ICI).
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American Chemical
Society, pp. 229 (ACS Symposium Series 42).
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid. Pestic. Biochem. Physiol., 6: 547-550.
FAO (1982) Report of the Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15 October,
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1985) Cyhalothrin. In: 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agricultural Organization of the United Nations,
pp. 173-185, 571-584 (FAO Plant production and protection Paper 67).
FAO/WHO (1986a) Cyhalothrin: In: 1986 Evaluations of some pesticide
residues in food, Part 1: Residues, Rome, Food and Agriculture Organization
of the United Nations, pp. 95-138 (FAO Plant Production and Protection
Paper 78).
FAO/WHO (1986b) Codex maximum limits for pesticide residues, 2nd ed., Rome,
Codex Alimentarius Commission, Food and Agriculture Organization of the
United Nations (CAC XIII).
FARRELLY, E., HAMER, M.J., & HILL, I.R. (1984) PP321: Toxicity to first
instar Daphnia magna (Unpublished proprietary report No. RJ0359B, submitted
to WHO by ICI).
FARRELLY, E., HAMER, M.J., & HILL, I.R. (1985) PP321: Toxicity of
formulations JF9509 and GFU383C to first instar Daphnia magna (Unpublished
proprietary report No. RJ0426B, submitted to WHO by ICI).
FITZPATRICK, R.D. (1985) PP321 dissipation in US soils - 1983 (Unpublished
report No. TMU1809, submitted to WHO by ICI).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous sensation
between synthetic pyrethroid insecticides. Contact dermatitis, 13: 140-147.
FORBES, S. & DUTTON, A.J. (1985) A small scale method for the determination
of pyrethroid insecticide residues in crops and soils. Pestic. Sci., 16:
404-408.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent class
antagonize GABA action at the crayfish neuromuscular junction. Neurosci.
Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of pyrethroid
action in the cockroach. Pestic. Biochem. Physiol., 15: 181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid toxicology:
Protective effects of Diazepam and phenobarbital in the mouse and the
cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
GIFAP (1981) Technical Monograph No. 4: Guidelines on pesticide residue
trials to provide residue data for the registration of pesticides and the
establishment of maximum residue limits, Brussels, International Group of
National Associations of Manufacturers of Agrochemical Products (GIFAP
D/1981/2537/3).
GIRENKO, D.B. & KLISENKO, M.A. (1984) Concentration and determination of
trace quantities of synthetic pyrethroids in the air. Gig. i Sanit., 3: 57-
59.
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4(6):
793-799.
GOUGH, H.J., COLLINS, I.G., EVERETT, C.J., & WILKINSON, W. (1984) PP321:
Acute contact and oral toxicity to honey bees (Apis mellifera) (Unpublished
proprietary report No. RJ0390B, submitted to WHO by ICI).
GOUGH, H.J., COLLINS, I.G., & WILKINSON, W. (1985) PP321: Field test of
toxicity to honey bees (Apis mellifera) on flowering oil-seed rape
(Brassica napus) (Unpublished proprietary report No. RJ0413B, submitted to
WHO by ICI).
HALL, J.S. & LEAHEY, J.P. (1983) Cyhalothrin: Fate in river water
(Unpublished proprietary report No. RJ0320B, submitted to WHO by ICI).
HAMER, M.J. & HILL, I.R. (1985) Cyhalothrin: The accumulation of
cyhalothrin and its degradation products by channel catfish and
Daphnia magna in a soil/water system (Unpublished proprietary
report No. RJ0427B, submitted to WHO by ICI).
HAMER, M.J., FARRELLY, E., & HILL, I.R. (1985a) PP321: Toxicity to Gammarus
pulex (Unpublished proprietary report No. RJ0414B, submitted to WHO by
ICI).
HAMER, M.J., FARRELLY, E., & HILL, I.R. (1985b) PP321: 21 day Daphnia magna
life-cycle study (Unpublished proprietary report No. RJ0451B, submitted to
WHO by ICI).
HARRISON, M.P. (1981) Cyhalothrin: The disposition and metabolism of [ 14 C]-
146814 in rats (Unpublished proprietary report No. 10/HD/007325, submitted
to WHO by ICI).
HARRISON, M.P. (1983) Cyhalothrin: The metabolism and disposition of 146814
in the rat: Part IV. Isolation and identification of the major urinary
metabolites derived from [14 C-benzyl]- or [14 C-cyclopropyl]-146814
following oral administration (Unpublished proprietary report No.
6/HC/005683, submitted to WHO by ICI).
HARRISON, M.P. (1984a) Cyhalothrin (146814): The metabolism and disposition
of 146814 in rats: Part II. Tissue residues derived from [14 C-benzyl] or
[14 C-cyclopropyl]-146814, after a single oral dose of 1 or 25 mg/kg
(Unpublished proprietary report No. KMR 002/2, submitted to WHO by ICI).
HARRISON, M.P. (1984b) Cyhalothrin: The metabolism and disposition of
[14 C]-146814 in rats: Part III. Studies to determine radioactive residues
in the rat following 14 days repeated oral administration (Unpublished
proprietary report No. 146814 KMR 002/03, submitted to WHO by ICI).
HARRISON, M.P. (1984c) Cyhalothrin (146814): The disposition and metabolism
of [14 C]-146814 in the dog (Unpublished proprietary report No. 146814 KMD
005, submitted to WHO by ICI).
HARRISON, M.P. (1984d) Cyhalothrin (146814): The metabolism, excretion and
residues in the cow after 7 days oral administration with two [14 C]-
labelled forms of 146814 at 1 mg/kg/day (Unpublished proprietary report No.
146814 KMC 006/007, submitted to WHO by ICI).
HART, D., BANHAM, P.B., CHART, I.S., EVANS, D.P., GORE, C.W., STONARD,
M.D., MORELAND, S., GODLEY, M.J., & ROBINSON, M. (1985) PP321: 90-day
feeding study in rats (Unpublished proprietary report No. CTL/P/1045,
submitted to WHO by ICI).
HART, T.B. (1984) Review of adverse skin reactions associated with the use
of synthetic pyrethroids (Unpublished proprietary report No. TMF2530,
submitted to WHO by ICI).
HENDERSON, C. & JACKSON, S.J. (1982) Cyhalothrin: Subacute dermal toxicity
study in rabbits (Unpublished proprietary report No. CTL/P/680, submitted
to WHO by ICI).
HEXT, P.M., BRAMMER, A., CHALMERS, D.T., CHART, I.S., GORE, C.W., PATE, I.,
& BANHAM, P.B. (1986) PP321: 1 year oral dosing study in dogs (Unpublished
proprietary report No. CTL/P/1316, submitted to WHO by ICI).
HILL, R.W. (1981) Determination of the acute toxicity of cyhalothrin
(PP563) to Rainbow trout (Salmo gairdneri) (Unpublished proprietary report
No. BL/B/2097, submitted to WHO by ICI).
HILL, R.W. (1984a) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) (Unpublished proprietary report No. BL/B/2405, submitted
to WHO by ICI).
HILL, R.W. (1984b) PP321: Determination of acute toxicity to Bluegill
sunfish (Lepomis macrochirus) (Unpublished proprietary report No.
BL/B/2406, submitted to WHO by ICI).
HILL, R.W. (1985a) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) of 2.5% w/w formulation (Unpublished proprietary report
No. BL/B/2603, submitted to WHO by ICI).
HILL, R.W. (1985b) PP321: Determination of acute toxicity of a 1 lb/US
gallon EC formulation to Rainbow trout (Salmo gairdneri) (Unpublished
proprietary report No. BL/B/2609, submitted to WHO by ICI).
HILL, R.W. (1985c) PP321: Determination of acute toxicity to Rainbow trout
(Salmo gairdneri) of a 5% EC formulation (Unpublished proprietary report
No. BL/B/2783, submitted to WHO by ICI).
HILL, R.W. (1985d) PP321: Determination of acute toxicity to Mirror carp
(Cyprinus carpio) of a 2.5% w/w formulation (Unpublished proprietary report
No. BL/B/2604, submitted to WHO by ICI).
HILL, R.W. (1985e) PP321: Determination of acute toxicity to Mirror carp
(Cyprinus carpio) of a 5% EC formulation (Unpublished proprietary report
No. BL/B/2784, submitted to WHO by ICI).
HILL, R.W. (1985f) PP321: Determination of acute toxicity of a 1 lb/US
gallon EC formulation to Bluegill sunfish (Lepomis macrochirus)
(Unpublished proprietary report No. BL/B/2610, submitted to WHO by ICI).
HILL, R.W. (1985g) PP321: Determination of acute toxicity to Sheepshead
minnow (Cyprinodon variegatus) (Unpublished proprietary report No.
BL/B/2615, submitted to WHO by ICI).
HILL, R.W., CAUNTER, J.E., & CUMMING, R.I. (1985) PP321: Determination of
the chronic toxicity to Sheepshead minnow (Cyprinodon variegatus) embryos
and larvae (Unpublished proprietary report No. BL/B/2677, submitted to WHO
by ICI).
IRVINE, L.F.H. (1981) Cyhalothrin: Oral (gavage) dominant lethal study in
the male mouse (Unpublished proprietary report of the Hazleton Laboratories
No. 2647-72/213, submitted to WHO by ICI).
JACKSON, S.J. (1981) Cyhalothrin: Eye irritation study in the rabbit
(Unpublished proprietary report No. CTL/T/1502, submitted to WHO by ICI).
JACKSON, S.J. & NIXON, J. (1981) C yhalothrin: Skin irritation studies in
the rabbit and rat (Unpublished proprietary report No. CTL/T/1504,
submitted to WHO by ICI).
KILLICK, M.E. (1981a) Cyhalothrin: Oral (gavage) teratology study in the
rat (Unpublished proprietary report of the Hazleton Laboratories No. 2661-
72/208, submitted to WHO by ICI).
KILLICK, M.E. (1981b) Cyhalothrin: Oral (gavage) teratology study in the
New Zealand white rabbit (Unpublished proprietary report of the Hazleton
Laboratories No. 2700-72/211, submitted to WHO by ICI).
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of pyrethroid
insecticides on the gamma-aminobutyric acid receptor-ionophore complex.
Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding sites.
Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor & Francis
Ltd, 440 pp.
LEAHEY, J.P. & FRENCH, D.A. (1986a) Quantification and characterisation of
radioactive residues found in soya leaves from plants treated with 14 C-
PP321 (Unpublished proprietary report No. RJ0507B, submitted to WHO by
ICI).
LEAHEY, J.P. & FRENCH, D.A. (1986b) Quantification and characterisation of
radioactive residues found in cotton leaves from plants treated with 14 C-
cyclopropane-labelled PP321 (Unpublished proprietary report No. JR0526B,
submitted to WHO by ICI).
LEAHEY, J.P. & FRENCH, D.A. (1986c) Quantification and characterisation of
radioactive residues in cotton leaves from plants treated 14 C-benzyl-
labelled PP321 (Unpublished proprietary report No. RJ0497B, submitted to
WHO by ICI).
LEAHEY, J.P. & PARKER, S. (1985) Cyhalothrin: Characterisation of residues
accumulated by carp continuously exposed to 14 C-cyhalothrin (Unpublished
proprietary report No. RJ0407B, submitted to WHO by ICI).
LEAHEY, J.P., FRENCH, D.A., & HEATH, J. (1985) PP321: Metabolism in a goat
(Unpublished proprietary Report No. RJ0435B, submitted to WHO by ICI).
LINDSAY, S., CHART, I.S., GODLEY, M.J., GORE, C.W., HALL, M., PRATT, I.,
ROBINSON, M., & STONARD, M. (1981) Cyhalothrin: 90-day feeding study in
rats (Unpublished proprietary report No. CTL/P/629, submitted to WHO by
ICI).
LINDSAY, S., DOE, J.E., GODLEY, M.J., HALL, M., PRATT, I., ROBINSON, M., &
STONARD, M.D. (1982) Cyhalothrin induced liver changes: Reversibility study
in male rats (Unpublished proprietary report No. CTL/P/668, submitted to
WHO by ICI).
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel modification
as the basis for the variation in the nerve membrane effects of pyrethroids
and DDT analogs. Pestic. Biochem. Physiol., 20: 203-216.
MILBURN, G.M., BANHAM, P., GODLEY, M.J., PIGOTT, G., & ROBINSON, M. (1984)
Cyhalothrin: Three generation reproduction study in the rat (Unpublished
proprietary report No. CTL/P/906, submitted to WHO by ICI).
MILONE, M.F. (1986) PP321: Unscheduled DNA synthesis in cultured Hela cells
(autoradiographic method) (Unpublished proprietary report of the Institute
di Ricerche Biomediche Antione Marxer No. M914, submitted to WHO by ICI).
MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2922.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare and
the environment. Proceedings of the Fifth International Congress on
Pesticide Chemistry, Kyoto, Japan, 29 August-4 September, 1982, Oxford, New
York, Pergamon Press (Vol. 1-4).
NIXON, J. & JACKSON, S.J. (1981a) Cyhalothrin: Acute toxicity (Unpublished
proprietary report No. CTL/T/1555, submitted to WHO by ICI).
NIXON, J. & JACKSON, S.J. (1981b) Cyhalothrin: Skin sensitisation study in
the guinea-pig (Unpublished proprietary report No. CTL/T/1552, submitted to
WHO by ICI).
PIGOTT, G.H., CHART, I.S., GODLEY, M.J., GORE, C.W., HOLLIS, K.J.,
ROBINSON, M., TAYLOR, K., & TINSTON, D.J. (1984) Cyhalothrin: Two year
feeding study in rats (Unpublished proprietary report No. CTL/P/980,
submitted to WHO by ICI).
PRITCHARD, V.K. (1984) PP321: Skin sensitisation study (Unpublished
proprietary report No. CTL/P/1054, submitted to WHO by ICI).
PRITCHARD, V.K. (1985a) PP321 and cyhalothrin: skin irritation study
(Unpublished proprietary report No. CTL/P/1139, submitted to WHO by ICI).
PRITCHARD, V.K. (1985b) PP321: Eye irritation study (Unpublished
proprietary report No. CTL/P/1207, submitted to WHO by ICI).
PROUT, M.S. (1984) Cyhalothrin: Bioaccumulation in the rat (Unpublished
proprietary report No. CTL/P/1014, submitted to WHO by ICI).
PROUT, M.S. & HOWARD, E.F. (1985) PP321: Comparative absorption study in
the rat (1 mg/kg) (Unpublished proprietary report No. CTL/P/1214, submitted
to WHO by ICI).
REYNOLDS, L.F. (1984) Cyhalothrin: Determination of acute toxicity to
Bluegill sunfish (Lepomis machrochirus) (Unpublished proprietary report No.
BL/B/2514, submitted to WHO by ICI).
RICHOLD, M., ALLEN, J.A., WILLIAMS, A., & RANSOME, S.J. (1981) Cell
transformation test for potential carcinogenicity of Y00102/010/005
(cyhalothrin (PP563)) (Unpublished proprietary report of the Huntingdon
Research Centre No. 391(B)/80948, submitted to WHO by ICI).
ROBERTS, N.L. & FAIRLEY, C. (1981) The acute oral toxicity (LD50) of
cyhalothrin to the Mallard duck (Unpublished proprietary report of the
Huntingdon Research Centre No. 373WL/801024, submitted to WHO by ICI).
ROBERTS, N.L. & FAIRLEY, C. (1984) The acute oral toxicity (LD50) of PP321
to the Mallard duck (Unpublished proprietary report of the Huntingdon
Research Centre No. 438BT/831011, submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., & WOODHOUSE, R.N. (1981a) The subacute dietary
toxicity (LC50 ) of cyhalothrin to the Mallard duck (Unpublished proprietary
report of the Huntingdon Research Centre No. 377WL/8120, submitted to WHO
by ICI).
ROBERTS, N.L., FAIRLEY, C., & WOODHOUSE, R.N. (1981b) The subacute dietary
toxicity (LC50 ) of cyhalothrin to the Bobwhite quail (Unpublished
proprietary report of the Huntingdon Research Centre No. 378WL/8179,
submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., HAKIN, B., PRENTICE, D.E., & WIGHT, D.G.D.
(1982) The acute oral toxicity (LD50 ) and neurotoxic effects of cyhalothrin
to the domestic hen (Unpublished proprietary report of the Huntingdon
Research Centre No. ICI/374 NT/81742, submitted to WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., & DAWE, I.S. (1985a) The subacute
dietary toxicity of PP321 to the Mallard duck (Unpublished proprietary
report of the Huntingdon Research Centre No. ISN 46BT/85171, submitted to
WHO by ICI).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., & DAWE, I.S. (1985b) The subacute
toxicity of PP321 to the Bobwhite quail (Unpublished proprietary report of
the Huntingdon Research Centre No. ISN 45BT/841287, submitted to WHO by
ICI).
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a nerve
muscle preparation of the clawed frog, Xenopus laevis. Pestic. Biochem.
Physiol., 25: 176-187.
SAPIETS, A. (1984a) Cyhalothrin: Storage stability of the residue on deep
frozen apple, cabbage and soil samples (Unpublished proprietary report No.
M3843B, submitted to WHO by ICI).
SAPIETS, A. (1984b) The determination of residues of PP321 in crops. A gas-
liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 81, submitted to WHO by
ICI).
SAPIETS, A. (1984c) The determination of residues of cyhalothrin
metabolites in crops. A gas-liquid chromatographic method (Unpublished
proprietary residue analytical method No. PPRAM 74, submitted to WHO by
ICI).
SAPIETS, A. (1985a) The determination of residues of cyhalothrin in crops.
A gas-liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 70, submitted to WHO by
ICI).
SAPIETS, A. (1985b) The determination of residues of cyhalothrin in water.
A gas-liquid chromatographic method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 69, submitted to WHO by
ICI).
SAPIETS, A. (1985c) PP321: Residue transfer study with dairy cows fed on a
diet containing the insecticide (Unpublished proprietary report No. M3936B,
submitted to WHO by ICI).
SAPIETS, A. (1986a) The determination of residues of PP321 in products of
animal origin. A GLC method using an internal standard (Unpublished
proprietary residue analytical method No. PPRAM 86/1, submitted to WHO by
ICI).
SAPIETS, A. (1986b) The determination of residues of PP321 in soil. A GLC
method using an internal standard (Unpublished proprietary residue
analytical method No. PPRAM 93, submitted to WHO by ICI).
SAPIETS, A., SWAINE, H., & TANDY, M.J. (1984) In: Zweig, G. & Sherma, J.,
ed. Cypermethrin (Chapter 2). Analytical methods for pesticides and plant
growth regulators: Vol XIII, Synthetic pyrethroids and other pesticides,
New York, London, San Francisco, Academic Press.
SHELDON, T., RICHARDSON, C.R., SHAW, J., & BARBER, G. (1984) An evaluation
of PP321 in the mouse micronucleus test (Unpublished proprietary report No.
CTL/P/1090, submitted to WHO by ICI).
SHELDON, T., HOWARD, C.A., & RICHARDSON, C.R. (1985) PP321: A cytogenetic
study in human lymphocytes in vitro (Unpublished proprietary report No.
CTL/P/1333, submitted to WHO by ICI).
SMART, L.E. & STEVENSON, J.H. (1982) Laboratory estimation of toxicity of
pyrethroid insecticides to honeybees: Relevance to hazard in the field. Bee
World, 63(4): 150-152.
SOUTHWOOD, J. (1984) PP321: Acute oral toxicity to the mouse (Unpublished
proprietary report No. CTL/P/1066, submitted to WHO by ICI).
SOUTHWOOD, J. (1985) PP321: Acute oral toxicity studies (Unpublished report
No. CTL/P/1102, submitted to WHO by ICI).
STEVENS, J.E.B. & BEWICK, D.W. (1985) PP563 and PP321: Leaching of PP563
and PP321 and their degradation products in soil columns (Unpublished
proprietary report No. RJ0408B, submitted to WHO by ICI).
TAKEDA CHEMICAL CO. LTD (1979) Test result of fish toxicity of PP-563
(Unpublished proprietary report, submitted to WHO by ICI).
THOMPSON, R.S. (1985) PP321: Determination of acute toxicity to Mysid
shrimps (Mysidopsis bahia) (Unpublished proprietary report No. BL/B/2635,
submitted to WHO by ICI).
THOMPSON, R.S. & WILLIAMS, T.D. (1985) PP321: Toxicity to the green alga
Selenastrum capricornutum (Unpublished proprietary report No. BL/B2584,
submitted to WHO by ICI).
TINSON, D.J., BANHAM, P.D., CHART, I.S., GORE, C.W., PRATT, I., SCALES,
M.D.C., & WEIGHT, T.M. (1984) PP563: 28-day feeding study in rats. Summary
report (Unpublished proprietary report No. CTL/P/1056, submitted to WHO by
ICI).
TRUEMAN, R.W. (1981) Cyhalothrin: Results from the Salmonella reverse
mutation assay (Unpublished proprietary report No. CTL/P/665, submitted to
WHO by ICI).
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies on
the effects of allethrin and DDT on the sodium channels in frog myelinated
nerve membrane. In: Insect neurobiology and pesticide action, London,
Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M. (1973) DDT-
like action of allethrin in the sensory nervous system of Xenopus laevis.
Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A. (1979) Effects of
insecticides on the sensory nervous system. In: Narashashi, T., ed.
Neurotoxicology of insecticides and pheromones, New York, London, Plenum
Publishing Corporation, pp. 183-210.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity relationships
of some pyrethroids in rats. Arch. Toxicol., 45: 325-329.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-dependent effects
of the pyrethroid insecticide decamethrin in frog myelinated nerve fibres.
Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a) Structure-
related effects of pyrethroid insecticides on the lateral-line sense organ
and on peripheral nerves of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel gating in
myelinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN DEN
BERCKEN, J. (1983) Temperature- and structure-dependent interaction of
pyrethroids with the sodium channels in the frog node of Ranvier. Biochem.
Biophys. Acta, 728: 73-82.
WHO (1979) Safe use of pesticides. Third report of the WHO Expert Committee
on Vector Biology and Control, Geneva, World Health Organization (WHO
Technical Report Series, No. 634).
WILLIAMS, T.D. & THOMPSON, R.S. (1981) Determination of the acute toxicity
of cyhalothrin (PP563) to Daphnia magna (Unpublished proprietary report No.
BL/B/2114, submitted to WHO by ICI).
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SAITO, H. (1984a) PP563
(cyhalothrin): Accumulation in fish (carp) in a flow-through water system
(Unpublished proprietary report of MITES No. 58-367, submitted to WHO by
ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984b) PP563
(cyhalothrin) formulation (5% WP): Acute toxicity to carp (Unpublished
proprietary report of MITES No. 58-367, submitted to WHO by ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984c) PP563
(cyhalothrin): Toxicity to daphnia and fish (carp) in the presence and
absence of soil (Unpublished proprietary report of MITES No. 58-367,
submitted to WHO by ICI).
YAMAUCHI, F., SHIGEOKA, T., YAMAGATA, T., & SATO, Y. (1984d) PP563
(cyhalothrin) formulation (5% WP): Acute toxicity to daphnia (Unpublished
proprietary report of MITES No. 58-367, submitted to WHO by ICI).
APPENDIX
On the basis of electrophysiological studies with peripheral
nerve preparations of frogs (Xenopus laevis; Rana temporaria, and
Rana escuelenta), it is possible to distinguish between 2 classes
of pyrethroid insecticides: (Type I and Type II). A similar
distinction between these 2 classes of pyrethroids has been made on
the basis of the symptoms of toxicity in mammals and insects (Van
den Bercken et al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980;
Glickman & Casida, 1982; Lawrence & Casida, 1982). The same
distinction was found in studies on cockroaches (Gammon et al.,
1981).
Based on the binding assay on the gamma-aminobutyric acid
(GABA) receptor-ionophore complex, synthetic pyrethroids can also
be classified into two types: the alpha-cyano-3-phenoxybenzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982;
Gammon & Casida, 1983; Lawrence & Casida, 1983; Lawrence et al.,
1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give
rise to pronounced repetitive activity in sense organs and in
sensory nerve fibres (Van den Bercken et al., 1973). At room
temperature, this repetitive activity usually consists of trains of
3-10 impulses and occasionally up to 25 impulses. Train duration
is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the
insecticide on neurotransmitter release or on the sensitivity of
the subsynaptic membrane, nor on the muscle fibre membrane.
Presynaptic repetitive firing was also observed in the sympathetic
ganglion treated with these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in sensory
nerve fibres, the pyrethroid-induced repetitive activity increases
dramatically as the temperature is lowered, and a decrease of 5 °C
in temperature may cause a more than 3-fold increase in the number
of repetitive impulses per train. This effect is easily reversed
by raising the temperature. The origin of this "negative
temperature coefficient" is not clear (Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve impulse. The
transitional state of the sodium channel is controlled by 2
separately acting gating mechanisms, referred to as the activation
gate and the inactivation gate. Since pyrethroids only appear to
affect the sodium current during depolarization, the rapid opening
of the activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is open,
the activation gate is restrained in the open position by the
pyrethroid molecule. While all pyrethroids have essentially the
same basic mechanism of action, however, the rate of relaxation
differs substantially for the various pyrethroids (Flannigan &
Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation
of the transient increase in sodium permeability of the nerve
membrane during excitation (Van den Bercken & Vijverberg, 1980).
Evidence so far available indicates that allethrin selectively
slows down the closing of the activation gate of a fraction of the
sodium channels that open during depolarization of the membrane.
The time constant of closing of the activation gate in the
allethrin-affected channels is about 100 milliseconds compared with
less than 100 microseconds in the normal sodium channel, i.e., it
is slowed down by a factor of more than 100. This results in a
marked prolongation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is directly
responsible for the repetitive activity induced by allethrin
(Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive
firing, and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral nervous
system of the frog. DDT also causes pronounced repetitive activity
in sense organs, in sensory nerve fibres, and in motor nerve
terminals, due to a prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation.
Recently, it was demonstrated that allethrin and DDT have
essentially the same effect on sodium channels in frog myelinated
nerve membrane. Both compounds slow down the rate of closing of a
fraction of the sodium channels that open on depolarization of the
membrane (Van den Bercken et al., 1973, 1979; Vijverberg et al.,
1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing after-potentials, and evoke repetitive firing with
minimal effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in
sodium permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl
alcohol (deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral line organ in the form of long-
lasting trains of impulses (Vijverberg et al., 1982a). Such a train
may last for up to 1 min and contains thousands of impulses. The
duration of the trains and the number of impulses per train
increase markedly on lowering the temperature. Cypermethrin does
not cause repetitive activity in myelinated nerve fibres. Instead,
this pyrethroid causes a frequency-dependant depression of the
nervous impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of depolarizing
after-potentials during train stimulation (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in
sodium permeability during excitation, presumably by slowing down
the closing of the activation gate of the sodium channel
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983). The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged sodium
current after cypermethrin is too small to induce repetitive
activity in nerve fibres, but is sufficient to cause the long-
lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a long-
lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
cray-fish, the Type II pyrethroids retain sodium channels in a
modified continuous open state persistently, depolarize the
membrane, and block the action potential without causing repetitive
firing (Lund & Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of
the Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant picrotoxin
(PTX). Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin
to rat brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible
relation between the Type II pyrethroid action and the GABA
receptor complex. The stereospecific correlation between the
toxicity of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand, [35S]- t-
butylbicyclophosphorothionate [35S]-TBPS.
Studies with 37 pyrethroids revealed an absolute correlation,
without any false positive or negative, between mouse intracerebral
toxicity and in vitro inhibition: all toxic cyano compounds
including deltamethrin, 1R, cis-cypermethrin, 1R, trans-cypermethrin,
and [2S,alpha]-fenval-erate were inhibitors, but their non-toxic
stereoisomers were not; non-cyano pyrethroids were much less potent
or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies
of pyrethroids were closely related to their mammalian toxicities.
The most toxic pyrethroids of Type II were the most potent
inhibitors of [3H]-Ro 5-4864 specific binding to rat brain
membranes. The [3H]-dihydropicrotoxin and [35S]-TBPS binding
studies with pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an interaction
with a GABA-regulated chloride ionophore-associated binding site.
Moreover, studies with [3H]-Ro 5-4864 support this hypothesis and,
in addition, indicate that the pyrethroid-binding site may be very
closely related to the convulsant benzodiazepine site of action
(Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin and
[2S, alphaS]-fenvalerate) increased the input resistance of
crayfish claw opener muscle fibres bathed in GABA. In contrast,
two non-insecticidal stereoisomers and Type I pyrethroids
(permethrin, resmethrin, allethrin) were inactive. Therefore,
cyanophenoxybenzyl pyrethroids appear to act on the GABA receptor-
ionophore complex (Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type II pyrethroids
(cypermethrin and deltamethrin) induced trains of repetitive
muscle action potentials without presynaptic repetitive activity.
However, an intermediate group of pyrethroids (1R-permethrin,
cyphenothrin, and fenvalerate) caused both types of effect. Thus,
in muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current. But no
clear distinction was observed between non-cyano and alpha-cyano
pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary
target site of pyrethroid insecticides in the vertebrate nervous
system is the sodium channel in the nerve membrane. Pyrethroids
without an alpha-cyano group (allethrin, d-phenothrin, permethrin,
and cismethrin) cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres,
and, in effect, nerve terminals. On the other hand, the alpha-
cyano pyrethroids cause a long-lasting prolongation of the
transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of
repetitive impulses in sense organs and a frequency-dependent
depression of the nerve impulse in nerve fibres. The difference in
effects between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-cyano
group on the phenoxybenzyl alcohol, indicates that it is this
alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation
and conduction are basically the same throughout the entire
nervous system, pyrethroids may also induce repetitive activity in
various parts of the brain. The difference in symptoms of
poisoning by alpha-cyano pyrethroids, compared with the classical
pyrethroids, is not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive activity
in sense organs and possibly in other parts of the nervous system,
which, in a more advance state of poisoning, may be accompanied by
a frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of
the nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane
is also the most important target site of pyrethroids in the
invertebrate nervous system (Wouters & Van den Bercken, 1978; WHO,
1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be
considered restricted to particular animal species, or to a certain
region of the nervous system. Although it has been established that
sense organs and nerve endings are the most vulnerable to the
action of pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions, and on the
chemical structure and physical characteristics of the pyrethroid
molecule (Vijverberg & Van den Bercken, 1982).
RESUME, EVALUATION, CONCLUSIONS, ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques, méthodes d'analyse
La cyhalothrine s'obtient en estérifiant l'acide (chloro-2
trifluoro-3,3,3 propényl-1)-3 diméthyl-2,2 cyclopropanecarb-
oxylique, par l'alcool alpha-cyanophénoxy-3 benzylique et elle
consiste en un mélange de quatre stéréoisomères formant deux paires
d'énantiomères. La lambda-cyhalothrine est l'une de ces paires
d'énantiomères et constitue la forme la plus active biologiquement.
La cyhalothrine de qualité technique est un liquide visqueux
d'un jaune brunâtre (point de fusion environ 10 °C) qui contient
plus de 90% de matière active. Elle est constituée de quatre
isomères cis dans la proportion de 1:1:1:1. Elle est insoluble
dans l'eau mais soluble dans divers solvants organiques, en
particulier les hydrocarbures aliphatiques et aromatiques. Elle
est stable à la lumière et à la chaleur et possède une faible
tension de vapeur.
La lambda-cyhalothrine technique est un solide beige (point de
fusion 49,2 °C) contenant plus de 90% de matière active. Le
rapport des énantiomères (Z) (1R, 3R) de l'ester S aux énantiomères
(Z) (1S, 3S) de l'ester R est de 1:1. Peu soluble dans l'eau, ce
produit est soluble dans divers solvants organiques et possède une
faible tension de vapeur. La cyhalothrine et la lambda-cyhalothrine
sont rapidement hydrolysées en milieu alcalin mais ne subissent
aucune hydrolyse en milieu neutre ou acide.
On dispose de méthodes confirmées pour la recherche et le
dosage des résidus de cyhalothrine et de lambda-cyhalothrine ainsi
que pour l'analyse des prélèvements effectués dans l'environnement
(limite inférieure de détection: 0,005 mg/kg).
1.2 Production et usage
La cyhalothrine a été mise au point en 1977. On l'utilise
principalement pour détruire diverses espèces nuisibles en santé
publique et santé publique vétérinaire ainsi qu'en agriculture
contre les ravageurs des fruits à pépins. La lambda-cyhalothrine
est utilisée essentiellement en agriculture pour la protection des
récoltes les plus variées et fait actuellement l'objet d'une mise
au point en vue d'être utilisée en santé publique.
On ne dispose d'aucune donnée sur les quantités produites.
1.3 Exposition humaine
Les résidus dans les produits alimentaires, par suite du
traitement des récoltes par la cyhalothrine et la lambda-
cyhalothrine ainsi que de leur utilisation en médecine vétérinaire
sont faibles, généralement inférieurs à 0,2 mg/kg. On ignore quel
est l'apport total d'origine alimentaire chez l'homme mais on peut
supposer que l'exposition de la population générale par cette voie
ne dépasse pas la DJA (0,02 mg/kg de poids corporel).
1.4 Exposition et destinée dans l'environnement
A la surface du sol et en solution aqueuse à pH 5, la lambda-
cyhalothrine se décompose sous l'effet du rayonnement solaire avec
une demi-vie d'environ 30 jours. Les principaux produits de
décomposition sont l'acide (chloro-2 trifluoro-3,3,3 propényl-1)-3
diméthyl-2,2 cyclopropanecarboxylique, l'amide correspondant et
l'acide phénoxy-3 benzoïque.
Dans le sol, la dégradation s'effectue principalement par
hydroxylation puis rupture de la liaison ester aboutissant à deux
produits principaux dont la dégradation se poursuit jusqu'à
obtention de dioxyde de carbone. La demi-vie initiale varie de 22 à
82 jours.
La cyhalothrine et la lambda-cyhalothrine sont adsorbées sur
les particules du sol et ne se déplacent pas dans l'environnement.
Sur les végétaux, la lambda-cyhalothrine se décompose à vitesse
modérée (demi-vie allant jusqu'à 40 jours) de sorte que les résidus
sont principalement constitués du composé initial. On trouve
également des métabolites en faibles quantités qui résultent d'un
certain nombre de réactions d'hydrolyse et d'oxydation.
On ne dispose d'aucune donnée sur les quantités effectivement
présentes dans l'environnement mais compte tenu du fait que ce
produit est relativement peu utilisé et à doses réduites, ces
quantités sont vraisemblablement faibles.
1.5 Absorption, métabolisme, et excrétion
Des études métaboliques ont été effectuées sur des rats, des
chiens, des vaches et des chèvres. Chez les rats et les chiens, on
a montré que la cyhalothrine administrée par voie orale était bien
absorbée, fortement métabolisée, puis éliminée sous forme de
conjugués polaires dans les urines. Chez le rat, on a constaté que
le taux de cyhalothrine tissulaire diminuait après cessation de
l'exposition. Dans les carcasses, les résidus étaient faibles
(moins de 5% de la dose au bout de sept jours) et consistaient
presque entièrement en cyhalothrine accumulée dans le tissu
adipeux. Les résidus présents dans les tissus adipeux étaient
éliminés avec une demi-vie de 23 jours.
Après administration par voie orale de cyhalothrine à des
vaches en lactation, on a constaté que le produit était rapidement
éliminé et qu'un état d'équilibre s'établissait au bout de trois
jours entre ingestion et élimination. Vingt-sept pour cent de la
dose étaient excrétés dans les urines, 50% dans les matières
fécales et 0,8% dans le lait. Les produits retrouvés dans les
urines étaient entièrement constitués de métabolites résultant du
clivage de l'ester et de leurs conjugués, alors que 60 à 70% des
produits marqués au 14C retrouvés dans les matières fécales
consistaient en cyhalothrine non métabolisée. Les résidus
tissulaires, mesurés 16 heures après l'administration de la
dernière dose, étaient faibles, la concentration la plus élevée
étant enregistrée dans le tissu adipeux. Les résidus marqués au 14C
retrouvés dans le lait et les tissus adipeux étaient presque
entièrement constitués de cyhalothrine non métabolisée, à
l'exclusion de tout autre composé.
Chez toutes les espèces mammaliennes étudiées, on a constaté
que la cyhalothrine subissait une forte métabolisation par clivage
de l'ester en acide cyclopropanecarboxylique et acide phénoxy-3
benzoïque et qu'elle était éliminée sous forme de conjugués.
Chez les poissons, les principaux résidus tissulaires
consistent en cyhalothrine non métabolisée à côté de quantités plus
faibles de produits résultant du clivage de l'ester.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Dans les conditions du laboratoire où la concentration est
constante, la cyhalothrine et la lambda-cyhalothrine se révèlent
très toxiques pour les poissons et les invertébrés aquatiques. Les
valeurs de la CL50 à 96 heures pour les poissons vont de 0,2 à 1,3
µg/litre, la CL50 à 48 heures pour les invertébrés aquatiques se
situant entre 0,008 et 0,04 µg/litre.
En étudiant au laboratoire l'accumulation de la cyhalothrine à
concentration constante, on a observé une absorption rapide par les
poissons (facteur d'accumulation d'environ 1000 à 2000). Toutefois
lorsqu'il y a un sol et des sédiments en suspension, on constate
une forte réduction du facteur de bioaccumulation, qui passe à 19
pour les poissons et à 194 pour les daphnies. Lorsqu'on remet les
poissons et les daphnies dans de l'eau propre, on constate une
diminution rapide des résidus, avec une demi-vie respective de 7 et
1 jours. Il est vraisemblable qu'en utilisation agricole normale,
la cyhalothrine et la lambda-cyhalothrine subsistent à faible
concentration dans l'eau. Etant donné que ces composés sont
rapidement adsorbés et dégradés dans les conditions naturelles, il
ne se pose en pratique aucun problème d'accumulation de résidus ni
de toxicité vis-à-vis des espèces aquatiques.
La cyhalothrine et la lambda-cyhalothrine sont pratiquement
inoffensives pour les oiseaux; la DL50 (dose unique) s'est révélée
supérieure à 3950 mg/kg pour toutes les espèces étudiées et la CL50
alimentaire à cinq jours la plus faible a été de 3948 mg/kg pour
des canards sauvages de huit jours qui recevaient de la lambda-
cyhalothrine dans leur alimentation.
Dans les conditions du laboratoire, la cyhalothrine et la
lambda-cyhalothrine sont toxiques pour les abeilles; la DL50 par
voie orale est de 0,97 µg de lambda-cyhalothrine par abeille.
Toutefois, le risque est probablement moins élevé en pratique étant
donné que les formulations actuellement en usage exercent une
action répulsive qui empêche les abeilles de butiner sur les
récoltes traitées. Lorsque les abeilles se remettent à butiner, on
ne constate pas d'augmentation importante de la mortalité.
1.7 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
La toxicité aiguë par voie orale de la cyhalothrine est modérée
chez les rats et les souris et faible chez les cobayes et les
lapins (les valeurs de la DL50 sont les suivantes: rat, 144-243
mg/kg; souris, 37-62 mg/kg; cobaye, plus de 5000 mg/kg; lapin, plus
de 1000 mg/kg). La toxicité aiguë par voie orale de la lambda-
cyhalothrine est plus forte que celle de la cyhalothrine (les
valeurs de la DL50 sont les suivantes: 56-79 mg/kg pour le rat et
20 mg/kg pour la souris). Par voie dermique, on obtient les
toxicités suivantes exprimées par la DL50: rat, 200-2000 mg/kg
(cyhalothrine), 632-696 mg/kg (lambda-cyhalothrine); lapin, plus
de 2000 mg/kg (cyhalothrine). La cyhalothrine et la lambda-
cyhalothrine sont des pyréthroïdes du type II avec pour signes
cliniques d'intoxication une ataxie, une démarche chancelante et
une hyperexcitabilité.
Chez le lapin, la cyhalothrine est modérément irritante pour
l'oeil et la lambda-cyhalothrine peu irritante; les deux composés
sont légèrement irritants pour la peau. La cyhalothrine n'est pas
irritante pour la peau chez le rat. Toutefois elle provoque une
sensibilisation cutanée modérée chez le cobaye. La lambda-
cyhalothrine n'a pas d'action sensibilisatrice sur la peau.
Lors d'une étude d'alimentation de 90 jours au cours de
laquelle des rats ont reçu de la cyhalothrine à des doses allant
jusqu'à 250 mg/kg de nourriture, on a observé une réduction du gain
de poids chez les mâles à la dose la plus forte. Des effets
marginaux sur la valeur moyenne de l'hématocrite ont été
enregistrés chez certains des groupes traités ainsi que quelques
modifications au niveau du foie que l'on a considérées comme
résultant d'une réaction d'adaptation. Lors d'une étude de 90
jours au cours de laquelle des rats ont reçu dans leur alimentation
de la lambda-cyhalothrine à des doses allant jusqu'à 250 mg/kg de
nourriture, on a observé une réduction du gain de poids chez les
deux sexes à la dose la plus forte. On a observé un certain nombre
d'effets sur le chimisme sanguin ainsi que des effets hépatiques
analogues à ceux qui avaient été observés avec la cyhalothrine. La
dose sans effet observable était de 50 mg/kg de nourriture.
Lors d'une étude de 26 semaines au cours de laquelle de la
cyhalothrine a été administrée par voie orale à des chiens à des
doses quotidiennes allant jusqu'à 10 mg/kg de poids corporel, on a
observé des signes d'intoxication de type pyréthroïde à la dose la
plus forte. La dose sans effet observable se situait à 2,5 mg/kg
de poids corporel. Une étude du même genre a été menée sur d'autres
chiens avec administration pendant 52 semaines de 3,5 mg de lambda-
cyhalothrine par kg de poids corporel et par jour. Des signes
cliniques d'intoxication de type pyréthroïde (signes neurologiques)
ont été observés chez tous les ani-maux à la dose quotidienne de
3,5 mg/kg de poids corporel. La dose sans effet observable se
situait à 0,5 mg/kg de poids corporel par jour.
Lors d'une étude de toxicité cutanée de 21 jours pratiquée sur
des lapins au moyen d'une solution de cyhalothrine dans le
polyéthylène-glycol à des doses quotidiennes allant jusqu'à 1000
mg/kg de poids corporel, on a constaté des signes cliniques
d'intoxication chez certains des animaux à la dose la plus forte.
Dans tous les groupes, y compris les témoins, on a observé une
irritation cutanée légère à sévère.
Deux études toxicologiques de 104 semaines ont été consacrées à
la cyhalothrine, l'une sur des rats et l'autre sur des souris.
L'étude sur le rat n'a révélé aucun effet oncogène à des doses
allant jusqu'à 250 mg/kg de nourriture (dose la plus forte
étudiée). La dose sans effet observable en ce qui concerne la
toxicité générale était de 50 mg/kg de nourriture (1,8 mg/kg de
poids corporel par jour). On a relevé une diminution du gain de
poids corporel chez les deux sexes à la dose de 250 mg/kg de
nourriture. Aucun effet oncogène n'a été observé chez la souris à
des doses allant jusqu'à 500 mg/kg de nourriture (dose la plus
forte étudiée). Les signes cliniques d'une intoxication de type
pyréthroïde ont été observés aux doses de 100 et 500 mg/kg de
nourriture, avec réduction du gain de poids corporel à cette
dernière dose. En ce qui concerne la toxicité générale, la dose
sans effet observable était de 20 mg/kg de nourriture (1,9 mg/kg de
poids corporel par jour). Aucun signe histologique de lésion du
système nerveux n'a été observé dans l'une ou l'autre de ces deux
études.
Lors d'une série d'épreuves in vivo et in vitro visant à
déceler des mutations géniques, des lésions chromosomiques et
autres effets génotoxiques, on n'a observé aucun effet de ce type
imputable à la cyhalothrine ou à la lambda-cyhalothrine.
Administrée par voie orale à des rats et à des lapins au cours de
la période cruciale de l'organogénèse, la cyhalothrine ne s'est
révélée ni embryotoxique ni tératogène à des doses quotidiennes qui
étaient toxiques pour la mère (15 mg/kg pour les rats et 30 mg/kg
pour les lapins, c'est-à-dire les deux doses les plus fortes
étudiées).
Lors d'une étude de reproduction portant sur trois générations,
des rats ont reçu de la cyhalothrine dans leur alimentation à des
doses allant jusqu'à 100 mg/kg de nourriture. A la dose de 100
mg/kg de nourriture on a observé une légère diminution de la taille
des portées et une réduction peu importante du gain de poids. En
ce qui concerne les effets sur la reproduction, la dose sans effet
observable était de 30 mg/kg de nourriture.
1.8 Effets sur l'homme
Aucun cas d'intoxication accidentelle n'a été décrit.
Des sensations subjectives au niveau de la face ont été
ressenties lors de la fabrication, de la formulation, du travail de
laboratoire et de l'utilisation sur le terrain. Cet effet ne dure
en général que quelques heures mais il arrive qu'il se prolonge
jusqu'à 72 heures après l'exposition; l'examen médical n'a pas
révélé d'anomalies neurologiques.
Ces sensations cutanées subjectives au niveau de la face, qui
sont ressenties par les personnes qui manipulent de la cyhalothrine
ou de la lambda-cyhalothrine sont, semble-t-il, provoquées par
l'excitation répétée des terminaisons nerveuses de la peau. On
peut les considérer comme un signal d'alarme qui indique une
surexposition de l'épiderme.
Rien n'indique que la cyhalothrine et la lambda-cyhalothrine
puissent avoir des effets nocifs pour l'homme lorsqu'on les utilise
dans les conditions et aux doses qui sont recommandées
actuellement.
2. Conclusions
a) Population générale: l'exposition de la population générale à la
cyhalothrine et à la lambda-cyhalothrine est vraisemblablement très
faible et ne présente probablement aucun danger lorsque ces
produits sont utilisés conformément aux recommandations.
b) Exposition professionnelle: moyennant de bonnes méthodes de
travail, des mesures d'hygiène et pour peu que l'on respecte les
précautions nécessaires, la cyhalothrine et la lambda-cyhalothrine
ne présentent vraisemblablement aucun danger pour les personnes
exposées de par leur profession.
c) Environnement: il ne semble pas que la cyhalothrine, la lambda-
cyhalothrine ou leurs produits de dégradation puissent atteindre
des concentrations nocives pour l'environnement lorsqu'elles sont
utilisées aux doses recommandées. Dans les conditions du
laboratoire, la cyhalothrine et la lambda-cyhalothrine sont très
toxiques pour les poissons, les arthropodes aquatiques et les
abeilles. Toutefois, en pratique, il ne semble pas qu'il puisse se
produire d'effets nocifs durables, lorsque ces insecticides sont
utilisés conformément aux recommandations.
3. Recommandations
Lorsque ces insecticides sont utilisés conformément aux
recommandations, leurs concentrations dans les denrées alimentaires
doivent être très faibles, toutefois il serait bon de le confirmer
en soumettant la cyhalothrine et la lambda-cyhalothrine à une
surveillance.
La cyhalothrine et la lambda-cyhalothrine sont utilisées
depuis des années et seuls des effets passagers ont été observés
lors d'expositions professionnelles; toute-fois il faut continuer à
surveiller l'exposition humaine.
RESUMEN, EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades físicas y químicas, y métodos
analíticos
La cihalotrina se obtiene esterificando ácido 3-(2-cloro-3,3,3-
trifluoroprop-1-enil)-2,2-dimetilciclopropanocarboxílico con
alcohol alpha-ciano-3-fenoxibencílico y se compone de una mezcla de
cuatro estereoisómeros. La lambda-cihalotrina se compone de un par
enantiomérico de isómeros y es la forma más activa biológicamente.
La cihalotrina de calidad técnica es un líquido viscoso, de
color entre amarillo y marrón (punto de fusión: aproximadamente 10
°C), que contiene más del 90% de material activo. Está compuesta
por cuatro isómeros cis en proporción 1:1:1:1. Aunque es insoluble
en el agua, es soluble en toda una gama de disolventes orgánicos
como los hidrocarburos alifáticos y aromáticos. Es estable respecto
de la luz y la temperatura y tiene una baja tensión de vapor.
La lambda-cihalotrina de calidad técnica es un sólido de color
café con leche (punto de fusión: 49,2 °C) que contiene más del 90%
de material activo. La razón enantiomérica del ( Z), (1R, 3R), S-
éster y el ( Z), (1S, 3S), R-éster es 1:1. Es apenas soluble en el
agua pero es soluble en toda una gama de disolventes orgánicos y
tiene una baja tensión de vapor. Tanto la cihalotrina como la
lambda-cihalotrina se hidrolizan rápidamente en medio alcalino pero
no en medio neutro o ácido.
Existen métodos bien establecidos para analizar la cihalotrina
y la lambda-cihalotrina presentes en residuos y en el medio
ambiente (la concentración detectable mínima es 0,005 mg/kg).
1.2 Producción y utilización
La cihalotrina comenzó a producirse en 1977. Se utiliza sobre
todo para combatir múltiples plagas en salud pública y en
veterinaria, pero también se emplea en agricultura contra las
plagas que atacan a los pomos. La lambda-cihalotrina se usa
principalmente como plaguicida agrícola aplicable a muy diversos
cultivos y se está preparando para emplearla en salud pública.
No se dispone de datos sobre los niveles de producción.
1.3 Exposición humana
Los residuos presentes en los alimentos de resultas de la
aplicación de cihalotrina y lamba-cihalotrina a los cultivos y en
veterinaria son bajos, por lo general inferiores a 0,2 mg/kg. No
se dispone de datos sobre la ingesta total en la dieta humana, pero
se puede suponer que la exposición dietética de la población
general no supera la IDA (0,02 mg/kg de peso corporal).
1.4 Exposición y transformación en el medio ambiente
En la superficie del suelo y en soluciones acuosas con un pH de
5, la lambda-cihalotrina se degrada a la luz del sol con una
semivida de unos 30 días. Los principales productos de esa
degradación son ácido 3-(2-cloro-3,3,3-trifluoroprop-1-enil)-2,2-
dimetilciclopropanocarboxílico, el derivado amídico de la
cihalotrina, y ácido 3-fenoxibenzoico.
La degradación en el suelo se produce primordialmente por
hidroxilación seguida de la ruptura del enlace éster para dar lugar
a dos principales productos de degradación, que siguen degradándose
hasta convertirse en bióxido de carbono. Las semividas iniciales
oscilan entre 22 y 82 días.
La cihalotrina y la lambda-cihalotrina se adsorben a las
partículas del suelo y no son móviles en el medio ambiente.
En las plantas, la lambda-cihalotrina se degrada a un ritmo
moderado (semivida de hasta 40 días), por lo que el principal
elemento constituyente de los residuos que en ellas se encuentran
es por lo común el compuesto inicial. Se hallan también niveles más
bajos de metabolitos, resultantes de diversas reacciones
hidrolíticas y oxidantes.
No se dispone de datos sobre los niveles efectivos en el medio
ambiente, pero dadas la escasa utilización habitual en la
actualidad y las reducidas tasas de aplicación, se supone que son
bajos.
1.5 Incorporación, metabolismo y excreción
Se han realizado estudios metabólicos en la rata, el perro, la
vaca y la cabra. En la rata y el perro se ha demostrado que la
cihalotrina administrada por vía oral se absorbe y se metaboliza
bien y se elimina en la orina, en forma de conjugados polares. En
los tejidos de las ratas, disminuyó la concentración de cihalotrina
al interrumpirse la exposición al compuesto. En los cadáveres de
las ratas se encontraron niveles reducidos de residuos (< 5% de la
dosis a los siete días), que se debían casi totalmente a la
cihalotrina contenida en las grasas. Los restos presentes en las
grasas se eliminaron con una semivida de 23 días.
La cihalotrina administrada por vía oral a vacas lactantes se
eliminó rápidamente, alcanzándose un equilibrio entre la ingestión
y la eliminación a los tres días. De la dosis total, el 27% se
excretó en la orina, el 50% en las heces, y el 0,8% en la leche.
El material presente en la orina estaba compuesto exclusivamente
por metabolitos de la disociación de los ésteres y por sus
conjugados, mientras que del 60% al 70% del material fecal marcado
con [14C] se identificó como cihalotrina no alterada. Dieciséis
horas después de la última dosis, los residuos tisulares eran
bajos, hallándose las concentraciones más elevadas en las grasas.
Los residuos marcados con [14C] presentes en la leche y los tejidos
adiposos constaban casi enteramente de cihalotrina no alterada, y
no se detectó ningún otro componente.
En todas las especies de mamíferos investigadas, se ha
descubierto que la cihalotrina se metaboliza ampliamente de
resultas de la ruptura del enlace éster para dar ácido
ciclopropanocarboxílico y ácido 3-fenoxibenzoico, y se elimina en
forma de conjugados.
En los peces, el principal residuo presente en los tejidos es
cihalotrina no alterada y hay niveles más bajos de los productos de
la ruptura del éster.
1.6 Efectos en los organismos del medio ambiente
En condiciones de laboratorio con concentraciones tóxicas
constantes, la cihalotrina y la lambda-cihalotrina son muy tóxicas
para los peces y los invertebrados acuáticos. El valor de las CL50
a las 96 horas para los peces oscila entre 0,2 y 1,3 µg/litro,
mientras que en el caso de los invertebrados acuáticos los valores
de la CL50 a las 48 horas van de 0,008 a 0,4 µg/litro.
Los estudios sobre la acumulación realizados en condiciones de
laboratorio con concentraciones constantes indican que, en los
peces, tiene lugar una rápida incorporación (factor de acumulación
aproximado de 1000 a 2000). No obstante, en presencia de suelo y
sedimentos en suspensión, los factores de bioacumulación quedan muy
reducidos (a 19 en el caso de los peces y a 194 en el caso de los
dáfnidos). Cuando se colocó en agua limpia a los peces y dáfnidos
expuestos, los residuos disminuyeron rápidamente, con semividas de
siete días y un día, respectivamente. Las concentraciones de
cihalotrina y lambda-cihalotrina que pueden hallarse en el agua de
resultas de su aplicación normal con fines agrícolas serán bajas.
Como el compuesto se adsorbe y se degrada rápidamente en
condiciones naturales, la acumulación de residuos o la toxicidad de
la cihalotrina y la lambda-cihalotrina para las especies acuáticas
no plantearán problemas prácticos.
La cihalotrina y la lambda-cihalotrina son prácticamente
inocuas para las aves; en todas las especies sometidas a prueba la
DL50 con una dosis única fue superior a 3950 mg/kg y la CL50
dietética más baja en cinco días fue de 3948 mg/kg (lambda-
cihalotrina administrada en el alimento a patos silvestres de ocho
días de edad).
En condiciones de laboratorio, la cihalotrina y la lambda-
cihalotrina son tóxicas para la abeja; la DL50 de la lambda-
cihalotrina por vía oral es de 0,97 µg/abeja. No obstante, en la
práctica, el riesgo es menor puesto que las formas actualmente
utilizadas tienen un efecto repelente que hace que las abejas
suspendan las actividades de búsqueda en los cultivos tratados.
Cuando éstas vuelven a emprenderse, no hay un aumento significativo
de la mortalidad de las abejas.
1.7 Efectos en animales de experimentación y en sistemas de
prueba in vitro
La toxicidad aguda de la cihalotrina administrada por vía oral
es moderada para las ratas y los ratones y baja para las cobayas y
los conejos (los valores de la DL50 son los siguientes: rata: 144-
243 mg/kg; ratón: 37-62 mg/kg; cobaya: > 5000 mg/kg; conejo: >
1000 mg/kg). La toxicidad aguda de la lambda-cihalotrina por vía
oral es mayor que la de la cihalotrina (los valores de la DL50 son:
56-79 mg/kg para la rata y 20 mg/kg para el ratón). La toxicidad
por vía cutánea (DL50) alcanza los siguientes valores: rata: 200-
2000 mg/kg (cihalotrina), 632-696 mg/kg (lambda-cihalotrina);
conejo: > 2000 mg/kg (cihalotrina). La cihalotrina y la lambda-
cihalotrina son piretroides de tipo II; entre los signos clínicos
figuran la ataxia, el andar vacilante y la hiperexcitabilidad.
En el conejo, la cihalotrina es un irritante ocular moderado y
la lambda-cihalotrina un irritante ocular leve; ambos irritan
levemente la piel. La cihalotrina no irrita la piel de la rata.
Sin embargo, sensibiliza moderadamente la piel de la cobaya. La
lambda-cihalotrina no sensibiliza la piel.
En un estudio de alimentación de 90 días en el que se
administró cihalotrina a ratas en dosis de hasta 250 mg/kg de
alimento, se observó una disminución del aumento del peso corporal
en los machos con la dosis de 250 mg. En algunos de los grupos
tratados, se señalaron efectos marginales en los volúmenes medios
de eritrocitos, así como ciertas alteraciones hepáticas, que se
consideraron una respuesta adaptativa. En otro estudio de
alimentación de 90 días, en el que se administró lambda-cihalotrina
a ratas en dosis de hasta 250 mg/kg de alimento, se observó en
ambos sexos una reducción del aumento del peso corporal con la
dosis de 250 mg. Se señalaron también algunos efectos en la
química clínica, así como efectos hepáticos análogos a los
observados con la cihalotrina. El nivel sin efecto observado fue
de 50 mg/kg de alimento.
En un estudio de 26 semanas en el que se administraron a perros
por vía oral dosis diarias de cihalotrina de hasta 10 mg/kg de peso
corporal, se observaron signos de toxicidad por piretroides con la
dosis de 10 mg. El nivel sin efecto observado fue de 2,5 mg/kg de
peso corporal al día. Se realizó otro estudio similar en el que se
administraron a perros, durante 52 semanas, dosis diarias de
lambda-cihalotrina de hasta 3,5 mg/kg de peso corporal. En todos
los animales que recibieron la dosis de 3,5 mg se observaron signos
clínicos de toxicidad por piretroides (signos neurológicos). El
nivel sin efecto observado fue de 0,5 mg/kg de peso corporal al
día.
En un estudio cutáneo de 21 días con conejos a los que se
aplicó cihalotrina en polietilénglicol en dosis diarias de hasta
1000 mg/kg, se observaron en algunos animales signos clínicos de
toxicidad con la dosis más alta. En todos los grupos, incluidos
los testigos, se señaló irritación de la piel de leve a grave.
Se realizaron dos estudios de alimentación de 104 semanas con
cihalotrina, uno con ratas y otro con ratones. En el estudio con
ratas, no se observaron efectos oncogénicos con dosis de hasta 250
mg/kg de alimento (la dosis más alta ensayada). En cuanto a la
toxicidad sistémica, el nivel sin efecto observado fue de 50 mg/kg
de alimento (1,8 mg/kg de peso corporal al día). Con dosis de 250
mg se señaló en ambos sexos una disminución del aumento del peso
corporal. En el estudio con ratones, no se observaron efectos
oncogénicos con dosis de hasta 500 mg/kg de alimento (la dosis más
alta ensayada). Con dosis de 100 y 500 mg/kg de alimento se
apreciaron signos clínicos de toxicidad por piretroides y, con
dosis de 500 mg, una disminución del aumento del peso corporal. En
cuanto a la toxicidad sistémica, el nivel sin efecto observado fue
de 20 mg/kg de alimento (1,9 mg/kg de peso corporal al día). En
ninguno de los dos estudios se obtuvieron datos histológicos que
indicaran la presencia de lesiones del sistema nervioso.
En una serie de pruebas in vivo e in vitro destinadas a
detectar mutaciones génicas, lesiones cromosómicas y otros efectos
genotóxicos, se obtuvieron resultados negativos con la cihalotrina
y la lambda-cihalotrina. La cihalotrina administrada por vía oral
a ratas y conejos durante el periodo de organogénesis principal no
resultó embriotóxica ni teratogénica en dosis que provocaron
reacciones tóxicas en las madres (15 mg/kg diarios para las ratas y
30 mg/kg diarios para los conejos, en ambos casos los niveles más
altos ensayados).
Se realizó un estudio sobre la reproducción en tres
generaciones de ratas a las que se administró cihalotrina en dosis
de hasta 100 mg/kg de alimento. Con la dosis de 100 mg se
observaron pequeñas reducciones del tamaño de las camadas y del
aumento de peso. En los aspectos relacionados con la reproducción,
el nivel sin efecto observado fue de 30 mg/kg de alimento.
1.8 Efectos en el ser humano
No se ha descrito ningún caso de intoxicación accidental.
En las actividades de fabricación, formulación y laboratorio y
durante el uso sobre el terreno, se han notificado sensaciones
faciales subjetivas. Este efecto sólo suele durar unas horas, pero
ocasionalmente persiste hasta 72 horas después de la exposición; el
examen médico no ha revelado ninguna anomalía neurológica.
Se cree que las sensaciones cutáneas faciales subjetivas que
pueden experimentar las personas que manipulan cihalotrina y
lambda-cihalotrina se deben a la repetida estimulación de las
terminaciones nerviosas de la piel. Pueden considerarse una señal
de alerta que indica que la piel ha sufrido una exposición
excesiva.
No hay ninguna indicación de que la cihalotrina y la lambda-
cihalotrina, utilizadas en las condiciones y con arreglo a las
tasas de aplicación actualmente recomendadas, tengan efectos
adversos en los seres humanos.
2. Conclusiones
(a) Población general: Se cree que la exposición de la población
general a la cihalotrina y la lambda-cihalotrina es muy baja y que
no es probable que represente un riesgo en las condiciones de uso
recomendadas.
(b) Exposición ocupacional: Con prácticas de trabajo, medidas de
higiene y precauciones de seguridad adecuadas, es poco probable que
la cihalotrina y la lambda-cihalotrina representen un riesgo para
las personas ocupacionalmente expuestas.
(c) Medio ambiente: No es probable que la cihalotrina y la lambda-
cihalotrina o los productos de su degradación alcancen niveles
ambientales que puedan producir efectos adversos, si se respetan
las tasas de aplicación recomendadas. En condiciones de
laboratorio, la cihalotrina y la lambda-cihalotrina son muy tóxicas
para los peces, los artrópodos acuáticos y las abejas. No
obstante, en la práctica, no es probable que se produzcan efectos
adversos duraderos en las condiciones de uso recomendadas.
3. Recomendaciones
Aunque se cree que los niveles dietéticos según el uso
recomendado son muy bajos, debe considerarse la posibilidad de
confirmar este extremo incluyendo la cihalotrina y la lambda-
cihalotrina en estudios de vigilancia.
Pese a que ambas sustancias se llevan utilizando varios años y
a que los efectos observados de la exposición ocupacional sólo han
sido transitorios, debe continuar la observación de la exposición
humana.
